
FENPYROXIMATE
First draft prepared by
K. Fujimori
Division of Pharmacology, Biological Safety Research Center, National
Institute of Health Sciences, Ministry of Health and Welfare, Tokyo,
Japan
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Acute delayed neurotoxicity
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Fenpyroximate, tert-butyl (E)-alpha-(1,3)-dimethyl-5-
phenoxy-1 H -pyrazol-4-yl methyleneamino-oxy)- para-toluate, is a
phenoxypyrazole acaricide for application to leaves. It is very active
against phytophagous mites, relatively less active against predacious
mites, and inactive against animal parasitic and soil mites. The only
insect against which it is active is Empoasca onukii (tea green
leafhopper) (Taninaka, 1993). Fenpyroximate inhibits mitochondrial
NADH-coenzyme Q reductase on the electron transport chain in
Tetrachynus urticae (two-spotted spider mite) and in rats (Motoba
et al., 1992).
Fenpyroximate was evaluated for the first time by the present
Meeting.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Groups of six male and six female Sprague-Dawley (Crl;CD)
rats were given single doses by gavage of 2 or 400 mg/kg bw of
[3-14C]-pyrazole-(radiochemical purity, 96.4-99.9%) or
[U-14C]-benzyl-fenpyroximate (radiochemical purity, 99.2-99.5%)
suspended in 1% aqueous Tween 80. Blood was collected from the tail
vein of five rats per group at various times up to 168 h after dosing.
In rats given 2 mg/kg bw, the concentration of radiolabel in blood
peaked within 1 h after dosing and reached a plateau, which was
sustained for about 18 h and was followed by a slow elimination phase
with a half-life of 6-9 h. In the group given 400 mg/kg bw, absorption
was delayed, and radiolabel was not detectable in blood within the
first 12 h after dosing. A nearly maximal level was achieved 12-24 h
after dosing, the plateau was sustained for 80-100 h and was followed
by an elimination phase with a half-life of 35-49 h (Table 1; Sharp,
1990a,b)
Groups of five male and five female Sprague-Dawley CD rats
received a single oral dose of 2 or 400 mg/kg bw 14C-pyrazole- or
14C-benzyl-labelled fenpyroximate. The recoveries of total radiolabel
within 168 h were 93-108%. The major route of excretion was the
faeces; 70-91% of the low dose and 75-80% of the high dose were found
in faeces, and 9-18% of the low dose and 10-12% of the high dose were
excreted in urine within 168 h. Excretion by both routes after the
high dose was time-dependent. There was little residual radiolabel
in tissues 168 h after treatment with 2 mg/kg bw; the highest
concentration was found in fat, which contained 0.01-0.03 µg/g
pyrazole- and 0.06-0.1 µg/g benzyl-labelled fenpyroximate. After
treatment with 400 mg/kg bw, the highest residual tissue
concentrations after 168 h were found in fat (3-21 µg/g), liver
(2-8 µg/g), and kidney (1-3 µg/g). Less than 0.1% of the low dose was
found in the gastric contents 24 h after treatment, but 53-64% of the
high dose remained after 24 h, and 0.3-2.6% was still observed at
168 h. The concentrations in blood 168 h after treatment with
400 mg/kg bw were very low (0.5 µg/g) for pyrazole-labelled
fenpyroximate and were not detectable for that labelled with benzyl.
There were no obvious changes in the tissue distributions of labelled
compound after pretreatment with unlabelled fenpyroximate, and there
was no significant sex-related difference. Further groups of five
males and five females received 14 consecutive daily doses of 2 mg/kg
bw unlabelled compound followed by a single dose of 14C-pyrazole- or
14C-benzyl-labelled compound. No significant difference in the
excretion pattern was observed between singly and repeatedly dosed
groups, and there was no significant difference between males and
females or with the pyrazole- and benzyl-labelled compounds (Sharp
1991a,b).
Table 1. Toxicokinetic parameters for radioactivity in blood of rats dosed with [14C-pyrazole]-
or [14C-benzyl]-fenpyroximate
Label Dose Sex Toxicokinetic parameter (mean + SD)
(mg/kg bw)
Cmax (µg/g) Tmax (h) Half-life (h) AUC (µg × h/ml)
Pyrazole 2 Male 0.15 ± 0.06 11.0 ± 8.3 8.9 3.49 ± 0.64
Female 0.18 ± 0.04 11.4 ± 4.5 8.9 3.82 ± 0.57
400 Male 4.67 ± 1.69 100.8 ± 26.3 48.7 377 ± 190
Female 4.69 ± 0.66 90.0 ± 12.0 45.3 411 ± 134
Benzyl 2 Male 0.10 ± 0.16 7.8 ± 1.6 6.1 1.80 ± 0.34
Female 0.18 ± 0.05 7.2 ± 3.4 7.9 3.01 ± 0.60
400 Male 5.10 ± 1.21 28.8 ± 10.7 47.0 425 ± 132
Female 8.88 ± 2.73 86.4 ± 27.4 35.4 728 ± 211
Total radioactivity, expressed as micrograms equivalent fenpyroximate; AUC, area under the
concentration-time curve
Groups of six male and six female Sprague-Dawley rats with
bile duct cannulae were given a single oral dose of 2 mg/kg of
14C-pyrazole- or 14C-benzyl-fenpyroximate. Within 48 h after
treatment with pyrazole-labelled fenpyroximate, 47% (females) to 55%
(males) of the radiolabel had been excreted in the bile, 5%, (males)
to 10% (females) in urine, and 17% (females) to 28% (males) in faeces.
Total excretion 48 h after treatment was about 88% for males and 73%
for females. The Tmax, Cmax, and half-lives of the radiolabel in the
blood of cannulated rats were similar to those of rats with no
cannulae. Within 48 h after oral administration of benzyl-labelled
fenpyroximate, 47% (females) to 51% (males) of the radiolabel had been
excreted in the bile, 6% (males) to 8% (females) in urine, and 28%
(females) to 40% (males) in faeces (Tsu-Han Li, 1991a,b).
Groups of four male Sprague-Dawley CD rats received single dermal
applications of 14C-pyrazole-fenpyroximate suspended in water at doses
of 0.1, 1.0, or 5.2 mg in 1 ml on a 10-cm2 area of skin for 0.5, 1,
2, 4, 10, or 24 h and were sacrificed at the end of the exposure
period, The concentration of radiolabel in blood was very low after
all applications. Excretion in urine was slight but increased with
duration of exposure; after 24 h of exposure, 0.7-0.9% of the applied
dose had been excreted. Radiolabel was found in faeces after 10 and
24 h of treatment, and faecal excretion was 1.4% at a dose of 1 mg,
0.5% at 10 mg, and 0.2% at 52 mg. These results suggest that
fenpyroximate is barely absorbed from the skin and is excreted via the
biliary-faecal and urinary routes (Mahon, 1993).
(b) Biotransformation
The proposed metabolic pathways for fenpyroximate in rats are
shown in Figure 1. Fenpyroximate is metabolized extensively by
hydrolytic cleavage of the oxime ether bond, hydrolysis of the
tert-butyl ester, oxidation of the tert-butyl, hydroxylation of
the phenoxy ring and 3-methyl, isomerization, N-demethylation, and
conjugation, producing a large number of metabolites. The major
metabolites identified are ( E)-4-[(1,3-dimethyl-5-phenoxypyrazol-
4-yl) methyleneaminooxymethyl] benzoic acid (C), (Z)-4-[(1,3-dimethyl-
5-phenoxypyrazol-4yl) methyleneaminooxymethyl] benzoic acid (D),
( E)-4-{[1,3-dimethyl-5-(4-hydroxyphenoxy) pyrazol-4-yl]
methyleneaminooxymethyl} benzoic acid ( E), 1,3-dimethyl-5-
phenoxypyrazole-4-carboxylic acid (H), 4-hydroxymethyl benzoic acid
(P), terephthalic acid (R), 4-cyano-1-methyl-5-phenoxypyrazole-
3-carboxylic acid (S), ( E)-2-{4-[(1,3-dimethyl-5-phenoxypyrazol-
4-yl) methyleneaminooxymethyl] benzoyloxy}-2-methylpropanoic acid (F),
( E)-2-{4-[1,3-dimethyl-5-(4-hydroxyphenoxy) pyrazol-4-yl]
methyleneaminooxymethyl} benzoyloxy]-2-methylpropionic acid (W), and
( E)-2-[{4-[3-hydroxymethyl-1-methyl-5-phenoxypyrazol-4-yl]
methyleneaminooxymethyl} benzoyloxy]-2-methylpropionic acid (X).
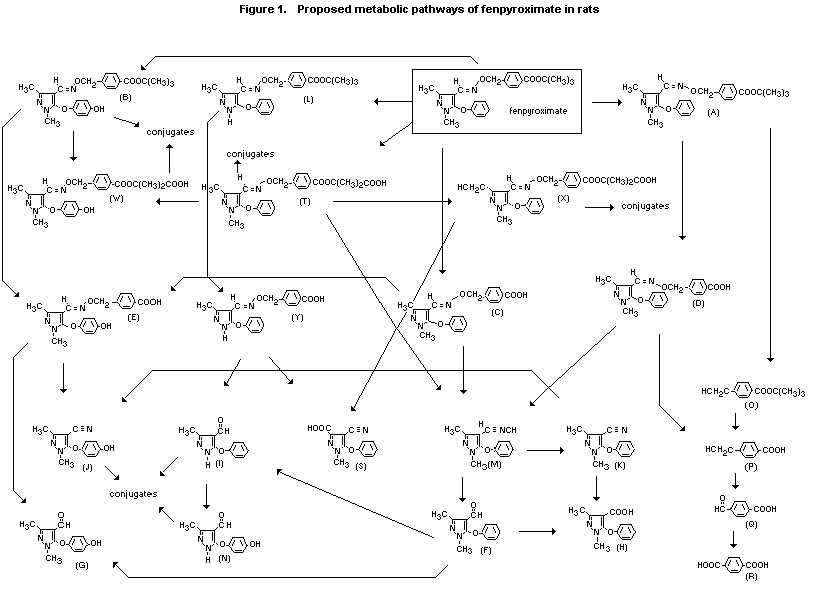 Groups of five male and five female Sprague-Dawley CD rats were
treated with single oral doses of 2 or 400 mg/kg bw 14C-pyrazole- or
14C-benzyl-labelled fenpyroximate, and urinary and faecal samples
were collected at 0-24, 24-48, 48-120, and 120-168 h. The samples from
each group were pooled for each time period. Radiolabel in faeces
after 0-48 h represented 80-87% of the dose of 2 mg/kg and 80% of the
dose of 400 mg/kg; parent fenpyroximate was found at 6-8% after
2 mg/kg and 50-52% after 400 mg/kg. No parent compound was found in
urine. Eleven urinary and 19 faecal metabolites were identified by
thin-layer co-chromatography with authentic samples. The major urinary
metabolites were H, S, and R (Figure 1), produced by hydrolytic
cleavage of the oxime ether bond between the benzyl moiety and the
pyrazole ring. The major faecal metabolites were C, D, and E, produced
by hydrolysis of the tert-butyl ester, and T, produced by oxidation
of the tert-butyl group. A Z isomer of fenpyroximate, A, was found
in faeces of rats treated with the high dose. The concentrations of
urinary metabolite H and faecal metabolites H and T were increased by
enzymatic hydrolysis of the excreta with ß-glucuronidase or sulfatase
(Sharp 1991a,b).
Groups of four male Sprague-Dawley (SLC) rats were treated with a
single oral dose of 1.5 mg/kg bw 14C-pyrazole- or 14C-benzoyl-
labelled fenpyroximate (radioactive purity, > 99%), and urinary and
faecal samples were collected for 0-72 h. Six urinary and 17 faecal
metabolites were identified by thin-layer co-chromatography with
authentic samples. Three of them, W, X, and V, were isolated from
faeces and identified by mass spectrometry and nuclear magnetic
resonance. The major urinary metabolites were H (7.3% of the dose),
S (2.5%), and R (3.8%). The major faecal metabolites were C (4.1-11.0%
of the dose), E (2.9-4.2%), and T (3.5-4.3%); P (7.5%) was found as a
precursor of R and W (2.0-9.7%) and X (3.3-4.5%) as hydroxylated
bodies of T. The concentrations of the urinary metabolites J and N and
the faecal metabolites B, T, W, X, and V were increased by enzymatic
hydrolysis of the excreta with ß-glucuronidase or sulfatase (Nishizawa
et al., 1993).
Groups of six male and six female Sprague-Dawley rats with
bile-duct cannulae were given a single oral dose of 2 mg/kg
14C-pyrazole-labelled fenpyroximate. No parent fenpyroximate was
found in bile, but metabolites C, D, E, F, G, H, I, J, M, N, and T and
conjugates of C, D, E, and H were found. Total radiolabel represented
less than 2% of the dose. The metabolic pathway proposed for
fenpyroximate in rats is cleavage of the ester bond, hydroxylation at
the phenoxypyrazole group, oxidation at the tert-butyl group, and
conjugation with sulfate and glucuronide (Tsu-Han Li, 1991a,b).
3. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of technical-grade (> 98%)
fenpyroximate and of a 5% suspension concentrate are summarized in
Table 2. Moderate oral toxicity was observed in rats and mice, with
LD50 values ranging from 245 to 520 mg/kg bw. Fenpyroximate was more
toxic to rats when administered by inhalation, with LC50 values
ranging from 0.21 to 0.36 mg/litre air. Signs of acute toxicity in
mice and rats included urinary and faecal staining, hypoactivity, and
hypopnoea. Decreased food consumption was observed in surviving
animals one week after dosing. Necropsy of animals found dead revealed
irritation and/or corrosive effects in the gastointestinal tract.
There were no signs of acute toxicity or dermal irritation after
dermal application of either formulation. Inhalation of active
ingredient or the formulation resulted in signs of respiratory
irritation (laboured breathing, rale, and gasping). The findings at
necropsy were unremarkable in groups exposed by whole-body inhalation,
but those in groups exposed by nose only included oedematous,
reddened, firm lungs and frothy fluid in the trachea.
(b) Short-term toxicity
Mice
In a preliminary study to establish the dose range for a
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice were given fenpyroximate (purity, 97.9%) mixed into
the diet at concentrations of 80, 400, or 2000 ppm, equal to 0, 10.8,
48.4, and 181.6 mg/kg bw per day in males and 0, 11.7, 50.4, and
170.0 mg/kg bw per day in females, for four weeks. There were no
deaths and no abnormal behavioural signs in any treated group.
Decreased body-weight gain was observed in males at 400 ppm and in
animals of each sex at 2000 ppm; food consumption and food efficiency
were reduced in animals at 2000 ppm. Haematological examination
revealed significant decreases in haemoglobin in animals of each sex,
of the haematocrit in males, and of the erythrocyte count in females,
and a significant decrease in total plasma protein and a significant
increase in aspartate aminotransferase in animals of each sex at
2000 ppm. The NOAEL in this study was 80 ppm, equal to 10.8 mg/kg bw
per day in males and 11.7 mg/kg bw per day in females (Takahashi,
1988).
In a four-week study to find the dose range for the
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice received fenpyroximate (purity, 97.9%) mixed into the
diet at a concentration of 0, 20, 100, or 500 ppm, equal to 0, 2.58,
12.9, and 53.2 mg/kg bw per day in males and 0, 3.07, 14.5, and
66.7 mg/kg bw per day in females. Slight reductions in body-weight
Table 2. Acute toxicity of fenpyroximate
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Fenpyroximate
ICR CD-1 mouse Male Orala 520 (334-706) 98 Blaszcak (1989a)
Female 440 (281-599)
Sprague-Dawley Male Orala 480 (298-662) 98 Blaszcak (1989b)
CD rat Female 245 (167-323)
Sprague-Dawley Male Dermalb > 2000 98 Blaszcak (1989c)
CD rat Female > 2000
Sprague-Dawley Male Inhalation 0.33 (0.16-0.68) 99 Hoffman (1989)
CD rat Female (whole-body)c 0.36 (0.24-0.54)
Sprague-Dawley Male Inhalation 0.21 (0.04-1.20) 98 Hoffman (1991a)
CD rat Female (nose-only)d 0.33 (0.20--0.55)
5% suspension concentrate
Sprague-Dawley Male Oral 7193 (2989-17 308) Mitchell (1990)
CD rat Female 6789 (4704-9797)
Sprague-Dawley Male Dermal > 4000 Mitchell (1991)
CD rat Female > 4000
Sprague-Dawley Male Inhalation 1.9 (1.2-3.1) Hoffman (1990)
CD rat Female (whole-body)e 2.4 (2.0-2.9)
Table 2. (con't)
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Sprague-Dawley Male Inhalation 5.1 (0.0-17.1) Hoffman (1991b)
CD rat Female (nose-only)f 3.4 (1 9-4.9)
a In Tween 80 and a 1% solution of Methocel (A4C)
b Applied on a gauze moistened with 0.9% saline
c Fenpyroximate containing white carbon (9:1); mean particle size, 4.2 µm; exposure time, 4 h
d Fenpyroximate containing white carbon (9:1); mean particle size, 2.8 µm; exposure time, 4 h
e Mean particle size, 3.3 µm; exposure time, 4 h
f Mean particle size, 5.1 µm; exposure time, 4 h
gain and in food consumption were observed in males at 500 ppm. There
were no abnormal clinical signs, no changes in haematological
parameters, and no changes in organ weights. The NOAEL in this study
was 100 ppm, equal to 12.9 mg/kg bw per day in males and > 500 ppm in
females (Takahashi, 1987).
Rats
Groups of 10 male and 10 female Sprague-Dawley CD rats received
fenpyroximate (purity, 99.0%) in the diet at concentrations of 0, 20,
100, or 500 ppm, equal to 0, 1.3, 6.6, or 35.2 mg/kg bw per day for
males and 0, 1.7, 8.3, or 38.6 mg/kg bw per day for females, for 13
weeks. There were no overt changes in physical condition or behaviour
and no abnormal ophthalmoscopic findings. Increased encrustations
of the muzzle in males and an increased incidence of hair loss
accompanied by encrustation of the skin in females were observed at
500 ppm, Significant reductions in body-weight gain at 100 and 500 ppm
and significant reductions in food consumption at 500 ppm were
observed in animals of each sex. A significant lowering of total
plasma protein level was observed in females at 100 ppm and in animals
of each sex at 500 ppm. A significant increase in relative liver body
weights and slight hypertrophy of hepatic cells were observed in
animals of each sex and a significant increase in alkaline phosphatase
activity in females at 500 ppm; a significantly lower volume and pH
value of urine were also observed at this dose. Erythrocyte
cholinesterase activity was significantly lowered in animals at
500 ppm, but the inhibition was less than 20% of control. The NOAEL in
this study was 20 ppm, equal to 1.30 mg/kg bw per day in males and
1.65 mg/kg bw per day in females, based on the lowered body-weight
gain in animals of each sex and a lowered plasma protein level in
females at 100 ppm (Aughton, 1987).
Fenpyroximate (purity, 99%) was applied to the dorsal skin of
groups of five male and five female Sprague-Dawley rats in a uniform
layer moistened with water at 0, 100, 300, or 1000 mg/kg bw for 6 h
per day for 21 consecutive days. Minor acanthosis was observed in all
treated and one control animal. All rats survived, and there were
no compound-related clinical abnormalities. Reductions in food
consumption and body-weight gain in animals of each sex and increases
in absolute liver weight in females were observed at 1000 mg/kg bw.
There were no compound-related changes in haematological or blood
chemical parameters. The NOAEL in this study was 300 mg/kg bw per day,
based on the reduction in body-weight gain in animals of each sex and
the increase in absolute liver weight in female at 1000 mg/kg bw
(Wilkinson et al., 1992).
Groups of four male and four female Sprague-Dawley CD rats were
exposed by nose-only inhalation to fenpyroximate (purity, 98.6%; mean
particle size, 3 µm) at a target concentration of 0, 2, 10, or
50 mg/m3 for 6 h per day, five days per week for four weeks. All
animals survived. The only treatment-related physical or behavioural
change was an increased incidence of laboured breathing during the
last three weeks of exposure to 50 mg/m3. Increase in absolute and
relative lung weight were observed in animals of each sex after four
weeks' exposure to 10 or 50 mg/m3, but these effects were not seen
two weeks after exposure. A higher incidence of mucosal changes in
the nasal passages (atrophy and metaplasia) were observed at
concentrations of 10 (in males) and 50 mg/m3 (males and females). The
NOAEL in this study was 2 mg/m3, based on an increase in lung weight
and an increased incidence of mucosal changes in the nasal passages
(Hoffman, 1991c).
Dogs
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.4%) in gelatin capsules at 0, 2, 10, or
50 mg/kg bw per day for 13 weeks. Two females given 50 mg/kg bw were
found moribund and were killed after five weeks of treatment.
Increased incidences of diarrhoea and emaciation were observed in
females at 10 mg/kg bw per day and in males at 50 mg/kg bw per day.
Electrocardiograms examined 2 and 24 h after treatment showed
bradycardia in males after six weeks of treatment at 10 or 50 mg/kg bw
per day and after 12 weeks of treatment at 50 mg/kg bw per day. There
were no treatment-related ocular lesions. Significant decreases in
total body-weight gain were observed in females at 10 mg/kg bw per
day and in animals of each sex at 50 mg/kg bw per day. The only
significant haematological change was a reduction in the total number
of leukocytes in females at 50 mg/kg bw per day after 6 and 12 weeks
of treatment. Histopathological examination showed fine cytoplasmic
vacuolation of cells in the renal medullary rays in two females at
50 mg/kg bw per day sacrificed before the end of treatment and in one
female killed at termination. There were no significant changes in
cholinesterase activity in plasma or erythrocytes. The NOAEL in this
study was 2 mg/kg bw per day, based on the reduction in body-weight
gain and clinical signs at 10 mg/kg bw per day (Broadmeadow, 1988).
