
d-PHENOTHRIN
EXPLANATION
Phenothrin was evaluated by the Joint Meeting in 1979 and 1980
and a temporary ADI was estimated in 1980 (Annex I, FAO/WHO 1980b,
1981b). It was reviewed in 1982 and 1984 (Annex I, FAO/WHO 1983b,
1985c). The rodent reproduction, chronic toxicity and oncogenicity
studies evaluated in 1980 were performed by Industrial Bio-Test
Laboratories (IBT). Repeat studies were required to be submitted by
1988. In 1984 a temporary ADI for d-phenothrin was estimated based on
6-months rat and dog studies. Data were submitted indicating that
metabolism and toxicity were similar for phenothrin and d-phenothrin
and that data for phenothrin can be used to support d-phenothrin. The
required data and some additional data have been submitted and are
summarized in this monograph addendum.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Rats
Studies were done using the 1R,cis- and 1R,trans- isomers
of phenothrin. Groups of 5 rats/sex were given either 14C-(1R,
Trans)-phenothrin or 14C-(1R, cis)-phenothrin as:
(1) a single oral dose at 4 mg/kg bw;
(2) a single oral dose at 200 mg/kg bw; or
(3) a single oral dose at 4 mg/kg bw 24 hours after the last of
14 daily oral doses of unlabelled material at the same
dose level.
With single doses of the trans- isomer at 4 or 200 mg/kg bw, 56-69%
of the administered radioactivity was recovered in faeces with 25-40%
in urine by 7 days after dosing. Following repeated doses with 4 mg/kg
bw, faecal excretion within 7 days decreased to 24-29% while urinary
excretion increased to 70-75%. With the cis- isomer, 80-87% of
single doses (4 or 200 mg/kg bw) was excreted in faeces and 11-18% in
urine within 7 days. Following repeated doses the amount excreted in
faeces decreased slightly to 72%, with 24% in urine. No sex
differences were apparent with either isomer.
Tissue levels were generally low under all the dosing regimens of
this study. The highest tissue residues were in fat. With the trans-
isomer fat residues were 2-10% lower than those with the cis-
isomer. Skin with hair, and carcass had the next highest residues -
possibly due to residual fat. Levels in other tissues were less than
10 ng phenothrin equivalents/gm tissue with single and repeated low
doses and less than 0.6 µg/gm tissue for the single high dose.
Fat levels were higher following repeated doses of 4 mg/kg bw
than following a single dose at this level with both isomers. Females
showed a larger increase than males, having lower tissue residues
following a single dose and higher tissue residues than males
following repeated doses (Isobe et al., 1987).
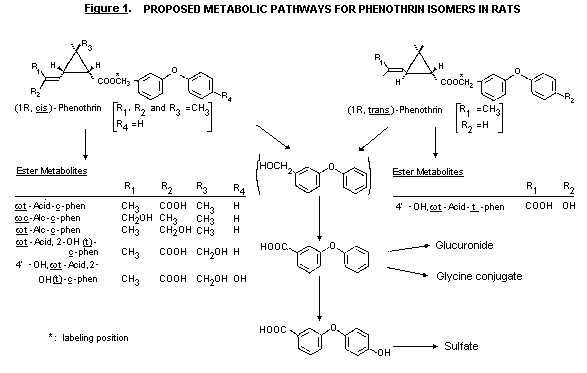
Biotransformation
The metabolism of the 1R,cis- and 1R,trans- isomers of
phenothrin was studied in the rat. In faeces a major proportion of the
excreted radioactivity was intact phenothrin. The authors stated that
this was considered to be unabsorbed material since intact phenothrin
is not excreted in bile. Phenothrin in faeces accounted for 14-16%
with the trans- and 17-25% with the cis- isomer of the repeated
4 mg/kg bw dose; 44-45% (trans) an 41-44% (cis) of the single
4 mg/kg bw dose; and 44-60% (trans.) and 50-59% (cis) of the
single 200 mg/kg bw dose.
Five faecal metabolites were identified for the cis- isomer.
These retained the ester linkage and were derived from oxidation
involving the alcohol moiety, the isobutenyl moiety and cyclopropane
ring of the acid moiety. These metabolites accounted for 0.8-9.2% of
the 14C dose.
Most of the urinary metabolites were derived following ester
cleavage. With both isomers a major metabolite was 4-OH-PB
acid-sulfate, accounting for 15-55% and 7-18% of the dose of
14C given as trans- and cis- isomer, respectively, by the three
dose regimens in both sexes. A metabolic pathway was proposed
(Isobe et al., 1987). (See Figure 1.)
Toxicological studies
Special studies on carcinogenicity
Mice
Groups of 50 male and 50 female B6C3F1 SPF mice were fed diets
containing 0, 300, 1000 or 3000 ppm d-phenothrin for 104 weeks.
Clinical observations, body weight gain, and food consumption were
recorded throughout the study. Ophtalmoscopic observations and urinary
analyses were performed at 6, 12, 18 and 24 months. At 104 weeks,
blood samples were taken from 10 animals/sex/dose for haematological
and clinical chemistry examinations. All survivors at 104 weeks were
sacrificed and organ weights were recorded. Histopathological
examination of about 40 tissues/organs was performed for all animals
sacrificed or dying during the study. Additional groups of 10, 10 and
20 mice/sex/dose were fed the same diets for 26, 53 or 78 weeks,
respectively. Blood samples were taken from 10 animals/sex/dose at
each of these time periods for haematological and blood chemistry
determinations. Organ weights were recorded at necropsy.
Histopathological examination was performed on about 40 tissues/organs
from mice sacrificed at 53 weeks.
Mortality was 2-6% at 78 weeks and 16-28% at termination in the
main study. The pattern of mortality did not show any relationship to
treatment. Effects due to treatment were reduced body weight gains in
males and increased liver/body weight ratios in both sexes in mice
given diet containing 3000 ppm d-phenothrin. Histopathologically,
"hepatocytic hypertrophy" (large hepatocytes) was observed in males
and females at 3000 ppm after 53 weeks of treatment but was not
observed to a significant degree after 104 weeks' treatment. This
observation may, therefore, have been of an adaptive effect.
Paradoxical changes in kidney/body weight ratios were observed at
3000 ppm: reduced in males, increased in females. There were no
histopathological changes in kidney to explain these differences. No
tumour type was observed to be statistically significantly increased
in any group. There was a slightly higher incidence of hepatocytic
adenomas and carcinomas in treated groups, but the incidences were not
strictly dose-related. There was also a slight increase in reported
metastases of hepatic carcinomas in males and females at 1000 and
3000 ppm. Most of the observed metastases were located only in lung.
Although the incidence of tumours did not indicate a carcinogenic
response, it would be valuable to have the metastatic lesions in lung
confirmed.
In this study no effects were observed at the lowest dose level
of 300 ppm (equal to 40.3 mg/kg bw/day) (Amyes et al., 1987).
Rats
Groups of 80 male and 80 female F344 SPF rats/dose level were
given diet containing d-phenothrin at levels of 0, 300, 1000 or
3000 ppm for 105 (males) or 118 (females) weeks. The rats were
separated into replicate groups of 40 animals/sex/dose to minimise
bias. Five rats/sex/dose/replicate were sacrificed at 52 weeks for
interim evaluation. Throughout the study clinical observations, body
weight gains, food consumption and ophthalmoscopic observations were
monitored. Blood samples were taken from 5 rats/sex/group/replicate on
weeks 26, 50, 78 and 104 for haematological and clinical chemistry
examinations. Urine samples were collected from the same animals for
analysis. Surviving males were sacrificed on week 106 and females on
week 119. Organ weights were recorded for these animals. All rats
which died or were sacrified were necropsied. Histopathological
examinations of about 40 tissues from 50 rats/sex/dose level dying
prior to or sacrificed at termination and from the 10 rats/sex/dose
sacrificed at 52 weeks were performed.
 Mortality was 42-56% in males and 14-36% in females at 104 weeks.
There was no increase in mortality as a result of treatment. There was
a slight but statistically significant reduction in body weight gain
during the first year of the study in females given 3000 ppm. This
group also had reduced alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels at weeks 25, 49 and 77. Males given
3000 ppm had reduced ALT activity at week 49 only. Liver/body weight
ratio was increased in females given 3000 ppm at week 52 only and in
males given 3000 ppm at weeks 52 and 105 (statistically significant
only at week 105). Histopathologically the only observation of note
was a slight increase in the incidence of hepatocytic hypertrophy in
males given 3000 ppm. There were no statistically significant
increases in the incidences of any tumour type. However, there were
increases in the incidence of preputial gland adenomas and carcinomas
in males. The incidence at 0, 300, 1000 and 3000 ppm were 1, 1, 1 and
4 adenomas and 0, 0, 1 and 3 carcinomas, respectively.
