
TEFLUBENZURON
First draft prepared by
J. Taylor and M. Watson,
Pesticides Safety Directorate,
Ministry of Agriculture, Fisheries and Food,
York, United Kingdom
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Embryotoxicity and teratogenicity
Genotoxicity
Special studies
Skin and eye irritation and skin sensitization
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Teflubenzuron is an insect growth regulator belonging to the
benzoyl urea group of compounds. It acts at the developmental stages
of insect pests, primarily via ingestion and by interfering with
chitin synthesis and the moulting process. It has an ovicidal effect
in some insects. Teflubenzuron was reviewed for the first time by
the present Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution and excretion
Rats
Groups of nine male and nine female Wistar rats received
teflubenzuron uniformly labelled with 14C in the aniline ring
(radiochemical purity, > 99%) at a dose of 25 mg/kg bw in dimethyl
sulfoxide, daily for seven consecutive days by gavage. Radioactive
residues in urine and faeces were assessed in four rats of each sex
during the dosing period and for eight days after the last dose. In
the other five animals of each sex, radioactivity was analysed in
organs and tissues 1, 6, 24, 48 and 120 h after dosing for seven
days. Radiolabel was rapidly excreted during and after the seven-day
dosing period, predominantly via the faeces. The mean levels in
urine and faeces over the treatment and eight-day depuration periods
showed no significant sex differences; 2-3% of the total radiolabel
was found in urine and 90-93% in faeces. At the end of the
depuration period, only 0.1% was found in the carcass. The total
radioactive residue recovered was 93-95% of the administered dose.
Assessment of radiolabel levels in rats between 1 and 120 h after
treatment showed no residue exceeding 0.5% of the administered dose
in any of the organs or tissues analysed (including fat) by 48 h
after the last treatment, with the exception of the liver
(0.1-0.2%). By 120 h, the level of radiolabel was less than 0.01% of
the total dose administered in virtually all organs and tissues
analysed, except liver, where the level was 0.05%.
14C-Teflubenzuron labelled in the aniline ring was thus rapidly
excreted, predominantly in the faeces, with no evidence of
significant bioaccumulation in organs or tissues (Schlüter, 1984).
Groups of five male and five female Wistar rats received
uniformly 14C-aniline ring-labelled teflubenzuron (radiochemical
purity, > 97%) in aqueous solutions of 1% Tylose and 1% Tween 80 by
gavage, according to different dosing regimes. Rats in the first
group were given one dose of 25 mg/kg bw, those in the second were
given one dose of 750 mg/kg bw, and those in the third group
received 14 daily doses of unlabelled teflubenzuron at 25 mg/kg bw
per day followed by the same dose of radiolabelled compound. Urine
and faeces were collected for eight days after treatment, after
which time the animals were killed. Excretion patterns were similar
in males and females at each dosing regimen. Most of the radiolabel
(91-95% of the total dose) was excreted in the faeces, predominantly
within 24 h, and only small quantities (0.2-0.9%) were found in
urine during eight days after treatment. Low levels of radiolabel
were detected in carcasses of animals (low-dose groups, 0.04-0.08%;
high-dose group, < 0.01% of the total dose). The total recoveries
of radioactive residue were 91-96%.
A preliminary experiment in one male and one female showed that
no radioactivity was present over 24 h in expired air of animals
treated orally with 25 mg/kg bw.
In another investigation, groups of five males and five females
received single oral doses of 25 or 750 mg/kg bw
14C-teflubenzuron, and blood samples were taken up to 168 h after
dosing. Plasma levels in animals given the low dose reached a
plateau after 1-8 h, at 0.38-0.46 µg/ml in males and 0.22-0.25 µg/ml
in females; the levels declined after 24 h and were < 0.01 µg/ml in
animals of each sex by 168 h. Blood levels of the active ingredient
were similar to the plasma levels in these animals. At 750 mg/kg bw,
the concentrations of radiolabel in plasma increased only slightly
(by four- to sixfold) in animals of each sex in comparison with the
30-fold increase in dose. In males, a mean peak concentration of
3.27 µg/ml was noted at 24 h; in females, the highest concentrations
were observed between 20 min and 8 h (0.98-1.43 µg/ml). Plasma
concentrations declined from the second day after dosing, reaching
0.06 µg/ml after seven days in males and 0.08 µg/ml in females. The
blood levels of the compound were slightly lower than the plasma
levels in these animals. The plasma concentrations observed were
likely to be a consequence of differences in overall absorption
rates at the low and high doses (Schlüter, 1986).
Groups of three male and three female Wistar rats received a
bile-duct cannula and then a single dose by gavage of 25 or 750
mg/kg bw 14C-aniline ring-labelled teflubenzuron (radiochemical
purity, > 98%) in an aqueous solution containing 1% Tylose and 1%
Tween 80. Animals given 25 mg/kg bw excreted 1.4% of the
administered dose in urine, 16% in bile and 46% in faeces between 0
and 48 h; about 0.4% of the dose was measured in liver, 23% in the
gastrointestinal tract and 1.6% in the remaining carcass at 48 h. A
total of 87-90% of the radioactivity was recovered. At 750 mg/kg bw,
0.4% of the administered dose was excreted in urine, 1.9% in bile
and 65% in faeces between 0 and 48 h; about 0.06% of the dose was
measured in liver, 19% in the gastrointestinal tract and 1.2% in the
remaining carcass at 48 h. A total of 86-88% of the radiolabel was
recovered. There was no significant sex difference in the excretion
pattern. The bile was a major route of excretion at the low dose but
a minor route at the high dose (Hawkins & Mayo, 1988).
The concentration of teflubenzuron in the plasma of groups of
five male and five female Wistar rats was determined after a 28-day
exposure to diets containing, 0, 500, 1000, 2000, 4000, 8000, 16 000
or 32 000 ppm teflubenzuron (purity, 92.4%), equivalent to 0 and
about 40-2500 mg/kg bw per day. Plasma concentrations were assayed
on day 28 by high-performance liquid chromatography (HPLC). The
concentrations were essentially constant for male rats receiving
dietary concentrations of > 1000 ppm and in females receiving
> 2000 ppm, at mean plateau levels of 0.38 µg/ml in males and
0.42 µg/ml in females. The results suggest that the absorption of
teflubenzuron from the diet by rats is saturated at 1000-2000 ppm.
There were no deaths and no overt signs of toxicity or effects on
food consumption, body weight, gross pathological signs or liver
weights that could be attributed to treatment (Ellgehausen et al.,
1986).
Plasma concentrations of teflubenzuron were determined at 4,
26, 52 and 104 weeks in Wistar rats fed diets containing 2500 or 10
000 ppm for 111 weeks. Time-related increases in plasma
concentrations were observed. The concentrations in males (0.18-0.79
µg/ml at 2500 ppm and 0.23-0.70 µg/ml at 10 000 ppm) showed no
evidence of a plateau between the two dose levels at weeks 26 and
52, although similar plasma concentrations were noted at weeks 4 and
104. In females, a plateau was seen between the two dose levels; the
concentrations were 0.18-0.60 µg/ml at 2500 ppm and 0.14-0.43 µg/ml
at 10 000 ppm (Tennekes et al., 1989).
Chickens
Laying hens were administered 14C-teflubenzuron orally twice
daily at a dose of 1.25 mg/kg bw per day for 7.5 days. The
administered dose was recovered rapidly and almost completely in
excreta. The residues of radioactivity in tissues 8 h after the last
dose were low; the highest levels (expressed as equivalents) were
found in bile (about 2.6 µg/g), liver (0.33 µg/g), fat (about 1.0
µg/g) and skin (0.45 µg/g). Minimal amounts of radiolabel were
eliminated in eggs, with the highest levels in yolk. Radioactivity
in the plasma reached a plateau (at 0.06-0.10 µg/ml) by day 4 of
treatment. The half-life of elimination of radioactive residue from
plasma was about two days (Cameron et al., 1987b).
Goats
14C-Aniline ring-labelled teflubenzuron was administered
orally to two lactating goats twice daily for 7.5 days to give a
total dose of 1 mg/kg bw per day. The animals were killed 8 h after
the final dose. The main route of elimination of radiolabel was via
the faeces, which, with the intestinal contents, accounted for about
99% of the total dose. Urinary excretion represented < 1% of the
administered dose. Radiolabel was found in bile at an equivalent
concentration of 1.3 µg/ml. Low concentrations of radiolabel were
found in plasma (with a plateau reached at day 4) and milk (day 5);
the highest equivalent concentrations were 0.008-0.010 µg/ml in
plasma and 0.010-0.015 µg/ml in milk. Low levels of radiolabel were
found in tissues; the highest were in liver (0-14% of the total
dose) and lung (0.02%) (Cameron et al., 1987a).
(b) Biotransformation
Rats
The metabolism of teflubenzuron was assessed in Wistar rats
that had been dosed orally with 25 mg/kg bw 14C-aniline
ring-labelled compound for seven consecutive days in the experiment
described above (Schlüter, 1984). The faeces was the major route of
elimination of radiolabel (about 90%). Metabolites in faeces were
identified by thin-layer chromatography (TLC). Most of the
radioactive residue in the faeces (70-75% of the administered dose)
consisted of unchanged teflubenzuron; the remainder consisted of at
least 15 unknown metabolites, none of which represented as much as
1% of the administered dose. Characterization of radioactive
residues in the urine, representing about 2.5% of the administered
dose, indicated the presence of three metabolites, each representing
< 1% of the dose. Two, which were structural isomers, were
identified by TLC and mass spectroscopy as hydroxylated products of
teflubenzuron (metabolites I and II, see Figure 1). The third
product, identified by mass spectroscopy, was formed by
dehalogenation of a fluoride atom with substitution by a hydroxide
group (metabolite III, see Figure 1). The position of the
substitution of the fluorine atom in the aniline ring could not be
established, as insufficient quantities of the metabolite were
available for nuclear magnetic resonance spectroscopy. There was no
significant difference between males and females in the metabolism
of teflubenzuron (Schlüter, 1985).
The biotransformation of teflubenzuron was investigated in the
urine and faeces of Wistar rats that had been given a single oral
dose of 25 or 750 mg/bw 14C-labelled compound or single doses of
25 mg/kg bw of unlabelled compound for 14 consecutive days followed
by a single dose of labelled compound. Most of the faecal
radioactive residue was found by TLC, HPLC and ultraviolet and mass
spectroscopy to be unchanged compound. Trace amounts of diverse,
mostly polar compounds were noted in each treatment group. One of
these compounds was identified as 3,5-dichloro-2,4-difluorophenyl
urea (metabolite IV, see Figure 1). TLC indicated that the low
levels of radiolabel in urine consisted mainly of very polar
compounds. Traces of the active ingredient were shown by HPLC and
ultraviolet spectroscopy to be present in urine, but this may have
been due to contamination by small particles of faeces. TLC and HPLC
of urine of animals treated at 750 mg/kg bw revealed the presence of
minor amounts of 3,5-dichloro-2,4-difluorophenyl urea (metabolite
IV). There were no significant differences in metabolism between
males and females or between animals that had and had not been
pre-treated with unlabelled teflubenzuron (Schlüter, 1986).
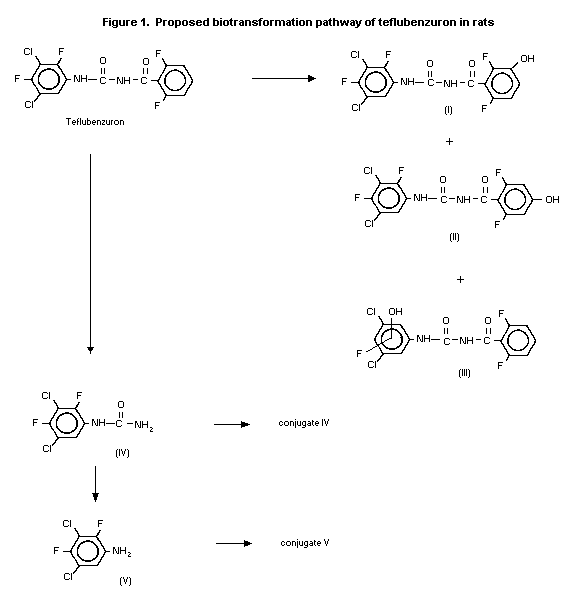 The nature of the excretion products in faecal extracts, bile
and urine from rats with bile cannulas that were given 25 or 750
mg/kg bw 14C-aniline ring-labelled teflubenzuron orally was
investigated by TLC. Most of the radiolabel extracted from 0-48-h
faeces of treated animals of each sex co-chromatographed with
teflubenzuron. In 0-48-h bile, most of the radiolabel was in
unidentified polar material. A minor biliary metabolite
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV, see Figure 1), and another co-chromatographed with
teflubenzuron. Hydrolytic treatment of 0-48-h bile extracts
indicated the presence of conjugates of 3,5-dichloro-2,4-
difluorophenyl urea (conjugate IV, see Figure 1), the corresponding
substituted aniline (conjugate V) and the meta-hydroxybenzoyl
derivative of teflubenzuron (metabolite I). The major radioactive
component in 0-48-h urine extracts from animals at 25 mg/kg bw was
unidentified polar material. Three minor urinary metabolites
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV), its corresponding substituted aniline metabolite
(metabolite V) and the meta-hydroxybenzoyl derivative (metabolite
I) of teflubenzuron (Hawkins & Mayo, 1988).
Chickens
Laying hens that had been administered a total of 1.25 mg/kg bw
per day of 14C-teflubenzuron by oral doses twice daily for 7.5
days were shown by TLC and HPLC to excrete mainly the parent
compound. Radiolabel in bile was in the form of a very polar
compound, which on treatment with ß-glucuronidase yielded a compound
with similar chromatographic characteristics to the
meta-hydroxybenzoyl derivative (metabolite I, see Figure 1) of
teflubenzuron (22% of radioactivity in bile). A compound with
identical HPLC retention characteristics to teflubenzuron was found
in fat, liver, plasma and egg yolk (Cameron et al., 1987b).
Further investigations of metabolites in chickens by mass
spectrometry showed that teflubenzuron was the major component of
excreta. Two components of liver and kidney extracts were observed
by TLC and HPLC; one had characteristics identical to those of
teflubenzuron, but the other could not be identified. A third
component of kidney extracts was shown to be 3,5-dichloro-2,4-
difluorophenyl urea (metabolite IV, see Figure 1). Further evidence
was provided for the presence of teflubenzuron in egg yolk and fat
(Cameron et al., 1988).
Goats
In lactating goats given oral doses twice daily to give a total
of 1 mg/kg bw per day 14C-teflubenzuron over 7.5 days, the major
faecal component was shown by TLC to be unchanged teflubenzon. About
41% of the biliary radiolabel had similar characteristics on HPLC
and TLC to the meta-hydroxybenzoyl derivative (metabolite I, see
Figure 1) of teflubenzuron after treatment with ß-glucuronidase. No
unchanged teflubenzuron was found in bile (Cameron et al., 1987a).
Mass spectrometry showed conclusively that the major faecal
component was teflubenzuron. In goat liver, the major radioactive
component was a polar compound, which was not considered to be a
conjugate since it was unaffected by treatment with deconjugating
enzymes. Traces of a metabolite that co-chromatographed with the
meta-hydroxybenzoyl derivative (metabolite I) of teflubenzuron
were found in liver extracts (Cameron et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of teflubenzuron
(purity, 96.5-97.5%) are summarized in Table 1. After oral
treatment, rats and mice showed only slight signs of toxicity,
including ruffled fur, dyspnoea, sedation and hunched posture, which
had resolved in all cases by 48-72 h. After intraperitoneal
administration, similar signs of toxicity were observed, which were
generally more severe and longer lasting. Deaths unrelated to
treatment were seen at 300 mg/kg bw, the lowest dose tested. There
was no evidence of toxicity after dermal treatment at 2000 mg/kg bw.
Slight dyspnoea and ruffled fur were the only findings after
inhalation, and these had resolved within 24 h. Teflubenzuron had no
clear effect on gross pathological findings in these studies.
Table 1. Acute toxicity of teflubenzuron in male and female rodents
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Mouse Oral > 5000 Ullman, 1983b
Rat Oral > 5000 Ullman et al., 1988
Rat Oral > 5000 Ullman, 1983a
Rat Dermal > 2000 Ullman, 1983c
Rat Intraperitonea] > 2000 Ullman, 1983d
Rat Inhalation > 5038 Ullman, 1983e
(b) Short-term toxicity
Mice
Groups of 12 male and 12 female CD-1 mice were administered
teflubenzuron (purity unspecified) at 0, 100, 1000 or 10 000 ppm in
the diet for 13 weeks, equal to 12, 115 or 1210 mg/kg bw per day in
males and 14, 143 or 1450 mg/kg bw per day in females. (The
techniques used to investigate biological parameters were not
specified.) Treatment appeared to have no effect on clinical signs
of toxicity, deaths, body-weight gain, food consumption or the
results of ophthalmoscopy, urinalysis and haematology. Clinical
chemical investigations revealed increased alanine aminotransferase
activity (in animals of each sex) and a raised cholesterol level (in
females) at 1000 and 10 000 ppm, and increased alkaline phosphatase
activity in females at 10 000 ppm. Necropsy revealed liver
enlargement in animals of each sex and dark-coloured livers in males
at 1000 and 10 000 ppm. Liver weights were increased in animals of
each sex at the middle and high doses. Histopathology revealed
centrilobular hepatocellular swelling in animals of each sex and
fatty change in males at 1000 and 10 000 ppm and microgranuloma in
males at 10 000 ppm. The NOAEL was 100 ppm, equal to 12 mg/kg bw per
day (Takahashi et al., 1987).
Rats
In a 28-day range-finding study, groups of five male and five
female Wistar rats were administered teflubenzuron (purity not
specified) in the diet at levels of 0, 100, 1000 or 10 000 ppm.
There were no deaths or signs of toxicity and no significant effects
on body-weight gain. Food consumption of males at the highest dose
level was slightly reduced. Haematology and urinalysis showed no
treatment-related findings. Clinical chemical analyses revealed
treatment-related increases in the activities of serum aspartate and
alanine aminotransferase in animals of each sex and of lactate
dehydrogenase in females at 1000 and 10 000 ppm. Gross pathology
revealed no treatment-related lesions. Histopathology and organ
weight measurements were not performed. The NOAEL was 100 ppm, equal
to 12 mg/kg bw per day (Suter, 1983).
Groups of 10 male and 10 female Wistar rats were administered
teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000 ppm in the
diet for 13 weeks. Additional groups of five male and five female
rats in the control and highest-dose groups were maintained on
control diet for a further four weeks after the 13-week treatment
phase to determine the reversibility of any findings. There were no
deaths and no treatment-related signs of toxicity. Body weights and
food consumption were not affected, and urinalysis, ophthalmoscopy
and haematology did not reveal any treatment-related findings.
Clinical chemical analyses showed increased activities of ornithine
carbamoyl transferase and alanine and aspartate aminotransferases in
animals of each sex at 1000 and 10 000 ppm at six weeks; males had
more significant increases and also an increase in alkaline
phosphatase activity at the highest dose. At 13 weeks, ornithine
carbamoyl transferase activity was increased in animals of each sex
at 10 000 ppm; some males had marked increases, and males also had
increased activities of alanine and aspartate aminotransferases and
slightly increased activities of lactate dehydrogenase and alkaline
phosphatase. At 17 weeks (after the four-week recovery phase),
clinical chemical parameters were not significantly altered in the
highest-dose group over those in the control group. Liver weights of
females and testicular weights of males were increased at the
highest dose at 13 weeks, but no treatment-related effects on organ
weights were observed at this dose at 17 weeks. Gross necropsy and
histopathology of animals at 13 and 17 weeks revealed no
treatment-related findings. The NOAEL was 100 ppm, equal to 8.0
mg/kg bw per day (Suter et al., 1987a).
The effects of treatment with aqueous solutions of five benzoyl
urea insecticides, including teflubenzuron, on haematological
parameters was investigated in 10 Wistar rats (sex not specified),
which were given 100 mg/kg bw per day of the active ingredient by
gavage for 28 days. Ten rats were used as controls. No overt signs
of toxicity were seen. Teflubenzuron slightly increased reticulocyte
counts; the increase was comparable to those seen after similar
treatment with diflubenzuron and hexaflumuron, but smaller than
those induced by flufenoxuron and triflumuron. No other
haematological parameters were affected by treatment with
teflubenzuron. Relatively minor effects on haemoglobin, mean
corpuscular haemoglobin concentration and methaemoglobin formation
were induced by some of the other insecticides (Tasheva & Hristeva,
1993).
Dogs
In a 28-day range-finding study, pairs of one male and one
female beagle dogs were fed diets containing 0, 100, 1000 or 10 000
ppm teflubenzuron (purity, 96.7%), equivalent to 2.5, 25 or 250
mg/kg bw per day. Treatment did not affect signs of toxicity, food
consumption or body weight, and haematology, clinical chemistry,
urinalysis and gross pathology revealed no significant
treatment-related effect. The NOAEL was 10 000 ppm, equivalent to
250 mg/kg bw per day (Bathe, 1983).
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000
ppm for 13 weeks. There were no deaths or signs of toxicity during
the study; food consumption and body-weight gain were unaffected,
and a hearing test, ophthalmoscopic examinations, haematology and
urinalysis showed no findings of toxicological significance.
Clinical chemical analyses revealed increased activities of alanine
and aspartate aminotransferases, alkaline phosphatase and ornithine
carbamoyl transferase in one male and one female at 10 000 ppm at
week 4; gamma-glutamyltranspeptidase activity was increased in the
same female. Alkaline phosphatase activity was raised in another
male and ornithine carbamoyl transferase activity increased in two
other males at the same dose. At week 8, alkaline phosphatase,
alanine aminotransferase and gamma-glutamyltrans-peptidase
activities were increased in one male at 10 000 ppm, but by week 13
only alanine aminotransferase activity was raised in this animal.