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.0%) in gelatin capsules at 0, 0.5, 1.5, 5,
or 15 mg/kg bw per day on seven days per week for 52 weeks. Clinical
observations were made daily, electrocardiograms were perfomed after
11, 24, and 50 weeks, haematological examinations were done after
12, 24, and 50 weeks, blood chemistry after 24 and 50 weeks, and
ophthalmoscopy after 49 weeks of treatment. There were no deaths.
Increased incidences of diarrhoea were observed during treatment in
males at 5 or 15 mg/kg bw per day and in females at 15 mg/kg bw per
day. Increased salivation was observed in females at 15 mg/kg bw per
day. Slight bradycardia was observed in animals of each sex at
15 mg/kg bw per day, which was significant in males at 11 and 50 weeks
of treatment. The overall body-weight gain throughout treatment was
significantly lower in males at 15 mg/kg bw per day, and a slightly
reduced total food consumption was observed. There were no treatment-
related haematological or ophthalmological findings. A slight but
significant lowering of total plasma protein level was observed in
males at 15 mg/kg bw per day after 50 weeks of treatment. There was no
change in erythrocyte acetylcholinesterase activity. The absolute and
relative prostatic weights were significantly increased in all treated
groups, but no histopathological changes were observed in the prostate
or in any other tissue examined. The NOAEL was 1.5 mg/kg bw per day,
based on the increased incidence of diarrhoea at 5 mg/kg bw per day
(Broadmeadow, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female ICR (Crj;CD) mice received
fenpyroximate (purity, 97.9-98.4%) mixed into the diet at
concentrations of 0, 25, 100, 400, or 800 ppm (overall mean
concentrations: 0, 24, 98, 388, and 776 ppm) for 18 months (78 weeks),
equal to 0, 2.4, 9.5, 38.0, or 69.6 mg/kg bw per day for males and 0,
2.5, 10.2, 41.5, or 73.1 mg/kg bw per day for females. There were no
treatment-related alterations in clinical signs. Body-weight gain and
food consumption were significantly lower than those of controls in
animals of each sex at 400 and 800 ppm. The only significant change in
haematological parameters was a decrease in the number of segmented
neutrophils at 500 ppm. A significant increase in the incidence of
emaciation was observed in males a t 500 ppm at the termination of
treatment. Histopathological examination revealed significant
increases in the incidence of ovarian atrophy in females at 400 and
800 ppm (25/50 in controls, 35/50 at 400 ppm, 35/50 at 800 ppm), but
these changes were morphologically similar to age-related changes seen
in female mice of this strain. There was no treatment-related increase
in the incidence of neoplasia. The NOAEL in this study was 100 ppm,
equal to 9.5 mg/kg bw per day in males and 10.2 mg/kg bw per day in
females, based on decreased body-weight gain and food consumption at
400 ppm (Takahashi, 1990).
Rats
Groups of 80 male and 80 female Sprague-Dawley CD rats
received fenpyroximate (purity, 97.1-97.2%) mixed into the diet at
concentrations of 0, 10, 25, 75, or 150 ppm for 104 weeks. The overall
doses achieved by the groups in which toxicity was assessed were 0,
0.40, 0.97, 3.08, and 6.18 mg/kg bw per day in males and 0, 0.48,
1.16, 3.79, and 7.57 mg/kg bw per day in females; those in the groups
for assessment of carcinogenicity were 0, 0.40, 0.97, 3.00, and
6.20 mg/kg bw per day in males and 0, 0.49, 1.21, 3.81, and 8.01 mg/kg
bw per day in females. Fifty rats of each sex per dose were assigned
for assessment of carcinogenicity and 30 of each sex per dose for
toxicity; 10 rats of each sex per dose were sacrificed at week 52 for
interim assessment. Blood samples were collected in weeks 24, 49, 76,
and 102 of treatment and urine samples in weeks 23, 49, 77, and 101.
Mortality after 104 weeks was 70, 72, 54, 60, and 52% among males and
58, 60, 50, 30, and 46% among females at 0,10, 25, 75, and 150 ppm,
respectively. There were no treatment-related adverse effects on
behaviour, appearance, or ophthalmoscopic parameters during treatment
or at termination. Absolute body weight was reduced after 104 weeks of
treatment, by 1-15% in males and 6-12% in females at 75 mg/kg bw per
day and by 11-28% in males and 17-22% in females at 150 mg/kg bw per
day. These changes were not statistically significant; however,
body-weight gains were significantly decreased in males at 75 ppm
and in animals of each sex at 150 ppm. Blood chemical analysis,
haematological examination, and urinalysis showed no treatment-related
findings. Macroscopic examination of animals used for toxicity
assessment revealed a significantly increased incidence of dark
popliteal lymph nodes in females at 150 ppm. Macroscopic examination
of animals for carcinogenicity revealed significantly increased
incidences of pituitary masses, masses that compressed the ventral
surface of the brain, and masses in the abdominal fat in males at
150 ppm, and increased incidences of uterine masses and distension of
the uterus in females at this dose. Histopathological examination
revealed higher incidences of non-neoplastic changes, which included
syncytial macrophages in the mesenteric lymph nodes of females in
the toxicity assessment group, and lesions associated with gastric
ulceration, compression of the brain due to pituitary neoplasia,
and pancreatic lobular degeneration in males and interstitial
proliferation of the ovary in females in the carcinogenicity
assessment groups. High incidences of pituitary adenoma were observed
in all groups, but the rates (males: 34/70, 36/57, 32/50, 37/54, and
36/69; females, 39/69, 50/60, 52/54, 41/47, and 43/66) were within the
historical control range (males, 34-70%; females, 67-86%). There was
no evidence of carcinogenicity. The NOAEL was 25 ppm, equal to
0.97 mg/kg bw per day in males, based on the lowered body-weight gain
at 75 ppm (Aughton, 1989).
(d) Reproductive toxicity
Rats
A preliminary range-finding study was conducted in which groups
of six male and six female Sprague-Dawley CD rats received
fenpyroximate (purity, 97.3%) mixed into the diet at concentrations of
10, 50, or 200 ppm for seven weeks, from 15 days before mating to day
4 post partum, for two generations. There were no treatment-related
changes in behaviour or appearance in F0 or F1 animals, and there
were no deaths. Significant reductions in body-weight gains were
observed in parental animals of each sex at 200 ppm, and food
consumption was reduced at this dose due to unpalatability (Higgins,
1988).
A two-generation (one litter per generation) study was conducted
with groups of 24 male and 24 female Sprague-Dawley CD rats that
received fenpyroximate (purity, 97.3%) mixed into the diet at
concentrations of 0, 10, 30, or 100 ppm, from 14 weeks before mating
up to weaning of the F1 generation (14 weeks before mating, three
weeks of mating, three weeks' gestation, 25 days' lactation) and then
from weaning of the F1 generation to weaning of the F2 generation.
The mean daily intakes of fenpyroximate were 0.67, 1.99, or 6.59 mg/kg
bw per day for F0 males 0.83, 2.44, or 8.60 mg/kg bw per day for F0
females, 0.78, 2.33, or 8.45 mg/kg bw per day for F1 males, and 0.96,
2.82, or 9.92 mg/kg bw per day for F1 females during the premating
period. There were no treatment-related abnormalities in behaviour or
appearance in F0, F1, or F2 animals throughout the study.
Significant reductions in body-weight gain were observed at 100 ppm in
males of the F0 and F1 generations throughout the study and in F0
females up to parturition. Food consumption was significantly reduced
in F0 males at 100 ppm during the period before mating. There were no
treatment-related changes in the oestrus cycle, mating performance
(percent mating, conception rate, or fertility index), length of
gestation, gestation index, or parturition index in F0 or F1
animals. Significantly reduced body-weight gains were seen during
lactation in F1 and F2 offspring at 100 ppm. There were no
treatment-related Changes in litter size, viability, sex ratio,
initial body weight at birth, or developmental indices (pinna
unfolding, hair growth, tooth eruption, or eye opening) in the F1 and
F2 generations. The absolute and relative weights of the testes and
epidymides were significantly increased at termination of the study in
F1 males fed 100 ppm, but the weights were only 10% greater than
those of controls, and macroscopic and histopathological examinations
revealed no treatment-related changes in the reproductive organs. The
NOAEL was 30 ppm, equal to 1.99 mg/kg bw per day in F0 males,
2.44 mg/kg bw per day in F0 females, 2.33 mg/kg bw per day in F1
males, and 2.82 mg/kg bw per day in F1 females, based on reductions
in body-weight gain in animals of each sex at 100 ppm (Higgins,
1989a).
(e) Developmental toxicity
Rats
Groups of 22 pregnant Sprague-Dawley CD rats received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxy-
methylcellulose and 0.1% Tween 80 by gavage at 0, 1, 5, or 25 mg/kg bw
per day on days 6-15 of gestation. Three females in the group at
5 mg/kg bw per day died due to a technical error; all surviving
animals were sacrificed on day 20 of gestation. A slight loss of body
weight after the first dose, a significant increase in water
consumption, and a slight reduction in food consumption were seen
during the first six days of treatment in rats at 25 mg/kg bw per day.
No other maternal toxicity was observed. There were no treatment-
related effects on litter parameters. Slight increases in the
incidences of skeletal variations were seen in fetuses in all treated
groups. The incidences of unilateral and bilateral additional 14th
thoracic rib(s) were 2.4 and 1.4% in controls, 5.2 and 4.8% at 1 mg/kg
bw per day, 5.0 and 1.7% at 5 mg/kg bw per day, 8.2 and 7.7% at
25 mg/kg bw per day, and 0-4.2 and 0-3.5%, in historical controls,
respectively. The increases were not dose-dependent, but the percent
incidence in fetuses and the number of litters with the skeletal
variation at the highest dose, 25 mg/kg bw per day, exceeded the
historical control range. There was no evidence of teratogenicity at
doses up to and including 25 mg/kg bw per day. The NOAEL in this study
was 5 mg/kg bw per day, based on slightly decreased body weight in
maternal rats and the increased incidence of skeletal variations in
fetuses at 25 mg/kg bw per day (Higgins, 1989b).
Rabbits
In a preliminary study, groups of four pregnant New Zealand white
rabbits received fenpyroximate (purity, 98.4%) suspended in 1% aqueous
carboxymethylcellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0,
2.5, or 5.0 mg/kg bw per day on days 6-19 of gestation. Maternal
toxicity was seen as marked reductions in body-weight gain and reduced
defaecation, food consumption, and water consumption at 5.0 mg/kg bw
per day. Increased post-implantation loss was also recorded at this
dose, as were reduced fetal weight and multiple anomalies (increased
incidences of small fetuses, oedema of head and/or ventral part of
neck, and thorax, fore- and hind-limb flexure). The NOAEL for
fetotoxicity was 2.5 mg/kg bw per day, based on reduced fetal weight
and art increased incidence of anomalies at 5.0 mg/kg bw per day
(Bailey, 1989).
Groups of 15 pregnant New Zealand white rabbits received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxymethyl-
cellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0, 2.5, or
5.0 mg/kg bw per day on days 6-19 of gestation. Two females receiving
2.5 mg/kg bw per day were killed when moribund; necropsy revealed a
respiratory tract infection in one (14 days after treatment) and
evidence of gastrointestinal tract disturbance in the other (26 days
after treatment). All surviving animals were sacrificed on day 29 of
gestation. A transient decrease in body weight and in food consumption
and an increased incidence of reduced defaecation were observed at
5.0 mg/kg bw per day; a significant decrease in body weight was
observed at 2.5 mg/kg bw per day. Slight increases in the rate of
abortion and of total litter loss were observed in rabbits at 2.5 and
5.0 mg/kg bw per day: 0/15 in controls, 0/15 at 1.0 mg/kg bw per day,
7.7% (1/13) at 2.5 mg/kg bw per day, 13.3% (2/15) at 5.0 mg/kg bw per
day, and 0-14.3% in historical controls. The changes in body weight
and abortion rate at 2.5 mg/kg bw per day were slight but dose-
dependent. There was no evidence of fetotoxicity or teratogenicity at
doses up to 5.0 mg/kg bw per day. The NOAEL for maternotoxicity was
2.5 mg/kg bw per day (King, 1989).
(f) Genotoxicity
The results of studies on technical-grade fenpyroximate (purity,
97.3%) are summarized in table 3. The studies include those for
point mutations, mutation of mammalian cells in vitro, chromosomal
aberrations, micronucleus formation in vivo, DNA repair ( rec
assay), and unscheduled DNA synthesis. All of the positive controls
used gave the expected responses. There was no evidence of
genotoxicity.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The primary ocular irritation potential of fenpyroximate (purity,
98.4%) as a fine powder was evaluated in male New Zealand white
rabbits by grading and scoring according to the method of Draize.
Changes due to irritation were observed in the conjunctivae of eyes
treated with 0.1 g of fenpyroximate for 1 h to two days after
treatment. Rinsing slightly reduced the severity of the changes.
Fenpyroximate is classified as a mild primary irritant (Kosaka,
1988a). A fenpyroximate formulation (5% suspension concentrate) was
moderately irritating to the eyes of female New Zealand white rabbits
receiving 0.1 ml (Teale, 1990a).
Fenpyroximate (purity, 98.4%) applied as a fine powder moistened
with distilled water was not irritating to the skin of male New
Zealand white rabbits, graded and scored according to the method of
Draize (Kosaka, 1988b). Little or no dermal irritation was seen after
application of 0.5 ml of the 5% suspension concentrate to female New
Zealand white rabbits (Hayes, 1990).
The dermal sensitizing potential of fenpyroximate (purity, 98.4%)
was studied in female Hartley guinea-pigs by the maximization test.
Fenpyroximate was given at 0.1 ml per animal at 5% by intradermal
injection and 25% by local application for induction and then given
at 25% by local application for challenge. The positive control was
2,4-dinitrochlorobenzene (at 0.1, 1, and 0.5%, respectively). The
positive control group was sensitized by 100% and the fenpyroximate-
treated group by 36%. Fenpyroximate thus had moderate dermal
sensitizing potential (Kosaka, 1988c).
The delayed dermal sensitizing potential of fenpyroximate
(purity, 98.4%) was studied in female Dunkin-Hartley guinea-pigs by
the method of Buehler. A dose of 0.5 ml of 50% aqueous fenpyroximate
was applied to the skin three times weekly for induction; the animals
were then challenged by applications of 50 or 25% w/w aqueous
Table 3. Results of tests for the genotoxicity of fenpyroximate
End-point Test system Concentration Purity Results Reference
or dose (solvent) (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-5000 µg/plate (DMSO) 97.3 Negative May (1988)
TA100, TA1535, TA1537,
TA1538 (± S9), E. coli WP2
uvrA (± S9)
Mutation Chinese hamster 0-330 µg/ml (acetone) 97.3 Negative Hodson-Walker
V79 lung cells (1988a)
hprt locus (± S9)
Chromosomal Human lymphocytes 0-20 µg/ml (acetone) 97.3 Negative Hodson-Walker
aberration (± S9) (1988b)
DNA repair B subtilis H17, M45 0-500 µg/disc (DMSO) 97.3 Negative Watanabe (1990)
(± S9)
Unscheduled DNA Fischer 344 rat primary 0-1.02 µg/ml (DMSO) 97.3 Negative Cifone (1989)
synthesis hepatocytes
In vivo
Micronucleus CD-1 mice 0-2000 mg/kg bw orally 97.3 Negative Hodson-Walker
formation (1988c)
DMSO, dimethyl sulfoxide; S9, 9000 × g supernatant
fenpyroximate two weeks after the third induction. The positive
control was 2,4-dinitrochlorobenzene (0.3% for induction, 0.05 and
0.025% for challenge). The positive control group showed the expected
response, but fenpyroximate had no effect (Teale, 1990b).
A 5% suspension of fenpyroximate had no delayed dermal
sensitizing potential in female Dunkin-Hartley guinea-pigs tested by
the method of Buehler (Teale, 1990c).
(ii) Acute delayed neurotoxicity
Twelve adult Sterling Ranger hybrid hens were administered
fenpyroximate (purity, 97.0%) suspended in 0.5% aqueous
methylcellulose by catheter at a dose of 5000 mg/kg bw twice, on days
1 and 22 after insemination. A single oral dose of tri- ortho-cresyl
phosphate was used as the positive control. All surviving birds were
sacrificed on days 43-45. No deaths, abnormal neurological signs, or
treatment-related lesions in the nervous system were observed in
treated hens, whereas the positive control caused high mortality,
locomotor impairment, and degenerative changes in the spinal cord and
upper cervical bulbs, resulting in delayed neurotoxic responses
(Cummins, 1989).
3. Observations in humans
The results of medical surveillance of workers manufacturing a 5%
formulation of fenpyroximate were reported. Ocular and dermal
irritation were seen in July and November 1990 and March 1991. The
concentrations of fenpyroximate in the air at eight to nine sites in
the working place were 0.117 mg/m3 in January 1991, 0.012 mg/m3 in
April 1991, 0.005 mg/m3 in November 1991, and 0.004 mg/m3 in January
1993; however, the actual exposure of the workers to fenpyroximate was
not determined (Nokata, 1992, 1994).
Comments
Fenpyroximate was relatively well absorbed by rats after oral
administration. Absorbed fenpyroximate was excreted predominantly via
the biliary route, with lesser amounts in urine. The residual levels
in organs and tissues after 168 h were low. There was no evidence of
bioaccumulation.
Fenpyroximate was extensively metabolized in rats; 23 metabolites
were identified. No parent compound was found in the urine;
metabolites found in the excreta represented 0-11% of the administered
dose. Multiple pathways have been proposed for the metabolism of
fenpyroximate, including oxidation, hydroxylation, demethylation,
hydrolysis, and isomerization.
Fenpyroximate has slight to moderate acute oral toxicity (oral
LD50 = 440-520 mg/kg bw in mice and 245-480 mg/kg bw in rats); it is
acutely toxic by inhalation (LC50 in rats = 0.21-0.36 mg/m3). WHO
has not classified fenpyroximate for acute toxicity.
In a 13-week study in rats in which fenpyroximate was
administered in the diet at concentrations of 0, 20, 100, or 500 ppm,
the NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, and was based on
decreased body-weight gain and lowered plasma protein levels in
females fed 100 ppm. In a 13-week study in dogs, fenpyroximate was
administered orally in capsules at doses of 0, 2, 10, or 50 mg/kg bw
per day. The NOAEL was 2 mg/kg bw per day, on the basis of decreased
body-weight gain and clinical signs, such as an increased incidence of
diarrhoea, emaciation, and slight bradycardia, at 10 mg/kg bw per day.
In a 52-week study of similar design, with doses of 0, 0.5, 1.5, 5, or
15 mg/kg bw per day, the NOAEL was 5 mg/kg bw per day, on the basis of
decreased body-weight gain and total protein levels at 15 mg/kg bw per
day.
In an 18-month study of carcinogenicity in mice given dietary
concentrations of 0, 25, 100, 400, or 800 ppm, the NOAEL was 100 ppm,
equal to 9.5 mg/kg bw per day, and was based on decreased body-weight
gain, absolute body weight, and food consumption in animals of each
sex at 400 ppm. There was no evidence of carcinogenicity. In a
104-week study in rats given dietary concentrations of 0, 10, 25, 75,
or 150 ppm, the NOAEL was 25 ppm, equal to 1 mg/kg bw per day, based
on decreased body weight and body-weight gain in males at 75 ppm.
There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats given dietary concentrations of 0, 10,
30, or 100 ppm, the NOAEL for maternal and developmental toxicity was
30 ppm, equal to 2 mg/kg bw per day, on the basis of decreased body-
weight gain in animals of the F0 and F1 generations at 100 ppm.
In a study of developmental toxicity in rats given doses of 0, 1,
5, or 25 mg/kg bw per day by gavage, the NOAEL for maternal toxicity
and fetotoxicity was 25 mg/kg bw per day, the highest dose tested.
There was no evidence of embryotoxicity or teratogenicity. In a study
of developmental toxicity in rabbits given doses of 0, 1, 2.5, or
5 mg/kg bw per day by gavage, there was no evidence of embryo- or
fetotoxicity or teratogenicity at any dose. The NOAEL for maternal
toxicity was 2.5 mg/kg bw per day on the basis of minor effects at
5 mg/kg bw per day.
Fenpyroximate has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that it
is not genotoxic.
Two high doses, given 21 days apart, to hens did not cause gait
abnormalities or morphological changes in the nervous system.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1 mg/kg bw per day for reductions in body-weight gain and
plasma protein concentration in the 104-week study in rats and a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 9.5 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 25 ppm, equal to 1 mg/kg bw per day (104-week study of
carcinogenicity)
30 ppm, equal to 2 mg/kg bw per day (study of reproductive
toxicity)
Rabbit: 2.5 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Dog: 5 mg/kg bw per day (52-week study of toxicity)
Estimate of acceptable daily intake/or humans
0-0. 01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenpyroximate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, lethality, mouse and rat LD50 = 245-520 mg/kg bw
Eye, irritation, rabbit Irritating
Eye, irritation, human Irritating
Skin, sensitization, guinea-pig Sensitizing
Dermal, lethality, rat LD50 > 2000 mg/kg bw
inhalation, lethality tat LC50 = 0.21-0.36 mg/m3
Medium-term (1-26 weeks) Repeated 21-day toxicity, dermal, rat NOAEL = 300 mg/kg bw per day based
on reduced body-weight gain and
increased liver weight
Repeated four-week inhalation, toxicity, rat NOAEL = 2 mg/m3 based on increased
lung weight and mucosal change in nasal
passages
Repeated 13-week dietary, toxicity, rat NOAEL = 1.3 mg/kg bw per day based
on reduced body-weight gain and
lowered plasma protein level
Dietary, two-generation reproductive NOAEL = 2 mg/kg bw per day for
and developmental toxicity, rat maternal and developmental toxicity
Gavage, developmental toxicity, rat, rabbit NOAEL = 2.5 mg/kg bw per day for
maternal toxicity; no fetotoxicity or
teratogenicity
Long-term (> one year) Repeated dietary, two-year toxicity and NOAEL = 1 mg/kg bw per day based on
carcinogenicity, mouse; rat reduced body-weight gain and lowered
body weight; no carcinogenicity
References
Aughton, P. (1987) NNI-850: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 89/NHH021/0972
from Life Science Research Ltd, Suffolk, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Aughton, P. (1989) NNI-850: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks. Unpublished
report No. 89/NHH034/0921 from Life Science Research Ltd,
Suffolk., United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Bailey, G.P. (1989) Preliminary teratology study in the rabbit.