In this study only the left preputial gland was examined
routinely for histopathology. The right preputial gland was examined
only when there were gross observations. However, the three carcinomas
in the high dose group were apparently not detected grossly and,
therefore, there may have been additional tumours in the right glands
that were not detected. Pathologists studying this gland have reported
that small tumours of the preputial gland cannot be detected when only
gross lesions are examined (Resnick & Ward, 1981). Incidences of
preputial tumours in F344 rats which have been published for the NTP
program in the United States indicate incidences of 2.2% with adenomas
and 2.7% with carcinomas among 2,320 control males in 2-year studies
and 0.4% with adenomas and 4.7% with carcinomas in 529 males in a
lifespan study (Solleveld et al., 1984). In one NTP study a total of
7 preputial gland tumours were observed in a group of 50 males
(untreated) but it is not indicated how many of the tumours were
adenomas or carcinomas (Haseman, 1983). The procedure for examining
this gland was not given in either of these studies. It appears likely
that in this gland adenomas progress to carcinomas (Resnick & Ward,
1981).
On the basis of this information the observed incidences of these
tumours do not appear of concern. However, it would be of interest to
have the preputial glands reexamined to determine the true incidence
of tumours in this study. In this study there was no apparent effect
at the 1000 ppm dose level (equivalent to 50 mg/kg bw/day) on any of
the examined parameters (Martin et al., 1987).
Special study on mutagenicity
The ability of d-phenothrin to cause chromosomal aberrations in
Chinese hamster ovary cells was tested in vitro. Based on the
results of a cytotoxicity test, 4 concentrations (2 × 10-5 to
2 × 10-4 M without S-9 mix, and 5 × 10-5 to 5 × 10-4 with S-9
mix) were tested. There was no increase above control levels in the
incidence of chromosomal aberrations at any of the dose levels tested.
Positive controls significantly increased the number of aberrations
observed (Kogiso et al., 1986).
The ability of d-phenothrin to induce unscheduled DNA synthesis
was tested in human cells (HeLa S3). Because no cytotoxicity was noted
with dose levels up to 4 mg/ml, concentrations tested were 0.25 to
4 mg/ml. No significant increases in uptake of tritiated thymidine
were observed following treatment with d-phenothrin in contrast to the
results with the positive controls. There was no evidence, under the
conditions of this test, that d-phenothrin induced unscheduled DNA
synthesis (Forster et al., 1984).
Special study on reproduction
Rats
In a two-generation reproduction study, groups of 30 male and 30
female Charles River CD (Sprague-Dawley-derived) rats were given diets
containing 0, 300, 1000 or 3000 ppm d-phenothrin (92.9% pure) during
growth, mating, gestation and lactation for 2 litters per generation.
The rats were mated on a 1:1 basis after 91 days of dietary exposure
to produce the F1a litter. The rats were subsequently remated to
produce the F1b litter. From the F1b litter 30 males and 30
females were selected to be parents to the F2a and F2b litters.
From the F2b litter 1 male and 1 female/litter/dose level were
maintained on test for 13 weeks.
Body weight gains of F0 rats given 3000 ppm were slightly lower
than controls during the pre-mating period. The difference in body
weight was maintained throughout the study but did not increase with
time. In the F1 parents there was a slight reduction in body weight
gain up to week 13 (pre-mating) at 3000 ppm which was maintained in
males but females approached control values during the second mating.
The F2b pups maintained for 13 weeks showed no effect on body weight
gain. There were no treatment-related effects on food consumption,
water intake, regularity of oestrus cycle, or reproductive performance
(fertility, gestation, litter size, viability, pup sex and weight).
Development of the F1b and F2b pups was normal as indicated by
auditory and visual responses, pinna unfolding, hair growth, tooth
eruption and eye opening. Liver/body weight ratios were increased in
F0 adult females, F1 adult females and F2 weanling males and
females given diet containing 3000 ppm of d-phenothrin. There was no
consistent histopathological change suggestive of a treatment-related
effect. In this study the first mating (F0->F1a) was mainly
consanguinous. There were no apparent differences in the results of
these matings and the subsequent non-consanguinous matings. The
offspring of this mating were not further used in the study so the
departure from protocol did not invalidate the study as a whole. The
authors concluded that d-phenothrin had no effect on reproductive
performance or development of Charles River rats at dietary levels of
up to 3000 ppm (equivalent to 150 mg/kg bw/day) over 2 generations.
The NOEL for parental effects was 1000 ppm (equivalent to 50 mg/kg
bw/day) (Tesh et al., 1987).
Short-term studies
Rats
Groups of 20 Sprague-Dawley weanling rats/sex/dose level were
given diets containing d-phenothrin (92.9% pure) at levels of 0, 1000,
3000 or 10000 ppm for 6 months. Additional groups of 10 rats/sex/dose
level were given the same diets for 3 months. No deaths occurred and
no clinical signs of toxicity or opthalmoscopic effects were observed.
A dietary level of 10000 ppm caused depressed body weight gain in the
first 3 months of feeding in both sexes, slight reduction of RBC
count, haematocrit and haemoglobin at 3 and 6 months in males, reduced
serum cholinesterase at 3 and 6 months in females and increased
liver/bw, kidney/bw and adrenal/bw ratios in both sexes. At 3000 ppm
liver/bw ratio was increased in both sexes at 3 and 6 months,
kidney/bw was increased in males at 3 months and adrenal/bw ratio was
increased in females at 3 months. Water intakes were reduced in males
at 3000 ppm and females at 10000 ppm and in both sexes serum albumen
levels and A/G ratios were increased and serum sodium levels were
reduced at 3 and 6 months at 10000 ppm. The biological relevance of
these observations was not established. No effects were observed on
food intake or urinary parameters and there were no treatment-related
histopathological lesions. The NOEL for this study was 1000 ppm (equal
to 55 mg/kg bw/day). (Murakami et al., 1981).
Dogs
Groups of 6 male and 6 female purebred beagle dogs (24-29 weeks
old) were given diets containing d-phenothrin at 0, 100, 300 or
1000 ppm for 6 months. The diets were prepared weekly. Two batches of
d-phenothrin were used: a 95.5% pure lot was used weeks 1-4 (week 4
for the 100 ppm diet); and a 91.3% pure lot was used weeks 4-26 (week
4 for the 300 and 1000 ppm diets). No deaths occurred and there were
no treatment-related effects or clinical signs of toxicity, food
consumption, haematology, urinalysis, ophthalmoscopic observations, or
gross or histopathology. Body weights were slightly (<10%) lower than
in controls in the 1000 ppm group (both sexes) throughout the study.
Serum alkaline phosphatase levels were elevated in both males and
females in the 1000 ppm group. Liver/body weight ratios were increased
in both males and females at 1000 ppm. The NOEL for this study is
considered to be 300 ppm (equal to 9.3 mg/kg bw/day based on food
intake and body weight). (Pence et al., 1981).
Groups of 4 male and 4 female beagle dogs/dose level were given
diet containing 0, 100, 300, 1000 or 3000 ppm d-phenothrin for 52
weeks. Clinical observations, body weight gain and food consumption
were monitored throughout the study. Blood and urine samples were
analyzed on weeks 13, 26, 39 and 52. All dogs were given
ophthalmoscopic examinations prior to initiation and at termination.
On week 52 all dogs were sacrificed and organ weights were recorded.
Histopathological examinations of about 40 tissues/organs from each
dog were performed.
No deaths occurred in the study. There were no treatment-related
effects on body weight gain, food consumption, haematology, urinalysis
or ophthalmoscopy. Among females emesis was slightly more frequent at
3000 ppm but was not unequivocally treatment-related. Alkaline
phosphatase levels were slightly increased in both males and females
at 3000 ppm but was statistically significant only in males on week 13
and was higher than the expected range only in males. There was a
tendency to reduced serum albumen levels and albumen/globulin ratios
at 10.00 and 3000 ppm but the levels were within the expected range at
the performing laboratory. Liver/body weight ratios were increased in
males and females at 3000 ppm but were statistically significant only
in females. Histopathologically there were increased incidences of the
diagnosis of microcyst in the pituitary in females at 3000 ppm, focal
degeneration accompanied by the presence of acicular crystalline
material in adrenal cortex in males at 3000 ppm and hepatocellular
enlargement in males and females at 3000 ppm. Focal degeneration in
adrenal cortex without crystalline material was observed in one male
at 1000 ppm. One male at 1000 ppm had slight diffuse hepatocellular
enlargement. The authors concluded that the NOEL for this study was
300 ppm (equal to 8.2 mg/kg bw/day) (Cox et al., 1987).
COMMENTS
d-Phenothrin is a mixture of predominantly 1R,cis- and
1R,trans- isomers (cis:trans ratio of 20:80) of the
chrysanthemic acid ester of 3-phenoxybenzyl alcohol.
Following either single or repeated doses of both the 1R,cis-
and 1R, trans- isomers of phenothrin, excretion was virtually
complete within 7 days after dosing. After a single dose of either
isomer the primary route of excretion was via the faeces, being higher
for the cis- isomer. After repeated doses of the cis- isomer the
faecal route was still the predominant route, although the proportion
recovered in faeces was slightly less. Urinary excretion was the
predominant route of elimination of the trans- isomer. Tissue levels
were low with residues primarily in the fat. Tissue levels were higher
following repeated doses than following a single dose. Much of the
material recovered from the faeces was intact phenothrin, which was
considered to be unabsorbed material. Faecal metabolites of the cis-
isomer retained the ester linkage and were derived from oxidations.
