
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 13
Carbon Monoxide
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme.
Published under the joint sponsorship of
the United Nations Environment Programme
and the World Health Organization
World Health Organization
Geneva, 1979
ISBN 92 4 154073 7
(c) World Health Organization 1979
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CARBON MONOXIDE
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.1.1. Properties and analytical methods
1.1.2. Sources of environmental pollution
1.1.3. Environmental levels
1.1.4. Effects on experimental animals
1.1.5. Effects on man
1.1.6. Evaluation of health risk
1.2. Recommendations for further studies
2. CHEMISTRY AND ANALYTICAL METHODS
2.1. Physical and chemical properties
2.2. Methods of measuring carbon monoxide in ambient air
2.3. Biological monitoring
3. SOURCES OF CARBON MONOXIDE IN THE ENVIRONMENT
3.1. Natural occurrence
3.2. Man-made sources
4. ENVIRONMENTAL DISTRIBUTION AND TRANSFORMATION
4.1. Atmospheric transport and diffusion
4.2. Environmental absorption and transformation
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1. Ambient air concentrations and exposures
5.2. Indoor concentrations and exposure
5.3. Occupational exposure
5.4. Carboxyhaemoglobin levels in the general population
6. METABOLISM
6.1. Endogenous carbon monoxide production
6.2. Absorption
6.3. Reactions with body tissues and fluids
6.4. Excretion
7. EFFECTS ON EXPERIMENTAL ANIMALS
7.1. Species differences
7.2. Cardiovascular system and blood
7.3. Central nervous system
7.4. Behavioural changes and work performance
7.5. Adaptation
7.6. Embryonal, fetal, neonatal, and teratogenic effects
7.7. Carcinogenicity and mutagenicity
7.8. Miscellaneous changes
7.9. Interactions
8. EFFECTS ON MAN
8.1. Healthy subjects
8.1.1. Behavioural changes
8.1.2. Work performance and exercise
8.1.3. Adaptation
8.1.4. Effects on the cardiovascular system and other
effects
8.1.5. Carboxyhaemoglobin levels resulting from exposure
to methane-derived halogenated hydrocarbons
8.1.6. Levels and effects of carboxyhaemoglobin resulting
from smoking
8.1.7. Interactions
8.2. High-risk groups
8.2.1. Individuals with cardiovascular and chronic
obstructive lung disease
8.2.2. Anaemic individuals
8.2.3. Embryo, fetus, neonate, and infants
8.2.4. Individuals living at high altitudes
8.3. Summary table
9. EVALUATION OF HEALTH RISKS
9.1. Introduction
9.2. Exposure
9.2.1. Assessment of exposure
9.2.2. Endogenous production
9.2.3. Outdoor environmental exposure
9.2.4. Indoor exposure
9.2.5. Exposures related to traffic
9.2.6. Occupational exposure
9.2.7. Tobacco smoking
9.2.8. Multiple exposures
9.3. Effects
9.3.1. Cardiovascular system
9.3.1.1 Development of atherosclerotic
cardiovascular disease
9.3.1.2 Acute effects on existing heart illness
9.3.1.3 Acute effects on existing vascular disease
9.3.2. Nervous system
9.3.3. Work capacity
9.4. Recommended exposure limits
9.4.1. General population exposure
9.4.2. Working population exposure
9.4.3. Derived limits for carbon monoxide concentrations
in air
REFERENCES
ANNEX 1
ANNEX 2
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CARBON MONOXIDE
Members
Dr H. Buchwald, Assistant Deputy Minister, Alberta Department of
Labour, Edmonton, Alberta, Canada (Chairman)
Dr V.A. Cizikov, Central Institute for Advanced Medical Training,
Moscow, USSR
Dr E. Haak, Physiology Branch, Clinical Studies Division, Health
Effects Research Laboratory, US Environmental Protection Agency,
Research Triangle Park, NC, USA
Dr P. Iordanidis, National Technical University of Athens, Athens,
Greece
Dr K. Ishikawa, Department of Public Health, School of Medicine, Chiba
University, Chiba, Japan
Dr V. Kodat, Hygiene Department, Ministry of Health of the Czech
Socialist Republic, Prague, Czechoslovakia (Vice-Chairman)
Dr K. Kurppa, Department of Occupational Medicine, Institute of
Occupational Health, Helsinki, Finland
Professor P.J. Lawther, Clinical Section, Medical Research Council
Toxicology Unit, St Bartholomew's Hospital Medical College,
London, England
Mr I.R.C. McDonald, Chemistry Division, Department of Scientific &
Industrial Research, Petone, New Zealand (Rapporteur)
Dr G. Winneke, Institute for Air Hygiene & Silicosis Research,
Düsseldorf, Federal Republic of Germany
Representatives of other organizations
Mr J. Janczak, Economic Commission for Europe, Geneva, Switzerland
Dr D. Djordjevic, International Labour Office, Geneva, Switzerland
Mr C. Satkunanthan, International Register of Potentially Toxic
Chemicals of the United Nations Environment Programme, Geneva,
Switzerland
Observers
Dr J.J. Vostal, Biomedical Science Department, General Motors Research
Laboratories, Warren, MI, USA
Secretariat
Mrs B. Goelzer, Scientist, Office of Occupational Health, Division of
Noncommunicable Disease, WHO, Geneva, Switzerland
Dr Y. Hasegawa, Medical Officer, Control of Environmental Pollution &
Hazards, Division of Environmental Health, WHO, Geneva,
Switzerland
Dr R. Horton, Senior Research Adviser, Health Effects Research
Laboratory, US Environmental Protection Agency, Research Triangle
Park, NC, USA (Temporary Adviser)
Dr J. Korneev, Scientist, Control of Environmental Pollution &
Hazards, Division of Environmental Health, WHO, Geneva,
Switzerland (Secretary)
Dr H. de Koning, Scientist, Control of Environmental Pollution &
Hazards, Division of Environmental Health, WHO, Geneva,
Switzerland
Dr M. Vandekar, Medical Officer, Pesticides Development & Safe Use,
Division of Vector Biology & Control, WHO, Geneva, Switzerland
Dr V.B. Vouk, Chief, Control of Environmental Pollution & Hazards,
Division of Environmental Health, WHO, Geneva, Switzerland
ENVIRONMENTAL HEALTH CRITERIA FOR CARBON MONOXIDE
A WHO Task Group on Environmental Health Criteria for Carbon
Monoxide met in Geneva from 11 to 17 October 1977. Dr V.B. Vouk,
Chief, Control of Environmental Pollution and Hazards, opened the
meeting on behalf of the Director-General. The Task Group reviewed and
revised the second draft of the criteria document and made an
evaluation of the health risks from exposure to carbon monoxide.
The first and second drafts were prepared by Dr S.M. Horvath of
the Institute of Environmental Studies, University of California,
Santa Barbara, USA. The comments on which the second draft was based
were received from the national focal points for the WHO Environmental
Health Criteria Programme in Bulgaria, Canada, Czechoslovakia, France,
Netherlands, Poland, USSR, and USA and from the International Labour
Organisation (ILO), Geneva, the Food and Agriculture Organization of
the United Nations (FAO), Rome, the United Nations Educational,
Scientific and Cultural Organization (UNESCO), Paris, the United
Nations Industrial Development Organization (UNIDO), Vienna, the
Permanent Commission and International Association on Occupational
Health, the Commission on Atmospheric Environment, International Union
of Pure and Applied Chemistry (IUPAC), and from the Pan American
Sanitary Engineering Center (CEPIS).
The collaboration of these national institutions, international
organizations and WHO collaborating centres is gratefully
acknowledged. Without their assistance this document would not have
been completed. The Secretariat wishes to thank, in particular,
Professor P.J. Lawther and Mr R.E. Waller of the Medical Research
Council Toxicology Unit, St Bartholomew's Hospital Medical College,
London, and Dr G. Winneke of the Institute for Air Hygiene and
Silicosis Research, Düsseldorf, for their help in the scientific
editing of the document.
This document is based primarily on original publications listed
in the reference section. However, several recent publications broadly
reviewing health aspects of carbon monoxide have also been used
including those of the Commission of the European Communities (1974),
NAS/NRC (1977), US Department of Health, Education and Welfare (1970,
1972), and Committee on the Challenges of Modern Society (1972).
Details of the WHO Environmental Health Criteria Programme,
including some of the terms frequently used in the documents, may be
found in the introduction to the publication "Environmental Health
Criteria 1 -- Mercury" published by the World Health Organization,
Geneva, 1976, and now available as a reprint.
The following conversion factorsa have been used in this document:
carbon monoxide 1 ppm = 1145 µg/m3 1 µg/m3 = 0.873 ppm
methylene chloride 1 ppm = 3480 µg/m3 1 µg/m3 = 0.288 ppm
nitrogen dioxide 1 ppm = 1880 µg/m3 1 µg/m3 = 0.532 ppm
ozone 1 ppm = 2000 µg/m3 1 µg/m3 = 0.500 ppm
peroxyacetyl nitrate 1 ppm = 5000 µg/m3 1 µg/m3 = 0.200 ppm
1 Torr = 1.333 × 102 pascals = 1 mmHg
a All conversion factors for atmospheric pollutants refer to 25°C
and 101 kPa (1 atm) pressure.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1 Summary
1.1.1 Properties and analytical methods
Carbon monoxide (CO) is a colourless, odourless, tasteless gas
that is slightly less dense than air. It is a product of incomplete
combustion of carbon-containing fuels and is also produced by some
industrial and biological processes. Its health significance as a
contaminant of air is largely due to the fact that it forms a strong
coordination bond with the iron atom of the protohaem complex in
haemoglobin forming carboxyhaemoglobin (HbCO) and thus impairs the
oxygen-carrying capacity of the blood. The dissociation of
oxyhaemoglobin is also altered by the presence in blood of
carboxyhaemoglobin so that the supply of oxygen to tissues is further
impaired. The affinity of haemoglobin for carbon monoxide is roughly
240 times that of its affinity for oxygen; the proportions of
carboxyhaemoglobin and oxyhaemoglobin in blood are largely dependent
on the partial pressures of carbon monoxide and oxygen. Carbon
monoxide is absorbed through the lungs and the concentrationa of
carboxyhaemoglobin in the blood at any time will depend on several
factors. When in equilibrium with ambient air, the carboxyhaemoglobin
content of the blood will depend mainly on the concentrations of
inspired carbon monoxide and oxygen. However, if equilibrium has not
been achieved, the carboxyhaemoglobin concentration will also depend
on the time of exposure, pulmonary ventilation, and the
carboxyhaemoglobin originally present before inhalation of the
contaminated air. Formulae exist by which these estimates can be made.
In addition to its reaction with haemoglobin, carbon monoxide combines
with myoglobin, cytochromes, and some enzymes; the health significance
of these reactions is not clearly understood but is likely to be of
less importance than that of the reaction of the gas with haemoglobin.
Methods available for the measurement of carbon monoxide in
ambient airb range from fully automated methods using the non-
dispersive infrared technique and gas chromatography to very simple
semiquantitative manual methods using detector tubes. Since the
formation of carboxyhaemoglobin in man is dependent on many factors
a Throughout the document, the word concentration refers to mass
concentration, unless otherwise stated.
b Selected Methods of Measuring Air Pollutants, WHO Offset
Publication No. 24 (1976) published under the joint sponsorship of
the United Nations Environment Programme and the World Health
Organization, Geneva.
including the variability of ambient air concentrations of carbon
monoxide, carboxyhaemoglobin concentrations should be measured rather
than calculated. Several relatively simple methods are available for
determining carbon monoxide either by analysis of the blood or of
alveolar air that is in equilibrium with the blood. Some of these
methods have been validated by careful comparative studies.
1.1.2 Sources of environmental pollution
At present, the significance of natural sources of carbon monoxide
for man is uncertain. Estimates of man-made carbon monoxide emissions
vary from 350 to 600 million tonnes per annum. By far the most
important source of carbon monoxide at breathing level is the exhaust
of petrol-powered motor vehicles. The emission rate depends on the
type of vehicle, its speed, and its mode of operation. Other sources
include heat and power generators, some industrial processes such as
the carbonization of fuel, and the incineration of refuse. Faulty
domestic cooking and heating appliances may be important sources that
are often overlooked.
1.1.3 Environmental levels
Natural background levels of carbon monoxide are low
(0.01-0.9 mg/m3 or 0.01-0.8 ppm). Carbon monoxide concentrations in
urban areas are closely related to motor traffic density and to
weather and vary greatly with time and distance from the sources. The
configuration of buildings is important and concentrations fall
sharply with increasing distance from the street.
There are usually well-marked diurnal patterns with peaks
corresponding to the morning and evening "rush hours". Data from Japan
and the USA show that 8-h mean concentrations of carbon monoxide are
generally less than 20 mg/m3 (17 ppm). However, maximum 8-h mean
concentrations of up to 60 mg/m3 (53 ppm) have occasionally been
recorded. Much higher relatively transient peaks may be observed in
still weather where there is traffic congestion, and high
concentrations can be found in confined spaces such as tunnels,
garages, and loading bays in which vehicles operate and in vehicles
with faulty exhaust systems. There may be relatively high pollution by
carbon monoxide in workplaces and in some homes with cooking and
heating appliances that are faulty or do not have flues.
By far the commonest cause of high carboxyhaemoglobin
concentrations in man is the smoking of tobacco and the inhalation of
the products by the smoker.
1.1.4 Effects on experimental animals
Many experiments on animals have yielded valuable information
about the effects of carbon monoxide. There is general agreement that
most animals die when carboxyhaemoglobin levels exceed about 70% and
that the rate of administration of the gas is important in determining
the outcome. It is also agreed that carboxyhaemoglobin levels
exceeding 50% are often associated with damage to organs including the
brain and the heart. When animals are exposed to lower concentrations,
the effects are more difficult to discern and may be manifested as
changes in metabolism and biochemistry, alterations in the blood, or
changes in behaviour. There is evidence that some animals adapt to
exposure to comparatively low concentrations of carbon monoxide. As
might be expected, the variability of reported results of experiments
increases as the effects become less marked and the need for
scrupulous experimental design and technique becomes more important.
Of particular importance is the interpretation of the claims of some
workers that continuous intermittent exposure of animals to
concentrations resulting in carboxyhaemoglobin levels of 10-20% can
produce demonstrable histological changes in the myocardium, blood
vessels, and central nervous system. There are claims that such
exposures affect cholesterol uptake in the aorta and the coronary
arteries. The relevance of these findings, if accepted as real, are
obvious for the aetiology of cardiovascular diseases in man and,
therefore, must be assessed with great caution. It is also important
to study carefully the reports of research workers who have failed to
find evidence of damage.
1.1.5 Effects on man
The effects on man of exposure to high concentrations of carbon
monoxide are well documented and the diagnosis, treatment, and
sequelae of acute carbon monoxide poisoning are adequately dealt with
in standard texts. Recently much attention has been paid to the
possible effects on function and structure of exposure to carbon
monoxide concentrations resulting in carboxyhaemoglobin levels of 10%
or less. Carbon monoxide acts primarily by interfering with oxygen
transport and as the central nervous system is more sensitive to
hypoxia than the other systems of the body, much work has been done on
the impairment of vigilance, perception, and the performance of fine
tasks following exposure to concentrations of carbon monoxide too low
to produce clinical signs or symptoms. Many common drugs, beverages,
food, and fatigue can alter alertness, efficiency, and dexterity and
reported observed effects of low concentrations of carbon monoxide are
difficult, if not impossible, to interpret when no account is given of
precautions taken in the experimental design to eliminate or assess
the separate effects of other stresses. Again, great attention must be
paid to reports of impeccable experiments that have failed to
reproduce effects already reported. There would seem to be some
justification for accepting the possibility that concentrations of
carboxyhaemoglobin exceeding 2.5% might be associated with some
impairment of vigilance and other modes of perception. However, it
cannot be emphasized too strongly that when assessing the significance
(health and social) of this, the effects that many other commonly
acceptable factors might have on the tests should be taken into
account. It is possible, even likely, that the damaged heart and
respiratory system are more prone to impairment by carbon monoxide
than the intact brain. Skeletal muscle is sensitive to hypoxia and
obviously its sensitivity is enhanced by arterial disease. However, of
much greater importance is the effect of carbon monoxide on the
ischaemic myocardium which is especially vulnerable to additional
hypoxia. Evidence has been reported of changes in cardiac function and
the time of onset of angina pectoris on exercise when
carboxyhaemoglobin levels exceed 2.5%. Changes in oxygen uptake and
transfer are theoretically possible at or below these levels, and thus
there will be some patients whose cardiac function is so impaired that
any further hypoxic stress from carbon monoxide or from other factors,
will be intolerable. Similarly, the gross hypoxia of all tissues seen
in cases of severe respiratory disease renders the body even more
susceptible to the effects of low concentrations of carbon monoxide.
It follows that there is reason to regard carbon monoxide in this
sense as a pollutant for which the "threshold" is that concentration
which would be in equilibrium with the carboxyhaemoglobin produced
endogenously by the breakdown of blood pigments. However, it must be
realized that at these extremes of illness other usually trivial
stresses, such as ambient and body temperatures, infection, noise, and
anxiety, may be of much greater importance.
In addition to patients with diseases of the heart and the lungs,
it is likely that other groups, such as the anaemic, elderly,
postoperative patients, or those with cerebrovascular arteriosclerosis
may be at special risk. The effects on the fetus in utero of carbon
monoxide, especially that derived from maternal smoking, are of
special interest. The effects of carbon monoxide on people living at
high altitudes are greater than on those living at sea level and this
added risk must be assessed. There is a distinct possibility that
healthy man may adapt to the mild hypoxia caused by carboxyhaemo-
globin levels of about 3-5% (or to even higher values) as he does to
high altitude. Many workers in industry, and even more smokers,
repeatedly have carboxyhaemoglobin values such as these and there have
been few attempts to correlate symptoms or pathological findings
specifically with these levels of carboxyhaemoglobin. There is little
evidence that exposure to comparatively low concentrations of carbon
monoxide causes disease though it is suspected of being an etiological
factor in the association of heart disease with smoking.
1.1.6 Evaluation of health risk
There can be no doubt that the assessment of risk of exposure to
the lower concentrations of carbon monoxide in inspired air in
populations containing the sick and the fit, the smoker and nonsmoker,
the very young and the very old, would be a complex, if not
impossible, task, even if the ambient concentration of carbon monoxide
remained constant in time and place. Rough guidance, therefore, is all
that is possible in the light of available evidence derived from sound
scientific work. Those responsible for the welfare of specially
susceptible groups must refer critically to the evidence from
published works and to that reviewed in this document and make their
special decisions. There is general agreement that any individual
should be protected from exposure to carbon monoxide that would result
in carboxyhaemoglobin levels of 5% for any but transient periods, and
that especially susceptible persons ought not to be subjected to
concentrations giving carboxyhaemoglobin levels exceeding 2.5%. Advice
concerning such subjects must depend on individual assessment of their
clinical status and of other environmental factors including the
demands of the tasks they have to perform (persons engaged in driving,
monotonous tasks, keeping watch, etc., though healthy, might require
special consideration). The real possibility that adaptation occurs
makes consideration of the smoker and industrially exposed worker
difficult; ethical as well as health factors might affect action but
it would seem reasonable to have the same limit of 5% carboxy-
haemoglobin for industrial workers as for the rest of the healthy
population. The smoker inflicts high carboxyhaemoglobin values on
himself, by choice; he ought to be told of the evidence that this
habit might be harmful and then be subject to the levels of protection
recommended above.
1.2 Recommendations for Further Studies
(a) Though some would maintain that there are enough data on
levels of carbon monoxide in urban air, there is a need for further
surveys of air and blood levels so that some more precise correlations
may be established. Various populations in various places should be
studied properly to assess the magnitude of the problem posed by
carbon monoxide in the air of towns, houses, and workplaces.
(b) Opinions concerning levels of carbon monoxide below which no
adverse effects are seen and above which impairment of mental or
bodily functions seems to occur are based on comparatively few data.
These have been obtained from experiments concerning vigilance tests
and other tests of perception and performance, or the effects of
carbon monoxide on exercising cardiac and skeletal muscle and on
symptoms in patients with cardiovascular disease. There is an obvious
need to provide further data concerning larger numbers of subjects in
soundly designed experiments, the results of which should be properly
analysed. By such means it is hoped that dose-response and
concentration-response relationships may be established. The continued
refinement and application of epidemiological techniques must
complement experimental work.
(c) There are few data for assessing the possible consequences of
exposure of man to long-term, low concentrations of carbon monoxide.
There is a need to evaluate the possible effects of such exposures and
to determine the role of adaptation.
(d) Evidence that carbon monoxide plays a role in the observed
deleterious effects of smoking has been produced by experiments on
animals but more work is needed to confirm and extend these findings.
The hazards to the fetus of maternal smoking need to be evaluated and
the susceptibility of the fetus to carbon monoxide from whatever
source needs to be studied.
(e) The effects of comparatively low levels of carboxyhaemoglobin
on such skills as driving, and on perception in other tasks, need
further careful investigation. Not only must the possibility of the
enhancement of effects of carbon monoxide by other commonly occurring
factors be assessed, but there is a need to compare the effects of
carbon monoxide on vigilance and performance with the effects of such
common agents as therapeutic drugs, alcohol, fatigue, and food.
Attention is drawn to the difficulties inherent in the design of such
tests as well as to the problems involved in the assessment of the
health and social significance of the results.
(f) The possible effects of carbon monoxide on people living and
working at high altitudes (including aircraft pilots) have received
too little attention. There is a need for further work on this
subject.
2. CHEMISTRY AND ANALYTICAL METHODS
2.1 Physical and Chemical Properties
Carbon monoxide (CO) is a colourless, odourless, and tasteless gas
which is commonly formed during the incomplete combustion of
carbonaceous material. It is slightly lighter than air and only
slightly soluble in water. Carbon monoxide absorbs electromagnetic
radiation in the infrared region with the main absorption band centred
at 4.67 µm; this property is used for the measurement of carbon
monoxide concentrations in air. Some other physical properties of
carbon monoxide are listed in Table 1.
Table 1. Physical properties of carbon monoxide
Relative molecular mass 28.01
Critical point -140.2°C at 34.5 atm (3.5 MPa)
Melting point -205.1°C
Boiling point -191.5°C
Density, at 0°C, 1 atm 1.250 g/litre
at 25°C, 1 arm 1.145 g/litre
Specific gravity relative to air 0.967
Solubility in water at 0°C, 1 atm 3.54 ml/100 ml
at 25°C, 1 atm 2.14 ml/100 ml
at 37°C, 1 atm 1.83 ml/100 mla
Conversion factors:
at 0°C, 1 atm 1 mg/m3 = 0.800 ppmb
1 ppm = 1.250 mg/m3
at 25°C, 1 atm 1 mg/m3 = 0.873 ppm
1 ppm = 1.145 mg/m3
a Value obtained by graphic or calculated interpolation (Altman et al., 1971).
b Parts per million by volume.
While carbon monoxide is chemically inert under normal conditions
of temperature and pressure (25°C; 1 atm (101 kPa)), it becomes
reactive at higher temperatures and can act as a strong reducing
agent. At 90°C, it reacts with iodine pentoxide to produce iodine
vapour. At 150°C, it also releases mercury vapour from mercury(II)
oxide. Both reactions are used in the analytical chemistry of carbon
monoxide. The oxidation of carbon monoxide to carbon dioxide (CO2)
is accelerated by metallic catalysts such as palladium on silica gel,
or by a mixture of manganese and copper oxides (Hopcalite).
In forming carboxyhaemoglobin (HbCO), carbon monoxide reacts with
the iron in protohaem -- a constituent of haemoglobin -- and forms
strong coordination bonds. Thus carboxyhaemoglobin is toxic because it
is about 200 times more stable than oxyhaemoglobin (HbO2). Carbon
monoxide also combines reversibly with myoglobin and cytochromes,
including P-450.
The environmental chemistry of carbon monoxide is discussed in
section 4.2.
2.2 Methods of Measuring Carbon Monoxide in Ambient Air
Three methods are most commonly used for the routine estimation of
carbon monoxide in air. These are the continuous analysis method based
upon nondispersive infrared absorption spectroscopy (NDIR); the semi-
continuous analysis method using gas chromatographic techniques and a
semiquantitative method employing detector-tubes. Other methods
include catalytic oxidation, electrochemical analysis, mercury
displacement, and the dual isotope technique (WHO, 1976).
In the NDIR method, infrared radiation is divided into two beams
that are directed through a reference and a sample cell, respectively.
Any carbon monoxide introduced into the sample cell will absorb
radiation at the characteristic band centred at 4.67 µg, causing the
detector to produce an output signal proportional to the concentration
of carbon monoxide in the sample cell. NDIR analysers are produced by
several manufacturers in the form of continuous, automated
instruments. Good commercial instruments have a detection limit of
about 1 mg/m3 (0.87 ppm). Carbon dioxide and water vapour interfere
but there are several techniques to minimize this interference.
In chromatographic methods, carbon monoxide is first separated
from water vapour, carbon dioxide, and hydrocarbons. It is then
catalytically reduced to methane and passed through a flame ionization
detector, the output signal of which is proportional to the carbon
monoxide concentration in the air sample. The most common
concentration range in commercial instruments is from about 1 to
350 mg/m3 (1-300 ppm) but others are available with a range of about
0.02 to 1.00 mg/m3 (0.017-0.87 ppm). Gas chromatography is
particularly suitable, when low concentrations of carbon monoxide have
to be measured with a high degree of specificity.
The detector tube method is very simple and can be used for
estimating concentrations above 5 mg/m3. Air is drawn through
specially manufactured tubes containing a chemical agent that changes
colour if carbon monoxide is present and can be used to estimate
concentrations. The advantages and limitations of detector tubes are
further discussed in a WHO manual (WHO, 1976).
A well known method is based on the measurement of the temperature
rise caused by the catalytic oxidation of carbon monoxide. The limit
of detection is about 1 mg/m3. Most hydrocarbons will interfere
unless removed (NAS/NRC, 1977). For measurements in ambient air, the
sensitivity may not always be sufficient.
Electrochemical analysers (Hersch, 1964, 1966) are based on the
liberation by carbon monoxide of iodine from iodine pentoxide (at
150°C), which is then reduced at the cathode of a galvanic cell. The
current developed is a measure of the carbon monoxide concentration
present in the air sample.
A further highly sensitive method is based on the reduction of
mercury(II) oxide by carbon monoxide at a temperature between 170 and
210°C. Mercury vapour generated during this reaction is determined by
absorption spectrophotometry at 253.7 nm. This method, as modified by
Seller & Junge (1970), has a reported detection limit of about
3 µg/m3.
The slight difference in the fluorescence spectra of 16CO and
18CO is used for carbon monoxide determination by the so-called dual
isotope fluorescence method. Instruments using this principle are
available with ranges of 0-20 mg/m3 (0-17.5 ppm) and 0-200 mg/m3
(0-175 ppm) with a reported detection limit of about 0.2 mg/m3
(0.17 ppm). Other pollutants present cause very little interference
(McClatchie et al., 1972).
An important part of any carbon-monoxide measurement procedure is
the calibration technique. Many publications deal with this topic and
the Deutsche Industrienormen Ausschuss (DIN) and the International
Standard Organization (ISO) have special groups for establishing
suitable calibration standards.
2.3 Biological Monitoring
Blood carboxyhaemoglobin can be satisfactorily determined in a
venous blood sample, which should be collected in a closed container
containing an anticoagulant (dry sodium heparin or di-sodium ethylene-
diaminotetracetic acid, EDTA). Blood samples may be preserved for
several days prior to analysis if kept cold (4°C) and in the dark.
Complete mixing of blood must be attained if carbon monoxide and
haemoglobin are to be measured separately. Total haemoglobin is
conveniently determined by conversion to cyanmethaemoglobin (Van
Kampen & Zijlstra, 1961), which is then determined spectrophoto-
metrically (Drabkin & Austin, 1935).
Various methods are available for the determination of carboxy-
haemoglobin by spectrophotometry or by the liberation of carbon
monoxide (WHO, 1976). One method consists of measuring the absorbance
at 4 wavelengths in the Soret region (390-440 nm) of a blood sample
diluted to about 1:70 with an aqueous solution of ammonia (Small et
al., 1971). Carboxyhaemoglobin and methaemoglobin are estimated from
absorbance values, and oxyhaemoglobin is obtained from the difference.
The method is precise at low carboxyhaemoglobin concentrations (up to
25% saturation). A very convenient method is the automated
differential spectrophotometer (Malenfant et al., 1968), which is
available commercially as CO-oximeter. Simultaneous absorbance
measurements are made to determine the three component system (reduced
haemoglobin, oxyhaemoglobin and carboxyhaemoglobin) contained in a
haemolysed blood sample. The signals are processed and displayed in a
digital form as haemoglobin (g/100 ml) and the percentage of
oxyhaemoglobin and carboxyhaemoglobin. A method has recently been
described (Rossi-Bernardi et al., 1977) for the simultaneous
determination of four haemoglobin derivatives (deoxyhaemoglobin,
oxyhaemoglobin, methaemoglobin, carboxyhaemoglobin) and total oxygen
contents of 10 µl of whole blood. The spectrophotometric method of
Commins & Lawther (1965), which has been validated by Lily et al.
(1972), has the advantage of requiring only 0.01 ml blood obtained
from a finger prick sample.
Gas chromatography on molecular sieves with a suitable detection
system is probably the most satisfactory procedure for the measurement
of carbon monoxide liberated from carboxyhaemoglobin. Carbon monoxide
liberation is achieved through acidification. The method described by
Sotnikov (1971) requires only 0.1 ml of blood and has a limit of
detection of 0.01 ml of carbon monoxide per 100 ml of blood. Dahms &
Horvath (1974) have proposed a very accurate method for estimating low
concentrations of carboxyhaemoglobin. Table 2 compares some techniques
for the analysis of carboxyhaemoglobin in blood.
Another approach to the estimation of exposure to carbon monoxide
is by the analysis of expired air. The subject takes a deep breath and
holds it for 20 sec. The first 350-500 ml of expired air (dead air
space) is discarded and the remaining gas (alveolar air) is collected
in an aluminized mylar bag for analysis, using an NDIR instrument. The
value of the alveolar technique, pioneered by Sjöstrand (1948), is
based on the assumption that, during breath-holding, the lung is a
closed vessel in which blood carboxyhaemoglobin equilibrates with lung
gas and that Haldane's relationship (see section 6) applies.
Theoretically, the slope of the straight line relating %
carboxyhaemoglobin to alveolar pCO in ppm should be approximately
0.155 at sea level for % carboxyhaemoglobin values equivalent to a
carbon monoxide concentration between 0 and 50 ppm, and progressively
lower for higher concentrations (Coburn et al., 1965). Values
approximating to this theoretical ratio have been found experimentally
(Forbes et al., 1945; Malenfant et al., 1968). Although the alveolar
air method is less precise than the direct measurement of
carboxyhaemoglobin in blood, it can be used in epidemiological studies
and for general monitoring (McFarland, 1973) but cannot be used on
persons with chronic pulmonary disease.
Table 2. Comparison of techniques for the analysis of carboxyhaemoglobin in blooda
Detection method Sample Resolutionb Sample CVc Reference
volume (ml/dl) analysis (%)
(ml) time
(min)
Gasometric
Van Slyke 1.0 0.3 15 6 Horvath & Roughton (1942)
syringe-capillary 0.5 0.02 30 2-4 Roughton & Root (1945)
Optical
spectrophotometric 2.0 0.006 30 1.8 Coburn et al. (1964)
spectrophotometric 0.1 0.08 10 Small et al. ( 1971 )
spectrophotometric 0.4 0.10 3 Maas et al. (1970)
spectrophotometric 0.01 0.10 20 Commins & Lawther (1965)
Chromatographic
thermal conductivity 1.0 0.005 20d 1.8 McCredie & Jose (1967)
flame ionization 0.1 0.002 20 1.8 Collison et al. (1968)
thermal conductivity 1.0 0.001 30 2.0 Ayres et al. (1966)
thermal conductivity 0.25 0.006 3 1.7 Dahms & Horvath (1974)
a Adapted from: Dahms & Horvath (1974).
b Smallest detectable difference between duplicate determinations.
c Coefficient of variation based on samples containing less than 2.0 ml of carbon monoxide
per decilitre.
d Best estimate.
3. SOURCES OF CARBON MONOXIDE IN THE ENVIRONMENT
3.1 Natural Occurrence
The amount of carbon monoxide produced globally by natural sources
is at present uncertain. Several investigators have estimated that
natural sources (primarily oxidation of methane in the atmosphere and
emissions from the oceans) produce about ten times as much carbon
monoxide as man-made sources (Spedding, 1974). On the other hand, a
recent study concluded that the natural production of carbon monoxide
was much smaller and might be somewhat less than the emissions from
man-made sources (Seiler, 1975). If this is the case, man-made
emissions of carbon monoxide may play an important role in the global
carbon monoxide cycle.
Several estimates of the production of carbon monoxide by
atmospheric reactions have been made. Stevens et al. (1972) estimated
that in the northern hemisphere alone, more than 3 × 109 metric
tonnes of carbon monoxide are produced annually by the oxidation of
methane and other organic constituents. McConnell et al. (1971)
considered that biologically produced methane could be the source of
approximately 2.5 × 109 metric tonnes of carbon monoxide annually.
Subsequently, it was calculated, by Weinstock & Nicki (1972), that the
oxidation of methane alone could produce twenty-five times the
quantity of carbon monoxide generated by man's activities. Estimates
by Levy (1972) indicated that the oxidation of methane was a much
larger source of carbon monoxide that man-made sources.
The surface layers of the ocean are a second major natural source
of carbon monoxide. Linnenbom et al. (1973) calculated an upper limit
of 220 × 106 metric tonnes of carbon monoxide emitted from the oceans.
Using a model of the flux of gases across the air-sea interface, Liss
& Slater (1974) estimated a total ocean flux of carbon monoxide of
43 × 106 metric tonnes per year.
Among other natural sources of carbon monoxide are forest and
grass fires, volcanoes, marsh gases, and electric storms. Some carbon
monoxide is also formed in the upper atmosphere (above 75 km) by the
photo-dissociation of carbon dioxide (Altshuller & Bufallini, 1965;
Bates & Witherspoon, 1952). Another natural source of carbon monoxide
is rainwater, where production of carbon monoxide in the clouds is
tentatively attributed to the photochemical oxidation of organic
matter or the slight dissociation of carbon dioxide induced by
electrical discharges or both (Swinnerton et al., 1971). Some carbon
monoxide is also formed during germination of seeds and seedling
growth (Siegel et al., 1962; Wilks, 1959), by the action of
microorganisms on plant flavonoids (Westlake et al., 1961), and in
higher plants (Delwiche, 1970). Kelp may be a significant source of
carbon monoxide. Chapman & Tocher (1966) reported that some float
cells of kelp contained carbon monoxide concentrations as high as
916 mg/m3 (800 ppm).
Carbon monoxide is produced in measurable quantities in man and
animals as a by-product of haem catabolism.
3.2 Man-Made Sources
According to Jaffe (1973), global emissions of man-made carbon
monoxide in 1970 amounted to 360 million tonnes. Seller (1975)
calculated the carbon monoxide emissions for 1973 as 600 million
tonnes. A breakdown of Jaffe's estimate according to the type of
source is shown in Table 3. The motor vehicle was by far the largest
contributor accounting for 55% of total emissions. Other
transportation sources, certain industrial processes, waste disposal
and miscellaneous burning activities were responsible for the
remaining carbon monoxide emissions.
Table 3. Estimated global man-made emissions of carbon monoxide,
1970a
Source Emissions (106 metric tonnes)
Mobile
Motor vehicles: gasoline 197
diesel 2
Aircraft 5
Watercraft 18
Railroads 2
Other (nonhighway) motor vehicles 26
Stationary
Coal combustion 4
Oil combustion 1
industrial processes 41
Refuse disposal 23
Miscellaneous 41
Total 360
a From: Jaffe (1973).
The tremendous increase in the number and use of motor vehicles
during the past 30 years has been accompanied by a rapid increase in
carbon monoxide emissions. In the USA for example, the emission of
carbon monoxide rose from approximately 73 million tonnes in 1940 to
about 100 million tonnes in 1970 (US Environmental Protection Agency,
1973a). In 1968, the upward trend was reversed because of the initial
impact of motor vehicle emission controls. The rate at which carbon
monoxide is emitted from motor vehicles varies not only with vehicle
but also with the mode of operation of the vehicle. The emissions of
carbon monoxide by other mobile sources are comparatively small;
however, the emissions from locomotives, boats, and aircraft may
create local problems.
Among the stationary sources, the burning of waste material and
certain industrial processes generate substantial amounts of carbon
monoxide. Petroleum refineries, iron foundries, kraft-pulp mills,
carbon-black plants, and sintering processes are the major sources.
Emission rates for some of these processes are given in Table 4. The
burning of refuse, either in incinerators or openly, is an important
source of carbon monoxide. If uncontrolled, the emission rate of
carbon monoxide from incinerators is about 17.5 kg per tonne of refuse
burned. If burned openly, the emission rates can vary from about 25 to
60 kg per tonne, depending upon the type of refuse (US Environmental
Protection Agency, 1973b). The combustion of fossil fuels in electric
generating plants, industries, and the home, while resulting in the
emission of smaller quantities of carbon monoxide individually, may
constitute a major source when combined.
Any industrial process or operation, where incomplete combustion
of carbonaceous material occurs, may easily be of importance as far as
occupational exposure to carbon monoxide is concerned. Smelting of
iron ore, gas production works, gasworks and coke ovens, distribution
and use of both natural gas and coal gas, automobile manufacturing,
garages, and service stations are among the most important sources for
occupational exposure to carbon monoxide (Ministry of Labour, 1965).
It should also be emphasized that tobacco smoke is a most
significant source of man-made carbon monoxide in a closed environment
and that the carboxyhaemoglobin levels found in smokers are
consistently higher than those in nonsmokers.
Table 4. Emission rates for carbon monoxide in selected industrial
processesa
Source Emissions (uncontrolled)
Petroleum refineries
Fluid catalytic cracking units 39.2 kg/103 litre
Moving bed catalytic cracking units 10.8 kg/103 litre
Steel mills
Blast furnaces--ore charge 875 kg/tonne
Sintering 22 kg/tonne
Basic oxygen furnace 69.5 kg/tonne
Gray iron foundries
Cupola 72.5 kg/tonne
Carbon black
Channel process 16 750 kg/tonne
Thermal process negligible
Furnace process 2650 kg/tonne
a From: US Environmental Protection Agency (1973b).
4. ENVIRONMENTAL DISTRIBUTION AND TRANSFORMATION
4.1 Atmospheric Transport and Diffusion
The ambient air concentrations of carbon monoxide at locations
removed from man-made sources are low and variable. Junge et al.
(1971) reported that background levels of carbon monoxide in the lower
atmosphere may range from 0.01 to 0.23 mg/m3 (0.009-0.2 ppm).
Concentrations have been reported of 0.025 to 0.9 mg/m3
(0.022-0.8 ppm) in North Pacific marine air; 0.04 to 0.9 mg/m3
(0.036-0.8 ppm) in rural areas of California; 0.07 to 0.30 mg/m3
(0.06-0.26 ppm) at Point Barrow, Alaska; 0.06 to 0.8 mg/m3
(0.05-0.7 ppm) in Greenland; and about 0.07 mg/m3 (0.06 ppm) in the
South Pacific (Cavanagh et al., 1969; Goldman et al., 1973; Robbins et
al., 1968; Robinson & Robbins, 1970). Seiler & Junge (1969) observed
similar average concentrations over the North and South Atlantic Ocean
(0.20 mg/m3 and 0.06 mg/m3 (0.18 and 0.05 ppm) respectively).
Background levels of carbon monoxide are influenced by the origin of
the air masses and vertical distributions of carbon monoxide have been
reported by Seiler & Junge (1969, 1970). They found consistent carbon
monoxide concentrations of 0.15 mg/m3 (0.13 ppm) at an altitude of
10 km in both northern and southern hemispheres. In the troposphere,
average concentrations of 0.11 mg/m3 (0.10 ppm) were observed,
while, in the stratosphere, the concentrations ranged from 0.03 to
0.06 mg/m3 (0.027-0.05 ppm). A recent study (Goldman et al., 1973)
showed that a gradual decrease in carbon monoxide concentrations
occurred with increasing altitude ranging from approximately
0.09 mg/m3 (0.08 ppm) at 4 km to 0.05 mg/m3 (0.04 ppm) at 15 km.
4.2 Environmental Absorption and Transformation
The residence time of carbon monoxide in the atmosphere is
believed to be approximately 0.2 years. Background levels do not
appear to be increasing, indicating the presence of various scavenging
and removal mechanisms (sinks). Oxidation in the atmosphere and take-
up by the soil, vegetation, and inland fresh waters have been
identified as the major removal mechanisms.
The oceans act as reservoirs for carbon monoxide, since
considerable quantities are dissolved in the water. Because of the
equilibrium that exists, carbon monoxide is dissolved or released
according to conditions depending on the partial pressure of carbon
monoxide in the atmosphere and on the water temperature.
The carbon monoxide produced at the earth's surface migrates by
diffusion and eddy currents to the troposphere and stratosphere where
it is oxidized to carbon dioxide (CO2 by the hydroxyl (OH) radical.
This process, however, can account for only a portion of the carbon
monoxide oxidation. Calvert (1973) suggested several possible
reactions of carbon monoxide with other pollutants also involving the
hydroxyl radical and Westberg et al. (1971) demonstrated that carbon
monoxide can accelerate both the oxidation of nitric acid (NO) to
nitrogen dioxide (NO2) and the rate of ozone formation.
Microorganisms can metabolize carbon monoxide and large quantities
of these organisms are present in the soil. Ingersoll et al. (1974)
showed that desert soils took up carbon monoxide at the lowest rates
and tropical soils at the highest. Cultivated soils had a lower carbon
monoxide uptake rate than uncultivated soils, presumably because there
is less organic matter in the surface layer.
It has been reported that some plant species can remove carbon
monoxide from the atmosphere by oxidation to carbon dioxide or by
conversion to methane (Bidwell & Fraser, 1972). However, Ingersoll et
al. (1974) could not measure carbon monoxide removal by any plants
tested in an artificial atmosphere containing a carbon monoxide
concentration of 115 mg/m3 (100 ppm). The process of plant
respiration as a carbon monoxide sink still requires considerable
further study.
It is believed that inland fresh waters may remove some carbon
monoxide from the atmosphere. Rainwater contains appreciable
quantities of carbon monoxide and the runoff into the lakes and rivers
may account for additional removal.
5. ENVIRONMENTAL LEVELS AND EXPOSURES
5.1 Ambient Air Concentrations and Exposures
Carbon monoxide concentrations in the ambient air have been
measured in many large urban areas for a number of years. Although a
substantial body of data is now available, it is still not possible to
assess the overall human exposure to carbon monoxide adequately. Both
the extreme temporal and spatial variability of carbon monoxide
concentrations and the small number of monitoring stations in each
urban area make the assessment of human exposure difficult. The
available data, however, provide some indication of the patterns and
trends of urban carbon monoxide concentrations.
Concentrations in urban areas usually follow a very pronounced
diurnal pattern, and, although influenced by factors such as location
and meteorological conditions, their values are closely correlated
with the amount of motor vehicle traffic. Thus, although the exact
shape of the curve representing the temporal variation in carbon
monoxide concentrations over a day, varies with the situation, it
usually shows two peaks, corresponding to the morning and evening
traffic rush hours. Such curves have been established by many authors
(Colucci & Begeman, 1969; Göthe et al., 1969; Waller et al., 1965), by
means of continuous carbon monoxide monitoring. In general, an initial
peak is detected between 07h00 and 09h00 coinciding with heavy morning
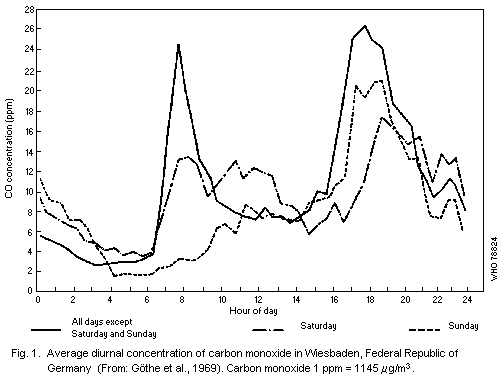
traffic, and another, in the late afternoon, as illustrated in Fig. 1.
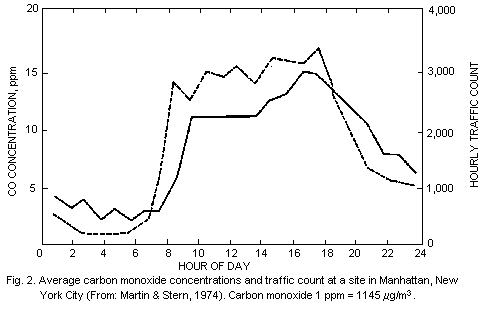
However, there may be exceptions to this typical pattern, as shown in
Fig. 2, which presents data from a monitoring station in New York City
(Martin & Stern, 1974). In this case, the traffic conditions were such
that carbon monoxide concentrations remained high during most of the
day. Because of changes in traffic patterns, the carbon monoxide
concentrations are usually lower at weekends than on weekdays and
follow a different diurnal pattern.
At any location, the concentration of carbon monoxide due to motor
vehicle traffic depends on the following specific variables:
(a) number of vehicles operating;
(b) engine characteristics of the operating vehicles (capacity,
gasoline or diesel, use of emission control devices);
(c) speed of traffic and gradient;
(d) temperature (as it affects the operating efficiency of the
engine).
More general variables include the meteorological conditions
(including wind speed and direction, and temperature gradients) and
the geometry of locality (shape and height of buildings, width of
street, etc.).
 Carbon monoxide concentrations are normally reported in terms of
8-h average concentrations. This averaging time has been used because
it takes from 4 to 12 h for the carboxyhaemoglobin levels in the human
body to reach equilibrium with the ambient carbon monoxide
concentrations. Two types of approaches have been used for calculating
the 8-h average (McMullen, 1975). One approach is to examine all
possible 8-h intervals and calculate a moving 8-h average (24, 8-h
averages each day). The other approach is to examine three
consecutive, nonoverlapping, 8-h intervals per day. It would appear
that the moving average approach offers some advantages in that it
approximates the human body's integrating response to cumulative
carbon monoxide exposure. In addition to the 8-h averages, carbon
monoxide concentrations are also reported in terms of other averaging
times and frequency distributions.
Carbon monoxide concentrations in the ambient air vary
considerably, not only among urban areas but within cities as well.
The maximum 8-h mean concentrations measured at more than 200
monitoring stations in the USA in 1973 ranged from less than
10 mg/m3 to 58 mg/m3 (8.7-51 ppm) with most of the values being
less than 30 mg/m3 (26 ppm) (Martin & Stern, 1974). The highest 8-h
mean concentration of 59 mg/m3 was observed at a monitoring station
in New York City. Data from a 38 station network in Japan showed that
the 8-h standard of 23 mg/m3 (20 ppm) was not exceeded (Environment
Agency, 1973). That carbon monoxide concentrations are extremely
variable within an urban area is illustrated by the maximum 8-h means
observed at monitoring stations in the metropolitan Los Angeles area
in 1973. They ranged from 7 mg/m3 (6 ppm) in the outlying areas to
49 mg/m3 (42.6 ppm) near the centre of the city. The maximum 1-h
mean concentrations exhibit a similar variability ranging from less
than 10 mg/m3 (8.7 ppm) in some areas to over 90 mg/m3 (78.3 ppm)
in others. The highest 1-h mean concentration observed in 1973 was
92 mg/m3 (80 ppm) at a monitoring station in Philadelphia; at more
than 80% of the stations the maximum 1-h concentration was below
50 mg/m3 43.5 ppm). In Japan the maximum 1-h concentrations ranged
from 5 mg/m3 to 48 mg/m3 (4.3-41.8 ppm).
Although the data presented above refer to measurements carried
out in the USA and Japan, it is indicated that very similar conditions
are prevalent in many parts of the world.
Annual average concentrations of carbon monoxide are not of much
value for assessing human exposure, although they do provide an
indication of the long-term trends. Annual average concentrations of
carbon monoxide in most locations fall well below 10 mg/m3 (8.7 ppm)
(Stewart et al., 1976).
Usually, the monitoring stations are located so that they can
provide representative information of air pollution within the
community. However, in certain areas, such as loading platforms and
underpasses, the concentrations found are much higher than those in
city streets. At Chicago post office loading platforms (Conlee et al.,
1967), ambient carbon monoxide concentrations ranged from 10 to
88 mg/m3 at various locations. Wright et al. (1975) determined the
levels of carbon monoxide encountered by pedestrians and street
workers in Toronto. They reported carbon monoxide levels ranging from
11 to 57 mg/m3, with much higher concentrations in poorly ventilated
underpasses and underground garages.
Industry also contributes to the pollution of the ambient air. Gas
generator plants (Nowara, 1975), smelters (Morel & Szemberg, 1971),
steelworks (Butt et al., 1974; Maziarka et al., 1974); plastics works
(Argirova, 1974, unpublished data)a, electric generating plants
(Grigorov et al., 1968), and mines (Notov, 1959) have all been
suggested, among other industrial activities, as sources of
environmental carbon monoxide pollution. Rural work places, especially
those involving intensive livestock production facilities, can provide
high ambient levels of carbon monoxide. Concentrations up to
136 mg/m3 (119 ppm) have been found in these conditions, with high
levels of carboxyhaemoglobin in both animals and workers (Long &
Donham, 1973).
5.2 Indoor Concentrations and Exposure
Carbon monoxide is widely generated indoors by heating, cooking,
and tobacco smoking. According to Yocom et al. (1971), gas heating
systems did not appear to affect indoor carbon monoxide
concentrations, but gas stoves, water heaters, and automobile activity
in attached garages could be major sources. These authors also
reported that carbon monoxide concentrations were generally unrelated
to outdoor levels. Obviously, peak concentrations in the kitchen were
observed during meal times, Sofoluwe (1968) reported extremely high
concentrations of carbon monoxide in Nigerian dwellings, where
firewood was used for cooking. During the preparation of meals,
average carbon monoxide concentrations were reported to be over
1000 mg/m3 (870 ppm) with peak levels as high as 3400 mg/m3
(2960 ppm). Sidorenko et al. (1970) found an indoor level of carbon
monoxide of 32.3 mg/m3 (28 ppm) some two and a half hours after
domestic gas combustion began, when there was no ventilation, but only
13.4 mg/m3 (11.6 ppm) when ventilation was provided. The use of
improved stoves resulted in better conditions (Sidorenko et al.,
1972).
a "Argirova, M. [Atmospheric air pollution by a plant processing
plastics.] In: Proceedings of the First National Conference on
Sanitary Chemistry of the Air, Sofia, Bulgaria, 26-27 November,
1974 (in Bulgarian).
Rench & Savage (1976) have also investigated winter levels of
carbon monoxide in the home. Outdoor concentrations were lower than
those found indoors. Age of building, type of appliance, heating
sources, and socio-economic status were statistically significantly
related to indoor levels of carbon monoxide, higher levels occurring
in the kitchens of old houses and in homes belonging to families in
lower income groups. Levels were also higher in kitchens with space
wall heaters compared with those with forced air, gravity feed, or hot
water heating systems. Places of recreation may be problem areas.
Excessive levels of carbon monoxide were found in ice-skating areas
where ice-resurfacing machines were used. Levels as high as
348 mg/m3 (304 ppm) were found in such an arena after complaints of
illness among children skating there were reported to the local health
department (Johnson et al., 1975). Improperly regulated space heaters
in such premises could also produce high concentrations of carbon
monoxide. This was recently reported to have occurred in an Alaskan
ice-skating arena.
That outdoor concentrations of carbon monoxide can sometimes
strongly influence indoor levels was illustrated in a study conducted
in New York City (Lee, 1972). As shown in Fig. 3, the concentrations
inside and outside an apartment building followed the same general
pattern and were closely related to nearby traffic flows.
Relatively high concentrations of carbon monoxide have also been
observed inside the passenger compartment of motor vehicles (Borst,
1970). Carbon monoxide may enter the compartment from faulty or
damaged exhaust systems or from the surrounding air, in road traffic.
The carbon monoxide level in the compartment is often higher than that
found outside the vehicle. Haagen-Smit (1966) found an average
concentration in a vehicle passenger compartment of 42 mg/m3
(36.5 ppm) on a Los Angeles freeway during the rush hour traffic
(higher concentrations have been reported by Aronow et al. (1972).
It should be emphasized that cigarette smoking is the most common
source of carbon monoxide for the general population (Table 5).
Exposure to smoking primarily affects the carboxyhaemoglobin level
of the smoker himself (Kahn et al., 1974; Landaw, 1973). In some
circumstances, such as poorly ventilated enclosed spaces, the tobacco
smoke may affect all of the occupants (section 8.1.6).
Carbon monoxide concentrations are normally reported in terms of
8-h average concentrations. This averaging time has been used because
it takes from 4 to 12 h for the carboxyhaemoglobin levels in the human
body to reach equilibrium with the ambient carbon monoxide
concentrations. Two types of approaches have been used for calculating
the 8-h average (McMullen, 1975). One approach is to examine all
possible 8-h intervals and calculate a moving 8-h average (24, 8-h
averages each day). The other approach is to examine three
consecutive, nonoverlapping, 8-h intervals per day. It would appear
that the moving average approach offers some advantages in that it
approximates the human body's integrating response to cumulative
carbon monoxide exposure. In addition to the 8-h averages, carbon
monoxide concentrations are also reported in terms of other averaging
times and frequency distributions.
Carbon monoxide concentrations in the ambient air vary
considerably, not only among urban areas but within cities as well.
The maximum 8-h mean concentrations measured at more than 200
monitoring stations in the USA in 1973 ranged from less than
10 mg/m3 to 58 mg/m3 (8.7-51 ppm) with most of the values being
less than 30 mg/m3 (26 ppm) (Martin & Stern, 1974). The highest 8-h
mean concentration of 59 mg/m3 was observed at a monitoring station
in New York City. Data from a 38 station network in Japan showed that
the 8-h standard of 23 mg/m3 (20 ppm) was not exceeded (Environment
Agency, 1973). That carbon monoxide concentrations are extremely
variable within an urban area is illustrated by the maximum 8-h means
observed at monitoring stations in the metropolitan Los Angeles area
in 1973. They ranged from 7 mg/m3 (6 ppm) in the outlying areas to
49 mg/m3 (42.6 ppm) near the centre of the city. The maximum 1-h
mean concentrations exhibit a similar variability ranging from less
than 10 mg/m3 (8.7 ppm) in some areas to over 90 mg/m3 (78.3 ppm)
in others. The highest 1-h mean concentration observed in 1973 was
92 mg/m3 (80 ppm) at a monitoring station in Philadelphia; at more
than 80% of the stations the maximum 1-h concentration was below
50 mg/m3 43.5 ppm). In Japan the maximum 1-h concentrations ranged
from 5 mg/m3 to 48 mg/m3 (4.3-41.8 ppm).
Although the data presented above refer to measurements carried
out in the USA and Japan, it is indicated that very similar conditions
are prevalent in many parts of the world.
Annual average concentrations of carbon monoxide are not of much
value for assessing human exposure, although they do provide an
indication of the long-term trends. Annual average concentrations of
carbon monoxide in most locations fall well below 10 mg/m3 (8.7 ppm)
(Stewart et al., 1976).
Usually, the monitoring stations are located so that they can
provide representative information of air pollution within the
community. However, in certain areas, such as loading platforms and
underpasses, the concentrations found are much higher than those in
city streets. At Chicago post office loading platforms (Conlee et al.,
1967), ambient carbon monoxide concentrations ranged from 10 to
88 mg/m3 at various locations. Wright et al. (1975) determined the
levels of carbon monoxide encountered by pedestrians and street
workers in Toronto. They reported carbon monoxide levels ranging from
11 to 57 mg/m3, with much higher concentrations in poorly ventilated
underpasses and underground garages.
Industry also contributes to the pollution of the ambient air. Gas
generator plants (Nowara, 1975), smelters (Morel & Szemberg, 1971),
steelworks (Butt et al., 1974; Maziarka et al., 1974); plastics works
(Argirova, 1974, unpublished data)a, electric generating plants
(Grigorov et al., 1968), and mines (Notov, 1959) have all been
suggested, among other industrial activities, as sources of
environmental carbon monoxide pollution. Rural work places, especially
those involving intensive livestock production facilities, can provide
high ambient levels of carbon monoxide. Concentrations up to
136 mg/m3 (119 ppm) have been found in these conditions, with high
levels of carboxyhaemoglobin in both animals and workers (Long &
Donham, 1973).
5.2 Indoor Concentrations and Exposure
Carbon monoxide is widely generated indoors by heating, cooking,
and tobacco smoking. According to Yocom et al. (1971), gas heating
systems did not appear to affect indoor carbon monoxide
concentrations, but gas stoves, water heaters, and automobile activity
in attached garages could be major sources. These authors also
reported that carbon monoxide concentrations were generally unrelated
to outdoor levels. Obviously, peak concentrations in the kitchen were
observed during meal times, Sofoluwe (1968) reported extremely high
concentrations of carbon monoxide in Nigerian dwellings, where
firewood was used for cooking. During the preparation of meals,
average carbon monoxide concentrations were reported to be over
1000 mg/m3 (870 ppm) with peak levels as high as 3400 mg/m3
(2960 ppm). Sidorenko et al. (1970) found an indoor level of carbon
monoxide of 32.3 mg/m3 (28 ppm) some two and a half hours after
domestic gas combustion began, when there was no ventilation, but only
13.4 mg/m3 (11.6 ppm) when ventilation was provided. The use of
improved stoves resulted in better conditions (Sidorenko et al.,
1972).
a "Argirova, M. [Atmospheric air pollution by a plant processing
plastics.] In: Proceedings of the First National Conference on
Sanitary Chemistry of the Air, Sofia, Bulgaria, 26-27 November,
1974 (in Bulgarian).
Rench & Savage (1976) have also investigated winter levels of
carbon monoxide in the home. Outdoor concentrations were lower than
those found indoors. Age of building, type of appliance, heating
sources, and socio-economic status were statistically significantly
related to indoor levels of carbon monoxide, higher levels occurring
in the kitchens of old houses and in homes belonging to families in
lower income groups. Levels were also higher in kitchens with space
wall heaters compared with those with forced air, gravity feed, or hot
water heating systems. Places of recreation may be problem areas.
Excessive levels of carbon monoxide were found in ice-skating areas
where ice-resurfacing machines were used. Levels as high as
348 mg/m3 (304 ppm) were found in such an arena after complaints of
illness among children skating there were reported to the local health
department (Johnson et al., 1975). Improperly regulated space heaters
in such premises could also produce high concentrations of carbon
monoxide. This was recently reported to have occurred in an Alaskan
ice-skating arena.
That outdoor concentrations of carbon monoxide can sometimes
strongly influence indoor levels was illustrated in a study conducted
in New York City (Lee, 1972). As shown in Fig. 3, the concentrations
inside and outside an apartment building followed the same general
pattern and were closely related to nearby traffic flows.
Relatively high concentrations of carbon monoxide have also been
observed inside the passenger compartment of motor vehicles (Borst,
1970). Carbon monoxide may enter the compartment from faulty or
damaged exhaust systems or from the surrounding air, in road traffic.
The carbon monoxide level in the compartment is often higher than that
found outside the vehicle. Haagen-Smit (1966) found an average
concentration in a vehicle passenger compartment of 42 mg/m3
(36.5 ppm) on a Los Angeles freeway during the rush hour traffic
(higher concentrations have been reported by Aronow et al. (1972).
It should be emphasized that cigarette smoking is the most common
source of carbon monoxide for the general population (Table 5).
Exposure to smoking primarily affects the carboxyhaemoglobin level
of the smoker himself (Kahn et al., 1974; Landaw, 1973). In some
circumstances, such as poorly ventilated enclosed spaces, the tobacco
smoke may affect all of the occupants (section 8.1.6).
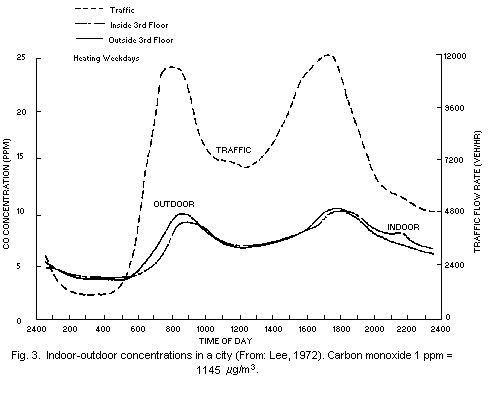 Table 5. Percentage carboxyhaemoglobin levels in smokers and
nonsmokersa
Description Nonsmokers Smokers
mean range mean range
UK pregnant women 1.1 3.6
Meat porters 1.6 5.1
Office workers 1.3 6.2
London office workers 1.12 0.1-2.7 5.5 2.2-13.0
29 000 USA blood donors 1.39 0.4-6.9 5.57 0.8-11.9
3311 California longshoremen 1.3 5.9
Munich population 2.36 7.38
Rural Bavarians 1.03 6.06
a From: Wakeham (1976).
5.3 Occupational Exposure
Many occupational groups are subject to high carbon monoxide
exposure. These include, traffic policemen, garage personnel, workers
in the metallurgical, petroleum, gas, and chemical industries, and
firefighters.
An average carbon monoxide level of 172 mg/m3 (149.6 ppm) was
recorded in the air of a Paris police garage between 07h30 and 08h00
and 205 mg/m3 (179 ppm) between 19h30 and 20h00 (Chovin, 1967).
Similarly, Trompeo et al. (1964) found an average level of 112 mg/m3
(97.4 ppm) in 12 underground garages in Rome; a maximum concentration
of 570 mg/m3 (498.5 ppm) was recorded. In similar studies in
enclosed garages with capacities of 300-500 vehicles, where the only
ventilation was via the entrance, the average concentration of carbon
monoxide for a period from 08h00 to 17h00 was 72 mg/m3 (62.6 ppm) in
the summer and 61 mg/m3 (53 ppm) in the winter (Ramsey, 1967a).
The same author (Ramsey, 1967b) reported that the carboxyhaemo-
globin levels of 14 nonsmoking parking garage employees, exposed to an
average concentration of carbon monoxide of 68 mg/m3 (59 ppm),
increased from 1.5 to 7.3%. He also noted that, in smokers exposed to
the same environment, initial carboxyhaemoglobin levels of 2.9% rose
to 9.3% at the end of the day. Smokers not exposed to this environment
had final levels of only 3.9%. Ramsey stated that occupational
exposure played a greater role than smoking in increasing
carboxyhaemoglobin levels. However, in a study of some 350 Canadian
garage and service station personnel, Buchwald (1969) found that
cigarette smoking was the more significant contributor to the high
levels of carboxyhaemoglobin measured. Of the smokers, 70% had levels
in excess of 5%, while a similar level was found in only 30% of the
nonsmokers. Breysse & Bovee (1969) used expired air samples to
determine exposure to carbon monoxide in stevedores, gasoline-powered
lift truck drivers, and winch operators. They made some 700 estimates
of carboxyhaemoglobin, almost 6% of which exceeded 10%. Seven percent
of the stevedores and 18% of the lift truck operators had
carboxyhaemoglobin levels over 10%. Smoking contributed substantially
to the attainment of these high levels. Carboxyhaemoglobin levels as
high as 10% were also found in dock workers in studies by Petrov
(1968). Inspectors at USA-Mexico border crossing stations were found
to be exposed to ambient levels of carbon monoxide that fluctuated
between 6 and 195 mg/m3 (5 and 170.5 ppm). During one hour of an
evening shift, ambient carbon monoxide averaged 131 mg/m3 (114 ppm).
Carboxyhaemoglobin levels of smokers and nonsmokers which were 4.0 and
1.4% respectively, prior to duty rose to 7.6 and 3.8% (Cohen et al.,
1971).
Data obtained by Balabaeva & Kalpazanov (1974) in studies on
traffic policemen in 4 large towns in Bulgaria were similar to those
Chovin (1967) obtained in his study on the exposure of Paris policemen
to carbon monoxide. However, Göthe et al. (1969) found relatively low
levels of carboxyhaemoglobin in Swedish traffic policemen. When blood
lead levels were studied in 50 London taxi drivers using their
carboxyhaemoglobin levels as an index of exposure to exhaust products,
carboxyhaemoglobin levels were higher in day drivers than in night
drivers and in smokers than in nonsmokers. Concentrations in all
groups ranged from 0.4 to 9.7%, and those in nonsmokers (day and
night) from 0.4 to 3.0%.
Direct measurement of carboxyhaemoglobin levels in firefighters
engaged in prolonged firefighting indicated that 10% had values
exceeding 10% (Gordon & Rogers, 1969). However, the high levels of
carboxyhaemoglobin found in the control (non-firefighting) groups make
these conclusions somewhat uncertain. Goldsmith's study (1970) of
longshoremen suggested that the expired alveolar concentrations of
carbon monoxide in smokers were age-related. For example, subjects
smoking one packet of 20 cigarettes per day in the 45-54 year age
group had an average alveolar value of 31 mg/m3 (27 ppm), while the
75-84 year age group had an average of only 16 mg/m3 (14.4 ppm).
Nonsmokers did not exhibit this age-related pattern, since even up to
84 years of age, alveolar concentrations remained at the same level
4 mg/m3 (3.6 ppm).
Exposure to low levels of carbon monoxide may have a significant
influence on the health and efficiency of these workers but this
awaits further study. Many other instances of occupational exposure
are available but most studies are complicated by the unknown or
unreported smoking habits of the workers under study (Grut, 1949).
5.4 Carboxyhaemoglobin Levels in the General Population
The most extensive study of blood carboxyhaemoglobin levels in the
general population was carried out by Stewart and his associates
(1973c and 1974), who took blood samples in 18 urban areas and in some
small towns in the States of New Hampshire and Vermont, USA. Similar
blood samples were evaluated for carboxyhaemoglobin levels by Kahn et
al. (1974), Davis & Gantner (1974), and Wallace et al. (1974) in
metropolitan St Louis. A total of 45 649 blood donors provided blood
for analysis. Stewart's subjects (29 000 including 1016 from St Louis)
were studied in March 1971 and Kahn's (16 649 subjects all from St
Louis) from October 1971 to October 1972. Stewart et al. concluded
that 45% of all the non-smoking donors exposed to ambient carbon
monoxide had a carboxyhaemoglobin saturation greater than 1.5%, while
Kahn's group reported a level of less than 1%.
6. METABOLISM
The primary factors that determine the final level of
carboxyhaemoglobin are: the amount of inspired carbon monoxide; minute
alveolar ventilation at rest and during exercise; endogenous carbon
monoxide production; blood volume; barometric pressure; and the
relative diffusion capability of the lungs. The rate of diffusion from
the alveoli and the binding of carbon monoxide with the blood
haemoglobin are the steps limiting the rate of uptake into the blood.
6.1 Endogenous Carbon Monoxide Production
Carbon monoxide can be produced endogenously from the catabolism
of pyrrole rings, originating from haemoglobin, myoglobin,
cytochromes, and other haem-containing pigments. Haem catabolism is
the main source of endogenous carbon monoxide production, but recent
in vitro investigations suggest additional endogenous sources, e.g.,
lipid peroxidation (Wolff & Bidlack, 1976).
The endogenous carboxyhaemoglobin level in man is estimated to be
about 0.1-1.0%. Increased production of endogenous carbon monoxide has
been found in haemolytic anaemias (Coburn et al., 1966), and could be
expected in haematomas and after exposure to certain toxic chemicals
capable of causing haemolysis. The liver is probably the major source
of endogenous carbon monoxide as a consequence of an increase in liver
cytochromes induced by certain drugs (Coburn, 1970a), or in porphyria
cutanea tarda (acquired or symptomatic porphyria) (White, 1970).
Similarly, bone marrow can become a major source of carbon monoxide in
haematological diseases, such as sideroblastic anaemia, being
characterized by ineffective erythropoiesis (White, 1970). In neonates
endogenous carbon monoxide can be markedly elevated, as well as in
females during the progesterone phase of the menstrual cycle
(Delivoria-Papadopoulos et al., 1970; Longo, 1970), and even more so
during pregnancy (see section 8.2.3). Another important cause of
carboxyhaemoglobin elevation is exposure to several methane-derived
halogenated hydrocarbons (see section 8.1.5).
6.2 Absorption
The classical absorption curves of Forbes et al. (1945) have been
re-evaluated for man at rest by Peterson & Stewart (1970) who exposed
volunteers to a variety of different concentrations of carbon monoxide
for periods ranging from 0.5 to 24 h. Using a regression approach,
they derived the following empirical relationship for blood carboxy-
haemoglobin as a function of ambient carbon monoxide concentration
and exposure time:
log10 y = 0.85753 log10 x + 0.62995 log10 t - 2.29519
where y = % carboxyhaemoglobin
x = carbon monoxide concentration in ppm
t = time in minutes
Although these new data come closer to presenting potential uptake in
individuals exposed to present-day ambient concentrations of carbon
monoxide, they do not apply to the uptake that would occur in active
man. Furthermore, they fit only the linear, non-steady-state portion
of the absorption curve. Ott & Mage (1974) have objected to the
Peterson & Stewart equation as being basically a static model. They
indicate that the use of averaging periods as long as 1 h compared
with 10-15 min introduces an error into recorded urban concentrations.
This error may be serious if many sharp, ambient peaks are present.
The data presented by Forbes et al. (1945) are still the only
experimental information available that takes ventilation into account
and, even so, this information is inadequate since the full range of
inspired, ventilatory volumes possible in exercising man was not
considered. Coburn et al. (1965) have developed an equation from which
it is possible to calculate blood carboxyhaemoglobin (Peterson &
Stewart, 1970) as a function of time, considering appropriate
physiological and physical factors. Their basic differential equation
is as follows:
d(CO) [HbCO] pCo2 1 pIco
= Vco - × × +
dt [HbO2] M 1 pB - 47 1 pB - 47
+ +
DL VA DL VA
d(CO)
where is the rate of change of CO in the body (ml/min)
dt
[HbCO] is the concentration of CO in the blood (ml CO/ml blood)
[HbO2] is the concentration of O2 in the blood (ml O2/ml blood)
DL is the diffusion capacity of the lung (ml/min/mmHg)
VA is the alveolar ventilation rate (ml/min)
pB is barometric pressure (mmHg)
pIco is inspired carbon monoxide pressure (mmHg)
pCo2 is the mean pulmonary capillary oxygen pressure (mmHg)
M is the Haldane constant (220-240 at pH 7.4)
Vco is the rate of endogenous CO production (ml/min)
One solution of the equation depends on the assumption that
pCo2, and HbO2 are constant and independent of HbCO. However,
HbO2 concentration depends upon HbCO in a complex way and, in
general, solution of the equation requires some special computer
techniques using a second approximation; these have been attempted and
general solutions are available (Coburn et al., 1965). Further
development of Coburn's concepts will undoubtedly improve the basis on
which theoretical uptakes can be calculated. For immediate, practical
purposes, calculations based on the Haldane formula (see section 6.3)
can be used.
Table 6 indicates the equilibrium percentage saturation of the
haemoglobin with carbon monoxide at various alveolar pressures of
carbon monoxide, calculated from the Haldane formula:a
% HbCO 230 pAco
=
% HbO2 pAo2
where pAco and pAo2, are the alveolar pressures of carbon
monoxide and oxygen respectively.
Table 6. Percent carboxyhaemoglobin versus carbon monoxide alveolar pressure
% HbCO mg/m3 ppm % in air Pa Torr
0.87 5.7 5 0.0005 0.506 0.0038
1.73 11.5 10 0.001 1.013 0.0076
3.45 23.0 20 0.002 2.026 0.0152
5.05 34.5 30 0.003 3.305 0.0248
6.63 46.0 40 0.004 4.052 0.0304
8.16 57.5 50 0.005 5.065 0.0380
9.63 69.0 60 0.006 6.078 0.0456
11.08 80 70 0.007 7.091 0.0532
12.46 92.0 80 0.008 8.104 0.0608
13.80 103.0 90 0.009 9.117 0.0684
15.11 114.5 100 0.010 10.130 0.0760
16.37 126.0 110 0.011 11.143 0.0836
17.60 130.0 120 0.012 12.156 0.0912
18.78 149.0 130 0.013 13.170 0.0988
19.95 160.0 140 0.014 14.183 0.1064
21.05 172.0 150 0.015 15.196 0.1140
22.15 183.0 160 0.016 16.209 0.1216
23.23 195.0 170 0.017 17.209 0.1291
24.26 206.0 180 0.018 18.235 0.1368
25.25 218.0 190 0.019 19.221 0.1442
26.22 229.0 200 0.020 20.261 0.1520
a The alveolar oxygen pressure is assumed to be 13 kPa (98 Torr)
6.3 Reactions with Body Tissues and Fluids
An adequate oxygen supply to maintain tissue metabolism is
provided by the integrated functioning of the respiratory and
cardiovascular systems to transport oxygen from the ambient air to the
various tissues of the body. Nearly all of the oxygen, except that
dissolved in plasma, is bound reversibly to the haemoglobin contained
within the erythrocytes. The most significant chemical characteristic
of carbon monoxide is that it also is reversibly bound by haemoglobin.
Therefore, it is a competitor with oxygen for the four binding sites
on the haemoglobin molecule.
The equilibrium constant M expresses the relative affinity of
haemoglobin for carbon monoxide and oxygen when the concentration of
reduced haemoglobin is minimal. This Haldane constant (Douglas et al.,
1912) is defined by the following equation:
HbCO pCO
= M x
HbO2 pO2
where pCO and pO2 represent the equilibrium partial gas
pressures: each pressure being the same in the erythrocytes or
haemoglobin solution as in the equilibrated gas phase. [HbCO] and
[HbO2] are the concentrations of carboxyhaemoglobin and
oxyhaemoglobin, respectively. The value of M is about 200 in most
species, in spite of the fact that carbon monoxide combines with
haemoglobin more slowly than oxygen. Carboxyhaemoglobin dissociates
very slowly due to the tight binding of carbon monoxide to
haemoglobin. Technically, it is not possible to measure the rate of
dissociation of carbon monoxide from partly saturated haemoglobin. The
dissociation velocity constant has been measured by only a few
investigators on sheep and human haemoglobin fully saturated with
carbon monoxide. Roughton (1970), however, has presented the most
comprehensive analysis of the interaction of carbon monoxide with
erythrocyte haemoglobin, showing clearly that, for man, M is higher
than commonly thought, i.e., it is more likely to be between 240 and
250; however, the value for M depends on the point of reference on
the dissociation curve.
Solution of Haldane's equation would give an approximate level of
carboxyhaemoglobin, e.g., exposure to ambient air containing carbon
monoxide levels of 28.7, 57.5, or 115 mg/m3 (25, 50, or 100 ppm)
would lead to carboxyhaemoglobin saturations of approximately 4.8, 9.2
and 16.3%, if the arterial oxygen pressure were 10.7 kPa (80 Torr).
The carbon monoxide enters the lungs with each breath and diffuses
across the alveolar-capillary membrane in a manner similar to oxygen.
If air with a constant concentration of carbon monoxide is breathed
for several hours, the rate of uptake of carbon monoxide decreases
approximately exponentially until an equilibrium state is attained in
which the partial pressure of carbon monoxide in the pulmonary
capillary blood is the same as that in the alveolar.
Oxygen transport in the blood is best described by the
oxyhaemoglobin dissociation curve (Fig. 4). In the presence of
carboxyhaemoglobin, this curve is no longer typically sigmoid but is
shifted to the left so that a lower oxygen pressure is present for the
same oxyhaemoglobin saturation compared with blood without
carboxyhaemoglobin (Roughton & Darling, 1944). Fig. 5 illustrates the
extent of the Haldane shift to the left more clearly than the typical
curves of Fig. 4. Mulhausen et al. (1968) illustrated this shift by
showing that the half saturation oxygen tension shifted from 3.6 to
3.1 kPa (26.7 to 23.2 Torr) in subjects who were intermittently
exposed to a high concentration of ambient carbon monoxide. Carbon
monoxide not only diminishes the total amount of oxygen available by
direct replacement of oxygen (Fig. 4) but also alters the dissociation
of the remaining oxygen so that it is held more tenaciously by
haemoglobin and released at lower oxygen tensions. The oxyhaemoglobin
curve in the presence of carboxyhaemoglobin progressively resembles
the simple oxygen dissociation curve of myoglobin. Myoglobin is a haem
compound with only one haem unit per molecule and does not exhibit
haem-haem interactions. It is possible that the combination of one or
more of the four haem groups in haemoglobin with carbon monoxide
decreases the haem-haem interactions of the remaining haem units and
results in a molecule approaching the behaviour of myoglobin.
Any consideration of the toxicity of carbon monoxide must include
not only the decrease in the oxygen carrying capacity of haemoglobin
but also the interference with oxygen release at the tissue level.
The venous pO2 values expected to result from various
carboxyhaemoglobin levels can be calculated (Forster, 1970; Permutt &
Fahri, 1969). If blood flow and metabolic rate remain constant,
equilibration with an ambient carbon monoxide concentration of
230 mg/m3 or 200 ppm (25% carboxyhaemoglobin) will lower venous
pO2 from 5.3 to less than 4 kPa (40 to less than 30 Torr). A
similar degree of venous hypoxaemia results from an ascent to an
altitude of 3658 metres or a 35% reduction in oxygen capacity in an
anaemic patient. It can also be calculated that, at 5%
carboxyhaemoglobin, there will be only a slight drop in the mixed
venous pO2. Even more valuable relationships can be obtained by
plotting oxygen content against the partial pressure of oxygen
(Roughton & Darling, 1944). The difference in oxygen content at
various percentages of carboxyhaemoglobin from 0 to 20 reveal the
minimal magnitude of the relative unavailability of oxygen due to the
Haldane effect (Fig. 4). It was this evaluation that led Roughton &
Darling to conclude that carboxyhaemoglobin concentrations of less
than 40% produce relatively easily compensated restrictions in the
amount of oxygen available for tissue delivery. This can only be
applied to subjects with normal respiratory and circulatory systems.
Table 5. Percentage carboxyhaemoglobin levels in smokers and
nonsmokersa
Description Nonsmokers Smokers
mean range mean range
UK pregnant women 1.1 3.6
Meat porters 1.6 5.1
Office workers 1.3 6.2
London office workers 1.12 0.1-2.7 5.5 2.2-13.0
29 000 USA blood donors 1.39 0.4-6.9 5.57 0.8-11.9
3311 California longshoremen 1.3 5.9
Munich population 2.36 7.38
Rural Bavarians 1.03 6.06
a From: Wakeham (1976).
5.3 Occupational Exposure
Many occupational groups are subject to high carbon monoxide
exposure. These include, traffic policemen, garage personnel, workers
in the metallurgical, petroleum, gas, and chemical industries, and
firefighters.
An average carbon monoxide level of 172 mg/m3 (149.6 ppm) was
recorded in the air of a Paris police garage between 07h30 and 08h00
and 205 mg/m3 (179 ppm) between 19h30 and 20h00 (Chovin, 1967).
Similarly, Trompeo et al. (1964) found an average level of 112 mg/m3
(97.4 ppm) in 12 underground garages in Rome; a maximum concentration
of 570 mg/m3 (498.5 ppm) was recorded. In similar studies in
enclosed garages with capacities of 300-500 vehicles, where the only
ventilation was via the entrance, the average concentration of carbon
monoxide for a period from 08h00 to 17h00 was 72 mg/m3 (62.6 ppm) in
the summer and 61 mg/m3 (53 ppm) in the winter (Ramsey, 1967a).
The same author (Ramsey, 1967b) reported that the carboxyhaemo-
globin levels of 14 nonsmoking parking garage employees, exposed to an
average concentration of carbon monoxide of 68 mg/m3 (59 ppm),
increased from 1.5 to 7.3%. He also noted that, in smokers exposed to
the same environment, initial carboxyhaemoglobin levels of 2.9% rose
to 9.3% at the end of the day. Smokers not exposed to this environment
had final levels of only 3.9%. Ramsey stated that occupational
exposure played a greater role than smoking in increasing
carboxyhaemoglobin levels. However, in a study of some 350 Canadian
garage and service station personnel, Buchwald (1969) found that
cigarette smoking was the more significant contributor to the high
levels of carboxyhaemoglobin measured. Of the smokers, 70% had levels
in excess of 5%, while a similar level was found in only 30% of the
nonsmokers. Breysse & Bovee (1969) used expired air samples to
determine exposure to carbon monoxide in stevedores, gasoline-powered
lift truck drivers, and winch operators. They made some 700 estimates
of carboxyhaemoglobin, almost 6% of which exceeded 10%. Seven percent
of the stevedores and 18% of the lift truck operators had
carboxyhaemoglobin levels over 10%. Smoking contributed substantially
to the attainment of these high levels. Carboxyhaemoglobin levels as
high as 10% were also found in dock workers in studies by Petrov
(1968). Inspectors at USA-Mexico border crossing stations were found
to be exposed to ambient levels of carbon monoxide that fluctuated
between 6 and 195 mg/m3 (5 and 170.5 ppm). During one hour of an
evening shift, ambient carbon monoxide averaged 131 mg/m3 (114 ppm).
Carboxyhaemoglobin levels of smokers and nonsmokers which were 4.0 and
1.4% respectively, prior to duty rose to 7.6 and 3.8% (Cohen et al.,
1971).
Data obtained by Balabaeva & Kalpazanov (1974) in studies on
traffic policemen in 4 large towns in Bulgaria were similar to those
Chovin (1967) obtained in his study on the exposure of Paris policemen
to carbon monoxide. However, Göthe et al. (1969) found relatively low
levels of carboxyhaemoglobin in Swedish traffic policemen. When blood
lead levels were studied in 50 London taxi drivers using their
carboxyhaemoglobin levels as an index of exposure to exhaust products,
carboxyhaemoglobin levels were higher in day drivers than in night
drivers and in smokers than in nonsmokers. Concentrations in all
groups ranged from 0.4 to 9.7%, and those in nonsmokers (day and
night) from 0.4 to 3.0%.
Direct measurement of carboxyhaemoglobin levels in firefighters
engaged in prolonged firefighting indicated that 10% had values
exceeding 10% (Gordon & Rogers, 1969). However, the high levels of
carboxyhaemoglobin found in the control (non-firefighting) groups make
these conclusions somewhat uncertain. Goldsmith's study (1970) of
longshoremen suggested that the expired alveolar concentrations of
carbon monoxide in smokers were age-related. For example, subjects
smoking one packet of 20 cigarettes per day in the 45-54 year age
group had an average alveolar value of 31 mg/m3 (27 ppm), while the
75-84 year age group had an average of only 16 mg/m3 (14.4 ppm).
Nonsmokers did not exhibit this age-related pattern, since even up to
84 years of age, alveolar concentrations remained at the same level
4 mg/m3 (3.6 ppm).
Exposure to low levels of carbon monoxide may have a significant
influence on the health and efficiency of these workers but this
awaits further study. Many other instances of occupational exposure
are available but most studies are complicated by the unknown or
unreported smoking habits of the workers under study (Grut, 1949).
5.4 Carboxyhaemoglobin Levels in the General Population
The most extensive study of blood carboxyhaemoglobin levels in the
general population was carried out by Stewart and his associates
(1973c and 1974), who took blood samples in 18 urban areas and in some
small towns in the States of New Hampshire and Vermont, USA. Similar
blood samples were evaluated for carboxyhaemoglobin levels by Kahn et
al. (1974), Davis & Gantner (1974), and Wallace et al. (1974) in
metropolitan St Louis. A total of 45 649 blood donors provided blood
for analysis. Stewart's subjects (29 000 including 1016 from St Louis)
were studied in March 1971 and Kahn's (16 649 subjects all from St
Louis) from October 1971 to October 1972. Stewart et al. concluded
that 45% of all the non-smoking donors exposed to ambient carbon
monoxide had a carboxyhaemoglobin saturation greater than 1.5%, while
Kahn's group reported a level of less than 1%.
6. METABOLISM
The primary factors that determine the final level of
carboxyhaemoglobin are: the amount of inspired carbon monoxide; minute
alveolar ventilation at rest and during exercise; endogenous carbon
monoxide production; blood volume; barometric pressure; and the
relative diffusion capability of the lungs. The rate of diffusion from
the alveoli and the binding of carbon monoxide with the blood
haemoglobin are the steps limiting the rate of uptake into the blood.
6.1 Endogenous Carbon Monoxide Production
Carbon monoxide can be produced endogenously from the catabolism
of pyrrole rings, originating from haemoglobin, myoglobin,
cytochromes, and other haem-containing pigments. Haem catabolism is
the main source of endogenous carbon monoxide production, but recent
in vitro investigations suggest additional endogenous sources, e.g.,
lipid peroxidation (Wolff & Bidlack, 1976).
The endogenous carboxyhaemoglobin level in man is estimated to be
about 0.1-1.0%. Increased production of endogenous carbon monoxide has
been found in haemolytic anaemias (Coburn et al., 1966), and could be
expected in haematomas and after exposure to certain toxic chemicals
capable of causing haemolysis. The liver is probably the major source
of endogenous carbon monoxide as a consequence of an increase in liver
cytochromes induced by certain drugs (Coburn, 1970a), or in porphyria
cutanea tarda (acquired or symptomatic porphyria) (White, 1970).
Similarly, bone marrow can become a major source of carbon monoxide in
haematological diseases, such as sideroblastic anaemia, being
characterized by ineffective erythropoiesis (White, 1970). In neonates
endogenous carbon monoxide can be markedly elevated, as well as in
females during the progesterone phase of the menstrual cycle
(Delivoria-Papadopoulos et al., 1970; Longo, 1970), and even more so
during pregnancy (see section 8.2.3). Another important cause of
carboxyhaemoglobin elevation is exposure to several methane-derived
halogenated hydrocarbons (see section 8.1.5).
6.2 Absorption
The classical absorption curves of Forbes et al. (1945) have been
re-evaluated for man at rest by Peterson & Stewart (1970) who exposed
volunteers to a variety of different concentrations of carbon monoxide
for periods ranging from 0.5 to 24 h. Using a regression approach,
they derived the following empirical relationship for blood carboxy-
haemoglobin as a function of ambient carbon monoxide concentration
and exposure time:
log10 y = 0.85753 log10 x + 0.62995 log10 t - 2.29519
where y = % carboxyhaemoglobin
x = carbon monoxide concentration in ppm
t = time in minutes
Although these new data come closer to presenting potential uptake in
individuals exposed to present-day ambient concentrations of carbon
monoxide, they do not apply to the uptake that would occur in active
man. Furthermore, they fit only the linear, non-steady-state portion
of the absorption curve. Ott & Mage (1974) have objected to the
Peterson & Stewart equation as being basically a static model. They
indicate that the use of averaging periods as long as 1 h compared
with 10-15 min introduces an error into recorded urban concentrations.
This error may be serious if many sharp, ambient peaks are present.
The data presented by Forbes et al. (1945) are still the only
experimental information available that takes ventilation into account
and, even so, this information is inadequate since the full range of
inspired, ventilatory volumes possible in exercising man was not
considered. Coburn et al. (1965) have developed an equation from which
it is possible to calculate blood carboxyhaemoglobin (Peterson &
Stewart, 1970) as a function of time, considering appropriate
physiological and physical factors. Their basic differential equation
is as follows:
d(CO) [HbCO] pCo2 1 pIco
= Vco - × × +
dt [HbO2] M 1 pB - 47 1 pB - 47
+ +
DL VA DL VA
d(CO)
where is the rate of change of CO in the body (ml/min)
dt
[HbCO] is the concentration of CO in the blood (ml CO/ml blood)
[HbO2] is the concentration of O2 in the blood (ml O2/ml blood)
DL is the diffusion capacity of the lung (ml/min/mmHg)
VA is the alveolar ventilation rate (ml/min)
pB is barometric pressure (mmHg)
pIco is inspired carbon monoxide pressure (mmHg)
pCo2 is the mean pulmonary capillary oxygen pressure (mmHg)
M is the Haldane constant (220-240 at pH 7.4)
Vco is the rate of endogenous CO production (ml/min)
One solution of the equation depends on the assumption that
pCo2, and HbO2 are constant and independent of HbCO. However,
HbO2 concentration depends upon HbCO in a complex way and, in
general, solution of the equation requires some special computer
techniques using a second approximation; these have been attempted and
general solutions are available (Coburn et al., 1965). Further
development of Coburn's concepts will undoubtedly improve the basis on
which theoretical uptakes can be calculated. For immediate, practical
purposes, calculations based on the Haldane formula (see section 6.3)
can be used.
Table 6 indicates the equilibrium percentage saturation of the
haemoglobin with carbon monoxide at various alveolar pressures of
carbon monoxide, calculated from the Haldane formula:a
% HbCO 230 pAco
=
% HbO2 pAo2
where pAco and pAo2, are the alveolar pressures of carbon
monoxide and oxygen respectively.
Table 6. Percent carboxyhaemoglobin versus carbon monoxide alveolar pressure
% HbCO mg/m3 ppm % in air Pa Torr
0.87 5.7 5 0.0005 0.506 0.0038
1.73 11.5 10 0.001 1.013 0.0076
3.45 23.0 20 0.002 2.026 0.0152
5.05 34.5 30 0.003 3.305 0.0248
6.63 46.0 40 0.004 4.052 0.0304
8.16 57.5 50 0.005 5.065 0.0380
9.63 69.0 60 0.006 6.078 0.0456
11.08 80 70 0.007 7.091 0.0532
12.46 92.0 80 0.008 8.104 0.0608
13.80 103.0 90 0.009 9.117 0.0684
15.11 114.5 100 0.010 10.130 0.0760
16.37 126.0 110 0.011 11.143 0.0836
17.60 130.0 120 0.012 12.156 0.0912
18.78 149.0 130 0.013 13.170 0.0988
19.95 160.0 140 0.014 14.183 0.1064
21.05 172.0 150 0.015 15.196 0.1140
22.15 183.0 160 0.016 16.209 0.1216
23.23 195.0 170 0.017 17.209 0.1291
24.26 206.0 180 0.018 18.235 0.1368
25.25 218.0 190 0.019 19.221 0.1442
26.22 229.0 200 0.020 20.261 0.1520
a The alveolar oxygen pressure is assumed to be 13 kPa (98 Torr)
6.3 Reactions with Body Tissues and Fluids
An adequate oxygen supply to maintain tissue metabolism is
provided by the integrated functioning of the respiratory and
cardiovascular systems to transport oxygen from the ambient air to the
various tissues of the body. Nearly all of the oxygen, except that
dissolved in plasma, is bound reversibly to the haemoglobin contained
within the erythrocytes. The most significant chemical characteristic
of carbon monoxide is that it also is reversibly bound by haemoglobin.
Therefore, it is a competitor with oxygen for the four binding sites
on the haemoglobin molecule.
The equilibrium constant M expresses the relative affinity of
haemoglobin for carbon monoxide and oxygen when the concentration of
reduced haemoglobin is minimal. This Haldane constant (Douglas et al.,
1912) is defined by the following equation:
HbCO pCO
= M x
HbO2 pO2
where pCO and pO2 represent the equilibrium partial gas
pressures: each pressure being the same in the erythrocytes or
haemoglobin solution as in the equilibrated gas phase. [HbCO] and
[HbO2] are the concentrations of carboxyhaemoglobin and
oxyhaemoglobin, respectively. The value of M is about 200 in most
species, in spite of the fact that carbon monoxide combines with
haemoglobin more slowly than oxygen. Carboxyhaemoglobin dissociates
very slowly due to the tight binding of carbon monoxide to
haemoglobin. Technically, it is not possible to measure the rate of
dissociation of carbon monoxide from partly saturated haemoglobin. The
dissociation velocity constant has been measured by only a few
investigators on sheep and human haemoglobin fully saturated with
carbon monoxide. Roughton (1970), however, has presented the most
comprehensive analysis of the interaction of carbon monoxide with
erythrocyte haemoglobin, showing clearly that, for man, M is higher
than commonly thought, i.e., it is more likely to be between 240 and
250; however, the value for M depends on the point of reference on
the dissociation curve.
Solution of Haldane's equation would give an approximate level of
carboxyhaemoglobin, e.g., exposure to ambient air containing carbon
monoxide levels of 28.7, 57.5, or 115 mg/m3 (25, 50, or 100 ppm)
would lead to carboxyhaemoglobin saturations of approximately 4.8, 9.2
and 16.3%, if the arterial oxygen pressure were 10.7 kPa (80 Torr).
The carbon monoxide enters the lungs with each breath and diffuses
across the alveolar-capillary membrane in a manner similar to oxygen.
If air with a constant concentration of carbon monoxide is breathed
for several hours, the rate of uptake of carbon monoxide decreases
approximately exponentially until an equilibrium state is attained in
which the partial pressure of carbon monoxide in the pulmonary
capillary blood is the same as that in the alveolar.
Oxygen transport in the blood is best described by the
oxyhaemoglobin dissociation curve (Fig. 4). In the presence of
carboxyhaemoglobin, this curve is no longer typically sigmoid but is
shifted to the left so that a lower oxygen pressure is present for the
same oxyhaemoglobin saturation compared with blood without
carboxyhaemoglobin (Roughton & Darling, 1944). Fig. 5 illustrates the
extent of the Haldane shift to the left more clearly than the typical
curves of Fig. 4. Mulhausen et al. (1968) illustrated this shift by
showing that the half saturation oxygen tension shifted from 3.6 to
3.1 kPa (26.7 to 23.2 Torr) in subjects who were intermittently
exposed to a high concentration of ambient carbon monoxide. Carbon
monoxide not only diminishes the total amount of oxygen available by
direct replacement of oxygen (Fig. 4) but also alters the dissociation
of the remaining oxygen so that it is held more tenaciously by
haemoglobin and released at lower oxygen tensions. The oxyhaemoglobin
curve in the presence of carboxyhaemoglobin progressively resembles
the simple oxygen dissociation curve of myoglobin. Myoglobin is a haem
compound with only one haem unit per molecule and does not exhibit
haem-haem interactions. It is possible that the combination of one or
more of the four haem groups in haemoglobin with carbon monoxide
decreases the haem-haem interactions of the remaining haem units and
results in a molecule approaching the behaviour of myoglobin.
Any consideration of the toxicity of carbon monoxide must include
not only the decrease in the oxygen carrying capacity of haemoglobin
but also the interference with oxygen release at the tissue level.
The venous pO2 values expected to result from various
carboxyhaemoglobin levels can be calculated (Forster, 1970; Permutt &
Fahri, 1969). If blood flow and metabolic rate remain constant,
equilibration with an ambient carbon monoxide concentration of
230 mg/m3 or 200 ppm (25% carboxyhaemoglobin) will lower venous
pO2 from 5.3 to less than 4 kPa (40 to less than 30 Torr). A
similar degree of venous hypoxaemia results from an ascent to an
altitude of 3658 metres or a 35% reduction in oxygen capacity in an
anaemic patient. It can also be calculated that, at 5%
carboxyhaemoglobin, there will be only a slight drop in the mixed
venous pO2. Even more valuable relationships can be obtained by
plotting oxygen content against the partial pressure of oxygen
(Roughton & Darling, 1944). The difference in oxygen content at
various percentages of carboxyhaemoglobin from 0 to 20 reveal the
minimal magnitude of the relative unavailability of oxygen due to the
Haldane effect (Fig. 4). It was this evaluation that led Roughton &
Darling to conclude that carboxyhaemoglobin concentrations of less
than 40% produce relatively easily compensated restrictions in the
amount of oxygen available for tissue delivery. This can only be
applied to subjects with normal respiratory and circulatory systems.
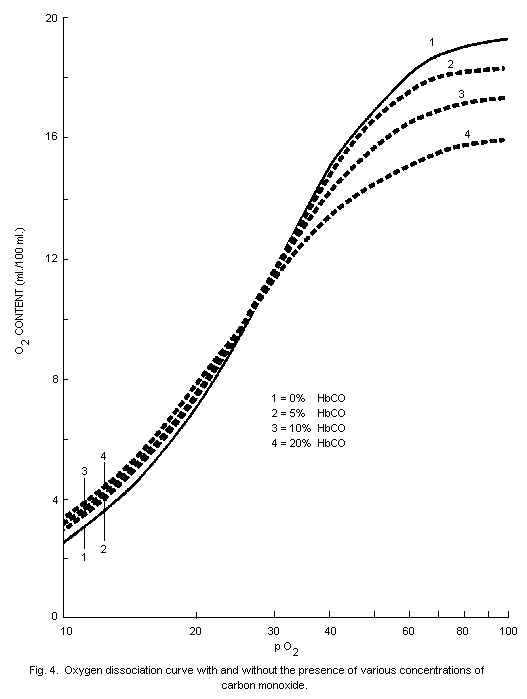
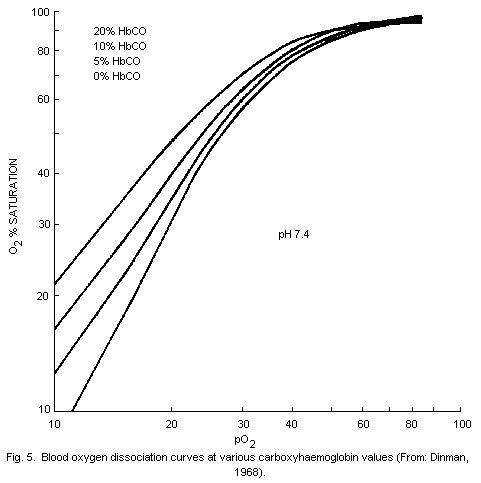 The small reductions in oxygen content at 5-10% carboxyhaemoglobin may
be quite critical for patients suffering from cardiovascular diseases
or chronic obstructive lung disease. Coburn et al. (1965) published a
detailed theoretical analysis of the physiology and variables that
determine blood carboxyhaemoglobin levels in man. The details of the
formulae used in these calculations are presented in section 6.2.
With regard to the intracellular effects of carbon monoxide,
consideration must be given to the interactions of all substances
within the tissue cells that are involved with oxygen delivery. Since
haemoglobin and myoglobin are structurally related, they react with
carbon monoxide in a similar manner. The function of myoglobin, in
vivo, may be to act as a reservoir for oxygen within the muscle
fibre. The carbon monoxide and oxygen equilibria of human myoglobin
has been studied in vitro and a hyperbolic oxygen dissociation curve
established (Rossi-Fanelli & Antonini, 1958). This curve, unlike that
for haemoglobin, is not affected by the hydrogen ion concentration,
the ionic strength, or the concentration of myoglobin. The relative
affinity constant, M, is approximately 40 but is still sufficient to
induce appreciable formation of carboxymyoglobin. Both Coburn et al.
(1970b, 1973) and Luomanmaki (1966) have studied the interrelation-
ships between carboxyhaemoglobin and carboxymyoglobin. Coburn, using
14CO, showed that identical carbon monoxide exposures can produce
different degrees of saturation of haemoglobin, depending upon the
partial pressures of oxygen in blood and tissue. Coburn (1970b)
determined the ratio of the carbon monoxide content in muscle to the
content in blood as a function of arterial pO2. This ratio, for
skeletal muscle, is approximately 1, but in myocardial tissue it was
found to be 3. When arterial pO2 fell below 5.3-4 kPa (40-30
Torr), carbon monoxide disappeared from the blood, presumably entering
the muscle. Considerable amounts of extravascular carbon monoxide are
stored in muscle. The higher ratio for cardiac tissue may be of
considerable significance. In an individual with a blood
carboxyhaemoglobin level of 10%, some 30% of cardiac myoglobin may be
saturated with carbon monoxide. Coburn and associates (1973) estimated
the mean pO2 of skeletal muscle and myocardium and found that they
were 0.8-1.1 and 0.5-0.8 kPa (6-8 and 4-6 Torr), respectively.
Although no final judgement can be made regarding the next lower
step involved in oxygen transport, i.e., the role of cytochromes a3
and P-450, the fact that, experimentally, they react with carbon
monoxide in the same way as other haem-containing substances suggests
that they may play a role in carbon monoxide poisoning. Available
evidence suggests that interactions between carbon monoxide and
cytochrome oxidases are of minor significance at the concentrations of
carbon monoxide found in community air pollution. All of the data on
the cytochromes have been obtained from in vitro experiments.
Whether similar events occur in vivo remains uncertain. The most
likely oxidase for in vivo inhibition is P-450. Cooper et al. (1965)
reported that the ratio of carbon monoxide to oxygen required for 50%
inhibition is close to 1, in contrast to a similar ratio of between
2.2 and 28 for cytochrome a3. Root (1965) believes that at a pCO
compatible with life, only nonsignificant blocking of the oxygen
consumption system occurs. In terms of the total distribution
throughout the body of an inhaled dose of carbon monoxide, the amounts
bound to these haemoproteins are small compared with haemoglobin and
myoglobin. A diagrammatic representation of the factors influencing
body carbon monoxide stores has been presented by Coburn (1970b). The
possible significance of the role of these haemoproteins lies in the
concept that, under conditions where tissue pO2 is decreased, the
affinity of intracellular haemoproteins for carbon monoxide may
increase.
6.4 Excretion
Adequate data are available on the rate of absorption of carbon
monoxide but there is considerably less information concerning the
rates of carbon monoxide egress from the lungs. The same factors that
determine how much carbon monoxide is taken up by the blood should
apply in reverse when clearance of carbon monoxide from blood is
considered. The primary factors involved are the amounts of carbon
monoxide and oxygen present, the magnitude of ventilation, and the
quality of the diffusion barrier. Age influences the quality of the
barrier and it appears that with advancing age the barrier becomes
"thicker" and there are fewer gas exchange membranes. Sedov et al.
(1971) presented data on the elimination of carbon monoxide at various
atmospheric pressures and ambient temperatures. Neither lower
barometric pressures nor high temperatures appreciably altered the
rates of elimination. It has been implied by Pace et al. (1950) that a
sex difference in elimination may also exist, a faster rate occurring
in females than in males, at least under the experimental conditions
of their study. Some sex differences in excretion have been reported
by Goldsmith et al. (1963).
Available evidence suggests that there is a biphasic decline in
the percentage of carboxyhaemoglobin in the arterial blood (Godin &
Shephard, 1972; Wagner et al., 1975). There is a rapid, initial,
exponential decline (distribution phase), probably related to the
distribution of carbon monoxide from the circulating blood to splenic
blood, myoglobin, and cytochrome enzymes. Elimination of carbon
monoxide through the lungs also occurs during this phase. The
distribution phase, which persists for the first 20-30 min, is
followed by a slower linear decline (elimination phase). This phase
probably reflects the rates of release of carbon monoxide from
haemoglobin and myoglobin, pulmonary diffusion, and ventilation, as
well as the fact that pCO decreases with time. Myhre (1974) found
that a similar biphasic excretion pattern occurred at an altitude of
1630 metres. However, he noted that the half-time of carboxyhaemo-
globin was much longer (5.5 h). After continuous exposure to carbon
monoxide for 49 h, 50% was eliminated in 30-180 min and 90% within
180-420 min (Tiunov & Kustov, 1964). Other investigators (Godin &
Shephard, 1972; Peterson & Stewart, 1970) reported exponential
carboxyhaemoglobin elimintation curves over many hours. However,
because of inadequate sampling in the early phase of elimination, they
were unable to observe the more rapid initial decline. The absolute
level of carboxyhaemoglobin, when the elimination studies began,
apparently modified the rate of disappearance of carbon monoxide from
the blood. Considerable individual variability has been observed and
the duration of exposure may also be an important factor. It has been
shown that prolonged exposure (more than 3 years) to concentrations of
carbon monoxide of 11.5-115 mg/m3 (10-100 ppm) results in a markedly
retarded elimination of carbon monoxide (Kodat, 1971).
In summary, discharge of carbon monoxide occurs rapidly at first
becoming slower with time and the lower the initial level of
carboxyhaemoglobin, the slower the rate of elimination. Apparently, no
studies have been made to determine elimination rates at the low
levels of carboxyhaemoglobin (2-4%) that might be present following
exposure to ambient concentrations of carbon monoxide.
Several procedures have been tested that could accelerate the
excretion of carbon monoxide from the blood of individuals with high
levels of carboxyhaemoglobin (40-70%). Pace et al. (1950) reported
that treatment of such individuals in a recompression chamber with an
oxygen level equivalent to 2.5 atmospheres (partial pressure of oxygen
equal to 253 kPa (1900 Torr) or an alveolar oxygen pressure of 239 kPa
(1801 Tort)) would facilitate removal of carbon monoxide. They
indicated that 1 h in such a chamber would result in the reduction of
the carboxyhaemoglobin level to 10 to 15% of the initial level. In a
revival of the old Henderson & Haggard treatment concept, Malorny et
al. (1962) evaluated the influence of breathing different gas mixtures
on the excretion of carbon monoxide in animals having high
carboxyhaemoglobin levels (60-74%). It was determined that 50% of
carbon monoxide could be excreted in 19 min if 5% carbon dioxide and
95% oxygen were breathed, compared with a time of 28 min for 100%
oxygen, and 41 min for ambient air. Another approach was suggested by
Agostini et al. (1974), who employed a total body asanguineous, hypo-
thermic procedure. These approaches to the removal of the body burden
of excessive amounts of carbon monoxide remain to be evaluated fully
in clinical trials.
7. EFFECTS ON EXPERIMENTAL ANIMALS
Great caution must be used in applying the resultsa obtained
from animal experiments to man. Nonetheless, animal studies have
provided valuable insight into both the potentially adverse effects of
carbon monoxide and the basic mechanisms by which this substance
influences physiological processes. However, many studies have used
extraordinarily high levels of carbon monoxide, rarely found in air.
These studies are not referred to in this document, since the toxic
effects of very high levels of carbon monoxide have been well
documented for both animals and man.
7.1 Species Differences
The oxygen dissociation curves for different animal species used
in carbon monoxide studies are not the same. There are also questions
concerning the relative affinities for carbon monoxide of the
haemoglobin in various animal species. Fodor & Winneke (1971.)
reported in vitro studies that showed different affinities for
different species, the highest being in man followed by rat, mouse,
and rabbit, in descending order. Klimisch et al. (1975) found the
following sequence of affinities: hamster, rat, pig, rabbit. Apart
from different affinities there may well be species differences with
respect to susceptibility to carbon monoxide. According to Alexandrov
(1973) certain mammals can be classified in order of decreasing carbon
monoxide susceptibility as follows: mouse, rat, cat, dog, guineapig,
and rabbit. This difference might, in part, be related to different
ventilation/body weight-ratios.
Species differences in reaction are ideally illustrated in the
studies by De Bias et al. (1972a,b, 1973) on dogs and cynomolgus
monkeys (Macaca fascicularis = M. irus). Chronic exposure (23 h per
day) over several months to 115 mg/m3 (100 ppm) resulted in
carboxyhaemoglobin levels of 14 and 12.4% in dogs, and monkeys,
respectively. The dogs (De Bias et al, 1972a) remained clinically in
good health with no untoward signs that could be interpreted as
induced by carbon monoxide. Serum enzymes, haematological variables,
and electrocardiograms did not change significantly. Carbon monoxide
exposure of normal monkeys resulted in myocardial effects.
Experimentally infarcted monkeys had greater P-wave amplitudes and
increased incidence of T-wave inversions than normal monkeys similarly
exposed. One important fact that may partly explain this difference
between dogs and monkeys is, that monkeys, like man, have end-
arteries, whereas dogs have a well developed collateral circulation.
a Animal studies have been reported in other sections of this
document wherever necessary. Consequently, some data on animals
(such as sheep) are not repeated in this section.
7.2 Cardiovascular System and Blood
Increase of coronary blood flow is the normal response of the
myocardium to carbon monoxide exposure. Permutt & Farhi (1969)
calculated theoretically, that, when carboxyhaemoglobin levels are
approximately 5%, an increase in coronary blood flow of about 20% more
than the resting level would be necessary to prevent coronary sinus
pO2 from falling below minimum levels. This calculation has been
confirmed experimentally by Adams et al. (1973) and Horvath (1973),
both of whom demonstrated approximately similar increases in coronary
blood flow in spite of using very different methods to increase
carboxyhaemoglobin levels.
There is considerable controversy concerning the cardiovascular
effects of carbon monoxide in dogs. Long-term exposures of animals to
carbon monoxide concentrations sufficiently high to produce
carboxyhaemoglobin levels in excess of 20% can induce pathological
changes in the heart and brain. As in acute high level intoxication in
man, serious sequelae develop. Lindenberg et al. (1961) exposed 8 dogs
to carbon monoxide concentrations of 115 mg/m3 (100 ppm). Four were
exposed continuously for 24 h a day, 7 days per week, for 6 weeks and
another 4 were exposed intermittently. All dogs had abnormal
electrocardiograms and some of the hearts showed histological
degeneration of muscle. In another study, Preziosi et al. (1970)
exposed dogs continuously to 115 mg/m3 for 6 weeks and reported
abnormal electrocardiograms, right and left heart dilatation, and
myocardial thinning. Histological examination showed older scarring in
some cases and fatty degeneration of heart muscle in others.
Carboxyhaemoglobin levels of 7.7 to 12% were lower than would be
predicted from the exposure. When 4 dogs were exposed intermittently
to a carbon monoxide concentration of 115 mg/m3 for 11 weeks,
central nervous system and cardiac effects were found (Lewey &
Drabkin, 1944).
Ehrich et al. (1944) also exposed 4 dogs to 115 mg/m3 on an
intermittent schedule. They observed that electrocardiographic changes
occurred at variable times during the 11 weeks of exposure. The gross
appearance of the hearts after exposure were normal but microscopic
examination revealed marked degenerative changes in individual fibres.
Lindenberg et al. (1961) exposed dogs to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) on the same schedule as for his
115 mg/m3 exposure studies. Carboxyhaemoglobin levels of 2.6-5.5%
were observed. No changes in haemoglobin levels or haematocrit were
noted. Electrocardiographic changes were observed in the third week of
exposure similar to, but less severe than, those observed in animals
exposed to the higher ambient level of carbon monoxide. Dogs were
exposed by Musselman et al. (1959) to a carbon monoxide concentration
of 57 mg/m3 for 24 h per day, 7 days per week, for 3 months. No
changes in the electrocardiogram or heart rates were observed.
Pathological examination of organs and tissues did not reveal any
changes after exposure. Experimental data have been presented by
Orellano et al. (1976) who claim that carbon monoxide injected
intraperitoneally into dogs is nontoxic. This study, as well as other
reports from these investigators, raise some intriguing questions as
to the mechanisms involved in carbon monoxide toxicity.
Continuous exposure of cynomolgus monkeys to a carbon monoxide
concentration of 115 mg/m3 resulted in demonstrable electro-
cardiographic effects in the myocardium of both normal monkeys
and monkeys with myocardial infarction (De Bias et al., 1972b, 1973).
The same investigators (De Bias et al., 1976) also studied the
susceptibility of the ventricles to induced fibrillation. Normal and
infarcted monkeys were exposed to a carbon monoxide concentration of
115 mg/m3 for 6 h. Application of high voltages were required to
produce fibrillation in normal monkeys in air but when infarcted
animals were exposed to carbon monoxide, the voltage level required
was very low. Animals with either infarction alone or carbon monoxide
exposure alone also required significantly less voltage to produce
fibrillation; when the two were combined, the effects were additive.
Intermittent exposure to carbon monoxide (30 min per h, 12 h per day,
over a period of 14 months) of cynomolgus monkeys fed either a normal
or a semipurified cholesterol diet did not result in myocardial
infarctions or electrocardiographic abnormalities (Malinow et al.,
1976). In these animals, the blood carboxyhaemoglobin concentration
reached 21.6% at the end of the daily period of breathing carbon
monoxide at 529 mg/m3 (460 ppm).
Carbon monoxide exposure did not increase the aortic and coronary
atherosclerosis induced in cynomolgus monkeys by cholesterol feeding.
Eckardt et al. (1972) exposed cynomolgus monkeys (22 h per day, 7 days
per week, for 2 years) to carbon monoxide concentrations of 23 or
75 mg/m3 (20 or 65 ppm). Carboxyhaemoglobin levels, which showed
considerable variation during the experimental period, ranged from 2.0
to 5.5% and 4.8 to 10.2% for low and high carbon monoxide ambient
concentrations, respectively. These levels of carboxyhaemoglobin did
not lead to compensatory increases in haematocrit, haemoglobin, or
erythrocyte counts nor to cardiac fibrosis or pathological effects in
the brain. Cholesterol-fed squirrel monkeys were exposed to carbon
monoxide at 229-344 mg/m3 (200-300 ppm) for several hours per day
for 7 months (Webster et al., (1968). No differences were found in
plasma cholesterol or in aortic and carotid atherosclerosis which
could be ascribed to carbon monoxide. However, the authors did observe
an increase in coronary atherosclerosis. Somewhat similar results were
later reported in studies on cynomolgus monkeys (Malinow et al.,
1976). Jones et al. (1971) exposed rats, guineapigs, dogs, and monkeys
to 58, 110, or 229 mg/m3 (51, 96 or 200 ppm) continuously for 90
days. Haematocrit and haemoglobin levels remained constant at the
lowest level of carbon monoxide exposure but were significantly
elevated at the two higher levels in all species except the dog.
Cynomolgus monkeys (Macaca irus) were exposed to a concentration of
286 mg/m3 (250 ppm) for 2 weeks by Thomsen (1974). In all the
exposed animals, the coronary arteries showed widening of the
subendothelial spaces in which cells with or without lipid droplets
were accumulating. He suggested that monkeys were more sensitive to
carbon monoxide than the rabbits studied by Astrup et al. (1967).
Experimental studies on rabbits exposed to relatively high
concentrations of carbon monoxide at 195-206 mg/m3 (170-180 ppm) for
extended periods indicated the presence of high levels of cholesterol
in the arteries or enhanced vascular disease (Astrup et al., 1970).
The lesions observed, which included subendothelial oedema, a gap
between endothelial cells, and increased infiltration of cells with
lipid droplets, might be early precursors of atherosclerotic disease.
However, such lesions occurred only in animals concurrently on a high
cholesterol or fat diet or on both. Exposure to carbon monoxide alone
induced some changes such as endothelial hypertrophy and
proliferation.
One experimental study on the effects of carbon monoxide on the
natural history of heart disease in the cynomolgus monkey has been
reported. De Bias et al. (1973) exposed animals to a carbon monoxide
concentration of 137 mg/m3 (120 ppm) for 24 weeks. The average
carboxyhaemoglobin level of 12.4% resulted in a polycythaemia with an
increase in haematocrit from 35 to 50%. All animals developed
increased P-wave amplitude and T-inversion which suggested nonspecific
myocardial stress rather than ischaemia. Animals in which an
experimental myocardial infarction was produced prior to exposure to
carbon monoxide had more marked electro-cardiographic changes than
animals breathing room air. In 1976, Ramsey & Casper reported that
erythrocytic 2,3-diphosphoglycerate (2,3-DPG) played neither a
compensatory nor an aggravating role in the hypoxia induced by the
presence of 20 or 30% carboxyhaemoglobin. Stupfel & Bouley (1970)
exposed mice and rats for 95 h per week to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) for either 1 to 3 months or for
their natural life expectancy of up to 2 years. A large number of
measurements were made during exposure followed by pathological
examination after death. The authors did not observe any important
effects of carbon monoxide exposure on the animals. In a study by
Penney et al. (1974a,b), the influence of hypoxic hypoxia on the
development of cardiac hypertrophy in the rat was compared with that
of carbon monoxide hypoxia. Exposure to various levels of carbon
monoxide resulted in hypertrophy of both the fight and left ventricles
in contrast with the right ventricle hypertrophy observed in response
to the hypoxic hypoxia stress.
Cardiac hypertrophy and a reduction in cytochrome oxidase levels
were demonstrated in chick embryos exposed to carbon monoxide for 144
and 168 h (Tumasonis & Baker, 1972). The resistance of young chickens
to carbon monoxide decreased with age. Body temperatures decreased
during exposure to carbon monoxide with the greatest fall in
temperature and the longest survival time occurring in the youngest
chickens. Total body asanguineous hypothermic perfusion (total body
exsanguination exchange transfusion) has been suggested as a
therapeutic measure for carbon monoxide poisoning (Agostini et al.,
1974). In an attempt to explain this beneficial effect, Ramirez et al.
(1974) compared the survival of normal dogs exposed to high levels of
carbon monoxide with that of acutely anaemic dogs transfused with
carboxyhaemoglobin blood to normal blood volumes. All normal dogs with
carboxyhaemoglobin levels of 54-100% died within 0.25-10 h but the
transfused animals, having a final mean carboxyhaemoglobin level of
80% after transfusion, survived. The authors suggested that hypoxic
anaemia was not the principal mechanism of carbon monoxide toxicity
but rather a blocking out of the energy supply on the cellular level,
governed by the cytochrome system.
Most of the investigations using rabbits for carbon monoxide
related research originated in Astrup's laboratories, where they first
demonstrated that low carboxyhaemoglobin levels enhanced the
development of atheromatosis (Astrup et al., 1967). Additional studies
(Hellung-Larsen et al., 1968; Thomsen & Kjeldsen, 1975) have shown
that lactate dehydrogenase isoenzymes (M subunits) increase and that a
higher incidence of focal intimal changes occur in rabbits exposed to
carbon monoxide. The myocardial ultrastructure of rabbits exposed to a
concentration of 206 mg/m3 (180 ppm) for at least 4 h showed
degenerative changes such as contraction bands, myofibrillar
disintegration, myelin body formation, and dehiscence of the
intercalated discs (Thomsen & Kjeldsen, 1974). Exposure of rabbits to
a similar concentration of carbon monoxide for 2 weeks resulted in
more extensive myocardial damage (Kjeldsen et al., 1974).
Astrup et al. (1970) furnished evidence that carbon monoxide
increases endothelial membrane permeability. They found that rabbits
exposed to carbon monoxide developed arterial lesions resulting in a
considerable accumulation of lipids. It has also been shown that human
coronary arteries exposed to carbon monoxide in vitro have a higher
uptake of cholesterol, although no significant changes in lipid
synthesis were observed (Sarma et al., 1975). Myocardial damage and
impaired myocardial performance have also been reported in animals and
man exposed to carbon monoxide (Ayres et al., 1970). Although there is
some evidence which suggests that exposure to carbon monoxide can
induce changes in blood vessels and the myocardium, there is also
evidence to the contrary (section 8.1).
7.3 Central Nervous System
Because of the brain's high oxygen demand, cerebral function
should be influenced at low carboxyhaemoglobin levels. However, data
concerning this are contradictory. Sensitivity to carbon monoxide may
follow a circadian rhythm (Stupfel, 1975; Stupfel et al., 1973).
Maximum sensitivity in rats occurred during the dark period of a
12-12 h light-dark cycle. Dyer & Annau (1976) could find no effect on
superior colliculus evoked potentials of rats until the level of
ambient carbon monoxide had reached 573 mg/m3 (500 ppm) in marked
contrast to Xintaras et al. (1966), who observed a 20% increase in the
amplitude of superior colliculus evoked potentials after only 1 h
exposure to 57 mg/m3 (50 ppm), and a 50% increase after a 2-h period
of exposure at this level. This study has, however, been criticized
for not taking into account the effects of dark adaptation on the
amplitude of visual evoked potentials in rats (Dyer & Annau, 1976).
In view of the conflicting reports it is of some interest to
examine the available data on cerebral pO2 tensions, cerebral
blood flow (CBF) and cerebral metabolism. Zorn (1972) studied the
effects of carbon monoxide inhalation on brain and liver pO2 using
platinum electrodes. Tissue pO2 fell in both organs, even at a
carboxyhaemoglobin concentration of 2%, and the fall was approximately
linear to increases in carboxyhaemoglobin. There was a decrease in
pO2 of 0.027-0.24 kPa (0.2-1.8 Torr) for each 1% fall in
oxyhaemoglobin percentage saturation. These data suggest that the
carbon monoxide influenced levels other than the intracellular level,
since if its effects were limited to this area then tissue pO2
would have been expected to increase. Similar studies were performed
by Weiss & Cohen (1974) on rat brain and muscle. They found a decrease
in cerebral cortical pO2 following inhalation of low levels of
carbon monoxide. Unfortunately, they did not measure
carboxyhaemoglobin levels in these rats but, in a group of sham-
operated animals exposed to similar levels of inhaled carbon monoxide,
carboxyhaemoglobin had increased to 3.3%. During progressive
administration of carbon monoxide to dogs, CBF did not increase until
carboxyhaemoglobin levels reached 20%. Thereafter, CBF increased
progressively and was double that in the controls when the
carboxyhaemoglobin level reached 50% (Häggendal et al., 1966). On the
other hand, Traystman (1976) did observe a progressive increase in CBF
in dogs even at very low carboxyhaemoglobin values. The lowest level
studied was 2.5%, which produced a slight but significant CBF
increase. Thus, Traystman did not believe in a threshold effect. The
effects were produced with both hypoxic hypoxia and carbon monoxide
hypoxia. It remains to be seen, how these data relate to cerebral
circulation in man.
The respiratory centre or arterial chemoreceptors were not
stimulated to increase respiratory minute volume even when
carboxyhaemoglobin levels were as high as 40% (Chiodi et al., 1941).
Mills & Edwards (1968) measured the frequency of electrical impulses
in the afferent nerves from the aortic and carotid chemoreceptors and
showed that administration of carbon monoxide did result in
chemoreceptor stimulation. The response appeared to have an
approximately linear relationship with the carboxyhaemoglobin
concentration (at least above 8%). These findings suggest that carbon
monoxide might stimulate breathing. Failure to observe an increased
minute volume may be explained by the fact that, in the presence of
carbon monoxide, the chemoreceptor stimulation was offset by hypoxic
depression of brain structures involved in breathing. There is some
evidence that this balancing between chemoreceptors and central
nervous depression is operative in anaemia (Santiago & Edelman, 1972).
Additional investigations are needed to clarify this effect of carbon
monoxide inhalation.
7.4 Behavioural Changes and Work Performance
In extensive studies on rabbits, guineapigs, rats, and mice
(Gadaskina, 1960; Ljublina, 1960; Rylova, 1960) exposure to a carbon
monoxide level of 30 mg/m3 (26 ppm), although not inducing any
morphological blood changes, did result in a number of unfavourable
physiological changes. Among these were decreased work capacity, poor
adjustment to postural shifts (orthostatic tests), and increased
thyroid activity. These changes were more evident during the initial
period of the exposure but reverted towards normal later, suggesting
some adaptation to carbon monoxide exposure.
7.5 Adaptation
Adaptation apparently can occur in animals exposed to moderate
concentrations of carbon monoxide (Gorbatov & Noro, 1948; Tiunov &
Kustov, 1969) as shown by their ability to tolerate, with apparent
ease, acute exposure to higher concentrations. Both Clark & Otis
(1952) and Tiunov & Kustov (1969) have demonstrated that, after long-
term exposure to carbon monoxide, animals developed tolerance to
short-term, high altitude exposure, and vice versa, an indication of
the development of a common adaptive mechanism. Acclimatization
continued despite a subsequent decrease in the initial elevation of
the haemoglobin concentration (Gorbatov & Noro, 1948). These
investigators noted an acclimatization effect in rats exposed daily to
0.4-0.5% carbon monoxide until loss of consciousness occurred. A
progressive improvement in tolerance time to unconsciousness was noted
so that by the eighth day of exposure a 3-fold improvement over the
time required on the first day had developed. In spite of apparent
acclimatization, the general condition of test animals became worse.
Unexpectedly, daily exposure to a carbon monoxide concentration of
1.0%, which on the first day necessitated a 5-min exposure prior to
unconsciousness, failed to induce any improvement in tolerance. A
possible correction of leftward shift of the oxygen dissociation by
alterations in the concentration of 2,3-diphosphoglycerate (inducing a
shift to the right) has been suggested. However, conflicting results
and the necessity to produce high levels of carboxyhaemoglobin negate
the possibilities of this beneficial effect (Astrup, 1970; Dinman et
al., 1970).
Evidence for adaptation to carbon monoxide is inconclusive.
Further studies appear to be warranted with special attention devoted
to studies using more realistic current ambient levels of carbon
monoxide, and also studies on the possible physiological cost of
adaptation, if it occurs.
7.6 Embryonal, Fetal, Neonatal, and Teratogenic Effects
Few studies have been made on the effects of carbon monoxide on
mammalian fetal growth and survival. Wells (1933) exposed pregnant
rats for 5-8 min to a carbon monoxide concentration of 1718 mg/m3
(1500 ppm) every other day during pregnancy. Maternal unconsciousness
and abortion or absorption of most of the fetuses resulted. Data
concerning carboxyhaemoglobin levels and numbers of animals studied
were not given. Rats were exposed to 0.34% carbon monoxide for 1 h
(carboxyhaemoglobin 60-70%) daily for a period of 3 months (Williams &
Smith, 1935). Among 7 pregnant females, the number of young per litter
was only half that of the controls and only 2 of the 13 newborns
survived to weaning age. Astrup et al. (1972) exposed rabbits during
their 30 days of pregnancy to carbon monoxide resulting in
carboxyhaemoglobin levels of either 9-19 or 16-18%. Neonatal mortality
in the 2 groups increased by 10 and 35%, respectively, compared with a
control value of 4.5%. Birth weights decreased by 12 and 17%,
respectively.
In their studies on the ewe and fetal lamb, Longo & Hill (1977)
indicated that fetal uptake and elimination of carbon monoxide was
relatively slow compared with that of the mother. They also reported
that, during steady-state conditions, fetal levels of
carboxyhaemoglobin were about 25% higher than maternal levels. These
results may be related to species differences, since Longo & Hill
(1970) found that the M valuesa for sheep maternal and fetal blood
were 218 and 216 respectively, while Engel et al. (1969) reported that
fetal haemoglobin had 20% less preferential binding of carbon monoxide
over oxygen than haemoglobin A.
There are very few studies on the teratogenicity of carbon
monoxide exposure. When fertilized chicken eggs were continuously
exposed to a carbon monoxide concentration of 747 mg/m3 (650 ppm)
for up to 18 days of incubation, the percentage of eggs hatching
decreased to 46% and developmental anomalies of the tibia and
metatarsal bones were noted (Baker & Tumasonis, 1972).
a M = Relative affinity of haemoglobin for carbon monoxide
compared with oxygen.
7.7 Carcinogenicity, and Mutagenicity
No evidence is available on carcinogenicity and mutagenicity in
relation to exposure to carbon monoxide.
7.8 Miscellaneous Changes
Kustov et al. (1972) exposed rats to carbon monoxide at 53 mg/m3
(46 ppm) and noted slower weight gains and an increase in haemoglobin.
Some enzyme systems were also found to have increased activity, when
rats were exposed to carbon monoxide (Pankow & Ponsold, 1972; Pankow
et al., 1974b). A slowing of in vivo metabolism of the drugs
hexobarbital and zoxazolamine, with prolongation of their
pharmacological effects has been reported in rats exposed to carbon
monoxide concentrations of 286-3435 mg/m3 (250-3000 ppm) (Montgomery
& Rubin, 1971). With prolonged exposure, these metabolic effects
became less pronounced and reverted to normal more quickly following
removal from the carbon monoxide environment. Sokal (1975) compared
the effect on blood pH and certain carbohydrate metabolic products
resulting from either a bolus administration or a fixed level of
inspired carbon monoxide, both resulting in equivalent final levels of
carboxyhaemoglobin. His data suggest that more intense biochemical
effects resulted following a gradual increase in carboxyhaemoglobin
levels compared with effects seen following rapid elevation of
carboxyhaemoglobin from the bolus. Data presented by Marks & Swiecicki
(1971) indicated that exposure to high levels of carbon monoxide
induced a stresslike response in the form of an elevation in
catecholamines. Swiecicki (1973) reported that increased
carboxyhaemoglobin levels stimulated the adrenergic system and
increased carbohydrate metabolism. He also noted that physical
training of the rat neither prevented nor reduced changes in
carbohydrate metabolism following carbon monoxide exposure and
vibration. Exposure of rats to 57 mg/m3 (50 ppm) for 5 h per day, 5
days per week, for 12 weeks produced an effect on trace metals at the
subcellular level, with a possible reduction in cellular respiration
and nucleoprotein synthesis.
