
AZINPHOS-METHYL
First draft prepared by Mr. M. Watson,
Ministry of Agriculture, Fisheries and Food,
Harpenden, Hertfordshire, United Kingdom
EXPLANATION
Azinphos-methyl was evaluated for acceptable daily intake by
previous Joint Meetings in 1965, 1968 and 1973 (Annex I, 3, 12, 22).
An ADI of 0 - 0.0025 mg/kg bw was established at the last evaluation.
Since that time additional information has become available and the
results of studies submitted to the present meeting are summarized in
this monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
The pharmacokinetic behaviour of carbonyl-14C-labelled
azinphos-methyl was investigated in rats. The material was almost
completely absorbed from the digestive tract, and irrespective of dose
and route of administration, 60 to 70% was eliminated in the urine and
25 to 35% in the faeces within 48 hours. Less than 0.1% of the
administered activity was eliminated with the respiratory air within
24 hours of dosing, and in rats with biliary fistulas around 30% of
the intravenously administered activity was eliminated in the bile
within 24 hours of dosing. Two days after dosing the total activity
content in the animal (excluding digestive tract) was less than 5% of
the administered dose; by 4 days this had declined to 2% and by 16
days to 1%. Six hours after dosing, the highest concentrations of
radioactivity were found in the organs of elimination (liver and
kidney) with relatively high concentrations found in blood. The
activity concentrations decayed rapidly in all organs up to 2 days
post dosing, but thereafter the activity was more slowly eliminated.
At 16 days after dosing the highest concentration was found in the
erythrocytes. In vitro studies, in which whole blood was incubated
with labelled parent compound, did not show any accumulation of
radioactivity in the blood constituents (Patzschke et al., 1976).
Dimethylthiophosphate (DMTP), one of the primary metabolites of
azinphos-methyl, was detected in the urine of rats following dermal
application of azinphos-methyl. A strong correlation was found
between the amount of pesticide applied and urinary DMTP levels
(Franklin et al., 1982).
Urinary DMTP levels were measured in workers applying
azinphos-methyl using orchard air-blast equipment and these data were
used to estimate exposure to azinphos-methyl. These estimates were
compared to exposure estimates derived by chemical analysis of patches
attached to the protective clothing. It was concluded in by the
author that urinary metabolite data provide a more reliable and
accurate estimate of exposure than patch data (Franklin et al.,
1986).
A sample of radiolabelled 14C azinphos methyl was applied to the
forearm of an unspecified number of human subjects and the urinary
excretion of radiolabel was quantified. Data obtained after
intravenous dosing was used to correct the skin penetration data for
incomplete urinary recovery. Using these data it was estimated that
dermal penetration approximated 16% of the applied dose (Feldmann &
Maibach, 1974).
The dermal penetration of azinphos-methyl through ventral forearm
skin in man was around 16% of the applied dose over a 24 hour exposure
period. This absorption increased by a factor of around 3.5 if the
application site was occluded and increased by a factor of around 3.8
when damaged skin was compared to intact skin (Webster & Maibach,
1985).
The pharmacokinetic behaviour of benzazimide was investigated in
rats using the ring-labelled 14C-compound. After oral administration
the 14C- activity was almost completely absorbed from the
gastrointestinal tract. Elimination of the activity took place
quickly, 24 hours after administration only 1.3% of the amount
administered was present in the animal not including the
gastrointestinal tract. More than 99% of the amount administered was
eliminated within 48 hours (54 to 66% in the urine and 33 to 45% via
the faeces) (Weber et al., 1980).
Biotransformation
The metabolism of azinphos-methyl was investigated by
administration of ring-UL-14C azinphos-methyl to male and female
Sprague-Dawley rats. The metabolic pathway of azinphos-methyl in rats
is proposed as detailed in Figure 1. Upon absorption, azinphos-methyl
is rapidly metabolized by mixed function oxidases and GSH-transferases
in the liver and other tissues, which results in the formation of
azinphos-methyl oxygen analog, mercaptomethylbenzazimide, glutathionyl
methylbenzazimide and desmethyl isoazinphos-methyl. Further
hydrolysis, methylation and oxidation of mercaptomethyl-benzazimide
forms benzazimide, methylthiomethylbenzazimide and its corresponding
oxidised metabolites. Hydrolysis of glutathionyl methyl-benzazimide
may result in the formation of cysteinylmethyl-benzazimide.
Subsequent oxidation of cysteinyl-methylbenzazimide forms its
corresponding sulfoxide and sulfone (Kao, 1988).
The rate of disappearance of azinphos-methyl effected by a
hepatic oxidative desulfurating system and a demethylating system was
investigated in liver homogenates from four different species (rat,
guinea pig, chicken and monkey). Azinphos-methyl was metabolized by
both systems and homogenates from all species were uniformly active
(Rao & McKinley, 1969).
Effects on enzymes and other biochemical parameters
The acute oral toxicity of azinphos-methyl, dissolved in
propylene glycol, was investigated in groups of female mice and the
effect of the oxime antidote, toxogonin (80 mg/kg bw
intraperitoneally, 15 minutes prior to oral dosing), was determined.
Antidote treatment reduced the toxicity of azinphos-methyl by
increasing the LD50 by a factor of 2 (Sterri et al., 1979).
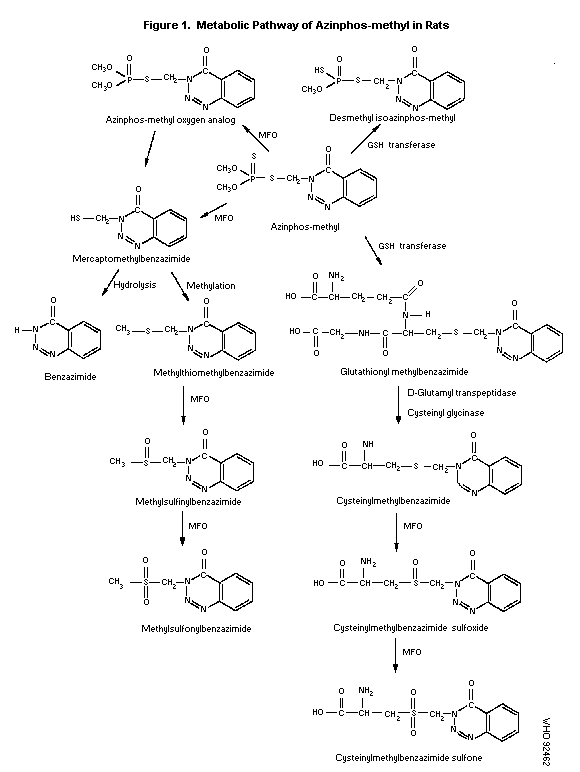 Table 1. Results of acute toxicity tests with azinphos-methyl and
related materials
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl Oral Rat DMSO m 5.6 Crawford (1974)
f 6.4
Benzazimide Oral Rat DMSO m 412
f 269
Methyl benzazimide Oral Rat DMSO m 330
f 330
Azinphos methyl Oral Rat Cremophor EL m 25.4 Flucke (1979)
Azinphos methyl Oral Rat Cremophor EL m 9.1 (fasted) Heimann (1981)
m 17.25 (non-fasted)
Azinphos methyl Oral Rat Cremophor EL m 6.7 (fasted) Heimann (1982)
m 12.8 (non-fasted)
Dermal Rat Cremophor EL m 225
f 155
Azinphos methyl Oral Rat Cremophor EL m 7.1 Heimann (1987)
Azinphos methyl Oral Rat CMC (fasted) m 19 Lamb et al. (1974)
f 16
m 19
(non-fasted) f 10
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Benzazimide Oral Rat CMC (fasted) m 576 Lamb (1974)
f 368
m 576
(non-fasted) f 487
Methyl benzazimide Oral Rat CMC (fasted) m 412
f 390
m 524
(non-fasted) f 460
Azinphos methyl Oral Rat Cremophor EL m 4.6 Mihail (1978)
f 4.4
Dermal Rat Cremophor EL m 2500-5000*
Oral Dog Cremophor EL m >10
Azinphos methyl Oral Rat Methylene chloride/ m 26 Pasquet et al. (1976)
Tween/80 Gum Arabic f 24
Dermal Rat Acetone/Ethanol/ f 90
Peanut Oil
Benzazimide Dermal Rabbit Tap water m >2000 Sheets (1988)
f >2000
Azinphos methyl 4 hr inhal Rat PEG/ETOH m 155 Shiotsuka (1987a)
f 132 (mg/m3)
Benzazimide 4 hr inhal Rat None m >1760* Shiotsuka (1987b)
(mg/m3)
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl 1 hr inhal Rat PEG/ETOH m 396 Shiotsuka (1988)
f 310 (mg/m3)
Azinphos methyl Oral Rat Cremophor EL m 15.6 Thyssen (1976a)
Azinphos methyl Oral Rat Cremophor EL m 16.75 Thyssen (1976b)
Methamidophos Oral Rat Cremophor EL m 31.9
Azinphos methyl +
Methamidophos Oral Rat Cremophor EL m 19.5
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977a)
Propoxur Oral Rat Cremophor EL m 39
Azinphos methyl +
Propoxur Oral Rat Cremophor EL m 29.3
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977b)
Azinphos ethyl Oral Rat Cremophor EL m 11.8
Azinphos methyl +
Azinphos ethyl Oral Rat Cremophor EL m 11.1
* sex not specified.
m male
f female
Toxicological studies
Acute Toxicity Studies
Results of acute toxicity tests with azinphos-methyl and related
materials are listed in Table 1.
Short-term studies
Rats
A 12-week inhalation study has been described in the published
literature. Groups of 10 male and 10 female Wistar rats were exposed
in inhalation chambers to mean aerosol concentrations in the air of 0
(control), 0.195, 1.24 and 4.72 mg/m3 azinphos methyl, for 6 hours
daily, 5 days per week, for 12 weeks. At the high dose level body
weight gain was depressed in males and erythrocyte cholinesterase
activity was inhibited in males and females. There was no other
evidence of any reaction to treatment. The NOAEL was 1.24 mg/m3
based on reduced weight gain in males at the high dose level
(Kimmerle, 1976).
Rabbits
In a dermal toxicity study in rabbits, azinphos-methyl was
applied for 6 hours per day, to the shaved dorsal and lateral skin, at
dose levels of 0 (control), 2, or 20 mg/kg bw, for 5 days per week for
3 weeks. Each group consisted of 6 males and 6 females, with the skin
left intact in 3 animals of each sex and abraded in the others.
Investigation of cholinesterase activity revealed a marginal
(approximately 30%) depression of erythrocyte activity, compared to
controls, in males and females treated with 20 mg/kg. Cholinesterase
activity in plasma and brain, and erythrocyte activity at 2 mg/kg,
remained undisturbed by treatment. There was no difference between
the groups with intact and abraded skin, and all other investigations
(clinical signs, measurement of food intake and weight gain, further
clinical chemistry, haematology and urinalysis and pathological
investigations including limited histopathology) revealed no
treatment-related changes. The NOAEL was 20 mg/kg bw, since only
erythrocyte cholinesterase activity was affected at this dose, the
highest dose used, with no effect on cholinesterase activity in brain
(Flucke & Schilde, 1980).
Dogs
In a 52 week toxicity study in beagle dogs, azinphos-methyl
(purity 91.9%) was administered, via the diet, to four groups, each of
4 males and 4 females, at dietary levels of 0 (control), 5, 25 or 125
ppm. Clinical signs of reaction to treatment were confined to a
higher incidence of diarrhoea in dogs receiving 125 ppm. Two males
receiving 125 ppm failed to gain weight during the course of the
study, but food intake remained unaffected by treatment. Haematology
and urinalysis revealed no indication of any reaction to treatment.
Clinical biochemistry tests revealed a depression of cholinesterase
activity in plasma and erythrocytes at 25 and 125 ppm and in brain at
termination at 125 ppm. There was also a very slight increase,
compared to controls, in liver cytochrome P450 and N-demethylase
activity at the high dose and a reduction in albumin levels.
Pathological investigations (macroscopic examination, organ weight
analysis and histopathology) revealed no evidence of any reaction to
treatment with azinphos-methyl. The NOAEL was 25 ppm (equal to
0.74 mg/kg bw/day) based on reduced weight gain and inhibition of
acetylcholinesterase activity in brain (Allen, et al., 1990).
Long-term/carcinogenicity studies
Mice
A bioassay of azinphos-methyl (purity 90% from manufacturing
specification) for possible carcinogenicity was conducted by the NCI.
The experiment involved administering the test material, in the feed,
to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats of each sex
were treated for 80 weeks, then observed for 34 or 35 weeks. Males
received time weighted average doses of 78 or 156 ppm, females
received 62.5 or 125 ppm. Matched controls consisted of 10 untreated
rats of each sex; pooled controls consisted of matched controls
combined with 95 male and 95 female untreated rats from similar
bioassays of 10 other chemicals. The mouse study was of similar
design; groups of 50 mice were treated for 80 weeks, then observed for
12 or 13 weeks, males received 31.3 or 62.5 ppm and females 62.5 or
125 ppm, matched controls consisted of 10 males and 10 females, pooled
controls 130 males and 120 females. Typical signs of organophosphate
intoxication (hyperactivity, tremors and dyspnoea) were observed in a
few animals of both species. Weight gain in treated males and high
dose females of both species was lower than in control animals. In
rats there was some evidence of decreased survival at the high dose
compared to controls but this was not seen in mice. In both sexes, at
all doses, survival to termination was adequate for assessment of
effects on late appearing tumours. The report concluded that in rats
the incidence of tumours of the pancreatic islets, and of follicular
cells in the thyroid in males suggested, but did not clearly
implicate, azinphos-methyl as a carcinogen in these animals. There
was no similar evidence in female rats and in mice of each sex there
was no increased incidence of tumours that could be related to the
administration of azinphos-methyl (National Cancer Institute, 1978).
In a carcinogenicity study of azinphos-methyl (purity 88.6%) in
mice, groups of 50 male and 50 female CD-1 mice received dietary
levels of 0 (control), 5, 20, or 40/80 ppm for two years. (The study
was initially started with 80 ppm as the high dietary level, but this
was reduced to 40 ppm after one week, due to severe reaction to
treatment, including mortality, at 80 ppm). Following the reduction
in the high dietary level, there were no clinical signs of reaction to
treatment and mortality remained unaffected by treatment. Weight gain
and food intake remained unaffected by treatment at dietary levels up
to and including 40 ppm. Haematological investigations revealed no
indication of any reaction to treatment. Measurement of
acetylcholinesterase activity revealed that at 5 ppm, activities in
plasma, erythrocyte and brain remained comparable with control values.
At 20 and 40 ppm there was a dose-related inhibition of cholinesterase
activity in plasma and erythrocytes. A similar effect was noted in
brain, except that males were only affected at 40 ppm, while females
exhibited a depression of brain cholinesterase activity at 20 and 40
ppm. Pathological investigations revealed no evidence of any reaction
to treatment, in particular there was no evidence of any carcinogenic
effect of azinphos-methyl. The NOAEL was 5 ppm (equal to
0.88 mg/kg bw/day) based on inhibition of cholinesterase in plasma,
erythrocytes and brain at 20 ppm (Hayes, 1985).
Rats
In a combined long-term toxicity and carcinogenicity study in
rats, groups of 60 male and 60 female Wistar rats received
azinphos-methyl (purity 87.2%) in the diet at levels of 0 (Control),
5, 15 or 45 ppm. From each group, 10 rats per sex were killed after
12 months, while all survivors were killed after 24 months continuous
treatment. There were no clinical signs of reaction to treatment and
survival was unaffected by azinphos-methyl. Weight gain of high dose
males was slightly less than controls but growth in other groups was
not affected by treatment. Clinical biochemistry (apart from
acetylcholinesterase investigations), haematology and urinalysis tests
revealed no indication of any reaction to treatment. Determinations
of acetylcholinesterase activities in erythrocytes, plasma and brain
revealed a marked inhibition, compared to controls, in males and
females from the high dose group (erythrocytes, plasma and brain) and
a less marked effect at 15 ppm (males: erythrocytes, females:
erythrocytes and plasma). Acetylcholinesterase activity in brain from
rats treated at 15 ppm and in erythrocytes, plasma and brain from rats
treated at 5 ppm, remained unaffected by treatment with
azinphos-methyl. Pathological examinations (including gross
examination, organ weight analysis and histological examination of
tissues) revealed no evidence of any reaction to treatment; in
particular there was no evidence of any carcinogenic effect of
azinphos-methyl. The NOAEL was 15 ppm (equal to 0.86 mg/kg bw/day)
based on effects on body weight gain and brain acetylcholinesterase
(Schmidt, 1987).
Reproduction studies
In a two generation (two litters per generation) reproduction
study in rats azinphos-methyl (purity 87.2%) was administered to
groups of 12 male and 24 female Wistar rats at dietary levels of 0
(control), 5, 15 or 45 ppm. At 15 and 45 ppm there was a decrease in
fertility of F0 rats and the total number of delivered pups. At 45
ppm there was an increased mortality of dams in the F0 generation and
reduced pup viability during lactation. As a consequence of these
effects only 5 females were available for mating in the F1b
generation. During mating of the F1b generation, fertility was again
adversely affected at 15 ppm but not to as great an extent as it was
during the F0 generation. At all stages of the study, there was no
evidence of treatment induced malformations and food intake remained
unaffected. Clinical signs of reaction to treatment, including
cholinergic signs, were seen at the high dose and weight gain was
adversely affected at 15 and 45 ppm. The NOAEL was 5 ppm, equal to
0.48 mg/kg bw/day, based on the adverse effects on fertility and body
weight gain seen at 15 and 45 ppm (Eiben & Janda, 1987).
A further study was conducted in order to investigate the effects
on reproductive performance noted in the study described above. The
objectives of this further, one generation study were to investigate
whether the slight effect on fertility at 15 ppm could be confirmed,
and, if reproducible, to determine whether the effect was attributable
to treatment of the male or the female and to determine if
reproductive effects were associated with treatment-induced inhibition
of cholinesterase activity. Azinphos-methyl (purity 92.0%) was
administered to groups of 18 male and 46 female Wistar rats, at
dietary levels of 0 (control), 5, 15, or 45 ppm. Treated males and
females were paired, and dams allowed to rear litters to weaning.
Additional treated males were paired with untreated females. At 15
ppm, when males and females were treated, the viability index was
reduced, largely confirming the results of the previous study.