Urinary metabolites were formed from both isomers following ester
cleavage after single or repeated dosing in both sexes.
In feeding studies in dogs, mice and rats, liver/body weight
ratios were increased. After one year in dogs and two years in rats
and mice large hepatocytes were observed. Histopathological changes
were also seen in the adrenals of dogs after one year, but not at 6
months. In mice there were slightly increased incidences of
hepatocellular adenomas (both sexes) and carcinomas (in females) in
all treated groups but these were not dose-related. Despite what were
reported to be pulmonary metastases of hepatic carcinomas in both
sexes at 1000 and 3000 ppm, the Meeting did not consider the compound
to be a hepatic carcinogen in this species. However, it was concerned
that the lung lesions be properly identified.
In rats there was an increase in the incidence of adenomas and
carcinomas of the preputial gland in males at 3000 ppm. Since only the
left gland was examined routinely, the true incidence of these tumours
is unknown. Although it would be of interest to have additional data
concerning these tumours, it was considered unlikely that the
incidences of preputial gland tumours were toxicologically relevant.
A two-generation reproduction study in rats was flawed by
consanguinous matings in the first mating period. However, subsequent
matings were non-consanguinous and the offspring of the consanguinous
matings were not used. Therefore, the study was considered acceptable.
No reproductive effects were observed at dose levels up to and
including 3000 ppm (equivalent to 150 mg/kg bw/day). The NOEL for
parental effects (body weight gain depression) was 1000 ppm
(equivalent to 50 mg/kg bw/day).
There was no increase in chromosomal aberrations in Chinese
hamster ovary cells or unscheduled DNA synthesis in human HeLa S3
cells.
Re-examination of the teratology studies in rabbits and mice
evaluated previously (1980 JMPR) indicated that in the mouse study
dosing was on days 7-12 of gestation. This period does not cover the
complete period of organogenesis. No other teratology study in a
rodent species was available. A rodent teratology study covering the
entire period of organogenesis is desirable.
TOXICOLOGICAL INFORMATION
LEVELS CAUSING TO TOXICOLOGICAL EFFECT
Mouse: 300 ppm in the diet, equal to 40.3 mg/kg bw/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw/day
Dog: 300 ppm in the diet, equal to 7.1 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.07 mg/kg bw for "d-phenothrin"
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Histopathological examination of all preputial glands from
the rat oncogenicity study.
2. Re-examination of the metastatic liver tumours in the lung
in the mouse oncogenicity study to determine whether they
represent true metastases.
3. A teratogenicity study in a rodent species.
REFERENCES
Amyes, S.J., Martin, P.A., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Oncogenicity and toxicity study
in mice. Unpublished report from Life Science Research. Submitted by
Sumitomo Chemical Co., Ltd.
Cox, R.H., Sutherland, J.D., Voelker, R.W., Alsaker, R.D., Vargas,
K.J., Lewis, S.A. & Hagen, W.J. 1987. Chronic Toxicity Study in Dogs
with Sumithrin -J.G.- Unpublished report from Hazleton Laboratories
Inc. Submitted by Sumitomo Chemical Co., Ltd.
Forster, R., Tipins, R.S., De Venezia, V. & Nunziata, A. 1984.
Unscheduled DNA synthesis in human cells. Cell Line: HeLa S3. Test
Substance: Sumithrin. Unpublished report from Life Sciences Research,
Roma Toxicology Center. Submitted by Sumitomo Chemical Co., Ltd.
Haseman, J.K. 1983. Patterns of Tumor Incidence in Two-Year Cancer
Bioassay Feeding Studies in Fischer 344 Rats. Fundamental and Applied
Toxicology 3:1-9.
Isobe, N., Matsunaga, H., Nakatsuka, I. & Yoshitake, A. 1987.
Metabolism of (1R, trans) and (1R, cis)-isomers of Phenothrin in
Rats. Unpublished report from Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Ito, K., Iwawaki, H. & Yoshitake, A. 1986.
In vitro chromosomal aberration test of S-2539F in Chinese hamster
ovary cells (CHO-K1). Unpublished report from Sumitomo Chemical Co.,
Ltd.
Martin, P.A., Amyes, S.J., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Combined Toxicity and
Oncogenicity Study in Rats. Unpublished Report from Life Science
Research, Submitted by Sumitomo Chemical Co., Ltd.
Murakami, M., Hiromori, T., Ito, S. & Hosokawa, S. 1981. Six Month
Oral Toxicity Study of S2539 Forte (SumithrinR) in Rats. Unpublished
report from Sumitomo Chemical Co., Ltd.
Pence, D.H., Hagen, W.H., Alsaker, R.D., Hastings, T.F., Dawkins,
B.G., Tacey, R.L. & Marshall, P.M. 1981. Subchronic Toxicity Study in
Dogs S2539-F. Unpublished report from Hazleton Laboratories Inc.
Submitted by Sumitomo Chemical Co., Ltd.
Resnik, G. & Ward, J.M. 1981. Morphology of Hyperplastic and
Neoplastic Lesions in the Clitoral and Preputial Gland of the F344
Rat. Veterinary Pathology 18:228-238.
Solleveld, H.A., Haseman, J.K. & McConnell, E.E. 1984. Natural History
of Body Weight Gain, Survival and Neoplasia in the F344 Rat. Journal
of the National Cancer Institute 72(4):929-940.
Tesh, J.M., Willoughby, C.R. & Fowler, J.S.L. 1987. Sumithrin: Effects
upon reproductive performance of rats treated continuously throughout
two successive generations. Unpublished report from Life Science
Research. Submitted by Sumitomo Chemical Co., Ltd.
Mortality was 42-56% in males and 14-36% in females at 104 weeks.
There was no increase in mortality as a result of treatment. There was
a slight but statistically significant reduction in body weight gain
during the first year of the study in females given 3000 ppm. This
group also had reduced alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels at weeks 25, 49 and 77. Males given
3000 ppm had reduced ALT activity at week 49 only. Liver/body weight
ratio was increased in females given 3000 ppm at week 52 only and in
males given 3000 ppm at weeks 52 and 105 (statistically significant
only at week 105). Histopathologically the only observation of note
was a slight increase in the incidence of hepatocytic hypertrophy in
males given 3000 ppm. There were no statistically significant
increases in the incidences of any tumour type. However, there were
increases in the incidence of preputial gland adenomas and carcinomas
in males. The incidence at 0, 300, 1000 and 3000 ppm were 1, 1, 1 and
4 adenomas and 0, 0, 1 and 3 carcinomas, respectively.
In this study only the left preputial gland was examined
routinely for histopathology. The right preputial gland was examined
only when there were gross observations. However, the three carcinomas
in the high dose group were apparently not detected grossly and,
therefore, there may have been additional tumours in the right glands
that were not detected. Pathologists studying this gland have reported
that small tumours of the preputial gland cannot be detected when only
gross lesions are examined (Resnick & Ward, 1981). Incidences of
preputial tumours in F344 rats which have been published for the NTP
program in the United States indicate incidences of 2.2% with adenomas
and 2.7% with carcinomas among 2,320 control males in 2-year studies
and 0.4% with adenomas and 4.7% with carcinomas in 529 males in a
lifespan study (Solleveld et al., 1984). In one NTP study a total of
7 preputial gland tumours were observed in a group of 50 males
(untreated) but it is not indicated how many of the tumours were
adenomas or carcinomas (Haseman, 1983). The procedure for examining
this gland was not given in either of these studies. It appears likely
that in this gland adenomas progress to carcinomas (Resnick & Ward,
1981).
On the basis of this information the observed incidences of these
tumours do not appear of concern. However, it would be of interest to
have the preputial glands reexamined to determine the true incidence
of tumours in this study. In this study there was no apparent effect
at the 1000 ppm dose level (equivalent to 50 mg/kg bw/day) on any of
the examined parameters (Martin et al., 1987).
Special study on mutagenicity
The ability of d-phenothrin to cause chromosomal aberrations in
Chinese hamster ovary cells was tested in vitro. Based on the
results of a cytotoxicity test, 4 concentrations (2 × 10-5 to
2 × 10-4 M without S-9 mix, and 5 × 10-5 to 5 × 10-4 with S-9
mix) were tested. There was no increase above control levels in the
incidence of chromosomal aberrations at any of the dose levels tested.
Positive controls significantly increased the number of aberrations
observed (Kogiso et al., 1986).
The ability of d-phenothrin to induce unscheduled DNA synthesis
was tested in human cells (HeLa S3). Because no cytotoxicity was noted
with dose levels up to 4 mg/ml, concentrations tested were 0.25 to
4 mg/ml. No significant increases in uptake of tritiated thymidine
were observed following treatment with d-phenothrin in contrast to the
results with the positive controls. There was no evidence, under the
conditions of this test, that d-phenothrin induced unscheduled DNA
synthesis (Forster et al., 1984).