Liver weights were slightly increased in animals of each sex at the
highest dose. Gross pathology revealed a firm liver with many
irregularities in the contour of the capsule in one male at 10 000
ppm. Isolated dark-red foci were noted in the pyloric area of the
stomachs of two dogs at the middle dose and two at the high dose.
The incidence of nodular foci in the pyloric or fundic area was also
increased at the high dose. Histopathological examination indicated
slight to moderate hepatotoxicity, resembling chronic active
hepatitis, in one male and one female at 10 000 ppm and slight
hepatotoxicity in one male at 100 ppm. Moderate centrilobular
hepatic necrosis was seen in another male at 100 ppm, and slight
round-cell infiltration was seen in one male at 1000 ppm and one at
10 000 ppm. Slight to moderate focal gastritis was noted in two
females at 10 000 ppm and in one at 1000 ppm. Follicular hyperplasia
of the pyloric mucosa in the stomach was noted in one control male
and in three males and three females at the highest dose. In view of
the possibly treatment-related histopathological findings in the
livers of males at 100 ppm, no NOAEL was identified in this study
(Bathe et al., 1984).
In a supplementary study designed to establish a clear NOAEL,
groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30 or 100 ppm,
equivalent to 0.75 or 2.5 mg/kg bw per day, for 13 weeks, and the
same parameters were assessed as in the first study. No
toxicologically significant effects were observed in either treated
group (Bathe et al., 1985).
The combined NOAEL in the two 13-week studies was 100 ppm,
equal to 4.1 mg/kg bw per day, on the basis of pathological findings
in the stomachs of dogs treated at 1000 ppm in the first study.
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30, 100 or 500 ppm
for 52 weeks. There were no deaths and no treatment-related signs of
toxicity; food consumption, body-weight gain, auditory perception
and ophthalmoscopic findings were not affected by treatment, and
haematology, clinical chemistry and urinalysis showed no effect.
Liver weights were increased in males treated at 500 ppm. Gross
necropsy and histopathological investigations revealed no other
treatment-related findings. The NOAEL was 100 ppm, equal to 3.2
mg/kg bw per day (Sachsse, 1986).
(c) Long-term toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, teflubenzuron (purity,
92.4%) was administered in the diet to groups of 60 male and 60
female NMRI mice at doses of 0, 15, 75 or 375 ppm, equal to 2.1, 10
or 54 mg/kg bw per day for males and 3.1, 15 or 72 mg/kg bw per day
for females. Ten animals of each sex in each group were killed at 12
months, and the remainder were killed at 18 months. Treatment did
not affect mortality or signs of toxicity, and ophthalmoscopy and
haematology showed no changes. Body-weight gain was reduced in males
at 375 ppm. Clinical chemical analyses revealed increased activities
of aspartate and alanine aminotransferases, lactate dehydrogenase,
alkaline phosphatase and ornithine carbamoyl transferase at 52 weeks
in males at 375 ppm. The alanine and aspartate aminotransferase and
ornithine carbamoyl transferase activities were still elevated at 78
weeks. Females treated at this dose had elevated alanine
aminotransferase and ornithine carbamoyl transferase activities at
week 52, and elevated alanine aminotransferase, lactate
dehydrogenase and ornithine carbamoyl transferase activities at week
78. Liver weights were increased at weeks 52 and 78 in males and
females at the highest dose and were slightly increased at week 78
in males at 75 ppm. Macroscopic investigations revealed an increased
incidence of hepatic nodules in males at 375 ppm and a dose-related
increase in the incidence of hepatic foci at week 78 in males at 75
and 375 ppm. Histopathological investigations indicated an increased
incidence of hepatocellular adenomas in males treated at 75 and 375
ppm at the time of the terminal kill: 6/50 in controls, 5/50 at 15
ppm, 11/50 at 75 ppm and 16/50 at 375 ppm. Contemporary historical
data on NMRI control mice from four long-term (84-110 weeks) studies
performed at the same laboratory indicated an incidence of
hepatocellular adenomas in control males of 4.5-16.4%, with a mean
incidence of 10.9%. Therefore, the incidence of hepatocellular
adenomas was increased above historical control values in animals
treated at 75 and 375 ppm.
Dose-related non-neoplastic hepatic changes were observed in
animals of each sex at the interim and terminal kills, which were
characterized mainly by various combinations of hepatocellular
hypertrophy, centrilobular to disseminated single-cell necrosis,
diffuse Kupffer-cell proliferation, disseminated phagocytic cell
foci, lipofuscin accumulation and patchy glycogen storage. These
changes were generally more pronounced in males. At the terminal
kill, the changes were moderate to severe in males treated at 75 and
375 ppm, and these animals also had an increased incidence of
hepatocellular nodular hyperplasia. Males at 15 ppm had increased
incidences of slight hepatocellular hypertrophy, single-cell
necrosis and phagocytic-cell foci. Similar non-neoplastic hepatic
changes were seen at the terminal kill in females at 375 ppm,
whereas only increased incidences of slight single-cell necrosis and
phagocytic-cell foci were noted at 75 ppm; at 15 ppm, slight
single-cell necrosis was seen. Slight to moderate bile duct
proliferation was seen in a dose-related fashion, and a decrease in
the amount of normal centrilobular fat storage was observed in males
at 75 and 375 ppm. Females at the highest dose had an increased
incidence of a patchy, coarse-droplet fatty change. At the interim
kill, males at 75 and 375 ppm had increased incidences of many of
the same non-neoplastic hepatic changes. At 15 ppm, increased
incidences of hepatocellular hypertrophy and single-cell necrosis
were seen. Females at 375 ppm had increased incidences of
phagocytic-cell foci and patchy fatty change at the interim kill. In
view of the treatment-related histopathological hepatic effects at
15 ppm, equal to 2.1 mg/kg bw per day, no NOAEL could be identified
in this study. Although there was an increased incidence of hepatic
effects observed at 15 ppm, they were not significantly increased in
terms of severity (Suter et al., 1987b).
Liver sections from males (comprising one routine slide and six
additional slides from each liver, with three sections per slide,
making a total of 21 sections) in the study described above were
re-evaluated by an independent pathologist, who concluded that the
development of hepatocellular nodules, adenomas and carcinomas in
males was not related to treatment with teflubenzuron. A possibly
dose-related increase in the incidence of hepatocellular nodules was
observed, however, in males on the basis of the pathologist's
diagnoses: 2/60 in controls, 0/60 at 15 ppm, 6/60 at 75 ppm and
12/60 at 375 ppm. In addition, a slight but non-significant increase
in the incidence of hepatocellular adenomas was noted in males at 75
and 375 ppm: 8/60 in controls, 5/60 at 15 ppm, 13/60 at 75 ppm and
13/60 at 375 ppm. The revised diagnoses were based on use of more
detailed diagnostic criteria for hepatocellular nodules and adenomas
than those described in the report of Suter et al. (1987b). The
pathologist's report concluded that teflubenzuron 'did not manifest
either tumorigenic effect (enhancement of hepatocellular adenomas)
or carcinogenic effect (enhancement of hepatocellular carcinomas)'
(Vesselinovitch, 1988). The Meeting concluded that this
re-evaluation could not override the report of the pathologist of
the original study.
Rats
In a 120-week study of toxicity and carcinogenicity, groups of
70 male and 70 female Wistar rats were administered teflubenzuron
(purity, 92.4%) in the diet at 0, 20, 100 or 500 ppm, equivalent to
1, 5 or 25 mg/kg bw per day. Ten rats of each sex in each group were
killed at 53 and 107 weeks and the remainder at 120 weeks. The mean
plasma concentrations of teflubenzuron determined in surviving
animals before the kill at week 107 were 0.02 µg/ml for animals of
each sex at 20 ppm, 0.06 µg/ml for males and 0.04 µg/ml for females
at 100 ppm and 0.26 µg/ml for males and 0.13 µg/ml for females at
500 ppm. Treatment did not affect mortality, signs of toxicity, body
weight, food consumption or the results of ophthalmoscopic or
hearing tests; haematology and urinalysis indicated no
treatment-related changes. Clinical chemical analyses revealed
increased activities of aspartate and alanine aminotransferases at
weeks 14, 26, 53 and 78 and increased alanine aminotransferase
activity at week 120 in males treated at 500 ppm. Ornithine
carbamoyl transferase activity was increased at weeks 53 and 78 in
males at 500 ppm. Liver weights were slightly increased in males at
this dose at week 120. Gross pathology showed no treatment-related
changes. Histopathology revealed a statistically significant
increase in the incidence of haemangiomas in mesenteric lymph nodes
of males treated at the highest dose (8/47 compared to 1/48 control
males). The incidence of this tumour in the concurrent control group
was generally lower than that in historical control groups, and the
incidence in males at the high dose was within the historical
control range. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per
day (Suter et al., 1987c).
In a supplementary study, three groups of 60 male and 60 female
Wistar rats were given teflubenzuron (purity, 92.4%) in the diet at
0, 2500 or 10 000 ppm, equivalent to 125 or 500 mg/kg bw per day.
Ten animals of each sex at each dose were killed at week 104, and 50
of each sex in each group at week 111. The plasma concentrations of
teflubenzuron were determined in five animals of each sex at each
dose after 4, 26, 52 and 104 weeks. Time-related increases in plasma
concentrations were observed in animals of each sex; the
concentrations in animals treated at 2500 and 10 000 ppm were
0.18-0.79 and 0.23-0.70 µg/ml, resepctively, in males and 0.18-0.60
and 0.14-0.43 µg/ml, respectively, in females. The survival rate of
males at 10 000 ppm at termination was significantly increased over
that in controls. There were no treatment-related clinical signs of
toxicity or effects on food consumption, but the body-weight gains
of females were slightly reduced at both doses. Haematology and
urinalysis showed no treatment-related findings. Increased
activities of plasma alanine and aspartate aminotransferases were
seen in males at 2500 and 10 000 ppm throughout treatment. Liver
weights were increased in males at the highest dose at week 104 and
to a lesser extent at week 111. Gross pathology revealed increased
incidences of diffuse, clay-coloured discoloration and focal and
multifocal discoloration in livers of males at the highest dose.
Histopathology revealed a number of treatment-related non-neoplastic
liver lesions: Increased incidences of foci of mixed and basophilic
cells were noted in males at 2500 and 10 000 ppm, and an increased
incidence of basophilic-cell foci was seen in females at 10 000 ppm.
The incidence of focal hepatocellular hyperplasia was increased in
males at the highest dose, and the incidences of fatty change and
centrilobular hepatocellular hypertrophy were increased in males and
females at 2500 and 10 000 ppm. Spongiosis hepatis was increased in
incidence in males at the highest dose. As treatment-related
non-neoplastic findings were seen at both doses, no NOAEL could be
identified in this study (Tennekes et al., 1989).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing teflubenzuron (purity, 92.4%) at 0, 20, 100 or 500
ppm for two generations, with one litter per generation. The F0
generation was kept for 10 weeks before mating and the F1
generation for about 12 weeks. Reproductive performance and effects
on adults and offspring were monitored daily throughout the study;
observations included body weight, food consumption, mating
performance, fertility rate, duration of gestation, pup hair growth,
pinna unfolding, tooth eruption, eye opening, litter size, pup
weight, pup mortality and sex ratio. F1 pups underwent functional
tests, comprising pupillary reflex, startle response and ability to
learn use of a water maze, and the sexual organs of the parental and
F1 animals that received the highest dose were examined by gross
necropsy and histopathology. There was no evidence of toxicity and
no effects on reproductive performance (Osterburg, 1986). The NOAEL
was > 500 ppm, equal to 40 mg/kg bw per day.
(e) Embryotoxicity and teratogenicity
Rats
Teflubenzuron (purity, 96.5%) was administered in 0.5% aqueous
carboxymethyl-cellulose to groups of 25 naturally inseminated female
Wistar rats by gavage on days 6-15 after mating at doses of 0, 10,
50 or 250 mg/kg bw per day. The dams were killed on day 20 of
gestation, and the fetuses were examined after caesarean section.
The doses were selected on the basis of the results of a
range-finding study of doses up to 250 mg/kg bw per day (Gleich,
1984). There were no treatment-related fetotoxic or teratogenic
effects or signs of maternal toxicity. The NOAEL for both dams and
fetuses was 250 mg/kg bw per day (Gleich et al., 1984a).
Groups of 25 naturally inseminated female Wistar rats received
teflubenzuron (purity, 92.1%) in 0.5% aqueous carboxymethylcellulose
by gavage at doses of 0, 100, 300 or 1000 mg/kg bw per day on days
7-17 of gestation. The dams were killed on day 20 of gestation, and
the fetuses examined after caesarean section. There were no
treatment-related signs of maternal toxicity or any treatment-
related teratogenic or fetotoxic effects. The NOAEL for both dams
and fetuses was 1000 mg/kg bw per day (Ishida et al., 1987).
Rabbits
Groups of 14-15 Himalayan rabbits proven to be pregnant were
administered teflubenzuron (purity, 96.5%) in 0.5% aqueous
carboxymethylcellulose at 0, 10, 50 or 250 mg/kg bw per day by
stomach tube on days 6-18 of gestation. The doses were based on the
results of a range-finding study in which rabbits received doses of
up to 250 mg/kg bw per day with no sign of toxicity and no effect on
fetuses. Caesarean sections were performed on the dams on day 29 of
gestation. There were no treatment-related effects on the dams or
fetuses. The NOAEL for maternal toxicity, fetotoxicity and
teratogenicity was 250 mg/kg bw per day (Gleich et al., 1984b).
A group of 22 naturally inseminated New Zealand white rabbits,
18 of which were proven to be pregnant, were administered
teflubenzuron (purity, 96.5%) in 0.5% aqueous carboxymethyl
cellulose at 1000 mg/kg bw per day by stomach tube on days 6-18 of
gestation. Sixteen rabbits (15 proven to be pregnant) treated with
the vehicle alone served as the control group. The dams were killed
on day 28 of gestation. The only sign of maternal toxicity possibly
related to treatment was noted at necropsy as an increased incidence
of 'grossly granulated cut surfaces of the liver' in dams treated at
1000 mg/kg bw per day (in 8/22 as compared with 1/16 controls).
There were no signs of treatment-related fetotoxicity or
teratogenicity at this dose. The NOAEL for maternal toxicity could
not be established conclusively in view of the possible effect on
the liver. The NOAEL for fetotoxicity and teratogenicity was 1000
mg/kg bw per day (Osterburg, 1987).
Four groups of four or five pregnant Himalayan rabbits were
given teflubenzuron (purity unspecified) in 0.5% aqueous
carboxymethylcellulose by gavage at doses of 0, 250 or 500 mg/kg bw
per day (two groups at the highest dose) on days 6-18 of gestation.
All animals except those in the second group at the highest dose
were killed on day 19. Their livers were immediately removed and
weighed, and the cytochrome P450 content and O- and
N-demethylase activities were assessed in homogenates; the numbers
of corpora lutea, living embryos and early resorptions were counted.
Animals in the second group at the high dose underwent caesarian
section on day 29 and their livers were examined in the same way as
described above. Fetuses were incubated for 24 h before being
examined for malformations. No toxicologically significant findings
were found, and there was no evidence of liver enzyme induction in
the dams (Gleich, 1985).
(f) Genotoxicity
The results of tests for the genotoxicity of teflubenzuron
(Table 2) revealed no evidence for mutagenicity or clastogenicity.
(g) Special studies
Skin and eye irritation and skin sensitization
The potential of teflubenzuron (purity, 96.5%) to irritate skin
was investigated in three New Zealand white rabbits by applying 0.5
g of the compound, moistened with tap water, to intact skin on the
clipped dorsum of each rabbit and keeping it under an occlusive
dressing for 4 h. There was no evidence of skin irritation over 72 h
(Ullman, 1983f).
The potential of teflubenzuron (purity, 96.5%) to irritate the
eye was investigated in three New Zealand white rabbits by
introducing 0.1 g of undiluted test material into the conjunctival
sac of the left eye of each animal. Slight conjunctival and scleral
redness was observed in each animal after 1 h. No signs of
irritation were seen at 24, 48 or 72 h (Ullman, 1983g).
Table 2. Results of tests for the genotoxicity of teflubenzuron
End-point Test object Concentration Purity Results Reference
of teflubenzuron (%)
In vitro
Reverse mutation S. typhimurium TA98, 125-5000 µg/plate NR Negativea Kramer, 1982
100, 1535, 1537, 1538 in DMSO
Point mutation at Chinese hamster V79 5-50 µg/ml 92.4 Negativea Heidemann, 1986
hprt locus cell line in DMSO
Chromosomal Chinese hamster V79 4, 25, 50 µg/ml 92.4 Negativea Heidemann, 1985
aberration cell line in DMSO
Unscheduled DNA Wistar CF HB male 1-100 µg/ml 92.4 Negativea Müller, 1986
synthesis rat hepatocytes in acetone
In vivo
Micronucleus NMRI mice 5000 mg/kg bw in 96.5 Negative Guenard, 1984
formation 2% carboxymethyl-
cellulose, sodium salt
DMSO, dimethyl sulfoxide; NR, not reported
a In the presence and absence of metabolic activation
The potential of teflubenzuron (purity, 93.5%) to sensitize
skin was investigated in the guinea-pig (Dunkin-Hartley)
maximization test, in which 10 animals were used as negative
controls and 20 as the test group. The results of two topical
challenges after intradermal or topical induction with the test
substance indicated that teflubenzuron did not sensitize skin under
these conditions (Ullman, 1984).
3. Observations in humans
No information was available.
Comments
In rats, teflubenzuron was absorbed only partially from the
gastrointestinal tract, absorption being dose-dependent and
saturable. Absorbed teflubenzuron was excreted mainly via the bile,
urinary excretion representing only a minor route. Faecal excretion
of absorbed and unabsorbed teflubenzuron was the main route. There
was no evidence of bioaccumulation in organs or tissues.
Teflubenzuron was eliminated largely unchanged in faeces,
although a number of unidentified minor metabolites were found.
Hydroxylated metabolites of teflubenzuron were found in urine in low
amounts. 3,5-Dichloro-2,4-difluorophenylurea and its corresponding
substituted aniline were observed in urine, indicating that cleavage
of the benzoylurea moiety had occurred. Conjugates of these
metabolites and the unconjugated 3,5-dichloro-2,4-difluorophenylurea
were detected in bile.
Teflubenzuron has low acute oral, dermal and inhalational
toxicity; it was more acutely toxic when administered by the
intraperitoneal route. WHO (1992) has classified teflubenzuron as
being unlikely to present an acute hazard in normal use.
In mice, rats and dogs given repeated doses in the diet, the
major target organ was the liver. Pathological and clinical chemical
findings of hepatotoxicity varied with species, dose and duration of
dosing. The indicators of hepatotoxicity included effects such as
increased activities of serum alanine aminotransferase, aspartate
aminotransferase, ornithine carbamoyl transferase, lactate
dehydrogenase and alkaline phosphatase, increased liver weight, and
hepatocellular necrosis, fatty change, hypertrophy and hyperplasia.
Haematological parameters generally remained unaltered by treatment.
In a 13-week study of toxicity in mice fed levels of 0, 100,
1000 or 10 000 ppm, effects indicative of hepatotoxicity were
observed at 1000 and 10 000 ppm. The NOAEL was 100 ppm, equal to
11.9 mg/kg bw per day. In a 28-day range-finding and a 13-week study
of toxicity, rats were fed diets containing 0, 100, 1000 or 10 000
ppm. The NOAEL was 100 ppm in each, equal to 11.7 and 8.0 mg/kg bw
per day, respectively, on the basis of indications of
hepatotoxicity. Two 13-week studies in dogs fed diets containing 0,
100, 1000 or 10 000 ppm and 0, 30 or 100 ppm indicated an NOAEL of
100 ppm, equal to 4.1 mg/kg bw per day, on the basis of focal
gastritis in dogs treated at 1000 ppm in the first study. The NOAEL
in the 13-week studies concurred with the NOAEL of 100 ppm, equal to
3.2 mg/kg bw per day, observed in a 52-week study in dogs, in which
liver weights were increased in males fed the highest level of 500
ppm.
Mice fed diets containing 0, 15, 75 or 375 ppm for 18 months in
a study of carcinogenicity showed non-neoplastic hepatotoxicity at
all doses. Changes observed in livers of mice at the lowest dose
were increased in incidence over those in controls but were not
increased in severity. The lowest dose of 15 ppm, equal to 2.1 mg/kg
bw per day, was the LOAEL. Histopathological investigations
indicated an increased incidence of hepatocellular adenomas in males
at 75 and 375 ppm, in comparison with rates in concurrent and
historical controls. This tumorigenic potential in mice was
considered not to be relevant to humans.
In a 120-week study of toxicity and carcinogenicity, rats were
fed diets containing 0, 20, 100 or 500 ppm teflubenzuron. The NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of
increased serum enzyme activities and liver weights in males. In a
supplementary study, rats were fed diets containing 0, 2500 or 10
000 ppm for 111 weeks. No NOAEL could be assigned because
non-neoplastic liver changes and increased serum enzyme activities
were seen at both doses. There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats fed dietary concentrations of 0, 20,
100 or 500 ppm, the NOAEL was 500 ppm, equal to 40 mg/kg bw per day,
on the basis of lack of toxicity or effects on reproductive
performance.
Two studies of teratogenicity in rats treated by gavage showed
no evidence of maternal toxicity, fetotoxicity or teratogenicity at
doses up to either 250 or 1000 mg/kg bw per day. In a study of
teratogenicity in rabbits, there was no evidence of maternal
toxicity, fetotoxicity or teratogenicity at doses of 0, 10, 50 or
250 mg/kg bw per day. A second study of teratogenicity, in rabbits
treated by gavage at 0 or 1000 mg/kg bw per day, showed no evidence
of fetotoxicity or teratogenicity. Effects possibly related to
treatment were noted at necropsy in the livers of some dams treated
at 1000 mg/kg bw per day. In another study, there was no evidence of
liver enzyme induction in pregnant rabbits treated by gavage with
doses of up to 500 mg/kg bw per day.