However, after treatment of male parental animals only, reproductive
parameters remained unaffected, even at 45 ppm. Investigations of
cholinesterase activity in parental animals revealed a depression in
activity in plasma and erythrocytes at all dose levels, and a
depression in activity in brain at 45 ppm in males and at 15 and 45
ppm in females. At 45 ppm, brain cholinesterase activity in pups was
also depressed. The NOAEL was 5 ppm, equal to 0.43 mg/kg bw/day,
based on the adverse effects on fertility and depression of brain
cholinesterase activity seen at 15 ppm (Holzum, 1990).
Special studies on delayed neurotoxicity
In a published report of experiments designed to investigate the
potential relationship between delayed neurotoxicity and copper
concentration in the serum of hens, it was reported that
azinphos-methyl failed to produce neurotoxic symptoms after either
single or repeated doses (Kimmerle & Loser, 1974).
In an acute delayed neurotoxicity test, azinphos-methyl (purity
85%)was administered twice, at the unprotected LD50 dose level of 330
mg/kg to a group of 30 white leghorn hens, with an interval of 21 days
between doses. Groups of untreated control, vehicle control and
positive control (TOCP 600 mg/kg bw) animals, each composed of 10
animals, were also included. Atropine was used for symptomatic
treatment after dosing. A total of 11 hens treated with
azinphos-methyl survived until termination. These animals appeared
normal during the last 12 or 13 days of the study, but exhibited
varying degrees of impaired locomotor activity soon after dosing.
Histopathological examinations indicated that azinphos-methyl did not
increase the incidence or severity of lesions in the nerve tissue
compared to untreated and vehicle controls. Investigations of
neuropathy target esterase activity were not included in the study
(Glaza, 1988).
Special studies on embryo/fetoxicity
Mice and rats
The effects of azinphos-methyl on development in rats and mice
were investigated in a series of experiments. On the basis of
preliminary toxicity studies doses of 0, 1.25, 2.5 and 5.0 mg/kg
bw/day were selected for developmental studies in both species, which
consisted of two phases. During the first phase, pregnant rats and
mice were treated for 10 days, starting on gestational day 6. During
the second phase, pregnant rats were treated from day 6 of gestation
to day 21 post partum. In the first phase, maternal toxicity was seen
only in rats receiving the high dose. When dams and fetuses were
examined (day 18 of gestation for mice, day 20 for rats) there was no
dose-related increase in anomalies or malformation in rats or mice.
In the second phase, dams in the high-dose group were more sensitive
to azinphos-methyl in the latter stages of gestation and signs of
anticholinesterase intoxication, including mortality, were observed.
As a result, only one litter (out of 13) survived to weaning in this
group. It was concluded that azinphos-methyl had little primary
effect on development in rats and mice (Short, et al, 1978; Short,
et al., 1980).
Rats
In a teratology study in rats, groups of 33 inseminated dams
received azinphos-methyl (purity 87.7%) from day 6 to day 15 of
gestation (day of insemination = day 0), at dose levels of
0 (control), 0.5, 1.0 or 2.0 mg/kg bw/day. From each group, 5 dams
were killed on day 16 of gestation and the remaining dams on day 20.
On day 16 of gestation, cholinesterase activities in plasma,
erythrocytes and brain were depressed, compared to controls, in dams
at the high dose only (fetal tissues were not examined). By day 20 of
gestation there was indication of recovery in cholinesterase activity
in all previously affected tissues and fetal brain cholinesterase
activity was comparable with control values. Azinphos-methyl did not
affect any maternal reproductive parameters and there was no
indication of treatment-related embryotoxicity, fetotoxicity or
teratogenicity at any dose level. The NOAEL for maternal toxicity was
1.0 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 16 of gestation (Kowalski et al., 1987).
Rabbits
In a teratology study in rabbits, groups of 11 or 12 pregnant
animals received daily oral doses of azinphos-methyl (purity 92.4%)
from day 6 to day 18 of gestation (day of insemination = day 0) at
levels of 0 (Control), 0.3, 1.0 or 3.0 mg/kg bw/day. Caesarean
section was carried out on day 29 of gestation. Azinphos-methyl
induced no evidence of maternal toxicity at any dose level and there
were no detectable effects on embryonic nor fetal development
(Machemer, 1975).
In a further teratology study in rabbits, groups of 20
inseminated does received daily oral doses of azinphos-methyl (purity
87.7%) from day 6 to day 18 of gestation (day of insemination = day 0)
at levels of 0 (Control), 1, 2.5 or 6 mg/kg bw/day. Ataxia in 4 high
dose does and tremors in 2 of these same animals represented clinical
signs of reaction to treatment.
Plasma and erythrocyte cholinesterase activity, on day 19 of
gestation, was depressed compared to controls at the mid and high
dose. By day 28 of gestation there was clear evidence of recovery in
plasma and erythrocyte cholinesterase activity, although activity in
brain was depressed, compared to controls, at the high dose.
Azinphos-methyl did not affect any maternal reproductive parameters
and there was no evidence of any treatment-related effect on
embryotoxicity, fetotoxicity or teratogenicity at any dose level. The
no observable adverse effect level for maternal toxicity was
2.5 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 28 of gestation (Clemens et al., 1988).
Special studies on genotoxicity
In Salmonella/microsome point mutation tests, azinphos-methyl
(purity >88.8%) showed no evidence of mutagenic activity which could
be classified as positive results in the tests. In one test there was
reproducible evidence of a slight dose-dependent increase in revertant
frequency in one test strain, but the increase was less than 2-fold
(Herbold, 1978; Herbold, 1988; Lawlor, 1987).
Azinphos-methyl (purity 91.1%) exhibited no mutagenic activity in
a reverse mutation test with Saccharomyces cerevisiae (Hoorn, 1983).
In Chinese hamster ovary cells in vitro, azinphos-methyl
induced chromosomal anomalies in a dose related fashion. Most
commonly observed were chromatid breaks and exchanges. In a test with
human lymphocytes in vitro, (purity 91.9%) there were no chromosomal
aberrations induced in the absence of S-9 mix but clear,
treatment-related variations were noted when azinphos-methyl was
tested in the presence of S-9 mix at cytotoxic concentrations. In an
investigation of ability to induce sister chromatid exchanges in
Chinese hamster V79 cells in vitro, azinphos-methyl was shown not to
increase the frequency of sister chromatid exchange, but did induce
some cell cycle delay (Alam, 1974; Herbold, 1986; Chen 1982 et al.,
1982a,b).
The potential of azinphos-methyl (purity 91.1%) to cause DNA
damage was assessed in Rosenkranz and Leifer's pol test employing two
E. coli strains which vary in regard to their repair systems for DNA
damage. The results showed that azinphos-methyl gave no indication of
any effect on DNA damage. In a primary rat hepatocyte unscheduled DNA
synthesis assay azinphos-methyl (purity 91.1%) did not induce
significant changes in the nuclear labelling of primary rat
hepatocytes and it was concluded that azinphos-methyl did not induce
DNA damage in this assay (Herbold, 1984; Myhr, 1983).
Mutagenic effects of azinphos-methyl (purity 92.3%) in vivo
were investigated in a micronucleus test and a dominant lethal test;
both in mice. In the micronucleus test two doses of azinphos-methyl
(2 x 2.5 or 2 x 5.0 mg/kg bw) were given 24 hours apart and a femoral
marrow smear was prepared 6 hours after the second dose; there was no
indication of any mutagenic effect. In the dominant lethal study male
mice received a single oral dose of 4 mg/kg bw azinphos-methyl and
were then mated with untreated females over 12 consecutive periods of
4 days. Fertility remained unaffected and there were no
treatment-related differences in implantation parameters (Herbold,
1979a,b).
An effort was made to evaluate the genotoxicity of a variety of
pesticides, with the specific objectives of comparing different
in vivo and in vitro assays, examining the spectrum of genetic
activity displayed by the selected pesticides and examining the test
results in relation to other biological and chemical features of the
pesticides. In this research programme azinphos-methyl has been
tested in a range of 14 mutagenicity tests, examining point or gene
mutations, DNA damage and chromosomal effects. Positive results for
azinphos-methyl were seen in only two tests: a forward mutation assay
in mouse lymphoma L5178Y cells (only in the presence of S-9 mix) and
a mitotic recombination assay in Saccharomyces cerevisiae strain D3.
Azinphos-methyl was negative in tests for point/gene mutation and DNA
damage in prokaryotes and showed no positive results in tests looking
at chromosomal effects (Waters et al., 1982).
Special studies on skin and eye irritation and sensitization
In a skin irritation study employing 6 rabbits, 24 hour exposure
to azinphos-methyl at intact and abraded skin sites did not cause any
signs of irritation. In an eye irritation study, exposure of the
conjunctiva of the eye to azinphos-methyl for 5 minutes (5 rabbits) or
24 hours (3 rabbits) caused no significant reaction (Thyssen & Lorke,
1981).
In a dermal irritation study utilizing 6 New Zealand white
rabbits, 0.5 g of benzazimide was moistened with water and kept in
contact with the shaved skin for 4 hours. At 30 and 60 minutes and
24, 48 and 72 hours after patch removal there was no evidence of
erythema or oedema at the treatment sites. Benzazimide is therefore
not a skin irritant in rabbits (Eigenberg, 1987).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Magnusson and Kligman
maximization test. The study revealed that azinphos-methyl had a
sensitizing effect in 95% of the test animals (Flucke, 1986).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Buehler patch test. By means
of a dermal application of a concentration of 25% sensitization was
induced in approximately 50% of the test animals (Porter et al.,
1987).
In another Buehler patch test dermal application of a
concentration of 12.5% induced sensitization in approximately 50% of
the test animals when challenged using a 6% concentration, but using
a challenge concentration of 0.6% failed to elicit any relevant skin
reactions (Heimann, 1987b).
Observations in humans
Employees working in the formulation of azinphos-methyl products
have been subjected to regular medical examinations and no general
impairment of health has been observed. In one isolated case it was
considered probable that contact with azinphos-methyl was the cause of
generalized dermatosis in an apparently hypersensitive, very dry skin
(Faul, 1981; Miksche, 1981).
Published reports from the pesticide incident monitoring system
in the United States of America and additional data from the state of
California in the USA have been reviewed. Between 1982 and 1988 a
small number of incidents have been reported annually (involving 5-12
persons each year) which have been definitely, probably or possibly
associated with azinphos-methyl either alone or in combination with
other pesticides. In addition, two incidents occurred in 1987, one
involving 26 people, the other involving 32 people. The first
involved spray drift in adverse weather conditions. The second
involved workers who experienced symptoms including headache, nausea,
weakness and vomiting upon entry to a field to pick peaches 3 days
after methomyl was applied to the crop and about 6 weeks after an
application of azinphos-methyl (US EPA, 1981; Mahler, 1991).
COMMENTS
The toxicokinetics of azinphos-methyl has been investigated
following oral administration in rats. It does not accumulate in body
tissues.
In a 52-week study in dogs, using dietary concentrations of 0, 5,
25 or 125 ppm the NOAEL was 25 ppm (equal to 0.74 mg/kg bw/day), based
on reduced body-weight gain and inhibition of acetylcholinesterase
activity in brain at 125 ppm.
Long-term/carcinogenicity studies in rats at dietary
concentrations of 0, 5, 15, or 45 ppm and in mice at 0, 5, 20 or 40
ppm showed that azinphos-methyl has no carcinogenic potential in
either species. These results clarified earlier equivocal findings in
rats in an NCI bioassay. The NOAEL in rats was 15 ppm (equal to
0.86 mg/kg bw/day), based on effects on brain acetylcholinesterase at
45 ppm. In mice the NOAEL was 5 ppm (equal to 0.88 mg/kg bw/day),
based on inhibition of cholinesterase in plasma, erythrocytes and
brain at 20 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 15 or 45 ppm, fertility and pup viability
during lactation were adversely affected, equivocally at 15 ppm and
markedly at 45 ppm. The NOAEL was 5 ppm, equal to 0.48 mg/kg bw/day.
Teratology studies in rats, mice and rabbits did not indicate
teratogenic effects at doses up to 2, 5 and 6 mg/kg bw/day
respectively.
The data from genotoxicity studies with azinphos-methyl were
conflicting. However, in vivo studies were negative, the positive
data being confined to some in vitro studies. After reviewing the
available information it was concluded that it is unlikely that
azinphos-methyl is genotoxic to humans.
Acute delayed neurotoxicity tests in hens with azinphos-methyl
gave negative results.
The 1973 JMPR reported that daily doses up to and around 0.3
mg/kg bw/day for 30 days in human volunteers had no effect on plasma
or erythrocyte cholinesterase activity. New data were not available
from occupational exposure or human volunteer studies with
azinphos-methyl. A review of the available literature and reports of
human poisoning with azinphos-methyl revealed no information relevant
to the estimation of the ADI.
Since the critical toxicological end-point was not
acetylcholinesterase inhibition, the human data were not appropriate
for estimation of the ADI, which was based on the NOAEL in the rat
multigeneration study in rats using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm (equal to 0.88 mg/kg bw/day)
Rat: 5 ppm (equal to 0.86 mg/kg bw/day) in a long-
term/carcinogenicity study
5 ppm (equal to 0.48 mg/kg bw/day) in a
multigeneration study
Dog: 25 ppm (equal to 0.74 mg/kg bw/day)
Human: 0.3 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans
REFERENCES
Alam, M.T., Corbeil, M., Chagnon, A., Kasatiya, S.S. (1974).
Chromosomal anomalies induced by the organic phosphate pesticide
guthion in Chinese hamster cells. Chromosoma (Berl.), 49: 77-86.
Allen, T.R., Frei, T., Janiak, T., Luetkemeier, H., Vogel, O.,
Biedermann, K., Wilson, J. (1990). 52-week oral toxicity (feeding)
study with azinphos-methyl in the dog. Unpublished report from
Research and Consulting Company AG, submitted by Bayer AG, Leverkusen,
Germany.
Chen, H.H., Sirianni, S.R., Huang, C.C. (1982a). Sister chromatid
exchanges and cell cycle delay in Chinese hamster V79 cells treated
with 9 organophosphorus compounds (8 pesticides and 1 defoliant).
Mutation Research, 103: 307-313.
Chen H.H., Sirianni, S.R., Huang, C.C. (1982b). Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environmental Mutagenesis, 4: 621-624.
Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1988). A teratology study
in the rabbit with azinphos-methyl. Unpublished report from Miles
Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to rats.
Unpublished report from Chemagro, submitted by Bayer AG, Leverkusen,
Germany.
Eiben, R., Janda, B. (1987). Azinphos-methyl - two generation study
on rats. Unpublished report from Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1987). Primary dermal irritation of Benzazimide in
albino rabbits. Unpublished report from Mobay Corporation, submitted
by Bayer AG, Leverkusen, Germany.
Faul, J. (1981). BBA request, effects on humans. Letter of 2 June
1981 from Bayer AG, Department DO Medical. Company internal letter,
submitted by Bayer AG, Leverkusen, Germany.
Feldmann, R.J., Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicology and Applied
Pharmacology, 28: 126-132.
Flucke, W. (1979). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Flucke, W. (1986). Azinphos-methyl - Study for skin sensitizing
effect in guinea pigs (Magnusson and Kligman maximization test).
Unpublished report from Bayer AG, Leverkusen, Germany.
Flucke, W., Schilde, B. (1980). Azinphos-methyl: Subacute cutaneous
toxicity to rabbits. Unpublished report from Bayer AG, Leverkusen,
Germany.
Franklin, C.A., Greenhalgh, R., Maibach, H.I. (1982). Pesticide
chemistry, human welfare and the environment. Volume 4,
pages 221-226. Pergamon Press, London, United Kingdom.
Franklin, C.A., Muir, N.I., Moody, R.P. (1986). The use of biological
monitoring in the estimation of exposure during the application of
pesticides. Toxicology Letters, 33: 127-136.
Glaza, S.M. (1988). Azinphos-methyl: Acute delayed neurotoxicity
study in the domestic fowl. Unpublished report from Hazleton
Laboratories America Inc, sponsored by Mobay Corporation, submitted by
Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985). Oncogenicity study of azinphos-methyl in mice.
Unpublished report from Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1981). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1982). Azinphos-methyl study of the acute oral and
dermal toxicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1987a). Determination of acute toxicity (LD50) of
azinphos methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987b). Azinphos-methyl: Study of skin sensitizing
effect on guinea pigs (Buehler patch test). Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1978). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1979a). Azinphos-methyl: micronucleus test on mice to
evaluate for possible mutagenic effects. Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1979b). Azinphos-methyl: dominant lethal study on male
mouse to test for mutagenic effects. Unpublished report from Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1984). Azinphos-methyl, Pol test on E. coli to
evaluate for potential DNA damage. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1986). Azinphos-methyl: cytogenic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report from Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Holzum, B. (1990). Azinphos-methyl: investigation of inhibition of
cholinesterase activity in plasma, erythrocytes and brain, in a
one-generation study. Unpublished report from Bayer AG, Leverkusen,
Germany.
Hoorn, A.J.W. (1983). Mutagenicity evaluation of azinphos-methyl in
the reverse mutation induction assay with Saccharomyces cerevisiae
strains S138 and S211. Unpublished report from Litton Bionetics,
Holland, submitted by Bayer AG, Leverkusen, Germany.
Kao, L.R.M. (1988). Disposition and metabolism of ring-UL-14C
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1987). A
teratology study with azinphos-methyl in the rat. Unpublished report
from Miles Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1976). Subchronic inhalation toxicity of
azinphos-methyl in rats. Arch. Toxicol., 35: 83-89.
Kimmerle, G.; Loser, E. (1974). Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environm. Qual. Safety, 3: 173-178.
Lamb, D.W., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to fasted and
non-fasted rats using CMC as the excipient. Unpublished report from
Chemagro, submitted by Bayer AG, Leverkusen, Germany.
Lawlor, T.E. (1987). Azinphos-methyl: Salmonella/microsome plate
incorporation mutagenicity assay. Unpublished report from
Microbiological Associates Inc., submitted by Bayer AG, Leverkusen,
Germany.
Lin, S.N., Chen, C.Y., Murphy, S.D., Caprioli, R.M. (1980).
Quantitative high-performance liquid chromatography and mass
spectrometry for the analysis of the in vitro metabolism of the
insecticide azinphos-methyl by rat liver homogenates. J. Agric. Food
Chem., 28: 85-88
Machemer, L. (1975). Azinphos-methyl: studies for embryonic and
teratogenic effects on rabbits following oral administration.
Unpublished report from Bayer AG, Leverkusen, Germany.