Special study on reproduction
Rats
In a two-generation reproduction study, groups of 30 male and 30
female Charles River CD (Sprague-Dawley-derived) rats were given diets
containing 0, 300, 1000 or 3000 ppm d-phenothrin (92.9% pure) during
growth, mating, gestation and lactation for 2 litters per generation.
The rats were mated on a 1:1 basis after 91 days of dietary exposure
to produce the F1a litter. The rats were subsequently remated to
produce the F1b litter. From the F1b litter 30 males and 30
females were selected to be parents to the F2a and F2b litters.
From the F2b litter 1 male and 1 female/litter/dose level were
maintained on test for 13 weeks.
Body weight gains of F0 rats given 3000 ppm were slightly lower
than controls during the pre-mating period. The difference in body
weight was maintained throughout the study but did not increase with
time. In the F1 parents there was a slight reduction in body weight
gain up to week 13 (pre-mating) at 3000 ppm which was maintained in
males but females approached control values during the second mating.
The F2b pups maintained for 13 weeks showed no effect on body weight
gain. There were no treatment-related effects on food consumption,
water intake, regularity of oestrus cycle, or reproductive performance
(fertility, gestation, litter size, viability, pup sex and weight).
Development of the F1b and F2b pups was normal as indicated by
auditory and visual responses, pinna unfolding, hair growth, tooth
eruption and eye opening. Liver/body weight ratios were increased in
F0 adult females, F1 adult females and F2 weanling males and
females given diet containing 3000 ppm of d-phenothrin. There was no
consistent histopathological change suggestive of a treatment-related
effect. In this study the first mating (F0->F1a) was mainly
consanguinous. There were no apparent differences in the results of
these matings and the subsequent non-consanguinous matings. The
offspring of this mating were not further used in the study so the
departure from protocol did not invalidate the study as a whole. The
authors concluded that d-phenothrin had no effect on reproductive
performance or development of Charles River rats at dietary levels of
up to 3000 ppm (equivalent to 150 mg/kg bw/day) over 2 generations.
The NOEL for parental effects was 1000 ppm (equivalent to 50 mg/kg
bw/day) (Tesh et al., 1987).
Short-term studies
Rats
Groups of 20 Sprague-Dawley weanling rats/sex/dose level were
given diets containing d-phenothrin (92.9% pure) at levels of 0, 1000,
3000 or 10000 ppm for 6 months. Additional groups of 10 rats/sex/dose
level were given the same diets for 3 months. No deaths occurred and
no clinical signs of toxicity or opthalmoscopic effects were observed.
A dietary level of 10000 ppm caused depressed body weight gain in the
first 3 months of feeding in both sexes, slight reduction of RBC
count, haematocrit and haemoglobin at 3 and 6 months in males, reduced
serum cholinesterase at 3 and 6 months in females and increased
liver/bw, kidney/bw and adrenal/bw ratios in both sexes. At 3000 ppm
liver/bw ratio was increased in both sexes at 3 and 6 months,
kidney/bw was increased in males at 3 months and adrenal/bw ratio was
increased in females at 3 months. Water intakes were reduced in males
at 3000 ppm and females at 10000 ppm and in both sexes serum albumen
levels and A/G ratios were increased and serum sodium levels were
reduced at 3 and 6 months at 10000 ppm. The biological relevance of
these observations was not established. No effects were observed on
food intake or urinary parameters and there were no treatment-related
histopathological lesions. The NOEL for this study was 1000 ppm (equal
to 55 mg/kg bw/day). (Murakami et al., 1981).
Dogs
Groups of 6 male and 6 female purebred beagle dogs (24-29 weeks
old) were given diets containing d-phenothrin at 0, 100, 300 or
1000 ppm for 6 months. The diets were prepared weekly. Two batches of
d-phenothrin were used: a 95.5% pure lot was used weeks 1-4 (week 4
for the 100 ppm diet); and a 91.3% pure lot was used weeks 4-26 (week
4 for the 300 and 1000 ppm diets). No deaths occurred and there were
no treatment-related effects or clinical signs of toxicity, food
consumption, haematology, urinalysis, ophthalmoscopic observations, or
gross or histopathology. Body weights were slightly (<10%) lower than
in controls in the 1000 ppm group (both sexes) throughout the study.
Serum alkaline phosphatase levels were elevated in both males and
females in the 1000 ppm group. Liver/body weight ratios were increased
in both males and females at 1000 ppm. The NOEL for this study is
considered to be 300 ppm (equal to 9.3 mg/kg bw/day based on food
intake and body weight). (Pence et al., 1981).
Groups of 4 male and 4 female beagle dogs/dose level were given
diet containing 0, 100, 300, 1000 or 3000 ppm d-phenothrin for 52
weeks. Clinical observations, body weight gain and food consumption
were monitored throughout the study. Blood and urine samples were
analyzed on weeks 13, 26, 39 and 52. All dogs were given
ophthalmoscopic examinations prior to initiation and at termination.
On week 52 all dogs were sacrificed and organ weights were recorded.
Histopathological examinations of about 40 tissues/organs from each
dog were performed.
No deaths occurred in the study. There were no treatment-related
effects on body weight gain, food consumption, haematology, urinalysis
or ophthalmoscopy. Among females emesis was slightly more frequent at
3000 ppm but was not unequivocally treatment-related. Alkaline
phosphatase levels were slightly increased in both males and females
at 3000 ppm but was statistically significant only in males on week 13
and was higher than the expected range only in males. There was a
tendency to reduced serum albumen levels and albumen/globulin ratios
at 10.00 and 3000 ppm but the levels were within the expected range at
the performing laboratory. Liver/body weight ratios were increased in
males and females at 3000 ppm but were statistically significant only
in females. Histopathologically there were increased incidences of the
diagnosis of microcyst in the pituitary in females at 3000 ppm, focal
degeneration accompanied by the presence of acicular crystalline
material in adrenal cortex in males at 3000 ppm and hepatocellular
enlargement in males and females at 3000 ppm. Focal degeneration in
adrenal cortex without crystalline material was observed in one male
at 1000 ppm. One male at 1000 ppm had slight diffuse hepatocellular
enlargement. The authors concluded that the NOEL for this study was
300 ppm (equal to 8.2 mg/kg bw/day) (Cox et al., 1987).
COMMENTS
d-Phenothrin is a mixture of predominantly 1R,cis- and
1R,trans- isomers (cis:trans ratio of 20:80) of the
chrysanthemic acid ester of 3-phenoxybenzyl alcohol.
Following either single or repeated doses of both the 1R,cis-
and 1R, trans- isomers of phenothrin, excretion was virtually
complete within 7 days after dosing. After a single dose of either
isomer the primary route of excretion was via the faeces, being higher
for the cis- isomer. After repeated doses of the cis- isomer the
faecal route was still the predominant route, although the proportion
recovered in faeces was slightly less. Urinary excretion was the
predominant route of elimination of the trans- isomer. Tissue levels
were low with residues primarily in the fat. Tissue levels were higher
following repeated doses than following a single dose. Much of the
material recovered from the faeces was intact phenothrin, which was
considered to be unabsorbed material. Faecal metabolites of the cis-
isomer retained the ester linkage and were derived from oxidations.
Urinary metabolites were formed from both isomers following ester
cleavage after single or repeated dosing in both sexes.
In feeding studies in dogs, mice and rats, liver/body weight
ratios were increased. After one year in dogs and two years in rats
and mice large hepatocytes were observed. Histopathological changes
were also seen in the adrenals of dogs after one year, but not at 6
months. In mice there were slightly increased incidences of
hepatocellular adenomas (both sexes) and carcinomas (in females) in
all treated groups but these were not dose-related. Despite what were
reported to be pulmonary metastases of hepatic carcinomas in both
sexes at 1000 and 3000 ppm, the Meeting did not consider the compound
to be a hepatic carcinogen in this species. However, it was concerned
that the lung lesions be properly identified.
In rats there was an increase in the incidence of adenomas and
carcinomas of the preputial gland in males at 3000 ppm. Since only the
left gland was examined routinely, the true incidence of these tumours
is unknown. Although it would be of interest to have additional data
concerning these tumours, it was considered unlikely that the
incidences of preputial gland tumours were toxicologically relevant.
A two-generation reproduction study in rats was flawed by
consanguinous matings in the first mating period. However, subsequent
matings were non-consanguinous and the offspring of the consanguinous
matings were not used. Therefore, the study was considered acceptable.
No reproductive effects were observed at dose levels up to and
including 3000 ppm (equivalent to 150 mg/kg bw/day). The NOEL for
parental effects (body weight gain depression) was 1000 ppm
(equivalent to 50 mg/kg bw/day).
There was no increase in chromosomal aberrations in Chinese
hamster ovary cells or unscheduled DNA synthesis in human HeLa S3
cells.
Re-examination of the teratology studies in rabbits and mice
evaluated previously (1980 JMPR) indicated that in the mouse study
dosing was on days 7-12 of gestation. This period does not cover the
complete period of organogenesis. No other teratology study in a
rodent species was available. A rodent teratology study covering the
entire period of organogenesis is desirable.
