
Phenol
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Main brand names, main trade names |
| 1.6 Main manufacturers, main importers |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Colour |
| 3.3.2 State/form |
| 3.3.3 Description |
| 3.4 Hazardous characteristics |
| 4. USES |
| 4.1 Uses |
| 4.1.1 Uses |
| 4.1.2 Description |
| 4.2 High risk circumstance of poisoning |
| 4.3 Occupationally exposed populations |
| 5. ROUTES OF EXPOSURE |
| 5.1 Oral |
| 5.2 Inhalation |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Other |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination and excretion |
| 7. TOXICOLOGY |
| 7.1 Mode of action |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.2.4 Workplace standards |
| 7.2.5 Acceptable daily intake (ADI) |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 8. TOXICOLOGICAL AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Material sampling plan |
| 8.1.1 Sampling and specimen collection |
| 8.1.1.1 Toxicological analyses |
| 8.1.1.2 Biomedical analyses |
| 8.1.1.3 Arterial blood gas analysis |
| 8.1.1.4 Haematological analyses |
| 8.1.1.5 Other (unspecified) analyses |
| 8.1.2 Storage of laboratory samples and specimens |
| 8.1.2.1 Toxicological analyses |
| 8.1.2.2 Biomedical analyses |
| 8.1.2.3 Arterial blood gas analysis |
| 8.1.2.4 Haematological analyses |
| 8.1.2.5 Other (unspecified) analyses |
| 8.1.3 Transport of laboratory samples and specimens |
| 8.1.3.1 Toxicological analyses |
| 8.1.3.2 Biomedical analyses |
| 8.1.3.3 Arterial blood gas analysis |
| 8.1.3.4 Haematological analyses |
| 8.1.3.5 Other (unspecified) analyses |
| 8.2 Toxicological analyses and their interpretation |
| 8.2.1 Tests on toxic ingredient(s) of material |
| 8.2.1.1 Simple qualitative test(s) |
| 8.2.1.2 Advanced qualitative confirmation test(s) |
| 8.2.1.3 Simple quantitative method(s) |
| 8.2.1.4 Advanced quantitative method(s) |
| 8.2.2 Tests for biological specimens |
| 8.2.2.1 Simple qualitative test(s) |
| 8.2.2.2 Advanced qualitative confirmation test(s) |
| 8.2.2.3 Simple quantitative method(s) |
| 8.2.2.4 Advanced quantitative method(s) |
| 8.2.2.5 Other dedicated method(s) |
| 8.2.3 Interpretation of toxicological analyses |
| 8.3 Biomedical investigations and their interpretation |
| 8.3.1 Biochemical analysis |
| 8.3.1.1 Blood, plasma or serum |
| 8.3.1.2 Urine |
| 8.3.1.3 Other fluids |
| 8.3.2 Arterial blood gas analyses |
| 8.3.3 Haematological analyses |
| 8.3.4 Interpretation of biomedical investigations |
| 8.4 Other biomedical (diagnostic) investigations and their interpretation |
| 8.5 Overall interpretation of all toxicological analyses and toxicological investigations |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin exposure |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 Central nervous system (CNS) |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 Skeletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Other |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ear, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reaction |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Other |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Life supportive procedures and symptomatic/specific treatment |
| 10.3 Decontamination |
| 10.4 Enhanced elimination |
| 10.5 Antidote treatment |
| 10.5.1 Adults |
| 10.5.2 Children |
| 10.6 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from the literature |
| 12. ADDITIONAL INFORMATION |
| 12.1 Specific preventative measures |
| 12.2 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
PHENOL
International Programme on Chemical Safety
Poisons Information Monograph 412
Chemical
1. NAME
1.1 Substance
Phenol
1.2 Group
Hydrocarbons, cyclic, alcohol
1.3 Synonyms
acidum carbolicum;
acidum phenolicum;
acidum phenylicum;
baker's P & S liquid ointment;
benzaphenol;
benzene phenol;
benzenol carbolic acid;
carbolic acid;
hydroxybenzene (IUPAC);
monohydroxybenzene;
monophenol;
NCI-C50124;
Oxybenzene;
phenic acid;
phenol alcohol;
phenyl hydrate;
phenyl hydroxide;
phenylic acid;
phenylic alcohol
1.4 Identification numbers
1.4.1 CAS number
108-95-2
1.4.2 Other numbers
NIOSH number: SJ 3325000
Hazchem code: 2X
DOT: 1671/2312/2821
1.5 Main brand names, main trade names
1.6 Main manufacturers, main importers
2. SUMMARY
2.1 Main risks and target organs
Phenol exerts a marked corrosive action on any tissue of
contact when ingested, inhaled or after skin exposure. Its
cellular uptake is both rapid and passive due to its
lipophilic character, and signs of systemic toxicity develop
soon after exposure. Phenol's main target organs are the
liver and kidney. It may also effect the respiratory and
cardiovascular systems.
2.2 Summary of clinical effects
After ingestion phenol produces burning pain and white
necrotic lesions in the mouth, oesophagus and stomach,
vomiting and bloody diarrhoea. After skin exposure, pain is
followed by numbness and the skin becomes blanched. The
systemic clinical effects of phenol are independent on the
route of exposure, they include: headache, dizziness,
hypotension, ventricular arrhythmia, shallow respiration,
cyanosis, pallor; excitation and convulsions may occur
initially, but it is quickly followed by unconsciousness. A
fall in body temperature and pulmonary oedema may occur.
Methemoglobinemia and hemolytic anemia have been reported
occasionally. The most important effects in short-term animal
studies are neurotoxicity, liver and kidney damage and
respiratory effects. The available data do not suggest a
strong potential for cumulative health effects from chronic
exposure.
2.3 Diagnosis
Phenol poisoning can be recognised by the characteristic
acrid odour on the breath or it can be detected in the urine,
which may be dark coloured. Therefore a urine sample and also
a blood sample should be taken. Phenol is corrosive to mucous
membranes, eyes and causes burns to the skin.
2.4 First aid measures and management principles
EYES: Immediately flush eye(s) with water (preferably
tepid) for at least 15 minutes.
INHALATION: Remove patient from the area of exposure to fresh
air. If unconscious, intubate with a cuffed endo-tracheal
tube and ventilate mechanically if necessary. If conscious,
place in Trendelenburg, left lateral position with succion
equipment ready. Treat convulsions, arrhythmias and
methaemoglobinemia according to Treatment Guides.
INGESTION: Do not induce vomiting. Do not dilute since it may
increase absorption. Gastric aspiration or lavage should be
weighed with risk. Give polyethylene glycol solution or
activated charcoal with sorbitol.
DERMAL: Wearing protective gloves remove contaminated
clothing immediately, flush excess chemical off the skin with
water if it is the only available liquid, but preferably
wash with polyethyleneglycol molecular weight 300 (Macrogol
300), isopropyl alcohol, industrial methylated spirits or
Golytely for at least 30 minutes.
In all cases of exposure the patient should be transferred to
a hospital as soon as possible.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Natural, obtained from coal tar, or as a degradation
product of benzene. Synthetic, made by fusing sodium
benzenesulfonate with NaOH, or by heating monochlorobenzene
with aqueous NaOH under high pressure (Windholz, 1983)
3.2 Chemical structure
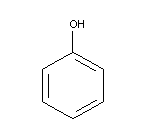 Chemical formula: C6H6O
Molecular weight: 94.11
3.3 Physical properties
3.3.1 Colour
Colourless or white (WHO, 1994).
3.3.2 State/form
Acicular-crystals, or white crystalline mass
(WHO, 1994).
3.3.3 Description
The crystals turn pink or red on exposure to
air and light, hastened in presence of alkalinity
(Windholz, 1983). Phenol has an acrid smell and a
sharp burning taste.
In the molten state, it is a clear, colourless liquid
with a low viscosity. It is soluble in most organic
solvents, and solubility is limited in aliphatic
solvents. Phenol's solubility. In water is limited
at room temperature, above 68°C it is entirely water
soluble. The vapour is heavier than air (WHO,
1994).
Melting point is 43°C, the commercial product has an
impurity that increases the melting point (Windholz,
1983).
Boiling point: 181.75°C
Flash point: 80°C (closed cup); 79°C to 85°C (open
cup)
Relative vapour density: 3.24 (WHO, 1994).
Explosive limits are 1.7% to 8.6% (Allen, 1991).
Vapour pressure at 20°C is 47 Pascal
pKa 10,0 at 25°C
pH of aqueous solutions is approximately 6.0
It is liquefied by mixing with about 8% water
(Windholz, 1983).
3.4 Hazardous characteristics
Autoignition temperature is 715°C. Phenol is a volatile,
combustible solid that when heated gives off flammable
vapours and carbon dioxide. Explosive or violent reactions
occur with acetylaldehyde; aluminium chloride plus
nitrobenzene; aluminium chloride-nitromethane; butadine;
calcium hypochlorite; peroxomonosulphuric acid;
peroxodisulphuric acid; sodium nitrate; and sodium
nitrate-trifluoroacetic acid (Allen, 1991).
Phenol is sensitive to oxidising agents. Splitting of the
hydrogen atom from the phenolic hydroxyl group is followed by
resonance stabilisation of the resulting phenyloxy radical.
The radical that is formed can be further oxidised. This
makes phenol suitable as an antioxidant, functioning as a
radical trapping agent (WHO, 1994). The taste threshold is
0.3 mg/L (0.00003%) in water (WHO, 1994).
4. USES
4.1 Uses
4.1.1 Uses
4.1.2 Description
The main use of phenol is as a feedstock for
phenolic resins, bisphenol A and caprolactam (an
intermediate in the production of nylon-6). It is used
in the manufacture of many products including
insulation materials, adhesives, lacquers, paint,
rubber, ink, dyes, illuminating gases, perfumes, soaps
and toys (IARC, 1989; WHO, 1994). Also used in
embalming and research laboratories. It is a product
of the decomposition of organic materials, liquid
manure, and the atmospheric degradation of
benzene.
It is found in some commercial disinfectants,
antiseptics, lotions and ointments. Phenol is active
against a wide range of microorganisms, and there are
some medical and pharmaceutical applications including
topical anaesthetic and ear drops, sclerosing agent.
It is also used in the treatment of ingrown nails in
the "nail matrix phenolization method" (Kimata et al.,
1995). Another medical application of phenol is its
use as a neurolytic agent, applied in order to relieve
spasms and chronic pain (Wood, 1978; Geller,
1997).
It is used in dermatology for chemical face peeling.
