
Brodifacoum
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Main brand names, main trade names |
| 1.6 Main manufacturers, main importers |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First-aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Colour |
| 3.3.2 State/form |
| 3.3.3 Description |
| 3.4 Hazardous characteristics |
| 4. USES |
| 4.1 Uses |
| 4.1.1 Uses |
| 4.1.2 Description |
| 4.2 High risk circumstance of poisoning |
| 4.3 Occupationally exposed populations |
| 5. ROUTES OF ENTRY |
| 5.1 Oral |
| 5.2 Inhalation |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Others |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination by route of exposure |
| 7. TOXICOLOGY |
| 7.1 Mode of Action |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.2.4 Workplace standards |
| 7.2.5 Acceptable daily intake (ADI) and other guideline levels |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Material sampling plan |
| 8.1.1 Sampling and specimen collection |
| 8.1.1.1 Toxicological analyses |
| 8.1.1.2 Biomedical analyses |
| 8.1.1.3 Arterial blood gas analysis |
| 8.1.1.4 Haematological analyses |
| 8.1.1.5 Other (unspecified) analyses |
| 8.1.2 Storage of laboratory samples and specimens |
| 8.1.2.1 Toxicological analyses |
| 8.1.2.2 Biomedical analyses |
| 8.1.2.3 Arterial blood gas analysis |
| 8.1.2.4 Haematological analyses |
| 8.1.2.5 Other (unspecified) analyses |
| 8.1.3 Transport of laboratory samples and specimens |
| 8.1.3.1 Toxicological analyses |
| 8.1.3.2 Biomedical analyses |
| 8.1.3.3 Arterial blood gas analysis |
| 8.1.3.4 Haematological analyses |
| 8.1.3.5 Other (unspecified) analyses |
| 8.2 Toxicological analyses and their interpretation |
| 8.2.1 Tests on toxic ingredient(s) of material |
| 8.2.1.1 Simple qualitative test(s) |
| 8.2.1.2 Advanced qualitative confirmation test(s) |
| 8.2.1.3 Simple quantitative method(s) |
| 8.2.1.4 Advanced quantitative method(s) |
| 8.2.2 Tests for biological specimens |
| 8.2.2.1 Simple qualitative test(s) |
| 8.2.2.2 Advanced qualitative confirmation test(s) |
| 8.2.2.3 Simple quantitative method(s) |
| 8.2.2.4 Advanced quantitative method(s) |
| 8.2.2.5 Other dedicated method(s) |
| 8.2.3 Interpretation of toxicological analyses |
| 8.3 Biomedical investigations and their interpretation |
| 8.3.1 Biochemical analysis |
| 8.3.1.1 Blood, plasma or serum |
| 8.3.1.2 Urine |
| 8.3.1.3 Other fluids |
| 8.3.2 Arterial blood gas analyses |
| 8.3.3 Haematological analyses |
| 8.3.4 Interpretation of biomedical investigations |
| 8.4 Other biomedical (diagnostic) investigations and their interpretation |
| 8.5 Overall interpretation of all toxicological analyses and toxicological investigations |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin exposure |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 Central Nervous System (CNS) |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 Skeletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Others |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ears, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reactions |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Others |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Life supportive procedures and symptomatic treatment |
| 10.3 Decontamination |
| 10.4 Enhanced elimination |
| 10.5 Antidote treatment |
| 10.5.1 Adults |
| 10.5.2 Children |
| 10.6 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from literature |
| 12. ADDITIONAL INFORMATION |
| 12.1 Specific preventive measures |
| 12.2 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
BRODIFACOUM
International Programme on Chemical Safety
Poisons Information Monograph 077
Chemical
1. NAME
1.1 Substance
Brodifacoum
1.2 Group
Coumarin derivative
1.3 Synonyms
3-[-(4'-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-1-
naphthyl]-4-3-[3-(4'-bromo[1,1'-biphenyl]-4-yl)-1,2,3,4-
tetrahydro-1-bromfenacoum;
super-warfarin
1.4 Identification numbers
1.4.1 CAS number
56073-10-0
1.4.2 Other numbers
1.5 Main brand names, main trade names
Klerat (Uruguay, UK)
Ratak (USA)
Talon (USA, New Zealand)
1.6 Main manufacturers, main importers
2. SUMMARY
2.1 Main risks and target organs
The target system is the haematological system, with
impairment of clotting. The main risks are associated with
potentially fatal gastrointestinal and intracerebral
haemorrhage.
2.2 Summary of clinical effects
If toxic amounts have been ingested, coagulation will be
impaired, with gum bleeding, epistaxis, ecchymosis,
haematomata, haematemesis, melenae, haematuria.
2.3 Diagnosis
The diagnosis is based on history of exposure (generally
by ingestion of a rodenticide); clinical evidence of
bleeding, which may appear even several days after exposure;
and abnormal prothrombin time.
A sample of the rodenticide should be kept for toxicological
analysis (if feasible). The most relevant biomedical
analysis is coagulation studies including:
* Determination of clotting factors
* Prothrombin time (PT)
* Activated partial thromboplastin time (PTT)
2.4 First-aid measures and management principles
All cases of brodifacoum ingestion should be taken to a
hospital for initial clinical and laboratory evalauation. In
ingestion was recent (within 3 to 6 hours) the following may
be recommended:
* Decontamination of the patient through gastric
lavage/emesis.
* Administration of activated charcoal followed by
cathartics.
Not all patients need to stay in the hospital but prolonged
clinical and analytical follow-up is mandatory.
If more than 24 hours have elapsed since ingestion,
decontamination measures are not effective and the patient
should be monitored closely using prothrombin time (PT) and
plasma thromboplastin time (PTT).