Unpublished report No. 89/NHH050/0393 from Life Science Research
Ltd, Suffolk United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989a) Acute oral toxicity study in mice/Test
material: NNI-850 Technical. Unpublished report No. 5066-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989b) Acute oral toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5065-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989c) Acute dermal toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5559-89 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Broadmeadow, A. (1988) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report
No. 89/NHH036/1111 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Broadmeadow, A. (1989) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report
No. 89/NHH037/0802 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Cifone, M.A. (1989) Mutagenicity test on NNI-850, technical grade in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. HLA 10753-0-447 from Hazleton Laboratories
America, Inc., Maryland, USA. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1989) NNI-850: Acute delayed neurotoxicity study in the
hen. Unpublished report No. 89/NHH054/0686 from Life Science
Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hayes, G. (1990) Primary skin irritation study, NNI-850 Flowable-R.
Unpublished report No. A/S/24651 from Toxicol Laboratories Ltd,
Herefordshire, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1988) NNI-850: Effects of dietary administration upon
reproductive performance in the rat; dosage range-finding study.
Unpublished report No. 88/NHH038/597 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989a) NNI-850: Reproductive performance study in rats
treated continuously through two successive generations.
Unpublished report No. 89/NHH043/0901 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989b) NNI-850: Teratology study in the rabbit.
Unpublished report No. 89/NHH053/0722 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988a) NNI-850: Investigation of mutagenic activity
at the HGPRT locus in a Chinese hamster V79 cell mutation system.
Unpublished report No. 89/NHH042/1060 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988b) In vitro assessment of the clastogenic
activity of NNI-850 in cultured human lymphocytes. Unpublished
report No. 90/NHH040/1369 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988c) NNI-850: Assessment of the clastogenic
action on bone marrow erythrocytes in the micronucleus test.
Unpublished report No. 89/NHH041/1059 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1989) An acute inhalation toxicity study of NNI-850 in
the rat. Unpublished report No. 88-8073 from Bio-dynamics Inc.,
New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Hoffman, G.M. (1990) An acute inhalation toxicity study of NNI-850
Flowable-R in the rat. Unpublished report No. 90-8253 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991a) An acute nose-only inhalation toxicity study of
NNI-850 in the rat. Unpublished report No. 90-8281 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991b) An acute nose-only inhalation toxicity study of
NNI-850 Flowable-R in the rat. Unpublished report No. 90-8282
from Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991c) A four week nose-only inhalation toxicity study
of NNI-850 in the rat. Unpublished report No. 90-8290 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
King, V.C. (1989) NNI-850: Teratology study in the rabbit. Unpublished
report No. 89/NHH051/0687 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Kosaka, T. (1988a) NNI-850: Primary eye irritation study in rabbits.
Unpublished report No. 87-0097 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988b) NNI-850: Primary dermal irritation study in
rabbits. Unpublished report No. 87-0098 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988c) NNI-850: Dermal sensitization study in guinea
pigs. Unpublished report No. 87-0099 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mahon, C.R. (1993) Dermal absorption of 14C-fenpyroximate following
a single dermal application to male Sprague-Dawley rats.
Unpublished report No. SC910039 from Battelle Columbus, Ohio,
USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
May, K. (1988) NNI-850 (technical grade): Assessment of mutagenic
potential in amino-acid auxotrophs of Salmonella typhimurium
and Escherichia coli (the Ames test). Unpublished report
No. 89/NHH039/1010 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Mitchell, J.M. (1990) Acute oral toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5795-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mitchell, J.M. (1991) Acute dermal toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5975-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Motoba, K., Suzuki, T. & Uchida, M. (1992) Effect of a new acaricide,
fenpyroximate, on energy metabolism and mitochondrial morphology
in adult female Tetracnychus urticae (two-spotted spider mite).
Pestic. Biochem. Physiol., 43, 37-44
Nishizawa, H., Motoba, K., Suzuki, T., Ohsima, T., Hamaguchi, H. &
Uchida, M. (1993) Metabolism of fenpyroximate in rats.
J. Pestic. Sci., 18, 59-66.
Nokata, M. (1992) Effect of fenpyroximate in human-eye and skin
irritation. Unpublished report No. H-4001 submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Nokata, M. (1994) Effect of fenpyroximate on factory workers.
Unpublished report No. H-4003 submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990a) Pharmacokinetics of a [pyrazole-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-104
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990b) Pharmacokinetics of a [benzyl-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-103
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991a) Metabolism and disposition of a [pyrazole-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-102 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991b) Metabolism and disposition of a [benzyl-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-101 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1987) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study Unpublished report No. IET
87-0034 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1988) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study. Unpublished report No. IET
87-0186 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1990) NNI-850: 18-Month oral oncogenicity study in
mice. Unpublished report No. 87-0036 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Taninaka, K. (1993) Fenpyroximate, a new acaricide. Agrochem. Jpn,
62, 15-17.
Teale, H.J (1990a) Primary eye irritation study, NNI-850 Flowable-R.
Unpublished report No. A/E/24652 from Toxicol Laboratories
Limited, Herefordshire, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Teale, H.J. (1990b) NNI-850 Technical/Delayed dermal sensitization
test in the guinea pig. Unpublished report No. A/B/22645 from
Toxicol Laboratories Ltd, Herefordshire, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Teale, H.J. (1990c) NNI-850 Flowable/Delayed dermal sensitization test
in the guinea pig. Unpublished report No. A/B/22646 from Toxicol
Laboratories Ltd, Herefordshire, United Kingdom. Submitted to WHO
by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Tsu-Han Li (1991a) [Benzyl-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-001 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Tsu-Han Li (1991b) [Pyrazole-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-002 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Watanabe, M. (1990) NNI-850: DNA repair test (rec-assay). Unpublished
report No. 88-0072 from the Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Wilkinson, G.E., Ryan, M.J & Peters, A.C. (1992) 21-Day repeated-dose
dermal toxicity study of fenpyroximate in the rat. Unpublished
report No. SC920009 from Battelle Columbus, Ohio, USA. Submitted
to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Groups of five male and five female Sprague-Dawley CD rats were
treated with single oral doses of 2 or 400 mg/kg bw 14C-pyrazole- or
14C-benzyl-labelled fenpyroximate, and urinary and faecal samples
were collected at 0-24, 24-48, 48-120, and 120-168 h. The samples from
each group were pooled for each time period. Radiolabel in faeces
after 0-48 h represented 80-87% of the dose of 2 mg/kg and 80% of the
dose of 400 mg/kg; parent fenpyroximate was found at 6-8% after
2 mg/kg and 50-52% after 400 mg/kg. No parent compound was found in
urine. Eleven urinary and 19 faecal metabolites were identified by
thin-layer co-chromatography with authentic samples. The major urinary
metabolites were H, S, and R (Figure 1), produced by hydrolytic
cleavage of the oxime ether bond between the benzyl moiety and the
pyrazole ring. The major faecal metabolites were C, D, and E, produced
by hydrolysis of the tert-butyl ester, and T, produced by oxidation
of the tert-butyl group. A Z isomer of fenpyroximate, A, was found
in faeces of rats treated with the high dose. The concentrations of
urinary metabolite H and faecal metabolites H and T were increased by
enzymatic hydrolysis of the excreta with ß-glucuronidase or sulfatase
(Sharp 1991a,b).
Groups of four male Sprague-Dawley (SLC) rats were treated with a
single oral dose of 1.5 mg/kg bw 14C-pyrazole- or 14C-benzoyl-
labelled fenpyroximate (radioactive purity, > 99%), and urinary and
faecal samples were collected for 0-72 h. Six urinary and 17 faecal
metabolites were identified by thin-layer co-chromatography with
authentic samples. Three of them, W, X, and V, were isolated from
faeces and identified by mass spectrometry and nuclear magnetic
resonance. The major urinary metabolites were H (7.3% of the dose),
S (2.5%), and R (3.8%). The major faecal metabolites were C (4.1-11.0%
of the dose), E (2.9-4.2%), and T (3.5-4.3%); P (7.5%) was found as a
precursor of R and W (2.0-9.7%) and X (3.3-4.5%) as hydroxylated
bodies of T. The concentrations of the urinary metabolites J and N and
the faecal metabolites B, T, W, X, and V were increased by enzymatic
hydrolysis of the excreta with ß-glucuronidase or sulfatase (Nishizawa
et al., 1993).
Groups of six male and six female Sprague-Dawley rats with
bile-duct cannulae were given a single oral dose of 2 mg/kg
14C-pyrazole-labelled fenpyroximate. No parent fenpyroximate was
found in bile, but metabolites C, D, E, F, G, H, I, J, M, N, and T and
conjugates of C, D, E, and H were found. Total radiolabel represented
less than 2% of the dose. The metabolic pathway proposed for
fenpyroximate in rats is cleavage of the ester bond, hydroxylation at
the phenoxypyrazole group, oxidation at the tert-butyl group, and
conjugation with sulfate and glucuronide (Tsu-Han Li, 1991a,b).
3. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of technical-grade (> 98%)
fenpyroximate and of a 5% suspension concentrate are summarized in
Table 2. Moderate oral toxicity was observed in rats and mice, with
LD50 values ranging from 245 to 520 mg/kg bw. Fenpyroximate was more
toxic to rats when administered by inhalation, with LC50 values
ranging from 0.21 to 0.36 mg/litre air. Signs of acute toxicity in
mice and rats included urinary and faecal staining, hypoactivity, and
hypopnoea. Decreased food consumption was observed in surviving
animals one week after dosing. Necropsy of animals found dead revealed
irritation and/or corrosive effects in the gastointestinal tract.
There were no signs of acute toxicity or dermal irritation after
dermal application of either formulation. Inhalation of active
ingredient or the formulation resulted in signs of respiratory
irritation (laboured breathing, rale, and gasping). The findings at
necropsy were unremarkable in groups exposed by whole-body inhalation,
but those in groups exposed by nose only included oedematous,
reddened, firm lungs and frothy fluid in the trachea.
(b) Short-term toxicity
Mice
In a preliminary study to establish the dose range for a
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice were given fenpyroximate (purity, 97.9%) mixed into
the diet at concentrations of 80, 400, or 2000 ppm, equal to 0, 10.8,
48.4, and 181.6 mg/kg bw per day in males and 0, 11.7, 50.4, and
170.0 mg/kg bw per day in females, for four weeks. There were no
deaths and no abnormal behavioural signs in any treated group.
Decreased body-weight gain was observed in males at 400 ppm and in
animals of each sex at 2000 ppm; food consumption and food efficiency
were reduced in animals at 2000 ppm. Haematological examination
revealed significant decreases in haemoglobin in animals of each sex,
of the haematocrit in males, and of the erythrocyte count in females,
and a significant decrease in total plasma protein and a significant
increase in aspartate aminotransferase in animals of each sex at
2000 ppm. The NOAEL in this study was 80 ppm, equal to 10.8 mg/kg bw
per day in males and 11.7 mg/kg bw per day in females (Takahashi,
1988).
In a four-week study to find the dose range for the
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice received fenpyroximate (purity, 97.9%) mixed into the
diet at a concentration of 0, 20, 100, or 500 ppm, equal to 0, 2.58,
12.9, and 53.2 mg/kg bw per day in males and 0, 3.07, 14.5, and
66.7 mg/kg bw per day in females. Slight reductions in body-weight
Table 2. Acute toxicity of fenpyroximate
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Fenpyroximate
ICR CD-1 mouse Male Orala 520 (334-706) 98 Blaszcak (1989a)
Female 440 (281-599)
Sprague-Dawley Male Orala 480 (298-662) 98 Blaszcak (1989b)
CD rat Female 245 (167-323)
Sprague-Dawley Male Dermalb > 2000 98 Blaszcak (1989c)
CD rat Female > 2000
Sprague-Dawley Male Inhalation 0.33 (0.16-0.68) 99 Hoffman (1989)
CD rat Female (whole-body)c 0.36 (0.24-0.54)
Sprague-Dawley Male Inhalation 0.21 (0.04-1.20) 98 Hoffman (1991a)
CD rat Female (nose-only)d 0.33 (0.20--0.55)
5% suspension concentrate
Sprague-Dawley Male Oral 7193 (2989-17 308) Mitchell (1990)
CD rat Female 6789 (4704-9797)
Sprague-Dawley Male Dermal > 4000 Mitchell (1991)
CD rat Female > 4000
Sprague-Dawley Male Inhalation 1.9 (1.2-3.1) Hoffman (1990)
CD rat Female (whole-body)e 2.4 (2.0-2.9)
Table 2. (con't)
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Sprague-Dawley Male Inhalation 5.1 (0.0-17.1) Hoffman (1991b)
CD rat Female (nose-only)f 3.4 (1 9-4.9)
a In Tween 80 and a 1% solution of Methocel (A4C)
b Applied on a gauze moistened with 0.9% saline
c Fenpyroximate containing white carbon (9:1); mean particle size, 4.2 µm; exposure time, 4 h
d Fenpyroximate containing white carbon (9:1); mean particle size, 2.8 µm; exposure time, 4 h
e Mean particle size, 3.3 µm; exposure time, 4 h
f Mean particle size, 5.1 µm; exposure time, 4 h
gain and in food consumption were observed in males at 500 ppm. There
were no abnormal clinical signs, no changes in haematological
parameters, and no changes in organ weights. The NOAEL in this study
was 100 ppm, equal to 12.9 mg/kg bw per day in males and > 500 ppm in
females (Takahashi, 1987).
Rats
Groups of 10 male and 10 female Sprague-Dawley CD rats received
fenpyroximate (purity, 99.0%) in the diet at concentrations of 0, 20,
100, or 500 ppm, equal to 0, 1.3, 6.6, or 35.2 mg/kg bw per day for
males and 0, 1.7, 8.3, or 38.6 mg/kg bw per day for females, for 13
weeks. There were no overt changes in physical condition or behaviour
and no abnormal ophthalmoscopic findings. Increased encrustations
of the muzzle in males and an increased incidence of hair loss
accompanied by encrustation of the skin in females were observed at
500 ppm, Significant reductions in body-weight gain at 100 and 500 ppm
and significant reductions in food consumption at 500 ppm were
observed in animals of each sex. A significant lowering of total
plasma protein level was observed in females at 100 ppm and in animals
of each sex at 500 ppm. A significant increase in relative liver body
weights and slight hypertrophy of hepatic cells were observed in
animals of each sex and a significant increase in alkaline phosphatase
activity in females at 500 ppm; a significantly lower volume and pH
value of urine were also observed at this dose. Erythrocyte
cholinesterase activity was significantly lowered in animals at
500 ppm, but the inhibition was less than 20% of control. The NOAEL in
this study was 20 ppm, equal to 1.30 mg/kg bw per day in males and
1.65 mg/kg bw per day in females, based on the lowered body-weight
gain in animals of each sex and a lowered plasma protein level in
females at 100 ppm (Aughton, 1987).
Fenpyroximate (purity, 99%) was applied to the dorsal skin of
groups of five male and five female Sprague-Dawley rats in a uniform
layer moistened with water at 0, 100, 300, or 1000 mg/kg bw for 6 h
per day for 21 consecutive days. Minor acanthosis was observed in all
treated and one control animal. All rats survived, and there were
no compound-related clinical abnormalities. Reductions in food
consumption and body-weight gain in animals of each sex and increases
in absolute liver weight in females were observed at 1000 mg/kg bw.
There were no compound-related changes in haematological or blood
chemical parameters. The NOAEL in this study was 300 mg/kg bw per day,
based on the reduction in body-weight gain in animals of each sex and
the increase in absolute liver weight in female at 1000 mg/kg bw
(Wilkinson et al., 1992).
Groups of four male and four female Sprague-Dawley CD rats were
exposed by nose-only inhalation to fenpyroximate (purity, 98.6%; mean
particle size, 3 µm) at a target concentration of 0, 2, 10, or
50 mg/m3 for 6 h per day, five days per week for four weeks. All
animals survived. The only treatment-related physical or behavioural
change was an increased incidence of laboured breathing during the
last three weeks of exposure to 50 mg/m3. Increase in absolute and
relative lung weight were observed in animals of each sex after four
weeks' exposure to 10 or 50 mg/m3, but these effects were not seen
two weeks after exposure. A higher incidence of mucosal changes in
the nasal passages (atrophy and metaplasia) were observed at
concentrations of 10 (in males) and 50 mg/m3 (males and females). The
NOAEL in this study was 2 mg/m3, based on an increase in lung weight
and an increased incidence of mucosal changes in the nasal passages
(Hoffman, 1991c).
Dogs
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.4%) in gelatin capsules at 0, 2, 10, or
50 mg/kg bw per day for 13 weeks. Two females given 50 mg/kg bw were
found moribund and were killed after five weeks of treatment.
Increased incidences of diarrhoea and emaciation were observed in
females at 10 mg/kg bw per day and in males at 50 mg/kg bw per day.
Electrocardiograms examined 2 and 24 h after treatment showed
bradycardia in males after six weeks of treatment at 10 or 50 mg/kg bw
per day and after 12 weeks of treatment at 50 mg/kg bw per day. There
were no treatment-related ocular lesions. Significant decreases in
total body-weight gain were observed in females at 10 mg/kg bw per
day and in animals of each sex at 50 mg/kg bw per day. The only
significant haematological change was a reduction in the total number
of leukocytes in females at 50 mg/kg bw per day after 6 and 12 weeks
of treatment. Histopathological examination showed fine cytoplasmic
vacuolation of cells in the renal medullary rays in two females at
50 mg/kg bw per day sacrificed before the end of treatment and in one
female killed at termination. There were no significant changes in
cholinesterase activity in plasma or erythrocytes. The NOAEL in this
study was 2 mg/kg bw per day, based on the reduction in body-weight
gain and clinical signs at 10 mg/kg bw per day (Broadmeadow, 1988).
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.0%) in gelatin capsules at 0, 0.5, 1.5, 5,
or 15 mg/kg bw per day on seven days per week for 52 weeks. Clinical
observations were made daily, electrocardiograms were perfomed after
11, 24, and 50 weeks, haematological examinations were done after
12, 24, and 50 weeks, blood chemistry after 24 and 50 weeks, and
ophthalmoscopy after 49 weeks of treatment. There were no deaths.
Increased incidences of diarrhoea were observed during treatment in
males at 5 or 15 mg/kg bw per day and in females at 15 mg/kg bw per
day. Increased salivation was observed in females at 15 mg/kg bw per
day. Slight bradycardia was observed in animals of each sex at
15 mg/kg bw per day, which was significant in males at 11 and 50 weeks
of treatment. The overall body-weight gain throughout treatment was
significantly lower in males at 15 mg/kg bw per day, and a slightly
reduced total food consumption was observed. There were no treatment-
related haematological or ophthalmological findings. A slight but
significant lowering of total plasma protein level was observed in
males at 15 mg/kg bw per day after 50 weeks of treatment. There was no
change in erythrocyte acetylcholinesterase activity. The absolute and
relative prostatic weights were significantly increased in all treated
groups, but no histopathological changes were observed in the prostate
or in any other tissue examined. The NOAEL was 1.5 mg/kg bw per day,
based on the increased incidence of diarrhoea at 5 mg/kg bw per day
(Broadmeadow, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female ICR (Crj;CD) mice received
fenpyroximate (purity, 97.9-98.4%) mixed into the diet at
concentrations of 0, 25, 100, 400, or 800 ppm (overall mean
concentrations: 0, 24, 98, 388, and 776 ppm) for 18 months (78 weeks),
equal to 0, 2.4, 9.5, 38.0, or 69.6 mg/kg bw per day for males and 0,
2.5, 10.2, 41.5, or 73.1 mg/kg bw per day for females. There were no
treatment-related alterations in clinical signs. Body-weight gain and
food consumption were significantly lower than those of controls in
animals of each sex at 400 and 800 ppm. The only significant change in
haematological parameters was a decrease in the number of segmented
neutrophils at 500 ppm. A significant increase in the incidence of
emaciation was observed in males a t 500 ppm at the termination of
treatment. Histopathological examination revealed significant
increases in the incidence of ovarian atrophy in females at 400 and
800 ppm (25/50 in controls, 35/50 at 400 ppm, 35/50 at 800 ppm), but
these changes were morphologically similar to age-related changes seen
in female mice of this strain. There was no treatment-related increase
in the incidence of neoplasia. The NOAEL in this study was 100 ppm,
equal to 9.5 mg/kg bw per day in males and 10.2 mg/kg bw per day in
females, based on decreased body-weight gain and food consumption at
400 ppm (Takahashi, 1990).
Rats
Groups of 80 male and 80 female Sprague-Dawley CD rats
received fenpyroximate (purity, 97.1-97.2%) mixed into the diet at
concentrations of 0, 10, 25, 75, or 150 ppm for 104 weeks. The overall
doses achieved by the groups in which toxicity was assessed were 0,
0.40, 0.97, 3.08, and 6.18 mg/kg bw per day in males and 0, 0.48,
1.16, 3.79, and 7.57 mg/kg bw per day in females; those in the groups
for assessment of carcinogenicity were 0, 0.40, 0.97, 3.00, and
6.20 mg/kg bw per day in males and 0, 0.49, 1.21, 3.81, and 8.01 mg/kg
bw per day in females. Fifty rats of each sex per dose were assigned
for assessment of carcinogenicity and 30 of each sex per dose for
toxicity; 10 rats of each sex per dose were sacrificed at week 52 for
interim assessment. Blood samples were collected in weeks 24, 49, 76,
and 102 of treatment and urine samples in weeks 23, 49, 77, and 101.
