
PROCYMIDONE
EXPLANATION
Procymidone {N-(3,5-dichlorophenyl)-1,2-dimethylcyclopropane-
1,2-dicarbox-imide} was submitted to the 1981 JMPR meeting for
evaluation (Annex 1, FAO/WHO 1982a). The data base for the
toxicological evaluation of the compound consisted of several IBT
studies which were essential to the estimation of an ADI for man.
The 1981 meeting required independent validation of the IBT studies
before an ADI could be established. Instead of validation of those
studies a complete new set of toxicology data was submitted to the
1989 JMPR for evaluation, which are summarized in this monograph.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mouse
Male ICR mice were given a single oral dose of 100 mg
[phenyl-14C]procymidone (purity more than 99%)/kg in corn oil.
Elimination of the radioactivity was very rapid, mainly in the urine
[after 1 day 92% (urine: 74%, feces 18%), and after 7 days 104%
(urine: 82%, feces 22%)]. 14C-levels in blood, brain, kidney,
liver and testis reached maxima within 2-8 hours (16.6, 27.4, 57.8,
67.1 and 17.3 mg/kg respectively) and decreased with half-lives of
9-10 hours (Shiba et al. 1988).
Rat
Wistar rats given a single oral dose (25 mg/kg bw) of
14C-procymidone (labelled at carbonyl carbon) or 3H-procymidone
(labelled at phenyl proton) excreted over 90% of the administered
radioactive dose (in the urine about 85% and in the feces about 8%)
within 2 days after dosing. Elimination was complete over a 7-day
period, less than 0.2% was evolved as CO2. No significant
difference in excretion rate was observed between sexes, pregnant
and non-pregnant females, or position of radiolabelling. Also
consecutive administration of 25 mg 14C-procymidone/kg bw for 7
days did not alter the excretion pattern significantly. A plateau
was reached after 3 days and during the administration period 80% of
the daily dose was excreted in the urine and 10% in the feces. At
the end of the treatment period, a total of about 92% of the
administered 14C dose was found in the excreta and elimination was
almost complete 7 days after treatment withdrawal (Mikami et al.
1979).
Whole body autoradiography in male rats given a single oral
dose of 25 mg 14C-procymidone/kg bw indicated that the
radioactivity 1 hr after treatment was high in the stomach, followed
by intestines, liver and kidneys. Hardly any radioactivity could be
detected 24 hr after treatment. 14C-levels in tissues and blood
after a single oral dose of 25 mg carbonyl 14C-procymidone/kg bw to
female rats (both pregnant and non-pregnant) peaked 6 hours after
treatment (highest concentration being noted in fat, pancreas,
spleen and adrenal gland) and then declined rapidly in all tissues
except fat. Nevertheless, 0.32 mg equivalent procymidone/kg fat was
present seven days after treatment. After repeated administration
of 25 mg 14C-procymidone/kg bw to non-pregnant female rats for 7
consecutive days the 14C level in fat reached a plateau (about
0.60 mg/kg) within 3 days and decreased rapidly after the end of the
treatment period (7 days). After a single oral administration of
25 mg 14C-procymidone/kg bw to pregnant rats, radioactivity was
found in fetal blood at similar levels as in maternal blood. It
disappeared promptly with the decrease of 14C in maternal blood
(Mikami et al. 1979).
Male Sprague-Dawley rats received a single oral dose of 100 mg
{phenyl-14C}procymidone (purity 99.5%)/kg. 14C-excretion was
rapid and predominantly in the urine {after 1 day 59% (urine 54%,
feces 6%) and after 7 days 96% (urine 84%, feces 13%)}. Maxima in
14C-levels in blood, brain, kidney, liver and testes (15.4, 25.8,
49.1, 66.5 and 11.0 mg/kg, respectively) were reached within 8-12
hours and decreased with half-lives of 9-12 hours (Shiba et al.
1988).
Biotransformation
Metabolic profiles in urine and feces after single or repeated
oral administration of 25 mg 14C-procymidone/kg bw to male and
female rats or after a single administration of 100 mg
14C-procymidone/kg bw to mice and rats indicated extensive
metabolism via hydroxylation at the methyl group followed by
oxidation to carboxylic acid and hydrolysis of the imide or amide
linkage (see Figure 1). The major excreted metabolites (counting
for 68.0-75.9% of the dose) were N-(3,5-dichlorophenyl)-1-carboxy-2-
methyl cyclopropane-1,2-dicarboximide (P5), and the products (P6 and
P7) yielded from P5 by hydrolysis at the imide. Small amounts of
3,5-dichloroaniline (P8) and 2-methylcyclopropane-1,1,2-
tricarboxylic acid (P9) as well as small amounts of unchanged
procymidone (only in the feces) were detected. There is no major
species difference in metabolism between rats and mice and
consecutive administration to rats did not alter the metabolic
profile (Mikami et al. 1979; Shiba et al. 1988).
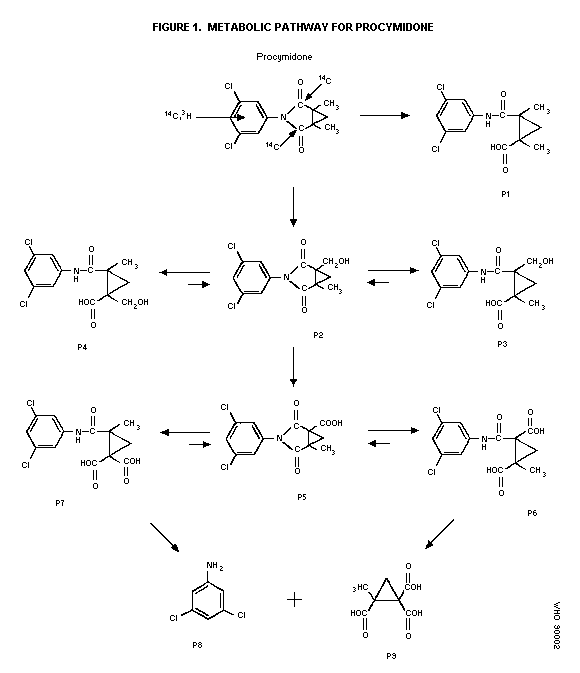 TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON PROCYMIDONE
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro
Ames test (both with S. typhimurium 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
and without metabolic TA98, TA100, ug/plate in (1)
activation TA1535, TA1538 DMSO
Ames test (both with S. typhimurium 10, 50, 100, 500 96.6 Negative Moriya et al. 1977
and without metabolic TA98, TA100, TA1535 ug/plate in (1)
activation TA1537, TA1538 DMSO
E. coli WP2 hcr
V79/HGPRT mutation Chinese hamster lung 7x10-4, 1.5x10-3 approx95 Negative Principe et al. 1980
assay (both with and cells (V79) 3x10-3, 6x10-3M (1)
without metabolic
activation)
SCE assay (both with Mouse embryo cells 10-6, 10-5, 96.9 Negative Suzuki et al. 1080
and without metabolic 10-4M in DMSO (1)
activation)
Rec-assay B. subtilis M45 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
rec H17 ug/disk in DMSO (1)
Rec-assay B. subtilis M45 20, 100, 200, 500 96.6 Negative Moriya et al. 1977
rec H17 1000, 2000 ug/disk (1)
in DMSO
TABLE 1 (contd.)
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro (contd)
Unscheduled DNA Human epithelial 6x10-6, 1x10-5 approx95 Negative Principe et al. 1980
synthesis test (both cells 6x10-4, 6x10-3M (1)
with and without in acetone
metabolic activation)
in vivo
Chromosomal aberration Male mice (ICR strain) 400, 800, 1600 96.9 Negative Hara et al. 1980
test mg/kg (i.p.) in (1)
corn oil
Host-mediated assay S. typhimurium 1000, 2000 mg/kg 96.3 Negative Suzuki & Miyamoto, 1976
G46 Male mice in DMSO (1)
(ICR strain)
Host-mediated assay S. typhimurium 200, 500 mg/kg x 2, 96.3 Negative Moriya et al. 1977
G46 Male mice in 5% Arabic gum (1)
(ICR strain)
(1) Positive control compounds gave positive responses.
Toxicological studies
Acute toxicity
The LD50 and LC50 values for the 50% dispersible powder
formulation for procymidone and some metabolites in various species
are given in Tables 2, 3 and 4, respectively.
TABLE 2. ACUTE TOXICITY OF 50% WETTABLE POWDER OF PROCYMIDONE
SPECIES SEX ROUTE LD50 REFERENCE
(mg/kg bw)
Mice M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-h exp.)
M i.p. 1750 Segawa, 1979
F i.p. 1650
Rat M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-hr exp.)
M&F inhal. >109 Kohda et al. 1976c
(4-hr exp.)
TABLE 3. ACUTE TOXICITY OF PROCYMIDONE
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Mouse M oral 7800 96.3 Kohda et al.
F 9100 96.3 1976a
M&F oral >5000 96.5 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
M i.p. 1560 96.3 Kohda et al.
F 1900 96.3 1976a
M i.p. 2030 96.5 Segawa, 1977
F 2050 96.5
Mouse M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
Rat M oral 6800 96.3 Kohda et al.
M 7700 96.3 1976a
M&F oral >5000 96.3 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
Rat M i.p. 850 96.3 Kohda et al.
F 730 96.3 1976a
M i.p. 1440 96.5 Segawa, 1977
F 1450 96.5
TABLE 3 (contd).
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Rat M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
M&F inhal. >1500 b) 99.5 Kohda et al.
1986
In all cases of suspensions of procymidone in corn oil were administered:
a) duration of exposure not indicated
b) 4-hr exposure to dust.
TABLE 4. ACUTE TOXICITY OF METABOLITES OF PROCYMIDONE IN MICE
TEST SEX ROUTE LD50 REFERENCE
COMPOUND (mg/kg bw)
3,5-DCAa M oral 900 Kohda et al. 1980
F oral 820
M s.c. 1300 Kohda et al. 1980
F s.c. 1250
DMPAb M oral 4200 Kohda et al. 1980
F oral 4650
DMPAb M s.c. 2100 Kohda et al. 1980
F s.c. 2650
SF-8748a M oral 1410 Kohda et al. 1980
F oral 1480
a) 3,5-DCA (P8) and SF-8748 (P1) were suspended or dissolved in
corn oil
b) DMPA (1,2-dimethylcyclopropane-dicarboxylic acid, plant and soil
metabolite) was suspended in 10% Tween 8O.
Short-term studies
Mice
Groups of male and female mice (ICR strain, 15/sex/group) were
fed diets containing 0, 50, 150 or 500 ppm procymidone (purity
96.9%) for three months. Mice were observed twice daily for changes
in appearance or behaviour, and body weight was recorded weekly.
Food and water consumption were measured once a week for 3
consecutive days. Hematological and biochemical studies as well as
opthalmological examinations were conducted after 3 months of
treatment. At the end of the treatment period surviving rats were
sacrificed and examined for gross pathology, organs were weighed and
histopathological examinations were carried out. There were no
compound related deaths, body weight changes, effects on food and
water consumption or clinical chemistry nor changes in
ophthalmological findings. Significantly decreased hematocrit and
erythrocyte values were observed in females at 500 ppm. Relative
kidney weight was significantly decreased in females at 150 and
500 ppm and relative adrenal weight was decreased (significantly but
not dose-relatedly) in all male dose groups. An increased relative
liver weight was observed in high dose males and females which was
not statistically significant. Hypertrophy of hepatocytes of the
liver was observed in males at 150 and 500 ppm and in females at
500 ppm. High dose females also showed an increased incidence of
dilated fundal-glands of the stomach. The NOAEL in this study is
50 ppm, equivalent to 7 mg/kg bw/day (Arai, 1980a).
Groups of ICR mice (20/group/sex) were fed diets containing 0,
50, 150 or 500 ppm procymidone (purity: 96.9%) in corn oil for 6
months. Observations included clinical examinations, mortality,
body weight, water and food consumption, ophthalmological,
hematological and biochemical examinations, macroscopy, organ weight
and histopathology. No dose-related deaths occurred. Body weight,
food and water consumption and ophthalmoscopy were not affected.
Leucocyte count was significantly decreased in males at 150 and
500 ppm and MCV values were significantly increased in females at
the same dose groups. Creatinine values were significantly
increased in males at 500 ppm. A tendency to an increased relative
liver weight was noted in females at 500 ppm. At histopathology an
increased incidence of atrophy of the testes was observed (2/19
(10.5%), 5/20 (25%), 6/20 (30%) and 10/20 (50%) in the control,
50 ppm, 150 ppm and 500 ppm group, respectively). This was
significant in the 500 ppm group only. The NOAEL in this study was
150 ppm, equivalent to 20 mg/kg bw/day (Arai, 1980b).
Groups of 20 male mice (Alpk/AP white) were fed diets
containing 0, 10, 30, 100 or 300 ppm procymidone (purity 99.8%) for
6 months. Observations included clinical signs, body weight, food
consumption, organ weight, macroscopy and histopathology of the
testes and the epididymes. The only findings observed were a not
dose-related increase in food consumption (means of 4 observations/
group) at 30, 100 and 300 ppm and a not dose related increase in
relative testes weight in all dose groups (significantly at 10 and
100 ppm), not associated with any histopathological change (Kinsey
et al. 1985).
Rat
Groups of rats (Sprague-Dawley, 12/sex/group) were fed diets
containing 0, 150, 500 or 1500 ppm procymidone (purity, 98.7%) for 6
months. Additional groups (15/sex/group) were fed diets containing
0 or 1500 ppm for 9 months, or 0 or 1500 ppm for 6 months and then
placed on a control diet for another 3 months. No compound-related
effects were observed on mortality, clinical signs, food and water
consumption and urinalysis. Body weight was decreased in females
(significantly at 1500 ppm) at all dose levels after 6 months and
male body weight was decreased at the highest dose after the same
period. After 9 months of feeding both male and female bodyweights
were significantly decreased at 1500 ppm. Significantly decreased
PCV and Hb values were observed in both sexes at 1500 ppm only after
6 months; ALAT levels were increased at 500 and 1500 ppm in both
sexes after 6 months. Relative liver weight was significantly
increased in females at 500 ppm (after 6 months) and in both sexes
at 1500 ppm after 6 and 9 months. Relative spleen weight was
significantly increased in females at 500 and 1500 ppm only after 6
months. There were also changes in several other organ weights,
possibly as a result of the change in body weight. An increased
incidence of swelling of the liver cell was observed in males at
1500 ppm after 6 (4/15) as well as after 9 months (2/15). The only
findings which were still evident after the 3 month recovery period
were a slightly decreased bodyweight in both sexes and an increased
relative liver weight in females at 1500 ppm. The NOAEL in this
study is 150 ppm, equivalent to 7.5 mg/kg bw/day (Kato et al.
1976).
Dog
Beagle dogs (6/sex/group) received by capsule at dose levels
0,20, 100 or 500 mg/kg bw/day procymidone (purity >95%) for 6
months. Mortality, body weight, food and water consumption,
ophthalmoscopy, haematology, urinalysis, organ weight, macroscopy
and histopathology were not affected by treatment. Dogs at
500 mg/kg bw/day had a higher incidence of emesis and diarrhea
(females only). Alkaline phosphatase activity was increased in
males and females at 500 mg/kg bw/day (during the second half of the
treatment period). At the same dose level BUN, glucose and calcium
(occasionally also at 100 mg/kg bw/day) levels were significantly
increased in males. Relative heart weight was significantly
decreased in high dose females. There was a tendency to an
increased liver weight in males and females and a decreased testes
weight in males at 500 mg/kg bw/day. The NOAEL in this study was
100 mg/kg bw/day (Nakashima et al. 1984).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
0, 30, 100, 300 or 1000 ppm procymidone (purity 99.8%) for 104
weeks. Satellite groups consisting of 40 mice/sex/group were
sacrificed at 6-month intervals (week 26, 52 and 78) for clinical
and pathology determinations. An additional 10 mice/sex were
assigned to the main control group for clinical pathology
determinations prior to the start of dosing. Observations included
mortality, clinical signs, body weight, food and water consumption,
ophthalmoscopy, hematology, clinical chemistry, urinalysis,
macroscopy, organ weights and histopathology. Survival rates at
termination varied from 64% to 88%. No treatment-related effects
were observed on mortality, body weight, food and water consumption,
ophthalmoscopy, hematology and urinalysis. A tendency to an
increased ALAT level was observed in both sexes at 1000 ppm.
Relative liver weight was significantly increased in males and
females at 1000 ppm and sometimes at 300 ppm. No effect was
observed on testes weight. An increased incidence of masses, pale
areas and raised areas in the livers was observed at 1000 ppm in
both males and females. An increased incidence of focal or
multifocal hepatocellular hyperplasia and fatty change (also in
300 ppm males) as well as changes in centrilobular cytomegaly were
noted in both sexes at 1000 ppm. Incidences for liver neoplastic
lesions are given in Table 5.
It can be concluded that a slight increase is found in female
hepatocellular adenoma and male hepatoblastoma. The NOAEL in this
study is 100 ppm (equivalent to 14.3 mg/kg bw) (Filler et al.
1988).
