
ANILAZINE
EXPLANATION
Anilazine is a fungicide used in controlling fungus diseases
which attack lawns and turf, cereals, coffee, and a wide variety of
vegetables and other crops. It is also used for the control of
potato and tomato leafspots. Anilazine was evaluated for the first
time by the present meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, Distribution and Excretion
Rats
Groups of male albino SD rats received single oral (0.5 or
5 mg/kg), intravenous (0.5 mg/kg), or intraduodenal (0.5 mg/kg doses
of [phenyl-UL-14C] anilazine (s.a. = 70.2 uCi/mg). Female rats
received only single oral doses (5 mg/kg bw of radioactive (RA)
material). Within 48 hours after oral dosing, both sexes eliminated
100% of the administered RA dose in the feces (85%) and urine (15%).
Less than 1 percent of the RA material remained within the body
after 2 days. Most of the renal elimination occurred over the first
6 hours of dosing whereas fecal elimination occurred mainly at the
6-24 hour time interval. Less than 0.001% of the administered RA
material was in expired CO2 over 24 hours. The total RA content of
the whole animal (including all organs and carcass, but excluding GI
tract and contents) was 7% of the administered dose at 1 hour, 4% at
8 hours, 0.5% after 3 days, and 0.2% after 10 days. The highest
concentrations of RA were found at 1 hour in blood (4.46 mcg/g),
kidney (6mcg/g), and liver (1.5 mcg/g), at 4 hours in the GI tract
(34.8 mcg/g) and at 8 hours in the rest of the carcass (0.28 mcg/g).
After 10 days, values of 0.4, 0.06, and 0.11 mcg/g were found in the
blood, kidneys, and liver, respectively. In all other organs and
tissues, the concentration was 0.007 mcg/g or less. No unusual
tissue distribution was observed.
Within 48 hours after i.v. injection, male rats eliminated 85%
of the administered RA dose in the feces (approximately 33%) and
urine (51%), while 15% remained in the body. The finding of RA in
the feces suggested that biliary excretion occurred. Most of the
renal elimination occurred over the first 6 hours, and most of the
fecal excretion over 6 to 24 hours.
After intraduodenal administration to male rats with cannulated
bile ducts, about 30% of the administered RA dose was absorbed from
the digestive tract within 8 hours, and 14% was excreted in the bile
over 24 hours (Eubler et al. 1979).
 Biotransformation
The metabolism of [phenyl-UL-14C]anilazine was characterized
over a 24-hour period in the urine and feces of albino SD rats given
a single oral dose of 5 mg/kg bw. About 16 to 82% of the
administered RA dose was excreted in urine and feces, respectively.
No parent compound was present in urine or feces. The major
identified metabolite was the hydroxylation product,
2-(2-chloroanilino)-s-triazinodione, which comprised about 25% of
the RA found in urine but was present only in insignificant amounts
in feces. Several other unknown polar metabolites, which were
predominantly acidic in nature, were found. In addition, a small
portion of the RA metabolite became less polar as a result of
treatment with diazomethane. The majority of the metabolite could
also be acetylated (Ecker, 1981). The proposed metabolic pathway
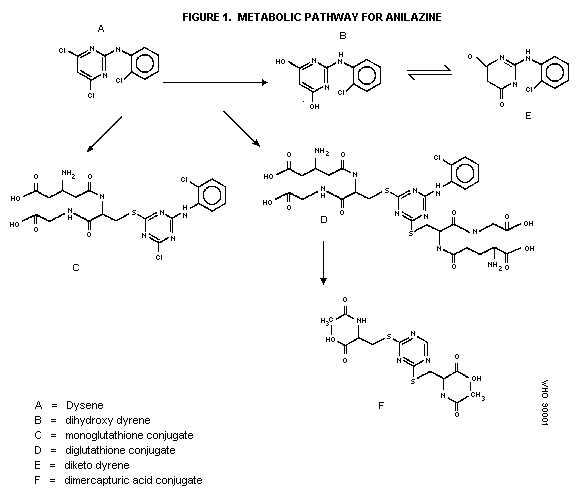
for anilazine in dairy goats is shown in Figure 1.
Another study in male rats comparing the metabolism of
[phenyl-UL-14C]anilazine and [triazine-UL-14C]anilazine (single
oral dose of 5 mg/kg) indicated that the urinary and fecal metabolic
patterns of both labels were identical, suggesting that the basic
structure of the two rings of the molecule were maintained during
biotransformation (Ecker, 1983).
TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON ANILAZINE
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Ames testa S. typhimurium 5-12,500 ug/plate 95.9% Negative Herbold, 1980a
TA98, TA100, in DMSO
TA1535, TA1537
Ames testa S. typhimurium 20-12,500 ug/plate Technical Negative Herbold, 1987
TA98, TA100, in DMSO 97.1%
TA1535, TA1537
Yeast testa S. cerevisiae 1-5000 ? Positive Jagannah, 1981
(Reverse mutation S138, S211 ug/incubation only at
assay tube in DMSO severely
cytoxic
levels
DNA damage in E. coli Pol A+/ 62.5-1000 ug/plate Technical Negative Herbold, 1981
E. colia Pol A- in DMSO 93.5%
CH-V79/HGPRT gene Chinese hamster 0.2-9 ug/mL in 97.3% Negative Miltenburger, 1986
mutation assaya cell line V79 DMSO
Mouse lymphoma L5178Y mouse 2.5-9 ug/mL in Technical Positive Witterland, 1984
forward mutation lymphoma cells DMSO 95.9% (nonactivated
assaya (TK+/-) only)
Unscheduled DNA Primary hepatocytes 0.049-9.8 ug/mL Technical Negative Myhr, 1983
synthesis from male F344 rats 98.6%
Mouse micronucleus Bone marrow 7500 mg/kg single 97.1% Negative Herbold, 1988a
test erythro-blasts oral dose
(NMRI mice)
TABLE 1 (contd.)
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Mouse micronucleus Bone marrow 100-200 mg/kg 95.9% Negative Herbold, 1980b
test erythro-blasts bw/day for 2 days
(NMRI mice)
Spot test in mice Coat pigment cells 3-30 mg/kg bw i.p. 97.3% Negative Herbold, 1986a
embryos to evaluate of crossbred on gestation day 10 in F1 mice
somatic gene changes C57B1/6J XT mice to pregnant females
Dominant lethal test Mouse (male) 5000 or 7500 mg/kg bw 97.3% Negative Herbold, 1988b
(single or oral dose)
Dominant lethal test Mouse (male) 500 mg/kg bw or 94% Negative Herbold, 1978
(single oral dose)
Chromosome aberration Chinese hamster 300-3000 mg/kg bw Technical Negative Völkner, 1987
test bone marrow (single oral dose)
Cytogenic testa Human lymphocyte 3-30 ug/ml in DMSO Technical Positive Herbold, 1986b
cultures (in vitro)
a with and without metabolic activation.
Toxicological studies
Acute toxicity
The acute toxicity of anilazine in several species is given in
Table 2. After oral administration to rats and cats, the most
common signs of toxicity were diarrhea and vomiting, respectively.
After dermal administration to rabbits, mild skin irritation
manifested as edema and erythema was observed. Anilazine was more
toxic by intraperitoneal injection than by other routes of
administration, suggesting poor enteral and percutaneous absorption.
TABLE 2. ACUTE TOXICITY OF ANILAZINE 1,2
LD50 LC50
SPECIES SEX ROUTE mg/kg/bwt (mg/l) REFERENCE
Mouse M oral approx 5000 - Flucke & Kimmerle, 1977
F 367 -
Mouse M s.c. > 5000 - Flucke & Kimmerle, 1977
F 3512 -
Rat M&F oral > 5000 - Flucke & Kimmerle, 1977
Hixson, 1979, 1980a
Heimann, 1985
M oral 3650 - Flucke, 1978
M&F i.p.3 71-137 - Flucke & Kimmerle, 1977
Heimann, 1984b
M&F inhal. - > 0.729 Sangha, 1984
M&F percutaneous > 5000 - Flucke & Kimmerle, 1977
Rabbit M&F dermal > 2000 - Hixson, 1980b, 1980c
Cat F oral > 500 - Flucke & Kimmerle, 1977
1 All studies performed using technical grade anilazine except for one LD50 study in
rats where 50% wettable powder was tested (Hixson, 1979), and two other LD50 where
formulated anilazine containing 40.5% a.i. was tested (Hixson, 1980a, 1980c).
2 The acute oral toxicity of dihydroxy-anilazine, a metabolite of anilazine was also
tested in rats. The LD50 value was > 5000 mg/kg (Heimann, 1984a).
3 The acute i.p. toxicity in male rats of anilazine (LD50 = 137 mg/kg) in combination
with triadimefon (LD50 = 317 mg/kg) was determined to be additive (LD50 of combination =
194 mg/kg) (Heimann, 1984b)
Short-term studies
Rats
SPF Wistar albino rats (20/sex/group) were administered
anilazine (technical grade, 92% pure) by gavage as an aqueous
emulsion at dose levels of 0, 30, 100, and 300 mg/kg bw/day for 28
days. The treatment period was followed by an additional 4-week
observation period. Half of the males in each group were sacrificed
at the end of the 28-day treatment period and the other half at the
end of the 4-week observation period. Hematology, clinical
chemistry, and urinalysis were performed in selected animals at each
time period prior to sacrifice. Animals were necropsied and
selected organs weighed. Histopathological examinations were
performed on 5 rats/sex/group from the 28-day treatment phase of the
study (16 tissues examined) and from 5 rats/sex/group from the
post-treatment observation phase of the study (3 tissues examined,
i.e., stomach, intestines, and esophagus).
During the 28-day treatment period, eight animals died (2/5
females at 100 mg/kg bw/day on day 11, and 1/5 males and 5/5 females
at 300 mg/kg bw/day on days 11-13). Gross pathology examination of
these animals indicated the presence of eroded abdominal and
thoracic viscera and lung lesions. Other changes seen in treated
animals were alterations in behaviour (apathy and dyspnea staring on
days 5-7 and lasting for 3 to 4 weeks) in females given 100 mg/kg
bw/day and in both sexes of rats at 300 mg/kg bw/day, and reductions
in body weight in males at 300 mg/kg bw/day on weeks 1 to 4. The
only observed treatment-related histological effect was
hyperkeratosis of the cutaneous mucosa of the proventricular part of
the stomach. The severity of the hyperkeratosis was also
dose-related, ranging from "trace" at the low-dose level; "minimal"
at the mid-dose level; and "moderate" at the high-dose level. The
finding was attributed to a local irritant effect of anilazine on
the mucosa. No clear treatment-related hematological, clinical
chemistry, urinalysis, or organ weight changes were observed during
the treatment period. None of the above described adverse effects
were seen in surviving rats similarly treated with anilazine but
allowed to recover for 4 additional weeks. The NOAEL for systemic
toxicity for anilazine, when administered by gavage to rats, was
30 mg/kg bw/day. However, stomach mucosal hyperkeratosis,
representing a local irritant effect, was seen at all of the tested
doses of anilazine (Flucke & Kaliner, 1978).
Groups of Sprague-Dawley CD rats (25 males and 25
females/group) received dietary concentrations of 0, 500, 2000, or
8000 ppm anilazine (93.5% pure) for 13 weeks. Five rats/sex/dose
group were sacrificed after 6 weeks.
No treatment-related deaths occurred. Body weight gain was
reduced over the study in mid- and high-dose males and in high-dose
females. Other changes were seen only in high-dose males and
females at the end of the study, and in many cases at the 6-week
interim kill period as well. These included clinical signs of
toxicity (piloerection, red-brown staining of fur around head and
back, rhinorrhea, and exophthalmos), and reduced food consumption,
increased alkaline phosphatase levels, reduction in lymphocytes,
creatinine and total protein levels, increases in the relative
weights of several organs (heart, brain, pituitary, gonads, adrenal
glands, thyroids, kidneys, and lungs), and various pathological
findings (pale appearing pancreas, foamy macrophage foci in the
lungs, testicular atrophy, lymph node hyperplasia, and enlarged
salivary glands with heightened secretory activity). As indicated
by histopathological analysis, the primary target organ of toxicity
appeared to be the forestomach. At the 6-week interim kill period,
a thickened forestomach mucosa was observed that was characterized
by dyskeratosis, acanthosis, hyperkeratosis, and edema in most of
the male and female rats examined at the mid- and high-dose levels.
At the 13-week terminal sacrifice period hyperkeratosis/hyperplasia
of the forestomach occurred at the mid- and high-dose levels at
incidences of 3/20 and 18/20, respectively, in male rats and 9/20
and 16/20, respectively, in females. These changes were accompanied
in some cases by ulcers and/or erosions of the forestomach, and were
considered to be a local irritant effect of anilazine on the mucosa.
The only finding at the lowest dose level was slight acanthosis and
hyperkeratosis of skin on the tails of female rats at the 13-week
sacrifice period (incidence: 2/20 controls; 5/20 low dose; 8/20
mid-dose, and 10/20 high dose). The findings occurred in male rats
only at the mid (1/20) and high (11/20) dose levels. The tail
lesions are of questionable biological significance. The NOAEL was
considered to be 25 mg/kg bw/day due to the observed reduction in
body weight gain in males and the local forestomach lesions in both
sexes (Goodyer, 1981).
Dogs
Purebred beagle dogs (4/sex/dose) were administered anilazine
(92.0% pure) orally (gelatin capsules) at dose levels of 0, 50, 150,
and 450 mg/kg bw/day for 13 weeks.
There were no effects of treatment on mortality, or on
patellar, flexor or extensor reflex activity or body temperature.
No adverse effects were observed with respect to ophthalmoscopic
examinations, or hematological or urinalysis parameters. Vomiting
and diarrhea several hours after dosing were prominent signs of
toxicity in both sexes administered all three dose levels. These
effects were dose-related and occurred throughout the study. Test
compound was frequently seen in vomitus and feces. The animals also
were agitated and ran back and forth in their cages before
defecation (the effect was attributed to possible intestinal spasms
evoked by the compound). Additional signs of toxicity were also
observed. Changes noted at both the 150 and 450 mg/kg bw/day levels
included reductions in pulse rate and slight drowsiness, reduced
serum albumin levels and increased plasma globulin levels (i.e.,
alpha-1, alpha-2, beta, and gamma globulin levels were increased),
and (in females), decreases in absolute and relative ovary weights.
Changes that occurred only at the 450 mg/kg bw/day dose level were
dull looking ungroomed hair coats, reductions in body weight gain
and food consumption, and reductions in total serum protein levels
and serum calcium levels. The absolute and relative weights of the
thymus gland were reduced, and thymus gland atrophy was observed
grossly, in high-dose males and females. Upon histological
examination, thymus gland atrophy was seen at all of the dose levels
tested in males and the highest dose in females. Histological
examination of the esophagus also revealed an increase in the
incidence of diffuse round cell infiltration of the mucosa at all
three dose levels. A NOAEL was not established in this study
(Hoffmann & Gröning, 1979).
Beagle dogs (4/sex/group) were administered anilazine
a.i.(91.5-94.4% pure) orally (gelatin capsules) at dose levels of 0,
10, 40, and 160 mg/kg bw/day for 18 months.
Treatment-related toxicological effects were produced by the
two highest dose levels of anilazine. Adverse findings that
occurred at 40 and 160 mg/kg bw/day in both males and females
included vomiting of the test substance with food, increases in
alpha-2, beta, and gamma globulins in plasma, and an increase in the
relative weight of the adrenal glands. Additional changes that were
seen in dogs of both sexes only at the highest dose level (160 mg/kg
bw/day) included salivation, loose-to-liquid feces, reduction in
bilirubin levels, and (in females) a decrease in the absolute weight
of the ovaries. High-dose animals also showed a tendency for a
decreased body weight gain which was due primarily to one male and
one female dog, although weight gain was also somewhat low in all of
the remaining dogs given the high dose level. One male dog in the
high-dose group was diagnosed with confluent bronchial pneumonia of
the right lung and suppurative bronchitis with increased blood
leukocytes and heightened hematopoiesis in bone marrow.
Esophagitis, gastritis, thymus involution, release of splenic
corpuscles and lymphadenitis were also noted in this animal. The
bronchial pneumonia was attributed to a bacterial or viral-induced
infection and was not considered to be compound-induced.
Anilazine did not produce treatment-related effects on
survival, vital signs (i.e., reflex activity, temperature, or pulse
rate) or food consumption, nor were adverse ophthalmological
findings observed. Urinalysis parameters were unchanged. No
treatment-related non-neoplastic or neoplastic lesions were evident.
The NOAEL was 10 mg/kg bw/day anilazine (Hoffmann et al. 1983;
Hoffmann et al. 1987; Hoffmann et al. 1988).
Long-term/carcinogenicity studies
Mice
Male and female Charles River CD-1 mice (50/sex/dose) were fed
diets containing 0, 50, 250, or 1250 ppm anilazine (93.5% pure) for
104 weeks. Sixteen additional mice/sex group served solely for the
collection of blood samples, and were not examined
histopathologically. The average dietary concentrations were
equivalent to 7.5, 37.5, and 290.0 mg/kg bw/day. Blood was collected
at 0, 27, 53, 79, and 103 weeks). The weights of liver, kidneys,
gonads, heart, and brain were obtained.
There were no effects of treatment on mortality, clinical signs
of toxicity, body weight, food consumption, hematology, or clinical
chemistry. Plasma glucose levels were elevated in occasional male
and female mice in the 250 and 1250 ppm groups. There were no
treatment-related effects on organ weights and no remarkable
findings at gross necropsy or after histopathological examination.
The incidences of Harderian gland adenomas were elevated in males
and females in the high-dose group. These tumour incidences,
however, were neither statistically significant nor above historical
control levels. On the basis of these findings the NOAEL in this
study exceeded 1250 ppm in the diet, equal to approximately
190.0 mg/kg bw/day in males and females (Goodyer, 1984a).
Rats
Male and female Sprague-Dawley CD rats (50/sex/group) were fed
anilazine (93.5% pure) for 104 weeks at levels of 0, 50, 330, or
2000 ppm. These concentrations were equivalent to approximately
2.5, 16.5 and 100 mg/kg bw/day. Ten additional rats/sex/dose were
used solely for the collection of blood samples; these animals were
not examined histopathologically. Blood was collected at 0, 26/27,
53, 79 and 103 weeks. Urinalysis studies, however, were not
performed. The weights of liver, kidney gonads, heart, brain,
adrenals, spleen, lungs, and thyroid glands were obtained.
