
PROFENOFOS
EXPLANATION
First draft prepared by Dr J.A. Quest,
US Environmental Protection Agency, Washington, D.C., USA
Profenofos is a broad spectrum organophosphate insecticide and
acaricide. Its mode of action is by inhibition of
acetylcholinesterase. Profenofos was evaluated for the first time by
the present meeting.
EVALUATION FOR ACCEPTABLE INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Rats
Four male and three female RAI rats received a single oral dose
of approximately 4.8 mg/kg bw of randomly ring-labelled-14C
profenofos (s.a. = 9.79 µCi/mg). Within 6 days essentially all of the
administered radioactive dose was eliminated in the urine (81.8% in
males and 96.4% in females) and faeces (15.7% in males and 2.5% in
females). Most of the urinary and faecal excretions occurred within
the first 24 hours of dosing. The excretion t1/2 was less than 8
hours for both sexes. Only minor amounts of radiolabelled material
were excreted in the expired CO2 (0.08% in males and 0.07% in
females). When the animals were sacrificed 6 days after dosing,
detectable amounts of residual radioactivity were found only in the
liver (0.013 ppm in males and 0.023 ppm in females) and kidney (0.007
ppm in males and 0.008 ppm in females), while radiolabel in other
tissues (fat, muscle, testis, ovary, brain) was below the limit of
detection or (blood) quantitation (Ifflaender, et al., 1974).
Male and female Harlan SD albino rats received single dermal
applications of ring-labelled 14C profenofos at doses of 0.5 mg/kg
(s.a. = 9.34 µCi/mg and 10 mg/kg (s.a. = 2.6 µCi/mg) in a 72-hour
balance study. Over the 72-hour absorption period, the total 14C
recoveries averaged 92% to 95% of each applied dose in each sex (80%
to 86% in urine, 2.2% to 3.9% in faeces, 0.09% to 1.8% in tissues,
0.06% or less in blood, 3% or less in treated skin, and 5% or less in
cage washings). Excretion in expired CO2 was negligible (less than
0.02%, as determined from a preliminary study using the highest dermal
dose). The calculated 50% absorption rates indicated that 14C-
profenofos was absorbed at nearly the same rate for males and females
regardless of the dose level; t1/2 absorption values were 17.9 and
15.0 hours after treatment with the low dose in males and females,
respectively, and 16.7 and 14.1 hours after treatment with the high
dose in males and females, respectively. The calculated 50% excretion
rates (urine was the major route of excretion) occurred 18.1 and 17.4
hours after treatment with the low dose in males and females,
respectively, and 23.2 and 18.7 hours after treatment with the high
dose in males and females, respectively. Fifty percent of 14C-
profenofos was excreted shortly after 50% had been absorbed indicating
that profenofos and its metabolites were rapidly excreted, i.e., there
was no lag time between absorption and excretion. Levels of
radioactivity in selected tissues (liver and kidney) and blood peaked
in 2 to 8 hours, plateaued by 8 hours, and declined rapidly by 72
hours (Williams, et al., 1984).
Hens
Two white Leghorn hens received oral doses of ring-labelled
14C-profenofos (s.a. = 19.1 µci/mg) for 14 consecutive days at a
dose rate equivalent to 5 mg/kg in the feed. A third hen served as an
untreated control. The total excretion over 14 days ranged from 81.3
to 85.2% of the radioactive dose (81-84% in excreta, 0.02% in tissues,
0.01% in blood, 0.21% in egg yolks, and 0.009% in egg whites). The
excretory plateau occurred after 5 to 9 days of dosing. The kidney
was the only tissue containing a notable amount of radioactivity
(equivalent to 0.054 to 0.073 mg/kg whereas negligible levels
(equivalent to 0.013 mg/kg or less) where found in liver, blood,
muscle, skin and fat (Oakes, et al., 1986).
Goats
A single goat received oral doses of ring-labelled 14C-
profenofos (s.a. = 26.2 µCi/mg) for 9 consecutive days at a level
equivalent to 5 mg/kg in the diet. A second goat served as an
untreated control. The total recovery of radioactivity over 9 days
was 97.8% of the radioactive dose (85.8% in urine, 4.4% in faeces,
5.8% in the rumen and intestinal contents, 1.0% in expired CO2, 1.0%
in milk, 0.9% in tissues, and 0.6% in blood). Based on urine, faeces,
and milk data, the radioactivity in excretions and secretions reached
a plateau by the second day of dosing. The levels of radioactivity in
tissues were the highest in the liver (0.096 mg/kg) and kidney (0.072
mg/kg), and lower in fat (0.018 mg/kg) and heart, brain and skeletal
muscle (0.004 mg/kg or less) (Thomas, et al., 1976).
Biotransformation
Rats
The metabolism of ring-labelled 14C-profenofos was studied over
a 24-hour period in the urine of RAI rats given a single oral dose of
approximately 4.8 mg/kg bw. Analysis of the urine via TLC in rats of
both sexes indicated complete degradation of profenofos as no
unchanged parent compound was present. Four metabolites were observed
in urine; the only one identified by TLC was the cleavage product, 4-
bromo-2-chlorophenol, which was found in negligible amounts in freshly
obtained urine. This metabolite did not appear in the initial
samples, indicating that other labile metabolites are cleaved to this
phenol (Ifflaender, et al., 1974).
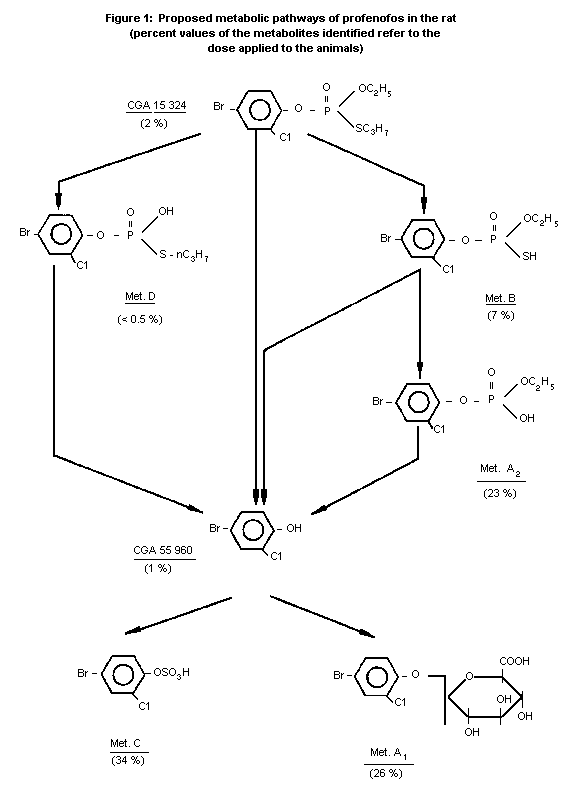
In a second study, the metabolism of ring-labelled 14C-
profenofos (s.a. = 26.2 µCi/mg) was investigated over a 2 day period
in the urine and faeces of eleven male RAI rats given a single oral
dose of 28.5 mg/kg bw. The material was readily absorbed from the gut
and excreted, with 90.4% and 3.6% of the administered dose excreted in
urine and faeces within 24 hours. The proposed metabolic pathways of
the parent compound, profenofos, in the urine and faeces of rats is
depicted in Figure 1. The main features of this scheme are as
follows:
1. Neither the intact parent compound profenofos (O-(-4-bromo-
2-chlorophenol)-O-ethyl-S-n-propyl phosphorothioate) nor its
corresponding phenol (4-bromo-2-chlorophenol) was detected
in freshly obtained urine.
2. The major metabolic pathway in urine involves the
depropylation of profenofos to Metabolite B, i.e., O-(4-
bromo-2-chlorophenol)-O-ethyl phosphorothioate (7%), which
is then desulfurated to Metabolite A2, i.e., 0-(4-bromo-2-
chlorophenol)-O-ethyl phosphate (23%). This, in turn,
undergoes cleavage at the phenyl-ester bond giving rise to
4-bromo-2-chlorophenol which is completely conjugated with
glucuronic acid to Metabolite A1, i.e., 4-bromo-2-
chlorophenol glucuronide (26%) and with sulfuric acid to
Metabolite C, i.e., 4-bromo-2-chlorophenol sulfate (34%).
3. A second but more minor metabolite pathway in urine involves
O-demethylation of profenofos to Metabolite D, i.e., O-(4-
bromo-2-chlorophenol)-s-propyl phosphorothioate (less than
0.5%). This also undergoes conversion to 4-bromo-2-
chlorophenol, and then to Metabolites A1 and C.
4. The faeces were reported to contain only small amounts of
the parent compound, profenofos (2%), and its corresponding
phenol, 4-bromo-2-chlorophenol (1%). Additional metabolites
were present in minute amounts (0.2% or less) but were not
identified. (Ifflaender and Mucke, 1976).
In a third study, male rats (Tif:RAI-f strain) dosed orally with
single doses of 0.19 or 1.80 mg/kg bw of ring-labelled 14C-
profenofos excreted 78% to 81% of the dose in the urine within 24
hours. Upon acidic hydrolysis, 96% of the urinary metabolites were
transformed to 4-bromo-2-chlorophenol. The main urinary metabolites
identified in this study were similar to those reported in Figure 1 by
Ifflaender and Mucke, 1976 (i.e., Metabolites B, A2, A, and C were
found in average distributions of 8%, 17%, 33%, and 38% of the urine
radioactivity, respectively). An exception was that a small amount
(approximately 7%) of unconjugated 4-bromo-2-chlorophenol was also
found in the present study. From these data, the authors determined
that the conversion factor to calculate the amount of profenofos taken
up orally from the amount of 4-bromo-2-chlorophenol determined in 0-24
hour urine after hydrolysis was 1.30 (for the 0.19 mg/kg dose) to 1.33
(for the 1.80 mg/kg dose). The data were independent of the dose
within the range tested, and could serve as a urinary monitoring
system for occupational exposure to profenofos (Mucke, 1986).
Hens
Two white Leghorn hens received oral doses of 5 ppm of ring-
labelled 14C-profenofos over 14 days at which time excreta samples
were selected for characterization of metabolites. Most of the
excreta residues were extractable (97%) and were divided between
organic soluble (66%) and aqueous soluble (31%) materials. Of the
organic soluble fraction, 10.6% was unchanged profenofos and 52.8% was
4-bromo-2-chlorophenol. The aqueous soluble fraction was
characterized as 4-bromo-2-chlorophenol (10.5%), its sulfate conjugate
(15.5%; referred to as Metabolite C in rats, above), 0-(4-bromo-2-
chlorophenol)-0-ethyl-phosphate (6.2%; referred to as Metabolite A2
in rats, above), and 4-bromo-2-chlorophenol glucuronide (2.6%;
referred to as Metabolite A1 in rats, above) (Oakes, et al., 1986).
Goats
A metabolism study in a single goat administered 5 mg/kg bw/day
of ring-labelled 14C-profenofos in the diet for 9 consecutive days
indicated that of the radioactivity in urine, 11% was 4-bromo-2-
chlorophenol. At least two other urinary metabolites were present,
one of which was unknown (less than 2%) and the other most probably a
sulfate conjugate (87%). A major part (87%) of the residues
identified in the liver was released as 4-bromo-2-chlorophenol upon
hydrolysis (Thomas, et al., 1976).
In Vitro studies
Incubation of 25 nmol of ring-labelled 14C-profenofos with
mouse liver microsomes containing NADPH resulted in the metabolic
formation of several products, including desthiopropylprofenofos,
despropylprofenofos, desethylprofenofos, and protein-bound
radiocarbon. Profenofos underwent little or no metabolism or
incubation with mouse liver microsomes without NADPH (Wing, et al.,
1984).
 Effects on enzymes and other biochemical parameters
Profenofos is stereospecifically converted to a more potent
inhibitor of acetylcholinesterase by mouse liver microsomal mixed-
function oxidase system. The chiral (-) isomer became a 34-fold
better inhibitor of acetylcholinesterase in vitro, while the less
toxic (+) isomer was deactivated by a factor of 2. Prior treatment
with mixed function oxidase inhibitors markedly decreased the
activation and also protected against brain acetylcholinesterase
inhibition and cholinergic symptoms resulting from (-) profenofos
administration in chicks (Wings, et al., 1983).
Toxicological studies
Acute studies
The acute toxicity of profenofos is given in Table 1. The
adverse signs of toxicity that were observed were generally similar
for each route of compound administration. These included non-
specific symptoms such as dyspnoea, exophthalmos, ruffled fur and
crooked body posture, and cholinergic symptoms such as sedation,
salivation, discharge from eyes and nose, trismus, tremors, and tonic-
clonic convulsions. The symptoms were reversible in surviving
animals.
Short-term studies
Rats
A feeding study was performed in which groups of 25 male and 25
female F344 rats received technical profenofos (90.6% a.i.) in the
daily diet for up to 13 weeks. Two control groups (basal diet) and
eleven dose groups (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300, and
1000 ppm) were used. The animals from one control group and the 0.3,
3, 30, and 300 ppm test groups (15/sex/dose level) were on test for 13
weeks. The animals in all of the other test groups were sacrificed at
weeks 2, 4 and 8 (5 to 15 rats/sex/group). The results indicated that
dose levels of profenofos of 10 to 1000 ppm produced a dose-related
inhibition of cholinesterase activity in plasma and erythrocytes (more
marked in females), that dose-levels of 100 to 1000 ppm produced
reduced food intake and a dose-related reduction in body weight gain,
and that dose-levels of 300 and 1000 ppm reduced brain cholinesterase
activity. These effects were observed in rats of both sexes.
Profenofos did not result in death of any of the treated animals. No
unusual signs of behavior or appearance were observed. Results of
haematology, clinical chemistry (excluding cholinesterase) and
urinalysis examinations were unremarkable. Necropsies performed at
the various sacrifice intervals and at termination of the study did
not reveal treatment-related gross changes.
Table 1: Acute toxicity of Profenofos1
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse M+F Oral 298 -- Bathe, 1974a
Rat M+F Oral 358-502 -- Bathe, 1974b
Kobel & Gfeffer, 1983
M+F Dermal 33002 -- Bathe, 1974c
M+F i.p. 585 -- Sachsse & Bathe, 1975
M+F inhalation -- 3-3.36 Sachsse & Ullmann, 1974a
(4hr)
Horath & Taylor, 1982
Chinese M+F Oral 153 -- Bath & Sachsse, 1979
hamster
Rabbit M+F Oral 700 -- Sachsse & Ullmann, 1974b
M+F Dermal 4722 -- Sachsse & Ullmann, 1974c
M+F Dermal 1313 -- Cannelongo, 1982a
M+F Dermal 25404 -- Kuhn, 1988
Dog M+F Oral >3005 -- Bathe, 1974d
1 All studies performed using technical grade profenofos
2 Intact skin, occlusive dressing (aluminum foil and plaster)
3 Intact and abraded skin, occlusive dressing (polyethylene film)
4 Intact skin, semipermeable dressing (orthopaedic stockinette)
5 Acute oral toxicity could not be determined in dogs due to vomiting
at doses of 300 mg/kg and higher.
Profenofos technical (purity unspecified) was administered by
inhalation (nose only exposure) to albino RAI rats for 21 days (6
hours/day, 5 days/week) at mean concentrations of 0, 68, 219 and 449
mg/m3 in air. Nine males and nine females per group were exposed.
Four males and 4 females of the control and 219 mg/m3 group were
kept for an additional 21 days without treatment (recovery group).
All rats of the 449 mg/m3 group (9/9M and 9/9F) and 1/9 females of
the 219 mg/m3 group died during the first week after showing
symptoms of exophthalmos, dyspnoea, tremor, ruffled fur, lateral
position, and irritation/ secretion of the mucous membranes of the
eyes. Microscopic exams of these rats revealed the presence of marked
congestion of the nasal mucous membranes, acute conjunctivitis, and
severe interstitial keratitis. In the remaining surviving animals of
the 68 mg/m3 and 219 mg/m3 groups there were reductions in food
consumption and body weight gain (seen in males throughout the study
and in females over the first 7 to 10 days of the study), reductions
in total plasma protein levels, and a dose-related inhibition of
cholinesterase activity in plasma, red blood cells, and brain. The
magnitude of cholinesterase inhibition ranged from -40% to -70% for
all 3 sites. In addition, alpha-1 globulin levels were reduced and
alpha-2 and beta globulin levels were increased in males of the 219
mg/m3 group. All of the above changes observed in the surviving
rats were reversible upon cessation of treatment, with the exception
of the reductions in plasma and red cell cholinesterase levels in rats
of the 219 mg/m3 group which remained about 25% below control levels
at the end of the 21-day recovery period. Finally, necropsy of the
surviving rats did not reveal any gross or microscopic pathological
changes, although the related weights of most organs were increased in
male rats due to the reduced weight gain incurred in these animals.
The NOAEL was less than 68 mg/m3 in air (lowest dose tested) in
males and females, based upon reductions in food consumption, weight
gain, and cholinesterase activity in plasma, red cells, and brain
(Ullmann, et al., 1977).
Rabbits
In a dermal toxicity study, profenofos technical (89.8% pure) was
diluted with polyethylene glycol and saline (70:30) to 2% and 5%
solutions and applied to the skin of KA46 rabbits (Himalayan strain;
3/sex/dose level) at doses of 0, 5, 20, and 100 mg/kg bw/day for 24
hours/day, 5 days/week, for 21 days. In the 0, 5, and 20 mg/kg bw/day
treatment groups, 1 rabbit/sex/dose level was kept for an additional
21 days without treatment (recovery group). All of the rabbits
treated dermally with 100 mg/kg bw/day of profenofos died within 6
days after dosing was initiated. These animals displayed moderate
erythema and oedema of the skin, reduced food intake and body weight
gain, various clinical signs of toxicity (dyspnoea, salivation,
tremors, ataxia, sedation, and curved position), and inhibition of
cholinesterase activity in plasma (-100%) and red cells (-50.3%) as
measured on day 4 prior to deaths. Histopathological examination of
the high dose animals showed focal hypertrophy, haemorrhages and fatty
changes of hepatocytes accompanied by necrosis of the liver
parenchyma, and slight to moderate atrophy of lymphoid and thymic
tissue. Microscopic examination of the skin at the site of dermal
application showed oedema, minute haemorrhages, small intradermal
pustules, focal acanthosis and parakeratosis. In the animals treated
with the lower doses (5 and 20 mg/kg bw/day) of profenofos the
observed toxic changes consisted of slight erythema and oedema at the
application site, and inhibition of cholinesterase activity in plasm,
red cells, and brain. These effects disappeared during the recovery
period. No other unusual findings were observed in the lower dose
group animals. The NOAEL was less than 5 mg/kg bw/day (lowest dose
tested) in male and female rabbits for short term dermal toxicity
(Ullmann, et al., 1976).
In a second dermal toxicity study, profenofos technical (92%
pure) was suspended in purified water containing 0.5% Tween 80 and
applied to the skin of albino rabbits (HAR:PC/CF (NZW) BR strain;
5/sex/dose level) at doses of 0, 0.05, 1 and 10 mg/kg bw/day for 6
hours/day, 5 days/week, for 21 days. No deaths occurred in any
treated animals. Profenofos administration produced slight erythema at
the application site in mid dose males and females, and in high dose
males. Additional changes that appeared to be treatment-related were
observed at the high dose level and included hyperactivity and soft
faeces in males and/or females, significant reductions of
cholinesterase activity (measured at terminal sacrifice) in plasma,
red blood cells, and brain, increases in serum bilirubin (males) and
gamma GT (females) levels, and a decrease in serum sodium levels
(males). No unusual histopathological findings occurred. The NOAEL
for subchronic dermal toxicity (excluding the occurrence of slight
dermal erythema at the mid dose) was 1 mg/kg bw/day in males and
females (Johnson, et al., 1984).
Dogs
In a 6-month study, groups of 7 male and 7 female pedigreed
beagles were fed diets containing 0, 0.2, 2, 100, or 500 ppm of
technical profenofos (88.1%-89.3% pure). One male and one female of
each group was kept for an additional 28 days on control diet
(recovery groups). The administered concentrations were calculated to
be equivalent to 0, 0.007, 0.05, 2.9, and 14.4 mg/kg bw/day in dogs of
both sexes. Standard examinations for clinical signs of toxicity,
ophthalmology, haematology, clinical chemistry (including plasma,
brain, and red cell cholinesterase activity and plasma and liver
carboxylesterase activities) and urinalysis parameters were performed
at regular intervals throughout the study. The animals were subjected
to a battery of neurological exams (e.g., muscle strength and tone,
reflexes, etc.), necropsied, and examined grossly for organ changes
and histologically for tissue changes.
Adverse findings were observed at dose levels of 2 ppm to 500 ppm
and consisted of cholinesterase inhibition in plasma (males and
females at 2, 100, and 500 ppm bw/day) and red cells (males and
females at 100 and 500 ppm), carboxylesterase inhibition in plasma and
liver (males at 100 and 500 ppm), reductions in erythrocyte,
haemoglobin and haematocrit levels (males and females at 500 ppm; also
occasionally at 100 ppm), and reduced food consumption (males at 500
ppm over weeks 0-3). The depressed haematological values were said to
be within physiological limits, but it was noted that they did not
increase with age as was the case in the control and lower dose group
animals. All those effects appeared to be reversible during the 4-
week recovery period. Aside from these changes, there were no other
compound-related effects.
Long-term/carcinogenicity studies
Mice
A long-term feeding study of profenofos technical (90.6% pure)
was performed in albino mice (HaM/ICR Swiss, CR-CD 1 strain) in which
dietary concentrations of 0, 1, 30, and 100 ppm were fed to 60
males/dose group for 85 weeks and 60 females/dose group for 96 weeks.
The original design of the study was for 104 weeks, but because
survival had reached 20% of the original number of mice in the mid-
dose males and in the high-dose females due to accidental causes, the
sacrifices were initiated early. An additional 5 mice/sex/dose were
studied at each dose level and were sacrificed after 52 weeks for the
determination of cholinesterase activity in erythrocytes, plasma and
brain; these animals were not examined histopathologically. The
administered concentrations were calculated to be equivalent to 0,
0.14, 4.5, and 14.2 mg/kg bw/day in males and 0, 0.19, 5.8, and 19.2
mg/kg bw/day in females.
There were no clinical signs of toxicity and no significant
effect of treatment on mortality. There were no treatment related
effects on food consumption or body weight gain, or at gross necropsy
or after histopathological examination. There were no increases in
tumors that appeared to be related to compound administration. With
respect to clinical laboratory examinations, a significant dose-
related inhibition of erythrocyte and plasma cholinesterase activity
(generally ranging from -38% to -76%) was observed in mid- and high-
dose males and females at week 53 (interim sacrifice animals) and at
the termination of the study (week 85 in males and week 97 in
females). In addition, brain cholinesterase activity was
significantly inhibited (about -25%) in high-dose females at the end
of the study, whereas a trend (not significant) for this effect was
observed in the males. The NOAEL for male and female mice was 1 ppm
in the diet (0.14 mg/kg bw/day in males and 0.19 mg/kg bw/day in
females) based upon cholinesterase inhibition (Burdock, et al.,
1981a).
Rats
Fischer 344 albino rats (60/sex/group) were fed profenofos
technical (90.6% pure) at doses of 0, 0.3, 10, and 100 ppm for 2
years. An additional 10 rats/sex were also included in the control
and high dose treatment groups, respectively. Of these, 5/sex/group
were sacrificed at 52 weeks (interim sacrifice), and 5/sex/group were
placed on control feed after 52 weeks so that recovery studies could
be conducted and these were then sacrificed during week 63 (recovery
animals). All of the animals in the study were examined
histopathologically. Plasma and erythrocyte cholinesterase activity
was determined in 10 rats/sex/dose group (main study group) at weeks
13, 26, 52, 78, and 105 and in the recovery animals at week 57. Brain
cholinesterase activity was determined in the interim and terminal
sacrifice animals at weeks 53 and 105. The administered
concentrations of profenofos were calculated to be equivalent to 0,
0.017, 0.56, and 5.7 mg/kg bw/day in males and 0, 0.02, 0.69, and 6.9
mg/kg bw/day in females.