TABLE 5. NON-NEOPLASTIC AND NEOPLASTIC INCIDENCES
LESIONS DOSE LEVEL
0 30 100 300 1000
M F M F M F M F M F
Neo-neoplastic
Liver
Centrilobular cytomegaly 0/49 0/49 0/48 0/50 0/49 2/49 11/49 25/50 17/46 36/50
Testes
Interstitial hyperplasia 2/50 0/49 1/50 7/49 12/49
Ovaries
Stromal hyperplasia 0/49 0/50 0/49 2/49 8/50
Neoplastic
Liver
Hepatocellular adenoma 7/50 1/50 11/50 1/40 12/50 0/48 9/49 3/50 10/49 7/50
Hepatocellular carcinoma 5/50 1/50 6/50 1/49 9/50 2/48 5/49 4/50 10/49 2/50
Hepatoblastoma 1/50 0/50 0/50 0/49 0/50 0/48 2/49 0/50 5/49 0/50
Testes
Interstitial cell tumours 1/50 1/50 0/50 10/49 16749
Rat
Groups of Osborne-Mendel rats (50/sex/group) were fed diets
containing 0, 100, 300, 1000 or 2000 ppm procymidone (purity 99.8%)
for 104 weeks. Satellite groups of rats (50/sex/group) were used in
interim (week 26, 65 and 78) evaluations of clinical pathology,
necropsy and organ weights. No compound-related effects were noted
on clinical signs, food and water consumption, mortality,
opthalmoscopy, hematology, biochemistry and urinalysis. At the end
of the study survival rates for males ranged from 22% to 34% while
for females it was 50% to 70%. Body weight gain was significantly
decreased at 1000 and 2000 ppm. At 300 ppm a decrease was found,
which was significant during a part of the study, and more
pronounced in males. Testosterone analysis showed increased levels
at 2000 ppm. At termination (and in most interim kills), relative
liver weight and relative testes weight were increased at 2000 ppm
in males and females. In females relative ovary weight, relative
kidney weight and relative brain weight were significantly increased
at 2000 ppm at the end of the study. An increased incidence of
hepatic centrilobular cytomegaly, testicular interstitial cell
hyperplasia and ovarian stromal hyperplasia as well as an increased
incidence of interstitial cell tumours were observed in the two
highest dose groups.
The NOAEL in this study is 100 ppm (4.6 mg/kg bw and 6.0 mg/kg
bw for males and females, respectively) (Keller et al. 1986).
Special study on affinity for androgen receptor
The binding ability to androgen receptors in prostate cytosols
from castreated mice and rats was examined in a competitive assay in
which the cytosols were incubated with [3H]dihydrotestosterone
(DHT) alone or together with increasing concentrations of
procymidone and some metabolites. The androgen receptors of rat and
mouse prostate have high specificity and high affinity for DHT while
procymidone possesses a low but distinct affinity. The relative
binding affinity (RBA) was 0.065% in rats and 0.070% in mice
compared to 14.2% and 0.058% in rats for cyproterone acetate and
flutamide (a non-steriodal anti-androgen for medical use),
respectively and 0% for some procymidone - soil or light -
degradation products (Murakami et al. 1988b).
Special study on effect upon testicular function
Mouse
Groups of 30 male ICR mice were fed diets containing 0, 1000,
5000 or 10000 ppm procymidone (purity 99.3%) for 13 weeks.
Observations included body weight, food consumption and organ weight
(seminal vesicles, epididymes, testes and prostate), serum
testosterone and LH (luteinizing hormone) levels, testosterone
levels in testes, LH levels in pituitary, in vitro production of
testosterone in testis stimulated by gonadotropin and testicular
binding of 125I-labelled human chorionic gonadotropin (hCG).
Testosterone and LH levels in serum and/or organs, and the
responsiveness of interstitial cells to hCG in vitro were enhanced
after 2 weeks. These changes (which indicate hypergonadotropism)
returned to control levels during the 3 month treatment period. The
binding affinity of hCG for LH/hCG receptor in mouse testes
decreased significantly (measured by an elevated dissociation
constant (Kd) at 5000 and 10000 ppm) during procymidone treatment.
The number of hCG binding sites in the testes was unchanged
(Murakami et al. 1988a).
Rat
In a study with the same protocol, groups of 12 male Sprague-
Dawley rats were fed diets containing 0, 700, 2000 or 6000 ppm
procymidone (purity 99.3% and 99.5%) Relative testes weight was
significantly increased at 6000 ppm during the 3 month treatment
period. Hormone levels (testosterone and LH) in serum and/or
organs, and the responsiveness of interstitial cells to hCG
in vitro were enhanced after 2 weeks. These effects were more
pronounced than in mice and were maintained over 3 months. The
binding affinity and the number of hCG binding sites in the testis
were unchanged (Murakami et al. 1988a).
Special studies on teratogenicity
Rat
Groups of 25 pregnant albino rats {Charles River (SD) BR} were
given oral daily doses of 0, 30, 100 or 300 mg procymidone (purity
99.6%)/kg bw in corn oil from day 6 through 15 of gestation. Dams
were observed for mortality, clinical signs, body weight and food
consumption. At day 20 of gestation, pups were delivered by cesarean
section. The number of corpora lutea, implantations, resorptions,
live and dead fetuses were recorded. Fetuses were weighed, sexed and
examined for external, visceral and skeletal malformations. There
were no effects observed on the dams and offspring except for a
slightly (not significantly) increased frequency of resorptions at
100 and 300 mg/kg bw. The NOAEL in this study is 300 mg/kg bw
(Pence et al. 1980).
Rabbit
Groups of 18 pregnant NZW rabbits were orally administered 0,
30, 150, 750, or 1000 mg procymidone (purity 99.6%)/kg bw in corn
oil from day 7-19 of gestation. All animals were observed for
clinical signs, mortality, bodyweight and food consumption. All
animals were sacrificed on gestation day 30, and the number of
corpora lutea, implantations, live and death fetuses were recorded.
Fetuses were weighed, sexed, inspected for external abnormalities
and examined for both internal and skeletal malformations. No
adverse effects of treatment were observed except for a slightly
increased number of fetuses with non-ossification of the 5th and 6th
sternebra at 1000 mg/kg bw. The NOAEL in this study was 750 mg/kg
bw (Wickramatne et al. 1988a).
Special studies on mutagenicity
Procymidone was negative in various mutagenicity assays. See
Table 1 for a summary of the studies considered.
Reproduction study
Rat
Groups of 30 male and 30 female rats (ALpk:APfSD) received 0,
50, 250 or 750 ppm procymidone (purity 99.5%) in the diet for 12
weeks before initial mating. Similar treatment was continued
throughout two litters and into a second generation which also
produced two litters maintained for 11 weeks. Observations were made
on general condition and behaviour, food consumption and body
weight. Reproduction parameters such as fertility indices, duration
of gestation, precoital interval, viability indices, litter size,
number and percent of live and dead foetuses, litter and pup weight
were studied. Autopsy and histopathological examinations of
selected organs were performed on all F0, F1 and F2 adults and
offspring. Liver and reproductive organs were weighed.
All high dose parental rats showed reductions in bodyweight
gain (significantly in F0 rats and F1 and F2 females), in food
consumption and in food efficiency. A slight effect on body weight
was observed at 250 ppm in F1 and F2 rats. In F1 parental males
(but not in F0) a significant loss of fertility was observed at
750 ppm. Infertile rats showed an abnormal appearance of external
genitalia (hypospadias). Litter weights (except the F2B litters)
were significantly decreased at 750 ppm. Parental relative liver
weight was significantly increased in F0, F1 and F2 generations
at 750 ppm and at 250 ppm also in F0 and F1 males and in F2
females. Relative testes weight was significantly increased in F0,
F1 and F2 males at 250 ppm as well as at 750 ppm. Penile
abnormalities were observed in F1 and F2 adults at 750 ppm
procymidone together with a decrease in prostate size. The
macroscopic diagnosis of hypospadias was confirmed histologically.
In the pituitary hypertrophy and hyperplasia of basophilic cells
were observed in F1 and F2 males at 750 ppm. No effects were
observed on spermatogenesis. High dose F1 males also showed an
increased incidence of bile duct proliferation in the liver.
Difficulties in sexing pups at birth were caused by a reduction
in ano- genital distance in male offspring at day 1 post partum at
750 ppm procymidone (in all litters). In F1B and F2B pups
relative liver weight was significantly increased at 250 (F2B only)
and 750 ppm and relative testes weight was significantly increased
at 250 and 750 ppm and slightly at 50 ppm. A significant decrease
was noted in relative weight of prostate and epididymes both at 250
(F1B only) and 750 ppm procymidone. Abnormalities of the penis
were also observed in F2A and F2B pups (see Table 2). No penile
abnormalities were detected in F1 and F2 litters killed at day 36
post partum. Glycogen depletion was observed in all livers from
F1B female pups at 750 ppm and in 4/5 male F1B pups. The NOAEL is
50 ppm in the diet, equivalent to 2.5 mg/kg bw (Wickramaratne
et al. 1988b).
Special study on serum hormone levels
Groups of 30 male rats were administered diets containing 0,
700, 2000 or 6000 ppm procymidone (purity 99.1%) for 3 months. Ten
rats/group were sacrificed after 14 days, 1 month and 3 months.
Observations included body weight as well as weights of testes,
epididymes, prostate and seminal vesicles, serum levels of
testosterone, LH (luteinizing hormone) and 17b- estradiol and
histopathology of testes, epididymes and plexus pampiniformis. At
6000 ppm body weight was significantly decreased (after 1 month also
at 2000 ppm) and testes weight was slightly increased. Testosterone
levels were significantly and dose-relatedly increased at all dose
levels and LH-levels were significantly increased at the highest
dose. The effects with procymidone were different from those with a
positive control (CdCl2), which caused decreased weight of testes,
epididymes, prostate and seminal vesicles and a marked depression of
testosterone levels. It is known that this CdC12 damages male
gonadal systems directly. In a second study groups of male rats
received 0, 100, 300, 700 or 2000 ppm procymidone for 6 months. Ten
rats/group were sacrificed after 1, 3 and 6 months. Testosterone
levels were significantly increased at 700 and 2000 ppm. The
no-hormonal-effect level is 300 ppm.
In a third study groups of male rats received 0 or 6000 ppm
procymidone for 1 month and were kept for a recovery period of 6
months to examine the reversibility of the hormonal changes. 10
Rats/group were sacrificed after 2 weeks, 1, 3 and 6 months. Levels
of testosterone and LH were normal after 1 month (Murakami et al.
1986).
Special studies on skin and eye irritation
Moistened technical procymidone (50 mg, purity 98% up) was
applied to the shaven skin of 5 male albino rabbits (Japanese
strain) for 4 hours. Slight congestion, lasting one hour, was
observed at the treated site of 2 animals. No other signs of
irritation were observed during a 7-day observation period (Kadota &
Miyamoto, 1976).
A moistened formulation of procymidone (500 mg, 50% water
dispersible powder) was applied to the intact and abraded skin of 6
male albino rabbits (Japanese strain) under occlusive conditions for
24 hours. Severe erythema (score 4) and slight edema (score 1) was
observed after 24 hours lasting for 72 hours. A 1:100 aqueous
suspension of the formulation did not induce any local reaction
(Matsubara et al. 1979).
Technical procymidone (50 mg, purity 98% up) instilled into one
eye of 5 female and 3 male albino rabbits (Japanese strain) caused
no irritant reactions during a 72-hour observation period with or
without washing, respectively (Kadota & Miyamoto, 1976).
A formulation of procymidone (100 mg, 50% water dispersible
powder) was instilled into one eye of 9 male albino rabbits
(Japanese strain). The eyes of three rabbits were washed 30 seconds
after treatment. The formulation was irritating to the unwashed eyes
and not irritating to the washed eyes. A 1:100 aqueous suspension of
the formulation did not cause any irritant reaction (Matsubara
et al. 1979).
Special studies on skin sensitization
A 1% or 5% solution of procymidone (purity 98%) in corn oil had
no sensitizing potential in male guinea pigs receiving 10
applications every other day followed by a challenge 2 weeks later
(Okuno et al. 1975).
A water dispersible powder of procymidone (containing 53% w/w
procymidone, purity 97.8%) was not sensitizing in a Buehler test
(Hara et al. 1979).
Observations in humans
Clinical examination records (taken two times a year, including
bodyweight, visual and hearing perception, chest X-ray, blood
pressure, urinary examination and diagnosis by questioning) of 20
male workers (being protected but without face guard or mask) who
have been engaged in the manufacturing of procymidone technical
grade and in the packing in drums for 3-4 years, were re-examined,
and the details and history of the operations were reviewed. After
re-examination of 5 clinical records of these 20 workers, no
abnormalities were observed in any examination item (Harada, 1983).
COMMENTS
After oral administration to mice and rats, procymidone was
rapidly excreted, primarily via the urine. The compound was oxidized
at the methyl group and hydrolyzed at the imide and amide linkages.
Neither procymidone nor its metabolites accumulated in the tissues
of mice or rats and repeated administration did not alter the
excretion pattern. After a single oral administration to pregnant
rats, the compound and/or its metabolites transferred to the fetus.
Procymidone has a low acute toxicity in the species examined.
In subchronic toxicity studies in mice, rats and dogs, the main
effects were increased liver weight and hepatocellular hyperplasia.
In the dog, the NOAEL was 100 mg/kg bw/day.
In a long-term feeding study in mice, a slightly increased
incidence in liver tumours was reported. The NOAEL was 100 ppm
(equivalent to 15 mg/kg/day). In a long-term feeding study in rats,
decreased weight gain and pathology of the male reproductive system
were observed at 1000 and 2000 ppm. The weight of the testes was
increased at 2000 ppm and at the two highest dose levels of 1000 and
2000 ppm, increased incidences of testicular interstitial cell
hyperplasia and interstitial cell tumours were observed. In this
study, the NOAEL was 300 ppm, equal to 14 mg/kg bw/day.
In a 2-generation, 2 litters per generation reproduction study
in rats, infertility and abnormalities of the male sexual organs
(hypospadias) were observed in adults and in pups at the highest
dose level of 750 ppm. At day 1 post-partum the male offspring
showed a reduction in ano-genital distance. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
In teratogenicity studies with rats and rabbits no embryotoxic
or teratogenic effects were found. The NOAELs were 30 mg/kg bw/day
and 750 mg/kg bw/day, respectively.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
In order to explain the effects on the testes, a number of
special studies were conducted. Procymidone had a low, but distinct
binding affinity for androgen receptors in rats and mice. High
doses had a clear effect on testicular function and hormone levels,
indicating trophic stimulation (resulting in hormonal imbalance) in
rats and mice. The effect in the mouse returned to normal during
the 3-months treatment period, while it remained in rats, where
interstitial hyperplasia and tumours were observed. In a special
study on the effects of procymidone on hormones in rats,
testosterone levels were increased at all dose levels (700, 2000 and
6000 ppm), while luteinizing hormone levels were increased only at
6000 ppm. In a study with lower dose levels, 300 ppm was the
no-hormonal-effect level. Rats were dosed for 1 month with
60000 ppm procymidone and hormonal recovery was studied. One month
after cessation of dosing, levels of testosterone and luteinizing
hormone had returned to normal.
It was concluded that both the effects on reproduction and the
induction of testicular tumours in the long-term rat study can be
explained by the effects of procymidone on the endocrine system.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 100 ppm in the diet, equal to 15 mg/kg bw/day
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg bw/day
Dog: 100 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arai, M., Hasegawa, R. & Ito, N. (1980a) Three-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report from Department of pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Arai, M., Hasegawa, R. & Ito, N. (1980b) Six-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report No. BT-00-0057 from Department of Pathology, Nagoya City
University Medical School, Nagoya, Japan. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Carlborg, F.W. (1987) Tumors observed in project 22254M.
Unpublished Report BT-81-0129 Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Filler, R., Maloney, D., Alsaker, R., Clinton, J., Parker, G.,
Thakur, A., Hohing, L. & Vanatta, P. (1988) Oral chronic toxicity
and oncogenicity study in mice/Sumilex. Unpublished Report No.
BT-81-0127 from Hazleton Laboratories America Inc. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1979) Skin sensitization test
of S7131 50% water dispersible powder in male quinea pigs.
Unpublished Report No. BT-00-0040 September 12, 1980 from Sumitomo
Research Department, Pesticides Division. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, M., Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Cytogenetic
test of procymidone in mouse bone marrow. Unpublished Report No.
BT-00-0043 from Sumitomo, Laboratory of Biochemistry and Toxicology,
Research Department. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Harada, T. (1983) A review of medical examinations of factory
workers possibly exposed to procymidone technical materials.
Unpublished Report dated 23-3-1983 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kadota, T. & Miyamoto, J. (1976) Eye and skin irritation study with
S-7131 technical material in albino rabbits. Unpublished report No.
BT-60-0004 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kato, T., Fujita, T. & Fukada, T. (1976) Toxicity of S-7131
Technical material to rats in prolonged dietary administration over
9 months. Unpublished Report BT-60-003 from Sumitomo Chemical Co.,
Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Keller, J.G., Fitzgerald, J., Sibinovic, F., Loeb, W.F., Cardy,
R.H., Clinton, J. & Wolfe, G.W. (1986) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumislex, Final Report
No. BT-61-0112. LBI project No. 22048-03/13 dated June, 1986, from
Litton Bionetics, Inc. Rockville, Maryland. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kinsey, J.G., Fitzgerald, J. Sibinovic, F., Loeb, W.F., Cardy, R.H.,
Clinton, J. & Wolfe, G.W. (1985) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumisclex. Unpublished Report
No. CTL/P/1146 dated 15-08-1985 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976a) Acute toxicity studies
with S- 7131 technical material in mice and rats. Unpublished Report
no. BT-60-0002 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976b) Acute oral and dermal
toxicities of Sumilex 50% wettable powder in mice and rats.
Unpublished Report No. BT-60-0006 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kodata, T. & Miyamoto, J. (1976c) Acute inhalation
toxicity study with S-7131 (Sumilex) 50% wettable powder in rats.
Unpublished Report No. BT-60-0007 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Nakamura, M., Kadota, T. & Miyamoto, J. (1980) Acute
oral and subcutaneous toxicity studies of three metabolites of
procymidone. Unpublished Report No. BT-00-0037 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kawaguchi., Watanabe, T., Suzuki, T., Kato, T. &
Miyamoto, J. (1986) Acute inhalation toxicity of Sumilex in rats.
Unpublished Report No. BT-60-0116 dated 25-6-1986 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
Primary eye and skin irritation tests of S-7131 50% water
dispersible powder in rabbits. Unpublished Report No. BT-90-0032
from Sumitomo Chemical Co., Ltd. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Mikami, N., Satogami, H. & Miyamoto, J. (1979) Metabolism of
procymidone in rats. J. Pest. Science 4, 165. Submitted as
published Report No. BM-90-0005 to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Moriya, M. & Kato, K. (1977) Mutagenicity of S-7131 in bacterial
test systems. Unpublished Report No. BT-71-0021 dated 25-10-1977
from Institute of Environmental Toxicology. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Koyama, Y., Okuno, Y., Hosokawa, S. & Miyamoto, J.
