
Glutethimide
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Main brand names, main trade names |
| 1.6 Main manufacturers, main importers |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Colour |
| 3.3.2 State/form |
| 3.3.3 Description |
| 3.4 Other characteristics |
| 3.4.1 Shelf-life of the substance |
| 3.4.2 Storage conditions |
| 4. USES |
| 4.1 Indications |
| 4.1.1 Indications |
| 4.1.2 Description |
| 4.2 Therapeutic dosage |
| 4.2.1 Adults |
| 4.2.2 Children |
| 4.3 Contraindications |
| 5. ROUTES OF ENTRY |
| 5.1 Oral |
| 5.2 Inhalation |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Other |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination by route of exposure |
| 7. PHARMACOLOGY AND TOXICOLOGY |
| 7.1 Mode of action |
| 7.1.1 Toxicodynamics |
| 7.1.2 Pharmacodynamics |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 7.7 Main adverse effects |
| 8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Material sampling plan |
| 8.1.1 Sampling and specimen collection |
| 8.1.1.1 Toxicological analyses |
| 8.1.1.2 Biomedical analyses |
| 8.1.1.3 Arterial blood gas analysis |
| 8.1.1.4 Haematological analyses |
| 8.1.1.5 Other (unspecified) analyses |
| 8.1.2 Storage of laboratory samples and specimens |
| 8.1.2.1 Toxicological analyses |
| 8.1.2.2 Biomedical analyses |
| 8.1.2.3 Arterial blood gas analysis |
| 8.1.2.4 Haematological analyses |
| 8.1.2.5 Other (unspecified) analyses |
| 8.1.3 Transport of laboratory samples and specimens |
| 8.1.3.1 Toxicological analyses |
| 8.1.3.2 Biomedical analyses |
| 8.1.3.3 Arterial blood gas analysis |
| 8.1.3.4 Haematological analyses |
| 8.1.3.5 Other (unspecified) analyses |
| 8.2 Toxicological analyses and their interpretation |
| 8.2.1 Tests on toxic ingredient(s) of material |
| 8.2.1.1 Simple qualitative test(s) |
| 8.2.1.2 Advanced qualitative confirmation test(s) |
| 8.2.1.3 Simple quantitative method(s) |
| 8.2.1.4 Advanced quantitative method(s) |
| 8.2.2 Tests for biological specimens |
| 8.2.2.1 Simple qualitative test(s) |
| 8.2.2.2 Advanced Qualitative Confirmation Test(s) |
| 8.2.2.3 Simple Quantitative Method(s) |
| 8.2.2.4 Advanced quantitative method(s) |
| 8.2.2.5 Other dedicated method(s) |
| 8.2.3 Interpretation of toxicological analyses |
| 8.3 Biomedical investigations and their interpretation |
| 8.3.1 Biochemical analysis |
| 8.3.1.1 Blood, plasma or serum |
| 8.3.1.2 Urine |
| 8.3.1.3 Other fluids |
| 8.3.2 Arterial blood gas analyses |
| 8.3.3 Haematological analyses |
| 8.3.4 Interpretation of biomedical investigations |
| 8.3.5 Interpretation of biological investigations |
| 8.4 Other biomedical (diagnostic) investigations and their interpretation |
| 8.5 Overall Interpretation of all toxicological analyses and toxicological investigations |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin exposure |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 Central Nervous System (CNS) |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 Skeletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Other |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ear, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reactions |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Other |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Life supportive procedures and symptomatic/specific treatment |
| 10.3 Decontamination |
| 10.4 Enhanced elimination |
| 10.5 Antidote treatment |
| 10.5.1 Adults |
| 10.5.2 Children |
| 10.6 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from literature |
| 12. ADDITIONAL INFORMATION |
| 12.1 Specific preventive measures |
| 12.2 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
GLUTETHIMIDE
International Programme on Chemical Safety
Poisons Information Monograph 246
Pharmaceutical
1. NAME
1.1 Substance
Glutethimide
1.2 Group
Minor psychotherapeutic, piperidinedione sedative and
hypnotic
1.3 Synonyms
2-ethyl-2-phenylglutarimide;
3-ethyl-3-phenyl-2,6-piperidinedione;
Alpha-ethyl-alpha-phenyl glutarimide.
1.4 Identification numbers
1.4.1 CAS number
77-21-4
1.4.2 Other numbers
No data available.
1.5 Main brand names, main trade names
Doridene, Doriden, Dorimide, Glimid, Elrodorm.
1.6 Main manufacturers, main importers
2. SUMMARY
2.1 Main risks and target organs
The main target organ is the central nervous system
causing coma with fluctuations in depth, and various degrees
of hypotension. Anticholinergic effects often occur.
2.2 Summary of clinical effects
At lower doses, acute intoxication may cause somnolence,
ataxia, tonic muscle spasms and abnormal reflexes. In severe
intoxication, hypotension, hypothermia, shock, coma,
respiratory depression and acidosis may occur. Effects on
other organs are usually secondary to coma and shock.
2.3 Diagnosis
Diagnosis is based mainly on the history of the patient
and clinical features observed (see 2.2) and also on
toxicological analyses.
Serum glutethimide is rarely measured since it is poorly
correlated with the clinical manifestation of the acute
poisoning and requires the use of advanced analytical
techniques.
2.4 First aid measures and management principles
If ingestion is recent and the patient is still fully
conscious with a normal pharyngeal reflex, induce vomiting.
The obtunded, comatose patient should be intubated before
gastric lavage is performed. Stomach emptying more than four
hours after ingestion is probably ineffective. Give
activated charcoal. Administer a cathartic.
Respiratory depression presents the greatest risk to the
patient. Ensure that oxygenation is adequate. Optimize airway
position of the patient, perform endotracheal intubation and
assist ventilation in severe cases. Blood gases should be
monitored in patients with patients with respiratory
failure.
Pneumonia must be treated with appropriate antibiotics.
Open and maintain an intravenous route. Give adequate fluids
to maintain diuresis of 2.5 to 3 L/day. Catheterize
bladder.
Severe hypotension should be treated with fluid replenishment
(dopamine or other vasoactive drugs might be needed).
In comatose patients, especially with signs of shock, renal
function should be monitored (renal output, plasma urea and
creatinine, electrolytes, acid-base balance). Pre-renal
uraemia occurs rarely but must be taken into
consideration.
Cerebral oedema (papilloedema) may require treatment with
mannitol.
Enhanced elimination procedures are not recommended: forced
diuresis is ineffective, and the efficacy of haemodialysis
(even using oil as dialysis fluid) and of haemoperfusion has
not been satisfactory proved.
Glutethimide is often ingested with other toxic substances:
mixed poisoning with codeine or paracetamol must always be
considered, especially in drug addicts.
Glutethimide is habit forming.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Synthetic.
3.2 Chemical structure
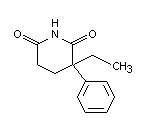 Chemical names: 2 ethyl-2-phenylglutarimide
alpha-ethyl-alpha-phenyl glutarimide
3-ethyl-3-phenylpiperidine-2,6-dione
Molecular weight: 217.26
Molecular formula: C13H15NO2
(Conversion of traditional units into SI: multiply the value
in mg/L by 4.603 to get the result in micromol per
litre.)
3.3 Physical properties
3.3.1 Colour
Colourless or white.
3.3.2 State/form
3.3.3 Description
Odourless and colourless crystals or white
crystalline powder, practically insoluble in water,
soluble one in five of ethanol, one in less than one
chloroform and one in 12 of ether, freely soluble in
acetone and ethyl acetate, soluble in methyl alcohol
(Reynolds, 1989).
Stability: at pH 5 the chemical half life was 28.3
years at 25蚓 and 1.02 months at pH 8, the
decomposition being due to hydrolysis.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
3.4.2 Storage conditions
Store in well closed, airtight containers,
protected from humidity and light (Wesolowski et al.,
1968).
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
Used as an hypnotic in insomnia but rarely as a
sedative, glutethimide was initially believed to be
almost free from side effects (Banen & Resnik, 1973).
However, further experience of its toxicity and
because its dependence liability (Sramek & Klajawal,
198l; Shamoian, 1975), glutethimide has been banned in
many countries and many companies have stopped
production.
4.2 Therapeutic dosage
4.2.1 Adults
The usual oral adult dose is 250 - 500 mg at
bedtime (Reynolds, 1989).
4.2.2 Children
It is not recommended for paediatric use (Reynolds,
1989).
4.3 Contraindications
Glutethimide is contraindicated in porphyria. Due to
its anti-muscarinic action, it should be given with great
care to patients with closed-angle glaucoma, prostatic
hypertrophy or urinary tract obstruction, and certain cardiac
arrhythmias. Alcohol enhances absorption and the hypnotic
effects of glutethimide. Like barbiturates, glutethimide
induces microsomal hepatic enzymes and enhances the
metabolism of coumarin anticoagulants and other drugs,
lowering their plasma concentrations. Chronic administration
of glutethimide may also enhance vitamin D metabolism
(Reynolds, 1989).
5. ROUTES OF ENTRY
5.1 Oral
This is the only likely route of administration in man.
5.2 Inhalation
Unknown.
5.3 Dermal
Unknown.
5.4 Eye
Unknown.
5.5 Parenteral
Unknown.
5.6 Other
Unknown.
6. KINETICS
6.1 Absorption by route of exposure
Oral: In six healthy volunteers given a dose of 500 mg,
absorption was irregular and peak plasma concentrations
occurred over one to six hours. However, in four of the six
subjects absorption was biphasic (Curry et al., 1971).
Erratic absorption may be due to the poor solubility of
glutethimide in water. The onset of sedation usually occurs
in 15 to 30 minutes (Baum et al., 1965).
Parenteral: following intraperitoneal administration of 70
mg/kg studies in the rat, most of drug was found in the brain
and spinal cord and other fat-containing tissues after 20
minutes (Keberle et al., 1962).
6.2 Distribution by route of exposure
Oral: following oral administration of 500 mg of
glutethimide to healthy subjects, peak plasma concentrations
of 2.85 to 7.05 駪/mL were achieved within two to six hours.
Plasma protein binding of glutethimide was about 50%. Mean
glutethimide concentrations in the breast milk of 13 nursing
mothers given 500 mg were: 0.27, 0.22, 0.12 and 0.04 駪/mL at
8, 12, 16 and 23 hours, respectively, but levels were
undetectable in one-third of the samples (Curry et al.,
1971).
Glutethimide is highly lipophilic and rapidly concentrates in
brain and adipose tissue (Hansen & Fischer, 1974).
6.3 Biological half-life by route of exposure
Oral: The elimination half-life is 10 to 12 hours
(Kastrup, 1987) but may increase in severe poisoning (Maher,
1970). In six healthy subjects, initial half-lives after
ingestion of 500 mg were 2.7 to 4.3 hours and subsequent
half-lives ranged from 5.1 to 22 hours (Curry et al.,
1971).
6.4 Metabolism
Glutethimide is partially metabolized by hydroxylation
into 4-hydroxy-2-ethyl-2-phenylglutarimide. In the mouse,
this appears to be twice as potent as the parent compound in
mice and is believed to contribute to prolonged coma
following overdosage (Hansen et al., 1975). Hydroxylated
metabolites are conjugated and excreted mainly in the urine
but also in bile (Keberle et al., 1962).
6.5 Elimination by route of exposure
Oral: glutethimide is inactivated by conjugation and
the metabolites are excreted in urine, only 2% of the parent
substance is excreted in urine, up to 2% of the dose has been
reported to be found in the faeces (Curry et al.,
1971).
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
7.1.2 Pharmacodynamics
Glutethimide directly blocks electron transfer
in cellular respiration (Reynolds, 1989).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
In adults, death has been reported
after 5 g. The usual lethal dose is 10 to 20
g, although survival after a dose of 28 g has
been reported (Skoutakis & Acchiardo,
1982).
7.2.1.2 Children
One 500 mg tablet may produce severe
toxicity in a small child (Sramek & Klajawal,
1981).
7.2.2 Relevant animal data
Not relevant.
7.2.3 Relevant in vitro data
Not relevant.
7.3 Carcinogenicity
Unknown.
7.4 Teratogenicity
There is no evidence of teratogenicity from therapeutic
use (Keberle et al., 1962).
7.5 Mutagenicity
Unknown.
7.6 Interactions
The effects of glutethimide are additive with those of
benzodiazepines, barbiturates, codeine and other CNS
depressants. Concomitant administration of antidepressants,
antiparkinsonian drugs or other anticholinergic agents may
cause additive anticholinergic effects such as urinary
retention, exacerbation of glaucoma, or adynamic ileus.
Ethanol enhances the effects of glutethimide.
Glutethimide induces the hepatic metabolism of some drugs,
such as dicoumarol derivatives, the dose of drugs taken
concomitantly may require adjustment (Hansten & Horn,
1989).
7.7 Main adverse effects
Common adverse effects are as follows: nausea, headache,
hangover, blurred vision, occasional skin rashes, blood
disorders (megaloblastic anaemia) (Pearson, 1965).
