
Salicylic acid
Salicylic acid
International Programme on Chemical Safety
Poisons Information Monograph 642
Pharmaceutical
1. NAME
1.1 Substance
Salicylic acid
1.2 Group
ATC Classification:
Analgesics,
Other Analgesic and Antipyretics,
Salicylic acid and derivatives
N02AB
1.3 Synonyms
Orthohydroxybenzoic acid;
2-hydroxybenzoic acid;
Acido Orthoxibenzoico;
Acidium Salicylicum;
Salizylsaure
1.4 Identification numbers
1.4.1 CAS number
69-72-7
1.4.2 Other numbers
RTECS/NIOSH: VO0525000
1.5 Brand names, Trade names
Salicylic Acid Collodion (B.P.). 12g/100 mL
Salicylic Acid Collodion (U.S.P.) 10g/100 mL
Salicylic Acid Paint (A.P.F.) Corn paint
Lactic and Salicylic Acid paint (A.P.F)
Salicylic Acid and Resorcinol Cream Aqueous (A.P.F)
Salicylic Acid and Sulphur Cream Aqueous (A.P.F)
Salicylic Acid Ear Drops (A.P.F)
Salicylic Acid Topical Foam (U.S.P.)
Salicylic Acid Gels (U.S.P)
Salicylic Acid Lotion (B.P.)
Salicylic Acid and Coal Tar (A.P.F)
Salicylic Acid Ointment Lotion (B.P.)
Salicylic Acid and Coal Tar Ointment (A.P.F)
Salicylic Acid and Sulphur Ointment (B.P.C)
Salicylic Acid Plaster (U.S.P)
Pyralvex( Norgine, UK),
Salactol (Dermal Labor. UK),
Verrugon (Pickles, UK),
Cuplex (Smith & Nephew Pharmaceuticals, UK),
Duofilm (Stiefel, UK),
Keralyt (Stiefel, UK),
Monophytol (Laboratories for Applied Biology, UK) (Reynolds,
1996)
1.6 Main manufacturers, main importers
See 1.5
2. SUMMARY
2.1 Main risks and target organs
The toxic effects of salicylic acid and salicylates are
complex. Main risks with oral therapeutic doses are mostly
gastrointestinal irritation. Hepatic encephalopathy (Reye's
Syndrome) has been reported in children who had taken aspirin
for treatment of viral infections such as influenza. Toxic
doses of salicylate stimulate the respiratory centre leading
to respiratory alkalosis. In severe intoxication, metabolic
acidosis, water and electrolyte loss occur as the principle
secondary consequences. Central nervous system toxicity
includes, tinnitus, hearing loss and in very severe cases
particularly in children convulsions and coma. Target organs
are central nervous system, lungs, kidneys and liver.
2.2 Summary of clinical effects
Following oral ingestion of salicylic acid (SA) and or
any other salicylate, nausea, vomiting, epigastric
discomfort, tinnitus, loss of hearing, sweating, flushing
(vasodilatation) tachypnoea and hyperpnoea are commonly
observed. Local gastrointestinal (GI) irritation of SA is
more marked than ASA (acetylsalicylic acid). In severe
intoxication irritability, tremor, blurred vision, mental
confusion, delirium, stupor, coma, fever, cerebral oedema and
cardio-respiratory arrest may occur. Central nervous system
toxicity and gastrointestinal haemorrhage are more common
after chronic (therapeutic) intoxication. A marked alteration
of acid-base balance from respiratory alkalosis to metabolic
acidosis may be observed. In severe salicylate intoxication
in adults, non cardiogenic pulmonary oedema, nonfocal
neurological abnormalities, unexplained ketosis and a
prolonged prothrombin time can occur. Skin eruption and
subconjuntival haemorrhage may be seen but marked
thrombocytopenia is rare. Methyl salicylate poisoning has the
odour of the drug which can be detected on the breath and in
the urine and vomit. Central nervous system stimulation,
intense hyperpnoea and hyperpyrexia are prominent features.
2.3 Diagnosis
Symptoms and signs of oral ingestion of SA overdose
usually commence by nausea, vomiting and epigastric
discomfort. However, in mild salicylism particularly with
therapeutic intoxication, tinnitus, loss of hearing,
dizziness, sweating, and flushing are more common. Severe
salicylate poisoning is associated with marked respiratory
and nervous system toxicity. Hyperventilation and respiratory
alkalosis are more common in adults, whereas hypoventilation
and metabolic acidosis are more commonly observed in
children. A rapid screening test for the presence of
salicylate in the urine may indicate the use of the drug.
Estimation of salicylate concentration in plasma/serum can
confirm the diagnosis and reveals the severity of
intoxication. A plasma salicylate concentration of 300 to 500
mg/L at 6 hour post-ingestion indicates a mild toxicity, 500
to 800 mg/L moderate toxicity and >800 mg/L is considered as
severe SA intoxication.
2.4 First aid measures and management principles
Inducing vomiting, gastric aspiration and lavage should
only be considered if large amounts of salicylate has been
ingested.
Enhancement of elimination using alkaline diuresis and
repeated doses of activated charcoal, correction of
dehydration, acidosis and electrolytes. In severe salicylate
intoxication, haemodialysis and or charcoal haemoperfusion is
indicated.
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Salicylic acid in the form of esters was found in
several plants, notably in wintergreen leaves and the bark of
sweet birch. It was made synthetically by heating sodium
phenolyate with carbon dioxide under pressure and microbial
oxidation of naphthalene (Windholz, 1983).
3.2 Chemical structure
Chemical name:
2-Hydroxy-benzoic acid
Molecular formula: C7H6O3
Molecular weight: 138.1
Chemical structure:
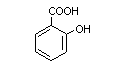 3.3 Physical properties
3.3.1 Colour
Colourless or white
3.3.2 State/form
Solid-crystals
3.3.3 Description
Colourless acicular crystals or a white
crystalline powder. The synthetic form is white but if
prepared from natural methyl salicylate, it may have a
slightly yellow or pink tint.
Salicylic acid is a white crystalline powder with a
sweetish acrid taste. If prepared from natural methyl
salicylate, it may have a faint mint like odour. It is
available in forms of ointments, cream, gel,
transdermal patches, liquids and plaster.
Salicylic acid is soluble 1 in 460 to 550 of water, 1
in 15 of boiling water, 1 in 3 to 4 in alcohol, 1 in 3
in ether and 1 in 45 in chloroform (Reynolds, 1996).
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Shelf-life is dependent on the manner of
storage. It should be stored in well closed containers
and protected from light (Reynolds, 1996).
3.4.2 Storage conditions
Store in well closed containers and protect
from light (Reynolds, 1996).
4. USES
4.1 Indications
4.1.1 Indications
Analgesic
Other analgesic/antipyretic
Salicylate; analgesic
4.1.2 Description
Salicylic acid has keratinolytic properties and
is applied topically in the treatment of
hyperkeratotic and scaling conditions such as
dandruff, ichthyosis and psoriasis. Initially a
concentration of 2% is used increasing to about 6% if
necessary.
It is often used in conjunction with many other
agents, such as benzoic acid, coal tar, resorcinol and
sulphur. Salicylic acid is also used in the form of
paint and in the form of collodion basis (10 to 17%)
or as a plaster (20 to 50%) to destroy warts and
corns. It also possesses fungicidal properties and is
used topically in the treatment of fungal skin
infections such as tinea (Reynolds, 1996).
4.2 Therapeutic dosage
4.2.1 Adults
The topical preparations such as transdermal
patches, gels, ointments, liquids, creams or plasters
are usually used in concentrations of 2.5 to 60% for
the treatment of psoriasis, warts and other keratinous
disorders (Chren & Bickers, 1990).
4.2.2 Children
Since salicylic acid is used topically, the
above concentration and dosage may be applied in
children with caution.
4.3 Contraindications
Due to the severe gastric irritation which salicylic
acid causes, it is no longer used orally. However, when used
topically it may cause an allergic contact rash in some
people. If applied to large areas of skin, it may be absorbed
into the blood stream and induce salicylism (Parish, 1991).
5. ROUTES OF ENTRY
5.1 Oral
Salicylic acid causes gastric irritation and thus,
there is no oral pharmaceutical available. However, Chinese
medicated oil (Koong yick Hung Far Oil) which contains 67%
methyl salicylate has been taken orally (Chan, 1996).
5.2 Inhalation
Not relevant.
5.3 Dermal
Salicylic acid is readily absorbed from the skin and may
induce toxicity (salicylism).
5.4 Eye
Unknown
5.5 Parenteral
Unknown
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Salicylic acid is readily absorbed from the skin and may
cause toxicity, particularly in children and the newborn.
Herbal products such as the chinese medicated oil which
contains methyl salicylate is also absorbed through
gastrointestinal mucosa following ingestion (Chan, 1996).
6.2 Distribution by route of exposure
About 50 to 80% of salicylate in the blood is bound to
plasma proteins, while the rest remains in the active ionized
state; protein binding is concentration dependent. Saturation
of binding sites lead to more free salicylate and increased
toxicity. The apparent volume of distribution is 0.1 to 0.2
L/kg. Acidosis increases the volume of distribution because
of the enhancement of tissue penetration of salicylate (Levy
& Tsuchiya, 1972).
6.3 Biological half-life by route of exposure
The plasma salicylate half-life following therapeutic
doses is 2 to 4.5 hours, but in overdosage, increases to 18
to 36 hours (Done, 1960). When the metabolic pathway is
saturated (conjugation with glycin), zero order kinetics
apply and thus the elimination half-life cannot be derived
correctly.
6.4 Metabolism
At low dosage, approximately 80% of salicylic acid is
metabolised in the liver. Conjugation with glycine, forms
salicyluric acid and when conjugated with glucuronic acid,
acyl and phenolic glucuronide are formed. Small amounts of
salicylic acid are also hydroxylated to gentisic acid. With
large doses, the kinetics switch from first order to zero
order (Michaelis-Menten) kinetics (Levy & Tsuchiya, 1972).
6.5 Elimination and excretion
Salicylates are excreted mainly by the kidney as
salicylic acid, salicyluric acid, salicylic glucuronides and
gentisic acid. The proportion excreted of each metabolite,
depends upon urinary pH. With urinary alkalinisation,
salicylic acid excretion is enhanced (Prescott et al., 1982).
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Marked hyperventilation occurs as a result of
direct stimulation of the respiratory centre. Indirect
stimulation of respiration is caused by an increased
production of CO2 as a result of salicylate-induced
uncoupling of oxidative phosphorylation. Respiratory
alkalosis develops as a result of the direct and
indirect stimulation of the respiratory centre. In an
attempt to compensate, bicarbonate accompanied by
sodium, potassium and water, is excreted in the urine.
Dehydration and hypokalemia result, but more
importantly, the loss of bicarbonate diminishes the
buffering capacity of the body and allows the
development of a metabolic acidosis (Proudfoot &
Brown, 1969; Davidson, 1971; Proudfoot, 1983). The
pyretic effect of toxic doses of salicylate is a
direct result of the uncoupling of oxidative
phosphorylation. High doses of salicylate have
additional toxic effects on the central nervous system
consisting of stimulation(including convulsions)
followed by depression, confusion, dizziness,
asterixis, delirium, psychosis, stupor and coma
(Anderson et al., 1976; Anderson, 1981). Very high
doses of salicylates have a depressive effect on the
medulla and may cause central respiratory paralysis as
well as sudden circulatory collapse secondary to
vasomotor depression. The loss of buffering capacity
and the effects of salicylate on carbohydrate, lipid
and protein metabolism lead to the development of a
metabolic acidosis or, more commonly in practice, a
mixed acid-base disturbance (Proudfoot & Brown, 1969;
Meredith & Vale, 1981; Proudfoot, 1983). Both hypo-
and hyper-glycaemia may occur in salicylate poisoning.