There were no clinical signs of toxicity and no effect of
treatment on mortality. There were no treatment-related effects on
body weight or food consumption throughout the study with the
exception of transient, parallel decreases in these parameters
during study week number 1 in males and females given 2000 ppm.
Hematology and clinical chemistry findings were generally
unremarkable. There was no treatment-related effect on organ
weights and no remarkable findings at gross necropsy or after
histopathological examination. There were no increases in tumours
that were treatment-related. The NOAEL exceeded 2000 ppm in the
diet, equivalent to more than 100 mg/kg bw/day in males and females
(Goodyer, 1984b).
Special carcinogenicity bioassays of anilazine in rodents (rats
and mice) were also performed by the National Cancer Institute but
they were not considered for toxicological evaluation (NCI, 1978).
However, since these studies were performed by Gulf South Research
Corporation and were not validated.
Reproduction study
Rats
Groups of male (15/sex/dose) and female (30/sex/dose) Sprague-
Dawley rats in each of three generations (F0, F1, and F2) were
fed diets containing 0, 50, 330, or 2000 ppm anilazine (93.5% pure).
The dietary concentrations were equivalent to 2.5, 16.5, and 100
mg/kg bw/day. After maturation periods of 15 weeks in the F0
generation and 18 weeks in the F1 and F2 generations, the parental
animals were allowed to rear their offspring to weaning (F0-F1a;
F1-F2a; F2-F3a). The animals for the F1 and F2 generations
were randomly selected from the F1a and F2a litters, avoiding
sibling matings.
No effects of treatment on mortality, clinical signs, food
consumption, or body weight of parental rats were noted. Although
slight inhibition of body weight gain was noted in F0 parental
males from week 5 until termination, the response was neither
statistically significant nor dose-related. The highest dietary
level of anilazine (2000 ppm) had an apparent adverse effect on the
libido of F0 males and the fertility (and possible fecundity) of
F0 females. This was indicated, respectively, by reductions in the
number of females that mated and the number that became pregnant in
comparison to controls. Although the respective differences were
not remarkable nor evident in subsequent generations (F1 and F2),
they were verified by a crossover mating experiment involving rats
from the control and high-dose groups. No adverse effects on the
reproductive parameters were evident at anilazine dietary levels of
330 and 50 ppm. No adverse changes occurred with respect to litter
size, viability, mean pup and litter weights, or physical (i.e.,
auditory response, pupillary reflex, visual placing response, grip
strength) and functional (i.e., onset and duration of pinna
unfolding, onset of hair growth, incisor eruption, and eye opening)
development of pups. No unusual pre- or post-weaning clinical
observations occurred. No treatment-related changes in organ weight
or pathological (gross and microscopic) findings were noted in
either parenteral rats or pups at necropsy (Irvine, 1984). The
NOAEL is 330 ppm, equivalent to 16.5 mg/kg bw/day.
Special studies on teratogenicity
Rats
Groups of female Long Evans FB-30 strain rats (21-22/dose) were
administered doses of 0, 30, 100, or 300 mg/kg bw/day anilazine, by
gavage, from days 6 to 25 of gestation (Day 0 = day of
insemination). Doses were based on a preliminary range-finding
study which demonstrated that a dose of 300 mg/kg bw/day for 10 days
had no apparent adverse activity. On gestation day 20, rats were
sacrificed and fetuses delivered by C-section. Approximately 30% of
the fetuses in each group were dissected and examined for visceral
anomalies and approximately 70% of the fetuses in each group were
cleared and stained for skeletal observations.
The 300 mg/kg bw/day dose of anilazine produced a significant
reduction in body weight gain in the dams during the treatment
period as well as throughout the entire gestation period. Body
weight gain, however, was not influenced by 30 or 100 mg/kg bw/day
anilazine. None of the doses produced changes in mortality, physical
appearance, or behaviour, or the fertilization ratio or pregnancy
ratio in the dams. Similarly, none of the doses appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. Anilazine was neither fetotoxic, embryotoxic, nor
teratogenic in rats at oral doses up to 300 mg/kg/day (highest dose
tested). Because of maternal toxicity (weight gain reduction) at
300 mg/kg bw/day, the NOAEL is 100 mg/kg bw/day by gavage (Machemer,
1978).
Groups of female Charles River Crl:CD-BR rats (28/dose) were
administered oral (gastric intubation) doses of 0, 150, 500, or
1500 mg/kg bw/day anilazine from days 6 to 15 of gestation. On
gestation day 20, rats were sacrificed and fetuses delivered by
C-section. Approximately one-half of the fetuses in each group were
dissected and examined for visceral anomalies, and the other half of
the fetuses in each group were cleared and stained for skeletal
observations.
Anilazine was maternally toxic at doses of 500 and 1500 mg/kg
bw/day. The adverse findings were: 1) an increased incidence of
loose and/or soft stools that often contained a creamy white
substance (seen in 28.6 and 100% of dams at 500 and 1500 mg/kg
bw/day, respectively); 2) significant reductions in body weight and
body weight gain in dams given 1500 mg/kg bw/day on gestation days 6
to 25 and 0 to 20; 3) a significant reduction in food consumption
in dams given 500 and 1500 mg/kg bw/day on gestation day 7 only; and
4) an increased incidence of thickened and coarse mucosa of the
nonglandular portion of the stomach (seen in 18 and 15% of dams
given 500 and 1500 mg/kg bw/day, respectively). This change was
characterized microscopically as epithelial hyperplasia (acanthosis)
and hyperkeratosis of the mucus membrane. In contrast to these
results, none of the doses of anilazine induced effects on embryo or
fetal development, and no teratogenic effects were observed. The
NOAEL was 150 mg/kg bw/day by intubation (Kowalski et al. 1988).
Rabbits
Groups of female Himalayan rabbits (13/dose) were administered
0, 5, 15 and 45 mg/kg bw/day anilazine (92.4% pure) from days 6 to
18 of gestation (Day 0 = day of insemination). The animals were
sacrificed on gestation day 29 and fetuses delivered by C-section.
All fetuses were necropsied and abdominal and thoracic organs as
well as the brain examined for visceral malformations. All fetuses
were examined for skeletal defects.
No treatment-related changes in mortality, appearance,
behaviour or weight gain were observed in the does. The
fertilization and pregnancy ratios were not adversely affected.
There were no statistically significant dose-related effects on the
number of implantations or corpora lutea, sex ratio, mean fetal
weights, mean placental weights, fetuses showing visceral
malformations, or numbers of fetuses with stunted growth. No
treatment-related skeletal abnormalities or malformations were
observed. The highest dose tested, 45 mg/kg bw/day by gavage, is a
NOAEL for maternal, embryo-, and fetotoxicity, and for
teratogenicity in rabbits (Roetz, 1981).
Special studies on mutagenicity
Anilazine was mutagenic in a cytogenetic study in human
lymphocyte cultures in vitro (both with and without metabolic
activation), in a mouse lymphoma forward mutation assay (without
metabolic activation only), and at severely cytotoxic levels in a
reverse mutation assay in yeast (both with and without metabolic
activation). In contrast, the chemical was negative in a variety of
the other in vitro and in vivo systems. The weight-of-the-
evidence indicates that anilazine is not genotoxic.
Special studies on eye and skin irritation
Instillation of 100 mg grade anilazine (40.5% a.i. formulation)
in the rabbit eye for 45 seconds produced reversible iritis, slight
erythema, chemosis, and discharge which lasted for up to 13 days
(Hixson, 1980b).
Anilazine (90 mg of a technical grade formulation) produced a
strong irritant effect (redness, swelling, and lacrimation) on the
rabbit eye when placed in the conjunctival sac for 24 hours. The
reactions were reversible within 14 days. No corrosive effect
occurred (Pauluhn, 1983).
Slight primary skin irritation (erythema and edema) was
observed when anilazine (500 mg technical) was applied to shaved
rabbit skin for 4 hours. The reactions were reversible within 7
days. No corrosive effect occurred (Pauluhn, 1983).
In a 3-week dermal toxicity study in rabbits, 0%, 1% (10 mg/ml)
and 4% (40 mg/mL) of anilazine was applied to shaved skin at a
volume of 0.5 ml/kg for 6 hours/day, 5X/week, for 3 weeks. In this
study, a formulation containing 497 grams a.i.(litre (= 38.4% w/w)
was used. Both concentrations of the compound produced dermal
changes consisting of slight erythema and cellular infiltration in
the subepidermal region; in addition, slight thickening of the
epidermis was noted 40 mg/ml. However, no systemic changes were
observed as noted from hematological, clinical chemistry and
urinalysis examinations, by measurements of body weight gain, and by
histopathological evaluation of selected organs and tissues. The
systemic NOAEL was calculated to be approximately 7.7 mg a.i./kg
bw/day. (Heimann & Schilde, 1985).
Special studies on skin sensitizing effect
Anilazine was shown to be a dermal sensitizer (i.e., to induce
redness and swelling) when tested in guinea pigs by the Buehler
Patch test (Heimann, 1988a; Heimann, 1988b), the Magnusson and
Kligman test (Heimann, 1982a), and in Klecak's open epicutaneous
test (Heimann, 1982b). The positive findings were obtained using
either pure and/or technical anilazine in induction concentrations
ranging from 0.03 to 5%, and challenge doses ranging from 0.03 to
1%. Negative results for sensitizing potential were obtained in one
study (Klecak's test) with pure anilazine at induction
concentrations of 0.01 to 0.1% and challenge doses of 0.01 to 0.3%
(Heimann, 1983).
Observations in humans
A group of farm workers employed on a single farm in the United
States arrived at a hospital emergency room with severe dermatitis
in July 1980. All were exposed to three pesticides in a tomato
field (anilazine, benomyl, and endosulfan). Seven of the workers
were available for patch testing to the three pesticides. Delayed
hypersensitivity (48-hour eczematous reaction) to 0.1% Dyrene
(anilazine) was demonstrated in 6 of the 7 affected workers. Nine
non-exposed adult volunteers who were used as control subjects had
negative patch tests to 0.1% Dyrene. The conclusion was reached
that anilazine caused allergic contact dermatitis (Schuman et al.
1980).
COMMENTS
Anilazine administered orally to rats was excreted primarily in
the feces via biliary excretion, and also in urine. Anilazine was
biotransformed in rats primarily to the hydroxylation product,
2-2-chloroanilino)-s-triazinedione, which was present only in
urine. A second biotransformation pathway involving conjugate
formation via the glutathione - mercapturic acid pathway was also
observed in goats and chickens. No unusual organ or tissue
localization of (14C)-labelled anilazine was observed.
Anilazine has a low acute toxicity in the species examined.
Subchronic studies in rats indicated that the stomach was the
primary target organ of toxicity; oral gavage doses of aqueous
anilazine emulsion of 30 mg/kg bw/day and a dietary level of
2000 ppm (equivalent to 100 mg/kg bw/day) or greater produced
forestomach changes characterized by acanthosis, hyperkeratosis, and
areas of ulceration and erosion. The NOAEL was 500 ppm (equivalent
to 25 mg/kg bw/day).
In an 18-month oral (capsule) toxicity study in dogs, anilazine
at doses of 40 and 160 mg/kg bw/day produced increases in alpha-1,
beta-2, and gamma-globulins in plasma, vomiting, and diarrhea in
males and females. These findings were also observed with oral
doses (capsules) of 50 to 450 mg/kg bw/day for 13 weeks in dogs.
The NOAEL in the 18-month study was 10 mg/kg bw/day.
In long-term studies in rats and mice, no treatment-related
increases in neoplasms were observed. The NOAELs were 1250 ppm
(equal to 188 mg/kg bw/day) in Cr CD-1 mice and 2000 ppm (equivalent
to 100 mg/kg bw/day) in SD-CD rats.
In a 3-generation, 2 litters per generation, reproduction study
in rats, reductions in male libido and female fertility occurred in
F0 generation animals at 2000 ppm in the diet (equivalent to
100 mg/kg bw/day). Although these effects were confirmed in
cross-mating experiments, they were not observed in subsequent
generations. The NOAEL was 330 ppm (equivalent to 16.5 mg/kg
bw/day).
In teratology studies in rats, the NOAEL was 150 mg/kg bw/day
based on maternal toxicity. Oral doses of 300 to 1500 mg/kg bw/day
produced soft/loose stools, weight loss, reduced food consumption
and acanthosis and hyperkeratosis of the stomach of dams, but had no
fetotoxic or teratogenic activity. The NOAEL was 45 mg/kg bw/day
(highest dose tested) in a rabbit teratology study.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 1250 ppm in the diet, equal to 188 mg/kg bw/day
Rat: 330 ppm in the diet, equivalent to 16.5 mg/kg bw/day
Dog: 10 mg/kg bw/day orally.
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ecker, W. (1981) [Phenyl-UL-14C]Anilazine: Characterization of
metabolites in rat urine and feces. Unpublished Report No. 10216
from Institute of Pharmacokinetics, Bayer AG. Submitted to WHO by
Bayer AG, Wuppertal, FRG.
Ecker, W. (1983) [Triazine-UL-14C]Anilazine: Characterization of
metabolites in rat urine; comparison of metabolite patterns after
administration of [Triazine-UL-14C]Anilazine and [Phenyl-UL-
14C]Anilazine. Unpublished Report No. 11610 from Institute of
Pharmacokinetics, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Eubler, H., Traber, J., Walk, R.A. (1979) Biokinetics of
14C-Anilazine on the rat. Unpublished Report No. A0197/1526 from
INBIFO Research, Cologne. Submitted to WHO by Bayer AG, Wuppertal,
FRG.
Flucke, W. (1978) Determination of acute toxicity (LD50);
refrigerator sample of June 21. Unpublished Report of August 11
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Flucke, W., Kaliner, G. (1978) Dyrene: subacute oral toxicity study
on rats. Unpublished Report No. 7844 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Flucke, W., Kimmerle, G. (1977) Dyrene: acute toxicity studies.
Unpublished Report No. 6761 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Goodyer, M.J. (1981) Anilazine: a 13-week oral (dietary
administration) toxicity study in the rat with a 6-week interim
kill. Unpublished Report No. 2660-262/23 from Hazleton
Laboratories Europe, Ltd., Harrogate, England. Submitted to WHO by
Bayer AG, Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984a) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the mouse.
Unpublished Report No. 3622-262/22 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984b) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the rat.
Unpublished Report No. 3686-262/24 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Heimann, K.G. (1982a) Technical dyrene: study of sensitization
effect on guinea pigs (maximization test of Magnusson and Kligman).
Unpublished Report No. 11059 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1982b) Dyrene: investigation of sensitizing effect
in the open epicutaneous test on guinea pigs. Unpublished Report
No. 10816 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1983) Dyrene pure active ingredient: study for
skin-sensitizing effect in the open epicutaneous test on guinea
pigs. Unpublished Report No. 12034 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50).
Unpublished Report of August 20 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984b) Anilazine and MEB 6447: study for combination
toxicity. Unpublished Report No. 12609 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1985) Determination of acute toxicity (LD50).
Unpublished Report of February 20 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1988a) Dyrene: study for skin-sensitizing effect on
guinea pigs (Buehler Patch Test). Unpublished Report No. 16419 from
Toxicology Division Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Heimann, K.G. (1988b) Dyrene Technical: study for skin-sensitizing
effect in the open epicutaneous test on guinea pigs (Buehler Patch
Test). Unpublished Report No. 16420 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G., Schilde, B. (1985) Dyrene: subacute study of dermal
toxicity to rabbits. Unpublished Report No. 14151 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1978) Dyrene: dominant lethal study on male mouse to
test for mutagenic effects. Unpublished Report No. 7448 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980a) Dyrene: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished Report No. 9172 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980b) Dyrene: micronucleus test on mouse to evaluate
dyrene for mutagenic potential. Unpublished Report No. 9565 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1981) Dyrene: PolA1 - test on E. coli to evaluate for
DNA damage. Unpublished Report No. 10149 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1986a) Spot test on cross-bred C57B1/6J x T stock
mouse embryos to evaluate for induced somatic changes in the genes
of the coat pigment cells. Unpublished Report No. 15041 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1986b) Dyrene: cytogenic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on chromosomes.
Unpublished Report No. 14188 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1987) Anizaline Tech. Salmonella/microsome test for
determination of point mutations. Unpublished Report No. 15780 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1988a) Anizaline: micronucleus test on the mouse for
clastogenic effects. Unpublished Report No. 16481 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1988b) Dyrene: dominant lethal test on the male mouse
to evaluate for mutagenic effects. Unpublished Report No. 16688
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Hixson, E.J. (1979) Acute oral toxicity study of Dyrene 50%
wettable powder to rats. Unpublished Report No. 68411 from Mobay
Chemical Corporation, Kansas City MO. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Hixson, E.J. (1980a) Acute oral toxicity study of Dyrene 4F to
rats. Unpublished Report No. 118 from Mobay Chemical Corporation,
Kansas City MO. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980b) Dermal irritation of Dyrene 4F. Unpublished
Report No. 123 from Mobay Chemical Corporation, Stillwell, KS.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980c) Acute oral toxicity of Dyrene 4F to rabbits.
Unpublished Report No. 126 from Mobay Chemical Corporation,
Stillwell, KS. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hoffmann, K., Gröning, P. (1979) Dyrene: subchronic toxicity study
on dogs (13-week oral administration in capsules). Unpublished
Report No. 8199 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer, AG, Wuppertal, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1983) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Unpublished Report No. 11613 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Bayerwerk, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1987) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1988) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Second Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Irvine, L.F.H. (1984) Anilazine: 3-generation oral (dietary
administration) reproduction study in the rat. Unpublished Report
No. 3744-262/26 from Hazleton Laboratories Europe, Ltd., Harrogate,
England. Submitted to WHO by Bayer AG, Institute of Toxicology,
Wuppertal, FRG.
Jagannath, D.R. (1981) Mutagenicity evaluation of Dyrene in the
saccharomyces reverse mutation assay. Unpublished Report No. R2088
from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
Kowalski, R.L., Clemens, G.R., Hartnagel, R.E. Jr (1988) A
teratology study with anilazine (Dyrene Technical) in the rat.
