

This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 68
First draft prepared by Mr Peter Watts, Toxicology Advice & Consulting Ltd, Sutton, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Tetrachloroethene.
(Concise international chemical assessment document ; 68)
Draft prepared by Peter Watts; edited by Marla Sheffer.
1. Tetrachloroethylene - adverse effects. 2. Tetrachloroethylene - toxicity.
3. Environmental exposure. 4. Risk assessment. I. Watts, Peter. II. Sheffer, Marla.
III. International Programme on Chemical Safety. IV. World Health Organization. V. Series.
ISBN 92 4 153068 5 (NLM Classification: QV 633)
ISBN 978 92 4 153068 2
ISSN 1020-6167
©World Health Organization 2006
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 3264; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and Swiss Agency for Environment, Forests and Landscape.
Technically and linguistically edited by Marla Sheffer, Ottawa, Canada, and printed by Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany
Concise International Chemical Assessment Documents (CICADs) are published by the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs have been developed from the Environmental Health Criteria documents (EHCs), more than 200 of which have been published since 1976 as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that:
|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e. a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD2 on tetrachloroethene was drafted by Toxicology Advice & Consulting Ltd based on four source documents. A report produced jointly by the Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards (de Raat, 2003) and the IARC evaluation of the carcinogenicity of tetrachloroethene (IARC, 1995) were used to draft most of the human health sections, and a USEPA (2003) discussion paper on neurotoxicity was used as the basis for sections on neurotoxicity. The environmental sections were drafted using the final draft EU Risk Assessment Report (Environment) (EC, 2001).3 Data identified as of 2001 (EC, 2001), 1995 (IARC, 1995), 2002 (USEPA, 2003), and 2002 (de Raat, 2003) were considered in the source documents. A comprehensive literature search of several online databases was conducted in May 2004 to identify any references published subsequent to those incorporated in the source documents. Information on the nature of the peer review and the availability of the source documents is presented in Appendix 2. Information on the peer review of this CICAD is presented in Appendix 3. This CICAD was first discussed as an international assessment at a meeting of the Final Review Board, held in Hanoi, Viet Nam, on 27 September – 1 October 2004. Participants of the Final Review Board meeting are listed in Appendix 4. Due to conflicting views on interpretation of data on critical end-points, the draft CICAD was referred to a WHO Consultative Group, which met at the United Kingdom Centre for Ecology and Hydrology in Monkswood, Cambridgeshire, on 25–27 April 2005. Participants of the Consultative Group meeting are listed in Appendix 5. The CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Nagpur, India, on 31 October – 3 November 2005. Participants of the Final Review Board meeting are listed in Appendix 6. The International Chemical Safety Card (ICSC 0076) for tetrachloroethene, produced by IPCS (2000), has also been reproduced in this document.
Tetrachloroethene (CAS No.
The most recent figures for annual production of tetrachloroethene in the EU and the USA are 164 000 and 160 000 tonnes, respectively, and apply to 1994 (EU) and 1998 (USA). Production in the EU and USA has approximately halved over the last 10–20 years. The major uses of tetrachloroethene are in the dry cleaning of textiles and as a chemical intermediate. Tetrachloroethene is also used in metal degreasing. Tetrachloroethene is released to the atmosphere during use, and the major portion of atmospheric releases is attributed to evaporative losses during dry cleaning.
Tetrachloroethene volatilizes readily from soil and surface water and undergoes degradation in air to produce phosgene, trichloroacetyl chloride, hydrogen chloride, carbon monoxide, and carbon dioxide. Its half-life in air is approximately 3–5 months. In water, it is resistant to abiotic and aerobic degradation, but it is biodegraded under anaerobic conditions to yield trichloroethene, dichloroethene, vinyl chloride, ethane, and ethene. It does not bioaccumulate to any significant extent in aquatic organisms. Tetrachloroethene is detected in outdoor air, usually at concentrations below 1–2 µg/m3. In Dutch homes, the median indoor air concentration was 4 µg/m3, with maxima of about 50–200 µg/m3. Concentrations can be much higher in buildings where dry cleaning operations are carried out. In drinking-water, tetrachloroethene concentrations are generally below 1–10 µg/l. Higher concentrations can occur in groundwater near polluted sites. Mean intake from food, drinking-water, and air is approximately 0.5–3 µg/kg body weight per day.
Tetrachloroethene is well absorbed by mammals following inhalation or oral exposure and is subsequently distributed mainly to adipose tissue, with smaller amounts found in the liver, brain, kidneys, and lungs. Dermal absorption may also occur. Humans and laboratory animals excrete most of the absorbed tetrachloroethene unchanged in expired air, with minor amounts excreted as urinary metabolites. Metabolism is more extensive in mice than in rats and humans. The major metabolite is trichloroacetic acid; minor metabolites include oxalic acid, dichloroacetic acid, ethylene glycol, trichloroacetyl amide, thioethers, and carbon dioxide. Oxidative metabolism (mediated by cytochrome P450) in the liver is the major pathway, leading to the formation of trichloroacetic acid. At higher exposures, this pathway becomes saturated, and a second pathway involving glutathione conjugation increases in significance. This pathway, which is more important in rats than in humans and mice, leads to the formation of S-(1,2,2-trichlorovinyl)-L-cysteine, which can be cleaved in the kidneys to yield cytotoxic and genotoxic metabolites. Reactive intermediates of both pathways can bind covalently to proteins and nucleic acids.
Neat tetrachloroethene was irritating to human and rabbit skin. The liquid caused only minimal irritation to the rabbit eye, and the vapour was irritating to the eyes and respiratory tract of exposed volunteers. In laboratory animals, acute inhalation and oral toxicities were low. In humans, acute accidental inhalation of unmeasured (but presumably high) concentrations of tetrachloroethene has induced CNS depression, dizziness, fatigue, loss of coordination, coma, reversible liver damage, and some deaths. Similar effects were observed in humans following acute ingestion at doses of about 70–90 mg/kg body weight.
Most of the available occupational studies involve people repeatedly exposed, predominantly to tetrachloroethene, but possibly also to other solvents, in the dry cleaning and electronics industries and during metal degreasing operations. Although information on the levels of individual exposures is lacking, average measured tetrachloroethene exposures were typically about 100 mg/m3. In these studies, there was some evidence of toxicity to the CNS and kidney. The neurotoxicity studies showed a common theme of disrupted visual spatial function and CNS cognitive processing of visual information. Although all of the occupational studies of neurotoxicity have limitations, the most informative study found deficits in behavioural tests at a mean exposure level of 83 mg/m3. In the most informative study on kidney effects, there were indications of injury to both tubular and glomerular regions of the kidney at a mean exposure level of 100 mg/m3. There was no clear evidence of liver toxicity in these studies.
On repeated exposure, liver, kidney, and the CNS are the major target organs in laboratory animals. Mice were more sensitive than rats to the liver toxicity of tetrachloroethene.
There is limited evidence that tetrachloroethene is a carcinogen in humans exposed occupationally. Available studies generally lack good information on exposure levels and on exposure to other solvents. The widespread use of tetrachloroethene in the dry cleaning industry did not begin until the 1960s; excess tumour incidence, if occupationally related, could be attributable in part to exposure conditions prior to the widespread use of tetrachloroethene. Where cancer mortality was examined among workers in dry cleaning establishments, elevated mortality was seen in relation to cancer of the oesophagus and cervix. There was some suggestion of an excess in kidney cancers. Three studies reported an excess of non-Hodgkin’s lymphoma, which was not statistically significant; in addition, there may have been multiple solvent exposure. General population and case–control studies gave no convincing evidence for any increased risk of total or specific cancers arising from exposure to tetrachloroethene in drinking-water.
Tetrachloroethene was clearly carcinogenic in laboratory animals. On repeated inhalation, it induced leukaemia in both sexes of F344 rats (in two studies) and malignant kidney tumours in male F344 rats in one study (of two). In inhalation studies, it induced malignant liver tumours in both sexes of B6C3F1 and BDF1 mice and benign Harderian gland tumours in BDF1 male mice. On repeated oral administration, tetrachloroethene induced malignant liver tumours in both sexes of B6C3F1 mice.
Tetrachloroethene has been fairly extensively examined for genotoxicity potential. In vivo, it did not cause chromosomal aberrations in the bone marrow of rats or mice or micronuclei in mouse bone marrow. Sperm abnormalities were not induced in rats or hamsters, but a low-purity grade increased the percentage of abnormal sperm in mice. Tetrachloroethene did not induce dominant lethal mutations in rats. In other assays, it did not damage DNA in the kidneys of rats or lungs of mice; however, transient DNA damage was reported in the liver and kidney of exposed mice. Tetrachloroethene did not induce sex-linked recessive lethal mutations in fruit flies. When tested in vitro, tetrachloroethene did not cause mutations in Ames bacterial assays, chromosome damage, or sister chromatid exchanges in hamster cells, mutations in mouse cells, or UDS in human, rat, or mouse cells. Although a few assays have produced positive results, a weight-of-evidence approach suggests that tetrachloroethene itself does not have significant in vivo genotoxic potential. Mammalian metabolites of tetrachloroethene have induced mutations in Ames assays.
Currently, no mechanisms have been proposed for the leukaemias and benign Harderian gland tumours induced in rats and male mice, respectively. Non-genotoxic mechanisms have been recognized for the formation of kidney tumours in male rats and liver tumours in mice for some chemicals. The available data on mode of action for tetrachloroethene are limited, and the dose–response data related to these recognized mechanisms are not consistent with the dose–response relationships for cancer induction by tetrachloroethene. In the absence of suitable supporting evidence to the contrary, it is concluded that the cancers produced by tetrachloroethene in rodents are of potential relevance to humans.
Some epidemiological studies of women occupationally exposed to tetrachloroethene have shown increased risks of spontaneous abortion; there is insufficient information to draw conclusions in respect of other adverse reproductive outcomes, such as decreased fertility and fetal malformations. Reproductive and developmental studies in rats, mice, and rabbits suggest that tetrachloroethene is fetotoxic at doses that also cause maternal toxicity. Several studies exposing pregnant rats and rabbits found no evidence of structural malformations in the offspring, but one such study in mice reported unspecified soft tissue malformations in the young (at a maternally toxic dose). Limited evidence is suggestive of slight changes in neurochemistry and CNS function in young rats and mice following exposure of the dams during pregnancy.
In occupationally exposed cohorts, the most consistent adverse finding was neurotoxicity; therefore, the most informative study on neurotoxic effects in exposed workers was used to derive a TC. The mean exposure level (83 mg/m3) was taken as a LOAEC. This was converted to an equivalent concentration for continuous exposure (20 mg/m3), and two uncertainty factors of 10 were applied (one to account for interindividual differences, the other because the selected concentration was a LOAEC rather than a NOAEC), to derive a TC of 0.2 mg/m3. For comparative purposes, a similar approach was used for studies reporting nephrotoxicity. The most informative study yielded a mean occupational exposure of 100 mg/m3, which generated a TC of 0.24 mg/m3, a value in good agreement with the TC protective against neurotoxic effects. Available data indicate that liver toxicity would occur only at exposures higher than those that affect the CNS and kidney. A TC for spontaneous abortions was not derived. However, the TC of 0.2 mg/m3 is more than 3 orders of magnitude lower than the exposure concentration that induced mild adverse effects in laboratory animals, and so it was considered to be protective against reproductive toxicity in humans.
The available information on oral exposure was inadequate for derivation of a TDI by the oral route. However, as tetrachloroethene is well absorbed after inhalation or ingestion and there is little evidence of first-pass metabolism, a PBPK model was used to derive a TDI. The model predicted that tetrachloroethene consumed in drinking-water at a dose level of 0.047 mg/kg body weight per day would yield an AUC in plasma similar to that from continuous exposure to tetrachloroethene at 0.2 mg/m3 in inhaled air. This oral figure was rounded to give a TDI of 50 µg/kg body weight.
Tetrachloroethene has induced several types of tumour in rats and mice. Currently, there is no convincing evidence that these tumours arise via modes of action that operate only in rodents, and hence their relevance to humans cannot be dismissed. Therefore, a BMC approach was used, and a BMC and its lower confidence limit (BMCL) were calculated for each animal tumour. Of the tumours observed in experimental animals, hepatocellular adenomas and carcinomas in male mice yield highest predicted risks. The TC derived above, 0.2 mg/m3, corresponds to a cumulative lifetime risk of 0.4 × 10−3 when a linear extrapolation is applied to the BMC10 as the point of departure.
Concentrations of tetrachloroethene in the atmosphere or indoor air in Europe and the USA are generally more than an order of magnitude lower than the TC, even in urban areas. In the vicinity of point sources, the observed concentrations also fall below the TC. In buildings where tetrachloroethene is used (notably dry cleaning facilities), concentrations clearly exceeding the TC have been measured. Drinking-water concentrations of tetrachloroethene in different countries in Europe are usually below 10 µg/l, leading to tetrachloroethene doses of below about 0.3 µg/kg body weight per day. This can be compared with the TDI of 50 µg/kg body weight. It should be noted that groundwater concentrations at polluted sites may exceed 1 mg/l.
For terrestrial organisms, the lowest PNEC was 10 µg/kg wet weight in soil. As this was higher than PECs, which ranged from 0.06 to 3.9 µg/kg, it was concluded that tetrachloroethene is unlikely to pose any significant risks to terrestrial organisms. For aquatic organisms, the lowest PNEC was 51 µg/l. PECs ranged from 0.002 to 9.1 µg/l, and so current tetrachloroethene exposures were considered to be a low risk to aquatic organisms. A similar conclusion was reached for sediment-dwelling organisms, where the lowest PNEC was calculated to be 277 mg/kg sediment, compared with a highest calculated PEC of 57 µg/kg. Tetrachlorethene was also considered unlikely to be a risk to microorganisms in sewage treatment processes, with lowest PNEC and highest PEC values of 11.2 mg/l and 16–23 µg/l, respectively. An additional risk assessment was carried out for plants exposed to atmospheric tetrachloroethene. The lowest PNEC was 8.2 µg/m3 air. PECs were generally below this value, but a higher value (36 µg/m3) was measured near a site where tetrachloroethene was produced and processed, leading to the conclusion that there is a need to limit risks of harm to plants from air emissions at such sites.
Tetrachloroethene (CAS No.
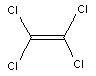
Figure 1: Chemical structure of tetrachloroethene.
At room temperature, tetrachloroethene is a clear, colourless liquid with an etheric odour. Selected physical/chemical properties are presented in Table 1.
Table 1: Physical and chemical properties of tetrachloroethene.
|
Property |
Valuea |
|
Boiling point (°C) at 101.3 kPa |
121.2 |
|
Vapour pressure (kPa) at 20 °C |
1.9 |
|
Water solubility (mg/l) at 25 °C |
150 |
|
Density (g/cm3) at 20 °C |
1.62 |
|
Henry’s law constant (Pa·m3/mol) at 20 °C |
2114 |
|
Log Kow |
2.53 |
a Data listed in source documents (EC, 2001; de Raat, 2003).
Additional properties are given in the International Chemical Safety Card (ICSC 0076) reproduced in this document.
The conversion factors4 for tetrachloroethene in air (at 20 °C and 101.3 kPa) are as follows:
1 ppm = 6.89 mg/m3
1 mg/m3 = 0.145 ppm
To prevent the slow decomposition of tetrachloroethene to trichloroacetyl chloride and phosgene by oxidation, low concentrations of stabilizers (including amines, epoxides, and phenols) are added. Suppliers have reported these as 2,3-epoxypropyl isopropylether (3 g/kg), 2,6-bis(1,1-dimethylethyl)-4-methylphenol (<0.1 g/kg), 2,4-di-tert-butylphenol (<0.05 g/kg), 4-methylmorpholine (<0.1 g/kg), diisopropylamine (<0.5 g/kg), tert-butyl glycidyl ether (<5 g/kg), and tert-amylphenol (<20 mg/kg) (EC, 2001).
Reported impurities include 1,1,1-trichloroethane (<100 mg/kg), carbon tetrachloride (<50 mg/kg), dichloromethane (<2 mg/kg), other chlorinated solvents (<50 mg/kg), trichloroethene (<50 mg/kg), and water (<50 mg/kg) (EC, 2001).
The source documents present several methods for determining tetrachloroethene concentrations in air but give no methods for other environmental media. The compound is always collected by adsorption. Sorbents used are activated charcoal or Tenax, the former being desorbed by elution with organic solvents (e.g. carbon disulfide), the latter by elution of the heated sorbent with an inert gas, followed by condensation. The desorbed material is fractionated by GC. Detection and quantification are based on FID or MS, while the identification of the compound is based on retention time and mass spectra. Several methods are outlined below. The most sensitive are NEN method 2948/2965 and IARC method 12.
ISO method 9486: (E) — A known volume of air is passed through a glass or metal tube packed with activated charcoal. The organic vapours are adsorbed onto the charcoal. The collected vapours are desorbed using a suitable solvent and analysed with a GC equipped with an FID or another suitable detector. This method can be used for the measurement of concentrations of airborne vapours of tetrachloroethene between approximately 1 and 1000 mg/m3 (about 0.2–200 ml/m3) when 10 litres of air are sampled. Organic components that have the same or nearly the same retention time as tetrachloroethene in the GC analysis will interfere. Proper selection of GC columns and programme conditions will minimize interference (ISO, 1991).
NEN method 2947/2964 — Air is drawn through a tube with two sections, both containing activated coconut charcoal to adsorb gaseous tetrachloroethene. The compound is subsequently desorbed with carbon disulfide (containing an internal standard) and is determined by GC, using FID. The method has been validated over a range of 2.5–1600 mg/m3 and has a detection limit of 238 µg/m3 (Dutch Normalisation Institute, 1999a, 2000a).
NEN method 2948/2965 — The sample is collected by adsorption on Tenax (200 mg) and analysed by thermal desorption of volatile components into a GC, using FID. The method has been validated over the range of 0.02–400 mg/m3 and has a detection limit of 0.1 µg/m3 (Dutch Normalisation Institute, 1999b, 2000b).
NEN method 2950 — The sample is collected on an indicator tube and analysed by reading the colour change. The method has been validated over a range of 140–1150 mg/m3. The coefficient of variation was 25% (Dutch Normalisation Institute, 1999c).
NIOSH methods 1003 and 3704 — Method 3704 is specific for tetrachloroethene in exhaled breath and air. Sampling is by gas bag or direct injection, with measurement using a portable GC with a PID. The method has an LOD of about 0.07 mg/m3 and is applicable over the 0.7–700 mg/m3 range (NIOSH, 1998). Method 1003 can be used for various halogenated hydrocarbons. The sampler is a solid sorbent tube containing coconut shell charcoal, and GC with FID is used to measure tetrachloroethene. The working range is 60–13 000 mg/m3, the LOD is 14 mg/m3, and the LOQ is 49 mg/m3 (NIOSH, 2003).
IARC method 5 — Air is drawn through a tube with two sections, both containing activated coconut charcoal to adsorb the gaseous compound. The compound is subsequently desorbed with carbon disulfide (containing an internal standard), followed by GC, using FID. A calibration curve is employed, and a correction curve is applied for desorption efficiency. This method has been validated over a range of 136–4060 mg/m3 using a 3-litre sample. The breakthrough volume is 21 litres at 2750 mg/m3. The detection limit depends on the analyte and lies normally in the useful range (MacKenzie Peers, 1985).
IARC method 12 — Air is drawn through a cartridge containing 1–2 g of Tenax. The cartridge is placed in a heated chamber and purged with an inert gas, which transfers the volatile compound from the cartridge onto a cold trap and subsequently onto a high-resolution (capillary) GC column, which is held at low temperature (e.g. −70 °C). The column temperature is then increased, and the component eluting from the column is identified and quantified by MS. Component identification is normally accomplished by a library search routine, using GC retention times and mass spectral characteristics. The limit of detection is generally in the order of 0.1–1.0 µg/m3 (Riggin, 1985).
BIA method 8690 — The Berufsgenossenschaftliches Institut für Arbeitssicherheit has published a method using Dräger active coal tubes, type B, and GC using FID. The limit of detection is 1.2 mg/m3 for an air volume of 40 litres (Schutz & Coenen, 1989).
The USEPA has also published useful methods for quantifying tetrachloroethene in air, specifically TO-1, TO-3, and TO-14A (USEPA, 1999). OSHA has published a validated method for determination in workplace atmospheres (OSHA, 1999).
For biological monitoring purposes, the concentrations of tetrachloroethene are determined in expired air or blood. Concentrations in expired air can be determined in the same manner as those in ambient air. Tetrachloroethene is removed from blood or tissues by evaporation or by extraction with organic solvents. Evaporated tetrachloroethene can be concentrated with Tenax before analysis with GC/MS or GC with ECD; analysis can also be performed without prior concentration (headspace analysis). The solvent extracts are also analysed by GC/MS or GC with ECD. Several methods are available, including the following.
IARC method 24 — This method can be used for the determination of tetrachloroethene in expired air. The breath sample is dried over calcium sulfate and led through a Tenax GC cartridge. The adsorbed tetrachloroethene is subsequently thermally desorbed and led into a GC/MS. The detection limit of the method is 0.33 µg/m3, and the linear range for the analysis depends mainly on the adsorption breakthrough volume and on the sensitivity of the MS (Pellizari et al., 1985b).
NIOSH method 3704 — Tetrachloroethene in exhaled air can be measured by this method. Sampling is by gas bag or direct injection, with measurement using a portable GC with a PID. The method has an LOD of about 0.07 mg/m3 and is applicable over the 0.7–700 mg/m3 range (NIOSH, 1998).
IARC method 25 — This method is suitable for the determination of tetrachloroethene in blood and tissues. The volatile tetrachloroethene is recovered from a blood sample by warming the sample and passing an inert gas over it. Tissues are first macerated in water, then treated in the same manner as blood. Tetrachloroethene is trapped on a Tenax GC cartridge, then recovered by thermal desorption and analysed by GC/MS. For a 10-ml blood sample, the limit of detection is about 3 ng/ml. Detection limits of about 6 ng/g are typical for 5-g tissue samples. Upper limits are approximately 100 times the lower limits (Pellizari et al., 1985a).
IARC method 27 — Tetrachloroethene concentrations in blood can be determined with this method. The specimen is extracted with n-hexane, and the concentration of tetrachloroethene in the organic phase is determined by GC, using ECD. The limit of detection is 5 µg/l (Pekari & Aitio, 1985).
DFG method 1 — For the determination of tetrachloroethene in blood, an organic matrix is prepared from the sample. The volatile compound is removed from the matrix by increasing the temperature. The headspace of the matrix is then analysed with GC, using ECD. The detection limit is 1.2 µg/l (Angerer & Schaller, 1991).
According to source documents (IARC, 1995; EC, 2001), natural production of tetrachloroethene by temperate, subtropical, and tropical algae and by one red microalga has been reported (Abrahamsson et al., 1994).
Atmospheric releases of tetrachloroethene can occur due to evaporative losses during dry cleaning. Other atmospheric emissions may result during manufacture, from use in metal degreasing, in production of fluorocarbons and other chemicals, in the textile industry, and in miscellaneous solvent-associated applications (ATSDR, 1997). Tetrachloroethene may also be disposed of to land and surface water (TRI, 2004).
Mainly as a result of industrial spillage, tetrachloroethene has been found in air, soil, surface water, seawater, sediments, drinking-water, aquatic organisms, and terrestrial organisms.
Annual production of tetrachloroethene in the USA was estimated to be about 350 000 tonnes in 1981 but had fallen to 169 000 tonnes by the mid-1990s (IARC, 1995). Demand (domestic production plus imports minus exports) for tetrachloroethene in the USA was 126 000 tonnes in 1996 and 143 000 tonnes in 1999 (NTP, 2002). In 1998, the USA produced 160 000 tonnes (of which 18 100 tonnes were exported) and imported 13 600 tonnes, giving a total demand of 155 500 tonnes (HSIA, 1999). The projected demand for 2003 in the USA was 153 000 tonnes (NTP, 2002). In 2004, total demand in the USA was estimated to be about 161 000 tonnes, of which about 16 300 tonnes were imported. An additional 18 600 tonnes were exported (HSIA, 2005). In the EU, total production capacity is 100 000–200 000 tonnes per annum, with an actual reported value in 1994 of 164 000 tonnes (European Chlorinated Solvent Association, personal communication, 1995, cited in EC, 2001). A graph on the Euro Chlor web site indicates that consumption fell from about 230 000 tonnes in 1990 to about 80 000 tonnes in 2004 (Euro Chlor, 2005). In 1994, 56 000 tonnes were exported (European Chlorinated Solvent Association, personal communication, 1996, cited in EC, 2001). For 1979, annual production was estimated to be 50 000–100 000 tonnes in Eastern Europe and about 55 000 tonnes in Japan. Germany, France, Italy, and the United Kingdom are major European producing countries, with Austria, Scandinavia, Spain, Switzerland, and Benelux producing lower amounts (IARC, 1995; EC, 2001; de Raat, 2003).
Currently, tetrachloroethene is produced mainly by oxychlorination, chlorination, and/or dehydrochlorination reactions of hydrocarbons or chlorinated hydrocarbons, most commonly the chlorination of propylene and the oxychlorination of 1,2-dichloroethane (Brooke et al., 1993).
Tetrachloroethene is used mainly as a solvent for dry cleaning and as a chemical intermediate, with additional use for vapour degreasing in metal cleaning. It is also used for processing and finishing in the textile industry, as an extraction solvent, as an anthelmintic, as a heat exchange fluid, in grain fumigation, and in the manufacture of fluorocarbons (EC, 2001; de Raat, 2003). In 1994, use in dry cleaning accounted for 38% of EU production volume, and tetrachloroethene accounted for about 90% of the total solvent used by the European dry cleaning industry (EC, 2001). In the USA in 1998–2000, 50% of tetrachloroethene was used for chemical intermediates, primarily as a basic intermediate in the production of HFC-134a, a popular alternative to CFC refrigerants. It is also used for the synthesis of HCFC-123 and HCFC-124, as well as HFC-125. Dry cleaning accounted for 21–25% of use in the USA, and automotive aerosols (brake cleaners) and metal degreasing each accounted for a further 10%. Tetrachloroethene is also used as an insulating fluid and cooling gas in electrical transformers, paint removers, printing inks, adhesive formulations, paper coatings, and aerosol formulations such as water repellents (HSIA, 1999; NTP, 2001; HSDB, 2003).
In 1990, about 53% of world demand for tetrachloroethene was for dry cleaning (cleaning fluid used by about 75% of all dry cleaners). Approximately 23% was used as a chemical intermediate, principally for the production of Freons, 13% for metal cleaning, and 11% for other uses (Linak et al., 1992; IARC, 1995).
It has been estimated that 80–85% of the tetrachloroethene used annually in the USA is released into the atmosphere (ATSDR, 1997), although the percentage is likely to be lower in the USA in present times, due to improved technology and regulatory restrictions. A major portion of the atmospheric releases is attributed to evaporative losses during dry cleaning. Other atmospheric emissions result from metal degreasing, production of fluorocarbons and other chemicals, use in the textile industry, and miscellaneous solvent-associated applications (ATSDR, 1997). Tetrachloroethene has to a large degree been used in small, geographically scattered, and possibly poorly controlled workplace settings and consequently has, in the USA, become a common contaminant at Superfund waste sites and has been a surface water and groundwater pollutant (NTP, 2001; Aschengrau et al., 2003). In the USA in 2002, the amount of tetrachloroethene estimated to have been released by manufacturing facilities was about 1300 tonnes. This was mainly on site as point source (625 tonnes) or fugitive (400 tonnes) air emissions. Land disposal accounted for about 45 tonnes. Disposal to surface waters was estimated to be about 0.36 tonnes (TRI, 2004).
The authors of the draft EU Risk Assessment Report (the final version of which was released after finalization of this CICAD) carried out a detailed consideration of environmental releases of tetrachloroethene resulting from production, use as a chemical intermediate, use in dry cleaning, use in metal cleaning operations, other uses, and disposal. Three separate scenarios were considered. In a local scenario, emissions to water and air were calculated for point sources and concentrations estimated close to the sources. This local assessment included production, use as an intermediate, dry cleaning, and metal cleaning. In the regional scenario, the EU Technical Guidance Document (ECB, 2003) was followed, with the assumption that the regional environment contained one large-scale production plant and one site using tetrachloroethene as an intermediate, together with 10% of the total EU activity for other uses and 10% of the estimated EU fugitive emissions due to landfill site disposal. The continental region scenario included the remaining releases from production sites, uses, and fugitive emissions from landfill sites. The amounts of tetrachloroethene released to the environment from various sources were summarized in a table, reproduced here as Table 2. These figures were used to calculate PECs (see section 11.2) (EC, 2001).
Table 2: Summary of environmental releases of tetrachloroethene.a
|
Scenario |
Environmental releases (kg/day) |
|||||
|
Continental |
Regional |
Local |
||||
|
Air |
Water |
Air |
Water |
Air |
Water |
|
|
Production and use as a chemical intermediateb |
35 |
0.66 |
602 |
0.67 |
733 |
0.81 |
|
Dry cleaning |
110 948 |
51 |
28 244 |
5.6 |
15.5 |
0.003 |
|
Metal cleaning |
31 068 |
346 |
3 452 |
38 |
42 |
0.48 |
|
Landfill |
7 397 |
822 |
||||
|
Total |
149 448 |
398 |
33 120 |
44 |
||
a From EC (2001).
b Regional and continental releases adjusted to 365 days per year.
Tetrachloroethene is distributed between environmental compartments by volatilization, precipitation, and adsorption. It is predominantly released and transported to the atmosphere. Based upon its environmental chemistry, computer models predict that the atmosphere will be the major sink for tetrachloroethene (EC, 2001).
Tests indicate that tetrachloroethene can be adsorbed onto soils of varying organic carbon contents, but amounts adsorbed are negligible, and tetrachloroethene is relatively mobile in groundwater in the absence of any removal processes. Tetrachloroethene can leach rapidly through sandy soil (0.0087% organic matter) into groundwater. In a bank filtration system, tetrachloroethene was rapidly transported to groundwater. It was estimated that only 0.01% was adsorbed to particulate matter (Zoeteman et al., 1980; Wilson et al., 1981; Schwarzenbach et al., 1983). Sorption of non-ionic compounds such as tetrachloroethene depends on the organic carbon content of the soils and sediments and on the nature of the organic matter. When measuring the adsorption coefficient of tetrachloroethene for different soil types, adsorption was highest with anthracite (organic carbon 80.1%) and lowest with lignite (organic carbon 18.5%) (Grathwohl, 1990).
The equilibrium constants were measured for four types of granular media: sandy loam soil, organic top soil, peat moss, and granular activated carbon. Adsorption increased as the carbon content increased, being least with the sandy loam soil (1% organic carbon) and highest with the granular activated carbon (74% organic carbon) (Biswas et al., 1992). Others found similar results upon measuring the adsorption and desorption of tetrachloroethene on a range of soils and clays. Sorption was found to be rapid in all cases and was highest in soils with a high organic carbon content (Doust & Huang, 1992).
Soil organic carbon/water adsorption partition coefficients (Koc) of 6.5 (over 24 h) and 7.3 (over 72 h) have been reported for tetrachloroethene in fine sand loam soil with aqueous solutions of 4.18–68.2 µg/l (Pignatello, 1990). Bentonite clay adsorbed 22% of tetrachloroethene from a solution containing 1 mg/l after 30 min. No further sorption was noted after this time. Peat moss adsorbed 40% of tetrachloroethene from a 1 mg/l solution in 10 min (Dilling et al., 1975). Reported log Koc values at 20 şC vary from 1.6 to 2.7 (Kenaga, 1980; Mabey et al., 1982; Giger et al., 1983; Friesel et al., 1984; Seip et al., 1986; Abdul et al., 1987; Lee et al., 1989; Zytner et al., 1989); a log Koc of 2.40 (251 l/kg) was taken as representative for tetrachloroethene in the EU risk assessment (EC, 2001). Using this log Koc value, the following partition coefficients were calculated for tetrachloroethene using the EU Technical Guidance Document (ECB, 2003) method:
|
Partition coefficient solid–water in suspended matter |
Kpsusp |
25.1 l/kg |
|
Partition coefficient solid–water in sediment |
Kpsed |
12.6 l/kg |
|
Partition coefficient solid–water in soil |
Kpsoil |
5.0 l/kg |
|
Soil–water partitioning coefficient |
Ksoil-water |
7.91 m3/m3 |
|
Suspended matter–water partitioning coefficient |
Ksusp-water |
7.18 m3/m3 |
|
Sediment–water partitioning coefficient |
Ksed-water |
7.08 m3/m3 |
Tetrachloroethene released to surface waters rapidly volatilizes to the atmosphere, at a rate dependent upon the degree of mixing in the water system. Removal is more rapid from water systems with a high degree of mixing, which depends upon water movements and wind speed. The evaporation half-life of tetrachloroethene from field measurements and theoretical considerations is in the order of 1–10 days in rivers and 10–30 days in lakes and ponds (ECETOC, 1999; EC, 2001). Using representative oxygen aeration rates for various bodies of water, half-lives for the evaporation of tetrachloroethene have been calculated as 5–12 days (pond), 3 h – 7 days (river), and 3.6–14 days (lake) (Lyman et al., 1981). In a study in which the evaporation of tetrachloroethene from a 1 mg/l solution was measured at ambient temperatures, the half-life was between 24 and 28 min with constant stirring at 200 rpm and was about 90 min with stirring for 15 s every 5 min (Dilling et al., 1975). In later experiments using the same technique, the measured half-life for evaporation was 20–27 min (Dilling, 1977).
Volatilization from water was reported as 0.18 µg/cm2 per hour (Wilson et al., 1981). The half-life for evaporation from water was reported as 3.2 min under stirring conditions (Chiou et al., 1980). Volatilization of tetrachloroethene was measured in a model mesocosm (a tank containing 13 m3 of seawater plus associated planktonic and microbial communities) that was mixed for 2 h, 4 times a day. The measured volatilization half-lives were 11 days in winter, 25 days in spring, and 14 days in summer (Wakeham et al., 1983).
Volatilization of tetrachloroethene from dry soil is likely to be rapid due to its high vapour pressure and low adsorption to soil. Volatilization from a sandy soil was reported as 0.103 µg/cm2 per hour (Wilson et al., 1981).
Tetrachloroethene has been detected in rainwater and has the potential to dissolve in atmospheric water droplets and be deposited by rainout. Trichloroacetic acid formed by the photodegradation of tetrachloroethene may be rained out, together with the hydrogen chloride formed. Trichloroacetic acid has been found in rainwater samples, soil samples, and spruce needles (EC, 2001).
A model (FUGMOD [OECD workshop] Mackay Level I) has been used to calculate the distribution of tetrachloroethene in the environment as follows: air (99.69%), water (0.23%), soil (0.07%), sediment (<0.01%), and biota (<0.01%). Using another model (FUGMOD [OECD workshop] Mackay Level III), the distribution of tetrachloroethene in the environment was calculated as follows: air (76.39%), water (23.32%), soil (0.06%), and sediment (0.23%). A release rate of 1000 kg/h was used in the Level III model. It was assumed that 90% of releases were to air and 10% to water (EC, 2001). The fate of tetrachloroethene in a wastewater treatment plant, as estimated with EUSES,5 is 91.2% to air, 6.54% to water, and 2.2% to sludge, with zero degradation (EC, 2001).
BCFs of approximately 40–50 have been reported for aquatic species with tetrachloroethene. For bluegill (Lepomis macrochirus) exposed to 3.43 µg/l for 21 days at 16 şC, a BCF of 49 was reported (Barrows et al., 1980). For rainbow trout (Oncorhynchus mykiss), a BCF of 40 was reported (Neely et al., 1974). Based on these data, no significant bioaccumulation of tetrachloroethene in fish is expected. BCF figures of 312 and 101 have been calculated for the marine microalgae Heterosigma akashiwo (dinoflagellate) and Skeletonema costatum (diatom), respectively (Wang et al., 1996).
The octanol–water partition coefficient (log Kow) value for tetrachloroethene is below 3, indicating a low potential for bioaccumulation. The BCF for fish is calculated as 28.2 by the EU Technical Guidance Document method (the current version of which is ECB, 2003), and this value was used in the EU risk assessment (EC, 2001).
Tetrachloroethene will react in the atmosphere with a number of photochemically produced species. The major removal process for tetrachloroethene from the atmosphere results from reaction with hydroxyl radicals. Atkinson (1985) reviewed the available data for this process and recommended the following value for the second-order reaction rate constant:
kOH = 9.64 × 10−12 exp(−1209/T) cm3/s per molecule
This gives a value for kOH of 1.23 × 10−13 cm3/s per molecule at T = 277 K (or 4 °C).
The atmospheric lifetime of tetrachloroethene due to reaction with hydroxyl radicals has been estimated to be around 0.43 years (WMO, 1991). The EU Technical Guidance Document (ECB, 2003) recommends a value for the atmospheric hydroxyl radical concentration of 5 × 105 molecules/cm3. Using this concentration, a half-life of about 3.2 months (lifetime = 4.6 months) is estimated for the reaction. The estimated half-life is long enough to allow transport of tetrachloroethene from the point of emission (ECETOC, 1999; EC, 2001).
The reaction with atmospheric chlorine atoms is thought to be the next most important atmospheric degradation mechanism for tetrachloroethene. The second-order rate constant for the reaction has been quoted (Nicovich et al., 1996) as:
kCl = 4.0 × 10−11 cm3/s per molecule
The actual concentration of chlorine radicals in the atmosphere is unknown. Concentrations of about 1000 molecules/cm3 have been suggested, but a study by Sidebottom & Franklin (1996) suggests that the actual concentration in the troposphere is generally close to zero and is at most 500 molecules/cm3. The half-life for the reaction between chlorine radicals and tetrachloroethene is estimated (for [Cl·] = 1000 or 500 molecules/ cm3) as 6–12 months (lifetime 9–17 months) (ECETOC, 1999; EC, 2001).
The overall lifetime for these two major processes combined is thought to be around 3 months (ECETOC, 1999), although the exact contribution of the reaction with chlorine atoms to the overall degradation of tetrachloroethene is uncertain.
Class & Ballschmiter (1987) measured the concentration of tetrachloroethene in the atmosphere at sites remote from any anthropogenic sources in the Northern and Southern hemispheres. The lifetime in the Northern Hemisphere was estimated as 0.46 years (5–6 months) and in the Southern Hemisphere as 0.18 years (2 months). These lifetimes were calculated based on estimated release rates and measured levels.
The lifetime for removal of tetrachloroethene by gas-phase photolysis has been calculated to be about 3 years in the troposphere. Direct photolysis is therefore thought to be of negligible importance compared with other tropospheric removal mechanisms (ECETOC, 1999; EC, 2001). Reactions of tetrachloroethene with other atmospheric species, such as ozone (k <3 × 10−20 cm3/s per molecule [Atkinson & Carter, 1984]; k <2 × 10−23 cm3/s per molecule [Franklin, 1994]), oxygen atoms (k(O3P) = 1.6 × 10−14 cm3/s per molecule, k(O1D) <5 × 10−10 cm3/s per molecule [Franklin, 1994]), nitrate radicals (k <1 × 10−16 cm3/s per molecule [Atkinson et al., 1992]; k <5.2 × 10−17 cm3/s per molecule [Franklin, 1994]), and hydroperoxy radicals (k <1 × 10−17 cm3/s per molecule [Franklin, 1994]), have been reported but are thought to be insignificant atmospheric degradation processes (estimated atmospheric lifetimes for these processes range from >5 to >1500 years) for tetrachloroethene (Franklin, 1994).
For the EU risk assessment, the reaction rates with hydroxyl and with chlorine radicals were combined to give an overall half-life of 96 days. This was used in the EUSES calculations (entered as the reaction rate with hydroxyl radicals that would give a half-life of 96 days) (EC, 2001).
In laboratory studies on the photochemical degradation of tetrachloroethene in air, the main products identified were phosgene, trichloroacetyl chloride, hydrogen chloride, carbon dioxide, and carbon monoxide, but other products, such as carbon tetrachloride, dichloroacetyl chloride, and chloroform, have also been detected (ECETOC, 1999). When tetrachloroethene (30 mg/m3) was irradiated with air containing nitrogen dioxide in a smog chamber for 140 min, around 7% of the tetrachloroethene reacted, forming carbon monoxide (0.31 mg/m3), ozone (0.27 mg/m3), hydrogen chloride (0.64 mg/m3), and phosgene (0.49 mg/m3). Trichloroacetyl chloride was also identified (Gay et al., 1976). After 7 days’ illumination with simulated sunlight, the product yields were around 70–85% phosgene and 8% carbon tetrachloride. The concentration of carbon tetrachloride continued to increase long after the tetrachloroethene had disappeared, indicating that it was formed from an intermediate substance, probably trichloroacetyl chloride (Singh et al., 1975). Others analysed the products formed during the reaction of tetrachloroethene with hydroxyl radicals. During the 2-h experiment, only about 10% of the tetrachloroethene reacted. The main products formed were phosgene (product yield 47–52%) and trichloroacetyl chloride (product yield 39–41%). When the experiment was repeated in the presence of a chlorine atom scavenger (ethane), there was a marked decrease in the amount of trichloroacetyl chloride formed (product yield <15%), indicating that trichloroacetyl chloride is formed by chlorine atom attack on the tetrachloroethene (Tuazon et al., 1988).
In a detailed study of the products from hydroxyl radical–initiated reactions of tetrachloroethene, the compound (181 mg/m3) was irradiated for 12 h in air (20% relative humidity) using hydrogen peroxide as the source of hydroxyl radicals. All of the tetrachloroethene reacted, producing trichloroacetyl chloride (46 mg/m3; 23.2% yield), carbon dioxide (9.7 mg/m3; 20% yield), carbon monoxide (5.9 mg/m3; 18% yield), phosgene (7.8 mg/m3; 7% yield), and carbon tetrachloride (126 µg/m3; 0.07% yield). The shapes of the degradation curves were consistent with two competing reactions — i.e. addition of hydroxyl radical to form phosgene and addition of chlorine atoms to form trichloroacetyl chloride, the latter becoming more prevalent with time as the chlorine atom concentration in the reaction chamber increased. Further experiments showed that carbon tetrachloride was formed by photolysis of trichloroacetyl chloride, and not directly from tetrachloroethene. The conversion of trichloroacetyl chloride to carbon tetrachloride was estimated to be about 0.1%, based on 24-h illumination (Itoh et al., 1994).
It is clear that two competing reactions occur in closed laboratory studies. The main products formed of relevance to the environment are phosgene, trichloroacetyl chloride, and carbon tetrachloride. Phosgene is derived from hydroxyl radical addition to tetrachloroethene. Trichloroacetyl chloride is derived from chlorine atom addition to tetrachloroethene, and carbon tetrachloride is formed as a result of further degradation of trichloroacetyl chloride. The main reaction pathways for the chlorine atom addition are shown below (EC, 2001; based on Sidebottom & Franklin, 1996 and Itoh et al., 1994):
Chlorine atom addition:
Cl2C=CCl2 + Cl· --> CCl3CCl2·
CCl3CCl2· + O2 --> CCl3CCl2O2·
CCl3CCl2O2· + NO --> CCl3CCl2O·
CCl3CCl2O· (85%) --> CCl3COCl + Cl·
CCl3CCl2O· (15%) --> COCl2 + CCl3· (--> COCl2)
CCl3COCl + hv --> Cl· + CCl3CO· --> CO + CCl3· --> --> COCl2
CCl3COCl (0.1%) + hv --> CCl4 + CO
It has been suggested that trichloroacetyl chloride is a major atmospheric degradation product of tetrachloroethene. Trichloroacetyl chloride can hydrolyse to form trichloroacetic acid, which can be washed out of the atmosphere (Reimann et al., 1996). Chloroacetic acids are toxic to many plants, and some have been used as herbicides. In laboratory experiments, seemingly large yields of trichloroacetyl chloride and carbon tetrachloride are formed; in the environment, however, this behaviour is likely to be modified. In laboratory studies, chlorine atoms formed during hydroxyl radical–initiated degradation of tetrachloroethene can build up in the test system, and so the chlorine addition pathway can effectively compete with hydroxyl radical addition, resulting in high yields of trichloroacetyl chloride (and subsequently carbon tetrachloride). In the environment, however, there are many other chemical species (e.g. hydrocarbons) that are capable of scavenging the reactive chlorine atoms, and so the proportion of tetrachloroethene reacting via this pathway will be much diminished in the environment. This has been demonstrated in laboratory studies in which a chlorine atom scavenger was added (EC, 2001).
The lifetime of tetrachloroethene in the troposphere is such that the fraction of emitted tetrachloroethene that enters the stratosphere is low (about 1% of atmospheric emissions). In the stratosphere, tetrachloroethene will be degraded by reaction with hydroxyl radicals. It may also undergo photolysis (ECETOC, 1999).
Degradation of tetrachloroethene in water by hydrolysis is very slow, with reported half-lives in the order of years (ECETOC, 1999). Tetrachloroethene may be removed from aquatic systems by photochemical reactions involving free radicals or electronically excited molecular species. These reactions are likely to compete with volatilization only in still, sunlit waters, where volatilization is limited by the available surface area for evaporation (ECETOC, 1999). Reductive pathways involving transition metals or their organic complexes may be significant in the presence of soils or sediments (ECETOC, 1999). No further information on these processes has been found (EC, 2001).
No degradation of tetrachloroethene was detected in a 190-h incubation with a mixed culture of methane-utilizing bacteria (Fogel et al., 1986) or when using a culture of the ammonium-oxidizing bacterium Nitrosomonas europaea (Vannelli et al., 1990). No degradation was observed in a modified shake flask, a closed bottle biodegradation test, or a river die-away study. A 21-day acclimation period was used in the closed bottle test and river die-away study (Mudder, 1982). No degradation of tetrachloroethene (at 9–74 µg/l initial concentrations) was observed in cultures containing a bacterial inoculum obtained from primary sewage effluent and incubated in the dark for 25 weeks at 20 şC (Bouwer et al., 1981). No degradation was observed when a sterile salt solution containing tetrachloroethene at 10–30 µg/l was applied continuously to an up-flow glass column containing inert material, seeded with primary sewage and operated at 22–23 şC under aerobic conditions for 2 years (Bouwer & McCarty, 1982). No degradation of tetrachloroethene was observed during the infiltration of river water to groundwater. Samples were taken from groundwater sites near a contaminated river over 1 year, and conditions were predominately aerobic (Schwarzenbach et al., 1983).
Although tetrachloroethene is persistent under aerobic conditions, 60–90% decreases in concentrations have been reported in studies using aerobic soil columns (Phelps et al., 1991; Enzien et al., 1994); this was possibly due to the presence of anaerobic niches within the column beds, although no specific evidence of anaerobic biodegradation was found (EC, 2001). One study, using a static culture, BOD-based flask method with an inoculum of domestic sewage sludge, indicated that tetrachloroethene may undergo primary degradation, the rate increasing with adaptation of the microorganisms. Losses of tetrachloroethene (initial concentration 5 mg/l) in four consecutive 7-day periods were 45%, 54%, 69%, and 87%, respectively. Volatilization accounted for the remainder (Tabak et al., 1981).
Tetrachloroethene undergoes anaerobic degradation by reductive dechlorination. Reported degradation products are trichloroethene, dichloroethene, vinyl chloride, ethene, and ethane and vary with the experimental conditions used. The inocula used in the majority of experiments were adapted, and degradation of tetrachloroethene was usually observed at elevated temperatures and in the presence of nutrients. Several methanogenic organisms were found to be capable of dechlorinating tetrachloroethene. The redox potential is important in determining the level of dechlorination. Anaerobic dechlorination takes place under methane- and sulfate-reducing conditions. For dechlorination to occur, an electron donor is usually required — for example, acetate or lactate (EC, 2001).
Reductive dechlorination of tetrachloroethene was studied in a fixed-bed column containing anaerobic river sediment and anaerobic granular sludge and continuously percolated with an anaerobic mineral medium. Reducing conditions were maintained in the column by the presence of sodium sulfide (10 mg/l), and lactate was used as an electron donor (1 mmol/l). The flow rate through the column was 15 ml/h at 20 şC in the dark. After adaptation, tetrachloroethene was dechlorinated stepwise via trichloroethene, cis-1,2-dichloroethene, and vinyl chloride to ethene. Ethene was then reduced to ethane within 24 h. The conversion of tetrachloroethene to ethane was 95–98% during a 24-h period at an initial concentration of 1.5 mg/l. Lowering the column temperature to 10 şC caused an initial decrease in the conversion to ethane; after 2 weeks at the lower temperature, however, only ethane and ethene were detected in the effluent (De Bruin et al., 1992).
Reductive dechlorination of tetrachloroethene was also studied in a methanogenic culture allowed to adapt for 115 days. Analysis of samples taken every 2 days between days 115 and 135 showed that freshly added tetrachloroethene was degraded to vinyl chloride (about two thirds) and ethane (about one third), with traces of trichloroethene and dichloroethene. After 170 days, tetrachloroethene was degraded to ethene (80%) and vinyl chloride (20%) within 2 days, even though methanogenic activity (as measured by methane production) had declined. When longer periods were allowed between additions of tetrachloroethene, it was found that the amount of vinyl chloride remaining after 4 days was <1% of the total products (DiStefano et al., 1991). In methanol- and hydrogen-fed mixed anaerobic cultures, tetrachloroethene was biodegraded to ethene (80%) and vinyl chloride (20%), with traces of trichloroethene and dichloroethene (DiStefano et al., 1992).
Testing of several anaerobic bacteria, including four strains of acetate-utilizing methanogens (Methanosarcria sp., Methanosarcria mazei, Methanosarcria acetivorans, and Methanothrix sp.), Desulfovibrio desulfuricans, Clostridium pasteurianium, Clostridium butyricum, and a pure-culture dehalogenator (Desulfomonile tiedjei; DCB-1), found that Methanosarcria sp., Methanosarcria mazei cultures, and DCB-1 could degrade tetrachloroethene to trichloroethene. The process by which methanogens dechlorinate tetrachloroethene is a co-metabolic process and appears to be dependent on the formation of methane from the carbon source (Fathepure et al., 1987; Fathepure & Boyd, 1988).
Fathepure & Tiedje (1994) studied the reductive dechlorination of tetrachloroethene at 35 şC in a continuously fed, up-flow biofilm reactor, inoculated with an enriched culture containing anaerobic Desulfomonile tiedjei bacteria. After steady-state conditions had being achieved (4 months), tetrachloroethene was added, and the column was left to acclimatize for 3–4 weeks. Degradation rates of between 78% and 86% were measured for tetrachloroethene concentrations of 0.26–1.0 mg/l, and trichloroethene and dichloroethene were found as degradation products.
Freedman & Gosset (1989) studied the anaerobic degradation of tetrachloroethene with microorganisms from a wastewater treatment plant. The organisms were adapted prior to use by anaerobic incubation at 35 şC with aqueous tetrachloroethene. When the added tetrachloroethene had been degraded, a sample of the culture was removed from each bottle and replaced by fresh medium and tetrachloroethene. Operating in this semi-continuous way, sixth-generation cultures were obtained. Redox conditions were maintained by the presence of Fe2+ ions. Ethene was reported as the main degradation product formed, with traces of trichloroethene and dichloroethene. Reductive dechlorination was found to occur only when methanol was used as co-metabolite. The addition of hydrogen to dechlorinating microcosms increased the dechlorination rate by about 500 times after 200 days.
Holliger et al. (1993) isolated a bacterium capable of growing on tetrachloroethene from an inoculum derived from anaerobic sediment and anaerobic granular sludge. Hydrogen or formate was necessary as electron donor for growth. Tetrachloroethene was degraded in the anaerobic packed-bed column from which the bacterium was derived. The main degradation product was ethane, and traces of cis-1,2-dichloroethene, trichloroethene, vinyl chloride, and ethene were detected.
Kästner (1991) found that reductive dechlorination of tetrachloroethene to cis-1,2-dichloroethene occurred upon the transfer from aerobic to anaerobic conditions. The cultures used contained aerobic isolates in a co-culture with a Bacillus sp. and a Desulfotomaculum sp. under conditions of limited oxygen supply. Degradation of tetrachloroethene occurred only in cultures that were initially aerobic but became anaerobic after a few days’ incubation. No degradation was observed in cultures that were anaerobic from the start. Transformation of tetrachloroethene required a decrease in redox potential of the system caused by sulfide formation from degradation of sulfur compounds present in the system.
Liang & Grbi-Gali (1993) studied the degradation of tetrachloroethene under methanogenic conditions using aquifer material obtained from contaminated sites. They incubated microcosms containing inocula and anaerobic mineral medium at 35 şC. Reducing conditions were maintained by the presence of Fe2+ ions. Trichloroethene, trans-1,2-dichloroethene, and vinyl chloride were detected as the degradation products.
Trichloroethene and cis-1,2-dichloroethene were detected as degradation products under both sulfate-reducing and methanogenic conditions in microcosms packed with soil containing tetrachloroethene at 11.5 mg/kg (Pavlostathis & Zhuang, 1993). In further studies, it was shown that reductive dechlorination of tetrachloroethene (1 mg/l) in a methanogenic culture derived from contaminated soil was influenced by temperature (peaked at 35 şC, then decreased above 45 şC) and acidity (maximal at pH 7) (Zhuang & Pavlostathis, 1995).
When microcosms prepared from aquifer solids and distilled water spiked with tetrachloroethene at 3 µmol/l were incubated in the dark, anaerobic biotransformation to trichloroethene and cis-1,2-dichloroethene was complete within 7 days (Ninomiya et al., 1994). In a continuous-flow fixed-film methanogenic column with a 2-day retention time, the tetrachloroethene concentration was anaerobically reduced from 20.5 mg/l to 4.4 µg/l (99.98% reduction). Trichloroethene, dichloroethene, and (mainly) vinyl chloride were identified as the degradation products. Mineralization to carbon dioxide was detected by tracer studies (Vogel & McCarty, 1985). In a deoxygenated anaerobic medium seeded with a methanogenic culture, tetrachloroethene (0.2 mg/l) was completely degraded (mainly to trichloroethene) within 8 weeks (Bouwer & McCarty, 1983). When studied using two continuous columns (in series) seeded by a primary sewage effluent and a methanogenic bacterial inoculum, respectively, steady-state removal of tetrachloroethene was 86% after a 10-week acclimation period. The columns were operated in the dark at 22–23 şC for 19 months (Bouwer & McCarty, 1983).
Suflita et al. (1988) compared biodegradation under methanogenic and sulfate-reducing conditions. In both instances, tetrachloroethene was dechlorinated by sequential reduction reactions to form mainly trichloroethene, dichloroethene, and vinyl chloride. Degradation was found to occur faster in the methanogenic cultures. Gibson & Sewell (1992) showed that dehalogenation occurs in the presence of a suitable electron donor, such as lactate and acetate. Trichloroethene and dichloroethene were detected as degradation products.
Reductive anaerobic dechlorination of tetrachloroethene can lead to the formation of vinyl chloride in groundwater, and the vinyl chloride detected in groundwater may be the result of tetrachloroethene degradation. However, several studies show that the process of dechlorination can continue under anaerobic conditions, with the end-products being ethene and ethane. Several other chlorinated solvents such as trichloroethene are also likely to break down in the environment to vinyl chloride. Therefore, while vinyl chloride may be formed from tetrachloroethene in groundwater, it is not possible to quantify the extent to which this process contributes to the levels of vinyl chloride found. Possible risks from this were not assessed in the EU risk assessment (EC, 2001). Some concerns have been expressed over the possible production of trichloroacetic acid in surface waters from the breakdown of tetrachloroethene. From the studies presented here, tetrachloroethene appears to be relatively stable to degradation in surface waters, with volatilization being the main removal process. In anaerobic environments, reductive dechlorination appears to be the most likely degradation pathway, producing trichloroethene, vinyl chloride, and ultimately ethene and ethane. No evidence for the formation of trichloroacetic acid from tetrachloroethene in water was found in the literature (EC, 2001).
The reactivity of tetrachloroethene in the troposphere has been reported as being low enough so as not to contribute significantly to tropospheric ozone formation and the related "photochemical smog". The photochemical ozone creation potential of tetrachloroethene in the troposphere is estimated as 1, expressed relative to 100 for ethene (a substance thought to be important in photochemical ozone production) (Derwent & Jenkin, 1990).
The small fraction of tetrachloroethene that enters the stratosphere (about 1% of atmospheric emissions) from the troposphere will be degraded by reaction with hydroxyl radicals, but some might undergo photolysis to yield products that may lead to ozone depletion. The actual impact is likely to be negligible compared with that of other ozone-depleting chemicals, such as CFCs and methyl chloroform (1,1,1-trichloroethane). The estimated stratospheric chlorine loading potential of tetrachloroethene is less than 0.01. Some of the degradation products of tetrachloroethene formed in the troposphere may enter the stratosphere and contribute to ozone depletion. The contribution that these products might make to ozone depletion has not been quantitatively assessed (ECETOC, 1999).
A source document (EC, 2001) has concluded that the reactivity of tetrachloroethene in the troposphere (the half-life is around 3–5 months) is such that it is not thought to contribute significantly to tropospheric ozone formation. Gas-phase photolysis and rainout are thought to be of negligible importance in the removal of tetrachloroethene from the troposphere. The lifetime of tetrachloroethene in the troposphere is such that the amount entering the stratosphere is low. Studies into stratospheric ozone depletion mention that tetrachloroethene is a possible ozone depleter, although its potential is significantly lower than that of other ozone-depleting chemicals. Degradation products of tetrachloroethene in the troposphere may enter the stratosphere; of these, carbon tetrachloride is a known ozone depleter. The amounts of carbon tetrachloride entering the stratosphere due to tetrachloroethene degradation are thought to be negligible when compared with other sources of carbon tetrachloride emissions. No data were found quantifying the contribution that tetrachloroethene makes to ozone depletion, either directly or indirectly via its degradation products. An expert working group on ozone depletion (WMO, 1991) considered that tetrachloroethene makes a negligible contribution to ozone depletion relative to other ozone-depleting chemicals, such as CFCs, HCFCs, carbon tetrachloride, and 1,1,1-trichloroethane. Tetrachloroethene is not expected to contribute significantly to global warming (EC, 2001).
The draft EU Risk Assessment Report concluded that the majority of measured levels are below 10 µg/m3 and, indeed, mostly below 1 µg/m3 (EC, 2001). A review of measured tetrachloroethene concentrations in Germany in 1988 found that concentrations in rural areas ranged between 0.5 and 2 µg/m3, and concentrations in urban areas ranged between 2 and 15 µg/m3 (BUA, 1993). The ATSDR in the USA concluded that background levels lie generally in the lower ppt range (1 ppt = 6.89 ng/m3) in rural and remote areas; values in the higher ppt and lower ppb range (1 ppb = 6.89 µg/m3) are found in urban and industrial areas and areas near point sources of pollution (ATSDR, 1997). The most recent modelling of exposure information for the USA by the USEPA is based on 1996 information and is published as part of the National Air Toxics Assessment. The results show that, for 95% of counties in the USA, the atmospheric level was 0.29 µg/m3 or below. The highest county level was 1.39 µg/m3 (USEPA, 2002). Earlier surveys of the air in nine cities in the USA showed concentrations between 0.2 and 52 µg/m3, with averages ranging from 2 to 4 µg/m3 (IPCS, 1984).
Global background concentrations measured in 1989 were 0.09 µg/m3 for the Northern Hemisphere and 0.02 µg/m3 for the Southern Hemisphere (Koppmann et al., 1993).
A median outdoor level of 2 µg/m3 was measured in a study in Holland (Lebret et al., 1986). In a study performed between October 1987 and September 1988 in an "urban canyon" in the centre of Turin, Italy (120 samples taken during 10 consecutive days, 24 h each, during approximately 1 year; 31 measurements during winter and 28 during summer), it was found that contamination of air was higher in winter than in summer, the mean atmospheric concentrations being either 8.70 µg/m3 and 4.75 µg/m3, respectively (Gilli et al., 1990a), or 13.6 µg/m3 and 4.75 µg/m3, respectively (Gilli et al., 1990b). It was found that the indoor/outdoor concentration ratio was higher in winter that in summer, median concentration ratios being 2.15 and 1.38, respectively (Gilli et al., 1990a,b).
Monitoring in the Rhine valley in 1996 showed a maximum value from one site (Freiburg) of 2.9 µg/m3; the highest 98th-percentile value for a sampling site was 1.8 µg/m3 at Karlsruhe (UMEG, 1997). Data from 1998 for the Nordrhein–Westfalen region, including the industrialized area between Dortmund and Cologne, showed a peak value of 2.4 µg/m3, with average values generally below 0.5 µg/m3 (LUA, 1999). Bruckmann et al. (1989) sampled air from 12 sites in Hamburg between April 1986 and April 1987 and characterized activities near the sites. Significantly higher concentrations were found at three sites: near a chemical laundry (dry cleaning), a rubber factory, and an industrial area with metalworking industry and small chemical factories. The highest yearly average concentration was 71 µg/m3, while the overall average concentration was 3.5 µg/m3 (Bruckmann et al., 1989). Surveys of the air in 14 cities in Germany showed average concentrations between 1.7 and 6.1 µg/m3 (IPCS, 1984).
Monitoring air concentrations near a tetrachloroethene production and processing facility over a 4-week period when both areas of activity were operating (24-h samples taken for 28 days) found an average daily mean concentration of 36 µg/m3 (EC, 2001).
In Germany, annual mean levels of 41 µg/m3 and 69 µg/m3 were detected downwind of a chemical laundry and a rubber factory, respectively (IPCS, 1984).
The median level of tetrachloroethene in about 400 Dutch homes was 4 µg/m3, while maximum levels varied between 49 and 205 µg/m3 (Lebret et al., 1986).
Levels can be much higher in buildings housing dry cleaning facilities. For example, sampling (over 100 samples) of air in six residential apartments in two buildings where dry cleaning was carried out on the ground floor revealed tetrachloroethene concentrations ranging from 50 to 6100 µg/m3, with means ranging from 358 to 2408 µg/m3. One month after the dry cleaning facilities ceased operating, atmospheric concentrations in the apartment air had declined substantially but still ranged from 10 to 800 µg/m3 (Schreiber et al., 2002).
Tetrachloroethene was reported in drinking-water in Germany at 1.3 µg/l (Lahl et al., 1981). More recently, figures for samples of drinking-water in Germany were given as <0.001 µg/l (51% of samples), 0.001–0.5 µg/l (40% of samples), and >0.5 µg/l (9% of samples) (Bauer, 1991). Levels in Finnish samples ranged up to 0.05 µg/l (Reunanen & Kroneld, 1982; Kroneld, 1986). Tetrachloroethene was detected in 454 of 2682 samples taken by 29 water companies in the United Kingdom. The detection limit ranged from 0.1 to 1 µg/l, and the maximum concentration detected was 12.2 µg/l. The range and mean were not given (personal communication to United Kingdom Environment Agency from United Kingdom Drinking Water Inspectorate, 1995, cited in EC, 2001).
Industrial disposal is the typical likely source of drinking-water contamination with tetrachloroethene. However, in the late 1960s through the early 1980s, tetrachloroethene leached into the drinking-water supplies of Cape Cod, Massachusetts, in the USA from an inner vinyl liner that was present in certain cement pipes (in which a slurry of a vinyl plastic and tetrachloroethene was used to coat the inside of the pipe before shipment), affecting 1000 km of the pipes. Typical levels in the water of affected towns in the region ranged from 1.60 to 7.75 mg/l in low-flow locations and from 1.5 to 80 µg/l in medium- and high-flow locations (Demond, 1982; Aschengrau et al., 2003).
Tetrachloroethene has been measured in surface (river) waters in Germany, Finland, the Netherlands, Italy, France, Switzerland, the United Kingdom, and the USA. Concentrations ranged from 0.01 to 168 µg/l, with levels typically below 5 µg/l (Reunanen & Kroneld, 1982; Ahel et al., 1984; Hellmann, 1984; Staples et al., 1985; Aggazzotti & Predieri, 1986; Kroneld, 1986; Van de Meent et al., 1986; Marchand et al., 1988; Van der Graff, 1988; Bohlen et al., 1989; Malle, 1990; RIVM, 1993; EC, 2001).
Analysis of coastal and estuarine waters of Germany, the United Kingdom, Sweden, France, Greece, and the Mediterranean revealed that tetrachloroethene concentrations were below 3 µg/l (Hellmann, 1984; Fytianos et al., 1985; Marchand et al., 1986, 1988; Van de Meent et al., 1986; Abrahamsson et al., 1989; Hurford et al., 1989; Dawes & Waldock, 1994; England and Wales National Rivers Authority, personal communication, 1995, cited in EC, 2001).