The former most probably due to an increased tissue
demand for glucose oxidation due to the uncoupling of
oxidative phosphorylation. Neuroglycopenia can occur
in the presence of normal blood glucose concentrations
(Thurston et al., 1970). If hepatic glycogen stores
are adequate, catecholamines production stimulates
glycogenolysis leading to hyperglycemia which can
persist for several days (Cotton & Fahlberg, 1964).
Salicylate intoxication is often accompanied by
hypothrombinaemia due to a warfarin like action of
salicylate on the vitamin K1 epoxide cycle, which
rarely causes clinical problems (Proudfoot, 1983).
7.1.2 Pharmacodynamics
Salicylic acid alleviates pain, lowers an
elevated body temperature and inflammation by
inhibiting the synthesis of prostaglandins that occur
in inflamed tissues. Salicylate inhibits the
conversion of arachidonic acid to the unstable
endoperoxide intermediate PG G2, which is catalyzed by
the enzyme cyclo oxygenase. Platelets are especially
susceptible to this action as they are incapable of
regenerating the enzyme, presumably they have little
or no capacity for protein biosynthesis (Brantmark et
al., 1981). Cyclo oxygenase(COX) is present in two
main isoforms. COX-1 is the isoform of the enzyme and
is present under normal physiological conditions. COX-
2 is the inducible isoform of the enzyme and is
induced in settings of inflammation (i.e. production
of eicosanoids and kinins). Inhibition of COX-1
results in unwanted side effects, particularly those
leading to gastric ulcer. Current pharmacological
research, in the NSAID field is centering on finding a
selective COX-2 inhibitor. Meloxicam is a selective
COX-2 inhibitor that has been marketed in France and
some other countries (Insel, 1996).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Since salicylic acid is available
only in topical preparations, human data on
toxicity have not been reported. However, the
chinese medicated oil (Koong Yick Hung Far
Oil), which contains 67% methyl salicylate
induced severe salicylate poisoning
(Chan,1996). Salicylic acid concentrations
above 800 mg/L after 6 hours post exposure is
severely toxic and may be lethal
(Balali-Mood, 1981).
7.2.1.2 Children
Salicylate intoxication is often
more serious in small children (<4 years)
than in older children, due to an early
development of a metabolic acidosis rather
than a respiratory alkalosis (Winters et al.,
1959).
7.2.2 Relevant animal data
None relevant.
7.2.3 Relevant in vitro data
None relevant.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
There is no evidence that moderate therapeutic doses of
salicylates cause fetal damage in human beings; however,
babies born to women who ingest salicylates for long periods
may have a significantly reduced mass at birth. In addition,
there is an increase in prenatal mortality, anaemia,
antepartum and postpartum haemorrhage, prolonged gestation
and complicated deliveries. These effects occur when
salicylates are administered during the third trimester, and
thus its use during this period of pregnancy should be
avoided (Insel, 1996).
7.5 Mutagenicity
No data available
7.6 Interactions
Salicylic acid is highly protein-bound and may increase
the unbound or free drug concentrations of other drugs such
as hypoglycemics, anticoagulants and methotrexate(an
antimetabolite chemotherapeutic drug), reaching toxic levels
of these agents. The uricosuric activity of phenylbutazone,
probenecide and sulphinpyrazone is strongly antagonized by
salicylate and maybe completely diminished by small doses due
to decreased tubular reabsorption of uric acid (Insel, 1996).
In a report of two cases of severe salicylate poisoning,
asystole occurred shortly after the intravenous
administration of diazepam (Berk & Anderson, 1989).
7.7 Main adverse effects
Salicylic acid is a gastric irritant and because of the
serious damage it may cause to the stomach lining, it has not
been used orally. Topical use of salicylic acid may induce
allergic contact dermatitis (Davies, 1985). Salicylic acid
may cause excessive drying and irritation in some people
(Parish, 1991). Some individuals, especially asthmatics
exhibit sensitivity to salicylates. Urticaria, angioneurotic
oedema, rhinitis, severe and even fatal paroxysmal,
bronchospasm and dyspnea may occur (Reynolds, 1996).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Screen residues or suspected
materials, plasma, urine and stomach
contents. Avoid sodium azide
preservation.
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (Unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Keep biological samples in a
refrigerator prior to analysis.
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (Unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special conditions.
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (Unspecified) analyses
8.2 Toxicological Analyses and Their Interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple Qualitative Test(s)
Trinder's reagent: Dissolve 40 g of
Hg(II) Cl2 in 850 mL of hot water. After
cooling down, add 120 mL of HCl 1 mol/L and
40 g of hydrated ferric nitrate. When the
ferric nitrate has been dissolved, add water
to 1000 mL. Add 0.1 mL Trinder's reagent to 1
mL of urine and mix. A violet colour
indicates the presence of salicylates. For
stomach contents and serum residues, boil a
portion of the specimen with 2 mL of
hydrochloric acid 0.1 mol/L for 10 minutes,
cool and filter, if necessary neutralise with
sodium hydroxide 0.1 mol/L. Add 100 Ál of
Trinders' reagent to the clear neutralized
solution. A violet colour indicates presence
of salicylate.
8.2.1.2 Advanced Qualitative Confirmation Test(s)
8.2.1.3 Simple Quantitative Method(s)
8.2.1.4 Advanced Quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple Qualitative Test(s)
8.2.2.2 Advanced Qualitative Confirmation Test(s)
8.2.2.3 Simple Quantitative Method(s)
8.2.2.4 Advanced Quantitative method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Platelet function and coagulation
tests, in order to estimate the degree of
inhibition of platelet aggregation and
hypoprothrombinaemia may be
required.
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
In adults with severe salicylate poisoning an
initial respiratory alkalosis is followed by metabolic
acidosis. In children, metabolic acidosis is more
common than in adults. Acid-base disturbances are
usually mixed in mild to moderate salicylate poisoning
(Proudfoot & Brown, 1969; Proudfoot, 1983).
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall interpretation of all toxicological analyses
and investigations
Following the qualitative test for salicylate, the
plasma/serum salicylate concentration should be determined on
admission, and at regular intervals to assess the severity of
intoxication, prognosis and approach to treatment. Salicylate
concentration may continue to rise even up to 24 hours post
ingestion (Balali-Mood, 1981). However, based on severe
intoxication plasma salicylate concentrations at 6 hours
after an overdose could be divided into three groups:
1. 300 to 500 mg/L mild toxicity
2. 500 to 800 mg/mL moderate toxicity
3. >800 mg/L severe toxicity (Proudfoot, 1983).
Clinical findings and acid-base disturbances should be
considered when interpreting the plasma salicylate
concentration and deciding upon management (Meredith & Vale,
1981). The Done nomogram categorizes the severity of
poisoning for single ingestion based on peak salicylate
concentrations. In patients with significant acidosis and in
patients who ingest multiple doses or sustained release
preparations, the Done nomogram will tend to underestimate
the severity of salicylate intoxication (Todd et al., 1981).
Development of the nomogram was based on the assumption that
salicylates are eliminated by a first order process, whereas
in fact they are partially eliminated through natural
processes. This may lead to overprediction of the severity
of toxicity. Therefore, the Done-nomogram is not commonly
used. Following ingestion of enteric-coated tablets, plasma
salicylate concentrations on admission are not a reliable
guide to the severity of poisoning. Salicylate concentration
may not peak until more than 12 hours after such an overdose
(Springer & Groll, 1980; Todd et al., 1981).
Hypokalemia, hypo/hyperglycaemia, hypocalcaemia and acid-base
disturbances may occur during salicylate poisoning. It is
therefore essential to investigate electrolyte (and if
possible osmolality) and arterial blood gas analysis. Other
biochemical analyses such as liver and kidney function tests
are required as clinically indicated (Cotton & Fahlbeg, 1964;
Balali-Mood, 1981; Proudfoot, 1983).
8.6 References
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Initial symptoms of salicylate poisoning may be
nausea and vomiting, epigastric pain and occasionally
haematemesis. Hyperventilation, sweating, flushing,
fever, irritability, tinnitus and loss of hearing are
the common clinical features of mild to moderate
salicylate intoxication. In severe intoxication,
hypoventilation, stupor, hallucination, convulsions,
papiloedema and coma particularly in children may
occur. Metabolic acidosis, non-cardiogenic pulmonary
oedema, hepatotoxicity and cardiac dysrhythmias may
also occur (Balali-Mood, 1981; Meredith & Vale, 1981).
9.1.2 Inhalation
No data available
9.1.3 Skin exposure
Severe poisoning has been reported as a result
of the use of salicylic acid ointment for
dermatological problems and in the treatment of skin
burns (Editorial of JAMA, 1964; Taylor & Halprin,
1975; Pluskwa et al., 1984).
9.1.4 Eye contact
No data available
9.1.5 Parenteral exposure
No data available
9.1.6 Other
None
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic salicylate poisoning occurred as a
result of excessive therapeutic administration over a
period of 12 hours or more were reported (Dove &
Jones, 1982). Metabolic pathways of salicylic acid
become saturated and thus plasma concentration
increases, producing toxicity. Small children are at
particular risk of overdose especially when fever,
sweating and tachycardia of salicylate intoxication
are attributed to the underlying illness and are used
as indications for increasing the dose (Proudfoot,
1983). Children may become intoxicated through breast
milk (Clark & Wilson, 1981). The presenting signs of
chronic salicylate poisoning can include metabolic
acidosis, hypoglycemia, lethargy, coma and fits
(English et al., 1996).
9.2.2 Inhalation
No data available
9.2.3 Skin exposure
Chronic usage of salicylic acid and or methyl
salicylate in skin and rheumatic diseases may cause
intoxication through percutaneous absorption. Life
threatening salicylate poisoning caused by
percutaneous absorption of salicylic acid (10%
ointment) in a 7-year-old boy with ichthyosis vulgaris
was reported (German et al., 1996). Application of
teething gels containing salicylic acid induced
intoxication (Paynter & Alexandre, 1979).
9.2.4 Eye contact
No data available
9.2.5 Parenteral exposure
No data available
9.2.6 Other
None
9.3 Course, prognosis, cause of death
If a large quantity of a salicylate has been taken,
nausea, vomiting, tinnitus, deafness, sweating,
vasodilatation and hyperventilation may develop. Acid-base
disturbances, electrolyte imbalance, non cardiogenic
pulmonary oedema, hypoventilation and hallucination, stupor,
irritability, coma and convulsions particularly in children
and older patients can proceed death. Loss of consciousness
in adults is very rare and when occurs indicates poor
prognosis. A review of 51 fatal cases of acute salicylate
poisoning in Ontario during 1983 and 1984, discovered that
salicylate was the most common cause of death, due to the
ingestion of single drugs. Autopsy results showed that 50% of
the patients had pulmonary abnormalities, 28% had lesions of
the gastrointestinal tract, 18% had nervous system
abnormalities and 25.6% had no pathological changes
(McGuigan, 1987). Mortality from chronic salicylate
intoxication is considerably higher (25%) than from acute
overdose (1 to 2%) (Anderson et al., 1976). Death is often
due to sudden cardiac arrest or occasionally due to multiple
complications following severe brain damage (Proudfoot,
1983).
9.4 Systemic description of clinical effects
9.4.1 Cardiovascular
Sudden cardiovascular collapse is a recognized
complication of salicylate poisoning (Anderson et
al.1976, Proudfoot et al, 1983). Two patients with
severe salicylate intoxication developed asystole
shortly after intravenous diazepam administration
(Berk & Anderson, 1989). Transient myocardial
dysfunction (global left ventricular shortening
fraction of 23%) with pulmonary edema was found in a
13-month-old boy with salicylate poisoning (Ralston et
al., 1995).
9.4.2 Respiratory
Non-cardiogenic pulmonary oedema may occur in
salicylate intoxicated patients who are over 30 years
of age. Cigarette smoking, chronic salicylate
ingestion, metabolic acidosis and the presence of
neurological symptoms and signs on admission are
strong risk factors for the subsequent development of
pulmonary oedema. The exact mechanism is unknown.
Three possible explanations are: (i) A direct toxic
effect on pulmonary microvasculature; (ii) Interaction
with endogenous mediators such as prostaglandins; and
(iii) A central nervous system mediated effect
(Walters et al., 1983).