Unpublished Report No. 1069 from Toxicology Department, Miles, Inc.,
USA. Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Machemer, L. (1978) Dyrene: evaluation for embryotoxic and
teratogenic effects on orally dosed rats. Unpublished Report
No. 7746 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Miltenberger, H.G. (2986) Anilazine Techn. Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with V79
cells. Unpublished Report No. R3821 from Laboratory for
Mutagenicity Testing, Technical University, Darmstadt. Submitted to
WHO by Bayer, AG, Wuppertal, FRG.
Myhr, B.C. (1983) Evaluation of Analazine Technical in the primary
rat hepatocyte unscheduled DNA synthesis assay. Unpublished Report
No. R2615 from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
NCI (1978) NCI (National Cancer Institute) Carcinogenesis technical
report series No. 104. Bioassay of anilazine for possible
carcinogenicity. US Department of Health, Education and Welfare
Publication No.(NIH) 78-1354.
Pauluhn, J. (1983) Dyrene (technical): study for irritant and
corrosive effect on skin and eye (rabbits). Unpublished Report
No. 11826 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Roetz, R. (1981) Dyrene: evaluation for embryotoxic and/or
teratogenic effects on the rabbit after oral administration.
Unpublished Report No. 10339 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Sangha, G.K. (1984) Acute inhalation toxicity study with Anilazine
(Dyrene) technical in rats. Unpublished Report No. 463 from Mobay
Chemical Corporation, Stillwell, KS. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Schuman, S.H., Dobson, R.L., Fingar, J.R. (1980) Dyrene dermatitis.
Lancet, page 1252, December 6, 1980.
Volkner, W. (1987) Chromosome aberration test in bone marrow cells
of the Chinese hamster with Anilazine Technical. Unpublished Report
No. R4100 from Cytotest Cell Research GmBH & Company, KG, Darmstadt,
FRG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Witterland, W.F. (1984) Mutagenicity evaluation of Anilazine
Technical in the mouse lymphoma forward mutation assay. Unpublished
Report No. R2831 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer AG, Wuppertal, FRG.
Biotransformation
The metabolism of [phenyl-UL-14C]anilazine was characterized
over a 24-hour period in the urine and feces of albino SD rats given
a single oral dose of 5 mg/kg bw. About 16 to 82% of the
administered RA dose was excreted in urine and feces, respectively.
No parent compound was present in urine or feces. The major
identified metabolite was the hydroxylation product,
2-(2-chloroanilino)-s-triazinodione, which comprised about 25% of
the RA found in urine but was present only in insignificant amounts
in feces. Several other unknown polar metabolites, which were
predominantly acidic in nature, were found. In addition, a small
portion of the RA metabolite became less polar as a result of
treatment with diazomethane. The majority of the metabolite could
also be acetylated (Ecker, 1981). The proposed metabolic pathway
for anilazine in dairy goats is shown in Figure 1.
Another study in male rats comparing the metabolism of
[phenyl-UL-14C]anilazine and [triazine-UL-14C]anilazine (single
oral dose of 5 mg/kg) indicated that the urinary and fecal metabolic
patterns of both labels were identical, suggesting that the basic
structure of the two rings of the molecule were maintained during
biotransformation (Ecker, 1983).
TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON ANILAZINE
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Ames testa S. typhimurium 5-12,500 ug/plate 95.9% Negative Herbold, 1980a
TA98, TA100, in DMSO
TA1535, TA1537
Ames testa S. typhimurium 20-12,500 ug/plate Technical Negative Herbold, 1987
TA98, TA100, in DMSO 97.1%
TA1535, TA1537
Yeast testa S. cerevisiae 1-5000 ? Positive Jagannah, 1981
(Reverse mutation S138, S211 ug/incubation only at
assay tube in DMSO severely
cytoxic
levels
DNA damage in E. coli Pol A+/ 62.5-1000 ug/plate Technical Negative Herbold, 1981
E. colia Pol A- in DMSO 93.5%
CH-V79/HGPRT gene Chinese hamster 0.2-9 ug/mL in 97.3% Negative Miltenburger, 1986
mutation assaya cell line V79 DMSO
Mouse lymphoma L5178Y mouse 2.5-9 ug/mL in Technical Positive Witterland, 1984
forward mutation lymphoma cells DMSO 95.9% (nonactivated
assaya (TK+/-) only)
Unscheduled DNA Primary hepatocytes 0.049-9.8 ug/mL Technical Negative Myhr, 1983
synthesis from male F344 rats 98.6%
Mouse micronucleus Bone marrow 7500 mg/kg single 97.1% Negative Herbold, 1988a
test erythro-blasts oral dose
(NMRI mice)
TABLE 1 (contd.)
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Mouse micronucleus Bone marrow 100-200 mg/kg 95.9% Negative Herbold, 1980b
test erythro-blasts bw/day for 2 days
(NMRI mice)
Spot test in mice Coat pigment cells 3-30 mg/kg bw i.p. 97.3% Negative Herbold, 1986a
embryos to evaluate of crossbred on gestation day 10 in F1 mice
somatic gene changes C57B1/6J XT mice to pregnant females
Dominant lethal test Mouse (male) 5000 or 7500 mg/kg bw 97.3% Negative Herbold, 1988b
(single or oral dose)
Dominant lethal test Mouse (male) 500 mg/kg bw or 94% Negative Herbold, 1978
(single oral dose)
Chromosome aberration Chinese hamster 300-3000 mg/kg bw Technical Negative Völkner, 1987
test bone marrow (single oral dose)
Cytogenic testa Human lymphocyte 3-30 ug/ml in DMSO Technical Positive Herbold, 1986b
cultures (in vitro)
a with and without metabolic activation.
Toxicological studies
Acute toxicity
The acute toxicity of anilazine in several species is given in
Table 2. After oral administration to rats and cats, the most
common signs of toxicity were diarrhea and vomiting, respectively.
After dermal administration to rabbits, mild skin irritation
manifested as edema and erythema was observed. Anilazine was more
toxic by intraperitoneal injection than by other routes of
administration, suggesting poor enteral and percutaneous absorption.
TABLE 2. ACUTE TOXICITY OF ANILAZINE 1,2
LD50 LC50
SPECIES SEX ROUTE mg/kg/bwt (mg/l) REFERENCE
Mouse M oral approx 5000 - Flucke & Kimmerle, 1977
F 367 -
Mouse M s.c. > 5000 - Flucke & Kimmerle, 1977
F 3512 -
Rat M&F oral > 5000 - Flucke & Kimmerle, 1977
Hixson, 1979, 1980a
Heimann, 1985
M oral 3650 - Flucke, 1978
M&F i.p.3 71-137 - Flucke & Kimmerle, 1977
Heimann, 1984b
M&F inhal. - > 0.729 Sangha, 1984
M&F percutaneous > 5000 - Flucke & Kimmerle, 1977
Rabbit M&F dermal > 2000 - Hixson, 1980b, 1980c
Cat F oral > 500 - Flucke & Kimmerle, 1977
1 All studies performed using technical grade anilazine except for one LD50 study in
rats where 50% wettable powder was tested (Hixson, 1979), and two other LD50 where
formulated anilazine containing 40.5% a.i. was tested (Hixson, 1980a, 1980c).
2 The acute oral toxicity of dihydroxy-anilazine, a metabolite of anilazine was also
tested in rats. The LD50 value was > 5000 mg/kg (Heimann, 1984a).
3 The acute i.p. toxicity in male rats of anilazine (LD50 = 137 mg/kg) in combination
with triadimefon (LD50 = 317 mg/kg) was determined to be additive (LD50 of combination =
194 mg/kg) (Heimann, 1984b)
Short-term studies
Rats
SPF Wistar albino rats (20/sex/group) were administered
anilazine (technical grade, 92% pure) by gavage as an aqueous
emulsion at dose levels of 0, 30, 100, and 300 mg/kg bw/day for 28
days. The treatment period was followed by an additional 4-week
observation period. Half of the males in each group were sacrificed
at the end of the 28-day treatment period and the other half at the
end of the 4-week observation period. Hematology, clinical
chemistry, and urinalysis were performed in selected animals at each
time period prior to sacrifice. Animals were necropsied and
selected organs weighed. Histopathological examinations were
performed on 5 rats/sex/group from the 28-day treatment phase of the
study (16 tissues examined) and from 5 rats/sex/group from the
post-treatment observation phase of the study (3 tissues examined,
i.e., stomach, intestines, and esophagus).
During the 28-day treatment period, eight animals died (2/5
females at 100 mg/kg bw/day on day 11, and 1/5 males and 5/5 females
at 300 mg/kg bw/day on days 11-13). Gross pathology examination of
these animals indicated the presence of eroded abdominal and
thoracic viscera and lung lesions. Other changes seen in treated
animals were alterations in behaviour (apathy and dyspnea staring on
days 5-7 and lasting for 3 to 4 weeks) in females given 100 mg/kg
bw/day and in both sexes of rats at 300 mg/kg bw/day, and reductions
in body weight in males at 300 mg/kg bw/day on weeks 1 to 4. The
only observed treatment-related histological effect was
hyperkeratosis of the cutaneous mucosa of the proventricular part of
the stomach. The severity of the hyperkeratosis was also
dose-related, ranging from "trace" at the low-dose level; "minimal"
at the mid-dose level; and "moderate" at the high-dose level. The
finding was attributed to a local irritant effect of anilazine on
the mucosa. No clear treatment-related hematological, clinical
chemistry, urinalysis, or organ weight changes were observed during
the treatment period. None of the above described adverse effects
were seen in surviving rats similarly treated with anilazine but
allowed to recover for 4 additional weeks. The NOAEL for systemic
toxicity for anilazine, when administered by gavage to rats, was
30 mg/kg bw/day. However, stomach mucosal hyperkeratosis,
representing a local irritant effect, was seen at all of the tested
doses of anilazine (Flucke & Kaliner, 1978).
Groups of Sprague-Dawley CD rats (25 males and 25
females/group) received dietary concentrations of 0, 500, 2000, or
8000 ppm anilazine (93.5% pure) for 13 weeks. Five rats/sex/dose
group were sacrificed after 6 weeks.
No treatment-related deaths occurred. Body weight gain was
reduced over the study in mid- and high-dose males and in high-dose
females. Other changes were seen only in high-dose males and
females at the end of the study, and in many cases at the 6-week
interim kill period as well. These included clinical signs of
toxicity (piloerection, red-brown staining of fur around head and
back, rhinorrhea, and exophthalmos), and reduced food consumption,
increased alkaline phosphatase levels, reduction in lymphocytes,
creatinine and total protein levels, increases in the relative
weights of several organs (heart, brain, pituitary, gonads, adrenal
glands, thyroids, kidneys, and lungs), and various pathological
findings (pale appearing pancreas, foamy macrophage foci in the
lungs, testicular atrophy, lymph node hyperplasia, and enlarged
salivary glands with heightened secretory activity). As indicated
by histopathological analysis, the primary target organ of toxicity
appeared to be the forestomach. At the 6-week interim kill period,
a thickened forestomach mucosa was observed that was characterized
by dyskeratosis, acanthosis, hyperkeratosis, and edema in most of
the male and female rats examined at the mid- and high-dose levels.
At the 13-week terminal sacrifice period hyperkeratosis/hyperplasia
of the forestomach occurred at the mid- and high-dose levels at
incidences of 3/20 and 18/20, respectively, in male rats and 9/20
and 16/20, respectively, in females. These changes were accompanied
in some cases by ulcers and/or erosions of the forestomach, and were
considered to be a local irritant effect of anilazine on the mucosa.
The only finding at the lowest dose level was slight acanthosis and
hyperkeratosis of skin on the tails of female rats at the 13-week
sacrifice period (incidence: 2/20 controls; 5/20 low dose; 8/20
mid-dose, and 10/20 high dose). The findings occurred in male rats
only at the mid (1/20) and high (11/20) dose levels. The tail
lesions are of questionable biological significance. The NOAEL was
considered to be 25 mg/kg bw/day due to the observed reduction in
body weight gain in males and the local forestomach lesions in both
sexes (Goodyer, 1981).
Dogs
Purebred beagle dogs (4/sex/dose) were administered anilazine
(92.0% pure) orally (gelatin capsules) at dose levels of 0, 50, 150,
and 450 mg/kg bw/day for 13 weeks.
There were no effects of treatment on mortality, or on
patellar, flexor or extensor reflex activity or body temperature.
No adverse effects were observed with respect to ophthalmoscopic
examinations, or hematological or urinalysis parameters. Vomiting
and diarrhea several hours after dosing were prominent signs of
toxicity in both sexes administered all three dose levels. These
effects were dose-related and occurred throughout the study. Test
compound was frequently seen in vomitus and feces. The animals also
were agitated and ran back and forth in their cages before
defecation (the effect was attributed to possible intestinal spasms
evoked by the compound). Additional signs of toxicity were also
observed. Changes noted at both the 150 and 450 mg/kg bw/day levels
included reductions in pulse rate and slight drowsiness, reduced
serum albumin levels and increased plasma globulin levels (i.e.,
alpha-1, alpha-2, beta, and gamma globulin levels were increased),
and (in females), decreases in absolute and relative ovary weights.
Changes that occurred only at the 450 mg/kg bw/day dose level were
dull looking ungroomed hair coats, reductions in body weight gain
and food consumption, and reductions in total serum protein levels
and serum calcium levels. The absolute and relative weights of the
thymus gland were reduced, and thymus gland atrophy was observed
grossly, in high-dose males and females. Upon histological
examination, thymus gland atrophy was seen at all of the dose levels
tested in males and the highest dose in females. Histological
examination of the esophagus also revealed an increase in the
incidence of diffuse round cell infiltration of the mucosa at all
three dose levels. A NOAEL was not established in this study
(Hoffmann & Gröning, 1979).
Beagle dogs (4/sex/group) were administered anilazine
a.i.(91.5-94.4% pure) orally (gelatin capsules) at dose levels of 0,
10, 40, and 160 mg/kg bw/day for 18 months.
Treatment-related toxicological effects were produced by the
two highest dose levels of anilazine. Adverse findings that
occurred at 40 and 160 mg/kg bw/day in both males and females
included vomiting of the test substance with food, increases in
alpha-2, beta, and gamma globulins in plasma, and an increase in the
relative weight of the adrenal glands. Additional changes that were
seen in dogs of both sexes only at the highest dose level (160 mg/kg
bw/day) included salivation, loose-to-liquid feces, reduction in
bilirubin levels, and (in females) a decrease in the absolute weight
of the ovaries. High-dose animals also showed a tendency for a
decreased body weight gain which was due primarily to one male and
one female dog, although weight gain was also somewhat low in all of
the remaining dogs given the high dose level. One male dog in the
high-dose group was diagnosed with confluent bronchial pneumonia of
the right lung and suppurative bronchitis with increased blood
leukocytes and heightened hematopoiesis in bone marrow.
Esophagitis, gastritis, thymus involution, release of splenic
corpuscles and lymphadenitis were also noted in this animal. The
bronchial pneumonia was attributed to a bacterial or viral-induced
infection and was not considered to be compound-induced.
Anilazine did not produce treatment-related effects on
survival, vital signs (i.e., reflex activity, temperature, or pulse
rate) or food consumption, nor were adverse ophthalmological
findings observed. Urinalysis parameters were unchanged. No
treatment-related non-neoplastic or neoplastic lesions were evident.
The NOAEL was 10 mg/kg bw/day anilazine (Hoffmann et al. 1983;
Hoffmann et al. 1987; Hoffmann et al. 1988).
Long-term/carcinogenicity studies
Mice
Male and female Charles River CD-1 mice (50/sex/dose) were fed
diets containing 0, 50, 250, or 1250 ppm anilazine (93.5% pure) for
104 weeks. Sixteen additional mice/sex group served solely for the
collection of blood samples, and were not examined
histopathologically. The average dietary concentrations were
equivalent to 7.5, 37.5, and 290.0 mg/kg bw/day. Blood was collected
at 0, 27, 53, 79, and 103 weeks). The weights of liver, kidneys,
gonads, heart, and brain were obtained.
There were no effects of treatment on mortality, clinical signs
of toxicity, body weight, food consumption, hematology, or clinical
chemistry. Plasma glucose levels were elevated in occasional male
and female mice in the 250 and 1250 ppm groups. There were no
treatment-related effects on organ weights and no remarkable
findings at gross necropsy or after histopathological examination.
The incidences of Harderian gland adenomas were elevated in males
and females in the high-dose group. These tumour incidences,
however, were neither statistically significant nor above historical
control levels. On the basis of these findings the NOAEL in this
study exceeded 1250 ppm in the diet, equal to approximately
190.0 mg/kg bw/day in males and females (Goodyer, 1984a).
Rats
Male and female Sprague-Dawley CD rats (50/sex/group) were fed
anilazine (93.5% pure) for 104 weeks at levels of 0, 50, 330, or
2000 ppm. These concentrations were equivalent to approximately
2.5, 16.5 and 100 mg/kg bw/day. Ten additional rats/sex/dose were
used solely for the collection of blood samples; these animals were
not examined histopathologically. Blood was collected at 0, 26/27,
53, 79 and 103 weeks. Urinalysis studies, however, were not
performed. The weights of liver, kidney gonads, heart, brain,
adrenals, spleen, lungs, and thyroid glands were obtained.
There were no clinical signs of toxicity and no effect of
treatment on mortality. There were no treatment-related effects on
body weight or food consumption throughout the study with the
exception of transient, parallel decreases in these parameters
during study week number 1 in males and females given 2000 ppm.
Hematology and clinical chemistry findings were generally
unremarkable. There was no treatment-related effect on organ
weights and no remarkable findings at gross necropsy or after
histopathological examination. There were no increases in tumours
that were treatment-related. The NOAEL exceeded 2000 ppm in the
diet, equivalent to more than 100 mg/kg bw/day in males and females
(Goodyer, 1984b).
Special carcinogenicity bioassays of anilazine in rodents (rats
and mice) were also performed by the National Cancer Institute but
they were not considered for toxicological evaluation (NCI, 1978).
However, since these studies were performed by Gulf South Research
Corporation and were not validated.
Reproduction study
Rats
Groups of male (15/sex/dose) and female (30/sex/dose) Sprague-
Dawley rats in each of three generations (F0, F1, and F2) were
fed diets containing 0, 50, 330, or 2000 ppm anilazine (93.5% pure).