There were no treatment-related clinical signs of toxicity,
deaths, or changes in body weight gain. No sustained changes in
clinical chemistry parameters occurred which were considered to be
treatment-related. A dose-related inhibition of plasma and
erythrocyte cholinesterase was observed in rats of both sexes at the
mid- and high-dose levels; these effects were considered to be
compound-related and were reversible as judged by their absence in the
recovery group animals. Brain cholinesterase was not altered by
profenofos in this study. Additional changes seen only at the high
dose level included an increase in food consumption in females, an
increase in relative thyroid gland weight in high dose males (seen in
interim sacrifice and recovery group animals but not in terminal
sacrifice animals, and not considered biologically significant), an
increase in thyroid gland perifollicular cell hyperplasia in high dose
males (i.e., 4/70 controls vs. 10/70 high dose), and an increase in
liver neoplastic nodules in high dose females (i.e., 1/70 controls;
3/60 low dose; 2/60 mid dose; and 6/70 high dose). No increase in
liver carcinomas occurred. The latter histopathological findings were
not considered to be compound-related changes. The NOAEL was 5.7
mg/kg bw/day (the highest dose tested).
Reproduction study
Groups of male (8/sex/dose) and female (16/sex/dose) albino rats
in each of three generations (F0, F1, and F2) were fed diets
containing 0, 0.2, 1.0, or 20 ppm profenofos (technical grade; 95.5%
pure). The dietary concentrations were equivalent to 0, 0.01, 0.05,
and 1.0 mg/kg bw/day. Parental animals were allowed to mature for 100
days, mate, and produce 2 litters. Eight males and 16 females from
the second litters were retained at weaning as parental animals for
the succeeding generations. The study was terminated following the
weaning of the F3b litters. Profenofos was administered
continuously through the experiment.
There were no compound related effects on body weight, mortality,
behavior, or various parameters measuring fertility (fecundity index,
male or female fertility index) in any of the parenteral animals.
Statistically significant reductions in cholinesterase occurred at 20
ppm in erythrocytes (F0, F1, and F2 males and females) and
plasma (F0 females). In contrast, statistically increased plasma
cholinesterase activity was seen in F0 females given 0.2 and 1 ppm
and F1 males given 1 ppm, and brain cholinesterase activity was
increased in F1 males and females given 1 and 20 ppm. No adverse
effects occurred in progeny with respect to litter size, viability,
body weight, cholinesterase activity in erythrocytes, plasma or brain,
or development throughout the study. Postmortem gross and
histopathologic examinations of parenteral animals and F3b weanlings
were unremarkable (IBTL, Inc., 1978). The NOAEL for reproductive
effects was greater than 1.0 mg/kg bw/day in the diet (highest dose
tested).
Special studies on embryo/fetotoxicity
Rats
Groups of 20-27 pregnant rats (strain unspecified) were
administered profenofos (technical grade; purity unspecified) via oral
gavage at dose levels of 0, 10, 30, and 60 mg/kg bw from days 6 to 15
of gestation (day 0 = day either spermatozoa or vaginal plug found).
On day 21 of gestation, all dams were sacrificed and fetuses delivered
by caesarean section. Maternal toxicity was evident in the high-dose
groups as indicated by a marked decrease in food consumption during
the period of treatment. No other adverse effects occurred in the
dams. Similarly, none of the doses of profenofos appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. The NOAEL for maternal toxicity was 30 mg/kg and that for
fetotoxicity/teratogenicity was 60 mg/kg by oral gavage (Fritz,
1974b).
Groups of 23-25 pregnant rats (JCL-SD strain) received profenofos
(technical grade; 95.8% purity) via intubation at dose levels of 0,
18, 35, or 70 mg/kg bw/day from days 7 to 17 of gestation (day =day
that a sperm plug was found). On day 21 of gestation, all dams were
sacrificed under ether anaesthesia and fetuses delivered by caesarean
section. The doses of profenofos selected for testing were based upon
the results of a preliminary range finding study in which 140 mg/kg
bw/day caused death in 5/6 treated rats during days 8 to 15 of
pregnancy. Profenofos administration was associated with increases in
body weight and water consumption in the dams at doses of 35 and 70
mg/kg bw/day on days 17 to 21, and an increased level of food
consumption at 70 mg/kg bw/day on days 14 to 21; these changes did not
appear to be deleterious. Although small increases in the weights of
several organs (heart, spleen, liver, and right kidney) were seen at
70 mg/kg bw/day, these were small in magnitude. No treatment-related
changes in mortality or behavior were observed, and no abnormal
findings were observed at gross necropsy. There were no adverse
effects on the offspring with respect to resorptions, sex ratios,
placental weights, body weights and lengths, or distribution of
fetuses within the uterine horns. External and visceral examinations
of fetuses were unremarkable. Skeletal examination of fetuses showed
increased incidences of progeny with holes in the xiphoid at the mid
and high doses (0% controls, 0% low dose, 18.8% mid dose, and 15.6%
high dose) and delayed ossification of vertebral arches at the high
dose (8.8% controls, 6.7% low dose, 0.5% mid dose, and 26.7%). No
historical control data was provided and it could not be determined if
the findings were all from one litter or from multiple litters. The
NOAEL for maternal and developmental toxicity appeared to be 70 mg/kg
bw/day by intubation (Sugiya et al., 1982).
Groups of 25 pregnant rats (Sim:(SD)fBR strain) received
profenofos (technical grade; 88.0% purity) via oral gavage at dose
levels of 0, 10, 30, 60, 90, and 120 mg/kg bw/day from days 6 to 15 of
gestation (day 0 = day either sperm or vaginal plug found). On day 21
of gestation, all dams were sacrificed using CO2 and fetuses
delivered by caesarean section. Maternal toxicity occurred at the
high dose level as evidenced by increased mortality, reduced food
consumption, and various clinical signs of toxicity (e.g.,
hypoactivity or tremors, ocular porphyrin discharge, diarrhoea,
dyspnoea, diuresis, and hypothermia). Two of the 4 dams that died
displayed these clinical signs whereas the other 2 did not. In
addition, 2 of the dams that died also showed scattered haemorrhages
in the stomach upon gross necropsy. None of the other doses tested
produced changes in the pregnancy ratio, percentage of live or dead
fetuses, number of resorptions, or live fetal weights or sex ratios.
Similarly none of the dose appeared to affect embryonic or fetal
development and no teratogenic effects were observed. The NOAEL for
maternal toxicity was 90 mg/kg bw/day (Harris and Holson, 1982).
Rabbits
Groups of 20 pregnant Chinchilla rabbits were administered
profenofos (technical grade; 89.5% purity) via intubation at dose
levels of 0, 5, 15, and 30 mg/kg bw from days 6 to 18 of gestation
(day 0 = day of mating). On day 28 of gestation, all dams were
sacrificed by cervical dislocation and fetuses delivered by caesarean
section. With the exception of a marginal reduction in food
consumption from day 6 onward there were no unusual effects of
profenofos on the does. In addition, no adverse prenatal effects,
malformations, or variations were observed. The NOAEL for both
maternal and development toxicity was 30 mg/kg bw/day by intubation
(Fritz, et al., 1979).
Groups of 16 pregnant New Zealand White rabbits were given
profenofos (technical grade; 90.8% purity) by oral gavage at dose
levels of 0, 30, 60, 90, and 175 mg/kg bw from days 6 to 18 of
gestation (day 0 = day of mating). On day 30 of gestation, all does
were euthanized and fetuses delivered by caesarean section. The doses
of profenofos selected for testing were based upon the results of a
preliminary range finding study in which doses up to 150 mg/kg bw did
not produce any signs of toxicity. Profenofos administration was
associated with reduced maternal weight gain and food consumption at
doses of 60 mg/kg bw or more, clinical signs of toxicity (e.g.
diarrhoea, soft stools, oral/perianal discharges) at 90 mg/kg bw or
more, and deaths in 9/16 (56.3%) of the does at 175 mg/kg bw. Many of
the does that died exhibited the above clinical signs of toxicity as
well as signs of pinpoint stomach haemorrhages and yellow-discolored
areas in the mesentery in the gastric region upon gross necropsy.
None of the doses tested produced changes in maternal pregnancy rates,
in prenatal effects (e.g., percentage of live fetuses and live
fetuses/litter, resorptions, litter size, fetal body weight, and sex
ratios), or in malformations or variations. The NOAEL for maternal
toxicity was 30 mg/kg bw/day and that for developmental toxicity was
175 mg/kg bw/day (Holson, 1983).
Special studies on eye and skin irritation
In two studies in rabbits, instillation of 0.1 ml of profenofos
(undiluted technical material) into the conjunctival sac for 30
seconds produced mildly irritating conjunctival reactions (redness and
chemosis) which generally lasted for periods of up to 24 to 48 hours
before dissipating (Sachsse and Ullmann, 1974e; Cannelongo, 1982d).
In one of the tests, 2 of 6 treated rabbits died within 3 days without
apparent symptoms (Sachsse and Ullmann, 1974e).
Profenofos (0.5 ml of undiluted technical grade formulation)
produced death in one rabbit and slight to severe erythema in 2 of 5
surviving rabbits when applied to shaved and abraded skin for 24
hours. The treated animals displayed toxic signs manifested as
lateral and curved position, asynchronisms of the extremities, muscle
spasms, and apathy (Sachsse and Ullmann, 1974d).
In a second skin irritation study, application of profenofos (0.5
ml of undiluted technical grade formulation) to intact and abraded
rabbit skin produced death in 6 of 6 animals studied within 24 to 72
hours. Slight skin irritation (erythema and oedema) was observed
(Cannelongo, 1982b).
Special studies on genotoxicity
Profenofos was negative in a variety of in vitro tests in
bacteria, yeast, and mammalian cell systems that evaluated potential
activity to produce gene mutations, gene conversion, mitotic crossing
over, non-disjunction, and unscheduled DNA synthesis. Profenofos was
also negative in two in vitro tests, a dominant lethal assay in mice
and a nucleus anomaly test in Chinese hamsters. However, the compound
was associated with the production of chromosome aberrations,
micronucleus induction, and sister chromatid exchanges in the bone
marrow at oral doses ranging from 36 to 216 mg/kg bw in a third
in vivo study in mice. The summary results of genotoxicity studies
with profenofos are presented in Table 2.
Special studies on skin sensitizing effect
Profenofos was examined for skin sensitizing effects in 2 studies
in guinea pigs. Negative results were obtained in one test when
animals received a series of 10 intracutaneous induction injections
followed 14 days later by a single challenge injection of profenofos
(0.1 ml of a 0.1% technical formulation). A vehicle control group was
also negative whereas dinitrochlorobenzene (positive control) produced
marked sensitization (Sachsse and Ullmann, 1974f). Positive results
for dermal sensitization (i.e., very light erythema or oedema) were
occasionally obtained in another test when animals received a series
of 11 intradermal induction injections followed 14 days later by a
single challenge injection (0.1 ml of 0.1% technical formulation) of
profenofos. Although 2,4-dinitrochlorobenzene (positive control) was
consistently active in this study, a concurrent negative control group
was not employed to facilitate an evaluation of the results
(Cannelongo, 1982d).
Special studies on neurotoxicity
Delayed neurotoxic effects of oral doses of 21.7, 46.4, and 60
mg/kg bw/day of technical profenofos were assessed in adult domestic
chickens. The compound was administered twice, 21 days apart. The
acute oral LD50 of the formulation was about 35 mg/kg bw. Only the
birds of the low dose group survived the two treatments with
profenofos. Signs of toxicity were also similar after both treatments
with profenofos (e.g., salivation, asynchronisms of the extremities,
curved position, apathy, and ruffled feathers); these were observed at
the mid and high doses levels after the initial treatment on day 0,
and also at the low-dose level after the second treatment on day 21.
Neither delayed neurotoxic symptoms nor histologic changes in spinal
cord or peripheral nerve were observed. A positive control group
receiving 1000 and 2150 mg/kg bw TOCP showed the expected reactions
(i.e., ataxia, deterioration of reflexes, and swelling, fragmentation
and disruption of myelin sheaths) (Krinke et al., 1974).
Table 2: Results of genotoxicity assays on profenofos.
Test System Test Object Concentration Purity Results Reference
of profenofos
Ames Test1 S. typhimurium 5,15,45,135, 405 µg/0.1 ml in DMSO ? Negative Arni and Muller, 1978
(reverse mutation) TA-98, TA-100,
TA-1535, TA-1537
Yeast Test1 S. cerevisae Nonactivated: 91.8% Negative Arni and Muller, 1982
(gene conversion, crossing (D7 strain) 12.5-500 µg/ml in DMSO
over, reverse mutation) Activated: 0.640 -10000 µg/ml in DMSO
Yeast Test1 S. cerevisae 39,156,625, 2500, 10000 µg/ml in DMSO 90.0% Negative Hool and Muller, 1986
(non-disjunction) (D61.M strain)
Mouse Lymphoma L5178Y mouse 0.078, 0.156, 0.313, 0.625 µg/ml in DMSO 91.8% Negative Strasser and Muller, 1982
Forward Mutation lymphoma cells
Assay1 (TK +/-)
DNA Repair Rat hepatocytes 0.016, 0.08, 0.4, 2 nl/ml 91.8% Negative Puri and Muller, 1982a
Test2 (UDS)3
DNA Repair Human fibroblasts 0.32, 1.6, 8, 40 nl/ml 91.8% Negative Puri and Muller, 1982b
Test2 (UDS)3
Dominant Lethal Test Mouse (male) 35, 100 mg/kg ? Negative Fritz, 1974a
(NMRI-derived) (single oral doses)
Nucleus Anomaly Test Chinese hamster 13, 26, 52 mg/kg (2 oral doses on 88.1% Negative Hool, et al., 1981
bone marrow consecutive days)
Table 2 (contd)
Test System Test Object Concentration Purity Results Reference
of profenofos
Somatic Cell Studies Male Swiss mouse 36, 162, 216 mg/kg (single oral doses) 72% Positive4 El Nahas, et al., 1988
in Mice (sister chromatid bone marrow
exchange, micronucleus,
chromosome aberration)
1 With and without metabolic activation.
2 No exogenous activation added.
3 UDS = unscheduled DNA synthesis.
4 Doses of 36-216 mg/kg produced increases in chromosome aberrations; doses of 162-216 mg/kg increased micronuclei formation and sister
chromatid exchanges.
In a second study, adult chickens received oral doses of 29.2,
58.5, 117, and 234 mg/kg bw of profenofos (38% EC) on days 0 and 21.
The acute oral LD50 of the formulation was 127.0 mg/kg bw. After
treatment on day 0, death occurred in 0%, 6.6%, 55%, and 100% of the
birds given doses of 29.2, 58.5, 117 and 234 mg/kg bw/day,
respectively. The increased mortality was accompanied by signs of
cholinesterase inhibition (i.e., weakness, lethargy, and anorexia).
After treatment on day 21, death occurred in 0%, 7.1%, and 67% of the
surviving birds given doses of 29.2, 58.5 and 117 mg/kg bw/day, and
similar signs of cholinesterase inhibition were observed. There were
no delayed neurotoxic symptoms or histologic changes in brain, spinal
cord, or sciatic nerves. A positive control group using 500 mg/kg bw
of TOCP exhibited clinical signs of delayed neurotoxicity (extreme
weakness of legs and wings) and neuropathological lesions in the
spinal cord and sciatic nerves (axonal degeneration and demyelination)
(Fletcher et al., 1977).
Profenofos (technical, 89.5% purity) was examined for delayed
neurotoxicity in a third study in adult chickens. In the first phase
of the study (day 0), birds received oral doses of 30 and 45.7 mg/kg
bw on the basis of preliminary acute toxicity studies in which an
acute LD50 dose of 45.7 mg/kg bw was determined. However, because
of increased mortality rates at these dose levels (70% at 30 mg/kg bw,
and 82% at 45.7 mg/kg bw), all surviving birds were redosed on day 21
at a new estimated oral LD50 of 17.1 mg/kg bw. At this dose level
of profenofos, a mortality rate of 5% was observed. The results
indicated that profenofos produced signs of lethargy and salivation in
treated birds, but no signs of delayed neurotoxicity or histological
lesions in the brain, spinal cord, or peripheral nerves. A positive
control group using 500 mg/kg bw of TOCP exhibited clinical signs of
delayed neurotoxicity (extreme weakness of legs and wings) and
lesions in the spinal cord and sciatic nerves (axonal degeneration and
demyelination) (Reinart et al., 1978).
Special studies on pesticide antagonistic agents
A protective effect of atropine given early after orally
administered profenofos in rats or intraperitoneally administered
profenofos in chicks and mice was demonstrated by a reduction in
mortality and toxic signs (e.g., salivation, tremors, sedation,
convulsions) typical of anticholinesterase exposure. The effect of
oximes was limited (Sachsse and Bathe, 1976; Gfeller and Kobel, 1974;
Glickman et al. 1984).
Special studies on pesticide interactions
No potentiating effects were found when mixtures of profenofos
and the organophosphates methidathion, methacrifos or diazinon were
given to rats in equitoxic doses (Sachsse and Bathe, 1977); (Sachsse
and Bathe, 1978).
Profenofos administered intraperitoneally to mice at 0.5 to 5.0
mg/kg bw strongly inhibited the liver microsomal esterase(s)
hydrolysing trans-permethrin. The intraperitoneal toxicity LD50
measured 24 hours after exposure of fenvalerate and malathion but not
that of trans-permethrin was greatly increased when the compounds were
given 1 hour after an intraperitoneal injection of 25 mg/kg bw of
profenofos (Gaughan et al., 1980).
Observations in humans
A group of six male spraymen, aged 20 to 25 years, was monitored
for whole blood cholinesterase activity over a period of 4 days while
treating fully grown cotton with a formulation containing 400 grams
profenofos and 40 grams cypermethrin per liter in organic solvents on
a plantation in the Multan region of Central Pakistan in September
1985. The application equipment consisted of hand-held battery
operated spinning disc devices. The workers wore heavy cotton shirts
with long sleeves, legs wrapped below the knees, and turbans which
were often used to cover the face. Cholinesterase activity was
measured at the end of each of the 4 work days after thorough washing
of the body. The results indicated that there was a tendency toward
inhibition of blood cholinesterase activity. Compared to baseline
pre-exposure cholinesterase readings, the average cholinesterase
levels were 103.3%, 84.8%, 85.6%, and 81.2% of control activities on
days 1, 2, 3, and 4, respectively. The lowest individual values were
72.7% and 73.1% of control levels on the fourth day in 2 of the 6
workers (Loosli, 1989).
No cases of poisonings in humans have been reported with either
the active ingredient per se or with profenofos formulations.
COMMENTS
Profenofos administered orally to rats was well absorbed and was
excreted primarily in the urine, but also in faeces. Profenofos was
biotransformed by a major pathway involving side chain depropylation,
desulfuration, and phenyl-ester bond cleavage to yield 4-bromo-2-
chlorophenol, and by a minor pathway involving side chain 0-de-
ethylation, and subsequent phenyl-ester bond cleavage to the above
phenol. Both pathways culminated in conjugation with glucuronic and
sulfuric acids. No unchanged parent compound was found in urine.
Faeces contained only minute amounts of the parent compound, the
intermediate phenol, and unidentified metabolites. A similar
absorption and excretion pattern was observed in hens and goats. No
unusual organ or tissue localization of 14C-labelled profenofos was
observed.
Profenofos has a moderate order of acute toxicity following oral
and dermal administration. It has been classified as moderately
hazardous by WHO (WHO, 1990).
In a dietary toxicity study in which rats were fed profenofos for
8-13 weeks, the NOAEL was 100 ppm (equivalent to 5 mg/kg bw/day) based
on the finding of brain cholinesterase inhibition at higher levels.
The observed reduction in erythrocyte cholinesterase at lower doses
was not considered to be of toxicological importance. Reduction in
food consumption and body weight gain were considered to be a
consequence of poor palatability of the compound.
In a six-month feeding study in dogs, profenofos caused
erythrocyte cholinesterase inhibition and plasma and liver carboxylase
inhibition at levels of 100 ppm or more. The highest dose level
tested, 500 ppm, resulted in reductions in erythrocyte counts,
haemoglobin and haematocrit levels and a decrease in food consumption.
All of the changes were reversible. No changes in brain
cholinesterase activity occurred. As noted above, enzyme inhibition
and reduced food consumption were not considered to be direct toxic
effects of profenofos administration. The NOAEL was 100 ppm (equal to
2.9 mg/kg bw/day) based upon the observed haematological changes.
In long-term feeding studies in mice and rats, no treatment-
related increases in the incidence of neoplasms was observed. In
mice, brain cholinesterase inhibition occurred at the highest dose
level tested, 100 ppm. The NOAEL was 30 ppm (equal to 5.8 mg/kg
bw/day in females) based on brain cholinesterase inhibition observed
at the next higher dose level in females. In rats erythrocyte
cholinesterase inhibition occurred at dietary levels of 10 and 100
ppm. The highest dose level of 100 ppm was also associated with an
increase in food consumption and a slightly elevated incidence of
parafollicular cell hyperplasia. This type of response is not seen in
man following exposure to chemicals. No effect on brain
cholinesterase was observed. The NOAEL was 100 ppm (equal to 5.7
mg/kg bw/day).
In a three-generation reproduction study in rats, reductions in
cholinesterase activity occurred in erythrocytes (F0, F1, and F2
males and females) at 20 ppm in the diet. This was the highest dose
tested. No changes in brain cholinesterase activity occurred. The
NOAEL for systemic toxicity and reproduction was 20 ppm (equivalent to
1.0 mg/kg bw/day) which was the highest dose tested.
In three teratology studies in rats the NOAELs for maternal
toxicity ranged from 30 to 90 mg/kg bw/day, based upon findings of
reduced food consumption, clinical signs of toxicity (e.g., diarrhoea,
dyspnoea, tremors, hypothermia) and increased mortality at higher dose
levels. No teratogenic activity was observed at dose up to and
including 120 mg/kg bw/day. The NOAEL for embryotoxicity/fetotoxicity
(based on an increased incidence of variants) was 18 mg/kg bw/day. In
two rabbit teratology studies, the NOAEL for maternal toxicity was 30
mg/kg bw/day, based upon findings of reduced food consumption and body
weight gain, the occurrence of soft stools and diarrhoea, and
increased mortality at higher dose levels. No embryotoxic, fetotoxic,
or teratogenic activity was observed at doses up to and including 175
mg/kg bw/day.
After reviewing all available in vitro and in vivo short-term
tests, the Meeting concluded that there was no evidence of
genotoxicity.
The Meeting based the ADI for profenofos on the NOAEL of 1 mg/kg
bw/day in the rat multigeneration study. Although there was no
evidence of any adverse effects at this dose level in the study, the
absence of information on reproductive parameters at higher levels
precluded the use of the NOAEL of 2.9 mg/kg bw/day in the 6-month
feeding study in dogs.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 30 ppm, equal to 5.8 mg/kg bw/day in females.
Rat: 20 ppm, equivalent to 1.0 mg/kg bw/day (reproduction).
Rat: 100 ppm, equal to 5.7 mg/kg bw/day in males (long-term
study).
Dog: 100 ppm, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in
the continued evaluation of the compound
Further observations in humans.
An additional multigeneration study in rats using higher doses.
REFERENCES
Arni, P. and Muller, D. (1978). Salmonella/mammalian-microsome test
with CGA 15'324 (Test for mutagenic properties in bacteria). Project
No.: 78/2531. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Arni, P. and Muller, D. (1982). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro with CGA 15'324 (Test for
mutagenic properties in yeast cells). Project No.: 911557.
Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Bathe, R. (1974a). Acute oral LD50 of technical CGA 15'324 in the
mouse. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974b). Acute oral LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974c). Acute dermal LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. (1974d). Acute oral LD50 of technical CGA 15'324 in the
dog. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. and Sachsse, K. (1979). Acute oral LD50 in the Chinese
hamster CGA 15'324. Project No.: 785329. Unpublished report from
Ciba-Geigy Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Kuper, C.H. (1981a). Twenty-four months carcinogenicity study in
mice, CGA 15'324. Project No.: 483-133. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Fieser, S. (1981b). Two-year chronic oral toxicity study in albino
rats, CGA 15/324 technical. Project No.: 483-134. Unpublished report
from Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B.F. (1982a). Rabbit acute dermal toxicity CGA 15'324
technical FL 811584. Project No.: 2460-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F., (1982b). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2462-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982c). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2461-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982d). Guinea pig sensitization CGA 15'324
technical FL 811584. Project No.: 2463-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
El Nahas, S.M., De Hondt, H.A., and Ramadan, H.A.I. (1988). In vivo
evaluation of the genotoxic potential of Curacron in somatic cells of
mice. Environ. Mol. Mutagen., 11(4): 515-522.
Fletcher, D., Gordon, D.E., Jenkins, D.H., Kennedy, G.L., Kinoshita,
F.K., and Phillips, B.M. (1977). Neurotoxicity study with CGA 15'324
38% E.C. in chickens. Project No.: IBT 8580-10426. Unpublished
report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL,
USA. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland and
validated by the United States Environmental Protection Agency.
Fritz, H. (1974a). Dominant lethal study on CGA 15'324 tech., mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No.: 327438. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H. (1974b). Reproductive study - technical CGA 15'324, rat,
segment II (test for teratogenic or embryotoxic effects). Project
No.: 22741900. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H., Becker, H., and Hess, R. (1979). Seg. II reproductive
study in rabbits, CGA 15'324 tech. Project No.: 785565. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gaughan, L.C., Engel, J.L., and Casida, J.E. (1980). Pesticide
interactions: Effects of organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology 14: 81-85.
Gfeller, W. and Kobel, W. (1984). CGA 15'324 technical, tentative and
antilethal study in the rat. Project No.: GU 820034. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gfeller, W., Kobel, W., Schaeppi, U., Zak, F., and Hess, R. (1981).
CGA 15'324 tech., 6-months toxicity study with dogs. Project No.:
790804. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Glickman, A.H., Wing, K.D., and Casida, J.E., (1984). Profenofos
insecticide bioactivation in relation to antidote action and the
stereospecificity and acetylcholinesterase inhibition, reactivation,
and aging. Toxicity and Applied Pharmacology, 73: 16-22.
Harris, S.B. and Holson, J.F. (1982). A teratology study of CGA
15'324 technical in albino rats. Project No.: 282009. Unpublished
report from Science Applications, Inc., La Jolla, CA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Holson, J.F. (1983). Teratology study (Seg II) in albino rabbits with
CGA 15'324 technical. Project No.: 283003. Unpublished report from
Science Applications, Inc., La Jolla, CA, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., and Muller, D. (1981). Nucleus anomaly test in
somatic interphase nuclei, CGA 15'324, Chinese hamster (test for
mutagenic effects on bone marrow cells). Project No.: 791557.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. and Muller, D. (1986). Test for non-disjunction on
Saccharomyces cerevisiae D61.M in vitro, CGA 15'324 tech. Project
No.: 850811. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Horath, L.L. and Taylor, G.D. (1982). Acute aerosol toxicity study in
rats of CGA 15'324 technical FL 811528. Project No.: 420-0921.
Unpublished report from Toxi-Genics, Inc., Decatur, IL, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
IBTL, Inc. (1978). Three generation reproduction study with CGA
15'324 technical in albino rats. Project No.: IBT 623-07924.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
Northbrook, IL, USA. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland. Validated by United States Environmental Protection
Agency.
Ifflaender, U. and Mucke, W. (1976). The metabolism of CGA 15'324 in
the rat. Project No.: 27/76. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Ifflaender, U., Mucke, W., and Esser, H.O. (1974). Distribution,
degradation and excretion of CGA 15'324 in the rat. Project No.:
16/74. Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Johnson, S., Tai, C.N., and Katz, R. (1984). Profenofos technical,
21-day dermal toxicity study in rabbits. Project No.: MIN 842008.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kobel, W. and Gfeller, W. (1983). Acute oral LD50 in the rat, CGA
15'324 tech. GU Project No.: 830188. Unpublished report from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Krinke, G., Ullmann, L., and Sachsse, K. (1974). Acute oral LD50
and neurotoxicity study of technical CGA 15'324 in the domestic fowl
(Gallus domesticus). Project No.: Siss 2850. Unpublished report from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1988). Acute dermal toxicity study in rabbits, profenofos
technical. Project No.: 5522-88. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Loosli, R. (1985). Project No.: 850910. Unpublished summary report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mucke, W. (1986). The renal excretion of U-14C-Phenyl CGA 15'324 by
male rats after oral administration (exposure monitoring). Project
No.: 13/86. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Oakes, T.L., Marco, G.J., and Ballantine, L. (1986). Metabolism of
14C-profenofos in chickens dosed at 5.0 ppm. Project No.: ABR-
86002. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Piccirillo, V.J. (1978). 90-day subacute oral toxicity study in rats,
CGA 15'324 technical. Project No.: 483-135. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. and Muller, D. (1982a). Autoradiographic DNA repair test on
rat hepatocytes, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811490. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Puri, E. and Muller, D. (1982b). Autoradiographic DNA repair test on
human fibroblasts, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811658. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Reinart, D., Fletcher, D., Arceo, R.J., and Gordon, D.E. (1978). 42-
day neurotoxicity study with CGA 15'324 technical in adult chickens.
Project No.: IBT 8580-11187. Unpublished report from Industrial
Bio-Test Laboratories, Inc., Decatur, IL. USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland and validated by the United States
Environmental Protection Agency.
Sachsse, K. and Bathe, R. (1975). Acute intraperitoneal LD50 in the
rat of technical CGA 15'324. Project No.: Siss 5048. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1976). The therapeutic activity of
pralidoxim (PAM) and TOXOGONIN\ with regard to CGA 15'324 in the rat.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1977). Potentiation study CGA 15'324
versus 2 insecticides CA 13'005 (methidathion) and G 24'480 (diazinon)
in the rat. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1978). Potentiation study CGA 20'168
versus 6 insecticides, C 177 (DDVP), C570 (phosphamidon), GS 13'005
(methidation), G 24'480 (diazinon), CGA 15'324 and malathion. Project
No.: 404478 - Siss 6526. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. and Ullmann, L. (1974a). Acute inhalation toxicity of
technical CGA 15'324 in the rat. Project No.: Siss 3647.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974b). Acute oral LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647 Unpublished report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974c). Acute dermal LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974d). Skin irritation in the rabbit
after single application of technical CGA 15'324. Project No.: Siss
3647. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974e). Irritation of technical CGA
15'324 in the rabbit eye. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974f). Skin sensitizing (contact
allergenic) effect in Guinea pigs of technical CGA 15'324. Project
No.: Siss 3647. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F.F. and Muller, D. (1982). L5178/TK+/- mouse lymphoma
mutagenicity test, CGA 15'324 (in vitro test for mutagenic
properties of chemical substances in mammalian cells). Project No.:
811491. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sugiya, J., Yoshida, K., Tamaki, Y., Yokota, M., Abo, Y., and
Kawakami, S. (1982). Teratogenicity in rats administered CGA 15'324
(profenofos) prenatally during the major organogenic period.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Thomas, R.D., Cassidy, J.E., and Marco, G.J. (1976). Metabolism and
balance study of 0-14C-CGA 15'324 in a lactating goat Project No.:
GAAC-76024. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1976). CGA 15'324 technical 21-day dermal toxicity study in rabbits.
Project No.: Siss 5119. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1977). CGA 15'324, 21-day inhalation study on the rat. Project No.:
Siss 5119. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, S.C., Marco, G.J., Simoneaux, B.J., and Ballantine, L.
(1984). Percutaneous absorption of 14C-profenofos in rats. Project
No.: ABR-84023. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1984).
Phosphorothiolate pesticides and related compounds: Oxidative
bioactivation and aging of the inhibited acetylcholinesterase.
Pesticide Biochemistry and Physiology, 21: 22-30.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1983). Oxidative
bioactivation of S-alkyl phosphorothiolate pesticides:
Stereospecificity of profenofos insecticide activation. Science, 219:
63-65.
Effects on enzymes and other biochemical parameters
Profenofos is stereospecifically converted to a more potent
inhibitor of acetylcholinesterase by mouse liver microsomal mixed-
function oxidase system. The chiral (-) isomer became a 34-fold
better inhibitor of acetylcholinesterase in vitro, while the less
toxic (+) isomer was deactivated by a factor of 2. Prior treatment
with mixed function oxidase inhibitors markedly decreased the
activation and also protected against brain acetylcholinesterase
inhibition and cholinergic symptoms resulting from (-) profenofos
administration in chicks (Wings, et al., 1983).
Toxicological studies
Acute studies
The acute toxicity of profenofos is given in Table 1. The
adverse signs of toxicity that were observed were generally similar
for each route of compound administration. These included non-
specific symptoms such as dyspnoea, exophthalmos, ruffled fur and
crooked body posture, and cholinergic symptoms such as sedation,
salivation, discharge from eyes and nose, trismus, tremors, and tonic-
clonic convulsions. The symptoms were reversible in surviving
animals.
Short-term studies
Rats
A feeding study was performed in which groups of 25 male and 25
female F344 rats received technical profenofos (90.6% a.i.) in the
daily diet for up to 13 weeks. Two control groups (basal diet) and
eleven dose groups (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300, and
1000 ppm) were used. The animals from one control group and the 0.3,
3, 30, and 300 ppm test groups (15/sex/dose level) were on test for 13
weeks. The animals in all of the other test groups were sacrificed at
weeks 2, 4 and 8 (5 to 15 rats/sex/group). The results indicated that
dose levels of profenofos of 10 to 1000 ppm produced a dose-related
inhibition of cholinesterase activity in plasma and erythrocytes (more
marked in females), that dose-levels of 100 to 1000 ppm produced
reduced food intake and a dose-related reduction in body weight gain,
and that dose-levels of 300 and 1000 ppm reduced brain cholinesterase
activity. These effects were observed in rats of both sexes.
Profenofos did not result in death of any of the treated animals. No
unusual signs of behavior or appearance were observed. Results of
haematology, clinical chemistry (excluding cholinesterase) and
urinalysis examinations were unremarkable. Necropsies performed at
the various sacrifice intervals and at termination of the study did
not reveal treatment-related gross changes.
Table 1: Acute toxicity of Profenofos1
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse M+F Oral 298 -- Bathe, 1974a
Rat M+F Oral 358-502 -- Bathe, 1974b
Kobel & Gfeffer, 1983
M+F Dermal 33002 -- Bathe, 1974c
M+F i.p. 585 -- Sachsse & Bathe, 1975
M+F inhalation -- 3-3.36 Sachsse & Ullmann, 1974a
(4hr)
Horath & Taylor, 1982
Chinese M+F Oral 153 -- Bath & Sachsse, 1979
hamster
Rabbit M+F Oral 700 -- Sachsse & Ullmann, 1974b
M+F Dermal 4722 -- Sachsse & Ullmann, 1974c
M+F Dermal 1313 -- Cannelongo, 1982a
M+F Dermal 25404 -- Kuhn, 1988
Dog M+F Oral >3005 -- Bathe, 1974d
1 All studies performed using technical grade profenofos
2 Intact skin, occlusive dressing (aluminum foil and plaster)
3 Intact and abraded skin, occlusive dressing (polyethylene film)
4 Intact skin, semipermeable dressing (orthopaedic stockinette)
5 Acute oral toxicity could not be determined in dogs due to vomiting
at doses of 300 mg/kg and higher.
Profenofos technical (purity unspecified) was administered by
inhalation (nose only exposure) to albino RAI rats for 21 days (6
hours/day, 5 days/week) at mean concentrations of 0, 68, 219 and 449
mg/m3 in air. Nine males and nine females per group were exposed.
Four males and 4 females of the control and 219 mg/m3 group were
kept for an additional 21 days without treatment (recovery group).
All rats of the 449 mg/m3 group (9/9M and 9/9F) and 1/9 females of
the 219 mg/m3 group died during the first week after showing
symptoms of exophthalmos, dyspnoea, tremor, ruffled fur, lateral
position, and irritation/ secretion of the mucous membranes of the
eyes. Microscopic exams of these rats revealed the presence of marked
congestion of the nasal mucous membranes, acute conjunctivitis, and
severe interstitial keratitis. In the remaining surviving animals of
the 68 mg/m3 and 219 mg/m3 groups there were reductions in food
consumption and body weight gain (seen in males throughout the study
and in females over the first 7 to 10 days of the study), reductions
in total plasma protein levels, and a dose-related inhibition of
cholinesterase activity in plasma, red blood cells, and brain. The
magnitude of cholinesterase inhibition ranged from -40% to -70% for
all 3 sites. In addition, alpha-1 globulin levels were reduced and
alpha-2 and beta globulin levels were increased in males of the 219
mg/m3 group. All of the above changes observed in the surviving
rats were reversible upon cessation of treatment, with the exception
of the reductions in plasma and red cell cholinesterase levels in rats
of the 219 mg/m3 group which remained about 25% below control levels
at the end of the 21-day recovery period. Finally, necropsy of the
surviving rats did not reveal any gross or microscopic pathological
changes, although the related weights of most organs were increased in
male rats due to the reduced weight gain incurred in these animals.
The NOAEL was less than 68 mg/m3 in air (lowest dose tested) in
males and females, based upon reductions in food consumption, weight
gain, and cholinesterase activity in plasma, red cells, and brain
(Ullmann, et al., 1977).
Rabbits
In a dermal toxicity study, profenofos technical (89.8% pure) was
diluted with polyethylene glycol and saline (70:30) to 2% and 5%
solutions and applied to the skin of KA46 rabbits (Himalayan strain;
3/sex/dose level) at doses of 0, 5, 20, and 100 mg/kg bw/day for 24
hours/day, 5 days/week, for 21 days. In the 0, 5, and 20 mg/kg bw/day
treatment groups, 1 rabbit/sex/dose level was kept for an additional
21 days without treatment (recovery group). All of the rabbits
treated dermally with 100 mg/kg bw/day of profenofos died within 6
days after dosing was initiated. These animals displayed moderate
erythema and oedema of the skin, reduced food intake and body weight
gain, various clinical signs of toxicity (dyspnoea, salivation,
tremors, ataxia, sedation, and curved position), and inhibition of
cholinesterase activity in plasma (-100%) and red cells (-50.3%) as
measured on day 4 prior to deaths. Histopathological examination of
the high dose animals showed focal hypertrophy, haemorrhages and fatty
changes of hepatocytes accompanied by necrosis of the liver
parenchyma, and slight to moderate atrophy of lymphoid and thymic
tissue. Microscopic examination of the skin at the site of dermal
application showed oedema, minute haemorrhages, small intradermal
pustules, focal acanthosis and parakeratosis. In the animals treated
with the lower doses (5 and 20 mg/kg bw/day) of profenofos the
observed toxic changes consisted of slight erythema and oedema at the
application site, and inhibition of cholinesterase activity in plasm,
red cells, and brain. These effects disappeared during the recovery
period. No other unusual findings were observed in the lower dose
group animals. The NOAEL was less than 5 mg/kg bw/day (lowest dose
tested) in male and female rabbits for short term dermal toxicity
(Ullmann, et al., 1976).
In a second dermal toxicity study, profenofos technical (92%
pure) was suspended in purified water containing 0.5% Tween 80 and
applied to the skin of albino rabbits (HAR:PC/CF (NZW) BR strain;
5/sex/dose level) at doses of 0, 0.05, 1 and 10 mg/kg bw/day for 6
hours/day, 5 days/week, for 21 days. No deaths occurred in any
treated animals. Profenofos administration produced slight erythema at
the application site in mid dose males and females, and in high dose
males. Additional changes that appeared to be treatment-related were
observed at the high dose level and included hyperactivity and soft
faeces in males and/or females, significant reductions of
cholinesterase activity (measured at terminal sacrifice) in plasma,
red blood cells, and brain, increases in serum bilirubin (males) and
gamma GT (females) levels, and a decrease in serum sodium levels
(males). No unusual histopathological findings occurred. The NOAEL
for subchronic dermal toxicity (excluding the occurrence of slight
dermal erythema at the mid dose) was 1 mg/kg bw/day in males and
females (Johnson, et al., 1984).
Dogs
In a 6-month study, groups of 7 male and 7 female pedigreed
beagles were fed diets containing 0, 0.2, 2, 100, or 500 ppm of
technical profenofos (88.1%-89.3% pure). One male and one female of
each group was kept for an additional 28 days on control diet
(recovery groups). The administered concentrations were calculated to
be equivalent to 0, 0.007, 0.05, 2.9, and 14.4 mg/kg bw/day in dogs of
both sexes. Standard examinations for clinical signs of toxicity,
ophthalmology, haematology, clinical chemistry (including plasma,
brain, and red cell cholinesterase activity and plasma and liver
carboxylesterase activities) and urinalysis parameters were performed
at regular intervals throughout the study. The animals were subjected
to a battery of neurological exams (e.g., muscle strength and tone,
reflexes, etc.), necropsied, and examined grossly for organ changes
and histologically for tissue changes.
Adverse findings were observed at dose levels of 2 ppm to 500 ppm
and consisted of cholinesterase inhibition in plasma (males and
females at 2, 100, and 500 ppm bw/day) and red cells (males and
females at 100 and 500 ppm), carboxylesterase inhibition in plasma and
liver (males at 100 and 500 ppm), reductions in erythrocyte,
haemoglobin and haematocrit levels (males and females at 500 ppm; also
occasionally at 100 ppm), and reduced food consumption (males at 500
ppm over weeks 0-3). The depressed haematological values were said to
be within physiological limits, but it was noted that they did not
increase with age as was the case in the control and lower dose group
animals. All those effects appeared to be reversible during the 4-
week recovery period. Aside from these changes, there were no other
compound-related effects.
Long-term/carcinogenicity studies
Mice
A long-term feeding study of profenofos technical (90.6% pure)
was performed in albino mice (HaM/ICR Swiss, CR-CD 1 strain) in which
dietary concentrations of 0, 1, 30, and 100 ppm were fed to 60
males/dose group for 85 weeks and 60 females/dose group for 96 weeks.
The original design of the study was for 104 weeks, but because
survival had reached 20% of the original number of mice in the mid-
dose males and in the high-dose females due to accidental causes, the
sacrifices were initiated early. An additional 5 mice/sex/dose were
studied at each dose level and were sacrificed after 52 weeks for the
determination of cholinesterase activity in erythrocytes, plasma and
brain; these animals were not examined histopathologically. The
administered concentrations were calculated to be equivalent to 0,
0.14, 4.5, and 14.2 mg/kg bw/day in males and 0, 0.19, 5.8, and 19.2
mg/kg bw/day in females.
There were no clinical signs of toxicity and no significant
effect of treatment on mortality. There were no treatment related
effects on food consumption or body weight gain, or at gross necropsy
or after histopathological examination. There were no increases in
tumors that appeared to be related to compound administration. With
respect to clinical laboratory examinations, a significant dose-
related inhibition of erythrocyte and plasma cholinesterase activity
(generally ranging from -38% to -76%) was observed in mid- and high-
dose males and females at week 53 (interim sacrifice animals) and at
the termination of the study (week 85 in males and week 97 in
females). In addition, brain cholinesterase activity was
significantly inhibited (about -25%) in high-dose females at the end
of the study, whereas a trend (not significant) for this effect was
observed in the males. The NOAEL for male and female mice was 1 ppm
in the diet (0.14 mg/kg bw/day in males and 0.19 mg/kg bw/day in
females) based upon cholinesterase inhibition (Burdock, et al.,
1981a).
Rats
Fischer 344 albino rats (60/sex/group) were fed profenofos
technical (90.6% pure) at doses of 0, 0.3, 10, and 100 ppm for 2
years. An additional 10 rats/sex were also included in the control
and high dose treatment groups, respectively. Of these, 5/sex/group
were sacrificed at 52 weeks (interim sacrifice), and 5/sex/group were
placed on control feed after 52 weeks so that recovery studies could
be conducted and these were then sacrificed during week 63 (recovery
animals). All of the animals in the study were examined
histopathologically. Plasma and erythrocyte cholinesterase activity
was determined in 10 rats/sex/dose group (main study group) at weeks
13, 26, 52, 78, and 105 and in the recovery animals at week 57. Brain
cholinesterase activity was determined in the interim and terminal
sacrifice animals at weeks 53 and 105. The administered
concentrations of profenofos were calculated to be equivalent to 0,
0.017, 0.56, and 5.7 mg/kg bw/day in males and 0, 0.02, 0.69, and 6.9
mg/kg bw/day in females.
There were no treatment-related clinical signs of toxicity,
deaths, or changes in body weight gain. No sustained changes in
clinical chemistry parameters occurred which were considered to be
treatment-related. A dose-related inhibition of plasma and
erythrocyte cholinesterase was observed in rats of both sexes at the
mid- and high-dose levels; these effects were considered to be
compound-related and were reversible as judged by their absence in the
recovery group animals. Brain cholinesterase was not altered by
profenofos in this study. Additional changes seen only at the high
dose level included an increase in food consumption in females, an
increase in relative thyroid gland weight in high dose males (seen in
interim sacrifice and recovery group animals but not in terminal
sacrifice animals, and not considered biologically significant), an
increase in thyroid gland perifollicular cell hyperplasia in high dose
males (i.e., 4/70 controls vs. 10/70 high dose), and an increase in
liver neoplastic nodules in high dose females (i.e., 1/70 controls;
3/60 low dose; 2/60 mid dose; and 6/70 high dose). No increase in
liver carcinomas occurred. The latter histopathological findings were
not considered to be compound-related changes. The NOAEL was 5.7
mg/kg bw/day (the highest dose tested).
Reproduction study
Groups of male (8/sex/dose) and female (16/sex/dose) albino rats
in each of three generations (F0, F1, and F2) were fed diets
containing 0, 0.2, 1.0, or 20 ppm profenofos (technical grade; 95.5%
pure). The dietary concentrations were equivalent to 0, 0.01, 0.05,
and 1.0 mg/kg bw/day. Parental animals were allowed to mature for 100
days, mate, and produce 2 litters. Eight males and 16 females from
the second litters were retained at weaning as parental animals for
the succeeding generations. The study was terminated following the
weaning of the F3b litters. Profenofos was administered
continuously through the experiment.
There were no compound related effects on body weight, mortality,
behavior, or various parameters measuring fertility (fecundity index,
male or female fertility index) in any of the parenteral animals.
Statistically significant reductions in cholinesterase occurred at 20
ppm in erythrocytes (F0, F1, and F2 males and females) and
plasma (F0 females). In contrast, statistically increased plasma
cholinesterase activity was seen in F0 females given 0.2 and 1 ppm
and F1 males given 1 ppm, and brain cholinesterase activity was
increased in F1 males and females given 1 and 20 ppm. No adverse
effects occurred in progeny with respect to litter size, viability,
body weight, cholinesterase activity in erythrocytes, plasma or brain,
or development throughout the study. Postmortem gross and
histopathologic examinations of parenteral animals and F3b weanlings
were unremarkable (IBTL, Inc., 1978). The NOAEL for reproductive
effects was greater than 1.0 mg/kg bw/day in the diet (highest dose
tested).
Special studies on embryo/fetotoxicity
Rats
Groups of 20-27 pregnant rats (strain unspecified) were
administered profenofos (technical grade; purity unspecified) via oral
gavage at dose levels of 0, 10, 30, and 60 mg/kg bw from days 6 to 15
of gestation (day 0 = day either spermatozoa or vaginal plug found).
On day 21 of gestation, all dams were sacrificed and fetuses delivered
by caesarean section. Maternal toxicity was evident in the high-dose
groups as indicated by a marked decrease in food consumption during
the period of treatment. No other adverse effects occurred in the
dams. Similarly, none of the doses of profenofos appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. The NOAEL for maternal toxicity was 30 mg/kg and that for
fetotoxicity/teratogenicity was 60 mg/kg by oral gavage (Fritz,
1974b).
