
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 139
PARTIALLY HALOGENATED CHLOROFLUOROCARBONS
(ETHANE DERIVATIVES)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
Draft prepared by Professor D. Beritc-Stahuljak and Professor
F. Valic (University of Azgreb, Croatia) using texts made
available by Dr R. Millischer (ATOCHEM, Paris, France),
Dr. S. Magda (Kali-Chemie, Hanover, Germany), Mr D.J. Tinston
(ICI Central Toxicology Laboratory, United Kingdom), Dr. H.J.
Trochimowicz (E.I. Du Pont de Nemours, Newark, Delaware, USA)
and Dr G.M. Rusch (Engineered Materials Sector, Allied-Signal Inc.,
Morristown, New Jersey, USA).
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Partially halogenated chlorofluorocarbons (ethane derivatives).
(Environmental health criteria ; 139)
1.Freons - adverse effects 2.Freons - toxicity
I.Series
ISBN 92 4 157139 X (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PARTIALLY HALOGENATED
CHLOROFLUOROCARBONS (ETHANE DERIVATIVES)
1. SUMMARY
1.1. Identity, physical and chemical properties, and analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism in laboratory animals and humans
1.6. Effects on laboratory mammals and in vitro test systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory and field
1.9. Evaluation and conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.1.1. Technical products
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels
3.2.2. Manufacturing processes
3.2.3. Loss during disposal, transport, storage and
accidents
3.3. Use patterns
3.3.1. Major uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Biodegradation and bioaccumulation
4.2. Environmental transformation and interaction with other
environmental factors
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water, food and other edible products
5.2. Human exposure
6. KINETICS AND METABOLISM
6.1. Animal studies
6.1.1. Absorption
6.1.2. Distribution
6.1.3. Metabolic transformation
6.1.3.1 General considerations
6.1.4. Covalent binding to macromolecules
6.1.5. Elimination
6.2. Human studies
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Acute oral toxicity
7.1.2. Acute inhalation toxicity
7.1.3. Acute dermal toxicity
7.2. Short-term inhalation exposure
7.3. Skin and eye irritation; sensitization
7.3.1. Skin and eye irritation
7.3.2. Skin sensitization
7.4. Long-term exposure
7.5. Reproduction, embryotoxicity, and teratogenicity
7.5.1. Reproduction
7.5.2. Embryotoxicity and teratogenicity
7.6. Mutagenicity
7.7. Carcinogenicity
7.8. Special studies - cardiovascular and respiratory effects
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Direct health effects
10.1.1. HCFC 141b
10.1.2. HCFC 142b
10.1.3. HCFC 132b
10.1.4. HCFC 133a
10.1.5. HCFC 123
10.1.6. HCFC 124
10.2. Health effects expected from a depletion of stratospheric
ozone
10.3. Effects on the environment
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1. Conclusions
11.2. Recommendations for protection of human health and the
environment
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR PARTIALLY
HALOGENATED CHLOROFLUOROCARBONS (ETHANE DERIVATIVES)
Members
Dr U. Andrae, Genetic Toxicology Group, Research Centre for
Environment and Health, Neuherberg, Germany
Professor D. Beritic-Stahuljak, Medical School, University of Zagreb,
Zagreb, Croatia
Dr J. Delic, Toxicology Unit, Health and Safety Executive, Bootle,
United Kingdom
Dr B. Gilbert, Technology Development Company (CODETEC) Cidade
Universitaria, Campinas, Brazil ( Joint Rapporteur)
Ms G. Hodson-Walker, Cell Biology Department, Life Science Research
Ltd., Eye, United Kingdom
Dr W. Jameson, National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA
Dr J. Kojima, Division of Environmental Chemistry, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr J. Sokal, Institute of Occupational Medicine, Sosnowiec, Poland
Dr S. Swierenga, Health and Welfare Canada, Ottawa, Canada ( Joint
Rapporteur)
Dr V. Vu, Oncology Branch, Office of Toxic Substances, US
Environmental Protection Agency, Washington, DC, USA ( Chairman)
Observers
Dr R. Millischer, Department of Toxicology, ATOCHEM, Paris, France
Dr H. Trochimowicz, E.I. Du Pont de Nemours & Co., Haskell Laboratory
for Toxicology and Industrial Medicine, Newark, Delaware, USA
Secretariat
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, Lyon, France
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland, also Vice-Rector, University of Zagreb,
Zagreb, Croatia ( Responsible Officer and Secretary)
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR PARTIALLY HALOGENATED
CHLOROFLUOROCARBONS (ETHANE DERIVATIVES)
A Task Group on Environmental Health Criteria for Partially
Halogenated Chlorofluorocarbons (Ethane Derivatives) met at the
British Industrial and Biological Research Association (BIBRA),
Carshalton, Surrey, United Kingdom, from 30 September to 5 October
1991. Dr S.D. Gangolli opened the meeting on behalf of the host
institute and greeted the participants on behalf of the Department of
Health. Professor F. Valic welcomed the participants on behalf of the
heads of the three cooperating organizations of the IPCS
(UNEP/ILO/WHO). The Task Group reviewed and revised the draft
monograph, made an evaluation of the direct and indirect risks for
human health from exposure to the partially halogenated
chlorofluorocarbons reviewed, and made recommendations for health
protection and further research.