4.2 High risk circumstance of poisoning
Deliberate, accidental or occupational exposure.
4.3 Occupationally exposed populations
Workers involved in the production of phenol by the
cumene process, or the production of phenol from
chlorobenzene are at risk. Phenol may be emitted into the air
during the processing of phenolic resins, production of
phenol and phenol derivatives, production of caprolactam,
production of cokes and insulation materials. Wood workers,
and workers at plywood plants are at risk of exposure. Phenol
is used in iron and steel foundries - in the manufacture of
moulds or kernels, or during the operation of an electric
furnace in a steel factory and also in coal gasification and
liquefication plants, bakelite factories, and synthetic fibre
and fibrous glass wool factories. In creosote impregnation
plants, with highest exposure levels during the cleaning of
creosote warming chambers. In embalming situations, where the
embalming solution contains high phenol concentrations
(Allen, 1991; WHO, 1994).
In chemical accidents, responders or health staff may be
exposed by direct contact, by vomitus, or by off-gassing of
contaminated clothing.
5. ROUTES OF EXPOSURE
5.1 Oral
Phenol is readily absorbed from the gastrointestinal
tract (WHO, 1994).
5.2 Inhalation
Phenol is readily absorbed from the lungs (Allen,
1991).
5.3 Dermal
When spilt on the skin, intact or abraded, it is rapidly
absorbed and may lead to systemic poisoning (Brooks &
Riviere, 1996).
5.4 Eye
Phenol is absorbed through the mucous membranes of the
eye (WHO, 1994).
5.5 Parenteral
Therapeutic use: phenol can be administered by
intrathecal injection to relieve pain and spasticity (Geller,
1997), and has been used as a sclerosing agent.
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Phenol is rapidly absorbed through skin, lungs, and the
gastrointestinal tract.
Eight humans exposed to 6 to 20 mg/m3 by inhalation for 8
hours absorbed 70 to 80% of the phenol dose.
Skin absorption of phenol vapour (5 to 25 mg/m3) occurs
rapidly. The absorption in 8 humans exposed to phenol vapour
at concentrations of 6 to 20 mg/m3 , by skin only, for 6
hours was also 70 to 80%. Concentrations between 5 and 10%
phenol denature epidermal protein and this can therefore
partly prevent absorption. The phenol-protein complex is not
stable and by dissociation of phenol the substance may exert
its action over a period of time. In an in vitro study with
human abdominal skin, 10.9% of the dose was absorbed. The
period of exposure and the concentration of phenol are both
factors that determine the extent of absorption, but the area
of skin exposed affects the extent of absorption more than
the concentration. A single dose of 25 mg/kg body weight
dermally administered to rats, pigs and sheep was more than
95% absorbed (WHO, 1994).
6.2 Distribution by route of exposure
Rapidly distributed to all tissues in exposed animals.
After a single oral administration of 207 mg/kg phenol to
rats, the highest concentration ratios between tissue and
plasma were found in the liver (42%), followed by spleen,
kidney, adrenal, thyroid and lungs, with a peak tissue level
occurring after 0.5 hours.
In rabbits 15 minutes after an oral dose of 0.5 g/kg, the
highest concentrations of phenol were in the liver, followed
by the CNS, lungs and blood. After 82 minutes the phenol was
relatively uniformly distributed in all tissues (WHO,
1994).
6.3 Biological half-life by route of exposure
The half-life of conjugated phenol in humans is 1 hour
(Leikin & Paloucek, 1996-7), but there have also been
reported half-lives of 4 to 5 hours in humans (WHO,
1994).
6.4 Metabolism
After oral uptake of phenol, there is a large first-pass
metabolism. It is unclear whether phenol also undergoes first
pass pulmonary metabolism, there have been conflicting
results (Dickenson & Taylor, 1996). The liver, lungs and the
gastrointestinal mucosa are the most important sites of
phenol metabolism (WHO, 1994). Conjugation with glucuronic
acid to phenyl glucuronide and sulphation to phenyl sulphate,
have been shown to be major metabolic pathways in several
species. A shift from sulphation to glucuronidation was
observed in rats after increasing the phenol doses, which is
thought to be due to a saturation of the overall sulphation
process, by the limited availability of
3-phosphoadenosine-5-phosphosulfate. The formation of
sulphate and glucuronic metabolites occurs in the
hepatocytes, and then transported to the bile or back into
the blood (Ballinger et al, 1995). In vitro studies have
shown the formation of the reactive metabolites 4,4'-biphenol
and diphenoquinone by neutrophils and activated leukocytes.
Both in vivo and in vitro tests have shown covalent binding
of phenol to tissue and plasma proteins, some phenol
metabolites also bind to proteins (WHO, 1994).
6.5 Elimination and excretion
Urinary (renal) excretion is the major route of phenol
elimination in animals and humans. The rate of excretion
varies with different species, dose and route of
administration. Three men after an oral administration of
0.01 mg/kg phenol, excreted 90% of the dose in the urine
within 24 hours, mainly as phenyl sulfate and phenyl
glucuronide. A minor part is eliminated in the faeces and
expired air. Urinary excretion of humans exposed to phenol
vapour via inhalation or skin, occurred with an excretion
rate constant of k 0.2/hour. On oxidation to quinones the
metabolites may tint the urine green.
The half life is estimated to be between 1 and 4.5 hours with
52% eliminated unchanged in the urine (Leikin & Paloucek,
1996-7). The natural presence of phenols in food and drug
metabolites, makes biological monitoring impossible. A minor
part is eliminated in expired air and faeces. (Reynolds,
1993; Ellenhorn & Barceloux, 1988)
7. TOXICOLOGY
7.1 Mode of action
Cellular uptake of phenol is due to its lipophilic
character. It denatures proteins (WHO, 1994). Phenol is known
to disrupt disulphide bridges in keratin in the skin (Brooks
& Riviere, 1996).
In vitro studies have shown the formation of the reactive
metabolites 4,4'-biphenol and diphenoquinone by neutrophils
and activated leukocytes. Both in vivo and in vitro tests
have shown covalent binding of phenol to tissue and plasma
protein, some phenol metabolites also bind to proteins (WHO,
1994). It produces coagulation necrosis.
The acute lethality of phenol, associated with exposure to
high dose concentrations, is customarily attributed to a
depressant effect on the CNS.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
The lethal dose ranges from 1 g to
15 g (Reynolds, 1993). Ingestion of 4.8 g
resulted in death after 10 minutes in one
person (Anderson, 1869).
However, an adult survived a 26.7 g dose
ingestion after a 15-day stormy hospital
course without permanent sequellae (Haddad et
al., 1979).
Survival has been reported with up to 350
mg/kg orally (Christiansen & Klaman,
1996).
7.2.1.2 Children
Children have died after the
application of 5 % phenol compresses
(Ellenhorn, 1996).
7.2.2 Relevant animal data
LD50 (oral) rodent values ranged from 300 to
600 mg/kg body weight.
LD50 (dermal) values for rats and rabbits range from
670 to1400 mg/kg body weight respectively.
LC50 (8 hour) for rats by inhalation was more than 900
mg/m3 (WHO, 1994).
In two multiple dose rat studies, NOAEL values
obtained were 40 mg/kg/day and 60 mg/kg /day and the
LOAEL values were 53 mg/kg/day and 120 mg/kg/day. In a
mouse study the NOAEL was 140 mg/kg/day and the LOAEL
was 280 mg/kg/day (WHO, 1994).
Chronic vapour exposures in rats ( 0.02 to 1ppm for 2
months) produced changes in the blood enzyme activity
and time for excitation of extensor muscles. At higher
exposures, phenol may lead to decrease in body weight.
In various animal species the inhalation of phenol
affected the lungs by causing hyperaemia, infarcts,
pneumonia, purulent bronchitis and hyperplasia of the
peribronchial tissues (WHO, 1994). There does not
appear to be a strong potential for cumulative health
effects from chronic exposure (WHO, 1994)
7.2.3 Relevant in vitro data
No data available.
7.2.4 Workplace standards
OSHA PEL: TWA 5 ppm (skin)
ACGIH TLV: TWA 5 ppm (skin)
IDLN: 100 ppm
DFG MAK: 5 ppm (19 mg/m3)
NIOSH REL: TWA 20 mg/m3; CL 60 mg/m3/15 minutes
(Sax & Lewis, 1989)
In Britain the occupational exposure standard is 19
mg/m3 (long term) and 38 mg/m3 (short term). In the
United States the permissible level is 19 mg/m3 and
the recommended is 20 mg/m3 (long-term) and the
maximum short term is 60 mg/m3 (Reynolds,
1993).
7.2.5 Acceptable daily intake (ADI)
A Task Group derived a tolerable daily intake
(TDI) using the lowest NOAEL's for kidney and
developmental effects in rats which is in the range of
12 to 40 mg/kg body weight /day. With an uncertainty
factor of 200, the range of 60 to 200 µg/kg/day was
recommended as the upper limit of the TDI (WHO,
1994)
The estimated maximal total daily intake of phenol for
a 70 kg individual is calculated to be 0.1 mg/kg body
weight per day (WHO, 1994).
However, according to IRIS (Integrated Risk
Information Sytem, 1996), the reference dose (Rfd) is
0.6 mg/kg/day, using the NOAEL (reduced fetal body
weight in rats) of 60 mg/kg/day, with an uncertainty
factor (Uf) of 100.
7.3 Carcinogenicity
An IARC review in 1989 found that the carcinogenicity
evidence for phenol was inadequate (group 3) (WHO, 1994). US
EPA classifies phenol in group D.
Two-stage carcinogenicity studies have shown that phenol,
applied repeatedly to mouse skin, has promoting activity
(WHO, 1994).
7.4 Teratogenicity
Phenol has been identified as a developmental toxicant
in studies with rats and mice (WHO, 1994).
7.5 Mutagenicity
The majority of bacterial mutagenicity tests have
demonstrated negative results. In mammalian cells, mutations,
chromosomal damage and DNA effects have been observed. Phenol
has shown no effect on intercellular communication in
cultured mammalian cells. The induction of micronuclei in
bone marrow cells of mice has been observed in some studies
at high doses. No micronuclei were observed in mice studies
at lower dose (IARC, 1989; WHO, 1994).
7.6 Interactions
No data available.