Guineapigs exposed to carbon monoxide concentrations of
1.7-30 mg/m3 (1.5-26 ppm) for 21 days, 8 h per day, did not show any
allergenic effects related to carbon monoxide exposure (Vinogradov et
al., 1974). Plasma leucine aminopeptidase (EC 5.4.11.1)a and
glutamic pyruvic transaminase (EC 2.4.1.2) activity, normally
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
increased by exposure to carbon tetrachloride, were further
potentiated when blood carboxyhaemoglobin levels were elevated. Pankow
et al. (1974a) also observed an additive effect on some enzymes with a
combination of alcohol and carbon monoxide giving a carboxyhaemoglobin
concentration of 50%.
Rondia (1970) observed a significant reduction of benzopyrene-
hydroxylase (EC 1.14.14.2) activity in the liver homogenates of rats
exposed to a carbon monoxide concentration of 70-150 mg/m3
(60-130 ppm) for only a few days. This finding might be interpreted as
meaning that carbon monoxide contributes to the induction of lung
cancer by lengthening the time of retention of carcinogens in the
lung. Additional work is necessary to clarify this important question.
7.9 Interactions
Much of the data reviewed by Pankow & Ponsfold (1974) concerning
the combined effects of carbon monoxide and other biologically active
agents are based on animal experiments. Because of the extreme
exposure conditions used in most of these studies, only a few of them
are directly relevant to the environmental exposure of man to carbon
monoxide.
The experimental evidence on the aggravation of carbon monoxide-
induced atherosclerosis in rabbits by dietary cholesterol has already
been mentioned (see section 7.2). No significant additive effects were
noted from ethanol when dogs exposed to a carbon monoxide
concentration of 115 mg/m3 (100 ppm) for 21 weeks, 5 days a week,
and 6 h per day were given a daily oral dose of 120 ml of a 15%
ethanol solution (Pecora, 1959). However, the excretion of total
lipoproteins was higher when carbon monoxide and ethanol treatments
were combined than with exposure to carbon monoxide alone. An
indication of interaction of sulfur dioxide and carbon monoxide was
given by Prohorov & Rogov (1959) in their experiments on rabbits. The
depressed activity of succinate dehydrogenase (EC 1.3.99.1) on heart,
liver, and kidney due to exposure to sulfur dioxide at 200 mg/m3
(76 ppm) was exacerbated by exposure to carbon monoxide at a level of
200-400 mg/m3 (174-348 ppm), for 3 h per day, over a 3-week period.
As for the combined effects of carbon monoxide and temperature, Tiunov
& Kustov (1969) showed clearly that carbon monoxide toxicity in mice
increased at temperatures above or below normal ambient levels.
8. EFFECTS ON MAN
8.1 Healthy Subjects
8.1.1 Behavioural changes
Demonstrable changes in the central nervous system (CNS) function
of subjects inadvertently exposed to high levels of ambient carbon
monoxide in illuminating gas and in automobile exhaust resulted in a
series of studies to determine psychomotor and psychological
aberrations in subjects having more modest blood levels of
carboxyhaemoglobin than those observed in the potentially moribund
patients. Deficiencies in earlier studies have been related to
inadequate understanding of the significance of behavioural changes,
the inability to distinguish between simple perceptual motor
performance and the more complex performance involving sustained
and/or selective attention, short-term memory, and decision making
among possible alternatives. Furthermore, the physiological mechanisms
involved in carbon monoxide intoxication were not appreciated and the
available physiological and psychological tools were not adequately
exploited. Even today, some physiological and behavioural studies
suffer from similar or other inadequacies, e.g., the failure to
measure blood carboxyhaemoglobin levels, the inability to distinguish
between the physiological effects of a carbon monoxide bolus of high
concentration or the slow, insidious increment in carboxyhaemoglobin
levels over time with lower inhaled concentrations, the amount of
carbon monoxide brought to or removed from the lungs by changes in
alveolar ventilatory volumes, and the small number of volunteers
examined. Other factors involve failure to provide control measures
for bias and effects of the experimental worker (by means of double-
blind administration), control periods so that task-learning effects
do not mask negative results, homogeneity of the groups labelled
"smokers" and "nonsmokers", and control of possible boredom and
fatigue effects, all essentially amounting to a failure to adopt a
proper experimental design that would produce statistically
significant information.
A reduction of logical memory and recognition was demonstrated by
Chalupa (1960) in individuals subjected to acute carbon monoxide
intoxication. However, these functions returned to normal. Sayers et
al. (1929) did not find any significant changes in 6 exposed men
despite carboxyhaemoglobin levels of approximately 20-30%;
observations included hand-eye coordination and steadiness, tapping
speed, arithmetic (continuous addition), location memory, and simple
reaction time. Simple sensory-motor times decreased by 10% in subjects
with carboxyhaemoglobin concentrations of approximately 6.2% (5.5-7%)
(Tiunov & Kustov, 1969). Simulated driving performance did not
deteriorate despite carboxyhaemoglobin levels of 25%, although a small
deterioration was observed when the carboxyhaemoglobin levels were
above 35% (Forbes et al., 1937). The first demonstrable influences of
carbon monoxide on higher CNS functions were noted by McFarland et al.
(1944) in conjunction with their altitude studies, when they observed
reduction of visual acuity at carboxyhaemoglobin levels as low as 5%.
These observations were extended by Halperin et al. (1959) when they
reported that visual function was impaired at carboxyhaemoglobin
levels as low as 4% and that impairment increased at higher levels.
More recently, McFarland et al. (1973) showed that, for glare
recovery, the dark adaptation final threshold values increased as
carboxyhaemoglobin levels rose from control to 6-17%. Peripheral
recognition tasks were not affected until levels reached 17%. They
also stated that central and peripheral complex tasks were not
influenced by low levels of carboxyhaemoglobin. Schulte (1963)
demonstrated that there was a decrease in performance in higher
intellectual processes that was observable when the carboxyhaemoglobin
level exceeded 5%; further deterioration was noted as the level
increased. These results were in direct contradiction to the negative
results obtained by Dorcus & Weigand (1929) who used a similar series
of tests but with subjects exposed for a shorter period. In studies by
Beard & Wertheim (1967), the ability to judge correctly slight
differences in successive short time intervals showed significant
impairment when carboxyhaemoglobin levels were approximately 2 to 3%
above basal levels. These findings, suggesting an altered mental
function, represent the lowest levels of carboxyhaemoglobin that
produce a significant alteration in behavioural performance. However,
attempts to replicate them have been less than satisfactory (O'Donnell
et al., 1971a,b; Stewart et al., 1973a) even though the subjects in
all these other studies attained higher levels of carboxyhaemoglobin.
Some of the discrepancies may be explained on the basis of differences
in protocol and in the environmental conditions under which the tests
were conducted. Some of the investigators designed their experiments
to minimize the factors of boredom and fatigue while others attempted
to minimize external influences and conducted their experiments for a
relatively long time.
However, the Beard & Wertheim study may actually be more relevant
to questions of vigilance and ideally should be discussed in this
context. Assessment of vigilance is the determination of an
individual's ability to detect small changes in his environment,
changes that take place at unpredictable times and so demand
continuous attention. In such monotonous tasks, subjects miss signals
that they would not have missed when starting the task. Such signals
are presented visually or aurally. Fodor & Winneke (1972) and Groll-
Knapp et al. (1972) used auditory signals for their vigilance task.
The former investigators used a white noise (frequency range from 20
to 20 000 Hz lasting 0.36 sec and repeated at 2-sec intervals. About 3
out of every 100 of these noises were slightly less intense and were
used as the signal to which the subjects responded by pressing a
button. Twelve nonsmokers (male and female) were tested at carbon
monoxide concentrations of 0 and 57 mg/m3 (0-50 ppm). They breathed
this concentration for 80 min prior to the first of three vigilance
tests. Carboxyhaemoglobin levels were estimated to be 2.3 and 3.1% at
the beginning and the end of the first vigilance test, respectively.
Subjects were likely to miss signals during this initial test. This
was not observed during the next two vigilance tests (total exposure
to carbon monoxide being 210 min with the carboxyhaemoglobin level
estimated to have finally reached 4.3%). These data suggest an initial
decrement in performance followed by a compensatory response. Groll-
Knapp et al. (1972) exposed 20 subjects for a 2-h period to carbon
monoxide concentrations of 0, 57, 115, or 172 mg/m3 (0, 50, 100,
150 ppm). There is some doubt as to which subjects, if any, were
smokers. Carboxyhaemoglobin levels were also estimated by these
investigators at the end of the test period to be 0, 3.0, 5.4, and
7.6%. Over the 90 min of the auditory test, some 200 paired tones were
given in which a weaker second tone was the signal. The mean number of
signals missed during the control test was 26. The number missed
increased in the presence of elevated carboxyhaemoglobin levels so
that 35, 40, and 44 misses occurred in environments containing carbon
monoxide levels of 57, 114, and 172 mg/m3, respectively. This
suggests a significant impairment when a concentration of 57 mg/m3
is inhaled. Winneke (1974) used a similar test in studies at carbon
monoxide concentrations of 0, 57, and 115 mg/m3. Results with all
levels of carbon monoxide exposure were negative, in marked contrast
to the previous data despite an estimated carboxyhaemoglobin level of
approximately 9% at the end of the 115 mg/m3 exposure.
Beard & Grandstaff (1975) examined the effect of carbon monoxide
exposure on a visual vigilance task. The signal was a shorter flash of
light than the non-signals. Following a 30-min control period, 9
subjects were exposed to carbon monoxide concentrations of 0, 57, 200,
or 286 mg/m3 (0, 50, 175, 250 ppm). Subjects exposed to room air
detected 73% of the signals presented to them in three vigils. In
environments containing carbon monoxide concentrations of 57 and
200 mg/m3, respectively, 64% of the signals were detected. These
differences were statistically significant at the 5% level. However,
exposure to a concentration of 286 mg/m3 yielded a correct
identification rate of about 70% that was not statistically
significant. They estimated blood carboxyhaemoglobin levels from
alveolar breath samples to be 1.8, 5.2, and 7.5%, respectively. The
alveolar samples were obtained 30 min after the exposures were
completed. Krotova & Muzyka (1974) studied subjects working for about
2 years in an environment containing carbon monoxide. Mean
carboxyhaemoglobin levels were approximately 3.2% before, and 4% after
work. Eleven of 56 workers reported a loss of vigilance. It has been
reported by Rummo & Sarlanis (1974) that, during a 2-h vigilance
driving simulator task, subjects with carboxyhaemoglobin levels of
6-8% were significantly slower in responding to lead car speed
changes. Horvath et al. (1971) also used a visual vigilance test and
were the only group of investigators that actually measured blood
levels of carboxyhaemoglobin. The vigilance task in these studies was
the detection of a light pulse that was slightly brighter than the
base level light pulse. A 1-h vigil was preceded by a short alerting
pre-test during which a randomly interspersed 10 of 60 light pulses
were the brighter signals. After a 1-min rest, the 60-min vigilance
task was begun. Only 40 of 1200 light pulses were signals. Ten of
these signals appeared randomly out of the 300 presented each 15 min.
Three levels of ambient carbon monoxide were used, 0, 30, and
127 mg/m3 (0, 26, and 111 ppm) with each subject serving as his own
control. Exposures were randomized, with 1 week elapsing between each
exposure. Fig. 6 illustrates the changes in blood levels of
carboxyhaemoglobin with time under all conditions and compares the
concentrations with the levels determined from the data obtained by
Forbes et al. (1945). The control group breathing filtered air (no
carbon monoxide present) had carboxyhaemoglobin levels of 0.9% before
and at the completion of the task. Exposure to a carbon monoxide
concentration of 30 mg/m3 led to a level of 1.6% after the first
hour (before the vigilance task) and 2.3% at the end. Carbon monoxide
exposure at 127 mg/m3 resulted in carboxyhaemoglobin levels of 4.2%
after the first hour and 6.6% at completion of the vigilance test.
Performance during the pre-test period gave approximately 88 correct
responses in all three conditions, carbon monoxide exposure having no
discernible effect. During the vigilance test itself, subjects
breathing a concentration of 127 mg/m3 made significantly fewer
correct responses (4.2-6.6% carboxyhaemoglobin) than the same subjects
breathing 0 or 27 mg/m3 (Fig. 7). It appeared that when the
carboxyhaemoglobin level was approximately 5%, a significant decrement
in performance occurred. It should be noted that the slight
improvement at the end of the test period probably represented the
usual alerting response observed whenever subjects estimate that the
task is completed. Recently, Winneke et al. (1976) attempted to
replicate this study without success. Although Horvath's experiments
were started with 15 alleged nonsmokers, pre-exposure blood samples
from 5 of the subjects showed carboxyhaemoglobin levels of almost 3%.
At the completion of all the exposures, these subjects admitted
smoking, thus confirming the blood levels. Data on these subjects were
not included in this analysis. When these smokers' data were analysed,
performance on the vigilance task showed no deterioration even though,
with exposure to a carbon monoxide concentration of 127 mg/m3,
carboxyhaemoglobin levels increased from an initial 2.8% to 5.1% after
the first hour and to 6.9% at the completion of the vigilance task
(O'Hanlon, 1975). There were too few subjects to permit more than a
suggestion that prior, continuous exposure to nonambient carbon
monoxide may result in some degree of questionable adaptation. Beard &
Wertheim's (1967) earlier indications that some deleterious
psychological effects would appear at carboxyhaemoglobin levels of
about 2% have not been confirmed or even replicated.
The design and experimental control in the major studies concerned
with this subject have been poor with errors of omission, and failure
to present finalized data. In the early studies of Forbes et al.
(1937), 5 subjects were exposed to a carbon monoxide concentration
The small reductions in oxygen content at 5-10% carboxyhaemoglobin may
be quite critical for patients suffering from cardiovascular diseases
or chronic obstructive lung disease. Coburn et al. (1965) published a
detailed theoretical analysis of the physiology and variables that
determine blood carboxyhaemoglobin levels in man. The details of the
formulae used in these calculations are presented in section 6.2.
With regard to the intracellular effects of carbon monoxide,
consideration must be given to the interactions of all substances
within the tissue cells that are involved with oxygen delivery. Since
haemoglobin and myoglobin are structurally related, they react with
carbon monoxide in a similar manner. The function of myoglobin, in
vivo, may be to act as a reservoir for oxygen within the muscle
fibre. The carbon monoxide and oxygen equilibria of human myoglobin
has been studied in vitro and a hyperbolic oxygen dissociation curve
established (Rossi-Fanelli & Antonini, 1958). This curve, unlike that
for haemoglobin, is not affected by the hydrogen ion concentration,
the ionic strength, or the concentration of myoglobin. The relative
affinity constant, M, is approximately 40 but is still sufficient to
induce appreciable formation of carboxymyoglobin. Both Coburn et al.
(1970b, 1973) and Luomanmaki (1966) have studied the interrelation-
ships between carboxyhaemoglobin and carboxymyoglobin. Coburn, using
14CO, showed that identical carbon monoxide exposures can produce
different degrees of saturation of haemoglobin, depending upon the
partial pressures of oxygen in blood and tissue. Coburn (1970b)
determined the ratio of the carbon monoxide content in muscle to the
content in blood as a function of arterial pO2. This ratio, for
skeletal muscle, is approximately 1, but in myocardial tissue it was
found to be 3. When arterial pO2 fell below 5.3-4 kPa (40-30
Torr), carbon monoxide disappeared from the blood, presumably entering
the muscle. Considerable amounts of extravascular carbon monoxide are
stored in muscle. The higher ratio for cardiac tissue may be of
considerable significance. In an individual with a blood
carboxyhaemoglobin level of 10%, some 30% of cardiac myoglobin may be
saturated with carbon monoxide. Coburn and associates (1973) estimated
the mean pO2 of skeletal muscle and myocardium and found that they
were 0.8-1.1 and 0.5-0.8 kPa (6-8 and 4-6 Torr), respectively.
Although no final judgement can be made regarding the next lower
step involved in oxygen transport, i.e., the role of cytochromes a3
and P-450, the fact that, experimentally, they react with carbon
monoxide in the same way as other haem-containing substances suggests
that they may play a role in carbon monoxide poisoning. Available
evidence suggests that interactions between carbon monoxide and
cytochrome oxidases are of minor significance at the concentrations of
carbon monoxide found in community air pollution. All of the data on
the cytochromes have been obtained from in vitro experiments.
Whether similar events occur in vivo remains uncertain. The most
likely oxidase for in vivo inhibition is P-450. Cooper et al. (1965)
reported that the ratio of carbon monoxide to oxygen required for 50%
inhibition is close to 1, in contrast to a similar ratio of between
2.2 and 28 for cytochrome a3. Root (1965) believes that at a pCO
compatible with life, only nonsignificant blocking of the oxygen
consumption system occurs. In terms of the total distribution
throughout the body of an inhaled dose of carbon monoxide, the amounts
bound to these haemoproteins are small compared with haemoglobin and
myoglobin. A diagrammatic representation of the factors influencing
body carbon monoxide stores has been presented by Coburn (1970b). The
possible significance of the role of these haemoproteins lies in the
concept that, under conditions where tissue pO2 is decreased, the
affinity of intracellular haemoproteins for carbon monoxide may
increase.
6.4 Excretion
Adequate data are available on the rate of absorption of carbon
monoxide but there is considerably less information concerning the
rates of carbon monoxide egress from the lungs. The same factors that
determine how much carbon monoxide is taken up by the blood should
apply in reverse when clearance of carbon monoxide from blood is
considered. The primary factors involved are the amounts of carbon
monoxide and oxygen present, the magnitude of ventilation, and the
quality of the diffusion barrier. Age influences the quality of the
barrier and it appears that with advancing age the barrier becomes
"thicker" and there are fewer gas exchange membranes. Sedov et al.
(1971) presented data on the elimination of carbon monoxide at various
atmospheric pressures and ambient temperatures. Neither lower
barometric pressures nor high temperatures appreciably altered the
rates of elimination. It has been implied by Pace et al. (1950) that a
sex difference in elimination may also exist, a faster rate occurring
in females than in males, at least under the experimental conditions
of their study. Some sex differences in excretion have been reported
by Goldsmith et al. (1963).
Available evidence suggests that there is a biphasic decline in
the percentage of carboxyhaemoglobin in the arterial blood (Godin &
Shephard, 1972; Wagner et al., 1975). There is a rapid, initial,
exponential decline (distribution phase), probably related to the
distribution of carbon monoxide from the circulating blood to splenic
blood, myoglobin, and cytochrome enzymes. Elimination of carbon
monoxide through the lungs also occurs during this phase. The
distribution phase, which persists for the first 20-30 min, is
followed by a slower linear decline (elimination phase). This phase
probably reflects the rates of release of carbon monoxide from
haemoglobin and myoglobin, pulmonary diffusion, and ventilation, as
well as the fact that pCO decreases with time. Myhre (1974) found
that a similar biphasic excretion pattern occurred at an altitude of
1630 metres. However, he noted that the half-time of carboxyhaemo-
globin was much longer (5.5 h). After continuous exposure to carbon
monoxide for 49 h, 50% was eliminated in 30-180 min and 90% within
180-420 min (Tiunov & Kustov, 1964). Other investigators (Godin &
Shephard, 1972; Peterson & Stewart, 1970) reported exponential
carboxyhaemoglobin elimintation curves over many hours. However,
because of inadequate sampling in the early phase of elimination, they
were unable to observe the more rapid initial decline. The absolute
level of carboxyhaemoglobin, when the elimination studies began,
apparently modified the rate of disappearance of carbon monoxide from
the blood. Considerable individual variability has been observed and
the duration of exposure may also be an important factor. It has been
shown that prolonged exposure (more than 3 years) to concentrations of
carbon monoxide of 11.5-115 mg/m3 (10-100 ppm) results in a markedly
retarded elimination of carbon monoxide (Kodat, 1971).
In summary, discharge of carbon monoxide occurs rapidly at first
becoming slower with time and the lower the initial level of
carboxyhaemoglobin, the slower the rate of elimination. Apparently, no
studies have been made to determine elimination rates at the low
levels of carboxyhaemoglobin (2-4%) that might be present following
exposure to ambient concentrations of carbon monoxide.
Several procedures have been tested that could accelerate the
excretion of carbon monoxide from the blood of individuals with high
levels of carboxyhaemoglobin (40-70%). Pace et al. (1950) reported
that treatment of such individuals in a recompression chamber with an
oxygen level equivalent to 2.5 atmospheres (partial pressure of oxygen
equal to 253 kPa (1900 Torr) or an alveolar oxygen pressure of 239 kPa
(1801 Tort)) would facilitate removal of carbon monoxide. They
indicated that 1 h in such a chamber would result in the reduction of
the carboxyhaemoglobin level to 10 to 15% of the initial level. In a
revival of the old Henderson & Haggard treatment concept, Malorny et
al. (1962) evaluated the influence of breathing different gas mixtures
on the excretion of carbon monoxide in animals having high
carboxyhaemoglobin levels (60-74%). It was determined that 50% of
carbon monoxide could be excreted in 19 min if 5% carbon dioxide and
95% oxygen were breathed, compared with a time of 28 min for 100%
oxygen, and 41 min for ambient air. Another approach was suggested by
Agostini et al. (1974), who employed a total body asanguineous, hypo-
thermic procedure. These approaches to the removal of the body burden
of excessive amounts of carbon monoxide remain to be evaluated fully
in clinical trials.
7. EFFECTS ON EXPERIMENTAL ANIMALS
Great caution must be used in applying the resultsa obtained
from animal experiments to man. Nonetheless, animal studies have
provided valuable insight into both the potentially adverse effects of
carbon monoxide and the basic mechanisms by which this substance
influences physiological processes. However, many studies have used
extraordinarily high levels of carbon monoxide, rarely found in air.
These studies are not referred to in this document, since the toxic
effects of very high levels of carbon monoxide have been well
documented for both animals and man.
7.1 Species Differences
The oxygen dissociation curves for different animal species used
in carbon monoxide studies are not the same. There are also questions
concerning the relative affinities for carbon monoxide of the
haemoglobin in various animal species. Fodor & Winneke (1971.)
reported in vitro studies that showed different affinities for
different species, the highest being in man followed by rat, mouse,
and rabbit, in descending order. Klimisch et al. (1975) found the
following sequence of affinities: hamster, rat, pig, rabbit. Apart
from different affinities there may well be species differences with
respect to susceptibility to carbon monoxide. According to Alexandrov
(1973) certain mammals can be classified in order of decreasing carbon
monoxide susceptibility as follows: mouse, rat, cat, dog, guineapig,
and rabbit. This difference might, in part, be related to different
ventilation/body weight-ratios.
Species differences in reaction are ideally illustrated in the
studies by De Bias et al. (1972a,b, 1973) on dogs and cynomolgus
monkeys (Macaca fascicularis = M. irus). Chronic exposure (23 h per
day) over several months to 115 mg/m3 (100 ppm) resulted in
carboxyhaemoglobin levels of 14 and 12.4% in dogs, and monkeys,
respectively. The dogs (De Bias et al, 1972a) remained clinically in
good health with no untoward signs that could be interpreted as
induced by carbon monoxide. Serum enzymes, haematological variables,
and electrocardiograms did not change significantly. Carbon monoxide
exposure of normal monkeys resulted in myocardial effects.
Experimentally infarcted monkeys had greater P-wave amplitudes and
increased incidence of T-wave inversions than normal monkeys similarly
exposed. One important fact that may partly explain this difference
between dogs and monkeys is, that monkeys, like man, have end-
arteries, whereas dogs have a well developed collateral circulation.
a Animal studies have been reported in other sections of this
document wherever necessary. Consequently, some data on animals
(such as sheep) are not repeated in this section.
7.2 Cardiovascular System and Blood
Increase of coronary blood flow is the normal response of the
myocardium to carbon monoxide exposure. Permutt & Farhi (1969)
calculated theoretically, that, when carboxyhaemoglobin levels are
approximately 5%, an increase in coronary blood flow of about 20% more
than the resting level would be necessary to prevent coronary sinus
pO2 from falling below minimum levels. This calculation has been
confirmed experimentally by Adams et al. (1973) and Horvath (1973),
both of whom demonstrated approximately similar increases in coronary
blood flow in spite of using very different methods to increase
carboxyhaemoglobin levels.
There is considerable controversy concerning the cardiovascular
effects of carbon monoxide in dogs. Long-term exposures of animals to
carbon monoxide concentrations sufficiently high to produce
carboxyhaemoglobin levels in excess of 20% can induce pathological
changes in the heart and brain. As in acute high level intoxication in
man, serious sequelae develop. Lindenberg et al. (1961) exposed 8 dogs
to carbon monoxide concentrations of 115 mg/m3 (100 ppm). Four were
exposed continuously for 24 h a day, 7 days per week, for 6 weeks and
another 4 were exposed intermittently. All dogs had abnormal
electrocardiograms and some of the hearts showed histological
degeneration of muscle. In another study, Preziosi et al. (1970)
exposed dogs continuously to 115 mg/m3 for 6 weeks and reported
abnormal electrocardiograms, right and left heart dilatation, and
myocardial thinning. Histological examination showed older scarring in
some cases and fatty degeneration of heart muscle in others.
Carboxyhaemoglobin levels of 7.7 to 12% were lower than would be
predicted from the exposure. When 4 dogs were exposed intermittently
to a carbon monoxide concentration of 115 mg/m3 for 11 weeks,
central nervous system and cardiac effects were found (Lewey &
Drabkin, 1944).
Ehrich et al. (1944) also exposed 4 dogs to 115 mg/m3 on an
intermittent schedule. They observed that electrocardiographic changes
occurred at variable times during the 11 weeks of exposure. The gross
appearance of the hearts after exposure were normal but microscopic
examination revealed marked degenerative changes in individual fibres.
Lindenberg et al. (1961) exposed dogs to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) on the same schedule as for his
115 mg/m3 exposure studies. Carboxyhaemoglobin levels of 2.6-5.5%
were observed. No changes in haemoglobin levels or haematocrit were
noted. Electrocardiographic changes were observed in the third week of
exposure similar to, but less severe than, those observed in animals
exposed to the higher ambient level of carbon monoxide. Dogs were
exposed by Musselman et al. (1959) to a carbon monoxide concentration
of 57 mg/m3 for 24 h per day, 7 days per week, for 3 months. No
changes in the electrocardiogram or heart rates were observed.
Pathological examination of organs and tissues did not reveal any
changes after exposure. Experimental data have been presented by
Orellano et al. (1976) who claim that carbon monoxide injected
intraperitoneally into dogs is nontoxic. This study, as well as other
reports from these investigators, raise some intriguing questions as
to the mechanisms involved in carbon monoxide toxicity.
Continuous exposure of cynomolgus monkeys to a carbon monoxide
concentration of 115 mg/m3 resulted in demonstrable electro-
cardiographic effects in the myocardium of both normal monkeys
and monkeys with myocardial infarction (De Bias et al., 1972b, 1973).
The same investigators (De Bias et al., 1976) also studied the
susceptibility of the ventricles to induced fibrillation. Normal and
infarcted monkeys were exposed to a carbon monoxide concentration of
115 mg/m3 for 6 h. Application of high voltages were required to
produce fibrillation in normal monkeys in air but when infarcted
animals were exposed to carbon monoxide, the voltage level required
was very low. Animals with either infarction alone or carbon monoxide
exposure alone also required significantly less voltage to produce
fibrillation; when the two were combined, the effects were additive.
Intermittent exposure to carbon monoxide (30 min per h, 12 h per day,
over a period of 14 months) of cynomolgus monkeys fed either a normal
or a semipurified cholesterol diet did not result in myocardial
infarctions or electrocardiographic abnormalities (Malinow et al.,
1976). In these animals, the blood carboxyhaemoglobin concentration
reached 21.6% at the end of the daily period of breathing carbon
monoxide at 529 mg/m3 (460 ppm).
Carbon monoxide exposure did not increase the aortic and coronary
atherosclerosis induced in cynomolgus monkeys by cholesterol feeding.
Eckardt et al. (1972) exposed cynomolgus monkeys (22 h per day, 7 days
per week, for 2 years) to carbon monoxide concentrations of 23 or
75 mg/m3 (20 or 65 ppm). Carboxyhaemoglobin levels, which showed
considerable variation during the experimental period, ranged from 2.0
to 5.5% and 4.8 to 10.2% for low and high carbon monoxide ambient
concentrations, respectively. These levels of carboxyhaemoglobin did
not lead to compensatory increases in haematocrit, haemoglobin, or
erythrocyte counts nor to cardiac fibrosis or pathological effects in
the brain. Cholesterol-fed squirrel monkeys were exposed to carbon
monoxide at 229-344 mg/m3 (200-300 ppm) for several hours per day
for 7 months (Webster et al., (1968). No differences were found in
plasma cholesterol or in aortic and carotid atherosclerosis which
could be ascribed to carbon monoxide. However, the authors did observe
an increase in coronary atherosclerosis. Somewhat similar results were
later reported in studies on cynomolgus monkeys (Malinow et al.,
1976). Jones et al. (1971) exposed rats, guineapigs, dogs, and monkeys
to 58, 110, or 229 mg/m3 (51, 96 or 200 ppm) continuously for 90
days. Haematocrit and haemoglobin levels remained constant at the
lowest level of carbon monoxide exposure but were significantly
elevated at the two higher levels in all species except the dog.
Cynomolgus monkeys (Macaca irus) were exposed to a concentration of
286 mg/m3 (250 ppm) for 2 weeks by Thomsen (1974). In all the
exposed animals, the coronary arteries showed widening of the
subendothelial spaces in which cells with or without lipid droplets
were accumulating. He suggested that monkeys were more sensitive to
carbon monoxide than the rabbits studied by Astrup et al. (1967).
Experimental studies on rabbits exposed to relatively high
concentrations of carbon monoxide at 195-206 mg/m3 (170-180 ppm) for
extended periods indicated the presence of high levels of cholesterol
in the arteries or enhanced vascular disease (Astrup et al., 1970).
The lesions observed, which included subendothelial oedema, a gap
between endothelial cells, and increased infiltration of cells with
lipid droplets, might be early precursors of atherosclerotic disease.
However, such lesions occurred only in animals concurrently on a high
cholesterol or fat diet or on both. Exposure to carbon monoxide alone
induced some changes such as endothelial hypertrophy and
proliferation.
One experimental study on the effects of carbon monoxide on the
natural history of heart disease in the cynomolgus monkey has been
reported. De Bias et al. (1973) exposed animals to a carbon monoxide
concentration of 137 mg/m3 (120 ppm) for 24 weeks. The average
carboxyhaemoglobin level of 12.4% resulted in a polycythaemia with an
increase in haematocrit from 35 to 50%. All animals developed
increased P-wave amplitude and T-inversion which suggested nonspecific
myocardial stress rather than ischaemia. Animals in which an
experimental myocardial infarction was produced prior to exposure to
carbon monoxide had more marked electro-cardiographic changes than
animals breathing room air. In 1976, Ramsey & Casper reported that
erythrocytic 2,3-diphosphoglycerate (2,3-DPG) played neither a
compensatory nor an aggravating role in the hypoxia induced by the
presence of 20 or 30% carboxyhaemoglobin. Stupfel & Bouley (1970)
exposed mice and rats for 95 h per week to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) for either 1 to 3 months or for
their natural life expectancy of up to 2 years. A large number of
measurements were made during exposure followed by pathological
examination after death. The authors did not observe any important
effects of carbon monoxide exposure on the animals. In a study by
Penney et al. (1974a,b), the influence of hypoxic hypoxia on the
development of cardiac hypertrophy in the rat was compared with that
of carbon monoxide hypoxia. Exposure to various levels of carbon
monoxide resulted in hypertrophy of both the fight and left ventricles
in contrast with the right ventricle hypertrophy observed in response
to the hypoxic hypoxia stress.
Cardiac hypertrophy and a reduction in cytochrome oxidase levels
were demonstrated in chick embryos exposed to carbon monoxide for 144
and 168 h (Tumasonis & Baker, 1972). The resistance of young chickens
to carbon monoxide decreased with age. Body temperatures decreased
during exposure to carbon monoxide with the greatest fall in
temperature and the longest survival time occurring in the youngest
chickens. Total body asanguineous hypothermic perfusion (total body
exsanguination exchange transfusion) has been suggested as a
therapeutic measure for carbon monoxide poisoning (Agostini et al.,
1974). In an attempt to explain this beneficial effect, Ramirez et al.
(1974) compared the survival of normal dogs exposed to high levels of
carbon monoxide with that of acutely anaemic dogs transfused with
carboxyhaemoglobin blood to normal blood volumes. All normal dogs with
carboxyhaemoglobin levels of 54-100% died within 0.25-10 h but the
transfused animals, having a final mean carboxyhaemoglobin level of
80% after transfusion, survived. The authors suggested that hypoxic
anaemia was not the principal mechanism of carbon monoxide toxicity
but rather a blocking out of the energy supply on the cellular level,
governed by the cytochrome system.
Most of the investigations using rabbits for carbon monoxide
related research originated in Astrup's laboratories, where they first
demonstrated that low carboxyhaemoglobin levels enhanced the
development of atheromatosis (Astrup et al., 1967). Additional studies
(Hellung-Larsen et al., 1968; Thomsen & Kjeldsen, 1975) have shown
that lactate dehydrogenase isoenzymes (M subunits) increase and that a
higher incidence of focal intimal changes occur in rabbits exposed to
carbon monoxide. The myocardial ultrastructure of rabbits exposed to a
concentration of 206 mg/m3 (180 ppm) for at least 4 h showed
degenerative changes such as contraction bands, myofibrillar
disintegration, myelin body formation, and dehiscence of the
intercalated discs (Thomsen & Kjeldsen, 1974). Exposure of rabbits to
a similar concentration of carbon monoxide for 2 weeks resulted in
more extensive myocardial damage (Kjeldsen et al., 1974).
Astrup et al. (1970) furnished evidence that carbon monoxide
increases endothelial membrane permeability. They found that rabbits
exposed to carbon monoxide developed arterial lesions resulting in a
considerable accumulation of lipids. It has also been shown that human
coronary arteries exposed to carbon monoxide in vitro have a higher
uptake of cholesterol, although no significant changes in lipid
synthesis were observed (Sarma et al., 1975). Myocardial damage and
impaired myocardial performance have also been reported in animals and
man exposed to carbon monoxide (Ayres et al., 1970). Although there is
some evidence which suggests that exposure to carbon monoxide can
induce changes in blood vessels and the myocardium, there is also
evidence to the contrary (section 8.1).
7.3 Central Nervous System
Because of the brain's high oxygen demand, cerebral function
should be influenced at low carboxyhaemoglobin levels. However, data
concerning this are contradictory. Sensitivity to carbon monoxide may
follow a circadian rhythm (Stupfel, 1975; Stupfel et al., 1973).
Maximum sensitivity in rats occurred during the dark period of a
12-12 h light-dark cycle. Dyer & Annau (1976) could find no effect on
superior colliculus evoked potentials of rats until the level of
ambient carbon monoxide had reached 573 mg/m3 (500 ppm) in marked
contrast to Xintaras et al. (1966), who observed a 20% increase in the
amplitude of superior colliculus evoked potentials after only 1 h
exposure to 57 mg/m3 (50 ppm), and a 50% increase after a 2-h period
of exposure at this level. This study has, however, been criticized
for not taking into account the effects of dark adaptation on the
amplitude of visual evoked potentials in rats (Dyer & Annau, 1976).
In view of the conflicting reports it is of some interest to
examine the available data on cerebral pO2 tensions, cerebral
blood flow (CBF) and cerebral metabolism. Zorn (1972) studied the
effects of carbon monoxide inhalation on brain and liver pO2 using
platinum electrodes. Tissue pO2 fell in both organs, even at a
carboxyhaemoglobin concentration of 2%, and the fall was approximately
linear to increases in carboxyhaemoglobin. There was a decrease in
pO2 of 0.027-0.24 kPa (0.2-1.8 Torr) for each 1% fall in
oxyhaemoglobin percentage saturation. These data suggest that the
carbon monoxide influenced levels other than the intracellular level,
since if its effects were limited to this area then tissue pO2
would have been expected to increase. Similar studies were performed
by Weiss & Cohen (1974) on rat brain and muscle. They found a decrease
in cerebral cortical pO2 following inhalation of low levels of
carbon monoxide. Unfortunately, they did not measure
carboxyhaemoglobin levels in these rats but, in a group of sham-
operated animals exposed to similar levels of inhaled carbon monoxide,
carboxyhaemoglobin had increased to 3.3%. During progressive
administration of carbon monoxide to dogs, CBF did not increase until
carboxyhaemoglobin levels reached 20%. Thereafter, CBF increased
progressively and was double that in the controls when the
carboxyhaemoglobin level reached 50% (Häggendal et al., 1966). On the
other hand, Traystman (1976) did observe a progressive increase in CBF
in dogs even at very low carboxyhaemoglobin values. The lowest level
studied was 2.5%, which produced a slight but significant CBF
increase. Thus, Traystman did not believe in a threshold effect. The
effects were produced with both hypoxic hypoxia and carbon monoxide
hypoxia. It remains to be seen, how these data relate to cerebral
circulation in man.
The respiratory centre or arterial chemoreceptors were not
stimulated to increase respiratory minute volume even when
carboxyhaemoglobin levels were as high as 40% (Chiodi et al., 1941).
Mills & Edwards (1968) measured the frequency of electrical impulses
in the afferent nerves from the aortic and carotid chemoreceptors and
showed that administration of carbon monoxide did result in
chemoreceptor stimulation. The response appeared to have an
approximately linear relationship with the carboxyhaemoglobin
concentration (at least above 8%). These findings suggest that carbon
monoxide might stimulate breathing. Failure to observe an increased
minute volume may be explained by the fact that, in the presence of
carbon monoxide, the chemoreceptor stimulation was offset by hypoxic
depression of brain structures involved in breathing. There is some
evidence that this balancing between chemoreceptors and central
nervous depression is operative in anaemia (Santiago & Edelman, 1972).
Additional investigations are needed to clarify this effect of carbon
monoxide inhalation.
7.4 Behavioural Changes and Work Performance
In extensive studies on rabbits, guineapigs, rats, and mice
(Gadaskina, 1960; Ljublina, 1960; Rylova, 1960) exposure to a carbon
monoxide level of 30 mg/m3 (26 ppm), although not inducing any
morphological blood changes, did result in a number of unfavourable
physiological changes. Among these were decreased work capacity, poor
adjustment to postural shifts (orthostatic tests), and increased
thyroid activity. These changes were more evident during the initial
period of the exposure but reverted towards normal later, suggesting
some adaptation to carbon monoxide exposure.
7.5 Adaptation
Adaptation apparently can occur in animals exposed to moderate
concentrations of carbon monoxide (Gorbatov & Noro, 1948; Tiunov &
Kustov, 1969) as shown by their ability to tolerate, with apparent
ease, acute exposure to higher concentrations. Both Clark & Otis
(1952) and Tiunov & Kustov (1969) have demonstrated that, after long-
term exposure to carbon monoxide, animals developed tolerance to
short-term, high altitude exposure, and vice versa, an indication of
the development of a common adaptive mechanism. Acclimatization
continued despite a subsequent decrease in the initial elevation of
the haemoglobin concentration (Gorbatov & Noro, 1948). These
investigators noted an acclimatization effect in rats exposed daily to
0.4-0.5% carbon monoxide until loss of consciousness occurred. A
progressive improvement in tolerance time to unconsciousness was noted
so that by the eighth day of exposure a 3-fold improvement over the
time required on the first day had developed. In spite of apparent
acclimatization, the general condition of test animals became worse.
Unexpectedly, daily exposure to a carbon monoxide concentration of
1.0%, which on the first day necessitated a 5-min exposure prior to
unconsciousness, failed to induce any improvement in tolerance. A
possible correction of leftward shift of the oxygen dissociation by
alterations in the concentration of 2,3-diphosphoglycerate (inducing a
shift to the right) has been suggested. However, conflicting results
and the necessity to produce high levels of carboxyhaemoglobin negate
the possibilities of this beneficial effect (Astrup, 1970; Dinman et
al., 1970).
Evidence for adaptation to carbon monoxide is inconclusive.
Further studies appear to be warranted with special attention devoted
to studies using more realistic current ambient levels of carbon
monoxide, and also studies on the possible physiological cost of
adaptation, if it occurs.
7.6 Embryonal, Fetal, Neonatal, and Teratogenic Effects
Few studies have been made on the effects of carbon monoxide on
mammalian fetal growth and survival. Wells (1933) exposed pregnant
rats for 5-8 min to a carbon monoxide concentration of 1718 mg/m3
(1500 ppm) every other day during pregnancy. Maternal unconsciousness
and abortion or absorption of most of the fetuses resulted. Data
concerning carboxyhaemoglobin levels and numbers of animals studied
were not given. Rats were exposed to 0.34% carbon monoxide for 1 h
(carboxyhaemoglobin 60-70%) daily for a period of 3 months (Williams &
Smith, 1935). Among 7 pregnant females, the number of young per litter
was only half that of the controls and only 2 of the 13 newborns
survived to weaning age. Astrup et al. (1972) exposed rabbits during
their 30 days of pregnancy to carbon monoxide resulting in
carboxyhaemoglobin levels of either 9-19 or 16-18%. Neonatal mortality
in the 2 groups increased by 10 and 35%, respectively, compared with a
control value of 4.5%. Birth weights decreased by 12 and 17%,
respectively.
In their studies on the ewe and fetal lamb, Longo & Hill (1977)
indicated that fetal uptake and elimination of carbon monoxide was
relatively slow compared with that of the mother. They also reported
that, during steady-state conditions, fetal levels of
carboxyhaemoglobin were about 25% higher than maternal levels. These
results may be related to species differences, since Longo & Hill
(1970) found that the M valuesa for sheep maternal and fetal blood
were 218 and 216 respectively, while Engel et al. (1969) reported that
fetal haemoglobin had 20% less preferential binding of carbon monoxide
over oxygen than haemoglobin A.
There are very few studies on the teratogenicity of carbon
monoxide exposure. When fertilized chicken eggs were continuously
exposed to a carbon monoxide concentration of 747 mg/m3 (650 ppm)
for up to 18 days of incubation, the percentage of eggs hatching
decreased to 46% and developmental anomalies of the tibia and
metatarsal bones were noted (Baker & Tumasonis, 1972).
a M = Relative affinity of haemoglobin for carbon monoxide
compared with oxygen.
7.7 Carcinogenicity, and Mutagenicity
No evidence is available on carcinogenicity and mutagenicity in
relation to exposure to carbon monoxide.
7.8 Miscellaneous Changes
Kustov et al. (1972) exposed rats to carbon monoxide at 53 mg/m3
(46 ppm) and noted slower weight gains and an increase in haemoglobin.
Some enzyme systems were also found to have increased activity, when
rats were exposed to carbon monoxide (Pankow & Ponsold, 1972; Pankow
et al., 1974b). A slowing of in vivo metabolism of the drugs
hexobarbital and zoxazolamine, with prolongation of their
pharmacological effects has been reported in rats exposed to carbon
monoxide concentrations of 286-3435 mg/m3 (250-3000 ppm) (Montgomery
& Rubin, 1971). With prolonged exposure, these metabolic effects
became less pronounced and reverted to normal more quickly following
removal from the carbon monoxide environment. Sokal (1975) compared
the effect on blood pH and certain carbohydrate metabolic products
resulting from either a bolus administration or a fixed level of
inspired carbon monoxide, both resulting in equivalent final levels of
carboxyhaemoglobin. His data suggest that more intense biochemical
effects resulted following a gradual increase in carboxyhaemoglobin
levels compared with effects seen following rapid elevation of
carboxyhaemoglobin from the bolus. Data presented by Marks & Swiecicki
(1971) indicated that exposure to high levels of carbon monoxide
induced a stresslike response in the form of an elevation in
catecholamines. Swiecicki (1973) reported that increased
carboxyhaemoglobin levels stimulated the adrenergic system and
increased carbohydrate metabolism. He also noted that physical
training of the rat neither prevented nor reduced changes in
carbohydrate metabolism following carbon monoxide exposure and
vibration. Exposure of rats to 57 mg/m3 (50 ppm) for 5 h per day, 5
days per week, for 12 weeks produced an effect on trace metals at the
subcellular level, with a possible reduction in cellular respiration
and nucleoprotein synthesis.
Guineapigs exposed to carbon monoxide concentrations of
1.7-30 mg/m3 (1.5-26 ppm) for 21 days, 8 h per day, did not show any
allergenic effects related to carbon monoxide exposure (Vinogradov et
al., 1974). Plasma leucine aminopeptidase (EC 5.4.11.1)a and
glutamic pyruvic transaminase (EC 2.4.1.2) activity, normally
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
increased by exposure to carbon tetrachloride, were further
potentiated when blood carboxyhaemoglobin levels were elevated. Pankow
et al. (1974a) also observed an additive effect on some enzymes with a
combination of alcohol and carbon monoxide giving a carboxyhaemoglobin
concentration of 50%.
Rondia (1970) observed a significant reduction of benzopyrene-
hydroxylase (EC 1.14.14.2) activity in the liver homogenates of rats
exposed to a carbon monoxide concentration of 70-150 mg/m3
(60-130 ppm) for only a few days. This finding might be interpreted as
meaning that carbon monoxide contributes to the induction of lung
cancer by lengthening the time of retention of carcinogens in the
lung. Additional work is necessary to clarify this important question.
7.9 Interactions
Much of the data reviewed by Pankow & Ponsfold (1974) concerning
the combined effects of carbon monoxide and other biologically active
agents are based on animal experiments. Because of the extreme
exposure conditions used in most of these studies, only a few of them
are directly relevant to the environmental exposure of man to carbon
monoxide.
The experimental evidence on the aggravation of carbon monoxide-
induced atherosclerosis in rabbits by dietary cholesterol has already
been mentioned (see section 7.2). No significant additive effects were
noted from ethanol when dogs exposed to a carbon monoxide
concentration of 115 mg/m3 (100 ppm) for 21 weeks, 5 days a week,
and 6 h per day were given a daily oral dose of 120 ml of a 15%
ethanol solution (Pecora, 1959). However, the excretion of total
lipoproteins was higher when carbon monoxide and ethanol treatments
were combined than with exposure to carbon monoxide alone. An
indication of interaction of sulfur dioxide and carbon monoxide was
given by Prohorov & Rogov (1959) in their experiments on rabbits. The
depressed activity of succinate dehydrogenase (EC 1.3.99.1) on heart,
liver, and kidney due to exposure to sulfur dioxide at 200 mg/m3
(76 ppm) was exacerbated by exposure to carbon monoxide at a level of
200-400 mg/m3 (174-348 ppm), for 3 h per day, over a 3-week period.
As for the combined effects of carbon monoxide and temperature, Tiunov
& Kustov (1969) showed clearly that carbon monoxide toxicity in mice
increased at temperatures above or below normal ambient levels.
8. EFFECTS ON MAN
8.1 Healthy Subjects
8.1.1 Behavioural changes
Demonstrable changes in the central nervous system (CNS) function
of subjects inadvertently exposed to high levels of ambient carbon
monoxide in illuminating gas and in automobile exhaust resulted in a
series of studies to determine psychomotor and psychological
aberrations in subjects having more modest blood levels of
carboxyhaemoglobin than those observed in the potentially moribund
patients. Deficiencies in earlier studies have been related to
inadequate understanding of the significance of behavioural changes,
the inability to distinguish between simple perceptual motor
performance and the more complex performance involving sustained
and/or selective attention, short-term memory, and decision making
among possible alternatives. Furthermore, the physiological mechanisms
involved in carbon monoxide intoxication were not appreciated and the
available physiological and psychological tools were not adequately
exploited. Even today, some physiological and behavioural studies
suffer from similar or other inadequacies, e.g., the failure to
measure blood carboxyhaemoglobin levels, the inability to distinguish
between the physiological effects of a carbon monoxide bolus of high
concentration or the slow, insidious increment in carboxyhaemoglobin
levels over time with lower inhaled concentrations, the amount of
carbon monoxide brought to or removed from the lungs by changes in
alveolar ventilatory volumes, and the small number of volunteers
examined. Other factors involve failure to provide control measures
for bias and effects of the experimental worker (by means of double-
blind administration), control periods so that task-learning effects
do not mask negative results, homogeneity of the groups labelled
"smokers" and "nonsmokers", and control of possible boredom and
fatigue effects, all essentially amounting to a failure to adopt a
proper experimental design that would produce statistically
significant information.
A reduction of logical memory and recognition was demonstrated by
Chalupa (1960) in individuals subjected to acute carbon monoxide
intoxication. However, these functions returned to normal. Sayers et
al. (1929) did not find any significant changes in 6 exposed men
despite carboxyhaemoglobin levels of approximately 20-30%;
observations included hand-eye coordination and steadiness, tapping
speed, arithmetic (continuous addition), location memory, and simple
reaction time. Simple sensory-motor times decreased by 10% in subjects
with carboxyhaemoglobin concentrations of approximately 6.2% (5.5-7%)
(Tiunov & Kustov, 1969). Simulated driving performance did not
deteriorate despite carboxyhaemoglobin levels of 25%, although a small
deterioration was observed when the carboxyhaemoglobin levels were
above 35% (Forbes et al., 1937). The first demonstrable influences of
carbon monoxide on higher CNS functions were noted by McFarland et al.
(1944) in conjunction with their altitude studies, when they observed
reduction of visual acuity at carboxyhaemoglobin levels as low as 5%.
These observations were extended by Halperin et al. (1959) when they
reported that visual function was impaired at carboxyhaemoglobin
levels as low as 4% and that impairment increased at higher levels.
More recently, McFarland et al. (1973) showed that, for glare
recovery, the dark adaptation final threshold values increased as
carboxyhaemoglobin levels rose from control to 6-17%. Peripheral
recognition tasks were not affected until levels reached 17%. They
also stated that central and peripheral complex tasks were not
influenced by low levels of carboxyhaemoglobin. Schulte (1963)
demonstrated that there was a decrease in performance in higher
intellectual processes that was observable when the carboxyhaemoglobin
level exceeded 5%; further deterioration was noted as the level
increased. These results were in direct contradiction to the negative
results obtained by Dorcus & Weigand (1929) who used a similar series
of tests but with subjects exposed for a shorter period. In studies by
Beard & Wertheim (1967), the ability to judge correctly slight
differences in successive short time intervals showed significant
impairment when carboxyhaemoglobin levels were approximately 2 to 3%
above basal levels. These findings, suggesting an altered mental
function, represent the lowest levels of carboxyhaemoglobin that
produce a significant alteration in behavioural performance. However,
attempts to replicate them have been less than satisfactory (O'Donnell
et al., 1971a,b; Stewart et al., 1973a) even though the subjects in
all these other studies attained higher levels of carboxyhaemoglobin.
Some of the discrepancies may be explained on the basis of differences
in protocol and in the environmental conditions under which the tests
were conducted. Some of the investigators designed their experiments
to minimize the factors of boredom and fatigue while others attempted
to minimize external influences and conducted their experiments for a
relatively long time.
However, the Beard & Wertheim study may actually be more relevant
to questions of vigilance and ideally should be discussed in this
context. Assessment of vigilance is the determination of an
individual's ability to detect small changes in his environment,
changes that take place at unpredictable times and so demand
continuous attention. In such monotonous tasks, subjects miss signals
that they would not have missed when starting the task. Such signals
are presented visually or aurally. Fodor & Winneke (1972) and Groll-
Knapp et al. (1972) used auditory signals for their vigilance task.
The former investigators used a white noise (frequency range from 20
to 20 000 Hz lasting 0.36 sec and repeated at 2-sec intervals. About 3
out of every 100 of these noises were slightly less intense and were
used as the signal to which the subjects responded by pressing a
button. Twelve nonsmokers (male and female) were tested at carbon
monoxide concentrations of 0 and 57 mg/m3 (0-50 ppm). They breathed
this concentration for 80 min prior to the first of three vigilance
tests. Carboxyhaemoglobin levels were estimated to be 2.3 and 3.1% at
the beginning and the end of the first vigilance test, respectively.
Subjects were likely to miss signals during this initial test. This
was not observed during the next two vigilance tests (total exposure
to carbon monoxide being 210 min with the carboxyhaemoglobin level
estimated to have finally reached 4.3%). These data suggest an initial
decrement in performance followed by a compensatory response. Groll-
Knapp et al. (1972) exposed 20 subjects for a 2-h period to carbon
monoxide concentrations of 0, 57, 115, or 172 mg/m3 (0, 50, 100,
150 ppm). There is some doubt as to which subjects, if any, were
smokers. Carboxyhaemoglobin levels were also estimated by these
investigators at the end of the test period to be 0, 3.0, 5.4, and
7.6%. Over the 90 min of the auditory test, some 200 paired tones were
given in which a weaker second tone was the signal. The mean number of
signals missed during the control test was 26. The number missed
increased in the presence of elevated carboxyhaemoglobin levels so
that 35, 40, and 44 misses occurred in environments containing carbon
monoxide levels of 57, 114, and 172 mg/m3, respectively. This
suggests a significant impairment when a concentration of 57 mg/m3
is inhaled. Winneke (1974) used a similar test in studies at carbon
monoxide concentrations of 0, 57, and 115 mg/m3. Results with all
levels of carbon monoxide exposure were negative, in marked contrast
to the previous data despite an estimated carboxyhaemoglobin level of
approximately 9% at the end of the 115 mg/m3 exposure.
Beard & Grandstaff (1975) examined the effect of carbon monoxide
exposure on a visual vigilance task. The signal was a shorter flash of
light than the non-signals. Following a 30-min control period, 9
subjects were exposed to carbon monoxide concentrations of 0, 57, 200,
or 286 mg/m3 (0, 50, 175, 250 ppm). Subjects exposed to room air
detected 73% of the signals presented to them in three vigils. In
environments containing carbon monoxide concentrations of 57 and
200 mg/m3, respectively, 64% of the signals were detected. These
differences were statistically significant at the 5% level. However,
exposure to a concentration of 286 mg/m3 yielded a correct
identification rate of about 70% that was not statistically
significant. They estimated blood carboxyhaemoglobin levels from
alveolar breath samples to be 1.8, 5.2, and 7.5%, respectively. The
alveolar samples were obtained 30 min after the exposures were
completed. Krotova & Muzyka (1974) studied subjects working for about
2 years in an environment containing carbon monoxide. Mean
carboxyhaemoglobin levels were approximately 3.2% before, and 4% after
work. Eleven of 56 workers reported a loss of vigilance. It has been
reported by Rummo & Sarlanis (1974) that, during a 2-h vigilance
driving simulator task, subjects with carboxyhaemoglobin levels of
6-8% were significantly slower in responding to lead car speed
changes. Horvath et al. (1971) also used a visual vigilance test and
were the only group of investigators that actually measured blood
levels of carboxyhaemoglobin. The vigilance task in these studies was
the detection of a light pulse that was slightly brighter than the
base level light pulse. A 1-h vigil was preceded by a short alerting
pre-test during which a randomly interspersed 10 of 60 light pulses
were the brighter signals. After a 1-min rest, the 60-min vigilance
task was begun. Only 40 of 1200 light pulses were signals. Ten of
these signals appeared randomly out of the 300 presented each 15 min.
Three levels of ambient carbon monoxide were used, 0, 30, and
127 mg/m3 (0, 26, and 111 ppm) with each subject serving as his own
control. Exposures were randomized, with 1 week elapsing between each
exposure. Fig. 6 illustrates the changes in blood levels of
carboxyhaemoglobin with time under all conditions and compares the
concentrations with the levels determined from the data obtained by
Forbes et al. (1945). The control group breathing filtered air (no
carbon monoxide present) had carboxyhaemoglobin levels of 0.9% before
and at the completion of the task. Exposure to a carbon monoxide
concentration of 30 mg/m3 led to a level of 1.6% after the first
hour (before the vigilance task) and 2.3% at the end. Carbon monoxide
exposure at 127 mg/m3 resulted in carboxyhaemoglobin levels of 4.2%
after the first hour and 6.6% at completion of the vigilance test.
Performance during the pre-test period gave approximately 88 correct
responses in all three conditions, carbon monoxide exposure having no
discernible effect. During the vigilance test itself, subjects
breathing a concentration of 127 mg/m3 made significantly fewer
correct responses (4.2-6.6% carboxyhaemoglobin) than the same subjects
breathing 0 or 27 mg/m3 (Fig. 7). It appeared that when the
carboxyhaemoglobin level was approximately 5%, a significant decrement
in performance occurred. It should be noted that the slight
improvement at the end of the test period probably represented the
usual alerting response observed whenever subjects estimate that the
task is completed. Recently, Winneke et al. (1976) attempted to
replicate this study without success. Although Horvath's experiments
were started with 15 alleged nonsmokers, pre-exposure blood samples
from 5 of the subjects showed carboxyhaemoglobin levels of almost 3%.
At the completion of all the exposures, these subjects admitted
smoking, thus confirming the blood levels. Data on these subjects were
not included in this analysis. When these smokers' data were analysed,
performance on the vigilance task showed no deterioration even though,
with exposure to a carbon monoxide concentration of 127 mg/m3,
carboxyhaemoglobin levels increased from an initial 2.8% to 5.1% after
the first hour and to 6.9% at the completion of the vigilance task
(O'Hanlon, 1975). There were too few subjects to permit more than a
suggestion that prior, continuous exposure to nonambient carbon
monoxide may result in some degree of questionable adaptation. Beard &
Wertheim's (1967) earlier indications that some deleterious
psychological effects would appear at carboxyhaemoglobin levels of
about 2% have not been confirmed or even replicated.
The design and experimental control in the major studies concerned
with this subject have been poor with errors of omission, and failure
to present finalized data. In the early studies of Forbes et al.
(1937), 5 subjects were exposed to a carbon monoxide concentration
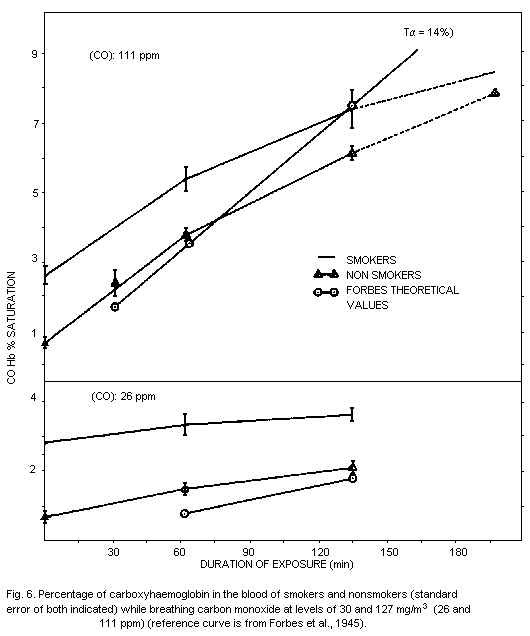
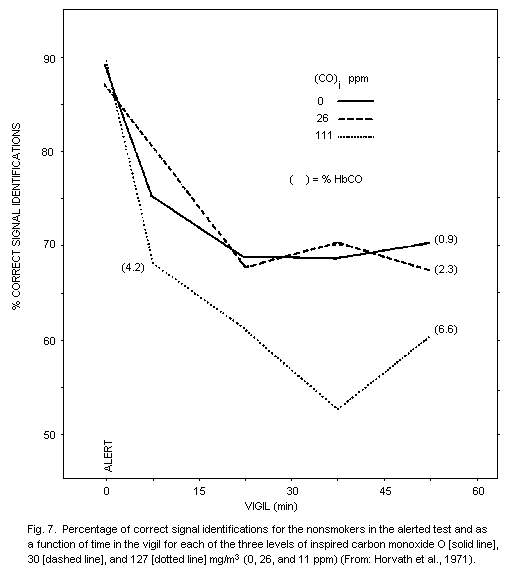 sufficient to raise carboxyhaemoglobin to a level as high as 30% and
their reaction times, coordination, and perceptual skill determined
within the context of a test of driving skill. They failed to present
adequate control data and did not consider the adaptation that occurs
following repetitive tests. McFarland (1973) also studied subjects
with relatively high carboxyhaemoglobin levels (17%) in actual driving
conditions. The exact effects on driving skills could not be
determined from the data presented. Studies by Ray & Rockwell (1970)
and Weir & Rockwell (1973) although first reported in 1970, are still
in a preliminary form and despite some interesting indications of
effects, the data cannot be really considered of value in determining
effects. A "standard driving simulator" was used by Wright et al.
(1973) with both smokers and nonsmokers as subjects (final
carboxyhaemoglobin levels were 5.6 and 7.0% respectively). They
suggested that a 3.4% increase in carboxyhaemoglobin was sufficient to
cause unsafe driving. While questions regarding the data raise doubts
as to the value of this interpretation, these conclusions should be
noted in view of the data reported in the vigilance studies. However,
a certain amount of caution must be applied to any extrapolation of
specific, behavioural changes and driving performance, since the
latter requires integration of many signals in a complex interaction
not measured in any of the simpler tasks used in most behavioural
testing.
The available information on reaction times and time
discrimination is presented in considerable detail in a report from
the National Research Council (NAS/NRC, 1977). Despite apparently
well-controlled studies on both of these variables, the negative and
positive effects reported make it impossible to form any valid
conclusionsa. It would appear that considerable additional effort,
using a larger number of subjects, more adequate control of
experimental conditions (especially control of boredom and fatigue),
direct determinations of carboxyhaemoglobin, and attention to the
potential effects of low levels of carboxyhaemoglobin are required
before any valid conclusions can be drawn.
Apparently, coordination, dexterity, steadiness, and tracking
ability were not influenced by a carbon monoxide concentration which
raised carboxyhaemoglobin to levels exceeding 20%. McFarland et al.
(1944) and Halperin et al. (1959) reported that carboxyhaemoglobin
a The studies by Beard & Wertheim (1967) suggesting a critical
carboxyhaemoglobin level of approximately 2% were evaluated under
vigilance and are not repeated here. Attempts by other
investigators (O'Donnell et al., 197 lb; Stewart et al., 1975) to
reproduce their results have not been successful.
levels of 4-5% resulted in impaired brightness discrimination. Their
findings have been confirmed by Beard & Wertheim (1967). However,
Ramsey (1973) was unable to reproduce these deleterious effects on
brightness discrimination. The effects of carbon monoxide exposure on
complex learned behaviour have been studied by a number of
investigator's. Exposure of firemen to a concentration of 115 mg/m3
(100 ppm) for various periods of time (Schulte, 1963) resulted in
considerable changes in the performance of a series of complex tasks.
In a test where subjects were required to underline all plural nouns
in prose passages, decreased performance was noted when the carboxy-
haemoglobin level was approximately 8%. The mean time to complete an
arithmetic test significantly increased at similar or slightly lower
carboxyhaemoglobin levels. This investigator may have underestimated
the carboxyhaemoglobin levels since the subjects, although mostly
smokers, had initial values close to zero. O'Donnell et al. (1971a)
studied the ability of 4 subjects to perform arithmetic problems
without pencil and paper. While the subjects required a longer time to
complete the answers (89.8 versus 98.6 sec) when carboxyhaemoglobin
levels were 5.9% and 12.7%, respectively, some questions as to the
experimental design of these studies and the limited number of
subjects used preclude full acceptance of the results. A deterioration
in the ability to learn meaningless syllables was found by Bender et
al. (1972), when the carboxyhaemoglobin levels were about 7%. Other
tests failed to show deterioration at these levels of
carboxyhaemoglobin.
O'Donnell et al. (1971a) sought to determine how overnight
exposure to carbon monoxide concentrations of 86.0 mg/m3 and
172 mg/m3 (75 and 150 ppm) (carboxyhaemoglobin levels up to 12.7%)
affected sleep and found small but unreliable changes that they
interpreted as a possible reduction in central nervous activation. A
significant reduction in REM (rapid eye movement) sleep in subjects of
both sexes exposed for 7 h to a carbon monoxide concentration of
115 mg/m3 (100 ppm) was reported by Groll-Knapp et al. (1976).
Earlier, Helmchen & Künkel (1964) reported changes in the rhythmic
after-potential fluctuations following photic excitation of the brain
during and following carbon monoxide exposure. However, in contrast,
Dinman (1969) analysed the photic responses in subjects with
carboxyhaemoglobin levels of 22% and 37% and did not find any changes
in latency or voltage following photic stimulation. Sul'ga (1962) did
not find any disturbances of the alpha rhythm in 2 subjects exposed to
a carbon monoxide concentration of 20 mg/m3 (17.4 ppm) for 15 min.
Carbon monoxide-induced visual invoked responses were reported by
Hosko (1970) and Stewart et al. (1973a) but only at carboxyhaemoglobin
levels of 20-28%. Stewart et al. (1973b) later reported that neither
the spontaneous nor the evoked electrical activity of the brain
exhibited significant changes attributable to carbon monoxide exposure
(carboxyhaemoglobin levels from 3.2% to 15.2%). Slow-wave brain
potentials (correlated to anticipatory responses) were measured by
Groll-Knapp et al. (1972) who noted a diminution in the height reached
by the anticipation wave and the extent of the drop seen after
response stimulus following exposure to a carbon monoxide
concentration of 172 mg/m3 (150 ppm). It appears that another
conical function test, critical flicker fusion frequency (CFFF) is not
influenced even by carboxyhaemoglobin levels of between 10% and 12.7%
(Guest et al., 1970; O'Donnell et al., 1971a; Ramsey, 1973; Winneke,
1974). Guest et al. (1970) also used an auditory analogue of CFFF, the
auditory flutter fusion threshold. This threshold was not affected by
a carboxyhaemoglobin level of 10%. The literature has been reviewed by
Grandstaff et al. (1975).
8.1.2 Work performance and exercise
Maximum exercise can increase the oxygen uptake of the whole body
by 20 or more times the resting uptake; at this level the oxygen
transport system will be maximally stressed. Indeed, Mitchell et al.
(1958) have suggested that the maximum sustained energy output is
determined by the capability of the cardiovascular system to transport
oxygen to the exercising muscle. Assuming this concept to be true, any
impairment of oxygen transport, such as can occur when
carboxyhaemoglobin is present could limit maximum aerobic capacity
( Vo2 max). In fact, it has been appreciated for some time that
individuals, having a large burden of carbon monoxide experience
difficulty in performing physical work. Subjects studied by Chiodi et
al. (1941) were unable to perform tasks requiring only low levels of
physical exertion when their blood levels of carboxyhaemoglobin
reached 40-50%. Several collapsed while attempting to perform routine
laboratory exercise tests. Roughton & Darling (1944) also suggested,
on theoretical grounds, that work capacity would be reduced to zero
when carboxyhaemoglobin levels approached 50%. An impaired performance
by competitive swimmers was associated with exposure to a carbon
monoxide level of 34 mg/m3 (30 ppm) originating from traffic
(MacMillan, 1969)a. Douze (1971) presented information on the
incidence of carbon monoxide poisoning due to the use of natural gas
heaters in Utrecht.
a Quoted by the US National Research Council, Division of Medical
Sciences, Committee on Effects of Atmospheric Contaminants on Human
Health and Welfare, 1969, p. 55.
There appears to be complete agreement that performance of light
to moderate work (up to 70% Vo2 max)b for a short period of time
is not significantly influenced by carboxyhaemoglobin levels as high
as 33%. All the submaximal exercise tests were of short duration
(5-60 min). Oxygen uptake during work was unchanged despite the
presence of carboxyhaemoglobin (Chevalier et al., 1966; Ekblom & Huot,
1972; Gliner et al., 1975; Mitchell et al., 1958; Nielsen, 1971;
Pirnay et al., 1971; Vogel & Gleser, 1972; Vogel et al., 1972). The
only clear indication of physiological load appeared to be a slight
increase in heart rate. Chevalier and associates (1963, 1966) studying
men carrying out light work for a period of 5 min, reported that while
the oxygen uptake was unaffected when the carboxyhaemoglobin level was
approximately 4% (estimated value), there was a significant increase
in oxygen debt when this was related to the total increased oxygen
uptake. Five subjects studied by Pirnay et al. (1971) performing work
for 15 min had an oxygen uptake of 1.5 litre per min. No changes in
oxygen uptake were found even though the carboxyhaemoglobin level
reached 15%. In a rather involved study, where carboxyhaemoglobin
levels fluctuated between 5% and 17%, Klausen et al. (1968) did not
find any differences in energy expenditure in relation to exposure
when subjects exercised for 15 min at 50% of their Vo2 max. It is
rather interesting that, despite the considerable variations in such
experimental conditions as the duration and magnitude of exercise, the
level of carboxyhaemoglobin and the method of administration of carbon
monoxide, and also the small numbers and limited age ranges of the
exposed subjects -- the results from all these studies were
essentially similar. Pirnay et al. (1971), Vogel & Gleser (1972), and
Vogel et al. (1972) reported consistently higher heart rates for given
selected submaximal work loads and increased ventilatory volume
exchange per unit of oxygen uptake.
Since populations may be exposed to polluted environments for long
periods, Gliner et al. (1975) studied the responses of 2 groups of 10
and 9 men, respectively (mean age 23.0 and 48~4 years) each of which
included 5 subjects who smoked. A work load of 35% Vo2 max was
selected (untrained men can work at this level for approximately 8 h
with minimum physiological changes), and the men walked for 4 h in an
environment containing a carbon monoxide concentration of 57 mg/m3
b This figure may be in error for all levels of carboxyhaemoglobin
above 5% since Vo2 max decreases with increasing level of
carboxyhaemoglobin and the initial percentages of Vo2 max were
apparently determined on the basis of a Vo2 max measured at
0.5% carboxyhaemoglobin for the fixed work loads used in the
studies. Thus, the highest percentage of Vo2 max reported (70%)
may have been as high as 91% and would represent hard work. Vo2
max is identical to the maximum aerobic capacity representing the
capability of the organism to take up oxygen.
(50 ppm). Final carboxyhaemoglobin levels were 5.3 and 6.1% for
nonsmokers and smokers, respectively. An additional study was
conducted on 4 men exposed to a carbon monoxide concentration of
115 mg/m3 (100 ppm). Final carboxyhaemoglobin levels for nonsmokers
and smokers were 10.3 and 13.2%, respectively. Ambient temperatures
were 25°C and 35°C, with a relative humidity of 30%. Cardiovascular
and respiratory variables were measured. The only significant change
was a higher heart rate in the carbon monoxide environment,
irrespective of age of subject (Fig. 8), confirming observations,
previously reported. Cardiac index remained constant at approximately
6 litres/min × m2 in both filtered air and in carbon monoxide
concentrations of 57-115 mg/m3 (50-100 ppm). The full significance
of this change in long-term performance in carbon monoxide polluted
environments is not apparent, at present.
The oxygen transport capacity of blood is reduced in the presence
of carboxyhaemoglobin. In short-term maximum exercise of several
minutes duration, where capacity for effort depends mainly on aerobic
metabolism, maximum aerobic capacity would be expected to diminish
approximately in proportion to the level of carboxyhaemoglobin present
in the blood. Such a diminution in Vo2 max, when the carboxyhaemo-
globin level is between 7% and 33% has been observed by a number of
investigators (Chiodi et al., 1941; Ekblom & Huot, 1972; Horvath et
al., 1975; Nielsen, 1971; Pirnay et al. 1971). In most of these
studies, bouts of exercise ranged from 2-6 min and the mode of
administration of carbon monoxide involved either breathing relatively
high concentrations of the gas or the administration of a bolus with
additional carbon monoxide to maintain the desired levels of
carboxyhaemoglobin. In some of these studies, the smoking habits of
the subjects were not identified.
In all of these studies, the levels of carboxyhaemoglobin were
considerably in excess of those anticipated to occur in men exposed to
the concentrations of carbon monoxide designated as limiting levels by
various governing bodies or even reported to occur in the outdoor air
of certain metropolitan areas. The initial studies by Horvath's group
(Drinkwater et al., 1974; Raven et al., 1974a, b) were made on
subjects breathing a carbon monoxide concentration of 57 mg/m3
(50 ppm) at one of 2 thermal environments, i.e., 25°C or 35°C with a
relative humidity of 20%. A walking test requiring some 15-24 min to
complete was carried out on a treadmill with progressively increasing
grade, in order to measure Vo2 max. The 2 populations consisted of
20 young (24+ years) and 16 middle-aged (48+ years) subjects with
equal numbers of smokers and nonsmokers in the young group and 7
smokers and 9 nonsmokers in the older group. The middle-aged subjects
demonstrated the anticipated decrease in Vo2 max associated with
advancing age. However, the middle-aged nonsmokers had a Vo2 max
that was about 27% greater than that of smokers of the same age. As
the test progressed, the carboxyhaemoglobin levels of nonsmokers
sufficient to raise carboxyhaemoglobin to a level as high as 30% and
their reaction times, coordination, and perceptual skill determined
within the context of a test of driving skill. They failed to present
adequate control data and did not consider the adaptation that occurs
following repetitive tests. McFarland (1973) also studied subjects
with relatively high carboxyhaemoglobin levels (17%) in actual driving
conditions. The exact effects on driving skills could not be
determined from the data presented. Studies by Ray & Rockwell (1970)
and Weir & Rockwell (1973) although first reported in 1970, are still
in a preliminary form and despite some interesting indications of
effects, the data cannot be really considered of value in determining
effects. A "standard driving simulator" was used by Wright et al.
(1973) with both smokers and nonsmokers as subjects (final
carboxyhaemoglobin levels were 5.6 and 7.0% respectively). They
suggested that a 3.4% increase in carboxyhaemoglobin was sufficient to
cause unsafe driving. While questions regarding the data raise doubts
as to the value of this interpretation, these conclusions should be
noted in view of the data reported in the vigilance studies. However,
a certain amount of caution must be applied to any extrapolation of
specific, behavioural changes and driving performance, since the
latter requires integration of many signals in a complex interaction
not measured in any of the simpler tasks used in most behavioural
testing.
The available information on reaction times and time
discrimination is presented in considerable detail in a report from
the National Research Council (NAS/NRC, 1977). Despite apparently
well-controlled studies on both of these variables, the negative and
positive effects reported make it impossible to form any valid
conclusionsa. It would appear that considerable additional effort,
using a larger number of subjects, more adequate control of
experimental conditions (especially control of boredom and fatigue),
direct determinations of carboxyhaemoglobin, and attention to the
potential effects of low levels of carboxyhaemoglobin are required
before any valid conclusions can be drawn.
Apparently, coordination, dexterity, steadiness, and tracking
ability were not influenced by a carbon monoxide concentration which
raised carboxyhaemoglobin to levels exceeding 20%. McFarland et al.
(1944) and Halperin et al. (1959) reported that carboxyhaemoglobin
a The studies by Beard & Wertheim (1967) suggesting a critical
carboxyhaemoglobin level of approximately 2% were evaluated under
vigilance and are not repeated here. Attempts by other
investigators (O'Donnell et al., 197 lb; Stewart et al., 1975) to
reproduce their results have not been successful.
levels of 4-5% resulted in impaired brightness discrimination. Their
findings have been confirmed by Beard & Wertheim (1967). However,
Ramsey (1973) was unable to reproduce these deleterious effects on
brightness discrimination. The effects of carbon monoxide exposure on
complex learned behaviour have been studied by a number of
investigator's. Exposure of firemen to a concentration of 115 mg/m3
(100 ppm) for various periods of time (Schulte, 1963) resulted in
considerable changes in the performance of a series of complex tasks.
In a test where subjects were required to underline all plural nouns
in prose passages, decreased performance was noted when the carboxy-
haemoglobin level was approximately 8%. The mean time to complete an
arithmetic test significantly increased at similar or slightly lower
carboxyhaemoglobin levels. This investigator may have underestimated
the carboxyhaemoglobin levels since the subjects, although mostly
smokers, had initial values close to zero. O'Donnell et al. (1971a)
studied the ability of 4 subjects to perform arithmetic problems
without pencil and paper. While the subjects required a longer time to
complete the answers (89.8 versus 98.6 sec) when carboxyhaemoglobin
levels were 5.9% and 12.7%, respectively, some questions as to the
experimental design of these studies and the limited number of
subjects used preclude full acceptance of the results. A deterioration
in the ability to learn meaningless syllables was found by Bender et
al. (1972), when the carboxyhaemoglobin levels were about 7%. Other
tests failed to show deterioration at these levels of
carboxyhaemoglobin.
O'Donnell et al. (1971a) sought to determine how overnight
exposure to carbon monoxide concentrations of 86.0 mg/m3 and
172 mg/m3 (75 and 150 ppm) (carboxyhaemoglobin levels up to 12.7%)
affected sleep and found small but unreliable changes that they
interpreted as a possible reduction in central nervous activation. A
significant reduction in REM (rapid eye movement) sleep in subjects of
both sexes exposed for 7 h to a carbon monoxide concentration of
115 mg/m3 (100 ppm) was reported by Groll-Knapp et al. (1976).
Earlier, Helmchen & Künkel (1964) reported changes in the rhythmic
after-potential fluctuations following photic excitation of the brain
during and following carbon monoxide exposure. However, in contrast,
Dinman (1969) analysed the photic responses in subjects with
carboxyhaemoglobin levels of 22% and 37% and did not find any changes
in latency or voltage following photic stimulation. Sul'ga (1962) did
not find any disturbances of the alpha rhythm in 2 subjects exposed to
a carbon monoxide concentration of 20 mg/m3 (17.4 ppm) for 15 min.
Carbon monoxide-induced visual invoked responses were reported by
Hosko (1970) and Stewart et al. (1973a) but only at carboxyhaemoglobin
levels of 20-28%. Stewart et al. (1973b) later reported that neither
the spontaneous nor the evoked electrical activity of the brain
exhibited significant changes attributable to carbon monoxide exposure
(carboxyhaemoglobin levels from 3.2% to 15.2%). Slow-wave brain
potentials (correlated to anticipatory responses) were measured by
Groll-Knapp et al. (1972) who noted a diminution in the height reached
by the anticipation wave and the extent of the drop seen after
response stimulus following exposure to a carbon monoxide
concentration of 172 mg/m3 (150 ppm). It appears that another
conical function test, critical flicker fusion frequency (CFFF) is not
influenced even by carboxyhaemoglobin levels of between 10% and 12.7%
(Guest et al., 1970; O'Donnell et al., 1971a; Ramsey, 1973; Winneke,
1974). Guest et al. (1970) also used an auditory analogue of CFFF, the
auditory flutter fusion threshold. This threshold was not affected by
a carboxyhaemoglobin level of 10%. The literature has been reviewed by
Grandstaff et al. (1975).
8.1.2 Work performance and exercise
Maximum exercise can increase the oxygen uptake of the whole body
by 20 or more times the resting uptake; at this level the oxygen
transport system will be maximally stressed. Indeed, Mitchell et al.
(1958) have suggested that the maximum sustained energy output is
determined by the capability of the cardiovascular system to transport
oxygen to the exercising muscle. Assuming this concept to be true, any
impairment of oxygen transport, such as can occur when
carboxyhaemoglobin is present could limit maximum aerobic capacity
( Vo2 max). In fact, it has been appreciated for some time that
individuals, having a large burden of carbon monoxide experience
difficulty in performing physical work. Subjects studied by Chiodi et
al. (1941) were unable to perform tasks requiring only low levels of
physical exertion when their blood levels of carboxyhaemoglobin
reached 40-50%. Several collapsed while attempting to perform routine
laboratory exercise tests. Roughton & Darling (1944) also suggested,
on theoretical grounds, that work capacity would be reduced to zero
when carboxyhaemoglobin levels approached 50%. An impaired performance
by competitive swimmers was associated with exposure to a carbon
monoxide level of 34 mg/m3 (30 ppm) originating from traffic
(MacMillan, 1969)a. Douze (1971) presented information on the
incidence of carbon monoxide poisoning due to the use of natural gas
heaters in Utrecht.
a Quoted by the US National Research Council, Division of Medical
Sciences, Committee on Effects of Atmospheric Contaminants on Human
Health and Welfare, 1969, p. 55.
There appears to be complete agreement that performance of light
to moderate work (up to 70% Vo2 max)b for a short period of time
is not significantly influenced by carboxyhaemoglobin levels as high
as 33%. All the submaximal exercise tests were of short duration
(5-60 min). Oxygen uptake during work was unchanged despite the
presence of carboxyhaemoglobin (Chevalier et al., 1966; Ekblom & Huot,
1972; Gliner et al., 1975; Mitchell et al., 1958; Nielsen, 1971;
Pirnay et al., 1971; Vogel & Gleser, 1972; Vogel et al., 1972). The
only clear indication of physiological load appeared to be a slight
increase in heart rate. Chevalier and associates (1963, 1966) studying
men carrying out light work for a period of 5 min, reported that while
the oxygen uptake was unaffected when the carboxyhaemoglobin level was
approximately 4% (estimated value), there was a significant increase
in oxygen debt when this was related to the total increased oxygen
uptake. Five subjects studied by Pirnay et al. (1971) performing work
for 15 min had an oxygen uptake of 1.5 litre per min. No changes in
oxygen uptake were found even though the carboxyhaemoglobin level
reached 15%. In a rather involved study, where carboxyhaemoglobin
levels fluctuated between 5% and 17%, Klausen et al. (1968) did not
find any differences in energy expenditure in relation to exposure
when subjects exercised for 15 min at 50% of their Vo2 max. It is
rather interesting that, despite the considerable variations in such
experimental conditions as the duration and magnitude of exercise, the
level of carboxyhaemoglobin and the method of administration of carbon
monoxide, and also the small numbers and limited age ranges of the
exposed subjects -- the results from all these studies were
essentially similar. Pirnay et al. (1971), Vogel & Gleser (1972), and
Vogel et al. (1972) reported consistently higher heart rates for given
selected submaximal work loads and increased ventilatory volume
exchange per unit of oxygen uptake.
Since populations may be exposed to polluted environments for long
periods, Gliner et al. (1975) studied the responses of 2 groups of 10
and 9 men, respectively (mean age 23.0 and 48~4 years) each of which
included 5 subjects who smoked. A work load of 35% Vo2 max was
selected (untrained men can work at this level for approximately 8 h
with minimum physiological changes), and the men walked for 4 h in an
environment containing a carbon monoxide concentration of 57 mg/m3
b This figure may be in error for all levels of carboxyhaemoglobin
above 5% since Vo2 max decreases with increasing level of
carboxyhaemoglobin and the initial percentages of Vo2 max were
apparently determined on the basis of a Vo2 max measured at
0.5% carboxyhaemoglobin for the fixed work loads used in the
studies. Thus, the highest percentage of Vo2 max reported (70%)
may have been as high as 91% and would represent hard work. Vo2
max is identical to the maximum aerobic capacity representing the
capability of the organism to take up oxygen.
(50 ppm). Final carboxyhaemoglobin levels were 5.3 and 6.1% for
nonsmokers and smokers, respectively. An additional study was
conducted on 4 men exposed to a carbon monoxide concentration of
115 mg/m3 (100 ppm). Final carboxyhaemoglobin levels for nonsmokers
and smokers were 10.3 and 13.2%, respectively. Ambient temperatures
were 25°C and 35°C, with a relative humidity of 30%. Cardiovascular
and respiratory variables were measured. The only significant change
was a higher heart rate in the carbon monoxide environment,
irrespective of age of subject (Fig. 8), confirming observations,
previously reported. Cardiac index remained constant at approximately
6 litres/min × m2 in both filtered air and in carbon monoxide
concentrations of 57-115 mg/m3 (50-100 ppm). The full significance
of this change in long-term performance in carbon monoxide polluted
environments is not apparent, at present.
The oxygen transport capacity of blood is reduced in the presence
of carboxyhaemoglobin. In short-term maximum exercise of several
minutes duration, where capacity for effort depends mainly on aerobic
metabolism, maximum aerobic capacity would be expected to diminish
approximately in proportion to the level of carboxyhaemoglobin present
in the blood. Such a diminution in Vo2 max, when the carboxyhaemo-
globin level is between 7% and 33% has been observed by a number of
investigators (Chiodi et al., 1941; Ekblom & Huot, 1972; Horvath et
al., 1975; Nielsen, 1971; Pirnay et al. 1971). In most of these
studies, bouts of exercise ranged from 2-6 min and the mode of
administration of carbon monoxide involved either breathing relatively
high concentrations of the gas or the administration of a bolus with
additional carbon monoxide to maintain the desired levels of
carboxyhaemoglobin. In some of these studies, the smoking habits of
the subjects were not identified.
In all of these studies, the levels of carboxyhaemoglobin were
considerably in excess of those anticipated to occur in men exposed to
the concentrations of carbon monoxide designated as limiting levels by
various governing bodies or even reported to occur in the outdoor air
of certain metropolitan areas. The initial studies by Horvath's group
(Drinkwater et al., 1974; Raven et al., 1974a, b) were made on
subjects breathing a carbon monoxide concentration of 57 mg/m3
(50 ppm) at one of 2 thermal environments, i.e., 25°C or 35°C with a
relative humidity of 20%. A walking test requiring some 15-24 min to
complete was carried out on a treadmill with progressively increasing
grade, in order to measure Vo2 max. The 2 populations consisted of
20 young (24+ years) and 16 middle-aged (48+ years) subjects with
equal numbers of smokers and nonsmokers in the young group and 7
smokers and 9 nonsmokers in the older group. The middle-aged subjects
demonstrated the anticipated decrease in Vo2 max associated with
advancing age. However, the middle-aged nonsmokers had a Vo2 max
that was about 27% greater than that of smokers of the same age. As
the test progressed, the carboxyhaemoglobin levels of nonsmokers
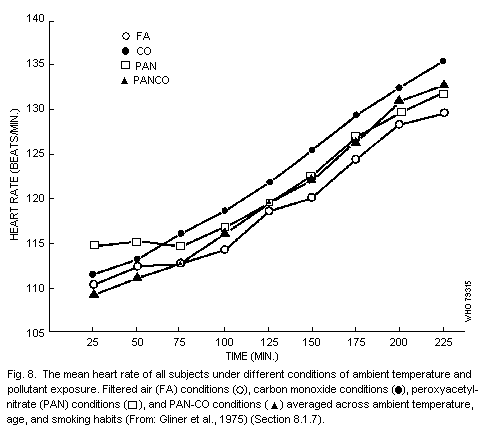 increased from 0.7% to approximately 2.8%, while those of smokers rose
from 2.6-3.2% to 4.1-4.5%. During control studies conducted on these
subjects while breathing filtered air, carboxyhaemoglobin levels
decreased in both smokers and nonsmokers. The results of these studies
(Drinkwater et al., 1974; Gliner et al., 1975; Raven et al., 1974a, b)
failed to demonstrate any reduction in Vo2, max. The decrement in
Vo2 max that occurred as a consequence of working in a hot
environment was greater than the changes observed while breathing
carbon monoxide. Other cardiovascular, respiratory, metabolic, and
temperature measurements made concurrently with the oxygen uptake
studies also failed to show any decrements associated with carbon
monoxide exposure. However, a decrease in absolute exercise time
consistently observed in nonsmoking subjects but not in smokers was
significantly related to carbon monoxide exposure. These observations
confirmed those found earlier by Ekblom & Huot (1972), although they
reported a surprisingly large (38%) decrease in work time at a
carboxyhaemoglobin level of 7%. Aronow & Cassidy (1975) have recently
reported a slight decrease in work time during a maximum exercise test
on 10 middle-aged (50.7 years) subjects. The only ischaemic S-T
segment depression occurred in one female subject. No electro-
cardiographic changes were observed in the subjects studied by
Horvath's group. Nielsen (1971) found that exercising subjects
developed higher internal body temperatures in the presence of carbon
monoxide than in its absence. Reductions in skin conductance suggested
a redistribution of the circulation to the working muscle and away
from the skin.
Horvath and co-workers had some doubts about the changes in
carboxyhaemoglobin levels in smokers and nonsmokers as well as the
lack of change in Vo2 max under the ambient and exercise
conditions employed. For their next series of studies (Dahms et al.,
1975), they developed a more precise method to regulate relatively low
levels of carboxyhaemoglobin (Fig. 9). It should be noted that a low
ambient level of carbon monoxide will reduce the rate of pulmonary
excretion of carbon monoxide particularly if the carboxyhaemoglobin
level is low. In these experiments, a double-blind study was again
used in which subjects breathed either filtered air or air containing
carbon monoxide which resulted in stable levels of carboxyhaemoglobin.
The data suggest that a critical level of carboxyhaemoglobin must be
present before significant physiological alterations can be
demonstrated. Statistically significant decreases in Vo2 max were
noted when carboxyhaemoglobin levels exceeded 4.3%. Although this was
a double-blind, randomized study in which neither the investigators
nor the subjects knew the composition of the air breathed, it was
subsequently determined that all subjects correctly identified the
experiment in which they had been exposed to the highest level of
ambient carbon monoxide. In all instances, they noted a heaviness in
the lower extremities and greater difficulty in performing the task.
increased from 0.7% to approximately 2.8%, while those of smokers rose
from 2.6-3.2% to 4.1-4.5%. During control studies conducted on these
subjects while breathing filtered air, carboxyhaemoglobin levels
decreased in both smokers and nonsmokers. The results of these studies
(Drinkwater et al., 1974; Gliner et al., 1975; Raven et al., 1974a, b)
failed to demonstrate any reduction in Vo2, max. The decrement in
Vo2 max that occurred as a consequence of working in a hot
environment was greater than the changes observed while breathing
carbon monoxide. Other cardiovascular, respiratory, metabolic, and
temperature measurements made concurrently with the oxygen uptake
studies also failed to show any decrements associated with carbon
monoxide exposure. However, a decrease in absolute exercise time
consistently observed in nonsmoking subjects but not in smokers was
significantly related to carbon monoxide exposure. These observations
confirmed those found earlier by Ekblom & Huot (1972), although they
reported a surprisingly large (38%) decrease in work time at a
carboxyhaemoglobin level of 7%. Aronow & Cassidy (1975) have recently
reported a slight decrease in work time during a maximum exercise test
on 10 middle-aged (50.7 years) subjects. The only ischaemic S-T
segment depression occurred in one female subject. No electro-
cardiographic changes were observed in the subjects studied by
Horvath's group. Nielsen (1971) found that exercising subjects
developed higher internal body temperatures in the presence of carbon
monoxide than in its absence. Reductions in skin conductance suggested
a redistribution of the circulation to the working muscle and away
from the skin.
Horvath and co-workers had some doubts about the changes in
carboxyhaemoglobin levels in smokers and nonsmokers as well as the
lack of change in Vo2 max under the ambient and exercise
conditions employed. For their next series of studies (Dahms et al.,
1975), they developed a more precise method to regulate relatively low
levels of carboxyhaemoglobin (Fig. 9). It should be noted that a low
ambient level of carbon monoxide will reduce the rate of pulmonary
excretion of carbon monoxide particularly if the carboxyhaemoglobin
level is low. In these experiments, a double-blind study was again
used in which subjects breathed either filtered air or air containing
carbon monoxide which resulted in stable levels of carboxyhaemoglobin.
The data suggest that a critical level of carboxyhaemoglobin must be
present before significant physiological alterations can be
demonstrated. Statistically significant decreases in Vo2 max were
noted when carboxyhaemoglobin levels exceeded 4.3%. Although this was
a double-blind, randomized study in which neither the investigators
nor the subjects knew the composition of the air breathed, it was
subsequently determined that all subjects correctly identified the
experiment in which they had been exposed to the highest level of
ambient carbon monoxide. In all instances, they noted a heaviness in
the lower extremities and greater difficulty in performing the task.
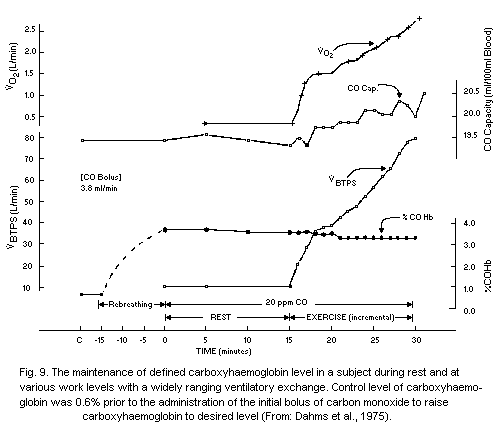 Data obtained by Horvath's group and others are summarized in
Fig. 10. There is a linear decline in Vo2 max when
carboxyhaemoglobin levels range from 4 to 33%. This can be expressed
as: % decrease in Vo2 max = 0.91 (% HbCO) + 2.2. It should be
noted that this does not apply to smokers in Horvath's series, who
frequently had carboxyhaemoglobin levels considerably in excess of
4-5% with no decrement in their respective Vo2 max values.
According to data available at present, carbon monoxide can modify
physiological responses. The level of blood carboxyhaemoglobin
required to induce these effects appears to be approximately 5%. The
carboxyhaemoglobin concentration is probably a more accurate
assessment of exposure than a statement of the exposure conditions,
ambient carbon monoxide concentrations, time, etc. Therefore,
physiological effects should be related to a carboxyhaemoglobin level
even though in some circumstances the method of exposure, i.e. rapid
loading versus slow loading may produce effects not evident from the
carboxyhaemoglobin concentration.
8.1.3 Adaptation
The implication that, in the presence of a clinical state of
chronic carbon monoxide poisoning, adaptation to carbon monoxide
occurs has not been verified. It would appear that such a state could
have been identified by studies on long-term heavy smokers. or
individuals exposed to environmental sources of carbon monoxide. Early
concern with carbon monoxide intoxication in England and Scandinavia
resulted in studies suggesting the possibility of such a condition
(Grut, 1949; Killick, 1940). However, doubts regarding the use of high
levels of inspired carbon monoxide (several hundred parts per million)
and inadequate experimental methods give rise to some scepticism about
the conclusions presented. Killick (1940), using herself as a subject,
reported that she developed acclimatization in the form of diminished
symptoms, slower heart rate, and the attainment of a lower
carboxyhaemoglobin equilibrium level following exposure to a given
inspired concentration of carbon monoxide. A similar finding
concerning the attainment of a different carboxyhaemoglobin
equilibrium following exposure to a fixed level of carbon monoxide in
the ambient air had been reported earlier by Haldane & Priestley
(1935). Additional information on other possible adaptation effects in
the pre-1940 literature can be found in Killick's review.
The changes indicated above, which have been reported as evidence
of adaptation, are probably related to compensatory haematological
changes. Some polycythaemia occurs as a response to chronic or
repeated exposure corresponding roughly to the ambient levels of
carbon monoxide. Brieger (1944) reported an increase in red cell mass
following exposure to 115 mg/m3 (100 ppm) and industrial workers
Data obtained by Horvath's group and others are summarized in
Fig. 10. There is a linear decline in Vo2 max when
carboxyhaemoglobin levels range from 4 to 33%. This can be expressed
as: % decrease in Vo2 max = 0.91 (% HbCO) + 2.2. It should be
noted that this does not apply to smokers in Horvath's series, who
frequently had carboxyhaemoglobin levels considerably in excess of
4-5% with no decrement in their respective Vo2 max values.
According to data available at present, carbon monoxide can modify
physiological responses. The level of blood carboxyhaemoglobin
required to induce these effects appears to be approximately 5%. The
carboxyhaemoglobin concentration is probably a more accurate
assessment of exposure than a statement of the exposure conditions,
ambient carbon monoxide concentrations, time, etc. Therefore,
physiological effects should be related to a carboxyhaemoglobin level
even though in some circumstances the method of exposure, i.e. rapid
loading versus slow loading may produce effects not evident from the
carboxyhaemoglobin concentration.
8.1.3 Adaptation
The implication that, in the presence of a clinical state of
chronic carbon monoxide poisoning, adaptation to carbon monoxide
occurs has not been verified. It would appear that such a state could
have been identified by studies on long-term heavy smokers. or
individuals exposed to environmental sources of carbon monoxide. Early
concern with carbon monoxide intoxication in England and Scandinavia
resulted in studies suggesting the possibility of such a condition
(Grut, 1949; Killick, 1940). However, doubts regarding the use of high
levels of inspired carbon monoxide (several hundred parts per million)
and inadequate experimental methods give rise to some scepticism about
the conclusions presented. Killick (1940), using herself as a subject,
reported that she developed acclimatization in the form of diminished
symptoms, slower heart rate, and the attainment of a lower
carboxyhaemoglobin equilibrium level following exposure to a given
inspired concentration of carbon monoxide. A similar finding
concerning the attainment of a different carboxyhaemoglobin
equilibrium following exposure to a fixed level of carbon monoxide in
the ambient air had been reported earlier by Haldane & Priestley
(1935). Additional information on other possible adaptation effects in
the pre-1940 literature can be found in Killick's review.
The changes indicated above, which have been reported as evidence
of adaptation, are probably related to compensatory haematological
changes. Some polycythaemia occurs as a response to chronic or
repeated exposure corresponding roughly to the ambient levels of
carbon monoxide. Brieger (1944) reported an increase in red cell mass
following exposure to 115 mg/m3 (100 ppm) and industrial workers
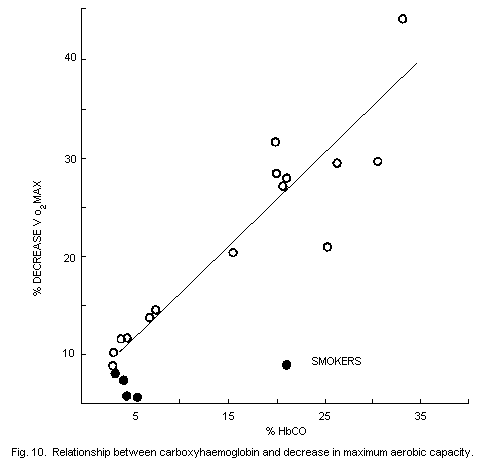 exposed to rather ill-defined levels of carbon monoxide have been
reported to be polycythaemic (Jenkins, 1932). Wilks et al. (1959)
believed that acclimatization was purely a function of increased red
cell mass.
The possibility that adaptation to carbon monoxide following
extensive exposure (as occurs in the case of adaptation to high
altitudes) could alter the position of the oxygen dissociation curve
appears to have been answered. Mulhausen et al. (1968) did not find
any change in the degree of left shift in the blood of individuals
exposed to carbon monoxide for a period of 8 days. Unfortunately, the
average carboxyhaemoglobin level of 13% was based on considerable
individual variations in carboxyhaemoglobin levels and periodic
exposure to relatively high concentrations of inhaled carbon monoxide.
Several investigators have sought evidence of a potential shift of the
curve back to the right. Red cell levels of 2,3-diphosphoglycerate
compounds are higher in individuals with anaemia and also during
residence at high altitudes (2,3-diphosphoglycerate is a
phosphorylated by-product of glycolysis). In the erythrocytes of man
and most other mammals, the molar concentration of this compound is
roughly equal to that of haemoglobin. Both it and some other organic
phosphates are bound rather strongly to deoxyhaemoglobin but have
little affinity for oxyhaemoglobin. Increases in 2,3-diphospho-
glycerate shift the effective oxygen affinity, i.e., there is a shift
of the oxyhaemoglobin dissociation to the right. Astrup (1970) found a
small decrease in erythrocyte 2,3-diphospho-glycerate in human
subjects with carboxyhaemoglobin levels maintained at 20% for 24 h.
Conversely, Dinman et al. (1970) found a small increase in 2,3-
diphosphoglycerate in human subjects after 3 h at an approximate
carboxyhaemoglobin level of 20% and in rats exposed to higher but
variable concentrations of carbon monoxide. A shift in the
dissociation curve does not appear to be an important adaptation
mechanism when carbon monoxide exposure lasts less than a few days.
8.1.4 Effects on the cardiovascular system and other effects
Functional heart disturbances (lability of blood pressure and
heart acceleration, extrasystoles, exacerbations of angina pectoris),
as well as temporary heart dilatation and cardiac asthma have been
reported in cases of acute carbon monoxide poisoning (Lazarev, 1965).
According to the same author, various changes were also seen in the
peripheral vascular system (vasodilation, stasis, vasopermeability
etc.). Lazarev (1965) also noted severe cardiovascular disturbances
such as heart acceleration, extra-systoles, pulse and blood pressure
lability (more often hypotension than hypertension) in groups of
workers exposed to carbon monoxide for long periods. Disturbances of
atrioventricular and interventricular conductance were observed after
1 to 1.5 years of exposure and even after cessation of contact with
carbon monoxide.
The first evidence of left ventricular abnormality was presented
by Corya et al. (1976) in 5 cases of nonfatal poisoning (carboxyhaemo-
globin level of 20%). Abnormal left ventricular wall motion was shown
by echocardiograph in 3 of the 5 cases. A similar number showed mitral
valve prolapse. A ballistocardiogram was used by Gorski (1962) to
demonstrate hypoxaemia of the myocardium in similar cases. Byczkowska
& Milan (1971) described functional kidney disturbances in a patient
poisoned with carbon monoxide. Clinical and physiological haemodynamic
studies on 2 groups (individuals in constant contact with carbon
monoxide and individuals having no evidence of chronic carbon monoxide
intoxication) were conducted by Zenkevic (1973). He noted considerable
cardiovascular abnormalities in the carbon monoxide-exposed group. A
study on cast-iron workers by Ejam-Berdjev (1973) also suggested a
larger frequency of cardiovascular, as well as central nervous system
disturbances in these workers, related to their increased blood levels
of carboxyhaemoglobin.
Evidence of a myocardosis was found in 18% of Japanese farmers
chronically exposed to a mean carbon monoxide concentration of
80 mg/m3 (70 ppm), (Komatsu, 1959). The exposure occurred as a
result of spending the winter months preparing hemp in enclosed
dwellings heated by charcoal fires. Following exposure, the farmers
exhibited symptoms of dizziness, palpitation, and congestive heart
failure. The diagnosis of a myocardosis was supported by clinical
evidence of congestive heart failure and ECG changes such as prolonged
QT interval, ST segment depression, and T-wave flattening.
Aleksieva & Dimitrova (1971) studied a large group of workers
exposed to a carbon monoxide concentration of 60 mg/m3 (52 ppm) and
reported changes in peripheral vessels suggesting impaired vascular
tone.
An additional hazard to patients, especially those undergoing
cardiovascular surgery, may develop during anaesthesia. Markedly
elevated carboxyhaemoglobin levels have been reported in patients
under cardiac bypass surgery (Middleton et al., 1965). This increase
could be related in part to the carbon monoxide present in transfused
blood and to the closed-circuit method of anaesthesia that precludes
the loss of endogenously produced carbon monoxide. This is also
important for infants undergoing transfusions (see sections 5.4 and
8.2.3).
8.1.5 Carboxyhaemoglobin levels resulting from exposure to
methane-derived halogenated hydrocarbons
The belated discovery that at least one chemical substance used in
industry and commerce is "degraded" within the body to carbon monoxide
has potentially significant epidemiological and clinical implications.
Methane-derived halogenated hydrocarbons have been widely used as
organic solvents, replacing carbon tetrachloride. A chance observation
(Stewart et al., 1972b) indicated that the inhalation of
dichloromethane (methylene chloride, CH2CI2) was followed by a
sustained elevation in carboxyhaemoglobin concentrations. Inhalation
of methylene chloride at a concentration of 1740-3480 mg/m3
(500-1000 ppm) (industrial TLVs for USA and USSR = 1740 mg/m3
(500 ppm) and 50 mg/m3 (14 ppm), respectively) for 1-2 h resulted in
carboxyhaemoglobin levels of more than 14% (Stewart et al., 1972a).
This elevation in carboxyhaemoglobin levels continued beyond the time
of exposure and gradually returned to normal during the next 24 h.
Nunes & Schoenborn (1973) demonstrated that the binding affinity of
carbon monoxide for haemoglobin increased in the presence of methylene
chloride. A number of studies have confirmed that methylene chloride
was metabolized to carbon monoxide (Divicenzo & Hamilton, 1975; Kubic
et al., 1974; Ratney et al., 1974; Roth et al., 1975). Roth et al.
(1975) noted that rabbits rarely succumbed to methylene chloride at a
concentration of 40 g/m3 (11 520 ppm) possibly because of saturation
of the pathways of methylene chloride metabolism and the rate of
carbon monoxide excretion. The mechanism by which methylene chloride
is metabolized to carbon monoxide has still to be elucidated. In
vitro studies (Ahmed et al., 1977; Hogan et al., 1976) suggest that
the mixed function oxygenase system of microsomes is responsible for
the metabolic conversion of methylene chloride to carbon monoxide.
Several investigators have studied the influence of methylene
chloride on physiological functions. Astrand et al. (1975) examined
the effects on work performance of exposure to concentrations of 870
and 1740 mg/m3 for four, 30-min periods but did not find any
impairment, apparently because carboxyhaemoglobin levels were low
(4%). Central nervous system depression was observed in some subjects
exposed to concentrations of 1740-3480 mg/m3 (500-1000 ppm) (Stewart
et al., 1972a).
In studies by Winneke (1974), the effects of exposure to ambient
levels of carbon monoxide of up to 115 mg/m3 (100 ppm) on vigilance
and CFFF were less marked than those resulting from exposure to
methylene chloride at 1044-2784 mg/m3 (300-800 ppm).
According to Stewart & Hake (1976) a potentially more dangerous
complication of exposure to methylene chloride is the sustained
carboxyhaemoglobin level that results from the metabolic production of
carbon monoxide from lipid stores of methylene chloride and continues
for many hours following exposure. The potential hazard of chemical
compounds that may be metabolized to carbon monoxide deserves further
investigation.
8.1.6 Levels and effects of carboxyhaemoglobin resulting from smoking
It would be extremely presumptive in a review of the effects of
ambient carbon monoxide to discuss all the possibilities arising from
the incomplete combustion of tobacco and paper. Many of the products
inhaled may produce subtle physiological and biochemical effects on
the smoker. Individuals breathing either pre-inhaled materials or the
smokers' exhaled products are affected to a much lesser degree than
the smoker (Russell et al., 1973; Srch, 1967). It is suggested that
those interested in the problems related to smoking tobacco,
carcinogenesis, and cardiovascular and pulmonary disease, refer to the
documents specifically concerned with these matters (Fletcher & Horn,
1970; Hammond, 1962; US Department of Health, Education and Welfare,
1973; WHO, 1975). Prospective and retrospective epidemiological
studies have, identified cigarette smoking as one of the major factors
in the development of coronary heart disease. The risk of developing
coronary heart disease for pipe and cigar smokers is apparently much
less than it is for cigarette smokers but more than for nonsmokers.
Furthermore, experimental studies suggest that tobacco smoking may
contribute to the development and aggravation of coronary heart
disease through the action of several independent or complementary
mechanisms, one of these being the formation of significant levels of
carboxyhaemoglobin. The role of carboxyhaemoglobin in cancer
development appears to be negligible and unproven. The possible
interaction of carbon monoxide and other constituents of smoke that
may occur in the lungs and other tissues and so induce pathological
changes remains to be elucidated since the basic chemistry has not
been adequately defined.
Kuller et al. (1975) in their epidemiological study in Baltimore,
USA, stated that, if there is an association between carbon monoxide
exposure and heart attacks, the significant exposures are probably
related to micro-environmental factors and cigarette smoking rather
than to community air pollution. They noted that relatively few heart
attacks occurred while an individual was smoking a cigarette. In Los
Angeles, USA (Cohen et al., 1969; Goldsmith & Landaw, 1968; Hexter &
Goldsmith, 1971), the case fatality rate for hospitalized myocardial
infarction (M.I.) patients was higher in areas with high ambient
levels of carbon monoxide (9-16 mg/m3 or 8-14 ppm) and was
positively correlated with ambient carbon monoxide levels. However,
there was no association between ambient carbon monoxide levels and
the admission rates per day. Wallace et al. (1974) concluded that
"from the human health hazard point of view, restriction or
elimination of cigarette smoke makes the most sense in terms of
protecting the atherosclerotic population and preventing a possible
future incidence of coronary heart disease due to chronic carbon
monoxide exposure". It has also been suggested by Astrup (1972) that
the risk of developing arterial diseases from intermittent exposure:
to carbon monoxide may be much higher for smokers than for nonsmokers.
Wald et al. (1973) and Ball & Turner (1974) came to similar
conclusions. In a study by Rissanen et al. (1972), cigarette smokers
had more advanced atherosclerosis than nonsmokers. An extensive review
and some experimental evidence for this viewpoint has been presented
by Kjeldsen (1969).
Smoking cigarettes resulted in higher carboxyhaemoglobin levels
than exposure to carbon monoxide levels present in street air
(Castleden & Cole, 1975; Göthe et al., 1969). Manual workers had lower
carboxyhaemoglobin levels than sedentary workers (both groups being
tobacco smokers), probably because of the increased ventilation
required by the occupations of the manual workers (Castleden & Cole,
1975; Summons & Coleman, 1974). Unless extreme experimental conditions
are considered (Russell et al., 1973; Srch, 1967), carbon monoxide
produced by passive smoking does not seem to present a health risk
(Hinds & First, 1975; Antweiler, 1975; Harke, 1975). Rylander (1974)
also concluded that carbon monoxide exposure through passive smoking
was negligible and that adverse effects upon health would not be
expected. The quantity of carbon monoxide actually entering the lung
depends upon the form in which the tobacco is smoked, the pattern of
smoking, and depth of inhalation. Very little carbon monoxide is
absorbed in the mouth and larynx (approximately 5%) so that most of
the carbon monoxide available for transfer to haemoglobin must reach
the alveoli in order to raise the level of carboxyhaemoglobin present
in the blood stream. Cigarette smokers inhale to a greater extent than
cigar smokers who, in turn, inhale more than pipe smokers, but there
are quite marked individual differences in this pattern. Heavy
cigarette smokers may have carboxyhaemoglobin levels as high as
15-17%. The carbon monoxide concentration in the mainstream smoke of
cigarettes (Table 7) is approximately 4% (V/V) (Fletcher & Horn, 1970;
Hoffman & Wynder, 1972; Wald & Howard, 1975). It has been estimated
that the cigarette smoker may be exposed to a carbon monoxide
concentration of 460-575 mg/m3 (400-500 ppm) for the approximately
6 min needed to smoke a cigarette. Landaw (1973) noted that the half-
time of carbon monoxide elimination in smokers was approximately
291 min. Fig. 11 illustrates the pattern of change in carboxyhaemo-
globin in a typical heavy cigarette smoker (Horvath, personal
communication). An indwelling venous catheter permitted the frequent
sampling of this smoker's blood. The subject smoked only during his
working hours. It should be noted that, by the time he began to smoke
the next day, he still had a body burden of 1.7%. Cigarette smokers
generally excrete carbon monoxide into the air rather than inhale it
from the ambient environment.
Table 7. Carbon monoxide (volume percent) in main stream smokea
Cigarette Cigar
(nonfilter) (filter) A (85 mm) B (85 mm) C (95 mm)
4.6 4.5 5.3 11.1 7.1
a From: Hoffman & Wynder (1972).
exposed to rather ill-defined levels of carbon monoxide have been
reported to be polycythaemic (Jenkins, 1932). Wilks et al. (1959)
believed that acclimatization was purely a function of increased red
cell mass.
The possibility that adaptation to carbon monoxide following
extensive exposure (as occurs in the case of adaptation to high
altitudes) could alter the position of the oxygen dissociation curve
appears to have been answered. Mulhausen et al. (1968) did not find
any change in the degree of left shift in the blood of individuals
exposed to carbon monoxide for a period of 8 days. Unfortunately, the
average carboxyhaemoglobin level of 13% was based on considerable
individual variations in carboxyhaemoglobin levels and periodic
exposure to relatively high concentrations of inhaled carbon monoxide.
Several investigators have sought evidence of a potential shift of the
curve back to the right. Red cell levels of 2,3-diphosphoglycerate
compounds are higher in individuals with anaemia and also during
residence at high altitudes (2,3-diphosphoglycerate is a
phosphorylated by-product of glycolysis). In the erythrocytes of man
and most other mammals, the molar concentration of this compound is
roughly equal to that of haemoglobin. Both it and some other organic
phosphates are bound rather strongly to deoxyhaemoglobin but have
little affinity for oxyhaemoglobin. Increases in 2,3-diphospho-
glycerate shift the effective oxygen affinity, i.e., there is a shift
of the oxyhaemoglobin dissociation to the right. Astrup (1970) found a
small decrease in erythrocyte 2,3-diphospho-glycerate in human
subjects with carboxyhaemoglobin levels maintained at 20% for 24 h.
Conversely, Dinman et al. (1970) found a small increase in 2,3-
diphosphoglycerate in human subjects after 3 h at an approximate
carboxyhaemoglobin level of 20% and in rats exposed to higher but
variable concentrations of carbon monoxide. A shift in the
dissociation curve does not appear to be an important adaptation
mechanism when carbon monoxide exposure lasts less than a few days.
8.1.4 Effects on the cardiovascular system and other effects
Functional heart disturbances (lability of blood pressure and
heart acceleration, extrasystoles, exacerbations of angina pectoris),
as well as temporary heart dilatation and cardiac asthma have been
reported in cases of acute carbon monoxide poisoning (Lazarev, 1965).
According to the same author, various changes were also seen in the
peripheral vascular system (vasodilation, stasis, vasopermeability
etc.). Lazarev (1965) also noted severe cardiovascular disturbances
such as heart acceleration, extra-systoles, pulse and blood pressure
lability (more often hypotension than hypertension) in groups of
workers exposed to carbon monoxide for long periods. Disturbances of
atrioventricular and interventricular conductance were observed after
1 to 1.5 years of exposure and even after cessation of contact with
carbon monoxide.
The first evidence of left ventricular abnormality was presented
by Corya et al. (1976) in 5 cases of nonfatal poisoning (carboxyhaemo-
globin level of 20%). Abnormal left ventricular wall motion was shown
by echocardiograph in 3 of the 5 cases. A similar number showed mitral
valve prolapse. A ballistocardiogram was used by Gorski (1962) to
demonstrate hypoxaemia of the myocardium in similar cases. Byczkowska
& Milan (1971) described functional kidney disturbances in a patient
poisoned with carbon monoxide. Clinical and physiological haemodynamic
studies on 2 groups (individuals in constant contact with carbon
monoxide and individuals having no evidence of chronic carbon monoxide
intoxication) were conducted by Zenkevic (1973). He noted considerable
cardiovascular abnormalities in the carbon monoxide-exposed group. A
study on cast-iron workers by Ejam-Berdjev (1973) also suggested a
larger frequency of cardiovascular, as well as central nervous system
disturbances in these workers, related to their increased blood levels
of carboxyhaemoglobin.
Evidence of a myocardosis was found in 18% of Japanese farmers
chronically exposed to a mean carbon monoxide concentration of
80 mg/m3 (70 ppm), (Komatsu, 1959). The exposure occurred as a
result of spending the winter months preparing hemp in enclosed
dwellings heated by charcoal fires. Following exposure, the farmers
exhibited symptoms of dizziness, palpitation, and congestive heart
failure. The diagnosis of a myocardosis was supported by clinical
evidence of congestive heart failure and ECG changes such as prolonged
QT interval, ST segment depression, and T-wave flattening.
Aleksieva & Dimitrova (1971) studied a large group of workers
exposed to a carbon monoxide concentration of 60 mg/m3 (52 ppm) and
reported changes in peripheral vessels suggesting impaired vascular
tone.
An additional hazard to patients, especially those undergoing
cardiovascular surgery, may develop during anaesthesia. Markedly
elevated carboxyhaemoglobin levels have been reported in patients
under cardiac bypass surgery (Middleton et al., 1965). This increase
could be related in part to the carbon monoxide present in transfused
blood and to the closed-circuit method of anaesthesia that precludes
the loss of endogenously produced carbon monoxide. This is also
important for infants undergoing transfusions (see sections 5.4 and
8.2.3).
8.1.5 Carboxyhaemoglobin levels resulting from exposure to
methane-derived halogenated hydrocarbons
The belated discovery that at least one chemical substance used in
industry and commerce is "degraded" within the body to carbon monoxide
has potentially significant epidemiological and clinical implications.
Methane-derived halogenated hydrocarbons have been widely used as
organic solvents, replacing carbon tetrachloride. A chance observation
(Stewart et al., 1972b) indicated that the inhalation of
dichloromethane (methylene chloride, CH2CI2) was followed by a
sustained elevation in carboxyhaemoglobin concentrations. Inhalation
of methylene chloride at a concentration of 1740-3480 mg/m3
(500-1000 ppm) (industrial TLVs for USA and USSR = 1740 mg/m3
(500 ppm) and 50 mg/m3 (14 ppm), respectively) for 1-2 h resulted in
carboxyhaemoglobin levels of more than 14% (Stewart et al., 1972a).
This elevation in carboxyhaemoglobin levels continued beyond the time
of exposure and gradually returned to normal during the next 24 h.
Nunes & Schoenborn (1973) demonstrated that the binding affinity of
carbon monoxide for haemoglobin increased in the presence of methylene
chloride. A number of studies have confirmed that methylene chloride
was metabolized to carbon monoxide (Divicenzo & Hamilton, 1975; Kubic
et al., 1974; Ratney et al., 1974; Roth et al., 1975). Roth et al.
(1975) noted that rabbits rarely succumbed to methylene chloride at a
concentration of 40 g/m3 (11 520 ppm) possibly because of saturation
of the pathways of methylene chloride metabolism and the rate of
carbon monoxide excretion. The mechanism by which methylene chloride
is metabolized to carbon monoxide has still to be elucidated. In
vitro studies (Ahmed et al., 1977; Hogan et al., 1976) suggest that
the mixed function oxygenase system of microsomes is responsible for
the metabolic conversion of methylene chloride to carbon monoxide.
Several investigators have studied the influence of methylene
chloride on physiological functions. Astrand et al. (1975) examined
the effects on work performance of exposure to concentrations of 870
and 1740 mg/m3 for four, 30-min periods but did not find any
impairment, apparently because carboxyhaemoglobin levels were low
(4%). Central nervous system depression was observed in some subjects
exposed to concentrations of 1740-3480 mg/m3 (500-1000 ppm) (Stewart
et al., 1972a).
In studies by Winneke (1974), the effects of exposure to ambient
levels of carbon monoxide of up to 115 mg/m3 (100 ppm) on vigilance
and CFFF were less marked than those resulting from exposure to
methylene chloride at 1044-2784 mg/m3 (300-800 ppm).
According to Stewart & Hake (1976) a potentially more dangerous
complication of exposure to methylene chloride is the sustained
carboxyhaemoglobin level that results from the metabolic production of
carbon monoxide from lipid stores of methylene chloride and continues
for many hours following exposure. The potential hazard of chemical
compounds that may be metabolized to carbon monoxide deserves further
investigation.
8.1.6 Levels and effects of carboxyhaemoglobin resulting from smoking
It would be extremely presumptive in a review of the effects of
ambient carbon monoxide to discuss all the possibilities arising from
the incomplete combustion of tobacco and paper. Many of the products
inhaled may produce subtle physiological and biochemical effects on
the smoker. Individuals breathing either pre-inhaled materials or the
smokers' exhaled products are affected to a much lesser degree than
the smoker (Russell et al., 1973; Srch, 1967). It is suggested that
those interested in the problems related to smoking tobacco,
carcinogenesis, and cardiovascular and pulmonary disease, refer to the
documents specifically concerned with these matters (Fletcher & Horn,
1970; Hammond, 1962; US Department of Health, Education and Welfare,
1973; WHO, 1975). Prospective and retrospective epidemiological
studies have, identified cigarette smoking as one of the major factors
in the development of coronary heart disease. The risk of developing
coronary heart disease for pipe and cigar smokers is apparently much
less than it is for cigarette smokers but more than for nonsmokers.
Furthermore, experimental studies suggest that tobacco smoking may
contribute to the development and aggravation of coronary heart
disease through the action of several independent or complementary
mechanisms, one of these being the formation of significant levels of
carboxyhaemoglobin. The role of carboxyhaemoglobin in cancer
development appears to be negligible and unproven. The possible
interaction of carbon monoxide and other constituents of smoke that
may occur in the lungs and other tissues and so induce pathological
changes remains to be elucidated since the basic chemistry has not
been adequately defined.
Kuller et al. (1975) in their epidemiological study in Baltimore,
USA, stated that, if there is an association between carbon monoxide
exposure and heart attacks, the significant exposures are probably
related to micro-environmental factors and cigarette smoking rather
than to community air pollution. They noted that relatively few heart
attacks occurred while an individual was smoking a cigarette. In Los
Angeles, USA (Cohen et al., 1969; Goldsmith & Landaw, 1968; Hexter &
Goldsmith, 1971), the case fatality rate for hospitalized myocardial
infarction (M.I.) patients was higher in areas with high ambient
levels of carbon monoxide (9-16 mg/m3 or 8-14 ppm) and was
positively correlated with ambient carbon monoxide levels. However,
there was no association between ambient carbon monoxide levels and
the admission rates per day. Wallace et al. (1974) concluded that
"from the human health hazard point of view, restriction or
elimination of cigarette smoke makes the most sense in terms of
protecting the atherosclerotic population and preventing a possible
future incidence of coronary heart disease due to chronic carbon
monoxide exposure". It has also been suggested by Astrup (1972) that
the risk of developing arterial diseases from intermittent exposure:
to carbon monoxide may be much higher for smokers than for nonsmokers.
Wald et al. (1973) and Ball & Turner (1974) came to similar
conclusions. In a study by Rissanen et al. (1972), cigarette smokers
had more advanced atherosclerosis than nonsmokers. An extensive review
and some experimental evidence for this viewpoint has been presented
by Kjeldsen (1969).
Smoking cigarettes resulted in higher carboxyhaemoglobin levels
than exposure to carbon monoxide levels present in street air
(Castleden & Cole, 1975; Göthe et al., 1969). Manual workers had lower
carboxyhaemoglobin levels than sedentary workers (both groups being
tobacco smokers), probably because of the increased ventilation
required by the occupations of the manual workers (Castleden & Cole,
1975; Summons & Coleman, 1974). Unless extreme experimental conditions
are considered (Russell et al., 1973; Srch, 1967), carbon monoxide
produced by passive smoking does not seem to present a health risk
(Hinds & First, 1975; Antweiler, 1975; Harke, 1975). Rylander (1974)
also concluded that carbon monoxide exposure through passive smoking
was negligible and that adverse effects upon health would not be
expected. The quantity of carbon monoxide actually entering the lung
depends upon the form in which the tobacco is smoked, the pattern of
smoking, and depth of inhalation. Very little carbon monoxide is
absorbed in the mouth and larynx (approximately 5%) so that most of
the carbon monoxide available for transfer to haemoglobin must reach
the alveoli in order to raise the level of carboxyhaemoglobin present
in the blood stream. Cigarette smokers inhale to a greater extent than
cigar smokers who, in turn, inhale more than pipe smokers, but there
are quite marked individual differences in this pattern. Heavy
cigarette smokers may have carboxyhaemoglobin levels as high as
15-17%. The carbon monoxide concentration in the mainstream smoke of
cigarettes (Table 7) is approximately 4% (V/V) (Fletcher & Horn, 1970;
Hoffman & Wynder, 1972; Wald & Howard, 1975). It has been estimated
that the cigarette smoker may be exposed to a carbon monoxide
concentration of 460-575 mg/m3 (400-500 ppm) for the approximately
6 min needed to smoke a cigarette. Landaw (1973) noted that the half-
time of carbon monoxide elimination in smokers was approximately
291 min. Fig. 11 illustrates the pattern of change in carboxyhaemo-
globin in a typical heavy cigarette smoker (Horvath, personal
communication). An indwelling venous catheter permitted the frequent
sampling of this smoker's blood. The subject smoked only during his
working hours. It should be noted that, by the time he began to smoke
the next day, he still had a body burden of 1.7%. Cigarette smokers
generally excrete carbon monoxide into the air rather than inhale it
from the ambient environment.
Table 7. Carbon monoxide (volume percent) in main stream smokea
Cigarette Cigar
(nonfilter) (filter) A (85 mm) B (85 mm) C (95 mm)
4.6 4.5 5.3 11.1 7.1
a From: Hoffman & Wynder (1972).
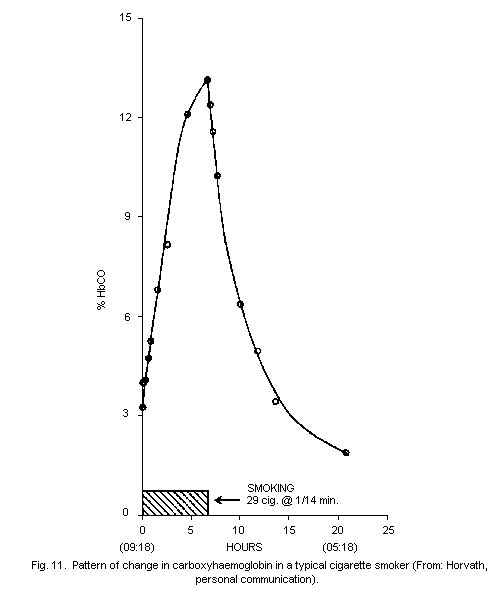 Dalhamn et al. (1968) determined the retention of cigarette smoke
components in the human lung. They found a 54% retention of carbon
monoxide and an 86-97% retention of all other compounds. Carbon
monoxide yields from cigarettes increased with puff volume and tobacco
moisture decreased with increased paper porosity, but remained
constant with puff duration (Robinson & Forbes, 1975). In studies by
Haebisch (1970), the carbon monoxide content of smoke increased as a
greater portion of the cigarette was consumed. Although Cohen et al.
(1971b) reported that different cigarette preparations did not result
in significant variations in the smoker's levels of
carboxyhaemoglobin, more recent studies (Gori, 1976; Turner et al.,
1974) suggested that so-called "low toxicity" cigarettes produced
significantly smaller amounts of carbon monoxide. However, the smoking
pattern of the individual smoker markedly altered the absolute amount
of carbon monoxide inhaled. Frankenhaeuser et al. (1971) reported
another response to cigarette smoking that may have important
consequences for the smoker. They observed a progressive increase in
adrenaline excretion with the number of cigarettes smoked. They also
found that, in moderate smokers, certain psychophysical performance
measures did not deteriorate when the subjects smoked, in contrast to
the decrement observed in nonsmoking conditions (Myrsten et al.,
1972). Aronow et al. (1971a) carried out cardiovascular measurements
on 8 volunteer anginal patients who, after abstaining for 12 h, smoked
3 cigarettes. One week later the same subjects were given carbon
monoxide to breathe so that the final carboxyhaemoglobin levels were
almost equivalent (3.90 and 3.86, respectively). As the patients were
smokers, their initial carboxyhaemoglobin levels were above 2%.
Catheterization of both the left and right ventricles permitted an
evaluation of the functions of the myocardium. The major differences
observed under the two conditions were as follows: cardiac output
decreased with carbon monoxide inhalation but did not change with
smoking; heart rate did not change with carbon monoxide inhalation but
increased with smoking; systolic and diastolic arterial pressures did
not change with carbon monoxide inhalation but increased with smoking;
left ventricular stroke work (dp/dt) decreased with inhalation but
did not change with smoking; left ventricle end-diastolic pressure
increased in both situations; and, finally, the partial pressure of
oxygen in arterial, mixed venous, and coronary sinus blood decreased
in patients inhaling carbon monoxide. These divergent effects need to
be further evaluated. The authors believed that the increased systemic
pressure and heart rates following cigarette smoking were related to
the nicotine in the cigarette.