Osteomalacia (Greenwood et al., 1973) peripheral neuropathy
and cerebral impairment (Nover, 1967) after prolonged use may
also occur. Glutethimide is a drug of abuse and may cause
dependence.
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: suspect materials
e.g. tablets, liquids
In case of ingestion:
Vomitus: total amount
Gastric aspirate: total amount
(or gastric lavage: first
portion: 100 mL)
Blood without additives: 10 mL
Urine: random specimen: 50 mL
8.1.1.2 Biomedical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses.
8.1.1.3 Arterial blood gas analysis
Heparinized arterial blood sample
(in severe cases).
8.1.1.4 Haematological analyses
Not necessary.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Store separated serum in
refrigerator (4蚓).
8.1.2.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
Not applicable.
8.1.2.5 Other (unspecified) analyses
Acute glutethimide poisoning is not
associated with specific biochemical effects
other than changes secondary to coma,
respiratory failure and shock. Routine
analyses for assessing the patient's general
clinical condition are necessary.
Chronic glutethimide abuse may lead to
conditions such as megaloblastic anaemia
(Pearson, 1965) or osteomalacia (Greenwood et
al., 1973). Bone marrow biopsy,
calcium/phosphate studies and serum
phosphatase determinations may be
necessary.
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
In acute glutethimide poisoning, an
isoelectric encephalogram may not indicate
brain death or a fatal prognosis
(Huttenlocher, 1963).
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
The presence of glutethimide in
materials can be inferred by a number of
simple colour tests. Details of the reagents
and procedures for these tests can be found
in Moffat et al. (1986). Results of colour
tests must be taken as presumptive only,
since many other drugs give similar
reactions, and the limitations of each are
given where these are known.
Koppanyi-Zwikker test. Dissolve
approximately 1 mg of the material in 1 mL
ethanol. Add 1 drop of 1% cobalt nitrate in
ethanol, followed by 10 無 pyrrolidine. A
violet reaction is given by compounds which
have >C=O and >NH groups adjacent within a
ring (i.e. by glutethimide and by
barbiturates). Note that hydrochloride salts
give a blue colour before addition of
pyrrolidine.
Liebermann's test. To 1 mg of material on a
white tile, add 2 drops 0.1% sodium nitrite
in concentrated sulphuric acid. A red colour
is produced by glutethimide, and by
phenobarbital, but not by other barbiturates.
Since many substances give a red colour with
sulphuric acid, all positive materials should
be re-tested with sulphuric acid.
Mercurous nitrate test. To freshly prepared
saturated mercurous nitrate add solid sodium
bicarbonate until effervescence ceases and
the precipitate becomes yellow. Shake before
use, and use within 1 hour. Dissolve a small
amount of test material in a minimum of
ethanol, and add one drop of reagent. A dark
grey / black colour within 2 minutes is given
by ring imides or sulphonamides with an
additional ring. The barbiturate reaction is
quicker and more intense than that of
glutethimide.
UV spectrophotometry gives rather more
specificity. Dissolve a portion of material
in ethanol to achieve an appropriate
instrument response. If necessary,
centrifuge or filter the mixture and analyse
the clear supernatant. The spectrum in
ethanol gives deltamax at 252 nm, 258 nm (A|
= 18) and 264 nm. Glutethimide is unstable
at alkaline pH, due to hydrolysis of the
glutarimide ring. Adjustment of the pH to
>11 (e.g. by addition of 4M NaOH or ammonium
hydroxide) to the ethanolic solution of
glutethimide results in a characteristic
decline in absorbance at 230 - 235 nm over a
time period of some 20 minutes.
Immunoassays for barbiturates (e.g. TDx
[Abbott Laboratories, Abbott Park, Illinois
60064 USA] or EMIT [Syva-Behring Diagnostics,
Cupertino, California 95014 USA]) do not
usually have sufficient cross-reactivity to
respond to glutethimide. However,
cross-reactivity varies between
manufacturers, and for polyclonal assays,
between lot numbers of the same kit. More
than 25 mg/L glutethimide is usually required
to obtain a positive result, corresponding to
a cross reactivity of less than 1%. Since
the concentration in a suspect material is
likely to exceed this concentration by
several-fold, it is always worth testing the
cross-reactivity of available kit by the
addition of known amounts of glutethimide to
drug free urine. Once the cut-off
concentration has been determined in this
way, the test substance dissolved in drug
free urine can then be examined.
Thin layer chromatography is highly
appropriate for identification of
glutethimide, and may be either an in-house
system or a commercially-available system
such as Toxi-Lab [Ansys Inc, Irvine,
California 92718, USA]. The material can be
dissolved in an organic solvent such as
methanol or dichloromethane and applied
directly to the plate. Using silica plates
without modifiers and standard systems, the
Rf of glutethimide is 0.75 on methanol /
concentrated ammonia (100: 1.2), and 0.62 on
ethyl acetate / methanol / ammonia (85:15:6).
Several locating reagents can be used.
Mercurous nitrate reagent is the most
specific, and gives a dark grey response with
a sensitivity of approximately 10 ng.
However, the purple response produced by
mercuric chloride-diphenylcarbazone reagent,
and the positive reaction to Dragendorff or
acidified iodoplatinate are also useful but
are less characteristic (Moffat et al.,
1986).
8.2.1.2 Advanced qualitative confirmation test(s)
Gas chromatography can be used after
dissolving the material in a small amount of
organic solvent (e.g. 10 mg in 10 mL
methanol). The Retention index for
glutethimide is 1836 on OV1, SE30, DB5 or
similar phases. Isothermal analysis may be
performed at about 220蚓, without the need
for derivatization. Flame ionization
detection gives adequate sensitivity (2 to 5
ng on column), and nitrogen-phosphorus
detection gives additional selectivity (see
for example Gold et al., 1974; Hansen &
Fischer, 1974; Flanagan & Berry, 1977). Mass
spectrometry can be applied to the gas
chromatographic identification of
glutethimide in suspect materials.
Characteristic fragmentation is achieved
without the need for derivatization, and the
most abundant ions are m/z 189, 132, 117,
160 and 217 (Kennedy et al., 1978).
HPLC may be used to identify glutethimide,
and most published methods involve reverse
phase chromatography with UV detection.
Dissolve a small amount of the suspect
material in the mobile phase, and filter if
necessary to obtain a clear supernatant.
Kabra et al. (1978) used a C18 column with a
mobile phase of acetonitrile / phosphate
buffer (300 無 1M KH2PO4 and 50 無 0.9 M
phosphoric acid in 1800 mL water) [215:785].
Using isocratic elution at 50蚓 glutethimide
was detected at 195 nm with a relative
retention of 0.55 to the internal standard
methylphenytoin. Svinarov & Dotchev (1989)
used a C8 column with a mobile phase of
acetonitrile / water (1:4), performing
isocratic elution at ambient temperature.
Glutethimide was detected at 208 nm with a
relative retention of 1.57 to the internal
standard tolylphenobarbital. Additional
confirmation of identity may be obtained by
performing a full scan analysis on the
appropriate portion of the HPLC
effluent.
8.2.1.3 Simple quantitative method(s)
Direct quantitative
spectrophotometric analysis of glutethimide
has been described. The decline in
absorbance in alkaline solution at 233 nm due
to hydrolysis of the glutarimide ring
directly correlates with the amount of
glutethimide present. The test is performed
by dissolving a small amount of material in
chloroform. Five volumes of the test
solution are mixed with one volume of 3M NaOH
solution. Absorbance at 233 nm is measured
at one and five minutes after addition of the
alkali. Alternatively, the difference in
absorbance at 233 nm at time zero and 20
minutes (by which time the degradation will
be completed) can be used. Quantitation is
performed by comparison to the analysis of
known amounts of glutethimide prepared
similarly. If a scanning spectrophotometer
is available, this test can be combined
effectively with the Broughton method for
barbiturate determination by following the
differential absorbance of pH 9.5 and pH 13
sample extracts over the wavelength range 220
to 320 nm (Dain & Trainer, 1970).
8.2.1.4 Advanced quantitative method(s)
Gas chromatography can be used after
dissolving the material in a small amount of
organic solvent (e.g. 10 mg in 10 mL
methanol). The retention index for
glutethimide is 1836 on OV1, SE30, DB5 or
similar phases. Isothermal analysis may be
performed at about 220蚓, without the need
for derivatization. Flame ionization
detection gives adequate sensitivity (2 to 5
ng), although nitrogen-phosphorus detection
gives additional selectivity. Quantitation
is performed by addition of an internal
standard (e.g. p-dimethylaminobenzaldehyde,
piperidone, p-hydroxybenzophenone or a
non-prescription barbiturate) and direct
comparison to known amounts of glutethimide
subjected to similar dilution. Using mass
spectrometry detection quantification of
glutethimide in materials is achieved in SIM
mode (using m/z 189; qualifier m/z 160)
without the need for derivatization (Kennedy
et al., 1978).
HPLC may be used to quantify glutethimide in
residues, and most published methods involve
reverse phase chromatography with UV
detection. The material should be dissolved
in mobile phase (e.g. 10 mg in 10 mL) and
filtered to provide a clear supernatant if
necessary. Kabra et al. (1978) used a C18
column with a mobile phase of acetonitrile /
phosphate buffer (300 無 1M KH2PO4 and 50
無 0.9 M phosphoric acid in 1800 mL water)
[215:785]. Using isocratic elution at 50蚓
glutethimide was detected at 195 nm with a
relative retention of 0.55 to the internal
standard methylphenytoin. Svinarov & Dotchev
(1989) used a C8 column with a mobile phase
of acetonitrile / water (1:4), performing
isocratic elution at ambient temperature.
Glutethimide was detected at 208 nm with a
relative retention of 1.57 to the internal
standard tolylphenobarbital. Quantitation is
achieved by comparison to known amounts of
glutethimide subjected to similar
dilution.
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
Commonly-available immunoassay kits
for barbiturate detection do not have
sufficient cross-reactivity to respond to
glutethimide or its metabolites in biological
specimens: sensitivity is usually less than
25 mg/L, which is less than 1% cross
reactivity (see under 8.2.1.1 above).
Direct UV methods may be applied to the
detection of glutethimide in gastric
contents, but are not useful for the analysis
of other fluids. Dilute a portion of gastric
contents in ethanol to achieve an appropriate
instrument response. The spectrum in ethanol
gives deltamax at 252 nm, 258 nm (A| = 18)
and 264 nm. Glutethimide and its common
metabolites are unstable in alkaline solution
(pH>11) due to hydrolysis of the glutarimide
ring. For the spectrophotometric
identification of glutethimide in urine or
serum, the drug must first be extracted from
the matrix at neutral pH into a polar solvent
(e.g. dichloromethane, ethyl acetate) to
maximize response from the metabolites.
After solvent evaporation, the residue is
taken up in water. The pH is adjusted to 13
by the addition of one part of 3M NaOH
solution to five parts of test solution.
Absorbance at 233 nm is monitored, where a
characteristic decline over a time period of
some 20 minutes will be observed. If a
scanning spectrophotometer is available, this
test can be combined effectively with the
Broughton method for barbiturate
determination by following the differential
absorbance of pH 9.5 and pH 13 sample
extracts over the wavelength range 220 to 320
nm (Dain & Trainer, 1970).
Thin layer chromatography can then be used
after extraction from the samples (urine or
gastric contents - 10 to 20 mL) into an
organic solvent at pH 5 to 7 (glutethimide is
unstable under alkaline conditions). The use
of a polar extraction solvent (e.g.
dichloromethane, ethyl acetate) ensures good
recovery of glutethimide and a number of
metabolites, whereas the use of a non-polar
solvent (e.g. hexane, petroleum ether)
excludes virtually all the metabolites from
the extraction. Concentration of the extract
may be performed by evaporation of the
solvent. Thin layer chromatography may be
either an in-house system or a
commercially-available system such as
Toxi-Lab [Ansys Inc, Irvine, California
92718, USA]. Using silica plates without
modifiers and standard solvent systems, the
Rf is 0.75 on methanol / concentrated ammonia
(100: 1.2), and 0.62 on ethyl acetate /
methanol / ammonia (85:15:6) (Moffat et al.,
1986). Chromatograms of urine samples
extracted with polar solvents typically show
up to six distinctive spots when eluted in
cyclohexane / ethanol (80:20). Rf values on
this system are: glutethimide 0.53;
4-hydroxyglutethimide 0.42; other
metabolites at 0.05, 0.13, 0.30 and 0.38
(Sunshine et al., 1969). Several locating
reagents can be used. Mercurous nitrate
reagent is the most specific, and gives a
dark grey response with a sensitivity in the
region of 1 mg/L in the original sample (50
ng on plate). However, the purple response
produced by mercuric
chloride-diphenylcarbazone reagent, and the
positive reaction to Dragendorff or acidified
iodoplatinate are also useful but are less
sensitive and less characteristic (Moffat et
al., 1986).
8.2.2.2 Advanced Qualitative Confirmation Test(s)
Gas chromatography can be used after
extraction into an organic solvent from a pH
adjusted to 4 to 7 using a phosphate buffer.