Mahler, L. (1991). Program Director, Pesticide Illness Surveillance
Program, Worker Health and Safety Branch, California Department of
Agriculture. Data provided to Mobay Corporation, submitted by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978). Azinphos-methyl acute toxicity studies.
Unpublished report from Bayer AG, Leverkusen, Germany.
Miksche, L. (1981). Information on effects in man/occupational
experience. Letter of 12 June 1981, from Bayer AG medical department.
Company internal letter, submitted by Bayer AG, Leverkusen, Germany.
Myhr, B.C. (1983). Evaluation of azinphos-methyl in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report from
Litton Bionetics USA, submitted by Bayer AG, Leverkusen, Germany.
National Cancer Institute (1978). Bioassay of azinphos-methyl for
possible carcinogenicity. Technical report series no 69.
Pasquet, J., Mazuret, A., Fournel, J., Koenig, F.H. (1976). Acute
oral and percutaneous toxicity of phosalone in the rat, in comparison
with azinphos-methyl and parathion. Toxicology and Applied
Pharmacology, 37: 85-92.
Patzschke, K., Wegner, L.A., Weber, H. (1976). Biokinetic
investigations of carbonyl-14C azinphos methyl in rats. Unpublished
report from Bayer AG, Leverkusen, Germany.
Porter, M.C., Craigo, R.E., Hartnagel, R.E. (1987). Dermal
sensitization evaluation of azinphos-methyl technical in the guinea
pig. Unpublished report from Miles Laboratories Inc., submitted by
Bayer AG, Leverkusen, Germany.
Rao, S.L N., McKinley, W.P. (1969). Metabolism of organophosphorus
insecticides by liver homogenates from different species. Can. J.
Biochem., 47: 1155-1159
Schmidt, W.M. (1987). Azinphos-methyl: study of chronic toxicity and
carcinogenicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1988). Acute dermal toxicity of technical grade
benzazimide to rabbits. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987a). Acute four-hour inhalation toxicity study
with azinphos-methyl in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b). Acute inhalation toxicity study with
benzazimide technical in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988). Acute one hour inhalation toxicity study with
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Short, R.D., Minor, J.L., Lee, C.C., Chernoff, N., Baron, R.L. (1980).
Developmental toxicity of azinphos-methyl in rats and mice. Arch.
Toxicol, 43: 177-186.
Short, R.D., Minor, J.L., Unger, T.M., Lee, C.C. (1978). Teratology
of azinphos-methyl. Unpublished report from Mid West Research
Institute, USA, contracted by US Environmental Protection Agency,
submitted by Bayer AG, Leverkusen, Germany.
Sterri, S.H., Rognerud, B., Fiskum, S.E., Lyngaas, S. (1979). Effect
of toxogonin and P2S on the toxicity of carbamates and
organophosphorus compounds. Acta Pharmacol. et Toxicol., 45: 9-15
Thyssen, J. (1976a). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Thyssen, J. (1976b). Studies to determine the toxic effects of the
simultaneous application of azinphos-methyl or azinphos-ethyl and
methamidophos. Unpublished report from Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977a). Study for combination toxicity of
azinphos-methyl and propoxur. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977b). Study for the combination toxicity of
azinphos-methyl and azinphos-ethyl. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J., Lorke (1981). Azinphos-methyl - study of the irritant
effects on the skin and mucous membranes (eye). Unpublished report
from Bayer AG, Leverkusen, Germany.
US EPA (1981). Summary of reported pesticide incidents involving
azinphos-methyl Pesticide incident monitoring system, report No. 469
submitted by Bayer AG, Leverkusen, Germany.
Waters, M.D., Sandhu, S.S., Simon, V.F., Mortelmans, K.E.,
Mitchell, A.A., Jorgenson, T.A., Jones, D.C.L., Valencia, R.,
Garrett, N.E. (1982). Study of pesticide genotoxicity. Genetic
Toxicology - an agricultural perspective, 21: 275 - 326.
Weber, H., Patzschke, K., Wegner, L.A. (1980). Biokinetic
investigation of ring-UL-14C benzazimide in rats. Unpublished report
from Mobay Corporation, submitted by Bayer AG, Leverkusen, Germany.
Webster, R.C.; Maibach, H.I. (1985). In vivo percutaneous
absorption and decontamination of pesticides in humans. Journal of
Toxicology and Environmental Health, 16: 25-37.
Table 1. Results of acute toxicity tests with azinphos-methyl and
related materials
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl Oral Rat DMSO m 5.6 Crawford (1974)
f 6.4
Benzazimide Oral Rat DMSO m 412
f 269
Methyl benzazimide Oral Rat DMSO m 330
f 330
Azinphos methyl Oral Rat Cremophor EL m 25.4 Flucke (1979)
Azinphos methyl Oral Rat Cremophor EL m 9.1 (fasted) Heimann (1981)
m 17.25 (non-fasted)
Azinphos methyl Oral Rat Cremophor EL m 6.7 (fasted) Heimann (1982)
m 12.8 (non-fasted)
Dermal Rat Cremophor EL m 225
f 155
Azinphos methyl Oral Rat Cremophor EL m 7.1 Heimann (1987)
Azinphos methyl Oral Rat CMC (fasted) m 19 Lamb et al. (1974)
f 16
m 19
(non-fasted) f 10
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Benzazimide Oral Rat CMC (fasted) m 576 Lamb (1974)
f 368
m 576
(non-fasted) f 487
Methyl benzazimide Oral Rat CMC (fasted) m 412
f 390
m 524
(non-fasted) f 460
Azinphos methyl Oral Rat Cremophor EL m 4.6 Mihail (1978)
f 4.4
Dermal Rat Cremophor EL m 2500-5000*
Oral Dog Cremophor EL m >10
Azinphos methyl Oral Rat Methylene chloride/ m 26 Pasquet et al. (1976)
Tween/80 Gum Arabic f 24
Dermal Rat Acetone/Ethanol/ f 90
Peanut Oil
Benzazimide Dermal Rabbit Tap water m >2000 Sheets (1988)
f >2000
Azinphos methyl 4 hr inhal Rat PEG/ETOH m 155 Shiotsuka (1987a)
f 132 (mg/m3)
Benzazimide 4 hr inhal Rat None m >1760* Shiotsuka (1987b)
(mg/m3)
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl 1 hr inhal Rat PEG/ETOH m 396 Shiotsuka (1988)
f 310 (mg/m3)
Azinphos methyl Oral Rat Cremophor EL m 15.6 Thyssen (1976a)
Azinphos methyl Oral Rat Cremophor EL m 16.75 Thyssen (1976b)
Methamidophos Oral Rat Cremophor EL m 31.9
Azinphos methyl +
Methamidophos Oral Rat Cremophor EL m 19.5
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977a)
Propoxur Oral Rat Cremophor EL m 39
Azinphos methyl +
Propoxur Oral Rat Cremophor EL m 29.3
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977b)
Azinphos ethyl Oral Rat Cremophor EL m 11.8
Azinphos methyl +
Azinphos ethyl Oral Rat Cremophor EL m 11.1
* sex not specified.
m male
f female
Toxicological studies
Acute Toxicity Studies
Results of acute toxicity tests with azinphos-methyl and related
materials are listed in Table 1.
Short-term studies
Rats
A 12-week inhalation study has been described in the published
literature. Groups of 10 male and 10 female Wistar rats were exposed
in inhalation chambers to mean aerosol concentrations in the air of 0
(control), 0.195, 1.24 and 4.72 mg/m3 azinphos methyl, for 6 hours
daily, 5 days per week, for 12 weeks. At the high dose level body
weight gain was depressed in males and erythrocyte cholinesterase
activity was inhibited in males and females. There was no other
evidence of any reaction to treatment. The NOAEL was 1.24 mg/m3
based on reduced weight gain in males at the high dose level
(Kimmerle, 1976).
Rabbits
In a dermal toxicity study in rabbits, azinphos-methyl was
applied for 6 hours per day, to the shaved dorsal and lateral skin, at
dose levels of 0 (control), 2, or 20 mg/kg bw, for 5 days per week for
3 weeks. Each group consisted of 6 males and 6 females, with the skin
left intact in 3 animals of each sex and abraded in the others.
Investigation of cholinesterase activity revealed a marginal
(approximately 30%) depression of erythrocyte activity, compared to
controls, in males and females treated with 20 mg/kg. Cholinesterase
activity in plasma and brain, and erythrocyte activity at 2 mg/kg,
remained undisturbed by treatment. There was no difference between
the groups with intact and abraded skin, and all other investigations
(clinical signs, measurement of food intake and weight gain, further
clinical chemistry, haematology and urinalysis and pathological
investigations including limited histopathology) revealed no
treatment-related changes. The NOAEL was 20 mg/kg bw, since only
erythrocyte cholinesterase activity was affected at this dose, the
highest dose used, with no effect on cholinesterase activity in brain
(Flucke & Schilde, 1980).
Dogs
In a 52 week toxicity study in beagle dogs, azinphos-methyl
(purity 91.9%) was administered, via the diet, to four groups, each of
4 males and 4 females, at dietary levels of 0 (control), 5, 25 or 125
ppm. Clinical signs of reaction to treatment were confined to a
higher incidence of diarrhoea in dogs receiving 125 ppm. Two males
receiving 125 ppm failed to gain weight during the course of the
study, but food intake remained unaffected by treatment. Haematology
and urinalysis revealed no indication of any reaction to treatment.
Clinical biochemistry tests revealed a depression of cholinesterase
activity in plasma and erythrocytes at 25 and 125 ppm and in brain at
termination at 125 ppm. There was also a very slight increase,
compared to controls, in liver cytochrome P450 and N-demethylase
activity at the high dose and a reduction in albumin levels.
Pathological investigations (macroscopic examination, organ weight
analysis and histopathology) revealed no evidence of any reaction to
treatment with azinphos-methyl. The NOAEL was 25 ppm (equal to
0.74 mg/kg bw/day) based on reduced weight gain and inhibition of
acetylcholinesterase activity in brain (Allen, et al., 1990).
Long-term/carcinogenicity studies
Mice
A bioassay of azinphos-methyl (purity 90% from manufacturing
specification) for possible carcinogenicity was conducted by the NCI.
The experiment involved administering the test material, in the feed,
to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats of each sex
were treated for 80 weeks, then observed for 34 or 35 weeks. Males
received time weighted average doses of 78 or 156 ppm, females
received 62.5 or 125 ppm. Matched controls consisted of 10 untreated
rats of each sex; pooled controls consisted of matched controls
combined with 95 male and 95 female untreated rats from similar
bioassays of 10 other chemicals. The mouse study was of similar
design; groups of 50 mice were treated for 80 weeks, then observed for
12 or 13 weeks, males received 31.3 or 62.5 ppm and females 62.5 or
125 ppm, matched controls consisted of 10 males and 10 females, pooled
controls 130 males and 120 females. Typical signs of organophosphate
intoxication (hyperactivity, tremors and dyspnoea) were observed in a
few animals of both species. Weight gain in treated males and high
dose females of both species was lower than in control animals. In
rats there was some evidence of decreased survival at the high dose
compared to controls but this was not seen in mice. In both sexes, at
all doses, survival to termination was adequate for assessment of
effects on late appearing tumours. The report concluded that in rats
the incidence of tumours of the pancreatic islets, and of follicular
cells in the thyroid in males suggested, but did not clearly
implicate, azinphos-methyl as a carcinogen in these animals. There
was no similar evidence in female rats and in mice of each sex there
was no increased incidence of tumours that could be related to the
administration of azinphos-methyl (National Cancer Institute, 1978).
In a carcinogenicity study of azinphos-methyl (purity 88.6%) in
mice, groups of 50 male and 50 female CD-1 mice received dietary
levels of 0 (control), 5, 20, or 40/80 ppm for two years. (The study
was initially started with 80 ppm as the high dietary level, but this
was reduced to 40 ppm after one week, due to severe reaction to
treatment, including mortality, at 80 ppm). Following the reduction
in the high dietary level, there were no clinical signs of reaction to
treatment and mortality remained unaffected by treatment. Weight gain
and food intake remained unaffected by treatment at dietary levels up
to and including 40 ppm. Haematological investigations revealed no
indication of any reaction to treatment. Measurement of
acetylcholinesterase activity revealed that at 5 ppm, activities in
plasma, erythrocyte and brain remained comparable with control values.
At 20 and 40 ppm there was a dose-related inhibition of cholinesterase
activity in plasma and erythrocytes. A similar effect was noted in
brain, except that males were only affected at 40 ppm, while females
exhibited a depression of brain cholinesterase activity at 20 and 40
ppm. Pathological investigations revealed no evidence of any reaction
to treatment, in particular there was no evidence of any carcinogenic
effect of azinphos-methyl. The NOAEL was 5 ppm (equal to
0.88 mg/kg bw/day) based on inhibition of cholinesterase in plasma,
erythrocytes and brain at 20 ppm (Hayes, 1985).
Rats
In a combined long-term toxicity and carcinogenicity study in
rats, groups of 60 male and 60 female Wistar rats received
azinphos-methyl (purity 87.2%) in the diet at levels of 0 (Control),
5, 15 or 45 ppm. From each group, 10 rats per sex were killed after
12 months, while all survivors were killed after 24 months continuous
treatment. There were no clinical signs of reaction to treatment and
survival was unaffected by azinphos-methyl. Weight gain of high dose
males was slightly less than controls but growth in other groups was
not affected by treatment. Clinical biochemistry (apart from
acetylcholinesterase investigations), haematology and urinalysis tests
revealed no indication of any reaction to treatment. Determinations
of acetylcholinesterase activities in erythrocytes, plasma and brain
revealed a marked inhibition, compared to controls, in males and
females from the high dose group (erythrocytes, plasma and brain) and
a less marked effect at 15 ppm (males: erythrocytes, females:
erythrocytes and plasma). Acetylcholinesterase activity in brain from
rats treated at 15 ppm and in erythrocytes, plasma and brain from rats
treated at 5 ppm, remained unaffected by treatment with
azinphos-methyl. Pathological examinations (including gross
examination, organ weight analysis and histological examination of
tissues) revealed no evidence of any reaction to treatment; in
particular there was no evidence of any carcinogenic effect of
azinphos-methyl. The NOAEL was 15 ppm (equal to 0.86 mg/kg bw/day)
based on effects on body weight gain and brain acetylcholinesterase
(Schmidt, 1987).
Reproduction studies
In a two generation (two litters per generation) reproduction
study in rats azinphos-methyl (purity 87.2%) was administered to
groups of 12 male and 24 female Wistar rats at dietary levels of 0
(control), 5, 15 or 45 ppm. At 15 and 45 ppm there was a decrease in
fertility of F0 rats and the total number of delivered pups. At 45
ppm there was an increased mortality of dams in the F0 generation and
reduced pup viability during lactation. As a consequence of these
effects only 5 females were available for mating in the F1b
generation. During mating of the F1b generation, fertility was again
adversely affected at 15 ppm but not to as great an extent as it was
during the F0 generation. At all stages of the study, there was no
evidence of treatment induced malformations and food intake remained
unaffected. Clinical signs of reaction to treatment, including
cholinergic signs, were seen at the high dose and weight gain was
adversely affected at 15 and 45 ppm. The NOAEL was 5 ppm, equal to
0.48 mg/kg bw/day, based on the adverse effects on fertility and body
weight gain seen at 15 and 45 ppm (Eiben & Janda, 1987).
A further study was conducted in order to investigate the effects
on reproductive performance noted in the study described above. The
objectives of this further, one generation study were to investigate
whether the slight effect on fertility at 15 ppm could be confirmed,
and, if reproducible, to determine whether the effect was attributable
to treatment of the male or the female and to determine if
reproductive effects were associated with treatment-induced inhibition
of cholinesterase activity. Azinphos-methyl (purity 92.0%) was
administered to groups of 18 male and 46 female Wistar rats, at
dietary levels of 0 (control), 5, 15, or 45 ppm. Treated males and
females were paired, and dams allowed to rear litters to weaning.
Additional treated males were paired with untreated females. At 15
ppm, when males and females were treated, the viability index was
reduced, largely confirming the results of the previous study.
However, after treatment of male parental animals only, reproductive
parameters remained unaffected, even at 45 ppm. Investigations of
cholinesterase activity in parental animals revealed a depression in
activity in plasma and erythrocytes at all dose levels, and a
depression in activity in brain at 45 ppm in males and at 15 and 45
ppm in females. At 45 ppm, brain cholinesterase activity in pups was
also depressed. The NOAEL was 5 ppm, equal to 0.43 mg/kg bw/day,
based on the adverse effects on fertility and depression of brain
cholinesterase activity seen at 15 ppm (Holzum, 1990).
Special studies on delayed neurotoxicity
In a published report of experiments designed to investigate the
potential relationship between delayed neurotoxicity and copper
concentration in the serum of hens, it was reported that
azinphos-methyl failed to produce neurotoxic symptoms after either
single or repeated doses (Kimmerle & Loser, 1974).
In an acute delayed neurotoxicity test, azinphos-methyl (purity
85%)was administered twice, at the unprotected LD50 dose level of 330
mg/kg to a group of 30 white leghorn hens, with an interval of 21 days
between doses. Groups of untreated control, vehicle control and
positive control (TOCP 600 mg/kg bw) animals, each composed of 10
animals, were also included. Atropine was used for symptomatic
treatment after dosing. A total of 11 hens treated with
azinphos-methyl survived until termination. These animals appeared
normal during the last 12 or 13 days of the study, but exhibited
varying degrees of impaired locomotor activity soon after dosing.
Histopathological examinations indicated that azinphos-methyl did not
increase the incidence or severity of lesions in the nerve tissue
compared to untreated and vehicle controls. Investigations of
neuropathy target esterase activity were not included in the study
(Glaza, 1988).
Special studies on embryo/fetoxicity
Mice and rats
The effects of azinphos-methyl on development in rats and mice
were investigated in a series of experiments. On the basis of
preliminary toxicity studies doses of 0, 1.25, 2.5 and 5.0 mg/kg
bw/day were selected for developmental studies in both species, which
consisted of two phases. During the first phase, pregnant rats and
mice were treated for 10 days, starting on gestational day 6. During
the second phase, pregnant rats were treated from day 6 of gestation
to day 21 post partum. In the first phase, maternal toxicity was seen
only in rats receiving the high dose. When dams and fetuses were
examined (day 18 of gestation for mice, day 20 for rats) there was no
dose-related increase in anomalies or malformation in rats or mice.