A critical problem arises in attempts to separate the effects of
carbon monoxide in cigarette smoking from those of other substances
present in the inhaled cigarette smoke. In further studies by Aronow
et al. (1971b), 10 male angina patients smoked lettuce leaf, non-
nicotine cigarettes, resulting in blood carboxyhaemoglobin levels of
7.8%. Heart rate and blood pressure were unaffected by smoking this
type of cigarette but again angina occurred earlier on effort. The
investigators suggested that the presence of carboxyhaemoglobin
following the smoking of cigarettes is the major factor in decreasing
exercise tolerance in subjects with angina pectoris.
It has been suggested by Ball & Turner (1974) that carbon monoxide
and nicotine from cigarette smoke may, by different mechanisms,
accelerate thrombus formation and the development of atherosclerosis.
Carbon monoxide reduces the amount of oxygen available to the
myocardium at the time when the work of the heart has been increased
by the absorption of nicotine. Kjeldsen (1969) reported that in a
group of 1000 Danish individuals a clear relationship between high
carboxyhaemoglobin concentrations after smoking and the occurrence of
atherosclerotic disease was observed. On the other hand, selective
evidence would suggest that carbon monoxide exposure might not be
related to the underlying atherosclerosis. Heavy cigarette smokers in
Japan, where diets are low in fat and cholesterol, do not appear to be
at high risk as regards heart attacks.
As Wald & Howard (1975) stated in their overall review on smoking,
carbon monoxide exposure, and arterial disease: "There is at present
only indirect evidence that carbon monoxide may be a cause of atheroma
in man" and "For the present, however, it is necessary to reserve
judgement on whether carbon monoxide is a cause of arterial disease,
while at the same time suspecting that it may be the principal agent
in tobacco smoke". Aronow and co-workers, as well as Anderson et al.
(1973) have shown that, in patients with ischaemic heart disease,
exercise-induced angina occurs earlier when the patients are exposed
to low levels of carbon monoxide (section 8.2.1). Carbon monoxide
exposure also exacerbates the pain of intermittent claudication and
the duration of effects in patients with this disease. The potential
deleterious influences of cigarette smoking and/or carbon monoxide
exposure on the pregnant woman, fetus, and neonate will be considered
in section 8.2.3. The only direct evidence that carbon monoxide
adversely influences fetal development was derived from studies
conducted on rabbits (Astrup et al., 1972).
A number of studies have suggested that cigarette smoking reduces
working capacity (Goldbarg et al., 1971; Krone et al., 1972; US
Department of Health, Education & Welfare, 1973) and, as presented in
section 8.1.2, this reduction has been directly related to the level
of carboxyhaemoglobin present in the exercising subject. In young
smokers, 21-30 years of age, no differences in maximal aerobic
capacity were observed in spite of reductions in vital capacity and
maximum breathing capacities (Raven et al, 1974a). Older smokers
(40-57 years of age) had significantly lower (27%), aerobic capacity
than nonsmokers of a similar age (Raven et al., 1974b). Younger
smokers had only a 6% lower aerobic capacity than nonsmokers of the
same age. There still remain some questions as to the possible role
played by materials other than carbon monoxide in cigarette smoke in
the reduction of aerobic capacity.
8.1.7 Interactions
There is only a small amount of data available on the combined
effects in man of carbon monoxide and other chemical or physical
agents. Experimental work, carried out by Horvath and his
collaborators (Drinkwater et al., 1974; Gliner et al., 1975; Raven et
al., 1974b), dealt with the combined effects of a concentration of
carbon monoxide of 57 mg/m3 (50 ppm) and peroxyacetylnitrate
1.4 mg/m3 (0.27 ppm) on the work capacity of healthy men. Combined
exposure to both pollutants did not produce greater effects than
exposure to carbon monoxide alone. Hackney et al. (1975) did not find
any consistent changes (synergistic or additive) in pulmonary
functions in a 2-h exposure of young male subjects to a combination of
pollutants namely ozone at 0.5 mg/m3 (0.25 ppm), nitrogen dioxide at
0.56 mg/m3 (0.30 ppm) and carbon monoxide at 34.5 mg/m3 (30 ppm).
As for the combined effects of carbon monoxide and physical agents,
there are occupational data suggesting additive effects of carbon
monoxide and heat (Vyskocil, 1957), and of carbon monoxide
(carboxyhaemoglobin levels up to 35%) and noise in workers exposed to
this stress combination for more than 10 years (Wagemann, 1960; Zorn,
1968).
There is a complete lack of information on the combined effects of
carbon monoxide and drugs or alcohol in man. Furthermore, it is quite
apparent that the question of interactions on the organism of carbon
monoxide and other air contaminants needs clarification.
8.2 High-Risk Groups
The limited clinical research on populations other than healthy,
normal subjects makes it difficult to identify with certainty the
groups that are at increased risk from exposure to carbon monoxide.
However, as will be discussed later in this section, one of these
groups includes individuals with known coronary heart disease. In view
of the susceptibility of this group to the hypoxic stress of carbon
monoxide exposure, it is implicit that other groups are also
potentially subject to increased risk including individuals with
cerebrovascular and peripheral vascular diseases, anaemias, and lung
diseases. In addition, hospitalized individuals suffering from tissue
hypoxia (e.g. shock) or those undergoing operations may be at
increased risk. Individuals with undetected or undiagnosed coronary
artery disease as well as the fetus in utero, the newborn, or, even
pregnant women may be assumed to be at increased risk because of the
anticipated reduced capacity to accommodate hypoxic stress or some
inherent sensitivity to hypoxia. Furthermore, other populations such
as those living at high altitudes, young children, or older adults may
also be at increased risk.
8.2.1 Individuals with cardiovascular and chronic obstructive lung
disease
This section will not be concerned with the potential pathological
effects of cigarette smoking on the development of cardiovascular
disease, chronic pulmonary obstructive disease, and cancer (section
8.1.6). No adequate evidence has been presented, as yet, that carbon
monoxide per se is directly involved in the pathogenesis of these
disorders. Only the potential influence of ambient carbon monoxide on
individuals at risk will be considered.
Epidemiological studies in Los Angeles County (Cohen et al., 1969;
Goldsmith & Landaw, 1968; Hexter & Goldsmith, 1971), have suggested
the possibility of increased mortality from myocardial infarction,
associated with high (9-16 mg/m3 or 8-14 ppm) atmospheric levels of
carbon monoxide. There have been some differences of opinion
concerning the interpretation of these data. A somewhat similar study,
at least in design, was completed in Baltimore by Kuller et al.
(1975). The Baltimore data did not indicate any apparent relationship
between either the incidence of myocardial infarction or sudden death
due to atherosclerotic heart disease and average 24-h ambient carbon
monoxide concentrations. Neither group of investigators was able to
detect a relationship between post mortem carboxyhaemoglobin levels
and causes of sudden death. In the latter study the diagnoses of
disease state were more precise and the population involved more
clearly defined. The ambient levels of carbon monoxide in Baltimore
appeared to be considerably lower than those reported for Los Angeles.
Thus, the possibility of an association between ambient carbon
monoxide and the incidence of myocardial infarction or sudden deaths
remains questionable. It is apparent that more comprehensive and
extensive epidemiological studies need to be conducted in order to
clarify this issue.
There are no adequate studies in man describing the relationship
between exposure to carbon monoxide and the rate of development of
atherosclerotic heart disease. Goldsmith & Aronow (1975) have reviewed
the available evidence.
The heart has a specialized circulatory system in which the
primary response to increased metabolic demands can only be secured by
an increased coronary blood flow. Even under no-stress conditions
(rest) there is an almost complete extraction, roughly 75-80%, of the
available oxygen supply. Adams et al. (1973) monitored conscious dogs
breathing a carbon monoxide concentration of 1718 mg/m3 (1500 ppm)
for a period of 30 min and were able to show a linear relationship
between carboxyhaemoglobin concentration and coronary blood flow. A
13% increase in coronary blood flow occurred at a carboxyhaemoglobin
level of 4% and, at a concentration of 20%, flow rate increased by
54%. Since measurements were not reported for the lower
carboxyhaemoglobin levels, the existence of a threshold could not be
determined. The observations of Mehmel et al. (1973) suggest that
increases in coronary blood flow are stimulated by shifts in the
oxyhaemoglobin dissociation curve. They demonstrated that increasing
pH from 7.4 to 7.6 decreased the P50 ( pO2 at half saturation of
haemoglobin) from 4 to 3.2 kPa (30 to 24 Torr) and increased coronary
blood flow by more than 20%. Earlier studies by Ayres et al. (1969,
1970) also indicated increased blood flow in response to the presence
of increased levels of carboxyhaemoglobin.
Ayres' studies of the haemodynamic and respiratory responses of
man during diagnostic coronary catheterization suggested that carbon
monoxide would have a significant effect on arterial pO2 in
patients with lung disease as well as in patients with certain
cardiovascular disorders.
Studies by Anderson et al. (1973) Aronow et al. (1972) and Aronow
& Isbell (1973) on patients with angina pectoris are listed in
Table 8. Aronow et al. (1972) studied the influence of riding in an
open car on a major Los Angeles freeway. Two trips were made, in one
of which the patients breathed compressed carbon monoxide-free air.
Carboxyhaemoglobin levels after this ride averaged 0.65%, in contrast
to the 5.08% observed in the trip in the open car. The trips were of
90 min duration and ambient carbon monoxide levels in the car averaged
61 mg/m3 (53 ppm). Exercise time, on a bicycle ergometer, to the
onset of angina, was determined prior to, and after the completion of
the exposure. Although no changes in the length of time of work to
onset of anginal pain were noted in the ride while breathing
compressed air, a significant reduction from a mean time of 247 to
174 sec was found when the carboxyhaemoglobin concentration was
elevated. Anginal pain also appeared to persist for a longer time
under these conditions. In a study by Anderson et al. (1973), patients
with stable angina walked on a treadmill. They then breathed air
containing 57 or 115 mg/m3 (50 or 100 ppm) intermittently while
resting for a period of 4 h, raising their carboxyhaemoglobin levels
to 2.9% and 4.5% respectively. The repeat exercise tests clearly
demonstrated a reduction in walking time to onset of angina. No
differences in time were observed at the 2 carboxyhaemoglobin levels
although the duration of the pain was longer at the higher level.
There appeared to be some additional depression of the ST segment but
the degree was not of a significant order. Other measures of cardiac
function-systolic time intervals, left ventricular ejection time, pre-
ejection period index, and pre-ejection peak to ejection time ratio
remained within normal limits.
Table 8. Exercise-induced angina and carbon monoxide (10 subjects per study)
Carboxyhaemoglobin Ambient CO Time to Reference
(%) mg/m3(ppm) angina
response
Initial Final
1.12 5.08 61a (53) Shortened Aronow et al (1972)
1.07 2.68 57b (50) Shortened Aronow & Isbell (1973)
1.40 2.90 57c (50) Shortened Anderson et al. (1973)
a Freeway trip.
b Continuous exposure for 2 h in laboratory,
c Intermittent exposure for 4 h in laboratory.
Another study by Aronow & Isbell (1973) was somewhat similar to
that of Anderson et al. (1973). Aronow and his co-workers exposed
patients (nonsmokers at the time of the test to a carbon monoxide
concentration of 57 mg/m3, resulting in a carboxyhaemoglobin
concentration of 2.68%. This study was also conducted as a double-
blind random trial with one day of breathing carbon monoxide and
another day breathing compressed carbon monoxide-free air. The angina
pectoris of all patients was documented by history and coronary
angiography. A 23% reduction in exercise time (bicycle) resulted
following the carbon monoxide exposure. No electro-cardiographic
changes were seen in these patients during any exercise period.
Plotting of Aronow's data suggests that there was a linear
relationship between carboxyhaemoglobin levels and the decrease in
time to angina.
This evidence suggests that a deleterious effect could occur at
carboxyhaemoglobin levels as low as 2.5% in certain subjects with
coronary heart diseases. The USA National Health Survey Examination
(US Environmental Protection Agency, 1975) reported that in the USA
there were 3 215 000 adults, aged 18-79 years, with definite coronary
heart disease and another 2 410 000 with suspected disease. Many of
these individuals, as well as others in the general population, have
carboxyhaemoglobin levels equal to, or above 2.5%. It would be rash to
even suggest that the above-mentioned studies implicate carbon
monoxide as a factor in determining the natural history of heart
disease in a community. It is even more dangerous to imply that
exposure to carbon monoxide increases the frequency or severity of
chest pain, or shortens life expectancy among patients with angina
pectoris or other clinical manifestations of heart disease. The
necessary epidemiological evidence for an association between the
frequency of episodes of angina pectoris and community ambient levels
of carbon monoxide is inadequate and additional information from more
and varied sources is required.
Patients with chronic obstructive pulmonary disease are probably
at high risk, although few studies on them have been reported. Any
increase in hypoxia could result in respiratory failure. However,
these individuals may absorb less carbon monoxide because of their
disease as the hypoxia may be compensated for by increased
erythropoiesis and a shift of the oxygen dissociation curve to the
right. An interesting approach to the evaluation of individuals at
risk from carbon monoxide and other pollutants can be found in a
publication of the US Environmental Protection Agency (US
Environmental Protection Agency, 1975). Ogawa et al. (1974) have
presented evidence on the development of pulmonary oedema and
discussed possible mechanisms of the role of carbon monoxide in the
disorder.
8.2.2 Anaemic individuals
The information available on the effects of carbon monoxide on
anaemic patients is still inadequate.
The oxygen dissociation curve of blood obtained from patients with
anaemia is shaped like the normal curve but is vertically compressed.
However, when curves from individuals with a 50% reduction in
haemoglobin content are compared with dissociation curves determined
in the presence of 50% carboxyhaemoglobin, there are striking
differences. Consequently, the tendency to make such comparisons is
likely to lead to erroneous deductions concerning effects occurring at
the tissue level. Brody & Coburn (1970) discussed these differences in
relation to arterial and venous pCO and pO2. Because the capacity
of the oxygen transport system is reduced in anaemic persons,
it can be assumed a priori that they could be more at risk from
carbon monoxide exposure than normal persons. Brody & Coburn (1970)
indicated that, if the oxygen content of the mixed venous blood is
abnormally low, as in anaemia or carbon monoxide poisoning, the effect
of the shunted blood in lowering arterial pO2 will be greater than
normal, and a small increase in the alveolar-arterial pressure
difference (AaDO2) will result. The change in the shape of the
oxyhaemoglobin curve due to the presence of carbon monoxide will also
increase the AaDO2. Furthermore, Brody & Coburn (1970) showed that
mild increases in carboxyhaemoglobin concentrations would have little
or no influence on the AaDO2 in normal subjects. However, in
patients with large intra-cardiac right-to-left shunts or with chronic
lung disease and regional variation in the ventilation perfusion ratio
( VA/ QA), the presence of carbon monoxide in the blood will increase
the AaDO2.
Tissue oxygenation may be involved initially because of the
anaemic state, since mixed venous pO2 is decreased (Cropp, 1970)
and the reduction in venous pO2 from a particular carboxyhaemo-
globin value is somewhat greater in anaemic than in normal subjects.
In patients with haemolytic anaemia and sickle cell disease (Engel et
al., 1971), the rate of endogenous carbon monoxide production from
haemoglobin catabolism is increased. Normal subjects produce
approximately 18 µmoles of carbon monoxide per hour, resulting in
carboxyhaemoglobin levels of 0.5 to 0.8%. Carbon monoxide production
in anaemic patients (Coburn et al., 1966; Logue et al., 1971) has been
reported to vary from 31 to 158 µmoles per hour, producing
carboxyhaemoglobin levels of 1.3-5.2%.
Anaemic subjects approach equilibrium levels of carboxyhaemoglobin
more rapidly than those with normal haemoglobin levels at any given
exposure to carbon monoxide. Exposure to a concentration of
22.9 mg/m3 (20 ppm) for approximately 4 h in an individual with a
haemoglobin level of 7 g/100 ml could result in a carboxyhaemoglobin
concentration of 4-5% compared with an anticipated level of 2.5% for
normal individuals. Exogenous carbon monoxide exposure of anaemic
individuals could result, in conjunction with higher endogenous
production, in their attaining critical levels of carboxyhaemoglobin
more rapidly than normal individuals.
8.2.3 Embryo, fetus, neonate, and infants
Pregnant mothers and their fetuses may be exposed acutely or
chronically to carbon monoxide either by maternal smoking or by
environmental pollution. The biological effects of carbon monoxide
exposure on fetal tissues during intrauterine development or during
the newborn period are far from clear.
Several studies (Astrup et al., 1972; MacMahon et al., 1966) have
demonstrated that babies delivered by mothers who smoke cigarettes
weigh less than those delivered by nonsmoking mothers. Relative
maternal, fetal, or placental hypoxia may be responsible, as suggested
by the observation that infants born at high altitudes also weigh less
than those born at sea level (Grahn & Kratchman, 1963). The New Mexico
State Department of Public Health (1975) provided additional
confirmation of the relationship between altitude and birth weights.
Mothers who smoked were reported to have carboxyhaemoglobin
concentrations ranging from 2 to 14%,. while concentrations in the
fetuses ranged from 2.4 to 9.8%. These values may not represent
conditions present during pregnancy, since these data were obtained
just prior to birth. Another factor that may produce differential
effects on the fetus is related to the endogenous production of carbon
monoxide by pregnant women. Delivoria-Papadopoulos et al., (1969)
indicated that nonsmoking pregnant women produced 0.9 ml of carbon
monoxide per hour in contrast to the nonpregnant female's production
of 0.4 ml reported by Longo (1970). Fetal production of endogenous
carbon monoxide accounted for 3% of the total carboxyhaemoglobin
present in the blood of a nonsmoking normal pregnant woman. The source
of the remainder is not well known but may be related to progesterone
levels (Delivoria-Papadopoulos et al., 1969). Even though
hyperventilation of pregnancy may partially compensate for the
increased carbon monoxide production in the absence of exogenous
exposure, the maternal carboxyhaemoglobin still remains about 13%
above that in nonpregnant women (Longo, 1970). It should be noted that
the post partum (24 h) female may be producing 3 times as much carbon
monoxide as a near term nonsmoking pregnant woman.
Behrman et al. (1971) measured carboxyhaemoglobin concentrations
in 25 relatively normal newborn infants in a downtown Chicago nursery
and found the mean value to be 6.98%. These investigators indicated
that absolute carboxyhaemoglobin levels were related to ambient levels
of carbon monoxide. Some doubts about this conclusion exist, since the
monitoring reference site was 2.4 km from the nursery; the
investigators did not report any untoward clinical effects from
exposure to these levels, and no consideration was given to the
possibility of increased endogenous carbon monoxide production in
these infants.
Of the several mechanisms that may account for the influence of
carbon monoxide on developing tissue, the most important is the
interference with tissue oxygenation. Carbon monoxide decreases the
capacity of haemoglobin to transport oxygen and shifts the oxygen
saturation curve to the left. The normal arterial pO2 supplying
fetal tissue is approximately 3.7 kPa (28 Torr). The shift to the left
will tend to further decrease the oxygen gradient from maternal to
fetal blood across the tissue. The decreased pO2 and the
diminished oxygen transport due to the presence of carboxyhaemoglobin
may also produce undesired influences on the fetus. One of the
possible mechanisms by which carbon monoxide or other components of
tobacco smoke may adversely influence fetal development is through
interference with the metabolic function of placental cells. These
cells have a role in metabolizing hormones as well as in the transport
of vitamins, carbohydrates, amino acids, and other substances through
their energy dependent processes. Tanaka (1965) reported that the
oxygen uptake of placental slices from mothers who smoked varied
inversely with maternal levels of carboxyhaemoglobin, being markedly
reduced when this level was higher than 7.0%. The preponderance of
evidence concerning maternal carboxyhaemoglobin levels, along with
fetal and perinatal exposure, tends to warrant the reduction of
exposure to exogenous carbon monoxide sources that might cause this
group to be at risk, to a minimum.
The potential toxicity of carbon monoxide present in transfused
blood has received little attention. Kandall et al. (1973) measured
carboxyhaemoglobin concentrations in donor blood and in relatively
healthy infants receiving exchange blood transfusions. The mean pre-
transfusion carboxyhaemoglobin concentration in 6 cases was 1.34%.
Donor blood contained a carboxyhaemoglobin concentration of 5.17%,
resulting in a mean value of 4.92% in the transfused infant. In one
infant transfused with blood containing a carboxyhaemoglobin
concentration of 8.87%, the resultant carboxyhaemoglobin value in the
infant was 7.43%. Although it was stated that the infants did not
appear to be adversely affected by the levels of carboxyhaemoglobin
reached during exchange transfusion, it should be noted that adverse
effects have been observed in adults at these levels. Furthermore, in
individuals whose oxygen transport system or cardiovascular reserve is
already compromised, the presence of additional carboxyhaemoglobin,
from transfused blood, may result in a further and more potentially
dangerous decrement in arterial, mixed venous, and coronary sinus
oxygen tensions. It should be recalled that some blood samples
collected from blood donors had carboxyhaemoglobin values that
exceeded 18%.
8.2.4 Individuals living at high altitudes
The effects of carbon monoxide and of hypoxia induced by high
altitude are similar. Carbon monoxide produces effects that aggravate
the oxygen deficiency present at high altitudes. When high altitude
and carbon monoxide exposures are combined (Table 9) the effects are
apparently additive. It should be noted, however, that decreased
pO2 in the air and increased carboxyhaemoglobin, produce different
physiological responses. They have different effects on blood pO2,
on the affinity of oxygen for haemoglobin, on the extent of
oxyhaemoglobin saturation (carbon monoxide hypoxaemia shifts the
oxyhaemoglobin dissociation curve to the left, and a decrease in
PAo2 shifts it to the right), and on ventilatory drive. These
effects have been discussed earlier.
The actual influence of a combination of increased carboxy-
haemoglobin and decreased oxyhaemoglobin has not been adequately
documented by experimental data. The few available studies refer
only to acute exposures to lower pO2 and raised pCO. The
most supportive information on the additive nature of this combination
originates from psychophysiological studies and even this information
is not very convincing. When Blackmore (1974) analysed the cause of
aircraft accidents in Britain, he found that carboxyhaemoglobin levels
provided valuable information in relation to altitude and sources of
carbon monoxide. The high levels of carbon monoxide found (up to 74%)
could be attributed to equipment failure, smoking, and fires. No data
are available on the effects of carbon monoxide on the native
inhabitants at high altitudes or on the reactions of these natives
when they are suddenly removed to sea level and possible high ambient
carbon monoxide concentrations.
Table 9. Approximate physiologically equivalent altitudes at
equilibrium with ambient carbon monoxide levelsa
Ambient CO concentration Actual altitude (metres)
mg/m3 ppm 0 (sea level) 1524 3048
Physiologically equivalent altitudes
with carboxyhaemoglobin
0 0 0 (sea level) 1524 3048
28.6 25 1829 2530 3962
67.3 50 3048 3658 4672
114.5 100 3749 4663 5486
a From: NAS/NRC (1977).
In their studies on altitude exposures of young males, McFarland
et al. (1944) showed that changes in visual threshold occurred at
carboxyhaemoglobin levels as low as 5% or at a simulated altitude of
2425 m. These observations were confirmed by Halperin et al. (1959),
who also noted that recovery from the detrimental effects on visual
function lagged behind the elimination of carbon monoxide. However,
the data given were sparse and the variability among the four subjects
was not given. Vollmer et al. (1946) studied the effects of carbon
monoxide at simulated altitudes of 3070 and 4555 m and reported that
there were no additive effects of carbon monoxide and altitude. They
suggested that the effects of carbon monoxide were masked by some
compensatory mechanisms. The data presented were not convincing.
However, Lilienthal & Fugitt (1946) indicated that a combination of
altitude (1540 m) and a carboxyhaemoglobin level of 5-9% induced a
decrease in flicker fusion frequency, although either one alone did
not have any effect. They also reported that the presence of 8-10%
carboxyhaemoglobin was effective in reducing altitude tolerance by
1215 m. During light activity at an altitude of 4875 m, carbon
monoxide uptake increased, probably owing to the hyperventilation at
altitude caused by the respiratory stimulus of decreased pO2
(Forbes et al., 1945). Evidence that carbon monoxide elimination was
similar at sea level and at altitudes up to 10 000 m was obtained by
several investigators (Gorodinsky et al., 1970; Sedov et al., 1971).
However, increased ambient temperatures up to 35°C and hard physical
work increased the rate of elimination (Vollmer et al., 1946). Pitts &
Pace (1947) stated that every 1% increase in carboxyhaemoglobin (up to
13%) was equivalent to a 109 m rise in altitude if the subjects were
at altitudes of 2100-3070 m. These observations were based on changes
in the heart rate response to work. A number of unanswered questions
arise from all these studies, which in general were obscured by such
factors as poor control and no identification of subjects who may have
been smokers.
Two groups of investigators have presented data comparing the
physiological responses of subjects to altitude and carbon monoxide
exposure where the hypoxaemia due to altitude and the presence of
carboxyhaemoglobin were approximately equal. In one study (Astrup &
Pauli, 1968), the carboxyhaemoglobin concentration was about 12%
(although the mode of exposure to carbon monoxide was such that
carboxyhaemoglobin ranged from 5% to 20% and the altitude study was
conducted at 3977 m). The second study (Sedov et al., 1971) compared
responses at an altitude of 4000 m and a carboxyhaemoglobin content of
20%. In both studies, carboxyhaemoglobin content was much in excess of
that anticipated for typical ambient pollution. However, they both
suggested that the effects attributable to carbon monoxide and to
altitude were equal.
8.3 Summary Table
Table 10 is a summary of controlled human studies that provide
useful information for evaluating the relationship between exposure to
carbon monoxide and its health effects.
Table 10. Summary of exposure-effect relationships
Exposure Reported effects Reference
(HbCO %)
(a) Behavioural changes
20 Essentially no impairment in time discrimination Stewart et al. (1973b)
(using Beard-Wertheim task)
11.3 No vigilance decrement (using Horvath task) Winneke et al. (1976)
9a No vigilance decrement (using Fodor-Winneke Winneke (1974)
task); no change in reaction time
8.4 No vigilance decrement (using their own Groll-Knapp et al. (1976)
vigilance task (1972))
7.6 Longer reaction times Ramsey (1973)
7.3 Disturbance in certain perceptual and cognitive Bender et al. (1972)
processes
5 Vigilance decrement Horvath et al. (1971)
4.5 Longer reaction times Ramsey (1972)
3.1b Initial vigilance decrement with subsequent Fodor & Winneke (1972)
normalization; no change in response latency
3b Vigilance decrement Groll-Knapp et al. (1972)
2b Impaired performance in time-discrimination Beard & Wertheim (1967)
(b) Changes in work performance
6.3 Decrease in maximal work time Ekblom & Huot (1972)
4.3 Decrease in a maximal oxygen uptake (VO2) Horvath et al. (1975)
(1.7c)
4.0 Decrease in mean exercise time until exhaustion Aronow & Cassidy (1975)
(0.6c)
2.5 Decrease in absolute exercise time in non- Drinkwater et al. (1974)
smokers
Table 10 (Cont'd)
Exposure Reported effects Reference
(HbCO %)
(c) Aggravation of symptoms in patients with cardiovascular disease
(1.1c)
5.1 Shortened time to angina response immediately Aronow et al. (1972)
after exposure
(1.1c)
2.9 Shortened time to angina response 2 h after Aronow et al. (1972)
(1.1c) exposure
2.9 Shortened time to angina response Anderson et al. (1973)
(1.1c)
2.8 Decrease in mean exercise time until onset of Aronow et al. (1974)
intermittent claudications
(1.0c)
2.7 Shortened time to angina response Aronow & Isbell (1973)
a Estimated values using the formula by Coburn et al. (1965).
b Estimated values using the formula by Peterson & Stewart (1970).
c HbCO % before exposure to CO.
9. EVALUATION OF HEALTH RISKS
9.1 Introduction
The acute toxicity of carbon monoxide has long been recognized and
is well documented. Much has been learned of the main sources of the
gas, its absorption, the kinetics of its reactions with blood, and the
biochemical and pathological consequences of poisoning by excessive
absorption. More recently, a great deal of attention has been paid to
the effects, demonstrable or suspected, of exposure to concentrations
much lower than those that cause definite poisoning. Such
concentrations are those commonly found in urban air (caused almost
wholly by traffic pollution) and indoors (caused by faulty ventilation
of heating or cooking appliances), but there has been much concern
with the effects of the gas on smokers, who inhale considerable
quantities of carbon monoxide with tobacco smoke. Since the main
source of carbon monoxide as an urban pollutant is the petrol engine,
the problems posed by the inhalation of relatively low concentrations
of the gas are likely to grow rather than diminish, as traffic becomes
denser and more widespread. The recognition of the importance of
pollution of the domestic environment is relatively recent and
deserves more study; the problems posed by smoking tobacco are common
and are, unfortunately, increasing. There is much published evidence,
some of which is of debatable value, that suggests that the
comparatively low concentrations of carboxyhaemoglobin produced by
exposure to pollution of the ambient air and the higher concentrations
usually associated with smoking, might cause demonstrable impairment
of vigilance, discrimination, and of the performance of fine tasks and
physical work in healthy subjects, and the exacerbation of symptoms
such as angina pectoris on effort in patients with cardiovascular
diseases. Likewise there is evidence, derived from experimentation on
animals, that chronic exposure to carbon monoxide leading to the
levels of carboxyhaemoglobin commonly found in smokers may, in
association with high cholesterol intakes, play a part in the genesis
of atherosclerosis. Moreover, there are reasons to suspect that
exposure to carbon monoxide may enhance the effects of other
pollutants, commonly administered therapeutic agents, socially
acceptable amounts of beverages such as alcohol, and other
environmental stresses. This section is intended as a brief assessment
of these topics in the hope that sound advice may be given on the need
to control levels of carbon monoxide in the ambient air.
9.2 Exposure
9.2.1 Assessment of exposure
Concentrations of carbon monoxide in air may be measured with
comparative ease by such methods as non-dispersive spectroscopy, gas
chromatography etc. But human body burdens of carboxyhaemoglobin
depend on many factors other than the partial pressure of carbon
monoxide in the inhaled air; among these factors are time of exposure,
pulmonary ventilation (which mainly depends on work done), and blood
volume. Since these quantities, especially ambient concentrations of
carbon monoxide, may vary widely, it is obviously difficult, if not
impossible at times, to calculate the likely body burden of
carboxyhaemoglobin in an exposed individual. There is, therefore, much
to be gained by sampling blood to obtain an integrated estimate of
carboxyhaemoglobin derived from all sources under various conditions
of exposure. It must be emphasized that the measurements of carbon
monoxide in air and in blood give complementary results and are not
merely alternative forms of monitoring. Methods of analysis are
discussed in sections 2.2 and 2.3.
There is a tendency to forget that the reaction between
haemoglobin and carbon monoxide is reversible and that, in a given
environment, a subject may acquire carbon monoxide, excrete it, or
remain in equilibrium with the ambient air depending on the carbon
monoxide concentration and the initial level of carboxyhaemoglobin in
the individual. The time taken to achieve equilibrium between blood
and ambient air depends on the initial carboxyhaemoglobin
concentrations as well as on the factors mentioned above. The rate of
excretion of carbon monoxide will depend not only on ambient air
levels, the initial carboxyhaemoglobin and on factors such as
pulmonary ventilation, but also on the partial pressure of oxygen in
inspired air, which might be introduced therapeutically to increase
elimination. Fig. 12 shows estimates of equilibrium times for various
ambient concentrations and levels of activity. The half-life for
excretion, at rest, is approximately 4“ h.
9.2.2 Endogenous production
The normal breakdown in the body of blood pigments produces carbon
monoxide to give endogenous carboxyhaemoglobin values of 0.1-1.0% and
normal blood is in equilibrium with carbon monoxide levels in air of
roughly 5 mg/m3 (4.3 ppm). These data could be used as a basis for
establishing air quality criteria for carbon monoxide. Various causes
of increased endogenous production of carbon monoxide are discussed in
section 6.1.
9.2.3 Outdoor environmental exposure
Natural sources of carbon monoxide (section 3.1) are of
considerable magnitude but are diffuse, and ambient air concentrations
at locations removed from man-made sources range from 0.01 to
0.9 mg/m3 (0.01 and 0.8 ppm) which is negligible in the context of this
report. By far the most important sources of carbon monoxide at
breathing level are petrol engine vehicle exhausts (section 3.2). The
diesel engine (compression ignition), when properly adjusted, emits
little carbon monoxide. The density, distribution, and mode of
operation of vehicles vary greatly and these and other factors, the
Dalhamn et al. (1968) determined the retention of cigarette smoke
components in the human lung. They found a 54% retention of carbon
monoxide and an 86-97% retention of all other compounds. Carbon
monoxide yields from cigarettes increased with puff volume and tobacco
moisture decreased with increased paper porosity, but remained
constant with puff duration (Robinson & Forbes, 1975). In studies by
Haebisch (1970), the carbon monoxide content of smoke increased as a
greater portion of the cigarette was consumed. Although Cohen et al.
(1971b) reported that different cigarette preparations did not result
in significant variations in the smoker's levels of
carboxyhaemoglobin, more recent studies (Gori, 1976; Turner et al.,
1974) suggested that so-called "low toxicity" cigarettes produced
significantly smaller amounts of carbon monoxide. However, the smoking
pattern of the individual smoker markedly altered the absolute amount
of carbon monoxide inhaled. Frankenhaeuser et al. (1971) reported
another response to cigarette smoking that may have important
consequences for the smoker. They observed a progressive increase in
adrenaline excretion with the number of cigarettes smoked. They also
found that, in moderate smokers, certain psychophysical performance
measures did not deteriorate when the subjects smoked, in contrast to
the decrement observed in nonsmoking conditions (Myrsten et al.,
1972). Aronow et al. (1971a) carried out cardiovascular measurements
on 8 volunteer anginal patients who, after abstaining for 12 h, smoked
3 cigarettes. One week later the same subjects were given carbon
monoxide to breathe so that the final carboxyhaemoglobin levels were
almost equivalent (3.90 and 3.86, respectively). As the patients were
smokers, their initial carboxyhaemoglobin levels were above 2%.
Catheterization of both the left and right ventricles permitted an
evaluation of the functions of the myocardium. The major differences
observed under the two conditions were as follows: cardiac output
decreased with carbon monoxide inhalation but did not change with
smoking; heart rate did not change with carbon monoxide inhalation but
increased with smoking; systolic and diastolic arterial pressures did
not change with carbon monoxide inhalation but increased with smoking;
left ventricular stroke work (dp/dt) decreased with inhalation but
did not change with smoking; left ventricle end-diastolic pressure
increased in both situations; and, finally, the partial pressure of
oxygen in arterial, mixed venous, and coronary sinus blood decreased
in patients inhaling carbon monoxide. These divergent effects need to
be further evaluated. The authors believed that the increased systemic
pressure and heart rates following cigarette smoking were related to
the nicotine in the cigarette.
A critical problem arises in attempts to separate the effects of
carbon monoxide in cigarette smoking from those of other substances
present in the inhaled cigarette smoke. In further studies by Aronow
et al. (1971b), 10 male angina patients smoked lettuce leaf, non-
nicotine cigarettes, resulting in blood carboxyhaemoglobin levels of
7.8%. Heart rate and blood pressure were unaffected by smoking this
type of cigarette but again angina occurred earlier on effort. The
investigators suggested that the presence of carboxyhaemoglobin
following the smoking of cigarettes is the major factor in decreasing
exercise tolerance in subjects with angina pectoris.
It has been suggested by Ball & Turner (1974) that carbon monoxide
and nicotine from cigarette smoke may, by different mechanisms,
accelerate thrombus formation and the development of atherosclerosis.
Carbon monoxide reduces the amount of oxygen available to the
myocardium at the time when the work of the heart has been increased
by the absorption of nicotine. Kjeldsen (1969) reported that in a
group of 1000 Danish individuals a clear relationship between high
carboxyhaemoglobin concentrations after smoking and the occurrence of
atherosclerotic disease was observed. On the other hand, selective
evidence would suggest that carbon monoxide exposure might not be
related to the underlying atherosclerosis. Heavy cigarette smokers in
Japan, where diets are low in fat and cholesterol, do not appear to be
at high risk as regards heart attacks.
As Wald & Howard (1975) stated in their overall review on smoking,
carbon monoxide exposure, and arterial disease: "There is at present
only indirect evidence that carbon monoxide may be a cause of atheroma
in man" and "For the present, however, it is necessary to reserve
judgement on whether carbon monoxide is a cause of arterial disease,
while at the same time suspecting that it may be the principal agent
in tobacco smoke". Aronow and co-workers, as well as Anderson et al.
(1973) have shown that, in patients with ischaemic heart disease,
exercise-induced angina occurs earlier when the patients are exposed
to low levels of carbon monoxide (section 8.2.1). Carbon monoxide
exposure also exacerbates the pain of intermittent claudication and
the duration of effects in patients with this disease. The potential
deleterious influences of cigarette smoking and/or carbon monoxide
exposure on the pregnant woman, fetus, and neonate will be considered
in section 8.2.3. The only direct evidence that carbon monoxide
adversely influences fetal development was derived from studies
conducted on rabbits (Astrup et al., 1972).
A number of studies have suggested that cigarette smoking reduces
working capacity (Goldbarg et al., 1971; Krone et al., 1972; US
Department of Health, Education & Welfare, 1973) and, as presented in
section 8.1.2, this reduction has been directly related to the level
of carboxyhaemoglobin present in the exercising subject. In young
smokers, 21-30 years of age, no differences in maximal aerobic
capacity were observed in spite of reductions in vital capacity and
maximum breathing capacities (Raven et al, 1974a). Older smokers
(40-57 years of age) had significantly lower (27%), aerobic capacity
than nonsmokers of a similar age (Raven et al., 1974b). Younger
smokers had only a 6% lower aerobic capacity than nonsmokers of the
same age. There still remain some questions as to the possible role
played by materials other than carbon monoxide in cigarette smoke in
the reduction of aerobic capacity.
8.1.7 Interactions
There is only a small amount of data available on the combined
effects in man of carbon monoxide and other chemical or physical
agents. Experimental work, carried out by Horvath and his
collaborators (Drinkwater et al., 1974; Gliner et al., 1975; Raven et
al., 1974b), dealt with the combined effects of a concentration of
carbon monoxide of 57 mg/m3 (50 ppm) and peroxyacetylnitrate
1.4 mg/m3 (0.27 ppm) on the work capacity of healthy men. Combined
exposure to both pollutants did not produce greater effects than
exposure to carbon monoxide alone. Hackney et al. (1975) did not find
any consistent changes (synergistic or additive) in pulmonary
functions in a 2-h exposure of young male subjects to a combination of
pollutants namely ozone at 0.5 mg/m3 (0.25 ppm), nitrogen dioxide at
0.56 mg/m3 (0.30 ppm) and carbon monoxide at 34.5 mg/m3 (30 ppm).
As for the combined effects of carbon monoxide and physical agents,
there are occupational data suggesting additive effects of carbon
monoxide and heat (Vyskocil, 1957), and of carbon monoxide
(carboxyhaemoglobin levels up to 35%) and noise in workers exposed to
this stress combination for more than 10 years (Wagemann, 1960; Zorn,
1968).
There is a complete lack of information on the combined effects of
carbon monoxide and drugs or alcohol in man. Furthermore, it is quite
apparent that the question of interactions on the organism of carbon
monoxide and other air contaminants needs clarification.
8.2 High-Risk Groups
The limited clinical research on populations other than healthy,
normal subjects makes it difficult to identify with certainty the
groups that are at increased risk from exposure to carbon monoxide.
However, as will be discussed later in this section, one of these
groups includes individuals with known coronary heart disease. In view
of the susceptibility of this group to the hypoxic stress of carbon
monoxide exposure, it is implicit that other groups are also
potentially subject to increased risk including individuals with
cerebrovascular and peripheral vascular diseases, anaemias, and lung
diseases. In addition, hospitalized individuals suffering from tissue
hypoxia (e.g. shock) or those undergoing operations may be at
increased risk. Individuals with undetected or undiagnosed coronary
artery disease as well as the fetus in utero, the newborn, or, even
pregnant women may be assumed to be at increased risk because of the
anticipated reduced capacity to accommodate hypoxic stress or some
inherent sensitivity to hypoxia. Furthermore, other populations such
as those living at high altitudes, young children, or older adults may
also be at increased risk.
8.2.1 Individuals with cardiovascular and chronic obstructive lung
disease
This section will not be concerned with the potential pathological
effects of cigarette smoking on the development of cardiovascular
disease, chronic pulmonary obstructive disease, and cancer (section
8.1.6). No adequate evidence has been presented, as yet, that carbon
monoxide per se is directly involved in the pathogenesis of these
disorders. Only the potential influence of ambient carbon monoxide on
individuals at risk will be considered.
Epidemiological studies in Los Angeles County (Cohen et al., 1969;
Goldsmith & Landaw, 1968; Hexter & Goldsmith, 1971), have suggested
the possibility of increased mortality from myocardial infarction,
associated with high (9-16 mg/m3 or 8-14 ppm) atmospheric levels of
carbon monoxide. There have been some differences of opinion
concerning the interpretation of these data. A somewhat similar study,
at least in design, was completed in Baltimore by Kuller et al.
(1975). The Baltimore data did not indicate any apparent relationship
between either the incidence of myocardial infarction or sudden death
due to atherosclerotic heart disease and average 24-h ambient carbon
monoxide concentrations. Neither group of investigators was able to
detect a relationship between post mortem carboxyhaemoglobin levels
and causes of sudden death. In the latter study the diagnoses of
disease state were more precise and the population involved more
clearly defined. The ambient levels of carbon monoxide in Baltimore
appeared to be considerably lower than those reported for Los Angeles.
Thus, the possibility of an association between ambient carbon
monoxide and the incidence of myocardial infarction or sudden deaths
remains questionable. It is apparent that more comprehensive and
extensive epidemiological studies need to be conducted in order to
clarify this issue.
There are no adequate studies in man describing the relationship
between exposure to carbon monoxide and the rate of development of
atherosclerotic heart disease. Goldsmith & Aronow (1975) have reviewed
the available evidence.
The heart has a specialized circulatory system in which the
primary response to increased metabolic demands can only be secured by
an increased coronary blood flow. Even under no-stress conditions
(rest) there is an almost complete extraction, roughly 75-80%, of the
available oxygen supply. Adams et al. (1973) monitored conscious dogs
breathing a carbon monoxide concentration of 1718 mg/m3 (1500 ppm)
for a period of 30 min and were able to show a linear relationship
between carboxyhaemoglobin concentration and coronary blood flow. A
13% increase in coronary blood flow occurred at a carboxyhaemoglobin
level of 4% and, at a concentration of 20%, flow rate increased by
54%. Since measurements were not reported for the lower
carboxyhaemoglobin levels, the existence of a threshold could not be
determined. The observations of Mehmel et al. (1973) suggest that
increases in coronary blood flow are stimulated by shifts in the
oxyhaemoglobin dissociation curve. They demonstrated that increasing
pH from 7.4 to 7.6 decreased the P50 ( pO2 at half saturation of
haemoglobin) from 4 to 3.2 kPa (30 to 24 Torr) and increased coronary
blood flow by more than 20%. Earlier studies by Ayres et al. (1969,
1970) also indicated increased blood flow in response to the presence
of increased levels of carboxyhaemoglobin.
Ayres' studies of the haemodynamic and respiratory responses of
man during diagnostic coronary catheterization suggested that carbon
monoxide would have a significant effect on arterial pO2 in
patients with lung disease as well as in patients with certain
cardiovascular disorders.
Studies by Anderson et al. (1973) Aronow et al. (1972) and Aronow
& Isbell (1973) on patients with angina pectoris are listed in
Table 8. Aronow et al. (1972) studied the influence of riding in an
open car on a major Los Angeles freeway. Two trips were made, in one
of which the patients breathed compressed carbon monoxide-free air.
Carboxyhaemoglobin levels after this ride averaged 0.65%, in contrast
to the 5.08% observed in the trip in the open car. The trips were of
90 min duration and ambient carbon monoxide levels in the car averaged
61 mg/m3 (53 ppm). Exercise time, on a bicycle ergometer, to the
onset of angina, was determined prior to, and after the completion of
the exposure. Although no changes in the length of time of work to
onset of anginal pain were noted in the ride while breathing
compressed air, a significant reduction from a mean time of 247 to
174 sec was found when the carboxyhaemoglobin concentration was
elevated. Anginal pain also appeared to persist for a longer time
under these conditions. In a study by Anderson et al. (1973), patients
with stable angina walked on a treadmill. They then breathed air
containing 57 or 115 mg/m3 (50 or 100 ppm) intermittently while
resting for a period of 4 h, raising their carboxyhaemoglobin levels
to 2.9% and 4.5% respectively. The repeat exercise tests clearly
demonstrated a reduction in walking time to onset of angina. No
differences in time were observed at the 2 carboxyhaemoglobin levels
although the duration of the pain was longer at the higher level.
There appeared to be some additional depression of the ST segment but
the degree was not of a significant order. Other measures of cardiac
function-systolic time intervals, left ventricular ejection time, pre-
ejection period index, and pre-ejection peak to ejection time ratio
remained within normal limits.
Table 8. Exercise-induced angina and carbon monoxide (10 subjects per study)
Carboxyhaemoglobin Ambient CO Time to Reference
(%) mg/m3(ppm) angina
response
Initial Final
1.12 5.08 61a (53) Shortened Aronow et al (1972)
1.07 2.68 57b (50) Shortened Aronow & Isbell (1973)
1.40 2.90 57c (50) Shortened Anderson et al. (1973)
a Freeway trip.
b Continuous exposure for 2 h in laboratory,
c Intermittent exposure for 4 h in laboratory.
Another study by Aronow & Isbell (1973) was somewhat similar to
that of Anderson et al. (1973). Aronow and his co-workers exposed
patients (nonsmokers at the time of the test to a carbon monoxide
concentration of 57 mg/m3, resulting in a carboxyhaemoglobin
concentration of 2.68%. This study was also conducted as a double-
blind random trial with one day of breathing carbon monoxide and
another day breathing compressed carbon monoxide-free air. The angina
pectoris of all patients was documented by history and coronary
angiography. A 23% reduction in exercise time (bicycle) resulted
following the carbon monoxide exposure. No electro-cardiographic
changes were seen in these patients during any exercise period.
Plotting of Aronow's data suggests that there was a linear
relationship between carboxyhaemoglobin levels and the decrease in
time to angina.
This evidence suggests that a deleterious effect could occur at
carboxyhaemoglobin levels as low as 2.5% in certain subjects with
coronary heart diseases. The USA National Health Survey Examination
(US Environmental Protection Agency, 1975) reported that in the USA
there were 3 215 000 adults, aged 18-79 years, with definite coronary
heart disease and another 2 410 000 with suspected disease. Many of
these individuals, as well as others in the general population, have
carboxyhaemoglobin levels equal to, or above 2.5%. It would be rash to
even suggest that the above-mentioned studies implicate carbon
monoxide as a factor in determining the natural history of heart
disease in a community. It is even more dangerous to imply that
exposure to carbon monoxide increases the frequency or severity of
chest pain, or shortens life expectancy among patients with angina
pectoris or other clinical manifestations of heart disease. The
necessary epidemiological evidence for an association between the
frequency of episodes of angina pectoris and community ambient levels
of carbon monoxide is inadequate and additional information from more
and varied sources is required.
Patients with chronic obstructive pulmonary disease are probably
at high risk, although few studies on them have been reported. Any
increase in hypoxia could result in respiratory failure. However,
these individuals may absorb less carbon monoxide because of their
disease as the hypoxia may be compensated for by increased
erythropoiesis and a shift of the oxygen dissociation curve to the
right. An interesting approach to the evaluation of individuals at
risk from carbon monoxide and other pollutants can be found in a
publication of the US Environmental Protection Agency (US
Environmental Protection Agency, 1975). Ogawa et al. (1974) have
presented evidence on the development of pulmonary oedema and
discussed possible mechanisms of the role of carbon monoxide in the
disorder.
8.2.2 Anaemic individuals
The information available on the effects of carbon monoxide on
anaemic patients is still inadequate.
The oxygen dissociation curve of blood obtained from patients with
anaemia is shaped like the normal curve but is vertically compressed.
However, when curves from individuals with a 50% reduction in
haemoglobin content are compared with dissociation curves determined
in the presence of 50% carboxyhaemoglobin, there are striking
differences. Consequently, the tendency to make such comparisons is
likely to lead to erroneous deductions concerning effects occurring at
the tissue level. Brody & Coburn (1970) discussed these differences in
relation to arterial and venous pCO and pO2. Because the capacity
of the oxygen transport system is reduced in anaemic persons,
it can be assumed a priori that they could be more at risk from
carbon monoxide exposure than normal persons. Brody & Coburn (1970)
indicated that, if the oxygen content of the mixed venous blood is
abnormally low, as in anaemia or carbon monoxide poisoning, the effect
of the shunted blood in lowering arterial pO2 will be greater than
normal, and a small increase in the alveolar-arterial pressure
difference (AaDO2) will result. The change in the shape of the
oxyhaemoglobin curve due to the presence of carbon monoxide will also
increase the AaDO2. Furthermore, Brody & Coburn (1970) showed that
mild increases in carboxyhaemoglobin concentrations would have little
or no influence on the AaDO2 in normal subjects. However, in
patients with large intra-cardiac right-to-left shunts or with chronic
lung disease and regional variation in the ventilation perfusion ratio
( VA/ QA), the presence of carbon monoxide in the blood will increase
the AaDO2.
Tissue oxygenation may be involved initially because of the
anaemic state, since mixed venous pO2 is decreased (Cropp, 1970)
and the reduction in venous pO2 from a particular carboxyhaemo-
globin value is somewhat greater in anaemic than in normal subjects.
In patients with haemolytic anaemia and sickle cell disease (Engel et
al., 1971), the rate of endogenous carbon monoxide production from
haemoglobin catabolism is increased. Normal subjects produce
approximately 18 µmoles of carbon monoxide per hour, resulting in
carboxyhaemoglobin levels of 0.5 to 0.8%. Carbon monoxide production
in anaemic patients (Coburn et al., 1966; Logue et al., 1971) has been
reported to vary from 31 to 158 µmoles per hour, producing
carboxyhaemoglobin levels of 1.3-5.2%.
Anaemic subjects approach equilibrium levels of carboxyhaemoglobin
more rapidly than those with normal haemoglobin levels at any given
exposure to carbon monoxide. Exposure to a concentration of
22.9 mg/m3 (20 ppm) for approximately 4 h in an individual with a
haemoglobin level of 7 g/100 ml could result in a carboxyhaemoglobin
concentration of 4-5% compared with an anticipated level of 2.5% for
normal individuals. Exogenous carbon monoxide exposure of anaemic
individuals could result, in conjunction with higher endogenous
production, in their attaining critical levels of carboxyhaemoglobin
more rapidly than normal individuals.
8.2.3 Embryo, fetus, neonate, and infants
Pregnant mothers and their fetuses may be exposed acutely or
chronically to carbon monoxide either by maternal smoking or by
environmental pollution. The biological effects of carbon monoxide
exposure on fetal tissues during intrauterine development or during
the newborn period are far from clear.
Several studies (Astrup et al., 1972; MacMahon et al., 1966) have
demonstrated that babies delivered by mothers who smoke cigarettes
weigh less than those delivered by nonsmoking mothers. Relative
maternal, fetal, or placental hypoxia may be responsible, as suggested
by the observation that infants born at high altitudes also weigh less
than those born at sea level (Grahn & Kratchman, 1963). The New Mexico
State Department of Public Health (1975) provided additional
confirmation of the relationship between altitude and birth weights.
Mothers who smoked were reported to have carboxyhaemoglobin
concentrations ranging from 2 to 14%,. while concentrations in the
fetuses ranged from 2.4 to 9.8%. These values may not represent
conditions present during pregnancy, since these data were obtained
just prior to birth. Another factor that may produce differential
effects on the fetus is related to the endogenous production of carbon
monoxide by pregnant women. Delivoria-Papadopoulos et al., (1969)
indicated that nonsmoking pregnant women produced 0.9 ml of carbon
monoxide per hour in contrast to the nonpregnant female's production
of 0.4 ml reported by Longo (1970). Fetal production of endogenous
carbon monoxide accounted for 3% of the total carboxyhaemoglobin
present in the blood of a nonsmoking normal pregnant woman. The source
of the remainder is not well known but may be related to progesterone
levels (Delivoria-Papadopoulos et al., 1969). Even though
hyperventilation of pregnancy may partially compensate for the
increased carbon monoxide production in the absence of exogenous
exposure, the maternal carboxyhaemoglobin still remains about 13%
above that in nonpregnant women (Longo, 1970). It should be noted that
the post partum (24 h) female may be producing 3 times as much carbon
monoxide as a near term nonsmoking pregnant woman.
Behrman et al. (1971) measured carboxyhaemoglobin concentrations
in 25 relatively normal newborn infants in a downtown Chicago nursery
and found the mean value to be 6.98%. These investigators indicated
that absolute carboxyhaemoglobin levels were related to ambient levels
of carbon monoxide. Some doubts about this conclusion exist, since the
monitoring reference site was 2.4 km from the nursery; the
investigators did not report any untoward clinical effects from
exposure to these levels, and no consideration was given to the
possibility of increased endogenous carbon monoxide production in
these infants.
Of the several mechanisms that may account for the influence of
carbon monoxide on developing tissue, the most important is the
interference with tissue oxygenation. Carbon monoxide decreases the
capacity of haemoglobin to transport oxygen and shifts the oxygen
saturation curve to the left. The normal arterial pO2 supplying
fetal tissue is approximately 3.7 kPa (28 Torr). The shift to the left
will tend to further decrease the oxygen gradient from maternal to
fetal blood across the tissue. The decreased pO2 and the
diminished oxygen transport due to the presence of carboxyhaemoglobin
may also produce undesired influences on the fetus. One of the
possible mechanisms by which carbon monoxide or other components of
tobacco smoke may adversely influence fetal development is through
interference with the metabolic function of placental cells. These
cells have a role in metabolizing hormones as well as in the transport
of vitamins, carbohydrates, amino acids, and other substances through
their energy dependent processes. Tanaka (1965) reported that the
oxygen uptake of placental slices from mothers who smoked varied
inversely with maternal levels of carboxyhaemoglobin, being markedly
reduced when this level was higher than 7.0%. The preponderance of
evidence concerning maternal carboxyhaemoglobin levels, along with
fetal and perinatal exposure, tends to warrant the reduction of
exposure to exogenous carbon monoxide sources that might cause this
group to be at risk, to a minimum.
The potential toxicity of carbon monoxide present in transfused
blood has received little attention. Kandall et al. (1973) measured
carboxyhaemoglobin concentrations in donor blood and in relatively
healthy infants receiving exchange blood transfusions. The mean pre-
transfusion carboxyhaemoglobin concentration in 6 cases was 1.34%.
Donor blood contained a carboxyhaemoglobin concentration of 5.17%,
resulting in a mean value of 4.92% in the transfused infant. In one
infant transfused with blood containing a carboxyhaemoglobin
concentration of 8.87%, the resultant carboxyhaemoglobin value in the
infant was 7.43%. Although it was stated that the infants did not
appear to be adversely affected by the levels of carboxyhaemoglobin
reached during exchange transfusion, it should be noted that adverse
effects have been observed in adults at these levels. Furthermore, in
individuals whose oxygen transport system or cardiovascular reserve is
already compromised, the presence of additional carboxyhaemoglobin,
from transfused blood, may result in a further and more potentially
dangerous decrement in arterial, mixed venous, and coronary sinus
oxygen tensions. It should be recalled that some blood samples
collected from blood donors had carboxyhaemoglobin values that
exceeded 18%.
8.2.4 Individuals living at high altitudes
The effects of carbon monoxide and of hypoxia induced by high
altitude are similar. Carbon monoxide produces effects that aggravate
the oxygen deficiency present at high altitudes. When high altitude
and carbon monoxide exposures are combined (Table 9) the effects are
apparently additive. It should be noted, however, that decreased
pO2 in the air and increased carboxyhaemoglobin, produce different
physiological responses. They have different effects on blood pO2,
on the affinity of oxygen for haemoglobin, on the extent of
oxyhaemoglobin saturation (carbon monoxide hypoxaemia shifts the
oxyhaemoglobin dissociation curve to the left, and a decrease in
PAo2 shifts it to the right), and on ventilatory drive. These
effects have been discussed earlier.
The actual influence of a combination of increased carboxy-
haemoglobin and decreased oxyhaemoglobin has not been adequately
documented by experimental data. The few available studies refer
only to acute exposures to lower pO2 and raised pCO. The
most supportive information on the additive nature of this combination
originates from psychophysiological studies and even this information
is not very convincing. When Blackmore (1974) analysed the cause of
aircraft accidents in Britain, he found that carboxyhaemoglobin levels
provided valuable information in relation to altitude and sources of
carbon monoxide. The high levels of carbon monoxide found (up to 74%)
could be attributed to equipment failure, smoking, and fires. No data
are available on the effects of carbon monoxide on the native
inhabitants at high altitudes or on the reactions of these natives
when they are suddenly removed to sea level and possible high ambient
carbon monoxide concentrations.
Table 9. Approximate physiologically equivalent altitudes at
equilibrium with ambient carbon monoxide levelsa
Ambient CO concentration Actual altitude (metres)
mg/m3 ppm 0 (sea level) 1524 3048
Physiologically equivalent altitudes
with carboxyhaemoglobin
0 0 0 (sea level) 1524 3048
28.6 25 1829 2530 3962
67.3 50 3048 3658 4672
114.5 100 3749 4663 5486
a From: NAS/NRC (1977).
In their studies on altitude exposures of young males, McFarland
et al. (1944) showed that changes in visual threshold occurred at
carboxyhaemoglobin levels as low as 5% or at a simulated altitude of
2425 m. These observations were confirmed by Halperin et al. (1959),
who also noted that recovery from the detrimental effects on visual
function lagged behind the elimination of carbon monoxide. However,
the data given were sparse and the variability among the four subjects
was not given. Vollmer et al. (1946) studied the effects of carbon
monoxide at simulated altitudes of 3070 and 4555 m and reported that
there were no additive effects of carbon monoxide and altitude. They
suggested that the effects of carbon monoxide were masked by some
compensatory mechanisms. The data presented were not convincing.
However, Lilienthal & Fugitt (1946) indicated that a combination of
altitude (1540 m) and a carboxyhaemoglobin level of 5-9% induced a
decrease in flicker fusion frequency, although either one alone did
not have any effect. They also reported that the presence of 8-10%
carboxyhaemoglobin was effective in reducing altitude tolerance by
1215 m. During light activity at an altitude of 4875 m, carbon
monoxide uptake increased, probably owing to the hyperventilation at
altitude caused by the respiratory stimulus of decreased pO2
(Forbes et al., 1945). Evidence that carbon monoxide elimination was
similar at sea level and at altitudes up to 10 000 m was obtained by
several investigators (Gorodinsky et al., 1970; Sedov et al., 1971).
However, increased ambient temperatures up to 35°C and hard physical
work increased the rate of elimination (Vollmer et al., 1946). Pitts &
Pace (1947) stated that every 1% increase in carboxyhaemoglobin (up to
13%) was equivalent to a 109 m rise in altitude if the subjects were
at altitudes of 2100-3070 m. These observations were based on changes
in the heart rate response to work. A number of unanswered questions
arise from all these studies, which in general were obscured by such
factors as poor control and no identification of subjects who may have
been smokers.
Two groups of investigators have presented data comparing the
physiological responses of subjects to altitude and carbon monoxide
exposure where the hypoxaemia due to altitude and the presence of
carboxyhaemoglobin were approximately equal. In one study (Astrup &
Pauli, 1968), the carboxyhaemoglobin concentration was about 12%
(although the mode of exposure to carbon monoxide was such that
carboxyhaemoglobin ranged from 5% to 20% and the altitude study was
conducted at 3977 m). The second study (Sedov et al., 1971) compared
responses at an altitude of 4000 m and a carboxyhaemoglobin content of
20%. In both studies, carboxyhaemoglobin content was much in excess of
that anticipated for typical ambient pollution. However, they both
suggested that the effects attributable to carbon monoxide and to
altitude were equal.
8.3 Summary Table
Table 10 is a summary of controlled human studies that provide
useful information for evaluating the relationship between exposure to
carbon monoxide and its health effects.
Table 10. Summary of exposure-effect relationships
Exposure Reported effects Reference
(HbCO %)
(a) Behavioural changes
20 Essentially no impairment in time discrimination Stewart et al. (1973b)
(using Beard-Wertheim task)
11.3 No vigilance decrement (using Horvath task) Winneke et al. (1976)
9a No vigilance decrement (using Fodor-Winneke Winneke (1974)
task); no change in reaction time
8.4 No vigilance decrement (using their own Groll-Knapp et al. (1976)
vigilance task (1972))
7.6 Longer reaction times Ramsey (1973)
7.3 Disturbance in certain perceptual and cognitive Bender et al. (1972)
processes
5 Vigilance decrement Horvath et al. (1971)
4.5 Longer reaction times Ramsey (1972)
3.1b Initial vigilance decrement with subsequent Fodor & Winneke (1972)
normalization; no change in response latency
3b Vigilance decrement Groll-Knapp et al. (1972)
2b Impaired performance in time-discrimination Beard & Wertheim (1967)
(b) Changes in work performance
6.3 Decrease in maximal work time Ekblom & Huot (1972)
4.3 Decrease in a maximal oxygen uptake (VO2) Horvath et al. (1975)
(1.7c)
4.0 Decrease in mean exercise time until exhaustion Aronow & Cassidy (1975)
(0.6c)
2.5 Decrease in absolute exercise time in non- Drinkwater et al. (1974)
smokers
Table 10 (Cont'd)
Exposure Reported effects Reference
(HbCO %)
(c) Aggravation of symptoms in patients with cardiovascular disease
(1.1c)
5.1 Shortened time to angina response immediately Aronow et al. (1972)
after exposure
(1.1c)
2.9 Shortened time to angina response 2 h after Aronow et al. (1972)
(1.1c) exposure
2.9 Shortened time to angina response Anderson et al. (1973)
(1.1c)
2.8 Decrease in mean exercise time until onset of Aronow et al. (1974)
intermittent claudications
(1.0c)
2.7 Shortened time to angina response Aronow & Isbell (1973)
a Estimated values using the formula by Coburn et al. (1965).
b Estimated values using the formula by Peterson & Stewart (1970).
c HbCO % before exposure to CO.
9. EVALUATION OF HEALTH RISKS
9.1 Introduction
The acute toxicity of carbon monoxide has long been recognized and
is well documented. Much has been learned of the main sources of the
gas, its absorption, the kinetics of its reactions with blood, and the
biochemical and pathological consequences of poisoning by excessive
absorption. More recently, a great deal of attention has been paid to
the effects, demonstrable or suspected, of exposure to concentrations
much lower than those that cause definite poisoning. Such
concentrations are those commonly found in urban air (caused almost
wholly by traffic pollution) and indoors (caused by faulty ventilation
of heating or cooking appliances), but there has been much concern
with the effects of the gas on smokers, who inhale considerable
quantities of carbon monoxide with tobacco smoke. Since the main
source of carbon monoxide as an urban pollutant is the petrol engine,
the problems posed by the inhalation of relatively low concentrations
of the gas are likely to grow rather than diminish, as traffic becomes
denser and more widespread. The recognition of the importance of
pollution of the domestic environment is relatively recent and
deserves more study; the problems posed by smoking tobacco are common
and are, unfortunately, increasing. There is much published evidence,
some of which is of debatable value, that suggests that the
comparatively low concentrations of carboxyhaemoglobin produced by
exposure to pollution of the ambient air and the higher concentrations
usually associated with smoking, might cause demonstrable impairment
of vigilance, discrimination, and of the performance of fine tasks and
physical work in healthy subjects, and the exacerbation of symptoms
such as angina pectoris on effort in patients with cardiovascular
diseases. Likewise there is evidence, derived from experimentation on
animals, that chronic exposure to carbon monoxide leading to the
levels of carboxyhaemoglobin commonly found in smokers may, in
association with high cholesterol intakes, play a part in the genesis
of atherosclerosis. Moreover, there are reasons to suspect that
exposure to carbon monoxide may enhance the effects of other
pollutants, commonly administered therapeutic agents, socially
acceptable amounts of beverages such as alcohol, and other
environmental stresses. This section is intended as a brief assessment
of these topics in the hope that sound advice may be given on the need
to control levels of carbon monoxide in the ambient air.
9.2 Exposure
9.2.1 Assessment of exposure
Concentrations of carbon monoxide in air may be measured with
comparative ease by such methods as non-dispersive spectroscopy, gas
chromatography etc. But human body burdens of carboxyhaemoglobin
depend on many factors other than the partial pressure of carbon
monoxide in the inhaled air; among these factors are time of exposure,
pulmonary ventilation (which mainly depends on work done), and blood
volume. Since these quantities, especially ambient concentrations of
carbon monoxide, may vary widely, it is obviously difficult, if not
impossible at times, to calculate the likely body burden of
carboxyhaemoglobin in an exposed individual. There is, therefore, much
to be gained by sampling blood to obtain an integrated estimate of
carboxyhaemoglobin derived from all sources under various conditions
of exposure. It must be emphasized that the measurements of carbon
monoxide in air and in blood give complementary results and are not
merely alternative forms of monitoring. Methods of analysis are
discussed in sections 2.2 and 2.3.
There is a tendency to forget that the reaction between
haemoglobin and carbon monoxide is reversible and that, in a given
environment, a subject may acquire carbon monoxide, excrete it, or
remain in equilibrium with the ambient air depending on the carbon
monoxide concentration and the initial level of carboxyhaemoglobin in
the individual. The time taken to achieve equilibrium between blood
and ambient air depends on the initial carboxyhaemoglobin
concentrations as well as on the factors mentioned above. The rate of
excretion of carbon monoxide will depend not only on ambient air
levels, the initial carboxyhaemoglobin and on factors such as
pulmonary ventilation, but also on the partial pressure of oxygen in
inspired air, which might be introduced therapeutically to increase
elimination. Fig. 12 shows estimates of equilibrium times for various
ambient concentrations and levels of activity. The half-life for
excretion, at rest, is approximately 4“ h.
9.2.2 Endogenous production
The normal breakdown in the body of blood pigments produces carbon
monoxide to give endogenous carboxyhaemoglobin values of 0.1-1.0% and
normal blood is in equilibrium with carbon monoxide levels in air of
roughly 5 mg/m3 (4.3 ppm). These data could be used as a basis for
establishing air quality criteria for carbon monoxide. Various causes
of increased endogenous production of carbon monoxide are discussed in
section 6.1.
9.2.3 Outdoor environmental exposure
Natural sources of carbon monoxide (section 3.1) are of
considerable magnitude but are diffuse, and ambient air concentrations
at locations removed from man-made sources range from 0.01 to
0.9 mg/m3 (0.01 and 0.8 ppm) which is negligible in the context of this
report. By far the most important sources of carbon monoxide at
breathing level are petrol engine vehicle exhausts (section 3.2). The
diesel engine (compression ignition), when properly adjusted, emits
little carbon monoxide. The density, distribution, and mode of
operation of vehicles vary greatly and these and other factors, the
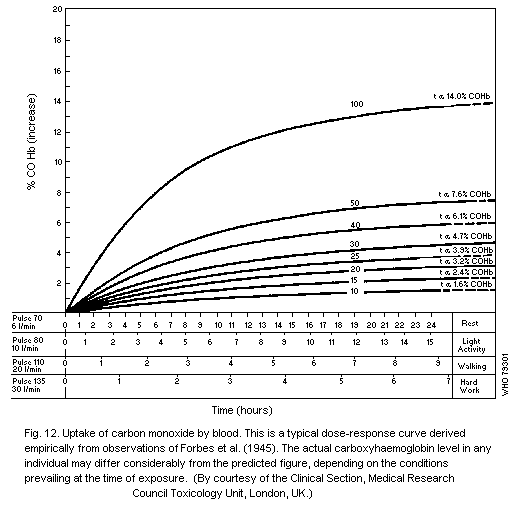 most important of which is the weather, produce great variations in
the concentrations of pollutants produced by traffic. Concentrations
fall steeply with distance from the street. However, distinct patterns
are often discernible (section 5.1). Concentrations for 8-h averaging
times are frequently used and quoted and usually vary from
<10 mg/m3 (8.7 ppm) to over 60 mg/m3 (52.2 ppm) but are mostly
<20 mg/m3 (17.6 ppm) in city streets. Away from heavy traffic, even
in towns, annual average concentrations are usually well under
10 mg/m3 (8.7 ppm). Obviously, in especially stagnant weather, very
heavy traffic may produce much higher levels. There is little
information about concentrations of carbon monoxide near large
stationary sources.
9.2.4 Indoor exposure
Carbon monoxide diffuses readily and, being relatively chemically
inert and not absorbed on surfaces, concentrations indoors are usually
similar to those found immediately outside. Not infrequently, however,
high concentrations may be found in kitchens and living rooms in which
there are coal, gas, or oil-fired cooking or heating appliances that
are maladjusted and inadequately vented to outside air; in some
countries, cases of acute and even fatal poisoning due to these causes
are not uncommon. The possible contribution of the domestic
environment must be noted in surveys. The smoking of tobacco indoors
can obviously increase the carbon monoxide concentration of the air
but recent work has shown that, before carbon monoxide reaches
significant levels, the irritation from the other constituents of
tobacco smoke becomes unacceptable if not actually intolerable.
9.2.5 Exposures related to traffic
In garages and tunnels, being in effect closed streets, pollution
by carbon monoxide can reach high levels. However, since transit time
in tunnels is relatively short, higher concentrations than those found
in streets are tolerable. Usually, there are monitoring instruments
that control ventilation and sound alarms if concentrations exceed
agreed values, which may vary from 115 to 570 mg/m3 (100-500 ppm)
depending on the use and length of the tunnel. High (sometimes lethal)
concentrations of carbon monoxide may accumulate inside motor vehicles
because of fractures in exhaust systems or other mechanical defects.
9.2.6 Occupational exposure
Traffic policemen, garage attendants, and drivers of taxis and
trucks are exposed to pollution from traffic and many studies have
shown a consequent increase in carboxyhaemoglobin levels (up to about
3% in nonsmokers), but there is much evidence that this increase may
be relatively undramatic, when compared with the manifest effects of
cigarette smoking. Exposure in certain industries, especially in iron
and steel works and in the manufacture of various gases, may be
relatively massive (in excess of 115 mg/m3 or 100 ppm), and high
carboxyhaemoglobin levels (>15% in nonsmokers) have been reported in
workers. Firemen may be exposed to very high concentrations of carbon
monoxide in fighting certain fires, but this exposure is obviously
episodic. These matters are discussed in section 5.3.
9.2.7 Tobacco smoking
The smoking of tobacco, especially in the form of cigarettes, has
been shown in many studies to be the major cause of raised
carboxyhaemoglobin levels in adult populations. Table 7 displays some
of this evidence and the topic is discussed in detail in section
8.1.6. Whereas carboxyhaemoglobin concentrations of 3% are rarely
found in nonsmokers exposed to town air, concentrations of 5-15% are
often found in smokers. It is important to remember that the effects
of smoking and exposure to town air are not simply additive and that
the resulting carboxyhaemoglobin levels will depend on other factors
already discussed.
9.2.8 Multiple exposures
Enough has been said to leave no doubt that carbon monoxide,
produced exogenously or endogenously, is a widespread pollutant
emanating from many sources to which people may be exposed in various
ways. This variety of exposure must be taken into account in the
interpretation of epidemiological surveys, the design of experiments,
and, above all, in giving advice about the fixing of air quality
criteria.
9.3 Effects
The main areas of concern that have arisen from acute or chronic
exposure to low levels of carbon monoxide in experimental and
epidemiological research in animals and man are: (a) its role in the
genesis of arteriosclerotic vascular diseases; (b) its role in the
aggravation of symptoms of cardiovascular diseases; (c) its
contribution to performance deficits in certain psychomotor tasks; and
(d) its role in limiting the working capacity of exercising man.
9.3.1 Cardiovascular system
9.3.1.1 Development of atherosclerotic cardiovascular disease
Extensive experimental work has been carried out over many years
on animals, mainly rabbits, showing that prolonged exposure to
moderate levels of carbon monoxide can produce atherosclerotic
changes, especially in the presence of high cholesterol levels (1-2%)
in the diet. The relevance of this work for man has not been
established. However, other animal work, and some epidemiological
studies of prolonged human exposures to elevated carbon monoxide
levels through smoking, occupation, or both, such as those carried out
in Denmark, Finland, and Japan, indicate the need for further
investigation of the possible role of carbon monoxide in the genesis
of atherosclerotic vascular changes in animals and man. The degree of
intermittency of exposure at various levels should be taken into
account as well as the possible contribution of other agents such as
nicotine and high-fat diets. There is some evidence of adaptation, but
such changes may not be entirely beneficial. None of the information,
currently available, is useful for the purpose of setting standards.
9.3.1.2 Acute effects on existing heart illness
The few existing epidemiological studies on the possible effects
of carbon monoxide on the severity or fatality of coronary occlusion
are insufficient to allow any conclusions. It is hoped that additional
work of this type will clarify matters.
Two carefully conducted human studies of the effects of low carbon
monoxide exposure and exercise on pain in volunteer patients with
angina pectoris offer valuable quantitative information. Although
limited in the number of patients studied, the findings are consistent
in the 2 investigations showing effects at carboxyhaemoglobin
concentrations of 2.5-3.0%. A third single-blind study revealed the
same detrimental effects in patients with angina pectoris when
exposure to traffic exhausts caused carboxyhaemoglobin levels to rise
to 5.1%. A no-adverse-effect level has not been established in these
observations, nor is it possible to determine whether there is a
graded response in this type of experiment. More work of a similar
nature would be useful to explore these questions.
9.3.1.3 Acute effects on existing vascular disease
One study, similar to those done on patients with angina, has been
carried out on patients with intermittent claudication from peripheral
vascular disease. Effects on pain with exercise were observed in the
same exposure range as with angina i.e., at carboxyhaemoglobin
concentrations of 2.5-3.1%, with a mean of 2.8%. Here, too, more data
of a similar kind are needed, preferably designed to provide dose-
response relationships.
9.3.2 Nervous system
As for the role of carbon monoxide in affecting psychomotor
functions, no definite conclusions can be drawn from the existing
data. The behavioural functions tested in such studies include
vigilance and psychomotor performance, visual acuity and sensitivity,
the ability to estimate time intervals, complex motor coordination as
tested by driving simulators, and different perceptual and mental
operationsa. Some workers observed detrimental effects at
carboxyhaemoglobin levels as low as 2%, whereas others were unable to
detect significant impairment even at levels from above 5% to about
20%. In evaluating these discrepancies, it should be mentioned, that
these behavioural functions are easily influenced by a number of other
factors besides carbon monoxide-induced hypoxia, e.g., degree of
sensory deprivation, compensatory abilities, drugs, temperature, time
of day, competition, etc.
9.3.3 Work capacity
That elevated carboxyhaemoglobin levels affect work capacity has
long been known. Levels of 40-50% will usually prevent working
entirely. Recent studies in the laboratory, on man, using maximum work
capacity or maximum aerobic capacity as indicators of performance,
have been carried out in relation to carboxyhaemoglobin levels. Here,
dose-response data are available for maximum effort. The limitation
appears at a carboxyhaemoglobin concentration of about 4% and
increases at higher levels. Lower exposure levels have been studied
and do not produce this effect. It should be noted that while levels
of carboxyhaemoglobin of 2.5-4%, did not reduce maximum work capacity,
they did reduce the length of time for which such effort could be
carried out. It is not known what specific levels of carboxy-
haemoglobin will reduce the capacity of individuals to perform at
ordinary work levels, such as 30-50% of their maximum capacity, for
prolonged periods of time.
9.4 Recommended Exposure Limits
It has already been stated that the major contributor to
carboxyhaemoglobin concentrations in the body is the smoking of
tobacco; however, in many of the experiments currently quoted to
justify the formulation of exposure limits, the smoking habits of the
subjects were not taken into account. Results of recent work suggest
that smokers and ex-smokers might be less sensitive to carbon monoxide
exposure than nonsmokers. In view of this suggestion, and because of
the deficiencies in the experiments mentioned, recommendations for
exposure limits should be confined to the protection of nonsmokers.
There is an urgent need for more work on possible adaptation following
a The possible importance of performance deficiencies resulting
from carbon monoxide is considerable, particularly in relation to
accidents at work, and while driving or flying. Further studies,
particularly of the vigilance type are urgently needed for a better
understanding of this problem.
exposure to carbon monoxide from smoking or from other sources. It is
also important to note that carboxyhaemo-globin levels have been the
measurement of exposure in most experimental work. Thus, it is
desirable to recommend the primary exposure limits in terms of
carboxyhaemoglobin, and follow this by comments on the derivation of
an appropriate air concentration equivalent.
9.4.1 General population exposure
Data used in arriving at a recommendation for an exposure limit
for the general population were mainly those obtained from the
exposure of subjects with cardiovascular illness to carbon monoxide in
conjunction with exercise. Agreement was not reached on a single
level. Thus, a range of carboxyhaemoglobin concentrations of 2.5-3.0%
is recommended for the protection of the general population including
those who have impaired health. The recommendation must be regarded as
tentative, since ideal dose-response or concentration-response
information is not yet available. However, it must also be recognized
that complete protection of all persons, at all times, cannot
reasonably be sought by environmental control alone. Persons who are
in should be educated by their physicians concerning their own
responsibility to avoid stressful exposures.
9.4.2 Working population exposure
Better quality data are available for recommending an exposure
limit for the working population. In this case, the Task Group
unanimously agreed on maintaining carboxyhaemoglobin levels below 5%,
on the basis of present knowledge, since working populations comprise
individuals who are assumed to be healthy, physiologically resilient,
and under regular supervision.
9.4.3 Derived limits for carbon monoxide concentrations in air
It is important, wherever possible, to have both biological and
environmental assessments of human exposure to pollutants. While the
biological measurements may be more relevant in relation to effects,
they may be more difficult to use in practice. For carbon monoxide,
the relationship between concentrations in air and carboxyhaemoglobin
levels is affected by several variables, including exposure time and
it is not easy to estimate. However, such estimates may be
sufficiently accurate for many practical purposes (for reviews see
Committee on the Challenges of Modern Society, 1972; Commission of the
European Communities, 1974; NAS/NRC, 1977; Winneke, 1977; Ott & Mage,
1978). It should be emphasized yet again that analyses of carbon
monoxide in air and of carboxyhaemoglobin in blood are complementary,
and should in no way be regarded as alternative methods of monitoring.
Obviously, air monitoring has its uses in the planning and
implementation of control measures, and for warning purposes, but such
measurements have limited value in estimating the actual human
exposure defined by carboxyhaemoglobin levels.
REFERENCES
ADAMS, J. D., ERICKSON, H. H., & STONE, H. L. (1973) Myocardial
metabolism during exposure to carbon monoxide in the conscious
dog. J. appl. Physiol., 34: 238-242.
AGOSTINI, J. C., RAMIREZ, R. G., ALBERT., S. N., GOLDBAUM, L. R., &
ABSOLON, K. B. (1974) Successful reversal of lethal carbon
monoxide intoxication by total body asanguineous hypothermic
perfusion. Surgery, 75: 213-219.
AHMED, A. E., KUBIC, V. L., & ANDERS, M. W. (1977) Metabolism of the
haloforms to carbon monoxide. I. In vitro studies. Drug
Metabol. Disposition, 5: 198-204.
ALEXANDROV, N. P. (1973) [Choice of experimental animals for
elaboration of standards for CO.] Gig. i Sanit., 11: 92-95 (in
Russian).
ALEKSIEVA, T. & DIMITROVA, M. (1971) [Some oscillographic changes in
workers exposed to chronic carbon monoxide effect.] Tr. Niotpz,
pp. 163-169 (in Bulgarian).
ALTMAN, P. L. & DITTMEN, D. S., ed. (1971) Respiration and
circulation, Bethesda, MD, Federation of the American Society of
Experimental Biology.
ALTSHULLER, A. P. & BUFALINI, J. J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ANDERSON E. W., ANDELMAN, R. J., STRAUCH, J. M., FORTUIN, N. J., &
KNELSON, J. H. (1973) Effect of low-level carbon monoxide exposure
on onset and duration of angina pectoris: a study in ten patients
with ischemic heart disease. Ann. intern. Med., 79: 46-50.
ANTWEILER, H. (1974) Physiological kinetics of CO inhalation.
In: Proceedings of the European Colloquium on Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg Commission of the European
Communities, Directorate General Scientific and Technical
Information Management, pp. 197-251 (in German with full English
translation).
ANTWEILER, H. (1975) [The reception and effect of carbon monoxide in
active and passive smokers.] Arbeitsmed. Sozialmed.
Präventivmed., 10: 245-248 (in German).
ARONOW, W. S. & CASSIDY, J. (1975) Effect of carbon monoxide on
maximal treadmill exercise: a study in normal persons. Ann.
intern. Med., 83: 496-499.
ARONOW, W. S. & ISBELL, M. W. (1973) Carbon monoxide effect on
exercise induced angina pectoris. Ann. intern. Med.,
79: 392-395.
ARONOW, W. S., CASSIDY, J., VANGROW, J. S., MARCH, H., KERN, J. C.,
GOLDSMITH, J. R., KHEMKA, M., PAGANO, J., & VAWTER, M. (1971a)
Effect of cigarette smoking and breathing carbon monoxide on
cardiovascular hemodynamics in anginal patients. Circulation,
50: 340-347.
ARONOW, W. S., DENDINGER, J., & ROKAW, S.N. (1971b) Heart rate and
carbon monoxide level after smoking high-, low-, and non-nicotine
cigarettes. Ann. intern. Med., 74: 697-702.
ARONOW, W. S, HARRIS, C. N., ISBELL, M. W., ROKAW, S. N., & IMPARATO,
B. (1972) Effect of freeway travel on angina pectoris. Ann.
intern. Med., 77: 669-676.
ARONOW, W. S., STEMMER, E. A., & ISBELL, M. W. (1974) Effect of carbon
monoxide exposure on intermittent claudication. Circulation,
49: 415-417.
ASTRAND, I., OVRUM, P., & CARLSSON, A. (1975) Exposure to methylene
chloride. I. Its concentration in alveolar air and blood during
rest and exercise and its metabolism. Scand. J. Work Environ.
Health, 1: 78-94.
ASTRUP, P. (1970) Intraerythrocytic 2,3-diphosphoglycerate and carbon
monoxide exposure. Ann. NY Acad. Sci., 174: 252-254.
ASTRUP, P. (1972) Some physiological and pathological effects of
moderate carbon monoxide exposure. Br. med. J., 4: 447-452.
ASTRUP, P. & PAULI, H. G. (1968) A comparison of prolonged exposure to
carbon monoxide and hypoxia in man. Scand. J. clin. lab. Invest.,
22 (Suppl. 103): 1-71.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1967) Enhancing influence of
carbon monoxide on the development of atheromatosis in
cholesterol-fed rabbits. J. atheroscler. Res., 7: 343-354.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1970) Effects of carbon
monoxide exposure on the arterial walls. Ann. NY Acad. Sci.,
174: 294-300.
ASTRUP, P., OLSEN, H. M., TROLLE, D., & KJELDSEN, K. (1972) Effect of
moderate carbon monoxide exposure on fetal development. Lancet,
2: 1220-1222.
AYRES, S. M., CRISCITIELLO, A., & GIANELLI, S., JR (1966)
Determination of blood carbon monoxide content by gas
chromatography. J. appl. Physiol., 21: 1368-1370.
AYRES, S. M., MUELLER, H. S., GREGORY, J. J., GIANELLI, S., JR, &
PENNY, J. L. (1969) Systemic and myocardial hemodynamic responses
to relatively small concentrations of carboxyhemoglobin (COHB).
Arch. environ. Health, 18: 699-709.
AYRES, S. M., GIANELLI, S., JR & MUELLER, H. (1970) Myocardial and
systemic responses to carboxyhemoglobin. Ann. NY. Acad. Sci.,
174: 268-293.
BAKER, F. D. & TUMASONIS, C. F. (1972) Carbon monoxide and avian
embryogenesis. Arch. environ. Health, 24: 53-61.
BALABAEVA, L. & KALPAZANOV, I. (1974) [Effect of smoking and length of
service on the content of carboxyhemoglobin in blood of traffic
policemen.] Hig. i Zdraveo., 5: 490-493 (in Bulgarian).
BALL, K. & TURNER, R. (1974) Smoking and the heart: the basis for
action. Lancet, 2 (7884): 822-826.
BATES, D. R. & WITHERSPOON, A. E. (1952) The photo-chemistry of some
minor constituents of the earth's atmosphere (CO2, CO, CH4,
N2O). Mon. not. R. Astron. Soc. (Lond.), 112: 101-124.
BEARD, R. R. & GRANDSTAFF, N. W. (1975) Carbon monoxide and human
functions. In: Weiss, P. & Laties, V. G., ed. Behavioral
Toxicology, New York, Plenum Press, pp. 1-26.
BEARD, R. R. & WERTHEIM, G. A. (1967) Behavioral impairment associated
with small doses of carbon monoxide. Am. J. public Health,
57: 2012-2022.
BEHRMAN, R. E., FISHER, D. E., & PATON, J. (1971) Air pollution in
nurseries: correlation with a decrease in oxygen-carrying capacity
of hemoglobin. J. Pediatr., 78: 1050-1054.
BENDER, W., GÖTHERT, M., & MALORNY, G. (1972) Effect of low carbon
monoxide concentrations on psychological functions. Staub-
Reinhalt. Luft., 32 (4): 54-60.
BIDWELL, R. G. S. & FRASER, D. E. (1972) Carbon monoxide uptake and
metabolism by leaves. Can. J. Bot., 50: 1435-1439.
BLACKMORE, D. J. (1974) Aircraft accident toxicology: UK experience
1967-1972. Aerosp. Med., 45: 987-994.
BORST, J. R. (1970) Acute and chronic carbon monoxide poisoning in
private motor-cars and lorries. Arts Auto, 36 (19): 1518-1523.
BREYSSE, P. A. & BOVEE, H. H. (1969) Use of expired air-carbon
monoxide for carboxyhemoglobin determinations in evaluating carbon
monoxide exposures resulting from the operation of gasoline fork
lift trucks in holds of ships. Am. Ind. Hyg. Assoc. J.,
30: 477-483.
BRIEGER, H. (1944) Carbon monoxide polycythemia. J. ind. Hyg.
Toxicol., 26: 321-327.
BRODY, J. S. & COBURN, R. F. (1970) Effects of elevated
carboxyhemoglobin on gas exchange in the lung. Ann. NY Acad.
Sci., 174: 255-260.
BUCHWALD, H. (1969) Exposure of garage and service station operatives
to carbon monoxide: a survey based on carboxyhemoglobin levels.
Am. Ind. Hyg. Assoc. J., 30: 570-575.
BUTT, J., DAVIES, G. M., JONES, J. G., & SINCLAIR, A. (1974)
Carboxyhaemoglobin levels in blast furnace workers. Ann. occup.
Hyg., 17: 57-63.
BYCZKOWSKA, Z. & MILAN, K. (1971) [Acute carbon monoxide poisoning
with temporary renal insufficiency.] Med. Pr., 22: 119-121
(in Polish).
CALVERT, J. G. (1973) Interactions of air pollutants. In: Proceedings
of the Conference on Health Effects of Air Pollutants, Oct.,
Washington, DC, National Academy of Sciences, National Research
Council, pp. 19-101 (Serial No. 93-15).
CASTLEDEN, C. M. & COLE, P. V. (1975) Carboxyhaemoglobin levels of
smokers and nonsmokers working in the city of London. Br. J. ind.
Med., 32: 115-118.
CAVANAGH, L. A., SCHADT, C. F., & ROBINSON, E. (1969) Atmospheric
hydrocarbon and carbon monoxide measurements at Point Barrow,
Alaska. Environ. Sci. Technol., 3: 251-257.
CHALUPA, B. (1960) [Disorders of memory in acute carbon monoxide
intoxications.] Prac. Lek., 12: 331-336 (in Polish).
CHAPMAN, D. J. & TOCHER, R. D. (1966) Occurrence and production of
carbon monoxide in some brown algae. Can. J. Bot.,
44: 1438-1442.
CHEVALIER, R. B., BOWERS, J. A., BONDURANT, S., & ROSS, J. C. (1963)
Circulatory and ventilatory effects of exercise in smokers and
nonsmokers. J. appl. Physiol., 18: 357-360.
CHEVALIER, R. B.. KRUMHOLZ, R. A., & ROSS, J. C. (1966) Reaction of
nonsmokers to carbon monoxide inhalation. Cardiopulmonary
responses at rest and during exercise. J. Am. Med. Assoc.,
198: 1061-1064.
CHIODI, H., DILL, D. B., CONSOLAZIO, F., & HORVATH, S. M. (1941)
Respiratory and circulatory responses to acute carbon monoxide
poisoning. Am. J. Physiol, 134: 683-693.
CHOVIN, P. (1967) Carbon monoxide: analysis of exhaust gas
investigations in Paris. Environ. Res., 1: 198-216.
CHOVIN, P. (1974) Comments on paper presented by H. Antweiler.
In: Proceedings of the European Colloquium Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg, Commission of the European
Communities Directorate General Scientific and Technical
Information Management, pp. 225-236.
CLARK, R. T., JR & OTIS, A. B. (1952) Comparative studies on
acclimatization of mice to carbon monoxide and to low oxygen.
Am. J. Physiol, 169: 285-294.
COBURN, R. F. (1970a) Enhancement by phenobarbital and
diphenylhydantoin of carbon monoxide production in normal man.
New Engl. J. Med., 283: 512-515.
COBURN, R. F. (1970b) The carbon monoxide body stores. Ann. NY Acad.
Sci., 174: 11-22.
COBURN, R. F., DANIELSON, G. K., BLAKEMORE, W. S., & FORSTER, R. E.
(1964) Carbon monoxide in blood: analytical method and sources of
error. J. appl. Physiol, 19: 510-515.
COBURN, R. F., FORSTER, R. E., & KANE, P. B. (1965) Considerations of
the physiological variables that determine the blood
carboxyhemoglobin in man. J. clin. Invest., 44: 1899-1910.
COBURN, R. F., WILLIAMS, W. J., & KAHN, P. B. (1966) Endogenous carbon
monoxide production in patients with hemolytic anemia. J. clin.
Invest., 45: 460-468.
COBURN, R. F., PLOEGMAKERS, F., GONDRIE, P., & ABBOUD, R. (1973)
Myocardial myoglobin oxygen tension. Am. J. Physiol.,
224: 870-876.
COHEN, S. I., DEANE, M., & GOLDSMITH, J. R. (1969) Carbon monoxide and
survival from myocardial infarction. Arch. environ. Health,
19: 510-517.
COHEN, S. I., DORION, G., GOLDSMITH, J. R., & PERMUTT, S. (1971a)
Carbon monoxide uptake by inspectors at a United States-Mexico
border station. Arch. environ. Health, 22: 47-54.
COHEN, S. I., PERKINS, N.M., URY, H. K., & GOLDSMITH, J. R. (1971b)
Carbon monoxide uptake in cigarette smoking. Arch. environ.
Health, 22: 55-60.
COLLISON, H. A., RODKEY, F. L., & O'NEAL, J. D. (1968) Determination
of carbon monoxide in blood by gas chromatography. Clin. Chem.,
14: 162-171.
COLUCCI, J. M. & BEGEMAN, C. R. (1969) Carbon monoxide in Detroit, New
York, and Los Angeles air. Environ. Sci. Technol., 3: 41-47.
COMMINS, B. T. & LAWTHER, P. J. (1965) A sensitive method for the
determination of carboxyhaemoglobin in a finger prick of blood.
Br. J. ind. Med., 22: 139-143.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) Proceedings of the
European Colloquium on Carbon Monoxide Environmental Pollution
and Public Health, Luxembourg, 17-19 December, 1973, Luxembourg
Directorate General Scientific and Technical Information and
Information Management, 437 pp.
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1972) Air pollution:
Air quality criteria for carbon monoxide, No. 10, 235 pp.
CONLEE, C. J., KENLINE, P. A, CUMMINS, R. L., & KONOPINSKI, V. J.
(1967) Motor vehicle exhaust at three selected sites. Arch.
environ. Health, 14: 429-446.
COOPER, D. Y., LEVIN, S., NARASIMHULU, S., & ROSENTHAL, O. (1965)
Photochemical action spectrum of the terminal oxidase of mixed
function oxidase systems. Science, 147: 400-402.
CORYA, B. C., BLACK, M. J., & MCHENRY, P. L. (1976) Echocardiographic
findings after acute carbon monoxide poisoning. Br. heart J.,
38: 712-717.
CROPP, G. J. A. (1970) Ventilation in anemia and polycythemia.
Can. J. Physiol. Pharmacol., 48: 382-393.
DAHMS, T. E. & HORVATH, S. M. (1974) Rapid, accurate technique for
determination of carbon monoxide in blood. Clin. Chem.,
20: 533-537.
DAHMS, T. E., HORVATH, S. M., & GRAY, D. J. (1975) Technique for
accurately producing desired carboxyhemoglobin levels during rest
and exercise. J. appl. Physiol., 38: 366-368.
DALHAMN, T., EDFORS, M.-L., & RYLANDER, R. (1968) Retention of
cigarette smoke components in human lungs. Arch. environ. Health,
17: 746-748.
DAVIS, G. L. & GANTNER, G. E.(1974) Carboxyhemoglobin in volunteer
blood donors. J. Am. Med. Assoc., 230: 996-997.
DE BIAS, D. A., BIRKHEAD, N. C., BANERJEE, C. M., KAZAL, L. A.,
HOLBURN, R. R., GREENE, C. H., HARRER, W. V., ROSENFELD, L. M,
MENDUKE, H., WILLIAMS, N., & FRIEDMAN, M. H. F. (1972a) The
effects of chronic exposure to carbon monoxide on the
cardiovascular and hematologic systems in dogs with experimental
myocardial infarction. Int. Arch. Arbeitsmed., 29: 253-267.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., FRIEDMAN, M. H. F.,
HARRER, W., HOLBURN, R., KAZAL, L., MENDUKE, H., ROSENFELD, L.,
WILLIAMS, N., & GREENE, C. H. (1972b) The effects of chronic
exposure to carbon monoxide (100 ppm) on the cardiovascular
system of monkeys, New York, Coordinating Research Council,
Inc., 54 pp. (PB 215-527).
BE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., HARRER, W. V., &
KAZAL, L. A. (1973) Carbon monoxide inhalation effects following
myocardial infarction in monkeys. Arch. environ. Health,
27: 161-167.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., GREEN, C. H., SCOTT,
D., & HARRER, W. V. (1976) Effects of carbon monoxide inhalation
on ventricular fibrillation. Arch. environ. Health, 31: 42-46.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., LONGO, L. D., & FORSTER, R.
E. (1969) Endogenous carbon monoxide of mother and fetus, 39th
Annual Meeting of the Society of Pediatrics Research, Atlantic
City, NJ, p. 148.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., & FORSTER, R. E. (1970)
Cyclical variation of rate of heme destruction and carbon monoxide
production in normal women. Physiologist, 13: 178 (abstract).
DELWICHE, C. C. (1970) Carbon monoxide production and utilization by
higher plants. Ann. NY Acad. Sci., 174: 116-121.
DINMAN, B. D. (1968) Pathophysiologic determinants of community air
quality standards for carbon monoxide. J. occup. Med.,
10: 446-456.
DINMAN, B. D. (1969) Effects of long-term exposure to carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behaviour, and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 25-27.
DINMAN, B. D, EATON, J. W., & BREWER, G. J. (1970) Effects of carbon
monoxide on DPG concentration in the erythrocyte. Ann. NY Acad.
Sci, 174: 246-251.
DIVINCENZO, G. D. & HAMILTON, M. L. (1975) Fate and disposition of
[14C]methylene chloride in the rat. Toxicol. appl. Pharmacol.,
32: 385-393.
DORCUS, R. M. & WEIGAND, G. E. (1929) The effect of exhaust gas on the
performance in certain psychological tests. J. gen. Psychol.,
2: 73-76.
DOUGLAS, C. G., HALDANE, J. S., & HALDANE, J. B. S. (1912) The laws of
combination of haemoglobin with carbon monoxide and oxygen.
J. Physiol. (Lond.), 44: 275-304.
DOUZE, J. M. C. (1971) [Carbon monoxide poisoning by natural gas.]
Ned. Tidjschr. Geneesk., 115 (36): 1487-1493.
DRABKIN, D. L. & AUSTIN, J. H. (1935) Spectrophotometric studies. II.
Preparation from washed blood cells; nitric oxide hemoglobin and
sulfhemoglobin. J. biol. Chem., 112: 51-65.
DRINKWATER, B. L., RAVEN, P. B., HORVATH, S. M., GLINER, J. A.,
RUHLING, R. O., BOLDUAN, N. W., & TAGUCHI, S. (1974) Air
pollution, exercise and heat stress. Arch. environ. Health,
28: 177-181.
DYER, R. S. & ANNAU, Z. (1976) Carbon monoxide and superior colliculus
evoked potentials. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Protection Agency, 9 pp.
ECKARDT, R. E., MACFARLAND, H. N., ALARIE, Y. C. E., & BUSYE, W. M.
(1972) The biological effect from long-term exposure of primates
to carbon monoxide. Arch. environ. Health., 25: 381-387.
EHRICH, W. E., BELLET, S., & LEWEY, F. H. (1944) Cardiac changes from
CO poisoning. Am. J. med. Sci., 208: 512-523.
EJAM-BERDJEV, A. K. (1973) [Effect of small CO concentrations on the
cardiovascular system of workers engaged in cast-iron moulding.]
Harkovskovo Med. Inst., 110: 185-186 (in Russian).
EJAM-BERDJEV, A. K. & ATAEV, B. A. (1973) [Effect of toxic factors
with low intensity on the functional state of the CNS of workers
engaged in cast-iron moulding.] Zdravoohr. Turkm., 10: 24-34
(in Russian).
EKBLOM, B. & HUOT, R. (1972) Response to submaximal and maximal
exercise at different levels of carboxyhaemoglobin. Acta Physiol.
Scand, 86: 474-482.
ENGEL, R. R., RODKEY, F. L., O'NEAL, J. D., & COLLISON, H. A. (1969)
Relative affinity of human fetal hemoglobin for carbon monoxide
and oxygen. Blood, 33: 37-45.
ENGEL, R. R., RODKEY, F. L., & KRILL, C. E., JR (1971)
Carboxyhemoglobin levels as an index of hemolysis. Pediatrics,
47: 723-730.
ENVIRONMENT AGENCY (1973) [Air Pollution in Japan. Air monitoring
data in fiscal year 1972.] Tokyo, Air Pollution Control
Division, Air Quality Bureau, pp. 206-210 (in Japanese).
FLETCHER, C. M. & HORN, D. (1970) Smoking and health. Chron. World
Health Organ., 24: 345-370.
FODOR, G. G. & WINNEKE, G. (1971) [Different affinity coefficients of
CO to hemoglobin for man and animals.] Zbl. Bakter. Parasit.
Infekt. Hyg., 224(1): 15 (in German).
FODOR, G. G. & WINNEKE, G. (1972) Effect of low CO concentrations on
resistance to monotony and on psychomotor capacity. Staub-
Reinhalt. Luft, 32(4): 46-54.
FORBES, W. H., DILL, D. B., DESILVA, H., & VAN DEVENTER, F. M. (1937)
Influence of moderate carbon monoxide poisoning upon ability to
drive automobiles. J. ind. Hyg. Toxicol., 19: 598-603.
FORBES, W. H., SARGENT, F., & ROUGHTON, F. J. W. (1945) The rate of
carbon monoxide uptake by normal men. Am. J. Physiol.,
143: 594-608.
FORSTER, R. E. (1970) Carbon monoxide and the partial pressure of
oxygen in tissue. Ann. NY Acad. Sci., 174: 233-241.
FRANKENHAEUSER, M., MYRSTEN, A.-L., POST, B., & JOHANSSON, G. (1971)
Behavioral and physiological effects of cigarette smoking in a
monotonous situation. Psychopharmacologia (Berl.), 22: 1-7.
GADASKINA, I. D. (1960) [Chronic poisoning by carbon monoxide and the
methods required for the experimental study of this problem.] In:
Letaveta, A. A. & Kanarevskoj, A. A., ed. Industrial Toxicology,
Moscow, Akademija Medicinskij Nauk SSSR, Institut Gigieny Truda I
Profzabolevanij, pp. 249-255 (in Russian).
GLINER, J. A., RAVEN, P. B., HORVATH, S. M., DRINKWATER, B. L., &
SUTTON, J. C. (1975) Man's physiologic response to long-term work
during thermal and pollutant stress. J. appl. Physiol.,
39: 628-632.
GODIN, G. & SHEPHARD, R. J. (1972) On the course of carbon monoxide
uptake and release. Respiration, 29: 317-329.
GOLDBARG, A. N., KRONE, R. J., & RESNEKOV, L. (1971) Effects of
cigarette smoking on hemodynamics at rest and during exercise. I.
Normal subjects. Chest, 60: 531-536.
GOLDMAN, A., MURCRAY, D. G., MURCRAY, F. H., WILLIAMS, W. J, BROOKS,
J. N., & BRADFORD, C. M. (1973) Vertical distribution of CO in the
atmosphere. J. geophys. Res., 78(24): 5273-5283.
GOLDSMITH, J. R. (1970) Contribution of motor vehicle exhaust,
industry and cigarette smoking to community carbon monoxide
exposures. Ann. NY Acad. Sci, 174: 122-134.
GOLDSMITH, J. R. & ARONOW, W. S. (1975) Carbon monoxide and coronary
heart disease: A review. Environ. Res., 10: 236-248.
GOLDSMITH, J. R. & LANDAW, S. A. (1968) Carbon monoxide and human
health. Science, 162: 1352-1359.
GOLDSMITH, J. R, TERZAGHI, J., & HACKNEY, J. D. (1963) Evaluation of
fluctuating carbon monoxide exposures. Arch. environ. Health,
7: 647-663.
GORBATOV, O. & NORO, L. (1948) On acclimatization in connection with
acute carbon monoxide poisonings. Acta Physiol. Scand.,
15: 77-87.
GORDON, G. S. & ROGERS, R. L. (1969) A report of medical findings of
project carbon monoxide, Washington, DC, Merkle Press.
GORI, G. B. (1976) Low-risk cigarettes: a prescription. Science,
194: 1243-1246.
GORODINSKY, S. M., SEDOV, A. V., MAZIN, A. N., GAZIEV, G. A,
KLEPTSOVA, A. P, & ZUKOVA, L. I. (1970) [Effect of reduced
barometric pressure on the elimination of gaseous and volatile
metabolites in man isolated in a diving suit.] Kosm. Biol. Med.,
2: 78-82 (in Russian).
GORSKI, J. (1962) [The ballistocardiogram in acute carbon-monoxide
poisoning.] Pol. Tyg. Lek., 17: 872-876 (in Polish).
GÖTHE, C. J., FRISTEDT, B., SUNDELL, L., KOLMODIN, B., EHRNER-SAMUEL,
H., & GÖTHE, K. (1969) Carbon monoxide hazard in city traffic-an
examination of traffic policemen in three Swedish towns. Arch.
environ. Health, 19: 310-314.
GRAHN, D. & KRATCHMAN, J. (1963) Variation in neonatal death rate and
birth weight in the United States and possible relations to
environmental radiation, geology and altitude. Am. J. hum.
Genet., 15: 329-352.
GRANDSTAFF, N. W., BEARD, R. R., & MORANDI, A. J. (1975) Review of
the neurobehavioral effects of carbon monoxide on humans,
Stanford, CA, Stanford University School of Medicine, 74 pp.
(USEPA Contract No. BU75 B001.)
GRIGOROV, L., KAZASOV, P., KURCATOVA, G., BALABAEVA, L., PAVLITZIAN,
T, VACEV, D., JAKIMOV, E., PETROVA, G., MAKSIMOVA, F., TZVETKOV,
T., BLASKOVA, D., DJOLOV, G., & Prof. BRESKOV. (1968) [Study of
the atmospheric air pollution in the region of the thermo-
electrical central "Maritza-Iztok", meteorological conditions and
their effects on the health of the population.] Izvestija NIHI,
3: 33 (in Bulgarian).
GROLL-KNAPP, E., WAGNER, H., HAUCK, H., & HAIDER, M. (1972) Effects of
low carbon monoxide concentrations on vigilance and computer-
analyzed brain potentials. Staub-Reinhalt. Luff, 32 (4): 64-68.
GROLL-KNAPP, E., HAIDER, M., HOLLER, H., JENKER, G., NEUBERGER, M., &
STIDL, H. (1976) Neuro- and psychophysiological effects of
moderate CO-doses. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Research Agency, 9 pp.
GRUT, A. (1949) Chronic carbon monoxide poisoning: a study in
occupational medicine, Copenhagen, Ejnar Munksgaard, 229 pp.
GUEST, A.D. L., DUNCAN, C., & LAWTHER, P. J. (1970) Carbon monoxide
and phenobarbitone: a comparison of effects on auditory flutter
fusion threshold and critical flicker fusion threshold.
Ergonomics (Lond.), 13(5): 587-594.
HAAGEN-SMIT, A. J. (1966) Carbon monoxide levels in city driving.
Arch. environ. Health, 12: 548-551.
HACKNEY, J. D, LINN, W. S., LAU, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. 3. Two hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HAEBISCH, H. (1970) [The cigarette as a source of carbon monoxide.]
Arch. Toxikol., 26: 251-261 (in German).
HÄGGENDAL, E., NILSSON, N.J., & NORBACH, B. (1966) Effect of blood
corpuscle concentration on cerebral blood flow. Acta Chir. Scand.
(Suppl.), 364: 3-12.
HALDANE, J. S. & PRIESTLEY, J. G. (1935) Respiration, New Haven,
Yale University Press, 493 pp.
HALPERIN, M. H., MCFARLAND, R. A., NIVEN, J. I., & ROUGHTON, F. J. W.
(1959) The time course of the effects of carbon monoxide on visual
thresholds. J. Physiol., 146: 583-593.
HAMMOND, E. C. (1962) The effects of smoking. Sci. Am., 207: 3-16.
HARKE, H. P. (1975) [Passive smokers and transport vehicles.]
Arbeitsmed. Sozialmed. Präventivmed., 10: 240-241 (in German).
HELLUNG-LARSEN, P., LAURSEN, T., KJELDSEN, K., & ASTRUP, P. (1968)
Lactate dehydrogenase isoenzymes of aortic tissue in rabbits
exposed to carbon monoxide. J. Atheroscler. Res., 8: 343-349.
HELMCHEN, H. & KUNKEL, H. (1964) [Findings concerning rhythmic after-
oscillations at optically evoked responses in EEG of man.] Arch.
Psychiatr. Z. Neurol., 205: 397-408 (in German).
HERSCH, P. (1964) Galvanic analysis. In: Reilley, C. A., ed. Advances
in analytical chemistry and instrumentation, New York,
Interscience Publishers, Vol. 3, pp. 183-249.
HERSCH, P. (1966) Process for measuring the carbon monoxide content of
a gas stream. Off. Gaz. US Pat. Off., 827(4): 1276-1277.
HEXTER, A. C. & GOLDSMITH, J. R. (1971) Carbon monoxide: association
of community air pollution with mortality. Science,
172: 265-267.
HINDS, W. C. & FIRST, M. W. (1975) Concentrations of nicotine and
tobacco smoke in public places. N. Engl. J. Med., 292
(16): 844-845.
HOFFMAN, D. & WYNDER, E. L. (1972) Smoke of cigarettes and little
cigars: an analytical comparison. Science, 178: 1197-1199.
HOGAN, G. K., SMITH, R. O., & CORNISH, H. H. (1976) Studies on the
microsomal conversion of dichloromethane to carbon monoxide.
Toxicol. appl. Pharmacol., 37: 112.
HORVATH, S. M. (1973) Effects of carbon monoxide on human behaviour.
In: Proceedings of the Conference on Health Effects of Air
Pollutants, 3-5 October, 1973, Washington, DC, National Academy
of Sciences, pp. 127-139 (Serial No. 93-15).
HORVATH, S. M. & ROUGHTON, F. J. W. (1942) Improvements in the
gasometric estimation of carbon monoxide in blood. J. biol.
Chem., 144: 747-755.
HORVATH, S. M., DAHMS, T. E., & O'HANLON, J. F. (1971) Carbon monoxide
and human vigilance: a deleterious effect of present urban
concentrations. Arch. environ. Health, 23: 343-347.
HORVATH, S. M., RAVEN, P. B., DAHMS, T. E., & GRAY, D. J. (1975)
Maximal aerobic capacity at different levels of carboxyhemoglobin.
J. appl. Physiol., 38: 300-303.
HOSKO, M. J. (1970) The effect of carbon monoxide on the visual evoked
response in man. Arch. environ. Health, 21: 174-180.
INGERSOLL, R. B., INMAN, R. E., & FISHER, W. R. (1974) Soil's
potential as a sink for atmospheric carbon monoxide. Tellus,
XXVI(1-2): 151-159.
JAFFE, L. S. (1973) Carbon monoxide in the biosphere: sources,
distributions and concentrations. J. geophys. Res.,
67: 5293-5305.
JENKINS, C. E. (1932) The haemoglobin concentration of workers
connected with internal combustion engines. J. Hyg.,
32: 406-408.
JOHNSON, C. J., MORAN, J. C., PAINE, S.C., ANDERSON, H. W., & BREYSSE,
P. A. (1975) Abatement of toxic levels of carbon monoxide in
Seattle ice-skating rinks. Am. J. public Health, 65: 1087-1090.
JONES, J. G. & WALTERS, D. H. (1962) A study of carboxyhaemoglobin
levels in employees at an integrated steelworks. Ann. occup.
Hyg., 5: 221-230.
JONES, R. A., STRICKLAND, J. A., STUNKARD, J. A., & SIEGEL, J. (1971)
Effects on experimental animals of long-term inhalation exposure
to carbon monoxide. Toxicol. appl. Pharmacol., 19: 46-53.
JONES, R. D., COMMINS, B. T., & CERNIK, A. A. (1972) Blood lead and
carboxyhaemoglobin levels in London taxi drivers. Lancet,
2: 302-303.
JUNGE, C., SEILER, W., & WARNECK, P. (1971) The atmospheric 12CO and
14CO budget. J. geophys. Res., 76(12): 2866-2879.
KAHN, A., RUTLEDGE, R. B., DAVIS, G. L., ALTES, J. A., GANTNER, G. E.,
THORNTON, C. A., & WALLACE, N. D. (1974) Carboxyhemoglobin sources
in the metropolitan St. Louis population. Arch. environ. Health,
29: 127-135.
KANDALL, S. R., LANDAW, S. A., & THALER, M. M. (1973) Carboxyhemo-
globin exchange between donors and recipients of blood
transfusions. J. Pediatr., 52: 716-718.
KILLICK, E. M. (1940) Carbon monoxide anoxemia Physiol. Rev.,
20(3): 313-344.
KJELDSEN, K. (1969) Smoking and atherosclerosis. Investigations on
the significance of the CO content in tobacco smoke in
atherogenesis, Copenhagen, Munksgaard, 142 pp.
KJELDSEN, K., THOMSEN, H. K., & ASTRUP, P. (1974) Effects of carbon
monoxide on myocardium. Circ. Res., 34: 339-348.
KLAUSEN, K., RASMUSSEN, B., GJELLEROD, H., MADSEN, H., & PETERSEN, E.
(1968) Circulation, metabolism and ventilation during prolonged
exposure to carbon monoxide and to high altitude. Scand. J. Clin.
Lab. Invest., 22 (Suppl. 103): 26-38.
KLIMISCH, H. J., CHEVALIER, H. J., HARKE, H. P., & DONTENWILL, W.
(1975) Uptake of carbon monoxide in blood of miniature pigs and
other mammals. Toxicology, 3: 301-310.
KODAT, V. ( 1971) Excretion of CO by workers on generatory station,
Candidate dissertation, Prague, 129 pp.
KOMATSU, E. (1959) [Shinshu myocardosis-a type of myocardosis caused
by carbon monoxide.] In: [Proceedings of the 15th General
Assembly of the Japan Medical Congress, Tokyo, Japan, 1-5 April,
1959.] pp. 343-348 (in Japanese).
KRONE, R. J., GOLDBARG, A. N., BALKOURA, M., SCHUESSLER, R., &
RESNEKOV, L. (1972) Effects of cigarette smoking at rest and
during exercise. II. Role of venous return. J. appl. Physiol,
32: 745-748.
KROTOVA, E. I. & MUZYKA, V. I. (1974) [Content of d-amino-levulinic
acid in erythrocytes of workers having contact with CO.] Gig. Tr.
i Prof. Zabol., 6: 43-44 (in Russian).
KUBIC, V. L., ANDERS, M. W., ENGEL, R. R., BARLOW, C. H., & CAUGHEY,
W. S. (1974) Metabolism of dihalomethanes to carbon monoxide.
Drug Metab. Disposition, 2(1): 53-57.
KULLER, L. H., RADFORD, E. P., SWIFT, D, PERPER, J., & FISHER, R.
(1975) Carbon monoxide and heart attacks. Arch. environ. Health,
30: 477-482.
KUSTOV, V. V., BELKIN, V. I., ABIDIN, B. I., OSTAPENKO, O. F.,
MALKUTA, A. N., & PODDUBNAJA, L. T. (1972) [Aspects of chronic
carbon monoxide poisoning in young animals.] Gig. Tr. i Prof
Zabol., 5: 50-52 (in Russian).
LANDAW, S. A. (1973) The effects of cigarette smoking on the total
body burden and excretion rates of carbon monoxide J. occup.
Med., 15(3): 231-240.
LAZAREV, N. V., ed. (1965) [Carbon monoxide.] In: [Toxic substances
in industry.] Vol. 2, pp. 198-218 (in Russian).
LEE, R. E. (1972) Indoor-outdoor carbon monoxide pollution study,
Washington, DC, US Environmental Protection Agency, 408 pp.
(EPA-R4-73-020).
LEVY, H. H. (1972) Photochemistry of the lower troposphere planet.
Space Sci., 20: 919-935.
LEWEY, F. H. & DRABKIN, D. L. (1944) Experimental chronic carbon
monoxide poisoning of dogs. Am. J. med. Sci., 208: 502-511.
LILIENTHAL, J. L., JR & FUGITT, C. H. (1946) The effect of low
concentrations of carboxyhemoglobin on the "altitude tolerance" of
man. Am. J. Physiol., 145: 359-364.
LILY, R. E. C., COLE, P. V., & HAWKINS, L. H. (1972)
Spectrophotometric measurements of carboxyhaemoglobin. Br. J.
ind. Med., 29: 454-457.
LINDENBERG, R., PREZIOSI, T., LEVY, D., & CHISTENSEN, M. K. (1961)
Experimental investigation of carbon monoxide toxicity. Presented
at: The Air Pollution Medical Research Conference, Los Angeles,
CA, 4-7 December, 1961.
LINNENBOM, V. J., SWINNERTON, J. W., & LAMONTAGNE, R. A. (1973) The
ocean as a source for atmospheric carbon monoxide. J. geophys.
Res., 78(24): 5333-5340.
LISS, P. S. & SLATER, P. G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LJUBLINA, E. I. (1960) [Shifts in some physiological functions of the
animal on subacute and chronic exposure to carbon monoxide.]
In: Letaveta, A. A. & Kanarevskoj, A. A., ed: [Industrial
Toxicology.] Moscow, Akademija Medicinskij Nauk SSSR, Institut
Gigieny Truda i Profzabolevanij, pp. 256-263 (in Russian).
LOGUE, G. L., ROSSE, W. F., SMITH, W. T., SALTZMAN, H. A., &
GUTTERMAN, L. A. (1971) Endogenous carbon monoxide production
measured by gas-phase analysis: an estimation of heme catabolic
rate. J. lab. clin. Med., 77: 867-876.
LONG, K. R. & DONHAM, K. J. (1973) Occupational exposure to CO in
selected rural work environments, Iowa City, IA, Institute of
Agricultural Medicine & Environmental Health, Department of
Preventive Medicine & Environmental Health, University of Iowa.
LONGO, L. D. (1970) Carbon monoxide in the pregnant mother and fetus
and its exchange across the placenta. Ann. NY Acad. Sci.,
174: 313-341.
LONGO, L. D. & HILL, E. P. (1977) Carbon monoxide uptake and
elimination in fetal and maternal sheep. Am. J. Physiol.,
232: H324-H330.
LUOMANMAKI, K. (1966) Studies on the metabolism of carbon monoxide.
Ann. med. exp. Biol. Fenn. (Helsinki), 44 (Suppl. 2): 1-55.
MAAS, A. H. J., HAMELINK, M. L., & DELEEUW, R. J. M. (1970) An
evaluation of the spectrophotometric determination of HbO2,
HbCO, and Hb in blood with the co-oximeter IL 182. Clin. Chim.
Acta, 29: 303-309.
MACMAHON, B., ALPERT, M., & SALBER, E. J. (1966) Infant weight and
parental smoking habits. Am. J. Epidemiol., 82: 247-261.
MALENFANT, A. L., GAMBINO, S. R., WARASKA, A. J., & ROE, E. I. (1968)
Spectrophotometric determination of hemoglobin concentration and
percent oxyhemoglobin and carboxyhemoglobin saturation. Clin.
Chem., 14: 789.
MALINOW, M. R., MCLAUGHLIN, P., DHINDSA, D. S., METCALFE, J., OCHSNER,
A. J., III, HILL, J., & MCNULTY, W. P. (1976) Failure of carbon
monoxide to induce myocardial infarction in cholesterol-fed
cynomolgus monkeys. Cardiovasc. Res., 10: 101-108.
MALORNY, G., MÜLLER-PLATHE, O., & STRAUB, M. (1962) [Disturbance of
acid-base balance in acute carbon monoxide poisoning and effect of
oxygen or carbon dioxide-oxygen mixtures.] Arch. exp. Pathol.
Pharmakol. (Naunyn-Schmiedeberg), 243: 439-458 (in German).
MARKS, E. & SWIECICKI, W. (1971) [Effect of carbon monoxide, vibration
and lowered atmospheric pressure upon cerebral neurosecretory
system and catecholamines level.] Med. Pr., 22: 336-341 (in
Polish).
MARTIN, W. & STERN, A. C. (1974) The world's air quality management
standards. Vol. 1. The air quality management standards of the
world, including United States federal standards, Washington,
DC, US Environmental Protection Agency, 383 pp.
MAZIARKA, S., BORKOWSKA, M., MISIAKIEWICZ, Z., STRUSINSKI, A., &
WYSZYNSKA, H. (1974) [Air pollution in the streets of Warsaw in
relation to surrounding buildings.] Roczn. Pzh., 25:1-5 (in
Polish).
MCCLATCHIE, E. A., COMPHER, A. B., & WILLIAMS, K. Y. (1972) A high
specificity carbon monoxide analyzer. Anal. Instrum. (Symp.),
10: 67-69.
MCCONNELL, J. C., MCELROY, M. B., & WOFSY, S.C. (1971) Natural sources
of atmospheric CO. Nature (Lond.), 233: 187-188.
MCCREDIE, R. M. & JOSE, A.D. (1967) Analysis of blood carbon monoxide
and oxygen by gas chromatography. J. appl. Physiol.,
22: 863-866.
MCFARLAND, R. A. (1973) Low level exposure to carbon monoxide and
driving performance. Arch. environ. Health, 27: 355-359.
MCFARLAND, R. A., ROUGHTON, F. J. W., HALPERIN, M. H., & NIVEN, J. I.
(1944) The effects of carbon monoxide and altitude on visual
thresholds. Aerosp. Med., 15: 381-394.
MCFARLAND, R. A., FORBES, W. H., STOUDT, H. W., DOUGHERTY, A., CROLEY,
T. J., MOORE, R. C., & NALWALK, T. J. (1973) A study of the
effects of low levels of carbon monoxide upon humans performing
driving tasks, Boston, Guggenheim Center of Aerospace Health &
Safety, Harvard School of Public Health, 88 pp.
MCMULLEN, T. B. (1975) Interpreting the eight-hour national ambient
air quality standard for carbon monoxide. J. Air Pollut. Control
Assoc., 25: 1009-1014.
MEHMEL, H. C., DUVELLEROY, M. A., & LAVER, M. B. (1973) Response of
coronary blood flow to pH-induced changes in hemoglobin-O2
affinity. J. appl. Physiol., 35: 485-489.
MIDDLETON, V., VAN POZNAK, A., ARTUSIO, J. F., JR, & SMITH, S. M.
(1965) Carbon monoxide accumulation in closed circle anesthesia
systems. Anesthesiology, 26: 715-719.
MILLS, E. & EDWARDS, M. W., JR (1968) Stimulation of aortic and
carotid chemoreceptors during carbon monoxide inhalation.
J. appl. Physiol., 25: 494-502.
MINISTRY OF LABOUR (1965) Carbon monoxide poisoning causes and
prevention, London, Her Majesty's Stationery Office, 72 pp.
(Safety, Health & Welfare Series No. 29).
MITCHELL, J. H., SPROULE, B. J., & CHAPMAN, C. B. (1958) The
physiological meaning of the maximal oxygen intake test. J. clin.
Invest., 37: 538-547.
MONTGOMERY, M. R. & RUBIN, R. J. (1971) The effect of carbon monoxide
inhalation on in vivo drug metabolism in the rat. J. Pharmacol.
exp. Ther., 179: 465-473.
MOREL, S. & SZEMBERG, T. (1971) [Air pollution in Ladle Bay.] Tech.
Sanit., 45: 353-355 (in Polish).
MULHAUSEN, R. O., ASTRUP, P., & MILLEMGAARD, K. (1968) Oxygen affinity
and acid-base status of human blood during exposure to hypoxia and
carbon monoxide. Scand. J. clin. lab. Invest., 22 (Suppl. 103):
9-15.
MUSSELMAN, N. P., GROFF, W. A., YEVICH, P. P., WILINSKY, F. T., WEEKS,
M. H., & OBERST, F. W. (1959) Continuous exposure of laboratory
animals to low concentration of carbon monoxide. Aerosp. Med.,
30: 524-529.
MYHRE, L. (1974) Rate of CO elimination in resting man. Fed. Proc,
33: 440.
MYRSTEN, A. L., POST, B., FRANKENHAEUSER, M., & JOHANSSON, G. (1972)
Changes in behavioural and physiological activation induced by
cigarette smoking in habitual smokers. Psychopharmacologia
(Berl.), 27: 305-312.
NAS/NRC (1977) Carbon monoxide, Washington, DC, National Academy of
Sciences, 268 pp.
NEW MEXICO STATE DEPARTMENT OF PUBLIC HEALTH (1975) Birth weight and
altitude, pp. 7-16.
NIELSEN, B. (1971) Thermoregulation during work in carbon monoxide
poisoning. Acta physiol. Scand, 82: 98-106.
NOTOV, A. (1959) [Acute poisonings with CO, SO2 and nitric gases in
mines.] Sb. Tr. VMI Plovidiv, 60: 404-411 (in Bulgarian).
NOWARA, M. (1975) [Evaluation of occupational exposure by means of
determining carbon monoxide in the air at work places and carbon
monoxide hemoglobin.] Med. Pr., 26: 351-360 (in Polish).
NUNES, A. C. & SCHOENBORN, B. P. (1973) Dichloromethane and myoglobin
function. Mol. Pharmacol., 9: 835-839.
O'DONNELL, R. D., CHIKOS, P., & THEODORE, J. (1971a) Effect of carbon
monoxide exposure on human sleep and psychomotor performance.
J. appl. Physiol., 31: 513-518.
O'DONNELL, R. D., MIKULKA, P., HEINIG, P., & THEODORE, J. (1971b) Low
level carbon monoxide exposure and human psychomotor performance.
Toxicol. appl. Pharmacol., 18: 593-602.
OGAWA, M, KATSURADA. K., & SUGIMOTO, I. (1974) Pulmonary edema in
acute carbon monoxide poisoning. Int. Arch. Arbeitsmed.,
33: 131-138.
O'HANLON, J. F. (1975) Preliminary studies of the effects of carbon
monoxide on vigilance in man. In: Weiss, B. & Laties, V. G., ed.
Behavioral toxicology, New York, Plenum Press, pp. 61-72.
ORELLANO, T., DERGAL, E., ALIJANI, M., BRIGGS, C., VASQUEZ, J.,
GOLDBAUM, L., & ABSOLON, K. B. (1976) Studies on the mechanism of
carbon monoxide toxicity. J. surg. Res., 20: 485-487.
OTT, W. & MAGE, D. (1974) Interpreting urban carbon monoxide
concentrations by means of a computerized blood COHb model.
J. Air Pollut. Control Assoc., 28(9): 911-916.
PACE, N., STRAJMAN, E., & WALKER, E. L. (1950) Acceleration of carbon
monoxide elimination in man by high pressure oxygen. Science,
111: 652-654.
PANKOW, D. & PONSOLD, W. (1972) Leucine aminopeptidase activity in
plasma of normal and carbon monoxide poisoned rats. Arch.
Toxikol., 29: 279-285.
PANKOW, D. & PONSOLD, W. (1974) [The combined effects of carbon
monoxide and other biologically active detrimental factors on the
organism.] Zeits. Ges. Hyg., 9: 561-571 (in German).
PANKOW, D., PONSOLD, W., & FRITZ, H. (1974a) Combined effects of
carbon monoxide and ethanol on the effects of leucine
aminopeptidase and glutamic-pyruvic transaminase in the plasma of
rats. Arch. Toxicol, 32: 331-340.
PANKOW, D., PONSOLD, W., & GRIMM, I. (1974b) [On the potentiation of
the hepatic toxicity of carbon tetrachloride by carbon monoxide.]
Dtsch. Ges. Wesen., 29: 853-856 (in German).
PECORA, L. J. (1959) Physiologic study of the summating effects of
ethyl alcohol and carbon monoxide. Am. Ind. Hyg. Assoc. J.,
20: 235-240.
PENNEY, D., BENJAMIN, M., & DUNHAM, E. (1974a) Effect of carbon
monoxide on cardiac weight as compared with altitude effects.
J. appl. Physiol, 37: 80-84.
PENNEY, D., DUNHAM, E., & BENJAMIN, M. (1974b) Chronic carbon monoxide
exposure: Time course of hemoglobin, heart weight and lactate
dehydrogenase isozyme changes. Toxicol. appl. Pharmacol,
28: 493-497.
PERMUTT, S. & FAHRI, L. (1969) Tissue hypoxia and carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behavior and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 18-24.
PETERSON, J. E. & STEWART, R. D. (1970) Absorption and elimination of
carbon monoxide by inactive young men. Arch. environ. Health,
21: 165-171.
PETROV, P. (1968) [Chronic CO intoxications in workers engaged in
loading and unloading processes.] Transp. Med. Vesti, 4: 24-27
(in Bulgarian).
PIRNAY, F., DUJARDIN, J., DEROANNE, R., & PETIT, J. M. (1971) Muscular
exercise during intoxication by carbon monoxide. J. appl.
Physiol., 31: 573-575.
PITTS, G. C. & PACE, N. (1947) The effects of blood carboxyhemoglobin
concentration on hypoxia tolerance. Am. J. Physiol.,
148: 139-151.