Summarizing several other reviews (e.g. IPCS, 1984; IARC, 1995), de Raat (2003) presented average and maximum concentrations in seawater of 0.012 and 2.6 µg/l, respectively. Surface water from the Atlantic Ocean was said to contain 0.0002–0.0008 µg/l. The highest concentration found in the surface waters of Lake St. Clair (Canada/Michigan, USA) was 0.47 µg/l (de Raat, 2003).
Rainwater samples in Germany, the Netherlands, Switzerland, the United Kingdom, and the USA contained tetrachloroethene at <0.005–0.15 µg/l; the highest figure was found in an industrial area (Kawamura & Kaplan, 1983; Atri, 1985; Van de Meent et al., 1986; Czuczwa et al., 1988; Kubin et al., 1989; Renner et al., 1990).
The EU Risk Assessment Report concluded that groundwater concentrations of tetrachloroethene vary widely. Although groundwater concentrations are generally higher than concentrations in surface water, this could reflect the fact that groundwater measurements tend to be taken where a problem (e.g. a spill) is thought to exist (EC, 2001). Groundwater levels are usually below 10 µg/l (Fielding et al., 1981; Trowborst, 1981; Fahmi, 1984; Aggazzotti & Predieri, 1986; Goodenkauf & Atkinson, 1986; Sagunski et al., 1987; Heil et al., 1989; Bauer, 1991; England and Wales National Rivers Authority, personal communication, 1995, cited in EC, 2001), but concentrations as high as 1300 µg/l have been reported for a contaminated site (Leschber et al., 1990).
One source document reported that tetrachloroethene has been measured at 0.01–46 µg/l groundwater in western Europe, and the maximum concentration reported in groundwater in the Netherlands was 22 µg/l (de Raat, 2003).
Tetrachloroethene has been measured in sediment samples at 1–50 µg/kg wet weight in Germany (Alberti, 1989) and at <5 µg/kg wet weight in the USA (Staples et al., 1985). One source document reports a maximum concentration of 4.8 mg/m3 in sediments (de Raat, 2003).
Samples of soil air taken in Germany contained tetrachloroethene at 2.1–4.5 µg/m3 (Frank et al., 1989).
The EU risk assessment reports tetrachloroethene concentrations in municipal wastewaters in Germany, the United Kingdom, France, Switzerland, and the USA. In Germany, concentrations in effluents ranged from 0.01 to 5.9 µg/l (Bohlen et al., 1989). In the United Kingdom, concentrations in effluent were typically up to 2 µg/l, with a maximum of 144 µg/l (Brown, 1978). The median concentration in a study in the USA was 5 µg/l (Staples et al., 1985). Swiss effluent samples contained 0.03–6.4 µg/l (means 0.16–1.0 µg/l) (Fahmi, 1984). Effluent concentrations are lower than influent concentrations. Influent and effluent concentrations were 15 µg/l and 1 µg/l, respectively, in a Swiss study (Fahmi, 1984). In samples taken in various regions of France, influent concentrations ranged from 1.05 to 23 µg/l, while concentrations in effluents ranged from not detectable to 8.5 µg/l; the detection limits were not disclosed (Marchand et al., 1988, 1989). One source document reports that the influent of a sewage treatment plant contained tetrachloroethene at 6.2 µg/l, while the effluent contained 3.9 and 4.2 µg/l before and after chlorination, respectively (de Raat, 2003).
Tetrachloroethene was measured at 2.8–10 µg/l in effluents from various industrial activities (ceiling coating material manufacture, metalworking, chemical product packaging, treatment of industrial effluents, surface treatment, wood preservation, and paint manufacture), at 7–29 µg/l in effluent from a car equipment manufacturing facility, and at 508 µg/l in a chemical industry effluent (DRIRE Franche Comté, 1996). Dry cleaning industry effluent in Finland contained 2.5–580 000 µg/l (geometric mean 2.5 µg/l, arithmetic mean 88 µg/l) (Finnish Environment Agency, personal communication, 1996, cited in EC, 2001).
De Raat (2003) presented a table (reproduced here as Table 3) of published data on tetrachloroethene concentrations in food. Being an adaptation of information from three review sources (IPCS, 1984; IARC, 1995; ATSDR, 1997), these figures might not reflect current concentrations.
Table 3: Concentration of tetrachloroethene in food products.a
|
Country |
Food samples |
Concentration (µg/kg) |
|
Switzerland |
Milk and meat products |
3–3490 |
|
United Kingdom |
Dairy products |
0.3–13 |
|
Meat |
0.9–5 |
|
|
Margarine |
7 |
|
|
Oils |
0.01–7 |
|
|
Instant coffee |
3 |
|
|
Tea |
3 |
|
|
Fruit and vegetables |
0.7–2 |
|
|
United Kingdom |
Olive oil (81 of 98 samples) |
<10 |
|
Olive oil (17 samples) |
1–17 |
|
|
Pennsylvania (USA), samples from a food processing plant |
Tap water |
0.0004 |
|
Chinese-style sauce |
0.002 |
|
|
Quince jelly |
0.0022 |
|
|
Crab apple jelly |
0.0025 |
|
|
Grape jelly |
0.0016 |
|
|
Chocolate sauce |
0.0036 |
|
|
USA |
93 of 231 samples |
13 (1–124) |
|
Cereals |
22 (1–108) |
|
|
Corn oil |
21 |
|
|
Pork and beans |
2 |
|
|
Peas |
2 |
|
|
Onion rings |
5 |
|
|
Fried potatoes |
9 |
|
|
Baked goods |
12 (3–48) |
|
|
Peanut butter |
3 |
|
|
Pecan nuts |
120 |
|
|
Milk chocolate |
20 |
|
|
Meat products |
13 (1–124) |
|
|
Baby foods |
2.5 (1–5) |
|
|
Bananas |
2 |
|
|
Grapes |
1 |
|
|
Avocados |
14 |
|
|
United Kingdom |
Fish |
0.3–11 |
|
Fish liver |
1–41 |
|
|
Molluscs (dry weight) |
4 (1–15) |
|
|
USA |
Clams |
3 |
|
Oysters |
10 |
|
|
Germany, supermarket near dry cleaning shop |
Margarine |
110 |
|
Herb butter |
7 |
|
|
Butter |
21 |
|
|
Flour |
25 |
|
|
Corn starch |
36 |
|
|
Cheese spread |
36 |
|
|
Germany, in dry cleaning shop |
Fruit sherbet |
2 |
|
Chocolate-coated ice cream |
1 330 |
|
|
Chocolate- and nut-coated ice cream |
4 450 |
|
|
Ice cream confection |
18 750 |
|
|
Germany, in apartment above dry cleaning shop |
Butter |
58 000 |
a As presented in source document (de Raat, 2003).
The most important routes of exposure to tetrachloroethene for members of the general population appear to be inhalation of the compound in ambient air and ingestion via drinking-water. Available data indicate that dermal exposure is not important for most people (de Raat, 2003).
An EU risk assessment for human health is being drafted. The current draft contains estimated figures for daily human intake, based on typical human consumption and inhalation rates. The total human dose of tetrachloroethene based on "background" exposure is estimated (using "reasonable worst-case assumptions") to be 0.43 µg/kg body weight per day, or 30 µg/day for a person weighing 70 kg. Equivalent estimated figures are higher for persons living near a manufacturer (19 µg/kg body weight per day) or above a dry cleaning establishment (1.67 mg/kg body weight per day) (EC, 2004).
In the USA, the average daily intake by the inhalation route, assuming ambient concentrations of 2.1–17.3 µg/m3 and inhalation of 20 m3 of air per day, is estimated to be 41–204 µg. The average daily intake from water, assuming concentrations of 0.3–3 µg/l and ingestion of 2 litres of water per day, is estimated to be 0.6–6 µg (ATSDR, 1997).
In Switzerland and Germany, total daily intakes via food were calculated to be 160 µg and 87 µg, respectively (IPCS, 1984).
The breath of residents living above 12 dry cleaning shops in the Netherlands was found to contain a mean tetrachloroethene concentration of 5 mg/m3, while the breath of residents living adjacent to the shops contained 1 mg/m3 (IPCS, 1984). Tetrachloroethene and trichloroacetic acid concentrations in blood and trichloroacetic acid concentrations in urine were determined primarily over the course of a week for 29 persons living in the vicinity of dry cleaning shops in Germany. The concentrations of tetrachloroethene in blood depended on the floor and the construction type of the building where the people resided, but not on the type of system used in the dry cleaning shops (Popp et al., 1992).
In Turin, Italy, blood samples of 30 volunteers (15 females, 15 males) contained a mean tetrachloroethene concentration of 1.33 µg/l during winter and 0.46 µg/l during summer (Gilli et al., 1990a) (see also section 6.1.1).
A study in Modena, Italy, reported tetrachloroethene levels in the ambient air of 30 homes of dry cleaners (located well away from the dry cleaning premises), alveolar air contemporaneously from the (36) dry cleaners, and samples of end-exhaled air (alveolar air) from 34 subjects who were not themselves occupationally exposed, but who were members of the households of dry cleaners. These were compared with samples from 41 members of the general population (located in the same district near the dry cleaners’ homes). Tetrachloroethene levels in dry cleaners’ homes were significantly higher than in control houses (geometric means: 265 vs 2 µg/m3, P < 0.001). Tetrachloroethene levels in the alveolar air exhaled by dry cleaners, their family members, and control subjects were statistically significantly different (geometric means: 5140, 225, and 3 µg/m3, respectively; P < 0.001) (Aggazzotti et al., 1994).
Due to the age of the reports, data in this section might not reflect current experience.
Exposure levels for organic solvents at Dutch workplaces were measured by the Dutch Ministry of Social Affairs and Employment. During cleaning activities in dry cleaning establishments, metal industries (cleaning machinery parts and degreasing activities), and offset-printing offices, breathing-zone air levels of up to 350 mg/m3, 270 mg/m3, and 110 mg/m3 were observed, respectively (Doorgeest et al., 1986).
A NIOSH (USA) survey (1977–1979) of 44 dry cleaning facilities showed exposures for machine operators to range from 30 to 1030 mg/m3. Geometric mean exposures for machine operators, pressers, and seamstresses and in front counter areas were 150, 23, 21, and 21 mg/m3, respectively. A study of the dry cleaning industry in the United Kingdom indicated exposure levels similar to those observed in American studies (ATSDR, 1997).
An 8-h TWA concentration of up to 4000 mg/m3 can occur in dry cleaning establishments. In the United Kingdom, over 90% of 493 8-h measurements in 131 dry cleaning establishments revealed concentrations below 680 mg/m3, and over 50% of these samples revealed concentrations below 200 mg/m3. Similar results were obtained in a survey of 46 dry cleaning establishments in Germany (IPCS, 1984).
At a railway works where tetrachloroethene was used as a cleaning agent, 94% of 104 8-h measurements exceeded 680 mg/m3, with peaks up to 1290 mg/m3 (IPCS, 1984).
A number of investigations have shown that tetrachloroethene is well absorbed by humans exposed by inhalation (Stewart et al., 1961b, 1970; Fernandez et al., 1976; Hake & Stewart, 1977; Monster et al., 1979, 1983; Benoit et al., 1985; Opdam & Smolders, 1986, 1987; Pezzagno et al., 1988), as would be predicted from the blood/air partition coefficient (Table 4). On exposure at 500 or 990 mg/m3, respiratory absorption was in excess of 90% at the start of exposure and fell to about 50% after 8 h of exposure (Monster et al., 1979). Absorption was 78–93% in volunteers exposed at 340–630 mg/m3 (Benoit et al., 1985).
Table 4: Blood/air, tissue/air, and tissue/blood human partition coefficients for tetrachloroethene.a
|
Blood/air and tissue/air partition coefficient |
Tissue/blood partition coefficient |
||
|
Blood/air |
12 |
||
|
Fat/air |
1450 |
Fat/blood |
125 |
|
Kidney/air |
59 |
Kidney/blood |
5 |
|
Muscle/air |
70 |
Muscle/blood |
6 |
|
Liver/air |
61 |
Liver/blood |
5 |
a From Gearhart et al. (1993).
An absorption figure of 50% was reported in rats exposed at 340 mg/m3 for 3 h (Dallas et al., 1994a). The amounts collected post-exposure (in exhaled air, urine, and carcass) in rats and mice exposed by inhalation suggest that absorption from the respiratory tract is considerable (Pegg et al., 1979; Schumann et al., 1980, 1982). Following a 4-h whole body exposure of rats at 3320 mg/m3, tetrachloroethene was found in the blood at 26 mg/l (Frantik et al., 1998).
Data on dermal absorption in humans are limited. A 3-min exposure of the forearm (27 cm2 area) of six volunteers to tetrachloroethene liquid resulted in an average dermal absorption rate of 0.68 mg/cm2 per minute (Kezic et al., 2001). It has been estimated that immersion of the hands and forearms in tetrachloroethene for 1 h would correspond to an inhalation exposure of 850 mg/m3 for 8 h. When five subjects immersed one thumb in tetrachloroethene for 40 min (inhalation was prevented), the mean tetrachloroethene concentration in exhaled air peaked at 2.1 mg/m3 within 10 min of the end of exposure and slowly decreased to 1.4 mg/m3 after 5 h (Stewart & Dodd, 1964). When two subjects immersed one hand in tetrachloroethene for 5 min (with prevention of inhalation), blood concentrations immediately after exposure were much higher in the immersed arm than in the non-immersed arm, and concentrations did not become similar until 2 h had passed, suggesting considerable local tissue absorption (Aitio et al., 1984). Upon exposure to the vapour, respiratory absorption seems to be much more important than dermal absorption. When three volunteers were exposed for 3.5 h at 4000 mg/m3 via the skin only, 48 mg was exhaled in the following 50 h. It was estimated that 4.2 g would have been exhaled following combined skin and respiratory exposure at 4000 mg/m3 for 3.5 h, suggesting that the inhalation route would account for about 99% of the total absorption in subjects exposed by both routes (Riihimäki & Pfäffli, 1978).
When 0.5 ml of tetrachloroethene was applied to the skin (2.9 cm2) of intact mice for 15 min, the total tetrachloroethene absorbed was estimated to be 177 µg, of which 173 µg was found in exhaled air. This amount equates to an absorption rate of 24.4 nmol/cm2 per minute (Tsuruta, 1975). Absorption in vitro appeared to be much slower, a figure of 0.067 nmol/cm2 per minute being calculated when tetrachloroethene was applied to isolated rat skin (Tsuruta, 1977).
Quantitative data on absorption in humans following ingestion are not available. However, the systemic toxicity and the presence of tetrachloroethene and its metabolites in the blood and urine of humans who accidentally ingested tetrachloroethene suggest that it is readily absorbed through the human gastrointestinal tract (Koppel et al., 1985; ATSDR, 1997).
Laboratory animal studies indicate rapid and extensive absorption of tetrachloroethene following oral administration (Daniel, 1963; Pegg et al., 1979; Frantz & Watanabe, 1983; Mitoma et al., 1985; Clement International Corporation, 1990; ATSDR, 1997).
In humans repeatedly exposed by inhalation, tetrachloroethene tends to accumulate in the adipose tissues, as would be predicted by the high fat/blood partition coefficient (Table 4). Evidence for this accumulation was seen in a study in which tetrachloroethene concentrations in exhaled air were higher in volunteers exposed at 700 mg/m3 for 7 h/day for 5 days than for 1 day, as tetrachloroethene released from fatty tissue contributed to exhaled compound on daily exposure. The long decay (tetrachloroethene was still present at more than 7 mg/m3 in exhaled air after 10 days) is also evidence of accumulation (Stewart et al., 1970). Prolonged decay was also seen in other, shorter studies (Guberan & Fernandez, 1974; Fernandez et al., 1976; Monster et al., 1979). Analysis of human tissues following fatal inhalation exposures found tetrachloroethene concentrations of 30–240 mg/kg in brain, kidneys, liver, and lungs (Lukaszewski, 1979; Levine et al., 1981).
Radioactivity was found mainly in the fat (especially the perirenal fat) in rats exposed by inhalation to radiolabelled tetrachloroethene at 1400 mg/m3, 6 h/day, for 4 days. Concentrations were reported to be 4495, 161, 143, 92, 74, and 31 nmol/g in perirenal fat, liver, cerebrum, cerebellum, lungs, and blood, respectively (Savolainen et al., 1977). This accumulation in the fat presumably reflects a combination of the low extent of metabolism, rather low vapour pressure, and high lipid/blood distribution coefficient (see also section 7.4). When rats and mice were exposed to radiolabelled tetrachloroethene at 70 mg/m3 for 6 h, the carcass 72 h later contained 3% of total recovered radiolabel. However, mice showed much (up to 9.2 times) higher binding to liver protein and excretion predominantly via the urine, whereas excretion was mainly by exhalation in rats. This reflects a much faster oxidative metabolism in mice (Schumann et al., 1980). On inhalation exposure of pregnant rats at 1500 mg/m3 for 8 h, mean tetrachloroethene concentrations in the maternal blood, fetal blood, and amniotic fluid were about 18, 12, and 6 "µl/ml". Respective figures for exposure at 8500 mg/m3 were 86, 25, and 18 "µl/ml" (Szakmáry et al., 1997). Following a 10-min inhalation exposure of pregnant mice to 14C-radiolabelled tetrachloroethene, there was a high uptake of radioactivity in the maternal body fat, brain, nasal mucosa, blood, and well perfused organs such as liver, kidney, and lung. Both volatile (unchanged compound) and non-volatile (metabolite) radioactivity reached embryonic and fetal tissue, particularly the liver and blood. Volatile radioactivity in the fetus was always lower than in the corresponding maternal tissues and was not detected by 4 h following exposure. Non-volatile radioactivity peaked at 4 h. Following exposure on day 11 of pregnancy, radioactivity was high in the neuroepithelium of the developing fetal brain. If dams were exposed on day 17 of pregnancy, levels in the fetal brain were lower than in other organs. Tetrachloroethene concentrations in the amniotic fluid were 6–14% of those in maternal blood. Concentrations of radiolabelled metabolite (trichloroacetic acid) peaked in the maternal plasma, amniotic fluid, and fetus at 4 h (Ghantous et al., 1986).
No good data are available on distribution in humans exposed dermally, but partition coefficients (Table 4) and the slow decay of tetrachloroethene concentrations in the exhaled air following dermal exposure to tetrachloroethene liquid or vapour suggest initial accumulation in fat (Stewart and Dodd, 1964; Riihimäki et al., 1978).
No data on distribution were found in laboratory animals exposed dermally.
No data on distribution were found in humans exposed orally.
In rats given radiolabelled tetrachloroethene at 1 or 500 mg/kg body weight by stomach tube, 3.3% and 1.2%, respectively, of the dose were found in the carcass 72 h later. At the lower dose, similar concentrations were found in the fat, liver, and kidneys, whereas the highest concentration was found in the fat at the 500 mg/kg body weight dose (Pegg et al., 1979). Marth (1987) identified two separate mechanisms of tetrachloroethene transport in mice after oral exposure: first, transport by the chylomicrons in the blood to the adipose tissue; second, adsorption to the phospholipid cell membrane of the red blood cells, leading to an increased fragility and premature destruction of these cells. Tetrachloroethene-loaded fragments of these cells are phagocytosed in the spleen, resulting in the accumulation of tetrachloroethene in this organ. Dallas et al. (1994b) investigated the time dependence of the tetrachloroethene concentrations in various organs after administration of 10 mg/kg body weight to male Sprague-Dawley rats and male beagle dogs by gavage. The dogs exhibited longer tissue and blood half-lives than did the rats. The species differences in toxicokinetic parameters (see Table 5) are presumably due to a markedly higher rate and magnitude of exhalation and metabolism in the rat.
Table 5: Toxicokinetic parameters in rat and dog after a single oral gavage tetrachloroethene treatment with 10 mg/kg body weight.
|
Tissue |
AUC (µg·min/ml) |
Half-time (min) |
Cmax (µg/g) |
Tmax (min) |
|
Dog |
||||
|
Liver |
1 851 |
2 448 |
6 |
60 |
|
Kidney |
1 606 |
1 572 |
5 |
60 |
|
Fat |
55 838 |
494 |
43 |
720 |
|
Heart |
1 849 |
1 775 |
6 |
60 |
|
Lung |
1 001 |
2 289 |
2 |
60 |
|
Muscle |
1 907 |
1 625 |
3 |
60 |
|
Brain |
3 238 |
4 641 |
11 |
60 |
|
Blood |
782 |
865 |
2 |
90 |
|
Rat |
||||
|
Liver |
1 673 |
331 |
12 |
10 |
|
Kidney |
1 057 |
395 |
6 |
10 |
|
Fat |
49 964 |
695 |
36 |
360 |
|
Heart |
806 |
396 |
3 |
15 |
|
Lung |
627 |
342 |
2 |
60 |
|
Muscle |
798 |
310 |
2 |
60 |
|
Brain |
1 377 |
327 |
5 |
15 |
|
Blood |
332 |
384 |
1 |
15 |
Cmax = maximum concentration; Tmax = time at which the maximum concentration is reached
Volunteer studies show that the major part (98–99%) of absorbed tetrachloroethene is excreted unchanged in exhaled air, regardless of exposure route (Stewart et al., 1961b, 1970; Stewart & Dodd, 1964; Guberan & Fernandez, 1974; Fernandez et al., 1976; Hake & Stewart, 1977; Riihimäki & Pfäffli, 1978; Monster, 1979; Monster et al., 1979; Benoit et al., 1985; Pezzagno et al., 1988). The major metabolite consistently detected in human blood and human urine is trichloroacetic acid (Ikeda et al., 1972; Fernandez et al., 1976; Ikeda, 1977; Monster et al., 1979, 1983; Ziglio et al., 1985; Monster, 1986; Skender et al., 1991; Popp et al., 1992). However, even trichloroacetic acid is formed only in small quantities, For example, exposure of dry cleaners and textile processing workers to tetrachloroethene at 340 mg/m3 for 8 h resulted in the excretion of less than 2% of the compound as urinary trichloroacetic acid, and saturation of biotransformation occurred upon respiratory exposure to 700 mg/m3 (Ohtsuki et al., 1983). Trichloroacetic acid could not be found in the urine of laundry workers exposed to 61–260 mg/m3, whereas tetrachloroethene could be detected in expired air (1.4–69 mg/m3) and blood (0.4–3.1 mg/l) 30 min after work (Lauwerys et al. 1983). Tetrachloroethene concentrations in blood and trichloroacetic acid concentrations in urine were determined (primarily over the course of a week) for 29 persons (16 females, 13 males, aged 6–76 years) living in the vicinity of nine dry cleaning shops and for 12 workers at the same shops in Germany. The urinary concentration of tetrachloroethene exceeded the German biological threshold limit value for workers (1 mg/l blood) in 2 of 29 residents (over the whole week in one case). The concentrations of tetrachloroethene in blood depended on the floor and the construction type of the building where these people lived, rather than on the type of system used in the dry cleaning shops. Five of the 12 dry cleaners were also found to have tetrachloroethene blood levels exceeding the 1 mg/l blood German biological threshold limit value, some by a considerable amount (Popp et al., 1992).
In addition to trichloroacetic acid, trichloroethanol has been reported in the urine of humans exposed to tetrachloroethene, but it seems probable that this trichloroethanol arises from the metabolism of trichloroethene (either due to concurrent occupational exposure to tetrachloroethene and trichloroethene or due to the common presence of trichloroethene as an impurity in tetrachloroethene, rather than from metabolism of tetrachloroethene). Ikeda et al. (1972) found trichloroacetic acid and trichloroethanol in similar amounts in the urine of workers exposed to tetrachloroethene at 140–480 mg/m3, while an increase in exposure to 1400–2800 mg/m3 resulted in a trichloroethanol/trichloroacetic acid ratio of 2:3. Others (Monster et al., 1983; Monster, 1986) found trichloroacetic acid in the blood and trichloroacetic acid and trichloroethanol in the urine of tetrachloroethene-exposed workers. Even rather low concentrations of trichloroethene in tetrachloroethene might lead to detectable trichloroethanol concentrations in urine, as trichloroethene is metabolized to a much greater extent than tetrachloroethene (75% vs 2%; Monster, 1979). Meuling & Ebens (1986) explained the presence of trichloroethanol in urine of laundry workers as due to trichloroethene as an impurity in tetrachloroethene, from the estimated exposure to this impurity and literature data on the formation rate of trichloroethanol from trichloroethene (Meuling & Ebens, 1986). In a study of metabolites of plasma and urinary trichloroacetic acid and trichloroethanol in 141 blood donors in Milan, Italy, exposed to trichloroethene and tetrachloroethene through measured environmental levels in drinking-water and air, the plasma levels of trichloroacetic acid (a common metabolite of both trichloroethene and tetrachloroethene) were in the range of previously found concentrations, while there was some doubt as to the degree of the contribution of trichloroethene as a metabolite of tetrachloroethene (Ziglio et al., 1985). No trichloroethanol was found in volunteers who inhaled 340 mg/m3 of pure tetrachloroethene vapour for 6 h (Berode et al., 1990).
An enhanced excretion of thioethers in occupationally exposed women might have arisen from conjugation between glutathione and an epoxide formed from tetrachloroethene. However, exposure to other compounds that give rise to an increase of thioethers could not be excluded (LaFuente & Mallol, 1986). GC/MS analysis of urine samples at the start and the end of the working week of four dry cleaning workers occupationally exposed to tetrachloroethene at 340 mg/m3 (two for 4 h/day, two for 8 h/day) identified trichloroacetic acid, trichloroethanol, and N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine. The concentrations of the latter compound were found to be more than 1000 times lower than those of the first two (2.2–14.6 pmol/mg creatinine vs 13.5–65 nmol/mg creatinine). Whereas the concentrations of trichloroacetic acid and trichloroethanol increased during the work week, those of N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine did not change. However, a clear increase was found for the concentrations of the latter compound when the daily working time increased from 4 to 8 h. Trichloroacetic acid and trichloroethanol were present in the urine of two of the exposed individuals. In two other individuals, only trichloroethanol was identified as a major urinary metabolite. The excretion of N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine in humans (although lower when compared with rats) indicates that glutathione-dependent bioactivation reactions are operative in humans and may be involved in the slight nephrotoxicity observed after occupational tetrachloroethene exposure (Birner et al., 1996).
Laboratory animals also eliminate most of the absorbed tetrachloroethene unchanged, although the amount metabolized is species dependent. As shown by the degree of binding of radioactive metabolites to liver protein, tetrachloroethene metabolism is much more extensive in mice than in other species studied (Schumann et al., 1980). A 6-h inhalation exposure at 70 mg/m3 led to the metabolism of 10.5 µmol/kg liver protein in rats compared with 89.5 µmol/kg liver protein in mice (Stott & Watanabe, 1982). As in humans, the major metabolite in laboratory animals is trichloroacetic acid. Several other minor metabolites have been found, including oxalic acid, dichloroacetic acid, ethylene glycol, trichloroacetyl amide, trichloroacetylaminoethanol, thioethers, and carbon dioxide. Further details are given in the source document (de Raat, 2003).
Volkel et al. (1998) described the dose-dependent excretion of trichloroacetic acid, dichloroacetic acid, and N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine in rats and humans after inhalation of tetrachloroethene. Six volunteers (three females aged 25–38 years and three males aged 38–72 years) were exposed to tetrachloroethene at 69, 140, and 280 mg/m3 for 6 h in a dynamic exposure chamber. Wistar rats (three per sex) were exposed to tetrachloroethene at up to 2800 mg/m3 in the chamber for 6 h. Male rats excreted more trichloroacetic acid and more N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine (103.7 nmol at 2800 mg/m3) than did female rats (31.5 nmol at 2800 mg/m3). N-Acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine was rapidly eliminated in urine by humans (half-life = 14.1 h) and rats (half-life = 7.5 h). Based on the urinary excretion of N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine, a potential substrate for the formation of reactive intermediates in the kidney, humans generated a significantly lower dose (3 nmol/kg body weight at 280 mg/m3) compared with rats (23 nmol/kg body weight) under identical exposure conditions. Additionally, rats excreted large amounts of dichloroacetic acid, which is presumably the product of beta-lyase-dependent metabolism of S-(1,2,2-trichlorovinyl)-L-cysteine in the kidney. Dichloroacetic acid was not detected in human urine. These data suggest that glutathione conjugate formation and beta-lyase-dependent activation of S-(1,2,2-trichlorovinyl)-L-cysteine in tetrachloroethene metabolism are significantly higher in rats than in humans (Volkel et al., 1998).
Two biotransformation pathways operate. The main pathway (Figure 2) is oxidative and occurs in the liver, the first step being epoxidation by cytochrome P450 to tetrachloro-oxirane, resulting in trichloroacetic acid as the major metabolite (Yllner, 1961; Costa & Ivanetich, 1980, 1984). Biotransformation via this pathway occurs mainly in the liver, which is the main target organ for tetrachloroethene’s toxicity and carcinogenicity. At higher exposures, a second pathway (Figure 3) operates in the liver (Dekant et al., 1985, 1986a,b; Odum & Green, 1986; Green, 1990; Green et al., 1990), the first step being the conjugation of tetrachloroethene with glutathione. This reaction is catalysed by glutathione transferase and leads eventually to S-(1,2,2-trichlorovinyl)-L-cysteine, which can be cleaved in the kidneys by beta-lyase into cytotoxic and genotoxic metabolites (Green & Odum, 1985; Dekant et al., 1986a,b, 1988; Anders, 1990; ECETOC, 1990; Green et al., 1990). Although quantitatively a minor pathway (Green, 1990; Green et al., 1990), it is important, as it offers a possible explanation for the kidney tumours in male rats.
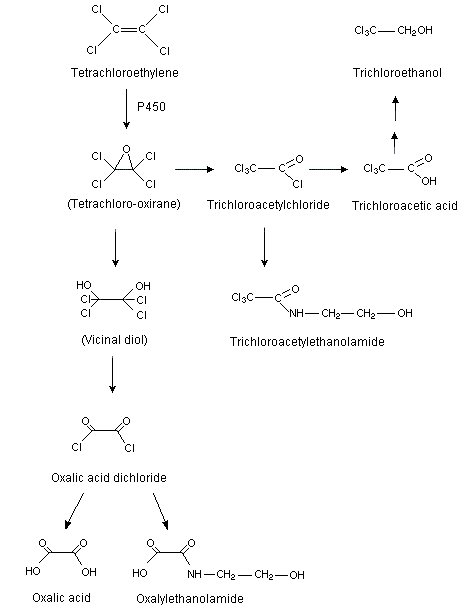
Figure 2: Oxidative biotransformation pathway of tetrachloroethene (de Raat, 2003).
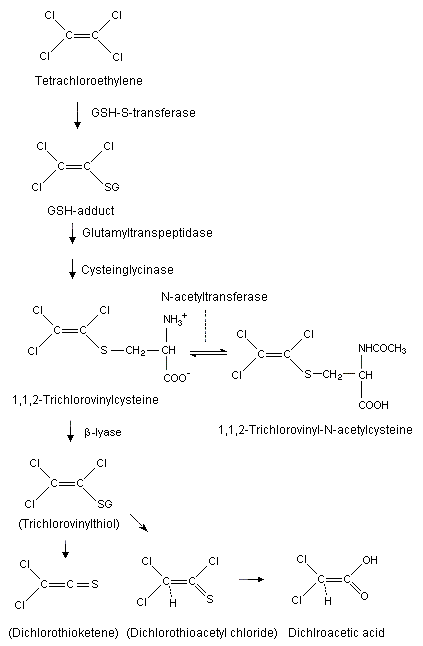
Figure 3: Conjugative biotransformation pathway of tetrachloroethene (de Raat, 2003).
Note that 1,2,2-trichlorovinylcysteine and 1,2,2-trichlorovinyl-N-acetylcysteine were
named as 1,1,2- compounds in this source document.
Glutathione-S-transferase-mediated formation of S-(1,2,2-trichlorovinyl)glutathione is the initial step in a sequence of reactions finally resulting in the formation of reactive intermediates in the rodent kidney. The enzymatic rates of formation of S-(1,2,2-trichlorovinyl)glutathione in liver and kidney subcellular fractions from rats, mice, and both sexes of humans (n = 11) have been compared. In microsomal fractions from the liver and kidney of all three species, enzymatic formation of S-(1,2,2-trichlorovinyl)glutathione from tetrachloroethene could not be observed. Additionally, the ability of subcellular fractions in the human liver to catalyse the formation of S-(1,2,2-trichlorovinyl)glutathione from tetrachloroethene is at least 2 orders of magnitude lower than that of rat liver. Sex-specific differences in the extent of hepatic conjugation of tetrachloroethene with glutathione, which may be relevant for nephrotoxicity, occur in rats (Dekant et al., 1998).
Pahler et al. (1998) described the generation of antibodies to di- and trichloroacetylated proteins and immunochemical detection of protein adducts in male Wistar rats treated with tetrachloroethene. The antibodies were successfully applied to detect modified proteins in subcellular fractions of liver and kidney from tetrachloroethene-treated rats, demonstrated by immunoblotting. Protein adduct formation from different tetrachloroethene metabolism pathways was confirmed by the observation that the majority of dichloroacetylated proteins were located in kidney mitochondria and trichloroacetylated proteins were located in liver microsomes.
Tetrachloroethene is primarily and slowly eliminated unchanged via exhaled air, irrespective of exposure route. Laboratory animal studies indicate that a fraction will be exhaled as carbon dioxide. Minor fractions will also be excreted in the urine as trichloroacetic acid and possibly trichloroethanol, oxalic acid, and mercapturic acid. In mice, a relatively large proportion of absorbed tetrachloroethene is eliminated as trichloroacetic acid, as a result of the more rapid oxidative metabolism in this species compared with other laboratory species and with humans (Schumann et al., 1980).
A number of studies that provide quantitative information about the elimination of tetrachloroethene in humans are summarized in a source document (de Raat, 2003). These (Fernandez et al., 1976; Monster & Houtkooper, 1979; Monster et al., 1979; Wallace et al., 1983; Walles, 1986) clearly show that the contribution of metabolites to the respiratory exhalation is small and insignificant. This elimination pattern is a result of the slow metabolism of tetrachloroethene, together with its air/blood and blood/fat partition coefficients (see Table 4) (Monster, 1979; Gearhart et al., 1993) and the poor perfusion of the adipose tissue (Monster, 1986). Generally, two elimination phases are discerned for solvents such as tetrachloroethene (Berlin et al., 1980). The first is a relatively rapid phase, which represents the clearance of the tetrachloroethene already present in the blood at the moment the exposure terminates. The rate-limiting factor of this phase is the air/blood partition coefficient. About 40–70% of absorbed tetrachloroethene is excreted unchanged in the first 24 h. When the tetrachloroethene already present in the blood is depleted, the blood/fat partition coefficient becomes the rate-limiting factor for the second phase, because it is much lower than the air/blood partition coefficient. Half-lives for respiratory elimination range from 1 to 72 h (de Raat, 2003).
The slow metabolism of tetrachloroethene allows absorption by the fat tissues before significant biotransformation to more polar components can occur. Furthermore, being much slower than the elimination of tetrachloroethene in the blood via the lungs, biotransformation does not contribute to any great extent to the elimination of the compound in the blood. Finally, the poor perfusion of the adipose tissue is assumed to be important in this context, slow elimination of tetrachloroethene from this tissue adding to the slow elimination in general (Monster, 1986). Monster et al. (1979) discerned a phase between the two mentioned here above. Analysis of their results led them to estimate a separate half-life for the muscles, between the relatively short one for the vessel-rich compartment of the body and the long one for the poorly perfused adipose tissue.
Several studies have investigated the feasibility of biological monitoring for tetrachloroethene, based on its concentration in exhaled air or blood and the concentration of trichloroacetic acid in blood or urine (Imamura & Ikeda, 1973; Guberan & Fernandez, 1974; Monster & Houtkooper, 1979; Lauwerys et al., 1983; Monster et al., 1983; Monster, 1986; Jang et al., 1993). The available data indicate that tetrachloroethene concentrations in blood and exhaled air give a reliable impression of the TWA exposure over the previous several days. Trichloroacetic acid is a less suitable indicator, as it is also the major metabolite of trichloroethene and trichloroethane, which are often present as impurities in tetrachloroethene or can be used in the same room as tetrachloroethene. Furthermore, the use of trichloroacetic acid may be hampered by interindividual differences in metabolism (de Raat, 2003).
The reliability of several biological indices for monitoring low atmospheric tetrachloroethene exposures was recently investigated in a group of 26 dry cleaners (16 men and 10 women) in seven small shops in urban Modena, Italy. Individual exposure was evaluated by personal air monitoring in the breathing zone for a complete work shift. The median concentration in air was 19.4 mg/m3 (mean 44.2 mg/m3; range 5.6–225 mg/m3). Biological monitoring was carried out in the last work shift of a working week. Alveolar air and blood were sampled at the end of this shift, and urine samples were collected during the afternoon of the last shift. Exposure (tetrachloroethene in air) was closely correlated with tetrachloroethene in alveolar air (r = 0.808) and tetrachloroethene in blood (r = 0.938; median blood level 335.5 µg/l), and less so with tetrachloroethene in urine (r = 0.667) (Gobba et al., 2003). The examined biological indices proved sensitive enough for biological monitoring of low exposure to tetrachloroethene and can give substantially similar information in terms of exposure evaluation. Most regressions were not truly linear throughout the whole range of air concentrations and cannot be extrapolated to substantially lower air concentration levels without corrections.
PBPK models for tetrachloroethene have been increasingly utilized for determining the influence of changes in specific parameters or physiological functions for interspecies extrapolations when data from humans are lacking (Hattis et al., 1990, 1993; Dallas et al., 1994a,b,c, 1995; Bois et al., 1996; Reitz et al., 1996; Jang & Droz, 1997; Lash & Parker, 2001). Jang & Droz (1997) measured metabolites of tetrachloroethene in exhaled air, venous blood, and urine in six male Caucasians and six male Orientals. Observations were compared with predictions, and the models were modified based on ethnic differences in physiological parameters. Differences as high as 20% were often found in the physiological parameters, including differences in average body weight, tissue volumes, and blood flows. Asians exhibited significantly lower peak trichloroacetic acid concentrations and AUC values in urine but higher tetrachloroethene concentrations in expired breath and blood than Caucasians (considered consistent with a slower rate of metabolism in Asians), and there were distributional differences between the two ethnic groups. The magnitude of the ethnic differences observed in this study was relatively small, and factors such as differences in body size and other physiological parameters, including differences in various enzyme systems in biotransformation, may all be contributing factors.
In vivo experiments in male F344 rats and B6C3F1 mice and in vitro studies in rats, mice, and humans have been used by Reitz et al. (1996) to develop and validate a "second-generation" PBPK model for tetrachloroethene. It was established that the relative ability to metabolize tetrachloroethene at low non-saturating concentrations is much higher in the mouse than in the rat on a per gram of liver basis; among the samples of human liver investigated, none had higher activity than the rat, and several had activities below the limit of detection.
Hattis et al. (1993) described a number of uncertainties in pharmacokinetic modelling utilizing 10 different human PBPK models for tetrachloroethene with data on absorption via inhalation and concentrations in alveolar air and venous blood. The most salient finding is that essentially all of the models show a time pattern of departures of predictions of air and blood levels related to experimental data that might be corrected by more sophisticated model structures incorporating either a) heterogeneity of the fat compartment with respect to either perfusion or partition coefficients or both or b) diffusion of tetrachloroethene between fat and muscle.
Bois et al. (1996) employed recent tools from population pharmacokinetics and Bayesian statistical inference to model the distribution and metabolism of tetrachloroethene in humans, deriving statistical distributions for the parameters of a physiological model for tetrachloroethene on the basis of data from Monster et al. (1979). An estimate of the relationship between tetrachloroethene exposure and fraction metabolized was obtained. The median population estimate for the fraction of inhaled tetrachloroethene that is metabolized at exposure levels exceeding current occupational standards is 1.5% (95% CI 0.52–4.1%). At levels approaching ambient inhalation exposure (0.007 mg/m3), the median estimate of the fraction metabolized is much higher, at 36% (95% CI 15–58%).
Additional detailed PBPK models for tetrachloroethene have been developed by Dallas et al. (1994a,b,c, 1995), focusing on partition coefficients and tissue distribution (Dallas et al., 1994b), tissue concentration–time data (Dallas et al., 1994c), prediction of systemic uptake and respiratory elimination (Dallas et al., 1994a), and predictions of differences due to species, dose, and exposure route (Dallas et al., 1995).
Acute inhalation toxicity in rats and mice is low. In rats, 6-h and 8-h LC50 values of 28 000 mg/m3 (Bonnet et al., 1980) and 35 000 mg/m3 (Pozzani et al., 1959), respectively, have been reported. In mice, reported 2-h, 4-h, and 6-h LC50 values were 40 000 mg/m3 (Friberg et al., 1953), 36 000 mg/m3 (Friberg et al., 1953), and 21 000 mg/m3 (Duprat & Bonnet, 1979), respectively. In NTP studies, rats and mice (five per sex per species) survived a 4-h exposure at 16 850 mg/m3, but deaths occurred at higher exposures (about 26 000 mg/m3 in rats and 18 000 mg/m3 in mice) (NTP, 1986).
The most notable effects of acute inhalation exposure are neurobehavioural and indicative of CNS depression (anaesthesia, hyperactivity and hypoactivity, hypotonia, loss of reflexes, drowsiness, trembling, ataxia, and stupor) (Pozzani et al., 1959; Bonnet et al., 1980; HSE, 1987; ATSDR, 1997). In NTP studies, rats and mice showed hypoactivity, ataxia, and anaesthesia when exposed for 4 h at about 16 000 mg/m3 and above (NTP, 1986). In one study, decreased motor activity was seen in mice exposed for 1 h at 600 mg/m3, the lowest concentration tested (Koppel et al., 1985). In another study, a 1-h exposure to 620 mg/m3 and above induced a concentration-dependent increase in motor activity in male mice during exposure, at all exposure levels; activity returned to normal levels several hours after exposure ceased (Kjellstrand et al., 1985a).
A weak effect on epinephrine-induced cardiac arrhythmias was reported in rabbits exposed at 36 000 mg/m3 for 1 h (Carlson, 1983). No such effect was seen in dogs exposed at up to 70 000 mg/m3 for 10 min (Reinhardt et al., 1973).
Liver toxicity (tissue changes and dysfunction) has been reported in rats and mice exposed by acute inhalation. Marginal increases in serum enzyme levels (AST, ALT, glucose-6-phosphatase, and ornithine carbamyl transferase) were seen when rats were exposed to tetrachloroethene at 3400 mg/m3 for 1 h. Marked increases were seen at 6900 mg/m3 (Drew et al., 1978). Cloudy swelling and diffusely distributed fat globules were found in the rat liver following exposure at a near-lethal concentration (Rowe et al., 1952). In mice, a 4-h exposure at 2800 mg/m3 and above produced dose-dependent increases in fatty infiltration and extractable fat. No effects were seen at 1400 mg/m3 (Kylin et al., 1963). Decreased liver ATP and marked, persistent increases in liver lipids and triglycerides resulted in mice exposed for 3 h at 5500 mg/m3 (Ogata et al., 1968) or for 4 h at 7400 mg/m3 (Ikeda et al., 1969). Following acute exposure at lethal concentrations, the mouse liver showed cloudy swelling, anisokaryosis, and Kupffer cell infiltration (Rowe et al., 1952).
Slight and irregular scattered necrotic and degenerative lesions were observed in the kidneys of mice exposed at 20 500 mg/m3 for 6 h (Gradiski et al., 1978; Duprat & Bonnet, 1979).
When mice were exposed to tetrachloroethene at 170 mg/m3 for 3 h, mortality from streptococcal pneumonia was increased and pulmonary bactericidal activity in response to inhaled Klebsiella pneumoniae was decreased, indicating possible immunotoxicity (Aranyi et al., 1986).
LD50 values in rats and mice range from 2.4 to 4.5 g/kg body weight (Pozzani et al., 1959; Withey & Hall, 1975; Hayes et al., 1986) and from 4.7 to 9.6 g/kg body weight (Dybing & Dybing, 1946; Wenzel & Gibson, 1951; Klaassen & Plaa, 1966), respectively, indicating a low acute toxicity by the oral route.
Although data are limited, there are indications that acute oral administration of tetrachloroethene can produce neurotoxicity and effects on the liver, kidneys, and spleen. In rats, a dose of 500 mg/kg body weight induced increases in liver weight and liver GGT activity. At this dose level, irreversible binding (not described further) was observed in the liver of rats and mice. Doses ranging from about 100 to 1200 mg/kg body weight were given to dogs, cats, foxes, cows, horses, and sheep. Reported effects (in one or more species) included ataxia, drowsiness, CNS depression, depression of heart rate, inflammation of the small intestines, fatty infiltration and haemosiderosis of the spleen, cell swelling, infiltration, cloudy swelling, and necrosis in the liver, and fatty infiltration, cloudy swelling, hyaline casts, atrophy, and vacuolization in the kidneys (Schlingman & Gruhzit, 1927; Christensen & Lynch, 1933; Carpenter, 1937; USEPA, 1986; HSE, 1987; ATSDR, 1997). Immunohistochemical staining techniques demonstrated the existence of trichloroacetylated protein adducts in the liver of mice (female MRL-lpr/lpr and MRL +/+ strains) given 830 mg/kg body weight by gavage. Adducts were localized to the centrilobular zones, where toxicity due to tetrachloroethene occurs (Green et al., 2001). In adult male SD rats gavaged with 480 mg/kg body weight, immediate testing of operant performance showed a transient rate decrease, recovering after 20–40 min, in three of six animals and a complete cessation of response in two of the six animals. No effect was seen at 160 mg/kg body weight. Tetrachloroethene concentrations increased rapidly after administration in blood, brain, fat, liver, and muscle. For the duration of the 90-min period of testing, blood tetrachloroethene levels were approximately linearly related to the administered dose, but brain tetrachloroethene levels were similar for both dose groups (Warren et al., 1996).
When pretreated with Aroclor-1254 (an inducer of mixed-function oxidases), urinary excretion of trichlorinated compounds by rats given tetrachloroethene at 0.75 ml/kg body weight was increased 5- to 7-fold. Aroclor-1254 pretreatment also led to an increase in serum AST, vacuolar degeneration, and necrosis. The study thus suggests that tetrachloroethene metabolites are the ultimate hepatotoxic agents. Pretreatment with another inducer (phenobarbitone) led to a similar increase in metabolism (based on urinary excretion of trichlorinated compounds) but did not increase hepatotoxicity (Moslen et al., 1977).
Covered 24-h application of undiluted tetrachloroethene to the skin of rabbits at 1.3, 2.5, 5, 10, or 20 g/kg body weight resulted in the deaths of 0/4, 1/4, 1/4, 1/4, and 2/4, respectively. Convulsions were noted at 20 g/kg body weight. The results suggest a low acute dermal toxicity (Wolf, 1956).
In an NTP study, no overt toxicity or microscopic effects on a comprehensive range of tissues were seen in F344 rats and B6C3F1 mice (groups of five per sex per species) exposed at 0, 690, 1400, or 2900 mg/m3 for 6 h/day, 5 days/week, for 2 weeks. At 6000 mg/m3, the mice showed fatty cytoplasmic hepatocyte vacuolation. At 12 000 mg/m3, there were deaths in both species, preceded by dyspnoea, hypoactivity, anaesthesia, and ataxia (NTP, 1986).
In a poorly reported study, no adverse effects were seen in seven guinea-pigs exposed (7 h) at 700 mg/m3 on 13 occasions over 17 days. At 1400 mg/m3 for 7 h/day on 14 occasions over 18 days, guinea-pigs showed depressed growth, increased liver weight, and slight fatty liver tissue degeneration. Overt behavioural effects, loss of body weight, and enlarged liver and kidneys (without tissue damage) were found in rats exposed at 11 000 mg/m3 for 7 h/day on 18 occasions over 25 days. Fatty degeneration of the liver was seen in guinea-pigs exposed at this concentration daily for 8 days. Severe CNS depression was observed in rats, rabbits, and guinea-pigs exposed at 17 000 mg/m3 for 7 h/day on 13–28 occasions over 18–39 days. All three species showed liver tissue changes, and cloudy swelling was additionally observed in the kidneys of guinea-pigs (Rowe et al., 1952).
In NTP studies, B6C3F1 mice and F344 rats (groups of 10 per sex per species) were exposed at about 0, 690, 1400, 2800, 5500, or 11 000 mg/m3 for 6 h/day, 5 days/ week, for 13 weeks. No overt signs of neurotoxicity were reported in the rats at any concentration or in mice at up to 1400 mg/m3. At 2800 mg/m3, mice were hunched and lacked movement; those in the 5500 mg/m3 group panted and appeared irritated; lack of coordination and loss of consciousness were seen at the highest exposure level (NTP, 1986).
A comprehensive neurotoxicological examination was carried out on 16-week-old F344 rats (12 per sex per group) exposed at 0, 340, 1400, or 5500 mg/m3 for 6 h/day, 5 days/week, for 13 weeks. Animals were monitored throughout the study for overt signs of neurotoxicity and subjected monthly to "expanded clinical observation", based on the USEPA functional observational battery. Grip performance was tested monthly. During week 14, an electrophysiological test battery was conducted, incorporating flash evoked potential, auditory brain stem response to clicks and to tone pips, somatosensory evoked potentials, caudal nerve action potentials, and rectal temperature. Waveforms were visually analysed. Comprehensive neuropathological examination of the brain, optic nerve, spinal cord and nerve roots, dorsal root ganglia, peripheral nerves, and skeletal muscles was carried out on five rats per sex at the highest exposure level. The only treatment-related effect was a subtle change (greater amplitude of the longer latency potential) in the flash evoked potential recorded for the visual cortex at 5500 mg/m3. The study NOAEC was 1400 mg/m3 (Mattsson et al., 1998).
In open-field tests, behavioural alteration (increased ambulation) was observed in male rats exposed by inhalation to tetrachloroethene of unspecified purity at 1400 mg/m3 for 6 h/day for 4 days. Ambulation was significantly increased 1 h, but not 17 h, after the last exposure. Biochemical changes in the brain following several additional exposures were reduced RNA content and increased nonspecific cholinesterase content. There was no histological examination of brain tissue, so these findings could not be correlated with brain structural damage (Savolainen et al., 1977).
The overt neurological effects of tetrachloroethene have prompted a series of investigations in rats, guinea-pigs, and gerbils (summarized in Table 6, reproduced from de Raat, 2003) into possible biochemical changes caused by this compound in the brain. Taken together, these suggest that long-term respiratory exposure to tetrachloroethene might lead to structural changes in the brain as a loss of brain cells (possibly glial cells), as partly reversible changes in the composition of cerebral membranes, and as interference in the metabolism of the structural proteins of brain cells. The observed biochemical changes were small, and their possible relationship with functional changes is unknown (de Raat, 2003).
Table 6: Neurochemical effects of respiratory exposure to tetrachloroethene.
|
Species |
Concentration (mg/m3) |
Exposure regimen |
Results |
Comments and details |
References |
|
Rat |
1400, 2800, and 5500 |
Continuous for 1 month |
Marked dose-related decrease of acetylcholine in the striatum. Slight, but not significant, changes observed in dopamine in the striatum, norepinephrine in the hypothalamus, and serotonin in the cortex and hippocampus. |
Honma et al. (1980a) |
|
|
Rat |
1400, 2800, and 5500 |
Continuous for 1 month |
Marked and dose-related increase of glutamine, threonine, and serine, while GABA decreased. |
Honma et al. (1980b) |
|
|
Mongolian gerbila |
830 |
Continuous for 12 months |
Small changes of fatty acid pattern of phospholipids. Decrease of long-chain linolenic acid–derived 22-carbon fatty acids. No changes in content/concentrations of protein, lipid phosphorus, or cholesterol in hippocampus and cerebral cortex. Decreased taurine content in hippocampus and cerebellum. Elevated glutamine in hippocampus. GABA levels, GABA uptake, and glutamine uptake normal. |
It is concluded that small changes are induced in membrane fatty acids at doses well below those causing anaesthesia. |
Kyrklund et al. (1984); Briving et al. (1986) |
|
Mongolian gerbil |
410 and 2200 |
Continuous for 3 months, followed by 4 months without exposure |
Decreased brain weight at 2200 mg/m3. Slightly increased concentrations of the astroglial protein S100 in hippocampus, cerebral occipital cortex, and cerebellum. S100 as well as DNA concentrations were decreased in the frontal cerebral cortex. Reduced DNA concentration in frontal cortex was seen at the lowest concentration (410 mg/m3). |
The results point to astroglial hypertrophy in hippocampus, cerebral occipital cortex, and cerebellum and atrophy, affecting the astroglial cells in the frontal cerebral cortex. |
Rosengren et al. (1986) |
|
Mongolian gerbil |
410 |
Continuous for 3 months, followed by 4 months without exposure |
Slight decrease of DNA concentration in frontal cerebral cortex. |
Indications of loss of neuronal/glial cells in the frontal cortex. The effect is not caused by metabolites but by tetrachloroethene itself. |
Karlsson et al. (1987) |
|
Mongolian gerbil |
2200 |
Continuous for 3 months |
Minor decrease of brain weight. Shift in fatty acids of ethanolamine phospholipids towards less saturated forms. |
Indicates slight changes in composition of cerebral membranes. Confirms earlier findings. |
Kyrklund et al. (1987) |
|
Rat |
2200 |
Continuous for 30 days |
Slight reduction of cholesterol and phospholipids in the brain. Shift in fatty acid composition of the brain. No such effects were observed for Freon and 1,1,1-trichloroethane. |
Indicates slight changes in composition of cerebral membranes. The effect is specific for tetrachloroethene. |
Kyrklund et al. (1988) |
|
Rat, guinea-pig (30 days pregnant), and Mongolian gerbil |
2200 and 1100 (guinea-pigs) |
Continuous for 30 days |
"Tendency towards decreased brain weight". Shift in fatty acid composition of the brain. No increased sensitivity during second half of gestation. |
The absence of an increased sensitivity during gestation might indicate (according to the authors) that membranes are not particularly sensitive to the effects of tetrachloroethene during their synthesis. |
Kyrklund & Haglid (1990) |
|
Rat |
2200 |
Continuous for 90 days, followed by a recovery of 30 days |
Slight shifts in fatty acid composition of brain phospholipids. Most changes normalized during post-exposure period. Slight persistent changes in brain cholesterol content. |
Indicates slight, partly reversible changes in composition of cerebral membranes. |
Kyrklund et al. (1990) |
|
Rat |
2100 and 4100 |
Continuous for 4 or 12 weeks |
Slower increase in brain weight at 4100 mg/m3 after 4 and 12 weeks. At highest dose after 12 weeks, decrease in DNA, total protein, and brain region weights in frontal cerebral cortex and brain stem, but not in hippocampus. Glial and neuronal cytoskeletal proteins decreased in frontal cortex at the highest dose. Glial proteins were decreased in frontal cortex, brain stem, and hippocampus (the three brain regions investigated) at the highest dose. No effects were found on neuronal enolase. NOEC was 2100 mg/m3. |
Indications for reduction in the number of brain cells, possibly glial cells, and interference with the metabolism of cytoskeletal elements in both glial and neuronal cells. |
Wang et al. (1993) |
a Meriones unguiculatus.
B6C3F1 mice and F344 rats (groups of 10 per sex per species) were exposed at about 0, 690, 1400, 2800, 5500, or 11 000 mg/m3 for 6 h/day, 5 days/week, for 13 weeks in an NTP study. No liver toxicity was seen at 690 mg/m3 in the mice (the rat liver was not examined at this exposure level). Rats exposed at 1400 mg/m3 and above showed only minimal to mild hepatic congestion. Mice exposed at 1400 mg/m3 had minimal mitotic changes, while at 2800 mg/m3 and above, there were minimal to mild hepatic leukocytic infiltration, centrilobular necrosis, and bile stasis (NTP, 1986).
A significant increase in relative liver weight was observed when mice were continuously exposed at 60 mg/m3 for 30 days; liver weight was doubled at 520 mg/m3. Liver cell hypertrophy and vacuolization were observed at 60 mg/m3, but the liver returned to normal when exposure ceased (Kjellstrand et al., 1984, 1985b). When mice were exposed at 1400 mg/m3 for 4 h/day, 6 days/week, for 8 weeks, massive, central infiltration of about 80% of the liver with fat was observed. Liver fat content doubled during the 1st week of exposure but did not increase any further afterwards. No cirrhosis or necrosis was reported (Kylin et al., 1965).
In another study, F344 rats and B6C3F1 mice were exposed at 1400 mg/m3, 6 h/day, for 28 days or at 2800 mg/m3, 6 h/day, for 14–28 days. Hepatic catalase activity was unaffected in both species. Peroxisome proliferation was not observed in rat liver or in the kidneys of either species, but the mouse liver showed lipid accumulation and peroxisome proliferation. Trichloroacetic acid was found to be a major metabolite, with peak blood levels in mice being 13 times higher than those observed in rats following a single 6-h exposure to tetrachloroethene at 1400 mg/m3. The difference in metabolism of tetrachloroethene to trichloroacetic acid in mice and rats leads to the species difference in hepatic peroxisome proliferation, which is believed to be the basis of the species difference in hepatocarcinogenicity (Odum et al., 1988).
In a poorly reported study, increased liver weight and a few small fatty vacuoles were seen in the liver when guinea-pigs were exposed at 700 mg/m3 for 7 h on 132 occasions over a 185-day period. Fatty liver degeneration occurred at 1400 mg/m3 (7-h exposures, 158 times over 220 days). No adverse effects were reported in rats (15 per sex), rabbits (2 per sex), or monkeys (2 per sex) given repeated 7-h exposures at 2800 mg/m3 for 7 h per exposure day. Total numbers of exposures were 130 in 183 days (rats), 159 in 222 days (rabbits), and 179 in 250 days (monkeys). Depressed growth and liver effects (increased weight, fatty degeneration, and slight cirrhosis) were seen in guinea-pigs exposed similarly on 169 days over a 236-day period (Rowe et al., 1952).
Increased serum levels of AST, ALT, and glutamic dehydrogenase, together with liver tissue damage, were seen in rabbits exposed at 19 000 mg/m3 for 4 h/day, 5 days/week, for 9 weeks (Mazza, 1972).
In an NTP study in which B6C3F1 mice and F344 rats (groups of 10 per sex per species) were exposed at 0 or about 690, 1400, 2800, 5500, or 11 000 mg/m3 for 6 h/day, 5 days/week, for 13 weeks, the mice showed minimal renal tubular karyomegaly at all concentrations except the lowest (incidences were 0/20, 0/20, 14/20, 20/20, 20/20, and 13/14 at 0, 690, 1400, 2800, 5500, and 11 000 mg/m3, respectively). No kidney lesions were seen in the rats (NTP, 1986).
Changes in concentrations of creatinine and para-aminohippuric acid in the urine of rabbits exposed at 16 000 mg/m3 for 4 h/day, 6 days/week, for 45 days led to the conclusion that tubular function was affected more than glomerular capacity (Brancaccio et al., 1971). The study has been criticized for the lack of reported detail (USEPA, 1986; HSE, 1987).
In another study, rats and mice were exposed at 1400 mg/m3, 6 h/day, for 28 days or at 2800 mg/m3, 6 h/day, for 14–28 days. No histopathological kidney changes were found. Slight indications of increased peroxisomal cyanide-insensitive palmitoyl CoA oxidation activity were obtained for female mice; catalase activity in the kidneys was unaffected in both species. The investigators concluded that peroxisome proliferation does not significantly contribute to the nephrotoxicity of tetrachloroethene, even in the relatively efficient trichloroacetic acid–producing mice (Odum et al., 1988).
No changes in kidney morphology, levels of alpha2u-globulin, or biochemical plasma and urinary markers of kidney toxicity were seen in male and female F344 rats exposed to tetrachloroethene at up to 5500 mg/m3 for 6 h/day for 28 days (Green, 1997). Male F344 rats exposed by inhalation at 2800 or 6900 mg/m3 for 28 days (in an investigation into the mechanism responsible for the induction of renal tumours in male rats) showed accumulation of protein droplets (alpha2u-globulin) in the P2 segment of the kidney proximal tubules at the higher concentration, but not at 2800 mg/m3 (Green et al., 1990).
Slight increases (not statistically significant) in adrenal hormones (both cortical and medullar) were seen in rabbits exposed at 15 000 mg/m3 for 1 h/day, 6 days/ week, for 15 days (Mazza & Brancaccio, 1971).
Plasma butyrylcholinesterase was approximately doubled in mice exposed continuously at 260 mg/m3 for 30 days. No effect was seen at 60 mg/m3 (Kjellstrand et al., 1984). A second study by the same scientists investigated whether this effect was caused by hepatotoxicity or had an endocrinological background. Mice were exposed continuously at 1000 mg/m3 for a month. The influence of castration and testosterone administration was also investigated. The results indicated that the effect on butyrylcholinesterase activity was not directly correlated with testosterone levels or with liver toxicity (Kjellstrand et al., 1985b). The significance of modified butyrylcholinesterase activity is unclear, because the biochemical/biological role of this enzyme is largely unknown. The correlation between butyrylcholinesterase activity and plasma acetylcholinesterase activity is poor (de Raat, 2003).
The effects of repeated tetrachloroethene exposure (930 mg/m3, 6 h/day, 5 days/week, for up to 7.5 weeks, or 1900 mg/m3 for up to 11.5 weeks, followed by an exposure-free period of 3 weeks) on a number of haematological parameters has been investigated in mice. In the peripheral blood, reductions of the lymphocyte, monocyte, and neutrophil counts were observed, followed by an almost complete regeneration during the exposure-free period. Reticulocytosis during and after exposure pointed to a compensatory reaction of the red blood cell system. No effects on the bone marrow pluripotent stem cells were seen. The number of erythroid-committed cells was suppressed, and slight indications for a disturbance of the granulocyte cell series were found (Seidel et al., 1992).
Liver cytochrome P450 concentrations were increased in rats given tetrachloroethene in the diet at a concentration of 25 mg/kg (about 1.3 mg/kg body weight per day) for 14 days (Kaemmerer et al., 1980).
No effects on histopathology and relative liver weight were seen in rats treated for 12 days by gavage with 500 mg/kg body weight per day (Schumann et al., 1982; Stott & Watanabe, 1982).
When Sprague-Dawley rats (20 per sex per group; 3–4 weeks old) were given tetrachloroethene in the drinking-water at nominal doses of 14, 400, or 1400 mg/kg body weight per day for 90 days (tetrachloroethene was present as emulsion droplets), there was no biochemical evidence of effects on the liver at the lowest dose level. A range of serum parameters (including LDH, ALT, AST, alkaline phosphatase, and BUN) were monitored. At 400 mg/kg body weight per day and above, there was an increase in serum 5'-nucleotidase activity (indicative of cholestasis). Liver weight (relative to body weight) was increased (figures not given) in both sexes at the top dose level, but liver weight relative to brain weight was unaffected. No gross liver tissue effects were seen; microscopic examination was not carried out (Hayes et al., 1986).
When tetrachloroethene dissolved in corn oil was administered by gavage to mice at 0, 20, 100, 200, 500, 1000, 1500, or 2000 mg/kg body weight per day, 5 days/week, for 6 weeks, there were no liver effects at 20 mg/kg body weight per day. At 100 mg/kg body weight per day and above, there was a dose-related increase of liver weight and accumulation of liver triglycerides. At 200 mg/kg body weight per day and higher doses, ALT was raised, and there was minimal to slight karyorrhexis and polyploidy and moderate degeneration (which increased in severity at higher doses). At 500 mg/kg body weight per day and above, liver glucose-6-phosphatase activity was reduced. The DNA content of the liver was normal at 200 mg/kg body weight per day but decreased by 17% at 1000 mg/kg body weight per day. The study included a detailed comparison of metabolism (determined as urinary excretion of trichloroacetic acid and trichloroethanol) and hepatotoxicity. Good linear relationships were found between urinary excretion of metabolites and ALT, serum glucose-6-phosphate, triglycerides, and liver weight, indicating that liver toxicity is caused by metabolites and not by tetrachloroethene itself (Buben & O’Flaherty, 1985).
Immunohistochemical staining techniques demonstrated the existence of trichloroacylated protein adducts in the liver of mice (female MRL-lpr/lpr and MRL +/+) given 830 mg/kg body weight, by gavage, every 4th day for 6 weeks. Adducts were localized to the centrilobular zones, where toxicity due to tetrachloroethene occurs (Green et al., 2001).