Treatment is based in the administration of vitamin K1
(phytomenadione) as indicated by the prothrombin time.
Fresh frozen plasma or whole blood are indicated in cases of
acute bleeding.
Close clinical observation is essential to detect occult
bleeding or life-threatening haemorrhage.
In cases of suspected serious ingestion, vitamin K1 is
indicated before signs and symptoms of haemorrhage
appear.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
This synthetic anticoagulant is obtained by the
condensation of 4-hydroxycoumarin with
3-(4'-bromodiphenyl-4-yl)-1,2,3,4-tetrahydronaphtol
(CCINFOdisc, 1989).
It was first introduced in 1975 to deal with the public
health problem of warfarin-resistant rodents (Lipton & Klaas,
1984).
3.2 Chemical structure
3-[3-(4'-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-1-naphtyl]
-4-hydroxcoumarin
3-[3-(4'-bromo[1,1'-biphenyl]-4-yl)-1,2,3,4-tetrahydro
-1-naphthalenyl]-4-hydroxy-2H-1-benzopyran-2-one
Molecular weight = 523.44
C31H23-BrO3 C 71.14%, H 4.43%, Br 15.27%, O 9.17%
Structural formula:
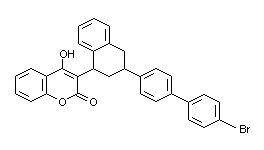 (Budivari, 1996)
Brodifacoum exists as cis and trans isomers that may be
separated by chromatography and identified by nuclear
magnetic resonance spectroscopy. The commercially available
preparation contains variable proportions of cis/trans
isomers as 50:50 and 70:30. There is no significant
difference in activity between the two isomers (ICI).
The lateral 4-bromo-biphenyl group provides greater stability
to the compound and overcomes the development of resistance
to the poison in the rat.
Note: Difenacoum is very similar to brodifacoum in its
chemical structure and anticoagulant effect (they are both
4-hydroxycoumarin derivatives).
3.3 Physical properties
3.3.1 Colour
Off-white to fawn powder
3.3.2 State/form
3.3.3 Description
Melting point: 228°C to 230°C
< 0.13 mPa (25°C).
Vapour pressure is less than 10 mg/l at 20°C.
Insoluble in water and ether, soluble in chloroform
and acetone, slightly soluble in: benzene, ethanol,
ethylglycerol acetate and polyethyleneglycol.
Brodifacoum is a weak acid which does not readily form
water-soluble salts. It does not lose activity after
30 days in direct sunlight.
3.4 Hazardous characteristics
Storage: commercial formulations are stable at least
for two years if protected from extreme temperatures and
sunlight. The container should be kept closed to maintain
activity of formulation (as a bait).
As a solid, it is stable under normal storage conditions. It
does not corrode commonly-used metallic objects. Forms amine
salts of limited solubility in water.
4. USES
4.1 Uses
4.1.1 Uses
4.1.2 Description
Rodenticide effective against
warfarin-resistant rats. It is usually sold as ready
to use bait in pellets, mini-pellets and waterproof
bait containing 0.005% brodifacoum (loose or in bait
packs).
Indicated for control of Norwegian rats, roof rats and
house mice in public, industrial and commercial
buildings, in residences and outdoor urban areas. The
application may be carried out by professional pest
control personnel orby the public in general.
Theoretically, the substance may be considered for
development as a therapeutic agent in anticoagulant
therapy, but the difficulty in reversal of effects
constitutes a major disadvantage (Jones et al.,
1984).
4.2 High risk circumstance of poisoning
This rodenticide is usually applied as a bait, and
possibilities of food or environmental contamination are
relatively low.
Poisoning occurs usually after accidental ingestion by small
children or suicide attempts by adults
4.3 Occupationally exposed populations
No specific data were found on risks of occupational
exposure in the manufacturing industry when handling the pure
substance.
5. ROUTES OF ENTRY
5.1 Oral
This is the commonest route of entry and the only one
described in the published literature.
5.2 Inhalation
No data available.
5.3 Dermal
Only animal data are available: acute dermal toxicity
for the male rabbit is 0.25 to 0.625 mg/kg, but if skin is
abraded the dermal toxic dose is 1.25 mg/kg (ICI Ltd).
5.4 Eye
No data available.
5.5 Parenteral
No data available.
5.6 Others
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Brodifacoum is readily absorbed in the gastrointestinal
tract.
6.2 Distribution by route of exposure
Brodifacoum and related compounds bind more strongly to
a lipophilic site in the liver than does warfarin. In the
poisoned rat, hepatic concentrations of the substance are 20
times higher than the serum concentrations (Bachman &
Sullivan, 1983).
6.3 Biological half-life by route of exposure
As with all super-warfarins, brodifacoum has a very long
plasma half-life. Metabolic studies in animals have shown a
half-life of approximately 24 days (Fitzgerald & Bronstein,
1987), 120 days in the dog (Lipton & Klaas, 1984), and 156
hours in the rat (Bachman & Sullivan, 1983).
Warfarin has a plasma half-life of 42 hours and, assuming a
normal liver function and diet, its anticoagulant effect
disappears in a few days. By contrast, the anticoagulant
effect of brodifacoum may last for more than 7 weeks in the
poisoned patient (Jones et al., 1984).
6.4 Metabolism
The metabolism of brodifacoum has not been fully
defined, but considering that it has a substituted
4-hydroxycoumarin ring structure, it is probably metabolized
in a similar manner to warfarin. It is hydroxylated to
inactive compounds by mixed function oxidase enzymes in
hepatic microsomes (Jones et al., 1984).