TOXICOLOGICAL INFORMATION
LEVELS CAUSING TO TOXICOLOGICAL EFFECT
Mouse: 300 ppm in the diet, equal to 40.3 mg/kg bw/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw/day
Dog: 300 ppm in the diet, equal to 7.1 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.07 mg/kg bw for "d-phenothrin"
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Histopathological examination of all preputial glands from
the rat oncogenicity study.
2. Re-examination of the metastatic liver tumours in the lung
in the mouse oncogenicity study to determine whether they
represent true metastases.
3. A teratogenicity study in a rodent species.
REFERENCES
Amyes, S.J., Martin, P.A., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Oncogenicity and toxicity study
in mice. Unpublished report from Life Science Research. Submitted by
Sumitomo Chemical Co., Ltd.
Cox, R.H., Sutherland, J.D., Voelker, R.W., Alsaker, R.D., Vargas,
K.J., Lewis, S.A. & Hagen, W.J. 1987. Chronic Toxicity Study in Dogs
with Sumithrin -J.G.- Unpublished report from Hazleton Laboratories
Inc. Submitted by Sumitomo Chemical Co., Ltd.
Forster, R., Tipins, R.S., De Venezia, V. & Nunziata, A. 1984.
Unscheduled DNA synthesis in human cells. Cell Line: HeLa S3. Test
Substance: Sumithrin. Unpublished report from Life Sciences Research,
Roma Toxicology Center. Submitted by Sumitomo Chemical Co., Ltd.
Haseman, J.K. 1983. Patterns of Tumor Incidence in Two-Year Cancer
Bioassay Feeding Studies in Fischer 344 Rats. Fundamental and Applied
Toxicology 3:1-9.
Isobe, N., Matsunaga, H., Nakatsuka, I. & Yoshitake, A. 1987.
Metabolism of (1R, trans) and (1R, cis)-isomers of Phenothrin in
Rats. Unpublished report from Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Ito, K., Iwawaki, H. & Yoshitake, A. 1986.
In vitro chromosomal aberration test of S-2539F in Chinese hamster
ovary cells (CHO-K1). Unpublished report from Sumitomo Chemical Co.,
Ltd.
Martin, P.A., Amyes, S.J., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Combined Toxicity and
Oncogenicity Study in Rats. Unpublished Report from Life Science
Research, Submitted by Sumitomo Chemical Co., Ltd.
Murakami, M., Hiromori, T., Ito, S. & Hosokawa, S. 1981. Six Month
Oral Toxicity Study of S2539 Forte (SumithrinR) in Rats. Unpublished
report from Sumitomo Chemical Co., Ltd.
Pence, D.H., Hagen, W.H., Alsaker, R.D., Hastings, T.F., Dawkins,
B.G., Tacey, R.L. & Marshall, P.M. 1981. Subchronic Toxicity Study in
Dogs S2539-F. Unpublished report from Hazleton Laboratories Inc.
Submitted by Sumitomo Chemical Co., Ltd.
Resnik, G. & Ward, J.M. 1981. Morphology of Hyperplastic and
Neoplastic Lesions in the Clitoral and Preputial Gland of the F344
Rat. Veterinary Pathology 18:228-238.
Solleveld, H.A., Haseman, J.K. & McConnell, E.E. 1984. Natural History
of Body Weight Gain, Survival and Neoplasia in the F344 Rat. Journal
of the National Cancer Institute 72(4):929-940.
Tesh, J.M., Willoughby, C.R. & Fowler, J.S.L. 1987. Sumithrin: Effects
upon reproductive performance of rats treated continuously throughout
two successive generations. Unpublished report from Life Science
Research. Submitted by Sumitomo Chemical Co., Ltd.
 Mortality was 42-56% in males and 14-36% in females at 104 weeks.
There was no increase in mortality as a result of treatment. There was
a slight but statistically significant reduction in body weight gain
during the first year of the study in females given 3000 ppm. This
group also had reduced alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels at weeks 25, 49 and 77. Males given
3000 ppm had reduced ALT activity at week 49 only. Liver/body weight
ratio was increased in females given 3000 ppm at week 52 only and in
males given 3000 ppm at weeks 52 and 105 (statistically significant
only at week 105). Histopathologically the only observation of note
was a slight increase in the incidence of hepatocytic hypertrophy in
males given 3000 ppm. There were no statistically significant
increases in the incidences of any tumour type. However, there were
increases in the incidence of preputial gland adenomas and carcinomas
in males. The incidence at 0, 300, 1000 and 3000 ppm were 1, 1, 1 and
4 adenomas and 0, 0, 1 and 3 carcinomas, respectively.
In this study only the left preputial gland was examined
routinely for histopathology. The right preputial gland was examined
only when there were gross observations. However, the three carcinomas
in the high dose group were apparently not detected grossly and,
therefore, there may have been additional tumours in the right glands
that were not detected. Pathologists studying this gland have reported
that small tumours of the preputial gland cannot be detected when only
gross lesions are examined (Resnick & Ward, 1981). Incidences of
preputial tumours in F344 rats which have been published for the NTP
program in the United States indicate incidences of 2.2% with adenomas
and 2.7% with carcinomas among 2,320 control males in 2-year studies
and 0.4% with adenomas and 4.7% with carcinomas in 529 males in a
lifespan study (Solleveld et al., 1984). In one NTP study a total of
7 preputial gland tumours were observed in a group of 50 males
(untreated) but it is not indicated how many of the tumours were
adenomas or carcinomas (Haseman, 1983). The procedure for examining
this gland was not given in either of these studies. It appears likely
that in this gland adenomas progress to carcinomas (Resnick & Ward,
1981).
On the basis of this information the observed incidences of these
tumours do not appear of concern. However, it would be of interest to
have the preputial glands reexamined to determine the true incidence
of tumours in this study. In this study there was no apparent effect
at the 1000 ppm dose level (equivalent to 50 mg/kg bw/day) on any of
the examined parameters (Martin et al., 1987).
Special study on mutagenicity
The ability of d-phenothrin to cause chromosomal aberrations in
Chinese hamster ovary cells was tested in vitro. Based on the
results of a cytotoxicity test, 4 concentrations (2 × 10-5 to
2 × 10-4 M without S-9 mix, and 5 × 10-5 to 5 × 10-4 with S-9
mix) were tested. There was no increase above control levels in the
incidence of chromosomal aberrations at any of the dose levels tested.
Positive controls significantly increased the number of aberrations
observed (Kogiso et al., 1986).
The ability of d-phenothrin to induce unscheduled DNA synthesis
was tested in human cells (HeLa S3). Because no cytotoxicity was noted
with dose levels up to 4 mg/ml, concentrations tested were 0.25 to
4 mg/ml. No significant increases in uptake of tritiated thymidine
were observed following treatment with d-phenothrin in contrast to the
results with the positive controls. There was no evidence, under the
conditions of this test, that d-phenothrin induced unscheduled DNA
synthesis (Forster et al., 1984).
Special study on reproduction
Rats
In a two-generation reproduction study, groups of 30 male and 30
female Charles River CD (Sprague-Dawley-derived) rats were given diets
containing 0, 300, 1000 or 3000 ppm d-phenothrin (92.9% pure) during
growth, mating, gestation and lactation for 2 litters per generation.
The rats were mated on a 1:1 basis after 91 days of dietary exposure
to produce the F1a litter. The rats were subsequently remated to
produce the F1b litter. From the F1b litter 30 males and 30
females were selected to be parents to the F2a and F2b litters.
From the F2b litter 1 male and 1 female/litter/dose level were
maintained on test for 13 weeks.
Body weight gains of F0 rats given 3000 ppm were slightly lower
than controls during the pre-mating period. The difference in body
weight was maintained throughout the study but did not increase with
time. In the F1 parents there was a slight reduction in body weight
gain up to week 13 (pre-mating) at 3000 ppm which was maintained in
males but females approached control values during the second mating.
The F2b pups maintained for 13 weeks showed no effect on body weight
gain. There were no treatment-related effects on food consumption,
water intake, regularity of oestrus cycle, or reproductive performance
(fertility, gestation, litter size, viability, pup sex and weight).
Development of the F1b and F2b pups was normal as indicated by
auditory and visual responses, pinna unfolding, hair growth, tooth
eruption and eye opening. Liver/body weight ratios were increased in
F0 adult females, F1 adult females and F2 weanling males and
females given diet containing 3000 ppm of d-phenothrin. There was no
consistent histopathological change suggestive of a treatment-related
effect. In this study the first mating (F0->F1a) was mainly
consanguinous. There were no apparent differences in the results of
these matings and the subsequent non-consanguinous matings. The
offspring of this mating were not further used in the study so the
departure from protocol did not invalidate the study as a whole. The
authors concluded that d-phenothrin had no effect on reproductive
performance or development of Charles River rats at dietary levels of
up to 3000 ppm (equivalent to 150 mg/kg bw/day) over 2 generations.
The NOEL for parental effects was 1000 ppm (equivalent to 50 mg/kg
bw/day) (Tesh et al., 1987).