To compare the hepatotoxicity of tetrachloroethene in mice and rats, animals were given 100, 250, 500, or 1000 mg/kg body weight per day for 11 days by gavage. All doses resulted in histopathological changes in the liver of mice (described as "accentuated lobular pattern with hepatocellular swelling in the centrilobular region"), while in rats there were only marginal effects at the highest dose (described as "altered staining ability of the hepatocytes in the centrilobular region"). The DNA concentration in the liver was reduced, and DNA synthesis was increased in mice at all doses, but not in rats (Schumann et al., 1980).
Cyanide-insensitive palmitoyl CoA oxidation (a sensitive measure of peroxisome proliferation) was increased 4.3-fold and 2.3-fold in the liver and kidneys of mice, respectively, by gavage administration of tetrachloroethene at 1000 mg/kg body weight per day for 10 days. In rats treated similarly, the increases were smaller (1.4-fold in liver; 1.7-fold in kidney) and not statistically significant (Goldsworthy & Popp, 1987).
When Sprague-Dawley rats (20 per sex per group; 3–4 weeks old) were given tetrachloroethene in the drinking-water at nominal doses of 14, 400, or 1400 mg/kg body weight per day for 90 days (tetrachloroethene was present as emulsion droplets), there were no effects on the kidney at the lowest dose level and no serum biochemistry changes indicative of kidney dysfunction at any dose level. Relative to body weight, kidney weight was increased (figures not given) at 400 mg/kg body weight per day and above, but kidney weight relative to brain weight was unaffected. No gross effects on the kidney were seen; microscopic examination was not carried out (Hayes et al., 1986).
Daily gavage dosing of rats at 500 mg/kg body weight per day for 4 weeks, in corn oil, induced increases in albuminuria (small but significant in females, up to 15-fold in males), urinary excretion of alpha2u-globulin (transient in males, marked in females), retinol binding protein, and N-acetylglucosaminidase. The urine contained increased amounts of protein (of low and high molecular weight in females and males, respectively). On microscopic examination, the kidneys showed a progressive increase in the number and size of hyaline droplets in the proximal convoluted epithelial cells, which was much more severe in the males. The results were seen as indicative of selective toxicity to tubular segment S2 (Bergamaschi et al., 1992).
On gavage treatment of F344 rats (three per sex per group) at 1000 mg/kg body weight per day for 10 days, only the males showed increases in renal alpha2u-globulin, protein droplet accumulation, and cell replication in the cytoplasm of the P2 segment of the proximal tubule. Comparable effects were observed in rats treated with pentachloroethane, but not in those treated with trichloroethene (Goldsworthy et al., 1988).
Increased alpha2u-globulin hyaline droplet formation was seen in the kidneys of male F344 rats given 1 g/kg body weight per day for 7 days (Potter et al., 1996).
Male F344 rats were exposed to tetrachloroethene by oral gavage (1500 mg/kg body weight per day for up to 42 days) in an investigation into the mechanism responsible for the induction of renal tumours in male rats. High doses of tetrachloroethene given by gavage were toxic to the rat kidney, causing increases in urinary markers of kidney damage. A marked accumulation of protein droplets (alpha2u-globulin) was seen in the P2 segment of the kidney proximal tubules (Green et al., 1990).
The neurotoxic potential of tetrachloroethene has been tested in young (3–4 weeks old) male Sprague-Dawley rats. Tetrachloroethene was given by gavage to groups of nine rats at 0, 5, or 50 mg/kg body weight per day, 5 days/week, for 8 weeks, and behavioural tests were initiated 3 days after the final dose. Nociception (tail immersion, hot-plate, increasing temperature hot-plate), locomotor activity (open field), and seizure induction (pentylenetetrazol-induced) were examined. Clinical behaviour during the treatment period was normal, but body weight gain was reduced by 10% in treated groups. Slightly but significantly (P < 0.001) slower responses were seen in all three nociceptive tests at both doses, but no dose–response was seen. Locomotion and rearing activity were reduced at both doses, the changes being statistically significant at the high dose. Both dose levels increased the seizure thresholds for myoclonic twitch and forelimb clonus (Chen et al., 2002).
When groups of 12 male NMRI mice (from 3–4 litters) were given tetrachloroethene at 5 or 320 mg/kg body weight per day on postnatal days 10–16 by gavage, locomotion, rearing, and total activity (measures of spontaneous motor activity) were unaffected on day 17. When the mice were 60 days old, statistically significant (P < 0.01) increases in locomotion and total activity were seen at both dose levels; the sizes of the effects were remarkably similar in the high and low dose groups, reducing confidence in the findings. Rearing activity was unaffected at the low dose and reduced (the opposite of what might be predicted from locomotion scores) at the high dose. Habituation, as defined by decreased activity over 1 h, in response to diminished novelty of the test chambers was attenuated in the tetrachloroethene-treated groups. The results provide some suggestion of neurodevelopmental toxicity in young mice resulting in persistent alterations in behaviour (Fredriksson et al., 1993).
Following gavage dosing at 50–1500 mg/kg body weight per day for 14 days, female F344 rats showed no adverse effects in a battery of neurotoxicity tests. However, a single dose of 150 mg/kg body weight induced increased lacrimation, agitation, and abnormal gait, decreased coordination and motor activity, and resulted in a lower response to an auditory stimulus. The results suggest behavioural adaptation (Moser et al., 1995).
When Sprague-Dawley rats (20 per sex per group; 3–4 weeks old) were given tetrachloroethene in the drinking-water at nominal doses of 14, 400, or 1400 mg/kg body weight per day for 90 days (tetrachloroethene was present as emulsion droplets), the blood picture and urine composition were normal. At 400 mg/kg body weight per day and above, growth was reduced (Hayes et al., 1986).
Increased thrombin and prothrombin times (without change in thrombocyte number) were noted in rats fed tetrachloroethene at 25 mg/kg in the diet for 14 days (Kaemmerer et al., 1982).
A source document (de Raat, 2003) summarizes studies from one group apparently showing histopathological effects in the spleen of mice given tetrachloroethene in the drinking-water resulting in doses of 0.05 and 0.1 mg/kg body weight per day for 7 weeks; no effects were observed in other examined organs (brain, liver, and kidneys). In the spleen, the following changes were observed: pulpa cords rich in erythrocytes, many blood formation centres in the red pulpa with megakaryocytes, and haemosiderin storage in the macrophages of the red pulpa. The proposed mechanism was premature erythrocyte disintegration, caused by interaction between apolar tetrachloroethene and the erythrocyte membrane. The spleen macrophages remove the erythrocyte fragments from the bloodstream and are thus subject to haemosiderin deposition. The exposure led to a reported decrease in growth and an increase in relative spleen weight. Effects on the haematopoietic system were reflected by clear increases in LDH activity and peripheral blood count and by microscopic examination of the bone marrow. Serum proteins were unaffected, but there was a change in the proportions of lipoproteins (high density, very low density, and low density), as well as a decrease in cholesterol that was assumed by the authors to be due to inhibition of hydroxymethylglutaryl CoA reductase (Marth et al., 1985a,b; Marth, 1987). It seems highly unlikely that such low doses would reduce body weight, and much higher doses were tolerated in other, longer studies.
In a high-quality study, F344 rats (50 per sex per dose) were exposed to tetrachloroethene (99.9% purity) at 0, 1400, or 2800 mg/m3 for 6 h/day, 5 days/week, for 103 weeks. Survival was reduced in the high-dose males. Tetrachloroethene-exposed rats showed high incidences of karyomegaly and cytomegaly in the proximal convoluted tubules of the kidneys. Hyaline droplets were not observed. Treatment also induced thrombosis in the nasal cavity (both sexes; considered secondary to leukaemia) and squamous cell metaplasia of the nasal cavity (only in males), adrenal medullar hyperplasia (only in males), adrenal cortical hyperplasia (only in females), and forestomach ulcers (only in males). The males showed renal tubular cell hyperplasia and increased incidences of tubular cell adenoma and adenocarcinoma (1/49, 3/49, and 4/50 in the 0, 1400, and 2800 mg/m3 groups, respectively). Although these increases were not statistically significant, the tubular cell adenocarcinomas (two in the top dose group) had not been seen previously at the study laboratory or other laboratories carrying out NTP bioassays (in total, about 2250 male rats of this strain). There were significant increases in mononuclear cell leukaemia in both sexes (28/50, 37/50, and 37/50 in males and 18/50, 30/50, and 29/50 in females at 0, 1400, and 2800 mg/m3, respectively) (NTP, 1986). The spontaneous incidence of this cancer in F344 rats is high and variable, so there is some uncertainty over the significance of the findings (HSE, 1987; ECETOC, 1990). However, NTP’s Board of Scientific Counselors considered the incidence of rat leukaemia to be a valid finding, because of the shorter time to the onset of the disease and its greater severity in the treated animals compared with the control animals (NTP, 1986; ATSDR, 1997).
Also as part of the NTP programme, B6C3F1 mice (50 per sex per group) were exposed to tetrachloroethene (99.9% purity) at 0, 700, or 1400 mg/m3 for 6 h/day, 5 days/week, for 103 weeks. Tetrachloroethene reduced survival, induced liver degeneration (vacuolization, necrosis, inflammation, and regenerative foci) (both sexes), kidney casts, tubular cell karyomegaly (both sexes), nephrosis (in females only), and acute passive lung congestion (both sexes). Both sexes showed a statistically significant increase of hepatocellular carcinoma (see Table 7), as well as metastasis to other organs (NTP, 1986).
Table 7: Hepatocellular neoplasms in B6C3F1 mice exposed to tetrachloroethene via inhalation.a
|
Control |
700 mg/m3 |
1400 mg/m3 |
||||
|
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Adenoma |
12/49 (24%) |
3/48 (6%) |
8/49 (16%) |
6/50 (12%) |
19/50 (38%) |
2/50 (4%) |
|
Carcinoma |
7/49 (14%) |
1/48 (2%) |
25/49 (51%) |
13/50 (26%) |
26/50 (58%) |
36/50 (72%) |
|
Adenoma or carcinoma combined |
17/49 (35%) |
4/48 (8%) |
31/49 (63%) |
17/50 (34%) |
41/50 (82%) |
38/50 (76%) |
a From NTP (1986).
In a study carried out at the Japan Bioassay Research Centre, F344 rats (groups of 50 per sex) were exposed to tetrachloroethene (>99% purity) at about 0, 340, 1400, or 4100 mg/m3 for 6 h/day, 5 days/week, for 104 weeks. All organs were examined microscopically. There were treatment-related increases in mononuclear cell leukaemia in both sexes (11/50, 14/50, 22/50, and 27/50 in males, and 10/50, 17/50, 16/50, and 19/50 in females, at 0, 340, 1400, and 4100 mg/m3, respectively). No other increases in tumour incidence were reported. The liver and kidney were microscopically normal at 340 mg/m3. Liver toxicity was limited to the males (increased incidences of spongiosis at 1400 mg/m3 and above, and hyperplasia at 4100 mg/m3). In the kidney, nuclear enlargement was seen in the proximal tubules of males at 1400 mg/m3 and above, while at 4100 mg/m3, both sexes showed increases in nuclear enlargement and atypical proximal tubular dilation. Males were much more susceptible than females to liver and kidney toxicity. For example, the incidences of spongiosis and hyperplasia in the liver in the top dose group were 16/50 and 13/50 in males compared with 0/50 and 1/50 in females. The incidences of enlargement and atypical tubular dilation at the highest concentration were 48/50 and 24/50 in males compared with 18/50 and 6/50 in females (Nagano et al., 1998a,b).
In a corresponding study in mice, groups of 50 males and 50 females (BDF1 strain) were exposed to tetrachloroethene (>99% purity) at about 0, 69, 340, or 1700 mg/m3 for 6 h/day, 5 days/week, for 104 weeks. All organs were examined microscopically. Dose-related increases were seen in the incidences of benign and malignant liver tumours in both sexes (see Table 8). In addition, the top-dose males had an increased incidence of benign tumours of the Harderian gland (2/50, 2/50, 2/50, and 8/50). Kidney pathology was limited to nuclear enlargement of the proximal tubule (at 340 mg/m3 and above in both sexes) and atypical proximal tubular dilation at 1700 mg/m3 in the females. There was no evidence of liver toxicity in either sex at 69 mg/m3, but at 340 mg/m3 and above, dose-dependent liver toxicity was seen (angiectasis, central degeneration, central and focal necrosis, hyperplasia [males only], clear cell focus, and basophilic cell focus) (Nagano et al., 1998a,b).
A more limited study in another strain of rats has been published as an abstract only (Rampy et al., 1978), but unpublished details and results were reported in an expert review (USEPA, 1986). Sprague-Dawley rats (96 per sex per group in exposed groups; 192 per sex in control group) were exposed to tetrachloroethene at 0, 2100, or 4100 mg/m3 for 6 h/day, 5 days/week, for 1 year, followed by an observation period of 19 months. Mortality was slightly increased in the 4100 mg/m3 males and was attributed to "spontaneous advanced chronic renal disease". Histopathology revealed increased numbers of inflammatory cells in the kidneys and focal progressive nephrosis in the exposed rats of both sexes at the highest exposure level. There were no obvious treatment-related effects on growth, haematology, urine analysis, clinical chemistry, cytogenetics, organ weights, gross tissue changes, or tumour incidence (Rampy et al., 1978). The relatively short exposure period would have limited the study’s ability to detect carcinogenic activity.
Table 8: Hepatocellular neoplasms in BDF1 mice exposed to tetrachloroethene via inhalation.a
|
Control |
69 mg/m3 |
340 mg/m3 |
1700 mg/m3 |
|||||
|
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Adenoma |
7/50 |
3/50 |
13/50 |
3/47 |
8/50 |
7/49 |
26/50 |
26/49 |
|
Carcinoma |
7/50 |
0/50 |
8/50 |
0/47 |
12/50 |
0/49 |
25/50 |
14/49 |
|
Adenoma or carcinoma combined |
13/50 |
3/50 |
21/50 |
3/47 |
19/50 |
7/49 |
40/50 |
33/49 |
|
Haemangioendothelioma (spleen) |
1/50 |
0/50 |
1/50 |
0/50 |
5/50 |
0/50 |
5/50 |
1/50 |
a From Nagano et al. (1998a,b).
In an NCI study, groups of 50 male and female B6C3F1 mice were given tetrachloroethene by gavage, in corn oil, on 5 days/week for 78 weeks. In males, the initial dose levels were 450 and 900 mg/kg body weight per day, while the females received 300 and 600 mg/kg body weight per day. After 11 weeks, these doses were increased to 550, 1100, 400, and 800 mg/kg body weight per day, respectively. Over the 78 weeks, this adjustment resulted in averages of 536 and 1072 mg/kg body weight per day for the males and 386 and 772 mg/kg body weight per day for the females. The exposure period was followed by an observation period of 12 weeks without further exposure. Adjusting for dosing on only 5 days/week and on 78 out of 90 weeks, the average lifetime exposures equated to 332 and 663 mg/kg body weight per day for the males and 239 and 478 mg/kg body weight per day for the females. Untreated and vehicle-treated control groups consisted of 20 animals of each sex. A dose-related increase in mortality was observed. Fifty per cent survival periods were 78, 43, 62, and 50 weeks for low- and high-dose males and low- and high-dose females, respectively, but were >90 weeks in all untreated and vehicle-treated control groups. Nearly all treated mice showed nephropathy, the probable cause of the high early mortality. The nephropathy consisted of degenerative changes in the proximal convoluted tubules at the junction of the cortex and the medulla, with cloudy swelling, fatty degeneration, and necrosis of the tubular epithelium and hyaline intraluminal casts. No treatment-related liver lesions were reported, despite other studies clearly showing tetrachloroethene liver toxicity in mice. The incidence of hepatocellular carcinoma increased from 0–10% in control groups (2/20 and 2/17 in vehicle-treated and untreated males, respectively; 0/20 and 2/20 in vehicle-treated and untreated females, respectively) to 40% (19/48) and 65% (32/49) in the low-dose females and males, respectively, and to 40% (19/48) and 56% (27/48) in the high-dose females and males, respectively. In a number of animals, the carcinomas metastasized to the lungs (1/49 of the low-dose females, 3/49 of the low-dose males, and 1/48 of the high-dose females) or to the kidneys (1/18 of the untreated males). Tumours appeared much earlier in the tetrachloroethene-treated groups than in the untreated and vehicle-treated control groups (NCI, 1977). The ATSDR noted the occurrence of pneumonia due to intercurrent infectious disease (ATSDR, 1997).
The NCI also studied carcinogenicity in groups of 50 male and 50 female Osborne-Mendel rats. The females received average doses of 474 or 949 mg/kg body weight per day, 5 days/week, for 78 weeks, by corn oil gavage, followed by a 32-week observation period. In the males, the average dose levels were 471 and 941 mg/kg body weight. The untreated and vehicle-treated control groups consisted of 20 animals of each sex. A high mortality was observed in the early part of the study (50% survival periods in males: 88, 72, and 44 weeks for control, low dose, and high dose, respectively; 50% survival period in females: 102, 66, and 74 weeks for control, low dose, and high dose, respectively), possibly due to toxic nephropathy (degenerative changes in proximal convoluted tubules, cloudy swelling, fatty degeneration, epithelial necrosis, and some hyaline cast-filled tubules). No indications of hepatotoxicity were obtained. At autopsy, about 80% of the treated animals (compared with 0% of controls) were affected by nephropathy. There was no evidence of an increase in tumour induction (NCI, 1977).
No increase in tumour incidence was observed in a limited study in which groups of 40 male and 40 female Sprague-Dawley rats were given 500 mg/kg body weight per day (by olive oil gavage), 4 or 5 days/week, for 104 weeks. Observation lasted to death (up to 141 weeks). The control groups consisted of 50 female and 50 male rats given the vehicle only. Only male rats (32%) showed kidney damage, reported as cytomegaly or karyomegaly in renal tubular cells (Maltoni & Cotti, 1986; see also ECETOC, 1990).
In a more recent study, tetrachloroethene was given by gavage in corn oil to 160 B6C3F1 males at 800 mg/kg body weight per day on 5 days/week for up to 76 weeks; mice were then killed. Control groups consisted of 50 untreated and 50 vehicle-dosed mice, and these were killed at various times between 76 and 134 weeks. At week 76, the percentages of mice with at least one liver carcinoma were 8%, 12%, and 32% in untreated, vehicle-treated, and test groups, respectively, and mean numbers of carcinoma were 0.09, 0.12, and 0.29, respectively. Corresponding figures for adenomas were 8%, 13%, and 80% and 0.90, 0.13, and 1.43, respectively. Foci of cellular alteration (presumed to be preneoplastic lesions) were common in the tetrachloroethene-treated group but rare in the control mice, and there was also mild to marked histological evidence of cytotoxicity in the treated group that was not apparent in the controls (Anna et al., 1994).
There was no increase in skin tumours when tetrachloroethene was applied to the skin of 30 female ICR Swiss mice at 18 or 54 mg/application (about 0.9 and 2.7 g/kg body weight, respectively), on 3 days/week, for at least 440 days (Van Duuren et al., 1979). The short exposure time, small numbers of animals, lack of treated males, and the restriction of examined tissues to the skin severely limit the ability of the study to detect carcinogenic activity.
Lung surface adenomas were not induced when tetrachloroethene was administered by intraperitoneal injection, 3 times weekly, to mice of a susceptible strain. Mice received either 80 mg/kg body weight on 14 occasions or 400 mg/kg body weight on 24 occasions and were killed 24 weeks after the first injection (Theiss et al., 1977; see also HSE, 1987).
One study (in an oral rat model) suggested that tetrachloroethene might have tumour promoting, but not initiating, properties. The rats were partially hepatectomized and subjected to various exposure regimens, and the livers were examined for enzyme altered foci (putative preneoplastic lesions, assumed potentially capable of developing into hepatocellular carcinomas). Initiating properties were investigated by scoring foci numbers after one intraperitoneal treatment with tetrachloroethene (maximum tolerated dose: 6 mmol/kg body weight), followed by treatment with phenobarbitone in drinking-water for 7 weeks; tetrachloroethene had no effect in this experiment. The number of foci occurring after one intraperitoneal treatment with diethylnitrosamine, followed by daily oral gavage (5 days/week for 7 weeks) of tetrachloroethene (6 mmol/kg body weight), was taken as an indicator for promoting activity. In this experiment, tetrachloroethene induced an increase in foci (Story et al., 1986). Other studies have failed to confirm tumour promoting ability in this model, using diethylnitrosamine as initiator (Holmberg et al., 1986; Lundberg et al., 1987). In one of these, tetrachloroethene was given by gavage at 1.1 g/kg body weight per day, 5 days/week, for 7 weeks, to partially hepatectomized rats following a dose of the initiator, and there was no effect on the number or volume of hepatic foci (Lundberg et al., 1987). A pulmonary tumour promoting assay in mice also failed to detect any such activity (Maronpot et al., 1986).
In a study reported only in limited detail, no initiating activity was seen when 30 female ICR Swiss mice were given a single dermal application of 163 mg tetrachloroethene followed by repeated treatment with phorbol myristate acetate for at least 428 days (Van Duuren et al., 1979).
Tetrachloroethene did not induce preneoplastic foci in the liver of neonatal female Wistar rats exposed by inhalation at 14 000 mg/m3 for 8 h/day, 5 days/week, for 10 weeks (Bolt et al., 1982).
According to a limited report, inhalation exposure at 2050 or 4100 mg/m3, 6 h/day, on 5 days/week for 12 months did not increase the incidence of chromosomal aberrations in the bone marrow of rats (three per sex per group) (Rampy et al., 1978). An equivocal result was reported in a bone marrow chromosome assay where rats (10 per sex per group) were exposed by inhalation at up to 3400 mg/m3, 7 h/day, for 1 or 5 days, but interpretation is hampered because the purity of the test compound was low (91.4%) (Beliles et al., 1980). Single or repeated (daily for 5 days) intraperitoneal injections of a sample of tetrachloroethene did not induce aberrations in the bone marrow chromosomes of mice. The doses were said to be 0.17 and 0.5 LD50, but inadequate study detail was given (Cerna & Kypenova, 1977). No micronuclei induction occurred in the bone marrow of mice given up to 2 g/kg body weight by intraperitoneal injection, but at 1 and 2 g/kg body weight, there was a significant dose-related increase in micronucleated cells in the liver of mice subjected to a partial hepatectomy (Murakiami & Horikawa, 1995).
Tetrachloroethene did not induce sperm head abnormalities in Chinese hamsters or rats, but a low-purity sample (91.4%) gave a positive result in mice (Beliles et al., 1980; Mennear, 1985). The studies in rats and mice involved exposure at 700 or 3400 mg/m3, 7 h/day, for 5 days, with examination of sperm 1, 4, and 10 weeks after the last exposure. At 4 weeks, the percentages of abnormal sperm in mice were 6.0%, 10.3%, and 19.7% at 0, 700, and 3400 mg/m3, respectively (Beliles et al., 1980).
Tetrachloroethene did not induce dominant lethal mutations in a study in which groups of 10 male Sprague-Dawley rats were exposed at 700 or 3400 mg/m3 for 7 h/day for 5 days. Following the 5-day exposure period, each male was mated with two females, replaced weekly, for 7 weeks. Females were killed on day 14 of pregnancy and examined for early fetal deaths. Group sizes of females were small (13–19), and higher exposures could probably have been tolerated by the males (Beliles et al., 1980).
Gavage administration of tetrachloroethene at 1000 mg/kg body weight did not induce UDS in the kidneys of rats. Increased replicative DNA synthesis was seen following repeated exposure at 1000 mg/kg body weight for 3 weeks (Goldsworthy et al., 1988).
Following intraperitoneal injection of tetrachloroethene (99.8% pure) at 0.65–1.3 g/kg body weight, single strand breaks were induced in the liver and kidneys, but not lungs, of male mice. The extent of breaks had returned to normal 23 h later, and the mechanism underlying their formation is uncertain. Tetrachloroethene was more potent than trichloroethene. As the latter is oxidized to trichloroacetic acid more rapidly and extensively than tetrachloroethene, DNA damage might not be due simply to oxidative biotransformation (Walles, 1986). When given at 1 g/kg body weight per day for 7 days by gavage to male F344 rats, tetrachloroethene did not induce DNA strand breaks in the kidney (Potter et al., 1996).
Following inhalation (4100 mg/m3 for 6 h) or gavage (500 mg/kg body weight) exposure of mice to radiolabelled tetrachloroethene, no radioactivity was found bound to hepatic DNA. However, the sensitivity of the test was relatively low (10–14.5 alkylations per million nucleotides) (Schumann et al., 1980). In a more sensitive assay (detection limit 0.13–0.94 adducts per million nucleotides), low levels of DNA binding were apparent in mouse liver 22 h after intraperitoneal injection of 14C-radiolabelled tetrachloroethene at 1.4 mg/kg body weight, and even lower levels were found in the rat liver and in the kidney and stomach of rats and mice. Binding to other macromolecules was much higher than binding to DNA (3- to 40-fold for protein, 30- to 2000-fold for RNA), and the DNA binding apparently detected might actually have reflected incorporation of radiolabelled carbon into the DNA during intermediary metabolism (Mazzullo et al., 1987).
Oxidative DNA damage (monitored by measuring 8-hydroxydeoxyguanosine in liver and lymphocyte DNA and in the urine) was not detected in male F344 rats 24 h following the administration of tetrachloroethene at 100, 500, or 1000 mg/kg body weight as a single intraperitoneal injection (Toraason et al., 1999).
Exposure of Drosophila melanogaster to tetrachloroethene by inhalation (up to 3400 mg/m3 for 7 h), feeding, or injection did not induce sex-linked recessive lethal mutations or effects upon the chromosomes (Beliles et al., 1980). Similarly, in NTP studies, sex-linked recessive lethal mutations were not induced by treating males (injection or feeding for 3 days before successive mating with untreated females) with a 4000 mg/l solution of tetrachloroethene (NTP, 1986).
In bacterial assays (including Ames tests), tetrachloroethene of a high purity has not given evidence of mutagenic activity (Greim et al., 1975; Bartsch et al., 1979; Hardin et al., 1981; Kringstad et al., 1981; Stanford Research Institute International, 1983; Williams & Shimada, 1983; Connor et al., 1985; NTP, 1986; Milman et al., 1988; Warner et al., 1988). Tetrachloroethene was tested in the liquid and vapour phases. As well as the usual Salmonella strains, the less commonly used Salmonella strains UTH 8413 and UTH 8414 have been tested, as has Escherichia coli K12. The influence of mammalian metabolism was investigated using rat, hamster, or mice liver homogenates (S9) from animals treated with either Aroclor-1254 or phenobarbital as inducers of hepatic biotransformation enzymes. In some of these studies, commercial or technical grades of tetrachloroethene were also tested and found to induce mutations. Because the same studies yielded negative results when highly purified tetrachloroethene was tested, it appears that impurities were responsible for the mutagenic responses. One study (a spot test) that produced a positive result (in S. typhimurium TA100) did not report the purity of the test compound (Cerna & Kypenova, 1977). When tetrachloroethene was preincubated with purified rat liver glutathione-S-transferases in the presence of glutathione and rat kidney fraction, the resulting product was mutagenic in the Ames test, apparently due to the formation of the genotoxic intermediate, S-(1,2,2-trichlorovinyl)cysteine (Vamvakas et al., 1989). According to a source document, tetrachloroethene was negative in an SOS chromotest using E. coli (de Raat, 2003).
Tetrachloroethene gave positive results in two host-mediated assays using bacteria and mice. However, the purity of test compound was low (91.4%) in one study (Beliles et al., 1980) and unreported in the second (Cerna & Kypenova, 1977), so conclusions cannot be drawn.
Tetrachloroethene did not induce point mutations, mitotic gene conversion, or mitotic gene recombination in Saccharomyces cerevisiae yeast (Bronzetti et al., 1983). Other yeast studies with various limitations (severe toxicity, lack of adequate positive controls, unknown purity) yielded negative, equivocal, or borderline results (HSE, 1987; ECETOC, 1990). A negative result was obtained in a host-mediated assay using yeast and mice and an unusual protocol (Bronzetti et al., 1983).
Tetrachloroethene did not induce chromosomal aberrations or sister chromatid exchanges in Chinese hamster ovary cells with or without S9 (NTP, 1986; Galloway et al., 1987).
When tested in a high-quality mouse lymphoma assay (using L5178Y cells), tetrachloroethene did not induce mutations, with or without S9 (NTP, 1986).
UDS assays in human lymphocytes, WI-38 human fibroblasts, and rat and mouse hepatocytes gave no evidence of DNA-damaging ability (Beliles et al., 1980; Williams & Shimada, 1983; Costa & Ivanetich, 1984; Milman et al., 1988). According to an abstract report, a positive UDS result was reported when a stabilized tetrachloroethene sample was tested at highly toxic doses in rat hepatocytes (Shimada et al., 1983).
In the absence of S9, tetrachloroethene did not induce cell transformation in two assays with BALB/c-3T3 cells (Tu et al., 1985; Milman et al., 1988) or in a third experiment using BHK 21/C13 cells (Milman et al., 1988). A positive result was scored in an assay using rat embryo cells F1706p108 infected with Rauscher leukaemia virus (Price et al., 1979).
Tetrachloroethene inhibited gap junction intercellular communication when incubated with rat liver cells (Benane et al., 1996).
Significant, dose-dependent increases in kinetochore-positive micronuclei were seen when tetrachloroethene was incubated with human cell lines engineered to express metabolizing enzymes (MCL-5, h2E1) (Doherty et al., 1996).
In a two-generation reproduction study, rats (24 per sex per group) were exposed by inhalation to 0, 700, 2100, or 7000 mg/m3 for 6 h/day, 5 days/week, for 11 weeks prior to mating, then daily until the end of mating. Females were then exposed daily until day 20 of pregnancy. When the F1 offspring were 6 days old, exposure was restarted and continued daily until selection of weanlings as second-generation parents. Selected weanlings were then exposed for 5 days/week for at least 11 weeks before mating. Three F2 litters were produced. For the F2A litter, dams and pups were treated as for the F1 litters, except that the 7000 mg/m3 group was not exposed during lactation (to prevent the sedation and parental neglect observed at this level in the first generation). The F2B litters were derived from matings that followed at least 2 weeks of daily exposure (0, 2100, or 7000 mg/m3 only); exposure of the dams then continued on days 1–20 of pregnancy, but with no exposure during lactation. Finally, the F2C litters were derived by mating control and 7000 mg/m3 males with unexposed females. Fertility and reproduction were unaffected at up to 2100 mg/m3. At 7000 mg/m3, maternal toxicity (reduced growth prior to mating and during pregnancy and lactation) and offspring toxicity (decreases in litter size, pup weight, and survival during lactation) were seen. No effects were seen in the F2C pups, indicating that changes were not male-mediated (Tinston, 1995).
Fertility and reproductive capacity were unaffected in groups of 10–12 female rats exposed to tetrachloroethene at 0, 500, 1600, or 3200 mg/m3 for 8 h/day, 5 days/week, for 28 weeks (Carpenter, 1937).
Oocytes taken from female rats following repeated inhalation at 12 000 mg/m3 for two 1-h exposures per day, 5 days/week, for 2 weeks had a slightly lower in vitro fertilizability. No such effect was seen when rats were given tetrachloroethene at 0.9% in the drinking-water for 2 weeks (a Tween vehicle was used to assist dissolution). However, tetrachloroethene reduced the percentage of females ovulating (53% vs 78%; P < 0.05) (Berger & Horner, 2003).
In the two-generation reproduction study described in section 8.5.1 in which rats were exposed by inhalation to 0, 700, 2100, or 7000 mg/m3 for 6 h/day, 5 days/week, for 11 weeks prior to mating and daily during mating, pregnancy, and (part of) lactation, no evidence of developmental toxicity was seen at up to 2100 mg/m3 (although examination was probably limited). Pup weight and survival were reduced at 7000 mg/m3, a concentration that also induced maternal neurotoxicity and nephrotoxicity. At 2100 mg/m3, a small reduction (6%) in absolute testicular weight was recorded in the F1 males, and a 16% reduction was found at 7000 mg/m3 (Tinston, 1995).
The developmental toxicity potential of tetrachloroethene was investigated by exposing small numbers (17 treated, 30 control) of rats to tetrachloroethene at 0 or 2100 mg/m3 for 7 h/day on days 6–15 of gestation. Maternal weight gain was slightly decreased in the tetrachloroethene group, but liver weight was unaffected. The numbers of litters, corpora lutea, implantation sites, and live fetuses and the sex ratio, fetus weight, and fetus length were unaffected. A small but significant increase in the resorption rate was observed (9% vs 4%) in the treated group. Examination for soft tissue anomalies and effects on the skeleton did not reveal any significant treatment-related effects. It was concluded that fetotoxicity was seen at a maternally toxic exposure, but there was no evidence of teratogenic effects (Schwetz et al., 1975).
No fetotoxicity or teratogenicity was seen when groups of 20 pregnant rats were exposed to tetrachloroethene at 3400 mg/m3 for 7 h/day 1) on days 0–18 of gestation, 2) for 3 weeks before mating and on days 0–18 of gestation, or 3) for 3 weeks before mating and on days 6–18 of gestation. The only maternal effects noted were slight increases in liver or kidney weights, and these were not seen consistently between groups. In a similar study with rabbits, the only treatment-related effect reported was an increase in placental abnormalities in the group exposed on days 7–21 of pregnancy (Beliles et al., 1980; Hardin et al., 1981).
One study gave some evidence that exposure to tetrachloroethene during pregnancy might affect CNS function in the offspring. Groups of 13–21 Sprague-Dawley rats were exposed at 700 mg/m3 on days 14–20 of gestation or at 6000 mg/m3 on days 7–13 or 14–20 of gestation. Maternal feed intake and weight gain were decreased in the group exposed at 6000 mg/m3 on days 7–13, but the liver and kidney were microscopically normal. The number of live-born pups was not affected by the treatment, and no mention of deformed pups was made. Behavioural tests found no adverse effects in the offspring of the mothers exposed at 700 mg/m3, but pointed to a decreased neuromuscular function (at 10–14 days of age) following maternal exposure at 6000 mg/m3 during days 7–13 of gestation. However, the pups born to dams exposed later during gestation performed better than controls in another test for neuromuscular function. Neurochemistry revealed a decrease of acetylcholine in the brains of 21-day-old offspring of both 6000 mg/m3 groups and of dopamine in the brains of 21-day-old offspring of the group exposed at 6000 mg/m3 on days 7–13. No neurochemical effects were observed in newborn rats. No effects on offspring brain histopathology were observed. On days 31–32, pups from the 6000 mg/m3 groups showed markedly higher activity in an open field test (Nelson et al., 1980).
When groups of 18–19 CFY rats were exposed at 0, 1500, 4500, or 8500 mg/m3 for 8 h/day throughout (days 0–21) pregnancy, 1500 mg/m3 induced no signs of maternal toxicity or significant effects on the fetuses. At 4500 mg/m3 and above, toxic effects were observed in the mothers (reduced growth and increased relative liver weight) and embryos/fetuses (increased reimplantation loss, reduced fetal weight, and increases in skeletal retardation and supernumerary ribs) (Szakmáry et al., 1997). Some offspring (it is not clear if these came from the same study) were given neurobehavioural tests after weaning until they were killed at 100 days of age. Generally, these tests gave normal results. There were a minimal and temporary decrease in exploratory activity (exposure concentrations not disclosed) and an increase in motor activity in the females on day 100. Sexual development was unaffected, and dissection revealed no increases in major or minor anomalies (Szakmáry et al., 1997).
In a screening protocol, treatment-related increases in embryo resorptions, malformations (small or no eyes), and postnatal deaths occurred when F344 rats were given tetrachloroethene by stomach tube at a maternally toxic dose (ataxia and reduced weight gain) of 900 mg/kg body weight per day on days 6–19 of pregnancy. Lower doses were not tested (Narotsky & Kavlock, 1995).
Developmental toxicity potential of tetrachloroethene was investigated by exposing small numbers (17 treated, 30 control) of mice to tetrachloroethene at 0 or 2100 mg/m3 for 7 h/day on days 6–15 of gestation. Tetrachloroethene induced increased maternal liver weight and reduced mean fetal weight. When expressed on a per litter basis (data were not analysed on a fetal basis), there were increases in delayed ossification of the skull and the sternebra, split sternebra, and subcutaneous oedema (Schwetz et al., 1975).
When C57BL mice were exposed at 0 (n = 77) or 1500 mg/m3 (n = 10) for 8 h/day throughout the organogenesis period of pregnancy, toxic effects were observed in the mothers exposed to tetrachloroethene (increased relative liver weight). Fetotoxic effects reported were a reduced number of live fetuses and an increase (from 0.8% to 14%) in unspecified visceral malformations (Szakmáry et al., 1997). The reason for such a large control group was not explained.
When male mice were given tetrachloroethene at 5 or 320 mg/kg body weight per day on postnatal days 10–16 by gavage, measures of spontaneous motor activity were normal on day 17, but increases in locomotion and total activity (P < 0.01) and other behavioural changes were seen at both dose levels on day 60. There was no clear evidence of a dose–response, and one change was in the direction opposite to that expected from other findings. However, the results provide some suggestion of neurodevelopmental toxicity in young mice resulting in persistent alterations in behaviour (Fredriksson et al., 1993). (See section 8.2.2.3 for further study details.)
When New Zealand rabbits were exposed to tetrachloroethene at 0 (n = 10) or 4500 mg/m3 (n = 16) for 8 h/day throughout the organogenesis period of pregnancy, toxic effects were observed in the mothers exposed to tetrachloroethene (reduced weight gain and increased relative liver weight). Fetotoxic effects reported were an increased postimplantation loss and an increase in the number of litters where all fetuses were reabsorbed. No evidence of teratogenicity was seen (Szakmáry et al., 1997).
When applied neat under cover to the skin of rabbits for 24 h, tetrachloroethene caused severe skin irritation (Duprat et al., 1976) and necrosis (Wolf, 1956). On 4-h contact with neat chemical, rabbit skin showed marked irritation but no corrosion (Van Beek, 1990). When applied (in glass ring depots) to guinea-pig skin, degenerative changes in the epidermis, junctional separation, and cellular infiltration in the dermis were seen in skin biopsies taken 0.25–16 h following application (Kronevi et al., 1981).
Minimal (4 on a scale of 110) irritation was reported when 0.1 ml neat tetrachloroethene was instilled into the rabbit eye (Duprat et al., 1976). When tetrachloroethene was directly sprayed into rabbit eyes, blepharospasm, a granular and optically irregular appearance of the corneal epithelium, and loss of patches of epithelium were observed, with complete recovery within 2 days (Grant, 1962).
No dermal sensitizing properties were observed in a study in which the split adjuvant technique was used. Only nine animals were tested, and reported detail of induction and challenge phases is inadequate (Rao et al., 1981).
Although tetrachloroethene has been investigated well for toxic properties upon respiratory exposure, no indications of irritation or sensitization of the respiratory tract have been obtained in laboratory animals (de Raat, 2003).
Sex and species differences in susceptibility to tetrachloroethene’s ability to cause kidney toxicity were examined by exposing isolated renal cells from rats and isolated renal mitochondria from rats and mice to tetrachloroethene and S-(1,2,2-trichlorovinyl)glutathione (the glutathione conjugate) at concentrations ranging from 0.1 to 10 mmol/l. Incubation of renal cortical cells from male F344 rats at tetrachloroethene concentrations of 0.1–1 mmol/l for up to 3 h induced time- and concentration-dependent increases in releases of LDH at all concentrations, whereas no increase was seen in cells from female rats at any concentration. A similar pattern was seen for the glutathione conjugate. LDH release was markedly increased from cells from the males at S-(1,2,2-trichlorovinyl)glutathione concentrations as low as 0.2 mmol/l for 2 h, whereas LDH release from female rat kidney cells was not significant below 0.5 mmol/l. In isolated rat kidney mitochondria, tetrachloroethene at 1 mmol/l had little or no effect on State 3 respiration (respiration in the presence of ADP), State 4 respiration (respiration in the absence of ADP), or overall energetic competence (State 3/State 4 ratio; respiratory control ratio, or RCR). In contrast, S-(1,2,2-trichlorovinyl)glutathione at 1 mmol/l produced a marked (>60%) reduction in State 3 respiration and a >50% reduction in RCR in mitochondria from male rats. Mitochondria from female rats were less sensitive, treatment with S-(1,2,2-trichlorovinyl)glutathione at 1 mmol/l inducing only a small decrease in State 3 respiration and having no effect on RCR. In mitochondria from mice, no obvious sex differences were seen. Tetrachloroethene and S-(1,2,2-trichlorovinyl)glutathione induced significant decreases in State 3 respiration and RCR. Tetrachloroethene boosted State 4 respiration in male mouse mitochondria, suggesting uncoupling of the mitochondria (Lash et al., 2002). Neither tetrachloroethene nor S-(1,2,2-trichlorovinyl)glutathione produced any significant effects on cytotoxicity or mitochondrial function in isolated hepatocytes from rats or isolated liver mitochondria from F344 rats or B6C3F1 mice, suggesting that the liver is not a major acute target for tetrachloroethene or its glutathione conjugate. Taken in sum, many of the species-, sex-, and tissue-dependent differences in toxicity of tetrachloroethene and S-(1,2,2-trichlorovinyl)glutathione that are observed in vivo are also observed in these in vitro models (Lash et al., 1998, 2002; Lash & Parker, 2001).
Rats have a much higher potential than mice (and humans) for producing reactive intermediates from the glutathione conjugate of tetrachloroethene (Dekant et al., 1998). The conjugate and its reactive metabolic products may be important in the development of kidney damage and tumours in rats (ATSDR, 1997). A particular type of kidney lesion, alpha2u-globulin nephropathy, is also seen in male rats treated with tetrachloroethene (Goldsworthy et al., 1988; Green et al., 1990; Potter et al., 1996; ATSDR, 1997). A large programme of mechanistic studies on a range of hydrocarbons that also commonly induce alpha2u-globulin nephropathy has led to widespread acceptance that this type of nephropathy is a response specific to the male rat and is not likely to be relevant to humans. However, of the seven criteria required for an agent to be considered as causing kidney tumours through the alpha2u-globulin-associated response in male rats (IARC, 1999), for tetrachloroethene, reversible binding to alpha2u-globulin has not been studied, and the dose–response relationship of the tumour outcome with the histopathological end-points does not support the concept.
Trichloroacetic acid, the major mammalian metabolite of tetrachloroethene, produces peroxisome proliferation (Odum et al., 1988) and tumours in the liver of mice (Herren-Freund et al., 1987) and might be in part responsible for the hepatic peroxisome proliferation (Odum et al., 1988) and liver tumours (NTP, 1986; Anna et al., 1994; Nagano et al., 1998a,b) seen in tetrachloroethene-exposed mice. Consistent with the possible role of trichloroacetic acid is the fact that, on inhalation exposure to tetrachloroethene, mice produced much higher blood levels of trichloroacetic acid than did rats (Odum et al., 1988), and peroxisome proliferation was observed in the mouse liver but not the rat liver (Odum et al., 1988); these observations demonstrate that there are likely to be major quantitative species differences in the dose–response of the liver toxicity. Others have also shown a peroxisome proliferative response in the liver of mice treated orally with tetrachloroethene, but no clear evidence of such a response in rats given similar treatment (Goldsworthy & Popp, 1987). As the mechanism of liver damage is believed to involve initial peroxisome proliferation of the liver as an early and critical step, qualitative species differences might also exist. The liver of humans is known to be largely resistant to the peroxisome proliferating action of a range of other chemicals that are powerful proliferators in rodents (ATSDR, 1997). Other studies have demonstrated that the mouse liver is more susceptible to toxic injury than the rat liver (Schumann et al., 1980). Of the key events in PPARalpha-dependent mode of action of carcinogenesis (Klaunig et al., 2003), peroxisome proliferation and inhibition of gap junction intercellular communication have been reported after exposure to tetrachloroethene. However, information is not available on the other known key events, namely PPARalpha-mediated regulation of genes encoding peroxisomal enzymes, cell proliferation, fatty acid metabolism, perturbation of cell proliferation and/or apoptosis, increased hepatocyte oxidative stress, Kupffer cell–mediated cell proliferation, or selective clonal expansion.
No increase in LDH release was seen when hepatocytes isolated from male and female F344 rats were incubated with tetrachloroethene or S-(1,2,2-trichlorovinyl)glutathione at up to 10 mmol/l for 3 h, in contrast to rat kidney cells (see above). This indicates that, in F344 rats, the liver is much less susceptible than the kidney to the acute toxicity of these chemicals (Lash et al., 2002).
Trichloroacetic acid, the tetrachloroethene metabolite, was a liver carcinogen when given to groups of 22 mice in their drinking-water at 5 g/l for 61 weeks. Hepatocellular carcinomas developed in 2 of 22 untreated controls and 7 of 22 treated mice. For hepatocellular adenomas, the incidences were 2/22 in controls and 8/22 in treated mice. This finding suggests that the liver carcinogenicity of tetrachloroethene in mice is caused by this major metabolite (Herren-Freund et al., 1987).
Ras oncogene activation does not seem to account for a high proportion of tetrachloroethene-induced B6C3F1 mouse liver tumours. Analysis of the oncogenes in 27 tetrachloroethene-related carcinomas (induced by gavage treatment on 5 days/week for 76 weeks) found 6 with H-ras codon 61, 1 with other H-ras, and 4 with K-ras mutations. The corresponding control figure for the H-ras codon mutation for 35 spontaneous carcinomas (derived mainly from historical data) was 26. For adenomas, the respective figures were 7, 1, and 3 for 26 tetrachloroethene-related carcinomas and 36 for 39 spontaneous tumours. The findings suggested that tetrachloroethene exposure provides a selective growth advantage to spontaneously occurring mutations in codon 61 of H-ras and at the same time is responsible for a small number of unique molecular lesions suggestive of either a random genotoxic mode of action or a nonspecific result of secondary DNA damage (Anna et al., 1994).
On a weight of evidence approach, tetrachloroethene does not appear to have significant genotoxic potential itself. However, certain of the possible metabolites are recognized Ames bacterial mutagens — e.g. the reductive pathway metabolites S-(1,2,2-trichlorovinyl)-L-cysteine (Green & Odum, 1985; Vamvakas et al., 1989) and N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine (Vamvakas et al., 1987) and tetrachloroethene oxide, a proposed epoxide/oxirane intermediate in oxidative metabolism (Kline et al., 1982).
The mechanisms underlying tetrachloroethene’s ability to cause CNS effects remain to be elucidated (ATSDR, 1997). Alterations in the fatty acid pattern of ethanolamine phosphoglyceride in the cerebral cortex, seen on subchronic exposure of rats, might play a role (Kyrklund et al., 1988, 1990).
Neat tetrachloroethene is irritating to human skin (Meyer, 1973; Nicolis & Helwig, 1973; Hake & Stewart, 1977; Metz et al., 1982). Extensive skin redness and blistering were seen in two workers who had lain unconscious in tetrachloroethene-soaked clothes for 0.5–5 h (Morgan, 1969; Ling & Lindsay, 1971).
De Raat (2003) mentions two cases of tetrachloroethene inducing allergic contact dermatitis, confirmed by patch testing with a 1% solution in olive oil (Vail, 1974). A woman who worked for 2 years in a dry cleaning establishment developed tetrachloroethene-dependent asthma, apparently caused by two separate high-exposure episodes (Palacek, 1970). An 18-year-old male student experienced an acute asthmatic episode of severe dyspnoea, cough, and chest tightness following a prolonged exposure to tetrachloroethene (Boulet, 1988). Asthma characterized by a history of high (and often single) exposure to an irritant product, with respiratory symptoms appearing shortly after the event and in the absence of a previous history of respiratory symptoms, is likely to be an irritant-induced response rather than immunologically based (Malo & Chan-Yeung, 2001). Anaphylactic reactions have been seen in children who ingested 0.25 ml/kg body weight per day for 2 days (Rabbini et al., 1985).
Transient, slight eye irritation was reported in volunteers exposed at 520–550 mg/m3 vapour within a few minutes (Stewart et al., 1961b) and within the first 2 h of exposure to the vapour at 690 mg/m3, but it subsided before the end of the 7-h exposure (Stewart et al., 1970). According to an early report, exposure of six volunteers at 570–900 mg/m3 for 1 h did not irritate the eyes. Mild nasal irritation occurred at 1500 mg/m3 for 2 h (Rowe et al., 1952) or 690 mg/m3 for 7 h (Stewart et al., 1970), but not at 730 mg/m3 for 1 h (Rowe et al., 1952). Exposure at 6400–8200 mg/m3 was severely and immediately irritating to the eyes and respiratory tract (Rowe et al., 1952).
Oral doses of up to several grams have been used to treat internal parasites. In such use, doses of about 4.5–6 g have caused vertigo, inebriation, giddiness, nausea, sleepiness, and loss of consciousness (Kendrick, 1929; Wright et al., 1937; Sandground, 1941; HSE, 1987). Severe psychosis (Haerer & Udelman, 1964) and fatalities (Goldbloom & Boyd, 1954; Lemburg et al., 1979) have been reported. In children, estimated intakes of 1.6–4.8 g/kg body weight have produced vomiting, gastrointestinal bleeding, shock, and even death in one case (EC, 2004).
Lightheadedness, loss of coordination, and altered liver function lasting for up to 63 days occurred in nine firemen exposed to high (unmeasured) levels of tetrachloroethene vapour for 3 min (Saland, 1967). A 7-h, virtually whole-body exposure resulted in loss of consciousness, coma, acute pulmonary oedema, and hypotension. Kidney and liver function were reported as normal (Patel et al., 1977). Impaired liver function (a transient increase in AST and a delayed elevation of urinary urobilinogen) was noted in a worker acutely exposed via inhalation to an anaesthetic concentration of tetrachloroethene (Stewart et al., 1961a; Stewart, 1969). Unconsciousness, a mild seizure, and some temporary liver and kidney damage resulted in a dry cleaning operator exposed by lying in a "pool" of tetrachloroethene for 12 h. Liver and kidney tests were normal after 21 days. Tetrachloroethene concentrations in breath decreased from about 4100 mg/m3 a few hours after exposure (the concentration immediately upon exposure was not measured, but must have been higher) in a bimodal way to 700 mg/m3 after about half a day, 70 mg/m3 after about 15 days, and 30 mg/m3 after about 25 days (Hake & Stewart, 1977). Fatalities due to acute inhalation exposure have been reported during repair in a dry cleaning shop and during recycling of dry cleaning tetrachloroethene by distillation (Lukaszewski, 1979; Levine et al., 1981).
Sensory changes and mild elation were reported by volunteers exposed to tetrachloroethene vapour at 3250 mg/m3 for 130 min. Lassitude, mental fogginess, and exhilaration were experienced at 6280 mg/m3 for 95 min, and inebriation resulted when this was increased to 10 000 mg/m3. Subjects found exposure to 13 400 mg/m3 intolerable (Carpenter, 1937). Apart from eye irritation (see section 9.1), no adverse effects were reported when six volunteers were exposed at 570–900 mg/m3 vapour for 1 h. Dizziness and sleepiness were felt at 1420–1620 mg/m3 for 0.75–2 h, while exposure to 1420–2450 mg/m3 for up to 2 h caused lightheadedness, a sense of irresponsibility, nausea, and impaired motor coordination (Rowe et al., 1952).
Stewart et al. (1970) exposed 16 volunteers for 7 h at 700 mg/m3 vapour (single exposure for 11 subjects, daily exposure for 5 days in five subjects). Haematological and biochemical parameters were measured in blood or serum, including the activities of AST, ALT, serum alkaline phosphatase, and serum LDH and a "complete" urine analysis, including urobilinogen, 17-ketosteroids, 17-hydroxycorticosteroids, catecholamines, and creatinine. Most subjects reported mild eye, nose, or throat irritation, frontal headache, flushing, sleepiness, and/or difficulty in speaking. These effects decreased upon repeated exposure, indicating adaptation.
No evidence of interaction was seen when volunteers (six per sex) were exposed to ethanol (up to 1.5 ml of 100 proof vodka per kg body weight) or diazepam (up to 10 mg/day, once a day) and to tetrachloroethene (up to 700 mg/m3), 5.5 h/day, for 11 weeks (Hake & Stewart, 1977; Stewart et al., 1977).
A source document (de Raat, 2003) presents a tabulated summary of cases (Hughes, 1954; Lob, 1957; Eberhardt & Freundt, 1966; Meckler & Phelps, 1966; Gold, 1969; Trense & Zimmerman, 1969; USEPA, 1986; Lorenz et al., 1990) where occupational exposure to tetrachloroethene induced toxic effects. Exposure was not usually quantified. Effects included fatigue, dizziness, headache, nausea, inebriation, vomiting, appetite loss, loss of coordination, sleepiness, impaired memory, irritability, jaundice, abnormal liver function, and liver tissue changes (biopsy or autopsy showed parenchymal cell degeneration, mononuclear cell focal collection, exaggeration of sinusoids, necrosis). In one case, a baby of a woman who worked as a dry cleaner (and whose breast milk contained tetrachloroethene at up to 10 mg/l) developed jaundice when breast fed (Anonymous, 1978).
A review on the carcinogenicity of tetrachloroethene used in dry cleaning operations was carried out by IARC in 1995. Based on limited evidence in humans and sufficient evidence in laboratory animals, IARC concluded that tetrachloroethene is probably carcinogenic to humans, classifying it in Group 2A. The human studies showed consistent positive associations between exposure to tetrachloroethene and the risks for oesophageal and cervical cancer and for non-Hodgkin’s lymphoma. "These associations appear unlikely to be due to chance, although confounding cannot be excluded and the total numbers in the cohort studies combined are relatively small" (IARC, 1995). In this CICAD, only key studies from IARC (1995), de Raat (2003), and more recent publications are summarized. The main findings of these studies are also presented briefly in Table 9.
Table 9: Summary of human carcinogenicity studies.
|
Subjects |
Organs/cancer type |
Indication of risk |
Reference |
|
1708 dry cleaning workers in the USA Exposed to tetrachloroethene (PCE) for at least 1 year before 1960 and followed up to 1996 625 exposed only to PCE [PCE-only] 1083 exposed to PCE and other solvents [PCE-plus] |
SMR [CI]; no. of cases [notes] |
Ruder et al. (2001) |
|
|
All cancer [cohort] |
1.25 [1.11–1.41] |
||
|
All cancer [PCE-only] |
1.08 [0.85–1.36] |
||
|
All cancer [PCE-plus] |
1.35 [1.16–1.55] |
||
|
Tongue [cohort] |
5.00 [1.62–11.68]; 5 |
||
|
Oesophagus [cohort] |
2.47 [1.35–4.14]; 14 [similar risks for PCE-only and PCE-plus groups] |
||
|
Intestine except rectum [cohort] |
1.48 [1.01–2.09]; 32 |
||
|
Trachea, bronchus, and lung [cohort] |
1.36 [1.05–1.73]; 65 |
||
|
Bladder and other urinary tract [cohort] |
2.22 [1.06–4.08]; 10 |
||
|
Cervix [cohort] |
1.95 [1.00–3.40]; 12 [similar risks for PCE-only and PCE-plus groups] |
||
|
Kidney [cohort] |
1.41 [0.46–3.30]; 5 |
||
|
5369 dry cleaning workers in the USA At least 1 year of employment between 1948 and end of 1993 |
SMR [CI]; no. of cases; notes |
Blair et al. (2003) |
|
|
All cancer |
1.2 [1.1–1.3] |
||
|
Oesophagus |
2.2 [1.5–3.3]; 26 2.1; "little or no exposure" 2.2; "medium/high exposure" |
||
|
Lung |
1.4 [1.1–1.6]; 125 |
||
|
Cervix |
1.6 [1.0–2.3]; 27 |
||
|
Larynx, bladder, and Hodgkin’s disease |
Increases, not statistically significant. For larynx, SMR 2.7 [1.0–5.8] for "medium/high exposure" |
||
|
671 white female laundry and dry cleaning workers in the USA Died in the period 1963–1977 |
PMR [CI] |
Katz & Jowett (1981) |
|
|
Kidney |
2.5 [1.0–5.2] |
||
|
Bladder |
1.9 [0.62–4.5] |
||
|
Skin |
2.6 [0.73–6.8] |
||
|
Cervix |
1.4 [0.68–2.6] |
||
|
Rectum |
1.3 [0.45–2.7] |
||
|
Lymphosarcoma |
1.8 [0.65–3.8] |
||
|
440 laundry and dry cleaning workers in the USA Died in the period 1975–1981 |
SMOR [CI]; no. of deaths |
Duh & Asal (1984) |
|
|
All cancer |
0.9 [0.7–1.2] |
||
|
Lung |
1.7 [1.2–2.5]; 37 |
||
|
Kidney |
3.8 [1.9–7.6]; 7 |
||
|
Cervix |
1.3 [0.3–5.3]; 2 |
||
|
Bladder and liver |
Deficits, not statistically significant |
||
|
Oesophagus |
No data given |
||
|
14 457 aircraft maintenance workers in the USA Died in the period 1952–1982 Employed at least for 1 year and exposed to over 20 different solvents |
SMR [CI]; no. of deaths |
Spirtas et al. (1991) |
|
|
Multiple myeloma (in women) |
1.7 [0.2–6.2]; 2 |
||
|
Non-Hodgkin’s lymphoma |
3.2 [0.87–8.1]; 4 |
||
|
No information on other cancers |
|||
|
10 600 Danish laundry and dry cleaning workers, aged 20–64 years; 10-year follow-up of Danish 1970 census information 510 cancer cases |
SIR [CI]; no of cases; notes |
Lynge & Thygesen (1990) |
|
|
All cancer |
1.0 |
||
|
Pancreas |
1.7 [1.1–2.6]; 22 |
||
|
Liver (women) [no increase in men] |
3.4 [1.4–7.0]; 7 |
||
|
Kidney, bladder, and cervix |
Small deficits, not statistically significant |
||
|
Non-Hodgkin’s lymphoma |
No increase (men showed a slight increase, O/E: 5/1.8; and women showed a slight deficit, O/E: 3/6) |
||
|
Oesophagus |
No data given |
||
|
10 600 Danish laundry and dry cleaning workers A nested case–control study of 17 cases of liver cancer (14 women, 3 men) and 16 of renal cancer (9 women, 7 men) that developed between 1970 and 1987 |
Liver |
None of the 17 cases worked in the dry cleaning industry |
Lynge et al. (1995) |
|
Kidney |
13/16 worked in laundries, 3 as dry cleaners; RR for dry cleaning workers 0.7, CI 0.2–2.6 |
||
|
849 Finnish workers (557 women) exposed to tetrachloroethene followed from 1967 to 1992 during which time there were 31 cancer cases |
SIR [CI]; no. of cases |
Anttila et al. (1995) |
|
|
All cancer |
0.9 [0.61–1.3] |
||
|
Cervix |
3.2 [0.39–11.6]; 2 |
||
|
Non-Hodgkin’s lymphoma |
3.8 [0.77–11.0]; 3 |
||
|
Pancreas |
3.1 [0.63–9.0]; 3 |
||
|
8163 deaths among former laundry and dry cleaning workers in the USA |
PMR [CI]; no. of deaths |
Walker et al. (1997) |
|
|
Oesophagus (black men) |
2.15 [1.11–3.76]; 12 |
||
|
Oesophagus (black women) |
1.84 [0.84–3.49]; 9 |
||
|
Oesophagus (white women) |
1.89 [0.51–4.83]; 4 |
||
|
Oesophagus (white men) |
0.75 [0.16–2.19]; 3 |
||
|
Larynx (white men) |
3.18 [1.17–6.93]; 6 |
||
|
Cervix (black women) |
1.18 [0.59–2.12]; 11 |
||
|
Cervix (white women) |
1.05 [0.46–2.08]; 8 |
||
|
Pancreas (black men) |
1.18 [0.32–3.02]; 4 |
||
|
Pancreas (white men) |
1.28 [0.58–2.43]; 9 |
||
|
Kidney |
Deficits (not significant) in white men, black men, and white women, slight excess (PMR 1.32) in black women |
||
|
Aircraft manufacturing workers A subcohort of 2631 employees "who had potential for routine exposure" to tetrachloroethene Employed for at least 1 year, on or after January 1960 to the end of 1996 No information was available on the levels of exposure |
SMR [CI]; no. of deaths; notes |
Boice et al. (1999) |
|
|
All cancer |
1.07 [0.90–1.26] |
||
|
Oesophagus |
1.47 [0.54–3.21]; 6 |
||
|
Stomach |
1.42 [0.57–2.93]; 7 |
||
|
Biliary passages and liver |
2.05 [0.83–4.23]; 7 |
||
|
Pancreas |
1.50 [0.72–2.76]; 10 |
||
|
Lung |
1.08 [0.79–1.44]; 46 |
||
|
Non-Hodgkin’s lymphoma |
1.70 [0.73–3.34]; 8 |
||
|
Cervix |
0 deaths |
||
|
Kidney |
0.69 [0.08–2.47]; 2 |
||
|
Bladder |
0.70 [0.09–2.53]; 2 |
||
|
Swedish dry cleaning, laundry, and ironing workers Occupation census 1960 and 1970 compared with cancer registry incidence data between 1971 and end of 1989 |
RR [CI]; no. of cases |
Travier et al. (2002) |
|
|
Hodgkin’s disease (men/women combined) |
2.69 [1.01–7.19]; 4 |
||
|
Leukaemia (women) |
2.53 [1.44–4.46]; 12 |
||
|
Laryngeal cancer (men) |
2.42 [0.91–6.45]; 4 |
||
|
Oesophagus |
0.34 [0.05–2.39]; 1 |
||
|
86 868 electronics factory workers in China, Province of Taiwan Factory operated between 1968 and 1992 Between 1985 and 1997, there were 316 cancer deaths Average exposure duration was only 1.6 years Wells nearby contaminated with tetrachloroethene and trichloroethylene |
SMR [CI]; no. of deaths; notes |
Chang et al. (2003) |
|
|
All cancer (females) |
1.00 |
||
|
All cancer (males) |
0.65 |
||
|
Kidney (women) |
1.18 [0.24–3.44]; 3 |
||
|
Kidney (men) |
0 deaths |
||
|
Oesophagus |
0 deaths |
||
|
Laundry, dry cleaning, and garment service workers in the USA Employment for >6 months |
OR [CI] |
Stemhagen et al. (1983) |
|
|
Liver (men) |
2.5 [1.0–6.1] |
||
|
Dry cleaning workers in the USA |
OR [CI] |
Suarez et al. (1989) |
|
|
Dry cleaning services |
Liver (men) |
0.98 [0.44–2.2] |
|
|
Dry cleaning operators |
Liver (men) |
0.55 [0.17–1.8] |
|
|
Case–control study in the USA, 80 liver cancer cases and 146 controls |
Liver |
No cases (and 4 controls) had worked in laundry and cleaning occupations for >6 months |
Austin et al. (1987) |
|
Swedish study, occupation in 1960 linked to cancer incidence data during 1960–1979 There were 7405 kidney cancer cases |
SIR, notes |
McLaughlin et al. (1987) |
|
|
Kidney (men) |
0.99 for working in dry cleaning and laundry establishment (18 cases) |
||
|
Kidney (women) |
0.86 for working in dry cleaning and laundry establishment (25 cases) |
||
|
Population-based German case–control study of 277 cases and 286 controls |
Renal cell cancer |
OR 2.52 [1.23–5.16] for exposure to "chlorinated solvents" |
Schlehofer et al. (1995) |
|
Population-based German case–control study of 935 cases and 4298 matched controls Exposure to tetrachloroethene assigned as medium, high, or substantial (substantial > high) |
Renal cell cancer, men |
OR [CI]; no. of cases |
Pesch et al. (2000) |
|
Medium exposure |
1.4 [1.1–1.7]; 154 |
||
|
High exposure |
1.1 [0.9–1.4]; 119 |
||
|
Substantial exposure |
1.4 [1.0–2.0]; 50 |
||
|
Renal cell cancer, women |
|||
|
Medium exposure |
0.7 [0.4–1.3]; 12 |
||
|
High exposure |
1.1 [ 0.7–1.9]; 19 |
||
|
Substantial exposure |
0.7 [0.3–2.2]; 4 |
||
|
Population-based case–control study in the USA |
Kidney (women) |
OR 2.8 [0.8–9.8] for dry cleaning as the predominant lifetime occupation (8 exposed cases, 1 exposed control) |
Asal et al. (1988) |
|
Kidney (men) |
OR 0.7 [0.2–2.3] for dry cleaning as the predominant lifetime occupation (3 exposed cases, 6 exposed controls) |
||
|
Canadian population-based multisite, case–control study (controls were people with cancer at other body sites) |
Kidney |
OR 2.0 [0.8–5.1] for employment in dry cleaning or laundry industry, for any duration, at least 5 years before disease onset |
Siemiatycki (1991); IARC (1995) |
|
Oesophagus |
None of the 99 oesophageal cancer cases had been a launderer or dry cleaner |
||
|
Australian population-based case–control study of renal cell cancer (489 cases), renal pelvic cancer (147 cases), and 523 controls |
OR [CI] (for any employment in dry cleaning) |
McCredie & Stewart (1993); IARC (1995) |
|
|
Renal cell cancer (men) |
2.7 [1.1–6.7] |
||
|
Renal cell cancer (women) |
2.5 [0.97–6.4] |
||
|
Renal pelvic cancer (men) |
6.1 [2.0–19] |
||
|
Renal pelvic cancer (women) |
4.7 [1.3–17] |
||
|
Danish population-based case–control study of 365 renal cell carcinoma cases and 396 controls |
OR [CI] for any employment in dry cleaning; no. of cases |
Mellemgaard et al. (1994) |
|
|
Kidney (males) |
2.3 [0.2–27]; 2 |
||
|
Kidney (females) |
2.9 [0.3–33]; 2 |
||
|
Population-based multisite, case–control study in the USA of 491 cases of cancer of the oral cavity and pharynx, 235 cases of laryngeal cancer, 404 cases of cancer of the oesophagus and gastric cardia, and 724 controls |
Larynx |
OR 2.7 [0.6–10.9]; 5 cases (risk increased with years employed in dry cleaning industry) |
Vaughan et al. (1997) |
|
Oesophagus |
OR 3.6 [0.5–27.0]; 2 cases, both employed in dry cleaning industry for a very short time |
||
|
Study of 672 women with breast cancer (diagnosed 1987–1993) and 66 controls from the same 8 towns in the USA as the cases Women were exposed to tetrachloroethene when it leached from the vinyl lining of water distribution pipes during the late 1960s through the early 1980s Relative delivered tetrachloroethene doses were estimated |
Breast |
OR 1.5–1.9 for the 75th percentile of delivered dose (0–15 years of latency) OR 1.3–2.8 for >90th percentile of delivered dose (0–15 years of latency) |
Aschengrau et al. (1998, 2003) |
|
Population-based multisite case–control study in the USA of colorectal cancer (326 cases), lung cancer (256 cases), brain cancer (37 cases), and pancreatic cancer (37 cases) Exposure to tetrachloroethene occurred when it leached from the vinyl lining of water distribution pipes during the late 1960s through the early 1980s |
Lung |
OR [CI] for exposure level above the 90th percentile |
Paulu et al. (1999) |
|
0 years of latency |
3.7 [1.0–11.7] |
||
|
Colon–rectum |
|
Assessing the possible carcinogenic effect of chemicals in epidemiological studies is often hampered by mixed exposures and by difficulties in defining the exposed populations, and this is applicable to tetrachloroethene. Tetrachloroethene has been mainly used in dry cleaning of textiles, and other chemicals, notably trichloroethene and hydrocarbon solvents, have been used for the same purposes. The periods of use of different solvents in dry cleaning overlap and vary between and within countries. In many studies of dry cleaning operations, workers in laundering are included, even though they are unlikely to be exposed to tetrachloroethene (unless both operations are done in the same shops), and the dilution of the "exposed" populations with these individuals, who are not actually exposed, decreases the sensitivity of such studies (de Raat, 2003).