Mortality after 104 weeks was 70, 72, 54, 60, and 52% among males and
58, 60, 50, 30, and 46% among females at 0,10, 25, 75, and 150 ppm,
respectively. There were no treatment-related adverse effects on
behaviour, appearance, or ophthalmoscopic parameters during treatment
or at termination. Absolute body weight was reduced after 104 weeks of
treatment, by 1-15% in males and 6-12% in females at 75 mg/kg bw per
day and by 11-28% in males and 17-22% in females at 150 mg/kg bw per
day. These changes were not statistically significant; however,
body-weight gains were significantly decreased in males at 75 ppm
and in animals of each sex at 150 ppm. Blood chemical analysis,
haematological examination, and urinalysis showed no treatment-related
findings. Macroscopic examination of animals used for toxicity
assessment revealed a significantly increased incidence of dark
popliteal lymph nodes in females at 150 ppm. Macroscopic examination
of animals for carcinogenicity revealed significantly increased
incidences of pituitary masses, masses that compressed the ventral
surface of the brain, and masses in the abdominal fat in males at
150 ppm, and increased incidences of uterine masses and distension of
the uterus in females at this dose. Histopathological examination
revealed higher incidences of non-neoplastic changes, which included
syncytial macrophages in the mesenteric lymph nodes of females in
the toxicity assessment group, and lesions associated with gastric
ulceration, compression of the brain due to pituitary neoplasia,
and pancreatic lobular degeneration in males and interstitial
proliferation of the ovary in females in the carcinogenicity
assessment groups. High incidences of pituitary adenoma were observed
in all groups, but the rates (males: 34/70, 36/57, 32/50, 37/54, and
36/69; females, 39/69, 50/60, 52/54, 41/47, and 43/66) were within the
historical control range (males, 34-70%; females, 67-86%). There was
no evidence of carcinogenicity. The NOAEL was 25 ppm, equal to
0.97 mg/kg bw per day in males, based on the lowered body-weight gain
at 75 ppm (Aughton, 1989).
(d) Reproductive toxicity
Rats
A preliminary range-finding study was conducted in which groups
of six male and six female Sprague-Dawley CD rats received
fenpyroximate (purity, 97.3%) mixed into the diet at concentrations of
10, 50, or 200 ppm for seven weeks, from 15 days before mating to day
4 post partum, for two generations. There were no treatment-related
changes in behaviour or appearance in F0 or F1 animals, and there
were no deaths. Significant reductions in body-weight gains were
observed in parental animals of each sex at 200 ppm, and food
consumption was reduced at this dose due to unpalatability (Higgins,
1988).
A two-generation (one litter per generation) study was conducted
with groups of 24 male and 24 female Sprague-Dawley CD rats that
received fenpyroximate (purity, 97.3%) mixed into the diet at
concentrations of 0, 10, 30, or 100 ppm, from 14 weeks before mating
up to weaning of the F1 generation (14 weeks before mating, three
weeks of mating, three weeks' gestation, 25 days' lactation) and then
from weaning of the F1 generation to weaning of the F2 generation.
The mean daily intakes of fenpyroximate were 0.67, 1.99, or 6.59 mg/kg
bw per day for F0 males 0.83, 2.44, or 8.60 mg/kg bw per day for F0
females, 0.78, 2.33, or 8.45 mg/kg bw per day for F1 males, and 0.96,
2.82, or 9.92 mg/kg bw per day for F1 females during the premating
period. There were no treatment-related abnormalities in behaviour or
appearance in F0, F1, or F2 animals throughout the study.
Significant reductions in body-weight gain were observed at 100 ppm in
males of the F0 and F1 generations throughout the study and in F0
females up to parturition. Food consumption was significantly reduced
in F0 males at 100 ppm during the period before mating. There were no
treatment-related changes in the oestrus cycle, mating performance
(percent mating, conception rate, or fertility index), length of
gestation, gestation index, or parturition index in F0 or F1
animals. Significantly reduced body-weight gains were seen during
lactation in F1 and F2 offspring at 100 ppm. There were no
treatment-related Changes in litter size, viability, sex ratio,
initial body weight at birth, or developmental indices (pinna
unfolding, hair growth, tooth eruption, or eye opening) in the F1 and
F2 generations. The absolute and relative weights of the testes and
epidymides were significantly increased at termination of the study in
F1 males fed 100 ppm, but the weights were only 10% greater than
those of controls, and macroscopic and histopathological examinations
revealed no treatment-related changes in the reproductive organs. The
NOAEL was 30 ppm, equal to 1.99 mg/kg bw per day in F0 males,
2.44 mg/kg bw per day in F0 females, 2.33 mg/kg bw per day in F1
males, and 2.82 mg/kg bw per day in F1 females, based on reductions
in body-weight gain in animals of each sex at 100 ppm (Higgins,
1989a).
(e) Developmental toxicity
Rats
Groups of 22 pregnant Sprague-Dawley CD rats received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxy-
methylcellulose and 0.1% Tween 80 by gavage at 0, 1, 5, or 25 mg/kg bw
per day on days 6-15 of gestation. Three females in the group at
5 mg/kg bw per day died due to a technical error; all surviving
animals were sacrificed on day 20 of gestation. A slight loss of body
weight after the first dose, a significant increase in water
consumption, and a slight reduction in food consumption were seen
during the first six days of treatment in rats at 25 mg/kg bw per day.
No other maternal toxicity was observed. There were no treatment-
related effects on litter parameters. Slight increases in the
incidences of skeletal variations were seen in fetuses in all treated
groups. The incidences of unilateral and bilateral additional 14th
thoracic rib(s) were 2.4 and 1.4% in controls, 5.2 and 4.8% at 1 mg/kg
bw per day, 5.0 and 1.7% at 5 mg/kg bw per day, 8.2 and 7.7% at
25 mg/kg bw per day, and 0-4.2 and 0-3.5%, in historical controls,
respectively. The increases were not dose-dependent, but the percent
incidence in fetuses and the number of litters with the skeletal
variation at the highest dose, 25 mg/kg bw per day, exceeded the
historical control range. There was no evidence of teratogenicity at
doses up to and including 25 mg/kg bw per day. The NOAEL in this study
was 5 mg/kg bw per day, based on slightly decreased body weight in
maternal rats and the increased incidence of skeletal variations in
fetuses at 25 mg/kg bw per day (Higgins, 1989b).
Rabbits
In a preliminary study, groups of four pregnant New Zealand white
rabbits received fenpyroximate (purity, 98.4%) suspended in 1% aqueous
carboxymethylcellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0,
2.5, or 5.0 mg/kg bw per day on days 6-19 of gestation. Maternal
toxicity was seen as marked reductions in body-weight gain and reduced
defaecation, food consumption, and water consumption at 5.0 mg/kg bw
per day. Increased post-implantation loss was also recorded at this
dose, as were reduced fetal weight and multiple anomalies (increased
incidences of small fetuses, oedema of head and/or ventral part of
neck, and thorax, fore- and hind-limb flexure). The NOAEL for
fetotoxicity was 2.5 mg/kg bw per day, based on reduced fetal weight
and art increased incidence of anomalies at 5.0 mg/kg bw per day
(Bailey, 1989).
Groups of 15 pregnant New Zealand white rabbits received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxymethyl-
cellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0, 2.5, or
5.0 mg/kg bw per day on days 6-19 of gestation. Two females receiving
2.5 mg/kg bw per day were killed when moribund; necropsy revealed a
respiratory tract infection in one (14 days after treatment) and
evidence of gastrointestinal tract disturbance in the other (26 days
after treatment). All surviving animals were sacrificed on day 29 of
gestation. A transient decrease in body weight and in food consumption
and an increased incidence of reduced defaecation were observed at
5.0 mg/kg bw per day; a significant decrease in body weight was
observed at 2.5 mg/kg bw per day. Slight increases in the rate of
abortion and of total litter loss were observed in rabbits at 2.5 and
5.0 mg/kg bw per day: 0/15 in controls, 0/15 at 1.0 mg/kg bw per day,
7.7% (1/13) at 2.5 mg/kg bw per day, 13.3% (2/15) at 5.0 mg/kg bw per
day, and 0-14.3% in historical controls. The changes in body weight
and abortion rate at 2.5 mg/kg bw per day were slight but dose-
dependent. There was no evidence of fetotoxicity or teratogenicity at
doses up to 5.0 mg/kg bw per day. The NOAEL for maternotoxicity was
2.5 mg/kg bw per day (King, 1989).
(f) Genotoxicity
The results of studies on technical-grade fenpyroximate (purity,
97.3%) are summarized in table 3. The studies include those for
point mutations, mutation of mammalian cells in vitro, chromosomal
aberrations, micronucleus formation in vivo, DNA repair ( rec
assay), and unscheduled DNA synthesis. All of the positive controls
used gave the expected responses. There was no evidence of
genotoxicity.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The primary ocular irritation potential of fenpyroximate (purity,
98.4%) as a fine powder was evaluated in male New Zealand white
rabbits by grading and scoring according to the method of Draize.
Changes due to irritation were observed in the conjunctivae of eyes
treated with 0.1 g of fenpyroximate for 1 h to two days after
treatment. Rinsing slightly reduced the severity of the changes.
Fenpyroximate is classified as a mild primary irritant (Kosaka,
1988a). A fenpyroximate formulation (5% suspension concentrate) was
moderately irritating to the eyes of female New Zealand white rabbits
receiving 0.1 ml (Teale, 1990a).
Fenpyroximate (purity, 98.4%) applied as a fine powder moistened
with distilled water was not irritating to the skin of male New
Zealand white rabbits, graded and scored according to the method of
Draize (Kosaka, 1988b). Little or no dermal irritation was seen after
application of 0.5 ml of the 5% suspension concentrate to female New
Zealand white rabbits (Hayes, 1990).
The dermal sensitizing potential of fenpyroximate (purity, 98.4%)
was studied in female Hartley guinea-pigs by the maximization test.
Fenpyroximate was given at 0.1 ml per animal at 5% by intradermal
injection and 25% by local application for induction and then given
at 25% by local application for challenge. The positive control was
2,4-dinitrochlorobenzene (at 0.1, 1, and 0.5%, respectively). The
positive control group was sensitized by 100% and the fenpyroximate-
treated group by 36%. Fenpyroximate thus had moderate dermal
sensitizing potential (Kosaka, 1988c).
The delayed dermal sensitizing potential of fenpyroximate
(purity, 98.4%) was studied in female Dunkin-Hartley guinea-pigs by
the method of Buehler. A dose of 0.5 ml of 50% aqueous fenpyroximate
was applied to the skin three times weekly for induction; the animals
were then challenged by applications of 50 or 25% w/w aqueous
Table 3. Results of tests for the genotoxicity of fenpyroximate
End-point Test system Concentration Purity Results Reference
or dose (solvent) (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-5000 µg/plate (DMSO) 97.3 Negative May (1988)
TA100, TA1535, TA1537,
TA1538 (± S9), E. coli WP2
uvrA (± S9)
Mutation Chinese hamster 0-330 µg/ml (acetone) 97.3 Negative Hodson-Walker
V79 lung cells (1988a)
hprt locus (± S9)
Chromosomal Human lymphocytes 0-20 µg/ml (acetone) 97.3 Negative Hodson-Walker
aberration (± S9) (1988b)
DNA repair B subtilis H17, M45 0-500 µg/disc (DMSO) 97.3 Negative Watanabe (1990)
(± S9)
Unscheduled DNA Fischer 344 rat primary 0-1.02 µg/ml (DMSO) 97.3 Negative Cifone (1989)
synthesis hepatocytes
In vivo
Micronucleus CD-1 mice 0-2000 mg/kg bw orally 97.3 Negative Hodson-Walker
formation (1988c)
DMSO, dimethyl sulfoxide; S9, 9000 × g supernatant
fenpyroximate two weeks after the third induction. The positive
control was 2,4-dinitrochlorobenzene (0.3% for induction, 0.05 and
0.025% for challenge). The positive control group showed the expected
response, but fenpyroximate had no effect (Teale, 1990b).
A 5% suspension of fenpyroximate had no delayed dermal
sensitizing potential in female Dunkin-Hartley guinea-pigs tested by
the method of Buehler (Teale, 1990c).
(ii) Acute delayed neurotoxicity
Twelve adult Sterling Ranger hybrid hens were administered
fenpyroximate (purity, 97.0%) suspended in 0.5% aqueous
methylcellulose by catheter at a dose of 5000 mg/kg bw twice, on days
1 and 22 after insemination. A single oral dose of tri- ortho-cresyl
phosphate was used as the positive control. All surviving birds were
sacrificed on days 43-45. No deaths, abnormal neurological signs, or
treatment-related lesions in the nervous system were observed in
treated hens, whereas the positive control caused high mortality,
locomotor impairment, and degenerative changes in the spinal cord and
upper cervical bulbs, resulting in delayed neurotoxic responses
(Cummins, 1989).
3. Observations in humans
The results of medical surveillance of workers manufacturing a 5%
formulation of fenpyroximate were reported. Ocular and dermal
irritation were seen in July and November 1990 and March 1991. The
concentrations of fenpyroximate in the air at eight to nine sites in
the working place were 0.117 mg/m3 in January 1991, 0.012 mg/m3 in
April 1991, 0.005 mg/m3 in November 1991, and 0.004 mg/m3 in January
1993; however, the actual exposure of the workers to fenpyroximate was
not determined (Nokata, 1992, 1994).
Comments
Fenpyroximate was relatively well absorbed by rats after oral
administration. Absorbed fenpyroximate was excreted predominantly via
the biliary route, with lesser amounts in urine. The residual levels
in organs and tissues after 168 h were low. There was no evidence of
bioaccumulation.
Fenpyroximate was extensively metabolized in rats; 23 metabolites
were identified. No parent compound was found in the urine;
metabolites found in the excreta represented 0-11% of the administered
dose. Multiple pathways have been proposed for the metabolism of
fenpyroximate, including oxidation, hydroxylation, demethylation,
hydrolysis, and isomerization.
Fenpyroximate has slight to moderate acute oral toxicity (oral
LD50 = 440-520 mg/kg bw in mice and 245-480 mg/kg bw in rats); it is
acutely toxic by inhalation (LC50 in rats = 0.21-0.36 mg/m3). WHO
has not classified fenpyroximate for acute toxicity.
In a 13-week study in rats in which fenpyroximate was
administered in the diet at concentrations of 0, 20, 100, or 500 ppm,
the NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, and was based on
decreased body-weight gain and lowered plasma protein levels in
females fed 100 ppm. In a 13-week study in dogs, fenpyroximate was
administered orally in capsules at doses of 0, 2, 10, or 50 mg/kg bw
per day. The NOAEL was 2 mg/kg bw per day, on the basis of decreased
body-weight gain and clinical signs, such as an increased incidence of
diarrhoea, emaciation, and slight bradycardia, at 10 mg/kg bw per day.
In a 52-week study of similar design, with doses of 0, 0.5, 1.5, 5, or
15 mg/kg bw per day, the NOAEL was 5 mg/kg bw per day, on the basis of
decreased body-weight gain and total protein levels at 15 mg/kg bw per
day.
In an 18-month study of carcinogenicity in mice given dietary
concentrations of 0, 25, 100, 400, or 800 ppm, the NOAEL was 100 ppm,
equal to 9.5 mg/kg bw per day, and was based on decreased body-weight
gain, absolute body weight, and food consumption in animals of each
sex at 400 ppm. There was no evidence of carcinogenicity. In a
104-week study in rats given dietary concentrations of 0, 10, 25, 75,
or 150 ppm, the NOAEL was 25 ppm, equal to 1 mg/kg bw per day, based
on decreased body weight and body-weight gain in males at 75 ppm.
There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats given dietary concentrations of 0, 10,
30, or 100 ppm, the NOAEL for maternal and developmental toxicity was
30 ppm, equal to 2 mg/kg bw per day, on the basis of decreased body-
weight gain in animals of the F0 and F1 generations at 100 ppm.
In a study of developmental toxicity in rats given doses of 0, 1,
5, or 25 mg/kg bw per day by gavage, the NOAEL for maternal toxicity
and fetotoxicity was 25 mg/kg bw per day, the highest dose tested.
There was no evidence of embryotoxicity or teratogenicity. In a study
of developmental toxicity in rabbits given doses of 0, 1, 2.5, or
5 mg/kg bw per day by gavage, there was no evidence of embryo- or
fetotoxicity or teratogenicity at any dose. The NOAEL for maternal
toxicity was 2.5 mg/kg bw per day on the basis of minor effects at
5 mg/kg bw per day.
Fenpyroximate has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that it
is not genotoxic.
Two high doses, given 21 days apart, to hens did not cause gait
abnormalities or morphological changes in the nervous system.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1 mg/kg bw per day for reductions in body-weight gain and
plasma protein concentration in the 104-week study in rats and a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 9.5 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 25 ppm, equal to 1 mg/kg bw per day (104-week study of
carcinogenicity)
30 ppm, equal to 2 mg/kg bw per day (study of reproductive
toxicity)
Rabbit: 2.5 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Dog: 5 mg/kg bw per day (52-week study of toxicity)
Estimate of acceptable daily intake/or humans
0-0. 01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenpyroximate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, lethality, mouse and rat LD50 = 245-520 mg/kg bw
Eye, irritation, rabbit Irritating
Eye, irritation, human Irritating
Skin, sensitization, guinea-pig Sensitizing
Dermal, lethality, rat LD50 > 2000 mg/kg bw
inhalation, lethality tat LC50 = 0.21-0.36 mg/m3
Medium-term (1-26 weeks) Repeated 21-day toxicity, dermal, rat NOAEL = 300 mg/kg bw per day based
on reduced body-weight gain and
increased liver weight
Repeated four-week inhalation, toxicity, rat NOAEL = 2 mg/m3 based on increased
lung weight and mucosal change in nasal
passages
Repeated 13-week dietary, toxicity, rat NOAEL = 1.3 mg/kg bw per day based
on reduced body-weight gain and
lowered plasma protein level
Dietary, two-generation reproductive NOAEL = 2 mg/kg bw per day for
and developmental toxicity, rat maternal and developmental toxicity
Gavage, developmental toxicity, rat, rabbit NOAEL = 2.5 mg/kg bw per day for
maternal toxicity; no fetotoxicity or
teratogenicity
Long-term (> one year) Repeated dietary, two-year toxicity and NOAEL = 1 mg/kg bw per day based on
carcinogenicity, mouse; rat reduced body-weight gain and lowered
body weight; no carcinogenicity
References
Aughton, P. (1987) NNI-850: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 89/NHH021/0972
from Life Science Research Ltd, Suffolk, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Aughton, P. (1989) NNI-850: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks. Unpublished
report No. 89/NHH034/0921 from Life Science Research Ltd,
Suffolk., United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Bailey, G.P. (1989) Preliminary teratology study in the rabbit.
Unpublished report No. 89/NHH050/0393 from Life Science Research
Ltd, Suffolk United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989a) Acute oral toxicity study in mice/Test
material: NNI-850 Technical. Unpublished report No. 5066-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989b) Acute oral toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5065-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989c) Acute dermal toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5559-89 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Broadmeadow, A. (1988) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report
No. 89/NHH036/1111 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Broadmeadow, A. (1989) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report
No. 89/NHH037/0802 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Cifone, M.A. (1989) Mutagenicity test on NNI-850, technical grade in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. HLA 10753-0-447 from Hazleton Laboratories
America, Inc., Maryland, USA. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1989) NNI-850: Acute delayed neurotoxicity study in the
hen. Unpublished report No. 89/NHH054/0686 from Life Science
Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hayes, G. (1990) Primary skin irritation study, NNI-850 Flowable-R.
Unpublished report No. A/S/24651 from Toxicol Laboratories Ltd,
Herefordshire, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1988) NNI-850: Effects of dietary administration upon
reproductive performance in the rat; dosage range-finding study.
Unpublished report No. 88/NHH038/597 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989a) NNI-850: Reproductive performance study in rats
treated continuously through two successive generations.
Unpublished report No. 89/NHH043/0901 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989b) NNI-850: Teratology study in the rabbit.
Unpublished report No. 89/NHH053/0722 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988a) NNI-850: Investigation of mutagenic activity
at the HGPRT locus in a Chinese hamster V79 cell mutation system.
Unpublished report No. 89/NHH042/1060 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988b) In vitro assessment of the clastogenic
activity of NNI-850 in cultured human lymphocytes. Unpublished
report No. 90/NHH040/1369 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988c) NNI-850: Assessment of the clastogenic
action on bone marrow erythrocytes in the micronucleus test.
Unpublished report No. 89/NHH041/1059 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1989) An acute inhalation toxicity study of NNI-850 in
the rat. Unpublished report No. 88-8073 from Bio-dynamics Inc.,
New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Hoffman, G.M. (1990) An acute inhalation toxicity study of NNI-850
Flowable-R in the rat. Unpublished report No. 90-8253 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991a) An acute nose-only inhalation toxicity study of
NNI-850 in the rat. Unpublished report No. 90-8281 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991b) An acute nose-only inhalation toxicity study of
NNI-850 Flowable-R in the rat. Unpublished report No. 90-8282
from Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991c) A four week nose-only inhalation toxicity study
of NNI-850 in the rat. Unpublished report No. 90-8290 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
King, V.C. (1989) NNI-850: Teratology study in the rabbit. Unpublished
report No. 89/NHH051/0687 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Kosaka, T. (1988a) NNI-850: Primary eye irritation study in rabbits.