The dietary concentrations were equivalent to 2.5, 16.5, and 100
mg/kg bw/day. After maturation periods of 15 weeks in the F0
generation and 18 weeks in the F1 and F2 generations, the parental
animals were allowed to rear their offspring to weaning (F0-F1a;
F1-F2a; F2-F3a). The animals for the F1 and F2 generations
were randomly selected from the F1a and F2a litters, avoiding
sibling matings.
No effects of treatment on mortality, clinical signs, food
consumption, or body weight of parental rats were noted. Although
slight inhibition of body weight gain was noted in F0 parental
males from week 5 until termination, the response was neither
statistically significant nor dose-related. The highest dietary
level of anilazine (2000 ppm) had an apparent adverse effect on the
libido of F0 males and the fertility (and possible fecundity) of
F0 females. This was indicated, respectively, by reductions in the
number of females that mated and the number that became pregnant in
comparison to controls. Although the respective differences were
not remarkable nor evident in subsequent generations (F1 and F2),
they were verified by a crossover mating experiment involving rats
from the control and high-dose groups. No adverse effects on the
reproductive parameters were evident at anilazine dietary levels of
330 and 50 ppm. No adverse changes occurred with respect to litter
size, viability, mean pup and litter weights, or physical (i.e.,
auditory response, pupillary reflex, visual placing response, grip
strength) and functional (i.e., onset and duration of pinna
unfolding, onset of hair growth, incisor eruption, and eye opening)
development of pups. No unusual pre- or post-weaning clinical
observations occurred. No treatment-related changes in organ weight
or pathological (gross and microscopic) findings were noted in
either parenteral rats or pups at necropsy (Irvine, 1984). The
NOAEL is 330 ppm, equivalent to 16.5 mg/kg bw/day.
Special studies on teratogenicity
Rats
Groups of female Long Evans FB-30 strain rats (21-22/dose) were
administered doses of 0, 30, 100, or 300 mg/kg bw/day anilazine, by
gavage, from days 6 to 25 of gestation (Day 0 = day of
insemination). Doses were based on a preliminary range-finding
study which demonstrated that a dose of 300 mg/kg bw/day for 10 days
had no apparent adverse activity. On gestation day 20, rats were
sacrificed and fetuses delivered by C-section. Approximately 30% of
the fetuses in each group were dissected and examined for visceral
anomalies and approximately 70% of the fetuses in each group were
cleared and stained for skeletal observations.
The 300 mg/kg bw/day dose of anilazine produced a significant
reduction in body weight gain in the dams during the treatment
period as well as throughout the entire gestation period. Body
weight gain, however, was not influenced by 30 or 100 mg/kg bw/day
anilazine. None of the doses produced changes in mortality, physical
appearance, or behaviour, or the fertilization ratio or pregnancy
ratio in the dams. Similarly, none of the doses appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. Anilazine was neither fetotoxic, embryotoxic, nor
teratogenic in rats at oral doses up to 300 mg/kg/day (highest dose
tested). Because of maternal toxicity (weight gain reduction) at
300 mg/kg bw/day, the NOAEL is 100 mg/kg bw/day by gavage (Machemer,
1978).
Groups of female Charles River Crl:CD-BR rats (28/dose) were
administered oral (gastric intubation) doses of 0, 150, 500, or
1500 mg/kg bw/day anilazine from days 6 to 15 of gestation. On
gestation day 20, rats were sacrificed and fetuses delivered by
C-section. Approximately one-half of the fetuses in each group were
dissected and examined for visceral anomalies, and the other half of
the fetuses in each group were cleared and stained for skeletal
observations.
Anilazine was maternally toxic at doses of 500 and 1500 mg/kg
bw/day. The adverse findings were: 1) an increased incidence of
loose and/or soft stools that often contained a creamy white
substance (seen in 28.6 and 100% of dams at 500 and 1500 mg/kg
bw/day, respectively); 2) significant reductions in body weight and
body weight gain in dams given 1500 mg/kg bw/day on gestation days 6
to 25 and 0 to 20; 3) a significant reduction in food consumption
in dams given 500 and 1500 mg/kg bw/day on gestation day 7 only; and
4) an increased incidence of thickened and coarse mucosa of the
nonglandular portion of the stomach (seen in 18 and 15% of dams
given 500 and 1500 mg/kg bw/day, respectively). This change was
characterized microscopically as epithelial hyperplasia (acanthosis)
and hyperkeratosis of the mucus membrane. In contrast to these
results, none of the doses of anilazine induced effects on embryo or
fetal development, and no teratogenic effects were observed. The
NOAEL was 150 mg/kg bw/day by intubation (Kowalski et al. 1988).
Rabbits
Groups of female Himalayan rabbits (13/dose) were administered
0, 5, 15 and 45 mg/kg bw/day anilazine (92.4% pure) from days 6 to
18 of gestation (Day 0 = day of insemination). The animals were
sacrificed on gestation day 29 and fetuses delivered by C-section.
All fetuses were necropsied and abdominal and thoracic organs as
well as the brain examined for visceral malformations. All fetuses
were examined for skeletal defects.
No treatment-related changes in mortality, appearance,
behaviour or weight gain were observed in the does. The
fertilization and pregnancy ratios were not adversely affected.
There were no statistically significant dose-related effects on the
number of implantations or corpora lutea, sex ratio, mean fetal
weights, mean placental weights, fetuses showing visceral
malformations, or numbers of fetuses with stunted growth. No
treatment-related skeletal abnormalities or malformations were
observed. The highest dose tested, 45 mg/kg bw/day by gavage, is a
NOAEL for maternal, embryo-, and fetotoxicity, and for
teratogenicity in rabbits (Roetz, 1981).
Special studies on mutagenicity
Anilazine was mutagenic in a cytogenetic study in human
lymphocyte cultures in vitro (both with and without metabolic
activation), in a mouse lymphoma forward mutation assay (without
metabolic activation only), and at severely cytotoxic levels in a
reverse mutation assay in yeast (both with and without metabolic
activation). In contrast, the chemical was negative in a variety of
the other in vitro and in vivo systems. The weight-of-the-
evidence indicates that anilazine is not genotoxic.
Special studies on eye and skin irritation
Instillation of 100 mg grade anilazine (40.5% a.i. formulation)
in the rabbit eye for 45 seconds produced reversible iritis, slight
erythema, chemosis, and discharge which lasted for up to 13 days
(Hixson, 1980b).
Anilazine (90 mg of a technical grade formulation) produced a
strong irritant effect (redness, swelling, and lacrimation) on the
rabbit eye when placed in the conjunctival sac for 24 hours. The
reactions were reversible within 14 days. No corrosive effect
occurred (Pauluhn, 1983).
Slight primary skin irritation (erythema and edema) was
observed when anilazine (500 mg technical) was applied to shaved
rabbit skin for 4 hours. The reactions were reversible within 7
days. No corrosive effect occurred (Pauluhn, 1983).
In a 3-week dermal toxicity study in rabbits, 0%, 1% (10 mg/ml)
and 4% (40 mg/mL) of anilazine was applied to shaved skin at a
volume of 0.5 ml/kg for 6 hours/day, 5X/week, for 3 weeks. In this
study, a formulation containing 497 grams a.i.(litre (= 38.4% w/w)
was used. Both concentrations of the compound produced dermal
changes consisting of slight erythema and cellular infiltration in
the subepidermal region; in addition, slight thickening of the
epidermis was noted 40 mg/ml. However, no systemic changes were
observed as noted from hematological, clinical chemistry and
urinalysis examinations, by measurements of body weight gain, and by
histopathological evaluation of selected organs and tissues. The
systemic NOAEL was calculated to be approximately 7.7 mg a.i./kg
bw/day. (Heimann & Schilde, 1985).
Special studies on skin sensitizing effect
Anilazine was shown to be a dermal sensitizer (i.e., to induce
redness and swelling) when tested in guinea pigs by the Buehler
Patch test (Heimann, 1988a; Heimann, 1988b), the Magnusson and
Kligman test (Heimann, 1982a), and in Klecak's open epicutaneous
test (Heimann, 1982b). The positive findings were obtained using
either pure and/or technical anilazine in induction concentrations
ranging from 0.03 to 5%, and challenge doses ranging from 0.03 to
1%. Negative results for sensitizing potential were obtained in one
study (Klecak's test) with pure anilazine at induction
concentrations of 0.01 to 0.1% and challenge doses of 0.01 to 0.3%
(Heimann, 1983).
Observations in humans
A group of farm workers employed on a single farm in the United
States arrived at a hospital emergency room with severe dermatitis
in July 1980. All were exposed to three pesticides in a tomato
field (anilazine, benomyl, and endosulfan). Seven of the workers
were available for patch testing to the three pesticides. Delayed
hypersensitivity (48-hour eczematous reaction) to 0.1% Dyrene
(anilazine) was demonstrated in 6 of the 7 affected workers. Nine
non-exposed adult volunteers who were used as control subjects had
negative patch tests to 0.1% Dyrene. The conclusion was reached
that anilazine caused allergic contact dermatitis (Schuman et al.
1980).
COMMENTS
Anilazine administered orally to rats was excreted primarily in
the feces via biliary excretion, and also in urine. Anilazine was
biotransformed in rats primarily to the hydroxylation product,
2-2-chloroanilino)-s-triazinedione, which was present only in
urine. A second biotransformation pathway involving conjugate
formation via the glutathione - mercapturic acid pathway was also
observed in goats and chickens. No unusual organ or tissue
localization of (14C)-labelled anilazine was observed.
Anilazine has a low acute toxicity in the species examined.
Subchronic studies in rats indicated that the stomach was the
primary target organ of toxicity; oral gavage doses of aqueous
anilazine emulsion of 30 mg/kg bw/day and a dietary level of
2000 ppm (equivalent to 100 mg/kg bw/day) or greater produced
forestomach changes characterized by acanthosis, hyperkeratosis, and
areas of ulceration and erosion. The NOAEL was 500 ppm (equivalent
to 25 mg/kg bw/day).
In an 18-month oral (capsule) toxicity study in dogs, anilazine
at doses of 40 and 160 mg/kg bw/day produced increases in alpha-1,
beta-2, and gamma-globulins in plasma, vomiting, and diarrhea in
males and females. These findings were also observed with oral
doses (capsules) of 50 to 450 mg/kg bw/day for 13 weeks in dogs.
The NOAEL in the 18-month study was 10 mg/kg bw/day.
In long-term studies in rats and mice, no treatment-related
increases in neoplasms were observed. The NOAELs were 1250 ppm
(equal to 188 mg/kg bw/day) in Cr CD-1 mice and 2000 ppm (equivalent
to 100 mg/kg bw/day) in SD-CD rats.
In a 3-generation, 2 litters per generation, reproduction study
in rats, reductions in male libido and female fertility occurred in
F0 generation animals at 2000 ppm in the diet (equivalent to
100 mg/kg bw/day). Although these effects were confirmed in
cross-mating experiments, they were not observed in subsequent
generations. The NOAEL was 330 ppm (equivalent to 16.5 mg/kg
bw/day).
In teratology studies in rats, the NOAEL was 150 mg/kg bw/day
based on maternal toxicity. Oral doses of 300 to 1500 mg/kg bw/day
produced soft/loose stools, weight loss, reduced food consumption
and acanthosis and hyperkeratosis of the stomach of dams, but had no
fetotoxic or teratogenic activity. The NOAEL was 45 mg/kg bw/day
(highest dose tested) in a rabbit teratology study.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 1250 ppm in the diet, equal to 188 mg/kg bw/day
Rat: 330 ppm in the diet, equivalent to 16.5 mg/kg bw/day
Dog: 10 mg/kg bw/day orally.
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ecker, W. (1981) [Phenyl-UL-14C]Anilazine: Characterization of
metabolites in rat urine and feces. Unpublished Report No. 10216
from Institute of Pharmacokinetics, Bayer AG. Submitted to WHO by
Bayer AG, Wuppertal, FRG.
Ecker, W. (1983) [Triazine-UL-14C]Anilazine: Characterization of
metabolites in rat urine; comparison of metabolite patterns after
administration of [Triazine-UL-14C]Anilazine and [Phenyl-UL-
14C]Anilazine. Unpublished Report No. 11610 from Institute of
Pharmacokinetics, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Eubler, H., Traber, J., Walk, R.A. (1979) Biokinetics of
14C-Anilazine on the rat. Unpublished Report No. A0197/1526 from
INBIFO Research, Cologne. Submitted to WHO by Bayer AG, Wuppertal,
FRG.
Flucke, W. (1978) Determination of acute toxicity (LD50);
refrigerator sample of June 21. Unpublished Report of August 11
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Flucke, W., Kaliner, G. (1978) Dyrene: subacute oral toxicity study
on rats. Unpublished Report No. 7844 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Flucke, W., Kimmerle, G. (1977) Dyrene: acute toxicity studies.
Unpublished Report No. 6761 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Goodyer, M.J. (1981) Anilazine: a 13-week oral (dietary
administration) toxicity study in the rat with a 6-week interim
kill. Unpublished Report No. 2660-262/23 from Hazleton
Laboratories Europe, Ltd., Harrogate, England. Submitted to WHO by
Bayer AG, Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984a) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the mouse.
Unpublished Report No. 3622-262/22 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984b) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the rat.
Unpublished Report No. 3686-262/24 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Heimann, K.G. (1982a) Technical dyrene: study of sensitization
effect on guinea pigs (maximization test of Magnusson and Kligman).
Unpublished Report No. 11059 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1982b) Dyrene: investigation of sensitizing effect
in the open epicutaneous test on guinea pigs. Unpublished Report
No. 10816 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1983) Dyrene pure active ingredient: study for
skin-sensitizing effect in the open epicutaneous test on guinea
pigs. Unpublished Report No. 12034 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50).
Unpublished Report of August 20 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984b) Anilazine and MEB 6447: study for combination
toxicity. Unpublished Report No. 12609 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1985) Determination of acute toxicity (LD50).
Unpublished Report of February 20 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1988a) Dyrene: study for skin-sensitizing effect on
guinea pigs (Buehler Patch Test). Unpublished Report No. 16419 from
Toxicology Division Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Heimann, K.G. (1988b) Dyrene Technical: study for skin-sensitizing
effect in the open epicutaneous test on guinea pigs (Buehler Patch
Test). Unpublished Report No. 16420 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G., Schilde, B. (1985) Dyrene: subacute study of dermal
toxicity to rabbits. Unpublished Report No. 14151 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1978) Dyrene: dominant lethal study on male mouse to
test for mutagenic effects. Unpublished Report No. 7448 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980a) Dyrene: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished Report No. 9172 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980b) Dyrene: micronucleus test on mouse to evaluate
dyrene for mutagenic potential. Unpublished Report No. 9565 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1981) Dyrene: PolA1 - test on E. coli to evaluate for
DNA damage. Unpublished Report No. 10149 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1986a) Spot test on cross-bred C57B1/6J x T stock
mouse embryos to evaluate for induced somatic changes in the genes
of the coat pigment cells. Unpublished Report No. 15041 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1986b) Dyrene: cytogenic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on chromosomes.
Unpublished Report No. 14188 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1987) Anizaline Tech. Salmonella/microsome test for
determination of point mutations. Unpublished Report No. 15780 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1988a) Anizaline: micronucleus test on the mouse for
clastogenic effects. Unpublished Report No. 16481 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1988b) Dyrene: dominant lethal test on the male mouse
to evaluate for mutagenic effects. Unpublished Report No. 16688
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Hixson, E.J. (1979) Acute oral toxicity study of Dyrene 50%
wettable powder to rats. Unpublished Report No. 68411 from Mobay
Chemical Corporation, Kansas City MO. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Hixson, E.J. (1980a) Acute oral toxicity study of Dyrene 4F to
rats. Unpublished Report No. 118 from Mobay Chemical Corporation,
Kansas City MO. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980b) Dermal irritation of Dyrene 4F. Unpublished
Report No. 123 from Mobay Chemical Corporation, Stillwell, KS.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980c) Acute oral toxicity of Dyrene 4F to rabbits.
Unpublished Report No. 126 from Mobay Chemical Corporation,
Stillwell, KS. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hoffmann, K., Gröning, P. (1979) Dyrene: subchronic toxicity study
on dogs (13-week oral administration in capsules). Unpublished
Report No. 8199 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer, AG, Wuppertal, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1983) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Unpublished Report No. 11613 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Bayerwerk, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1987) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1988) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Second Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Irvine, L.F.H. (1984) Anilazine: 3-generation oral (dietary
administration) reproduction study in the rat. Unpublished Report
No. 3744-262/26 from Hazleton Laboratories Europe, Ltd., Harrogate,
England. Submitted to WHO by Bayer AG, Institute of Toxicology,
Wuppertal, FRG.
Jagannath, D.R. (1981) Mutagenicity evaluation of Dyrene in the
saccharomyces reverse mutation assay. Unpublished Report No. R2088
from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
Kowalski, R.L., Clemens, G.R., Hartnagel, R.E. Jr (1988) A
teratology study with anilazine (Dyrene Technical) in the rat.
Unpublished Report No. 1069 from Toxicology Department, Miles, Inc.,
USA. Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Machemer, L. (1978) Dyrene: evaluation for embryotoxic and
teratogenic effects on orally dosed rats. Unpublished Report
No. 7746 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Miltenberger, H.G. (2986) Anilazine Techn. Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with V79
cells. Unpublished Report No. R3821 from Laboratory for
Mutagenicity Testing, Technical University, Darmstadt. Submitted to
WHO by Bayer, AG, Wuppertal, FRG.
Myhr, B.C. (1983) Evaluation of Analazine Technical in the primary
rat hepatocyte unscheduled DNA synthesis assay. Unpublished Report
No. R2615 from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
NCI (1978) NCI (National Cancer Institute) Carcinogenesis technical
report series No. 104. Bioassay of anilazine for possible
carcinogenicity. US Department of Health, Education and Welfare
Publication No.(NIH) 78-1354.
Pauluhn, J. (1983) Dyrene (technical): study for irritant and
corrosive effect on skin and eye (rabbits). Unpublished Report
No. 11826 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Roetz, R. (1981) Dyrene: evaluation for embryotoxic and/or
teratogenic effects on the rabbit after oral administration.