Groups of 23-25 pregnant rats (JCL-SD strain) received profenofos
(technical grade; 95.8% purity) via intubation at dose levels of 0,
18, 35, or 70 mg/kg bw/day from days 7 to 17 of gestation (day =day
that a sperm plug was found). On day 21 of gestation, all dams were
sacrificed under ether anaesthesia and fetuses delivered by caesarean
section. The doses of profenofos selected for testing were based upon
the results of a preliminary range finding study in which 140 mg/kg
bw/day caused death in 5/6 treated rats during days 8 to 15 of
pregnancy. Profenofos administration was associated with increases in
body weight and water consumption in the dams at doses of 35 and 70
mg/kg bw/day on days 17 to 21, and an increased level of food
consumption at 70 mg/kg bw/day on days 14 to 21; these changes did not
appear to be deleterious. Although small increases in the weights of
several organs (heart, spleen, liver, and right kidney) were seen at
70 mg/kg bw/day, these were small in magnitude. No treatment-related
changes in mortality or behavior were observed, and no abnormal
findings were observed at gross necropsy. There were no adverse
effects on the offspring with respect to resorptions, sex ratios,
placental weights, body weights and lengths, or distribution of
fetuses within the uterine horns. External and visceral examinations
of fetuses were unremarkable. Skeletal examination of fetuses showed
increased incidences of progeny with holes in the xiphoid at the mid
and high doses (0% controls, 0% low dose, 18.8% mid dose, and 15.6%
high dose) and delayed ossification of vertebral arches at the high
dose (8.8% controls, 6.7% low dose, 0.5% mid dose, and 26.7%). No
historical control data was provided and it could not be determined if
the findings were all from one litter or from multiple litters. The
NOAEL for maternal and developmental toxicity appeared to be 70 mg/kg
bw/day by intubation (Sugiya et al., 1982).
Groups of 25 pregnant rats (Sim:(SD)fBR strain) received
profenofos (technical grade; 88.0% purity) via oral gavage at dose
levels of 0, 10, 30, 60, 90, and 120 mg/kg bw/day from days 6 to 15 of
gestation (day 0 = day either sperm or vaginal plug found). On day 21
of gestation, all dams were sacrificed using CO2 and fetuses
delivered by caesarean section. Maternal toxicity occurred at the
high dose level as evidenced by increased mortality, reduced food
consumption, and various clinical signs of toxicity (e.g.,
hypoactivity or tremors, ocular porphyrin discharge, diarrhoea,
dyspnoea, diuresis, and hypothermia). Two of the 4 dams that died
displayed these clinical signs whereas the other 2 did not. In
addition, 2 of the dams that died also showed scattered haemorrhages
in the stomach upon gross necropsy. None of the other doses tested
produced changes in the pregnancy ratio, percentage of live or dead
fetuses, number of resorptions, or live fetal weights or sex ratios.
Similarly none of the dose appeared to affect embryonic or fetal
development and no teratogenic effects were observed. The NOAEL for
maternal toxicity was 90 mg/kg bw/day (Harris and Holson, 1982).
Rabbits
Groups of 20 pregnant Chinchilla rabbits were administered
profenofos (technical grade; 89.5% purity) via intubation at dose
levels of 0, 5, 15, and 30 mg/kg bw from days 6 to 18 of gestation
(day 0 = day of mating). On day 28 of gestation, all dams were
sacrificed by cervical dislocation and fetuses delivered by caesarean
section. With the exception of a marginal reduction in food
consumption from day 6 onward there were no unusual effects of
profenofos on the does. In addition, no adverse prenatal effects,
malformations, or variations were observed. The NOAEL for both
maternal and development toxicity was 30 mg/kg bw/day by intubation
(Fritz, et al., 1979).
Groups of 16 pregnant New Zealand White rabbits were given
profenofos (technical grade; 90.8% purity) by oral gavage at dose
levels of 0, 30, 60, 90, and 175 mg/kg bw from days 6 to 18 of
gestation (day 0 = day of mating). On day 30 of gestation, all does
were euthanized and fetuses delivered by caesarean section. The doses
of profenofos selected for testing were based upon the results of a
preliminary range finding study in which doses up to 150 mg/kg bw did
not produce any signs of toxicity. Profenofos administration was
associated with reduced maternal weight gain and food consumption at
doses of 60 mg/kg bw or more, clinical signs of toxicity (e.g.
diarrhoea, soft stools, oral/perianal discharges) at 90 mg/kg bw or
more, and deaths in 9/16 (56.3%) of the does at 175 mg/kg bw. Many of
the does that died exhibited the above clinical signs of toxicity as
well as signs of pinpoint stomach haemorrhages and yellow-discolored
areas in the mesentery in the gastric region upon gross necropsy.
None of the doses tested produced changes in maternal pregnancy rates,
in prenatal effects (e.g., percentage of live fetuses and live
fetuses/litter, resorptions, litter size, fetal body weight, and sex
ratios), or in malformations or variations. The NOAEL for maternal
toxicity was 30 mg/kg bw/day and that for developmental toxicity was
175 mg/kg bw/day (Holson, 1983).
Special studies on eye and skin irritation
In two studies in rabbits, instillation of 0.1 ml of profenofos
(undiluted technical material) into the conjunctival sac for 30
seconds produced mildly irritating conjunctival reactions (redness and
chemosis) which generally lasted for periods of up to 24 to 48 hours
before dissipating (Sachsse and Ullmann, 1974e; Cannelongo, 1982d).
In one of the tests, 2 of 6 treated rabbits died within 3 days without
apparent symptoms (Sachsse and Ullmann, 1974e).
Profenofos (0.5 ml of undiluted technical grade formulation)
produced death in one rabbit and slight to severe erythema in 2 of 5
surviving rabbits when applied to shaved and abraded skin for 24
hours. The treated animals displayed toxic signs manifested as
lateral and curved position, asynchronisms of the extremities, muscle
spasms, and apathy (Sachsse and Ullmann, 1974d).
In a second skin irritation study, application of profenofos (0.5
ml of undiluted technical grade formulation) to intact and abraded
rabbit skin produced death in 6 of 6 animals studied within 24 to 72
hours. Slight skin irritation (erythema and oedema) was observed
(Cannelongo, 1982b).
Special studies on genotoxicity
Profenofos was negative in a variety of in vitro tests in
bacteria, yeast, and mammalian cell systems that evaluated potential
activity to produce gene mutations, gene conversion, mitotic crossing
over, non-disjunction, and unscheduled DNA synthesis. Profenofos was
also negative in two in vitro tests, a dominant lethal assay in mice
and a nucleus anomaly test in Chinese hamsters. However, the compound
was associated with the production of chromosome aberrations,
micronucleus induction, and sister chromatid exchanges in the bone
marrow at oral doses ranging from 36 to 216 mg/kg bw in a third
in vivo study in mice. The summary results of genotoxicity studies
with profenofos are presented in Table 2.
Special studies on skin sensitizing effect
Profenofos was examined for skin sensitizing effects in 2 studies
in guinea pigs. Negative results were obtained in one test when
animals received a series of 10 intracutaneous induction injections
followed 14 days later by a single challenge injection of profenofos
(0.1 ml of a 0.1% technical formulation). A vehicle control group was
also negative whereas dinitrochlorobenzene (positive control) produced
marked sensitization (Sachsse and Ullmann, 1974f). Positive results
for dermal sensitization (i.e., very light erythema or oedema) were
occasionally obtained in another test when animals received a series
of 11 intradermal induction injections followed 14 days later by a
single challenge injection (0.1 ml of 0.1% technical formulation) of
profenofos. Although 2,4-dinitrochlorobenzene (positive control) was
consistently active in this study, a concurrent negative control group
was not employed to facilitate an evaluation of the results
(Cannelongo, 1982d).
Special studies on neurotoxicity
Delayed neurotoxic effects of oral doses of 21.7, 46.4, and 60
mg/kg bw/day of technical profenofos were assessed in adult domestic
chickens. The compound was administered twice, 21 days apart. The
acute oral LD50 of the formulation was about 35 mg/kg bw. Only the
birds of the low dose group survived the two treatments with
profenofos. Signs of toxicity were also similar after both treatments
with profenofos (e.g., salivation, asynchronisms of the extremities,
curved position, apathy, and ruffled feathers); these were observed at
the mid and high doses levels after the initial treatment on day 0,
and also at the low-dose level after the second treatment on day 21.
Neither delayed neurotoxic symptoms nor histologic changes in spinal
cord or peripheral nerve were observed. A positive control group
receiving 1000 and 2150 mg/kg bw TOCP showed the expected reactions
(i.e., ataxia, deterioration of reflexes, and swelling, fragmentation
and disruption of myelin sheaths) (Krinke et al., 1974).
Table 2: Results of genotoxicity assays on profenofos.
Test System Test Object Concentration Purity Results Reference
of profenofos
Ames Test1 S. typhimurium 5,15,45,135, 405 µg/0.1 ml in DMSO ? Negative Arni and Muller, 1978
(reverse mutation) TA-98, TA-100,
TA-1535, TA-1537
Yeast Test1 S. cerevisae Nonactivated: 91.8% Negative Arni and Muller, 1982
(gene conversion, crossing (D7 strain) 12.5-500 µg/ml in DMSO
over, reverse mutation) Activated: 0.640 -10000 µg/ml in DMSO
Yeast Test1 S. cerevisae 39,156,625, 2500, 10000 µg/ml in DMSO 90.0% Negative Hool and Muller, 1986
(non-disjunction) (D61.M strain)
Mouse Lymphoma L5178Y mouse 0.078, 0.156, 0.313, 0.625 µg/ml in DMSO 91.8% Negative Strasser and Muller, 1982
Forward Mutation lymphoma cells
Assay1 (TK +/-)
DNA Repair Rat hepatocytes 0.016, 0.08, 0.4, 2 nl/ml 91.8% Negative Puri and Muller, 1982a
Test2 (UDS)3
DNA Repair Human fibroblasts 0.32, 1.6, 8, 40 nl/ml 91.8% Negative Puri and Muller, 1982b
Test2 (UDS)3
Dominant Lethal Test Mouse (male) 35, 100 mg/kg ? Negative Fritz, 1974a
(NMRI-derived) (single oral doses)
Nucleus Anomaly Test Chinese hamster 13, 26, 52 mg/kg (2 oral doses on 88.1% Negative Hool, et al., 1981
bone marrow consecutive days)
Table 2 (contd)
Test System Test Object Concentration Purity Results Reference
of profenofos
Somatic Cell Studies Male Swiss mouse 36, 162, 216 mg/kg (single oral doses) 72% Positive4 El Nahas, et al., 1988
in Mice (sister chromatid bone marrow
exchange, micronucleus,
chromosome aberration)
1 With and without metabolic activation.
2 No exogenous activation added.
3 UDS = unscheduled DNA synthesis.
4 Doses of 36-216 mg/kg produced increases in chromosome aberrations; doses of 162-216 mg/kg increased micronuclei formation and sister
chromatid exchanges.
In a second study, adult chickens received oral doses of 29.2,
58.5, 117, and 234 mg/kg bw of profenofos (38% EC) on days 0 and 21.
The acute oral LD50 of the formulation was 127.0 mg/kg bw. After
treatment on day 0, death occurred in 0%, 6.6%, 55%, and 100% of the
birds given doses of 29.2, 58.5, 117 and 234 mg/kg bw/day,
respectively. The increased mortality was accompanied by signs of
cholinesterase inhibition (i.e., weakness, lethargy, and anorexia).
After treatment on day 21, death occurred in 0%, 7.1%, and 67% of the
surviving birds given doses of 29.2, 58.5 and 117 mg/kg bw/day, and
similar signs of cholinesterase inhibition were observed. There were
no delayed neurotoxic symptoms or histologic changes in brain, spinal
cord, or sciatic nerves. A positive control group using 500 mg/kg bw
of TOCP exhibited clinical signs of delayed neurotoxicity (extreme
weakness of legs and wings) and neuropathological lesions in the
spinal cord and sciatic nerves (axonal degeneration and demyelination)
(Fletcher et al., 1977).
Profenofos (technical, 89.5% purity) was examined for delayed
neurotoxicity in a third study in adult chickens. In the first phase
of the study (day 0), birds received oral doses of 30 and 45.7 mg/kg
bw on the basis of preliminary acute toxicity studies in which an
acute LD50 dose of 45.7 mg/kg bw was determined. However, because
of increased mortality rates at these dose levels (70% at 30 mg/kg bw,
and 82% at 45.7 mg/kg bw), all surviving birds were redosed on day 21
at a new estimated oral LD50 of 17.1 mg/kg bw. At this dose level
of profenofos, a mortality rate of 5% was observed. The results
indicated that profenofos produced signs of lethargy and salivation in
treated birds, but no signs of delayed neurotoxicity or histological
lesions in the brain, spinal cord, or peripheral nerves. A positive
control group using 500 mg/kg bw of TOCP exhibited clinical signs of
delayed neurotoxicity (extreme weakness of legs and wings) and
lesions in the spinal cord and sciatic nerves (axonal degeneration and
demyelination) (Reinart et al., 1978).
Special studies on pesticide antagonistic agents
A protective effect of atropine given early after orally
administered profenofos in rats or intraperitoneally administered
profenofos in chicks and mice was demonstrated by a reduction in
mortality and toxic signs (e.g., salivation, tremors, sedation,
convulsions) typical of anticholinesterase exposure. The effect of
oximes was limited (Sachsse and Bathe, 1976; Gfeller and Kobel, 1974;
Glickman et al. 1984).
Special studies on pesticide interactions
No potentiating effects were found when mixtures of profenofos
and the organophosphates methidathion, methacrifos or diazinon were
given to rats in equitoxic doses (Sachsse and Bathe, 1977); (Sachsse
and Bathe, 1978).
Profenofos administered intraperitoneally to mice at 0.5 to 5.0
mg/kg bw strongly inhibited the liver microsomal esterase(s)
hydrolysing trans-permethrin. The intraperitoneal toxicity LD50
measured 24 hours after exposure of fenvalerate and malathion but not
that of trans-permethrin was greatly increased when the compounds were
given 1 hour after an intraperitoneal injection of 25 mg/kg bw of
profenofos (Gaughan et al., 1980).
Observations in humans
A group of six male spraymen, aged 20 to 25 years, was monitored
for whole blood cholinesterase activity over a period of 4 days while
treating fully grown cotton with a formulation containing 400 grams
profenofos and 40 grams cypermethrin per liter in organic solvents on
a plantation in the Multan region of Central Pakistan in September
1985. The application equipment consisted of hand-held battery
operated spinning disc devices. The workers wore heavy cotton shirts
with long sleeves, legs wrapped below the knees, and turbans which
were often used to cover the face. Cholinesterase activity was
measured at the end of each of the 4 work days after thorough washing
of the body. The results indicated that there was a tendency toward
inhibition of blood cholinesterase activity. Compared to baseline
pre-exposure cholinesterase readings, the average cholinesterase
levels were 103.3%, 84.8%, 85.6%, and 81.2% of control activities on
days 1, 2, 3, and 4, respectively. The lowest individual values were
72.7% and 73.1% of control levels on the fourth day in 2 of the 6
workers (Loosli, 1989).
No cases of poisonings in humans have been reported with either
the active ingredient per se or with profenofos formulations.
COMMENTS
Profenofos administered orally to rats was well absorbed and was
excreted primarily in the urine, but also in faeces. Profenofos was
biotransformed by a major pathway involving side chain depropylation,
desulfuration, and phenyl-ester bond cleavage to yield 4-bromo-2-
chlorophenol, and by a minor pathway involving side chain 0-de-
ethylation, and subsequent phenyl-ester bond cleavage to the above
phenol. Both pathways culminated in conjugation with glucuronic and
sulfuric acids. No unchanged parent compound was found in urine.
Faeces contained only minute amounts of the parent compound, the
intermediate phenol, and unidentified metabolites. A similar
absorption and excretion pattern was observed in hens and goats. No
unusual organ or tissue localization of 14C-labelled profenofos was
observed.
Profenofos has a moderate order of acute toxicity following oral
and dermal administration. It has been classified as moderately
hazardous by WHO (WHO, 1990).
In a dietary toxicity study in which rats were fed profenofos for
8-13 weeks, the NOAEL was 100 ppm (equivalent to 5 mg/kg bw/day) based
on the finding of brain cholinesterase inhibition at higher levels.
The observed reduction in erythrocyte cholinesterase at lower doses
was not considered to be of toxicological importance. Reduction in
food consumption and body weight gain were considered to be a
consequence of poor palatability of the compound.
In a six-month feeding study in dogs, profenofos caused
erythrocyte cholinesterase inhibition and plasma and liver carboxylase
inhibition at levels of 100 ppm or more. The highest dose level
tested, 500 ppm, resulted in reductions in erythrocyte counts,
haemoglobin and haematocrit levels and a decrease in food consumption.
All of the changes were reversible. No changes in brain
cholinesterase activity occurred. As noted above, enzyme inhibition
and reduced food consumption were not considered to be direct toxic
effects of profenofos administration. The NOAEL was 100 ppm (equal to
2.9 mg/kg bw/day) based upon the observed haematological changes.
In long-term feeding studies in mice and rats, no treatment-
related increases in the incidence of neoplasms was observed. In
mice, brain cholinesterase inhibition occurred at the highest dose
level tested, 100 ppm. The NOAEL was 30 ppm (equal to 5.8 mg/kg
bw/day in females) based on brain cholinesterase inhibition observed
at the next higher dose level in females. In rats erythrocyte
cholinesterase inhibition occurred at dietary levels of 10 and 100
ppm. The highest dose level of 100 ppm was also associated with an
increase in food consumption and a slightly elevated incidence of
parafollicular cell hyperplasia. This type of response is not seen in
man following exposure to chemicals. No effect on brain
cholinesterase was observed. The NOAEL was 100 ppm (equal to 5.7
mg/kg bw/day).
In a three-generation reproduction study in rats, reductions in
cholinesterase activity occurred in erythrocytes (F0, F1, and F2
males and females) at 20 ppm in the diet. This was the highest dose
tested. No changes in brain cholinesterase activity occurred. The
NOAEL for systemic toxicity and reproduction was 20 ppm (equivalent to
1.0 mg/kg bw/day) which was the highest dose tested.
In three teratology studies in rats the NOAELs for maternal
toxicity ranged from 30 to 90 mg/kg bw/day, based upon findings of
reduced food consumption, clinical signs of toxicity (e.g., diarrhoea,
dyspnoea, tremors, hypothermia) and increased mortality at higher dose
levels. No teratogenic activity was observed at dose up to and
including 120 mg/kg bw/day. The NOAEL for embryotoxicity/fetotoxicity
(based on an increased incidence of variants) was 18 mg/kg bw/day. In
two rabbit teratology studies, the NOAEL for maternal toxicity was 30
mg/kg bw/day, based upon findings of reduced food consumption and body
weight gain, the occurrence of soft stools and diarrhoea, and
increased mortality at higher dose levels. No embryotoxic, fetotoxic,
or teratogenic activity was observed at doses up to and including 175
mg/kg bw/day.
After reviewing all available in vitro and in vivo short-term
tests, the Meeting concluded that there was no evidence of
genotoxicity.
The Meeting based the ADI for profenofos on the NOAEL of 1 mg/kg
bw/day in the rat multigeneration study. Although there was no
evidence of any adverse effects at this dose level in the study, the
absence of information on reproductive parameters at higher levels
precluded the use of the NOAEL of 2.9 mg/kg bw/day in the 6-month
feeding study in dogs.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 30 ppm, equal to 5.8 mg/kg bw/day in females.
Rat: 20 ppm, equivalent to 1.0 mg/kg bw/day (reproduction).
Rat: 100 ppm, equal to 5.7 mg/kg bw/day in males (long-term
study).
Dog: 100 ppm, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in
the continued evaluation of the compound
Further observations in humans.
An additional multigeneration study in rats using higher doses.
REFERENCES
Arni, P. and Muller, D. (1978). Salmonella/mammalian-microsome test
with CGA 15'324 (Test for mutagenic properties in bacteria). Project
No.: 78/2531. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Arni, P. and Muller, D. (1982). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro with CGA 15'324 (Test for
mutagenic properties in yeast cells). Project No.: 911557.
Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Bathe, R. (1974a). Acute oral LD50 of technical CGA 15'324 in the
mouse. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974b). Acute oral LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974c). Acute dermal LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. (1974d). Acute oral LD50 of technical CGA 15'324 in the
dog. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. and Sachsse, K. (1979). Acute oral LD50 in the Chinese
hamster CGA 15'324. Project No.: 785329. Unpublished report from
Ciba-Geigy Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Kuper, C.H. (1981a). Twenty-four months carcinogenicity study in
mice, CGA 15'324. Project No.: 483-133. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Fieser, S. (1981b). Two-year chronic oral toxicity study in albino
rats, CGA 15/324 technical. Project No.: 483-134. Unpublished report
from Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B.F. (1982a). Rabbit acute dermal toxicity CGA 15'324
technical FL 811584. Project No.: 2460-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F., (1982b). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2462-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982c). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2461-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982d). Guinea pig sensitization CGA 15'324
technical FL 811584. Project No.: 2463-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
El Nahas, S.M., De Hondt, H.A., and Ramadan, H.A.I. (1988). In vivo
evaluation of the genotoxic potential of Curacron in somatic cells of
mice. Environ. Mol. Mutagen., 11(4): 515-522.
Fletcher, D., Gordon, D.E., Jenkins, D.H., Kennedy, G.L., Kinoshita,
F.K., and Phillips, B.M. (1977). Neurotoxicity study with CGA 15'324
38% E.C. in chickens. Project No.: IBT 8580-10426. Unpublished
report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL,
USA. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland and
validated by the United States Environmental Protection Agency.
Fritz, H. (1974a). Dominant lethal study on CGA 15'324 tech., mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No.: 327438. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H. (1974b). Reproductive study - technical CGA 15'324, rat,
segment II (test for teratogenic or embryotoxic effects). Project
No.: 22741900. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H., Becker, H., and Hess, R. (1979). Seg. II reproductive
study in rabbits, CGA 15'324 tech. Project No.: 785565. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gaughan, L.C., Engel, J.L., and Casida, J.E. (1980). Pesticide
interactions: Effects of organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology 14: 81-85.
Gfeller, W. and Kobel, W. (1984). CGA 15'324 technical, tentative and
antilethal study in the rat. Project No.: GU 820034. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gfeller, W., Kobel, W., Schaeppi, U., Zak, F., and Hess, R. (1981).
CGA 15'324 tech., 6-months toxicity study with dogs. Project No.:
790804. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Glickman, A.H., Wing, K.D., and Casida, J.E., (1984). Profenofos
insecticide bioactivation in relation to antidote action and the
stereospecificity and acetylcholinesterase inhibition, reactivation,
and aging. Toxicity and Applied Pharmacology, 73: 16-22.
Harris, S.B. and Holson, J.F. (1982). A teratology study of CGA
15'324 technical in albino rats. Project No.: 282009. Unpublished
report from Science Applications, Inc., La Jolla, CA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Holson, J.F. (1983). Teratology study (Seg II) in albino rabbits with
CGA 15'324 technical. Project No.: 283003. Unpublished report from
Science Applications, Inc., La Jolla, CA, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., and Muller, D. (1981). Nucleus anomaly test in
somatic interphase nuclei, CGA 15'324, Chinese hamster (test for
mutagenic effects on bone marrow cells). Project No.: 791557.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. and Muller, D. (1986). Test for non-disjunction on
Saccharomyces cerevisiae D61.M in vitro, CGA 15'324 tech. Project
No.: 850811. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Horath, L.L. and Taylor, G.D. (1982). Acute aerosol toxicity study in
rats of CGA 15'324 technical FL 811528. Project No.: 420-0921.
Unpublished report from Toxi-Genics, Inc., Decatur, IL, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
IBTL, Inc. (1978). Three generation reproduction study with CGA
15'324 technical in albino rats. Project No.: IBT 623-07924.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
Northbrook, IL, USA. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland. Validated by United States Environmental Protection
Agency.