Four early mortality studies by occupation in the United Kingdom, Washington and Wisconsin (USA), and British Columbia (Canada) reported elevated mortality of workers in laundry or dry cleaning operations from cancers of stomach (in two studies), intestine and rectum, trachea, bronchus, and lung (in two studies), lip, all digestive tract, buccal cavity, pharynx, connective tissues, oesophagus, and urinary bladder (IARC, 1995). Liver cancer among laundry workers and dry cleaners has been studied in more detail following reports (Hernberg et al., 1984, 1988) of an elevated risk of hepatic cancer among persons exposed to chlorinated solvents.
NIOSH has studied the cancer mortality among dry cleaning workers from four labour unions in the USA; results have been reported in several successive reports (Kaplan, 1980; Brown & Kaplan, 1987; Ruder et al., 1994, 2001). In the most recent report, a cohort of 1708 dry cleaning workers, exposed to tetrachloroethene for at least 1 year before 1960, was followed until the end of 1996. The follow-up was 96% successful; 625 workers had worked in shops where only tetrachloroethene was used [PCE-only], while 1083 had been exposed to tetrachloroethene and other solvents [PCE-plus]. SMRs, using national rates to derive the expected numbers of deaths, were calculated by duration of employment in dry cleaning shops using tetrachloroethene (1–5 years or 5+ years) and by latency periods (<20 or 20+ years). The SMR for all causes of death was 1.03 (95% CI 0.97–1.10). The SMR for all cancer for the entire cohort was 1.25 (95% CI 1.11–1.41) and increased with time of exposure and with latency. However, it was higher in the PCE-plus group (SMR 1.35, 95% CI 1.16–1.55) than in the PCE-only group (SMR 1.08, 95% CI 0.85–1.36). Cancer risks were elevated in the whole cohort for cancer of the tongue (SMR 5.00, 95% CI 1.62–11.68; 5 cases), oesophagus (SMR 2.47, 95% CI 1.35–4.14; 14 cases), intestine except rectum (SMR 1.48, 95% CI 1.01–2.09; 32 cases), trachea, bronchus, and lung (SMR 1.36, 95% CI 1.05–1.73; 65 cases), bladder and other urinary (SMR 2.22, 95% CI 1.06–4.08; 10 cases), and uterine cervix (SMR 1.95, 95% CI 1.00–3.40; 12 cases). The excess risk for oesophageal cancer death was seen in all four (white, black, men, women) gender/race categories. For cancer of the liver and biliary tract, there was a (statistically not significant) deficit; for cancer of the kidney, the SMR was 1.41 (95% CI 0.46–3.30; 5 cases). Small numbers did not allow analysis by duration of exposure and latency for most sites, but for cancers of oesophagus and bladder, the risk increased with both latency and duration of exposure. The risk of dying from cervical cancer increased with increasing duration of exposure. For the oesophagus and cervix, the increased risks were similar for the PCE-only and PCE-plus cohort. All bladder cancer deaths occurred in the PCE-plus cohort. The authors concluded that although important lifestyle and socioeconomic risk factors exist for both cervical and oesophageal cancer mortality, excesses of these sites in the PCE-only cohort and among workers with longer duration of PCE exposure suggest an association with PCE exposure (Ruder et al., 2001).
NCI has studied cancer mortality in a cohort of union members of dry cleaning workers in Missouri, USA (Blair et al., 1979, 1986, 1990, 2003). In the most recent update, underlying and contributing causes of death were analysed among 5369 union members with at least 1 year of employment between 1948 and 31 December 1993. The follow-up was 88% complete. The SMR from all causes was 1.0 (95% CI 1.0–1.1), and that from all cancer 1.2 (95% CI 1.1–1.3). A statistically significantly elevated mortality was observed for cancers of the oesophagus (SMR 2.2, 95% CI 1.5–3.3; 26 deaths), lung (SMR 1.4, 95% CI 1.1–1.6; 125 deaths), and uterine cervix (SMR 1.6, 95% CI 1.0–2.3; 27 deaths); excesses that were not statistically significant were observed for cancers of the larynx and bladder as well as Hodgkin’s disease. There was a (statistically not significant) deficit for liver cancer. The risk of oesophageal cancer was 2.1 for "little or no exposure" and 2.2 for "medium/high exposure"; the risk was elevated for white men, black men, and white women, although it was statistically significant only for black men (who accounted for 18 of the 26 deaths from this cause). The SMR for laryngeal cancer was statistically significant for those with "medium/high exposure" (2.7, 95% CI 1.0–5.8). Highest risk for cervix cancer was observed in the low exposure group. Overall cancer mortality rates and mortality rates for cancer of the oesophagus, larynx, lung, kidney, and cervix were similar among workers entering the cohort before and after 1960, the time when tetrachloroethene use became predominant. SMRs by years in union showed no difference by duration for any cause of death, except bladder cancer, where the SMR was 2.1 for less than the median of 4.4 years and 0.9 for greater than the median (Blair et al., 2003).
In a death certificate study in Wisconsin, USA, PMRs were calculated for 25 causes of death for 671 white female laundry and dry cleaning workers who died in the period 1963–1977. Elevated PMRs, in comparison with low-wage women of the state, were found for cancer of the kidney (PMR 2.5, 95% CI 1.0–5.2), bladder (PMR 1.9, 95% CI 0.62–4.5), skin (PMR 2.6, 95% CI 0.73–6.8), uterine cervix (PMR 1.4, 95% CI 0.68–2.6), and rectum (PMR 1.3, 95% CI 0.45–2.7), as well as for lymphosarcoma (PMR 1.8, 95% CI 0.65–3.8).6 Laundry workers have less exposure to organic solvents, and their inclusion in the analysis would have reduced the sensitivity of the study. Earlier exposure to other dry cleaning solvents may have contributed to the elevation of the PMRs for some cancers (Katz & Jowett, 1981).
SMORs were calculated from death certificates for a group of 440 laundry and dry cleaning workers in Oklahoma, USA, deceased in the period 1975–1981, with stratification on sex, race, and age at death. In comparison with national rates in 1978, no excess risk was found for all cancers (SMOR 0.9, 95% CI 0.7–1.2), while the SMORs were statistically significantly increased for cancers of the lung (SMOR 1.7, 95% CI 1.2–2.5; 37 cases) and kidney (SMOR 3.8, 95% CI 1.9–7.6; 7 cases). There were two deaths from cervical cancer (SMOR 1.3, 95% CI 0.3–5.3); statistically not significant deficits were observed for bladder and liver cancers. Oesophageal cancer was not mentioned. The sensitivity of the study was reduced by the inclusion of deceased laundry workers, and petroleum solvents accounted for over 50% of the solvents used (Duh & Asal, 1984).
In a mortality analysis between 1952 and 1982 among 14 457 aircraft maintenance workers who had been employed at least for a year and exposed to over 20 different solvents, information was provided on the association between exposure to tetrachloroethene and deaths due to multiple myeloma or non-Hodgkin’s lymphoma. Two deaths from multiple myeloma (0.12 expected) in women and four deaths from non-Hodgkin’s lymphoma (SMR 3.2, 95% CI 0.87–8.1) in men and women were observed. Information on other cancer sites (and tetrachloroethene) was not given. In the facilities, Stoddard solvent, carbon tetrachloride, trichloroethene, and 1,1,1-trichloroethane had been the main solvents used (Spirtas et al., 1991).
Lynge & Thygesen (1990) linked the Danish 1970 population census information on occupation with 10-year follow-up information on cancer incidence from the Danish Occupational Cancer Registry for a study of cancer risks among persons aged 20–64 years and engaged in laundry and dry cleaning. The number of total cancer cases was 510, with 502 expected. An excess of pancreatic cancer (SIR 1.7, 95% CI 1.1–2.6; 22 cases) was seen; there was also an excess of primary liver cancer among women (SIR 3.4, 95% CI 1.4–7.0; 7 cases), but not among men. For cancers of the kidney, bladder, and uterine cervix, small (statistically not significant) deficits were observed. There was no increase in non-Hodgkin’s lymphoma; no mention was made of oesophageal cancer (Lynge & Thygesen, 1990). In a follow-up case–control study, it was observed that all 17 primary liver cancer cases had worked in laundries (and none as dry cleaners), while only 74% of the referents had done so, suggesting that exposure to tetrachloroethene (which is limited to dry cleaning shops) does not explain the excess liver cancer. Similarly, the case–referent study did not support tetrachloroethene as the causative agent for the kidney cancer (RR 0.7, 95% CI 0.2–2.6; 3 cases) (Lynge et al., 1995).
A Finnish cohort of workers was biologically monitored for exposure to tetrachloroethene, trichloroethene, and trichloroethane; 849 workers were exposed to tetrachloroethene. They were followed from 1967 to 1992 for cancer incidence. A total of 31 cancer cases was observed (SIR 0.9, 95% CI 0.61–1.3), and no significant excesses were observed for any cancer site. There were two cases of cervical cancer (SIR 3.2, 95% CI 0.39–11.6) and three cases of non-Hodgkin’s lymphoma. The workers probably had also been exposed to petroleum solvents (Anttila et al., 1995).
In an analysis of the National Occupational Mortality Surveillance database in the USA, which collects information on causes of death and occupation from death certificates, Walker and co-workers (1997) analysed the proportional cancer mortality among 8163 former laundry and dry cleaning workers in 28 states of the USA. Black men had higher PMRs for total cancer (PMR 1.30, 95% CI 1.05–1.59) and for oesophageal cancer (PMR 2.15, 95% CI 1.11–3.76; 12 deaths) when compared with the general population in the same states. In the subpopulation of white men, cancer of the larynx yielded a higher PMR (3.18, 95% CI 1.17–6.93; 6 deaths). Among women (609 deaths for black women, 659 for white women), no significantly elevated cancer PMRs were observed; PMRs for cervical cancer were 1.18 (95% CI 0.59–2.12) for black women and 1.05 (95% CI 0.46–2.08) for white women. For cancer of the kidney, (statistically not significant) deficits were observed for white and black men and for white women. Only three liver cancer cases were seen. For pancreatic cancer, PMRs were 1.18 (95% CI 0.32–3.02; 4 deaths) in black men and 1.28 (95% CI 0.58–2.43; 9 deaths) in white men, while (not statistically significant) deficits were seen in women. Cancer experience of dry cleaning and laundry workers was not analysed separately (Walker et al., 1997).
A study on the mortality among aircraft manufacturing workers in California, USA (Boice et al., 1999), identified a subcohort of 2631 employees "who had potential for routine exposure" to tetrachloroethene. The mortality within the cohort was studied for workers employed for at least 1 year on or after January 1960 to the end of the year 1996; the follow-up was 99% complete, and death certificates were obtained for 98% of the deceased. For the subcohort, the overall mortality was lower than expected (SMR 0.90, 95% CI 0.82–0.98), while cancer mortality had an SMR of 1.07 (95% CI 0.90–1.26). For no organ site was a significant excess observed; non-significant excesses were observed for oesophagus (SMR 1.47, 95% CI 0.54–3.21; 6 deaths), stomach (SMR 1.42, 95% CI 0.57–2.93; 7 deaths), biliary passages and liver (SMR 2.05, 95% CI 0.83–4.23; 7 deaths), pancreas (SMR 1.50, 95% CI 0.72–2.76; 10 deaths), lung (SMR 1.08, 95% CI 0.79–1.44; 46 deaths), and non-Hodgkin’s lymphoma (SMR 1.70, 95% CI 0.73–3.34; 8 deaths). There were (not statistically significant) deficits for cancer of the kidney, bladder, and uterine cervix (2, 2, and 0 deaths, respectively). No information was available on the levels of exposure.
Travier et al. (2002) investigated cancer incidence among dry cleaning, laundry, and ironing workers in Sweden by linking census information on occupation in 1960 and 1970 to cancer registry incidence data between 1971 and 1989, in comparison with the population who had never worked in these industries. The relative risk for all cancer was close to unity; statistically significant excesses were observed for Hodgkin’s disease in men/ women combined (RR 2.69, 95% CI 1.01–7.19; 4 cases) and leukaemia in women (RR 2.53, 95% CI 1.44–4.46; 12 cases), while there was a non-significant increase in laryngeal cancer in the men (RR 2.42, 95% CI 0.91–6.45; 4 cases).
No evidence of any association between chlorinated organic solvents and any cancer was found in a retrospective cohort study of cancer mortality among 86 868 workers at an electronics factory in northern Taiwan, China. The factory operated from 1968 to 1992, and wells nearby were known to be contaminated with tetrachloroethene and trichloroethene. Vital status and cause of death were determined from January 1985 to December 1997, and SMRs were calculated based on national rates. Person-years at risk exceeded 1 million, and there were 316 cancer deaths among 1357 deaths in total. Deaths from (any) cancer were as expected (SMR 1.00) in females and lower than expected (SMR 0.65) in males. No individual cancer type was significantly increased in either male or female exposed workers when compared with the general population in Taiwan, China. The average age at the end of the follow-up of the cohort was 40 years, and average duration of employment was just 1.6 years. No data on exposure levels were given, and exposures to other chemicals were not discussed. No liver or oesophageal cancers occurred, and there were only three kidney cancer deaths (Chang et al., 2003).
In a population-based case–control study on occupational factors and liver cancer in New Jersey, USA (Stemhagen et al., 1983), employment for >6 months in laundry, dry cleaning, and garment services was associated with an increased risk of primary liver cell cancer (OR 2.5, 95% CI 1.0–6.1) in males.
In a death certificate study in Texas, USA (Suarez et al., 1989), on occupational factors and primary liver cancer in males, usual occupation in dry cleaning services (OR 0.98, 95% CI 0.44–2.2) or as a dry cleaning operator (OR 0.55, 95% CI 0.17–1.8) was not associated with an elevated risk.
A case–control study of 80 cases of hepatocellular cancer and 146 controls found that none of the cases and four of the controls had >6 months of employment in laundry and cleaning occupations. The study therefore provided no evidence of an association between such employment and liver cancer (Austin et al., 1987).
In a hypothesis-generating study, McLaughlin et al. (1987) linked the Swedish census data on occupation in 1960 to cancer incidence data during 1960–1979 from the Cancer–Environment Registry. Among 7405 cases of renal cancer, 18 males (SIR 0.99) and 25 females (SIR 0.86) had been employed in laundry and dry cleaning establishments. The analysis was not carried out for dry cleaners only (McLaughlin et al., 1987).
In a population-based case–control study conducted in Germany, including 277 renal cell carcinoma cases and 286 controls (response rates 85% in cases and 75% in controls), an OR of 2.52 (95% CI 1.23–5.16) for "chlorinated solvents" was detected in men. The category chlorinated solvents included tetrachloroethene and tetrachlorocarbonate (Schlehofer et al., 1995).
Another large population-based case–control study was conducted in Germany on renal cell carcinoma. Exposure assessment was done by expert rating with two job exposure matrices. Response rates were 81% (cases) and 75% (controls). Overall, 935 incident renal cell carcinoma cases and 4298 controls were interviewed. An OR of 1.4 (95% CI 1.1–1.7) was observed in men with medium levels of exposure to tetrachloroethene, whereas ORs of 1.1 (95% CI 0.9–1.4) and 1.4 (95% CI 1.0–2.0) were observed for high levels and for substantial levels, respectively (based on the "German job exposure matrix"; exposure categories medium, high, and substantial were defined as the 30th, 60th, and 90th percentiles of the exposure index among exposed controls). No association was observed in women (Pesch et al., 2000).
In a population-based case–control study of kidney cancer in the USA, an increased risk was observed for females whose "predominant lifetime occupation" had been dry cleaning (OR 2.8, 95% CI 0.8–9.8, eight exposed cases, one exposed control), but not for men (OR 0.7, 95% CI 0.2–2.3, three exposed cases, six exposed controls) (figures adjusted for age, weight, and smoking) (Asal et al., 1988).
An elevated risk of kidney cancer for employment in laundry or dry cleaning (OR 2.0, 95% CI 0.8–5.1)7 for any duration of time 5 years before disease onset was reported in a population-based, multisite case–control study in Canada. None of the 99 oesophageal cancer cases had been a launderer or dry cleaner. Controls had cancer at other sites, and the results were adjusted for age, race, income, and smoking (Siemiatycki, 1991; IARC, 1995).
In a population-based case–control study in Australia (McCredie & Stewart, 1993; IARC, 1995), any employment in dry cleaning was associated with increased risks of renal cell cancer (OR 2.7, 95% CI 1.1–6.7 for males; OR 2.5, 95% CI 0.97–6.4 for females) and renal pelvic cancer (OR 6.1, 95% CI 2.0–19 for males; OR 4.7, 95% CI 1.3–17 for females). The analysis adjusted for age, sex, and smoking.
In an evaluation of occupational factors associated with renal cell cancer in a population-based case–control study in Denmark, any employment in dry cleaning was reported to be associated with an increased risk in both males and females. These risks were based on small numbers and were not statistically significant (OR 2.3, 95% CI 0.2–27 for males; OR 2.9, 95% CI 0.3–33 for females; two cases for each sex) (Mellemgaard et al., 1994).
In a population-based case–control study in western Washington, USA, information was collected on lifetime history of occupation and use of alcohol and tobacco for 491 incident cases of cancer of the oral cavity and pharynx, 235 cases of laryngeal cancer, 404 cases of cancer of the oesophagus and gastric cardia, and 724 controls selected by random digit dialling. A statistically not significant excess was observed for laryngeal cancer (OR 2.7, 95% CI 0.6–10.9; five cases) and for squamous cell oesophageal cancer (OR 3.6, 95% CI 0.5–27.0; two cases); for laryngeal cancer, the risk increased with years employed in the dry cleaning industry. The two cases of oesophageal cancer had both worked in dry cleaning "for only a short time" (Vaughan et al., 1997).
A number of studies investigating cancer and drinking-water have been published (Isacson et al., 1985; Lagakos et al., 1986; Aschengrau et al., 1993; Cohn et al., 1994; Vartiainen et al., 1997; all summarized in IARC, 1995). One source document concluded that no consistent pattern of risk for any specific cancer was observed in four of these studies; the fifth study showed a significantly increased risk of leukaemia, but this was based on only two cases (IARC, 1995).
Aschengrau et al. (2003) recently reported a case–control study further evaluating earlier suggested associations (Aschengrau et al., 1998) between breast cancer and tetrachloroethene exposure from drinking-water. In the 2003 publication, the cases (n = 672) were women from eight towns in the Cape Cod region of Massachusetts, USA, who had been diagnosed with breast cancer between 1987 and 1993. Controls (n = 616) were demographically similar women from the same towns. Women were exposed to tetrachloroethene when it leached from the vinyl lining of water distribution pipes during the late 1960s through the early 1980s. A relative delivered dose of tetrachloroethene that entered a home was estimated using an algorithm that considered residential history, water flow, and pipe characteristics. Small to moderate elevations in risk were observed among women whose exposure levels were above the 75th and 90th percentiles when 0–15 years of latency were considered (adjusted ORs, 1.5–1.9 for the 75th percentile; 1.3–2.8 for the >90th percentile).
A related population-based case–control study evaluated the relationship between cancer of the colon–rectum (n = 326), lung (n = 256), brain (n = 37), and pancreas (n = 37) and drinking-water exposure to tetrachloroethene among residents of five upper Cape Cod towns (Barnstable, Bourne, Falmouth, Mashpee, and Sandwich) in Massachusetts, USA, who were diagnosed during 1983–1986. Adjusted ORs for lung cancer were elevated among subjects whose exposure level was above the 90th percentile whether or not a latency period was assumed (ORs and 95% CIs: 3.7 (1.0–11.7), 3.3 (0.6–13.4), 6.2 (1.1–31.6), and 19.3 (2.5–141.7) for 0, 5, 7, and 9 years of latency, respectively). The adjusted ORs for colon–rectum cancer were moderately elevated among exposed subjects, as more years of latency were assumed (ORs and 95% CIs: 1.7 (0.8–3.8) and 2.0 (0.6–5.8) for 11 and 13 years of latency, respectively) (Paulu et al., 1999).
The frequencies of chromosomal aberrations and sister chromatid exchanges in the lymphocytes were similar in nine unexposed workers, in six factory workers exposed to tetrachloroethene at 200–1500 mg/m3 (geometric mean 630 mg/m3 in 25 determinations), and in four workers exposed at 70–280 mg/m3. The differences in exposure were confirmed by urine analysis. Workers in the high exposure group were involved in degreasing with tetrachloroethene; the low exposure group worked in the same workshop but did not use tetrachloroethene directly. Technical tetrachloroethene was the sole solvent used in the workshop. Personal air sampling and urine analysis were carried out for exposure assessment (Ikeda & Koizumi, 1980). The value of the study is deemed limited by the ATSDR (1997) because of the small number of subjects involved and the wide exposure range.
The frequency of lymphocytes with chromosomal aberrations (excluding gaps) was twice as high in nine women employed in dry cleaning shops in Berlin, Germany, than in nine women with office jobs. The dry cleaning group was exposed to tetrachloroethene at 144–348 mg/m3. The difference was more marked when gaps were included in the analysis. Dicentric chromosomes occurred 13 times as often in the cells of the exposed groups. The tetrachloroethene was contaminated with 0.11–0.43% (by volume) of trichloroethene (Fender, 1993).
Levels of 8-hydroxydeoxyguanosine in the leukocytes (a measure of oxidative DNA damage; values expressed as ng/mg deoxyguanosine) were 8.1 ± 3.6 in female dry cleaners exposed to tetrachloroethene (at unspecified concentrations), 16.0 ± 7.3 in female launderers without occupational exposure to tetrachloroethene, 11.8 ± 5.9 in black women controls, and 17.8 ± 7.4 in white women controls. Concentrations of 8-hydroxydeoxyguanosine in the urine (an indicator of DNA repair) did not differ between groups. Overall, the results showed no evidence of increased oxidative DNA damage in dry cleaners occupationally exposed to tetrachloroethene (Toraason et al., 2003).
Similar frequencies of sister chromatid exchanges were found in 27 Japanese (14 men, 13 women) who had worked as dry cleaning workers (geometric mean personal 8-h TWA tetrachloroethene exposure 69 mg/m3) for a mean of about 41 months and in 26 controls matched for age, sex, smoking habits, and residence (Seiji et al., 1990). No effects were found on the frequency of chromosomal aberrations and sister chromatid exchanges in lymphocytes in 38 workers in dry cleaning shops, compared with 45 controls. The possible effects of smoking were taken into account (Böttger & Elstermeier, 1989).
Studies of women exposed to tetrachloroethene in dry cleaning shops and other settings have been generally consistent in showing an increase in the rate of spontaneous abortion; however, other solvents were also present in most of these workplaces. The studies have been less able to evaluate effects on other reproductive outcomes, such as stillbirths, low birth weight, and congenital malformations. A few studies of limited size have suggested disturbances in sperm quality and fertility among male dry cleaners (IARC, 1995).
In a study of over 294 000 pregnancies in Finland in 1973–1976, census data were linked with hospital discharge records to assess the association between parental occupations and occupational exposure and spontaneous abortions. There were 416 pregnancies among dry cleaning and laundry workers; the rate of spontaneous abortion was higher in this group than among other service workers (adjusted OR 1.5, 95% CI 1.1–2.0). Dry cleaners (who would have been exposed to tetrachloroethene) were not evaluated separately (Lindbohm et al., 1984).
Obstetrical history was obtained by interview for 67 women working (for an average of 20 years) in 53 dry cleaning shops in Rome, Italy. Of the total of 102 pregnancies reported, 46 occurred during times when the women were not involved in dry cleaning and 56 when they were involved in dry cleaning. Five spontaneous abortions were reported by women when exposed to tetrachloroethene, one when they were not. The difference was not statistically significant (P < 0.10), but the study power was low. Small numbers of events precluded analysis of other reproductive outcomes (Bosco et al., 1987).
Kyyrönen et al. (1989) studied the pregnancy outcome in 1973–1983 among 5700 female Finnish dry cleaning and laundry workers, identified from union membership files and employers’ records for employees. Out of 247 spontaneous abortion cases, 130 finally were included in the analysis, together with 289 controls. Dry cleaning was associated with an increased risk of spontaneous abortion (OR 4.9, 95% CI 1.3–20). High exposure (exposure assessed by questionnaire) to tetrachloroethene was also associated with an increased risk of spontaneous abortion (OR 3.4, CI 1.0–11.2, adjusted for use of other solvents, heavy lifting at work, and alcohol use). The analysis of malformations was limited to 24 cases and 93 controls, and no excess was observed in relation to tetrachloroethene exposure during the first trimester (OR 0.8, 95% CI 0.2–3.5).
A series of nested case–referent studies examined the pregnancy outcome, as indicated by hospital discharge records in 1973–1983, in more than 18 000 women from the four Nordic countries who had worked in any dry cleaning shop or laundry. Information on exposure during pregnancy was collected by interview or questionnaire from the employers (Norway) or from the women (the other three countries). Overall, an elevated risk of spontaneous abortion was observed. For low and high exposure to tetrachloroethene, adjusted ORs were 1.2 (95% CI 0.74–1.9) and 2.9 (95% CI 0.98–8.4), respectively. However, the increased risk was limited to Finland; the results of the Finnish part of the study were separately reported by Kyyrönen et al. (1989) (see above). No association was observed between malformations, stillbirths, or low birth weight and exposure to tetrachloroethene among dry cleaning workers in Scandinavia, but the numbers of these events were small (38, 13, and 13, respectively) (Olsen et al., 1990).
In a small case–control study of spontaneous abortion in Finnish subjects biologically monitored for solvent exposure, 8/73 cases and 15/167 controls were exposed to tetrachloroethene. This population is likely to have overlapped with that of the Kyyrönen et al. (1989) study. The OR found for tetrachloroethene exposure (regardless of industry) in the study was 1.4 (95% CI 0.5–4.2, 8 cases and 15 controls) for all exposures, 0.5 (95% CI 0.1–2.9, 3 cases and 9 controls) for low exposure, and 2.5 (95% CI 0.6–10.5, 5 cases and 6 controls) for high exposure. For dry cleaners, the OR increased to 2.7 (95% CI 0.7–11.2, 4 cases and 5 controls). These figures were not statistically significant, but the study was small (Lindbohm et al., 1990).
A further case–referent study investigated the effects of reported maternal exposure to solvents (during the first 20 weeks of pregnancy) on spontaneous abortion in California, USA. Cases (n = 852) were women 18 years of age or older who had a spontaneous abortion for which a pathology specimen was submitted to one of the 11 hospital laboratories in Santa Clara County between June 1986 and February 1987. For each case, two controls were randomly selected among county residents who had had a live birth. Exposure and confounders were assessed by telephone interviews. Five cases reported exposure to tetrachloroethene, versus two controls, which resulted in a crude OR of 4.7 (95% CI 1.1–21.1). The OR and CI were small-sample estimates calculated by Haldane’s method and Fisher’s exact test. Four of the cases also reported exposure to trichloroethene (Windham et al., 1991).
The United Kingdom’s Health and Safety Executive commissioned an investigation into the maternity histories of 3110 women currently or previously employed in dry cleaning shops or laundry units at the time of their pregnancy or in the 3 months before conception. No increased risk of spontaneous abortion was seen in dry cleaning workers when compared with laundry workers. Within the dry cleaners, a higher spontaneous abortion rate was seen in those described as "operators" compared with "non-operators" (17.1% vs 11.6%, OR 1.63, 95% CI 1.01–2.66; figures adjusted for maternal age, pregnancy order, and year of birth). Operators were assumed to have had higher exposure to tetrachloroethene. For women employed as "non-operators", the risk of spontaneous abortion was no greater than for pregnancies conceived while women were not employed in either the dry cleaning or laundry industry (Doyle et al., 1997).
A case–control study nested within a cohort of 6000 male Finnish workers monitored biologically for exposure to six organic solvents (styrene, toluene, xylene, tetrachloroethene, trichloroethene, and 1,1,1-trichloroethane) found no association between paternal exposure to tetrachloroethene and spontaneous abortion (120 cases, 251 controls; crude OR 0.5, 95% CI 0.2–1.5) or congenital malformations. Cases were identified from the national Hospital Discharge Register. Data on paternal preconception exposure were obtained by questionnaire and from a register of biological exposure measurements of the Finnish Institute for Occupational Health from the period 1965–1983. There was a significant association between spontaneous abortion and paternal exposure to "solvents in general" but not to tetrachloroethene (OR 0.5, 95% CI 0.2–1.5, 4 in the cases, 17 in the referents). The power of the study is low due to the small numbers of spontaneous abortions and especially congenital malformations observed (Taskinen et al., 1989).
Reproductive outcome was examined in 17 female partners of dry cleaners exposed to tetrachloroethene and compared with outcome in the 32 female partners of laundry workers not exposed to dry cleaning solvents, in a small study in California, USA. No differences were found for number of pregnancies, number of live births, and rates of spontaneous abortion. Standardized fertility ratios were almost identical for the two groups. However, the partners of the dry cleaners "were more than twice as likely to have a history of attempting to become pregnant for more than 12 months or to have sought care for an infertility problem". Cox proportional models indicated that the per-cycle pregnancy rate of the partners of dry cleaners was only half that of the partners of laundry workers, after controlling for other potential confounders (estimated pregnancy rate per cycle ratio 0.54, 95% CI 0.23–1.27). Furthermore, partners of laundry workers were more likely than partners of dry cleaners to become pregnant within the first two cycles of trying to conceive. Interpretation of the study is limited by the very small sample size (Eskenazi et al., 1991a).
When semen quality of 34 dry cleaners was compared with that of 48 laundry workers in California, USA, the semen quality of both groups was within normal limits ("by standard clinical measurements"), but certain differences suggested a possible effect of tetrachloroethene exposure on semen quality. The tetrachloroethene group showed a higher number of round sperm (P < 0.002; dose related; an effect associated with infertility), broader sperm (P < 0.02; dose related), and a greater amplitude of lateral head displacement (P < 0.09; dose related). It was not clear whether the described subtle effects on sperm quality are due to exposure to tetrachloroethene alone or to a mixture of compounds. It is not known whether these slight effects would affect fertility (Eskenazi et al., 1991b).
Assessment of proteinuria, albuminuria, urine lysozyme activity, and urine beta-glucuronidase activity in 57 (mostly female) workers from 29 dry cleaning shops and in control groups (16–50 females and 30–65 males) identified significant increases in the last two parameters, suggesting "very weak and tubular, rather than glomerular" damage. The tetrachloroethene exposure concentration in the dry cleaning workers was estimated (from biological monitoring) to be 70 mg/m3 (Franchini et al., 1983).
Urinary beta2-microglobulin, albumin, and retinol binding protein levels were similar in 24 female and 2 male dry cleaning shop workers in Belgium, when compared with unexposed subjects (31 females, 2 males). The TWA tetrachloroethene exposure (estimated by personal air monitoring, analysis of breath and blood for tetrachloroethene, and urine analysis for trichloroacetic acid) in the dry cleaning workers varied from 61 to 260 mg/m3, with a mean for all samples of 143 mg/m3. The study suggested that if the blood concentration of tetrachloroethene does not exceed 1 mg/l, 16 h after the end of exposure, the TWA exposure is likely to have been below 340 mg/m3. It was suggested that exposure to such levels for an average of 6 years did not seem to exert any adverse effect on the CNS, liver, or kidneys (Lauwerys et al., 1983).
Urinary levels of beta2-microglobulin, creatinine, glucose, lysozyme, LDH, and total proteins were determined in 16 female workers from five dry cleaning shops and in 13 unexposed (control) females. TWA tetrachloroethene exposure (assessed by personal air monitoring) varied between 9 and 799 mg/m3 (mean 157 mg/m3 TWA). The only significant effect of exposure was increased lysozyme activity. No correlation was found with either concentration or duration of exposure. The investigators concluded that "in view of these limitations and results of other authors, the existence of a chronic nephropathy at low exposure levels remains very hypothetical" (Vyskocil et al., 1990).
A collaborative European cross-sectional study assessed renal effects in 50 dry cleaning workers (41 women, 9 men) who had been exposed to tetrachloroethene for an average of 10 years. Tetrachloroethene was measured in blood samples collected during the working day and in air samples collected during 4-h periods randomly selected over the working week. Atmospheric concentrations ranged from "traces" to about 590 mg/m3, with a mean of 100 mg/m3. Blood concentrations ranged from 9 to 900 µg/l (mean 143 µg/l). The control group consisted of 50 blood donors, matched for sex and age, who had no history of tetrachloroethene exposure. Renal function was assessed using markers in blood (creatinine, beta2-microglobulin, antiglomerular basement membrane antibodies, and laminin fragments) and urine (total protein, albumin, transferrin, immunoglobulin G, beta2-microglobulin, retinol binding protein, brush border antigens BBA, BB50, HF5, prostaglandins PGF1alpha, PGE2, PGF2alpha, thromboxane B2, Tamm-Horsfall glycoprotein, glycosaminoglycans, N-acetyl-beta-D-glucosaminidase activity, alkaline phosphatase activity, and fibronectin). Mean values of virtually all urinary markers were higher in the exposed group, and statistically significant increases were observed for albumin and transferrin (high molecular weight proteins), the three brush border antigens, fibronectin, and alkaline phosphatase. The increases in excreted glycoprotein and glycosaminoglycans also approached significance. In serum, there were statistically significant increases in laminin fragments and antiglomerular basement membrane antibodies. Serum creatinine and beta2-microglobulin values overlapped in the two groups, indicating the absence of major impairment of kidney function. In the control group, only 3/50 had >3 abnormal values, compared with 13/50 exposed workers. Increased high molecular weight proteins associated with markers of tubular alterations were seen in 17/50 exposed workers compared with 1/50 controls. Although there was no apparent correlation between duration or intensity of exposure and renal damage, the results suggest tetrachloroethene-induced glomerular and tubular changes that might represent an early stage of progressive renal disease (Mutti et al., 1992).
Verplanke et al. (1999) investigated the effects of tetrachloroethene exposure on the kidney in 82 exposed workers (from four dry cleaning shops) and 19 non-exposed workers (from laundries or areas of dry cleaning shops where tetrachloroethene was not used) in the Netherlands. Tetrachloroethene was determined in alveolar air samples, leading to an estimated mean 8-h TWA exposure of 7.9 mg/m3 (range 1–221 mg/m3). A chronic dose index was estimated from data on the current tetrachloroethene dose and the occupational history of the individual subjects. The mean chronic dose index in the exposed group was 400 months·mg/m3 (range, 12–4882 months·mg/m3). Effects on the renal tubules were assessed with the indicator parameters: N-acetyl-beta-D-glucosaminidase, beta-galactosidase, alanine aminopeptidase, and retinol binding protein in urine. The tubular parameter retinol binding protein was the only parameter increased in the exposed group compared with the non-exposed group.
Trevisan and co-workers (2000) compared urine total solutes and proteins, angiotensin converting enzyme, N-acetyl-beta-D-glucosaminidase, and glutamine synthetase, as markers of effect, among 40 dry cleaning and 45 female ironing shop workers. The average tetrachloroethene concentration in the air in the dry cleaning shops was 59.7 mg/m3. No statistically significant difference was observed between the two groups, but a significant correlation was observed between urinary glutamine synthetase activity and exposure to tetrachloroethene.
In a study among 192 dry cleaners at 14 dry cleaning shops in the USA, no relationship was observed between the concentration of tetrachloroethene in exhaled breath and urinary ratios of total urinary protein, albumin, and N-acetyl-beta-D-glucosaminidase to creatinine. The average estimated concentration of tetrachloroethene in the workroom air was 100 mg/m3, and exposure duration was 11.6 years. There was no unexposed control group (Solet & Robins, 1991).
A German study considered 113 workers in an engine repair shop who had used tetrachloroethene in manual degreasing operations for an average period of 11.5 years (range 2–24 years), although at the time of the study only 8 were still in contact with it, and then only occasionally. The average tetrachloroethene exposure concentration (presumably 8-h reference period) for these 8 workers was about 240 mg/m3, although 15% of the 105 individual measurements exceeded 690 mg/m3, up to a peak of 1700 mg/m3. All the other workers had ceased to be exposed to tetrachloroethene for 2 years at the time of the investigations. Renal function was assessed by various (low sensitivity) markers (concentration test, phenol red test, serum urea and creatinine, urinalysis) and found to be within the "normal" range in all cases, both for the total group and for a series of subgroups. There was no correlation between individual values and length of exposure to tetrachloroethene (Essing et al., 1974a,b).
Acute renal failure occurred in a man who accidentally ingested 75 g tetrachloroethene. Renal biopsy 19 days later revealed acute tubular necrosis with aggregation of calcium-rich crystals in the tubular lumen. Following repeated dialysis, renal function gradually returned to normal (Choi et al., 2003).
Serum levels of ALT and GGT were similar in 24 female and 2 male dry cleaning shop workers, when compared with unexposed subjects (31 females, 2 males). The TWA tetrachloroethene exposure (estimated by personal air monitoring, analysis of breath and blood for tetrachloroethene, and urine analysis for trichloroacetic acid) in the dry cleaning workers varied from 61 to 260 mg/m3, with a mean for all samples of 143 mg/m3 (Lauwerys et al., 1983).
Gennari et al. (1992) studied GGT isoenzyme patterns in 141 tetrachloroethene-exposed workers and 130 controls. The exposed group (124 females, 17 males, aged 20–58 years; mean 43.0 years) were employed for a mean of 12.3 years in 47 small laundries and dry cleaning shops in Bologna, Italy, exposed only to tetrachloroethene, while the control group was drawn from among staff and students of the University of Bologna. Based on measurement of trichloroacetic acid in urine after at least 5 consecutive days of exposure, tetrachloroethene exposure in the "exposed" group was estimated to be below 340 mg/m3 (mean 76 ± 27 mg/m3). Total GGT activity was higher in the exposed group (12.36 ± 6.90 vs 8.76 ± 4.94 U/l, P < 0.01 by Mann & Whitney U test), as was the GGT-2 isoenzyme fraction (6.79 ± 5.74 vs 3.48 ± 3.29 U/l, P < 0.01). When total GGT was ranked by activity (0–8, 9–16, 17–24, 25–32 U/l), the frequency distribution was significantly different, more of the controls being distributed among the lower ranks. One GGT fraction (GGT-4), considered to reflect hepatobiliary impairment, was seen only in the exposed group. No correlation existed between serum GGT activity and tetrachloroethene exposure level or duration. No differences in activity of other enzymes measured (ALT, AST, alkaline phosphatase, LDH, 5'-nucleotidase) were mentioned (Gennari et al., 1992).
Hepatic ultrasonography findings and serum enzyme activities were compared for 29 (17 male, 12 female) dry cleaners exposed exclusively to tetrachloroethene over the previous 5 years and 29 (14 male, 15 female) laundry workers not exposed to solvents during a specified 6-month period, in Washington, USA. A subgroup of 19 of the dry cleaners had a mean 8-h TWA exposure to tetrachloroethene of about 110 mg/m3 (range 2.8–570 mg/m3) as assessed by badge dosimetry. The dry cleaning and laundry groups were similar for weight, education, and (at most moderate) alcohol intake, but there were differences in mean age (46 and 38 years, respectively), duration of employment (20 and 5 years), and ethnic background: 24% of the dry cleaners were Asian, 14% were black, and none was Hispanic, compared with 7%, 3%, and 14% of the laundry workers, respectively. There were no significant differences in mean serum levels of bilirubin, AST and ALT, or GGT between the two groups. Increased ALT activities (all less than 1.5 times the upper limit of the normal reference range) were found in 5 of 27 dry cleaners compared with only 1 of 26 laundry workers, but there were no such differences for the other enzymes. In contrast, diffuse hepatic parenchymal changes in echogenicity, as determined by ultrasonography, were found more commonly in dry cleaners than in laundry workers (18/27 vs 10/26; P < 0.05). The pattern of sonographic readings was interpreted in a blinded manner as "normal", "mild", or "moderate to severe" parenchymal change. Nine, 13, and 5 of the 27 dry cleaners evaluated fell into these groups, respectively, compared with 16, 4, and 6 for 26 laundry workers. Thus, the main difference between the two occupations was in the "normal" and "mild" categories. Ten of 26 control subjects were considered to give abnormal responses, and the six moderate to severe changes detected in this group (more than among the dry cleaners) suggest some significant background confounding factor. The results for the tetrachloroethene-exposed workers were also subdivided in several ways: according to the current national TWA exposure (above or below 100 mg/m3), cumulative years of exposure (above or below 10 years), and the nature of the dry cleaning equipment in operation at the time of the study (new "dry-to-dry" compared with old "dry-to-dry" or wet transfer operation). This subdivision revealed some statistically significant response trends across the exposure groups, although these trends were not always convincing. Interpretation was complicated because contemporary tetrachloroethene exposure levels and operations provided no information on previous exposures (both to tetrachloroethene and to other solvents), there were ethnic and age differences between the two groups, and the reliability of hepatic ultrasonography in relation to toxicology is unclear (Brodkin et al., 1995).
Liver function tests were carried out on 113 German workers in an engine repair shop who had used tetrachloroethene in manual degreasing operations for an average period of 11.5 years (range 2–24 years). At the time of the study, only eight were still exposed, and then only occasionally. The average tetrachloroethene exposure concentration for these eight workers was about 240 m3, although 15% of the 105 individual measurements exceeded 690 mg/m3, up to a peak of 1700 mg/m3. The other workers had not been exposed to tetrachloroethene in the 2 years before the investigation. Serum aminotransferase activities were found to correlate with alcohol consumption but not with exposure to tetrachloroethene (Essing et al., 1974a,b). In what appears to be another report of the same workers, no liver damage, based on biochemical tests and some biopsy samples, was detected in a group of 106 workers in a railway repair shop where tetrachloroethene was used to clean metal parts. All had been exposed for at least 2 years. Atmospheric concentrations often exceeded 2800 mg/m3, with about 75% of measurements lying between 1.4 and 340 mg/m3 (Essing, 1975).
No significant differences in serum indicators of liver function (aminotransferases, bilirubin, GGT, alkaline phosphatase, BUN) were seen between 56 workers (27 women, 29 men; mean tetrachloroethene exposure time 3 years) from three dry cleaning shops in China and 69 unexposed workshop controls (37 women, 32 men). The male dry cleaning workers were on average 4 years younger than the male controls, and the women were 4.9 years older than the female controls. Passive air sampling revealed a geometric mean 8-h TWA tetrachloroethene concentration of 140 mg/m3 and a TWA concentration range of 28–670 mg/m3 (Cai et al., 1991).
This section contains mainly abbreviated versions of the descriptions of studies summarized in a source document, in which additional study information can be found (USEPA, 2003).
An extensive description of the neurotoxicological studies in humans is available in the source document (USEPA, 2003).
Eleven healthy adults were exposed at 690 mg/m3 for 7 h, and a further five subjects were exposed daily for 5 days. Three subjects had difficulty in maintaining equilibrium in the Romberg test within the first 3 h but performed normally when given a second chance. Performance on the other tests was not impaired. An additional subject, exposed during the 3rd day of testing, showed a slight deterioration in his Romberg test and complained of slight dizziness and slight impairment of his intellectual faculties after 1 h of exposure (Stewart et al., 1970).
A review article summarized controlled exposure studies primarily funded by NIOSH. As part of a 5-week study, 3–4 healthy men were exposed 1, 3, or 7.5 h/day, 5 days/week, to tetrachloroethene at about 0, 140, 690, or 1000 mg/m3. Complaints of symptoms were not related to tetrachloroethene exposure. EEG recordings made during exposure suggested altered patterns indicative of cortical depression in males and females exposed to tetrachloroethene at 690 mg/m3 for 7.5 h. Recordings of visual evoked responses and equilibrium tests were normal in men and women. Men were given neurobehavioural tests of cognitive function, motor function, motor/cognitive function, and time estimation; performance was not statistically significantly affected by exposure. The performance of men on a second test of motor function (Flanagan coordination) was statistically significantly decreased (P < 0.05) when compared with the response at 0 mg/m3 on at least 1 day during the weeks of tetrachloroethene exposure at 690 and 1000 mg/m3 (Hake & Stewart, 1977). Another experiment examined, in six men and six women, interactions on behavioural and neurological function associated with inhaled tetrachloroethene and oral doses of alcohol or diazepam, a tranquillizer. Individuals were typically exposed for 5.5 h to 0 mg/m3 on Monday or Tuesday, 690 mg/m3 on Wednesday and Friday, and 170 mg/m3 on Thursday during each of the 11 weeks of exposure and were given a placebo capsule, alcohol, diazepam, or nothing during each period. Exposure to tetrachloroethene at 170 or 690 mg/m3 for 5.5 h did not increase the prevalence of symptoms or alter the subjects’ mood. Exposure did not significantly reduce performance on two equilibrium tests and two neurobehavioural tests of motor function. At 690 mg/m3, there was a statistically significant decrease (P < 0.05) in scores on a third test of motor function (Flanagan coordination test) on some days of exposure. Statistical analyses performed by the investigators revealed no effect of tetrachloroethene exposure on EEGs (Hake & Stewart, 1977; Stewart et al., 1977).
In a single-blind study, neurophysiological measurements were made on 12 subjects exposed to tetrachloroethene at about 69 mg/m3 and on 10 subjects exposed to 340 mg/m3, for 4 h/day on 4 consecutive days. There was no unexposed control group. The visual evoked potential patterns of subjects during the 3rd hour of exposure to 340 mg/m3 on days 1, 2, 3, and 4 of exposure were significantly different (P < 0.05) from those measured on the control day, and the differences became progressively greater on successive exposure days. Visual evoked potential patterns in subjects during exposure to 69 mg/m3 were different from their patterns on the control day, but the differences were not statistically significant (P > 0.05). There were significant differences (P < 0.05) between the visual evoked potential patterns of subjects exposed to 69 mg/m3 and those exposed to 340 mg/m3. Data on contrast sensitivity indicated greater effects at 340 mg/m3 than at 69 mg/m3; effects were most pronounced on the last day of exposure. However, statistical analysis was not reported, and the data are limited by the small number of subjects. There were no indications of peripheral hearing loss at either exposure level. There were significant post-exposure performance deficits (P < 0.05) among subjects exposed to 340 mg/m3 when compared with the group exposed to 69 mg/m3 in tests of motor/cognitive function and motor function, and there was a near-significant difference (P = 0.09) on a test of motor function (Altmann et al., 1990, 1992).
After a 10-min exposure to tetrachloroethene at 1000 mg/m3, which produced a blood level of 3 mg/l, volunteers showed a slight (but significant) increase in resistance to attention failures under conditions of monotony in the vigilance performance test. A similar effect can be produced by exposure to low concentrations of other solvents and to low blood levels of ethanol (Beneš et al., 1986).
Lauwerys et al. (1983) studied 26 workers (24 women and 2 men) occupationally exposed to tetrachloroethene in six dry cleaning shops in Belgium for a mean of 6.4 years. The controls (31 women and 2 men) worked in a chocolate factory or an occupational health service and reported no occupational exposure to solvents. Personal air sampling revealed a mean tetrachloroethene concentration (8-h TWA) of about 140 mg/m3 (TWA concentration range approximately 62–260 mg/m3). Seventeen of 22 symptoms of nervous system disturbances were more prevalent among the dry cleaners than among unexposed controls. However, none of the differences was statistically significant, and there was no relationship with exposure duration. None of the mean scores of the dry cleaning workers on the four neurobehavioural tests were significantly lower than those of the control group, and the prevalence of abnormal scores (those beyond the 5th or 95th percentile of the control group) did not vary significantly between the two groups (Lauwerys et al., 1983).
Seeber (1989) administered a number of neuropsychological tests to 44 German dry cleaning workers with high tetrachloroethene exposure (39 women, 5 men), 57 dry cleaners with low exposure (50 women, 7 men), and 84 controls (employees of department stores and hotels) without exposure (64 women, 20 men). Air monitoring revealed that mean tetrachloroethene concentrations (8-h TWA) for the low and high exposure groups were approximately 83 ± 55 mg/m3 and 370 ± 120 mg/m3, respectively. The mean durations of occupational exposure for the low and high exposure groups were 11.8 and 10.6 years, respectively. Subjects were administered standardized tests of symptoms and personality, tests of sensorimotor function (including finger tapping and aiming), and dexterity tests. Threshold of perceptual speed was assessed by recognition of stimuli flashed briefly on a screen. Choice reaction time was determined using "nine light and tone stimuli". Subtests of the Wechsler Intelligence Test (digit span, digit symbol, and cancellations) were used, as was recognition of words, faces, and digits. Intelligence was assessed using a logical thinking subtest. Stratified analysis was used to control for various group differences. Both exposed groups performed significantly (P < 0.01) worse than controls in tests for the threshold of perceptual speed and choice reaction times and had lower scores in tests of attention (digit reproduction and digit symbol) and visual scanning and memory (cancellation), but there were no significant differences between the low and high exposure groups. Exposed groups reported more neurological signs and emotional liability, but the scores were statistically significantly different only in the low exposure group. There were no differences between groups on the other tests. Controlling for group differences in alcohol consumption did not alter any test results (Seeber, 1989).
CNS effects were assessed in 56 workers (27 women, 29 men; mean tetrachloroethene exposure time 3 years) from three dry cleaning shops in China and in 69 unexposed workshop controls (37 women, 32 men). Passive air sampling revealed a geometric mean 8-h TWA tetrachloroethene concentration of 140 mg/m3 and a TWA concentration range of about 28–670 mg/m3. Five symptoms (dizziness, drunken feeling, floating sensation, heavy feeling in head, facial flushes) were significantly more prevalent among the dry cleaning workers (Cai et al., 1991).
Nakatsuka et al. (1992) evaluated colour vision in 64 dry cleaning workers from the same shops studied by Cai et al. (1991). Passive sampling revealed a geometric mean air concentration of tetrachloroethene (averaging time not reported) of 90 mg/m3. Lanthony’s new colour test found no significant difference between the groups, and there were no distinct cases of colour vision loss among the dry cleaning workers (Nakatsuka et al., 1992).
Ferroni et al. (1992) evaluated neuroendocrine and neurobehavioural effects of tetrachloroethene exposure among all 60 female dry cleaners who worked (mean duration 10 years) in shops in a small town near Parma, Italy. Controls (n = 30) were hospital workers who cleaned clothes using a water-based process, with no organic solvent exposure. The groups were similar in age, physical characteristics, and smoking habits, but alcohol intake was about 5% higher (P < 0.03) in the control group. Air sampling found a median 4-h TWA tetrachloroethene concentration of about 100 mg/m3 and a TWA concentration range of approximately 7–460 mg/m3. The dry cleaners showed significantly reduced performance in three tests (simple reaction time, vigilance, and stress). Additionally, the mean serum level of prolactin was significantly higher in the workers than in the matched controls. None of the three measures of exposure (duration of exposure and air or blood concentration of tetrachloroethene) was significantly associated with decreased test scores or increased serum prolactin levels among the dry cleaners (Ferroni et al., 1992).
Colour vision was assessed in 35 randomly selected dry cleaners who had worked for at least 1 year (mean 8.8 years) in dry cleaning shops in Modena, Italy. The controls were 35 Modena factory workers without occupational or hobby exposure to solvents or other neurotoxic chemicals, matched by gender, age, alcohol consumption, and cigarette use. For all dry cleaners, the mean 8-h TWA tetrachloroethene concentration was 41 mg/m3, and the TWA concentration range was about 2.8–210 mg/m3 (from passive sampling of personal air). The mean 8-h TWA concentration was only slightly higher (48 mg/m3) for the 22 operators than for the 13 ironers (34 mg/m3). Only 3 dry cleaning workers, as opposed to 13 controls, scored a perfect test score (P < 0.01). Mistakes were made mainly in the blue-yellow range (an effect associated with solvent exposure). Overall, the workers showed poorer performance on the test compared with controls. There was also a statistically significant positive correlation (P < 0.01) between TWA air concentrations and total errors (r = 0.52). Total errors were not associated with two other measures of tetrachloroethene exposure (mean duration and an integrated index of exposure, yearly TWA level) (Cavalleri et al., 1994).
Colour vision in 33 of the 35 dry cleaners and ironers (two had retired) examined by Cavalleri et al. (1994) was re-examined after 2 years by Gobba et al. (1998). Tetrachloroethene concentration had increased during the 2-year period for 19 subjects, identified as Group A, and had decreased for 14 subjects. For the 33 workers overall, exposure was only slightly changed over the 2-year period (geometric mean, from 17 to 13 mg/m3). Colour vision had deteriorated between the two surveys for the entire group, a reflection of the colour vision loss among Group A subjects, whose exposure had increased in the second survey. Perception of the blue-yellow range of colour was most affected. Colour vision performance for the entire group was related significantly to age (r = 0.45) and tetrachloroethene concentration (r = 0.39). For subjects who experienced lower exposure concentrations by the second survey, the error score had not changed (Gobba et al., 1998).
Complaints of fatigue and confusion, accompanied by cognitive deficits in memory, motor, visuospatial, and executive function, were noted in four patients diagnosed with tetrachloroethene encephalopathy (induced by 10–16 years of exposure as a dry cleaner in three cases and following mistaken home treatment of wood in the fourth case). Exposure data were not available. The investigators assessed the performance of 65 dry cleaning workers (35 men, 30 women) in neurobehavioural tests designed to detect the same impairments noted in the clinical cases. Workers in the low, moderate, and high groups had been employed for 2.1, 3.9, and 14.6 years, respectively. The three groups were also characterized by estimates of current exposure (low, medium, and high), corresponding to mean tetrachloroethene air concentrations (8-h TWA) of about 76, 160, and 280 mg/m3. Scores of the dry cleaning workers with high chronic exposure were statistically significantly lower (P < 0.01) than those of the workers with low chronic exposure in three tests of visual reproduction, pattern memory, and pattern recognition. These impairments of visually mediated function were consistent with the impairment of visuospatial functions observed in the four patients diagnosed with tetrachloroethene encephalopathy. Among complaints by the dry cleaning workers, only the number of complaints of dizziness from standing up rapidly and "solvent-induced dizziness" over the previous 3 months was significantly elevated in the high exposure group. Effects on visuospatial function were consistently found in subjects employed as operators for an average of 14.6 years and exposed to an estimated tetrachloroethene 8-h TWA air concentration of 280 mg/m3 (Echeverria et al., 1995).
Neurophysiological and neurobehavioural techniques were used to assess the effects of long-term residential exposures to tetrachloroethene in 5 males and 9 females living in Mulheim, Germany, from a population of 92 subjects living in neighbourhoods close to dry cleaning facilities. Selected subjects had a tetrachloroethene blood level above 0.002 mg/l, had lived above or next to a dry cleaning facility for at least 1 year, and had no occupational exposure to organic solvents. A control group (9 men, 14 women), matched for age and sex, consisted mainly of staff of a public health office or an institute for environmental hygiene, with no reported history of solvent exposure. The investigators were employed at these two organizations and therefore would have been administering the tests to co-workers, which raises concerns over potential bias. For the exposed subjects, indoor air sampling indicated that the mean (7-day TWA) air concentration was 4.8 mg/m3 and the median was 1.4 mg/m3. After adjusting for covariates and possible confounders, the mean scores of exposed subjects were statistically significantly worse on three neurobehavioural tests. No differences were observed in the finger tapping or eye–hand coordination tests, which are measures of fine motor function, on visual evoked potential, which would be expected to be less sensitive than direct measurement of visual function, or on vibration perception at the ankle using a tuning fork (Altmann et al., 1995).
A possible effect of tetrachloroethene exposure on vocal reaction times was assessed by examining 35 dry cleaners and 39 unexposed controls, matched for age and education. Exposure was assessed only by a "grab sample" technique and indicated a median concentration of tetrachloroethene of 55 mg/m3 (range 14–940 mg/m3). An index of cumulative exposure to tetrachloroethene was also developed. The exposed group had statistically significantly longer mean reaction times and/or vocalization durations, and statistically significant positive correlations were observed between cumulative tetrachloroethene exposure and immediate and delayed tasks (r = 0.69 and r = 0.73, respectively) (Spinatonda et al., 1997).
Schreiber et al. (2002) reported the findings from studies that assessed neurological function in two potentially exposed populations in the USA: apartment residents (two buildings in New York City that each contained a dry cleaning business) and day care employees (in a building in Albany, New York, that also housed a dry cleaning business). The residential study considered 17 subjects (11 adults aged 20–50 years, 2 adults over 60 years, and 4 children) from six families that had resided in one of the buildings for an average of 5.8 years. Control subjects from among New York State Department of Health employees in Albany were age- and gender-matched to exposed apartment residents. The selection of controls provides a potential for bias in this residential study. First, the controls were from Albany, which is some 240 km from New York City, where the cases lived. Second, the investigators would have known whether they were studying cases or controls (because of the distance, the tests were presumably carried out at different facilities and at Albany were being administered to co-workers). In the day care study, there were nine adults in the exposed group. Controls were age- and gender-matched subjects from several groups — acquaintances of the exposed participants, local retail shop employees, New York State Department of Health employees, or staff from other local day care centres. It is unclear how many were New York State Department of Health employees. For the residential study, median concentrations in air samples, which were taken during the day during active periods of dry cleaning, were 1.4 mg/m3 (mean 2.5 mg/m3; range 0.69–6.2 mg/m3). At the time of visual testing, the median tetrachloroethene concentration was 0.62 mg/m3 (mean 1.2 mg/m3; range 0.069–5.4 mg/m3). Atmospheric monitoring of the day care facility before closure of the dry cleaning business showed concentrations ranging from 1.9 to 2.4 mg/m3, with median and mean concentrations of 2.2 mg/m3. At the time of visual testing, 5 weeks after removal of the dry cleaning machines, concentrations approached background values (range 0.0083–0.056 mg/m3). Visual acuity did not differ between exposed and control groups. Group mean scores for visual contrast sensitivity across spatial frequencies were statistically significantly lower in exposed residents and day care employees than in respective controls. An exposure–response analysis did not show an association between poorer performance and increasing tetrachloroethene concentration. The spatial vision of the four children was poor enough that it would likely affect normal activities. In the residential study, exposed subjects were retested twice, 6–10 and 17–21 months after closure of the dry cleaning facility. Visual contrast sensitivity performance appeared to worsen over successive examinations (although statistical comparisons were not performed). Colour vision assessment revealed that the exposed groups made more errors than respective controls, but the differences were not statistically significant (Schreiber et al., 2002).
Feeling tone checklist, Wechsler digit span, Wechsler digit symbol, Neisser letter search, critical flicker fusion, Santa Ana dexterity, choice reaction time, and simple reaction time tests were carried out on 18 dry cleaners (9 males and 9 females; the exposed group) and on 9 laundry workers (the control group). The mean TWA tetrachloroethene concentration for the dry cleaning group (based on breath samples and environmental monitoring for the 5 days of testing) was 120 mg/m3, while the men (who are often machine operators) were exposed to a mean TWA concentration of 220 mg/m3. Although significant differences were found for 2 of the 11 measured end-points, multiple regression analysis suggested that these effects were probably due to exposure to Stoddard solvent (Tuttle et al., 1977).
No evidence of neurological effects was found in a study of 106 workers in a railway repair shop, where tetrachloroethene was used to clean metal parts. All had been exposed for at least 2 years. Atmospheric concentrations often exceeded 2800 mg/m3, with about 75% of measurements lying between 1.4 and 340 mg/m3 (Essing, 1975).
A review noted that an increased risk of IHD had been reported in two out of four cohort studies of dry cleaners, compared with national or regional reference populations (Mundt et al., 2003). This was somewhat surprising, because most mortality cohort studies of workers exposed to air pollutants have shown a decreased risk for IHD when these groups have been compared with national death rates, due to the well known healthy worker effect (explained by the lack of comparability between workers and the general population, which will include sick and disabled people unable to work) (McMichael, 1976). It has been proposed that IHD might result via a low-grade inflammation, with release of mediators capable of increasing blood coagulability (Seaton et al., 1995; Sjögren, 1997). Of the studies reporting a possible increase in IHD risk in dry cleaners, one examined workers with only tetrachloroethene exposure and found an increased risk (SMR 1.27, 95% CI 1.02–1.55; 93 deaths); there was only a comparatively low risk for lung cancer (RR 1.17) and bladder cancer (n = 0), indicating that the increase was unlikely to be due to tobacco smoking (Ruder et al., 2001). The other study of dry cleaners found no increased risk of IHD over the whole follow-up period (RR 1.0, 95% CI 1.0–1.1); a slightly increased risk (RR 1.1, 95% CI 1.0–1.3) was reported for the 1979–1991 period of follow-up (Blair et al., 2003). Other determinants may explain these observations, such as imbalances regarding job strain and effort–reward.
In a cross-sectional study of dry cleaners, a subgroup was exposed to tetrachloroethene at a mean concentration of 335 mg/m3 (range 84–750 mg/m3). This group had a higher total leukocyte count compared with blood donors as a possible expression of an inflammatory process (Andrys et al., 1997). An increased total leukocyte count is an established risk indicator for IHD (Danesh et al., 1998). Studies of tetrachloroethene exposure and its relation to other inflammatory and IHD markers such as C-reactive protein and fibrinogen (Danesh et al., 1998) are needed in the future in order to further explore these relations.
Toxicity data are available for aquatic organisms (microorganisms, algae, invertebrates, and fish), for pond studies, and for terrestrial organisms (soil bacteria, plants, invertebrates, and mammals). Data in this section are mainly taken from a source document, which generally did not report 95% CIs (EC, 2001).
