
HEXYTHIAZOX
First draft prepared by
Mrs.Dr.E.M. den Tonkelaar,
Mrs.J.E.M. van Koten-Vermeulen,
National Institute of Public Health
and Environmental Protection,
Bilthoven, The Netherlands
(4RS,5RS)-5-(4-chlorophenyl)-N-cyclohexyl-4-methyl-2-oxo-
1,3-thiazolidine-3-carboxamide.
EXPLANATION
Hexythiazox is a specific acaricide used in controlling many
kinds of phytophagous mites. It acts as an ovicide, larvicide and
nymphicide. It was reviewed for the first time at the present
meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
Biological data
Biochemical aspects
Absorption, distribution and excretion
Five male and five female CR Fischer rats were given via a
stomach tube a single oral dose of 10 mg/kg bw [thiazolidine-5-14C]
hexythiazox (purity >99%) in DMSO. Blood, urine and faeces were
collected periodically for 72 hours. Rats were sacrificed and tissues
were collected and analyzed for radioactivity. Maximal plasma
concentrations were observed 4 hours after dosing (2.22 and 2.55 mg/kg
in males and females, respectively) and decreased rapidly thereafter
to approximately 0.1 mg/kg in both sexes after 72 hours. A total of
30% of the radioactivity was excreted in the urine in both sexes, but
a slightly larger proportion was eliminated in the faeces of male rats
(66%) than in female rats (60%). About 4% and 10% was recovered in
the carcass and tissues of males and females, respectively. Highest
tissue concentrations were observed in fat, adrenals and digestive
organs and their contents, liver and ovaries (Soeda, 1985).
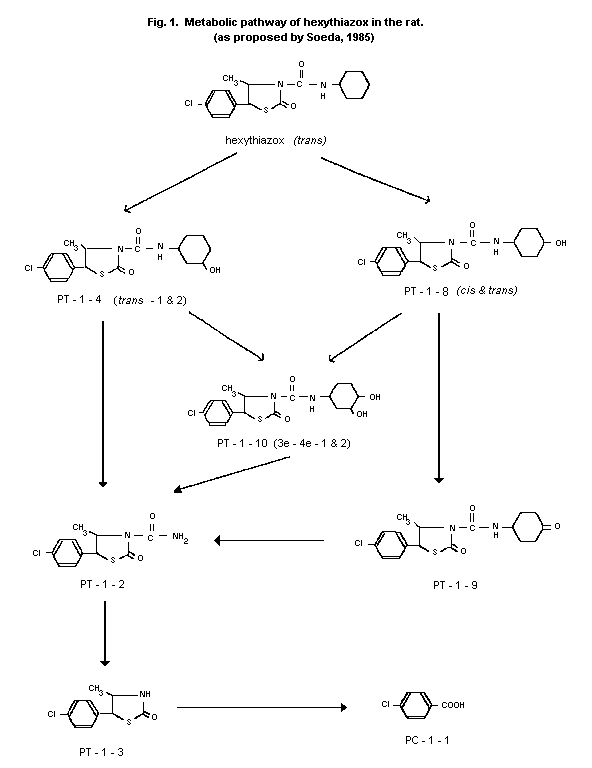
Biotransformation
After the oral administration of 10 mg/kg bw [thiazolidine-
5-14C] hexythiazox to 5 male and 5 female rats (see above), urine and
faeces were analyzed by HPLC-cochromatography and spectrometry. About
28% to 60% of the administered radioactivity was excreted in the urine
and faeces, respectively. Twenty percent of the dose was excreted as
parent compound, mainly in the faeces. The main metabolite in urine
and faeces was 5-(4-chlorophenyl)-N-( cis-4-hydroxylcyclohexyl)-
4-methyl-trans-2-oxothiazolidine-3-carboxamide {PT-1-8 ( cis)}. A
number of other metabolites were detected in smaller quantities. The
levels of bound 14C-residues were evaluated by polar solvent
extraction of liver and fat tissue. The largest part of the extracted
radioactivity in the fat was unchanged parent compound.
Biotransformation occurred via oxidation of the cyclohexane ring,
followed by splitting off of this ring and finally of the thiazolidone
ring. However, only approximately 10% of the radioactivity in the
urine and 50% in the faeces could be identified. A proposed metabolic
pathway is given in Figure 1 (Soeda, 1985).
 Toxicological studies
Acute toxicity
The LD50 and LC50 for hexythiazox, the 10% WP formulation and
certain metabolites, are given in Tables 1, 2 and 3, respectively.
No signs of toxicity were observed after acute oral and dermal
exposure to hexythiazox.
Short-term studies
Mice
Male and female B6C3F1 mice (10/sex/group) were fed diets
containing 0, 50, 300, 1800 or 10 800 ppm hexythiazox (purity 98.3%)
for 28 days. No compound-related effects were observed on mortality,
clinical signs, food consumption and food efficiency, haematology, nor
macroscopy. Male body weight gain was decreased at 1800 and 10 800
ppm hexythiazox. Total cholesterol levels decreased in male and
female mice at 10 800 ppm and in males also at 1800 ppm. Urine
specific gravity was increased in high-dose males. Relative liver
weight was increased in male and female mice at 1800 and 10 800 ppm.
At histopathology an increased incidence of swollen liver cells was
observed in high-dose males (also at 1800 ppm) and females. The NOAEL
in this study is 300 ppm, equal to 55.1 mg/kg bw (males) and 62.9
mg/kg bw (females) (Takaori & Nishibe, 1983a).
Rats
Groups of Fischer rats (20/sex/group) received a diet containing
0, 10, 70, 500 or 3500 ppm hexythiazox (98.3%) for 13 weeks.
Observations included clinical signs, food and water consumption,
haematology, biochemistry, cholinesterase activity, urinalysis,
macroscopy, organ weights and histopathology. Growth was depressed in
both sexes at 3500 ppm and in females also at 500 ppm. In high-dose
males Hb, PCV, RBC and MCV were decreased and platelet count was
increased. In high-dose females MCHC and MCH (also at 500 ppm) were
decreased. At 3500 ppm hexythiazox total cholesterol, albumin (in
males also at 500 ppm), protein and calcium were increased in both
sexes. ALP and ASAT were increased in high-dose females. Protein in
urine was increased in males at 3500 ppm. Relative liver (also at 500
ppm), kidney, adrenal and gonad weight were increased and spleen
weight decreased at 3500 ppm in both sexes. At 70 ppm liver weight was
still slightly increased in females. Female brain weight was increased
at 500 and 3500 ppm. At histopathology swelling of hepatocytes in the
central zone was observed in both sexes at the highest dose. Fatty
degeneration of the zona fasciculata of the adrenal cortex was
observed at dose levels of 500 ppm and greater. The NOAEL is 70 ppm,
equal to 8.1 mg/kg bw/day (males) and 5.3 mg/kg bw/day (females)
(Takaori & Nishibe, 1983b).
Table 1. Acute toxicity of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Mouse M&F oral 98.3% > 5000 Saika & Nishibe (1983a)
M&F s.c. 98.2% > 5000 Inoue (1987a)
M&F i.p. 98.2% > 5000 Inoue (1987b)
Rat M&F oral 98.3% > 5000 Saika & Nishibe (1983b)
M&F s.c. 98.2% > 5000 Inoue (1987c)
M&F i.p. 98.2% > 5000 Inoue (1985)
M&F inhl.a 98.3% > 2.0 Saika & Nishibe (1983c)
M&F dermal 98.3% > 5000 Saika & Nishibe (1983d)
Dog M&F oral 98.3% > 5000 Saika & Nishibe (1984)
a 4 hour exposure
Table 2. Acute toxicity of 10% wettable powder of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Rat M&F oral > 5000 Saika & Nishibe (1983e)
M&F inhal.a > 2.9 Saika & Nishibe (1983f)
M&F dermal > 5000 Saika & Nishibe (1983g)
a 4 hour exposure
Table 3. Acute toxicity of metabolites of hexythiazox in rats
Metabolite Sex Route Purity LD50 References
(mg/kg bw)
PT-1-2 M oral > 99% 2321 Saika & Nishibe (1985)
F 1079
PT-1-3 M oral > 99% 494 Saika & Nishibe (1985)
F 341
PT-1-4 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans-2)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(cis)
Dogs
In a range-finding study groups of Beagle dogs (2/sex/group) were
fed diets containing 0, 125, 500, 2000 or 8000 ppm hexythiazox (purity
97.7%) for 4 weeks. No effects were observed on clinical signs nor
mortality. Body weight gain was decreased in females and food
consumption in males at 8000 ppm. Relative liver weight was increased
in females at 2000 and 8000 ppm and slightly increased in high-dose
males (Spicer, 1984a).
Male and female Beagle dogs (4/sex/group) received a diet
containing 0, 100, 500 or 5000 ppm hexythiazox (purity 97.7%) for 12
months. Observations were made for clinical signs, body weight, food
consumption and food efficiency, ophthalmology, haematology,
biochemistry, cholinesterase activity and urinalysis. The weights of
10 organs/animal were recorded. Gross necropsy and histo-pathology
were carried out. ALP was increased in both sexes at 5000 ppm and
ALAT was increased in females at the same dose. Total protein was
decreased in males at 500 and 5000 ppm and in females at 5000 ppm.
Phosphorus was decreased in females 500 and 5000 ppm. Relative
adrenal weight was increased in high-dose dogs and relative liver
weight was increased in high-dose males. At histopathology
adrenocortical hypertrophy occurred in male and female dogs at 500 and
5000 ppm. Hepatocellular hypertrophy was observed in high-dose dogs.
The NOAEL in this study is 100 ppm, equal to 2.87 mg/kg (males) and
3.17 mg/kg (females) (Spicer, 1984b).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) received a diet
containing 0, 40, 250 or 1500 ppm hexythiazox (purity 98.2%) for 104
weeks. An additional 30 mice (10/sex/group) served for the collection
of blood samples after 26, 52 and 79 weeks. Observations included
clinical signs, mortality, body weight, food consumption and food
efficiency, haematology, clinical chemistry, urinalysis, macroscopy,
organ weight (after 26, 52, 78 and 104 weeks) and histopathology
(after 52 and 104 weeks). Body weight gain and absolute body weights
were decreased at 250 and 1500 ppm in male mice only. During the
second half of the study body weight gain was also significantly
decreased at 40 ppm. Various red blood cell parameters were decreased
during the first 78 weeks at the highest dose. Relative liver weight
was increased in males and females at 1500 ppm during the whole study.
After 104 weeks relative weights of brain, heart, kidneys, adrenals
and testes were increased in male mice. Relative brain, kidneys,
adrenals and testes weight were increased in males at 250 ppm.
Histopathology of the liver after 52 weeks showed an increased
incidence of fatty degeneration and hypertrophy of the hepatocytes
with nuclear pleomorphism in males at 1500 ppm which was only
marginally increased at 250 ppm. At the end of the study the incidence
of hyperplastic nodes consisting of proliferating hepatocytes was
increased in both sexes at the highest dose (incidences (52+104 weeks
60 rats): 20%, 20%, 25% and 50% for males and 5%, 8%, 6% and 27% for
females at 0, 40, 250, and 1500 ppm, respectively). At the same dose
an increase in the incidence of hepatocellular adenomas or carcinomas
was also observed (incidences: adenomas: 20%, 25%, 20% and 33% for
males and 10%, 2%, 5% and 17% for females; carcinomas: 13%, 13%, 17%
and 13% for males and 0%, 5%, 5% and 8% for females both at 0, 40,
250, and 1500 ppm hexythiazox). In 3 males at 1500 ppm
haemangiopericytoma was observed in the liver. This incidence was
within the historical control range. At the lowest dose level of 40
ppm (equal to 6.7 in males and 8.4 in females) there was still a
decreased body weight gain (Inoue & Enomoto, 1984).
Because of the observed increase of liver tumours an additional
histological examination was performed on the livers of mice
(10/sex/group) sacrificed after 78 weeks. In high-dose females the
incidence of nuclear pleomorphism of the liver cells and fatty
degeneration were increased. The tumour incidences were not enhanced
(Enomoto, 1985).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 60, 430, or 3000 ppm hexythiazox (purity 99%) for 104
weeks. Additional groups of 10 rats/sex/group were treated at the same
dose levels and were used for interim sacrifice at 12 months. The
observations included clinical signs, body weight, food consumption,
ophthalmology, haematology, biochemistry, urinalysis, macroscopy,
organ weight and histopathology. At the end of the study the
incidence of internal abdominal masses and swollen testicles and
non-descended testicles was high in all males but was observed to a
greater degree at 430 and 3000 ppm hexythiazox. Mean body weight was
decreased in high dose males and females and slightly at 430 ppm. Food
consumption was increased in both sexes at 3000 ppm and in females at
430 ppm. ALP, ALAT, ASAT and potassium levels were decreased in
high-dose females. At the end of the study most relative organ weights
(including the liver) were increased in both sexes at 3000 ppm. At
430 ppm relative brain, heart, lung and spleen weights were still
increased in males. No treatment-related histopathological changes
were observed. The tumour incidence was not enhanced. The NOAEL in
this study is 60 ppm, equal to 3.2 mg/kg bw (males) and 4.02 mg/kg bw
(females) (Spicer, 1984c).
Reproduction studies
In a two-generation reproduction study (2 litters/generation)
groups of SPF Wistar rats (30/sex/group) were given diets containing
0, 60, 400 or 2400 ppm hexythiazox (purity 98.2%). Observations
included clinical signs, body weight, food consumption, food
efficiency, reproductive findings, organ weight and histopathology.
Five dams/group were sacrificed on day 21 of pregnancy for teratogenic
examinations.
Body weight and food consumption were decreased in high dose F0
parents. An increased number of delivered F1 pups was observed in
the second mating at 400 and 2400 ppm hexythiazox. At 400 and 2400
ppm the relative weights of liver, kidney, adrenals (only at 2400 ppm)
and testes (no dose relationship) were increased in F0 adults. A
tendency to an increased mean number of implantations and total
resorptions was observed in the F0 generation at 400 and 2400 ppm.