Ifflaender, U. and Mucke, W. (1976). The metabolism of CGA 15'324 in
the rat. Project No.: 27/76. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Ifflaender, U., Mucke, W., and Esser, H.O. (1974). Distribution,
degradation and excretion of CGA 15'324 in the rat. Project No.:
16/74. Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Johnson, S., Tai, C.N., and Katz, R. (1984). Profenofos technical,
21-day dermal toxicity study in rabbits. Project No.: MIN 842008.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kobel, W. and Gfeller, W. (1983). Acute oral LD50 in the rat, CGA
15'324 tech. GU Project No.: 830188. Unpublished report from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Krinke, G., Ullmann, L., and Sachsse, K. (1974). Acute oral LD50
and neurotoxicity study of technical CGA 15'324 in the domestic fowl
(Gallus domesticus). Project No.: Siss 2850. Unpublished report from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1988). Acute dermal toxicity study in rabbits, profenofos
technical. Project No.: 5522-88. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Loosli, R. (1985). Project No.: 850910. Unpublished summary report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mucke, W. (1986). The renal excretion of U-14C-Phenyl CGA 15'324 by
male rats after oral administration (exposure monitoring). Project
No.: 13/86. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Oakes, T.L., Marco, G.J., and Ballantine, L. (1986). Metabolism of
14C-profenofos in chickens dosed at 5.0 ppm. Project No.: ABR-
86002. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Piccirillo, V.J. (1978). 90-day subacute oral toxicity study in rats,
CGA 15'324 technical. Project No.: 483-135. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. and Muller, D. (1982a). Autoradiographic DNA repair test on
rat hepatocytes, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811490. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Puri, E. and Muller, D. (1982b). Autoradiographic DNA repair test on
human fibroblasts, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811658. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Reinart, D., Fletcher, D., Arceo, R.J., and Gordon, D.E. (1978). 42-
day neurotoxicity study with CGA 15'324 technical in adult chickens.
Project No.: IBT 8580-11187. Unpublished report from Industrial
Bio-Test Laboratories, Inc., Decatur, IL. USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland and validated by the United States
Environmental Protection Agency.
Sachsse, K. and Bathe, R. (1975). Acute intraperitoneal LD50 in the
rat of technical CGA 15'324. Project No.: Siss 5048. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1976). The therapeutic activity of
pralidoxim (PAM) and TOXOGONIN\ with regard to CGA 15'324 in the rat.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1977). Potentiation study CGA 15'324
versus 2 insecticides CA 13'005 (methidathion) and G 24'480 (diazinon)
in the rat. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1978). Potentiation study CGA 20'168
versus 6 insecticides, C 177 (DDVP), C570 (phosphamidon), GS 13'005
(methidation), G 24'480 (diazinon), CGA 15'324 and malathion. Project
No.: 404478 - Siss 6526. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. and Ullmann, L. (1974a). Acute inhalation toxicity of
technical CGA 15'324 in the rat. Project No.: Siss 3647.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974b). Acute oral LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647 Unpublished report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974c). Acute dermal LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974d). Skin irritation in the rabbit
after single application of technical CGA 15'324. Project No.: Siss
3647. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974e). Irritation of technical CGA
15'324 in the rabbit eye. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974f). Skin sensitizing (contact
allergenic) effect in Guinea pigs of technical CGA 15'324. Project
No.: Siss 3647. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F.F. and Muller, D. (1982). L5178/TK+/- mouse lymphoma
mutagenicity test, CGA 15'324 (in vitro test for mutagenic
properties of chemical substances in mammalian cells). Project No.:
811491. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sugiya, J., Yoshida, K., Tamaki, Y., Yokota, M., Abo, Y., and
Kawakami, S. (1982). Teratogenicity in rats administered CGA 15'324
(profenofos) prenatally during the major organogenic period.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Thomas, R.D., Cassidy, J.E., and Marco, G.J. (1976). Metabolism and
balance study of 0-14C-CGA 15'324 in a lactating goat Project No.:
GAAC-76024. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1976). CGA 15'324 technical 21-day dermal toxicity study in rabbits.
Project No.: Siss 5119. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1977). CGA 15'324, 21-day inhalation study on the rat. Project No.:
Siss 5119. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, S.C., Marco, G.J., Simoneaux, B.J., and Ballantine, L.
(1984). Percutaneous absorption of 14C-profenofos in rats. Project
No.: ABR-84023. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1984).
Phosphorothiolate pesticides and related compounds: Oxidative
bioactivation and aging of the inhibited acetylcholinesterase.
Pesticide Biochemistry and Physiology, 21: 22-30.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1983). Oxidative
bioactivation of S-alkyl phosphorothiolate pesticides:
Stereospecificity of profenofos insecticide activation. Science, 219:
63-65.
See Also:
Toxicological Abbreviations
 Effects on enzymes and other biochemical parameters
Profenofos is stereospecifically converted to a more potent
inhibitor of acetylcholinesterase by mouse liver microsomal mixed-
function oxidase system. The chiral (-) isomer became a 34-fold
better inhibitor of acetylcholinesterase in vitro, while the less
toxic (+) isomer was deactivated by a factor of 2. Prior treatment
with mixed function oxidase inhibitors markedly decreased the
activation and also protected against brain acetylcholinesterase
inhibition and cholinergic symptoms resulting from (-) profenofos
administration in chicks (Wings, et al., 1983).
Toxicological studies
Acute studies
The acute toxicity of profenofos is given in Table 1. The
adverse signs of toxicity that were observed were generally similar
for each route of compound administration. These included non-
specific symptoms such as dyspnoea, exophthalmos, ruffled fur and
crooked body posture, and cholinergic symptoms such as sedation,
salivation, discharge from eyes and nose, trismus, tremors, and tonic-
clonic convulsions. The symptoms were reversible in surviving
animals.
Short-term studies
Rats
A feeding study was performed in which groups of 25 male and 25
female F344 rats received technical profenofos (90.6% a.i.) in the
daily diet for up to 13 weeks. Two control groups (basal diet) and
eleven dose groups (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300, and
1000 ppm) were used. The animals from one control group and the 0.3,
3, 30, and 300 ppm test groups (15/sex/dose level) were on test for 13
weeks. The animals in all of the other test groups were sacrificed at
weeks 2, 4 and 8 (5 to 15 rats/sex/group). The results indicated that
dose levels of profenofos of 10 to 1000 ppm produced a dose-related
inhibition of cholinesterase activity in plasma and erythrocytes (more
marked in females), that dose-levels of 100 to 1000 ppm produced
reduced food intake and a dose-related reduction in body weight gain,
and that dose-levels of 300 and 1000 ppm reduced brain cholinesterase
activity. These effects were observed in rats of both sexes.
Profenofos did not result in death of any of the treated animals. No
unusual signs of behavior or appearance were observed. Results of
haematology, clinical chemistry (excluding cholinesterase) and
urinalysis examinations were unremarkable. Necropsies performed at
the various sacrifice intervals and at termination of the study did
not reveal treatment-related gross changes.
Table 1: Acute toxicity of Profenofos1
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse M+F Oral 298 -- Bathe, 1974a
Rat M+F Oral 358-502 -- Bathe, 1974b
Kobel & Gfeffer, 1983
M+F Dermal 33002 -- Bathe, 1974c
M+F i.p. 585 -- Sachsse & Bathe, 1975
M+F inhalation -- 3-3.36 Sachsse & Ullmann, 1974a
(4hr)
Horath & Taylor, 1982
Chinese M+F Oral 153 -- Bath & Sachsse, 1979
hamster
Rabbit M+F Oral 700 -- Sachsse & Ullmann, 1974b
M+F Dermal 4722 -- Sachsse & Ullmann, 1974c
M+F Dermal 1313 -- Cannelongo, 1982a
M+F Dermal 25404 -- Kuhn, 1988
Dog M+F Oral >3005 -- Bathe, 1974d
1 All studies performed using technical grade profenofos
2 Intact skin, occlusive dressing (aluminum foil and plaster)
3 Intact and abraded skin, occlusive dressing (polyethylene film)
4 Intact skin, semipermeable dressing (orthopaedic stockinette)
5 Acute oral toxicity could not be determined in dogs due to vomiting
at doses of 300 mg/kg and higher.
Profenofos technical (purity unspecified) was administered by
inhalation (nose only exposure) to albino RAI rats for 21 days (6
hours/day, 5 days/week) at mean concentrations of 0, 68, 219 and 449
mg/m3 in air. Nine males and nine females per group were exposed.
Four males and 4 females of the control and 219 mg/m3 group were
kept for an additional 21 days without treatment (recovery group).
All rats of the 449 mg/m3 group (9/9M and 9/9F) and 1/9 females of
the 219 mg/m3 group died during the first week after showing
symptoms of exophthalmos, dyspnoea, tremor, ruffled fur, lateral
position, and irritation/ secretion of the mucous membranes of the
eyes. Microscopic exams of these rats revealed the presence of marked
congestion of the nasal mucous membranes, acute conjunctivitis, and
severe interstitial keratitis. In the remaining surviving animals of
the 68 mg/m3 and 219 mg/m3 groups there were reductions in food
consumption and body weight gain (seen in males throughout the study
and in females over the first 7 to 10 days of the study), reductions
in total plasma protein levels, and a dose-related inhibition of
cholinesterase activity in plasma, red blood cells, and brain. The
magnitude of cholinesterase inhibition ranged from -40% to -70% for
all 3 sites. In addition, alpha-1 globulin levels were reduced and
alpha-2 and beta globulin levels were increased in males of the 219
mg/m3 group. All of the above changes observed in the surviving
rats were reversible upon cessation of treatment, with the exception
of the reductions in plasma and red cell cholinesterase levels in rats
of the 219 mg/m3 group which remained about 25% below control levels
at the end of the 21-day recovery period. Finally, necropsy of the
surviving rats did not reveal any gross or microscopic pathological
changes, although the related weights of most organs were increased in
male rats due to the reduced weight gain incurred in these animals.
The NOAEL was less than 68 mg/m3 in air (lowest dose tested) in
males and females, based upon reductions in food consumption, weight
gain, and cholinesterase activity in plasma, red cells, and brain
(Ullmann, et al., 1977).
Rabbits
In a dermal toxicity study, profenofos technical (89.8% pure) was
diluted with polyethylene glycol and saline (70:30) to 2% and 5%
solutions and applied to the skin of KA46 rabbits (Himalayan strain;
3/sex/dose level) at doses of 0, 5, 20, and 100 mg/kg bw/day for 24
hours/day, 5 days/week, for 21 days. In the 0, 5, and 20 mg/kg bw/day
treatment groups, 1 rabbit/sex/dose level was kept for an additional
21 days without treatment (recovery group). All of the rabbits
treated dermally with 100 mg/kg bw/day of profenofos died within 6
days after dosing was initiated. These animals displayed moderate
erythema and oedema of the skin, reduced food intake and body weight
gain, various clinical signs of toxicity (dyspnoea, salivation,
tremors, ataxia, sedation, and curved position), and inhibition of
cholinesterase activity in plasma (-100%) and red cells (-50.3%) as
measured on day 4 prior to deaths. Histopathological examination of
the high dose animals showed focal hypertrophy, haemorrhages and fatty
changes of hepatocytes accompanied by necrosis of the liver
parenchyma, and slight to moderate atrophy of lymphoid and thymic
tissue. Microscopic examination of the skin at the site of dermal
application showed oedema, minute haemorrhages, small intradermal
pustules, focal acanthosis and parakeratosis. In the animals treated
with the lower doses (5 and 20 mg/kg bw/day) of profenofos the
observed toxic changes consisted of slight erythema and oedema at the
application site, and inhibition of cholinesterase activity in plasm,
red cells, and brain. These effects disappeared during the recovery
period. No other unusual findings were observed in the lower dose
group animals. The NOAEL was less than 5 mg/kg bw/day (lowest dose
tested) in male and female rabbits for short term dermal toxicity
(Ullmann, et al., 1976).
In a second dermal toxicity study, profenofos technical (92%
pure) was suspended in purified water containing 0.5% Tween 80 and
applied to the skin of albino rabbits (HAR:PC/CF (NZW) BR strain;
5/sex/dose level) at doses of 0, 0.05, 1 and 10 mg/kg bw/day for 6
hours/day, 5 days/week, for 21 days. No deaths occurred in any
treated animals. Profenofos administration produced slight erythema at
the application site in mid dose males and females, and in high dose
males. Additional changes that appeared to be treatment-related were
observed at the high dose level and included hyperactivity and soft
faeces in males and/or females, significant reductions of
cholinesterase activity (measured at terminal sacrifice) in plasma,
red blood cells, and brain, increases in serum bilirubin (males) and
gamma GT (females) levels, and a decrease in serum sodium levels
(males). No unusual histopathological findings occurred. The NOAEL
for subchronic dermal toxicity (excluding the occurrence of slight
dermal erythema at the mid dose) was 1 mg/kg bw/day in males and
females (Johnson, et al., 1984).
Dogs
In a 6-month study, groups of 7 male and 7 female pedigreed
beagles were fed diets containing 0, 0.2, 2, 100, or 500 ppm of
technical profenofos (88.1%-89.3% pure). One male and one female of
each group was kept for an additional 28 days on control diet
(recovery groups). The administered concentrations were calculated to
be equivalent to 0, 0.007, 0.05, 2.9, and 14.4 mg/kg bw/day in dogs of
both sexes. Standard examinations for clinical signs of toxicity,
ophthalmology, haematology, clinical chemistry (including plasma,
brain, and red cell cholinesterase activity and plasma and liver
carboxylesterase activities) and urinalysis parameters were performed
at regular intervals throughout the study. The animals were subjected
to a battery of neurological exams (e.g., muscle strength and tone,
reflexes, etc.), necropsied, and examined grossly for organ changes
and histologically for tissue changes.
Adverse findings were observed at dose levels of 2 ppm to 500 ppm
and consisted of cholinesterase inhibition in plasma (males and
females at 2, 100, and 500 ppm bw/day) and red cells (males and
females at 100 and 500 ppm), carboxylesterase inhibition in plasma and
liver (males at 100 and 500 ppm), reductions in erythrocyte,
haemoglobin and haematocrit levels (males and females at 500 ppm; also
occasionally at 100 ppm), and reduced food consumption (males at 500
ppm over weeks 0-3). The depressed haematological values were said to
be within physiological limits, but it was noted that they did not
increase with age as was the case in the control and lower dose group
animals. All those effects appeared to be reversible during the 4-
week recovery period. Aside from these changes, there were no other
compound-related effects.
Long-term/carcinogenicity studies
Mice
A long-term feeding study of profenofos technical (90.6% pure)
was performed in albino mice (HaM/ICR Swiss, CR-CD 1 strain) in which
dietary concentrations of 0, 1, 30, and 100 ppm were fed to 60
males/dose group for 85 weeks and 60 females/dose group for 96 weeks.
The original design of the study was for 104 weeks, but because
survival had reached 20% of the original number of mice in the mid-
dose males and in the high-dose females due to accidental causes, the
sacrifices were initiated early. An additional 5 mice/sex/dose were
studied at each dose level and were sacrificed after 52 weeks for the
determination of cholinesterase activity in erythrocytes, plasma and
brain; these animals were not examined histopathologically. The
administered concentrations were calculated to be equivalent to 0,
0.14, 4.5, and 14.2 mg/kg bw/day in males and 0, 0.19, 5.8, and 19.2
mg/kg bw/day in females.
There were no clinical signs of toxicity and no significant
effect of treatment on mortality. There were no treatment related
effects on food consumption or body weight gain, or at gross necropsy
or after histopathological examination. There were no increases in
tumors that appeared to be related to compound administration. With
respect to clinical laboratory examinations, a significant dose-
related inhibition of erythrocyte and plasma cholinesterase activity
(generally ranging from -38% to -76%) was observed in mid- and high-
dose males and females at week 53 (interim sacrifice animals) and at
the termination of the study (week 85 in males and week 97 in
females). In addition, brain cholinesterase activity was
significantly inhibited (about -25%) in high-dose females at the end
of the study, whereas a trend (not significant) for this effect was
observed in the males. The NOAEL for male and female mice was 1 ppm
in the diet (0.14 mg/kg bw/day in males and 0.19 mg/kg bw/day in
females) based upon cholinesterase inhibition (Burdock, et al.,
1981a).
Rats
Fischer 344 albino rats (60/sex/group) were fed profenofos
technical (90.6% pure) at doses of 0, 0.3, 10, and 100 ppm for 2
years. An additional 10 rats/sex were also included in the control
and high dose treatment groups, respectively. Of these, 5/sex/group
were sacrificed at 52 weeks (interim sacrifice), and 5/sex/group were
placed on control feed after 52 weeks so that recovery studies could
be conducted and these were then sacrificed during week 63 (recovery
animals). All of the animals in the study were examined
histopathologically. Plasma and erythrocyte cholinesterase activity
was determined in 10 rats/sex/dose group (main study group) at weeks
13, 26, 52, 78, and 105 and in the recovery animals at week 57. Brain
cholinesterase activity was determined in the interim and terminal
sacrifice animals at weeks 53 and 105. The administered
concentrations of profenofos were calculated to be equivalent to 0,
0.017, 0.56, and 5.7 mg/kg bw/day in males and 0, 0.02, 0.69, and 6.9
mg/kg bw/day in females.
There were no treatment-related clinical signs of toxicity,
deaths, or changes in body weight gain. No sustained changes in
clinical chemistry parameters occurred which were considered to be
treatment-related. A dose-related inhibition of plasma and
erythrocyte cholinesterase was observed in rats of both sexes at the
mid- and high-dose levels; these effects were considered to be
compound-related and were reversible as judged by their absence in the
recovery group animals. Brain cholinesterase was not altered by
profenofos in this study. Additional changes seen only at the high
dose level included an increase in food consumption in females, an
increase in relative thyroid gland weight in high dose males (seen in
interim sacrifice and recovery group animals but not in terminal
sacrifice animals, and not considered biologically significant), an
increase in thyroid gland perifollicular cell hyperplasia in high dose
males (i.e., 4/70 controls vs. 10/70 high dose), and an increase in
liver neoplastic nodules in high dose females (i.e., 1/70 controls;
3/60 low dose; 2/60 mid dose; and 6/70 high dose). No increase in
liver carcinomas occurred. The latter histopathological findings were
not considered to be compound-related changes. The NOAEL was 5.7
mg/kg bw/day (the highest dose tested).
Reproduction study
Groups of male (8/sex/dose) and female (16/sex/dose) albino rats
in each of three generations (F0, F1, and F2) were fed diets
containing 0, 0.2, 1.0, or 20 ppm profenofos (technical grade; 95.5%
pure). The dietary concentrations were equivalent to 0, 0.01, 0.05,
and 1.0 mg/kg bw/day. Parental animals were allowed to mature for 100
days, mate, and produce 2 litters. Eight males and 16 females from
the second litters were retained at weaning as parental animals for
the succeeding generations. The study was terminated following the
weaning of the F3b litters. Profenofos was administered
continuously through the experiment.
There were no compound related effects on body weight, mortality,
behavior, or various parameters measuring fertility (fecundity index,
male or female fertility index) in any of the parenteral animals.
Statistically significant reductions in cholinesterase occurred at 20
ppm in erythrocytes (F0, F1, and F2 males and females) and
plasma (F0 females). In contrast, statistically increased plasma
cholinesterase activity was seen in F0 females given 0.2 and 1 ppm
and F1 males given 1 ppm, and brain cholinesterase activity was
increased in F1 males and females given 1 and 20 ppm. No adverse
effects occurred in progeny with respect to litter size, viability,
body weight, cholinesterase activity in erythrocytes, plasma or brain,
or development throughout the study. Postmortem gross and
histopathologic examinations of parenteral animals and F3b weanlings
were unremarkable (IBTL, Inc., 1978). The NOAEL for reproductive
effects was greater than 1.0 mg/kg bw/day in the diet (highest dose
tested).
Special studies on embryo/fetotoxicity
Rats
Groups of 20-27 pregnant rats (strain unspecified) were
administered profenofos (technical grade; purity unspecified) via oral
gavage at dose levels of 0, 10, 30, and 60 mg/kg bw from days 6 to 15
of gestation (day 0 = day either spermatozoa or vaginal plug found).
On day 21 of gestation, all dams were sacrificed and fetuses delivered
by caesarean section. Maternal toxicity was evident in the high-dose
groups as indicated by a marked decrease in food consumption during
the period of treatment. No other adverse effects occurred in the
dams. Similarly, none of the doses of profenofos appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. The NOAEL for maternal toxicity was 30 mg/kg and that for
fetotoxicity/teratogenicity was 60 mg/kg by oral gavage (Fritz,
1974b).
Groups of 23-25 pregnant rats (JCL-SD strain) received profenofos
(technical grade; 95.8% purity) via intubation at dose levels of 0,
18, 35, or 70 mg/kg bw/day from days 7 to 17 of gestation (day =day
that a sperm plug was found). On day 21 of gestation, all dams were
sacrificed under ether anaesthesia and fetuses delivered by caesarean
section. The doses of profenofos selected for testing were based upon
the results of a preliminary range finding study in which 140 mg/kg
bw/day caused death in 5/6 treated rats during days 8 to 15 of
pregnancy. Profenofos administration was associated with increases in
body weight and water consumption in the dams at doses of 35 and 70
mg/kg bw/day on days 17 to 21, and an increased level of food
consumption at 70 mg/kg bw/day on days 14 to 21; these changes did not
appear to be deleterious. Although small increases in the weights of
several organs (heart, spleen, liver, and right kidney) were seen at
70 mg/kg bw/day, these were small in magnitude. No treatment-related
changes in mortality or behavior were observed, and no abnormal
findings were observed at gross necropsy. There were no adverse
effects on the offspring with respect to resorptions, sex ratios,
placental weights, body weights and lengths, or distribution of
fetuses within the uterine horns. External and visceral examinations
of fetuses were unremarkable. Skeletal examination of fetuses showed
increased incidences of progeny with holes in the xiphoid at the mid
and high doses (0% controls, 0% low dose, 18.8% mid dose, and 15.6%
high dose) and delayed ossification of vertebral arches at the high
dose (8.8% controls, 6.7% low dose, 0.5% mid dose, and 26.7%). No
historical control data was provided and it could not be determined if
the findings were all from one litter or from multiple litters. The
NOAEL for maternal and developmental toxicity appeared to be 70 mg/kg
bw/day by intubation (Sugiya et al., 1982).
Groups of 25 pregnant rats (Sim:(SD)fBR strain) received
profenofos (technical grade; 88.0% purity) via oral gavage at dose
levels of 0, 10, 30, 60, 90, and 120 mg/kg bw/day from days 6 to 15 of
gestation (day 0 = day either sperm or vaginal plug found). On day 21
of gestation, all dams were sacrificed using CO2 and fetuses
delivered by caesarean section. Maternal toxicity occurred at the
high dose level as evidenced by increased mortality, reduced food
consumption, and various clinical signs of toxicity (e.g.,
hypoactivity or tremors, ocular porphyrin discharge, diarrhoea,
dyspnoea, diuresis, and hypothermia). Two of the 4 dams that died
displayed these clinical signs whereas the other 2 did not. In
addition, 2 of the dams that died also showed scattered haemorrhages
in the stomach upon gross necropsy. None of the other doses tested
produced changes in the pregnancy ratio, percentage of live or dead
fetuses, number of resorptions, or live fetal weights or sex ratios.
Similarly none of the dose appeared to affect embryonic or fetal
development and no teratogenic effects were observed. The NOAEL for
maternal toxicity was 90 mg/kg bw/day (Harris and Holson, 1982).
Rabbits
Groups of 20 pregnant Chinchilla rabbits were administered
profenofos (technical grade; 89.5% purity) via intubation at dose
levels of 0, 5, 15, and 30 mg/kg bw from days 6 to 18 of gestation
(day 0 = day of mating). On day 28 of gestation, all dams were
sacrificed by cervical dislocation and fetuses delivered by caesarean
section. With the exception of a marginal reduction in food
consumption from day 6 onward there were no unusual effects of
profenofos on the does. In addition, no adverse prenatal effects,
malformations, or variations were observed. The NOAEL for both
maternal and development toxicity was 30 mg/kg bw/day by intubation
(Fritz, et al., 1979).