The literature contains values of 68 mg/l as a 10-min EC10 for inhibition of bioluminescence in Photobacterium phosphoreum (Bazin et al., 1987), a 24-h EC50 of 100 mg/l in Tetrahymena pyriformis (Yoshioka et al., 1986), and a 24-h EC50 of 112 mg/l in Nitrosomonas sp. bacteria (Blum & Speece, 1991). All of the studies reported appear to be valid, but the test with P. phosphoreum is not relevant for microorganisms in treatment plants. The other tests are suitable for use in the risk assessment and indicate 50% toxic effects at about 100 mg/l (EC, 2001).
A 72-h EC50 (cell multiplication inhibition) of 3.64 mg/l was reported for the freshwater alga Chlamydomonas reinhardtii by Brack & Rottler (1994). The test conditions were fully described, and the test can be considered valid. In the same study, Brack & Rottler (1994) reported a 72-h EC10 of 1.77 mg/l for this alga. The EU Technical Guidance Document (ECB, 2003) suggests that this EC10 may be considered as a NOEC and that, for algae, a 72-h NOEC may be considered as a long-term study result. A 96-h NOEC of 816 mg/l was reported in the freshwater alga Selenastrum capricornutum (USEPA, 1980).
A 48-h NOEC of 1 mg/l is reported by Erickson & Hawkins (1980) for estuarine phytoplankton. No indication is given as to the species tested; therefore, the result is not considered valid (EC, 2001). Other studies, in marine diatoms, report an EC50 (exposure time unspecified) of 10.5 mg/l in Phaeodactylum tricornutum (Pearson & McConnell, 1975), a 4-h EC10 of >36 mg/l in Haematococcus pluvialis (Knie et al., 1983), a 96-h EC50 of 500 mg/l in Skeletonema costatum (USEPA, 1980), and a 7-day EC50 of >16 mg/l in Skeletonema costatum (Erickson & Freeman, 1978).
The lowest 48-h EC50 reported for Daphnia magna is 8.5 mg/l (Richter et al., 1983) and is based on measured concentrations. The test conditions were fully described for this result, and the test is therefore considered valid. A 24-h EC50 of 3.2 mg/l is reported by Bazin et al. (1987) based upon nominal concentrations. No details are given as to the test method, so this study was considered not valid (EC, 2001). Others have reported a 24-h EC50 value of 123–176 mg/l (Bringmann & Kühn, 1982) and 48-h EC50 figures for the water flea of 18–22 mg/l (LeBlanc, 1980; Knie et al., 1983).
For other aquatic invertebrates, a 3-h EC50 of 1.8 mg/l is reported for Monia macrocopa (Yoshioka et al., 1986), and a 48-h EC50 of 3.5 mg/l is reported for Elminius modestus (Pearson & McConnell, 1975). Both of these test results are based upon nominal concentrations; the test methods used are also poorly described, and therefore these results are considered not valid (EC, 2001). Other acute toxicity values include a 96-h EC50 of 10.2 mg/l in Mysidopsis bahia (USEPA, 1980) and a 48-h EC50 of 30.8 mg/l in Tanytarsus dissimilis (Call et al., 1983).
In longer-term studies, a 28-day NOEC based upon reproduction of 0.51 mg/l was reported in Daphnia magna (Richter et al., 1983). The test concentrations were measured and the test conditions are fully described, and so the result is considered as valid (EC, 2001). A 28-day NOEC for reproduction and length was reported to be 1.11 mg/l (Call et al., 1983). A lower value of 0.45 mg/l is reported for Mysidopsis bahia (USEPA, 1980); no indication is given as to the test conditions, and the result is therefore considered not valid (EC, 2001).
In field studies, tetrachloroethene was added to a natural pond at nominal concentrations of 25 and 250 mg/l. The initial concentrations were measured at 0.44 mg/l and 1.2 mg/l, respectively, and were below the detection limit (0.1 mg/l) after 7 weeks. Adverse effects were noted in the phytoplankton and zooplankton communities under the conditions of the experiment. The numbers of Daphnia declined to zero within 1 day at the higher concentration and within 3.5 days at the lower concentration. Some of the plankton communities were unaffected by exposure, some increased in number, and others decreased in number during the study. Changes were also observed in the untreated control areas, where Spyrogyra sp. died out within 5–7 days (Lay et al., 1984).
In a follow-up study, an experimental pond was divided into four subsystems (enclosures) using open-ended polyvinyl chloride cylinders sunk into the pond sediment. Two enclosures were continuously dosed with tetrachloroethene for 11 weeks. The concentrations in the water were measured regularly and reached the target levels of 0.8 and 1.6 mg/l after 5 days. Primary production in the treated enclosures showed a decrease in the short term, but a marked increase from the 2nd week. Within this group, some individual species disappeared, while others increased in number. The population of copepods was greatly reduced at the higher treatment level; at the lower treatment level, an initial decrease was followed by an increase in reproduction rate towards the end of the experiment. Among rotifers, an initial increase in population for some species was followed by their later disappearance (Lay & Herrmann, 1989)
These field studies show that tetrachloroethene can have an impact on aquatic ecosystems. It is not possible to derive a NOEC from either of these studies, but they are considered as supporting information in the derivation of the PNEC (EC, 2001).
The most sensitive freshwater species in acute toxicity tests appears to be Japanese medaka (Oryzias latipes), with a 48-h LC50 of 1.6 mg/l (Yoshioka et al., 1986). However, the reported effect concentration appears to be based upon nominal concentrations in the test solution, and it is unclear whether appropriate measures were taken to minimize evaporation of tetrachloroethene from the test solution, and so the study was considered invalid (EC, 2001). The next most sensitive species appears to be rainbow trout (Oncorhynchus mykiss), with a 96-h LC50 of 5 mg/l. This study was considered to be valid because the test conditions were fully described and the effect concentration was based upon measured levels (Shubat et al., 1982). Other 96-h LC50 values reported include 5 mg/l in dab (Limanda limanda) (Pearson & McConnell, 1975), 8.4 mg/l in American flagfish (Jordanella floridae) (Smith et al., 1991), 13 mg/l in bluegill (Lepomis macrochirus) (Buccafusco et al., 1981), 13.4–23.8 mg/l in fathead minnow (Pimephales promelas) (Alexander et al., 1978; Walbridge et al., 1983; Broderius & Kahl, 1985), 29–52 mg/l in sheepshead minnow (Cyprinodon variegatus) (Heitmuller et al., 1981), and 130 mg/l in ide (Leuciscus idus) (Knie et al., 1983). In Japanese medaka, a 96-h LC50 for 1-day-old egg viability was 27 mg/l (95% CI 19.5–32.9 mg/l), and the NOEC was 2.19 mg/l (95% CI 0.80–3.84 mg/l) (Spencer et al., 2002). A 7-day LC50 value of 17.8 mg/l was reported for guppy (Poecilia reticulata) (Könemann, 1981).
In chronic toxicity tests, Mexican molly (Poecilia sphenops) appears to be the most sensitive species, with a 60-day LOEC of 1.6 mg/l (Lökle et al., 1983). This study was not considered valid, because no measures appear to have been taken to monitor concentrations or to minimize evaporation of tetrachloroethene from the test solution; the resultant effect concentrations are based on nominal concentrations (EC, 2001). The next most sensitive species appears to be American flagfish, with a 28-day NOEC based on fry survival of 2.34 mg/l. In this study, a 10-day NOEC of 1.99 mg/l based upon larval survival was also reported (Smith et al., 1991). These results are considered to be valid, as test conditions were fully reported and the effect concentrations are based upon measured levels.
Since the EU risk assessment was drafted, a study has been published in which 1-day-old eggs of Japanese medaka were exposed (static renewal method) for 10 days at 0, 1.5, 3, 6, 12, or 25 mg/l. Even at the lowest exposure level (1.5 mg/l), tetrachloroethene induced reduced hatching and a significant number of developmental effects (abnormal development of the circulatory system, yolk sac oedema, pericardial oedema, scoliosis, haemorrhaging, blood pooling, and defects in heart morphology). The severity of these abnormalities was concentration dependent (Spencer et al., 2002).
No data on wild-living terrestrial mammals were identified. However, mammalian species have been extensively tested in the laboratory, and the results are reviewed in section 8. The effects observed vary with species and occurred at much higher concentrations than have been measured or are predicted to arise in the environment, and it is thus unlikely that adverse effects will be noted under typical conditions (EC, 2001).
The acute toxicity of tetrachloroethene to earthworms (Eisenia foetida) has been tested using OECD Guideline No. 207. The medium was an artificial soil (10% peat, 20% kaolin clay, and 70% industrial sand) with a pH of 6. The exposure period was extended from 14 days (as recommended in the Guideline) to 28 days. Production of cocoons was observed in addition to mortality. To overcome the problem of evaporation, tetrachloroethene was tested in closed containers supplied with sufficient oxygen for the worms to breathe, and the soil and substrate were replaced weekly. The 14-day LC50 was 100–320 mg/kg, the 28-day NOEC (based upon cocoons) was <18 mg/kg, and the 28-day NOEC (based upon appearance) was 18–32 mg/kg (Vonk et al., 1986).
A second study of the acute toxicity of tetrachloroethene to earthworms (Eisenia foetida) using OECD Guideline No. 207 has been reported. The earthworms were 2 months old and weighed 246–585 mg. They were exposed to tetrachloroethene in glass jars containing an artificial soil (10% peat, 20% kaolin clay, and 70% industrial sand) with a pH of 6 and absolute water content of 34%. The exposure period was 14 days at 20 şC. Mortality and changes in biomass, behaviour, and morphology were recorded, and the LC50 was determined by probit analysis. The highest test concentration causing no mortalities or changes in weight and behaviour was 577 mg/kg, the lowest test concentration causing 100% mortality was >1000 mg/kg, and the LC50 was determined as 945 mg/kg. At 1000 mg/kg, worms refused to crawl into the substrate. As reported concentrations were nominal and no precautions were taken to prevent evaporation of tetrachloroethene, these results may underestimate actual toxicity (Römbke et al., 1991).
Tetrachloroethene toxicity has been studied in the carabid beetle (Poecilus cupreus). Beetles were exposed for 14 days in sand (99.7% silicon dioxide) moistened to 70% of its holding capacity with water containing tetrachloroethene (1.25 mg/l, equivalent to 5 mg/kg sand). Mortality and behavioural changes were observed. There was then a 6-day rest period, followed by exposure to tetrachloroethene for a further 11 days, with tetrachloroethene applications (3 mg/kg) occurring every 2 days. In the acute tests, no mortality or behavioural changes were observed, although there was an 18% reduction in feeding rate. In the chronic study, no mortality or behavioural changes were observed, although a 14% reduction in feeding rate was observed (Römbke et al., 1993).
An acute toxicity test and a reproduction test were carried out in the soil-dwelling springtail (Folsomia candida). The test was modified by using a standard soil (LUFA Speyer) instead of an artificial soil. In the acute test, the springtails were exposed for 24 h to tetrachloroethene concentrations of 0.1, 1.0, 10, 100, or 1000 mg/kg dry weight, and the 24-h EC50 was calculated as 113 mg/kg (Heimann & Härle, 1993). The organic matter content of the test soil was reported as 0.7%, so the 24-h EC50 was 549 mg/kg when converted to a standard organic matter content (3.4%). Problems with the reproduction test results (mortality in the control tests) resulted in them being regarded as invalid (EC, 2001)
Vonk et al. (1986) studied the effect of tetrachloroethene on microorganisms responsible for soil respiration, ammonification, and nitrification. Short-term oxygen consumption was measured with a Warburg respirometer. A loam soil and a humic sand were used in the test. Measurements were taken with and without glucose added as an external source of carbon (basal and stimulated soil respiration). Nitrification was measured by addition of ammonium sulfate to the soils and monitoring the conversion of ammoniacal nitrogen. A NOEC of <2000 mg/kg wet weight was determined for soil respiration, and NOECs of <40 mg/kg wet weight and <0.1 mg/kg wet weight were determined for nitrification with humic sand and loam soil, respectively.
Kanazawa & Filip (1987) studied the effect of tetrachloroethene on soil biomass and microbial counts in an arable brown soil (pH 6.8, carbon 1.44%, nitrogen 0.12%). This was air dried, large particles were discarded, and water was added to obtain a soil moisture content of 50% of the soil maximum. The soil was placed in flasks, which were closed with rubber stoppers. Tetrachloroethene was then added to the soil (test concentrations 0.1, 1, and 10 mg/kg). The samples were incubated for 8 weeks in the dark at 25 şC. Samples were taken at 3, 7, 14, 28, and 56 days and analysed microbiologically and for soil biomass. All tested tetrachloroethene concentrations decreased the amount of soil biomass, the greatest effect being observed at 10 mg/kg. Very little effect on the population of soil fungi was observed at 0.1 and 1 mg/kg levels, but at 10 mg/kg, fungal growth was inhibited. Both copiotrophic and oligotrophic aerobic soil bacteria were inhibited at 10 mg/kg after 3 days, but this was followed by an increase in the populations up to 28 days. The organic matter content of the soil was reported as 1.44%.
The effect of tetrachloroethene on the dehydrogenase activity of soil microorganisms was studied by Danneberg (1993). Two concentrations were tested: 0.5 and 5 mg/kg dry weight. There was an initial increase (42–62%) in dehydrogenase activity, followed by a decrease (11–18%) after 14 days and an increase (6–13%) after 28 days. The data showed no consistent effect.
A source document (EC, 2001) briefly reports a 16-h EC10 in Pseudomonas putida of >45 mg/l (Knie et al., 1983).
In a study of effects on the early developmental stage of oats8 (Avena sativa), germinated plants were exposed for 16 days to tetrachloroethene at 1, 10, 100, or 1000 mg/kg dry weight in a standard soil. The 16-day NOEC (growth) was 100 mg/kg, the 16-day NOEC (sublethal effects) was 1 mg/kg, and the 16-day EC50 (growth) was 580 mg/kg (Bauer & Dietze, 1992). The organic matter content of the test soil was reported as 2.29%. This gives a 16-day NOEC (growth) of 148 mg/kg, a 16-day NOEC (sublethal effects) of 1.48 mg/kg, and a 16-day EC50 (growth) of 861 mg/kg when converted to a standard organic matter content (EC, 2001).
Cuttings of a hybrid poplar (Populus deltoides × nigra DN34) were exposed to tetrachloroethene in hydroponic solutions in closed vessels, to reduce volatilization and maintain concentrations. Solutions were replaced every 2 days, and the concentrations were confirmed by analysis. The mass of the cuttings was determined after 2 weeks of exposure. The use of water by the plants was also monitored at 2-day intervals as a measure of the transpiration rate. The results were presented as the concentration that resulted in no increase in the mass of the plants over the 2-week period (45 mg/l) and as the concentration producing a 50% reduction in the transpiration rate over the same period (38 mg/l) (Dietz & Schnoor, 2001).
Bleaching of chlorophyll from sun-exposed needle surfaces was seen when a 10-year-old spruce (Picea sp.) tree was exposed to trichloroethene and tetrachloroethene in the Black Forest. Exposures were uncontrolled, the substances being allowed to evaporate from a bottle below the tree. The effect occurred in needles on the upper face of twigs and only during sunny periods, with partial recovery of damaged needles during cloudy periods. Needles on shaded twigs remained dark green. Similar symptoms were seen on the sun-exposed leaves of a hornbeam shrub (Carpinus betulus) at a distance of 2 m from the conifer. It was suggested that the combined action of the chloroethenes and UV light was required. As UV is attenuated at lower altitudes by smog, etc., the effect is observed only at higher altitudes (Frank & Frank, 1985). On further investigation, a 5-h exposure of single spruce tree needles to airborne tetrachloroethene under direct irradiation caused the needles to change colour from dark green to a dirty brown green. The concentrations of pigments in the exposed needles were found to be reduced, particularly chlorophyll-a and beta-carotene (Frank & Frank, 1986). The radiation used may have been sufficiently energetic to cause direct photolysis of the substance. Also, exposure to UV alone led to a reduction in one of the pigments studied. Therefore, it is possible that the needles were under stress as a result of the UV exposure alone (EC, 2001).
In a further experiment (reported only in a progress report to the Bavarian State Ministry), acute phytotoxicity (earlier and heavier yellowing of the directly irradiated needles) was seen when 3-year-old potted spruce trees were exposed at 130 µg/m3 for 1–2 months in exposure chambers. Continuing exposure led to heavy needle loss and eventually the deaths of the experimental trees. The lighting conditions appear likely to include higher levels of UV than under natural conditions, and there were also problems of pests on the trees, indicating non-ideal growing conditions (unreferenced study described in EC, 2001).
As a result of the above conifer studies, a more comprehensive study was carried out to establish a no-effect concentration for plants exposed to tetrachloroethene through the air (PRI, 2000). To represent a range of European flora, the study was carried out on bean (Phaseolus vulgaris), wheat (Triticum aestivum), kale (Brassica oleracea), Norway spruce (Picea abies), Scotch pine (Pinus sylvestris), European beech (Fagus sylvatica), white clover (Trifolium repens), purple moor grass (Molinia caerulea), European blueberry or bilberry (Vaccinium myrtillus), haircap moss (Polytrichum formosum), Schreber’s moss (Pleurozium schreberi), and goose neck moss (Rhytidiadelphus squarrosus). Plants were exposed in open-top chambers at measured, seasonal average concentrations ranging from 7 to 2140 µg/m3 for 1.5–6 months (depending upon species), and then monitored over the winter for delayed effects. Plants were evaluated for injured and senescent leaves (wilting and yellowing), numbers of flowers, pods, and berries, stem diameters (trees), and plant height. Chlorophyll was measured in beech and kale, as they did not show any overt signs of foliar injury (PRI, 2000).
NOEC values reported in the study are summarized in Table 10. Where a concentration–response relationship could be established, a logistic curve was fitted to the data. The NOEC was derived as the concentration at which the curve crossed the lower 95% confidence limit of the control response (the asymptote to the curve). Two experiments were carried out on beans and clover. The first exposures of beans in the spring showed strong effects of tetrachloroethene, with the pod yield reduced to zero at the three highest concentrations. In a repeat experiment in the summer, no adverse effects were seen at any concentration. Clover was not affected as strongly in the spring experiment and was also not affected at any concentration in the summer repeat. From the observations on foliar injury, there did not appear to be any significant effects at concentrations up to about 100 µg/m3. The lowest concentration at which most species showed noticeable effects (>20% of the surface area affected) was 257 µg/m3 (PRI, 2000).
Table 10: NOECs for adverse effects of tetrachloroethene in different plant species, as reported in PRI (2000).a
|
Plant species |
Representative variable |
NOEC (µg/m3) |
|
Seed/fruit production |
||
|
Bean I (Phaseolus vulgaris) |
Pod dry weight |
46b |
|
Bean II (Phaseolus vulgaris) |
Pod dry weight |
>2056 |
|
Wheat (Triticum aestivum) |
Ear dry weight |
747–1966c |
|
Kale (Brassica oleracea) |
Shoot dry weight |
>1955 |
|
Blueberry/bilberry (Vaccinium myrtillus) |
Weight of berries |
252–1009c |
|
Growth/timber production |
||
|
Scotch pine (Pinus sylvestris) |
Stem diameter |
319b |
|
Norway spruce (Picea abies) |
Stem diameter |
387b |
|
European beech (Fagus sylvatica) |
Stem diameter |
>2104 |
|
Growth/survival |
||
|
Haircap moss (Polytrichum formosum) |
Regrowth |
>2101 |
|
Schreber’s moss (Pleurozium schreberi) |
Regrowth |
>2101 |
|
Goose neck moss (Rhytidiadelphus squarrosus) |
Regrowth |
>2101 |
|
Clover I (Trifolium repens) |
Shoot dry weight |
1034b |
|
Clover II (Trifolium repens) |
Shoot dry weight |
>2179 |
|
Purple moor grass (Molinia caerulea) |
Shoot dry weight |
>2029 |
|
a |
Adapted from EC (2001). |
|
b |
NOECs derived from the exposure–response relationships as the concentration at which the curve crossed the lower 95% confidence limit of the control response. |
|
c |
Dose–response could not be established, but there was a significant difference between two treatments. |
Criticism of the NOEC derivation process used in the study report (PRI, 2000) led to a re-evaluation of the data (EC, 2001). Where a concentration–response relationship could be established, EC10 values were estimated for the same end-points indicated in Table 10, together with the ear dry weight for wheat. For bean, wheat, and clover, an EC10 value with reasonable confidence intervals could be established. The large degree of background biological variation with pine and spruce meant that this approach was unreliable, as shown by the very wide confidence limits obtained (over 2 orders of magnitude on either side for spruce). The resulting values are shown in Table 11.
Table 11: EC10 values for adverse effects of tetrachloroethene in plants, as reassessed by EC (2001).
|
Plant species and effect |
EC10 (µg/m3) |
95th percentile confidence limits |
|
Bean (Phaseolus vulgaris), pod dry weight |
48 |
31–74 |
|
Wheat (Triticum aestivum), ear dry weight |
1239 |
312–4912 |
|
Norway spruce (Picea abies), increase in stem diameter |
14 |
0.1–1442 |
|
Scotch pine (Pinus sylvestris), increase in stem diameter |
43 |
1.6–1990 |
|
Clover (Trifolium repens), shoot dry weight |
543 |
89–3317 |
NOECs were also estimated by establishing the highest concentration tested where the effect in the exposed group was not significantly different from that in the controls. This allowed some of the other end-points to be considered, such as foliar injury and senescence. As many of the end-points monitored as could be considered were included, and the results for the most sensitive end-point for each species are included in Table 12. For the bean, the value from the analysis of the dose–response relationship was retained. There was no strict NOEC for the bean, as effects were noted at the lowest test concentration (82 µg/m3). The logistic curve-fitting method and the EC10 approach gave similar figures (46 and 48 µg/m3), and the lower of these values was used. For clover, the two methods again gave similar results, but in this case the lower value is the EC10 result, and this was used. As noted above, the EC10 values estimated for pine and spruce are not considered to be reliable in view of the wide confidence limits obtained. Values for foliar injury were lower than those derived from the curve-fitting method and so were used for these two species (EC, 2001).
Table 12: NOEC values for the most sensitive end-point for each plant species, as reassessed by EC (2001).
|
Plant species |
End-point |
NOEC (µg/m3) |
|
Bean (Phaseolus vulgaris) |
Pod dry weight |
46 |
|
Wheat (Triticum aestivum) |
Ear dry weight |
747 |
|
Kale (Brassica oleracea) |
Stem dry weight |
758 |
|
Spruce (Picea abies) |
Foliar injury |
109 |
|
Pine (Pinus silvestris) |
Foliar injury |
109 |
|
Beech (Fagus sylvatica) |
Foliar injury |
750 |
|
White clover (Trifolium repens) |
Shoot dry weight |
543 |
|
Purple moor grass (Molinia caerulea) |
Senescence |
109 |
|
Blueberry/bilberry (Vaccinium myrtillus) |
Senescence |
109 |
|
Haircap moss (Polytrichum formosum) |
Post-exposure growth |
2101 |
|
Schreber’s moss (Pleurozium schreberi) |
Post-exposure growth |
984 |
|
Goose neck moss (Rhytidiadelphus squarrosus) |
Post-exposure growth |
2101 |
Trichloroacetic acid was found in significant amounts in all four species analysed (pine, spruce, bean, and kale). The highest concentrations were found in conifer needles, with levels up to 1000 times those reported for samples collected in the field. Although the levels of trichloroacetic acid in the biomass increased with the exposure concentration of tetrachloroethene, there was not a linear relationship. Trichloroacetic acid might be responsible for inducing the effects seen in the plants. Trichloroacetic acid formation presumably takes place in the plants after uptake of tetrachloroethene from the air (EC, 2001).
The human epidemiological data on all health effects of tetrachloroethene involve people exposed predominantly to tetrachloroethene in the dry cleaning industry or exposed during the use of tetrachloroethene as one of perhaps several solvents in the electronics industry or in metal degreasing operations. Additional studies involving exposures to tetrachloroethene in finished drinking-water involved exposures to much lower levels — and to a variety of other chemicals — and were not considered influential for the purposes of this CICAD. Thus, studies of different health effects were performed on similar cohorts, and any systematic bias would affect all end-points and most studies. Other general limitations were noted. Most studies did not provide information on dose–response; thus, effects seen were considered to result from typical measured tetrachloroethene exposures in dry cleaning operations of about 100 mg/m3. In occupational studies, it is often not possible to distinguish acute effects from chronic effects. In some studies, information on dry cleaners and laundry workers was included together; laundry workers might not have been exposed to tetrachloroethene, and combining in this manner would likely reduce the power of the studies to detect any effect. Nonetheless, the studies as a whole were considered fairly typical of occupational studies.
The toxicological properties of tetrachloroethene have been investigated in humans and laboratory animals. The key toxicological end-points appear to be neurotoxicity, nephrotoxicity, hepatotoxicity, reproductive/developmental toxicity, and carcinogenicity.
Tetrachloroethene is absorbed following inhalation, dermal, and oral exposure in laboratory animals and humans and, once in the body, distributes widely and concentrates in fat. The majority of absorbed tetrachloroethene is excreted unchanged in exhaled breath. A small percentage of tetrachloroethene is metabolized in humans and laboratory animals, with the exact proportion differing among species. Mice appear to metabolize more absorbed tetrachloroethene than rats or humans. Two metabolic pathways have been shown to operate in both humans and laboratory animals. The major pathway is cytochrome P450-dependent oxidation of tetrachloroethene, through a postulated epoxide intermediate (tetrachloro-oxirane), primarily to trichloroacetic acid. A second pathway involves conjugation of tetrachloroethene with glutathione catalysed by glutathione-S-transferases and is associated with generation of reactive metabolites via initial cleavage to S-(1,2,2-trichlorovinyl)-L-cysteine and further degradation of the cysteine conjugate by beta-lyase to a reactive dithioketene and ultimately dichloroacetic acid.
In humans, acute accidental inhalation of high (but unmeasured) concentrations of tetrachloroethene has induced CNS depression, dizziness, fatigue, headache, loss of coordination, unconsciousness, narcosis, and liver damage; some deaths have occurred. Survivors showed reversible and relatively minor effects on liver function. Human data on tetrachloroethene ingestion are limited. When used as a deworming agent, oral doses of about 70–90 mg/kg body weight have led to CNS effects (including dizziness, inebriation, nausea, psychosis, and loss of consciousness), and some fatalities have occurred. A loss of consciousness and temporary liver and kidney damage were reported in a worker who lay for 12 h in a pool of tetrachloroethene; presumably the dermal and inhalation routes could have contributed to these effects.
In laboratory rodents, acute inhalation and oral toxicity are low. In rats and mice, LC50 values are above 20 000 mg/m3, and oral LD50 values are in excess of 2 g/kg body weight. CNS depression was the major overt sign noted in treated animals. Liver and kidney toxicity were also observed.
Tetrachloroethene is irritating to the skin in humans and rabbits, but corrosion has not been observed. Slight, transient eye irritation was reported by volunteers exposed at about 520 mg/m3 vapour. In rabbits, instillation of liquid tetrachloroethene produced only minimal irritation. Mild nasal irritation was reported by volunteers exposed at 1500 mg/m3 for 2 h and at 690 mg/m3 for 7 h (but not at 730 mg/m3 for 1 h). Asthma symptoms have been reported in two cases.
There is substantial information on tetrachloroethene effects in laboratory animals repeatedly exposed by inhalation and oral routes. Liver and kidney are the main target organs, and there are species- and sex-related differences in susceptibility.
Liver weight was unaffected in mice given 20 mg/kg body weight per day, 5 days/week, for 6 weeks, but increased at 100 mg/kg body weight per day (Buben & O’Flaherty, 1985). In rats, liver weight was unaffected at 400 mg/kg body weight per day for 90 days, but increased at 1400 mg/kg body weight per day (Hayes et al., 1986). Microscopic liver changes were seen in mice given 100 mg/kg body weight per day for 11 days, whereas there were no changes at 500 mg/kg body weight per day and only minimal changes at 1000 mg/kg body weight per day in rats (Schumann et al., 1980). At 1000 mg/kg body weight per day for 10 days, a 4.3-fold increase in cyanide-insensitive palmitoyl CoA oxidation (an indicator of peroxisome proliferation) was seen in mice, compared with just a 1.4-fold increase in rats treated similarly (Goldsworthy & Popp, 1987).
No liver toxicity was seen in mice inhaling tetrachloroethene at 690 mg/m3, 6 h/day, 5 days/week, for 13 weeks (NTP, 1986). Mouse liver showed peroxisome proliferation at 1400 mg/m3, 6 h/day, for 28 days, (Odum et al., 1988), and liver necrosis was seen at 2800 mg/m3 for 13 weeks (NTP, 1986). Rats showed only minimal to mild hepatic congestion when exposed at 1400–11 000 mg/m3 for 13 weeks (NTP, 1986).
No kidney toxicity was seen in male or female Sprague-Dawley rats given 14 mg/kg body weight per day for 13 weeks, but kidney weight was increased at and above 400 mg/kg body weight per day (Hayes et al., 1986). Gavage at 1000–1500 mg/kg body weight per day for 7–42 days in F344 rats resulted in accumulation of alpha2u-globulin hyaline droplets in the kidneys of the males, but not the females (Goldsworthy et al., 1988; Green et al., 1990; Potter et al., 1996). Kidney toxicity was seen in mice given 386 (females) or 536 (males) mg/kg body weight per day, 5 days/week, for 78 weeks (NCI, 1977).
No kidney effects were seen in male or female F344 rats exposed at up to 5500 mg/m3 air, 6 h/day, for 28 days (Green, 1997). However, males exposed at 6900 mg/m3 for 28 days showed alpha2u-globulin droplet accumulation in the kidneys (Green et al., 1990). No kidney lesions were seen in male or female F344 rats exposed at up to 11 000 mg/m3 for 13 weeks. In mice, the NOEC in this study was 690 mg/m3, with minimal renal tubular effects at 1400 mg/m3 and above (NTP, 1986).
A NOAEC for neurological effects of 1400 mg/m3 (6 h/day, 5 days/week; equivalent to 250 mg/m3 for continuous exposure) was identified for rats in a comprehensive 13-week neurotoxicity study, with a possible effect (a change in flash evoked potential) at 5500 mg/m3 (Mattsson et al., 1998). When gerbils were exposed continuously (24 h/day, 7 days/week) at 410 mg/m3 for 3 months (a rather severe exposure regimen), there were changes in the brain indicative of possible loss of glial cells from the frontal cerebral cortex (Rosengren et al., 1986). Rats exposed continuously at about 2100 mg/m3 for 1–4 months showed biochemical and structural changes in the brain, including possible loss of glial cells (Wang et al., 1993).
Several studies have investigated effects on kidney function in exposed workers (mostly dry cleaners). Overall, there is some evidence that tetrachloroethene can induce glomerular and tubular kidney damage. It is difficult to determine the lowest concentrations capable of inducing adverse effects. One study (Verplanke et al., 1999) reported an increase in urinary retinol binding protein in a group of 82 workers whose estimated mean 8-h TWA exposure was 7.9 mg/m3. However, the range of TWA exposures in this study was wide (1–221 mg/m3), and no effects on this or other urinary proteins were seen in other studies at higher (143 and 157 mg/m3) mean exposures (Lauwerys et al., 1983; Vyskocil et al., 1990). (Although Vyskocil et al. [1990] did not specifically measure retinol binding protein, concentrations of other urinary proteins showed no changes.) The most informative of the available studies assessed a wide range of serum and urinary markers for renal function in 50 dry cleaning workers exposed at a mean concentration of 100 mg/m3 for an average of 10 years. Mean values of nearly all urinary markers were higher in the exposed group, and several of these differences were statistically significant. Although no correlation between renal changes and duration or intensity of exposure was apparent, the changes might represent an early stage of progressive renal disease (Mutti et al., 1992).
Possible effects on liver function have been examined in several studies of tetrachloroethene-exposed workers. No clear evidence of tetrachloroethene-induced effects in these workers was seen.
Tetrachloroethene is a CNS depressant, with symptoms occurring in subjects exposed in chamber studies at about 690 mg/m3 (Stewart et al., 1970, 1977; Hake & Stewart, 1977). Deficits in vision, motor/cognitive, and motor function occurred in a randomized chamber study at 340 mg/m3 (Altmann et al., 1990). Among the occupational studies, the most informative study found deficits in neurobehavioural tests at a mean exposure of 83 mg/m3 and, in the same study, at a mean exposure of 370 mg/m3 (Seeber, 1989). Effects in a test of colour discrimination were also seen in an occupational study at similar or slightly lower levels; however, this result was difficult to interpret (Cavalleri et al., 1994). Effects have been reported following residential exposure to very low tetrachloroethene concentrations (Altmann et al., 1995; Schreiber et al., 2002), but questions remain about the designs of the studies, especially with regard to selection of controls (see section 9.8.2 for details).
The data from occupational studies of neurotoxicity do not permit a distinction between acute effects and effects of repeated exposures. Potential limitations of studies in this area include lack of or inappropriate control groups, non-blinding of investigators to subject status in some studies, lack of exposure–response relationships, inconsistency of results between studies, and compounding by previous solvent exposures. There have been no formal studies to evaluate the risk of chronic disabling disease (if any).
There is limited evidence for the role of tetrachloroethene in human cancer. The available studies generally lack good information on exposure levels and on exposure to other solvents. The widespread use of tetrachloroethene in the dry cleaning industry did not begin until the 1960s; excess tumour incidence, if occupationally related, could be attributable in part to exposure conditions prior to the widespread use of tetrachloroethene. Where cancer mortality was examined among workers in dry cleaning establishments, elevated mortality was seen in relation to cancer of the oesophagus and cervix. There was some suggestion of an excess in kidney cancers, but no evidence of an increased risk of liver cancer. The risk of non-Hodgkin’s lymphoma among workers who used tetrachloroethene was found to be elevated in three studies, but the increases were not statistically significant. Furthermore, the workers may have had multiple solvent exposure.
A number of published general population and case–control studies attempted to investigate the potential role of tetrachloroethene in drinking-water in human cancer. These gave no convincing evidence for an increased risk of total or specific cancers arising from exposure to tetrachloroethene in drinking-water (Isacson et al., 1985; Lagakos et al., 1986; Aschengrau et al., 1993, 1998, 2003; Cohn et al., 1994; Vartiainen et al., 1997).
Tetrachloroethene was clearly carcinogenic in laboratory animal studies, producing leukaemias in both sexes of F344 rats (NTP, 1986; Nagano et al., 1998a,b) and a small increase in kidney tumours in male rats in one study (NTP, 1986) but not in another (Nagano et al., 1998a,b). It induced liver tumours in both sexes of B6C3F1 mice (NCI, 1977; NTP, 1986; Anna et al., 1994) and BDF1 mice (Nagano et al., 1998a,b), and benign Harderian gland tumours in male BDF1 mice (Nagano et al., 1998a,b). Leukaemias were found at an elevated incidence only in a rat strain with a high spontaneous rate, renal tumours were found in only one of the two rat studies, and the incidence of Harderian gland tumours was increased only at the top dose in one mouse study. The most consistent observation is the increase in liver tumours in mice.
The genotoxicity of tetrachloroethene has been fairly well investigated in experimental test systems. There was no clear evidence of activity in bacterial (Ames) tests or in a mouse lymphoma gene mutation assay. Other, more limited, in vitro and several in vivo tests have also produced negative results. Weight of evidence suggests that tetrachloroethene itself is not genotoxic in conventional in vivo and in vitro assays. However, a predicted metabolite of the oxidative pathway (tetrachloroethene oxide) and known metabolites of the glutathione conjugation pathway (S-(1,2,2-trichlorovinyl)-L-cysteine and N-acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine) have demonstrated activity in Ames bacterial mutagenicity assays. Human studies have generally been uninformative in regard to the genotoxicity of tetrachloroethene.
Chronic inhalation of tetrachloroethene resulted in increased incidences of leukaemias in rats in two studies and benign Harderian gland tumours in mice in one study. The relevance of these findings for humans is unclear. The IPCS conceptual framework for evaluating a mode of action for animal cancers (Sonich-Mullin et al., 2001) was applied. In addition, the ILSI/RSI human relevance framework for evaluating the human relevance of laboratory animal tumours (Meek et al., 2003) was consulted. No modes of action operating only in rodents have been proposed for the induction of these two types of tumours. When the data are insufficient to confidently postulate a mode of action for test animals, the animal tumour data are presumed to be relevant for human risk assessment (Meek et al., 2003).
In one of two chronic inhalation studies in rats, a small number of kidney tumours in males were attributed to tetrachloroethene exposure. It has been suggested that this response may be due to binding of tetrachloroethene or a metabolite with a male rat-specific protein, alpha2u-globulin, with tumours arising from a cycle of persistent renal tubular cell cytotoxicity and regeneration. However, application of the IARC criteria for alpha2u-globulin-associated rat kidney tumours (IARC, 1999) led to the rejection of this hypothesis, since renal accumulation of alpha2u-globulin has been shown only at tetrachloroethene exposure concentrations higher than those inducing tumours, and the renal toxicity of tetrachloroethene is not restricted to male rats. An alternative postulated mode of action involves the induction of renal tubular cell tumours from exposure to genotoxic and cytotoxic metabolites from beta-lyase-catalysed metabolism of S-(1,2,2-trichlorovinyl)-L-cysteine. This intermediate is a metabolite of the glutathione conjugation pathway, a pathway that has been shown to operate in rodents and, at reduced rates, in humans.
In the literature, it has been suggested that the liver tumours observed in several inhalation and oral studies in both sexes of mice chronically exposed to tetrachloroethene might be associated with induction of peroxisome proliferation. This mode of action has been established to occur in rodents, and mice in particular, in response to a variety of chemical exposures, including to trichloroacetic acid, a metabolite of tetrachloroethene. Accordingly, the IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis was applied. This framework considers the available data on the postulated mode of action, key events, dose–response relationship, temporal association, strength, consistency and specificity of the association between tumour response and key events, biological plausibility and coherence, and the possible existence of other modes of action (Sonich-Mullin et al., 2001). In this case, the proposed mode of action involves peroxisome proliferation in mouse liver. Guidance on applying the IPCS conceptual framework specifically to peroxisome proliferating chemicals has been published (Klaunig et al., 2003). In the case of tetrachloroethene and mouse liver tumours, experimental data are lacking to support several of the key events in the proposed mode of action. Additionally, exposures to tetrachloroethene shown to cause liver tumours in mice are lower than those required to demonstrate measurable peroxisome proliferation in the mouse liver. Another plausible but unproven mode of action might involve reactive intermediates generated from the oxidative metabolism of tetrachloroethene in the liver. Dichloroacetic acid is a minor metabolite of tetrachloroethene and can induce liver tumours in the absence of peroxisome proliferation. Present knowledge is thus inadequate to determine with confidence the mode of action by which tetrachloroethene induced tumours in the liver of mice. Consequently, the possible relevance to humans cannot be determined currently and, by default, cannot be dismissed.
In summary, non-genotoxic mechanisms have been recognized for the formation of kidney tumours in male rats and liver tumours in mice for some chemicals. The available data on mode of action for tetrachloroethene are limited, and the dose–response data related to these recognized mechanisms are not consistent with the dose–response relationships for cancer induction by tetrachloroethene. In the absence of suitable supporting evidence to the contrary, it must be concluded that the cancers produced by tetrachloroethene in rodents are of potential relevance to humans.
An adequate two-generation study in rats found no evidence that tetrachloroethene adversely affects fertility or mating performance. Exposure at 7000 mg/m3 for 6 h/day, 5 days/week, caused slight toxicity to the parental rats, together with reductions in litter size, pup survival, and pup body weight gain. A NOAEC of 2100 mg/m3 was identified for these effects. However, testicular weight was reduced in the F1 generation at 700 mg/m3 (Tinston, 1995).
The developmental toxicity of tetrachloroethene by the inhalation route has been investigated in rats, mice, and rabbits. The studies gave no evidence that inhaled tetrachloroethene induces structural malformations. Studies in rats and rabbits gave no evidence of developmental toxicity at exposure concentrations of 2100–3400 mg/m3 (6–8 h/day). Two mouse studies provided some limited evidence of slight developmental toxicity at 1500–2100 mg/m3 (7–8 h/day), manifested as a very slight developmental delay, in the absence of overt maternal toxicity (Schwetz et al., 1975), and more severe fetal effects at a maternally toxic exposure concentration (Szakmáry et al., 1997).
Some studies of women occupationally exposed to tetrachloroethene in the dry cleaning industry have shown increased rates of spontaneous abortion (e.g. Lindbohm et al., 1984; Kyyrönen et al., 1989; Olsen et al., 1990; Windham et al., 1991). There is insufficient evidence to reach conclusions about other adverse reproductive outcomes, such as decreased fertility and fetal malformations.
The key end-points associated with human exposure to tetrachloroethene are neurotoxicity, kidney toxicity, liver toxicity, reproductive/developmental toxicity, and cancer.
The recent USEPA neurotoxicity review evaluated the entirety of reported effects and grouped the responses by affected domain. The significant findings of the various studies had a common theme of disruption of visual spatial function and CNS (cognitive) processing of visual information, similar in some respects to the effects induced by other solvents (including toluene, styrene, and mixed solvents). None of the occupational studies was considered to be without limitations. They were designed to identify a hazard and not a no-effect concentration. In some studies, investigators were not "blinded" as to subject status. All studies were relatively small and used a cross-sectional design (where the potential for selection bias and exposure misclassification is higher than in longitudinal studies). Some studies gave inadequate details relating to selection of controls, behavioural test procedures, and results. The occupational cohort study of Seeber (1989) was among the largest and had two exposure groups with fairly well defined exposures measured by sampling personal and workroom air. Both groups had in excess of 10 years of mean exposure. Alcohol consumption was accounted for. A number of neurophysiological tests were administered, including standardized tests of symptoms and personality, sensorimotor function, dexterity, perceptual speed, and choice reaction time. In addition, Wechsler intelligence test subsets, logical thinking tests, and recognition tests were given. The scientists administering the neurobehavioural tests were blinded as to exposure status. Therefore, this was selected as the key study on which to base a TC for inhalation. This study identified a mean LOAEC of 83 mg/m3. This is equivalent to 20 mg/m3 for continuous exposure (83 × 8/24 × 5/7). Application of two uncertainty factors of 10 (to take account of interindividual differences and the fact that this is a LOAEC, not a NOAEC) generates a TC of 0.2 mg/m3. Setting a TC using the study TWA exposure is a precautionary approach, as effects might be the result of occasional high exposures.
With regard to nephrotoxicity, studies of biomarkers of renal injury have suggested that both tubular and glomerular regions of the kidney are affected in occupational cohorts exposed to tetrachloroethene. However, there is a large discrepancy between the LOAECs identified in two of the better studies for the different markers of tubular damage (Mutti et al., 1992; Verplanke et al., 1999). The Mutti et al. (1992) study was selected as the more appropriate (despite the wide range of tetrachloroethene exposures in the exposed group) for derivation of a TC. The findings of Verplanke et al. (1999) were discounted, because several other studies failed to find evidence of similar tubular injury at much higher average exposures to tetrachloroethene. Mutti et al. (1992) identified a mean LOAEC of 100 mg/m3. Converting this figure to continuous exposure and applying two uncertainty factors of 10 (as described above) yield a TC of 0.24 mg/m3, which is essentially the same figure as derived for neurotoxicity.
Evidence for possible effects on the liver in exposed cohorts is less convincing than that for neurotoxicity and nephrotoxicity. The key study on liver effects was identified as Gennari et al. (1992). As effects on the liver would be induced only at higher exposures than those causing effects on the CNS and kidney, the TCs derived to protect the kidneys and CNS would also protect against liver toxicity.
It was not possible to derive a NOEC for increased risk of spontaneous abortions from the available human data. Nevertheless, it was considered that the risk would be insignificant at the TC of 0.2 mg/m3, based on the high concentrations needed to induce any reproductive effects in laboratory animals. In the most sensitive species (mice), there was only limited evidence of a slight delay in development at 1500 mg/m3, 8 h/day, for which the equivalent continuous exposure concentration (500 mg/m3) is 2500 times higher than the TC. Rats and rabbits were less sensitive than mice. It was felt that the TC of 0.2 mg/m3, derived from studies of neurotoxicity, would be sufficient to ensure that a significant risk of spontaneous abortions would be unlikely.
There is clear evidence that tetrachloroethene is carcinogenic in rodents and limited evidence of carcinogenicity in humans exposed occupationally. Although tetrachloroethene is not mutagenic in standard bacterial tests and shows little evidence of genotoxicity in other assays, there is some evidence to support the involvement of genotoxic metabolites in the induction of tumours in laboratory animals. Therefore, it was decided to calculate a BMC and BMCL for each animal tumour and use these figures as a point of departure for linear low-dose extrapolation (for details, see Appendix 7).
Highest risk estimates come from the hepatocellular adenomas or carcinomas in male mice from the Nagano et al. (1998a,b) study. The BMC10 and BMCL10 were 56 and 20 mg/m3, giving unit risks of 1.8 × 10−3 and 5.2 × 10−3/(mg/m3), respectively, using the multistage model. Linear extrapolation from these points of departure yields risks of 0.4 × 10−3 and 1 × 10−3 at the TC of 0.2 mg/m3 derived above, respectively.
The database for derivation of a TDI for oral exposures to tetrachloroethene was inadequate. Tetrachloroethene is well absorbed by oral and inhalation routes of exposure, and first-pass metabolism by the liver is relatively insignificant following oral absorption. In addition, the toxicity of tetrachloroethene is qualitatively similar after exposure via the inhalation and oral routes. Therefore, a TDI was derived using a PBPK model (for details, see Appendix 8). Oral administration of tetrachloroethene at 0.047 mg/kg body weight in drinking-water, divided in eight equal doses, was estimated to result in an AUC (a measure of systemic exposure) for plasma tetrachloroethene similar to that from continuous exposure to the TC of 0.2 mg/m3. This value was rounded to a TDI of 0.05 mg/kg body weight.
Laboratory animal studies suggest that the young might be a sensitive group. Neurotoxic effects resulting in behavioural changes were seen in young (3–4 weeks old) rats given 5 mg/kg body weight per day, 5 days/ week, for 8 weeks by gavage (Chen et al., 2002) and in 10- to 16-day-old mice given 5 mg/kg body weight per day for 6 days by gavage (Fredriksson et al., 1993) (although a lack of dose–response and/or consistency in direction of reported effects was noted in these studies). In humans (children, age not given), ingestion of up to 4.8 g/kg body weight has induced effects (including anaphylactic reactions, shock, gastrointestinal bleeding, and even death in one case) (Rabbini et al., 1985; EC, 2004). A case is reported of a baby of a woman working as a dry cleaner who developed jaundice when breast fed (breast milk contained tetrachloroethene at up to 10 mg/l) (Anonymous, 1978). Young adults (age not given) developed neurotoxic effects (neurobehavioural effects indicative of CNS depression) following inhalation exposure to tetrachloroethene at 340 mg/m3 (Altmann et al., 1990, 1992).
Measured concentrations of tetrachloroethene in the atmosphere or in indoor air in Europe and the USA are generally more than an order of magnitude lower than the TC, even in urban areas. In the vicinity of point sources, the observed concentrations also fall below the TC. In buildings where tetrachloroethene is used (notably dry cleaning facilities), concentrations clearly exceeding the TC have been measured.
Drinking-water concentrations of tetrachloroethene in different countries in Europe are usually below 1–10 µg/l, leading to daily doses of about 0.3 µg/kg body weight. This can be compared with the TDI of 50 µg/kg body weight. It should be noted that groundwater concentrations at polluted sites may exceed 1 mg/l.
In general, many of the human studies lack good information on exposure levels, and concurrent exposure to other solvents is commonly suspected but not well documented.
Epidemiological studies have suggested a possible association between cancer and occupational exposure to tetrachloroethene. Most of the better-quality human cancer studies were based on mortality rather than incidence. There is considerable difficulty in classifying haematopoietic cancers.
Tetrachloroethene induced leukaemia in rats, liver and Harderian gland cancers in mice, and, at high doses, kidney tumours in male Fischer 344 rats. The mechanisms of induction for these tumours are unclear. The relevance of the rat kidney and mouse liver tumours to humans is unknown but has not been disproved.
Certain known or postulated mammalian metabolites of tetrachloroethene are mutagenic in the Ames test. It is uncertain whether these are related to the observed rodent tumours.
There is uncertainty over the significance of the apparent kidney toxicity — e.g. few adequate studies, lack of dose–response, uncertainty over relevance of end-points.
It is unclear whether exposure to tetrachloroethene is the cause of the increased risk of spontaneous abortion observed in women employed in the dry cleaning industries. Good data on birth weight, fertility, and fetal malformation rates were not identified.
Setting TCs involved the use of study TWA exposures, although the actual measured exposures varied widely. Thus, there is some uncertainty over whether the effects reflect TWA exposures or result from occasional high exposures. The approach taken is precautionary.
It is unknown whether the neurological effects observed in the key study (Seeber, 1989) are the result of short- or long-term exposure. It is also unknown whether the neurological effects are transitory.
Persistent signs of possible neurotoxicity were obtained in two laboratory studies in which rats were given tetrachloroethene orally when very young, at doses lower than those that were effective in adults. It is uncertain whether this is a significant or real finding. If this holds true for humans, infants and young children might be especially sensitive to the neurotoxicity of tetrachloroethene.
The possible immunotoxicity potential of tetrachloroethene cannot be judged using the current, limited data set.
Assessment has been carried out for terrestrial species, aquatic species, sediment-dwelling organisms, and microorganisms in sewage treatment processes.
For most existing chemicals, the pool of data from which to predict ecosystem effects is limited. In these circumstances, empirically derived assessment factors are used to calculate a PNEC. The PNEC is the level below which the probabilities suggest that an adverse environmental effect will not occur. It is not intended to be a level below which the chemical is considered as safe. The PNEC is calculated by dividing a selected toxicity value, such as the lowest NOEC, L(E)C50, or L(E)C10 value by the appropriate assessment factor. For each end-point, a PEC (a conservative estimated exposure value) is selected, divided by the PNEC to give a conservative quotient (PEC/PNEC) for each assessment end-point in order to determine whether there is a potential ecological risk. If these quotients are less than 1, then it can be concluded that tetrachloroethene is unlikely to pose a significant risk to the environment (EC, 2001).
The quantities considered to be produced and used in the EU for the risk assessment were largely based on data from 1994. It is recognized that some changes have occurred to the overall tonnages and the relative amounts in each area since this time. This needs to be kept in mind in interpreting the conclusions. These changes are most likely to affect the regional concentrations. However, the local concentration estimates are based on more specific information relating to emissions and/or to the sizes of sites using tetrachloroethene, and these are considered less likely to have changed significantly (EC, 2001).
For tetrachloroethene, enough terrestrial toxicity data are reported to allow the PNEC to be derived from actual data, although these are of questionable validity. Long-term studies have been conducted with three trophic levels/species: invertebrates (Eisenia foetida), plants (Avena sativa), and soil-dwelling bacteria. The lowest NOEC reported is for nitrification in a loam, with a NOEC of <0.1 mg/kg wet weight. Applying an assessment factor of 10 results in a PNECsoil of 0.01 mg/kg wet weight. The EU Risk Assessment Report also derived an alternative PNEC for terrestrial species, based on a PNECaquatic species. This derivation used an equilibrium partitioning method, which is described in detail in the EU Technical Guidance Document (ECB, 2003). The starting point for the derivation was the LOAEC of 38 mg/l for a terrestrial plant (a hybrid poplar) exposed to tetrachloroethene in a hydroponic system (Dietz & Schnoor, 2001). Although the exposure of plants in hydroponic solutions is not a soil exposure, it could be considered as equivalent to exposure through the pore water. This study took precautions to reduce the possible volatilization from the exposures. As the 2-week exposures could be considered as acute tests for this species, an assessment factor of 1000 was applied to give a PNEC value of 38 µg/l water. Using standard equations given in the EU Technical Guidance Document (ECB, 2003), this equated to a PNEC for terrestrial species of 0.18 mg/kg soil, or 0.24 mg/kg soil wet weight. The PNEC derived from actual soil organism toxicity data (0.01 mg/kg wet weight) is lower than this PNEC derived from aquatic species data, and so the former was used in the risk assessment.
PEClocal values for tetrachloroethene in soil were higher than PECregional and PECcontinental values (for derivation of PECs, see EC, 2001). PEClocal values from production/processing, dry cleaning, and metal cleaning are 3.9, 0.06, and 2.5 µg/kg, respectively. The PEC/ PNEC ratios range from 0.006 to 0.4. As these are all less than 1, it is unlikely that tetrachloroethene will be a risk to terrestrial organisms (EC, 2001).
The risk assessment also considered the possible impact of trichloroacetic acid produced by atmospheric degradation of tetrachloroethene. Trichloroacetic acid might be the main degradation product to show adverse effects on terrestrial species. The PNECsoil for trichloroacetic acid is 2.4 µg/kg dry weight (see EC, 2001). Based on measured levels in soils at a few sites, a risk is identified for certain regions (e.g. the Black Forest in Germany). However, there is uncertainty about the quantitative (and causal) relationship between soil trichloroacetic acid and tetrachloroethene in the atmosphere. Based on the conservative assumption that soil trichloroacetic acid arises predominantly from tetrachloroethene degradation and enhanced deposition under certain geographical conditions, some member states of the EU have proposed that there is a need to limit risks from trichloroacetic acid at certain localities. Other member states consider the current database inadequate to conclude that tetrachloroethene is the sole source of the high soil trichloroacetic acid levels in certain localities. They consider that trichloroacetic acid levels on a regional scale are of potential concern but that further work is needed to make a quantitative link with tetrachloroethene and to investigate the geographical extent of the problem. The work should take the form of widespread monitoring of soil, air, and rainwater for trichloroacetic acid and tetrachloroethene, including a full mass balance study at an appropriate number of sites. An isotope fingerprinting analysis on the trichloroacetic acid found in soil from at least one site with high levels should also be attempted, as should a test for possible natural formation in soil under natural conditions (EC, 2001).
For tetrachloroethene, short-term L(E)C50s from validated sources are reported for fish, invertebrates, and algae. From validated data, the lowest LC50 (96-h) for freshwater fish is 5 mg/l (rainbow trout, Oncorhynchus mykiss); for invertebrates, the lowest EC50 (48-h) is 8.5 mg/l (Daphnia magna); and for algae, the lowest EC50 (72-h) is 3.64 mg/l (Chlamydomonas reinhardtii).
In addition to short-term toxicity data, longer-term toxicity data are reported for fish, Daphnia, and algae. For fish, a 10-day NOEC of 1.99 mg/l and a 28-day NOEC of 2.34 mg/l are reported for the larvae and fry survival of American flagfish (Jordanella floridae), respectively. Although these studies did not follow recognized testing protocols for chronic tests as described in the EU Technical Guidance Document (ECB, 2003) on testing strategies, they do cover three different life cycle stages for fish, including the early stages. Taken together, the results are considered to give sufficient evidence on the chronic effects in fish. For Daphnia, a 28-day NOEC based upon reproduction of 0.51 mg/l is reported from validated sources. For freshwater algae, a 72-h EC10 of 1.77 mg/l is reported, and this is taken as a long-term NOEC value.
An assessment factor of 10 may be applied when three long-term NOECs have been determined from different trophic levels and one of these long-term NOECs is from the species with the lowest L(E)C50 from the short-term tests. When the assessment factor of 10 is applied to the 28-day NOEC for Daphnia magna of 0.51 mg/l, a PNEC of 51 µg/l is obtained. This value is an order of magnitude below the concentrations at which effects were seen in field studies.
Table 13 summarizes PECs and measured levels from the EU risk assessment, along with PEC/PNEC ratios. PECs in water were calculated using the EU Technical Guidance Document (ECB, 2003) and appropriate computer modelling programs (EC, 2001). When the PECs and PNEC are compared, the PEC/PNEC ratio is less than 1 for all life cycle stages and for each use category. On the basis of the PECs and measured data, tetrachloroethene should not present a risk to the aquatic (surface water) environment. The risk to groundwater was not addressed in this assessment. It was concluded that there is no concern for regional surface water concentrations of trichloroacetic acid formed through the degradation of tetrachloroethene in air (EC, 2001).
Table 13: PECs and measured levels of tetrachloroethene in water.
|
Parameter |
PEC (surface water) (µg/l) |
PEC/PNEC |
|
PEClocal (production and processing sites) |
||
|
Site A |
0.02 (direct, based on detection limit) |
0.000 4 |
|
Site B |
0.01 (via wastewater treatment plants) |
0.000 2 |
|
Site C |
5 (direct) |
0.098 |
|
Site D |
0.085 (direct) |
0.017 |
|
Site E |
9.1 (direct) |
0.18 |
|
Site F |
4.2 (direct) |
0.082 |
|
Dry cleaning |
0.02 |
0.000 39 |
|
Metal cleaning |
1.6 |
0.03 |
|
PECregional and PECcontinental |
||
|
Regional |
0.011 |
0.000 2 |
|
Continental |
0.0016 |
0.000 03 |
|
Measured levels |
||
|
Surface water (realistic worst case based on a number of measurements) |
5 |
0.1 |
No data were found on the toxic effects of tetrachloroethene on sediment-dwelling organisms, and so the equilibrium partitioning method was used to calculate a PNECsediment as an initial screen. The PNECsediment was calculated to be 277 mg/kg wet weight (EC, 2001), based on the following equation (from ECB, 2003):
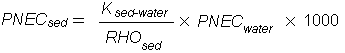
where:
Ksed-water (the sediment–water partition coefficient) is 7.08 m3/m3
RHOsed (the bulk density of wet sediment) is 1300 kg/m3
PNECwater is 51 µg/l (i.e. PNECaquatic organisms).
By using this method, only uptake via the water phase is taken into account, and it should be noted that uptake may also occur via ingestion of sediment. The highest PEClocal(sediment) is calculated (ECB, 2003) as 57 µg/kg, and the maximum measured level is 50 µg/kg. When these values are compared with the PNEC, the PEC/PNEC ratio is less than 1; it is therefore unlikely that tetrachloroethene is a risk to sediment-dwelling organisms (EC, 2001).
Since chemicals can have an adverse effect on microbial activity in wastewater treatment plants, a PNECmicroorganisms was derived. The PNEC should be calculated at the concentration at which significant effects occur, and data from short-term measurements equivalent to the retention time of the chemical in the plants are preferable. The assessment factor to be used depends upon the microbial effect data available. If the test has been performed on nitrifying bacteria, the effect concentration may be compared directly with the effluent concentration. For other tests, assessment factors in the range of 10–100 may be applied. For tetrachloroethene, toxic effects upon microorganisms are observed at about 100 mg/l. Toxic effects are observed with Nitrosomonas sp., which are nitrifying bacteria. This test is thought to be more relevant for wastewater treatment plants than the test with Tetrahymena pyriformis. A 24-h EC50 of 112 mg/l was reported; the EU Technical Guidance Document (ECB, 2003) recommends that this be divided by an assessment factor of 10, giving a PNECmicroorganisms for tetrachloroethene of 11.2 mg/l.
The highest PECsewage treatment plants is calculated (ECB, 2003) as 16 µg/l, and the maximum measured level for municipal wastewater treatment plants is 23 µg/l. When these values are compared with the PNEC, the PEC/ PNEC ratio is less than 1, and so it is unlikely that tetrachloroethene is a risk to microorganisms in the wastewater treatment plants (EC, 2001).
The EU Risk Assessment Report rapporteur (EC, 2001) carried out a separate assessment on risks to plants from atmospheric tetrachloroethene. There is no established framework within the EU Technical Guidance Document (ECB, 2003) with which to generate a PNEC for plants. Instead, the available test information was used to derive a PNEC, taking into account the characteristics of the test conditions and the nature of the organisms tested. The PNEC was derived from a study considered valid in regard to exposure conditions, concentrations of exposure, etc. Results were available on a wide range of species, covering trees, crops, ornamental/wild plants, and mosses. A range of parameters were monitored as end-points, and the responses show a wide range of sensitivities. NOECs were reported in section 10.2.4.
The conventional approach to deriving a PNEC is to identify the lowest NOEC and to apply an assessment factor. The lowest NOEC from the study (from the revised NOEC values included in Table 12) is that for the bean (Phaseolus vulgaris) from the spring exposure, at 46 µg/m3. The same species shows a NOEC of >2056 µg/m3 later in the year for the same end-point. A different treatment of the data for the spring exposure gave an EC10 value of 48 µg/m3 (see Table 11). Although the control plants in this experiment were exposed to a low level of tetrachloroethene (due to minor contamination), the historical controls for beans in these chambers show that the beans were not significantly affected. The very wide confidence limits obtained when deriving EC10 values for spruce and pine mean that these results are not considered reliable. Hence, the lowest valid NOEC is taken to be that for the bean, at 46 µg/m3.
In considering what assessment factor to apply to this result, a number of aspects need to be included. In this test, the exposure conditions were very close to those in the field, only differing in the requirement for containment to maintain the exposure concentrations. Exposure extended for the complete post-emergence lifetime of the bean. The exposure was repeated in a different season; a plausible explanation of the difference in sensitivities between the two exposures was provided, suggesting that the exposure resulting in the NOEC of 46 µg/m3 was under the most sensitive conditions. The end-point on which the NOEC is based is the production of seed pods, which as well as being a measure of yield is also a measure of reproduction. Although not stated explicitly, it can be assumed that the reduced biomass of the seed pods resulted from reduction in weight of the seeds rather than reduction in numbers of seeds developing; that is, the seeds were fertile but grew less as a result of treatment. The effect, therefore, is consistent with other findings on other species showing reduced photosynthetic capacity with a resulting reduction in growth, and there is no suggestion of adverse reproductive effects. The study as a whole looked at 12 species of plants chosen to cover the important groups and those previously considered to be potentially susceptible. Taken together, the range of species covered and the near-field exposure conditions cover at least some of the usual uncertainty in extrapolating from experiments to the environment, and so the use of an assessment factor less than 10 on the NOEC from beans could be considered. There were no previous examples to follow in this case, so as a conservative approach, an assessment factor of 5 was proposed. This led to a PNEC of 9.2 µg/m3.
Initial concerns over effects of tetrachloroethene related to pine and spruce trees, and these were included in the study together with a third tree species (beech). Measurements on spruce and pine showed that the higher concentrations of tetrachloroethene affected their growth (stem girth and height), and NOECs of 319 µg/m3 (pine) and 387 µg/m3 (spruce) were determined by the logistic curve-fitting method. Observations on foliar damage indicated effects at lower concentrations, with a NOEC of 109 µg/m3 for both spruce and pine. Relative to the life span of the trees, the exposure period used was short. However, it covered a complete growing season, and the trees were overwintered to look for effects the following spring. The trees were young (3-year-old saplings), whereas effects have been noted in older needles in other studies. Against this, there is evidence that immature foliage is more sensitive than mature foliage. The youngest needle age class is the most important for the growth of the plants, with at least 70% of the photosynthetic capacity being present in the needles of the most recent year, even in species that have needles up to 7 years old. Again, exposure conditions were considered to be very close to those in the field. Balancing these aspects, but taking into account the relatively short proportion of the lifetime exposed, it was considered prudent to apply an assessment factor to the NOECs for trees. In the absence of a firm indication of what factor to apply, a value of 10 was applied to the lowest of the three NOECs, giving a PNEC of 11 µg/m3. This was based on the yellowing of needles, and it is not clear how significant this degree of yellowing is for the survival or growth of the trees, so this is to some degree conservative (EC, 2001).
In a third approach to deriving a PNEC, a statistical extrapolation method was used. The revised set of NOEC values contains actual values for 10 species. For two species, there were no effects at the highest concentration tested; in these cases, the limit value of the highest tested concentration was used. The Aldenberg & Slob (1993) method, assuming a log-normal distribution, gave an HC5 (50%) value — the hazardous concentration to protect 95% of species with 50% confidence — of 41 µg/m3. The fit of the data set to the assumed distribution is not rejected by the Kolmogorov-Smirnov test. For comparison, the data set without the two unbounded values (so including 10 values) gives an HC5 (50%) of 37.5 µg/m3. An assessment factor of 5 was considered appropriate. The number of values available meets the requirements of the method from a statistical viewpoint. In terms of the representation of species, criteria developed for aquatic organisms are not directly applicable, since only plants are being considered here. The data set includes 12 species, covering a range of types of plant considered to be reasonably representative of those in the EU. The number is less than the 15 species indicated as preferred for aquatic species; however, plants should be a more homogeneous group than aquatic organisms as a whole. The most sensitive of the measured end-points has been taken in each case. The end-points are mostly related to growth (although the foliar effects are at a level below growth as such) or reproduction (pod weights, etc.). Against this, there is little experience in this area. The data set also includes two "unbounded NOEC’ values for moss species. Therefore, a conservative approach is taken in using an assessment factor of 5 on the HC5. This gives a PNEC of 8.2 µg/m3 (EC, 2001).