8. TOXICOLOGICAL AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method(s)
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall interpretation of all toxicological analyses and
toxicological investigations
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
After swallowing a significant concentrated
dose, an intense burning of the mouth and throat is
felt (necrosis of the skin and mucous membranes of the
throat), and pain in the abdominal area, with
gastrointestinal irritation including nausea,
vomiting, sweating and diarrhoea. The face is usually
pale and sweaty, the pupils may be contracted or
dilated; cyanosis is usually marked; the pulse is
usually weak and slow, occasionally it may be racing;
respiration may initially be increased in rate, but
later decreased in rate and magnitude; body
temperature may fluctuate. Excitation may occur
initially, but it is quickly followed by
unconsciousness. Occasionally isolated twitching of
muscles or convulsions may be observed. Acute renal
failure can develop resulting from systemic
absorption. Ingestion is usually fatal (Foxall et al.,
1989; Allen, 1991; Reynolds, 1993; WHO, 1994).
9.1.2 Inhalation
Phenol vapours are irritating to the upper
respiratory tract. Ocular and nasal irritation,
tremors and incoordination were reported in rats
exposed to phenol via inhalation to 906 mg/m3 for 8
hours (WHO, 1994). Wheezing may occur. Other symptoms
associated with inhalation include anorexia, weight
loss, headache, salivation, vertigo (WHO, 1994) and
dark urine (dark/brown/green) (Leikin & Paloucek,
1996-7).
9.1.3 Skin exposure
Phenol is a local anaesthetic, so upon initial
contact, no pain is felt. By the time pain is felt,
serious burns and absorption through the skin may have
occurred (Allen, 1991). Local damage to the skin
includes erythema, inflammation, and necrosis. The
effects are worse when the application sites are
bandaged (WHO, 1994). A white, brown or red
discolouration of the skin may occur (Leikin &
Paloucek 1996-7). Systemic intoxication can occur from
absorption (WHO, 1994); roughly 50 % of all reported
cases have a fatal outcome (Horch et al., 1994).
In the Kligman maximization test phenol did not cause
sensitisation in 24 human volunteers (Kligman,
1966).
9.1.4 Eye contact
Phenol is an eye irritant (WHO, 1994).
Solutions can be corrosive to the eyes, and can cause
severe ocular damage including corneal
opacification.
9.1.5 Parenteral exposure
After intraperitoneal and subcutaneous doses of
phenol, tremors, convulsions, coma and death have been
reported (WHO, 1994). Injection of 30 mL of 89% phenol
instead of 10% for celiac plexus nerve block resulted
in coma, hypotension, respiratory insufficiency and
ventricular tachycardia (Christiansen & Klaman,
1996).
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
Severe gastrointestinal irritation,
cardiovascular, CNS and respiratory effects,
hypothermia and decreased body weight. Brown or
discoloured urine has also been observed in chronic
poisoning (Goldfrank & Bresnitz, 1990).
Repeated oral exposure for several weeks (estimated
intake 10 to 240 mg/day) resulted in mouth sores,
diarrhea and dark urine. Examination 6 months after
the exposure revealed no residual effects (Baker et
al., 1978).
9.2.2 Inhalation
There does not appear to be a strong potential
for cumulative health effects from chronic exposure
(WHO, 1994)
9.2.3 Skin exposure
Chronic doses may result in onychronosis
(yellowing of the skin) and skin eruption (WHO, 1994).
Death has been observed from repeated application of
small doses (Olson, 1994).
In former times phenol 5 to 10% was used as a skin
disinfectant giving rise to the "carbolic marasmus"
characterized by anorexia, headache, vertigo,
salivation, dark urine and increased skin and scleral
pigmentation (Merliss, 1972).
9.2.4 Eye contact
No data available
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Hypotension, renal failure, apnea, laryngeal oedema and
ARDS can develop soon after exposure leading to death (WHO,
1994). Coma and seizures usually occur within minutes to a
few hours after exposure. Toxic effects may be delayed up to
18 hours.
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Heart rate at first increases and then becomes
slow and irregular. Blood pressure at first increases
slightly, then falls markedly (hypotension) (WHO,
1994). Cardiovascular collapse, atrial and ventricular
arrythmias have been reported (Leikin & Paloucek,
1996-7). Deep venous thrombosis has been reported
following injection of phenol (WHO, 1994).
Cardiac dysrhythmias have been observed in skin
peeling and nerve blockade (Forrest & Ramage, 1987;
Lober, 1987; Sorkin, 1988; Gaudy et al., 1993; Lalanne
et al., 1994; Zamponi & French, 1994).
9.4.2 Respiratory
Respiration may initially be increased in rate,
but later decreased in rate. Pulmonary oedema,
wheezing, coughing, dyspnea, pneumonia are common
signs (Leikin & Paloucek, 1996-7). The cause of death
from phenol exposure is often respiratory failure
(WHO, 1994).
9.4.3 Neurological
9.4.3.1 Central nervous system (CNS)
Initial signs and symptoms include
headache, dizziness and tinnitus. Seizures,
coma, respiratory depression and death may
ensue quickly. Coma and seizures usually
occur within minutes to a few hours after
exposure or a delay of up to 18 hours. Phenol
may also cause demyelination and axonal
damage of peripheral nerves (WHO,
1994).
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
A decrease in body temperature has
been reported (WHO, 1994).
9.4.3.4 Skeletal and smooth muscle
Locomotor activity reduced at 244 mg
phenol/kg body weight in female Fischer-344
rats (WHO, 1994). Chronic exposure in rats
lead to changes in the time for excitation of
extensor muscles.
9.4.4 Gastrointestinal
Symptoms include diarrhoea, salivation,
vomiting, ulceration and haemorrhage (Leikin &
Paloucek, 1996-7). Corrosive damage may involve the
entire gastrointestinal tract.
9.4.5 Hepatic
Hepatic necrosis was observed in two(out of
six) female Fischer-344 rats when given 244 mg
phenol/kg body weight (WHO, 1994).
9.4.6 Urinary
9.4.6.1 Renal
Renal failure has been reported in
acute poisoning. Urinalysis may reveal a
green to brown discolouration of the urine
with albuminuria. Nephritis is reported
(Leikin & Paloucek, 1996-7).
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Increased incidence of preimplantation loss and
early postnatal death in the offspring of rats (WHO,
1994).
9.4.8 Dermatological
Chronic doses may result in ochronosis
(yellowing of the skin) and skin eruption (WHO,
1994).
9.4.9 Eye, ear, nose, throat: local effects
The fumes are irritating to the eyes and
affects the pupil's response to light (miosis) (WHO,
1994). Solutions can be corrosive to the eyes, and can
cause severe ocular damage including corneal
opacification. Lymph production in the conjunctiva may
be increased and will leave the cornea white and
hypesthetic (Jaeger, 1987).
Necrosis of the mucous membranes of the throat.
Phenol applied to the inner ear round window of
Sprague-Dawley rats caused morphological damage to the
organ of Corti in the basal coil. The outer hair cells
appeared to be more sensitive to phenol and as a
result of the damage, impairment of inner ear function
was noted which was permanent for higher frequencies.
One experiment in female mice lead to an increase in
ear thickness (WHO, 1994)
9.4.10 Haematological
Heinz body haemolytic anaemia and
hyperbilirubinemia have been reported occasionally
(WHO, 1994).
9.4.11 Immunological
There are no studies in humans. For four weeks
groups of five male CD-1 mice were given drinking
water containing 0, 4.7, 19.5 or 95.2 mg phenol/L.
Total and differential leukocyte counts were
unaffected. The highest dose suppressed the
stimulation of cultured splenic lymphocytes by the
B-cell mitogen lipopolysaccharide, the T-cell mitogen
phytohaemagglutinin, and the T and B-cell mitogen
pokeweed, but not by concanavatin. Suppression of the
animal's antibody production in response to a
T-cell-dependent antigen, occurred at the mid and high
doses (WHO, 1994).
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Metabolic acidosis (WHO, 1994)
9.4.12.2 Fluid and electrolyte disturbances
Fluid loss secondary to burns or shock.
9.4.12.3 Others
No data available.
9.4.13 Allergic reaction
No data available.
9.4.14 Other clinical effects
No data available
9.4.15 Special risks
Phenol has been shown to be a developmental
toxicant in rats and mice (WHO, 1994).
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
When spilt on the skin or in the eyes, there should be
an immediate washing with water (preferably tepid) for at
least 10 minutes. If available wash with polyethyleneglycol
molecular weight 300 (Macrogol 300), isopropyl alcohol,
industrial methylated spirits or Goletely (PEG 3550) for at
least 30 minutes (Horch et al., 1994).
Do not induce vomiting. Dilution may increase absorption.
Gastric lavage should be carefully weighed against the risk
of complications. Recommended gastric lavage fluids are
polyethylene glycol, water, following administration of
activated charcoal, or vegetable oils, such as olive oil,
castor oil or cottonseed oil (WHO, 1994).
Treatment is mainly supportive. If there is a systemic
intoxication, monitor the respiration and the level of
oxygenation, the blood pressure and ECG, the level of
methaemoglobinemia, the hepatic and renal functions. Control
convulsions and cardiac arrhythmias according to the
Treatment Guides.
10.2 Life supportive procedures and symptomatic/specific treatment
Make a proper assessment of airway, breathing,
circulation and neurological status.
Maintain a clear airway.
If unconscious give artificial respiration.
If the patient has breathing difficulties, put them in a
sitting position.
Monitor vital signs.
Monitor blood pressure and ECG.
Monitor fluid and electrolyte balance.
Monitor acid-base balance.
Control cardiac dysrhythmias with appropriate drug
regimen.
Control convulsions with appropriate drug regimen.
10.3 Decontamination
Remove and discard contaminated clothing.
Irrigate exposed eyes with copious amounts of water.
Wash skin with copious amounts of water or preferably if
available wash with polyethyleneglycol molecular weight 300
(Macrogol 300), isopropyl alcohol, industrial methylated
spirits or Goletely (PEG 3550) for at least 30 minutes.
Do not induce vomiting, empty stomach by aspiration followed
by polyethylene glycol or activated charcoal with
cathartic.
Endoscopy.
10.4 Enhanced elimination
If acute renal failure occurs in phenol poisoning,
dialysis should probably not be used alone, but in
conjunction with charcoal hemoperfusion. Without renal
failure the use of charcoal hemoperfusion may also be useful,
when the patient has been exposed to 15 to 20 g of phenol
(Christiansen & Klaman, 1996)
10.5 Antidote treatment
10.5.1 Adults
No antidote available.
10.5.2 Children
No antidote available.
10.6 Management discussion
No data available.