(1986) Study on serum hormone levels in rats treated with S-7131.
Revised Final Report No. BT-60-0113 dated 27-7-1986 from Takarazuka
Research Center. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Murakami, M., Ineyama, M., Yamada, T., Ito, M., Koyama, Y., Kimura,
J., Hosokawa, S., Yoshitake, A. & Yamada, H. (1988a). Effect on
testicular function of male rats and mice in subacute administration
of procymidone. Unpublished Report No. BT-80-0130 dated 23-6-1988
from Takarazuka Research Center. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Ineyama, M., Hosokawa, S., Yoshitake, W., & Yamada, H.
(1998b). The affinity of procymidone to androgen receptor in rats
and mice. Unpublished Report No. Bt-80-0131 dated 23-6-1988 from
Takarazuka Research Center. Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Nakashima, N., Ebino, K., Tuda, S., Harada, T. & Kitazawa, T. (1984)
A 6- month subchronic toxicity study of Sumilex in beagles.
Unpublished Report No. BT-41-0091 dated January 1984, from the
Institute of Environmental Toxicology Kodaira, Tokyo 187. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Okuno, Y., Kadota, K. & Miyamoto, J. (1975) Skin sensitization
study with S- 7131 in guinea pigs. Unpublished Report No. BT-60-0005
dated 14-4-1975 from Research Department Pesticides Division.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Pence, O.H., Hoberman, A.M., Andrews, J.P., Durloo, R.S. & Mossburg,
P.A. (1980) Teratology study in rats S-7131 (technical), final
report. Unpublished Report No. BT-01-0048 dated 19-12-1980 from
Hazleton Laboratories America, Inc. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Principe, P. Monaco, M. & Nunziata, A. (1980) Report on
mutagenicity experiment on the substance sumisclex (procymidone).
Unpublished Report No. BT-01-0050 from Centro Ricerca Farmaceutica
s.p.a. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka,
Japan.
Segawa, T. (1977) Acute oral, subcutaneous, intraperitoneal and
dermal toxicities of S-7131 in rats and mice. Unpublished Report
No. BT-71- 0053 dated 7-12-1977 from Hiroshima University School of
Medicine. Submitted to WHO by Sumitomo Chemical Co., Osaka, Japan.
Segawa, T. (1979) Acute intraperitoneal toxicity of S-7131 50%
water- dispersible powder in mice. Unpublished Report No. BT-91-0030
dated 24-3-1979. Hiroshima University School of Medicine. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Shiba, K., Iba, K. Kimura, K. Yoshitake, A. & Yamada, H. (1988)
Comparative metabolism of procymidone in rats and mice. Unpublished
Report No. BM-80-0019 from Takarazuka Research Center. Submitted to
WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1976) Studies on mutagenicity of S-7131
with bacterial systems. Unpublished Report No. BT-60-0011 submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Effect of procymidone
on sister chromatid exchanges (SCE) in cultured mouse embryo cells.
Unpublished Report No. BT-00-0042 dated September 1980, Laboratory
of Biochemistry and Toxicology Research Department. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Wickramaratne, G.A. de S., Kinsey, D.L., Banham, P.B., Greenwood,
M.R. & Pigott, G.H. (1988a) Procymidone: teratogenicity study in
the rabbit. Unpublished Report No. CTL/P/1812 dated 1-6-1988 from
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Wickramaratne, G.A. de S., Milburn, G.M. Banham, P.B., Greenwood,
M.R., Moreland, S. & Pigott, G.H. (1988b) Procymidone:
multigeneration reproduction study in the rat. Unpublished Report
No. CTL/P/1954 dated 1-7-1988 from Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, Enmgland. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON PROCYMIDONE
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro
Ames test (both with S. typhimurium 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
and without metabolic TA98, TA100, ug/plate in (1)
activation TA1535, TA1538 DMSO
Ames test (both with S. typhimurium 10, 50, 100, 500 96.6 Negative Moriya et al. 1977
and without metabolic TA98, TA100, TA1535 ug/plate in (1)
activation TA1537, TA1538 DMSO
E. coli WP2 hcr
V79/HGPRT mutation Chinese hamster lung 7x10-4, 1.5x10-3 approx95 Negative Principe et al. 1980
assay (both with and cells (V79) 3x10-3, 6x10-3M (1)
without metabolic
activation)
SCE assay (both with Mouse embryo cells 10-6, 10-5, 96.9 Negative Suzuki et al. 1080
and without metabolic 10-4M in DMSO (1)
activation)
Rec-assay B. subtilis M45 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
rec H17 ug/disk in DMSO (1)
Rec-assay B. subtilis M45 20, 100, 200, 500 96.6 Negative Moriya et al. 1977
rec H17 1000, 2000 ug/disk (1)
in DMSO
TABLE 1 (contd.)
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro (contd)
Unscheduled DNA Human epithelial 6x10-6, 1x10-5 approx95 Negative Principe et al. 1980
synthesis test (both cells 6x10-4, 6x10-3M (1)
with and without in acetone
metabolic activation)
in vivo
Chromosomal aberration Male mice (ICR strain) 400, 800, 1600 96.9 Negative Hara et al. 1980
test mg/kg (i.p.) in (1)
corn oil
Host-mediated assay S. typhimurium 1000, 2000 mg/kg 96.3 Negative Suzuki & Miyamoto, 1976
G46 Male mice in DMSO (1)
(ICR strain)
Host-mediated assay S. typhimurium 200, 500 mg/kg x 2, 96.3 Negative Moriya et al. 1977
G46 Male mice in 5% Arabic gum (1)
(ICR strain)
(1) Positive control compounds gave positive responses.
Toxicological studies
Acute toxicity
The LD50 and LC50 values for the 50% dispersible powder
formulation for procymidone and some metabolites in various species
are given in Tables 2, 3 and 4, respectively.
TABLE 2. ACUTE TOXICITY OF 50% WETTABLE POWDER OF PROCYMIDONE
SPECIES SEX ROUTE LD50 REFERENCE
(mg/kg bw)
Mice M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-h exp.)
M i.p. 1750 Segawa, 1979
F i.p. 1650
Rat M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-hr exp.)
M&F inhal. >109 Kohda et al. 1976c
(4-hr exp.)
TABLE 3. ACUTE TOXICITY OF PROCYMIDONE
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Mouse M oral 7800 96.3 Kohda et al.
F 9100 96.3 1976a
M&F oral >5000 96.5 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
M i.p. 1560 96.3 Kohda et al.
F 1900 96.3 1976a
M i.p. 2030 96.5 Segawa, 1977
F 2050 96.5
Mouse M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
Rat M oral 6800 96.3 Kohda et al.
M 7700 96.3 1976a
M&F oral >5000 96.3 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
Rat M i.p. 850 96.3 Kohda et al.
F 730 96.3 1976a
M i.p. 1440 96.5 Segawa, 1977
F 1450 96.5
TABLE 3 (contd).
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Rat M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
M&F inhal. >1500 b) 99.5 Kohda et al.
1986
In all cases of suspensions of procymidone in corn oil were administered:
a) duration of exposure not indicated
b) 4-hr exposure to dust.
TABLE 4. ACUTE TOXICITY OF METABOLITES OF PROCYMIDONE IN MICE
TEST SEX ROUTE LD50 REFERENCE
COMPOUND (mg/kg bw)
3,5-DCAa M oral 900 Kohda et al. 1980
F oral 820
M s.c. 1300 Kohda et al. 1980
F s.c. 1250
DMPAb M oral 4200 Kohda et al. 1980
F oral 4650
DMPAb M s.c. 2100 Kohda et al. 1980
F s.c. 2650
SF-8748a M oral 1410 Kohda et al. 1980
F oral 1480
a) 3,5-DCA (P8) and SF-8748 (P1) were suspended or dissolved in
corn oil
b) DMPA (1,2-dimethylcyclopropane-dicarboxylic acid, plant and soil
metabolite) was suspended in 10% Tween 8O.
Short-term studies
Mice
Groups of male and female mice (ICR strain, 15/sex/group) were
fed diets containing 0, 50, 150 or 500 ppm procymidone (purity
96.9%) for three months. Mice were observed twice daily for changes
in appearance or behaviour, and body weight was recorded weekly.
Food and water consumption were measured once a week for 3
consecutive days. Hematological and biochemical studies as well as
opthalmological examinations were conducted after 3 months of
treatment. At the end of the treatment period surviving rats were
sacrificed and examined for gross pathology, organs were weighed and
histopathological examinations were carried out. There were no
compound related deaths, body weight changes, effects on food and
water consumption or clinical chemistry nor changes in
ophthalmological findings. Significantly decreased hematocrit and
erythrocyte values were observed in females at 500 ppm. Relative
kidney weight was significantly decreased in females at 150 and
500 ppm and relative adrenal weight was decreased (significantly but
not dose-relatedly) in all male dose groups. An increased relative
liver weight was observed in high dose males and females which was
not statistically significant. Hypertrophy of hepatocytes of the
liver was observed in males at 150 and 500 ppm and in females at
500 ppm. High dose females also showed an increased incidence of
dilated fundal-glands of the stomach. The NOAEL in this study is
50 ppm, equivalent to 7 mg/kg bw/day (Arai, 1980a).
Groups of ICR mice (20/group/sex) were fed diets containing 0,
50, 150 or 500 ppm procymidone (purity: 96.9%) in corn oil for 6
months. Observations included clinical examinations, mortality,
body weight, water and food consumption, ophthalmological,
hematological and biochemical examinations, macroscopy, organ weight
and histopathology. No dose-related deaths occurred. Body weight,
food and water consumption and ophthalmoscopy were not affected.
Leucocyte count was significantly decreased in males at 150 and
500 ppm and MCV values were significantly increased in females at
the same dose groups. Creatinine values were significantly
increased in males at 500 ppm. A tendency to an increased relative
liver weight was noted in females at 500 ppm. At histopathology an
increased incidence of atrophy of the testes was observed (2/19
(10.5%), 5/20 (25%), 6/20 (30%) and 10/20 (50%) in the control,
50 ppm, 150 ppm and 500 ppm group, respectively). This was
significant in the 500 ppm group only. The NOAEL in this study was
150 ppm, equivalent to 20 mg/kg bw/day (Arai, 1980b).
Groups of 20 male mice (Alpk/AP white) were fed diets
containing 0, 10, 30, 100 or 300 ppm procymidone (purity 99.8%) for
6 months. Observations included clinical signs, body weight, food
consumption, organ weight, macroscopy and histopathology of the
testes and the epididymes. The only findings observed were a not
dose-related increase in food consumption (means of 4 observations/
group) at 30, 100 and 300 ppm and a not dose related increase in
relative testes weight in all dose groups (significantly at 10 and
100 ppm), not associated with any histopathological change (Kinsey
et al. 1985).
Rat
Groups of rats (Sprague-Dawley, 12/sex/group) were fed diets
containing 0, 150, 500 or 1500 ppm procymidone (purity, 98.7%) for 6
months. Additional groups (15/sex/group) were fed diets containing
0 or 1500 ppm for 9 months, or 0 or 1500 ppm for 6 months and then
placed on a control diet for another 3 months. No compound-related
effects were observed on mortality, clinical signs, food and water
consumption and urinalysis. Body weight was decreased in females
(significantly at 1500 ppm) at all dose levels after 6 months and
male body weight was decreased at the highest dose after the same
period. After 9 months of feeding both male and female bodyweights
were significantly decreased at 1500 ppm. Significantly decreased
PCV and Hb values were observed in both sexes at 1500 ppm only after
6 months; ALAT levels were increased at 500 and 1500 ppm in both
sexes after 6 months. Relative liver weight was significantly
increased in females at 500 ppm (after 6 months) and in both sexes
at 1500 ppm after 6 and 9 months. Relative spleen weight was
significantly increased in females at 500 and 1500 ppm only after 6
months. There were also changes in several other organ weights,
possibly as a result of the change in body weight. An increased
incidence of swelling of the liver cell was observed in males at
1500 ppm after 6 (4/15) as well as after 9 months (2/15). The only
findings which were still evident after the 3 month recovery period
were a slightly decreased bodyweight in both sexes and an increased
relative liver weight in females at 1500 ppm. The NOAEL in this
study is 150 ppm, equivalent to 7.5 mg/kg bw/day (Kato et al.
1976).
Dog
Beagle dogs (6/sex/group) received by capsule at dose levels
0,20, 100 or 500 mg/kg bw/day procymidone (purity >95%) for 6
months. Mortality, body weight, food and water consumption,
ophthalmoscopy, haematology, urinalysis, organ weight, macroscopy
and histopathology were not affected by treatment. Dogs at
500 mg/kg bw/day had a higher incidence of emesis and diarrhea
(females only). Alkaline phosphatase activity was increased in
males and females at 500 mg/kg bw/day (during the second half of the
treatment period). At the same dose level BUN, glucose and calcium
(occasionally also at 100 mg/kg bw/day) levels were significantly
increased in males. Relative heart weight was significantly
decreased in high dose females. There was a tendency to an
increased liver weight in males and females and a decreased testes
weight in males at 500 mg/kg bw/day. The NOAEL in this study was
100 mg/kg bw/day (Nakashima et al. 1984).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
0, 30, 100, 300 or 1000 ppm procymidone (purity 99.8%) for 104
weeks. Satellite groups consisting of 40 mice/sex/group were
sacrificed at 6-month intervals (week 26, 52 and 78) for clinical
and pathology determinations. An additional 10 mice/sex were
assigned to the main control group for clinical pathology
determinations prior to the start of dosing. Observations included
mortality, clinical signs, body weight, food and water consumption,
ophthalmoscopy, hematology, clinical chemistry, urinalysis,
macroscopy, organ weights and histopathology. Survival rates at
termination varied from 64% to 88%. No treatment-related effects
were observed on mortality, body weight, food and water consumption,
ophthalmoscopy, hematology and urinalysis. A tendency to an
increased ALAT level was observed in both sexes at 1000 ppm.
Relative liver weight was significantly increased in males and
females at 1000 ppm and sometimes at 300 ppm. No effect was
observed on testes weight. An increased incidence of masses, pale
areas and raised areas in the livers was observed at 1000 ppm in
both males and females. An increased incidence of focal or
multifocal hepatocellular hyperplasia and fatty change (also in
300 ppm males) as well as changes in centrilobular cytomegaly were
noted in both sexes at 1000 ppm. Incidences for liver neoplastic
lesions are given in Table 5.
It can be concluded that a slight increase is found in female
hepatocellular adenoma and male hepatoblastoma. The NOAEL in this
study is 100 ppm (equivalent to 14.3 mg/kg bw) (Filler et al.
1988).
TABLE 5. NON-NEOPLASTIC AND NEOPLASTIC INCIDENCES
LESIONS DOSE LEVEL
0 30 100 300 1000
M F M F M F M F M F
Neo-neoplastic
Liver
Centrilobular cytomegaly 0/49 0/49 0/48 0/50 0/49 2/49 11/49 25/50 17/46 36/50
Testes
Interstitial hyperplasia 2/50 0/49 1/50 7/49 12/49
Ovaries
Stromal hyperplasia 0/49 0/50 0/49 2/49 8/50
Neoplastic
Liver
Hepatocellular adenoma 7/50 1/50 11/50 1/40 12/50 0/48 9/49 3/50 10/49 7/50
Hepatocellular carcinoma 5/50 1/50 6/50 1/49 9/50 2/48 5/49 4/50 10/49 2/50
Hepatoblastoma 1/50 0/50 0/50 0/49 0/50 0/48 2/49 0/50 5/49 0/50
Testes
Interstitial cell tumours 1/50 1/50 0/50 10/49 16749
Rat
Groups of Osborne-Mendel rats (50/sex/group) were fed diets
containing 0, 100, 300, 1000 or 2000 ppm procymidone (purity 99.8%)
for 104 weeks. Satellite groups of rats (50/sex/group) were used in
interim (week 26, 65 and 78) evaluations of clinical pathology,
necropsy and organ weights. No compound-related effects were noted
on clinical signs, food and water consumption, mortality,
opthalmoscopy, hematology, biochemistry and urinalysis. At the end
of the study survival rates for males ranged from 22% to 34% while
for females it was 50% to 70%. Body weight gain was significantly
decreased at 1000 and 2000 ppm. At 300 ppm a decrease was found,
which was significant during a part of the study, and more
pronounced in males. Testosterone analysis showed increased levels
at 2000 ppm. At termination (and in most interim kills), relative
liver weight and relative testes weight were increased at 2000 ppm
in males and females. In females relative ovary weight, relative
kidney weight and relative brain weight were significantly increased
at 2000 ppm at the end of the study. An increased incidence of
hepatic centrilobular cytomegaly, testicular interstitial cell
hyperplasia and ovarian stromal hyperplasia as well as an increased
incidence of interstitial cell tumours were observed in the two
highest dose groups.
The NOAEL in this study is 100 ppm (4.6 mg/kg bw and 6.0 mg/kg
bw for males and females, respectively) (Keller et al. 1986).
Special study on affinity for androgen receptor
The binding ability to androgen receptors in prostate cytosols
from castreated mice and rats was examined in a competitive assay in
which the cytosols were incubated with [3H]dihydrotestosterone
(DHT) alone or together with increasing concentrations of
procymidone and some metabolites. The androgen receptors of rat and
mouse prostate have high specificity and high affinity for DHT while
procymidone possesses a low but distinct affinity. The relative
binding affinity (RBA) was 0.065% in rats and 0.070% in mice
compared to 14.2% and 0.058% in rats for cyproterone acetate and
flutamide (a non-steriodal anti-androgen for medical use),
respectively and 0% for some procymidone - soil or light -
degradation products (Murakami et al. 1988b).