Teflubenzuron has been adequately tested for genotoxicity in
vivo and in vitro in a range of assays. The Meeting concluded
that it was not genotoxic.
An ADI was allocated on the basis of the LOAEL of 15 ppm, equal
to 2.1 mg/kg bw per day, in the 18-month study of carcinogenicity in
mice. A 200-fold safety factor was applied since no NOAEL was
identified in this study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 11.9 mg/kg bw per day (13-week
study of toxicity)
Rat: 100 ppm, equal to 4.8 mg/kg bw per day (120-week
study of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
1000 mg/kg bw per day (study of teratogenicity,
maternal and fetal toxicity)
Rabbit: 1000 mg/kg bw per day (fetal toxicity in a study of
teratogenicity)
250 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Lowest-observed-adverse-effect level
Mouse: 15 ppm, equal to 2.1 mg/kg bw per day (18-month study
of carcinogenicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Bathe, R. (1983) 28-Day oral toxicity (feeding) study with CME 134
in beagle dogs. Project 017212. Document No. 134AB-432-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.,
London, United Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1984) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 018865. Document No. 134AB-433-007. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1985) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 040702. Document No. 134AB-433-008. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987a) The disposition
of [14C]-CME 134 in the lactating goat. Report No. 4278A. Document
No. 134AX-652-001. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987b) The disposition
of [14C]-CME 134 in the laying hen. Report No. 4278B. Unpublished
report from Inveresk Research International Ltd, Musselburgh,
Scotland, United Kingdom. Submitted to WHO by Shell International
Chemical Co. Ltd.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1988)
Further identification of [14C]-CME 134 metabolites in the hen.
Unpublished report from Inveresk Research International Ltd,
Musselburgh, Scotland, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1989)
Further identification of [14C]-CME 134 metabolites in the goat.
Report No. 4490A. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ellgehausen, K. (1986) 28-Day dietary study with CME 134 in the rat
for proof of absorption. Project 059973. Document No. 134AB-651-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Gleich, J. (1984) CME 134. Dosifindungsversuch für eine Prufung auf
embryotoxische Wirkung an Ratten nach oraler Applikation. Report No.
4/7/84. Document No 134AB-451-001. Unpublished report from Institute
of Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J. (1985) CME 134. Supplementary study on embryotoxicity and
liver enzyme induction in Himalayan rabbits. Report No. 4/65/85.
Document No. 134AB-451-005. Unpublished report from Institute of
Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G. & Unkelbach, H. (1984a) CME 134.
Embryotoxicity study in rats after oral administration. Report No.
4/29/84. Document No. 134AB-451-003. Unpublished report from
Institute of Toxicology, E. Merck, Darmstadt, Germany. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G., Unkelbach, H.D. & Hofmann, A. (1984b) CME
134. Embryotoxicity study in rabbits after oral administration.
Report No. 4/63/84. Document No. 134AB-451-004. Unpublished report
from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Guenard, J. (1984) Mouse micronucleus assay with CME 134. Project
025672. Unpublished report from Resarch and Consulting Co. Ag,
Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Hawkins, D.R. & Mayo, B.C. (1988) The biliary excretion and
metabolism of [14C]-CME 134. Report No. HRC/CMK 17/871263. Document
No. 134AX-651-010. Unpublished report from Huntingdon Research
Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1985) CME 134. Chromosome aberrations in cells of
Chinese hamster cell line V79. Study LMP 135C. Document No.
134AB-457-003. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1986) CME 134. Detection of gene mutations in somatic
mammalian cells in culture: HGPRT-test with V79 cells. Study LMP
135B. Document No. 134AC-457-005. Unpublished report from the
Laboratory for Mutagenicity Testing, Darmstadt, Germany. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ishida, S., Yamazaki, E., Ikeya, M. & Suzuki, K. (1987)
Teratological study in rats treated orally with teflubenzuron. Study
No. R-128. TZ-432-001. Unpublished report from Bozo Research Centre
Ltd, Tokyo, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Kramer, P.J. (1982) CME 134. In vitro assessment for mutagenic
potential in bacteria with and without addition of a metabolizing
system. Experiment T12 568. Document No. 134AA-457-001. Unpublished
report from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Müller, E. (1986) CME 134. Unscheduled DNA synthesis in hepatocytes
of male rats in vitro (UDS test). Study LMP 135A. Document No.
134AC-457-006. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Osterburg, I. (1986) Two generation oral (dietary administration)
reproduction toxicity study in the rat. Project No. 460/1. Document
No. 134AB-453-002. Unpublished report from Hazleton Laboratories,
Münster, Germany. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Osterburg, I. (1987) CME 134. Oral (gavage) teratogenicity limit
test in the rabbit. Project No. 460/13. Document No. 134AB-451-009.
Unpublished report from Hazleton Laboratories, Münster, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Sachsse, K. (1986) 52-Week oral (feeding) toxicity study with CME
134 in beagle dogs. Project 034828. Document No. 134AB-437-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Schlüter, H. (1984) Initial investigations on the biokinetics of
CME134 in the rat. Document No. 134AA-651-001. Unpublished report
from Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1985) Investigations on the metabolism of CME134 in
the rat. Document No. 134AA-651-012. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1986) The biokinetics and metabolism of [14C]-CME 134
in the rat. Document No. 134AX-651-007. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P. (1983) 28 Day range-finding study with CME 134 in rats.
Project 017201. Document No. 134AB-432-002. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Horst, K., Luetkemeier, H., Chevalier, J. & Terrier, C.
(1987a) 13-Week subchronic toxicity (feeding) study with CME 134 in
the rat. Project 018843. Document No. 134AB-433-006. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Westen, H. & Terrier, C.
(1987b) 18-Month oncogenicity (feeding) study with CME 134 in mice.
Project 027810. Document No. 134AB-455-003. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Schlotke, B., Ellgehausen,
H., Terrier, Ch. (1987c) 120-week chronic toxicity and oncogenicity
study with CME 134 in the rat. Project 027472. Document No
134AB-437-006. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Takahashi, K., Saitoh, T., Miyaoka, S., Maita, K. & Goloh, M. (1987)
13 Weeks subacute toxicity study with teflubenzuron in mice. Report
T-15. Document no. 134AB-433-005. Unpublished report from Kodaira
Laboratory, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Tasheva, M. & Hristeva, V. (1993) Comparative study on the effects
of five benzoylphenylurea insecticides on haematological parameters
in rats. J. Appl. Toxicol., 13, 67-68.
Tennekes, H., Stucki, P., Luetkemeier, H., Biedermann, K., Bloch,
M., Chevalier, H., Vogel, O. & Terrier, C. (1989) Chronic toxicity
and oncogenicity (feeding) study with CME 134 in the rat. Project
064192. Document No. 134AB-437-009. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983a) Acute oral toxicity study (LD50) with CME 134
in rats. Project 019596. Document No. 134AB-421-004. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983b) Acute oral toxicity study (LD50) with CME 134
in mice. Project 025571. Document No. 134 AB-421-005. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983c) Acute dermal toxicity study (LD50) with CME 134
in rats. Project 025593. Document No. 134 AB-422-001. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983d) Acute intraperitoneal toxicity study (LD50)
with CME 134 in rats. Project 025582. Document No. 134AB-424-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L. (1983e) 4-Hour dust-aerosol inhalation toxicity study
(LD50) with CME 134 in rats. Project 025626. Document No.
134AB-423-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983f) Primary skin irritation study following a single
4-hour occlusive application with CME 134 in the rabbit. Project
025604. Document No. 134AB-465-001. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983g) Primary eye irritation study after single
application with CME 134 in the rabbit. Project 025615. Document No.
134AB-466-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1984) Test for delayed hypersensitivity in the albino
guinea-pig with CME 134 Project 034817. Document No. 134AB-467-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L., Sacher, R. & Vogel, O. (1988) Acute oral toxicity study
with teflubenzuron in rats. Project 206741, Document No.
134AC-421-009. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Vesselinovitch, S. (1988) Histologic evaluation of liver tissues
originating from potential carcinogenicity bioassay studies of CME
134, Batch DW 44/83 in NMRI mice. Unpublished report from the
Departments of Pathology and Radiology, University of Chicago, IL,
USA. Submitted to WHO by Shell Chemical Co. Ltd, London, United
Kingdom.
The nature of the excretion products in faecal extracts, bile
and urine from rats with bile cannulas that were given 25 or 750
mg/kg bw 14C-aniline ring-labelled teflubenzuron orally was
investigated by TLC. Most of the radiolabel extracted from 0-48-h
faeces of treated animals of each sex co-chromatographed with
teflubenzuron. In 0-48-h bile, most of the radiolabel was in
unidentified polar material. A minor biliary metabolite
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV, see Figure 1), and another co-chromatographed with
teflubenzuron. Hydrolytic treatment of 0-48-h bile extracts
indicated the presence of conjugates of 3,5-dichloro-2,4-
difluorophenyl urea (conjugate IV, see Figure 1), the corresponding
substituted aniline (conjugate V) and the meta-hydroxybenzoyl
derivative of teflubenzuron (metabolite I). The major radioactive
component in 0-48-h urine extracts from animals at 25 mg/kg bw was
unidentified polar material. Three minor urinary metabolites
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV), its corresponding substituted aniline metabolite
(metabolite V) and the meta-hydroxybenzoyl derivative (metabolite
I) of teflubenzuron (Hawkins & Mayo, 1988).
Chickens
Laying hens that had been administered a total of 1.25 mg/kg bw
per day of 14C-teflubenzuron by oral doses twice daily for 7.5
days were shown by TLC and HPLC to excrete mainly the parent
compound. Radiolabel in bile was in the form of a very polar
compound, which on treatment with ß-glucuronidase yielded a compound
with similar chromatographic characteristics to the
meta-hydroxybenzoyl derivative (metabolite I, see Figure 1) of
teflubenzuron (22% of radioactivity in bile). A compound with
identical HPLC retention characteristics to teflubenzuron was found
in fat, liver, plasma and egg yolk (Cameron et al., 1987b).
Further investigations of metabolites in chickens by mass
spectrometry showed that teflubenzuron was the major component of
excreta. Two components of liver and kidney extracts were observed
by TLC and HPLC; one had characteristics identical to those of
teflubenzuron, but the other could not be identified. A third
component of kidney extracts was shown to be 3,5-dichloro-2,4-
difluorophenyl urea (metabolite IV, see Figure 1). Further evidence
was provided for the presence of teflubenzuron in egg yolk and fat
(Cameron et al., 1988).
Goats
In lactating goats given oral doses twice daily to give a total
of 1 mg/kg bw per day 14C-teflubenzuron over 7.5 days, the major
faecal component was shown by TLC to be unchanged teflubenzon. About
41% of the biliary radiolabel had similar characteristics on HPLC
and TLC to the meta-hydroxybenzoyl derivative (metabolite I, see
Figure 1) of teflubenzuron after treatment with ß-glucuronidase. No
unchanged teflubenzuron was found in bile (Cameron et al., 1987a).
Mass spectrometry showed conclusively that the major faecal
component was teflubenzuron. In goat liver, the major radioactive
component was a polar compound, which was not considered to be a
conjugate since it was unaffected by treatment with deconjugating
enzymes. Traces of a metabolite that co-chromatographed with the
meta-hydroxybenzoyl derivative (metabolite I) of teflubenzuron
were found in liver extracts (Cameron et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of teflubenzuron
(purity, 96.5-97.5%) are summarized in Table 1. After oral
treatment, rats and mice showed only slight signs of toxicity,
including ruffled fur, dyspnoea, sedation and hunched posture, which
had resolved in all cases by 48-72 h. After intraperitoneal
administration, similar signs of toxicity were observed, which were
generally more severe and longer lasting. Deaths unrelated to
treatment were seen at 300 mg/kg bw, the lowest dose tested. There
was no evidence of toxicity after dermal treatment at 2000 mg/kg bw.
Slight dyspnoea and ruffled fur were the only findings after
inhalation, and these had resolved within 24 h. Teflubenzuron had no
clear effect on gross pathological findings in these studies.
Table 1. Acute toxicity of teflubenzuron in male and female rodents
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Mouse Oral > 5000 Ullman, 1983b
Rat Oral > 5000 Ullman et al., 1988
Rat Oral > 5000 Ullman, 1983a
Rat Dermal > 2000 Ullman, 1983c
Rat Intraperitonea] > 2000 Ullman, 1983d
Rat Inhalation > 5038 Ullman, 1983e
(b) Short-term toxicity
Mice
Groups of 12 male and 12 female CD-1 mice were administered
teflubenzuron (purity unspecified) at 0, 100, 1000 or 10 000 ppm in
the diet for 13 weeks, equal to 12, 115 or 1210 mg/kg bw per day in
males and 14, 143 or 1450 mg/kg bw per day in females. (The
techniques used to investigate biological parameters were not
specified.) Treatment appeared to have no effect on clinical signs
of toxicity, deaths, body-weight gain, food consumption or the
results of ophthalmoscopy, urinalysis and haematology. Clinical
chemical investigations revealed increased alanine aminotransferase
activity (in animals of each sex) and a raised cholesterol level (in
females) at 1000 and 10 000 ppm, and increased alkaline phosphatase
activity in females at 10 000 ppm. Necropsy revealed liver
enlargement in animals of each sex and dark-coloured livers in males
at 1000 and 10 000 ppm. Liver weights were increased in animals of
each sex at the middle and high doses. Histopathology revealed
centrilobular hepatocellular swelling in animals of each sex and
fatty change in males at 1000 and 10 000 ppm and microgranuloma in
males at 10 000 ppm. The NOAEL was 100 ppm, equal to 12 mg/kg bw per
day (Takahashi et al., 1987).
Rats
In a 28-day range-finding study, groups of five male and five
female Wistar rats were administered teflubenzuron (purity not
specified) in the diet at levels of 0, 100, 1000 or 10 000 ppm.
There were no deaths or signs of toxicity and no significant effects
on body-weight gain. Food consumption of males at the highest dose
level was slightly reduced. Haematology and urinalysis showed no
treatment-related findings. Clinical chemical analyses revealed
treatment-related increases in the activities of serum aspartate and
alanine aminotransferase in animals of each sex and of lactate
dehydrogenase in females at 1000 and 10 000 ppm. Gross pathology
revealed no treatment-related lesions. Histopathology and organ
weight measurements were not performed. The NOAEL was 100 ppm, equal
to 12 mg/kg bw per day (Suter, 1983).
Groups of 10 male and 10 female Wistar rats were administered
teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000 ppm in the
diet for 13 weeks. Additional groups of five male and five female
rats in the control and highest-dose groups were maintained on
control diet for a further four weeks after the 13-week treatment
phase to determine the reversibility of any findings. There were no
deaths and no treatment-related signs of toxicity. Body weights and
food consumption were not affected, and urinalysis, ophthalmoscopy
and haematology did not reveal any treatment-related findings.
Clinical chemical analyses showed increased activities of ornithine
carbamoyl transferase and alanine and aspartate aminotransferases in
animals of each sex at 1000 and 10 000 ppm at six weeks; males had
more significant increases and also an increase in alkaline
phosphatase activity at the highest dose. At 13 weeks, ornithine
carbamoyl transferase activity was increased in animals of each sex
at 10 000 ppm; some males had marked increases, and males also had
increased activities of alanine and aspartate aminotransferases and
slightly increased activities of lactate dehydrogenase and alkaline
phosphatase. At 17 weeks (after the four-week recovery phase),
clinical chemical parameters were not significantly altered in the
highest-dose group over those in the control group. Liver weights of
females and testicular weights of males were increased at the
highest dose at 13 weeks, but no treatment-related effects on organ
weights were observed at this dose at 17 weeks. Gross necropsy and
histopathology of animals at 13 and 17 weeks revealed no
treatment-related findings. The NOAEL was 100 ppm, equal to 8.0
mg/kg bw per day (Suter et al., 1987a).
The effects of treatment with aqueous solutions of five benzoyl
urea insecticides, including teflubenzuron, on haematological
parameters was investigated in 10 Wistar rats (sex not specified),
which were given 100 mg/kg bw per day of the active ingredient by
gavage for 28 days. Ten rats were used as controls. No overt signs
of toxicity were seen. Teflubenzuron slightly increased reticulocyte
counts; the increase was comparable to those seen after similar
treatment with diflubenzuron and hexaflumuron, but smaller than
those induced by flufenoxuron and triflumuron. No other
haematological parameters were affected by treatment with
teflubenzuron. Relatively minor effects on haemoglobin, mean
corpuscular haemoglobin concentration and methaemoglobin formation
were induced by some of the other insecticides (Tasheva & Hristeva,
1993).
Dogs
In a 28-day range-finding study, pairs of one male and one
female beagle dogs were fed diets containing 0, 100, 1000 or 10 000
ppm teflubenzuron (purity, 96.7%), equivalent to 2.5, 25 or 250
mg/kg bw per day. Treatment did not affect signs of toxicity, food
consumption or body weight, and haematology, clinical chemistry,
urinalysis and gross pathology revealed no significant
treatment-related effect. The NOAEL was 10 000 ppm, equivalent to
250 mg/kg bw per day (Bathe, 1983).
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000
ppm for 13 weeks. There were no deaths or signs of toxicity during
the study; food consumption and body-weight gain were unaffected,
and a hearing test, ophthalmoscopic examinations, haematology and
urinalysis showed no findings of toxicological significance.
Clinical chemical analyses revealed increased activities of alanine
and aspartate aminotransferases, alkaline phosphatase and ornithine
carbamoyl transferase in one male and one female at 10 000 ppm at
week 4; gamma-glutamyltranspeptidase activity was increased in the
same female. Alkaline phosphatase activity was raised in another
male and ornithine carbamoyl transferase activity increased in two
other males at the same dose. At week 8, alkaline phosphatase,
alanine aminotransferase and gamma-glutamyltrans-peptidase
activities were increased in one male at 10 000 ppm, but by week 13
only alanine aminotransferase activity was raised in this animal.
Liver weights were slightly increased in animals of each sex at the
highest dose. Gross pathology revealed a firm liver with many
irregularities in the contour of the capsule in one male at 10 000
ppm. Isolated dark-red foci were noted in the pyloric area of the
stomachs of two dogs at the middle dose and two at the high dose.
The incidence of nodular foci in the pyloric or fundic area was also
increased at the high dose. Histopathological examination indicated
slight to moderate hepatotoxicity, resembling chronic active
hepatitis, in one male and one female at 10 000 ppm and slight
hepatotoxicity in one male at 100 ppm. Moderate centrilobular
hepatic necrosis was seen in another male at 100 ppm, and slight
round-cell infiltration was seen in one male at 1000 ppm and one at
10 000 ppm. Slight to moderate focal gastritis was noted in two
females at 10 000 ppm and in one at 1000 ppm. Follicular hyperplasia
of the pyloric mucosa in the stomach was noted in one control male
and in three males and three females at the highest dose. In view of
the possibly treatment-related histopathological findings in the
livers of males at 100 ppm, no NOAEL was identified in this study
(Bathe et al., 1984).
In a supplementary study designed to establish a clear NOAEL,
groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30 or 100 ppm,
equivalent to 0.75 or 2.5 mg/kg bw per day, for 13 weeks, and the
same parameters were assessed as in the first study. No
toxicologically significant effects were observed in either treated
group (Bathe et al., 1985).
The combined NOAEL in the two 13-week studies was 100 ppm,
equal to 4.1 mg/kg bw per day, on the basis of pathological findings
in the stomachs of dogs treated at 1000 ppm in the first study.
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30, 100 or 500 ppm
for 52 weeks. There were no deaths and no treatment-related signs of
toxicity; food consumption, body-weight gain, auditory perception
and ophthalmoscopic findings were not affected by treatment, and
haematology, clinical chemistry and urinalysis showed no effect.
Liver weights were increased in males treated at 500 ppm. Gross
necropsy and histopathological investigations revealed no other
treatment-related findings. The NOAEL was 100 ppm, equal to 3.2
mg/kg bw per day (Sachsse, 1986).
(c) Long-term toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, teflubenzuron (purity,
92.4%) was administered in the diet to groups of 60 male and 60
female NMRI mice at doses of 0, 15, 75 or 375 ppm, equal to 2.1, 10
or 54 mg/kg bw per day for males and 3.1, 15 or 72 mg/kg bw per day
for females. Ten animals of each sex in each group were killed at 12
months, and the remainder were killed at 18 months. Treatment did
not affect mortality or signs of toxicity, and ophthalmoscopy and
haematology showed no changes. Body-weight gain was reduced in males
at 375 ppm. Clinical chemical analyses revealed increased activities
of aspartate and alanine aminotransferases, lactate dehydrogenase,
alkaline phosphatase and ornithine carbamoyl transferase at 52 weeks
in males at 375 ppm. The alanine and aspartate aminotransferase and
ornithine carbamoyl transferase activities were still elevated at 78
weeks. Females treated at this dose had elevated alanine
aminotransferase and ornithine carbamoyl transferase activities at
week 52, and elevated alanine aminotransferase, lactate
dehydrogenase and ornithine carbamoyl transferase activities at week
78. Liver weights were increased at weeks 52 and 78 in males and
females at the highest dose and were slightly increased at week 78
in males at 75 ppm. Macroscopic investigations revealed an increased
incidence of hepatic nodules in males at 375 ppm and a dose-related
increase in the incidence of hepatic foci at week 78 in males at 75
and 375 ppm. Histopathological investigations indicated an increased
incidence of hepatocellular adenomas in males treated at 75 and 375
ppm at the time of the terminal kill: 6/50 in controls, 5/50 at 15
ppm, 11/50 at 75 ppm and 16/50 at 375 ppm. Contemporary historical
data on NMRI control mice from four long-term (84-110 weeks) studies
performed at the same laboratory indicated an incidence of
hepatocellular adenomas in control males of 4.5-16.4%, with a mean
incidence of 10.9%. Therefore, the incidence of hepatocellular
adenomas was increased above historical control values in animals
treated at 75 and 375 ppm.