PREZIOSI, T. J., LINDENBERG, R., LEVY, D., & CHRISTENSON, M. (1970) An
experimental investigation in animals of the functional and
morphological effects of single and repeated exposures to high and
low concentrations of carbon monoxide. Ann. NY Acad. Sci,
174: 369-384.
PROHOROV, Y. D. & ROGOV, A. A. (1959) [The pathohistological and
histochemical changes in the organs of rabbits subjected to a
prolonged action of carbon monoxide, sulfur dioxide and their
mixtures.] Gig. i Sanit., 24: 22-26 (in Russian).
RAMIREZ, R. G., ALBERT, S. N., AGOSTINI, J. C., BASU, A. P., GOLDBAUM,
L. R., & ABSOLON, K. B. (1974) Lack of toxicity of transfused
carboxyhemoglobin red blood cells and carbon monoxide inhalations.
Surg. Forum, 25: 165-168.
RAMSEY, J. M. (1967a) Carbon monoxide exposures in parking garages.
Bull. environ. Contam. Toxicol., 2: 182-190.
RAMSEY, J. M. (1967b) Carboxyhemoglobinemia in parking garage
employees. Arch. environ. Health, 15: 580-583.
RAMSEY, J. M. (1972) Carbon monoxide, tissue hypoxia, and sensory
psychomotor response in hypoxaemic subjects. Clin. Sci.,
42: 619-625.
RAMSEY, J. M. (1973) Effects of single exposures of carbon monoxide on
sensory and psychomotor response. Am. Ind. Hyg. Assoc. J.,
34: 212-216.
RAMSEY, J. M. & CASPER, P. W., JR (1976) Effect of carbon monoxide
exposures on erythrocyte 2,3-DPG in rabbits. J. appl. Physiol.,
41: 689-692.
RATNEY, R. S., WEGMAN, D. H., & ELKINS, H. B. (1974) In vivo
conversion of methylene chloride to carbon monoxide. Arch.
environ. Health, 28: 223-226.
RAVEN, P. B., DRINKWATER, B. L., HORVATH, S. M., RUHLING, R. O.,
GLINER, J. A., SUTTON, J. C., BOLDUAN, N. W. (1947a) Age, smoking
habits, heat stress and their interactive effects with carbon
monoxide and peroxyacetyl nitrate on man's aerobic power. Int. J.
Biometeorol., 18: 222-232.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N. W.,
TAGUCHI, S., GLINER, J. A., & HORVATH, S. M. (1974b) Effect of
carbon monoxide and peroxyacetyl nitrate on man's maximal aerobic
capacity. J. appl. Physiol., 36: 288-293.
RAY, A.M. & ROCKWELL, T. H. (1970) An exploratory study of automobile
driving performance under the influence of low levels of
carboxyhemoglobin. Ann. NY Acad. Sci., 174: 396-407.
RENCH, J. D. & SAVAGE, E. P. (1976) Carbon monoxide in the home
environment. J. environ. Health, 39: 104-106.
RISSANEN, V., PYÖRÄLÄ, K., & HEINONEN, O. P. (1972) Cigarette smoking
in relation to coronary and aortic atherosclerosis. Acta pathol.
microbiol. Scand. Sect. A, 80: 491-500.
ROBBINS, R. C., BORG, K. M., & ROBINSON, E. (1968) Carbon monoxide in
the atmosphere. J. Air Pollut. Control Assoc., 18: 106-110.
ROBINSON, J. C. & FORBES, W. F. (1975) The role of carbon monoxide in
cigarette smoking. Arch. environ. Health, 30: 425-434.
ROBINSON, E. & ROBBINS, R. C. (1970) Atmospheric background
concentrations of carbon monoxide. Ann. NY Acad. Sci.,
174: 89-95.
RONDIA, D. (1970) Abaissement de l'activité de la benzopyrene-
hydroxylase hepatique in vivo apres inhalation d'oxyde de
carbone. C. R. Acad. Sci. (Paris), 271: 617-619.
ROOT, W. S. (1965) Carbon monoxide. In: Fenn, W. O. & Rahn, H., ed.
Handbook of physiology, respiration 2, Washington, DC, American
Physiological Society, pp. 1087-1098.
ROSSI-BERNARDI, L., PERRELLA, M., LUZZANA, M., SAMAJA, M., & RAFFAELE,
I. (1977) Simultaneous determination of hemoglobin derivatives,
oxygen content, oxygen capacity, and oxygen saturation in 10 µl of
whole blood. Clin. Chem., 23: 1215-1225.
ROSSI-FANELLI, A. & ANTONINI, E. (1958) Studies on the oxygen and
carbon monoxide equilibria of human myoglobin. Arch. Biochem.
Biophys., 77: 478-492.
ROTH, R. P., DREW, R. T., Lo, R. J., & FOUTS, J. R. (1975)
Dichloromethane inhalation, carboxyhemoglobin concentrations, and
drug metabolizing enzymes in rabbits. Toxicol. appl. Pharmacol.,
33: 427-437.
ROUGHTON, F. J. W. (1970) The equilibrium of carbon monoxide with
human hemoglobin in whole blood. Ann. NY Acad. Sci.,
174: 177-188.
ROUGHTON, F. J. W. & DARLING, R. C. (1944) The effect of carbon
monoxide on the oxyhemoglobin dissociation curve. Am. J.
Physiol., 141: 17-31.
ROUGHTON, F. J. W. & ROOT, W. S. (1945) The estimation of small
amounts of carbon monoxide in blood. J. biol. Chem.,
160: 123-133.
RUMMO, N. & SARLANIS, K. (1974) The effect of carbon monoxide on
several measures of vigilance in a simulated driving task.
J. Saf Res., 6: 126-130.
RUSSELL, M. A. H., COLE, P. V., & BROWN, E. (1973) Absorption by non-
smokers of carbon monoxide from room air polluted by tobacco
smoke. Lancet, 1: 576-579.
RYLANDER, R. (1974) Environmental tobacco smoke effects on the non-
smoker. Report from a workshop. Scand J. resp. Dis.,
(Suppl. 91): 90 pp.
RYLOVA, M. L. (1960) [The application of integral methods of
investigation to determine the effect of repeated exposure to
carbon monoxide in animals.] In: Letaveta, A. A. & Kanarevskoj, A.
A., ed. [Industrial toxicology.] Moscow, Akademija Medicinskij
Nauk SSSR, Institut Gigieny Truda i Profzabolevanij, pp. 264-270
(in Russian).
SAMMONS, J. H. & COLEMAN, R. L. (1974) Firefighters' occupational
exposure to carbon monoxide. J. occup. Med., 16: 543-546.
SANTIAGO, T. V. & EDELMAN, N. H. (1972) Effect of anemia on the
ventilatory response to transient hypoxia. Fed. Proc.,
31: 371 (Abstract No. 864).
SARMA, J. S. M., TILLMANS, H., IKEDA, S., & BINS, R. J. (1975) The
effect of carbon monoxide on lipid metabolism of human coronary
arteries. Atherosclerosis, 22: 193-198.
SAYERS, R. R., YANT, W. P., LEVY, E., & FULTON, W. B. (1929) Effect
of repeated daily exposure of several hours to small amounts of
automobile exhaust gas, Washington, DC, Government Printing
Office, 49 pp. (Public Health Bull. No. 186).
SCHULTE, J. H. (1963) Effects of mild carbon monoxide intoxication.
Arch. environ. Health, 7: 524-530.
SEDOV, A. V., ZUKOVA, L. I., & MAZNEVA, G. E. (1971) [Carbon monoxide
excretion by the human organism.] Gig. Tr. i Prof Zabol.,
9: 36-39 (in Russian).
SEILER, W. (1975) The cycle of carbon monoxide in the atmosphere.
In: International Conference on Environmental Sensing and
Assessment, Las Vegas, NV, 14-19 September, New York, Institute
of Electrical and Electronics Engineers, Vol. 2, 6 pp.
SEILER, W. & JUNGE, C. E. (1969) Decrease of carbon monoxide mixing
ratio above the polar tropopause. Tellus, 21(3): 447-449.
SEILER, W. & JUNGLE, C. E. (1970) Carbon monoxide in the atmosphere.
J. geophys. Res., 75(12): 2217-2226.
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA,. M. A., & TARASOVA, L. N.
(1970) [New data on the concentration of the products of the
incomplete combustion of domestic gas in the air of premises
provided with a gas supply.] In: [Problems of current interest in
hygiene.] Moscow, Akademija Medicinskij Nauk SSSR, Institute of
Genetics and Community Hygiene, pp. 137-142 (in Russian).
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA, M. A., KLEBANOVA, V. A.,
OSINCEVA, V. P., & NAZARENKO, A. F. (1972) [The experimental basis
for measures to improve the quality of the air in flats provided
with a gas supply.] Gig. i Sanit., 7: 24-28 (in Russian).
SIEGEL, S. M., RENWICK, G., & ROSEN, L. A. (1962) Formation of carbon
monoxide during seed germination and seedling growth. Science,
137: 683-684.
SJÖSTRAND, T. (1948) A method for the determination of
carboxyhaemoglobin concentrations by analysis of the alveolar
air. Acta Physiol. Scand., 16: 201-210.
SMALL, K. A., RADFORD, E. P., FRAZIER, J. M., RODKEY, F. L., &
COLLISON, H. A. (1971) A rapid method for simultaneous measurement
of carboxy- and methemoglobin in blood. J. appl. Physiol.,
31: 154-160.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumonia. Arch. environ. Health, 16: 670-672.
SOKAL, J. A. (1975) Lack of the correlation between biochemical
effects on rats and blood carboxyhemoglobin concentrations in
various conditions of single acute exposure to carbon monoxide.
Arch. Toxicol, 34: 331-336.
SOTNIKOV, E. E. (1971) [Determination of carbon monoxide in the blood
by a gas chromatographic method.] Gig. i Sanit., 6: 61-64 (in
Russian).
SPEDDING, D. J. (1974) Air pollution, Oxford, Clarendon Press,
pp. 58-62.
SRCH, M. (1967) [The significance of carbon monoxide in cigarette
smoke in passenger car interiors.] Dtsch. Z. ges. gerichtl. Med,
60: 80-89 (in German).
STEVENS, C. M., KROUT, L., WALLING, D., VENTERS, A., ENGELKEMEIR, A.,
& Ross, L. E. (1972) The isotopic composition of atmospheric
carbon monoxide. Earth planet. Sci. Lett, 16: 147-165.
STEWART, R. D. & HAKE, C. L. (1976) Paint remover hazard. J. Am. Med.
Assoc., 235: 398-401.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. C. (1972a) Experimental human exposure to
methylene chloride. Arch. environ. Health, 26: 342-348.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. D. (1972b) Carboxyhemoglobin elevation after
exposure to dichloromethane. Science, 176: 295-296.
STEWART, R. D., NEWTON, P. E., HOSKO, M. J., & PETERSON, J. E. (1973a)
Effect of carbon monoxide on time perception. Arch. environ.
Health, 27: 155-160.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973b) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWARD, E. D.,
KALBFLEICH, J. H., VAN YSERLOO, B., & RIMM, A. A. (1973c)
Carboxyhemoglobin concentrations in blood from donors in Chicago,
Milwaukee, New York, and Los Angeles. Science, 182: 1362-1364.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWART, E. D.,
KALBFLEICH, J. D., VAN YSERLOO, B., & RIMM, A. A. (1974)
Carboxyhaemoglobin tests in American blood donors. JAMA,
229: 1187-1195.
STEWART, R. D, NEWTON, P. E., HOSKO, M. J., PETERSON, J. E., &
MELLENDER, J. W. (1975) The effect of carbon monoxide on time
perception, manual coordination, inspection, and arithmetic.
In: Weiss, B. & Laties, V. G., ed. Behavioral toxicology, New
York, Plenum Press, pp. 29-60.
STEWART, R. D., HAKE, C. L., WU, A., STEWART, T. A., & KALBFLEISCH, J.
H. (1976) Carboxyhemoglobin trend in Chicago blood donors,
1970-1974. Arch. environ. Health, 31: 280-286.
STUPFEL, M. (1975) Rhythms and air pollution. Chronobiologia,
11: 105-115.
STUPFEL, M. & BOULEY, G. (1970) Physiological and biochemical effects
on rats and mice exposed to small concentrations of carbon
monoxide for long periods. Ann. NY Acad. Sci. 174: 342-368.
STUPFEL, M., MAGNIER, M., & ROMARY, F. (1973) Rythme circadien de
l'action de l'oxide de carbone sur l'emission du gaz carbonique
par le rat en groupe. C. R. Acad. Sci. (Paris) Série D,
276: 1009-1012.
SUL'GA, T. M. (1962) [New data for the hygienic evaluation of CO in
air.] In: Ryazanov, V. A., ed. [ Maximal permissible
concentrations of air pollution.] Moscow, Medgiz, pp. 128-145
(in Russian).
SWIECICKI, W. (1973) [The effect of vibration and physical training on
carbohydrate metabolism in rats intoxicated with carbon monoxide.]
Med. Pr., 34: 399-405 (in Polish).
SWINNERTON, J. W., LAMONTAGNE, R. A., & LINNENBOM, V. J. (1971) Carbon
monoxide in rain water. Science, 172: 943-945.
TANAKA, M. (1965) [Studies on the etiological mechanism of fetal
development disorders caused by maternal smoking habits during
pregnancy.] J. Jpn. Obstet. Gynecol. Soc., 17: 1107-1114 (in
Japanese, English translation in J. Obstet. Gynecol. Soc.,
14: 45 (1967)).
THOMSEN, H. K. (1974) Carbon monoxide-induced artherosclerosis in
primates. Atherosclerosis, 20: 233-240.
THOMSEN, H. K. & KJELDSEN, K. (1974) Threshold limit for carbon
monoxide-induced myocardial damage. Arch. environ. Health,
29: 73-79.
THOMSEN, H. K. & KJELDSEN, K. (1975) Aortic intimal injury in rabbits.
Arch. environ. Health, 30: 604-607.
TIUNOV. A. & KUSTOV, V. V. (1969) [Toxicology of carbon monoxide.]
Leningrad, Medicina, pp. 81-217 (in Russian).
TRAYSTMAN, R. J. (1976) Effects of carbon monoxide hypoxia and hypoxic
hypoxia on cerebral circulation. In: Fourth International
Congress on Event Related Slow Potentials of the Brain,
Hendersonville, NC, April 4-10, 1976, Research Triangle Park,
NC, US Environmental Protection Agency, 9 pp.
TROMPEO, G., TURLETTl, G., & GIARRUSSO, O. T. (1964) [Concentration of
carbon monoxide in underground garages.] Rass. Med. Ind.,
33: 392-393 (in Italian).
TUMASONIS, C. F. & BAKER, F. D. (1972) Influence of carbon monoxide
upon some respiratory enzymes of the chick embryo. Bull. environ.
Contain. Toxicol., 8: 113-119.
TURNER, J. A. McM., SILLETT, R. W., & BALL, K. P. (1974) Some effects
of changing to low-tar and low-nicotine cigarettes. Lancet,
2:737-739.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Air quality
criteria for carbon monoxide, Washington, DC, US DHEW Public
Health Service, National Air Pollution Control Administration.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1972) Criteria for a
recommended standard. Occupational exposure to carbon monoxide,
Washington, DC, US DHEW Health Services and Mental Health,
National Institute for Occupational Safety and Health, 120 pp.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1973) The Health
Consequences of Smoking, Washington, DC, US DHEW, Public Health
Service, pp. 367-381.
US ENVIRONMENTAL PROTECTION AGENCY (1973a) The national air
monitoring program: Air quality and emission trends, Annual
report, Vol. 1, Research Triangle Park, NC, USEPA, 182 pp.
(EPA 450/1-73-001-A).
US ENVIRONMENTAL PROTECTION AGENCY (1973b) Compilation of air
pollution emission factors 2nd ed., Research Triangle Park, NC,
USEPA, 288 pp. (Report No. AP42).
US ENVIRONMENTAL PROTECTION AGENCY (1975) Human health damages from
mobile source air pollution: A delphic study, Research Triangle
Park, NC, USEPA (Project No. 07AAC-OS).
VAN KAMPEN, E. J. & ZIJLSTRA, W. G. (1961) Standardization of
hemoglobinometry. II. The hemiglobincyanide method. Clin. Chim.
Acta, 6: 538-544.
VINOGRADOV, G. I., CERNICENKO, I. A., & MAKARENKO, E. M. (1974) [The
allergenic activity of motor vehicle gases.] Gig. i Sanit.,
8: 10-13 (in Russian).
VOGEL, J. A. & GLESER, M. A. (1972) Effect of carbon monoxide on
oxygen transport during exercise. J. appl. Physiol.,
32: 234-239.
VOGEL, J. A., GLESER, M. A., WHEELER, R. C., & WHITTEN, B. K. (1972)
Carbon monoxide and physical work capacity. Arch. environ.
Health, 24: 198-203.
VOLLMER, E. P., KING, B. G, BIRREN, J. E., & FISHER, M. B. (1946) The
effects of carbon monoxide on three types of performance at
simulated altitudes of 10,000 and 15,000 feet. J. exp. Psychol.,
36: 244-251.
VYSKOCIL, J. (1957) Neurohumoral regulation in acute and chronic
carbon monoxide poisoning. Int. Arch. Gewerbepathol. Gewerbehyg.,
15: 457-472.
WAGEMANN, W. (1960) [The otological picture in carbon monoxide
poisoning.] Z. Laringol. Rhinol. Otol., 39: 691-708 (in German).
WAGNER, J. A., HORVATH, S. M., & DAHMS, T. E. (1975) Carbon monoxide
elimination. Respir. Physiol, 23: 41-47.
WAKEHAM, H. R. R. (1976) Environmental carbon monoxide from cigarette
smoking, Sixth International Tobacco Scientific Congress, Tokyo,
Japan, November 18, 1976, 17 pp.
WALD, N. & HOWARD, S. (1975) Smoking, carbon monoxide and arterial
disease. Ann. occup. Hyg., 18: 1-14.
WALD, N., HOWARD, S., SMITH, P. G., & KJELDSEN, K. (1973) Association
between atherosclerotic diseases and carboxyhaemoglobin levels in
tobacco smokers. Br. med. J., 1: 761-765.
WALLACE, N. D., DAVIS, G. L., RUTLEDGE, R. B., & KAHN, A. (1974)
Smoking and carboxyhemoglobin in the St. Louis metropolitan
population. Arch. environ. Health, 29: 136-142.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WEBSTER, W. S., CLARKSON, T. B., & LOFLAND, H. B. (1968) Carbon
monoxide-aggravated atherosclerosis in the squirrel monkey. Exp.
mol. Pathol., 13: 36-50.
WEINSTOCK, B. & NIKI, H. (1972) Carbon monoxide balance in nature.
Science, 176: 290-292.
WEIR, F. W. & ROCKWELL, T. H. (1973) An investigation of the effects
of carbon monoxide on humans in the driving task, Columbus, OH,
Ohio State University Research Foundation, 170 pp. (Contract No.
68-02-0329 and CRC-APARC Project CAPM-9-69).
WEISS, H. R. & COHEN, J. A. (1974) Effects of low levels of carbon
monoxide on rat brain and muscle tissue pO2. Environ.
Physiol. Biochem., 4: 31-49.
WELLS, L. L. (1933) The prenatal effect of carbon monoxide on albino
rats and the resulting neuropathology. Biologist, 15: 80-81.
WESTBERG, K, COHEN, N., & WILSON, K. W. (1971) Carbon monoxide: its
role in photochemical smog formation. Science, 171: 1013-1015.
WESTLAKE, D. W., ROXBURGH, J. M., & TALBOT, G. (1961) Microbial
production of carbon monoxide from flavonoids. Nature (Lond.),
189: 510-511.
WHITE, P. (1970) Carbon monoxide production and heme catabolism. Ann.
NY Acad. Sci., 174: 23-31.
WILKS, S. S. (1959) Carbon monoxide in green plants. Science,
129: 964-966.
WILKS, S. S., TOMASHEFSKI, J. F., & CLARKE, R. T., JR (1959)
Physiological effects of chronic exposure to carbon monoxide.
J. appl. Physiol., 14: 305-310.
WILLIAMS, I. R. & SMITH, E. (1935) Blood picture, reproduction, and
general condition during daily exposure to illuminating gas.
Am. J. Physiol., 110: 611-615.
WINNEKE, G. (1974) Behavioral effects of methylene chloride and carbon
monoxide as assessed by sensory and psychomotor performance. In:
Xintaras, C., Johnson, B., & deGroot, I., ed. Behavioral
toxicology: early detection of occupational hazards, Washington,
DC, US Government Printing Office, pp. 130-144 (New Publ. No.
N10511).
WINNEKE, G. (1977) [Studies on marginal values for carbon monoxide.]
Lufthyg. Silkoseforsch., 10: 75-90 (in German).
WINNEKE, G., FODOR, G. G., & SCHLIPKÖTER, H. W. (1976) Effects of low
level carbon monoxide exposure and monotony on vigilance and
vertex evoked potentials. In: Proceedings of the fourth
international congress on event-related slow potentials of the
brain. Hendersonville, NC, April 4-10, 1976, Research Triangle
Park, NC, US Environmental Protection Agency, 5 pp.
WOLFE, D. G. & BIDLACK, W. R. (1976) The formation of carbon monoxide
during peroxidation of microsomal lipids. Biochem. Biophys. res.
Commun., 73:850-857.
WHO (1975) WHO Technical Report Series, No. 568 (Smoking and its
effects on health), 100 pp.
WHO (1976) Selected methods of measuring air pollutants, Geneva,
WHO, 112 pp. (Offset Publication No. 24).
WRIGHT, G. R., JEWCZYK, S., ONROT, J., TOMLINSON, P., & SHEPHARD, R.
J. (1975) Carbon monoxide in the urban atmosphere. Arch. environ.
Health, 30: 123-129.
WRIGHT, G. W., RANDELL, P., & SHEPHARD, D.. R. J. (1973) Carbon
monoxide and driving skills. Arch. environ. Health, 27: 349-354.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response technique
in air pollution toxicology. Toxicol appl. Pharmacol., 8: 77-87.
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor-outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
ZENKEVIC, E. C. (1973) [Hemodynamic changes under the effect of CO
displayed adaptation.] Sb. Probl. Adapt. Gig. Tr., pp. 67-72
(in Russian).
ZORN, H. (1968) [Carbon dioxide-oxygen mixture or pure oxygen for
respiration in the case of carbon monoxide intoxication.] Dtsch.
Med. Wochenschr., 93: 1536 (in German).
ZORN, H. (1972) The partial oxygen pressure in the brain and liver at
subtoxic concentrations of carbon monoxide. Staub-Reinhalt. Luff,
32: 24-29.
Annex 1
THE RELATIONSHIP BETWEEN CARBON MONOXIDE CONCENTRATION IN AIR AND
CARBOXYHAEMOGLOBINa
(1) Several empirical equations have been proposed for estimating
carboxyhaemoglobin levels from environmental exposure conditions.
These equations were based on controlled exposures of human
volunteers. Most of them referred to subjects at rest, or performing
sedentary activities or light work. The simplest empirical equations
described carboxyhaemoglobin levels as a linear function of carbon
monoxide concentration in the inspired air [CO], and of exposure time
(t) (Forbes et al., 1945; Pace et al., 1946). They were applicable
only within a limited range of exposure conditions. Hanks & Farquhar
(1969) and Peterson & Stewart (1970) compiled empirical equations
involving more complex functional relationships that had a wider
application.
(2) In addition, models have been proposed that relate carboxyhaemo-
globin levels to both environmental exposure conditions and a number
of physiological variables such as blood volume, VB, endogenous
carbon monoxide production, Vco, diffusion capacity of the lung,
DL, and alveolar ventilation rate, VA. The best known model,
developed by Coburn et al. (1965), has been briefly discussed in
section 6.2. A more recent model, that took into account the dynamic
condition of urban carbon monoxide concentrations, was suggested by
Ott & Mage (1978) (p. 118).
(3) A simple linear relationship, proposed by Forbes et al. (1945),
linked the increase in the carboxyhaemoglobin level with the carbon
monoxide concentration in air, [CO](ppm) and exposure time, t(min):
a "This annex has been prepared by the Secretariat and has not been
reviewed by the Task Group, with the exception of Table 3.
Equations are presented in the form given in the original studies
and no attempt has been made to change to SI units. The Secretariat
wishes to express its appreciation to Dr H. Buchwald (Canada),
Chairman of the Task Group, for providing information contained in
Tables 3 and 4 and to Dr R. Horton, Dr D. J. McKee, USEPA, and
Dr V. Armstrong, Health and Welfare, Canada for reviewing the
annexes.
[HbCO](%) = k × [CO] × t (1)
where k was a constant equal to 0.0003 for "an individual at rest"
( VA = 6 litre/min, pulse rate 70), 0.0005 for "light activity"
( VA = 9.5 litre/min, pulse rate 80), 0.0008 for "light work"
(50 watts, VA = 18 litre/min, pulse rate 110), and 0.0011 for
"heavy work"a (about 100 watts, VA = 30 litre/min, pulse rate 135).
This equation was based on sets of controlled exposure
observations on human volunteers. Exposure concentrations ranged from
100 to 20 000 ppm (0.01 to 2.0%), and exposure times up to 6 h. At a
[CO] of 100 ppm, the equation holds only up to delta[HbCO] of about
7%. At 1000 ppm, the equation is applicable up to 30% [HbCO].
The equation of Forbes et al. was used by the California State
Department of Health for estimating [HbCO] after shorter periods of
exposure to carbon monoxide concentrations higher than 100 ppm in
individuals at rest or engaged in light work (Goldsmith & Landow,
1978).
(4) The empirical equation suggested by Pace et al. (1945) was derived
from controlled carbon monoxide exposures of 32 volunteers, aged 18-40
years. The concentrations of carbon monoxide in the inspired air
varied from 90 to 21 800 ppm, and the subjects were either sitting
quietly or walking on a level treadmill (about 4.3 km/h, VA about
19.2 litre/min). The exposure times ranged from 20 to 300 min. The
equation was also linear, but it took into account the alveolar
ventilation rate, VA, and the blood volume, VB:
[CO](ppm) × VA (litre/min) × t(min)
DELTA[HbCO](%) = (2)
4650 × VB(litre)
If it is assumed that the blood volume equals 5.51 and
VA = 6 litre/min (rest or light sedentary activity) or 18 litre/min
(light physical work), the equation takes the same form as that of
Forbes et al, but k values are somewhat lower (0.00023 and 0.00070,
respectively).
(5) Hanks & Farquhar (1969) also conducted carefully controlled
exposure studies on sedentary volunteers, and expressed their results
by the equation:
[HbCO](%) = 0.147[CO](1 - e-0.00289 t) (3)
where [CO] is in ppm and t in min. The equation was valid for subjects
having a ventilation rate of about 6 litre/min.
a VA for heavy physical work may be as high as 60 litre/min.
According to Chovin (1974), the "coefficient of (pulmonary)
ventilation" K could be introduced into equation (3), which then
became applicable to subjects performing various degrees of activity.
Chovin's modified equation read:
K
- t
0.147
[HbCO](%) = 0.147[CO](1 - e ) (4)
where t was in hours. For subjects at rest, K = 0.025, and for those
performing heavy physical work, K = 0.065. For intermediate degrees of
activity, Chovin proposed K values of 0.035, 0.045, and 0.055.
(6) Another empirical equation, referred to on p. 36, was suggested by
Peterson & Stewart (1970). It could also be written in the following
form:
[HbCO](%) = 0.0051[CO]0.858 × t0.63 (5)
where [CO] is in ppm, and t in min. The equation was based on
controlled human exposure data. The volunteers (18 healthy graduate
students) were exposed to carbon monoxide concentrations of <1, 25,
50, 100, 200, 500, and 1000 ppm for periods ranging from 30 min to
24 h, while performing strictly sedentary activities. Like linear
equations, this equation needed to be used with caution, since it did
not yield a finite [HbCO] value for an infinite exposure time. It was
used in developing US national ambient air quality standards, and is
strictly valid for constant [CO] and for shorter periods of exposure
(Ott & Mage, 1978).
(7) A model proposed by Coburn et al. (1965) has been discussed on
pp. 36 and 37. It is valid for male subjects only.a A solution of
their differential equation has been provided in the original paper; it
assumes that the mean pulmonary capillary oxygen pressure ( pCo2)
and the concentration of oxyhaemoglobin [HbO2] are constant and
independent of carboxyhaemoglobin levels [HbCO]. However, the
oxyhaemoglobin level depends on carboxyhaemoglobin concentrations in a
complex way. Solutions of the differential equation taking this into
account are available, and their application is illustrated in the
NAS/NRC (1977) document. Nevertheless, for most practical purposes,
the solution given in the original paper appears to be adequate. It
has been used, for example, by Peterson & Stewart (1970) and in the
criteria document of the National Institute of Occupational Safety and
Health (US Department of Health, Education and Welfare, 1972). A
useful form of this solution is given in the document of the Committee
on the Challenges of Modern Society (1972):
a Endogenous CO production may be different for females due to
menstruation, pregnancy, and other metabolic factors.
1
[HbCO] t = {e- kt(A[HbCO]o - VcoB - [CO]) + VcoB + [CO]} (6)
A
where [HbCO] t and [HbCO]o are the carboxyhaemoglobin levels at
times
pCo2 1 ( pB - 47)
t and t = 0, [CO] = pIco, A = , B = ( + ) and
M[HbO2] DL VA
A
k =
VB.B
the symbols used to define [CO], A, and B are explained on p. 36. The
relationships between [CO] in ppm and pIco, and between [HbCO]% and
[HbCO] in ml CO/ml blood are:
pIco × 106
[CO](ppm) = ,
pB
and
[HbCO](%) = 497.5 [HbCO](ml CO/ml blood)
(8) Ott & Mage (1978) designed a model that took into consideration
the dynamic characteristics of urban carbon monoxide concentrations.
The differential equation describing it reads:
d[HbCO]
tau dt + [HbCO] - ß = alpha[CO] (7)
O < [CO] < 100 ppm
where ß was the endogenous level of blood carboxyhaemoglobin and was
assumed to be 0.5%, alpha was assumed to be 0.15, and tau = 2.49h.
The main conclusion of the authors was that [CO] in ambient air
should be reported for averaging periods of 10-15 min, if the
monitoring stations were located near heavy traffic or on congested
streets. In such cases, sharp carbon monoxide peaks of short duration
might occur fairly often, and concentrations reported with longer
averaging periods, e.g., 1 h or more, might introduce an error of up
to 21% in the estimated [HbCO].
(9) Several empirical equations are compared with Coburn's model in
Table 1, for persons at rest ( VA - 6 litre/min) or performing light
physical work ( VA = 18 litre/min), at a carbon monoxide exposure
concentration of 100 ppm. For subjects performing light work, Hanks &
Farquhar's equation has been used in Chovin's modification with
K = 0.060. The following assumptions have been made in calculating
[HbCO]t values from Coburn's equation (6): [HbCO] = 0.5% or
0.001 ml CO/ml blood, Vco = 0.007 ml/min, VB = 5500 ml,
pCo2 = 100 mmHg, [HbO2] = 0.2 ml O2/ml blood, M = 218,
pB - 47 = 713 mmHg; sedentary subjects: DL = 40 ml/min/mmHg,
VA = 6000 ml/min; light work: DL = 40 ml/min/mmHg,
VA = 18 000 ml/min. For all empirical equations, [HbCO]o = 0.5%
has been added to the calculated values of [HbCO].
Table 1 shows clearly that Hanks & Farquhar's equation agrees best
with Coburn's model. Peterson & Stewart's equation gives values that
are higher than the other equations up to 6 h of exposure; then it
gives lower results than Forbes' equation. At a carbon monoxide
concentration of 100 ppm, Pace's equation gives lower [HbCO] values
for subjects at rest than the other equations, up to 7 h of exposure;
for subjects performing light work, it is applicable up to 2 h of
exposure. For subjects at rest, Forbe's equation is applicable up to
6 h, and for persons performing light work, up to 2 h.
(10) Table 2 shows [HbCO] values predicted by Coburn's model.a The
assumptions are the same as those specified in paragraph 9. For heavy
work, it has been assumed that DL = 60 ml/min/mmHg and that
VA=30 000 ml/min.
(11) Guidelines on exposure conditions that would prevent
carboxyhaemoglobin levels exceeding 2.5-3% in general nonsmoking
populations are given in Table 3.
These guidelines were reviewed by the Task Group. Comparison with
Tables 1 and 2 indicates the degree of protection provided, if these
guidelines are applied, both for sedentary individuals and for persons
performing light work. The exposure guidelines for 8-24 h have been
added by the Secretariat, after the Task Group meeting, to facilitate
comparison with national air quality standards.
(12) Guidelines on exposure conditions, which would prevent carboxy-
haemoglobin levels exceeding 5% in nonsmoking occupational groups, are
shown in Table 4. They were prepared, after the meeting, by Dr
Buchwald, at the request of the Secretariat, and have not been
reviewed by the Task Group. Guidelines for heavy work have been
suggested by the Secretariat, also after the meeting. Heavy work has
been defined by DL = 60 ml/min/mmHg and VA = 30 000 ml/min. The
degree of protection provided by these guidelines is indicated in
columns 3 and 4 of Table 4. Column 3 shows carbon monoxide
concentrations that would produce 5% HbCO within exposure times given
a The Secretariat wishes to thank Dr M. A. Vouk, University
Computing Centre, Zagreb, Yugoslavia, for programming Coburn's
equations and providing computer printouts.
in column 2, for light and heavy work, respectively. Column 4 provides
"safety factors" obtained by dividing the concentrations in column 3
by concentrations in column 1. Unless otherwise indicated, the
guidelines given in Table 4 should be considered as desirable
conditions rather than maximum acceptable limits.
Table 1. [HbCO](%) predicted by different empirical equations and by the model of Coburn et al. (1965).
Exposure to a carbon monoxide concentration of 115 mg/m3 (100 ppm)
Time Subjects at rest Subjects performing light work
(min)
F P H PS C F P H C
15 1.0 0.8 1.1 2.0 1.2 1.7 1.6 1.9 2.0
30 1.4 1.0 1.7 2.8 1.8 2.9 2.6 3.2 3.3
45 1.9 1.5 2.3 3.4 2.4 4.1 3.6 4.4 4.6
60 2.3 1.9 2.8 4.0 3.0 5.3 4.7 5.4 5.7
120 4.1 3.3 4.7 5.9 5.0 10.1 8.9 8.7 9.2
180 5.9 4.5 6.4 7.5 6.8 14.9 13.1 10.9 11.6
240 7.7 6.0 7.8 8.9 8.3 19.7 17.3 12.3 13.2
300 9.0 7.4 9.0 10.1 9.6 24.5 21.5 13.3 14.1
360 10.8 8.8 10.0 11.3 10.7 29.3 25.7 13.9 15.1
420 12.6 10.2 10.8 12.4 11.6 34.1 29.9 14.3 15.6
480 14.4 11.5 11.5 13.5 12.4 38.9 34.1 14.6 15.9
F = Forbes et al. (1945), P = Pace et al. (1946), H = Hanks & Farquhar (1969),
PS = Peterson & Stewart (1970), C = Coburn et al. (1965).
Table 2. [HbCO] values predicted from Coburn et al. (1965) model
Time 200 ppm 100 ppm 75 ppm 50 ppm
S L H S L H S L H S L H
15 min 1.8 3.5 5.2 1.2 2.0 2.8 1.0 1.6 2.2 0.82 1.2 1.6
30 min 3.1 6.2 9.2 1.8 3.3 4.8 1.5 2.6 3.7 1.1 1.9 2.6
45 min 4.3 8.7 12.6 2.4 4.6 6.5 1.9 3.5 4.9 1.4 2.5 3.4
60 min 5.5 11.0 15.5 3.0 5.7 7.9 2.3 4.3 6.0 1.7 3.0 4.1
90 min 7.7 14.9 20.2 4.0 7.6 10.2 3.1 5.8 7.7 2.2 4.0 5.2
2h 9.7 18.1 23.7 5.0 9.2 11.9 3.9 7.0 9.0 2.7 4.7 6.1
4h 16.3 26.2 30.4 8.3 13.2 16.3 6.3 10.0 11.5 4.4 6.9 7.7
6h 21.1 30.0 32.4 10.7 15.1 16.2 8.1 11.3 12.2 5.5 7.6 8.2
8h 24.5 31.7 32.9 12.4 15.9 16.5 9.4 12.0 12.4 6.4 8.0 8.3
24h 32.7 33.2 33.2 16.5 16.7 16.6 12.4 12.5 12.5 8.4 8.4 8.3
infinity 33.4 33.2 33.2 16.8 16.7 16.6 12.7 12.5 12.5 8.5 8.4 8.3
Time 35 ppm 25 ppm 10 ppm 5 ppm
S L H S L H S L H S L H
15 min 0.72 1.0 1.3 0.66 0.84 1.0 0.55 0.61 0.67 0.52 0.54 0.56
30 min 0.93 1.4 1.9 0.80 1.2 1.5 0.61 0.72 0.82 0.54 0.57 0.60
45 min 1.1 1.9 2.5 0.95 1.4 1.9 0.66 0.81 0.95 0.56 0.61 0.64
60 min 1.3 2.2 3.0 1.1 1.7 2.2 0.71 0.90 1.1 0.58 0.63 0.68
90 min 1.7 2.9 3.7 1.3 2.1 2.7 0.80 1.1 1.2 0.62 0.69 0.74
2 h 2.0 3.4 4.3 1.6 2.5 3.1 0.89 1.2 1.4 0.66 0.73 0.78
4 h 3.2 4.7 5.4 2.4 3.4 3.9 1.2 1.5 1.6 0.77 0.84 0.86
6 h 4.0 5.4 5.7 2.9 3.9 4.1 1.4 1.6 1.7 0.85 0.88 0.88
8 h 4.5 5.7 5.8 3.3 4.1 4.2 1.5 1.7 1.7 0.91 0.91 0.89
24 h 5.9 5.9 5.9 4.3 4.2 4.2 1.9 1.8 1.7 1.05 0.93 0.89
infinity 6.0 5.9 5.9 4.4 4.2 4.2 1.9 1.8 1.7 1.06 0.93 0.89
S = sedentary subjects, L = light physical work, H = heavy physical work, all as defined in sections 9 and 10.
Table 3. Guidelines for exposure conditions to prevent carboxyhaemoglobin levels exceeding
2.5-3% in nonsmoking populations
(a) A ceiling or maximum permitted exposure of 115 mg/m3 (100 ppm) for periods of exposure
not exceeding 15 min (No exposure over 115 mg/m3 (100 ppm) permitted, even for very
short time periods).
(b) A time-weighted average exposure of 55 mg/m3 (50 ppm) for periods of exposure not
exceeding 30 min.
(c) A time-weighted average exposure of 29 mg/m3 (25 ppm) for periods of exposure not
exceeding one h.
(d) A time-weighted average exposure of 15 mg/m3 (13 ppm) for periods of exposure of more
than one h.
(e) A time-weighted average exposure of 11.5 mg/m3 (10 ppm) for periods of exposure of
8-24 h.a
a Suggested by the Secretariat.
Table 4. Guidelines for exposure conditions that would prevent carboxyhaemoglobin levels
exceeding 5% in nonsmoking occupational groups performing light and heavy
physical work.
Concentration Exposure time not to Concentrations that Safety factor
be exceeded would produce 5% HbCOc
ppm mg/m3
Light Heavy Light Heavy Light Heavy
worka workb work work work work
200d 230 15 min - 298 - 1.5 -
100e,f 115 30 min 15 min 157 193 1.6 1.9
75f 86 60 min 30 min 87 105 1.2 1.4
50f 55 90 min 60 min 64 62 1.3 1.2
35f 40 4 h 2 h 37 41 1.1 1.2
25f 29 8 h 8 h 31 30 1.2 1.2
a Limits suggested by Dr Buchwald.
b Limits suggested by the Secretariat.
c Calculated from Coburn's equation (6).
d Short-term limit or maximum permissible concentration for light work.
e Short-term limit or maximum permissible concentration for heavy work.
f Time weighted average.
REFERENCES
HANKS, T. G. & FARQUHAR, R. D. (1969) Analysis of human performance
capabilities as a function of exposure to carbon monoxide.
(SystMed Corporation Report R 9001, Contract PH-22-68-31).
PACE, N., CONSOLAZIO, W. V., WHITE, W. A. JR. & BEHNKE, A. R. (1945)
Formulation of principal factors affecting the rate of uptake of
carbon monoxide by man. Am. J. Physiol., 147: 352-359.
All other references are included in the list of references for
the main body of the document (pp. 98-114).
Annex 2
SELECTED NATIONAL AMBIENT AIR QUALITY STANDARDS AND OCCUPATIONAL
EXPOSURE STANDARDS FOR CARBON MONOXIDEa
1. NATIONAL AMBIENT AIR QUALITY STANDARDS
1.1 Canada (1974)
National air quality objectives
Desirable concentrations:
(a) 0-6 mg/m3 (0-5 ppm)b average concentration over an 8-h
period.
(b) 0-15 mg/m3 (0-13 ppm) average concentration over a one-h
period.
Acceptable concentrations:
(a) 6-15 mg/m3 (5-13 ppm) average concentration over an 8-h
period.
(b) 15-35 mg/m3 (13-31 ppm) average concentration over a one-h
period.
Method of measurement:
Nondispersive infrared spectrometry, Report No. EPS 1-AP-73-1.
Source: Velma Ouellet (1978) The Clean Air Act--Compilation of
Regulations and Guidelines, Ottawa, Environment Canada (Report EPS
1-AP-78-2).
In addition to the desirable and acceptable concentrations listed,
a tolerable range of 15-20 mg/m3 average concentration over a
continuous 8-h period was prescribed in 1978.
Source: Canada Gazette (1978) Part 2, Volume 112, No. 3 (February 8).
a Prepared by the Secretariat.
b Concentrations in alternative units have been added by the
Secretariat to facilitate comparison of national quality standards.
1.2 Federal Republic of Germany (1974)
In the Federal Republic of Germany, immissionsc (Immissionen)
are legally defined as "air pollutants, noise, vibrations, light,
heat, radiations, and analogous environmental factors affecting human
beings, animals, plants, or other objects". "As a rule, air pollutants
occurring at a height of 1.5 metres above ground or at the upper limit
of vegetation or at a distance of 1.5 metres from the surface of a
building shall be considered active air pollutants."
"Immission standards are the values for long-term exposure (IW 1)
and short-term exposure (IW 2)."
The following immission standards have been established for carbon
monoxide:
Long-term exposure (IW 1): 10.0 mg/m3 (9 ppm).
Short-term exposure (IW 2): 30.0 mg/m3 (26 ppm).
Method of measurement: VDI 2455, Sheet 1 (August 1970) and 2455,
Sheet 2 guidelines (October 1970).
"Characteristic value I 1 for comparison with IW 1 shall be the
arithmetic mean of all individual data for a measurement area.
Characteristic value I 2 for comparison with IW 2 shall be the 95%
value for the cumulative frequency distribution of all individual
values for a measurement area; this may be also calculated with the
formula I 2 = x + ts where x is the arithmetic mean of individual
data for a measurement area,
2SUM(x - xi)2
s = + SQRT ,
2z - 1
xi = individual data which are greater than x, z = number of
individual data, which are greater than x, and t = 1.64 for the 95%
confidence level."
Source: Federal Minister of the Interior (1974) Technical
Instructions for Maintaining Air Purity, 28 August 1974, Bonn.
c Immission A German term for which there is no simple English
equivalent. Immissions are to be distinguished from emissions
(Emissionen), which are defined as air pollutants, noise,
vibrations, light, heat, radiations, and analogous phenomena
originating from an installation (Federal Republic of Germany, Law
on Protection against Emissions, 15 March 1974).
1.3 Japan (1973)
Ambient air quality standards are defined as follows:
(a) Average of hourly values in 8 consecutive hours shall not
exceed 20 ppm (23 mg/m3).
(b) Daily average of hourly values shall not exceed 10 ppm
(11 mg/m3).
These standards do not apply to industrial zones, roadways, and
other areas or places where people do not usually live.
Source: Environment Agency (1978) Environmental Laws and
Regulations in Japan (II) Air. Tokyo.
1.4 Union of Soviet Socialist Republics (1971)
Maximum permissible (single exposure) concentration for carbon
monoxide is 3 mg/m3 (2.6 ppm). This concentration should not provoke
reflex (i.e., subsensory) reactions in human organisms.
Maximum permissible (24-hour average) concentration for carbon
monoxide is 1.0 mg/m3 (0.9 ppm). This concentration should not have
either a direct or indirect harmful effect on man, for unlimited in
time, continuous exposure, 24 hours a day.
Method not specified.
Source: Krotov, Ju. A. (1975) Maximum permissible concentrations
of harmful substances in air and water, Himija, Moscow.
1.5 United States of America (1971)
"The national primary and secondary ambient air quality standards
for carbon monoxide, measured by the reference method described in
Appendix C to this part, or by an equivalent method, are:
(a) 10 milligrams per cubic meter (9 ppm) -- maximum 8-hour
concentration not to be exceeded more than once per year.
(b) 40 milligrams per cubic meter (35 ppm) -- maximum 1-hour
concentration not to be exceeded more than once per year."
Reference method for the continuous measurement of carbon monoxide
in the atmosphere is nondispersive infrared spectrometry.
Source: Federal Register, 36 (228), Thursday, November 25, 1971,
Washington DC, pp. 22385 and 22391-22392.
1.6 Other Member States
For further information on national ambient air quality standards
for carbon monoxide, the reader is referred to W. Martin & A. C. Stern
(1974) The world's air quality management standards, Washington, DC,
US Environmental Protection Agency (EPA-650/a-75-001-a).
2. OCCUPATIONAL EXPOSURE LIMITS
An occupational exposure limit for carbon monoxide of 50 ppm or
55 mg/m3 has been set in the following Member States: Australia,
Belgium, Finland, Federal Republic of Germany, Italy, Japan,
Netherlands, Switzerland, the USA, and Yugoslavia.
Bulgaria and the USSR have established a limit of 20 mg/m3
(17 ppm), Hungary and Poland, 30 mg/m3 (26 ppm) and Sweden,
40 mg/m3 (35 ppm). Czechoslovakia's standard includes average and
maximum values of 30 mg/m3 (26 ppm) and 150 mg/m3 (130 ppm),
respectively. Other standards that include both average and maximum
values are those of the German Democratic Republic, (35 and
110 mg/m3) (30 and 96 ppm) and Romania, (30 and 50 mg/m3) (26 and
44 ppm).
These values should be interpreted in terms of definitions of
occupational exposure limits, which may be different in different
countries. The reader is referred to the International Labour Office
publication: Occupational Exposure Limits for Airborne Toxic
Substances, Occupational Safety and Health Series 37, ILO, Geneva,
1977. The values given in paragraphs 1 and 2 have been extracted from
this publication.
The US National Institute of Occupational Safety and Health
(NIOSH, 1972) has recommended environmental exposure limits of 35 ppm
(40 mg/m3) (time weighted average) and 200 ppm (229 mg/m3)
(ceiling) (Summary of NIOSH Recommendations for Occupational Health
Standards, July, 1978).
most important of which is the weather, produce great variations in
the concentrations of pollutants produced by traffic. Concentrations
fall steeply with distance from the street. However, distinct patterns
are often discernible (section 5.1). Concentrations for 8-h averaging
times are frequently used and quoted and usually vary from
<10 mg/m3 (8.7 ppm) to over 60 mg/m3 (52.2 ppm) but are mostly
<20 mg/m3 (17.6 ppm) in city streets. Away from heavy traffic, even
in towns, annual average concentrations are usually well under
10 mg/m3 (8.7 ppm). Obviously, in especially stagnant weather, very
heavy traffic may produce much higher levels. There is little
information about concentrations of carbon monoxide near large
stationary sources.
9.2.4 Indoor exposure
Carbon monoxide diffuses readily and, being relatively chemically
inert and not absorbed on surfaces, concentrations indoors are usually
similar to those found immediately outside. Not infrequently, however,
high concentrations may be found in kitchens and living rooms in which
there are coal, gas, or oil-fired cooking or heating appliances that
are maladjusted and inadequately vented to outside air; in some
countries, cases of acute and even fatal poisoning due to these causes
are not uncommon. The possible contribution of the domestic
environment must be noted in surveys. The smoking of tobacco indoors
can obviously increase the carbon monoxide concentration of the air
but recent work has shown that, before carbon monoxide reaches
significant levels, the irritation from the other constituents of
tobacco smoke becomes unacceptable if not actually intolerable.
9.2.5 Exposures related to traffic
In garages and tunnels, being in effect closed streets, pollution
by carbon monoxide can reach high levels. However, since transit time
in tunnels is relatively short, higher concentrations than those found
in streets are tolerable. Usually, there are monitoring instruments
that control ventilation and sound alarms if concentrations exceed
agreed values, which may vary from 115 to 570 mg/m3 (100-500 ppm)
depending on the use and length of the tunnel. High (sometimes lethal)
concentrations of carbon monoxide may accumulate inside motor vehicles
because of fractures in exhaust systems or other mechanical defects.
9.2.6 Occupational exposure
Traffic policemen, garage attendants, and drivers of taxis and
trucks are exposed to pollution from traffic and many studies have
shown a consequent increase in carboxyhaemoglobin levels (up to about
3% in nonsmokers), but there is much evidence that this increase may
be relatively undramatic, when compared with the manifest effects of
cigarette smoking. Exposure in certain industries, especially in iron
and steel works and in the manufacture of various gases, may be
relatively massive (in excess of 115 mg/m3 or 100 ppm), and high
carboxyhaemoglobin levels (>15% in nonsmokers) have been reported in
workers. Firemen may be exposed to very high concentrations of carbon
monoxide in fighting certain fires, but this exposure is obviously
episodic. These matters are discussed in section 5.3.
9.2.7 Tobacco smoking
The smoking of tobacco, especially in the form of cigarettes, has
been shown in many studies to be the major cause of raised
carboxyhaemoglobin levels in adult populations. Table 7 displays some
of this evidence and the topic is discussed in detail in section
8.1.6. Whereas carboxyhaemoglobin concentrations of 3% are rarely
found in nonsmokers exposed to town air, concentrations of 5-15% are
often found in smokers. It is important to remember that the effects
of smoking and exposure to town air are not simply additive and that
the resulting carboxyhaemoglobin levels will depend on other factors
already discussed.
9.2.8 Multiple exposures
Enough has been said to leave no doubt that carbon monoxide,
produced exogenously or endogenously, is a widespread pollutant
emanating from many sources to which people may be exposed in various
ways. This variety of exposure must be taken into account in the
interpretation of epidemiological surveys, the design of experiments,
and, above all, in giving advice about the fixing of air quality
criteria.
9.3 Effects
The main areas of concern that have arisen from acute or chronic
exposure to low levels of carbon monoxide in experimental and
epidemiological research in animals and man are: (a) its role in the
genesis of arteriosclerotic vascular diseases; (b) its role in the
aggravation of symptoms of cardiovascular diseases; (c) its
contribution to performance deficits in certain psychomotor tasks; and
(d) its role in limiting the working capacity of exercising man.
9.3.1 Cardiovascular system
9.3.1.1 Development of atherosclerotic cardiovascular disease
Extensive experimental work has been carried out over many years
on animals, mainly rabbits, showing that prolonged exposure to
moderate levels of carbon monoxide can produce atherosclerotic
changes, especially in the presence of high cholesterol levels (1-2%)
in the diet. The relevance of this work for man has not been
established. However, other animal work, and some epidemiological
studies of prolonged human exposures to elevated carbon monoxide
levels through smoking, occupation, or both, such as those carried out
in Denmark, Finland, and Japan, indicate the need for further
investigation of the possible role of carbon monoxide in the genesis
of atherosclerotic vascular changes in animals and man. The degree of
intermittency of exposure at various levels should be taken into
account as well as the possible contribution of other agents such as
nicotine and high-fat diets. There is some evidence of adaptation, but
such changes may not be entirely beneficial. None of the information,
currently available, is useful for the purpose of setting standards.
9.3.1.2 Acute effects on existing heart illness
The few existing epidemiological studies on the possible effects
of carbon monoxide on the severity or fatality of coronary occlusion
are insufficient to allow any conclusions. It is hoped that additional
work of this type will clarify matters.
Two carefully conducted human studies of the effects of low carbon
monoxide exposure and exercise on pain in volunteer patients with
angina pectoris offer valuable quantitative information. Although
limited in the number of patients studied, the findings are consistent
in the 2 investigations showing effects at carboxyhaemoglobin
concentrations of 2.5-3.0%. A third single-blind study revealed the
same detrimental effects in patients with angina pectoris when
exposure to traffic exhausts caused carboxyhaemoglobin levels to rise
to 5.1%. A no-adverse-effect level has not been established in these
observations, nor is it possible to determine whether there is a
graded response in this type of experiment. More work of a similar
nature would be useful to explore these questions.
9.3.1.3 Acute effects on existing vascular disease
One study, similar to those done on patients with angina, has been
carried out on patients with intermittent claudication from peripheral
vascular disease. Effects on pain with exercise were observed in the
same exposure range as with angina i.e., at carboxyhaemoglobin
concentrations of 2.5-3.1%, with a mean of 2.8%. Here, too, more data
of a similar kind are needed, preferably designed to provide dose-
response relationships.
9.3.2 Nervous system
As for the role of carbon monoxide in affecting psychomotor
functions, no definite conclusions can be drawn from the existing
data. The behavioural functions tested in such studies include
vigilance and psychomotor performance, visual acuity and sensitivity,
the ability to estimate time intervals, complex motor coordination as
tested by driving simulators, and different perceptual and mental
operationsa. Some workers observed detrimental effects at
carboxyhaemoglobin levels as low as 2%, whereas others were unable to
detect significant impairment even at levels from above 5% to about
20%. In evaluating these discrepancies, it should be mentioned, that
these behavioural functions are easily influenced by a number of other
factors besides carbon monoxide-induced hypoxia, e.g., degree of
sensory deprivation, compensatory abilities, drugs, temperature, time
of day, competition, etc.
9.3.3 Work capacity
That elevated carboxyhaemoglobin levels affect work capacity has
long been known. Levels of 40-50% will usually prevent working
entirely. Recent studies in the laboratory, on man, using maximum work
capacity or maximum aerobic capacity as indicators of performance,
have been carried out in relation to carboxyhaemoglobin levels. Here,
dose-response data are available for maximum effort. The limitation
appears at a carboxyhaemoglobin concentration of about 4% and
increases at higher levels. Lower exposure levels have been studied
and do not produce this effect. It should be noted that while levels
of carboxyhaemoglobin of 2.5-4%, did not reduce maximum work capacity,
they did reduce the length of time for which such effort could be
carried out. It is not known what specific levels of carboxy-
haemoglobin will reduce the capacity of individuals to perform at
ordinary work levels, such as 30-50% of their maximum capacity, for
prolonged periods of time.
9.4 Recommended Exposure Limits
It has already been stated that the major contributor to
carboxyhaemoglobin concentrations in the body is the smoking of
tobacco; however, in many of the experiments currently quoted to
justify the formulation of exposure limits, the smoking habits of the
subjects were not taken into account. Results of recent work suggest
that smokers and ex-smokers might be less sensitive to carbon monoxide
exposure than nonsmokers. In view of this suggestion, and because of
the deficiencies in the experiments mentioned, recommendations for
exposure limits should be confined to the protection of nonsmokers.
There is an urgent need for more work on possible adaptation following
a The possible importance of performance deficiencies resulting
from carbon monoxide is considerable, particularly in relation to
accidents at work, and while driving or flying. Further studies,
particularly of the vigilance type are urgently needed for a better
understanding of this problem.
exposure to carbon monoxide from smoking or from other sources. It is
also important to note that carboxyhaemo-globin levels have been the
measurement of exposure in most experimental work. Thus, it is
desirable to recommend the primary exposure limits in terms of
carboxyhaemoglobin, and follow this by comments on the derivation of
an appropriate air concentration equivalent.
9.4.1 General population exposure
Data used in arriving at a recommendation for an exposure limit
for the general population were mainly those obtained from the
exposure of subjects with cardiovascular illness to carbon monoxide in
conjunction with exercise. Agreement was not reached on a single
level. Thus, a range of carboxyhaemoglobin concentrations of 2.5-3.0%
is recommended for the protection of the general population including
those who have impaired health. The recommendation must be regarded as
tentative, since ideal dose-response or concentration-response
information is not yet available. However, it must also be recognized
that complete protection of all persons, at all times, cannot
reasonably be sought by environmental control alone. Persons who are
in should be educated by their physicians concerning their own
responsibility to avoid stressful exposures.
9.4.2 Working population exposure
Better quality data are available for recommending an exposure
limit for the working population. In this case, the Task Group
unanimously agreed on maintaining carboxyhaemoglobin levels below 5%,
on the basis of present knowledge, since working populations comprise
individuals who are assumed to be healthy, physiologically resilient,
and under regular supervision.
9.4.3 Derived limits for carbon monoxide concentrations in air
It is important, wherever possible, to have both biological and
environmental assessments of human exposure to pollutants. While the
biological measurements may be more relevant in relation to effects,
they may be more difficult to use in practice. For carbon monoxide,
the relationship between concentrations in air and carboxyhaemoglobin
levels is affected by several variables, including exposure time and
it is not easy to estimate. However, such estimates may be
sufficiently accurate for many practical purposes (for reviews see
Committee on the Challenges of Modern Society, 1972; Commission of the
European Communities, 1974; NAS/NRC, 1977; Winneke, 1977; Ott & Mage,
1978). It should be emphasized yet again that analyses of carbon
monoxide in air and of carboxyhaemoglobin in blood are complementary,
and should in no way be regarded as alternative methods of monitoring.
Obviously, air monitoring has its uses in the planning and
implementation of control measures, and for warning purposes, but such
measurements have limited value in estimating the actual human
exposure defined by carboxyhaemoglobin levels.
REFERENCES
ADAMS, J. D., ERICKSON, H. H., & STONE, H. L. (1973) Myocardial
metabolism during exposure to carbon monoxide in the conscious
dog. J. appl. Physiol., 34: 238-242.
AGOSTINI, J. C., RAMIREZ, R. G., ALBERT., S. N., GOLDBAUM, L. R., &
ABSOLON, K. B. (1974) Successful reversal of lethal carbon
monoxide intoxication by total body asanguineous hypothermic
perfusion. Surgery, 75: 213-219.
AHMED, A. E., KUBIC, V. L., & ANDERS, M. W. (1977) Metabolism of the
haloforms to carbon monoxide. I. In vitro studies. Drug
Metabol. Disposition, 5: 198-204.
ALEXANDROV, N. P. (1973) [Choice of experimental animals for
elaboration of standards for CO.] Gig. i Sanit., 11: 92-95 (in
Russian).
ALEKSIEVA, T. & DIMITROVA, M. (1971) [Some oscillographic changes in
workers exposed to chronic carbon monoxide effect.] Tr. Niotpz,
pp. 163-169 (in Bulgarian).
ALTMAN, P. L. & DITTMEN, D. S., ed. (1971) Respiration and
circulation, Bethesda, MD, Federation of the American Society of
Experimental Biology.
ALTSHULLER, A. P. & BUFALINI, J. J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ANDERSON E. W., ANDELMAN, R. J., STRAUCH, J. M., FORTUIN, N. J., &
KNELSON, J. H. (1973) Effect of low-level carbon monoxide exposure
on onset and duration of angina pectoris: a study in ten patients
with ischemic heart disease. Ann. intern. Med., 79: 46-50.
ANTWEILER, H. (1974) Physiological kinetics of CO inhalation.
In: Proceedings of the European Colloquium on Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg Commission of the European
Communities, Directorate General Scientific and Technical
Information Management, pp. 197-251 (in German with full English
translation).
ANTWEILER, H. (1975) [The reception and effect of carbon monoxide in
active and passive smokers.] Arbeitsmed. Sozialmed.
Präventivmed., 10: 245-248 (in German).
ARONOW, W. S. & CASSIDY, J. (1975) Effect of carbon monoxide on
maximal treadmill exercise: a study in normal persons. Ann.
intern. Med., 83: 496-499.
ARONOW, W. S. & ISBELL, M. W. (1973) Carbon monoxide effect on
exercise induced angina pectoris. Ann. intern. Med.,
79: 392-395.
ARONOW, W. S., CASSIDY, J., VANGROW, J. S., MARCH, H., KERN, J. C.,
GOLDSMITH, J. R., KHEMKA, M., PAGANO, J., & VAWTER, M. (1971a)
Effect of cigarette smoking and breathing carbon monoxide on
cardiovascular hemodynamics in anginal patients. Circulation,
50: 340-347.
ARONOW, W. S., DENDINGER, J., & ROKAW, S.N. (1971b) Heart rate and
carbon monoxide level after smoking high-, low-, and non-nicotine
cigarettes. Ann. intern. Med., 74: 697-702.
ARONOW, W. S, HARRIS, C. N., ISBELL, M. W., ROKAW, S. N., & IMPARATO,
B. (1972) Effect of freeway travel on angina pectoris. Ann.
intern. Med., 77: 669-676.
ARONOW, W. S., STEMMER, E. A., & ISBELL, M. W. (1974) Effect of carbon
monoxide exposure on intermittent claudication. Circulation,
49: 415-417.
ASTRAND, I., OVRUM, P., & CARLSSON, A. (1975) Exposure to methylene
chloride. I. Its concentration in alveolar air and blood during
rest and exercise and its metabolism. Scand. J. Work Environ.
Health, 1: 78-94.
ASTRUP, P. (1970) Intraerythrocytic 2,3-diphosphoglycerate and carbon
monoxide exposure. Ann. NY Acad. Sci., 174: 252-254.
ASTRUP, P. (1972) Some physiological and pathological effects of
moderate carbon monoxide exposure. Br. med. J., 4: 447-452.
ASTRUP, P. & PAULI, H. G. (1968) A comparison of prolonged exposure to
carbon monoxide and hypoxia in man. Scand. J. clin. lab. Invest.,
22 (Suppl. 103): 1-71.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1967) Enhancing influence of
carbon monoxide on the development of atheromatosis in
cholesterol-fed rabbits. J. atheroscler. Res., 7: 343-354.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1970) Effects of carbon
monoxide exposure on the arterial walls. Ann. NY Acad. Sci.,
174: 294-300.
ASTRUP, P., OLSEN, H. M., TROLLE, D., & KJELDSEN, K. (1972) Effect of
moderate carbon monoxide exposure on fetal development. Lancet,
2: 1220-1222.
AYRES, S. M., CRISCITIELLO, A., & GIANELLI, S., JR (1966)
Determination of blood carbon monoxide content by gas
chromatography. J. appl. Physiol., 21: 1368-1370.
AYRES, S. M., MUELLER, H. S., GREGORY, J. J., GIANELLI, S., JR, &
PENNY, J. L. (1969) Systemic and myocardial hemodynamic responses
to relatively small concentrations of carboxyhemoglobin (COHB).
Arch. environ. Health, 18: 699-709.
AYRES, S. M., GIANELLI, S., JR & MUELLER, H. (1970) Myocardial and
systemic responses to carboxyhemoglobin. Ann. NY. Acad. Sci.,
174: 268-293.
BAKER, F. D. & TUMASONIS, C. F. (1972) Carbon monoxide and avian
embryogenesis. Arch. environ. Health, 24: 53-61.
BALABAEVA, L. & KALPAZANOV, I. (1974) [Effect of smoking and length of
service on the content of carboxyhemoglobin in blood of traffic
policemen.] Hig. i Zdraveo., 5: 490-493 (in Bulgarian).
BALL, K. & TURNER, R. (1974) Smoking and the heart: the basis for
action. Lancet, 2 (7884): 822-826.
BATES, D. R. & WITHERSPOON, A. E. (1952) The photo-chemistry of some
minor constituents of the earth's atmosphere (CO2, CO, CH4,
N2O). Mon. not. R. Astron. Soc. (Lond.), 112: 101-124.
BEARD, R. R. & GRANDSTAFF, N. W. (1975) Carbon monoxide and human
functions. In: Weiss, P. & Laties, V. G., ed. Behavioral
Toxicology, New York, Plenum Press, pp. 1-26.
BEARD, R. R. & WERTHEIM, G. A. (1967) Behavioral impairment associated
with small doses of carbon monoxide. Am. J. public Health,
57: 2012-2022.
BEHRMAN, R. E., FISHER, D. E., & PATON, J. (1971) Air pollution in
nurseries: correlation with a decrease in oxygen-carrying capacity
of hemoglobin. J. Pediatr., 78: 1050-1054.
BENDER, W., GÖTHERT, M., & MALORNY, G. (1972) Effect of low carbon
monoxide concentrations on psychological functions. Staub-
Reinhalt. Luft., 32 (4): 54-60.
BIDWELL, R. G. S. & FRASER, D. E. (1972) Carbon monoxide uptake and
metabolism by leaves. Can. J. Bot., 50: 1435-1439.
BLACKMORE, D. J. (1974) Aircraft accident toxicology: UK experience
1967-1972. Aerosp. Med., 45: 987-994.
BORST, J. R. (1970) Acute and chronic carbon monoxide poisoning in
private motor-cars and lorries. Arts Auto, 36 (19): 1518-1523.
BREYSSE, P. A. & BOVEE, H. H. (1969) Use of expired air-carbon
monoxide for carboxyhemoglobin determinations in evaluating carbon
monoxide exposures resulting from the operation of gasoline fork
lift trucks in holds of ships. Am. Ind. Hyg. Assoc. J.,
30: 477-483.
BRIEGER, H. (1944) Carbon monoxide polycythemia. J. ind. Hyg.
Toxicol., 26: 321-327.
BRODY, J. S. & COBURN, R. F. (1970) Effects of elevated
carboxyhemoglobin on gas exchange in the lung. Ann. NY Acad.
Sci., 174: 255-260.
BUCHWALD, H. (1969) Exposure of garage and service station operatives
to carbon monoxide: a survey based on carboxyhemoglobin levels.
Am. Ind. Hyg. Assoc. J., 30: 570-575.
BUTT, J., DAVIES, G. M., JONES, J. G., & SINCLAIR, A. (1974)
Carboxyhaemoglobin levels in blast furnace workers. Ann. occup.
Hyg., 17: 57-63.
BYCZKOWSKA, Z. & MILAN, K. (1971) [Acute carbon monoxide poisoning
with temporary renal insufficiency.] Med. Pr., 22: 119-121
(in Polish).
CALVERT, J. G. (1973) Interactions of air pollutants. In: Proceedings
of the Conference on Health Effects of Air Pollutants, Oct.,
Washington, DC, National Academy of Sciences, National Research
Council, pp. 19-101 (Serial No. 93-15).
CASTLEDEN, C. M. & COLE, P. V. (1975) Carboxyhaemoglobin levels of
smokers and nonsmokers working in the city of London. Br. J. ind.
Med., 32: 115-118.
CAVANAGH, L. A., SCHADT, C. F., & ROBINSON, E. (1969) Atmospheric
hydrocarbon and carbon monoxide measurements at Point Barrow,
Alaska. Environ. Sci. Technol., 3: 251-257.
CHALUPA, B. (1960) [Disorders of memory in acute carbon monoxide
intoxications.] Prac. Lek., 12: 331-336 (in Polish).
CHAPMAN, D. J. & TOCHER, R. D. (1966) Occurrence and production of
carbon monoxide in some brown algae. Can. J. Bot.,
44: 1438-1442.
CHEVALIER, R. B., BOWERS, J. A., BONDURANT, S., & ROSS, J. C. (1963)
Circulatory and ventilatory effects of exercise in smokers and
nonsmokers. J. appl. Physiol., 18: 357-360.
CHEVALIER, R. B.. KRUMHOLZ, R. A., & ROSS, J. C. (1966) Reaction of
nonsmokers to carbon monoxide inhalation. Cardiopulmonary
responses at rest and during exercise. J. Am. Med. Assoc.,
198: 1061-1064.
CHIODI, H., DILL, D. B., CONSOLAZIO, F., & HORVATH, S. M. (1941)
Respiratory and circulatory responses to acute carbon monoxide
poisoning. Am. J. Physiol, 134: 683-693.
CHOVIN, P. (1967) Carbon monoxide: analysis of exhaust gas
investigations in Paris. Environ. Res., 1: 198-216.
CHOVIN, P. (1974) Comments on paper presented by H. Antweiler.
In: Proceedings of the European Colloquium Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg, Commission of the European
Communities Directorate General Scientific and Technical
Information Management, pp. 225-236.
CLARK, R. T., JR & OTIS, A. B. (1952) Comparative studies on
acclimatization of mice to carbon monoxide and to low oxygen.
Am. J. Physiol, 169: 285-294.
COBURN, R. F. (1970a) Enhancement by phenobarbital and
diphenylhydantoin of carbon monoxide production in normal man.
New Engl. J. Med., 283: 512-515.
COBURN, R. F. (1970b) The carbon monoxide body stores. Ann. NY Acad.
Sci., 174: 11-22.
COBURN, R. F., DANIELSON, G. K., BLAKEMORE, W. S., & FORSTER, R. E.
(1964) Carbon monoxide in blood: analytical method and sources of
error. J. appl. Physiol, 19: 510-515.
COBURN, R. F., FORSTER, R. E., & KANE, P. B. (1965) Considerations of
the physiological variables that determine the blood
carboxyhemoglobin in man. J. clin. Invest., 44: 1899-1910.
COBURN, R. F., WILLIAMS, W. J., & KAHN, P. B. (1966) Endogenous carbon
monoxide production in patients with hemolytic anemia. J. clin.
Invest., 45: 460-468.
COBURN, R. F., PLOEGMAKERS, F., GONDRIE, P., & ABBOUD, R. (1973)
Myocardial myoglobin oxygen tension. Am. J. Physiol.,
224: 870-876.
COHEN, S. I., DEANE, M., & GOLDSMITH, J. R. (1969) Carbon monoxide and
survival from myocardial infarction. Arch. environ. Health,
19: 510-517.
COHEN, S. I., DORION, G., GOLDSMITH, J. R., & PERMUTT, S. (1971a)
Carbon monoxide uptake by inspectors at a United States-Mexico
border station. Arch. environ. Health, 22: 47-54.
COHEN, S. I., PERKINS, N.M., URY, H. K., & GOLDSMITH, J. R. (1971b)
Carbon monoxide uptake in cigarette smoking. Arch. environ.
Health, 22: 55-60.
COLLISON, H. A., RODKEY, F. L., & O'NEAL, J. D. (1968) Determination
of carbon monoxide in blood by gas chromatography. Clin. Chem.,
14: 162-171.
COLUCCI, J. M. & BEGEMAN, C. R. (1969) Carbon monoxide in Detroit, New
York, and Los Angeles air. Environ. Sci. Technol., 3: 41-47.
COMMINS, B. T. & LAWTHER, P. J. (1965) A sensitive method for the
determination of carboxyhaemoglobin in a finger prick of blood.
Br. J. ind. Med., 22: 139-143.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) Proceedings of the
European Colloquium on Carbon Monoxide Environmental Pollution
and Public Health, Luxembourg, 17-19 December, 1973, Luxembourg
Directorate General Scientific and Technical Information and
Information Management, 437 pp.
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1972) Air pollution:
Air quality criteria for carbon monoxide, No. 10, 235 pp.
CONLEE, C. J., KENLINE, P. A, CUMMINS, R. L., & KONOPINSKI, V. J.
(1967) Motor vehicle exhaust at three selected sites. Arch.
environ. Health, 14: 429-446.
COOPER, D. Y., LEVIN, S., NARASIMHULU, S., & ROSENTHAL, O. (1965)
Photochemical action spectrum of the terminal oxidase of mixed
function oxidase systems. Science, 147: 400-402.
CORYA, B. C., BLACK, M. J., & MCHENRY, P. L. (1976) Echocardiographic
findings after acute carbon monoxide poisoning. Br. heart J.,
38: 712-717.
CROPP, G. J. A. (1970) Ventilation in anemia and polycythemia.
Can. J. Physiol. Pharmacol., 48: 382-393.
DAHMS, T. E. & HORVATH, S. M. (1974) Rapid, accurate technique for
determination of carbon monoxide in blood. Clin. Chem.,
20: 533-537.
DAHMS, T. E., HORVATH, S. M., & GRAY, D. J. (1975) Technique for
accurately producing desired carboxyhemoglobin levels during rest
and exercise. J. appl. Physiol., 38: 366-368.
DALHAMN, T., EDFORS, M.-L., & RYLANDER, R. (1968) Retention of
cigarette smoke components in human lungs. Arch. environ. Health,
17: 746-748.
DAVIS, G. L. & GANTNER, G. E.(1974) Carboxyhemoglobin in volunteer
blood donors. J. Am. Med. Assoc., 230: 996-997.
DE BIAS, D. A., BIRKHEAD, N. C., BANERJEE, C. M., KAZAL, L. A.,
HOLBURN, R. R., GREENE, C. H., HARRER, W. V., ROSENFELD, L. M,
MENDUKE, H., WILLIAMS, N., & FRIEDMAN, M. H. F. (1972a) The
effects of chronic exposure to carbon monoxide on the
cardiovascular and hematologic systems in dogs with experimental
myocardial infarction. Int. Arch. Arbeitsmed., 29: 253-267.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., FRIEDMAN, M. H. F.,
HARRER, W., HOLBURN, R., KAZAL, L., MENDUKE, H., ROSENFELD, L.,
WILLIAMS, N., & GREENE, C. H. (1972b) The effects of chronic
exposure to carbon monoxide (100 ppm) on the cardiovascular
system of monkeys, New York, Coordinating Research Council,
Inc., 54 pp. (PB 215-527).
BE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., HARRER, W. V., &
KAZAL, L. A. (1973) Carbon monoxide inhalation effects following
myocardial infarction in monkeys. Arch. environ. Health,
27: 161-167.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., GREEN, C. H., SCOTT,
D., & HARRER, W. V. (1976) Effects of carbon monoxide inhalation
on ventricular fibrillation. Arch. environ. Health, 31: 42-46.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., LONGO, L. D., & FORSTER, R.
E. (1969) Endogenous carbon monoxide of mother and fetus, 39th
Annual Meeting of the Society of Pediatrics Research, Atlantic
City, NJ, p. 148.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., & FORSTER, R. E. (1970)
Cyclical variation of rate of heme destruction and carbon monoxide
production in normal women. Physiologist, 13: 178 (abstract).
DELWICHE, C. C. (1970) Carbon monoxide production and utilization by
higher plants. Ann. NY Acad. Sci., 174: 116-121.
DINMAN, B. D. (1968) Pathophysiologic determinants of community air
quality standards for carbon monoxide. J. occup. Med.,
10: 446-456.
DINMAN, B. D. (1969) Effects of long-term exposure to carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behaviour, and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 25-27.
DINMAN, B. D, EATON, J. W., & BREWER, G. J. (1970) Effects of carbon
monoxide on DPG concentration in the erythrocyte. Ann. NY Acad.
Sci, 174: 246-251.
DIVINCENZO, G. D. & HAMILTON, M. L. (1975) Fate and disposition of
[14C]methylene chloride in the rat. Toxicol. appl. Pharmacol.,
32: 385-393.
DORCUS, R. M. & WEIGAND, G. E. (1929) The effect of exhaust gas on the
performance in certain psychological tests. J. gen. Psychol.,
2: 73-76.
DOUGLAS, C. G., HALDANE, J. S., & HALDANE, J. B. S. (1912) The laws of
combination of haemoglobin with carbon monoxide and oxygen.
J. Physiol. (Lond.), 44: 275-304.
DOUZE, J. M. C. (1971) [Carbon monoxide poisoning by natural gas.]
Ned. Tidjschr. Geneesk., 115 (36): 1487-1493.
DRABKIN, D. L. & AUSTIN, J. H. (1935) Spectrophotometric studies. II.
Preparation from washed blood cells; nitric oxide hemoglobin and
sulfhemoglobin. J. biol. Chem., 112: 51-65.
DRINKWATER, B. L., RAVEN, P. B., HORVATH, S. M., GLINER, J. A.,
RUHLING, R. O., BOLDUAN, N. W., & TAGUCHI, S. (1974) Air
pollution, exercise and heat stress. Arch. environ. Health,
28: 177-181.
DYER, R. S. & ANNAU, Z. (1976) Carbon monoxide and superior colliculus
evoked potentials. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Protection Agency, 9 pp.
ECKARDT, R. E., MACFARLAND, H. N., ALARIE, Y. C. E., & BUSYE, W. M.
(1972) The biological effect from long-term exposure of primates
to carbon monoxide. Arch. environ. Health., 25: 381-387.
EHRICH, W. E., BELLET, S., & LEWEY, F. H. (1944) Cardiac changes from
CO poisoning. Am. J. med. Sci., 208: 512-523.
EJAM-BERDJEV, A. K. (1973) [Effect of small CO concentrations on the
cardiovascular system of workers engaged in cast-iron moulding.]
Harkovskovo Med. Inst., 110: 185-186 (in Russian).
EJAM-BERDJEV, A. K. & ATAEV, B. A. (1973) [Effect of toxic factors
with low intensity on the functional state of the CNS of workers
engaged in cast-iron moulding.] Zdravoohr. Turkm., 10: 24-34
(in Russian).
EKBLOM, B. & HUOT, R. (1972) Response to submaximal and maximal
exercise at different levels of carboxyhaemoglobin. Acta Physiol.
Scand, 86: 474-482.
ENGEL, R. R., RODKEY, F. L., O'NEAL, J. D., & COLLISON, H. A. (1969)
Relative affinity of human fetal hemoglobin for carbon monoxide
and oxygen. Blood, 33: 37-45.
ENGEL, R. R., RODKEY, F. L., & KRILL, C. E., JR (1971)
Carboxyhemoglobin levels as an index of hemolysis. Pediatrics,
47: 723-730.
ENVIRONMENT AGENCY (1973) [Air Pollution in Japan. Air monitoring
data in fiscal year 1972.] Tokyo, Air Pollution Control
Division, Air Quality Bureau, pp. 206-210 (in Japanese).
FLETCHER, C. M. & HORN, D. (1970) Smoking and health. Chron. World
Health Organ., 24: 345-370.
FODOR, G. G. & WINNEKE, G. (1971) [Different affinity coefficients of
CO to hemoglobin for man and animals.] Zbl. Bakter. Parasit.
Infekt. Hyg., 224(1): 15 (in German).
FODOR, G. G. & WINNEKE, G. (1972) Effect of low CO concentrations on
resistance to monotony and on psychomotor capacity. Staub-
Reinhalt. Luft, 32(4): 46-54.
FORBES, W. H., DILL, D. B., DESILVA, H., & VAN DEVENTER, F. M. (1937)
Influence of moderate carbon monoxide poisoning upon ability to
drive automobiles. J. ind. Hyg. Toxicol., 19: 598-603.
FORBES, W. H., SARGENT, F., & ROUGHTON, F. J. W. (1945) The rate of
carbon monoxide uptake by normal men. Am. J. Physiol.,
143: 594-608.
FORSTER, R. E. (1970) Carbon monoxide and the partial pressure of
oxygen in tissue. Ann. NY Acad. Sci., 174: 233-241.
FRANKENHAEUSER, M., MYRSTEN, A.-L., POST, B., & JOHANSSON, G. (1971)
Behavioral and physiological effects of cigarette smoking in a
monotonous situation. Psychopharmacologia (Berl.), 22: 1-7.
GADASKINA, I. D. (1960) [Chronic poisoning by carbon monoxide and the
methods required for the experimental study of this problem.] In:
Letaveta, A. A. & Kanarevskoj, A. A., ed. Industrial Toxicology,
Moscow, Akademija Medicinskij Nauk SSSR, Institut Gigieny Truda I
Profzabolevanij, pp. 249-255 (in Russian).
GLINER, J. A., RAVEN, P. B., HORVATH, S. M., DRINKWATER, B. L., &
SUTTON, J. C. (1975) Man's physiologic response to long-term work
during thermal and pollutant stress. J. appl. Physiol.,
39: 628-632.
GODIN, G. & SHEPHARD, R. J. (1972) On the course of carbon monoxide
uptake and release. Respiration, 29: 317-329.
GOLDBARG, A. N., KRONE, R. J., & RESNEKOV, L. (1971) Effects of
cigarette smoking on hemodynamics at rest and during exercise. I.
Normal subjects. Chest, 60: 531-536.
GOLDMAN, A., MURCRAY, D. G., MURCRAY, F. H., WILLIAMS, W. J, BROOKS,
J. N., & BRADFORD, C. M. (1973) Vertical distribution of CO in the
atmosphere. J. geophys. Res., 78(24): 5273-5283.
GOLDSMITH, J. R. (1970) Contribution of motor vehicle exhaust,
industry and cigarette smoking to community carbon monoxide
exposures. Ann. NY Acad. Sci, 174: 122-134.
GOLDSMITH, J. R. & ARONOW, W. S. (1975) Carbon monoxide and coronary
heart disease: A review. Environ. Res., 10: 236-248.
GOLDSMITH, J. R. & LANDAW, S. A. (1968) Carbon monoxide and human
health. Science, 162: 1352-1359.
GOLDSMITH, J. R, TERZAGHI, J., & HACKNEY, J. D. (1963) Evaluation of
fluctuating carbon monoxide exposures. Arch. environ. Health,
7: 647-663.
GORBATOV, O. & NORO, L. (1948) On acclimatization in connection with
acute carbon monoxide poisonings. Acta Physiol. Scand.,
15: 77-87.
GORDON, G. S. & ROGERS, R. L. (1969) A report of medical findings of
project carbon monoxide, Washington, DC, Merkle Press.
GORI, G. B. (1976) Low-risk cigarettes: a prescription. Science,
194: 1243-1246.
GORODINSKY, S. M., SEDOV, A. V., MAZIN, A. N., GAZIEV, G. A,
KLEPTSOVA, A. P, & ZUKOVA, L. I. (1970) [Effect of reduced
barometric pressure on the elimination of gaseous and volatile
metabolites in man isolated in a diving suit.] Kosm. Biol. Med.,
2: 78-82 (in Russian).
GORSKI, J. (1962) [The ballistocardiogram in acute carbon-monoxide
poisoning.] Pol. Tyg. Lek., 17: 872-876 (in Polish).
GÖTHE, C. J., FRISTEDT, B., SUNDELL, L., KOLMODIN, B., EHRNER-SAMUEL,
H., & GÖTHE, K. (1969) Carbon monoxide hazard in city traffic-an
examination of traffic policemen in three Swedish towns. Arch.
environ. Health, 19: 310-314.
GRAHN, D. & KRATCHMAN, J. (1963) Variation in neonatal death rate and
birth weight in the United States and possible relations to
environmental radiation, geology and altitude. Am. J. hum.
Genet., 15: 329-352.
GRANDSTAFF, N. W., BEARD, R. R., & MORANDI, A. J. (1975) Review of
the neurobehavioral effects of carbon monoxide on humans,
Stanford, CA, Stanford University School of Medicine, 74 pp.
(USEPA Contract No. BU75 B001.)
GRIGOROV, L., KAZASOV, P., KURCATOVA, G., BALABAEVA, L., PAVLITZIAN,
T, VACEV, D., JAKIMOV, E., PETROVA, G., MAKSIMOVA, F., TZVETKOV,
T., BLASKOVA, D., DJOLOV, G., & Prof. BRESKOV. (1968) [Study of
the atmospheric air pollution in the region of the thermo-
electrical central "Maritza-Iztok", meteorological conditions and
their effects on the health of the population.] Izvestija NIHI,
3: 33 (in Bulgarian).
GROLL-KNAPP, E., WAGNER, H., HAUCK, H., & HAIDER, M. (1972) Effects of
low carbon monoxide concentrations on vigilance and computer-
analyzed brain potentials. Staub-Reinhalt. Luff, 32 (4): 64-68.
GROLL-KNAPP, E., HAIDER, M., HOLLER, H., JENKER, G., NEUBERGER, M., &
STIDL, H. (1976) Neuro- and psychophysiological effects of
moderate CO-doses. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Research Agency, 9 pp.
GRUT, A. (1949) Chronic carbon monoxide poisoning: a study in
occupational medicine, Copenhagen, Ejnar Munksgaard, 229 pp.
GUEST, A.D. L., DUNCAN, C., & LAWTHER, P. J. (1970) Carbon monoxide
and phenobarbitone: a comparison of effects on auditory flutter
fusion threshold and critical flicker fusion threshold.
Ergonomics (Lond.), 13(5): 587-594.
HAAGEN-SMIT, A. J. (1966) Carbon monoxide levels in city driving.
Arch. environ. Health, 12: 548-551.
HACKNEY, J. D, LINN, W. S., LAU, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. 3. Two hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HAEBISCH, H. (1970) [The cigarette as a source of carbon monoxide.]
Arch. Toxikol., 26: 251-261 (in German).
HÄGGENDAL, E., NILSSON, N.J., & NORBACH, B. (1966) Effect of blood
corpuscle concentration on cerebral blood flow. Acta Chir. Scand.
(Suppl.), 364: 3-12.
HALDANE, J. S. & PRIESTLEY, J. G. (1935) Respiration, New Haven,
Yale University Press, 493 pp.
HALPERIN, M. H., MCFARLAND, R. A., NIVEN, J. I., & ROUGHTON, F. J. W.
(1959) The time course of the effects of carbon monoxide on visual
thresholds. J. Physiol., 146: 583-593.
HAMMOND, E. C. (1962) The effects of smoking. Sci. Am., 207: 3-16.
HARKE, H. P. (1975) [Passive smokers and transport vehicles.]
Arbeitsmed. Sozialmed. Präventivmed., 10: 240-241 (in German).
HELLUNG-LARSEN, P., LAURSEN, T., KJELDSEN, K., & ASTRUP, P. (1968)
Lactate dehydrogenase isoenzymes of aortic tissue in rabbits
exposed to carbon monoxide. J. Atheroscler. Res., 8: 343-349.
HELMCHEN, H. & KUNKEL, H. (1964) [Findings concerning rhythmic after-
oscillations at optically evoked responses in EEG of man.] Arch.
Psychiatr. Z. Neurol., 205: 397-408 (in German).
HERSCH, P. (1964) Galvanic analysis. In: Reilley, C. A., ed. Advances
in analytical chemistry and instrumentation, New York,
Interscience Publishers, Vol. 3, pp. 183-249.
HERSCH, P. (1966) Process for measuring the carbon monoxide content of
a gas stream. Off. Gaz. US Pat. Off., 827(4): 1276-1277.
HEXTER, A. C. & GOLDSMITH, J. R. (1971) Carbon monoxide: association
of community air pollution with mortality. Science,
172: 265-267.
HINDS, W. C. & FIRST, M. W. (1975) Concentrations of nicotine and
tobacco smoke in public places. N. Engl. J. Med., 292
(16): 844-845.
HOFFMAN, D. & WYNDER, E. L. (1972) Smoke of cigarettes and little
cigars: an analytical comparison. Science, 178: 1197-1199.
HOGAN, G. K., SMITH, R. O., & CORNISH, H. H. (1976) Studies on the
microsomal conversion of dichloromethane to carbon monoxide.
Toxicol. appl. Pharmacol., 37: 112.
HORVATH, S. M. (1973) Effects of carbon monoxide on human behaviour.
In: Proceedings of the Conference on Health Effects of Air
Pollutants, 3-5 October, 1973, Washington, DC, National Academy
of Sciences, pp. 127-139 (Serial No. 93-15).
HORVATH, S. M. & ROUGHTON, F. J. W. (1942) Improvements in the
gasometric estimation of carbon monoxide in blood. J. biol.
Chem., 144: 747-755.
HORVATH, S. M., DAHMS, T. E., & O'HANLON, J. F. (1971) Carbon monoxide
and human vigilance: a deleterious effect of present urban
concentrations. Arch. environ. Health, 23: 343-347.
HORVATH, S. M., RAVEN, P. B., DAHMS, T. E., & GRAY, D. J. (1975)
Maximal aerobic capacity at different levels of carboxyhemoglobin.
J. appl. Physiol., 38: 300-303.
HOSKO, M. J. (1970) The effect of carbon monoxide on the visual evoked
response in man. Arch. environ. Health, 21: 174-180.
INGERSOLL, R. B., INMAN, R. E., & FISHER, W. R. (1974) Soil's
potential as a sink for atmospheric carbon monoxide. Tellus,
XXVI(1-2): 151-159.
JAFFE, L. S. (1973) Carbon monoxide in the biosphere: sources,
distributions and concentrations. J. geophys. Res.,
67: 5293-5305.
JENKINS, C. E. (1932) The haemoglobin concentration of workers
connected with internal combustion engines. J. Hyg.,
32: 406-408.
JOHNSON, C. J., MORAN, J. C., PAINE, S.C., ANDERSON, H. W., & BREYSSE,
P. A. (1975) Abatement of toxic levels of carbon monoxide in
Seattle ice-skating rinks. Am. J. public Health, 65: 1087-1090.
JONES, J. G. & WALTERS, D. H. (1962) A study of carboxyhaemoglobin
levels in employees at an integrated steelworks. Ann. occup.
Hyg., 5: 221-230.
JONES, R. A., STRICKLAND, J. A., STUNKARD, J. A., & SIEGEL, J. (1971)
Effects on experimental animals of long-term inhalation exposure
to carbon monoxide. Toxicol. appl. Pharmacol., 19: 46-53.
JONES, R. D., COMMINS, B. T., & CERNIK, A. A. (1972) Blood lead and
carboxyhaemoglobin levels in London taxi drivers. Lancet,
2: 302-303.
JUNGE, C., SEILER, W., & WARNECK, P. (1971) The atmospheric 12CO and
14CO budget. J. geophys. Res., 76(12): 2866-2879.
KAHN, A., RUTLEDGE, R. B., DAVIS, G. L., ALTES, J. A., GANTNER, G. E.,
THORNTON, C. A., & WALLACE, N. D. (1974) Carboxyhemoglobin sources
in the metropolitan St. Louis population. Arch. environ. Health,
29: 127-135.
KANDALL, S. R., LANDAW, S. A., & THALER, M. M. (1973) Carboxyhemo-
globin exchange between donors and recipients of blood
transfusions. J. Pediatr., 52: 716-718.
KILLICK, E. M. (1940) Carbon monoxide anoxemia Physiol. Rev.,
20(3): 313-344.
KJELDSEN, K. (1969) Smoking and atherosclerosis. Investigations on
the significance of the CO content in tobacco smoke in
atherogenesis, Copenhagen, Munksgaard, 142 pp.
KJELDSEN, K., THOMSEN, H. K., & ASTRUP, P. (1974) Effects of carbon
monoxide on myocardium. Circ. Res., 34: 339-348.
KLAUSEN, K., RASMUSSEN, B., GJELLEROD, H., MADSEN, H., & PETERSEN, E.
(1968) Circulation, metabolism and ventilation during prolonged
exposure to carbon monoxide and to high altitude. Scand. J. Clin.
Lab. Invest., 22 (Suppl. 103): 26-38.
KLIMISCH, H. J., CHEVALIER, H. J., HARKE, H. P., & DONTENWILL, W.
(1975) Uptake of carbon monoxide in blood of miniature pigs and
other mammals. Toxicology, 3: 301-310.
KODAT, V. ( 1971) Excretion of CO by workers on generatory station,
Candidate dissertation, Prague, 129 pp.
KOMATSU, E. (1959) [Shinshu myocardosis-a type of myocardosis caused
by carbon monoxide.] In: [Proceedings of the 15th General
Assembly of the Japan Medical Congress, Tokyo, Japan, 1-5 April,
1959.] pp. 343-348 (in Japanese).
KRONE, R. J., GOLDBARG, A. N., BALKOURA, M., SCHUESSLER, R., &
RESNEKOV, L. (1972) Effects of cigarette smoking at rest and
during exercise. II. Role of venous return. J. appl. Physiol,
32: 745-748.
KROTOVA, E. I. & MUZYKA, V. I. (1974) [Content of d-amino-levulinic
acid in erythrocytes of workers having contact with CO.] Gig. Tr.
i Prof. Zabol., 6: 43-44 (in Russian).
KUBIC, V. L., ANDERS, M. W., ENGEL, R. R., BARLOW, C. H., & CAUGHEY,
W. S. (1974) Metabolism of dihalomethanes to carbon monoxide.
Drug Metab. Disposition, 2(1): 53-57.
KULLER, L. H., RADFORD, E. P., SWIFT, D, PERPER, J., & FISHER, R.
(1975) Carbon monoxide and heart attacks. Arch. environ. Health,
30: 477-482.
KUSTOV, V. V., BELKIN, V. I., ABIDIN, B. I., OSTAPENKO, O. F.,
MALKUTA, A. N., & PODDUBNAJA, L. T. (1972) [Aspects of chronic
carbon monoxide poisoning in young animals.] Gig. Tr. i Prof
Zabol., 5: 50-52 (in Russian).
LANDAW, S. A. (1973) The effects of cigarette smoking on the total
body burden and excretion rates of carbon monoxide J. occup.
Med., 15(3): 231-240.
LAZAREV, N. V., ed. (1965) [Carbon monoxide.] In: [Toxic substances
in industry.] Vol. 2, pp. 198-218 (in Russian).
LEE, R. E. (1972) Indoor-outdoor carbon monoxide pollution study,
Washington, DC, US Environmental Protection Agency, 408 pp.
(EPA-R4-73-020).
LEVY, H. H. (1972) Photochemistry of the lower troposphere planet.
Space Sci., 20: 919-935.
LEWEY, F. H. & DRABKIN, D. L. (1944) Experimental chronic carbon
monoxide poisoning of dogs. Am. J. med. Sci., 208: 502-511.
LILIENTHAL, J. L., JR & FUGITT, C. H. (1946) The effect of low
concentrations of carboxyhemoglobin on the "altitude tolerance" of
man. Am. J. Physiol., 145: 359-364.
LILY, R. E. C., COLE, P. V., & HAWKINS, L. H. (1972)
Spectrophotometric measurements of carboxyhaemoglobin. Br. J.
ind. Med., 29: 454-457.
LINDENBERG, R., PREZIOSI, T., LEVY, D., & CHISTENSEN, M. K. (1961)
Experimental investigation of carbon monoxide toxicity. Presented
at: The Air Pollution Medical Research Conference, Los Angeles,
CA, 4-7 December, 1961.
LINNENBOM, V. J., SWINNERTON, J. W., & LAMONTAGNE, R. A. (1973) The
ocean as a source for atmospheric carbon monoxide. J. geophys.
Res., 78(24): 5333-5340.
LISS, P. S. & SLATER, P. G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LJUBLINA, E. I. (1960) [Shifts in some physiological functions of the
animal on subacute and chronic exposure to carbon monoxide.]
In: Letaveta, A. A. & Kanarevskoj, A. A., ed: [Industrial
Toxicology.] Moscow, Akademija Medicinskij Nauk SSSR, Institut
Gigieny Truda i Profzabolevanij, pp. 256-263 (in Russian).
LOGUE, G. L., ROSSE, W. F., SMITH, W. T., SALTZMAN, H. A., &
GUTTERMAN, L. A. (1971) Endogenous carbon monoxide production
measured by gas-phase analysis: an estimation of heme catabolic
rate. J. lab. clin. Med., 77: 867-876.
LONG, K. R. & DONHAM, K. J. (1973) Occupational exposure to CO in
selected rural work environments, Iowa City, IA, Institute of
Agricultural Medicine & Environmental Health, Department of
Preventive Medicine & Environmental Health, University of Iowa.
LONGO, L. D. (1970) Carbon monoxide in the pregnant mother and fetus
and its exchange across the placenta. Ann. NY Acad. Sci.,
174: 313-341.
LONGO, L. D. & HILL, E. P. (1977) Carbon monoxide uptake and
elimination in fetal and maternal sheep. Am. J. Physiol.,
232: H324-H330.
LUOMANMAKI, K. (1966) Studies on the metabolism of carbon monoxide.
Ann. med. exp. Biol. Fenn. (Helsinki), 44 (Suppl. 2): 1-55.
MAAS, A. H. J., HAMELINK, M. L., & DELEEUW, R. J. M. (1970) An
evaluation of the spectrophotometric determination of HbO2,
HbCO, and Hb in blood with the co-oximeter IL 182. Clin. Chim.
Acta, 29: 303-309.
MACMAHON, B., ALPERT, M., & SALBER, E. J. (1966) Infant weight and
parental smoking habits. Am. J. Epidemiol., 82: 247-261.
MALENFANT, A. L., GAMBINO, S. R., WARASKA, A. J., & ROE, E. I. (1968)
Spectrophotometric determination of hemoglobin concentration and
percent oxyhemoglobin and carboxyhemoglobin saturation. Clin.
Chem., 14: 789.
MALINOW, M. R., MCLAUGHLIN, P., DHINDSA, D. S., METCALFE, J., OCHSNER,
A. J., III, HILL, J., & MCNULTY, W. P. (1976) Failure of carbon
monoxide to induce myocardial infarction in cholesterol-fed
cynomolgus monkeys. Cardiovasc. Res., 10: 101-108.
MALORNY, G., MÜLLER-PLATHE, O., & STRAUB, M. (1962) [Disturbance of
acid-base balance in acute carbon monoxide poisoning and effect of
oxygen or carbon dioxide-oxygen mixtures.] Arch. exp. Pathol.
Pharmakol. (Naunyn-Schmiedeberg), 243: 439-458 (in German).
MARKS, E. & SWIECICKI, W. (1971) [Effect of carbon monoxide, vibration
and lowered atmospheric pressure upon cerebral neurosecretory
system and catecholamines level.] Med. Pr., 22: 336-341 (in
Polish).
MARTIN, W. & STERN, A. C. (1974) The world's air quality management
standards. Vol. 1. The air quality management standards of the
world, including United States federal standards, Washington,
DC, US Environmental Protection Agency, 383 pp.
MAZIARKA, S., BORKOWSKA, M., MISIAKIEWICZ, Z., STRUSINSKI, A., &
WYSZYNSKA, H. (1974) [Air pollution in the streets of Warsaw in
relation to surrounding buildings.] Roczn. Pzh., 25:1-5 (in
Polish).
MCCLATCHIE, E. A., COMPHER, A. B., & WILLIAMS, K. Y. (1972) A high
specificity carbon monoxide analyzer. Anal. Instrum. (Symp.),
10: 67-69.
MCCONNELL, J. C., MCELROY, M. B., & WOFSY, S.C. (1971) Natural sources
of atmospheric CO. Nature (Lond.), 233: 187-188.
MCCREDIE, R. M. & JOSE, A.D. (1967) Analysis of blood carbon monoxide
and oxygen by gas chromatography. J. appl. Physiol.,
22: 863-866.
MCFARLAND, R. A. (1973) Low level exposure to carbon monoxide and
driving performance. Arch. environ. Health, 27: 355-359.
MCFARLAND, R. A., ROUGHTON, F. J. W., HALPERIN, M. H., & NIVEN, J. I.
(1944) The effects of carbon monoxide and altitude on visual
thresholds. Aerosp. Med., 15: 381-394.
MCFARLAND, R. A., FORBES, W. H., STOUDT, H. W., DOUGHERTY, A., CROLEY,
T. J., MOORE, R. C., & NALWALK, T. J. (1973) A study of the
effects of low levels of carbon monoxide upon humans performing
driving tasks, Boston, Guggenheim Center of Aerospace Health &
Safety, Harvard School of Public Health, 88 pp.
MCMULLEN, T. B. (1975) Interpreting the eight-hour national ambient
air quality standard for carbon monoxide. J. Air Pollut. Control
Assoc., 25: 1009-1014.
MEHMEL, H. C., DUVELLEROY, M. A., & LAVER, M. B. (1973) Response of
coronary blood flow to pH-induced changes in hemoglobin-O2
affinity. J. appl. Physiol., 35: 485-489.
MIDDLETON, V., VAN POZNAK, A., ARTUSIO, J. F., JR, & SMITH, S. M.
(1965) Carbon monoxide accumulation in closed circle anesthesia
systems. Anesthesiology, 26: 715-719.
MILLS, E. & EDWARDS, M. W., JR (1968) Stimulation of aortic and
carotid chemoreceptors during carbon monoxide inhalation.
J. appl. Physiol., 25: 494-502.
MINISTRY OF LABOUR (1965) Carbon monoxide poisoning causes and
prevention, London, Her Majesty's Stationery Office, 72 pp.
(Safety, Health & Welfare Series No. 29).
MITCHELL, J. H., SPROULE, B. J., & CHAPMAN, C. B. (1958) The
physiological meaning of the maximal oxygen intake test. J. clin.
Invest., 37: 538-547.
MONTGOMERY, M. R. & RUBIN, R. J. (1971) The effect of carbon monoxide
inhalation on in vivo drug metabolism in the rat. J. Pharmacol.
exp. Ther., 179: 465-473.
MOREL, S. & SZEMBERG, T. (1971) [Air pollution in Ladle Bay.] Tech.
Sanit., 45: 353-355 (in Polish).
MULHAUSEN, R. O., ASTRUP, P., & MILLEMGAARD, K. (1968) Oxygen affinity
and acid-base status of human blood during exposure to hypoxia and
carbon monoxide. Scand. J. clin. lab. Invest., 22 (Suppl. 103):
9-15.
MUSSELMAN, N. P., GROFF, W. A., YEVICH, P. P., WILINSKY, F. T., WEEKS,
M. H., & OBERST, F. W. (1959) Continuous exposure of laboratory
animals to low concentration of carbon monoxide. Aerosp. Med.,
30: 524-529.
MYHRE, L. (1974) Rate of CO elimination in resting man. Fed. Proc,
33: 440.
MYRSTEN, A. L., POST, B., FRANKENHAEUSER, M., & JOHANSSON, G. (1972)
Changes in behavioural and physiological activation induced by
cigarette smoking in habitual smokers. Psychopharmacologia
(Berl.), 27: 305-312.
NAS/NRC (1977) Carbon monoxide, Washington, DC, National Academy of
Sciences, 268 pp.
NEW MEXICO STATE DEPARTMENT OF PUBLIC HEALTH (1975) Birth weight and
altitude, pp. 7-16.
NIELSEN, B. (1971) Thermoregulation during work in carbon monoxide
poisoning. Acta physiol. Scand, 82: 98-106.
NOTOV, A. (1959) [Acute poisonings with CO, SO2 and nitric gases in
mines.] Sb. Tr. VMI Plovidiv, 60: 404-411 (in Bulgarian).
NOWARA, M. (1975) [Evaluation of occupational exposure by means of
determining carbon monoxide in the air at work places and carbon
monoxide hemoglobin.] Med. Pr., 26: 351-360 (in Polish).
NUNES, A. C. & SCHOENBORN, B. P. (1973) Dichloromethane and myoglobin
function. Mol. Pharmacol., 9: 835-839.
O'DONNELL, R. D., CHIKOS, P., & THEODORE, J. (1971a) Effect of carbon
monoxide exposure on human sleep and psychomotor performance.
J. appl. Physiol., 31: 513-518.
O'DONNELL, R. D., MIKULKA, P., HEINIG, P., & THEODORE, J. (1971b) Low
level carbon monoxide exposure and human psychomotor performance.
Toxicol. appl. Pharmacol., 18: 593-602.
OGAWA, M, KATSURADA. K., & SUGIMOTO, I. (1974) Pulmonary edema in
acute carbon monoxide poisoning. Int. Arch. Arbeitsmed.,
33: 131-138.
O'HANLON, J. F. (1975) Preliminary studies of the effects of carbon
monoxide on vigilance in man. In: Weiss, B. & Laties, V. G., ed.
Behavioral toxicology, New York, Plenum Press, pp. 61-72.
ORELLANO, T., DERGAL, E., ALIJANI, M., BRIGGS, C., VASQUEZ, J.,
GOLDBAUM, L., & ABSOLON, K. B. (1976) Studies on the mechanism of
carbon monoxide toxicity. J. surg. Res., 20: 485-487.
OTT, W. & MAGE, D. (1974) Interpreting urban carbon monoxide
concentrations by means of a computerized blood COHb model.
J. Air Pollut. Control Assoc., 28(9): 911-916.
PACE, N., STRAJMAN, E., & WALKER, E. L. (1950) Acceleration of carbon
monoxide elimination in man by high pressure oxygen. Science,
111: 652-654.
PANKOW, D. & PONSOLD, W. (1972) Leucine aminopeptidase activity in
plasma of normal and carbon monoxide poisoned rats. Arch.
Toxikol., 29: 279-285.
PANKOW, D. & PONSOLD, W. (1974) [The combined effects of carbon
monoxide and other biologically active detrimental factors on the
organism.] Zeits. Ges. Hyg., 9: 561-571 (in German).
PANKOW, D., PONSOLD, W., & FRITZ, H. (1974a) Combined effects of
carbon monoxide and ethanol on the effects of leucine
aminopeptidase and glutamic-pyruvic transaminase in the plasma of
rats. Arch. Toxicol, 32: 331-340.
PANKOW, D., PONSOLD, W., & GRIMM, I. (1974b) [On the potentiation of
the hepatic toxicity of carbon tetrachloride by carbon monoxide.]
Dtsch. Ges. Wesen., 29: 853-856 (in German).
PECORA, L. J. (1959) Physiologic study of the summating effects of
ethyl alcohol and carbon monoxide. Am. Ind. Hyg. Assoc. J.,
20: 235-240.
PENNEY, D., BENJAMIN, M., & DUNHAM, E. (1974a) Effect of carbon
monoxide on cardiac weight as compared with altitude effects.
J. appl. Physiol, 37: 80-84.
PENNEY, D., DUNHAM, E., & BENJAMIN, M. (1974b) Chronic carbon monoxide
exposure: Time course of hemoglobin, heart weight and lactate
dehydrogenase isozyme changes. Toxicol. appl. Pharmacol,
28: 493-497.
PERMUTT, S. & FAHRI, L. (1969) Tissue hypoxia and carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behavior and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 18-24.
PETERSON, J. E. & STEWART, R. D. (1970) Absorption and elimination of
carbon monoxide by inactive young men. Arch. environ. Health,
21: 165-171.
PETROV, P. (1968) [Chronic CO intoxications in workers engaged in
loading and unloading processes.] Transp. Med. Vesti, 4: 24-27
(in Bulgarian).
PIRNAY, F., DUJARDIN, J., DEROANNE, R., & PETIT, J. M. (1971) Muscular
exercise during intoxication by carbon monoxide. J. appl.
Physiol., 31: 573-575.
PITTS, G. C. & PACE, N. (1947) The effects of blood carboxyhemoglobin
concentration on hypoxia tolerance. Am. J. Physiol.,
148: 139-151.
PREZIOSI, T. J., LINDENBERG, R., LEVY, D., & CHRISTENSON, M. (1970) An
experimental investigation in animals of the functional and
morphological effects of single and repeated exposures to high and
low concentrations of carbon monoxide. Ann. NY Acad. Sci,
174: 369-384.
PROHOROV, Y. D. & ROGOV, A. A. (1959) [The pathohistological and
histochemical changes in the organs of rabbits subjected to a
prolonged action of carbon monoxide, sulfur dioxide and their
mixtures.] Gig. i Sanit., 24: 22-26 (in Russian).
RAMIREZ, R. G., ALBERT, S. N., AGOSTINI, J. C., BASU, A. P., GOLDBAUM,
L. R., & ABSOLON, K. B. (1974) Lack of toxicity of transfused
carboxyhemoglobin red blood cells and carbon monoxide inhalations.
Surg. Forum, 25: 165-168.
RAMSEY, J. M. (1967a) Carbon monoxide exposures in parking garages.
Bull. environ. Contam. Toxicol., 2: 182-190.
RAMSEY, J. M. (1967b) Carboxyhemoglobinemia in parking garage
employees. Arch. environ. Health, 15: 580-583.
RAMSEY, J. M. (1972) Carbon monoxide, tissue hypoxia, and sensory
psychomotor response in hypoxaemic subjects. Clin. Sci.,
42: 619-625.
RAMSEY, J. M. (1973) Effects of single exposures of carbon monoxide on
sensory and psychomotor response. Am. Ind. Hyg. Assoc. J.,
34: 212-216.
RAMSEY, J. M. & CASPER, P. W., JR (1976) Effect of carbon monoxide
exposures on erythrocyte 2,3-DPG in rabbits. J. appl. Physiol.,
41: 689-692.
RATNEY, R. S., WEGMAN, D. H., & ELKINS, H. B. (1974) In vivo
conversion of methylene chloride to carbon monoxide. Arch.
environ. Health, 28: 223-226.
RAVEN, P. B., DRINKWATER, B. L., HORVATH, S. M., RUHLING, R. O.,
GLINER, J. A., SUTTON, J. C., BOLDUAN, N. W. (1947a) Age, smoking
habits, heat stress and their interactive effects with carbon
monoxide and peroxyacetyl nitrate on man's aerobic power. Int. J.
Biometeorol., 18: 222-232.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N. W.,
TAGUCHI, S., GLINER, J. A., & HORVATH, S. M. (1974b) Effect of
carbon monoxide and peroxyacetyl nitrate on man's maximal aerobic
capacity. J. appl. Physiol., 36: 288-293.
RAY, A.M. & ROCKWELL, T. H. (1970) An exploratory study of automobile
driving performance under the influence of low levels of
carboxyhemoglobin. Ann. NY Acad. Sci., 174: 396-407.
RENCH, J. D. & SAVAGE, E. P. (1976) Carbon monoxide in the home
environment. J. environ. Health, 39: 104-106.
RISSANEN, V., PYÖRÄLÄ, K., & HEINONEN, O. P. (1972) Cigarette smoking
in relation to coronary and aortic atherosclerosis. Acta pathol.
microbiol. Scand. Sect. A, 80: 491-500.
ROBBINS, R. C., BORG, K. M., & ROBINSON, E. (1968) Carbon monoxide in
the atmosphere. J. Air Pollut. Control Assoc., 18: 106-110.
ROBINSON, J. C. & FORBES, W. F. (1975) The role of carbon monoxide in
cigarette smoking. Arch. environ. Health, 30: 425-434.
ROBINSON, E. & ROBBINS, R. C. (1970) Atmospheric background
concentrations of carbon monoxide. Ann. NY Acad. Sci.,
174: 89-95.
RONDIA, D. (1970) Abaissement de l'activité de la benzopyrene-
hydroxylase hepatique in vivo apres inhalation d'oxyde de
carbone. C. R. Acad. Sci. (Paris), 271: 617-619.
ROOT, W. S. (1965) Carbon monoxide. In: Fenn, W. O. & Rahn, H., ed.
Handbook of physiology, respiration 2, Washington, DC, American
Physiological Society, pp. 1087-1098.
ROSSI-BERNARDI, L., PERRELLA, M., LUZZANA, M., SAMAJA, M., & RAFFAELE,
I. (1977) Simultaneous determination of hemoglobin derivatives,
oxygen content, oxygen capacity, and oxygen saturation in 10 µl of
whole blood. Clin. Chem., 23: 1215-1225.
ROSSI-FANELLI, A. & ANTONINI, E. (1958) Studies on the oxygen and
carbon monoxide equilibria of human myoglobin. Arch. Biochem.
Biophys., 77: 478-492.
ROTH, R. P., DREW, R. T., Lo, R. J., & FOUTS, J. R. (1975)
Dichloromethane inhalation, carboxyhemoglobin concentrations, and
drug metabolizing enzymes in rabbits. Toxicol. appl. Pharmacol.,
33: 427-437.
ROUGHTON, F. J. W. (1970) The equilibrium of carbon monoxide with
human hemoglobin in whole blood. Ann. NY Acad. Sci.,
174: 177-188.
ROUGHTON, F. J. W. & DARLING, R. C. (1944) The effect of carbon
monoxide on the oxyhemoglobin dissociation curve. Am. J.
Physiol., 141: 17-31.
ROUGHTON, F. J. W. & ROOT, W. S. (1945) The estimation of small
amounts of carbon monoxide in blood. J. biol. Chem.,
160: 123-133.
RUMMO, N. & SARLANIS, K. (1974) The effect of carbon monoxide on
several measures of vigilance in a simulated driving task.
J. Saf Res., 6: 126-130.
RUSSELL, M. A. H., COLE, P. V., & BROWN, E. (1973) Absorption by non-
smokers of carbon monoxide from room air polluted by tobacco
smoke. Lancet, 1: 576-579.
RYLANDER, R. (1974) Environmental tobacco smoke effects on the non-
smoker. Report from a workshop. Scand J. resp. Dis.,
(Suppl. 91): 90 pp.
RYLOVA, M. L. (1960) [The application of integral methods of
investigation to determine the effect of repeated exposure to
carbon monoxide in animals.] In: Letaveta, A. A. & Kanarevskoj, A.
A., ed. [Industrial toxicology.] Moscow, Akademija Medicinskij
Nauk SSSR, Institut Gigieny Truda i Profzabolevanij, pp. 264-270
(in Russian).
SAMMONS, J. H. & COLEMAN, R. L. (1974) Firefighters' occupational
exposure to carbon monoxide. J. occup. Med., 16: 543-546.
SANTIAGO, T. V. & EDELMAN, N. H. (1972) Effect of anemia on the
ventilatory response to transient hypoxia. Fed. Proc.,
31: 371 (Abstract No. 864).
SARMA, J. S. M., TILLMANS, H., IKEDA, S., & BINS, R. J. (1975) The
effect of carbon monoxide on lipid metabolism of human coronary
arteries. Atherosclerosis, 22: 193-198.
SAYERS, R. R., YANT, W. P., LEVY, E., & FULTON, W. B. (1929) Effect
of repeated daily exposure of several hours to small amounts of
automobile exhaust gas, Washington, DC, Government Printing
Office, 49 pp. (Public Health Bull. No. 186).
SCHULTE, J. H. (1963) Effects of mild carbon monoxide intoxication.
Arch. environ. Health, 7: 524-530.
SEDOV, A. V., ZUKOVA, L. I., & MAZNEVA, G. E. (1971) [Carbon monoxide
excretion by the human organism.] Gig. Tr. i Prof Zabol.,
9: 36-39 (in Russian).
SEILER, W. (1975) The cycle of carbon monoxide in the atmosphere.
In: International Conference on Environmental Sensing and
Assessment, Las Vegas, NV, 14-19 September, New York, Institute
of Electrical and Electronics Engineers, Vol. 2, 6 pp.
SEILER, W. & JUNGE, C. E. (1969) Decrease of carbon monoxide mixing
ratio above the polar tropopause. Tellus, 21(3): 447-449.
SEILER, W. & JUNGLE, C. E. (1970) Carbon monoxide in the atmosphere.
J. geophys. Res., 75(12): 2217-2226.
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA,. M. A., & TARASOVA, L. N.
(1970) [New data on the concentration of the products of the
incomplete combustion of domestic gas in the air of premises
provided with a gas supply.] In: [Problems of current interest in
hygiene.] Moscow, Akademija Medicinskij Nauk SSSR, Institute of
Genetics and Community Hygiene, pp. 137-142 (in Russian).
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA, M. A., KLEBANOVA, V. A.,
OSINCEVA, V. P., & NAZARENKO, A. F. (1972) [The experimental basis
for measures to improve the quality of the air in flats provided
with a gas supply.] Gig. i Sanit., 7: 24-28 (in Russian).
SIEGEL, S. M., RENWICK, G., & ROSEN, L. A. (1962) Formation of carbon
monoxide during seed germination and seedling growth. Science,
137: 683-684.
SJÖSTRAND, T. (1948) A method for the determination of
carboxyhaemoglobin concentrations by analysis of the alveolar
air. Acta Physiol. Scand., 16: 201-210.
SMALL, K. A., RADFORD, E. P., FRAZIER, J. M., RODKEY, F. L., &
COLLISON, H. A. (1971) A rapid method for simultaneous measurement
of carboxy- and methemoglobin in blood. J. appl. Physiol.,
31: 154-160.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumonia. Arch. environ. Health, 16: 670-672.
SOKAL, J. A. (1975) Lack of the correlation between biochemical
effects on rats and blood carboxyhemoglobin concentrations in
various conditions of single acute exposure to carbon monoxide.
Arch. Toxicol, 34: 331-336.
SOTNIKOV, E. E. (1971) [Determination of carbon monoxide in the blood
by a gas chromatographic method.] Gig. i Sanit., 6: 61-64 (in
Russian).
SPEDDING, D. J. (1974) Air pollution, Oxford, Clarendon Press,
pp. 58-62.
SRCH, M. (1967) [The significance of carbon monoxide in cigarette
smoke in passenger car interiors.] Dtsch. Z. ges. gerichtl. Med,
60: 80-89 (in German).
STEVENS, C. M., KROUT, L., WALLING, D., VENTERS, A., ENGELKEMEIR, A.,
& Ross, L. E. (1972) The isotopic composition of atmospheric
carbon monoxide. Earth planet. Sci. Lett, 16: 147-165.
STEWART, R. D. & HAKE, C. L. (1976) Paint remover hazard. J. Am. Med.
Assoc., 235: 398-401.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. C. (1972a) Experimental human exposure to
methylene chloride. Arch. environ. Health, 26: 342-348.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. D. (1972b) Carboxyhemoglobin elevation after
exposure to dichloromethane. Science, 176: 295-296.
STEWART, R. D., NEWTON, P. E., HOSKO, M. J., & PETERSON, J. E. (1973a)
Effect of carbon monoxide on time perception. Arch. environ.
Health, 27: 155-160.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973b) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWARD, E. D.,
KALBFLEICH, J. H., VAN YSERLOO, B., & RIMM, A. A. (1973c)
Carboxyhemoglobin concentrations in blood from donors in Chicago,
Milwaukee, New York, and Los Angeles. Science, 182: 1362-1364.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWART, E. D.,
KALBFLEICH, J. D., VAN YSERLOO, B., & RIMM, A. A. (1974)
Carboxyhaemoglobin tests in American blood donors. JAMA,
229: 1187-1195.
STEWART, R. D, NEWTON, P. E., HOSKO, M. J., PETERSON, J. E., &
MELLENDER, J. W. (1975) The effect of carbon monoxide on time
perception, manual coordination, inspection, and arithmetic.
In: Weiss, B. & Laties, V. G., ed. Behavioral toxicology, New
York, Plenum Press, pp. 29-60.
STEWART, R. D., HAKE, C. L., WU, A., STEWART, T. A., & KALBFLEISCH, J.
H. (1976) Carboxyhemoglobin trend in Chicago blood donors,
1970-1974. Arch. environ. Health, 31: 280-286.
STUPFEL, M. (1975) Rhythms and air pollution. Chronobiologia,
11: 105-115.
STUPFEL, M. & BOULEY, G. (1970) Physiological and biochemical effects
on rats and mice exposed to small concentrations of carbon
monoxide for long periods. Ann. NY Acad. Sci. 174: 342-368.
STUPFEL, M., MAGNIER, M., & ROMARY, F. (1973) Rythme circadien de
l'action de l'oxide de carbone sur l'emission du gaz carbonique
par le rat en groupe. C. R. Acad. Sci. (Paris) Série D,
276: 1009-1012.
SUL'GA, T. M. (1962) [New data for the hygienic evaluation of CO in
air.] In: Ryazanov, V. A., ed. [ Maximal permissible
concentrations of air pollution.] Moscow, Medgiz, pp. 128-145
(in Russian).
SWIECICKI, W. (1973) [The effect of vibration and physical training on
carbohydrate metabolism in rats intoxicated with carbon monoxide.]
Med. Pr., 34: 399-405 (in Polish).
SWINNERTON, J. W., LAMONTAGNE, R. A., & LINNENBOM, V. J. (1971) Carbon
monoxide in rain water. Science, 172: 943-945.
TANAKA, M. (1965) [Studies on the etiological mechanism of fetal
development disorders caused by maternal smoking habits during
pregnancy.] J. Jpn. Obstet. Gynecol. Soc., 17: 1107-1114 (in
Japanese, English translation in J. Obstet. Gynecol. Soc.,
14: 45 (1967)).
THOMSEN, H. K. (1974) Carbon monoxide-induced artherosclerosis in
primates. Atherosclerosis, 20: 233-240.
THOMSEN, H. K. & KJELDSEN, K. (1974) Threshold limit for carbon
monoxide-induced myocardial damage. Arch. environ. Health,
29: 73-79.
THOMSEN, H. K. & KJELDSEN, K. (1975) Aortic intimal injury in rabbits.
Arch. environ. Health, 30: 604-607.
TIUNOV. A. & KUSTOV, V. V. (1969) [Toxicology of carbon monoxide.]
Leningrad, Medicina, pp. 81-217 (in Russian).
TRAYSTMAN, R. J. (1976) Effects of carbon monoxide hypoxia and hypoxic
hypoxia on cerebral circulation. In: Fourth International
Congress on Event Related Slow Potentials of the Brain,
Hendersonville, NC, April 4-10, 1976, Research Triangle Park,
NC, US Environmental Protection Agency, 9 pp.
TROMPEO, G., TURLETTl, G., & GIARRUSSO, O. T. (1964) [Concentration of
carbon monoxide in underground garages.] Rass. Med. Ind.,
33: 392-393 (in Italian).
TUMASONIS, C. F. & BAKER, F. D. (1972) Influence of carbon monoxide
upon some respiratory enzymes of the chick embryo. Bull. environ.
Contain. Toxicol., 8: 113-119.
TURNER, J. A. McM., SILLETT, R. W., & BALL, K. P. (1974) Some effects
of changing to low-tar and low-nicotine cigarettes. Lancet,
2:737-739.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Air quality
criteria for carbon monoxide, Washington, DC, US DHEW Public
Health Service, National Air Pollution Control Administration.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1972) Criteria for a
recommended standard. Occupational exposure to carbon monoxide,
Washington, DC, US DHEW Health Services and Mental Health,
National Institute for Occupational Safety and Health, 120 pp.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1973) The Health
Consequences of Smoking, Washington, DC, US DHEW, Public Health
Service, pp. 367-381.
US ENVIRONMENTAL PROTECTION AGENCY (1973a) The national air
monitoring program: Air quality and emission trends, Annual
report, Vol. 1, Research Triangle Park, NC, USEPA, 182 pp.
(EPA 450/1-73-001-A).
US ENVIRONMENTAL PROTECTION AGENCY (1973b) Compilation of air
pollution emission factors 2nd ed., Research Triangle Park, NC,
USEPA, 288 pp. (Report No. AP42).
US ENVIRONMENTAL PROTECTION AGENCY (1975) Human health damages from
mobile source air pollution: A delphic study, Research Triangle
Park, NC, USEPA (Project No. 07AAC-OS).
VAN KAMPEN, E. J. & ZIJLSTRA, W. G. (1961) Standardization of
hemoglobinometry. II. The hemiglobincyanide method. Clin. Chim.
Acta, 6: 538-544.
VINOGRADOV, G. I., CERNICENKO, I. A., & MAKARENKO, E. M. (1974) [The
allergenic activity of motor vehicle gases.] Gig. i Sanit.,
8: 10-13 (in Russian).
VOGEL, J. A. & GLESER, M. A. (1972) Effect of carbon monoxide on
oxygen transport during exercise. J. appl. Physiol.,
32: 234-239.
VOGEL, J. A., GLESER, M. A., WHEELER, R. C., & WHITTEN, B. K. (1972)
Carbon monoxide and physical work capacity. Arch. environ.
Health, 24: 198-203.
VOLLMER, E. P., KING, B. G, BIRREN, J. E., & FISHER, M. B. (1946) The
effects of carbon monoxide on three types of performance at
simulated altitudes of 10,000 and 15,000 feet. J. exp. Psychol.,
36: 244-251.
VYSKOCIL, J. (1957) Neurohumoral regulation in acute and chronic
carbon monoxide poisoning. Int. Arch. Gewerbepathol. Gewerbehyg.,
15: 457-472.
WAGEMANN, W. (1960) [The otological picture in carbon monoxide
poisoning.] Z. Laringol. Rhinol. Otol., 39: 691-708 (in German).
WAGNER, J. A., HORVATH, S. M., & DAHMS, T. E. (1975) Carbon monoxide
elimination. Respir. Physiol, 23: 41-47.
WAKEHAM, H. R. R. (1976) Environmental carbon monoxide from cigarette
smoking, Sixth International Tobacco Scientific Congress, Tokyo,
Japan, November 18, 1976, 17 pp.
WALD, N. & HOWARD, S. (1975) Smoking, carbon monoxide and arterial
disease. Ann. occup. Hyg., 18: 1-14.
WALD, N., HOWARD, S., SMITH, P. G., & KJELDSEN, K. (1973) Association
between atherosclerotic diseases and carboxyhaemoglobin levels in
tobacco smokers. Br. med. J., 1: 761-765.
WALLACE, N. D., DAVIS, G. L., RUTLEDGE, R. B., & KAHN, A. (1974)
Smoking and carboxyhemoglobin in the St. Louis metropolitan
population. Arch. environ. Health, 29: 136-142.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WEBSTER, W. S., CLARKSON, T. B., & LOFLAND, H. B. (1968) Carbon
monoxide-aggravated atherosclerosis in the squirrel monkey. Exp.
mol. Pathol., 13: 36-50.
WEINSTOCK, B. & NIKI, H. (1972) Carbon monoxide balance in nature.
Science, 176: 290-292.
WEIR, F. W. & ROCKWELL, T. H. (1973) An investigation of the effects
of carbon monoxide on humans in the driving task, Columbus, OH,
Ohio State University Research Foundation, 170 pp. (Contract No.
68-02-0329 and CRC-APARC Project CAPM-9-69).
WEISS, H. R. & COHEN, J. A. (1974) Effects of low levels of carbon
monoxide on rat brain and muscle tissue pO2. Environ.
Physiol. Biochem., 4: 31-49.
WELLS, L. L. (1933) The prenatal effect of carbon monoxide on albino
rats and the resulting neuropathology. Biologist, 15: 80-81.
WESTBERG, K, COHEN, N., & WILSON, K. W. (1971) Carbon monoxide: its
role in photochemical smog formation. Science, 171: 1013-1015.
WESTLAKE, D. W., ROXBURGH, J. M., & TALBOT, G. (1961) Microbial
production of carbon monoxide from flavonoids. Nature (Lond.),
189: 510-511.
WHITE, P. (1970) Carbon monoxide production and heme catabolism. Ann.
NY Acad. Sci., 174: 23-31.
WILKS, S. S. (1959) Carbon monoxide in green plants. Science,
129: 964-966.
WILKS, S. S., TOMASHEFSKI, J. F., & CLARKE, R. T., JR (1959)
Physiological effects of chronic exposure to carbon monoxide.
J. appl. Physiol., 14: 305-310.
WILLIAMS, I. R. & SMITH, E. (1935) Blood picture, reproduction, and
general condition during daily exposure to illuminating gas.
Am. J. Physiol., 110: 611-615.
WINNEKE, G. (1974) Behavioral effects of methylene chloride and carbon
monoxide as assessed by sensory and psychomotor performance. In:
Xintaras, C., Johnson, B., & deGroot, I., ed. Behavioral
toxicology: early detection of occupational hazards, Washington,
DC, US Government Printing Office, pp. 130-144 (New Publ. No.
N10511).
WINNEKE, G. (1977) [Studies on marginal values for carbon monoxide.]
Lufthyg. Silkoseforsch., 10: 75-90 (in German).
WINNEKE, G., FODOR, G. G., & SCHLIPKÖTER, H. W. (1976) Effects of low
level carbon monoxide exposure and monotony on vigilance and
vertex evoked potentials. In: Proceedings of the fourth
international congress on event-related slow potentials of the
brain. Hendersonville, NC, April 4-10, 1976, Research Triangle
Park, NC, US Environmental Protection Agency, 5 pp.
WOLFE, D. G. & BIDLACK, W. R. (1976) The formation of carbon monoxide
during peroxidation of microsomal lipids. Biochem. Biophys. res.
Commun., 73:850-857.