Unpublished Report No. 10339 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Sangha, G.K. (1984) Acute inhalation toxicity study with Anilazine
(Dyrene) technical in rats. Unpublished Report No. 463 from Mobay
Chemical Corporation, Stillwell, KS. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Schuman, S.H., Dobson, R.L., Fingar, J.R. (1980) Dyrene dermatitis.
Lancet, page 1252, December 6, 1980.
Volkner, W. (1987) Chromosome aberration test in bone marrow cells
of the Chinese hamster with Anilazine Technical. Unpublished Report
No. R4100 from Cytotest Cell Research GmBH & Company, KG, Darmstadt,
FRG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Witterland, W.F. (1984) Mutagenicity evaluation of Anilazine
Technical in the mouse lymphoma forward mutation assay. Unpublished
Report No. R2831 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer AG, Wuppertal, FRG.
See Also:
Toxicological Abbreviations
 Biotransformation
The metabolism of [phenyl-UL-14C]anilazine was characterized
over a 24-hour period in the urine and feces of albino SD rats given
a single oral dose of 5 mg/kg bw. About 16 to 82% of the
administered RA dose was excreted in urine and feces, respectively.
No parent compound was present in urine or feces. The major
identified metabolite was the hydroxylation product,
2-(2-chloroanilino)-s-triazinodione, which comprised about 25% of
the RA found in urine but was present only in insignificant amounts
in feces. Several other unknown polar metabolites, which were
predominantly acidic in nature, were found. In addition, a small
portion of the RA metabolite became less polar as a result of
treatment with diazomethane. The majority of the metabolite could
also be acetylated (Ecker, 1981). The proposed metabolic pathway
for anilazine in dairy goats is shown in Figure 1.
Another study in male rats comparing the metabolism of
[phenyl-UL-14C]anilazine and [triazine-UL-14C]anilazine (single
oral dose of 5 mg/kg) indicated that the urinary and fecal metabolic
patterns of both labels were identical, suggesting that the basic
structure of the two rings of the molecule were maintained during
biotransformation (Ecker, 1983).
TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON ANILAZINE
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Ames testa S. typhimurium 5-12,500 ug/plate 95.9% Negative Herbold, 1980a
TA98, TA100, in DMSO
TA1535, TA1537
Ames testa S. typhimurium 20-12,500 ug/plate Technical Negative Herbold, 1987
TA98, TA100, in DMSO 97.1%
TA1535, TA1537
Yeast testa S. cerevisiae 1-5000 ? Positive Jagannah, 1981
(Reverse mutation S138, S211 ug/incubation only at
assay tube in DMSO severely
cytoxic
levels
DNA damage in E. coli Pol A+/ 62.5-1000 ug/plate Technical Negative Herbold, 1981
E. colia Pol A- in DMSO 93.5%
CH-V79/HGPRT gene Chinese hamster 0.2-9 ug/mL in 97.3% Negative Miltenburger, 1986
mutation assaya cell line V79 DMSO
Mouse lymphoma L5178Y mouse 2.5-9 ug/mL in Technical Positive Witterland, 1984
forward mutation lymphoma cells DMSO 95.9% (nonactivated
assaya (TK+/-) only)
Unscheduled DNA Primary hepatocytes 0.049-9.8 ug/mL Technical Negative Myhr, 1983
synthesis from male F344 rats 98.6%
Mouse micronucleus Bone marrow 7500 mg/kg single 97.1% Negative Herbold, 1988a
test erythro-blasts oral dose
(NMRI mice)
TABLE 1 (contd.)
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Mouse micronucleus Bone marrow 100-200 mg/kg 95.9% Negative Herbold, 1980b
test erythro-blasts bw/day for 2 days
(NMRI mice)
Spot test in mice Coat pigment cells 3-30 mg/kg bw i.p. 97.3% Negative Herbold, 1986a
embryos to evaluate of crossbred on gestation day 10 in F1 mice
somatic gene changes C57B1/6J XT mice to pregnant females
Dominant lethal test Mouse (male) 5000 or 7500 mg/kg bw 97.3% Negative Herbold, 1988b
(single or oral dose)
Dominant lethal test Mouse (male) 500 mg/kg bw or 94% Negative Herbold, 1978
(single oral dose)
Chromosome aberration Chinese hamster 300-3000 mg/kg bw Technical Negative Völkner, 1987
test bone marrow (single oral dose)
Cytogenic testa Human lymphocyte 3-30 ug/ml in DMSO Technical Positive Herbold, 1986b
cultures (in vitro)
a with and without metabolic activation.
Toxicological studies
Acute toxicity
The acute toxicity of anilazine in several species is given in
Table 2. After oral administration to rats and cats, the most
common signs of toxicity were diarrhea and vomiting, respectively.
After dermal administration to rabbits, mild skin irritation
manifested as edema and erythema was observed. Anilazine was more
toxic by intraperitoneal injection than by other routes of
administration, suggesting poor enteral and percutaneous absorption.
TABLE 2. ACUTE TOXICITY OF ANILAZINE 1,2
LD50 LC50
SPECIES SEX ROUTE mg/kg/bwt (mg/l) REFERENCE
Mouse M oral approx 5000 - Flucke & Kimmerle, 1977
F 367 -
Mouse M s.c. > 5000 - Flucke & Kimmerle, 1977
F 3512 -
Rat M&F oral > 5000 - Flucke & Kimmerle, 1977
Hixson, 1979, 1980a
Heimann, 1985
M oral 3650 - Flucke, 1978
M&F i.p.3 71-137 - Flucke & Kimmerle, 1977
Heimann, 1984b
M&F inhal. - > 0.729 Sangha, 1984
M&F percutaneous > 5000 - Flucke & Kimmerle, 1977
Rabbit M&F dermal > 2000 - Hixson, 1980b, 1980c
Cat F oral > 500 - Flucke & Kimmerle, 1977
1 All studies performed using technical grade anilazine except for one LD50 study in
rats where 50% wettable powder was tested (Hixson, 1979), and two other LD50 where
formulated anilazine containing 40.5% a.i. was tested (Hixson, 1980a, 1980c).
2 The acute oral toxicity of dihydroxy-anilazine, a metabolite of anilazine was also
tested in rats. The LD50 value was > 5000 mg/kg (Heimann, 1984a).
3 The acute i.p. toxicity in male rats of anilazine (LD50 = 137 mg/kg) in combination
with triadimefon (LD50 = 317 mg/kg) was determined to be additive (LD50 of combination =
194 mg/kg) (Heimann, 1984b)
Short-term studies
Rats
SPF Wistar albino rats (20/sex/group) were administered
anilazine (technical grade, 92% pure) by gavage as an aqueous
emulsion at dose levels of 0, 30, 100, and 300 mg/kg bw/day for 28
days. The treatment period was followed by an additional 4-week
observation period. Half of the males in each group were sacrificed
at the end of the 28-day treatment period and the other half at the
end of the 4-week observation period. Hematology, clinical
chemistry, and urinalysis were performed in selected animals at each
time period prior to sacrifice. Animals were necropsied and
selected organs weighed. Histopathological examinations were
performed on 5 rats/sex/group from the 28-day treatment phase of the
study (16 tissues examined) and from 5 rats/sex/group from the
post-treatment observation phase of the study (3 tissues examined,
i.e., stomach, intestines, and esophagus).
During the 28-day treatment period, eight animals died (2/5
females at 100 mg/kg bw/day on day 11, and 1/5 males and 5/5 females
at 300 mg/kg bw/day on days 11-13). Gross pathology examination of
these animals indicated the presence of eroded abdominal and
thoracic viscera and lung lesions. Other changes seen in treated
animals were alterations in behaviour (apathy and dyspnea staring on
days 5-7 and lasting for 3 to 4 weeks) in females given 100 mg/kg
bw/day and in both sexes of rats at 300 mg/kg bw/day, and reductions
in body weight in males at 300 mg/kg bw/day on weeks 1 to 4. The
only observed treatment-related histological effect was
hyperkeratosis of the cutaneous mucosa of the proventricular part of
the stomach. The severity of the hyperkeratosis was also
dose-related, ranging from "trace" at the low-dose level; "minimal"
at the mid-dose level; and "moderate" at the high-dose level. The
finding was attributed to a local irritant effect of anilazine on
the mucosa. No clear treatment-related hematological, clinical
chemistry, urinalysis, or organ weight changes were observed during
the treatment period. None of the above described adverse effects
were seen in surviving rats similarly treated with anilazine but
allowed to recover for 4 additional weeks. The NOAEL for systemic
toxicity for anilazine, when administered by gavage to rats, was
30 mg/kg bw/day. However, stomach mucosal hyperkeratosis,
representing a local irritant effect, was seen at all of the tested
doses of anilazine (Flucke & Kaliner, 1978).
Groups of Sprague-Dawley CD rats (25 males and 25
females/group) received dietary concentrations of 0, 500, 2000, or
8000 ppm anilazine (93.5% pure) for 13 weeks. Five rats/sex/dose
group were sacrificed after 6 weeks.
No treatment-related deaths occurred. Body weight gain was
reduced over the study in mid- and high-dose males and in high-dose
females. Other changes were seen only in high-dose males and
females at the end of the study, and in many cases at the 6-week
interim kill period as well. These included clinical signs of
toxicity (piloerection, red-brown staining of fur around head and
back, rhinorrhea, and exophthalmos), and reduced food consumption,
increased alkaline phosphatase levels, reduction in lymphocytes,
creatinine and total protein levels, increases in the relative
weights of several organs (heart, brain, pituitary, gonads, adrenal
glands, thyroids, kidneys, and lungs), and various pathological
findings (pale appearing pancreas, foamy macrophage foci in the
lungs, testicular atrophy, lymph node hyperplasia, and enlarged
salivary glands with heightened secretory activity). As indicated
by histopathological analysis, the primary target organ of toxicity
appeared to be the forestomach. At the 6-week interim kill period,
a thickened forestomach mucosa was observed that was characterized
by dyskeratosis, acanthosis, hyperkeratosis, and edema in most of
the male and female rats examined at the mid- and high-dose levels.
At the 13-week terminal sacrifice period hyperkeratosis/hyperplasia
of the forestomach occurred at the mid- and high-dose levels at
incidences of 3/20 and 18/20, respectively, in male rats and 9/20
and 16/20, respectively, in females. These changes were accompanied
in some cases by ulcers and/or erosions of the forestomach, and were
considered to be a local irritant effect of anilazine on the mucosa.
The only finding at the lowest dose level was slight acanthosis and
hyperkeratosis of skin on the tails of female rats at the 13-week
sacrifice period (incidence: 2/20 controls; 5/20 low dose; 8/20
mid-dose, and 10/20 high dose). The findings occurred in male rats
only at the mid (1/20) and high (11/20) dose levels. The tail
lesions are of questionable biological significance. The NOAEL was
considered to be 25 mg/kg bw/day due to the observed reduction in
body weight gain in males and the local forestomach lesions in both
sexes (Goodyer, 1981).
Dogs
Purebred beagle dogs (4/sex/dose) were administered anilazine
(92.0% pure) orally (gelatin capsules) at dose levels of 0, 50, 150,
and 450 mg/kg bw/day for 13 weeks.
There were no effects of treatment on mortality, or on
patellar, flexor or extensor reflex activity or body temperature.
No adverse effects were observed with respect to ophthalmoscopic
examinations, or hematological or urinalysis parameters. Vomiting
and diarrhea several hours after dosing were prominent signs of
toxicity in both sexes administered all three dose levels. These
effects were dose-related and occurred throughout the study. Test
compound was frequently seen in vomitus and feces. The animals also
were agitated and ran back and forth in their cages before
defecation (the effect was attributed to possible intestinal spasms
evoked by the compound). Additional signs of toxicity were also
observed. Changes noted at both the 150 and 450 mg/kg bw/day levels
included reductions in pulse rate and slight drowsiness, reduced
serum albumin levels and increased plasma globulin levels (i.e.,
alpha-1, alpha-2, beta, and gamma globulin levels were increased),
and (in females), decreases in absolute and relative ovary weights.
Changes that occurred only at the 450 mg/kg bw/day dose level were
dull looking ungroomed hair coats, reductions in body weight gain
and food consumption, and reductions in total serum protein levels
and serum calcium levels. The absolute and relative weights of the
thymus gland were reduced, and thymus gland atrophy was observed
grossly, in high-dose males and females. Upon histological
examination, thymus gland atrophy was seen at all of the dose levels
tested in males and the highest dose in females. Histological
examination of the esophagus also revealed an increase in the
incidence of diffuse round cell infiltration of the mucosa at all
three dose levels. A NOAEL was not established in this study
(Hoffmann & Gröning, 1979).
Beagle dogs (4/sex/group) were administered anilazine
a.i.(91.5-94.4% pure) orally (gelatin capsules) at dose levels of 0,
10, 40, and 160 mg/kg bw/day for 18 months.
Treatment-related toxicological effects were produced by the
two highest dose levels of anilazine. Adverse findings that
occurred at 40 and 160 mg/kg bw/day in both males and females
included vomiting of the test substance with food, increases in
alpha-2, beta, and gamma globulins in plasma, and an increase in the
relative weight of the adrenal glands. Additional changes that were
seen in dogs of both sexes only at the highest dose level (160 mg/kg
bw/day) included salivation, loose-to-liquid feces, reduction in
bilirubin levels, and (in females) a decrease in the absolute weight
of the ovaries. High-dose animals also showed a tendency for a
decreased body weight gain which was due primarily to one male and
one female dog, although weight gain was also somewhat low in all of
the remaining dogs given the high dose level. One male dog in the
high-dose group was diagnosed with confluent bronchial pneumonia of
the right lung and suppurative bronchitis with increased blood
leukocytes and heightened hematopoiesis in bone marrow.
Esophagitis, gastritis, thymus involution, release of splenic
corpuscles and lymphadenitis were also noted in this animal. The
bronchial pneumonia was attributed to a bacterial or viral-induced
infection and was not considered to be compound-induced.
Anilazine did not produce treatment-related effects on
survival, vital signs (i.e., reflex activity, temperature, or pulse
rate) or food consumption, nor were adverse ophthalmological
findings observed. Urinalysis parameters were unchanged. No
treatment-related non-neoplastic or neoplastic lesions were evident.
The NOAEL was 10 mg/kg bw/day anilazine (Hoffmann et al. 1983;
Hoffmann et al. 1987; Hoffmann et al. 1988).
Long-term/carcinogenicity studies
Mice
Male and female Charles River CD-1 mice (50/sex/dose) were fed
diets containing 0, 50, 250, or 1250 ppm anilazine (93.5% pure) for
104 weeks. Sixteen additional mice/sex group served solely for the
collection of blood samples, and were not examined
histopathologically. The average dietary concentrations were
equivalent to 7.5, 37.5, and 290.0 mg/kg bw/day. Blood was collected
at 0, 27, 53, 79, and 103 weeks). The weights of liver, kidneys,
gonads, heart, and brain were obtained.
There were no effects of treatment on mortality, clinical signs
of toxicity, body weight, food consumption, hematology, or clinical
chemistry. Plasma glucose levels were elevated in occasional male
and female mice in the 250 and 1250 ppm groups. There were no
treatment-related effects on organ weights and no remarkable
findings at gross necropsy or after histopathological examination.
The incidences of Harderian gland adenomas were elevated in males
and females in the high-dose group. These tumour incidences,
however, were neither statistically significant nor above historical
control levels. On the basis of these findings the NOAEL in this
study exceeded 1250 ppm in the diet, equal to approximately
190.0 mg/kg bw/day in males and females (Goodyer, 1984a).
Rats
Male and female Sprague-Dawley CD rats (50/sex/group) were fed
anilazine (93.5% pure) for 104 weeks at levels of 0, 50, 330, or
2000 ppm. These concentrations were equivalent to approximately
2.5, 16.5 and 100 mg/kg bw/day. Ten additional rats/sex/dose were
used solely for the collection of blood samples; these animals were
not examined histopathologically. Blood was collected at 0, 26/27,
53, 79 and 103 weeks. Urinalysis studies, however, were not
performed. The weights of liver, kidney gonads, heart, brain,
adrenals, spleen, lungs, and thyroid glands were obtained.
There were no clinical signs of toxicity and no effect of
treatment on mortality. There were no treatment-related effects on
body weight or food consumption throughout the study with the
exception of transient, parallel decreases in these parameters
during study week number 1 in males and females given 2000 ppm.
Hematology and clinical chemistry findings were generally
unremarkable. There was no treatment-related effect on organ
weights and no remarkable findings at gross necropsy or after
histopathological examination. There were no increases in tumours
that were treatment-related. The NOAEL exceeded 2000 ppm in the
diet, equivalent to more than 100 mg/kg bw/day in males and females
(Goodyer, 1984b).
Special carcinogenicity bioassays of anilazine in rodents (rats
and mice) were also performed by the National Cancer Institute but
they were not considered for toxicological evaluation (NCI, 1978).
However, since these studies were performed by Gulf South Research
Corporation and were not validated.
Reproduction study
Rats
Groups of male (15/sex/dose) and female (30/sex/dose) Sprague-
Dawley rats in each of three generations (F0, F1, and F2) were
fed diets containing 0, 50, 330, or 2000 ppm anilazine (93.5% pure).
The dietary concentrations were equivalent to 2.5, 16.5, and 100
mg/kg bw/day. After maturation periods of 15 weeks in the F0
generation and 18 weeks in the F1 and F2 generations, the parental
animals were allowed to rear their offspring to weaning (F0-F1a;
F1-F2a; F2-F3a). The animals for the F1 and F2 generations
were randomly selected from the F1a and F2a litters, avoiding
sibling matings.
No effects of treatment on mortality, clinical signs, food
consumption, or body weight of parental rats were noted. Although
slight inhibition of body weight gain was noted in F0 parental
males from week 5 until termination, the response was neither
statistically significant nor dose-related. The highest dietary
level of anilazine (2000 ppm) had an apparent adverse effect on the
libido of F0 males and the fertility (and possible fecundity) of
F0 females. This was indicated, respectively, by reductions in the
number of females that mated and the number that became pregnant in
comparison to controls. Although the respective differences were
not remarkable nor evident in subsequent generations (F1 and F2),
they were verified by a crossover mating experiment involving rats
from the control and high-dose groups. No adverse effects on the
reproductive parameters were evident at anilazine dietary levels of
330 and 50 ppm. No adverse changes occurred with respect to litter
size, viability, mean pup and litter weights, or physical (i.e.,
auditory response, pupillary reflex, visual placing response, grip
strength) and functional (i.e., onset and duration of pinna
unfolding, onset of hair growth, incisor eruption, and eye opening)
development of pups. No unusual pre- or post-weaning clinical
observations occurred. No treatment-related changes in organ weight
or pathological (gross and microscopic) findings were noted in
either parenteral rats or pups at necropsy (Irvine, 1984). The
NOAEL is 330 ppm, equivalent to 16.5 mg/kg bw/day.