11. ILLUSTRATIVE CASES
11.1 Case reports from the literature
Occupational - Male
A 27-year-old male spilt 80% phenol on both knees and arrived
at the emergency department 30 to 60 minutes after the
spillage. Both legs had been washed with copious amounts of
water and he had also undergone further irrigation and
application of glycerin cream, but continued to be in pain.
Further irrigation with 6 litres of saline followed and then
it was suggested to irrigate with polyethylene glycol. By
mistake Golytely (PEG 3550) was used. The patient reported an
immediate soothing of the pain and his long term recovery was
favourable (Wahl et al., 1995).
Accidental Injection
A 50-year-old woman inadvertently received a 30 ml dose of
89% phenol (26.9 g, 0.44 mg/kg body weight). At 23 minutes
post- injection she was unresponsive, at 27 minutes she had
respiratory distress and was intubated. At 62 minutes she
developed shock and dopamine was started followed by
epinephrine and neosynephrine. At the same time she developed
ventricular tachycardia and was treated with lidocaine. 4
hours after the injection she was comatose with a blood
pressure of 70/58, while receiving large doses of
vasopressors. At 4.5 hours charcoal hemoperfusion was
started, and given for 6 hours and 20 minutes. Her clinical
status improved with the perfusion and she made a complete
recovery. The perfusion enhanced the elimination of free and
total phenol (Christiansen & Klaman, 1996).
Five Year Acute Exposure Study
A five year evaluation of acute exposure to phenol
disinfectant (26%) studied 80 cases with an age range of 1 to
78 years, 75% under 5 years old. There were 60 oral only
exposures, 7 dermal only, 12 oral/dermal and 1 inhalation.
65% were assessed at the emergency department and 33% were
admitted. 14% of oral exposures developed rapid CNS
depression, without seizures, and 2 patients developed coma
after ingestion. Burns occurred in 17 oral exposures and 5
dermal. 17 patients underwent endoscopy. Urine colour change
was noted in 5 patients following ingestion. There were no
cardiovascular complications, or oliguria and anuria. In all
cases there were complete recoveries (Spiller et al.,
1993).
Accidental Ingestion - Male Alcoholic, Addict
A report from the Invercargill hospital in New Zealand early
in 1997, reports a 29 year old male, alcoholic, addict, who
drank 30 mL of 88% phenol from a bottle he found (26.4 g/75
kg = 350 mg/kg). He had a cardiac arrest requiring DC
cardioversion, and developed renal failure secondary to
rhabdomolysis. He developed a denuded oesophagus, large,
superficial, antral ulceration and received continuous
haemofiltration and endoscopy. His urine output only
recommenced 14 days after the ingestion and his renal
function is expected to return to normal (NZNPIC, 1997).
Ingestion - Female
Fatal case reports include a 21 year old female who drank 10
to 20 g of phenol. She went into a deep coma with partial
areflexia, a heart rate of 140 and dilated pupils, and had a
cardiac arrest 60 minutes post ingestion. She received
repeated gastric lavage with water, glycerin and animal
charcoal, but died from pulmonary oedema and shock
(Stajduhar-Carie, 1968).
Occupational - Fatal Dermal Exposure
A 17-year-old male had 30% phenol (as industrial waste)
splashed on his face, neck and right trunk. He was washed
with water, but 30 minutes later he had a seizure and died.
His blood phenol level was 2.7 mg/dL. An autopsy showed red
areas to 15% of his skin area, and pulmonary oedema. (WHO,
1994)
Accidental Immersion
A man was partially submerged in a solution of 20% phenol in
dichloromethane for a few seconds. He immediately showered ,
but collapsed, his extremities were cold and he had 50% burns
to his body. He developed acute renal failure. Anuria
followed, with a rise in plasma creatinine, but treatment
with intravenous furosemide and haemodyalisis (daily for
seven days, then with decreasing intervals for a further 18
days), allowed adequate urinary volumes to be produced. He
also had respiratory distress, treated in intensive care. A
spill of 80-100% phenol on the hip, thigh and scrotum with a
contact duration of 20 minutes lead to death, while a
reported spill of 43.5% to the lower half of the body
resulted in shock. A spill of 4 to 5 litres on the upper
half of the body of 78%, with a contact duration of 2 to 5
minutes resulted in a coma (WHO, 1994).
Contaminated Drinking Water
A chemical spill in Wisconsin in 1974 contaminated
groundwater which was being used as drinking water. One month
later there were complaints of health effects, and six months
later medical histories were taken from 100 people. The
estimated daily exposure was 10 to 240 mg phenol/person. A
significantly significant increase in diarrhoea, mouth sores,
dark urine and burning of the mouth was found.
Gastrointestinal illnesses were also reported in North Wales,
when a river used for the preparation of drinking water was
contaminated with phenol, and when chlorinated, various
chlorophenols formed (WHO, 1994)
Accidental Ingestion
An outpatient was mistakenly given in a measured container
one ounce of 89 % phenol. The patient immediatly clutched her
throat and collapsed. Within 30 minutes she had an
unrecordable blood pressure and sustained respiratory arrest.
During endotracheal intubation in the ED the mouth and
hypopharynx were noted to be white. A "lamp oil" odour was
noted while ventilating the patient with a bag mask. The
patient experienced ventricular tachycardia one hour after
ingestion and resuscitation was effected by cardioversion.
Over the first 24 hours she exhibited ventricular arrhythmia,
seizures and metabolic acidosis. (Haddad et al., 1979)
Child Exposure
A 10-year-old boy had a solution of 40% phenol and 0.8%
croton oil in hexachlorophene soap and water applied to a
large nevus covering his 1.9% of his body surface whilst
under anaesthesia. After 55 minutes of treatment, multifocal
and coupled premature ventricular complexes were detected by
ECG. An intravenous infusion of 250 mg bretylium sulfate
suppressed the dysrhythmia (WHO, 1994).
12. ADDITIONAL INFORMATION
12.1 Specific preventative measures
Phenol should be kept in a tightly closed container, in
a cool, dry place, away from heat, flame and oxidising
agents. It is light sensitive and should be kept in the dark
(WHO, 1994).
Protective clothing should will be appropriate to the amount
and form of the phenol being handled. It should be handled
wearing an approved respirator; viton, butyl rubber or
neoprene gloves (not nitrile or PVA gloves), safety goggles
and other protective clothing. Safety showers and
polyethylene glycol 300 should be near where phenol is being
handled.(Allen, 1991)
12.2 Other
Phenol is not likely to persist in air, soil or sewage,
sea or surface water. It readily reacts photochemically, is
rapidly biodegraded aerobically to mainly carbon dioxide, and
anaerobic biodegradation occurs also at a slower rate. Low
removal rates of phenol in ground water and soil may occur
e.g. following spills, with subsequent inhibition of the
microbial populations. Phenol is toxic to aquatic organisms:
the lowest EC50 for water organisms is estimated to be 3.1
mg/L. The lowest chronic NOEC is estimated to be 0.2
(g/L).
13. REFERENCES
Allen R, Ed. (1991) Chemical Safety data Sheets, Volume 4b:
Toxic Chemicals (m-z ). Royal Society of Chemistry, Cambridge.
Printed by Staples Printers Rochester Ltd, Kent.
Anderson W (1869) fatal misadventure with carbolic acid. Lancet,
1: 179
Baker EL, Landrigan PJ, Bertozzi PE, Field PH, Basteyns BJ,
Skinner HG (1978) Phenol poisoning due to contaminated drinking
water. Arch Environ Health, 83: 98-94
Ballinger LN, Cross SE & Roberts MS (1995) Availability and Mean
Transit Times of Phenol and its Metabolites in the Isolated
Perfused Rat Liver: Normal and Retrograde Studies Using Tracer
Concentrations of Phenol. J Pharm Pharmacol, 47: 949-956.
Brooks JD & Riviere JE (1996) Quantitative Percutaneous Absorption
and Cutaneous Distribution of Binary Mixtures of Phenol and
para-Nitrophenol in Isolated Perfused Porcine Skin. Fundamental
and Applied Toxicology, 32: 233-243.
Christiansen RG & Klaman RN (1996) Successful Treatment of Phenol
Poisoning With Charcoal Hemoperfusion. Veterinary and Human
Toxicology, 38(1): 27-28
Dickenson A & Taylor G (1996) Pulmonary First-Pass and
Steady-State Metabolism of Phenols. Pharmaceutical Research,
13(5): 744-748.
Forrest T & Ramage DT (1987) Cardiac dysrhythmia after subtrigonal
phenol. Anaesthesia, 42: 77-778
Foxall PJD, Bending MR, Gartland KPR & Nicholson JK (1989) Acute
renal failure following accidental cutaneous absorption of phenol:
application of NMR urinalysis to monitor the disease process.
Human Toxicol, 9: 491-496
Gaudy JH, Tricot Z, Sezeur A (1993) [Serious heart rate disorders
following perioperative splanchnic phenol nerve block]. Cand J
Anaesth, 40: 357-359 (in French)
Geller AS (1997) Botulinum Toxin A. Archives of Physical Medicine
and Rehabilitation, 78: 233
Goldfrank LR & Bresnitz EA (1990) Toxicologic emergencies, 4th ed,
East Norwalk, Appleton & Lange
Haddad LM, Dimond KA, Schweistris JE (1979) Case report: phenol
poisoning. Burns, 20: 45-50
Horch R, Spilker G, Stark GB (1994) Phenol burns and intoxication.
Burns, 20: 45-50
IARC (1989) Phenol. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research
on Cancer, pp 263-287 (IARC Monographs on the evaluation of
carcinogenic risks to humans, volume 47)
Jaeger RW (1987) Poisoning Emergencies: A Primer. St Louis, MO.
The Catholic Health Association of the United States.
Kimata Y, Uetake M, Tsukada S & Harii K (1995) Follow-up Study of
Patients Treated for Ingrown Nails with the Nail Matrix
Phenolization Method. Plastic and Reconstructive Surgery, 95(4):
719-724
Kligman AM (1966) The identification of contact allergens by human
assays III The maximum test: a procedure for screening and rating
contact sensitizers. J Invest Dermatol, 47: 393-409
Lalanne B, Baubion O, Sezeur A, Tricot C, Gaudy JH (1994)
[Circulatory arrest after splanchnic neurolysis with phenol in
unresectable cancer of the pancreas]. Annales de chirurgie, 48:
1025-1028 (in French)
Leikin JB & Paloucek FP (1996-1997) Poisoning and Toxicology
Handbook. 2nd edition American Pharmaceutical Association.
Lexi-Comp Ltd.