At histopathology a higher incidence of proteinaceous casts in the
kidneys was observed in F0 male rats at 2400 ppm. The number of live
born F1b pups was increased at 400 and 2400 ppm and pup weight was
decreased from day 4 to 28 of lactation at these dose levels. A delay
in hairgrowth of abdomen was observed in pups at all treated groups of
the first generation. In F1 parents, body weight was decreased at
2400 ppm and in males occasionally also at 400 ppm. Food consumption
was decreased at the highest dose. The mean number of implants,
implants/corpora lutea and number of live fetuses were increased in
the 2400 ppm F1 group. Relative weights of brain (males only),
liver, kidneys, adrenals (males only) and ovaries were increased in
high dose F1 parents. F2b pup weight was decreased at 2400 ppm
hexythiazox. A delay in abdominal hair growth and eye-opening were
observed in F2b pups at 2400 ppm. Body weight and food consumption
(females only) of F1 adults were decreased at the highest dose. F1
adults showed an increased relative weight of ovaries, adrenal, liver
and kidney (males only) and showed a decreased spleen weight at 2400
ppm. Liver and kidney weight were also increased in males at 400 ppm.
An increase in the appearance of proteinaceous casts was observed in
the kidneys of high-dose males. Malformations were not observed. The
NOAEL in this study is 60 ppm (equal to a range between 4.22 and 5.34
mg/kg bw, depending on sex and generation) (Okugi & Enomoto, 1984).
Special studies on embryotoxicity and/or teratogenicity
Rats
Pregnant Sprague-Dawley rats (24/group) received by gavage 0,
240, 720 or 2160 mg hexythiazox (purity 98.3%)/kg bw from day 7
through 17 of gestation. The rats were sacrificed on day 21.
Observations were made for body weight, food and water consumption,
number of implantations, number of live and dead fetuses, number of
early and late resorptions and number of corpora lutea. The fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. Female body weight gain and occasionally food
consumption were decreased at 720 and 2160 mg/kg bw/day. Relative
ovarian weight was increased at the two highest dose levels. The
ossification of the metatarsal bones was significantly delayed at 720
and 2160 mg/kg bw/day. The NOAEL for maternal and embryotoxicity was
240 mg/kg bw/day. There was no evidence for hexythiazox-induced
irreversible structural effects (Gotoh & Nishibe, 1983).
Rabbits
Four groups of 15 pregnant New Zealand white rabbits received by
gavage 0, 120, 360, or 1080 mg hexythiazox (purity 97.7%)/kg from day
6 to 18 of gestation. All dams were sacrificed on day 28 of
gestation. No significant changes were observed in mortality,
maternal body weight and food consumption, uterus weight, number and
location of viable fetuses, early and late resorptions, number of
total implantations nor corpora lutea and organ weight. Two dams died,
1 at 120 mg/kg bw/day and 1 at 360 mg/kg bw/day. A slightly greater
number of fetuses with skeletal variations (overlapping of the
vertebral arch, asymmetry of the sternebrae) was observed at 1080
mg/kg bw/day. The NOAEL in this study is 360 mg/kg bw/day. No
irreversible structural effects were observed (Gotoh & Nihsibe, 1984).
Special studies on mutagenicity
Hexythiazox was negative in various mutagenicity assays (see
Table 1). Ames tests were conducted with 8 plant metabolites (some
also occurring in the rat): PT-1-2. PT-1-3, PT-1-4( trans-2),
PT-1-5(1), PT-1-6( trans-2), PT-1-8( cis), (PT-1-8( trans) and
PT-1-9. All metabolites gave negative results in the tests using
S. typhimurium (TA1535, TA100, TA1538, TA98 and TA1537) as well as
Escherichia coli (WP2 uvra) (Sasaki & Nishibe, 1985).
Special studies on pharmacology
Mice
Doses > 1000 mg hexythiazox (purity 98.3%) given
intraperitoneally to 3 male S1c:ddY mice delayed the sleeping time
induced by sodium pentobarbital. Effects were observed as soon as 15
minutes after hexythiazox administration. Hexythiazox also prolonged
the time at which convulsions induced by pentetrazole or strychnine
began. Intestinal motility and gastric secretion in mice were not
affected by the administration of up to 150 mg hexythiazox iv nor up
to 3000 mg/kg (oral), respectively (Souma & Nishibe, 1985).
Rats
Blood coagulation time in S1c:Wistar rats was decreased after
administration of 3000 mg hexythiazox in saline, but haemolysis was
not observed. I.p. administration of doses as high as 2500 mg
hexythiazox/kg had no effect on rat body temperature nor on gastric
secretion. No effect on the tension of the muscle twitch induced by
the indirect stimulation was observed after i.v. injection of 50 or
100 mg hexythiazox/kg (Souma & Nishibe, 1985).
Table 4. Results of mutagenicity assays on hexythiazox
Test system Test object Concentration Purity (%) Results References
In vitro
Ames testa,b S. typhimurium 100-6400 µg/plate 97.7 Negative Inoue (1983a)
TA 100, TA98, in DMSO
TA1535, TA1537,
TA1538
E coli, WP2 uvra
Yeast testa,b S. cerevisiae 312-10 000 µg/ml 98.4 Negative Jagannath (1984)
(reverse mutation) S138, S211 in DMSO
(mitotic gene S. cerevisiae 1-10 000 µg/plate 98.4 Negative
conversion) D4
V79/HGPRTa,b Chinese hamster 9.38-150 µg/ml 98.6 Negative Seeberg (1986)
mutation assay V79 cells in DMSO
Chromosome Chinese hamster 1.5-50 µg/ml 98.4 Negative Galloway (1984)
aberration ovary cells
assaya
Rec-assay B. subtilis 400-3200 µg/plate 97.7 Negative Inoue (1983b)
H17(rec+) in DMSO
M45(rec-)
Unscheduled DNA Rat hepatocytes 2.5-100 µg/ml 98.4 Negative Cifone (1985)
synthesis (250 µg/ml lethal)
Table 4 (contd).
Test system Test object Concentration Purity (%) Results References
In vivo
Cytogenic testa,b Chinese hamster 1000, 2000 or 4000 98.6 Negative Mosesso (1986)
bone marrow mg/kg p.o.
Micronucleus test Male and female 0.1, 0.5 and 1.0 98.4 Negative Ivett (1984)
CD-1 bone marrow g/kg p.o.
cells
a without metabolic activation
b with metabolic activation
Rabbits
Hypotension and hypoventilation were observed after the iv
injection of 50 mg hexythiazox/kg once weekly (Souma & Nishibe, 1985).
Guinea-pig
High concentrations of hexythiazox inhibited the contraction of
isolated ileum induced by acetylcholine, histamine and barium chloride
(Souma & Nishibe, 1985).
Special studies on sensitization
Hexythiazox (purity 98.3%) as well as a 10% WP formulation did
not exhibit a sensitizing effect in the maximization test (Magnusson
& Kligman) in female Hartley guinea-pigs (Souma & Nishibe, 1983a;
1983d).
Special studies on skin and eye irritation
A dose of 0.5 g hexythiazox (purity 98.3%) and a 10% WP
formulation moistened with water was applied under occlusive
conditions to the clipped back skin of 6 male New Zealand White
rabbits for 4 hours. No skin irritation was observed up to 72 hours
after application (Souma & Nishibe, 1983b; 1983e).
Applications of 0.1 g hexythiazox (purity 98.3%) and a 10% WP
formulation into the eyes of 6 male New Zealand White rabbits caused
slight irritation (redness of the conjunctivae). No irritation
effects were observed 48 hours post-application (Souma & Nishibe,
1983c; 1983f).
Observation in humans
Health examinations (twice a year) were performed on a group of
16 to 26 workers in a Japanese manufacturing plant. The health
examination consisted of analysis for gamma-GTP (once a year), Hb,
haematocrit, erythrocytes and urinalysis (protein, glucose and
urobilinogen). No adverse effects were observed on the examined
parameters after three years of continuous exposure to hexythiazox
(Motogi, 1987).
COMMENTS
After oral administration to rats, hexythiazox was eliminated
rapidly, two-thirds via the faeces and one-third via the urine. Low
levels were recovered in the tissues and organs, with the highest
concentrations in the fat, liver, adrenals and in the gastrointestinal
tract and its contents. In the faeces about 20% was excreted as the
parent compound. Many metabolites were found in the urine and faeces,
although only 10% of those in the urine and 50% of those in the faeces
could be identified. Metabolism occurs via oxidation, and finally
cleavage, of the cyclohexane ring.
The compound shows low acute toxicity in the species examined.
WHO has classified hexythiazox as unlikely to present acute hazard in
normal use.
Short-term administration of hexythiazox to rats and dogs
revealed the liver and the adrenals as target organs. In a 90-day
study in rats the NOAEL was 70 ppm, equal to 4.9 and 5.3 mg/kg bw/day
for males and females respectively. In a one-year study in dogs the
NOAEL was 100 ppm, equal to 2.9 mg/kg bw/day in males and 3.2 mg/kg
bw/day in females.
In a 2-year feeding study in mice at dietary concentrations of 0,
40, 250, or 1500 ppm there was an increased incidence of liver nodules
in both sexes at 1500 ppm. The incidences of liver adenomas and
hepatocarcinomas were increased in females at 1500 ppm. Three
haemangiopericytomas in the liver were observed in males at 1500 ppm.
These were subsequently re-evaluated as hepatoblastomas. At the
lowest concentration of 40 ppm, equal to 6.7 and 8.4 mg/kg bw/day in
males and females respectively, reduced body-weight gain in male
animals was observed. A NOAEL was not established.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 60, 430, or 3000 ppm, there were no
treatment-related increases in neoplasias. There was a reduction in
body-weight gain and an increase in some organ weights at 430 ppm.
The NOAEL was 60 ppm, equal to 3.2 and 4.2 mg/kg bw/day for males and
females respectively.
In a 2-generation, 2-litters-per-generation reproduction study in
rats at 0, 60, 400, or 2400 ppm, no adverse effects on reproduction
were found. The NOAEL was 60 ppm, equivalent to 3 mg/kg bw/day, based
on increased liver and kidney weights at 400 ppm.
Maternal toxicity (reduced weight gains) and embryotoxicity
(delayed development) were observed in a teratogenicity study in rats
at doses of 0, 240, 720, or 2160 mg/kg bw/day. The NOAEL was 240
mg/kg bw/day. In a teratogenicity study in rabbits at doses of 0,
120, 360 or 1080 mg/kg bw/day, slight embryotoxicity was observed at
the highest dose. The NOAEL was 360 mg/kg bw/day. No teratogenic
effects were observed in either species.
After reviewing all available in vitro and in vivo short-term
tests on hexythiazox it was concluded that there was no evidence of
genotoxicity.
An ADI was established which was based on NOAELs in the 2-year
feeding and reproduction studies in rats and a 1-year study in dogs,
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 60 ppm in the diet, equal to 3.2 mg/kg bw/day
(2-year study)
Rat: 60 ppm in the diet, equivalent to 3 mg/kg bw/day
(reproduction study)
Dog: 100 ppm in the diet, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Cifone, M.A. (1985) Evaluation of NA-73 technical lot no. SCF-05 in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished second amended report 20991 (RD-8556) from Litton
Bionetics, Inc. Kensington, Maryland, USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Enomoto, M. (1985) Supplement to the chronic feeding and oncogenicity
studies in mice with NA-73. Histological findings of the liver of mice
sacrificed at 78 weeks. Unpublished Report 527 (RD-8534) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Galloway, S.M. (1984) Mutagenic evaluation of NA-73 Technical lot no.
SCF-05. in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished amended report 20990 (RD-8435N) from Litton Bionetics,
Inc. Kensington, Maryland 20895, USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1983) Teratogenicity study of NA-73 in rats.
Unpublished toxicology report 0118 (RD-83104) from Biomedical research
department of Nisso Institute for Life Science. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1984) Teratogenicity study of NA-73 in
rabbits. Unpublished toxicology report 0126 (RD-8454) from Biomedical
Research Department of Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1983a) NA-73: Mutagenicity test in bacteria. Unpublished
Report 364 (RD-83107) from Biosafety Research Center, Foods, Drugs and
Pesticides (AN-PYO Center). Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Inoue, H. (1983b) NA-73: Rec-assay with Bacillus subtilis strains
H 17 (rec+) and M 45-). Unpublished Report 363 (RD-83106) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1985) Acute intraperitoneal toxicity study in rats with
NA-73. Unpublished Report 623 (RD-8582) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987a) Acute subcutaneous toxicity study in mice with
NA-73. Unpublished Report 989 (RD-8759) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987b) Acute intraperitoneal toxicity study in mice with
NA-73. Unpublished Report 990 (RD-8758) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987c) Acute subcutaneous toxicity study in rats with
NA-73. Unpublished Report 991 (RD-8757) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H & Enomoto, M. (1984) Chronic feeding and oncogenicity
studies in mice with NA-73. Unpublished Report 483 (RD-84107) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Ivett, J.L. (1984) Mutagenicity evaluation of NA-73 technical in the
in vivo mouse micronucleus assay. Unpublished Report 20996
(RD-8436N) from Litton Bionetics, Inc. Kensington, Maryland, USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Jagannath, D.R. (1984) Mutagenicity evaluation of NA-73 in the mitotic
gene conversion and reverse mutation induction in yeast strains D4,
S138 and S211 plate test. Litton Bionetics, Inc. Submitted to WHO by
Nippon Soda Co. Ltd., Tokyo, Japan. Unpublished Report RD-8434N.