Groups of 16 pregnant New Zealand White rabbits were given
profenofos (technical grade; 90.8% purity) by oral gavage at dose
levels of 0, 30, 60, 90, and 175 mg/kg bw from days 6 to 18 of
gestation (day 0 = day of mating). On day 30 of gestation, all does
were euthanized and fetuses delivered by caesarean section. The doses
of profenofos selected for testing were based upon the results of a
preliminary range finding study in which doses up to 150 mg/kg bw did
not produce any signs of toxicity. Profenofos administration was
associated with reduced maternal weight gain and food consumption at
doses of 60 mg/kg bw or more, clinical signs of toxicity (e.g.
diarrhoea, soft stools, oral/perianal discharges) at 90 mg/kg bw or
more, and deaths in 9/16 (56.3%) of the does at 175 mg/kg bw. Many of
the does that died exhibited the above clinical signs of toxicity as
well as signs of pinpoint stomach haemorrhages and yellow-discolored
areas in the mesentery in the gastric region upon gross necropsy.
None of the doses tested produced changes in maternal pregnancy rates,
in prenatal effects (e.g., percentage of live fetuses and live
fetuses/litter, resorptions, litter size, fetal body weight, and sex
ratios), or in malformations or variations. The NOAEL for maternal
toxicity was 30 mg/kg bw/day and that for developmental toxicity was
175 mg/kg bw/day (Holson, 1983).
Special studies on eye and skin irritation
In two studies in rabbits, instillation of 0.1 ml of profenofos
(undiluted technical material) into the conjunctival sac for 30
seconds produced mildly irritating conjunctival reactions (redness and
chemosis) which generally lasted for periods of up to 24 to 48 hours
before dissipating (Sachsse and Ullmann, 1974e; Cannelongo, 1982d).
In one of the tests, 2 of 6 treated rabbits died within 3 days without
apparent symptoms (Sachsse and Ullmann, 1974e).
Profenofos (0.5 ml of undiluted technical grade formulation)
produced death in one rabbit and slight to severe erythema in 2 of 5
surviving rabbits when applied to shaved and abraded skin for 24
hours. The treated animals displayed toxic signs manifested as
lateral and curved position, asynchronisms of the extremities, muscle
spasms, and apathy (Sachsse and Ullmann, 1974d).
In a second skin irritation study, application of profenofos (0.5
ml of undiluted technical grade formulation) to intact and abraded
rabbit skin produced death in 6 of 6 animals studied within 24 to 72
hours. Slight skin irritation (erythema and oedema) was observed
(Cannelongo, 1982b).
Special studies on genotoxicity
Profenofos was negative in a variety of in vitro tests in
bacteria, yeast, and mammalian cell systems that evaluated potential
activity to produce gene mutations, gene conversion, mitotic crossing
over, non-disjunction, and unscheduled DNA synthesis. Profenofos was
also negative in two in vitro tests, a dominant lethal assay in mice
and a nucleus anomaly test in Chinese hamsters. However, the compound
was associated with the production of chromosome aberrations,
micronucleus induction, and sister chromatid exchanges in the bone
marrow at oral doses ranging from 36 to 216 mg/kg bw in a third
in vivo study in mice. The summary results of genotoxicity studies
with profenofos are presented in Table 2.
Special studies on skin sensitizing effect
Profenofos was examined for skin sensitizing effects in 2 studies
in guinea pigs. Negative results were obtained in one test when
animals received a series of 10 intracutaneous induction injections
followed 14 days later by a single challenge injection of profenofos
(0.1 ml of a 0.1% technical formulation). A vehicle control group was
also negative whereas dinitrochlorobenzene (positive control) produced
marked sensitization (Sachsse and Ullmann, 1974f). Positive results
for dermal sensitization (i.e., very light erythema or oedema) were
occasionally obtained in another test when animals received a series
of 11 intradermal induction injections followed 14 days later by a
single challenge injection (0.1 ml of 0.1% technical formulation) of
profenofos. Although 2,4-dinitrochlorobenzene (positive control) was
consistently active in this study, a concurrent negative control group
was not employed to facilitate an evaluation of the results
(Cannelongo, 1982d).
Special studies on neurotoxicity
Delayed neurotoxic effects of oral doses of 21.7, 46.4, and 60
mg/kg bw/day of technical profenofos were assessed in adult domestic
chickens. The compound was administered twice, 21 days apart. The
acute oral LD50 of the formulation was about 35 mg/kg bw. Only the
birds of the low dose group survived the two treatments with
profenofos. Signs of toxicity were also similar after both treatments
with profenofos (e.g., salivation, asynchronisms of the extremities,
curved position, apathy, and ruffled feathers); these were observed at
the mid and high doses levels after the initial treatment on day 0,
and also at the low-dose level after the second treatment on day 21.
Neither delayed neurotoxic symptoms nor histologic changes in spinal
cord or peripheral nerve were observed. A positive control group
receiving 1000 and 2150 mg/kg bw TOCP showed the expected reactions
(i.e., ataxia, deterioration of reflexes, and swelling, fragmentation
and disruption of myelin sheaths) (Krinke et al., 1974).
Table 2: Results of genotoxicity assays on profenofos.
Test System Test Object Concentration Purity Results Reference
of profenofos
Ames Test1 S. typhimurium 5,15,45,135, 405 µg/0.1 ml in DMSO ? Negative Arni and Muller, 1978
(reverse mutation) TA-98, TA-100,
TA-1535, TA-1537
Yeast Test1 S. cerevisae Nonactivated: 91.8% Negative Arni and Muller, 1982
(gene conversion, crossing (D7 strain) 12.5-500 µg/ml in DMSO
over, reverse mutation) Activated: 0.640 -10000 µg/ml in DMSO
Yeast Test1 S. cerevisae 39,156,625, 2500, 10000 µg/ml in DMSO 90.0% Negative Hool and Muller, 1986
(non-disjunction) (D61.M strain)
Mouse Lymphoma L5178Y mouse 0.078, 0.156, 0.313, 0.625 µg/ml in DMSO 91.8% Negative Strasser and Muller, 1982
Forward Mutation lymphoma cells
Assay1 (TK +/-)
DNA Repair Rat hepatocytes 0.016, 0.08, 0.4, 2 nl/ml 91.8% Negative Puri and Muller, 1982a
Test2 (UDS)3
DNA Repair Human fibroblasts 0.32, 1.6, 8, 40 nl/ml 91.8% Negative Puri and Muller, 1982b
Test2 (UDS)3
Dominant Lethal Test Mouse (male) 35, 100 mg/kg ? Negative Fritz, 1974a
(NMRI-derived) (single oral doses)
Nucleus Anomaly Test Chinese hamster 13, 26, 52 mg/kg (2 oral doses on 88.1% Negative Hool, et al., 1981
bone marrow consecutive days)
Table 2 (contd)
Test System Test Object Concentration Purity Results Reference
of profenofos
Somatic Cell Studies Male Swiss mouse 36, 162, 216 mg/kg (single oral doses) 72% Positive4 El Nahas, et al., 1988
in Mice (sister chromatid bone marrow
exchange, micronucleus,
chromosome aberration)
1 With and without metabolic activation.
2 No exogenous activation added.
3 UDS = unscheduled DNA synthesis.
4 Doses of 36-216 mg/kg produced increases in chromosome aberrations; doses of 162-216 mg/kg increased micronuclei formation and sister
chromatid exchanges.
In a second study, adult chickens received oral doses of 29.2,
58.5, 117, and 234 mg/kg bw of profenofos (38% EC) on days 0 and 21.
The acute oral LD50 of the formulation was 127.0 mg/kg bw. After
treatment on day 0, death occurred in 0%, 6.6%, 55%, and 100% of the
birds given doses of 29.2, 58.5, 117 and 234 mg/kg bw/day,
respectively. The increased mortality was accompanied by signs of
cholinesterase inhibition (i.e., weakness, lethargy, and anorexia).
After treatment on day 21, death occurred in 0%, 7.1%, and 67% of the
surviving birds given doses of 29.2, 58.5 and 117 mg/kg bw/day, and
similar signs of cholinesterase inhibition were observed. There were
no delayed neurotoxic symptoms or histologic changes in brain, spinal
cord, or sciatic nerves. A positive control group using 500 mg/kg bw
of TOCP exhibited clinical signs of delayed neurotoxicity (extreme
weakness of legs and wings) and neuropathological lesions in the
spinal cord and sciatic nerves (axonal degeneration and demyelination)
(Fletcher et al., 1977).
Profenofos (technical, 89.5% purity) was examined for delayed
neurotoxicity in a third study in adult chickens. In the first phase
of the study (day 0), birds received oral doses of 30 and 45.7 mg/kg
bw on the basis of preliminary acute toxicity studies in which an
acute LD50 dose of 45.7 mg/kg bw was determined. However, because
of increased mortality rates at these dose levels (70% at 30 mg/kg bw,
and 82% at 45.7 mg/kg bw), all surviving birds were redosed on day 21
at a new estimated oral LD50 of 17.1 mg/kg bw. At this dose level
of profenofos, a mortality rate of 5% was observed. The results
indicated that profenofos produced signs of lethargy and salivation in
treated birds, but no signs of delayed neurotoxicity or histological
lesions in the brain, spinal cord, or peripheral nerves. A positive
control group using 500 mg/kg bw of TOCP exhibited clinical signs of
delayed neurotoxicity (extreme weakness of legs and wings) and
lesions in the spinal cord and sciatic nerves (axonal degeneration and
demyelination) (Reinart et al., 1978).
Special studies on pesticide antagonistic agents
A protective effect of atropine given early after orally
administered profenofos in rats or intraperitoneally administered
profenofos in chicks and mice was demonstrated by a reduction in
mortality and toxic signs (e.g., salivation, tremors, sedation,
convulsions) typical of anticholinesterase exposure. The effect of
oximes was limited (Sachsse and Bathe, 1976; Gfeller and Kobel, 1974;
Glickman et al. 1984).
Special studies on pesticide interactions
No potentiating effects were found when mixtures of profenofos
and the organophosphates methidathion, methacrifos or diazinon were
given to rats in equitoxic doses (Sachsse and Bathe, 1977); (Sachsse
and Bathe, 1978).
Profenofos administered intraperitoneally to mice at 0.5 to 5.0
mg/kg bw strongly inhibited the liver microsomal esterase(s)
hydrolysing trans-permethrin. The intraperitoneal toxicity LD50
measured 24 hours after exposure of fenvalerate and malathion but not
that of trans-permethrin was greatly increased when the compounds were
given 1 hour after an intraperitoneal injection of 25 mg/kg bw of
profenofos (Gaughan et al., 1980).
Observations in humans
A group of six male spraymen, aged 20 to 25 years, was monitored
for whole blood cholinesterase activity over a period of 4 days while
treating fully grown cotton with a formulation containing 400 grams
profenofos and 40 grams cypermethrin per liter in organic solvents on
a plantation in the Multan region of Central Pakistan in September
1985. The application equipment consisted of hand-held battery
operated spinning disc devices. The workers wore heavy cotton shirts
with long sleeves, legs wrapped below the knees, and turbans which
were often used to cover the face. Cholinesterase activity was
measured at the end of each of the 4 work days after thorough washing
of the body. The results indicated that there was a tendency toward
inhibition of blood cholinesterase activity. Compared to baseline
pre-exposure cholinesterase readings, the average cholinesterase
levels were 103.3%, 84.8%, 85.6%, and 81.2% of control activities on
days 1, 2, 3, and 4, respectively. The lowest individual values were
72.7% and 73.1% of control levels on the fourth day in 2 of the 6
workers (Loosli, 1989).
No cases of poisonings in humans have been reported with either
the active ingredient per se or with profenofos formulations.
COMMENTS
Profenofos administered orally to rats was well absorbed and was
excreted primarily in the urine, but also in faeces. Profenofos was
biotransformed by a major pathway involving side chain depropylation,
desulfuration, and phenyl-ester bond cleavage to yield 4-bromo-2-
chlorophenol, and by a minor pathway involving side chain 0-de-
ethylation, and subsequent phenyl-ester bond cleavage to the above
phenol. Both pathways culminated in conjugation with glucuronic and
sulfuric acids. No unchanged parent compound was found in urine.
Faeces contained only minute amounts of the parent compound, the
intermediate phenol, and unidentified metabolites. A similar
absorption and excretion pattern was observed in hens and goats. No
unusual organ or tissue localization of 14C-labelled profenofos was
observed.
Profenofos has a moderate order of acute toxicity following oral
and dermal administration. It has been classified as moderately
hazardous by WHO (WHO, 1990).
In a dietary toxicity study in which rats were fed profenofos for
8-13 weeks, the NOAEL was 100 ppm (equivalent to 5 mg/kg bw/day) based
on the finding of brain cholinesterase inhibition at higher levels.
The observed reduction in erythrocyte cholinesterase at lower doses
was not considered to be of toxicological importance. Reduction in
food consumption and body weight gain were considered to be a
consequence of poor palatability of the compound.
In a six-month feeding study in dogs, profenofos caused
erythrocyte cholinesterase inhibition and plasma and liver carboxylase
inhibition at levels of 100 ppm or more. The highest dose level
tested, 500 ppm, resulted in reductions in erythrocyte counts,
haemoglobin and haematocrit levels and a decrease in food consumption.
All of the changes were reversible. No changes in brain
cholinesterase activity occurred. As noted above, enzyme inhibition
and reduced food consumption were not considered to be direct toxic
effects of profenofos administration. The NOAEL was 100 ppm (equal to
2.9 mg/kg bw/day) based upon the observed haematological changes.
In long-term feeding studies in mice and rats, no treatment-
related increases in the incidence of neoplasms was observed. In
mice, brain cholinesterase inhibition occurred at the highest dose
level tested, 100 ppm. The NOAEL was 30 ppm (equal to 5.8 mg/kg
bw/day in females) based on brain cholinesterase inhibition observed
at the next higher dose level in females. In rats erythrocyte
cholinesterase inhibition occurred at dietary levels of 10 and 100
ppm. The highest dose level of 100 ppm was also associated with an
increase in food consumption and a slightly elevated incidence of
parafollicular cell hyperplasia. This type of response is not seen in
man following exposure to chemicals. No effect on brain
cholinesterase was observed. The NOAEL was 100 ppm (equal to 5.7
mg/kg bw/day).
In a three-generation reproduction study in rats, reductions in
cholinesterase activity occurred in erythrocytes (F0, F1, and F2
males and females) at 20 ppm in the diet. This was the highest dose
tested. No changes in brain cholinesterase activity occurred. The
NOAEL for systemic toxicity and reproduction was 20 ppm (equivalent to
1.0 mg/kg bw/day) which was the highest dose tested.
In three teratology studies in rats the NOAELs for maternal
toxicity ranged from 30 to 90 mg/kg bw/day, based upon findings of
reduced food consumption, clinical signs of toxicity (e.g., diarrhoea,
dyspnoea, tremors, hypothermia) and increased mortality at higher dose
levels. No teratogenic activity was observed at dose up to and
including 120 mg/kg bw/day. The NOAEL for embryotoxicity/fetotoxicity
(based on an increased incidence of variants) was 18 mg/kg bw/day. In
two rabbit teratology studies, the NOAEL for maternal toxicity was 30
mg/kg bw/day, based upon findings of reduced food consumption and body
weight gain, the occurrence of soft stools and diarrhoea, and
increased mortality at higher dose levels. No embryotoxic, fetotoxic,
or teratogenic activity was observed at doses up to and including 175
mg/kg bw/day.
After reviewing all available in vitro and in vivo short-term
tests, the Meeting concluded that there was no evidence of
genotoxicity.
The Meeting based the ADI for profenofos on the NOAEL of 1 mg/kg
bw/day in the rat multigeneration study. Although there was no
evidence of any adverse effects at this dose level in the study, the
absence of information on reproductive parameters at higher levels
precluded the use of the NOAEL of 2.9 mg/kg bw/day in the 6-month
feeding study in dogs.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 30 ppm, equal to 5.8 mg/kg bw/day in females.
Rat: 20 ppm, equivalent to 1.0 mg/kg bw/day (reproduction).
Rat: 100 ppm, equal to 5.7 mg/kg bw/day in males (long-term
study).
Dog: 100 ppm, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in
the continued evaluation of the compound
Further observations in humans.
An additional multigeneration study in rats using higher doses.
REFERENCES
Arni, P. and Muller, D. (1978). Salmonella/mammalian-microsome test
with CGA 15'324 (Test for mutagenic properties in bacteria). Project
No.: 78/2531. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Arni, P. and Muller, D. (1982). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro with CGA 15'324 (Test for
mutagenic properties in yeast cells). Project No.: 911557.
Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Bathe, R. (1974a). Acute oral LD50 of technical CGA 15'324 in the
mouse. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974b). Acute oral LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974c). Acute dermal LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. (1974d). Acute oral LD50 of technical CGA 15'324 in the
dog. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. and Sachsse, K. (1979). Acute oral LD50 in the Chinese
hamster CGA 15'324. Project No.: 785329. Unpublished report from
Ciba-Geigy Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Kuper, C.H. (1981a). Twenty-four months carcinogenicity study in
mice, CGA 15'324. Project No.: 483-133. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Fieser, S. (1981b). Two-year chronic oral toxicity study in albino
rats, CGA 15/324 technical. Project No.: 483-134. Unpublished report
from Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B.F. (1982a). Rabbit acute dermal toxicity CGA 15'324
technical FL 811584. Project No.: 2460-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F., (1982b). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2462-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982c). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2461-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982d). Guinea pig sensitization CGA 15'324
technical FL 811584. Project No.: 2463-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
El Nahas, S.M., De Hondt, H.A., and Ramadan, H.A.I. (1988). In vivo
evaluation of the genotoxic potential of Curacron in somatic cells of
mice. Environ. Mol. Mutagen., 11(4): 515-522.
Fletcher, D., Gordon, D.E., Jenkins, D.H., Kennedy, G.L., Kinoshita,
F.K., and Phillips, B.M. (1977). Neurotoxicity study with CGA 15'324
38% E.C. in chickens. Project No.: IBT 8580-10426. Unpublished
report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL,
USA. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland and
validated by the United States Environmental Protection Agency.
Fritz, H. (1974a). Dominant lethal study on CGA 15'324 tech., mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No.: 327438. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H. (1974b). Reproductive study - technical CGA 15'324, rat,
segment II (test for teratogenic or embryotoxic effects). Project
No.: 22741900. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H., Becker, H., and Hess, R. (1979). Seg. II reproductive
study in rabbits, CGA 15'324 tech. Project No.: 785565. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gaughan, L.C., Engel, J.L., and Casida, J.E. (1980). Pesticide
interactions: Effects of organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology 14: 81-85.
Gfeller, W. and Kobel, W. (1984). CGA 15'324 technical, tentative and
antilethal study in the rat. Project No.: GU 820034. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gfeller, W., Kobel, W., Schaeppi, U., Zak, F., and Hess, R. (1981).
CGA 15'324 tech., 6-months toxicity study with dogs. Project No.:
790804. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Glickman, A.H., Wing, K.D., and Casida, J.E., (1984). Profenofos
insecticide bioactivation in relation to antidote action and the
stereospecificity and acetylcholinesterase inhibition, reactivation,
and aging. Toxicity and Applied Pharmacology, 73: 16-22.
Harris, S.B. and Holson, J.F. (1982). A teratology study of CGA
15'324 technical in albino rats. Project No.: 282009. Unpublished
report from Science Applications, Inc., La Jolla, CA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Holson, J.F. (1983). Teratology study (Seg II) in albino rabbits with
CGA 15'324 technical. Project No.: 283003. Unpublished report from
Science Applications, Inc., La Jolla, CA, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., and Muller, D. (1981). Nucleus anomaly test in
somatic interphase nuclei, CGA 15'324, Chinese hamster (test for
mutagenic effects on bone marrow cells). Project No.: 791557.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. and Muller, D. (1986). Test for non-disjunction on
Saccharomyces cerevisiae D61.M in vitro, CGA 15'324 tech. Project
No.: 850811. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Horath, L.L. and Taylor, G.D. (1982). Acute aerosol toxicity study in
rats of CGA 15'324 technical FL 811528. Project No.: 420-0921.
Unpublished report from Toxi-Genics, Inc., Decatur, IL, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
IBTL, Inc. (1978). Three generation reproduction study with CGA
15'324 technical in albino rats. Project No.: IBT 623-07924.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
Northbrook, IL, USA. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland. Validated by United States Environmental Protection
Agency.
Ifflaender, U. and Mucke, W. (1976). The metabolism of CGA 15'324 in
the rat. Project No.: 27/76. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Ifflaender, U., Mucke, W., and Esser, H.O. (1974). Distribution,
degradation and excretion of CGA 15'324 in the rat. Project No.:
16/74. Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Johnson, S., Tai, C.N., and Katz, R. (1984). Profenofos technical,
21-day dermal toxicity study in rabbits. Project No.: MIN 842008.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kobel, W. and Gfeller, W. (1983). Acute oral LD50 in the rat, CGA
15'324 tech. GU Project No.: 830188. Unpublished report from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Krinke, G., Ullmann, L., and Sachsse, K. (1974). Acute oral LD50
and neurotoxicity study of technical CGA 15'324 in the domestic fowl
(Gallus domesticus). Project No.: Siss 2850. Unpublished report from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1988). Acute dermal toxicity study in rabbits, profenofos
technical. Project No.: 5522-88. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Loosli, R. (1985). Project No.: 850910. Unpublished summary report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mucke, W. (1986). The renal excretion of U-14C-Phenyl CGA 15'324 by
male rats after oral administration (exposure monitoring). Project
No.: 13/86. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Oakes, T.L., Marco, G.J., and Ballantine, L. (1986). Metabolism of
14C-profenofos in chickens dosed at 5.0 ppm. Project No.: ABR-
86002. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Piccirillo, V.J. (1978). 90-day subacute oral toxicity study in rats,
CGA 15'324 technical. Project No.: 483-135. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. and Muller, D. (1982a). Autoradiographic DNA repair test on
rat hepatocytes, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811490. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Puri, E. and Muller, D. (1982b). Autoradiographic DNA repair test on
human fibroblasts, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811658. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Reinart, D., Fletcher, D., Arceo, R.J., and Gordon, D.E. (1978). 42-
day neurotoxicity study with CGA 15'324 technical in adult chickens.
Project No.: IBT 8580-11187. Unpublished report from Industrial
Bio-Test Laboratories, Inc., Decatur, IL. USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland and validated by the United States
Environmental Protection Agency.
Sachsse, K. and Bathe, R. (1975). Acute intraperitoneal LD50 in the
rat of technical CGA 15'324. Project No.: Siss 5048. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1976). The therapeutic activity of
pralidoxim (PAM) and TOXOGONIN\ with regard to CGA 15'324 in the rat.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1977). Potentiation study CGA 15'324
versus 2 insecticides CA 13'005 (methidathion) and G 24'480 (diazinon)
in the rat. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1978). Potentiation study CGA 20'168
versus 6 insecticides, C 177 (DDVP), C570 (phosphamidon), GS 13'005
(methidation), G 24'480 (diazinon), CGA 15'324 and malathion. Project
No.: 404478 - Siss 6526. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. and Ullmann, L. (1974a). Acute inhalation toxicity of
technical CGA 15'324 in the rat. Project No.: Siss 3647.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974b). Acute oral LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647 Unpublished report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974c). Acute dermal LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974d). Skin irritation in the rabbit
after single application of technical CGA 15'324. Project No.: Siss
3647. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974e). Irritation of technical CGA
15'324 in the rabbit eye. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974f). Skin sensitizing (contact
allergenic) effect in Guinea pigs of technical CGA 15'324. Project
No.: Siss 3647. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F.F. and Muller, D. (1982). L5178/TK+/- mouse lymphoma
mutagenicity test, CGA 15'324 (in vitro test for mutagenic
properties of chemical substances in mammalian cells). Project No.:
811491. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sugiya, J., Yoshida, K., Tamaki, Y., Yokota, M., Abo, Y., and
Kawakami, S. (1982). Teratogenicity in rats administered CGA 15'324
(profenofos) prenatally during the major organogenic period.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Thomas, R.D., Cassidy, J.E., and Marco, G.J. (1976). Metabolism and
balance study of 0-14C-CGA 15'324 in a lactating goat Project No.:
GAAC-76024. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1976). CGA 15'324 technical 21-day dermal toxicity study in rabbits.