Special studies on teratogenicity
Rats
Groups of female Long Evans FB-30 strain rats (21-22/dose) were
administered doses of 0, 30, 100, or 300 mg/kg bw/day anilazine, by
gavage, from days 6 to 25 of gestation (Day 0 = day of
insemination). Doses were based on a preliminary range-finding
study which demonstrated that a dose of 300 mg/kg bw/day for 10 days
had no apparent adverse activity. On gestation day 20, rats were
sacrificed and fetuses delivered by C-section. Approximately 30% of
the fetuses in each group were dissected and examined for visceral
anomalies and approximately 70% of the fetuses in each group were
cleared and stained for skeletal observations.
The 300 mg/kg bw/day dose of anilazine produced a significant
reduction in body weight gain in the dams during the treatment
period as well as throughout the entire gestation period. Body
weight gain, however, was not influenced by 30 or 100 mg/kg bw/day
anilazine. None of the doses produced changes in mortality, physical
appearance, or behaviour, or the fertilization ratio or pregnancy
ratio in the dams. Similarly, none of the doses appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. Anilazine was neither fetotoxic, embryotoxic, nor
teratogenic in rats at oral doses up to 300 mg/kg/day (highest dose
tested). Because of maternal toxicity (weight gain reduction) at
300 mg/kg bw/day, the NOAEL is 100 mg/kg bw/day by gavage (Machemer,
1978).
Groups of female Charles River Crl:CD-BR rats (28/dose) were
administered oral (gastric intubation) doses of 0, 150, 500, or
1500 mg/kg bw/day anilazine from days 6 to 15 of gestation. On
gestation day 20, rats were sacrificed and fetuses delivered by
C-section. Approximately one-half of the fetuses in each group were
dissected and examined for visceral anomalies, and the other half of
the fetuses in each group were cleared and stained for skeletal
observations.
Anilazine was maternally toxic at doses of 500 and 1500 mg/kg
bw/day. The adverse findings were: 1) an increased incidence of
loose and/or soft stools that often contained a creamy white
substance (seen in 28.6 and 100% of dams at 500 and 1500 mg/kg
bw/day, respectively); 2) significant reductions in body weight and
body weight gain in dams given 1500 mg/kg bw/day on gestation days 6
to 25 and 0 to 20; 3) a significant reduction in food consumption
in dams given 500 and 1500 mg/kg bw/day on gestation day 7 only; and
4) an increased incidence of thickened and coarse mucosa of the
nonglandular portion of the stomach (seen in 18 and 15% of dams
given 500 and 1500 mg/kg bw/day, respectively). This change was
characterized microscopically as epithelial hyperplasia (acanthosis)
and hyperkeratosis of the mucus membrane. In contrast to these
results, none of the doses of anilazine induced effects on embryo or
fetal development, and no teratogenic effects were observed. The
NOAEL was 150 mg/kg bw/day by intubation (Kowalski et al. 1988).
Rabbits
Groups of female Himalayan rabbits (13/dose) were administered
0, 5, 15 and 45 mg/kg bw/day anilazine (92.4% pure) from days 6 to
18 of gestation (Day 0 = day of insemination). The animals were
sacrificed on gestation day 29 and fetuses delivered by C-section.
All fetuses were necropsied and abdominal and thoracic organs as
well as the brain examined for visceral malformations. All fetuses
were examined for skeletal defects.
No treatment-related changes in mortality, appearance,
behaviour or weight gain were observed in the does. The
fertilization and pregnancy ratios were not adversely affected.
There were no statistically significant dose-related effects on the
number of implantations or corpora lutea, sex ratio, mean fetal
weights, mean placental weights, fetuses showing visceral
malformations, or numbers of fetuses with stunted growth. No
treatment-related skeletal abnormalities or malformations were
observed. The highest dose tested, 45 mg/kg bw/day by gavage, is a
NOAEL for maternal, embryo-, and fetotoxicity, and for
teratogenicity in rabbits (Roetz, 1981).
Special studies on mutagenicity
Anilazine was mutagenic in a cytogenetic study in human
lymphocyte cultures in vitro (both with and without metabolic
activation), in a mouse lymphoma forward mutation assay (without
metabolic activation only), and at severely cytotoxic levels in a
reverse mutation assay in yeast (both with and without metabolic
activation). In contrast, the chemical was negative in a variety of
the other in vitro and in vivo systems. The weight-of-the-
evidence indicates that anilazine is not genotoxic.
Special studies on eye and skin irritation
Instillation of 100 mg grade anilazine (40.5% a.i. formulation)
in the rabbit eye for 45 seconds produced reversible iritis, slight
erythema, chemosis, and discharge which lasted for up to 13 days
(Hixson, 1980b).
Anilazine (90 mg of a technical grade formulation) produced a
strong irritant effect (redness, swelling, and lacrimation) on the
rabbit eye when placed in the conjunctival sac for 24 hours. The
reactions were reversible within 14 days. No corrosive effect
occurred (Pauluhn, 1983).
Slight primary skin irritation (erythema and edema) was
observed when anilazine (500 mg technical) was applied to shaved
rabbit skin for 4 hours. The reactions were reversible within 7
days. No corrosive effect occurred (Pauluhn, 1983).
In a 3-week dermal toxicity study in rabbits, 0%, 1% (10 mg/ml)
and 4% (40 mg/mL) of anilazine was applied to shaved skin at a
volume of 0.5 ml/kg for 6 hours/day, 5X/week, for 3 weeks. In this
study, a formulation containing 497 grams a.i.(litre (= 38.4% w/w)
was used. Both concentrations of the compound produced dermal
changes consisting of slight erythema and cellular infiltration in
the subepidermal region; in addition, slight thickening of the
epidermis was noted 40 mg/ml. However, no systemic changes were
observed as noted from hematological, clinical chemistry and
urinalysis examinations, by measurements of body weight gain, and by
histopathological evaluation of selected organs and tissues. The
systemic NOAEL was calculated to be approximately 7.7 mg a.i./kg
bw/day. (Heimann & Schilde, 1985).
Special studies on skin sensitizing effect
Anilazine was shown to be a dermal sensitizer (i.e., to induce
redness and swelling) when tested in guinea pigs by the Buehler
Patch test (Heimann, 1988a; Heimann, 1988b), the Magnusson and
Kligman test (Heimann, 1982a), and in Klecak's open epicutaneous
test (Heimann, 1982b). The positive findings were obtained using
either pure and/or technical anilazine in induction concentrations
ranging from 0.03 to 5%, and challenge doses ranging from 0.03 to
1%. Negative results for sensitizing potential were obtained in one
study (Klecak's test) with pure anilazine at induction
concentrations of 0.01 to 0.1% and challenge doses of 0.01 to 0.3%
(Heimann, 1983).
Observations in humans
A group of farm workers employed on a single farm in the United
States arrived at a hospital emergency room with severe dermatitis
in July 1980. All were exposed to three pesticides in a tomato
field (anilazine, benomyl, and endosulfan). Seven of the workers
were available for patch testing to the three pesticides. Delayed
hypersensitivity (48-hour eczematous reaction) to 0.1% Dyrene
(anilazine) was demonstrated in 6 of the 7 affected workers. Nine
non-exposed adult volunteers who were used as control subjects had
negative patch tests to 0.1% Dyrene. The conclusion was reached
that anilazine caused allergic contact dermatitis (Schuman et al.
1980).
COMMENTS
Anilazine administered orally to rats was excreted primarily in
the feces via biliary excretion, and also in urine. Anilazine was
biotransformed in rats primarily to the hydroxylation product,
2-2-chloroanilino)-s-triazinedione, which was present only in
urine. A second biotransformation pathway involving conjugate
formation via the glutathione - mercapturic acid pathway was also
observed in goats and chickens. No unusual organ or tissue
localization of (14C)-labelled anilazine was observed.
Anilazine has a low acute toxicity in the species examined.
Subchronic studies in rats indicated that the stomach was the
primary target organ of toxicity; oral gavage doses of aqueous
anilazine emulsion of 30 mg/kg bw/day and a dietary level of
2000 ppm (equivalent to 100 mg/kg bw/day) or greater produced
forestomach changes characterized by acanthosis, hyperkeratosis, and
areas of ulceration and erosion. The NOAEL was 500 ppm (equivalent
to 25 mg/kg bw/day).
In an 18-month oral (capsule) toxicity study in dogs, anilazine
at doses of 40 and 160 mg/kg bw/day produced increases in alpha-1,
beta-2, and gamma-globulins in plasma, vomiting, and diarrhea in
males and females. These findings were also observed with oral
doses (capsules) of 50 to 450 mg/kg bw/day for 13 weeks in dogs.
The NOAEL in the 18-month study was 10 mg/kg bw/day.
In long-term studies in rats and mice, no treatment-related
increases in neoplasms were observed. The NOAELs were 1250 ppm
(equal to 188 mg/kg bw/day) in Cr CD-1 mice and 2000 ppm (equivalent
to 100 mg/kg bw/day) in SD-CD rats.
In a 3-generation, 2 litters per generation, reproduction study
in rats, reductions in male libido and female fertility occurred in
F0 generation animals at 2000 ppm in the diet (equivalent to
100 mg/kg bw/day). Although these effects were confirmed in
cross-mating experiments, they were not observed in subsequent
generations. The NOAEL was 330 ppm (equivalent to 16.5 mg/kg
bw/day).
In teratology studies in rats, the NOAEL was 150 mg/kg bw/day
based on maternal toxicity. Oral doses of 300 to 1500 mg/kg bw/day
produced soft/loose stools, weight loss, reduced food consumption
and acanthosis and hyperkeratosis of the stomach of dams, but had no
fetotoxic or teratogenic activity. The NOAEL was 45 mg/kg bw/day
(highest dose tested) in a rabbit teratology study.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 1250 ppm in the diet, equal to 188 mg/kg bw/day
Rat: 330 ppm in the diet, equivalent to 16.5 mg/kg bw/day
Dog: 10 mg/kg bw/day orally.
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ecker, W. (1981) [Phenyl-UL-14C]Anilazine: Characterization of
metabolites in rat urine and feces. Unpublished Report No. 10216
from Institute of Pharmacokinetics, Bayer AG. Submitted to WHO by
Bayer AG, Wuppertal, FRG.
Ecker, W. (1983) [Triazine-UL-14C]Anilazine: Characterization of
metabolites in rat urine; comparison of metabolite patterns after
administration of [Triazine-UL-14C]Anilazine and [Phenyl-UL-
14C]Anilazine. Unpublished Report No. 11610 from Institute of
Pharmacokinetics, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Eubler, H., Traber, J., Walk, R.A. (1979) Biokinetics of
14C-Anilazine on the rat. Unpublished Report No. A0197/1526 from
INBIFO Research, Cologne. Submitted to WHO by Bayer AG, Wuppertal,
FRG.
Flucke, W. (1978) Determination of acute toxicity (LD50);
refrigerator sample of June 21. Unpublished Report of August 11
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Flucke, W., Kaliner, G. (1978) Dyrene: subacute oral toxicity study
on rats. Unpublished Report No. 7844 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Flucke, W., Kimmerle, G. (1977) Dyrene: acute toxicity studies.
Unpublished Report No. 6761 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Goodyer, M.J. (1981) Anilazine: a 13-week oral (dietary
administration) toxicity study in the rat with a 6-week interim
kill. Unpublished Report No. 2660-262/23 from Hazleton
Laboratories Europe, Ltd., Harrogate, England. Submitted to WHO by
Bayer AG, Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984a) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the mouse.
Unpublished Report No. 3622-262/22 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984b) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the rat.
Unpublished Report No. 3686-262/24 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Heimann, K.G. (1982a) Technical dyrene: study of sensitization
effect on guinea pigs (maximization test of Magnusson and Kligman).
Unpublished Report No. 11059 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1982b) Dyrene: investigation of sensitizing effect
in the open epicutaneous test on guinea pigs. Unpublished Report
No. 10816 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1983) Dyrene pure active ingredient: study for
skin-sensitizing effect in the open epicutaneous test on guinea
pigs. Unpublished Report No. 12034 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50).
Unpublished Report of August 20 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984b) Anilazine and MEB 6447: study for combination
toxicity. Unpublished Report No. 12609 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1985) Determination of acute toxicity (LD50).
Unpublished Report of February 20 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1988a) Dyrene: study for skin-sensitizing effect on
guinea pigs (Buehler Patch Test). Unpublished Report No. 16419 from
Toxicology Division Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Heimann, K.G. (1988b) Dyrene Technical: study for skin-sensitizing
effect in the open epicutaneous test on guinea pigs (Buehler Patch
Test). Unpublished Report No. 16420 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G., Schilde, B. (1985) Dyrene: subacute study of dermal
toxicity to rabbits. Unpublished Report No. 14151 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1978) Dyrene: dominant lethal study on male mouse to
test for mutagenic effects. Unpublished Report No. 7448 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980a) Dyrene: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished Report No. 9172 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980b) Dyrene: micronucleus test on mouse to evaluate
dyrene for mutagenic potential. Unpublished Report No. 9565 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1981) Dyrene: PolA1 - test on E. coli to evaluate for
DNA damage. Unpublished Report No. 10149 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1986a) Spot test on cross-bred C57B1/6J x T stock
mouse embryos to evaluate for induced somatic changes in the genes
of the coat pigment cells. Unpublished Report No. 15041 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1986b) Dyrene: cytogenic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on chromosomes.
Unpublished Report No. 14188 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1987) Anizaline Tech. Salmonella/microsome test for
determination of point mutations. Unpublished Report No. 15780 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1988a) Anizaline: micronucleus test on the mouse for
clastogenic effects. Unpublished Report No. 16481 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1988b) Dyrene: dominant lethal test on the male mouse
to evaluate for mutagenic effects. Unpublished Report No. 16688
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Hixson, E.J. (1979) Acute oral toxicity study of Dyrene 50%
wettable powder to rats. Unpublished Report No. 68411 from Mobay
Chemical Corporation, Kansas City MO. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Hixson, E.J. (1980a) Acute oral toxicity study of Dyrene 4F to
rats. Unpublished Report No. 118 from Mobay Chemical Corporation,
Kansas City MO. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980b) Dermal irritation of Dyrene 4F. Unpublished
Report No. 123 from Mobay Chemical Corporation, Stillwell, KS.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980c) Acute oral toxicity of Dyrene 4F to rabbits.
Unpublished Report No. 126 from Mobay Chemical Corporation,
Stillwell, KS. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hoffmann, K., Gröning, P. (1979) Dyrene: subchronic toxicity study
on dogs (13-week oral administration in capsules). Unpublished
Report No. 8199 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer, AG, Wuppertal, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1983) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Unpublished Report No. 11613 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Bayerwerk, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1987) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1988) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Second Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Irvine, L.F.H. (1984) Anilazine: 3-generation oral (dietary
administration) reproduction study in the rat. Unpublished Report
No. 3744-262/26 from Hazleton Laboratories Europe, Ltd., Harrogate,
England. Submitted to WHO by Bayer AG, Institute of Toxicology,
Wuppertal, FRG.
Jagannath, D.R. (1981) Mutagenicity evaluation of Dyrene in the
saccharomyces reverse mutation assay. Unpublished Report No. R2088
from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
Kowalski, R.L., Clemens, G.R., Hartnagel, R.E. Jr (1988) A
teratology study with anilazine (Dyrene Technical) in the rat.
Unpublished Report No. 1069 from Toxicology Department, Miles, Inc.,
USA. Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Machemer, L. (1978) Dyrene: evaluation for embryotoxic and
teratogenic effects on orally dosed rats. Unpublished Report
No. 7746 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Miltenberger, H.G. (2986) Anilazine Techn. Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with V79
cells. Unpublished Report No. R3821 from Laboratory for
Mutagenicity Testing, Technical University, Darmstadt. Submitted to
WHO by Bayer, AG, Wuppertal, FRG.
Myhr, B.C. (1983) Evaluation of Analazine Technical in the primary
rat hepatocyte unscheduled DNA synthesis assay. Unpublished Report
No. R2615 from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
NCI (1978) NCI (National Cancer Institute) Carcinogenesis technical
report series No. 104. Bioassay of anilazine for possible
carcinogenicity. US Department of Health, Education and Welfare
Publication No.(NIH) 78-1354.
Pauluhn, J. (1983) Dyrene (technical): study for irritant and
corrosive effect on skin and eye (rabbits). Unpublished Report
No. 11826 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Roetz, R. (1981) Dyrene: evaluation for embryotoxic and/or
teratogenic effects on the rabbit after oral administration.
Unpublished Report No. 10339 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Sangha, G.K. (1984) Acute inhalation toxicity study with Anilazine
(Dyrene) technical in rats. Unpublished Report No. 463 from Mobay
Chemical Corporation, Stillwell, KS. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Schuman, S.H., Dobson, R.L., Fingar, J.R. (1980) Dyrene dermatitis.
Lancet, page 1252, December 6, 1980.
Volkner, W. (1987) Chromosome aberration test in bone marrow cells
of the Chinese hamster with Anilazine Technical. Unpublished Report
No. R4100 from Cytotest Cell Research GmBH & Company, KG, Darmstadt,
FRG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Witterland, W.F. (1984) Mutagenicity evaluation of Anilazine
Technical in the mouse lymphoma forward mutation assay. Unpublished
Report No. R2831 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer AG, Wuppertal, FRG.
Biotransformation
The metabolism of [phenyl-UL-14C]anilazine was characterized
over a 24-hour period in the urine and feces of albino SD rats given
a single oral dose of 5 mg/kg bw. About 16 to 82% of the
administered RA dose was excreted in urine and feces, respectively.
No parent compound was present in urine or feces. The major
identified metabolite was the hydroxylation product,
2-(2-chloroanilino)-s-triazinodione, which comprised about 25% of
the RA found in urine but was present only in insignificant amounts
in feces. Several other unknown polar metabolites, which were
predominantly acidic in nature, were found. In addition, a small
portion of the RA metabolite became less polar as a result of
treatment with diazomethane. The majority of the metabolite could
also be acetylated (Ecker, 1981). The proposed metabolic pathway
for anilazine in dairy goats is shown in Figure 1.