Unpublished report No. 87-0097 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988b) NNI-850: Primary dermal irritation study in
rabbits. Unpublished report No. 87-0098 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988c) NNI-850: Dermal sensitization study in guinea
pigs. Unpublished report No. 87-0099 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mahon, C.R. (1993) Dermal absorption of 14C-fenpyroximate following
a single dermal application to male Sprague-Dawley rats.
Unpublished report No. SC910039 from Battelle Columbus, Ohio,
USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
May, K. (1988) NNI-850 (technical grade): Assessment of mutagenic
potential in amino-acid auxotrophs of Salmonella typhimurium
and Escherichia coli (the Ames test). Unpublished report
No. 89/NHH039/1010 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Mitchell, J.M. (1990) Acute oral toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5795-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mitchell, J.M. (1991) Acute dermal toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5975-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Motoba, K., Suzuki, T. & Uchida, M. (1992) Effect of a new acaricide,
fenpyroximate, on energy metabolism and mitochondrial morphology
in adult female Tetracnychus urticae (two-spotted spider mite).
Pestic. Biochem. Physiol., 43, 37-44
Nishizawa, H., Motoba, K., Suzuki, T., Ohsima, T., Hamaguchi, H. &
Uchida, M. (1993) Metabolism of fenpyroximate in rats.
J. Pestic. Sci., 18, 59-66.
Nokata, M. (1992) Effect of fenpyroximate in human-eye and skin
irritation. Unpublished report No. H-4001 submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Nokata, M. (1994) Effect of fenpyroximate on factory workers.
Unpublished report No. H-4003 submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990a) Pharmacokinetics of a [pyrazole-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-104
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990b) Pharmacokinetics of a [benzyl-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-103
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991a) Metabolism and disposition of a [pyrazole-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-102 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991b) Metabolism and disposition of a [benzyl-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-101 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1987) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study Unpublished report No. IET
87-0034 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1988) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study. Unpublished report No. IET
87-0186 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1990) NNI-850: 18-Month oral oncogenicity study in
mice. Unpublished report No. 87-0036 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Taninaka, K. (1993) Fenpyroximate, a new acaricide. Agrochem. Jpn,
62, 15-17.
Teale, H.J (1990a) Primary eye irritation study, NNI-850 Flowable-R.
Unpublished report No. A/E/24652 from Toxicol Laboratories
Limited, Herefordshire, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Teale, H.J. (1990b) NNI-850 Technical/Delayed dermal sensitization
test in the guinea pig. Unpublished report No. A/B/22645 from
Toxicol Laboratories Ltd, Herefordshire, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Teale, H.J. (1990c) NNI-850 Flowable/Delayed dermal sensitization test
in the guinea pig. Unpublished report No. A/B/22646 from Toxicol
Laboratories Ltd, Herefordshire, United Kingdom. Submitted to WHO
by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Tsu-Han Li (1991a) [Benzyl-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-001 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Tsu-Han Li (1991b) [Pyrazole-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-002 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Watanabe, M. (1990) NNI-850: DNA repair test (rec-assay). Unpublished
report No. 88-0072 from the Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Wilkinson, G.E., Ryan, M.J & Peters, A.C. (1992) 21-Day repeated-dose
dermal toxicity study of fenpyroximate in the rat. Unpublished
report No. SC920009 from Battelle Columbus, Ohio, USA. Submitted
to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
See Also:
Toxicological Abbreviations
 Groups of five male and five female Sprague-Dawley CD rats were
treated with single oral doses of 2 or 400 mg/kg bw 14C-pyrazole- or
14C-benzyl-labelled fenpyroximate, and urinary and faecal samples
were collected at 0-24, 24-48, 48-120, and 120-168 h. The samples from
each group were pooled for each time period. Radiolabel in faeces
after 0-48 h represented 80-87% of the dose of 2 mg/kg and 80% of the
dose of 400 mg/kg; parent fenpyroximate was found at 6-8% after
2 mg/kg and 50-52% after 400 mg/kg. No parent compound was found in
urine. Eleven urinary and 19 faecal metabolites were identified by
thin-layer co-chromatography with authentic samples. The major urinary
metabolites were H, S, and R (Figure 1), produced by hydrolytic
cleavage of the oxime ether bond between the benzyl moiety and the
pyrazole ring. The major faecal metabolites were C, D, and E, produced
by hydrolysis of the tert-butyl ester, and T, produced by oxidation
of the tert-butyl group. A Z isomer of fenpyroximate, A, was found
in faeces of rats treated with the high dose. The concentrations of
urinary metabolite H and faecal metabolites H and T were increased by
enzymatic hydrolysis of the excreta with ß-glucuronidase or sulfatase
(Sharp 1991a,b).
Groups of four male Sprague-Dawley (SLC) rats were treated with a
single oral dose of 1.5 mg/kg bw 14C-pyrazole- or 14C-benzoyl-
labelled fenpyroximate (radioactive purity, > 99%), and urinary and
faecal samples were collected for 0-72 h. Six urinary and 17 faecal
metabolites were identified by thin-layer co-chromatography with
authentic samples. Three of them, W, X, and V, were isolated from
faeces and identified by mass spectrometry and nuclear magnetic
resonance. The major urinary metabolites were H (7.3% of the dose),
S (2.5%), and R (3.8%). The major faecal metabolites were C (4.1-11.0%
of the dose), E (2.9-4.2%), and T (3.5-4.3%); P (7.5%) was found as a
precursor of R and W (2.0-9.7%) and X (3.3-4.5%) as hydroxylated
bodies of T. The concentrations of the urinary metabolites J and N and
the faecal metabolites B, T, W, X, and V were increased by enzymatic
hydrolysis of the excreta with ß-glucuronidase or sulfatase (Nishizawa
et al., 1993).
Groups of six male and six female Sprague-Dawley rats with
bile-duct cannulae were given a single oral dose of 2 mg/kg
14C-pyrazole-labelled fenpyroximate. No parent fenpyroximate was
found in bile, but metabolites C, D, E, F, G, H, I, J, M, N, and T and
conjugates of C, D, E, and H were found. Total radiolabel represented
less than 2% of the dose. The metabolic pathway proposed for
fenpyroximate in rats is cleavage of the ester bond, hydroxylation at
the phenoxypyrazole group, oxidation at the tert-butyl group, and
conjugation with sulfate and glucuronide (Tsu-Han Li, 1991a,b).
3. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of technical-grade (> 98%)
fenpyroximate and of a 5% suspension concentrate are summarized in
Table 2. Moderate oral toxicity was observed in rats and mice, with
LD50 values ranging from 245 to 520 mg/kg bw. Fenpyroximate was more
toxic to rats when administered by inhalation, with LC50 values
ranging from 0.21 to 0.36 mg/litre air. Signs of acute toxicity in
mice and rats included urinary and faecal staining, hypoactivity, and
hypopnoea. Decreased food consumption was observed in surviving
animals one week after dosing. Necropsy of animals found dead revealed
irritation and/or corrosive effects in the gastointestinal tract.
There were no signs of acute toxicity or dermal irritation after
dermal application of either formulation. Inhalation of active
ingredient or the formulation resulted in signs of respiratory
irritation (laboured breathing, rale, and gasping). The findings at
necropsy were unremarkable in groups exposed by whole-body inhalation,
but those in groups exposed by nose only included oedematous,
reddened, firm lungs and frothy fluid in the trachea.
(b) Short-term toxicity
Mice
In a preliminary study to establish the dose range for a
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice were given fenpyroximate (purity, 97.9%) mixed into
the diet at concentrations of 80, 400, or 2000 ppm, equal to 0, 10.8,
48.4, and 181.6 mg/kg bw per day in males and 0, 11.7, 50.4, and
170.0 mg/kg bw per day in females, for four weeks. There were no
deaths and no abnormal behavioural signs in any treated group.
Decreased body-weight gain was observed in males at 400 ppm and in
animals of each sex at 2000 ppm; food consumption and food efficiency
were reduced in animals at 2000 ppm. Haematological examination
revealed significant decreases in haemoglobin in animals of each sex,
of the haematocrit in males, and of the erythrocyte count in females,
and a significant decrease in total plasma protein and a significant
increase in aspartate aminotransferase in animals of each sex at
2000 ppm. The NOAEL in this study was 80 ppm, equal to 10.8 mg/kg bw
per day in males and 11.7 mg/kg bw per day in females (Takahashi,
1988).
In a four-week study to find the dose range for the
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice received fenpyroximate (purity, 97.9%) mixed into the
diet at a concentration of 0, 20, 100, or 500 ppm, equal to 0, 2.58,
12.9, and 53.2 mg/kg bw per day in males and 0, 3.07, 14.5, and
66.7 mg/kg bw per day in females. Slight reductions in body-weight
Table 2. Acute toxicity of fenpyroximate
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Fenpyroximate
ICR CD-1 mouse Male Orala 520 (334-706) 98 Blaszcak (1989a)
Female 440 (281-599)
Sprague-Dawley Male Orala 480 (298-662) 98 Blaszcak (1989b)
CD rat Female 245 (167-323)
Sprague-Dawley Male Dermalb > 2000 98 Blaszcak (1989c)
CD rat Female > 2000
Sprague-Dawley Male Inhalation 0.33 (0.16-0.68) 99 Hoffman (1989)
CD rat Female (whole-body)c 0.36 (0.24-0.54)
Sprague-Dawley Male Inhalation 0.21 (0.04-1.20) 98 Hoffman (1991a)
CD rat Female (nose-only)d 0.33 (0.20--0.55)
5% suspension concentrate
Sprague-Dawley Male Oral 7193 (2989-17 308) Mitchell (1990)
CD rat Female 6789 (4704-9797)
Sprague-Dawley Male Dermal > 4000 Mitchell (1991)
CD rat Female > 4000
Sprague-Dawley Male Inhalation 1.9 (1.2-3.1) Hoffman (1990)
CD rat Female (whole-body)e 2.4 (2.0-2.9)
Table 2. (con't)
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Sprague-Dawley Male Inhalation 5.1 (0.0-17.1) Hoffman (1991b)
CD rat Female (nose-only)f 3.4 (1 9-4.9)
a In Tween 80 and a 1% solution of Methocel (A4C)
b Applied on a gauze moistened with 0.9% saline
c Fenpyroximate containing white carbon (9:1); mean particle size, 4.2 µm; exposure time, 4 h
d Fenpyroximate containing white carbon (9:1); mean particle size, 2.8 µm; exposure time, 4 h
e Mean particle size, 3.3 µm; exposure time, 4 h
f Mean particle size, 5.1 µm; exposure time, 4 h
gain and in food consumption were observed in males at 500 ppm. There
were no abnormal clinical signs, no changes in haematological
parameters, and no changes in organ weights. The NOAEL in this study
was 100 ppm, equal to 12.9 mg/kg bw per day in males and > 500 ppm in
females (Takahashi, 1987).
Rats
Groups of 10 male and 10 female Sprague-Dawley CD rats received
fenpyroximate (purity, 99.0%) in the diet at concentrations of 0, 20,
100, or 500 ppm, equal to 0, 1.3, 6.6, or 35.2 mg/kg bw per day for
males and 0, 1.7, 8.3, or 38.6 mg/kg bw per day for females, for 13
weeks. There were no overt changes in physical condition or behaviour
and no abnormal ophthalmoscopic findings. Increased encrustations
of the muzzle in males and an increased incidence of hair loss
accompanied by encrustation of the skin in females were observed at
500 ppm, Significant reductions in body-weight gain at 100 and 500 ppm
and significant reductions in food consumption at 500 ppm were
observed in animals of each sex. A significant lowering of total
plasma protein level was observed in females at 100 ppm and in animals
of each sex at 500 ppm. A significant increase in relative liver body
weights and slight hypertrophy of hepatic cells were observed in
animals of each sex and a significant increase in alkaline phosphatase
activity in females at 500 ppm; a significantly lower volume and pH
value of urine were also observed at this dose. Erythrocyte
cholinesterase activity was significantly lowered in animals at
500 ppm, but the inhibition was less than 20% of control. The NOAEL in
this study was 20 ppm, equal to 1.30 mg/kg bw per day in males and
1.65 mg/kg bw per day in females, based on the lowered body-weight
gain in animals of each sex and a lowered plasma protein level in
females at 100 ppm (Aughton, 1987).
Fenpyroximate (purity, 99%) was applied to the dorsal skin of
groups of five male and five female Sprague-Dawley rats in a uniform
layer moistened with water at 0, 100, 300, or 1000 mg/kg bw for 6 h
per day for 21 consecutive days. Minor acanthosis was observed in all
treated and one control animal. All rats survived, and there were
no compound-related clinical abnormalities. Reductions in food
consumption and body-weight gain in animals of each sex and increases
in absolute liver weight in females were observed at 1000 mg/kg bw.
There were no compound-related changes in haematological or blood
chemical parameters. The NOAEL in this study was 300 mg/kg bw per day,
based on the reduction in body-weight gain in animals of each sex and
the increase in absolute liver weight in female at 1000 mg/kg bw
(Wilkinson et al., 1992).
Groups of four male and four female Sprague-Dawley CD rats were
exposed by nose-only inhalation to fenpyroximate (purity, 98.6%; mean
particle size, 3 µm) at a target concentration of 0, 2, 10, or
50 mg/m3 for 6 h per day, five days per week for four weeks. All
animals survived. The only treatment-related physical or behavioural
change was an increased incidence of laboured breathing during the
last three weeks of exposure to 50 mg/m3. Increase in absolute and
relative lung weight were observed in animals of each sex after four
weeks' exposure to 10 or 50 mg/m3, but these effects were not seen
two weeks after exposure. A higher incidence of mucosal changes in
the nasal passages (atrophy and metaplasia) were observed at
concentrations of 10 (in males) and 50 mg/m3 (males and females). The
NOAEL in this study was 2 mg/m3, based on an increase in lung weight
and an increased incidence of mucosal changes in the nasal passages
(Hoffman, 1991c).
Dogs
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.4%) in gelatin capsules at 0, 2, 10, or
50 mg/kg bw per day for 13 weeks. Two females given 50 mg/kg bw were
found moribund and were killed after five weeks of treatment.
Increased incidences of diarrhoea and emaciation were observed in
females at 10 mg/kg bw per day and in males at 50 mg/kg bw per day.
Electrocardiograms examined 2 and 24 h after treatment showed
bradycardia in males after six weeks of treatment at 10 or 50 mg/kg bw
per day and after 12 weeks of treatment at 50 mg/kg bw per day. There
were no treatment-related ocular lesions. Significant decreases in
total body-weight gain were observed in females at 10 mg/kg bw per
day and in animals of each sex at 50 mg/kg bw per day. The only
significant haematological change was a reduction in the total number
of leukocytes in females at 50 mg/kg bw per day after 6 and 12 weeks
of treatment. Histopathological examination showed fine cytoplasmic
vacuolation of cells in the renal medullary rays in two females at
50 mg/kg bw per day sacrificed before the end of treatment and in one
female killed at termination. There were no significant changes in
cholinesterase activity in plasma or erythrocytes. The NOAEL in this
study was 2 mg/kg bw per day, based on the reduction in body-weight
gain and clinical signs at 10 mg/kg bw per day (Broadmeadow, 1988).
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.0%) in gelatin capsules at 0, 0.5, 1.5, 5,
or 15 mg/kg bw per day on seven days per week for 52 weeks. Clinical
observations were made daily, electrocardiograms were perfomed after
11, 24, and 50 weeks, haematological examinations were done after
12, 24, and 50 weeks, blood chemistry after 24 and 50 weeks, and
ophthalmoscopy after 49 weeks of treatment. There were no deaths.
Increased incidences of diarrhoea were observed during treatment in
males at 5 or 15 mg/kg bw per day and in females at 15 mg/kg bw per
day. Increased salivation was observed in females at 15 mg/kg bw per
day. Slight bradycardia was observed in animals of each sex at
15 mg/kg bw per day, which was significant in males at 11 and 50 weeks
of treatment. The overall body-weight gain throughout treatment was
significantly lower in males at 15 mg/kg bw per day, and a slightly
reduced total food consumption was observed. There were no treatment-
related haematological or ophthalmological findings. A slight but
significant lowering of total plasma protein level was observed in
males at 15 mg/kg bw per day after 50 weeks of treatment. There was no
change in erythrocyte acetylcholinesterase activity. The absolute and
relative prostatic weights were significantly increased in all treated
groups, but no histopathological changes were observed in the prostate
or in any other tissue examined. The NOAEL was 1.5 mg/kg bw per day,
based on the increased incidence of diarrhoea at 5 mg/kg bw per day
(Broadmeadow, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female ICR (Crj;CD) mice received
fenpyroximate (purity, 97.9-98.4%) mixed into the diet at
concentrations of 0, 25, 100, 400, or 800 ppm (overall mean
concentrations: 0, 24, 98, 388, and 776 ppm) for 18 months (78 weeks),
equal to 0, 2.4, 9.5, 38.0, or 69.6 mg/kg bw per day for males and 0,
2.5, 10.2, 41.5, or 73.1 mg/kg bw per day for females. There were no
treatment-related alterations in clinical signs. Body-weight gain and
food consumption were significantly lower than those of controls in
animals of each sex at 400 and 800 ppm. The only significant change in
haematological parameters was a decrease in the number of segmented
neutrophils at 500 ppm. A significant increase in the incidence of
emaciation was observed in males a t 500 ppm at the termination of
treatment. Histopathological examination revealed significant
increases in the incidence of ovarian atrophy in females at 400 and
800 ppm (25/50 in controls, 35/50 at 400 ppm, 35/50 at 800 ppm), but
these changes were morphologically similar to age-related changes seen
in female mice of this strain. There was no treatment-related increase
in the incidence of neoplasia. The NOAEL in this study was 100 ppm,
equal to 9.5 mg/kg bw per day in males and 10.2 mg/kg bw per day in
females, based on decreased body-weight gain and food consumption at
400 ppm (Takahashi, 1990).
Rats
Groups of 80 male and 80 female Sprague-Dawley CD rats
received fenpyroximate (purity, 97.1-97.2%) mixed into the diet at
concentrations of 0, 10, 25, 75, or 150 ppm for 104 weeks. The overall
doses achieved by the groups in which toxicity was assessed were 0,
0.40, 0.97, 3.08, and 6.18 mg/kg bw per day in males and 0, 0.48,
1.16, 3.79, and 7.57 mg/kg bw per day in females; those in the groups
for assessment of carcinogenicity were 0, 0.40, 0.97, 3.00, and
6.20 mg/kg bw per day in males and 0, 0.49, 1.21, 3.81, and 8.01 mg/kg
bw per day in females. Fifty rats of each sex per dose were assigned
for assessment of carcinogenicity and 30 of each sex per dose for
toxicity; 10 rats of each sex per dose were sacrificed at week 52 for
interim assessment. Blood samples were collected in weeks 24, 49, 76,
and 102 of treatment and urine samples in weeks 23, 49, 77, and 101.
Mortality after 104 weeks was 70, 72, 54, 60, and 52% among males and
58, 60, 50, 30, and 46% among females at 0,10, 25, 75, and 150 ppm,
respectively. There were no treatment-related adverse effects on
behaviour, appearance, or ophthalmoscopic parameters during treatment
or at termination. Absolute body weight was reduced after 104 weeks of
treatment, by 1-15% in males and 6-12% in females at 75 mg/kg bw per
day and by 11-28% in males and 17-22% in females at 150 mg/kg bw per
day. These changes were not statistically significant; however,
body-weight gains were significantly decreased in males at 75 ppm
and in animals of each sex at 150 ppm. Blood chemical analysis,
haematological examination, and urinalysis showed no treatment-related
findings. Macroscopic examination of animals used for toxicity
assessment revealed a significantly increased incidence of dark
popliteal lymph nodes in females at 150 ppm. Macroscopic examination
of animals for carcinogenicity revealed significantly increased
incidences of pituitary masses, masses that compressed the ventral
surface of the brain, and masses in the abdominal fat in males at
150 ppm, and increased incidences of uterine masses and distension of
the uterus in females at this dose. Histopathological examination
revealed higher incidences of non-neoplastic changes, which included
syncytial macrophages in the mesenteric lymph nodes of females in
the toxicity assessment group, and lesions associated with gastric
ulceration, compression of the brain due to pituitary neoplasia,
and pancreatic lobular degeneration in males and interstitial
proliferation of the ovary in females in the carcinogenicity
assessment groups. High incidences of pituitary adenoma were observed
in all groups, but the rates (males: 34/70, 36/57, 32/50, 37/54, and
36/69; females, 39/69, 50/60, 52/54, 41/47, and 43/66) were within the
historical control range (males, 34-70%; females, 67-86%). There was
no evidence of carcinogenicity. The NOAEL was 25 ppm, equal to
0.97 mg/kg bw per day in males, based on the lowered body-weight gain
at 75 ppm (Aughton, 1989).
(d) Reproductive toxicity
Rats
A preliminary range-finding study was conducted in which groups
of six male and six female Sprague-Dawley CD rats received
fenpyroximate (purity, 97.3%) mixed into the diet at concentrations of
10, 50, or 200 ppm for seven weeks, from 15 days before mating to day
4 post partum, for two generations. There were no treatment-related
changes in behaviour or appearance in F0 or F1 animals, and there
were no deaths. Significant reductions in body-weight gains were
observed in parental animals of each sex at 200 ppm, and food
consumption was reduced at this dose due to unpalatability (Higgins,
1988).
A two-generation (one litter per generation) study was conducted
with groups of 24 male and 24 female Sprague-Dawley CD rats that
received fenpyroximate (purity, 97.3%) mixed into the diet at
concentrations of 0, 10, 30, or 100 ppm, from 14 weeks before mating
up to weaning of the F1 generation (14 weeks before mating, three
weeks of mating, three weeks' gestation, 25 days' lactation) and then
from weaning of the F1 generation to weaning of the F2 generation.