The use of a polar solvent (e.g.
dichloromethane or ethyl acetate) ensures
good recovery of glutethimide and
metabolites, whereas the use of a non-polar
solvent (e.g. hexane or petroleum ether)
excludes the extraction of up to 90% of the
metabolites. The recovery of metabolites from
urine can be greatly enhanced by incubation
of the sample with glucuronidase prior to
extraction (e.g. at pH5 for one hour at
50蚓). Isothermal analysis may be performed
at about 220蚓, without the need for
derivatization. Urine extracted with polar
solvents typically show up to six peaks in
addition to glutethimide: two elute before,
and four elute after glutethimide. The major
urinary metabolites are immediately adjacent
to the glutethimide peak. The retention
index for glutethimide is 1836 on OV1, SE30,
DB5 or similar phases; the major metabolites
4-hydroxyglutethimide and 2-phenylglutarimide
run at 1875 and 1778 respectively. Flame
ionization detection gives adequate
sensitivity (2 to 5 ng), and
nitrogen-phosphorus detection gives
additional selectivity, but does not give
improved sensitivity. Mass
spectrophotometric detection can be applied
to the gas chromatographic detection of
glutethimide and several metabolites in
plasma and urine. Characteristic
fragmentation of glutethimide,
2-phenylglutarimide and desethylglutethimide
is achieved without the need for
derivatization, although the hydroxylated
metabolites are chromatographed as
trifluoroacetate derivatives (Kennedy et al.,
1978).
HPLC may be used to identify glutethimide,
and most published methods involve reverse
phase chromatography with UV detection, and
do not discuss analysis of urine specimens.
The drug must first be extracted from the
specimen, and the precipitation of plasma
with an equal volume of acetonitrile as
described by Kabra et al. (1978) is easily
performed and reliable. Kabra et al. (1978)
used a C18 column with a mobile phase of
acetonitrile / phosphate buffer (300 無 1M
KH2PO4 and 50 無 0.9 M phosphoric acid in
1800 mL water) [215:785]. Using isocratic
elution at 50蚓 glutethimide was detected at
195 nm with a relative retention of 0.55 to
the internal standard methylphenytoin.
Svinarov & Dotchev (1989) used a C8 column
with a mobile phase of acetonitrile / water
(1:4), performing isocratic elution at
ambient temperature. Glutethimide was
detected at 208 nm with a relative retention
of 1.57 to the internal standard
tolylphenobarbital. Neither method shows
chromatograms from specimens taken following
glutethimide ingestion, nor give mention of
glutethimide metabolites. Additional
confirmation of identity may be obtained by
performing a full scan analysis on the
appropriate portion of the HPLC effluent or
incorporating a diode array
detector.
8.2.2.3 Simple Quantitative Method(s)
Quantitative spectrophotometric
analysis of glutethimide in biological fluids
has been described. The analysis is based on
the observation that the decline in
absorbance in alkaline solution at 230 nm,
due to hydrolysis of the glutarimide ring
directly correlates with the amount of
glutethimide present. The test is performed
by extracting the drug from the matrix at
neutral pH (at pH 5.5 the recovery of
barbiturates and glutethimide is higher, but
this introduces the possibility of
co-extraction of salicylates which will
interfere with the absorption spectrum).
Care should be taken to select a non-polar
solvent such as hexane or petroleum ether, as
the use of a polar solvent co-extracts
glutethimide metabolites which will interfere
with the analysis and produce falsely
elevated results, particularly in the later
stages of intoxication. Some methods which
use dichloromethane as extraction solvent
employ a washing step with NaOH to remove
co-extracted metabolites, but it is thought
that contact with the alkali initiates the
degradation process and makes the timing of
the assay critical. The extract is
evaporated to dryness and the residue is
taken up in water (methods which use ethanol
as the reconstitution solvent suffer
interference from the dissolution of fatty
deposits from serum). Five volumes of the
test solution are mixed with one volume of 3M
NaOH solution. Absorbance at 233 nm is
measured at one and five minutes after
addition of the alkali. Alternatively, the
difference in absorbance at 233 nm at time
zero and 20 minutes (by which time the
degradation will be completed) can be used.
Quantitation is performed by comparison to
the analysis of known amounts of glutethimide
prepared in a similar matrix and extracted
similarly. (Dain & Trainer, 1970). A
modification of this procedure is given by
Finkle (1975). If a scanning
spectrophotometer is available, this test can
be combined effectively with the Broughton
method for barbiturate determination by
following the differential absorbance of pH
9.5 and pH 13 sample extracts over the
wavelength range 220 to 320 nm (Dain &
Trainer, 1970).
8.2.2.4 Advanced quantitative method(s)
Gas chromatography methods for
quantitation of glutethimide and its major
metabolites in serum have been described. In
general, most of the methods which have been
described for screening of barbiturates and
hypnotics in serum can be applied to the
analysis of glutethimide. However, all of
the published dedicated gas chromatography
methods for glutethimide pre-date the use of
capillary columns which offer superior
separation over standard packed columns and
improved sensitivity for polar metabolites.
After the addition of a suitable internal
standard (e.g. p-dimethylaminobenzaldehyde,
piperidone, p-hydroxybenzophenone or a
non-prescription barbiturate), the drug is
extracted into an organic solvent from a pH
adjusted to 4 to 7 using a phosphate buffer.
If non-polar solvents such as hexane are
used, metabolites will not be extracted: the
use of polar solvents (e.g. dichloromethane
or ethyl acetate) ensures that metabolites
are also extracted. Gold et al. (1974)
report the presence of up to six metabolites
in sera from poisoned patients, only three of
which were present in significant quantities.
Chromatography can be performed isothermally
at about 220蚓, on a number of common packed
column phases (OV1, SE30, OV225, Carbowax
20M, PolyA-103) without the need for
derivatization: DB5 is a useful capillary
column equivalent. The retention index for
glutethimide is 1836 on OV1, SE30, DB5 or
similar phases; the major metabolites
4-hydroxyglutethimide and 2-phenylglutarimide
run at 1875 and 1778 respectively. Flame
ionization detection gives adequate
sensitivity (2 to 5 ng), and
nitrogen-phosphorus detection gives
additional selectivity, but does not improve
sensitivity. Quantitative analysis can be
performed by comparison to known amounts of
glutethimide dissolved in aqueous solution or
preferably plasma / serum and extracted
similarly. Using 0.2 mL of serum a
sensitivity of 1 mg/L should easily be
achieved. For example see the methods
described by Gold et al., 1974; Hansen &
Fischer, 1974; Flanagan & Berry, 1977. When
analysing plasma using a packed column and
FID, care should be taken to exclude
interference from co-eluting endogenous fatty
acids. The use of a capillary column or a
more selective detector (nitrogen-phosphorus)
alleviates this problem. Mass Spectrometry in
SIM mode has been used with gas
chromatography for quantification of
glutethimide and its metabolites in plasma
and urine. Glutethimide, 2-phenylglutarimide
and dehydroglutethimide are quantified
directly, while the hydroxylated metabolites
are chromatographed following derivatization
with trifluoroacetic anhydride (Kennedy et
al., 1978).
HPLC methods are described for quantitative
analysis of glutethimide in plasma. Most
published methods involve reverse phase
chromatography with UV detection, and give a
sensitivity of 1 mg/L using a 100 無 sample
volume. The drug must first be extracted from
the specimen, and the precipitation of plasma
with an equal volume of acetonitrile as
described by Kabra et al. (1978) is easily
performed and reliable. Kabra et al. (1978)
used a C18 column with a mobile phase of
acetonitrile / phosphate buffer (300 無 1M
KH2PO4 and 50 無 0.9 M phosphoric acid in
1800 mL water) [215:785]. Using isocratic
elution at 50蚓 glutethimide was detected at
195 nm with a relative retention of 0.55 to
the internal standard methylphenytoin.
Svinarov & Dotchev (1989) used a C8 column
with a mobile phase of acetonitrile / water
(1:4), performing isocratic elution at
ambient temperature. Glutethimide was
detected at 208 nm with a relative retention
of 1.57 to the internal standard
tolylphenobarbital. Neither method shows
chromatograms of specimens taken following
glutethimide ingestion, nor gives mention of
glutethimide metabolites. Analysis of
patient samples by HPLC must therefore be
undertaken with due attention to the
possibility of interference from co-extracted
metabolites. Quantitation is performed by
comparison to samples of drug free plasma to
which known amounts of glutethimide have been
added and treated similarly.
8.2.2.5 Other dedicated method(s)
Not applicable.
8.2.3 Interpretation of toxicological analyses
There is considerable variation in individual
response to a given plasma glutethimide concentration.
Contribution to the overall clinical picture can be
made by co-ingested medications, the amount of toxic
metabolites produced, the degree of tissue
distribution, underlying medical conditions, presence
of infective agents etc. As a guide, the following
table shows typical concentrations of glutethimide in
serum.
mg/L 痠ol/L
After single oral dose (1 to 6 hours) 3 - 7 14 - 32
Steady-state in therapy <4 <18
Toxicity apparent (coma, convulsions,
pulmonary oedema) 10 46
Potentially fatal (deep coma,
sudden apnoea) 30 138
After therapeutic doses, peak concentrations of the
active metabolite 4-hydroxyglutethimide are in the
range 4 to 6 mg/L at about 24 hours. After overdose,
4-hydroxyglutethimide accumulates in plasma, rising to
several times the concentration of the parent
compound, and peaking on the second day, where after
it declines in parallel to glutethimide. There is
disagreement over whether fluctuations in
4-hydroxyglutethimide may be responsible for, or
contribute to the cyclical and prolonged coma seen
after overdose (Gold et al., 1974; Hansen et al.,
1975; Curry et al., 1987). There are insufficient data
to determine the clinical significance of the
concentrations of the other active metabolites
2-phenylglutarimide and the gamma-butyrolactone
derivative.
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Sodium, potassium, chloride
Alanine aminotransferase, aspartate
transaminase
Glucose, urea, creatinine
8.3.1.2 Urine
Not applicable.
8.3.1.3 Other fluids
No dedicated test.
8.3.2 Arterial blood gas analyses
pH, pCO2, pO2, HCO3- concentration, base
excess, O2-saturation.
8.3.3 Haematological analyses
Not applicable.
8.3.4 Interpretation of biomedical investigations
Not applicable.
8.3.5 Interpretation of biological investigations
Acute glutethimide poisoning is not associated
with specific biochemical effects other than changes
secondary to coma, respiratory failure and shock.
Routine analyses for assessing the patient's general
clinical condition are necessary.
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
There are no special precautions to be taken for sample
collection for biomedical or toxicological analyses. Acute
glutethimide poisoning is not associated with specific
biochemical effects other than changes secondary to coma,
respiratory failure and shock. Routine analyses for
assessing the patient's general clinical condition are
necessary.
Presumptive tests on toxic ingredients of materials can be
performed by colourimetric, spectrophotometric or thin layer
chromatographic techniques. Gas chromatography of
glutethimide (flame ionization detection) is not difficult,
and is much more specific.
Specific identification of the causative agent as
glutethimide in cases of hypnotic intoxication is useful
since the clinical course of glutethimide poisoning is more
complicated, and its management is more difficult, than that
of the barbiturates. Measurement of serum concentrations of
glutethimide may be useful in cases where coma is prolonged
or symptoms are particularly severe.
Measurement of glutethimide in biological materials is
possible after extraction into an organic solvent, and
metabolites will be co-extracted if polar solvents are used.
Metabolites (particularly 4-hydroxyglutethimide) are seen by
most advanced techniques. Qualitative analysis is most
easily performed by thin layer chromatography. Gas
chromatography allows for both qualitative and quantitative
analysis, and derivatization is not required; flame
ionization detection gives adequate sensitivity for most
applications. Gas chromatography / mass spectrometry has
been used where confirmatory testing is required. HPLC has
not been widely used.
Typical concentrations of glutethimide in serum are:
mg/L 痠ol/L
After single oral dose (1 to 6 hours) 3 - 7 14 - 32
Steady-state in therapy <4 <18
Toxicity apparent (coma, convulsions,
pulmonary oedema) 10 46
Potentially fatal (deep coma, sudden
apnoea) 30 138
The metabolite 4-hydroxyglutethimide may contribute to the
clinical and toxic effects of glutethimide, but there are
insufficient data to determine the clinical significance of
the concentrations of the metabolites 4-hydroxyglutethimide,
2-phenylglutarimide and gamma-butyrolactone, all of which
have known pharmacological activity.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Ingestion is the only route by which acute
poisoning may occur. Mild intoxication with small
doses results in somnolence, ataxia, tonic muscle
spasms, abnormal reflexes.
In severe intoxication coma, hypotension, hypothermia,
shock, respiratory depression and cerebral oedema may
occur.
Signs from other organs and systems are usually
secondary to coma and shock.
9.1.2 Inhalation
Unknown.
9.1.3 Skin exposure
Unknown.
9.1.4 Eye contact
Unknown.
9.1.5 Parenteral exposure
Unknown.
9.1.6 Other
Unknown.