Short-term studies
Rats
Groups of 20 Sprague-Dawley weanling rats/sex/dose level were
given diets containing d-phenothrin (92.9% pure) at levels of 0, 1000,
3000 or 10000 ppm for 6 months. Additional groups of 10 rats/sex/dose
level were given the same diets for 3 months. No deaths occurred and
no clinical signs of toxicity or opthalmoscopic effects were observed.
A dietary level of 10000 ppm caused depressed body weight gain in the
first 3 months of feeding in both sexes, slight reduction of RBC
count, haematocrit and haemoglobin at 3 and 6 months in males, reduced
serum cholinesterase at 3 and 6 months in females and increased
liver/bw, kidney/bw and adrenal/bw ratios in both sexes. At 3000 ppm
liver/bw ratio was increased in both sexes at 3 and 6 months,
kidney/bw was increased in males at 3 months and adrenal/bw ratio was
increased in females at 3 months. Water intakes were reduced in males
at 3000 ppm and females at 10000 ppm and in both sexes serum albumen
levels and A/G ratios were increased and serum sodium levels were
reduced at 3 and 6 months at 10000 ppm. The biological relevance of
these observations was not established. No effects were observed on
food intake or urinary parameters and there were no treatment-related
histopathological lesions. The NOEL for this study was 1000 ppm (equal
to 55 mg/kg bw/day). (Murakami et al., 1981).
Dogs
Groups of 6 male and 6 female purebred beagle dogs (24-29 weeks
old) were given diets containing d-phenothrin at 0, 100, 300 or
1000 ppm for 6 months. The diets were prepared weekly. Two batches of
d-phenothrin were used: a 95.5% pure lot was used weeks 1-4 (week 4
for the 100 ppm diet); and a 91.3% pure lot was used weeks 4-26 (week
4 for the 300 and 1000 ppm diets). No deaths occurred and there were
no treatment-related effects or clinical signs of toxicity, food
consumption, haematology, urinalysis, ophthalmoscopic observations, or
gross or histopathology. Body weights were slightly (<10%) lower than
in controls in the 1000 ppm group (both sexes) throughout the study.
Serum alkaline phosphatase levels were elevated in both males and
females in the 1000 ppm group. Liver/body weight ratios were increased
in both males and females at 1000 ppm. The NOEL for this study is
considered to be 300 ppm (equal to 9.3 mg/kg bw/day based on food
intake and body weight). (Pence et al., 1981).
Groups of 4 male and 4 female beagle dogs/dose level were given
diet containing 0, 100, 300, 1000 or 3000 ppm d-phenothrin for 52
weeks. Clinical observations, body weight gain and food consumption
were monitored throughout the study. Blood and urine samples were
analyzed on weeks 13, 26, 39 and 52. All dogs were given
ophthalmoscopic examinations prior to initiation and at termination.
On week 52 all dogs were sacrificed and organ weights were recorded.
Histopathological examinations of about 40 tissues/organs from each
dog were performed.
No deaths occurred in the study. There were no treatment-related
effects on body weight gain, food consumption, haematology, urinalysis
or ophthalmoscopy. Among females emesis was slightly more frequent at
3000 ppm but was not unequivocally treatment-related. Alkaline
phosphatase levels were slightly increased in both males and females
at 3000 ppm but was statistically significant only in males on week 13
and was higher than the expected range only in males. There was a
tendency to reduced serum albumen levels and albumen/globulin ratios
at 10.00 and 3000 ppm but the levels were within the expected range at
the performing laboratory. Liver/body weight ratios were increased in
males and females at 3000 ppm but were statistically significant only
in females. Histopathologically there were increased incidences of the
diagnosis of microcyst in the pituitary in females at 3000 ppm, focal
degeneration accompanied by the presence of acicular crystalline
material in adrenal cortex in males at 3000 ppm and hepatocellular
enlargement in males and females at 3000 ppm. Focal degeneration in
adrenal cortex without crystalline material was observed in one male
at 1000 ppm. One male at 1000 ppm had slight diffuse hepatocellular
enlargement. The authors concluded that the NOEL for this study was
300 ppm (equal to 8.2 mg/kg bw/day) (Cox et al., 1987).
COMMENTS
d-Phenothrin is a mixture of predominantly 1R,cis- and
1R,trans- isomers (cis:trans ratio of 20:80) of the
chrysanthemic acid ester of 3-phenoxybenzyl alcohol.
Following either single or repeated doses of both the 1R,cis-
and 1R, trans- isomers of phenothrin, excretion was virtually
complete within 7 days after dosing. After a single dose of either
isomer the primary route of excretion was via the faeces, being higher
for the cis- isomer. After repeated doses of the cis- isomer the
faecal route was still the predominant route, although the proportion
recovered in faeces was slightly less. Urinary excretion was the
predominant route of elimination of the trans- isomer. Tissue levels
were low with residues primarily in the fat. Tissue levels were higher
following repeated doses than following a single dose. Much of the
material recovered from the faeces was intact phenothrin, which was
considered to be unabsorbed material. Faecal metabolites of the cis-
isomer retained the ester linkage and were derived from oxidations.
Urinary metabolites were formed from both isomers following ester
cleavage after single or repeated dosing in both sexes.
In feeding studies in dogs, mice and rats, liver/body weight
ratios were increased. After one year in dogs and two years in rats
and mice large hepatocytes were observed. Histopathological changes
were also seen in the adrenals of dogs after one year, but not at 6
months. In mice there were slightly increased incidences of
hepatocellular adenomas (both sexes) and carcinomas (in females) in
all treated groups but these were not dose-related. Despite what were
reported to be pulmonary metastases of hepatic carcinomas in both
sexes at 1000 and 3000 ppm, the Meeting did not consider the compound
to be a hepatic carcinogen in this species. However, it was concerned
that the lung lesions be properly identified.
In rats there was an increase in the incidence of adenomas and
carcinomas of the preputial gland in males at 3000 ppm. Since only the
left gland was examined routinely, the true incidence of these tumours
is unknown. Although it would be of interest to have additional data
concerning these tumours, it was considered unlikely that the
incidences of preputial gland tumours were toxicologically relevant.
A two-generation reproduction study in rats was flawed by
consanguinous matings in the first mating period. However, subsequent
matings were non-consanguinous and the offspring of the consanguinous
matings were not used. Therefore, the study was considered acceptable.
No reproductive effects were observed at dose levels up to and
including 3000 ppm (equivalent to 150 mg/kg bw/day). The NOEL for
parental effects (body weight gain depression) was 1000 ppm
(equivalent to 50 mg/kg bw/day).
There was no increase in chromosomal aberrations in Chinese
hamster ovary cells or unscheduled DNA synthesis in human HeLa S3
cells.
Re-examination of the teratology studies in rabbits and mice
evaluated previously (1980 JMPR) indicated that in the mouse study
dosing was on days 7-12 of gestation. This period does not cover the
complete period of organogenesis. No other teratology study in a
rodent species was available. A rodent teratology study covering the
entire period of organogenesis is desirable.
TOXICOLOGICAL INFORMATION
LEVELS CAUSING TO TOXICOLOGICAL EFFECT
Mouse: 300 ppm in the diet, equal to 40.3 mg/kg bw/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw/day
Dog: 300 ppm in the diet, equal to 7.1 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.07 mg/kg bw for "d-phenothrin"
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Histopathological examination of all preputial glands from
the rat oncogenicity study.
2. Re-examination of the metastatic liver tumours in the lung
in the mouse oncogenicity study to determine whether they
represent true metastases.
3. A teratogenicity study in a rodent species.
REFERENCES
Amyes, S.J., Martin, P.A., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Oncogenicity and toxicity study
in mice. Unpublished report from Life Science Research. Submitted by
Sumitomo Chemical Co., Ltd.
Cox, R.H., Sutherland, J.D., Voelker, R.W., Alsaker, R.D., Vargas,
K.J., Lewis, S.A. & Hagen, W.J. 1987. Chronic Toxicity Study in Dogs
with Sumithrin -J.G.- Unpublished report from Hazleton Laboratories
Inc. Submitted by Sumitomo Chemical Co., Ltd.
Forster, R., Tipins, R.S., De Venezia, V. & Nunziata, A. 1984.
Unscheduled DNA synthesis in human cells. Cell Line: HeLa S3. Test
Substance: Sumithrin. Unpublished report from Life Sciences Research,
Roma Toxicology Center. Submitted by Sumitomo Chemical Co., Ltd.
Haseman, J.K. 1983. Patterns of Tumor Incidence in Two-Year Cancer
Bioassay Feeding Studies in Fischer 344 Rats. Fundamental and Applied
Toxicology 3:1-9.
Isobe, N., Matsunaga, H., Nakatsuka, I. & Yoshitake, A. 1987.
Metabolism of (1R, trans) and (1R, cis)-isomers of Phenothrin in
Rats. Unpublished report from Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Ito, K., Iwawaki, H. & Yoshitake, A. 1986.