Mosesso, P. (1986) Chinese hamster bone marrow metaphase analysis
( in vivo cytogenetics). Test substance: NA 73. Unpublished LSR-RTC
Report 063015-M-04086 (RD-8696) from Life Science Research Roma
Toxicology Centre. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Motogi, S. (1987) Human handling experiences from plant employees in
hexythiazox production. Unpublished Report (RD-8749) from
Environmental Control and Safety Department, Nihongi Plant, Japan.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Okugi, M. & Enomoto, M. (1984) NA-73: Two-generation reproduction
study in rats (experiment 221). Unpublished Report 453 (RD-84108) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983a) Acute oral toxicity study of NA-73 in
mice. Unpublished toxicology report 0107 (RD-8393) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983b) Acute oral toxicity study of NA-73 in
rats. Unpublished toxicology report 0092 (RD-8394) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983c) Acute inhalation toxicity study of
NA-73 in rats. Unpublished toxicology report 0094 (RD-8396) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983d) Acute dermal toxicity study of NA-73
in rats. Unpublished toxicology report 0093 (RD-8395) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983e) Acute oral toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0123 (RD-83112) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983f) Acute inhalation toxicity study of
NA-73 10% WP in rats. Unpublished toxicology report 0121 (RD-83114)
from Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983g) Acute dermal toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0119 (RD-83113) from
Biomedical Research Department, Nisso institute for life science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1984) Acute oral toxicity study of NA-73 in
dogs. Unpublished toxicology report 0125 (RD-8428) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1985) Acute oral toxicity study of main
metabolites of NA-73 in Rats. Unpublished report 0181 (RD-8570) from
Toxicology Group, Nippon Soda Ltd. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Sasaki, T. & Nishibe, T. (1985) Reverse mutation study of main
metabolites of NA-73. Unpublished Report 0185 (RD-8571) from
Toxicology Group, Soda Ltd. Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Seeberg, A.H. (1986) Gene mutation in Chinese hamster V79 cells test
substance: NA 73. Unpublished Report 063014-M-03986 (RD-8695) from
Life Science Research Roma Toxicology Centre. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Soeda, Y. (1985) Metabolism of NA-73 in rats (group B). Unpublished
Report EC-9 (RD-8520N) from Environmental Toxicology Laboratory of
Nippon Soda Ltd. Submitted to WHO by Nippon Soda Co., Ltd. Tokyo,
Japan.
Souma, S. & Nishibe, T. (1983a) Delayed contact hypersensitivity
study of NA-73 in guinea pigs. Unpublished toxicology report 0097
(RD-8399) from Biomedical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983b) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report 0095 (RD-8398) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983c) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report 0096 (RD-8397) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983d) Delayed contact hypersensitivity
study of NA-73 10%WP in guinea pigs. Unpublished toxicology report
(RD-83117) from Bio-medical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983e) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report (RD-83116) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983f) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report (RD-83115) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1985) General pharmacology study of NA-73.
Unpublished toxicology report 0186 (RD-85104) from Environmental
Toxicology Laboratory, Nippon Soda Co., Ltd. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984a) Four week dietary range-finding study in dogs
with NA-73. Unpublished Report (RD-84105) from International Research
and Development Corporation, Michigan USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984b) One year dietary study of NA-73 in dogs.
Unpublished Report (RD-8433) from International Research and
Development Corporation, Michigan USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984c) Lifetime (24-month) dietary toxicity and
oncogenicity study of NA-73 in rats. Unpublished Report (RD-84106)
from International Research and Development Corporation, Michigan USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983a) Cumulative toxicity study of NA-73
in mice. Unpublished Report (RD-83100) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983b) Subchronic feeding study of NA-73
in rats. Unpublished Report (RD-83101) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
Toxicological studies
Acute toxicity
The LD50 and LC50 for hexythiazox, the 10% WP formulation and
certain metabolites, are given in Tables 1, 2 and 3, respectively.
No signs of toxicity were observed after acute oral and dermal
exposure to hexythiazox.
Short-term studies
Mice
Male and female B6C3F1 mice (10/sex/group) were fed diets
containing 0, 50, 300, 1800 or 10 800 ppm hexythiazox (purity 98.3%)
for 28 days. No compound-related effects were observed on mortality,
clinical signs, food consumption and food efficiency, haematology, nor
macroscopy. Male body weight gain was decreased at 1800 and 10 800
ppm hexythiazox. Total cholesterol levels decreased in male and
female mice at 10 800 ppm and in males also at 1800 ppm. Urine
specific gravity was increased in high-dose males. Relative liver
weight was increased in male and female mice at 1800 and 10 800 ppm.
At histopathology an increased incidence of swollen liver cells was
observed in high-dose males (also at 1800 ppm) and females. The NOAEL
in this study is 300 ppm, equal to 55.1 mg/kg bw (males) and 62.9
mg/kg bw (females) (Takaori & Nishibe, 1983a).
Rats
Groups of Fischer rats (20/sex/group) received a diet containing
0, 10, 70, 500 or 3500 ppm hexythiazox (98.3%) for 13 weeks.
Observations included clinical signs, food and water consumption,
haematology, biochemistry, cholinesterase activity, urinalysis,
macroscopy, organ weights and histopathology. Growth was depressed in
both sexes at 3500 ppm and in females also at 500 ppm. In high-dose
males Hb, PCV, RBC and MCV were decreased and platelet count was
increased. In high-dose females MCHC and MCH (also at 500 ppm) were
decreased. At 3500 ppm hexythiazox total cholesterol, albumin (in
males also at 500 ppm), protein and calcium were increased in both
sexes. ALP and ASAT were increased in high-dose females. Protein in
urine was increased in males at 3500 ppm. Relative liver (also at 500
ppm), kidney, adrenal and gonad weight were increased and spleen
weight decreased at 3500 ppm in both sexes. At 70 ppm liver weight was
still slightly increased in females. Female brain weight was increased
at 500 and 3500 ppm. At histopathology swelling of hepatocytes in the
central zone was observed in both sexes at the highest dose. Fatty
degeneration of the zona fasciculata of the adrenal cortex was
observed at dose levels of 500 ppm and greater. The NOAEL is 70 ppm,
equal to 8.1 mg/kg bw/day (males) and 5.3 mg/kg bw/day (females)
(Takaori & Nishibe, 1983b).
Table 1. Acute toxicity of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Mouse M&F oral 98.3% > 5000 Saika & Nishibe (1983a)
M&F s.c. 98.2% > 5000 Inoue (1987a)
M&F i.p. 98.2% > 5000 Inoue (1987b)
Rat M&F oral 98.3% > 5000 Saika & Nishibe (1983b)
M&F s.c. 98.2% > 5000 Inoue (1987c)
M&F i.p. 98.2% > 5000 Inoue (1985)
M&F inhl.a 98.3% > 2.0 Saika & Nishibe (1983c)
M&F dermal 98.3% > 5000 Saika & Nishibe (1983d)
Dog M&F oral 98.3% > 5000 Saika & Nishibe (1984)
a 4 hour exposure
Table 2. Acute toxicity of 10% wettable powder of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Rat M&F oral > 5000 Saika & Nishibe (1983e)
M&F inhal.a > 2.9 Saika & Nishibe (1983f)
M&F dermal > 5000 Saika & Nishibe (1983g)
a 4 hour exposure
Table 3. Acute toxicity of metabolites of hexythiazox in rats
Metabolite Sex Route Purity LD50 References
(mg/kg bw)
PT-1-2 M oral > 99% 2321 Saika & Nishibe (1985)
F 1079
PT-1-3 M oral > 99% 494 Saika & Nishibe (1985)
F 341
PT-1-4 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans-2)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(cis)
Dogs
In a range-finding study groups of Beagle dogs (2/sex/group) were
fed diets containing 0, 125, 500, 2000 or 8000 ppm hexythiazox (purity
97.7%) for 4 weeks. No effects were observed on clinical signs nor
mortality. Body weight gain was decreased in females and food
consumption in males at 8000 ppm. Relative liver weight was increased
in females at 2000 and 8000 ppm and slightly increased in high-dose
males (Spicer, 1984a).
Male and female Beagle dogs (4/sex/group) received a diet
containing 0, 100, 500 or 5000 ppm hexythiazox (purity 97.7%) for 12
months. Observations were made for clinical signs, body weight, food
consumption and food efficiency, ophthalmology, haematology,
biochemistry, cholinesterase activity and urinalysis. The weights of
10 organs/animal were recorded. Gross necropsy and histo-pathology
were carried out. ALP was increased in both sexes at 5000 ppm and
ALAT was increased in females at the same dose. Total protein was
decreased in males at 500 and 5000 ppm and in females at 5000 ppm.
Phosphorus was decreased in females 500 and 5000 ppm. Relative
adrenal weight was increased in high-dose dogs and relative liver
weight was increased in high-dose males. At histopathology
adrenocortical hypertrophy occurred in male and female dogs at 500 and
5000 ppm. Hepatocellular hypertrophy was observed in high-dose dogs.
The NOAEL in this study is 100 ppm, equal to 2.87 mg/kg (males) and
3.17 mg/kg (females) (Spicer, 1984b).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) received a diet
containing 0, 40, 250 or 1500 ppm hexythiazox (purity 98.2%) for 104
weeks. An additional 30 mice (10/sex/group) served for the collection
of blood samples after 26, 52 and 79 weeks. Observations included
clinical signs, mortality, body weight, food consumption and food
efficiency, haematology, clinical chemistry, urinalysis, macroscopy,
organ weight (after 26, 52, 78 and 104 weeks) and histopathology
(after 52 and 104 weeks). Body weight gain and absolute body weights
were decreased at 250 and 1500 ppm in male mice only. During the
second half of the study body weight gain was also significantly
decreased at 40 ppm. Various red blood cell parameters were decreased
during the first 78 weeks at the highest dose. Relative liver weight
was increased in males and females at 1500 ppm during the whole study.
After 104 weeks relative weights of brain, heart, kidneys, adrenals
and testes were increased in male mice. Relative brain, kidneys,
adrenals and testes weight were increased in males at 250 ppm.
Histopathology of the liver after 52 weeks showed an increased
incidence of fatty degeneration and hypertrophy of the hepatocytes
with nuclear pleomorphism in males at 1500 ppm which was only
marginally increased at 250 ppm. At the end of the study the incidence
of hyperplastic nodes consisting of proliferating hepatocytes was
increased in both sexes at the highest dose (incidences (52+104 weeks
60 rats): 20%, 20%, 25% and 50% for males and 5%, 8%, 6% and 27% for
females at 0, 40, 250, and 1500 ppm, respectively). At the same dose
an increase in the incidence of hepatocellular adenomas or carcinomas
was also observed (incidences: adenomas: 20%, 25%, 20% and 33% for
males and 10%, 2%, 5% and 17% for females; carcinomas: 13%, 13%, 17%
and 13% for males and 0%, 5%, 5% and 8% for females both at 0, 40,
250, and 1500 ppm hexythiazox). In 3 males at 1500 ppm
haemangiopericytoma was observed in the liver. This incidence was
within the historical control range. At the lowest dose level of 40
ppm (equal to 6.7 in males and 8.4 in females) there was still a
decreased body weight gain (Inoue & Enomoto, 1984).
Because of the observed increase of liver tumours an additional
histological examination was performed on the livers of mice
(10/sex/group) sacrificed after 78 weeks. In high-dose females the
incidence of nuclear pleomorphism of the liver cells and fatty
degeneration were increased. The tumour incidences were not enhanced
(Enomoto, 1985).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 60, 430, or 3000 ppm hexythiazox (purity 99%) for 104
weeks. Additional groups of 10 rats/sex/group were treated at the same
dose levels and were used for interim sacrifice at 12 months. The
observations included clinical signs, body weight, food consumption,
ophthalmology, haematology, biochemistry, urinalysis, macroscopy,
organ weight and histopathology. At the end of the study the
incidence of internal abdominal masses and swollen testicles and
non-descended testicles was high in all males but was observed to a
greater degree at 430 and 3000 ppm hexythiazox. Mean body weight was
decreased in high dose males and females and slightly at 430 ppm. Food
consumption was increased in both sexes at 3000 ppm and in females at
430 ppm. ALP, ALAT, ASAT and potassium levels were decreased in
high-dose females. At the end of the study most relative organ weights
(including the liver) were increased in both sexes at 3000 ppm. At
430 ppm relative brain, heart, lung and spleen weights were still
increased in males. No treatment-related histopathological changes
were observed. The tumour incidence was not enhanced. The NOAEL in
this study is 60 ppm, equal to 3.2 mg/kg bw (males) and 4.02 mg/kg bw
(females) (Spicer, 1984c).
Reproduction studies
In a two-generation reproduction study (2 litters/generation)
groups of SPF Wistar rats (30/sex/group) were given diets containing
0, 60, 400 or 2400 ppm hexythiazox (purity 98.2%). Observations
included clinical signs, body weight, food consumption, food
efficiency, reproductive findings, organ weight and histopathology.
Five dams/group were sacrificed on day 21 of pregnancy for teratogenic
examinations.
Body weight and food consumption were decreased in high dose F0
parents. An increased number of delivered F1 pups was observed in
the second mating at 400 and 2400 ppm hexythiazox. At 400 and 2400
ppm the relative weights of liver, kidney, adrenals (only at 2400 ppm)
and testes (no dose relationship) were increased in F0 adults. A
tendency to an increased mean number of implantations and total
resorptions was observed in the F0 generation at 400 and 2400 ppm.
At histopathology a higher incidence of proteinaceous casts in the
kidneys was observed in F0 male rats at 2400 ppm. The number of live
born F1b pups was increased at 400 and 2400 ppm and pup weight was
decreased from day 4 to 28 of lactation at these dose levels. A delay
in hairgrowth of abdomen was observed in pups at all treated groups of
the first generation. In F1 parents, body weight was decreased at
2400 ppm and in males occasionally also at 400 ppm. Food consumption
was decreased at the highest dose. The mean number of implants,
implants/corpora lutea and number of live fetuses were increased in
the 2400 ppm F1 group. Relative weights of brain (males only),
liver, kidneys, adrenals (males only) and ovaries were increased in
high dose F1 parents. F2b pup weight was decreased at 2400 ppm
hexythiazox. A delay in abdominal hair growth and eye-opening were
observed in F2b pups at 2400 ppm. Body weight and food consumption
(females only) of F1 adults were decreased at the highest dose. F1
adults showed an increased relative weight of ovaries, adrenal, liver
and kidney (males only) and showed a decreased spleen weight at 2400
ppm. Liver and kidney weight were also increased in males at 400 ppm.
An increase in the appearance of proteinaceous casts was observed in
the kidneys of high-dose males. Malformations were not observed. The
NOAEL in this study is 60 ppm (equal to a range between 4.22 and 5.34
mg/kg bw, depending on sex and generation) (Okugi & Enomoto, 1984).
Special studies on embryotoxicity and/or teratogenicity
Rats
Pregnant Sprague-Dawley rats (24/group) received by gavage 0,
240, 720 or 2160 mg hexythiazox (purity 98.3%)/kg bw from day 7
through 17 of gestation. The rats were sacrificed on day 21.