Lober CW (1987) Chemexfoliation. Indications and cautions. J Am
Acad Dermat, 17: 109-112
Merliss RR (1972) Phenol marasmus. J Occup Med, 14: 55-56
New Zealand National Poisons and Hazardous Chemical Information
Centre (1997): Report Form, Call Number 90101.
Olson KR (1994) Poisoning and drug overdose, 2nd Ed, Appleton and
Lange, Norwalk CT, USA
Reynolds JEF Ed. (1993) Martindale, The Extra Pharmacopoeia, 30th
ed. London. The Pharmaceutical Press.
Sax NI & Lewis RJ (1989) Dangerous properties of industrial
materials. 7th Ed. Van Nostrand Reinhold, New York.
Sorkin MJ (1988) Cardiac arrhythmias during phenol face peel. J
Dermatol Surg Oncol, 14: 477
Spiller HA, Quadrani-Kushner DA & Cleveland P (1993) A Five Year
Evaluation of Acute Exposures To Phenol Disinfectant. Journal of
Toxicology and Clinical Toxicology, 31: 307-313.
Stajduhar-Carie J (1968) Acute Phenol Poisoning: Singular Findings
in a Lethal Case. Journal of Forensic Medicine, 15: 41-42.
Wahl M, Lipscomb J & McAllister K (1995) Phenol Burn
Decontaminated with Golytely. Journal of Toxicology and Clinical
Toxicology, 33: 494
WHO (1994) IPCS Health and Safety Guide No. 88. Published by WHO.
Printed by Wissenschsftliche Verlagsgesellschaft, Stuttgart
WHO (1994) IPCS Environmental Health Criteria for Phenol (161 )
First draft prepared by Ms G.K. Montizan. Published by WHO.
Printed in Finland.
Windholz M, Ed. (1983) The Merck Index. An encyclopaedia of
chemicals, drugs and biologicals , 10th ed. Rahway, New Jersey,
Merck and Co., Inc
Wood KA (1978) The use of phenol as a neurolytic agent: a review.
Pain, 5: 205-225
Zamponi GW & French RJ (1994) Arrhythmias during phenol therapies:
a specific action on cardiac sodium channels? (letter).
Circulation, 89: 914
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Author: Rachael Inder
Pharmacology Department
School of Medical Sciences
Otago University
Dunedin
New Zealand
E-mail: rachael.inder@stonebow.otago.ac.nz
Date: April 1997
Reviewer: MO Rambourg Schepens
Centre Anti-Poisons de Champagne Ardenne
Centre Hospitalier Universitaire
51092 Reims Cedex
France
E-mail: marie-odile.rambourg@wanadoo.fr
Date: August 1997
Peer
review: Rio de Janeiro, Brazil, September 1997
(Group members: R. McKeown, W.A. Temple, W.A. Watson)
Update: Ad van Heijst
Bosch en Duin
Netherlands
Date: March 1998
Accepted: London, United Kingdom, March 1998
(Group members: R.E. Ferner, A. van Heijst, M.
Mathieu-Nolf, A.J. Nantel, M.O. Rambourg Schepens
(coordinator))
Editor: Mrs J. Duménil
International Programme on Chemical Safety
Date: October 1999
Chemical formula: C6H6O
Molecular weight: 94.11
3.3 Physical properties
3.3.1 Colour
Colourless or white (WHO, 1994).
3.3.2 State/form
Acicular-crystals, or white crystalline mass
(WHO, 1994).
3.3.3 Description
The crystals turn pink or red on exposure to
air and light, hastened in presence of alkalinity
(Windholz, 1983). Phenol has an acrid smell and a
sharp burning taste.
In the molten state, it is a clear, colourless liquid
with a low viscosity. It is soluble in most organic
solvents, and solubility is limited in aliphatic
solvents. Phenol's solubility. In water is limited
at room temperature, above 68°C it is entirely water
soluble. The vapour is heavier than air (WHO,
1994).
Melting point is 43°C, the commercial product has an
impurity that increases the melting point (Windholz,
1983).
Boiling point: 181.75°C
Flash point: 80°C (closed cup); 79°C to 85°C (open
cup)
Relative vapour density: 3.24 (WHO, 1994).
Explosive limits are 1.7% to 8.6% (Allen, 1991).
Vapour pressure at 20°C is 47 Pascal
pKa 10,0 at 25°C
pH of aqueous solutions is approximately 6.0
It is liquefied by mixing with about 8% water
(Windholz, 1983).
3.4 Hazardous characteristics
Autoignition temperature is 715°C. Phenol is a volatile,
combustible solid that when heated gives off flammable
vapours and carbon dioxide. Explosive or violent reactions
occur with acetylaldehyde; aluminium chloride plus
nitrobenzene; aluminium chloride-nitromethane; butadine;
calcium hypochlorite; peroxomonosulphuric acid;
peroxodisulphuric acid; sodium nitrate; and sodium
nitrate-trifluoroacetic acid (Allen, 1991).
Phenol is sensitive to oxidising agents. Splitting of the
hydrogen atom from the phenolic hydroxyl group is followed by
resonance stabilisation of the resulting phenyloxy radical.
The radical that is formed can be further oxidised. This
makes phenol suitable as an antioxidant, functioning as a
radical trapping agent (WHO, 1994). The taste threshold is
0.3 mg/L (0.00003%) in water (WHO, 1994).
4. USES
4.1 Uses
4.1.1 Uses
4.1.2 Description
The main use of phenol is as a feedstock for
phenolic resins, bisphenol A and caprolactam (an
intermediate in the production of nylon-6). It is used
in the manufacture of many products including
insulation materials, adhesives, lacquers, paint,
rubber, ink, dyes, illuminating gases, perfumes, soaps
and toys (IARC, 1989; WHO, 1994). Also used in
embalming and research laboratories. It is a product
of the decomposition of organic materials, liquid
manure, and the atmospheric degradation of
benzene.
It is found in some commercial disinfectants,
antiseptics, lotions and ointments. Phenol is active
against a wide range of microorganisms, and there are
some medical and pharmaceutical applications including
topical anaesthetic and ear drops, sclerosing agent.
It is also used in the treatment of ingrown nails in
the "nail matrix phenolization method" (Kimata et al.,
1995). Another medical application of phenol is its
use as a neurolytic agent, applied in order to relieve
spasms and chronic pain (Wood, 1978; Geller,
1997).
It is used in dermatology for chemical face peeling.
4.2 High risk circumstance of poisoning
Deliberate, accidental or occupational exposure.
4.3 Occupationally exposed populations
Workers involved in the production of phenol by the
cumene process, or the production of phenol from
chlorobenzene are at risk. Phenol may be emitted into the air
during the processing of phenolic resins, production of
phenol and phenol derivatives, production of caprolactam,
production of cokes and insulation materials. Wood workers,
and workers at plywood plants are at risk of exposure. Phenol
is used in iron and steel foundries - in the manufacture of
moulds or kernels, or during the operation of an electric
furnace in a steel factory and also in coal gasification and
liquefication plants, bakelite factories, and synthetic fibre
and fibrous glass wool factories. In creosote impregnation
plants, with highest exposure levels during the cleaning of
creosote warming chambers. In embalming situations, where the
embalming solution contains high phenol concentrations
(Allen, 1991; WHO, 1994).
In chemical accidents, responders or health staff may be
exposed by direct contact, by vomitus, or by off-gassing of
contaminated clothing.
5. ROUTES OF EXPOSURE
5.1 Oral
Phenol is readily absorbed from the gastrointestinal
tract (WHO, 1994).
5.2 Inhalation
Phenol is readily absorbed from the lungs (Allen,
1991).
5.3 Dermal
When spilt on the skin, intact or abraded, it is rapidly
absorbed and may lead to systemic poisoning (Brooks &
Riviere, 1996).
5.4 Eye
Phenol is absorbed through the mucous membranes of the
eye (WHO, 1994).
5.5 Parenteral
Therapeutic use: phenol can be administered by
intrathecal injection to relieve pain and spasticity (Geller,
1997), and has been used as a sclerosing agent.
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Phenol is rapidly absorbed through skin, lungs, and the
gastrointestinal tract.
Eight humans exposed to 6 to 20 mg/m3 by inhalation for 8
hours absorbed 70 to 80% of the phenol dose.
Skin absorption of phenol vapour (5 to 25 mg/m3) occurs
rapidly. The absorption in 8 humans exposed to phenol vapour
at concentrations of 6 to 20 mg/m3 , by skin only, for 6
hours was also 70 to 80%. Concentrations between 5 and 10%
phenol denature epidermal protein and this can therefore
partly prevent absorption. The phenol-protein complex is not
stable and by dissociation of phenol the substance may exert
its action over a period of time. In an in vitro study with
human abdominal skin, 10.9% of the dose was absorbed. The
period of exposure and the concentration of phenol are both
factors that determine the extent of absorption, but the area
of skin exposed affects the extent of absorption more than
the concentration. A single dose of 25 mg/kg body weight
dermally administered to rats, pigs and sheep was more than
95% absorbed (WHO, 1994).
6.2 Distribution by route of exposure
Rapidly distributed to all tissues in exposed animals.
After a single oral administration of 207 mg/kg phenol to
rats, the highest concentration ratios between tissue and
plasma were found in the liver (42%), followed by spleen,
kidney, adrenal, thyroid and lungs, with a peak tissue level
occurring after 0.5 hours.
In rabbits 15 minutes after an oral dose of 0.5 g/kg, the
highest concentrations of phenol were in the liver, followed
by the CNS, lungs and blood. After 82 minutes the phenol was
relatively uniformly distributed in all tissues (WHO,
1994).
6.3 Biological half-life by route of exposure
The half-life of conjugated phenol in humans is 1 hour
(Leikin & Paloucek, 1996-7), but there have also been
reported half-lives of 4 to 5 hours in humans (WHO,
1994).
6.4 Metabolism
After oral uptake of phenol, there is a large first-pass
metabolism. It is unclear whether phenol also undergoes first
pass pulmonary metabolism, there have been conflicting
results (Dickenson & Taylor, 1996). The liver, lungs and the
gastrointestinal mucosa are the most important sites of
phenol metabolism (WHO, 1994). Conjugation with glucuronic
acid to phenyl glucuronide and sulphation to phenyl sulphate,
have been shown to be major metabolic pathways in several
species. A shift from sulphation to glucuronidation was
observed in rats after increasing the phenol doses, which is
thought to be due to a saturation of the overall sulphation
process, by the limited availability of
3-phosphoadenosine-5-phosphosulfate. The formation of
sulphate and glucuronic metabolites occurs in the
hepatocytes, and then transported to the bile or back into
the blood (Ballinger et al, 1995). In vitro studies have
shown the formation of the reactive metabolites 4,4'-biphenol
and diphenoquinone by neutrophils and activated leukocytes.