The draft was prepared by Professor D. Beritic-Stahuljak and Professor
F. Valic, using the texts made available by Dr R. Millischer, ATOCHEM,
Paris, France (HCFC 141b), Dr S. Magda, Kali-Chemie, Hanover, Germany
(HCFC 142b), Mr D.J. Tinston, Central Toxicology Laboratory, ICI,
Alderley Park, United Kingdom (HCFC 133a), Dr H.J. Trochimowicz, E.I.
Du Pont de Nemours, Newark, Delaware, USA (HCFC 132b), and Dr G.M.
Rusch, Engineered Materials Sector, Allied-Signal Inc., Morristown,
New Jersey, USA (HCFC 123 and HCFC 124).
Professor F. Valic was responsible for the overall scientific content
and for the organization of the meeting, and Dr P.G. Jenkins, IPCS,
for the technical editing of the monograph.
INTRODUCTION
The global concern over the depletion of the stratospheric ozone layer
by active chlorine from fully halogenated chlorofluorocarbons resulted
in the development of the Vienna Convention for the Protection of the
Ozone Layer, adopted in March 1985, and its Montreal Protocol on
Substances that Deplete the Ozone Layer, signed in 1987. The agreement
required a freeze in the production and use of the fully halogenated
chlorofluorocarbons 11, 12, 113, 114 and 115 at l986 levels by
mid-1989, a 20% reduction from 1 July 1993 and a further 30% reduction
from 1 July 1998. Sixty-seven countries and the European Economic
Community have signed the Protocol. A total phase-out of 15 fully
halogenated chlorofluorocarbons by the year 2000 was agreed by the
Parties to the Protocol in June 1990.
This phase-out has created an urgent need for acceptable substitute
chemicals. These should have similar properties to the
chlorofluorocarbons included in the Protocol, but their
ozone-depleting potentials and possibly global-warming potentials
should be lower, and their atmospheric residence times shorter. In
addition, the substitute chemicals should not pose an unreasonable
risk to human health or the environment.
The hydrogenated partially halogenated chlorofluorocarbons constitute
a class of chemicals being considered as substitutes. The
ozone-depleting potentials and the global-warming potentials of the
partially halogenated chlorofluorocarbons are considerably lower than
those of the fully halogenated chlorofluorocarbons, and their
atmospheric residence times are shorter. Therefore, the partially
halogenated chlorofluorocarbons for which the toxicity evaluations
suggest no unreasonable health risks could be considered as possible
substitutes for the unacceptable fully halogenated
chlorofluorocarbons, particularly in the case of those for which the
production should be technologically feasible. The evaluation of two
partially halogenated methane derivatives of chlorofluorocarbons
(hydrochlorofluorocarbons 21 and 22) has been completed and published
as monograph No. 126 in the WHO Environmental Health Criteria series
(WHO, 1991). The present monograph evaluates six partially halogenated
ethane derivatives of chlorofluorocarbons (hydrochlorofluorocarbons
141b, 142b, 132b, 133a, 123 and 124).
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
This monograph concerns six hydrochlorofluorocarbons (HCFCs)
derived from the partial substitution of the hydrogen atoms in ethane
with both fluorine and chlorine atoms. The compounds considered in
this report are 1,1-dichloro-1-fluoroethane (HCFC 141b), 1-chloro-1,1-
difluoroethane (HCFC 142b), 1,2-dichloro-1,1-difluoroethane (HCFC
132b), 1-chloro-2,2,2-trifluoroethane (HCFC 133a), 1,1-dichloro-2,2,2-
trifluoroethane (HCFC 123) and 1-chloro-1,2,2,2-tetrafluoroethane
(HCFC 124).
Under normal temperatures and pressures these compounds are
flammable (HCFC 142b) or non-flammable gases (HCFC 133a, HCFC 124) or
non-flammable volatile liquids (HCFC 141b, HCFC 132b, HCFC 123). They
are colourless and the majority are practically odourless or have a
faint ethereal odour (HCFC 141b and HCFC 123). They are slightly or
moderately soluble in water and miscible with many organic solvents.
Analytical methods available for the determination of these
hydrochlorofluorocarbons include gas chromatography with flame
ionization and electron capture detection. Relatively high
concentrations in air can be monitored by single-beam photometry.