WHO (1975) WHO Technical Report Series, No. 568 (Smoking and its
effects on health), 100 pp.
WHO (1976) Selected methods of measuring air pollutants, Geneva,
WHO, 112 pp. (Offset Publication No. 24).
WRIGHT, G. R., JEWCZYK, S., ONROT, J., TOMLINSON, P., & SHEPHARD, R.
J. (1975) Carbon monoxide in the urban atmosphere. Arch. environ.
Health, 30: 123-129.
WRIGHT, G. W., RANDELL, P., & SHEPHARD, D.. R. J. (1973) Carbon
monoxide and driving skills. Arch. environ. Health, 27: 349-354.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response technique
in air pollution toxicology. Toxicol appl. Pharmacol., 8: 77-87.
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor-outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
ZENKEVIC, E. C. (1973) [Hemodynamic changes under the effect of CO
displayed adaptation.] Sb. Probl. Adapt. Gig. Tr., pp. 67-72
(in Russian).
ZORN, H. (1968) [Carbon dioxide-oxygen mixture or pure oxygen for
respiration in the case of carbon monoxide intoxication.] Dtsch.
Med. Wochenschr., 93: 1536 (in German).
ZORN, H. (1972) The partial oxygen pressure in the brain and liver at
subtoxic concentrations of carbon monoxide. Staub-Reinhalt. Luff,
32: 24-29.
Annex 1
THE RELATIONSHIP BETWEEN CARBON MONOXIDE CONCENTRATION IN AIR AND
CARBOXYHAEMOGLOBINa
(1) Several empirical equations have been proposed for estimating
carboxyhaemoglobin levels from environmental exposure conditions.
These equations were based on controlled exposures of human
volunteers. Most of them referred to subjects at rest, or performing
sedentary activities or light work. The simplest empirical equations
described carboxyhaemoglobin levels as a linear function of carbon
monoxide concentration in the inspired air [CO], and of exposure time
(t) (Forbes et al., 1945; Pace et al., 1946). They were applicable
only within a limited range of exposure conditions. Hanks & Farquhar
(1969) and Peterson & Stewart (1970) compiled empirical equations
involving more complex functional relationships that had a wider
application.
(2) In addition, models have been proposed that relate carboxyhaemo-
globin levels to both environmental exposure conditions and a number
of physiological variables such as blood volume, VB, endogenous
carbon monoxide production, Vco, diffusion capacity of the lung,
DL, and alveolar ventilation rate, VA. The best known model,
developed by Coburn et al. (1965), has been briefly discussed in
section 6.2. A more recent model, that took into account the dynamic
condition of urban carbon monoxide concentrations, was suggested by
Ott & Mage (1978) (p. 118).
(3) A simple linear relationship, proposed by Forbes et al. (1945),
linked the increase in the carboxyhaemoglobin level with the carbon
monoxide concentration in air, [CO](ppm) and exposure time, t(min):
a "This annex has been prepared by the Secretariat and has not been
reviewed by the Task Group, with the exception of Table 3.
Equations are presented in the form given in the original studies
and no attempt has been made to change to SI units. The Secretariat
wishes to express its appreciation to Dr H. Buchwald (Canada),
Chairman of the Task Group, for providing information contained in
Tables 3 and 4 and to Dr R. Horton, Dr D. J. McKee, USEPA, and
Dr V. Armstrong, Health and Welfare, Canada for reviewing the
annexes.
[HbCO](%) = k × [CO] × t (1)
where k was a constant equal to 0.0003 for "an individual at rest"
( VA = 6 litre/min, pulse rate 70), 0.0005 for "light activity"
( VA = 9.5 litre/min, pulse rate 80), 0.0008 for "light work"
(50 watts, VA = 18 litre/min, pulse rate 110), and 0.0011 for
"heavy work"a (about 100 watts, VA = 30 litre/min, pulse rate 135).
This equation was based on sets of controlled exposure
observations on human volunteers. Exposure concentrations ranged from
100 to 20 000 ppm (0.01 to 2.0%), and exposure times up to 6 h. At a
[CO] of 100 ppm, the equation holds only up to delta[HbCO] of about
7%. At 1000 ppm, the equation is applicable up to 30% [HbCO].
The equation of Forbes et al. was used by the California State
Department of Health for estimating [HbCO] after shorter periods of
exposure to carbon monoxide concentrations higher than 100 ppm in
individuals at rest or engaged in light work (Goldsmith & Landow,
1978).
(4) The empirical equation suggested by Pace et al. (1945) was derived
from controlled carbon monoxide exposures of 32 volunteers, aged 18-40
years. The concentrations of carbon monoxide in the inspired air
varied from 90 to 21 800 ppm, and the subjects were either sitting
quietly or walking on a level treadmill (about 4.3 km/h, VA about
19.2 litre/min). The exposure times ranged from 20 to 300 min. The
equation was also linear, but it took into account the alveolar
ventilation rate, VA, and the blood volume, VB:
[CO](ppm) × VA (litre/min) × t(min)
DELTA[HbCO](%) = (2)
4650 × VB(litre)
If it is assumed that the blood volume equals 5.51 and
VA = 6 litre/min (rest or light sedentary activity) or 18 litre/min
(light physical work), the equation takes the same form as that of
Forbes et al, but k values are somewhat lower (0.00023 and 0.00070,
respectively).
(5) Hanks & Farquhar (1969) also conducted carefully controlled
exposure studies on sedentary volunteers, and expressed their results
by the equation:
[HbCO](%) = 0.147[CO](1 - e-0.00289 t) (3)
where [CO] is in ppm and t in min. The equation was valid for subjects
having a ventilation rate of about 6 litre/min.
a VA for heavy physical work may be as high as 60 litre/min.
According to Chovin (1974), the "coefficient of (pulmonary)
ventilation" K could be introduced into equation (3), which then
became applicable to subjects performing various degrees of activity.
Chovin's modified equation read:
K
- t
0.147
[HbCO](%) = 0.147[CO](1 - e ) (4)
where t was in hours. For subjects at rest, K = 0.025, and for those
performing heavy physical work, K = 0.065. For intermediate degrees of
activity, Chovin proposed K values of 0.035, 0.045, and 0.055.
(6) Another empirical equation, referred to on p. 36, was suggested by
Peterson & Stewart (1970). It could also be written in the following
form:
[HbCO](%) = 0.0051[CO]0.858 × t0.63 (5)
where [CO] is in ppm, and t in min. The equation was based on
controlled human exposure data. The volunteers (18 healthy graduate
students) were exposed to carbon monoxide concentrations of <1, 25,
50, 100, 200, 500, and 1000 ppm for periods ranging from 30 min to
24 h, while performing strictly sedentary activities. Like linear
equations, this equation needed to be used with caution, since it did
not yield a finite [HbCO] value for an infinite exposure time. It was
used in developing US national ambient air quality standards, and is
strictly valid for constant [CO] and for shorter periods of exposure
(Ott & Mage, 1978).
(7) A model proposed by Coburn et al. (1965) has been discussed on
pp. 36 and 37. It is valid for male subjects only.a A solution of
their differential equation has been provided in the original paper; it
assumes that the mean pulmonary capillary oxygen pressure ( pCo2)
and the concentration of oxyhaemoglobin [HbO2] are constant and
independent of carboxyhaemoglobin levels [HbCO]. However, the
oxyhaemoglobin level depends on carboxyhaemoglobin concentrations in a
complex way. Solutions of the differential equation taking this into
account are available, and their application is illustrated in the
NAS/NRC (1977) document. Nevertheless, for most practical purposes,
the solution given in the original paper appears to be adequate. It
has been used, for example, by Peterson & Stewart (1970) and in the
criteria document of the National Institute of Occupational Safety and
Health (US Department of Health, Education and Welfare, 1972). A
useful form of this solution is given in the document of the Committee
on the Challenges of Modern Society (1972):
a Endogenous CO production may be different for females due to
menstruation, pregnancy, and other metabolic factors.
1
[HbCO] t = {e- kt(A[HbCO]o - VcoB - [CO]) + VcoB + [CO]} (6)
A
where [HbCO] t and [HbCO]o are the carboxyhaemoglobin levels at
times
pCo2 1 ( pB - 47)
t and t = 0, [CO] = pIco, A = , B = ( + ) and
M[HbO2] DL VA
A
k =
VB.B
the symbols used to define [CO], A, and B are explained on p. 36. The
relationships between [CO] in ppm and pIco, and between [HbCO]% and
[HbCO] in ml CO/ml blood are:
pIco × 106
[CO](ppm) = ,
pB
and
[HbCO](%) = 497.5 [HbCO](ml CO/ml blood)
(8) Ott & Mage (1978) designed a model that took into consideration
the dynamic characteristics of urban carbon monoxide concentrations.
The differential equation describing it reads:
d[HbCO]
tau dt + [HbCO] - ß = alpha[CO] (7)
O < [CO] < 100 ppm
where ß was the endogenous level of blood carboxyhaemoglobin and was
assumed to be 0.5%, alpha was assumed to be 0.15, and tau = 2.49h.
The main conclusion of the authors was that [CO] in ambient air
should be reported for averaging periods of 10-15 min, if the
monitoring stations were located near heavy traffic or on congested
streets. In such cases, sharp carbon monoxide peaks of short duration
might occur fairly often, and concentrations reported with longer
averaging periods, e.g., 1 h or more, might introduce an error of up
to 21% in the estimated [HbCO].
(9) Several empirical equations are compared with Coburn's model in
Table 1, for persons at rest ( VA - 6 litre/min) or performing light
physical work ( VA = 18 litre/min), at a carbon monoxide exposure
concentration of 100 ppm. For subjects performing light work, Hanks &
Farquhar's equation has been used in Chovin's modification with
K = 0.060. The following assumptions have been made in calculating
[HbCO]t values from Coburn's equation (6): [HbCO] = 0.5% or
0.001 ml CO/ml blood, Vco = 0.007 ml/min, VB = 5500 ml,
pCo2 = 100 mmHg, [HbO2] = 0.2 ml O2/ml blood, M = 218,
pB - 47 = 713 mmHg; sedentary subjects: DL = 40 ml/min/mmHg,
VA = 6000 ml/min; light work: DL = 40 ml/min/mmHg,
VA = 18 000 ml/min. For all empirical equations, [HbCO]o = 0.5%
has been added to the calculated values of [HbCO].
Table 1 shows clearly that Hanks & Farquhar's equation agrees best
with Coburn's model. Peterson & Stewart's equation gives values that
are higher than the other equations up to 6 h of exposure; then it
gives lower results than Forbes' equation. At a carbon monoxide
concentration of 100 ppm, Pace's equation gives lower [HbCO] values
for subjects at rest than the other equations, up to 7 h of exposure;
for subjects performing light work, it is applicable up to 2 h of
exposure. For subjects at rest, Forbe's equation is applicable up to
6 h, and for persons performing light work, up to 2 h.
(10) Table 2 shows [HbCO] values predicted by Coburn's model.a The
assumptions are the same as those specified in paragraph 9. For heavy
work, it has been assumed that DL = 60 ml/min/mmHg and that
VA=30 000 ml/min.
(11) Guidelines on exposure conditions that would prevent
carboxyhaemoglobin levels exceeding 2.5-3% in general nonsmoking
populations are given in Table 3.
These guidelines were reviewed by the Task Group. Comparison with
Tables 1 and 2 indicates the degree of protection provided, if these
guidelines are applied, both for sedentary individuals and for persons
performing light work. The exposure guidelines for 8-24 h have been
added by the Secretariat, after the Task Group meeting, to facilitate
comparison with national air quality standards.
(12) Guidelines on exposure conditions, which would prevent carboxy-
haemoglobin levels exceeding 5% in nonsmoking occupational groups, are
shown in Table 4. They were prepared, after the meeting, by Dr
Buchwald, at the request of the Secretariat, and have not been
reviewed by the Task Group. Guidelines for heavy work have been
suggested by the Secretariat, also after the meeting. Heavy work has
been defined by DL = 60 ml/min/mmHg and VA = 30 000 ml/min. The
degree of protection provided by these guidelines is indicated in
columns 3 and 4 of Table 4. Column 3 shows carbon monoxide
concentrations that would produce 5% HbCO within exposure times given
a The Secretariat wishes to thank Dr M. A. Vouk, University
Computing Centre, Zagreb, Yugoslavia, for programming Coburn's
equations and providing computer printouts.
in column 2, for light and heavy work, respectively. Column 4 provides
"safety factors" obtained by dividing the concentrations in column 3
by concentrations in column 1. Unless otherwise indicated, the
guidelines given in Table 4 should be considered as desirable
conditions rather than maximum acceptable limits.
Table 1. [HbCO](%) predicted by different empirical equations and by the model of Coburn et al. (1965).
Exposure to a carbon monoxide concentration of 115 mg/m3 (100 ppm)
Time Subjects at rest Subjects performing light work
(min)
F P H PS C F P H C
15 1.0 0.8 1.1 2.0 1.2 1.7 1.6 1.9 2.0
30 1.4 1.0 1.7 2.8 1.8 2.9 2.6 3.2 3.3
45 1.9 1.5 2.3 3.4 2.4 4.1 3.6 4.4 4.6
60 2.3 1.9 2.8 4.0 3.0 5.3 4.7 5.4 5.7
120 4.1 3.3 4.7 5.9 5.0 10.1 8.9 8.7 9.2
180 5.9 4.5 6.4 7.5 6.8 14.9 13.1 10.9 11.6
240 7.7 6.0 7.8 8.9 8.3 19.7 17.3 12.3 13.2
300 9.0 7.4 9.0 10.1 9.6 24.5 21.5 13.3 14.1
360 10.8 8.8 10.0 11.3 10.7 29.3 25.7 13.9 15.1
420 12.6 10.2 10.8 12.4 11.6 34.1 29.9 14.3 15.6
480 14.4 11.5 11.5 13.5 12.4 38.9 34.1 14.6 15.9
F = Forbes et al. (1945), P = Pace et al. (1946), H = Hanks & Farquhar (1969),
PS = Peterson & Stewart (1970), C = Coburn et al. (1965).
Table 2. [HbCO] values predicted from Coburn et al. (1965) model
Time 200 ppm 100 ppm 75 ppm 50 ppm
S L H S L H S L H S L H
15 min 1.8 3.5 5.2 1.2 2.0 2.8 1.0 1.6 2.2 0.82 1.2 1.6
30 min 3.1 6.2 9.2 1.8 3.3 4.8 1.5 2.6 3.7 1.1 1.9 2.6
45 min 4.3 8.7 12.6 2.4 4.6 6.5 1.9 3.5 4.9 1.4 2.5 3.4
60 min 5.5 11.0 15.5 3.0 5.7 7.9 2.3 4.3 6.0 1.7 3.0 4.1
90 min 7.7 14.9 20.2 4.0 7.6 10.2 3.1 5.8 7.7 2.2 4.0 5.2
2h 9.7 18.1 23.7 5.0 9.2 11.9 3.9 7.0 9.0 2.7 4.7 6.1
4h 16.3 26.2 30.4 8.3 13.2 16.3 6.3 10.0 11.5 4.4 6.9 7.7
6h 21.1 30.0 32.4 10.7 15.1 16.2 8.1 11.3 12.2 5.5 7.6 8.2
8h 24.5 31.7 32.9 12.4 15.9 16.5 9.4 12.0 12.4 6.4 8.0 8.3
24h 32.7 33.2 33.2 16.5 16.7 16.6 12.4 12.5 12.5 8.4 8.4 8.3
infinity 33.4 33.2 33.2 16.8 16.7 16.6 12.7 12.5 12.5 8.5 8.4 8.3
Time 35 ppm 25 ppm 10 ppm 5 ppm
S L H S L H S L H S L H
15 min 0.72 1.0 1.3 0.66 0.84 1.0 0.55 0.61 0.67 0.52 0.54 0.56
30 min 0.93 1.4 1.9 0.80 1.2 1.5 0.61 0.72 0.82 0.54 0.57 0.60
45 min 1.1 1.9 2.5 0.95 1.4 1.9 0.66 0.81 0.95 0.56 0.61 0.64
60 min 1.3 2.2 3.0 1.1 1.7 2.2 0.71 0.90 1.1 0.58 0.63 0.68
90 min 1.7 2.9 3.7 1.3 2.1 2.7 0.80 1.1 1.2 0.62 0.69 0.74
2 h 2.0 3.4 4.3 1.6 2.5 3.1 0.89 1.2 1.4 0.66 0.73 0.78
4 h 3.2 4.7 5.4 2.4 3.4 3.9 1.2 1.5 1.6 0.77 0.84 0.86
6 h 4.0 5.4 5.7 2.9 3.9 4.1 1.4 1.6 1.7 0.85 0.88 0.88
8 h 4.5 5.7 5.8 3.3 4.1 4.2 1.5 1.7 1.7 0.91 0.91 0.89
24 h 5.9 5.9 5.9 4.3 4.2 4.2 1.9 1.8 1.7 1.05 0.93 0.89
infinity 6.0 5.9 5.9 4.4 4.2 4.2 1.9 1.8 1.7 1.06 0.93 0.89
S = sedentary subjects, L = light physical work, H = heavy physical work, all as defined in sections 9 and 10.
Table 3. Guidelines for exposure conditions to prevent carboxyhaemoglobin levels exceeding
2.5-3% in nonsmoking populations
(a) A ceiling or maximum permitted exposure of 115 mg/m3 (100 ppm) for periods of exposure
not exceeding 15 min (No exposure over 115 mg/m3 (100 ppm) permitted, even for very
short time periods).
(b) A time-weighted average exposure of 55 mg/m3 (50 ppm) for periods of exposure not
exceeding 30 min.
(c) A time-weighted average exposure of 29 mg/m3 (25 ppm) for periods of exposure not
exceeding one h.
(d) A time-weighted average exposure of 15 mg/m3 (13 ppm) for periods of exposure of more
than one h.
(e) A time-weighted average exposure of 11.5 mg/m3 (10 ppm) for periods of exposure of
8-24 h.a
a Suggested by the Secretariat.
Table 4. Guidelines for exposure conditions that would prevent carboxyhaemoglobin levels
exceeding 5% in nonsmoking occupational groups performing light and heavy
physical work.
Concentration Exposure time not to Concentrations that Safety factor
be exceeded would produce 5% HbCOc
ppm mg/m3
Light Heavy Light Heavy Light Heavy
worka workb work work work work
200d 230 15 min - 298 - 1.5 -
100e,f 115 30 min 15 min 157 193 1.6 1.9
75f 86 60 min 30 min 87 105 1.2 1.4
50f 55 90 min 60 min 64 62 1.3 1.2
35f 40 4 h 2 h 37 41 1.1 1.2
25f 29 8 h 8 h 31 30 1.2 1.2
a Limits suggested by Dr Buchwald.
b Limits suggested by the Secretariat.
c Calculated from Coburn's equation (6).
d Short-term limit or maximum permissible concentration for light work.
e Short-term limit or maximum permissible concentration for heavy work.
f Time weighted average.
REFERENCES
HANKS, T. G. & FARQUHAR, R. D. (1969) Analysis of human performance
capabilities as a function of exposure to carbon monoxide.
(SystMed Corporation Report R 9001, Contract PH-22-68-31).
PACE, N., CONSOLAZIO, W. V., WHITE, W. A. JR. & BEHNKE, A. R. (1945)
Formulation of principal factors affecting the rate of uptake of
carbon monoxide by man. Am. J. Physiol., 147: 352-359.
All other references are included in the list of references for
the main body of the document (pp. 98-114).
Annex 2
SELECTED NATIONAL AMBIENT AIR QUALITY STANDARDS AND OCCUPATIONAL
EXPOSURE STANDARDS FOR CARBON MONOXIDEa
1. NATIONAL AMBIENT AIR QUALITY STANDARDS
1.1 Canada (1974)
National air quality objectives
Desirable concentrations:
(a) 0-6 mg/m3 (0-5 ppm)b average concentration over an 8-h
period.
(b) 0-15 mg/m3 (0-13 ppm) average concentration over a one-h
period.
Acceptable concentrations:
(a) 6-15 mg/m3 (5-13 ppm) average concentration over an 8-h
period.
(b) 15-35 mg/m3 (13-31 ppm) average concentration over a one-h
period.
Method of measurement:
Nondispersive infrared spectrometry, Report No. EPS 1-AP-73-1.
Source: Velma Ouellet (1978) The Clean Air Act--Compilation of
Regulations and Guidelines, Ottawa, Environment Canada (Report EPS
1-AP-78-2).
In addition to the desirable and acceptable concentrations listed,
a tolerable range of 15-20 mg/m3 average concentration over a
continuous 8-h period was prescribed in 1978.
Source: Canada Gazette (1978) Part 2, Volume 112, No. 3 (February 8).
a Prepared by the Secretariat.
b Concentrations in alternative units have been added by the
Secretariat to facilitate comparison of national quality standards.
1.2 Federal Republic of Germany (1974)
In the Federal Republic of Germany, immissionsc (Immissionen)
are legally defined as "air pollutants, noise, vibrations, light,
heat, radiations, and analogous environmental factors affecting human
beings, animals, plants, or other objects". "As a rule, air pollutants
occurring at a height of 1.5 metres above ground or at the upper limit
of vegetation or at a distance of 1.5 metres from the surface of a
building shall be considered active air pollutants."
"Immission standards are the values for long-term exposure (IW 1)
and short-term exposure (IW 2)."
The following immission standards have been established for carbon
monoxide:
Long-term exposure (IW 1): 10.0 mg/m3 (9 ppm).
Short-term exposure (IW 2): 30.0 mg/m3 (26 ppm).
Method of measurement: VDI 2455, Sheet 1 (August 1970) and 2455,
Sheet 2 guidelines (October 1970).
"Characteristic value I 1 for comparison with IW 1 shall be the
arithmetic mean of all individual data for a measurement area.
Characteristic value I 2 for comparison with IW 2 shall be the 95%
value for the cumulative frequency distribution of all individual
values for a measurement area; this may be also calculated with the
formula I 2 = x + ts where x is the arithmetic mean of individual
data for a measurement area,
2SUM(x - xi)2
s = + SQRT ,
2z - 1
xi = individual data which are greater than x, z = number of
individual data, which are greater than x, and t = 1.64 for the 95%
confidence level."
Source: Federal Minister of the Interior (1974) Technical
Instructions for Maintaining Air Purity, 28 August 1974, Bonn.
c Immission A German term for which there is no simple English
equivalent. Immissions are to be distinguished from emissions
(Emissionen), which are defined as air pollutants, noise,
vibrations, light, heat, radiations, and analogous phenomena
originating from an installation (Federal Republic of Germany, Law
on Protection against Emissions, 15 March 1974).
1.3 Japan (1973)
Ambient air quality standards are defined as follows:
(a) Average of hourly values in 8 consecutive hours shall not
exceed 20 ppm (23 mg/m3).
(b) Daily average of hourly values shall not exceed 10 ppm
(11 mg/m3).
These standards do not apply to industrial zones, roadways, and
other areas or places where people do not usually live.
Source: Environment Agency (1978) Environmental Laws and
Regulations in Japan (II) Air. Tokyo.
1.4 Union of Soviet Socialist Republics (1971)
Maximum permissible (single exposure) concentration for carbon
monoxide is 3 mg/m3 (2.6 ppm). This concentration should not provoke
reflex (i.e., subsensory) reactions in human organisms.
Maximum permissible (24-hour average) concentration for carbon
monoxide is 1.0 mg/m3 (0.9 ppm). This concentration should not have
either a direct or indirect harmful effect on man, for unlimited in
time, continuous exposure, 24 hours a day.
Method not specified.
Source: Krotov, Ju. A. (1975) Maximum permissible concentrations
of harmful substances in air and water, Himija, Moscow.
1.5 United States of America (1971)
"The national primary and secondary ambient air quality standards
for carbon monoxide, measured by the reference method described in
Appendix C to this part, or by an equivalent method, are:
(a) 10 milligrams per cubic meter (9 ppm) -- maximum 8-hour
concentration not to be exceeded more than once per year.
(b) 40 milligrams per cubic meter (35 ppm) -- maximum 1-hour
concentration not to be exceeded more than once per year."
Reference method for the continuous measurement of carbon monoxide
in the atmosphere is nondispersive infrared spectrometry.
Source: Federal Register, 36 (228), Thursday, November 25, 1971,
Washington DC, pp. 22385 and 22391-22392.
1.6 Other Member States
For further information on national ambient air quality standards
for carbon monoxide, the reader is referred to W. Martin & A. C. Stern
(1974) The world's air quality management standards, Washington, DC,
US Environmental Protection Agency (EPA-650/a-75-001-a).
2. OCCUPATIONAL EXPOSURE LIMITS
An occupational exposure limit for carbon monoxide of 50 ppm or
55 mg/m3 has been set in the following Member States: Australia,
Belgium, Finland, Federal Republic of Germany, Italy, Japan,
Netherlands, Switzerland, the USA, and Yugoslavia.
Bulgaria and the USSR have established a limit of 20 mg/m3
(17 ppm), Hungary and Poland, 30 mg/m3 (26 ppm) and Sweden,
40 mg/m3 (35 ppm). Czechoslovakia's standard includes average and
maximum values of 30 mg/m3 (26 ppm) and 150 mg/m3 (130 ppm),
respectively. Other standards that include both average and maximum
values are those of the German Democratic Republic, (35 and
110 mg/m3) (30 and 96 ppm) and Romania, (30 and 50 mg/m3) (26 and
44 ppm).
These values should be interpreted in terms of definitions of
occupational exposure limits, which may be different in different
countries. The reader is referred to the International Labour Office
publication: Occupational Exposure Limits for Airborne Toxic
Substances, Occupational Safety and Health Series 37, ILO, Geneva,
1977. The values given in paragraphs 1 and 2 have been extracted from
this publication.
The US National Institute of Occupational Safety and Health
(NIOSH, 1972) has recommended environmental exposure limits of 35 ppm
(40 mg/m3) (time weighted average) and 200 ppm (229 mg/m3)
(ceiling) (Summary of NIOSH Recommendations for Occupational Health
Standards, July, 1978).

 Carbon monoxide concentrations are normally reported in terms of
8-h average concentrations. This averaging time has been used because
it takes from 4 to 12 h for the carboxyhaemoglobin levels in the human
body to reach equilibrium with the ambient carbon monoxide
concentrations. Two types of approaches have been used for calculating
the 8-h average (McMullen, 1975). One approach is to examine all
possible 8-h intervals and calculate a moving 8-h average (24, 8-h
averages each day). The other approach is to examine three
consecutive, nonoverlapping, 8-h intervals per day. It would appear
that the moving average approach offers some advantages in that it
approximates the human body's integrating response to cumulative
carbon monoxide exposure. In addition to the 8-h averages, carbon
monoxide concentrations are also reported in terms of other averaging
times and frequency distributions.
Carbon monoxide concentrations in the ambient air vary
considerably, not only among urban areas but within cities as well.
The maximum 8-h mean concentrations measured at more than 200
monitoring stations in the USA in 1973 ranged from less than
10 mg/m3 to 58 mg/m3 (8.7-51 ppm) with most of the values being
less than 30 mg/m3 (26 ppm) (Martin & Stern, 1974). The highest 8-h
mean concentration of 59 mg/m3 was observed at a monitoring station
in New York City. Data from a 38 station network in Japan showed that
the 8-h standard of 23 mg/m3 (20 ppm) was not exceeded (Environment
Agency, 1973). That carbon monoxide concentrations are extremely
variable within an urban area is illustrated by the maximum 8-h means
observed at monitoring stations in the metropolitan Los Angeles area
in 1973. They ranged from 7 mg/m3 (6 ppm) in the outlying areas to
49 mg/m3 (42.6 ppm) near the centre of the city. The maximum 1-h
mean concentrations exhibit a similar variability ranging from less
than 10 mg/m3 (8.7 ppm) in some areas to over 90 mg/m3 (78.3 ppm)
in others. The highest 1-h mean concentration observed in 1973 was
92 mg/m3 (80 ppm) at a monitoring station in Philadelphia; at more
than 80% of the stations the maximum 1-h concentration was below
50 mg/m3 43.5 ppm). In Japan the maximum 1-h concentrations ranged
from 5 mg/m3 to 48 mg/m3 (4.3-41.8 ppm).
Although the data presented above refer to measurements carried
out in the USA and Japan, it is indicated that very similar conditions
are prevalent in many parts of the world.
Annual average concentrations of carbon monoxide are not of much
value for assessing human exposure, although they do provide an
indication of the long-term trends. Annual average concentrations of
carbon monoxide in most locations fall well below 10 mg/m3 (8.7 ppm)
(Stewart et al., 1976).
Usually, the monitoring stations are located so that they can
provide representative information of air pollution within the
community. However, in certain areas, such as loading platforms and
underpasses, the concentrations found are much higher than those in
city streets. At Chicago post office loading platforms (Conlee et al.,
1967), ambient carbon monoxide concentrations ranged from 10 to
88 mg/m3 at various locations. Wright et al. (1975) determined the
levels of carbon monoxide encountered by pedestrians and street
workers in Toronto. They reported carbon monoxide levels ranging from
11 to 57 mg/m3, with much higher concentrations in poorly ventilated
underpasses and underground garages.
Industry also contributes to the pollution of the ambient air. Gas
generator plants (Nowara, 1975), smelters (Morel & Szemberg, 1971),
steelworks (Butt et al., 1974; Maziarka et al., 1974); plastics works
(Argirova, 1974, unpublished data)a, electric generating plants
(Grigorov et al., 1968), and mines (Notov, 1959) have all been
suggested, among other industrial activities, as sources of
environmental carbon monoxide pollution. Rural work places, especially
those involving intensive livestock production facilities, can provide
high ambient levels of carbon monoxide. Concentrations up to
136 mg/m3 (119 ppm) have been found in these conditions, with high
levels of carboxyhaemoglobin in both animals and workers (Long &
Donham, 1973).
5.2 Indoor Concentrations and Exposure
Carbon monoxide is widely generated indoors by heating, cooking,
and tobacco smoking. According to Yocom et al. (1971), gas heating
systems did not appear to affect indoor carbon monoxide
concentrations, but gas stoves, water heaters, and automobile activity
in attached garages could be major sources. These authors also
reported that carbon monoxide concentrations were generally unrelated
to outdoor levels. Obviously, peak concentrations in the kitchen were
observed during meal times, Sofoluwe (1968) reported extremely high
concentrations of carbon monoxide in Nigerian dwellings, where
firewood was used for cooking. During the preparation of meals,
average carbon monoxide concentrations were reported to be over
1000 mg/m3 (870 ppm) with peak levels as high as 3400 mg/m3
(2960 ppm). Sidorenko et al. (1970) found an indoor level of carbon
monoxide of 32.3 mg/m3 (28 ppm) some two and a half hours after
domestic gas combustion began, when there was no ventilation, but only
13.4 mg/m3 (11.6 ppm) when ventilation was provided. The use of
improved stoves resulted in better conditions (Sidorenko et al.,
1972).
a "Argirova, M. [Atmospheric air pollution by a plant processing
plastics.] In: Proceedings of the First National Conference on
Sanitary Chemistry of the Air, Sofia, Bulgaria, 26-27 November,
1974 (in Bulgarian).
Rench & Savage (1976) have also investigated winter levels of
carbon monoxide in the home. Outdoor concentrations were lower than
those found indoors. Age of building, type of appliance, heating
sources, and socio-economic status were statistically significantly
related to indoor levels of carbon monoxide, higher levels occurring
in the kitchens of old houses and in homes belonging to families in
lower income groups. Levels were also higher in kitchens with space
wall heaters compared with those with forced air, gravity feed, or hot
water heating systems. Places of recreation may be problem areas.
Excessive levels of carbon monoxide were found in ice-skating areas
where ice-resurfacing machines were used. Levels as high as
348 mg/m3 (304 ppm) were found in such an arena after complaints of
illness among children skating there were reported to the local health
department (Johnson et al., 1975). Improperly regulated space heaters
in such premises could also produce high concentrations of carbon
monoxide. This was recently reported to have occurred in an Alaskan
ice-skating arena.
That outdoor concentrations of carbon monoxide can sometimes
strongly influence indoor levels was illustrated in a study conducted
in New York City (Lee, 1972). As shown in Fig. 3, the concentrations
inside and outside an apartment building followed the same general
pattern and were closely related to nearby traffic flows.
Relatively high concentrations of carbon monoxide have also been
observed inside the passenger compartment of motor vehicles (Borst,
1970). Carbon monoxide may enter the compartment from faulty or
damaged exhaust systems or from the surrounding air, in road traffic.
The carbon monoxide level in the compartment is often higher than that
found outside the vehicle. Haagen-Smit (1966) found an average
concentration in a vehicle passenger compartment of 42 mg/m3
(36.5 ppm) on a Los Angeles freeway during the rush hour traffic
(higher concentrations have been reported by Aronow et al. (1972).
It should be emphasized that cigarette smoking is the most common
source of carbon monoxide for the general population (Table 5).
Exposure to smoking primarily affects the carboxyhaemoglobin level
of the smoker himself (Kahn et al., 1974; Landaw, 1973). In some
circumstances, such as poorly ventilated enclosed spaces, the tobacco
smoke may affect all of the occupants (section 8.1.6).
Carbon monoxide concentrations are normally reported in terms of
8-h average concentrations. This averaging time has been used because
it takes from 4 to 12 h for the carboxyhaemoglobin levels in the human
body to reach equilibrium with the ambient carbon monoxide
concentrations. Two types of approaches have been used for calculating
the 8-h average (McMullen, 1975). One approach is to examine all
possible 8-h intervals and calculate a moving 8-h average (24, 8-h
averages each day). The other approach is to examine three
consecutive, nonoverlapping, 8-h intervals per day. It would appear
that the moving average approach offers some advantages in that it
approximates the human body's integrating response to cumulative
carbon monoxide exposure. In addition to the 8-h averages, carbon
monoxide concentrations are also reported in terms of other averaging
times and frequency distributions.
Carbon monoxide concentrations in the ambient air vary
considerably, not only among urban areas but within cities as well.
The maximum 8-h mean concentrations measured at more than 200
monitoring stations in the USA in 1973 ranged from less than
10 mg/m3 to 58 mg/m3 (8.7-51 ppm) with most of the values being
less than 30 mg/m3 (26 ppm) (Martin & Stern, 1974). The highest 8-h
mean concentration of 59 mg/m3 was observed at a monitoring station
in New York City. Data from a 38 station network in Japan showed that
the 8-h standard of 23 mg/m3 (20 ppm) was not exceeded (Environment
Agency, 1973). That carbon monoxide concentrations are extremely
variable within an urban area is illustrated by the maximum 8-h means
observed at monitoring stations in the metropolitan Los Angeles area
in 1973. They ranged from 7 mg/m3 (6 ppm) in the outlying areas to
49 mg/m3 (42.6 ppm) near the centre of the city. The maximum 1-h
mean concentrations exhibit a similar variability ranging from less
than 10 mg/m3 (8.7 ppm) in some areas to over 90 mg/m3 (78.3 ppm)
in others. The highest 1-h mean concentration observed in 1973 was
92 mg/m3 (80 ppm) at a monitoring station in Philadelphia; at more
than 80% of the stations the maximum 1-h concentration was below
50 mg/m3 43.5 ppm). In Japan the maximum 1-h concentrations ranged
from 5 mg/m3 to 48 mg/m3 (4.3-41.8 ppm).
Although the data presented above refer to measurements carried
out in the USA and Japan, it is indicated that very similar conditions
are prevalent in many parts of the world.
Annual average concentrations of carbon monoxide are not of much
value for assessing human exposure, although they do provide an
indication of the long-term trends. Annual average concentrations of
carbon monoxide in most locations fall well below 10 mg/m3 (8.7 ppm)
(Stewart et al., 1976).
Usually, the monitoring stations are located so that they can
provide representative information of air pollution within the
community. However, in certain areas, such as loading platforms and
underpasses, the concentrations found are much higher than those in
city streets. At Chicago post office loading platforms (Conlee et al.,
1967), ambient carbon monoxide concentrations ranged from 10 to
88 mg/m3 at various locations. Wright et al. (1975) determined the
levels of carbon monoxide encountered by pedestrians and street
workers in Toronto. They reported carbon monoxide levels ranging from
11 to 57 mg/m3, with much higher concentrations in poorly ventilated
underpasses and underground garages.
Industry also contributes to the pollution of the ambient air. Gas
generator plants (Nowara, 1975), smelters (Morel & Szemberg, 1971),
steelworks (Butt et al., 1974; Maziarka et al., 1974); plastics works
(Argirova, 1974, unpublished data)a, electric generating plants
(Grigorov et al., 1968), and mines (Notov, 1959) have all been
suggested, among other industrial activities, as sources of
environmental carbon monoxide pollution. Rural work places, especially
those involving intensive livestock production facilities, can provide
high ambient levels of carbon monoxide. Concentrations up to
136 mg/m3 (119 ppm) have been found in these conditions, with high
levels of carboxyhaemoglobin in both animals and workers (Long &
Donham, 1973).
5.2 Indoor Concentrations and Exposure
Carbon monoxide is widely generated indoors by heating, cooking,
and tobacco smoking. According to Yocom et al. (1971), gas heating
systems did not appear to affect indoor carbon monoxide
concentrations, but gas stoves, water heaters, and automobile activity
in attached garages could be major sources. These authors also
reported that carbon monoxide concentrations were generally unrelated
to outdoor levels. Obviously, peak concentrations in the kitchen were
observed during meal times, Sofoluwe (1968) reported extremely high
concentrations of carbon monoxide in Nigerian dwellings, where
firewood was used for cooking. During the preparation of meals,
average carbon monoxide concentrations were reported to be over
1000 mg/m3 (870 ppm) with peak levels as high as 3400 mg/m3
(2960 ppm). Sidorenko et al. (1970) found an indoor level of carbon
monoxide of 32.3 mg/m3 (28 ppm) some two and a half hours after
domestic gas combustion began, when there was no ventilation, but only
13.4 mg/m3 (11.6 ppm) when ventilation was provided. The use of
improved stoves resulted in better conditions (Sidorenko et al.,
1972).
a "Argirova, M. [Atmospheric air pollution by a plant processing
plastics.] In: Proceedings of the First National Conference on
Sanitary Chemistry of the Air, Sofia, Bulgaria, 26-27 November,
1974 (in Bulgarian).
Rench & Savage (1976) have also investigated winter levels of
carbon monoxide in the home. Outdoor concentrations were lower than
those found indoors. Age of building, type of appliance, heating
sources, and socio-economic status were statistically significantly
related to indoor levels of carbon monoxide, higher levels occurring
in the kitchens of old houses and in homes belonging to families in
lower income groups. Levels were also higher in kitchens with space
wall heaters compared with those with forced air, gravity feed, or hot
water heating systems. Places of recreation may be problem areas.
Excessive levels of carbon monoxide were found in ice-skating areas
where ice-resurfacing machines were used. Levels as high as
348 mg/m3 (304 ppm) were found in such an arena after complaints of
illness among children skating there were reported to the local health
department (Johnson et al., 1975). Improperly regulated space heaters
in such premises could also produce high concentrations of carbon
monoxide. This was recently reported to have occurred in an Alaskan
ice-skating arena.
That outdoor concentrations of carbon monoxide can sometimes
strongly influence indoor levels was illustrated in a study conducted
in New York City (Lee, 1972). As shown in Fig. 3, the concentrations
inside and outside an apartment building followed the same general
pattern and were closely related to nearby traffic flows.
Relatively high concentrations of carbon monoxide have also been
observed inside the passenger compartment of motor vehicles (Borst,
1970). Carbon monoxide may enter the compartment from faulty or
damaged exhaust systems or from the surrounding air, in road traffic.
The carbon monoxide level in the compartment is often higher than that
found outside the vehicle. Haagen-Smit (1966) found an average
concentration in a vehicle passenger compartment of 42 mg/m3
(36.5 ppm) on a Los Angeles freeway during the rush hour traffic
(higher concentrations have been reported by Aronow et al. (1972).
It should be emphasized that cigarette smoking is the most common
source of carbon monoxide for the general population (Table 5).
Exposure to smoking primarily affects the carboxyhaemoglobin level
of the smoker himself (Kahn et al., 1974; Landaw, 1973). In some
circumstances, such as poorly ventilated enclosed spaces, the tobacco
smoke may affect all of the occupants (section 8.1.6).
 Table 5. Percentage carboxyhaemoglobin levels in smokers and
nonsmokersa
Description Nonsmokers Smokers
mean range mean range
UK pregnant women 1.1 3.6
Meat porters 1.6 5.1
Office workers 1.3 6.2
London office workers 1.12 0.1-2.7 5.5 2.2-13.0
29 000 USA blood donors 1.39 0.4-6.9 5.57 0.8-11.9
3311 California longshoremen 1.3 5.9
Munich population 2.36 7.38
Rural Bavarians 1.03 6.06
a From: Wakeham (1976).
5.3 Occupational Exposure
Many occupational groups are subject to high carbon monoxide
exposure. These include, traffic policemen, garage personnel, workers
in the metallurgical, petroleum, gas, and chemical industries, and
firefighters.
An average carbon monoxide level of 172 mg/m3 (149.6 ppm) was
recorded in the air of a Paris police garage between 07h30 and 08h00
and 205 mg/m3 (179 ppm) between 19h30 and 20h00 (Chovin, 1967).
Similarly, Trompeo et al. (1964) found an average level of 112 mg/m3
(97.4 ppm) in 12 underground garages in Rome; a maximum concentration
of 570 mg/m3 (498.5 ppm) was recorded. In similar studies in
enclosed garages with capacities of 300-500 vehicles, where the only
ventilation was via the entrance, the average concentration of carbon
monoxide for a period from 08h00 to 17h00 was 72 mg/m3 (62.6 ppm) in
the summer and 61 mg/m3 (53 ppm) in the winter (Ramsey, 1967a).
The same author (Ramsey, 1967b) reported that the carboxyhaemo-
globin levels of 14 nonsmoking parking garage employees, exposed to an
average concentration of carbon monoxide of 68 mg/m3 (59 ppm),
increased from 1.5 to 7.3%. He also noted that, in smokers exposed to
the same environment, initial carboxyhaemoglobin levels of 2.9% rose
to 9.3% at the end of the day. Smokers not exposed to this environment
had final levels of only 3.9%. Ramsey stated that occupational
exposure played a greater role than smoking in increasing
carboxyhaemoglobin levels. However, in a study of some 350 Canadian
garage and service station personnel, Buchwald (1969) found that
cigarette smoking was the more significant contributor to the high
levels of carboxyhaemoglobin measured. Of the smokers, 70% had levels
in excess of 5%, while a similar level was found in only 30% of the
nonsmokers. Breysse & Bovee (1969) used expired air samples to
determine exposure to carbon monoxide in stevedores, gasoline-powered
lift truck drivers, and winch operators. They made some 700 estimates
of carboxyhaemoglobin, almost 6% of which exceeded 10%. Seven percent
of the stevedores and 18% of the lift truck operators had
carboxyhaemoglobin levels over 10%. Smoking contributed substantially
to the attainment of these high levels. Carboxyhaemoglobin levels as
high as 10% were also found in dock workers in studies by Petrov
(1968). Inspectors at USA-Mexico border crossing stations were found
to be exposed to ambient levels of carbon monoxide that fluctuated
between 6 and 195 mg/m3 (5 and 170.5 ppm). During one hour of an
evening shift, ambient carbon monoxide averaged 131 mg/m3 (114 ppm).
Carboxyhaemoglobin levels of smokers and nonsmokers which were 4.0 and
1.4% respectively, prior to duty rose to 7.6 and 3.8% (Cohen et al.,
1971).
Data obtained by Balabaeva & Kalpazanov (1974) in studies on
traffic policemen in 4 large towns in Bulgaria were similar to those
Chovin (1967) obtained in his study on the exposure of Paris policemen
to carbon monoxide. However, Göthe et al. (1969) found relatively low
levels of carboxyhaemoglobin in Swedish traffic policemen. When blood
lead levels were studied in 50 London taxi drivers using their
carboxyhaemoglobin levels as an index of exposure to exhaust products,
carboxyhaemoglobin levels were higher in day drivers than in night
drivers and in smokers than in nonsmokers. Concentrations in all
groups ranged from 0.4 to 9.7%, and those in nonsmokers (day and
night) from 0.4 to 3.0%.
Direct measurement of carboxyhaemoglobin levels in firefighters
engaged in prolonged firefighting indicated that 10% had values
exceeding 10% (Gordon & Rogers, 1969). However, the high levels of
carboxyhaemoglobin found in the control (non-firefighting) groups make
these conclusions somewhat uncertain. Goldsmith's study (1970) of
longshoremen suggested that the expired alveolar concentrations of
carbon monoxide in smokers were age-related. For example, subjects
smoking one packet of 20 cigarettes per day in the 45-54 year age
group had an average alveolar value of 31 mg/m3 (27 ppm), while the
75-84 year age group had an average of only 16 mg/m3 (14.4 ppm).
Nonsmokers did not exhibit this age-related pattern, since even up to
84 years of age, alveolar concentrations remained at the same level
4 mg/m3 (3.6 ppm).
Exposure to low levels of carbon monoxide may have a significant
influence on the health and efficiency of these workers but this
awaits further study. Many other instances of occupational exposure
are available but most studies are complicated by the unknown or
unreported smoking habits of the workers under study (Grut, 1949).
5.4 Carboxyhaemoglobin Levels in the General Population
The most extensive study of blood carboxyhaemoglobin levels in the
general population was carried out by Stewart and his associates
(1973c and 1974), who took blood samples in 18 urban areas and in some
small towns in the States of New Hampshire and Vermont, USA. Similar
blood samples were evaluated for carboxyhaemoglobin levels by Kahn et
al. (1974), Davis & Gantner (1974), and Wallace et al. (1974) in
metropolitan St Louis. A total of 45 649 blood donors provided blood
for analysis. Stewart's subjects (29 000 including 1016 from St Louis)
were studied in March 1971 and Kahn's (16 649 subjects all from St
Louis) from October 1971 to October 1972. Stewart et al. concluded
that 45% of all the non-smoking donors exposed to ambient carbon
monoxide had a carboxyhaemoglobin saturation greater than 1.5%, while
Kahn's group reported a level of less than 1%.
6. METABOLISM
The primary factors that determine the final level of
carboxyhaemoglobin are: the amount of inspired carbon monoxide; minute
alveolar ventilation at rest and during exercise; endogenous carbon
monoxide production; blood volume; barometric pressure; and the
relative diffusion capability of the lungs. The rate of diffusion from
the alveoli and the binding of carbon monoxide with the blood
haemoglobin are the steps limiting the rate of uptake into the blood.
6.1 Endogenous Carbon Monoxide Production
Carbon monoxide can be produced endogenously from the catabolism
of pyrrole rings, originating from haemoglobin, myoglobin,
cytochromes, and other haem-containing pigments. Haem catabolism is
the main source of endogenous carbon monoxide production, but recent
in vitro investigations suggest additional endogenous sources, e.g.,
lipid peroxidation (Wolff & Bidlack, 1976).
The endogenous carboxyhaemoglobin level in man is estimated to be
about 0.1-1.0%. Increased production of endogenous carbon monoxide has
been found in haemolytic anaemias (Coburn et al., 1966), and could be
expected in haematomas and after exposure to certain toxic chemicals
capable of causing haemolysis. The liver is probably the major source
of endogenous carbon monoxide as a consequence of an increase in liver
cytochromes induced by certain drugs (Coburn, 1970a), or in porphyria
cutanea tarda (acquired or symptomatic porphyria) (White, 1970).
Similarly, bone marrow can become a major source of carbon monoxide in
haematological diseases, such as sideroblastic anaemia, being
characterized by ineffective erythropoiesis (White, 1970). In neonates
endogenous carbon monoxide can be markedly elevated, as well as in
females during the progesterone phase of the menstrual cycle
(Delivoria-Papadopoulos et al., 1970; Longo, 1970), and even more so
during pregnancy (see section 8.2.3). Another important cause of
carboxyhaemoglobin elevation is exposure to several methane-derived
halogenated hydrocarbons (see section 8.1.5).
6.2 Absorption
The classical absorption curves of Forbes et al. (1945) have been
re-evaluated for man at rest by Peterson & Stewart (1970) who exposed
volunteers to a variety of different concentrations of carbon monoxide
for periods ranging from 0.5 to 24 h. Using a regression approach,
they derived the following empirical relationship for blood carboxy-
haemoglobin as a function of ambient carbon monoxide concentration
and exposure time:
log10 y = 0.85753 log10 x + 0.62995 log10 t - 2.29519
where y = % carboxyhaemoglobin
x = carbon monoxide concentration in ppm
t = time in minutes
Although these new data come closer to presenting potential uptake in
individuals exposed to present-day ambient concentrations of carbon
monoxide, they do not apply to the uptake that would occur in active
man. Furthermore, they fit only the linear, non-steady-state portion
of the absorption curve. Ott & Mage (1974) have objected to the
Peterson & Stewart equation as being basically a static model. They
indicate that the use of averaging periods as long as 1 h compared
with 10-15 min introduces an error into recorded urban concentrations.
This error may be serious if many sharp, ambient peaks are present.
The data presented by Forbes et al. (1945) are still the only
experimental information available that takes ventilation into account
and, even so, this information is inadequate since the full range of
inspired, ventilatory volumes possible in exercising man was not
considered. Coburn et al. (1965) have developed an equation from which
it is possible to calculate blood carboxyhaemoglobin (Peterson &
Stewart, 1970) as a function of time, considering appropriate
physiological and physical factors. Their basic differential equation
is as follows:
d(CO) [HbCO] pCo2 1 pIco
= Vco - × × +
dt [HbO2] M 1 pB - 47 1 pB - 47
+ +
DL VA DL VA
d(CO)
where is the rate of change of CO in the body (ml/min)
dt
[HbCO] is the concentration of CO in the blood (ml CO/ml blood)
[HbO2] is the concentration of O2 in the blood (ml O2/ml blood)
DL is the diffusion capacity of the lung (ml/min/mmHg)
VA is the alveolar ventilation rate (ml/min)
pB is barometric pressure (mmHg)
pIco is inspired carbon monoxide pressure (mmHg)
pCo2 is the mean pulmonary capillary oxygen pressure (mmHg)
M is the Haldane constant (220-240 at pH 7.4)
Vco is the rate of endogenous CO production (ml/min)
One solution of the equation depends on the assumption that
pCo2, and HbO2 are constant and independent of HbCO. However,
HbO2 concentration depends upon HbCO in a complex way and, in
general, solution of the equation requires some special computer
techniques using a second approximation; these have been attempted and
general solutions are available (Coburn et al., 1965). Further
development of Coburn's concepts will undoubtedly improve the basis on
which theoretical uptakes can be calculated. For immediate, practical
purposes, calculations based on the Haldane formula (see section 6.3)
can be used.
Table 6 indicates the equilibrium percentage saturation of the
haemoglobin with carbon monoxide at various alveolar pressures of
carbon monoxide, calculated from the Haldane formula:a
% HbCO 230 pAco
=
% HbO2 pAo2
where pAco and pAo2, are the alveolar pressures of carbon
monoxide and oxygen respectively.
Table 6. Percent carboxyhaemoglobin versus carbon monoxide alveolar pressure
% HbCO mg/m3 ppm % in air Pa Torr
0.87 5.7 5 0.0005 0.506 0.0038
1.73 11.5 10 0.001 1.013 0.0076
3.45 23.0 20 0.002 2.026 0.0152
5.05 34.5 30 0.003 3.305 0.0248
6.63 46.0 40 0.004 4.052 0.0304
8.16 57.5 50 0.005 5.065 0.0380
9.63 69.0 60 0.006 6.078 0.0456
11.08 80 70 0.007 7.091 0.0532
12.46 92.0 80 0.008 8.104 0.0608
13.80 103.0 90 0.009 9.117 0.0684
15.11 114.5 100 0.010 10.130 0.0760
16.37 126.0 110 0.011 11.143 0.0836
17.60 130.0 120 0.012 12.156 0.0912
18.78 149.0 130 0.013 13.170 0.0988
19.95 160.0 140 0.014 14.183 0.1064
21.05 172.0 150 0.015 15.196 0.1140
22.15 183.0 160 0.016 16.209 0.1216
23.23 195.0 170 0.017 17.209 0.1291
24.26 206.0 180 0.018 18.235 0.1368
25.25 218.0 190 0.019 19.221 0.1442
26.22 229.0 200 0.020 20.261 0.1520
a The alveolar oxygen pressure is assumed to be 13 kPa (98 Torr)
6.3 Reactions with Body Tissues and Fluids
An adequate oxygen supply to maintain tissue metabolism is
provided by the integrated functioning of the respiratory and
cardiovascular systems to transport oxygen from the ambient air to the
various tissues of the body. Nearly all of the oxygen, except that
dissolved in plasma, is bound reversibly to the haemoglobin contained
within the erythrocytes. The most significant chemical characteristic
of carbon monoxide is that it also is reversibly bound by haemoglobin.
Therefore, it is a competitor with oxygen for the four binding sites
on the haemoglobin molecule.
The equilibrium constant M expresses the relative affinity of
haemoglobin for carbon monoxide and oxygen when the concentration of
reduced haemoglobin is minimal. This Haldane constant (Douglas et al.,
1912) is defined by the following equation:
HbCO pCO
= M x
HbO2 pO2
where pCO and pO2 represent the equilibrium partial gas
pressures: each pressure being the same in the erythrocytes or
haemoglobin solution as in the equilibrated gas phase. [HbCO] and
[HbO2] are the concentrations of carboxyhaemoglobin and
oxyhaemoglobin, respectively. The value of M is about 200 in most
species, in spite of the fact that carbon monoxide combines with
haemoglobin more slowly than oxygen. Carboxyhaemoglobin dissociates
very slowly due to the tight binding of carbon monoxide to
haemoglobin. Technically, it is not possible to measure the rate of
dissociation of carbon monoxide from partly saturated haemoglobin. The
dissociation velocity constant has been measured by only a few
investigators on sheep and human haemoglobin fully saturated with
carbon monoxide. Roughton (1970), however, has presented the most
comprehensive analysis of the interaction of carbon monoxide with
erythrocyte haemoglobin, showing clearly that, for man, M is higher
than commonly thought, i.e., it is more likely to be between 240 and
250; however, the value for M depends on the point of reference on
the dissociation curve.
Solution of Haldane's equation would give an approximate level of
carboxyhaemoglobin, e.g., exposure to ambient air containing carbon
monoxide levels of 28.7, 57.5, or 115 mg/m3 (25, 50, or 100 ppm)
would lead to carboxyhaemoglobin saturations of approximately 4.8, 9.2
and 16.3%, if the arterial oxygen pressure were 10.7 kPa (80 Torr).
The carbon monoxide enters the lungs with each breath and diffuses
across the alveolar-capillary membrane in a manner similar to oxygen.
If air with a constant concentration of carbon monoxide is breathed
for several hours, the rate of uptake of carbon monoxide decreases
approximately exponentially until an equilibrium state is attained in
which the partial pressure of carbon monoxide in the pulmonary
capillary blood is the same as that in the alveolar.
Oxygen transport in the blood is best described by the
oxyhaemoglobin dissociation curve (Fig. 4). In the presence of
carboxyhaemoglobin, this curve is no longer typically sigmoid but is
shifted to the left so that a lower oxygen pressure is present for the
same oxyhaemoglobin saturation compared with blood without
carboxyhaemoglobin (Roughton & Darling, 1944). Fig. 5 illustrates the
extent of the Haldane shift to the left more clearly than the typical
curves of Fig. 4. Mulhausen et al. (1968) illustrated this shift by
showing that the half saturation oxygen tension shifted from 3.6 to
3.1 kPa (26.7 to 23.2 Torr) in subjects who were intermittently
exposed to a high concentration of ambient carbon monoxide. Carbon
monoxide not only diminishes the total amount of oxygen available by
direct replacement of oxygen (Fig. 4) but also alters the dissociation
of the remaining oxygen so that it is held more tenaciously by
haemoglobin and released at lower oxygen tensions. The oxyhaemoglobin
curve in the presence of carboxyhaemoglobin progressively resembles
the simple oxygen dissociation curve of myoglobin. Myoglobin is a haem
compound with only one haem unit per molecule and does not exhibit
haem-haem interactions. It is possible that the combination of one or
more of the four haem groups in haemoglobin with carbon monoxide
decreases the haem-haem interactions of the remaining haem units and
results in a molecule approaching the behaviour of myoglobin.
Any consideration of the toxicity of carbon monoxide must include
not only the decrease in the oxygen carrying capacity of haemoglobin
but also the interference with oxygen release at the tissue level.
The venous pO2 values expected to result from various
carboxyhaemoglobin levels can be calculated (Forster, 1970; Permutt &
Fahri, 1969). If blood flow and metabolic rate remain constant,
equilibration with an ambient carbon monoxide concentration of
230 mg/m3 or 200 ppm (25% carboxyhaemoglobin) will lower venous
pO2 from 5.3 to less than 4 kPa (40 to less than 30 Torr). A
similar degree of venous hypoxaemia results from an ascent to an
altitude of 3658 metres or a 35% reduction in oxygen capacity in an
anaemic patient. It can also be calculated that, at 5%
carboxyhaemoglobin, there will be only a slight drop in the mixed
venous pO2. Even more valuable relationships can be obtained by
plotting oxygen content against the partial pressure of oxygen
(Roughton & Darling, 1944). The difference in oxygen content at
various percentages of carboxyhaemoglobin from 0 to 20 reveal the
minimal magnitude of the relative unavailability of oxygen due to the
Haldane effect (Fig. 4). It was this evaluation that led Roughton &
Darling to conclude that carboxyhaemoglobin concentrations of less
than 40% produce relatively easily compensated restrictions in the
amount of oxygen available for tissue delivery. This can only be
applied to subjects with normal respiratory and circulatory systems.
Table 5. Percentage carboxyhaemoglobin levels in smokers and
nonsmokersa
Description Nonsmokers Smokers
mean range mean range
UK pregnant women 1.1 3.6
Meat porters 1.6 5.1
Office workers 1.3 6.2
London office workers 1.12 0.1-2.7 5.5 2.2-13.0
29 000 USA blood donors 1.39 0.4-6.9 5.57 0.8-11.9
3311 California longshoremen 1.3 5.9
Munich population 2.36 7.38
Rural Bavarians 1.03 6.06
a From: Wakeham (1976).
5.3 Occupational Exposure
Many occupational groups are subject to high carbon monoxide
exposure. These include, traffic policemen, garage personnel, workers
in the metallurgical, petroleum, gas, and chemical industries, and
firefighters.
An average carbon monoxide level of 172 mg/m3 (149.6 ppm) was
recorded in the air of a Paris police garage between 07h30 and 08h00
and 205 mg/m3 (179 ppm) between 19h30 and 20h00 (Chovin, 1967).
Similarly, Trompeo et al. (1964) found an average level of 112 mg/m3
(97.4 ppm) in 12 underground garages in Rome; a maximum concentration
of 570 mg/m3 (498.5 ppm) was recorded. In similar studies in
enclosed garages with capacities of 300-500 vehicles, where the only
ventilation was via the entrance, the average concentration of carbon
monoxide for a period from 08h00 to 17h00 was 72 mg/m3 (62.6 ppm) in
the summer and 61 mg/m3 (53 ppm) in the winter (Ramsey, 1967a).
The same author (Ramsey, 1967b) reported that the carboxyhaemo-
globin levels of 14 nonsmoking parking garage employees, exposed to an
average concentration of carbon monoxide of 68 mg/m3 (59 ppm),
increased from 1.5 to 7.3%. He also noted that, in smokers exposed to
the same environment, initial carboxyhaemoglobin levels of 2.9% rose
to 9.3% at the end of the day. Smokers not exposed to this environment
had final levels of only 3.9%. Ramsey stated that occupational
exposure played a greater role than smoking in increasing
carboxyhaemoglobin levels. However, in a study of some 350 Canadian
garage and service station personnel, Buchwald (1969) found that
cigarette smoking was the more significant contributor to the high
levels of carboxyhaemoglobin measured. Of the smokers, 70% had levels
in excess of 5%, while a similar level was found in only 30% of the
nonsmokers. Breysse & Bovee (1969) used expired air samples to
determine exposure to carbon monoxide in stevedores, gasoline-powered
lift truck drivers, and winch operators. They made some 700 estimates
of carboxyhaemoglobin, almost 6% of which exceeded 10%. Seven percent
of the stevedores and 18% of the lift truck operators had
carboxyhaemoglobin levels over 10%. Smoking contributed substantially
to the attainment of these high levels. Carboxyhaemoglobin levels as
high as 10% were also found in dock workers in studies by Petrov
(1968). Inspectors at USA-Mexico border crossing stations were found
to be exposed to ambient levels of carbon monoxide that fluctuated
between 6 and 195 mg/m3 (5 and 170.5 ppm). During one hour of an
evening shift, ambient carbon monoxide averaged 131 mg/m3 (114 ppm).
Carboxyhaemoglobin levels of smokers and nonsmokers which were 4.0 and
1.4% respectively, prior to duty rose to 7.6 and 3.8% (Cohen et al.,
1971).
Data obtained by Balabaeva & Kalpazanov (1974) in studies on
traffic policemen in 4 large towns in Bulgaria were similar to those
Chovin (1967) obtained in his study on the exposure of Paris policemen
to carbon monoxide. However, Göthe et al. (1969) found relatively low
levels of carboxyhaemoglobin in Swedish traffic policemen. When blood
lead levels were studied in 50 London taxi drivers using their
carboxyhaemoglobin levels as an index of exposure to exhaust products,
carboxyhaemoglobin levels were higher in day drivers than in night
drivers and in smokers than in nonsmokers. Concentrations in all
groups ranged from 0.4 to 9.7%, and those in nonsmokers (day and
night) from 0.4 to 3.0%.
Direct measurement of carboxyhaemoglobin levels in firefighters
engaged in prolonged firefighting indicated that 10% had values
exceeding 10% (Gordon & Rogers, 1969). However, the high levels of
carboxyhaemoglobin found in the control (non-firefighting) groups make
these conclusions somewhat uncertain. Goldsmith's study (1970) of
longshoremen suggested that the expired alveolar concentrations of
carbon monoxide in smokers were age-related. For example, subjects
smoking one packet of 20 cigarettes per day in the 45-54 year age
group had an average alveolar value of 31 mg/m3 (27 ppm), while the
75-84 year age group had an average of only 16 mg/m3 (14.4 ppm).
Nonsmokers did not exhibit this age-related pattern, since even up to
84 years of age, alveolar concentrations remained at the same level
4 mg/m3 (3.6 ppm).
Exposure to low levels of carbon monoxide may have a significant
influence on the health and efficiency of these workers but this
awaits further study. Many other instances of occupational exposure
are available but most studies are complicated by the unknown or
unreported smoking habits of the workers under study (Grut, 1949).
5.4 Carboxyhaemoglobin Levels in the General Population
The most extensive study of blood carboxyhaemoglobin levels in the
general population was carried out by Stewart and his associates
(1973c and 1974), who took blood samples in 18 urban areas and in some
small towns in the States of New Hampshire and Vermont, USA. Similar
blood samples were evaluated for carboxyhaemoglobin levels by Kahn et
al. (1974), Davis & Gantner (1974), and Wallace et al. (1974) in
metropolitan St Louis. A total of 45 649 blood donors provided blood
for analysis. Stewart's subjects (29 000 including 1016 from St Louis)
were studied in March 1971 and Kahn's (16 649 subjects all from St
Louis) from October 1971 to October 1972. Stewart et al. concluded
that 45% of all the non-smoking donors exposed to ambient carbon
monoxide had a carboxyhaemoglobin saturation greater than 1.5%, while
Kahn's group reported a level of less than 1%.
6. METABOLISM
The primary factors that determine the final level of
carboxyhaemoglobin are: the amount of inspired carbon monoxide; minute
alveolar ventilation at rest and during exercise; endogenous carbon
monoxide production; blood volume; barometric pressure; and the
relative diffusion capability of the lungs. The rate of diffusion from
the alveoli and the binding of carbon monoxide with the blood
haemoglobin are the steps limiting the rate of uptake into the blood.
6.1 Endogenous Carbon Monoxide Production
Carbon monoxide can be produced endogenously from the catabolism
of pyrrole rings, originating from haemoglobin, myoglobin,
cytochromes, and other haem-containing pigments. Haem catabolism is
the main source of endogenous carbon monoxide production, but recent
in vitro investigations suggest additional endogenous sources, e.g.,
lipid peroxidation (Wolff & Bidlack, 1976).
The endogenous carboxyhaemoglobin level in man is estimated to be
about 0.1-1.0%. Increased production of endogenous carbon monoxide has
been found in haemolytic anaemias (Coburn et al., 1966), and could be
expected in haematomas and after exposure to certain toxic chemicals
capable of causing haemolysis. The liver is probably the major source
of endogenous carbon monoxide as a consequence of an increase in liver
cytochromes induced by certain drugs (Coburn, 1970a), or in porphyria
cutanea tarda (acquired or symptomatic porphyria) (White, 1970).
Similarly, bone marrow can become a major source of carbon monoxide in
haematological diseases, such as sideroblastic anaemia, being
characterized by ineffective erythropoiesis (White, 1970). In neonates
endogenous carbon monoxide can be markedly elevated, as well as in
females during the progesterone phase of the menstrual cycle
(Delivoria-Papadopoulos et al., 1970; Longo, 1970), and even more so
during pregnancy (see section 8.2.3). Another important cause of
carboxyhaemoglobin elevation is exposure to several methane-derived
halogenated hydrocarbons (see section 8.1.5).
6.2 Absorption
The classical absorption curves of Forbes et al. (1945) have been
re-evaluated for man at rest by Peterson & Stewart (1970) who exposed
volunteers to a variety of different concentrations of carbon monoxide
for periods ranging from 0.5 to 24 h. Using a regression approach,
they derived the following empirical relationship for blood carboxy-
haemoglobin as a function of ambient carbon monoxide concentration
and exposure time:
log10 y = 0.85753 log10 x + 0.62995 log10 t - 2.29519
where y = % carboxyhaemoglobin
x = carbon monoxide concentration in ppm
t = time in minutes
Although these new data come closer to presenting potential uptake in
individuals exposed to present-day ambient concentrations of carbon
monoxide, they do not apply to the uptake that would occur in active
man. Furthermore, they fit only the linear, non-steady-state portion
of the absorption curve. Ott & Mage (1974) have objected to the
Peterson & Stewart equation as being basically a static model. They
indicate that the use of averaging periods as long as 1 h compared
with 10-15 min introduces an error into recorded urban concentrations.
This error may be serious if many sharp, ambient peaks are present.
The data presented by Forbes et al. (1945) are still the only
experimental information available that takes ventilation into account
and, even so, this information is inadequate since the full range of
inspired, ventilatory volumes possible in exercising man was not
considered. Coburn et al. (1965) have developed an equation from which
it is possible to calculate blood carboxyhaemoglobin (Peterson &
Stewart, 1970) as a function of time, considering appropriate
physiological and physical factors. Their basic differential equation
is as follows:
d(CO) [HbCO] pCo2 1 pIco
= Vco - × × +
dt [HbO2] M 1 pB - 47 1 pB - 47
+ +
DL VA DL VA
d(CO)
where is the rate of change of CO in the body (ml/min)
dt
[HbCO] is the concentration of CO in the blood (ml CO/ml blood)
[HbO2] is the concentration of O2 in the blood (ml O2/ml blood)
DL is the diffusion capacity of the lung (ml/min/mmHg)
VA is the alveolar ventilation rate (ml/min)
pB is barometric pressure (mmHg)
pIco is inspired carbon monoxide pressure (mmHg)
pCo2 is the mean pulmonary capillary oxygen pressure (mmHg)
M is the Haldane constant (220-240 at pH 7.4)
Vco is the rate of endogenous CO production (ml/min)
One solution of the equation depends on the assumption that
pCo2, and HbO2 are constant and independent of HbCO. However,
HbO2 concentration depends upon HbCO in a complex way and, in
general, solution of the equation requires some special computer
techniques using a second approximation; these have been attempted and
general solutions are available (Coburn et al., 1965). Further
development of Coburn's concepts will undoubtedly improve the basis on
which theoretical uptakes can be calculated. For immediate, practical
purposes, calculations based on the Haldane formula (see section 6.3)
can be used.
Table 6 indicates the equilibrium percentage saturation of the
haemoglobin with carbon monoxide at various alveolar pressures of
carbon monoxide, calculated from the Haldane formula:a
% HbCO 230 pAco
=
% HbO2 pAo2
where pAco and pAo2, are the alveolar pressures of carbon
monoxide and oxygen respectively.
Table 6. Percent carboxyhaemoglobin versus carbon monoxide alveolar pressure
% HbCO mg/m3 ppm % in air Pa Torr
0.87 5.7 5 0.0005 0.506 0.0038
1.73 11.5 10 0.001 1.013 0.0076
3.45 23.0 20 0.002 2.026 0.0152
5.05 34.5 30 0.003 3.305 0.0248
6.63 46.0 40 0.004 4.052 0.0304
8.16 57.5 50 0.005 5.065 0.0380
9.63 69.0 60 0.006 6.078 0.0456
11.08 80 70 0.007 7.091 0.0532
12.46 92.0 80 0.008 8.104 0.0608
13.80 103.0 90 0.009 9.117 0.0684
15.11 114.5 100 0.010 10.130 0.0760
16.37 126.0 110 0.011 11.143 0.0836
17.60 130.0 120 0.012 12.156 0.0912
18.78 149.0 130 0.013 13.170 0.0988
19.95 160.0 140 0.014 14.183 0.1064
21.05 172.0 150 0.015 15.196 0.1140
22.15 183.0 160 0.016 16.209 0.1216
23.23 195.0 170 0.017 17.209 0.1291
24.26 206.0 180 0.018 18.235 0.1368
25.25 218.0 190 0.019 19.221 0.1442
26.22 229.0 200 0.020 20.261 0.1520
a The alveolar oxygen pressure is assumed to be 13 kPa (98 Torr)
6.3 Reactions with Body Tissues and Fluids
An adequate oxygen supply to maintain tissue metabolism is
provided by the integrated functioning of the respiratory and
cardiovascular systems to transport oxygen from the ambient air to the
various tissues of the body. Nearly all of the oxygen, except that
dissolved in plasma, is bound reversibly to the haemoglobin contained
within the erythrocytes. The most significant chemical characteristic
of carbon monoxide is that it also is reversibly bound by haemoglobin.
Therefore, it is a competitor with oxygen for the four binding sites
on the haemoglobin molecule.
The equilibrium constant M expresses the relative affinity of
haemoglobin for carbon monoxide and oxygen when the concentration of
reduced haemoglobin is minimal. This Haldane constant (Douglas et al.,
1912) is defined by the following equation:
HbCO pCO
= M x
HbO2 pO2
where pCO and pO2 represent the equilibrium partial gas
pressures: each pressure being the same in the erythrocytes or
haemoglobin solution as in the equilibrated gas phase. [HbCO] and
[HbO2] are the concentrations of carboxyhaemoglobin and
oxyhaemoglobin, respectively. The value of M is about 200 in most
species, in spite of the fact that carbon monoxide combines with
haemoglobin more slowly than oxygen. Carboxyhaemoglobin dissociates
very slowly due to the tight binding of carbon monoxide to
haemoglobin. Technically, it is not possible to measure the rate of
dissociation of carbon monoxide from partly saturated haemoglobin. The
dissociation velocity constant has been measured by only a few
investigators on sheep and human haemoglobin fully saturated with
carbon monoxide. Roughton (1970), however, has presented the most
comprehensive analysis of the interaction of carbon monoxide with
erythrocyte haemoglobin, showing clearly that, for man, M is higher
than commonly thought, i.e., it is more likely to be between 240 and
250; however, the value for M depends on the point of reference on
the dissociation curve.
Solution of Haldane's equation would give an approximate level of
carboxyhaemoglobin, e.g., exposure to ambient air containing carbon
monoxide levels of 28.7, 57.5, or 115 mg/m3 (25, 50, or 100 ppm)
would lead to carboxyhaemoglobin saturations of approximately 4.8, 9.2
and 16.3%, if the arterial oxygen pressure were 10.7 kPa (80 Torr).
The carbon monoxide enters the lungs with each breath and diffuses
across the alveolar-capillary membrane in a manner similar to oxygen.
If air with a constant concentration of carbon monoxide is breathed
for several hours, the rate of uptake of carbon monoxide decreases
approximately exponentially until an equilibrium state is attained in
which the partial pressure of carbon monoxide in the pulmonary
capillary blood is the same as that in the alveolar.
Oxygen transport in the blood is best described by the
oxyhaemoglobin dissociation curve (Fig. 4). In the presence of
carboxyhaemoglobin, this curve is no longer typically sigmoid but is
shifted to the left so that a lower oxygen pressure is present for the
same oxyhaemoglobin saturation compared with blood without
carboxyhaemoglobin (Roughton & Darling, 1944). Fig. 5 illustrates the
extent of the Haldane shift to the left more clearly than the typical
curves of Fig. 4. Mulhausen et al. (1968) illustrated this shift by
showing that the half saturation oxygen tension shifted from 3.6 to
3.1 kPa (26.7 to 23.2 Torr) in subjects who were intermittently
exposed to a high concentration of ambient carbon monoxide. Carbon
monoxide not only diminishes the total amount of oxygen available by
direct replacement of oxygen (Fig. 4) but also alters the dissociation
of the remaining oxygen so that it is held more tenaciously by
haemoglobin and released at lower oxygen tensions. The oxyhaemoglobin
curve in the presence of carboxyhaemoglobin progressively resembles
the simple oxygen dissociation curve of myoglobin. Myoglobin is a haem
compound with only one haem unit per molecule and does not exhibit
haem-haem interactions. It is possible that the combination of one or
more of the four haem groups in haemoglobin with carbon monoxide
decreases the haem-haem interactions of the remaining haem units and
results in a molecule approaching the behaviour of myoglobin.
Any consideration of the toxicity of carbon monoxide must include
not only the decrease in the oxygen carrying capacity of haemoglobin
but also the interference with oxygen release at the tissue level.
The venous pO2 values expected to result from various
carboxyhaemoglobin levels can be calculated (Forster, 1970; Permutt &
Fahri, 1969). If blood flow and metabolic rate remain constant,
equilibration with an ambient carbon monoxide concentration of
230 mg/m3 or 200 ppm (25% carboxyhaemoglobin) will lower venous
pO2 from 5.3 to less than 4 kPa (40 to less than 30 Torr). A
similar degree of venous hypoxaemia results from an ascent to an
altitude of 3658 metres or a 35% reduction in oxygen capacity in an
anaemic patient. It can also be calculated that, at 5%
carboxyhaemoglobin, there will be only a slight drop in the mixed
venous pO2. Even more valuable relationships can be obtained by
plotting oxygen content against the partial pressure of oxygen
(Roughton & Darling, 1944). The difference in oxygen content at
various percentages of carboxyhaemoglobin from 0 to 20 reveal the
minimal magnitude of the relative unavailability of oxygen due to the
Haldane effect (Fig. 4). It was this evaluation that led Roughton &
Darling to conclude that carboxyhaemoglobin concentrations of less
than 40% produce relatively easily compensated restrictions in the
amount of oxygen available for tissue delivery. This can only be
applied to subjects with normal respiratory and circulatory systems.

 The small reductions in oxygen content at 5-10% carboxyhaemoglobin may
be quite critical for patients suffering from cardiovascular diseases
or chronic obstructive lung disease. Coburn et al. (1965) published a
detailed theoretical analysis of the physiology and variables that
determine blood carboxyhaemoglobin levels in man. The details of the
formulae used in these calculations are presented in section 6.2.
With regard to the intracellular effects of carbon monoxide,
consideration must be given to the interactions of all substances
within the tissue cells that are involved with oxygen delivery. Since
haemoglobin and myoglobin are structurally related, they react with
carbon monoxide in a similar manner. The function of myoglobin, in
vivo, may be to act as a reservoir for oxygen within the muscle
fibre. The carbon monoxide and oxygen equilibria of human myoglobin
has been studied in vitro and a hyperbolic oxygen dissociation curve
established (Rossi-Fanelli & Antonini, 1958). This curve, unlike that
for haemoglobin, is not affected by the hydrogen ion concentration,
the ionic strength, or the concentration of myoglobin. The relative
affinity constant, M, is approximately 40 but is still sufficient to
induce appreciable formation of carboxymyoglobin. Both Coburn et al.
(1970b, 1973) and Luomanmaki (1966) have studied the interrelation-
ships between carboxyhaemoglobin and carboxymyoglobin. Coburn, using
14CO, showed that identical carbon monoxide exposures can produce
different degrees of saturation of haemoglobin, depending upon the
partial pressures of oxygen in blood and tissue. Coburn (1970b)
determined the ratio of the carbon monoxide content in muscle to the
content in blood as a function of arterial pO2. This ratio, for
skeletal muscle, is approximately 1, but in myocardial tissue it was
found to be 3. When arterial pO2 fell below 5.3-4 kPa (40-30
Torr), carbon monoxide disappeared from the blood, presumably entering
the muscle. Considerable amounts of extravascular carbon monoxide are
stored in muscle. The higher ratio for cardiac tissue may be of
considerable significance. In an individual with a blood
carboxyhaemoglobin level of 10%, some 30% of cardiac myoglobin may be
saturated with carbon monoxide. Coburn and associates (1973) estimated
the mean pO2 of skeletal muscle and myocardium and found that they
were 0.8-1.1 and 0.5-0.8 kPa (6-8 and 4-6 Torr), respectively.
Although no final judgement can be made regarding the next lower
step involved in oxygen transport, i.e., the role of cytochromes a3
and P-450, the fact that, experimentally, they react with carbon
monoxide in the same way as other haem-containing substances suggests
that they may play a role in carbon monoxide poisoning. Available
evidence suggests that interactions between carbon monoxide and
cytochrome oxidases are of minor significance at the concentrations of
carbon monoxide found in community air pollution. All of the data on
the cytochromes have been obtained from in vitro experiments.
Whether similar events occur in vivo remains uncertain. The most
likely oxidase for in vivo inhibition is P-450. Cooper et al. (1965)
reported that the ratio of carbon monoxide to oxygen required for 50%
inhibition is close to 1, in contrast to a similar ratio of between
2.2 and 28 for cytochrome a3. Root (1965) believes that at a pCO
compatible with life, only nonsignificant blocking of the oxygen
consumption system occurs. In terms of the total distribution
throughout the body of an inhaled dose of carbon monoxide, the amounts
bound to these haemoproteins are small compared with haemoglobin and
myoglobin. A diagrammatic representation of the factors influencing
body carbon monoxide stores has been presented by Coburn (1970b). The
possible significance of the role of these haemoproteins lies in the
concept that, under conditions where tissue pO2 is decreased, the
affinity of intracellular haemoproteins for carbon monoxide may
increase.
6.4 Excretion
Adequate data are available on the rate of absorption of carbon
monoxide but there is considerably less information concerning the
rates of carbon monoxide egress from the lungs. The same factors that
determine how much carbon monoxide is taken up by the blood should
apply in reverse when clearance of carbon monoxide from blood is
considered. The primary factors involved are the amounts of carbon
monoxide and oxygen present, the magnitude of ventilation, and the
quality of the diffusion barrier. Age influences the quality of the
barrier and it appears that with advancing age the barrier becomes
"thicker" and there are fewer gas exchange membranes. Sedov et al.
(1971) presented data on the elimination of carbon monoxide at various
atmospheric pressures and ambient temperatures. Neither lower
barometric pressures nor high temperatures appreciably altered the
rates of elimination. It has been implied by Pace et al. (1950) that a
sex difference in elimination may also exist, a faster rate occurring
in females than in males, at least under the experimental conditions
of their study. Some sex differences in excretion have been reported
by Goldsmith et al. (1963).
Available evidence suggests that there is a biphasic decline in
the percentage of carboxyhaemoglobin in the arterial blood (Godin &
Shephard, 1972; Wagner et al., 1975). There is a rapid, initial,
exponential decline (distribution phase), probably related to the
distribution of carbon monoxide from the circulating blood to splenic
blood, myoglobin, and cytochrome enzymes. Elimination of carbon
monoxide through the lungs also occurs during this phase. The
distribution phase, which persists for the first 20-30 min, is
followed by a slower linear decline (elimination phase). This phase
probably reflects the rates of release of carbon monoxide from
haemoglobin and myoglobin, pulmonary diffusion, and ventilation, as
well as the fact that pCO decreases with time. Myhre (1974) found
that a similar biphasic excretion pattern occurred at an altitude of
1630 metres. However, he noted that the half-time of carboxyhaemo-
globin was much longer (5.5 h). After continuous exposure to carbon
monoxide for 49 h, 50% was eliminated in 30-180 min and 90% within
180-420 min (Tiunov & Kustov, 1964). Other investigators (Godin &
Shephard, 1972; Peterson & Stewart, 1970) reported exponential
carboxyhaemoglobin elimintation curves over many hours. However,
because of inadequate sampling in the early phase of elimination, they
were unable to observe the more rapid initial decline. The absolute
level of carboxyhaemoglobin, when the elimination studies began,
apparently modified the rate of disappearance of carbon monoxide from
the blood. Considerable individual variability has been observed and
the duration of exposure may also be an important factor. It has been
shown that prolonged exposure (more than 3 years) to concentrations of
carbon monoxide of 11.5-115 mg/m3 (10-100 ppm) results in a markedly
retarded elimination of carbon monoxide (Kodat, 1971).
In summary, discharge of carbon monoxide occurs rapidly at first
becoming slower with time and the lower the initial level of
carboxyhaemoglobin, the slower the rate of elimination. Apparently, no
studies have been made to determine elimination rates at the low
levels of carboxyhaemoglobin (2-4%) that might be present following
exposure to ambient concentrations of carbon monoxide.
Several procedures have been tested that could accelerate the
excretion of carbon monoxide from the blood of individuals with high
levels of carboxyhaemoglobin (40-70%). Pace et al. (1950) reported
that treatment of such individuals in a recompression chamber with an
oxygen level equivalent to 2.5 atmospheres (partial pressure of oxygen
equal to 253 kPa (1900 Torr) or an alveolar oxygen pressure of 239 kPa
(1801 Tort)) would facilitate removal of carbon monoxide. They
indicated that 1 h in such a chamber would result in the reduction of
the carboxyhaemoglobin level to 10 to 15% of the initial level. In a
revival of the old Henderson & Haggard treatment concept, Malorny et
al. (1962) evaluated the influence of breathing different gas mixtures
on the excretion of carbon monoxide in animals having high
carboxyhaemoglobin levels (60-74%). It was determined that 50% of
carbon monoxide could be excreted in 19 min if 5% carbon dioxide and
95% oxygen were breathed, compared with a time of 28 min for 100%
oxygen, and 41 min for ambient air. Another approach was suggested by
Agostini et al. (1974), who employed a total body asanguineous, hypo-
thermic procedure. These approaches to the removal of the body burden
of excessive amounts of carbon monoxide remain to be evaluated fully
in clinical trials.
7. EFFECTS ON EXPERIMENTAL ANIMALS
Great caution must be used in applying the resultsa obtained
from animal experiments to man. Nonetheless, animal studies have
provided valuable insight into both the potentially adverse effects of
carbon monoxide and the basic mechanisms by which this substance
influences physiological processes. However, many studies have used
extraordinarily high levels of carbon monoxide, rarely found in air.
These studies are not referred to in this document, since the toxic
effects of very high levels of carbon monoxide have been well
documented for both animals and man.
7.1 Species Differences
The oxygen dissociation curves for different animal species used
in carbon monoxide studies are not the same. There are also questions
concerning the relative affinities for carbon monoxide of the
haemoglobin in various animal species. Fodor & Winneke (1971.)
reported in vitro studies that showed different affinities for
different species, the highest being in man followed by rat, mouse,
and rabbit, in descending order. Klimisch et al. (1975) found the
following sequence of affinities: hamster, rat, pig, rabbit. Apart
from different affinities there may well be species differences with
respect to susceptibility to carbon monoxide. According to Alexandrov
(1973) certain mammals can be classified in order of decreasing carbon
monoxide susceptibility as follows: mouse, rat, cat, dog, guineapig,
and rabbit. This difference might, in part, be related to different
ventilation/body weight-ratios.
Species differences in reaction are ideally illustrated in the
studies by De Bias et al. (1972a,b, 1973) on dogs and cynomolgus
monkeys (Macaca fascicularis = M. irus). Chronic exposure (23 h per
day) over several months to 115 mg/m3 (100 ppm) resulted in
carboxyhaemoglobin levels of 14 and 12.4% in dogs, and monkeys,
respectively. The dogs (De Bias et al, 1972a) remained clinically in
good health with no untoward signs that could be interpreted as
induced by carbon monoxide. Serum enzymes, haematological variables,
and electrocardiograms did not change significantly. Carbon monoxide
exposure of normal monkeys resulted in myocardial effects.
Experimentally infarcted monkeys had greater P-wave amplitudes and
increased incidence of T-wave inversions than normal monkeys similarly
exposed. One important fact that may partly explain this difference
between dogs and monkeys is, that monkeys, like man, have end-
arteries, whereas dogs have a well developed collateral circulation.
a Animal studies have been reported in other sections of this
document wherever necessary. Consequently, some data on animals
(such as sheep) are not repeated in this section.
7.2 Cardiovascular System and Blood
Increase of coronary blood flow is the normal response of the
myocardium to carbon monoxide exposure. Permutt & Farhi (1969)
calculated theoretically, that, when carboxyhaemoglobin levels are
approximately 5%, an increase in coronary blood flow of about 20% more
than the resting level would be necessary to prevent coronary sinus
pO2 from falling below minimum levels. This calculation has been
confirmed experimentally by Adams et al. (1973) and Horvath (1973),
both of whom demonstrated approximately similar increases in coronary
blood flow in spite of using very different methods to increase
carboxyhaemoglobin levels.
There is considerable controversy concerning the cardiovascular
effects of carbon monoxide in dogs. Long-term exposures of animals to
carbon monoxide concentrations sufficiently high to produce
carboxyhaemoglobin levels in excess of 20% can induce pathological
changes in the heart and brain. As in acute high level intoxication in
man, serious sequelae develop. Lindenberg et al. (1961) exposed 8 dogs
to carbon monoxide concentrations of 115 mg/m3 (100 ppm). Four were
exposed continuously for 24 h a day, 7 days per week, for 6 weeks and
another 4 were exposed intermittently. All dogs had abnormal
electrocardiograms and some of the hearts showed histological
degeneration of muscle. In another study, Preziosi et al. (1970)
exposed dogs continuously to 115 mg/m3 for 6 weeks and reported
abnormal electrocardiograms, right and left heart dilatation, and
myocardial thinning. Histological examination showed older scarring in
some cases and fatty degeneration of heart muscle in others.
Carboxyhaemoglobin levels of 7.7 to 12% were lower than would be
predicted from the exposure. When 4 dogs were exposed intermittently
to a carbon monoxide concentration of 115 mg/m3 for 11 weeks,
central nervous system and cardiac effects were found (Lewey &
Drabkin, 1944).
Ehrich et al. (1944) also exposed 4 dogs to 115 mg/m3 on an
intermittent schedule. They observed that electrocardiographic changes
occurred at variable times during the 11 weeks of exposure. The gross
appearance of the hearts after exposure were normal but microscopic
examination revealed marked degenerative changes in individual fibres.
Lindenberg et al. (1961) exposed dogs to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) on the same schedule as for his
115 mg/m3 exposure studies. Carboxyhaemoglobin levels of 2.6-5.5%
were observed. No changes in haemoglobin levels or haematocrit were
noted. Electrocardiographic changes were observed in the third week of
exposure similar to, but less severe than, those observed in animals
exposed to the higher ambient level of carbon monoxide. Dogs were
exposed by Musselman et al. (1959) to a carbon monoxide concentration
of 57 mg/m3 for 24 h per day, 7 days per week, for 3 months. No
changes in the electrocardiogram or heart rates were observed.
Pathological examination of organs and tissues did not reveal any
changes after exposure. Experimental data have been presented by
Orellano et al. (1976) who claim that carbon monoxide injected
intraperitoneally into dogs is nontoxic. This study, as well as other
reports from these investigators, raise some intriguing questions as
to the mechanisms involved in carbon monoxide toxicity.
Continuous exposure of cynomolgus monkeys to a carbon monoxide
concentration of 115 mg/m3 resulted in demonstrable electro-
cardiographic effects in the myocardium of both normal monkeys
and monkeys with myocardial infarction (De Bias et al., 1972b, 1973).
The same investigators (De Bias et al., 1976) also studied the
susceptibility of the ventricles to induced fibrillation. Normal and
infarcted monkeys were exposed to a carbon monoxide concentration of
115 mg/m3 for 6 h. Application of high voltages were required to
produce fibrillation in normal monkeys in air but when infarcted
animals were exposed to carbon monoxide, the voltage level required
was very low. Animals with either infarction alone or carbon monoxide
exposure alone also required significantly less voltage to produce
fibrillation; when the two were combined, the effects were additive.
Intermittent exposure to carbon monoxide (30 min per h, 12 h per day,
over a period of 14 months) of cynomolgus monkeys fed either a normal
or a semipurified cholesterol diet did not result in myocardial
infarctions or electrocardiographic abnormalities (Malinow et al.,
1976). In these animals, the blood carboxyhaemoglobin concentration
reached 21.6% at the end of the daily period of breathing carbon
monoxide at 529 mg/m3 (460 ppm).
Carbon monoxide exposure did not increase the aortic and coronary
atherosclerosis induced in cynomolgus monkeys by cholesterol feeding.
Eckardt et al. (1972) exposed cynomolgus monkeys (22 h per day, 7 days
per week, for 2 years) to carbon monoxide concentrations of 23 or
75 mg/m3 (20 or 65 ppm). Carboxyhaemoglobin levels, which showed
considerable variation during the experimental period, ranged from 2.0
to 5.5% and 4.8 to 10.2% for low and high carbon monoxide ambient
concentrations, respectively. These levels of carboxyhaemoglobin did
not lead to compensatory increases in haematocrit, haemoglobin, or
erythrocyte counts nor to cardiac fibrosis or pathological effects in
the brain. Cholesterol-fed squirrel monkeys were exposed to carbon
monoxide at 229-344 mg/m3 (200-300 ppm) for several hours per day
for 7 months (Webster et al., (1968). No differences were found in
plasma cholesterol or in aortic and carotid atherosclerosis which
could be ascribed to carbon monoxide. However, the authors did observe
an increase in coronary atherosclerosis. Somewhat similar results were
later reported in studies on cynomolgus monkeys (Malinow et al.,
1976). Jones et al. (1971) exposed rats, guineapigs, dogs, and monkeys
to 58, 110, or 229 mg/m3 (51, 96 or 200 ppm) continuously for 90
days. Haematocrit and haemoglobin levels remained constant at the
lowest level of carbon monoxide exposure but were significantly
elevated at the two higher levels in all species except the dog.
Cynomolgus monkeys (Macaca irus) were exposed to a concentration of
286 mg/m3 (250 ppm) for 2 weeks by Thomsen (1974). In all the
exposed animals, the coronary arteries showed widening of the
subendothelial spaces in which cells with or without lipid droplets
were accumulating. He suggested that monkeys were more sensitive to
carbon monoxide than the rabbits studied by Astrup et al. (1967).
Experimental studies on rabbits exposed to relatively high
concentrations of carbon monoxide at 195-206 mg/m3 (170-180 ppm) for
extended periods indicated the presence of high levels of cholesterol
in the arteries or enhanced vascular disease (Astrup et al., 1970).
The lesions observed, which included subendothelial oedema, a gap
between endothelial cells, and increased infiltration of cells with
lipid droplets, might be early precursors of atherosclerotic disease.
However, such lesions occurred only in animals concurrently on a high
cholesterol or fat diet or on both. Exposure to carbon monoxide alone
induced some changes such as endothelial hypertrophy and
proliferation.
One experimental study on the effects of carbon monoxide on the
natural history of heart disease in the cynomolgus monkey has been
reported. De Bias et al. (1973) exposed animals to a carbon monoxide
concentration of 137 mg/m3 (120 ppm) for 24 weeks. The average
carboxyhaemoglobin level of 12.4% resulted in a polycythaemia with an
increase in haematocrit from 35 to 50%. All animals developed
increased P-wave amplitude and T-inversion which suggested nonspecific
myocardial stress rather than ischaemia. Animals in which an
experimental myocardial infarction was produced prior to exposure to
carbon monoxide had more marked electro-cardiographic changes than
animals breathing room air. In 1976, Ramsey & Casper reported that
erythrocytic 2,3-diphosphoglycerate (2,3-DPG) played neither a
compensatory nor an aggravating role in the hypoxia induced by the
presence of 20 or 30% carboxyhaemoglobin. Stupfel & Bouley (1970)
exposed mice and rats for 95 h per week to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) for either 1 to 3 months or for
their natural life expectancy of up to 2 years. A large number of
measurements were made during exposure followed by pathological
examination after death. The authors did not observe any important
effects of carbon monoxide exposure on the animals. In a study by
Penney et al. (1974a,b), the influence of hypoxic hypoxia on the
development of cardiac hypertrophy in the rat was compared with that
of carbon monoxide hypoxia. Exposure to various levels of carbon
monoxide resulted in hypertrophy of both the fight and left ventricles
in contrast with the right ventricle hypertrophy observed in response
to the hypoxic hypoxia stress.
Cardiac hypertrophy and a reduction in cytochrome oxidase levels
were demonstrated in chick embryos exposed to carbon monoxide for 144
and 168 h (Tumasonis & Baker, 1972). The resistance of young chickens
to carbon monoxide decreased with age. Body temperatures decreased
during exposure to carbon monoxide with the greatest fall in
temperature and the longest survival time occurring in the youngest
chickens. Total body asanguineous hypothermic perfusion (total body
exsanguination exchange transfusion) has been suggested as a
therapeutic measure for carbon monoxide poisoning (Agostini et al.,
1974). In an attempt to explain this beneficial effect, Ramirez et al.
(1974) compared the survival of normal dogs exposed to high levels of
carbon monoxide with that of acutely anaemic dogs transfused with
carboxyhaemoglobin blood to normal blood volumes. All normal dogs with
carboxyhaemoglobin levels of 54-100% died within 0.25-10 h but the
transfused animals, having a final mean carboxyhaemoglobin level of
80% after transfusion, survived. The authors suggested that hypoxic
anaemia was not the principal mechanism of carbon monoxide toxicity
but rather a blocking out of the energy supply on the cellular level,
governed by the cytochrome system.
Most of the investigations using rabbits for carbon monoxide
related research originated in Astrup's laboratories, where they first
demonstrated that low carboxyhaemoglobin levels enhanced the
development of atheromatosis (Astrup et al., 1967). Additional studies
(Hellung-Larsen et al., 1968; Thomsen & Kjeldsen, 1975) have shown
that lactate dehydrogenase isoenzymes (M subunits) increase and that a
higher incidence of focal intimal changes occur in rabbits exposed to
carbon monoxide. The myocardial ultrastructure of rabbits exposed to a
concentration of 206 mg/m3 (180 ppm) for at least 4 h showed
degenerative changes such as contraction bands, myofibrillar
disintegration, myelin body formation, and dehiscence of the
intercalated discs (Thomsen & Kjeldsen, 1974). Exposure of rabbits to
a similar concentration of carbon monoxide for 2 weeks resulted in
more extensive myocardial damage (Kjeldsen et al., 1974).
Astrup et al. (1970) furnished evidence that carbon monoxide
increases endothelial membrane permeability. They found that rabbits
exposed to carbon monoxide developed arterial lesions resulting in a
considerable accumulation of lipids. It has also been shown that human
coronary arteries exposed to carbon monoxide in vitro have a higher
uptake of cholesterol, although no significant changes in lipid
synthesis were observed (Sarma et al., 1975). Myocardial damage and
impaired myocardial performance have also been reported in animals and
man exposed to carbon monoxide (Ayres et al., 1970). Although there is
some evidence which suggests that exposure to carbon monoxide can
induce changes in blood vessels and the myocardium, there is also
evidence to the contrary (section 8.1).
7.3 Central Nervous System
Because of the brain's high oxygen demand, cerebral function
should be influenced at low carboxyhaemoglobin levels. However, data
concerning this are contradictory. Sensitivity to carbon monoxide may
follow a circadian rhythm (Stupfel, 1975; Stupfel et al., 1973).
Maximum sensitivity in rats occurred during the dark period of a
12-12 h light-dark cycle. Dyer & Annau (1976) could find no effect on
superior colliculus evoked potentials of rats until the level of
ambient carbon monoxide had reached 573 mg/m3 (500 ppm) in marked
contrast to Xintaras et al. (1966), who observed a 20% increase in the
amplitude of superior colliculus evoked potentials after only 1 h
exposure to 57 mg/m3 (50 ppm), and a 50% increase after a 2-h period
of exposure at this level. This study has, however, been criticized
for not taking into account the effects of dark adaptation on the
amplitude of visual evoked potentials in rats (Dyer & Annau, 1976).
In view of the conflicting reports it is of some interest to
examine the available data on cerebral pO2 tensions, cerebral
blood flow (CBF) and cerebral metabolism. Zorn (1972) studied the
effects of carbon monoxide inhalation on brain and liver pO2 using
platinum electrodes. Tissue pO2 fell in both organs, even at a
carboxyhaemoglobin concentration of 2%, and the fall was approximately
linear to increases in carboxyhaemoglobin. There was a decrease in
pO2 of 0.027-0.24 kPa (0.2-1.8 Torr) for each 1% fall in
oxyhaemoglobin percentage saturation. These data suggest that the
carbon monoxide influenced levels other than the intracellular level,
since if its effects were limited to this area then tissue pO2
would have been expected to increase. Similar studies were performed
by Weiss & Cohen (1974) on rat brain and muscle. They found a decrease
in cerebral cortical pO2 following inhalation of low levels of
carbon monoxide. Unfortunately, they did not measure
carboxyhaemoglobin levels in these rats but, in a group of sham-
operated animals exposed to similar levels of inhaled carbon monoxide,
carboxyhaemoglobin had increased to 3.3%. During progressive
administration of carbon monoxide to dogs, CBF did not increase until
carboxyhaemoglobin levels reached 20%. Thereafter, CBF increased
progressively and was double that in the controls when the
carboxyhaemoglobin level reached 50% (Häggendal et al., 1966). On the
other hand, Traystman (1976) did observe a progressive increase in CBF
in dogs even at very low carboxyhaemoglobin values. The lowest level
studied was 2.5%, which produced a slight but significant CBF
increase. Thus, Traystman did not believe in a threshold effect. The
effects were produced with both hypoxic hypoxia and carbon monoxide
hypoxia. It remains to be seen, how these data relate to cerebral
circulation in man.
The respiratory centre or arterial chemoreceptors were not
stimulated to increase respiratory minute volume even when
carboxyhaemoglobin levels were as high as 40% (Chiodi et al., 1941).
Mills & Edwards (1968) measured the frequency of electrical impulses
in the afferent nerves from the aortic and carotid chemoreceptors and
showed that administration of carbon monoxide did result in
chemoreceptor stimulation. The response appeared to have an
approximately linear relationship with the carboxyhaemoglobin
concentration (at least above 8%). These findings suggest that carbon
monoxide might stimulate breathing. Failure to observe an increased
minute volume may be explained by the fact that, in the presence of
carbon monoxide, the chemoreceptor stimulation was offset by hypoxic
depression of brain structures involved in breathing. There is some
evidence that this balancing between chemoreceptors and central
nervous depression is operative in anaemia (Santiago & Edelman, 1972).
Additional investigations are needed to clarify this effect of carbon
monoxide inhalation.
7.4 Behavioural Changes and Work Performance
In extensive studies on rabbits, guineapigs, rats, and mice
(Gadaskina, 1960; Ljublina, 1960; Rylova, 1960) exposure to a carbon
monoxide level of 30 mg/m3 (26 ppm), although not inducing any
morphological blood changes, did result in a number of unfavourable
physiological changes. Among these were decreased work capacity, poor
adjustment to postural shifts (orthostatic tests), and increased
thyroid activity. These changes were more evident during the initial
period of the exposure but reverted towards normal later, suggesting
some adaptation to carbon monoxide exposure.
7.5 Adaptation
Adaptation apparently can occur in animals exposed to moderate
concentrations of carbon monoxide (Gorbatov & Noro, 1948; Tiunov &
Kustov, 1969) as shown by their ability to tolerate, with apparent
ease, acute exposure to higher concentrations. Both Clark & Otis
(1952) and Tiunov & Kustov (1969) have demonstrated that, after long-
term exposure to carbon monoxide, animals developed tolerance to
short-term, high altitude exposure, and vice versa, an indication of
the development of a common adaptive mechanism. Acclimatization
continued despite a subsequent decrease in the initial elevation of
the haemoglobin concentration (Gorbatov & Noro, 1948). These
investigators noted an acclimatization effect in rats exposed daily to
0.4-0.5% carbon monoxide until loss of consciousness occurred. A
progressive improvement in tolerance time to unconsciousness was noted
so that by the eighth day of exposure a 3-fold improvement over the
time required on the first day had developed. In spite of apparent
acclimatization, the general condition of test animals became worse.
Unexpectedly, daily exposure to a carbon monoxide concentration of
1.0%, which on the first day necessitated a 5-min exposure prior to
unconsciousness, failed to induce any improvement in tolerance. A
possible correction of leftward shift of the oxygen dissociation by
alterations in the concentration of 2,3-diphosphoglycerate (inducing a
shift to the right) has been suggested. However, conflicting results
and the necessity to produce high levels of carboxyhaemoglobin negate
the possibilities of this beneficial effect (Astrup, 1970; Dinman et
al., 1970).
Evidence for adaptation to carbon monoxide is inconclusive.
Further studies appear to be warranted with special attention devoted
to studies using more realistic current ambient levels of carbon
monoxide, and also studies on the possible physiological cost of
adaptation, if it occurs.
7.6 Embryonal, Fetal, Neonatal, and Teratogenic Effects
Few studies have been made on the effects of carbon monoxide on
mammalian fetal growth and survival. Wells (1933) exposed pregnant
rats for 5-8 min to a carbon monoxide concentration of 1718 mg/m3
(1500 ppm) every other day during pregnancy. Maternal unconsciousness
and abortion or absorption of most of the fetuses resulted. Data
concerning carboxyhaemoglobin levels and numbers of animals studied
were not given. Rats were exposed to 0.34% carbon monoxide for 1 h
(carboxyhaemoglobin 60-70%) daily for a period of 3 months (Williams &
Smith, 1935). Among 7 pregnant females, the number of young per litter
was only half that of the controls and only 2 of the 13 newborns
survived to weaning age. Astrup et al. (1972) exposed rabbits during
their 30 days of pregnancy to carbon monoxide resulting in
carboxyhaemoglobin levels of either 9-19 or 16-18%. Neonatal mortality
in the 2 groups increased by 10 and 35%, respectively, compared with a
control value of 4.5%. Birth weights decreased by 12 and 17%,
respectively.
In their studies on the ewe and fetal lamb, Longo & Hill (1977)
indicated that fetal uptake and elimination of carbon monoxide was
relatively slow compared with that of the mother. They also reported
that, during steady-state conditions, fetal levels of
carboxyhaemoglobin were about 25% higher than maternal levels. These
results may be related to species differences, since Longo & Hill
(1970) found that the M valuesa for sheep maternal and fetal blood
were 218 and 216 respectively, while Engel et al. (1969) reported that
fetal haemoglobin had 20% less preferential binding of carbon monoxide
over oxygen than haemoglobin A.
There are very few studies on the teratogenicity of carbon
monoxide exposure. When fertilized chicken eggs were continuously
exposed to a carbon monoxide concentration of 747 mg/m3 (650 ppm)
for up to 18 days of incubation, the percentage of eggs hatching
decreased to 46% and developmental anomalies of the tibia and
metatarsal bones were noted (Baker & Tumasonis, 1972).
a M = Relative affinity of haemoglobin for carbon monoxide
compared with oxygen.
7.7 Carcinogenicity, and Mutagenicity
No evidence is available on carcinogenicity and mutagenicity in
relation to exposure to carbon monoxide.
7.8 Miscellaneous Changes
Kustov et al. (1972) exposed rats to carbon monoxide at 53 mg/m3
(46 ppm) and noted slower weight gains and an increase in haemoglobin.
Some enzyme systems were also found to have increased activity, when
rats were exposed to carbon monoxide (Pankow & Ponsold, 1972; Pankow
et al., 1974b). A slowing of in vivo metabolism of the drugs
hexobarbital and zoxazolamine, with prolongation of their
pharmacological effects has been reported in rats exposed to carbon
monoxide concentrations of 286-3435 mg/m3 (250-3000 ppm) (Montgomery
& Rubin, 1971). With prolonged exposure, these metabolic effects
became less pronounced and reverted to normal more quickly following
removal from the carbon monoxide environment. Sokal (1975) compared
the effect on blood pH and certain carbohydrate metabolic products
resulting from either a bolus administration or a fixed level of
inspired carbon monoxide, both resulting in equivalent final levels of
carboxyhaemoglobin. His data suggest that more intense biochemical
effects resulted following a gradual increase in carboxyhaemoglobin
levels compared with effects seen following rapid elevation of
carboxyhaemoglobin from the bolus. Data presented by Marks & Swiecicki
(1971) indicated that exposure to high levels of carbon monoxide
induced a stresslike response in the form of an elevation in
catecholamines. Swiecicki (1973) reported that increased
carboxyhaemoglobin levels stimulated the adrenergic system and
increased carbohydrate metabolism. He also noted that physical
training of the rat neither prevented nor reduced changes in
carbohydrate metabolism following carbon monoxide exposure and
vibration. Exposure of rats to 57 mg/m3 (50 ppm) for 5 h per day, 5
days per week, for 12 weeks produced an effect on trace metals at the
subcellular level, with a possible reduction in cellular respiration
and nucleoprotein synthesis.
Guineapigs exposed to carbon monoxide concentrations of
1.7-30 mg/m3 (1.5-26 ppm) for 21 days, 8 h per day, did not show any
allergenic effects related to carbon monoxide exposure (Vinogradov et
al., 1974). Plasma leucine aminopeptidase (EC 5.4.11.1)a and
glutamic pyruvic transaminase (EC 2.4.1.2) activity, normally
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
increased by exposure to carbon tetrachloride, were further
potentiated when blood carboxyhaemoglobin levels were elevated. Pankow
et al. (1974a) also observed an additive effect on some enzymes with a
combination of alcohol and carbon monoxide giving a carboxyhaemoglobin
concentration of 50%.
Rondia (1970) observed a significant reduction of benzopyrene-
hydroxylase (EC 1.14.14.2) activity in the liver homogenates of rats
exposed to a carbon monoxide concentration of 70-150 mg/m3
(60-130 ppm) for only a few days. This finding might be interpreted as
meaning that carbon monoxide contributes to the induction of lung
cancer by lengthening the time of retention of carcinogens in the
lung. Additional work is necessary to clarify this important question.
7.9 Interactions
Much of the data reviewed by Pankow & Ponsfold (1974) concerning
the combined effects of carbon monoxide and other biologically active
agents are based on animal experiments. Because of the extreme
exposure conditions used in most of these studies, only a few of them
are directly relevant to the environmental exposure of man to carbon
monoxide.
The experimental evidence on the aggravation of carbon monoxide-
induced atherosclerosis in rabbits by dietary cholesterol has already
been mentioned (see section 7.2). No significant additive effects were
noted from ethanol when dogs exposed to a carbon monoxide
concentration of 115 mg/m3 (100 ppm) for 21 weeks, 5 days a week,
and 6 h per day were given a daily oral dose of 120 ml of a 15%
ethanol solution (Pecora, 1959). However, the excretion of total
lipoproteins was higher when carbon monoxide and ethanol treatments
were combined than with exposure to carbon monoxide alone. An
indication of interaction of sulfur dioxide and carbon monoxide was
given by Prohorov & Rogov (1959) in their experiments on rabbits. The
depressed activity of succinate dehydrogenase (EC 1.3.99.1) on heart,
liver, and kidney due to exposure to sulfur dioxide at 200 mg/m3
(76 ppm) was exacerbated by exposure to carbon monoxide at a level of
200-400 mg/m3 (174-348 ppm), for 3 h per day, over a 3-week period.
As for the combined effects of carbon monoxide and temperature, Tiunov
& Kustov (1969) showed clearly that carbon monoxide toxicity in mice
increased at temperatures above or below normal ambient levels.
8. EFFECTS ON MAN
8.1 Healthy Subjects
8.1.1 Behavioural changes
Demonstrable changes in the central nervous system (CNS) function
of subjects inadvertently exposed to high levels of ambient carbon
monoxide in illuminating gas and in automobile exhaust resulted in a
series of studies to determine psychomotor and psychological
aberrations in subjects having more modest blood levels of
carboxyhaemoglobin than those observed in the potentially moribund
patients. Deficiencies in earlier studies have been related to
inadequate understanding of the significance of behavioural changes,
the inability to distinguish between simple perceptual motor
performance and the more complex performance involving sustained
and/or selective attention, short-term memory, and decision making
among possible alternatives. Furthermore, the physiological mechanisms
involved in carbon monoxide intoxication were not appreciated and the
available physiological and psychological tools were not adequately
exploited. Even today, some physiological and behavioural studies
suffer from similar or other inadequacies, e.g., the failure to
measure blood carboxyhaemoglobin levels, the inability to distinguish
between the physiological effects of a carbon monoxide bolus of high
concentration or the slow, insidious increment in carboxyhaemoglobin
levels over time with lower inhaled concentrations, the amount of
carbon monoxide brought to or removed from the lungs by changes in
alveolar ventilatory volumes, and the small number of volunteers
examined. Other factors involve failure to provide control measures
for bias and effects of the experimental worker (by means of double-
blind administration), control periods so that task-learning effects
do not mask negative results, homogeneity of the groups labelled
"smokers" and "nonsmokers", and control of possible boredom and
fatigue effects, all essentially amounting to a failure to adopt a
proper experimental design that would produce statistically
significant information.
A reduction of logical memory and recognition was demonstrated by
Chalupa (1960) in individuals subjected to acute carbon monoxide
intoxication. However, these functions returned to normal. Sayers et
al. (1929) did not find any significant changes in 6 exposed men
despite carboxyhaemoglobin levels of approximately 20-30%;
observations included hand-eye coordination and steadiness, tapping
speed, arithmetic (continuous addition), location memory, and simple
reaction time. Simple sensory-motor times decreased by 10% in subjects
with carboxyhaemoglobin concentrations of approximately 6.2% (5.5-7%)
(Tiunov & Kustov, 1969). Simulated driving performance did not
deteriorate despite carboxyhaemoglobin levels of 25%, although a small
deterioration was observed when the carboxyhaemoglobin levels were
above 35% (Forbes et al., 1937). The first demonstrable influences of
carbon monoxide on higher CNS functions were noted by McFarland et al.
(1944) in conjunction with their altitude studies, when they observed
reduction of visual acuity at carboxyhaemoglobin levels as low as 5%.
These observations were extended by Halperin et al. (1959) when they
reported that visual function was impaired at carboxyhaemoglobin
levels as low as 4% and that impairment increased at higher levels.
More recently, McFarland et al. (1973) showed that, for glare
recovery, the dark adaptation final threshold values increased as
carboxyhaemoglobin levels rose from control to 6-17%. Peripheral
recognition tasks were not affected until levels reached 17%. They
also stated that central and peripheral complex tasks were not
influenced by low levels of carboxyhaemoglobin. Schulte (1963)
demonstrated that there was a decrease in performance in higher
intellectual processes that was observable when the carboxyhaemoglobin
level exceeded 5%; further deterioration was noted as the level
increased. These results were in direct contradiction to the negative
results obtained by Dorcus & Weigand (1929) who used a similar series
of tests but with subjects exposed for a shorter period. In studies by
Beard & Wertheim (1967), the ability to judge correctly slight
differences in successive short time intervals showed significant
impairment when carboxyhaemoglobin levels were approximately 2 to 3%
above basal levels. These findings, suggesting an altered mental
function, represent the lowest levels of carboxyhaemoglobin that
produce a significant alteration in behavioural performance. However,
attempts to replicate them have been less than satisfactory (O'Donnell
et al., 1971a,b; Stewart et al., 1973a) even though the subjects in
all these other studies attained higher levels of carboxyhaemoglobin.
Some of the discrepancies may be explained on the basis of differences
in protocol and in the environmental conditions under which the tests
were conducted. Some of the investigators designed their experiments
to minimize the factors of boredom and fatigue while others attempted
to minimize external influences and conducted their experiments for a
relatively long time.
However, the Beard & Wertheim study may actually be more relevant
to questions of vigilance and ideally should be discussed in this
context. Assessment of vigilance is the determination of an
individual's ability to detect small changes in his environment,
changes that take place at unpredictable times and so demand
continuous attention. In such monotonous tasks, subjects miss signals
that they would not have missed when starting the task. Such signals
are presented visually or aurally. Fodor & Winneke (1972) and Groll-
Knapp et al. (1972) used auditory signals for their vigilance task.
The former investigators used a white noise (frequency range from 20
to 20 000 Hz lasting 0.36 sec and repeated at 2-sec intervals. About 3
out of every 100 of these noises were slightly less intense and were
used as the signal to which the subjects responded by pressing a
button. Twelve nonsmokers (male and female) were tested at carbon
monoxide concentrations of 0 and 57 mg/m3 (0-50 ppm). They breathed
this concentration for 80 min prior to the first of three vigilance
tests. Carboxyhaemoglobin levels were estimated to be 2.3 and 3.1% at
the beginning and the end of the first vigilance test, respectively.
Subjects were likely to miss signals during this initial test. This
was not observed during the next two vigilance tests (total exposure
to carbon monoxide being 210 min with the carboxyhaemoglobin level
estimated to have finally reached 4.3%). These data suggest an initial
decrement in performance followed by a compensatory response. Groll-
Knapp et al. (1972) exposed 20 subjects for a 2-h period to carbon
monoxide concentrations of 0, 57, 115, or 172 mg/m3 (0, 50, 100,
150 ppm). There is some doubt as to which subjects, if any, were
smokers. Carboxyhaemoglobin levels were also estimated by these
investigators at the end of the test period to be 0, 3.0, 5.4, and
7.6%. Over the 90 min of the auditory test, some 200 paired tones were
given in which a weaker second tone was the signal. The mean number of
signals missed during the control test was 26. The number missed
increased in the presence of elevated carboxyhaemoglobin levels so
that 35, 40, and 44 misses occurred in environments containing carbon
monoxide levels of 57, 114, and 172 mg/m3, respectively. This
suggests a significant impairment when a concentration of 57 mg/m3
is inhaled. Winneke (1974) used a similar test in studies at carbon
monoxide concentrations of 0, 57, and 115 mg/m3. Results with all
levels of carbon monoxide exposure were negative, in marked contrast
to the previous data despite an estimated carboxyhaemoglobin level of
approximately 9% at the end of the 115 mg/m3 exposure.
Beard & Grandstaff (1975) examined the effect of carbon monoxide
exposure on a visual vigilance task. The signal was a shorter flash of
light than the non-signals. Following a 30-min control period, 9
subjects were exposed to carbon monoxide concentrations of 0, 57, 200,
or 286 mg/m3 (0, 50, 175, 250 ppm). Subjects exposed to room air
detected 73% of the signals presented to them in three vigils. In
environments containing carbon monoxide concentrations of 57 and
200 mg/m3, respectively, 64% of the signals were detected. These
differences were statistically significant at the 5% level. However,
exposure to a concentration of 286 mg/m3 yielded a correct
identification rate of about 70% that was not statistically
significant. They estimated blood carboxyhaemoglobin levels from
alveolar breath samples to be 1.8, 5.2, and 7.5%, respectively. The
alveolar samples were obtained 30 min after the exposures were
completed. Krotova & Muzyka (1974) studied subjects working for about
2 years in an environment containing carbon monoxide. Mean
carboxyhaemoglobin levels were approximately 3.2% before, and 4% after
work. Eleven of 56 workers reported a loss of vigilance. It has been
reported by Rummo & Sarlanis (1974) that, during a 2-h vigilance
driving simulator task, subjects with carboxyhaemoglobin levels of
6-8% were significantly slower in responding to lead car speed
changes. Horvath et al. (1971) also used a visual vigilance test and
were the only group of investigators that actually measured blood
levels of carboxyhaemoglobin. The vigilance task in these studies was
the detection of a light pulse that was slightly brighter than the
base level light pulse. A 1-h vigil was preceded by a short alerting
pre-test during which a randomly interspersed 10 of 60 light pulses
were the brighter signals. After a 1-min rest, the 60-min vigilance
task was begun. Only 40 of 1200 light pulses were signals. Ten of
these signals appeared randomly out of the 300 presented each 15 min.
Three levels of ambient carbon monoxide were used, 0, 30, and
127 mg/m3 (0, 26, and 111 ppm) with each subject serving as his own
control. Exposures were randomized, with 1 week elapsing between each
exposure. Fig. 6 illustrates the changes in blood levels of
carboxyhaemoglobin with time under all conditions and compares the
concentrations with the levels determined from the data obtained by
Forbes et al. (1945). The control group breathing filtered air (no
carbon monoxide present) had carboxyhaemoglobin levels of 0.9% before
and at the completion of the task. Exposure to a carbon monoxide
concentration of 30 mg/m3 led to a level of 1.6% after the first
hour (before the vigilance task) and 2.3% at the end. Carbon monoxide
exposure at 127 mg/m3 resulted in carboxyhaemoglobin levels of 4.2%
after the first hour and 6.6% at completion of the vigilance test.
Performance during the pre-test period gave approximately 88 correct
responses in all three conditions, carbon monoxide exposure having no
discernible effect. During the vigilance test itself, subjects
breathing a concentration of 127 mg/m3 made significantly fewer
correct responses (4.2-6.6% carboxyhaemoglobin) than the same subjects
breathing 0 or 27 mg/m3 (Fig. 7). It appeared that when the
carboxyhaemoglobin level was approximately 5%, a significant decrement
in performance occurred. It should be noted that the slight
improvement at the end of the test period probably represented the
usual alerting response observed whenever subjects estimate that the
task is completed. Recently, Winneke et al. (1976) attempted to
replicate this study without success. Although Horvath's experiments
were started with 15 alleged nonsmokers, pre-exposure blood samples
from 5 of the subjects showed carboxyhaemoglobin levels of almost 3%.
At the completion of all the exposures, these subjects admitted
smoking, thus confirming the blood levels. Data on these subjects were
not included in this analysis. When these smokers' data were analysed,
performance on the vigilance task showed no deterioration even though,
with exposure to a carbon monoxide concentration of 127 mg/m3,
carboxyhaemoglobin levels increased from an initial 2.8% to 5.1% after
the first hour and to 6.9% at the completion of the vigilance task
(O'Hanlon, 1975). There were too few subjects to permit more than a
suggestion that prior, continuous exposure to nonambient carbon
monoxide may result in some degree of questionable adaptation. Beard &
Wertheim's (1967) earlier indications that some deleterious
psychological effects would appear at carboxyhaemoglobin levels of
about 2% have not been confirmed or even replicated.
The design and experimental control in the major studies concerned
with this subject have been poor with errors of omission, and failure
to present finalized data. In the early studies of Forbes et al.
(1937), 5 subjects were exposed to a carbon monoxide concentration
The small reductions in oxygen content at 5-10% carboxyhaemoglobin may
be quite critical for patients suffering from cardiovascular diseases
or chronic obstructive lung disease. Coburn et al. (1965) published a
detailed theoretical analysis of the physiology and variables that
determine blood carboxyhaemoglobin levels in man. The details of the
formulae used in these calculations are presented in section 6.2.
With regard to the intracellular effects of carbon monoxide,
consideration must be given to the interactions of all substances
within the tissue cells that are involved with oxygen delivery. Since
haemoglobin and myoglobin are structurally related, they react with
carbon monoxide in a similar manner. The function of myoglobin, in
vivo, may be to act as a reservoir for oxygen within the muscle
fibre. The carbon monoxide and oxygen equilibria of human myoglobin
has been studied in vitro and a hyperbolic oxygen dissociation curve
established (Rossi-Fanelli & Antonini, 1958). This curve, unlike that
for haemoglobin, is not affected by the hydrogen ion concentration,
the ionic strength, or the concentration of myoglobin. The relative
affinity constant, M, is approximately 40 but is still sufficient to
induce appreciable formation of carboxymyoglobin. Both Coburn et al.
(1970b, 1973) and Luomanmaki (1966) have studied the interrelation-
ships between carboxyhaemoglobin and carboxymyoglobin. Coburn, using
14CO, showed that identical carbon monoxide exposures can produce
different degrees of saturation of haemoglobin, depending upon the
partial pressures of oxygen in blood and tissue. Coburn (1970b)
determined the ratio of the carbon monoxide content in muscle to the
content in blood as a function of arterial pO2. This ratio, for
skeletal muscle, is approximately 1, but in myocardial tissue it was
found to be 3. When arterial pO2 fell below 5.3-4 kPa (40-30
Torr), carbon monoxide disappeared from the blood, presumably entering
the muscle. Considerable amounts of extravascular carbon monoxide are
stored in muscle. The higher ratio for cardiac tissue may be of
considerable significance. In an individual with a blood
carboxyhaemoglobin level of 10%, some 30% of cardiac myoglobin may be
saturated with carbon monoxide. Coburn and associates (1973) estimated
the mean pO2 of skeletal muscle and myocardium and found that they
were 0.8-1.1 and 0.5-0.8 kPa (6-8 and 4-6 Torr), respectively.
Although no final judgement can be made regarding the next lower
step involved in oxygen transport, i.e., the role of cytochromes a3
and P-450, the fact that, experimentally, they react with carbon
monoxide in the same way as other haem-containing substances suggests
that they may play a role in carbon monoxide poisoning. Available
evidence suggests that interactions between carbon monoxide and
cytochrome oxidases are of minor significance at the concentrations of
carbon monoxide found in community air pollution. All of the data on
the cytochromes have been obtained from in vitro experiments.
Whether similar events occur in vivo remains uncertain. The most
likely oxidase for in vivo inhibition is P-450. Cooper et al. (1965)
reported that the ratio of carbon monoxide to oxygen required for 50%
inhibition is close to 1, in contrast to a similar ratio of between
2.2 and 28 for cytochrome a3. Root (1965) believes that at a pCO
compatible with life, only nonsignificant blocking of the oxygen
consumption system occurs. In terms of the total distribution
throughout the body of an inhaled dose of carbon monoxide, the amounts
bound to these haemoproteins are small compared with haemoglobin and
myoglobin. A diagrammatic representation of the factors influencing
body carbon monoxide stores has been presented by Coburn (1970b). The
possible significance of the role of these haemoproteins lies in the
concept that, under conditions where tissue pO2 is decreased, the
affinity of intracellular haemoproteins for carbon monoxide may
increase.
6.4 Excretion
Adequate data are available on the rate of absorption of carbon
monoxide but there is considerably less information concerning the
rates of carbon monoxide egress from the lungs. The same factors that
determine how much carbon monoxide is taken up by the blood should
apply in reverse when clearance of carbon monoxide from blood is
considered. The primary factors involved are the amounts of carbon
monoxide and oxygen present, the magnitude of ventilation, and the
quality of the diffusion barrier. Age influences the quality of the
barrier and it appears that with advancing age the barrier becomes
"thicker" and there are fewer gas exchange membranes. Sedov et al.
(1971) presented data on the elimination of carbon monoxide at various
atmospheric pressures and ambient temperatures. Neither lower
barometric pressures nor high temperatures appreciably altered the
rates of elimination. It has been implied by Pace et al. (1950) that a
sex difference in elimination may also exist, a faster rate occurring
in females than in males, at least under the experimental conditions
of their study. Some sex differences in excretion have been reported
by Goldsmith et al. (1963).
Available evidence suggests that there is a biphasic decline in
the percentage of carboxyhaemoglobin in the arterial blood (Godin &
Shephard, 1972; Wagner et al., 1975). There is a rapid, initial,
exponential decline (distribution phase), probably related to the
distribution of carbon monoxide from the circulating blood to splenic
blood, myoglobin, and cytochrome enzymes. Elimination of carbon
monoxide through the lungs also occurs during this phase. The
distribution phase, which persists for the first 20-30 min, is
followed by a slower linear decline (elimination phase). This phase
probably reflects the rates of release of carbon monoxide from
haemoglobin and myoglobin, pulmonary diffusion, and ventilation, as
well as the fact that pCO decreases with time. Myhre (1974) found
that a similar biphasic excretion pattern occurred at an altitude of
1630 metres. However, he noted that the half-time of carboxyhaemo-
globin was much longer (5.5 h). After continuous exposure to carbon
monoxide for 49 h, 50% was eliminated in 30-180 min and 90% within
180-420 min (Tiunov & Kustov, 1964). Other investigators (Godin &
Shephard, 1972; Peterson & Stewart, 1970) reported exponential
carboxyhaemoglobin elimintation curves over many hours. However,
because of inadequate sampling in the early phase of elimination, they
were unable to observe the more rapid initial decline. The absolute
level of carboxyhaemoglobin, when the elimination studies began,
apparently modified the rate of disappearance of carbon monoxide from
the blood. Considerable individual variability has been observed and
the duration of exposure may also be an important factor. It has been
shown that prolonged exposure (more than 3 years) to concentrations of
carbon monoxide of 11.5-115 mg/m3 (10-100 ppm) results in a markedly
retarded elimination of carbon monoxide (Kodat, 1971).
In summary, discharge of carbon monoxide occurs rapidly at first
becoming slower with time and the lower the initial level of
carboxyhaemoglobin, the slower the rate of elimination. Apparently, no
studies have been made to determine elimination rates at the low
levels of carboxyhaemoglobin (2-4%) that might be present following
exposure to ambient concentrations of carbon monoxide.
Several procedures have been tested that could accelerate the
excretion of carbon monoxide from the blood of individuals with high
levels of carboxyhaemoglobin (40-70%). Pace et al. (1950) reported
that treatment of such individuals in a recompression chamber with an
oxygen level equivalent to 2.5 atmospheres (partial pressure of oxygen
equal to 253 kPa (1900 Torr) or an alveolar oxygen pressure of 239 kPa
(1801 Tort)) would facilitate removal of carbon monoxide. They
indicated that 1 h in such a chamber would result in the reduction of
the carboxyhaemoglobin level to 10 to 15% of the initial level. In a
revival of the old Henderson & Haggard treatment concept, Malorny et
al. (1962) evaluated the influence of breathing different gas mixtures
on the excretion of carbon monoxide in animals having high
carboxyhaemoglobin levels (60-74%). It was determined that 50% of
carbon monoxide could be excreted in 19 min if 5% carbon dioxide and
95% oxygen were breathed, compared with a time of 28 min for 100%
oxygen, and 41 min for ambient air. Another approach was suggested by
Agostini et al. (1974), who employed a total body asanguineous, hypo-
thermic procedure. These approaches to the removal of the body burden
of excessive amounts of carbon monoxide remain to be evaluated fully
in clinical trials.
7. EFFECTS ON EXPERIMENTAL ANIMALS
Great caution must be used in applying the resultsa obtained
from animal experiments to man. Nonetheless, animal studies have
provided valuable insight into both the potentially adverse effects of
carbon monoxide and the basic mechanisms by which this substance
influences physiological processes. However, many studies have used
extraordinarily high levels of carbon monoxide, rarely found in air.
These studies are not referred to in this document, since the toxic
effects of very high levels of carbon monoxide have been well
documented for both animals and man.