The mean daily intakes of fenpyroximate were 0.67, 1.99, or 6.59 mg/kg
bw per day for F0 males 0.83, 2.44, or 8.60 mg/kg bw per day for F0
females, 0.78, 2.33, or 8.45 mg/kg bw per day for F1 males, and 0.96,
2.82, or 9.92 mg/kg bw per day for F1 females during the premating
period. There were no treatment-related abnormalities in behaviour or
appearance in F0, F1, or F2 animals throughout the study.
Significant reductions in body-weight gain were observed at 100 ppm in
males of the F0 and F1 generations throughout the study and in F0
females up to parturition. Food consumption was significantly reduced
in F0 males at 100 ppm during the period before mating. There were no
treatment-related changes in the oestrus cycle, mating performance
(percent mating, conception rate, or fertility index), length of
gestation, gestation index, or parturition index in F0 or F1
animals. Significantly reduced body-weight gains were seen during
lactation in F1 and F2 offspring at 100 ppm. There were no
treatment-related Changes in litter size, viability, sex ratio,
initial body weight at birth, or developmental indices (pinna
unfolding, hair growth, tooth eruption, or eye opening) in the F1 and
F2 generations. The absolute and relative weights of the testes and
epidymides were significantly increased at termination of the study in
F1 males fed 100 ppm, but the weights were only 10% greater than
those of controls, and macroscopic and histopathological examinations
revealed no treatment-related changes in the reproductive organs. The
NOAEL was 30 ppm, equal to 1.99 mg/kg bw per day in F0 males,
2.44 mg/kg bw per day in F0 females, 2.33 mg/kg bw per day in F1
males, and 2.82 mg/kg bw per day in F1 females, based on reductions
in body-weight gain in animals of each sex at 100 ppm (Higgins,
1989a).
(e) Developmental toxicity
Rats
Groups of 22 pregnant Sprague-Dawley CD rats received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxy-
methylcellulose and 0.1% Tween 80 by gavage at 0, 1, 5, or 25 mg/kg bw
per day on days 6-15 of gestation. Three females in the group at
5 mg/kg bw per day died due to a technical error; all surviving
animals were sacrificed on day 20 of gestation. A slight loss of body
weight after the first dose, a significant increase in water
consumption, and a slight reduction in food consumption were seen
during the first six days of treatment in rats at 25 mg/kg bw per day.
No other maternal toxicity was observed. There were no treatment-
related effects on litter parameters. Slight increases in the
incidences of skeletal variations were seen in fetuses in all treated
groups. The incidences of unilateral and bilateral additional 14th
thoracic rib(s) were 2.4 and 1.4% in controls, 5.2 and 4.8% at 1 mg/kg
bw per day, 5.0 and 1.7% at 5 mg/kg bw per day, 8.2 and 7.7% at
25 mg/kg bw per day, and 0-4.2 and 0-3.5%, in historical controls,
respectively. The increases were not dose-dependent, but the percent
incidence in fetuses and the number of litters with the skeletal
variation at the highest dose, 25 mg/kg bw per day, exceeded the
historical control range. There was no evidence of teratogenicity at
doses up to and including 25 mg/kg bw per day. The NOAEL in this study
was 5 mg/kg bw per day, based on slightly decreased body weight in
maternal rats and the increased incidence of skeletal variations in
fetuses at 25 mg/kg bw per day (Higgins, 1989b).
Rabbits
In a preliminary study, groups of four pregnant New Zealand white
rabbits received fenpyroximate (purity, 98.4%) suspended in 1% aqueous
carboxymethylcellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0,
2.5, or 5.0 mg/kg bw per day on days 6-19 of gestation. Maternal
toxicity was seen as marked reductions in body-weight gain and reduced
defaecation, food consumption, and water consumption at 5.0 mg/kg bw
per day. Increased post-implantation loss was also recorded at this
dose, as were reduced fetal weight and multiple anomalies (increased
incidences of small fetuses, oedema of head and/or ventral part of
neck, and thorax, fore- and hind-limb flexure). The NOAEL for
fetotoxicity was 2.5 mg/kg bw per day, based on reduced fetal weight
and art increased incidence of anomalies at 5.0 mg/kg bw per day
(Bailey, 1989).
Groups of 15 pregnant New Zealand white rabbits received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxymethyl-
cellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0, 2.5, or
5.0 mg/kg bw per day on days 6-19 of gestation. Two females receiving
2.5 mg/kg bw per day were killed when moribund; necropsy revealed a
respiratory tract infection in one (14 days after treatment) and
evidence of gastrointestinal tract disturbance in the other (26 days
after treatment). All surviving animals were sacrificed on day 29 of
gestation. A transient decrease in body weight and in food consumption
and an increased incidence of reduced defaecation were observed at
5.0 mg/kg bw per day; a significant decrease in body weight was
observed at 2.5 mg/kg bw per day. Slight increases in the rate of
abortion and of total litter loss were observed in rabbits at 2.5 and
5.0 mg/kg bw per day: 0/15 in controls, 0/15 at 1.0 mg/kg bw per day,
7.7% (1/13) at 2.5 mg/kg bw per day, 13.3% (2/15) at 5.0 mg/kg bw per
day, and 0-14.3% in historical controls. The changes in body weight
and abortion rate at 2.5 mg/kg bw per day were slight but dose-
dependent. There was no evidence of fetotoxicity or teratogenicity at
doses up to 5.0 mg/kg bw per day. The NOAEL for maternotoxicity was
2.5 mg/kg bw per day (King, 1989).
(f) Genotoxicity
The results of studies on technical-grade fenpyroximate (purity,
97.3%) are summarized in table 3. The studies include those for
point mutations, mutation of mammalian cells in vitro, chromosomal
aberrations, micronucleus formation in vivo, DNA repair ( rec
assay), and unscheduled DNA synthesis. All of the positive controls
used gave the expected responses. There was no evidence of
genotoxicity.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The primary ocular irritation potential of fenpyroximate (purity,
98.4%) as a fine powder was evaluated in male New Zealand white
rabbits by grading and scoring according to the method of Draize.
Changes due to irritation were observed in the conjunctivae of eyes
treated with 0.1 g of fenpyroximate for 1 h to two days after
treatment. Rinsing slightly reduced the severity of the changes.
Fenpyroximate is classified as a mild primary irritant (Kosaka,
1988a). A fenpyroximate formulation (5% suspension concentrate) was
moderately irritating to the eyes of female New Zealand white rabbits
receiving 0.1 ml (Teale, 1990a).
Fenpyroximate (purity, 98.4%) applied as a fine powder moistened
with distilled water was not irritating to the skin of male New
Zealand white rabbits, graded and scored according to the method of
Draize (Kosaka, 1988b). Little or no dermal irritation was seen after
application of 0.5 ml of the 5% suspension concentrate to female New
Zealand white rabbits (Hayes, 1990).
The dermal sensitizing potential of fenpyroximate (purity, 98.4%)
was studied in female Hartley guinea-pigs by the maximization test.
Fenpyroximate was given at 0.1 ml per animal at 5% by intradermal
injection and 25% by local application for induction and then given
at 25% by local application for challenge. The positive control was
2,4-dinitrochlorobenzene (at 0.1, 1, and 0.5%, respectively). The
positive control group was sensitized by 100% and the fenpyroximate-
treated group by 36%. Fenpyroximate thus had moderate dermal
sensitizing potential (Kosaka, 1988c).
The delayed dermal sensitizing potential of fenpyroximate
(purity, 98.4%) was studied in female Dunkin-Hartley guinea-pigs by
the method of Buehler. A dose of 0.5 ml of 50% aqueous fenpyroximate
was applied to the skin three times weekly for induction; the animals
were then challenged by applications of 50 or 25% w/w aqueous
Table 3. Results of tests for the genotoxicity of fenpyroximate
End-point Test system Concentration Purity Results Reference
or dose (solvent) (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-5000 µg/plate (DMSO) 97.3 Negative May (1988)
TA100, TA1535, TA1537,
TA1538 (± S9), E. coli WP2
uvrA (± S9)
Mutation Chinese hamster 0-330 µg/ml (acetone) 97.3 Negative Hodson-Walker
V79 lung cells (1988a)
hprt locus (± S9)
Chromosomal Human lymphocytes 0-20 µg/ml (acetone) 97.3 Negative Hodson-Walker
aberration (± S9) (1988b)
DNA repair B subtilis H17, M45 0-500 µg/disc (DMSO) 97.3 Negative Watanabe (1990)
(± S9)
Unscheduled DNA Fischer 344 rat primary 0-1.02 µg/ml (DMSO) 97.3 Negative Cifone (1989)
synthesis hepatocytes
In vivo
Micronucleus CD-1 mice 0-2000 mg/kg bw orally 97.3 Negative Hodson-Walker
formation (1988c)
DMSO, dimethyl sulfoxide; S9, 9000 × g supernatant
fenpyroximate two weeks after the third induction. The positive
control was 2,4-dinitrochlorobenzene (0.3% for induction, 0.05 and
0.025% for challenge). The positive control group showed the expected
response, but fenpyroximate had no effect (Teale, 1990b).
A 5% suspension of fenpyroximate had no delayed dermal
sensitizing potential in female Dunkin-Hartley guinea-pigs tested by
the method of Buehler (Teale, 1990c).
(ii) Acute delayed neurotoxicity
Twelve adult Sterling Ranger hybrid hens were administered
fenpyroximate (purity, 97.0%) suspended in 0.5% aqueous
methylcellulose by catheter at a dose of 5000 mg/kg bw twice, on days
1 and 22 after insemination. A single oral dose of tri- ortho-cresyl
phosphate was used as the positive control. All surviving birds were
sacrificed on days 43-45. No deaths, abnormal neurological signs, or
treatment-related lesions in the nervous system were observed in
treated hens, whereas the positive control caused high mortality,
locomotor impairment, and degenerative changes in the spinal cord and
upper cervical bulbs, resulting in delayed neurotoxic responses
(Cummins, 1989).
3. Observations in humans
The results of medical surveillance of workers manufacturing a 5%
formulation of fenpyroximate were reported. Ocular and dermal
irritation were seen in July and November 1990 and March 1991. The
concentrations of fenpyroximate in the air at eight to nine sites in
the working place were 0.117 mg/m3 in January 1991, 0.012 mg/m3 in
April 1991, 0.005 mg/m3 in November 1991, and 0.004 mg/m3 in January
1993; however, the actual exposure of the workers to fenpyroximate was
not determined (Nokata, 1992, 1994).
Comments
Fenpyroximate was relatively well absorbed by rats after oral
administration. Absorbed fenpyroximate was excreted predominantly via
the biliary route, with lesser amounts in urine. The residual levels
in organs and tissues after 168 h were low. There was no evidence of
bioaccumulation.
Fenpyroximate was extensively metabolized in rats; 23 metabolites
were identified. No parent compound was found in the urine;
metabolites found in the excreta represented 0-11% of the administered
dose. Multiple pathways have been proposed for the metabolism of
fenpyroximate, including oxidation, hydroxylation, demethylation,
hydrolysis, and isomerization.
Fenpyroximate has slight to moderate acute oral toxicity (oral
LD50 = 440-520 mg/kg bw in mice and 245-480 mg/kg bw in rats); it is
acutely toxic by inhalation (LC50 in rats = 0.21-0.36 mg/m3). WHO
has not classified fenpyroximate for acute toxicity.
In a 13-week study in rats in which fenpyroximate was
administered in the diet at concentrations of 0, 20, 100, or 500 ppm,
the NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, and was based on
decreased body-weight gain and lowered plasma protein levels in
females fed 100 ppm. In a 13-week study in dogs, fenpyroximate was
administered orally in capsules at doses of 0, 2, 10, or 50 mg/kg bw
per day. The NOAEL was 2 mg/kg bw per day, on the basis of decreased
body-weight gain and clinical signs, such as an increased incidence of
diarrhoea, emaciation, and slight bradycardia, at 10 mg/kg bw per day.
In a 52-week study of similar design, with doses of 0, 0.5, 1.5, 5, or
15 mg/kg bw per day, the NOAEL was 5 mg/kg bw per day, on the basis of
decreased body-weight gain and total protein levels at 15 mg/kg bw per
day.
In an 18-month study of carcinogenicity in mice given dietary
concentrations of 0, 25, 100, 400, or 800 ppm, the NOAEL was 100 ppm,
equal to 9.5 mg/kg bw per day, and was based on decreased body-weight
gain, absolute body weight, and food consumption in animals of each
sex at 400 ppm. There was no evidence of carcinogenicity. In a
104-week study in rats given dietary concentrations of 0, 10, 25, 75,
or 150 ppm, the NOAEL was 25 ppm, equal to 1 mg/kg bw per day, based
on decreased body weight and body-weight gain in males at 75 ppm.
There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats given dietary concentrations of 0, 10,
30, or 100 ppm, the NOAEL for maternal and developmental toxicity was
30 ppm, equal to 2 mg/kg bw per day, on the basis of decreased body-
weight gain in animals of the F0 and F1 generations at 100 ppm.
In a study of developmental toxicity in rats given doses of 0, 1,
5, or 25 mg/kg bw per day by gavage, the NOAEL for maternal toxicity
and fetotoxicity was 25 mg/kg bw per day, the highest dose tested.
There was no evidence of embryotoxicity or teratogenicity. In a study
of developmental toxicity in rabbits given doses of 0, 1, 2.5, or
5 mg/kg bw per day by gavage, there was no evidence of embryo- or
fetotoxicity or teratogenicity at any dose. The NOAEL for maternal
toxicity was 2.5 mg/kg bw per day on the basis of minor effects at
5 mg/kg bw per day.
Fenpyroximate has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that it
is not genotoxic.
Two high doses, given 21 days apart, to hens did not cause gait
abnormalities or morphological changes in the nervous system.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1 mg/kg bw per day for reductions in body-weight gain and
plasma protein concentration in the 104-week study in rats and a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 9.5 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 25 ppm, equal to 1 mg/kg bw per day (104-week study of
carcinogenicity)
30 ppm, equal to 2 mg/kg bw per day (study of reproductive
toxicity)
Rabbit: 2.5 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Dog: 5 mg/kg bw per day (52-week study of toxicity)
Estimate of acceptable daily intake/or humans
0-0. 01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenpyroximate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, lethality, mouse and rat LD50 = 245-520 mg/kg bw
Eye, irritation, rabbit Irritating
Eye, irritation, human Irritating
Skin, sensitization, guinea-pig Sensitizing
Dermal, lethality, rat LD50 > 2000 mg/kg bw
inhalation, lethality tat LC50 = 0.21-0.36 mg/m3
Medium-term (1-26 weeks) Repeated 21-day toxicity, dermal, rat NOAEL = 300 mg/kg bw per day based
on reduced body-weight gain and
increased liver weight
Repeated four-week inhalation, toxicity, rat NOAEL = 2 mg/m3 based on increased
lung weight and mucosal change in nasal
passages
Repeated 13-week dietary, toxicity, rat NOAEL = 1.3 mg/kg bw per day based
on reduced body-weight gain and
lowered plasma protein level
Dietary, two-generation reproductive NOAEL = 2 mg/kg bw per day for
and developmental toxicity, rat maternal and developmental toxicity
Gavage, developmental toxicity, rat, rabbit NOAEL = 2.5 mg/kg bw per day for
maternal toxicity; no fetotoxicity or
teratogenicity
Long-term (> one year) Repeated dietary, two-year toxicity and NOAEL = 1 mg/kg bw per day based on
carcinogenicity, mouse; rat reduced body-weight gain and lowered
body weight; no carcinogenicity
References
Aughton, P. (1987) NNI-850: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 89/NHH021/0972
from Life Science Research Ltd, Suffolk, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Aughton, P. (1989) NNI-850: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks. Unpublished
report No. 89/NHH034/0921 from Life Science Research Ltd,
Suffolk., United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Bailey, G.P. (1989) Preliminary teratology study in the rabbit.
Unpublished report No. 89/NHH050/0393 from Life Science Research
Ltd, Suffolk United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989a) Acute oral toxicity study in mice/Test
material: NNI-850 Technical. Unpublished report No. 5066-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989b) Acute oral toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5065-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989c) Acute dermal toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5559-89 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Broadmeadow, A. (1988) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report
No. 89/NHH036/1111 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Broadmeadow, A. (1989) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report
No. 89/NHH037/0802 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Cifone, M.A. (1989) Mutagenicity test on NNI-850, technical grade in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. HLA 10753-0-447 from Hazleton Laboratories
America, Inc., Maryland, USA. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1989) NNI-850: Acute delayed neurotoxicity study in the
hen. Unpublished report No. 89/NHH054/0686 from Life Science
Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hayes, G. (1990) Primary skin irritation study, NNI-850 Flowable-R.
Unpublished report No. A/S/24651 from Toxicol Laboratories Ltd,
Herefordshire, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1988) NNI-850: Effects of dietary administration upon
reproductive performance in the rat; dosage range-finding study.
Unpublished report No. 88/NHH038/597 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989a) NNI-850: Reproductive performance study in rats
treated continuously through two successive generations.
Unpublished report No. 89/NHH043/0901 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989b) NNI-850: Teratology study in the rabbit.
Unpublished report No. 89/NHH053/0722 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988a) NNI-850: Investigation of mutagenic activity
at the HGPRT locus in a Chinese hamster V79 cell mutation system.
Unpublished report No. 89/NHH042/1060 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988b) In vitro assessment of the clastogenic
activity of NNI-850 in cultured human lymphocytes. Unpublished
report No. 90/NHH040/1369 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988c) NNI-850: Assessment of the clastogenic
action on bone marrow erythrocytes in the micronucleus test.
Unpublished report No. 89/NHH041/1059 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1989) An acute inhalation toxicity study of NNI-850 in
the rat. Unpublished report No. 88-8073 from Bio-dynamics Inc.,
New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Hoffman, G.M. (1990) An acute inhalation toxicity study of NNI-850
Flowable-R in the rat. Unpublished report No. 90-8253 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991a) An acute nose-only inhalation toxicity study of
NNI-850 in the rat. Unpublished report No. 90-8281 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991b) An acute nose-only inhalation toxicity study of
NNI-850 Flowable-R in the rat. Unpublished report No. 90-8282
from Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991c) A four week nose-only inhalation toxicity study
of NNI-850 in the rat. Unpublished report No. 90-8290 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
King, V.C. (1989) NNI-850: Teratology study in the rabbit. Unpublished
report No. 89/NHH051/0687 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Kosaka, T. (1988a) NNI-850: Primary eye irritation study in rabbits.
Unpublished report No. 87-0097 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988b) NNI-850: Primary dermal irritation study in
rabbits. Unpublished report No. 87-0098 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988c) NNI-850: Dermal sensitization study in guinea
pigs. Unpublished report No. 87-0099 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mahon, C.R. (1993) Dermal absorption of 14C-fenpyroximate following
a single dermal application to male Sprague-Dawley rats.
Unpublished report No. SC910039 from Battelle Columbus, Ohio,
USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
May, K. (1988) NNI-850 (technical grade): Assessment of mutagenic
potential in amino-acid auxotrophs of Salmonella typhimurium
and Escherichia coli (the Ames test). Unpublished report
No. 89/NHH039/1010 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Mitchell, J.M. (1990) Acute oral toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5795-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mitchell, J.M. (1991) Acute dermal toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5975-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Motoba, K., Suzuki, T. & Uchida, M. (1992) Effect of a new acaricide,
fenpyroximate, on energy metabolism and mitochondrial morphology
in adult female Tetracnychus urticae (two-spotted spider mite).
Pestic. Biochem. Physiol., 43, 37-44
Nishizawa, H., Motoba, K., Suzuki, T., Ohsima, T., Hamaguchi, H. &
Uchida, M. (1993) Metabolism of fenpyroximate in rats.
J. Pestic. Sci., 18, 59-66.
Nokata, M. (1992) Effect of fenpyroximate in human-eye and skin
irritation. Unpublished report No. H-4001 submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Nokata, M. (1994) Effect of fenpyroximate on factory workers.
Unpublished report No. H-4003 submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990a) Pharmacokinetics of a [pyrazole-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-104
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990b) Pharmacokinetics of a [benzyl-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-103
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991a) Metabolism and disposition of a [pyrazole-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-102 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991b) Metabolism and disposition of a [benzyl-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-101 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1987) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study Unpublished report No. IET
87-0034 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1988) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study. Unpublished report No. IET
87-0186 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1990) NNI-850: 18-Month oral oncogenicity study in
mice. Unpublished report No. 87-0036 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Taninaka, K. (1993) Fenpyroximate, a new acaricide. Agrochem. Jpn,
62, 15-17.
Teale, H.J (1990a) Primary eye irritation study, NNI-850 Flowable-R.