In the second phase, dams in the high-dose group were more sensitive
to azinphos-methyl in the latter stages of gestation and signs of
anticholinesterase intoxication, including mortality, were observed.
As a result, only one litter (out of 13) survived to weaning in this
group. It was concluded that azinphos-methyl had little primary
effect on development in rats and mice (Short, et al, 1978; Short,
et al., 1980).
Rats
In a teratology study in rats, groups of 33 inseminated dams
received azinphos-methyl (purity 87.7%) from day 6 to day 15 of
gestation (day of insemination = day 0), at dose levels of
0 (control), 0.5, 1.0 or 2.0 mg/kg bw/day. From each group, 5 dams
were killed on day 16 of gestation and the remaining dams on day 20.
On day 16 of gestation, cholinesterase activities in plasma,
erythrocytes and brain were depressed, compared to controls, in dams
at the high dose only (fetal tissues were not examined). By day 20 of
gestation there was indication of recovery in cholinesterase activity
in all previously affected tissues and fetal brain cholinesterase
activity was comparable with control values. Azinphos-methyl did not
affect any maternal reproductive parameters and there was no
indication of treatment-related embryotoxicity, fetotoxicity or
teratogenicity at any dose level. The NOAEL for maternal toxicity was
1.0 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 16 of gestation (Kowalski et al., 1987).
Rabbits
In a teratology study in rabbits, groups of 11 or 12 pregnant
animals received daily oral doses of azinphos-methyl (purity 92.4%)
from day 6 to day 18 of gestation (day of insemination = day 0) at
levels of 0 (Control), 0.3, 1.0 or 3.0 mg/kg bw/day. Caesarean
section was carried out on day 29 of gestation. Azinphos-methyl
induced no evidence of maternal toxicity at any dose level and there
were no detectable effects on embryonic nor fetal development
(Machemer, 1975).
In a further teratology study in rabbits, groups of 20
inseminated does received daily oral doses of azinphos-methyl (purity
87.7%) from day 6 to day 18 of gestation (day of insemination = day 0)
at levels of 0 (Control), 1, 2.5 or 6 mg/kg bw/day. Ataxia in 4 high
dose does and tremors in 2 of these same animals represented clinical
signs of reaction to treatment.
Plasma and erythrocyte cholinesterase activity, on day 19 of
gestation, was depressed compared to controls at the mid and high
dose. By day 28 of gestation there was clear evidence of recovery in
plasma and erythrocyte cholinesterase activity, although activity in
brain was depressed, compared to controls, at the high dose.
Azinphos-methyl did not affect any maternal reproductive parameters
and there was no evidence of any treatment-related effect on
embryotoxicity, fetotoxicity or teratogenicity at any dose level. The
no observable adverse effect level for maternal toxicity was
2.5 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 28 of gestation (Clemens et al., 1988).
Special studies on genotoxicity
In Salmonella/microsome point mutation tests, azinphos-methyl
(purity >88.8%) showed no evidence of mutagenic activity which could
be classified as positive results in the tests. In one test there was
reproducible evidence of a slight dose-dependent increase in revertant
frequency in one test strain, but the increase was less than 2-fold
(Herbold, 1978; Herbold, 1988; Lawlor, 1987).
Azinphos-methyl (purity 91.1%) exhibited no mutagenic activity in
a reverse mutation test with Saccharomyces cerevisiae (Hoorn, 1983).
In Chinese hamster ovary cells in vitro, azinphos-methyl
induced chromosomal anomalies in a dose related fashion. Most
commonly observed were chromatid breaks and exchanges. In a test with
human lymphocytes in vitro, (purity 91.9%) there were no chromosomal
aberrations induced in the absence of S-9 mix but clear,
treatment-related variations were noted when azinphos-methyl was
tested in the presence of S-9 mix at cytotoxic concentrations. In an
investigation of ability to induce sister chromatid exchanges in
Chinese hamster V79 cells in vitro, azinphos-methyl was shown not to
increase the frequency of sister chromatid exchange, but did induce
some cell cycle delay (Alam, 1974; Herbold, 1986; Chen 1982 et al.,
1982a,b).
The potential of azinphos-methyl (purity 91.1%) to cause DNA
damage was assessed in Rosenkranz and Leifer's pol test employing two
E. coli strains which vary in regard to their repair systems for DNA
damage. The results showed that azinphos-methyl gave no indication of
any effect on DNA damage. In a primary rat hepatocyte unscheduled DNA
synthesis assay azinphos-methyl (purity 91.1%) did not induce
significant changes in the nuclear labelling of primary rat
hepatocytes and it was concluded that azinphos-methyl did not induce
DNA damage in this assay (Herbold, 1984; Myhr, 1983).
Mutagenic effects of azinphos-methyl (purity 92.3%) in vivo
were investigated in a micronucleus test and a dominant lethal test;
both in mice. In the micronucleus test two doses of azinphos-methyl
(2 x 2.5 or 2 x 5.0 mg/kg bw) were given 24 hours apart and a femoral
marrow smear was prepared 6 hours after the second dose; there was no
indication of any mutagenic effect. In the dominant lethal study male
mice received a single oral dose of 4 mg/kg bw azinphos-methyl and
were then mated with untreated females over 12 consecutive periods of
4 days. Fertility remained unaffected and there were no
treatment-related differences in implantation parameters (Herbold,
1979a,b).
An effort was made to evaluate the genotoxicity of a variety of
pesticides, with the specific objectives of comparing different
in vivo and in vitro assays, examining the spectrum of genetic
activity displayed by the selected pesticides and examining the test
results in relation to other biological and chemical features of the
pesticides. In this research programme azinphos-methyl has been
tested in a range of 14 mutagenicity tests, examining point or gene
mutations, DNA damage and chromosomal effects. Positive results for
azinphos-methyl were seen in only two tests: a forward mutation assay
in mouse lymphoma L5178Y cells (only in the presence of S-9 mix) and
a mitotic recombination assay in Saccharomyces cerevisiae strain D3.
Azinphos-methyl was negative in tests for point/gene mutation and DNA
damage in prokaryotes and showed no positive results in tests looking
at chromosomal effects (Waters et al., 1982).
Special studies on skin and eye irritation and sensitization
In a skin irritation study employing 6 rabbits, 24 hour exposure
to azinphos-methyl at intact and abraded skin sites did not cause any
signs of irritation. In an eye irritation study, exposure of the
conjunctiva of the eye to azinphos-methyl for 5 minutes (5 rabbits) or
24 hours (3 rabbits) caused no significant reaction (Thyssen & Lorke,
1981).
In a dermal irritation study utilizing 6 New Zealand white
rabbits, 0.5 g of benzazimide was moistened with water and kept in
contact with the shaved skin for 4 hours. At 30 and 60 minutes and
24, 48 and 72 hours after patch removal there was no evidence of
erythema or oedema at the treatment sites. Benzazimide is therefore
not a skin irritant in rabbits (Eigenberg, 1987).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Magnusson and Kligman
maximization test. The study revealed that azinphos-methyl had a
sensitizing effect in 95% of the test animals (Flucke, 1986).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Buehler patch test. By means
of a dermal application of a concentration of 25% sensitization was
induced in approximately 50% of the test animals (Porter et al.,
1987).
In another Buehler patch test dermal application of a
concentration of 12.5% induced sensitization in approximately 50% of
the test animals when challenged using a 6% concentration, but using
a challenge concentration of 0.6% failed to elicit any relevant skin
reactions (Heimann, 1987b).
Observations in humans
Employees working in the formulation of azinphos-methyl products
have been subjected to regular medical examinations and no general
impairment of health has been observed. In one isolated case it was
considered probable that contact with azinphos-methyl was the cause of
generalized dermatosis in an apparently hypersensitive, very dry skin
(Faul, 1981; Miksche, 1981).
Published reports from the pesticide incident monitoring system
in the United States of America and additional data from the state of
California in the USA have been reviewed. Between 1982 and 1988 a
small number of incidents have been reported annually (involving 5-12
persons each year) which have been definitely, probably or possibly
associated with azinphos-methyl either alone or in combination with
other pesticides. In addition, two incidents occurred in 1987, one
involving 26 people, the other involving 32 people. The first
involved spray drift in adverse weather conditions. The second
involved workers who experienced symptoms including headache, nausea,
weakness and vomiting upon entry to a field to pick peaches 3 days
after methomyl was applied to the crop and about 6 weeks after an
application of azinphos-methyl (US EPA, 1981; Mahler, 1991).
COMMENTS
The toxicokinetics of azinphos-methyl has been investigated
following oral administration in rats. It does not accumulate in body
tissues.
In a 52-week study in dogs, using dietary concentrations of 0, 5,
25 or 125 ppm the NOAEL was 25 ppm (equal to 0.74 mg/kg bw/day), based
on reduced body-weight gain and inhibition of acetylcholinesterase
activity in brain at 125 ppm.
Long-term/carcinogenicity studies in rats at dietary
concentrations of 0, 5, 15, or 45 ppm and in mice at 0, 5, 20 or 40
ppm showed that azinphos-methyl has no carcinogenic potential in
either species. These results clarified earlier equivocal findings in
rats in an NCI bioassay. The NOAEL in rats was 15 ppm (equal to
0.86 mg/kg bw/day), based on effects on brain acetylcholinesterase at
45 ppm. In mice the NOAEL was 5 ppm (equal to 0.88 mg/kg bw/day),
based on inhibition of cholinesterase in plasma, erythrocytes and
brain at 20 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 15 or 45 ppm, fertility and pup viability
during lactation were adversely affected, equivocally at 15 ppm and
markedly at 45 ppm. The NOAEL was 5 ppm, equal to 0.48 mg/kg bw/day.
Teratology studies in rats, mice and rabbits did not indicate
teratogenic effects at doses up to 2, 5 and 6 mg/kg bw/day
respectively.
The data from genotoxicity studies with azinphos-methyl were
conflicting. However, in vivo studies were negative, the positive
data being confined to some in vitro studies. After reviewing the
available information it was concluded that it is unlikely that
azinphos-methyl is genotoxic to humans.
Acute delayed neurotoxicity tests in hens with azinphos-methyl
gave negative results.
The 1973 JMPR reported that daily doses up to and around 0.3
mg/kg bw/day for 30 days in human volunteers had no effect on plasma
or erythrocyte cholinesterase activity. New data were not available
from occupational exposure or human volunteer studies with
azinphos-methyl. A review of the available literature and reports of
human poisoning with azinphos-methyl revealed no information relevant
to the estimation of the ADI.
Since the critical toxicological end-point was not
acetylcholinesterase inhibition, the human data were not appropriate
for estimation of the ADI, which was based on the NOAEL in the rat
multigeneration study in rats using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm (equal to 0.88 mg/kg bw/day)
Rat: 5 ppm (equal to 0.86 mg/kg bw/day) in a long-
term/carcinogenicity study
5 ppm (equal to 0.48 mg/kg bw/day) in a
multigeneration study
Dog: 25 ppm (equal to 0.74 mg/kg bw/day)
Human: 0.3 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans
REFERENCES
Alam, M.T., Corbeil, M., Chagnon, A., Kasatiya, S.S. (1974).
Chromosomal anomalies induced by the organic phosphate pesticide
guthion in Chinese hamster cells. Chromosoma (Berl.), 49: 77-86.
Allen, T.R., Frei, T., Janiak, T., Luetkemeier, H., Vogel, O.,
Biedermann, K., Wilson, J. (1990). 52-week oral toxicity (feeding)
study with azinphos-methyl in the dog. Unpublished report from
Research and Consulting Company AG, submitted by Bayer AG, Leverkusen,
Germany.
Chen, H.H., Sirianni, S.R., Huang, C.C. (1982a). Sister chromatid
exchanges and cell cycle delay in Chinese hamster V79 cells treated
with 9 organophosphorus compounds (8 pesticides and 1 defoliant).
Mutation Research, 103: 307-313.
Chen H.H., Sirianni, S.R., Huang, C.C. (1982b). Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environmental Mutagenesis, 4: 621-624.
Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1988). A teratology study
in the rabbit with azinphos-methyl. Unpublished report from Miles
Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to rats.
Unpublished report from Chemagro, submitted by Bayer AG, Leverkusen,
Germany.
Eiben, R., Janda, B. (1987). Azinphos-methyl - two generation study
on rats. Unpublished report from Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1987). Primary dermal irritation of Benzazimide in
albino rabbits. Unpublished report from Mobay Corporation, submitted
by Bayer AG, Leverkusen, Germany.
Faul, J. (1981). BBA request, effects on humans. Letter of 2 June
1981 from Bayer AG, Department DO Medical. Company internal letter,
submitted by Bayer AG, Leverkusen, Germany.
Feldmann, R.J., Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicology and Applied
Pharmacology, 28: 126-132.
Flucke, W. (1979). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Flucke, W. (1986). Azinphos-methyl - Study for skin sensitizing
effect in guinea pigs (Magnusson and Kligman maximization test).
Unpublished report from Bayer AG, Leverkusen, Germany.
Flucke, W., Schilde, B. (1980). Azinphos-methyl: Subacute cutaneous
toxicity to rabbits. Unpublished report from Bayer AG, Leverkusen,
Germany.
Franklin, C.A., Greenhalgh, R., Maibach, H.I. (1982). Pesticide
chemistry, human welfare and the environment. Volume 4,
pages 221-226. Pergamon Press, London, United Kingdom.
Franklin, C.A., Muir, N.I., Moody, R.P. (1986). The use of biological
monitoring in the estimation of exposure during the application of
pesticides. Toxicology Letters, 33: 127-136.
Glaza, S.M. (1988). Azinphos-methyl: Acute delayed neurotoxicity
study in the domestic fowl. Unpublished report from Hazleton
Laboratories America Inc, sponsored by Mobay Corporation, submitted by
Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985). Oncogenicity study of azinphos-methyl in mice.
Unpublished report from Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1981). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1982). Azinphos-methyl study of the acute oral and
dermal toxicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1987a). Determination of acute toxicity (LD50) of
azinphos methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987b). Azinphos-methyl: Study of skin sensitizing
effect on guinea pigs (Buehler patch test). Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1978). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1979a). Azinphos-methyl: micronucleus test on mice to
evaluate for possible mutagenic effects. Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1979b). Azinphos-methyl: dominant lethal study on male
mouse to test for mutagenic effects. Unpublished report from Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1984). Azinphos-methyl, Pol test on E. coli to
evaluate for potential DNA damage. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1986). Azinphos-methyl: cytogenic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report from Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Holzum, B. (1990). Azinphos-methyl: investigation of inhibition of
cholinesterase activity in plasma, erythrocytes and brain, in a
one-generation study. Unpublished report from Bayer AG, Leverkusen,
Germany.
Hoorn, A.J.W. (1983). Mutagenicity evaluation of azinphos-methyl in
the reverse mutation induction assay with Saccharomyces cerevisiae
strains S138 and S211. Unpublished report from Litton Bionetics,
Holland, submitted by Bayer AG, Leverkusen, Germany.
Kao, L.R.M. (1988). Disposition and metabolism of ring-UL-14C
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1987). A
teratology study with azinphos-methyl in the rat. Unpublished report
from Miles Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1976). Subchronic inhalation toxicity of
azinphos-methyl in rats. Arch. Toxicol., 35: 83-89.
Kimmerle, G.; Loser, E. (1974). Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environm. Qual. Safety, 3: 173-178.
Lamb, D.W., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to fasted and
non-fasted rats using CMC as the excipient. Unpublished report from
Chemagro, submitted by Bayer AG, Leverkusen, Germany.
Lawlor, T.E. (1987). Azinphos-methyl: Salmonella/microsome plate
incorporation mutagenicity assay. Unpublished report from
Microbiological Associates Inc., submitted by Bayer AG, Leverkusen,
Germany.
Lin, S.N., Chen, C.Y., Murphy, S.D., Caprioli, R.M. (1980).
Quantitative high-performance liquid chromatography and mass
spectrometry for the analysis of the in vitro metabolism of the
insecticide azinphos-methyl by rat liver homogenates. J. Agric. Food
Chem., 28: 85-88
Machemer, L. (1975). Azinphos-methyl: studies for embryonic and
teratogenic effects on rabbits following oral administration.
Unpublished report from Bayer AG, Leverkusen, Germany.
Mahler, L. (1991). Program Director, Pesticide Illness Surveillance
Program, Worker Health and Safety Branch, California Department of
Agriculture. Data provided to Mobay Corporation, submitted by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978). Azinphos-methyl acute toxicity studies.
Unpublished report from Bayer AG, Leverkusen, Germany.
Miksche, L. (1981). Information on effects in man/occupational
experience. Letter of 12 June 1981, from Bayer AG medical department.
Company internal letter, submitted by Bayer AG, Leverkusen, Germany.
Myhr, B.C. (1983). Evaluation of azinphos-methyl in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report from
Litton Bionetics USA, submitted by Bayer AG, Leverkusen, Germany.
National Cancer Institute (1978). Bioassay of azinphos-methyl for
possible carcinogenicity. Technical report series no 69.
Pasquet, J., Mazuret, A., Fournel, J., Koenig, F.H. (1976). Acute
oral and percutaneous toxicity of phosalone in the rat, in comparison
with azinphos-methyl and parathion. Toxicology and Applied
Pharmacology, 37: 85-92.
Patzschke, K., Wegner, L.A., Weber, H. (1976). Biokinetic
investigations of carbonyl-14C azinphos methyl in rats. Unpublished
report from Bayer AG, Leverkusen, Germany.
Porter, M.C., Craigo, R.E., Hartnagel, R.E. (1987). Dermal
sensitization evaluation of azinphos-methyl technical in the guinea
pig. Unpublished report from Miles Laboratories Inc., submitted by
Bayer AG, Leverkusen, Germany.
Rao, S.L N., McKinley, W.P. (1969). Metabolism of organophosphorus
insecticides by liver homogenates from different species. Can. J.
Biochem., 47: 1155-1159
Schmidt, W.M. (1987). Azinphos-methyl: study of chronic toxicity and
carcinogenicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1988). Acute dermal toxicity of technical grade
benzazimide to rabbits. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987a). Acute four-hour inhalation toxicity study
with azinphos-methyl in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b). Acute inhalation toxicity study with
benzazimide technical in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988). Acute one hour inhalation toxicity study with
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Short, R.D., Minor, J.L., Lee, C.C., Chernoff, N., Baron, R.L. (1980).
Developmental toxicity of azinphos-methyl in rats and mice. Arch.
Toxicol, 43: 177-186.