Phenobarbital is known to increase the activity of the
hepatocellular microsomal enzyme system, and would increase
brodifacoum's metabolism as it does with warfarin. In the
animal, it has been demonstrated that pretreatment with
phenobarbital decreases the anticoagulant effect of
brodifacoum (Bachman & Sullivan, 1983).
6.5 Elimination by route of exposure
The elimination of brodifacoum is probably similar to
that of warfarin: compounds resulting from the hydroxylation
in the hepatocellular mixed function oxidases system are
excreted in the urine (Jones et al., 1984).
7. TOXICOLOGY
7.1 Mode of Action
Brodifacoum acts by inhibiting the vitamin K epoxide
reductase in the vitamin K1-epoxide cycle (Park et al.,
1979), impeding the cyclic regeneration of vitamin K1,
resulting in hypoprothrombinemia. Under physiological
conditions, the oxidation of vitamin K in the hepatocyte is
coupled to a carboxylation step essential for activation of
prothrombin factors from inactive precursors. Brodifacoum
produces hypoprothrombinaemia because the coupled
carboxylationreaction is inhibited (Lipton & Klaas, 1984). If
therapeutic doses of vitamin K1 are given, additional
substrate becomes available to resume the cycle and continue
the carboxylation process, reversing the
hypoprothrombinaemia(Lipton & Klaas, 1984).
The superwarfarins produce a diminution of the vitamin
K-dependent carboxylation of glutamic acid residues in
prothrombin factor precursors. This effect is 100 times
greater on molar basis than that of warfarin. This, with
brodifacoum's very long plasma half-life, results in a potent
anticoagulant effect.
A longitudinal analysis of alterations in specific
coagulation factors in a poisoning case reported by Hoffman
et al. (1988) demonstrated a profound decrease of factors II,
VII, IX and X, lasting at least 43 days post-ingestion.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
The ingestion of thirty 50 g
packages of brodifacoum over a two-day period
by a 31-year-old psychotic woman produced
generalized ecchymosis and abortion. The
estimated ingested dose was 75 mg (Lipton &
Klaas, 1984).
A 17-year-old male adolescent ingested
approximately 7.5 mg (0.12 mg/kg) of
brodifacoum and was admitted to the hospital
with flank pain and important haematuria,
followed by epistaxis and gum bleeding.
Therapy with vitamin K1 had to be maintained
for over 50 days (Jones et al., 1984).
The ingestion of 1 mg of brodifacoum in an
adult produced bleeding that persisted for
more than 2 months (Chong et al., 1986).
No clearly defined toxic dose has been
established in the human, and few clinical
reports are available.
7.2.1.2 Children
Very few reports are available
specifying the toxic dose, although ingestion
of long-acting rodenticides have been
reported in children.
Anticoagulation has been detected but without
clinical symptomatology in most cases
(Smolinske et al., 1987).
7.2.2 Relevant animal data
LD50 oral (female rat) 0.37 to 0.68 mg/kg
LD50 oral (beagle dog) 0.25 to 1 mg/kg
LD50 oral (cat) estimated as 25 mg/kg.
The acute dermal toxicity (male rabbits) with intact
skin was estimated to be 0.25 to 0.625 mg/kg. When
the skin is abraded, the dose is 1.25 mg/kg (ICI
Ltd).
Brodifacoum is slightly irritant when applied to the
skin of rabbits and to the eye.
7.2.3 Relevant in vitro data
No data available.
7.2.4 Workplace standards
No data available.
7.2.5 Acceptable daily intake (ADI) and other guideline levels
No data available.
7.3 Carcinogenicity
No effect has been demonstrated with technical
brodifacoum in carcinogenicity tests (ICI Ltd.).
7.4 Teratogenicity
Studies in rats and rabbits have demonstrated no
fetotoxic embryotoxic or teratogenic effects.
7.5 Mutagenicity
No data available.
7.6 Interactions
Warfarin interacts with many drugs but, although a
similar pattern is expected with brodifacoum, no specific
data were found.
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method(s)
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall interpretation of all toxicological analyses and
toxicological investigations
Collect a sample of the product blood/urine/faeces.
Biomedical analysis
Prothrombin time (PT) monitoring is essential. For the first
5 to 6 days it should be done at least daily, and repeated
even if results were normal uponadmission. Prothrombin time
may initially be normal, even in a severe poisoning because
vitamin K-dependent clotting factors have a long half-life
and are still circulating several hours after brodifacoum
starts its effect.
Activated partial thromboplastin time (PTT) is abnormal.
Coagulation tests in general may be normal (e.g. coagulation
time, thromboelastogram).
Haemoglobin: Red blood count
Urinalysis (haematuria): Stools
Toxicological analysis
Although a sensitive HPLC technique exists, it is not
clinically useful.
Other investigations
Specific organ evaluation for bleeding as clinically
indicated.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Ingestion of brodifacoum is initially
asymptomatic, and may continue as such even with
prolonged alterations in prothrombin time. No
gastrointestinal tract or other symptomatology
occurs.
Coagulation disturbances may become evident a few days
after ingestion, and may be detected only by
laboratory studies. In severe poisoning,
gum-bleeding, epistaxis, petechiae, ecchymoses,
haematomata, blood in urine and faeces, and genital
haemorrhage may occur. Internal bleeding and cerebral
haemorrhage may complicate the patient's
prognosis.
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
The commercial product is not irritating to the
skin. Animal studies showed that the pure substance
is absorbed through the skin.
9.1.4 Eye contact
Slightly irritant.