9.2 Chronic poisoning
9.2.1 Ingestion
Ingestion is the only route of glutethimide
administration in humans. Prolonged use of the drug
may cause peripheral neuropathy (Nover, 1967),
hypocalcaemia (Ober et al., 1981) and osteomalacia
(Greenwood et al., 1973). Acute abstinence syndrome
following glutethimide withdrawal has been described
(Johnson & Van Buren, 1962). Chronic ingestion of
high doses is associated with impaired memory,
inability to concentrate, ataxia, tremors,
hyporeflexia, slurring of speech, and convulsions
(Reynolds, 1989).
9.2.2 Inhalation
Unknown.
9.2.3 Skin exposure
Unknown.
9.2.4 Eye contact
Unknown.
9.2.5 Parenteral exposure
Unknown.
9.2.6 Other
Unknown.
9.3 Course, prognosis, cause of death
Any acute poisoning without loss of consciousness may be
regarded as mild and the patient is not at risk.
The occurrence of coma, hypotension, hypothermia, shock,
respiratory depression and complications such as pneumonia
mean that glutethimide poisoning is potentially serious.
However, death is unusual provided the patient is admitted to
intensive care when needed. Cerebral oedema may be fatal
(Wright & Roscoe, 1970).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Hypotension, shock and tachycardia have been
observed. Unexplained dysrhythmias may be due to the
antimuscarinic effects of the drug or low plasma
calcium concentrations (Wright & Roscoe, 1970, Chazan
& Garella, 1971).
9.4.2 Respiratory
Respiratory depression with intermittent apnea
and or arrest may occur in very severe cases.
Pneumonia due to aspiration and pulmonary oedema have
been reported (Wright & Roscoe, 1970, Chazan &
Garella, 1971).
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Various degrees of CNS depression
may occur, ranging from lethargy to deep
coma. Cerebral oedema, intracranial
haemorrhage, tonic muscle spasms and
hyperreflexia may occur. Truncal ataxia has
been reported in acute glutethimide
intoxication in children (Huttenlocher,
1963).
9.4.3.2 Peripheral nervous system
Peripheral neuropathy and diplopia
has been reported following chronic
use.
9.4.3.3 Autonomic nervous system
Glutethimide has
antimuscarinic/anticholinergic activity,
tachycardia, dryness of mouth, mydriasis,
irritability, urinary retention and
constipation.
9.4.3.4 Skeletal and smooth muscle
Tonic muscle spasm and paralytic
ileus (adynamic ileus) may also be
observed.
9.4.4 Gastrointestinal
Gastrointestinal atony due to parasympatholytic
activity may occur (Chazan & Garella, 1971).
9.4.5 Hepatic
No direct effects are known.
9.4.6 Urinary
9.4.6.1 Renal
With the exception of possible
pre-renal uraemia due to severe hypotension,
no other renal effects occur (Wright &
Roscoe, 1970; Chartier, 1983).
9.4.6.2 Other
Urinary retention may occur due to
the anticholinergic effect of
glutethimide.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
Bullous changes resembling those seen in
barbiturate poisoning (Burdon & Cade, 1979) and
erythematous vesicles (Leavell et al., 1972) have been
described.
9.4.9 Eye, ear, nose, throat: local effects
Mydriasis and papilloedema have been observed
(Wright & Roscoe, 1970).
9.4.10 Haematological
Significant methaemoglobinemia has been
reported rarely (Filippini, 1965); in a further case,
megaloblastic anaemia, thrombocytopenia and aplastic
anaemia occurred (Pearson, 1965).
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Acid base disturbances may occur
secondary to coma or shock.
9.4.12.2 Fluid and electrolyte disturbances
Hypocalcaemia has been described
(Crawshaw, 1968).
9.4.12.3 Others
Hypothermia has been described
(Skoutakis & Acchiardo, 1982; Ozdemir &
Tannenberg, 1972).
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Glutethimide readily crosses the placenta and
may cause neonatal respiratory depression (Kurtz et
al., 1966, Reveri et al., 1977) and neonatal
withdrawal symptoms (Asnes & Lamb, 1969).
Eight to 12 hours after a maternal does of 500 mg of
glutethimide, a peak concentration of 270 nanogram per
mL in breast milk has been reported (Curry et al.,
1971).
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with mild poisoning who are only sedated need
little or no treatment. Emptying the stomach by emesis
and/or lavage should be done within the first four hours
after ingestion if the clinical condition of the patient
allows it. If the patient is obtunded, gastric lavage should
be performed after endotracheal intubation.
Coma associated with shock is the most important feature of
severe poisoning. Treatment is symptomatic.
There is no specific antidotes. Procedures to enhance
elimination are not recommended: forced diuresis has been
shown to be ineffective; haemodialysis (even using oil as
dialyzing fluid) and haemoperfusion have not proven to be
effective.
10.2 Life supportive procedures and symptomatic/specific treatment
Patients with mild signs of overdose do not need
special treatment but should be under continuous clinical
observation, especially during the early stages of poisoning.
Intestinal absorption of additional amounts of glutethimide
may unpredictably precipitate deep coma requiring intensive
care.
Severe poisoning with coma always needs intensive care. It
is essential to maintain a clear airway and provide oxygen.
Perform endotracheal intubation and support ventilation.
Frequent change of the position of the patient and vigorous
physiotherapy are indicated to prevent pneumonia and
pulmonary infarctions. Pneumonia must be treated with
appropriate antibiotics.
Maintain one central or peripheral intravenous route.
Administer intravenous fluids in amounts adequate to maintain
daily diuresis of two to three litres. Urinary
catheterization is necessary in the comatose patient to
measure hourly urine output and obtain urine samples.
In case of significant hypotension, hypovolaemia must be
considered as a possible cause and be corrected. If
hypotension is severe, infusion of dopamine may be required
(2 to 5 痢/kg/minute, not more than 10 痢/kg/minute).
Monitoring of central venous pressure, and if possible
pulmonary artery pressures is indicated (Swan-Ganz
catheter).
Papilledema or other signs of cerebral edema (Wright &
Roscoe, 1970) may indicate the need for mannitol 20%.
Correct acidosis.
10.3 Decontamination
Since ingestion is the route of poisoning only
decontamination of the gastrointestinal tract should be
considered.
Induce vomiting and perform gastric lavage within the first
four hours following ingestion, but only in the conscious
patient. If the patient is drowsy or comatose, gastric
lavage should be done after endotracheal intubation.
Activated charcoal and cathartics should be given, unless
contraindicated (Ellenhorn & Barceloux, 1988).
10.4 Enhanced elimination
Forced diuresis does to enhance elimination of
glutethimide (Wright & Roscoe, 1970). Haemodialysis (even
using oil as the dialyzing fluid), (Chazan & Garella, 1971);
and haemoperfusion through charcoal column (Koffler et al.,
1978) or resin column (Raja, 1986) have not proved effective.
Experience at the Warsaw Poison Centre with oil haemodialysis
and charcoal haemoperfusion is not convincing.
10.5 Antidote treatment
10.5.1 Adults
No antidote available.
10.5.2 Children
No antidote available.
10.6 Management discussion
Supportive treatment is essential for the successful
management of acute glutethimide poisoning.
The prognosis is favourable even in severe cases but the
possibility of mixed poisoning must always be
considered.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
The most recent publications have reviewed many cases
of acute poisoning and their conclusions are included in this
monograph (Wright & Roscoe, 1970; Chazan & Garella, 1971).
Short descriptions and discussion of particular cases will be
presented under 11.2. Only two cases of chronic poisoning
are mentioned here.
Case 1 (Nover, 1967): A 37 year-old woman was treated with
glutethimide, up to 5 g per day, for five years. She
developed sensory neuropathy with glove-and-stocking
paraesthesias and noted poor recent memory and calculating
ability. She felt very weak, was unable to stand or walk
unaided and complained of ataxia. Neurological examination
found absent position, vibration, light touch, and pin prick
sensations distally in all four extremities. Decreased nerve
conduction velocity was found. Glutethimide was withdrawn
over 20 days despite a grand mal seizure occurring when the
patient was changed to phenobarbital. Even two weeks after
the complete withdrawal of glutethimide the patient was
unable to walk or stand without assistance and complained of
paraesthesias. Sensory findings were somewhat improved but
some symptoms and signs persisted for several months.
Case 2 (Pearson, 1965): A 47-year-old man was treated with
100 to 400 mg of glutethimide for five years and developed
megaloblastic anaemia with haemoglobin value as low as 6 g/dL
and megaloblastic hyperplasia in the bone marrow.
Discontinuation of glutethimide and administration of folic
acid resulted in normalization of the peripheral blood and
bone marrow.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Since there are safer hypnotic drugs currently
available, it seems, there is no reason to continue the use
of glutethimide.
12.2 Other
No data available.
13. REFERENCES
Asnes RS & Lamb JM (1969) Neonatal respiratory depression
secondary to maternal analgesics; treated by exchange
transfusion. Pediatrics 43: 94.
Banen DM & Resnick O (1973) Lorazepam v. glutethimide as a
sleep-inducing agent for the geriatric patient. J Am Geriat Soc,
21: 507.
Baum G, Bill CES, Freeling P et al. (1965) Sedation with a new
non-barbiturate compound. Practitioner 195: 366.
Burdon JGW & Cade JF (1979) Barbiturate burns caused by
glutethimide. Med J Aust, 1: 101.
Chartier DM (1983) Glutethimide and codeine overdose. Emerg
Nursing 9: 307.
Chazan JA & Garella S (1971) Glutethimide intoxication. A
prospective study of 70 patients treated conservatively without
hemodialysis. Arch Int Med, 128: 215.
Crawshaw JA (1968) Hypocalcaemia in glutethimide overdose.
Practitioner, 200: 739.
Curry SH, Riddal D, Gordan JS et al. (1971) Disposition of
glutethimide in man. Clin Pharmacol Ther, 12: 849.
Curry SC, Hubbard JM, Gerkin R, Selden B, Ryan PJ, Meinhart R,
Hagner D (1987) Lack of correlation between plasma
4-hydroxyglutethimide and severity of coma in acute glutethimide
poisoning: A case report and brief review of the literature. Med
Toxicol, 2: 309-316.
Dain D, Trainer TD (1970) Simultaneous spectrophotometric
determination of glutethimide and barbiturates. Clin Chem, 16:
318-321.
Ellenhorn MS & Barceloux DG (1988) Medical Toxicology. Elsevier
Science Publishing Co., New York.
Filippini VL (1965) Methemoglobinaemie bei Doriden-Intoxication.
Schweiz Med Wschr, 95: 1618.
Finkle BS (1975) In: Sunshine I ed. Methodology for Analytical
Toxicology. Cleveland Ohio, CRC Press, pp178-180.
Flanagan RJ, Berry DJ (1977) Routine analysis of barbiturates and
some other hypnotic drugs in blood plasma as an aid to the
diagnosis of acute poisoning. J Chromatogr, 131: 131-146.
Gold M, Tassoni E, Etzl E, Mathew G (1974) Concentration of
glutethimide and associated compounds in human serum and
cerebrospinal fluid after drug overdose. Clin Chem, 20: 195-
199.
Greenwood RH, Prunty FT & Silver J (1973) Osteomalacia after
prolonged glutethimide administration. B Med J, 1: 643.
Hansen AR & Fischer LJ (1974) Gas-chromatographic simultaneous
analysis for glutethimide and an active hydroxylated metabolite in
tissues, plasma and urine. Clin Chem, 20: 236.
Hansen AR, Kennedy KA, Ambre JJ et al. (1975) Glutethimide
poisoning; a metabolite contributes to morbidity and mortality. N
Engl J Med, 292: 250.
Hansten PD & Horn JR (1989) Drug interactions, 6th ed., Lea &
Febiger, Philadelphia, PA.
Huttenlocher PR (1963) Accidental glutethimide intoxication in
children. N Eng J Med, 269: 38.
Johnson FA & Van Buren HC (1962) Abstinence syndrome following
glutethimide intoxication. JAMA, 180: 1024.
Kabra PM, Koo HY, Marton LJ (1978) Simultaneous
liquid-chromatographic determination of 12 common sedatives and
hypnotics in serum. Clin Chem, 24: 657-662.
Kastrup EK (ed) (1987) Facts and comparisons. JB Lippincott Co,
St Louis, MO, 87: 269b.
Keberle H, Hoffmann K & Bernhard K (1962) The metabolism of
glutethimide (Doriden). Experientia, 18: 105.
Kennedy KA, Ambre JJ, Fischer LJ (1978) A selected ion monitoring
method for glutethimide and six metabolites: Application to blood
and urine from humans intoxicated with glutethimide. Biomed Mass
Spec, 5: 679-685.
Koffler A, Bernstein H, La Sette A et al. (1978) Fixed-bed
charcoal hemoperfusion. Treatment of drug overdose. Arch Intern
Med, 138: 1691.
Kurtz GG, Michael EF, Morosi HJ et al. (1966) Hemodialysis during
pregnancy. Arch Intern Med, 118: 30.
Leavell VM, Coyer JR & Taylor RJ (1972) Dermographism and
erythematous lines in glutethimide overdose. Arch Derm, 106:
724.
Maher JF (1970) Determinants of serum half-life of glutethimide
in intoxicated patients. J Pharmacol Exp Ther, 174: 450.