Short, R.D., Minor, J.L., Unger, T.M., Lee, C.C. (1978). Teratology
of azinphos-methyl. Unpublished report from Mid West Research
Institute, USA, contracted by US Environmental Protection Agency,
submitted by Bayer AG, Leverkusen, Germany.
Sterri, S.H., Rognerud, B., Fiskum, S.E., Lyngaas, S. (1979). Effect
of toxogonin and P2S on the toxicity of carbamates and
organophosphorus compounds. Acta Pharmacol. et Toxicol., 45: 9-15
Thyssen, J. (1976a). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Thyssen, J. (1976b). Studies to determine the toxic effects of the
simultaneous application of azinphos-methyl or azinphos-ethyl and
methamidophos. Unpublished report from Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977a). Study for combination toxicity of
azinphos-methyl and propoxur. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977b). Study for the combination toxicity of
azinphos-methyl and azinphos-ethyl. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J., Lorke (1981). Azinphos-methyl - study of the irritant
effects on the skin and mucous membranes (eye). Unpublished report
from Bayer AG, Leverkusen, Germany.
US EPA (1981). Summary of reported pesticide incidents involving
azinphos-methyl Pesticide incident monitoring system, report No. 469
submitted by Bayer AG, Leverkusen, Germany.
Waters, M.D., Sandhu, S.S., Simon, V.F., Mortelmans, K.E.,
Mitchell, A.A., Jorgenson, T.A., Jones, D.C.L., Valencia, R.,
Garrett, N.E. (1982). Study of pesticide genotoxicity. Genetic
Toxicology - an agricultural perspective, 21: 275 - 326.
Weber, H., Patzschke, K., Wegner, L.A. (1980). Biokinetic
investigation of ring-UL-14C benzazimide in rats. Unpublished report
from Mobay Corporation, submitted by Bayer AG, Leverkusen, Germany.
Webster, R.C.; Maibach, H.I. (1985). In vivo percutaneous
absorption and decontamination of pesticides in humans. Journal of
Toxicology and Environmental Health, 16: 25-37.
 Table 1. Results of acute toxicity tests with azinphos-methyl and
related materials
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl Oral Rat DMSO m 5.6 Crawford (1974)
f 6.4
Benzazimide Oral Rat DMSO m 412
f 269
Methyl benzazimide Oral Rat DMSO m 330
f 330
Azinphos methyl Oral Rat Cremophor EL m 25.4 Flucke (1979)
Azinphos methyl Oral Rat Cremophor EL m 9.1 (fasted) Heimann (1981)
m 17.25 (non-fasted)
Azinphos methyl Oral Rat Cremophor EL m 6.7 (fasted) Heimann (1982)
m 12.8 (non-fasted)
Dermal Rat Cremophor EL m 225
f 155
Azinphos methyl Oral Rat Cremophor EL m 7.1 Heimann (1987)
Azinphos methyl Oral Rat CMC (fasted) m 19 Lamb et al. (1974)
f 16
m 19
(non-fasted) f 10
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Benzazimide Oral Rat CMC (fasted) m 576 Lamb (1974)
f 368
m 576
(non-fasted) f 487
Methyl benzazimide Oral Rat CMC (fasted) m 412
f 390
m 524
(non-fasted) f 460
Azinphos methyl Oral Rat Cremophor EL m 4.6 Mihail (1978)
f 4.4
Dermal Rat Cremophor EL m 2500-5000*
Oral Dog Cremophor EL m >10
Azinphos methyl Oral Rat Methylene chloride/ m 26 Pasquet et al. (1976)
Tween/80 Gum Arabic f 24
Dermal Rat Acetone/Ethanol/ f 90
Peanut Oil
Benzazimide Dermal Rabbit Tap water m >2000 Sheets (1988)
f >2000
Azinphos methyl 4 hr inhal Rat PEG/ETOH m 155 Shiotsuka (1987a)
f 132 (mg/m3)
Benzazimide 4 hr inhal Rat None m >1760* Shiotsuka (1987b)
(mg/m3)
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl 1 hr inhal Rat PEG/ETOH m 396 Shiotsuka (1988)
f 310 (mg/m3)
Azinphos methyl Oral Rat Cremophor EL m 15.6 Thyssen (1976a)
Azinphos methyl Oral Rat Cremophor EL m 16.75 Thyssen (1976b)
Methamidophos Oral Rat Cremophor EL m 31.9
Azinphos methyl +
Methamidophos Oral Rat Cremophor EL m 19.5
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977a)
Propoxur Oral Rat Cremophor EL m 39
Azinphos methyl +
Propoxur Oral Rat Cremophor EL m 29.3
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977b)
Azinphos ethyl Oral Rat Cremophor EL m 11.8
Azinphos methyl +
Azinphos ethyl Oral Rat Cremophor EL m 11.1
* sex not specified.
m male
f female
Toxicological studies
Acute Toxicity Studies
Results of acute toxicity tests with azinphos-methyl and related
materials are listed in Table 1.
Short-term studies
Rats
A 12-week inhalation study has been described in the published
literature. Groups of 10 male and 10 female Wistar rats were exposed
in inhalation chambers to mean aerosol concentrations in the air of 0
(control), 0.195, 1.24 and 4.72 mg/m3 azinphos methyl, for 6 hours
daily, 5 days per week, for 12 weeks. At the high dose level body
weight gain was depressed in males and erythrocyte cholinesterase
activity was inhibited in males and females. There was no other
evidence of any reaction to treatment. The NOAEL was 1.24 mg/m3
based on reduced weight gain in males at the high dose level
(Kimmerle, 1976).
Rabbits
In a dermal toxicity study in rabbits, azinphos-methyl was
applied for 6 hours per day, to the shaved dorsal and lateral skin, at
dose levels of 0 (control), 2, or 20 mg/kg bw, for 5 days per week for
3 weeks. Each group consisted of 6 males and 6 females, with the skin
left intact in 3 animals of each sex and abraded in the others.
Investigation of cholinesterase activity revealed a marginal
(approximately 30%) depression of erythrocyte activity, compared to
controls, in males and females treated with 20 mg/kg. Cholinesterase
activity in plasma and brain, and erythrocyte activity at 2 mg/kg,
remained undisturbed by treatment. There was no difference between
the groups with intact and abraded skin, and all other investigations
(clinical signs, measurement of food intake and weight gain, further
clinical chemistry, haematology and urinalysis and pathological
investigations including limited histopathology) revealed no
treatment-related changes. The NOAEL was 20 mg/kg bw, since only
erythrocyte cholinesterase activity was affected at this dose, the
highest dose used, with no effect on cholinesterase activity in brain
(Flucke & Schilde, 1980).
Dogs
In a 52 week toxicity study in beagle dogs, azinphos-methyl
(purity 91.9%) was administered, via the diet, to four groups, each of
4 males and 4 females, at dietary levels of 0 (control), 5, 25 or 125
ppm. Clinical signs of reaction to treatment were confined to a
higher incidence of diarrhoea in dogs receiving 125 ppm. Two males
receiving 125 ppm failed to gain weight during the course of the
study, but food intake remained unaffected by treatment. Haematology
and urinalysis revealed no indication of any reaction to treatment.
Clinical biochemistry tests revealed a depression of cholinesterase
activity in plasma and erythrocytes at 25 and 125 ppm and in brain at
termination at 125 ppm. There was also a very slight increase,
compared to controls, in liver cytochrome P450 and N-demethylase
activity at the high dose and a reduction in albumin levels.
Pathological investigations (macroscopic examination, organ weight
analysis and histopathology) revealed no evidence of any reaction to
treatment with azinphos-methyl. The NOAEL was 25 ppm (equal to
0.74 mg/kg bw/day) based on reduced weight gain and inhibition of
acetylcholinesterase activity in brain (Allen, et al., 1990).
Long-term/carcinogenicity studies
Mice
A bioassay of azinphos-methyl (purity 90% from manufacturing
specification) for possible carcinogenicity was conducted by the NCI.
The experiment involved administering the test material, in the feed,
to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats of each sex
were treated for 80 weeks, then observed for 34 or 35 weeks. Males
received time weighted average doses of 78 or 156 ppm, females
received 62.5 or 125 ppm. Matched controls consisted of 10 untreated
rats of each sex; pooled controls consisted of matched controls
combined with 95 male and 95 female untreated rats from similar
bioassays of 10 other chemicals. The mouse study was of similar
design; groups of 50 mice were treated for 80 weeks, then observed for
12 or 13 weeks, males received 31.3 or 62.5 ppm and females 62.5 or
125 ppm, matched controls consisted of 10 males and 10 females, pooled
controls 130 males and 120 females. Typical signs of organophosphate
intoxication (hyperactivity, tremors and dyspnoea) were observed in a
few animals of both species. Weight gain in treated males and high
dose females of both species was lower than in control animals. In
rats there was some evidence of decreased survival at the high dose
compared to controls but this was not seen in mice. In both sexes, at
all doses, survival to termination was adequate for assessment of
effects on late appearing tumours. The report concluded that in rats
the incidence of tumours of the pancreatic islets, and of follicular
cells in the thyroid in males suggested, but did not clearly
implicate, azinphos-methyl as a carcinogen in these animals. There
was no similar evidence in female rats and in mice of each sex there
was no increased incidence of tumours that could be related to the
administration of azinphos-methyl (National Cancer Institute, 1978).
In a carcinogenicity study of azinphos-methyl (purity 88.6%) in
mice, groups of 50 male and 50 female CD-1 mice received dietary
levels of 0 (control), 5, 20, or 40/80 ppm for two years. (The study
was initially started with 80 ppm as the high dietary level, but this
was reduced to 40 ppm after one week, due to severe reaction to
treatment, including mortality, at 80 ppm). Following the reduction
in the high dietary level, there were no clinical signs of reaction to
treatment and mortality remained unaffected by treatment. Weight gain
and food intake remained unaffected by treatment at dietary levels up
to and including 40 ppm. Haematological investigations revealed no
indication of any reaction to treatment. Measurement of
acetylcholinesterase activity revealed that at 5 ppm, activities in
plasma, erythrocyte and brain remained comparable with control values.
At 20 and 40 ppm there was a dose-related inhibition of cholinesterase
activity in plasma and erythrocytes. A similar effect was noted in
brain, except that males were only affected at 40 ppm, while females
exhibited a depression of brain cholinesterase activity at 20 and 40
ppm. Pathological investigations revealed no evidence of any reaction
to treatment, in particular there was no evidence of any carcinogenic
effect of azinphos-methyl. The NOAEL was 5 ppm (equal to
0.88 mg/kg bw/day) based on inhibition of cholinesterase in plasma,
erythrocytes and brain at 20 ppm (Hayes, 1985).
Rats
In a combined long-term toxicity and carcinogenicity study in
rats, groups of 60 male and 60 female Wistar rats received
azinphos-methyl (purity 87.2%) in the diet at levels of 0 (Control),
5, 15 or 45 ppm. From each group, 10 rats per sex were killed after
12 months, while all survivors were killed after 24 months continuous
treatment. There were no clinical signs of reaction to treatment and
survival was unaffected by azinphos-methyl. Weight gain of high dose
males was slightly less than controls but growth in other groups was
not affected by treatment. Clinical biochemistry (apart from
acetylcholinesterase investigations), haematology and urinalysis tests
revealed no indication of any reaction to treatment. Determinations
of acetylcholinesterase activities in erythrocytes, plasma and brain
revealed a marked inhibition, compared to controls, in males and
females from the high dose group (erythrocytes, plasma and brain) and
a less marked effect at 15 ppm (males: erythrocytes, females:
erythrocytes and plasma). Acetylcholinesterase activity in brain from
rats treated at 15 ppm and in erythrocytes, plasma and brain from rats
treated at 5 ppm, remained unaffected by treatment with
azinphos-methyl. Pathological examinations (including gross
examination, organ weight analysis and histological examination of
tissues) revealed no evidence of any reaction to treatment; in
particular there was no evidence of any carcinogenic effect of
azinphos-methyl. The NOAEL was 15 ppm (equal to 0.86 mg/kg bw/day)
based on effects on body weight gain and brain acetylcholinesterase
(Schmidt, 1987).
Reproduction studies
In a two generation (two litters per generation) reproduction
study in rats azinphos-methyl (purity 87.2%) was administered to
groups of 12 male and 24 female Wistar rats at dietary levels of 0
(control), 5, 15 or 45 ppm. At 15 and 45 ppm there was a decrease in
fertility of F0 rats and the total number of delivered pups. At 45
ppm there was an increased mortality of dams in the F0 generation and
reduced pup viability during lactation. As a consequence of these
effects only 5 females were available for mating in the F1b
generation. During mating of the F1b generation, fertility was again
adversely affected at 15 ppm but not to as great an extent as it was
during the F0 generation. At all stages of the study, there was no
evidence of treatment induced malformations and food intake remained
unaffected. Clinical signs of reaction to treatment, including
cholinergic signs, were seen at the high dose and weight gain was
adversely affected at 15 and 45 ppm. The NOAEL was 5 ppm, equal to
0.48 mg/kg bw/day, based on the adverse effects on fertility and body
weight gain seen at 15 and 45 ppm (Eiben & Janda, 1987).
A further study was conducted in order to investigate the effects
on reproductive performance noted in the study described above. The
objectives of this further, one generation study were to investigate
whether the slight effect on fertility at 15 ppm could be confirmed,
and, if reproducible, to determine whether the effect was attributable
to treatment of the male or the female and to determine if
reproductive effects were associated with treatment-induced inhibition
of cholinesterase activity. Azinphos-methyl (purity 92.0%) was
administered to groups of 18 male and 46 female Wistar rats, at
dietary levels of 0 (control), 5, 15, or 45 ppm. Treated males and
females were paired, and dams allowed to rear litters to weaning.
Additional treated males were paired with untreated females. At 15
ppm, when males and females were treated, the viability index was
reduced, largely confirming the results of the previous study.
However, after treatment of male parental animals only, reproductive
parameters remained unaffected, even at 45 ppm. Investigations of
cholinesterase activity in parental animals revealed a depression in
activity in plasma and erythrocytes at all dose levels, and a
depression in activity in brain at 45 ppm in males and at 15 and 45
ppm in females. At 45 ppm, brain cholinesterase activity in pups was
also depressed. The NOAEL was 5 ppm, equal to 0.43 mg/kg bw/day,
based on the adverse effects on fertility and depression of brain
cholinesterase activity seen at 15 ppm (Holzum, 1990).
Special studies on delayed neurotoxicity
In a published report of experiments designed to investigate the
potential relationship between delayed neurotoxicity and copper
concentration in the serum of hens, it was reported that
azinphos-methyl failed to produce neurotoxic symptoms after either
single or repeated doses (Kimmerle & Loser, 1974).
In an acute delayed neurotoxicity test, azinphos-methyl (purity
85%)was administered twice, at the unprotected LD50 dose level of 330
mg/kg to a group of 30 white leghorn hens, with an interval of 21 days
between doses. Groups of untreated control, vehicle control and
positive control (TOCP 600 mg/kg bw) animals, each composed of 10
animals, were also included. Atropine was used for symptomatic
treatment after dosing. A total of 11 hens treated with
azinphos-methyl survived until termination. These animals appeared
normal during the last 12 or 13 days of the study, but exhibited
varying degrees of impaired locomotor activity soon after dosing.
Histopathological examinations indicated that azinphos-methyl did not
increase the incidence or severity of lesions in the nerve tissue
compared to untreated and vehicle controls. Investigations of
neuropathy target esterase activity were not included in the study
(Glaza, 1988).
Special studies on embryo/fetoxicity
Mice and rats
The effects of azinphos-methyl on development in rats and mice
were investigated in a series of experiments. On the basis of
preliminary toxicity studies doses of 0, 1.25, 2.5 and 5.0 mg/kg
bw/day were selected for developmental studies in both species, which
consisted of two phases. During the first phase, pregnant rats and
mice were treated for 10 days, starting on gestational day 6. During
the second phase, pregnant rats were treated from day 6 of gestation
to day 21 post partum. In the first phase, maternal toxicity was seen
only in rats receiving the high dose. When dams and fetuses were
examined (day 18 of gestation for mice, day 20 for rats) there was no
dose-related increase in anomalies or malformation in rats or mice.
In the second phase, dams in the high-dose group were more sensitive
to azinphos-methyl in the latter stages of gestation and signs of
anticholinesterase intoxication, including mortality, were observed.
As a result, only one litter (out of 13) survived to weaning in this
group. It was concluded that azinphos-methyl had little primary
effect on development in rats and mice (Short, et al, 1978; Short,
et al., 1980).
Rats
In a teratology study in rats, groups of 33 inseminated dams
received azinphos-methyl (purity 87.7%) from day 6 to day 15 of
gestation (day of insemination = day 0), at dose levels of
0 (control), 0.5, 1.0 or 2.0 mg/kg bw/day. From each group, 5 dams
were killed on day 16 of gestation and the remaining dams on day 20.
On day 16 of gestation, cholinesterase activities in plasma,
erythrocytes and brain were depressed, compared to controls, in dams
at the high dose only (fetal tissues were not examined). By day 20 of
gestation there was indication of recovery in cholinesterase activity
in all previously affected tissues and fetal brain cholinesterase
activity was comparable with control values. Azinphos-methyl did not
affect any maternal reproductive parameters and there was no
indication of treatment-related embryotoxicity, fetotoxicity or
teratogenicity at any dose level. The NOAEL for maternal toxicity was
1.0 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 16 of gestation (Kowalski et al., 1987).
Rabbits
In a teratology study in rabbits, groups of 11 or 12 pregnant
animals received daily oral doses of azinphos-methyl (purity 92.4%)
from day 6 to day 18 of gestation (day of insemination = day 0) at
levels of 0 (Control), 0.3, 1.0 or 3.0 mg/kg bw/day. Caesarean
section was carried out on day 29 of gestation. Azinphos-methyl
induced no evidence of maternal toxicity at any dose level and there
were no detectable effects on embryonic nor fetal development
(Machemer, 1975).
In a further teratology study in rabbits, groups of 20
inseminated does received daily oral doses of azinphos-methyl (purity
87.7%) from day 6 to day 18 of gestation (day of insemination = day 0)
at levels of 0 (Control), 1, 2.5 or 6 mg/kg bw/day. Ataxia in 4 high
dose does and tremors in 2 of these same animals represented clinical
signs of reaction to treatment.
Plasma and erythrocyte cholinesterase activity, on day 19 of
gestation, was depressed compared to controls at the mid and high
dose. By day 28 of gestation there was clear evidence of recovery in
plasma and erythrocyte cholinesterase activity, although activity in
brain was depressed, compared to controls, at the high dose.