Dose-related non-neoplastic hepatic changes were observed in
animals of each sex at the interim and terminal kills, which were
characterized mainly by various combinations of hepatocellular
hypertrophy, centrilobular to disseminated single-cell necrosis,
diffuse Kupffer-cell proliferation, disseminated phagocytic cell
foci, lipofuscin accumulation and patchy glycogen storage. These
changes were generally more pronounced in males. At the terminal
kill, the changes were moderate to severe in males treated at 75 and
375 ppm, and these animals also had an increased incidence of
hepatocellular nodular hyperplasia. Males at 15 ppm had increased
incidences of slight hepatocellular hypertrophy, single-cell
necrosis and phagocytic-cell foci. Similar non-neoplastic hepatic
changes were seen at the terminal kill in females at 375 ppm,
whereas only increased incidences of slight single-cell necrosis and
phagocytic-cell foci were noted at 75 ppm; at 15 ppm, slight
single-cell necrosis was seen. Slight to moderate bile duct
proliferation was seen in a dose-related fashion, and a decrease in
the amount of normal centrilobular fat storage was observed in males
at 75 and 375 ppm. Females at the highest dose had an increased
incidence of a patchy, coarse-droplet fatty change. At the interim
kill, males at 75 and 375 ppm had increased incidences of many of
the same non-neoplastic hepatic changes. At 15 ppm, increased
incidences of hepatocellular hypertrophy and single-cell necrosis
were seen. Females at 375 ppm had increased incidences of
phagocytic-cell foci and patchy fatty change at the interim kill. In
view of the treatment-related histopathological hepatic effects at
15 ppm, equal to 2.1 mg/kg bw per day, no NOAEL could be identified
in this study. Although there was an increased incidence of hepatic
effects observed at 15 ppm, they were not significantly increased in
terms of severity (Suter et al., 1987b).
Liver sections from males (comprising one routine slide and six
additional slides from each liver, with three sections per slide,
making a total of 21 sections) in the study described above were
re-evaluated by an independent pathologist, who concluded that the
development of hepatocellular nodules, adenomas and carcinomas in
males was not related to treatment with teflubenzuron. A possibly
dose-related increase in the incidence of hepatocellular nodules was
observed, however, in males on the basis of the pathologist's
diagnoses: 2/60 in controls, 0/60 at 15 ppm, 6/60 at 75 ppm and
12/60 at 375 ppm. In addition, a slight but non-significant increase
in the incidence of hepatocellular adenomas was noted in males at 75
and 375 ppm: 8/60 in controls, 5/60 at 15 ppm, 13/60 at 75 ppm and
13/60 at 375 ppm. The revised diagnoses were based on use of more
detailed diagnostic criteria for hepatocellular nodules and adenomas
than those described in the report of Suter et al. (1987b). The
pathologist's report concluded that teflubenzuron 'did not manifest
either tumorigenic effect (enhancement of hepatocellular adenomas)
or carcinogenic effect (enhancement of hepatocellular carcinomas)'
(Vesselinovitch, 1988). The Meeting concluded that this
re-evaluation could not override the report of the pathologist of
the original study.
Rats
In a 120-week study of toxicity and carcinogenicity, groups of
70 male and 70 female Wistar rats were administered teflubenzuron
(purity, 92.4%) in the diet at 0, 20, 100 or 500 ppm, equivalent to
1, 5 or 25 mg/kg bw per day. Ten rats of each sex in each group were
killed at 53 and 107 weeks and the remainder at 120 weeks. The mean
plasma concentrations of teflubenzuron determined in surviving
animals before the kill at week 107 were 0.02 µg/ml for animals of
each sex at 20 ppm, 0.06 µg/ml for males and 0.04 µg/ml for females
at 100 ppm and 0.26 µg/ml for males and 0.13 µg/ml for females at
500 ppm. Treatment did not affect mortality, signs of toxicity, body
weight, food consumption or the results of ophthalmoscopic or
hearing tests; haematology and urinalysis indicated no
treatment-related changes. Clinical chemical analyses revealed
increased activities of aspartate and alanine aminotransferases at
weeks 14, 26, 53 and 78 and increased alanine aminotransferase
activity at week 120 in males treated at 500 ppm. Ornithine
carbamoyl transferase activity was increased at weeks 53 and 78 in
males at 500 ppm. Liver weights were slightly increased in males at
this dose at week 120. Gross pathology showed no treatment-related
changes. Histopathology revealed a statistically significant
increase in the incidence of haemangiomas in mesenteric lymph nodes
of males treated at the highest dose (8/47 compared to 1/48 control
males). The incidence of this tumour in the concurrent control group
was generally lower than that in historical control groups, and the
incidence in males at the high dose was within the historical
control range. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per
day (Suter et al., 1987c).
In a supplementary study, three groups of 60 male and 60 female
Wistar rats were given teflubenzuron (purity, 92.4%) in the diet at
0, 2500 or 10 000 ppm, equivalent to 125 or 500 mg/kg bw per day.
Ten animals of each sex at each dose were killed at week 104, and 50
of each sex in each group at week 111. The plasma concentrations of
teflubenzuron were determined in five animals of each sex at each
dose after 4, 26, 52 and 104 weeks. Time-related increases in plasma
concentrations were observed in animals of each sex; the
concentrations in animals treated at 2500 and 10 000 ppm were
0.18-0.79 and 0.23-0.70 µg/ml, resepctively, in males and 0.18-0.60
and 0.14-0.43 µg/ml, respectively, in females. The survival rate of
males at 10 000 ppm at termination was significantly increased over
that in controls. There were no treatment-related clinical signs of
toxicity or effects on food consumption, but the body-weight gains
of females were slightly reduced at both doses. Haematology and
urinalysis showed no treatment-related findings. Increased
activities of plasma alanine and aspartate aminotransferases were
seen in males at 2500 and 10 000 ppm throughout treatment. Liver
weights were increased in males at the highest dose at week 104 and
to a lesser extent at week 111. Gross pathology revealed increased
incidences of diffuse, clay-coloured discoloration and focal and
multifocal discoloration in livers of males at the highest dose.
Histopathology revealed a number of treatment-related non-neoplastic
liver lesions: Increased incidences of foci of mixed and basophilic
cells were noted in males at 2500 and 10 000 ppm, and an increased
incidence of basophilic-cell foci was seen in females at 10 000 ppm.
The incidence of focal hepatocellular hyperplasia was increased in
males at the highest dose, and the incidences of fatty change and
centrilobular hepatocellular hypertrophy were increased in males and
females at 2500 and 10 000 ppm. Spongiosis hepatis was increased in
incidence in males at the highest dose. As treatment-related
non-neoplastic findings were seen at both doses, no NOAEL could be
identified in this study (Tennekes et al., 1989).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing teflubenzuron (purity, 92.4%) at 0, 20, 100 or 500
ppm for two generations, with one litter per generation. The F0
generation was kept for 10 weeks before mating and the F1
generation for about 12 weeks. Reproductive performance and effects
on adults and offspring were monitored daily throughout the study;
observations included body weight, food consumption, mating
performance, fertility rate, duration of gestation, pup hair growth,
pinna unfolding, tooth eruption, eye opening, litter size, pup
weight, pup mortality and sex ratio. F1 pups underwent functional
tests, comprising pupillary reflex, startle response and ability to
learn use of a water maze, and the sexual organs of the parental and
F1 animals that received the highest dose were examined by gross
necropsy and histopathology. There was no evidence of toxicity and
no effects on reproductive performance (Osterburg, 1986). The NOAEL
was > 500 ppm, equal to 40 mg/kg bw per day.
(e) Embryotoxicity and teratogenicity
Rats
Teflubenzuron (purity, 96.5%) was administered in 0.5% aqueous
carboxymethyl-cellulose to groups of 25 naturally inseminated female
Wistar rats by gavage on days 6-15 after mating at doses of 0, 10,
50 or 250 mg/kg bw per day. The dams were killed on day 20 of
gestation, and the fetuses were examined after caesarean section.
The doses were selected on the basis of the results of a
range-finding study of doses up to 250 mg/kg bw per day (Gleich,
1984). There were no treatment-related fetotoxic or teratogenic
effects or signs of maternal toxicity. The NOAEL for both dams and
fetuses was 250 mg/kg bw per day (Gleich et al., 1984a).
Groups of 25 naturally inseminated female Wistar rats received
teflubenzuron (purity, 92.1%) in 0.5% aqueous carboxymethylcellulose
by gavage at doses of 0, 100, 300 or 1000 mg/kg bw per day on days
7-17 of gestation. The dams were killed on day 20 of gestation, and
the fetuses examined after caesarean section. There were no
treatment-related signs of maternal toxicity or any treatment-
related teratogenic or fetotoxic effects. The NOAEL for both dams
and fetuses was 1000 mg/kg bw per day (Ishida et al., 1987).
Rabbits
Groups of 14-15 Himalayan rabbits proven to be pregnant were
administered teflubenzuron (purity, 96.5%) in 0.5% aqueous
carboxymethylcellulose at 0, 10, 50 or 250 mg/kg bw per day by
stomach tube on days 6-18 of gestation. The doses were based on the
results of a range-finding study in which rabbits received doses of
up to 250 mg/kg bw per day with no sign of toxicity and no effect on
fetuses. Caesarean sections were performed on the dams on day 29 of
gestation. There were no treatment-related effects on the dams or
fetuses. The NOAEL for maternal toxicity, fetotoxicity and
teratogenicity was 250 mg/kg bw per day (Gleich et al., 1984b).
A group of 22 naturally inseminated New Zealand white rabbits,
18 of which were proven to be pregnant, were administered
teflubenzuron (purity, 96.5%) in 0.5% aqueous carboxymethyl
cellulose at 1000 mg/kg bw per day by stomach tube on days 6-18 of
gestation. Sixteen rabbits (15 proven to be pregnant) treated with
the vehicle alone served as the control group. The dams were killed
on day 28 of gestation. The only sign of maternal toxicity possibly
related to treatment was noted at necropsy as an increased incidence
of 'grossly granulated cut surfaces of the liver' in dams treated at
1000 mg/kg bw per day (in 8/22 as compared with 1/16 controls).
There were no signs of treatment-related fetotoxicity or
teratogenicity at this dose. The NOAEL for maternal toxicity could
not be established conclusively in view of the possible effect on
the liver. The NOAEL for fetotoxicity and teratogenicity was 1000
mg/kg bw per day (Osterburg, 1987).
Four groups of four or five pregnant Himalayan rabbits were
given teflubenzuron (purity unspecified) in 0.5% aqueous
carboxymethylcellulose by gavage at doses of 0, 250 or 500 mg/kg bw
per day (two groups at the highest dose) on days 6-18 of gestation.
All animals except those in the second group at the highest dose
were killed on day 19. Their livers were immediately removed and
weighed, and the cytochrome P450 content and O- and
N-demethylase activities were assessed in homogenates; the numbers
of corpora lutea, living embryos and early resorptions were counted.
Animals in the second group at the high dose underwent caesarian
section on day 29 and their livers were examined in the same way as
described above. Fetuses were incubated for 24 h before being
examined for malformations. No toxicologically significant findings
were found, and there was no evidence of liver enzyme induction in
the dams (Gleich, 1985).
(f) Genotoxicity
The results of tests for the genotoxicity of teflubenzuron
(Table 2) revealed no evidence for mutagenicity or clastogenicity.
(g) Special studies
Skin and eye irritation and skin sensitization
The potential of teflubenzuron (purity, 96.5%) to irritate skin
was investigated in three New Zealand white rabbits by applying 0.5
g of the compound, moistened with tap water, to intact skin on the
clipped dorsum of each rabbit and keeping it under an occlusive
dressing for 4 h. There was no evidence of skin irritation over 72 h
(Ullman, 1983f).
The potential of teflubenzuron (purity, 96.5%) to irritate the
eye was investigated in three New Zealand white rabbits by
introducing 0.1 g of undiluted test material into the conjunctival
sac of the left eye of each animal. Slight conjunctival and scleral
redness was observed in each animal after 1 h. No signs of
irritation were seen at 24, 48 or 72 h (Ullman, 1983g).
Table 2. Results of tests for the genotoxicity of teflubenzuron
End-point Test object Concentration Purity Results Reference
of teflubenzuron (%)
In vitro
Reverse mutation S. typhimurium TA98, 125-5000 µg/plate NR Negativea Kramer, 1982
100, 1535, 1537, 1538 in DMSO
Point mutation at Chinese hamster V79 5-50 µg/ml 92.4 Negativea Heidemann, 1986
hprt locus cell line in DMSO
Chromosomal Chinese hamster V79 4, 25, 50 µg/ml 92.4 Negativea Heidemann, 1985
aberration cell line in DMSO
Unscheduled DNA Wistar CF HB male 1-100 µg/ml 92.4 Negativea Müller, 1986
synthesis rat hepatocytes in acetone
In vivo
Micronucleus NMRI mice 5000 mg/kg bw in 96.5 Negative Guenard, 1984
formation 2% carboxymethyl-
cellulose, sodium salt
DMSO, dimethyl sulfoxide; NR, not reported
a In the presence and absence of metabolic activation
The potential of teflubenzuron (purity, 93.5%) to sensitize
skin was investigated in the guinea-pig (Dunkin-Hartley)
maximization test, in which 10 animals were used as negative
controls and 20 as the test group. The results of two topical
challenges after intradermal or topical induction with the test
substance indicated that teflubenzuron did not sensitize skin under
these conditions (Ullman, 1984).
3. Observations in humans
No information was available.
Comments
In rats, teflubenzuron was absorbed only partially from the
gastrointestinal tract, absorption being dose-dependent and
saturable. Absorbed teflubenzuron was excreted mainly via the bile,
urinary excretion representing only a minor route. Faecal excretion
of absorbed and unabsorbed teflubenzuron was the main route. There
was no evidence of bioaccumulation in organs or tissues.
Teflubenzuron was eliminated largely unchanged in faeces,
although a number of unidentified minor metabolites were found.
Hydroxylated metabolites of teflubenzuron were found in urine in low
amounts. 3,5-Dichloro-2,4-difluorophenylurea and its corresponding
substituted aniline were observed in urine, indicating that cleavage
of the benzoylurea moiety had occurred. Conjugates of these
metabolites and the unconjugated 3,5-dichloro-2,4-difluorophenylurea
were detected in bile.
Teflubenzuron has low acute oral, dermal and inhalational
toxicity; it was more acutely toxic when administered by the
intraperitoneal route. WHO (1992) has classified teflubenzuron as
being unlikely to present an acute hazard in normal use.
In mice, rats and dogs given repeated doses in the diet, the
major target organ was the liver. Pathological and clinical chemical
findings of hepatotoxicity varied with species, dose and duration of
dosing. The indicators of hepatotoxicity included effects such as
increased activities of serum alanine aminotransferase, aspartate
aminotransferase, ornithine carbamoyl transferase, lactate
dehydrogenase and alkaline phosphatase, increased liver weight, and
hepatocellular necrosis, fatty change, hypertrophy and hyperplasia.
Haematological parameters generally remained unaltered by treatment.
In a 13-week study of toxicity in mice fed levels of 0, 100,
1000 or 10 000 ppm, effects indicative of hepatotoxicity were
observed at 1000 and 10 000 ppm. The NOAEL was 100 ppm, equal to
11.9 mg/kg bw per day. In a 28-day range-finding and a 13-week study
of toxicity, rats were fed diets containing 0, 100, 1000 or 10 000
ppm. The NOAEL was 100 ppm in each, equal to 11.7 and 8.0 mg/kg bw
per day, respectively, on the basis of indications of
hepatotoxicity. Two 13-week studies in dogs fed diets containing 0,
100, 1000 or 10 000 ppm and 0, 30 or 100 ppm indicated an NOAEL of
100 ppm, equal to 4.1 mg/kg bw per day, on the basis of focal
gastritis in dogs treated at 1000 ppm in the first study. The NOAEL
in the 13-week studies concurred with the NOAEL of 100 ppm, equal to
3.2 mg/kg bw per day, observed in a 52-week study in dogs, in which
liver weights were increased in males fed the highest level of 500
ppm.
Mice fed diets containing 0, 15, 75 or 375 ppm for 18 months in
a study of carcinogenicity showed non-neoplastic hepatotoxicity at
all doses. Changes observed in livers of mice at the lowest dose
were increased in incidence over those in controls but were not
increased in severity. The lowest dose of 15 ppm, equal to 2.1 mg/kg
bw per day, was the LOAEL. Histopathological investigations
indicated an increased incidence of hepatocellular adenomas in males
at 75 and 375 ppm, in comparison with rates in concurrent and
historical controls. This tumorigenic potential in mice was
considered not to be relevant to humans.
In a 120-week study of toxicity and carcinogenicity, rats were
fed diets containing 0, 20, 100 or 500 ppm teflubenzuron. The NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of
increased serum enzyme activities and liver weights in males. In a
supplementary study, rats were fed diets containing 0, 2500 or 10
000 ppm for 111 weeks. No NOAEL could be assigned because
non-neoplastic liver changes and increased serum enzyme activities
were seen at both doses. There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats fed dietary concentrations of 0, 20,
100 or 500 ppm, the NOAEL was 500 ppm, equal to 40 mg/kg bw per day,
on the basis of lack of toxicity or effects on reproductive
performance.
Two studies of teratogenicity in rats treated by gavage showed
no evidence of maternal toxicity, fetotoxicity or teratogenicity at
doses up to either 250 or 1000 mg/kg bw per day. In a study of
teratogenicity in rabbits, there was no evidence of maternal
toxicity, fetotoxicity or teratogenicity at doses of 0, 10, 50 or
250 mg/kg bw per day. A second study of teratogenicity, in rabbits
treated by gavage at 0 or 1000 mg/kg bw per day, showed no evidence
of fetotoxicity or teratogenicity. Effects possibly related to
treatment were noted at necropsy in the livers of some dams treated
at 1000 mg/kg bw per day. In another study, there was no evidence of
liver enzyme induction in pregnant rabbits treated by gavage with
doses of up to 500 mg/kg bw per day.
Teflubenzuron has been adequately tested for genotoxicity in
vivo and in vitro in a range of assays. The Meeting concluded
that it was not genotoxic.
An ADI was allocated on the basis of the LOAEL of 15 ppm, equal
to 2.1 mg/kg bw per day, in the 18-month study of carcinogenicity in
mice. A 200-fold safety factor was applied since no NOAEL was
identified in this study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 11.9 mg/kg bw per day (13-week
study of toxicity)
Rat: 100 ppm, equal to 4.8 mg/kg bw per day (120-week
study of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
1000 mg/kg bw per day (study of teratogenicity,
maternal and fetal toxicity)
Rabbit: 1000 mg/kg bw per day (fetal toxicity in a study of
teratogenicity)
250 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Lowest-observed-adverse-effect level
Mouse: 15 ppm, equal to 2.1 mg/kg bw per day (18-month study
of carcinogenicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Bathe, R. (1983) 28-Day oral toxicity (feeding) study with CME 134
in beagle dogs. Project 017212. Document No. 134AB-432-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.,
London, United Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1984) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 018865. Document No. 134AB-433-007. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1985) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 040702. Document No. 134AB-433-008. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987a) The disposition
of [14C]-CME 134 in the lactating goat. Report No. 4278A. Document
No. 134AX-652-001. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987b) The disposition
of [14C]-CME 134 in the laying hen. Report No. 4278B. Unpublished
report from Inveresk Research International Ltd, Musselburgh,
Scotland, United Kingdom. Submitted to WHO by Shell International
Chemical Co. Ltd.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1988)
Further identification of [14C]-CME 134 metabolites in the hen.
Unpublished report from Inveresk Research International Ltd,
Musselburgh, Scotland, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1989)
Further identification of [14C]-CME 134 metabolites in the goat.
Report No. 4490A. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ellgehausen, K. (1986) 28-Day dietary study with CME 134 in the rat
for proof of absorption. Project 059973. Document No. 134AB-651-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Gleich, J. (1984) CME 134. Dosifindungsversuch für eine Prufung auf
embryotoxische Wirkung an Ratten nach oraler Applikation. Report No.
4/7/84. Document No 134AB-451-001. Unpublished report from Institute
of Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J. (1985) CME 134. Supplementary study on embryotoxicity and
liver enzyme induction in Himalayan rabbits. Report No. 4/65/85.
Document No. 134AB-451-005. Unpublished report from Institute of
Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G. & Unkelbach, H. (1984a) CME 134.
Embryotoxicity study in rats after oral administration. Report No.
4/29/84. Document No. 134AB-451-003. Unpublished report from
Institute of Toxicology, E. Merck, Darmstadt, Germany. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G., Unkelbach, H.D. & Hofmann, A. (1984b) CME
134. Embryotoxicity study in rabbits after oral administration.
Report No. 4/63/84. Document No. 134AB-451-004. Unpublished report
from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Guenard, J. (1984) Mouse micronucleus assay with CME 134. Project
025672. Unpublished report from Resarch and Consulting Co. Ag,
Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Hawkins, D.R. & Mayo, B.C. (1988) The biliary excretion and
metabolism of [14C]-CME 134. Report No. HRC/CMK 17/871263. Document
No. 134AX-651-010. Unpublished report from Huntingdon Research
Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1985) CME 134. Chromosome aberrations in cells of
Chinese hamster cell line V79. Study LMP 135C. Document No.