Moffat AC, Jackson JV, Moss MS, & Widdop B eds. (1986) Clarke's
Isolation and Identification of Drugs. London, Pharmaceutical
Press.
Nover R (1967) Persistent neuropathy following chronic use of
glutethimide. Clin Pharm Ther, 8: 283.
Ober KP, Hennessy JF & Hellman RM (1981) Severe hypocalcemia
associated with chronic glutethimide addiction. Am J Psych, 138:
1239.
Ozdemir AI & Tannenberg AM (1972) Peritoneal and hemodialysis for
acute glutethimide overdosages. NY State J Med, 72: 2076.
Pearson D (1965) Megaloblastic anaemia due to glutethimide.
Lancet, 1: 110.
Raja RM (1986) Resin hemoperfusion for drug intoxication - an
update. Int J Artif Org, 9: 319.
Reveri M, Pyatis SP & Pildes RS (1977) Neonatal withdrawal
symptoms associated with glutethimide (Doriden) addiction in the
mother during pregnancy. Clin Pediatr, 16: 424.
Reynolds JEF (ed.) (1989) Martindale: The Extra Pharmacopoeia,
29th Ed.
Shamoian CA (1975) Codeine and glutethimide; euphoric, addicting
combination. NY State J Med, 75: 97.
Skoutakis VA & Acchiardo SR (1982) Glutethimide intoxication.
Clin Toxicol Consultant, 4: 18.
Sramek JJ & Klajawal A (1981) Loads. N Engl J Med, 305: 231.
Sunshine I, Maes R, Faracci R (1969) Determination of
glutethimide (Doriden) and its metabolites in biologic specimens.
Clin Chem, 14: 595-609.
Svinarov DA, Dotchev DC (1989) Simultaneous
liquid-chromatographic determination of some bronchodilators,
anticonvulsants, chloramphenicol, and hypnotic agents, with
Chromosorb P columns used for sample preparation. Clin Chem 1989,
35: 1615-1618.
Wesolowski JW et al. (1968) J Pharm Sci, 87: 811.
Wright N & Roscoe PR (1970) Acute glutethimide poisoning.
Conservative treatment of 31 patients. JAMA, 214: 1704.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES),
COMPLETE ADDRESS(ES)
Author: Janusz Szajewski, MD
Warsaw Poison Control Centre
Szpital Praski III Oddzial
Chorob Wewnetrznych
Al. Swierczewskiego 67
03-701 Warsaw
Poland
Date: August 1992
Peer
Review: London, United Kingdom, September 1992
(Members of the Group: M. Balali-Mood, J. Szajewski,
O. Kasilo, A. Wong, J.F. Deng, J. Higa, S.
Shintani)
IPCS
update: May 1994
Author
Section 8: Dr S. Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editor: Mrs J. Dum幯il
International Programme on Chemical Safety
Date: May 1999
Chemical names: 2 ethyl-2-phenylglutarimide
alpha-ethyl-alpha-phenyl glutarimide
3-ethyl-3-phenylpiperidine-2,6-dione
Molecular weight: 217.26
Molecular formula: C13H15NO2
(Conversion of traditional units into SI: multiply the value
in mg/L by 4.603 to get the result in micromol per
litre.)
3.3 Physical properties
3.3.1 Colour
Colourless or white.
3.3.2 State/form
3.3.3 Description
Odourless and colourless crystals or white
crystalline powder, practically insoluble in water,
soluble one in five of ethanol, one in less than one
chloroform and one in 12 of ether, freely soluble in
acetone and ethyl acetate, soluble in methyl alcohol
(Reynolds, 1989).
Stability: at pH 5 the chemical half life was 28.3
years at 25蚓 and 1.02 months at pH 8, the
decomposition being due to hydrolysis.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
3.4.2 Storage conditions
Store in well closed, airtight containers,
protected from humidity and light (Wesolowski et al.,
1968).
4. USES
4.1 Indications
4.1.1 Indications
4.1.2 Description
Used as an hypnotic in insomnia but rarely as a
sedative, glutethimide was initially believed to be
almost free from side effects (Banen & Resnik, 1973).
However, further experience of its toxicity and
because its dependence liability (Sramek & Klajawal,
198l; Shamoian, 1975), glutethimide has been banned in
many countries and many companies have stopped
production.
4.2 Therapeutic dosage
4.2.1 Adults
The usual oral adult dose is 250 - 500 mg at
bedtime (Reynolds, 1989).
4.2.2 Children
It is not recommended for paediatric use (Reynolds,
1989).
4.3 Contraindications
Glutethimide is contraindicated in porphyria. Due to
its anti-muscarinic action, it should be given with great
care to patients with closed-angle glaucoma, prostatic
hypertrophy or urinary tract obstruction, and certain cardiac
arrhythmias. Alcohol enhances absorption and the hypnotic
effects of glutethimide. Like barbiturates, glutethimide
induces microsomal hepatic enzymes and enhances the
metabolism of coumarin anticoagulants and other drugs,
lowering their plasma concentrations. Chronic administration
of glutethimide may also enhance vitamin D metabolism
(Reynolds, 1989).
5. ROUTES OF ENTRY
5.1 Oral
This is the only likely route of administration in man.
5.2 Inhalation
Unknown.
5.3 Dermal
Unknown.
5.4 Eye
Unknown.
5.5 Parenteral
Unknown.
5.6 Other
Unknown.
6. KINETICS
6.1 Absorption by route of exposure
Oral: In six healthy volunteers given a dose of 500 mg,
absorption was irregular and peak plasma concentrations
occurred over one to six hours. However, in four of the six
subjects absorption was biphasic (Curry et al., 1971).
Erratic absorption may be due to the poor solubility of
glutethimide in water. The onset of sedation usually occurs
in 15 to 30 minutes (Baum et al., 1965).
Parenteral: following intraperitoneal administration of 70
mg/kg studies in the rat, most of drug was found in the brain
and spinal cord and other fat-containing tissues after 20
minutes (Keberle et al., 1962).
6.2 Distribution by route of exposure
Oral: following oral administration of 500 mg of
glutethimide to healthy subjects, peak plasma concentrations
of 2.85 to 7.05 駪/mL were achieved within two to six hours.
Plasma protein binding of glutethimide was about 50%. Mean
glutethimide concentrations in the breast milk of 13 nursing
mothers given 500 mg were: 0.27, 0.22, 0.12 and 0.04 駪/mL at
8, 12, 16 and 23 hours, respectively, but levels were
undetectable in one-third of the samples (Curry et al.,
1971).
Glutethimide is highly lipophilic and rapidly concentrates in
brain and adipose tissue (Hansen & Fischer, 1974).
6.3 Biological half-life by route of exposure
Oral: The elimination half-life is 10 to 12 hours
(Kastrup, 1987) but may increase in severe poisoning (Maher,
1970). In six healthy subjects, initial half-lives after
ingestion of 500 mg were 2.7 to 4.3 hours and subsequent
half-lives ranged from 5.1 to 22 hours (Curry et al.,
1971).
6.4 Metabolism
Glutethimide is partially metabolized by hydroxylation
into 4-hydroxy-2-ethyl-2-phenylglutarimide. In the mouse,
this appears to be twice as potent as the parent compound in
mice and is believed to contribute to prolonged coma
following overdosage (Hansen et al., 1975). Hydroxylated
metabolites are conjugated and excreted mainly in the urine
but also in bile (Keberle et al., 1962).
6.5 Elimination by route of exposure
Oral: glutethimide is inactivated by conjugation and
the metabolites are excreted in urine, only 2% of the parent
substance is excreted in urine, up to 2% of the dose has been
reported to be found in the faeces (Curry et al.,
1971).
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
7.1.2 Pharmacodynamics
Glutethimide directly blocks electron transfer
in cellular respiration (Reynolds, 1989).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
In adults, death has been reported
after 5 g. The usual lethal dose is 10 to 20
g, although survival after a dose of 28 g has
been reported (Skoutakis & Acchiardo,
1982).
7.2.1.2 Children
One 500 mg tablet may produce severe
toxicity in a small child (Sramek & Klajawal,
1981).
7.2.2 Relevant animal data
Not relevant.
7.2.3 Relevant in vitro data
Not relevant.
7.3 Carcinogenicity
Unknown.
7.4 Teratogenicity
There is no evidence of teratogenicity from therapeutic
use (Keberle et al., 1962).
7.5 Mutagenicity
Unknown.
7.6 Interactions
The effects of glutethimide are additive with those of
benzodiazepines, barbiturates, codeine and other CNS
depressants. Concomitant administration of antidepressants,
antiparkinsonian drugs or other anticholinergic agents may
cause additive anticholinergic effects such as urinary
retention, exacerbation of glaucoma, or adynamic ileus.
Ethanol enhances the effects of glutethimide.
Glutethimide induces the hepatic metabolism of some drugs,
such as dicoumarol derivatives, the dose of drugs taken
concomitantly may require adjustment (Hansten & Horn,
1989).
7.7 Main adverse effects
Common adverse effects are as follows: nausea, headache,
hangover, blurred vision, occasional skin rashes, blood
disorders (megaloblastic anaemia) (Pearson, 1965).
Osteomalacia (Greenwood et al., 1973) peripheral neuropathy
and cerebral impairment (Nover, 1967) after prolonged use may
also occur. Glutethimide is a drug of abuse and may cause
dependence.
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Toxic ingredient: suspect materials
e.g. tablets, liquids
In case of ingestion:
Vomitus: total amount
Gastric aspirate: total amount
(or gastric lavage: first
portion: 100 mL)
Blood without additives: 10 mL
Urine: random specimen: 50 mL
8.1.1.2 Biomedical analyses
Plasma (lithium heparin as
anticoagulant) or serum and urine for
standard biochemical analyses.
8.1.1.3 Arterial blood gas analysis
Heparinized arterial blood sample
(in severe cases).
8.1.1.4 Haematological analyses
Not necessary.
8.1.1.5 Other (unspecified) analyses
No further materials.
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Store separated serum in
refrigerator (4蚓).
8.1.2.2 Biomedical analyses
No special requirements, but as
usually performed.
8.1.2.3 Arterial blood gas analysis
No special requirements, but as
usually performed.
8.1.2.4 Haematological analyses
Not applicable.
8.1.2.5 Other (unspecified) analyses
Acute glutethimide poisoning is not
associated with specific biochemical effects
other than changes secondary to coma,
respiratory failure and shock. Routine
analyses for assessing the patient's general
clinical condition are necessary.
Chronic glutethimide abuse may lead to
conditions such as megaloblastic anaemia
(Pearson, 1965) or osteomalacia (Greenwood et
al., 1973). Bone marrow biopsy,
calcium/phosphate studies and serum
phosphatase determinations may be
necessary.
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
8.1.3.2 Biomedical analyses
In acute glutethimide poisoning, an
isoelectric encephalogram may not indicate
brain death or a fatal prognosis
(Huttenlocher, 1963).
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (unspecified) analyses
8.2 Toxicological analyses and their interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple qualitative test(s)
The presence of glutethimide in
materials can be inferred by a number of
simple colour tests. Details of the reagents
and procedures for these tests can be found
in Moffat et al. (1986). Results of colour
tests must be taken as presumptive only,
since many other drugs give similar
reactions, and the limitations of each are
given where these are known.
Koppanyi-Zwikker test. Dissolve
approximately 1 mg of the material in 1 mL
ethanol. Add 1 drop of 1% cobalt nitrate in
ethanol, followed by 10 無 pyrrolidine. A
violet reaction is given by compounds which
have >C=O and >NH groups adjacent within a
ring (i.e. by glutethimide and by
barbiturates). Note that hydrochloride salts
give a blue colour before addition of
pyrrolidine.
Liebermann's test. To 1 mg of material on a
white tile, add 2 drops 0.1% sodium nitrite
in concentrated sulphuric acid. A red colour
is produced by glutethimide, and by
phenobarbital, but not by other barbiturates.
Since many substances give a red colour with
sulphuric acid, all positive materials should
be re-tested with sulphuric acid.
Mercurous nitrate test. To freshly prepared
saturated mercurous nitrate add solid sodium
bicarbonate until effervescence ceases and
the precipitate becomes yellow. Shake before
use, and use within 1 hour. Dissolve a small
amount of test material in a minimum of
ethanol, and add one drop of reagent. A dark
grey / black colour within 2 minutes is given
by ring imides or sulphonamides with an
additional ring. The barbiturate reaction is
quicker and more intense than that of
glutethimide.
UV spectrophotometry gives rather more
specificity. Dissolve a portion of material
in ethanol to achieve an appropriate
instrument response. If necessary,
centrifuge or filter the mixture and analyse
the clear supernatant. The spectrum in
ethanol gives deltamax at 252 nm, 258 nm (A|
= 18) and 264 nm. Glutethimide is unstable
at alkaline pH, due to hydrolysis of the
glutarimide ring. Adjustment of the pH to
>11 (e.g. by addition of 4M NaOH or ammonium
hydroxide) to the ethanolic solution of
glutethimide results in a characteristic
decline in absorbance at 230 - 235 nm over a
time period of some 20 minutes.