Azinphos-methyl did not affect any maternal reproductive parameters
and there was no evidence of any treatment-related effect on
embryotoxicity, fetotoxicity or teratogenicity at any dose level. The
no observable adverse effect level for maternal toxicity was
2.5 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 28 of gestation (Clemens et al., 1988).
Special studies on genotoxicity
In Salmonella/microsome point mutation tests, azinphos-methyl
(purity >88.8%) showed no evidence of mutagenic activity which could
be classified as positive results in the tests. In one test there was
reproducible evidence of a slight dose-dependent increase in revertant
frequency in one test strain, but the increase was less than 2-fold
(Herbold, 1978; Herbold, 1988; Lawlor, 1987).
Azinphos-methyl (purity 91.1%) exhibited no mutagenic activity in
a reverse mutation test with Saccharomyces cerevisiae (Hoorn, 1983).
In Chinese hamster ovary cells in vitro, azinphos-methyl
induced chromosomal anomalies in a dose related fashion. Most
commonly observed were chromatid breaks and exchanges. In a test with
human lymphocytes in vitro, (purity 91.9%) there were no chromosomal
aberrations induced in the absence of S-9 mix but clear,
treatment-related variations were noted when azinphos-methyl was
tested in the presence of S-9 mix at cytotoxic concentrations. In an
investigation of ability to induce sister chromatid exchanges in
Chinese hamster V79 cells in vitro, azinphos-methyl was shown not to
increase the frequency of sister chromatid exchange, but did induce
some cell cycle delay (Alam, 1974; Herbold, 1986; Chen 1982 et al.,
1982a,b).
The potential of azinphos-methyl (purity 91.1%) to cause DNA
damage was assessed in Rosenkranz and Leifer's pol test employing two
E. coli strains which vary in regard to their repair systems for DNA
damage. The results showed that azinphos-methyl gave no indication of
any effect on DNA damage. In a primary rat hepatocyte unscheduled DNA
synthesis assay azinphos-methyl (purity 91.1%) did not induce
significant changes in the nuclear labelling of primary rat
hepatocytes and it was concluded that azinphos-methyl did not induce
DNA damage in this assay (Herbold, 1984; Myhr, 1983).
Mutagenic effects of azinphos-methyl (purity 92.3%) in vivo
were investigated in a micronucleus test and a dominant lethal test;
both in mice. In the micronucleus test two doses of azinphos-methyl
(2 x 2.5 or 2 x 5.0 mg/kg bw) were given 24 hours apart and a femoral
marrow smear was prepared 6 hours after the second dose; there was no
indication of any mutagenic effect. In the dominant lethal study male
mice received a single oral dose of 4 mg/kg bw azinphos-methyl and
were then mated with untreated females over 12 consecutive periods of
4 days. Fertility remained unaffected and there were no
treatment-related differences in implantation parameters (Herbold,
1979a,b).
An effort was made to evaluate the genotoxicity of a variety of
pesticides, with the specific objectives of comparing different
in vivo and in vitro assays, examining the spectrum of genetic
activity displayed by the selected pesticides and examining the test
results in relation to other biological and chemical features of the
pesticides. In this research programme azinphos-methyl has been
tested in a range of 14 mutagenicity tests, examining point or gene
mutations, DNA damage and chromosomal effects. Positive results for
azinphos-methyl were seen in only two tests: a forward mutation assay
in mouse lymphoma L5178Y cells (only in the presence of S-9 mix) and
a mitotic recombination assay in Saccharomyces cerevisiae strain D3.
Azinphos-methyl was negative in tests for point/gene mutation and DNA
damage in prokaryotes and showed no positive results in tests looking
at chromosomal effects (Waters et al., 1982).
Special studies on skin and eye irritation and sensitization
In a skin irritation study employing 6 rabbits, 24 hour exposure
to azinphos-methyl at intact and abraded skin sites did not cause any
signs of irritation. In an eye irritation study, exposure of the
conjunctiva of the eye to azinphos-methyl for 5 minutes (5 rabbits) or
24 hours (3 rabbits) caused no significant reaction (Thyssen & Lorke,
1981).
In a dermal irritation study utilizing 6 New Zealand white
rabbits, 0.5 g of benzazimide was moistened with water and kept in
contact with the shaved skin for 4 hours. At 30 and 60 minutes and
24, 48 and 72 hours after patch removal there was no evidence of
erythema or oedema at the treatment sites. Benzazimide is therefore
not a skin irritant in rabbits (Eigenberg, 1987).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Magnusson and Kligman
maximization test. The study revealed that azinphos-methyl had a
sensitizing effect in 95% of the test animals (Flucke, 1986).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Buehler patch test. By means
of a dermal application of a concentration of 25% sensitization was
induced in approximately 50% of the test animals (Porter et al.,
1987).
In another Buehler patch test dermal application of a
concentration of 12.5% induced sensitization in approximately 50% of
the test animals when challenged using a 6% concentration, but using
a challenge concentration of 0.6% failed to elicit any relevant skin
reactions (Heimann, 1987b).
Observations in humans
Employees working in the formulation of azinphos-methyl products
have been subjected to regular medical examinations and no general
impairment of health has been observed. In one isolated case it was
considered probable that contact with azinphos-methyl was the cause of
generalized dermatosis in an apparently hypersensitive, very dry skin
(Faul, 1981; Miksche, 1981).
Published reports from the pesticide incident monitoring system
in the United States of America and additional data from the state of
California in the USA have been reviewed. Between 1982 and 1988 a
small number of incidents have been reported annually (involving 5-12
persons each year) which have been definitely, probably or possibly
associated with azinphos-methyl either alone or in combination with
other pesticides. In addition, two incidents occurred in 1987, one
involving 26 people, the other involving 32 people. The first
involved spray drift in adverse weather conditions. The second
involved workers who experienced symptoms including headache, nausea,
weakness and vomiting upon entry to a field to pick peaches 3 days
after methomyl was applied to the crop and about 6 weeks after an
application of azinphos-methyl (US EPA, 1981; Mahler, 1991).
COMMENTS
The toxicokinetics of azinphos-methyl has been investigated
following oral administration in rats. It does not accumulate in body
tissues.
In a 52-week study in dogs, using dietary concentrations of 0, 5,
25 or 125 ppm the NOAEL was 25 ppm (equal to 0.74 mg/kg bw/day), based
on reduced body-weight gain and inhibition of acetylcholinesterase
activity in brain at 125 ppm.
Long-term/carcinogenicity studies in rats at dietary
concentrations of 0, 5, 15, or 45 ppm and in mice at 0, 5, 20 or 40
ppm showed that azinphos-methyl has no carcinogenic potential in
either species. These results clarified earlier equivocal findings in
rats in an NCI bioassay. The NOAEL in rats was 15 ppm (equal to
0.86 mg/kg bw/day), based on effects on brain acetylcholinesterase at
45 ppm. In mice the NOAEL was 5 ppm (equal to 0.88 mg/kg bw/day),
based on inhibition of cholinesterase in plasma, erythrocytes and
brain at 20 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 15 or 45 ppm, fertility and pup viability
during lactation were adversely affected, equivocally at 15 ppm and
markedly at 45 ppm. The NOAEL was 5 ppm, equal to 0.48 mg/kg bw/day.
Teratology studies in rats, mice and rabbits did not indicate
teratogenic effects at doses up to 2, 5 and 6 mg/kg bw/day
respectively.
The data from genotoxicity studies with azinphos-methyl were
conflicting. However, in vivo studies were negative, the positive
data being confined to some in vitro studies. After reviewing the
available information it was concluded that it is unlikely that
azinphos-methyl is genotoxic to humans.
Acute delayed neurotoxicity tests in hens with azinphos-methyl
gave negative results.
The 1973 JMPR reported that daily doses up to and around 0.3
mg/kg bw/day for 30 days in human volunteers had no effect on plasma
or erythrocyte cholinesterase activity. New data were not available
from occupational exposure or human volunteer studies with
azinphos-methyl. A review of the available literature and reports of
human poisoning with azinphos-methyl revealed no information relevant
to the estimation of the ADI.
Since the critical toxicological end-point was not
acetylcholinesterase inhibition, the human data were not appropriate
for estimation of the ADI, which was based on the NOAEL in the rat
multigeneration study in rats using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm (equal to 0.88 mg/kg bw/day)
Rat: 5 ppm (equal to 0.86 mg/kg bw/day) in a long-
term/carcinogenicity study
5 ppm (equal to 0.48 mg/kg bw/day) in a
multigeneration study
Dog: 25 ppm (equal to 0.74 mg/kg bw/day)
Human: 0.3 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans
REFERENCES
Alam, M.T., Corbeil, M., Chagnon, A., Kasatiya, S.S. (1974).
Chromosomal anomalies induced by the organic phosphate pesticide
guthion in Chinese hamster cells. Chromosoma (Berl.), 49: 77-86.
Allen, T.R., Frei, T., Janiak, T., Luetkemeier, H., Vogel, O.,
Biedermann, K., Wilson, J. (1990). 52-week oral toxicity (feeding)
study with azinphos-methyl in the dog. Unpublished report from
Research and Consulting Company AG, submitted by Bayer AG, Leverkusen,
Germany.
Chen, H.H., Sirianni, S.R., Huang, C.C. (1982a). Sister chromatid
exchanges and cell cycle delay in Chinese hamster V79 cells treated
with 9 organophosphorus compounds (8 pesticides and 1 defoliant).
Mutation Research, 103: 307-313.
Chen H.H., Sirianni, S.R., Huang, C.C. (1982b). Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environmental Mutagenesis, 4: 621-624.
Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1988). A teratology study
in the rabbit with azinphos-methyl. Unpublished report from Miles
Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to rats.
Unpublished report from Chemagro, submitted by Bayer AG, Leverkusen,
Germany.
Eiben, R., Janda, B. (1987). Azinphos-methyl - two generation study
on rats. Unpublished report from Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1987). Primary dermal irritation of Benzazimide in
albino rabbits. Unpublished report from Mobay Corporation, submitted
by Bayer AG, Leverkusen, Germany.
Faul, J. (1981). BBA request, effects on humans. Letter of 2 June
1981 from Bayer AG, Department DO Medical. Company internal letter,
submitted by Bayer AG, Leverkusen, Germany.
Feldmann, R.J., Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicology and Applied
Pharmacology, 28: 126-132.
Flucke, W. (1979). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Flucke, W. (1986). Azinphos-methyl - Study for skin sensitizing
effect in guinea pigs (Magnusson and Kligman maximization test).
Unpublished report from Bayer AG, Leverkusen, Germany.
Flucke, W., Schilde, B. (1980). Azinphos-methyl: Subacute cutaneous
toxicity to rabbits. Unpublished report from Bayer AG, Leverkusen,
Germany.
Franklin, C.A., Greenhalgh, R., Maibach, H.I. (1982). Pesticide
chemistry, human welfare and the environment. Volume 4,
pages 221-226. Pergamon Press, London, United Kingdom.
Franklin, C.A., Muir, N.I., Moody, R.P. (1986). The use of biological
monitoring in the estimation of exposure during the application of
pesticides. Toxicology Letters, 33: 127-136.
Glaza, S.M. (1988). Azinphos-methyl: Acute delayed neurotoxicity
study in the domestic fowl. Unpublished report from Hazleton
Laboratories America Inc, sponsored by Mobay Corporation, submitted by
Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985). Oncogenicity study of azinphos-methyl in mice.
Unpublished report from Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1981). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1982). Azinphos-methyl study of the acute oral and
dermal toxicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1987a). Determination of acute toxicity (LD50) of
azinphos methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987b). Azinphos-methyl: Study of skin sensitizing
effect on guinea pigs (Buehler patch test). Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1978). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1979a). Azinphos-methyl: micronucleus test on mice to
evaluate for possible mutagenic effects. Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1979b). Azinphos-methyl: dominant lethal study on male
mouse to test for mutagenic effects. Unpublished report from Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1984). Azinphos-methyl, Pol test on E. coli to
evaluate for potential DNA damage. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1986). Azinphos-methyl: cytogenic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report from Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Holzum, B. (1990). Azinphos-methyl: investigation of inhibition of
cholinesterase activity in plasma, erythrocytes and brain, in a
one-generation study. Unpublished report from Bayer AG, Leverkusen,
Germany.
Hoorn, A.J.W. (1983). Mutagenicity evaluation of azinphos-methyl in
the reverse mutation induction assay with Saccharomyces cerevisiae
strains S138 and S211. Unpublished report from Litton Bionetics,
Holland, submitted by Bayer AG, Leverkusen, Germany.
Kao, L.R.M. (1988). Disposition and metabolism of ring-UL-14C
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1987). A
teratology study with azinphos-methyl in the rat. Unpublished report
from Miles Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1976). Subchronic inhalation toxicity of
azinphos-methyl in rats. Arch. Toxicol., 35: 83-89.
Kimmerle, G.; Loser, E. (1974). Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environm. Qual. Safety, 3: 173-178.
Lamb, D.W., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to fasted and
non-fasted rats using CMC as the excipient. Unpublished report from
Chemagro, submitted by Bayer AG, Leverkusen, Germany.
Lawlor, T.E. (1987). Azinphos-methyl: Salmonella/microsome plate
incorporation mutagenicity assay. Unpublished report from
Microbiological Associates Inc., submitted by Bayer AG, Leverkusen,
Germany.
Lin, S.N., Chen, C.Y., Murphy, S.D., Caprioli, R.M. (1980).
Quantitative high-performance liquid chromatography and mass
spectrometry for the analysis of the in vitro metabolism of the
insecticide azinphos-methyl by rat liver homogenates. J. Agric. Food
Chem., 28: 85-88
Machemer, L. (1975). Azinphos-methyl: studies for embryonic and
teratogenic effects on rabbits following oral administration.
Unpublished report from Bayer AG, Leverkusen, Germany.
Mahler, L. (1991). Program Director, Pesticide Illness Surveillance
Program, Worker Health and Safety Branch, California Department of
Agriculture. Data provided to Mobay Corporation, submitted by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978). Azinphos-methyl acute toxicity studies.
Unpublished report from Bayer AG, Leverkusen, Germany.
Miksche, L. (1981). Information on effects in man/occupational
experience. Letter of 12 June 1981, from Bayer AG medical department.
Company internal letter, submitted by Bayer AG, Leverkusen, Germany.
Myhr, B.C. (1983). Evaluation of azinphos-methyl in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report from
Litton Bionetics USA, submitted by Bayer AG, Leverkusen, Germany.
National Cancer Institute (1978). Bioassay of azinphos-methyl for
possible carcinogenicity. Technical report series no 69.
Pasquet, J., Mazuret, A., Fournel, J., Koenig, F.H. (1976). Acute
oral and percutaneous toxicity of phosalone in the rat, in comparison
with azinphos-methyl and parathion. Toxicology and Applied
Pharmacology, 37: 85-92.
Patzschke, K., Wegner, L.A., Weber, H. (1976). Biokinetic
investigations of carbonyl-14C azinphos methyl in rats. Unpublished
report from Bayer AG, Leverkusen, Germany.
Porter, M.C., Craigo, R.E., Hartnagel, R.E. (1987). Dermal
sensitization evaluation of azinphos-methyl technical in the guinea
pig. Unpublished report from Miles Laboratories Inc., submitted by
Bayer AG, Leverkusen, Germany.
Rao, S.L N., McKinley, W.P. (1969). Metabolism of organophosphorus
insecticides by liver homogenates from different species. Can. J.
Biochem., 47: 1155-1159
Schmidt, W.M. (1987). Azinphos-methyl: study of chronic toxicity and
carcinogenicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1988). Acute dermal toxicity of technical grade
benzazimide to rabbits. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987a). Acute four-hour inhalation toxicity study
with azinphos-methyl in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b). Acute inhalation toxicity study with
benzazimide technical in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988). Acute one hour inhalation toxicity study with
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Short, R.D., Minor, J.L., Lee, C.C., Chernoff, N., Baron, R.L. (1980).
Developmental toxicity of azinphos-methyl in rats and mice. Arch.
Toxicol, 43: 177-186.
Short, R.D., Minor, J.L., Unger, T.M., Lee, C.C. (1978). Teratology
of azinphos-methyl. Unpublished report from Mid West Research
Institute, USA, contracted by US Environmental Protection Agency,
submitted by Bayer AG, Leverkusen, Germany.
Sterri, S.H., Rognerud, B., Fiskum, S.E., Lyngaas, S. (1979). Effect
of toxogonin and P2S on the toxicity of carbamates and
organophosphorus compounds. Acta Pharmacol. et Toxicol., 45: 9-15
Thyssen, J. (1976a). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Thyssen, J. (1976b). Studies to determine the toxic effects of the
simultaneous application of azinphos-methyl or azinphos-ethyl and
methamidophos. Unpublished report from Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977a). Study for combination toxicity of
azinphos-methyl and propoxur. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977b). Study for the combination toxicity of
azinphos-methyl and azinphos-ethyl. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J., Lorke (1981). Azinphos-methyl - study of the irritant
effects on the skin and mucous membranes (eye). Unpublished report
from Bayer AG, Leverkusen, Germany.
US EPA (1981). Summary of reported pesticide incidents involving
azinphos-methyl Pesticide incident monitoring system, report No. 469
submitted by Bayer AG, Leverkusen, Germany.
Waters, M.D., Sandhu, S.S., Simon, V.F., Mortelmans, K.E.,
Mitchell, A.A., Jorgenson, T.A., Jones, D.C.L., Valencia, R.,
Garrett, N.E. (1982). Study of pesticide genotoxicity. Genetic
Toxicology - an agricultural perspective, 21: 275 - 326.
Weber, H., Patzschke, K., Wegner, L.A. (1980). Biokinetic
investigation of ring-UL-14C benzazimide in rats. Unpublished report
from Mobay Corporation, submitted by Bayer AG, Leverkusen, Germany.
Webster, R.C.; Maibach, H.I. (1985). In vivo percutaneous
absorption and decontamination of pesticides in humans. Journal of
Toxicology and Environmental Health, 16: 25-37.