134AB-457-003. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1986) CME 134. Detection of gene mutations in somatic
mammalian cells in culture: HGPRT-test with V79 cells. Study LMP
135B. Document No. 134AC-457-005. Unpublished report from the
Laboratory for Mutagenicity Testing, Darmstadt, Germany. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ishida, S., Yamazaki, E., Ikeya, M. & Suzuki, K. (1987)
Teratological study in rats treated orally with teflubenzuron. Study
No. R-128. TZ-432-001. Unpublished report from Bozo Research Centre
Ltd, Tokyo, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Kramer, P.J. (1982) CME 134. In vitro assessment for mutagenic
potential in bacteria with and without addition of a metabolizing
system. Experiment T12 568. Document No. 134AA-457-001. Unpublished
report from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Müller, E. (1986) CME 134. Unscheduled DNA synthesis in hepatocytes
of male rats in vitro (UDS test). Study LMP 135A. Document No.
134AC-457-006. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Osterburg, I. (1986) Two generation oral (dietary administration)
reproduction toxicity study in the rat. Project No. 460/1. Document
No. 134AB-453-002. Unpublished report from Hazleton Laboratories,
Münster, Germany. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Osterburg, I. (1987) CME 134. Oral (gavage) teratogenicity limit
test in the rabbit. Project No. 460/13. Document No. 134AB-451-009.
Unpublished report from Hazleton Laboratories, Münster, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Sachsse, K. (1986) 52-Week oral (feeding) toxicity study with CME
134 in beagle dogs. Project 034828. Document No. 134AB-437-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Schlüter, H. (1984) Initial investigations on the biokinetics of
CME134 in the rat. Document No. 134AA-651-001. Unpublished report
from Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1985) Investigations on the metabolism of CME134 in
the rat. Document No. 134AA-651-012. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1986) The biokinetics and metabolism of [14C]-CME 134
in the rat. Document No. 134AX-651-007. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P. (1983) 28 Day range-finding study with CME 134 in rats.
Project 017201. Document No. 134AB-432-002. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Horst, K., Luetkemeier, H., Chevalier, J. & Terrier, C.
(1987a) 13-Week subchronic toxicity (feeding) study with CME 134 in
the rat. Project 018843. Document No. 134AB-433-006. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Westen, H. & Terrier, C.
(1987b) 18-Month oncogenicity (feeding) study with CME 134 in mice.
Project 027810. Document No. 134AB-455-003. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Schlotke, B., Ellgehausen,
H., Terrier, Ch. (1987c) 120-week chronic toxicity and oncogenicity
study with CME 134 in the rat. Project 027472. Document No
134AB-437-006. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Takahashi, K., Saitoh, T., Miyaoka, S., Maita, K. & Goloh, M. (1987)
13 Weeks subacute toxicity study with teflubenzuron in mice. Report
T-15. Document no. 134AB-433-005. Unpublished report from Kodaira
Laboratory, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Tasheva, M. & Hristeva, V. (1993) Comparative study on the effects
of five benzoylphenylurea insecticides on haematological parameters
in rats. J. Appl. Toxicol., 13, 67-68.
Tennekes, H., Stucki, P., Luetkemeier, H., Biedermann, K., Bloch,
M., Chevalier, H., Vogel, O. & Terrier, C. (1989) Chronic toxicity
and oncogenicity (feeding) study with CME 134 in the rat. Project
064192. Document No. 134AB-437-009. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983a) Acute oral toxicity study (LD50) with CME 134
in rats. Project 019596. Document No. 134AB-421-004. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983b) Acute oral toxicity study (LD50) with CME 134
in mice. Project 025571. Document No. 134 AB-421-005. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983c) Acute dermal toxicity study (LD50) with CME 134
in rats. Project 025593. Document No. 134 AB-422-001. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983d) Acute intraperitoneal toxicity study (LD50)
with CME 134 in rats. Project 025582. Document No. 134AB-424-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L. (1983e) 4-Hour dust-aerosol inhalation toxicity study
(LD50) with CME 134 in rats. Project 025626. Document No.
134AB-423-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983f) Primary skin irritation study following a single
4-hour occlusive application with CME 134 in the rabbit. Project
025604. Document No. 134AB-465-001. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983g) Primary eye irritation study after single
application with CME 134 in the rabbit. Project 025615. Document No.
134AB-466-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1984) Test for delayed hypersensitivity in the albino
guinea-pig with CME 134 Project 034817. Document No. 134AB-467-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L., Sacher, R. & Vogel, O. (1988) Acute oral toxicity study
with teflubenzuron in rats. Project 206741, Document No.
134AC-421-009. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Vesselinovitch, S. (1988) Histologic evaluation of liver tissues
originating from potential carcinogenicity bioassay studies of CME
134, Batch DW 44/83 in NMRI mice. Unpublished report from the
Departments of Pathology and Radiology, University of Chicago, IL,
USA. Submitted to WHO by Shell Chemical Co. Ltd, London, United
Kingdom.
See Also:
Toxicological Abbreviations
 The nature of the excretion products in faecal extracts, bile
and urine from rats with bile cannulas that were given 25 or 750
mg/kg bw 14C-aniline ring-labelled teflubenzuron orally was
investigated by TLC. Most of the radiolabel extracted from 0-48-h
faeces of treated animals of each sex co-chromatographed with
teflubenzuron. In 0-48-h bile, most of the radiolabel was in
unidentified polar material. A minor biliary metabolite
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV, see Figure 1), and another co-chromatographed with
teflubenzuron. Hydrolytic treatment of 0-48-h bile extracts
indicated the presence of conjugates of 3,5-dichloro-2,4-
difluorophenyl urea (conjugate IV, see Figure 1), the corresponding
substituted aniline (conjugate V) and the meta-hydroxybenzoyl
derivative of teflubenzuron (metabolite I). The major radioactive
component in 0-48-h urine extracts from animals at 25 mg/kg bw was
unidentified polar material. Three minor urinary metabolites
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV), its corresponding substituted aniline metabolite
(metabolite V) and the meta-hydroxybenzoyl derivative (metabolite
I) of teflubenzuron (Hawkins & Mayo, 1988).
Chickens
Laying hens that had been administered a total of 1.25 mg/kg bw
per day of 14C-teflubenzuron by oral doses twice daily for 7.5
days were shown by TLC and HPLC to excrete mainly the parent
compound. Radiolabel in bile was in the form of a very polar
compound, which on treatment with ß-glucuronidase yielded a compound
with similar chromatographic characteristics to the
meta-hydroxybenzoyl derivative (metabolite I, see Figure 1) of
teflubenzuron (22% of radioactivity in bile). A compound with
identical HPLC retention characteristics to teflubenzuron was found
in fat, liver, plasma and egg yolk (Cameron et al., 1987b).
Further investigations of metabolites in chickens by mass
spectrometry showed that teflubenzuron was the major component of
excreta. Two components of liver and kidney extracts were observed
by TLC and HPLC; one had characteristics identical to those of
teflubenzuron, but the other could not be identified. A third
component of kidney extracts was shown to be 3,5-dichloro-2,4-
difluorophenyl urea (metabolite IV, see Figure 1). Further evidence
was provided for the presence of teflubenzuron in egg yolk and fat
(Cameron et al., 1988).
Goats
In lactating goats given oral doses twice daily to give a total
of 1 mg/kg bw per day 14C-teflubenzuron over 7.5 days, the major
faecal component was shown by TLC to be unchanged teflubenzon. About
41% of the biliary radiolabel had similar characteristics on HPLC
and TLC to the meta-hydroxybenzoyl derivative (metabolite I, see
Figure 1) of teflubenzuron after treatment with ß-glucuronidase. No
unchanged teflubenzuron was found in bile (Cameron et al., 1987a).
Mass spectrometry showed conclusively that the major faecal
component was teflubenzuron. In goat liver, the major radioactive
component was a polar compound, which was not considered to be a
conjugate since it was unaffected by treatment with deconjugating
enzymes. Traces of a metabolite that co-chromatographed with the
meta-hydroxybenzoyl derivative (metabolite I) of teflubenzuron
were found in liver extracts (Cameron et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of teflubenzuron
(purity, 96.5-97.5%) are summarized in Table 1. After oral
treatment, rats and mice showed only slight signs of toxicity,
including ruffled fur, dyspnoea, sedation and hunched posture, which
had resolved in all cases by 48-72 h. After intraperitoneal
administration, similar signs of toxicity were observed, which were
generally more severe and longer lasting. Deaths unrelated to
treatment were seen at 300 mg/kg bw, the lowest dose tested. There
was no evidence of toxicity after dermal treatment at 2000 mg/kg bw.
Slight dyspnoea and ruffled fur were the only findings after
inhalation, and these had resolved within 24 h. Teflubenzuron had no
clear effect on gross pathological findings in these studies.
Table 1. Acute toxicity of teflubenzuron in male and female rodents
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Mouse Oral > 5000 Ullman, 1983b
Rat Oral > 5000 Ullman et al., 1988
Rat Oral > 5000 Ullman, 1983a
Rat Dermal > 2000 Ullman, 1983c
Rat Intraperitonea] > 2000 Ullman, 1983d
Rat Inhalation > 5038 Ullman, 1983e
(b) Short-term toxicity
Mice
Groups of 12 male and 12 female CD-1 mice were administered
teflubenzuron (purity unspecified) at 0, 100, 1000 or 10 000 ppm in
the diet for 13 weeks, equal to 12, 115 or 1210 mg/kg bw per day in
males and 14, 143 or 1450 mg/kg bw per day in females. (The
techniques used to investigate biological parameters were not
specified.) Treatment appeared to have no effect on clinical signs
of toxicity, deaths, body-weight gain, food consumption or the
results of ophthalmoscopy, urinalysis and haematology. Clinical
chemical investigations revealed increased alanine aminotransferase
activity (in animals of each sex) and a raised cholesterol level (in
females) at 1000 and 10 000 ppm, and increased alkaline phosphatase
activity in females at 10 000 ppm. Necropsy revealed liver
enlargement in animals of each sex and dark-coloured livers in males
at 1000 and 10 000 ppm. Liver weights were increased in animals of
each sex at the middle and high doses. Histopathology revealed
centrilobular hepatocellular swelling in animals of each sex and
fatty change in males at 1000 and 10 000 ppm and microgranuloma in
males at 10 000 ppm. The NOAEL was 100 ppm, equal to 12 mg/kg bw per
day (Takahashi et al., 1987).
Rats
In a 28-day range-finding study, groups of five male and five
female Wistar rats were administered teflubenzuron (purity not
specified) in the diet at levels of 0, 100, 1000 or 10 000 ppm.
There were no deaths or signs of toxicity and no significant effects
on body-weight gain. Food consumption of males at the highest dose
level was slightly reduced. Haematology and urinalysis showed no
treatment-related findings. Clinical chemical analyses revealed
treatment-related increases in the activities of serum aspartate and
alanine aminotransferase in animals of each sex and of lactate
dehydrogenase in females at 1000 and 10 000 ppm. Gross pathology
revealed no treatment-related lesions. Histopathology and organ
weight measurements were not performed. The NOAEL was 100 ppm, equal
to 12 mg/kg bw per day (Suter, 1983).
Groups of 10 male and 10 female Wistar rats were administered
teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000 ppm in the
diet for 13 weeks. Additional groups of five male and five female
rats in the control and highest-dose groups were maintained on
control diet for a further four weeks after the 13-week treatment
phase to determine the reversibility of any findings. There were no
deaths and no treatment-related signs of toxicity. Body weights and
food consumption were not affected, and urinalysis, ophthalmoscopy
and haematology did not reveal any treatment-related findings.
Clinical chemical analyses showed increased activities of ornithine
carbamoyl transferase and alanine and aspartate aminotransferases in
animals of each sex at 1000 and 10 000 ppm at six weeks; males had
more significant increases and also an increase in alkaline
phosphatase activity at the highest dose. At 13 weeks, ornithine
carbamoyl transferase activity was increased in animals of each sex
at 10 000 ppm; some males had marked increases, and males also had
increased activities of alanine and aspartate aminotransferases and
slightly increased activities of lactate dehydrogenase and alkaline
phosphatase. At 17 weeks (after the four-week recovery phase),
clinical chemical parameters were not significantly altered in the
highest-dose group over those in the control group. Liver weights of
females and testicular weights of males were increased at the
highest dose at 13 weeks, but no treatment-related effects on organ
weights were observed at this dose at 17 weeks. Gross necropsy and
histopathology of animals at 13 and 17 weeks revealed no
treatment-related findings. The NOAEL was 100 ppm, equal to 8.0
mg/kg bw per day (Suter et al., 1987a).
The effects of treatment with aqueous solutions of five benzoyl
urea insecticides, including teflubenzuron, on haematological
parameters was investigated in 10 Wistar rats (sex not specified),
which were given 100 mg/kg bw per day of the active ingredient by
gavage for 28 days. Ten rats were used as controls. No overt signs
of toxicity were seen. Teflubenzuron slightly increased reticulocyte
counts; the increase was comparable to those seen after similar
treatment with diflubenzuron and hexaflumuron, but smaller than
those induced by flufenoxuron and triflumuron. No other
haematological parameters were affected by treatment with
teflubenzuron. Relatively minor effects on haemoglobin, mean
corpuscular haemoglobin concentration and methaemoglobin formation
were induced by some of the other insecticides (Tasheva & Hristeva,
1993).
Dogs
In a 28-day range-finding study, pairs of one male and one
female beagle dogs were fed diets containing 0, 100, 1000 or 10 000
ppm teflubenzuron (purity, 96.7%), equivalent to 2.5, 25 or 250
mg/kg bw per day. Treatment did not affect signs of toxicity, food
consumption or body weight, and haematology, clinical chemistry,
urinalysis and gross pathology revealed no significant
treatment-related effect. The NOAEL was 10 000 ppm, equivalent to
250 mg/kg bw per day (Bathe, 1983).
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000
ppm for 13 weeks. There were no deaths or signs of toxicity during
the study; food consumption and body-weight gain were unaffected,
and a hearing test, ophthalmoscopic examinations, haematology and
urinalysis showed no findings of toxicological significance.
Clinical chemical analyses revealed increased activities of alanine
and aspartate aminotransferases, alkaline phosphatase and ornithine
carbamoyl transferase in one male and one female at 10 000 ppm at
week 4; gamma-glutamyltranspeptidase activity was increased in the
same female. Alkaline phosphatase activity was raised in another
male and ornithine carbamoyl transferase activity increased in two
other males at the same dose. At week 8, alkaline phosphatase,
alanine aminotransferase and gamma-glutamyltrans-peptidase
activities were increased in one male at 10 000 ppm, but by week 13
only alanine aminotransferase activity was raised in this animal.
Liver weights were slightly increased in animals of each sex at the
highest dose. Gross pathology revealed a firm liver with many
irregularities in the contour of the capsule in one male at 10 000
ppm. Isolated dark-red foci were noted in the pyloric area of the
stomachs of two dogs at the middle dose and two at the high dose.
The incidence of nodular foci in the pyloric or fundic area was also
increased at the high dose. Histopathological examination indicated
slight to moderate hepatotoxicity, resembling chronic active
hepatitis, in one male and one female at 10 000 ppm and slight
hepatotoxicity in one male at 100 ppm. Moderate centrilobular
hepatic necrosis was seen in another male at 100 ppm, and slight
round-cell infiltration was seen in one male at 1000 ppm and one at
10 000 ppm. Slight to moderate focal gastritis was noted in two
females at 10 000 ppm and in one at 1000 ppm. Follicular hyperplasia
of the pyloric mucosa in the stomach was noted in one control male
and in three males and three females at the highest dose. In view of
the possibly treatment-related histopathological findings in the
livers of males at 100 ppm, no NOAEL was identified in this study
(Bathe et al., 1984).
In a supplementary study designed to establish a clear NOAEL,
groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30 or 100 ppm,
equivalent to 0.75 or 2.5 mg/kg bw per day, for 13 weeks, and the
same parameters were assessed as in the first study. No
toxicologically significant effects were observed in either treated
group (Bathe et al., 1985).
The combined NOAEL in the two 13-week studies was 100 ppm,
equal to 4.1 mg/kg bw per day, on the basis of pathological findings
in the stomachs of dogs treated at 1000 ppm in the first study.
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30, 100 or 500 ppm
for 52 weeks. There were no deaths and no treatment-related signs of
toxicity; food consumption, body-weight gain, auditory perception
and ophthalmoscopic findings were not affected by treatment, and
haematology, clinical chemistry and urinalysis showed no effect.
Liver weights were increased in males treated at 500 ppm. Gross
necropsy and histopathological investigations revealed no other
treatment-related findings. The NOAEL was 100 ppm, equal to 3.2
mg/kg bw per day (Sachsse, 1986).
(c) Long-term toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, teflubenzuron (purity,
92.4%) was administered in the diet to groups of 60 male and 60
female NMRI mice at doses of 0, 15, 75 or 375 ppm, equal to 2.1, 10
or 54 mg/kg bw per day for males and 3.1, 15 or 72 mg/kg bw per day
for females. Ten animals of each sex in each group were killed at 12
months, and the remainder were killed at 18 months. Treatment did
not affect mortality or signs of toxicity, and ophthalmoscopy and
haematology showed no changes. Body-weight gain was reduced in males
at 375 ppm. Clinical chemical analyses revealed increased activities
of aspartate and alanine aminotransferases, lactate dehydrogenase,
alkaline phosphatase and ornithine carbamoyl transferase at 52 weeks
in males at 375 ppm. The alanine and aspartate aminotransferase and
ornithine carbamoyl transferase activities were still elevated at 78
weeks. Females treated at this dose had elevated alanine
aminotransferase and ornithine carbamoyl transferase activities at
week 52, and elevated alanine aminotransferase, lactate
dehydrogenase and ornithine carbamoyl transferase activities at week
78. Liver weights were increased at weeks 52 and 78 in males and
females at the highest dose and were slightly increased at week 78
in males at 75 ppm. Macroscopic investigations revealed an increased
incidence of hepatic nodules in males at 375 ppm and a dose-related
increase in the incidence of hepatic foci at week 78 in males at 75
and 375 ppm. Histopathological investigations indicated an increased
incidence of hepatocellular adenomas in males treated at 75 and 375
ppm at the time of the terminal kill: 6/50 in controls, 5/50 at 15
ppm, 11/50 at 75 ppm and 16/50 at 375 ppm. Contemporary historical
data on NMRI control mice from four long-term (84-110 weeks) studies
performed at the same laboratory indicated an incidence of
hepatocellular adenomas in control males of 4.5-16.4%, with a mean
incidence of 10.9%. Therefore, the incidence of hepatocellular
adenomas was increased above historical control values in animals
treated at 75 and 375 ppm.
Dose-related non-neoplastic hepatic changes were observed in
animals of each sex at the interim and terminal kills, which were
characterized mainly by various combinations of hepatocellular
hypertrophy, centrilobular to disseminated single-cell necrosis,
diffuse Kupffer-cell proliferation, disseminated phagocytic cell
foci, lipofuscin accumulation and patchy glycogen storage. These
changes were generally more pronounced in males. At the terminal
kill, the changes were moderate to severe in males treated at 75 and
375 ppm, and these animals also had an increased incidence of
hepatocellular nodular hyperplasia. Males at 15 ppm had increased
incidences of slight hepatocellular hypertrophy, single-cell
necrosis and phagocytic-cell foci. Similar non-neoplastic hepatic
changes were seen at the terminal kill in females at 375 ppm,
whereas only increased incidences of slight single-cell necrosis and
phagocytic-cell foci were noted at 75 ppm; at 15 ppm, slight
single-cell necrosis was seen. Slight to moderate bile duct
proliferation was seen in a dose-related fashion, and a decrease in
the amount of normal centrilobular fat storage was observed in males
at 75 and 375 ppm. Females at the highest dose had an increased
incidence of a patchy, coarse-droplet fatty change. At the interim
kill, males at 75 and 375 ppm had increased incidences of many of
the same non-neoplastic hepatic changes. At 15 ppm, increased
incidences of hepatocellular hypertrophy and single-cell necrosis
were seen. Females at 375 ppm had increased incidences of
phagocytic-cell foci and patchy fatty change at the interim kill. In
view of the treatment-related histopathological hepatic effects at
15 ppm, equal to 2.1 mg/kg bw per day, no NOAEL could be identified
in this study. Although there was an increased incidence of hepatic
effects observed at 15 ppm, they were not significantly increased in
terms of severity (Suter et al., 1987b).
Liver sections from males (comprising one routine slide and six
additional slides from each liver, with three sections per slide,
making a total of 21 sections) in the study described above were
re-evaluated by an independent pathologist, who concluded that the
development of hepatocellular nodules, adenomas and carcinomas in
males was not related to treatment with teflubenzuron. A possibly
dose-related increase in the incidence of hepatocellular nodules was
observed, however, in males on the basis of the pathologist's
diagnoses: 2/60 in controls, 0/60 at 15 ppm, 6/60 at 75 ppm and
12/60 at 375 ppm. In addition, a slight but non-significant increase
in the incidence of hepatocellular adenomas was noted in males at 75
and 375 ppm: 8/60 in controls, 5/60 at 15 ppm, 13/60 at 75 ppm and
13/60 at 375 ppm. The revised diagnoses were based on use of more
detailed diagnostic criteria for hepatocellular nodules and adenomas
than those described in the report of Suter et al. (1987b). The
pathologist's report concluded that teflubenzuron 'did not manifest
either tumorigenic effect (enhancement of hepatocellular adenomas)
or carcinogenic effect (enhancement of hepatocellular carcinomas)'
(Vesselinovitch, 1988). The Meeting concluded that this
re-evaluation could not override the report of the pathologist of
the original study.