Project No.: Siss 5119. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1977). CGA 15'324, 21-day inhalation study on the rat. Project No.:
Siss 5119. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, S.C., Marco, G.J., Simoneaux, B.J., and Ballantine, L.
(1984). Percutaneous absorption of 14C-profenofos in rats. Project
No.: ABR-84023. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1984).
Phosphorothiolate pesticides and related compounds: Oxidative
bioactivation and aging of the inhibited acetylcholinesterase.
Pesticide Biochemistry and Physiology, 21: 22-30.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1983). Oxidative
bioactivation of S-alkyl phosphorothiolate pesticides:
Stereospecificity of profenofos insecticide activation. Science, 219:
63-65.
Effects on enzymes and other biochemical parameters
Profenofos is stereospecifically converted to a more potent
inhibitor of acetylcholinesterase by mouse liver microsomal mixed-
function oxidase system. The chiral (-) isomer became a 34-fold
better inhibitor of acetylcholinesterase in vitro, while the less
toxic (+) isomer was deactivated by a factor of 2. Prior treatment
with mixed function oxidase inhibitors markedly decreased the
activation and also protected against brain acetylcholinesterase
inhibition and cholinergic symptoms resulting from (-) profenofos
administration in chicks (Wings, et al., 1983).
Toxicological studies
Acute studies
The acute toxicity of profenofos is given in Table 1. The
adverse signs of toxicity that were observed were generally similar
for each route of compound administration. These included non-
specific symptoms such as dyspnoea, exophthalmos, ruffled fur and
crooked body posture, and cholinergic symptoms such as sedation,
salivation, discharge from eyes and nose, trismus, tremors, and tonic-
clonic convulsions. The symptoms were reversible in surviving
animals.
Short-term studies
Rats
A feeding study was performed in which groups of 25 male and 25
female F344 rats received technical profenofos (90.6% a.i.) in the
daily diet for up to 13 weeks. Two control groups (basal diet) and
eleven dose groups (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100, 300, and
1000 ppm) were used. The animals from one control group and the 0.3,
3, 30, and 300 ppm test groups (15/sex/dose level) were on test for 13
weeks. The animals in all of the other test groups were sacrificed at
weeks 2, 4 and 8 (5 to 15 rats/sex/group). The results indicated that
dose levels of profenofos of 10 to 1000 ppm produced a dose-related
inhibition of cholinesterase activity in plasma and erythrocytes (more
marked in females), that dose-levels of 100 to 1000 ppm produced
reduced food intake and a dose-related reduction in body weight gain,
and that dose-levels of 300 and 1000 ppm reduced brain cholinesterase
activity. These effects were observed in rats of both sexes.
Profenofos did not result in death of any of the treated animals. No
unusual signs of behavior or appearance were observed. Results of
haematology, clinical chemistry (excluding cholinesterase) and
urinalysis examinations were unremarkable. Necropsies performed at
the various sacrifice intervals and at termination of the study did
not reveal treatment-related gross changes.
Table 1: Acute toxicity of Profenofos1
Species Sex Route LD50 LC50 Reference
(mg/kg bw) (mg/l)
Mouse M+F Oral 298 -- Bathe, 1974a
Rat M+F Oral 358-502 -- Bathe, 1974b
Kobel & Gfeffer, 1983
M+F Dermal 33002 -- Bathe, 1974c
M+F i.p. 585 -- Sachsse & Bathe, 1975
M+F inhalation -- 3-3.36 Sachsse & Ullmann, 1974a
(4hr)
Horath & Taylor, 1982
Chinese M+F Oral 153 -- Bath & Sachsse, 1979
hamster
Rabbit M+F Oral 700 -- Sachsse & Ullmann, 1974b
M+F Dermal 4722 -- Sachsse & Ullmann, 1974c
M+F Dermal 1313 -- Cannelongo, 1982a
M+F Dermal 25404 -- Kuhn, 1988
Dog M+F Oral >3005 -- Bathe, 1974d
1 All studies performed using technical grade profenofos
2 Intact skin, occlusive dressing (aluminum foil and plaster)
3 Intact and abraded skin, occlusive dressing (polyethylene film)
4 Intact skin, semipermeable dressing (orthopaedic stockinette)
5 Acute oral toxicity could not be determined in dogs due to vomiting
at doses of 300 mg/kg and higher.
Profenofos technical (purity unspecified) was administered by
inhalation (nose only exposure) to albino RAI rats for 21 days (6
hours/day, 5 days/week) at mean concentrations of 0, 68, 219 and 449
mg/m3 in air. Nine males and nine females per group were exposed.
Four males and 4 females of the control and 219 mg/m3 group were
kept for an additional 21 days without treatment (recovery group).
All rats of the 449 mg/m3 group (9/9M and 9/9F) and 1/9 females of
the 219 mg/m3 group died during the first week after showing
symptoms of exophthalmos, dyspnoea, tremor, ruffled fur, lateral
position, and irritation/ secretion of the mucous membranes of the
eyes. Microscopic exams of these rats revealed the presence of marked
congestion of the nasal mucous membranes, acute conjunctivitis, and
severe interstitial keratitis. In the remaining surviving animals of
the 68 mg/m3 and 219 mg/m3 groups there were reductions in food
consumption and body weight gain (seen in males throughout the study
and in females over the first 7 to 10 days of the study), reductions
in total plasma protein levels, and a dose-related inhibition of
cholinesterase activity in plasma, red blood cells, and brain. The
magnitude of cholinesterase inhibition ranged from -40% to -70% for
all 3 sites. In addition, alpha-1 globulin levels were reduced and
alpha-2 and beta globulin levels were increased in males of the 219
mg/m3 group. All of the above changes observed in the surviving
rats were reversible upon cessation of treatment, with the exception
of the reductions in plasma and red cell cholinesterase levels in rats
of the 219 mg/m3 group which remained about 25% below control levels
at the end of the 21-day recovery period. Finally, necropsy of the
surviving rats did not reveal any gross or microscopic pathological
changes, although the related weights of most organs were increased in
male rats due to the reduced weight gain incurred in these animals.
The NOAEL was less than 68 mg/m3 in air (lowest dose tested) in
males and females, based upon reductions in food consumption, weight
gain, and cholinesterase activity in plasma, red cells, and brain
(Ullmann, et al., 1977).
Rabbits
In a dermal toxicity study, profenofos technical (89.8% pure) was
diluted with polyethylene glycol and saline (70:30) to 2% and 5%
solutions and applied to the skin of KA46 rabbits (Himalayan strain;
3/sex/dose level) at doses of 0, 5, 20, and 100 mg/kg bw/day for 24
hours/day, 5 days/week, for 21 days. In the 0, 5, and 20 mg/kg bw/day
treatment groups, 1 rabbit/sex/dose level was kept for an additional
21 days without treatment (recovery group). All of the rabbits
treated dermally with 100 mg/kg bw/day of profenofos died within 6
days after dosing was initiated. These animals displayed moderate
erythema and oedema of the skin, reduced food intake and body weight
gain, various clinical signs of toxicity (dyspnoea, salivation,
tremors, ataxia, sedation, and curved position), and inhibition of
cholinesterase activity in plasma (-100%) and red cells (-50.3%) as
measured on day 4 prior to deaths. Histopathological examination of
the high dose animals showed focal hypertrophy, haemorrhages and fatty
changes of hepatocytes accompanied by necrosis of the liver
parenchyma, and slight to moderate atrophy of lymphoid and thymic
tissue. Microscopic examination of the skin at the site of dermal
application showed oedema, minute haemorrhages, small intradermal
pustules, focal acanthosis and parakeratosis. In the animals treated
with the lower doses (5 and 20 mg/kg bw/day) of profenofos the
observed toxic changes consisted of slight erythema and oedema at the
application site, and inhibition of cholinesterase activity in plasm,
red cells, and brain. These effects disappeared during the recovery
period. No other unusual findings were observed in the lower dose
group animals. The NOAEL was less than 5 mg/kg bw/day (lowest dose
tested) in male and female rabbits for short term dermal toxicity
(Ullmann, et al., 1976).
In a second dermal toxicity study, profenofos technical (92%
pure) was suspended in purified water containing 0.5% Tween 80 and
applied to the skin of albino rabbits (HAR:PC/CF (NZW) BR strain;
5/sex/dose level) at doses of 0, 0.05, 1 and 10 mg/kg bw/day for 6
hours/day, 5 days/week, for 21 days. No deaths occurred in any
treated animals. Profenofos administration produced slight erythema at
the application site in mid dose males and females, and in high dose
males. Additional changes that appeared to be treatment-related were
observed at the high dose level and included hyperactivity and soft
faeces in males and/or females, significant reductions of
cholinesterase activity (measured at terminal sacrifice) in plasma,
red blood cells, and brain, increases in serum bilirubin (males) and
gamma GT (females) levels, and a decrease in serum sodium levels
(males). No unusual histopathological findings occurred. The NOAEL
for subchronic dermal toxicity (excluding the occurrence of slight
dermal erythema at the mid dose) was 1 mg/kg bw/day in males and
females (Johnson, et al., 1984).
Dogs
In a 6-month study, groups of 7 male and 7 female pedigreed
beagles were fed diets containing 0, 0.2, 2, 100, or 500 ppm of
technical profenofos (88.1%-89.3% pure). One male and one female of
each group was kept for an additional 28 days on control diet
(recovery groups). The administered concentrations were calculated to
be equivalent to 0, 0.007, 0.05, 2.9, and 14.4 mg/kg bw/day in dogs of
both sexes. Standard examinations for clinical signs of toxicity,
ophthalmology, haematology, clinical chemistry (including plasma,
brain, and red cell cholinesterase activity and plasma and liver
carboxylesterase activities) and urinalysis parameters were performed
at regular intervals throughout the study. The animals were subjected
to a battery of neurological exams (e.g., muscle strength and tone,
reflexes, etc.), necropsied, and examined grossly for organ changes
and histologically for tissue changes.
Adverse findings were observed at dose levels of 2 ppm to 500 ppm
and consisted of cholinesterase inhibition in plasma (males and
females at 2, 100, and 500 ppm bw/day) and red cells (males and
females at 100 and 500 ppm), carboxylesterase inhibition in plasma and
liver (males at 100 and 500 ppm), reductions in erythrocyte,
haemoglobin and haematocrit levels (males and females at 500 ppm; also
occasionally at 100 ppm), and reduced food consumption (males at 500
ppm over weeks 0-3). The depressed haematological values were said to
be within physiological limits, but it was noted that they did not
increase with age as was the case in the control and lower dose group
animals. All those effects appeared to be reversible during the 4-
week recovery period. Aside from these changes, there were no other
compound-related effects.
Long-term/carcinogenicity studies
Mice
A long-term feeding study of profenofos technical (90.6% pure)
was performed in albino mice (HaM/ICR Swiss, CR-CD 1 strain) in which
dietary concentrations of 0, 1, 30, and 100 ppm were fed to 60
males/dose group for 85 weeks and 60 females/dose group for 96 weeks.
The original design of the study was for 104 weeks, but because
survival had reached 20% of the original number of mice in the mid-
dose males and in the high-dose females due to accidental causes, the
sacrifices were initiated early. An additional 5 mice/sex/dose were
studied at each dose level and were sacrificed after 52 weeks for the
determination of cholinesterase activity in erythrocytes, plasma and
brain; these animals were not examined histopathologically. The
administered concentrations were calculated to be equivalent to 0,
0.14, 4.5, and 14.2 mg/kg bw/day in males and 0, 0.19, 5.8, and 19.2
mg/kg bw/day in females.
There were no clinical signs of toxicity and no significant
effect of treatment on mortality. There were no treatment related
effects on food consumption or body weight gain, or at gross necropsy
or after histopathological examination. There were no increases in
tumors that appeared to be related to compound administration. With
respect to clinical laboratory examinations, a significant dose-
related inhibition of erythrocyte and plasma cholinesterase activity
(generally ranging from -38% to -76%) was observed in mid- and high-
dose males and females at week 53 (interim sacrifice animals) and at
the termination of the study (week 85 in males and week 97 in
females). In addition, brain cholinesterase activity was
significantly inhibited (about -25%) in high-dose females at the end
of the study, whereas a trend (not significant) for this effect was
observed in the males. The NOAEL for male and female mice was 1 ppm
in the diet (0.14 mg/kg bw/day in males and 0.19 mg/kg bw/day in
females) based upon cholinesterase inhibition (Burdock, et al.,
1981a).
Rats
Fischer 344 albino rats (60/sex/group) were fed profenofos
technical (90.6% pure) at doses of 0, 0.3, 10, and 100 ppm for 2
years. An additional 10 rats/sex were also included in the control
and high dose treatment groups, respectively. Of these, 5/sex/group
were sacrificed at 52 weeks (interim sacrifice), and 5/sex/group were
placed on control feed after 52 weeks so that recovery studies could
be conducted and these were then sacrificed during week 63 (recovery
animals). All of the animals in the study were examined
histopathologically. Plasma and erythrocyte cholinesterase activity
was determined in 10 rats/sex/dose group (main study group) at weeks
13, 26, 52, 78, and 105 and in the recovery animals at week 57. Brain
cholinesterase activity was determined in the interim and terminal
sacrifice animals at weeks 53 and 105. The administered
concentrations of profenofos were calculated to be equivalent to 0,
0.017, 0.56, and 5.7 mg/kg bw/day in males and 0, 0.02, 0.69, and 6.9
mg/kg bw/day in females.
There were no treatment-related clinical signs of toxicity,
deaths, or changes in body weight gain. No sustained changes in
clinical chemistry parameters occurred which were considered to be
treatment-related. A dose-related inhibition of plasma and
erythrocyte cholinesterase was observed in rats of both sexes at the
mid- and high-dose levels; these effects were considered to be
compound-related and were reversible as judged by their absence in the
recovery group animals. Brain cholinesterase was not altered by
profenofos in this study. Additional changes seen only at the high
dose level included an increase in food consumption in females, an
increase in relative thyroid gland weight in high dose males (seen in
interim sacrifice and recovery group animals but not in terminal
sacrifice animals, and not considered biologically significant), an
increase in thyroid gland perifollicular cell hyperplasia in high dose
males (i.e., 4/70 controls vs. 10/70 high dose), and an increase in
liver neoplastic nodules in high dose females (i.e., 1/70 controls;
3/60 low dose; 2/60 mid dose; and 6/70 high dose). No increase in
liver carcinomas occurred. The latter histopathological findings were
not considered to be compound-related changes. The NOAEL was 5.7
mg/kg bw/day (the highest dose tested).
Reproduction study
Groups of male (8/sex/dose) and female (16/sex/dose) albino rats
in each of three generations (F0, F1, and F2) were fed diets
containing 0, 0.2, 1.0, or 20 ppm profenofos (technical grade; 95.5%
pure). The dietary concentrations were equivalent to 0, 0.01, 0.05,
and 1.0 mg/kg bw/day. Parental animals were allowed to mature for 100
days, mate, and produce 2 litters. Eight males and 16 females from
the second litters were retained at weaning as parental animals for
the succeeding generations. The study was terminated following the
weaning of the F3b litters. Profenofos was administered
continuously through the experiment.
There were no compound related effects on body weight, mortality,
behavior, or various parameters measuring fertility (fecundity index,
male or female fertility index) in any of the parenteral animals.
Statistically significant reductions in cholinesterase occurred at 20
ppm in erythrocytes (F0, F1, and F2 males and females) and
plasma (F0 females). In contrast, statistically increased plasma
cholinesterase activity was seen in F0 females given 0.2 and 1 ppm
and F1 males given 1 ppm, and brain cholinesterase activity was
increased in F1 males and females given 1 and 20 ppm. No adverse
effects occurred in progeny with respect to litter size, viability,
body weight, cholinesterase activity in erythrocytes, plasma or brain,
or development throughout the study. Postmortem gross and
histopathologic examinations of parenteral animals and F3b weanlings
were unremarkable (IBTL, Inc., 1978). The NOAEL for reproductive
effects was greater than 1.0 mg/kg bw/day in the diet (highest dose
tested).
Special studies on embryo/fetotoxicity
Rats
Groups of 20-27 pregnant rats (strain unspecified) were
administered profenofos (technical grade; purity unspecified) via oral
gavage at dose levels of 0, 10, 30, and 60 mg/kg bw from days 6 to 15
of gestation (day 0 = day either spermatozoa or vaginal plug found).
On day 21 of gestation, all dams were sacrificed and fetuses delivered
by caesarean section. Maternal toxicity was evident in the high-dose
groups as indicated by a marked decrease in food consumption during
the period of treatment. No other adverse effects occurred in the
dams. Similarly, none of the doses of profenofos appeared to affect
embryonic or fetal development and no teratogenic effects were
observed. The NOAEL for maternal toxicity was 30 mg/kg and that for
fetotoxicity/teratogenicity was 60 mg/kg by oral gavage (Fritz,
1974b).
Groups of 23-25 pregnant rats (JCL-SD strain) received profenofos
(technical grade; 95.8% purity) via intubation at dose levels of 0,
18, 35, or 70 mg/kg bw/day from days 7 to 17 of gestation (day =day
that a sperm plug was found). On day 21 of gestation, all dams were
sacrificed under ether anaesthesia and fetuses delivered by caesarean
section. The doses of profenofos selected for testing were based upon
the results of a preliminary range finding study in which 140 mg/kg
bw/day caused death in 5/6 treated rats during days 8 to 15 of
pregnancy. Profenofos administration was associated with increases in
body weight and water consumption in the dams at doses of 35 and 70
mg/kg bw/day on days 17 to 21, and an increased level of food
consumption at 70 mg/kg bw/day on days 14 to 21; these changes did not
appear to be deleterious. Although small increases in the weights of
several organs (heart, spleen, liver, and right kidney) were seen at
70 mg/kg bw/day, these were small in magnitude. No treatment-related
changes in mortality or behavior were observed, and no abnormal
findings were observed at gross necropsy. There were no adverse
effects on the offspring with respect to resorptions, sex ratios,
placental weights, body weights and lengths, or distribution of
fetuses within the uterine horns. External and visceral examinations
of fetuses were unremarkable. Skeletal examination of fetuses showed
increased incidences of progeny with holes in the xiphoid at the mid
and high doses (0% controls, 0% low dose, 18.8% mid dose, and 15.6%
high dose) and delayed ossification of vertebral arches at the high
dose (8.8% controls, 6.7% low dose, 0.5% mid dose, and 26.7%). No
historical control data was provided and it could not be determined if
the findings were all from one litter or from multiple litters. The
NOAEL for maternal and developmental toxicity appeared to be 70 mg/kg
bw/day by intubation (Sugiya et al., 1982).
Groups of 25 pregnant rats (Sim:(SD)fBR strain) received
profenofos (technical grade; 88.0% purity) via oral gavage at dose
levels of 0, 10, 30, 60, 90, and 120 mg/kg bw/day from days 6 to 15 of
gestation (day 0 = day either sperm or vaginal plug found). On day 21
of gestation, all dams were sacrificed using CO2 and fetuses
delivered by caesarean section. Maternal toxicity occurred at the
high dose level as evidenced by increased mortality, reduced food
consumption, and various clinical signs of toxicity (e.g.,
hypoactivity or tremors, ocular porphyrin discharge, diarrhoea,
dyspnoea, diuresis, and hypothermia). Two of the 4 dams that died
displayed these clinical signs whereas the other 2 did not. In
addition, 2 of the dams that died also showed scattered haemorrhages
in the stomach upon gross necropsy. None of the other doses tested
produced changes in the pregnancy ratio, percentage of live or dead
fetuses, number of resorptions, or live fetal weights or sex ratios.
Similarly none of the dose appeared to affect embryonic or fetal
development and no teratogenic effects were observed. The NOAEL for
maternal toxicity was 90 mg/kg bw/day (Harris and Holson, 1982).
Rabbits
Groups of 20 pregnant Chinchilla rabbits were administered
profenofos (technical grade; 89.5% purity) via intubation at dose
levels of 0, 5, 15, and 30 mg/kg bw from days 6 to 18 of gestation
(day 0 = day of mating). On day 28 of gestation, all dams were
sacrificed by cervical dislocation and fetuses delivered by caesarean
section. With the exception of a marginal reduction in food
consumption from day 6 onward there were no unusual effects of
profenofos on the does. In addition, no adverse prenatal effects,
malformations, or variations were observed. The NOAEL for both
maternal and development toxicity was 30 mg/kg bw/day by intubation
(Fritz, et al., 1979).
Groups of 16 pregnant New Zealand White rabbits were given
profenofos (technical grade; 90.8% purity) by oral gavage at dose
levels of 0, 30, 60, 90, and 175 mg/kg bw from days 6 to 18 of
gestation (day 0 = day of mating). On day 30 of gestation, all does
were euthanized and fetuses delivered by caesarean section. The doses
of profenofos selected for testing were based upon the results of a
preliminary range finding study in which doses up to 150 mg/kg bw did
not produce any signs of toxicity. Profenofos administration was
associated with reduced maternal weight gain and food consumption at
doses of 60 mg/kg bw or more, clinical signs of toxicity (e.g.
diarrhoea, soft stools, oral/perianal discharges) at 90 mg/kg bw or
more, and deaths in 9/16 (56.3%) of the does at 175 mg/kg bw. Many of
the does that died exhibited the above clinical signs of toxicity as
well as signs of pinpoint stomach haemorrhages and yellow-discolored
areas in the mesentery in the gastric region upon gross necropsy.
None of the doses tested produced changes in maternal pregnancy rates,
in prenatal effects (e.g., percentage of live fetuses and live
fetuses/litter, resorptions, litter size, fetal body weight, and sex
ratios), or in malformations or variations. The NOAEL for maternal
toxicity was 30 mg/kg bw/day and that for developmental toxicity was
175 mg/kg bw/day (Holson, 1983).
Special studies on eye and skin irritation
In two studies in rabbits, instillation of 0.1 ml of profenofos
(undiluted technical material) into the conjunctival sac for 30
seconds produced mildly irritating conjunctival reactions (redness and
chemosis) which generally lasted for periods of up to 24 to 48 hours
before dissipating (Sachsse and Ullmann, 1974e; Cannelongo, 1982d).
In one of the tests, 2 of 6 treated rabbits died within 3 days without
apparent symptoms (Sachsse and Ullmann, 1974e).
Profenofos (0.5 ml of undiluted technical grade formulation)
produced death in one rabbit and slight to severe erythema in 2 of 5
surviving rabbits when applied to shaved and abraded skin for 24
hours. The treated animals displayed toxic signs manifested as
lateral and curved position, asynchronisms of the extremities, muscle
spasms, and apathy (Sachsse and Ullmann, 1974d).
In a second skin irritation study, application of profenofos (0.5
ml of undiluted technical grade formulation) to intact and abraded
rabbit skin produced death in 6 of 6 animals studied within 24 to 72
hours. Slight skin irritation (erythema and oedema) was observed
(Cannelongo, 1982b).
Special studies on genotoxicity
Profenofos was negative in a variety of in vitro tests in
bacteria, yeast, and mammalian cell systems that evaluated potential
activity to produce gene mutations, gene conversion, mitotic crossing
over, non-disjunction, and unscheduled DNA synthesis. Profenofos was
also negative in two in vitro tests, a dominant lethal assay in mice
and a nucleus anomaly test in Chinese hamsters. However, the compound
was associated with the production of chromosome aberrations,
micronucleus induction, and sister chromatid exchanges in the bone
marrow at oral doses ranging from 36 to 216 mg/kg bw in a third
in vivo study in mice. The summary results of genotoxicity studies
with profenofos are presented in Table 2.
Special studies on skin sensitizing effect
Profenofos was examined for skin sensitizing effects in 2 studies
in guinea pigs. Negative results were obtained in one test when
animals received a series of 10 intracutaneous induction injections
followed 14 days later by a single challenge injection of profenofos
(0.1 ml of a 0.1% technical formulation). A vehicle control group was
also negative whereas dinitrochlorobenzene (positive control) produced
marked sensitization (Sachsse and Ullmann, 1974f). Positive results
for dermal sensitization (i.e., very light erythema or oedema) were
occasionally obtained in another test when animals received a series
of 11 intradermal induction injections followed 14 days later by a
single challenge injection (0.1 ml of 0.1% technical formulation) of
profenofos. Although 2,4-dinitrochlorobenzene (positive control) was
consistently active in this study, a concurrent negative control group
was not employed to facilitate an evaluation of the results
(Cannelongo, 1982d).