Table 1. Results of acute toxicity tests with azinphos-methyl and
related materials
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl Oral Rat DMSO m 5.6 Crawford (1974)
f 6.4
Benzazimide Oral Rat DMSO m 412
f 269
Methyl benzazimide Oral Rat DMSO m 330
f 330
Azinphos methyl Oral Rat Cremophor EL m 25.4 Flucke (1979)
Azinphos methyl Oral Rat Cremophor EL m 9.1 (fasted) Heimann (1981)
m 17.25 (non-fasted)
Azinphos methyl Oral Rat Cremophor EL m 6.7 (fasted) Heimann (1982)
m 12.8 (non-fasted)
Dermal Rat Cremophor EL m 225
f 155
Azinphos methyl Oral Rat Cremophor EL m 7.1 Heimann (1987)
Azinphos methyl Oral Rat CMC (fasted) m 19 Lamb et al. (1974)
f 16
m 19
(non-fasted) f 10
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Benzazimide Oral Rat CMC (fasted) m 576 Lamb (1974)
f 368
m 576
(non-fasted) f 487
Methyl benzazimide Oral Rat CMC (fasted) m 412
f 390
m 524
(non-fasted) f 460
Azinphos methyl Oral Rat Cremophor EL m 4.6 Mihail (1978)
f 4.4
Dermal Rat Cremophor EL m 2500-5000*
Oral Dog Cremophor EL m >10
Azinphos methyl Oral Rat Methylene chloride/ m 26 Pasquet et al. (1976)
Tween/80 Gum Arabic f 24
Dermal Rat Acetone/Ethanol/ f 90
Peanut Oil
Benzazimide Dermal Rabbit Tap water m >2000 Sheets (1988)
f >2000
Azinphos methyl 4 hr inhal Rat PEG/ETOH m 155 Shiotsuka (1987a)
f 132 (mg/m3)
Benzazimide 4 hr inhal Rat None m >1760* Shiotsuka (1987b)
(mg/m3)
Table 1 (contd).
Test material Route Species Vehicle LD50 (mg/kg bw) Reference
Azinphos methyl 1 hr inhal Rat PEG/ETOH m 396 Shiotsuka (1988)
f 310 (mg/m3)
Azinphos methyl Oral Rat Cremophor EL m 15.6 Thyssen (1976a)
Azinphos methyl Oral Rat Cremophor EL m 16.75 Thyssen (1976b)
Methamidophos Oral Rat Cremophor EL m 31.9
Azinphos methyl +
Methamidophos Oral Rat Cremophor EL m 19.5
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977a)
Propoxur Oral Rat Cremophor EL m 39
Azinphos methyl +
Propoxur Oral Rat Cremophor EL m 29.3
Azinphos methyl Oral Rat Cremophor EL m 9.7 Thyssen (1977b)
Azinphos ethyl Oral Rat Cremophor EL m 11.8
Azinphos methyl +
Azinphos ethyl Oral Rat Cremophor EL m 11.1
* sex not specified.
m male
f female
Toxicological studies
Acute Toxicity Studies
Results of acute toxicity tests with azinphos-methyl and related
materials are listed in Table 1.
Short-term studies
Rats
A 12-week inhalation study has been described in the published
literature. Groups of 10 male and 10 female Wistar rats were exposed
in inhalation chambers to mean aerosol concentrations in the air of 0
(control), 0.195, 1.24 and 4.72 mg/m3 azinphos methyl, for 6 hours
daily, 5 days per week, for 12 weeks. At the high dose level body
weight gain was depressed in males and erythrocyte cholinesterase
activity was inhibited in males and females. There was no other
evidence of any reaction to treatment. The NOAEL was 1.24 mg/m3
based on reduced weight gain in males at the high dose level
(Kimmerle, 1976).
Rabbits
In a dermal toxicity study in rabbits, azinphos-methyl was
applied for 6 hours per day, to the shaved dorsal and lateral skin, at
dose levels of 0 (control), 2, or 20 mg/kg bw, for 5 days per week for
3 weeks. Each group consisted of 6 males and 6 females, with the skin
left intact in 3 animals of each sex and abraded in the others.
Investigation of cholinesterase activity revealed a marginal
(approximately 30%) depression of erythrocyte activity, compared to
controls, in males and females treated with 20 mg/kg. Cholinesterase
activity in plasma and brain, and erythrocyte activity at 2 mg/kg,
remained undisturbed by treatment. There was no difference between
the groups with intact and abraded skin, and all other investigations
(clinical signs, measurement of food intake and weight gain, further
clinical chemistry, haematology and urinalysis and pathological
investigations including limited histopathology) revealed no
treatment-related changes. The NOAEL was 20 mg/kg bw, since only
erythrocyte cholinesterase activity was affected at this dose, the
highest dose used, with no effect on cholinesterase activity in brain
(Flucke & Schilde, 1980).
Dogs
In a 52 week toxicity study in beagle dogs, azinphos-methyl
(purity 91.9%) was administered, via the diet, to four groups, each of
4 males and 4 females, at dietary levels of 0 (control), 5, 25 or 125
ppm. Clinical signs of reaction to treatment were confined to a
higher incidence of diarrhoea in dogs receiving 125 ppm. Two males
receiving 125 ppm failed to gain weight during the course of the
study, but food intake remained unaffected by treatment. Haematology
and urinalysis revealed no indication of any reaction to treatment.
Clinical biochemistry tests revealed a depression of cholinesterase
activity in plasma and erythrocytes at 25 and 125 ppm and in brain at
termination at 125 ppm. There was also a very slight increase,
compared to controls, in liver cytochrome P450 and N-demethylase
activity at the high dose and a reduction in albumin levels.
Pathological investigations (macroscopic examination, organ weight
analysis and histopathology) revealed no evidence of any reaction to
treatment with azinphos-methyl. The NOAEL was 25 ppm (equal to
0.74 mg/kg bw/day) based on reduced weight gain and inhibition of
acetylcholinesterase activity in brain (Allen, et al., 1990).
Long-term/carcinogenicity studies
Mice
A bioassay of azinphos-methyl (purity 90% from manufacturing
specification) for possible carcinogenicity was conducted by the NCI.
The experiment involved administering the test material, in the feed,
to Osborne-Mendel rats and B6C3F1 mice. Groups of 50 rats of each sex
were treated for 80 weeks, then observed for 34 or 35 weeks. Males
received time weighted average doses of 78 or 156 ppm, females
received 62.5 or 125 ppm. Matched controls consisted of 10 untreated
rats of each sex; pooled controls consisted of matched controls
combined with 95 male and 95 female untreated rats from similar
bioassays of 10 other chemicals. The mouse study was of similar
design; groups of 50 mice were treated for 80 weeks, then observed for
12 or 13 weeks, males received 31.3 or 62.5 ppm and females 62.5 or
125 ppm, matched controls consisted of 10 males and 10 females, pooled
controls 130 males and 120 females. Typical signs of organophosphate
intoxication (hyperactivity, tremors and dyspnoea) were observed in a
few animals of both species. Weight gain in treated males and high
dose females of both species was lower than in control animals. In
rats there was some evidence of decreased survival at the high dose
compared to controls but this was not seen in mice. In both sexes, at
all doses, survival to termination was adequate for assessment of
effects on late appearing tumours. The report concluded that in rats
the incidence of tumours of the pancreatic islets, and of follicular
cells in the thyroid in males suggested, but did not clearly
implicate, azinphos-methyl as a carcinogen in these animals. There
was no similar evidence in female rats and in mice of each sex there
was no increased incidence of tumours that could be related to the
administration of azinphos-methyl (National Cancer Institute, 1978).
In a carcinogenicity study of azinphos-methyl (purity 88.6%) in
mice, groups of 50 male and 50 female CD-1 mice received dietary
levels of 0 (control), 5, 20, or 40/80 ppm for two years. (The study
was initially started with 80 ppm as the high dietary level, but this
was reduced to 40 ppm after one week, due to severe reaction to
treatment, including mortality, at 80 ppm). Following the reduction
in the high dietary level, there were no clinical signs of reaction to
treatment and mortality remained unaffected by treatment. Weight gain
and food intake remained unaffected by treatment at dietary levels up
to and including 40 ppm. Haematological investigations revealed no
indication of any reaction to treatment. Measurement of
acetylcholinesterase activity revealed that at 5 ppm, activities in
plasma, erythrocyte and brain remained comparable with control values.
At 20 and 40 ppm there was a dose-related inhibition of cholinesterase
activity in plasma and erythrocytes. A similar effect was noted in
brain, except that males were only affected at 40 ppm, while females
exhibited a depression of brain cholinesterase activity at 20 and 40
ppm. Pathological investigations revealed no evidence of any reaction
to treatment, in particular there was no evidence of any carcinogenic
effect of azinphos-methyl. The NOAEL was 5 ppm (equal to
0.88 mg/kg bw/day) based on inhibition of cholinesterase in plasma,
erythrocytes and brain at 20 ppm (Hayes, 1985).
Rats
In a combined long-term toxicity and carcinogenicity study in
rats, groups of 60 male and 60 female Wistar rats received
azinphos-methyl (purity 87.2%) in the diet at levels of 0 (Control),
5, 15 or 45 ppm. From each group, 10 rats per sex were killed after
12 months, while all survivors were killed after 24 months continuous
treatment. There were no clinical signs of reaction to treatment and
survival was unaffected by azinphos-methyl. Weight gain of high dose
males was slightly less than controls but growth in other groups was
not affected by treatment. Clinical biochemistry (apart from
acetylcholinesterase investigations), haematology and urinalysis tests
revealed no indication of any reaction to treatment. Determinations
of acetylcholinesterase activities in erythrocytes, plasma and brain
revealed a marked inhibition, compared to controls, in males and
females from the high dose group (erythrocytes, plasma and brain) and
a less marked effect at 15 ppm (males: erythrocytes, females:
erythrocytes and plasma). Acetylcholinesterase activity in brain from
rats treated at 15 ppm and in erythrocytes, plasma and brain from rats
treated at 5 ppm, remained unaffected by treatment with
azinphos-methyl. Pathological examinations (including gross
examination, organ weight analysis and histological examination of
tissues) revealed no evidence of any reaction to treatment; in
particular there was no evidence of any carcinogenic effect of
azinphos-methyl. The NOAEL was 15 ppm (equal to 0.86 mg/kg bw/day)
based on effects on body weight gain and brain acetylcholinesterase
(Schmidt, 1987).
Reproduction studies
In a two generation (two litters per generation) reproduction
study in rats azinphos-methyl (purity 87.2%) was administered to
groups of 12 male and 24 female Wistar rats at dietary levels of 0
(control), 5, 15 or 45 ppm. At 15 and 45 ppm there was a decrease in
fertility of F0 rats and the total number of delivered pups. At 45
ppm there was an increased mortality of dams in the F0 generation and
reduced pup viability during lactation. As a consequence of these
effects only 5 females were available for mating in the F1b
generation. During mating of the F1b generation, fertility was again
adversely affected at 15 ppm but not to as great an extent as it was
during the F0 generation. At all stages of the study, there was no
evidence of treatment induced malformations and food intake remained
unaffected. Clinical signs of reaction to treatment, including
cholinergic signs, were seen at the high dose and weight gain was
adversely affected at 15 and 45 ppm. The NOAEL was 5 ppm, equal to
0.48 mg/kg bw/day, based on the adverse effects on fertility and body
weight gain seen at 15 and 45 ppm (Eiben & Janda, 1987).
A further study was conducted in order to investigate the effects
on reproductive performance noted in the study described above. The
objectives of this further, one generation study were to investigate
whether the slight effect on fertility at 15 ppm could be confirmed,
and, if reproducible, to determine whether the effect was attributable
to treatment of the male or the female and to determine if
reproductive effects were associated with treatment-induced inhibition
of cholinesterase activity. Azinphos-methyl (purity 92.0%) was
administered to groups of 18 male and 46 female Wistar rats, at
dietary levels of 0 (control), 5, 15, or 45 ppm. Treated males and
females were paired, and dams allowed to rear litters to weaning.
Additional treated males were paired with untreated females. At 15
ppm, when males and females were treated, the viability index was
reduced, largely confirming the results of the previous study.
However, after treatment of male parental animals only, reproductive
parameters remained unaffected, even at 45 ppm. Investigations of
cholinesterase activity in parental animals revealed a depression in
activity in plasma and erythrocytes at all dose levels, and a
depression in activity in brain at 45 ppm in males and at 15 and 45
ppm in females. At 45 ppm, brain cholinesterase activity in pups was
also depressed. The NOAEL was 5 ppm, equal to 0.43 mg/kg bw/day,
based on the adverse effects on fertility and depression of brain
cholinesterase activity seen at 15 ppm (Holzum, 1990).
Special studies on delayed neurotoxicity
In a published report of experiments designed to investigate the
potential relationship between delayed neurotoxicity and copper
concentration in the serum of hens, it was reported that
azinphos-methyl failed to produce neurotoxic symptoms after either
single or repeated doses (Kimmerle & Loser, 1974).
In an acute delayed neurotoxicity test, azinphos-methyl (purity
85%)was administered twice, at the unprotected LD50 dose level of 330
mg/kg to a group of 30 white leghorn hens, with an interval of 21 days
between doses. Groups of untreated control, vehicle control and
positive control (TOCP 600 mg/kg bw) animals, each composed of 10
animals, were also included. Atropine was used for symptomatic
treatment after dosing. A total of 11 hens treated with
azinphos-methyl survived until termination. These animals appeared
normal during the last 12 or 13 days of the study, but exhibited
varying degrees of impaired locomotor activity soon after dosing.
Histopathological examinations indicated that azinphos-methyl did not
increase the incidence or severity of lesions in the nerve tissue
compared to untreated and vehicle controls. Investigations of
neuropathy target esterase activity were not included in the study
(Glaza, 1988).
Special studies on embryo/fetoxicity
Mice and rats
The effects of azinphos-methyl on development in rats and mice
were investigated in a series of experiments. On the basis of
preliminary toxicity studies doses of 0, 1.25, 2.5 and 5.0 mg/kg
bw/day were selected for developmental studies in both species, which
consisted of two phases. During the first phase, pregnant rats and
mice were treated for 10 days, starting on gestational day 6. During
the second phase, pregnant rats were treated from day 6 of gestation
to day 21 post partum. In the first phase, maternal toxicity was seen
only in rats receiving the high dose. When dams and fetuses were
examined (day 18 of gestation for mice, day 20 for rats) there was no
dose-related increase in anomalies or malformation in rats or mice.
In the second phase, dams in the high-dose group were more sensitive
to azinphos-methyl in the latter stages of gestation and signs of
anticholinesterase intoxication, including mortality, were observed.
As a result, only one litter (out of 13) survived to weaning in this
group. It was concluded that azinphos-methyl had little primary
effect on development in rats and mice (Short, et al, 1978; Short,
et al., 1980).
Rats
In a teratology study in rats, groups of 33 inseminated dams
received azinphos-methyl (purity 87.7%) from day 6 to day 15 of
gestation (day of insemination = day 0), at dose levels of
0 (control), 0.5, 1.0 or 2.0 mg/kg bw/day. From each group, 5 dams
were killed on day 16 of gestation and the remaining dams on day 20.
On day 16 of gestation, cholinesterase activities in plasma,
erythrocytes and brain were depressed, compared to controls, in dams
at the high dose only (fetal tissues were not examined). By day 20 of
gestation there was indication of recovery in cholinesterase activity
in all previously affected tissues and fetal brain cholinesterase
activity was comparable with control values. Azinphos-methyl did not
affect any maternal reproductive parameters and there was no
indication of treatment-related embryotoxicity, fetotoxicity or
teratogenicity at any dose level. The NOAEL for maternal toxicity was
1.0 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 16 of gestation (Kowalski et al., 1987).
Rabbits
In a teratology study in rabbits, groups of 11 or 12 pregnant
animals received daily oral doses of azinphos-methyl (purity 92.4%)
from day 6 to day 18 of gestation (day of insemination = day 0) at
levels of 0 (Control), 0.3, 1.0 or 3.0 mg/kg bw/day. Caesarean
section was carried out on day 29 of gestation. Azinphos-methyl
induced no evidence of maternal toxicity at any dose level and there
were no detectable effects on embryonic nor fetal development
(Machemer, 1975).
In a further teratology study in rabbits, groups of 20
inseminated does received daily oral doses of azinphos-methyl (purity
87.7%) from day 6 to day 18 of gestation (day of insemination = day 0)
at levels of 0 (Control), 1, 2.5 or 6 mg/kg bw/day. Ataxia in 4 high
dose does and tremors in 2 of these same animals represented clinical
signs of reaction to treatment.
Plasma and erythrocyte cholinesterase activity, on day 19 of
gestation, was depressed compared to controls at the mid and high
dose. By day 28 of gestation there was clear evidence of recovery in
plasma and erythrocyte cholinesterase activity, although activity in
brain was depressed, compared to controls, at the high dose.
Azinphos-methyl did not affect any maternal reproductive parameters
and there was no evidence of any treatment-related effect on
embryotoxicity, fetotoxicity or teratogenicity at any dose level. The
no observable adverse effect level for maternal toxicity was
2.5 mg/kg bw/day, based on the inhibition of brain cholinesterase
activity seen on day 28 of gestation (Clemens et al., 1988).
Special studies on genotoxicity
In Salmonella/microsome point mutation tests, azinphos-methyl
(purity >88.8%) showed no evidence of mutagenic activity which could
be classified as positive results in the tests. In one test there was
reproducible evidence of a slight dose-dependent increase in revertant
frequency in one test strain, but the increase was less than 2-fold
(Herbold, 1978; Herbold, 1988; Lawlor, 1987).
Azinphos-methyl (purity 91.1%) exhibited no mutagenic activity in
a reverse mutation test with Saccharomyces cerevisiae (Hoorn, 1983).
In Chinese hamster ovary cells in vitro, azinphos-methyl
induced chromosomal anomalies in a dose related fashion. Most
commonly observed were chromatid breaks and exchanges. In a test with
human lymphocytes in vitro, (purity 91.9%) there were no chromosomal
aberrations induced in the absence of S-9 mix but clear,
treatment-related variations were noted when azinphos-methyl was
tested in the presence of S-9 mix at cytotoxic concentrations. In an
investigation of ability to induce sister chromatid exchanges in
Chinese hamster V79 cells in vitro, azinphos-methyl was shown not to
increase the frequency of sister chromatid exchange, but did induce
some cell cycle delay (Alam, 1974; Herbold, 1986; Chen 1982 et al.,
1982a,b).