9.1.5 Parenteral exposure
No data available.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
Ingestion of brodifacoum may be repeated,
leading to severe poisoning (Basehore & Mowry,
1987).
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
The course of poisoning is characteristically long.
Alterations of coagulation parameters and clinical symptoms
of bleeding may be maintained for several days if no
treatment is provided.
The prognosis is poor in cases with internal bleeding or
intracerebral haemorrhage, and also in patients with previous
haematological illnesses or renal insufficiency.
Death is uncommon (Basehore & Mowry, 1987).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
No direct cardiotoxic effects have been
described in man.
9.4.2 Respiratory
No effects have been reported but theoretically
haemoptysis would be a possibility.
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Intracerebral haemorrhage has been
described as a secondary complication
(Basehore & Mowry, 1987).
9.4.3.2 Peripheral nervous system
No effects have been reported.
9.4.3.3 Autonomic nervous system
No effects have been reported.
9.4.3.4 Skeletal and smooth muscle
Muscular haematoma may occur,
especially on the elbows, knees and buttocks
(CCINFOdisc, 1989).
9.4.4 Gastrointestinal
Haematemesis and melena may occur. Abdominal
and flank pain have been reported after
intra-abdominal bleeding (Jones et al., 1984).
9.4.5 Hepatic
Although the liver is the site of metabolism of
brodifacoum, no observable clinical effects are
apparent, except coagulopathy.
9.4.6 Urinary
9.4.6.1 Renal
Haematuria may be clinically evident
or detected only by the laboratory (Jones et
al., 1984). Urinary tract haemorrhage has
been reported (Hollinger & Pastoor,
1993).
9.4.6.2 Others
No data available.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
Petechial rashes may be seen.
9.4.9 Eye, ears, nose, throat: local effects
Epistaxis and gum bleeding.
9.4.10 Haematological
Coagulation is impaired after ingestion of
significant quantities of brodifacoum. The usual
haematological symptoms are: gum bleeding, epistaxis,
ecchymoses, haematoma, and haematuria; there may be
internal bleeding.
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
No data available.
9.4.12.2 Fluid and electrolyte disturbances
No data available.
9.4.12.3 Others
No data available.
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
One case of abortion has been reported by
Lipton & Klaas (1984). Data on breast milk secretion
are not available and therefore breast feeding should
not be recommended. Individuals with bleeding
diatheses may be at higher risk.
9.5 Others
9.6 Summary
10. MANAGEMENT
10.1 General principles
In all cases of brodifacoum ingestion, the patient
(especially children) should be clinically monitored by daily
laboratory studies for at least 4 or 5 days.
Gastric lavage or emesis is indicated if the ingested amount
is uncertain or possibly toxic. Administration of activated
charcoal and cathartics is useful. If initial coagulation
disorders have been detected, the prothrombin time should be
monitored (for at least 60 days). The treatment is based
upon the administration of vitamin K1. Fresh frozen plasma
or whole blood should be given in severe cases of poisoning
in order to provide clotting factors.
Clinical observation should be very strict in order to
diagnose any possibility of bleeding.
10.2 Life supportive procedures and symptomatic treatment
Life-saving procedures are indicated if serious
complications arise.
Symptomatic treatment for bleeding is the administration of
fresh frozen plasma or fresh whole blood for correcting
coagulopathy.
10.3 Decontamination
If more than 24 hours have elapsed decontamination
measures are not effective.
Emesis or gastric lavage may be effective if attempted within
3 to 6 hours of ingestion.
Administration of activated charcoal and cathartics may be
considered.
10.4 Enhanced elimination
Enhancement of elimination is not indicated.
10.5 Antidote treatment
10.5.1 Adults
Phytomenadione (vitamin K1) should be
administered. If prothrombin time is significantly
reduced vitamin K1 should be administered
intravenously starting with 10 mg each 6 h (40
mg/day). The intramuscular route may be chosen under
appropriate clinical settings. The dosage should be
adjusted according to the prothrombin time.
10.5.2 Children
Indication and dosage is the same as in adults.
10.6 Management discussion
There is no consensus on the appropriate administration
schemes for vitamin K. Some authors give oral vitamin K
prophylactically in cases where ingestion is uncertain. The
usual route for treatment is intravenous, although in
somecases it has been given intramuscularly or
subcutaneously. The use of phenobarbital as a hepatic
microsomal enzyme inducer is controversial.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
Lipton & Klaas (1984) reported a case of a psychotic
31-year-old woman who ingested thirty 50 g packages of
commercial rodenticide containing brodifacoum. Five days
later, she was admitted to hospital with slash marks on both
wrists and blood clots in the gums. Laboratory results were:
haemoglobin 12.2 g/dL, Ht 37.9%, WBC 5.6/mm3, platelets
390.000, prothrombin time 72s (control = 12 s), activated
partial thromboplastin time greater than 100s (normal = 25 to
35s), fibrinogen 380 mg/dL. Urinalysis was negative for
blood and protein. Treatment was started with 25 mg of
vitamin K1 and fresh frozen plasma. The dose of vitamin K1
was increased to 125 mg per day after little improvement in
the prothrombin time. Phenobarbital was also administered as
an hepatic enzyme inducer. This case was complicated by a
complete and apparently spontaneous abortion.
Basehore & Mowry (1987) reported a patient who, following
chronic ingestion of the rodenticide, was admitted to
hospital with nose-bleeding, haematuria and multiple
ecchymoses. Laboratory studies showed: prothrombin time = 60
seconds (normal = 12.4), partial prothrombin time = 79.2
seconds (normal = 27), bleeding time 15 minutes. Treatment
was started with vitamin K1 and frozen fresh plasma. The
patient was discharged 9 days afterwards with almost normal
prothrombin time and partial thromboplastin time and
prescribed oral phytomenadione. Three weeks later, he
presented with status epilepticus; intradural haemorrhage was
diagnosed by CAT-Scan. Prothrombin time was again over 60
seconds. He deteriorated and died 4 hours after
admission.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
As any other pesticide, these rodenticides should be
kept out of the reach of children and irresponsible persons.