Immunoassays for barbiturates (e.g. TDx
[Abbott Laboratories, Abbott Park, Illinois
60064 USA] or EMIT [Syva-Behring Diagnostics,
Cupertino, California 95014 USA]) do not
usually have sufficient cross-reactivity to
respond to glutethimide. However,
cross-reactivity varies between
manufacturers, and for polyclonal assays,
between lot numbers of the same kit. More
than 25 mg/L glutethimide is usually required
to obtain a positive result, corresponding to
a cross reactivity of less than 1%. Since
the concentration in a suspect material is
likely to exceed this concentration by
several-fold, it is always worth testing the
cross-reactivity of available kit by the
addition of known amounts of glutethimide to
drug free urine. Once the cut-off
concentration has been determined in this
way, the test substance dissolved in drug
free urine can then be examined.
Thin layer chromatography is highly
appropriate for identification of
glutethimide, and may be either an in-house
system or a commercially-available system
such as Toxi-Lab [Ansys Inc, Irvine,
California 92718, USA]. The material can be
dissolved in an organic solvent such as
methanol or dichloromethane and applied
directly to the plate. Using silica plates
without modifiers and standard systems, the
Rf of glutethimide is 0.75 on methanol /
concentrated ammonia (100: 1.2), and 0.62 on
ethyl acetate / methanol / ammonia (85:15:6).
Several locating reagents can be used.
Mercurous nitrate reagent is the most
specific, and gives a dark grey response with
a sensitivity of approximately 10 ng.
However, the purple response produced by
mercuric chloride-diphenylcarbazone reagent,
and the positive reaction to Dragendorff or
acidified iodoplatinate are also useful but
are less characteristic (Moffat et al.,
1986).
8.2.1.2 Advanced qualitative confirmation test(s)
Gas chromatography can be used after
dissolving the material in a small amount of
organic solvent (e.g. 10 mg in 10 mL
methanol). The Retention index for
glutethimide is 1836 on OV1, SE30, DB5 or
similar phases. Isothermal analysis may be
performed at about 220蚓, without the need
for derivatization. Flame ionization
detection gives adequate sensitivity (2 to 5
ng on column), and nitrogen-phosphorus
detection gives additional selectivity (see
for example Gold et al., 1974; Hansen &
Fischer, 1974; Flanagan & Berry, 1977). Mass
spectrometry can be applied to the gas
chromatographic identification of
glutethimide in suspect materials.
Characteristic fragmentation is achieved
without the need for derivatization, and the
most abundant ions are m/z 189, 132, 117,
160 and 217 (Kennedy et al., 1978).
HPLC may be used to identify glutethimide,
and most published methods involve reverse
phase chromatography with UV detection.
Dissolve a small amount of the suspect
material in the mobile phase, and filter if
necessary to obtain a clear supernatant.
Kabra et al. (1978) used a C18 column with a
mobile phase of acetonitrile / phosphate
buffer (300 無 1M KH2PO4 and 50 無 0.9 M
phosphoric acid in 1800 mL water) [215:785].
Using isocratic elution at 50蚓 glutethimide
was detected at 195 nm with a relative
retention of 0.55 to the internal standard
methylphenytoin. Svinarov & Dotchev (1989)
used a C8 column with a mobile phase of
acetonitrile / water (1:4), performing
isocratic elution at ambient temperature.
Glutethimide was detected at 208 nm with a
relative retention of 1.57 to the internal
standard tolylphenobarbital. Additional
confirmation of identity may be obtained by
performing a full scan analysis on the
appropriate portion of the HPLC
effluent.
8.2.1.3 Simple quantitative method(s)
Direct quantitative
spectrophotometric analysis of glutethimide
has been described. The decline in
absorbance in alkaline solution at 233 nm due
to hydrolysis of the glutarimide ring
directly correlates with the amount of
glutethimide present. The test is performed
by dissolving a small amount of material in
chloroform. Five volumes of the test
solution are mixed with one volume of 3M NaOH
solution. Absorbance at 233 nm is measured
at one and five minutes after addition of the
alkali. Alternatively, the difference in
absorbance at 233 nm at time zero and 20
minutes (by which time the degradation will
be completed) can be used. Quantitation is
performed by comparison to the analysis of
known amounts of glutethimide prepared
similarly. If a scanning spectrophotometer
is available, this test can be combined
effectively with the Broughton method for
barbiturate determination by following the
differential absorbance of pH 9.5 and pH 13
sample extracts over the wavelength range 220
to 320 nm (Dain & Trainer, 1970).
8.2.1.4 Advanced quantitative method(s)
Gas chromatography can be used after
dissolving the material in a small amount of
organic solvent (e.g. 10 mg in 10 mL
methanol). The retention index for
glutethimide is 1836 on OV1, SE30, DB5 or
similar phases. Isothermal analysis may be
performed at about 220蚓, without the need
for derivatization. Flame ionization
detection gives adequate sensitivity (2 to 5
ng), although nitrogen-phosphorus detection
gives additional selectivity. Quantitation
is performed by addition of an internal
standard (e.g. p-dimethylaminobenzaldehyde,
piperidone, p-hydroxybenzophenone or a
non-prescription barbiturate) and direct
comparison to known amounts of glutethimide
subjected to similar dilution. Using mass
spectrometry detection quantification of
glutethimide in materials is achieved in SIM
mode (using m/z 189; qualifier m/z 160)
without the need for derivatization (Kennedy
et al., 1978).
HPLC may be used to quantify glutethimide in
residues, and most published methods involve
reverse phase chromatography with UV
detection. The material should be dissolved
in mobile phase (e.g. 10 mg in 10 mL) and
filtered to provide a clear supernatant if
necessary. Kabra et al. (1978) used a C18
column with a mobile phase of acetonitrile /
phosphate buffer (300 無 1M KH2PO4 and 50
無 0.9 M phosphoric acid in 1800 mL water)
[215:785]. Using isocratic elution at 50蚓
glutethimide was detected at 195 nm with a
relative retention of 0.55 to the internal
standard methylphenytoin. Svinarov & Dotchev
(1989) used a C8 column with a mobile phase
of acetonitrile / water (1:4), performing
isocratic elution at ambient temperature.
Glutethimide was detected at 208 nm with a
relative retention of 1.57 to the internal
standard tolylphenobarbital. Quantitation is
achieved by comparison to known amounts of
glutethimide subjected to similar
dilution.
8.2.2 Tests for biological specimens
8.2.2.1 Simple qualitative test(s)
Commonly-available immunoassay kits
for barbiturate detection do not have
sufficient cross-reactivity to respond to
glutethimide or its metabolites in biological
specimens: sensitivity is usually less than
25 mg/L, which is less than 1% cross
reactivity (see under 8.2.1.1 above).
Direct UV methods may be applied to the
detection of glutethimide in gastric
contents, but are not useful for the analysis
of other fluids. Dilute a portion of gastric
contents in ethanol to achieve an appropriate
instrument response. The spectrum in ethanol
gives deltamax at 252 nm, 258 nm (A| = 18)
and 264 nm. Glutethimide and its common
metabolites are unstable in alkaline solution
(pH>11) due to hydrolysis of the glutarimide
ring. For the spectrophotometric
identification of glutethimide in urine or
serum, the drug must first be extracted from
the matrix at neutral pH into a polar solvent
(e.g. dichloromethane, ethyl acetate) to
maximize response from the metabolites.
After solvent evaporation, the residue is
taken up in water. The pH is adjusted to 13
by the addition of one part of 3M NaOH
solution to five parts of test solution.
Absorbance at 233 nm is monitored, where a
characteristic decline over a time period of
some 20 minutes will be observed. If a
scanning spectrophotometer is available, this
test can be combined effectively with the
Broughton method for barbiturate
determination by following the differential
absorbance of pH 9.5 and pH 13 sample
extracts over the wavelength range 220 to 320
nm (Dain & Trainer, 1970).
Thin layer chromatography can then be used
after extraction from the samples (urine or
gastric contents - 10 to 20 mL) into an
organic solvent at pH 5 to 7 (glutethimide is
unstable under alkaline conditions). The use
of a polar extraction solvent (e.g.
dichloromethane, ethyl acetate) ensures good
recovery of glutethimide and a number of
metabolites, whereas the use of a non-polar
solvent (e.g. hexane, petroleum ether)
excludes virtually all the metabolites from
the extraction. Concentration of the extract
may be performed by evaporation of the
solvent. Thin layer chromatography may be
either an in-house system or a
commercially-available system such as
Toxi-Lab [Ansys Inc, Irvine, California
92718, USA]. Using silica plates without
modifiers and standard solvent systems, the
Rf is 0.75 on methanol / concentrated ammonia
(100: 1.2), and 0.62 on ethyl acetate /
methanol / ammonia (85:15:6) (Moffat et al.,
1986). Chromatograms of urine samples
extracted with polar solvents typically show
up to six distinctive spots when eluted in
cyclohexane / ethanol (80:20). Rf values on
this system are: glutethimide 0.53;
4-hydroxyglutethimide 0.42; other
metabolites at 0.05, 0.13, 0.30 and 0.38
(Sunshine et al., 1969). Several locating
reagents can be used. Mercurous nitrate
reagent is the most specific, and gives a
dark grey response with a sensitivity in the
region of 1 mg/L in the original sample (50
ng on plate). However, the purple response
produced by mercuric
chloride-diphenylcarbazone reagent, and the
positive reaction to Dragendorff or acidified
iodoplatinate are also useful but are less
sensitive and less characteristic (Moffat et
al., 1986).
8.2.2.2 Advanced Qualitative Confirmation Test(s)
Gas chromatography can be used after
extraction into an organic solvent from a pH
adjusted to 4 to 7 using a phosphate buffer.
The use of a polar solvent (e.g.
dichloromethane or ethyl acetate) ensures
good recovery of glutethimide and
metabolites, whereas the use of a non-polar
solvent (e.g. hexane or petroleum ether)
excludes the extraction of up to 90% of the
metabolites. The recovery of metabolites from
urine can be greatly enhanced by incubation
of the sample with glucuronidase prior to
extraction (e.g. at pH5 for one hour at
50蚓). Isothermal analysis may be performed
at about 220蚓, without the need for
derivatization. Urine extracted with polar
solvents typically show up to six peaks in
addition to glutethimide: two elute before,
and four elute after glutethimide. The major
urinary metabolites are immediately adjacent
to the glutethimide peak. The retention
index for glutethimide is 1836 on OV1, SE30,
DB5 or similar phases; the major metabolites
4-hydroxyglutethimide and 2-phenylglutarimide
run at 1875 and 1778 respectively. Flame
ionization detection gives adequate
sensitivity (2 to 5 ng), and
nitrogen-phosphorus detection gives
additional selectivity, but does not give
improved sensitivity. Mass
spectrophotometric detection can be applied
to the gas chromatographic detection of
glutethimide and several metabolites in
plasma and urine. Characteristic
fragmentation of glutethimide,
2-phenylglutarimide and desethylglutethimide
is achieved without the need for
derivatization, although the hydroxylated
metabolites are chromatographed as
trifluoroacetate derivatives (Kennedy et al.,
1978).
HPLC may be used to identify glutethimide,
and most published methods involve reverse
phase chromatography with UV detection, and
do not discuss analysis of urine specimens.
The drug must first be extracted from the
specimen, and the precipitation of plasma
with an equal volume of acetonitrile as
described by Kabra et al. (1978) is easily
performed and reliable. Kabra et al. (1978)
used a C18 column with a mobile phase of
acetonitrile / phosphate buffer (300 無 1M
KH2PO4 and 50 無 0.9 M phosphoric acid in
1800 mL water) [215:785]. Using isocratic
elution at 50蚓 glutethimide was detected at
195 nm with a relative retention of 0.55 to
the internal standard methylphenytoin.
Svinarov & Dotchev (1989) used a C8 column
with a mobile phase of acetonitrile / water
(1:4), performing isocratic elution at
ambient temperature. Glutethimide was
detected at 208 nm with a relative retention
of 1.57 to the internal standard
tolylphenobarbital. Neither method shows
chromatograms from specimens taken following
glutethimide ingestion, nor give mention of
glutethimide metabolites. Additional
confirmation of identity may be obtained by
performing a full scan analysis on the
appropriate portion of the HPLC effluent or
incorporating a diode array
detector.
8.2.2.3 Simple Quantitative Method(s)
Quantitative spectrophotometric
analysis of glutethimide in biological fluids
has been described. The analysis is based on
the observation that the decline in
absorbance in alkaline solution at 230 nm,
due to hydrolysis of the glutarimide ring
directly correlates with the amount of
glutethimide present. The test is performed
by extracting the drug from the matrix at
neutral pH (at pH 5.5 the recovery of
barbiturates and glutethimide is higher, but
this introduces the possibility of
co-extraction of salicylates which will
interfere with the absorption spectrum).