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
In moderate to severe salicylate
intoxication, CNS stimulation (irritability
and convulsions) followed by depression,
confusion, dizziness, delirium, psychosis,
asterixis, stupor and coma occur usually when
metabolic acidosis is the dominant acid-base
abnormality (Proudfoot & Brown, 1969). These
features are thought to be due to reduced
ionisation of salicylic acid and a shift of
salicylate from the plasma into the brain.
Very high doses of salicylate have a
depressive effect on the medulla and may
cause central respiratory paralysis as well
as sudden circulatory collapse, secondary to
vasomotor depression (Proudfoot,
1983).
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
No data available.
9.4.3.4 Skeletal and smooth muscle
No data available.
9.4.4 Gastrointestinal
Ingestion of salicylate may result in
epigastric discomfort, nausea and vomiting. Salicylic
acid has more irritant effects on gastric mucosa than
acetylsalicylic acid and thus has been withdrawn from
oral administration for many years. Acetylsalicylic
acid may also cause gastric irritation. Perforated
peptic ulcer also occurs extremely rarely (Robins et
al., 1985).
9.4.5 Hepatic
Hepatotoxicity may occur both after the
therapeutic use of salicylate or following its
overdosage. Liver biopsy revealed acute hepatocellular
necrosis with periportal inflammation and fatty
changes in hepatocytes (Wofe et al.,1974). Hepatic
encephalopathy (Reye's Syndrome) has been reported in
children taking aspirin for treatment of viral
infections such as influenza.
9.4.6 Urinary
9.4.6.1 Renal
Oliguria sometimes occurs, which is
mostly due to dehydration(Temple et al,
1976). Renal failure may rarely occur in
individuals without volume depletion, pre-
existing renal and or systemic diseases (Rupp
et al., 1983).
9.4.6.2 Other
None.
9.4.7 Endocrine and reproductive systems
High doses of salicylate cause release of
adrenaline from the adrenal medulla; this is thought
to be partly responsible for the observed
hypoglycaemia due to glycogenolysis that sometimes
occurs. Large doses of salicylate stimulate
corticosteroids secretion by the adrenal cortex
(Temple, 1981).
9.4.8 Dermatological
Toxic epidermal necrosis in 13 patients
associated with the use of salicylate have been
reported (Lowney et al., 1967).
9.4.9 Eye, ear, nose, throat: local effects
Eye: Transient myopia occurred in a patient
following ingestion of 2.7 g acetylsalicylic acid
(Sandford-Smith, 1974). Bilateral subconjunctival
haemorrhage has been reported (Black & Bensinger,
1982).
Ear: Tinnitus and hearing loss caused by salicylate in
overdose are due to increased labyrinthine pressure
(Waltner, 1955). It may also be due to an effect on
the hair cells of the cochlea. There is a relationship
between the hearing loss and the plasma salicylate
concentration (Meyers et al., 1965).
There has been no reports on the local effects of
salicylate on the nose and throat.
9.4.10 Haematological
Salicylates prolong the bleeding time due to
inhibition of collagen glucosyltransferase present in
membranes of platelets. As a result, the adherence of
platelets to connective tissue or collagen fibres is
diminished. Salicylate overdose reduces the
concentration of vitamin K-dependent coagulation
factors, particularly prothrombin (Brantmark et al.,
1981).
9.4.11 Immunological
Salicylates have the capacity to supress a
variety of antigen-antibody reactions such as: the
inhibition of antibody production, of antigen-antibody
aggregation and of antigen induced release of
histamine. Salicylates also induce a non specific
stabilization of capillary permeability during
immunological insults. The concentration of salicylate
causing this effect is high, and their relationship
to the antirheumatic efficacy of salicylates is yet to
be determined.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Respiratory alkalosis is more often
observed in adults than in children.
Metabolic acidosis develops sooner in
children than in adults (Winters et al.,
1959; Proudfoot & Brown, 1969). However, in
severe salicylate poisoning in adults,
metabolic acidosis may also occur. High
concentrations of salicylate may lower
bicarbonate by not more than 2 to 3 mmols.
The acidosis is not therefore due to the
presence of salicylic acid itself. The
principle cause is competitive inhibition of
NAD+-dependent dehydrogenases including
lactate and oxoglutarate dehydrogenase and
the other oxidative enzymes such as succinate
dehydrogenase (Grisolia et al., 1969).
Salicylate enhances entry and oxidation of
fatty acids in liver cells,leading to
increased ketogenesis. Competitive inhibition
of amino acyl-tRNA synthetases in pairs and
amino acid incorporation results in
amino-acidaemia (Smith & Dawkins, 1971).
Dehydration and vasomotor depression results
in poor renal perfusion and accumulation of
sulphuric and phosphoric acids (Tenney &
Miller, 1995; Winters et al., 1959).
9.4.12.2 Fluid and electrolyte disturbances
Increased renal secretion of
sodium, potassium and water accompanies loss
of bicarbonate in the urine. Fluid loss also
results from vomiting, sweating and
hyperventilation. Dehydration is commonly
associated with hypernatraemia. Water loss
may be considerable from 2 to 3 L/m2 surface
area in moderate to severe poisoning and up
to 6 L/m2 in severely poisoned patients
(Temple, 1978).
9.4.12.3 Others
(1) Oxidative Phosphorylation
The uncoupling of oxidative phosphorylation
by salicylate results in the inhibition of a
number of ATP-dependent reactions and an
increase in O2 uptake and CO2 production.
(2) Nitrogen compound metabolism
Toxic doses of salicylate cause a significant
nitrogen imbalance, characterized by amino
aciduria, though this is due in part to
stimulation of active tubular absorption
because of reduced ATP formation.
(3) Fat metabolism
Salicylates enhance oxidation of fatty acids
in muscle, liver and other tissues together
with a decrease of concentrations of plasma,
free fatty acids, phospholipids and
cholesterol (Insel, 1996).
9.4.13 Allergic reactions
Some people particularly asthmatics, exhibit
marked sensitivity to salicylate, resulting in various
reactions including urticaria and other skin
eruptions, angioneuritis, oedema, rhinitis and severe
and even fatal paroxysmal bronchospasm and dyspnea,
hypotension, shock and syncope (Reynolds, 1996).
Despite the fact that the symptoms (such as the ones
mentioned above) resemble anaphylaxis, this reaction
does not appear to be immunological in nature. It may
be a shunt towards the lipoxygenase pathway leading to
an increased production of leukotrienes and other
inflammatory mediators. Although, this hypothesis is
unproved and it does not explain why only a minority
of patients with asthma or other predisposing
conditions display the reaction. Even so, results in a
small number of patients suggest that blockade of
5-lipoxygenase with the drug zileuton may prevent
symptoms and signs of aspirin intolerance (Insel,
1996).
9.4.14 Other clinical effects
The relationship between the use of salicylate
and Reye's syndrome in children and adolescents
(mostly 5 to 15 years) has been demonstrated by
epidemiological studies (Sullivan-Bolyai & Corey,
1981).
9.4.15 Special risks
Salicylate intoxication may occur through
placental transfer(Lynd, 1976) and breast milk (Clark
& Wilson, 1981).
9.5 Other
None.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Management of acute salicylic acid poisoning includes
prevention of absorption, correction of acid-base, fluid and
electrolyte imbalance and enhancing of the drug elimination.
Respiratory alkalosis needs no specific treatment, but severe
acidosis requires at least a partial correction with sodium
bicarbonate. Hypokalaemia may be aggravated by administration
of sodium bicarbonate. Thus, potassium may need repletion.
However, if large amounts of water and electrolytes are given
to the patient, the sodium and water load may precipitate
pulmonary oedema (Balali-Mood, 1981; Proudfoot, 1983).
Sedative and depressive drugs must be avoided. Tetany may be
corrected with the use of calcium gluconate(Meredith & Vale,
1981). Due to the delayed effects of salicylate overdose, the
patient must be kept under observation for at least 24 hours
(Balali-Mood, 1981).
10.2 Life supportive procedures and symptomatic/specific treatment
If non-cardiogenic pulmonary oedema occurs, mechanical
ventilation with positive end-expiratory pressure (PEEP) may
be required. Correction of electrolyte and acid-base
disturbances is essential. ECG monitoring and in very severe
cases intensive care therapy is needed. Sponging and any
other physical countermeasure for hyperpyrexia may be
required. Antipyretics must be avoided (Balali-Mood, 1981;
Proudfoot, 1983).
10.3 Decontamination
Inducing emesis and when the patient reaches the
hospital, gastric aspiration and lavage, is only recommend if
the patient ingested large amounts of salicylate (American
Academy of Clinical Toxicology and the European Association
of Poisons Centres and Clinical Toxicologists, 1997). It is
not necessary if a non-oral route of entry such as dermal
absorption, caused intoxication.
Although activated charcoal proved to be equally effective as
emesis and gastric lavage in volunteers (Danel & Henry,
1988), it may only be exploited therapeutically if the
patient presents soon after ingestion. Repeated doses of
activated charcoal (50 to 75 g immediately and 1 g/kg 4
hourly) will increase the non renal elimination of salicylate
and will greatly diminish the plasma half-life (Hillman &
Prescott, 1985). Although multiple doses of activated
charcoal in pigs following high dose intravenous aspirin
administration did not enhance salicylate clearance (Johnson
et al., 1995), it was found effective in the poisoned
patients (Montoya-Carbera et al., 1995). Addition of sodium
sulphate as a saline catharatic to activated charcoal was
found to have no effect on the prevention of salicylate in
six healthy volunteers (Sketsis et al., 1982). However, it
may be used as a cathartic in a single dose with the initial
dose of plain activated charcoal to prevent constipation,
although sorbitol may be considered as a safer cathartic to
be used.
10.4 Enhanced elimination
Forced alkaline diuresis was employed in the management
of salicylate poisoning (Lawson et al., 1969; Berg, 1977).
Fluid retention may occur during forced diuresis and increase
the risk of pulmonary oedema in severe salicylate
intoxication (Heffner & Sahn, 1981; Balali-Mood, 1981). It is
now recognised that the urine pH is of far greater
importance than the volume of urine excreted (Balali-Mood,
1981; Prescott et al., 1982). To achieve maximum excretion of
salicylate, a urine pH of more than 8 is required
(Balali-Mood , 1981). Urinary alkalinisation requires close
supervision in the ward/intensive-care unit. In patients with
cardiac and or renal impairment, and in those who are in
shock, haemodialysis/haemoperfusion should be considered.
Haemodialysis should also be considered in severely poisoned
patients with features of central nervous system toxicity,
pulmonary oedema, cerebral oedema and in cases of plasma
salicylate concentrations of more than 800 mg/L (Proudfoot,
1983). Haemodialysis is preferred to haemoperfusion because
it corrects acid-base and electrolyte abnormalities more
rapidly and may avoid the need for the administration of
large amounts of sodium bicarbonate (Winchester et al.,
1981). Peritoneal dialysis is less effective than alkaline
diuresis and is two to three times less effective than
haemodialysis, and its use is not recommended (Winchester et
al., 1977).
10.5 Antidote treatment
10.5.1 Adults
There is no specific antidote.
10.5.2 Children
There is no specific antidote.
10.7 Management discussion
Salicylate poisoning particularly in chronic cases may
be missed. Systemic inflammatory response syndrome (SIRS) is
characterised by body temperature abnormalities, tachypnea or
hyperventilation, tachycardia, and leukocytosis or
leukopenia. Chronic salicylate poisoning should be considered
as a cause of SIRS in the absence of a source of infection,
since survival depends on prompt diagnosis and management
(Chalasani et al., 1996). Chronic salicylate poisoning
particularly those with metabolic acidosis, hypoglycaemia,
lethargy, coma and fits in malaria endemic areas may mimic
severe malaria as was investigated in Kenya (English et al.,
1996). Although the clinical value of screening for
salicylates in acute poisoning was emphasized (Chan et al.,
1995), determination of plasma salicylate concentration is
required to confirm the diagnosis and to estimate the
severity of salicylate intoxication. Prevention of absorption
by emesis, gastric aspiration and lavage after ingestion may
be required. Estimation of serial plasma salicylate poisoning
following overdosage in adults revealed that the peak
concentration may be delayed up to 24 hours post ingestion
(Balali-Mood, 1981). Alkalization of the urine (pH> 8) and
repeated doses of activated charcoal will enhance salicylate
elimination (Balali-Mood, 1981; Prescott et al., 1982;
Hillman & Prescott, 1985). In severe salicylate intoxication
(plasma salicylate concentrations >800 mg/L), haemodialysis
is also recommended.