1.2 Sources of human and environmental exposure
The hydrochlorofluorocarbons reviewed in this monograph are not
known to occur as natural products. Due to the fact that these
compounds are not produced commercially on a large scale for end use,
there is little human exposure or release to the environment. Some of
these compounds may be used in the future as substitutes for fully
halogenated chlorofluorocarbons (e.g., CFC 11, CFC 12, and CFC 113).
HCFCs 133a and 142b are intermediates in the manufacture of other
fluorinated products. HCFC 133a is an in vivo metabolite of the
anaesthetic halothane.
1.3 Environmental transport, distribution and transformation
Data on biodegradation in the environment is limited to studies
of HCFCs 141b and 142b, which have been shown to be not readily
biodegradable by microorganisms. Little information on log
octanol/water partition coefficients is available, but that for HCFC
141b is 2.3 and so bioaccumulation of this hydrochlorofluorocarbon is
unlikely. In the troposphere these compounds are mainly decomposed by
reactions with hydroxy radicals. Their atmospheric lifetimes (relative
to an atmospheric lifetime of methyl chloroform of 6.3 years) lie
between 1.6 years (HCFC 123) and 19.1 years (HCFC 142b). (The
atmospheric lifetime of CFC 11 is 75, CFC 12 is 110, and
CFC 113 is 90 years). With the exception of HCFC 133a, for which there
are no values, the ozone-depleting and global-warming potentials of
these compounds are less than or equal to one-tenth of that of CFC 11,
the fully halogenated chlorofluorocarbon with the highest
ozone-depleting and global-warming potential (HCFC 142b, for which the
global-warming potential is approximately one-third that of CFC 11, is
an exception).
1.4 Environmental levels and human exposure
As HCFCs 141b, 132b, 133a, 123 and 124 are not yet in large-scale
commercial production and HCFC 142b is only used as an intermediate,
these substances are not released significantly into the environment.
There are therefore no data on environmental levels or human exposure.
1.5 Kinetics and metabolism in laboratory animals and humans
No data are available on the toxicokinetics in humans of any of
the HCFCs reviewed.
1.5.1 HCFC 141b
Results from toxicity studies suggest that absorption of HCFC
141b takes place across the respiratory epithelium. No information is
available on the distribution of HCFC 141b in mammals. In recent
in vitro single-exposure studies in rats, 2,2-dichloro-2-fluoro-
ethyl glucuronide and 2,2-dichloro-2-fluoroacetic acid were identified
in the urine.
A pilot study for absorption and metabolism of HCFC 141b in rats
exposed to its vapour suggested that metabolic transformation occurs
only to a very small extent.
An in vitro study indicated that HCFC 141b is dechlorinated to
a limited extent by hepatic microsomes.
1.5.2 HCFC 142b
There is no information on toxicokinetics for HCFC 142b. From
animal toxicity studies it can be inferred that absorption takes
place. An in vitro study suggested that dechlorination may occur.
1.5.3 HCFC 132b
In a metabolism study using intraperitoneal administration of
HCFC 132b to rats, 2-chloro-2,2-difluoroethylglucuronide,
chlorodifluoroacetaldehyde (hydrated and conjugated) and
chlorodifluoroacetic acid were identified in the urine. Formation and
excretion of chlorodifluoroacetic acid were increased after repeated
injection of the animals with HCFC 132b. In vitro experiments using
rat liver microsomes suggested the involvement of cytochrome P-450
IIEI in the initial hydroxylation step. No evidence for covalent
binding of fluorinated metabolites to liver proteins has been
observed.
1.5.4 HCFC 133a
No information is available on the toxicokinetics of HCFC 133a.
That absorption occurs following exposure of animals can be inferred
from the toxic effects seen in various studies. Dechlorination of HCFC
133a has been observed in vitro.
1.5.5 HCFC 123
There are no toxicokinetic data on HCFC 123. Absorption, however,
can be inferred from systemic effects and the elevated urinary
fluoride levels seen in toxicity studies in rats. HCFC 123 has been
shown to undergo metabolic transformation in rats. The extent of
metabolism is not known, but trifluoroacetic acid (TFA) has been
identified as a major urinary metabolite, in addition to fluoride.
Covalent binding to liver protein has been demonstrated for HCFC 123.
1.5.6 HCFC 124
There are no data on the kinetics and metabolism of HCFC 124. It
may be inferred from inhalation toxicity studies that absorption of
HCFC 124 occurs in the respiratory tract.
1.6 Effects on laboratory mammals and in vitro test systems
1.6.1 HCFC 141b
The acute oral toxicity of HCFC 141b is low. No signs of toxicity
were observed after rats were dosed with 5 g/kg.