These three approaches give similar results: 9.2, 11, and 8.2 µg/m3; thus, the lowest (8.2 µg/m3) was selected as the PNEC for plants exposed through the air for risk assessment (EC, 2001).
The expert assessment considered three separate aspects in the risk characterization for atmospheric effects of tetrachloroethene: direct effects of tetrachloroethene on plants, consequences of atmospheric degradation to produce toxic metabolites, and potential for ozone formation or depletion.
First, the PNEC was compared with calculated and measured levels in the environment. The highest value, measured for a production and processing site and considered to represent a reasonable worst case, was 36 µg/m3, giving a PEC/PNEC ratio of 4.4 (i.e. above 1). (EU [2001] also calculated PEC values for other production sites and found that these were below the PNEC. The calculated PEC values for dry cleaning [4.4 µg/m3], metal cleaning [7.7 µg/m3], and the regional background [0.88 µg/m3] all led to PEC/PNEC ratios below 1 [0.54, 0.94, and 0.11, respectively].)
Second, tetrachloroethene reacts with species such as hydroxyl radicals and chlorine radicals in the troposphere, leading to the formation of acidic degradation products.
The third aspect relates to more indirect effects, such as low-level ozone formation and stratospheric ozone depletion. The reactivity of tetrachloroethene in the troposphere (the half-life is around 3–5 months) is such that it is not thought to contribute significantly to tropospheric ozone formation. Gas-phase photolysis and rainout are thought to be of negligible importance in the removal of tetrachloroethene from the troposphere. The lifetime of tetrachloroethene in the troposphere is such that the amount entering the stratosphere is low. Studies into stratospheric ozone depletion mention that tetrachloroethene is a possible ozone depleter, although its potential is significantly lower than that of other ozone-depleting chemicals. Degradation products of tetrachloroethene in the troposphere may enter the stratosphere; of these, carbon tetrachloride is a known ozone depleter. The amounts of carbon tetrachloride entering the stratosphere due to tetrachloroethene degradation are thought to be negligible when compared with other sources of carbon tetrachloride emissions. No data were found quantifying the contribution that tetrachloroethene makes to ozone depletion either directly or indirectly via its degradation products. An expert working group on ozone depletion (WMO, 1991) considered that tetrachloroethene makes a negligible contribution to ozone depletion relative to other ozone-depleting chemicals such as CFCs, HCFCs, carbon tetrachloride, and 1,1,1-trichloroethane. Tetrachloroethene is not expected to contribute significantly to global warming (EC, 2001).
It was concluded that there is a need for limiting the risks of harm to plants from air emissions of tetrachloroethene at sites where this compound is produced and processed as an intermediate. However, it was noted that at only one site was the PEC/PNEC ratio greater than 1. There did not appear to be a risk to plants from the use of tetrachloroethene in dry cleaning or metal cleaning processes (EC, 2001).
It is unclear to what extent (if any) tetrachloroethene is the sole or major source of the high soil trichloroacetic acid levels in certain localities.
IARC has classified tetrachloroethene in Group 2A — probably carcinogenic to humans — on the basis of limited evidence in humans and sufficient evidence in experimental animals (IARC, 1995).
WHO has recommended an oral TDI for tetrachloroethene of 14 µg/kg body weight, based on the dose–response of the liver pathology seen in subchronic studies in mice (Buben & O’Flaherty, 1985) and rats (Hayes et al., 1986). An uncertainty factor of 1000, made up of constituent factors of 10 each for inter- and intraspecies variation and 10 "for carcinogenic potential", was applied to the NOAEL for liver toxicity of 14 mg/kg body weight per day to derive the TDI. An additional uncertainty factor to reflect the short duration of the key studies was considered unnecessary "in view of the database on tetrachloroethene and considerations regarding the application of the dose via drinking-water in one of the two critical studies" (WHO, 2003).
An air quality guideline for tetrachloroethene of 0.25 mg/m3 has been recommended by a WHO Working Group. The chosen critical study involved the long-term exposure of dry cleaning workers, which suggested mild effects on the kidney at a median concentration of 102 mg/m3 (Mutti et al., 1992). This LOAEC was divided by 4.2 to convert it from occupational to continuous exposure (168 vs 40 h per week), and then an uncertainty factor of 100 was applied. The uncertainty factor was made up of two component factors of 10, one for the use of a LOAEC (instead of a NOAEC), and the other to take account of interindividual variations in susceptibility (WHO, 2000).
Abdul AS, Gibson TL, Rai DN (1987) Statistical correlations for predicting the partition coefficient for non-polar organic contaminants between aquifer organic carbon and water. Hazardous Waste and Hazardous Materials, 4(3):211–222 [cited in EC, 2001].
Abrahamsson K, Dryssen D, Jogebrant G, Krysell M (1989) Halocarbon concentrations in Askeröfjorden related to the water exchange and inputs from the petrochemical site at Stenungsund. Vatten, 45:3–8 [cited in IARC, 1995; EC, 2001].
Abrahamsson K, Ekdahl A, Collen J, Pedersén M (1994) Marine algae — A source of trichloroethylene and perchloroethylene. Limnology and Oceanography, 40(7):1321–1326 [cited in EC, 2001].
Aggazzotti G, Predieri G (1986) Survey of volatile halogenated organics (VHO) in Italy. Levels of VHO in drinking waters, surface waters and swimming pools. Water Research, 20(8):959–963.
Aggazzotti G, Fantuzzi G, Predieri G, Righi E, Moscardelli S (1994) Indoor exposure to perchloroethylene (PCE) in individuals living with dry-cleaning workers. Science of the Total Environment, 156:133–137.
Ahel M, Giger W, Molnar-Kubica E, Schaffner C (1984) Organic micropollutants in surface waters of the Glatt valley, Switzerland. Analysis of organic micropollutants in water. Brussels, Commission of the European Communities, pp. 280–288 (EUR 8518) [cited in EC, 2001].
Aitio A, Pekari K, Jarvisalo J (1984) Skin absorption as a source of error in biological monitoring. Scandinavian Journal of Work, Environment and Health, 10:317–320 [cited in de Raat, 2003].
Alberti J (1989) Analysierbarkeit von halogenorganischen Verbindungen in Gewässern. VDI-Berichte, 745:373–393 [cited in EC, 2001].
Aldenberg T, Slob W (1993) Confidence limits for hazardous concentrations based on logistically distributed NOEC toxicity data. Ecotoxicology and Environmental Safety, 25:48–63.
Alexander HC, McCarty WM, Bartlett EA (1978) Toxicity of perchloroethylene, trichloroethylene, 1,1,1-trichloroethylene and methylene chloride to fathead minnows. Bulletin of Environmental Contamination and Toxicology, 20:344–352 [cited in EC, 2001].
Altmann L, Bottger A, Wiegand H (1990) Neurophysiological and psychophysical measurements reveal effects of acute low-level organic solvent exposure in humans. International Archives of Occupational and Environmental Health, 62:493–499 [cited in de Raat, 2003].
Altmann L, Wiegand H, Bottger A, Elstermeier F, Winneke G (1992) Neurobehavioral and neurophysiological outcomes of acute repeated perchloroethylene exposure. Applied Psychology, An International Review, 41(3):269–279.
Altmann L, Neuhann HF, Kramer U, Witten J, Jermann, E (1995) Neurobehavioral and neurophysiological outcomes of chronic low-level tetrachloroethylene exposure measured in neighborhoods of dry cleaning shops. Environmental Research, 69:83–89.
Anders MW (1990) Glutathione-dependent bioactivation of xenobiotics: Implications for mutagenicity and carcinogenicity. In: Xenobiotics and cancer: implications for chemical carcinogenesis and cancer chemotherapy: proceedings of the 21st international symposium of the Princess Takamatsu Cancer Research Fund, Tokyo. Tokyo, Japan Scientific Societies Press, pp. 89–99 [cited in de Raat, 2003].
Andrys C, Hanovcova I, Chylkova V, Tejral J, Eminger S, Prochazkova J (1997) Immunological monitoring of dry-cleaning shop workers — exposure to tetrachloroethylene. Central European Journal of Public Health, 5:136–142.
Angerer J, Schaller KH, eds (1991) Analyses of hazardous substances in biological materials. Vol. 3. Weinheim, VCH Verlagsgesellschaft mbH.
Anna CH, Maronpot RR, Pereira MA, Foley JF, Malarkey DE, Anderson MW (1994) Ras proto-oncogene activation in dichloroacetic acid-, trichloroethylene- and tetrachloroethylene-induced liver tumours in B6C3F1 mice. Carcinogenesis, 15:2255–2261.
Anonymous (1978) Dry-cleaned [sic] breast milk poisons baby. Medical World News, 19:6 [cited in de Raat, 2003].
Anttila A, Pukkala E, Sallmen M, Hernberg S, Hemminki K (1995) Cancer incidence among Finnish workers exposed to halogenated hydrocarbons. Journal of Occupational and Environmental Medicine, 37:797–806 [cited in de Raat, 2003].
Aranyi C, O’Shea WJ, Graham JA (1986) The effects of inhalation of organic chemical air contaminants on murine host defences. Fundamental and Applied Toxicology, 6:713–720 [cited in de Raat, 2003].
Asal NR, Geyer JR, Risser DR, Lee ET, Kadamani S, Cheng N (1988) Risk factors in renal cell carcinoma. II. Medical history, occupation, multivariate analysis, and conclusions. Cancer Detection and Prevention, 13:263–279.
Aschengrau A, Ozonoff D, Paulu C, Coogan P, Vezina R, Heeren T, Zhang Y (1993) Cancer risk and tetrachloroethylene-contaminated drinking water in Massachusetts. Archives of Environmental Health, 48:284–292 [cited in de Raat, 2003].
Aschengrau A, Paulu C, Ozonoff D (1998) Tetrachloroethylene-contaminated drinking water and the risk of breast cancer. Environmental Health Perspectives, 106:947–953.
Aschengrau A, Rogers S, Ozonoff A (2003) Perchloroethylene-contaminated drinking water and the risk of breast cancer: additional results from Cape Cod, Massachusetts, USA. Environmental Health Perspectives, 111(2):167–173.
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chemical Reviews, 85:69–201 [cited in EC, 2001].
Atkinson R, Carter WPL (1984) Kinetics and mechanisms of the gas phase reactions of ozone with organic compounds under atmospheric conditions. Chemical Reviews, 84:69–201.
Atkinson R, Baulch DL, Cox RA, Hampson RF Jr, Kerr JA, Troe J (1992) Evaluated kinetic and photochemical data for atmospheric chemistry. Supplement IV. Atmospheric Environment, 26A:1187–1230 [cited in EC, 2001].
Atri FR (1985) Chlorierte Kohlenwasserstoffe in der Umwelt I. Stuttgart, Fischer (Schriftenreihe Verein WaBoLu-Hygiene 60) [cited in EC, 2001].
ATSDR (1997) Toxicological profile for tetrachloroethylene (update). Atlanta, GA, United States Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR/TP-97/18) [cited in de Raat, 2003].
Austin H, Delzell E, Grufferman S, Levine R, Morrison AS, Stolley PD, Cole P (1987) Case–control study of hepatocellular carcinoma, occupation and chemical exposures. Journal of Occupational Medicine, 29:665–669.
Barrows ME, Petrocelli SR, Macek KJ, Carroll JJ (1980) Bioconcentration and elimination of selected water pollutants by the bluegill sunfish (Lepomis macrochirus). In: Haque R, ed. Dynamics, exposure and hazard assessment of toxic chemicals. Ann Arbor, MI, Ann Arbor Science, pp. 379–392 [cited in EC, 2001].
Bartsch H, Malaveille C, Barbin A (1979) Mutagenic and alkylating metabolites of halo-ethylenes, chlorobutadiene and dichlorobutenes produced by rodent or human liver tissues. Evidence for oxirane formation by P450 linked microsomal mono-oxygenases. Archives of Toxicology, 41:249–277 [cited in de Raat, 2003].
Bauer C, Dietze C (1992) Phytotoxizitätstest en eniner monokotylen pflanzenart (Hafer, Avena sativa L) mit tetrachloroethen mach dem verfahrensvorschlag ‘Phytotoxizitätest an einer monokotylen pflanzenart (Avena sativa L) und einer dikotylen pflanzenart (Brassica rapa ssp. Rapa Metzg)’. Geneva, Battelle Europe [cited in EC, 2001].
Bauer U (1991) Occurrence of tetrachloroethylene in the Federal Republic of Germany. Chemosphere, 23(11–12):1777–1781.
Bazin C, Chambon P, Bonnefille M, Larbaigt G (1987) Compared sensitivity of luminescent marine bacteria (Photobacterium phosphoreum) and Daphnia bioassays. Sciences de l’eau, 6:403–413 [cited in EC, 2001].
Beliles RP, Brusick DJ, Mecler FJ (1980) Teratogenic–mutagenic risk of workplace contaminants: Trichloroethylene, perchloroethylene and carbon disulfide. Washington, DC, United States Department of Health, Education and Welfare (Contract No. 210-77-0074) [cited in de Raat, 2003].
Benane SG, Blackman CF, House DE (1996) Effect of perchloroethylene and its metabolites on intercellular communication in clone 9 rat liver cells. Journal of Toxicology and Environmental Health, 48:427–437.
Beneš V, Frantik E, Horváth M, Kožená L (1986) Vigilance performance paralleled with blood levels of solvents: acetone, styrene, tetrachloroethylene. Activitas Nervosa Superior, 28:234–236.
Benoit FM, Davidson WR, Lovett AM, Nacson S, Ngo A (1985) Breath analysis by API/MS — human exposure to volatile organic solvents. International Archives of Occupational and Environmental Health, 55:113–120 [cited in de Raat, 2003].
Bergamaschi E, Mutti A, Bocchi MC, Alinovi R, Olivetti G, Ghiggeri GM, Franchini I (1992) Rat model of perchloroethylene-induced renal dysfunction. Environmental Research, 59:427–439 [cited in de Raat, 2003].
Berger T, Horner CM (2003) In vivo exposure of female rats to toxicants may affect oocyte quality. Reproductive Toxicology, 17:273–281.
Berlin M, Gage JC, Gullberg B, Holm S, Knutsson P, Eng C, Tunek A (1980) Breath concentration as an index of the health risk from benzene. Studies on the accumulation and clearance of inhaled benzene. Scandinavian Journal of Work, Environment and Health, 6:104–111 [cited in de Raat, 2003].
Berode M, Boillat MA, Guillemin MP, Wu MM, Savolainen H (1990) Demethylation pathways in caffeine metabolism as indicators of variability in 1,1,1-trichloroethane oxidation in man. Pharmacology and Toxicology, 67:41–46.
Birner G, Rutkowska A, Dekant W (1996) N-Acetyl-S-(1,2,2-trichlorovinyl)-L-cysteine and 2,2,2-trichloroethanol. Two novel metabolites of tetrachloroethylene in humans after occupational exposure. Drug Metabolism and Disposition, 24:41–48 [cited in de Raat, 2003].
Biswas N, Zytner RG, Jatindr BK (1992) Model for predicting PCE desorption from contaminated soils. Water Environment Research, 64(2):170–178 [cited in EC, 2001].
Blair A, Decoufle P, Grauman D (1979) Causes of death among laundry and dry-cleaning workers. American Journal of Public Health, 69:508–511.
Blair A, Tolbert PE, Thomas T, Grauman D (1986) Mortality among dry cleaners. La Medicina del Lavoro, 77:82–83 (abstract).
Blair A, Stewart PA, Tolbert PE, Grauman D, Moran FX, Vaught J, Rayner J (1990) Cancer and other causes of death among a cohort of dry cleaners. British Journal of Industrial Medicine, 47:162–168.
Blair A, Petralia SA, Stewart PA (2003) Extended mortality follow-up of a cohort of dry cleaners. Annals of Epidemiology, 13:50–56.
Blum DJW, Speece RE (1991) Quantitative structure activity relationships for chemical toxicity to environmental bacteria. Ecotoxicology and Environmental Safety, 22:198–224.
Bohlen H, Hicke K, Stöbel AO, Zierott M, Thiemann W (1989) Die Belastung der Unterweser im bremischen Raum mit Halogenorganika und Phosphorsäureestern I. Vom Wasser, 72:185–197 [cited in EC, 2001].
Boice JD Jr, Marano DE, Fryzek JP, Sadler CJ, McLaughlin JK (1999) Mortality among aircraft manufacturing workers. Occupational and Environmental Medicine, 56:581–597.
Bois FY, Gelman A, Jiang J, Maszle DR, Zeise L, Alexeef G (1996) Population toxicokinetics of tetrachloroethylene. Archives of Toxicology, 70:347–355.
Bolt HM, Laib RJ, Filser JG (1982) Reactive metabolites and carcinogenicity of halogenated ethylenes. Biochemical Pharmacology, 31:1–4.
Bonnet P, Francin J-M, Gradski D, Raoult G, Zissu D (1980) Determination of the lethal concentration-50 of the principal chlorinated hydrocarbons in the rat. Archives des Maladies Professionnelles de Medecine du Travail et de Sécurité Sociale, 41:317–321 [cited in de Raat, 2003].
Bosco MG, Figa-Talamanca I, Salerno S (1987) Health and reproductive status of female workers in dry cleaning shops. International Archives of Occupational and Environmental Health, 59:295–301.
Böttger A, Elstermeier F (1989) Belastung der Bevölkerung durch organische Lösungsmittel. Umwelt Bundesamtes, 31 May 1989 (Final report on Project No. 10606058) [cited in de Raat, 2003].
Boulet L-P (1988) Increases in airway responsiveness following acute exposure to respiratory irritants. Reactive airway dysfunction syndrome or occupational asthma? Chest, 94:476–481.
Bouwer EJ, McCarty PL (1982) Removal of trace chlorinated organic compounds by activated carbon and fixed-film bacteria. Environmental Science and Technology, 16(2):836–843 [cited in EC, 2001].
Bouwer EJ, McCarty PL (1983) Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Applied and Environmental Microbiology, 45(5):1286–1294 [cited in EC, 2001].
Bouwer EJ, Rittmann BE, McCarty PL (1981) Anaerobic degradation of halogenated 1- and 2-carbon organic compounds. Environmental Science and Technology, 15(5):596–599 [cited in EC, 2001].
Brack W, Rottler H (1994) Toxicity testing of highly volatile chemicals with green algae. Environmental Science and Pollution Research International, 1(4):223–228.
Brancaccio A, Mazza V, DiPaolo R (1971) Renal function in experimental tetrachloroethylene poisoning. Folia Medica (Naples, Italy), 54:233–237 [cited in de Raat, 2003].
Bringmann G, Kühn R (1982) Ergebnisse der Schadwirkung wassergefährdender Stoffe gegen Daphnia magna in einem weiterentwickelten stanardisierten Testverfahren. Zeitschrift für Wasser und Abwasser Forschung, 15(1):1–6 [cited in EC, 2001].
Briving C, Jacobson I, Hamberger A, Kjellstrand P, Haglid KG, Rosengren LE (1986) Chronic effects of perchloroethylene and trichloroethylene on the gerbil brain amino acids and glutathione. Neurotoxicology, 7:101–108.
Broderius S, Kahl M (1985) Acute toxicity of organic chemical mixtures to the fathead minnow. Aquatic Toxicology, 6:307–322 [cited in EC, 2001].
Brodkin CA, Daniell W, Checkoway H, Echeverria D, Johnson J, Wang K, Sohaey R, Green D, Redlich C, Gretch D, Rosenstock L (1995) Hepatic ultrasonic changes in workers exposed to perchloroethylene. Occupational and Environmental Medicine, 52:679–685.
Bronzetti G, Bauer C, Corsi C, Del Carratore R, Galli A, Nieri R, Paolini M (1983) Genetic and biochemical studies on perchloroethylene in vitro and in vivo. Mutation Research, 116:323–331 [cited in de Raat, 2003].
Brooke DN, Crookes MJ, Howe PD (1993) Environmental hazard assessment: Tetrachloroethylene. Watford, Building Research Establishment [cited in EC, 2001].
Brown D (1978) Chlorinated solvents in sewage works. Effluent and Water Treatment Journal, 18(3):110–117.
Brown DP, Kaplan SD (1987) Retrospective cohort mortality study of dry cleaner workers using perchloroethylene. Journal of Occupational Medicine, 29:535–541 [cited in de Raat, 2003].
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicology and Industrial Health, 13:407–484.
Bruckmann P, Kersten W, Hagedorn B, Ball M, Paepke O, Funcke W, Theisen J (1989) Immissionsmessungen halgenierter organischer Verbindungen in Hamburg. VDI-Berichte, 745:209–234 [cited in EC, 2001].
BUA (1993) Tetrachloroethylene. GDCh-Advisory Committee on Existing Chemicals of Environmental Relevance. Stuttgart, S. Hirzel (BUA Report 139) [cited in EC, 2001].
Buben J, O’Flaherty E (1985) Delineation of the role of metabolism in the hepatotoxicity of trichloroethylene and perchloroethylene: a dose effect study. Toxicology and Applied Pharmacology, 78:105–122 [cited in de Raat, 2003].
Buccafusco RJ, Ells SJ, LeBlanc GA (1981) Acute toxicity of priority pollutants to bluegill (Lepomis macrochirus). Bulletin of Environmental Contamination and Toxicology, 26(4):446–452 [cited in EC, 2001].
Cai SX, Huang MY, Chen Z, Liu YT, Jin C, Watanabe T, Nakatsuka H, Seiji K, Inoue O, Ikeda M (1991) Subjective symptom increase among dry-cleaning workers exposed to tetrachloroethylene vapor. Industrial Health, 29:111–121.
Call DJ, Brooke LT, Ahmad N, Richter JE (1983) Toxicity and metabolism studies with EPA priority pollutants and related chemicals in freshwater organisms. Washington, DC, United States Environmental Protection Agency (EPA 600/3-83-095; NTIS PB83-283665) [cited in EC, 2001].
Carlson GP (1983) Epinephrine-induced cardiac arrhythmias in rabbits exposed to tetrachloroethylene. Toxicology Letters, 19:113–117 [cited in de Raat, 2003].
Carpenter CP (1937) The chronic toxicity of tetrachloroethylene. Journal of Industrial Hygiene and Toxicology, 19:323–336 [cited in de Raat, 2003].
Cavalleri A, Gobba F, Paltrinieri M, Fantuzzi G, Righi E, Aggazzotti G (1994) Perchloroethylene exposure can induce colour vision loss. Neuroscience Letters, 179:162–166.
Cerna N, Kypenova H (1977) Mutagenic activity of chloroethylenes analysed by screening system tests. Mutation Research, 46:214–215 [cited in de Raat, 2003].
Chang YM, Tai CF, Yang SW, Chen CJ, Shih TS, Lin RS, Liou SH (2003) A cohort mortality study of workers exposed to chlorinated organic solvents in Taiwan. Annals of Epidemiology, 13:652–660.
Chen H-H, Chan M-H, Fu S-H (2002) Behavioural effects of tetrachloroethylene exposure in rats: acute and subchronic studies. Toxicology, 170:201–209.
Chiou CT, Freed VH, Peters LJ, Kohnert RL (1980) Evaporation of solutes from water. Environment International, 3:231–236 [cited in EC, 2001].
Choi YH, Kim N, Seo YS, Choi SJ, Yang JO, Lee EY, Hong SY, Lee HS (2003) ARF requiring hemodialysis after accidental perchloroethylene ingestion. American Journal of Kidney Disease, 41:E11.
Christensen BV, Lynch HJ (1933) The effect of anthelminthics on the host. I. Tetrachloroethylene. II. Hexylresorcinol. Journal of Pharmacology and Experimental Therapeutics, 48:311–316 [cited in de Raat, 2003].
Class T, Ballschmiter K (1987) Global baseline pollution studies. X. Atmospheric halocarbons: global budget estimations for tetrachloroethene, 1,2-dichloroethane, 1,1,1,2-tetrachloroethane and hexachlorobutadiene. Estimation of the hydroxyl radical concentrations in the troposphere of the northern and southern hemisphere. Fresenius’ Zeitschrift für Analytische Chemie, 327:198–204 [cited in EC, 2001].
Clement International Corporation (1990) Tetrachloroethylene (AAMRL-TR-90-072). Development and validation of methods for applying pharmacokinetic data in risk assessment. Vol. III. Wright-Patterson Air Force Base, Ohio, Harry G. Armstrong Aerospace Medical Research Laboratory [cited in de Raat, 2003].
Cohn P, Klotz J, Bove F, Berkowitz M, Fagliano J (1994) Drinking water contamination and the incidence of leukemia and non-Hodgkin’s lymphoma. Environmental Health Perspectives, 102:556–561 [cited in de Raat, 2003].
Connor TH, Theiss JC, Hanna HA, Monteith DK, Matney TS (1985) Genotoxicity of organic chemical frequently found in the air of mobile homes. Toxicology Letters, 25:33–40 [cited in de Raat, 2003].
Costa AK, Ivanetich KM (1980) Tetrachloroethylene metabolism by the hepatic microsomal cytochrome P-450 system. Biochemical Pharmacology, 29:2863–2869 [cited in de Raat, 2003].
Costa AK, Ivanetich KM (1984) Chlorinated ethylenes: their metabolism and effect on DNA repair in rat hepatocytes. Carcinogenesis, 5:1629–1636 [cited in de Raat, 2003].
Czuczwa J, Leuenberger C, Giger W (1988) Seasonal and temporal changes of organic compounds in rain and snow. Atmospheric Environment, 22(5):907–916 [cited in EC, 2001].
Dallas CE, Muralidhara S, Chen XM, Ramanathan R, Varkonyi P, Gallo JM, Bruckner JV (1994a) Use of a physiologically based model to predict systemic uptake and respiratory elimination of perchloroethylene. Toxicology and Applied Pharmacology, 128:60–68.
Dallas CE, Chen XM, Muralidhara S, Varkonyi P, Tackett RL, Bruckner JV (1994b) Use of tissue disposition data from rats and dogs to determine species differences in input parameters for a physiological model for perchloroethylene. Environmental Research, 67:54–67 [cited in de Raat, 2003].
Dallas CE, Chen XM, O’Barr K, Muralidhara S, Varkonyi P, Bruckner JV (1994c) Development of a physiologically based pharmacokinetic model for perchloroethylene using tissue concentration–time data. Toxicology and Applied Pharmacology, 128:50–59.
Dallas CE, Chen XM, Murildahara S, Varkonyi P, Tackett RL, Bruckner JV (1995) Physiologically based pharmacokinetic model useful in prediction of the influence of species, dose and exposure route on perchloroethylene pharmacokinetics. Journal of Toxicology and Environmental Health, 44:301–317.
Danesh J, Collins R, Appleby P, Peto R (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease. Meta-analyses of prospective studies. Journal of the American Medical Association, 279:1477–1482.
Daniel JW (1963) The metabolism of 36Cl-labelled trichloroethylene and tetrachloroethylene in the rat. Biochemical Pharmacology, 12:705–802 [cited in de Raat, 2003].
Danneberg G (1993) Auswirkungen von tetrachlorethen auf die aktivität der bodenmikroflora. Geneva, Battelle Europe [cited in EC, 2001].
Dawes VJ, Waldock MJ (1994) Measurement of volatile organic compounds at UK national monitoring plan stations. Marine Pollution Bulletin, 28(5):291–298.
De Bruin WP, Kotterman MJJ, Posthumus MA, Schraa G, Zehnder AJB (1992) Complete biological reductive transformation of tetrachloroethylene to ethane. Applied and Environmental Microbiology, 56(6):1996–2000 [cited in EC, 2001].
Dekant W, Haug R, Henschler D (1985) Absorption, elimination and metabolism of tetrachloroethylene. Nauyn-Schmiedeberg’s Archives of Pharmacology, 329:R24 (abstract) [cited in de Raat, 2003].
Dekant W, Metzler M, Henschler D (1986a) Identification of S-1,2,2-trichlorovinyl-N-acetylcysteine as urinary metabolite to tetrachloroethylene bioactivation through glutathione conjugation as a possible explanation for its nephrotoxicity. Journal of Biochemical Toxicology, 1:57–72 [cited in de Raat, 2003].
Dekant W, Vamvakas S, Berthold K, Schmidt S, Wild D, Henschler D (1986b) Bacterial beta-lyase mediated cleavage and mutagenicity of cysteine conjugates derived from the nephrocarcinogenic alkanes trichloroethylene, tetrachloroethylene and hexachlorobutadiene. Chemico-Biological Interactions, 60:31–45 [cited in de Raat, 2003].
Dekant W, Berthold K, Vamvakas S, Henschler D, Anders MW (1988) Thioacylating intermediates as metabolites of S-(1,2,2-dichlorovinyl)-L-cysteine and S-(1,2,2-trichlorovinyl)-L-cysteine formed by cysteine conjugate beta-lyase. Chemical Research in Toxicology, 1:175–178 [cited in de Raat, 2003].
Dekant W, Birner G, Werner M, Parker J (1998) Glutathione conjugation of perchloroethylene in subcellular fractions from rodent and human liver and kidney. Chemico-Biological Interactions, 116:31–43.
Demond AH (1982) A source of tetrachloroethylene in the drinking water of New England: An evaluation of the toxicity of tetrachloroethylene and the prediction of its leaching rates from vinyl-lined asbestos-cement pipe [MS Thesis]. Cambridge, MA, Massachusetts Institute of Technology.
de Raat K (2003) The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards. 133. Tetrachloroethylene (PER). Stockholm, National Institute for Working Life (Arbete och Hälsa NR 2003:14; ISBN 91-7045-695-X).
Derwent RG, Jenkin ME (1990) Hydrocarbon involvement in photochemical ozone formation in Europe. Harwell, United Kingdom Atomic Energy Authority, May (Report No. AERE R 13736).
Dietz AC, Schnoor JL (2001) Phytotoxicity of chlorinated aliphatics to hybrid poplar (Populus deltoides × nigra DN34). Environmental Toxicology and Chemistry, 20(2):389–393 [cited in EC, 2001].
Dilling WL (1977) Interphase transfer processes. II. Evaporation rates of chloromethanes, ethanes, ethylenes, propanes and propylenes from dilute aqueous solutions. Comparisons with theoretical predictions. Environmental Science and Technology, 11(4):405–409 [cited in EC, 2001].
Dilling WL, Tefertiller NB, Kallos GJ (1975) Evaporation rates and reactivities of methylene chloride, chloroform, 1,1,1-trichloroethane, trichloroethylene, tetrachloroethylene and other chlorinated compounds. Environmental Science and Technology, 9(9):833–838 [cited in EC, 2001].
DiStefano TD, Gossett JM, Zinder SH (1991) Reductive dechlorination of high concentrations of tetrachloroethylene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Applied and Environmental Microbiology, 57(8):2287–2292 [cited in EC, 2001].
DiStefano TD, Gosset JM, Zinder SH (1992) Hydrogen as an electron donor for dechlorination of tetrachloroethylene by an anaerobic mixed culture. Applied and Environmental Microbiology, 58(11):3622–3629 [cited in EC, 2001].
Doherty AT, Ellard S, Parry EM, Parry JM (1996) An investigation into the activation and deactivation of chlorinated hydrocarbons to genotoxins in metabolically competent human cells. Mutagenesis, 11:247–274.
Doorgeest T, Meijer PB, De Mik G (1986) Chronische Effecten Tengevolge van Blootstelling aan Organische Oplosmiddelen. Voorburg, Directorate General of Labour, Ministry of Social Affairs and Employment (Report No. S29-1) [cited in de Raat, 2003].
Doust HG, Huang J-C (1992) The fate and transport of hazardous chemicals in the subsurface environment. Water Science and Technology, 25(1):169–176.
Doyle P, Roman E, Beral V, Brookes M (1997) Spontaneous abortion in dry cleaning workers potentially exposed to perchloroethylene. Occupational and Environmental Medicine, 54:848–853.
Drew RT, Patel JM, Lin F-N (1978) Changes in serum enzymes in rats after inhalation of organic solvents singly and in combination. Toxicology and Applied Pharmacology, 45:809–819 [cited in de Raat, 2003].
DRIRE Franche Comté (1996) Inventaire des rejets de substances toxiques dans les eaux (octobre 1993 – avril 1995). Unpublished report [cited in EC, 2001].
Duh R-W, Asal NR (1984) Mortality among laundry and dry-cleaning workers in Oklahoma. American Journal of Public Health, 74:1278–1280 [cited in de Raat, 2003].
Duprat P, Delsant L, Gradiski D (1976) Irritant potency of the principal aliphatic chloride solvents on the skin and ocular mucous membranes of rabbits. European Journal of Toxicology, 3:171–177 [cited in de Raat, 2003].
Duprat P, Bonnet P (1979) Study on the organic lesions in the mouse produced by chlorinated aliphatic solvents administered by inhalation. INRS Bulletin de documentation, 1103:73–101 [cited in de Raat, 2003].
Dutch Normalisation Institute (1999a) NEN2947: Air quality, Occupational atmosphere; General method for determination of toxic damp or gases by active sampling using absorption tube, liquid desorption and gas chromatography. Delft, Dutch Normalisation Institute [cited in de Raat, 2003].
Dutch Normalisation Institute (1999b) NEN2948: Air quality, Occupational atmosphere; General method for determination of toxic damp or gases by active sampling using absorption tube, thermic desorption and gas chromatography. Delft, Dutch Normalisation Institute [cited in de Raat, 2003].
Dutch Normalisation Institute (1999c) NEN2950: Air quality, Occupational atmosphere; General method for determination of toxic damp or gases by gas diffusion sampling using direct readable dosimetry. Delft, Dutch Normalisation Institute [cited in de Raat, 2003].
Dutch Normalisation Institute (2000a) NEN2964: Air quality, workplace atmosphere; Determination of the concentration of gaseous chlorinated hydrocarbons by active sorbent tube sampling, liquid desorption and gas chromatography. Delft, Dutch Normalisation Institute [cited in de Raat, 2003].
Dutch Normalisation Institute (2000b) NEN2965: Air quality, workplace atmosphere; Determination of the concentration of gaseous chlorinated hydrocarbons by active sorbent tube sampling, thermal desorption and gas chromatography. Delft, Dutch Normalisation Institute [cited in de Raat, 2003].
Dybing F, Dybing O (1946) The toxic effect of tetrachloromethane and tetrachloroethylene in oily solution. Acta Pharmacologica, 2:223–226 [cited in de Raat, 2003].
Eberhardt H, Freundt KJ (1966) Perchloräthylenvergiftung. Archives of Toxicology, 21:338 [cited in de Raat, 2003].
EC (2001) Draft European Union risk assessment report. Tetrachloroethylene. CAS No: 127-81-4 [sic], EINECS No: 204-825-9. Draft final environmental report. Luxembourg, European Commission, August.9
EC (2004) European Union risk assessment report. Tetrachloroethylene. CAS No: 127-81-4 [sic], EINECS No: 204-825-9. Draft human health report. Luxembourg, European Commission, May.
ECB (2003) Technical guidance document on risk assessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances, Commission Regulation (EC) No 1488/94 on risk assessment for existing substances, Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Ispra, European Commission, Joint Research Centre, European Chemicals Bureau, Institute for Health and Consumer Protection (http://ecb.jrc.it/home.php?CONTENU=/TechnicalGuidanceDocument/sommaire.php).
ECETOC (1990) Tetrachloroethylene: Assessment of human carcinogenic hazard. Brussels, European Centre for Ecotoxicology and Toxicology of Chemicals, 70 pp. (Technical Report No. 37).
ECETOC (1999) Tetrachloroethylene. Brussels, European Centre for Ecotoxicology and Toxicology of Chemicals (Joint Assessment of Commodity Chemicals No. 39).
Echeverria D, White RF, Sampaio C (1995) A behavioural evaluation of PCE exposure in patients and dry cleaners: a possible relationship between clinical and preclinical effects. Journal of Occupational and Environmental Medicine, 37:667 [cited in de Raat, 2003].
Enzien MV, Picardal F, Hazen TC, Arnold RG, Fliermans CB (1994) Reductive dechlorination of trichloroethylene and tetrachloroethylene under aerobic conditions in a sediment column. Applied and Environmental Microbiology, 60(6):2200–2204 [cited in EC, 2001].
Erickson SJ, Freeman AE (1978) Toxicity screening of fifteen chlorinated and brominated compounds using four species of marine plankton. In: Jolley RL, ed. Water chlorination: Environmental impact and health effects. Vol. 2. Ann Arbor, MI, Ann Arbor Science, pp. 307–311.
Erickson SJ, Hawkins CE (1980) Effects of halogenated organic compounds on photosynthesis in estuarine phytoplankton. Bulletin of Environmental Contamination and Toxicology, 24:910–915 [cited in EC, 2001].
Eskenazi B, Fenster L, Hudes M, Wyrobek AJ, Katz DF, Gerson J, Rempel DM (1991a) A study of the effect of perchloroethylene exposure on the reproductive outcomes of wives of dry-cleaning workers. American Journal of Industrial Medicine, 20:593–600.
Eskenazi B, Wyrobek AJ, Fenster L, Katz DF, Sadler M, Lee J, Hudes M, Rempel DM (1991b) A study of the effect of perchloroethylene exposure on semen quality in dry cleaning workers. American Journal of Industrial Medicine, 20:575–591.
Essing H-G (1975) Feldstudie zur Frage der Hepato- und Nephrotoxizität des Perchloräthylens nach langjähriger berufliche Exposition. In: Schriftenreihe Arbeitsmedizin, Sozialmedizin, Präventivmedizin. Stuttgart, AW Gentner Verlag, p. 59 (ISBN 3872472038) [cited in de Raat, 2003].
Essing H-G, Schacke G, Konig H, et al. (1974a) Investigations on hepatic function in repair shop workers with long-term exposure to perchloroethylene. Der Arztliche Dienst, 35:33–40.
Essing H-G, Schacke G, Bekmann H, et al. (1974b) Investigations on renal function in repair shop workers with long term exposure to perchloroethylene. Der Arztliche Dienst, 35:65–72.
Euro Chlor (2005) Western European consumption (http://www.eurochlor.org/consumption).
Fahrni H-P (1984) Leichtflüchtige chlorierte kohlenwasserstoffe in schweizer gewässern. Gaz-Eaux-Eaux Usées, 64(11):689–695 [cited in EC, 2001].
Fathepure BZ, Boyd SA (1988) Dependence of tetrachloroethylene dechlorination on methanogenic substrate consumption by Methanosarcina sp. strain DCM. Applied and Environmental Microbiology, 54(12):2976–2980 [cited in EC, 2001].
Fathepure BZ, Tiedje JM (1994) Reductive dechlorination of tetrachloroethylene by a chlorobenzoate-enriched biofilm reactor. Environmental Science and Technology, 28(4):746–752 [cited in EC, 2001].
Fathepure BZ, Nengu JP, Boyd SA (1987) Anaerobic bacteria that dechlorinate perchloroethene. Applied and Environmental Microbiology, 53(11):2671–2674.
Fender H (1993) Chromosomenanalytische Undersuchungen bei Textilreinigern. In: Arndt D, Obe G, eds. Cytogenetische Methoden im Rahmen des Populationsmonitoring. München, MMV Verlag, pp. 71–76 [cited in de Raat, 2003].
Fernandez J, Guberan E, Caperos J (1976) Experimental human exposure to tetrachloroethylene vapor and inhalation in breath after inhalation. American Industrial Hygiene Association Journal, 37:143–150 [cited in de Raat, 2003].
Ferroni C, Selis L, Mutti A, Folli D, Bergamaschi E, Franchini I (1992) Neurobehavioral and neuroendocrine effects of occupational exposure to perchloroethylene. Neurotoxicology, 13:243–247 [cited in de Raat, 2003].
Fielding M, Gibson TM, James HA (1981) Levels of trichloroethylene, tetrachloroethylene, and p-dichlorobenzene in groundwaters. Environmental Technology Letters, 2:545–550 [cited in EC, 2001].
Fogel MM, Taddeo AR, Fogel S (1986) Biodegradation of chlorinated ethenes by a methane-utilizing mixed culture. Applied and Environmental Microbiology, 51(4):720–724 [cited in EC, 2001].
Franchini I, Cavatorta A, Falzoi M, Lucertini S, Mutti A (1983) Early indicators of renal damage in workers exposed to organic solvents. International Archives of Occupational and Environmental Health, 52:1–9 [cited in de Raat, 2003].
Frank H, Frank W (1985) Chlorophyll-bleaching by atmospheric pollutants and sunlight. Naturwissenschaften, 72:139–141 [cited in EC, 2001].
Frank H, Frank W (1986) Photochemical activation of chloroethenes leading to destruction of photosynthetic pigments. Experientia, 42:1267–1269 [cited in EC, 2001].
Frank H, Frank W, Thiel D (1989) C1- and C2-halocarbons in soil–air of forests. Atmospheric Environment, 23(6):1333–1335.
Franklin J (1994) The atmospheric degradation and impact of perchloroethylene. Toxicology and Environmental Chemistry, 46:169–182 [cited in EC, 2001].
Frantik E, Vodičková L, Hornychová M, Nosek M (1998) Relative subnarcotic potency of solvents predicted by partition co-efficients.
Central European Journal of Occupational and Environmental Medicine, 4:25–35.Frantz SW, Watanabe PG (1983) Tetrachloroethylene: Balance and tissue distribution in male Sprague-Dawley rats by drinking water administration. Toxicology and Applied Pharmacology, 69:66–72 [cited in de Raat, 2003].
Fredriksson A, Danielsson BRG, Eriksson P (1993) Altered behaviour in adult mice orally exposed to tri- and tetrachloroethylene as neonates. Toxicology Letters, 66:13–19.
Freedman DL, Gosset JM (1989) Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Applied and Environmental Microbiology, 55(9):2144–2151 [cited in EC, 2001].
Friberg L, Kylin B, Nyström A (1953) Toxicities of trichloroethylene and tetrachloroethylene and Fujiwara’s pyridine–alkali reaction. Acta Pharmacologica et Toxicologica, 9:303–312 [cited in de Raat, 2003].
Friesel P, Milde G, Steiner B (1984) Interactions of halogenated hydrocarbons with soils. Fresenius’ Zeitschrift für Analytische Chemie, 319:160–164 [cited in EC, 2001].
Fytianos K, Vasilikiotis G, Weil L (1985) Identification and determination of some trace organic compounds in coastal seawater of northern Greece. Bulletin of Environmental Contamination and Toxicology, 34(3):390–395 [cited in EC, 2001].
Galloway SM, Armstrong MJ, Reuben C, Colman S, Brown B, Cannon C, Bloom AD, Nakamura F, Ahmed M, Duk S, Rimpo J, Margolin BH, Resnick MA, Anderson B, Zeiger E (1987) Chromosome aberrations and sister chromatid exchanges in Chinese hamster ovary cells: evaluations of 108 chemicals. Environmental and Molecular Mutagenesis, 10(Suppl. 10):1–175 [cited in de Raat, 2003].
Gay BW Jr, Hanst PL, Bufalini JJ, Noonan RC (1976) Atmospheric oxidation of chlorinated ethylenes. Environmental Science and Technology, 10:58–67 [cited in EC, 2001].
Gearhart JM, Mahle DA, Greene RJ, Seckel CS, Flemming CD, Fisher JW, Clewell HJ III (1993) Variability of physiologically based pharmacokinetic (PBPK) model parameters and their effects on PBPK model predictions in a risk assessment for perchloroethylene (PCE). Toxicology Letters, 68:131–144 [cited in de Raat, 2003].
Gennari P, Naldi M, Motta R, Nucci MC, Giacomini C, Violante FS, Raffi GB (1992) Gammaglutamyltransferase isoenzyme pattern in workers exposed to tetrachloroethylene. American Journal of Industrial Medicine, 21:661–671 [cited in de Raat, 2003].
Ghantous H, Danielsson BR, Dencker L, Gorczak J, Vesterberg O (1986) Trichloroacetic acid accumulates in murine amniotic fluid after tri- and tetrachloroethylene inhalation. Acta Pharmacologica et Toxicologica, 58:105–114 [cited in de Raat, 2003].
Gibson SA, Sewell GW (1992) Stimulation of reductive dechlorination of tetrachloroethylene in anaerobic aquifer microcosms by addition of short-chain organic acids or alcohols. Applied and Environmental Microbiology, 58(4):1392–1393 [cited in EC, 2001].
Giger W, Schwarzenbach RP, Höhn E, Schellenberg K, Schneider JK, Wasmer HR, Westall J, Zorbrist J (1983) Das verhalten organischer wasser-inhaltsstoffe bei der grunwasserbidung und in Grunwasser. Gas-Wasser-Abwasser, 63(9):517–531 [cited in EC, 2001].
Gilli G, Bono R, Scursatone E (1990a) Volatile halogenated hydrocarbons in urban atmosphere and in human blood. Archives of Environmental Health, 45:101–106 [cited in de Raat, 2003].
Gilli G, Scursatone E, Bono R, Natale P, Grosa M (1990b) An overview of atmospheric pollution in Italy before the use of new gasoline. Science of the Total Environment, 93:51–56 [cited in EC, 2001].
Gobba F, Righi E, Fantuzzi G, Predieri G, Cavazzuti L, Aggazzotti G (1998) Two-year evaluation of perchlorothylene-induced color-vision loss. Archives of Environmental Health, 53:196–198.
Gobba F, Righi E, Fantuzzi G, Roccatto G, Aggazzotti G (2003) Perchloroethylene in alveolar air, blood, and urine as biologic indices of low-level exposure. Journal of Occupational and Environmental Medicine, 45:1152–1157.
Gold JH (1969) Chronic perchloroethylene poisoning. Canadian Psychiatric Association Journal, 14:627–630 [cited in de Raat, 2003].
Goldbloom AA, Boyd LJ (1954) Tetrachloroethylene fatality. Case report of a patient with infectious (virus) hepatitis and hookworm infestion. Industrial Medicine and Surgery, 23:116–119 [cited in de Raat, 2003].
Goldsworthy TL, Popp JA (1987) Chlorinated hydrocarbon induced peroxisomal enzyme activity in relation to species and organ carcinogenicity. Toxicology and Applied Pharmacology, 88:225–233 [cited in de Raat, 2003].
Goldsworthy TL, Smith-Oliver T, Loury DJ (1988) Assessment of chlorinated hydrocarbon-induced genotoxicity and cell replication in rat kidney cells. Environmental and Molecular Mutagenesis, 11(Suppl. 11):39 [cited in de Raat, 2003].
Goodenkauf O, Atkinson JC (1986) Occurrence of volatile organic chemicals in Nebraska ground water. Ground Water, 24(2):231–233.
Gradiski D, Bonnet P, Raoult G (1978) Comparative acute inhalation study of the principal chlorinated aliphatic solvents. Archives des Maladies Professionnelles de Medecine du Travail et de Sécurité Sociale, 39:249–257 [cited in de Raat, 2003].
Grant WM (1962) Toxicology of the eye. Springfield, IL, Charles C. Thomas [cited in de Raat, 2003].
Grathwohl P (1990) Influence of organic matter from soil and sediments from various origins on the sorption of some chlorinated aliphatic hydrocarbons: Implications on Koc correlations. Environmental Science and Technology, 24(11):1687–1693 [cited in EC, 2001].
Green SM, Kahn MF, Kaphalia BS, Ansari GA (2001) Immunohistochemical localization of trichloroacylated protein adducts in tetrachloroethene-treated mice. Journal of Toxicology and Environmental Health, 63:145–157.
Green T (1990) Species differences in carcinogenicity: the role of metabolism in human risk evaluation. Teratogenesis, Carcinogenesis, Mutagenesis, 10:103–113 [cited in de Raat, 2003].
Green T (1997) Tetrachloroethylene: 28 day inhalation study to investigate effects on rat liver and kidney. Alderley Park, Macclesfield, Cheshire, Zeneca Central Toxicology Laboratory (Report CTL/R/1325) [cited in EC, 2004].
Green T, Odum J (1985) Structure/activity studies of the nephrotoxic and mutagenic action of cysteine conjugates of chloro- and fluoroalkenes. Chemico-Biological Interactions, 54:15–31 [cited in de Raat, 2003].
Green T, Odum J, Nash JA, Foster JR (1990) Perchloroethylene-induced rat kidney tumors: An investigation of the mechanisms involved and their relevance to humans. Toxicology and Applied Pharmacology, 103:77–89 [cited in de Raat, 2003].
Greim H, Bonse G, Radwan Z, Reichert D, Henschler D (1975) Mutagenicity in vitro and potential carcinogenicity of chlorinated ethylenes as a function of metabolic oxirane formation. Biochemical Pharmacology, 24:2013–2017 [cited in de Raat, 2003].
Guberan E, Fernandez J (1974) Control of industrial exposure to tetrachloroethylene by measuring alveolar concentrations: Theoretical approach using a mathematical model. British Journal of Industrial Medicine, 31:159–167 [cited in de Raat, 2003].
Haerer AF, Udelman HD (1964) Acute brain syndrome secondary to tetrachloroethylene ingestion. American Journal of Psychiatry, 12:78–79 [cited in de Raat, 2003].
Hake CL, Stewart RD (1977) Human exposure to tetrachloroethylene: Inhalation and skin contact. Environmental Health Perspectives, 21:231–238.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, Niemeier RW (1981) Testing of selected workplace chemicals for teratogenic potential. Scandinavian Journal of Work, Environment and Health, 7(Suppl. 4):66–75 [cited in de Raat, 2003].
Hattis D, White P, Marmorstein L, Koch P (1990) Uncertainties in pharmacokinetic modelling for perchloroethylene. I. Comparison of model structure, parameters, and predictions for low-dose metabolism rates for models derived by different authors. Risk Analysis, 10(3):449–458.
Hattis D, White P, Koch P (1993) Uncertainties in pharmacokinetic modelling for perchloroethylene: II. Comparison of model predictions with data for a variety of different parameters. Risk Analysis, 13:599–610.
Hayes JR, Condie LW, Borcelleca JF (1986) The subchronic toxicity of tetrachloroethylene (perchloroethylene) administered in the drinking water of rats. Fundamental and Applied Toxicology, 7:119–125.
Heil H, Eikmann T, Einbrodt HJ, König H, Lahl U, Zeschmar-Lahl B (1989) Konsequenzen aus dem Altlastfall Bielefeld-Brake. Vom Wasser, 72:321–348 [cited in EC, 2001].
Heimann D, Härle M (1993) Auwirkungen von tetrachloroethen auf Folsomia candida. Geneva, Battelle Europe.
Heitmuller PT, Hollister TA, Parrish PR (1981) Acute toxicity of 54 industrial chemicals to sheepshead minnows (Cyprinodon variegatus). Bulletin of Environmental Contamination and Toxicology, 27:596–604 [cited in EC, 2001].
Hellmann H (1984) Leichtflüchtige Chlorkohlenwasserstoffein den gewässern der Bundesrepublik Deutschland-Auftreten und Bilanz. Haustechnik-Bauphysik-Umwelttechnik-Gesundheits-Igenieur, 105:269–278 [cited in EC, 2001].
Hernberg S, Korkala ML, Asikainen U, Riala R (1984) Primary liver cancer and exposure to solvents. International Archives of Occupational and Environmental Health, 54:147–153.
Hernberg S, Kauppinen T, Riala R, Korkala ML, Asikainen U (1988) Increased risk for primary liver cancer among women exposed to solvents. Scandinavian Journal of Work, Environment and Health,14:356–365.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The carcinogenicity of trichloroethylene and its metabolites trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicology and Applied Pharmacology, 90:183–189 [cited in de Raat, 2003].
Holliger C, Schraa G, Stams AJM, Zehnder AJB (1993) A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Applied and Environmental Microbiology, 59(9):2991–2997 [cited in EC, 2001].
Holmberg B, Ekström T, Högberg J, Lundberg I, Nilsson K, Walles S, Warholm M (1986) Five industrial chemicals investigated for tumor promoting activity in the rat liver. Toxicology Letters, Suppl. 31:197 (abstract P14-7) [cited in de Raat, 2003].
Honma T, Hasegawa H, Sato M, Sudo A (1980a) Changes of free amino acid content in rat brain after exposure to trichloroethylene and tetrachloroethylene. Industrial Health, 18:1–8 [cited in de Raat, 2003].
Honma T, Sudo A, Miyagawa M, Sato M, Hasegawa H (1980b) Effects of exposure to trichloroethylene and tetrachloroethylene on the contents of acetylcholine, dopamine, norepinephrine and serotonin in rat brain. Industrial Health, 18:171–178 [cited in de Raat, 2003].
HSDB (2003) Hazardous Substances Data Bank. Bethesda, MD, United States National Library of Medicine (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB).
HSE (1987) Tetrachloroethylene (tetrachloroethene, perchloroethylene): Toxicity review 17. London, Health and Safety Executive, HMSO Publications Centre [cited in de Raat, 2003].
HSIA (1999) Perchloroethylene white paper. Arlington, VA, Halogenated Solvents Industry Alliance, Inc. (http://www.hsia.org).
HSIA (2005) Perchloroethylene white paper. Arlington, VA, Halogenated Solvents Industry Alliance, Inc., August (http://www.hsia.org/white_papers/perc%20wp%202005.htm).
Hughes JP (1954) Hazardous exposure to some so called safe solvents. Journal of the American Medical Association, 156:234–237 [cited in de Raat, 2003].
Hurford H, Law RJ, Payne AP, Fileman TW (1989) Concentrations of chemicals in the North Sea arising from discharges from chemical tankers. Oil Chemical Pollution, 5:391–410 [cited in EC, 2001].
IARC (1995) Dry cleaning, some chlorinated solvents and other industrial compounds. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 63).
IARC (1999) Consensus report. In: Capen CC, Dybing E, Rice JM, Wilbourn JD, eds. Species differences in thyroid, kidney and urinary bladder carcinogenesis. Lyon, International Agency for Research on Cancer, pp. 1–14 (IARC Scientific Publications 147).
Ikeda M (1977) Metabolism of trichloroethylene and tetrachloroethylene in human subjects. Environmental Health Perspectives, 21:239–245 [cited in de Raat, 2003].
Ikeda M, Koizumi A (1980) Cytogenetic and cytokinetic investigation on lymphocytes from workers occupationally exposed to tetrachloroethylene. In: Holmstedt B, Lauwerijs R, Mercier M, Roberfroid M, eds. Mechanisms of toxicity and hazard evaluation. Amsterdam, Elsevier/North Holland Press [cited in de Raat, 2003].
Ikeda T, Nagano C, Okada A (1969) Hepatotoxic effects of trichloroethylene and perchloroethylene in the rat and mouse. Igaku to Seibutsugako, 79:123–129 [EPA translation TR-78-0135; cited in de Raat, 2003].
Ikeda M, Otsuji H, Imamura T, Komoike Y (1972) Urinary excretion of total trichloro-compounds, trichloroethanol, and trichloroacetic acid as a measure of exposure to trichloroethylene and tetrachloroethylene. British Journal of Industrial Medicine, 29:328–333 [cited in de Raat, 2003].
Imamura T, Ikeda M (1973) Lower fiducial limit of urinary metabolite level as an index of excessive exposure to industrial chemicals. British Journal of Industrial Medicine, 30:289–292 [cited in de Raat, 2003].
IPCS (1984) Tetrachloroethylene. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 31) [cited in de Raat, 2003].
IPCS (2000) International Chemical Safety Card — Tetrachloroethylene. Geneva, World Health Organization, International Programme on Chemical Safety, April 2000 (ICSC 0076).
Isacson P, Bean JA, Splinter R, Olson DB, Kohler J (1985) Drinking water and cancer incidence in Iowa. III. Association of cancer with indices of contamination. American Journal of Epidemiology, 121:856–869 [cited in de Raat, 2003].
ISO (1991) Workplace air — Determination of vaporous chlorinated hydrocarbons — Charcoal tube/solvent desorption/gas chromatographic method. Geneva, International Organization for Standardization (International Standard ISO 9486:1991 (E)) [cited in de Raat, 2003].
Itoh N, Kutsuna S, Ibusuki TI (1994) A product study of the OH radical initiated oxidation of perchloroethylene and trichloroethylene. Chemosphere, 28:2029–2040 [cited in EC, 2001].
Jang J-Y, Droz PO (1997) Ethnic differences in biological monitoring of several organic solvents. II. A simulation study with a physiologically based pharmacokinetic model. International Archives of Occupational and Environmental Health, 70:41–50.
Jang J-Y, Kang SK, Chung HK (1993) Biological exposure indices of organic solvents for Korean workers. International Archives of Occupational and Environmental Health, 65:S219–S222 [cited in de Raat, 2003].
JISA (1993) Carcinogenicity study of tetrachloroethylene by inhalation in rats and mice. Data No. 3-1. Kanagawa, Japan Industrial Safety Association (available from USEPA-IRIS Information Desk).
Kaemmerer K, Fink J, Kietzman M (1982) Studies on the pharmacodynamics of perchloroethylene. Tieraerztliche Umschau, 63:171–182 [cited in de Raat, 2003].
Kanazawa S, Filip Z (1987) Effects of trichloroethylene, tetrachloroethylene and dichloromethane on soil biomass and microbial counts. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene B, 184:24–33 [cited in EC, 2001].
Kaplan SD (1980) Dry-cleaner worker exposed to perchloroethylene; A retrospective cohort mortality study. Cincinnati, OH, United States Department of Health, Education and Welfare (Contract No. 210-77-0094) [cited in de Raat, 2003].
Karlsson JE, Rosengren LE, Kjellstrand P, Haglid KG (1987) Effects of low-dose inhalation of three chlorinated aliphatic organic solvents on deoxyribonucleic acid in the gerbil brain. Scandinavian Journal of Work, Environment and Health, 13:453–458 [cited in de Raat, 2003].
Kästner M (1991) Reductive dechlorination of tri- and tetrachloroethylenes depends on transition from aerobic to anaerobic conditions. Applied and Environmental Microbiology, 57(7):2039–2046 [cited in EC, 2001].
Katz RM, Jowett D (1981) Female laundry and dry cleaning workers in Wisconsin: a mortality analysis. American Journal of Public Health, 71:305–307 [cited in de Raat, 2003].
Kawamura K, Kaplan IR (1983) Organic compounds in the rainwater of Los Angeles. Environmental Science and Technology, 17(8):497–501 [cited in EC, 2001].
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption coefficients of pesticides and other chemicals. Ecotoxicology and Environmental Safety, 4(1):26–38 [cited in EC, 2001].
Kendrick JF (1929) Treatment of hookworm disease with tetrachloroethylene. American Journal of Tropical Medicine, 438–488 [cited in de Raat, 2003].
Kezic S, Monster AC, van de Gevel IA, Kruse J, Opdam JJG, Verberk MM (2001) Dermal absorption of neat liquid solvents on brief exposures in volunteers. American Industrial Hygiene Association Journal, 62:12–18.
Kjellstrand P, Holmquist B, Kanje M (1984) Perchloroethylene: Effects on body and organ weights and plasma butyrylcholinesterase activity in mice. Acta Pharmacologica et Toxicologica, 54:414–424 [cited in de Raat, 2003].
Kjellstrand P, Holmquist B, Jonsson I, Romare S, Mansson L (1985a) Effects of organic solvents on motor activity in mice. Toxicology, 35:35–46.
Kjellstrand P, Bjerkemo M, Adler-Maihofer M, Holmquist B (1985b) Effects of solvent exposure on testosterone levels and butyrylcholinesterase activity in mice. Acta Pharmacologica et Toxicologica, 57:242–249 [cited in de Raat, 2003].
Klaassen CD, Plaa GL (1966) Relative effects of various chlorinated hydrocarbons on liver and kidney function in mice. Toxicology and Applied Pharmacology, 9:139–151 [cited in de Raat, 2003].
Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JG, Roberts RA, Fenner-Crisp PA (2003) PPARalpha agonist-induced rodent tumors: Modes of action and human relevance. Critical Reviews in Toxicology, 33:655–780.
Kline SA, McCoy EC, Rosenkranz HS, Van Duuren BL (1982) Mutagenicity of chloroalkene epoxides in bacterial systems. Mutation Research, 101:115–125.
Knie J, Hälke A, Juhnke I, Schiller W (1983) Ergbnisse der Untersuchungen von chemischen Stoffen mit vier Biotests. Deutsches Gewässerkundliche Mitteilungen, 3:77–79.
Könemann H (1981) Quantitative structure–activity relationships in fish toxicity studies. Part 1: Relationships for 50 industrial pollutants. Toxicology, 19:209–221 [cited in EC, 2001].
Koppel C, Arndt I, Arendt U, Koeppe P (1985) Acute tetrachloroethylene poisoning: Blood elimination kinetics during hyperventilation therapy. Journal of Toxicology and Clinical Toxicology, 23:103–115 [cited in de Raat, 2003].
Koppmann R, Johnen FJ, Plass-Dülmer C, Rudolph J (1993) Distribution of methylchloride, dichloromethane, trichloroethene and tetrachloroethene over the north and south Atlantic. Journal of Geophysical Research, 98(D11):20 517–20 526 [cited in EC, 2001].
Kringstad KP, Ljungquist PO, De Sousa F, Stromberg LM (1981) Identification and mutagenic properties of some chlorinated aliphatic hydrocarbons in spent liquor from kraft pulp chlorination. Environmental Science and Technology, 15:562–566 [cited in de Raat, 2003].
Kroneld R (1986) Volatile halocarbons in water. Bulletin of Environmental Contamination and Toxicology, 37(5):677–685 [cited in EC, 2001].
Kronevi T, Wahlberg JE, Holmberg B (1981) Skin pathology following epicutaneous exposure to seven organic solvents. International Journal of Tissue Reactions, 3:21–30 [cited in de Raat, 2003].
Kubin D, Bächmann K, Kessel M (1989) Langzeitmessungen chlorierter Kohlenwasserstoffe in der Atmosphäre. VDI-Berichte, 745:257–265 [cited in EC, 2001].
Kylin B, Reichard H, Sümegi I, Yllner S (1963) Hepatotoxicity of inhaled trichloroethylene, tetrachloroethylene and chloroform. Single exposure. Acta Pharmacologica et Toxicologica, 20:16– 26 [cited in de Raat, 2003].
Kylin B, Sumegi I, Yllner S (1965) Hepatotoxicity of trichloroethylene and tetrachloroethylene. Longterm exposure. Acta Pharmacologica et Toxicologica, 22:379–385 [cited in de Raat, 2003].
Kyrklund T, Haglid KG (1990) Brain lipid changes after organic solvent exposure. Uppsala Journal of Medical Sciences, Supplement, 48:267–277 [cited in de Raat, 2003].
Kyrklund T, Alling C, Kjellstrand P, Haglid KH (1984) Chronic effects of perchloroethylene on the composition of lipid and acyl groups in the cerebral cortex and hippocampus of the gerbil. Toxicology Letters, 22:343–349 [cited in de Raat, 2003].
Kyrklund T, Kjellstrand P, Haglid KG (1987) Lipid composition and fatty acid pattern of the gerbil brain after exposure to perchloroethylene. Archives of Toxicology, 60:397–400 [cited in de Raat, 2003].
Kyrklund T, Kjellstrand P, Haglid KG (1988) Effects of exposure of Freon 11, 1,1,1-trichloroethane or perchloroethylene on the lipid and fatty-acid composition of rat cerebral cortex. Scandinavian Journal of Work, Environment and Health, 14:91–94 [cited in de Raat, 2003].
Kyrklund T, Kjellstrand P, Haglid KG (1990) Long-term exposure of rats to perchloroethylene, with and without a post-exposure solvent-free recovery period: effects on brain lipids. Toxicology Letters, 52:279–285 [cited in de Raat, 2003].
Kyyrönen P, Taskinen H, Lindbohm ML, Hemminki K, Heinonen OP (1989) Spontaneous abortions and congenital malformations among women exposed to tetrachloroethylene in dry cleaning. Journal of Epidemiology and Community Health, 43:346–351.
LaFuente A, Mallol J (1986) Thioethers in urine during occupational exposure to tetrachloroethylene. British Journal of Industrial Medicine, 43:68–69 [cited in de Raat, 2003].
Lagakos SW, Wessen BJ, Zelen M (1986) Analysis of contaminated well water and health effects in Woburn, Massachusetts. Journal of the American Statistical Association, 81:583–596 [cited in de Raat, 2003].
Lahl U, Cetinkaya M, Düszeln J, Stachel B, Thiemann W (1981) Health risks from volatile halogenated hydrocarbons. Science of the Total Environment, 20:171–189.
Lash LH, Parker JC (2001) Hepatic and renal toxicities associated with perchloroethylene. Pharmacological Reviews, 53:177–208.