Observations were made for body weight, food and water consumption,
number of implantations, number of live and dead fetuses, number of
early and late resorptions and number of corpora lutea. The fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. Female body weight gain and occasionally food
consumption were decreased at 720 and 2160 mg/kg bw/day. Relative
ovarian weight was increased at the two highest dose levels. The
ossification of the metatarsal bones was significantly delayed at 720
and 2160 mg/kg bw/day. The NOAEL for maternal and embryotoxicity was
240 mg/kg bw/day. There was no evidence for hexythiazox-induced
irreversible structural effects (Gotoh & Nishibe, 1983).
Rabbits
Four groups of 15 pregnant New Zealand white rabbits received by
gavage 0, 120, 360, or 1080 mg hexythiazox (purity 97.7%)/kg from day
6 to 18 of gestation. All dams were sacrificed on day 28 of
gestation. No significant changes were observed in mortality,
maternal body weight and food consumption, uterus weight, number and
location of viable fetuses, early and late resorptions, number of
total implantations nor corpora lutea and organ weight. Two dams died,
1 at 120 mg/kg bw/day and 1 at 360 mg/kg bw/day. A slightly greater
number of fetuses with skeletal variations (overlapping of the
vertebral arch, asymmetry of the sternebrae) was observed at 1080
mg/kg bw/day. The NOAEL in this study is 360 mg/kg bw/day. No
irreversible structural effects were observed (Gotoh & Nihsibe, 1984).
Special studies on mutagenicity
Hexythiazox was negative in various mutagenicity assays (see
Table 1). Ames tests were conducted with 8 plant metabolites (some
also occurring in the rat): PT-1-2. PT-1-3, PT-1-4( trans-2),
PT-1-5(1), PT-1-6( trans-2), PT-1-8( cis), (PT-1-8( trans) and
PT-1-9. All metabolites gave negative results in the tests using
S. typhimurium (TA1535, TA100, TA1538, TA98 and TA1537) as well as
Escherichia coli (WP2 uvra) (Sasaki & Nishibe, 1985).
Special studies on pharmacology
Mice
Doses > 1000 mg hexythiazox (purity 98.3%) given
intraperitoneally to 3 male S1c:ddY mice delayed the sleeping time
induced by sodium pentobarbital. Effects were observed as soon as 15
minutes after hexythiazox administration. Hexythiazox also prolonged
the time at which convulsions induced by pentetrazole or strychnine
began. Intestinal motility and gastric secretion in mice were not
affected by the administration of up to 150 mg hexythiazox iv nor up
to 3000 mg/kg (oral), respectively (Souma & Nishibe, 1985).
Rats
Blood coagulation time in S1c:Wistar rats was decreased after
administration of 3000 mg hexythiazox in saline, but haemolysis was
not observed. I.p. administration of doses as high as 2500 mg
hexythiazox/kg had no effect on rat body temperature nor on gastric
secretion. No effect on the tension of the muscle twitch induced by
the indirect stimulation was observed after i.v. injection of 50 or
100 mg hexythiazox/kg (Souma & Nishibe, 1985).
Table 4. Results of mutagenicity assays on hexythiazox
Test system Test object Concentration Purity (%) Results References
In vitro
Ames testa,b S. typhimurium 100-6400 µg/plate 97.7 Negative Inoue (1983a)
TA 100, TA98, in DMSO
TA1535, TA1537,
TA1538
E coli, WP2 uvra
Yeast testa,b S. cerevisiae 312-10 000 µg/ml 98.4 Negative Jagannath (1984)
(reverse mutation) S138, S211 in DMSO
(mitotic gene S. cerevisiae 1-10 000 µg/plate 98.4 Negative
conversion) D4
V79/HGPRTa,b Chinese hamster 9.38-150 µg/ml 98.6 Negative Seeberg (1986)
mutation assay V79 cells in DMSO
Chromosome Chinese hamster 1.5-50 µg/ml 98.4 Negative Galloway (1984)
aberration ovary cells
assaya
Rec-assay B. subtilis 400-3200 µg/plate 97.7 Negative Inoue (1983b)
H17(rec+) in DMSO
M45(rec-)
Unscheduled DNA Rat hepatocytes 2.5-100 µg/ml 98.4 Negative Cifone (1985)
synthesis (250 µg/ml lethal)
Table 4 (contd).
Test system Test object Concentration Purity (%) Results References
In vivo
Cytogenic testa,b Chinese hamster 1000, 2000 or 4000 98.6 Negative Mosesso (1986)
bone marrow mg/kg p.o.
Micronucleus test Male and female 0.1, 0.5 and 1.0 98.4 Negative Ivett (1984)
CD-1 bone marrow g/kg p.o.
cells
a without metabolic activation
b with metabolic activation
Rabbits
Hypotension and hypoventilation were observed after the iv
injection of 50 mg hexythiazox/kg once weekly (Souma & Nishibe, 1985).
Guinea-pig
High concentrations of hexythiazox inhibited the contraction of
isolated ileum induced by acetylcholine, histamine and barium chloride
(Souma & Nishibe, 1985).
Special studies on sensitization
Hexythiazox (purity 98.3%) as well as a 10% WP formulation did
not exhibit a sensitizing effect in the maximization test (Magnusson
& Kligman) in female Hartley guinea-pigs (Souma & Nishibe, 1983a;
1983d).
Special studies on skin and eye irritation
A dose of 0.5 g hexythiazox (purity 98.3%) and a 10% WP
formulation moistened with water was applied under occlusive
conditions to the clipped back skin of 6 male New Zealand White
rabbits for 4 hours. No skin irritation was observed up to 72 hours
after application (Souma & Nishibe, 1983b; 1983e).
Applications of 0.1 g hexythiazox (purity 98.3%) and a 10% WP
formulation into the eyes of 6 male New Zealand White rabbits caused
slight irritation (redness of the conjunctivae). No irritation
effects were observed 48 hours post-application (Souma & Nishibe,
1983c; 1983f).
Observation in humans
Health examinations (twice a year) were performed on a group of
16 to 26 workers in a Japanese manufacturing plant. The health
examination consisted of analysis for gamma-GTP (once a year), Hb,
haematocrit, erythrocytes and urinalysis (protein, glucose and
urobilinogen). No adverse effects were observed on the examined
parameters after three years of continuous exposure to hexythiazox
(Motogi, 1987).
COMMENTS
After oral administration to rats, hexythiazox was eliminated
rapidly, two-thirds via the faeces and one-third via the urine. Low
levels were recovered in the tissues and organs, with the highest
concentrations in the fat, liver, adrenals and in the gastrointestinal
tract and its contents. In the faeces about 20% was excreted as the
parent compound. Many metabolites were found in the urine and faeces,
although only 10% of those in the urine and 50% of those in the faeces
could be identified. Metabolism occurs via oxidation, and finally
cleavage, of the cyclohexane ring.
The compound shows low acute toxicity in the species examined.
WHO has classified hexythiazox as unlikely to present acute hazard in
normal use.
Short-term administration of hexythiazox to rats and dogs
revealed the liver and the adrenals as target organs. In a 90-day
study in rats the NOAEL was 70 ppm, equal to 4.9 and 5.3 mg/kg bw/day
for males and females respectively. In a one-year study in dogs the
NOAEL was 100 ppm, equal to 2.9 mg/kg bw/day in males and 3.2 mg/kg
bw/day in females.
In a 2-year feeding study in mice at dietary concentrations of 0,
40, 250, or 1500 ppm there was an increased incidence of liver nodules
in both sexes at 1500 ppm. The incidences of liver adenomas and
hepatocarcinomas were increased in females at 1500 ppm. Three
haemangiopericytomas in the liver were observed in males at 1500 ppm.
These were subsequently re-evaluated as hepatoblastomas. At the
lowest concentration of 40 ppm, equal to 6.7 and 8.4 mg/kg bw/day in
males and females respectively, reduced body-weight gain in male
animals was observed. A NOAEL was not established.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 60, 430, or 3000 ppm, there were no
treatment-related increases in neoplasias. There was a reduction in
body-weight gain and an increase in some organ weights at 430 ppm.
The NOAEL was 60 ppm, equal to 3.2 and 4.2 mg/kg bw/day for males and
females respectively.
In a 2-generation, 2-litters-per-generation reproduction study in
rats at 0, 60, 400, or 2400 ppm, no adverse effects on reproduction
were found. The NOAEL was 60 ppm, equivalent to 3 mg/kg bw/day, based
on increased liver and kidney weights at 400 ppm.
Maternal toxicity (reduced weight gains) and embryotoxicity
(delayed development) were observed in a teratogenicity study in rats
at doses of 0, 240, 720, or 2160 mg/kg bw/day. The NOAEL was 240
mg/kg bw/day. In a teratogenicity study in rabbits at doses of 0,
120, 360 or 1080 mg/kg bw/day, slight embryotoxicity was observed at
the highest dose. The NOAEL was 360 mg/kg bw/day. No teratogenic
effects were observed in either species.
After reviewing all available in vitro and in vivo short-term
tests on hexythiazox it was concluded that there was no evidence of
genotoxicity.
An ADI was established which was based on NOAELs in the 2-year
feeding and reproduction studies in rats and a 1-year study in dogs,
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 60 ppm in the diet, equal to 3.2 mg/kg bw/day
(2-year study)
Rat: 60 ppm in the diet, equivalent to 3 mg/kg bw/day
(reproduction study)
Dog: 100 ppm in the diet, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Cifone, M.A. (1985) Evaluation of NA-73 technical lot no. SCF-05 in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished second amended report 20991 (RD-8556) from Litton
Bionetics, Inc. Kensington, Maryland, USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Enomoto, M. (1985) Supplement to the chronic feeding and oncogenicity
studies in mice with NA-73. Histological findings of the liver of mice
sacrificed at 78 weeks. Unpublished Report 527 (RD-8534) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Galloway, S.M. (1984) Mutagenic evaluation of NA-73 Technical lot no.
SCF-05. in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished amended report 20990 (RD-8435N) from Litton Bionetics,
Inc. Kensington, Maryland 20895, USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1983) Teratogenicity study of NA-73 in rats.
Unpublished toxicology report 0118 (RD-83104) from Biomedical research
department of Nisso Institute for Life Science. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1984) Teratogenicity study of NA-73 in
rabbits. Unpublished toxicology report 0126 (RD-8454) from Biomedical
Research Department of Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1983a) NA-73: Mutagenicity test in bacteria. Unpublished
Report 364 (RD-83107) from Biosafety Research Center, Foods, Drugs and
Pesticides (AN-PYO Center). Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Inoue, H. (1983b) NA-73: Rec-assay with Bacillus subtilis strains
H 17 (rec+) and M 45-). Unpublished Report 363 (RD-83106) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1985) Acute intraperitoneal toxicity study in rats with
NA-73. Unpublished Report 623 (RD-8582) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987a) Acute subcutaneous toxicity study in mice with
NA-73. Unpublished Report 989 (RD-8759) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987b) Acute intraperitoneal toxicity study in mice with
NA-73. Unpublished Report 990 (RD-8758) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987c) Acute subcutaneous toxicity study in rats with
NA-73. Unpublished Report 991 (RD-8757) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H & Enomoto, M. (1984) Chronic feeding and oncogenicity
studies in mice with NA-73. Unpublished Report 483 (RD-84107) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Ivett, J.L. (1984) Mutagenicity evaluation of NA-73 technical in the
in vivo mouse micronucleus assay. Unpublished Report 20996
(RD-8436N) from Litton Bionetics, Inc. Kensington, Maryland, USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Jagannath, D.R. (1984) Mutagenicity evaluation of NA-73 in the mitotic
gene conversion and reverse mutation induction in yeast strains D4,
S138 and S211 plate test. Litton Bionetics, Inc. Submitted to WHO by
Nippon Soda Co. Ltd., Tokyo, Japan. Unpublished Report RD-8434N.
Mosesso, P. (1986) Chinese hamster bone marrow metaphase analysis
( in vivo cytogenetics). Test substance: NA 73. Unpublished LSR-RTC
Report 063015-M-04086 (RD-8696) from Life Science Research Roma
Toxicology Centre. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Motogi, S. (1987) Human handling experiences from plant employees in
hexythiazox production. Unpublished Report (RD-8749) from
Environmental Control and Safety Department, Nihongi Plant, Japan.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Okugi, M. & Enomoto, M. (1984) NA-73: Two-generation reproduction
study in rats (experiment 221). Unpublished Report 453 (RD-84108) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983a) Acute oral toxicity study of NA-73 in
mice. Unpublished toxicology report 0107 (RD-8393) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983b) Acute oral toxicity study of NA-73 in
rats. Unpublished toxicology report 0092 (RD-8394) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983c) Acute inhalation toxicity study of
NA-73 in rats. Unpublished toxicology report 0094 (RD-8396) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983d) Acute dermal toxicity study of NA-73
in rats. Unpublished toxicology report 0093 (RD-8395) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983e) Acute oral toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0123 (RD-83112) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983f) Acute inhalation toxicity study of
NA-73 10% WP in rats. Unpublished toxicology report 0121 (RD-83114)
from Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983g) Acute dermal toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0119 (RD-83113) from
Biomedical Research Department, Nisso institute for life science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1984) Acute oral toxicity study of NA-73 in
dogs. Unpublished toxicology report 0125 (RD-8428) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1985) Acute oral toxicity study of main
metabolites of NA-73 in Rats. Unpublished report 0181 (RD-8570) from
Toxicology Group, Nippon Soda Ltd. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Sasaki, T. & Nishibe, T. (1985) Reverse mutation study of main
metabolites of NA-73. Unpublished Report 0185 (RD-8571) from
Toxicology Group, Soda Ltd. Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Seeberg, A.H. (1986) Gene mutation in Chinese hamster V79 cells test
substance: NA 73. Unpublished Report 063014-M-03986 (RD-8695) from
Life Science Research Roma Toxicology Centre. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Soeda, Y. (1985) Metabolism of NA-73 in rats (group B). Unpublished
Report EC-9 (RD-8520N) from Environmental Toxicology Laboratory of
Nippon Soda Ltd. Submitted to WHO by Nippon Soda Co., Ltd. Tokyo,
Japan.
Souma, S. & Nishibe, T. (1983a) Delayed contact hypersensitivity
study of NA-73 in guinea pigs. Unpublished toxicology report 0097
(RD-8399) from Biomedical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983b) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report 0095 (RD-8398) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983c) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report 0096 (RD-8397) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983d) Delayed contact hypersensitivity
study of NA-73 10%WP in guinea pigs. Unpublished toxicology report
(RD-83117) from Bio-medical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983e) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report (RD-83116) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983f) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report (RD-83115) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1985) General pharmacology study of NA-73.