Use of superwarfarin, such as brodifacoum, should be
restricted to agronomical application and not widely
advertised or sold without restriction for household
use.
12.2 Other
No data available.
13. REFERENCES
Bachman KA & Sullivan TJ (1983) Dispositional and
pharmacodynamics characteristics of brodifacoum in
warfarin-sensitive rats. Pharmacology, 27(5): 251-288.
Basehore LM & Mowry JM (1987) Death following ingestion of
superwarfarin rodenticide: A case report. Vet & Human Toxicology,
29 (6 Dec.).
Budivari S (ed) (1996) The Merck Index, An Encyclopedia of
Chemicals, Drugs and Biologicals, 12th ed., Merck & Co., Inc.,
Whitehouse Station, NJ, USA.
CCINFO (1989)
Chong L, Chan W, Ho C (1986) A case of superwarfarin poisoning.
Scand J Haematol, 36: 314-315.
Fitzgerald K & Bronstein AC (1987). Comparison of first and
second generation anticoagulant rodenticide poisonings: fourteen
canine cases. Abstracts from the AACT/AAPCC/ABMT/CAPCC scientific
meetings. Sept. 27.
Hoffman RS, Smilkstein MJ, Goldfrank LR (1988) "Evaluation of
coagulation factor abnormalities in long-acting anticoagulant
overdose". J Toxicol Clin Toxicol, 26 (3-4): 48.
Hollinger B & Pastoor TP (1993). Case management and plasma
half-life in a case of brodifacoum poisoning. Arch Intern Med, 153
(16):1925-8
Jones E, Growe GH, Naiman SC (1984) Prolonged anticoagulation in
rat poisoning. JAMA, 252: 3005-3007.
ICI Ltd. - Technical Information
Lipton RA & Klaas EM (1984) Human ingestion of superwarfarin
rodenticide resulting in a prolonged anticoagulant effect. JAMA,
Dec 7., vol. 252, No. 21.
Park BK et al. (1979) A study of the effect of anticoagulants on
(3H) vitamin K1 metabolism and prothrombin complex activity in the
rabbit. Biochem Pharmacol, 28 (8): 1323-9.
Smolinske SC, Sherger DS, Kearus PS & Rumack BH (1987) Long
acting coagulant rodenticide ingestion in children. Vet & Hum
Toxicology, 29 (6 Dec.).
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors: Dr C. Dell Acqua
Dr J. Pronczuk
CIAT
Piso 7, Hospital de Clinicas
Av. Italia s/n
Montevideo
Uruguay
Tel: 598-2-470300 and 598-2-804000
Fax: 598-2-470300
Date: February 1990
Peer
review: London, United Kingdom, March 1990.
Informal Consultation, post JMPR meeting, September
1991
Original
Editor: Dr S. Chaplin, Newcastle-upon-Tyne, United Kingdom
(September 1991)
Update: International Programme on Chemical Safety (May
1992)
Editor: Mrs J. Duménil, International Programme on Chemical
Safety
(July 1999)
(Budivari, 1996)
Brodifacoum exists as cis and trans isomers that may be
separated by chromatography and identified by nuclear
magnetic resonance spectroscopy. The commercially available
preparation contains variable proportions of cis/trans
isomers as 50:50 and 70:30. There is no significant
difference in activity between the two isomers (ICI).
The lateral 4-bromo-biphenyl group provides greater stability
to the compound and overcomes the development of resistance
to the poison in the rat.
Note: Difenacoum is very similar to brodifacoum in its
chemical structure and anticoagulant effect (they are both
4-hydroxycoumarin derivatives).
3.3 Physical properties
3.3.1 Colour
Off-white to fawn powder
3.3.2 State/form
3.3.3 Description
Melting point: 228°C to 230°C
< 0.13 mPa (25°C).
Vapour pressure is less than 10 mg/l at 20°C.
Insoluble in water and ether, soluble in chloroform
and acetone, slightly soluble in: benzene, ethanol,
ethylglycerol acetate and polyethyleneglycol.
Brodifacoum is a weak acid which does not readily form
water-soluble salts. It does not lose activity after
30 days in direct sunlight.
3.4 Hazardous characteristics
Storage: commercial formulations are stable at least
for two years if protected from extreme temperatures and
sunlight. The container should be kept closed to maintain
activity of formulation (as a bait).
As a solid, it is stable under normal storage conditions. It
does not corrode commonly-used metallic objects. Forms amine
salts of limited solubility in water.
4. USES
4.1 Uses
4.1.1 Uses
4.1.2 Description
Rodenticide effective against
warfarin-resistant rats. It is usually sold as ready
to use bait in pellets, mini-pellets and waterproof
bait containing 0.005% brodifacoum (loose or in bait
packs).
Indicated for control of Norwegian rats, roof rats and
house mice in public, industrial and commercial
buildings, in residences and outdoor urban areas. The
application may be carried out by professional pest
control personnel orby the public in general.
Theoretically, the substance may be considered for
development as a therapeutic agent in anticoagulant
therapy, but the difficulty in reversal of effects
constitutes a major disadvantage (Jones et al.,
1984).
4.2 High risk circumstance of poisoning
This rodenticide is usually applied as a bait, and
possibilities of food or environmental contamination are
relatively low.