Care should be taken to select a non-polar
solvent such as hexane or petroleum ether, as
the use of a polar solvent co-extracts
glutethimide metabolites which will interfere
with the analysis and produce falsely
elevated results, particularly in the later
stages of intoxication. Some methods which
use dichloromethane as extraction solvent
employ a washing step with NaOH to remove
co-extracted metabolites, but it is thought
that contact with the alkali initiates the
degradation process and makes the timing of
the assay critical. The extract is
evaporated to dryness and the residue is
taken up in water (methods which use ethanol
as the reconstitution solvent suffer
interference from the dissolution of fatty
deposits from serum). Five volumes of the
test solution are mixed with one volume of 3M
NaOH solution. Absorbance at 233 nm is
measured at one and five minutes after
addition of the alkali. Alternatively, the
difference in absorbance at 233 nm at time
zero and 20 minutes (by which time the
degradation will be completed) can be used.
Quantitation is performed by comparison to
the analysis of known amounts of glutethimide
prepared in a similar matrix and extracted
similarly. (Dain & Trainer, 1970). A
modification of this procedure is given by
Finkle (1975). If a scanning
spectrophotometer is available, this test can
be combined effectively with the Broughton
method for barbiturate determination by
following the differential absorbance of pH
9.5 and pH 13 sample extracts over the
wavelength range 220 to 320 nm (Dain &
Trainer, 1970).
8.2.2.4 Advanced quantitative method(s)
Gas chromatography methods for
quantitation of glutethimide and its major
metabolites in serum have been described. In
general, most of the methods which have been
described for screening of barbiturates and
hypnotics in serum can be applied to the
analysis of glutethimide. However, all of
the published dedicated gas chromatography
methods for glutethimide pre-date the use of
capillary columns which offer superior
separation over standard packed columns and
improved sensitivity for polar metabolites.
After the addition of a suitable internal
standard (e.g. p-dimethylaminobenzaldehyde,
piperidone, p-hydroxybenzophenone or a
non-prescription barbiturate), the drug is
extracted into an organic solvent from a pH
adjusted to 4 to 7 using a phosphate buffer.
If non-polar solvents such as hexane are
used, metabolites will not be extracted: the
use of polar solvents (e.g. dichloromethane
or ethyl acetate) ensures that metabolites
are also extracted. Gold et al. (1974)
report the presence of up to six metabolites
in sera from poisoned patients, only three of
which were present in significant quantities.
Chromatography can be performed isothermally
at about 220蚓, on a number of common packed
column phases (OV1, SE30, OV225, Carbowax
20M, PolyA-103) without the need for
derivatization: DB5 is a useful capillary
column equivalent. The retention index for
glutethimide is 1836 on OV1, SE30, DB5 or
similar phases; the major metabolites
4-hydroxyglutethimide and 2-phenylglutarimide
run at 1875 and 1778 respectively. Flame
ionization detection gives adequate
sensitivity (2 to 5 ng), and
nitrogen-phosphorus detection gives
additional selectivity, but does not improve
sensitivity. Quantitative analysis can be
performed by comparison to known amounts of
glutethimide dissolved in aqueous solution or
preferably plasma / serum and extracted
similarly. Using 0.2 mL of serum a
sensitivity of 1 mg/L should easily be
achieved. For example see the methods
described by Gold et al., 1974; Hansen &
Fischer, 1974; Flanagan & Berry, 1977. When
analysing plasma using a packed column and
FID, care should be taken to exclude
interference from co-eluting endogenous fatty
acids. The use of a capillary column or a
more selective detector (nitrogen-phosphorus)
alleviates this problem. Mass Spectrometry in
SIM mode has been used with gas
chromatography for quantification of
glutethimide and its metabolites in plasma
and urine. Glutethimide, 2-phenylglutarimide
and dehydroglutethimide are quantified
directly, while the hydroxylated metabolites
are chromatographed following derivatization
with trifluoroacetic anhydride (Kennedy et
al., 1978).
HPLC methods are described for quantitative
analysis of glutethimide in plasma. Most
published methods involve reverse phase
chromatography with UV detection, and give a
sensitivity of 1 mg/L using a 100 無 sample
volume. The drug must first be extracted from
the specimen, and the precipitation of plasma
with an equal volume of acetonitrile as
described by Kabra et al. (1978) is easily
performed and reliable. Kabra et al. (1978)
used a C18 column with a mobile phase of
acetonitrile / phosphate buffer (300 無 1M
KH2PO4 and 50 無 0.9 M phosphoric acid in
1800 mL water) [215:785]. Using isocratic
elution at 50蚓 glutethimide was detected at
195 nm with a relative retention of 0.55 to
the internal standard methylphenytoin.
Svinarov & Dotchev (1989) used a C8 column
with a mobile phase of acetonitrile / water
(1:4), performing isocratic elution at
ambient temperature. Glutethimide was
detected at 208 nm with a relative retention
of 1.57 to the internal standard
tolylphenobarbital. Neither method shows
chromatograms of specimens taken following
glutethimide ingestion, nor gives mention of
glutethimide metabolites. Analysis of
patient samples by HPLC must therefore be
undertaken with due attention to the
possibility of interference from co-extracted
metabolites. Quantitation is performed by
comparison to samples of drug free plasma to
which known amounts of glutethimide have been
added and treated similarly.
8.2.2.5 Other dedicated method(s)
Not applicable.
8.2.3 Interpretation of toxicological analyses
There is considerable variation in individual
response to a given plasma glutethimide concentration.
Contribution to the overall clinical picture can be
made by co-ingested medications, the amount of toxic
metabolites produced, the degree of tissue
distribution, underlying medical conditions, presence
of infective agents etc. As a guide, the following
table shows typical concentrations of glutethimide in
serum.
mg/L 痠ol/L
After single oral dose (1 to 6 hours) 3 - 7 14 - 32
Steady-state in therapy <4 <18
Toxicity apparent (coma, convulsions,
pulmonary oedema) 10 46
Potentially fatal (deep coma,
sudden apnoea) 30 138
After therapeutic doses, peak concentrations of the
active metabolite 4-hydroxyglutethimide are in the
range 4 to 6 mg/L at about 24 hours. After overdose,
4-hydroxyglutethimide accumulates in plasma, rising to
several times the concentration of the parent
compound, and peaking on the second day, where after
it declines in parallel to glutethimide. There is
disagreement over whether fluctuations in
4-hydroxyglutethimide may be responsible for, or
contribute to the cyclical and prolonged coma seen
after overdose (Gold et al., 1974; Hansen et al.,
1975; Curry et al., 1987). There are insufficient data
to determine the clinical significance of the
concentrations of the other active metabolites
2-phenylglutarimide and the gamma-butyrolactone
derivative.
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Sodium, potassium, chloride
Alanine aminotransferase, aspartate
transaminase
Glucose, urea, creatinine
8.3.1.2 Urine
Not applicable.
8.3.1.3 Other fluids
No dedicated test.
8.3.2 Arterial blood gas analyses
pH, pCO2, pO2, HCO3- concentration, base
excess, O2-saturation.
8.3.3 Haematological analyses
Not applicable.
8.3.4 Interpretation of biomedical investigations
Not applicable.
8.3.5 Interpretation of biological investigations
Acute glutethimide poisoning is not associated
with specific biochemical effects other than changes
secondary to coma, respiratory failure and shock.
Routine analyses for assessing the patient's general
clinical condition are necessary.
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall Interpretation of all toxicological analyses and
toxicological investigations
There are no special precautions to be taken for sample
collection for biomedical or toxicological analyses. Acute
glutethimide poisoning is not associated with specific
biochemical effects other than changes secondary to coma,
respiratory failure and shock. Routine analyses for
assessing the patient's general clinical condition are
necessary.
Presumptive tests on toxic ingredients of materials can be
performed by colourimetric, spectrophotometric or thin layer
chromatographic techniques. Gas chromatography of
glutethimide (flame ionization detection) is not difficult,
and is much more specific.
Specific identification of the causative agent as
glutethimide in cases of hypnotic intoxication is useful
since the clinical course of glutethimide poisoning is more
complicated, and its management is more difficult, than that
of the barbiturates. Measurement of serum concentrations of
glutethimide may be useful in cases where coma is prolonged
or symptoms are particularly severe.
Measurement of glutethimide in biological materials is
possible after extraction into an organic solvent, and
metabolites will be co-extracted if polar solvents are used.
Metabolites (particularly 4-hydroxyglutethimide) are seen by
most advanced techniques. Qualitative analysis is most
easily performed by thin layer chromatography. Gas
chromatography allows for both qualitative and quantitative
analysis, and derivatization is not required; flame
ionization detection gives adequate sensitivity for most
applications. Gas chromatography / mass spectrometry has
been used where confirmatory testing is required. HPLC has
not been widely used.
Typical concentrations of glutethimide in serum are:
mg/L 痠ol/L
After single oral dose (1 to 6 hours) 3 - 7 14 - 32
Steady-state in therapy <4 <18
Toxicity apparent (coma, convulsions,
pulmonary oedema) 10 46
Potentially fatal (deep coma, sudden
apnoea) 30 138
The metabolite 4-hydroxyglutethimide may contribute to the
clinical and toxic effects of glutethimide, but there are
insufficient data to determine the clinical significance of
the concentrations of the metabolites 4-hydroxyglutethimide,
2-phenylglutarimide and gamma-butyrolactone, all of which
have known pharmacological activity.
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Ingestion is the only route by which acute
poisoning may occur. Mild intoxication with small
doses results in somnolence, ataxia, tonic muscle
spasms, abnormal reflexes.
In severe intoxication coma, hypotension, hypothermia,
shock, respiratory depression and cerebral oedema may
occur.
Signs from other organs and systems are usually
secondary to coma and shock.
9.1.2 Inhalation
Unknown.
9.1.3 Skin exposure
Unknown.
9.1.4 Eye contact
Unknown.
9.1.5 Parenteral exposure
Unknown.
9.1.6 Other
Unknown.
9.2 Chronic poisoning
9.2.1 Ingestion
Ingestion is the only route of glutethimide
administration in humans. Prolonged use of the drug
may cause peripheral neuropathy (Nover, 1967),
hypocalcaemia (Ober et al., 1981) and osteomalacia
(Greenwood et al., 1973). Acute abstinence syndrome
following glutethimide withdrawal has been described
(Johnson & Van Buren, 1962). Chronic ingestion of
high doses is associated with impaired memory,
inability to concentrate, ataxia, tremors,
hyporeflexia, slurring of speech, and convulsions
(Reynolds, 1989).
9.2.2 Inhalation
Unknown.
9.2.3 Skin exposure
Unknown.
9.2.4 Eye contact
Unknown.
9.2.5 Parenteral exposure
Unknown.
9.2.6 Other
Unknown.
9.3 Course, prognosis, cause of death
Any acute poisoning without loss of consciousness may be
regarded as mild and the patient is not at risk.
The occurrence of coma, hypotension, hypothermia, shock,
respiratory depression and complications such as pneumonia
mean that glutethimide poisoning is potentially serious.
However, death is unusual provided the patient is admitted to
intensive care when needed. Cerebral oedema may be fatal
(Wright & Roscoe, 1970).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Hypotension, shock and tachycardia have been
observed. Unexplained dysrhythmias may be due to the
antimuscarinic effects of the drug or low plasma
calcium concentrations (Wright & Roscoe, 1970, Chazan
& Garella, 1971).
9.4.2 Respiratory
Respiratory depression with intermittent apnea
and or arrest may occur in very severe cases.
Pneumonia due to aspiration and pulmonary oedema have
been reported (Wright & Roscoe, 1970, Chazan &
Garella, 1971).
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
Various degrees of CNS depression
may occur, ranging from lethargy to deep
coma. Cerebral oedema, intracranial
haemorrhage, tonic muscle spasms and
hyperreflexia may occur. Truncal ataxia has
been reported in acute glutethimide
intoxication in children (Huttenlocher,
1963).
9.4.3.2 Peripheral nervous system
Peripheral neuropathy and diplopia
has been reported following chronic
use.
9.4.3.3 Autonomic nervous system
Glutethimide has
antimuscarinic/anticholinergic activity,
tachycardia, dryness of mouth, mydriasis,
irritability, urinary retention and
constipation.
9.4.3.4 Skeletal and smooth muscle
Tonic muscle spasm and paralytic
ileus (adynamic ileus) may also be
observed.
9.4.4 Gastrointestinal
Gastrointestinal atony due to parasympatholytic
activity may occur (Chazan & Garella, 1971).
9.4.5 Hepatic
No direct effects are known.
9.4.6 Urinary
9.4.6.1 Renal
With the exception of possible
pre-renal uraemia due to severe hypotension,
no other renal effects occur (Wright &
Roscoe, 1970; Chartier, 1983).
9.4.6.2 Other
Urinary retention may occur due to
the anticholinergic effect of
glutethimide.
9.4.7 Endocrine and reproductive systems
No data available.
9.4.8 Dermatological
Bullous changes resembling those seen in
barbiturate poisoning (Burdon & Cade, 1979) and
erythematous vesicles (Leavell et al., 1972) have been
described.
9.4.9 Eye, ear, nose, throat: local effects
Mydriasis and papilloedema have been observed
(Wright & Roscoe, 1970).