Special study on effect upon testicular function
Mouse
Groups of 30 male ICR mice were fed diets containing 0, 1000,
5000 or 10000 ppm procymidone (purity 99.3%) for 13 weeks.
Observations included body weight, food consumption and organ weight
(seminal vesicles, epididymes, testes and prostate), serum
testosterone and LH (luteinizing hormone) levels, testosterone
levels in testes, LH levels in pituitary, in vitro production of
testosterone in testis stimulated by gonadotropin and testicular
binding of 125I-labelled human chorionic gonadotropin (hCG).
Testosterone and LH levels in serum and/or organs, and the
responsiveness of interstitial cells to hCG in vitro were enhanced
after 2 weeks. These changes (which indicate hypergonadotropism)
returned to control levels during the 3 month treatment period. The
binding affinity of hCG for LH/hCG receptor in mouse testes
decreased significantly (measured by an elevated dissociation
constant (Kd) at 5000 and 10000 ppm) during procymidone treatment.
The number of hCG binding sites in the testes was unchanged
(Murakami et al. 1988a).
Rat
In a study with the same protocol, groups of 12 male Sprague-
Dawley rats were fed diets containing 0, 700, 2000 or 6000 ppm
procymidone (purity 99.3% and 99.5%) Relative testes weight was
significantly increased at 6000 ppm during the 3 month treatment
period. Hormone levels (testosterone and LH) in serum and/or
organs, and the responsiveness of interstitial cells to hCG
in vitro were enhanced after 2 weeks. These effects were more
pronounced than in mice and were maintained over 3 months. The
binding affinity and the number of hCG binding sites in the testis
were unchanged (Murakami et al. 1988a).
Special studies on teratogenicity
Rat
Groups of 25 pregnant albino rats {Charles River (SD) BR} were
given oral daily doses of 0, 30, 100 or 300 mg procymidone (purity
99.6%)/kg bw in corn oil from day 6 through 15 of gestation. Dams
were observed for mortality, clinical signs, body weight and food
consumption. At day 20 of gestation, pups were delivered by cesarean
section. The number of corpora lutea, implantations, resorptions,
live and dead fetuses were recorded. Fetuses were weighed, sexed and
examined for external, visceral and skeletal malformations. There
were no effects observed on the dams and offspring except for a
slightly (not significantly) increased frequency of resorptions at
100 and 300 mg/kg bw. The NOAEL in this study is 300 mg/kg bw
(Pence et al. 1980).
Rabbit
Groups of 18 pregnant NZW rabbits were orally administered 0,
30, 150, 750, or 1000 mg procymidone (purity 99.6%)/kg bw in corn
oil from day 7-19 of gestation. All animals were observed for
clinical signs, mortality, bodyweight and food consumption. All
animals were sacrificed on gestation day 30, and the number of
corpora lutea, implantations, live and death fetuses were recorded.
Fetuses were weighed, sexed, inspected for external abnormalities
and examined for both internal and skeletal malformations. No
adverse effects of treatment were observed except for a slightly
increased number of fetuses with non-ossification of the 5th and 6th
sternebra at 1000 mg/kg bw. The NOAEL in this study was 750 mg/kg
bw (Wickramatne et al. 1988a).
Special studies on mutagenicity
Procymidone was negative in various mutagenicity assays. See
Table 1 for a summary of the studies considered.
Reproduction study
Rat
Groups of 30 male and 30 female rats (ALpk:APfSD) received 0,
50, 250 or 750 ppm procymidone (purity 99.5%) in the diet for 12
weeks before initial mating. Similar treatment was continued
throughout two litters and into a second generation which also
produced two litters maintained for 11 weeks. Observations were made
on general condition and behaviour, food consumption and body
weight. Reproduction parameters such as fertility indices, duration
of gestation, precoital interval, viability indices, litter size,
number and percent of live and dead foetuses, litter and pup weight
were studied. Autopsy and histopathological examinations of
selected organs were performed on all F0, F1 and F2 adults and
offspring. Liver and reproductive organs were weighed.
All high dose parental rats showed reductions in bodyweight
gain (significantly in F0 rats and F1 and F2 females), in food
consumption and in food efficiency. A slight effect on body weight
was observed at 250 ppm in F1 and F2 rats. In F1 parental males
(but not in F0) a significant loss of fertility was observed at
750 ppm. Infertile rats showed an abnormal appearance of external
genitalia (hypospadias). Litter weights (except the F2B litters)
were significantly decreased at 750 ppm. Parental relative liver
weight was significantly increased in F0, F1 and F2 generations
at 750 ppm and at 250 ppm also in F0 and F1 males and in F2
females. Relative testes weight was significantly increased in F0,
F1 and F2 males at 250 ppm as well as at 750 ppm. Penile
abnormalities were observed in F1 and F2 adults at 750 ppm
procymidone together with a decrease in prostate size. The
macroscopic diagnosis of hypospadias was confirmed histologically.
In the pituitary hypertrophy and hyperplasia of basophilic cells
were observed in F1 and F2 males at 750 ppm. No effects were
observed on spermatogenesis. High dose F1 males also showed an
increased incidence of bile duct proliferation in the liver.
Difficulties in sexing pups at birth were caused by a reduction
in ano- genital distance in male offspring at day 1 post partum at
750 ppm procymidone (in all litters). In F1B and F2B pups
relative liver weight was significantly increased at 250 (F2B only)
and 750 ppm and relative testes weight was significantly increased
at 250 and 750 ppm and slightly at 50 ppm. A significant decrease
was noted in relative weight of prostate and epididymes both at 250
(F1B only) and 750 ppm procymidone. Abnormalities of the penis
were also observed in F2A and F2B pups (see Table 2). No penile
abnormalities were detected in F1 and F2 litters killed at day 36
post partum. Glycogen depletion was observed in all livers from
F1B female pups at 750 ppm and in 4/5 male F1B pups. The NOAEL is
50 ppm in the diet, equivalent to 2.5 mg/kg bw (Wickramaratne
et al. 1988b).
Special study on serum hormone levels
Groups of 30 male rats were administered diets containing 0,
700, 2000 or 6000 ppm procymidone (purity 99.1%) for 3 months. Ten
rats/group were sacrificed after 14 days, 1 month and 3 months.
Observations included body weight as well as weights of testes,
epididymes, prostate and seminal vesicles, serum levels of
testosterone, LH (luteinizing hormone) and 17b- estradiol and
histopathology of testes, epididymes and plexus pampiniformis. At
6000 ppm body weight was significantly decreased (after 1 month also
at 2000 ppm) and testes weight was slightly increased. Testosterone
levels were significantly and dose-relatedly increased at all dose
levels and LH-levels were significantly increased at the highest
dose. The effects with procymidone were different from those with a
positive control (CdCl2), which caused decreased weight of testes,
epididymes, prostate and seminal vesicles and a marked depression of
testosterone levels. It is known that this CdC12 damages male
gonadal systems directly. In a second study groups of male rats
received 0, 100, 300, 700 or 2000 ppm procymidone for 6 months. Ten
rats/group were sacrificed after 1, 3 and 6 months. Testosterone
levels were significantly increased at 700 and 2000 ppm. The
no-hormonal-effect level is 300 ppm.
In a third study groups of male rats received 0 or 6000 ppm
procymidone for 1 month and were kept for a recovery period of 6
months to examine the reversibility of the hormonal changes. 10
Rats/group were sacrificed after 2 weeks, 1, 3 and 6 months. Levels
of testosterone and LH were normal after 1 month (Murakami et al.
1986).
Special studies on skin and eye irritation
Moistened technical procymidone (50 mg, purity 98% up) was
applied to the shaven skin of 5 male albino rabbits (Japanese
strain) for 4 hours. Slight congestion, lasting one hour, was
observed at the treated site of 2 animals. No other signs of
irritation were observed during a 7-day observation period (Kadota &
Miyamoto, 1976).
A moistened formulation of procymidone (500 mg, 50% water
dispersible powder) was applied to the intact and abraded skin of 6
male albino rabbits (Japanese strain) under occlusive conditions for
24 hours. Severe erythema (score 4) and slight edema (score 1) was
observed after 24 hours lasting for 72 hours. A 1:100 aqueous
suspension of the formulation did not induce any local reaction
(Matsubara et al. 1979).
Technical procymidone (50 mg, purity 98% up) instilled into one
eye of 5 female and 3 male albino rabbits (Japanese strain) caused
no irritant reactions during a 72-hour observation period with or
without washing, respectively (Kadota & Miyamoto, 1976).
A formulation of procymidone (100 mg, 50% water dispersible
powder) was instilled into one eye of 9 male albino rabbits
(Japanese strain). The eyes of three rabbits were washed 30 seconds
after treatment. The formulation was irritating to the unwashed eyes
and not irritating to the washed eyes. A 1:100 aqueous suspension of
the formulation did not cause any irritant reaction (Matsubara
et al. 1979).
Special studies on skin sensitization
A 1% or 5% solution of procymidone (purity 98%) in corn oil had
no sensitizing potential in male guinea pigs receiving 10
applications every other day followed by a challenge 2 weeks later
(Okuno et al. 1975).
A water dispersible powder of procymidone (containing 53% w/w
procymidone, purity 97.8%) was not sensitizing in a Buehler test
(Hara et al. 1979).
Observations in humans
Clinical examination records (taken two times a year, including
bodyweight, visual and hearing perception, chest X-ray, blood
pressure, urinary examination and diagnosis by questioning) of 20
male workers (being protected but without face guard or mask) who
have been engaged in the manufacturing of procymidone technical
grade and in the packing in drums for 3-4 years, were re-examined,
and the details and history of the operations were reviewed. After
re-examination of 5 clinical records of these 20 workers, no
abnormalities were observed in any examination item (Harada, 1983).
COMMENTS
After oral administration to mice and rats, procymidone was
rapidly excreted, primarily via the urine. The compound was oxidized
at the methyl group and hydrolyzed at the imide and amide linkages.
Neither procymidone nor its metabolites accumulated in the tissues
of mice or rats and repeated administration did not alter the
excretion pattern. After a single oral administration to pregnant
rats, the compound and/or its metabolites transferred to the fetus.
Procymidone has a low acute toxicity in the species examined.
In subchronic toxicity studies in mice, rats and dogs, the main
effects were increased liver weight and hepatocellular hyperplasia.
In the dog, the NOAEL was 100 mg/kg bw/day.
In a long-term feeding study in mice, a slightly increased
incidence in liver tumours was reported. The NOAEL was 100 ppm
(equivalent to 15 mg/kg/day). In a long-term feeding study in rats,
decreased weight gain and pathology of the male reproductive system
were observed at 1000 and 2000 ppm. The weight of the testes was
increased at 2000 ppm and at the two highest dose levels of 1000 and
2000 ppm, increased incidences of testicular interstitial cell
hyperplasia and interstitial cell tumours were observed. In this
study, the NOAEL was 300 ppm, equal to 14 mg/kg bw/day.
In a 2-generation, 2 litters per generation reproduction study
in rats, infertility and abnormalities of the male sexual organs
(hypospadias) were observed in adults and in pups at the highest
dose level of 750 ppm. At day 1 post-partum the male offspring
showed a reduction in ano-genital distance. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
In teratogenicity studies with rats and rabbits no embryotoxic
or teratogenic effects were found. The NOAELs were 30 mg/kg bw/day
and 750 mg/kg bw/day, respectively.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
In order to explain the effects on the testes, a number of
special studies were conducted. Procymidone had a low, but distinct
binding affinity for androgen receptors in rats and mice. High
doses had a clear effect on testicular function and hormone levels,
indicating trophic stimulation (resulting in hormonal imbalance) in
rats and mice. The effect in the mouse returned to normal during
the 3-months treatment period, while it remained in rats, where
interstitial hyperplasia and tumours were observed. In a special
study on the effects of procymidone on hormones in rats,
testosterone levels were increased at all dose levels (700, 2000 and
6000 ppm), while luteinizing hormone levels were increased only at
6000 ppm. In a study with lower dose levels, 300 ppm was the
no-hormonal-effect level. Rats were dosed for 1 month with
60000 ppm procymidone and hormonal recovery was studied. One month
after cessation of dosing, levels of testosterone and luteinizing
hormone had returned to normal.
It was concluded that both the effects on reproduction and the
induction of testicular tumours in the long-term rat study can be
explained by the effects of procymidone on the endocrine system.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 100 ppm in the diet, equal to 15 mg/kg bw/day
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg bw/day
Dog: 100 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arai, M., Hasegawa, R. & Ito, N. (1980a) Three-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report from Department of pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Arai, M., Hasegawa, R. & Ito, N. (1980b) Six-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report No. BT-00-0057 from Department of Pathology, Nagoya City
University Medical School, Nagoya, Japan. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Carlborg, F.W. (1987) Tumors observed in project 22254M.
Unpublished Report BT-81-0129 Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Filler, R., Maloney, D., Alsaker, R., Clinton, J., Parker, G.,
Thakur, A., Hohing, L. & Vanatta, P. (1988) Oral chronic toxicity
and oncogenicity study in mice/Sumilex. Unpublished Report No.
BT-81-0127 from Hazleton Laboratories America Inc. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1979) Skin sensitization test
of S7131 50% water dispersible powder in male quinea pigs.
Unpublished Report No. BT-00-0040 September 12, 1980 from Sumitomo
Research Department, Pesticides Division. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, M., Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Cytogenetic
test of procymidone in mouse bone marrow. Unpublished Report No.
BT-00-0043 from Sumitomo, Laboratory of Biochemistry and Toxicology,
Research Department. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Harada, T. (1983) A review of medical examinations of factory
workers possibly exposed to procymidone technical materials.
Unpublished Report dated 23-3-1983 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kadota, T. & Miyamoto, J. (1976) Eye and skin irritation study with
S-7131 technical material in albino rabbits. Unpublished report No.
BT-60-0004 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kato, T., Fujita, T. & Fukada, T. (1976) Toxicity of S-7131
Technical material to rats in prolonged dietary administration over
9 months. Unpublished Report BT-60-003 from Sumitomo Chemical Co.,
Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Keller, J.G., Fitzgerald, J., Sibinovic, F., Loeb, W.F., Cardy,
R.H., Clinton, J. & Wolfe, G.W. (1986) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumislex, Final Report
No. BT-61-0112. LBI project No. 22048-03/13 dated June, 1986, from
Litton Bionetics, Inc. Rockville, Maryland. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kinsey, J.G., Fitzgerald, J. Sibinovic, F., Loeb, W.F., Cardy, R.H.,
Clinton, J. & Wolfe, G.W. (1985) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumisclex. Unpublished Report
No. CTL/P/1146 dated 15-08-1985 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976a) Acute toxicity studies
with S- 7131 technical material in mice and rats. Unpublished Report
no. BT-60-0002 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976b) Acute oral and dermal
toxicities of Sumilex 50% wettable powder in mice and rats.
Unpublished Report No. BT-60-0006 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kodata, T. & Miyamoto, J. (1976c) Acute inhalation
toxicity study with S-7131 (Sumilex) 50% wettable powder in rats.
Unpublished Report No. BT-60-0007 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Nakamura, M., Kadota, T. & Miyamoto, J. (1980) Acute
oral and subcutaneous toxicity studies of three metabolites of
procymidone. Unpublished Report No. BT-00-0037 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kawaguchi., Watanabe, T., Suzuki, T., Kato, T. &
Miyamoto, J. (1986) Acute inhalation toxicity of Sumilex in rats.
Unpublished Report No. BT-60-0116 dated 25-6-1986 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
Primary eye and skin irritation tests of S-7131 50% water
dispersible powder in rabbits. Unpublished Report No. BT-90-0032
from Sumitomo Chemical Co., Ltd. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Mikami, N., Satogami, H. & Miyamoto, J. (1979) Metabolism of
procymidone in rats. J. Pest. Science 4, 165. Submitted as
published Report No. BM-90-0005 to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Moriya, M. & Kato, K. (1977) Mutagenicity of S-7131 in bacterial
test systems. Unpublished Report No. BT-71-0021 dated 25-10-1977
from Institute of Environmental Toxicology. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Koyama, Y., Okuno, Y., Hosokawa, S. & Miyamoto, J.
(1986) Study on serum hormone levels in rats treated with S-7131.
Revised Final Report No. BT-60-0113 dated 27-7-1986 from Takarazuka
Research Center. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Murakami, M., Ineyama, M., Yamada, T., Ito, M., Koyama, Y., Kimura,
J., Hosokawa, S., Yoshitake, A. & Yamada, H. (1988a). Effect on
testicular function of male rats and mice in subacute administration
of procymidone. Unpublished Report No. BT-80-0130 dated 23-6-1988
from Takarazuka Research Center. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Ineyama, M., Hosokawa, S., Yoshitake, W., & Yamada, H.
(1998b). The affinity of procymidone to androgen receptor in rats
and mice. Unpublished Report No. Bt-80-0131 dated 23-6-1988 from
Takarazuka Research Center. Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Nakashima, N., Ebino, K., Tuda, S., Harada, T. & Kitazawa, T. (1984)
A 6- month subchronic toxicity study of Sumilex in beagles.
Unpublished Report No. BT-41-0091 dated January 1984, from the
Institute of Environmental Toxicology Kodaira, Tokyo 187. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Okuno, Y., Kadota, K. & Miyamoto, J. (1975) Skin sensitization
study with S- 7131 in guinea pigs. Unpublished Report No. BT-60-0005
dated 14-4-1975 from Research Department Pesticides Division.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Pence, O.H., Hoberman, A.M., Andrews, J.P., Durloo, R.S. & Mossburg,
P.A. (1980) Teratology study in rats S-7131 (technical), final
report. Unpublished Report No. BT-01-0048 dated 19-12-1980 from
Hazleton Laboratories America, Inc. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Principe, P. Monaco, M. & Nunziata, A. (1980) Report on
mutagenicity experiment on the substance sumisclex (procymidone).
Unpublished Report No. BT-01-0050 from Centro Ricerca Farmaceutica
s.p.a. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka,
Japan.