Poisoning occurs usually after accidental ingestion by small
children or suicide attempts by adults
4.3 Occupationally exposed populations
No specific data were found on risks of occupational
exposure in the manufacturing industry when handling the pure
substance.
5. ROUTES OF ENTRY
5.1 Oral
This is the commonest route of entry and the only one
described in the published literature.
5.2 Inhalation
No data available.
5.3 Dermal
Only animal data are available: acute dermal toxicity
for the male rabbit is 0.25 to 0.625 mg/kg, but if skin is
abraded the dermal toxic dose is 1.25 mg/kg (ICI Ltd).
5.4 Eye
No data available.
5.5 Parenteral
No data available.
5.6 Others
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Brodifacoum is readily absorbed in the gastrointestinal
tract.
6.2 Distribution by route of exposure
Brodifacoum and related compounds bind more strongly to
a lipophilic site in the liver than does warfarin. In the
poisoned rat, hepatic concentrations of the substance are 20
times higher than the serum concentrations (Bachman &
Sullivan, 1983).
6.3 Biological half-life by route of exposure
As with all super-warfarins, brodifacoum has a very long
plasma half-life. Metabolic studies in animals have shown a
half-life of approximately 24 days (Fitzgerald & Bronstein,
1987), 120 days in the dog (Lipton & Klaas, 1984), and 156
hours in the rat (Bachman & Sullivan, 1983).
Warfarin has a plasma half-life of 42 hours and, assuming a
normal liver function and diet, its anticoagulant effect
disappears in a few days. By contrast, the anticoagulant
effect of brodifacoum may last for more than 7 weeks in the
poisoned patient (Jones et al., 1984).
6.4 Metabolism
The metabolism of brodifacoum has not been fully
defined, but considering that it has a substituted
4-hydroxycoumarin ring structure, it is probably metabolized
in a similar manner to warfarin. It is hydroxylated to
inactive compounds by mixed function oxidase enzymes in
hepatic microsomes (Jones et al., 1984).
Phenobarbital is known to increase the activity of the
hepatocellular microsomal enzyme system, and would increase
brodifacoum's metabolism as it does with warfarin. In the
animal, it has been demonstrated that pretreatment with
phenobarbital decreases the anticoagulant effect of
brodifacoum (Bachman & Sullivan, 1983).
6.5 Elimination by route of exposure
The elimination of brodifacoum is probably similar to
that of warfarin: compounds resulting from the hydroxylation
in the hepatocellular mixed function oxidases system are
excreted in the urine (Jones et al., 1984).
7. TOXICOLOGY
7.1 Mode of Action
Brodifacoum acts by inhibiting the vitamin K epoxide
reductase in the vitamin K1-epoxide cycle (Park et al.,
1979), impeding the cyclic regeneration of vitamin K1,
resulting in hypoprothrombinemia. Under physiological
conditions, the oxidation of vitamin K in the hepatocyte is
coupled to a carboxylation step essential for activation of
prothrombin factors from inactive precursors. Brodifacoum
produces hypoprothrombinaemia because the coupled
carboxylationreaction is inhibited (Lipton & Klaas, 1984). If
therapeutic doses of vitamin K1 are given, additional
substrate becomes available to resume the cycle and continue
the carboxylation process, reversing the
hypoprothrombinaemia(Lipton & Klaas, 1984).
The superwarfarins produce a diminution of the vitamin
K-dependent carboxylation of glutamic acid residues in
prothrombin factor precursors. This effect is 100 times
greater on molar basis than that of warfarin. This, with
brodifacoum's very long plasma half-life, results in a potent
anticoagulant effect.
A longitudinal analysis of alterations in specific
coagulation factors in a poisoning case reported by Hoffman
et al. (1988) demonstrated a profound decrease of factors II,
VII, IX and X, lasting at least 43 days post-ingestion.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
The ingestion of thirty 50 g
packages of brodifacoum over a two-day period
by a 31-year-old psychotic woman produced
generalized ecchymosis and abortion. The
estimated ingested dose was 75 mg (Lipton &
Klaas, 1984).
A 17-year-old male adolescent ingested
approximately 7.5 mg (0.12 mg/kg) of
brodifacoum and was admitted to the hospital
with flank pain and important haematuria,
followed by epistaxis and gum bleeding.
Therapy with vitamin K1 had to be maintained
for over 50 days (Jones et al., 1984).
The ingestion of 1 mg of brodifacoum in an
adult produced bleeding that persisted for
more than 2 months (Chong et al., 1986).
No clearly defined toxic dose has been
established in the human, and few clinical
reports are available.
7.2.1.2 Children
Very few reports are available
specifying the toxic dose, although ingestion
of long-acting rodenticides have been
reported in children.
Anticoagulation has been detected but without
clinical symptomatology in most cases
(Smolinske et al., 1987).
7.2.2 Relevant animal data
LD50 oral (female rat) 0.37 to 0.68 mg/kg
LD50 oral (beagle dog) 0.25 to 1 mg/kg
LD50 oral (cat) estimated as 25 mg/kg.
The acute dermal toxicity (male rabbits) with intact
skin was estimated to be 0.25 to 0.625 mg/kg. When
the skin is abraded, the dose is 1.25 mg/kg (ICI
Ltd).
Brodifacoum is slightly irritant when applied to the
skin of rabbits and to the eye.
7.2.3 Relevant in vitro data
No data available.
7.2.4 Workplace standards
No data available.
7.2.5 Acceptable daily intake (ADI) and other guideline levels
No data available.