Unpublished report No. A/E/24652 from Toxicol Laboratories
Limited, Herefordshire, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Teale, H.J. (1990b) NNI-850 Technical/Delayed dermal sensitization
test in the guinea pig. Unpublished report No. A/B/22645 from
Toxicol Laboratories Ltd, Herefordshire, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Teale, H.J. (1990c) NNI-850 Flowable/Delayed dermal sensitization test
in the guinea pig. Unpublished report No. A/B/22646 from Toxicol
Laboratories Ltd, Herefordshire, United Kingdom. Submitted to WHO
by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Tsu-Han Li (1991a) [Benzyl-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-001 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Tsu-Han Li (1991b) [Pyrazole-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-002 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Watanabe, M. (1990) NNI-850: DNA repair test (rec-assay). Unpublished
report No. 88-0072 from the Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Wilkinson, G.E., Ryan, M.J & Peters, A.C. (1992) 21-Day repeated-dose
dermal toxicity study of fenpyroximate in the rat. Unpublished
report No. SC920009 from Battelle Columbus, Ohio, USA. Submitted
to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Groups of five male and five female Sprague-Dawley CD rats were
treated with single oral doses of 2 or 400 mg/kg bw 14C-pyrazole- or
14C-benzyl-labelled fenpyroximate, and urinary and faecal samples
were collected at 0-24, 24-48, 48-120, and 120-168 h. The samples from
each group were pooled for each time period. Radiolabel in faeces
after 0-48 h represented 80-87% of the dose of 2 mg/kg and 80% of the
dose of 400 mg/kg; parent fenpyroximate was found at 6-8% after
2 mg/kg and 50-52% after 400 mg/kg. No parent compound was found in
urine. Eleven urinary and 19 faecal metabolites were identified by
thin-layer co-chromatography with authentic samples. The major urinary
metabolites were H, S, and R (Figure 1), produced by hydrolytic
cleavage of the oxime ether bond between the benzyl moiety and the
pyrazole ring. The major faecal metabolites were C, D, and E, produced
by hydrolysis of the tert-butyl ester, and T, produced by oxidation
of the tert-butyl group. A Z isomer of fenpyroximate, A, was found
in faeces of rats treated with the high dose. The concentrations of
urinary metabolite H and faecal metabolites H and T were increased by
enzymatic hydrolysis of the excreta with ß-glucuronidase or sulfatase
(Sharp 1991a,b).
Groups of four male Sprague-Dawley (SLC) rats were treated with a
single oral dose of 1.5 mg/kg bw 14C-pyrazole- or 14C-benzoyl-
labelled fenpyroximate (radioactive purity, > 99%), and urinary and
faecal samples were collected for 0-72 h. Six urinary and 17 faecal
metabolites were identified by thin-layer co-chromatography with
authentic samples. Three of them, W, X, and V, were isolated from
faeces and identified by mass spectrometry and nuclear magnetic
resonance. The major urinary metabolites were H (7.3% of the dose),
S (2.5%), and R (3.8%). The major faecal metabolites were C (4.1-11.0%
of the dose), E (2.9-4.2%), and T (3.5-4.3%); P (7.5%) was found as a
precursor of R and W (2.0-9.7%) and X (3.3-4.5%) as hydroxylated
bodies of T. The concentrations of the urinary metabolites J and N and
the faecal metabolites B, T, W, X, and V were increased by enzymatic
hydrolysis of the excreta with ß-glucuronidase or sulfatase (Nishizawa
et al., 1993).
Groups of six male and six female Sprague-Dawley rats with
bile-duct cannulae were given a single oral dose of 2 mg/kg
14C-pyrazole-labelled fenpyroximate. No parent fenpyroximate was
found in bile, but metabolites C, D, E, F, G, H, I, J, M, N, and T and
conjugates of C, D, E, and H were found. Total radiolabel represented
less than 2% of the dose. The metabolic pathway proposed for
fenpyroximate in rats is cleavage of the ester bond, hydroxylation at
the phenoxypyrazole group, oxidation at the tert-butyl group, and
conjugation with sulfate and glucuronide (Tsu-Han Li, 1991a,b).
3. Toxicological studies
(a) Acute toxicity
Studies of the acute toxicity of technical-grade (> 98%)
fenpyroximate and of a 5% suspension concentrate are summarized in
Table 2. Moderate oral toxicity was observed in rats and mice, with
LD50 values ranging from 245 to 520 mg/kg bw. Fenpyroximate was more
toxic to rats when administered by inhalation, with LC50 values
ranging from 0.21 to 0.36 mg/litre air. Signs of acute toxicity in
mice and rats included urinary and faecal staining, hypoactivity, and
hypopnoea. Decreased food consumption was observed in surviving
animals one week after dosing. Necropsy of animals found dead revealed
irritation and/or corrosive effects in the gastointestinal tract.
There were no signs of acute toxicity or dermal irritation after
dermal application of either formulation. Inhalation of active
ingredient or the formulation resulted in signs of respiratory
irritation (laboured breathing, rale, and gasping). The findings at
necropsy were unremarkable in groups exposed by whole-body inhalation,
but those in groups exposed by nose only included oedematous,
reddened, firm lungs and frothy fluid in the trachea.
(b) Short-term toxicity
Mice
In a preliminary study to establish the dose range for a
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice were given fenpyroximate (purity, 97.9%) mixed into
the diet at concentrations of 80, 400, or 2000 ppm, equal to 0, 10.8,
48.4, and 181.6 mg/kg bw per day in males and 0, 11.7, 50.4, and
170.0 mg/kg bw per day in females, for four weeks. There were no
deaths and no abnormal behavioural signs in any treated group.
Decreased body-weight gain was observed in males at 400 ppm and in
animals of each sex at 2000 ppm; food consumption and food efficiency
were reduced in animals at 2000 ppm. Haematological examination
revealed significant decreases in haemoglobin in animals of each sex,
of the haematocrit in males, and of the erythrocyte count in females,
and a significant decrease in total plasma protein and a significant
increase in aspartate aminotransferase in animals of each sex at
2000 ppm. The NOAEL in this study was 80 ppm, equal to 10.8 mg/kg bw
per day in males and 11.7 mg/kg bw per day in females (Takahashi,
1988).
In a four-week study to find the dose range for the
carcinogenicity study, groups of nine male and nine female ICR
(Crj:CD-1) mice received fenpyroximate (purity, 97.9%) mixed into the
diet at a concentration of 0, 20, 100, or 500 ppm, equal to 0, 2.58,
12.9, and 53.2 mg/kg bw per day in males and 0, 3.07, 14.5, and
66.7 mg/kg bw per day in females. Slight reductions in body-weight
Table 2. Acute toxicity of fenpyroximate
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Fenpyroximate
ICR CD-1 mouse Male Orala 520 (334-706) 98 Blaszcak (1989a)
Female 440 (281-599)
Sprague-Dawley Male Orala 480 (298-662) 98 Blaszcak (1989b)
CD rat Female 245 (167-323)
Sprague-Dawley Male Dermalb > 2000 98 Blaszcak (1989c)
CD rat Female > 2000
Sprague-Dawley Male Inhalation 0.33 (0.16-0.68) 99 Hoffman (1989)
CD rat Female (whole-body)c 0.36 (0.24-0.54)
Sprague-Dawley Male Inhalation 0.21 (0.04-1.20) 98 Hoffman (1991a)
CD rat Female (nose-only)d 0.33 (0.20--0.55)
5% suspension concentrate
Sprague-Dawley Male Oral 7193 (2989-17 308) Mitchell (1990)
CD rat Female 6789 (4704-9797)
Sprague-Dawley Male Dermal > 4000 Mitchell (1991)
CD rat Female > 4000
Sprague-Dawley Male Inhalation 1.9 (1.2-3.1) Hoffman (1990)
CD rat Female (whole-body)e 2.4 (2.0-2.9)
Table 2. (con't)
Species Sex Route LD50 or LC50 Purity Reference
(mg/kg bw or (%)
mg/litre air)
(confidence interval)
Sprague-Dawley Male Inhalation 5.1 (0.0-17.1) Hoffman (1991b)
CD rat Female (nose-only)f 3.4 (1 9-4.9)
a In Tween 80 and a 1% solution of Methocel (A4C)
b Applied on a gauze moistened with 0.9% saline
c Fenpyroximate containing white carbon (9:1); mean particle size, 4.2 µm; exposure time, 4 h
d Fenpyroximate containing white carbon (9:1); mean particle size, 2.8 µm; exposure time, 4 h
e Mean particle size, 3.3 µm; exposure time, 4 h
f Mean particle size, 5.1 µm; exposure time, 4 h
gain and in food consumption were observed in males at 500 ppm. There
were no abnormal clinical signs, no changes in haematological
parameters, and no changes in organ weights. The NOAEL in this study
was 100 ppm, equal to 12.9 mg/kg bw per day in males and > 500 ppm in
females (Takahashi, 1987).
Rats
Groups of 10 male and 10 female Sprague-Dawley CD rats received
fenpyroximate (purity, 99.0%) in the diet at concentrations of 0, 20,
100, or 500 ppm, equal to 0, 1.3, 6.6, or 35.2 mg/kg bw per day for
males and 0, 1.7, 8.3, or 38.6 mg/kg bw per day for females, for 13
weeks. There were no overt changes in physical condition or behaviour
and no abnormal ophthalmoscopic findings. Increased encrustations
of the muzzle in males and an increased incidence of hair loss
accompanied by encrustation of the skin in females were observed at
500 ppm, Significant reductions in body-weight gain at 100 and 500 ppm
and significant reductions in food consumption at 500 ppm were
observed in animals of each sex. A significant lowering of total
plasma protein level was observed in females at 100 ppm and in animals
of each sex at 500 ppm. A significant increase in relative liver body
weights and slight hypertrophy of hepatic cells were observed in
animals of each sex and a significant increase in alkaline phosphatase
activity in females at 500 ppm; a significantly lower volume and pH
value of urine were also observed at this dose. Erythrocyte
cholinesterase activity was significantly lowered in animals at
500 ppm, but the inhibition was less than 20% of control. The NOAEL in
this study was 20 ppm, equal to 1.30 mg/kg bw per day in males and
1.65 mg/kg bw per day in females, based on the lowered body-weight
gain in animals of each sex and a lowered plasma protein level in
females at 100 ppm (Aughton, 1987).
Fenpyroximate (purity, 99%) was applied to the dorsal skin of
groups of five male and five female Sprague-Dawley rats in a uniform
layer moistened with water at 0, 100, 300, or 1000 mg/kg bw for 6 h
per day for 21 consecutive days. Minor acanthosis was observed in all
treated and one control animal. All rats survived, and there were
no compound-related clinical abnormalities. Reductions in food
consumption and body-weight gain in animals of each sex and increases
in absolute liver weight in females were observed at 1000 mg/kg bw.
There were no compound-related changes in haematological or blood
chemical parameters. The NOAEL in this study was 300 mg/kg bw per day,
based on the reduction in body-weight gain in animals of each sex and
the increase in absolute liver weight in female at 1000 mg/kg bw
(Wilkinson et al., 1992).
Groups of four male and four female Sprague-Dawley CD rats were
exposed by nose-only inhalation to fenpyroximate (purity, 98.6%; mean
particle size, 3 µm) at a target concentration of 0, 2, 10, or
50 mg/m3 for 6 h per day, five days per week for four weeks. All
animals survived. The only treatment-related physical or behavioural
change was an increased incidence of laboured breathing during the
last three weeks of exposure to 50 mg/m3. Increase in absolute and
relative lung weight were observed in animals of each sex after four
weeks' exposure to 10 or 50 mg/m3, but these effects were not seen
two weeks after exposure. A higher incidence of mucosal changes in
the nasal passages (atrophy and metaplasia) were observed at
concentrations of 10 (in males) and 50 mg/m3 (males and females). The
NOAEL in this study was 2 mg/m3, based on an increase in lung weight
and an increased incidence of mucosal changes in the nasal passages
(Hoffman, 1991c).
Dogs
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.4%) in gelatin capsules at 0, 2, 10, or
50 mg/kg bw per day for 13 weeks. Two females given 50 mg/kg bw were
found moribund and were killed after five weeks of treatment.
Increased incidences of diarrhoea and emaciation were observed in
females at 10 mg/kg bw per day and in males at 50 mg/kg bw per day.
Electrocardiograms examined 2 and 24 h after treatment showed
bradycardia in males after six weeks of treatment at 10 or 50 mg/kg bw
per day and after 12 weeks of treatment at 50 mg/kg bw per day. There
were no treatment-related ocular lesions. Significant decreases in
total body-weight gain were observed in females at 10 mg/kg bw per
day and in animals of each sex at 50 mg/kg bw per day. The only
significant haematological change was a reduction in the total number
of leukocytes in females at 50 mg/kg bw per day after 6 and 12 weeks
of treatment. Histopathological examination showed fine cytoplasmic
vacuolation of cells in the renal medullary rays in two females at
50 mg/kg bw per day sacrificed before the end of treatment and in one
female killed at termination. There were no significant changes in
cholinesterase activity in plasma or erythrocytes. The NOAEL in this
study was 2 mg/kg bw per day, based on the reduction in body-weight
gain and clinical signs at 10 mg/kg bw per day (Broadmeadow, 1988).
Groups of four male and four female beagle dogs received
fenpyroximate (purity, 98.0%) in gelatin capsules at 0, 0.5, 1.5, 5,
or 15 mg/kg bw per day on seven days per week for 52 weeks. Clinical
observations were made daily, electrocardiograms were perfomed after
11, 24, and 50 weeks, haematological examinations were done after
12, 24, and 50 weeks, blood chemistry after 24 and 50 weeks, and
ophthalmoscopy after 49 weeks of treatment. There were no deaths.
Increased incidences of diarrhoea were observed during treatment in
males at 5 or 15 mg/kg bw per day and in females at 15 mg/kg bw per
day. Increased salivation was observed in females at 15 mg/kg bw per
day. Slight bradycardia was observed in animals of each sex at
15 mg/kg bw per day, which was significant in males at 11 and 50 weeks
of treatment. The overall body-weight gain throughout treatment was
significantly lower in males at 15 mg/kg bw per day, and a slightly
reduced total food consumption was observed. There were no treatment-
related haematological or ophthalmological findings. A slight but
significant lowering of total plasma protein level was observed in
males at 15 mg/kg bw per day after 50 weeks of treatment. There was no
change in erythrocyte acetylcholinesterase activity. The absolute and
relative prostatic weights were significantly increased in all treated
groups, but no histopathological changes were observed in the prostate
or in any other tissue examined. The NOAEL was 1.5 mg/kg bw per day,
based on the increased incidence of diarrhoea at 5 mg/kg bw per day
(Broadmeadow, 1989).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 50 male and 50 female ICR (Crj;CD) mice received
fenpyroximate (purity, 97.9-98.4%) mixed into the diet at
concentrations of 0, 25, 100, 400, or 800 ppm (overall mean
concentrations: 0, 24, 98, 388, and 776 ppm) for 18 months (78 weeks),
equal to 0, 2.4, 9.5, 38.0, or 69.6 mg/kg bw per day for males and 0,
2.5, 10.2, 41.5, or 73.1 mg/kg bw per day for females. There were no
treatment-related alterations in clinical signs. Body-weight gain and
food consumption were significantly lower than those of controls in
animals of each sex at 400 and 800 ppm. The only significant change in
haematological parameters was a decrease in the number of segmented
neutrophils at 500 ppm. A significant increase in the incidence of
emaciation was observed in males a t 500 ppm at the termination of
treatment. Histopathological examination revealed significant
increases in the incidence of ovarian atrophy in females at 400 and
800 ppm (25/50 in controls, 35/50 at 400 ppm, 35/50 at 800 ppm), but
these changes were morphologically similar to age-related changes seen
in female mice of this strain. There was no treatment-related increase
in the incidence of neoplasia. The NOAEL in this study was 100 ppm,
equal to 9.5 mg/kg bw per day in males and 10.2 mg/kg bw per day in
females, based on decreased body-weight gain and food consumption at
400 ppm (Takahashi, 1990).
Rats
Groups of 80 male and 80 female Sprague-Dawley CD rats
received fenpyroximate (purity, 97.1-97.2%) mixed into the diet at
concentrations of 0, 10, 25, 75, or 150 ppm for 104 weeks. The overall
doses achieved by the groups in which toxicity was assessed were 0,
0.40, 0.97, 3.08, and 6.18 mg/kg bw per day in males and 0, 0.48,
1.16, 3.79, and 7.57 mg/kg bw per day in females; those in the groups
for assessment of carcinogenicity were 0, 0.40, 0.97, 3.00, and
6.20 mg/kg bw per day in males and 0, 0.49, 1.21, 3.81, and 8.01 mg/kg
bw per day in females. Fifty rats of each sex per dose were assigned
for assessment of carcinogenicity and 30 of each sex per dose for
toxicity; 10 rats of each sex per dose were sacrificed at week 52 for
interim assessment. Blood samples were collected in weeks 24, 49, 76,
and 102 of treatment and urine samples in weeks 23, 49, 77, and 101.
Mortality after 104 weeks was 70, 72, 54, 60, and 52% among males and
58, 60, 50, 30, and 46% among females at 0,10, 25, 75, and 150 ppm,
respectively. There were no treatment-related adverse effects on
behaviour, appearance, or ophthalmoscopic parameters during treatment
or at termination. Absolute body weight was reduced after 104 weeks of
treatment, by 1-15% in males and 6-12% in females at 75 mg/kg bw per
day and by 11-28% in males and 17-22% in females at 150 mg/kg bw per
day. These changes were not statistically significant; however,
body-weight gains were significantly decreased in males at 75 ppm
and in animals of each sex at 150 ppm. Blood chemical analysis,
haematological examination, and urinalysis showed no treatment-related
findings. Macroscopic examination of animals used for toxicity
assessment revealed a significantly increased incidence of dark
popliteal lymph nodes in females at 150 ppm. Macroscopic examination
of animals for carcinogenicity revealed significantly increased
incidences of pituitary masses, masses that compressed the ventral
surface of the brain, and masses in the abdominal fat in males at
150 ppm, and increased incidences of uterine masses and distension of
the uterus in females at this dose. Histopathological examination
revealed higher incidences of non-neoplastic changes, which included
syncytial macrophages in the mesenteric lymph nodes of females in
the toxicity assessment group, and lesions associated with gastric
ulceration, compression of the brain due to pituitary neoplasia,
and pancreatic lobular degeneration in males and interstitial
proliferation of the ovary in females in the carcinogenicity
assessment groups. High incidences of pituitary adenoma were observed
in all groups, but the rates (males: 34/70, 36/57, 32/50, 37/54, and
36/69; females, 39/69, 50/60, 52/54, 41/47, and 43/66) were within the
historical control range (males, 34-70%; females, 67-86%). There was
no evidence of carcinogenicity. The NOAEL was 25 ppm, equal to
0.97 mg/kg bw per day in males, based on the lowered body-weight gain
at 75 ppm (Aughton, 1989).
(d) Reproductive toxicity
Rats
A preliminary range-finding study was conducted in which groups
of six male and six female Sprague-Dawley CD rats received
fenpyroximate (purity, 97.3%) mixed into the diet at concentrations of
10, 50, or 200 ppm for seven weeks, from 15 days before mating to day
4 post partum, for two generations. There were no treatment-related
changes in behaviour or appearance in F0 or F1 animals, and there
were no deaths. Significant reductions in body-weight gains were
observed in parental animals of each sex at 200 ppm, and food
consumption was reduced at this dose due to unpalatability (Higgins,
1988).
A two-generation (one litter per generation) study was conducted
with groups of 24 male and 24 female Sprague-Dawley CD rats that
received fenpyroximate (purity, 97.3%) mixed into the diet at
concentrations of 0, 10, 30, or 100 ppm, from 14 weeks before mating
up to weaning of the F1 generation (14 weeks before mating, three
weeks of mating, three weeks' gestation, 25 days' lactation) and then
from weaning of the F1 generation to weaning of the F2 generation.
The mean daily intakes of fenpyroximate were 0.67, 1.99, or 6.59 mg/kg
bw per day for F0 males 0.83, 2.44, or 8.60 mg/kg bw per day for F0
females, 0.78, 2.33, or 8.45 mg/kg bw per day for F1 males, and 0.96,
2.82, or 9.92 mg/kg bw per day for F1 females during the premating
period. There were no treatment-related abnormalities in behaviour or
appearance in F0, F1, or F2 animals throughout the study.
Significant reductions in body-weight gain were observed at 100 ppm in
males of the F0 and F1 generations throughout the study and in F0
females up to parturition. Food consumption was significantly reduced
in F0 males at 100 ppm during the period before mating. There were no
treatment-related changes in the oestrus cycle, mating performance
(percent mating, conception rate, or fertility index), length of
gestation, gestation index, or parturition index in F0 or F1
animals. Significantly reduced body-weight gains were seen during
lactation in F1 and F2 offspring at 100 ppm. There were no
treatment-related Changes in litter size, viability, sex ratio,
initial body weight at birth, or developmental indices (pinna
unfolding, hair growth, tooth eruption, or eye opening) in the F1 and
F2 generations. The absolute and relative weights of the testes and
epidymides were significantly increased at termination of the study in
F1 males fed 100 ppm, but the weights were only 10% greater than
those of controls, and macroscopic and histopathological examinations
revealed no treatment-related changes in the reproductive organs. The
NOAEL was 30 ppm, equal to 1.99 mg/kg bw per day in F0 males,
2.44 mg/kg bw per day in F0 females, 2.33 mg/kg bw per day in F1
males, and 2.82 mg/kg bw per day in F1 females, based on reductions
in body-weight gain in animals of each sex at 100 ppm (Higgins,
1989a).
(e) Developmental toxicity
Rats
Groups of 22 pregnant Sprague-Dawley CD rats received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxy-
methylcellulose and 0.1% Tween 80 by gavage at 0, 1, 5, or 25 mg/kg bw
per day on days 6-15 of gestation. Three females in the group at
5 mg/kg bw per day died due to a technical error; all surviving
animals were sacrificed on day 20 of gestation. A slight loss of body
weight after the first dose, a significant increase in water
consumption, and a slight reduction in food consumption were seen
during the first six days of treatment in rats at 25 mg/kg bw per day.
No other maternal toxicity was observed. There were no treatment-
related effects on litter parameters. Slight increases in the
incidences of skeletal variations were seen in fetuses in all treated
groups. The incidences of unilateral and bilateral additional 14th
thoracic rib(s) were 2.4 and 1.4% in controls, 5.2 and 4.8% at 1 mg/kg
bw per day, 5.0 and 1.7% at 5 mg/kg bw per day, 8.2 and 7.7% at
25 mg/kg bw per day, and 0-4.2 and 0-3.5%, in historical controls,
respectively. The increases were not dose-dependent, but the percent
incidence in fetuses and the number of litters with the skeletal
variation at the highest dose, 25 mg/kg bw per day, exceeded the
historical control range. There was no evidence of teratogenicity at
doses up to and including 25 mg/kg bw per day. The NOAEL in this study
was 5 mg/kg bw per day, based on slightly decreased body weight in
maternal rats and the increased incidence of skeletal variations in
fetuses at 25 mg/kg bw per day (Higgins, 1989b).