Special studies on neurotoxicity
Delayed neurotoxic effects of oral doses of 21.7, 46.4, and 60
mg/kg bw/day of technical profenofos were assessed in adult domestic
chickens. The compound was administered twice, 21 days apart. The
acute oral LD50 of the formulation was about 35 mg/kg bw. Only the
birds of the low dose group survived the two treatments with
profenofos. Signs of toxicity were also similar after both treatments
with profenofos (e.g., salivation, asynchronisms of the extremities,
curved position, apathy, and ruffled feathers); these were observed at
the mid and high doses levels after the initial treatment on day 0,
and also at the low-dose level after the second treatment on day 21.
Neither delayed neurotoxic symptoms nor histologic changes in spinal
cord or peripheral nerve were observed. A positive control group
receiving 1000 and 2150 mg/kg bw TOCP showed the expected reactions
(i.e., ataxia, deterioration of reflexes, and swelling, fragmentation
and disruption of myelin sheaths) (Krinke et al., 1974).
Table 2: Results of genotoxicity assays on profenofos.
Test System Test Object Concentration Purity Results Reference
of profenofos
Ames Test1 S. typhimurium 5,15,45,135, 405 µg/0.1 ml in DMSO ? Negative Arni and Muller, 1978
(reverse mutation) TA-98, TA-100,
TA-1535, TA-1537
Yeast Test1 S. cerevisae Nonactivated: 91.8% Negative Arni and Muller, 1982
(gene conversion, crossing (D7 strain) 12.5-500 µg/ml in DMSO
over, reverse mutation) Activated: 0.640 -10000 µg/ml in DMSO
Yeast Test1 S. cerevisae 39,156,625, 2500, 10000 µg/ml in DMSO 90.0% Negative Hool and Muller, 1986
(non-disjunction) (D61.M strain)
Mouse Lymphoma L5178Y mouse 0.078, 0.156, 0.313, 0.625 µg/ml in DMSO 91.8% Negative Strasser and Muller, 1982
Forward Mutation lymphoma cells
Assay1 (TK +/-)
DNA Repair Rat hepatocytes 0.016, 0.08, 0.4, 2 nl/ml 91.8% Negative Puri and Muller, 1982a
Test2 (UDS)3
DNA Repair Human fibroblasts 0.32, 1.6, 8, 40 nl/ml 91.8% Negative Puri and Muller, 1982b
Test2 (UDS)3
Dominant Lethal Test Mouse (male) 35, 100 mg/kg ? Negative Fritz, 1974a
(NMRI-derived) (single oral doses)
Nucleus Anomaly Test Chinese hamster 13, 26, 52 mg/kg (2 oral doses on 88.1% Negative Hool, et al., 1981
bone marrow consecutive days)
Table 2 (contd)
Test System Test Object Concentration Purity Results Reference
of profenofos
Somatic Cell Studies Male Swiss mouse 36, 162, 216 mg/kg (single oral doses) 72% Positive4 El Nahas, et al., 1988
in Mice (sister chromatid bone marrow
exchange, micronucleus,
chromosome aberration)
1 With and without metabolic activation.
2 No exogenous activation added.
3 UDS = unscheduled DNA synthesis.
4 Doses of 36-216 mg/kg produced increases in chromosome aberrations; doses of 162-216 mg/kg increased micronuclei formation and sister
chromatid exchanges.
In a second study, adult chickens received oral doses of 29.2,
58.5, 117, and 234 mg/kg bw of profenofos (38% EC) on days 0 and 21.
The acute oral LD50 of the formulation was 127.0 mg/kg bw. After
treatment on day 0, death occurred in 0%, 6.6%, 55%, and 100% of the
birds given doses of 29.2, 58.5, 117 and 234 mg/kg bw/day,
respectively. The increased mortality was accompanied by signs of
cholinesterase inhibition (i.e., weakness, lethargy, and anorexia).
After treatment on day 21, death occurred in 0%, 7.1%, and 67% of the
surviving birds given doses of 29.2, 58.5 and 117 mg/kg bw/day, and
similar signs of cholinesterase inhibition were observed. There were
no delayed neurotoxic symptoms or histologic changes in brain, spinal
cord, or sciatic nerves. A positive control group using 500 mg/kg bw
of TOCP exhibited clinical signs of delayed neurotoxicity (extreme
weakness of legs and wings) and neuropathological lesions in the
spinal cord and sciatic nerves (axonal degeneration and demyelination)
(Fletcher et al., 1977).
Profenofos (technical, 89.5% purity) was examined for delayed
neurotoxicity in a third study in adult chickens. In the first phase
of the study (day 0), birds received oral doses of 30 and 45.7 mg/kg
bw on the basis of preliminary acute toxicity studies in which an
acute LD50 dose of 45.7 mg/kg bw was determined. However, because
of increased mortality rates at these dose levels (70% at 30 mg/kg bw,
and 82% at 45.7 mg/kg bw), all surviving birds were redosed on day 21
at a new estimated oral LD50 of 17.1 mg/kg bw. At this dose level
of profenofos, a mortality rate of 5% was observed. The results
indicated that profenofos produced signs of lethargy and salivation in
treated birds, but no signs of delayed neurotoxicity or histological
lesions in the brain, spinal cord, or peripheral nerves. A positive
control group using 500 mg/kg bw of TOCP exhibited clinical signs of
delayed neurotoxicity (extreme weakness of legs and wings) and
lesions in the spinal cord and sciatic nerves (axonal degeneration and
demyelination) (Reinart et al., 1978).
Special studies on pesticide antagonistic agents
A protective effect of atropine given early after orally
administered profenofos in rats or intraperitoneally administered
profenofos in chicks and mice was demonstrated by a reduction in
mortality and toxic signs (e.g., salivation, tremors, sedation,
convulsions) typical of anticholinesterase exposure. The effect of
oximes was limited (Sachsse and Bathe, 1976; Gfeller and Kobel, 1974;
Glickman et al. 1984).
Special studies on pesticide interactions
No potentiating effects were found when mixtures of profenofos
and the organophosphates methidathion, methacrifos or diazinon were
given to rats in equitoxic doses (Sachsse and Bathe, 1977); (Sachsse
and Bathe, 1978).
Profenofos administered intraperitoneally to mice at 0.5 to 5.0
mg/kg bw strongly inhibited the liver microsomal esterase(s)
hydrolysing trans-permethrin. The intraperitoneal toxicity LD50
measured 24 hours after exposure of fenvalerate and malathion but not
that of trans-permethrin was greatly increased when the compounds were
given 1 hour after an intraperitoneal injection of 25 mg/kg bw of
profenofos (Gaughan et al., 1980).
Observations in humans
A group of six male spraymen, aged 20 to 25 years, was monitored
for whole blood cholinesterase activity over a period of 4 days while
treating fully grown cotton with a formulation containing 400 grams
profenofos and 40 grams cypermethrin per liter in organic solvents on
a plantation in the Multan region of Central Pakistan in September
1985. The application equipment consisted of hand-held battery
operated spinning disc devices. The workers wore heavy cotton shirts
with long sleeves, legs wrapped below the knees, and turbans which
were often used to cover the face. Cholinesterase activity was
measured at the end of each of the 4 work days after thorough washing
of the body. The results indicated that there was a tendency toward
inhibition of blood cholinesterase activity. Compared to baseline
pre-exposure cholinesterase readings, the average cholinesterase
levels were 103.3%, 84.8%, 85.6%, and 81.2% of control activities on
days 1, 2, 3, and 4, respectively. The lowest individual values were
72.7% and 73.1% of control levels on the fourth day in 2 of the 6
workers (Loosli, 1989).
No cases of poisonings in humans have been reported with either
the active ingredient per se or with profenofos formulations.
COMMENTS
Profenofos administered orally to rats was well absorbed and was
excreted primarily in the urine, but also in faeces. Profenofos was
biotransformed by a major pathway involving side chain depropylation,
desulfuration, and phenyl-ester bond cleavage to yield 4-bromo-2-
chlorophenol, and by a minor pathway involving side chain 0-de-
ethylation, and subsequent phenyl-ester bond cleavage to the above
phenol. Both pathways culminated in conjugation with glucuronic and
sulfuric acids. No unchanged parent compound was found in urine.
Faeces contained only minute amounts of the parent compound, the
intermediate phenol, and unidentified metabolites. A similar
absorption and excretion pattern was observed in hens and goats. No
unusual organ or tissue localization of 14C-labelled profenofos was
observed.
Profenofos has a moderate order of acute toxicity following oral
and dermal administration. It has been classified as moderately
hazardous by WHO (WHO, 1990).
In a dietary toxicity study in which rats were fed profenofos for
8-13 weeks, the NOAEL was 100 ppm (equivalent to 5 mg/kg bw/day) based
on the finding of brain cholinesterase inhibition at higher levels.
The observed reduction in erythrocyte cholinesterase at lower doses
was not considered to be of toxicological importance. Reduction in
food consumption and body weight gain were considered to be a
consequence of poor palatability of the compound.
In a six-month feeding study in dogs, profenofos caused
erythrocyte cholinesterase inhibition and plasma and liver carboxylase
inhibition at levels of 100 ppm or more. The highest dose level
tested, 500 ppm, resulted in reductions in erythrocyte counts,
haemoglobin and haematocrit levels and a decrease in food consumption.
All of the changes were reversible. No changes in brain
cholinesterase activity occurred. As noted above, enzyme inhibition
and reduced food consumption were not considered to be direct toxic
effects of profenofos administration. The NOAEL was 100 ppm (equal to
2.9 mg/kg bw/day) based upon the observed haematological changes.
In long-term feeding studies in mice and rats, no treatment-
related increases in the incidence of neoplasms was observed. In
mice, brain cholinesterase inhibition occurred at the highest dose
level tested, 100 ppm. The NOAEL was 30 ppm (equal to 5.8 mg/kg
bw/day in females) based on brain cholinesterase inhibition observed
at the next higher dose level in females. In rats erythrocyte
cholinesterase inhibition occurred at dietary levels of 10 and 100
ppm. The highest dose level of 100 ppm was also associated with an
increase in food consumption and a slightly elevated incidence of
parafollicular cell hyperplasia. This type of response is not seen in
man following exposure to chemicals. No effect on brain
cholinesterase was observed. The NOAEL was 100 ppm (equal to 5.7
mg/kg bw/day).
In a three-generation reproduction study in rats, reductions in
cholinesterase activity occurred in erythrocytes (F0, F1, and F2
males and females) at 20 ppm in the diet. This was the highest dose
tested. No changes in brain cholinesterase activity occurred. The
NOAEL for systemic toxicity and reproduction was 20 ppm (equivalent to
1.0 mg/kg bw/day) which was the highest dose tested.
In three teratology studies in rats the NOAELs for maternal
toxicity ranged from 30 to 90 mg/kg bw/day, based upon findings of
reduced food consumption, clinical signs of toxicity (e.g., diarrhoea,
dyspnoea, tremors, hypothermia) and increased mortality at higher dose
levels. No teratogenic activity was observed at dose up to and
including 120 mg/kg bw/day. The NOAEL for embryotoxicity/fetotoxicity
(based on an increased incidence of variants) was 18 mg/kg bw/day. In
two rabbit teratology studies, the NOAEL for maternal toxicity was 30
mg/kg bw/day, based upon findings of reduced food consumption and body
weight gain, the occurrence of soft stools and diarrhoea, and
increased mortality at higher dose levels. No embryotoxic, fetotoxic,
or teratogenic activity was observed at doses up to and including 175
mg/kg bw/day.
After reviewing all available in vitro and in vivo short-term
tests, the Meeting concluded that there was no evidence of
genotoxicity.
The Meeting based the ADI for profenofos on the NOAEL of 1 mg/kg
bw/day in the rat multigeneration study. Although there was no
evidence of any adverse effects at this dose level in the study, the
absence of information on reproductive parameters at higher levels
precluded the use of the NOAEL of 2.9 mg/kg bw/day in the 6-month
feeding study in dogs.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 30 ppm, equal to 5.8 mg/kg bw/day in females.
Rat: 20 ppm, equivalent to 1.0 mg/kg bw/day (reproduction).
Rat: 100 ppm, equal to 5.7 mg/kg bw/day in males (long-term
study).
Dog: 100 ppm, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw.
Studies which will provide information valuable in
the continued evaluation of the compound
Further observations in humans.
An additional multigeneration study in rats using higher doses.
REFERENCES
Arni, P. and Muller, D. (1978). Salmonella/mammalian-microsome test
with CGA 15'324 (Test for mutagenic properties in bacteria). Project
No.: 78/2531. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Arni, P. and Muller, D. (1982). Saccharomyces cerevisiae D7/mammalian
microsome mutagenicity test in vitro with CGA 15'324 (Test for
mutagenic properties in yeast cells). Project No.: 911557.
Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted
to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Bathe, R. (1974a). Acute oral LD50 of technical CGA 15'324 in the
mouse. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974b). Acute oral LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Bathe, R. (1974c). Acute dermal LD50 of technical CGA 15'324 in the
rat. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. (1974d). Acute oral LD50 of technical CGA 15'324 in the
dog. Project No.: Siss 3647. Unpublished report from Ciba-Geigy
Ltd., Basle Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Bathe, R. and Sachsse, K. (1979). Acute oral LD50 in the Chinese
hamster CGA 15'324. Project No.: 785329. Unpublished report from
Ciba-Geigy Ltd, Basle Switzerland. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Kuper, C.H. (1981a). Twenty-four months carcinogenicity study in
mice, CGA 15'324. Project No.: 483-133. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Burdock, G.A., Dudeck, L.E., Alsacker, R.D., Suchmann-Purvis, D., and
Fieser, S. (1981b). Two-year chronic oral toxicity study in albino
rats, CGA 15/324 technical. Project No.: 483-134. Unpublished report
from Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Cannelongo, B.F. (1982a). Rabbit acute dermal toxicity CGA 15'324
technical FL 811584. Project No.: 2460-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F., (1982b). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2462-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982c). Rabbit eye irritation CGA 15'324 technical
FL 811584. Project No.: 2461-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
Cannelongo, B.F. (1982d). Guinea pig sensitization CGA 15'324
technical FL 811584. Project No.: 2463-81. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd, Basle, Switzerland.
El Nahas, S.M., De Hondt, H.A., and Ramadan, H.A.I. (1988). In vivo
evaluation of the genotoxic potential of Curacron in somatic cells of
mice. Environ. Mol. Mutagen., 11(4): 515-522.
Fletcher, D., Gordon, D.E., Jenkins, D.H., Kennedy, G.L., Kinoshita,
F.K., and Phillips, B.M. (1977). Neurotoxicity study with CGA 15'324
38% E.C. in chickens. Project No.: IBT 8580-10426. Unpublished
report from Industrial Bio-Test Laboratories, Inc., Northbrook, IL,
USA. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland and
validated by the United States Environmental Protection Agency.
Fritz, H. (1974a). Dominant lethal study on CGA 15'324 tech., mouse
(test for cytotoxic or mutagenic effects on male germinal cells).
Project No.: 327438. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H. (1974b). Reproductive study - technical CGA 15'324, rat,
segment II (test for teratogenic or embryotoxic effects). Project
No.: 22741900. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Fritz, H., Becker, H., and Hess, R. (1979). Seg. II reproductive
study in rabbits, CGA 15'324 tech. Project No.: 785565. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gaughan, L.C., Engel, J.L., and Casida, J.E. (1980). Pesticide
interactions: Effects of organophosphorus pesticides on the
metabolism, toxicity and persistence of selected pyrethroid
insecticides. Pesticide Biochemistry and Physiology 14: 81-85.
Gfeller, W. and Kobel, W. (1984). CGA 15'324 technical, tentative and
antilethal study in the rat. Project No.: GU 820034. Unpublished
report from Ciba-Geigy Ltd, Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd, Basle, Switzerland.
Gfeller, W., Kobel, W., Schaeppi, U., Zak, F., and Hess, R. (1981).
CGA 15'324 tech., 6-months toxicity study with dogs. Project No.:
790804. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Glickman, A.H., Wing, K.D., and Casida, J.E., (1984). Profenofos
insecticide bioactivation in relation to antidote action and the
stereospecificity and acetylcholinesterase inhibition, reactivation,
and aging. Toxicity and Applied Pharmacology, 73: 16-22.
Harris, S.B. and Holson, J.F. (1982). A teratology study of CGA
15'324 technical in albino rats. Project No.: 282009. Unpublished
report from Science Applications, Inc., La Jolla, CA, USA. Submitted
to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Holson, J.F. (1983). Teratology study (Seg II) in albino rabbits with
CGA 15'324 technical. Project No.: 283003. Unpublished report from
Science Applications, Inc., La Jolla, CA, USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G., Langauer, M., and Muller, D. (1981). Nucleus anomaly test in
somatic interphase nuclei, CGA 15'324, Chinese hamster (test for
mutagenic effects on bone marrow cells). Project No.: 791557.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Hool, G. and Muller, D. (1986). Test for non-disjunction on
Saccharomyces cerevisiae D61.M in vitro, CGA 15'324 tech. Project
No.: 850811. Unpublished report from Ciba-Geigy Ltd, Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Horath, L.L. and Taylor, G.D. (1982). Acute aerosol toxicity study in
rats of CGA 15'324 technical FL 811528. Project No.: 420-0921.
Unpublished report from Toxi-Genics, Inc., Decatur, IL, USA.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
IBTL, Inc. (1978). Three generation reproduction study with CGA
15'324 technical in albino rats. Project No.: IBT 623-07924.
Unpublished report from Industrial Bio-Test Laboratories, Inc.,
Northbrook, IL, USA. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland. Validated by United States Environmental Protection
Agency.
Ifflaender, U. and Mucke, W. (1976). The metabolism of CGA 15'324 in
the rat. Project No.: 27/76. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd, Basle,
Switzerland.
Ifflaender, U., Mucke, W., and Esser, H.O. (1974). Distribution,
degradation and excretion of CGA 15'324 in the rat. Project No.:
16/74. Unpublished report from Ciba-Geigy Ltd, Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd, Basle, Switzerland.
Johnson, S., Tai, C.N., and Katz, R. (1984). Profenofos technical,
21-day dermal toxicity study in rabbits. Project No.: MIN 842008.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Kobel, W. and Gfeller, W. (1983). Acute oral LD50 in the rat, CGA
15'324 tech. GU Project No.: 830188. Unpublished report from Ciba-
Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd.,
Basle, Switzerland.
Krinke, G., Ullmann, L., and Sachsse, K. (1974). Acute oral LD50
and neurotoxicity study of technical CGA 15'324 in the domestic fowl
(Gallus domesticus). Project No.: Siss 2850. Unpublished report from
Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Kuhn, J.O. (1988). Acute dermal toxicity study in rabbits, profenofos
technical. Project No.: 5522-88. Unpublished report from
Stillmeadow, Inc., Houston, TX, USA. Submitted to WHO by Ciba-Geigy
Ltd., Basle, Switzerland.
Loosli, R. (1985). Project No.: 850910. Unpublished summary report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Mucke, W. (1986). The renal excretion of U-14C-Phenyl CGA 15'324 by
male rats after oral administration (exposure monitoring). Project
No.: 13/86. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Oakes, T.L., Marco, G.J., and Ballantine, L. (1986). Metabolism of
14C-profenofos in chickens dosed at 5.0 ppm. Project No.: ABR-
86002. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Piccirillo, V.J. (1978). 90-day subacute oral toxicity study in rats,
CGA 15'324 technical. Project No.: 483-135. Unpublished report from
Hazleton Laboratories America, Inc., Vienna, VA, USA. Submitted to
WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Puri, E. and Muller, D. (1982a). Autoradiographic DNA repair test on
rat hepatocytes, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811490. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Puri, E. and Muller, D. (1982b). Autoradiographic DNA repair test on
human fibroblasts, CGA 15'324, (in vitro test for DNA damaging
properties). Project No.: 811658. Unpublished report from Ciba-Geigy
Ltd., Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Reinart, D., Fletcher, D., Arceo, R.J., and Gordon, D.E. (1978). 42-
day neurotoxicity study with CGA 15'324 technical in adult chickens.
Project No.: IBT 8580-11187. Unpublished report from Industrial
Bio-Test Laboratories, Inc., Decatur, IL. USA. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland and validated by the United States
Environmental Protection Agency.
Sachsse, K. and Bathe, R. (1975). Acute intraperitoneal LD50 in the
rat of technical CGA 15'324. Project No.: Siss 5048. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1976). The therapeutic activity of
pralidoxim (PAM) and TOXOGONIN\ with regard to CGA 15'324 in the rat.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1977). Potentiation study CGA 15'324
versus 2 insecticides CA 13'005 (methidathion) and G 24'480 (diazinon)
in the rat. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Bathe, R. (1978). Potentiation study CGA 20'168
versus 6 insecticides, C 177 (DDVP), C570 (phosphamidon), GS 13'005
(methidation), G 24'480 (diazinon), CGA 15'324 and malathion. Project
No.: 404478 - Siss 6526. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Sachsse, K. and Ullmann, L. (1974a). Acute inhalation toxicity of
technical CGA 15'324 in the rat. Project No.: Siss 3647.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974b). Acute oral LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647 Unpublished report
from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by Ciba-
Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974c). Acute dermal LD50 of technical
CGA 15'324 in the rabbit. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974d). Skin irritation in the rabbit
after single application of technical CGA 15'324. Project No.: Siss
3647. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974e). Irritation of technical CGA
15'324 in the rabbit eye. Project No.: Siss 3647. Unpublished
report from Ciba-Geigy Ltd., Basle, Switzerland. Submitted to WHO by
Ciba-Geigy Ltd., Basle, Switzerland.
Sachsse, K. and Ullmann, L. (1974f). Skin sensitizing (contact
allergenic) effect in Guinea pigs of technical CGA 15'324. Project
No.: Siss 3647. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Strasser, F.F. and Muller, D. (1982). L5178/TK+/- mouse lymphoma
mutagenicity test, CGA 15'324 (in vitro test for mutagenic
properties of chemical substances in mammalian cells). Project No.:
811491. Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Sugiya, J., Yoshida, K., Tamaki, Y., Yokota, M., Abo, Y., and
Kawakami, S. (1982). Teratogenicity in rats administered CGA 15'324
(profenofos) prenatally during the major organogenic period.
Unpublished report from Ciba-Geigy Ltd., Basle, Switzerland.
Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Thomas, R.D., Cassidy, J.E., and Marco, G.J. (1976). Metabolism and
balance study of 0-14C-CGA 15'324 in a lactating goat Project No.:
GAAC-76024. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1976). CGA 15'324 technical 21-day dermal toxicity study in rabbits.
Project No.: Siss 5119. Unpublished report from Ciba-Geigy Ltd.,
Basle, Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle,
Switzerland.
Ullmann, L., Luetkemeier, H., Sachsse, K., Zak, F., and Hess, R.
(1977). CGA 15'324, 21-day inhalation study on the rat. Project No.:
Siss 5119. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
WHO (1990). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1990-1991 (WHO/PCS/90.1).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Williams, S.C., Marco, G.J., Simoneaux, B.J., and Ballantine, L.
(1984). Percutaneous absorption of 14C-profenofos in rats. Project
No.: ABR-84023. Unpublished report from Ciba-Geigy Ltd., Basle,
Switzerland. Submitted to WHO by Ciba-Geigy Ltd., Basle, Switzerland.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1984).
Phosphorothiolate pesticides and related compounds: Oxidative
bioactivation and aging of the inhibited acetylcholinesterase.
Pesticide Biochemistry and Physiology, 21: 22-30.
Wing, K.D., Glickman, A.H., and Casida, J.E. (1983). Oxidative
bioactivation of S-alkyl phosphorothiolate pesticides:
Stereospecificity of profenofos insecticide activation. Science, 219:
63-65.