11. ILLUSTRATIVE CASES
11.1 Case report from literature
Salicylate poisoning has been very common and thousands
of cases have been reported in the literature since the early
20th century. Cases of salicylic acid dermal absorption which
induced intoxication particularly in children have also been
reported (Davies et al., 1979; Clark & Wilson, 1981).
Salicylic acid intoxication caused by teething ointment was
also reported (Paynter & Alexander, 1979).
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Since salicylic acid is only available in external
usage forms care should be taken to use it under supervision.
Drugs must be kept in a safe place, not to be reached by
children. Parents and patients need to be warned of the
potential risks of chronic usage of salicylic acid
preparations.
12.2 Other
None.
13. REFERENCES
Alvan G, Bergman U, Gustafsson LL, (1981) . High unbound
fraction of salicylate in plasma during intoxication. Br J Clin
Pharmacol, 11:625-6.
American Academy of Clinical Toxicology and European Association
of Poisons Centres and Clinical Toxicologists (1997) Position
Statement: Gastric Lavage. Clinical Toxicology 35(7):711-19.
Anderson RJ, Potts DE, Gabow PA, Rumack BH, Schrier RW, (1976).
Unrecognised adult salicylate intoxication. Ann Intern Med,
85:745-8.
Anderson RJ, (1981). Asterixis as a manifestation of salicylate
toxicity. Ann Intern Med, 95:188-9.
Balali-Mood M, (1981). Effects of forced alkaline diuresis on
salicylate distribution and elimination following overdosage, Ph.D
thesis, University of Edinburgh, 132-270.
Berg KJ, (1977). Acute acetylsalicylic acid poisoning. Treatment
with forced alkaline diuresis and diuretics. Eur J Clin Pharmacol,
12:111-6.
Berk WA, Anderson JC, (1989). Salicylate associated asystole:
report of two cases. Am J Med, 86:505-6.
Black RA, Bensinger RE, (1982). Bilateral subconjunctival
haemorrhage after acetylsalicylic acid overdose. Ann Opthalmol,
14:1024-5.
Borga O, Odar-Cederlof I, Ringbeger VA, Norlin A, (1976). Protein
binding of salicylate in uremic and normal plasma. Clin Pharm
Ther, 20:464-75.
Boldly DAR, Bensinger RE, (1982). Bilateral subconjunctival
hemorrhage after acetylsalicylic acid overdose. Ann Opthamol,
14:1024-5.
Brantmark B, Hedner U, Melander A et al, (1981). Salicylate
inhibition of antiplatelet effect of aspirin. Lancet, 2:1349.
Chalasani N, Roman J, Jurado RL, (1996). Systemic inflammatory
response syndrome caused by chronic salicylate intoxication. South
Med J, 89: 479-82.
Chan TY, (1996). Medicated oil and severe salicylate poisoning:
quantifying the risk based on methyl salicylate content and bottle
size. Vet Hum Toxicol 38:133-4.
Chan TY, Chan ay, Ho CS, Critchley JA, (1995). The clinical value
of screening for salicylates in acute poisoning. Vet Hum Toxicol
37: 37-8.
Chren MM, Bickers DR, (1990). Dermatological pharmacology
in:Goodman & Gilman(eds) Pharmacological Basis of Therapeutics,
Pergamon press, New York, p.1587.
Clark JH, Wilson WG, (1981). A 16-day-old breast-fed infant with
metabolic acidosis caused by salicylate. Clin Pediatr,
20:53-4.
Cotton EK, Fahlberg VI, (1964). Hypoglycaemia with salicylate
poisoning. A report of two cases. Am J Dis Children, 108:171-3
Danel V, Henry JA, (1988). Activated charcoal, emesis, and gastric
lavage in aspirin overdose. Br Med J, 296:1507
Davies DM, (1985). Skin disorders in:Textbook of adverse drug
reactions. 3rd edition. Oxford University press. pp484.
Davies MG, Vella Briffa D, Greaves MW, (1979). Systemic toxictiy
from topically applied salicylic acid. Br Med J, 1:661.
Davidson C, (1971). Salicylate metabolism in man. Ann. N.Y. Acad.
Sci., 179: 249-268.
Done AK, (1960). Salicylate intoxication. Significance of
measurements of salicylate in blood in cases of acute ingestion.
Pediatrics, 26:800-7.
Dove DJ, Jones T, (1982). Delayed coma associated with salicylate
intoxication, J Pediatr, 100:493-6.
Editorial, (1964). Percutaneous salicylic acid intoxication. JAMA,
190:1064.
English M, Marsh V, Amukoya E, Lowe B, Murphy S, Marsh K, (1996).
Lancet 347: 1736-7.
Gaudrealt P, Temple AR, Lovejoy FH, (1982). The relative severity
of acute versus chronic salicylate poisoning in children. A
clinical comparison. Pediatrics, 70:566-9.
Germann R, Schindera I, Kuch M, Seitz U, Altmeyer S, Schindera F,
(1996). Life threatening salicylate poisoning caused by cutaneous
absorption in severe icthyosis vulgaris. Hautarzt 47:624-7.
Grisolia S, Mendelson J, Diedrich D, (1969). Inactivation of
enzymes by therapeutic concentrations of aspirin and salicylate.
Nature, 223:79.
Heffner JE, Sahn SA, (1981). Salicylate induced pulmonary oedema.
Ann Intern Med, 95:405-9.
Hillman RJ, Prescott LF, (1985). Treatment of salicylate poisoning
with repeated oral charcoal. Br Med J, 291:1472.
Insel PA, (1996).Analgesic-antipyretics and anti-inflammatory
agents and drugs employed in the treatment of gout in: Goodman &
Gilman Pharmacological basis of Therapeutics.eds. Hardman JG. &
Limbird LE. McGraw Hill, New York, pp 617-657.
Johnson D, Eppler J, Giersbrecht E, Verjee Z, Rais A, Wiggins T,
Fragga C, (1995). Effect of multiple-dose activated charcoal on
the clearance of high dose intravenous aspirin in a procine model.
Ann Emerg Med, 26: 569-74.
Johnson PN, Welch DW, (1984). Methylsalicylate/aspirin
(salicylate) equivalence: Who do you trust? Vet Human Toxicol,
26:317-8.
Kaplan EH, Kennedy J, Davies J, (1954). Effects of salicylate and
other benzoates on oxidative enzymes of the tricarboxcylic acid
cycle in rat tissue homogenates. Arch Biochem Biophys, 51:47.
Lawson AAH, Proudfoot AT, Brown SS et al, (1969). Forced diuresis
in the treatment of acute salicylate poisoning in adults. Quart J
Med, 38:31-48.
Levy G, Tsuchiya T, (1972). Darvon poisoning with delayed
salicylism: a case report. Pediatrics, 49:610-611.
Lowney ED, Baublis JV, Kery GM, Harrel ER, McKenzie AR, (1967).
The scaled skin syndrome in small chlildren. Arch Dermatol, 95:
359-69.
Lynd PA, Andreasen AC, Wyatt RJ, (1976). Intrauterine salicylate
intoxication in a newborn. Clin Pediatrics, 15:912-3.
McGuigan MA, (1987). A two-year review of salicylate deaths in
Ontario. Arch Intern Med, 147:510-2.
Meredith TJ, Vale JA, (1981). Salicylate poisoning in: Poisoning
Diagnosis and Treatment, Vale and Meredith Eds, (Update Books,
London): pp 97-103.
Montoya-Cabrera MA, Escalate-Galido P, Saucera-Garcia JM, (1995).
Treatment of acute poisoning caused by carbamazepine, digoxin and
acetyl salicylic acid with repeated doses of activated charcoal.
Gac Med Mex, 131: 349-54.
Meyers EN, Bernstein JM, Fostiropoloous G, (1965). Salicylate
ototoxictiy: a clinical study. N Engl J Med, 273:587-90.
Parish P, (1991). Skin Diseases in:Medical Treatments-The benefits
and risks, Penguin Books. London, 1024-41.
Paynter AS, Alexander FW, (1979). Salicylate intoxication caused
by teething ointment. Lancet, 2:1132.
Pluskwa F, Couturier M, Kirsh JM, Fourgeaux B, Campinos L, Duval
G, (1984). Intoxication, salicylee, grave par vou transcutanee
chez un brule. La Presse Med, 13:1391.
Prescott LF, Balai-Mood M, Critchley JAJH, Johnstone AF, Proudfoot
AT, (1982). Diuresis or urinary alkalinisation for salicylate
poisoning. Br Med J, 285:1383-6.
Proudfoot AT, Brown SS, (1969). Acidaemia and salicylate poisoning
in adults. Br Med J, 2:547-50.
Proudfoot AT, (1983). Toxicity of salicylates. Am J Med, 14:99-
103.
Ralston ME, Pearigan PD, Ponaman ML, Erickson LC, (1995).
Transient myocardial dysfunction in a child with salicylate
toxicity. J Emerg Med, 13: 657-9.
Reynolds JEF (ed) (1996). Salicylic acid in: Martindale The Extra
Pharmacopoeia, 31st Edition. The Royal Pharmaceutical Society,
London, pp 1093.
Reye RDK, Morgan G, Baral J, (1963). Encephalopathy and fatty
degeneration of the viscera. A disease entity in childhood.
Lancet, 11:749-53.
Robins JB, Turnbull JA, Robertson C, (1985). Gastric perforation
after acute aspirin overdose. Hum Toxicol, 4:527-8.
Rupp DJ, Seaton RD, Wiegmann TB, (1983). Acute polyuric renal
failure after aspirin intoxication. Arch Int Med, 143:1237-8.
Sandford-Smith JH, (1974). Transient myopia occurred in a patient
following the ingestion of 2.7g of aspirin. Br J Opthal,
58:698.
Smith MJH, Dawkins PD, (1971). Salicylate and enzymes. J Pharm
Pharmacol, 23:729.
Springer DJ, Groll A, (1980). Poisoning with enteric coated
acetylsalicylic acid complicating gastric acid outlet
obstruction. Can Assoc J, 122:1032-3.
Sullivan-Bolyai JZ, Corey L, (1981). Epidemiology of Reye
syndrome. Epidemiologic Rev, 3:1-26.
Taylor JR, Halprin KM, (1975). Percutaneous absorption of
salicylic acid. Arch Dermatol, 111:740-3.
Temple AR, (1978). Pathophysiology of aspirin overdosage toxicity,
with implications for management. Pediatrics, 62
(suppl):873-6.
Temple AR, (1981). Acute and chronic effects of aspirin toxicity
and their treatment. Arch Intern Med, 141:364-9.
Temple AR, George DJ, Done AK, Thompson JA, (1976). Salicylate
poisoning complicated by fluid retention. Clin Toxicol,
9:61-8.
Tenney SM, Miller RM, (1955). The respiratory and circulatory
actions of salicylate. Am J Med, 19:495-508.
Thurston JH, Pollock PG, Warren SK, Jones EM, (1970). Reduced
brain glucose with normal plasma glucose in salicylate poisoning.
J Clin Invest, 49:2139.
Todd PJ, Sills JA, Harris F, Cowen JM, (1981). Problems with
overdose of sustained-release aspirin. Lancet, 1:777.
Vale JA, Buckley GM, Meredith TJ, (1985). Algorithm for modified
alkaline diuresis in salicylate-induced poisoning. Br Med J,
290:155.