Unpublished toxicology report 0186 (RD-85104) from Environmental
Toxicology Laboratory, Nippon Soda Co., Ltd. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984a) Four week dietary range-finding study in dogs
with NA-73. Unpublished Report (RD-84105) from International Research
and Development Corporation, Michigan USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984b) One year dietary study of NA-73 in dogs.
Unpublished Report (RD-8433) from International Research and
Development Corporation, Michigan USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984c) Lifetime (24-month) dietary toxicity and
oncogenicity study of NA-73 in rats. Unpublished Report (RD-84106)
from International Research and Development Corporation, Michigan USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983a) Cumulative toxicity study of NA-73
in mice. Unpublished Report (RD-83100) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983b) Subchronic feeding study of NA-73
in rats. Unpublished Report (RD-83101) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
See Also:
Toxicological Abbreviations
 Toxicological studies
Acute toxicity
The LD50 and LC50 for hexythiazox, the 10% WP formulation and
certain metabolites, are given in Tables 1, 2 and 3, respectively.
No signs of toxicity were observed after acute oral and dermal
exposure to hexythiazox.
Short-term studies
Mice
Male and female B6C3F1 mice (10/sex/group) were fed diets
containing 0, 50, 300, 1800 or 10 800 ppm hexythiazox (purity 98.3%)
for 28 days. No compound-related effects were observed on mortality,
clinical signs, food consumption and food efficiency, haematology, nor
macroscopy. Male body weight gain was decreased at 1800 and 10 800
ppm hexythiazox. Total cholesterol levels decreased in male and
female mice at 10 800 ppm and in males also at 1800 ppm. Urine
specific gravity was increased in high-dose males. Relative liver
weight was increased in male and female mice at 1800 and 10 800 ppm.
At histopathology an increased incidence of swollen liver cells was
observed in high-dose males (also at 1800 ppm) and females. The NOAEL
in this study is 300 ppm, equal to 55.1 mg/kg bw (males) and 62.9
mg/kg bw (females) (Takaori & Nishibe, 1983a).
Rats
Groups of Fischer rats (20/sex/group) received a diet containing
0, 10, 70, 500 or 3500 ppm hexythiazox (98.3%) for 13 weeks.
Observations included clinical signs, food and water consumption,
haematology, biochemistry, cholinesterase activity, urinalysis,
macroscopy, organ weights and histopathology. Growth was depressed in
both sexes at 3500 ppm and in females also at 500 ppm. In high-dose
males Hb, PCV, RBC and MCV were decreased and platelet count was
increased. In high-dose females MCHC and MCH (also at 500 ppm) were
decreased. At 3500 ppm hexythiazox total cholesterol, albumin (in
males also at 500 ppm), protein and calcium were increased in both
sexes. ALP and ASAT were increased in high-dose females. Protein in
urine was increased in males at 3500 ppm. Relative liver (also at 500
ppm), kidney, adrenal and gonad weight were increased and spleen
weight decreased at 3500 ppm in both sexes. At 70 ppm liver weight was
still slightly increased in females. Female brain weight was increased
at 500 and 3500 ppm. At histopathology swelling of hepatocytes in the
central zone was observed in both sexes at the highest dose. Fatty
degeneration of the zona fasciculata of the adrenal cortex was
observed at dose levels of 500 ppm and greater. The NOAEL is 70 ppm,
equal to 8.1 mg/kg bw/day (males) and 5.3 mg/kg bw/day (females)
(Takaori & Nishibe, 1983b).
Table 1. Acute toxicity of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Mouse M&F oral 98.3% > 5000 Saika & Nishibe (1983a)
M&F s.c. 98.2% > 5000 Inoue (1987a)
M&F i.p. 98.2% > 5000 Inoue (1987b)
Rat M&F oral 98.3% > 5000 Saika & Nishibe (1983b)
M&F s.c. 98.2% > 5000 Inoue (1987c)
M&F i.p. 98.2% > 5000 Inoue (1985)
M&F inhl.a 98.3% > 2.0 Saika & Nishibe (1983c)
M&F dermal 98.3% > 5000 Saika & Nishibe (1983d)
Dog M&F oral 98.3% > 5000 Saika & Nishibe (1984)
a 4 hour exposure
Table 2. Acute toxicity of 10% wettable powder of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Rat M&F oral > 5000 Saika & Nishibe (1983e)
M&F inhal.a > 2.9 Saika & Nishibe (1983f)
M&F dermal > 5000 Saika & Nishibe (1983g)
a 4 hour exposure
Table 3. Acute toxicity of metabolites of hexythiazox in rats
Metabolite Sex Route Purity LD50 References
(mg/kg bw)
PT-1-2 M oral > 99% 2321 Saika & Nishibe (1985)
F 1079
PT-1-3 M oral > 99% 494 Saika & Nishibe (1985)
F 341
PT-1-4 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans-2)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(cis)
Dogs
In a range-finding study groups of Beagle dogs (2/sex/group) were
fed diets containing 0, 125, 500, 2000 or 8000 ppm hexythiazox (purity
97.7%) for 4 weeks. No effects were observed on clinical signs nor
mortality. Body weight gain was decreased in females and food
consumption in males at 8000 ppm. Relative liver weight was increased
in females at 2000 and 8000 ppm and slightly increased in high-dose
males (Spicer, 1984a).
Male and female Beagle dogs (4/sex/group) received a diet
containing 0, 100, 500 or 5000 ppm hexythiazox (purity 97.7%) for 12
months. Observations were made for clinical signs, body weight, food
consumption and food efficiency, ophthalmology, haematology,
biochemistry, cholinesterase activity and urinalysis. The weights of
10 organs/animal were recorded. Gross necropsy and histo-pathology
were carried out. ALP was increased in both sexes at 5000 ppm and
ALAT was increased in females at the same dose. Total protein was
decreased in males at 500 and 5000 ppm and in females at 5000 ppm.
Phosphorus was decreased in females 500 and 5000 ppm. Relative
adrenal weight was increased in high-dose dogs and relative liver
weight was increased in high-dose males. At histopathology
adrenocortical hypertrophy occurred in male and female dogs at 500 and
5000 ppm. Hepatocellular hypertrophy was observed in high-dose dogs.
The NOAEL in this study is 100 ppm, equal to 2.87 mg/kg (males) and
3.17 mg/kg (females) (Spicer, 1984b).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) received a diet
containing 0, 40, 250 or 1500 ppm hexythiazox (purity 98.2%) for 104
weeks. An additional 30 mice (10/sex/group) served for the collection
of blood samples after 26, 52 and 79 weeks. Observations included
clinical signs, mortality, body weight, food consumption and food
efficiency, haematology, clinical chemistry, urinalysis, macroscopy,
organ weight (after 26, 52, 78 and 104 weeks) and histopathology
(after 52 and 104 weeks). Body weight gain and absolute body weights
were decreased at 250 and 1500 ppm in male mice only. During the
second half of the study body weight gain was also significantly
decreased at 40 ppm. Various red blood cell parameters were decreased
during the first 78 weeks at the highest dose. Relative liver weight
was increased in males and females at 1500 ppm during the whole study.
After 104 weeks relative weights of brain, heart, kidneys, adrenals
and testes were increased in male mice. Relative brain, kidneys,
adrenals and testes weight were increased in males at 250 ppm.
Histopathology of the liver after 52 weeks showed an increased
incidence of fatty degeneration and hypertrophy of the hepatocytes
with nuclear pleomorphism in males at 1500 ppm which was only
marginally increased at 250 ppm. At the end of the study the incidence
of hyperplastic nodes consisting of proliferating hepatocytes was
increased in both sexes at the highest dose (incidences (52+104 weeks
60 rats): 20%, 20%, 25% and 50% for males and 5%, 8%, 6% and 27% for
females at 0, 40, 250, and 1500 ppm, respectively). At the same dose
an increase in the incidence of hepatocellular adenomas or carcinomas
was also observed (incidences: adenomas: 20%, 25%, 20% and 33% for
males and 10%, 2%, 5% and 17% for females; carcinomas: 13%, 13%, 17%
and 13% for males and 0%, 5%, 5% and 8% for females both at 0, 40,
250, and 1500 ppm hexythiazox). In 3 males at 1500 ppm
haemangiopericytoma was observed in the liver. This incidence was
within the historical control range. At the lowest dose level of 40
ppm (equal to 6.7 in males and 8.4 in females) there was still a
decreased body weight gain (Inoue & Enomoto, 1984).
Because of the observed increase of liver tumours an additional
histological examination was performed on the livers of mice
(10/sex/group) sacrificed after 78 weeks. In high-dose females the
incidence of nuclear pleomorphism of the liver cells and fatty
degeneration were increased. The tumour incidences were not enhanced
(Enomoto, 1985).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 60, 430, or 3000 ppm hexythiazox (purity 99%) for 104
weeks. Additional groups of 10 rats/sex/group were treated at the same
dose levels and were used for interim sacrifice at 12 months. The
observations included clinical signs, body weight, food consumption,
ophthalmology, haematology, biochemistry, urinalysis, macroscopy,
organ weight and histopathology. At the end of the study the
incidence of internal abdominal masses and swollen testicles and
non-descended testicles was high in all males but was observed to a
greater degree at 430 and 3000 ppm hexythiazox. Mean body weight was
decreased in high dose males and females and slightly at 430 ppm. Food
consumption was increased in both sexes at 3000 ppm and in females at
430 ppm. ALP, ALAT, ASAT and potassium levels were decreased in
high-dose females. At the end of the study most relative organ weights
(including the liver) were increased in both sexes at 3000 ppm. At
430 ppm relative brain, heart, lung and spleen weights were still
increased in males. No treatment-related histopathological changes
were observed. The tumour incidence was not enhanced. The NOAEL in
this study is 60 ppm, equal to 3.2 mg/kg bw (males) and 4.02 mg/kg bw
(females) (Spicer, 1984c).
Reproduction studies
In a two-generation reproduction study (2 litters/generation)
groups of SPF Wistar rats (30/sex/group) were given diets containing
0, 60, 400 or 2400 ppm hexythiazox (purity 98.2%). Observations
included clinical signs, body weight, food consumption, food
efficiency, reproductive findings, organ weight and histopathology.
Five dams/group were sacrificed on day 21 of pregnancy for teratogenic
examinations.
Body weight and food consumption were decreased in high dose F0
parents. An increased number of delivered F1 pups was observed in
the second mating at 400 and 2400 ppm hexythiazox. At 400 and 2400
ppm the relative weights of liver, kidney, adrenals (only at 2400 ppm)
and testes (no dose relationship) were increased in F0 adults. A
tendency to an increased mean number of implantations and total
resorptions was observed in the F0 generation at 400 and 2400 ppm.
At histopathology a higher incidence of proteinaceous casts in the
kidneys was observed in F0 male rats at 2400 ppm. The number of live
born F1b pups was increased at 400 and 2400 ppm and pup weight was
decreased from day 4 to 28 of lactation at these dose levels. A delay
in hairgrowth of abdomen was observed in pups at all treated groups of
the first generation. In F1 parents, body weight was decreased at
2400 ppm and in males occasionally also at 400 ppm. Food consumption
was decreased at the highest dose. The mean number of implants,
implants/corpora lutea and number of live fetuses were increased in
the 2400 ppm F1 group. Relative weights of brain (males only),
liver, kidneys, adrenals (males only) and ovaries were increased in
high dose F1 parents. F2b pup weight was decreased at 2400 ppm
hexythiazox. A delay in abdominal hair growth and eye-opening were
observed in F2b pups at 2400 ppm. Body weight and food consumption
(females only) of F1 adults were decreased at the highest dose. F1
adults showed an increased relative weight of ovaries, adrenal, liver
and kidney (males only) and showed a decreased spleen weight at 2400
ppm. Liver and kidney weight were also increased in males at 400 ppm.
An increase in the appearance of proteinaceous casts was observed in
the kidneys of high-dose males. Malformations were not observed. The
NOAEL in this study is 60 ppm (equal to a range between 4.22 and 5.34
mg/kg bw, depending on sex and generation) (Okugi & Enomoto, 1984).
Special studies on embryotoxicity and/or teratogenicity
Rats
Pregnant Sprague-Dawley rats (24/group) received by gavage 0,
240, 720 or 2160 mg hexythiazox (purity 98.3%)/kg bw from day 7
through 17 of gestation. The rats were sacrificed on day 21.
Observations were made for body weight, food and water consumption,
number of implantations, number of live and dead fetuses, number of
early and late resorptions and number of corpora lutea. The fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. Female body weight gain and occasionally food
consumption were decreased at 720 and 2160 mg/kg bw/day. Relative
ovarian weight was increased at the two highest dose levels. The
ossification of the metatarsal bones was significantly delayed at 720
and 2160 mg/kg bw/day. The NOAEL for maternal and embryotoxicity was
240 mg/kg bw/day. There was no evidence for hexythiazox-induced
irreversible structural effects (Gotoh & Nishibe, 1983).
Rabbits
Four groups of 15 pregnant New Zealand white rabbits received by
gavage 0, 120, 360, or 1080 mg hexythiazox (purity 97.7%)/kg from day
6 to 18 of gestation. All dams were sacrificed on day 28 of
gestation. No significant changes were observed in mortality,
maternal body weight and food consumption, uterus weight, number and
location of viable fetuses, early and late resorptions, number of
total implantations nor corpora lutea and organ weight. Two dams died,
1 at 120 mg/kg bw/day and 1 at 360 mg/kg bw/day. A slightly greater
number of fetuses with skeletal variations (overlapping of the
vertebral arch, asymmetry of the sternebrae) was observed at 1080
mg/kg bw/day. The NOAEL in this study is 360 mg/kg bw/day. No
irreversible structural effects were observed (Gotoh & Nihsibe, 1984).
Special studies on mutagenicity
Hexythiazox was negative in various mutagenicity assays (see
Table 1). Ames tests were conducted with 8 plant metabolites (some
also occurring in the rat): PT-1-2. PT-1-3, PT-1-4( trans-2),
PT-1-5(1), PT-1-6( trans-2), PT-1-8( cis), (PT-1-8( trans) and
PT-1-9. All metabolites gave negative results in the tests using
S. typhimurium (TA1535, TA100, TA1538, TA98 and TA1537) as well as
Escherichia coli (WP2 uvra) (Sasaki & Nishibe, 1985).