Rats
In a 120-week study of toxicity and carcinogenicity, groups of
70 male and 70 female Wistar rats were administered teflubenzuron
(purity, 92.4%) in the diet at 0, 20, 100 or 500 ppm, equivalent to
1, 5 or 25 mg/kg bw per day. Ten rats of each sex in each group were
killed at 53 and 107 weeks and the remainder at 120 weeks. The mean
plasma concentrations of teflubenzuron determined in surviving
animals before the kill at week 107 were 0.02 µg/ml for animals of
each sex at 20 ppm, 0.06 µg/ml for males and 0.04 µg/ml for females
at 100 ppm and 0.26 µg/ml for males and 0.13 µg/ml for females at
500 ppm. Treatment did not affect mortality, signs of toxicity, body
weight, food consumption or the results of ophthalmoscopic or
hearing tests; haematology and urinalysis indicated no
treatment-related changes. Clinical chemical analyses revealed
increased activities of aspartate and alanine aminotransferases at
weeks 14, 26, 53 and 78 and increased alanine aminotransferase
activity at week 120 in males treated at 500 ppm. Ornithine
carbamoyl transferase activity was increased at weeks 53 and 78 in
males at 500 ppm. Liver weights were slightly increased in males at
this dose at week 120. Gross pathology showed no treatment-related
changes. Histopathology revealed a statistically significant
increase in the incidence of haemangiomas in mesenteric lymph nodes
of males treated at the highest dose (8/47 compared to 1/48 control
males). The incidence of this tumour in the concurrent control group
was generally lower than that in historical control groups, and the
incidence in males at the high dose was within the historical
control range. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per
day (Suter et al., 1987c).
In a supplementary study, three groups of 60 male and 60 female
Wistar rats were given teflubenzuron (purity, 92.4%) in the diet at
0, 2500 or 10 000 ppm, equivalent to 125 or 500 mg/kg bw per day.
Ten animals of each sex at each dose were killed at week 104, and 50
of each sex in each group at week 111. The plasma concentrations of
teflubenzuron were determined in five animals of each sex at each
dose after 4, 26, 52 and 104 weeks. Time-related increases in plasma
concentrations were observed in animals of each sex; the
concentrations in animals treated at 2500 and 10 000 ppm were
0.18-0.79 and 0.23-0.70 µg/ml, resepctively, in males and 0.18-0.60
and 0.14-0.43 µg/ml, respectively, in females. The survival rate of
males at 10 000 ppm at termination was significantly increased over
that in controls. There were no treatment-related clinical signs of
toxicity or effects on food consumption, but the body-weight gains
of females were slightly reduced at both doses. Haematology and
urinalysis showed no treatment-related findings. Increased
activities of plasma alanine and aspartate aminotransferases were
seen in males at 2500 and 10 000 ppm throughout treatment. Liver
weights were increased in males at the highest dose at week 104 and
to a lesser extent at week 111. Gross pathology revealed increased
incidences of diffuse, clay-coloured discoloration and focal and
multifocal discoloration in livers of males at the highest dose.
Histopathology revealed a number of treatment-related non-neoplastic
liver lesions: Increased incidences of foci of mixed and basophilic
cells were noted in males at 2500 and 10 000 ppm, and an increased
incidence of basophilic-cell foci was seen in females at 10 000 ppm.
The incidence of focal hepatocellular hyperplasia was increased in
males at the highest dose, and the incidences of fatty change and
centrilobular hepatocellular hypertrophy were increased in males and
females at 2500 and 10 000 ppm. Spongiosis hepatis was increased in
incidence in males at the highest dose. As treatment-related
non-neoplastic findings were seen at both doses, no NOAEL could be
identified in this study (Tennekes et al., 1989).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing teflubenzuron (purity, 92.4%) at 0, 20, 100 or 500
ppm for two generations, with one litter per generation. The F0
generation was kept for 10 weeks before mating and the F1
generation for about 12 weeks. Reproductive performance and effects
on adults and offspring were monitored daily throughout the study;
observations included body weight, food consumption, mating
performance, fertility rate, duration of gestation, pup hair growth,
pinna unfolding, tooth eruption, eye opening, litter size, pup
weight, pup mortality and sex ratio. F1 pups underwent functional
tests, comprising pupillary reflex, startle response and ability to
learn use of a water maze, and the sexual organs of the parental and
F1 animals that received the highest dose were examined by gross
necropsy and histopathology. There was no evidence of toxicity and
no effects on reproductive performance (Osterburg, 1986). The NOAEL
was > 500 ppm, equal to 40 mg/kg bw per day.
(e) Embryotoxicity and teratogenicity
Rats
Teflubenzuron (purity, 96.5%) was administered in 0.5% aqueous
carboxymethyl-cellulose to groups of 25 naturally inseminated female
Wistar rats by gavage on days 6-15 after mating at doses of 0, 10,
50 or 250 mg/kg bw per day. The dams were killed on day 20 of
gestation, and the fetuses were examined after caesarean section.
The doses were selected on the basis of the results of a
range-finding study of doses up to 250 mg/kg bw per day (Gleich,
1984). There were no treatment-related fetotoxic or teratogenic
effects or signs of maternal toxicity. The NOAEL for both dams and
fetuses was 250 mg/kg bw per day (Gleich et al., 1984a).
Groups of 25 naturally inseminated female Wistar rats received
teflubenzuron (purity, 92.1%) in 0.5% aqueous carboxymethylcellulose
by gavage at doses of 0, 100, 300 or 1000 mg/kg bw per day on days
7-17 of gestation. The dams were killed on day 20 of gestation, and
the fetuses examined after caesarean section. There were no
treatment-related signs of maternal toxicity or any treatment-
related teratogenic or fetotoxic effects. The NOAEL for both dams
and fetuses was 1000 mg/kg bw per day (Ishida et al., 1987).
Rabbits
Groups of 14-15 Himalayan rabbits proven to be pregnant were
administered teflubenzuron (purity, 96.5%) in 0.5% aqueous
carboxymethylcellulose at 0, 10, 50 or 250 mg/kg bw per day by
stomach tube on days 6-18 of gestation. The doses were based on the
results of a range-finding study in which rabbits received doses of
up to 250 mg/kg bw per day with no sign of toxicity and no effect on
fetuses. Caesarean sections were performed on the dams on day 29 of
gestation. There were no treatment-related effects on the dams or
fetuses. The NOAEL for maternal toxicity, fetotoxicity and
teratogenicity was 250 mg/kg bw per day (Gleich et al., 1984b).
A group of 22 naturally inseminated New Zealand white rabbits,
18 of which were proven to be pregnant, were administered
teflubenzuron (purity, 96.5%) in 0.5% aqueous carboxymethyl
cellulose at 1000 mg/kg bw per day by stomach tube on days 6-18 of
gestation. Sixteen rabbits (15 proven to be pregnant) treated with
the vehicle alone served as the control group. The dams were killed
on day 28 of gestation. The only sign of maternal toxicity possibly
related to treatment was noted at necropsy as an increased incidence
of 'grossly granulated cut surfaces of the liver' in dams treated at
1000 mg/kg bw per day (in 8/22 as compared with 1/16 controls).
There were no signs of treatment-related fetotoxicity or
teratogenicity at this dose. The NOAEL for maternal toxicity could
not be established conclusively in view of the possible effect on
the liver. The NOAEL for fetotoxicity and teratogenicity was 1000
mg/kg bw per day (Osterburg, 1987).
Four groups of four or five pregnant Himalayan rabbits were
given teflubenzuron (purity unspecified) in 0.5% aqueous
carboxymethylcellulose by gavage at doses of 0, 250 or 500 mg/kg bw
per day (two groups at the highest dose) on days 6-18 of gestation.
All animals except those in the second group at the highest dose
were killed on day 19. Their livers were immediately removed and
weighed, and the cytochrome P450 content and O- and
N-demethylase activities were assessed in homogenates; the numbers
of corpora lutea, living embryos and early resorptions were counted.
Animals in the second group at the high dose underwent caesarian
section on day 29 and their livers were examined in the same way as
described above. Fetuses were incubated for 24 h before being
examined for malformations. No toxicologically significant findings
were found, and there was no evidence of liver enzyme induction in
the dams (Gleich, 1985).
(f) Genotoxicity
The results of tests for the genotoxicity of teflubenzuron
(Table 2) revealed no evidence for mutagenicity or clastogenicity.
(g) Special studies
Skin and eye irritation and skin sensitization
The potential of teflubenzuron (purity, 96.5%) to irritate skin
was investigated in three New Zealand white rabbits by applying 0.5
g of the compound, moistened with tap water, to intact skin on the
clipped dorsum of each rabbit and keeping it under an occlusive
dressing for 4 h. There was no evidence of skin irritation over 72 h
(Ullman, 1983f).
The potential of teflubenzuron (purity, 96.5%) to irritate the
eye was investigated in three New Zealand white rabbits by
introducing 0.1 g of undiluted test material into the conjunctival
sac of the left eye of each animal. Slight conjunctival and scleral
redness was observed in each animal after 1 h. No signs of
irritation were seen at 24, 48 or 72 h (Ullman, 1983g).
Table 2. Results of tests for the genotoxicity of teflubenzuron
End-point Test object Concentration Purity Results Reference
of teflubenzuron (%)
In vitro
Reverse mutation S. typhimurium TA98, 125-5000 µg/plate NR Negativea Kramer, 1982
100, 1535, 1537, 1538 in DMSO
Point mutation at Chinese hamster V79 5-50 µg/ml 92.4 Negativea Heidemann, 1986
hprt locus cell line in DMSO
Chromosomal Chinese hamster V79 4, 25, 50 µg/ml 92.4 Negativea Heidemann, 1985
aberration cell line in DMSO
Unscheduled DNA Wistar CF HB male 1-100 µg/ml 92.4 Negativea Müller, 1986
synthesis rat hepatocytes in acetone
In vivo
Micronucleus NMRI mice 5000 mg/kg bw in 96.5 Negative Guenard, 1984
formation 2% carboxymethyl-
cellulose, sodium salt
DMSO, dimethyl sulfoxide; NR, not reported
a In the presence and absence of metabolic activation
The potential of teflubenzuron (purity, 93.5%) to sensitize
skin was investigated in the guinea-pig (Dunkin-Hartley)
maximization test, in which 10 animals were used as negative
controls and 20 as the test group. The results of two topical
challenges after intradermal or topical induction with the test
substance indicated that teflubenzuron did not sensitize skin under
these conditions (Ullman, 1984).
3. Observations in humans
No information was available.
Comments
In rats, teflubenzuron was absorbed only partially from the
gastrointestinal tract, absorption being dose-dependent and
saturable. Absorbed teflubenzuron was excreted mainly via the bile,
urinary excretion representing only a minor route. Faecal excretion
of absorbed and unabsorbed teflubenzuron was the main route. There
was no evidence of bioaccumulation in organs or tissues.
Teflubenzuron was eliminated largely unchanged in faeces,
although a number of unidentified minor metabolites were found.
Hydroxylated metabolites of teflubenzuron were found in urine in low
amounts. 3,5-Dichloro-2,4-difluorophenylurea and its corresponding
substituted aniline were observed in urine, indicating that cleavage
of the benzoylurea moiety had occurred. Conjugates of these
metabolites and the unconjugated 3,5-dichloro-2,4-difluorophenylurea
were detected in bile.
Teflubenzuron has low acute oral, dermal and inhalational
toxicity; it was more acutely toxic when administered by the
intraperitoneal route. WHO (1992) has classified teflubenzuron as
being unlikely to present an acute hazard in normal use.
In mice, rats and dogs given repeated doses in the diet, the
major target organ was the liver. Pathological and clinical chemical
findings of hepatotoxicity varied with species, dose and duration of
dosing. The indicators of hepatotoxicity included effects such as
increased activities of serum alanine aminotransferase, aspartate
aminotransferase, ornithine carbamoyl transferase, lactate
dehydrogenase and alkaline phosphatase, increased liver weight, and
hepatocellular necrosis, fatty change, hypertrophy and hyperplasia.
Haematological parameters generally remained unaltered by treatment.
In a 13-week study of toxicity in mice fed levels of 0, 100,
1000 or 10 000 ppm, effects indicative of hepatotoxicity were
observed at 1000 and 10 000 ppm. The NOAEL was 100 ppm, equal to
11.9 mg/kg bw per day. In a 28-day range-finding and a 13-week study
of toxicity, rats were fed diets containing 0, 100, 1000 or 10 000
ppm. The NOAEL was 100 ppm in each, equal to 11.7 and 8.0 mg/kg bw
per day, respectively, on the basis of indications of
hepatotoxicity. Two 13-week studies in dogs fed diets containing 0,
100, 1000 or 10 000 ppm and 0, 30 or 100 ppm indicated an NOAEL of
100 ppm, equal to 4.1 mg/kg bw per day, on the basis of focal
gastritis in dogs treated at 1000 ppm in the first study. The NOAEL
in the 13-week studies concurred with the NOAEL of 100 ppm, equal to
3.2 mg/kg bw per day, observed in a 52-week study in dogs, in which
liver weights were increased in males fed the highest level of 500
ppm.
Mice fed diets containing 0, 15, 75 or 375 ppm for 18 months in
a study of carcinogenicity showed non-neoplastic hepatotoxicity at
all doses. Changes observed in livers of mice at the lowest dose
were increased in incidence over those in controls but were not
increased in severity. The lowest dose of 15 ppm, equal to 2.1 mg/kg
bw per day, was the LOAEL. Histopathological investigations
indicated an increased incidence of hepatocellular adenomas in males
at 75 and 375 ppm, in comparison with rates in concurrent and
historical controls. This tumorigenic potential in mice was
considered not to be relevant to humans.
In a 120-week study of toxicity and carcinogenicity, rats were
fed diets containing 0, 20, 100 or 500 ppm teflubenzuron. The NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of
increased serum enzyme activities and liver weights in males. In a
supplementary study, rats were fed diets containing 0, 2500 or 10
000 ppm for 111 weeks. No NOAEL could be assigned because
non-neoplastic liver changes and increased serum enzyme activities
were seen at both doses. There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats fed dietary concentrations of 0, 20,
100 or 500 ppm, the NOAEL was 500 ppm, equal to 40 mg/kg bw per day,
on the basis of lack of toxicity or effects on reproductive
performance.
Two studies of teratogenicity in rats treated by gavage showed
no evidence of maternal toxicity, fetotoxicity or teratogenicity at
doses up to either 250 or 1000 mg/kg bw per day. In a study of
teratogenicity in rabbits, there was no evidence of maternal
toxicity, fetotoxicity or teratogenicity at doses of 0, 10, 50 or
250 mg/kg bw per day. A second study of teratogenicity, in rabbits
treated by gavage at 0 or 1000 mg/kg bw per day, showed no evidence
of fetotoxicity or teratogenicity. Effects possibly related to
treatment were noted at necropsy in the livers of some dams treated
at 1000 mg/kg bw per day. In another study, there was no evidence of
liver enzyme induction in pregnant rabbits treated by gavage with
doses of up to 500 mg/kg bw per day.
Teflubenzuron has been adequately tested for genotoxicity in
vivo and in vitro in a range of assays. The Meeting concluded
that it was not genotoxic.
An ADI was allocated on the basis of the LOAEL of 15 ppm, equal
to 2.1 mg/kg bw per day, in the 18-month study of carcinogenicity in
mice. A 200-fold safety factor was applied since no NOAEL was
identified in this study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 11.9 mg/kg bw per day (13-week
study of toxicity)
Rat: 100 ppm, equal to 4.8 mg/kg bw per day (120-week
study of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
1000 mg/kg bw per day (study of teratogenicity,
maternal and fetal toxicity)
Rabbit: 1000 mg/kg bw per day (fetal toxicity in a study of
teratogenicity)
250 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Lowest-observed-adverse-effect level
Mouse: 15 ppm, equal to 2.1 mg/kg bw per day (18-month study
of carcinogenicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Bathe, R. (1983) 28-Day oral toxicity (feeding) study with CME 134
in beagle dogs. Project 017212. Document No. 134AB-432-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.,
London, United Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1984) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 018865. Document No. 134AB-433-007. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1985) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 040702. Document No. 134AB-433-008. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987a) The disposition
of [14C]-CME 134 in the lactating goat. Report No. 4278A. Document
No. 134AX-652-001. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987b) The disposition
of [14C]-CME 134 in the laying hen. Report No. 4278B. Unpublished
report from Inveresk Research International Ltd, Musselburgh,
Scotland, United Kingdom. Submitted to WHO by Shell International
Chemical Co. Ltd.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1988)
Further identification of [14C]-CME 134 metabolites in the hen.
Unpublished report from Inveresk Research International Ltd,
Musselburgh, Scotland, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1989)
Further identification of [14C]-CME 134 metabolites in the goat.
Report No. 4490A. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ellgehausen, K. (1986) 28-Day dietary study with CME 134 in the rat
for proof of absorption. Project 059973. Document No. 134AB-651-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Gleich, J. (1984) CME 134. Dosifindungsversuch für eine Prufung auf
embryotoxische Wirkung an Ratten nach oraler Applikation. Report No.
4/7/84. Document No 134AB-451-001. Unpublished report from Institute
of Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J. (1985) CME 134. Supplementary study on embryotoxicity and
liver enzyme induction in Himalayan rabbits. Report No. 4/65/85.
Document No. 134AB-451-005. Unpublished report from Institute of
Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G. & Unkelbach, H. (1984a) CME 134.
Embryotoxicity study in rats after oral administration. Report No.
4/29/84. Document No. 134AB-451-003. Unpublished report from
Institute of Toxicology, E. Merck, Darmstadt, Germany. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G., Unkelbach, H.D. & Hofmann, A. (1984b) CME
134. Embryotoxicity study in rabbits after oral administration.
Report No. 4/63/84. Document No. 134AB-451-004. Unpublished report
from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Guenard, J. (1984) Mouse micronucleus assay with CME 134. Project
025672. Unpublished report from Resarch and Consulting Co. Ag,
Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Hawkins, D.R. & Mayo, B.C. (1988) The biliary excretion and
metabolism of [14C]-CME 134. Report No. HRC/CMK 17/871263. Document
No. 134AX-651-010. Unpublished report from Huntingdon Research
Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1985) CME 134. Chromosome aberrations in cells of
Chinese hamster cell line V79. Study LMP 135C. Document No.
134AB-457-003. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1986) CME 134. Detection of gene mutations in somatic
mammalian cells in culture: HGPRT-test with V79 cells. Study LMP
135B. Document No. 134AC-457-005. Unpublished report from the
Laboratory for Mutagenicity Testing, Darmstadt, Germany. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ishida, S., Yamazaki, E., Ikeya, M. & Suzuki, K. (1987)
Teratological study in rats treated orally with teflubenzuron. Study
No. R-128. TZ-432-001. Unpublished report from Bozo Research Centre
Ltd, Tokyo, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Kramer, P.J. (1982) CME 134. In vitro assessment for mutagenic
potential in bacteria with and without addition of a metabolizing
system. Experiment T12 568. Document No. 134AA-457-001. Unpublished
report from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Müller, E. (1986) CME 134. Unscheduled DNA synthesis in hepatocytes
of male rats in vitro (UDS test). Study LMP 135A. Document No.
134AC-457-006. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Osterburg, I. (1986) Two generation oral (dietary administration)
reproduction toxicity study in the rat. Project No. 460/1. Document
No. 134AB-453-002. Unpublished report from Hazleton Laboratories,
Münster, Germany. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Osterburg, I. (1987) CME 134. Oral (gavage) teratogenicity limit
test in the rabbit. Project No. 460/13. Document No. 134AB-451-009.
Unpublished report from Hazleton Laboratories, Münster, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Sachsse, K. (1986) 52-Week oral (feeding) toxicity study with CME
134 in beagle dogs. Project 034828. Document No. 134AB-437-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Schlüter, H. (1984) Initial investigations on the biokinetics of
CME134 in the rat. Document No. 134AA-651-001. Unpublished report
from Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1985) Investigations on the metabolism of CME134 in
the rat. Document No. 134AA-651-012. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1986) The biokinetics and metabolism of [14C]-CME 134
in the rat. Document No. 134AX-651-007. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P. (1983) 28 Day range-finding study with CME 134 in rats.
Project 017201. Document No. 134AB-432-002. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Horst, K., Luetkemeier, H., Chevalier, J. & Terrier, C.
(1987a) 13-Week subchronic toxicity (feeding) study with CME 134 in
the rat. Project 018843. Document No. 134AB-433-006. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Westen, H. & Terrier, C.
(1987b) 18-Month oncogenicity (feeding) study with CME 134 in mice.
Project 027810. Document No. 134AB-455-003. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Schlotke, B., Ellgehausen,
H., Terrier, Ch. (1987c) 120-week chronic toxicity and oncogenicity
study with CME 134 in the rat. Project 027472. Document No
134AB-437-006. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Takahashi, K., Saitoh, T., Miyaoka, S., Maita, K. & Goloh, M. (1987)
13 Weeks subacute toxicity study with teflubenzuron in mice. Report
T-15. Document no. 134AB-433-005. Unpublished report from Kodaira
Laboratory, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Tasheva, M. & Hristeva, V. (1993) Comparative study on the effects
of five benzoylphenylurea insecticides on haematological parameters
in rats. J. Appl. Toxicol., 13, 67-68.
Tennekes, H., Stucki, P., Luetkemeier, H., Biedermann, K., Bloch,
M., Chevalier, H., Vogel, O. & Terrier, C. (1989) Chronic toxicity
and oncogenicity (feeding) study with CME 134 in the rat. Project
064192. Document No. 134AB-437-009. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983a) Acute oral toxicity study (LD50) with CME 134
in rats. Project 019596. Document No. 134AB-421-004. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983b) Acute oral toxicity study (LD50) with CME 134
in mice. Project 025571. Document No. 134 AB-421-005. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983c) Acute dermal toxicity study (LD50) with CME 134
in rats. Project 025593. Document No. 134 AB-422-001. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983d) Acute intraperitoneal toxicity study (LD50)
with CME 134 in rats. Project 025582. Document No. 134AB-424-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L. (1983e) 4-Hour dust-aerosol inhalation toxicity study
(LD50) with CME 134 in rats. Project 025626. Document No.
134AB-423-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983f) Primary skin irritation study following a single
4-hour occlusive application with CME 134 in the rabbit. Project
025604. Document No. 134AB-465-001. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983g) Primary eye irritation study after single
application with CME 134 in the rabbit. Project 025615. Document No.