7.3 Carcinogenicity
No effect has been demonstrated with technical
brodifacoum in carcinogenicity tests (ICI Ltd.).
7.4 Teratogenicity
Studies in rats and rabbits have demonstrated no
fetotoxic embryotoxic or teratogenic effects.
7.5 Mutagenicity
No data available.
7.6 Interactions
Warfarin interacts with many drugs but, although a
similar pattern is expected with brodifacoum, no specific
data were found.
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method(s)
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall interpretation of all toxicological analyses and
toxicological investigations
Collect a sample of the product blood/urine/faeces.
Biomedical analysis
Prothrombin time (PT) monitoring is essential. For the first
5 to 6 days it should be done at least daily, and repeated
even if results were normal uponadmission. Prothrombin time
may initially be normal, even in a severe poisoning because
vitamin K-dependent clotting factors have a long half-life
and are still circulating several hours after brodifacoum
starts its effect.
Activated partial thromboplastin time (PTT) is abnormal.
Coagulation tests in general may be normal (e.g. coagulation
time, thromboelastogram).
Haemoglobin: Red blood count
Urinalysis (haematuria): Stools
Toxicological analysis
Although a sensitive HPLC technique exists, it is not
clinically useful.
Other investigations
Specific organ evaluation for bleeding as clinically
indicated.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Ingestion of brodifacoum is initially
asymptomatic, and may continue as such even with
prolonged alterations in prothrombin time. No
gastrointestinal tract or other symptomatology
occurs.
Coagulation disturbances may become evident a few days
after ingestion, and may be detected only by
laboratory studies. In severe poisoning,
gum-bleeding, epistaxis, petechiae, ecchymoses,
haematomata, blood in urine and faeces, and genital
haemorrhage may occur. Internal bleeding and cerebral
haemorrhage may complicate the patient's
prognosis.
9.1.2 Inhalation
No data available.
9.1.3 Skin exposure
The commercial product is not irritating to the
skin. Animal studies showed that the pure substance
is absorbed through the skin.
9.1.4 Eye contact
Slightly irritant.
9.1.5 Parenteral exposure
No data available.
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
Ingestion of brodifacoum may be repeated,
leading to severe poisoning (Basehore & Mowry,
1987).
9.2.2 Inhalation
No data available.
9.2.3 Skin exposure
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
The course of poisoning is characteristically long.
Alterations of coagulation parameters and clinical symptoms
of bleeding may be maintained for several days if no
treatment is provided.
The prognosis is poor in cases with internal bleeding or
intracerebral haemorrhage, and also in patients with previous
haematological illnesses or renal insufficiency.
Death is uncommon (Basehore & Mowry, 1987).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
No direct cardiotoxic effects have been
described in man.
9.4.2 Respiratory
No effects have been reported but theoretically
haemoptysis would be a possibility.
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Intracerebral haemorrhage has been
described as a secondary complication
(Basehore & Mowry, 1987).
9.4.3.2 Peripheral nervous system
No effects have been reported.
9.4.3.3 Autonomic nervous system
No effects have been reported.
9.4.3.4 Skeletal and smooth muscle
Muscular haematoma may occur,
especially on the elbows, knees and buttocks
(CCINFOdisc, 1989).
9.4.4 Gastrointestinal
Haematemesis and melena may occur. Abdominal
and flank pain have been reported after
intra-abdominal bleeding (Jones et al., 1984).
9.4.5 Hepatic
Although the liver is the site of metabolism of
brodifacoum, no observable clinical effects are
apparent, except coagulopathy.
9.4.6 Urinary
9.4.6.1 Renal
Haematuria may be clinically evident
or detected only by the laboratory (Jones et
al., 1984). Urinary tract haemorrhage has
been reported (Hollinger & Pastoor,
1993).
9.4.6.2 Others
No data available.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
Petechial rashes may be seen.
9.4.9 Eye, ears, nose, throat: local effects
Epistaxis and gum bleeding.
9.4.10 Haematological
Coagulation is impaired after ingestion of
significant quantities of brodifacoum. The usual
haematological symptoms are: gum bleeding, epistaxis,
ecchymoses, haematoma, and haematuria; there may be
internal bleeding.
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
No data available.
9.4.12.2 Fluid and electrolyte disturbances
No data available.
9.4.12.3 Others
No data available.
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
One case of abortion has been reported by
Lipton & Klaas (1984). Data on breast milk secretion
are not available and therefore breast feeding should
not be recommended. Individuals with bleeding
diatheses may be at higher risk.
9.5 Others
9.6 Summary
10. MANAGEMENT
10.1 General principles
In all cases of brodifacoum ingestion, the patient
(especially children) should be clinically monitored by daily
laboratory studies for at least 4 or 5 days.
Gastric lavage or emesis is indicated if the ingested amount
is uncertain or possibly toxic. Administration of activated
charcoal and cathartics is useful. If initial coagulation
disorders have been detected, the prothrombin time should be
monitored (for at least 60 days). The treatment is based
upon the administration of vitamin K1. Fresh frozen plasma
or whole blood should be given in severe cases of poisoning
in order to provide clotting factors.
Clinical observation should be very strict in order to
diagnose any possibility of bleeding.
10.2 Life supportive procedures and symptomatic treatment
Life-saving procedures are indicated if serious
complications arise.
Symptomatic treatment for bleeding is the administration of
fresh frozen plasma or fresh whole blood for correcting
coagulopathy.
10.3 Decontamination
If more than 24 hours have elapsed decontamination
measures are not effective.
Emesis or gastric lavage may be effective if attempted within
3 to 6 hours of ingestion.
Administration of activated charcoal and cathartics may be
considered.