Segawa, T. (1977) Acute oral, subcutaneous, intraperitoneal and
dermal toxicities of S-7131 in rats and mice. Unpublished Report
No. BT-71- 0053 dated 7-12-1977 from Hiroshima University School of
Medicine. Submitted to WHO by Sumitomo Chemical Co., Osaka, Japan.
Segawa, T. (1979) Acute intraperitoneal toxicity of S-7131 50%
water- dispersible powder in mice. Unpublished Report No. BT-91-0030
dated 24-3-1979. Hiroshima University School of Medicine. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Shiba, K., Iba, K. Kimura, K. Yoshitake, A. & Yamada, H. (1988)
Comparative metabolism of procymidone in rats and mice. Unpublished
Report No. BM-80-0019 from Takarazuka Research Center. Submitted to
WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1976) Studies on mutagenicity of S-7131
with bacterial systems. Unpublished Report No. BT-60-0011 submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Effect of procymidone
on sister chromatid exchanges (SCE) in cultured mouse embryo cells.
Unpublished Report No. BT-00-0042 dated September 1980, Laboratory
of Biochemistry and Toxicology Research Department. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Wickramaratne, G.A. de S., Kinsey, D.L., Banham, P.B., Greenwood,
M.R. & Pigott, G.H. (1988a) Procymidone: teratogenicity study in
the rabbit. Unpublished Report No. CTL/P/1812 dated 1-6-1988 from
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Wickramaratne, G.A. de S., Milburn, G.M. Banham, P.B., Greenwood,
M.R., Moreland, S. & Pigott, G.H. (1988b) Procymidone:
multigeneration reproduction study in the rat. Unpublished Report
No. CTL/P/1954 dated 1-7-1988 from Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, Enmgland. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
See Also:
Toxicological Abbreviations
Procymidone (Pesticide residues in food: 1981 evaluations)
 TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON PROCYMIDONE
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro
Ames test (both with S. typhimurium 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
and without metabolic TA98, TA100, ug/plate in (1)
activation TA1535, TA1538 DMSO
Ames test (both with S. typhimurium 10, 50, 100, 500 96.6 Negative Moriya et al. 1977
and without metabolic TA98, TA100, TA1535 ug/plate in (1)
activation TA1537, TA1538 DMSO
E. coli WP2 hcr
V79/HGPRT mutation Chinese hamster lung 7x10-4, 1.5x10-3 approx95 Negative Principe et al. 1980
assay (both with and cells (V79) 3x10-3, 6x10-3M (1)
without metabolic
activation)
SCE assay (both with Mouse embryo cells 10-6, 10-5, 96.9 Negative Suzuki et al. 1080
and without metabolic 10-4M in DMSO (1)
activation)
Rec-assay B. subtilis M45 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
rec H17 ug/disk in DMSO (1)
Rec-assay B. subtilis M45 20, 100, 200, 500 96.6 Negative Moriya et al. 1977
rec H17 1000, 2000 ug/disk (1)
in DMSO
TABLE 1 (contd.)
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro (contd)
Unscheduled DNA Human epithelial 6x10-6, 1x10-5 approx95 Negative Principe et al. 1980
synthesis test (both cells 6x10-4, 6x10-3M (1)
with and without in acetone
metabolic activation)
in vivo
Chromosomal aberration Male mice (ICR strain) 400, 800, 1600 96.9 Negative Hara et al. 1980
test mg/kg (i.p.) in (1)
corn oil
Host-mediated assay S. typhimurium 1000, 2000 mg/kg 96.3 Negative Suzuki & Miyamoto, 1976
G46 Male mice in DMSO (1)
(ICR strain)
Host-mediated assay S. typhimurium 200, 500 mg/kg x 2, 96.3 Negative Moriya et al. 1977
G46 Male mice in 5% Arabic gum (1)
(ICR strain)
(1) Positive control compounds gave positive responses.
Toxicological studies
Acute toxicity
The LD50 and LC50 values for the 50% dispersible powder
formulation for procymidone and some metabolites in various species
are given in Tables 2, 3 and 4, respectively.
TABLE 2. ACUTE TOXICITY OF 50% WETTABLE POWDER OF PROCYMIDONE
SPECIES SEX ROUTE LD50 REFERENCE
(mg/kg bw)
Mice M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-h exp.)
M i.p. 1750 Segawa, 1979
F i.p. 1650
Rat M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-hr exp.)
M&F inhal. >109 Kohda et al. 1976c
(4-hr exp.)
TABLE 3. ACUTE TOXICITY OF PROCYMIDONE
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Mouse M oral 7800 96.3 Kohda et al.
F 9100 96.3 1976a
M&F oral >5000 96.5 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
M i.p. 1560 96.3 Kohda et al.
F 1900 96.3 1976a
M i.p. 2030 96.5 Segawa, 1977
F 2050 96.5
Mouse M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
Rat M oral 6800 96.3 Kohda et al.
M 7700 96.3 1976a
M&F oral >5000 96.3 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
Rat M i.p. 850 96.3 Kohda et al.
F 730 96.3 1976a
M i.p. 1440 96.5 Segawa, 1977
F 1450 96.5
TABLE 3 (contd).
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Rat M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
M&F inhal. >1500 b) 99.5 Kohda et al.
1986
In all cases of suspensions of procymidone in corn oil were administered:
a) duration of exposure not indicated
b) 4-hr exposure to dust.
TABLE 4. ACUTE TOXICITY OF METABOLITES OF PROCYMIDONE IN MICE
TEST SEX ROUTE LD50 REFERENCE
COMPOUND (mg/kg bw)
3,5-DCAa M oral 900 Kohda et al. 1980
F oral 820
M s.c. 1300 Kohda et al. 1980
F s.c. 1250
DMPAb M oral 4200 Kohda et al. 1980
F oral 4650
DMPAb M s.c. 2100 Kohda et al. 1980
F s.c. 2650
SF-8748a M oral 1410 Kohda et al. 1980
F oral 1480
a) 3,5-DCA (P8) and SF-8748 (P1) were suspended or dissolved in
corn oil
b) DMPA (1,2-dimethylcyclopropane-dicarboxylic acid, plant and soil
metabolite) was suspended in 10% Tween 8O.
Short-term studies
Mice
Groups of male and female mice (ICR strain, 15/sex/group) were
fed diets containing 0, 50, 150 or 500 ppm procymidone (purity
96.9%) for three months. Mice were observed twice daily for changes
in appearance or behaviour, and body weight was recorded weekly.
Food and water consumption were measured once a week for 3
consecutive days. Hematological and biochemical studies as well as
opthalmological examinations were conducted after 3 months of
treatment. At the end of the treatment period surviving rats were
sacrificed and examined for gross pathology, organs were weighed and
histopathological examinations were carried out. There were no
compound related deaths, body weight changes, effects on food and
water consumption or clinical chemistry nor changes in
ophthalmological findings. Significantly decreased hematocrit and
erythrocyte values were observed in females at 500 ppm. Relative
kidney weight was significantly decreased in females at 150 and
500 ppm and relative adrenal weight was decreased (significantly but
not dose-relatedly) in all male dose groups. An increased relative
liver weight was observed in high dose males and females which was
not statistically significant. Hypertrophy of hepatocytes of the
liver was observed in males at 150 and 500 ppm and in females at
500 ppm. High dose females also showed an increased incidence of
dilated fundal-glands of the stomach. The NOAEL in this study is
50 ppm, equivalent to 7 mg/kg bw/day (Arai, 1980a).
Groups of ICR mice (20/group/sex) were fed diets containing 0,
50, 150 or 500 ppm procymidone (purity: 96.9%) in corn oil for 6
months. Observations included clinical examinations, mortality,
body weight, water and food consumption, ophthalmological,
hematological and biochemical examinations, macroscopy, organ weight
and histopathology. No dose-related deaths occurred. Body weight,
food and water consumption and ophthalmoscopy were not affected.
Leucocyte count was significantly decreased in males at 150 and
500 ppm and MCV values were significantly increased in females at
the same dose groups. Creatinine values were significantly
increased in males at 500 ppm. A tendency to an increased relative
liver weight was noted in females at 500 ppm. At histopathology an
increased incidence of atrophy of the testes was observed (2/19
(10.5%), 5/20 (25%), 6/20 (30%) and 10/20 (50%) in the control,
50 ppm, 150 ppm and 500 ppm group, respectively). This was
significant in the 500 ppm group only. The NOAEL in this study was
150 ppm, equivalent to 20 mg/kg bw/day (Arai, 1980b).
Groups of 20 male mice (Alpk/AP white) were fed diets
containing 0, 10, 30, 100 or 300 ppm procymidone (purity 99.8%) for
6 months. Observations included clinical signs, body weight, food
consumption, organ weight, macroscopy and histopathology of the
testes and the epididymes. The only findings observed were a not
dose-related increase in food consumption (means of 4 observations/
group) at 30, 100 and 300 ppm and a not dose related increase in
relative testes weight in all dose groups (significantly at 10 and
100 ppm), not associated with any histopathological change (Kinsey
et al. 1985).
Rat
Groups of rats (Sprague-Dawley, 12/sex/group) were fed diets
containing 0, 150, 500 or 1500 ppm procymidone (purity, 98.7%) for 6
months. Additional groups (15/sex/group) were fed diets containing
0 or 1500 ppm for 9 months, or 0 or 1500 ppm for 6 months and then
placed on a control diet for another 3 months. No compound-related
effects were observed on mortality, clinical signs, food and water
consumption and urinalysis. Body weight was decreased in females
(significantly at 1500 ppm) at all dose levels after 6 months and
male body weight was decreased at the highest dose after the same
period. After 9 months of feeding both male and female bodyweights
were significantly decreased at 1500 ppm. Significantly decreased
PCV and Hb values were observed in both sexes at 1500 ppm only after
6 months; ALAT levels were increased at 500 and 1500 ppm in both
sexes after 6 months. Relative liver weight was significantly
increased in females at 500 ppm (after 6 months) and in both sexes
at 1500 ppm after 6 and 9 months. Relative spleen weight was
significantly increased in females at 500 and 1500 ppm only after 6
months. There were also changes in several other organ weights,
possibly as a result of the change in body weight. An increased
incidence of swelling of the liver cell was observed in males at
1500 ppm after 6 (4/15) as well as after 9 months (2/15). The only
findings which were still evident after the 3 month recovery period
were a slightly decreased bodyweight in both sexes and an increased
relative liver weight in females at 1500 ppm. The NOAEL in this
study is 150 ppm, equivalent to 7.5 mg/kg bw/day (Kato et al.
1976).
Dog
Beagle dogs (6/sex/group) received by capsule at dose levels
0,20, 100 or 500 mg/kg bw/day procymidone (purity >95%) for 6
months. Mortality, body weight, food and water consumption,
ophthalmoscopy, haematology, urinalysis, organ weight, macroscopy
and histopathology were not affected by treatment. Dogs at
500 mg/kg bw/day had a higher incidence of emesis and diarrhea
(females only). Alkaline phosphatase activity was increased in
males and females at 500 mg/kg bw/day (during the second half of the
treatment period). At the same dose level BUN, glucose and calcium
(occasionally also at 100 mg/kg bw/day) levels were significantly
increased in males. Relative heart weight was significantly
decreased in high dose females. There was a tendency to an
increased liver weight in males and females and a decreased testes
weight in males at 500 mg/kg bw/day. The NOAEL in this study was
100 mg/kg bw/day (Nakashima et al. 1984).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
0, 30, 100, 300 or 1000 ppm procymidone (purity 99.8%) for 104
weeks. Satellite groups consisting of 40 mice/sex/group were
sacrificed at 6-month intervals (week 26, 52 and 78) for clinical
and pathology determinations. An additional 10 mice/sex were
assigned to the main control group for clinical pathology
determinations prior to the start of dosing. Observations included
mortality, clinical signs, body weight, food and water consumption,
ophthalmoscopy, hematology, clinical chemistry, urinalysis,
macroscopy, organ weights and histopathology. Survival rates at
termination varied from 64% to 88%. No treatment-related effects
were observed on mortality, body weight, food and water consumption,
ophthalmoscopy, hematology and urinalysis. A tendency to an
increased ALAT level was observed in both sexes at 1000 ppm.
Relative liver weight was significantly increased in males and
females at 1000 ppm and sometimes at 300 ppm. No effect was
observed on testes weight. An increased incidence of masses, pale
areas and raised areas in the livers was observed at 1000 ppm in
both males and females. An increased incidence of focal or
multifocal hepatocellular hyperplasia and fatty change (also in
300 ppm males) as well as changes in centrilobular cytomegaly were
noted in both sexes at 1000 ppm. Incidences for liver neoplastic
lesions are given in Table 5.
It can be concluded that a slight increase is found in female
hepatocellular adenoma and male hepatoblastoma. The NOAEL in this
study is 100 ppm (equivalent to 14.3 mg/kg bw) (Filler et al.
1988).
TABLE 5. NON-NEOPLASTIC AND NEOPLASTIC INCIDENCES
LESIONS DOSE LEVEL
0 30 100 300 1000
M F M F M F M F M F
Neo-neoplastic
Liver
Centrilobular cytomegaly 0/49 0/49 0/48 0/50 0/49 2/49 11/49 25/50 17/46 36/50
Testes
Interstitial hyperplasia 2/50 0/49 1/50 7/49 12/49
Ovaries
Stromal hyperplasia 0/49 0/50 0/49 2/49 8/50
Neoplastic
Liver
Hepatocellular adenoma 7/50 1/50 11/50 1/40 12/50 0/48 9/49 3/50 10/49 7/50
Hepatocellular carcinoma 5/50 1/50 6/50 1/49 9/50 2/48 5/49 4/50 10/49 2/50
Hepatoblastoma 1/50 0/50 0/50 0/49 0/50 0/48 2/49 0/50 5/49 0/50
Testes
Interstitial cell tumours 1/50 1/50 0/50 10/49 16749
Rat
Groups of Osborne-Mendel rats (50/sex/group) were fed diets
containing 0, 100, 300, 1000 or 2000 ppm procymidone (purity 99.8%)
for 104 weeks. Satellite groups of rats (50/sex/group) were used in
interim (week 26, 65 and 78) evaluations of clinical pathology,
necropsy and organ weights. No compound-related effects were noted
on clinical signs, food and water consumption, mortality,
opthalmoscopy, hematology, biochemistry and urinalysis. At the end
of the study survival rates for males ranged from 22% to 34% while
for females it was 50% to 70%. Body weight gain was significantly
decreased at 1000 and 2000 ppm. At 300 ppm a decrease was found,
which was significant during a part of the study, and more
pronounced in males. Testosterone analysis showed increased levels
at 2000 ppm. At termination (and in most interim kills), relative
liver weight and relative testes weight were increased at 2000 ppm
in males and females. In females relative ovary weight, relative
kidney weight and relative brain weight were significantly increased
at 2000 ppm at the end of the study. An increased incidence of
hepatic centrilobular cytomegaly, testicular interstitial cell
hyperplasia and ovarian stromal hyperplasia as well as an increased
incidence of interstitial cell tumours were observed in the two
highest dose groups.
The NOAEL in this study is 100 ppm (4.6 mg/kg bw and 6.0 mg/kg
bw for males and females, respectively) (Keller et al. 1986).
Special study on affinity for androgen receptor
The binding ability to androgen receptors in prostate cytosols
from castreated mice and rats was examined in a competitive assay in
which the cytosols were incubated with [3H]dihydrotestosterone
(DHT) alone or together with increasing concentrations of
procymidone and some metabolites. The androgen receptors of rat and
mouse prostate have high specificity and high affinity for DHT while
procymidone possesses a low but distinct affinity. The relative
binding affinity (RBA) was 0.065% in rats and 0.070% in mice
compared to 14.2% and 0.058% in rats for cyproterone acetate and
flutamide (a non-steriodal anti-androgen for medical use),
respectively and 0% for some procymidone - soil or light -
degradation products (Murakami et al. 1988b).
Special study on effect upon testicular function
Mouse
Groups of 30 male ICR mice were fed diets containing 0, 1000,
5000 or 10000 ppm procymidone (purity 99.3%) for 13 weeks.
Observations included body weight, food consumption and organ weight
(seminal vesicles, epididymes, testes and prostate), serum
testosterone and LH (luteinizing hormone) levels, testosterone
levels in testes, LH levels in pituitary, in vitro production of
testosterone in testis stimulated by gonadotropin and testicular
binding of 125I-labelled human chorionic gonadotropin (hCG).
Testosterone and LH levels in serum and/or organs, and the
responsiveness of interstitial cells to hCG in vitro were enhanced
after 2 weeks. These changes (which indicate hypergonadotropism)
returned to control levels during the 3 month treatment period. The
binding affinity of hCG for LH/hCG receptor in mouse testes
decreased significantly (measured by an elevated dissociation
constant (Kd) at 5000 and 10000 ppm) during procymidone treatment.
The number of hCG binding sites in the testes was unchanged
(Murakami et al. 1988a).
Rat
In a study with the same protocol, groups of 12 male Sprague-
Dawley rats were fed diets containing 0, 700, 2000 or 6000 ppm
procymidone (purity 99.3% and 99.5%) Relative testes weight was
significantly increased at 6000 ppm during the 3 month treatment
period. Hormone levels (testosterone and LH) in serum and/or
organs, and the responsiveness of interstitial cells to hCG
in vitro were enhanced after 2 weeks. These effects were more
pronounced than in mice and were maintained over 3 months. The
binding affinity and the number of hCG binding sites in the testis
were unchanged (Murakami et al. 1988a).
Special studies on teratogenicity
Rat
Groups of 25 pregnant albino rats {Charles River (SD) BR} were
given oral daily doses of 0, 30, 100 or 300 mg procymidone (purity
99.6%)/kg bw in corn oil from day 6 through 15 of gestation. Dams
were observed for mortality, clinical signs, body weight and food
consumption. At day 20 of gestation, pups were delivered by cesarean
section. The number of corpora lutea, implantations, resorptions,
live and dead fetuses were recorded. Fetuses were weighed, sexed and
examined for external, visceral and skeletal malformations. There
were no effects observed on the dams and offspring except for a
slightly (not significantly) increased frequency of resorptions at
100 and 300 mg/kg bw. The NOAEL in this study is 300 mg/kg bw
(Pence et al. 1980).