Special studies on pharmacology
Mice
Doses > 1000 mg hexythiazox (purity 98.3%) given
intraperitoneally to 3 male S1c:ddY mice delayed the sleeping time
induced by sodium pentobarbital. Effects were observed as soon as 15
minutes after hexythiazox administration. Hexythiazox also prolonged
the time at which convulsions induced by pentetrazole or strychnine
began. Intestinal motility and gastric secretion in mice were not
affected by the administration of up to 150 mg hexythiazox iv nor up
to 3000 mg/kg (oral), respectively (Souma & Nishibe, 1985).
Rats
Blood coagulation time in S1c:Wistar rats was decreased after
administration of 3000 mg hexythiazox in saline, but haemolysis was
not observed. I.p. administration of doses as high as 2500 mg
hexythiazox/kg had no effect on rat body temperature nor on gastric
secretion. No effect on the tension of the muscle twitch induced by
the indirect stimulation was observed after i.v. injection of 50 or
100 mg hexythiazox/kg (Souma & Nishibe, 1985).
Table 4. Results of mutagenicity assays on hexythiazox
Test system Test object Concentration Purity (%) Results References
In vitro
Ames testa,b S. typhimurium 100-6400 µg/plate 97.7 Negative Inoue (1983a)
TA 100, TA98, in DMSO
TA1535, TA1537,
TA1538
E coli, WP2 uvra
Yeast testa,b S. cerevisiae 312-10 000 µg/ml 98.4 Negative Jagannath (1984)
(reverse mutation) S138, S211 in DMSO
(mitotic gene S. cerevisiae 1-10 000 µg/plate 98.4 Negative
conversion) D4
V79/HGPRTa,b Chinese hamster 9.38-150 µg/ml 98.6 Negative Seeberg (1986)
mutation assay V79 cells in DMSO
Chromosome Chinese hamster 1.5-50 µg/ml 98.4 Negative Galloway (1984)
aberration ovary cells
assaya
Rec-assay B. subtilis 400-3200 µg/plate 97.7 Negative Inoue (1983b)
H17(rec+) in DMSO
M45(rec-)
Unscheduled DNA Rat hepatocytes 2.5-100 µg/ml 98.4 Negative Cifone (1985)
synthesis (250 µg/ml lethal)
Table 4 (contd).
Test system Test object Concentration Purity (%) Results References
In vivo
Cytogenic testa,b Chinese hamster 1000, 2000 or 4000 98.6 Negative Mosesso (1986)
bone marrow mg/kg p.o.
Micronucleus test Male and female 0.1, 0.5 and 1.0 98.4 Negative Ivett (1984)
CD-1 bone marrow g/kg p.o.
cells
a without metabolic activation
b with metabolic activation
Rabbits
Hypotension and hypoventilation were observed after the iv
injection of 50 mg hexythiazox/kg once weekly (Souma & Nishibe, 1985).
Guinea-pig
High concentrations of hexythiazox inhibited the contraction of
isolated ileum induced by acetylcholine, histamine and barium chloride
(Souma & Nishibe, 1985).
Special studies on sensitization
Hexythiazox (purity 98.3%) as well as a 10% WP formulation did
not exhibit a sensitizing effect in the maximization test (Magnusson
& Kligman) in female Hartley guinea-pigs (Souma & Nishibe, 1983a;
1983d).
Special studies on skin and eye irritation
A dose of 0.5 g hexythiazox (purity 98.3%) and a 10% WP
formulation moistened with water was applied under occlusive
conditions to the clipped back skin of 6 male New Zealand White
rabbits for 4 hours. No skin irritation was observed up to 72 hours
after application (Souma & Nishibe, 1983b; 1983e).
Applications of 0.1 g hexythiazox (purity 98.3%) and a 10% WP
formulation into the eyes of 6 male New Zealand White rabbits caused
slight irritation (redness of the conjunctivae). No irritation
effects were observed 48 hours post-application (Souma & Nishibe,
1983c; 1983f).
Observation in humans
Health examinations (twice a year) were performed on a group of
16 to 26 workers in a Japanese manufacturing plant. The health
examination consisted of analysis for gamma-GTP (once a year), Hb,
haematocrit, erythrocytes and urinalysis (protein, glucose and
urobilinogen). No adverse effects were observed on the examined
parameters after three years of continuous exposure to hexythiazox
(Motogi, 1987).
COMMENTS
After oral administration to rats, hexythiazox was eliminated
rapidly, two-thirds via the faeces and one-third via the urine. Low
levels were recovered in the tissues and organs, with the highest
concentrations in the fat, liver, adrenals and in the gastrointestinal
tract and its contents. In the faeces about 20% was excreted as the
parent compound. Many metabolites were found in the urine and faeces,
although only 10% of those in the urine and 50% of those in the faeces
could be identified. Metabolism occurs via oxidation, and finally
cleavage, of the cyclohexane ring.
The compound shows low acute toxicity in the species examined.
WHO has classified hexythiazox as unlikely to present acute hazard in
normal use.
Short-term administration of hexythiazox to rats and dogs
revealed the liver and the adrenals as target organs. In a 90-day
study in rats the NOAEL was 70 ppm, equal to 4.9 and 5.3 mg/kg bw/day
for males and females respectively. In a one-year study in dogs the
NOAEL was 100 ppm, equal to 2.9 mg/kg bw/day in males and 3.2 mg/kg
bw/day in females.
In a 2-year feeding study in mice at dietary concentrations of 0,
40, 250, or 1500 ppm there was an increased incidence of liver nodules
in both sexes at 1500 ppm. The incidences of liver adenomas and
hepatocarcinomas were increased in females at 1500 ppm. Three
haemangiopericytomas in the liver were observed in males at 1500 ppm.
These were subsequently re-evaluated as hepatoblastomas. At the
lowest concentration of 40 ppm, equal to 6.7 and 8.4 mg/kg bw/day in
males and females respectively, reduced body-weight gain in male
animals was observed. A NOAEL was not established.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 60, 430, or 3000 ppm, there were no
treatment-related increases in neoplasias. There was a reduction in
body-weight gain and an increase in some organ weights at 430 ppm.
The NOAEL was 60 ppm, equal to 3.2 and 4.2 mg/kg bw/day for males and
females respectively.
In a 2-generation, 2-litters-per-generation reproduction study in
rats at 0, 60, 400, or 2400 ppm, no adverse effects on reproduction
were found. The NOAEL was 60 ppm, equivalent to 3 mg/kg bw/day, based
on increased liver and kidney weights at 400 ppm.
Maternal toxicity (reduced weight gains) and embryotoxicity
(delayed development) were observed in a teratogenicity study in rats
at doses of 0, 240, 720, or 2160 mg/kg bw/day. The NOAEL was 240
mg/kg bw/day. In a teratogenicity study in rabbits at doses of 0,
120, 360 or 1080 mg/kg bw/day, slight embryotoxicity was observed at
the highest dose. The NOAEL was 360 mg/kg bw/day. No teratogenic
effects were observed in either species.
After reviewing all available in vitro and in vivo short-term
tests on hexythiazox it was concluded that there was no evidence of
genotoxicity.
An ADI was established which was based on NOAELs in the 2-year
feeding and reproduction studies in rats and a 1-year study in dogs,
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 60 ppm in the diet, equal to 3.2 mg/kg bw/day
(2-year study)
Rat: 60 ppm in the diet, equivalent to 3 mg/kg bw/day
(reproduction study)
Dog: 100 ppm in the diet, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Cifone, M.A. (1985) Evaluation of NA-73 technical lot no. SCF-05 in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished second amended report 20991 (RD-8556) from Litton
Bionetics, Inc. Kensington, Maryland, USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Enomoto, M. (1985) Supplement to the chronic feeding and oncogenicity
studies in mice with NA-73. Histological findings of the liver of mice
sacrificed at 78 weeks. Unpublished Report 527 (RD-8534) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Galloway, S.M. (1984) Mutagenic evaluation of NA-73 Technical lot no.
SCF-05. in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished amended report 20990 (RD-8435N) from Litton Bionetics,
Inc. Kensington, Maryland 20895, USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1983) Teratogenicity study of NA-73 in rats.
Unpublished toxicology report 0118 (RD-83104) from Biomedical research
department of Nisso Institute for Life Science. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1984) Teratogenicity study of NA-73 in
rabbits. Unpublished toxicology report 0126 (RD-8454) from Biomedical
Research Department of Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1983a) NA-73: Mutagenicity test in bacteria. Unpublished
Report 364 (RD-83107) from Biosafety Research Center, Foods, Drugs and
Pesticides (AN-PYO Center). Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Inoue, H. (1983b) NA-73: Rec-assay with Bacillus subtilis strains
H 17 (rec+) and M 45-). Unpublished Report 363 (RD-83106) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1985) Acute intraperitoneal toxicity study in rats with
NA-73. Unpublished Report 623 (RD-8582) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987a) Acute subcutaneous toxicity study in mice with
NA-73. Unpublished Report 989 (RD-8759) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987b) Acute intraperitoneal toxicity study in mice with
NA-73. Unpublished Report 990 (RD-8758) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987c) Acute subcutaneous toxicity study in rats with
NA-73. Unpublished Report 991 (RD-8757) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H & Enomoto, M. (1984) Chronic feeding and oncogenicity
studies in mice with NA-73. Unpublished Report 483 (RD-84107) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Ivett, J.L. (1984) Mutagenicity evaluation of NA-73 technical in the
in vivo mouse micronucleus assay. Unpublished Report 20996
(RD-8436N) from Litton Bionetics, Inc. Kensington, Maryland, USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Jagannath, D.R. (1984) Mutagenicity evaluation of NA-73 in the mitotic
gene conversion and reverse mutation induction in yeast strains D4,
S138 and S211 plate test. Litton Bionetics, Inc. Submitted to WHO by
Nippon Soda Co. Ltd., Tokyo, Japan. Unpublished Report RD-8434N.
Mosesso, P. (1986) Chinese hamster bone marrow metaphase analysis
( in vivo cytogenetics). Test substance: NA 73. Unpublished LSR-RTC
Report 063015-M-04086 (RD-8696) from Life Science Research Roma
Toxicology Centre. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Motogi, S. (1987) Human handling experiences from plant employees in
hexythiazox production. Unpublished Report (RD-8749) from
Environmental Control and Safety Department, Nihongi Plant, Japan.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Okugi, M. & Enomoto, M. (1984) NA-73: Two-generation reproduction
study in rats (experiment 221). Unpublished Report 453 (RD-84108) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983a) Acute oral toxicity study of NA-73 in
mice. Unpublished toxicology report 0107 (RD-8393) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983b) Acute oral toxicity study of NA-73 in
rats. Unpublished toxicology report 0092 (RD-8394) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983c) Acute inhalation toxicity study of
NA-73 in rats. Unpublished toxicology report 0094 (RD-8396) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983d) Acute dermal toxicity study of NA-73
in rats. Unpublished toxicology report 0093 (RD-8395) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983e) Acute oral toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0123 (RD-83112) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983f) Acute inhalation toxicity study of
NA-73 10% WP in rats. Unpublished toxicology report 0121 (RD-83114)
from Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983g) Acute dermal toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0119 (RD-83113) from
Biomedical Research Department, Nisso institute for life science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1984) Acute oral toxicity study of NA-73 in
dogs. Unpublished toxicology report 0125 (RD-8428) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1985) Acute oral toxicity study of main
metabolites of NA-73 in Rats. Unpublished report 0181 (RD-8570) from
Toxicology Group, Nippon Soda Ltd. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Sasaki, T. & Nishibe, T. (1985) Reverse mutation study of main
metabolites of NA-73. Unpublished Report 0185 (RD-8571) from
Toxicology Group, Soda Ltd. Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Seeberg, A.H. (1986) Gene mutation in Chinese hamster V79 cells test
substance: NA 73. Unpublished Report 063014-M-03986 (RD-8695) from
Life Science Research Roma Toxicology Centre. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Soeda, Y. (1985) Metabolism of NA-73 in rats (group B). Unpublished
Report EC-9 (RD-8520N) from Environmental Toxicology Laboratory of
Nippon Soda Ltd. Submitted to WHO by Nippon Soda Co., Ltd. Tokyo,
Japan.
Souma, S. & Nishibe, T. (1983a) Delayed contact hypersensitivity
study of NA-73 in guinea pigs. Unpublished toxicology report 0097
(RD-8399) from Biomedical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983b) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report 0095 (RD-8398) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983c) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report 0096 (RD-8397) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983d) Delayed contact hypersensitivity
study of NA-73 10%WP in guinea pigs. Unpublished toxicology report
(RD-83117) from Bio-medical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983e) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report (RD-83116) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983f) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report (RD-83115) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1985) General pharmacology study of NA-73.
Unpublished toxicology report 0186 (RD-85104) from Environmental
Toxicology Laboratory, Nippon Soda Co., Ltd. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984a) Four week dietary range-finding study in dogs
with NA-73. Unpublished Report (RD-84105) from International Research
and Development Corporation, Michigan USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984b) One year dietary study of NA-73 in dogs.
Unpublished Report (RD-8433) from International Research and
Development Corporation, Michigan USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984c) Lifetime (24-month) dietary toxicity and
oncogenicity study of NA-73 in rats. Unpublished Report (RD-84106)
from International Research and Development Corporation, Michigan USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983a) Cumulative toxicity study of NA-73
in mice. Unpublished Report (RD-83100) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983b) Subchronic feeding study of NA-73
in rats. Unpublished Report (RD-83101) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
Toxicological studies
Acute toxicity
The LD50 and LC50 for hexythiazox, the 10% WP formulation and
certain metabolites, are given in Tables 1, 2 and 3, respectively.
No signs of toxicity were observed after acute oral and dermal
exposure to hexythiazox.
Short-term studies
Mice
Male and female B6C3F1 mice (10/sex/group) were fed diets
containing 0, 50, 300, 1800 or 10 800 ppm hexythiazox (purity 98.3%)
for 28 days. No compound-related effects were observed on mortality,
clinical signs, food consumption and food efficiency, haematology, nor
macroscopy. Male body weight gain was decreased at 1800 and 10 800
ppm hexythiazox. Total cholesterol levels decreased in male and
female mice at 10 800 ppm and in males also at 1800 ppm. Urine
specific gravity was increased in high-dose males. Relative liver
weight was increased in male and female mice at 1800 and 10 800 ppm.