Rabbits
In a preliminary study, groups of four pregnant New Zealand white
rabbits received fenpyroximate (purity, 98.4%) suspended in 1% aqueous
carboxymethylcellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0,
2.5, or 5.0 mg/kg bw per day on days 6-19 of gestation. Maternal
toxicity was seen as marked reductions in body-weight gain and reduced
defaecation, food consumption, and water consumption at 5.0 mg/kg bw
per day. Increased post-implantation loss was also recorded at this
dose, as were reduced fetal weight and multiple anomalies (increased
incidences of small fetuses, oedema of head and/or ventral part of
neck, and thorax, fore- and hind-limb flexure). The NOAEL for
fetotoxicity was 2.5 mg/kg bw per day, based on reduced fetal weight
and art increased incidence of anomalies at 5.0 mg/kg bw per day
(Bailey, 1989).
Groups of 15 pregnant New Zealand white rabbits received
fenpyroximate (purity, 97.6%) suspended in 1% aqueous carboxymethyl-
cellulose and 0.1% Tween 80 by gavage at doses of 0, 1.0, 2.5, or
5.0 mg/kg bw per day on days 6-19 of gestation. Two females receiving
2.5 mg/kg bw per day were killed when moribund; necropsy revealed a
respiratory tract infection in one (14 days after treatment) and
evidence of gastrointestinal tract disturbance in the other (26 days
after treatment). All surviving animals were sacrificed on day 29 of
gestation. A transient decrease in body weight and in food consumption
and an increased incidence of reduced defaecation were observed at
5.0 mg/kg bw per day; a significant decrease in body weight was
observed at 2.5 mg/kg bw per day. Slight increases in the rate of
abortion and of total litter loss were observed in rabbits at 2.5 and
5.0 mg/kg bw per day: 0/15 in controls, 0/15 at 1.0 mg/kg bw per day,
7.7% (1/13) at 2.5 mg/kg bw per day, 13.3% (2/15) at 5.0 mg/kg bw per
day, and 0-14.3% in historical controls. The changes in body weight
and abortion rate at 2.5 mg/kg bw per day were slight but dose-
dependent. There was no evidence of fetotoxicity or teratogenicity at
doses up to 5.0 mg/kg bw per day. The NOAEL for maternotoxicity was
2.5 mg/kg bw per day (King, 1989).
(f) Genotoxicity
The results of studies on technical-grade fenpyroximate (purity,
97.3%) are summarized in table 3. The studies include those for
point mutations, mutation of mammalian cells in vitro, chromosomal
aberrations, micronucleus formation in vivo, DNA repair ( rec
assay), and unscheduled DNA synthesis. All of the positive controls
used gave the expected responses. There was no evidence of
genotoxicity.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
The primary ocular irritation potential of fenpyroximate (purity,
98.4%) as a fine powder was evaluated in male New Zealand white
rabbits by grading and scoring according to the method of Draize.
Changes due to irritation were observed in the conjunctivae of eyes
treated with 0.1 g of fenpyroximate for 1 h to two days after
treatment. Rinsing slightly reduced the severity of the changes.
Fenpyroximate is classified as a mild primary irritant (Kosaka,
1988a). A fenpyroximate formulation (5% suspension concentrate) was
moderately irritating to the eyes of female New Zealand white rabbits
receiving 0.1 ml (Teale, 1990a).
Fenpyroximate (purity, 98.4%) applied as a fine powder moistened
with distilled water was not irritating to the skin of male New
Zealand white rabbits, graded and scored according to the method of
Draize (Kosaka, 1988b). Little or no dermal irritation was seen after
application of 0.5 ml of the 5% suspension concentrate to female New
Zealand white rabbits (Hayes, 1990).
The dermal sensitizing potential of fenpyroximate (purity, 98.4%)
was studied in female Hartley guinea-pigs by the maximization test.
Fenpyroximate was given at 0.1 ml per animal at 5% by intradermal
injection and 25% by local application for induction and then given
at 25% by local application for challenge. The positive control was
2,4-dinitrochlorobenzene (at 0.1, 1, and 0.5%, respectively). The
positive control group was sensitized by 100% and the fenpyroximate-
treated group by 36%. Fenpyroximate thus had moderate dermal
sensitizing potential (Kosaka, 1988c).
The delayed dermal sensitizing potential of fenpyroximate
(purity, 98.4%) was studied in female Dunkin-Hartley guinea-pigs by
the method of Buehler. A dose of 0.5 ml of 50% aqueous fenpyroximate
was applied to the skin three times weekly for induction; the animals
were then challenged by applications of 50 or 25% w/w aqueous
Table 3. Results of tests for the genotoxicity of fenpyroximate
End-point Test system Concentration Purity Results Reference
or dose (solvent) (%)
In vitro
Reverse mutation S. typhimurium TA98, 0-5000 µg/plate (DMSO) 97.3 Negative May (1988)
TA100, TA1535, TA1537,
TA1538 (± S9), E. coli WP2
uvrA (± S9)
Mutation Chinese hamster 0-330 µg/ml (acetone) 97.3 Negative Hodson-Walker
V79 lung cells (1988a)
hprt locus (± S9)
Chromosomal Human lymphocytes 0-20 µg/ml (acetone) 97.3 Negative Hodson-Walker
aberration (± S9) (1988b)
DNA repair B subtilis H17, M45 0-500 µg/disc (DMSO) 97.3 Negative Watanabe (1990)
(± S9)
Unscheduled DNA Fischer 344 rat primary 0-1.02 µg/ml (DMSO) 97.3 Negative Cifone (1989)
synthesis hepatocytes
In vivo
Micronucleus CD-1 mice 0-2000 mg/kg bw orally 97.3 Negative Hodson-Walker
formation (1988c)
DMSO, dimethyl sulfoxide; S9, 9000 × g supernatant
fenpyroximate two weeks after the third induction. The positive
control was 2,4-dinitrochlorobenzene (0.3% for induction, 0.05 and
0.025% for challenge). The positive control group showed the expected
response, but fenpyroximate had no effect (Teale, 1990b).
A 5% suspension of fenpyroximate had no delayed dermal
sensitizing potential in female Dunkin-Hartley guinea-pigs tested by
the method of Buehler (Teale, 1990c).
(ii) Acute delayed neurotoxicity
Twelve adult Sterling Ranger hybrid hens were administered
fenpyroximate (purity, 97.0%) suspended in 0.5% aqueous
methylcellulose by catheter at a dose of 5000 mg/kg bw twice, on days
1 and 22 after insemination. A single oral dose of tri- ortho-cresyl
phosphate was used as the positive control. All surviving birds were
sacrificed on days 43-45. No deaths, abnormal neurological signs, or
treatment-related lesions in the nervous system were observed in
treated hens, whereas the positive control caused high mortality,
locomotor impairment, and degenerative changes in the spinal cord and
upper cervical bulbs, resulting in delayed neurotoxic responses
(Cummins, 1989).
3. Observations in humans
The results of medical surveillance of workers manufacturing a 5%
formulation of fenpyroximate were reported. Ocular and dermal
irritation were seen in July and November 1990 and March 1991. The
concentrations of fenpyroximate in the air at eight to nine sites in
the working place were 0.117 mg/m3 in January 1991, 0.012 mg/m3 in
April 1991, 0.005 mg/m3 in November 1991, and 0.004 mg/m3 in January
1993; however, the actual exposure of the workers to fenpyroximate was
not determined (Nokata, 1992, 1994).
Comments
Fenpyroximate was relatively well absorbed by rats after oral
administration. Absorbed fenpyroximate was excreted predominantly via
the biliary route, with lesser amounts in urine. The residual levels
in organs and tissues after 168 h were low. There was no evidence of
bioaccumulation.
Fenpyroximate was extensively metabolized in rats; 23 metabolites
were identified. No parent compound was found in the urine;
metabolites found in the excreta represented 0-11% of the administered
dose. Multiple pathways have been proposed for the metabolism of
fenpyroximate, including oxidation, hydroxylation, demethylation,
hydrolysis, and isomerization.
Fenpyroximate has slight to moderate acute oral toxicity (oral
LD50 = 440-520 mg/kg bw in mice and 245-480 mg/kg bw in rats); it is
acutely toxic by inhalation (LC50 in rats = 0.21-0.36 mg/m3). WHO
has not classified fenpyroximate for acute toxicity.
In a 13-week study in rats in which fenpyroximate was
administered in the diet at concentrations of 0, 20, 100, or 500 ppm,
the NOAEL was 20 ppm, equal to 1.3 mg/kg bw per day, and was based on
decreased body-weight gain and lowered plasma protein levels in
females fed 100 ppm. In a 13-week study in dogs, fenpyroximate was
administered orally in capsules at doses of 0, 2, 10, or 50 mg/kg bw
per day. The NOAEL was 2 mg/kg bw per day, on the basis of decreased
body-weight gain and clinical signs, such as an increased incidence of
diarrhoea, emaciation, and slight bradycardia, at 10 mg/kg bw per day.
In a 52-week study of similar design, with doses of 0, 0.5, 1.5, 5, or
15 mg/kg bw per day, the NOAEL was 5 mg/kg bw per day, on the basis of
decreased body-weight gain and total protein levels at 15 mg/kg bw per
day.
In an 18-month study of carcinogenicity in mice given dietary
concentrations of 0, 25, 100, 400, or 800 ppm, the NOAEL was 100 ppm,
equal to 9.5 mg/kg bw per day, and was based on decreased body-weight
gain, absolute body weight, and food consumption in animals of each
sex at 400 ppm. There was no evidence of carcinogenicity. In a
104-week study in rats given dietary concentrations of 0, 10, 25, 75,
or 150 ppm, the NOAEL was 25 ppm, equal to 1 mg/kg bw per day, based
on decreased body weight and body-weight gain in males at 75 ppm.
There was no evidence of carcinogenicity.
In a two-generation (one litter per generation) study of
reproductive toxicity in rats given dietary concentrations of 0, 10,
30, or 100 ppm, the NOAEL for maternal and developmental toxicity was
30 ppm, equal to 2 mg/kg bw per day, on the basis of decreased body-
weight gain in animals of the F0 and F1 generations at 100 ppm.
In a study of developmental toxicity in rats given doses of 0, 1,
5, or 25 mg/kg bw per day by gavage, the NOAEL for maternal toxicity
and fetotoxicity was 25 mg/kg bw per day, the highest dose tested.
There was no evidence of embryotoxicity or teratogenicity. In a study
of developmental toxicity in rabbits given doses of 0, 1, 2.5, or
5 mg/kg bw per day by gavage, there was no evidence of embryo- or
fetotoxicity or teratogenicity at any dose. The NOAEL for maternal
toxicity was 2.5 mg/kg bw per day on the basis of minor effects at
5 mg/kg bw per day.
Fenpyroximate has been adequately tested for genotoxicity in a
range of tests in vitro and in vivo. The Meeting concluded that it
is not genotoxic.
Two high doses, given 21 days apart, to hens did not cause gait
abnormalities or morphological changes in the nervous system.
An ADI of 0-0.01 mg/kg bw was established on the basis of the
NOAEL of 1 mg/kg bw per day for reductions in body-weight gain and
plasma protein concentration in the 104-week study in rats and a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 100 ppm, equal to 9.5 mg/kg bw per day (18-month study of
carcinogenicity)
Rat: 25 ppm, equal to 1 mg/kg bw per day (104-week study of
carcinogenicity)
30 ppm, equal to 2 mg/kg bw per day (study of reproductive
toxicity)
Rabbit: 2.5 mg/kg bw per day (maternal toxicity in study of
developmental toxicity)
Dog: 5 mg/kg bw per day (52-week study of toxicity)
Estimate of acceptable daily intake/or humans
0-0. 01 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to fenpyroximate
Exposure Relevant route, study type, species Results, remarks
Short-term (1-7 days) Oral, lethality, mouse and rat LD50 = 245-520 mg/kg bw
Eye, irritation, rabbit Irritating
Eye, irritation, human Irritating
Skin, sensitization, guinea-pig Sensitizing
Dermal, lethality, rat LD50 > 2000 mg/kg bw
inhalation, lethality tat LC50 = 0.21-0.36 mg/m3
Medium-term (1-26 weeks) Repeated 21-day toxicity, dermal, rat NOAEL = 300 mg/kg bw per day based
on reduced body-weight gain and
increased liver weight
Repeated four-week inhalation, toxicity, rat NOAEL = 2 mg/m3 based on increased
lung weight and mucosal change in nasal
passages
Repeated 13-week dietary, toxicity, rat NOAEL = 1.3 mg/kg bw per day based
on reduced body-weight gain and
lowered plasma protein level
Dietary, two-generation reproductive NOAEL = 2 mg/kg bw per day for
and developmental toxicity, rat maternal and developmental toxicity
Gavage, developmental toxicity, rat, rabbit NOAEL = 2.5 mg/kg bw per day for
maternal toxicity; no fetotoxicity or
teratogenicity
Long-term (> one year) Repeated dietary, two-year toxicity and NOAEL = 1 mg/kg bw per day based on
carcinogenicity, mouse; rat reduced body-weight gain and lowered
body weight; no carcinogenicity
References
Aughton, P. (1987) NNI-850: Toxicity study by dietary administration
to CD rats for 13 weeks. Unpublished report No. 89/NHH021/0972
from Life Science Research Ltd, Suffolk, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Aughton, P. (1989) NNI-850: Combined oncogenicity and toxicity study
by dietary administration to CD rats for 104 weeks. Unpublished
report No. 89/NHH034/0921 from Life Science Research Ltd,
Suffolk., United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Bailey, G.P. (1989) Preliminary teratology study in the rabbit.
Unpublished report No. 89/NHH050/0393 from Life Science Research
Ltd, Suffolk United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989a) Acute oral toxicity study in mice/Test
material: NNI-850 Technical. Unpublished report No. 5066-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989b) Acute oral toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5065-88 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Blaszcak, D.L. (1989c) Acute dermal toxicity study in rats/Test
material: NNI-850 Technical. Unpublished report No. 5559-89 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Broadmeadow, A. (1988) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 13 weeks. Unpublished report
No. 89/NHH036/1111 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Broadmeadow, A. (1989) NNI-850: Toxicity study by oral (capsule)
administration to beagle dogs for 52 weeks. Unpublished report
No. 89/NHH037/0802 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Cifone, M.A. (1989) Mutagenicity test on NNI-850, technical grade in
the rat primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report No. HLA 10753-0-447 from Hazleton Laboratories
America, Inc., Maryland, USA. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Cummins, H.A. (1989) NNI-850: Acute delayed neurotoxicity study in the
hen. Unpublished report No. 89/NHH054/0686 from Life Science
Research Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hayes, G. (1990) Primary skin irritation study, NNI-850 Flowable-R.
Unpublished report No. A/S/24651 from Toxicol Laboratories Ltd,
Herefordshire, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1988) NNI-850: Effects of dietary administration upon
reproductive performance in the rat; dosage range-finding study.
Unpublished report No. 88/NHH038/597 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989a) NNI-850: Reproductive performance study in rats
treated continuously through two successive generations.
Unpublished report No. 89/NHH043/0901 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Higgins, C. (1989b) NNI-850: Teratology study in the rabbit.
Unpublished report No. 89/NHH053/0722 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988a) NNI-850: Investigation of mutagenic activity
at the HGPRT locus in a Chinese hamster V79 cell mutation system.
Unpublished report No. 89/NHH042/1060 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988b) In vitro assessment of the clastogenic
activity of NNI-850 in cultured human lymphocytes. Unpublished
report No. 90/NHH040/1369 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Hodson-Walker, G. (1988c) NNI-850: Assessment of the clastogenic
action on bone marrow erythrocytes in the micronucleus test.
Unpublished report No. 89/NHH041/1059 from Life Science Research
Ltd, Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1989) An acute inhalation toxicity study of NNI-850 in
the rat. Unpublished report No. 88-8073 from Bio-dynamics Inc.,
New Jersey, USA. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Hoffman, G.M. (1990) An acute inhalation toxicity study of NNI-850
Flowable-R in the rat. Unpublished report No. 90-8253 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991a) An acute nose-only inhalation toxicity study of
NNI-850 in the rat. Unpublished report No. 90-8281 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991b) An acute nose-only inhalation toxicity study of
NNI-850 Flowable-R in the rat. Unpublished report No. 90-8282
from Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Hoffman, G.M. (1991c) A four week nose-only inhalation toxicity study
of NNI-850 in the rat. Unpublished report No. 90-8290 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
King, V.C. (1989) NNI-850: Teratology study in the rabbit. Unpublished
report No. 89/NHH051/0687 from Life Science Research Ltd,
Suffolk, United Kingdom. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Kosaka, T. (1988a) NNI-850: Primary eye irritation study in rabbits.
Unpublished report No. 87-0097 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988b) NNI-850: Primary dermal irritation study in
rabbits. Unpublished report No. 87-0098 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Kosaka, T. (1988c) NNI-850: Dermal sensitization study in guinea
pigs. Unpublished report No. 87-0099 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mahon, C.R. (1993) Dermal absorption of 14C-fenpyroximate following
a single dermal application to male Sprague-Dawley rats.
Unpublished report No. SC910039 from Battelle Columbus, Ohio,
USA. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
May, K. (1988) NNI-850 (technical grade): Assessment of mutagenic
potential in amino-acid auxotrophs of Salmonella typhimurium
and Escherichia coli (the Ames test). Unpublished report
No. 89/NHH039/1010 from Life Science Research Ltd, Suffolk,
United Kingdom. Submitted to WHO by Nihon Nohyaku Co., Ltd,
Tokyo, Japan.
Mitchell, J.M. (1990) Acute oral toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5795-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Mitchell, J.M. (1991) Acute dermal toxicity study in rats. Test
material: NNI-850 Flowable-R. Unpublished report No. 5975-90 from
Bio-dynamics Inc., New Jersey, USA. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Motoba, K., Suzuki, T. & Uchida, M. (1992) Effect of a new acaricide,
fenpyroximate, on energy metabolism and mitochondrial morphology
in adult female Tetracnychus urticae (two-spotted spider mite).
Pestic. Biochem. Physiol., 43, 37-44
Nishizawa, H., Motoba, K., Suzuki, T., Ohsima, T., Hamaguchi, H. &
Uchida, M. (1993) Metabolism of fenpyroximate in rats.
J. Pestic. Sci., 18, 59-66.
Nokata, M. (1992) Effect of fenpyroximate in human-eye and skin
irritation. Unpublished report No. H-4001 submitted to WHO by
Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Nokata, M. (1994) Effect of fenpyroximate on factory workers.
Unpublished report No. H-4003 submitted to WHO by Nihon Nohyaku
Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990a) Pharmacokinetics of a [pyrazole-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-104
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1990b) Pharmacokinetics of a [benzyl-14C] NNI-850 in
rats (high and low doses). Unpublished report No. HLA 6283-103
from Hazleton Laboratories America, Inc., Maryland, USA.
Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991a) Metabolism and disposition of a [pyrazole-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-102 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Sharp, D.E. (1991b) Metabolism and disposition of a [benzyl-14C]
NNI-850 in rats. Unpublished report No. HLA 6283-101 from
Hazleton Laboratories America, Inc., Maryland, USA. Submitted to
WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1987) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study Unpublished report No. IET
87-0034 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1988) NNI-850: 18-Month oral oncogenicity study in
mice. 4 week dose range finding study. Unpublished report No. IET
87-0186 from the Institute of Environmental Toxicology, Tokyo,
Japan. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
Takahashi, K. (1990) NNI-850: 18-Month oral oncogenicity study in
mice. Unpublished report No. 87-0036 from the Institute of
Environmental Toxicology, Tokyo, Japan. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Taninaka, K. (1993) Fenpyroximate, a new acaricide. Agrochem. Jpn,
62, 15-17.
Teale, H.J (1990a) Primary eye irritation study, NNI-850 Flowable-R.
Unpublished report No. A/E/24652 from Toxicol Laboratories
Limited, Herefordshire, United Kingdom. Submitted to WHO by Nihon
Nohyaku Co., Ltd, Tokyo, Japan.
Teale, H.J. (1990b) NNI-850 Technical/Delayed dermal sensitization
test in the guinea pig. Unpublished report No. A/B/22645 from
Toxicol Laboratories Ltd, Herefordshire, United Kingdom.
Submitted to WHO by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Teale, H.J. (1990c) NNI-850 Flowable/Delayed dermal sensitization test
in the guinea pig. Unpublished report No. A/B/22646 from Toxicol
Laboratories Ltd, Herefordshire, United Kingdom. Submitted to WHO
by Nihon Nohyaku Co, Ltd, Tokyo, Japan.
Tsu-Han Li (1991a) [Benzyl-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-001 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Tsu-Han Li (1991b) [Pyrazole-14C] fenpyroximate: Biliary excretion in
the rat. Unpublished report No. 7189-002 from Southern Research
Institute. Submitted to WHO by Nihon Nohyaku Co., Ltd, Tokyo,
Japan.
Watanabe, M. (1990) NNI-850: DNA repair test (rec-assay). Unpublished
report No. 88-0072 from the Institute of Environmental
Toxicology, Tokyo, Japan. Submitted to WHO by Nihon Nohyaku Co.,
Ltd, Tokyo, Japan.
Wilkinson, G.E., Ryan, M.J & Peters, A.C. (1992) 21-Day repeated-dose
dermal toxicity study of fenpyroximate in the rat. Unpublished
report No. SC920009 from Battelle Columbus, Ohio, USA. Submitted
to WHO by Nihon Nohyaku Co., Ltd, Tokyo, Japan.