134AB-466-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1984) Test for delayed hypersensitivity in the albino
guinea-pig with CME 134 Project 034817. Document No. 134AB-467-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L., Sacher, R. & Vogel, O. (1988) Acute oral toxicity study
with teflubenzuron in rats. Project 206741, Document No.
134AC-421-009. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Vesselinovitch, S. (1988) Histologic evaluation of liver tissues
originating from potential carcinogenicity bioassay studies of CME
134, Batch DW 44/83 in NMRI mice. Unpublished report from the
Departments of Pathology and Radiology, University of Chicago, IL,
USA. Submitted to WHO by Shell Chemical Co. Ltd, London, United
Kingdom.
The nature of the excretion products in faecal extracts, bile
and urine from rats with bile cannulas that were given 25 or 750
mg/kg bw 14C-aniline ring-labelled teflubenzuron orally was
investigated by TLC. Most of the radiolabel extracted from 0-48-h
faeces of treated animals of each sex co-chromatographed with
teflubenzuron. In 0-48-h bile, most of the radiolabel was in
unidentified polar material. A minor biliary metabolite
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV, see Figure 1), and another co-chromatographed with
teflubenzuron. Hydrolytic treatment of 0-48-h bile extracts
indicated the presence of conjugates of 3,5-dichloro-2,4-
difluorophenyl urea (conjugate IV, see Figure 1), the corresponding
substituted aniline (conjugate V) and the meta-hydroxybenzoyl
derivative of teflubenzuron (metabolite I). The major radioactive
component in 0-48-h urine extracts from animals at 25 mg/kg bw was
unidentified polar material. Three minor urinary metabolites
co-chromatographed with 3,5-dichloro-2,4-difluorophenyl urea
(metabolite IV), its corresponding substituted aniline metabolite
(metabolite V) and the meta-hydroxybenzoyl derivative (metabolite
I) of teflubenzuron (Hawkins & Mayo, 1988).
Chickens
Laying hens that had been administered a total of 1.25 mg/kg bw
per day of 14C-teflubenzuron by oral doses twice daily for 7.5
days were shown by TLC and HPLC to excrete mainly the parent
compound. Radiolabel in bile was in the form of a very polar
compound, which on treatment with ß-glucuronidase yielded a compound
with similar chromatographic characteristics to the
meta-hydroxybenzoyl derivative (metabolite I, see Figure 1) of
teflubenzuron (22% of radioactivity in bile). A compound with
identical HPLC retention characteristics to teflubenzuron was found
in fat, liver, plasma and egg yolk (Cameron et al., 1987b).
Further investigations of metabolites in chickens by mass
spectrometry showed that teflubenzuron was the major component of
excreta. Two components of liver and kidney extracts were observed
by TLC and HPLC; one had characteristics identical to those of
teflubenzuron, but the other could not be identified. A third
component of kidney extracts was shown to be 3,5-dichloro-2,4-
difluorophenyl urea (metabolite IV, see Figure 1). Further evidence
was provided for the presence of teflubenzuron in egg yolk and fat
(Cameron et al., 1988).
Goats
In lactating goats given oral doses twice daily to give a total
of 1 mg/kg bw per day 14C-teflubenzuron over 7.5 days, the major
faecal component was shown by TLC to be unchanged teflubenzon. About
41% of the biliary radiolabel had similar characteristics on HPLC
and TLC to the meta-hydroxybenzoyl derivative (metabolite I, see
Figure 1) of teflubenzuron after treatment with ß-glucuronidase. No
unchanged teflubenzuron was found in bile (Cameron et al., 1987a).
Mass spectrometry showed conclusively that the major faecal
component was teflubenzuron. In goat liver, the major radioactive
component was a polar compound, which was not considered to be a
conjugate since it was unaffected by treatment with deconjugating
enzymes. Traces of a metabolite that co-chromatographed with the
meta-hydroxybenzoyl derivative (metabolite I) of teflubenzuron
were found in liver extracts (Cameron et al., 1989).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of teflubenzuron
(purity, 96.5-97.5%) are summarized in Table 1. After oral
treatment, rats and mice showed only slight signs of toxicity,
including ruffled fur, dyspnoea, sedation and hunched posture, which
had resolved in all cases by 48-72 h. After intraperitoneal
administration, similar signs of toxicity were observed, which were
generally more severe and longer lasting. Deaths unrelated to
treatment were seen at 300 mg/kg bw, the lowest dose tested. There
was no evidence of toxicity after dermal treatment at 2000 mg/kg bw.
Slight dyspnoea and ruffled fur were the only findings after
inhalation, and these had resolved within 24 h. Teflubenzuron had no
clear effect on gross pathological findings in these studies.
Table 1. Acute toxicity of teflubenzuron in male and female rodents
Species Route LD50 (mg/kg bw) Reference
or LC50 (mg/m3)
Mouse Oral > 5000 Ullman, 1983b
Rat Oral > 5000 Ullman et al., 1988
Rat Oral > 5000 Ullman, 1983a
Rat Dermal > 2000 Ullman, 1983c
Rat Intraperitonea] > 2000 Ullman, 1983d
Rat Inhalation > 5038 Ullman, 1983e
(b) Short-term toxicity
Mice
Groups of 12 male and 12 female CD-1 mice were administered
teflubenzuron (purity unspecified) at 0, 100, 1000 or 10 000 ppm in
the diet for 13 weeks, equal to 12, 115 or 1210 mg/kg bw per day in
males and 14, 143 or 1450 mg/kg bw per day in females. (The
techniques used to investigate biological parameters were not
specified.) Treatment appeared to have no effect on clinical signs
of toxicity, deaths, body-weight gain, food consumption or the
results of ophthalmoscopy, urinalysis and haematology. Clinical
chemical investigations revealed increased alanine aminotransferase
activity (in animals of each sex) and a raised cholesterol level (in
females) at 1000 and 10 000 ppm, and increased alkaline phosphatase
activity in females at 10 000 ppm. Necropsy revealed liver
enlargement in animals of each sex and dark-coloured livers in males
at 1000 and 10 000 ppm. Liver weights were increased in animals of
each sex at the middle and high doses. Histopathology revealed
centrilobular hepatocellular swelling in animals of each sex and
fatty change in males at 1000 and 10 000 ppm and microgranuloma in
males at 10 000 ppm. The NOAEL was 100 ppm, equal to 12 mg/kg bw per
day (Takahashi et al., 1987).
Rats
In a 28-day range-finding study, groups of five male and five
female Wistar rats were administered teflubenzuron (purity not
specified) in the diet at levels of 0, 100, 1000 or 10 000 ppm.
There were no deaths or signs of toxicity and no significant effects
on body-weight gain. Food consumption of males at the highest dose
level was slightly reduced. Haematology and urinalysis showed no
treatment-related findings. Clinical chemical analyses revealed
treatment-related increases in the activities of serum aspartate and
alanine aminotransferase in animals of each sex and of lactate
dehydrogenase in females at 1000 and 10 000 ppm. Gross pathology
revealed no treatment-related lesions. Histopathology and organ
weight measurements were not performed. The NOAEL was 100 ppm, equal
to 12 mg/kg bw per day (Suter, 1983).
Groups of 10 male and 10 female Wistar rats were administered
teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000 ppm in the
diet for 13 weeks. Additional groups of five male and five female
rats in the control and highest-dose groups were maintained on
control diet for a further four weeks after the 13-week treatment
phase to determine the reversibility of any findings. There were no
deaths and no treatment-related signs of toxicity. Body weights and
food consumption were not affected, and urinalysis, ophthalmoscopy
and haematology did not reveal any treatment-related findings.
Clinical chemical analyses showed increased activities of ornithine
carbamoyl transferase and alanine and aspartate aminotransferases in
animals of each sex at 1000 and 10 000 ppm at six weeks; males had
more significant increases and also an increase in alkaline
phosphatase activity at the highest dose. At 13 weeks, ornithine
carbamoyl transferase activity was increased in animals of each sex
at 10 000 ppm; some males had marked increases, and males also had
increased activities of alanine and aspartate aminotransferases and
slightly increased activities of lactate dehydrogenase and alkaline
phosphatase. At 17 weeks (after the four-week recovery phase),
clinical chemical parameters were not significantly altered in the
highest-dose group over those in the control group. Liver weights of
females and testicular weights of males were increased at the
highest dose at 13 weeks, but no treatment-related effects on organ
weights were observed at this dose at 17 weeks. Gross necropsy and
histopathology of animals at 13 and 17 weeks revealed no
treatment-related findings. The NOAEL was 100 ppm, equal to 8.0
mg/kg bw per day (Suter et al., 1987a).
The effects of treatment with aqueous solutions of five benzoyl
urea insecticides, including teflubenzuron, on haematological
parameters was investigated in 10 Wistar rats (sex not specified),
which were given 100 mg/kg bw per day of the active ingredient by
gavage for 28 days. Ten rats were used as controls. No overt signs
of toxicity were seen. Teflubenzuron slightly increased reticulocyte
counts; the increase was comparable to those seen after similar
treatment with diflubenzuron and hexaflumuron, but smaller than
those induced by flufenoxuron and triflumuron. No other
haematological parameters were affected by treatment with
teflubenzuron. Relatively minor effects on haemoglobin, mean
corpuscular haemoglobin concentration and methaemoglobin formation
were induced by some of the other insecticides (Tasheva & Hristeva,
1993).
Dogs
In a 28-day range-finding study, pairs of one male and one
female beagle dogs were fed diets containing 0, 100, 1000 or 10 000
ppm teflubenzuron (purity, 96.7%), equivalent to 2.5, 25 or 250
mg/kg bw per day. Treatment did not affect signs of toxicity, food
consumption or body weight, and haematology, clinical chemistry,
urinalysis and gross pathology revealed no significant
treatment-related effect. The NOAEL was 10 000 ppm, equivalent to
250 mg/kg bw per day (Bathe, 1983).
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 96.5%) at 0, 100, 1000 or 10 000
ppm for 13 weeks. There were no deaths or signs of toxicity during
the study; food consumption and body-weight gain were unaffected,
and a hearing test, ophthalmoscopic examinations, haematology and
urinalysis showed no findings of toxicological significance.
Clinical chemical analyses revealed increased activities of alanine
and aspartate aminotransferases, alkaline phosphatase and ornithine
carbamoyl transferase in one male and one female at 10 000 ppm at
week 4; gamma-glutamyltranspeptidase activity was increased in the
same female. Alkaline phosphatase activity was raised in another
male and ornithine carbamoyl transferase activity increased in two
other males at the same dose. At week 8, alkaline phosphatase,
alanine aminotransferase and gamma-glutamyltrans-peptidase
activities were increased in one male at 10 000 ppm, but by week 13
only alanine aminotransferase activity was raised in this animal.
Liver weights were slightly increased in animals of each sex at the
highest dose. Gross pathology revealed a firm liver with many
irregularities in the contour of the capsule in one male at 10 000
ppm. Isolated dark-red foci were noted in the pyloric area of the
stomachs of two dogs at the middle dose and two at the high dose.
The incidence of nodular foci in the pyloric or fundic area was also
increased at the high dose. Histopathological examination indicated
slight to moderate hepatotoxicity, resembling chronic active
hepatitis, in one male and one female at 10 000 ppm and slight
hepatotoxicity in one male at 100 ppm. Moderate centrilobular
hepatic necrosis was seen in another male at 100 ppm, and slight
round-cell infiltration was seen in one male at 1000 ppm and one at
10 000 ppm. Slight to moderate focal gastritis was noted in two
females at 10 000 ppm and in one at 1000 ppm. Follicular hyperplasia
of the pyloric mucosa in the stomach was noted in one control male
and in three males and three females at the highest dose. In view of
the possibly treatment-related histopathological findings in the
livers of males at 100 ppm, no NOAEL was identified in this study
(Bathe et al., 1984).
In a supplementary study designed to establish a clear NOAEL,
groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30 or 100 ppm,
equivalent to 0.75 or 2.5 mg/kg bw per day, for 13 weeks, and the
same parameters were assessed as in the first study. No
toxicologically significant effects were observed in either treated
group (Bathe et al., 1985).
The combined NOAEL in the two 13-week studies was 100 ppm,
equal to 4.1 mg/kg bw per day, on the basis of pathological findings
in the stomachs of dogs treated at 1000 ppm in the first study.
Groups of four male and four female beagle dogs were fed diets
containing teflubenzuron (purity, 92.4%) at 0, 30, 100 or 500 ppm
for 52 weeks. There were no deaths and no treatment-related signs of
toxicity; food consumption, body-weight gain, auditory perception
and ophthalmoscopic findings were not affected by treatment, and
haematology, clinical chemistry and urinalysis showed no effect.
Liver weights were increased in males treated at 500 ppm. Gross
necropsy and histopathological investigations revealed no other
treatment-related findings. The NOAEL was 100 ppm, equal to 3.2
mg/kg bw per day (Sachsse, 1986).
(c) Long-term toxicity and carcinogenicity
Mice
In an 18-month study of carcinogenicity, teflubenzuron (purity,
92.4%) was administered in the diet to groups of 60 male and 60
female NMRI mice at doses of 0, 15, 75 or 375 ppm, equal to 2.1, 10
or 54 mg/kg bw per day for males and 3.1, 15 or 72 mg/kg bw per day
for females. Ten animals of each sex in each group were killed at 12
months, and the remainder were killed at 18 months. Treatment did
not affect mortality or signs of toxicity, and ophthalmoscopy and
haematology showed no changes. Body-weight gain was reduced in males
at 375 ppm. Clinical chemical analyses revealed increased activities
of aspartate and alanine aminotransferases, lactate dehydrogenase,
alkaline phosphatase and ornithine carbamoyl transferase at 52 weeks
in males at 375 ppm. The alanine and aspartate aminotransferase and
ornithine carbamoyl transferase activities were still elevated at 78
weeks. Females treated at this dose had elevated alanine
aminotransferase and ornithine carbamoyl transferase activities at
week 52, and elevated alanine aminotransferase, lactate
dehydrogenase and ornithine carbamoyl transferase activities at week
78. Liver weights were increased at weeks 52 and 78 in males and
females at the highest dose and were slightly increased at week 78
in males at 75 ppm. Macroscopic investigations revealed an increased
incidence of hepatic nodules in males at 375 ppm and a dose-related
increase in the incidence of hepatic foci at week 78 in males at 75
and 375 ppm. Histopathological investigations indicated an increased
incidence of hepatocellular adenomas in males treated at 75 and 375
ppm at the time of the terminal kill: 6/50 in controls, 5/50 at 15
ppm, 11/50 at 75 ppm and 16/50 at 375 ppm. Contemporary historical
data on NMRI control mice from four long-term (84-110 weeks) studies
performed at the same laboratory indicated an incidence of
hepatocellular adenomas in control males of 4.5-16.4%, with a mean
incidence of 10.9%. Therefore, the incidence of hepatocellular
adenomas was increased above historical control values in animals
treated at 75 and 375 ppm.
Dose-related non-neoplastic hepatic changes were observed in
animals of each sex at the interim and terminal kills, which were
characterized mainly by various combinations of hepatocellular
hypertrophy, centrilobular to disseminated single-cell necrosis,
diffuse Kupffer-cell proliferation, disseminated phagocytic cell
foci, lipofuscin accumulation and patchy glycogen storage. These
changes were generally more pronounced in males. At the terminal
kill, the changes were moderate to severe in males treated at 75 and
375 ppm, and these animals also had an increased incidence of
hepatocellular nodular hyperplasia. Males at 15 ppm had increased
incidences of slight hepatocellular hypertrophy, single-cell
necrosis and phagocytic-cell foci. Similar non-neoplastic hepatic
changes were seen at the terminal kill in females at 375 ppm,
whereas only increased incidences of slight single-cell necrosis and
phagocytic-cell foci were noted at 75 ppm; at 15 ppm, slight
single-cell necrosis was seen. Slight to moderate bile duct
proliferation was seen in a dose-related fashion, and a decrease in
the amount of normal centrilobular fat storage was observed in males
at 75 and 375 ppm. Females at the highest dose had an increased
incidence of a patchy, coarse-droplet fatty change. At the interim
kill, males at 75 and 375 ppm had increased incidences of many of
the same non-neoplastic hepatic changes. At 15 ppm, increased
incidences of hepatocellular hypertrophy and single-cell necrosis
were seen. Females at 375 ppm had increased incidences of
phagocytic-cell foci and patchy fatty change at the interim kill. In
view of the treatment-related histopathological hepatic effects at
15 ppm, equal to 2.1 mg/kg bw per day, no NOAEL could be identified
in this study. Although there was an increased incidence of hepatic
effects observed at 15 ppm, they were not significantly increased in
terms of severity (Suter et al., 1987b).
Liver sections from males (comprising one routine slide and six
additional slides from each liver, with three sections per slide,
making a total of 21 sections) in the study described above were
re-evaluated by an independent pathologist, who concluded that the
development of hepatocellular nodules, adenomas and carcinomas in
males was not related to treatment with teflubenzuron. A possibly
dose-related increase in the incidence of hepatocellular nodules was
observed, however, in males on the basis of the pathologist's
diagnoses: 2/60 in controls, 0/60 at 15 ppm, 6/60 at 75 ppm and
12/60 at 375 ppm. In addition, a slight but non-significant increase
in the incidence of hepatocellular adenomas was noted in males at 75
and 375 ppm: 8/60 in controls, 5/60 at 15 ppm, 13/60 at 75 ppm and
13/60 at 375 ppm. The revised diagnoses were based on use of more
detailed diagnostic criteria for hepatocellular nodules and adenomas
than those described in the report of Suter et al. (1987b). The
pathologist's report concluded that teflubenzuron 'did not manifest
either tumorigenic effect (enhancement of hepatocellular adenomas)
or carcinogenic effect (enhancement of hepatocellular carcinomas)'
(Vesselinovitch, 1988). The Meeting concluded that this
re-evaluation could not override the report of the pathologist of
the original study.
Rats
In a 120-week study of toxicity and carcinogenicity, groups of
70 male and 70 female Wistar rats were administered teflubenzuron
(purity, 92.4%) in the diet at 0, 20, 100 or 500 ppm, equivalent to
1, 5 or 25 mg/kg bw per day. Ten rats of each sex in each group were
killed at 53 and 107 weeks and the remainder at 120 weeks. The mean
plasma concentrations of teflubenzuron determined in surviving
animals before the kill at week 107 were 0.02 µg/ml for animals of
each sex at 20 ppm, 0.06 µg/ml for males and 0.04 µg/ml for females
at 100 ppm and 0.26 µg/ml for males and 0.13 µg/ml for females at
500 ppm. Treatment did not affect mortality, signs of toxicity, body
weight, food consumption or the results of ophthalmoscopic or
hearing tests; haematology and urinalysis indicated no
treatment-related changes. Clinical chemical analyses revealed
increased activities of aspartate and alanine aminotransferases at
weeks 14, 26, 53 and 78 and increased alanine aminotransferase
activity at week 120 in males treated at 500 ppm. Ornithine
carbamoyl transferase activity was increased at weeks 53 and 78 in
males at 500 ppm. Liver weights were slightly increased in males at
this dose at week 120. Gross pathology showed no treatment-related
changes. Histopathology revealed a statistically significant
increase in the incidence of haemangiomas in mesenteric lymph nodes
of males treated at the highest dose (8/47 compared to 1/48 control
males). The incidence of this tumour in the concurrent control group
was generally lower than that in historical control groups, and the
incidence in males at the high dose was within the historical
control range. The NOAEL was 100 ppm, equivalent to 5 mg/kg bw per
day (Suter et al., 1987c).
In a supplementary study, three groups of 60 male and 60 female
Wistar rats were given teflubenzuron (purity, 92.4%) in the diet at
0, 2500 or 10 000 ppm, equivalent to 125 or 500 mg/kg bw per day.
Ten animals of each sex at each dose were killed at week 104, and 50
of each sex in each group at week 111. The plasma concentrations of
teflubenzuron were determined in five animals of each sex at each
dose after 4, 26, 52 and 104 weeks. Time-related increases in plasma
concentrations were observed in animals of each sex; the
concentrations in animals treated at 2500 and 10 000 ppm were
0.18-0.79 and 0.23-0.70 µg/ml, resepctively, in males and 0.18-0.60
and 0.14-0.43 µg/ml, respectively, in females. The survival rate of
males at 10 000 ppm at termination was significantly increased over
that in controls. There were no treatment-related clinical signs of
toxicity or effects on food consumption, but the body-weight gains
of females were slightly reduced at both doses. Haematology and
urinalysis showed no treatment-related findings. Increased
activities of plasma alanine and aspartate aminotransferases were
seen in males at 2500 and 10 000 ppm throughout treatment. Liver
weights were increased in males at the highest dose at week 104 and
to a lesser extent at week 111. Gross pathology revealed increased
incidences of diffuse, clay-coloured discoloration and focal and
multifocal discoloration in livers of males at the highest dose.
Histopathology revealed a number of treatment-related non-neoplastic
liver lesions: Increased incidences of foci of mixed and basophilic
cells were noted in males at 2500 and 10 000 ppm, and an increased
incidence of basophilic-cell foci was seen in females at 10 000 ppm.
The incidence of focal hepatocellular hyperplasia was increased in
males at the highest dose, and the incidences of fatty change and
centrilobular hepatocellular hypertrophy were increased in males and
females at 2500 and 10 000 ppm. Spongiosis hepatis was increased in
incidence in males at the highest dose. As treatment-related
non-neoplastic findings were seen at both doses, no NOAEL could be
identified in this study (Tennekes et al., 1989).