The potential of azinphos-methyl (purity 91.1%) to cause DNA
damage was assessed in Rosenkranz and Leifer's pol test employing two
E. coli strains which vary in regard to their repair systems for DNA
damage. The results showed that azinphos-methyl gave no indication of
any effect on DNA damage. In a primary rat hepatocyte unscheduled DNA
synthesis assay azinphos-methyl (purity 91.1%) did not induce
significant changes in the nuclear labelling of primary rat
hepatocytes and it was concluded that azinphos-methyl did not induce
DNA damage in this assay (Herbold, 1984; Myhr, 1983).
Mutagenic effects of azinphos-methyl (purity 92.3%) in vivo
were investigated in a micronucleus test and a dominant lethal test;
both in mice. In the micronucleus test two doses of azinphos-methyl
(2 x 2.5 or 2 x 5.0 mg/kg bw) were given 24 hours apart and a femoral
marrow smear was prepared 6 hours after the second dose; there was no
indication of any mutagenic effect. In the dominant lethal study male
mice received a single oral dose of 4 mg/kg bw azinphos-methyl and
were then mated with untreated females over 12 consecutive periods of
4 days. Fertility remained unaffected and there were no
treatment-related differences in implantation parameters (Herbold,
1979a,b).
An effort was made to evaluate the genotoxicity of a variety of
pesticides, with the specific objectives of comparing different
in vivo and in vitro assays, examining the spectrum of genetic
activity displayed by the selected pesticides and examining the test
results in relation to other biological and chemical features of the
pesticides. In this research programme azinphos-methyl has been
tested in a range of 14 mutagenicity tests, examining point or gene
mutations, DNA damage and chromosomal effects. Positive results for
azinphos-methyl were seen in only two tests: a forward mutation assay
in mouse lymphoma L5178Y cells (only in the presence of S-9 mix) and
a mitotic recombination assay in Saccharomyces cerevisiae strain D3.
Azinphos-methyl was negative in tests for point/gene mutation and DNA
damage in prokaryotes and showed no positive results in tests looking
at chromosomal effects (Waters et al., 1982).
Special studies on skin and eye irritation and sensitization
In a skin irritation study employing 6 rabbits, 24 hour exposure
to azinphos-methyl at intact and abraded skin sites did not cause any
signs of irritation. In an eye irritation study, exposure of the
conjunctiva of the eye to azinphos-methyl for 5 minutes (5 rabbits) or
24 hours (3 rabbits) caused no significant reaction (Thyssen & Lorke,
1981).
In a dermal irritation study utilizing 6 New Zealand white
rabbits, 0.5 g of benzazimide was moistened with water and kept in
contact with the shaved skin for 4 hours. At 30 and 60 minutes and
24, 48 and 72 hours after patch removal there was no evidence of
erythema or oedema at the treatment sites. Benzazimide is therefore
not a skin irritant in rabbits (Eigenberg, 1987).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Magnusson and Kligman
maximization test. The study revealed that azinphos-methyl had a
sensitizing effect in 95% of the test animals (Flucke, 1986).
The skin sensitizing potential of azinphos-methyl was
investigated in guinea pigs, using the Buehler patch test. By means
of a dermal application of a concentration of 25% sensitization was
induced in approximately 50% of the test animals (Porter et al.,
1987).
In another Buehler patch test dermal application of a
concentration of 12.5% induced sensitization in approximately 50% of
the test animals when challenged using a 6% concentration, but using
a challenge concentration of 0.6% failed to elicit any relevant skin
reactions (Heimann, 1987b).
Observations in humans
Employees working in the formulation of azinphos-methyl products
have been subjected to regular medical examinations and no general
impairment of health has been observed. In one isolated case it was
considered probable that contact with azinphos-methyl was the cause of
generalized dermatosis in an apparently hypersensitive, very dry skin
(Faul, 1981; Miksche, 1981).
Published reports from the pesticide incident monitoring system
in the United States of America and additional data from the state of
California in the USA have been reviewed. Between 1982 and 1988 a
small number of incidents have been reported annually (involving 5-12
persons each year) which have been definitely, probably or possibly
associated with azinphos-methyl either alone or in combination with
other pesticides. In addition, two incidents occurred in 1987, one
involving 26 people, the other involving 32 people. The first
involved spray drift in adverse weather conditions. The second
involved workers who experienced symptoms including headache, nausea,
weakness and vomiting upon entry to a field to pick peaches 3 days
after methomyl was applied to the crop and about 6 weeks after an
application of azinphos-methyl (US EPA, 1981; Mahler, 1991).
COMMENTS
The toxicokinetics of azinphos-methyl has been investigated
following oral administration in rats. It does not accumulate in body
tissues.
In a 52-week study in dogs, using dietary concentrations of 0, 5,
25 or 125 ppm the NOAEL was 25 ppm (equal to 0.74 mg/kg bw/day), based
on reduced body-weight gain and inhibition of acetylcholinesterase
activity in brain at 125 ppm.
Long-term/carcinogenicity studies in rats at dietary
concentrations of 0, 5, 15, or 45 ppm and in mice at 0, 5, 20 or 40
ppm showed that azinphos-methyl has no carcinogenic potential in
either species. These results clarified earlier equivocal findings in
rats in an NCI bioassay. The NOAEL in rats was 15 ppm (equal to
0.86 mg/kg bw/day), based on effects on brain acetylcholinesterase at
45 ppm. In mice the NOAEL was 5 ppm (equal to 0.88 mg/kg bw/day),
based on inhibition of cholinesterase in plasma, erythrocytes and
brain at 20 ppm.
In a two-generation reproduction study in rats at dietary
concentrations of 0, 5, 15 or 45 ppm, fertility and pup viability
during lactation were adversely affected, equivocally at 15 ppm and
markedly at 45 ppm. The NOAEL was 5 ppm, equal to 0.48 mg/kg bw/day.
Teratology studies in rats, mice and rabbits did not indicate
teratogenic effects at doses up to 2, 5 and 6 mg/kg bw/day
respectively.
The data from genotoxicity studies with azinphos-methyl were
conflicting. However, in vivo studies were negative, the positive
data being confined to some in vitro studies. After reviewing the
available information it was concluded that it is unlikely that
azinphos-methyl is genotoxic to humans.
Acute delayed neurotoxicity tests in hens with azinphos-methyl
gave negative results.
The 1973 JMPR reported that daily doses up to and around 0.3
mg/kg bw/day for 30 days in human volunteers had no effect on plasma
or erythrocyte cholinesterase activity. New data were not available
from occupational exposure or human volunteer studies with
azinphos-methyl. A review of the available literature and reports of
human poisoning with azinphos-methyl revealed no information relevant
to the estimation of the ADI.
Since the critical toxicological end-point was not
acetylcholinesterase inhibition, the human data were not appropriate
for estimation of the ADI, which was based on the NOAEL in the rat
multigeneration study in rats using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 5 ppm (equal to 0.88 mg/kg bw/day)
Rat: 5 ppm (equal to 0.86 mg/kg bw/day) in a long-
term/carcinogenicity study
5 ppm (equal to 0.48 mg/kg bw/day) in a
multigeneration study
Dog: 25 ppm (equal to 0.74 mg/kg bw/day)
Human: 0.3 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Further observations in humans
REFERENCES
Alam, M.T., Corbeil, M., Chagnon, A., Kasatiya, S.S. (1974).
Chromosomal anomalies induced by the organic phosphate pesticide
guthion in Chinese hamster cells. Chromosoma (Berl.), 49: 77-86.
Allen, T.R., Frei, T., Janiak, T., Luetkemeier, H., Vogel, O.,
Biedermann, K., Wilson, J. (1990). 52-week oral toxicity (feeding)
study with azinphos-methyl in the dog. Unpublished report from
Research and Consulting Company AG, submitted by Bayer AG, Leverkusen,
Germany.
Chen, H.H., Sirianni, S.R., Huang, C.C. (1982a). Sister chromatid
exchanges and cell cycle delay in Chinese hamster V79 cells treated
with 9 organophosphorus compounds (8 pesticides and 1 defoliant).
Mutation Research, 103: 307-313.
Chen H.H., Sirianni, S.R., Huang, C.C. (1982b). Sister chromatid
exchanges in Chinese hamster cells treated with seventeen
organophosphorus compounds in the presence of a metabolic activation
system. Environmental Mutagenesis, 4: 621-624.
Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1988). A teratology study
in the rabbit with azinphos-methyl. Unpublished report from Miles
Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to rats.
Unpublished report from Chemagro, submitted by Bayer AG, Leverkusen,
Germany.
Eiben, R., Janda, B. (1987). Azinphos-methyl - two generation study
on rats. Unpublished report from Bayer AG, Leverkusen, Germany.
Eigenberg, D.A. (1987). Primary dermal irritation of Benzazimide in
albino rabbits. Unpublished report from Mobay Corporation, submitted
by Bayer AG, Leverkusen, Germany.
Faul, J. (1981). BBA request, effects on humans. Letter of 2 June
1981 from Bayer AG, Department DO Medical. Company internal letter,
submitted by Bayer AG, Leverkusen, Germany.
Feldmann, R.J., Maibach, H.I. (1974). Percutaneous penetration of
some pesticides and herbicides in man. Toxicology and Applied
Pharmacology, 28: 126-132.
Flucke, W. (1979). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Flucke, W. (1986). Azinphos-methyl - Study for skin sensitizing
effect in guinea pigs (Magnusson and Kligman maximization test).
Unpublished report from Bayer AG, Leverkusen, Germany.
Flucke, W., Schilde, B. (1980). Azinphos-methyl: Subacute cutaneous
toxicity to rabbits. Unpublished report from Bayer AG, Leverkusen,
Germany.
Franklin, C.A., Greenhalgh, R., Maibach, H.I. (1982). Pesticide
chemistry, human welfare and the environment. Volume 4,
pages 221-226. Pergamon Press, London, United Kingdom.
Franklin, C.A., Muir, N.I., Moody, R.P. (1986). The use of biological
monitoring in the estimation of exposure during the application of
pesticides. Toxicology Letters, 33: 127-136.
Glaza, S.M. (1988). Azinphos-methyl: Acute delayed neurotoxicity
study in the domestic fowl. Unpublished report from Hazleton
Laboratories America Inc, sponsored by Mobay Corporation, submitted by
Bayer AG, Leverkusen, Germany.
Hayes, R.H. (1985). Oncogenicity study of azinphos-methyl in mice.
Unpublished report from Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1981). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1982). Azinphos-methyl study of the acute oral and
dermal toxicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Heimann, K.G. (1987a). Determination of acute toxicity (LD50) of
azinphos methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Heimann, K.G. (1987b). Azinphos-methyl: Study of skin sensitizing
effect on guinea pigs (Buehler patch test). Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1978). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1979a). Azinphos-methyl: micronucleus test on mice to
evaluate for possible mutagenic effects. Unpublished report from
Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1979b). Azinphos-methyl: dominant lethal study on male
mouse to test for mutagenic effects. Unpublished report from Bayer
AG, Leverkusen, Germany.
Herbold, B.A. (1984). Azinphos-methyl, Pol test on E. coli to
evaluate for potential DNA damage. Unpublished report from Bayer AG,
Leverkusen, Germany.
Herbold, B.A. (1986). Azinphos-methyl: cytogenic study with human
lymphocyte cultures in vitro to evaluate for harmful effect on
chromosomes. Unpublished report from Bayer AG, Leverkusen, Germany.
Herbold, B.A. (1988). Azinphos-methyl: Salmonella/microsome test to
evaluate for point mutation. Unpublished report from Bayer AG,
Leverkusen, Germany.
Holzum, B. (1990). Azinphos-methyl: investigation of inhibition of
cholinesterase activity in plasma, erythrocytes and brain, in a
one-generation study. Unpublished report from Bayer AG, Leverkusen,
Germany.
Hoorn, A.J.W. (1983). Mutagenicity evaluation of azinphos-methyl in
the reverse mutation induction assay with Saccharomyces cerevisiae
strains S138 and S211. Unpublished report from Litton Bionetics,
Holland, submitted by Bayer AG, Leverkusen, Germany.
Kao, L.R.M. (1988). Disposition and metabolism of ring-UL-14C
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Kowalski, R.L., Clemens, G.R., Bare, J.J., Hartnagel, R.E. (1987). A
teratology study with azinphos-methyl in the rat. Unpublished report
from Miles Inc, sponsored by Mobay Corporation, submitted by Bayer AG,
Leverkusen, Germany.
Kimmerle, G. (1976). Subchronic inhalation toxicity of
azinphos-methyl in rats. Arch. Toxicol., 35: 83-89.
Kimmerle, G.; Loser, E. (1974). Delayed neurotoxicity of
organophosphorus compounds and copper concentration in the serum of
hens. Environm. Qual. Safety, 3: 173-178.
Lamb, D.W., Anderson, R.H. (1974). The acute oral toxicity of
azinphos-methyl, benzazimide and methyl benzazimide to fasted and
non-fasted rats using CMC as the excipient. Unpublished report from
Chemagro, submitted by Bayer AG, Leverkusen, Germany.
Lawlor, T.E. (1987). Azinphos-methyl: Salmonella/microsome plate
incorporation mutagenicity assay. Unpublished report from
Microbiological Associates Inc., submitted by Bayer AG, Leverkusen,
Germany.
Lin, S.N., Chen, C.Y., Murphy, S.D., Caprioli, R.M. (1980).
Quantitative high-performance liquid chromatography and mass
spectrometry for the analysis of the in vitro metabolism of the
insecticide azinphos-methyl by rat liver homogenates. J. Agric. Food
Chem., 28: 85-88
Machemer, L. (1975). Azinphos-methyl: studies for embryonic and
teratogenic effects on rabbits following oral administration.
Unpublished report from Bayer AG, Leverkusen, Germany.
Mahler, L. (1991). Program Director, Pesticide Illness Surveillance
Program, Worker Health and Safety Branch, California Department of
Agriculture. Data provided to Mobay Corporation, submitted by Bayer
AG, Leverkusen, Germany.
Mihail, F. (1978). Azinphos-methyl acute toxicity studies.
Unpublished report from Bayer AG, Leverkusen, Germany.
Miksche, L. (1981). Information on effects in man/occupational
experience. Letter of 12 June 1981, from Bayer AG medical department.
Company internal letter, submitted by Bayer AG, Leverkusen, Germany.
Myhr, B.C. (1983). Evaluation of azinphos-methyl in the primary rat
hepatocyte unscheduled DNA synthesis assay. Unpublished report from
Litton Bionetics USA, submitted by Bayer AG, Leverkusen, Germany.
National Cancer Institute (1978). Bioassay of azinphos-methyl for
possible carcinogenicity. Technical report series no 69.
Pasquet, J., Mazuret, A., Fournel, J., Koenig, F.H. (1976). Acute
oral and percutaneous toxicity of phosalone in the rat, in comparison
with azinphos-methyl and parathion. Toxicology and Applied
Pharmacology, 37: 85-92.
Patzschke, K., Wegner, L.A., Weber, H. (1976). Biokinetic
investigations of carbonyl-14C azinphos methyl in rats. Unpublished
report from Bayer AG, Leverkusen, Germany.
Porter, M.C., Craigo, R.E., Hartnagel, R.E. (1987). Dermal
sensitization evaluation of azinphos-methyl technical in the guinea
pig. Unpublished report from Miles Laboratories Inc., submitted by
Bayer AG, Leverkusen, Germany.
Rao, S.L N., McKinley, W.P. (1969). Metabolism of organophosphorus
insecticides by liver homogenates from different species. Can. J.
Biochem., 47: 1155-1159
Schmidt, W.M. (1987). Azinphos-methyl: study of chronic toxicity and
carcinogenicity to rats. Unpublished report from Bayer AG,
Leverkusen, Germany.
Sheets, L.P. (1988). Acute dermal toxicity of technical grade
benzazimide to rabbits. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987a). Acute four-hour inhalation toxicity study
with azinphos-methyl in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1987b). Acute inhalation toxicity study with
benzazimide technical in rats. Unpublished report from Mobay
Corporation, submitted by Bayer AG, Leverkusen, Germany.
Shiotsuka, R.N. (1988). Acute one hour inhalation toxicity study with
azinphos-methyl in rats. Unpublished report from Mobay Corporation,
submitted by Bayer AG, Leverkusen, Germany.
Short, R.D., Minor, J.L., Lee, C.C., Chernoff, N., Baron, R.L. (1980).
Developmental toxicity of azinphos-methyl in rats and mice. Arch.
Toxicol, 43: 177-186.
Short, R.D., Minor, J.L., Unger, T.M., Lee, C.C. (1978). Teratology
of azinphos-methyl. Unpublished report from Mid West Research
Institute, USA, contracted by US Environmental Protection Agency,
submitted by Bayer AG, Leverkusen, Germany.
Sterri, S.H., Rognerud, B., Fiskum, S.E., Lyngaas, S. (1979). Effect
of toxogonin and P2S on the toxicity of carbamates and
organophosphorus compounds. Acta Pharmacol. et Toxicol., 45: 9-15
Thyssen, J. (1976a). Determination of acute toxicity (LD50) of
azinphos-methyl. Unpublished report from Bayer AG, Leverkusen,
Germany.
Thyssen, J. (1976b). Studies to determine the toxic effects of the
simultaneous application of azinphos-methyl or azinphos-ethyl and
methamidophos. Unpublished report from Bayer AG, Leverkusen, Germany.
Thyssen, J. (1977a). Study for combination toxicity of
azinphos-methyl and propoxur. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J. (1977b). Study for the combination toxicity of
azinphos-methyl and azinphos-ethyl. Unpublished report from Bayer AG,
Leverkusen, Germany.
Thyssen, J., Lorke (1981). Azinphos-methyl - study of the irritant
effects on the skin and mucous membranes (eye). Unpublished report
from Bayer AG, Leverkusen, Germany.
US EPA (1981). Summary of reported pesticide incidents involving
azinphos-methyl Pesticide incident monitoring system, report No. 469
submitted by Bayer AG, Leverkusen, Germany.
Waters, M.D., Sandhu, S.S., Simon, V.F., Mortelmans, K.E.,
Mitchell, A.A., Jorgenson, T.A., Jones, D.C.L., Valencia, R.,
Garrett, N.E. (1982). Study of pesticide genotoxicity. Genetic
Toxicology - an agricultural perspective, 21: 275 - 326.
Weber, H., Patzschke, K., Wegner, L.A. (1980). Biokinetic
investigation of ring-UL-14C benzazimide in rats. Unpublished report
from Mobay Corporation, submitted by Bayer AG, Leverkusen, Germany.
Webster, R.C.; Maibach, H.I. (1985). In vivo percutaneous
absorption and decontamination of pesticides in humans. Journal of
Toxicology and Environmental Health, 16: 25-37.