Both in vivo and in vitro tests have shown covalent binding
of phenol to tissue and plasma proteins, some phenol
metabolites also bind to proteins (WHO, 1994).
6.5 Elimination and excretion
Urinary (renal) excretion is the major route of phenol
elimination in animals and humans. The rate of excretion
varies with different species, dose and route of
administration. Three men after an oral administration of
0.01 mg/kg phenol, excreted 90% of the dose in the urine
within 24 hours, mainly as phenyl sulfate and phenyl
glucuronide. A minor part is eliminated in the faeces and
expired air. Urinary excretion of humans exposed to phenol
vapour via inhalation or skin, occurred with an excretion
rate constant of k 0.2/hour. On oxidation to quinones the
metabolites may tint the urine green.
The half life is estimated to be between 1 and 4.5 hours with
52% eliminated unchanged in the urine (Leikin & Paloucek,
1996-7). The natural presence of phenols in food and drug
metabolites, makes biological monitoring impossible. A minor
part is eliminated in expired air and faeces. (Reynolds,
1993; Ellenhorn & Barceloux, 1988)
7. TOXICOLOGY
7.1 Mode of action
Cellular uptake of phenol is due to its lipophilic
character. It denatures proteins (WHO, 1994). Phenol is known
to disrupt disulphide bridges in keratin in the skin (Brooks
& Riviere, 1996).
In vitro studies have shown the formation of the reactive
metabolites 4,4'-biphenol and diphenoquinone by neutrophils
and activated leukocytes. Both in vivo and in vitro tests
have shown covalent binding of phenol to tissue and plasma
protein, some phenol metabolites also bind to proteins (WHO,
1994). It produces coagulation necrosis.
The acute lethality of phenol, associated with exposure to
high dose concentrations, is customarily attributed to a
depressant effect on the CNS.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
The lethal dose ranges from 1 g to
15 g (Reynolds, 1993). Ingestion of 4.8 g
resulted in death after 10 minutes in one
person (Anderson, 1869).
However, an adult survived a 26.7 g dose
ingestion after a 15-day stormy hospital
course without permanent sequellae (Haddad et
al., 1979).
Survival has been reported with up to 350
mg/kg orally (Christiansen & Klaman,
1996).
7.2.1.2 Children
Children have died after the
application of 5 % phenol compresses
(Ellenhorn, 1996).
7.2.2 Relevant animal data
LD50 (oral) rodent values ranged from 300 to
600 mg/kg body weight.
LD50 (dermal) values for rats and rabbits range from
670 to1400 mg/kg body weight respectively.
LC50 (8 hour) for rats by inhalation was more than 900
mg/m3 (WHO, 1994).
In two multiple dose rat studies, NOAEL values
obtained were 40 mg/kg/day and 60 mg/kg /day and the
LOAEL values were 53 mg/kg/day and 120 mg/kg/day. In a
mouse study the NOAEL was 140 mg/kg/day and the LOAEL
was 280 mg/kg/day (WHO, 1994).
Chronic vapour exposures in rats ( 0.02 to 1ppm for 2
months) produced changes in the blood enzyme activity
and time for excitation of extensor muscles. At higher
exposures, phenol may lead to decrease in body weight.
In various animal species the inhalation of phenol
affected the lungs by causing hyperaemia, infarcts,
pneumonia, purulent bronchitis and hyperplasia of the
peribronchial tissues (WHO, 1994). There does not
appear to be a strong potential for cumulative health
effects from chronic exposure (WHO, 1994)
7.2.3 Relevant in vitro data
No data available.
7.2.4 Workplace standards
OSHA PEL: TWA 5 ppm (skin)
ACGIH TLV: TWA 5 ppm (skin)
IDLN: 100 ppm
DFG MAK: 5 ppm (19 mg/m3)
NIOSH REL: TWA 20 mg/m3; CL 60 mg/m3/15 minutes
(Sax & Lewis, 1989)
In Britain the occupational exposure standard is 19
mg/m3 (long term) and 38 mg/m3 (short term). In the
United States the permissible level is 19 mg/m3 and
the recommended is 20 mg/m3 (long-term) and the
maximum short term is 60 mg/m3 (Reynolds,
1993).
7.2.5 Acceptable daily intake (ADI)
A Task Group derived a tolerable daily intake
(TDI) using the lowest NOAEL's for kidney and
developmental effects in rats which is in the range of
12 to 40 mg/kg body weight /day. With an uncertainty
factor of 200, the range of 60 to 200 µg/kg/day was
recommended as the upper limit of the TDI (WHO,
1994)
The estimated maximal total daily intake of phenol for
a 70 kg individual is calculated to be 0.1 mg/kg body
weight per day (WHO, 1994).
However, according to IRIS (Integrated Risk
Information Sytem, 1996), the reference dose (Rfd) is
0.6 mg/kg/day, using the NOAEL (reduced fetal body
weight in rats) of 60 mg/kg/day, with an uncertainty
factor (Uf) of 100.
7.3 Carcinogenicity
An IARC review in 1989 found that the carcinogenicity
evidence for phenol was inadequate (group 3) (WHO, 1994). US
EPA classifies phenol in group D.
Two-stage carcinogenicity studies have shown that phenol,
applied repeatedly to mouse skin, has promoting activity
(WHO, 1994).
7.4 Teratogenicity
Phenol has been identified as a developmental toxicant
in studies with rats and mice (WHO, 1994).
7.5 Mutagenicity
The majority of bacterial mutagenicity tests have
demonstrated negative results. In mammalian cells, mutations,
chromosomal damage and DNA effects have been observed. Phenol
has shown no effect on intercellular communication in
cultured mammalian cells. The induction of micronuclei in
bone marrow cells of mice has been observed in some studies
at high doses. No micronuclei were observed in mice studies
at lower dose (IARC, 1989; WHO, 1994).
7.6 Interactions
No data available.
8. TOXICOLOGICAL AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
8.2.1.2 Advanced qualitative confirmation test(s)
8.2.1.3 Simple quantitative method(s)
8.2.1.4 Advanced quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
8.2.2.2 Advanced qualitative confirmation test(s)
8.2.2.3 Simple quantitative method(s)
8.2.2.4 Advanced quantitative method(s)
8.2.2.5 Other dedicated method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall interpretation of all toxicological analyses and
toxicological investigations
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
After swallowing a significant concentrated
dose, an intense burning of the mouth and throat is
felt (necrosis of the skin and mucous membranes of the
throat), and pain in the abdominal area, with
gastrointestinal irritation including nausea,
vomiting, sweating and diarrhoea. The face is usually
pale and sweaty, the pupils may be contracted or
dilated; cyanosis is usually marked; the pulse is
usually weak and slow, occasionally it may be racing;
respiration may initially be increased in rate, but
later decreased in rate and magnitude; body
temperature may fluctuate. Excitation may occur
initially, but it is quickly followed by
unconsciousness. Occasionally isolated twitching of
muscles or convulsions may be observed. Acute renal
failure can develop resulting from systemic
absorption. Ingestion is usually fatal (Foxall et al.,
1989; Allen, 1991; Reynolds, 1993; WHO, 1994).
9.1.2 Inhalation
Phenol vapours are irritating to the upper
respiratory tract. Ocular and nasal irritation,
tremors and incoordination were reported in rats
exposed to phenol via inhalation to 906 mg/m3 for 8
hours (WHO, 1994). Wheezing may occur. Other symptoms
associated with inhalation include anorexia, weight
loss, headache, salivation, vertigo (WHO, 1994) and
dark urine (dark/brown/green) (Leikin & Paloucek,
1996-7).
9.1.3 Skin exposure
Phenol is a local anaesthetic, so upon initial
contact, no pain is felt. By the time pain is felt,
serious burns and absorption through the skin may have
occurred (Allen, 1991). Local damage to the skin
includes erythema, inflammation, and necrosis. The
effects are worse when the application sites are
bandaged (WHO, 1994). A white, brown or red
discolouration of the skin may occur (Leikin &
Paloucek 1996-7). Systemic intoxication can occur from
absorption (WHO, 1994); roughly 50 % of all reported
cases have a fatal outcome (Horch et al., 1994).
In the Kligman maximization test phenol did not cause
sensitisation in 24 human volunteers (Kligman,
1966).
9.1.4 Eye contact
Phenol is an eye irritant (WHO, 1994).
Solutions can be corrosive to the eyes, and can cause
severe ocular damage including corneal
opacification.
9.1.5 Parenteral exposure
After intraperitoneal and subcutaneous doses of
phenol, tremors, convulsions, coma and death have been
reported (WHO, 1994). Injection of 30 mL of 89% phenol
instead of 10% for celiac plexus nerve block resulted
in coma, hypotension, respiratory insufficiency and
ventricular tachycardia (Christiansen & Klaman,
1996).
9.1.6 Other
No data available.
9.2 Chronic poisoning
9.2.1 Ingestion
Severe gastrointestinal irritation,
cardiovascular, CNS and respiratory effects,
hypothermia and decreased body weight. Brown or
discoloured urine has also been observed in chronic
poisoning (Goldfrank & Bresnitz, 1990).
Repeated oral exposure for several weeks (estimated
intake 10 to 240 mg/day) resulted in mouth sores,
diarrhea and dark urine. Examination 6 months after
the exposure revealed no residual effects (Baker et
al., 1978).
9.2.2 Inhalation
There does not appear to be a strong potential
for cumulative health effects from chronic exposure
(WHO, 1994)
9.2.3 Skin exposure
Chronic doses may result in onychronosis
(yellowing of the skin) and skin eruption (WHO, 1994).
Death has been observed from repeated application of
small doses (Olson, 1994).
In former times phenol 5 to 10% was used as a skin
disinfectant giving rise to the "carbolic marasmus"
characterized by anorexia, headache, vertigo,
salivation, dark urine and increased skin and scleral
pigmentation (Merliss, 1972).
9.2.4 Eye contact
No data available
9.2.5 Parenteral exposure
No data available.
9.2.6 Other
No data available.
9.3 Course, prognosis, cause of death
Hypotension, renal failure, apnea, laryngeal oedema and
ARDS can develop soon after exposure leading to death (WHO,
1994). Coma and seizures usually occur within minutes to a
few hours after exposure. Toxic effects may be delayed up to
18 hours.