7.1 Species Differences
The oxygen dissociation curves for different animal species used
in carbon monoxide studies are not the same. There are also questions
concerning the relative affinities for carbon monoxide of the
haemoglobin in various animal species. Fodor & Winneke (1971.)
reported in vitro studies that showed different affinities for
different species, the highest being in man followed by rat, mouse,
and rabbit, in descending order. Klimisch et al. (1975) found the
following sequence of affinities: hamster, rat, pig, rabbit. Apart
from different affinities there may well be species differences with
respect to susceptibility to carbon monoxide. According to Alexandrov
(1973) certain mammals can be classified in order of decreasing carbon
monoxide susceptibility as follows: mouse, rat, cat, dog, guineapig,
and rabbit. This difference might, in part, be related to different
ventilation/body weight-ratios.
Species differences in reaction are ideally illustrated in the
studies by De Bias et al. (1972a,b, 1973) on dogs and cynomolgus
monkeys (Macaca fascicularis = M. irus). Chronic exposure (23 h per
day) over several months to 115 mg/m3 (100 ppm) resulted in
carboxyhaemoglobin levels of 14 and 12.4% in dogs, and monkeys,
respectively. The dogs (De Bias et al, 1972a) remained clinically in
good health with no untoward signs that could be interpreted as
induced by carbon monoxide. Serum enzymes, haematological variables,
and electrocardiograms did not change significantly. Carbon monoxide
exposure of normal monkeys resulted in myocardial effects.
Experimentally infarcted monkeys had greater P-wave amplitudes and
increased incidence of T-wave inversions than normal monkeys similarly
exposed. One important fact that may partly explain this difference
between dogs and monkeys is, that monkeys, like man, have end-
arteries, whereas dogs have a well developed collateral circulation.
a Animal studies have been reported in other sections of this
document wherever necessary. Consequently, some data on animals
(such as sheep) are not repeated in this section.
7.2 Cardiovascular System and Blood
Increase of coronary blood flow is the normal response of the
myocardium to carbon monoxide exposure. Permutt & Farhi (1969)
calculated theoretically, that, when carboxyhaemoglobin levels are
approximately 5%, an increase in coronary blood flow of about 20% more
than the resting level would be necessary to prevent coronary sinus
pO2 from falling below minimum levels. This calculation has been
confirmed experimentally by Adams et al. (1973) and Horvath (1973),
both of whom demonstrated approximately similar increases in coronary
blood flow in spite of using very different methods to increase
carboxyhaemoglobin levels.
There is considerable controversy concerning the cardiovascular
effects of carbon monoxide in dogs. Long-term exposures of animals to
carbon monoxide concentrations sufficiently high to produce
carboxyhaemoglobin levels in excess of 20% can induce pathological
changes in the heart and brain. As in acute high level intoxication in
man, serious sequelae develop. Lindenberg et al. (1961) exposed 8 dogs
to carbon monoxide concentrations of 115 mg/m3 (100 ppm). Four were
exposed continuously for 24 h a day, 7 days per week, for 6 weeks and
another 4 were exposed intermittently. All dogs had abnormal
electrocardiograms and some of the hearts showed histological
degeneration of muscle. In another study, Preziosi et al. (1970)
exposed dogs continuously to 115 mg/m3 for 6 weeks and reported
abnormal electrocardiograms, right and left heart dilatation, and
myocardial thinning. Histological examination showed older scarring in
some cases and fatty degeneration of heart muscle in others.
Carboxyhaemoglobin levels of 7.7 to 12% were lower than would be
predicted from the exposure. When 4 dogs were exposed intermittently
to a carbon monoxide concentration of 115 mg/m3 for 11 weeks,
central nervous system and cardiac effects were found (Lewey &
Drabkin, 1944).
Ehrich et al. (1944) also exposed 4 dogs to 115 mg/m3 on an
intermittent schedule. They observed that electrocardiographic changes
occurred at variable times during the 11 weeks of exposure. The gross
appearance of the hearts after exposure were normal but microscopic
examination revealed marked degenerative changes in individual fibres.
Lindenberg et al. (1961) exposed dogs to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) on the same schedule as for his
115 mg/m3 exposure studies. Carboxyhaemoglobin levels of 2.6-5.5%
were observed. No changes in haemoglobin levels or haematocrit were
noted. Electrocardiographic changes were observed in the third week of
exposure similar to, but less severe than, those observed in animals
exposed to the higher ambient level of carbon monoxide. Dogs were
exposed by Musselman et al. (1959) to a carbon monoxide concentration
of 57 mg/m3 for 24 h per day, 7 days per week, for 3 months. No
changes in the electrocardiogram or heart rates were observed.
Pathological examination of organs and tissues did not reveal any
changes after exposure. Experimental data have been presented by
Orellano et al. (1976) who claim that carbon monoxide injected
intraperitoneally into dogs is nontoxic. This study, as well as other
reports from these investigators, raise some intriguing questions as
to the mechanisms involved in carbon monoxide toxicity.
Continuous exposure of cynomolgus monkeys to a carbon monoxide
concentration of 115 mg/m3 resulted in demonstrable electro-
cardiographic effects in the myocardium of both normal monkeys
and monkeys with myocardial infarction (De Bias et al., 1972b, 1973).
The same investigators (De Bias et al., 1976) also studied the
susceptibility of the ventricles to induced fibrillation. Normal and
infarcted monkeys were exposed to a carbon monoxide concentration of
115 mg/m3 for 6 h. Application of high voltages were required to
produce fibrillation in normal monkeys in air but when infarcted
animals were exposed to carbon monoxide, the voltage level required
was very low. Animals with either infarction alone or carbon monoxide
exposure alone also required significantly less voltage to produce
fibrillation; when the two were combined, the effects were additive.
Intermittent exposure to carbon monoxide (30 min per h, 12 h per day,
over a period of 14 months) of cynomolgus monkeys fed either a normal
or a semipurified cholesterol diet did not result in myocardial
infarctions or electrocardiographic abnormalities (Malinow et al.,
1976). In these animals, the blood carboxyhaemoglobin concentration
reached 21.6% at the end of the daily period of breathing carbon
monoxide at 529 mg/m3 (460 ppm).
Carbon monoxide exposure did not increase the aortic and coronary
atherosclerosis induced in cynomolgus monkeys by cholesterol feeding.
Eckardt et al. (1972) exposed cynomolgus monkeys (22 h per day, 7 days
per week, for 2 years) to carbon monoxide concentrations of 23 or
75 mg/m3 (20 or 65 ppm). Carboxyhaemoglobin levels, which showed
considerable variation during the experimental period, ranged from 2.0
to 5.5% and 4.8 to 10.2% for low and high carbon monoxide ambient
concentrations, respectively. These levels of carboxyhaemoglobin did
not lead to compensatory increases in haematocrit, haemoglobin, or
erythrocyte counts nor to cardiac fibrosis or pathological effects in
the brain. Cholesterol-fed squirrel monkeys were exposed to carbon
monoxide at 229-344 mg/m3 (200-300 ppm) for several hours per day
for 7 months (Webster et al., (1968). No differences were found in
plasma cholesterol or in aortic and carotid atherosclerosis which
could be ascribed to carbon monoxide. However, the authors did observe
an increase in coronary atherosclerosis. Somewhat similar results were
later reported in studies on cynomolgus monkeys (Malinow et al.,
1976). Jones et al. (1971) exposed rats, guineapigs, dogs, and monkeys
to 58, 110, or 229 mg/m3 (51, 96 or 200 ppm) continuously for 90
days. Haematocrit and haemoglobin levels remained constant at the
lowest level of carbon monoxide exposure but were significantly
elevated at the two higher levels in all species except the dog.
Cynomolgus monkeys (Macaca irus) were exposed to a concentration of
286 mg/m3 (250 ppm) for 2 weeks by Thomsen (1974). In all the
exposed animals, the coronary arteries showed widening of the
subendothelial spaces in which cells with or without lipid droplets
were accumulating. He suggested that monkeys were more sensitive to
carbon monoxide than the rabbits studied by Astrup et al. (1967).
Experimental studies on rabbits exposed to relatively high
concentrations of carbon monoxide at 195-206 mg/m3 (170-180 ppm) for
extended periods indicated the presence of high levels of cholesterol
in the arteries or enhanced vascular disease (Astrup et al., 1970).
The lesions observed, which included subendothelial oedema, a gap
between endothelial cells, and increased infiltration of cells with
lipid droplets, might be early precursors of atherosclerotic disease.
However, such lesions occurred only in animals concurrently on a high
cholesterol or fat diet or on both. Exposure to carbon monoxide alone
induced some changes such as endothelial hypertrophy and
proliferation.
One experimental study on the effects of carbon monoxide on the
natural history of heart disease in the cynomolgus monkey has been
reported. De Bias et al. (1973) exposed animals to a carbon monoxide
concentration of 137 mg/m3 (120 ppm) for 24 weeks. The average
carboxyhaemoglobin level of 12.4% resulted in a polycythaemia with an
increase in haematocrit from 35 to 50%. All animals developed
increased P-wave amplitude and T-inversion which suggested nonspecific
myocardial stress rather than ischaemia. Animals in which an
experimental myocardial infarction was produced prior to exposure to
carbon monoxide had more marked electro-cardiographic changes than
animals breathing room air. In 1976, Ramsey & Casper reported that
erythrocytic 2,3-diphosphoglycerate (2,3-DPG) played neither a
compensatory nor an aggravating role in the hypoxia induced by the
presence of 20 or 30% carboxyhaemoglobin. Stupfel & Bouley (1970)
exposed mice and rats for 95 h per week to a carbon monoxide
concentration of 57 mg/m3 (50 ppm) for either 1 to 3 months or for
their natural life expectancy of up to 2 years. A large number of
measurements were made during exposure followed by pathological
examination after death. The authors did not observe any important
effects of carbon monoxide exposure on the animals. In a study by
Penney et al. (1974a,b), the influence of hypoxic hypoxia on the
development of cardiac hypertrophy in the rat was compared with that
of carbon monoxide hypoxia. Exposure to various levels of carbon
monoxide resulted in hypertrophy of both the fight and left ventricles
in contrast with the right ventricle hypertrophy observed in response
to the hypoxic hypoxia stress.
Cardiac hypertrophy and a reduction in cytochrome oxidase levels
were demonstrated in chick embryos exposed to carbon monoxide for 144
and 168 h (Tumasonis & Baker, 1972). The resistance of young chickens
to carbon monoxide decreased with age. Body temperatures decreased
during exposure to carbon monoxide with the greatest fall in
temperature and the longest survival time occurring in the youngest
chickens. Total body asanguineous hypothermic perfusion (total body
exsanguination exchange transfusion) has been suggested as a
therapeutic measure for carbon monoxide poisoning (Agostini et al.,
1974). In an attempt to explain this beneficial effect, Ramirez et al.
(1974) compared the survival of normal dogs exposed to high levels of
carbon monoxide with that of acutely anaemic dogs transfused with
carboxyhaemoglobin blood to normal blood volumes. All normal dogs with
carboxyhaemoglobin levels of 54-100% died within 0.25-10 h but the
transfused animals, having a final mean carboxyhaemoglobin level of
80% after transfusion, survived. The authors suggested that hypoxic
anaemia was not the principal mechanism of carbon monoxide toxicity
but rather a blocking out of the energy supply on the cellular level,
governed by the cytochrome system.
Most of the investigations using rabbits for carbon monoxide
related research originated in Astrup's laboratories, where they first
demonstrated that low carboxyhaemoglobin levels enhanced the
development of atheromatosis (Astrup et al., 1967). Additional studies
(Hellung-Larsen et al., 1968; Thomsen & Kjeldsen, 1975) have shown
that lactate dehydrogenase isoenzymes (M subunits) increase and that a
higher incidence of focal intimal changes occur in rabbits exposed to
carbon monoxide. The myocardial ultrastructure of rabbits exposed to a
concentration of 206 mg/m3 (180 ppm) for at least 4 h showed
degenerative changes such as contraction bands, myofibrillar
disintegration, myelin body formation, and dehiscence of the
intercalated discs (Thomsen & Kjeldsen, 1974). Exposure of rabbits to
a similar concentration of carbon monoxide for 2 weeks resulted in
more extensive myocardial damage (Kjeldsen et al., 1974).
Astrup et al. (1970) furnished evidence that carbon monoxide
increases endothelial membrane permeability. They found that rabbits
exposed to carbon monoxide developed arterial lesions resulting in a
considerable accumulation of lipids. It has also been shown that human
coronary arteries exposed to carbon monoxide in vitro have a higher
uptake of cholesterol, although no significant changes in lipid
synthesis were observed (Sarma et al., 1975). Myocardial damage and
impaired myocardial performance have also been reported in animals and
man exposed to carbon monoxide (Ayres et al., 1970). Although there is
some evidence which suggests that exposure to carbon monoxide can
induce changes in blood vessels and the myocardium, there is also
evidence to the contrary (section 8.1).
7.3 Central Nervous System
Because of the brain's high oxygen demand, cerebral function
should be influenced at low carboxyhaemoglobin levels. However, data
concerning this are contradictory. Sensitivity to carbon monoxide may
follow a circadian rhythm (Stupfel, 1975; Stupfel et al., 1973).
Maximum sensitivity in rats occurred during the dark period of a
12-12 h light-dark cycle. Dyer & Annau (1976) could find no effect on
superior colliculus evoked potentials of rats until the level of
ambient carbon monoxide had reached 573 mg/m3 (500 ppm) in marked
contrast to Xintaras et al. (1966), who observed a 20% increase in the
amplitude of superior colliculus evoked potentials after only 1 h
exposure to 57 mg/m3 (50 ppm), and a 50% increase after a 2-h period
of exposure at this level. This study has, however, been criticized
for not taking into account the effects of dark adaptation on the
amplitude of visual evoked potentials in rats (Dyer & Annau, 1976).
In view of the conflicting reports it is of some interest to
examine the available data on cerebral pO2 tensions, cerebral
blood flow (CBF) and cerebral metabolism. Zorn (1972) studied the
effects of carbon monoxide inhalation on brain and liver pO2 using
platinum electrodes. Tissue pO2 fell in both organs, even at a
carboxyhaemoglobin concentration of 2%, and the fall was approximately
linear to increases in carboxyhaemoglobin. There was a decrease in
pO2 of 0.027-0.24 kPa (0.2-1.8 Torr) for each 1% fall in
oxyhaemoglobin percentage saturation. These data suggest that the
carbon monoxide influenced levels other than the intracellular level,
since if its effects were limited to this area then tissue pO2
would have been expected to increase. Similar studies were performed
by Weiss & Cohen (1974) on rat brain and muscle. They found a decrease
in cerebral cortical pO2 following inhalation of low levels of
carbon monoxide. Unfortunately, they did not measure
carboxyhaemoglobin levels in these rats but, in a group of sham-
operated animals exposed to similar levels of inhaled carbon monoxide,
carboxyhaemoglobin had increased to 3.3%. During progressive
administration of carbon monoxide to dogs, CBF did not increase until
carboxyhaemoglobin levels reached 20%. Thereafter, CBF increased
progressively and was double that in the controls when the
carboxyhaemoglobin level reached 50% (Häggendal et al., 1966). On the
other hand, Traystman (1976) did observe a progressive increase in CBF
in dogs even at very low carboxyhaemoglobin values. The lowest level
studied was 2.5%, which produced a slight but significant CBF
increase. Thus, Traystman did not believe in a threshold effect. The
effects were produced with both hypoxic hypoxia and carbon monoxide
hypoxia. It remains to be seen, how these data relate to cerebral
circulation in man.
The respiratory centre or arterial chemoreceptors were not
stimulated to increase respiratory minute volume even when
carboxyhaemoglobin levels were as high as 40% (Chiodi et al., 1941).
Mills & Edwards (1968) measured the frequency of electrical impulses
in the afferent nerves from the aortic and carotid chemoreceptors and
showed that administration of carbon monoxide did result in
chemoreceptor stimulation. The response appeared to have an
approximately linear relationship with the carboxyhaemoglobin
concentration (at least above 8%). These findings suggest that carbon
monoxide might stimulate breathing. Failure to observe an increased
minute volume may be explained by the fact that, in the presence of
carbon monoxide, the chemoreceptor stimulation was offset by hypoxic
depression of brain structures involved in breathing. There is some
evidence that this balancing between chemoreceptors and central
nervous depression is operative in anaemia (Santiago & Edelman, 1972).
Additional investigations are needed to clarify this effect of carbon
monoxide inhalation.
7.4 Behavioural Changes and Work Performance
In extensive studies on rabbits, guineapigs, rats, and mice
(Gadaskina, 1960; Ljublina, 1960; Rylova, 1960) exposure to a carbon
monoxide level of 30 mg/m3 (26 ppm), although not inducing any
morphological blood changes, did result in a number of unfavourable
physiological changes. Among these were decreased work capacity, poor
adjustment to postural shifts (orthostatic tests), and increased
thyroid activity. These changes were more evident during the initial
period of the exposure but reverted towards normal later, suggesting
some adaptation to carbon monoxide exposure.
7.5 Adaptation
Adaptation apparently can occur in animals exposed to moderate
concentrations of carbon monoxide (Gorbatov & Noro, 1948; Tiunov &
Kustov, 1969) as shown by their ability to tolerate, with apparent
ease, acute exposure to higher concentrations. Both Clark & Otis
(1952) and Tiunov & Kustov (1969) have demonstrated that, after long-
term exposure to carbon monoxide, animals developed tolerance to
short-term, high altitude exposure, and vice versa, an indication of
the development of a common adaptive mechanism. Acclimatization
continued despite a subsequent decrease in the initial elevation of
the haemoglobin concentration (Gorbatov & Noro, 1948). These
investigators noted an acclimatization effect in rats exposed daily to
0.4-0.5% carbon monoxide until loss of consciousness occurred. A
progressive improvement in tolerance time to unconsciousness was noted
so that by the eighth day of exposure a 3-fold improvement over the
time required on the first day had developed. In spite of apparent
acclimatization, the general condition of test animals became worse.
Unexpectedly, daily exposure to a carbon monoxide concentration of
1.0%, which on the first day necessitated a 5-min exposure prior to
unconsciousness, failed to induce any improvement in tolerance. A
possible correction of leftward shift of the oxygen dissociation by
alterations in the concentration of 2,3-diphosphoglycerate (inducing a
shift to the right) has been suggested. However, conflicting results
and the necessity to produce high levels of carboxyhaemoglobin negate
the possibilities of this beneficial effect (Astrup, 1970; Dinman et
al., 1970).
Evidence for adaptation to carbon monoxide is inconclusive.
Further studies appear to be warranted with special attention devoted
to studies using more realistic current ambient levels of carbon
monoxide, and also studies on the possible physiological cost of
adaptation, if it occurs.
7.6 Embryonal, Fetal, Neonatal, and Teratogenic Effects
Few studies have been made on the effects of carbon monoxide on
mammalian fetal growth and survival. Wells (1933) exposed pregnant
rats for 5-8 min to a carbon monoxide concentration of 1718 mg/m3
(1500 ppm) every other day during pregnancy. Maternal unconsciousness
and abortion or absorption of most of the fetuses resulted. Data
concerning carboxyhaemoglobin levels and numbers of animals studied
were not given. Rats were exposed to 0.34% carbon monoxide for 1 h
(carboxyhaemoglobin 60-70%) daily for a period of 3 months (Williams &
Smith, 1935). Among 7 pregnant females, the number of young per litter
was only half that of the controls and only 2 of the 13 newborns
survived to weaning age. Astrup et al. (1972) exposed rabbits during
their 30 days of pregnancy to carbon monoxide resulting in
carboxyhaemoglobin levels of either 9-19 or 16-18%. Neonatal mortality
in the 2 groups increased by 10 and 35%, respectively, compared with a
control value of 4.5%. Birth weights decreased by 12 and 17%,
respectively.
In their studies on the ewe and fetal lamb, Longo & Hill (1977)
indicated that fetal uptake and elimination of carbon monoxide was
relatively slow compared with that of the mother. They also reported
that, during steady-state conditions, fetal levels of
carboxyhaemoglobin were about 25% higher than maternal levels. These
results may be related to species differences, since Longo & Hill
(1970) found that the M valuesa for sheep maternal and fetal blood
were 218 and 216 respectively, while Engel et al. (1969) reported that
fetal haemoglobin had 20% less preferential binding of carbon monoxide
over oxygen than haemoglobin A.
There are very few studies on the teratogenicity of carbon
monoxide exposure. When fertilized chicken eggs were continuously
exposed to a carbon monoxide concentration of 747 mg/m3 (650 ppm)
for up to 18 days of incubation, the percentage of eggs hatching
decreased to 46% and developmental anomalies of the tibia and
metatarsal bones were noted (Baker & Tumasonis, 1972).
a M = Relative affinity of haemoglobin for carbon monoxide
compared with oxygen.
7.7 Carcinogenicity, and Mutagenicity
No evidence is available on carcinogenicity and mutagenicity in
relation to exposure to carbon monoxide.
7.8 Miscellaneous Changes
Kustov et al. (1972) exposed rats to carbon monoxide at 53 mg/m3
(46 ppm) and noted slower weight gains and an increase in haemoglobin.
Some enzyme systems were also found to have increased activity, when
rats were exposed to carbon monoxide (Pankow & Ponsold, 1972; Pankow
et al., 1974b). A slowing of in vivo metabolism of the drugs
hexobarbital and zoxazolamine, with prolongation of their
pharmacological effects has been reported in rats exposed to carbon
monoxide concentrations of 286-3435 mg/m3 (250-3000 ppm) (Montgomery
& Rubin, 1971). With prolonged exposure, these metabolic effects
became less pronounced and reverted to normal more quickly following
removal from the carbon monoxide environment. Sokal (1975) compared
the effect on blood pH and certain carbohydrate metabolic products
resulting from either a bolus administration or a fixed level of
inspired carbon monoxide, both resulting in equivalent final levels of
carboxyhaemoglobin. His data suggest that more intense biochemical
effects resulted following a gradual increase in carboxyhaemoglobin
levels compared with effects seen following rapid elevation of
carboxyhaemoglobin from the bolus. Data presented by Marks & Swiecicki
(1971) indicated that exposure to high levels of carbon monoxide
induced a stresslike response in the form of an elevation in
catecholamines. Swiecicki (1973) reported that increased
carboxyhaemoglobin levels stimulated the adrenergic system and
increased carbohydrate metabolism. He also noted that physical
training of the rat neither prevented nor reduced changes in
carbohydrate metabolism following carbon monoxide exposure and
vibration. Exposure of rats to 57 mg/m3 (50 ppm) for 5 h per day, 5
days per week, for 12 weeks produced an effect on trace metals at the
subcellular level, with a possible reduction in cellular respiration
and nucleoprotein synthesis.
Guineapigs exposed to carbon monoxide concentrations of
1.7-30 mg/m3 (1.5-26 ppm) for 21 days, 8 h per day, did not show any
allergenic effects related to carbon monoxide exposure (Vinogradov et
al., 1974). Plasma leucine aminopeptidase (EC 5.4.11.1)a and
glutamic pyruvic transaminase (EC 2.4.1.2) activity, normally
a The numbers within parentheses following the names of enzymes are
those assigned by the Enzyme Commission of the Joint IUPAC-IUB
Commission on Biochemical Nomenclature.
increased by exposure to carbon tetrachloride, were further
potentiated when blood carboxyhaemoglobin levels were elevated. Pankow
et al. (1974a) also observed an additive effect on some enzymes with a
combination of alcohol and carbon monoxide giving a carboxyhaemoglobin
concentration of 50%.
Rondia (1970) observed a significant reduction of benzopyrene-
hydroxylase (EC 1.14.14.2) activity in the liver homogenates of rats
exposed to a carbon monoxide concentration of 70-150 mg/m3
(60-130 ppm) for only a few days. This finding might be interpreted as
meaning that carbon monoxide contributes to the induction of lung
cancer by lengthening the time of retention of carcinogens in the
lung. Additional work is necessary to clarify this important question.
7.9 Interactions
Much of the data reviewed by Pankow & Ponsfold (1974) concerning
the combined effects of carbon monoxide and other biologically active
agents are based on animal experiments. Because of the extreme
exposure conditions used in most of these studies, only a few of them
are directly relevant to the environmental exposure of man to carbon
monoxide.
The experimental evidence on the aggravation of carbon monoxide-
induced atherosclerosis in rabbits by dietary cholesterol has already
been mentioned (see section 7.2). No significant additive effects were
noted from ethanol when dogs exposed to a carbon monoxide
concentration of 115 mg/m3 (100 ppm) for 21 weeks, 5 days a week,
and 6 h per day were given a daily oral dose of 120 ml of a 15%
ethanol solution (Pecora, 1959). However, the excretion of total
lipoproteins was higher when carbon monoxide and ethanol treatments
were combined than with exposure to carbon monoxide alone. An
indication of interaction of sulfur dioxide and carbon monoxide was
given by Prohorov & Rogov (1959) in their experiments on rabbits. The
depressed activity of succinate dehydrogenase (EC 1.3.99.1) on heart,
liver, and kidney due to exposure to sulfur dioxide at 200 mg/m3
(76 ppm) was exacerbated by exposure to carbon monoxide at a level of
200-400 mg/m3 (174-348 ppm), for 3 h per day, over a 3-week period.
As for the combined effects of carbon monoxide and temperature, Tiunov
& Kustov (1969) showed clearly that carbon monoxide toxicity in mice
increased at temperatures above or below normal ambient levels.
8. EFFECTS ON MAN
8.1 Healthy Subjects
8.1.1 Behavioural changes
Demonstrable changes in the central nervous system (CNS) function
of subjects inadvertently exposed to high levels of ambient carbon
monoxide in illuminating gas and in automobile exhaust resulted in a
series of studies to determine psychomotor and psychological
aberrations in subjects having more modest blood levels of
carboxyhaemoglobin than those observed in the potentially moribund
patients. Deficiencies in earlier studies have been related to
inadequate understanding of the significance of behavioural changes,
the inability to distinguish between simple perceptual motor
performance and the more complex performance involving sustained
and/or selective attention, short-term memory, and decision making
among possible alternatives. Furthermore, the physiological mechanisms
involved in carbon monoxide intoxication were not appreciated and the
available physiological and psychological tools were not adequately
exploited. Even today, some physiological and behavioural studies
suffer from similar or other inadequacies, e.g., the failure to
measure blood carboxyhaemoglobin levels, the inability to distinguish
between the physiological effects of a carbon monoxide bolus of high
concentration or the slow, insidious increment in carboxyhaemoglobin
levels over time with lower inhaled concentrations, the amount of
carbon monoxide brought to or removed from the lungs by changes in
alveolar ventilatory volumes, and the small number of volunteers
examined. Other factors involve failure to provide control measures
for bias and effects of the experimental worker (by means of double-
blind administration), control periods so that task-learning effects
do not mask negative results, homogeneity of the groups labelled
"smokers" and "nonsmokers", and control of possible boredom and
fatigue effects, all essentially amounting to a failure to adopt a
proper experimental design that would produce statistically
significant information.
A reduction of logical memory and recognition was demonstrated by
Chalupa (1960) in individuals subjected to acute carbon monoxide
intoxication. However, these functions returned to normal. Sayers et
al. (1929) did not find any significant changes in 6 exposed men
despite carboxyhaemoglobin levels of approximately 20-30%;
observations included hand-eye coordination and steadiness, tapping
speed, arithmetic (continuous addition), location memory, and simple
reaction time. Simple sensory-motor times decreased by 10% in subjects
with carboxyhaemoglobin concentrations of approximately 6.2% (5.5-7%)
(Tiunov & Kustov, 1969). Simulated driving performance did not
deteriorate despite carboxyhaemoglobin levels of 25%, although a small
deterioration was observed when the carboxyhaemoglobin levels were
above 35% (Forbes et al., 1937). The first demonstrable influences of
carbon monoxide on higher CNS functions were noted by McFarland et al.
(1944) in conjunction with their altitude studies, when they observed
reduction of visual acuity at carboxyhaemoglobin levels as low as 5%.
These observations were extended by Halperin et al. (1959) when they
reported that visual function was impaired at carboxyhaemoglobin
levels as low as 4% and that impairment increased at higher levels.
More recently, McFarland et al. (1973) showed that, for glare
recovery, the dark adaptation final threshold values increased as
carboxyhaemoglobin levels rose from control to 6-17%. Peripheral
recognition tasks were not affected until levels reached 17%. They
also stated that central and peripheral complex tasks were not
influenced by low levels of carboxyhaemoglobin. Schulte (1963)
demonstrated that there was a decrease in performance in higher
intellectual processes that was observable when the carboxyhaemoglobin
level exceeded 5%; further deterioration was noted as the level
increased. These results were in direct contradiction to the negative
results obtained by Dorcus & Weigand (1929) who used a similar series
of tests but with subjects exposed for a shorter period. In studies by
Beard & Wertheim (1967), the ability to judge correctly slight
differences in successive short time intervals showed significant
impairment when carboxyhaemoglobin levels were approximately 2 to 3%
above basal levels. These findings, suggesting an altered mental
function, represent the lowest levels of carboxyhaemoglobin that
produce a significant alteration in behavioural performance. However,
attempts to replicate them have been less than satisfactory (O'Donnell
et al., 1971a,b; Stewart et al., 1973a) even though the subjects in
all these other studies attained higher levels of carboxyhaemoglobin.
Some of the discrepancies may be explained on the basis of differences
in protocol and in the environmental conditions under which the tests
were conducted. Some of the investigators designed their experiments
to minimize the factors of boredom and fatigue while others attempted
to minimize external influences and conducted their experiments for a
relatively long time.
However, the Beard & Wertheim study may actually be more relevant
to questions of vigilance and ideally should be discussed in this
context. Assessment of vigilance is the determination of an
individual's ability to detect small changes in his environment,
changes that take place at unpredictable times and so demand
continuous attention. In such monotonous tasks, subjects miss signals
that they would not have missed when starting the task. Such signals
are presented visually or aurally. Fodor & Winneke (1972) and Groll-
Knapp et al. (1972) used auditory signals for their vigilance task.
The former investigators used a white noise (frequency range from 20
to 20 000 Hz lasting 0.36 sec and repeated at 2-sec intervals. About 3
out of every 100 of these noises were slightly less intense and were
used as the signal to which the subjects responded by pressing a
button. Twelve nonsmokers (male and female) were tested at carbon
monoxide concentrations of 0 and 57 mg/m3 (0-50 ppm). They breathed
this concentration for 80 min prior to the first of three vigilance
tests. Carboxyhaemoglobin levels were estimated to be 2.3 and 3.1% at
the beginning and the end of the first vigilance test, respectively.
Subjects were likely to miss signals during this initial test. This
was not observed during the next two vigilance tests (total exposure
to carbon monoxide being 210 min with the carboxyhaemoglobin level
estimated to have finally reached 4.3%). These data suggest an initial
decrement in performance followed by a compensatory response. Groll-
Knapp et al. (1972) exposed 20 subjects for a 2-h period to carbon
monoxide concentrations of 0, 57, 115, or 172 mg/m3 (0, 50, 100,
150 ppm). There is some doubt as to which subjects, if any, were
smokers. Carboxyhaemoglobin levels were also estimated by these
investigators at the end of the test period to be 0, 3.0, 5.4, and
7.6%. Over the 90 min of the auditory test, some 200 paired tones were
given in which a weaker second tone was the signal. The mean number of
signals missed during the control test was 26. The number missed
increased in the presence of elevated carboxyhaemoglobin levels so
that 35, 40, and 44 misses occurred in environments containing carbon
monoxide levels of 57, 114, and 172 mg/m3, respectively. This
suggests a significant impairment when a concentration of 57 mg/m3
is inhaled. Winneke (1974) used a similar test in studies at carbon
monoxide concentrations of 0, 57, and 115 mg/m3. Results with all
levels of carbon monoxide exposure were negative, in marked contrast
to the previous data despite an estimated carboxyhaemoglobin level of
approximately 9% at the end of the 115 mg/m3 exposure.
Beard & Grandstaff (1975) examined the effect of carbon monoxide
exposure on a visual vigilance task. The signal was a shorter flash of
light than the non-signals. Following a 30-min control period, 9
subjects were exposed to carbon monoxide concentrations of 0, 57, 200,
or 286 mg/m3 (0, 50, 175, 250 ppm). Subjects exposed to room air
detected 73% of the signals presented to them in three vigils. In
environments containing carbon monoxide concentrations of 57 and
200 mg/m3, respectively, 64% of the signals were detected. These
differences were statistically significant at the 5% level. However,
exposure to a concentration of 286 mg/m3 yielded a correct
identification rate of about 70% that was not statistically
significant. They estimated blood carboxyhaemoglobin levels from
alveolar breath samples to be 1.8, 5.2, and 7.5%, respectively. The
alveolar samples were obtained 30 min after the exposures were
completed. Krotova & Muzyka (1974) studied subjects working for about
2 years in an environment containing carbon monoxide. Mean
carboxyhaemoglobin levels were approximately 3.2% before, and 4% after
work. Eleven of 56 workers reported a loss of vigilance. It has been
reported by Rummo & Sarlanis (1974) that, during a 2-h vigilance
driving simulator task, subjects with carboxyhaemoglobin levels of
6-8% were significantly slower in responding to lead car speed
changes. Horvath et al. (1971) also used a visual vigilance test and
were the only group of investigators that actually measured blood
levels of carboxyhaemoglobin. The vigilance task in these studies was
the detection of a light pulse that was slightly brighter than the
base level light pulse. A 1-h vigil was preceded by a short alerting
pre-test during which a randomly interspersed 10 of 60 light pulses
were the brighter signals. After a 1-min rest, the 60-min vigilance
task was begun. Only 40 of 1200 light pulses were signals. Ten of
these signals appeared randomly out of the 300 presented each 15 min.
Three levels of ambient carbon monoxide were used, 0, 30, and
127 mg/m3 (0, 26, and 111 ppm) with each subject serving as his own
control. Exposures were randomized, with 1 week elapsing between each
exposure. Fig. 6 illustrates the changes in blood levels of
carboxyhaemoglobin with time under all conditions and compares the
concentrations with the levels determined from the data obtained by
Forbes et al. (1945). The control group breathing filtered air (no
carbon monoxide present) had carboxyhaemoglobin levels of 0.9% before
and at the completion of the task. Exposure to a carbon monoxide
concentration of 30 mg/m3 led to a level of 1.6% after the first
hour (before the vigilance task) and 2.3% at the end. Carbon monoxide
exposure at 127 mg/m3 resulted in carboxyhaemoglobin levels of 4.2%
after the first hour and 6.6% at completion of the vigilance test.
Performance during the pre-test period gave approximately 88 correct
responses in all three conditions, carbon monoxide exposure having no
discernible effect. During the vigilance test itself, subjects
breathing a concentration of 127 mg/m3 made significantly fewer
correct responses (4.2-6.6% carboxyhaemoglobin) than the same subjects
breathing 0 or 27 mg/m3 (Fig. 7). It appeared that when the
carboxyhaemoglobin level was approximately 5%, a significant decrement
in performance occurred. It should be noted that the slight
improvement at the end of the test period probably represented the
usual alerting response observed whenever subjects estimate that the
task is completed. Recently, Winneke et al. (1976) attempted to
replicate this study without success. Although Horvath's experiments
were started with 15 alleged nonsmokers, pre-exposure blood samples
from 5 of the subjects showed carboxyhaemoglobin levels of almost 3%.
At the completion of all the exposures, these subjects admitted
smoking, thus confirming the blood levels. Data on these subjects were
not included in this analysis. When these smokers' data were analysed,
performance on the vigilance task showed no deterioration even though,
with exposure to a carbon monoxide concentration of 127 mg/m3,
carboxyhaemoglobin levels increased from an initial 2.8% to 5.1% after
the first hour and to 6.9% at the completion of the vigilance task
(O'Hanlon, 1975). There were too few subjects to permit more than a
suggestion that prior, continuous exposure to nonambient carbon
monoxide may result in some degree of questionable adaptation. Beard &
Wertheim's (1967) earlier indications that some deleterious
psychological effects would appear at carboxyhaemoglobin levels of
about 2% have not been confirmed or even replicated.
The design and experimental control in the major studies concerned
with this subject have been poor with errors of omission, and failure
to present finalized data. In the early studies of Forbes et al.
(1937), 5 subjects were exposed to a carbon monoxide concentration

 sufficient to raise carboxyhaemoglobin to a level as high as 30% and
their reaction times, coordination, and perceptual skill determined
within the context of a test of driving skill. They failed to present
adequate control data and did not consider the adaptation that occurs
following repetitive tests. McFarland (1973) also studied subjects
with relatively high carboxyhaemoglobin levels (17%) in actual driving
conditions. The exact effects on driving skills could not be
determined from the data presented. Studies by Ray & Rockwell (1970)
and Weir & Rockwell (1973) although first reported in 1970, are still
in a preliminary form and despite some interesting indications of
effects, the data cannot be really considered of value in determining
effects. A "standard driving simulator" was used by Wright et al.
(1973) with both smokers and nonsmokers as subjects (final
carboxyhaemoglobin levels were 5.6 and 7.0% respectively). They
suggested that a 3.4% increase in carboxyhaemoglobin was sufficient to
cause unsafe driving. While questions regarding the data raise doubts
as to the value of this interpretation, these conclusions should be
noted in view of the data reported in the vigilance studies. However,
a certain amount of caution must be applied to any extrapolation of
specific, behavioural changes and driving performance, since the
latter requires integration of many signals in a complex interaction
not measured in any of the simpler tasks used in most behavioural
testing.
The available information on reaction times and time
discrimination is presented in considerable detail in a report from
the National Research Council (NAS/NRC, 1977). Despite apparently
well-controlled studies on both of these variables, the negative and
positive effects reported make it impossible to form any valid
conclusionsa. It would appear that considerable additional effort,
using a larger number of subjects, more adequate control of
experimental conditions (especially control of boredom and fatigue),
direct determinations of carboxyhaemoglobin, and attention to the
potential effects of low levels of carboxyhaemoglobin are required
before any valid conclusions can be drawn.
Apparently, coordination, dexterity, steadiness, and tracking
ability were not influenced by a carbon monoxide concentration which
raised carboxyhaemoglobin to levels exceeding 20%. McFarland et al.
(1944) and Halperin et al. (1959) reported that carboxyhaemoglobin
a The studies by Beard & Wertheim (1967) suggesting a critical
carboxyhaemoglobin level of approximately 2% were evaluated under
vigilance and are not repeated here. Attempts by other
investigators (O'Donnell et al., 197 lb; Stewart et al., 1975) to
reproduce their results have not been successful.
levels of 4-5% resulted in impaired brightness discrimination. Their
findings have been confirmed by Beard & Wertheim (1967). However,
Ramsey (1973) was unable to reproduce these deleterious effects on
brightness discrimination. The effects of carbon monoxide exposure on
complex learned behaviour have been studied by a number of
investigator's. Exposure of firemen to a concentration of 115 mg/m3
(100 ppm) for various periods of time (Schulte, 1963) resulted in
considerable changes in the performance of a series of complex tasks.
In a test where subjects were required to underline all plural nouns
in prose passages, decreased performance was noted when the carboxy-
haemoglobin level was approximately 8%. The mean time to complete an
arithmetic test significantly increased at similar or slightly lower
carboxyhaemoglobin levels. This investigator may have underestimated
the carboxyhaemoglobin levels since the subjects, although mostly
smokers, had initial values close to zero. O'Donnell et al. (1971a)
studied the ability of 4 subjects to perform arithmetic problems
without pencil and paper. While the subjects required a longer time to
complete the answers (89.8 versus 98.6 sec) when carboxyhaemoglobin
levels were 5.9% and 12.7%, respectively, some questions as to the
experimental design of these studies and the limited number of
subjects used preclude full acceptance of the results. A deterioration
in the ability to learn meaningless syllables was found by Bender et
al. (1972), when the carboxyhaemoglobin levels were about 7%. Other
tests failed to show deterioration at these levels of
carboxyhaemoglobin.
O'Donnell et al. (1971a) sought to determine how overnight
exposure to carbon monoxide concentrations of 86.0 mg/m3 and
172 mg/m3 (75 and 150 ppm) (carboxyhaemoglobin levels up to 12.7%)
affected sleep and found small but unreliable changes that they
interpreted as a possible reduction in central nervous activation. A
significant reduction in REM (rapid eye movement) sleep in subjects of
both sexes exposed for 7 h to a carbon monoxide concentration of
115 mg/m3 (100 ppm) was reported by Groll-Knapp et al. (1976).
Earlier, Helmchen & Künkel (1964) reported changes in the rhythmic
after-potential fluctuations following photic excitation of the brain
during and following carbon monoxide exposure. However, in contrast,
Dinman (1969) analysed the photic responses in subjects with
carboxyhaemoglobin levels of 22% and 37% and did not find any changes
in latency or voltage following photic stimulation. Sul'ga (1962) did
not find any disturbances of the alpha rhythm in 2 subjects exposed to
a carbon monoxide concentration of 20 mg/m3 (17.4 ppm) for 15 min.
Carbon monoxide-induced visual invoked responses were reported by
Hosko (1970) and Stewart et al. (1973a) but only at carboxyhaemoglobin
levels of 20-28%. Stewart et al. (1973b) later reported that neither
the spontaneous nor the evoked electrical activity of the brain
exhibited significant changes attributable to carbon monoxide exposure
(carboxyhaemoglobin levels from 3.2% to 15.2%). Slow-wave brain
potentials (correlated to anticipatory responses) were measured by
Groll-Knapp et al. (1972) who noted a diminution in the height reached
by the anticipation wave and the extent of the drop seen after
response stimulus following exposure to a carbon monoxide
concentration of 172 mg/m3 (150 ppm). It appears that another
conical function test, critical flicker fusion frequency (CFFF) is not
influenced even by carboxyhaemoglobin levels of between 10% and 12.7%
(Guest et al., 1970; O'Donnell et al., 1971a; Ramsey, 1973; Winneke,
1974). Guest et al. (1970) also used an auditory analogue of CFFF, the
auditory flutter fusion threshold. This threshold was not affected by
a carboxyhaemoglobin level of 10%. The literature has been reviewed by
Grandstaff et al. (1975).
8.1.2 Work performance and exercise
Maximum exercise can increase the oxygen uptake of the whole body
by 20 or more times the resting uptake; at this level the oxygen
transport system will be maximally stressed. Indeed, Mitchell et al.
(1958) have suggested that the maximum sustained energy output is
determined by the capability of the cardiovascular system to transport
oxygen to the exercising muscle. Assuming this concept to be true, any
impairment of oxygen transport, such as can occur when
carboxyhaemoglobin is present could limit maximum aerobic capacity
( Vo2 max). In fact, it has been appreciated for some time that
individuals, having a large burden of carbon monoxide experience
difficulty in performing physical work. Subjects studied by Chiodi et
al. (1941) were unable to perform tasks requiring only low levels of
physical exertion when their blood levels of carboxyhaemoglobin
reached 40-50%. Several collapsed while attempting to perform routine
laboratory exercise tests. Roughton & Darling (1944) also suggested,
on theoretical grounds, that work capacity would be reduced to zero
when carboxyhaemoglobin levels approached 50%. An impaired performance
by competitive swimmers was associated with exposure to a carbon
monoxide level of 34 mg/m3 (30 ppm) originating from traffic
(MacMillan, 1969)a. Douze (1971) presented information on the
incidence of carbon monoxide poisoning due to the use of natural gas
heaters in Utrecht.
a Quoted by the US National Research Council, Division of Medical
Sciences, Committee on Effects of Atmospheric Contaminants on Human
Health and Welfare, 1969, p. 55.
There appears to be complete agreement that performance of light
to moderate work (up to 70% Vo2 max)b for a short period of time
is not significantly influenced by carboxyhaemoglobin levels as high
as 33%. All the submaximal exercise tests were of short duration
(5-60 min). Oxygen uptake during work was unchanged despite the
presence of carboxyhaemoglobin (Chevalier et al., 1966; Ekblom & Huot,
1972; Gliner et al., 1975; Mitchell et al., 1958; Nielsen, 1971;
Pirnay et al., 1971; Vogel & Gleser, 1972; Vogel et al., 1972). The
only clear indication of physiological load appeared to be a slight
increase in heart rate. Chevalier and associates (1963, 1966) studying
men carrying out light work for a period of 5 min, reported that while
the oxygen uptake was unaffected when the carboxyhaemoglobin level was
approximately 4% (estimated value), there was a significant increase
in oxygen debt when this was related to the total increased oxygen
uptake. Five subjects studied by Pirnay et al. (1971) performing work
for 15 min had an oxygen uptake of 1.5 litre per min. No changes in
oxygen uptake were found even though the carboxyhaemoglobin level
reached 15%. In a rather involved study, where carboxyhaemoglobin
levels fluctuated between 5% and 17%, Klausen et al. (1968) did not
find any differences in energy expenditure in relation to exposure
when subjects exercised for 15 min at 50% of their Vo2 max. It is
rather interesting that, despite the considerable variations in such
experimental conditions as the duration and magnitude of exercise, the
level of carboxyhaemoglobin and the method of administration of carbon
monoxide, and also the small numbers and limited age ranges of the
exposed subjects -- the results from all these studies were
essentially similar. Pirnay et al. (1971), Vogel & Gleser (1972), and
Vogel et al. (1972) reported consistently higher heart rates for given
selected submaximal work loads and increased ventilatory volume
exchange per unit of oxygen uptake.
Since populations may be exposed to polluted environments for long
periods, Gliner et al. (1975) studied the responses of 2 groups of 10
and 9 men, respectively (mean age 23.0 and 48~4 years) each of which
included 5 subjects who smoked. A work load of 35% Vo2 max was
selected (untrained men can work at this level for approximately 8 h
with minimum physiological changes), and the men walked for 4 h in an
environment containing a carbon monoxide concentration of 57 mg/m3
b This figure may be in error for all levels of carboxyhaemoglobin
above 5% since Vo2 max decreases with increasing level of
carboxyhaemoglobin and the initial percentages of Vo2 max were
apparently determined on the basis of a Vo2 max measured at
0.5% carboxyhaemoglobin for the fixed work loads used in the
studies. Thus, the highest percentage of Vo2 max reported (70%)
may have been as high as 91% and would represent hard work. Vo2
max is identical to the maximum aerobic capacity representing the
capability of the organism to take up oxygen.
(50 ppm). Final carboxyhaemoglobin levels were 5.3 and 6.1% for
nonsmokers and smokers, respectively. An additional study was
conducted on 4 men exposed to a carbon monoxide concentration of
115 mg/m3 (100 ppm). Final carboxyhaemoglobin levels for nonsmokers
and smokers were 10.3 and 13.2%, respectively. Ambient temperatures
were 25°C and 35°C, with a relative humidity of 30%. Cardiovascular
and respiratory variables were measured. The only significant change
was a higher heart rate in the carbon monoxide environment,
irrespective of age of subject (Fig. 8), confirming observations,
previously reported. Cardiac index remained constant at approximately
6 litres/min × m2 in both filtered air and in carbon monoxide
concentrations of 57-115 mg/m3 (50-100 ppm). The full significance
of this change in long-term performance in carbon monoxide polluted
environments is not apparent, at present.
The oxygen transport capacity of blood is reduced in the presence
of carboxyhaemoglobin. In short-term maximum exercise of several
minutes duration, where capacity for effort depends mainly on aerobic
metabolism, maximum aerobic capacity would be expected to diminish
approximately in proportion to the level of carboxyhaemoglobin present
in the blood. Such a diminution in Vo2 max, when the carboxyhaemo-
globin level is between 7% and 33% has been observed by a number of
investigators (Chiodi et al., 1941; Ekblom & Huot, 1972; Horvath et
al., 1975; Nielsen, 1971; Pirnay et al. 1971). In most of these
studies, bouts of exercise ranged from 2-6 min and the mode of
administration of carbon monoxide involved either breathing relatively
high concentrations of the gas or the administration of a bolus with
additional carbon monoxide to maintain the desired levels of
carboxyhaemoglobin. In some of these studies, the smoking habits of
the subjects were not identified.
In all of these studies, the levels of carboxyhaemoglobin were
considerably in excess of those anticipated to occur in men exposed to
the concentrations of carbon monoxide designated as limiting levels by
various governing bodies or even reported to occur in the outdoor air
of certain metropolitan areas. The initial studies by Horvath's group
(Drinkwater et al., 1974; Raven et al., 1974a, b) were made on
subjects breathing a carbon monoxide concentration of 57 mg/m3
(50 ppm) at one of 2 thermal environments, i.e., 25°C or 35°C with a
relative humidity of 20%. A walking test requiring some 15-24 min to
complete was carried out on a treadmill with progressively increasing
grade, in order to measure Vo2 max. The 2 populations consisted of
20 young (24+ years) and 16 middle-aged (48+ years) subjects with
equal numbers of smokers and nonsmokers in the young group and 7
smokers and 9 nonsmokers in the older group. The middle-aged subjects
demonstrated the anticipated decrease in Vo2 max associated with
advancing age. However, the middle-aged nonsmokers had a Vo2 max
that was about 27% greater than that of smokers of the same age. As
the test progressed, the carboxyhaemoglobin levels of nonsmokers
sufficient to raise carboxyhaemoglobin to a level as high as 30% and
their reaction times, coordination, and perceptual skill determined
within the context of a test of driving skill. They failed to present
adequate control data and did not consider the adaptation that occurs
following repetitive tests. McFarland (1973) also studied subjects
with relatively high carboxyhaemoglobin levels (17%) in actual driving
conditions. The exact effects on driving skills could not be
determined from the data presented. Studies by Ray & Rockwell (1970)
and Weir & Rockwell (1973) although first reported in 1970, are still
in a preliminary form and despite some interesting indications of
effects, the data cannot be really considered of value in determining
effects. A "standard driving simulator" was used by Wright et al.
(1973) with both smokers and nonsmokers as subjects (final
carboxyhaemoglobin levels were 5.6 and 7.0% respectively). They
suggested that a 3.4% increase in carboxyhaemoglobin was sufficient to
cause unsafe driving. While questions regarding the data raise doubts
as to the value of this interpretation, these conclusions should be
noted in view of the data reported in the vigilance studies. However,
a certain amount of caution must be applied to any extrapolation of
specific, behavioural changes and driving performance, since the
latter requires integration of many signals in a complex interaction
not measured in any of the simpler tasks used in most behavioural
testing.
The available information on reaction times and time
discrimination is presented in considerable detail in a report from
the National Research Council (NAS/NRC, 1977). Despite apparently
well-controlled studies on both of these variables, the negative and
positive effects reported make it impossible to form any valid
conclusionsa. It would appear that considerable additional effort,
using a larger number of subjects, more adequate control of
experimental conditions (especially control of boredom and fatigue),
direct determinations of carboxyhaemoglobin, and attention to the
potential effects of low levels of carboxyhaemoglobin are required
before any valid conclusions can be drawn.
Apparently, coordination, dexterity, steadiness, and tracking
ability were not influenced by a carbon monoxide concentration which
raised carboxyhaemoglobin to levels exceeding 20%. McFarland et al.
(1944) and Halperin et al. (1959) reported that carboxyhaemoglobin
a The studies by Beard & Wertheim (1967) suggesting a critical
carboxyhaemoglobin level of approximately 2% were evaluated under
vigilance and are not repeated here. Attempts by other
investigators (O'Donnell et al., 197 lb; Stewart et al., 1975) to
reproduce their results have not been successful.
levels of 4-5% resulted in impaired brightness discrimination. Their
findings have been confirmed by Beard & Wertheim (1967). However,
Ramsey (1973) was unable to reproduce these deleterious effects on
brightness discrimination. The effects of carbon monoxide exposure on
complex learned behaviour have been studied by a number of
investigator's. Exposure of firemen to a concentration of 115 mg/m3
(100 ppm) for various periods of time (Schulte, 1963) resulted in
considerable changes in the performance of a series of complex tasks.
In a test where subjects were required to underline all plural nouns
in prose passages, decreased performance was noted when the carboxy-
haemoglobin level was approximately 8%. The mean time to complete an
arithmetic test significantly increased at similar or slightly lower
carboxyhaemoglobin levels. This investigator may have underestimated
the carboxyhaemoglobin levels since the subjects, although mostly
smokers, had initial values close to zero. O'Donnell et al. (1971a)
studied the ability of 4 subjects to perform arithmetic problems
without pencil and paper. While the subjects required a longer time to
complete the answers (89.8 versus 98.6 sec) when carboxyhaemoglobin
levels were 5.9% and 12.7%, respectively, some questions as to the
experimental design of these studies and the limited number of
subjects used preclude full acceptance of the results. A deterioration
in the ability to learn meaningless syllables was found by Bender et
al. (1972), when the carboxyhaemoglobin levels were about 7%. Other
tests failed to show deterioration at these levels of
carboxyhaemoglobin.
O'Donnell et al. (1971a) sought to determine how overnight
exposure to carbon monoxide concentrations of 86.0 mg/m3 and
172 mg/m3 (75 and 150 ppm) (carboxyhaemoglobin levels up to 12.7%)
affected sleep and found small but unreliable changes that they
interpreted as a possible reduction in central nervous activation. A
significant reduction in REM (rapid eye movement) sleep in subjects of
both sexes exposed for 7 h to a carbon monoxide concentration of
115 mg/m3 (100 ppm) was reported by Groll-Knapp et al. (1976).
Earlier, Helmchen & Künkel (1964) reported changes in the rhythmic
after-potential fluctuations following photic excitation of the brain
during and following carbon monoxide exposure. However, in contrast,
Dinman (1969) analysed the photic responses in subjects with
carboxyhaemoglobin levels of 22% and 37% and did not find any changes
in latency or voltage following photic stimulation. Sul'ga (1962) did
not find any disturbances of the alpha rhythm in 2 subjects exposed to
a carbon monoxide concentration of 20 mg/m3 (17.4 ppm) for 15 min.
Carbon monoxide-induced visual invoked responses were reported by
Hosko (1970) and Stewart et al. (1973a) but only at carboxyhaemoglobin
levels of 20-28%. Stewart et al. (1973b) later reported that neither
the spontaneous nor the evoked electrical activity of the brain
exhibited significant changes attributable to carbon monoxide exposure
(carboxyhaemoglobin levels from 3.2% to 15.2%). Slow-wave brain
potentials (correlated to anticipatory responses) were measured by
Groll-Knapp et al. (1972) who noted a diminution in the height reached
by the anticipation wave and the extent of the drop seen after
response stimulus following exposure to a carbon monoxide
concentration of 172 mg/m3 (150 ppm). It appears that another
conical function test, critical flicker fusion frequency (CFFF) is not
influenced even by carboxyhaemoglobin levels of between 10% and 12.7%
(Guest et al., 1970; O'Donnell et al., 1971a; Ramsey, 1973; Winneke,
1974). Guest et al. (1970) also used an auditory analogue of CFFF, the
auditory flutter fusion threshold. This threshold was not affected by
a carboxyhaemoglobin level of 10%. The literature has been reviewed by
Grandstaff et al. (1975).
8.1.2 Work performance and exercise
Maximum exercise can increase the oxygen uptake of the whole body
by 20 or more times the resting uptake; at this level the oxygen
transport system will be maximally stressed. Indeed, Mitchell et al.
(1958) have suggested that the maximum sustained energy output is
determined by the capability of the cardiovascular system to transport
oxygen to the exercising muscle. Assuming this concept to be true, any
impairment of oxygen transport, such as can occur when
carboxyhaemoglobin is present could limit maximum aerobic capacity
( Vo2 max). In fact, it has been appreciated for some time that
individuals, having a large burden of carbon monoxide experience
difficulty in performing physical work. Subjects studied by Chiodi et
al. (1941) were unable to perform tasks requiring only low levels of
physical exertion when their blood levels of carboxyhaemoglobin
reached 40-50%. Several collapsed while attempting to perform routine
laboratory exercise tests. Roughton & Darling (1944) also suggested,
on theoretical grounds, that work capacity would be reduced to zero
when carboxyhaemoglobin levels approached 50%. An impaired performance
by competitive swimmers was associated with exposure to a carbon
monoxide level of 34 mg/m3 (30 ppm) originating from traffic
(MacMillan, 1969)a. Douze (1971) presented information on the
incidence of carbon monoxide poisoning due to the use of natural gas
heaters in Utrecht.
a Quoted by the US National Research Council, Division of Medical
Sciences, Committee on Effects of Atmospheric Contaminants on Human
Health and Welfare, 1969, p. 55.
There appears to be complete agreement that performance of light
to moderate work (up to 70% Vo2 max)b for a short period of time
is not significantly influenced by carboxyhaemoglobin levels as high
as 33%. All the submaximal exercise tests were of short duration
(5-60 min). Oxygen uptake during work was unchanged despite the
presence of carboxyhaemoglobin (Chevalier et al., 1966; Ekblom & Huot,
1972; Gliner et al., 1975; Mitchell et al., 1958; Nielsen, 1971;
Pirnay et al., 1971; Vogel & Gleser, 1972; Vogel et al., 1972). The
only clear indication of physiological load appeared to be a slight
increase in heart rate. Chevalier and associates (1963, 1966) studying
men carrying out light work for a period of 5 min, reported that while
the oxygen uptake was unaffected when the carboxyhaemoglobin level was
approximately 4% (estimated value), there was a significant increase
in oxygen debt when this was related to the total increased oxygen
uptake. Five subjects studied by Pirnay et al. (1971) performing work
for 15 min had an oxygen uptake of 1.5 litre per min. No changes in
oxygen uptake were found even though the carboxyhaemoglobin level
reached 15%. In a rather involved study, where carboxyhaemoglobin
levels fluctuated between 5% and 17%, Klausen et al. (1968) did not
find any differences in energy expenditure in relation to exposure
when subjects exercised for 15 min at 50% of their Vo2 max. It is
rather interesting that, despite the considerable variations in such
experimental conditions as the duration and magnitude of exercise, the
level of carboxyhaemoglobin and the method of administration of carbon
monoxide, and also the small numbers and limited age ranges of the
exposed subjects -- the results from all these studies were
essentially similar. Pirnay et al. (1971), Vogel & Gleser (1972), and
Vogel et al. (1972) reported consistently higher heart rates for given
selected submaximal work loads and increased ventilatory volume
exchange per unit of oxygen uptake.
Since populations may be exposed to polluted environments for long
periods, Gliner et al. (1975) studied the responses of 2 groups of 10
and 9 men, respectively (mean age 23.0 and 48~4 years) each of which
included 5 subjects who smoked. A work load of 35% Vo2 max was
selected (untrained men can work at this level for approximately 8 h
with minimum physiological changes), and the men walked for 4 h in an
environment containing a carbon monoxide concentration of 57 mg/m3
b This figure may be in error for all levels of carboxyhaemoglobin
above 5% since Vo2 max decreases with increasing level of
carboxyhaemoglobin and the initial percentages of Vo2 max were
apparently determined on the basis of a Vo2 max measured at
0.5% carboxyhaemoglobin for the fixed work loads used in the
studies. Thus, the highest percentage of Vo2 max reported (70%)
may have been as high as 91% and would represent hard work. Vo2
max is identical to the maximum aerobic capacity representing the
capability of the organism to take up oxygen.
(50 ppm). Final carboxyhaemoglobin levels were 5.3 and 6.1% for
nonsmokers and smokers, respectively. An additional study was
conducted on 4 men exposed to a carbon monoxide concentration of
115 mg/m3 (100 ppm). Final carboxyhaemoglobin levels for nonsmokers
and smokers were 10.3 and 13.2%, respectively. Ambient temperatures
were 25°C and 35°C, with a relative humidity of 30%. Cardiovascular
and respiratory variables were measured. The only significant change
was a higher heart rate in the carbon monoxide environment,
irrespective of age of subject (Fig. 8), confirming observations,
previously reported. Cardiac index remained constant at approximately
6 litres/min × m2 in both filtered air and in carbon monoxide
concentrations of 57-115 mg/m3 (50-100 ppm). The full significance
of this change in long-term performance in carbon monoxide polluted
environments is not apparent, at present.
The oxygen transport capacity of blood is reduced in the presence
of carboxyhaemoglobin. In short-term maximum exercise of several
minutes duration, where capacity for effort depends mainly on aerobic
metabolism, maximum aerobic capacity would be expected to diminish
approximately in proportion to the level of carboxyhaemoglobin present
in the blood. Such a diminution in Vo2 max, when the carboxyhaemo-
globin level is between 7% and 33% has been observed by a number of
investigators (Chiodi et al., 1941; Ekblom & Huot, 1972; Horvath et
al., 1975; Nielsen, 1971; Pirnay et al. 1971). In most of these
studies, bouts of exercise ranged from 2-6 min and the mode of
administration of carbon monoxide involved either breathing relatively
high concentrations of the gas or the administration of a bolus with
additional carbon monoxide to maintain the desired levels of
carboxyhaemoglobin. In some of these studies, the smoking habits of
the subjects were not identified.
In all of these studies, the levels of carboxyhaemoglobin were
considerably in excess of those anticipated to occur in men exposed to
the concentrations of carbon monoxide designated as limiting levels by
various governing bodies or even reported to occur in the outdoor air
of certain metropolitan areas. The initial studies by Horvath's group
(Drinkwater et al., 1974; Raven et al., 1974a, b) were made on
subjects breathing a carbon monoxide concentration of 57 mg/m3
(50 ppm) at one of 2 thermal environments, i.e., 25°C or 35°C with a
relative humidity of 20%. A walking test requiring some 15-24 min to
complete was carried out on a treadmill with progressively increasing
grade, in order to measure Vo2 max. The 2 populations consisted of
20 young (24+ years) and 16 middle-aged (48+ years) subjects with
equal numbers of smokers and nonsmokers in the young group and 7
smokers and 9 nonsmokers in the older group. The middle-aged subjects
demonstrated the anticipated decrease in Vo2 max associated with
advancing age. However, the middle-aged nonsmokers had a Vo2 max
that was about 27% greater than that of smokers of the same age. As
the test progressed, the carboxyhaemoglobin levels of nonsmokers
 increased from 0.7% to approximately 2.8%, while those of smokers rose
from 2.6-3.2% to 4.1-4.5%. During control studies conducted on these
subjects while breathing filtered air, carboxyhaemoglobin levels
decreased in both smokers and nonsmokers. The results of these studies
(Drinkwater et al., 1974; Gliner et al., 1975; Raven et al., 1974a, b)
failed to demonstrate any reduction in Vo2, max. The decrement in
Vo2 max that occurred as a consequence of working in a hot
environment was greater than the changes observed while breathing
carbon monoxide. Other cardiovascular, respiratory, metabolic, and
temperature measurements made concurrently with the oxygen uptake
studies also failed to show any decrements associated with carbon
monoxide exposure. However, a decrease in absolute exercise time
consistently observed in nonsmoking subjects but not in smokers was
significantly related to carbon monoxide exposure. These observations
confirmed those found earlier by Ekblom & Huot (1972), although they
reported a surprisingly large (38%) decrease in work time at a
carboxyhaemoglobin level of 7%. Aronow & Cassidy (1975) have recently
reported a slight decrease in work time during a maximum exercise test
on 10 middle-aged (50.7 years) subjects. The only ischaemic S-T
segment depression occurred in one female subject. No electro-
cardiographic changes were observed in the subjects studied by
Horvath's group. Nielsen (1971) found that exercising subjects
developed higher internal body temperatures in the presence of carbon
monoxide than in its absence. Reductions in skin conductance suggested
a redistribution of the circulation to the working muscle and away
from the skin.
Horvath and co-workers had some doubts about the changes in
carboxyhaemoglobin levels in smokers and nonsmokers as well as the
lack of change in Vo2 max under the ambient and exercise
conditions employed. For their next series of studies (Dahms et al.,
1975), they developed a more precise method to regulate relatively low
levels of carboxyhaemoglobin (Fig. 9). It should be noted that a low
ambient level of carbon monoxide will reduce the rate of pulmonary
excretion of carbon monoxide particularly if the carboxyhaemoglobin
level is low. In these experiments, a double-blind study was again
used in which subjects breathed either filtered air or air containing
carbon monoxide which resulted in stable levels of carboxyhaemoglobin.
The data suggest that a critical level of carboxyhaemoglobin must be
present before significant physiological alterations can be
demonstrated. Statistically significant decreases in Vo2 max were
noted when carboxyhaemoglobin levels exceeded 4.3%. Although this was
a double-blind, randomized study in which neither the investigators
nor the subjects knew the composition of the air breathed, it was
subsequently determined that all subjects correctly identified the
experiment in which they had been exposed to the highest level of
ambient carbon monoxide. In all instances, they noted a heaviness in
the lower extremities and greater difficulty in performing the task.
increased from 0.7% to approximately 2.8%, while those of smokers rose
from 2.6-3.2% to 4.1-4.5%. During control studies conducted on these
subjects while breathing filtered air, carboxyhaemoglobin levels
decreased in both smokers and nonsmokers. The results of these studies
(Drinkwater et al., 1974; Gliner et al., 1975; Raven et al., 1974a, b)
failed to demonstrate any reduction in Vo2, max. The decrement in
Vo2 max that occurred as a consequence of working in a hot
environment was greater than the changes observed while breathing
carbon monoxide. Other cardiovascular, respiratory, metabolic, and
temperature measurements made concurrently with the oxygen uptake
studies also failed to show any decrements associated with carbon
monoxide exposure. However, a decrease in absolute exercise time
consistently observed in nonsmoking subjects but not in smokers was
significantly related to carbon monoxide exposure. These observations
confirmed those found earlier by Ekblom & Huot (1972), although they
reported a surprisingly large (38%) decrease in work time at a
carboxyhaemoglobin level of 7%. Aronow & Cassidy (1975) have recently
reported a slight decrease in work time during a maximum exercise test
on 10 middle-aged (50.7 years) subjects. The only ischaemic S-T
segment depression occurred in one female subject. No electro-
cardiographic changes were observed in the subjects studied by
Horvath's group. Nielsen (1971) found that exercising subjects
developed higher internal body temperatures in the presence of carbon
monoxide than in its absence. Reductions in skin conductance suggested
a redistribution of the circulation to the working muscle and away
from the skin.
Horvath and co-workers had some doubts about the changes in
carboxyhaemoglobin levels in smokers and nonsmokers as well as the
lack of change in Vo2 max under the ambient and exercise
conditions employed. For their next series of studies (Dahms et al.,
1975), they developed a more precise method to regulate relatively low
levels of carboxyhaemoglobin (Fig. 9). It should be noted that a low
ambient level of carbon monoxide will reduce the rate of pulmonary
excretion of carbon monoxide particularly if the carboxyhaemoglobin
level is low. In these experiments, a double-blind study was again
used in which subjects breathed either filtered air or air containing
carbon monoxide which resulted in stable levels of carboxyhaemoglobin.
The data suggest that a critical level of carboxyhaemoglobin must be
present before significant physiological alterations can be
demonstrated. Statistically significant decreases in Vo2 max were
noted when carboxyhaemoglobin levels exceeded 4.3%. Although this was
a double-blind, randomized study in which neither the investigators
nor the subjects knew the composition of the air breathed, it was
subsequently determined that all subjects correctly identified the
experiment in which they had been exposed to the highest level of
ambient carbon monoxide. In all instances, they noted a heaviness in
the lower extremities and greater difficulty in performing the task.
 Data obtained by Horvath's group and others are summarized in
Fig. 10. There is a linear decline in Vo2 max when
carboxyhaemoglobin levels range from 4 to 33%. This can be expressed
as: % decrease in Vo2 max = 0.91 (% HbCO) + 2.2. It should be
noted that this does not apply to smokers in Horvath's series, who
frequently had carboxyhaemoglobin levels considerably in excess of
4-5% with no decrement in their respective Vo2 max values.
According to data available at present, carbon monoxide can modify
physiological responses. The level of blood carboxyhaemoglobin
required to induce these effects appears to be approximately 5%. The
carboxyhaemoglobin concentration is probably a more accurate
assessment of exposure than a statement of the exposure conditions,
ambient carbon monoxide concentrations, time, etc. Therefore,
physiological effects should be related to a carboxyhaemoglobin level
even though in some circumstances the method of exposure, i.e. rapid
loading versus slow loading may produce effects not evident from the
carboxyhaemoglobin concentration.
8.1.3 Adaptation
The implication that, in the presence of a clinical state of
chronic carbon monoxide poisoning, adaptation to carbon monoxide
occurs has not been verified. It would appear that such a state could
have been identified by studies on long-term heavy smokers. or
individuals exposed to environmental sources of carbon monoxide. Early
concern with carbon monoxide intoxication in England and Scandinavia
resulted in studies suggesting the possibility of such a condition
(Grut, 1949; Killick, 1940). However, doubts regarding the use of high
levels of inspired carbon monoxide (several hundred parts per million)
and inadequate experimental methods give rise to some scepticism about
the conclusions presented. Killick (1940), using herself as a subject,
reported that she developed acclimatization in the form of diminished
symptoms, slower heart rate, and the attainment of a lower
carboxyhaemoglobin equilibrium level following exposure to a given
inspired concentration of carbon monoxide. A similar finding
concerning the attainment of a different carboxyhaemoglobin
equilibrium following exposure to a fixed level of carbon monoxide in
the ambient air had been reported earlier by Haldane & Priestley
(1935). Additional information on other possible adaptation effects in
the pre-1940 literature can be found in Killick's review.
The changes indicated above, which have been reported as evidence
of adaptation, are probably related to compensatory haematological
changes. Some polycythaemia occurs as a response to chronic or
repeated exposure corresponding roughly to the ambient levels of
carbon monoxide. Brieger (1944) reported an increase in red cell mass
following exposure to 115 mg/m3 (100 ppm) and industrial workers
Data obtained by Horvath's group and others are summarized in
Fig. 10. There is a linear decline in Vo2 max when
carboxyhaemoglobin levels range from 4 to 33%. This can be expressed
as: % decrease in Vo2 max = 0.91 (% HbCO) + 2.2. It should be
noted that this does not apply to smokers in Horvath's series, who
frequently had carboxyhaemoglobin levels considerably in excess of
4-5% with no decrement in their respective Vo2 max values.
According to data available at present, carbon monoxide can modify
physiological responses. The level of blood carboxyhaemoglobin
required to induce these effects appears to be approximately 5%. The
carboxyhaemoglobin concentration is probably a more accurate
assessment of exposure than a statement of the exposure conditions,
ambient carbon monoxide concentrations, time, etc. Therefore,
physiological effects should be related to a carboxyhaemoglobin level
even though in some circumstances the method of exposure, i.e. rapid
loading versus slow loading may produce effects not evident from the
carboxyhaemoglobin concentration.
8.1.3 Adaptation
The implication that, in the presence of a clinical state of
chronic carbon monoxide poisoning, adaptation to carbon monoxide
occurs has not been verified. It would appear that such a state could
have been identified by studies on long-term heavy smokers. or
individuals exposed to environmental sources of carbon monoxide. Early
concern with carbon monoxide intoxication in England and Scandinavia
resulted in studies suggesting the possibility of such a condition
(Grut, 1949; Killick, 1940). However, doubts regarding the use of high
levels of inspired carbon monoxide (several hundred parts per million)
and inadequate experimental methods give rise to some scepticism about
the conclusions presented. Killick (1940), using herself as a subject,
reported that she developed acclimatization in the form of diminished
symptoms, slower heart rate, and the attainment of a lower
carboxyhaemoglobin equilibrium level following exposure to a given
inspired concentration of carbon monoxide. A similar finding
concerning the attainment of a different carboxyhaemoglobin
equilibrium following exposure to a fixed level of carbon monoxide in
the ambient air had been reported earlier by Haldane & Priestley
(1935). Additional information on other possible adaptation effects in
the pre-1940 literature can be found in Killick's review.
The changes indicated above, which have been reported as evidence
of adaptation, are probably related to compensatory haematological
changes. Some polycythaemia occurs as a response to chronic or
repeated exposure corresponding roughly to the ambient levels of
carbon monoxide. Brieger (1944) reported an increase in red cell mass
following exposure to 115 mg/m3 (100 ppm) and industrial workers
 exposed to rather ill-defined levels of carbon monoxide have been
reported to be polycythaemic (Jenkins, 1932). Wilks et al. (1959)
believed that acclimatization was purely a function of increased red
cell mass.
The possibility that adaptation to carbon monoxide following
extensive exposure (as occurs in the case of adaptation to high
altitudes) could alter the position of the oxygen dissociation curve
appears to have been answered. Mulhausen et al. (1968) did not find
any change in the degree of left shift in the blood of individuals
exposed to carbon monoxide for a period of 8 days. Unfortunately, the
average carboxyhaemoglobin level of 13% was based on considerable
individual variations in carboxyhaemoglobin levels and periodic
exposure to relatively high concentrations of inhaled carbon monoxide.
Several investigators have sought evidence of a potential shift of the
curve back to the right. Red cell levels of 2,3-diphosphoglycerate
compounds are higher in individuals with anaemia and also during
residence at high altitudes (2,3-diphosphoglycerate is a
phosphorylated by-product of glycolysis). In the erythrocytes of man
and most other mammals, the molar concentration of this compound is
roughly equal to that of haemoglobin. Both it and some other organic
phosphates are bound rather strongly to deoxyhaemoglobin but have
little affinity for oxyhaemoglobin. Increases in 2,3-diphospho-
glycerate shift the effective oxygen affinity, i.e., there is a shift
of the oxyhaemoglobin dissociation to the right. Astrup (1970) found a
small decrease in erythrocyte 2,3-diphospho-glycerate in human
subjects with carboxyhaemoglobin levels maintained at 20% for 24 h.
Conversely, Dinman et al. (1970) found a small increase in 2,3-
diphosphoglycerate in human subjects after 3 h at an approximate
carboxyhaemoglobin level of 20% and in rats exposed to higher but
variable concentrations of carbon monoxide. A shift in the
dissociation curve does not appear to be an important adaptation
mechanism when carbon monoxide exposure lasts less than a few days.
8.1.4 Effects on the cardiovascular system and other effects
Functional heart disturbances (lability of blood pressure and
heart acceleration, extrasystoles, exacerbations of angina pectoris),
as well as temporary heart dilatation and cardiac asthma have been
reported in cases of acute carbon monoxide poisoning (Lazarev, 1965).
According to the same author, various changes were also seen in the
peripheral vascular system (vasodilation, stasis, vasopermeability
etc.). Lazarev (1965) also noted severe cardiovascular disturbances
such as heart acceleration, extra-systoles, pulse and blood pressure
lability (more often hypotension than hypertension) in groups of
workers exposed to carbon monoxide for long periods. Disturbances of
atrioventricular and interventricular conductance were observed after
1 to 1.5 years of exposure and even after cessation of contact with
carbon monoxide.
The first evidence of left ventricular abnormality was presented
by Corya et al. (1976) in 5 cases of nonfatal poisoning (carboxyhaemo-
globin level of 20%). Abnormal left ventricular wall motion was shown
by echocardiograph in 3 of the 5 cases. A similar number showed mitral
valve prolapse. A ballistocardiogram was used by Gorski (1962) to
demonstrate hypoxaemia of the myocardium in similar cases. Byczkowska
& Milan (1971) described functional kidney disturbances in a patient
poisoned with carbon monoxide. Clinical and physiological haemodynamic
studies on 2 groups (individuals in constant contact with carbon
monoxide and individuals having no evidence of chronic carbon monoxide
intoxication) were conducted by Zenkevic (1973). He noted considerable
cardiovascular abnormalities in the carbon monoxide-exposed group. A
study on cast-iron workers by Ejam-Berdjev (1973) also suggested a
larger frequency of cardiovascular, as well as central nervous system
disturbances in these workers, related to their increased blood levels
of carboxyhaemoglobin.
Evidence of a myocardosis was found in 18% of Japanese farmers
chronically exposed to a mean carbon monoxide concentration of
80 mg/m3 (70 ppm), (Komatsu, 1959). The exposure occurred as a
result of spending the winter months preparing hemp in enclosed
dwellings heated by charcoal fires. Following exposure, the farmers
exhibited symptoms of dizziness, palpitation, and congestive heart
failure. The diagnosis of a myocardosis was supported by clinical
evidence of congestive heart failure and ECG changes such as prolonged
QT interval, ST segment depression, and T-wave flattening.
Aleksieva & Dimitrova (1971) studied a large group of workers
exposed to a carbon monoxide concentration of 60 mg/m3 (52 ppm) and
reported changes in peripheral vessels suggesting impaired vascular
tone.
An additional hazard to patients, especially those undergoing
cardiovascular surgery, may develop during anaesthesia. Markedly
elevated carboxyhaemoglobin levels have been reported in patients
under cardiac bypass surgery (Middleton et al., 1965). This increase
could be related in part to the carbon monoxide present in transfused
blood and to the closed-circuit method of anaesthesia that precludes
the loss of endogenously produced carbon monoxide. This is also
important for infants undergoing transfusions (see sections 5.4 and
8.2.3).
8.1.5 Carboxyhaemoglobin levels resulting from exposure to
methane-derived halogenated hydrocarbons
The belated discovery that at least one chemical substance used in
industry and commerce is "degraded" within the body to carbon monoxide
has potentially significant epidemiological and clinical implications.
Methane-derived halogenated hydrocarbons have been widely used as
organic solvents, replacing carbon tetrachloride. A chance observation
(Stewart et al., 1972b) indicated that the inhalation of
dichloromethane (methylene chloride, CH2CI2) was followed by a
sustained elevation in carboxyhaemoglobin concentrations. Inhalation
of methylene chloride at a concentration of 1740-3480 mg/m3
(500-1000 ppm) (industrial TLVs for USA and USSR = 1740 mg/m3
(500 ppm) and 50 mg/m3 (14 ppm), respectively) for 1-2 h resulted in
carboxyhaemoglobin levels of more than 14% (Stewart et al., 1972a).
This elevation in carboxyhaemoglobin levels continued beyond the time
of exposure and gradually returned to normal during the next 24 h.
Nunes & Schoenborn (1973) demonstrated that the binding affinity of
carbon monoxide for haemoglobin increased in the presence of methylene
chloride. A number of studies have confirmed that methylene chloride
was metabolized to carbon monoxide (Divicenzo & Hamilton, 1975; Kubic
et al., 1974; Ratney et al., 1974; Roth et al., 1975). Roth et al.
(1975) noted that rabbits rarely succumbed to methylene chloride at a
concentration of 40 g/m3 (11 520 ppm) possibly because of saturation
of the pathways of methylene chloride metabolism and the rate of
carbon monoxide excretion. The mechanism by which methylene chloride
is metabolized to carbon monoxide has still to be elucidated. In
vitro studies (Ahmed et al., 1977; Hogan et al., 1976) suggest that
the mixed function oxygenase system of microsomes is responsible for
the metabolic conversion of methylene chloride to carbon monoxide.
Several investigators have studied the influence of methylene
chloride on physiological functions. Astrand et al. (1975) examined
the effects on work performance of exposure to concentrations of 870
and 1740 mg/m3 for four, 30-min periods but did not find any
impairment, apparently because carboxyhaemoglobin levels were low
(4%). Central nervous system depression was observed in some subjects
exposed to concentrations of 1740-3480 mg/m3 (500-1000 ppm) (Stewart
et al., 1972a).
In studies by Winneke (1974), the effects of exposure to ambient
levels of carbon monoxide of up to 115 mg/m3 (100 ppm) on vigilance
and CFFF were less marked than those resulting from exposure to
methylene chloride at 1044-2784 mg/m3 (300-800 ppm).
According to Stewart & Hake (1976) a potentially more dangerous
complication of exposure to methylene chloride is the sustained
carboxyhaemoglobin level that results from the metabolic production of
carbon monoxide from lipid stores of methylene chloride and continues
for many hours following exposure. The potential hazard of chemical
compounds that may be metabolized to carbon monoxide deserves further
investigation.
8.1.6 Levels and effects of carboxyhaemoglobin resulting from smoking
It would be extremely presumptive in a review of the effects of
ambient carbon monoxide to discuss all the possibilities arising from
the incomplete combustion of tobacco and paper. Many of the products
inhaled may produce subtle physiological and biochemical effects on
the smoker. Individuals breathing either pre-inhaled materials or the
smokers' exhaled products are affected to a much lesser degree than
the smoker (Russell et al., 1973; Srch, 1967). It is suggested that
those interested in the problems related to smoking tobacco,
carcinogenesis, and cardiovascular and pulmonary disease, refer to the
documents specifically concerned with these matters (Fletcher & Horn,
1970; Hammond, 1962; US Department of Health, Education and Welfare,
1973; WHO, 1975). Prospective and retrospective epidemiological
studies have, identified cigarette smoking as one of the major factors
in the development of coronary heart disease. The risk of developing
coronary heart disease for pipe and cigar smokers is apparently much
less than it is for cigarette smokers but more than for nonsmokers.
Furthermore, experimental studies suggest that tobacco smoking may
contribute to the development and aggravation of coronary heart
disease through the action of several independent or complementary
mechanisms, one of these being the formation of significant levels of
carboxyhaemoglobin. The role of carboxyhaemoglobin in cancer
development appears to be negligible and unproven. The possible
interaction of carbon monoxide and other constituents of smoke that
may occur in the lungs and other tissues and so induce pathological
changes remains to be elucidated since the basic chemistry has not
been adequately defined.
Kuller et al. (1975) in their epidemiological study in Baltimore,
USA, stated that, if there is an association between carbon monoxide
exposure and heart attacks, the significant exposures are probably
related to micro-environmental factors and cigarette smoking rather
than to community air pollution. They noted that relatively few heart
attacks occurred while an individual was smoking a cigarette. In Los
Angeles, USA (Cohen et al., 1969; Goldsmith & Landaw, 1968; Hexter &
Goldsmith, 1971), the case fatality rate for hospitalized myocardial
infarction (M.I.) patients was higher in areas with high ambient
levels of carbon monoxide (9-16 mg/m3 or 8-14 ppm) and was
positively correlated with ambient carbon monoxide levels. However,
there was no association between ambient carbon monoxide levels and
the admission rates per day. Wallace et al. (1974) concluded that
"from the human health hazard point of view, restriction or
elimination of cigarette smoke makes the most sense in terms of
protecting the atherosclerotic population and preventing a possible
future incidence of coronary heart disease due to chronic carbon
monoxide exposure". It has also been suggested by Astrup (1972) that
the risk of developing arterial diseases from intermittent exposure:
to carbon monoxide may be much higher for smokers than for nonsmokers.
Wald et al. (1973) and Ball & Turner (1974) came to similar
conclusions. In a study by Rissanen et al. (1972), cigarette smokers
had more advanced atherosclerosis than nonsmokers. An extensive review
and some experimental evidence for this viewpoint has been presented
by Kjeldsen (1969).
Smoking cigarettes resulted in higher carboxyhaemoglobin levels
than exposure to carbon monoxide levels present in street air
(Castleden & Cole, 1975; Göthe et al., 1969). Manual workers had lower
carboxyhaemoglobin levels than sedentary workers (both groups being
tobacco smokers), probably because of the increased ventilation
required by the occupations of the manual workers (Castleden & Cole,
1975; Summons & Coleman, 1974). Unless extreme experimental conditions
are considered (Russell et al., 1973; Srch, 1967), carbon monoxide
produced by passive smoking does not seem to present a health risk
(Hinds & First, 1975; Antweiler, 1975; Harke, 1975). Rylander (1974)
also concluded that carbon monoxide exposure through passive smoking
was negligible and that adverse effects upon health would not be
expected. The quantity of carbon monoxide actually entering the lung
depends upon the form in which the tobacco is smoked, the pattern of
smoking, and depth of inhalation. Very little carbon monoxide is
absorbed in the mouth and larynx (approximately 5%) so that most of
the carbon monoxide available for transfer to haemoglobin must reach
the alveoli in order to raise the level of carboxyhaemoglobin present
in the blood stream. Cigarette smokers inhale to a greater extent than
cigar smokers who, in turn, inhale more than pipe smokers, but there
are quite marked individual differences in this pattern. Heavy
cigarette smokers may have carboxyhaemoglobin levels as high as
15-17%. The carbon monoxide concentration in the mainstream smoke of
cigarettes (Table 7) is approximately 4% (V/V) (Fletcher & Horn, 1970;
Hoffman & Wynder, 1972; Wald & Howard, 1975). It has been estimated
that the cigarette smoker may be exposed to a carbon monoxide
concentration of 460-575 mg/m3 (400-500 ppm) for the approximately
6 min needed to smoke a cigarette. Landaw (1973) noted that the half-
time of carbon monoxide elimination in smokers was approximately
291 min. Fig. 11 illustrates the pattern of change in carboxyhaemo-
globin in a typical heavy cigarette smoker (Horvath, personal
communication). An indwelling venous catheter permitted the frequent
sampling of this smoker's blood. The subject smoked only during his
working hours. It should be noted that, by the time he began to smoke
the next day, he still had a body burden of 1.7%. Cigarette smokers
generally excrete carbon monoxide into the air rather than inhale it
from the ambient environment.
Table 7. Carbon monoxide (volume percent) in main stream smokea
Cigarette Cigar
(nonfilter) (filter) A (85 mm) B (85 mm) C (95 mm)
4.6 4.5 5.3 11.1 7.1
a From: Hoffman & Wynder (1972).
exposed to rather ill-defined levels of carbon monoxide have been
reported to be polycythaemic (Jenkins, 1932). Wilks et al. (1959)
believed that acclimatization was purely a function of increased red
cell mass.
The possibility that adaptation to carbon monoxide following
extensive exposure (as occurs in the case of adaptation to high
altitudes) could alter the position of the oxygen dissociation curve
appears to have been answered. Mulhausen et al. (1968) did not find
any change in the degree of left shift in the blood of individuals
exposed to carbon monoxide for a period of 8 days. Unfortunately, the
average carboxyhaemoglobin level of 13% was based on considerable
individual variations in carboxyhaemoglobin levels and periodic
exposure to relatively high concentrations of inhaled carbon monoxide.
Several investigators have sought evidence of a potential shift of the
curve back to the right. Red cell levels of 2,3-diphosphoglycerate
compounds are higher in individuals with anaemia and also during
residence at high altitudes (2,3-diphosphoglycerate is a
phosphorylated by-product of glycolysis). In the erythrocytes of man
and most other mammals, the molar concentration of this compound is
roughly equal to that of haemoglobin. Both it and some other organic
phosphates are bound rather strongly to deoxyhaemoglobin but have
little affinity for oxyhaemoglobin. Increases in 2,3-diphospho-
glycerate shift the effective oxygen affinity, i.e., there is a shift
of the oxyhaemoglobin dissociation to the right. Astrup (1970) found a
small decrease in erythrocyte 2,3-diphospho-glycerate in human
subjects with carboxyhaemoglobin levels maintained at 20% for 24 h.
Conversely, Dinman et al. (1970) found a small increase in 2,3-
diphosphoglycerate in human subjects after 3 h at an approximate
carboxyhaemoglobin level of 20% and in rats exposed to higher but
variable concentrations of carbon monoxide. A shift in the
dissociation curve does not appear to be an important adaptation
mechanism when carbon monoxide exposure lasts less than a few days.
8.1.4 Effects on the cardiovascular system and other effects
Functional heart disturbances (lability of blood pressure and
heart acceleration, extrasystoles, exacerbations of angina pectoris),
as well as temporary heart dilatation and cardiac asthma have been
reported in cases of acute carbon monoxide poisoning (Lazarev, 1965).
According to the same author, various changes were also seen in the
peripheral vascular system (vasodilation, stasis, vasopermeability
etc.). Lazarev (1965) also noted severe cardiovascular disturbances
such as heart acceleration, extra-systoles, pulse and blood pressure
lability (more often hypotension than hypertension) in groups of
workers exposed to carbon monoxide for long periods. Disturbances of
atrioventricular and interventricular conductance were observed after
1 to 1.5 years of exposure and even after cessation of contact with
carbon monoxide.
The first evidence of left ventricular abnormality was presented
by Corya et al. (1976) in 5 cases of nonfatal poisoning (carboxyhaemo-
globin level of 20%). Abnormal left ventricular wall motion was shown
by echocardiograph in 3 of the 5 cases. A similar number showed mitral
valve prolapse. A ballistocardiogram was used by Gorski (1962) to
demonstrate hypoxaemia of the myocardium in similar cases. Byczkowska
& Milan (1971) described functional kidney disturbances in a patient
poisoned with carbon monoxide. Clinical and physiological haemodynamic
studies on 2 groups (individuals in constant contact with carbon
monoxide and individuals having no evidence of chronic carbon monoxide
intoxication) were conducted by Zenkevic (1973). He noted considerable
cardiovascular abnormalities in the carbon monoxide-exposed group. A
study on cast-iron workers by Ejam-Berdjev (1973) also suggested a
larger frequency of cardiovascular, as well as central nervous system
disturbances in these workers, related to their increased blood levels
of carboxyhaemoglobin.
Evidence of a myocardosis was found in 18% of Japanese farmers
chronically exposed to a mean carbon monoxide concentration of
80 mg/m3 (70 ppm), (Komatsu, 1959). The exposure occurred as a
result of spending the winter months preparing hemp in enclosed
dwellings heated by charcoal fires. Following exposure, the farmers
exhibited symptoms of dizziness, palpitation, and congestive heart
failure. The diagnosis of a myocardosis was supported by clinical
evidence of congestive heart failure and ECG changes such as prolonged
QT interval, ST segment depression, and T-wave flattening.
Aleksieva & Dimitrova (1971) studied a large group of workers
exposed to a carbon monoxide concentration of 60 mg/m3 (52 ppm) and
reported changes in peripheral vessels suggesting impaired vascular
tone.
An additional hazard to patients, especially those undergoing
cardiovascular surgery, may develop during anaesthesia. Markedly
elevated carboxyhaemoglobin levels have been reported in patients
under cardiac bypass surgery (Middleton et al., 1965). This increase
could be related in part to the carbon monoxide present in transfused
blood and to the closed-circuit method of anaesthesia that precludes
the loss of endogenously produced carbon monoxide. This is also
important for infants undergoing transfusions (see sections 5.4 and
8.2.3).
8.1.5 Carboxyhaemoglobin levels resulting from exposure to
methane-derived halogenated hydrocarbons
The belated discovery that at least one chemical substance used in
industry and commerce is "degraded" within the body to carbon monoxide
has potentially significant epidemiological and clinical implications.
Methane-derived halogenated hydrocarbons have been widely used as
organic solvents, replacing carbon tetrachloride. A chance observation
(Stewart et al., 1972b) indicated that the inhalation of
dichloromethane (methylene chloride, CH2CI2) was followed by a
sustained elevation in carboxyhaemoglobin concentrations. Inhalation
of methylene chloride at a concentration of 1740-3480 mg/m3
(500-1000 ppm) (industrial TLVs for USA and USSR = 1740 mg/m3
(500 ppm) and 50 mg/m3 (14 ppm), respectively) for 1-2 h resulted in
carboxyhaemoglobin levels of more than 14% (Stewart et al., 1972a).
This elevation in carboxyhaemoglobin levels continued beyond the time
of exposure and gradually returned to normal during the next 24 h.
Nunes & Schoenborn (1973) demonstrated that the binding affinity of
carbon monoxide for haemoglobin increased in the presence of methylene
chloride. A number of studies have confirmed that methylene chloride
was metabolized to carbon monoxide (Divicenzo & Hamilton, 1975; Kubic
et al., 1974; Ratney et al., 1974; Roth et al., 1975). Roth et al.
(1975) noted that rabbits rarely succumbed to methylene chloride at a
concentration of 40 g/m3 (11 520 ppm) possibly because of saturation
of the pathways of methylene chloride metabolism and the rate of
carbon monoxide excretion. The mechanism by which methylene chloride
is metabolized to carbon monoxide has still to be elucidated. In
vitro studies (Ahmed et al., 1977; Hogan et al., 1976) suggest that
the mixed function oxygenase system of microsomes is responsible for
the metabolic conversion of methylene chloride to carbon monoxide.
Several investigators have studied the influence of methylene
chloride on physiological functions. Astrand et al. (1975) examined
the effects on work performance of exposure to concentrations of 870
and 1740 mg/m3 for four, 30-min periods but did not find any
impairment, apparently because carboxyhaemoglobin levels were low
(4%). Central nervous system depression was observed in some subjects
exposed to concentrations of 1740-3480 mg/m3 (500-1000 ppm) (Stewart
et al., 1972a).
In studies by Winneke (1974), the effects of exposure to ambient
levels of carbon monoxide of up to 115 mg/m3 (100 ppm) on vigilance
and CFFF were less marked than those resulting from exposure to
methylene chloride at 1044-2784 mg/m3 (300-800 ppm).
According to Stewart & Hake (1976) a potentially more dangerous
complication of exposure to methylene chloride is the sustained
carboxyhaemoglobin level that results from the metabolic production of
carbon monoxide from lipid stores of methylene chloride and continues
for many hours following exposure. The potential hazard of chemical
compounds that may be metabolized to carbon monoxide deserves further
investigation.
8.1.6 Levels and effects of carboxyhaemoglobin resulting from smoking
It would be extremely presumptive in a review of the effects of
ambient carbon monoxide to discuss all the possibilities arising from
the incomplete combustion of tobacco and paper. Many of the products
inhaled may produce subtle physiological and biochemical effects on
the smoker. Individuals breathing either pre-inhaled materials or the
smokers' exhaled products are affected to a much lesser degree than
the smoker (Russell et al., 1973; Srch, 1967). It is suggested that
those interested in the problems related to smoking tobacco,
carcinogenesis, and cardiovascular and pulmonary disease, refer to the
documents specifically concerned with these matters (Fletcher & Horn,
1970; Hammond, 1962; US Department of Health, Education and Welfare,
1973; WHO, 1975). Prospective and retrospective epidemiological
studies have, identified cigarette smoking as one of the major factors
in the development of coronary heart disease. The risk of developing
coronary heart disease for pipe and cigar smokers is apparently much
less than it is for cigarette smokers but more than for nonsmokers.
Furthermore, experimental studies suggest that tobacco smoking may
contribute to the development and aggravation of coronary heart
disease through the action of several independent or complementary
mechanisms, one of these being the formation of significant levels of
carboxyhaemoglobin. The role of carboxyhaemoglobin in cancer
development appears to be negligible and unproven. The possible
interaction of carbon monoxide and other constituents of smoke that
may occur in the lungs and other tissues and so induce pathological
changes remains to be elucidated since the basic chemistry has not
been adequately defined.
Kuller et al. (1975) in their epidemiological study in Baltimore,
USA, stated that, if there is an association between carbon monoxide
exposure and heart attacks, the significant exposures are probably
related to micro-environmental factors and cigarette smoking rather
than to community air pollution. They noted that relatively few heart
attacks occurred while an individual was smoking a cigarette. In Los
Angeles, USA (Cohen et al., 1969; Goldsmith & Landaw, 1968; Hexter &
Goldsmith, 1971), the case fatality rate for hospitalized myocardial
infarction (M.I.) patients was higher in areas with high ambient
levels of carbon monoxide (9-16 mg/m3 or 8-14 ppm) and was
positively correlated with ambient carbon monoxide levels. However,
there was no association between ambient carbon monoxide levels and
the admission rates per day. Wallace et al. (1974) concluded that
"from the human health hazard point of view, restriction or
elimination of cigarette smoke makes the most sense in terms of
protecting the atherosclerotic population and preventing a possible
future incidence of coronary heart disease due to chronic carbon
monoxide exposure". It has also been suggested by Astrup (1972) that
the risk of developing arterial diseases from intermittent exposure:
to carbon monoxide may be much higher for smokers than for nonsmokers.
Wald et al. (1973) and Ball & Turner (1974) came to similar
conclusions. In a study by Rissanen et al. (1972), cigarette smokers
had more advanced atherosclerosis than nonsmokers. An extensive review
and some experimental evidence for this viewpoint has been presented
by Kjeldsen (1969).
Smoking cigarettes resulted in higher carboxyhaemoglobin levels
than exposure to carbon monoxide levels present in street air
(Castleden & Cole, 1975; Göthe et al., 1969). Manual workers had lower
carboxyhaemoglobin levels than sedentary workers (both groups being
tobacco smokers), probably because of the increased ventilation
required by the occupations of the manual workers (Castleden & Cole,
1975; Summons & Coleman, 1974). Unless extreme experimental conditions
are considered (Russell et al., 1973; Srch, 1967), carbon monoxide
produced by passive smoking does not seem to present a health risk
(Hinds & First, 1975; Antweiler, 1975; Harke, 1975). Rylander (1974)
also concluded that carbon monoxide exposure through passive smoking
was negligible and that adverse effects upon health would not be
expected. The quantity of carbon monoxide actually entering the lung
depends upon the form in which the tobacco is smoked, the pattern of
smoking, and depth of inhalation. Very little carbon monoxide is
absorbed in the mouth and larynx (approximately 5%) so that most of
the carbon monoxide available for transfer to haemoglobin must reach
the alveoli in order to raise the level of carboxyhaemoglobin present
in the blood stream. Cigarette smokers inhale to a greater extent than
cigar smokers who, in turn, inhale more than pipe smokers, but there
are quite marked individual differences in this pattern. Heavy
cigarette smokers may have carboxyhaemoglobin levels as high as
15-17%. The carbon monoxide concentration in the mainstream smoke of
cigarettes (Table 7) is approximately 4% (V/V) (Fletcher & Horn, 1970;
Hoffman & Wynder, 1972; Wald & Howard, 1975). It has been estimated
that the cigarette smoker may be exposed to a carbon monoxide
concentration of 460-575 mg/m3 (400-500 ppm) for the approximately
6 min needed to smoke a cigarette. Landaw (1973) noted that the half-
time of carbon monoxide elimination in smokers was approximately
291 min. Fig. 11 illustrates the pattern of change in carboxyhaemo-
globin in a typical heavy cigarette smoker (Horvath, personal
communication). An indwelling venous catheter permitted the frequent
sampling of this smoker's blood. The subject smoked only during his
working hours. It should be noted that, by the time he began to smoke
the next day, he still had a body burden of 1.7%. Cigarette smokers
generally excrete carbon monoxide into the air rather than inhale it
from the ambient environment.
Table 7. Carbon monoxide (volume percent) in main stream smokea
Cigarette Cigar
(nonfilter) (filter) A (85 mm) B (85 mm) C (95 mm)
4.6 4.5 5.3 11.1 7.1
a From: Hoffman & Wynder (1972).
 Dalhamn et al. (1968) determined the retention of cigarette smoke
components in the human lung. They found a 54% retention of carbon
monoxide and an 86-97% retention of all other compounds. Carbon
monoxide yields from cigarettes increased with puff volume and tobacco
moisture decreased with increased paper porosity, but remained
constant with puff duration (Robinson & Forbes, 1975). In studies by
Haebisch (1970), the carbon monoxide content of smoke increased as a
greater portion of the cigarette was consumed. Although Cohen et al.
(1971b) reported that different cigarette preparations did not result
in significant variations in the smoker's levels of
carboxyhaemoglobin, more recent studies (Gori, 1976; Turner et al.,
1974) suggested that so-called "low toxicity" cigarettes produced
significantly smaller amounts of carbon monoxide. However, the smoking
pattern of the individual smoker markedly altered the absolute amount
of carbon monoxide inhaled. Frankenhaeuser et al. (1971) reported
another response to cigarette smoking that may have important
consequences for the smoker. They observed a progressive increase in
adrenaline excretion with the number of cigarettes smoked. They also
found that, in moderate smokers, certain psychophysical performance
measures did not deteriorate when the subjects smoked, in contrast to
the decrement observed in nonsmoking conditions (Myrsten et al.,
1972). Aronow et al. (1971a) carried out cardiovascular measurements
on 8 volunteer anginal patients who, after abstaining for 12 h, smoked
3 cigarettes. One week later the same subjects were given carbon
monoxide to breathe so that the final carboxyhaemoglobin levels were
almost equivalent (3.90 and 3.86, respectively). As the patients were
smokers, their initial carboxyhaemoglobin levels were above 2%.
Catheterization of both the left and right ventricles permitted an
evaluation of the functions of the myocardium. The major differences
observed under the two conditions were as follows: cardiac output
decreased with carbon monoxide inhalation but did not change with
smoking; heart rate did not change with carbon monoxide inhalation but
increased with smoking; systolic and diastolic arterial pressures did
not change with carbon monoxide inhalation but increased with smoking;
left ventricular stroke work (dp/dt) decreased with inhalation but
did not change with smoking; left ventricle end-diastolic pressure
increased in both situations; and, finally, the partial pressure of
oxygen in arterial, mixed venous, and coronary sinus blood decreased
in patients inhaling carbon monoxide. These divergent effects need to
be further evaluated. The authors believed that the increased systemic
pressure and heart rates following cigarette smoking were related to
the nicotine in the cigarette.
A critical problem arises in attempts to separate the effects of
carbon monoxide in cigarette smoking from those of other substances
present in the inhaled cigarette smoke. In further studies by Aronow
et al. (1971b), 10 male angina patients smoked lettuce leaf, non-
nicotine cigarettes, resulting in blood carboxyhaemoglobin levels of
7.8%. Heart rate and blood pressure were unaffected by smoking this
type of cigarette but again angina occurred earlier on effort. The
investigators suggested that the presence of carboxyhaemoglobin
following the smoking of cigarettes is the major factor in decreasing
exercise tolerance in subjects with angina pectoris.
It has been suggested by Ball & Turner (1974) that carbon monoxide
and nicotine from cigarette smoke may, by different mechanisms,
accelerate thrombus formation and the development of atherosclerosis.
Carbon monoxide reduces the amount of oxygen available to the
myocardium at the time when the work of the heart has been increased
by the absorption of nicotine. Kjeldsen (1969) reported that in a
group of 1000 Danish individuals a clear relationship between high
carboxyhaemoglobin concentrations after smoking and the occurrence of
atherosclerotic disease was observed. On the other hand, selective
evidence would suggest that carbon monoxide exposure might not be
related to the underlying atherosclerosis. Heavy cigarette smokers in
Japan, where diets are low in fat and cholesterol, do not appear to be
at high risk as regards heart attacks.
As Wald & Howard (1975) stated in their overall review on smoking,
carbon monoxide exposure, and arterial disease: "There is at present
only indirect evidence that carbon monoxide may be a cause of atheroma
in man" and "For the present, however, it is necessary to reserve
judgement on whether carbon monoxide is a cause of arterial disease,
while at the same time suspecting that it may be the principal agent
in tobacco smoke". Aronow and co-workers, as well as Anderson et al.
(1973) have shown that, in patients with ischaemic heart disease,
exercise-induced angina occurs earlier when the patients are exposed
to low levels of carbon monoxide (section 8.2.1). Carbon monoxide
exposure also exacerbates the pain of intermittent claudication and
the duration of effects in patients with this disease. The potential
deleterious influences of cigarette smoking and/or carbon monoxide
exposure on the pregnant woman, fetus, and neonate will be considered
in section 8.2.3. The only direct evidence that carbon monoxide
adversely influences fetal development was derived from studies
conducted on rabbits (Astrup et al., 1972).
A number of studies have suggested that cigarette smoking reduces
working capacity (Goldbarg et al., 1971; Krone et al., 1972; US
Department of Health, Education & Welfare, 1973) and, as presented in
section 8.1.2, this reduction has been directly related to the level
of carboxyhaemoglobin present in the exercising subject. In young
smokers, 21-30 years of age, no differences in maximal aerobic
capacity were observed in spite of reductions in vital capacity and
maximum breathing capacities (Raven et al, 1974a). Older smokers
(40-57 years of age) had significantly lower (27%), aerobic capacity
than nonsmokers of a similar age (Raven et al., 1974b). Younger
smokers had only a 6% lower aerobic capacity than nonsmokers of the
same age. There still remain some questions as to the possible role
played by materials other than carbon monoxide in cigarette smoke in
the reduction of aerobic capacity.
8.1.7 Interactions
There is only a small amount of data available on the combined
effects in man of carbon monoxide and other chemical or physical
agents. Experimental work, carried out by Horvath and his
collaborators (Drinkwater et al., 1974; Gliner et al., 1975; Raven et
al., 1974b), dealt with the combined effects of a concentration of
carbon monoxide of 57 mg/m3 (50 ppm) and peroxyacetylnitrate
1.4 mg/m3 (0.27 ppm) on the work capacity of healthy men. Combined
exposure to both pollutants did not produce greater effects than
exposure to carbon monoxide alone. Hackney et al. (1975) did not find
any consistent changes (synergistic or additive) in pulmonary
functions in a 2-h exposure of young male subjects to a combination of
pollutants namely ozone at 0.5 mg/m3 (0.25 ppm), nitrogen dioxide at
0.56 mg/m3 (0.30 ppm) and carbon monoxide at 34.5 mg/m3 (30 ppm).
As for the combined effects of carbon monoxide and physical agents,
there are occupational data suggesting additive effects of carbon
monoxide and heat (Vyskocil, 1957), and of carbon monoxide
(carboxyhaemoglobin levels up to 35%) and noise in workers exposed to
this stress combination for more than 10 years (Wagemann, 1960; Zorn,
1968).
There is a complete lack of information on the combined effects of
carbon monoxide and drugs or alcohol in man. Furthermore, it is quite
apparent that the question of interactions on the organism of carbon
monoxide and other air contaminants needs clarification.
8.2 High-Risk Groups
The limited clinical research on populations other than healthy,
normal subjects makes it difficult to identify with certainty the
groups that are at increased risk from exposure to carbon monoxide.
However, as will be discussed later in this section, one of these
groups includes individuals with known coronary heart disease. In view
of the susceptibility of this group to the hypoxic stress of carbon
monoxide exposure, it is implicit that other groups are also
potentially subject to increased risk including individuals with
cerebrovascular and peripheral vascular diseases, anaemias, and lung
diseases. In addition, hospitalized individuals suffering from tissue
hypoxia (e.g. shock) or those undergoing operations may be at
increased risk. Individuals with undetected or undiagnosed coronary
artery disease as well as the fetus in utero, the newborn, or, even
pregnant women may be assumed to be at increased risk because of the
anticipated reduced capacity to accommodate hypoxic stress or some
inherent sensitivity to hypoxia. Furthermore, other populations such
as those living at high altitudes, young children, or older adults may
also be at increased risk.
8.2.1 Individuals with cardiovascular and chronic obstructive lung
disease
This section will not be concerned with the potential pathological
effects of cigarette smoking on the development of cardiovascular
disease, chronic pulmonary obstructive disease, and cancer (section
8.1.6). No adequate evidence has been presented, as yet, that carbon
monoxide per se is directly involved in the pathogenesis of these
disorders. Only the potential influence of ambient carbon monoxide on
individuals at risk will be considered.
Epidemiological studies in Los Angeles County (Cohen et al., 1969;
Goldsmith & Landaw, 1968; Hexter & Goldsmith, 1971), have suggested
the possibility of increased mortality from myocardial infarction,
associated with high (9-16 mg/m3 or 8-14 ppm) atmospheric levels of
carbon monoxide. There have been some differences of opinion
concerning the interpretation of these data. A somewhat similar study,
at least in design, was completed in Baltimore by Kuller et al.
(1975). The Baltimore data did not indicate any apparent relationship
between either the incidence of myocardial infarction or sudden death
due to atherosclerotic heart disease and average 24-h ambient carbon
monoxide concentrations. Neither group of investigators was able to
detect a relationship between post mortem carboxyhaemoglobin levels
and causes of sudden death. In the latter study the diagnoses of
disease state were more precise and the population involved more
clearly defined. The ambient levels of carbon monoxide in Baltimore
appeared to be considerably lower than those reported for Los Angeles.
Thus, the possibility of an association between ambient carbon
monoxide and the incidence of myocardial infarction or sudden deaths
remains questionable. It is apparent that more comprehensive and
extensive epidemiological studies need to be conducted in order to
clarify this issue.
There are no adequate studies in man describing the relationship
between exposure to carbon monoxide and the rate of development of
atherosclerotic heart disease. Goldsmith & Aronow (1975) have reviewed
the available evidence.
The heart has a specialized circulatory system in which the
primary response to increased metabolic demands can only be secured by
an increased coronary blood flow. Even under no-stress conditions
(rest) there is an almost complete extraction, roughly 75-80%, of the
available oxygen supply. Adams et al. (1973) monitored conscious dogs
breathing a carbon monoxide concentration of 1718 mg/m3 (1500 ppm)
for a period of 30 min and were able to show a linear relationship
between carboxyhaemoglobin concentration and coronary blood flow. A
13% increase in coronary blood flow occurred at a carboxyhaemoglobin
level of 4% and, at a concentration of 20%, flow rate increased by
54%. Since measurements were not reported for the lower
carboxyhaemoglobin levels, the existence of a threshold could not be
determined. The observations of Mehmel et al. (1973) suggest that
increases in coronary blood flow are stimulated by shifts in the
oxyhaemoglobin dissociation curve. They demonstrated that increasing
pH from 7.4 to 7.6 decreased the P50 ( pO2 at half saturation of
haemoglobin) from 4 to 3.2 kPa (30 to 24 Torr) and increased coronary
blood flow by more than 20%. Earlier studies by Ayres et al. (1969,
1970) also indicated increased blood flow in response to the presence
of increased levels of carboxyhaemoglobin.
Ayres' studies of the haemodynamic and respiratory responses of
man during diagnostic coronary catheterization suggested that carbon
monoxide would have a significant effect on arterial pO2 in
patients with lung disease as well as in patients with certain
cardiovascular disorders.
Studies by Anderson et al. (1973) Aronow et al. (1972) and Aronow
& Isbell (1973) on patients with angina pectoris are listed in
Table 8. Aronow et al. (1972) studied the influence of riding in an
open car on a major Los Angeles freeway. Two trips were made, in one
of which the patients breathed compressed carbon monoxide-free air.
Carboxyhaemoglobin levels after this ride averaged 0.65%, in contrast
to the 5.08% observed in the trip in the open car. The trips were of
90 min duration and ambient carbon monoxide levels in the car averaged
61 mg/m3 (53 ppm). Exercise time, on a bicycle ergometer, to the
onset of angina, was determined prior to, and after the completion of
the exposure. Although no changes in the length of time of work to
onset of anginal pain were noted in the ride while breathing
compressed air, a significant reduction from a mean time of 247 to
174 sec was found when the carboxyhaemoglobin concentration was
elevated. Anginal pain also appeared to persist for a longer time
under these conditions. In a study by Anderson et al. (1973), patients
with stable angina walked on a treadmill. They then breathed air
containing 57 or 115 mg/m3 (50 or 100 ppm) intermittently while
resting for a period of 4 h, raising their carboxyhaemoglobin levels
to 2.9% and 4.5% respectively. The repeat exercise tests clearly
demonstrated a reduction in walking time to onset of angina. No
differences in time were observed at the 2 carboxyhaemoglobin levels
although the duration of the pain was longer at the higher level.
There appeared to be some additional depression of the ST segment but
the degree was not of a significant order. Other measures of cardiac
function-systolic time intervals, left ventricular ejection time, pre-
ejection period index, and pre-ejection peak to ejection time ratio
remained within normal limits.
Table 8. Exercise-induced angina and carbon monoxide (10 subjects per study)
Carboxyhaemoglobin Ambient CO Time to Reference
(%) mg/m3(ppm) angina
response
Initial Final
1.12 5.08 61a (53) Shortened Aronow et al (1972)
1.07 2.68 57b (50) Shortened Aronow & Isbell (1973)
1.40 2.90 57c (50) Shortened Anderson et al. (1973)
a Freeway trip.
b Continuous exposure for 2 h in laboratory,
c Intermittent exposure for 4 h in laboratory.
Another study by Aronow & Isbell (1973) was somewhat similar to
that of Anderson et al. (1973). Aronow and his co-workers exposed
patients (nonsmokers at the time of the test to a carbon monoxide
concentration of 57 mg/m3, resulting in a carboxyhaemoglobin
concentration of 2.68%. This study was also conducted as a double-
blind random trial with one day of breathing carbon monoxide and
another day breathing compressed carbon monoxide-free air. The angina
pectoris of all patients was documented by history and coronary
angiography. A 23% reduction in exercise time (bicycle) resulted
following the carbon monoxide exposure. No electro-cardiographic
changes were seen in these patients during any exercise period.
Plotting of Aronow's data suggests that there was a linear
relationship between carboxyhaemoglobin levels and the decrease in
time to angina.
This evidence suggests that a deleterious effect could occur at
carboxyhaemoglobin levels as low as 2.5% in certain subjects with
coronary heart diseases. The USA National Health Survey Examination
(US Environmental Protection Agency, 1975) reported that in the USA
there were 3 215 000 adults, aged 18-79 years, with definite coronary
heart disease and another 2 410 000 with suspected disease. Many of
these individuals, as well as others in the general population, have
carboxyhaemoglobin levels equal to, or above 2.5%. It would be rash to
even suggest that the above-mentioned studies implicate carbon
monoxide as a factor in determining the natural history of heart
disease in a community. It is even more dangerous to imply that
exposure to carbon monoxide increases the frequency or severity of
chest pain, or shortens life expectancy among patients with angina
pectoris or other clinical manifestations of heart disease. The
necessary epidemiological evidence for an association between the
frequency of episodes of angina pectoris and community ambient levels
of carbon monoxide is inadequate and additional information from more
and varied sources is required.
Patients with chronic obstructive pulmonary disease are probably
at high risk, although few studies on them have been reported. Any
increase in hypoxia could result in respiratory failure. However,
these individuals may absorb less carbon monoxide because of their
disease as the hypoxia may be compensated for by increased
erythropoiesis and a shift of the oxygen dissociation curve to the
right. An interesting approach to the evaluation of individuals at
risk from carbon monoxide and other pollutants can be found in a
publication of the US Environmental Protection Agency (US
Environmental Protection Agency, 1975). Ogawa et al. (1974) have
presented evidence on the development of pulmonary oedema and
discussed possible mechanisms of the role of carbon monoxide in the
disorder.
8.2.2 Anaemic individuals
The information available on the effects of carbon monoxide on
anaemic patients is still inadequate.
The oxygen dissociation curve of blood obtained from patients with
anaemia is shaped like the normal curve but is vertically compressed.
However, when curves from individuals with a 50% reduction in
haemoglobin content are compared with dissociation curves determined
in the presence of 50% carboxyhaemoglobin, there are striking
differences. Consequently, the tendency to make such comparisons is
likely to lead to erroneous deductions concerning effects occurring at
the tissue level. Brody & Coburn (1970) discussed these differences in
relation to arterial and venous pCO and pO2. Because the capacity
of the oxygen transport system is reduced in anaemic persons,
it can be assumed a priori that they could be more at risk from
carbon monoxide exposure than normal persons. Brody & Coburn (1970)
indicated that, if the oxygen content of the mixed venous blood is
abnormally low, as in anaemia or carbon monoxide poisoning, the effect
of the shunted blood in lowering arterial pO2 will be greater than
normal, and a small increase in the alveolar-arterial pressure
difference (AaDO2) will result. The change in the shape of the
oxyhaemoglobin curve due to the presence of carbon monoxide will also
increase the AaDO2. Furthermore, Brody & Coburn (1970) showed that
mild increases in carboxyhaemoglobin concentrations would have little
or no influence on the AaDO2 in normal subjects. However, in
patients with large intra-cardiac right-to-left shunts or with chronic
lung disease and regional variation in the ventilation perfusion ratio
( VA/ QA), the presence of carbon monoxide in the blood will increase
the AaDO2.
Tissue oxygenation may be involved initially because of the
anaemic state, since mixed venous pO2 is decreased (Cropp, 1970)
and the reduction in venous pO2 from a particular carboxyhaemo-
globin value is somewhat greater in anaemic than in normal subjects.
In patients with haemolytic anaemia and sickle cell disease (Engel et
al., 1971), the rate of endogenous carbon monoxide production from
haemoglobin catabolism is increased. Normal subjects produce
approximately 18 µmoles of carbon monoxide per hour, resulting in
carboxyhaemoglobin levels of 0.5 to 0.8%. Carbon monoxide production
in anaemic patients (Coburn et al., 1966; Logue et al., 1971) has been
reported to vary from 31 to 158 µmoles per hour, producing
carboxyhaemoglobin levels of 1.3-5.2%.
Anaemic subjects approach equilibrium levels of carboxyhaemoglobin
more rapidly than those with normal haemoglobin levels at any given
exposure to carbon monoxide. Exposure to a concentration of
22.9 mg/m3 (20 ppm) for approximately 4 h in an individual with a
haemoglobin level of 7 g/100 ml could result in a carboxyhaemoglobin
concentration of 4-5% compared with an anticipated level of 2.5% for
normal individuals. Exogenous carbon monoxide exposure of anaemic
individuals could result, in conjunction with higher endogenous
production, in their attaining critical levels of carboxyhaemoglobin
more rapidly than normal individuals.
8.2.3 Embryo, fetus, neonate, and infants
Pregnant mothers and their fetuses may be exposed acutely or
chronically to carbon monoxide either by maternal smoking or by
environmental pollution. The biological effects of carbon monoxide
exposure on fetal tissues during intrauterine development or during
the newborn period are far from clear.
Several studies (Astrup et al., 1972; MacMahon et al., 1966) have
demonstrated that babies delivered by mothers who smoke cigarettes
weigh less than those delivered by nonsmoking mothers. Relative
maternal, fetal, or placental hypoxia may be responsible, as suggested
by the observation that infants born at high altitudes also weigh less
than those born at sea level (Grahn & Kratchman, 1963). The New Mexico
State Department of Public Health (1975) provided additional
confirmation of the relationship between altitude and birth weights.
Mothers who smoked were reported to have carboxyhaemoglobin
concentrations ranging from 2 to 14%,. while concentrations in the
fetuses ranged from 2.4 to 9.8%. These values may not represent
conditions present during pregnancy, since these data were obtained
just prior to birth. Another factor that may produce differential
effects on the fetus is related to the endogenous production of carbon
monoxide by pregnant women. Delivoria-Papadopoulos et al., (1969)
indicated that nonsmoking pregnant women produced 0.9 ml of carbon
monoxide per hour in contrast to the nonpregnant female's production
of 0.4 ml reported by Longo (1970). Fetal production of endogenous
carbon monoxide accounted for 3% of the total carboxyhaemoglobin
present in the blood of a nonsmoking normal pregnant woman. The source
of the remainder is not well known but may be related to progesterone
levels (Delivoria-Papadopoulos et al., 1969). Even though
hyperventilation of pregnancy may partially compensate for the
increased carbon monoxide production in the absence of exogenous
exposure, the maternal carboxyhaemoglobin still remains about 13%
above that in nonpregnant women (Longo, 1970). It should be noted that
the post partum (24 h) female may be producing 3 times as much carbon
monoxide as a near term nonsmoking pregnant woman.
Behrman et al. (1971) measured carboxyhaemoglobin concentrations
in 25 relatively normal newborn infants in a downtown Chicago nursery
and found the mean value to be 6.98%. These investigators indicated
that absolute carboxyhaemoglobin levels were related to ambient levels
of carbon monoxide. Some doubts about this conclusion exist, since the
monitoring reference site was 2.4 km from the nursery; the
investigators did not report any untoward clinical effects from
exposure to these levels, and no consideration was given to the
possibility of increased endogenous carbon monoxide production in
these infants.
Of the several mechanisms that may account for the influence of
carbon monoxide on developing tissue, the most important is the
interference with tissue oxygenation. Carbon monoxide decreases the
capacity of haemoglobin to transport oxygen and shifts the oxygen
saturation curve to the left. The normal arterial pO2 supplying
fetal tissue is approximately 3.7 kPa (28 Torr). The shift to the left
will tend to further decrease the oxygen gradient from maternal to
fetal blood across the tissue. The decreased pO2 and the
diminished oxygen transport due to the presence of carboxyhaemoglobin
may also produce undesired influences on the fetus. One of the
possible mechanisms by which carbon monoxide or other components of
tobacco smoke may adversely influence fetal development is through
interference with the metabolic function of placental cells. These
cells have a role in metabolizing hormones as well as in the transport
of vitamins, carbohydrates, amino acids, and other substances through
their energy dependent processes. Tanaka (1965) reported that the
oxygen uptake of placental slices from mothers who smoked varied
inversely with maternal levels of carboxyhaemoglobin, being markedly
reduced when this level was higher than 7.0%. The preponderance of
evidence concerning maternal carboxyhaemoglobin levels, along with
fetal and perinatal exposure, tends to warrant the reduction of
exposure to exogenous carbon monoxide sources that might cause this
group to be at risk, to a minimum.
The potential toxicity of carbon monoxide present in transfused
blood has received little attention. Kandall et al. (1973) measured
carboxyhaemoglobin concentrations in donor blood and in relatively
healthy infants receiving exchange blood transfusions. The mean pre-
transfusion carboxyhaemoglobin concentration in 6 cases was 1.34%.
Donor blood contained a carboxyhaemoglobin concentration of 5.17%,
resulting in a mean value of 4.92% in the transfused infant. In one
infant transfused with blood containing a carboxyhaemoglobin
concentration of 8.87%, the resultant carboxyhaemoglobin value in the
infant was 7.43%. Although it was stated that the infants did not
appear to be adversely affected by the levels of carboxyhaemoglobin
reached during exchange transfusion, it should be noted that adverse
effects have been observed in adults at these levels. Furthermore, in
individuals whose oxygen transport system or cardiovascular reserve is
already compromised, the presence of additional carboxyhaemoglobin,
from transfused blood, may result in a further and more potentially
dangerous decrement in arterial, mixed venous, and coronary sinus
oxygen tensions. It should be recalled that some blood samples
collected from blood donors had carboxyhaemoglobin values that
exceeded 18%.
8.2.4 Individuals living at high altitudes
The effects of carbon monoxide and of hypoxia induced by high
altitude are similar. Carbon monoxide produces effects that aggravate
the oxygen deficiency present at high altitudes. When high altitude
and carbon monoxide exposures are combined (Table 9) the effects are
apparently additive. It should be noted, however, that decreased
pO2 in the air and increased carboxyhaemoglobin, produce different
physiological responses. They have different effects on blood pO2,
on the affinity of oxygen for haemoglobin, on the extent of
oxyhaemoglobin saturation (carbon monoxide hypoxaemia shifts the
oxyhaemoglobin dissociation curve to the left, and a decrease in
PAo2 shifts it to the right), and on ventilatory drive. These
effects have been discussed earlier.
The actual influence of a combination of increased carboxy-
haemoglobin and decreased oxyhaemoglobin has not been adequately
documented by experimental data. The few available studies refer
only to acute exposures to lower pO2 and raised pCO. The
most supportive information on the additive nature of this combination
originates from psychophysiological studies and even this information
is not very convincing. When Blackmore (1974) analysed the cause of
aircraft accidents in Britain, he found that carboxyhaemoglobin levels
provided valuable information in relation to altitude and sources of
carbon monoxide. The high levels of carbon monoxide found (up to 74%)
could be attributed to equipment failure, smoking, and fires. No data
are available on the effects of carbon monoxide on the native
inhabitants at high altitudes or on the reactions of these natives
when they are suddenly removed to sea level and possible high ambient
carbon monoxide concentrations.
Table 9. Approximate physiologically equivalent altitudes at
equilibrium with ambient carbon monoxide levelsa
Ambient CO concentration Actual altitude (metres)
mg/m3 ppm 0 (sea level) 1524 3048
Physiologically equivalent altitudes
with carboxyhaemoglobin
0 0 0 (sea level) 1524 3048
28.6 25 1829 2530 3962
67.3 50 3048 3658 4672
114.5 100 3749 4663 5486
a From: NAS/NRC (1977).
In their studies on altitude exposures of young males, McFarland
et al. (1944) showed that changes in visual threshold occurred at
carboxyhaemoglobin levels as low as 5% or at a simulated altitude of
2425 m. These observations were confirmed by Halperin et al. (1959),
who also noted that recovery from the detrimental effects on visual
function lagged behind the elimination of carbon monoxide. However,
the data given were sparse and the variability among the four subjects
was not given. Vollmer et al. (1946) studied the effects of carbon
monoxide at simulated altitudes of 3070 and 4555 m and reported that
there were no additive effects of carbon monoxide and altitude. They
suggested that the effects of carbon monoxide were masked by some
compensatory mechanisms. The data presented were not convincing.
However, Lilienthal & Fugitt (1946) indicated that a combination of
altitude (1540 m) and a carboxyhaemoglobin level of 5-9% induced a
decrease in flicker fusion frequency, although either one alone did
not have any effect. They also reported that the presence of 8-10%
carboxyhaemoglobin was effective in reducing altitude tolerance by
1215 m. During light activity at an altitude of 4875 m, carbon
monoxide uptake increased, probably owing to the hyperventilation at
altitude caused by the respiratory stimulus of decreased pO2
(Forbes et al., 1945). Evidence that carbon monoxide elimination was
similar at sea level and at altitudes up to 10 000 m was obtained by
several investigators (Gorodinsky et al., 1970; Sedov et al., 1971).
However, increased ambient temperatures up to 35°C and hard physical
work increased the rate of elimination (Vollmer et al., 1946). Pitts &
Pace (1947) stated that every 1% increase in carboxyhaemoglobin (up to
13%) was equivalent to a 109 m rise in altitude if the subjects were
at altitudes of 2100-3070 m. These observations were based on changes
in the heart rate response to work. A number of unanswered questions
arise from all these studies, which in general were obscured by such
factors as poor control and no identification of subjects who may have
been smokers.
Two groups of investigators have presented data comparing the
physiological responses of subjects to altitude and carbon monoxide
exposure where the hypoxaemia due to altitude and the presence of
carboxyhaemoglobin were approximately equal. In one study (Astrup &
Pauli, 1968), the carboxyhaemoglobin concentration was about 12%
(although the mode of exposure to carbon monoxide was such that
carboxyhaemoglobin ranged from 5% to 20% and the altitude study was
conducted at 3977 m). The second study (Sedov et al., 1971) compared
responses at an altitude of 4000 m and a carboxyhaemoglobin content of
20%. In both studies, carboxyhaemoglobin content was much in excess of
that anticipated for typical ambient pollution. However, they both
suggested that the effects attributable to carbon monoxide and to
altitude were equal.
8.3 Summary Table
Table 10 is a summary of controlled human studies that provide
useful information for evaluating the relationship between exposure to
carbon monoxide and its health effects.
Table 10. Summary of exposure-effect relationships
Exposure Reported effects Reference
(HbCO %)
(a) Behavioural changes
20 Essentially no impairment in time discrimination Stewart et al. (1973b)
(using Beard-Wertheim task)
11.3 No vigilance decrement (using Horvath task) Winneke et al. (1976)
9a No vigilance decrement (using Fodor-Winneke Winneke (1974)
task); no change in reaction time
8.4 No vigilance decrement (using their own Groll-Knapp et al. (1976)
vigilance task (1972))
7.6 Longer reaction times Ramsey (1973)
7.3 Disturbance in certain perceptual and cognitive Bender et al. (1972)
processes
5 Vigilance decrement Horvath et al. (1971)
4.5 Longer reaction times Ramsey (1972)
3.1b Initial vigilance decrement with subsequent Fodor & Winneke (1972)
normalization; no change in response latency
3b Vigilance decrement Groll-Knapp et al. (1972)
2b Impaired performance in time-discrimination Beard & Wertheim (1967)
(b) Changes in work performance
6.3 Decrease in maximal work time Ekblom & Huot (1972)
4.3 Decrease in a maximal oxygen uptake (VO2) Horvath et al. (1975)
(1.7c)
4.0 Decrease in mean exercise time until exhaustion Aronow & Cassidy (1975)
(0.6c)
2.5 Decrease in absolute exercise time in non- Drinkwater et al. (1974)
smokers
Table 10 (Cont'd)
Exposure Reported effects Reference
(HbCO %)
(c) Aggravation of symptoms in patients with cardiovascular disease
(1.1c)
5.1 Shortened time to angina response immediately Aronow et al. (1972)
after exposure
(1.1c)
2.9 Shortened time to angina response 2 h after Aronow et al. (1972)
(1.1c) exposure
2.9 Shortened time to angina response Anderson et al. (1973)
(1.1c)
2.8 Decrease in mean exercise time until onset of Aronow et al. (1974)
intermittent claudications
(1.0c)
2.7 Shortened time to angina response Aronow & Isbell (1973)
a Estimated values using the formula by Coburn et al. (1965).
b Estimated values using the formula by Peterson & Stewart (1970).
c HbCO % before exposure to CO.