Another study in male rats comparing the metabolism of
[phenyl-UL-14C]anilazine and [triazine-UL-14C]anilazine (single
oral dose of 5 mg/kg) indicated that the urinary and fecal metabolic
patterns of both labels were identical, suggesting that the basic
structure of the two rings of the molecule were maintained during
biotransformation (Ecker, 1983).
TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON ANILAZINE
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Ames testa S. typhimurium 5-12,500 ug/plate 95.9% Negative Herbold, 1980a
TA98, TA100, in DMSO
TA1535, TA1537
Ames testa S. typhimurium 20-12,500 ug/plate Technical Negative Herbold, 1987
TA98, TA100, in DMSO 97.1%
TA1535, TA1537
Yeast testa S. cerevisiae 1-5000 ? Positive Jagannah, 1981
(Reverse mutation S138, S211 ug/incubation only at
assay tube in DMSO severely
cytoxic
levels
DNA damage in E. coli Pol A+/ 62.5-1000 ug/plate Technical Negative Herbold, 1981
E. colia Pol A- in DMSO 93.5%
CH-V79/HGPRT gene Chinese hamster 0.2-9 ug/mL in 97.3% Negative Miltenburger, 1986
mutation assaya cell line V79 DMSO
Mouse lymphoma L5178Y mouse 2.5-9 ug/mL in Technical Positive Witterland, 1984
forward mutation lymphoma cells DMSO 95.9% (nonactivated
assaya (TK+/-) only)
Unscheduled DNA Primary hepatocytes 0.049-9.8 ug/mL Technical Negative Myhr, 1983
synthesis from male F344 rats 98.6%
Mouse micronucleus Bone marrow 7500 mg/kg single 97.1% Negative Herbold, 1988a
test erythro-blasts oral dose
(NMRI mice)
TABLE 1 (contd.)
CONCENTRATION
TEST SYSTEM TEST OBJECT OF ANILAZINE PURITY RESULTS REFERENCE
Mouse micronucleus Bone marrow 100-200 mg/kg 95.9% Negative Herbold, 1980b
test erythro-blasts bw/day for 2 days
(NMRI mice)
Spot test in mice Coat pigment cells 3-30 mg/kg bw i.p. 97.3% Negative Herbold, 1986a
embryos to evaluate of crossbred on gestation day 10 in F1 mice
somatic gene changes C57B1/6J XT mice to pregnant females
Dominant lethal test Mouse (male) 5000 or 7500 mg/kg bw 97.3% Negative Herbold, 1988b
(single or oral dose)
Dominant lethal test Mouse (male) 500 mg/kg bw or 94% Negative Herbold, 1978
(single oral dose)
Chromosome aberration Chinese hamster 300-3000 mg/kg bw Technical Negative Völkner, 1987
test bone marrow (single oral dose)
Cytogenic testa Human lymphocyte 3-30 ug/ml in DMSO Technical Positive Herbold, 1986b
cultures (in vitro)
a with and without metabolic activation.
Toxicological studies
Acute toxicity
The acute toxicity of anilazine in several species is given in
Table 2. After oral administration to rats and cats, the most
common signs of toxicity were diarrhea and vomiting, respectively.
After dermal administration to rabbits, mild skin irritation
manifested as edema and erythema was observed. Anilazine was more
toxic by intraperitoneal injection than by other routes of
administration, suggesting poor enteral and percutaneous absorption.
TABLE 2. ACUTE TOXICITY OF ANILAZINE 1,2
LD50 LC50
SPECIES SEX ROUTE mg/kg/bwt (mg/l) REFERENCE
Mouse M oral approx 5000 - Flucke & Kimmerle, 1977
F 367 -
Mouse M s.c. > 5000 - Flucke & Kimmerle, 1977
F 3512 -
Rat M&F oral > 5000 - Flucke & Kimmerle, 1977
Hixson, 1979, 1980a
Heimann, 1985
M oral 3650 - Flucke, 1978
M&F i.p.3 71-137 - Flucke & Kimmerle, 1977
Heimann, 1984b
M&F inhal. - > 0.729 Sangha, 1984
M&F percutaneous > 5000 - Flucke & Kimmerle, 1977
Rabbit M&F dermal > 2000 - Hixson, 1980b, 1980c
Cat F oral > 500 - Flucke & Kimmerle, 1977
1 All studies performed using technical grade anilazine except for one LD50 study in
rats where 50% wettable powder was tested (Hixson, 1979), and two other LD50 where
formulated anilazine containing 40.5% a.i. was tested (Hixson, 1980a, 1980c).
2 The acute oral toxicity of dihydroxy-anilazine, a metabolite of anilazine was also
tested in rats. The LD50 value was > 5000 mg/kg (Heimann, 1984a).
3 The acute i.p. toxicity in male rats of anilazine (LD50 = 137 mg/kg) in combination
with triadimefon (LD50 = 317 mg/kg) was determined to be additive (LD50 of combination =
194 mg/kg) (Heimann, 1984b)
Short-term studies
Rats
SPF Wistar albino rats (20/sex/group) were administered
anilazine (technical grade, 92% pure) by gavage as an aqueous
emulsion at dose levels of 0, 30, 100, and 300 mg/kg bw/day for 28
days. The treatment period was followed by an additional 4-week
observation period. Half of the males in each group were sacrificed
at the end of the 28-day treatment period and the other half at the
end of the 4-week observation period. Hematology, clinical
chemistry, and urinalysis were performed in selected animals at each
time period prior to sacrifice. Animals were necropsied and
selected organs weighed. Histopathological examinations were
performed on 5 rats/sex/group from the 28-day treatment phase of the
study (16 tissues examined) and from 5 rats/sex/group from the
post-treatment observation phase of the study (3 tissues examined,
i.e., stomach, intestines, and esophagus).
During the 28-day treatment period, eight animals died (2/5
females at 100 mg/kg bw/day on day 11, and 1/5 males and 5/5 females
at 300 mg/kg bw/day on days 11-13). Gross pathology examination of
these animals indicated the presence of eroded abdominal and
thoracic viscera and lung lesions. Other changes seen in treated
animals were alterations in behaviour (apathy and dyspnea staring on
days 5-7 and lasting for 3 to 4 weeks) in females given 100 mg/kg
bw/day and in both sexes of rats at 300 mg/kg bw/day, and reductions
in body weight in males at 300 mg/kg bw/day on weeks 1 to 4. The
only observed treatment-related histological effect was
hyperkeratosis of the cutaneous mucosa of the proventricular part of
the stomach. The severity of the hyperkeratosis was also
dose-related, ranging from "trace" at the low-dose level; "minimal"
at the mid-dose level; and "moderate" at the high-dose level. The
finding was attributed to a local irritant effect of anilazine on
the mucosa. No clear treatment-related hematological, clinical
chemistry, urinalysis, or organ weight changes were observed during
the treatment period. None of the above described adverse effects
were seen in surviving rats similarly treated with anilazine but
allowed to recover for 4 additional weeks. The NOAEL for systemic
toxicity for anilazine, when administered by gavage to rats, was
30 mg/kg bw/day. However, stomach mucosal hyperkeratosis,
representing a local irritant effect, was seen at all of the tested
doses of anilazine (Flucke & Kaliner, 1978).
Groups of Sprague-Dawley CD rats (25 males and 25
females/group) received dietary concentrations of 0, 500, 2000, or
8000 ppm anilazine (93.5% pure) for 13 weeks. Five rats/sex/dose
group were sacrificed after 6 weeks.
No treatment-related deaths occurred. Body weight gain was
reduced over the study in mid- and high-dose males and in high-dose
females. Other changes were seen only in high-dose males and
females at the end of the study, and in many cases at the 6-week
interim kill period as well. These included clinical signs of
toxicity (piloerection, red-brown staining of fur around head and
back, rhinorrhea, and exophthalmos), and reduced food consumption,
increased alkaline phosphatase levels, reduction in lymphocytes,
creatinine and total protein levels, increases in the relative
weights of several organs (heart, brain, pituitary, gonads, adrenal
glands, thyroids, kidneys, and lungs), and various pathological
findings (pale appearing pancreas, foamy macrophage foci in the
lungs, testicular atrophy, lymph node hyperplasia, and enlarged
salivary glands with heightened secretory activity). As indicated
by histopathological analysis, the primary target organ of toxicity
appeared to be the forestomach. At the 6-week interim kill period,
a thickened forestomach mucosa was observed that was characterized
by dyskeratosis, acanthosis, hyperkeratosis, and edema in most of
the male and female rats examined at the mid- and high-dose levels.
At the 13-week terminal sacrifice period hyperkeratosis/hyperplasia
of the forestomach occurred at the mid- and high-dose levels at
incidences of 3/20 and 18/20, respectively, in male rats and 9/20
and 16/20, respectively, in females. These changes were accompanied
in some cases by ulcers and/or erosions of the forestomach, and were
considered to be a local irritant effect of anilazine on the mucosa.
The only finding at the lowest dose level was slight acanthosis and
hyperkeratosis of skin on the tails of female rats at the 13-week
sacrifice period (incidence: 2/20 controls; 5/20 low dose; 8/20
mid-dose, and 10/20 high dose). The findings occurred in male rats
only at the mid (1/20) and high (11/20) dose levels. The tail
lesions are of questionable biological significance. The NOAEL was
considered to be 25 mg/kg bw/day due to the observed reduction in
body weight gain in males and the local forestomach lesions in both
sexes (Goodyer, 1981).
Dogs
Purebred beagle dogs (4/sex/dose) were administered anilazine
(92.0% pure) orally (gelatin capsules) at dose levels of 0, 50, 150,
and 450 mg/kg bw/day for 13 weeks.
There were no effects of treatment on mortality, or on
patellar, flexor or extensor reflex activity or body temperature.
No adverse effects were observed with respect to ophthalmoscopic
examinations, or hematological or urinalysis parameters. Vomiting
and diarrhea several hours after dosing were prominent signs of
toxicity in both sexes administered all three dose levels. These
effects were dose-related and occurred throughout the study. Test
compound was frequently seen in vomitus and feces. The animals also
were agitated and ran back and forth in their cages before
defecation (the effect was attributed to possible intestinal spasms
evoked by the compound). Additional signs of toxicity were also
observed. Changes noted at both the 150 and 450 mg/kg bw/day levels
included reductions in pulse rate and slight drowsiness, reduced
serum albumin levels and increased plasma globulin levels (i.e.,
alpha-1, alpha-2, beta, and gamma globulin levels were increased),
and (in females), decreases in absolute and relative ovary weights.
Changes that occurred only at the 450 mg/kg bw/day dose level were
dull looking ungroomed hair coats, reductions in body weight gain
and food consumption, and reductions in total serum protein levels
and serum calcium levels. The absolute and relative weights of the
thymus gland were reduced, and thymus gland atrophy was observed
grossly, in high-dose males and females. Upon histological
examination, thymus gland atrophy was seen at all of the dose levels
tested in males and the highest dose in females. Histological
examination of the esophagus also revealed an increase in the
incidence of diffuse round cell infiltration of the mucosa at all
three dose levels. A NOAEL was not established in this study
(Hoffmann & Gröning, 1979).
Beagle dogs (4/sex/group) were administered anilazine
a.i.(91.5-94.4% pure) orally (gelatin capsules) at dose levels of 0,
10, 40, and 160 mg/kg bw/day for 18 months.
Treatment-related toxicological effects were produced by the
two highest dose levels of anilazine. Adverse findings that
occurred at 40 and 160 mg/kg bw/day in both males and females
included vomiting of the test substance with food, increases in
alpha-2, beta, and gamma globulins in plasma, and an increase in the
relative weight of the adrenal glands. Additional changes that were
seen in dogs of both sexes only at the highest dose level (160 mg/kg
bw/day) included salivation, loose-to-liquid feces, reduction in
bilirubin levels, and (in females) a decrease in the absolute weight
of the ovaries. High-dose animals also showed a tendency for a
decreased body weight gain which was due primarily to one male and
one female dog, although weight gain was also somewhat low in all of
the remaining dogs given the high dose level. One male dog in the
high-dose group was diagnosed with confluent bronchial pneumonia of
the right lung and suppurative bronchitis with increased blood
leukocytes and heightened hematopoiesis in bone marrow.
Esophagitis, gastritis, thymus involution, release of splenic
corpuscles and lymphadenitis were also noted in this animal. The
bronchial pneumonia was attributed to a bacterial or viral-induced
infection and was not considered to be compound-induced.
Anilazine did not produce treatment-related effects on
survival, vital signs (i.e., reflex activity, temperature, or pulse
rate) or food consumption, nor were adverse ophthalmological
findings observed. Urinalysis parameters were unchanged. No
treatment-related non-neoplastic or neoplastic lesions were evident.
The NOAEL was 10 mg/kg bw/day anilazine (Hoffmann et al. 1983;
Hoffmann et al. 1987; Hoffmann et al. 1988).
Long-term/carcinogenicity studies
Mice
Male and female Charles River CD-1 mice (50/sex/dose) were fed
diets containing 0, 50, 250, or 1250 ppm anilazine (93.5% pure) for
104 weeks. Sixteen additional mice/sex group served solely for the
collection of blood samples, and were not examined
histopathologically. The average dietary concentrations were
equivalent to 7.5, 37.5, and 290.0 mg/kg bw/day. Blood was collected
at 0, 27, 53, 79, and 103 weeks). The weights of liver, kidneys,
gonads, heart, and brain were obtained.
There were no effects of treatment on mortality, clinical signs
of toxicity, body weight, food consumption, hematology, or clinical
chemistry. Plasma glucose levels were elevated in occasional male
and female mice in the 250 and 1250 ppm groups. There were no
treatment-related effects on organ weights and no remarkable
findings at gross necropsy or after histopathological examination.
The incidences of Harderian gland adenomas were elevated in males
and females in the high-dose group. These tumour incidences,
however, were neither statistically significant nor above historical
control levels. On the basis of these findings the NOAEL in this
study exceeded 1250 ppm in the diet, equal to approximately
190.0 mg/kg bw/day in males and females (Goodyer, 1984a).
Rats
Male and female Sprague-Dawley CD rats (50/sex/group) were fed
anilazine (93.5% pure) for 104 weeks at levels of 0, 50, 330, or
2000 ppm. These concentrations were equivalent to approximately
2.5, 16.5 and 100 mg/kg bw/day. Ten additional rats/sex/dose were
used solely for the collection of blood samples; these animals were
not examined histopathologically. Blood was collected at 0, 26/27,
53, 79 and 103 weeks. Urinalysis studies, however, were not
performed. The weights of liver, kidney gonads, heart, brain,
adrenals, spleen, lungs, and thyroid glands were obtained.
There were no clinical signs of toxicity and no effect of
treatment on mortality. There were no treatment-related effects on
body weight or food consumption throughout the study with the
exception of transient, parallel decreases in these parameters
during study week number 1 in males and females given 2000 ppm.
Hematology and clinical chemistry findings were generally
unremarkable. There was no treatment-related effect on organ
weights and no remarkable findings at gross necropsy or after
histopathological examination. There were no increases in tumours
that were treatment-related. The NOAEL exceeded 2000 ppm in the
diet, equivalent to more than 100 mg/kg bw/day in males and females
(Goodyer, 1984b).
Special carcinogenicity bioassays of anilazine in rodents (rats
and mice) were also performed by the National Cancer Institute but
they were not considered for toxicological evaluation (NCI, 1978).
However, since these studies were performed by Gulf South Research
Corporation and were not validated.
Reproduction study
Rats
Groups of male (15/sex/dose) and female (30/sex/dose) Sprague-
Dawley rats in each of three generations (F0, F1, and F2) were
fed diets containing 0, 50, 330, or 2000 ppm anilazine (93.5% pure).
The dietary concentrations were equivalent to 2.5, 16.5, and 100
mg/kg bw/day. After maturation periods of 15 weeks in the F0
generation and 18 weeks in the F1 and F2 generations, the parental
animals were allowed to rear their offspring to weaning (F0-F1a;
F1-F2a; F2-F3a). The animals for the F1 and F2 generations
were randomly selected from the F1a and F2a litters, avoiding
sibling matings.
No effects of treatment on mortality, clinical signs, food
consumption, or body weight of parental rats were noted. Although
slight inhibition of body weight gain was noted in F0 parental
males from week 5 until termination, the response was neither
statistically significant nor dose-related. The highest dietary
level of anilazine (2000 ppm) had an apparent adverse effect on the
libido of F0 males and the fertility (and possible fecundity) of
F0 females. This was indicated, respectively, by reductions in the
number of females that mated and the number that became pregnant in
comparison to controls. Although the respective differences were
not remarkable nor evident in subsequent generations (F1 and F2),
they were verified by a crossover mating experiment involving rats
from the control and high-dose groups. No adverse effects on the
reproductive parameters were evident at anilazine dietary levels of
330 and 50 ppm. No adverse changes occurred with respect to litter
size, viability, mean pup and litter weights, or physical (i.e.,
auditory response, pupillary reflex, visual placing response, grip
strength) and functional (i.e., onset and duration of pinna
unfolding, onset of hair growth, incisor eruption, and eye opening)
development of pups. No unusual pre- or post-weaning clinical
observations occurred. No treatment-related changes in organ weight
or pathological (gross and microscopic) findings were noted in
either parenteral rats or pups at necropsy (Irvine, 1984). The
NOAEL is 330 ppm, equivalent to 16.5 mg/kg bw/day.
Special studies on teratogenicity
Rats
Groups of female Long Evans FB-30 strain rats (21-22/dose) were
administered doses of 0, 30, 100, or 300 mg/kg bw/day anilazine, by
gavage, from days 6 to 25 of gestation (Day 0 = day of
insemination). Doses were based on a preliminary range-finding
study which demonstrated that a dose of 300 mg/kg bw/day for 10 days
had no apparent adverse activity. On gestation day 20, rats were
sacrificed and fetuses delivered by C-section. Approximately 30% of
the fetuses in each group were dissected and examined for visceral
anomalies and approximately 70% of the fetuses in each group were
cleared and stained for skeletal observations.