Rabbit
Groups of 18 pregnant NZW rabbits were orally administered 0,
30, 150, 750, or 1000 mg procymidone (purity 99.6%)/kg bw in corn
oil from day 7-19 of gestation. All animals were observed for
clinical signs, mortality, bodyweight and food consumption. All
animals were sacrificed on gestation day 30, and the number of
corpora lutea, implantations, live and death fetuses were recorded.
Fetuses were weighed, sexed, inspected for external abnormalities
and examined for both internal and skeletal malformations. No
adverse effects of treatment were observed except for a slightly
increased number of fetuses with non-ossification of the 5th and 6th
sternebra at 1000 mg/kg bw. The NOAEL in this study was 750 mg/kg
bw (Wickramatne et al. 1988a).
Special studies on mutagenicity
Procymidone was negative in various mutagenicity assays. See
Table 1 for a summary of the studies considered.
Reproduction study
Rat
Groups of 30 male and 30 female rats (ALpk:APfSD) received 0,
50, 250 or 750 ppm procymidone (purity 99.5%) in the diet for 12
weeks before initial mating. Similar treatment was continued
throughout two litters and into a second generation which also
produced two litters maintained for 11 weeks. Observations were made
on general condition and behaviour, food consumption and body
weight. Reproduction parameters such as fertility indices, duration
of gestation, precoital interval, viability indices, litter size,
number and percent of live and dead foetuses, litter and pup weight
were studied. Autopsy and histopathological examinations of
selected organs were performed on all F0, F1 and F2 adults and
offspring. Liver and reproductive organs were weighed.
All high dose parental rats showed reductions in bodyweight
gain (significantly in F0 rats and F1 and F2 females), in food
consumption and in food efficiency. A slight effect on body weight
was observed at 250 ppm in F1 and F2 rats. In F1 parental males
(but not in F0) a significant loss of fertility was observed at
750 ppm. Infertile rats showed an abnormal appearance of external
genitalia (hypospadias). Litter weights (except the F2B litters)
were significantly decreased at 750 ppm. Parental relative liver
weight was significantly increased in F0, F1 and F2 generations
at 750 ppm and at 250 ppm also in F0 and F1 males and in F2
females. Relative testes weight was significantly increased in F0,
F1 and F2 males at 250 ppm as well as at 750 ppm. Penile
abnormalities were observed in F1 and F2 adults at 750 ppm
procymidone together with a decrease in prostate size. The
macroscopic diagnosis of hypospadias was confirmed histologically.
In the pituitary hypertrophy and hyperplasia of basophilic cells
were observed in F1 and F2 males at 750 ppm. No effects were
observed on spermatogenesis. High dose F1 males also showed an
increased incidence of bile duct proliferation in the liver.
Difficulties in sexing pups at birth were caused by a reduction
in ano- genital distance in male offspring at day 1 post partum at
750 ppm procymidone (in all litters). In F1B and F2B pups
relative liver weight was significantly increased at 250 (F2B only)
and 750 ppm and relative testes weight was significantly increased
at 250 and 750 ppm and slightly at 50 ppm. A significant decrease
was noted in relative weight of prostate and epididymes both at 250
(F1B only) and 750 ppm procymidone. Abnormalities of the penis
were also observed in F2A and F2B pups (see Table 2). No penile
abnormalities were detected in F1 and F2 litters killed at day 36
post partum. Glycogen depletion was observed in all livers from
F1B female pups at 750 ppm and in 4/5 male F1B pups. The NOAEL is
50 ppm in the diet, equivalent to 2.5 mg/kg bw (Wickramaratne
et al. 1988b).
Special study on serum hormone levels
Groups of 30 male rats were administered diets containing 0,
700, 2000 or 6000 ppm procymidone (purity 99.1%) for 3 months. Ten
rats/group were sacrificed after 14 days, 1 month and 3 months.
Observations included body weight as well as weights of testes,
epididymes, prostate and seminal vesicles, serum levels of
testosterone, LH (luteinizing hormone) and 17b- estradiol and
histopathology of testes, epididymes and plexus pampiniformis. At
6000 ppm body weight was significantly decreased (after 1 month also
at 2000 ppm) and testes weight was slightly increased. Testosterone
levels were significantly and dose-relatedly increased at all dose
levels and LH-levels were significantly increased at the highest
dose. The effects with procymidone were different from those with a
positive control (CdCl2), which caused decreased weight of testes,
epididymes, prostate and seminal vesicles and a marked depression of
testosterone levels. It is known that this CdC12 damages male
gonadal systems directly. In a second study groups of male rats
received 0, 100, 300, 700 or 2000 ppm procymidone for 6 months. Ten
rats/group were sacrificed after 1, 3 and 6 months. Testosterone
levels were significantly increased at 700 and 2000 ppm. The
no-hormonal-effect level is 300 ppm.
In a third study groups of male rats received 0 or 6000 ppm
procymidone for 1 month and were kept for a recovery period of 6
months to examine the reversibility of the hormonal changes. 10
Rats/group were sacrificed after 2 weeks, 1, 3 and 6 months. Levels
of testosterone and LH were normal after 1 month (Murakami et al.
1986).
Special studies on skin and eye irritation
Moistened technical procymidone (50 mg, purity 98% up) was
applied to the shaven skin of 5 male albino rabbits (Japanese
strain) for 4 hours. Slight congestion, lasting one hour, was
observed at the treated site of 2 animals. No other signs of
irritation were observed during a 7-day observation period (Kadota &
Miyamoto, 1976).
A moistened formulation of procymidone (500 mg, 50% water
dispersible powder) was applied to the intact and abraded skin of 6
male albino rabbits (Japanese strain) under occlusive conditions for
24 hours. Severe erythema (score 4) and slight edema (score 1) was
observed after 24 hours lasting for 72 hours. A 1:100 aqueous
suspension of the formulation did not induce any local reaction
(Matsubara et al. 1979).
Technical procymidone (50 mg, purity 98% up) instilled into one
eye of 5 female and 3 male albino rabbits (Japanese strain) caused
no irritant reactions during a 72-hour observation period with or
without washing, respectively (Kadota & Miyamoto, 1976).
A formulation of procymidone (100 mg, 50% water dispersible
powder) was instilled into one eye of 9 male albino rabbits
(Japanese strain). The eyes of three rabbits were washed 30 seconds
after treatment. The formulation was irritating to the unwashed eyes
and not irritating to the washed eyes. A 1:100 aqueous suspension of
the formulation did not cause any irritant reaction (Matsubara
et al. 1979).
Special studies on skin sensitization
A 1% or 5% solution of procymidone (purity 98%) in corn oil had
no sensitizing potential in male guinea pigs receiving 10
applications every other day followed by a challenge 2 weeks later
(Okuno et al. 1975).
A water dispersible powder of procymidone (containing 53% w/w
procymidone, purity 97.8%) was not sensitizing in a Buehler test
(Hara et al. 1979).
Observations in humans
Clinical examination records (taken two times a year, including
bodyweight, visual and hearing perception, chest X-ray, blood
pressure, urinary examination and diagnosis by questioning) of 20
male workers (being protected but without face guard or mask) who
have been engaged in the manufacturing of procymidone technical
grade and in the packing in drums for 3-4 years, were re-examined,
and the details and history of the operations were reviewed. After
re-examination of 5 clinical records of these 20 workers, no
abnormalities were observed in any examination item (Harada, 1983).
COMMENTS
After oral administration to mice and rats, procymidone was
rapidly excreted, primarily via the urine. The compound was oxidized
at the methyl group and hydrolyzed at the imide and amide linkages.
Neither procymidone nor its metabolites accumulated in the tissues
of mice or rats and repeated administration did not alter the
excretion pattern. After a single oral administration to pregnant
rats, the compound and/or its metabolites transferred to the fetus.
Procymidone has a low acute toxicity in the species examined.
In subchronic toxicity studies in mice, rats and dogs, the main
effects were increased liver weight and hepatocellular hyperplasia.
In the dog, the NOAEL was 100 mg/kg bw/day.
In a long-term feeding study in mice, a slightly increased
incidence in liver tumours was reported. The NOAEL was 100 ppm
(equivalent to 15 mg/kg/day). In a long-term feeding study in rats,
decreased weight gain and pathology of the male reproductive system
were observed at 1000 and 2000 ppm. The weight of the testes was
increased at 2000 ppm and at the two highest dose levels of 1000 and
2000 ppm, increased incidences of testicular interstitial cell
hyperplasia and interstitial cell tumours were observed. In this
study, the NOAEL was 300 ppm, equal to 14 mg/kg bw/day.
In a 2-generation, 2 litters per generation reproduction study
in rats, infertility and abnormalities of the male sexual organs
(hypospadias) were observed in adults and in pups at the highest
dose level of 750 ppm. At day 1 post-partum the male offspring
showed a reduction in ano-genital distance. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
In teratogenicity studies with rats and rabbits no embryotoxic
or teratogenic effects were found. The NOAELs were 30 mg/kg bw/day
and 750 mg/kg bw/day, respectively.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
In order to explain the effects on the testes, a number of
special studies were conducted. Procymidone had a low, but distinct
binding affinity for androgen receptors in rats and mice. High
doses had a clear effect on testicular function and hormone levels,
indicating trophic stimulation (resulting in hormonal imbalance) in
rats and mice. The effect in the mouse returned to normal during
the 3-months treatment period, while it remained in rats, where
interstitial hyperplasia and tumours were observed. In a special
study on the effects of procymidone on hormones in rats,
testosterone levels were increased at all dose levels (700, 2000 and
6000 ppm), while luteinizing hormone levels were increased only at
6000 ppm. In a study with lower dose levels, 300 ppm was the
no-hormonal-effect level. Rats were dosed for 1 month with
60000 ppm procymidone and hormonal recovery was studied. One month
after cessation of dosing, levels of testosterone and luteinizing
hormone had returned to normal.
It was concluded that both the effects on reproduction and the
induction of testicular tumours in the long-term rat study can be
explained by the effects of procymidone on the endocrine system.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 100 ppm in the diet, equal to 15 mg/kg bw/day
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg bw/day
Dog: 100 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arai, M., Hasegawa, R. & Ito, N. (1980a) Three-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report from Department of pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Arai, M., Hasegawa, R. & Ito, N. (1980b) Six-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report No. BT-00-0057 from Department of Pathology, Nagoya City
University Medical School, Nagoya, Japan. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Carlborg, F.W. (1987) Tumors observed in project 22254M.
Unpublished Report BT-81-0129 Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Filler, R., Maloney, D., Alsaker, R., Clinton, J., Parker, G.,
Thakur, A., Hohing, L. & Vanatta, P. (1988) Oral chronic toxicity
and oncogenicity study in mice/Sumilex. Unpublished Report No.
BT-81-0127 from Hazleton Laboratories America Inc. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1979) Skin sensitization test
of S7131 50% water dispersible powder in male quinea pigs.
Unpublished Report No. BT-00-0040 September 12, 1980 from Sumitomo
Research Department, Pesticides Division. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, M., Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Cytogenetic
test of procymidone in mouse bone marrow. Unpublished Report No.
BT-00-0043 from Sumitomo, Laboratory of Biochemistry and Toxicology,
Research Department. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Harada, T. (1983) A review of medical examinations of factory
workers possibly exposed to procymidone technical materials.
Unpublished Report dated 23-3-1983 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kadota, T. & Miyamoto, J. (1976) Eye and skin irritation study with
S-7131 technical material in albino rabbits. Unpublished report No.
BT-60-0004 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kato, T., Fujita, T. & Fukada, T. (1976) Toxicity of S-7131
Technical material to rats in prolonged dietary administration over
9 months. Unpublished Report BT-60-003 from Sumitomo Chemical Co.,
Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Keller, J.G., Fitzgerald, J., Sibinovic, F., Loeb, W.F., Cardy,
R.H., Clinton, J. & Wolfe, G.W. (1986) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumislex, Final Report
No. BT-61-0112. LBI project No. 22048-03/13 dated June, 1986, from
Litton Bionetics, Inc. Rockville, Maryland. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kinsey, J.G., Fitzgerald, J. Sibinovic, F., Loeb, W.F., Cardy, R.H.,
Clinton, J. & Wolfe, G.W. (1985) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumisclex. Unpublished Report
No. CTL/P/1146 dated 15-08-1985 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976a) Acute toxicity studies
with S- 7131 technical material in mice and rats. Unpublished Report
no. BT-60-0002 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976b) Acute oral and dermal
toxicities of Sumilex 50% wettable powder in mice and rats.
Unpublished Report No. BT-60-0006 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kodata, T. & Miyamoto, J. (1976c) Acute inhalation
toxicity study with S-7131 (Sumilex) 50% wettable powder in rats.
Unpublished Report No. BT-60-0007 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Nakamura, M., Kadota, T. & Miyamoto, J. (1980) Acute
oral and subcutaneous toxicity studies of three metabolites of
procymidone. Unpublished Report No. BT-00-0037 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kawaguchi., Watanabe, T., Suzuki, T., Kato, T. &
Miyamoto, J. (1986) Acute inhalation toxicity of Sumilex in rats.
Unpublished Report No. BT-60-0116 dated 25-6-1986 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
Primary eye and skin irritation tests of S-7131 50% water
dispersible powder in rabbits. Unpublished Report No. BT-90-0032
from Sumitomo Chemical Co., Ltd. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Mikami, N., Satogami, H. & Miyamoto, J. (1979) Metabolism of
procymidone in rats. J. Pest. Science 4, 165. Submitted as
published Report No. BM-90-0005 to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Moriya, M. & Kato, K. (1977) Mutagenicity of S-7131 in bacterial
test systems. Unpublished Report No. BT-71-0021 dated 25-10-1977
from Institute of Environmental Toxicology. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Koyama, Y., Okuno, Y., Hosokawa, S. & Miyamoto, J.
(1986) Study on serum hormone levels in rats treated with S-7131.
Revised Final Report No. BT-60-0113 dated 27-7-1986 from Takarazuka
Research Center. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Murakami, M., Ineyama, M., Yamada, T., Ito, M., Koyama, Y., Kimura,
J., Hosokawa, S., Yoshitake, A. & Yamada, H. (1988a). Effect on
testicular function of male rats and mice in subacute administration
of procymidone. Unpublished Report No. BT-80-0130 dated 23-6-1988
from Takarazuka Research Center. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Ineyama, M., Hosokawa, S., Yoshitake, W., & Yamada, H.
(1998b). The affinity of procymidone to androgen receptor in rats
and mice. Unpublished Report No. Bt-80-0131 dated 23-6-1988 from
Takarazuka Research Center. Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Nakashima, N., Ebino, K., Tuda, S., Harada, T. & Kitazawa, T. (1984)
A 6- month subchronic toxicity study of Sumilex in beagles.
Unpublished Report No. BT-41-0091 dated January 1984, from the
Institute of Environmental Toxicology Kodaira, Tokyo 187. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Okuno, Y., Kadota, K. & Miyamoto, J. (1975) Skin sensitization
study with S- 7131 in guinea pigs. Unpublished Report No. BT-60-0005
dated 14-4-1975 from Research Department Pesticides Division.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Pence, O.H., Hoberman, A.M., Andrews, J.P., Durloo, R.S. & Mossburg,
P.A. (1980) Teratology study in rats S-7131 (technical), final
report. Unpublished Report No. BT-01-0048 dated 19-12-1980 from
Hazleton Laboratories America, Inc. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Principe, P. Monaco, M. & Nunziata, A. (1980) Report on
mutagenicity experiment on the substance sumisclex (procymidone).
Unpublished Report No. BT-01-0050 from Centro Ricerca Farmaceutica
s.p.a. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka,
Japan.
Segawa, T. (1977) Acute oral, subcutaneous, intraperitoneal and
dermal toxicities of S-7131 in rats and mice. Unpublished Report
No. BT-71- 0053 dated 7-12-1977 from Hiroshima University School of
Medicine. Submitted to WHO by Sumitomo Chemical Co., Osaka, Japan.
Segawa, T. (1979) Acute intraperitoneal toxicity of S-7131 50%
water- dispersible powder in mice. Unpublished Report No. BT-91-0030
dated 24-3-1979. Hiroshima University School of Medicine. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Shiba, K., Iba, K. Kimura, K. Yoshitake, A. & Yamada, H. (1988)
Comparative metabolism of procymidone in rats and mice. Unpublished
Report No. BM-80-0019 from Takarazuka Research Center. Submitted to
WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1976) Studies on mutagenicity of S-7131
with bacterial systems. Unpublished Report No. BT-60-0011 submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Effect of procymidone
on sister chromatid exchanges (SCE) in cultured mouse embryo cells.
Unpublished Report No. BT-00-0042 dated September 1980, Laboratory
of Biochemistry and Toxicology Research Department. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Wickramaratne, G.A. de S., Kinsey, D.L., Banham, P.B., Greenwood,
M.R. & Pigott, G.H. (1988a) Procymidone: teratogenicity study in
the rabbit. Unpublished Report No. CTL/P/1812 dated 1-6-1988 from
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Wickramaratne, G.A. de S., Milburn, G.M. Banham, P.B., Greenwood,
M.R., Moreland, S. & Pigott, G.H. (1988b) Procymidone:
multigeneration reproduction study in the rat. Unpublished Report
No. CTL/P/1954 dated 1-7-1988 from Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, Enmgland. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
TABLE 1. RESULTS OF MUTAGENICITY ASSAYS ON PROCYMIDONE
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro
Ames test (both with S. typhimurium 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
and without metabolic TA98, TA100, ug/plate in (1)
activation TA1535, TA1538 DMSO
Ames test (both with S. typhimurium 10, 50, 100, 500 96.6 Negative Moriya et al. 1977
and without metabolic TA98, TA100, TA1535 ug/plate in (1)
activation TA1537, TA1538 DMSO
E. coli WP2 hcr
V79/HGPRT mutation Chinese hamster lung 7x10-4, 1.5x10-3 approx95 Negative Principe et al. 1980
assay (both with and cells (V79) 3x10-3, 6x10-3M (1)
without metabolic
activation)
SCE assay (both with Mouse embryo cells 10-6, 10-5, 96.9 Negative Suzuki et al. 1080
and without metabolic 10-4M in DMSO (1)
activation)
Rec-assay B. subtilis M45 10, 100, 1000 96.3 Negative Suzuki & Miyamoto, 1976
rec H17 ug/disk in DMSO (1)
Rec-assay B. subtilis M45 20, 100, 200, 500 96.6 Negative Moriya et al. 1977
rec H17 1000, 2000 ug/disk (1)
in DMSO
TABLE 1 (contd.)