Walters JS, Woodring JH, Stelling CB, Rosenbaum HD, (1983).
Salicylate-induced pulmonary edema. Radiology, 146:289-93.
Waltner JG, (1955). The effect of salicylates on the inner ear.
Ann Otol Rhino Lar, 64:617-22.
Winchester JF, Gelfland MC, Knepshield JH, Schreiner GE, (1977).
Dialysis and hemoperfusion of poisons and drugs. Transactions of
the American society of Artificial and internal organs,
23:762-842.
Windholz M, (1983). Salicylic acid. The Merck Index, Merck & Co
Inc. Rahway, N.J., USA. 10th edition, 8190.
Winchester JF, Gelfland MC, Helliwell M, Vale JA, Goulding R,
Schreiner GE, (1981). Extracorporeal treatment of salicylate or
acetaminophen poisoning.Is there a role? Arch Intern Med,
141:370-4.
Winters RW, White JS, Ordway NK, (1959). Disturbances of acid-base
equilibrium in salicylate intoxication. Pediatrics, 23:260-85.
Wofe JD, Metger AL, Goldstein RC, (1974). Aspirin hepatitis. Ann
Intern Med, 80:74-6.
Young RSK, Torreti D, Williams RH et al, (1984). Reye's syndrome
associated with long-term aspirin overdose. Ann Emerg Med, 16:434.
14. AUTHORS
Authors: Mahdi Balali-Mood & Kia Balali-Mood
Poisons Control Centre
Imam Reza Hospital
Faculty of Medicine
Mashhad University of Medical Sciences
Mashhad 91735
Islamic Republic of Iran
Tel: +98-51-889301/98973
Fax: +98-51-883714/93038
Date: August 1996
Reviewer: Bill Watson, Kansas City, USA
Date: 1996
Peer review: INTOX Meeting, London, UK, March, 1998
Editor: Dr M.Ruse (September, 1998)
3.3 Physical properties
3.3.1 Colour
Colourless or white
3.3.2 State/form
Solid-crystals
3.3.3 Description
Colourless acicular crystals or a white
crystalline powder. The synthetic form is white but if
prepared from natural methyl salicylate, it may have a
slightly yellow or pink tint.
Salicylic acid is a white crystalline powder with a
sweetish acrid taste. If prepared from natural methyl
salicylate, it may have a faint mint like odour. It is
available in forms of ointments, cream, gel,
transdermal patches, liquids and plaster.
Salicylic acid is soluble 1 in 460 to 550 of water, 1
in 15 of boiling water, 1 in 3 to 4 in alcohol, 1 in 3
in ether and 1 in 45 in chloroform (Reynolds, 1996).
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Shelf-life is dependent on the manner of
storage. It should be stored in well closed containers
and protected from light (Reynolds, 1996).
3.4.2 Storage conditions
Store in well closed containers and protect
from light (Reynolds, 1996).
4. USES
4.1 Indications
4.1.1 Indications
Analgesic
Other analgesic/antipyretic
Salicylate; analgesic
4.1.2 Description
Salicylic acid has keratinolytic properties and
is applied topically in the treatment of
hyperkeratotic and scaling conditions such as
dandruff, ichthyosis and psoriasis. Initially a
concentration of 2% is used increasing to about 6% if
necessary.
It is often used in conjunction with many other
agents, such as benzoic acid, coal tar, resorcinol and
sulphur. Salicylic acid is also used in the form of
paint and in the form of collodion basis (10 to 17%)
or as a plaster (20 to 50%) to destroy warts and
corns. It also possesses fungicidal properties and is
used topically in the treatment of fungal skin
infections such as tinea (Reynolds, 1996).
4.2 Therapeutic dosage
4.2.1 Adults
The topical preparations such as transdermal
patches, gels, ointments, liquids, creams or plasters
are usually used in concentrations of 2.5 to 60% for
the treatment of psoriasis, warts and other keratinous
disorders (Chren & Bickers, 1990).
4.2.2 Children
Since salicylic acid is used topically, the
above concentration and dosage may be applied in
children with caution.
4.3 Contraindications
Due to the severe gastric irritation which salicylic
acid causes, it is no longer used orally. However, when used
topically it may cause an allergic contact rash in some
people. If applied to large areas of skin, it may be absorbed
into the blood stream and induce salicylism (Parish, 1991).
5. ROUTES OF ENTRY
5.1 Oral
Salicylic acid causes gastric irritation and thus,
there is no oral pharmaceutical available. However, Chinese
medicated oil (Koong yick Hung Far Oil) which contains 67%
methyl salicylate has been taken orally (Chan, 1996).
5.2 Inhalation
Not relevant.
5.3 Dermal
Salicylic acid is readily absorbed from the skin and may
induce toxicity (salicylism).
5.4 Eye
Unknown
5.5 Parenteral
Unknown
5.6 Other
No data available.
6. KINETICS
6.1 Absorption by route of exposure
Salicylic acid is readily absorbed from the skin and may
cause toxicity, particularly in children and the newborn.
Herbal products such as the chinese medicated oil which
contains methyl salicylate is also absorbed through
gastrointestinal mucosa following ingestion (Chan, 1996).
6.2 Distribution by route of exposure
About 50 to 80% of salicylate in the blood is bound to
plasma proteins, while the rest remains in the active ionized
state; protein binding is concentration dependent. Saturation
of binding sites lead to more free salicylate and increased
toxicity. The apparent volume of distribution is 0.1 to 0.2
L/kg. Acidosis increases the volume of distribution because
of the enhancement of tissue penetration of salicylate (Levy
& Tsuchiya, 1972).
6.3 Biological half-life by route of exposure
The plasma salicylate half-life following therapeutic
doses is 2 to 4.5 hours, but in overdosage, increases to 18
to 36 hours (Done, 1960). When the metabolic pathway is
saturated (conjugation with glycin), zero order kinetics
apply and thus the elimination half-life cannot be derived
correctly.
6.4 Metabolism
At low dosage, approximately 80% of salicylic acid is
metabolised in the liver. Conjugation with glycine, forms
salicyluric acid and when conjugated with glucuronic acid,
acyl and phenolic glucuronide are formed. Small amounts of
salicylic acid are also hydroxylated to gentisic acid. With
large doses, the kinetics switch from first order to zero
order (Michaelis-Menten) kinetics (Levy & Tsuchiya, 1972).
6.5 Elimination and excretion
Salicylates are excreted mainly by the kidney as
salicylic acid, salicyluric acid, salicylic glucuronides and
gentisic acid. The proportion excreted of each metabolite,
depends upon urinary pH. With urinary alkalinisation,
salicylic acid excretion is enhanced (Prescott et al., 1982).
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Marked hyperventilation occurs as a result of
direct stimulation of the respiratory centre. Indirect
stimulation of respiration is caused by an increased
production of CO2 as a result of salicylate-induced
uncoupling of oxidative phosphorylation. Respiratory
alkalosis develops as a result of the direct and
indirect stimulation of the respiratory centre. In an
attempt to compensate, bicarbonate accompanied by
sodium, potassium and water, is excreted in the urine.
Dehydration and hypokalemia result, but more
importantly, the loss of bicarbonate diminishes the
buffering capacity of the body and allows the
development of a metabolic acidosis (Proudfoot &
Brown, 1969; Davidson, 1971; Proudfoot, 1983). The
pyretic effect of toxic doses of salicylate is a
direct result of the uncoupling of oxidative
phosphorylation. High doses of salicylate have
additional toxic effects on the central nervous system
consisting of stimulation(including convulsions)
followed by depression, confusion, dizziness,
asterixis, delirium, psychosis, stupor and coma
(Anderson et al., 1976; Anderson, 1981). Very high
doses of salicylates have a depressive effect on the
medulla and may cause central respiratory paralysis as
well as sudden circulatory collapse secondary to
vasomotor depression. The loss of buffering capacity
and the effects of salicylate on carbohydrate, lipid
and protein metabolism lead to the development of a
metabolic acidosis or, more commonly in practice, a
mixed acid-base disturbance (Proudfoot & Brown, 1969;
Meredith & Vale, 1981; Proudfoot, 1983). Both hypo-
and hyper-glycaemia may occur in salicylate poisoning.
The former most probably due to an increased tissue
demand for glucose oxidation due to the uncoupling of
oxidative phosphorylation. Neuroglycopenia can occur
in the presence of normal blood glucose concentrations
(Thurston et al., 1970). If hepatic glycogen stores
are adequate, catecholamines production stimulates
glycogenolysis leading to hyperglycemia which can
persist for several days (Cotton & Fahlberg, 1964).
Salicylate intoxication is often accompanied by
hypothrombinaemia due to a warfarin like action of
salicylate on the vitamin K1 epoxide cycle, which
rarely causes clinical problems (Proudfoot, 1983).
7.1.2 Pharmacodynamics
Salicylic acid alleviates pain, lowers an
elevated body temperature and inflammation by
inhibiting the synthesis of prostaglandins that occur
in inflamed tissues. Salicylate inhibits the
conversion of arachidonic acid to the unstable
endoperoxide intermediate PG G2, which is catalyzed by
the enzyme cyclo oxygenase. Platelets are especially
susceptible to this action as they are incapable of
regenerating the enzyme, presumably they have little
or no capacity for protein biosynthesis (Brantmark et
al., 1981). Cyclo oxygenase(COX) is present in two
main isoforms. COX-1 is the isoform of the enzyme and
is present under normal physiological conditions. COX-
2 is the inducible isoform of the enzyme and is
induced in settings of inflammation (i.e. production
of eicosanoids and kinins). Inhibition of COX-1
results in unwanted side effects, particularly those
leading to gastric ulcer. Current pharmacological
research, in the NSAID field is centering on finding a
selective COX-2 inhibitor. Meloxicam is a selective
COX-2 inhibitor that has been marketed in France and
some other countries (Insel, 1996).
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Since salicylic acid is available
only in topical preparations, human data on
toxicity have not been reported. However, the
chinese medicated oil (Koong Yick Hung Far
Oil), which contains 67% methyl salicylate
induced severe salicylate poisoning
(Chan,1996). Salicylic acid concentrations
above 800 mg/L after 6 hours post exposure is
severely toxic and may be lethal
(Balali-Mood, 1981).
7.2.1.2 Children
Salicylate intoxication is often
more serious in small children (<4 years)
than in older children, due to an early
development of a metabolic acidosis rather
than a respiratory alkalosis (Winters et al.,
1959).
7.2.2 Relevant animal data
None relevant.
7.2.3 Relevant in vitro data
None relevant.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
There is no evidence that moderate therapeutic doses of
salicylates cause fetal damage in human beings; however,
babies born to women who ingest salicylates for long periods
may have a significantly reduced mass at birth. In addition,
there is an increase in prenatal mortality, anaemia,
antepartum and postpartum haemorrhage, prolonged gestation
and complicated deliveries. These effects occur when
salicylates are administered during the third trimester, and
thus its use during this period of pregnancy should be
avoided (Insel, 1996).
7.5 Mutagenicity
No data available
7.6 Interactions
Salicylic acid is highly protein-bound and may increase
the unbound or free drug concentrations of other drugs such
as hypoglycemics, anticoagulants and methotrexate(an
antimetabolite chemotherapeutic drug), reaching toxic levels
of these agents. The uricosuric activity of phenylbutazone,
probenecide and sulphinpyrazone is strongly antagonized by
salicylate and maybe completely diminished by small doses due
to decreased tubular reabsorption of uric acid (Insel, 1996).
In a report of two cases of severe salicylate poisoning,
asystole occurred shortly after the intravenous
administration of diazepam (Berk & Anderson, 1989).
7.7 Main adverse effects
Salicylic acid is a gastric irritant and because of the
serious damage it may cause to the stomach lining, it has not
been used orally. Topical use of salicylic acid may induce
allergic contact dermatitis (Davies, 1985). Salicylic acid
may cause excessive drying and irritation in some people
(Parish, 1991). Some individuals, especially asthmatics
exhibit sensitivity to salicylates. Urticaria, angioneurotic
oedema, rhinitis, severe and even fatal paroxysmal,
bronchospasm and dyspnea may occur (Reynolds, 1996).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Material sampling plan
8.1.1 Sampling and specimen collection
8.1.1.1 Toxicological analyses
Screen residues or suspected
materials, plasma, urine and stomach
contents. Avoid sodium azide
preservation.