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Heart rate at first increases and then becomes
slow and irregular. Blood pressure at first increases
slightly, then falls markedly (hypotension) (WHO,
1994). Cardiovascular collapse, atrial and ventricular
arrythmias have been reported (Leikin & Paloucek,
1996-7). Deep venous thrombosis has been reported
following injection of phenol (WHO, 1994).
Cardiac dysrhythmias have been observed in skin
peeling and nerve blockade (Forrest & Ramage, 1987;
Lober, 1987; Sorkin, 1988; Gaudy et al., 1993; Lalanne
et al., 1994; Zamponi & French, 1994).
9.4.2 Respiratory
Respiration may initially be increased in rate,
but later decreased in rate. Pulmonary oedema,
wheezing, coughing, dyspnea, pneumonia are common
signs (Leikin & Paloucek, 1996-7). The cause of death
from phenol exposure is often respiratory failure
(WHO, 1994).
9.4.3 Neurological
9.4.3.1 Central nervous system (CNS)
Initial signs and symptoms include
headache, dizziness and tinnitus. Seizures,
coma, respiratory depression and death may
ensue quickly. Coma and seizures usually
occur within minutes to a few hours after
exposure or a delay of up to 18 hours. Phenol
may also cause demyelination and axonal
damage of peripheral nerves (WHO,
1994).
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
A decrease in body temperature has
been reported (WHO, 1994).
9.4.3.4 Skeletal and smooth muscle
Locomotor activity reduced at 244 mg
phenol/kg body weight in female Fischer-344
rats (WHO, 1994). Chronic exposure in rats
lead to changes in the time for excitation of
extensor muscles.
9.4.4 Gastrointestinal
Symptoms include diarrhoea, salivation,
vomiting, ulceration and haemorrhage (Leikin &
Paloucek, 1996-7). Corrosive damage may involve the
entire gastrointestinal tract.
9.4.5 Hepatic
Hepatic necrosis was observed in two(out of
six) female Fischer-344 rats when given 244 mg
phenol/kg body weight (WHO, 1994).
9.4.6 Urinary
9.4.6.1 Renal
Renal failure has been reported in
acute poisoning. Urinalysis may reveal a
green to brown discolouration of the urine
with albuminuria. Nephritis is reported
(Leikin & Paloucek, 1996-7).
9.4.6.2 Other
No data available.
9.4.7 Endocrine and reproductive systems
Increased incidence of preimplantation loss and
early postnatal death in the offspring of rats (WHO,
1994).
9.4.8 Dermatological
Chronic doses may result in ochronosis
(yellowing of the skin) and skin eruption (WHO,
1994).
9.4.9 Eye, ear, nose, throat: local effects
The fumes are irritating to the eyes and
affects the pupil's response to light (miosis) (WHO,
1994). Solutions can be corrosive to the eyes, and can
cause severe ocular damage including corneal
opacification. Lymph production in the conjunctiva may
be increased and will leave the cornea white and
hypesthetic (Jaeger, 1987).
Necrosis of the mucous membranes of the throat.
Phenol applied to the inner ear round window of
Sprague-Dawley rats caused morphological damage to the
organ of Corti in the basal coil. The outer hair cells
appeared to be more sensitive to phenol and as a
result of the damage, impairment of inner ear function
was noted which was permanent for higher frequencies.
One experiment in female mice lead to an increase in
ear thickness (WHO, 1994)
9.4.10 Haematological
Heinz body haemolytic anaemia and
hyperbilirubinemia have been reported occasionally
(WHO, 1994).
9.4.11 Immunological
There are no studies in humans. For four weeks
groups of five male CD-1 mice were given drinking
water containing 0, 4.7, 19.5 or 95.2 mg phenol/L.
Total and differential leukocyte counts were
unaffected. The highest dose suppressed the
stimulation of cultured splenic lymphocytes by the
B-cell mitogen lipopolysaccharide, the T-cell mitogen
phytohaemagglutinin, and the T and B-cell mitogen
pokeweed, but not by concanavatin. Suppression of the
animal's antibody production in response to a
T-cell-dependent antigen, occurred at the mid and high
doses (WHO, 1994).
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Metabolic acidosis (WHO, 1994)
9.4.12.2 Fluid and electrolyte disturbances
Fluid loss secondary to burns or shock.
9.4.12.3 Others
No data available.
9.4.13 Allergic reaction
No data available.
9.4.14 Other clinical effects
No data available
9.4.15 Special risks
Phenol has been shown to be a developmental
toxicant in rats and mice (WHO, 1994).
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
When spilt on the skin or in the eyes, there should be
an immediate washing with water (preferably tepid) for at
least 10 minutes. If available wash with polyethyleneglycol
molecular weight 300 (Macrogol 300), isopropyl alcohol,
industrial methylated spirits or Goletely (PEG 3550) for at
least 30 minutes (Horch et al., 1994).
Do not induce vomiting. Dilution may increase absorption.
Gastric lavage should be carefully weighed against the risk
of complications. Recommended gastric lavage fluids are
polyethylene glycol, water, following administration of
activated charcoal, or vegetable oils, such as olive oil,
castor oil or cottonseed oil (WHO, 1994).
Treatment is mainly supportive. If there is a systemic
intoxication, monitor the respiration and the level of
oxygenation, the blood pressure and ECG, the level of
methaemoglobinemia, the hepatic and renal functions. Control
convulsions and cardiac arrhythmias according to the
Treatment Guides.
10.2 Life supportive procedures and symptomatic/specific treatment
Make a proper assessment of airway, breathing,
circulation and neurological status.
Maintain a clear airway.
If unconscious give artificial respiration.
If the patient has breathing difficulties, put them in a
sitting position.
Monitor vital signs.
Monitor blood pressure and ECG.
Monitor fluid and electrolyte balance.
Monitor acid-base balance.
Control cardiac dysrhythmias with appropriate drug
regimen.
Control convulsions with appropriate drug regimen.
10.3 Decontamination
Remove and discard contaminated clothing.
Irrigate exposed eyes with copious amounts of water.
Wash skin with copious amounts of water or preferably if
available wash with polyethyleneglycol molecular weight 300
(Macrogol 300), isopropyl alcohol, industrial methylated
spirits or Goletely (PEG 3550) for at least 30 minutes.
Do not induce vomiting, empty stomach by aspiration followed
by polyethylene glycol or activated charcoal with
cathartic.
Endoscopy.
10.4 Enhanced elimination
If acute renal failure occurs in phenol poisoning,
dialysis should probably not be used alone, but in
conjunction with charcoal hemoperfusion. Without renal
failure the use of charcoal hemoperfusion may also be useful,
when the patient has been exposed to 15 to 20 g of phenol
(Christiansen & Klaman, 1996)
10.5 Antidote treatment
10.5.1 Adults
No antidote available.
10.5.2 Children
No antidote available.
10.6 Management discussion
No data available.
11. ILLUSTRATIVE CASES
11.1 Case reports from the literature
Occupational - Male
A 27-year-old male spilt 80% phenol on both knees and arrived
at the emergency department 30 to 60 minutes after the
spillage. Both legs had been washed with copious amounts of
water and he had also undergone further irrigation and
application of glycerin cream, but continued to be in pain.
Further irrigation with 6 litres of saline followed and then
it was suggested to irrigate with polyethylene glycol. By
mistake Golytely (PEG 3550) was used. The patient reported an
immediate soothing of the pain and his long term recovery was
favourable (Wahl et al., 1995).
Accidental Injection
A 50-year-old woman inadvertently received a 30 ml dose of
89% phenol (26.9 g, 0.44 mg/kg body weight). At 23 minutes
post- injection she was unresponsive, at 27 minutes she had
respiratory distress and was intubated. At 62 minutes she
developed shock and dopamine was started followed by
epinephrine and neosynephrine. At the same time she developed
ventricular tachycardia and was treated with lidocaine. 4
hours after the injection she was comatose with a blood
pressure of 70/58, while receiving large doses of
vasopressors. At 4.5 hours charcoal hemoperfusion was
started, and given for 6 hours and 20 minutes. Her clinical
status improved with the perfusion and she made a complete
recovery. The perfusion enhanced the elimination of free and
total phenol (Christiansen & Klaman, 1996).
Five Year Acute Exposure Study
A five year evaluation of acute exposure to phenol
disinfectant (26%) studied 80 cases with an age range of 1 to
78 years, 75% under 5 years old. There were 60 oral only
exposures, 7 dermal only, 12 oral/dermal and 1 inhalation.
65% were assessed at the emergency department and 33% were
admitted. 14% of oral exposures developed rapid CNS
depression, without seizures, and 2 patients developed coma
after ingestion. Burns occurred in 17 oral exposures and 5
dermal. 17 patients underwent endoscopy. Urine colour change
was noted in 5 patients following ingestion. There were no
cardiovascular complications, or oliguria and anuria. In all
cases there were complete recoveries (Spiller et al.,
1993).
Accidental Ingestion - Male Alcoholic, Addict
A report from the Invercargill hospital in New Zealand early
in 1997, reports a 29 year old male, alcoholic, addict, who
drank 30 mL of 88% phenol from a bottle he found (26.4 g/75
kg = 350 mg/kg). He had a cardiac arrest requiring DC
cardioversion, and developed renal failure secondary to
rhabdomolysis. He developed a denuded oesophagus, large,
superficial, antral ulceration and received continuous
haemofiltration and endoscopy. His urine output only
recommenced 14 days after the ingestion and his renal
function is expected to return to normal (NZNPIC, 1997).
Ingestion - Female
Fatal case reports include a 21 year old female who drank 10
to 20 g of phenol. She went into a deep coma with partial
areflexia, a heart rate of 140 and dilated pupils, and had a
cardiac arrest 60 minutes post ingestion. She received
repeated gastric lavage with water, glycerin and animal
charcoal, but died from pulmonary oedema and shock
(Stajduhar-Carie, 1968).
Occupational - Fatal Dermal Exposure
A 17-year-old male had 30% phenol (as industrial waste)
splashed on his face, neck and right trunk. He was washed
with water, but 30 minutes later he had a seizure and died.