(d) Reproductive toxicity
Rats
Groups of 25 male and 25 female Sprague-Dawley rats were fed
diets containing teflubenzuron (purity, 92.4%) at 0, 20, 100 or 500
ppm for two generations, with one litter per generation. The F0
generation was kept for 10 weeks before mating and the F1
generation for about 12 weeks. Reproductive performance and effects
on adults and offspring were monitored daily throughout the study;
observations included body weight, food consumption, mating
performance, fertility rate, duration of gestation, pup hair growth,
pinna unfolding, tooth eruption, eye opening, litter size, pup
weight, pup mortality and sex ratio. F1 pups underwent functional
tests, comprising pupillary reflex, startle response and ability to
learn use of a water maze, and the sexual organs of the parental and
F1 animals that received the highest dose were examined by gross
necropsy and histopathology. There was no evidence of toxicity and
no effects on reproductive performance (Osterburg, 1986). The NOAEL
was > 500 ppm, equal to 40 mg/kg bw per day.
(e) Embryotoxicity and teratogenicity
Rats
Teflubenzuron (purity, 96.5%) was administered in 0.5% aqueous
carboxymethyl-cellulose to groups of 25 naturally inseminated female
Wistar rats by gavage on days 6-15 after mating at doses of 0, 10,
50 or 250 mg/kg bw per day. The dams were killed on day 20 of
gestation, and the fetuses were examined after caesarean section.
The doses were selected on the basis of the results of a
range-finding study of doses up to 250 mg/kg bw per day (Gleich,
1984). There were no treatment-related fetotoxic or teratogenic
effects or signs of maternal toxicity. The NOAEL for both dams and
fetuses was 250 mg/kg bw per day (Gleich et al., 1984a).
Groups of 25 naturally inseminated female Wistar rats received
teflubenzuron (purity, 92.1%) in 0.5% aqueous carboxymethylcellulose
by gavage at doses of 0, 100, 300 or 1000 mg/kg bw per day on days
7-17 of gestation. The dams were killed on day 20 of gestation, and
the fetuses examined after caesarean section. There were no
treatment-related signs of maternal toxicity or any treatment-
related teratogenic or fetotoxic effects. The NOAEL for both dams
and fetuses was 1000 mg/kg bw per day (Ishida et al., 1987).
Rabbits
Groups of 14-15 Himalayan rabbits proven to be pregnant were
administered teflubenzuron (purity, 96.5%) in 0.5% aqueous
carboxymethylcellulose at 0, 10, 50 or 250 mg/kg bw per day by
stomach tube on days 6-18 of gestation. The doses were based on the
results of a range-finding study in which rabbits received doses of
up to 250 mg/kg bw per day with no sign of toxicity and no effect on
fetuses. Caesarean sections were performed on the dams on day 29 of
gestation. There were no treatment-related effects on the dams or
fetuses. The NOAEL for maternal toxicity, fetotoxicity and
teratogenicity was 250 mg/kg bw per day (Gleich et al., 1984b).
A group of 22 naturally inseminated New Zealand white rabbits,
18 of which were proven to be pregnant, were administered
teflubenzuron (purity, 96.5%) in 0.5% aqueous carboxymethyl
cellulose at 1000 mg/kg bw per day by stomach tube on days 6-18 of
gestation. Sixteen rabbits (15 proven to be pregnant) treated with
the vehicle alone served as the control group. The dams were killed
on day 28 of gestation. The only sign of maternal toxicity possibly
related to treatment was noted at necropsy as an increased incidence
of 'grossly granulated cut surfaces of the liver' in dams treated at
1000 mg/kg bw per day (in 8/22 as compared with 1/16 controls).
There were no signs of treatment-related fetotoxicity or
teratogenicity at this dose. The NOAEL for maternal toxicity could
not be established conclusively in view of the possible effect on
the liver. The NOAEL for fetotoxicity and teratogenicity was 1000
mg/kg bw per day (Osterburg, 1987).
Four groups of four or five pregnant Himalayan rabbits were
given teflubenzuron (purity unspecified) in 0.5% aqueous
carboxymethylcellulose by gavage at doses of 0, 250 or 500 mg/kg bw
per day (two groups at the highest dose) on days 6-18 of gestation.
All animals except those in the second group at the highest dose
were killed on day 19. Their livers were immediately removed and
weighed, and the cytochrome P450 content and O- and
N-demethylase activities were assessed in homogenates; the numbers
of corpora lutea, living embryos and early resorptions were counted.
Animals in the second group at the high dose underwent caesarian
section on day 29 and their livers were examined in the same way as
described above. Fetuses were incubated for 24 h before being
examined for malformations. No toxicologically significant findings
were found, and there was no evidence of liver enzyme induction in
the dams (Gleich, 1985).
(f) Genotoxicity
The results of tests for the genotoxicity of teflubenzuron
(Table 2) revealed no evidence for mutagenicity or clastogenicity.
(g) Special studies
Skin and eye irritation and skin sensitization
The potential of teflubenzuron (purity, 96.5%) to irritate skin
was investigated in three New Zealand white rabbits by applying 0.5
g of the compound, moistened with tap water, to intact skin on the
clipped dorsum of each rabbit and keeping it under an occlusive
dressing for 4 h. There was no evidence of skin irritation over 72 h
(Ullman, 1983f).
The potential of teflubenzuron (purity, 96.5%) to irritate the
eye was investigated in three New Zealand white rabbits by
introducing 0.1 g of undiluted test material into the conjunctival
sac of the left eye of each animal. Slight conjunctival and scleral
redness was observed in each animal after 1 h. No signs of
irritation were seen at 24, 48 or 72 h (Ullman, 1983g).
Table 2. Results of tests for the genotoxicity of teflubenzuron
End-point Test object Concentration Purity Results Reference
of teflubenzuron (%)
In vitro
Reverse mutation S. typhimurium TA98, 125-5000 µg/plate NR Negativea Kramer, 1982
100, 1535, 1537, 1538 in DMSO
Point mutation at Chinese hamster V79 5-50 µg/ml 92.4 Negativea Heidemann, 1986
hprt locus cell line in DMSO
Chromosomal Chinese hamster V79 4, 25, 50 µg/ml 92.4 Negativea Heidemann, 1985
aberration cell line in DMSO
Unscheduled DNA Wistar CF HB male 1-100 µg/ml 92.4 Negativea Müller, 1986
synthesis rat hepatocytes in acetone
In vivo
Micronucleus NMRI mice 5000 mg/kg bw in 96.5 Negative Guenard, 1984
formation 2% carboxymethyl-
cellulose, sodium salt
DMSO, dimethyl sulfoxide; NR, not reported
a In the presence and absence of metabolic activation
The potential of teflubenzuron (purity, 93.5%) to sensitize
skin was investigated in the guinea-pig (Dunkin-Hartley)
maximization test, in which 10 animals were used as negative
controls and 20 as the test group. The results of two topical
challenges after intradermal or topical induction with the test
substance indicated that teflubenzuron did not sensitize skin under
these conditions (Ullman, 1984).
3. Observations in humans
No information was available.
Comments
In rats, teflubenzuron was absorbed only partially from the
gastrointestinal tract, absorption being dose-dependent and
saturable. Absorbed teflubenzuron was excreted mainly via the bile,
urinary excretion representing only a minor route. Faecal excretion
of absorbed and unabsorbed teflubenzuron was the main route. There
was no evidence of bioaccumulation in organs or tissues.
Teflubenzuron was eliminated largely unchanged in faeces,
although a number of unidentified minor metabolites were found.
Hydroxylated metabolites of teflubenzuron were found in urine in low
amounts. 3,5-Dichloro-2,4-difluorophenylurea and its corresponding
substituted aniline were observed in urine, indicating that cleavage
of the benzoylurea moiety had occurred. Conjugates of these
metabolites and the unconjugated 3,5-dichloro-2,4-difluorophenylurea
were detected in bile.
Teflubenzuron has low acute oral, dermal and inhalational
toxicity; it was more acutely toxic when administered by the
intraperitoneal route. WHO (1992) has classified teflubenzuron as
being unlikely to present an acute hazard in normal use.
In mice, rats and dogs given repeated doses in the diet, the
major target organ was the liver. Pathological and clinical chemical
findings of hepatotoxicity varied with species, dose and duration of
dosing. The indicators of hepatotoxicity included effects such as
increased activities of serum alanine aminotransferase, aspartate
aminotransferase, ornithine carbamoyl transferase, lactate
dehydrogenase and alkaline phosphatase, increased liver weight, and
hepatocellular necrosis, fatty change, hypertrophy and hyperplasia.
Haematological parameters generally remained unaltered by treatment.
In a 13-week study of toxicity in mice fed levels of 0, 100,
1000 or 10 000 ppm, effects indicative of hepatotoxicity were
observed at 1000 and 10 000 ppm. The NOAEL was 100 ppm, equal to
11.9 mg/kg bw per day. In a 28-day range-finding and a 13-week study
of toxicity, rats were fed diets containing 0, 100, 1000 or 10 000
ppm. The NOAEL was 100 ppm in each, equal to 11.7 and 8.0 mg/kg bw
per day, respectively, on the basis of indications of
hepatotoxicity. Two 13-week studies in dogs fed diets containing 0,
100, 1000 or 10 000 ppm and 0, 30 or 100 ppm indicated an NOAEL of
100 ppm, equal to 4.1 mg/kg bw per day, on the basis of focal
gastritis in dogs treated at 1000 ppm in the first study. The NOAEL
in the 13-week studies concurred with the NOAEL of 100 ppm, equal to
3.2 mg/kg bw per day, observed in a 52-week study in dogs, in which
liver weights were increased in males fed the highest level of 500
ppm.
Mice fed diets containing 0, 15, 75 or 375 ppm for 18 months in
a study of carcinogenicity showed non-neoplastic hepatotoxicity at
all doses. Changes observed in livers of mice at the lowest dose
were increased in incidence over those in controls but were not
increased in severity. The lowest dose of 15 ppm, equal to 2.1 mg/kg
bw per day, was the LOAEL. Histopathological investigations
indicated an increased incidence of hepatocellular adenomas in males
at 75 and 375 ppm, in comparison with rates in concurrent and
historical controls. This tumorigenic potential in mice was
considered not to be relevant to humans.
In a 120-week study of toxicity and carcinogenicity, rats were
fed diets containing 0, 20, 100 or 500 ppm teflubenzuron. The NOAEL
was 100 ppm, equal to 4.8 mg/kg bw per day, on the basis of
increased serum enzyme activities and liver weights in males. In a
supplementary study, rats were fed diets containing 0, 2500 or 10
000 ppm for 111 weeks. No NOAEL could be assigned because
non-neoplastic liver changes and increased serum enzyme activities
were seen at both doses. There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats fed dietary concentrations of 0, 20,
100 or 500 ppm, the NOAEL was 500 ppm, equal to 40 mg/kg bw per day,
on the basis of lack of toxicity or effects on reproductive
performance.
Two studies of teratogenicity in rats treated by gavage showed
no evidence of maternal toxicity, fetotoxicity or teratogenicity at
doses up to either 250 or 1000 mg/kg bw per day. In a study of
teratogenicity in rabbits, there was no evidence of maternal
toxicity, fetotoxicity or teratogenicity at doses of 0, 10, 50 or
250 mg/kg bw per day. A second study of teratogenicity, in rabbits
treated by gavage at 0 or 1000 mg/kg bw per day, showed no evidence
of fetotoxicity or teratogenicity. Effects possibly related to
treatment were noted at necropsy in the livers of some dams treated
at 1000 mg/kg bw per day. In another study, there was no evidence of
liver enzyme induction in pregnant rabbits treated by gavage with
doses of up to 500 mg/kg bw per day.
Teflubenzuron has been adequately tested for genotoxicity in
vivo and in vitro in a range of assays. The Meeting concluded
that it was not genotoxic.
An ADI was allocated on the basis of the LOAEL of 15 ppm, equal
to 2.1 mg/kg bw per day, in the 18-month study of carcinogenicity in
mice. A 200-fold safety factor was applied since no NOAEL was
identified in this study.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 11.9 mg/kg bw per day (13-week
study of toxicity)
Rat: 100 ppm, equal to 4.8 mg/kg bw per day (120-week
study of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (two-generation
study of reproductive toxicity)
1000 mg/kg bw per day (study of teratogenicity,
maternal and fetal toxicity)
Rabbit: 1000 mg/kg bw per day (fetal toxicity in a study of
teratogenicity)
250 mg/kg bw per day (maternal toxicity in a study of
teratogenicity)
Dog: 100 ppm, equal to 3.2 mg/kg bw per day (one-year
study of toxicity)
Lowest-observed-adverse-effect level
Mouse: 15 ppm, equal to 2.1 mg/kg bw per day (18-month study
of carcinogenicity)
Estimate of acceptable daily intake for humans
0-0.01 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Bathe, R. (1983) 28-Day oral toxicity (feeding) study with CME 134
in beagle dogs. Project 017212. Document No. 134AB-432-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.,
London, United Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1984) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 018865. Document No. 134AB-433-007. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Bathe, R., Frei, T., Luetkemeier, H., Schlotke, B. & Terrier, C.
(1985) 13-Week oral (feeding) toxicity study with CME 134 in beagle
dogs. Project 040702. Document No. 134AB-433-008. Unpublished report
from Research and Consulting Co. Ag, Itingen, Switzerland. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987a) The disposition
of [14C]-CME 134 in the lactating goat. Report No. 4278A. Document
No. 134AX-652-001. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Cameron, B.D., O'Brien, J.W. & Young, C.G. (1987b) The disposition
of [14C]-CME 134 in the laying hen. Report No. 4278B. Unpublished
report from Inveresk Research International Ltd, Musselburgh,
Scotland, United Kingdom. Submitted to WHO by Shell International
Chemical Co. Ltd.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1988)
Further identification of [14C]-CME 134 metabolites in the hen.
Unpublished report from Inveresk Research International Ltd,
Musselburgh, Scotland, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Cameron, B.D., O'Brien, J.W., Young, C.G. & McGuire, G.M. (1989)
Further identification of [14C]-CME 134 metabolites in the goat.
Report No. 4490A. Unpublished report from Inveresk Research
International Ltd, Musselburgh, Scotland, United Kingdom. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ellgehausen, K. (1986) 28-Day dietary study with CME 134 in the rat
for proof of absorption. Project 059973. Document No. 134AB-651-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Gleich, J. (1984) CME 134. Dosifindungsversuch für eine Prufung auf
embryotoxische Wirkung an Ratten nach oraler Applikation. Report No.
4/7/84. Document No 134AB-451-001. Unpublished report from Institute
of Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J. (1985) CME 134. Supplementary study on embryotoxicity and
liver enzyme induction in Himalayan rabbits. Report No. 4/65/85.
Document No. 134AB-451-005. Unpublished report from Institute of
Toxicology, E. Merck, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G. & Unkelbach, H. (1984a) CME 134.
Embryotoxicity study in rats after oral administration. Report No.
4/29/84. Document No. 134AB-451-003. Unpublished report from
Institute of Toxicology, E. Merck, Darmstadt, Germany. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Gleich, J., Weisze, G., Unkelbach, H.D. & Hofmann, A. (1984b) CME
134. Embryotoxicity study in rabbits after oral administration.
Report No. 4/63/84. Document No. 134AB-451-004. Unpublished report
from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Guenard, J. (1984) Mouse micronucleus assay with CME 134. Project
025672. Unpublished report from Resarch and Consulting Co. Ag,
Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Hawkins, D.R. & Mayo, B.C. (1988) The biliary excretion and
metabolism of [14C]-CME 134. Report No. HRC/CMK 17/871263. Document
No. 134AX-651-010. Unpublished report from Huntingdon Research
Centre Ltd, Huntingdon, United Kingdom. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1985) CME 134. Chromosome aberrations in cells of
Chinese hamster cell line V79. Study LMP 135C. Document No.
134AB-457-003. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Heidemann, A. (1986) CME 134. Detection of gene mutations in somatic
mammalian cells in culture: HGPRT-test with V79 cells. Study LMP
135B. Document No. 134AC-457-005. Unpublished report from the
Laboratory for Mutagenicity Testing, Darmstadt, Germany. Submitted
to WHO by Shell International Chemical Co. Ltd, London, United
Kingdom.
Ishida, S., Yamazaki, E., Ikeya, M. & Suzuki, K. (1987)
Teratological study in rats treated orally with teflubenzuron. Study
No. R-128. TZ-432-001. Unpublished report from Bozo Research Centre
Ltd, Tokyo, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Kramer, P.J. (1982) CME 134. In vitro assessment for mutagenic
potential in bacteria with and without addition of a metabolizing
system. Experiment T12 568. Document No. 134AA-457-001. Unpublished
report from Institute of Toxicology, E. Merck, Darmstadt, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Müller, E. (1986) CME 134. Unscheduled DNA synthesis in hepatocytes
of male rats in vitro (UDS test). Study LMP 135A. Document No.
134AC-457-006. Unpublished report from the Laboratory for
Mutagenicity Testing, Darmstadt, Germany. Submitted to WHO by Shell
International Chemical Co. Ltd, London, United Kingdom.
Osterburg, I. (1986) Two generation oral (dietary administration)
reproduction toxicity study in the rat. Project No. 460/1. Document
No. 134AB-453-002. Unpublished report from Hazleton Laboratories,
Münster, Germany. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Osterburg, I. (1987) CME 134. Oral (gavage) teratogenicity limit
test in the rabbit. Project No. 460/13. Document No. 134AB-451-009.
Unpublished report from Hazleton Laboratories, Münster, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Sachsse, K. (1986) 52-Week oral (feeding) toxicity study with CME
134 in beagle dogs. Project 034828. Document No. 134AB-437-005.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Schlüter, H. (1984) Initial investigations on the biokinetics of
CME134 in the rat. Document No. 134AA-651-001. Unpublished report
from Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1985) Investigations on the metabolism of CME134 in
the rat. Document No. 134AA-651-012. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Schlüter, H. (1986) The biokinetics and metabolism of [14C]-CME 134
in the rat. Document No. 134AX-651-007. Unpublished report from
Celamerck GmbH, Biochemical Laboratory, Ingelheim, Germany.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P. (1983) 28 Day range-finding study with CME 134 in rats.
Project 017201. Document No. 134AB-432-002. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Horst, K., Luetkemeier, H., Chevalier, J. & Terrier, C.
(1987a) 13-Week subchronic toxicity (feeding) study with CME 134 in
the rat. Project 018843. Document No. 134AB-433-006. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Westen, H. & Terrier, C.
(1987b) 18-Month oncogenicity (feeding) study with CME 134 in mice.
Project 027810. Document No. 134AB-455-003. Unpublished report from
Research and Consulting Co. Ag, Itingen, Switzerland. Submitted to
WHO by Shell International Chemical Co. Ltd, London, United Kingdom.
Suter, P., Dewert, H., Luetkemeier, H., Schlotke, B., Ellgehausen,
H., Terrier, Ch. (1987c) 120-week chronic toxicity and oncogenicity
study with CME 134 in the rat. Project 027472. Document No
134AB-437-006. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Takahashi, K., Saitoh, T., Miyaoka, S., Maita, K. & Goloh, M. (1987)
13 Weeks subacute toxicity study with teflubenzuron in mice. Report
T-15. Document no. 134AB-433-005. Unpublished report from Kodaira
Laboratory, Japan. Submitted to WHO by Shell International Chemical
Co. Ltd, London, United Kingdom.
Tasheva, M. & Hristeva, V. (1993) Comparative study on the effects
of five benzoylphenylurea insecticides on haematological parameters
in rats. J. Appl. Toxicol., 13, 67-68.
Tennekes, H., Stucki, P., Luetkemeier, H., Biedermann, K., Bloch,
M., Chevalier, H., Vogel, O. & Terrier, C. (1989) Chronic toxicity
and oncogenicity (feeding) study with CME 134 in the rat. Project
064192. Document No. 134AB-437-009. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983a) Acute oral toxicity study (LD50) with CME 134
in rats. Project 019596. Document No. 134AB-421-004. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983b) Acute oral toxicity study (LD50) with CME 134
in mice. Project 025571. Document No. 134 AB-421-005. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983c) Acute dermal toxicity study (LD50) with CME 134
in rats. Project 025593. Document No. 134 AB-422-001. Unpublished
report from Research and Consulting Co. Ag, Itingen, Switzerland.
Submitted to WHO by Shell International Chemical Co. Ltd, London,
United Kingdom.
Ullman, L. (1983d) Acute intraperitoneal toxicity study (LD50)
with CME 134 in rats. Project 025582. Document No. 134AB-424-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L. (1983e) 4-Hour dust-aerosol inhalation toxicity study
(LD50) with CME 134 in rats. Project 025626. Document No.
134AB-423-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983f) Primary skin irritation study following a single
4-hour occlusive application with CME 134 in the rabbit. Project
025604. Document No. 134AB-465-001. Unpublished report from Research
and Consulting Co. Ag, Itingen, Switzerland. Submitted to WHO by
Shell International Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1983g) Primary eye irritation study after single
application with CME 134 in the rabbit. Project 025615. Document No.
134AB-466-001. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Ullman, L. (1984) Test for delayed hypersensitivity in the albino
guinea-pig with CME 134 Project 034817. Document No. 134AB-467-001.
Unpublished report from Research and Consulting Co. Ag, Itingen,
Switzerland. Submitted to WHO by Shell International Chemical Co.
Ltd, London, United Kingdom.
Ullman, L., Sacher, R. & Vogel, O. (1988) Acute oral toxicity study
with teflubenzuron in rats. Project 206741, Document No.
134AC-421-009. Unpublished report from Research and Consulting Co.
Ag, Itingen, Switzerland. Submitted to WHO by Shell International
Chemical Co. Ltd, London, United Kingdom.
Vesselinovitch, S. (1988) Histologic evaluation of liver tissues
originating from potential carcinogenicity bioassay studies of CME
134, Batch DW 44/83 in NMRI mice. Unpublished report from the
Departments of Pathology and Radiology, University of Chicago, IL,
USA. Submitted to WHO by Shell Chemical Co. Ltd, London, United
Kingdom.