8.1.1.2 Biomedical analyses
8.1.1.3 Arterial blood gas analysis
8.1.1.4 Haematological analyses
8.1.1.5 Other (Unspecified) analyses
8.1.2 Storage of laboratory samples and specimens
8.1.2.1 Toxicological analyses
Keep biological samples in a
refrigerator prior to analysis.
8.1.2.2 Biomedical analyses
8.1.2.3 Arterial blood gas analysis
8.1.2.4 Haematological analyses
8.1.2.5 Other (Unspecified) analyses
8.1.3 Transport of laboratory samples and specimens
8.1.3.1 Toxicological analyses
No special conditions.
8.1.3.2 Biomedical analyses
8.1.3.3 Arterial blood gas analysis
8.1.3.4 Haematological analyses
8.1.3.5 Other (Unspecified) analyses
8.2 Toxicological Analyses and Their Interpretation
8.2.1 Tests on toxic ingredient(s) of material
8.2.1.1 Simple Qualitative Test(s)
Trinder's reagent: Dissolve 40 g of
Hg(II) Cl2 in 850 mL of hot water. After
cooling down, add 120 mL of HCl 1 mol/L and
40 g of hydrated ferric nitrate. When the
ferric nitrate has been dissolved, add water
to 1000 mL. Add 0.1 mL Trinder's reagent to 1
mL of urine and mix. A violet colour
indicates the presence of salicylates. For
stomach contents and serum residues, boil a
portion of the specimen with 2 mL of
hydrochloric acid 0.1 mol/L for 10 minutes,
cool and filter, if necessary neutralise with
sodium hydroxide 0.1 mol/L. Add 100 Ál of
Trinders' reagent to the clear neutralized
solution. A violet colour indicates presence
of salicylate.
8.2.1.2 Advanced Qualitative Confirmation Test(s)
8.2.1.3 Simple Quantitative Method(s)
8.2.1.4 Advanced Quantitative method(s)
8.2.2 Tests for biological specimens
8.2.2.1 Simple Qualitative Test(s)
8.2.2.2 Advanced Qualitative Confirmation Test(s)
8.2.2.3 Simple Quantitative Method(s)
8.2.2.4 Advanced Quantitative method(s)
8.2.3 Interpretation of toxicological analyses
8.3 Biomedical investigations and their interpretation
8.3.1 Biochemical analysis
8.3.1.1 Blood, plasma or serum
Platelet function and coagulation
tests, in order to estimate the degree of
inhibition of platelet aggregation and
hypoprothrombinaemia may be
required.
8.3.1.2 Urine
8.3.1.3 Other fluids
8.3.2 Arterial blood gas analyses
In adults with severe salicylate poisoning an
initial respiratory alkalosis is followed by metabolic
acidosis. In children, metabolic acidosis is more
common than in adults. Acid-base disturbances are
usually mixed in mild to moderate salicylate poisoning
(Proudfoot & Brown, 1969; Proudfoot, 1983).
8.3.3 Haematological analyses
8.3.4 Interpretation of biomedical investigations
8.4 Other biomedical (diagnostic) investigations and their
interpretation
8.5 Overall interpretation of all toxicological analyses
and investigations
Following the qualitative test for salicylate, the
plasma/serum salicylate concentration should be determined on
admission, and at regular intervals to assess the severity of
intoxication, prognosis and approach to treatment. Salicylate
concentration may continue to rise even up to 24 hours post
ingestion (Balali-Mood, 1981). However, based on severe
intoxication plasma salicylate concentrations at 6 hours
after an overdose could be divided into three groups:
1. 300 to 500 mg/L mild toxicity
2. 500 to 800 mg/mL moderate toxicity
3. >800 mg/L severe toxicity (Proudfoot, 1983).
Clinical findings and acid-base disturbances should be
considered when interpreting the plasma salicylate
concentration and deciding upon management (Meredith & Vale,
1981). The Done nomogram categorizes the severity of
poisoning for single ingestion based on peak salicylate
concentrations. In patients with significant acidosis and in
patients who ingest multiple doses or sustained release
preparations, the Done nomogram will tend to underestimate
the severity of salicylate intoxication (Todd et al., 1981).
Development of the nomogram was based on the assumption that
salicylates are eliminated by a first order process, whereas
in fact they are partially eliminated through natural
processes. This may lead to overprediction of the severity
of toxicity. Therefore, the Done-nomogram is not commonly
used. Following ingestion of enteric-coated tablets, plasma
salicylate concentrations on admission are not a reliable
guide to the severity of poisoning. Salicylate concentration
may not peak until more than 12 hours after such an overdose
(Springer & Groll, 1980; Todd et al., 1981).
Hypokalemia, hypo/hyperglycaemia, hypocalcaemia and acid-base
disturbances may occur during salicylate poisoning. It is
therefore essential to investigate electrolyte (and if
possible osmolality) and arterial blood gas analysis. Other
biochemical analyses such as liver and kidney function tests
are required as clinically indicated (Cotton & Fahlbeg, 1964;
Balali-Mood, 1981; Proudfoot, 1983).
8.6 References
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Initial symptoms of salicylate poisoning may be
nausea and vomiting, epigastric pain and occasionally
haematemesis. Hyperventilation, sweating, flushing,
fever, irritability, tinnitus and loss of hearing are
the common clinical features of mild to moderate
salicylate intoxication. In severe intoxication,
hypoventilation, stupor, hallucination, convulsions,
papiloedema and coma particularly in children may
occur. Metabolic acidosis, non-cardiogenic pulmonary
oedema, hepatotoxicity and cardiac dysrhythmias may
also occur (Balali-Mood, 1981; Meredith & Vale, 1981).
9.1.2 Inhalation
No data available
9.1.3 Skin exposure
Severe poisoning has been reported as a result
of the use of salicylic acid ointment for
dermatological problems and in the treatment of skin
burns (Editorial of JAMA, 1964; Taylor & Halprin,
1975; Pluskwa et al., 1984).
9.1.4 Eye contact
No data available
9.1.5 Parenteral exposure
No data available
9.1.6 Other
None
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic salicylate poisoning occurred as a
result of excessive therapeutic administration over a
period of 12 hours or more were reported (Dove &
Jones, 1982). Metabolic pathways of salicylic acid
become saturated and thus plasma concentration
increases, producing toxicity. Small children are at
particular risk of overdose especially when fever,
sweating and tachycardia of salicylate intoxication
are attributed to the underlying illness and are used
as indications for increasing the dose (Proudfoot,
1983). Children may become intoxicated through breast
milk (Clark & Wilson, 1981). The presenting signs of
chronic salicylate poisoning can include metabolic
acidosis, hypoglycemia, lethargy, coma and fits
(English et al., 1996).
9.2.2 Inhalation
No data available
9.2.3 Skin exposure
Chronic usage of salicylic acid and or methyl
salicylate in skin and rheumatic diseases may cause
intoxication through percutaneous absorption. Life
threatening salicylate poisoning caused by
percutaneous absorption of salicylic acid (10%
ointment) in a 7-year-old boy with ichthyosis vulgaris
was reported (German et al., 1996). Application of
teething gels containing salicylic acid induced
intoxication (Paynter & Alexandre, 1979).
9.2.4 Eye contact
No data available
9.2.5 Parenteral exposure
No data available
9.2.6 Other
None
9.3 Course, prognosis, cause of death
If a large quantity of a salicylate has been taken,
nausea, vomiting, tinnitus, deafness, sweating,
vasodilatation and hyperventilation may develop. Acid-base
disturbances, electrolyte imbalance, non cardiogenic
pulmonary oedema, hypoventilation and hallucination, stupor,
irritability, coma and convulsions particularly in children
and older patients can proceed death. Loss of consciousness
in adults is very rare and when occurs indicates poor
prognosis. A review of 51 fatal cases of acute salicylate
poisoning in Ontario during 1983 and 1984, discovered that
salicylate was the most common cause of death, due to the
ingestion of single drugs. Autopsy results showed that 50% of
the patients had pulmonary abnormalities, 28% had lesions of
the gastrointestinal tract, 18% had nervous system
abnormalities and 25.6% had no pathological changes
(McGuigan, 1987). Mortality from chronic salicylate
intoxication is considerably higher (25%) than from acute
overdose (1 to 2%) (Anderson et al., 1976). Death is often
due to sudden cardiac arrest or occasionally due to multiple
complications following severe brain damage (Proudfoot,
1983).
9.4 Systemic description of clinical effects
9.4.1 Cardiovascular
Sudden cardiovascular collapse is a recognized
complication of salicylate poisoning (Anderson et
al.1976, Proudfoot et al, 1983). Two patients with
severe salicylate intoxication developed asystole
shortly after intravenous diazepam administration
(Berk & Anderson, 1989). Transient myocardial
dysfunction (global left ventricular shortening
fraction of 23%) with pulmonary edema was found in a
13-month-old boy with salicylate poisoning (Ralston et
al., 1995).
9.4.2 Respiratory
Non-cardiogenic pulmonary oedema may occur in
salicylate intoxicated patients who are over 30 years
of age. Cigarette smoking, chronic salicylate
ingestion, metabolic acidosis and the presence of
neurological symptoms and signs on admission are
strong risk factors for the subsequent development of
pulmonary oedema. The exact mechanism is unknown.
Three possible explanations are: (i) A direct toxic
effect on pulmonary microvasculature; (ii) Interaction
with endogenous mediators such as prostaglandins; and
(iii) A central nervous system mediated effect
(Walters et al., 1983).
9.4.3 Neurological
9.4.3.1 Central Nervous System (CNS)
In moderate to severe salicylate
intoxication, CNS stimulation (irritability
and convulsions) followed by depression,
confusion, dizziness, delirium, psychosis,
asterixis, stupor and coma occur usually when
metabolic acidosis is the dominant acid-base
abnormality (Proudfoot & Brown, 1969). These
features are thought to be due to reduced
ionisation of salicylic acid and a shift of
salicylate from the plasma into the brain.
Very high doses of salicylate have a
depressive effect on the medulla and may
cause central respiratory paralysis as well
as sudden circulatory collapse, secondary to
vasomotor depression (Proudfoot,
1983).
9.4.3.2 Peripheral nervous system
No data available.
9.4.3.3 Autonomic nervous system
No data available.
9.4.3.4 Skeletal and smooth muscle
No data available.
9.4.4 Gastrointestinal
Ingestion of salicylate may result in
epigastric discomfort, nausea and vomiting. Salicylic
acid has more irritant effects on gastric mucosa than
acetylsalicylic acid and thus has been withdrawn from
oral administration for many years. Acetylsalicylic
acid may also cause gastric irritation. Perforated
peptic ulcer also occurs extremely rarely (Robins et
al., 1985).
9.4.5 Hepatic
Hepatotoxicity may occur both after the
therapeutic use of salicylate or following its
overdosage. Liver biopsy revealed acute hepatocellular
necrosis with periportal inflammation and fatty
changes in hepatocytes (Wofe et al.,1974). Hepatic
encephalopathy (Reye's Syndrome) has been reported in
children taking aspirin for treatment of viral
infections such as influenza.
9.4.6 Urinary
9.4.6.1 Renal
Oliguria sometimes occurs, which is
mostly due to dehydration(Temple et al,
1976). Renal failure may rarely occur in
individuals without volume depletion, pre-
existing renal and or systemic diseases (Rupp
et al., 1983).
9.4.6.2 Other
None.
9.4.7 Endocrine and reproductive systems
High doses of salicylate cause release of
adrenaline from the adrenal medulla; this is thought
to be partly responsible for the observed
hypoglycaemia due to glycogenolysis that sometimes
occurs. Large doses of salicylate stimulate
corticosteroids secretion by the adrenal cortex
(Temple, 1981).