His blood phenol level was 2.7 mg/dL. An autopsy showed red
areas to 15% of his skin area, and pulmonary oedema. (WHO,
1994)
Accidental Immersion
A man was partially submerged in a solution of 20% phenol in
dichloromethane for a few seconds. He immediately showered ,
but collapsed, his extremities were cold and he had 50% burns
to his body. He developed acute renal failure. Anuria
followed, with a rise in plasma creatinine, but treatment
with intravenous furosemide and haemodyalisis (daily for
seven days, then with decreasing intervals for a further 18
days), allowed adequate urinary volumes to be produced. He
also had respiratory distress, treated in intensive care. A
spill of 80-100% phenol on the hip, thigh and scrotum with a
contact duration of 20 minutes lead to death, while a
reported spill of 43.5% to the lower half of the body
resulted in shock. A spill of 4 to 5 litres on the upper
half of the body of 78%, with a contact duration of 2 to 5
minutes resulted in a coma (WHO, 1994).
Contaminated Drinking Water
A chemical spill in Wisconsin in 1974 contaminated
groundwater which was being used as drinking water. One month
later there were complaints of health effects, and six months
later medical histories were taken from 100 people. The
estimated daily exposure was 10 to 240 mg phenol/person. A
significantly significant increase in diarrhoea, mouth sores,
dark urine and burning of the mouth was found.
Gastrointestinal illnesses were also reported in North Wales,
when a river used for the preparation of drinking water was
contaminated with phenol, and when chlorinated, various
chlorophenols formed (WHO, 1994)
Accidental Ingestion
An outpatient was mistakenly given in a measured container
one ounce of 89 % phenol. The patient immediatly clutched her
throat and collapsed. Within 30 minutes she had an
unrecordable blood pressure and sustained respiratory arrest.
During endotracheal intubation in the ED the mouth and
hypopharynx were noted to be white. A "lamp oil" odour was
noted while ventilating the patient with a bag mask. The
patient experienced ventricular tachycardia one hour after
ingestion and resuscitation was effected by cardioversion.
Over the first 24 hours she exhibited ventricular arrhythmia,
seizures and metabolic acidosis. (Haddad et al., 1979)
Child Exposure
A 10-year-old boy had a solution of 40% phenol and 0.8%
croton oil in hexachlorophene soap and water applied to a
large nevus covering his 1.9% of his body surface whilst
under anaesthesia. After 55 minutes of treatment, multifocal
and coupled premature ventricular complexes were detected by
ECG. An intravenous infusion of 250 mg bretylium sulfate
suppressed the dysrhythmia (WHO, 1994).
12. ADDITIONAL INFORMATION
12.1 Specific preventative measures
Phenol should be kept in a tightly closed container, in
a cool, dry place, away from heat, flame and oxidising
agents. It is light sensitive and should be kept in the dark
(WHO, 1994).
Protective clothing should will be appropriate to the amount
and form of the phenol being handled. It should be handled
wearing an approved respirator; viton, butyl rubber or
neoprene gloves (not nitrile or PVA gloves), safety goggles
and other protective clothing. Safety showers and
polyethylene glycol 300 should be near where phenol is being
handled.(Allen, 1991)
12.2 Other
Phenol is not likely to persist in air, soil or sewage,
sea or surface water. It readily reacts photochemically, is
rapidly biodegraded aerobically to mainly carbon dioxide, and
anaerobic biodegradation occurs also at a slower rate. Low
removal rates of phenol in ground water and soil may occur
e.g. following spills, with subsequent inhibition of the
microbial populations. Phenol is toxic to aquatic organisms:
the lowest EC50 for water organisms is estimated to be 3.1
mg/L. The lowest chronic NOEC is estimated to be 0.2
(g/L).
13. REFERENCES
Allen R, Ed. (1991) Chemical Safety data Sheets, Volume 4b:
Toxic Chemicals (m-z ). Royal Society of Chemistry, Cambridge.
Printed by Staples Printers Rochester Ltd, Kent.
Anderson W (1869) fatal misadventure with carbolic acid. Lancet,
1: 179
Baker EL, Landrigan PJ, Bertozzi PE, Field PH, Basteyns BJ,
Skinner HG (1978) Phenol poisoning due to contaminated drinking
water. Arch Environ Health, 83: 98-94
Ballinger LN, Cross SE & Roberts MS (1995) Availability and Mean
Transit Times of Phenol and its Metabolites in the Isolated
Perfused Rat Liver: Normal and Retrograde Studies Using Tracer
Concentrations of Phenol. J Pharm Pharmacol, 47: 949-956.
Brooks JD & Riviere JE (1996) Quantitative Percutaneous Absorption
and Cutaneous Distribution of Binary Mixtures of Phenol and
para-Nitrophenol in Isolated Perfused Porcine Skin. Fundamental
and Applied Toxicology, 32: 233-243.
Christiansen RG & Klaman RN (1996) Successful Treatment of Phenol
Poisoning With Charcoal Hemoperfusion. Veterinary and Human
Toxicology, 38(1): 27-28
Dickenson A & Taylor G (1996) Pulmonary First-Pass and
Steady-State Metabolism of Phenols. Pharmaceutical Research,
13(5): 744-748.
Forrest T & Ramage DT (1987) Cardiac dysrhythmia after subtrigonal
phenol. Anaesthesia, 42: 77-778
Foxall PJD, Bending MR, Gartland KPR & Nicholson JK (1989) Acute
renal failure following accidental cutaneous absorption of phenol:
application of NMR urinalysis to monitor the disease process.
Human Toxicol, 9: 491-496
Gaudy JH, Tricot Z, Sezeur A (1993) [Serious heart rate disorders
following perioperative splanchnic phenol nerve block]. Cand J
Anaesth, 40: 357-359 (in French)
Geller AS (1997) Botulinum Toxin A. Archives of Physical Medicine
and Rehabilitation, 78: 233
Goldfrank LR & Bresnitz EA (1990) Toxicologic emergencies, 4th ed,
East Norwalk, Appleton & Lange
Haddad LM, Dimond KA, Schweistris JE (1979) Case report: phenol
poisoning. Burns, 20: 45-50
Horch R, Spilker G, Stark GB (1994) Phenol burns and intoxication.
Burns, 20: 45-50
IARC (1989) Phenol. In: Some organic solvents, resin monomers and
related compounds, pigments and occupational exposures in paint
manufacture and painting. Lyon, International Agency for Research
on Cancer, pp 263-287 (IARC Monographs on the evaluation of
carcinogenic risks to humans, volume 47)
Jaeger RW (1987) Poisoning Emergencies: A Primer. St Louis, MO.
The Catholic Health Association of the United States.
Kimata Y, Uetake M, Tsukada S & Harii K (1995) Follow-up Study of
Patients Treated for Ingrown Nails with the Nail Matrix
Phenolization Method. Plastic and Reconstructive Surgery, 95(4):
719-724
Kligman AM (1966) The identification of contact allergens by human
assays III The maximum test: a procedure for screening and rating
contact sensitizers. J Invest Dermatol, 47: 393-409
Lalanne B, Baubion O, Sezeur A, Tricot C, Gaudy JH (1994)
[Circulatory arrest after splanchnic neurolysis with phenol in
unresectable cancer of the pancreas]. Annales de chirurgie, 48:
1025-1028 (in French)
Leikin JB & Paloucek FP (1996-1997) Poisoning and Toxicology
Handbook. 2nd edition American Pharmaceutical Association.
Lexi-Comp Ltd.
Lober CW (1987) Chemexfoliation. Indications and cautions. J Am
Acad Dermat, 17: 109-112
Merliss RR (1972) Phenol marasmus. J Occup Med, 14: 55-56
New Zealand National Poisons and Hazardous Chemical Information
Centre (1997): Report Form, Call Number 90101.
Olson KR (1994) Poisoning and drug overdose, 2nd Ed, Appleton and
Lange, Norwalk CT, USA
Reynolds JEF Ed. (1993) Martindale, The Extra Pharmacopoeia, 30th
ed. London. The Pharmaceutical Press.
Sax NI & Lewis RJ (1989) Dangerous properties of industrial
materials. 7th Ed. Van Nostrand Reinhold, New York.
Sorkin MJ (1988) Cardiac arrhythmias during phenol face peel. J
Dermatol Surg Oncol, 14: 477
Spiller HA, Quadrani-Kushner DA & Cleveland P (1993) A Five Year
Evaluation of Acute Exposures To Phenol Disinfectant. Journal of
Toxicology and Clinical Toxicology, 31: 307-313.
Stajduhar-Carie J (1968) Acute Phenol Poisoning: Singular Findings
in a Lethal Case. Journal of Forensic Medicine, 15: 41-42.
Wahl M, Lipscomb J & McAllister K (1995) Phenol Burn
Decontaminated with Golytely. Journal of Toxicology and Clinical
Toxicology, 33: 494
WHO (1994) IPCS Health and Safety Guide No. 88. Published by WHO.
Printed by Wissenschsftliche Verlagsgesellschaft, Stuttgart
WHO (1994) IPCS Environmental Health Criteria for Phenol (161 )
First draft prepared by Ms G.K. Montizan. Published by WHO.
Printed in Finland.
Windholz M, Ed. (1983) The Merck Index. An encyclopaedia of
chemicals, drugs and biologicals , 10th ed. Rahway, New Jersey,
Merck and Co., Inc
Wood KA (1978) The use of phenol as a neurolytic agent: a review.
Pain, 5: 205-225
Zamponi GW & French RJ (1994) Arrhythmias during phenol therapies:
a specific action on cardiac sodium channels? (letter).
Circulation, 89: 914
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Author: Rachael Inder
Pharmacology Department
School of Medical Sciences
Otago University
Dunedin
New Zealand
E-mail: rachael.inder@stonebow.otago.ac.nz
Date: April 1997
Reviewer: MO Rambourg Schepens
Centre Anti-Poisons de Champagne Ardenne
Centre Hospitalier Universitaire
51092 Reims Cedex
France
E-mail: marie-odile.rambourg@wanadoo.fr
Date: August 1997
Peer
review: Rio de Janeiro, Brazil, September 1997
(Group members: R. McKeown, W.A. Temple, W.A. Watson)
Update: Ad van Heijst
Bosch en Duin
Netherlands
Date: March 1998
Accepted: London, United Kingdom, March 1998
(Group members: R.E. Ferner, A. van Heijst, M.
Mathieu-Nolf, A.J. Nantel, M.O. Rambourg Schepens
(coordinator))
Editor: Mrs J. Duménil
International Programme on Chemical Safety
Date: October 1999
See Also:
Toxicological Abbreviations
Phenol (EHC 161, 1994)
Phenol (HSG 88, 1994)
Phenol (ICSC)
PHENOL (JECFA Evaluation)
Phenol (IARC Summary & Evaluation, Volume 71, 1999)