Lash LH, Quia W, Putt DA, Desai K, Elfarra AA, Sicuria R, Parker JC (1998) Glutathione conjugation of perchloroethylene in rats and mice in vitro: sex-, species-, and tissue-dependent differences. Toxicology and Applied Pharmacology, 150:49–57.
Lash LH, Qian W, Putt DA, Hueni SE, Elfarra AA, Sicuri AR, Parker JC (2002) Renal toxicity of perchloroethylene and S-(1,2,2-trichlorovinyl)glutathione in rats and mice: sex- and species-dependent differences. Toxicology and Applied Pharmacology, 179:163–171.
Lauwerys R, Herbrand J, Buchet JP, Bernard A, Gaussin J (1983) Health surveillance of workers exposed to tetrachloroethylene in dry-cleaning shops. International Archives of Occupational and Environmental Health, 52:69–77 [cited in de Raat, 2003].
Lay JP, Herrmann ME (1989) Wirkungen auf Ökosysteme des Süßwassers – Eine Fallstudie mit Tetrachlorethen. VDI-Berichte, 745:585–600 [cited in EC, 2001].
Lay JP, Schauerte W, Klein W, Korte F (1984) Influence of tetrachloroethylene on the biota of aquatic systems: Toxicity to phyto- and zooplankton species in compartments of a natural pond. Archives of Environmental Contamination and Toxicology, 13(2):135–142 [cited in EC, 2001].
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea (Daphnia magna). Bulletin of Environmental Contamination and Toxicology, 24(5):684–691 [cited in EC, 2001].
Lebret E, Van de Wiel HJ, Bos HP, Noij D, Boleij JSM (1986) Volatile organic compounds in Dutch homes. Environment International, 12:323–332 [cited in de Raat, 2003].
Lee JF, Crum JR, Boyd SA (1989) Enhanced retention of organic contaminants by soils exchanged with organic cations. Environmental Science and Technology, 23(11):1365–1372 [cited in EC, 2001].
Lemburg P, Sprock I, Bretschneider A, Storm W, Gobel U (1979) A new concept of therapy in accidental intoxications with halogenated hydrocarbons. Veterinary and Human Toxicology, 21:37–40 [cited in de Raat, 2003].
Leschber R, Mergler-Voelkl R, Nerger M (1990) Soil and groundwater contamination by low boiling chlorinated hydrocarbons in Berlin. International Journal of Environmental Analytical Chemistry, 39:159–164 [cited in EC, 2001].
Levine B, Fierro MF, Goza SW, Valentour JC (1981) A tetrachloroethylene fatality. Journal of Forensic Science, 26:206–209 [cited in de Raat, 2003].
Liang LN, Grbi-Gali D (1993) Biotransformation of chlorinated aliphatic solvents in the presence of aromatic compounds under methanogenic conditions. Environmental Toxicology and Chemistry, 12:1377–1393 [cited in EC, 2001].
Linak E, Leder A, Yoshida Y (1992) Chlorinated solvents. In: Chemical economics handbook. Menlo Park, CA, SRI International, pp. 632.3000–632.3002.
Lindbohm M-L, Hemminki K, Kyyrönen P (1984) Parental occupational exposure and spontaneous abortions in Finland. American Journal of Epidemiology, 120:370–378.
Lindbohm ML, Taskinen H, Sallmen M, Hemminki K (1990) Spontaneous abortions among women exposed to organic solvents. American Journal of Industrial Medicine, 17:449–463.
Ling S, Lindsay WA (1971) Perchloroethylene burns. British Medical Journal, 3:115 [cited in de Raat, 2003].
Lob M (1957) The dangers of perchloroethylene. Internationales Archiv für Gewerbepathologie und Gewerbehygiene, 16:45–52 [cited in de Raat, 2003].
Lökle DM, Schecter AJ, Christian JJ (1983) Effects of chloroform, tetrachloroethylene and trichloroethylene on survival, growth and liver of Poecilia sphenops. Bulletin of Environmental Contamination and Toxicology, 30:199–205.
Lorenz H, Weber E, Walter G, Haaß A, Steigerwald F, Buchter A (1990) Nachweis von Hirnschädigungen durch Tetrachlorethen. Zentralblatt für Arbeitsmedizin, 40:355–364 [cited in de Raat, 2003].
LUA (1999) Luftqualität in Nordrhein-Westfalen. Landesumweltamt Essen (LUQS-Jahresbericht 1998) [cited in EC, 2001].
Lukaszewski T (1979) Acute tetrachloroethylene fatality. Clinical Toxicology, 15:411–415 [cited in de Raat, 2003].
Lundberg I, Högberg J, Kronevi T, Holmberg B (1987) Three industrial solvents investigated for tumor promoting activity in the rat liver. Cancer Letters, 36:29–33 [cited in de Raat, 2003].
Lyman WL, Reehl WF, Rosenblatt DH (1981) Handbook of chemical property estimation methods: environmental behaviour of organic compounds. New York, NY, McGraw-Hill Inc., pp. 15.17–15.33.
Lynge E, Thygesen L (1990) Primary liver cancer among women in laundry and dry-cleaning work in Denmark. Scandinavian Journal of Work, Environment and Health, 16:108–112.
Lynge E, Carstensen B, Andersen O (1995) Primary liver cancer and renal cell carcinoma in laundry and dry-cleaning workers in Denmark. Scandinavian Journal of Work, Environment and Health, 21:293–295.
Mabey WR, Smith JH, Podoll RT, Johnson HL, Mill T, Chou TW, Gates J, Waight Partridge I, Jaber H, Vandenberg D (1982) Aquatic fate data for organic priority pollutants. Washington, DC, United States Environmental Protection Agency (EPA Report 440/4-81-014) [cited in EC, 2001].
MacKenzie Peers A (1985) Method 5. The determination of trichloroethylene and tetrachloroethylene in air. In: Fishbein L, O’Neill IK, eds. Environmental carcinogens — Selected methods of analysis. Vol. 7. Some halogenated hydrocarbons. Lyon, International Agency for Research on Cancer (IARC Scientific Publications 68) [cited in de Raat, 2003].
Malle KG (1990) Vergleich von Elbe und Rhein-Gegenüberstellung der Gütedaten. Umweltwissenschaften und Schadstoff-Forschung Zeitschrift für Umweltchemie und Ökotoxikologie, 2(2):92–96 [cited in EC, 2001].
Malo J-L, Chan-Yeung M (2001) Occupational asthma. Journal of Allergy and Clinical Immunology, 108:317–328.
Maltoni C, Cotti G (1986) Results of long-term carcinogenicity bioassays of Sprague-Dawley rats administrated by ingestion. Acta Oncologica, 1:11–26 [cited in de Raat, 2003].
Marchand M, Capris JC, Tronczynski J, Marty JC, Scribe P, Saliot A (1986) Processus de transport et flux des hydrocarbures et hydrocarbures halogénés dans l’estuaire de la Loire. Rapports et Proces-Verbaux des Réunions, Conseil International pour l’Exploration de la Mer, 186:361–374 [cited in EC, 2001].
Marchand M, Caprais JC, Pignet P (1988) Hydrocarbons and halogenated hydrocarbons in coastal waters of the Western Mediterranean (France). Marine Environmental Research, 25:131–159 [cited in EC, 2001].
Marchand M, Caprais JC, Pignet P, Porot V (1989) Organic pollutants in urban sewage and pollutant inputs to the marine environment. Application to the French shoreline. Water Research, 23(4):461–470.
Maronpot RR, Shimkin MB, Witschi HP, Smith LH, Cline JM (1986) Strain A mouse pulmonary tumor test results for chemicals previously tested in the National Cancer Institute carcinogenicity tests. Journal of the National Cancer Institute, 76:1101–1112 [cited in de Raat, 2003].
Marth E (1987) Metabolic changes following oral exposure to tetrachloroethylene in subtoxic concentrations. Archives of Toxicology, 60:293–299 [cited in de Raat, 2003].
Marth E, Stunzer D, Binder H, Mose JR (1985a) Tetrachloroethylene; A study of the effect of low concentrations of 1,1,2,2-tetrachloroethylene on the organism [sic] of the mouse. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene B, 181:525–540 [cited in de Raat, 2003].
Marth E, Stunzer D, Binder H, Mose JR (1985b) Tetrachloroethylene; A study of the effect of low concentrations of 1,1,2,2-tetrachloroethylene on the organism [sic] of the mouse. Examinations of tetrachloroethylene residues in various organs and establishment of histologic changes of the examined organ. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene B,181:541–547 [cited in de Raat, 2003].
Mattsson JL, Albee RR, Yano BL, Bradley GJ, Spencer PJ (1998) Neurotoxicologic examination of rats exposed to 1,1,2,2-tetrachloroethylene (perchloroethylene) vapour for 13 weeks. Neurotoxicology and Teratology, 20:83–98.
Mazza V (1972) Enzymatic changes in experimental tetrachloroethylene poisoning. Folia Medica (Naples, Italy), 55:373–381 [cited in de Raat, 2003].
Mazza V, Brancaccio A (1971) Adrenal cortical and medullar hormones in experimental tetrachloroethylene poisoning. Folia Medica (Naples, Italy), 54:204–207 [cited in de Raat, 2003].
Mazzullo M, Grilli S, Lattanzi G, Prodi G, Turina MP, Colacci A (1987) Evidence of DNA binding activity of perchloroethylene. Research Communications in Chemical and Pathological Pharmacology, 58:215–235.
McCredie M, Stewart JH (1993) Risk factors for kidney cancer in New South Wales. IV. Occupation. British Journal of Industrial Medicine, 50:349–354 [cited in IARC, 1995].
McLaughlin JK, Malker HS, Stone BJ, Weiner JA, Malker BK, Ericsson JL, Blot WJ, Fraumeni JF Jr (1987) Occupational risks for renal cancer in Sweden. British Journal of Industrial Medicine, 44:119–123 [cited in de Raat, 2003].
McMichael AJ (1976) Standardized mortality ratios and the "healthy worker effect": Scratching beneath the surface. Journal of Occupational Medicine,18:165–168.
Meckler LC, Phelps DK (1966) Liver disease secondary to tetrachloroethylene exposure. Journal of the American Medical Association, 197:144–145 [cited in de Raat, 2003].
Meek ME, Bucher JR, Cohen SM, Dellarco V, Hill RN, Lehman-McKeeman LD, Longfellow DG, Pastoor T, Seed J, Patton DE (2003) A framework for human relevance analysis of information on carcinogenic modes of action. CRC Critical Reviews in Toxicology, 33(6):591–653.
Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH (1994) Occupational risk factors for renal-cell carcinoma in Denmark. Scandinavian Journal of Work, Environment and Health, 20:160–165.
Mennear JH (1985) NTP technical report on the toxicology and carcinogenesis studies of tetrachloroethylene (perchloroethylene) in F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle Park, NC, United States Department of Health and Human Services, National Toxicology Program (DHHS-NIH Report 85-2657) [cited in de Raat, 2003].
Metz V, Graben N, Bock KD (1982) Symptoms and differential therapy of tetra-(=per-)chloroethylene poisoning by ingestion or inhalation. Die Medizinische Welt, 33:892–894 [cited in de Raat, 2003].
Meuling WJA, Ebens R (1986) Biological monitoring of tetrachloroethylene in a laundry facility. Rijswijk, Medical Biological Laboratory TNO (MBL 1986-24A) (in Dutch) [cited in de Raat, 2003].
Meyer HJ (1973) Bronchopulmonary changes induced by trichloroethylene and other halogenated hydrocarbons. Bronches, 23:113–124 [cited in de Raat, 2003].
Milman HA, Story DL, Ricio ES (1988) Rat liver foci and in vitro assays to detect initiating and promoting effects of chlorinated ethanes and ethylenes. Annals of the New York Academy of Sciences, 534:521–530 [cited in de Raat, 2003].
Mitoma C, Steeger T, Jackson SE, Wheeler KP, Rogers JH, Milman HA (1985) Metabolic disposition study of chlorinated hydrocarbons in rats and mice. Drug and Chemical Toxicology, 8:183–194 [cited in de Raat, 2003].
Monster AC (1979) Difference in uptake, elimination, and metabolism in exposure to trichloroethylene, 1,1,1-trichloroethane, and tetrachloroethylene. International Archives of Occupational and Environmental Health, 42:311–317 [cited in de Raat, 2003].
Monster AC (1986) Biological monitoring of chlorinated hydrocarbon vapors. Journal of Occupational Medicine, 28:583–588 [cited in de Raat, 2003].
Monster AC, Houtkooper JM (1979) Estimation of individual uptake of trichloroethylene, 1,1,1-trichloroethane and tetrachloroethylene from biological parameters. International Archives of Occupational and Environmental Health, 42:319–323 [cited in de Raat, 2003].
Monster AC, Boersma G, Steenweg H (1979) Kinetics of tetrachloroethylene in volunteers: Influence of exposure concentration and work load. International Archives of Occupational and Environmental Health, 42:303–309 [cited in de Raat, 2003].
Monster A, Regouin-Peeters W, van Schijndel A, van der Tuin J (1983) Biological monitoring of occupational exposure to tetrachloroethene. Scandinavian Journal of Work, Environment and Health, 9:273–281 [cited in de Raat, 2003].
Morgan B (1969) Dangers of perchloroethylene. British Medical Journal, 2:513 [cited in de Raat, 2003].
Moser VC, Cheek BM, MacPhail RC (1995) A multidisciplinary approach to toxicological screening: III. Neurobehavioral toxicity. Journal of Toxicology and Environmental Health, 45:173–210.
Moslen M, Reynolds E, Szabo S (1977) Enhancement of metabolism and hepatotoxicity of trichloroethylene and perchloroethylene. Biochemical Pharmacology, 26:369–375 [cited in de Raat, 2003].
Mudder TI (1982) Development of empirical structure biodegradability relationships and biodegradability test protocol for volatile and slightly soluble priority pollutants. In: American Chemical Society environmental chemistry conference proceedings 1982, Kansas, MO, pp. 52–53 [cited in EU, 2001].
Mundt KA, Birk T, Burch MT (2003) Critical review of the epidemiological literature on occupational exposure to perchloroethylene and cancer. International Archives of Occupational and Environmental Health, 76:473–491.
Murakiami K, Horikawa K (1995) The induction of micronuclei in mice hepatocytes and reticulocytes by tetrachloroethylene. Chemosphere, 31:3733–3739.
Mutti A, Alinovi R, Bergamaschi E, Biagini C, Cavazzini S, Franchini I, Lauwerys RR, Bernard AM, Roels H, Gelpi E, Rosello J, Ramis I, Price RG, Taylor SA, De Broe M, Nuyts GD, Stolte H, Fels LM, Herbort C (1992) Nephropathies and exposure to perchloroethylene in dry-cleaners. Lancet, 340:189–193.
Nagano K, Nishizawa T, Yamamoto S, Matsushima T (1998a) Inhalation carcinogenesis studies of six halogenated hydrocarbons in rats and mice. In: Chiyotani K, Hosada Y, Aizawa Y, eds. Advances in the prevention of occupational respiratory diseases. Proceedings of the 9th international conference on occupational respiratory diseases, Kyoto, 13–16 October 1997. Elsevier, Amsterdam.
Nagano K, Nishizawa T, Yamamoto S, Matsushima T (1998b) Brief summary of the report to the Ministry of Labour on "Toxicology and carcinogenesis studies of tetrachloroethylene in F344/DuCrj rats and Crj:BDF1 mice (two-year inhalation studies)". Submitted in May 2005 to WHO by Dr K. Nagano, Division of Experimental Toxicology, Japan Bioassay Research Center, Japanese Industrial Safety and Health Association, Kanagawa (in Japanese).
Nakatsuka H, Watanabe T, Takeuchi Y, Hisanaga N, Shibata E, Suzuki H, Huang MY, Chen Z, Qu QS, Ikeda M (1992) Absence of blue-yellow color vision loss among workers exposed to toluene or tetrachloroethylene, mostly at levels below occupational exposure limits. International Archives of Occupational and Environmental Health, 64:113–117.
Narotsky MG, Kavlock RJ (1995) A multidisciplinary approach to toxicological screening: II. Development toxicity. Journal of Toxicology and Environmental Health, 45:145–171.
NCI (1977) Bioassay of tetrachloroethylene for possible carcinogenicity. Cincinnati, OH, United States Department of Health, Education and Welfare, National Cancer Institute (NCI TR 23) [cited in de Raat, 2003].
Neely WB, Branson DR, Blau GE (1974) Partition coefficient to measure bioconcentration potential of organic chemicals in fish. Environmental Science and Technology, 8(13):1113–1115 [cited in EC, 2001].
Nelson BK, Taylor BJ, Setzer JV, Hornung RW (1980) Behavioral teratology of perchloroethylene in rats. Journal of Environmental Pathology and Toxicology, 3:233–250 [cited in de Raat, 2003].
Nicolis GD, Helwig EB (1973) Exfoliate dermatitis. A clinicopathological study of 135 cases. Archives of Dermatology, 108:788–797 [cited in de Raat, 2003].
Nicovich JM, Wand S, McKee ML, Wine PH (1996) Kinetics and thermochemistry of the Cl(2Pj) + C2Cl4 association reaction. Journal of Physical Chemistry, 100:680–688 [cited in EC, 2001].
Ninomiya K, Saki M, Ohba E, Kashiwagi N (1994) Kinetic model for the biotransformation of tetrachloroethylene in groundwater. Water Science and Technology, 30(7):3–18.
NIOSH (1998) Perchloroethylene (portable GC) in exhaled breath and air. Method 3704. In: NIOSH manual of analytical methods. Atlanta, GA, United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (http://www.cdc.gov/niosh/nmam/method-2000.html).
NIOSH (2003) Hydrocarbons, halogenated. Method 1003. In: NIOSH manual of analytical methods. Atlanta, GA, United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (http://www.cdc.gov/niosh/nmam/method-2000.html).
NTP (1986) Toxicology and carcinogenesis studies of tetrachloroethylene (perchloroethylene) (CAS No.
NTP (2001) Tetrachloroethylene. In: Report on carcinogens, 9th ed. Research Triangle Park, NC, United States Department of Health and Human Services, National Toxicology Program.
NTP (2002) Tetrachloroethylene (perchloroethylene). In: Report on carcinogens, 10th ed. Research Triangle Park, NC, United States Department of Health and Human Services, National Toxicology Program.
Odum J, Green T (1986) Perchloroethylene metabolism by glutathione pathway. Toxicologist, 7:1077 [cited in de Raat, 2003].
Odum J, Green T, Foster JR, Hext PM (1988) The role of trichloroacetic acid and peroxisome proliferation in the differences in carcinogenicity of perchloroethylene in the mouse and rat. Toxicology and Applied Pharmacology, 92:103–112 [cited in de Raat, 2003].
Ogata M, Tomokuni K, Watanabe S (1968) ATP and lipid contents in the liver of mice after inhalation of chlorinated hydrocarbons. Industrial Health, 6:116–119 [cited in de Raat, 2003].
Ohtsuki T, Sato K, Koizumi A, Kumai M, Ikeda M (1983) Limited capacity of humans to metabolize tetrachloroethylene. International Archives of Occupational and Environmental Health, 51:381–390 [cited in de Raat, 2003].
Olsen J, Hemminki K, Ahlborg G, Bjerkedal T, Kyyronen P, Taskinen H, Lindbohm ML, Heinonen OP, Brandt L, Kolstad H, Halvorsen BA, Egenæs J (1990) Low birthweight, congenital malformations, and spontaneous abortions among dry-cleaning workers in Scandinavia. Scandinavian Journal of Work, Environment and Health, 16:163–168.
Opdam JJG, Smolders FJF (1986) Alveolar sampling and fast kinetics of tetrachloroethene in man. I. Alveolar sampling. British Journal of Industrial Medicine, 43:814–824 [cited in de Raat, 2003].
Opdam JJG, Smolders FJF (1987) Alveolar sampling and fast kinetics of tetrachloroethene in man. II. Fast kinetics. British Journal of Industrial Medicine, 44:26–34 [cited in de Raat, 2003].
OSHA (1999) Tetrachloroethylene/trichloroethylene. Method 1001. Washington, DC, United States Department of Labor, Occupational Health and Safety Administration (http://www.osha.gov/dts/sltc/methods/mdt/mdt1001/1001.html).
Pahler A, Birner G, Parker J, Dekant W (1998) Generation of antibodies to di- and trichloroacetylated proteins and immunochemical detection of protein adducts in rats treated with perchloroethylene. Chemical Research in Toxicology, 11:995–1004.
Palacek I (1970) So-called chemical asthma. Arhiv za Higijenu Rada i Toksikologiju, 21:161–166 [cited in de Raat, 2003].
Patel R, Janakiraman N, Towne WD (1977) Pulmonary edema due to tetrachloroethylene. Environmental Health Perspectives, 21:247–249 [cited in de Raat, 2003].
Paulu C, Aschengrau A, Ozonoff A (1999) Tetrachloroethylene-contaminated drinking water in Massachusetts and the risk of colon–rectum, lung and other cancers. Environmental Health Perspectives, 107:265–271.
Pavlostathis SG, Zhuang P (1993) Reductive dechlorination of chloroalkenes in microcosms developed with a field contaminated soil. Chemosphere, 27(4):585–595 [cited in EC, 2001].
Pearson CR, McConnell G (1975) Chlorinated C1 and C2 hydrocarbons in the marine environment. Proceedings of the Royal Society of London, Series B, 189:305–332.
Pegg DG, Zempel JA, Braun WH, Watanabe PG (1979) Disposition of tetrachloro(14C)ethylene following oral and inhalation exposure in rats. Toxicology and Applied Pharmacology, 51:465–474 [cited in de Raat, 2003].
Pekari K, Aitio A (1985) Method 27; Determination of tetrachloroethylene in blood. In: Fishbein L, O’Neill IK, eds. Environmental carcinogens — Selected methods of analysis. Vol. 7. Some halogenated hydrocarbons. Lyon, International Agency for Research on Cancer (IARC Scientific Publications 68) [cited in de Raat, 2003].
Pellizari ED, Sheldon LS, Bursey JT (1985a) Method 25; GC/MS determination of volatile halocarbons in blood and tissue. In: Fishbein L, O’Neill IK, eds. Environmental carcinogens — Selected methods of analysis. Vol. 7. Some halogenated hydrocarbons. Lyon, International Agency for Research on Cancer (IARC Scientific Publications 68) [cited in de Raat, 2003].
Pellizari ED, Zweidinger RA, Sheldon LS (1985b) Method 24; GC/MS determination of volatile hydrocarbons in breath samples. In: Fishbein L, O’Neill IK, eds. Environmental carcinogens — Selected methods of analysis. Vol. 7. Some halogenated hydrocarbons. Lyon, International Agency for Research on Cancer (IARC Scientific Publications 68) [cited in de Raat, 2003].
Pesch B, Haerting J, Ranft U, Klimpel A, Oelschlagel B, Schill W, MURC Study Group (2000) Occupational risk factors for urothelial carcinoma: agent-specific results from a case–control study in Germany. International Journal of Epidemiology, 29:238–247.
Pezzagno G, Imbriani M, Ghittori S, Capodaglio E (1988) Urinary concentration, environmental concentration, and respiratory uptake of some solvents: effect of the work load. American Industrial Hygiene Association Journal, 49:546–552 [cited in de Raat, 2003].
Phelps TJ, Niedzielski JJ, Malachowsky KJ, Schram RM, Herbes SE, White DC (1991) Biodegradation of mixed-organic wastes by microbial consortia in continuous-recycle expanded bed reactors. Environmental Science and Technology, 25(8):1461–1465 [cited in EC, 2001].
Pignatello JJ (1990) Slowly reversible sorption of aliphatic halocarbons in soils. Formation of residual fractions. Environmental Toxicology and Chemistry, 9:1107–1115 [cited in EC, 2001].
Popp W, Muller G, Baltes-Schmitz B, Wehner B, Vahrenholz C, Schmieding W, Benninghoff M, Norpoth K (1992) Concentrations of tetrachloroethene in blood and trichloroacetic acid in urine in workers and neighbours of dry-cleaning shops. International Archives of Occupational and Environmental Health, 63:393–395 [cited in de Raat, 2003].
Potter CL, Chang LW, DeAngelo AB, Daniel FB (1996) Effects of four trihalomethanes on DNA strand breaks, renal hyaline droplet formation and serum testosterone in male F-344 rats. Cancer Letters, 106:235–242.
Pozzani UC, Weil CS, Carpenter CP (1959) The toxicological basis of threshold limit values: 5. The experimental inhalation of vapour mixture by rats, with notes upon the relationship between single dose inhalation and single dose oral data. American Industrial Hygiene Association Journal, 20:364–369 [cited in de Raat, 2003].
PRI (2000) Assessing the chronic effects of atmospheric tetrachloroethylene (PER) on plants. Wageningen, Plant Research International (Note 48) [cited in EC, 2001].
Price PJ, Hassett CM, Mansfield JL (1979) Transforming activities of trichloroethylene and proposed industrial alternatives. In Vitro, 14:290–293 [cited in de Raat, 2003].
Rabbini GH, Gilman RH, Kabir I, Mondel G (1985) The treatment of Fasciolopsis buski infection in children: a comparison of thiabendazole, mebendazole, levamisole, pyrantel pamoate, hexylresorcinol and tetrachloroethylene. Transactions of the Royal Society of Tropical Medicine and Hygiene, 79:513–515 [cited in de Raat, 2003].
Rampy LW, Quast JF, Balmer MF (1978) Results of long-term inhalation toxicity study on rats of perchloroethylene (tetrachloroethylene) formulation. In: Plaa GL, Duncan WAM, eds. Proceedings of the first international congress on toxicology. New York, NY, Academic Press [cited in de Raat, 2003].
Ramsey JC, Andersen ME (1984) A physiologically based description of the inhalation pharmacokinetics of styrene in rats and humans. Toxicology and Applied Pharmacology, 73:159–175.
Rao HV, Brown DR (1993) A physiologically based pharmacokinetic assessment of tetrachloroethylene in groundwater for a bathing and showering determination. Risk Analysis, 13:37–49.
Rao KS, Betso JE, Olson KJ (1981) A collection of guinea pig sensitisation test results — grouped by chemical class. Drug and Chemical Toxicology, 4:331–351 [cited in de Raat, 2003].
Reimann S, Grob K, Frank H (1996) Chloroacetic acids in rainwater. Environmental Science and Technology, 30:2340–2344 [cited in EC, 2001].
Reinhardt CF, Mullin LS, Maxfield ME (1973) Epinephrine-induced cardiac arrhythmia potential of some common industrial solvents. Journal of Occupational Medicine, 15:953–955 [cited in de Raat, 2003].
Reitz RH, Gargas ML, Mendrala AL, Schumann AM (1996) In vivo and in vitro studies of perchloroethylene metabolism for physiologically based pharmacokinetics modeling in rats, mice and humans. Toxicology and Applied Pharmacology, 136:289–306.
Renner I, Schleyer R, Mühlhausen D (1990) Gefährdung der grundwasserqualität durch anthropogene organische Luftverunreinigungen. VDI-Bericht, 837:705–727 [cited in EC, 2001].
Reunanen M, Kroneld R (1982) Determination of volatile halocarbons in raw and drinking water, human serum, and urine by electron capture GC. Journal of Chromatographic Science, 20:449–454 [cited in EC, 2001].
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic toxicity of some chlorinated benzenes, chlorinated ethanes and tetrachloroethylene to Daphnia magna. Archives of Environmental Contamination and Toxicology, 12(6):679–684 [cited in EC, 2001].
Riggin RM (1985) Method 12. Determination of volatile organic compounds in ambient air using Tenax adsorption and gas chromatography/mass spectrometry. In: Fishbein L, O’Neill IK, eds. Environmental carcinogens — Selected methods of analysis. Vol. 7. Some halogenated hydrocarbons. Lyon, International Agency for Research on Cancer (IARC Scientific Publications 68) [cited in de Raat, 2003].
Riihimäki V, Pfäffli P (1978) Percutaneous absorption of solvent vapours in man. Scandinavian Journal of Work, Environment and Health, 4:73–85 [cited in de Raat, 2003].
RIVM (1993) Vluchtige gechloreerde koolwaterstoffen. Bilthoven, National Institute for Public Health and the Environment.
Römbke J, Bauer C, Hilt J (1991) Study of the acute toxicity for the earthworm of tetrachloroethene. According to the OECD Guideline for testing of chemicals No. 207. Battelle Institute [cited in EC, 2001].
Römbke J, Heimann D, Bauer C, Vickus P (1993) Study of the acute toxicity for Poecilus cupreus (Carabidae) for tetrachloroethene. Battelle Institute [cited in EC, 2001].
Rosengren LE, Kjellstrand P, Haglid KG (1986) Tetrachloroethylene: levels of DNA and S-100 in the gerbil CNS after chronic exposure. Neurobehavioural Toxicology and Teratology, 8:201–206 [cited in de Raat, 2003].
Rowe VK, McCollister D, Spencer HC, Adams EM, Irish DD (1952) Vapor toxicity of tetrachloroethylene for laboratory animals and human subjects. AMA Archives of Industrial Hygiene and Occupational Medicine, 5:566–579 [cited in de Raat, 2003].
Ruder AM, Ward EM, Brown DP (1994) Cancer mortality in female and male dry-cleaning workers. Journal of Occupational Medicine, 36:867–874.
Ruder AM, Ward EM, Brown DP (2001) Mortality in dry-cleaning workers: an update. American Journal of Industrial Medicine, 39:121–132.
Sagunski H, Hajimiragha H, Fischer U, Böttger A (1987) Grundwasserverunreinigungen durch Halogenkohlenwasserstoffe im Raum Düsselodorf. Forum Städte-Hygiene, 38:75–76.
Saland G (1967) Accidental exposure to perchloroethylene. New York State Journal of Medicine, 67:2359–2361 [cited in de Raat, 2003].
Sandground JH (1941) Coma following medication with tetrachloroethylene. Journal of the American Medical Association, 117:440–441 [cited in de Raat, 2003].
Savolainen H, Pfäffli P, Tengen M, Vainio H (1977) Biochemical and behavioural effects of inhalation exposure to tetrachloroethylene and dichloromethane. Journal of Neuropathology and Experimental Neurology, 36:941–949 [cited in de Raat, 2003].
Schlehofer B, Heuer C, Blettner M, Niehoff D, Wahrendorf J (1995) Occupation, smoking and demographic factors, and renal cell carcinoma in Germany. International Journal of Epidemiology, 24(1):51–57.
Schlingman AS, Gruhzit OM (1927) Studies on the toxicity of tetrachloroethylene, a new anthelminthic. Journal of the American Veterinary Medical Association, 71:189–209 [cited in de Raat, 2003].
Schreiber JS, Hudnell HK, Geller AM, House DE, Aldous KM, Force MS, Langguth KW, Prohonic EJ, Parker JC (2002) Apartment residents’ and day care workers’ exposures to tetrachloroethylene (perc) and deficits in visual contrast sensitivity. Environmental Health Perspectives, 110:655–664.
Schumann AM, Quast JF, Watanabe PG (1980) The pharmacokinetics and macromolecular interactions of perchloroethylene in rats as related to oncogenicity. Toxicology and Applied Pharmacology, 55:207–219 [cited in de Raat, 2003].
Schumann AM, Watanabe PG, Reitz RH, Gehring PJ (1982) The importance of pharmacokinetic and macromolecular events as they relate to mechanisms of tumorigenicity and risk assessment. In: Plaa G, Hewitt WR, eds. Toxicology of the liver. New York, NY, Raven Press (Target Organ Toxicology Series) [cited in de Raat, 2003].
Schutz A, Coenen W (1989) Tetrachlorethylen. In: Messung von Gefahrstoffe, BIA-Arbeitsmappe. Bielefeld, Berufsgenossenschaftliches Institut für Arbeitssicherheit (BIA) des Hauptverbandes des gewerblichen Berufsgenossenschaft e.V, Erich Schmidt Verlag [cited in de Raat, 2003].
Schwarzenbach RP, Giger W, Höhn E, Schneider JK (1983) Behaviour of organic compounds during infiltration of river water to groundwater, field studies. Environmental Science and Technology, 17(8):472–479 [cited in EC, 2001].
Schwetz BA, Leong BKJ, Gehring PJ (1975) The effect of maternally inhaled trichloroethylene, perchloroethylene, methyl chloroform, and methylene chloride on embryonal and fetal development in mice and rats. Toxicology and Applied Pharmacology, 32:84–96 [cited in de Raat, 2003].
Seaton A, MacNee W, Donaldson K, Goddon D (1995) Particulate air pollution and acute health effects. Lancet, 345:176–178.
Seeber A (1989) Neurobehavioral toxicity of long-term exposure to tetrachloroethylene. Neurotoxicology and Teratology, 11:579–583.
Seidel HJ, Weber L, Barthel E (1992) Hematological toxicity of tetrachloroethylene in mice. Archives of Toxicology, 66:228–230 [cited in de Raat, 2003].
Seiji K, Jin C, Watanabe T, Nakatsuka H, Ikeda M (1990) Sister chromatid exchanges in peripheral lymphocytes of workers exposed to benzene, trichloroethylene and tetrachloroethylene, with reference to smoking habits. International Archives of Occupational and Environmental Health, 62:171–176.
Seip HM, Abstad J, Carlberg GE, Martinsen K, Skaane R (1986) Measurement of mobility of organic compounds in soil. Science of the Total Environment, 50:87–101 [cited in EC, 2001].
Shimada T, Swanson A, Lever P (1983) The evaluation for genotoxicity of several halogenated solvents. Environmental Mutagenesis, 5:447 [cited in de Raat, 2003].
Shubat PJ, Poirier SH, Knuth ML, Brooke LT (1982) Acute toxicity of tetrachlorethylene and trichlorethylene with dimethylformamide to rainbow trout (Salmo gairdneri). Bulletin of Environmental Contamination and Toxicology, 28(1):7–10.
Sidebottom H, Franklin J (1996) The atmospheric fate and impact of hydrochlorofluorocarbons and chlorinated solvents. Pure and Applied Chemistry, 68:1757–1769 [cited in EC, 2001].
Siemiatycki J (1991) Risk factors for cancer in the workplace. Boca Raton, FL, CRC Press [cited in IARC, 1995].
Singh HB, Lillian D, Appleby A, Lobban L (1975) Atmospheric formation of carbon tetrachloride from tetrachloroethylene. Environmental Letters, 10:253–256 [cited in EC, 2001].
Sjögren B (1997) Occupational exposure to dust: inflammation and ischaemic heart disease. Occupational and Environmental Medicine, 54:466–469.
Skender LJ, Karacic V, Prpic-Majic D (1991) A comparative study of human levels of trichloroethylene and tetrachloroethylene after occupational exposure. Archives of Environmental Health, 46:174–178 [cited in de Raat, 2003].
Smith AD, Bharath A, Mallard C, Orr D, Smith K, Sutton JA, Vukmanich J, McCarty LS, Ozburn GW (1991) The acute and chronic toxicity of ten chlorinated organic compounds to the American flagfish (Jordanella floridae). Archives of Environmental Contamination and Toxicology, 20:94–102 [cited in EC, 2001].
Solet D, Robins TG (1991) Renal function in dry cleaning workers exposed to perchloroethylene. American Journal of Industrial Medicine, 20:601–614.
Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, Meek E, Rice JM, Younes M (2001) IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regulatory and Toxicological Pharmacology, 34(2):146–152.
Spencer HB, Hussein WR, Tchounwou PB (2002) Effects of tetrachloroethylene on the viability and development of embryos of the Japanese medaka, Oryzias latipes. Archives of Environmental Contamination and Toxicology, 42:463–469.
Spinatonda G, Colombo R, Capodaglio EM, Imbriani M, Pasetti C, Minuco G, Pinelli P (1997) [Study on speech production processes: application for a group of subjects chronically exposed to organic solvents (part II).] La Medicina del Lavoro, 19:85–88 (in Italian).
Spirtas R, Stewart PA, Lee JS (1991) Retrospective cohort mortality study of workers at an aircraft maintenance facility. I. Epidemiological results. British Journal of Industrial Medicine, 48:515–530 [cited in de Raat, 2003].
Stanford Research Institute International (1983) Salmonella test results on tetrachloroethylene. Prepared for Dr. Harry Milman, project officer. Unpublished, cited in USEPA (1986) [cited in de Raat, 2003].
Staples CA, Werner AF, Hoogheem TJ (1985) Assessment of priority pollutant concentrations in the United States using STORET database. Environmental Toxicology and Chemistry, 4:131–142 [cited in EC, 2001].
Stemhagen A, Slade J, Altman R, Bill J (1983) Occupational risk factors and liver cancer. A retrospective case–control study of primary liver cancer in New Jersey. American Journal of Epidemiology, 117:443–454.
Stewart RD (1969) Acute tetrachloroethylene intoxication. Journal of the American Medical Association, 208:1490–1492 [cited in de Raat, 2003].
Stewart RD, Dodd HC (1964) Absorption of carbon tetrachloride, trichloroethylene, tetrachloroethylene, methylene chloride, and 1,1,1-trichloroethane through the human skin. American Industrial Hygiene Association Journal, 25:439–446 [cited in de Raat, 2003].
Stewart RD, Erley MS, Schaffer BS, Kalbfleisch J, Newton PE (1961a) Accidental vapor exposure to anesthetic concentrations of a solvent containing tetrachloroethylene. Industrial Medicine and Surgery, 30:327–330 [cited in de Raat, 2003].
Stewart RD, Gay HH, Erley DS, Hake CL, Schaffer AW (1961b) Human exposure to tetrachloroethylene vapor. Archives of Environmental Health, 2:40–46 [cited in de Raat, 2003].
Stewart RD, Baretta ED, Dodd HC, Torkelson TR (1970) Experimental human exposure to tetrachloroethylene. Archives of Environmental Health, 20:225–229 [cited in de Raat, 2003].
Stewart RD, Hake CL, Wu A, Kalbleisch J, Newton PE, Marlow SK, Vucicevic-Salama M (1977) Effects of perchloroethylene drug interactions on behavior and neurological function. Washington, DC, Department of Health, Education, and Welfare (DHEW (NIOSH) Publication No. 77-191) (available from the USEPA-IRIS Information Desk) [cited in USEPA, 2003].
Story DL, Meierhenry EF, Tyson CA, Milman HA (1986) Differences in rat-liver enzyme-altered foci produced by chlorinated aliphatics and phenobarbital. Toxicology and Industrial Health, 2:351–362 [cited in de Raat, 2003].
Stott WT, Watanabe PG (1982) Differentiation of genetic versus epigenetic mechanisms of toxicity and its application to risk assessment. Drug Metabolism Reviews, 13:853–873 [cited in de Raat, 2003].
Suarez L, Weiss NS, Martin J (1989) Primary liver cancer death and occupation in Texas. American Journal of Industrial Medicine, 15:167–175.
Suflita JM, Gibson SA, Beeman RE (1988) Anaerobic biotransformations of pollutant chemicals in aquifers. Journal of Industrial Microbiology, 3:179–194 [cited in EC, 2001].
Szakmáry É, Ungváry G, Tátrai E (1997) The offspring-damaging effect of tetrachloroethylene in rats, mice and rabbits. Central European Journal of Occupational and Environmental Medicine, 3:31–39.
Tabak HH, Quave SA, Mashni CI, Barth EF (1981) Biodegradability studies with organic priority pollutant compounds. Journal of the Water Pollution Control Federation, 53(10):1503–1518 [cited in EC, 2001].
Taskinen H, Anttila A, Lindbohm ML, Sallmen M, Hemminki K (1989) Spontaneous abortions and congenital malformations among the wives of men occupationally exposed to organic solvents. Scandinavian Journal of Work, Environment and Health, 15:345–352.
Theiss JC, Stoner GD, Shimkin MB (1977) Test for carcinogenicity of organic contaminants of United States drinking waters by pulmonary tumour response in A mice. Cancer Research, 37:2717–2720 [cited in de Raat, 2003].
Tinston DJ (1995) Perchloroethylene: multigeneration inhalation study in the rat. Report of Zeneca Central Toxicology Laboratory. Alderley Park, Macclesfield, Cheshire, Zeneca Central Toxicology Laboratory (Report No. CTL/P/4097).
Toraason M, Clark J, Dankovic D, Mathias P, Skaggs S, Walker C, Werren D (1999) Oxidative stress and DNA damage in Fischer rats following acute exposure to trichloroethylene or perchloroethylene. Toxicology, 138:43–53.
Toraason M, Butler MA, Ruder A, Forrester C, Taylor L, Ashley DL, Mathias P, Marlow KL, Cheever KL, Kreig E, Wey H (2003) Effect of perchloroethylene, smoking and race on oxidative DNA damage in female dry cleaners. Mutation Research, 539:9–18.
Travier N, Gridley G, De Roos AJ, Plato N, Moradi T, Boffetta P (2002) Cancer incidence of dry cleaning, laundry and ironing workers in Sweden. Scandinavian Journal of Work, Environment and Health, 28:341–348.
Trense E, Zimmerman H (1969) Fatal inhalation poisoning with chronically acting tetrachloroethylene vapours. Zentralblatt für Arbeitsmedizin und Arbeitsschutz, 19:131–137 [cited in de Raat, 2003].
Trevisan A, Macca I, Rui F, Carrieri M, Bartolucci GB, Manno M (2000) Kidney and liver biomarkers in female dry-cleaning workers exposed to perchloroethylene. Biomarkers, 5:399–409.
TRI (2004) Toxics Release Inventory (TRI) Program. Washington, DC, United States Environmental Protection Agency (http://www.epa.gov/tri).
Trowborst T (1981) Groundwater pollution by volatile halogenated hydrocarbons; sources of pollution and methods to estimate their relevance. Science of the Total Environment, 21:41–46 [cited in EC, 2001].
Tsuruta H (1975) Percutaneous absorption of organic solvents; (1) Comparative study of the in vivo percutaneous absorption of chlorinated solvents in mice. Industrial Health, 13:227–236 [cited in de Raat, 2003].
Tsuruta H (1977) Percutaneous absorption of organic solvents; (2) A method for measuring penetration rate of chlorinated solvents through excised rat skin. Industrial Health, 15:131–139 [cited in de Raat, 2003].
Tu AS, Murray TA, Hatch KM (1985) In vitro transformation of BALB/c-3T3 cells by chlorinated ethanes and ethylenes. Cancer Letters, 28:85–92 [cited in de Raat, 2003].
Tuazon EC, Atkinson R, Aschmann SM, Goodman MA, Winer AM (1988) Atmospheric reactions of chloroethenes with the OH radical. International Journal of Chemical Kinetics, 20:241–165 [cited in EC, 2001].
Tuttle TC, Wood GD, Crether CB, Johnson, BL, Xintaras C (1977) A behavioral and neurological evaluation of dry cleaners exposed to perchloroethylene. Cincinnati, OH, United States Department of Health, Education, and Welfare, Public Health Service, National Institute for Occupational Safety and Health (Contribution No. HSM 99-73-35).
UMEG (1997) Jahresbericht 1996 der Gesellschaft für Umweltmessungen und Umwelterhebungen mbH (UMEG), commissioned by the Ministry for Environment and Traffic ("Umwelt und Verkehr"). Baden-Württemberg, August [cited in EC, 2001].
USEPA (1980) Ambient water quality criteria for tetrachloroethylene. Washington, DC, United States Environmental Protection Agency (EPA 440/5-80-073; NTIS PB81-117830) [cited in EC, 2001].
USEPA (1986) Drinking water criteria document for tetrachloroethylene (final). Springfield, IL, United States Environmental Protection Agency; United States Department of Commerce, National Technical Information Service (PB86-118114) [cited in de Raat, 2003].
USEPA (1994) Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. Washington, DC, United States Environmental Protection Agency, Office of Health and Environmental Assessment, Office of Research and Development (EPA/600/8-90/066F).
USEPA (1999) Air toxic methods. Washington, DC, United States Environmental Protection Agency, Technology Transfer Network, Ambient Monitoring Technology Information Centre (http://www.EPA.gov/ttn/amtic/airtox.html).
USEPA (2000) Benchmark dose technical guidance document [external review draft]. Washington, DC, United States Environmental Protection Agency, Risk Assessment Forum (EPA/630/R-00/001; http://www.epa.gov/ncea/raf).
USEPA (2002) The national-scale air toxics assessment. Washington, DC, United States Environmental Protection Agency, Technology Transfer Network, National Air Toxics Assessment (http://www.epa.gov/ttn/atw/nata/).
USEPA (2003) Neurotoxicity of tetrachloroethylene (perchloroethylene): Discussion paper. External review draft. Washington, DC, United States Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, October (EPA/600/P-03/005A; http://www.epa.gov/ncea).
USEPA (2005) Toxicological review of tetrachloroethylene (perchloroethylene) (CAS No.
Vail JT (1974) False-negative reaction to patch testing with volatile compounds [letter]. Archives of Dermatology, 110:130 [cited in de Raat, 2003].
Vamvakas S, Dekant W, Berthold K, Schmidt S, Wild D, Henschler D (1987) Enzymatic transformation of mercapturic acids derived from halogenated alkenes to reactive and mutagenic intermediates. Biochemical Pharmacology, 36:2741–2748.
Vamvakas S, Herkenhoff M, Dekant W, Henschler D (1989) Mutagenicity of tetrachloroethene in the Ames test: Metabolic activation by conjugation with glutathione. Journal of Biochemical Toxicology, 4:21–27.
Van Beek L (1990) Investigation of a possibility to reduce the use of rabbits in skin irritation tests; experiments with dichloromethane, trichloroethylene, tetrachloroethylene and 1,1,1-trichloroethane. Zeist, TNO-CIVO Institute (Report V89-265) (cited in EC, 2004).
Van de Meent D, den Hollaner HA, Pool WG, Vredenbregt MJ, van Oers HAM, de Greef E, Luijten JA (1986) Organic micropollutants in Dutch coastal waters. Water Science and Technology, 18:73–81 [cited in EC, 2001].
Van der Graff S (1988) Dynamik leichtflüchtiger halogenischer Verbindungen auf Kläranlagen. Münchner Beitrage zur Abwasser-, Fischerie- und Flussbiologie, 42:151–175.
Van Duuren BL, Goldschmidt BM, Loewengart G, Smith AC, Melchionne S, Seldman I, Roth D (1979) Carcinogenicity of halogenated olefinic and aliphatic hydrocarbons in mice. Journal of the National Cancer Institute, 63:1433–1439 [cited in de Raat, 2003].
Vannelli T, Logan M, Arciero DM, Hooper AB (1990) Degradation of halogenated aliphatic compounds by the ammonia-oxidising bacterium Nitrosomonas europaea. Applied and Environmental Microbiology, 56:1169–1171 [cited in EC, 2001].
Vartiainen T, Pukkula E, Rienoja T, Haeseler E (1997) Population exposure to tri- and tetrachloroethene and cancer risk: two cases of drinking water pollution. Chemosphere, 27:1171–1181 [cited in de Raat, 2003].
Vaughan TL, Stewart PA, Davis S, Thomas DB (1997) Work in dry cleaning and the incidence of cancer of the oral cavity, larynx, and oesophagus. Occupational and Environmental Medicine, 54:692–695 [cited in de Raat, 2003].
Verplanke A, Leummens MHL, Herber RFM (1999) Occupational exposure to tetrachloroethene and its effects on the kidneys. Journal of Occupational and Environmental Medicine, 41:11–16.
Vogel TM, McCarty PL (1985) Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Applied and Environmental Microbiology, 49(5):1080–1083 [cited in EC, 2001].
Volkel W, Friedewald M, Lederer E, Pahler A, Parker J, Dekant W (1998) Biotransformation of perchloroethylene: dose dependent excretion of trichloroacetic acid, dichloroacetic acid, and N-acetyl-S-(trichlorovinyl)-L-cysteine in rats and humans after inhalation. Toxicology and Applied Pharmacology, 153:20–27.
Vonk JW, Adema DMM, Barug D (1986) Comparison of the effects of several chemicals on microorganisms, higher plants and earthworms. In: Assink JW, van de Brink WJ, eds. First international TNO conference on contaminated soil, 11–15 November 1985, Utrecht, The Netherlands. Hingham, MA, Kluwer Academic Publishers [cited in EC, 2001].
Vyskocil A, Emminger S, Tejral J, Fiala Z, Ettlerova E, Cermanova A (1990) Study on kidney function in female workers exposed to perchloroethylene. Human Experimental Toxicology, 9:377–380 [cited in de Raat, 2003].
Wakeham SG, Davis AC, Karas JL (1983) Mesocosm experiments to determine the fate and persistence of volatile organic compounds in coastal seawater. Environmental Science and Technology, 17:611–617 [cited in EC, 2001].
Walbridge CT, Fiandt JT, Phipps GL, Holcombe GW (1983) Acute toxicity of ten chlorinated aliphatic hydrocarbons to the fathead minnow (Pimephales promelas). Archives of Environmental Contamination and Toxicology, 12:661–666 [cited in EC, 2001].
Walker JT, Burnett CA, Lalich NR, Sestito JP, Halperin WE (1997) Cancer mortality among laundry and dry cleaning workers. American Journal of Industrial Medicine, 32:614–619 [cited in de Raat, 2003].
Wallace L, Pellizari ED, Hartwell TD, Sparacino C, Zelon H (1983) Personal exposure to volatile organics and other compounds indoors and outdoors — the TEAM study. In: Proceedings of the 76th annual national conference of the Air Pollution Control Association, Atlanta, GA, 19–24 June 1983 (Paper 83.912) [cited in de Raat, 2003].
Walles SAS (1986) Induction of single-strand breaks in DNA of mice by trichloroethylene and tetrachloroethylene. Toxicology Letters, 31:31–35 [cited in de Raat, 2003].
Wang S, Karlsson JE, Kyrklund T, Haglid K (1993) Perchloroethylene-induced reduction in glial and neuronal cell marker proteins in rat brain. Pharmacology & Toxicology, 72:273–278 [cited in de Raat, 2003].
Wang X, Harada S, Watanabe M, Koshikawa H, Sato K, Kimura T (1996) Determination of bioconcentration potential of tetrachloroethylene in marine algae by 13C. Chemosphere, 33(5):865–877 [cited in EC, 2001].
Ward RC, Travis CC, Hetrick DM, Andersen ME, Gargas ML (1988) Pharmacokinetics of tetrachloroethylene. Toxicology and Applied Pharmacology, 93:108–117.
Warner JR, Hughes TJ, Claxton LD (1988) Mutagenicity of 16 volatile organic chemicals in a vaporization technique with Salmonella typhimurium TA100. Environmental and Molecular Mutagenesis, 11(Suppl. 11):111 [cited in de Raat, 2003].
Warren DA, Reigle TG, Muralidhara S, Dallas CE (1996) Schedule-controlled operant behavior of rats following oral administration of perchloroethylene: time course and relationship to blood and brain solvent levels. Journal of Toxicology and Environmental Health, 47:345–362.
Wenzel DG, Gibson RD (1951) A study of the toxicity and the anthelminthic activity of n-butylidene chloride. Journal of Pharmacology, 3:169–176 [cited in de Raat, 2003].
WHO (2000) Air quality guidelines for Europe, 2nd ed. Geneva, World Health Organization (WHO Regional Publications, European Series No. 91).
WHO (2003) Tetrachloroethene in drinking-water. Background document for development of WHO Guidelines for drinking-water quality. Geneva, World Health Organization (WHO/SDE/WSH/03.04/23; http://www.who.int/water_sanitation_health/dwq/chemicals/tetrachloroethene.pdf).
Williams GM, Shimada T (1983) Evaluation of several halogenated ethane and ethylene compounds for genotoxicity. Final report for PPG Industries, Pittsburgh, PA. Unpublished [cited in de Raat, 2003].
Wilson JT, Enfield CG, Dunlap WJ, Cosby RL, Foster DA, Baskin LB (1981) Transport and fate of selected organic pollutants in sandy soil. Journal of Environmental Quality, 10(4):501–506 [cited in EC, 2001].
Windham GC, Shusterman D, Swan SH, Fenster L, Eskenazi B (1991) Exposure to organic solvents and adverse pregnancy outcome. American Journal of Industrial Medicine, 20:241–259 [cited in de Raat, 2003].
Withey RJ, Hall JW (1975) The joint toxic action of perchloroethylene with benzene in rats. Toxicology, 4:5–15 [cited in de Raat, 2003].
WMO (1991) Scientific assessment of ozone depletion: 1991. Geneva, World Meteorological Organisation (Global Ozone Research and Monitoring Project Report 25) [cited in EC, 2001].
Wolf MA (1956) Results of skin absorption studies on carbon tetrachloride, ethylene dichloride, tetrachloroethylene, trichloroethylene and chlorethene. Report for the Dow Chemical Company (cited in EC, 2004).
Wright WH, Bozicevick J, Gordon LS (1937) Studies on oxyuriasis. V. Therapy with single doses of tetrachloroethylene. Journal of the American Medical Association, 109:570–573 [cited in de Raat, 2003].
Yllner S (1961) Urinary metabolites of 14C-tetrachloroethylene in mice. Nature, 191:820 [cited in de Raat, 2003].
Yoshioka Y, Ose Y, Sato T (1986) Correlation of the five test methods to assess chemical toxicity and relation to physical properties. Ecotoxicology and Environmental Safety, 12(1):15–21 [cited in EC, 2001].
Zhuang P, Pavlostathis SG (1995) Effect of temperature, pH and electron donor on the microbial reductive dechlorination of chloroalkenes. Chemosphere, 31(6):3537–3548.
Zoeteman BCJ, Harmsen K, Linders JBHJ, Morra CFH, Sloof W (1980) Persistent organic pollutants in river water and groundwater of the Netherlands. Chemosphere, 9:231–249 [cited in EC, 2001].
Ziglio G, Beltramelli G, Pregliasco F, Ferrari G (1985) Metabolites of chlorinated solvents in blood and urine of subjects exposed at environmental level. Science of the Total Environment, 47:473–477 [cited in de Raat, 2003].
Zytner R, Biswas N, Bewtra JK (1989) Adsorption and desorption of perchloroethylene in soils, peat moss, and granular activated carbon. Canadian Journal of Civil Engineering, 16:798–806 [cited in EC, 2001].
|
ADP |
adenosine diphosphate |
|
ALT |
alanine aminotransferase (SGPT) |
|
AST |
aspartate aminotransferase (SGOT) |
|
ATP |
adenosine triphosphate |
|
ATSDR |
Agency for Toxic Substances and Disease Registry (USA) |
|
AUC |
area under the curve |
|
BCF |
bioconcentration factor |
|
BIA |
Berufsgenossenschaftliches Institut für Arbeitssicherheit |
|
BMC |
benchmark concentration |
|
BMC10 |
concentration associated with a 10% increase in the absolute risk of seeing an "adverse" response |
|
BMCL |
lower confidence limit on the benchmark concentration |
|
BMCL10 |
lower confidence limit on the concentration associated with a 10% increase in the absolute risk of seeing an "adverse" response |
|
BMDS |
Benchmark Dose Software |
|
BOD |
biological oxygen demand |
|
BUN |
blood urea nitrogen |
|
CAS |
Chemical Abstracts Service |
|
CCRIS |
Chemical Carcinogenesis Research Information System |
|
CFC |
chlorofluorocarbon |
|
CI |
confidence interval |
|
CICAD |
Concise International Chemical Assessment Document |
|
CNS |
central nervous system |
|
CoA |
coenzyme A |
|
DART |
Developmental & Reproductive Toxicology |
|
DECOS |
Dutch Expert Committee on Occupational Standards |
|
DFG |
Deutsche Forschungsgemeinschaft |
|
DNA |
deoxyribonucleic acid |
|
EC |
European Commission |
|
EC10 |
effective concentration for 10% of test species |
|
EC50 |
median effective concentration |
|
ECD |
electron capture detection |
|
EEG |
electroencephalogram |
|
EHC |
Environmental Health Criteria |
|
EMIC |
Environmental Mutagen Information Center |
|
ETIC |
Environmental Teratology Information Center |
|
EU |
European Union |
|
EUSES |
European Union System for the Evaluation of Substances |
|
FAO |
Food and Agriculture Organization of the United Nations |
|
FID |
flame ionization detection |
|
FUGMOD |
fugacity model |
|
GABA |
gamma-aminobutyric acid |
|
GC |
gas chromatograph/chromatography |
|
GENE-TOX |
Genetic Toxicology |
|
GGT |
gamma-glutamyltranspeptidase |
|
HC5 (50%) |
hazardous concentration to protect 95% of species with 50% confidence |
|
HCFC |
hydrochlorofluorocarbon |
|
HFC |
hydrofluorocarbon |
|
HSDB |
Hazardous Substances Data Bank |
|
IARC |
International Agency for Research on Cancer |
|
ICSC |
International Chemical Safety Card |
|
IHD |
ischaemic heart disease |
|
ILSI |
International Life Sciences Institute |
|
IOMC |
Inter-Organization Programme for the Sound Management of Chemicals |
|
IPCS |
International Programme on Chemical Safety |
|
IRIS |
Integrated Risk Information System |
|
ISO |
International Organization for Standardization |
|
JECFA |
Joint FAO/WHO Expert Committee on Food Additives |
|
JMPR |
Joint FAO/WHO Meeting on Pesticide Residues |
|
Koc |
soil organic carbon/water adsorption partition coefficient |
|
Kow |
octanol–water partition coefficient |
|
LC50 |
median lethal concentration |
|
LD50 |
median lethal dose |
|
LDH |
lactate dehydrogenase |
|
LOAEC |
lowest-observed-adverse-effect concentration |
|
LOD |
limit of detection |
|
LOEC |
lowest-observed-effect concentration |
|
LOQ |
limit of quantification |
|
MS |
mass spectrometry |
|
NCI |
National Cancer Institute (USA) |
|
NEG |
Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals |
|
NEN |
Dutch Normalisation Institute |
|
NIOSH |
National Institute for Occupational Safety and Health (USA) |
|
NOAEC |
no-observed-adverse-effect concentration |
|
NOEC |
no-observed-effect concentration |
|
NTP |
National Toxicology Program (USA) |
|
O/E |
observed/expected |
|
OECD |
Organisation for Economic Co-operation and Development |
|
OR |
odds ratio |
|
OSHA |
Occupational Safety and Health Administration (USA) |
|
PBPK |
physiologically based pharmacokinetic |
|
PCE |
tetrachloroethene |
|
PEC |
predicted environmental concentration |
|
PER |
tetrachloroethene |
|
PERC |
tetrachloroethene |
|
PID |
photoionization detector |
|
PMR |
proportional mortality ratio |
|
PNEC |
predicted no-effect concentration |
|
PPARalpha |
peroxisome proliferator activated receptor-alpha |
|
ppb |
parts per billion |
|
ppm |
parts per million |
|
ppt |
parts per trillion |
|
RCR |
respiratory control ratio (State 3/State 4 ratio) |
|
RNA |
ribonucleic acid |
|
rpm |
revolutions per minute |
|
RR |
relative risk |
|
RSI |
Risk Science Institute |
|
RTECS |
Registry of Toxic Effects of Chemical Substances |
|
SGOT |
serum glutamic–oxaloacetic transaminase |
|
SGPT |
serum glutamic–pyruvic transaminase |
|
SI |
International System of Units (Système international d’unités) |
|
SIDS |
screening information data set |
|
SIR |
standardized incidence ratio |
|
SMOR |
standardized mortality odds ratio |
|
SMR |
standardized mortality ratio |
|
TC |
tolerable concentration |
|
TDI |
tolerable daily intake |
|
TSCA |
Toxic Substances Control Act Chemical Inventory Database (USA) |
|
TWA |
time-weighted average |
|
UDS |
unscheduled DNA synthesis |
|
USA |
United States of America |
|
USEPA |
United States Environmental Protection Agency |
|
UV |
ultraviolet |
|
WHO |
World Health Organization |
de Raat K (2003) The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals and the Dutch Expert Committee on Occupational Standards. 133. Tetrachloroethylene (PER). Stockholm, National Institute for Working Life (Arbete och Hälsa NR 2003:14; ISBN 91-7045-695-X).
The human health sections were produced primarily from this report, produced under an agreement signed by the Dutch Expert Committee on Occupational Standards (DECOS) of the Health Council of the Netherlands and the Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals (NEG). The purpose of the agreement is to write joint scientific criteria documents that can be used by the national regulatory authorities in both the Netherlands and the Nordic countries.
This document on human health effects of tetrachloroethene was written by Karel de Raat, TNO Food and Nutrition Research, the Netherlands, and was reviewed by DECOS as well as by NEG. The joint document is published separately by DECOS and NEG, and the NEG version (adapted to the requirements of NEG and the format of Arbete och Hälsa) was used in preparation of this CICAD. The editorial work and technical editing were carried out by Jill Järnberg, scientific secretary of NEG, at the National Institute for Working Life in Sweden. The Nordic Council of Ministers was acknowledged by G.J. Mulder and G. Johanson, Chairmen of DECOS and NEG, respectively, for financial support of the project.
IARC (1995) Dry cleaning, some chlorinated solvents and other industrial compounds. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 63).
Approximately 1 year in advance of a meeting of a working group, the topics of the monographs are announced and participants are selected by IARC staff in consultation with other experts. Subsequently, relevant biological and epidemiological data are collected by IARC from recognized sources of information on carcinogenesis, including data storage and retrieval systems, such as MEDLINE and TOXLINE, and EMIC and ETIC for data on genetic and related effects and reproductive and developmental effects, respectively.
For chemicals and some complex mixtures, the major collection of data and the preparation of first drafts of the sections on chemical and physical properties, on analysis, on production and use, and on occurrence are carried out under a separate contract funded by the United States NCI. Representatives from industrial associations may assist in the preparation of sections on production and use. Information on production and trade is obtained from governmental and trade publications and, in some cases, by direct contact with industries. Separate production data on some agents may not be available because their publication could disclose confidential information. Information on uses may be obtained from published sources but is often complemented by direct contact with manufacturers. Efforts are made to supplement this information with data from other national and international sources.
Six months before the meeting, the material obtained is sent to meeting participants or is used by IARC staff to prepare sections for the first drafts of monographs. The first drafts are compiled by IARC staff and sent, prior to the meeting, to all participants of the Working Group for review.
The Working Group meets in Lyon for 7–8 days to discuss and finalize the texts of the monographs and to formulate the evaluations. After the meeting, the master copy of each monograph is verified by consulting the original literature, edited, and prepared for publication. The aim is to publish monographs within 6 months of the Working Group meeting.
The available studies are summarized by the Working Group, with particular regard to certain defined qualitative aspects, as discussed in the source document. In general, numerical findings are indicated as they appear in the original report; units are converted when necessary for easier comparison. The Working Group may conduct additional analyses of the published data and use them in its assessment of the evidence; the results of such supplementary analyses are given in square brackets. When an important aspect of a study, directly impinging on its interpretation, should be brought to the attention of the reader, a comment is given in square brackets.
IARC Working Group participants
Members
A. Abbondandolo, National Institute for Research on Cancer, Genoa, Italy; O. Axelson, University Hospital, Linköping, Sweden; S. Cordier, INSERM, Villejuif, France; W. Dekant, University of Würzburg, Würzburg, Germany; E. Dybing, National Institute of Public Health, Oslo, Norway; J. Fajen, National Institute for Occupational Safety and Health, Cincinnati, OH, USA; G.R. Howe, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; A. Huici-Montagud, National Center of Working Conditions, Barcelona, Spain; Y. Konishi, Nara Medical University, Nara, Japan; H. Kromhout, Wageningen Agricultural University, Wageningen, Netherlands; L.S. Levy, University of Birmingham, Birmingham, United Kingdom; E. Lynge, Danish Cancer Society, Copenhagen, Denmark; R.L. Melnick, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA; H. Norppa, Institute of Occupational Health, Helsinki, Finland; S. Olin, International Life Sciences Institute, Washington, DC, USA; C. Rosenberg, Institute of Occupational Health, Helsinki, Finland; N.H. Stacey, Worksafe Australia, Sydney, Australia; S. Vamvakas, University of Würzburg, Würzburg, Germany.
Representatives/observers
J. Sontag, National Cancer Institute, Bethesda, MD, USA; N.S. Weiss, University of Washington, Seattle, WA, USA; D.G. Farrar, ICI Chemicals and Polymer Ltd, Cheshire, United Kingdom; E. de Pauw, Health and Safety Directorate, European Commission, Luxembourg, Grand Duchy of Luxembourg; D. Burch, University of Toronto, Toronto, Ontario, Canada; J.C. Parker, United States Environmental Protection Agency, Washington, DC, USA.