9. EVALUATION OF HEALTH RISKS
9.1 Introduction
The acute toxicity of carbon monoxide has long been recognized and
is well documented. Much has been learned of the main sources of the
gas, its absorption, the kinetics of its reactions with blood, and the
biochemical and pathological consequences of poisoning by excessive
absorption. More recently, a great deal of attention has been paid to
the effects, demonstrable or suspected, of exposure to concentrations
much lower than those that cause definite poisoning. Such
concentrations are those commonly found in urban air (caused almost
wholly by traffic pollution) and indoors (caused by faulty ventilation
of heating or cooking appliances), but there has been much concern
with the effects of the gas on smokers, who inhale considerable
quantities of carbon monoxide with tobacco smoke. Since the main
source of carbon monoxide as an urban pollutant is the petrol engine,
the problems posed by the inhalation of relatively low concentrations
of the gas are likely to grow rather than diminish, as traffic becomes
denser and more widespread. The recognition of the importance of
pollution of the domestic environment is relatively recent and
deserves more study; the problems posed by smoking tobacco are common
and are, unfortunately, increasing. There is much published evidence,
some of which is of debatable value, that suggests that the
comparatively low concentrations of carboxyhaemoglobin produced by
exposure to pollution of the ambient air and the higher concentrations
usually associated with smoking, might cause demonstrable impairment
of vigilance, discrimination, and of the performance of fine tasks and
physical work in healthy subjects, and the exacerbation of symptoms
such as angina pectoris on effort in patients with cardiovascular
diseases. Likewise there is evidence, derived from experimentation on
animals, that chronic exposure to carbon monoxide leading to the
levels of carboxyhaemoglobin commonly found in smokers may, in
association with high cholesterol intakes, play a part in the genesis
of atherosclerosis. Moreover, there are reasons to suspect that
exposure to carbon monoxide may enhance the effects of other
pollutants, commonly administered therapeutic agents, socially
acceptable amounts of beverages such as alcohol, and other
environmental stresses. This section is intended as a brief assessment
of these topics in the hope that sound advice may be given on the need
to control levels of carbon monoxide in the ambient air.
9.2 Exposure
9.2.1 Assessment of exposure
Concentrations of carbon monoxide in air may be measured with
comparative ease by such methods as non-dispersive spectroscopy, gas
chromatography etc. But human body burdens of carboxyhaemoglobin
depend on many factors other than the partial pressure of carbon
monoxide in the inhaled air; among these factors are time of exposure,
pulmonary ventilation (which mainly depends on work done), and blood
volume. Since these quantities, especially ambient concentrations of
carbon monoxide, may vary widely, it is obviously difficult, if not
impossible at times, to calculate the likely body burden of
carboxyhaemoglobin in an exposed individual. There is, therefore, much
to be gained by sampling blood to obtain an integrated estimate of
carboxyhaemoglobin derived from all sources under various conditions
of exposure. It must be emphasized that the measurements of carbon
monoxide in air and in blood give complementary results and are not
merely alternative forms of monitoring. Methods of analysis are
discussed in sections 2.2 and 2.3.
There is a tendency to forget that the reaction between
haemoglobin and carbon monoxide is reversible and that, in a given
environment, a subject may acquire carbon monoxide, excrete it, or
remain in equilibrium with the ambient air depending on the carbon
monoxide concentration and the initial level of carboxyhaemoglobin in
the individual. The time taken to achieve equilibrium between blood
and ambient air depends on the initial carboxyhaemoglobin
concentrations as well as on the factors mentioned above. The rate of
excretion of carbon monoxide will depend not only on ambient air
levels, the initial carboxyhaemoglobin and on factors such as
pulmonary ventilation, but also on the partial pressure of oxygen in
inspired air, which might be introduced therapeutically to increase
elimination. Fig. 12 shows estimates of equilibrium times for various
ambient concentrations and levels of activity. The half-life for
excretion, at rest, is approximately 4“ h.
9.2.2 Endogenous production
The normal breakdown in the body of blood pigments produces carbon
monoxide to give endogenous carboxyhaemoglobin values of 0.1-1.0% and
normal blood is in equilibrium with carbon monoxide levels in air of
roughly 5 mg/m3 (4.3 ppm). These data could be used as a basis for
establishing air quality criteria for carbon monoxide. Various causes
of increased endogenous production of carbon monoxide are discussed in
section 6.1.
9.2.3 Outdoor environmental exposure
Natural sources of carbon monoxide (section 3.1) are of
considerable magnitude but are diffuse, and ambient air concentrations
at locations removed from man-made sources range from 0.01 to
0.9 mg/m3 (0.01 and 0.8 ppm) which is negligible in the context of this
report. By far the most important sources of carbon monoxide at
breathing level are petrol engine vehicle exhausts (section 3.2). The
diesel engine (compression ignition), when properly adjusted, emits
little carbon monoxide. The density, distribution, and mode of
operation of vehicles vary greatly and these and other factors, the
Dalhamn et al. (1968) determined the retention of cigarette smoke
components in the human lung. They found a 54% retention of carbon
monoxide and an 86-97% retention of all other compounds. Carbon
monoxide yields from cigarettes increased with puff volume and tobacco
moisture decreased with increased paper porosity, but remained
constant with puff duration (Robinson & Forbes, 1975). In studies by
Haebisch (1970), the carbon monoxide content of smoke increased as a
greater portion of the cigarette was consumed. Although Cohen et al.
(1971b) reported that different cigarette preparations did not result
in significant variations in the smoker's levels of
carboxyhaemoglobin, more recent studies (Gori, 1976; Turner et al.,
1974) suggested that so-called "low toxicity" cigarettes produced
significantly smaller amounts of carbon monoxide. However, the smoking
pattern of the individual smoker markedly altered the absolute amount
of carbon monoxide inhaled. Frankenhaeuser et al. (1971) reported
another response to cigarette smoking that may have important
consequences for the smoker. They observed a progressive increase in
adrenaline excretion with the number of cigarettes smoked. They also
found that, in moderate smokers, certain psychophysical performance
measures did not deteriorate when the subjects smoked, in contrast to
the decrement observed in nonsmoking conditions (Myrsten et al.,
1972). Aronow et al. (1971a) carried out cardiovascular measurements
on 8 volunteer anginal patients who, after abstaining for 12 h, smoked
3 cigarettes. One week later the same subjects were given carbon
monoxide to breathe so that the final carboxyhaemoglobin levels were
almost equivalent (3.90 and 3.86, respectively). As the patients were
smokers, their initial carboxyhaemoglobin levels were above 2%.
Catheterization of both the left and right ventricles permitted an
evaluation of the functions of the myocardium. The major differences
observed under the two conditions were as follows: cardiac output
decreased with carbon monoxide inhalation but did not change with
smoking; heart rate did not change with carbon monoxide inhalation but
increased with smoking; systolic and diastolic arterial pressures did
not change with carbon monoxide inhalation but increased with smoking;
left ventricular stroke work (dp/dt) decreased with inhalation but
did not change with smoking; left ventricle end-diastolic pressure
increased in both situations; and, finally, the partial pressure of
oxygen in arterial, mixed venous, and coronary sinus blood decreased
in patients inhaling carbon monoxide. These divergent effects need to
be further evaluated. The authors believed that the increased systemic
pressure and heart rates following cigarette smoking were related to
the nicotine in the cigarette.
A critical problem arises in attempts to separate the effects of
carbon monoxide in cigarette smoking from those of other substances
present in the inhaled cigarette smoke. In further studies by Aronow
et al. (1971b), 10 male angina patients smoked lettuce leaf, non-
nicotine cigarettes, resulting in blood carboxyhaemoglobin levels of
7.8%. Heart rate and blood pressure were unaffected by smoking this
type of cigarette but again angina occurred earlier on effort. The
investigators suggested that the presence of carboxyhaemoglobin
following the smoking of cigarettes is the major factor in decreasing
exercise tolerance in subjects with angina pectoris.
It has been suggested by Ball & Turner (1974) that carbon monoxide
and nicotine from cigarette smoke may, by different mechanisms,
accelerate thrombus formation and the development of atherosclerosis.
Carbon monoxide reduces the amount of oxygen available to the
myocardium at the time when the work of the heart has been increased
by the absorption of nicotine. Kjeldsen (1969) reported that in a
group of 1000 Danish individuals a clear relationship between high
carboxyhaemoglobin concentrations after smoking and the occurrence of
atherosclerotic disease was observed. On the other hand, selective
evidence would suggest that carbon monoxide exposure might not be
related to the underlying atherosclerosis. Heavy cigarette smokers in
Japan, where diets are low in fat and cholesterol, do not appear to be
at high risk as regards heart attacks.
As Wald & Howard (1975) stated in their overall review on smoking,
carbon monoxide exposure, and arterial disease: "There is at present
only indirect evidence that carbon monoxide may be a cause of atheroma
in man" and "For the present, however, it is necessary to reserve
judgement on whether carbon monoxide is a cause of arterial disease,
while at the same time suspecting that it may be the principal agent
in tobacco smoke". Aronow and co-workers, as well as Anderson et al.
(1973) have shown that, in patients with ischaemic heart disease,
exercise-induced angina occurs earlier when the patients are exposed
to low levels of carbon monoxide (section 8.2.1). Carbon monoxide
exposure also exacerbates the pain of intermittent claudication and
the duration of effects in patients with this disease. The potential
deleterious influences of cigarette smoking and/or carbon monoxide
exposure on the pregnant woman, fetus, and neonate will be considered
in section 8.2.3. The only direct evidence that carbon monoxide
adversely influences fetal development was derived from studies
conducted on rabbits (Astrup et al., 1972).
A number of studies have suggested that cigarette smoking reduces
working capacity (Goldbarg et al., 1971; Krone et al., 1972; US
Department of Health, Education & Welfare, 1973) and, as presented in
section 8.1.2, this reduction has been directly related to the level
of carboxyhaemoglobin present in the exercising subject. In young
smokers, 21-30 years of age, no differences in maximal aerobic
capacity were observed in spite of reductions in vital capacity and
maximum breathing capacities (Raven et al, 1974a). Older smokers
(40-57 years of age) had significantly lower (27%), aerobic capacity
than nonsmokers of a similar age (Raven et al., 1974b). Younger
smokers had only a 6% lower aerobic capacity than nonsmokers of the
same age. There still remain some questions as to the possible role
played by materials other than carbon monoxide in cigarette smoke in
the reduction of aerobic capacity.
8.1.7 Interactions
There is only a small amount of data available on the combined
effects in man of carbon monoxide and other chemical or physical
agents. Experimental work, carried out by Horvath and his
collaborators (Drinkwater et al., 1974; Gliner et al., 1975; Raven et
al., 1974b), dealt with the combined effects of a concentration of
carbon monoxide of 57 mg/m3 (50 ppm) and peroxyacetylnitrate
1.4 mg/m3 (0.27 ppm) on the work capacity of healthy men. Combined
exposure to both pollutants did not produce greater effects than
exposure to carbon monoxide alone. Hackney et al. (1975) did not find
any consistent changes (synergistic or additive) in pulmonary
functions in a 2-h exposure of young male subjects to a combination of
pollutants namely ozone at 0.5 mg/m3 (0.25 ppm), nitrogen dioxide at
0.56 mg/m3 (0.30 ppm) and carbon monoxide at 34.5 mg/m3 (30 ppm).
As for the combined effects of carbon monoxide and physical agents,
there are occupational data suggesting additive effects of carbon
monoxide and heat (Vyskocil, 1957), and of carbon monoxide
(carboxyhaemoglobin levels up to 35%) and noise in workers exposed to
this stress combination for more than 10 years (Wagemann, 1960; Zorn,
1968).
There is a complete lack of information on the combined effects of
carbon monoxide and drugs or alcohol in man. Furthermore, it is quite
apparent that the question of interactions on the organism of carbon
monoxide and other air contaminants needs clarification.
8.2 High-Risk Groups
The limited clinical research on populations other than healthy,
normal subjects makes it difficult to identify with certainty the
groups that are at increased risk from exposure to carbon monoxide.
However, as will be discussed later in this section, one of these
groups includes individuals with known coronary heart disease. In view
of the susceptibility of this group to the hypoxic stress of carbon
monoxide exposure, it is implicit that other groups are also
potentially subject to increased risk including individuals with
cerebrovascular and peripheral vascular diseases, anaemias, and lung
diseases. In addition, hospitalized individuals suffering from tissue
hypoxia (e.g. shock) or those undergoing operations may be at
increased risk. Individuals with undetected or undiagnosed coronary
artery disease as well as the fetus in utero, the newborn, or, even
pregnant women may be assumed to be at increased risk because of the
anticipated reduced capacity to accommodate hypoxic stress or some
inherent sensitivity to hypoxia. Furthermore, other populations such
as those living at high altitudes, young children, or older adults may
also be at increased risk.
8.2.1 Individuals with cardiovascular and chronic obstructive lung
disease
This section will not be concerned with the potential pathological
effects of cigarette smoking on the development of cardiovascular
disease, chronic pulmonary obstructive disease, and cancer (section
8.1.6). No adequate evidence has been presented, as yet, that carbon
monoxide per se is directly involved in the pathogenesis of these
disorders. Only the potential influence of ambient carbon monoxide on
individuals at risk will be considered.
Epidemiological studies in Los Angeles County (Cohen et al., 1969;
Goldsmith & Landaw, 1968; Hexter & Goldsmith, 1971), have suggested
the possibility of increased mortality from myocardial infarction,
associated with high (9-16 mg/m3 or 8-14 ppm) atmospheric levels of
carbon monoxide. There have been some differences of opinion
concerning the interpretation of these data. A somewhat similar study,
at least in design, was completed in Baltimore by Kuller et al.
(1975). The Baltimore data did not indicate any apparent relationship
between either the incidence of myocardial infarction or sudden death
due to atherosclerotic heart disease and average 24-h ambient carbon
monoxide concentrations. Neither group of investigators was able to
detect a relationship between post mortem carboxyhaemoglobin levels
and causes of sudden death. In the latter study the diagnoses of
disease state were more precise and the population involved more
clearly defined. The ambient levels of carbon monoxide in Baltimore
appeared to be considerably lower than those reported for Los Angeles.
Thus, the possibility of an association between ambient carbon
monoxide and the incidence of myocardial infarction or sudden deaths
remains questionable. It is apparent that more comprehensive and
extensive epidemiological studies need to be conducted in order to
clarify this issue.
There are no adequate studies in man describing the relationship
between exposure to carbon monoxide and the rate of development of
atherosclerotic heart disease. Goldsmith & Aronow (1975) have reviewed
the available evidence.
The heart has a specialized circulatory system in which the
primary response to increased metabolic demands can only be secured by
an increased coronary blood flow. Even under no-stress conditions
(rest) there is an almost complete extraction, roughly 75-80%, of the
available oxygen supply. Adams et al. (1973) monitored conscious dogs
breathing a carbon monoxide concentration of 1718 mg/m3 (1500 ppm)
for a period of 30 min and were able to show a linear relationship
between carboxyhaemoglobin concentration and coronary blood flow. A
13% increase in coronary blood flow occurred at a carboxyhaemoglobin
level of 4% and, at a concentration of 20%, flow rate increased by
54%. Since measurements were not reported for the lower
carboxyhaemoglobin levels, the existence of a threshold could not be
determined. The observations of Mehmel et al. (1973) suggest that
increases in coronary blood flow are stimulated by shifts in the
oxyhaemoglobin dissociation curve. They demonstrated that increasing
pH from 7.4 to 7.6 decreased the P50 ( pO2 at half saturation of
haemoglobin) from 4 to 3.2 kPa (30 to 24 Torr) and increased coronary
blood flow by more than 20%. Earlier studies by Ayres et al. (1969,
1970) also indicated increased blood flow in response to the presence
of increased levels of carboxyhaemoglobin.
Ayres' studies of the haemodynamic and respiratory responses of
man during diagnostic coronary catheterization suggested that carbon
monoxide would have a significant effect on arterial pO2 in
patients with lung disease as well as in patients with certain
cardiovascular disorders.
Studies by Anderson et al. (1973) Aronow et al. (1972) and Aronow
& Isbell (1973) on patients with angina pectoris are listed in
Table 8. Aronow et al. (1972) studied the influence of riding in an
open car on a major Los Angeles freeway. Two trips were made, in one
of which the patients breathed compressed carbon monoxide-free air.
Carboxyhaemoglobin levels after this ride averaged 0.65%, in contrast
to the 5.08% observed in the trip in the open car. The trips were of
90 min duration and ambient carbon monoxide levels in the car averaged
61 mg/m3 (53 ppm). Exercise time, on a bicycle ergometer, to the
onset of angina, was determined prior to, and after the completion of
the exposure. Although no changes in the length of time of work to
onset of anginal pain were noted in the ride while breathing
compressed air, a significant reduction from a mean time of 247 to
174 sec was found when the carboxyhaemoglobin concentration was
elevated. Anginal pain also appeared to persist for a longer time
under these conditions. In a study by Anderson et al. (1973), patients
with stable angina walked on a treadmill. They then breathed air
containing 57 or 115 mg/m3 (50 or 100 ppm) intermittently while
resting for a period of 4 h, raising their carboxyhaemoglobin levels
to 2.9% and 4.5% respectively. The repeat exercise tests clearly
demonstrated a reduction in walking time to onset of angina. No
differences in time were observed at the 2 carboxyhaemoglobin levels
although the duration of the pain was longer at the higher level.
There appeared to be some additional depression of the ST segment but
the degree was not of a significant order. Other measures of cardiac
function-systolic time intervals, left ventricular ejection time, pre-
ejection period index, and pre-ejection peak to ejection time ratio
remained within normal limits.
Table 8. Exercise-induced angina and carbon monoxide (10 subjects per study)
Carboxyhaemoglobin Ambient CO Time to Reference
(%) mg/m3(ppm) angina
response
Initial Final
1.12 5.08 61a (53) Shortened Aronow et al (1972)
1.07 2.68 57b (50) Shortened Aronow & Isbell (1973)
1.40 2.90 57c (50) Shortened Anderson et al. (1973)
a Freeway trip.
b Continuous exposure for 2 h in laboratory,
c Intermittent exposure for 4 h in laboratory.
Another study by Aronow & Isbell (1973) was somewhat similar to
that of Anderson et al. (1973). Aronow and his co-workers exposed
patients (nonsmokers at the time of the test to a carbon monoxide
concentration of 57 mg/m3, resulting in a carboxyhaemoglobin
concentration of 2.68%. This study was also conducted as a double-
blind random trial with one day of breathing carbon monoxide and
another day breathing compressed carbon monoxide-free air. The angina
pectoris of all patients was documented by history and coronary
angiography. A 23% reduction in exercise time (bicycle) resulted
following the carbon monoxide exposure. No electro-cardiographic
changes were seen in these patients during any exercise period.
Plotting of Aronow's data suggests that there was a linear
relationship between carboxyhaemoglobin levels and the decrease in
time to angina.
This evidence suggests that a deleterious effect could occur at
carboxyhaemoglobin levels as low as 2.5% in certain subjects with
coronary heart diseases. The USA National Health Survey Examination
(US Environmental Protection Agency, 1975) reported that in the USA
there were 3 215 000 adults, aged 18-79 years, with definite coronary
heart disease and another 2 410 000 with suspected disease. Many of
these individuals, as well as others in the general population, have
carboxyhaemoglobin levels equal to, or above 2.5%. It would be rash to
even suggest that the above-mentioned studies implicate carbon
monoxide as a factor in determining the natural history of heart
disease in a community. It is even more dangerous to imply that
exposure to carbon monoxide increases the frequency or severity of
chest pain, or shortens life expectancy among patients with angina
pectoris or other clinical manifestations of heart disease. The
necessary epidemiological evidence for an association between the
frequency of episodes of angina pectoris and community ambient levels
of carbon monoxide is inadequate and additional information from more
and varied sources is required.
Patients with chronic obstructive pulmonary disease are probably
at high risk, although few studies on them have been reported. Any
increase in hypoxia could result in respiratory failure. However,
these individuals may absorb less carbon monoxide because of their
disease as the hypoxia may be compensated for by increased
erythropoiesis and a shift of the oxygen dissociation curve to the
right. An interesting approach to the evaluation of individuals at
risk from carbon monoxide and other pollutants can be found in a
publication of the US Environmental Protection Agency (US
Environmental Protection Agency, 1975). Ogawa et al. (1974) have
presented evidence on the development of pulmonary oedema and
discussed possible mechanisms of the role of carbon monoxide in the
disorder.
8.2.2 Anaemic individuals
The information available on the effects of carbon monoxide on
anaemic patients is still inadequate.
The oxygen dissociation curve of blood obtained from patients with
anaemia is shaped like the normal curve but is vertically compressed.
However, when curves from individuals with a 50% reduction in
haemoglobin content are compared with dissociation curves determined
in the presence of 50% carboxyhaemoglobin, there are striking
differences. Consequently, the tendency to make such comparisons is
likely to lead to erroneous deductions concerning effects occurring at
the tissue level. Brody & Coburn (1970) discussed these differences in
relation to arterial and venous pCO and pO2. Because the capacity
of the oxygen transport system is reduced in anaemic persons,
it can be assumed a priori that they could be more at risk from
carbon monoxide exposure than normal persons. Brody & Coburn (1970)
indicated that, if the oxygen content of the mixed venous blood is
abnormally low, as in anaemia or carbon monoxide poisoning, the effect
of the shunted blood in lowering arterial pO2 will be greater than
normal, and a small increase in the alveolar-arterial pressure
difference (AaDO2) will result. The change in the shape of the
oxyhaemoglobin curve due to the presence of carbon monoxide will also
increase the AaDO2. Furthermore, Brody & Coburn (1970) showed that
mild increases in carboxyhaemoglobin concentrations would have little
or no influence on the AaDO2 in normal subjects. However, in
patients with large intra-cardiac right-to-left shunts or with chronic
lung disease and regional variation in the ventilation perfusion ratio
( VA/ QA), the presence of carbon monoxide in the blood will increase
the AaDO2.
Tissue oxygenation may be involved initially because of the
anaemic state, since mixed venous pO2 is decreased (Cropp, 1970)
and the reduction in venous pO2 from a particular carboxyhaemo-
globin value is somewhat greater in anaemic than in normal subjects.
In patients with haemolytic anaemia and sickle cell disease (Engel et
al., 1971), the rate of endogenous carbon monoxide production from
haemoglobin catabolism is increased. Normal subjects produce
approximately 18 µmoles of carbon monoxide per hour, resulting in
carboxyhaemoglobin levels of 0.5 to 0.8%. Carbon monoxide production
in anaemic patients (Coburn et al., 1966; Logue et al., 1971) has been
reported to vary from 31 to 158 µmoles per hour, producing
carboxyhaemoglobin levels of 1.3-5.2%.
Anaemic subjects approach equilibrium levels of carboxyhaemoglobin
more rapidly than those with normal haemoglobin levels at any given
exposure to carbon monoxide. Exposure to a concentration of
22.9 mg/m3 (20 ppm) for approximately 4 h in an individual with a
haemoglobin level of 7 g/100 ml could result in a carboxyhaemoglobin
concentration of 4-5% compared with an anticipated level of 2.5% for
normal individuals. Exogenous carbon monoxide exposure of anaemic
individuals could result, in conjunction with higher endogenous
production, in their attaining critical levels of carboxyhaemoglobin
more rapidly than normal individuals.
8.2.3 Embryo, fetus, neonate, and infants
Pregnant mothers and their fetuses may be exposed acutely or
chronically to carbon monoxide either by maternal smoking or by
environmental pollution. The biological effects of carbon monoxide
exposure on fetal tissues during intrauterine development or during
the newborn period are far from clear.
Several studies (Astrup et al., 1972; MacMahon et al., 1966) have
demonstrated that babies delivered by mothers who smoke cigarettes
weigh less than those delivered by nonsmoking mothers. Relative
maternal, fetal, or placental hypoxia may be responsible, as suggested
by the observation that infants born at high altitudes also weigh less
than those born at sea level (Grahn & Kratchman, 1963). The New Mexico
State Department of Public Health (1975) provided additional
confirmation of the relationship between altitude and birth weights.
Mothers who smoked were reported to have carboxyhaemoglobin
concentrations ranging from 2 to 14%,. while concentrations in the
fetuses ranged from 2.4 to 9.8%. These values may not represent
conditions present during pregnancy, since these data were obtained
just prior to birth. Another factor that may produce differential
effects on the fetus is related to the endogenous production of carbon
monoxide by pregnant women. Delivoria-Papadopoulos et al., (1969)
indicated that nonsmoking pregnant women produced 0.9 ml of carbon
monoxide per hour in contrast to the nonpregnant female's production
of 0.4 ml reported by Longo (1970). Fetal production of endogenous
carbon monoxide accounted for 3% of the total carboxyhaemoglobin
present in the blood of a nonsmoking normal pregnant woman. The source
of the remainder is not well known but may be related to progesterone
levels (Delivoria-Papadopoulos et al., 1969). Even though
hyperventilation of pregnancy may partially compensate for the
increased carbon monoxide production in the absence of exogenous
exposure, the maternal carboxyhaemoglobin still remains about 13%
above that in nonpregnant women (Longo, 1970). It should be noted that
the post partum (24 h) female may be producing 3 times as much carbon
monoxide as a near term nonsmoking pregnant woman.
Behrman et al. (1971) measured carboxyhaemoglobin concentrations
in 25 relatively normal newborn infants in a downtown Chicago nursery
and found the mean value to be 6.98%. These investigators indicated
that absolute carboxyhaemoglobin levels were related to ambient levels
of carbon monoxide. Some doubts about this conclusion exist, since the
monitoring reference site was 2.4 km from the nursery; the
investigators did not report any untoward clinical effects from
exposure to these levels, and no consideration was given to the
possibility of increased endogenous carbon monoxide production in
these infants.
Of the several mechanisms that may account for the influence of
carbon monoxide on developing tissue, the most important is the
interference with tissue oxygenation. Carbon monoxide decreases the
capacity of haemoglobin to transport oxygen and shifts the oxygen
saturation curve to the left. The normal arterial pO2 supplying
fetal tissue is approximately 3.7 kPa (28 Torr). The shift to the left
will tend to further decrease the oxygen gradient from maternal to
fetal blood across the tissue. The decreased pO2 and the
diminished oxygen transport due to the presence of carboxyhaemoglobin
may also produce undesired influences on the fetus. One of the
possible mechanisms by which carbon monoxide or other components of
tobacco smoke may adversely influence fetal development is through
interference with the metabolic function of placental cells. These
cells have a role in metabolizing hormones as well as in the transport
of vitamins, carbohydrates, amino acids, and other substances through
their energy dependent processes. Tanaka (1965) reported that the
oxygen uptake of placental slices from mothers who smoked varied
inversely with maternal levels of carboxyhaemoglobin, being markedly
reduced when this level was higher than 7.0%. The preponderance of
evidence concerning maternal carboxyhaemoglobin levels, along with
fetal and perinatal exposure, tends to warrant the reduction of
exposure to exogenous carbon monoxide sources that might cause this
group to be at risk, to a minimum.
The potential toxicity of carbon monoxide present in transfused
blood has received little attention. Kandall et al. (1973) measured
carboxyhaemoglobin concentrations in donor blood and in relatively
healthy infants receiving exchange blood transfusions. The mean pre-
transfusion carboxyhaemoglobin concentration in 6 cases was 1.34%.
Donor blood contained a carboxyhaemoglobin concentration of 5.17%,
resulting in a mean value of 4.92% in the transfused infant. In one
infant transfused with blood containing a carboxyhaemoglobin
concentration of 8.87%, the resultant carboxyhaemoglobin value in the
infant was 7.43%. Although it was stated that the infants did not
appear to be adversely affected by the levels of carboxyhaemoglobin
reached during exchange transfusion, it should be noted that adverse
effects have been observed in adults at these levels. Furthermore, in
individuals whose oxygen transport system or cardiovascular reserve is
already compromised, the presence of additional carboxyhaemoglobin,
from transfused blood, may result in a further and more potentially
dangerous decrement in arterial, mixed venous, and coronary sinus
oxygen tensions. It should be recalled that some blood samples
collected from blood donors had carboxyhaemoglobin values that
exceeded 18%.
8.2.4 Individuals living at high altitudes
The effects of carbon monoxide and of hypoxia induced by high
altitude are similar. Carbon monoxide produces effects that aggravate
the oxygen deficiency present at high altitudes. When high altitude
and carbon monoxide exposures are combined (Table 9) the effects are
apparently additive. It should be noted, however, that decreased
pO2 in the air and increased carboxyhaemoglobin, produce different
physiological responses. They have different effects on blood pO2,
on the affinity of oxygen for haemoglobin, on the extent of
oxyhaemoglobin saturation (carbon monoxide hypoxaemia shifts the
oxyhaemoglobin dissociation curve to the left, and a decrease in
PAo2 shifts it to the right), and on ventilatory drive. These
effects have been discussed earlier.
The actual influence of a combination of increased carboxy-
haemoglobin and decreased oxyhaemoglobin has not been adequately
documented by experimental data. The few available studies refer
only to acute exposures to lower pO2 and raised pCO. The
most supportive information on the additive nature of this combination
originates from psychophysiological studies and even this information
is not very convincing. When Blackmore (1974) analysed the cause of
aircraft accidents in Britain, he found that carboxyhaemoglobin levels
provided valuable information in relation to altitude and sources of
carbon monoxide. The high levels of carbon monoxide found (up to 74%)
could be attributed to equipment failure, smoking, and fires. No data
are available on the effects of carbon monoxide on the native
inhabitants at high altitudes or on the reactions of these natives
when they are suddenly removed to sea level and possible high ambient
carbon monoxide concentrations.
Table 9. Approximate physiologically equivalent altitudes at
equilibrium with ambient carbon monoxide levelsa
Ambient CO concentration Actual altitude (metres)
mg/m3 ppm 0 (sea level) 1524 3048
Physiologically equivalent altitudes
with carboxyhaemoglobin
0 0 0 (sea level) 1524 3048
28.6 25 1829 2530 3962
67.3 50 3048 3658 4672
114.5 100 3749 4663 5486
a From: NAS/NRC (1977).
In their studies on altitude exposures of young males, McFarland
et al. (1944) showed that changes in visual threshold occurred at
carboxyhaemoglobin levels as low as 5% or at a simulated altitude of
2425 m. These observations were confirmed by Halperin et al. (1959),
who also noted that recovery from the detrimental effects on visual
function lagged behind the elimination of carbon monoxide. However,
the data given were sparse and the variability among the four subjects
was not given. Vollmer et al. (1946) studied the effects of carbon
monoxide at simulated altitudes of 3070 and 4555 m and reported that
there were no additive effects of carbon monoxide and altitude. They
suggested that the effects of carbon monoxide were masked by some
compensatory mechanisms. The data presented were not convincing.
However, Lilienthal & Fugitt (1946) indicated that a combination of
altitude (1540 m) and a carboxyhaemoglobin level of 5-9% induced a
decrease in flicker fusion frequency, although either one alone did
not have any effect. They also reported that the presence of 8-10%
carboxyhaemoglobin was effective in reducing altitude tolerance by
1215 m. During light activity at an altitude of 4875 m, carbon
monoxide uptake increased, probably owing to the hyperventilation at
altitude caused by the respiratory stimulus of decreased pO2
(Forbes et al., 1945). Evidence that carbon monoxide elimination was
similar at sea level and at altitudes up to 10 000 m was obtained by
several investigators (Gorodinsky et al., 1970; Sedov et al., 1971).
However, increased ambient temperatures up to 35°C and hard physical
work increased the rate of elimination (Vollmer et al., 1946). Pitts &
Pace (1947) stated that every 1% increase in carboxyhaemoglobin (up to
13%) was equivalent to a 109 m rise in altitude if the subjects were
at altitudes of 2100-3070 m. These observations were based on changes
in the heart rate response to work. A number of unanswered questions
arise from all these studies, which in general were obscured by such
factors as poor control and no identification of subjects who may have
been smokers.
Two groups of investigators have presented data comparing the
physiological responses of subjects to altitude and carbon monoxide
exposure where the hypoxaemia due to altitude and the presence of
carboxyhaemoglobin were approximately equal. In one study (Astrup &
Pauli, 1968), the carboxyhaemoglobin concentration was about 12%
(although the mode of exposure to carbon monoxide was such that
carboxyhaemoglobin ranged from 5% to 20% and the altitude study was
conducted at 3977 m). The second study (Sedov et al., 1971) compared
responses at an altitude of 4000 m and a carboxyhaemoglobin content of
20%. In both studies, carboxyhaemoglobin content was much in excess of
that anticipated for typical ambient pollution. However, they both
suggested that the effects attributable to carbon monoxide and to
altitude were equal.
8.3 Summary Table
Table 10 is a summary of controlled human studies that provide
useful information for evaluating the relationship between exposure to
carbon monoxide and its health effects.
Table 10. Summary of exposure-effect relationships
Exposure Reported effects Reference
(HbCO %)
(a) Behavioural changes
20 Essentially no impairment in time discrimination Stewart et al. (1973b)
(using Beard-Wertheim task)
11.3 No vigilance decrement (using Horvath task) Winneke et al. (1976)
9a No vigilance decrement (using Fodor-Winneke Winneke (1974)
task); no change in reaction time
8.4 No vigilance decrement (using their own Groll-Knapp et al. (1976)
vigilance task (1972))
7.6 Longer reaction times Ramsey (1973)
7.3 Disturbance in certain perceptual and cognitive Bender et al. (1972)
processes
5 Vigilance decrement Horvath et al. (1971)
4.5 Longer reaction times Ramsey (1972)
3.1b Initial vigilance decrement with subsequent Fodor & Winneke (1972)
normalization; no change in response latency
3b Vigilance decrement Groll-Knapp et al. (1972)
2b Impaired performance in time-discrimination Beard & Wertheim (1967)
(b) Changes in work performance
6.3 Decrease in maximal work time Ekblom & Huot (1972)
4.3 Decrease in a maximal oxygen uptake (VO2) Horvath et al. (1975)
(1.7c)
4.0 Decrease in mean exercise time until exhaustion Aronow & Cassidy (1975)
(0.6c)
2.5 Decrease in absolute exercise time in non- Drinkwater et al. (1974)
smokers
Table 10 (Cont'd)
Exposure Reported effects Reference
(HbCO %)
(c) Aggravation of symptoms in patients with cardiovascular disease
(1.1c)
5.1 Shortened time to angina response immediately Aronow et al. (1972)
after exposure
(1.1c)
2.9 Shortened time to angina response 2 h after Aronow et al. (1972)
(1.1c) exposure
2.9 Shortened time to angina response Anderson et al. (1973)
(1.1c)
2.8 Decrease in mean exercise time until onset of Aronow et al. (1974)
intermittent claudications
(1.0c)
2.7 Shortened time to angina response Aronow & Isbell (1973)
a Estimated values using the formula by Coburn et al. (1965).
b Estimated values using the formula by Peterson & Stewart (1970).
c HbCO % before exposure to CO.
9. EVALUATION OF HEALTH RISKS
9.1 Introduction
The acute toxicity of carbon monoxide has long been recognized and
is well documented. Much has been learned of the main sources of the
gas, its absorption, the kinetics of its reactions with blood, and the
biochemical and pathological consequences of poisoning by excessive
absorption. More recently, a great deal of attention has been paid to
the effects, demonstrable or suspected, of exposure to concentrations
much lower than those that cause definite poisoning. Such
concentrations are those commonly found in urban air (caused almost
wholly by traffic pollution) and indoors (caused by faulty ventilation
of heating or cooking appliances), but there has been much concern
with the effects of the gas on smokers, who inhale considerable
quantities of carbon monoxide with tobacco smoke. Since the main
source of carbon monoxide as an urban pollutant is the petrol engine,
the problems posed by the inhalation of relatively low concentrations
of the gas are likely to grow rather than diminish, as traffic becomes
denser and more widespread. The recognition of the importance of
pollution of the domestic environment is relatively recent and
deserves more study; the problems posed by smoking tobacco are common
and are, unfortunately, increasing. There is much published evidence,
some of which is of debatable value, that suggests that the
comparatively low concentrations of carboxyhaemoglobin produced by
exposure to pollution of the ambient air and the higher concentrations
usually associated with smoking, might cause demonstrable impairment
of vigilance, discrimination, and of the performance of fine tasks and
physical work in healthy subjects, and the exacerbation of symptoms
such as angina pectoris on effort in patients with cardiovascular
diseases. Likewise there is evidence, derived from experimentation on
animals, that chronic exposure to carbon monoxide leading to the
levels of carboxyhaemoglobin commonly found in smokers may, in
association with high cholesterol intakes, play a part in the genesis
of atherosclerosis. Moreover, there are reasons to suspect that
exposure to carbon monoxide may enhance the effects of other
pollutants, commonly administered therapeutic agents, socially
acceptable amounts of beverages such as alcohol, and other
environmental stresses. This section is intended as a brief assessment
of these topics in the hope that sound advice may be given on the need
to control levels of carbon monoxide in the ambient air.
9.2 Exposure
9.2.1 Assessment of exposure
Concentrations of carbon monoxide in air may be measured with
comparative ease by such methods as non-dispersive spectroscopy, gas
chromatography etc. But human body burdens of carboxyhaemoglobin
depend on many factors other than the partial pressure of carbon
monoxide in the inhaled air; among these factors are time of exposure,
pulmonary ventilation (which mainly depends on work done), and blood
volume. Since these quantities, especially ambient concentrations of
carbon monoxide, may vary widely, it is obviously difficult, if not
impossible at times, to calculate the likely body burden of
carboxyhaemoglobin in an exposed individual. There is, therefore, much
to be gained by sampling blood to obtain an integrated estimate of
carboxyhaemoglobin derived from all sources under various conditions
of exposure. It must be emphasized that the measurements of carbon
monoxide in air and in blood give complementary results and are not
merely alternative forms of monitoring. Methods of analysis are
discussed in sections 2.2 and 2.3.
There is a tendency to forget that the reaction between
haemoglobin and carbon monoxide is reversible and that, in a given
environment, a subject may acquire carbon monoxide, excrete it, or
remain in equilibrium with the ambient air depending on the carbon
monoxide concentration and the initial level of carboxyhaemoglobin in
the individual. The time taken to achieve equilibrium between blood
and ambient air depends on the initial carboxyhaemoglobin
concentrations as well as on the factors mentioned above. The rate of
excretion of carbon monoxide will depend not only on ambient air
levels, the initial carboxyhaemoglobin and on factors such as
pulmonary ventilation, but also on the partial pressure of oxygen in
inspired air, which might be introduced therapeutically to increase
elimination. Fig. 12 shows estimates of equilibrium times for various
ambient concentrations and levels of activity. The half-life for
excretion, at rest, is approximately 4“ h.
9.2.2 Endogenous production
The normal breakdown in the body of blood pigments produces carbon
monoxide to give endogenous carboxyhaemoglobin values of 0.1-1.0% and
normal blood is in equilibrium with carbon monoxide levels in air of
roughly 5 mg/m3 (4.3 ppm). These data could be used as a basis for
establishing air quality criteria for carbon monoxide. Various causes
of increased endogenous production of carbon monoxide are discussed in
section 6.1.
9.2.3 Outdoor environmental exposure
Natural sources of carbon monoxide (section 3.1) are of
considerable magnitude but are diffuse, and ambient air concentrations
at locations removed from man-made sources range from 0.01 to
0.9 mg/m3 (0.01 and 0.8 ppm) which is negligible in the context of this
report. By far the most important sources of carbon monoxide at
breathing level are petrol engine vehicle exhausts (section 3.2). The
diesel engine (compression ignition), when properly adjusted, emits
little carbon monoxide. The density, distribution, and mode of
operation of vehicles vary greatly and these and other factors, the
 most important of which is the weather, produce great variations in
the concentrations of pollutants produced by traffic. Concentrations
fall steeply with distance from the street. However, distinct patterns
are often discernible (section 5.1). Concentrations for 8-h averaging
times are frequently used and quoted and usually vary from
<10 mg/m3 (8.7 ppm) to over 60 mg/m3 (52.2 ppm) but are mostly
<20 mg/m3 (17.6 ppm) in city streets. Away from heavy traffic, even
in towns, annual average concentrations are usually well under
10 mg/m3 (8.7 ppm). Obviously, in especially stagnant weather, very
heavy traffic may produce much higher levels. There is little
information about concentrations of carbon monoxide near large
stationary sources.
9.2.4 Indoor exposure
Carbon monoxide diffuses readily and, being relatively chemically
inert and not absorbed on surfaces, concentrations indoors are usually
similar to those found immediately outside. Not infrequently, however,
high concentrations may be found in kitchens and living rooms in which
there are coal, gas, or oil-fired cooking or heating appliances that
are maladjusted and inadequately vented to outside air; in some
countries, cases of acute and even fatal poisoning due to these causes
are not uncommon. The possible contribution of the domestic
environment must be noted in surveys. The smoking of tobacco indoors
can obviously increase the carbon monoxide concentration of the air
but recent work has shown that, before carbon monoxide reaches
significant levels, the irritation from the other constituents of
tobacco smoke becomes unacceptable if not actually intolerable.
9.2.5 Exposures related to traffic
In garages and tunnels, being in effect closed streets, pollution
by carbon monoxide can reach high levels. However, since transit time
in tunnels is relatively short, higher concentrations than those found
in streets are tolerable. Usually, there are monitoring instruments
that control ventilation and sound alarms if concentrations exceed
agreed values, which may vary from 115 to 570 mg/m3 (100-500 ppm)
depending on the use and length of the tunnel. High (sometimes lethal)
concentrations of carbon monoxide may accumulate inside motor vehicles
because of fractures in exhaust systems or other mechanical defects.
9.2.6 Occupational exposure
Traffic policemen, garage attendants, and drivers of taxis and
trucks are exposed to pollution from traffic and many studies have
shown a consequent increase in carboxyhaemoglobin levels (up to about
3% in nonsmokers), but there is much evidence that this increase may
be relatively undramatic, when compared with the manifest effects of
cigarette smoking. Exposure in certain industries, especially in iron
and steel works and in the manufacture of various gases, may be
relatively massive (in excess of 115 mg/m3 or 100 ppm), and high
carboxyhaemoglobin levels (>15% in nonsmokers) have been reported in
workers. Firemen may be exposed to very high concentrations of carbon
monoxide in fighting certain fires, but this exposure is obviously
episodic. These matters are discussed in section 5.3.
9.2.7 Tobacco smoking
The smoking of tobacco, especially in the form of cigarettes, has
been shown in many studies to be the major cause of raised
carboxyhaemoglobin levels in adult populations. Table 7 displays some
of this evidence and the topic is discussed in detail in section
8.1.6. Whereas carboxyhaemoglobin concentrations of 3% are rarely
found in nonsmokers exposed to town air, concentrations of 5-15% are
often found in smokers. It is important to remember that the effects
of smoking and exposure to town air are not simply additive and that
the resulting carboxyhaemoglobin levels will depend on other factors
already discussed.
9.2.8 Multiple exposures
Enough has been said to leave no doubt that carbon monoxide,
produced exogenously or endogenously, is a widespread pollutant
emanating from many sources to which people may be exposed in various
ways. This variety of exposure must be taken into account in the
interpretation of epidemiological surveys, the design of experiments,
and, above all, in giving advice about the fixing of air quality
criteria.
9.3 Effects
The main areas of concern that have arisen from acute or chronic
exposure to low levels of carbon monoxide in experimental and
epidemiological research in animals and man are: (a) its role in the
genesis of arteriosclerotic vascular diseases; (b) its role in the
aggravation of symptoms of cardiovascular diseases; (c) its
contribution to performance deficits in certain psychomotor tasks; and
(d) its role in limiting the working capacity of exercising man.
9.3.1 Cardiovascular system
9.3.1.1 Development of atherosclerotic cardiovascular disease
Extensive experimental work has been carried out over many years
on animals, mainly rabbits, showing that prolonged exposure to
moderate levels of carbon monoxide can produce atherosclerotic
changes, especially in the presence of high cholesterol levels (1-2%)
in the diet. The relevance of this work for man has not been
established. However, other animal work, and some epidemiological
studies of prolonged human exposures to elevated carbon monoxide
levels through smoking, occupation, or both, such as those carried out
in Denmark, Finland, and Japan, indicate the need for further
investigation of the possible role of carbon monoxide in the genesis
of atherosclerotic vascular changes in animals and man. The degree of
intermittency of exposure at various levels should be taken into
account as well as the possible contribution of other agents such as
nicotine and high-fat diets. There is some evidence of adaptation, but
such changes may not be entirely beneficial. None of the information,
currently available, is useful for the purpose of setting standards.
9.3.1.2 Acute effects on existing heart illness
The few existing epidemiological studies on the possible effects
of carbon monoxide on the severity or fatality of coronary occlusion
are insufficient to allow any conclusions. It is hoped that additional
work of this type will clarify matters.
Two carefully conducted human studies of the effects of low carbon
monoxide exposure and exercise on pain in volunteer patients with
angina pectoris offer valuable quantitative information. Although
limited in the number of patients studied, the findings are consistent
in the 2 investigations showing effects at carboxyhaemoglobin
concentrations of 2.5-3.0%. A third single-blind study revealed the
same detrimental effects in patients with angina pectoris when
exposure to traffic exhausts caused carboxyhaemoglobin levels to rise
to 5.1%. A no-adverse-effect level has not been established in these
observations, nor is it possible to determine whether there is a
graded response in this type of experiment. More work of a similar
nature would be useful to explore these questions.
9.3.1.3 Acute effects on existing vascular disease
One study, similar to those done on patients with angina, has been
carried out on patients with intermittent claudication from peripheral
vascular disease. Effects on pain with exercise were observed in the
same exposure range as with angina i.e., at carboxyhaemoglobin
concentrations of 2.5-3.1%, with a mean of 2.8%. Here, too, more data
of a similar kind are needed, preferably designed to provide dose-
response relationships.
9.3.2 Nervous system
As for the role of carbon monoxide in affecting psychomotor
functions, no definite conclusions can be drawn from the existing
data. The behavioural functions tested in such studies include
vigilance and psychomotor performance, visual acuity and sensitivity,
the ability to estimate time intervals, complex motor coordination as
tested by driving simulators, and different perceptual and mental
operationsa. Some workers observed detrimental effects at
carboxyhaemoglobin levels as low as 2%, whereas others were unable to
detect significant impairment even at levels from above 5% to about
20%. In evaluating these discrepancies, it should be mentioned, that
these behavioural functions are easily influenced by a number of other
factors besides carbon monoxide-induced hypoxia, e.g., degree of
sensory deprivation, compensatory abilities, drugs, temperature, time
of day, competition, etc.
9.3.3 Work capacity
That elevated carboxyhaemoglobin levels affect work capacity has
long been known. Levels of 40-50% will usually prevent working
entirely. Recent studies in the laboratory, on man, using maximum work
capacity or maximum aerobic capacity as indicators of performance,
have been carried out in relation to carboxyhaemoglobin levels. Here,
dose-response data are available for maximum effort. The limitation
appears at a carboxyhaemoglobin concentration of about 4% and
increases at higher levels. Lower exposure levels have been studied
and do not produce this effect. It should be noted that while levels
of carboxyhaemoglobin of 2.5-4%, did not reduce maximum work capacity,
they did reduce the length of time for which such effort could be
carried out. It is not known what specific levels of carboxy-
haemoglobin will reduce the capacity of individuals to perform at
ordinary work levels, such as 30-50% of their maximum capacity, for
prolonged periods of time.
9.4 Recommended Exposure Limits
It has already been stated that the major contributor to
carboxyhaemoglobin concentrations in the body is the smoking of
tobacco; however, in many of the experiments currently quoted to
justify the formulation of exposure limits, the smoking habits of the
subjects were not taken into account. Results of recent work suggest
that smokers and ex-smokers might be less sensitive to carbon monoxide
exposure than nonsmokers. In view of this suggestion, and because of
the deficiencies in the experiments mentioned, recommendations for
exposure limits should be confined to the protection of nonsmokers.
There is an urgent need for more work on possible adaptation following
a The possible importance of performance deficiencies resulting
from carbon monoxide is considerable, particularly in relation to
accidents at work, and while driving or flying. Further studies,
particularly of the vigilance type are urgently needed for a better
understanding of this problem.
exposure to carbon monoxide from smoking or from other sources. It is
also important to note that carboxyhaemo-globin levels have been the
measurement of exposure in most experimental work. Thus, it is
desirable to recommend the primary exposure limits in terms of
carboxyhaemoglobin, and follow this by comments on the derivation of
an appropriate air concentration equivalent.
9.4.1 General population exposure
Data used in arriving at a recommendation for an exposure limit
for the general population were mainly those obtained from the
exposure of subjects with cardiovascular illness to carbon monoxide in
conjunction with exercise. Agreement was not reached on a single
level. Thus, a range of carboxyhaemoglobin concentrations of 2.5-3.0%
is recommended for the protection of the general population including
those who have impaired health. The recommendation must be regarded as
tentative, since ideal dose-response or concentration-response
information is not yet available. However, it must also be recognized
that complete protection of all persons, at all times, cannot
reasonably be sought by environmental control alone. Persons who are
in should be educated by their physicians concerning their own
responsibility to avoid stressful exposures.
9.4.2 Working population exposure
Better quality data are available for recommending an exposure
limit for the working population. In this case, the Task Group
unanimously agreed on maintaining carboxyhaemoglobin levels below 5%,
on the basis of present knowledge, since working populations comprise
individuals who are assumed to be healthy, physiologically resilient,
and under regular supervision.
9.4.3 Derived limits for carbon monoxide concentrations in air
It is important, wherever possible, to have both biological and
environmental assessments of human exposure to pollutants. While the
biological measurements may be more relevant in relation to effects,
they may be more difficult to use in practice. For carbon monoxide,
the relationship between concentrations in air and carboxyhaemoglobin
levels is affected by several variables, including exposure time and
it is not easy to estimate. However, such estimates may be
sufficiently accurate for many practical purposes (for reviews see
Committee on the Challenges of Modern Society, 1972; Commission of the
European Communities, 1974; NAS/NRC, 1977; Winneke, 1977; Ott & Mage,
1978). It should be emphasized yet again that analyses of carbon
monoxide in air and of carboxyhaemoglobin in blood are complementary,
and should in no way be regarded as alternative methods of monitoring.
Obviously, air monitoring has its uses in the planning and
implementation of control measures, and for warning purposes, but such
measurements have limited value in estimating the actual human
exposure defined by carboxyhaemoglobin levels.
REFERENCES
ADAMS, J. D., ERICKSON, H. H., & STONE, H. L. (1973) Myocardial
metabolism during exposure to carbon monoxide in the conscious
dog. J. appl. Physiol., 34: 238-242.
AGOSTINI, J. C., RAMIREZ, R. G., ALBERT., S. N., GOLDBAUM, L. R., &
ABSOLON, K. B. (1974) Successful reversal of lethal carbon
monoxide intoxication by total body asanguineous hypothermic
perfusion. Surgery, 75: 213-219.
AHMED, A. E., KUBIC, V. L., & ANDERS, M. W. (1977) Metabolism of the
haloforms to carbon monoxide. I. In vitro studies. Drug
Metabol. Disposition, 5: 198-204.
ALEXANDROV, N. P. (1973) [Choice of experimental animals for
elaboration of standards for CO.] Gig. i Sanit., 11: 92-95 (in
Russian).
ALEKSIEVA, T. & DIMITROVA, M. (1971) [Some oscillographic changes in
workers exposed to chronic carbon monoxide effect.] Tr. Niotpz,
pp. 163-169 (in Bulgarian).
ALTMAN, P. L. & DITTMEN, D. S., ed. (1971) Respiration and
circulation, Bethesda, MD, Federation of the American Society of
Experimental Biology.
ALTSHULLER, A. P. & BUFALINI, J. J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ANDERSON E. W., ANDELMAN, R. J., STRAUCH, J. M., FORTUIN, N. J., &
KNELSON, J. H. (1973) Effect of low-level carbon monoxide exposure
on onset and duration of angina pectoris: a study in ten patients
with ischemic heart disease. Ann. intern. Med., 79: 46-50.
ANTWEILER, H. (1974) Physiological kinetics of CO inhalation.
In: Proceedings of the European Colloquium on Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg Commission of the European
Communities, Directorate General Scientific and Technical
Information Management, pp. 197-251 (in German with full English
translation).
ANTWEILER, H. (1975) [The reception and effect of carbon monoxide in
active and passive smokers.] Arbeitsmed. Sozialmed.
Präventivmed., 10: 245-248 (in German).
ARONOW, W. S. & CASSIDY, J. (1975) Effect of carbon monoxide on
maximal treadmill exercise: a study in normal persons. Ann.
intern. Med., 83: 496-499.
ARONOW, W. S. & ISBELL, M. W. (1973) Carbon monoxide effect on
exercise induced angina pectoris. Ann. intern. Med.,
79: 392-395.
ARONOW, W. S., CASSIDY, J., VANGROW, J. S., MARCH, H., KERN, J. C.,
GOLDSMITH, J. R., KHEMKA, M., PAGANO, J., & VAWTER, M. (1971a)
Effect of cigarette smoking and breathing carbon monoxide on
cardiovascular hemodynamics in anginal patients. Circulation,
50: 340-347.
ARONOW, W. S., DENDINGER, J., & ROKAW, S.N. (1971b) Heart rate and
carbon monoxide level after smoking high-, low-, and non-nicotine
cigarettes. Ann. intern. Med., 74: 697-702.
ARONOW, W. S, HARRIS, C. N., ISBELL, M. W., ROKAW, S. N., & IMPARATO,
B. (1972) Effect of freeway travel on angina pectoris. Ann.
intern. Med., 77: 669-676.
ARONOW, W. S., STEMMER, E. A., & ISBELL, M. W. (1974) Effect of carbon
monoxide exposure on intermittent claudication. Circulation,
49: 415-417.
ASTRAND, I., OVRUM, P., & CARLSSON, A. (1975) Exposure to methylene
chloride. I. Its concentration in alveolar air and blood during
rest and exercise and its metabolism. Scand. J. Work Environ.
Health, 1: 78-94.
ASTRUP, P. (1970) Intraerythrocytic 2,3-diphosphoglycerate and carbon
monoxide exposure. Ann. NY Acad. Sci., 174: 252-254.
ASTRUP, P. (1972) Some physiological and pathological effects of
moderate carbon monoxide exposure. Br. med. J., 4: 447-452.
ASTRUP, P. & PAULI, H. G. (1968) A comparison of prolonged exposure to
carbon monoxide and hypoxia in man. Scand. J. clin. lab. Invest.,
22 (Suppl. 103): 1-71.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1967) Enhancing influence of
carbon monoxide on the development of atheromatosis in
cholesterol-fed rabbits. J. atheroscler. Res., 7: 343-354.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1970) Effects of carbon
monoxide exposure on the arterial walls. Ann. NY Acad. Sci.,
174: 294-300.
ASTRUP, P., OLSEN, H. M., TROLLE, D., & KJELDSEN, K. (1972) Effect of
moderate carbon monoxide exposure on fetal development. Lancet,
2: 1220-1222.
AYRES, S. M., CRISCITIELLO, A., & GIANELLI, S., JR (1966)
Determination of blood carbon monoxide content by gas
chromatography. J. appl. Physiol., 21: 1368-1370.
AYRES, S. M., MUELLER, H. S., GREGORY, J. J., GIANELLI, S., JR, &
PENNY, J. L. (1969) Systemic and myocardial hemodynamic responses
to relatively small concentrations of carboxyhemoglobin (COHB).
Arch. environ. Health, 18: 699-709.
AYRES, S. M., GIANELLI, S., JR & MUELLER, H. (1970) Myocardial and
systemic responses to carboxyhemoglobin. Ann. NY. Acad. Sci.,
174: 268-293.
BAKER, F. D. & TUMASONIS, C. F. (1972) Carbon monoxide and avian
embryogenesis. Arch. environ. Health, 24: 53-61.
BALABAEVA, L. & KALPAZANOV, I. (1974) [Effect of smoking and length of
service on the content of carboxyhemoglobin in blood of traffic
policemen.] Hig. i Zdraveo., 5: 490-493 (in Bulgarian).
BALL, K. & TURNER, R. (1974) Smoking and the heart: the basis for
action. Lancet, 2 (7884): 822-826.
BATES, D. R. & WITHERSPOON, A. E. (1952) The photo-chemistry of some
minor constituents of the earth's atmosphere (CO2, CO, CH4,
N2O). Mon. not. R. Astron. Soc. (Lond.), 112: 101-124.
BEARD, R. R. & GRANDSTAFF, N. W. (1975) Carbon monoxide and human
functions. In: Weiss, P. & Laties, V. G., ed. Behavioral
Toxicology, New York, Plenum Press, pp. 1-26.
BEARD, R. R. & WERTHEIM, G. A. (1967) Behavioral impairment associated
with small doses of carbon monoxide. Am. J. public Health,
57: 2012-2022.
BEHRMAN, R. E., FISHER, D. E., & PATON, J. (1971) Air pollution in
nurseries: correlation with a decrease in oxygen-carrying capacity
of hemoglobin. J. Pediatr., 78: 1050-1054.
BENDER, W., GÖTHERT, M., & MALORNY, G. (1972) Effect of low carbon
monoxide concentrations on psychological functions. Staub-
Reinhalt. Luft., 32 (4): 54-60.
BIDWELL, R. G. S. & FRASER, D. E. (1972) Carbon monoxide uptake and
metabolism by leaves. Can. J. Bot., 50: 1435-1439.
BLACKMORE, D. J. (1974) Aircraft accident toxicology: UK experience
1967-1972. Aerosp. Med., 45: 987-994.
BORST, J. R. (1970) Acute and chronic carbon monoxide poisoning in
private motor-cars and lorries. Arts Auto, 36 (19): 1518-1523.
BREYSSE, P. A. & BOVEE, H. H. (1969) Use of expired air-carbon
monoxide for carboxyhemoglobin determinations in evaluating carbon
monoxide exposures resulting from the operation of gasoline fork
lift trucks in holds of ships. Am. Ind. Hyg. Assoc. J.,
30: 477-483.
BRIEGER, H. (1944) Carbon monoxide polycythemia. J. ind. Hyg.
Toxicol., 26: 321-327.
BRODY, J. S. & COBURN, R. F. (1970) Effects of elevated
carboxyhemoglobin on gas exchange in the lung. Ann. NY Acad.
Sci., 174: 255-260.
BUCHWALD, H. (1969) Exposure of garage and service station operatives
to carbon monoxide: a survey based on carboxyhemoglobin levels.
Am. Ind. Hyg. Assoc. J., 30: 570-575.
BUTT, J., DAVIES, G. M., JONES, J. G., & SINCLAIR, A. (1974)
Carboxyhaemoglobin levels in blast furnace workers. Ann. occup.
Hyg., 17: 57-63.
BYCZKOWSKA, Z. & MILAN, K. (1971) [Acute carbon monoxide poisoning
with temporary renal insufficiency.] Med. Pr., 22: 119-121
(in Polish).
CALVERT, J. G. (1973) Interactions of air pollutants. In: Proceedings
of the Conference on Health Effects of Air Pollutants, Oct.,
Washington, DC, National Academy of Sciences, National Research
Council, pp. 19-101 (Serial No. 93-15).
CASTLEDEN, C. M. & COLE, P. V. (1975) Carboxyhaemoglobin levels of
smokers and nonsmokers working in the city of London. Br. J. ind.
Med., 32: 115-118.
CAVANAGH, L. A., SCHADT, C. F., & ROBINSON, E. (1969) Atmospheric
hydrocarbon and carbon monoxide measurements at Point Barrow,
Alaska. Environ. Sci. Technol., 3: 251-257.
CHALUPA, B. (1960) [Disorders of memory in acute carbon monoxide
intoxications.] Prac. Lek., 12: 331-336 (in Polish).
CHAPMAN, D. J. & TOCHER, R. D. (1966) Occurrence and production of
carbon monoxide in some brown algae. Can. J. Bot.,
44: 1438-1442.
CHEVALIER, R. B., BOWERS, J. A., BONDURANT, S., & ROSS, J. C. (1963)
Circulatory and ventilatory effects of exercise in smokers and
nonsmokers. J. appl. Physiol., 18: 357-360.
CHEVALIER, R. B.. KRUMHOLZ, R. A., & ROSS, J. C. (1966) Reaction of
nonsmokers to carbon monoxide inhalation. Cardiopulmonary
responses at rest and during exercise. J. Am. Med. Assoc.,
198: 1061-1064.
CHIODI, H., DILL, D. B., CONSOLAZIO, F., & HORVATH, S. M. (1941)
Respiratory and circulatory responses to acute carbon monoxide
poisoning. Am. J. Physiol, 134: 683-693.
CHOVIN, P. (1967) Carbon monoxide: analysis of exhaust gas
investigations in Paris. Environ. Res., 1: 198-216.
CHOVIN, P. (1974) Comments on paper presented by H. Antweiler.
In: Proceedings of the European Colloquium Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg, Commission of the European
Communities Directorate General Scientific and Technical
Information Management, pp. 225-236.
CLARK, R. T., JR & OTIS, A. B. (1952) Comparative studies on
acclimatization of mice to carbon monoxide and to low oxygen.
Am. J. Physiol, 169: 285-294.
COBURN, R. F. (1970a) Enhancement by phenobarbital and
diphenylhydantoin of carbon monoxide production in normal man.
New Engl. J. Med., 283: 512-515.
COBURN, R. F. (1970b) The carbon monoxide body stores. Ann. NY Acad.
Sci., 174: 11-22.
COBURN, R. F., DANIELSON, G. K., BLAKEMORE, W. S., & FORSTER, R. E.
(1964) Carbon monoxide in blood: analytical method and sources of
error. J. appl. Physiol, 19: 510-515.
COBURN, R. F., FORSTER, R. E., & KANE, P. B. (1965) Considerations of
the physiological variables that determine the blood
carboxyhemoglobin in man. J. clin. Invest., 44: 1899-1910.
COBURN, R. F., WILLIAMS, W. J., & KAHN, P. B. (1966) Endogenous carbon
monoxide production in patients with hemolytic anemia. J. clin.
Invest., 45: 460-468.
COBURN, R. F., PLOEGMAKERS, F., GONDRIE, P., & ABBOUD, R. (1973)
Myocardial myoglobin oxygen tension. Am. J. Physiol.,
224: 870-876.
COHEN, S. I., DEANE, M., & GOLDSMITH, J. R. (1969) Carbon monoxide and
survival from myocardial infarction. Arch. environ. Health,
19: 510-517.
COHEN, S. I., DORION, G., GOLDSMITH, J. R., & PERMUTT, S. (1971a)
Carbon monoxide uptake by inspectors at a United States-Mexico
border station. Arch. environ. Health, 22: 47-54.
COHEN, S. I., PERKINS, N.M., URY, H. K., & GOLDSMITH, J. R. (1971b)
Carbon monoxide uptake in cigarette smoking. Arch. environ.
Health, 22: 55-60.
COLLISON, H. A., RODKEY, F. L., & O'NEAL, J. D. (1968) Determination
of carbon monoxide in blood by gas chromatography. Clin. Chem.,
14: 162-171.
COLUCCI, J. M. & BEGEMAN, C. R. (1969) Carbon monoxide in Detroit, New
York, and Los Angeles air. Environ. Sci. Technol., 3: 41-47.
COMMINS, B. T. & LAWTHER, P. J. (1965) A sensitive method for the
determination of carboxyhaemoglobin in a finger prick of blood.
Br. J. ind. Med., 22: 139-143.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) Proceedings of the
European Colloquium on Carbon Monoxide Environmental Pollution
and Public Health, Luxembourg, 17-19 December, 1973, Luxembourg
Directorate General Scientific and Technical Information and
Information Management, 437 pp.
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1972) Air pollution:
Air quality criteria for carbon monoxide, No. 10, 235 pp.
CONLEE, C. J., KENLINE, P. A, CUMMINS, R. L., & KONOPINSKI, V. J.
(1967) Motor vehicle exhaust at three selected sites. Arch.
environ. Health, 14: 429-446.
COOPER, D. Y., LEVIN, S., NARASIMHULU, S., & ROSENTHAL, O. (1965)
Photochemical action spectrum of the terminal oxidase of mixed
function oxidase systems. Science, 147: 400-402.
CORYA, B. C., BLACK, M. J., & MCHENRY, P. L. (1976) Echocardiographic
findings after acute carbon monoxide poisoning. Br. heart J.,
38: 712-717.
CROPP, G. J. A. (1970) Ventilation in anemia and polycythemia.
Can. J. Physiol. Pharmacol., 48: 382-393.
DAHMS, T. E. & HORVATH, S. M. (1974) Rapid, accurate technique for
determination of carbon monoxide in blood. Clin. Chem.,
20: 533-537.
DAHMS, T. E., HORVATH, S. M., & GRAY, D. J. (1975) Technique for
accurately producing desired carboxyhemoglobin levels during rest
and exercise. J. appl. Physiol., 38: 366-368.
DALHAMN, T., EDFORS, M.-L., & RYLANDER, R. (1968) Retention of
cigarette smoke components in human lungs. Arch. environ. Health,
17: 746-748.
DAVIS, G. L. & GANTNER, G. E.(1974) Carboxyhemoglobin in volunteer
blood donors. J. Am. Med. Assoc., 230: 996-997.
DE BIAS, D. A., BIRKHEAD, N. C., BANERJEE, C. M., KAZAL, L. A.,
HOLBURN, R. R., GREENE, C. H., HARRER, W. V., ROSENFELD, L. M,
MENDUKE, H., WILLIAMS, N., & FRIEDMAN, M. H. F. (1972a) The
effects of chronic exposure to carbon monoxide on the
cardiovascular and hematologic systems in dogs with experimental
myocardial infarction. Int. Arch. Arbeitsmed., 29: 253-267.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., FRIEDMAN, M. H. F.,
HARRER, W., HOLBURN, R., KAZAL, L., MENDUKE, H., ROSENFELD, L.,
WILLIAMS, N., & GREENE, C. H. (1972b) The effects of chronic
exposure to carbon monoxide (100 ppm) on the cardiovascular
system of monkeys, New York, Coordinating Research Council,
Inc., 54 pp. (PB 215-527).
BE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., HARRER, W. V., &
KAZAL, L. A. (1973) Carbon monoxide inhalation effects following
myocardial infarction in monkeys. Arch. environ. Health,
27: 161-167.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., GREEN, C. H., SCOTT,
D., & HARRER, W. V. (1976) Effects of carbon monoxide inhalation
on ventricular fibrillation. Arch. environ. Health, 31: 42-46.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., LONGO, L. D., & FORSTER, R.
E. (1969) Endogenous carbon monoxide of mother and fetus, 39th
Annual Meeting of the Society of Pediatrics Research, Atlantic
City, NJ, p. 148.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., & FORSTER, R. E. (1970)
Cyclical variation of rate of heme destruction and carbon monoxide
production in normal women. Physiologist, 13: 178 (abstract).
DELWICHE, C. C. (1970) Carbon monoxide production and utilization by
higher plants. Ann. NY Acad. Sci., 174: 116-121.
DINMAN, B. D. (1968) Pathophysiologic determinants of community air
quality standards for carbon monoxide. J. occup. Med.,
10: 446-456.
DINMAN, B. D. (1969) Effects of long-term exposure to carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behaviour, and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 25-27.
DINMAN, B. D, EATON, J. W., & BREWER, G. J. (1970) Effects of carbon
monoxide on DPG concentration in the erythrocyte. Ann. NY Acad.
Sci, 174: 246-251.
DIVINCENZO, G. D. & HAMILTON, M. L. (1975) Fate and disposition of
[14C]methylene chloride in the rat. Toxicol. appl. Pharmacol.,
32: 385-393.
DORCUS, R. M. & WEIGAND, G. E. (1929) The effect of exhaust gas on the
performance in certain psychological tests. J. gen. Psychol.,
2: 73-76.
DOUGLAS, C. G., HALDANE, J. S., & HALDANE, J. B. S. (1912) The laws of
combination of haemoglobin with carbon monoxide and oxygen.
J. Physiol. (Lond.), 44: 275-304.
DOUZE, J. M. C. (1971) [Carbon monoxide poisoning by natural gas.]
Ned. Tidjschr. Geneesk., 115 (36): 1487-1493.
DRABKIN, D. L. & AUSTIN, J. H. (1935) Spectrophotometric studies. II.
Preparation from washed blood cells; nitric oxide hemoglobin and
sulfhemoglobin. J. biol. Chem., 112: 51-65.
DRINKWATER, B. L., RAVEN, P. B., HORVATH, S. M., GLINER, J. A.,
RUHLING, R. O., BOLDUAN, N. W., & TAGUCHI, S. (1974) Air
pollution, exercise and heat stress. Arch. environ. Health,
28: 177-181.
DYER, R. S. & ANNAU, Z. (1976) Carbon monoxide and superior colliculus
evoked potentials. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Protection Agency, 9 pp.
ECKARDT, R. E., MACFARLAND, H. N., ALARIE, Y. C. E., & BUSYE, W. M.
(1972) The biological effect from long-term exposure of primates
to carbon monoxide. Arch. environ. Health., 25: 381-387.
EHRICH, W. E., BELLET, S., & LEWEY, F. H. (1944) Cardiac changes from
CO poisoning. Am. J. med. Sci., 208: 512-523.
EJAM-BERDJEV, A. K. (1973) [Effect of small CO concentrations on the
cardiovascular system of workers engaged in cast-iron moulding.]
Harkovskovo Med. Inst., 110: 185-186 (in Russian).
EJAM-BERDJEV, A. K. & ATAEV, B. A. (1973) [Effect of toxic factors
with low intensity on the functional state of the CNS of workers
engaged in cast-iron moulding.] Zdravoohr. Turkm., 10: 24-34
(in Russian).
EKBLOM, B. & HUOT, R. (1972) Response to submaximal and maximal
exercise at different levels of carboxyhaemoglobin. Acta Physiol.
Scand, 86: 474-482.
ENGEL, R. R., RODKEY, F. L., O'NEAL, J. D., & COLLISON, H. A. (1969)
Relative affinity of human fetal hemoglobin for carbon monoxide
and oxygen. Blood, 33: 37-45.
ENGEL, R. R., RODKEY, F. L., & KRILL, C. E., JR (1971)
Carboxyhemoglobin levels as an index of hemolysis. Pediatrics,
47: 723-730.
ENVIRONMENT AGENCY (1973) [Air Pollution in Japan. Air monitoring
data in fiscal year 1972.] Tokyo, Air Pollution Control
Division, Air Quality Bureau, pp. 206-210 (in Japanese).
FLETCHER, C. M. & HORN, D. (1970) Smoking and health. Chron. World
Health Organ., 24: 345-370.
FODOR, G. G. & WINNEKE, G. (1971) [Different affinity coefficients of
CO to hemoglobin for man and animals.] Zbl. Bakter. Parasit.
Infekt. Hyg., 224(1): 15 (in German).
FODOR, G. G. & WINNEKE, G. (1972) Effect of low CO concentrations on
resistance to monotony and on psychomotor capacity. Staub-
Reinhalt. Luft, 32(4): 46-54.
FORBES, W. H., DILL, D. B., DESILVA, H., & VAN DEVENTER, F. M. (1937)
Influence of moderate carbon monoxide poisoning upon ability to
drive automobiles. J. ind. Hyg. Toxicol., 19: 598-603.
FORBES, W. H., SARGENT, F., & ROUGHTON, F. J. W. (1945) The rate of
carbon monoxide uptake by normal men. Am. J. Physiol.,
143: 594-608.
FORSTER, R. E. (1970) Carbon monoxide and the partial pressure of
oxygen in tissue. Ann. NY Acad. Sci., 174: 233-241.
FRANKENHAEUSER, M., MYRSTEN, A.-L., POST, B., & JOHANSSON, G. (1971)
Behavioral and physiological effects of cigarette smoking in a
monotonous situation. Psychopharmacologia (Berl.), 22: 1-7.
GADASKINA, I. D. (1960) [Chronic poisoning by carbon monoxide and the
methods required for the experimental study of this problem.] In:
Letaveta, A. A. & Kanarevskoj, A. A., ed. Industrial Toxicology,
Moscow, Akademija Medicinskij Nauk SSSR, Institut Gigieny Truda I
Profzabolevanij, pp. 249-255 (in Russian).
GLINER, J. A., RAVEN, P. B., HORVATH, S. M., DRINKWATER, B. L., &
SUTTON, J. C. (1975) Man's physiologic response to long-term work
during thermal and pollutant stress. J. appl. Physiol.,
39: 628-632.
GODIN, G. & SHEPHARD, R. J. (1972) On the course of carbon monoxide
uptake and release. Respiration, 29: 317-329.
GOLDBARG, A. N., KRONE, R. J., & RESNEKOV, L. (1971) Effects of
cigarette smoking on hemodynamics at rest and during exercise. I.
Normal subjects. Chest, 60: 531-536.
GOLDMAN, A., MURCRAY, D. G., MURCRAY, F. H., WILLIAMS, W. J, BROOKS,
J. N., & BRADFORD, C. M. (1973) Vertical distribution of CO in the
atmosphere. J. geophys. Res., 78(24): 5273-5283.
GOLDSMITH, J. R. (1970) Contribution of motor vehicle exhaust,
industry and cigarette smoking to community carbon monoxide
exposures. Ann. NY Acad. Sci, 174: 122-134.
GOLDSMITH, J. R. & ARONOW, W. S. (1975) Carbon monoxide and coronary
heart disease: A review. Environ. Res., 10: 236-248.
GOLDSMITH, J. R. & LANDAW, S. A. (1968) Carbon monoxide and human
health. Science, 162: 1352-1359.
GOLDSMITH, J. R, TERZAGHI, J., & HACKNEY, J. D. (1963) Evaluation of
fluctuating carbon monoxide exposures. Arch. environ. Health,
7: 647-663.
GORBATOV, O. & NORO, L. (1948) On acclimatization in connection with
acute carbon monoxide poisonings. Acta Physiol. Scand.,
15: 77-87.
GORDON, G. S. & ROGERS, R. L. (1969) A report of medical findings of
project carbon monoxide, Washington, DC, Merkle Press.
GORI, G. B. (1976) Low-risk cigarettes: a prescription. Science,
194: 1243-1246.
GORODINSKY, S. M., SEDOV, A. V., MAZIN, A. N., GAZIEV, G. A,
KLEPTSOVA, A. P, & ZUKOVA, L. I. (1970) [Effect of reduced
barometric pressure on the elimination of gaseous and volatile
metabolites in man isolated in a diving suit.] Kosm. Biol. Med.,
2: 78-82 (in Russian).
GORSKI, J. (1962) [The ballistocardiogram in acute carbon-monoxide
poisoning.] Pol. Tyg. Lek., 17: 872-876 (in Polish).
GÖTHE, C. J., FRISTEDT, B., SUNDELL, L., KOLMODIN, B., EHRNER-SAMUEL,
H., & GÖTHE, K. (1969) Carbon monoxide hazard in city traffic-an
examination of traffic policemen in three Swedish towns. Arch.
environ. Health, 19: 310-314.
GRAHN, D. & KRATCHMAN, J. (1963) Variation in neonatal death rate and
birth weight in the United States and possible relations to
environmental radiation, geology and altitude. Am. J. hum.
Genet., 15: 329-352.
GRANDSTAFF, N. W., BEARD, R. R., & MORANDI, A. J. (1975) Review of
the neurobehavioral effects of carbon monoxide on humans,
Stanford, CA, Stanford University School of Medicine, 74 pp.
(USEPA Contract No. BU75 B001.)
GRIGOROV, L., KAZASOV, P., KURCATOVA, G., BALABAEVA, L., PAVLITZIAN,
T, VACEV, D., JAKIMOV, E., PETROVA, G., MAKSIMOVA, F., TZVETKOV,
T., BLASKOVA, D., DJOLOV, G., & Prof. BRESKOV. (1968) [Study of
the atmospheric air pollution in the region of the thermo-
electrical central "Maritza-Iztok", meteorological conditions and
their effects on the health of the population.] Izvestija NIHI,
3: 33 (in Bulgarian).
GROLL-KNAPP, E., WAGNER, H., HAUCK, H., & HAIDER, M. (1972) Effects of
low carbon monoxide concentrations on vigilance and computer-
analyzed brain potentials. Staub-Reinhalt. Luff, 32 (4): 64-68.
GROLL-KNAPP, E., HAIDER, M., HOLLER, H., JENKER, G., NEUBERGER, M., &
STIDL, H. (1976) Neuro- and psychophysiological effects of
moderate CO-doses. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Research Agency, 9 pp.
GRUT, A. (1949) Chronic carbon monoxide poisoning: a study in
occupational medicine, Copenhagen, Ejnar Munksgaard, 229 pp.
GUEST, A.D. L., DUNCAN, C., & LAWTHER, P. J. (1970) Carbon monoxide
and phenobarbitone: a comparison of effects on auditory flutter
fusion threshold and critical flicker fusion threshold.
Ergonomics (Lond.), 13(5): 587-594.
HAAGEN-SMIT, A. J. (1966) Carbon monoxide levels in city driving.
Arch. environ. Health, 12: 548-551.
HACKNEY, J. D, LINN, W. S., LAU, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. 3. Two hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HAEBISCH, H. (1970) [The cigarette as a source of carbon monoxide.]
Arch. Toxikol., 26: 251-261 (in German).
HÄGGENDAL, E., NILSSON, N.J., & NORBACH, B. (1966) Effect of blood
corpuscle concentration on cerebral blood flow. Acta Chir. Scand.
(Suppl.), 364: 3-12.
HALDANE, J. S. & PRIESTLEY, J. G. (1935) Respiration, New Haven,
Yale University Press, 493 pp.
HALPERIN, M. H., MCFARLAND, R. A., NIVEN, J. I., & ROUGHTON, F. J. W.
(1959) The time course of the effects of carbon monoxide on visual
thresholds. J. Physiol., 146: 583-593.
HAMMOND, E. C. (1962) The effects of smoking. Sci. Am., 207: 3-16.
HARKE, H. P. (1975) [Passive smokers and transport vehicles.]
Arbeitsmed. Sozialmed. Präventivmed., 10: 240-241 (in German).
HELLUNG-LARSEN, P., LAURSEN, T., KJELDSEN, K., & ASTRUP, P. (1968)
Lactate dehydrogenase isoenzymes of aortic tissue in rabbits
exposed to carbon monoxide. J. Atheroscler. Res., 8: 343-349.
HELMCHEN, H. & KUNKEL, H. (1964) [Findings concerning rhythmic after-
oscillations at optically evoked responses in EEG of man.] Arch.
Psychiatr. Z. Neurol., 205: 397-408 (in German).
HERSCH, P. (1964) Galvanic analysis. In: Reilley, C. A., ed. Advances
in analytical chemistry and instrumentation, New York,
Interscience Publishers, Vol. 3, pp. 183-249.
HERSCH, P. (1966) Process for measuring the carbon monoxide content of
a gas stream. Off. Gaz. US Pat. Off., 827(4): 1276-1277.
HEXTER, A. C. & GOLDSMITH, J. R. (1971) Carbon monoxide: association
of community air pollution with mortality. Science,
172: 265-267.
HINDS, W. C. & FIRST, M. W. (1975) Concentrations of nicotine and
tobacco smoke in public places. N. Engl. J. Med., 292
(16): 844-845.
HOFFMAN, D. & WYNDER, E. L. (1972) Smoke of cigarettes and little
cigars: an analytical comparison. Science, 178: 1197-1199.
HOGAN, G. K., SMITH, R. O., & CORNISH, H. H. (1976) Studies on the
microsomal conversion of dichloromethane to carbon monoxide.
Toxicol. appl. Pharmacol., 37: 112.
HORVATH, S. M. (1973) Effects of carbon monoxide on human behaviour.
In: Proceedings of the Conference on Health Effects of Air
Pollutants, 3-5 October, 1973, Washington, DC, National Academy
of Sciences, pp. 127-139 (Serial No. 93-15).
HORVATH, S. M. & ROUGHTON, F. J. W. (1942) Improvements in the
gasometric estimation of carbon monoxide in blood. J. biol.
Chem., 144: 747-755.
HORVATH, S. M., DAHMS, T. E., & O'HANLON, J. F. (1971) Carbon monoxide
and human vigilance: a deleterious effect of present urban
concentrations. Arch. environ. Health, 23: 343-347.
HORVATH, S. M., RAVEN, P. B., DAHMS, T. E., & GRAY, D. J. (1975)
Maximal aerobic capacity at different levels of carboxyhemoglobin.
J. appl. Physiol., 38: 300-303.
HOSKO, M. J. (1970) The effect of carbon monoxide on the visual evoked
response in man. Arch. environ. Health, 21: 174-180.
INGERSOLL, R. B., INMAN, R. E., & FISHER, W. R. (1974) Soil's
potential as a sink for atmospheric carbon monoxide. Tellus,
XXVI(1-2): 151-159.
JAFFE, L. S. (1973) Carbon monoxide in the biosphere: sources,
distributions and concentrations. J. geophys. Res.,
67: 5293-5305.
JENKINS, C. E. (1932) The haemoglobin concentration of workers
connected with internal combustion engines. J. Hyg.,
32: 406-408.
JOHNSON, C. J., MORAN, J. C., PAINE, S.C., ANDERSON, H. W., & BREYSSE,
P. A. (1975) Abatement of toxic levels of carbon monoxide in
Seattle ice-skating rinks. Am. J. public Health, 65: 1087-1090.
JONES, J. G. & WALTERS, D. H. (1962) A study of carboxyhaemoglobin
levels in employees at an integrated steelworks. Ann. occup.
Hyg., 5: 221-230.
JONES, R. A., STRICKLAND, J. A., STUNKARD, J. A., & SIEGEL, J. (1971)
Effects on experimental animals of long-term inhalation exposure
to carbon monoxide. Toxicol. appl. Pharmacol., 19: 46-53.
JONES, R. D., COMMINS, B. T., & CERNIK, A. A. (1972) Blood lead and
carboxyhaemoglobin levels in London taxi drivers. Lancet,
2: 302-303.
JUNGE, C., SEILER, W., & WARNECK, P. (1971) The atmospheric 12CO and
14CO budget. J. geophys. Res., 76(12): 2866-2879.
KAHN, A., RUTLEDGE, R. B., DAVIS, G. L., ALTES, J. A., GANTNER, G. E.,
THORNTON, C. A., & WALLACE, N. D. (1974) Carboxyhemoglobin sources
in the metropolitan St. Louis population. Arch. environ. Health,
29: 127-135.
KANDALL, S. R., LANDAW, S. A., & THALER, M. M. (1973) Carboxyhemo-
globin exchange between donors and recipients of blood
transfusions. J. Pediatr., 52: 716-718.
KILLICK, E. M. (1940) Carbon monoxide anoxemia Physiol. Rev.,
20(3): 313-344.
KJELDSEN, K. (1969) Smoking and atherosclerosis. Investigations on
the significance of the CO content in tobacco smoke in
atherogenesis, Copenhagen, Munksgaard, 142 pp.
KJELDSEN, K., THOMSEN, H. K., & ASTRUP, P. (1974) Effects of carbon
monoxide on myocardium. Circ. Res., 34: 339-348.
KLAUSEN, K., RASMUSSEN, B., GJELLEROD, H., MADSEN, H., & PETERSEN, E.
(1968) Circulation, metabolism and ventilation during prolonged
exposure to carbon monoxide and to high altitude. Scand. J. Clin.
Lab. Invest., 22 (Suppl. 103): 26-38.
KLIMISCH, H. J., CHEVALIER, H. J., HARKE, H. P., & DONTENWILL, W.
(1975) Uptake of carbon monoxide in blood of miniature pigs and
other mammals. Toxicology, 3: 301-310.
KODAT, V. ( 1971) Excretion of CO by workers on generatory station,
Candidate dissertation, Prague, 129 pp.
KOMATSU, E. (1959) [Shinshu myocardosis-a type of myocardosis caused
by carbon monoxide.] In: [Proceedings of the 15th General
Assembly of the Japan Medical Congress, Tokyo, Japan, 1-5 April,
1959.] pp. 343-348 (in Japanese).
KRONE, R. J., GOLDBARG, A. N., BALKOURA, M., SCHUESSLER, R., &
RESNEKOV, L. (1972) Effects of cigarette smoking at rest and
during exercise. II. Role of venous return. J. appl. Physiol,
32: 745-748.
KROTOVA, E. I. & MUZYKA, V. I. (1974) [Content of d-amino-levulinic
acid in erythrocytes of workers having contact with CO.] Gig. Tr.
i Prof. Zabol., 6: 43-44 (in Russian).
KUBIC, V. L., ANDERS, M. W., ENGEL, R. R., BARLOW, C. H., & CAUGHEY,
W. S. (1974) Metabolism of dihalomethanes to carbon monoxide.
Drug Metab. Disposition, 2(1): 53-57.
KULLER, L. H., RADFORD, E. P., SWIFT, D, PERPER, J., & FISHER, R.
(1975) Carbon monoxide and heart attacks. Arch. environ. Health,
30: 477-482.
KUSTOV, V. V., BELKIN, V. I., ABIDIN, B. I., OSTAPENKO, O. F.,
MALKUTA, A. N., & PODDUBNAJA, L. T. (1972) [Aspects of chronic
carbon monoxide poisoning in young animals.] Gig. Tr. i Prof
Zabol., 5: 50-52 (in Russian).
LANDAW, S. A. (1973) The effects of cigarette smoking on the total
body burden and excretion rates of carbon monoxide J. occup.
Med., 15(3): 231-240.
LAZAREV, N. V., ed. (1965) [Carbon monoxide.] In: [Toxic substances
in industry.] Vol. 2, pp. 198-218 (in Russian).
LEE, R. E. (1972) Indoor-outdoor carbon monoxide pollution study,
Washington, DC, US Environmental Protection Agency, 408 pp.
(EPA-R4-73-020).
LEVY, H. H. (1972) Photochemistry of the lower troposphere planet.
Space Sci., 20: 919-935.
LEWEY, F. H. & DRABKIN, D. L. (1944) Experimental chronic carbon
monoxide poisoning of dogs. Am. J. med. Sci., 208: 502-511.
LILIENTHAL, J. L., JR & FUGITT, C. H. (1946) The effect of low
concentrations of carboxyhemoglobin on the "altitude tolerance" of
man. Am. J. Physiol., 145: 359-364.
LILY, R. E. C., COLE, P. V., & HAWKINS, L. H. (1972)
Spectrophotometric measurements of carboxyhaemoglobin. Br. J.
ind. Med., 29: 454-457.
LINDENBERG, R., PREZIOSI, T., LEVY, D., & CHISTENSEN, M. K. (1961)
Experimental investigation of carbon monoxide toxicity. Presented
at: The Air Pollution Medical Research Conference, Los Angeles,
CA, 4-7 December, 1961.
LINNENBOM, V. J., SWINNERTON, J. W., & LAMONTAGNE, R. A. (1973) The
ocean as a source for atmospheric carbon monoxide. J. geophys.
Res., 78(24): 5333-5340.
LISS, P. S. & SLATER, P. G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LJUBLINA, E. I. (1960) [Shifts in some physiological functions of the
animal on subacute and chronic exposure to carbon monoxide.]
In: Letaveta, A. A. & Kanarevskoj, A. A., ed: [Industrial
Toxicology.] Moscow, Akademija Medicinskij Nauk SSSR, Institut
Gigieny Truda i Profzabolevanij, pp. 256-263 (in Russian).
LOGUE, G. L., ROSSE, W. F., SMITH, W. T., SALTZMAN, H. A., &
GUTTERMAN, L. A. (1971) Endogenous carbon monoxide production
measured by gas-phase analysis: an estimation of heme catabolic
rate. J. lab. clin. Med., 77: 867-876.
LONG, K. R. & DONHAM, K. J. (1973) Occupational exposure to CO in
selected rural work environments, Iowa City, IA, Institute of
Agricultural Medicine & Environmental Health, Department of
Preventive Medicine & Environmental Health, University of Iowa.
LONGO, L. D. (1970) Carbon monoxide in the pregnant mother and fetus
and its exchange across the placenta. Ann. NY Acad. Sci.,
174: 313-341.
LONGO, L. D. & HILL, E. P. (1977) Carbon monoxide uptake and
elimination in fetal and maternal sheep. Am. J. Physiol.,
232: H324-H330.
LUOMANMAKI, K. (1966) Studies on the metabolism of carbon monoxide.
Ann. med. exp. Biol. Fenn. (Helsinki), 44 (Suppl. 2): 1-55.
MAAS, A. H. J., HAMELINK, M. L., & DELEEUW, R. J. M. (1970) An
evaluation of the spectrophotometric determination of HbO2,
HbCO, and Hb in blood with the co-oximeter IL 182. Clin. Chim.
Acta, 29: 303-309.
MACMAHON, B., ALPERT, M., & SALBER, E. J. (1966) Infant weight and
parental smoking habits. Am. J. Epidemiol., 82: 247-261.
MALENFANT, A. L., GAMBINO, S. R., WARASKA, A. J., & ROE, E. I. (1968)
Spectrophotometric determination of hemoglobin concentration and
percent oxyhemoglobin and carboxyhemoglobin saturation. Clin.
Chem., 14: 789.
MALINOW, M. R., MCLAUGHLIN, P., DHINDSA, D. S., METCALFE, J., OCHSNER,
A. J., III, HILL, J., & MCNULTY, W. P. (1976) Failure of carbon
monoxide to induce myocardial infarction in cholesterol-fed
cynomolgus monkeys. Cardiovasc. Res., 10: 101-108.
MALORNY, G., MÜLLER-PLATHE, O., & STRAUB, M. (1962) [Disturbance of
acid-base balance in acute carbon monoxide poisoning and effect of
oxygen or carbon dioxide-oxygen mixtures.] Arch. exp. Pathol.
Pharmakol. (Naunyn-Schmiedeberg), 243: 439-458 (in German).
MARKS, E. & SWIECICKI, W. (1971) [Effect of carbon monoxide, vibration
and lowered atmospheric pressure upon cerebral neurosecretory
system and catecholamines level.] Med. Pr., 22: 336-341 (in
Polish).
MARTIN, W. & STERN, A. C. (1974) The world's air quality management
standards. Vol. 1. The air quality management standards of the
world, including United States federal standards, Washington,
DC, US Environmental Protection Agency, 383 pp.
MAZIARKA, S., BORKOWSKA, M., MISIAKIEWICZ, Z., STRUSINSKI, A., &
WYSZYNSKA, H. (1974) [Air pollution in the streets of Warsaw in
relation to surrounding buildings.] Roczn. Pzh., 25:1-5 (in
Polish).
MCCLATCHIE, E. A., COMPHER, A. B., & WILLIAMS, K. Y. (1972) A high
specificity carbon monoxide analyzer. Anal. Instrum. (Symp.),
10: 67-69.
MCCONNELL, J. C., MCELROY, M. B., & WOFSY, S.C. (1971) Natural sources
of atmospheric CO. Nature (Lond.), 233: 187-188.
MCCREDIE, R. M. & JOSE, A.D. (1967) Analysis of blood carbon monoxide
and oxygen by gas chromatography. J. appl. Physiol.,
22: 863-866.
MCFARLAND, R. A. (1973) Low level exposure to carbon monoxide and
driving performance. Arch. environ. Health, 27: 355-359.
MCFARLAND, R. A., ROUGHTON, F. J. W., HALPERIN, M. H., & NIVEN, J. I.
(1944) The effects of carbon monoxide and altitude on visual
thresholds. Aerosp. Med., 15: 381-394.
MCFARLAND, R. A., FORBES, W. H., STOUDT, H. W., DOUGHERTY, A., CROLEY,
T. J., MOORE, R. C., & NALWALK, T. J. (1973) A study of the
effects of low levels of carbon monoxide upon humans performing
driving tasks, Boston, Guggenheim Center of Aerospace Health &
Safety, Harvard School of Public Health, 88 pp.
MCMULLEN, T. B. (1975) Interpreting the eight-hour national ambient
air quality standard for carbon monoxide. J. Air Pollut. Control
Assoc., 25: 1009-1014.
MEHMEL, H. C., DUVELLEROY, M. A., & LAVER, M. B. (1973) Response of
coronary blood flow to pH-induced changes in hemoglobin-O2
affinity. J. appl. Physiol., 35: 485-489.
MIDDLETON, V., VAN POZNAK, A., ARTUSIO, J. F., JR, & SMITH, S. M.
(1965) Carbon monoxide accumulation in closed circle anesthesia
systems. Anesthesiology, 26: 715-719.
MILLS, E. & EDWARDS, M. W., JR (1968) Stimulation of aortic and
carotid chemoreceptors during carbon monoxide inhalation.
J. appl. Physiol., 25: 494-502.
MINISTRY OF LABOUR (1965) Carbon monoxide poisoning causes and
prevention, London, Her Majesty's Stationery Office, 72 pp.
(Safety, Health & Welfare Series No. 29).
MITCHELL, J. H., SPROULE, B. J., & CHAPMAN, C. B. (1958) The
physiological meaning of the maximal oxygen intake test. J. clin.
Invest., 37: 538-547.
MONTGOMERY, M. R. & RUBIN, R. J. (1971) The effect of carbon monoxide
inhalation on in vivo drug metabolism in the rat. J. Pharmacol.
exp. Ther., 179: 465-473.
MOREL, S. & SZEMBERG, T. (1971) [Air pollution in Ladle Bay.] Tech.
Sanit., 45: 353-355 (in Polish).
MULHAUSEN, R. O., ASTRUP, P., & MILLEMGAARD, K. (1968) Oxygen affinity
and acid-base status of human blood during exposure to hypoxia and
carbon monoxide. Scand. J. clin. lab. Invest., 22 (Suppl. 103):
9-15.
MUSSELMAN, N. P., GROFF, W. A., YEVICH, P. P., WILINSKY, F. T., WEEKS,
M. H., & OBERST, F. W. (1959) Continuous exposure of laboratory
animals to low concentration of carbon monoxide. Aerosp. Med.,
30: 524-529.
MYHRE, L. (1974) Rate of CO elimination in resting man. Fed. Proc,
33: 440.
MYRSTEN, A. L., POST, B., FRANKENHAEUSER, M., & JOHANSSON, G. (1972)
Changes in behavioural and physiological activation induced by
cigarette smoking in habitual smokers. Psychopharmacologia
(Berl.), 27: 305-312.
NAS/NRC (1977) Carbon monoxide, Washington, DC, National Academy of
Sciences, 268 pp.
NEW MEXICO STATE DEPARTMENT OF PUBLIC HEALTH (1975) Birth weight and
altitude, pp. 7-16.
NIELSEN, B. (1971) Thermoregulation during work in carbon monoxide
poisoning. Acta physiol. Scand, 82: 98-106.
NOTOV, A. (1959) [Acute poisonings with CO, SO2 and nitric gases in
mines.] Sb. Tr. VMI Plovidiv, 60: 404-411 (in Bulgarian).
NOWARA, M. (1975) [Evaluation of occupational exposure by means of
determining carbon monoxide in the air at work places and carbon
monoxide hemoglobin.] Med. Pr., 26: 351-360 (in Polish).
NUNES, A. C. & SCHOENBORN, B. P. (1973) Dichloromethane and myoglobin
function. Mol. Pharmacol., 9: 835-839.
O'DONNELL, R. D., CHIKOS, P., & THEODORE, J. (1971a) Effect of carbon
monoxide exposure on human sleep and psychomotor performance.
J. appl. Physiol., 31: 513-518.
O'DONNELL, R. D., MIKULKA, P., HEINIG, P., & THEODORE, J. (1971b) Low
level carbon monoxide exposure and human psychomotor performance.
Toxicol. appl. Pharmacol., 18: 593-602.
OGAWA, M, KATSURADA. K., & SUGIMOTO, I. (1974) Pulmonary edema in
acute carbon monoxide poisoning. Int. Arch. Arbeitsmed.,
33: 131-138.
O'HANLON, J. F. (1975) Preliminary studies of the effects of carbon
monoxide on vigilance in man. In: Weiss, B. & Laties, V. G., ed.
Behavioral toxicology, New York, Plenum Press, pp. 61-72.
ORELLANO, T., DERGAL, E., ALIJANI, M., BRIGGS, C., VASQUEZ, J.,
GOLDBAUM, L., & ABSOLON, K. B. (1976) Studies on the mechanism of
carbon monoxide toxicity. J. surg. Res., 20: 485-487.
OTT, W. & MAGE, D. (1974) Interpreting urban carbon monoxide
concentrations by means of a computerized blood COHb model.
J. Air Pollut. Control Assoc., 28(9): 911-916.
PACE, N., STRAJMAN, E., & WALKER, E. L. (1950) Acceleration of carbon
monoxide elimination in man by high pressure oxygen. Science,
111: 652-654.
PANKOW, D. & PONSOLD, W. (1972) Leucine aminopeptidase activity in
plasma of normal and carbon monoxide poisoned rats. Arch.
Toxikol., 29: 279-285.
PANKOW, D. & PONSOLD, W. (1974) [The combined effects of carbon
monoxide and other biologically active detrimental factors on the
organism.] Zeits. Ges. Hyg., 9: 561-571 (in German).
PANKOW, D., PONSOLD, W., & FRITZ, H. (1974a) Combined effects of
carbon monoxide and ethanol on the effects of leucine
aminopeptidase and glutamic-pyruvic transaminase in the plasma of
rats. Arch. Toxicol, 32: 331-340.
PANKOW, D., PONSOLD, W., & GRIMM, I. (1974b) [On the potentiation of
the hepatic toxicity of carbon tetrachloride by carbon monoxide.]
Dtsch. Ges. Wesen., 29: 853-856 (in German).
PECORA, L. J. (1959) Physiologic study of the summating effects of
ethyl alcohol and carbon monoxide. Am. Ind. Hyg. Assoc. J.,
20: 235-240.
PENNEY, D., BENJAMIN, M., & DUNHAM, E. (1974a) Effect of carbon
monoxide on cardiac weight as compared with altitude effects.
J. appl. Physiol, 37: 80-84.
PENNEY, D., DUNHAM, E., & BENJAMIN, M. (1974b) Chronic carbon monoxide
exposure: Time course of hemoglobin, heart weight and lactate
dehydrogenase isozyme changes. Toxicol. appl. Pharmacol,
28: 493-497.
PERMUTT, S. & FAHRI, L. (1969) Tissue hypoxia and carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behavior and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 18-24.
PETERSON, J. E. & STEWART, R. D. (1970) Absorption and elimination of
carbon monoxide by inactive young men. Arch. environ. Health,
21: 165-171.
PETROV, P. (1968) [Chronic CO intoxications in workers engaged in
loading and unloading processes.] Transp. Med. Vesti, 4: 24-27
(in Bulgarian).
PIRNAY, F., DUJARDIN, J., DEROANNE, R., & PETIT, J. M. (1971) Muscular
exercise during intoxication by carbon monoxide. J. appl.
Physiol., 31: 573-575.
PITTS, G. C. & PACE, N. (1947) The effects of blood carboxyhemoglobin
concentration on hypoxia tolerance. Am. J. Physiol.,
148: 139-151.
PREZIOSI, T. J., LINDENBERG, R., LEVY, D., & CHRISTENSON, M. (1970) An
experimental investigation in animals of the functional and
morphological effects of single and repeated exposures to high and
low concentrations of carbon monoxide. Ann. NY Acad. Sci,
174: 369-384.
PROHOROV, Y. D. & ROGOV, A. A. (1959) [The pathohistological and
histochemical changes in the organs of rabbits subjected to a
prolonged action of carbon monoxide, sulfur dioxide and their
mixtures.] Gig. i Sanit., 24: 22-26 (in Russian).
RAMIREZ, R. G., ALBERT, S. N., AGOSTINI, J. C., BASU, A. P., GOLDBAUM,
L. R., & ABSOLON, K. B. (1974) Lack of toxicity of transfused
carboxyhemoglobin red blood cells and carbon monoxide inhalations.
Surg. Forum, 25: 165-168.
RAMSEY, J. M. (1967a) Carbon monoxide exposures in parking garages.
Bull. environ. Contam. Toxicol., 2: 182-190.
RAMSEY, J. M. (1967b) Carboxyhemoglobinemia in parking garage
employees. Arch. environ. Health, 15: 580-583.
RAMSEY, J. M. (1972) Carbon monoxide, tissue hypoxia, and sensory
psychomotor response in hypoxaemic subjects. Clin. Sci.,
42: 619-625.
RAMSEY, J. M. (1973) Effects of single exposures of carbon monoxide on
sensory and psychomotor response. Am. Ind. Hyg. Assoc. J.,
34: 212-216.
RAMSEY, J. M. & CASPER, P. W., JR (1976) Effect of carbon monoxide
exposures on erythrocyte 2,3-DPG in rabbits. J. appl. Physiol.,
41: 689-692.
RATNEY, R. S., WEGMAN, D. H., & ELKINS, H. B. (1974) In vivo
conversion of methylene chloride to carbon monoxide. Arch.
environ. Health, 28: 223-226.
RAVEN, P. B., DRINKWATER, B. L., HORVATH, S. M., RUHLING, R. O.,
GLINER, J. A., SUTTON, J. C., BOLDUAN, N. W. (1947a) Age, smoking
habits, heat stress and their interactive effects with carbon
monoxide and peroxyacetyl nitrate on man's aerobic power. Int. J.
Biometeorol., 18: 222-232.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N. W.,
TAGUCHI, S., GLINER, J. A., & HORVATH, S. M. (1974b) Effect of
carbon monoxide and peroxyacetyl nitrate on man's maximal aerobic
capacity. J. appl. Physiol., 36: 288-293.
RAY, A.M. & ROCKWELL, T. H. (1970) An exploratory study of automobile
driving performance under the influence of low levels of
carboxyhemoglobin. Ann. NY Acad. Sci., 174: 396-407.
RENCH, J. D. & SAVAGE, E. P. (1976) Carbon monoxide in the home
environment. J. environ. Health, 39: 104-106.
RISSANEN, V., PYÖRÄLÄ, K., & HEINONEN, O. P. (1972) Cigarette smoking
in relation to coronary and aortic atherosclerosis. Acta pathol.
microbiol. Scand. Sect. A, 80: 491-500.
ROBBINS, R. C., BORG, K. M., & ROBINSON, E. (1968) Carbon monoxide in
the atmosphere. J. Air Pollut. Control Assoc., 18: 106-110.
ROBINSON, J. C. & FORBES, W. F. (1975) The role of carbon monoxide in
cigarette smoking. Arch. environ. Health, 30: 425-434.
ROBINSON, E. & ROBBINS, R. C. (1970) Atmospheric background
concentrations of carbon monoxide. Ann. NY Acad. Sci.,
174: 89-95.
RONDIA, D. (1970) Abaissement de l'activité de la benzopyrene-
hydroxylase hepatique in vivo apres inhalation d'oxyde de
carbone. C. R. Acad. Sci. (Paris), 271: 617-619.
ROOT, W. S. (1965) Carbon monoxide. In: Fenn, W. O. & Rahn, H., ed.
Handbook of physiology, respiration 2, Washington, DC, American
Physiological Society, pp. 1087-1098.
ROSSI-BERNARDI, L., PERRELLA, M., LUZZANA, M., SAMAJA, M., & RAFFAELE,
I. (1977) Simultaneous determination of hemoglobin derivatives,
oxygen content, oxygen capacity, and oxygen saturation in 10 µl of
whole blood. Clin. Chem., 23: 1215-1225.
ROSSI-FANELLI, A. & ANTONINI, E. (1958) Studies on the oxygen and
carbon monoxide equilibria of human myoglobin. Arch. Biochem.
Biophys., 77: 478-492.
ROTH, R. P., DREW, R. T., Lo, R. J., & FOUTS, J. R. (1975)
Dichloromethane inhalation, carboxyhemoglobin concentrations, and
drug metabolizing enzymes in rabbits. Toxicol. appl. Pharmacol.,
33: 427-437.
ROUGHTON, F. J. W. (1970) The equilibrium of carbon monoxide with
human hemoglobin in whole blood. Ann. NY Acad. Sci.,
174: 177-188.
ROUGHTON, F. J. W. & DARLING, R. C. (1944) The effect of carbon
monoxide on the oxyhemoglobin dissociation curve. Am. J.
Physiol., 141: 17-31.
ROUGHTON, F. J. W. & ROOT, W. S. (1945) The estimation of small
amounts of carbon monoxide in blood. J. biol. Chem.,
160: 123-133.
RUMMO, N. & SARLANIS, K. (1974) The effect of carbon monoxide on
several measures of vigilance in a simulated driving task.
J. Saf Res., 6: 126-130.
RUSSELL, M. A. H., COLE, P. V., & BROWN, E. (1973) Absorption by non-
smokers of carbon monoxide from room air polluted by tobacco
smoke. Lancet, 1: 576-579.
RYLANDER, R. (1974) Environmental tobacco smoke effects on the non-
smoker. Report from a workshop. Scand J. resp. Dis.,
(Suppl. 91): 90 pp.
RYLOVA, M. L. (1960) [The application of integral methods of
investigation to determine the effect of repeated exposure to
carbon monoxide in animals.] In: Letaveta, A. A. & Kanarevskoj, A.
A., ed. [Industrial toxicology.] Moscow, Akademija Medicinskij
Nauk SSSR, Institut Gigieny Truda i Profzabolevanij, pp. 264-270
(in Russian).
SAMMONS, J. H. & COLEMAN, R. L. (1974) Firefighters' occupational
exposure to carbon monoxide. J. occup. Med., 16: 543-546.
SANTIAGO, T. V. & EDELMAN, N. H. (1972) Effect of anemia on the
ventilatory response to transient hypoxia. Fed. Proc.,
31: 371 (Abstract No. 864).
SARMA, J. S. M., TILLMANS, H., IKEDA, S., & BINS, R. J. (1975) The
effect of carbon monoxide on lipid metabolism of human coronary
arteries. Atherosclerosis, 22: 193-198.
SAYERS, R. R., YANT, W. P., LEVY, E., & FULTON, W. B. (1929) Effect
of repeated daily exposure of several hours to small amounts of
automobile exhaust gas, Washington, DC, Government Printing
Office, 49 pp. (Public Health Bull. No. 186).
SCHULTE, J. H. (1963) Effects of mild carbon monoxide intoxication.
Arch. environ. Health, 7: 524-530.
SEDOV, A. V., ZUKOVA, L. I., & MAZNEVA, G. E. (1971) [Carbon monoxide
excretion by the human organism.] Gig. Tr. i Prof Zabol.,
9: 36-39 (in Russian).
SEILER, W. (1975) The cycle of carbon monoxide in the atmosphere.
In: International Conference on Environmental Sensing and
Assessment, Las Vegas, NV, 14-19 September, New York, Institute
of Electrical and Electronics Engineers, Vol. 2, 6 pp.
SEILER, W. & JUNGE, C. E. (1969) Decrease of carbon monoxide mixing
ratio above the polar tropopause. Tellus, 21(3): 447-449.
SEILER, W. & JUNGLE, C. E. (1970) Carbon monoxide in the atmosphere.
J. geophys. Res., 75(12): 2217-2226.
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA,. M. A., & TARASOVA, L. N.
(1970) [New data on the concentration of the products of the
incomplete combustion of domestic gas in the air of premises
provided with a gas supply.] In: [Problems of current interest in
hygiene.] Moscow, Akademija Medicinskij Nauk SSSR, Institute of
Genetics and Community Hygiene, pp. 137-142 (in Russian).
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA, M. A., KLEBANOVA, V. A.,
OSINCEVA, V. P., & NAZARENKO, A. F. (1972) [The experimental basis
for measures to improve the quality of the air in flats provided
with a gas supply.] Gig. i Sanit., 7: 24-28 (in Russian).
SIEGEL, S. M., RENWICK, G., & ROSEN, L. A. (1962) Formation of carbon
monoxide during seed germination and seedling growth. Science,
137: 683-684.
SJÖSTRAND, T. (1948) A method for the determination of
carboxyhaemoglobin concentrations by analysis of the alveolar
air. Acta Physiol. Scand., 16: 201-210.
SMALL, K. A., RADFORD, E. P., FRAZIER, J. M., RODKEY, F. L., &
COLLISON, H. A. (1971) A rapid method for simultaneous measurement
of carboxy- and methemoglobin in blood. J. appl. Physiol.,
31: 154-160.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumonia. Arch. environ. Health, 16: 670-672.
SOKAL, J. A. (1975) Lack of the correlation between biochemical
effects on rats and blood carboxyhemoglobin concentrations in
various conditions of single acute exposure to carbon monoxide.
Arch. Toxicol, 34: 331-336.
SOTNIKOV, E. E. (1971) [Determination of carbon monoxide in the blood
by a gas chromatographic method.] Gig. i Sanit., 6: 61-64 (in
Russian).
SPEDDING, D. J. (1974) Air pollution, Oxford, Clarendon Press,
pp. 58-62.
SRCH, M. (1967) [The significance of carbon monoxide in cigarette
smoke in passenger car interiors.] Dtsch. Z. ges. gerichtl. Med,
60: 80-89 (in German).
STEVENS, C. M., KROUT, L., WALLING, D., VENTERS, A., ENGELKEMEIR, A.,
& Ross, L. E. (1972) The isotopic composition of atmospheric
carbon monoxide. Earth planet. Sci. Lett, 16: 147-165.
STEWART, R. D. & HAKE, C. L. (1976) Paint remover hazard. J. Am. Med.
Assoc., 235: 398-401.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. C. (1972a) Experimental human exposure to
methylene chloride. Arch. environ. Health, 26: 342-348.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. D. (1972b) Carboxyhemoglobin elevation after
exposure to dichloromethane. Science, 176: 295-296.
STEWART, R. D., NEWTON, P. E., HOSKO, M. J., & PETERSON, J. E. (1973a)
Effect of carbon monoxide on time perception. Arch. environ.
Health, 27: 155-160.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973b) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWARD, E. D.,
KALBFLEICH, J. H., VAN YSERLOO, B., & RIMM, A. A. (1973c)
Carboxyhemoglobin concentrations in blood from donors in Chicago,
Milwaukee, New York, and Los Angeles. Science, 182: 1362-1364.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWART, E. D.,
KALBFLEICH, J. D., VAN YSERLOO, B., & RIMM, A. A. (1974)
Carboxyhaemoglobin tests in American blood donors. JAMA,
229: 1187-1195.
STEWART, R. D, NEWTON, P. E., HOSKO, M. J., PETERSON, J. E., &
MELLENDER, J. W. (1975) The effect of carbon monoxide on time
perception, manual coordination, inspection, and arithmetic.
In: Weiss, B. & Laties, V. G., ed. Behavioral toxicology, New
York, Plenum Press, pp. 29-60.
STEWART, R. D., HAKE, C. L., WU, A., STEWART, T. A., & KALBFLEISCH, J.
H. (1976) Carboxyhemoglobin trend in Chicago blood donors,
1970-1974. Arch. environ. Health, 31: 280-286.
STUPFEL, M. (1975) Rhythms and air pollution. Chronobiologia,
11: 105-115.
STUPFEL, M. & BOULEY, G. (1970) Physiological and biochemical effects
on rats and mice exposed to small concentrations of carbon
monoxide for long periods. Ann. NY Acad. Sci. 174: 342-368.
STUPFEL, M., MAGNIER, M., & ROMARY, F. (1973) Rythme circadien de
l'action de l'oxide de carbone sur l'emission du gaz carbonique
par le rat en groupe. C. R. Acad. Sci. (Paris) Série D,
276: 1009-1012.
SUL'GA, T. M. (1962) [New data for the hygienic evaluation of CO in
air.] In: Ryazanov, V. A., ed. [ Maximal permissible
concentrations of air pollution.] Moscow, Medgiz, pp. 128-145
(in Russian).
SWIECICKI, W. (1973) [The effect of vibration and physical training on
carbohydrate metabolism in rats intoxicated with carbon monoxide.]
Med. Pr., 34: 399-405 (in Polish).
SWINNERTON, J. W., LAMONTAGNE, R. A., & LINNENBOM, V. J. (1971) Carbon
monoxide in rain water. Science, 172: 943-945.
TANAKA, M. (1965) [Studies on the etiological mechanism of fetal
development disorders caused by maternal smoking habits during
pregnancy.] J. Jpn. Obstet. Gynecol. Soc., 17: 1107-1114 (in
Japanese, English translation in J. Obstet. Gynecol. Soc.,
14: 45 (1967)).
THOMSEN, H. K. (1974) Carbon monoxide-induced artherosclerosis in
primates. Atherosclerosis, 20: 233-240.
THOMSEN, H. K. & KJELDSEN, K. (1974) Threshold limit for carbon
monoxide-induced myocardial damage. Arch. environ. Health,
29: 73-79.
THOMSEN, H. K. & KJELDSEN, K. (1975) Aortic intimal injury in rabbits.
Arch. environ. Health, 30: 604-607.
TIUNOV. A. & KUSTOV, V. V. (1969) [Toxicology of carbon monoxide.]
Leningrad, Medicina, pp. 81-217 (in Russian).
TRAYSTMAN, R. J. (1976) Effects of carbon monoxide hypoxia and hypoxic
hypoxia on cerebral circulation. In: Fourth International
Congress on Event Related Slow Potentials of the Brain,
Hendersonville, NC, April 4-10, 1976, Research Triangle Park,
NC, US Environmental Protection Agency, 9 pp.
TROMPEO, G., TURLETTl, G., & GIARRUSSO, O. T. (1964) [Concentration of
carbon monoxide in underground garages.] Rass. Med. Ind.,
33: 392-393 (in Italian).
TUMASONIS, C. F. & BAKER, F. D. (1972) Influence of carbon monoxide
upon some respiratory enzymes of the chick embryo. Bull. environ.
Contain. Toxicol., 8: 113-119.
TURNER, J. A. McM., SILLETT, R. W., & BALL, K. P. (1974) Some effects
of changing to low-tar and low-nicotine cigarettes. Lancet,
2:737-739.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Air quality
criteria for carbon monoxide, Washington, DC, US DHEW Public
Health Service, National Air Pollution Control Administration.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1972) Criteria for a
recommended standard. Occupational exposure to carbon monoxide,
Washington, DC, US DHEW Health Services and Mental Health,
National Institute for Occupational Safety and Health, 120 pp.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1973) The Health
Consequences of Smoking, Washington, DC, US DHEW, Public Health
Service, pp. 367-381.
US ENVIRONMENTAL PROTECTION AGENCY (1973a) The national air
monitoring program: Air quality and emission trends, Annual
report, Vol. 1, Research Triangle Park, NC, USEPA, 182 pp.
(EPA 450/1-73-001-A).
US ENVIRONMENTAL PROTECTION AGENCY (1973b) Compilation of air
pollution emission factors 2nd ed., Research Triangle Park, NC,
USEPA, 288 pp. (Report No. AP42).
US ENVIRONMENTAL PROTECTION AGENCY (1975) Human health damages from
mobile source air pollution: A delphic study, Research Triangle
Park, NC, USEPA (Project No. 07AAC-OS).
VAN KAMPEN, E. J. & ZIJLSTRA, W. G. (1961) Standardization of
hemoglobinometry. II. The hemiglobincyanide method. Clin. Chim.
Acta, 6: 538-544.
VINOGRADOV, G. I., CERNICENKO, I. A., & MAKARENKO, E. M. (1974) [The
allergenic activity of motor vehicle gases.] Gig. i Sanit.,
8: 10-13 (in Russian).
VOGEL, J. A. & GLESER, M. A. (1972) Effect of carbon monoxide on
oxygen transport during exercise. J. appl. Physiol.,
32: 234-239.
VOGEL, J. A., GLESER, M. A., WHEELER, R. C., & WHITTEN, B. K. (1972)
Carbon monoxide and physical work capacity. Arch. environ.
Health, 24: 198-203.
VOLLMER, E. P., KING, B. G, BIRREN, J. E., & FISHER, M. B. (1946) The
effects of carbon monoxide on three types of performance at
simulated altitudes of 10,000 and 15,000 feet. J. exp. Psychol.,
36: 244-251.
VYSKOCIL, J. (1957) Neurohumoral regulation in acute and chronic
carbon monoxide poisoning. Int. Arch. Gewerbepathol. Gewerbehyg.,
15: 457-472.
WAGEMANN, W. (1960) [The otological picture in carbon monoxide
poisoning.] Z. Laringol. Rhinol. Otol., 39: 691-708 (in German).
WAGNER, J. A., HORVATH, S. M., & DAHMS, T. E. (1975) Carbon monoxide
elimination. Respir. Physiol, 23: 41-47.
WAKEHAM, H. R. R. (1976) Environmental carbon monoxide from cigarette
smoking, Sixth International Tobacco Scientific Congress, Tokyo,
Japan, November 18, 1976, 17 pp.
WALD, N. & HOWARD, S. (1975) Smoking, carbon monoxide and arterial
disease. Ann. occup. Hyg., 18: 1-14.
WALD, N., HOWARD, S., SMITH, P. G., & KJELDSEN, K. (1973) Association
between atherosclerotic diseases and carboxyhaemoglobin levels in
tobacco smokers. Br. med. J., 1: 761-765.
WALLACE, N. D., DAVIS, G. L., RUTLEDGE, R. B., & KAHN, A. (1974)
Smoking and carboxyhemoglobin in the St. Louis metropolitan
population. Arch. environ. Health, 29: 136-142.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WEBSTER, W. S., CLARKSON, T. B., & LOFLAND, H. B. (1968) Carbon
monoxide-aggravated atherosclerosis in the squirrel monkey. Exp.
mol. Pathol., 13: 36-50.
WEINSTOCK, B. & NIKI, H. (1972) Carbon monoxide balance in nature.
Science, 176: 290-292.
WEIR, F. W. & ROCKWELL, T. H. (1973) An investigation of the effects
of carbon monoxide on humans in the driving task, Columbus, OH,
Ohio State University Research Foundation, 170 pp. (Contract No.
68-02-0329 and CRC-APARC Project CAPM-9-69).
WEISS, H. R. & COHEN, J. A. (1974) Effects of low levels of carbon
monoxide on rat brain and muscle tissue pO2. Environ.
Physiol. Biochem., 4: 31-49.
WELLS, L. L. (1933) The prenatal effect of carbon monoxide on albino
rats and the resulting neuropathology. Biologist, 15: 80-81.
WESTBERG, K, COHEN, N., & WILSON, K. W. (1971) Carbon monoxide: its
role in photochemical smog formation. Science, 171: 1013-1015.
WESTLAKE, D. W., ROXBURGH, J. M., & TALBOT, G. (1961) Microbial
production of carbon monoxide from flavonoids. Nature (Lond.),
189: 510-511.
WHITE, P. (1970) Carbon monoxide production and heme catabolism. Ann.
NY Acad. Sci., 174: 23-31.
WILKS, S. S. (1959) Carbon monoxide in green plants. Science,
129: 964-966.
WILKS, S. S., TOMASHEFSKI, J. F., & CLARKE, R. T., JR (1959)
Physiological effects of chronic exposure to carbon monoxide.
J. appl. Physiol., 14: 305-310.
WILLIAMS, I. R. & SMITH, E. (1935) Blood picture, reproduction, and
general condition during daily exposure to illuminating gas.
Am. J. Physiol., 110: 611-615.
WINNEKE, G. (1974) Behavioral effects of methylene chloride and carbon
monoxide as assessed by sensory and psychomotor performance. In:
Xintaras, C., Johnson, B., & deGroot, I., ed. Behavioral
toxicology: early detection of occupational hazards, Washington,
DC, US Government Printing Office, pp. 130-144 (New Publ. No.
N10511).
WINNEKE, G. (1977) [Studies on marginal values for carbon monoxide.]
Lufthyg. Silkoseforsch., 10: 75-90 (in German).
WINNEKE, G., FODOR, G. G., & SCHLIPKÖTER, H. W. (1976) Effects of low
level carbon monoxide exposure and monotony on vigilance and
vertex evoked potentials. In: Proceedings of the fourth
international congress on event-related slow potentials of the
brain. Hendersonville, NC, April 4-10, 1976, Research Triangle
Park, NC, US Environmental Protection Agency, 5 pp.
WOLFE, D. G. & BIDLACK, W. R. (1976) The formation of carbon monoxide
during peroxidation of microsomal lipids. Biochem. Biophys. res.
Commun., 73:850-857.
WHO (1975) WHO Technical Report Series, No. 568 (Smoking and its
effects on health), 100 pp.
WHO (1976) Selected methods of measuring air pollutants, Geneva,
WHO, 112 pp. (Offset Publication No. 24).
WRIGHT, G. R., JEWCZYK, S., ONROT, J., TOMLINSON, P., & SHEPHARD, R.
J. (1975) Carbon monoxide in the urban atmosphere. Arch. environ.
Health, 30: 123-129.
WRIGHT, G. W., RANDELL, P., & SHEPHARD, D.. R. J. (1973) Carbon
monoxide and driving skills. Arch. environ. Health, 27: 349-354.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response technique
in air pollution toxicology. Toxicol appl. Pharmacol., 8: 77-87.
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor-outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
ZENKEVIC, E. C. (1973) [Hemodynamic changes under the effect of CO
displayed adaptation.] Sb. Probl. Adapt. Gig. Tr., pp. 67-72
(in Russian).
ZORN, H. (1968) [Carbon dioxide-oxygen mixture or pure oxygen for
respiration in the case of carbon monoxide intoxication.] Dtsch.
Med. Wochenschr., 93: 1536 (in German).
ZORN, H. (1972) The partial oxygen pressure in the brain and liver at
subtoxic concentrations of carbon monoxide. Staub-Reinhalt. Luff,
32: 24-29.
Annex 1
THE RELATIONSHIP BETWEEN CARBON MONOXIDE CONCENTRATION IN AIR AND
CARBOXYHAEMOGLOBINa
(1) Several empirical equations have been proposed for estimating
carboxyhaemoglobin levels from environmental exposure conditions.
These equations were based on controlled exposures of human
volunteers. Most of them referred to subjects at rest, or performing
sedentary activities or light work. The simplest empirical equations
described carboxyhaemoglobin levels as a linear function of carbon
monoxide concentration in the inspired air [CO], and of exposure time
(t) (Forbes et al., 1945; Pace et al., 1946). They were applicable
only within a limited range of exposure conditions. Hanks & Farquhar
(1969) and Peterson & Stewart (1970) compiled empirical equations
involving more complex functional relationships that had a wider
application.
(2) In addition, models have been proposed that relate carboxyhaemo-
globin levels to both environmental exposure conditions and a number
of physiological variables such as blood volume, VB, endogenous
carbon monoxide production, Vco, diffusion capacity of the lung,
DL, and alveolar ventilation rate, VA. The best known model,
developed by Coburn et al. (1965), has been briefly discussed in
section 6.2. A more recent model, that took into account the dynamic
condition of urban carbon monoxide concentrations, was suggested by
Ott & Mage (1978) (p. 118).
(3) A simple linear relationship, proposed by Forbes et al. (1945),
linked the increase in the carboxyhaemoglobin level with the carbon
monoxide concentration in air, [CO](ppm) and exposure time, t(min):
a "This annex has been prepared by the Secretariat and has not been
reviewed by the Task Group, with the exception of Table 3.
Equations are presented in the form given in the original studies
and no attempt has been made to change to SI units. The Secretariat
wishes to express its appreciation to Dr H. Buchwald (Canada),
Chairman of the Task Group, for providing information contained in
Tables 3 and 4 and to Dr R. Horton, Dr D. J. McKee, USEPA, and
Dr V. Armstrong, Health and Welfare, Canada for reviewing the
annexes.
[HbCO](%) = k × [CO] × t (1)
where k was a constant equal to 0.0003 for "an individual at rest"
( VA = 6 litre/min, pulse rate 70), 0.0005 for "light activity"
( VA = 9.5 litre/min, pulse rate 80), 0.0008 for "light work"
(50 watts, VA = 18 litre/min, pulse rate 110), and 0.0011 for
"heavy work"a (about 100 watts, VA = 30 litre/min, pulse rate 135).
This equation was based on sets of controlled exposure
observations on human volunteers. Exposure concentrations ranged from
100 to 20 000 ppm (0.01 to 2.0%), and exposure times up to 6 h. At a
[CO] of 100 ppm, the equation holds only up to delta[HbCO] of about
7%. At 1000 ppm, the equation is applicable up to 30% [HbCO].
The equation of Forbes et al. was used by the California State
Department of Health for estimating [HbCO] after shorter periods of
exposure to carbon monoxide concentrations higher than 100 ppm in
individuals at rest or engaged in light work (Goldsmith & Landow,
1978).
(4) The empirical equation suggested by Pace et al. (1945) was derived
from controlled carbon monoxide exposures of 32 volunteers, aged 18-40
years. The concentrations of carbon monoxide in the inspired air
varied from 90 to 21 800 ppm, and the subjects were either sitting
quietly or walking on a level treadmill (about 4.3 km/h, VA about
19.2 litre/min). The exposure times ranged from 20 to 300 min. The
equation was also linear, but it took into account the alveolar
ventilation rate, VA, and the blood volume, VB:
[CO](ppm) × VA (litre/min) × t(min)
DELTA[HbCO](%) = (2)
4650 × VB(litre)
If it is assumed that the blood volume equals 5.51 and
VA = 6 litre/min (rest or light sedentary activity) or 18 litre/min
(light physical work), the equation takes the same form as that of
Forbes et al, but k values are somewhat lower (0.00023 and 0.00070,
respectively).
(5) Hanks & Farquhar (1969) also conducted carefully controlled
exposure studies on sedentary volunteers, and expressed their results
by the equation:
[HbCO](%) = 0.147[CO](1 - e-0.00289 t) (3)
where [CO] is in ppm and t in min. The equation was valid for subjects
having a ventilation rate of about 6 litre/min.
a VA for heavy physical work may be as high as 60 litre/min.
According to Chovin (1974), the "coefficient of (pulmonary)
ventilation" K could be introduced into equation (3), which then
became applicable to subjects performing various degrees of activity.
Chovin's modified equation read:
K
- t
0.147
[HbCO](%) = 0.147[CO](1 - e ) (4)
where t was in hours. For subjects at rest, K = 0.025, and for those
performing heavy physical work, K = 0.065. For intermediate degrees of
activity, Chovin proposed K values of 0.035, 0.045, and 0.055.
(6) Another empirical equation, referred to on p. 36, was suggested by
Peterson & Stewart (1970). It could also be written in the following
form:
[HbCO](%) = 0.0051[CO]0.858 × t0.63 (5)
where [CO] is in ppm, and t in min. The equation was based on
controlled human exposure data. The volunteers (18 healthy graduate
students) were exposed to carbon monoxide concentrations of <1, 25,
50, 100, 200, 500, and 1000 ppm for periods ranging from 30 min to
24 h, while performing strictly sedentary activities. Like linear
equations, this equation needed to be used with caution, since it did
not yield a finite [HbCO] value for an infinite exposure time. It was
used in developing US national ambient air quality standards, and is
strictly valid for constant [CO] and for shorter periods of exposure
(Ott & Mage, 1978).