In vitro chromosomal aberration test of S-2539F in Chinese hamster
ovary cells (CHO-K1). Unpublished report from Sumitomo Chemical Co.,
Ltd.
Martin, P.A., Amyes, S.J., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Combined Toxicity and
Oncogenicity Study in Rats. Unpublished Report from Life Science
Research, Submitted by Sumitomo Chemical Co., Ltd.
Murakami, M., Hiromori, T., Ito, S. & Hosokawa, S. 1981. Six Month
Oral Toxicity Study of S2539 Forte (SumithrinR) in Rats. Unpublished
report from Sumitomo Chemical Co., Ltd.
Pence, D.H., Hagen, W.H., Alsaker, R.D., Hastings, T.F., Dawkins,
B.G., Tacey, R.L. & Marshall, P.M. 1981. Subchronic Toxicity Study in
Dogs S2539-F. Unpublished report from Hazleton Laboratories Inc.
Submitted by Sumitomo Chemical Co., Ltd.
Resnik, G. & Ward, J.M. 1981. Morphology of Hyperplastic and
Neoplastic Lesions in the Clitoral and Preputial Gland of the F344
Rat. Veterinary Pathology 18:228-238.
Solleveld, H.A., Haseman, J.K. & McConnell, E.E. 1984. Natural History
of Body Weight Gain, Survival and Neoplasia in the F344 Rat. Journal
of the National Cancer Institute 72(4):929-940.
Tesh, J.M., Willoughby, C.R. & Fowler, J.S.L. 1987. Sumithrin: Effects
upon reproductive performance of rats treated continuously throughout
two successive generations. Unpublished report from Life Science
Research. Submitted by Sumitomo Chemical Co., Ltd.
Mortality was 42-56% in males and 14-36% in females at 104 weeks.
There was no increase in mortality as a result of treatment. There was
a slight but statistically significant reduction in body weight gain
during the first year of the study in females given 3000 ppm. This
group also had reduced alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels at weeks 25, 49 and 77. Males given
3000 ppm had reduced ALT activity at week 49 only. Liver/body weight
ratio was increased in females given 3000 ppm at week 52 only and in
males given 3000 ppm at weeks 52 and 105 (statistically significant
only at week 105). Histopathologically the only observation of note
was a slight increase in the incidence of hepatocytic hypertrophy in
males given 3000 ppm. There were no statistically significant
increases in the incidences of any tumour type. However, there were
increases in the incidence of preputial gland adenomas and carcinomas
in males. The incidence at 0, 300, 1000 and 3000 ppm were 1, 1, 1 and
4 adenomas and 0, 0, 1 and 3 carcinomas, respectively.
In this study only the left preputial gland was examined
routinely for histopathology. The right preputial gland was examined
only when there were gross observations. However, the three carcinomas
in the high dose group were apparently not detected grossly and,
therefore, there may have been additional tumours in the right glands
that were not detected. Pathologists studying this gland have reported
that small tumours of the preputial gland cannot be detected when only
gross lesions are examined (Resnick & Ward, 1981). Incidences of
preputial tumours in F344 rats which have been published for the NTP
program in the United States indicate incidences of 2.2% with adenomas
and 2.7% with carcinomas among 2,320 control males in 2-year studies
and 0.4% with adenomas and 4.7% with carcinomas in 529 males in a
lifespan study (Solleveld et al., 1984). In one NTP study a total of
7 preputial gland tumours were observed in a group of 50 males
(untreated) but it is not indicated how many of the tumours were
adenomas or carcinomas (Haseman, 1983). The procedure for examining
this gland was not given in either of these studies. It appears likely
that in this gland adenomas progress to carcinomas (Resnick & Ward,
1981).
On the basis of this information the observed incidences of these
tumours do not appear of concern. However, it would be of interest to
have the preputial glands reexamined to determine the true incidence
of tumours in this study. In this study there was no apparent effect
at the 1000 ppm dose level (equivalent to 50 mg/kg bw/day) on any of
the examined parameters (Martin et al., 1987).
Special study on mutagenicity
The ability of d-phenothrin to cause chromosomal aberrations in
Chinese hamster ovary cells was tested in vitro. Based on the
results of a cytotoxicity test, 4 concentrations (2 × 10-5 to
2 × 10-4 M without S-9 mix, and 5 × 10-5 to 5 × 10-4 with S-9
mix) were tested. There was no increase above control levels in the
incidence of chromosomal aberrations at any of the dose levels tested.
Positive controls significantly increased the number of aberrations
observed (Kogiso et al., 1986).
The ability of d-phenothrin to induce unscheduled DNA synthesis
was tested in human cells (HeLa S3). Because no cytotoxicity was noted
with dose levels up to 4 mg/ml, concentrations tested were 0.25 to
4 mg/ml. No significant increases in uptake of tritiated thymidine
were observed following treatment with d-phenothrin in contrast to the
results with the positive controls. There was no evidence, under the
conditions of this test, that d-phenothrin induced unscheduled DNA
synthesis (Forster et al., 1984).
Special study on reproduction
Rats
In a two-generation reproduction study, groups of 30 male and 30
female Charles River CD (Sprague-Dawley-derived) rats were given diets
containing 0, 300, 1000 or 3000 ppm d-phenothrin (92.9% pure) during
growth, mating, gestation and lactation for 2 litters per generation.
The rats were mated on a 1:1 basis after 91 days of dietary exposure
to produce the F1a litter. The rats were subsequently remated to
produce the F1b litter. From the F1b litter 30 males and 30
females were selected to be parents to the F2a and F2b litters.
From the F2b litter 1 male and 1 female/litter/dose level were
maintained on test for 13 weeks.
Body weight gains of F0 rats given 3000 ppm were slightly lower
than controls during the pre-mating period. The difference in body
weight was maintained throughout the study but did not increase with
time. In the F1 parents there was a slight reduction in body weight
gain up to week 13 (pre-mating) at 3000 ppm which was maintained in
males but females approached control values during the second mating.
The F2b pups maintained for 13 weeks showed no effect on body weight
gain. There were no treatment-related effects on food consumption,
water intake, regularity of oestrus cycle, or reproductive performance
(fertility, gestation, litter size, viability, pup sex and weight).
Development of the F1b and F2b pups was normal as indicated by
auditory and visual responses, pinna unfolding, hair growth, tooth
eruption and eye opening. Liver/body weight ratios were increased in
F0 adult females, F1 adult females and F2 weanling males and
females given diet containing 3000 ppm of d-phenothrin. There was no
consistent histopathological change suggestive of a treatment-related
effect. In this study the first mating (F0->F1a) was mainly
consanguinous. There were no apparent differences in the results of
these matings and the subsequent non-consanguinous matings. The
offspring of this mating were not further used in the study so the
departure from protocol did not invalidate the study as a whole. The
authors concluded that d-phenothrin had no effect on reproductive
performance or development of Charles River rats at dietary levels of
up to 3000 ppm (equivalent to 150 mg/kg bw/day) over 2 generations.
The NOEL for parental effects was 1000 ppm (equivalent to 50 mg/kg
bw/day) (Tesh et al., 1987).
Short-term studies
Rats
Groups of 20 Sprague-Dawley weanling rats/sex/dose level were
given diets containing d-phenothrin (92.9% pure) at levels of 0, 1000,
3000 or 10000 ppm for 6 months. Additional groups of 10 rats/sex/dose
level were given the same diets for 3 months. No deaths occurred and
no clinical signs of toxicity or opthalmoscopic effects were observed.
A dietary level of 10000 ppm caused depressed body weight gain in the
first 3 months of feeding in both sexes, slight reduction of RBC
count, haematocrit and haemoglobin at 3 and 6 months in males, reduced
serum cholinesterase at 3 and 6 months in females and increased
liver/bw, kidney/bw and adrenal/bw ratios in both sexes. At 3000 ppm
liver/bw ratio was increased in both sexes at 3 and 6 months,
kidney/bw was increased in males at 3 months and adrenal/bw ratio was
increased in females at 3 months. Water intakes were reduced in males
at 3000 ppm and females at 10000 ppm and in both sexes serum albumen
levels and A/G ratios were increased and serum sodium levels were
reduced at 3 and 6 months at 10000 ppm. The biological relevance of
these observations was not established. No effects were observed on
food intake or urinary parameters and there were no treatment-related
histopathological lesions. The NOEL for this study was 1000 ppm (equal
to 55 mg/kg bw/day). (Murakami et al., 1981).
Dogs
Groups of 6 male and 6 female purebred beagle dogs (24-29 weeks
old) were given diets containing d-phenothrin at 0, 100, 300 or
1000 ppm for 6 months. The diets were prepared weekly. Two batches of
d-phenothrin were used: a 95.5% pure lot was used weeks 1-4 (week 4
for the 100 ppm diet); and a 91.3% pure lot was used weeks 4-26 (week
4 for the 300 and 1000 ppm diets). No deaths occurred and there were
no treatment-related effects or clinical signs of toxicity, food
consumption, haematology, urinalysis, ophthalmoscopic observations, or
gross or histopathology. Body weights were slightly (<10%) lower than
in controls in the 1000 ppm group (both sexes) throughout the study.