CONCENTRATION PURITY
TEST SYSTEM TEST OBJECT OF PROCYMIDONE % RESULTS REFERENCE
in vitro (contd)
Unscheduled DNA Human epithelial 6x10-6, 1x10-5 approx95 Negative Principe et al. 1980
synthesis test (both cells 6x10-4, 6x10-3M (1)
with and without in acetone
metabolic activation)
in vivo
Chromosomal aberration Male mice (ICR strain) 400, 800, 1600 96.9 Negative Hara et al. 1980
test mg/kg (i.p.) in (1)
corn oil
Host-mediated assay S. typhimurium 1000, 2000 mg/kg 96.3 Negative Suzuki & Miyamoto, 1976
G46 Male mice in DMSO (1)
(ICR strain)
Host-mediated assay S. typhimurium 200, 500 mg/kg x 2, 96.3 Negative Moriya et al. 1977
G46 Male mice in 5% Arabic gum (1)
(ICR strain)
(1) Positive control compounds gave positive responses.
Toxicological studies
Acute toxicity
The LD50 and LC50 values for the 50% dispersible powder
formulation for procymidone and some metabolites in various species
are given in Tables 2, 3 and 4, respectively.
TABLE 2. ACUTE TOXICITY OF 50% WETTABLE POWDER OF PROCYMIDONE
SPECIES SEX ROUTE LD50 REFERENCE
(mg/kg bw)
Mice M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-h exp.)
M i.p. 1750 Segawa, 1979
F i.p. 1650
Rat M&F oral >10000 Kohda et al. 1976b
M&F dermal >10000 Kohda et al. 1976b
(24-hr exp.)
M&F inhal. >109 Kohda et al. 1976c
(4-hr exp.)
TABLE 3. ACUTE TOXICITY OF PROCYMIDONE
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Mouse M oral 7800 96.3 Kohda et al.
F 9100 96.3 1976a
M&F oral >5000 96.5 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
M i.p. 1560 96.3 Kohda et al.
F 1900 96.3 1976a
M i.p. 2030 96.5 Segawa, 1977
F 2050 96.5
Mouse M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
Rat M oral 6800 96.3 Kohda et al.
M 7700 96.3 1976a
M&F oral >5000 96.3 Segawa, 1977
M&F dermal >2500 a) 96.3 Kohda et al.
1976a
M&F dermal >5000 96.5 Segawa, 1977
Rat M i.p. 850 96.3 Kohda et al.
F 730 96.3 1976a
M i.p. 1440 96.5 Segawa, 1977
F 1450 96.5
TABLE 3 (contd).
SPECIES SEX ROUTE LD50 LC50 PURITY REFERENCE
(mg/kg bw) (mg/m3) %
Rat M&F s.c. >10000 96.3 Kohda et al.
1976a
M&F s.c. >10000 96.5 Segawa, 1977
M&F inhal. >1500 b) 99.5 Kohda et al.
1986
In all cases of suspensions of procymidone in corn oil were administered:
a) duration of exposure not indicated
b) 4-hr exposure to dust.
TABLE 4. ACUTE TOXICITY OF METABOLITES OF PROCYMIDONE IN MICE
TEST SEX ROUTE LD50 REFERENCE
COMPOUND (mg/kg bw)
3,5-DCAa M oral 900 Kohda et al. 1980
F oral 820
M s.c. 1300 Kohda et al. 1980
F s.c. 1250
DMPAb M oral 4200 Kohda et al. 1980
F oral 4650
DMPAb M s.c. 2100 Kohda et al. 1980
F s.c. 2650
SF-8748a M oral 1410 Kohda et al. 1980
F oral 1480
a) 3,5-DCA (P8) and SF-8748 (P1) were suspended or dissolved in
corn oil
b) DMPA (1,2-dimethylcyclopropane-dicarboxylic acid, plant and soil
metabolite) was suspended in 10% Tween 8O.
Short-term studies
Mice
Groups of male and female mice (ICR strain, 15/sex/group) were
fed diets containing 0, 50, 150 or 500 ppm procymidone (purity
96.9%) for three months. Mice were observed twice daily for changes
in appearance or behaviour, and body weight was recorded weekly.
Food and water consumption were measured once a week for 3
consecutive days. Hematological and biochemical studies as well as
opthalmological examinations were conducted after 3 months of
treatment. At the end of the treatment period surviving rats were
sacrificed and examined for gross pathology, organs were weighed and
histopathological examinations were carried out. There were no
compound related deaths, body weight changes, effects on food and
water consumption or clinical chemistry nor changes in
ophthalmological findings. Significantly decreased hematocrit and
erythrocyte values were observed in females at 500 ppm. Relative
kidney weight was significantly decreased in females at 150 and
500 ppm and relative adrenal weight was decreased (significantly but
not dose-relatedly) in all male dose groups. An increased relative
liver weight was observed in high dose males and females which was
not statistically significant. Hypertrophy of hepatocytes of the
liver was observed in males at 150 and 500 ppm and in females at
500 ppm. High dose females also showed an increased incidence of
dilated fundal-glands of the stomach. The NOAEL in this study is
50 ppm, equivalent to 7 mg/kg bw/day (Arai, 1980a).
Groups of ICR mice (20/group/sex) were fed diets containing 0,
50, 150 or 500 ppm procymidone (purity: 96.9%) in corn oil for 6
months. Observations included clinical examinations, mortality,
body weight, water and food consumption, ophthalmological,
hematological and biochemical examinations, macroscopy, organ weight
and histopathology. No dose-related deaths occurred. Body weight,
food and water consumption and ophthalmoscopy were not affected.
Leucocyte count was significantly decreased in males at 150 and
500 ppm and MCV values were significantly increased in females at
the same dose groups. Creatinine values were significantly
increased in males at 500 ppm. A tendency to an increased relative
liver weight was noted in females at 500 ppm. At histopathology an
increased incidence of atrophy of the testes was observed (2/19
(10.5%), 5/20 (25%), 6/20 (30%) and 10/20 (50%) in the control,
50 ppm, 150 ppm and 500 ppm group, respectively). This was
significant in the 500 ppm group only. The NOAEL in this study was
150 ppm, equivalent to 20 mg/kg bw/day (Arai, 1980b).
Groups of 20 male mice (Alpk/AP white) were fed diets
containing 0, 10, 30, 100 or 300 ppm procymidone (purity 99.8%) for
6 months. Observations included clinical signs, body weight, food
consumption, organ weight, macroscopy and histopathology of the
testes and the epididymes. The only findings observed were a not
dose-related increase in food consumption (means of 4 observations/
group) at 30, 100 and 300 ppm and a not dose related increase in
relative testes weight in all dose groups (significantly at 10 and
100 ppm), not associated with any histopathological change (Kinsey
et al. 1985).
Rat
Groups of rats (Sprague-Dawley, 12/sex/group) were fed diets
containing 0, 150, 500 or 1500 ppm procymidone (purity, 98.7%) for 6
months. Additional groups (15/sex/group) were fed diets containing
0 or 1500 ppm for 9 months, or 0 or 1500 ppm for 6 months and then
placed on a control diet for another 3 months. No compound-related
effects were observed on mortality, clinical signs, food and water
consumption and urinalysis. Body weight was decreased in females
(significantly at 1500 ppm) at all dose levels after 6 months and
male body weight was decreased at the highest dose after the same
period. After 9 months of feeding both male and female bodyweights
were significantly decreased at 1500 ppm. Significantly decreased
PCV and Hb values were observed in both sexes at 1500 ppm only after
6 months; ALAT levels were increased at 500 and 1500 ppm in both
sexes after 6 months. Relative liver weight was significantly
increased in females at 500 ppm (after 6 months) and in both sexes
at 1500 ppm after 6 and 9 months. Relative spleen weight was
significantly increased in females at 500 and 1500 ppm only after 6
months. There were also changes in several other organ weights,
possibly as a result of the change in body weight. An increased
incidence of swelling of the liver cell was observed in males at
1500 ppm after 6 (4/15) as well as after 9 months (2/15). The only
findings which were still evident after the 3 month recovery period
were a slightly decreased bodyweight in both sexes and an increased
relative liver weight in females at 1500 ppm. The NOAEL in this
study is 150 ppm, equivalent to 7.5 mg/kg bw/day (Kato et al.
1976).
Dog
Beagle dogs (6/sex/group) received by capsule at dose levels
0,20, 100 or 500 mg/kg bw/day procymidone (purity >95%) for 6
months. Mortality, body weight, food and water consumption,
ophthalmoscopy, haematology, urinalysis, organ weight, macroscopy
and histopathology were not affected by treatment. Dogs at
500 mg/kg bw/day had a higher incidence of emesis and diarrhea
(females only). Alkaline phosphatase activity was increased in
males and females at 500 mg/kg bw/day (during the second half of the
treatment period). At the same dose level BUN, glucose and calcium
(occasionally also at 100 mg/kg bw/day) levels were significantly
increased in males. Relative heart weight was significantly
decreased in high dose females. There was a tendency to an
increased liver weight in males and females and a decreased testes
weight in males at 500 mg/kg bw/day. The NOAEL in this study was
100 mg/kg bw/day (Nakashima et al. 1984).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) were fed diets containing
0, 30, 100, 300 or 1000 ppm procymidone (purity 99.8%) for 104
weeks. Satellite groups consisting of 40 mice/sex/group were
sacrificed at 6-month intervals (week 26, 52 and 78) for clinical
and pathology determinations. An additional 10 mice/sex were
assigned to the main control group for clinical pathology
determinations prior to the start of dosing. Observations included
mortality, clinical signs, body weight, food and water consumption,
ophthalmoscopy, hematology, clinical chemistry, urinalysis,
macroscopy, organ weights and histopathology. Survival rates at
termination varied from 64% to 88%. No treatment-related effects
were observed on mortality, body weight, food and water consumption,
ophthalmoscopy, hematology and urinalysis. A tendency to an
increased ALAT level was observed in both sexes at 1000 ppm.
Relative liver weight was significantly increased in males and
females at 1000 ppm and sometimes at 300 ppm. No effect was
observed on testes weight. An increased incidence of masses, pale
areas and raised areas in the livers was observed at 1000 ppm in
both males and females. An increased incidence of focal or
multifocal hepatocellular hyperplasia and fatty change (also in
300 ppm males) as well as changes in centrilobular cytomegaly were
noted in both sexes at 1000 ppm. Incidences for liver neoplastic
lesions are given in Table 5.
It can be concluded that a slight increase is found in female
hepatocellular adenoma and male hepatoblastoma. The NOAEL in this
study is 100 ppm (equivalent to 14.3 mg/kg bw) (Filler et al.
1988).
TABLE 5. NON-NEOPLASTIC AND NEOPLASTIC INCIDENCES
LESIONS DOSE LEVEL
0 30 100 300 1000
M F M F M F M F M F
Neo-neoplastic
Liver
Centrilobular cytomegaly 0/49 0/49 0/48 0/50 0/49 2/49 11/49 25/50 17/46 36/50
Testes
Interstitial hyperplasia 2/50 0/49 1/50 7/49 12/49
Ovaries
Stromal hyperplasia 0/49 0/50 0/49 2/49 8/50
Neoplastic
Liver
Hepatocellular adenoma 7/50 1/50 11/50 1/40 12/50 0/48 9/49 3/50 10/49 7/50
Hepatocellular carcinoma 5/50 1/50 6/50 1/49 9/50 2/48 5/49 4/50 10/49 2/50
Hepatoblastoma 1/50 0/50 0/50 0/49 0/50 0/48 2/49 0/50 5/49 0/50
Testes
Interstitial cell tumours 1/50 1/50 0/50 10/49 16749
Rat
Groups of Osborne-Mendel rats (50/sex/group) were fed diets
containing 0, 100, 300, 1000 or 2000 ppm procymidone (purity 99.8%)
for 104 weeks. Satellite groups of rats (50/sex/group) were used in
interim (week 26, 65 and 78) evaluations of clinical pathology,
necropsy and organ weights. No compound-related effects were noted
on clinical signs, food and water consumption, mortality,
opthalmoscopy, hematology, biochemistry and urinalysis. At the end
of the study survival rates for males ranged from 22% to 34% while
for females it was 50% to 70%. Body weight gain was significantly
decreased at 1000 and 2000 ppm. At 300 ppm a decrease was found,
which was significant during a part of the study, and more
pronounced in males. Testosterone analysis showed increased levels
at 2000 ppm. At termination (and in most interim kills), relative
liver weight and relative testes weight were increased at 2000 ppm
in males and females. In females relative ovary weight, relative
kidney weight and relative brain weight were significantly increased
at 2000 ppm at the end of the study. An increased incidence of
hepatic centrilobular cytomegaly, testicular interstitial cell
hyperplasia and ovarian stromal hyperplasia as well as an increased
incidence of interstitial cell tumours were observed in the two
highest dose groups.
The NOAEL in this study is 100 ppm (4.6 mg/kg bw and 6.0 mg/kg
bw for males and females, respectively) (Keller et al. 1986).
Special study on affinity for androgen receptor
The binding ability to androgen receptors in prostate cytosols
from castreated mice and rats was examined in a competitive assay in
which the cytosols were incubated with [3H]dihydrotestosterone
(DHT) alone or together with increasing concentrations of
procymidone and some metabolites. The androgen receptors of rat and
mouse prostate have high specificity and high affinity for DHT while
procymidone possesses a low but distinct affinity. The relative
binding affinity (RBA) was 0.065% in rats and 0.070% in mice
compared to 14.2% and 0.058% in rats for cyproterone acetate and
flutamide (a non-steriodal anti-androgen for medical use),
respectively and 0% for some procymidone - soil or light -
degradation products (Murakami et al. 1988b).
Special study on effect upon testicular function
Mouse
Groups of 30 male ICR mice were fed diets containing 0, 1000,
5000 or 10000 ppm procymidone (purity 99.3%) for 13 weeks.
Observations included body weight, food consumption and organ weight
(seminal vesicles, epididymes, testes and prostate), serum
testosterone and LH (luteinizing hormone) levels, testosterone
levels in testes, LH levels in pituitary, in vitro production of
testosterone in testis stimulated by gonadotropin and testicular
binding of 125I-labelled human chorionic gonadotropin (hCG).
Testosterone and LH levels in serum and/or organs, and the
responsiveness of interstitial cells to hCG in vitro were enhanced
after 2 weeks. These changes (which indicate hypergonadotropism)
returned to control levels during the 3 month treatment period. The
binding affinity of hCG for LH/hCG receptor in mouse testes
decreased significantly (measured by an elevated dissociation
constant (Kd) at 5000 and 10000 ppm) during procymidone treatment.
The number of hCG binding sites in the testes was unchanged
(Murakami et al. 1988a).
Rat
In a study with the same protocol, groups of 12 male Sprague-
Dawley rats were fed diets containing 0, 700, 2000 or 6000 ppm
procymidone (purity 99.3% and 99.5%) Relative testes weight was
significantly increased at 6000 ppm during the 3 month treatment
period. Hormone levels (testosterone and LH) in serum and/or
organs, and the responsiveness of interstitial cells to hCG
in vitro were enhanced after 2 weeks. These effects were more
pronounced than in mice and were maintained over 3 months. The
binding affinity and the number of hCG binding sites in the testis
were unchanged (Murakami et al. 1988a).
Special studies on teratogenicity
Rat
Groups of 25 pregnant albino rats {Charles River (SD) BR} were
given oral daily doses of 0, 30, 100 or 300 mg procymidone (purity
99.6%)/kg bw in corn oil from day 6 through 15 of gestation. Dams
were observed for mortality, clinical signs, body weight and food
consumption. At day 20 of gestation, pups were delivered by cesarean
section. The number of corpora lutea, implantations, resorptions,
live and dead fetuses were recorded. Fetuses were weighed, sexed and
examined for external, visceral and skeletal malformations. There
were no effects observed on the dams and offspring except for a
slightly (not significantly) increased frequency of resorptions at
100 and 300 mg/kg bw. The NOAEL in this study is 300 mg/kg bw
(Pence et al. 1980).
Rabbit
Groups of 18 pregnant NZW rabbits were orally administered 0,
30, 150, 750, or 1000 mg procymidone (purity 99.6%)/kg bw in corn
oil from day 7-19 of gestation. All animals were observed for
clinical signs, mortality, bodyweight and food consumption. All
animals were sacrificed on gestation day 30, and the number of
corpora lutea, implantations, live and death fetuses were recorded.
Fetuses were weighed, sexed, inspected for external abnormalities
and examined for both internal and skeletal malformations. No
adverse effects of treatment were observed except for a slightly
increased number of fetuses with non-ossification of the 5th and 6th
sternebra at 1000 mg/kg bw. The NOAEL in this study was 750 mg/kg
bw (Wickramatne et al. 1988a).
Special studies on mutagenicity
Procymidone was negative in various mutagenicity assays. See
Table 1 for a summary of the studies considered.
Reproduction study
Rat
Groups of 30 male and 30 female rats (ALpk:APfSD) received 0,
50, 250 or 750 ppm procymidone (purity 99.5%) in the diet for 12
weeks before initial mating. Similar treatment was continued
throughout two litters and into a second generation which also
produced two litters maintained for 11 weeks. Observations were made
on general condition and behaviour, food consumption and body
weight. Reproduction parameters such as fertility indices, duration
of gestation, precoital interval, viability indices, litter size,
number and percent of live and dead foetuses, litter and pup weight
were studied. Autopsy and histopathological examinations of
selected organs were performed on all F0, F1 and F2 adults and
offspring. Liver and reproductive organs were weighed.