(7) A model proposed by Coburn et al. (1965) has been discussed on
pp. 36 and 37. It is valid for male subjects only.a A solution of
their differential equation has been provided in the original paper; it
assumes that the mean pulmonary capillary oxygen pressure ( pCo2)
and the concentration of oxyhaemoglobin [HbO2] are constant and
independent of carboxyhaemoglobin levels [HbCO]. However, the
oxyhaemoglobin level depends on carboxyhaemoglobin concentrations in a
complex way. Solutions of the differential equation taking this into
account are available, and their application is illustrated in the
NAS/NRC (1977) document. Nevertheless, for most practical purposes,
the solution given in the original paper appears to be adequate. It
has been used, for example, by Peterson & Stewart (1970) and in the
criteria document of the National Institute of Occupational Safety and
Health (US Department of Health, Education and Welfare, 1972). A
useful form of this solution is given in the document of the Committee
on the Challenges of Modern Society (1972):
a Endogenous CO production may be different for females due to
menstruation, pregnancy, and other metabolic factors.
1
[HbCO] t = {e- kt(A[HbCO]o - VcoB - [CO]) + VcoB + [CO]} (6)
A
where [HbCO] t and [HbCO]o are the carboxyhaemoglobin levels at
times
pCo2 1 ( pB - 47)
t and t = 0, [CO] = pIco, A = , B = ( + ) and
M[HbO2] DL VA
A
k =
VB.B
the symbols used to define [CO], A, and B are explained on p. 36. The
relationships between [CO] in ppm and pIco, and between [HbCO]% and
[HbCO] in ml CO/ml blood are:
pIco × 106
[CO](ppm) = ,
pB
and
[HbCO](%) = 497.5 [HbCO](ml CO/ml blood)
(8) Ott & Mage (1978) designed a model that took into consideration
the dynamic characteristics of urban carbon monoxide concentrations.
The differential equation describing it reads:
d[HbCO]
tau dt + [HbCO] - ß = alpha[CO] (7)
O < [CO] < 100 ppm
where ß was the endogenous level of blood carboxyhaemoglobin and was
assumed to be 0.5%, alpha was assumed to be 0.15, and tau = 2.49h.
The main conclusion of the authors was that [CO] in ambient air
should be reported for averaging periods of 10-15 min, if the
monitoring stations were located near heavy traffic or on congested
streets. In such cases, sharp carbon monoxide peaks of short duration
might occur fairly often, and concentrations reported with longer
averaging periods, e.g., 1 h or more, might introduce an error of up
to 21% in the estimated [HbCO].
(9) Several empirical equations are compared with Coburn's model in
Table 1, for persons at rest ( VA - 6 litre/min) or performing light
physical work ( VA = 18 litre/min), at a carbon monoxide exposure
concentration of 100 ppm. For subjects performing light work, Hanks &
Farquhar's equation has been used in Chovin's modification with
K = 0.060. The following assumptions have been made in calculating
[HbCO]t values from Coburn's equation (6): [HbCO] = 0.5% or
0.001 ml CO/ml blood, Vco = 0.007 ml/min, VB = 5500 ml,
pCo2 = 100 mmHg, [HbO2] = 0.2 ml O2/ml blood, M = 218,
pB - 47 = 713 mmHg; sedentary subjects: DL = 40 ml/min/mmHg,
VA = 6000 ml/min; light work: DL = 40 ml/min/mmHg,
VA = 18 000 ml/min. For all empirical equations, [HbCO]o = 0.5%
has been added to the calculated values of [HbCO].
Table 1 shows clearly that Hanks & Farquhar's equation agrees best
with Coburn's model. Peterson & Stewart's equation gives values that
are higher than the other equations up to 6 h of exposure; then it
gives lower results than Forbes' equation. At a carbon monoxide
concentration of 100 ppm, Pace's equation gives lower [HbCO] values
for subjects at rest than the other equations, up to 7 h of exposure;
for subjects performing light work, it is applicable up to 2 h of
exposure. For subjects at rest, Forbe's equation is applicable up to
6 h, and for persons performing light work, up to 2 h.
(10) Table 2 shows [HbCO] values predicted by Coburn's model.a The
assumptions are the same as those specified in paragraph 9. For heavy
work, it has been assumed that DL = 60 ml/min/mmHg and that
VA=30 000 ml/min.
(11) Guidelines on exposure conditions that would prevent
carboxyhaemoglobin levels exceeding 2.5-3% in general nonsmoking
populations are given in Table 3.
These guidelines were reviewed by the Task Group. Comparison with
Tables 1 and 2 indicates the degree of protection provided, if these
guidelines are applied, both for sedentary individuals and for persons
performing light work. The exposure guidelines for 8-24 h have been
added by the Secretariat, after the Task Group meeting, to facilitate
comparison with national air quality standards.
(12) Guidelines on exposure conditions, which would prevent carboxy-
haemoglobin levels exceeding 5% in nonsmoking occupational groups, are
shown in Table 4. They were prepared, after the meeting, by Dr
Buchwald, at the request of the Secretariat, and have not been
reviewed by the Task Group. Guidelines for heavy work have been
suggested by the Secretariat, also after the meeting. Heavy work has
been defined by DL = 60 ml/min/mmHg and VA = 30 000 ml/min. The
degree of protection provided by these guidelines is indicated in
columns 3 and 4 of Table 4. Column 3 shows carbon monoxide
concentrations that would produce 5% HbCO within exposure times given
a The Secretariat wishes to thank Dr M. A. Vouk, University
Computing Centre, Zagreb, Yugoslavia, for programming Coburn's
equations and providing computer printouts.
in column 2, for light and heavy work, respectively. Column 4 provides
"safety factors" obtained by dividing the concentrations in column 3
by concentrations in column 1. Unless otherwise indicated, the
guidelines given in Table 4 should be considered as desirable
conditions rather than maximum acceptable limits.
Table 1. [HbCO](%) predicted by different empirical equations and by the model of Coburn et al. (1965).
Exposure to a carbon monoxide concentration of 115 mg/m3 (100 ppm)
Time Subjects at rest Subjects performing light work
(min)
F P H PS C F P H C
15 1.0 0.8 1.1 2.0 1.2 1.7 1.6 1.9 2.0
30 1.4 1.0 1.7 2.8 1.8 2.9 2.6 3.2 3.3
45 1.9 1.5 2.3 3.4 2.4 4.1 3.6 4.4 4.6
60 2.3 1.9 2.8 4.0 3.0 5.3 4.7 5.4 5.7
120 4.1 3.3 4.7 5.9 5.0 10.1 8.9 8.7 9.2
180 5.9 4.5 6.4 7.5 6.8 14.9 13.1 10.9 11.6
240 7.7 6.0 7.8 8.9 8.3 19.7 17.3 12.3 13.2
300 9.0 7.4 9.0 10.1 9.6 24.5 21.5 13.3 14.1
360 10.8 8.8 10.0 11.3 10.7 29.3 25.7 13.9 15.1
420 12.6 10.2 10.8 12.4 11.6 34.1 29.9 14.3 15.6
480 14.4 11.5 11.5 13.5 12.4 38.9 34.1 14.6 15.9
F = Forbes et al. (1945), P = Pace et al. (1946), H = Hanks & Farquhar (1969),
PS = Peterson & Stewart (1970), C = Coburn et al. (1965).
Table 2. [HbCO] values predicted from Coburn et al. (1965) model
Time 200 ppm 100 ppm 75 ppm 50 ppm
S L H S L H S L H S L H
15 min 1.8 3.5 5.2 1.2 2.0 2.8 1.0 1.6 2.2 0.82 1.2 1.6
30 min 3.1 6.2 9.2 1.8 3.3 4.8 1.5 2.6 3.7 1.1 1.9 2.6
45 min 4.3 8.7 12.6 2.4 4.6 6.5 1.9 3.5 4.9 1.4 2.5 3.4
60 min 5.5 11.0 15.5 3.0 5.7 7.9 2.3 4.3 6.0 1.7 3.0 4.1
90 min 7.7 14.9 20.2 4.0 7.6 10.2 3.1 5.8 7.7 2.2 4.0 5.2
2h 9.7 18.1 23.7 5.0 9.2 11.9 3.9 7.0 9.0 2.7 4.7 6.1
4h 16.3 26.2 30.4 8.3 13.2 16.3 6.3 10.0 11.5 4.4 6.9 7.7
6h 21.1 30.0 32.4 10.7 15.1 16.2 8.1 11.3 12.2 5.5 7.6 8.2
8h 24.5 31.7 32.9 12.4 15.9 16.5 9.4 12.0 12.4 6.4 8.0 8.3
24h 32.7 33.2 33.2 16.5 16.7 16.6 12.4 12.5 12.5 8.4 8.4 8.3
infinity 33.4 33.2 33.2 16.8 16.7 16.6 12.7 12.5 12.5 8.5 8.4 8.3
Time 35 ppm 25 ppm 10 ppm 5 ppm
S L H S L H S L H S L H
15 min 0.72 1.0 1.3 0.66 0.84 1.0 0.55 0.61 0.67 0.52 0.54 0.56
30 min 0.93 1.4 1.9 0.80 1.2 1.5 0.61 0.72 0.82 0.54 0.57 0.60
45 min 1.1 1.9 2.5 0.95 1.4 1.9 0.66 0.81 0.95 0.56 0.61 0.64
60 min 1.3 2.2 3.0 1.1 1.7 2.2 0.71 0.90 1.1 0.58 0.63 0.68
90 min 1.7 2.9 3.7 1.3 2.1 2.7 0.80 1.1 1.2 0.62 0.69 0.74
2 h 2.0 3.4 4.3 1.6 2.5 3.1 0.89 1.2 1.4 0.66 0.73 0.78
4 h 3.2 4.7 5.4 2.4 3.4 3.9 1.2 1.5 1.6 0.77 0.84 0.86
6 h 4.0 5.4 5.7 2.9 3.9 4.1 1.4 1.6 1.7 0.85 0.88 0.88
8 h 4.5 5.7 5.8 3.3 4.1 4.2 1.5 1.7 1.7 0.91 0.91 0.89
24 h 5.9 5.9 5.9 4.3 4.2 4.2 1.9 1.8 1.7 1.05 0.93 0.89
infinity 6.0 5.9 5.9 4.4 4.2 4.2 1.9 1.8 1.7 1.06 0.93 0.89
S = sedentary subjects, L = light physical work, H = heavy physical work, all as defined in sections 9 and 10.
Table 3. Guidelines for exposure conditions to prevent carboxyhaemoglobin levels exceeding
2.5-3% in nonsmoking populations
(a) A ceiling or maximum permitted exposure of 115 mg/m3 (100 ppm) for periods of exposure
not exceeding 15 min (No exposure over 115 mg/m3 (100 ppm) permitted, even for very
short time periods).
(b) A time-weighted average exposure of 55 mg/m3 (50 ppm) for periods of exposure not
exceeding 30 min.
(c) A time-weighted average exposure of 29 mg/m3 (25 ppm) for periods of exposure not
exceeding one h.
(d) A time-weighted average exposure of 15 mg/m3 (13 ppm) for periods of exposure of more
than one h.
(e) A time-weighted average exposure of 11.5 mg/m3 (10 ppm) for periods of exposure of
8-24 h.a
a Suggested by the Secretariat.
Table 4. Guidelines for exposure conditions that would prevent carboxyhaemoglobin levels
exceeding 5% in nonsmoking occupational groups performing light and heavy
physical work.
Concentration Exposure time not to Concentrations that Safety factor
be exceeded would produce 5% HbCOc
ppm mg/m3
Light Heavy Light Heavy Light Heavy
worka workb work work work work
200d 230 15 min - 298 - 1.5 -
100e,f 115 30 min 15 min 157 193 1.6 1.9
75f 86 60 min 30 min 87 105 1.2 1.4
50f 55 90 min 60 min 64 62 1.3 1.2
35f 40 4 h 2 h 37 41 1.1 1.2
25f 29 8 h 8 h 31 30 1.2 1.2
a Limits suggested by Dr Buchwald.
b Limits suggested by the Secretariat.
c Calculated from Coburn's equation (6).
d Short-term limit or maximum permissible concentration for light work.
e Short-term limit or maximum permissible concentration for heavy work.
f Time weighted average.
REFERENCES
HANKS, T. G. & FARQUHAR, R. D. (1969) Analysis of human performance
capabilities as a function of exposure to carbon monoxide.
(SystMed Corporation Report R 9001, Contract PH-22-68-31).
PACE, N., CONSOLAZIO, W. V., WHITE, W. A. JR. & BEHNKE, A. R. (1945)
Formulation of principal factors affecting the rate of uptake of
carbon monoxide by man. Am. J. Physiol., 147: 352-359.
All other references are included in the list of references for
the main body of the document (pp. 98-114).
Annex 2
SELECTED NATIONAL AMBIENT AIR QUALITY STANDARDS AND OCCUPATIONAL
EXPOSURE STANDARDS FOR CARBON MONOXIDEa
1. NATIONAL AMBIENT AIR QUALITY STANDARDS
1.1 Canada (1974)
National air quality objectives
Desirable concentrations:
(a) 0-6 mg/m3 (0-5 ppm)b average concentration over an 8-h
period.
(b) 0-15 mg/m3 (0-13 ppm) average concentration over a one-h
period.
Acceptable concentrations:
(a) 6-15 mg/m3 (5-13 ppm) average concentration over an 8-h
period.
(b) 15-35 mg/m3 (13-31 ppm) average concentration over a one-h
period.
Method of measurement:
Nondispersive infrared spectrometry, Report No. EPS 1-AP-73-1.
Source: Velma Ouellet (1978) The Clean Air Act--Compilation of
Regulations and Guidelines, Ottawa, Environment Canada (Report EPS
1-AP-78-2).
In addition to the desirable and acceptable concentrations listed,
a tolerable range of 15-20 mg/m3 average concentration over a
continuous 8-h period was prescribed in 1978.
Source: Canada Gazette (1978) Part 2, Volume 112, No. 3 (February 8).
a Prepared by the Secretariat.
b Concentrations in alternative units have been added by the
Secretariat to facilitate comparison of national quality standards.
1.2 Federal Republic of Germany (1974)
In the Federal Republic of Germany, immissionsc (Immissionen)
are legally defined as "air pollutants, noise, vibrations, light,
heat, radiations, and analogous environmental factors affecting human
beings, animals, plants, or other objects". "As a rule, air pollutants
occurring at a height of 1.5 metres above ground or at the upper limit
of vegetation or at a distance of 1.5 metres from the surface of a
building shall be considered active air pollutants."
"Immission standards are the values for long-term exposure (IW 1)
and short-term exposure (IW 2)."
The following immission standards have been established for carbon
monoxide:
Long-term exposure (IW 1): 10.0 mg/m3 (9 ppm).
Short-term exposure (IW 2): 30.0 mg/m3 (26 ppm).
Method of measurement: VDI 2455, Sheet 1 (August 1970) and 2455,
Sheet 2 guidelines (October 1970).
"Characteristic value I 1 for comparison with IW 1 shall be the
arithmetic mean of all individual data for a measurement area.
Characteristic value I 2 for comparison with IW 2 shall be the 95%
value for the cumulative frequency distribution of all individual
values for a measurement area; this may be also calculated with the
formula I 2 = x + ts where x is the arithmetic mean of individual
data for a measurement area,
2SUM(x - xi)2
s = + SQRT ,
2z - 1
xi = individual data which are greater than x, z = number of
individual data, which are greater than x, and t = 1.64 for the 95%
confidence level."
Source: Federal Minister of the Interior (1974) Technical
Instructions for Maintaining Air Purity, 28 August 1974, Bonn.
c Immission A German term for which there is no simple English
equivalent. Immissions are to be distinguished from emissions
(Emissionen), which are defined as air pollutants, noise,
vibrations, light, heat, radiations, and analogous phenomena
originating from an installation (Federal Republic of Germany, Law
on Protection against Emissions, 15 March 1974).
1.3 Japan (1973)
Ambient air quality standards are defined as follows:
(a) Average of hourly values in 8 consecutive hours shall not
exceed 20 ppm (23 mg/m3).
(b) Daily average of hourly values shall not exceed 10 ppm
(11 mg/m3).
These standards do not apply to industrial zones, roadways, and
other areas or places where people do not usually live.
Source: Environment Agency (1978) Environmental Laws and
Regulations in Japan (II) Air. Tokyo.
1.4 Union of Soviet Socialist Republics (1971)
Maximum permissible (single exposure) concentration for carbon
monoxide is 3 mg/m3 (2.6 ppm). This concentration should not provoke
reflex (i.e., subsensory) reactions in human organisms.
Maximum permissible (24-hour average) concentration for carbon
monoxide is 1.0 mg/m3 (0.9 ppm). This concentration should not have
either a direct or indirect harmful effect on man, for unlimited in
time, continuous exposure, 24 hours a day.
Method not specified.
Source: Krotov, Ju. A. (1975) Maximum permissible concentrations
of harmful substances in air and water, Himija, Moscow.
1.5 United States of America (1971)
"The national primary and secondary ambient air quality standards
for carbon monoxide, measured by the reference method described in
Appendix C to this part, or by an equivalent method, are:
(a) 10 milligrams per cubic meter (9 ppm) -- maximum 8-hour
concentration not to be exceeded more than once per year.
(b) 40 milligrams per cubic meter (35 ppm) -- maximum 1-hour
concentration not to be exceeded more than once per year."
Reference method for the continuous measurement of carbon monoxide
in the atmosphere is nondispersive infrared spectrometry.
Source: Federal Register, 36 (228), Thursday, November 25, 1971,
Washington DC, pp. 22385 and 22391-22392.
1.6 Other Member States
For further information on national ambient air quality standards
for carbon monoxide, the reader is referred to W. Martin & A. C. Stern
(1974) The world's air quality management standards, Washington, DC,
US Environmental Protection Agency (EPA-650/a-75-001-a).
2. OCCUPATIONAL EXPOSURE LIMITS
An occupational exposure limit for carbon monoxide of 50 ppm or
55 mg/m3 has been set in the following Member States: Australia,
Belgium, Finland, Federal Republic of Germany, Italy, Japan,
Netherlands, Switzerland, the USA, and Yugoslavia.
Bulgaria and the USSR have established a limit of 20 mg/m3
(17 ppm), Hungary and Poland, 30 mg/m3 (26 ppm) and Sweden,
40 mg/m3 (35 ppm). Czechoslovakia's standard includes average and
maximum values of 30 mg/m3 (26 ppm) and 150 mg/m3 (130 ppm),
respectively. Other standards that include both average and maximum
values are those of the German Democratic Republic, (35 and
110 mg/m3) (30 and 96 ppm) and Romania, (30 and 50 mg/m3) (26 and
44 ppm).
These values should be interpreted in terms of definitions of
occupational exposure limits, which may be different in different
countries. The reader is referred to the International Labour Office
publication: Occupational Exposure Limits for Airborne Toxic
Substances, Occupational Safety and Health Series 37, ILO, Geneva,
1977. The values given in paragraphs 1 and 2 have been extracted from
this publication.
The US National Institute of Occupational Safety and Health
(NIOSH, 1972) has recommended environmental exposure limits of 35 ppm
(40 mg/m3) (time weighted average) and 200 ppm (229 mg/m3)
(ceiling) (Summary of NIOSH Recommendations for Occupational Health
Standards, July, 1978).
most important of which is the weather, produce great variations in
the concentrations of pollutants produced by traffic. Concentrations
fall steeply with distance from the street. However, distinct patterns
are often discernible (section 5.1). Concentrations for 8-h averaging
times are frequently used and quoted and usually vary from
<10 mg/m3 (8.7 ppm) to over 60 mg/m3 (52.2 ppm) but are mostly
<20 mg/m3 (17.6 ppm) in city streets. Away from heavy traffic, even
in towns, annual average concentrations are usually well under
10 mg/m3 (8.7 ppm). Obviously, in especially stagnant weather, very
heavy traffic may produce much higher levels. There is little
information about concentrations of carbon monoxide near large
stationary sources.
9.2.4 Indoor exposure
Carbon monoxide diffuses readily and, being relatively chemically
inert and not absorbed on surfaces, concentrations indoors are usually
similar to those found immediately outside. Not infrequently, however,
high concentrations may be found in kitchens and living rooms in which
there are coal, gas, or oil-fired cooking or heating appliances that
are maladjusted and inadequately vented to outside air; in some
countries, cases of acute and even fatal poisoning due to these causes
are not uncommon. The possible contribution of the domestic
environment must be noted in surveys. The smoking of tobacco indoors
can obviously increase the carbon monoxide concentration of the air
but recent work has shown that, before carbon monoxide reaches
significant levels, the irritation from the other constituents of
tobacco smoke becomes unacceptable if not actually intolerable.
9.2.5 Exposures related to traffic
In garages and tunnels, being in effect closed streets, pollution
by carbon monoxide can reach high levels. However, since transit time
in tunnels is relatively short, higher concentrations than those found
in streets are tolerable. Usually, there are monitoring instruments
that control ventilation and sound alarms if concentrations exceed
agreed values, which may vary from 115 to 570 mg/m3 (100-500 ppm)
depending on the use and length of the tunnel. High (sometimes lethal)
concentrations of carbon monoxide may accumulate inside motor vehicles
because of fractures in exhaust systems or other mechanical defects.
9.2.6 Occupational exposure
Traffic policemen, garage attendants, and drivers of taxis and
trucks are exposed to pollution from traffic and many studies have
shown a consequent increase in carboxyhaemoglobin levels (up to about
3% in nonsmokers), but there is much evidence that this increase may
be relatively undramatic, when compared with the manifest effects of
cigarette smoking. Exposure in certain industries, especially in iron
and steel works and in the manufacture of various gases, may be
relatively massive (in excess of 115 mg/m3 or 100 ppm), and high
carboxyhaemoglobin levels (>15% in nonsmokers) have been reported in
workers. Firemen may be exposed to very high concentrations of carbon
monoxide in fighting certain fires, but this exposure is obviously
episodic. These matters are discussed in section 5.3.
9.2.7 Tobacco smoking
The smoking of tobacco, especially in the form of cigarettes, has
been shown in many studies to be the major cause of raised
carboxyhaemoglobin levels in adult populations. Table 7 displays some
of this evidence and the topic is discussed in detail in section
8.1.6. Whereas carboxyhaemoglobin concentrations of 3% are rarely
found in nonsmokers exposed to town air, concentrations of 5-15% are
often found in smokers. It is important to remember that the effects
of smoking and exposure to town air are not simply additive and that
the resulting carboxyhaemoglobin levels will depend on other factors
already discussed.
9.2.8 Multiple exposures
Enough has been said to leave no doubt that carbon monoxide,
produced exogenously or endogenously, is a widespread pollutant
emanating from many sources to which people may be exposed in various
ways. This variety of exposure must be taken into account in the
interpretation of epidemiological surveys, the design of experiments,
and, above all, in giving advice about the fixing of air quality
criteria.
9.3 Effects
The main areas of concern that have arisen from acute or chronic
exposure to low levels of carbon monoxide in experimental and
epidemiological research in animals and man are: (a) its role in the
genesis of arteriosclerotic vascular diseases; (b) its role in the
aggravation of symptoms of cardiovascular diseases; (c) its
contribution to performance deficits in certain psychomotor tasks; and
(d) its role in limiting the working capacity of exercising man.
9.3.1 Cardiovascular system
9.3.1.1 Development of atherosclerotic cardiovascular disease
Extensive experimental work has been carried out over many years
on animals, mainly rabbits, showing that prolonged exposure to
moderate levels of carbon monoxide can produce atherosclerotic
changes, especially in the presence of high cholesterol levels (1-2%)
in the diet. The relevance of this work for man has not been
established. However, other animal work, and some epidemiological
studies of prolonged human exposures to elevated carbon monoxide
levels through smoking, occupation, or both, such as those carried out
in Denmark, Finland, and Japan, indicate the need for further
investigation of the possible role of carbon monoxide in the genesis
of atherosclerotic vascular changes in animals and man. The degree of
intermittency of exposure at various levels should be taken into
account as well as the possible contribution of other agents such as
nicotine and high-fat diets. There is some evidence of adaptation, but
such changes may not be entirely beneficial. None of the information,
currently available, is useful for the purpose of setting standards.
9.3.1.2 Acute effects on existing heart illness
The few existing epidemiological studies on the possible effects
of carbon monoxide on the severity or fatality of coronary occlusion
are insufficient to allow any conclusions. It is hoped that additional
work of this type will clarify matters.
Two carefully conducted human studies of the effects of low carbon
monoxide exposure and exercise on pain in volunteer patients with
angina pectoris offer valuable quantitative information. Although
limited in the number of patients studied, the findings are consistent
in the 2 investigations showing effects at carboxyhaemoglobin
concentrations of 2.5-3.0%. A third single-blind study revealed the
same detrimental effects in patients with angina pectoris when
exposure to traffic exhausts caused carboxyhaemoglobin levels to rise
to 5.1%. A no-adverse-effect level has not been established in these
observations, nor is it possible to determine whether there is a
graded response in this type of experiment. More work of a similar
nature would be useful to explore these questions.
9.3.1.3 Acute effects on existing vascular disease
One study, similar to those done on patients with angina, has been
carried out on patients with intermittent claudication from peripheral
vascular disease. Effects on pain with exercise were observed in the
same exposure range as with angina i.e., at carboxyhaemoglobin
concentrations of 2.5-3.1%, with a mean of 2.8%. Here, too, more data
of a similar kind are needed, preferably designed to provide dose-
response relationships.
9.3.2 Nervous system
As for the role of carbon monoxide in affecting psychomotor
functions, no definite conclusions can be drawn from the existing
data. The behavioural functions tested in such studies include
vigilance and psychomotor performance, visual acuity and sensitivity,
the ability to estimate time intervals, complex motor coordination as
tested by driving simulators, and different perceptual and mental
operationsa. Some workers observed detrimental effects at
carboxyhaemoglobin levels as low as 2%, whereas others were unable to
detect significant impairment even at levels from above 5% to about
20%. In evaluating these discrepancies, it should be mentioned, that
these behavioural functions are easily influenced by a number of other
factors besides carbon monoxide-induced hypoxia, e.g., degree of
sensory deprivation, compensatory abilities, drugs, temperature, time
of day, competition, etc.
9.3.3 Work capacity
That elevated carboxyhaemoglobin levels affect work capacity has
long been known. Levels of 40-50% will usually prevent working
entirely. Recent studies in the laboratory, on man, using maximum work
capacity or maximum aerobic capacity as indicators of performance,
have been carried out in relation to carboxyhaemoglobin levels. Here,
dose-response data are available for maximum effort. The limitation
appears at a carboxyhaemoglobin concentration of about 4% and
increases at higher levels. Lower exposure levels have been studied
and do not produce this effect. It should be noted that while levels
of carboxyhaemoglobin of 2.5-4%, did not reduce maximum work capacity,
they did reduce the length of time for which such effort could be
carried out. It is not known what specific levels of carboxy-
haemoglobin will reduce the capacity of individuals to perform at
ordinary work levels, such as 30-50% of their maximum capacity, for
prolonged periods of time.
9.4 Recommended Exposure Limits
It has already been stated that the major contributor to
carboxyhaemoglobin concentrations in the body is the smoking of
tobacco; however, in many of the experiments currently quoted to
justify the formulation of exposure limits, the smoking habits of the
subjects were not taken into account. Results of recent work suggest
that smokers and ex-smokers might be less sensitive to carbon monoxide
exposure than nonsmokers. In view of this suggestion, and because of
the deficiencies in the experiments mentioned, recommendations for
exposure limits should be confined to the protection of nonsmokers.
There is an urgent need for more work on possible adaptation following
a The possible importance of performance deficiencies resulting
from carbon monoxide is considerable, particularly in relation to
accidents at work, and while driving or flying. Further studies,
particularly of the vigilance type are urgently needed for a better
understanding of this problem.
exposure to carbon monoxide from smoking or from other sources. It is
also important to note that carboxyhaemo-globin levels have been the
measurement of exposure in most experimental work. Thus, it is
desirable to recommend the primary exposure limits in terms of
carboxyhaemoglobin, and follow this by comments on the derivation of
an appropriate air concentration equivalent.
9.4.1 General population exposure
Data used in arriving at a recommendation for an exposure limit
for the general population were mainly those obtained from the
exposure of subjects with cardiovascular illness to carbon monoxide in
conjunction with exercise. Agreement was not reached on a single
level. Thus, a range of carboxyhaemoglobin concentrations of 2.5-3.0%
is recommended for the protection of the general population including
those who have impaired health. The recommendation must be regarded as
tentative, since ideal dose-response or concentration-response
information is not yet available. However, it must also be recognized
that complete protection of all persons, at all times, cannot
reasonably be sought by environmental control alone. Persons who are
in should be educated by their physicians concerning their own
responsibility to avoid stressful exposures.
9.4.2 Working population exposure
Better quality data are available for recommending an exposure
limit for the working population. In this case, the Task Group
unanimously agreed on maintaining carboxyhaemoglobin levels below 5%,
on the basis of present knowledge, since working populations comprise
individuals who are assumed to be healthy, physiologically resilient,
and under regular supervision.
9.4.3 Derived limits for carbon monoxide concentrations in air
It is important, wherever possible, to have both biological and
environmental assessments of human exposure to pollutants. While the
biological measurements may be more relevant in relation to effects,
they may be more difficult to use in practice. For carbon monoxide,
the relationship between concentrations in air and carboxyhaemoglobin
levels is affected by several variables, including exposure time and
it is not easy to estimate. However, such estimates may be
sufficiently accurate for many practical purposes (for reviews see
Committee on the Challenges of Modern Society, 1972; Commission of the
European Communities, 1974; NAS/NRC, 1977; Winneke, 1977; Ott & Mage,
1978). It should be emphasized yet again that analyses of carbon
monoxide in air and of carboxyhaemoglobin in blood are complementary,
and should in no way be regarded as alternative methods of monitoring.
Obviously, air monitoring has its uses in the planning and
implementation of control measures, and for warning purposes, but such
measurements have limited value in estimating the actual human
exposure defined by carboxyhaemoglobin levels.
REFERENCES
ADAMS, J. D., ERICKSON, H. H., & STONE, H. L. (1973) Myocardial
metabolism during exposure to carbon monoxide in the conscious
dog. J. appl. Physiol., 34: 238-242.
AGOSTINI, J. C., RAMIREZ, R. G., ALBERT., S. N., GOLDBAUM, L. R., &
ABSOLON, K. B. (1974) Successful reversal of lethal carbon
monoxide intoxication by total body asanguineous hypothermic
perfusion. Surgery, 75: 213-219.
AHMED, A. E., KUBIC, V. L., & ANDERS, M. W. (1977) Metabolism of the
haloforms to carbon monoxide. I. In vitro studies. Drug
Metabol. Disposition, 5: 198-204.
ALEXANDROV, N. P. (1973) [Choice of experimental animals for
elaboration of standards for CO.] Gig. i Sanit., 11: 92-95 (in
Russian).
ALEKSIEVA, T. & DIMITROVA, M. (1971) [Some oscillographic changes in
workers exposed to chronic carbon monoxide effect.] Tr. Niotpz,
pp. 163-169 (in Bulgarian).
ALTMAN, P. L. & DITTMEN, D. S., ed. (1971) Respiration and
circulation, Bethesda, MD, Federation of the American Society of
Experimental Biology.
ALTSHULLER, A. P. & BUFALINI, J. J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ANDERSON E. W., ANDELMAN, R. J., STRAUCH, J. M., FORTUIN, N. J., &
KNELSON, J. H. (1973) Effect of low-level carbon monoxide exposure
on onset and duration of angina pectoris: a study in ten patients
with ischemic heart disease. Ann. intern. Med., 79: 46-50.
ANTWEILER, H. (1974) Physiological kinetics of CO inhalation.
In: Proceedings of the European Colloquium on Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg Commission of the European
Communities, Directorate General Scientific and Technical
Information Management, pp. 197-251 (in German with full English
translation).
ANTWEILER, H. (1975) [The reception and effect of carbon monoxide in
active and passive smokers.] Arbeitsmed. Sozialmed.
Präventivmed., 10: 245-248 (in German).
ARONOW, W. S. & CASSIDY, J. (1975) Effect of carbon monoxide on
maximal treadmill exercise: a study in normal persons. Ann.
intern. Med., 83: 496-499.
ARONOW, W. S. & ISBELL, M. W. (1973) Carbon monoxide effect on
exercise induced angina pectoris. Ann. intern. Med.,
79: 392-395.
ARONOW, W. S., CASSIDY, J., VANGROW, J. S., MARCH, H., KERN, J. C.,
GOLDSMITH, J. R., KHEMKA, M., PAGANO, J., & VAWTER, M. (1971a)
Effect of cigarette smoking and breathing carbon monoxide on
cardiovascular hemodynamics in anginal patients. Circulation,
50: 340-347.
ARONOW, W. S., DENDINGER, J., & ROKAW, S.N. (1971b) Heart rate and
carbon monoxide level after smoking high-, low-, and non-nicotine
cigarettes. Ann. intern. Med., 74: 697-702.
ARONOW, W. S, HARRIS, C. N., ISBELL, M. W., ROKAW, S. N., & IMPARATO,
B. (1972) Effect of freeway travel on angina pectoris. Ann.
intern. Med., 77: 669-676.
ARONOW, W. S., STEMMER, E. A., & ISBELL, M. W. (1974) Effect of carbon
monoxide exposure on intermittent claudication. Circulation,
49: 415-417.
ASTRAND, I., OVRUM, P., & CARLSSON, A. (1975) Exposure to methylene
chloride. I. Its concentration in alveolar air and blood during
rest and exercise and its metabolism. Scand. J. Work Environ.
Health, 1: 78-94.
ASTRUP, P. (1970) Intraerythrocytic 2,3-diphosphoglycerate and carbon
monoxide exposure. Ann. NY Acad. Sci., 174: 252-254.
ASTRUP, P. (1972) Some physiological and pathological effects of
moderate carbon monoxide exposure. Br. med. J., 4: 447-452.
ASTRUP, P. & PAULI, H. G. (1968) A comparison of prolonged exposure to
carbon monoxide and hypoxia in man. Scand. J. clin. lab. Invest.,
22 (Suppl. 103): 1-71.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1967) Enhancing influence of
carbon monoxide on the development of atheromatosis in
cholesterol-fed rabbits. J. atheroscler. Res., 7: 343-354.
ASTRUP, P., KJELDSEN, K., & WANSTRUP, J. (1970) Effects of carbon
monoxide exposure on the arterial walls. Ann. NY Acad. Sci.,
174: 294-300.
ASTRUP, P., OLSEN, H. M., TROLLE, D., & KJELDSEN, K. (1972) Effect of
moderate carbon monoxide exposure on fetal development. Lancet,
2: 1220-1222.
AYRES, S. M., CRISCITIELLO, A., & GIANELLI, S., JR (1966)
Determination of blood carbon monoxide content by gas
chromatography. J. appl. Physiol., 21: 1368-1370.
AYRES, S. M., MUELLER, H. S., GREGORY, J. J., GIANELLI, S., JR, &
PENNY, J. L. (1969) Systemic and myocardial hemodynamic responses
to relatively small concentrations of carboxyhemoglobin (COHB).
Arch. environ. Health, 18: 699-709.
AYRES, S. M., GIANELLI, S., JR & MUELLER, H. (1970) Myocardial and
systemic responses to carboxyhemoglobin. Ann. NY. Acad. Sci.,
174: 268-293.
BAKER, F. D. & TUMASONIS, C. F. (1972) Carbon monoxide and avian
embryogenesis. Arch. environ. Health, 24: 53-61.
BALABAEVA, L. & KALPAZANOV, I. (1974) [Effect of smoking and length of
service on the content of carboxyhemoglobin in blood of traffic
policemen.] Hig. i Zdraveo., 5: 490-493 (in Bulgarian).
BALL, K. & TURNER, R. (1974) Smoking and the heart: the basis for
action. Lancet, 2 (7884): 822-826.
BATES, D. R. & WITHERSPOON, A. E. (1952) The photo-chemistry of some
minor constituents of the earth's atmosphere (CO2, CO, CH4,
N2O). Mon. not. R. Astron. Soc. (Lond.), 112: 101-124.
BEARD, R. R. & GRANDSTAFF, N. W. (1975) Carbon monoxide and human
functions. In: Weiss, P. & Laties, V. G., ed. Behavioral
Toxicology, New York, Plenum Press, pp. 1-26.
BEARD, R. R. & WERTHEIM, G. A. (1967) Behavioral impairment associated
with small doses of carbon monoxide. Am. J. public Health,
57: 2012-2022.
BEHRMAN, R. E., FISHER, D. E., & PATON, J. (1971) Air pollution in
nurseries: correlation with a decrease in oxygen-carrying capacity
of hemoglobin. J. Pediatr., 78: 1050-1054.
BENDER, W., GÖTHERT, M., & MALORNY, G. (1972) Effect of low carbon
monoxide concentrations on psychological functions. Staub-
Reinhalt. Luft., 32 (4): 54-60.
BIDWELL, R. G. S. & FRASER, D. E. (1972) Carbon monoxide uptake and
metabolism by leaves. Can. J. Bot., 50: 1435-1439.
BLACKMORE, D. J. (1974) Aircraft accident toxicology: UK experience
1967-1972. Aerosp. Med., 45: 987-994.
BORST, J. R. (1970) Acute and chronic carbon monoxide poisoning in
private motor-cars and lorries. Arts Auto, 36 (19): 1518-1523.
BREYSSE, P. A. & BOVEE, H. H. (1969) Use of expired air-carbon
monoxide for carboxyhemoglobin determinations in evaluating carbon
monoxide exposures resulting from the operation of gasoline fork
lift trucks in holds of ships. Am. Ind. Hyg. Assoc. J.,
30: 477-483.
BRIEGER, H. (1944) Carbon monoxide polycythemia. J. ind. Hyg.
Toxicol., 26: 321-327.
BRODY, J. S. & COBURN, R. F. (1970) Effects of elevated
carboxyhemoglobin on gas exchange in the lung. Ann. NY Acad.
Sci., 174: 255-260.
BUCHWALD, H. (1969) Exposure of garage and service station operatives
to carbon monoxide: a survey based on carboxyhemoglobin levels.
Am. Ind. Hyg. Assoc. J., 30: 570-575.
BUTT, J., DAVIES, G. M., JONES, J. G., & SINCLAIR, A. (1974)
Carboxyhaemoglobin levels in blast furnace workers. Ann. occup.
Hyg., 17: 57-63.
BYCZKOWSKA, Z. & MILAN, K. (1971) [Acute carbon monoxide poisoning
with temporary renal insufficiency.] Med. Pr., 22: 119-121
(in Polish).
CALVERT, J. G. (1973) Interactions of air pollutants. In: Proceedings
of the Conference on Health Effects of Air Pollutants, Oct.,
Washington, DC, National Academy of Sciences, National Research
Council, pp. 19-101 (Serial No. 93-15).
CASTLEDEN, C. M. & COLE, P. V. (1975) Carboxyhaemoglobin levels of
smokers and nonsmokers working in the city of London. Br. J. ind.
Med., 32: 115-118.
CAVANAGH, L. A., SCHADT, C. F., & ROBINSON, E. (1969) Atmospheric
hydrocarbon and carbon monoxide measurements at Point Barrow,
Alaska. Environ. Sci. Technol., 3: 251-257.
CHALUPA, B. (1960) [Disorders of memory in acute carbon monoxide
intoxications.] Prac. Lek., 12: 331-336 (in Polish).
CHAPMAN, D. J. & TOCHER, R. D. (1966) Occurrence and production of
carbon monoxide in some brown algae. Can. J. Bot.,
44: 1438-1442.
CHEVALIER, R. B., BOWERS, J. A., BONDURANT, S., & ROSS, J. C. (1963)
Circulatory and ventilatory effects of exercise in smokers and
nonsmokers. J. appl. Physiol., 18: 357-360.
CHEVALIER, R. B.. KRUMHOLZ, R. A., & ROSS, J. C. (1966) Reaction of
nonsmokers to carbon monoxide inhalation. Cardiopulmonary
responses at rest and during exercise. J. Am. Med. Assoc.,
198: 1061-1064.
CHIODI, H., DILL, D. B., CONSOLAZIO, F., & HORVATH, S. M. (1941)
Respiratory and circulatory responses to acute carbon monoxide
poisoning. Am. J. Physiol, 134: 683-693.
CHOVIN, P. (1967) Carbon monoxide: analysis of exhaust gas
investigations in Paris. Environ. Res., 1: 198-216.
CHOVIN, P. (1974) Comments on paper presented by H. Antweiler.
In: Proceedings of the European Colloquium Carbon Monoxide
Environmental Pollution and Public Health, Luxembourg, 17-19
December, 1973, Luxembourg, Commission of the European
Communities Directorate General Scientific and Technical
Information Management, pp. 225-236.
CLARK, R. T., JR & OTIS, A. B. (1952) Comparative studies on
acclimatization of mice to carbon monoxide and to low oxygen.
Am. J. Physiol, 169: 285-294.
COBURN, R. F. (1970a) Enhancement by phenobarbital and
diphenylhydantoin of carbon monoxide production in normal man.
New Engl. J. Med., 283: 512-515.
COBURN, R. F. (1970b) The carbon monoxide body stores. Ann. NY Acad.
Sci., 174: 11-22.
COBURN, R. F., DANIELSON, G. K., BLAKEMORE, W. S., & FORSTER, R. E.
(1964) Carbon monoxide in blood: analytical method and sources of
error. J. appl. Physiol, 19: 510-515.
COBURN, R. F., FORSTER, R. E., & KANE, P. B. (1965) Considerations of
the physiological variables that determine the blood
carboxyhemoglobin in man. J. clin. Invest., 44: 1899-1910.
COBURN, R. F., WILLIAMS, W. J., & KAHN, P. B. (1966) Endogenous carbon
monoxide production in patients with hemolytic anemia. J. clin.
Invest., 45: 460-468.
COBURN, R. F., PLOEGMAKERS, F., GONDRIE, P., & ABBOUD, R. (1973)
Myocardial myoglobin oxygen tension. Am. J. Physiol.,
224: 870-876.
COHEN, S. I., DEANE, M., & GOLDSMITH, J. R. (1969) Carbon monoxide and
survival from myocardial infarction. Arch. environ. Health,
19: 510-517.
COHEN, S. I., DORION, G., GOLDSMITH, J. R., & PERMUTT, S. (1971a)
Carbon monoxide uptake by inspectors at a United States-Mexico
border station. Arch. environ. Health, 22: 47-54.
COHEN, S. I., PERKINS, N.M., URY, H. K., & GOLDSMITH, J. R. (1971b)
Carbon monoxide uptake in cigarette smoking. Arch. environ.
Health, 22: 55-60.
COLLISON, H. A., RODKEY, F. L., & O'NEAL, J. D. (1968) Determination
of carbon monoxide in blood by gas chromatography. Clin. Chem.,
14: 162-171.
COLUCCI, J. M. & BEGEMAN, C. R. (1969) Carbon monoxide in Detroit, New
York, and Los Angeles air. Environ. Sci. Technol., 3: 41-47.
COMMINS, B. T. & LAWTHER, P. J. (1965) A sensitive method for the
determination of carboxyhaemoglobin in a finger prick of blood.
Br. J. ind. Med., 22: 139-143.
COMMISSION OF THE EUROPEAN COMMUNITIES (1974) Proceedings of the
European Colloquium on Carbon Monoxide Environmental Pollution
and Public Health, Luxembourg, 17-19 December, 1973, Luxembourg
Directorate General Scientific and Technical Information and
Information Management, 437 pp.
COMMITTEE ON THE CHALLENGES OF MODERN SOCIETY (1972) Air pollution:
Air quality criteria for carbon monoxide, No. 10, 235 pp.
CONLEE, C. J., KENLINE, P. A, CUMMINS, R. L., & KONOPINSKI, V. J.
(1967) Motor vehicle exhaust at three selected sites. Arch.
environ. Health, 14: 429-446.
COOPER, D. Y., LEVIN, S., NARASIMHULU, S., & ROSENTHAL, O. (1965)
Photochemical action spectrum of the terminal oxidase of mixed
function oxidase systems. Science, 147: 400-402.
CORYA, B. C., BLACK, M. J., & MCHENRY, P. L. (1976) Echocardiographic
findings after acute carbon monoxide poisoning. Br. heart J.,
38: 712-717.
CROPP, G. J. A. (1970) Ventilation in anemia and polycythemia.
Can. J. Physiol. Pharmacol., 48: 382-393.
DAHMS, T. E. & HORVATH, S. M. (1974) Rapid, accurate technique for
determination of carbon monoxide in blood. Clin. Chem.,
20: 533-537.
DAHMS, T. E., HORVATH, S. M., & GRAY, D. J. (1975) Technique for
accurately producing desired carboxyhemoglobin levels during rest
and exercise. J. appl. Physiol., 38: 366-368.
DALHAMN, T., EDFORS, M.-L., & RYLANDER, R. (1968) Retention of
cigarette smoke components in human lungs. Arch. environ. Health,
17: 746-748.
DAVIS, G. L. & GANTNER, G. E.(1974) Carboxyhemoglobin in volunteer
blood donors. J. Am. Med. Assoc., 230: 996-997.
DE BIAS, D. A., BIRKHEAD, N. C., BANERJEE, C. M., KAZAL, L. A.,
HOLBURN, R. R., GREENE, C. H., HARRER, W. V., ROSENFELD, L. M,
MENDUKE, H., WILLIAMS, N., & FRIEDMAN, M. H. F. (1972a) The
effects of chronic exposure to carbon monoxide on the
cardiovascular and hematologic systems in dogs with experimental
myocardial infarction. Int. Arch. Arbeitsmed., 29: 253-267.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., FRIEDMAN, M. H. F.,
HARRER, W., HOLBURN, R., KAZAL, L., MENDUKE, H., ROSENFELD, L.,
WILLIAMS, N., & GREENE, C. H. (1972b) The effects of chronic
exposure to carbon monoxide (100 ppm) on the cardiovascular
system of monkeys, New York, Coordinating Research Council,
Inc., 54 pp. (PB 215-527).
BE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., HARRER, W. V., &
KAZAL, L. A. (1973) Carbon monoxide inhalation effects following
myocardial infarction in monkeys. Arch. environ. Health,
27: 161-167.
DE BIAS, D. A., BANERJEE, C. M., BIRKHEAD, N. C., GREEN, C. H., SCOTT,
D., & HARRER, W. V. (1976) Effects of carbon monoxide inhalation
on ventricular fibrillation. Arch. environ. Health, 31: 42-46.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., LONGO, L. D., & FORSTER, R.
E. (1969) Endogenous carbon monoxide of mother and fetus, 39th
Annual Meeting of the Society of Pediatrics Research, Atlantic
City, NJ, p. 148.
DELIVORIA-PAPADOPOULOS, M., COBURN, R. F., & FORSTER, R. E. (1970)
Cyclical variation of rate of heme destruction and carbon monoxide
production in normal women. Physiologist, 13: 178 (abstract).
DELWICHE, C. C. (1970) Carbon monoxide production and utilization by
higher plants. Ann. NY Acad. Sci., 174: 116-121.
DINMAN, B. D. (1968) Pathophysiologic determinants of community air
quality standards for carbon monoxide. J. occup. Med.,
10: 446-456.
DINMAN, B. D. (1969) Effects of long-term exposure to carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behaviour, and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 25-27.
DINMAN, B. D, EATON, J. W., & BREWER, G. J. (1970) Effects of carbon
monoxide on DPG concentration in the erythrocyte. Ann. NY Acad.
Sci, 174: 246-251.
DIVINCENZO, G. D. & HAMILTON, M. L. (1975) Fate and disposition of
[14C]methylene chloride in the rat. Toxicol. appl. Pharmacol.,
32: 385-393.
DORCUS, R. M. & WEIGAND, G. E. (1929) The effect of exhaust gas on the
performance in certain psychological tests. J. gen. Psychol.,
2: 73-76.
DOUGLAS, C. G., HALDANE, J. S., & HALDANE, J. B. S. (1912) The laws of
combination of haemoglobin with carbon monoxide and oxygen.
J. Physiol. (Lond.), 44: 275-304.
DOUZE, J. M. C. (1971) [Carbon monoxide poisoning by natural gas.]
Ned. Tidjschr. Geneesk., 115 (36): 1487-1493.
DRABKIN, D. L. & AUSTIN, J. H. (1935) Spectrophotometric studies. II.
Preparation from washed blood cells; nitric oxide hemoglobin and
sulfhemoglobin. J. biol. Chem., 112: 51-65.
DRINKWATER, B. L., RAVEN, P. B., HORVATH, S. M., GLINER, J. A.,
RUHLING, R. O., BOLDUAN, N. W., & TAGUCHI, S. (1974) Air
pollution, exercise and heat stress. Arch. environ. Health,
28: 177-181.
DYER, R. S. & ANNAU, Z. (1976) Carbon monoxide and superior colliculus
evoked potentials. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Protection Agency, 9 pp.
ECKARDT, R. E., MACFARLAND, H. N., ALARIE, Y. C. E., & BUSYE, W. M.
(1972) The biological effect from long-term exposure of primates
to carbon monoxide. Arch. environ. Health., 25: 381-387.
EHRICH, W. E., BELLET, S., & LEWEY, F. H. (1944) Cardiac changes from
CO poisoning. Am. J. med. Sci., 208: 512-523.
EJAM-BERDJEV, A. K. (1973) [Effect of small CO concentrations on the
cardiovascular system of workers engaged in cast-iron moulding.]
Harkovskovo Med. Inst., 110: 185-186 (in Russian).
EJAM-BERDJEV, A. K. & ATAEV, B. A. (1973) [Effect of toxic factors
with low intensity on the functional state of the CNS of workers
engaged in cast-iron moulding.] Zdravoohr. Turkm., 10: 24-34
(in Russian).
EKBLOM, B. & HUOT, R. (1972) Response to submaximal and maximal
exercise at different levels of carboxyhaemoglobin. Acta Physiol.
Scand, 86: 474-482.
ENGEL, R. R., RODKEY, F. L., O'NEAL, J. D., & COLLISON, H. A. (1969)
Relative affinity of human fetal hemoglobin for carbon monoxide
and oxygen. Blood, 33: 37-45.
ENGEL, R. R., RODKEY, F. L., & KRILL, C. E., JR (1971)
Carboxyhemoglobin levels as an index of hemolysis. Pediatrics,
47: 723-730.
ENVIRONMENT AGENCY (1973) [Air Pollution in Japan. Air monitoring
data in fiscal year 1972.] Tokyo, Air Pollution Control
Division, Air Quality Bureau, pp. 206-210 (in Japanese).
FLETCHER, C. M. & HORN, D. (1970) Smoking and health. Chron. World
Health Organ., 24: 345-370.
FODOR, G. G. & WINNEKE, G. (1971) [Different affinity coefficients of
CO to hemoglobin for man and animals.] Zbl. Bakter. Parasit.
Infekt. Hyg., 224(1): 15 (in German).
FODOR, G. G. & WINNEKE, G. (1972) Effect of low CO concentrations on
resistance to monotony and on psychomotor capacity. Staub-
Reinhalt. Luft, 32(4): 46-54.
FORBES, W. H., DILL, D. B., DESILVA, H., & VAN DEVENTER, F. M. (1937)
Influence of moderate carbon monoxide poisoning upon ability to
drive automobiles. J. ind. Hyg. Toxicol., 19: 598-603.
FORBES, W. H., SARGENT, F., & ROUGHTON, F. J. W. (1945) The rate of
carbon monoxide uptake by normal men. Am. J. Physiol.,
143: 594-608.
FORSTER, R. E. (1970) Carbon monoxide and the partial pressure of
oxygen in tissue. Ann. NY Acad. Sci., 174: 233-241.
FRANKENHAEUSER, M., MYRSTEN, A.-L., POST, B., & JOHANSSON, G. (1971)
Behavioral and physiological effects of cigarette smoking in a
monotonous situation. Psychopharmacologia (Berl.), 22: 1-7.
GADASKINA, I. D. (1960) [Chronic poisoning by carbon monoxide and the
methods required for the experimental study of this problem.] In:
Letaveta, A. A. & Kanarevskoj, A. A., ed. Industrial Toxicology,
Moscow, Akademija Medicinskij Nauk SSSR, Institut Gigieny Truda I
Profzabolevanij, pp. 249-255 (in Russian).
GLINER, J. A., RAVEN, P. B., HORVATH, S. M., DRINKWATER, B. L., &
SUTTON, J. C. (1975) Man's physiologic response to long-term work
during thermal and pollutant stress. J. appl. Physiol.,
39: 628-632.
GODIN, G. & SHEPHARD, R. J. (1972) On the course of carbon monoxide
uptake and release. Respiration, 29: 317-329.
GOLDBARG, A. N., KRONE, R. J., & RESNEKOV, L. (1971) Effects of
cigarette smoking on hemodynamics at rest and during exercise. I.
Normal subjects. Chest, 60: 531-536.
GOLDMAN, A., MURCRAY, D. G., MURCRAY, F. H., WILLIAMS, W. J, BROOKS,
J. N., & BRADFORD, C. M. (1973) Vertical distribution of CO in the
atmosphere. J. geophys. Res., 78(24): 5273-5283.
GOLDSMITH, J. R. (1970) Contribution of motor vehicle exhaust,
industry and cigarette smoking to community carbon monoxide
exposures. Ann. NY Acad. Sci, 174: 122-134.
GOLDSMITH, J. R. & ARONOW, W. S. (1975) Carbon monoxide and coronary
heart disease: A review. Environ. Res., 10: 236-248.
GOLDSMITH, J. R. & LANDAW, S. A. (1968) Carbon monoxide and human
health. Science, 162: 1352-1359.
GOLDSMITH, J. R, TERZAGHI, J., & HACKNEY, J. D. (1963) Evaluation of
fluctuating carbon monoxide exposures. Arch. environ. Health,
7: 647-663.
GORBATOV, O. & NORO, L. (1948) On acclimatization in connection with
acute carbon monoxide poisonings. Acta Physiol. Scand.,
15: 77-87.
GORDON, G. S. & ROGERS, R. L. (1969) A report of medical findings of
project carbon monoxide, Washington, DC, Merkle Press.
GORI, G. B. (1976) Low-risk cigarettes: a prescription. Science,
194: 1243-1246.
GORODINSKY, S. M., SEDOV, A. V., MAZIN, A. N., GAZIEV, G. A,
KLEPTSOVA, A. P, & ZUKOVA, L. I. (1970) [Effect of reduced
barometric pressure on the elimination of gaseous and volatile
metabolites in man isolated in a diving suit.] Kosm. Biol. Med.,
2: 78-82 (in Russian).
GORSKI, J. (1962) [The ballistocardiogram in acute carbon-monoxide
poisoning.] Pol. Tyg. Lek., 17: 872-876 (in Polish).
GÖTHE, C. J., FRISTEDT, B., SUNDELL, L., KOLMODIN, B., EHRNER-SAMUEL,
H., & GÖTHE, K. (1969) Carbon monoxide hazard in city traffic-an
examination of traffic policemen in three Swedish towns. Arch.
environ. Health, 19: 310-314.
GRAHN, D. & KRATCHMAN, J. (1963) Variation in neonatal death rate and
birth weight in the United States and possible relations to
environmental radiation, geology and altitude. Am. J. hum.
Genet., 15: 329-352.
GRANDSTAFF, N. W., BEARD, R. R., & MORANDI, A. J. (1975) Review of
the neurobehavioral effects of carbon monoxide on humans,
Stanford, CA, Stanford University School of Medicine, 74 pp.
(USEPA Contract No. BU75 B001.)
GRIGOROV, L., KAZASOV, P., KURCATOVA, G., BALABAEVA, L., PAVLITZIAN,
T, VACEV, D., JAKIMOV, E., PETROVA, G., MAKSIMOVA, F., TZVETKOV,
T., BLASKOVA, D., DJOLOV, G., & Prof. BRESKOV. (1968) [Study of
the atmospheric air pollution in the region of the thermo-
electrical central "Maritza-Iztok", meteorological conditions and
their effects on the health of the population.] Izvestija NIHI,
3: 33 (in Bulgarian).
GROLL-KNAPP, E., WAGNER, H., HAUCK, H., & HAIDER, M. (1972) Effects of
low carbon monoxide concentrations on vigilance and computer-
analyzed brain potentials. Staub-Reinhalt. Luff, 32 (4): 64-68.
GROLL-KNAPP, E., HAIDER, M., HOLLER, H., JENKER, G., NEUBERGER, M., &
STIDL, H. (1976) Neuro- and psychophysiological effects of
moderate CO-doses. In: Fourth International Congress on Event
Related Slow Potentials of the Brain, Hendersonville, NC, April
4-10, 1976, Research Triangle Park, NC, US Environmental
Research Agency, 9 pp.
GRUT, A. (1949) Chronic carbon monoxide poisoning: a study in
occupational medicine, Copenhagen, Ejnar Munksgaard, 229 pp.
GUEST, A.D. L., DUNCAN, C., & LAWTHER, P. J. (1970) Carbon monoxide
and phenobarbitone: a comparison of effects on auditory flutter
fusion threshold and critical flicker fusion threshold.
Ergonomics (Lond.), 13(5): 587-594.
HAAGEN-SMIT, A. J. (1966) Carbon monoxide levels in city driving.
Arch. environ. Health, 12: 548-551.
HACKNEY, J. D, LINN, W. S., LAU, D.C., KARUZA, S. K., GREENBERG, H.,
BUCKLEY, R. D., & PEDERSEN, E. E. (1975) Experimental studies on
human health effects of air pollutants. 3. Two hour exposure to
ozone alone and in combination with other pollutant gases. Arch.
environ. Health, 30: 385-390.
HAEBISCH, H. (1970) [The cigarette as a source of carbon monoxide.]
Arch. Toxikol., 26: 251-261 (in German).
HÄGGENDAL, E., NILSSON, N.J., & NORBACH, B. (1966) Effect of blood
corpuscle concentration on cerebral blood flow. Acta Chir. Scand.
(Suppl.), 364: 3-12.
HALDANE, J. S. & PRIESTLEY, J. G. (1935) Respiration, New Haven,
Yale University Press, 493 pp.
HALPERIN, M. H., MCFARLAND, R. A., NIVEN, J. I., & ROUGHTON, F. J. W.
(1959) The time course of the effects of carbon monoxide on visual
thresholds. J. Physiol., 146: 583-593.
HAMMOND, E. C. (1962) The effects of smoking. Sci. Am., 207: 3-16.
HARKE, H. P. (1975) [Passive smokers and transport vehicles.]
Arbeitsmed. Sozialmed. Präventivmed., 10: 240-241 (in German).
HELLUNG-LARSEN, P., LAURSEN, T., KJELDSEN, K., & ASTRUP, P. (1968)
Lactate dehydrogenase isoenzymes of aortic tissue in rabbits
exposed to carbon monoxide. J. Atheroscler. Res., 8: 343-349.
HELMCHEN, H. & KUNKEL, H. (1964) [Findings concerning rhythmic after-
oscillations at optically evoked responses in EEG of man.] Arch.
Psychiatr. Z. Neurol., 205: 397-408 (in German).
HERSCH, P. (1964) Galvanic analysis. In: Reilley, C. A., ed. Advances
in analytical chemistry and instrumentation, New York,
Interscience Publishers, Vol. 3, pp. 183-249.
HERSCH, P. (1966) Process for measuring the carbon monoxide content of
a gas stream. Off. Gaz. US Pat. Off., 827(4): 1276-1277.
HEXTER, A. C. & GOLDSMITH, J. R. (1971) Carbon monoxide: association
of community air pollution with mortality. Science,
172: 265-267.
HINDS, W. C. & FIRST, M. W. (1975) Concentrations of nicotine and
tobacco smoke in public places. N. Engl. J. Med., 292
(16): 844-845.
HOFFMAN, D. & WYNDER, E. L. (1972) Smoke of cigarettes and little
cigars: an analytical comparison. Science, 178: 1197-1199.
HOGAN, G. K., SMITH, R. O., & CORNISH, H. H. (1976) Studies on the
microsomal conversion of dichloromethane to carbon monoxide.
Toxicol. appl. Pharmacol., 37: 112.
HORVATH, S. M. (1973) Effects of carbon monoxide on human behaviour.
In: Proceedings of the Conference on Health Effects of Air
Pollutants, 3-5 October, 1973, Washington, DC, National Academy
of Sciences, pp. 127-139 (Serial No. 93-15).
HORVATH, S. M. & ROUGHTON, F. J. W. (1942) Improvements in the
gasometric estimation of carbon monoxide in blood. J. biol.
Chem., 144: 747-755.
HORVATH, S. M., DAHMS, T. E., & O'HANLON, J. F. (1971) Carbon monoxide
and human vigilance: a deleterious effect of present urban
concentrations. Arch. environ. Health, 23: 343-347.
HORVATH, S. M., RAVEN, P. B., DAHMS, T. E., & GRAY, D. J. (1975)
Maximal aerobic capacity at different levels of carboxyhemoglobin.
J. appl. Physiol., 38: 300-303.
HOSKO, M. J. (1970) The effect of carbon monoxide on the visual evoked
response in man. Arch. environ. Health, 21: 174-180.
INGERSOLL, R. B., INMAN, R. E., & FISHER, W. R. (1974) Soil's
potential as a sink for atmospheric carbon monoxide. Tellus,
XXVI(1-2): 151-159.
JAFFE, L. S. (1973) Carbon monoxide in the biosphere: sources,
distributions and concentrations. J. geophys. Res.,
67: 5293-5305.
JENKINS, C. E. (1932) The haemoglobin concentration of workers
connected with internal combustion engines. J. Hyg.,
32: 406-408.
JOHNSON, C. J., MORAN, J. C., PAINE, S.C., ANDERSON, H. W., & BREYSSE,
P. A. (1975) Abatement of toxic levels of carbon monoxide in
Seattle ice-skating rinks. Am. J. public Health, 65: 1087-1090.
JONES, J. G. & WALTERS, D. H. (1962) A study of carboxyhaemoglobin
levels in employees at an integrated steelworks. Ann. occup.
Hyg., 5: 221-230.
JONES, R. A., STRICKLAND, J. A., STUNKARD, J. A., & SIEGEL, J. (1971)
Effects on experimental animals of long-term inhalation exposure
to carbon monoxide. Toxicol. appl. Pharmacol., 19: 46-53.
JONES, R. D., COMMINS, B. T., & CERNIK, A. A. (1972) Blood lead and
carboxyhaemoglobin levels in London taxi drivers. Lancet,
2: 302-303.
JUNGE, C., SEILER, W., & WARNECK, P. (1971) The atmospheric 12CO and
14CO budget. J. geophys. Res., 76(12): 2866-2879.
KAHN, A., RUTLEDGE, R. B., DAVIS, G. L., ALTES, J. A., GANTNER, G. E.,
THORNTON, C. A., & WALLACE, N. D. (1974) Carboxyhemoglobin sources
in the metropolitan St. Louis population. Arch. environ. Health,
29: 127-135.
KANDALL, S. R., LANDAW, S. A., & THALER, M. M. (1973) Carboxyhemo-
globin exchange between donors and recipients of blood
transfusions. J. Pediatr., 52: 716-718.
KILLICK, E. M. (1940) Carbon monoxide anoxemia Physiol. Rev.,
20(3): 313-344.
KJELDSEN, K. (1969) Smoking and atherosclerosis. Investigations on
the significance of the CO content in tobacco smoke in
atherogenesis, Copenhagen, Munksgaard, 142 pp.
KJELDSEN, K., THOMSEN, H. K., & ASTRUP, P. (1974) Effects of carbon
monoxide on myocardium. Circ. Res., 34: 339-348.
KLAUSEN, K., RASMUSSEN, B., GJELLEROD, H., MADSEN, H., & PETERSEN, E.
(1968) Circulation, metabolism and ventilation during prolonged
exposure to carbon monoxide and to high altitude. Scand. J. Clin.
Lab. Invest., 22 (Suppl. 103): 26-38.
KLIMISCH, H. J., CHEVALIER, H. J., HARKE, H. P., & DONTENWILL, W.
(1975) Uptake of carbon monoxide in blood of miniature pigs and
other mammals. Toxicology, 3: 301-310.
KODAT, V. ( 1971) Excretion of CO by workers on generatory station,
Candidate dissertation, Prague, 129 pp.
KOMATSU, E. (1959) [Shinshu myocardosis-a type of myocardosis caused
by carbon monoxide.] In: [Proceedings of the 15th General
Assembly of the Japan Medical Congress, Tokyo, Japan, 1-5 April,
1959.] pp. 343-348 (in Japanese).
KRONE, R. J., GOLDBARG, A. N., BALKOURA, M., SCHUESSLER, R., &
RESNEKOV, L. (1972) Effects of cigarette smoking at rest and
during exercise. II. Role of venous return. J. appl. Physiol,
32: 745-748.
KROTOVA, E. I. & MUZYKA, V. I. (1974) [Content of d-amino-levulinic
acid in erythrocytes of workers having contact with CO.] Gig. Tr.
i Prof. Zabol., 6: 43-44 (in Russian).
KUBIC, V. L., ANDERS, M. W., ENGEL, R. R., BARLOW, C. H., & CAUGHEY,
W. S. (1974) Metabolism of dihalomethanes to carbon monoxide.
Drug Metab. Disposition, 2(1): 53-57.
KULLER, L. H., RADFORD, E. P., SWIFT, D, PERPER, J., & FISHER, R.
(1975) Carbon monoxide and heart attacks. Arch. environ. Health,
30: 477-482.
KUSTOV, V. V., BELKIN, V. I., ABIDIN, B. I., OSTAPENKO, O. F.,
MALKUTA, A. N., & PODDUBNAJA, L. T. (1972) [Aspects of chronic
carbon monoxide poisoning in young animals.] Gig. Tr. i Prof
Zabol., 5: 50-52 (in Russian).
LANDAW, S. A. (1973) The effects of cigarette smoking on the total
body burden and excretion rates of carbon monoxide J. occup.
Med., 15(3): 231-240.
LAZAREV, N. V., ed. (1965) [Carbon monoxide.] In: [Toxic substances
in industry.] Vol. 2, pp. 198-218 (in Russian).
LEE, R. E. (1972) Indoor-outdoor carbon monoxide pollution study,
Washington, DC, US Environmental Protection Agency, 408 pp.
(EPA-R4-73-020).
LEVY, H. H. (1972) Photochemistry of the lower troposphere planet.
Space Sci., 20: 919-935.
LEWEY, F. H. & DRABKIN, D. L. (1944) Experimental chronic carbon
monoxide poisoning of dogs. Am. J. med. Sci., 208: 502-511.
LILIENTHAL, J. L., JR & FUGITT, C. H. (1946) The effect of low
concentrations of carboxyhemoglobin on the "altitude tolerance" of
man. Am. J. Physiol., 145: 359-364.
LILY, R. E. C., COLE, P. V., & HAWKINS, L. H. (1972)
Spectrophotometric measurements of carboxyhaemoglobin. Br. J.
ind. Med., 29: 454-457.
LINDENBERG, R., PREZIOSI, T., LEVY, D., & CHISTENSEN, M. K. (1961)
Experimental investigation of carbon monoxide toxicity. Presented
at: The Air Pollution Medical Research Conference, Los Angeles,
CA, 4-7 December, 1961.
LINNENBOM, V. J., SWINNERTON, J. W., & LAMONTAGNE, R. A. (1973) The
ocean as a source for atmospheric carbon monoxide. J. geophys.
Res., 78(24): 5333-5340.
LISS, P. S. & SLATER, P. G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LJUBLINA, E. I. (1960) [Shifts in some physiological functions of the
animal on subacute and chronic exposure to carbon monoxide.]
In: Letaveta, A. A. & Kanarevskoj, A. A., ed: [Industrial
Toxicology.] Moscow, Akademija Medicinskij Nauk SSSR, Institut
Gigieny Truda i Profzabolevanij, pp. 256-263 (in Russian).
LOGUE, G. L., ROSSE, W. F., SMITH, W. T., SALTZMAN, H. A., &
GUTTERMAN, L. A. (1971) Endogenous carbon monoxide production
measured by gas-phase analysis: an estimation of heme catabolic
rate. J. lab. clin. Med., 77: 867-876.
LONG, K. R. & DONHAM, K. J. (1973) Occupational exposure to CO in
selected rural work environments, Iowa City, IA, Institute of
Agricultural Medicine & Environmental Health, Department of
Preventive Medicine & Environmental Health, University of Iowa.
LONGO, L. D. (1970) Carbon monoxide in the pregnant mother and fetus
and its exchange across the placenta. Ann. NY Acad. Sci.,
174: 313-341.
LONGO, L. D. & HILL, E. P. (1977) Carbon monoxide uptake and
elimination in fetal and maternal sheep. Am. J. Physiol.,
232: H324-H330.
LUOMANMAKI, K. (1966) Studies on the metabolism of carbon monoxide.
Ann. med. exp. Biol. Fenn. (Helsinki), 44 (Suppl. 2): 1-55.
MAAS, A. H. J., HAMELINK, M. L., & DELEEUW, R. J. M. (1970) An
evaluation of the spectrophotometric determination of HbO2,
HbCO, and Hb in blood with the co-oximeter IL 182. Clin. Chim.
Acta, 29: 303-309.
MACMAHON, B., ALPERT, M., & SALBER, E. J. (1966) Infant weight and
parental smoking habits. Am. J. Epidemiol., 82: 247-261.
MALENFANT, A. L., GAMBINO, S. R., WARASKA, A. J., & ROE, E. I. (1968)
Spectrophotometric determination of hemoglobin concentration and
percent oxyhemoglobin and carboxyhemoglobin saturation. Clin.
Chem., 14: 789.
MALINOW, M. R., MCLAUGHLIN, P., DHINDSA, D. S., METCALFE, J., OCHSNER,
A. J., III, HILL, J., & MCNULTY, W. P. (1976) Failure of carbon
monoxide to induce myocardial infarction in cholesterol-fed
cynomolgus monkeys. Cardiovasc. Res., 10: 101-108.
MALORNY, G., MÜLLER-PLATHE, O., & STRAUB, M. (1962) [Disturbance of
acid-base balance in acute carbon monoxide poisoning and effect of
oxygen or carbon dioxide-oxygen mixtures.] Arch. exp. Pathol.
Pharmakol. (Naunyn-Schmiedeberg), 243: 439-458 (in German).
MARKS, E. & SWIECICKI, W. (1971) [Effect of carbon monoxide, vibration
and lowered atmospheric pressure upon cerebral neurosecretory
system and catecholamines level.] Med. Pr., 22: 336-341 (in
Polish).
MARTIN, W. & STERN, A. C. (1974) The world's air quality management
standards. Vol. 1. The air quality management standards of the
world, including United States federal standards, Washington,
DC, US Environmental Protection Agency, 383 pp.
MAZIARKA, S., BORKOWSKA, M., MISIAKIEWICZ, Z., STRUSINSKI, A., &
WYSZYNSKA, H. (1974) [Air pollution in the streets of Warsaw in
relation to surrounding buildings.] Roczn. Pzh., 25:1-5 (in
Polish).
MCCLATCHIE, E. A., COMPHER, A. B., & WILLIAMS, K. Y. (1972) A high
specificity carbon monoxide analyzer. Anal. Instrum. (Symp.),
10: 67-69.
MCCONNELL, J. C., MCELROY, M. B., & WOFSY, S.C. (1971) Natural sources
of atmospheric CO. Nature (Lond.), 233: 187-188.
MCCREDIE, R. M. & JOSE, A.D. (1967) Analysis of blood carbon monoxide
and oxygen by gas chromatography. J. appl. Physiol.,
22: 863-866.
MCFARLAND, R. A. (1973) Low level exposure to carbon monoxide and
driving performance. Arch. environ. Health, 27: 355-359.
MCFARLAND, R. A., ROUGHTON, F. J. W., HALPERIN, M. H., & NIVEN, J. I.
(1944) The effects of carbon monoxide and altitude on visual
thresholds. Aerosp. Med., 15: 381-394.
MCFARLAND, R. A., FORBES, W. H., STOUDT, H. W., DOUGHERTY, A., CROLEY,
T. J., MOORE, R. C., & NALWALK, T. J. (1973) A study of the
effects of low levels of carbon monoxide upon humans performing
driving tasks, Boston, Guggenheim Center of Aerospace Health &
Safety, Harvard School of Public Health, 88 pp.
MCMULLEN, T. B. (1975) Interpreting the eight-hour national ambient
air quality standard for carbon monoxide. J. Air Pollut. Control
Assoc., 25: 1009-1014.
MEHMEL, H. C., DUVELLEROY, M. A., & LAVER, M. B. (1973) Response of
coronary blood flow to pH-induced changes in hemoglobin-O2
affinity. J. appl. Physiol., 35: 485-489.
MIDDLETON, V., VAN POZNAK, A., ARTUSIO, J. F., JR, & SMITH, S. M.
(1965) Carbon monoxide accumulation in closed circle anesthesia
systems. Anesthesiology, 26: 715-719.
MILLS, E. & EDWARDS, M. W., JR (1968) Stimulation of aortic and
carotid chemoreceptors during carbon monoxide inhalation.
J. appl. Physiol., 25: 494-502.
MINISTRY OF LABOUR (1965) Carbon monoxide poisoning causes and
prevention, London, Her Majesty's Stationery Office, 72 pp.
(Safety, Health & Welfare Series No. 29).
MITCHELL, J. H., SPROULE, B. J., & CHAPMAN, C. B. (1958) The
physiological meaning of the maximal oxygen intake test. J. clin.
Invest., 37: 538-547.
MONTGOMERY, M. R. & RUBIN, R. J. (1971) The effect of carbon monoxide
inhalation on in vivo drug metabolism in the rat. J. Pharmacol.
exp. Ther., 179: 465-473.
MOREL, S. & SZEMBERG, T. (1971) [Air pollution in Ladle Bay.] Tech.
Sanit., 45: 353-355 (in Polish).
MULHAUSEN, R. O., ASTRUP, P., & MILLEMGAARD, K. (1968) Oxygen affinity
and acid-base status of human blood during exposure to hypoxia and
carbon monoxide. Scand. J. clin. lab. Invest., 22 (Suppl. 103):
9-15.
MUSSELMAN, N. P., GROFF, W. A., YEVICH, P. P., WILINSKY, F. T., WEEKS,
M. H., & OBERST, F. W. (1959) Continuous exposure of laboratory
animals to low concentration of carbon monoxide. Aerosp. Med.,
30: 524-529.
MYHRE, L. (1974) Rate of CO elimination in resting man. Fed. Proc,
33: 440.
MYRSTEN, A. L., POST, B., FRANKENHAEUSER, M., & JOHANSSON, G. (1972)
Changes in behavioural and physiological activation induced by
cigarette smoking in habitual smokers. Psychopharmacologia
(Berl.), 27: 305-312.
NAS/NRC (1977) Carbon monoxide, Washington, DC, National Academy of
Sciences, 268 pp.
NEW MEXICO STATE DEPARTMENT OF PUBLIC HEALTH (1975) Birth weight and
altitude, pp. 7-16.
NIELSEN, B. (1971) Thermoregulation during work in carbon monoxide
poisoning. Acta physiol. Scand, 82: 98-106.
NOTOV, A. (1959) [Acute poisonings with CO, SO2 and nitric gases in
mines.] Sb. Tr. VMI Plovidiv, 60: 404-411 (in Bulgarian).
NOWARA, M. (1975) [Evaluation of occupational exposure by means of
determining carbon monoxide in the air at work places and carbon
monoxide hemoglobin.] Med. Pr., 26: 351-360 (in Polish).
NUNES, A. C. & SCHOENBORN, B. P. (1973) Dichloromethane and myoglobin
function. Mol. Pharmacol., 9: 835-839.
O'DONNELL, R. D., CHIKOS, P., & THEODORE, J. (1971a) Effect of carbon
monoxide exposure on human sleep and psychomotor performance.
J. appl. Physiol., 31: 513-518.
O'DONNELL, R. D., MIKULKA, P., HEINIG, P., & THEODORE, J. (1971b) Low
level carbon monoxide exposure and human psychomotor performance.
Toxicol. appl. Pharmacol., 18: 593-602.
OGAWA, M, KATSURADA. K., & SUGIMOTO, I. (1974) Pulmonary edema in
acute carbon monoxide poisoning. Int. Arch. Arbeitsmed.,
33: 131-138.
O'HANLON, J. F. (1975) Preliminary studies of the effects of carbon
monoxide on vigilance in man. In: Weiss, B. & Laties, V. G., ed.
Behavioral toxicology, New York, Plenum Press, pp. 61-72.
ORELLANO, T., DERGAL, E., ALIJANI, M., BRIGGS, C., VASQUEZ, J.,
GOLDBAUM, L., & ABSOLON, K. B. (1976) Studies on the mechanism of
carbon monoxide toxicity. J. surg. Res., 20: 485-487.
OTT, W. & MAGE, D. (1974) Interpreting urban carbon monoxide
concentrations by means of a computerized blood COHb model.
J. Air Pollut. Control Assoc., 28(9): 911-916.
PACE, N., STRAJMAN, E., & WALKER, E. L. (1950) Acceleration of carbon
monoxide elimination in man by high pressure oxygen. Science,
111: 652-654.
PANKOW, D. & PONSOLD, W. (1972) Leucine aminopeptidase activity in
plasma of normal and carbon monoxide poisoned rats. Arch.
Toxikol., 29: 279-285.
PANKOW, D. & PONSOLD, W. (1974) [The combined effects of carbon
monoxide and other biologically active detrimental factors on the
organism.] Zeits. Ges. Hyg., 9: 561-571 (in German).
PANKOW, D., PONSOLD, W., & FRITZ, H. (1974a) Combined effects of
carbon monoxide and ethanol on the effects of leucine
aminopeptidase and glutamic-pyruvic transaminase in the plasma of
rats. Arch. Toxicol, 32: 331-340.
PANKOW, D., PONSOLD, W., & GRIMM, I. (1974b) [On the potentiation of
the hepatic toxicity of carbon tetrachloride by carbon monoxide.]
Dtsch. Ges. Wesen., 29: 853-856 (in German).
PECORA, L. J. (1959) Physiologic study of the summating effects of
ethyl alcohol and carbon monoxide. Am. Ind. Hyg. Assoc. J.,
20: 235-240.
PENNEY, D., BENJAMIN, M., & DUNHAM, E. (1974a) Effect of carbon
monoxide on cardiac weight as compared with altitude effects.
J. appl. Physiol, 37: 80-84.
PENNEY, D., DUNHAM, E., & BENJAMIN, M. (1974b) Chronic carbon monoxide
exposure: Time course of hemoglobin, heart weight and lactate
dehydrogenase isozyme changes. Toxicol. appl. Pharmacol,
28: 493-497.
PERMUTT, S. & FAHRI, L. (1969) Tissue hypoxia and carbon monoxide.
In: Effects of chronic exposure to low levels of carbon monoxide
on human health, behavior and performance, Washington, DC,
National Academy of Sciences, National Academy of Engineering,
pp. 18-24.
PETERSON, J. E. & STEWART, R. D. (1970) Absorption and elimination of
carbon monoxide by inactive young men. Arch. environ. Health,
21: 165-171.
PETROV, P. (1968) [Chronic CO intoxications in workers engaged in
loading and unloading processes.] Transp. Med. Vesti, 4: 24-27
(in Bulgarian).
PIRNAY, F., DUJARDIN, J., DEROANNE, R., & PETIT, J. M. (1971) Muscular
exercise during intoxication by carbon monoxide. J. appl.
Physiol., 31: 573-575.
PITTS, G. C. & PACE, N. (1947) The effects of blood carboxyhemoglobin
concentration on hypoxia tolerance. Am. J. Physiol.,
148: 139-151.
PREZIOSI, T. J., LINDENBERG, R., LEVY, D., & CHRISTENSON, M. (1970) An
experimental investigation in animals of the functional and
morphological effects of single and repeated exposures to high and
low concentrations of carbon monoxide. Ann. NY Acad. Sci,
174: 369-384.
PROHOROV, Y. D. & ROGOV, A. A. (1959) [The pathohistological and
histochemical changes in the organs of rabbits subjected to a
prolonged action of carbon monoxide, sulfur dioxide and their
mixtures.] Gig. i Sanit., 24: 22-26 (in Russian).
RAMIREZ, R. G., ALBERT, S. N., AGOSTINI, J. C., BASU, A. P., GOLDBAUM,
L. R., & ABSOLON, K. B. (1974) Lack of toxicity of transfused
carboxyhemoglobin red blood cells and carbon monoxide inhalations.
Surg. Forum, 25: 165-168.
RAMSEY, J. M. (1967a) Carbon monoxide exposures in parking garages.
Bull. environ. Contam. Toxicol., 2: 182-190.
RAMSEY, J. M. (1967b) Carboxyhemoglobinemia in parking garage
employees. Arch. environ. Health, 15: 580-583.
RAMSEY, J. M. (1972) Carbon monoxide, tissue hypoxia, and sensory
psychomotor response in hypoxaemic subjects. Clin. Sci.,
42: 619-625.
RAMSEY, J. M. (1973) Effects of single exposures of carbon monoxide on
sensory and psychomotor response. Am. Ind. Hyg. Assoc. J.,
34: 212-216.
RAMSEY, J. M. & CASPER, P. W., JR (1976) Effect of carbon monoxide
exposures on erythrocyte 2,3-DPG in rabbits. J. appl. Physiol.,
41: 689-692.
RATNEY, R. S., WEGMAN, D. H., & ELKINS, H. B. (1974) In vivo
conversion of methylene chloride to carbon monoxide. Arch.
environ. Health, 28: 223-226.
RAVEN, P. B., DRINKWATER, B. L., HORVATH, S. M., RUHLING, R. O.,
GLINER, J. A., SUTTON, J. C., BOLDUAN, N. W. (1947a) Age, smoking
habits, heat stress and their interactive effects with carbon
monoxide and peroxyacetyl nitrate on man's aerobic power. Int. J.
Biometeorol., 18: 222-232.
RAVEN, P. B., DRINKWATER, B. L., RUHLING, R. O., BOLDUAN, N. W.,
TAGUCHI, S., GLINER, J. A., & HORVATH, S. M. (1974b) Effect of
carbon monoxide and peroxyacetyl nitrate on man's maximal aerobic
capacity. J. appl. Physiol., 36: 288-293.
RAY, A.M. & ROCKWELL, T. H. (1970) An exploratory study of automobile
driving performance under the influence of low levels of
carboxyhemoglobin. Ann. NY Acad. Sci., 174: 396-407.
RENCH, J. D. & SAVAGE, E. P. (1976) Carbon monoxide in the home
environment. J. environ. Health, 39: 104-106.
RISSANEN, V., PYÖRÄLÄ, K., & HEINONEN, O. P. (1972) Cigarette smoking
in relation to coronary and aortic atherosclerosis. Acta pathol.
microbiol. Scand. Sect. A, 80: 491-500.
ROBBINS, R. C., BORG, K. M., & ROBINSON, E. (1968) Carbon monoxide in
the atmosphere. J. Air Pollut. Control Assoc., 18: 106-110.
ROBINSON, J. C. & FORBES, W. F. (1975) The role of carbon monoxide in
cigarette smoking. Arch. environ. Health, 30: 425-434.
ROBINSON, E. & ROBBINS, R. C. (1970) Atmospheric background
concentrations of carbon monoxide. Ann. NY Acad. Sci.,
174: 89-95.
RONDIA, D. (1970) Abaissement de l'activité de la benzopyrene-
hydroxylase hepatique in vivo apres inhalation d'oxyde de
carbone. C. R. Acad. Sci. (Paris), 271: 617-619.
ROOT, W. S. (1965) Carbon monoxide. In: Fenn, W. O. & Rahn, H., ed.
Handbook of physiology, respiration 2, Washington, DC, American
Physiological Society, pp. 1087-1098.
ROSSI-BERNARDI, L., PERRELLA, M., LUZZANA, M., SAMAJA, M., & RAFFAELE,
I. (1977) Simultaneous determination of hemoglobin derivatives,
oxygen content, oxygen capacity, and oxygen saturation in 10 µl of
whole blood. Clin. Chem., 23: 1215-1225.
ROSSI-FANELLI, A. & ANTONINI, E. (1958) Studies on the oxygen and
carbon monoxide equilibria of human myoglobin. Arch. Biochem.
Biophys., 77: 478-492.
ROTH, R. P., DREW, R. T., Lo, R. J., & FOUTS, J. R. (1975)
Dichloromethane inhalation, carboxyhemoglobin concentrations, and
drug metabolizing enzymes in rabbits. Toxicol. appl. Pharmacol.,
33: 427-437.
ROUGHTON, F. J. W. (1970) The equilibrium of carbon monoxide with
human hemoglobin in whole blood. Ann. NY Acad. Sci.,
174: 177-188.
ROUGHTON, F. J. W. & DARLING, R. C. (1944) The effect of carbon
monoxide on the oxyhemoglobin dissociation curve. Am. J.
Physiol., 141: 17-31.
ROUGHTON, F. J. W. & ROOT, W. S. (1945) The estimation of small
amounts of carbon monoxide in blood. J. biol. Chem.,
160: 123-133.
RUMMO, N. & SARLANIS, K. (1974) The effect of carbon monoxide on
several measures of vigilance in a simulated driving task.
J. Saf Res., 6: 126-130.
RUSSELL, M. A. H., COLE, P. V., & BROWN, E. (1973) Absorption by non-
smokers of carbon monoxide from room air polluted by tobacco
smoke. Lancet, 1: 576-579.
RYLANDER, R. (1974) Environmental tobacco smoke effects on the non-
smoker. Report from a workshop. Scand J. resp. Dis.,
(Suppl. 91): 90 pp.
RYLOVA, M. L. (1960) [The application of integral methods of
investigation to determine the effect of repeated exposure to
carbon monoxide in animals.] In: Letaveta, A. A. & Kanarevskoj, A.
A., ed. [Industrial toxicology.] Moscow, Akademija Medicinskij
Nauk SSSR, Institut Gigieny Truda i Profzabolevanij, pp. 264-270
(in Russian).
SAMMONS, J. H. & COLEMAN, R. L. (1974) Firefighters' occupational
exposure to carbon monoxide. J. occup. Med., 16: 543-546.
SANTIAGO, T. V. & EDELMAN, N. H. (1972) Effect of anemia on the
ventilatory response to transient hypoxia. Fed. Proc.,
31: 371 (Abstract No. 864).
SARMA, J. S. M., TILLMANS, H., IKEDA, S., & BINS, R. J. (1975) The
effect of carbon monoxide on lipid metabolism of human coronary
arteries. Atherosclerosis, 22: 193-198.
SAYERS, R. R., YANT, W. P., LEVY, E., & FULTON, W. B. (1929) Effect
of repeated daily exposure of several hours to small amounts of
automobile exhaust gas, Washington, DC, Government Printing
Office, 49 pp. (Public Health Bull. No. 186).
SCHULTE, J. H. (1963) Effects of mild carbon monoxide intoxication.
Arch. environ. Health, 7: 524-530.
SEDOV, A. V., ZUKOVA, L. I., & MAZNEVA, G. E. (1971) [Carbon monoxide
excretion by the human organism.] Gig. Tr. i Prof Zabol.,
9: 36-39 (in Russian).
SEILER, W. (1975) The cycle of carbon monoxide in the atmosphere.
In: International Conference on Environmental Sensing and
Assessment, Las Vegas, NV, 14-19 September, New York, Institute
of Electrical and Electronics Engineers, Vol. 2, 6 pp.
SEILER, W. & JUNGE, C. E. (1969) Decrease of carbon monoxide mixing
ratio above the polar tropopause. Tellus, 21(3): 447-449.
SEILER, W. & JUNGLE, C. E. (1970) Carbon monoxide in the atmosphere.
J. geophys. Res., 75(12): 2217-2226.
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA,. M. A., & TARASOVA, L. N.
(1970) [New data on the concentration of the products of the
incomplete combustion of domestic gas in the air of premises
provided with a gas supply.] In: [Problems of current interest in
hygiene.] Moscow, Akademija Medicinskij Nauk SSSR, Institute of
Genetics and Community Hygiene, pp. 137-142 (in Russian).
SIDORENKO, G. I., LAMPERT, F. F., PINIGINA, M. A., KLEBANOVA, V. A.,
OSINCEVA, V. P., & NAZARENKO, A. F. (1972) [The experimental basis
for measures to improve the quality of the air in flats provided
with a gas supply.] Gig. i Sanit., 7: 24-28 (in Russian).
SIEGEL, S. M., RENWICK, G., & ROSEN, L. A. (1962) Formation of carbon
monoxide during seed germination and seedling growth. Science,
137: 683-684.
SJÖSTRAND, T. (1948) A method for the determination of
carboxyhaemoglobin concentrations by analysis of the alveolar
air. Acta Physiol. Scand., 16: 201-210.
SMALL, K. A., RADFORD, E. P., FRAZIER, J. M., RODKEY, F. L., &
COLLISON, H. A. (1971) A rapid method for simultaneous measurement
of carboxy- and methemoglobin in blood. J. appl. Physiol.,
31: 154-160.
SOFOLUWE, G. O. (1968) Smoke pollution in dwellings of infants with
bronchopneumonia. Arch. environ. Health, 16: 670-672.
SOKAL, J. A. (1975) Lack of the correlation between biochemical
effects on rats and blood carboxyhemoglobin concentrations in
various conditions of single acute exposure to carbon monoxide.
Arch. Toxicol, 34: 331-336.
SOTNIKOV, E. E. (1971) [Determination of carbon monoxide in the blood
by a gas chromatographic method.] Gig. i Sanit., 6: 61-64 (in
Russian).
SPEDDING, D. J. (1974) Air pollution, Oxford, Clarendon Press,
pp. 58-62.
SRCH, M. (1967) [The significance of carbon monoxide in cigarette
smoke in passenger car interiors.] Dtsch. Z. ges. gerichtl. Med,
60: 80-89 (in German).
STEVENS, C. M., KROUT, L., WALLING, D., VENTERS, A., ENGELKEMEIR, A.,
& Ross, L. E. (1972) The isotopic composition of atmospheric
carbon monoxide. Earth planet. Sci. Lett, 16: 147-165.
STEWART, R. D. & HAKE, C. L. (1976) Paint remover hazard. J. Am. Med.
Assoc., 235: 398-401.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. C. (1972a) Experimental human exposure to
methylene chloride. Arch. environ. Health, 26: 342-348.
STEWART, R. D., FISHER, T. N., HOSKO, M. J., PETERSON, J. E., BARETTA,
E. D., & DODD, H. D. (1972b) Carboxyhemoglobin elevation after
exposure to dichloromethane. Science, 176: 295-296.
STEWART, R. D., NEWTON, P. E., HOSKO, M. J., & PETERSON, J. E. (1973a)
Effect of carbon monoxide on time perception. Arch. environ.
Health, 27: 155-160.
STEWART, R. D., PETERSON, J. E., FISHER, T. N., HOSKO, M. J., BARETTA,
E. D., DODD, H. C., & HERRMANN, A. A. (1973b) Experimental human
exposure to high concentrations of carbon monoxide. Arch.
environ. Health, 26: 1-7.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWARD, E. D.,
KALBFLEICH, J. H., VAN YSERLOO, B., & RIMM, A. A. (1973c)
Carboxyhemoglobin concentrations in blood from donors in Chicago,
Milwaukee, New York, and Los Angeles. Science, 182: 1362-1364.
STEWART, R. D., BARETTA, E. D., PLATTE, L. R., STEWART, E. D.,
KALBFLEICH, J. D., VAN YSERLOO, B., & RIMM, A. A. (1974)
Carboxyhaemoglobin tests in American blood donors. JAMA,
229: 1187-1195.
STEWART, R. D, NEWTON, P. E., HOSKO, M. J., PETERSON, J. E., &
MELLENDER, J. W. (1975) The effect of carbon monoxide on time
perception, manual coordination, inspection, and arithmetic.
In: Weiss, B. & Laties, V. G., ed. Behavioral toxicology, New
York, Plenum Press, pp. 29-60.
STEWART, R. D., HAKE, C. L., WU, A., STEWART, T. A., & KALBFLEISCH, J.
H. (1976) Carboxyhemoglobin trend in Chicago blood donors,
1970-1974. Arch. environ. Health, 31: 280-286.
STUPFEL, M. (1975) Rhythms and air pollution. Chronobiologia,
11: 105-115.
STUPFEL, M. & BOULEY, G. (1970) Physiological and biochemical effects
on rats and mice exposed to small concentrations of carbon
monoxide for long periods. Ann. NY Acad. Sci. 174: 342-368.
STUPFEL, M., MAGNIER, M., & ROMARY, F. (1973) Rythme circadien de
l'action de l'oxide de carbone sur l'emission du gaz carbonique
par le rat en groupe. C. R. Acad. Sci. (Paris) Série D,
276: 1009-1012.
SUL'GA, T. M. (1962) [New data for the hygienic evaluation of CO in
air.] In: Ryazanov, V. A., ed. [ Maximal permissible
concentrations of air pollution.] Moscow, Medgiz, pp. 128-145
(in Russian).
SWIECICKI, W. (1973) [The effect of vibration and physical training on
carbohydrate metabolism in rats intoxicated with carbon monoxide.]
Med. Pr., 34: 399-405 (in Polish).
SWINNERTON, J. W., LAMONTAGNE, R. A., & LINNENBOM, V. J. (1971) Carbon
monoxide in rain water. Science, 172: 943-945.
TANAKA, M. (1965) [Studies on the etiological mechanism of fetal
development disorders caused by maternal smoking habits during
pregnancy.] J. Jpn. Obstet. Gynecol. Soc., 17: 1107-1114 (in
Japanese, English translation in J. Obstet. Gynecol. Soc.,
14: 45 (1967)).
THOMSEN, H. K. (1974) Carbon monoxide-induced artherosclerosis in
primates. Atherosclerosis, 20: 233-240.
THOMSEN, H. K. & KJELDSEN, K. (1974) Threshold limit for carbon
monoxide-induced myocardial damage. Arch. environ. Health,
29: 73-79.
THOMSEN, H. K. & KJELDSEN, K. (1975) Aortic intimal injury in rabbits.
Arch. environ. Health, 30: 604-607.
TIUNOV. A. & KUSTOV, V. V. (1969) [Toxicology of carbon monoxide.]
Leningrad, Medicina, pp. 81-217 (in Russian).
TRAYSTMAN, R. J. (1976) Effects of carbon monoxide hypoxia and hypoxic
hypoxia on cerebral circulation. In: Fourth International
Congress on Event Related Slow Potentials of the Brain,
Hendersonville, NC, April 4-10, 1976, Research Triangle Park,
NC, US Environmental Protection Agency, 9 pp.
TROMPEO, G., TURLETTl, G., & GIARRUSSO, O. T. (1964) [Concentration of
carbon monoxide in underground garages.] Rass. Med. Ind.,
33: 392-393 (in Italian).
TUMASONIS, C. F. & BAKER, F. D. (1972) Influence of carbon monoxide
upon some respiratory enzymes of the chick embryo. Bull. environ.
Contain. Toxicol., 8: 113-119.
TURNER, J. A. McM., SILLETT, R. W., & BALL, K. P. (1974) Some effects
of changing to low-tar and low-nicotine cigarettes. Lancet,
2:737-739.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1970) Air quality
criteria for carbon monoxide, Washington, DC, US DHEW Public
Health Service, National Air Pollution Control Administration.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1972) Criteria for a
recommended standard. Occupational exposure to carbon monoxide,
Washington, DC, US DHEW Health Services and Mental Health,
National Institute for Occupational Safety and Health, 120 pp.
US DEPARTMENT OF HEALTH, EDUCATION AND WELFARE (1973) The Health
Consequences of Smoking, Washington, DC, US DHEW, Public Health
Service, pp. 367-381.
US ENVIRONMENTAL PROTECTION AGENCY (1973a) The national air
monitoring program: Air quality and emission trends, Annual
report, Vol. 1, Research Triangle Park, NC, USEPA, 182 pp.
(EPA 450/1-73-001-A).
US ENVIRONMENTAL PROTECTION AGENCY (1973b) Compilation of air
pollution emission factors 2nd ed., Research Triangle Park, NC,
USEPA, 288 pp. (Report No. AP42).
US ENVIRONMENTAL PROTECTION AGENCY (1975) Human health damages from
mobile source air pollution: A delphic study, Research Triangle
Park, NC, USEPA (Project No. 07AAC-OS).
VAN KAMPEN, E. J. & ZIJLSTRA, W. G. (1961) Standardization of
hemoglobinometry. II. The hemiglobincyanide method. Clin. Chim.
Acta, 6: 538-544.
VINOGRADOV, G. I., CERNICENKO, I. A., & MAKARENKO, E. M. (1974) [The
allergenic activity of motor vehicle gases.] Gig. i Sanit.,
8: 10-13 (in Russian).
VOGEL, J. A. & GLESER, M. A. (1972) Effect of carbon monoxide on
oxygen transport during exercise. J. appl. Physiol.,
32: 234-239.
VOGEL, J. A., GLESER, M. A., WHEELER, R. C., & WHITTEN, B. K. (1972)
Carbon monoxide and physical work capacity. Arch. environ.
Health, 24: 198-203.
VOLLMER, E. P., KING, B. G, BIRREN, J. E., & FISHER, M. B. (1946) The
effects of carbon monoxide on three types of performance at
simulated altitudes of 10,000 and 15,000 feet. J. exp. Psychol.,
36: 244-251.
VYSKOCIL, J. (1957) Neurohumoral regulation in acute and chronic
carbon monoxide poisoning. Int. Arch. Gewerbepathol. Gewerbehyg.,
15: 457-472.
WAGEMANN, W. (1960) [The otological picture in carbon monoxide
poisoning.] Z. Laringol. Rhinol. Otol., 39: 691-708 (in German).
WAGNER, J. A., HORVATH, S. M., & DAHMS, T. E. (1975) Carbon monoxide
elimination. Respir. Physiol, 23: 41-47.
WAKEHAM, H. R. R. (1976) Environmental carbon monoxide from cigarette
smoking, Sixth International Tobacco Scientific Congress, Tokyo,
Japan, November 18, 1976, 17 pp.
WALD, N. & HOWARD, S. (1975) Smoking, carbon monoxide and arterial
disease. Ann. occup. Hyg., 18: 1-14.
WALD, N., HOWARD, S., SMITH, P. G., & KJELDSEN, K. (1973) Association
between atherosclerotic diseases and carboxyhaemoglobin levels in
tobacco smokers. Br. med. J., 1: 761-765.
WALLACE, N. D., DAVIS, G. L., RUTLEDGE, R. B., & KAHN, A. (1974)
Smoking and carboxyhemoglobin in the St. Louis metropolitan
population. Arch. environ. Health, 29: 136-142.
WALLER, R. E., COMMINS, B. T., & LAWTHER, P. J. (1965) Air pollution
in a city street. Br. J. ind. Med., 22: 128-138.
WEBSTER, W. S., CLARKSON, T. B., & LOFLAND, H. B. (1968) Carbon
monoxide-aggravated atherosclerosis in the squirrel monkey. Exp.
mol. Pathol., 13: 36-50.
WEINSTOCK, B. & NIKI, H. (1972) Carbon monoxide balance in nature.
Science, 176: 290-292.
WEIR, F. W. & ROCKWELL, T. H. (1973) An investigation of the effects
of carbon monoxide on humans in the driving task, Columbus, OH,
Ohio State University Research Foundation, 170 pp. (Contract No.
68-02-0329 and CRC-APARC Project CAPM-9-69).
WEISS, H. R. & COHEN, J. A. (1974) Effects of low levels of carbon
monoxide on rat brain and muscle tissue pO2. Environ.
Physiol. Biochem., 4: 31-49.
WELLS, L. L. (1933) The prenatal effect of carbon monoxide on albino
rats and the resulting neuropathology. Biologist, 15: 80-81.
WESTBERG, K, COHEN, N., & WILSON, K. W. (1971) Carbon monoxide: its
role in photochemical smog formation. Science, 171: 1013-1015.
WESTLAKE, D. W., ROXBURGH, J. M., & TALBOT, G. (1961) Microbial
production of carbon monoxide from flavonoids. Nature (Lond.),
189: 510-511.
WHITE, P. (1970) Carbon monoxide production and heme catabolism. Ann.
NY Acad. Sci., 174: 23-31.
WILKS, S. S. (1959) Carbon monoxide in green plants. Science,
129: 964-966.
WILKS, S. S., TOMASHEFSKI, J. F., & CLARKE, R. T., JR (1959)
Physiological effects of chronic exposure to carbon monoxide.
J. appl. Physiol., 14: 305-310.
WILLIAMS, I. R. & SMITH, E. (1935) Blood picture, reproduction, and
general condition during daily exposure to illuminating gas.
Am. J. Physiol., 110: 611-615.
WINNEKE, G. (1974) Behavioral effects of methylene chloride and carbon
monoxide as assessed by sensory and psychomotor performance. In:
Xintaras, C., Johnson, B., & deGroot, I., ed. Behavioral
toxicology: early detection of occupational hazards, Washington,
DC, US Government Printing Office, pp. 130-144 (New Publ. No.
N10511).
WINNEKE, G. (1977) [Studies on marginal values for carbon monoxide.]
Lufthyg. Silkoseforsch., 10: 75-90 (in German).
WINNEKE, G., FODOR, G. G., & SCHLIPKÖTER, H. W. (1976) Effects of low
level carbon monoxide exposure and monotony on vigilance and
vertex evoked potentials. In: Proceedings of the fourth
international congress on event-related slow potentials of the
brain. Hendersonville, NC, April 4-10, 1976, Research Triangle
Park, NC, US Environmental Protection Agency, 5 pp.
WOLFE, D. G. & BIDLACK, W. R. (1976) The formation of carbon monoxide
during peroxidation of microsomal lipids. Biochem. Biophys. res.
Commun., 73:850-857.
WHO (1975) WHO Technical Report Series, No. 568 (Smoking and its
effects on health), 100 pp.
WHO (1976) Selected methods of measuring air pollutants, Geneva,
WHO, 112 pp. (Offset Publication No. 24).
WRIGHT, G. R., JEWCZYK, S., ONROT, J., TOMLINSON, P., & SHEPHARD, R.
J. (1975) Carbon monoxide in the urban atmosphere. Arch. environ.
Health, 30: 123-129.
WRIGHT, G. W., RANDELL, P., & SHEPHARD, D.. R. J. (1973) Carbon
monoxide and driving skills. Arch. environ. Health, 27: 349-354.
XINTARAS, C., JOHNSON, B. L., ULRICH, C. E., TERRILL, R. E., &
SOBECKI, M. F. (1966) Application of the evoked response technique
in air pollution toxicology. Toxicol appl. Pharmacol., 8: 77-87.
YOCOM, J. E., CLINK, W. L., & COTE, W. A. (1971) Indoor-outdoor air
quality relationships. J. Air Pollut. Control Assoc.,
21: 251-259.
ZENKEVIC, E. C. (1973) [Hemodynamic changes under the effect of CO
displayed adaptation.] Sb. Probl. Adapt. Gig. Tr., pp. 67-72
(in Russian).
ZORN, H. (1968) [Carbon dioxide-oxygen mixture or pure oxygen for
respiration in the case of carbon monoxide intoxication.] Dtsch.
Med. Wochenschr., 93: 1536 (in German).
ZORN, H. (1972) The partial oxygen pressure in the brain and liver at
subtoxic concentrations of carbon monoxide. Staub-Reinhalt. Luff,
32: 24-29.
Annex 1
THE RELATIONSHIP BETWEEN CARBON MONOXIDE CONCENTRATION IN AIR AND
CARBOXYHAEMOGLOBINa
(1) Several empirical equations have been proposed for estimating
carboxyhaemoglobin levels from environmental exposure conditions.
These equations were based on controlled exposures of human
volunteers. Most of them referred to subjects at rest, or performing
sedentary activities or light work. The simplest empirical equations
described carboxyhaemoglobin levels as a linear function of carbon
monoxide concentration in the inspired air [CO], and of exposure time
(t) (Forbes et al., 1945; Pace et al., 1946). They were applicable
only within a limited range of exposure conditions. Hanks & Farquhar
(1969) and Peterson & Stewart (1970) compiled empirical equations
involving more complex functional relationships that had a wider
application.
(2) In addition, models have been proposed that relate carboxyhaemo-
globin levels to both environmental exposure conditions and a number
of physiological variables such as blood volume, VB, endogenous
carbon monoxide production, Vco, diffusion capacity of the lung,
DL, and alveolar ventilation rate, VA. The best known model,
developed by Coburn et al. (1965), has been briefly discussed in
section 6.2. A more recent model, that took into account the dynamic
condition of urban carbon monoxide concentrations, was suggested by
Ott & Mage (1978) (p. 118).
(3) A simple linear relationship, proposed by Forbes et al. (1945),
linked the increase in the carboxyhaemoglobin level with the carbon
monoxide concentration in air, [CO](ppm) and exposure time, t(min):
a "This annex has been prepared by the Secretariat and has not been
reviewed by the Task Group, with the exception of Table 3.
Equations are presented in the form given in the original studies
and no attempt has been made to change to SI units. The Secretariat
wishes to express its appreciation to Dr H. Buchwald (Canada),
Chairman of the Task Group, for providing information contained in
Tables 3 and 4 and to Dr R. Horton, Dr D. J. McKee, USEPA, and
Dr V. Armstrong, Health and Welfare, Canada for reviewing the
annexes.
[HbCO](%) = k × [CO] × t (1)
where k was a constant equal to 0.0003 for "an individual at rest"
( VA = 6 litre/min, pulse rate 70), 0.0005 for "light activity"
( VA = 9.5 litre/min, pulse rate 80), 0.0008 for "light work"
(50 watts, VA = 18 litre/min, pulse rate 110), and 0.0011 for
"heavy work"a (about 100 watts, VA = 30 litre/min, pulse rate 135).
This equation was based on sets of controlled exposure
observations on human volunteers. Exposure concentrations ranged from
100 to 20 000 ppm (0.01 to 2.0%), and exposure times up to 6 h. At a
[CO] of 100 ppm, the equation holds only up to delta[HbCO] of about
7%. At 1000 ppm, the equation is applicable up to 30% [HbCO].
The equation of Forbes et al. was used by the California State
Department of Health for estimating [HbCO] after shorter periods of
exposure to carbon monoxide concentrations higher than 100 ppm in
individuals at rest or engaged in light work (Goldsmith & Landow,
1978).
(4) The empirical equation suggested by Pace et al. (1945) was derived
from controlled carbon monoxide exposures of 32 volunteers, aged 18-40
years. The concentrations of carbon monoxide in the inspired air
varied from 90 to 21 800 ppm, and the subjects were either sitting
quietly or walking on a level treadmill (about 4.3 km/h, VA about
19.2 litre/min). The exposure times ranged from 20 to 300 min. The
equation was also linear, but it took into account the alveolar
ventilation rate, VA, and the blood volume, VB:
[CO](ppm) × VA (litre/min) × t(min)
DELTA[HbCO](%) = (2)
4650 × VB(litre)
If it is assumed that the blood volume equals 5.51 and
VA = 6 litre/min (rest or light sedentary activity) or 18 litre/min
(light physical work), the equation takes the same form as that of
Forbes et al, but k values are somewhat lower (0.00023 and 0.00070,
respectively).
(5) Hanks & Farquhar (1969) also conducted carefully controlled
exposure studies on sedentary volunteers, and expressed their results
by the equation:
[HbCO](%) = 0.147[CO](1 - e-0.00289 t) (3)
where [CO] is in ppm and t in min. The equation was valid for subjects
having a ventilation rate of about 6 litre/min.
a VA for heavy physical work may be as high as 60 litre/min.
According to Chovin (1974), the "coefficient of (pulmonary)
ventilation" K could be introduced into equation (3), which then
became applicable to subjects performing various degrees of activity.
Chovin's modified equation read:
K
- t
0.147
[HbCO](%) = 0.147[CO](1 - e ) (4)
where t was in hours. For subjects at rest, K = 0.025, and for those
performing heavy physical work, K = 0.065. For intermediate degrees of
activity, Chovin proposed K values of 0.035, 0.045, and 0.055.
(6) Another empirical equation, referred to on p. 36, was suggested by
Peterson & Stewart (1970). It could also be written in the following
form:
[HbCO](%) = 0.0051[CO]0.858 × t0.63 (5)
where [CO] is in ppm, and t in min. The equation was based on
controlled human exposure data. The volunteers (18 healthy graduate
students) were exposed to carbon monoxide concentrations of <1, 25,
50, 100, 200, 500, and 1000 ppm for periods ranging from 30 min to
24 h, while performing strictly sedentary activities. Like linear
equations, this equation needed to be used with caution, since it did
not yield a finite [HbCO] value for an infinite exposure time. It was
used in developing US national ambient air quality standards, and is
strictly valid for constant [CO] and for shorter periods of exposure
(Ott & Mage, 1978).
(7) A model proposed by Coburn et al. (1965) has been discussed on
pp. 36 and 37. It is valid for male subjects only.a A solution of
their differential equation has been provided in the original paper; it
assumes that the mean pulmonary capillary oxygen pressure ( pCo2)
and the concentration of oxyhaemoglobin [HbO2] are constant and
independent of carboxyhaemoglobin levels [HbCO]. However, the
oxyhaemoglobin level depends on carboxyhaemoglobin concentrations in a
complex way. Solutions of the differential equation taking this into
account are available, and their application is illustrated in the
NAS/NRC (1977) document. Nevertheless, for most practical purposes,
the solution given in the original paper appears to be adequate. It
has been used, for example, by Peterson & Stewart (1970) and in the
criteria document of the National Institute of Occupational Safety and
Health (US Department of Health, Education and Welfare, 1972). A
useful form of this solution is given in the document of the Committee
on the Challenges of Modern Society (1972):
a Endogenous CO production may be different for females due to
menstruation, pregnancy, and other metabolic factors.
1
[HbCO] t = {e- kt(A[HbCO]o - VcoB - [CO]) + VcoB + [CO]} (6)
A
where [HbCO] t and [HbCO]o are the carboxyhaemoglobin levels at
times
pCo2 1 ( pB - 47)
t and t = 0, [CO] = pIco, A = , B = ( + ) and
M[HbO2] DL VA
A
k =
VB.B
the symbols used to define [CO], A, and B are explained on p. 36. The
relationships between [CO] in ppm and pIco, and between [HbCO]% and
[HbCO] in ml CO/ml blood are:
pIco × 106
[CO](ppm) = ,
pB
and
[HbCO](%) = 497.5 [HbCO](ml CO/ml blood)
(8) Ott & Mage (1978) designed a model that took into consideration
the dynamic characteristics of urban carbon monoxide concentrations.
The differential equation describing it reads:
d[HbCO]
tau dt + [HbCO] - ß = alpha[CO] (7)
O < [CO] < 100 ppm
where ß was the endogenous level of blood carboxyhaemoglobin and was
assumed to be 0.5%, alpha was assumed to be 0.15, and tau = 2.49h.
The main conclusion of the authors was that [CO] in ambient air
should be reported for averaging periods of 10-15 min, if the
monitoring stations were located near heavy traffic or on congested
streets. In such cases, sharp carbon monoxide peaks of short duration
might occur fairly often, and concentrations reported with longer
averaging periods, e.g., 1 h or more, might introduce an error of up
to 21% in the estimated [HbCO].
(9) Several empirical equations are compared with Coburn's model in
Table 1, for persons at rest ( VA - 6 litre/min) or performing light
physical work ( VA = 18 litre/min), at a carbon monoxide exposure
concentration of 100 ppm. For subjects performing light work, Hanks &
Farquhar's equation has been used in Chovin's modification with
K = 0.060. The following assumptions have been made in calculating
[HbCO]t values from Coburn's equation (6): [HbCO] = 0.5% or
0.001 ml CO/ml blood, Vco = 0.007 ml/min, VB = 5500 ml,
pCo2 = 100 mmHg, [HbO2] = 0.2 ml O2/ml blood, M = 218,
pB - 47 = 713 mmHg; sedentary subjects: DL = 40 ml/min/mmHg,
VA = 6000 ml/min; light work: DL = 40 ml/min/mmHg,
VA = 18 000 ml/min. For all empirical equations, [HbCO]o = 0.5%
has been added to the calculated values of [HbCO].
Table 1 shows clearly that Hanks & Farquhar's equation agrees best
with Coburn's model. Peterson & Stewart's equation gives values that
are higher than the other equations up to 6 h of exposure; then it
gives lower results than Forbes' equation. At a carbon monoxide
concentration of 100 ppm, Pace's equation gives lower [HbCO] values
for subjects at rest than the other equations, up to 7 h of exposure;
for subjects performing light work, it is applicable up to 2 h of
exposure. For subjects at rest, Forbe's equation is applicable up to
6 h, and for persons performing light work, up to 2 h.
(10) Table 2 shows [HbCO] values predicted by Coburn's model.a The
assumptions are the same as those specified in paragraph 9. For heavy
work, it has been assumed that DL = 60 ml/min/mmHg and that
VA=30 000 ml/min.
(11) Guidelines on exposure conditions that would prevent
carboxyhaemoglobin levels exceeding 2.5-3% in general nonsmoking
populations are given in Table 3.
These guidelines were reviewed by the Task Group. Comparison with
Tables 1 and 2 indicates the degree of protection provided, if these
guidelines are applied, both for sedentary individuals and for persons
performing light work. The exposure guidelines for 8-24 h have been
added by the Secretariat, after the Task Group meeting, to facilitate
comparison with national air quality standards.
(12) Guidelines on exposure conditions, which would prevent carboxy-
haemoglobin levels exceeding 5% in nonsmoking occupational groups, are
shown in Table 4. They were prepared, after the meeting, by Dr
Buchwald, at the request of the Secretariat, and have not been
reviewed by the Task Group. Guidelines for heavy work have been
suggested by the Secretariat, also after the meeting. Heavy work has
been defined by DL = 60 ml/min/mmHg and VA = 30 000 ml/min. The
degree of protection provided by these guidelines is indicated in
columns 3 and 4 of Table 4. Column 3 shows carbon monoxide
concentrations that would produce 5% HbCO within exposure times given
a The Secretariat wishes to thank Dr M. A. Vouk, University
Computing Centre, Zagreb, Yugoslavia, for programming Coburn's
equations and providing computer printouts.
in column 2, for light and heavy work, respectively. Column 4 provides
"safety factors" obtained by dividing the concentrations in column 3
by concentrations in column 1. Unless otherwise indicated, the
guidelines given in Table 4 should be considered as desirable
conditions rather than maximum acceptable limits.
Table 1. [HbCO](%) predicted by different empirical equations and by the model of Coburn et al. (1965).
Exposure to a carbon monoxide concentration of 115 mg/m3 (100 ppm)
Time Subjects at rest Subjects performing light work
(min)
F P H PS C F P H C
15 1.0 0.8 1.1 2.0 1.2 1.7 1.6 1.9 2.0
30 1.4 1.0 1.7 2.8 1.8 2.9 2.6 3.2 3.3
45 1.9 1.5 2.3 3.4 2.4 4.1 3.6 4.4 4.6
60 2.3 1.9 2.8 4.0 3.0 5.3 4.7 5.4 5.7
120 4.1 3.3 4.7 5.9 5.0 10.1 8.9 8.7 9.2
180 5.9 4.5 6.4 7.5 6.8 14.9 13.1 10.9 11.6
240 7.7 6.0 7.8 8.9 8.3 19.7 17.3 12.3 13.2
300 9.0 7.4 9.0 10.1 9.6 24.5 21.5 13.3 14.1
360 10.8 8.8 10.0 11.3 10.7 29.3 25.7 13.9 15.1
420 12.6 10.2 10.8 12.4 11.6 34.1 29.9 14.3 15.6
480 14.4 11.5 11.5 13.5 12.4 38.9 34.1 14.6 15.9
F = Forbes et al. (1945), P = Pace et al. (1946), H = Hanks & Farquhar (1969),
PS = Peterson & Stewart (1970), C = Coburn et al. (1965).
Table 2. [HbCO] values predicted from Coburn et al. (1965) model
Time 200 ppm 100 ppm 75 ppm 50 ppm
S L H S L H S L H S L H
15 min 1.8 3.5 5.2 1.2 2.0 2.8 1.0 1.6 2.2 0.82 1.2 1.6
30 min 3.1 6.2 9.2 1.8 3.3 4.8 1.5 2.6 3.7 1.1 1.9 2.6
45 min 4.3 8.7 12.6 2.4 4.6 6.5 1.9 3.5 4.9 1.4 2.5 3.4
60 min 5.5 11.0 15.5 3.0 5.7 7.9 2.3 4.3 6.0 1.7 3.0 4.1
90 min 7.7 14.9 20.2 4.0 7.6 10.2 3.1 5.8 7.7 2.2 4.0 5.2
2h 9.7 18.1 23.7 5.0 9.2 11.9 3.9 7.0 9.0 2.7 4.7 6.1
4h 16.3 26.2 30.4 8.3 13.2 16.3 6.3 10.0 11.5 4.4 6.9 7.7
6h 21.1 30.0 32.4 10.7 15.1 16.2 8.1 11.3 12.2 5.5 7.6 8.2
8h 24.5 31.7 32.9 12.4 15.9 16.5 9.4 12.0 12.4 6.4 8.0 8.3
24h 32.7 33.2 33.2 16.5 16.7 16.6 12.4 12.5 12.5 8.4 8.4 8.3
infinity 33.4 33.2 33.2 16.8 16.7 16.6 12.7 12.5 12.5 8.5 8.4 8.3
Time 35 ppm 25 ppm 10 ppm 5 ppm
S L H S L H S L H S L H
15 min 0.72 1.0 1.3 0.66 0.84 1.0 0.55 0.61 0.67 0.52 0.54 0.56
30 min 0.93 1.4 1.9 0.80 1.2 1.5 0.61 0.72 0.82 0.54 0.57 0.60
45 min 1.1 1.9 2.5 0.95 1.4 1.9 0.66 0.81 0.95 0.56 0.61 0.64
60 min 1.3 2.2 3.0 1.1 1.7 2.2 0.71 0.90 1.1 0.58 0.63 0.68
90 min 1.7 2.9 3.7 1.3 2.1 2.7 0.80 1.1 1.2 0.62 0.69 0.74
2 h 2.0 3.4 4.3 1.6 2.5 3.1 0.89 1.2 1.4 0.66 0.73 0.78
4 h 3.2 4.7 5.4 2.4 3.4 3.9 1.2 1.5 1.6 0.77 0.84 0.86
6 h 4.0 5.4 5.7 2.9 3.9 4.1 1.4 1.6 1.7 0.85 0.88 0.88
8 h 4.5 5.7 5.8 3.3 4.1 4.2 1.5 1.7 1.7 0.91 0.91 0.89
24 h 5.9 5.9 5.9 4.3 4.2 4.2 1.9 1.8 1.7 1.05 0.93 0.89
infinity 6.0 5.9 5.9 4.4 4.2 4.2 1.9 1.8 1.7 1.06 0.93 0.89
S = sedentary subjects, L = light physical work, H = heavy physical work, all as defined in sections 9 and 10.
Table 3. Guidelines for exposure conditions to prevent carboxyhaemoglobin levels exceeding
2.5-3% in nonsmoking populations
(a) A ceiling or maximum permitted exposure of 115 mg/m3 (100 ppm) for periods of exposure
not exceeding 15 min (No exposure over 115 mg/m3 (100 ppm) permitted, even for very
short time periods).
(b) A time-weighted average exposure of 55 mg/m3 (50 ppm) for periods of exposure not
exceeding 30 min.
(c) A time-weighted average exposure of 29 mg/m3 (25 ppm) for periods of exposure not
exceeding one h.
(d) A time-weighted average exposure of 15 mg/m3 (13 ppm) for periods of exposure of more
than one h.
(e) A time-weighted average exposure of 11.5 mg/m3 (10 ppm) for periods of exposure of
8-24 h.a
a Suggested by the Secretariat.
Table 4. Guidelines for exposure conditions that would prevent carboxyhaemoglobin levels
exceeding 5% in nonsmoking occupational groups performing light and heavy
physical work.
Concentration Exposure time not to Concentrations that Safety factor
be exceeded would produce 5% HbCOc
ppm mg/m3
Light Heavy Light Heavy Light Heavy
worka workb work work work work
200d 230 15 min - 298 - 1.5 -
100e,f 115 30 min 15 min 157 193 1.6 1.9
75f 86 60 min 30 min 87 105 1.2 1.4
50f 55 90 min 60 min 64 62 1.3 1.2
35f 40 4 h 2 h 37 41 1.1 1.2
25f 29 8 h 8 h 31 30 1.2 1.2
a Limits suggested by Dr Buchwald.
b Limits suggested by the Secretariat.
c Calculated from Coburn's equation (6).
d Short-term limit or maximum permissible concentration for light work.
e Short-term limit or maximum permissible concentration for heavy work.
f Time weighted average.
REFERENCES
HANKS, T. G. & FARQUHAR, R. D. (1969) Analysis of human performance
capabilities as a function of exposure to carbon monoxide.
(SystMed Corporation Report R 9001, Contract PH-22-68-31).
PACE, N., CONSOLAZIO, W. V., WHITE, W. A. JR. & BEHNKE, A. R. (1945)
Formulation of principal factors affecting the rate of uptake of
carbon monoxide by man. Am. J. Physiol., 147: 352-359.
All other references are included in the list of references for
the main body of the document (pp. 98-114).
Annex 2
SELECTED NATIONAL AMBIENT AIR QUALITY STANDARDS AND OCCUPATIONAL
EXPOSURE STANDARDS FOR CARBON MONOXIDEa
1. NATIONAL AMBIENT AIR QUALITY STANDARDS
1.1 Canada (1974)
National air quality objectives
Desirable concentrations:
(a) 0-6 mg/m3 (0-5 ppm)b average concentration over an 8-h
period.
(b) 0-15 mg/m3 (0-13 ppm) average concentration over a one-h
period.
Acceptable concentrations:
(a) 6-15 mg/m3 (5-13 ppm) average concentration over an 8-h
period.
(b) 15-35 mg/m3 (13-31 ppm) average concentration over a one-h
period.
Method of measurement:
Nondispersive infrared spectrometry, Report No. EPS 1-AP-73-1.
Source: Velma Ouellet (1978) The Clean Air Act--Compilation of
Regulations and Guidelines, Ottawa, Environment Canada (Report EPS
1-AP-78-2).
In addition to the desirable and acceptable concentrations listed,
a tolerable range of 15-20 mg/m3 average concentration over a
continuous 8-h period was prescribed in 1978.
Source: Canada Gazette (1978) Part 2, Volume 112, No. 3 (February 8).
a Prepared by the Secretariat.
b Concentrations in alternative units have been added by the
Secretariat to facilitate comparison of national quality standards.
1.2 Federal Republic of Germany (1974)
In the Federal Republic of Germany, immissionsc (Immissionen)
are legally defined as "air pollutants, noise, vibrations, light,
heat, radiations, and analogous environmental factors affecting human
beings, animals, plants, or other objects". "As a rule, air pollutants
occurring at a height of 1.5 metres above ground or at the upper limit
of vegetation or at a distance of 1.5 metres from the surface of a
building shall be considered active air pollutants."
"Immission standards are the values for long-term exposure (IW 1)
and short-term exposure (IW 2)."
The following immission standards have been established for carbon
monoxide:
Long-term exposure (IW 1): 10.0 mg/m3 (9 ppm).
Short-term exposure (IW 2): 30.0 mg/m3 (26 ppm).
Method of measurement: VDI 2455, Sheet 1 (August 1970) and 2455,
Sheet 2 guidelines (October 1970).
"Characteristic value I 1 for comparison with IW 1 shall be the
arithmetic mean of all individual data for a measurement area.
Characteristic value I 2 for comparison with IW 2 shall be the 95%
value for the cumulative frequency distribution of all individual
values for a measurement area; this may be also calculated with the
formula I 2 = x + ts where x is the arithmetic mean of individual
data for a measurement area,
2SUM(x - xi)2
s = + SQRT ,
2z - 1
xi = individual data which are greater than x, z = number of
individual data, which are greater than x, and t = 1.64 for the 95%
confidence level."
Source: Federal Minister of the Interior (1974) Technical
Instructions for Maintaining Air Purity, 28 August 1974, Bonn.
c Immission A German term for which there is no simple English
equivalent. Immissions are to be distinguished from emissions
(Emissionen), which are defined as air pollutants, noise,
vibrations, light, heat, radiations, and analogous phenomena
originating from an installation (Federal Republic of Germany, Law
on Protection against Emissions, 15 March 1974).
1.3 Japan (1973)
Ambient air quality standards are defined as follows:
(a) Average of hourly values in 8 consecutive hours shall not
exceed 20 ppm (23 mg/m3).
(b) Daily average of hourly values shall not exceed 10 ppm
(11 mg/m3).
These standards do not apply to industrial zones, roadways, and
other areas or places where people do not usually live.
Source: Environment Agency (1978) Environmental Laws and
Regulations in Japan (II) Air. Tokyo.
1.4 Union of Soviet Socialist Republics (1971)
Maximum permissible (single exposure) concentration for carbon
monoxide is 3 mg/m3 (2.6 ppm). This concentration should not provoke
reflex (i.e., subsensory) reactions in human organisms.
Maximum permissible (24-hour average) concentration for carbon
monoxide is 1.0 mg/m3 (0.9 ppm). This concentration should not have
either a direct or indirect harmful effect on man, for unlimited in
time, continuous exposure, 24 hours a day.
Method not specified.
Source: Krotov, Ju. A. (1975) Maximum permissible concentrations
of harmful substances in air and water, Himija, Moscow.
1.5 United States of America (1971)
"The national primary and secondary ambient air quality standards
for carbon monoxide, measured by the reference method described in
Appendix C to this part, or by an equivalent method, are:
(a) 10 milligrams per cubic meter (9 ppm) -- maximum 8-hour
concentration not to be exceeded more than once per year.
(b) 40 milligrams per cubic meter (35 ppm) -- maximum 1-hour
concentration not to be exceeded more than once per year."
Reference method for the continuous measurement of carbon monoxide
in the atmosphere is nondispersive infrared spectrometry.
Source: Federal Register, 36 (228), Thursday, November 25, 1971,
Washington DC, pp. 22385 and 22391-22392.
1.6 Other Member States
For further information on national ambient air quality standards
for carbon monoxide, the reader is referred to W. Martin & A. C. Stern
(1974) The world's air quality management standards, Washington, DC,
US Environmental Protection Agency (EPA-650/a-75-001-a).
2. OCCUPATIONAL EXPOSURE LIMITS
An occupational exposure limit for carbon monoxide of 50 ppm or
55 mg/m3 has been set in the following Member States: Australia,
Belgium, Finland, Federal Republic of Germany, Italy, Japan,
Netherlands, Switzerland, the USA, and Yugoslavia.
Bulgaria and the USSR have established a limit of 20 mg/m3
(17 ppm), Hungary and Poland, 30 mg/m3 (26 ppm) and Sweden,
40 mg/m3 (35 ppm). Czechoslovakia's standard includes average and
maximum values of 30 mg/m3 (26 ppm) and 150 mg/m3 (130 ppm),
respectively. Other standards that include both average and maximum
values are those of the German Democratic Republic, (35 and
110 mg/m3) (30 and 96 ppm) and Romania, (30 and 50 mg/m3) (26 and
44 ppm).
These values should be interpreted in terms of definitions of
occupational exposure limits, which may be different in different
countries. The reader is referred to the International Labour Office
publication: Occupational Exposure Limits for Airborne Toxic
Substances, Occupational Safety and Health Series 37, ILO, Geneva,
1977. The values given in paragraphs 1 and 2 have been extracted from
this publication.
The US National Institute of Occupational Safety and Health
(NIOSH, 1972) has recommended environmental exposure limits of 35 ppm
(40 mg/m3) (time weighted average) and 200 ppm (229 mg/m3)
(ceiling) (Summary of NIOSH Recommendations for Occupational Health
Standards, July, 1978).