Serum alkaline phosphatase levels were elevated in both males and
females in the 1000 ppm group. Liver/body weight ratios were increased
in both males and females at 1000 ppm. The NOEL for this study is
considered to be 300 ppm (equal to 9.3 mg/kg bw/day based on food
intake and body weight). (Pence et al., 1981).
Groups of 4 male and 4 female beagle dogs/dose level were given
diet containing 0, 100, 300, 1000 or 3000 ppm d-phenothrin for 52
weeks. Clinical observations, body weight gain and food consumption
were monitored throughout the study. Blood and urine samples were
analyzed on weeks 13, 26, 39 and 52. All dogs were given
ophthalmoscopic examinations prior to initiation and at termination.
On week 52 all dogs were sacrificed and organ weights were recorded.
Histopathological examinations of about 40 tissues/organs from each
dog were performed.
No deaths occurred in the study. There were no treatment-related
effects on body weight gain, food consumption, haematology, urinalysis
or ophthalmoscopy. Among females emesis was slightly more frequent at
3000 ppm but was not unequivocally treatment-related. Alkaline
phosphatase levels were slightly increased in both males and females
at 3000 ppm but was statistically significant only in males on week 13
and was higher than the expected range only in males. There was a
tendency to reduced serum albumen levels and albumen/globulin ratios
at 10.00 and 3000 ppm but the levels were within the expected range at
the performing laboratory. Liver/body weight ratios were increased in
males and females at 3000 ppm but were statistically significant only
in females. Histopathologically there were increased incidences of the
diagnosis of microcyst in the pituitary in females at 3000 ppm, focal
degeneration accompanied by the presence of acicular crystalline
material in adrenal cortex in males at 3000 ppm and hepatocellular
enlargement in males and females at 3000 ppm. Focal degeneration in
adrenal cortex without crystalline material was observed in one male
at 1000 ppm. One male at 1000 ppm had slight diffuse hepatocellular
enlargement. The authors concluded that the NOEL for this study was
300 ppm (equal to 8.2 mg/kg bw/day) (Cox et al., 1987).
COMMENTS
d-Phenothrin is a mixture of predominantly 1R,cis- and
1R,trans- isomers (cis:trans ratio of 20:80) of the
chrysanthemic acid ester of 3-phenoxybenzyl alcohol.
Following either single or repeated doses of both the 1R,cis-
and 1R, trans- isomers of phenothrin, excretion was virtually
complete within 7 days after dosing. After a single dose of either
isomer the primary route of excretion was via the faeces, being higher
for the cis- isomer. After repeated doses of the cis- isomer the
faecal route was still the predominant route, although the proportion
recovered in faeces was slightly less. Urinary excretion was the
predominant route of elimination of the trans- isomer. Tissue levels
were low with residues primarily in the fat. Tissue levels were higher
following repeated doses than following a single dose. Much of the
material recovered from the faeces was intact phenothrin, which was
considered to be unabsorbed material. Faecal metabolites of the cis-
isomer retained the ester linkage and were derived from oxidations.
Urinary metabolites were formed from both isomers following ester
cleavage after single or repeated dosing in both sexes.
In feeding studies in dogs, mice and rats, liver/body weight
ratios were increased. After one year in dogs and two years in rats
and mice large hepatocytes were observed. Histopathological changes
were also seen in the adrenals of dogs after one year, but not at 6
months. In mice there were slightly increased incidences of
hepatocellular adenomas (both sexes) and carcinomas (in females) in
all treated groups but these were not dose-related. Despite what were
reported to be pulmonary metastases of hepatic carcinomas in both
sexes at 1000 and 3000 ppm, the Meeting did not consider the compound
to be a hepatic carcinogen in this species. However, it was concerned
that the lung lesions be properly identified.
In rats there was an increase in the incidence of adenomas and
carcinomas of the preputial gland in males at 3000 ppm. Since only the
left gland was examined routinely, the true incidence of these tumours
is unknown. Although it would be of interest to have additional data
concerning these tumours, it was considered unlikely that the
incidences of preputial gland tumours were toxicologically relevant.
A two-generation reproduction study in rats was flawed by
consanguinous matings in the first mating period. However, subsequent
matings were non-consanguinous and the offspring of the consanguinous
matings were not used. Therefore, the study was considered acceptable.
No reproductive effects were observed at dose levels up to and
including 3000 ppm (equivalent to 150 mg/kg bw/day). The NOEL for
parental effects (body weight gain depression) was 1000 ppm
(equivalent to 50 mg/kg bw/day).
There was no increase in chromosomal aberrations in Chinese
hamster ovary cells or unscheduled DNA synthesis in human HeLa S3
cells.
Re-examination of the teratology studies in rabbits and mice
evaluated previously (1980 JMPR) indicated that in the mouse study
dosing was on days 7-12 of gestation. This period does not cover the
complete period of organogenesis. No other teratology study in a
rodent species was available. A rodent teratology study covering the
entire period of organogenesis is desirable.
TOXICOLOGICAL INFORMATION
LEVELS CAUSING TO TOXICOLOGICAL EFFECT
Mouse: 300 ppm in the diet, equal to 40.3 mg/kg bw/day
Rat: 1000 ppm in the diet, equivalent to 50 mg/kg bw/day
Dog: 300 ppm in the diet, equal to 7.1 mg/kg bw/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.07 mg/kg bw for "d-phenothrin"
STUDIES WHICH WILL PROVIDE INFORMATION VALUABLE FOR THE CONTINUED
EVALUATION OF THE COMPOUND
1. Histopathological examination of all preputial glands from
the rat oncogenicity study.
2. Re-examination of the metastatic liver tumours in the lung
in the mouse oncogenicity study to determine whether they
represent true metastases.
3. A teratogenicity study in a rodent species.
REFERENCES
Amyes, S.J., Martin, P.A., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Oncogenicity and toxicity study
in mice. Unpublished report from Life Science Research. Submitted by
Sumitomo Chemical Co., Ltd.
Cox, R.H., Sutherland, J.D., Voelker, R.W., Alsaker, R.D., Vargas,
K.J., Lewis, S.A. & Hagen, W.J. 1987. Chronic Toxicity Study in Dogs
with Sumithrin -J.G.- Unpublished report from Hazleton Laboratories
Inc. Submitted by Sumitomo Chemical Co., Ltd.
Forster, R., Tipins, R.S., De Venezia, V. & Nunziata, A. 1984.
Unscheduled DNA synthesis in human cells. Cell Line: HeLa S3. Test
Substance: Sumithrin. Unpublished report from Life Sciences Research,
Roma Toxicology Center. Submitted by Sumitomo Chemical Co., Ltd.
Haseman, J.K. 1983. Patterns of Tumor Incidence in Two-Year Cancer
Bioassay Feeding Studies in Fischer 344 Rats. Fundamental and Applied
Toxicology 3:1-9.
Isobe, N., Matsunaga, H., Nakatsuka, I. & Yoshitake, A. 1987.
Metabolism of (1R, trans) and (1R, cis)-isomers of Phenothrin in
Rats. Unpublished report from Sumitomo Chemical Co., Ltd.
Kogiso, S., Hara, M., Ito, K., Iwawaki, H. & Yoshitake, A. 1986.
In vitro chromosomal aberration test of S-2539F in Chinese hamster
ovary cells (CHO-K1). Unpublished report from Sumitomo Chemical Co.,
Ltd.
Martin, P.A., Amyes, S.J., Ashby, R., Lee, P., Brown, P.M., Fowler,
J.S.L. & Finn, J.P. 1987. Sumithrin: Combined Toxicity and
Oncogenicity Study in Rats. Unpublished Report from Life Science
Research, Submitted by Sumitomo Chemical Co., Ltd.
Murakami, M., Hiromori, T., Ito, S. & Hosokawa, S. 1981. Six Month
Oral Toxicity Study of S2539 Forte (SumithrinR) in Rats. Unpublished
report from Sumitomo Chemical Co., Ltd.
Pence, D.H., Hagen, W.H., Alsaker, R.D., Hastings, T.F., Dawkins,
B.G., Tacey, R.L. & Marshall, P.M. 1981. Subchronic Toxicity Study in
Dogs S2539-F. Unpublished report from Hazleton Laboratories Inc.
Submitted by Sumitomo Chemical Co., Ltd.
Resnik, G. & Ward, J.M. 1981. Morphology of Hyperplastic and
Neoplastic Lesions in the Clitoral and Preputial Gland of the F344
Rat. Veterinary Pathology 18:228-238.
Solleveld, H.A., Haseman, J.K. & McConnell, E.E. 1984. Natural History
of Body Weight Gain, Survival and Neoplasia in the F344 Rat. Journal
of the National Cancer Institute 72(4):929-940.
Tesh, J.M., Willoughby, C.R. & Fowler, J.S.L. 1987. Sumithrin: Effects
upon reproductive performance of rats treated continuously throughout
two successive generations. Unpublished report from Life Science
Research. Submitted by Sumitomo Chemical Co., Ltd.