At histopathology an increased incidence of swollen liver cells was
observed in high-dose males (also at 1800 ppm) and females. The NOAEL
in this study is 300 ppm, equal to 55.1 mg/kg bw (males) and 62.9
mg/kg bw (females) (Takaori & Nishibe, 1983a).
Rats
Groups of Fischer rats (20/sex/group) received a diet containing
0, 10, 70, 500 or 3500 ppm hexythiazox (98.3%) for 13 weeks.
Observations included clinical signs, food and water consumption,
haematology, biochemistry, cholinesterase activity, urinalysis,
macroscopy, organ weights and histopathology. Growth was depressed in
both sexes at 3500 ppm and in females also at 500 ppm. In high-dose
males Hb, PCV, RBC and MCV were decreased and platelet count was
increased. In high-dose females MCHC and MCH (also at 500 ppm) were
decreased. At 3500 ppm hexythiazox total cholesterol, albumin (in
males also at 500 ppm), protein and calcium were increased in both
sexes. ALP and ASAT were increased in high-dose females. Protein in
urine was increased in males at 3500 ppm. Relative liver (also at 500
ppm), kidney, adrenal and gonad weight were increased and spleen
weight decreased at 3500 ppm in both sexes. At 70 ppm liver weight was
still slightly increased in females. Female brain weight was increased
at 500 and 3500 ppm. At histopathology swelling of hepatocytes in the
central zone was observed in both sexes at the highest dose. Fatty
degeneration of the zona fasciculata of the adrenal cortex was
observed at dose levels of 500 ppm and greater. The NOAEL is 70 ppm,
equal to 8.1 mg/kg bw/day (males) and 5.3 mg/kg bw/day (females)
(Takaori & Nishibe, 1983b).
Table 1. Acute toxicity of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Mouse M&F oral 98.3% > 5000 Saika & Nishibe (1983a)
M&F s.c. 98.2% > 5000 Inoue (1987a)
M&F i.p. 98.2% > 5000 Inoue (1987b)
Rat M&F oral 98.3% > 5000 Saika & Nishibe (1983b)
M&F s.c. 98.2% > 5000 Inoue (1987c)
M&F i.p. 98.2% > 5000 Inoue (1985)
M&F inhl.a 98.3% > 2.0 Saika & Nishibe (1983c)
M&F dermal 98.3% > 5000 Saika & Nishibe (1983d)
Dog M&F oral 98.3% > 5000 Saika & Nishibe (1984)
a 4 hour exposure
Table 2. Acute toxicity of 10% wettable powder of hexythiazox
Species Sex Route Purity LD50 LC50 References
(mg/kg bw) (mg/l)
Rat M&F oral > 5000 Saika & Nishibe (1983e)
M&F inhal.a > 2.9 Saika & Nishibe (1983f)
M&F dermal > 5000 Saika & Nishibe (1983g)
a 4 hour exposure
Table 3. Acute toxicity of metabolites of hexythiazox in rats
Metabolite Sex Route Purity LD50 References
(mg/kg bw)
PT-1-2 M oral > 99% 2321 Saika & Nishibe (1985)
F 1079
PT-1-3 M oral > 99% 494 Saika & Nishibe (1985)
F 341
PT-1-4 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans-2)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(trans)
PT-1-8 M&F oral > 99% > 5000 Saika & Nishibe (1985)
(cis)
Dogs
In a range-finding study groups of Beagle dogs (2/sex/group) were
fed diets containing 0, 125, 500, 2000 or 8000 ppm hexythiazox (purity
97.7%) for 4 weeks. No effects were observed on clinical signs nor
mortality. Body weight gain was decreased in females and food
consumption in males at 8000 ppm. Relative liver weight was increased
in females at 2000 and 8000 ppm and slightly increased in high-dose
males (Spicer, 1984a).
Male and female Beagle dogs (4/sex/group) received a diet
containing 0, 100, 500 or 5000 ppm hexythiazox (purity 97.7%) for 12
months. Observations were made for clinical signs, body weight, food
consumption and food efficiency, ophthalmology, haematology,
biochemistry, cholinesterase activity and urinalysis. The weights of
10 organs/animal were recorded. Gross necropsy and histo-pathology
were carried out. ALP was increased in both sexes at 5000 ppm and
ALAT was increased in females at the same dose. Total protein was
decreased in males at 500 and 5000 ppm and in females at 5000 ppm.
Phosphorus was decreased in females 500 and 5000 ppm. Relative
adrenal weight was increased in high-dose dogs and relative liver
weight was increased in high-dose males. At histopathology
adrenocortical hypertrophy occurred in male and female dogs at 500 and
5000 ppm. Hepatocellular hypertrophy was observed in high-dose dogs.
The NOAEL in this study is 100 ppm, equal to 2.87 mg/kg (males) and
3.17 mg/kg (females) (Spicer, 1984b).
Long-term/carcinogenicity studies
Mice
Groups of B6C3F1 mice (50/sex/group) received a diet
containing 0, 40, 250 or 1500 ppm hexythiazox (purity 98.2%) for 104
weeks. An additional 30 mice (10/sex/group) served for the collection
of blood samples after 26, 52 and 79 weeks. Observations included
clinical signs, mortality, body weight, food consumption and food
efficiency, haematology, clinical chemistry, urinalysis, macroscopy,
organ weight (after 26, 52, 78 and 104 weeks) and histopathology
(after 52 and 104 weeks). Body weight gain and absolute body weights
were decreased at 250 and 1500 ppm in male mice only. During the
second half of the study body weight gain was also significantly
decreased at 40 ppm. Various red blood cell parameters were decreased
during the first 78 weeks at the highest dose. Relative liver weight
was increased in males and females at 1500 ppm during the whole study.
After 104 weeks relative weights of brain, heart, kidneys, adrenals
and testes were increased in male mice. Relative brain, kidneys,
adrenals and testes weight were increased in males at 250 ppm.
Histopathology of the liver after 52 weeks showed an increased
incidence of fatty degeneration and hypertrophy of the hepatocytes
with nuclear pleomorphism in males at 1500 ppm which was only
marginally increased at 250 ppm. At the end of the study the incidence
of hyperplastic nodes consisting of proliferating hepatocytes was
increased in both sexes at the highest dose (incidences (52+104 weeks
60 rats): 20%, 20%, 25% and 50% for males and 5%, 8%, 6% and 27% for
females at 0, 40, 250, and 1500 ppm, respectively). At the same dose
an increase in the incidence of hepatocellular adenomas or carcinomas
was also observed (incidences: adenomas: 20%, 25%, 20% and 33% for
males and 10%, 2%, 5% and 17% for females; carcinomas: 13%, 13%, 17%
and 13% for males and 0%, 5%, 5% and 8% for females both at 0, 40,
250, and 1500 ppm hexythiazox). In 3 males at 1500 ppm
haemangiopericytoma was observed in the liver. This incidence was
within the historical control range. At the lowest dose level of 40
ppm (equal to 6.7 in males and 8.4 in females) there was still a
decreased body weight gain (Inoue & Enomoto, 1984).
Because of the observed increase of liver tumours an additional
histological examination was performed on the livers of mice
(10/sex/group) sacrificed after 78 weeks. In high-dose females the
incidence of nuclear pleomorphism of the liver cells and fatty
degeneration were increased. The tumour incidences were not enhanced
(Enomoto, 1985).
Rats
Groups of Fischer 344 rats (50/sex/group) were fed diets
containing 0, 60, 430, or 3000 ppm hexythiazox (purity 99%) for 104
weeks. Additional groups of 10 rats/sex/group were treated at the same
dose levels and were used for interim sacrifice at 12 months. The
observations included clinical signs, body weight, food consumption,
ophthalmology, haematology, biochemistry, urinalysis, macroscopy,
organ weight and histopathology. At the end of the study the
incidence of internal abdominal masses and swollen testicles and
non-descended testicles was high in all males but was observed to a
greater degree at 430 and 3000 ppm hexythiazox. Mean body weight was
decreased in high dose males and females and slightly at 430 ppm. Food
consumption was increased in both sexes at 3000 ppm and in females at
430 ppm. ALP, ALAT, ASAT and potassium levels were decreased in
high-dose females. At the end of the study most relative organ weights
(including the liver) were increased in both sexes at 3000 ppm. At
430 ppm relative brain, heart, lung and spleen weights were still
increased in males. No treatment-related histopathological changes
were observed. The tumour incidence was not enhanced. The NOAEL in
this study is 60 ppm, equal to 3.2 mg/kg bw (males) and 4.02 mg/kg bw
(females) (Spicer, 1984c).
Reproduction studies
In a two-generation reproduction study (2 litters/generation)
groups of SPF Wistar rats (30/sex/group) were given diets containing
0, 60, 400 or 2400 ppm hexythiazox (purity 98.2%). Observations
included clinical signs, body weight, food consumption, food
efficiency, reproductive findings, organ weight and histopathology.
Five dams/group were sacrificed on day 21 of pregnancy for teratogenic
examinations.
Body weight and food consumption were decreased in high dose F0
parents. An increased number of delivered F1 pups was observed in
the second mating at 400 and 2400 ppm hexythiazox. At 400 and 2400
ppm the relative weights of liver, kidney, adrenals (only at 2400 ppm)
and testes (no dose relationship) were increased in F0 adults. A
tendency to an increased mean number of implantations and total
resorptions was observed in the F0 generation at 400 and 2400 ppm.
At histopathology a higher incidence of proteinaceous casts in the
kidneys was observed in F0 male rats at 2400 ppm. The number of live
born F1b pups was increased at 400 and 2400 ppm and pup weight was
decreased from day 4 to 28 of lactation at these dose levels. A delay
in hairgrowth of abdomen was observed in pups at all treated groups of
the first generation. In F1 parents, body weight was decreased at
2400 ppm and in males occasionally also at 400 ppm. Food consumption
was decreased at the highest dose. The mean number of implants,
implants/corpora lutea and number of live fetuses were increased in
the 2400 ppm F1 group. Relative weights of brain (males only),
liver, kidneys, adrenals (males only) and ovaries were increased in
high dose F1 parents. F2b pup weight was decreased at 2400 ppm
hexythiazox. A delay in abdominal hair growth and eye-opening were
observed in F2b pups at 2400 ppm. Body weight and food consumption
(females only) of F1 adults were decreased at the highest dose. F1
adults showed an increased relative weight of ovaries, adrenal, liver
and kidney (males only) and showed a decreased spleen weight at 2400
ppm. Liver and kidney weight were also increased in males at 400 ppm.
An increase in the appearance of proteinaceous casts was observed in
the kidneys of high-dose males. Malformations were not observed. The
NOAEL in this study is 60 ppm (equal to a range between 4.22 and 5.34
mg/kg bw, depending on sex and generation) (Okugi & Enomoto, 1984).
Special studies on embryotoxicity and/or teratogenicity
Rats
Pregnant Sprague-Dawley rats (24/group) received by gavage 0,
240, 720 or 2160 mg hexythiazox (purity 98.3%)/kg bw from day 7
through 17 of gestation. The rats were sacrificed on day 21.
Observations were made for body weight, food and water consumption,
number of implantations, number of live and dead fetuses, number of
early and late resorptions and number of corpora lutea. The fetuses
were weighed and examined for external, visceral and skeletal
abnormalities. Female body weight gain and occasionally food
consumption were decreased at 720 and 2160 mg/kg bw/day. Relative
ovarian weight was increased at the two highest dose levels. The
ossification of the metatarsal bones was significantly delayed at 720
and 2160 mg/kg bw/day. The NOAEL for maternal and embryotoxicity was
240 mg/kg bw/day. There was no evidence for hexythiazox-induced
irreversible structural effects (Gotoh & Nishibe, 1983).
Rabbits
Four groups of 15 pregnant New Zealand white rabbits received by
gavage 0, 120, 360, or 1080 mg hexythiazox (purity 97.7%)/kg from day
6 to 18 of gestation. All dams were sacrificed on day 28 of
gestation. No significant changes were observed in mortality,
maternal body weight and food consumption, uterus weight, number and
location of viable fetuses, early and late resorptions, number of
total implantations nor corpora lutea and organ weight. Two dams died,
1 at 120 mg/kg bw/day and 1 at 360 mg/kg bw/day. A slightly greater
number of fetuses with skeletal variations (overlapping of the
vertebral arch, asymmetry of the sternebrae) was observed at 1080
mg/kg bw/day. The NOAEL in this study is 360 mg/kg bw/day. No
irreversible structural effects were observed (Gotoh & Nihsibe, 1984).
Special studies on mutagenicity
Hexythiazox was negative in various mutagenicity assays (see
Table 1). Ames tests were conducted with 8 plant metabolites (some
also occurring in the rat): PT-1-2. PT-1-3, PT-1-4( trans-2),
PT-1-5(1), PT-1-6( trans-2), PT-1-8( cis), (PT-1-8( trans) and
PT-1-9. All metabolites gave negative results in the tests using
S. typhimurium (TA1535, TA100, TA1538, TA98 and TA1537) as well as
Escherichia coli (WP2 uvra) (Sasaki & Nishibe, 1985).
Special studies on pharmacology
Mice
Doses > 1000 mg hexythiazox (purity 98.3%) given
intraperitoneally to 3 male S1c:ddY mice delayed the sleeping time
induced by sodium pentobarbital. Effects were observed as soon as 15
minutes after hexythiazox administration. Hexythiazox also prolonged
the time at which convulsions induced by pentetrazole or strychnine
began. Intestinal motility and gastric secretion in mice were not
affected by the administration of up to 150 mg hexythiazox iv nor up
to 3000 mg/kg (oral), respectively (Souma & Nishibe, 1985).
Rats
Blood coagulation time in S1c:Wistar rats was decreased after
administration of 3000 mg hexythiazox in saline, but haemolysis was
not observed. I.p. administration of doses as high as 2500 mg
hexythiazox/kg had no effect on rat body temperature nor on gastric
secretion. No effect on the tension of the muscle twitch induced by
the indirect stimulation was observed after i.v. injection of 50 or
100 mg hexythiazox/kg (Souma & Nishibe, 1985).