9.4.8 Dermatological
Toxic epidermal necrosis in 13 patients
associated with the use of salicylate have been
reported (Lowney et al., 1967).
9.4.9 Eye, ear, nose, throat: local effects
Eye: Transient myopia occurred in a patient
following ingestion of 2.7 g acetylsalicylic acid
(Sandford-Smith, 1974). Bilateral subconjunctival
haemorrhage has been reported (Black & Bensinger,
1982).
Ear: Tinnitus and hearing loss caused by salicylate in
overdose are due to increased labyrinthine pressure
(Waltner, 1955). It may also be due to an effect on
the hair cells of the cochlea. There is a relationship
between the hearing loss and the plasma salicylate
concentration (Meyers et al., 1965).
There has been no reports on the local effects of
salicylate on the nose and throat.
9.4.10 Haematological
Salicylates prolong the bleeding time due to
inhibition of collagen glucosyltransferase present in
membranes of platelets. As a result, the adherence of
platelets to connective tissue or collagen fibres is
diminished. Salicylate overdose reduces the
concentration of vitamin K-dependent coagulation
factors, particularly prothrombin (Brantmark et al.,
1981).
9.4.11 Immunological
Salicylates have the capacity to supress a
variety of antigen-antibody reactions such as: the
inhibition of antibody production, of antigen-antibody
aggregation and of antigen induced release of
histamine. Salicylates also induce a non specific
stabilization of capillary permeability during
immunological insults. The concentration of salicylate
causing this effect is high, and their relationship
to the antirheumatic efficacy of salicylates is yet to
be determined.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Respiratory alkalosis is more often
observed in adults than in children.
Metabolic acidosis develops sooner in
children than in adults (Winters et al.,
1959; Proudfoot & Brown, 1969). However, in
severe salicylate poisoning in adults,
metabolic acidosis may also occur. High
concentrations of salicylate may lower
bicarbonate by not more than 2 to 3 mmols.
The acidosis is not therefore due to the
presence of salicylic acid itself. The
principle cause is competitive inhibition of
NAD+-dependent dehydrogenases including
lactate and oxoglutarate dehydrogenase and
the other oxidative enzymes such as succinate
dehydrogenase (Grisolia et al., 1969).
Salicylate enhances entry and oxidation of
fatty acids in liver cells,leading to
increased ketogenesis. Competitive inhibition
of amino acyl-tRNA synthetases in pairs and
amino acid incorporation results in
amino-acidaemia (Smith & Dawkins, 1971).
Dehydration and vasomotor depression results
in poor renal perfusion and accumulation of
sulphuric and phosphoric acids (Tenney &
Miller, 1995; Winters et al., 1959).
9.4.12.2 Fluid and electrolyte disturbances
Increased renal secretion of
sodium, potassium and water accompanies loss
of bicarbonate in the urine. Fluid loss also
results from vomiting, sweating and
hyperventilation. Dehydration is commonly
associated with hypernatraemia. Water loss
may be considerable from 2 to 3 L/m2 surface
area in moderate to severe poisoning and up
to 6 L/m2 in severely poisoned patients
(Temple, 1978).
9.4.12.3 Others
(1) Oxidative Phosphorylation
The uncoupling of oxidative phosphorylation
by salicylate results in the inhibition of a
number of ATP-dependent reactions and an
increase in O2 uptake and CO2 production.
(2) Nitrogen compound metabolism
Toxic doses of salicylate cause a significant
nitrogen imbalance, characterized by amino
aciduria, though this is due in part to
stimulation of active tubular absorption
because of reduced ATP formation.
(3) Fat metabolism
Salicylates enhance oxidation of fatty acids
in muscle, liver and other tissues together
with a decrease of concentrations of plasma,
free fatty acids, phospholipids and
cholesterol (Insel, 1996).
9.4.13 Allergic reactions
Some people particularly asthmatics, exhibit
marked sensitivity to salicylate, resulting in various
reactions including urticaria and other skin
eruptions, angioneuritis, oedema, rhinitis and severe
and even fatal paroxysmal bronchospasm and dyspnea,
hypotension, shock and syncope (Reynolds, 1996).
Despite the fact that the symptoms (such as the ones
mentioned above) resemble anaphylaxis, this reaction
does not appear to be immunological in nature. It may
be a shunt towards the lipoxygenase pathway leading to
an increased production of leukotrienes and other
inflammatory mediators. Although, this hypothesis is
unproved and it does not explain why only a minority
of patients with asthma or other predisposing
conditions display the reaction. Even so, results in a
small number of patients suggest that blockade of
5-lipoxygenase with the drug zileuton may prevent
symptoms and signs of aspirin intolerance (Insel,
1996).
9.4.14 Other clinical effects
The relationship between the use of salicylate
and Reye's syndrome in children and adolescents
(mostly 5 to 15 years) has been demonstrated by
epidemiological studies (Sullivan-Bolyai & Corey,
1981).
9.4.15 Special risks
Salicylate intoxication may occur through
placental transfer(Lynd, 1976) and breast milk (Clark
& Wilson, 1981).
9.5 Other
None.
9.6 Summary
10. MANAGEMENT
10.1 General principles
Management of acute salicylic acid poisoning includes
prevention of absorption, correction of acid-base, fluid and
electrolyte imbalance and enhancing of the drug elimination.
Respiratory alkalosis needs no specific treatment, but severe
acidosis requires at least a partial correction with sodium
bicarbonate. Hypokalaemia may be aggravated by administration
of sodium bicarbonate. Thus, potassium may need repletion.
However, if large amounts of water and electrolytes are given
to the patient, the sodium and water load may precipitate
pulmonary oedema (Balali-Mood, 1981; Proudfoot, 1983).
Sedative and depressive drugs must be avoided. Tetany may be
corrected with the use of calcium gluconate(Meredith & Vale,
1981). Due to the delayed effects of salicylate overdose, the
patient must be kept under observation for at least 24 hours
(Balali-Mood, 1981).
10.2 Life supportive procedures and symptomatic/specific treatment
If non-cardiogenic pulmonary oedema occurs, mechanical
ventilation with positive end-expiratory pressure (PEEP) may
be required. Correction of electrolyte and acid-base
disturbances is essential. ECG monitoring and in very severe
cases intensive care therapy is needed. Sponging and any
other physical countermeasure for hyperpyrexia may be
required. Antipyretics must be avoided (Balali-Mood, 1981;
Proudfoot, 1983).
10.3 Decontamination
Inducing emesis and when the patient reaches the
hospital, gastric aspiration and lavage, is only recommend if
the patient ingested large amounts of salicylate (American
Academy of Clinical Toxicology and the European Association
of Poisons Centres and Clinical Toxicologists, 1997). It is
not necessary if a non-oral route of entry such as dermal
absorption, caused intoxication.
Although activated charcoal proved to be equally effective as
emesis and gastric lavage in volunteers (Danel & Henry,
1988), it may only be exploited therapeutically if the
patient presents soon after ingestion. Repeated doses of
activated charcoal (50 to 75 g immediately and 1 g/kg 4
hourly) will increase the non renal elimination of salicylate
and will greatly diminish the plasma half-life (Hillman &
Prescott, 1985). Although multiple doses of activated
charcoal in pigs following high dose intravenous aspirin
administration did not enhance salicylate clearance (Johnson
et al., 1995), it was found effective in the poisoned
patients (Montoya-Carbera et al., 1995). Addition of sodium
sulphate as a saline catharatic to activated charcoal was
found to have no effect on the prevention of salicylate in
six healthy volunteers (Sketsis et al., 1982). However, it
may be used as a cathartic in a single dose with the initial
dose of plain activated charcoal to prevent constipation,
although sorbitol may be considered as a safer cathartic to
be used.
10.4 Enhanced elimination
Forced alkaline diuresis was employed in the management
of salicylate poisoning (Lawson et al., 1969; Berg, 1977).
Fluid retention may occur during forced diuresis and increase
the risk of pulmonary oedema in severe salicylate
intoxication (Heffner & Sahn, 1981; Balali-Mood, 1981). It is
now recognised that the urine pH is of far greater
importance than the volume of urine excreted (Balali-Mood,
1981; Prescott et al., 1982). To achieve maximum excretion of
salicylate, a urine pH of more than 8 is required
(Balali-Mood , 1981). Urinary alkalinisation requires close
supervision in the ward/intensive-care unit. In patients with
cardiac and or renal impairment, and in those who are in
shock, haemodialysis/haemoperfusion should be considered.
Haemodialysis should also be considered in severely poisoned
patients with features of central nervous system toxicity,
pulmonary oedema, cerebral oedema and in cases of plasma
salicylate concentrations of more than 800 mg/L (Proudfoot,
1983). Haemodialysis is preferred to haemoperfusion because
it corrects acid-base and electrolyte abnormalities more
rapidly and may avoid the need for the administration of
large amounts of sodium bicarbonate (Winchester et al.,
1981). Peritoneal dialysis is less effective than alkaline
diuresis and is two to three times less effective than
haemodialysis, and its use is not recommended (Winchester et
al., 1977).
10.5 Antidote treatment
10.5.1 Adults
There is no specific antidote.
10.5.2 Children
There is no specific antidote.
10.7 Management discussion
Salicylate poisoning particularly in chronic cases may
be missed. Systemic inflammatory response syndrome (SIRS) is
characterised by body temperature abnormalities, tachypnea or
hyperventilation, tachycardia, and leukocytosis or
leukopenia. Chronic salicylate poisoning should be considered
as a cause of SIRS in the absence of a source of infection,
since survival depends on prompt diagnosis and management
(Chalasani et al., 1996). Chronic salicylate poisoning
particularly those with metabolic acidosis, hypoglycaemia,
lethargy, coma and fits in malaria endemic areas may mimic
severe malaria as was investigated in Kenya (English et al.,
1996). Although the clinical value of screening for
salicylates in acute poisoning was emphasized (Chan et al.,
1995), determination of plasma salicylate concentration is
required to confirm the diagnosis and to estimate the
severity of salicylate intoxication. Prevention of absorption
by emesis, gastric aspiration and lavage after ingestion may
be required. Estimation of serial plasma salicylate poisoning
following overdosage in adults revealed that the peak
concentration may be delayed up to 24 hours post ingestion
(Balali-Mood, 1981). Alkalization of the urine (pH> 8) and
repeated doses of activated charcoal will enhance salicylate
elimination (Balali-Mood, 1981; Prescott et al., 1982;
Hillman & Prescott, 1985). In severe salicylate intoxication
(plasma salicylate concentrations >800 mg/L), haemodialysis
is also recommended.
11. ILLUSTRATIVE CASES
11.1 Case report from literature
Salicylate poisoning has been very common and thousands
of cases have been reported in the literature since the early
20th century. Cases of salicylic acid dermal absorption which
induced intoxication particularly in children have also been
reported (Davies et al., 1979; Clark & Wilson, 1981).
Salicylic acid intoxication caused by teething ointment was
also reported (Paynter & Alexander, 1979).
12. ADDITIONAL INFORMATION
12.1 Specific preventive measures
Since salicylic acid is only available in external
usage forms care should be taken to use it under supervision.
Drugs must be kept in a safe place, not to be reached by
children. Parents and patients need to be warned of the
potential risks of chronic usage of salicylic acid
preparations.
12.2 Other
None.
13. REFERENCES
Alvan G, Bergman U, Gustafsson LL, (1981) . High unbound
fraction of salicylate in plasma during intoxication. Br J Clin
Pharmacol, 11:625-6.
American Academy of Clinical Toxicology and European Association
of Poisons Centres and Clinical Toxicologists (1997) Position
Statement: Gastric Lavage. Clinical Toxicology 35(7):711-19.
Anderson RJ, Potts DE, Gabow PA, Rumack BH, Schrier RW, (1976).
Unrecognised adult salicylate intoxication. Ann Intern Med,
85:745-8.
Anderson RJ, (1981). Asterixis as a manifestation of salicylate
toxicity. Ann Intern Med, 95:188-9.