10.4 Enhanced elimination
Enhancement of elimination is not indicated.
10.5 Antidote treatment
10.5.1 Adults
Phytomenadione (vitamin K1) should be
administered. If prothrombin time is significantly
reduced vitamin K1 should be administered
intravenously starting with 10 mg each 6 h (40
mg/day). The intramuscular route may be chosen under
appropriate clinical settings. The dosage should be
adjusted according to the prothrombin time.
10.5.2 Children
Indication and dosage is the same as in adults.
10.6 Management discussion
There is no consensus on the appropriate administration
schemes for vitamin K. Some authors give oral vitamin K
prophylactically in cases where ingestion is uncertain. The
usual route for treatment is intravenous, although in
somecases it has been given intramuscularly or
subcutaneously. The use of phenobarbital as a hepatic
microsomal enzyme inducer is controversial.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
Lipton & Klaas (1984) reported a case of a psychotic
31-year-old woman who ingested thirty 50 g packages of
commercial rodenticide containing brodifacoum. Five days
later, she was admitted to hospital with slash marks on both
wrists and blood clots in the gums. Laboratory results were:
haemoglobin 12.2 g/dL, Ht 37.9%, WBC 5.6/mm3, platelets
390.000, prothrombin time 72s (control = 12 s), activated
partial thromboplastin time greater than 100s (normal = 25 to
35s), fibrinogen 380 mg/dL. Urinalysis was negative for
blood and protein. Treatment was started with 25 mg of
vitamin K1 and fresh frozen plasma. The dose of vitamin K1
was increased to 125 mg per day after little improvement in
the prothrombin time. Phenobarbital was also administered as
an hepatic enzyme inducer. This case was complicated by a
complete and apparently spontaneous abortion.
Basehore & Mowry (1987) reported a patient who, following
chronic ingestion of the rodenticide, was admitted to
hospital with nose-bleeding, haematuria and multiple
ecchymoses. Laboratory studies showed: prothrombin time = 60
seconds (normal = 12.4), partial prothrombin time = 79.2
seconds (normal = 27), bleeding time 15 minutes. Treatment
was started with vitamin K1 and frozen fresh plasma. The
patient was discharged 9 days afterwards with almost normal
prothrombin time and partial thromboplastin time and
prescribed oral phytomenadione. Three weeks later, he
presented with status epilepticus; intradural haemorrhage was
diagnosed by CAT-Scan. Prothrombin time was again over 60
seconds. He deteriorated and died 4 hours after
admission.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
As any other pesticide, these rodenticides should be
kept out of the reach of children and irresponsible persons.
Use of superwarfarin, such as brodifacoum, should be
restricted to agronomical application and not widely
advertised or sold without restriction for household
use.
12.2 Other
No data available.
13. REFERENCES
Bachman KA & Sullivan TJ (1983) Dispositional and
pharmacodynamics characteristics of brodifacoum in
warfarin-sensitive rats. Pharmacology, 27(5): 251-288.
Basehore LM & Mowry JM (1987) Death following ingestion of
superwarfarin rodenticide: A case report. Vet & Human Toxicology,
29 (6 Dec.).
Budivari S (ed) (1996) The Merck Index, An Encyclopedia of
Chemicals, Drugs and Biologicals, 12th ed., Merck & Co., Inc.,
Whitehouse Station, NJ, USA.
CCINFO (1989)
Chong L, Chan W, Ho C (1986) A case of superwarfarin poisoning.
Scand J Haematol, 36: 314-315.
Fitzgerald K & Bronstein AC (1987). Comparison of first and
second generation anticoagulant rodenticide poisonings: fourteen
canine cases. Abstracts from the AACT/AAPCC/ABMT/CAPCC scientific
meetings. Sept. 27.
Hoffman RS, Smilkstein MJ, Goldfrank LR (1988) "Evaluation of
coagulation factor abnormalities in long-acting anticoagulant
overdose". J Toxicol Clin Toxicol, 26 (3-4): 48.
Hollinger B & Pastoor TP (1993). Case management and plasma
half-life in a case of brodifacoum poisoning. Arch Intern Med, 153
(16):1925-8
Jones E, Growe GH, Naiman SC (1984) Prolonged anticoagulation in
rat poisoning. JAMA, 252: 3005-3007.
ICI Ltd. - Technical Information
Lipton RA & Klaas EM (1984) Human ingestion of superwarfarin
rodenticide resulting in a prolonged anticoagulant effect. JAMA,
Dec 7., vol. 252, No. 21.
Park BK et al. (1979) A study of the effect of anticoagulants on
(3H) vitamin K1 metabolism and prothrombin complex activity in the
rabbit. Biochem Pharmacol, 28 (8): 1323-9.
Smolinske SC, Sherger DS, Kearus PS & Rumack BH (1987) Long
acting coagulant rodenticide ingestion in children. Vet & Hum
Toxicology, 29 (6 Dec.).
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors: Dr C. Dell Acqua
Dr J. Pronczuk
CIAT
Piso 7, Hospital de Clinicas
Av. Italia s/n
Montevideo
Uruguay
Tel: 598-2-470300 and 598-2-804000
Fax: 598-2-470300
Date: February 1990
Peer
review: London, United Kingdom, March 1990.
Informal Consultation, post JMPR meeting, September
1991
Original
Editor: Dr S. Chaplin, Newcastle-upon-Tyne, United Kingdom
(September 1991)
Update: International Programme on Chemical Safety (May
1992)
Editor: Mrs J. Duménil, International Programme on Chemical
Safety
(July 1999)
See Also:
Toxicological Abbreviations
Brodifacoum (HSG 93, 1995)