All high dose parental rats showed reductions in bodyweight
gain (significantly in F0 rats and F1 and F2 females), in food
consumption and in food efficiency. A slight effect on body weight
was observed at 250 ppm in F1 and F2 rats. In F1 parental males
(but not in F0) a significant loss of fertility was observed at
750 ppm. Infertile rats showed an abnormal appearance of external
genitalia (hypospadias). Litter weights (except the F2B litters)
were significantly decreased at 750 ppm. Parental relative liver
weight was significantly increased in F0, F1 and F2 generations
at 750 ppm and at 250 ppm also in F0 and F1 males and in F2
females. Relative testes weight was significantly increased in F0,
F1 and F2 males at 250 ppm as well as at 750 ppm. Penile
abnormalities were observed in F1 and F2 adults at 750 ppm
procymidone together with a decrease in prostate size. The
macroscopic diagnosis of hypospadias was confirmed histologically.
In the pituitary hypertrophy and hyperplasia of basophilic cells
were observed in F1 and F2 males at 750 ppm. No effects were
observed on spermatogenesis. High dose F1 males also showed an
increased incidence of bile duct proliferation in the liver.
Difficulties in sexing pups at birth were caused by a reduction
in ano- genital distance in male offspring at day 1 post partum at
750 ppm procymidone (in all litters). In F1B and F2B pups
relative liver weight was significantly increased at 250 (F2B only)
and 750 ppm and relative testes weight was significantly increased
at 250 and 750 ppm and slightly at 50 ppm. A significant decrease
was noted in relative weight of prostate and epididymes both at 250
(F1B only) and 750 ppm procymidone. Abnormalities of the penis
were also observed in F2A and F2B pups (see Table 2). No penile
abnormalities were detected in F1 and F2 litters killed at day 36
post partum. Glycogen depletion was observed in all livers from
F1B female pups at 750 ppm and in 4/5 male F1B pups. The NOAEL is
50 ppm in the diet, equivalent to 2.5 mg/kg bw (Wickramaratne
et al. 1988b).
Special study on serum hormone levels
Groups of 30 male rats were administered diets containing 0,
700, 2000 or 6000 ppm procymidone (purity 99.1%) for 3 months. Ten
rats/group were sacrificed after 14 days, 1 month and 3 months.
Observations included body weight as well as weights of testes,
epididymes, prostate and seminal vesicles, serum levels of
testosterone, LH (luteinizing hormone) and 17b- estradiol and
histopathology of testes, epididymes and plexus pampiniformis. At
6000 ppm body weight was significantly decreased (after 1 month also
at 2000 ppm) and testes weight was slightly increased. Testosterone
levels were significantly and dose-relatedly increased at all dose
levels and LH-levels were significantly increased at the highest
dose. The effects with procymidone were different from those with a
positive control (CdCl2), which caused decreased weight of testes,
epididymes, prostate and seminal vesicles and a marked depression of
testosterone levels. It is known that this CdC12 damages male
gonadal systems directly. In a second study groups of male rats
received 0, 100, 300, 700 or 2000 ppm procymidone for 6 months. Ten
rats/group were sacrificed after 1, 3 and 6 months. Testosterone
levels were significantly increased at 700 and 2000 ppm. The
no-hormonal-effect level is 300 ppm.
In a third study groups of male rats received 0 or 6000 ppm
procymidone for 1 month and were kept for a recovery period of 6
months to examine the reversibility of the hormonal changes. 10
Rats/group were sacrificed after 2 weeks, 1, 3 and 6 months. Levels
of testosterone and LH were normal after 1 month (Murakami et al.
1986).
Special studies on skin and eye irritation
Moistened technical procymidone (50 mg, purity 98% up) was
applied to the shaven skin of 5 male albino rabbits (Japanese
strain) for 4 hours. Slight congestion, lasting one hour, was
observed at the treated site of 2 animals. No other signs of
irritation were observed during a 7-day observation period (Kadota &
Miyamoto, 1976).
A moistened formulation of procymidone (500 mg, 50% water
dispersible powder) was applied to the intact and abraded skin of 6
male albino rabbits (Japanese strain) under occlusive conditions for
24 hours. Severe erythema (score 4) and slight edema (score 1) was
observed after 24 hours lasting for 72 hours. A 1:100 aqueous
suspension of the formulation did not induce any local reaction
(Matsubara et al. 1979).
Technical procymidone (50 mg, purity 98% up) instilled into one
eye of 5 female and 3 male albino rabbits (Japanese strain) caused
no irritant reactions during a 72-hour observation period with or
without washing, respectively (Kadota & Miyamoto, 1976).
A formulation of procymidone (100 mg, 50% water dispersible
powder) was instilled into one eye of 9 male albino rabbits
(Japanese strain). The eyes of three rabbits were washed 30 seconds
after treatment. The formulation was irritating to the unwashed eyes
and not irritating to the washed eyes. A 1:100 aqueous suspension of
the formulation did not cause any irritant reaction (Matsubara
et al. 1979).
Special studies on skin sensitization
A 1% or 5% solution of procymidone (purity 98%) in corn oil had
no sensitizing potential in male guinea pigs receiving 10
applications every other day followed by a challenge 2 weeks later
(Okuno et al. 1975).
A water dispersible powder of procymidone (containing 53% w/w
procymidone, purity 97.8%) was not sensitizing in a Buehler test
(Hara et al. 1979).
Observations in humans
Clinical examination records (taken two times a year, including
bodyweight, visual and hearing perception, chest X-ray, blood
pressure, urinary examination and diagnosis by questioning) of 20
male workers (being protected but without face guard or mask) who
have been engaged in the manufacturing of procymidone technical
grade and in the packing in drums for 3-4 years, were re-examined,
and the details and history of the operations were reviewed. After
re-examination of 5 clinical records of these 20 workers, no
abnormalities were observed in any examination item (Harada, 1983).
COMMENTS
After oral administration to mice and rats, procymidone was
rapidly excreted, primarily via the urine. The compound was oxidized
at the methyl group and hydrolyzed at the imide and amide linkages.
Neither procymidone nor its metabolites accumulated in the tissues
of mice or rats and repeated administration did not alter the
excretion pattern. After a single oral administration to pregnant
rats, the compound and/or its metabolites transferred to the fetus.
Procymidone has a low acute toxicity in the species examined.
In subchronic toxicity studies in mice, rats and dogs, the main
effects were increased liver weight and hepatocellular hyperplasia.
In the dog, the NOAEL was 100 mg/kg bw/day.
In a long-term feeding study in mice, a slightly increased
incidence in liver tumours was reported. The NOAEL was 100 ppm
(equivalent to 15 mg/kg/day). In a long-term feeding study in rats,
decreased weight gain and pathology of the male reproductive system
were observed at 1000 and 2000 ppm. The weight of the testes was
increased at 2000 ppm and at the two highest dose levels of 1000 and
2000 ppm, increased incidences of testicular interstitial cell
hyperplasia and interstitial cell tumours were observed. In this
study, the NOAEL was 300 ppm, equal to 14 mg/kg bw/day.
In a 2-generation, 2 litters per generation reproduction study
in rats, infertility and abnormalities of the male sexual organs
(hypospadias) were observed in adults and in pups at the highest
dose level of 750 ppm. At day 1 post-partum the male offspring
showed a reduction in ano-genital distance. The NOAEL was 250 ppm,
equivalent to 12.5 mg/kg bw/day.
In teratogenicity studies with rats and rabbits no embryotoxic
or teratogenic effects were found. The NOAELs were 30 mg/kg bw/day
and 750 mg/kg bw/day, respectively.
After reviewing all available in vitro and in vivo
short-term tests, the Meeting concluded that there was no evidence
of genotoxicity.
In order to explain the effects on the testes, a number of
special studies were conducted. Procymidone had a low, but distinct
binding affinity for androgen receptors in rats and mice. High
doses had a clear effect on testicular function and hormone levels,
indicating trophic stimulation (resulting in hormonal imbalance) in
rats and mice. The effect in the mouse returned to normal during
the 3-months treatment period, while it remained in rats, where
interstitial hyperplasia and tumours were observed. In a special
study on the effects of procymidone on hormones in rats,
testosterone levels were increased at all dose levels (700, 2000 and
6000 ppm), while luteinizing hormone levels were increased only at
6000 ppm. In a study with lower dose levels, 300 ppm was the
no-hormonal-effect level. Rats were dosed for 1 month with
60000 ppm procymidone and hormonal recovery was studied. One month
after cessation of dosing, levels of testosterone and luteinizing
hormone had returned to normal.
It was concluded that both the effects on reproduction and the
induction of testicular tumours in the long-term rat study can be
explained by the effects of procymidone on the endocrine system.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 100 ppm in the diet, equal to 15 mg/kg bw/day
Rat: 250 ppm in the diet, equivalent to 12.5 mg/kg bw/day
Dog: 100 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.2 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
Arai, M., Hasegawa, R. & Ito, N. (1980a) Three-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report from Department of pathology, Nagoya City University Medical
School, Nagoya, Japan. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Arai, M., Hasegawa, R. & Ito, N. (1980b) Six-month subacute
toxicity study of S-7131 (Sumilex, Sumisclex) in mice. Unpublished
Report No. BT-00-0057 from Department of Pathology, Nagoya City
University Medical School, Nagoya, Japan. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Carlborg, F.W. (1987) Tumors observed in project 22254M.
Unpublished Report BT-81-0129 Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Filler, R., Maloney, D., Alsaker, R., Clinton, J., Parker, G.,
Thakur, A., Hohing, L. & Vanatta, P. (1988) Oral chronic toxicity
and oncogenicity study in mice/Sumilex. Unpublished Report No.
BT-81-0127 from Hazleton Laboratories America Inc. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, S., Suzuki, T. & Miyamoto, J. (1979) Skin sensitization test
of S7131 50% water dispersible powder in male quinea pigs.
Unpublished Report No. BT-00-0040 September 12, 1980 from Sumitomo
Research Department, Pesticides Division. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Hara, M., Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Cytogenetic
test of procymidone in mouse bone marrow. Unpublished Report No.
BT-00-0043 from Sumitomo, Laboratory of Biochemistry and Toxicology,
Research Department. Submitted to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Harada, T. (1983) A review of medical examinations of factory
workers possibly exposed to procymidone technical materials.
Unpublished Report dated 23-3-1983 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kadota, T. & Miyamoto, J. (1976) Eye and skin irritation study with
S-7131 technical material in albino rabbits. Unpublished report No.
BT-60-0004 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kato, T., Fujita, T. & Fukada, T. (1976) Toxicity of S-7131
Technical material to rats in prolonged dietary administration over
9 months. Unpublished Report BT-60-003 from Sumitomo Chemical Co.,
Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Keller, J.G., Fitzgerald, J., Sibinovic, F., Loeb, W.F., Cardy,
R.H., Clinton, J. & Wolfe, G.W. (1986) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumislex, Final Report
No. BT-61-0112. LBI project No. 22048-03/13 dated June, 1986, from
Litton Bionetics, Inc. Rockville, Maryland. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kinsey, J.G., Fitzgerald, J. Sibinovic, F., Loeb, W.F., Cardy, R.H.,
Clinton, J. & Wolfe, G.W. (1985) Oral chronic toxicity and
oncogenicity study in rats, TB0100 Sumisclex. Unpublished Report
No. CTL/P/1146 dated 15-08-1985 from Imperial Chemical Industries
PLC, Central Toxicology Laboratory, Alderley Park, Macclesfield,
Cheshire, UK. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976a) Acute toxicity studies
with S- 7131 technical material in mice and rats. Unpublished Report
no. BT-60-0002 from Sumitomo Chemical Co., Ltd. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kadota, T. & Miyamoto, J. (1976b) Acute oral and dermal
toxicities of Sumilex 50% wettable powder in mice and rats.
Unpublished Report No. BT-60-0006 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Kodata, T. & Miyamoto, J. (1976c) Acute inhalation
toxicity study with S-7131 (Sumilex) 50% wettable powder in rats.
Unpublished Report No. BT-60-0007 from Sumitomo Chemical Co., Ltd.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Kohda, H., Nakamura, M., Kadota, T. & Miyamoto, J. (1980) Acute
oral and subcutaneous toxicity studies of three metabolites of
procymidone. Unpublished Report No. BT-00-0037 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Kohda, H., Kawaguchi., Watanabe, T., Suzuki, T., Kato, T. &
Miyamoto, J. (1986) Acute inhalation toxicity of Sumilex in rats.
Unpublished Report No. BT-60-0116 dated 25-6-1986 from Sumitomo
Chemical Co., Ltd. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Matsubara, T., Hara, S., Suzuki, T., Kadota, T. & Miyamoto, J.
Primary eye and skin irritation tests of S-7131 50% water
dispersible powder in rabbits. Unpublished Report No. BT-90-0032
from Sumitomo Chemical Co., Ltd. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Mikami, N., Satogami, H. & Miyamoto, J. (1979) Metabolism of
procymidone in rats. J. Pest. Science 4, 165. Submitted as
published Report No. BM-90-0005 to WHO by Sumitomo Chemical Co.,
Ltd., Osaka, Japan.
Moriya, M. & Kato, K. (1977) Mutagenicity of S-7131 in bacterial
test systems. Unpublished Report No. BT-71-0021 dated 25-10-1977
from Institute of Environmental Toxicology. Submitted to WHO by
Sumitomo Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Koyama, Y., Okuno, Y., Hosokawa, S. & Miyamoto, J.
(1986) Study on serum hormone levels in rats treated with S-7131.
Revised Final Report No. BT-60-0113 dated 27-7-1986 from Takarazuka
Research Center. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Murakami, M., Ineyama, M., Yamada, T., Ito, M., Koyama, Y., Kimura,
J., Hosokawa, S., Yoshitake, A. & Yamada, H. (1988a). Effect on
testicular function of male rats and mice in subacute administration
of procymidone. Unpublished Report No. BT-80-0130 dated 23-6-1988
from Takarazuka Research Center. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Murakami, M., Ineyama, M., Hosokawa, S., Yoshitake, W., & Yamada, H.
(1998b). The affinity of procymidone to androgen receptor in rats
and mice. Unpublished Report No. Bt-80-0131 dated 23-6-1988 from
Takarazuka Research Center. Submitted to WHO by Sumitomo Chemical
Co., Ltd., Osaka, Japan.
Nakashima, N., Ebino, K., Tuda, S., Harada, T. & Kitazawa, T. (1984)
A 6- month subchronic toxicity study of Sumilex in beagles.
Unpublished Report No. BT-41-0091 dated January 1984, from the
Institute of Environmental Toxicology Kodaira, Tokyo 187. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Okuno, Y., Kadota, K. & Miyamoto, J. (1975) Skin sensitization
study with S- 7131 in guinea pigs. Unpublished Report No. BT-60-0005
dated 14-4-1975 from Research Department Pesticides Division.
Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Pence, O.H., Hoberman, A.M., Andrews, J.P., Durloo, R.S. & Mossburg,
P.A. (1980) Teratology study in rats S-7131 (technical), final
report. Unpublished Report No. BT-01-0048 dated 19-12-1980 from
Hazleton Laboratories America, Inc. Submitted to WHO by Sumitomo
Chemical Co., Ltd., Osaka, Japan.
Principe, P. Monaco, M. & Nunziata, A. (1980) Report on
mutagenicity experiment on the substance sumisclex (procymidone).
Unpublished Report No. BT-01-0050 from Centro Ricerca Farmaceutica
s.p.a. Submitted to WHO by Sumitomo Chemical Co., Ltd., Osaka,
Japan.
Segawa, T. (1977) Acute oral, subcutaneous, intraperitoneal and
dermal toxicities of S-7131 in rats and mice. Unpublished Report
No. BT-71- 0053 dated 7-12-1977 from Hiroshima University School of
Medicine. Submitted to WHO by Sumitomo Chemical Co., Osaka, Japan.
Segawa, T. (1979) Acute intraperitoneal toxicity of S-7131 50%
water- dispersible powder in mice. Unpublished Report No. BT-91-0030
dated 24-3-1979. Hiroshima University School of Medicine. Submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Shiba, K., Iba, K. Kimura, K. Yoshitake, A. & Yamada, H. (1988)
Comparative metabolism of procymidone in rats and mice. Unpublished
Report No. BM-80-0019 from Takarazuka Research Center. Submitted to
WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H. & Miyamoto, J. (1976) Studies on mutagenicity of S-7131
with bacterial systems. Unpublished Report No. BT-60-0011 submitted
to WHO by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Suzuki, H., Ohkawa, H. & Miyamoto, J. (1980) Effect of procymidone
on sister chromatid exchanges (SCE) in cultured mouse embryo cells.
Unpublished Report No. BT-00-0042 dated September 1980, Laboratory
of Biochemistry and Toxicology Research Department. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
Wickramaratne, G.A. de S., Kinsey, D.L., Banham, P.B., Greenwood,
M.R. & Pigott, G.H. (1988a) Procymidone: teratogenicity study in
the rabbit. Unpublished Report No. CTL/P/1812 dated 1-6-1988 from
Imperial Chemical Industries PLC, Alderley Park, Macclesfield,
Cheshire, England. Submitted to WHO by Sumitomo Chemical Co., Ltd.,
Osaka, Japan.
Wickramaratne, G.A. de S., Milburn, G.M. Banham, P.B., Greenwood,
M.R., Moreland, S. & Pigott, G.H. (1988b) Procymidone:
multigeneration reproduction study in the rat. Unpublished Report
No. CTL/P/1954 dated 1-7-1988 from Imperial Chemical Industries PLC,
Alderley Park, Macclesfield, Cheshire, Enmgland. Submitted to WHO
by Sumitomo Chemical Co., Ltd., Osaka, Japan.