Balali-Mood M, (1981). Effects of forced alkaline diuresis on
salicylate distribution and elimination following overdosage, Ph.D
thesis, University of Edinburgh, 132-270.
Berg KJ, (1977). Acute acetylsalicylic acid poisoning. Treatment
with forced alkaline diuresis and diuretics. Eur J Clin Pharmacol,
12:111-6.
Berk WA, Anderson JC, (1989). Salicylate associated asystole:
report of two cases. Am J Med, 86:505-6.
Black RA, Bensinger RE, (1982). Bilateral subconjunctival
haemorrhage after acetylsalicylic acid overdose. Ann Opthalmol,
14:1024-5.
Borga O, Odar-Cederlof I, Ringbeger VA, Norlin A, (1976). Protein
binding of salicylate in uremic and normal plasma. Clin Pharm
Ther, 20:464-75.
Boldly DAR, Bensinger RE, (1982). Bilateral subconjunctival
hemorrhage after acetylsalicylic acid overdose. Ann Opthamol,
14:1024-5.
Brantmark B, Hedner U, Melander A et al, (1981). Salicylate
inhibition of antiplatelet effect of aspirin. Lancet, 2:1349.
Chalasani N, Roman J, Jurado RL, (1996). Systemic inflammatory
response syndrome caused by chronic salicylate intoxication. South
Med J, 89: 479-82.
Chan TY, (1996). Medicated oil and severe salicylate poisoning:
quantifying the risk based on methyl salicylate content and bottle
size. Vet Hum Toxicol 38:133-4.
Chan TY, Chan ay, Ho CS, Critchley JA, (1995). The clinical value
of screening for salicylates in acute poisoning. Vet Hum Toxicol
37: 37-8.
Chren MM, Bickers DR, (1990). Dermatological pharmacology
in:Goodman & Gilman(eds) Pharmacological Basis of Therapeutics,
Pergamon press, New York, p.1587.
Clark JH, Wilson WG, (1981). A 16-day-old breast-fed infant with
metabolic acidosis caused by salicylate. Clin Pediatr,
20:53-4.
Cotton EK, Fahlberg VI, (1964). Hypoglycaemia with salicylate
poisoning. A report of two cases. Am J Dis Children, 108:171-3
Danel V, Henry JA, (1988). Activated charcoal, emesis, and gastric
lavage in aspirin overdose. Br Med J, 296:1507
Davies DM, (1985). Skin disorders in:Textbook of adverse drug
reactions. 3rd edition. Oxford University press. pp484.
Davies MG, Vella Briffa D, Greaves MW, (1979). Systemic toxictiy
from topically applied salicylic acid. Br Med J, 1:661.
Davidson C, (1971). Salicylate metabolism in man. Ann. N.Y. Acad.
Sci., 179: 249-268.
Done AK, (1960). Salicylate intoxication. Significance of
measurements of salicylate in blood in cases of acute ingestion.
Pediatrics, 26:800-7.
Dove DJ, Jones T, (1982). Delayed coma associated with salicylate
intoxication, J Pediatr, 100:493-6.
Editorial, (1964). Percutaneous salicylic acid intoxication. JAMA,
190:1064.
English M, Marsh V, Amukoya E, Lowe B, Murphy S, Marsh K, (1996).
Lancet 347: 1736-7.
Gaudrealt P, Temple AR, Lovejoy FH, (1982). The relative severity
of acute versus chronic salicylate poisoning in children. A
clinical comparison. Pediatrics, 70:566-9.
Germann R, Schindera I, Kuch M, Seitz U, Altmeyer S, Schindera F,
(1996). Life threatening salicylate poisoning caused by cutaneous
absorption in severe icthyosis vulgaris. Hautarzt 47:624-7.
Grisolia S, Mendelson J, Diedrich D, (1969). Inactivation of
enzymes by therapeutic concentrations of aspirin and salicylate.
Nature, 223:79.
Heffner JE, Sahn SA, (1981). Salicylate induced pulmonary oedema.
Ann Intern Med, 95:405-9.
Hillman RJ, Prescott LF, (1985). Treatment of salicylate poisoning
with repeated oral charcoal. Br Med J, 291:1472.
Insel PA, (1996).Analgesic-antipyretics and anti-inflammatory
agents and drugs employed in the treatment of gout in: Goodman &
Gilman Pharmacological basis of Therapeutics.eds. Hardman JG. &
Limbird LE. McGraw Hill, New York, pp 617-657.
Johnson D, Eppler J, Giersbrecht E, Verjee Z, Rais A, Wiggins T,
Fragga C, (1995). Effect of multiple-dose activated charcoal on
the clearance of high dose intravenous aspirin in a procine model.
Ann Emerg Med, 26: 569-74.
Johnson PN, Welch DW, (1984). Methylsalicylate/aspirin
(salicylate) equivalence: Who do you trust? Vet Human Toxicol,
26:317-8.
Kaplan EH, Kennedy J, Davies J, (1954). Effects of salicylate and
other benzoates on oxidative enzymes of the tricarboxcylic acid
cycle in rat tissue homogenates. Arch Biochem Biophys, 51:47.
Lawson AAH, Proudfoot AT, Brown SS et al, (1969). Forced diuresis
in the treatment of acute salicylate poisoning in adults. Quart J
Med, 38:31-48.
Levy G, Tsuchiya T, (1972). Darvon poisoning with delayed
salicylism: a case report. Pediatrics, 49:610-611.
Lowney ED, Baublis JV, Kery GM, Harrel ER, McKenzie AR, (1967).
The scaled skin syndrome in small chlildren. Arch Dermatol, 95:
359-69.
Lynd PA, Andreasen AC, Wyatt RJ, (1976). Intrauterine salicylate
intoxication in a newborn. Clin Pediatrics, 15:912-3.
McGuigan MA, (1987). A two-year review of salicylate deaths in
Ontario. Arch Intern Med, 147:510-2.
Meredith TJ, Vale JA, (1981). Salicylate poisoning in: Poisoning
Diagnosis and Treatment, Vale and Meredith Eds, (Update Books,
London): pp 97-103.
Montoya-Cabrera MA, Escalate-Galido P, Saucera-Garcia JM, (1995).
Treatment of acute poisoning caused by carbamazepine, digoxin and
acetyl salicylic acid with repeated doses of activated charcoal.
Gac Med Mex, 131: 349-54.
Meyers EN, Bernstein JM, Fostiropoloous G, (1965). Salicylate
ototoxictiy: a clinical study. N Engl J Med, 273:587-90.
Parish P, (1991). Skin Diseases in:Medical Treatments-The benefits
and risks, Penguin Books. London, 1024-41.
Paynter AS, Alexander FW, (1979). Salicylate intoxication caused
by teething ointment. Lancet, 2:1132.
Pluskwa F, Couturier M, Kirsh JM, Fourgeaux B, Campinos L, Duval
G, (1984). Intoxication, salicylee, grave par vou transcutanee
chez un brule. La Presse Med, 13:1391.
Prescott LF, Balai-Mood M, Critchley JAJH, Johnstone AF, Proudfoot
AT, (1982). Diuresis or urinary alkalinisation for salicylate
poisoning. Br Med J, 285:1383-6.
Proudfoot AT, Brown SS, (1969). Acidaemia and salicylate poisoning
in adults. Br Med J, 2:547-50.
Proudfoot AT, (1983). Toxicity of salicylates. Am J Med, 14:99-
103.
Ralston ME, Pearigan PD, Ponaman ML, Erickson LC, (1995).
Transient myocardial dysfunction in a child with salicylate
toxicity. J Emerg Med, 13: 657-9.
Reynolds JEF (ed) (1996). Salicylic acid in: Martindale The Extra
Pharmacopoeia, 31st Edition. The Royal Pharmaceutical Society,
London, pp 1093.
Reye RDK, Morgan G, Baral J, (1963). Encephalopathy and fatty
degeneration of the viscera. A disease entity in childhood.
Lancet, 11:749-53.
Robins JB, Turnbull JA, Robertson C, (1985). Gastric perforation
after acute aspirin overdose. Hum Toxicol, 4:527-8.
Rupp DJ, Seaton RD, Wiegmann TB, (1983). Acute polyuric renal
failure after aspirin intoxication. Arch Int Med, 143:1237-8.
Sandford-Smith JH, (1974). Transient myopia occurred in a patient
following the ingestion of 2.7g of aspirin. Br J Opthal,
58:698.
Smith MJH, Dawkins PD, (1971). Salicylate and enzymes. J Pharm
Pharmacol, 23:729.
Springer DJ, Groll A, (1980). Poisoning with enteric coated
acetylsalicylic acid complicating gastric acid outlet
obstruction. Can Assoc J, 122:1032-3.
Sullivan-Bolyai JZ, Corey L, (1981). Epidemiology of Reye
syndrome. Epidemiologic Rev, 3:1-26.
Taylor JR, Halprin KM, (1975). Percutaneous absorption of
salicylic acid. Arch Dermatol, 111:740-3.
Temple AR, (1978). Pathophysiology of aspirin overdosage toxicity,
with implications for management. Pediatrics, 62
(suppl):873-6.
Temple AR, (1981). Acute and chronic effects of aspirin toxicity
and their treatment. Arch Intern Med, 141:364-9.
Temple AR, George DJ, Done AK, Thompson JA, (1976). Salicylate
poisoning complicated by fluid retention. Clin Toxicol,
9:61-8.
Tenney SM, Miller RM, (1955). The respiratory and circulatory
actions of salicylate. Am J Med, 19:495-508.
Thurston JH, Pollock PG, Warren SK, Jones EM, (1970). Reduced
brain glucose with normal plasma glucose in salicylate poisoning.
J Clin Invest, 49:2139.
Todd PJ, Sills JA, Harris F, Cowen JM, (1981). Problems with
overdose of sustained-release aspirin. Lancet, 1:777.
Vale JA, Buckley GM, Meredith TJ, (1985). Algorithm for modified
alkaline diuresis in salicylate-induced poisoning. Br Med J,
290:155.
Walters JS, Woodring JH, Stelling CB, Rosenbaum HD, (1983).
Salicylate-induced pulmonary edema. Radiology, 146:289-93.
Waltner JG, (1955). The effect of salicylates on the inner ear.
Ann Otol Rhino Lar, 64:617-22.
Winchester JF, Gelfland MC, Knepshield JH, Schreiner GE, (1977).
Dialysis and hemoperfusion of poisons and drugs. Transactions of
the American society of Artificial and internal organs,
23:762-842.
Windholz M, (1983). Salicylic acid. The Merck Index, Merck & Co
Inc. Rahway, N.J., USA. 10th edition, 8190.
Winchester JF, Gelfland MC, Helliwell M, Vale JA, Goulding R,
Schreiner GE, (1981). Extracorporeal treatment of salicylate or
acetaminophen poisoning.Is there a role? Arch Intern Med,
141:370-4.
Winters RW, White JS, Ordway NK, (1959). Disturbances of acid-base
equilibrium in salicylate intoxication. Pediatrics, 23:260-85.
Wofe JD, Metger AL, Goldstein RC, (1974). Aspirin hepatitis. Ann
Intern Med, 80:74-6.
Young RSK, Torreti D, Williams RH et al, (1984). Reye's syndrome
associated with long-term aspirin overdose. Ann Emerg Med, 16:434.
14. AUTHORS
Authors: Mahdi Balali-Mood & Kia Balali-Mood
Poisons Control Centre
Imam Reza Hospital
Faculty of Medicine
Mashhad University of Medical Sciences
Mashhad 91735
Islamic Republic of Iran
Tel: +98-51-889301/98973
Fax: +98-51-883714/93038
Date: August 1996
Reviewer: Bill Watson, Kansas City, USA
Date: 1996
Peer review: INTOX Meeting, London, UK, March, 1998
Editor: Dr M.Ruse (September, 1998)
 3.3 Physical properties
3.3.1 Colour
3.3 Physical properties
3.3.1 Colour

 3.3 Physical properties
3.3.1 Colour
3.3 Physical properties
3.3.1 Colour