The 300 mg/kg bw/day dose of anilazine produced a significant
reduction in body weight gain in the dams during the treatment
period as well as throughout the entire gestation period. Body
weight gain, however, was not influenced by 30 or 100 mg/kg bw/day
anilazine. None of the doses produced changes in mortality, physical
appearance, or behaviour, or the fertilization ratio or pregnancy
ratio in the dams. Similarly, none of the doses appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. Anilazine was neither fetotoxic, embryotoxic, nor
teratogenic in rats at oral doses up to 300 mg/kg/day (highest dose
tested). Because of maternal toxicity (weight gain reduction) at
300 mg/kg bw/day, the NOAEL is 100 mg/kg bw/day by gavage (Machemer,
1978).
Groups of female Charles River Crl:CD-BR rats (28/dose) were
administered oral (gastric intubation) doses of 0, 150, 500, or
1500 mg/kg bw/day anilazine from days 6 to 15 of gestation. On
gestation day 20, rats were sacrificed and fetuses delivered by
C-section. Approximately one-half of the fetuses in each group were
dissected and examined for visceral anomalies, and the other half of
the fetuses in each group were cleared and stained for skeletal
observations.
Anilazine was maternally toxic at doses of 500 and 1500 mg/kg
bw/day. The adverse findings were: 1) an increased incidence of
loose and/or soft stools that often contained a creamy white
substance (seen in 28.6 and 100% of dams at 500 and 1500 mg/kg
bw/day, respectively); 2) significant reductions in body weight and
body weight gain in dams given 1500 mg/kg bw/day on gestation days 6
to 25 and 0 to 20; 3) a significant reduction in food consumption
in dams given 500 and 1500 mg/kg bw/day on gestation day 7 only; and
4) an increased incidence of thickened and coarse mucosa of the
nonglandular portion of the stomach (seen in 18 and 15% of dams
given 500 and 1500 mg/kg bw/day, respectively). This change was
characterized microscopically as epithelial hyperplasia (acanthosis)
and hyperkeratosis of the mucus membrane. In contrast to these
results, none of the doses of anilazine induced effects on embryo or
fetal development, and no teratogenic effects were observed. The
NOAEL was 150 mg/kg bw/day by intubation (Kowalski et al. 1988).
Rabbits
Groups of female Himalayan rabbits (13/dose) were administered
0, 5, 15 and 45 mg/kg bw/day anilazine (92.4% pure) from days 6 to
18 of gestation (Day 0 = day of insemination). The animals were
sacrificed on gestation day 29 and fetuses delivered by C-section.
All fetuses were necropsied and abdominal and thoracic organs as
well as the brain examined for visceral malformations. All fetuses
were examined for skeletal defects.
No treatment-related changes in mortality, appearance,
behaviour or weight gain were observed in the does. The
fertilization and pregnancy ratios were not adversely affected.
There were no statistically significant dose-related effects on the
number of implantations or corpora lutea, sex ratio, mean fetal
weights, mean placental weights, fetuses showing visceral
malformations, or numbers of fetuses with stunted growth. No
treatment-related skeletal abnormalities or malformations were
observed. The highest dose tested, 45 mg/kg bw/day by gavage, is a
NOAEL for maternal, embryo-, and fetotoxicity, and for
teratogenicity in rabbits (Roetz, 1981).
Special studies on mutagenicity
Anilazine was mutagenic in a cytogenetic study in human
lymphocyte cultures in vitro (both with and without metabolic
activation), in a mouse lymphoma forward mutation assay (without
metabolic activation only), and at severely cytotoxic levels in a
reverse mutation assay in yeast (both with and without metabolic
activation). In contrast, the chemical was negative in a variety of
the other in vitro and in vivo systems. The weight-of-the-
evidence indicates that anilazine is not genotoxic.
Special studies on eye and skin irritation
Instillation of 100 mg grade anilazine (40.5% a.i. formulation)
in the rabbit eye for 45 seconds produced reversible iritis, slight
erythema, chemosis, and discharge which lasted for up to 13 days
(Hixson, 1980b).
Anilazine (90 mg of a technical grade formulation) produced a
strong irritant effect (redness, swelling, and lacrimation) on the
rabbit eye when placed in the conjunctival sac for 24 hours. The
reactions were reversible within 14 days. No corrosive effect
occurred (Pauluhn, 1983).
Slight primary skin irritation (erythema and edema) was
observed when anilazine (500 mg technical) was applied to shaved
rabbit skin for 4 hours. The reactions were reversible within 7
days. No corrosive effect occurred (Pauluhn, 1983).
In a 3-week dermal toxicity study in rabbits, 0%, 1% (10 mg/ml)
and 4% (40 mg/mL) of anilazine was applied to shaved skin at a
volume of 0.5 ml/kg for 6 hours/day, 5X/week, for 3 weeks. In this
study, a formulation containing 497 grams a.i.(litre (= 38.4% w/w)
was used. Both concentrations of the compound produced dermal
changes consisting of slight erythema and cellular infiltration in
the subepidermal region; in addition, slight thickening of the
epidermis was noted 40 mg/ml. However, no systemic changes were
observed as noted from hematological, clinical chemistry and
urinalysis examinations, by measurements of body weight gain, and by
histopathological evaluation of selected organs and tissues. The
systemic NOAEL was calculated to be approximately 7.7 mg a.i./kg
bw/day. (Heimann & Schilde, 1985).
Special studies on skin sensitizing effect
Anilazine was shown to be a dermal sensitizer (i.e., to induce
redness and swelling) when tested in guinea pigs by the Buehler
Patch test (Heimann, 1988a; Heimann, 1988b), the Magnusson and
Kligman test (Heimann, 1982a), and in Klecak's open epicutaneous
test (Heimann, 1982b). The positive findings were obtained using
either pure and/or technical anilazine in induction concentrations
ranging from 0.03 to 5%, and challenge doses ranging from 0.03 to
1%. Negative results for sensitizing potential were obtained in one
study (Klecak's test) with pure anilazine at induction
concentrations of 0.01 to 0.1% and challenge doses of 0.01 to 0.3%
(Heimann, 1983).
Observations in humans
A group of farm workers employed on a single farm in the United
States arrived at a hospital emergency room with severe dermatitis
in July 1980. All were exposed to three pesticides in a tomato
field (anilazine, benomyl, and endosulfan). Seven of the workers
were available for patch testing to the three pesticides. Delayed
hypersensitivity (48-hour eczematous reaction) to 0.1% Dyrene
(anilazine) was demonstrated in 6 of the 7 affected workers. Nine
non-exposed adult volunteers who were used as control subjects had
negative patch tests to 0.1% Dyrene. The conclusion was reached
that anilazine caused allergic contact dermatitis (Schuman et al.
1980).
COMMENTS
Anilazine administered orally to rats was excreted primarily in
the feces via biliary excretion, and also in urine. Anilazine was
biotransformed in rats primarily to the hydroxylation product,
2-2-chloroanilino)-s-triazinedione, which was present only in
urine. A second biotransformation pathway involving conjugate
formation via the glutathione - mercapturic acid pathway was also
observed in goats and chickens. No unusual organ or tissue
localization of (14C)-labelled anilazine was observed.
Anilazine has a low acute toxicity in the species examined.
Subchronic studies in rats indicated that the stomach was the
primary target organ of toxicity; oral gavage doses of aqueous
anilazine emulsion of 30 mg/kg bw/day and a dietary level of
2000 ppm (equivalent to 100 mg/kg bw/day) or greater produced
forestomach changes characterized by acanthosis, hyperkeratosis, and
areas of ulceration and erosion. The NOAEL was 500 ppm (equivalent
to 25 mg/kg bw/day).
In an 18-month oral (capsule) toxicity study in dogs, anilazine
at doses of 40 and 160 mg/kg bw/day produced increases in alpha-1,
beta-2, and gamma-globulins in plasma, vomiting, and diarrhea in
males and females. These findings were also observed with oral
doses (capsules) of 50 to 450 mg/kg bw/day for 13 weeks in dogs.
The NOAEL in the 18-month study was 10 mg/kg bw/day.
In long-term studies in rats and mice, no treatment-related
increases in neoplasms were observed. The NOAELs were 1250 ppm
(equal to 188 mg/kg bw/day) in Cr CD-1 mice and 2000 ppm (equivalent
to 100 mg/kg bw/day) in SD-CD rats.
In a 3-generation, 2 litters per generation, reproduction study
in rats, reductions in male libido and female fertility occurred in
F0 generation animals at 2000 ppm in the diet (equivalent to
100 mg/kg bw/day). Although these effects were confirmed in
cross-mating experiments, they were not observed in subsequent
generations. The NOAEL was 330 ppm (equivalent to 16.5 mg/kg
bw/day).
In teratology studies in rats, the NOAEL was 150 mg/kg bw/day
based on maternal toxicity. Oral doses of 300 to 1500 mg/kg bw/day
produced soft/loose stools, weight loss, reduced food consumption
and acanthosis and hyperkeratosis of the stomach of dams, but had no
fetotoxic or teratogenic activity. The NOAEL was 45 mg/kg bw/day
(highest dose tested) in a rabbit teratology study.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 1250 ppm in the diet, equal to 188 mg/kg bw/day
Rat: 330 ppm in the diet, equivalent to 16.5 mg/kg bw/day
Dog: 10 mg/kg bw/day orally.
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw.
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
REFERENCES
Ecker, W. (1981) [Phenyl-UL-14C]Anilazine: Characterization of
metabolites in rat urine and feces. Unpublished Report No. 10216
from Institute of Pharmacokinetics, Bayer AG. Submitted to WHO by
Bayer AG, Wuppertal, FRG.
Ecker, W. (1983) [Triazine-UL-14C]Anilazine: Characterization of
metabolites in rat urine; comparison of metabolite patterns after
administration of [Triazine-UL-14C]Anilazine and [Phenyl-UL-
14C]Anilazine. Unpublished Report No. 11610 from Institute of
Pharmacokinetics, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Eubler, H., Traber, J., Walk, R.A. (1979) Biokinetics of
14C-Anilazine on the rat. Unpublished Report No. A0197/1526 from
INBIFO Research, Cologne. Submitted to WHO by Bayer AG, Wuppertal,
FRG.
Flucke, W. (1978) Determination of acute toxicity (LD50);
refrigerator sample of June 21. Unpublished Report of August 11
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Flucke, W., Kaliner, G. (1978) Dyrene: subacute oral toxicity study
on rats. Unpublished Report No. 7844 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Flucke, W., Kimmerle, G. (1977) Dyrene: acute toxicity studies.
Unpublished Report No. 6761 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Goodyer, M.J. (1981) Anilazine: a 13-week oral (dietary
administration) toxicity study in the rat with a 6-week interim
kill. Unpublished Report No. 2660-262/23 from Hazleton
Laboratories Europe, Ltd., Harrogate, England. Submitted to WHO by
Bayer AG, Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984a) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the mouse.
Unpublished Report No. 3622-262/22 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Goodyer, M.J. (1984b) Anilazine: 104-week oral (dietary
administration) carcinogenicity and toxicity study in the rat.
Unpublished Report No. 3686-262/24 from Hazleton Laboratories
Europe, Ltd., Harrogate, England. Submitted to WHO by Bayer AG,
Institut of Toxikologie, Wuppertal, FRG.
Heimann, K.G. (1982a) Technical dyrene: study of sensitization
effect on guinea pigs (maximization test of Magnusson and Kligman).
Unpublished Report No. 11059 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1982b) Dyrene: investigation of sensitizing effect
in the open epicutaneous test on guinea pigs. Unpublished Report
No. 10816 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1983) Dyrene pure active ingredient: study for
skin-sensitizing effect in the open epicutaneous test on guinea
pigs. Unpublished Report No. 12034 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984a) Determination of acute toxicity (LD50).
Unpublished Report of August 20 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1984b) Anilazine and MEB 6447: study for combination
toxicity. Unpublished Report No. 12609 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1985) Determination of acute toxicity (LD50).
Unpublished Report of February 20 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G. (1988a) Dyrene: study for skin-sensitizing effect on
guinea pigs (Buehler Patch Test). Unpublished Report No. 16419 from
Toxicology Division Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Heimann, K.G. (1988b) Dyrene Technical: study for skin-sensitizing
effect in the open epicutaneous test on guinea pigs (Buehler Patch
Test). Unpublished Report No. 16420 from Institute of Toxicology,
Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Heimann, K.G., Schilde, B. (1985) Dyrene: subacute study of dermal
toxicity to rabbits. Unpublished Report No. 14151 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1978) Dyrene: dominant lethal study on male mouse to
test for mutagenic effects. Unpublished Report No. 7448 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980a) Dyrene: Salmonella/microsome test for detection
of point-mutagenic effects. Unpublished Report No. 9172 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1980b) Dyrene: micronucleus test on mouse to evaluate
dyrene for mutagenic potential. Unpublished Report No. 9565 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1981) Dyrene: PolA1 - test on E. coli to evaluate for
DNA damage. Unpublished Report No. 10149 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1986a) Spot test on cross-bred C57B1/6J x T stock
mouse embryos to evaluate for induced somatic changes in the genes
of the coat pigment cells. Unpublished Report No. 15041 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1986b) Dyrene: cytogenic study with human lymphocyte
cultures in vitro to evaluate for harmful effects on chromosomes.
Unpublished Report No. 14188 from Institute of Toxicology, Bayer
AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1987) Anizaline Tech. Salmonella/microsome test for
determination of point mutations. Unpublished Report No. 15780 from
Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Herbold, B. (1988a) Anizaline: micronucleus test on the mouse for
clastogenic effects. Unpublished Report No. 16481 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Herbold, B. (1988b) Dyrene: dominant lethal test on the male mouse
to evaluate for mutagenic effects. Unpublished Report No. 16688
from Institute of Toxicology, Bayer AG. Submitted to WHO by Bayer
AG, Wuppertal, FRG.
Hixson, E.J. (1979) Acute oral toxicity study of Dyrene 50%
wettable powder to rats. Unpublished Report No. 68411 from Mobay
Chemical Corporation, Kansas City MO. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Hixson, E.J. (1980a) Acute oral toxicity study of Dyrene 4F to
rats. Unpublished Report No. 118 from Mobay Chemical Corporation,
Kansas City MO. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980b) Dermal irritation of Dyrene 4F. Unpublished
Report No. 123 from Mobay Chemical Corporation, Stillwell, KS.
Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hixson, E.J. (1980c) Acute oral toxicity of Dyrene 4F to rabbits.
Unpublished Report No. 126 from Mobay Chemical Corporation,
Stillwell, KS. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Hoffmann, K., Gröning, P. (1979) Dyrene: subchronic toxicity study
on dogs (13-week oral administration in capsules). Unpublished
Report No. 8199 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer, AG, Wuppertal, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1983) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Unpublished Report No. 11613 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Bayerwerk, FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1987) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Hoffmann, K., Luckhaus, G., Janda, B. (1988) Dyrene: chronic
toxicity to dogs on oral administration (18-month capsule study).
Second Addendum to Unpublished Report No. 11613 from Institute of
Toxicology, Bayer AG. Submitted to WHO by Bayer, AG, Bayerwerk,
FRG.
Irvine, L.F.H. (1984) Anilazine: 3-generation oral (dietary
administration) reproduction study in the rat. Unpublished Report
No. 3744-262/26 from Hazleton Laboratories Europe, Ltd., Harrogate,
England. Submitted to WHO by Bayer AG, Institute of Toxicology,
Wuppertal, FRG.
Jagannath, D.R. (1981) Mutagenicity evaluation of Dyrene in the
saccharomyces reverse mutation assay. Unpublished Report No. R2088
from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
Kowalski, R.L., Clemens, G.R., Hartnagel, R.E. Jr (1988) A
teratology study with anilazine (Dyrene Technical) in the rat.
Unpublished Report No. 1069 from Toxicology Department, Miles, Inc.,
USA. Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Machemer, L. (1978) Dyrene: evaluation for embryotoxic and
teratogenic effects on orally dosed rats. Unpublished Report
No. 7746 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Miltenberger, H.G. (2986) Anilazine Techn. Detection of gene
mutations in somatic mammalian cells in culture: HGPRT-test with V79
cells. Unpublished Report No. R3821 from Laboratory for
Mutagenicity Testing, Technical University, Darmstadt. Submitted to
WHO by Bayer, AG, Wuppertal, FRG.
Myhr, B.C. (1983) Evaluation of Analazine Technical in the primary
rat hepatocyte unscheduled DNA synthesis assay. Unpublished Report
No. R2615 from Litton Bionetics, USA. Submitted to WHO by Bayer, AG,
Wuppertal, FRG.
NCI (1978) NCI (National Cancer Institute) Carcinogenesis technical
report series No. 104. Bioassay of anilazine for possible
carcinogenicity. US Department of Health, Education and Welfare
Publication No.(NIH) 78-1354.
Pauluhn, J. (1983) Dyrene (technical): study for irritant and
corrosive effect on skin and eye (rabbits). Unpublished Report
No. 11826 from Institute of Toxicology, Bayer AG. Submitted to WHO
by Bayer, AG, Wuppertal, FRG.
Roetz, R. (1981) Dyrene: evaluation for embryotoxic and/or
teratogenic effects on the rabbit after oral administration.
Unpublished Report No. 10339 from Institute of Toxicology, Bayer AG.
Submitted to WHO by Bayer, AG, Wuppertal, FRG.
Sangha, G.K. (1984) Acute inhalation toxicity study with Anilazine
(Dyrene) technical in rats. Unpublished Report No. 463 from Mobay
Chemical Corporation, Stillwell, KS. Submitted to WHO by Bayer AG,
Wuppertal, FRG.
Schuman, S.H., Dobson, R.L., Fingar, J.R. (1980) Dyrene dermatitis.
Lancet, page 1252, December 6, 1980.
Volkner, W. (1987) Chromosome aberration test in bone marrow cells
of the Chinese hamster with Anilazine Technical. Unpublished Report
No. R4100 from Cytotest Cell Research GmBH & Company, KG, Darmstadt,
FRG. Submitted to WHO by Bayer AG, Wuppertal, FRG.
Witterland, W.F. (1984) Mutagenicity evaluation of Anilazine
Technical in the mouse lymphoma forward mutation assay. Unpublished
Report No. R2831 from Institute of Toxicology, Bayer AG. Submitted
to WHO by Bayer AG, Wuppertal, FRG.