Table 4. Results of mutagenicity assays on hexythiazox
Test system Test object Concentration Purity (%) Results References
In vitro
Ames testa,b S. typhimurium 100-6400 µg/plate 97.7 Negative Inoue (1983a)
TA 100, TA98, in DMSO
TA1535, TA1537,
TA1538
E coli, WP2 uvra
Yeast testa,b S. cerevisiae 312-10 000 µg/ml 98.4 Negative Jagannath (1984)
(reverse mutation) S138, S211 in DMSO
(mitotic gene S. cerevisiae 1-10 000 µg/plate 98.4 Negative
conversion) D4
V79/HGPRTa,b Chinese hamster 9.38-150 µg/ml 98.6 Negative Seeberg (1986)
mutation assay V79 cells in DMSO
Chromosome Chinese hamster 1.5-50 µg/ml 98.4 Negative Galloway (1984)
aberration ovary cells
assaya
Rec-assay B. subtilis 400-3200 µg/plate 97.7 Negative Inoue (1983b)
H17(rec+) in DMSO
M45(rec-)
Unscheduled DNA Rat hepatocytes 2.5-100 µg/ml 98.4 Negative Cifone (1985)
synthesis (250 µg/ml lethal)
Table 4 (contd).
Test system Test object Concentration Purity (%) Results References
In vivo
Cytogenic testa,b Chinese hamster 1000, 2000 or 4000 98.6 Negative Mosesso (1986)
bone marrow mg/kg p.o.
Micronucleus test Male and female 0.1, 0.5 and 1.0 98.4 Negative Ivett (1984)
CD-1 bone marrow g/kg p.o.
cells
a without metabolic activation
b with metabolic activation
Rabbits
Hypotension and hypoventilation were observed after the iv
injection of 50 mg hexythiazox/kg once weekly (Souma & Nishibe, 1985).
Guinea-pig
High concentrations of hexythiazox inhibited the contraction of
isolated ileum induced by acetylcholine, histamine and barium chloride
(Souma & Nishibe, 1985).
Special studies on sensitization
Hexythiazox (purity 98.3%) as well as a 10% WP formulation did
not exhibit a sensitizing effect in the maximization test (Magnusson
& Kligman) in female Hartley guinea-pigs (Souma & Nishibe, 1983a;
1983d).
Special studies on skin and eye irritation
A dose of 0.5 g hexythiazox (purity 98.3%) and a 10% WP
formulation moistened with water was applied under occlusive
conditions to the clipped back skin of 6 male New Zealand White
rabbits for 4 hours. No skin irritation was observed up to 72 hours
after application (Souma & Nishibe, 1983b; 1983e).
Applications of 0.1 g hexythiazox (purity 98.3%) and a 10% WP
formulation into the eyes of 6 male New Zealand White rabbits caused
slight irritation (redness of the conjunctivae). No irritation
effects were observed 48 hours post-application (Souma & Nishibe,
1983c; 1983f).
Observation in humans
Health examinations (twice a year) were performed on a group of
16 to 26 workers in a Japanese manufacturing plant. The health
examination consisted of analysis for gamma-GTP (once a year), Hb,
haematocrit, erythrocytes and urinalysis (protein, glucose and
urobilinogen). No adverse effects were observed on the examined
parameters after three years of continuous exposure to hexythiazox
(Motogi, 1987).
COMMENTS
After oral administration to rats, hexythiazox was eliminated
rapidly, two-thirds via the faeces and one-third via the urine. Low
levels were recovered in the tissues and organs, with the highest
concentrations in the fat, liver, adrenals and in the gastrointestinal
tract and its contents. In the faeces about 20% was excreted as the
parent compound. Many metabolites were found in the urine and faeces,
although only 10% of those in the urine and 50% of those in the faeces
could be identified. Metabolism occurs via oxidation, and finally
cleavage, of the cyclohexane ring.
The compound shows low acute toxicity in the species examined.
WHO has classified hexythiazox as unlikely to present acute hazard in
normal use.
Short-term administration of hexythiazox to rats and dogs
revealed the liver and the adrenals as target organs. In a 90-day
study in rats the NOAEL was 70 ppm, equal to 4.9 and 5.3 mg/kg bw/day
for males and females respectively. In a one-year study in dogs the
NOAEL was 100 ppm, equal to 2.9 mg/kg bw/day in males and 3.2 mg/kg
bw/day in females.
In a 2-year feeding study in mice at dietary concentrations of 0,
40, 250, or 1500 ppm there was an increased incidence of liver nodules
in both sexes at 1500 ppm. The incidences of liver adenomas and
hepatocarcinomas were increased in females at 1500 ppm. Three
haemangiopericytomas in the liver were observed in males at 1500 ppm.
These were subsequently re-evaluated as hepatoblastomas. At the
lowest concentration of 40 ppm, equal to 6.7 and 8.4 mg/kg bw/day in
males and females respectively, reduced body-weight gain in male
animals was observed. A NOAEL was not established.
In a long-term carcinogenicity study in rats at dietary
concentrations of 0, 60, 430, or 3000 ppm, there were no
treatment-related increases in neoplasias. There was a reduction in
body-weight gain and an increase in some organ weights at 430 ppm.
The NOAEL was 60 ppm, equal to 3.2 and 4.2 mg/kg bw/day for males and
females respectively.
In a 2-generation, 2-litters-per-generation reproduction study in
rats at 0, 60, 400, or 2400 ppm, no adverse effects on reproduction
were found. The NOAEL was 60 ppm, equivalent to 3 mg/kg bw/day, based
on increased liver and kidney weights at 400 ppm.
Maternal toxicity (reduced weight gains) and embryotoxicity
(delayed development) were observed in a teratogenicity study in rats
at doses of 0, 240, 720, or 2160 mg/kg bw/day. The NOAEL was 240
mg/kg bw/day. In a teratogenicity study in rabbits at doses of 0,
120, 360 or 1080 mg/kg bw/day, slight embryotoxicity was observed at
the highest dose. The NOAEL was 360 mg/kg bw/day. No teratogenic
effects were observed in either species.
After reviewing all available in vitro and in vivo short-term
tests on hexythiazox it was concluded that there was no evidence of
genotoxicity.
An ADI was established which was based on NOAELs in the 2-year
feeding and reproduction studies in rats and a 1-year study in dogs,
using a 100-fold safety factor.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 60 ppm in the diet, equal to 3.2 mg/kg bw/day
(2-year study)
Rat: 60 ppm in the diet, equivalent to 3 mg/kg bw/day
(reproduction study)
Dog: 100 ppm in the diet, equal to 2.9 mg/kg bw/day
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Studies which will provide information valuable in the
continued evaluation of the compound
Observations in humans.
REFERENCES
Cifone, M.A. (1985) Evaluation of NA-73 technical lot no. SCF-05 in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished second amended report 20991 (RD-8556) from Litton
Bionetics, Inc. Kensington, Maryland, USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Enomoto, M. (1985) Supplement to the chronic feeding and oncogenicity
studies in mice with NA-73. Histological findings of the liver of mice
sacrificed at 78 weeks. Unpublished Report 527 (RD-8534) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Galloway, S.M. (1984) Mutagenic evaluation of NA-73 Technical lot no.
SCF-05. in an in vitro cytogenetic assay measuring chromosome
aberration frequencies in Chinese hamster ovary (CHO) cells.
Unpublished amended report 20990 (RD-8435N) from Litton Bionetics,
Inc. Kensington, Maryland 20895, USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1983) Teratogenicity study of NA-73 in rats.
Unpublished toxicology report 0118 (RD-83104) from Biomedical research
department of Nisso Institute for Life Science. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Gotoh, K. & Nishibe, T. (1984) Teratogenicity study of NA-73 in
rabbits. Unpublished toxicology report 0126 (RD-8454) from Biomedical
Research Department of Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1983a) NA-73: Mutagenicity test in bacteria. Unpublished
Report 364 (RD-83107) from Biosafety Research Center, Foods, Drugs and
Pesticides (AN-PYO Center). Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Inoue, H. (1983b) NA-73: Rec-assay with Bacillus subtilis strains
H 17 (rec+) and M 45-). Unpublished Report 363 (RD-83106) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center). Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1985) Acute intraperitoneal toxicity study in rats with
NA-73. Unpublished Report 623 (RD-8582) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987a) Acute subcutaneous toxicity study in mice with
NA-73. Unpublished Report 989 (RD-8759) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987b) Acute intraperitoneal toxicity study in mice with
NA-73. Unpublished Report 990 (RD-8758) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H. (1987c) Acute subcutaneous toxicity study in rats with
NA-73. Unpublished Report 991 (RD-8757) from Biosafety Research
Center, Foods, Drugs and Pesticides (AN-PYO Center), Japan. Submitted
to WHO by Nippon Soda Ltd. Tokyo, Japan.
Inoue, H & Enomoto, M. (1984) Chronic feeding and oncogenicity
studies in mice with NA-73. Unpublished Report 483 (RD-84107) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Ivett, J.L. (1984) Mutagenicity evaluation of NA-73 technical in the
in vivo mouse micronucleus assay. Unpublished Report 20996
(RD-8436N) from Litton Bionetics, Inc. Kensington, Maryland, USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Jagannath, D.R. (1984) Mutagenicity evaluation of NA-73 in the mitotic
gene conversion and reverse mutation induction in yeast strains D4,
S138 and S211 plate test. Litton Bionetics, Inc. Submitted to WHO by
Nippon Soda Co. Ltd., Tokyo, Japan. Unpublished Report RD-8434N.
Mosesso, P. (1986) Chinese hamster bone marrow metaphase analysis
( in vivo cytogenetics). Test substance: NA 73. Unpublished LSR-RTC
Report 063015-M-04086 (RD-8696) from Life Science Research Roma
Toxicology Centre. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Motogi, S. (1987) Human handling experiences from plant employees in
hexythiazox production. Unpublished Report (RD-8749) from
Environmental Control and Safety Department, Nihongi Plant, Japan.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Okugi, M. & Enomoto, M. (1984) NA-73: Two-generation reproduction
study in rats (experiment 221). Unpublished Report 453 (RD-84108) from
Biosafety Research Center, Foods, Drugs and Pesticides (AN-PYO
Center), Japan. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983a) Acute oral toxicity study of NA-73 in
mice. Unpublished toxicology report 0107 (RD-8393) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983b) Acute oral toxicity study of NA-73 in
rats. Unpublished toxicology report 0092 (RD-8394) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983c) Acute inhalation toxicity study of
NA-73 in rats. Unpublished toxicology report 0094 (RD-8396) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983d) Acute dermal toxicity study of NA-73
in rats. Unpublished toxicology report 0093 (RD-8395) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983e) Acute oral toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0123 (RD-83112) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983f) Acute inhalation toxicity study of
NA-73 10% WP in rats. Unpublished toxicology report 0121 (RD-83114)
from Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1983g) Acute dermal toxicity study of NA-73
10% WP in rats. Unpublished toxicology report 0119 (RD-83113) from
Biomedical Research Department, Nisso institute for life science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1984) Acute oral toxicity study of NA-73 in
dogs. Unpublished toxicology report 0125 (RD-8428) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Saika, O. & Nishibe, T. (1985) Acute oral toxicity study of main
metabolites of NA-73 in Rats. Unpublished report 0181 (RD-8570) from
Toxicology Group, Nippon Soda Ltd. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Sasaki, T. & Nishibe, T. (1985) Reverse mutation study of main
metabolites of NA-73. Unpublished Report 0185 (RD-8571) from
Toxicology Group, Soda Ltd. Submitted to WHO by Nippon Soda Ltd.
Tokyo, Japan.
Seeberg, A.H. (1986) Gene mutation in Chinese hamster V79 cells test
substance: NA 73. Unpublished Report 063014-M-03986 (RD-8695) from
Life Science Research Roma Toxicology Centre. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Soeda, Y. (1985) Metabolism of NA-73 in rats (group B). Unpublished
Report EC-9 (RD-8520N) from Environmental Toxicology Laboratory of
Nippon Soda Ltd. Submitted to WHO by Nippon Soda Co., Ltd. Tokyo,
Japan.
Souma, S. & Nishibe, T. (1983a) Delayed contact hypersensitivity
study of NA-73 in guinea pigs. Unpublished toxicology report 0097
(RD-8399) from Biomedical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983b) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report 0095 (RD-8398) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983c) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report 0096 (RD-8397) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983d) Delayed contact hypersensitivity
study of NA-73 10%WP in guinea pigs. Unpublished toxicology report
(RD-83117) from Bio-medical Research Department, Nisso Institute for
Life Science. Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983e) Primary dermal irritation study of
NA-73 in rabbits. Unpublished toxicology report (RD-83116) from
Biomedical Research Department, Nisso Institute for Life Science.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1983f) Primary eye irritation study of NA-73
in rabbits. Unpublished toxicology report (RD-83115) from Biomedical
Research Department, Nisso Institute for Life Science. Submitted to
WHO by Nippon Soda Ltd. Tokyo, Japan.
Souma, S. & Nishibe, T. (1985) General pharmacology study of NA-73.
Unpublished toxicology report 0186 (RD-85104) from Environmental
Toxicology Laboratory, Nippon Soda Co., Ltd. Submitted to WHO by
Nippon Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984a) Four week dietary range-finding study in dogs
with NA-73. Unpublished Report (RD-84105) from International Research
and Development Corporation, Michigan USA. Submitted to WHO by Nippon
Soda Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984b) One year dietary study of NA-73 in dogs.
Unpublished Report (RD-8433) from International Research and
Development Corporation, Michigan USA. Submitted to WHO by Nippon Soda
Ltd. Tokyo, Japan.
Spicer, E.J.F. (1984c) Lifetime (24-month) dietary toxicity and
oncogenicity study of NA-73 in rats. Unpublished Report (RD-84106)
from International Research and Development Corporation, Michigan USA.
Submitted to WHO by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983a) Cumulative toxicity study of NA-73
in mice. Unpublished Report (RD-83100) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
Takaori, H. & Nishibe, T. (1983b) Subchronic feeding study of NA-73
in rats. Unpublished Report (RD-83101) from Biomedical Research
Department, Nisso Institute for Life Science, Japan. Submitted to WHO
by Nippon Soda Ltd. Tokyo, Japan.