9.4.10 Haematological
Significant methaemoglobinemia has been
reported rarely (Filippini, 1965); in a further case,
megaloblastic anaemia, thrombocytopenia and aplastic
anaemia occurred (Pearson, 1965).
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Acid base disturbances may occur
secondary to coma or shock.
9.4.12.2 Fluid and electrolyte disturbances
Hypocalcaemia has been described
(Crawshaw, 1968).
9.4.12.3 Others
Hypothermia has been described
(Skoutakis & Acchiardo, 1982; Ozdemir &
Tannenberg, 1972).
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Glutethimide readily crosses the placenta and
may cause neonatal respiratory depression (Kurtz et
al., 1966, Reveri et al., 1977) and neonatal
withdrawal symptoms (Asnes & Lamb, 1969).
Eight to 12 hours after a maternal does of 500 mg of
glutethimide, a peak concentration of 270 nanogram per
mL in breast milk has been reported (Curry et al.,
1971).
9.5 Other
No data available.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Patients with mild poisoning who are only sedated need
little or no treatment. Emptying the stomach by emesis
and/or lavage should be done within the first four hours
after ingestion if the clinical condition of the patient
allows it. If the patient is obtunded, gastric lavage should
be performed after endotracheal intubation.
Coma associated with shock is the most important feature of
severe poisoning. Treatment is symptomatic.
There is no specific antidotes. Procedures to enhance
elimination are not recommended: forced diuresis has been
shown to be ineffective; haemodialysis (even using oil as
dialyzing fluid) and haemoperfusion have not proven to be
effective.
10.2 Life supportive procedures and symptomatic/specific treatment
Patients with mild signs of overdose do not need
special treatment but should be under continuous clinical
observation, especially during the early stages of poisoning.
Intestinal absorption of additional amounts of glutethimide
may unpredictably precipitate deep coma requiring intensive
care.
Severe poisoning with coma always needs intensive care. It
is essential to maintain a clear airway and provide oxygen.
Perform endotracheal intubation and support ventilation.
Frequent change of the position of the patient and vigorous
physiotherapy are indicated to prevent pneumonia and
pulmonary infarctions. Pneumonia must be treated with
appropriate antibiotics.
Maintain one central or peripheral intravenous route.
Administer intravenous fluids in amounts adequate to maintain
daily diuresis of two to three litres. Urinary
catheterization is necessary in the comatose patient to
measure hourly urine output and obtain urine samples.
In case of significant hypotension, hypovolaemia must be
considered as a possible cause and be corrected. If
hypotension is severe, infusion of dopamine may be required
(2 to 5 痢/kg/minute, not more than 10 痢/kg/minute).
Monitoring of central venous pressure, and if possible
pulmonary artery pressures is indicated (Swan-Ganz
catheter).
Papilledema or other signs of cerebral edema (Wright &
Roscoe, 1970) may indicate the need for mannitol 20%.
Correct acidosis.
10.3 Decontamination
Since ingestion is the route of poisoning only
decontamination of the gastrointestinal tract should be
considered.
Induce vomiting and perform gastric lavage within the first
four hours following ingestion, but only in the conscious
patient. If the patient is drowsy or comatose, gastric
lavage should be done after endotracheal intubation.
Activated charcoal and cathartics should be given, unless
contraindicated (Ellenhorn & Barceloux, 1988).
10.4 Enhanced elimination
Forced diuresis does to enhance elimination of
glutethimide (Wright & Roscoe, 1970). Haemodialysis (even
using oil as the dialyzing fluid), (Chazan & Garella, 1971);
and haemoperfusion through charcoal column (Koffler et al.,
1978) or resin column (Raja, 1986) have not proved effective.
Experience at the Warsaw Poison Centre with oil haemodialysis
and charcoal haemoperfusion is not convincing.
10.5 Antidote treatment
10.5.1 Adults
No antidote available.
10.5.2 Children
No antidote available.
10.6 Management discussion
Supportive treatment is essential for the successful
management of acute glutethimide poisoning.
The prognosis is favourable even in severe cases but the
possibility of mixed poisoning must always be
considered.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
The most recent publications have reviewed many cases
of acute poisoning and their conclusions are included in this
monograph (Wright & Roscoe, 1970; Chazan & Garella, 1971).
Short descriptions and discussion of particular cases will be
presented under 11.2. Only two cases of chronic poisoning
are mentioned here.
Case 1 (Nover, 1967): A 37 year-old woman was treated with
glutethimide, up to 5 g per day, for five years. She
developed sensory neuropathy with glove-and-stocking
paraesthesias and noted poor recent memory and calculating
ability. She felt very weak, was unable to stand or walk
unaided and complained of ataxia. Neurological examination
found absent position, vibration, light touch, and pin prick
sensations distally in all four extremities. Decreased nerve
conduction velocity was found. Glutethimide was withdrawn
over 20 days despite a grand mal seizure occurring when the
patient was changed to phenobarbital. Even two weeks after
the complete withdrawal of glutethimide the patient was
unable to walk or stand without assistance and complained of
paraesthesias. Sensory findings were somewhat improved but
some symptoms and signs persisted for several months.
Case 2 (Pearson, 1965): A 47-year-old man was treated with
100 to 400 mg of glutethimide for five years and developed
megaloblastic anaemia with haemoglobin value as low as 6 g/dL
and megaloblastic hyperplasia in the bone marrow.
Discontinuation of glutethimide and administration of folic
acid resulted in normalization of the peripheral blood and
bone marrow.
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Since there are safer hypnotic drugs currently
available, it seems, there is no reason to continue the use
of glutethimide.
12.2 Other
No data available.
13. REFERENCES
Asnes RS & Lamb JM (1969) Neonatal respiratory depression
secondary to maternal analgesics; treated by exchange
transfusion. Pediatrics 43: 94.
Banen DM & Resnick O (1973) Lorazepam v. glutethimide as a
sleep-inducing agent for the geriatric patient. J Am Geriat Soc,
21: 507.
Baum G, Bill CES, Freeling P et al. (1965) Sedation with a new
non-barbiturate compound. Practitioner 195: 366.
Burdon JGW & Cade JF (1979) Barbiturate burns caused by
glutethimide. Med J Aust, 1: 101.
Chartier DM (1983) Glutethimide and codeine overdose. Emerg
Nursing 9: 307.
Chazan JA & Garella S (1971) Glutethimide intoxication. A
prospective study of 70 patients treated conservatively without
hemodialysis. Arch Int Med, 128: 215.
Crawshaw JA (1968) Hypocalcaemia in glutethimide overdose.
Practitioner, 200: 739.
Curry SH, Riddal D, Gordan JS et al. (1971) Disposition of
glutethimide in man. Clin Pharmacol Ther, 12: 849.
Curry SC, Hubbard JM, Gerkin R, Selden B, Ryan PJ, Meinhart R,
Hagner D (1987) Lack of correlation between plasma
4-hydroxyglutethimide and severity of coma in acute glutethimide
poisoning: A case report and brief review of the literature. Med
Toxicol, 2: 309-316.
Dain D, Trainer TD (1970) Simultaneous spectrophotometric
determination of glutethimide and barbiturates. Clin Chem, 16:
318-321.
Ellenhorn MS & Barceloux DG (1988) Medical Toxicology. Elsevier
Science Publishing Co., New York.
Filippini VL (1965) Methemoglobinaemie bei Doriden-Intoxication.
Schweiz Med Wschr, 95: 1618.
Finkle BS (1975) In: Sunshine I ed. Methodology for Analytical
Toxicology. Cleveland Ohio, CRC Press, pp178-180.
Flanagan RJ, Berry DJ (1977) Routine analysis of barbiturates and
some other hypnotic drugs in blood plasma as an aid to the
diagnosis of acute poisoning. J Chromatogr, 131: 131-146.
Gold M, Tassoni E, Etzl E, Mathew G (1974) Concentration of
glutethimide and associated compounds in human serum and
cerebrospinal fluid after drug overdose. Clin Chem, 20: 195-
199.
Greenwood RH, Prunty FT & Silver J (1973) Osteomalacia after
prolonged glutethimide administration. B Med J, 1: 643.
Hansen AR & Fischer LJ (1974) Gas-chromatographic simultaneous
analysis for glutethimide and an active hydroxylated metabolite in
tissues, plasma and urine. Clin Chem, 20: 236.
Hansen AR, Kennedy KA, Ambre JJ et al. (1975) Glutethimide
poisoning; a metabolite contributes to morbidity and mortality. N
Engl J Med, 292: 250.
Hansten PD & Horn JR (1989) Drug interactions, 6th ed., Lea &
Febiger, Philadelphia, PA.
Huttenlocher PR (1963) Accidental glutethimide intoxication in
children. N Eng J Med, 269: 38.
Johnson FA & Van Buren HC (1962) Abstinence syndrome following
glutethimide intoxication. JAMA, 180: 1024.
Kabra PM, Koo HY, Marton LJ (1978) Simultaneous
liquid-chromatographic determination of 12 common sedatives and
hypnotics in serum. Clin Chem, 24: 657-662.
Kastrup EK (ed) (1987) Facts and comparisons. JB Lippincott Co,
St Louis, MO, 87: 269b.
Keberle H, Hoffmann K & Bernhard K (1962) The metabolism of
glutethimide (Doriden). Experientia, 18: 105.
Kennedy KA, Ambre JJ, Fischer LJ (1978) A selected ion monitoring
method for glutethimide and six metabolites: Application to blood
and urine from humans intoxicated with glutethimide. Biomed Mass
Spec, 5: 679-685.
Koffler A, Bernstein H, La Sette A et al. (1978) Fixed-bed
charcoal hemoperfusion. Treatment of drug overdose. Arch Intern
Med, 138: 1691.
Kurtz GG, Michael EF, Morosi HJ et al. (1966) Hemodialysis during
pregnancy. Arch Intern Med, 118: 30.
Leavell VM, Coyer JR & Taylor RJ (1972) Dermographism and
erythematous lines in glutethimide overdose. Arch Derm, 106:
724.
Maher JF (1970) Determinants of serum half-life of glutethimide
in intoxicated patients. J Pharmacol Exp Ther, 174: 450.
Moffat AC, Jackson JV, Moss MS, & Widdop B eds. (1986) Clarke's
Isolation and Identification of Drugs. London, Pharmaceutical
Press.
Nover R (1967) Persistent neuropathy following chronic use of
glutethimide. Clin Pharm Ther, 8: 283.
Ober KP, Hennessy JF & Hellman RM (1981) Severe hypocalcemia
associated with chronic glutethimide addiction. Am J Psych, 138:
1239.
Ozdemir AI & Tannenberg AM (1972) Peritoneal and hemodialysis for
acute glutethimide overdosages. NY State J Med, 72: 2076.
Pearson D (1965) Megaloblastic anaemia due to glutethimide.
Lancet, 1: 110.
Raja RM (1986) Resin hemoperfusion for drug intoxication - an
update. Int J Artif Org, 9: 319.
Reveri M, Pyatis SP & Pildes RS (1977) Neonatal withdrawal
symptoms associated with glutethimide (Doriden) addiction in the
mother during pregnancy. Clin Pediatr, 16: 424.
Reynolds JEF (ed.) (1989) Martindale: The Extra Pharmacopoeia,
29th Ed.
Shamoian CA (1975) Codeine and glutethimide; euphoric, addicting
combination. NY State J Med, 75: 97.
Skoutakis VA & Acchiardo SR (1982) Glutethimide intoxication.
Clin Toxicol Consultant, 4: 18.
Sramek JJ & Klajawal A (1981) Loads. N Engl J Med, 305: 231.
Sunshine I, Maes R, Faracci R (1969) Determination of
glutethimide (Doriden) and its metabolites in biologic specimens.
Clin Chem, 14: 595-609.
Svinarov DA, Dotchev DC (1989) Simultaneous
liquid-chromatographic determination of some bronchodilators,
anticonvulsants, chloramphenicol, and hypnotic agents, with
Chromosorb P columns used for sample preparation. Clin Chem 1989,
35: 1615-1618.
Wesolowski JW et al. (1968) J Pharm Sci, 87: 811.
Wright N & Roscoe PR (1970) Acute glutethimide poisoning.
Conservative treatment of 31 patients. JAMA, 214: 1704.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES),
COMPLETE ADDRESS(ES)
Author: Janusz Szajewski, MD
Warsaw Poison Control Centre
Szpital Praski III Oddzial
Chorob Wewnetrznych
Al. Swierczewskiego 67
03-701 Warsaw
Poland
Date: August 1992
Peer
Review: London, United Kingdom, September 1992
(Members of the Group: M. Balali-Mood, J. Szajewski,
O. Kasilo, A. Wong, J.F. Deng, J. Higa, S.
Shintani)
IPCS
update: May 1994
Author
Section 8: Dr S. Dawling
Center for Clinical Toxicology
Vanderbilt University Medical Center
501 Oxford House
1161 21st Avenue South
Nashville, TN 37232-4632
United States of America
Tel: 1-615-9360760
Fax: 1-615-9360756
E-mail: sheila.dawling@mcmail.vanderbilt.edu
Date: March 1998
Editor: Mrs J. Dum幯il
International Programme on Chemical Safety
Date: May 1999