IARC Secretariat
P. Boffetta, A. Dufournet, M. Friesen, M.-J. Ghess, E. Heseltine, V. Krutovskikh, M. Lang, D. McGregor, D. Mietton, H. Møller, A. Mylvaganam, C. Partensky, S. Ruiz, P. Webb, J. Wilbourn, H. Yamasaki
The summary and evaluation of the carcinogenicity of tetrachloroethene are available at: http://www.iarc.fr/
USEPA (2003) Neurotoxicity of tetrachloroethylene (perchloroethylene): Discussion paper. External review draft. Washington, DC, United States Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, October (EPA/600/P-03/005A; http://www.epa.gov/ncea).
This paper is a background document for a meeting of neurotoxicity experts to discuss the CNS effects of exposure to tetrachloroethene. The document reviews the literature on neurological testing of people exposed to tetrachloroethene occupationally in dry cleaning facilities and of people living near dry cleaning facilities. It also reviews the neurobehavioural studies of laboratory animals exposed to tetrachloroethene via inhalation. The report describes impairment of visual information processing and other adverse neurobehavioural effects in several studies of employees working in dry cleaning facilities using tetrachloroethene. Two studies of people living near dry cleaning facilities have also shown neurological effects; their exposures have been at lower concentrations than for the workers, and the specific neurological tests used in the residential studies have been different. The expert panel discusses issues centring on the question of whether this limited information at lower exposures is strong enough to infer that low concentrations of tetrachloroethene are a hazard to the general population.
Contact Information:
Robert E. McGaughy
E-mail at: mcgaughy.robert@epa.gov
Available at: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=75193
EC (2001) Draft European Union risk assessment report. Tetrachloroethylene. CAS No: 127-81-4 [sic], EINECS No: 204-825-9. Draft final environmental report. Luxembourg, European Commission, August.
The environmental health sections were prepared from the draft EU Risk Assessment Report, which was available via http://ecb.jrc.it/existing-chemicals/ on the Internet. This document was prepared by the United Kingdom rapporteur on behalf of the EU. The scientific work on the environmental part was prepared by the Building Research Establishment Ltd, under contract to the rapporteur. The contact points for this draft report are:
Contact point (health): Health & Safety Executive, Industrial Chemicals Unit, Magdalen House, Stanley Precinct, Bootle, Merseyside, United Kingdom L20 3QZ
and
Contact point (environment): Environment Agency, Chemicals Assessment Section, Ecotoxicology & Hazardous Substances National Centre, Isis House, Howbery Park, Wallingford, Oxfordshire, United Kingdom OX10 8BD.
The review of the environmental report by Member State Technical Experts was finalized in July 2001; the final report was issued just before this CICAD was finalized and is available at http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/REPORT/tetraENVreport021.pdf.
The Draft EU Risk Assessment Report was produced in accordance with Council Regulation (EEC) 793/93 on the evaluation and control of the risks of "existing" substances. Regulation 793/93 provides a systematic framework for the evaluation of the risks to human health and the environment of these substances if they are produced or imported into the Community in volumes above 10 tonnes per year.
There are four overall stages in the Regulation for reducing the risks: data collection, priority setting, risk assessment, and risk reduction. Data provided by industry are used by Member States and the Commission services to determine the priority of the substances that need to be assessed. For each substance on a priority list, a Member State volunteers to act as "Rapporteur", undertaking the in-depth risk assessment and recommending a strategy to limit the risks of exposure to the substance, if necessary.
The methods for carrying out an in-depth risk assessment at Community level are laid down in Commission Regulation (EC) 1488/94, which is supported by a technical guidance document. Normally, the "Rapporteur" and individual companies producing, importing, and/or using the chemicals work closely together to develop a draft Risk Assessment Report, which is then presented to Competent Group of Member State experts for endorsement. Observers from industry, consumer organizations, trade unions, environmental organizations, and certain international organizations are also invited to attend the meetings. The Risk Assessment Report is then peer reviewed by the Scientific Committee on Toxicity, Eco-toxicity and the Environment, which gives its opinion to the EC on the quality of the risk assessment.
This Draft Risk Assessment Report was discussed by Competent Group of Member State experts with the aim of reaching consensus. During such discussions, it is understood that the scientific interpretation of the underlying information may change, more information may be included, and even the conclusions reached may change. Competent Group of Member State experts seek as wide a distribution of these drafts as possible, in order to assure as complete and accurate an information basis as possible. The information contained in the Draft Risk Assessment Report therefore does not necessarily provide a sound basis for decision-making regarding the hazards, exposures, or risks associated with the priority substance. This Draft Risk Assessment Report is the responsibility of the Member State rapporteur. In order to avoid possible misinterpretations or misuse of the findings in this draft, anyone wishing to cite, quote, or copy this report must obtain the permission of the Member State rapporteur beforehand.
* * * * *
In May 2004, a comprehensive literature search was conducted by Toxicology Advice & Consulting Ltd in order to identify critical data published since publication of the source documents. Databases searched included ChemIDplus (the ChemIDplus system searches and/or identifies literature from a wide range of online databases and databanks, including ATSDR, CANCERLIT, CCRIS, DART/ETIC, GENE-TOX, HSDB, IRIS, MEDLINE, TOXLINE Core, TOXLINE Special, and TSCA); INCHEM (the INCHEM database consolidates information from a number of intergovernmental organizations, including JECFA, JMPR, IARC, EHC monographs, and SIDS); RTECS; and USEPA Toxicological Profiles.
A substantial amount of information has been published on tetrachloroethene during the period from 2002 to May 2004. However, judging from information presented in the above sources (usually only a title or abstract), few new papers appear to be critical in regard to the preparation of this CICAD. Critical papers were purchased, assessed, and included in the CICAD, where appropriate, by Toxicology Advice & Consulting Ltd. In the late stages of CICAD preparation, a number of papers were kindly lent by BIBRA Information Services Ltd of Sutton, Surrey, United Kingdom.
The draft CICAD on tetrachloroethene was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. The draft document prepared by the Consultative Group was sent to peer review to those reviewers who had earlier commented on the sections on the evaluation of health effects. Comments were received from:
R. Benson, United States Environmental Protection Agency, Denver, CO, USA
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
V. Cogliano, International Agency for Research on Cancer, Lyon, France
I. Desi, University of Szeged, Szeged, Hungary
P.H. Dugard, Halogenated Solvents Industry Alliance, Inc., Arlington, VA, USA
G. Fan, Australian Government Department of the Environment and Heritage, Canberra, Australian Capital Territory, Australia
L. Fishbein, Fairfax, VA, USA
E. Frantik, National Institute of Public Health, Prague, Czech Republic
H. Gibb, Sciences International Inc., Alexandria, VA, USA
R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
S. Humphrey, Center for Food Safety and Applied Nutrition, Food and Drug Administration, College Park, MD, USA
R. McGaughy, National Center for Environmental Assessment, United States Environmental Protection Agency, Washington, DC, USA
M.E. Meek, Health Canada, Ottawa, Ontario, Canada
Peter W. Preuss, National Center for Environmental Assessment, United States Environmental Protection Agency, Washington, DC, USA
V. Riihimäki, Finnish Institute of Occupational Health, Helsinki, Finland
M. Rio, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
H. Savolainen, Ministry of Social Affairs & Health, Tampere, Finland
P.A. Schulte, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
U. Stenius, Karolinska Institute, Stockholm, Sweden
M.H. Sweeney, United States Embassy, Hanoi, Viet Nam
G. Ungvary, National Centre for Public Health, Budapest, Hungary
S. Zaluzny, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
K. Ziegler-Skylakakis, European Commission, Luxembourg
Hanoi, Viet Nam
28 September – 1 October 2004
Members
Mr D.T. Bai, Centre of Environmental Protection & Chemical Safety, Institute of Industrial Chemistry, Hanoi, Viet Nam
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Mr P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centres for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health of József Fodor National Centre of Public Health, Budapest, Hungary
Ms C.W. Fang, National Institute of Occupational Safety and Health Malaysia, Selangor, Malaysia
Dr L. Fishbein, Fairfax, VA, USA
Dr L. Fruchtengarten, Poison Control Center of São Paulo, São Paulo, Brazil
Dr C.L. Geraci, Document Development Branch, Centers for Disease Control and Prevention / National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr H. Gibb, Sciences International Inc., Alexandria, VA, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment (BfR), Berlin, Germany
Mr P. Howe, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Health Sciences, Tokyo, Japan
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany
Dr S. Kunarattanapruke, Food & Drug Administration, Ministry of Public Health, Nonthaburi, Thailand
Dr Y. Liang, Department of Occupational Health, Fudan University School of Public Health, Shanghai, China
Ms M.E. Meek, Existing Substances Division, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Food and Drug Quality Control Laboratories, Ministry of Health and Medical Education, Tehran, Islamic Republic of Iran
Dr J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
Dr M.H. Sweeney, United States Embassy, Hanoi, Viet Nam
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Centre for Ecology and Hydrology, Monks Wood, United Kingdom
25–27 April 2005
Participants
J. Bucher, National Toxicology Program, USA (Rapporteur)
N. Cherry, University of Alberta, Canada
S. Dobson, Centre for Ecology and Hydrology, United Kingdom (Chair)
R.F. Hertel, Federal Institute for Risk Assessment, Germany (Vice Chair)
R. McGaughy, National Center for Environmental Assessment, Environmental Protection Agency, USA
A. Renwick, University of Southampton, United Kingdom
V. Riihimäki, Institute of Occupational Health, Finland
P. Vineis, Imperial College, United Kingdom
E. Ward, American Cancer Society, USA
P. Watts, Toxicology Advice & Consulting Ltd, United Kingdom
Resource person
C. Scott, National Center for Environmental Assessment, Environmental Protection Agency, USA
Secretariat
A. Aitio, International Programme on Chemical Safety, World Health Organization, Switzerland
Nagpur, India
31 October – 3 November 2005
Members
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nagpur, India
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Mr P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centres for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr L. Fishbein, Fairfax, VA, USA
Dr L. Fruchtengarten, Poison Control Center of São Paulo, São Paulo, Brazil
Dr H. Gibb, Sciences International Inc., Alexandria, VA, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment (BfR), Berlin, Germany
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Ms K. Hughes, Health Canada, Ottawa, Ontario, Canada
Dr D. Kanungo, Directorate General of Health Services, New Delhi, India
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Experimental Medicine, Hanover, Germany
Dr G. Kong, Hanyang University, Seoul, Republic of Korea
Dr J. Rischer, Agency for Toxic Substances and Disease Registry, Centres for Disease Control and Prevention, Chamblee, GA, USA
Dr O. Sabzevari, Tehran University of Medical Sciences, Tehran, Islamic Republic of Iran
Dr R. Sonawane, National Centre for Environmental Assessment, Environmental Protection Agency, Washington, DC, USA
Dr J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
Dr M.H. Sweeney, United States Embassy, Hanoi, Viet Nam
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
Dr Y. Zheng, National Institute for Occupational Health & Poison Control, Beijing, People’s Republic of China
Dr K. Ziegler-Skylakakis, Secretariat of the Commission for the Investigation of Health Hazards of Chemical Compounds in the Workplace Area (MAK Commission), Freising-Weihenstephan, Germany
Observer
Mr P. Ashford, Resorcinol Task Force, Wotton-under-edge, Gloucestershire, United Kingdom
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Ms L. Onyon, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr M. Shibatsuji, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
The maximum likelihood and 95% lower bound of the point of departure were calculated using the multistage model in BMDS version 1.3.2 (USEPA, 2000) to analyse the NTP (1986) and the JISA (1993) data (Tables A7-1 and A7-2). The risk estimates and model parameters are presented in Table A7-3.
Table A7-1: Tumour incidence in mice exposed to tetrachloroethene.
|
Bioassay |
Sex |
Doses/exposures |
Survival-adjusted tumour incidencea (%) |
|
|
Administered |
Continuous equivalent |
|||
|
Hepatocellular adenomas and carcinomas |
||||
|
NCI (1977)b B6C3F1 mice Gavage: 5 days/week, 78 weeks |
Male |
Vehicle control 450 mg/kg body weight per dayc 900 mg/kg body weight per dayc |
0 332 mg/kg body weight per dayd 663 mg/kg body weight per dayd |
2/20 (10) 32/48 (67) 27/45 (60) |
|
Female |
Vehicle control 300 mg/kg body weight per dayc 600 mg/kg body weight per dayc |
0 239 mg/kg body weight per dayd 478 mg/kg body weight per dayd |
0/20 (0) 19/48 (40) 19/45 (42) |
|
|
NTP (1986) B6C3F1 mice Inhalation: 6 h/day, 5 days/week, 104 weeks |
Male |
0 ppme 100 ppm 200 ppm |
0 18 ppm 36 ppm |
17/49 (35) 31/47 (70) 41/50 (82) |
|
Female |
0 ppm 100 ppm 200 ppm |
0 18 ppm 36 ppm |
4/45 (9) 17/42 (40) 38/48 (79) |
|
|
JISA (1993) Crj:BDF1 mice Inhalation: 6 h/day, 5 days/week, 104 weeks |
Male |
0 ppm 10 ppm 50 ppm 250 ppm |
0 1.8 ppm 9.0 ppm 45 ppm |
13/46 (28) 21/49 (43) 19/48 (40) 40/49 (82) |
|
Female |
0 ppm 10 ppm 50 ppm 250 ppm |
0 1.8 ppm 9.0 ppm 45 ppm |
3/50 (6) 3/47 (6) 7/48 (15) 33/49 (67) |
|
|
Malignant haemangiosarcomas, liver or spleen |
||||
|
JISA (1993) Crj:BDF1 mice Inhalation: 6 h/day, 5 days/week, 104 weeks |
Male |
0 ppm 10 ppm 50 ppm 250 ppm |
0 1.8 ppm 9.0 ppm 45 ppm |
2/46 (4) 1/49 (2) 6/48 (13) 9/49 (18) |
|
a |
Animals dying before the first appearance of the tumour of interest but no later than week 52 were omitted from the totals because these animals were presumed not to have had adequate time on study to develop these tumours. |
|
b |
No adenomas were reported in this study. Because hepatic adenomas and carcinomas are considered part of the same continuum of tumour development, and adenomas have been distinguished from carcinomas only on the basis of size, the correspondence of this observation to the other studies is not clear. |
|
c |
450, 900, 300 and 600 mg/kg body weight per day doses were increased to 550, 1100, 400 and 800 mg/kg body weight per day, respectively, after 11 weeks. |
|
d |
Continuous equivalent dose = Cumulative dose (mg/kg body weight per day) / (total days on study) = {[(initial dose level × 11 weeks) + (increased dose level × 67 weeks)] / 90 weeks} × (5 days / 7 days). |
|
e |
1 ppm = 6.89 mg/m3 at 20 °C and 101.3 kPa. |
Table A7-2: Incidence of mononuclear cell leukaemia, kidney tumours, and brain gliomas in rats exposed to tetrachloroethene by inhalation.a
|
Bioassay |
Sex |
Exposure concentration (ppm)b |
Survival-adjusted tumour incidencec (%) |
|
|
Administered |
Continuous equivalent |
|||
|
Mononuclear cell leukaemia |
||||
|
NTP (1986) F344/N rats Inhalation: 6 h/day, 5 days/week, 104 weeks |
Male |
0 200 400 |
0 36 72 |
28/50 (56) 37/48 (77) 37/50 (74) |
|
Female |
0 200 400 |
0 36 72 |
18/50 (36) 30/50 (60) 29/50 (58) |
|
|
JISA (1993) F344/DuCrj rats Inhalation: 6 h/day, 5 days/week, 104 weeks |
Male |
0 50 200 600 |
0 9 36 108 |
11/50 (22) 14/50 (28) 22/50 (44) 27/50 (54) |
|
Female |
0 50 200 600 |
0 9 36 108 |
10/50 (20) 17/50 (34) 16/50 (32) 19/50 (38) |
|
|
Kidney tumours: tubular cell adenoma or adenocarcinoma |
||||
|
NTP (1986) |
Male |
0 200 400 |
0 36 71 |
1/49 (2) 3/47 (6) 4/50 (8) |
|
JISA (1993) |
Male |
0 50 200 600 |
0 9 36 110 |
1/50 (2) 2/50 (4) 1/50 (2) 2/50 (4) |
|
Brain gliomas |
||||
|
NTP (1986) |
Male |
0 200 400 |
0 36 71 |
1/50 (29 0/48 (0) 4/50 (8) |
|
JISA (1993) |
Male |
0 50 200 600 |
0 9 36 110 |
2/50 (4) 0/50 (0) 0/50 (0) 0/50 (0) |
|
a |
From NTP (1986) and JISA (1993). |
|
b |
1 ppm = 6.89 mg/m3 at 20 °C and 101.3 kPa. |
|
c |
Animals dying before the first appearance of the tumour of interest but no later than week 52 were omitted from the totals because these animals were presumed not to have had adequate time on study to develop these tumours. |
Table A7-3: Dose–response summary and cancer risk estimates using continuous equivalent administered tetrachloroethene levels as dosimeter.a
|
Tumour type |
Source |
Modelling summaryb |
Maximum likelihood estimatec |
Lower boundc |
|||
|
MLE dose coefficients |
p-value |
POD (ppm) |
Unit risk (10−3/ppm) |
POD (ppm) |
Unit risk (10−3/ppm) |
||
|
Hepatocellular adenomas or carcinomas, male mice |
JISA (1993) |
q0 = 0.34 |
0.17 |
BMC10 = 8.1 |
12 |
BMCL10 = 2.8 |
36 |
|
q1 = 1.3 × 10−2 |
|||||||
|
q3 = 7.8 × 10−6 |
|||||||
|
Hepatocellular adenomas or carcinomas, female mice |
JISA (1993) |
q0 = 0.056 |
0.83 |
BMC05 = 5.4 |
9.3 |
BMCL05 = 2.1 |
24 |
|
q1 = 0.0076 |
|||||||
|
q2 = 3.6 × 10−4 |
|||||||
|
Haemangioendotheliomas, spleen or liver, male mice |
JISA (1993) |
q0 = 0.076 |
0.32 |
BMC01 = 2.4 |
4.2 |
BMCL01 = 1.4 |
7.1 |
|
q1 = 0.0031 |
|||||||
|
Mononuclear cell leukaemia, male rats |
JISA (1993) |
q0 = 0.21 |
0.51 |
BMC05 = 10 |
5.0 |
BMCL05 = 6.4 |
7.9 |
|
q1 = 0.0051 |
|||||||
|
Mononuclear cell leukaemia, female rats |
JISA (1993) |
q0 = 0.26 |
0.68 |
BMC05 = 29 |
1.7 |
BMCL05 = 13 |
3.9 |
|
q1 = 0.0017 |
|||||||
|
Kidney tubular cell adenoma or adenocarcinoma, male rats |
NTP (1986) |
q0 = 0.022 |
0.75 |
BMC05 = 53 |
0.94 |
BMCL05 = 24 |
2.1 |
|
q3 = 9.6 × 10−4 |
|||||||
|
BMCx = concentration at x% effect (extra risk) level; BMCLx = 95% lower bound on concentration at x% effect (extra risk) level; MLE = maximum likelihood estimate; POD = point of departure |
|
|
a |
From NTP (1986) and JISA (1993). |
|
b |
Using dose coefficients in terms of administered ppm of tetrachloroethene adjusted to continuous equivalent exposure, consistent with reference concentration methodology (USEPA, 1994), and the multistage model, extra risk: |
|
c |
1 ppm = 6.89 mg/m3 at 20 °C and 101.3 kPa. |
The implementation of the Rao & Brown (1993) model follows the PBPK model structure of Ramsey & Andersen (1984). The Rao & Brown (1993) model is composed of five compartments: poorly perfused tissues, well perfused tissues, fat, liver, and brain. In the implementation of the Rao & Brown (1993) model in our analysis, there is no separate skin compartment. The compartments are assumed to be homogeneous, and distribution is limited by blood flow. The metabolism of tetrachloroethene is modelled by a Michaelis-Menten term in the differential equation for the liver compartment. The kinetics or transformation of the metabolites are not modelled. The simulation is represented by the following equations:
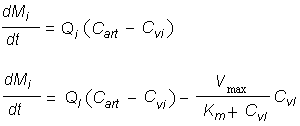
where:
|
i |
= |
compartments other than liver, |
|
l |
= |
liver, |
|
Mi |
= |
mass of tetrachloroethene in the compartment, |
|
Cvi |
= |
venous concentration of tetrachloroethene at the exit from compartment I, |
|
Cart |
= |
arterial concentration of tetrachloroethene, and |
|
Qi |
= |
blood flow rate into the ith compartment. |
Pulmonary exchange is represented by:
Qalv (Cinh – Calv ) = Qtot (Cart – Cven )
Cart = hba Calv
Cexh = 0.67Calv 0.33Cinh
where:
|
Cinh |
= |
inhaled concentrations of the chemical, |
|
Cexh |
= |
exhaled concentrations of the chemical, |
|
Calv |
= |
alveolar concentrations of the chemical, |
|
Cven |
= |
venous concentrations of the chemical, |
|
Cart |
= |
arterial concentrations of the chemical, |
|
hba |
= |
blood/air partition coefficient, |
|
Qtot |
= |
total blood flow rate (equal to the cardiac output), and |
|
Qalv |
= |
alveolar ventilation rate (which is different from the inspiratory flow rate because of the respiratory dead space). The alveolar ventilation rate and cardiac output (the ratio that is referred to as the ventilation-to-perfusion ratio) increase with activity, but at different rates. |
For oral exposures, the gastric route was added by assuming "first-pass" metabolism — i.e. by assuming that all tetrachloroethene ingested is transported directly to the liver, the metabolizing organ. A separate PBPK compartment for the stomach was therefore not necessary. The absorption of tetrachloroethene in the stomach was modelled as a first-order process with an absorption rate constant, ka. Then the mass balance equation for the liver may be modified to have an additional source term, as follows:
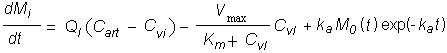
M0 is the amount of tetrachloroethene ingested and is itself a function of time. In our simulations, tetrachloroethene was administered via drinking-water as a series of boluses.
Most human PBPK models have been implemented to investigate inhalation exposure and do not incorporate gastric absorption rate constants. Values in the literature for the gastric absorption rate vary widely. Ward et al. (1988) reported a gastric absorption rate constant in mice of 0.5 l/h. Dallas et al. (1995) reported oral absorption rate constants in rats and dogs as 1.5 and 20.4 l/h, respectively, obtained by fitting blood concentrations following oral gavage. For our modelling purposes, we chose a gastric absorption rate constant of 1.6 l/h. This predicts a reasonably rapid gastric absorption consistent with the data. We found that the resulting blood concentrations of tetrachloroethene are not particularly sensitive to larger values of this parameter. Simulations of gastric absorption of tetrachloroethene were carried out for humans for use in route-to-route extrapolation. Because these simulations were at low exposures and because of first-pass metabolism effects, the uncertainty in the gastric absorption rate constant is not likely to significantly affect the results of the extrapolation. Increasing the gastric absorption rate constant to 20 l/h results in an approximately 2-fold increase in peak blood concentration. Changing this parameter does not substantially impact the elimination profile. The parameter sets used in this modelling effort are shown in Table A8-1.
For inhalation exposures, ventilation rate is a key parameter. In rodents, ventilation rate (VE) was calculated as a function of body weight using the following equations (USEPA, 1994):
For mice: VE (l/min) = e0.326+1.05 ln(w)
For rats: VE (l/min) = e−0.578+0.821 ln(w)
where w is body weight in kilograms and ln represents the natural log operation. These equations provide total ventilation rate. The alveolar ventilation rate is the total ventilation rate less the volume of air that is inhaled through the physiological dead space (total effective volume not involved in gas exchange) in a given time. For the rats and mice and for resting inspiratory rates (7.5 l/min) in humans, Qalv ≈ 0.67 VE (Brown et al., 1997). For the exercising individual (24–49 l/min), Qalv increases up to 0.8 VE (Brown et al., 1997). For the ventilation rates covered in this document, it was considered reasonable to use the relationship Qalv ≈ 0.67 VE throughout. These values represent reasonable physiological values, recognizing that there is substantial variation. The alveolar ventilation rate corresponding to the resting inhaled minute volume is 5.5 l/min. However, USEPA typically assumes a total ventilation rate of 13.8 l/min for a 70-kg human. Thus, unless otherwise stated, the calculations presented in this assessment assume an alveolar ventilation rate of 9.3 l/min.
The results of PBPK simulations of oral exposure to tetrachloroethene are shown in Figure A8-1. In these simulations, tetrachloroethene was orally delivered via drinking-water in nine bolus doses spaced 2 h apart within 16 h, followed by 8 h of no dosing. Because tetrachloroethene concentrations and the rate of metabolism were found to be negligible at the end of the 24-h period, it was determined adequate to terminate the simulation after 24 h.
Table A8-1. Parameters for tetrachloroethene PBPK modelling.
|
Parameter |
Human model: Rao & Brown (1993) |
|
Body weight (BW) (kg) |
70 |
|
Cardiac output (l/h) |
430 |
|
Alveolar ventilation (l/h) |
558 |
|
Tissue volumesa (%) |
|
|
Rapidly perfused |
1.7 |
|
Slowly perfused |
57 |
|
Fat |
23.1 |
|
Brain |
2 |
|
Liver |
3.4 |
|
Blood flow (% cardiac output) |
|
|
Rapidly perfused |
41 |
|
Slowly perfused |
19 |
|
Fat |
5 |
|
Brain |
11 |
|
Liver |
24 |
|
Partition (tissue/blood) |
|
|
Rapidly perfused |
3.72 |
|
Slowly perfused |
1.06 |
|
Fat |
86.6 |
|
Brain |
3.72 |
|
Liver |
3.72 |
|
Blood/air |
10.3 |
|
Metabolic parameters |
|
|
Vmax (mg/h) |
6.77 |
|
Km (mg/l) |
4.56 |
|
Gastric absorption rate |
|
|
ka (1/h) |
1.6 |
a A density of 0.92 and 1 g/cm3 was used for fat and for other compartments, respectively.
According to the Rao & Brown (1993) model,10 the venous blood tetrachloroethene AUC resulting from continuous exposure to tetrachloroethene at 0.2 mg/m3 is 2.93 mg/l × min, corresponding to a 24-h TWA concentration of 20 µg/l. An assumption of the amount of water consumed is also not necessary, because blood concentrations of tetrachloroethene are solely dependent on the amount of compound ingested during each drinking episode. The model predicts that a total dose of 0.047 mg/kg body weight per day results in the blood tetrachloroethene concentration depicted as a continuous line in Figure A8-1, which shows the same AUC (and 24-h TWA concentration) as (dashed line) continuous inhalation exposure to 0.2 mg/m3.
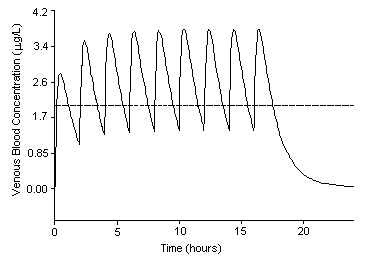
Figure A8-1. Time course of venous blood concentration in humans
as predicted by the Rao & Brown (1993)
PBPK model for ingested tetrachloroethene.
|
A total of 3.2 mg of tetrachloroethene was orally delivered via drinking-water in nine bolus doses spaced 2 h apart for a duration of 16 h, followed by 8 h of no dosing. The dashed line indicates the steady-state blood concentration (2 µg/l) due to inhaled tetrachloroethene at a 0.2 mg/m3 exposure concentration that results in the same AUC |
Le présent CICAD11 relatif au tétrachloréthylène (également appelé perchloréthylène) a été établi par Toxicology Advice & Consulting Ltd à partir de quatre documents de base. La plupart des sections consacrées aux effets sur la santé humaine reprennent les éléments d’un rapport rédigé conjointement par le Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals (Groupe nordique d’experts chargé de la documentation relative aux risques pour la santé imputables aux produits chimiques) et le Dutch Expert Committee on Occupational Standards (Comité néerlandais d’experts sur les normes en matière de santé au travail) (de Raat, 2003); elles s’inspirent également d’une évaluation de la cancérogénicité du tétrachloréthylène effectuée par le CIRC (IARC, 1995). Par ailleurs un document de travail de l’USEPA (Agence des Etats-Unis pour la protection de l’environnement) a été utilisé pour la rédaction des sections consacrées à la neurotoxicité de ce composé (USEPA, 2003). Les parties portant sur l’environnement s’inspirent de la version finale du EU Risk Assessment Report (environment) (Rapport de l’Union européenne sur l’évaluation du risque (environnement) (EC, 2001).12 Les documents de base prennent en compte les données existantes jusqu’à l’année 2001 (EC, 2001), 1995 (IARC, 1995), 2002 (USEPA, 2003) et 2002 (de Raat, 2003). Une recherche bibliographique exhaustive a été conduite en mai 2004 sur plusieurs bases de données en ligne afin de retrouver les références postérieures à celles qui ont servi à l’établissement des documents de base. Des renseignements sur la disponibilité des documents de base et sur la nature de leur examen par des pairs sont donnés à l’appendice 2. L’appendice 3 donne des indications sur l’examen par des pairs du présent CICAD. Ce CICAD a tout d’abord été examiné en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Hanoi (Viet Nam) du 27 septembre au 1er octobre 2004. La liste des participants à cette réunion figure à l’appendice 4. En raison d’opinions divergentes concernant l’interprétation des données relatives à des points d’aboutissement essentiels de l’action toxique du composé, la version préliminaire du CICAD a été soumise à un Groupe consultatif de l’OMS qui s’est réuni au Centre for Ecology and Hydrology de Monkswood (Cambridgeshire) du 25 au 27 avril 2005. La liste des membres de ce groupe consultatif figure à l’appendice 5. Le CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Nagpur (Inde) du 31 octobre au 3 novembre 2005. La liste des participants à cette réunion figure à l’appendice 6. La Fiche internationale sur la sécurité chimique du tétrachloréthylène (ICSC 0076) établie par le Programme international sur la sécurité chimique (IPCS, 2000) est également reproduite dans le présent document.
Le tétrachloréthylène (No CAS
En ce qui concerne la production annuelle de tétrachloréthylène dans l’Union européenne et aux Etats-Unis, les chiffres les plus récents sont respectivement égaux à 164 000 et 160 000 tonnes et correspondent à l’année 1994 pour l’Union européenne et 1998 pour les Etats-Unis. Au cours des 10 à 20 dernières années, la production a baissé environ de moitié tant aux Etats-Unis que dans l’Union européenne. Le tétrachloréthylène est principalement utilisé pour le nettoyage à sec des textiles et comme intermédiaire de synthèse. On s’en sert également pour le dégraissage des métaux. Lors de son utilisation, ce composé est libéré dans l’atmosphère, en majeure partie par évaporation lors du nettoyage à sec.
Le tétrachloréthylène se volatilise facilement à partir du sol et des eaux superficielles et il se décompose dans l’air pour donner du phosgène, du chlorure de trichloracétyle, du chlorure d’hydrogène ainsi que du monoxyde et du dioxyde de carbone. Sa demi-vie dans l’atmosphère est d’environ 3 à 5 mois. Dans l’eau, il résiste à la dégradation abiotique ou aérobie mais se décompose en anaérobiose pour donner du trichloréthylène, du dichloréthylène, du chlorure de vinyle, de l’éthane et de l’éthylène. Il ne s’accumule pas en quantités importantes dans les organismes aquatiques. On peut déceler sa présence dans l’air extérieur à des concentrations généralement inférieures à 1-2 µg/m3. Aux Pays-Bas, sa concentration médiane dans l’air intérieur des lieux d’habitation est égale à 4 µg/m3, avec une teneur maximale d’environ 50 à 200 µg/m3. Dans l’air d’un immeuble où l’on procède à des opérations de nettoyage à sec, sa concentration peut être beaucoup plus élevée. Dans l’eau destinée à la consommation, la concentration du tétrachloréthylène est généralement inférieure à 1-10 µg/l. On peut mettre en évidence des concentrations plus élevées dans les eaux souterraines à proximité de sites pollués. Les quantités moyennes absorbées quotidiennement à partir de la nourriture, de l’eau de boisson et de l’air inspiré sont approximativement comprises entre 0,5 et 3 µg/kg de poids corporel par jour.
Le tétrachloréthylène est bien absorbé par les mammifères en cas d’inhalation ou d’ingestion, après quoi il se répartit principalement dans les tissus adipeux et en moindre proportion dans le foie, l’encéphale, les reins et les poumons. Il peut également y avoir résorption transcutanée. Chez l’Homme et les animaux de laboratoire, la molécule est en majeure partie excrétée sans modification dans l’air expiré, avec de petites quantités présentes dans les urines sous forme de métabolites. Chez la souris, la métabolisation est plus importante que chez le rat ou l’Homme. L’acide trichloracétique est le principal métabolite à côté d’autres métabolites mineurs tels que l’acide oxalique, l’acide dichloracétique, l’éthylène-glycol, le trichloracétylamide, divers thioéthers et le dioxyde de carbone. La principale voie métabolique est la voie oxydative intrahépatique (par l’intermédiaire du cytochrome P450); c’est elle qui conduit à la formation d’acide trichloracétique. En cas de forte exposition, il y a saturation de cette voie métabolique qui est alors relayée par une seconde voie comportant une conjugaison avec le glutathion. Cette dernière voie, plus importante chez le rat que chez l’Homme et la souris, conduit à la formation de S-(1,2,2-trichlorovinyl)-L-cystéine qui, par clivage au niveau du rein, donne naissance à des métabolite cytotoxiques et génotoxiques. Les intermédiaires réactifs engendrés au cours de ces deux processus peuvent former des liaisons covalentes avec les protéines et les acides nucléiques.
Non dilué, le tétrachloréthylène se révèle irritant pour l’épiderme de l’Homme et du lapin. Le liquide ne provoque qu’une irritation minime de la muqueuse oculaire du lapin, mais chez des volontaires exposés, les vapeurs se sont montrées irritantes pour les yeux et les voies respiratoires. Chez les animaux de laboratoire, la toxicité aiguë par voie respiratoire ou orale reste faible. Chez l’Homme, on a constaté les effets suivants dans des cas d’intoxication accidentelle aiguë consécutive à l’inhalation de tétrachloréthylène à une concentration inconnue (mais probablement forte) : dépression du système nerveux central, vertiges, fatigue intense, perte de la coordination, coma, lésions hépatiques réversibles et, parfois, la mort. Des effets analogues ont été observés chez des sujets humains après ingestion d’une dose d’environ 70 à 90 mg/kg de poids corporel.
La plupart des études de santé au travail dont on peut disposer portent sur des employés de l’industrie du nettoyage à sec et de l’électronique ou sur des personnes procédant au dégraissage de métaux et qui sont exposées de manière répétée principalement au tétrachloréthylène, mais peut-être aussi à d’autres solvants. On ignore le niveau d’exposition individuel de ces employés, mais en moyenne, le niveau d’exposition mesuré est habituellement d’environ 100 mg/m3. Ces études ont révélé la présence de signes de toxicité au niveau du système nerveux central et du rein. Les études de neurotoxicité mettent systématiquement en évidence une perturbation de la perception spatiale visuelle et du traitement de l’information visuelle par le cerveau. Quelles que soient les faiblesses de toutes ces études de neurotoxicité en milieu professionnel, la plus instructive d’entre elles révèle qu’il y a une baisse de performance dans les tests de comportement lorsque l’exposition moyenne est égale à 83 mg/m3. L’étude la plus instructive au sujet des effets néphrotoxiques a permis de déceler des signes de lésions tubulaires et glomérulaires lorsque l’exposition moyenne était égale à 100 mg/m3. Aucun signe manifeste d’hépatotoxicité n’a été observé lors de ces études.
En cas d’expositions à répétition, ce sont le foie, le rein et le système nerveux central qui constituent les principaux organes cibles chez les animaux de laboratoire. La souris est plus sensible que le rat aux effets hépatotoxiques du tétrachloréthylène.
Les éléments de preuve en faveur d’une cancérogénicité du composé pour les sujets humains exposés en milieu professionnel sont limitées. Les études existantes ne sont généralement pas très informatives au sujet du niveau d’exposition ou de l’exposition à d’autres solvants. La généralisation de l’usage du tétrachloréthylène dans l’industrie du nettoyage à sec remonte seulement au début des années 1960; l’excès d’incidence des tumeurs, s’il est imputable à l’activité professionnelle, pourrait s’expliquer en partie par les conditions d’exposition du personnel antérieurement à l’usage généralisé de ce produit. L’étude de la mortalité par cancer parmi le personnel des établissements de nettoyage à sec fait ressortir une mortalité élevée due à des cancers de l’śsophage et du col de l’utérus. Il y aurait également, selon certaines indications, un excès de cancers du rein. Trois études font également état d’un excès de lymphomes non hodgkiniens, mais qui n’est pas statistiquement significatif. Par ailleurs, il peut y avoir eu exposition à plusieurs solvants. Ni les enquêtes menées dans la population générale, ni les études cas-témoins n’ont fourni de preuves convaincantes d’un quelconque accroissement du risque de cancer en général ou de cancers de localisation précise qui soit attribuable à une exposition au tétrachloréthylène par l’intermédiaire de l’eau de boisson.
L’expérimentation animale montre que le tétrachloréthylène est indiscutablement cancérogène. L’inhalation répétée du produit a provoqué des leucémies chez des rats F344 des deux sexes (selon deux études) et des tumeurs malignes du foie chez les rats mâles de cette même souche dans une étude sur deux. Après exposition par inhalation, des tumeurs malignes du foie ont également été observées chez des souris B6C3F1 et BDF1 des deux sexes ainsi que des tumeurs bénignes de la glande de Harder chez les mâles BDF1. Une exposition répétée au tétrachloréthylène par la voie orale a provoqué la formation de tumeurs malignes du foie chez des souris B6C3F1 des deux sexes.
La possibilité d’une génotoxicité du tétrachloréthylène a été assez largement étudiée. In vivo, ce composé ne provoque pas d’aberrations chromosomiques dans la moelle osseuse du rat ou de la souris, ni la formation de micronoyaux dans la moelle osseuse de la souris. Chez le rat et le hamster, il n’entraîne pas non plus d’anomalies au niveau des spermatozoïdes, mais des spermatozoïdes anormaux ont été observés en plus grande proportion après exposition de souris à un produit de pureté médiocre. Le tétrachloréthylène ne provoque pas de mutations létales dominantes chez le rat. D’autres types de tests ont montré qu’il n’induit pas de lésions de l’ADN au niveau rénal chez le rat ou pulmonaire chez la souris; toutefois, on a constaté la présence de lésions passagères de l’ADN au niveau du foie et du rein chez les souris exposées. Chez la drosophile, le composé ne provoque pas de mutations létales récessives liées au sexe. In vitro, le test d’Ames ne révèle pas de mutations chez les bactéries et on ne constate pas non plus de lésions chromosomiques ou d’échanges de chromatides sśurs dans des cellules de hamster. Le composé ne provoque pas de mutations dans des cellules murines et n’entraîne pas de synthèse non programmée de l’ADN dans des cellules de sujets humains, de rats ou de souris. Malgré quelques résultats positifs, l’examen des données disponibles incite à penser que le tétrachloréthylène, n’a pas, en tant que tel, d’activité génotoxique in vivo. Le test d’Ames a permis de mettre en évidence une activité mutagène des métabolites du tétrachloréthylène produits par l’organisme de mammifères.
Pour l’instant, aucun mécanisme n’a été proposé pour expliquer les leucémies et les tumeurs bénignes de la glande de Harder observées respectivement chez le rat et la souris mâle. On admet l’existence d’un mode d’action non génotoxique en ce qui concerne la formation de tumeurs rénales chez le rat mâle et de tumeurs hépatiques chez les souris sous l’action de certaines substances chimiques. Les données relatives au mode d’action du tétrachloréthylène sont limitées et en ce qui concerne ces types reconnus de mécanisme, les données obtenues ne cadrent pas avec les relations dose-réponse établies pour le processus de cancérisation sous l’action du tétrachloréthylène. Faute d’une preuve suffisante du contraire, on est amené à conclure que l’observation de cancers chez des rongeurs exposés au tétrachloréthylène est à prendre en compte eu égard à la santé humaine.
Quelques études épidémiologiques portant sur des femmes exposées au tétrachloréthylène en milieu professionnel montrent qu’elles courent un risque plus élevé d’avortement spontané; on ne dispose pas de données suffisantes pour pouvoir conclure au sujet d’autres effets indésirables sur la reproduction comme une diminution de la fécondité ou des malformations fśtales. Des études sur la reproduction et le développement effectuées chez le rat, la souris et le lapin donnent à penser que le composé est foetotoxique à des doses également toxiques pour la mère. Lors de plusieurs études au cours desquelles des rattes et des lapines gravides ont été exposées au tétrachloréthylène, on n’a pas observé de signes de malformations structurelles dans leur descendance, mais les auteurs d’une étude de ce genre portant sur la souris font état de malformations non précisées au niveau des tissus mous chez les souriceaux (à des doses toxiques pour la mère). On dispose également de données limitées indiquant de légères modifications affectant les paramètres neurochimiques et les fonctions centrales chez les ratons et les souriceaux lorsque la mère a été exposée pendant la gestation.
C’est la toxicité qui est l’effet indésirable le plus systématiquement observé dans des cohortes de sujets professionnellement exposés au perchloréthylène. C’est pour cette raison que l’on a utilisé les résultats de l’étude la plus instructive à ce sujet pour déterminer la concentration tolérable (TC). Le niveau d’exposition moyen (83 mg/m3) a été choisi comme valeur de la LOAEC (dose la plus faible produisant un effet indésirable observable). On en a ensuite tiré la valeur de la concentration équivalente correspondant à une exposition continue (20 mg/m3) puis, après application de deux facteurs d’incertitude égaux à 10 (l’un pour prendre en considération les différences interindividuelles et l’autre pour tenir compte du fait que l’on est parti de la LOAEC et non de la NOAEC ou concentration sans effet indésirable observable), on est parvenu à une valeur de la concentration tolérable égale à 0,2 mg/m3. Pour permettre des comparaisons, on a procédé de la même manière dans le cas des études faisant état d’effets néphrotoxiques. L’étude la plus instructive indique une exposition professionnelle moyenne de 100 mg/m3, d’où l’on tire une valeur de 0,24 mg/m3 pour la concentration tolérable, en bon accord avec la concentration tolérable dans le cas des effets neurotoxiques. Les données disponibles montrent qu’une hépatoxicité ne se manifesterait que pour une exposition supérieure à celle qui entraîne des effets sur le SNC ou le rein. On n’a pas calculé de concentration tolérable dans le cas des avortements spontanés. Quoi qu’il en soit, la concentra``tion tolérable de 0,2 mg/m3 est inférieure de plus de 3 ordres de grandeur à celle qui produit de légers effets indésirables chez les animaux de laboratoire, de sorte que l’on considère qu’elle est protectrice vis-à-vis des effets toxiques sur la reproduction humaine.
S’agissant de l’exposition par la voie orale, les données disponibles sont insuffisantes pour permettre d’établir la valeur de la dose journalière tolérable par cette voie d’absorption. Toutefois, comme le composé est bien résorbé par inhalation ou ingestion et qu’il n’y a guère de signes d’un métabolisme de premier passage, on a utilisé un modèle PBPK (modèle pharmacocinétique à base physiologique) pour établir la valeur de cette dose journalière. Selon ce modèle, l’ingestion de tétrachloréthylène présent dans l’eau de boisson à raison de 0,047 mg/kg de poids corporel par jour, donnerait une ASC plasmatique similaire à celle que l’on obtiendrait en cas d’exposition continue à une concentration de tétrachloréthylène dans l’air inhalé égale à 0,2 mg/m3. Cette valeur relative à la voie orale a été arrondie pour donner une dose journalière tolérable égale à 50 µg/kg de poids corporel.
Le tétrachloréthylène provoque plusieurs types de tumeurs chez le rat et la souris. On ne possède actuellement aucune preuve convaincante que ces tumeurs apparaissent selon un mécanisme qui serait propre aux rongeurs, aussi ne peut-on en minimiser l’importance pour la santé humaine. On a s’est donc basé sur la concentration de référence (BMC) et calculé cette concentration et sa limite de confiance inférieure pour chaque type de tumeur. De toutes les tumeurs révélées par l’expérimentation animale, ce sont les adénomes et les carcinomes hépatocellulaires observés chez la souris mâle qui comportent le degré de risque prévisible le plus élevé. La concentration tolérable de 0,2 mg/m3 évoquée plus haut correspond à un risque cumulé de 0,4 × 10−3 sur toute la durée de la vie lorsqu’on effectue une extrapolation linéaire en prenant la BMC10 comme point de départ.
En Europe et aux Etats-Unis, les concentrations de tétrachloréthylène dans l’atmosphère ou l’air à l’intérieur des bâtiments sont généralement inférieures de plus d’un ordre de grandeur à la concentration tolérable, même en milieu urbain. A proximité des sources ponctuelles de tétrachloréthylène, les concentrations observées restent également inférieures à la TC. Dans les bâtiments où l’on utilise ce produit (notamment dans les ateliers de nettoyage à sec), on a mesuré des concentrations nettement supérieures à la concentration tolérable. Dans l’eau de boisson des divers pays d’Europe, la concentration du tétrachloréthylène est généralement inférieure à 10 µg/l, ce qui donne une dose journalière inférieure à environ 0,3 µg/kg de poids corporel. Cette valeur est à rapprocher de la dose journalière tolérable, fixée à 50 µg/kg de poids corporel. A noter que sur les sites pollués, la concentration dans les eaux souterraines peut dépasser 1 mg/l.
Pour les organismes terrestres, la concentration minimale prédite sans effet (PNEC) est de 10 µg/kg de poids humide dans le sol. Comme cette valeur est supérieure à la concentration prédite dans l’environnement (PEC), qui va de 0,06 à 3,9 µg/kg, on en a conclu que le tétrachloréthylène ne représente pas un risque important pour les organismes terrestres. S’agissant des organismes aquatiques, la concentration minimale prédite sans effet est de 51 µg/l. Etant donné que la concentration prédite dans l’environnement va de 0,002 à 9,1 µg/l, on estime que l’exposition au tétrachloréthylène ne représente pour instant qu’un faible risque pour les organismes aquatiques. Même conclusion en ce qui concerne les organismes sédimenticoles, puisque la PNEC est de 277 mg/kg de sédiment, contre 57 µg/kg pour la valeur la plus forte de la PEC. On estime également que le tétrachloréthylène ne devrait pas représenter de risque pour les microorganismes qui interviennent dans le traitement des effluents, puisque la valeur minimale de la PNEC et la valeur maximale de la PEC sont dans ce cas respectivement égales à 11,2 mg/l et 16-23 µg/l. On a procédé à une évaluation supplémentaire du risque pour les végétaux exposés au tétrachloréthylène présent dans l’atmosphère. Le calcul de la PNEC minimale donne une valeur de 8,2 µg/m3 d’air. Les valeurs de la PEC sont généralement inférieures à ce chiffre, mais une mesure effectuée à proximité d’un site de production et de transformation du tétrachloréthylène a fourni une valeur plus élevée (36 µg/m3), ce qui amène à conclure qu’il faut limiter le risque que représentent les émissions dans l’atmosphère de ce composé pour les végétaux présents dans le voisinage de tels sites.
Este CICAD13 sobre el tetracloroeteno fue preparado por Toxicology Advice & Consulting Ltd basándose en cuatro documentos originales. Se utilizó un informe producido por el Grupo de Expertos Nórdicos para la Documentación de los Criterios relativos a los Riesgos para la Salud de los Productos Químicos en colaboración con el Comité de Expertos Neerlandeses en Normas del Trabajo (de Raat, 2003) y la evaluación de la carcinogenicidad del tetracloroeteno del IARC (IARC, 1995) para redactar la mayor parte de las secciones relativas a la salud humana y un documento de debate del EPA de los Estados Unidos (USEPA, 2003) sobre la neurotoxicidad como base para las secciones sobre este tema. Las secciones relativas al medio ambiente se prepararon a partir del proyecto final de Informe de Evaluación de los Riesgos (Medio Ambiente) de la Unión Europea (EC, 2001).14 En los documentos originales se examinaron los datos obtenidos hasta 2001 (EC, 2001), 1995 (IARC, 1995), 2002 (USEPA, 2003) y 2002 (de Raat, 2003). En mayo de 2004 se realizó una búsqueda bibliográfica amplia de varias bases de datos en línea para identificar cualquier referencia publicada después de las incorporadas a los documentos originales. La información sobre el carácter del examen colegiado y la disponibilidad de los documentos originales se presenta en el apéndice 2. La información sobre el examen colegiado de este CICAD figura en el apéndice 3. Este CICAD se examinó en primer lugar como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Hanoi (Viet Nam) del 27 de septiembre al 1ş de octubre de 2004. La lista de participantes en esta reunión figura en el apéndice 4. Debido a las opiniones divergentes sobre la interpretación de los datos relativos a los efectos críticos finales, el proyecto de CICAD se remitió a un grupo consultivo de la OMS, que se reunió en el Centro de Ecología e Hidrología de Monks Wood, Cambridgeshire (Reino Unido) del 25 al 27 de abril de 2005. La lista de participantes en esta reunión aparece en el apéndice 5. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Nagpur (India) del 31 de octubre al 3 de noviembre de 2005. La lista de participantes en esta reunión figura en el apéndice 6. También se reproduce en este documento la Ficha internacional de seguridad química para el tetracloroeteno (ICSC 0076), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000).
El tetracloroeteno (CAS Nş
Las cifras más recientes para la producción anual de tetracloroeteno en la Unión Europea y los Estados Unidos son, respectivamente, de 164 000 y 160 000 toneladas, y corresponden a 1994 (Unión Europea) y 1998 (Estados Unidos). La producción en la Unión Europea y los Estados Unidos se ha reducido aproximadamente a la mitad durante los 10-20 últimos años. Las principales aplicaciones del tetracloroeteno son en la limpieza en seco de textiles y como intermediario químico. Se utiliza también para el desengrasado de metales. El tetracloroeteno se libera en la atmósfera durante su utilización y la porción principal de las emisiones atmosféricas se debe a pérdidas por evaporación durante la limpieza en seco.
El tetracloroeteno se volatiliza fácilmente a partir del suelo y las aguas superficiales y se degrada en el aire para producir fosgeno, cloruro de tricloroacetilo, cloruro de hidrógeno, monóxido de carbono y anhídrido carbónico. Su semivida en el aire es de unos 3-5 meses. En el agua resiste la degradación abiótica y aerobia, pero se biodegrada en condiciones anaerobias para formar tricloroeteno, dicloroeteno, cloruro de vinilo, etano y eteno. Su bioacumulación en los organismos acuáticos no es significativa. El tetracloroeteno se detecta en el aire exterior, normalmente a concentraciones inferiores a 1-2 µg/m3. En los hogares neerlandeses, la concentración media en el aire de espacios cerrados era de 4 µg/m3, con valores máximos de alrededor de 50-200 µg/m3. Las concentraciones pueden ser mucho más elevadas en los edificios donde se efectúan operaciones de limpieza en seco. En el agua de bebida, las concentraciones de tetracloroeteno suelen estar por debajo de 1-10 µg/l. Se pueden encontrar concentraciones más altas en aguas subterráneas próximas a lugares contaminados. La ingesta media a partir de los alimentos, el agua de bebida y el aire es de unos 0,5-3 µg/kg de peso corporal al día.
Los mamíferos absorben bien el tetracloroeteno tras la inhalación o la exposición oral y a continuación se distribuye principalmente en el tejido adiposo, detectándose cantidades más pequeñas en el hígado, el cerebro, el riñón y el pulmón. También se puede producir absorción cutánea. Las personas y los animales de laboratorio excretan la mayor parte del tetracloroeteno absorbido de manera inalterada en el aire expirado, con pequeñas cantidades como metabolitos urinarios. El metabolismo es más amplio en los ratones que en las ratas y las personas. El metabolito principal es el ácido tricloroacético; son metabolitos secundarios el ácido oxálico, el ácido dicloroacético, el etilenglicol, la tricloroacetilamida, algunos tioéteres y el anhídrido carbónico. El metabolismo oxidativo (mediado por el citocromo P450) en el hígado es la vía más importante, dando lugar a la formación de ácido tricloroacético. Con exposiciones más elevadas esta vía se satura y adquiere más importancia una segunda vía, de conjugación con el glutatión. Esta vía, más importante en las ratas que en las personas y los ratones, determina la formación de S-(1,2,2-triclorovinil)-cisteína, que se puede dividir en el riñón para producir metabolitos citotóxicos y genotóxicos. Los intermediarios reactivos de ambas vías se pueden unir mediante enlace covalente con las proteínas y los ácidos nucleicos.
El tetracloroeteno puro era irritante cutáneo para las personas y los conejos. El líquido sólo provocaba una irritación ocular mínima en los conejos y el vapor era irritante para los ojos y el tracto respiratorio de los voluntarios expuestos. En los animales de laboratorio, la toxicidad aguda por inhalación y por vía oral fue baja. En las personas, la inhalación aguda accidental de concentraciones no medidas (pero presumiblemente altas) de tetracloroeteno indujo depresión del sistema nervioso central, vértigo, fatiga, pérdida de coordinación, coma, daños hepáticos reversibles y algunas muertes. Se observaron efectos semejantes en las personas tras la ingestión aguda de dosis de unos 70-90 mg/kg de peso corporal.
La mayor parte de los estudios ocupacionales corresponden a personas expuestas repetidamente, en particular al tetracloroeteno, pero posiblemente también a otros disolventes, en las industrias de la limpieza en seco y la electrónica y durante las operaciones de desengrasado de metales. Aunque se carece de información sobre los niveles de exposición individuales, las exposiciones medias medidas al tetracloroeteno solían ser de unos 100 mg/m3. En estos estudios se obtuvieron algunas pruebas de toxicidad para el sistema nervioso central y el riñón. Los estudios de neurotoxicidad pusieron de manifiesto un efecto común de perturbación de la función visual espacial y de la elaboración cognoscitiva de la información visual en el sistema nervioso central. Aunque todos los estudios ocupacionales de neurotoxicidad tienen limitaciones, en el estudio más ilustrativo se detectaron deficiencias en las pruebas de comportamiento con un nivel medio de exposición de 83 mg/m3. En el estudio más ilustrativo sobre los efectos en el riñón había indicios de daños en las regiones tanto tubulares como glomerulares del riñón con un nivel medio de exposición de 100 mg/m3. En estos estudios no se encontraron pruebas de toxicidad hepática.
Con exposiciones repetidas, el hígado, el riñón y el sistema nervioso central son los principales órganos destinatarios en los animales de laboratorio. Los ratones eran más sensibles que las ratas a la toxicidad hepática del tetracloroeteno.
Hay pruebas limitadas de que el tetracloroeteno es carcinogénico en las personas expuestas en el lugar de trabajo. Los estudios disponibles suelen carecer de buena información sobre los niveles de exposición y sobre la exposición a otros disolventes. El uso generalizado de tetracloroeteno en la industria de la limpieza en seco no comenzó hasta los años sesenta; si el exceso de incidencia de tumores estaba relacionado con la actividad ocupacional, se podía atribuir en parte a las condiciones de exposición antes del uso generalizado del tetracloroeteno. Cuando se examinó la mortalidad por cáncer de los trabajadores de los establecimientos de limpieza en seco, se observó una mortalidad elevada en relación con el cáncer de esófago y de cuello uterino. Hubo algunas indicaciones de un exceso de casos de cáncer de riñón. En tres estudios se notificó un exceso de linfoma no Hodgking, que no era importante desde un punto de vista estadístico; además, puede haber habido exposición a disolventes múltiples. Los estudios sobre la población general y los de casos y testigos no aportaron pruebas convincentes de ningún aumento del riesgo de cáncer total o específico derivado de la exposición al tetracloroeteno en el agua de bebida.
El tetracloroeteno era claramente carcinogénico en los animales de laboratorio. Con inhalaciones repetidas indujo leucemia en ratas F344 de ambos sexos (en dos estudios) y tumores renales malignos en ratas F344 macho en un estudio (de dos). En estudios de inhalación, indujo la formación de tumores malignos de hígado en ratones B6C3F1 y BDF1 de ambos sexos y tumores benignos en la glándula de Harder de ratones BDF1 macho. Con dosis repetidas administradas por vía oral, el tetracloroeteno indujo tumores hepáticos malignos en ratones B6C3F1 de ambos sexos.
Se ha examinado bastante ampliamente el potencial genotóxico del tetracloroeteno. In vivo no provocó aberraciones cromosómicas en la médula ósea de ratas o ratones ni micronúcleos en la médula ósea del ratón. Tampoco indujo anomalías en el esperma de ratas o hámsteres, pero con un grado de pureza bajo aumentó el porcentaje de esperma anormal en los ratones. El tetracloroeteno no indujo mutaciones dominantes letales en las ratas. En otras pruebas no produjo daños en el ADN del riñón de las ratas o el pulmón de los ratones; sin embargo, se notificaron daños transitorios del ADN en el hígado y el riñón de los ratones expuestos. No indujo mutaciones recesivas letales ligadas al sexo en las moscas de la fruta. Cuando se sometió a pruebas in vitro, el tetracloroeteno no produjo mutaciones en las pruebas bacterianas de Ames, daños en los cromosomas o intercambios de cromátidas hermanas en las células de hámster, mutaciones en las células de ratón o síntesis de ADN no programado en las células de personas, ratas o ratones. Aunque en un pequeño número de pruebas se obtuvieron resultados positivos, un método de valor probatorio parece indicar que el tetracloroeteno en sí no tiene un potencial genotóxico significativo in vivo. Los metabolitos del tetracloroeteno en los mamíferos han inducido mutaciones en pruebas de Ames.
Por el momento no se han propuesto mecanismos para la inducción de leucemia y tumores en las glándulas de Harder en ratas y ratones macho, respectivamente. Se han reconocido mecanismos no genotóxicos para la formación de tumores de riñón en las ratas macho y de hígado en los ratones debido a algunas sustancias químicas. Los datos disponibles sobre el mecanismo de acción del tetracloroeteno son limitados y los datos relativos a la relación dosis-respuesta para estos mecanismos reconocidos no son compatibles con las relaciones dosis-respuesta para la inducción de cáncer por el tetracloroeteno. En ausencia de pruebas adecuadas que demuestren lo contrario, se llega a la conclusión de que el cáncer producido por el tetracloroeteno en los roedores tiene una importancia potencial para las personas.
Algunos estudios epidemiológicos de mujeres expuestas al tetracloroeteno en el lugar de trabajo han puesto de manifiesto un mayor riesgo de aborto espontáneo; la información disponible no es suficiente para sacar conclusiones con respecto a otros resultados reproductivos adversos, como la disminución de la fecundidad y la aparición de malformaciones fetales. Los estudios reproductivos y del desarrollo en ratas, ratones y conejos parecen indicar que el tetracloroeteno es fetotóxico en dosis que también provocan toxicidad materna. En varios estudios de exposición de ratas y conejas preñadas no se encontraron pruebas de malformaciones estructurales en la descendencia, pero en uno de esos estudios con ratones se notificaron malformaciones inespecíficas en los tejidos blandos de las crías (con una dosis tóxica para la madre). Hay pruebas limitadas que parecen indicar cambios ligeros en la neuroquímica y la función del sistema nervioso central de las ratas y ratones jóvenes tras la exposición de las madres durante la gestación.
El resultado adverso más sistemático en cohortes expuestas en el lugar de trabajo fue la neurotoxicidad; por consiguiente, para obtener una concentración tolerable se utilizó el estudio más ilustrativo sobre los efectos neurotóxicos en trabajadores expuestos. Se tomó como LOAEC el nivel medio de exposición (83 mg/m3). Este valor se convirtió en una concentración equivalente para la exposición continua (20 mg/m3) y se aplicaron dos factores de incertidumbre de 10 (uno para tener en cuenta las diferencias interindividuales y el otro porque la concentración seleccionada fue una LOAEC en lugar de una NOAEC) para obtener una concentración tolerable de 0,2 mg/m3. Se utilizó un sistema similar con fines de comparación para los estudios de nefrotoxicidad. En el estudio más ilustrativo se obtuvo una exposición ocupacional media de 100 mg/m3, que generó una concentración tolerable de 0,24 mg/m3, valor compatible con los efectos protectores de la concentración tolerable frente a los neurotóxicos. Los datos disponibles indican que sólo se produciría toxicidad hepática con exposiciones superiores a las que afectan al sistema nervioso central y el riñón. No se obtuvo una concentración tolerable para los abortos espontáneos. Sin embargo, la concentración tolerable de 0,2 mg/m3 es inferior en más de tres órdenes de magnitud a la de exposición que indujo efectos adversos ligeros en animales de laboratorio, por lo que se consideró protectora frente a la toxicidad reproductiva en las personas.
La información disponible sobre la exposición oral era insuficiente para obtener una ingesta diaria tolerable por vía oral. Sin embargo, como el tetracloroeteno se absorbe bien tras la inhalación o la ingestión y hay pocas pruebas de metabolismo de primer paso, se utilizó un modelo farmacocinético con una base fisiológica para obtener una ingesta diaria tolerable. El modelo predecía que el tetracloroeteno consumido en el agua de bebida con una dosis de 0,047 mg/kg de peso corporal al día produciría una zona bajo la curva en el plasma semejante a la obtenida a partir de la exposición continua a una concentración de tetracloroeteno en el aire inhalado de 0,2 mg/m3. Esta cifra por vía oral se redondeó para obtener una ingesta diaria tolerable de 50 µg/kg de peso corporal.
El tetracloroeteno ha inducido varios tipos de tumores en ratas y ratones. Por el momento no hay pruebas convincentes de que estos tumores se deriven de mecanismos de acción que funcionan sólo en los roedores, por lo que no se puede desestimar su importancia para las personas. Además, se utilizó un enfoque de concentración de referencia y se calculó una concentración de referencia y su límite de confianza más bajo para cada tumor de los animales. De los tumores observados en animales experimentales, los riesgos pronosticados más altos fueron de adenomas hepatocelulares y carcinomas en los ratones macho. La concentración tolerable obtenida mas arriba, de 0,2 mg/m3, corresponde a un riesgo acumulativo durante toda la vida de 0,4 × 10−3 cuando se aplica una extrapolación lineal a la concentración asociada con un aumento del 10 por ciento en el riesgo absoluto de encontrar una respuesta "adversa".
Las concentraciones de tetracloroeteno en la atmósfera o en el aire de recintos cerrados en Europa y los Estados Unidos suelen ser más de un orden de magnitud inferiores a la concentración tolerable, incluso en zonas urbanas. En las proximidades de fuentes puntuales, las concentraciones observadas quedan por debajo de la concentración tolerable. En los edificios en los que se utiliza tetracloroeteno (en particular en las instalaciones de limpieza en seco), se han medido concentraciones que rebasan claramente la concentración tolerable. Las concentraciones de tetracloroeteno en el agua de bebida en diferentes países de Europa suelen ser inferiores a 10 µg/l, lo que da lugar a dosis de tetracloroeteno por debajo de un nivel aproximado de 0,3 µg/kg de peso corporal al día. Esto es comparable con la ingesta diaria tolerable de 50 µg/kg de peso corporal. Hay que señalar que las concentraciones en el agua subterránea de lugares contaminados pueden ser superiores a 1 mg/l.
Para los organismos terrestres, la concentración prevista sin efectos (PNEC) más baja fue de 10 µg/kg de peso húmedo en el suelo. Como ésta era superior a las concentraciones previstas en el medio ambiente (PEC), que oscilaban entre 0,06 y 3,9 µg/kg, se llegó a la conclusión de que era poco probable que el tetracloroeteno representase un riesgo importante para los organismos terrestres. Para los organismos acuáticos, la PNEC más baja fue de 51 µg/l. Las PEC oscilaban entre 0,002 y 9,1 µg/l, por lo que se consideraba que las exposiciones actuales al tetracloroeteno tenían un riesgo bajo para los organismos acuáticos. Se llegó a una conclusión semejante para los organismos de los sedimentos, donde se calculó que la PNEC más baja era de 277 mg/kg de sedimento, en comparación con una PEC máxima calculada de 57 µg/kg. También se consideraba que era poco probable que el tetracloroeteno representara un riesgo para los microorganismos en los procesos de tratamiento de las aguas residuales, con los valores más bajos de la PNEC y más altos de la PEC de 11,2 mg/l y 16-23 µg/l, respectivamente. Se realizó una evaluación adicional del riesgo para las plantas expuestas al tetracloroeteno atmosférico. La PNEC más baja fue de 8,2 µg/m3 de aire. Las PEC se mantuvieron en general por debajo de este valor, aunque se midió un valor más alto (36 µg/m3) cerca de un lugar donde se producía y elaboraba tetracloroeteno, por lo que se llegó a la conclusión de que era necesario limitar los riesgos de daño para las plantas a partir de las emisiones al aire en esos lugares.
ENDNOTES:
See Also:
Toxicological Abbreviations