In acute inhalation studies in rats and mice, central nervous
system (CNS) depression, anaesthesia and death were observed at high
exposure levels. No treatment-related macroscopic or histopathological
effects were observed. The 4-h LC50 reported for rats in one study
was 295 g/m3, and the 2-h LC50 in mice was reported to be 151
g/m3 in another study. In rats, the lowest concentration inducing
lethality was reported to be 242 g/m3 for 6 h.
No mortality in rats or rabbits was observed after dermal
exposure to 2 g/kg.
No marked toxicity was observed in short-term inhalation studies
at exposures ranging from 10 to 97 g/m3 and lasting up to 90 days.
Effects seen included reduced body weight gain, "slight
biochemical changes" and CNS depression. A no-observed-effect level
was not achieved in the 90-day study.
HCFC 141b did not produce signs of dermal irritation in rabbits,
or eye irritation in one of the two studies performed. In the second
study, a "mild" irritant response in the eye was observed. No skin
sensitization was observed in guinea-pigs.
A 2-generation reproduction study with HCFC 141b is currently in
progress. In developmental studies, increased incidences of
subcutaneous oedema and haemorrhaging in the fetuses and of embryonal
deaths were observed, but only at the maternally toxic concentration
of 97 g/m3 in a rat study. There were no teratogenic effects. No
treatment-related effects on embryo or fetal development were observed
in a rabbit study.
HCFC 141b was not mutagenic in a bacterial DNA repair assay and
produced conflicting results in other bacterial mutation tests. It had
no effect on V79 cells in the hprt locus assay. Chromosome
aberrations were observed after in vitro treatment of Chinese
hamster ovary (CHO) cells, but this was not reflected in an in vitro
human lymphocyte study. Two in vivo micronucleus assays in mice were
also negative.
A combined chronic inhalation toxicity/carcinogenicity study on
rats is in progress.
HCFC 141b exhibits cardiac sensitization potential to exogenous
adrenaline in dogs. The lowest concentrations of HCFC 141b inducing
responses were 24 and 48 g/m3 in dogs and monkeys, respectively.
1.6.2 HCFC 142b
Orally administered HCFC 142b produced only slight signs of
toxicity in rats at single doses of up to 5 g/kg.
Single inhalation exposure of rats to 525 g/m3 for 4 h killed
approximately 50% of the animals. Other studies with shorter duration
exposures yielded LC50 values in excess of 1000 g/m3.
Repeated inhalation exposure studies did not produce any adverse
responses in rats at a concentration of 41 g/m3 (6 h/day, 5 days per
week for 90 days). At much higher dose levels, death in rats was
associated with severe pulmonary irritation.
There are no reports of studies with HCFC 142b on skin and eye
irritation or skin sensitization. In cardiac sensitization experiments
(using exogenous adrenaline), mice, dogs and monkeys were tested. Dogs
were most sensitive; the NOEL was 102.5 g/m3 for a 5-min exposure,
while 205 g/m3 (also a 5-min exposure) induced cardiac arrhythmia.
There has been a single long-term study reported, in which rats
(130 males and 110 females per group) were exposed to HCFC 142b at 4,
41 and 82 g/m3 for 6 h/day, 5 days/week, for up to 104 weeks. No
treatment-related effects were observed in any of the parameters
studied, which included haematology, blood and urine chemistry and
histopathology. No significant treatment-related changes in tumour
incidence were reported.
No conventional studies have investigated the effect of HCFC 142b
on reproduction, but no effect on male fertility was observed in a
dominant lethal study. Two rat teratogenicity tests have been
performed. In one teratogenicity study, Sprague-Dawley rats were
exposed to 4 and 41 g/m3 (6 h/day from day 3 to day 15 of
pregnancy), while in the other study, Sprague-Dawley rats were exposed
to 13 and 39 g/m3 (6 h/day from day 6 to day 15 of pregnancy). No
teratogenic effects were noted. Reduced ossification was observed in
small numbers of fetuses at both dose levels in the latter study, but
not in the former.
HCFC 142b induces mutations in bacteria, but there is a lack of
data from genotoxicity assays with cultured mammalian cells. In vivo
assays did not show any increases in chromosomal aberrations in bone
marrow or dominant lethal effects in male rats.
1.6.3 HCFC 132b
The acute oral toxicity of HCFC 132b in the rat is low. The
lowest dose at which mortality was observed was 25 g/kg. After oral
dosing with 2 g/kg, depression of the autonomic and the central
nervous system was observed, together with effects on motor
coordination, motor activity and muscle tone. In males, swollen livers
and reduced liver weights were noted.
The acute inhalation toxicity of HCFC 132b is characterized by
anaesthesia at high exposure levels. The lowest dose at which
mortality was observed in rats during a 4-h exposure was 110 g/m3.
In mice, the LC50 for a 30-min exposure was 269 g/m3; anaesthesia
occurred at 71 g/m3. In one study, decreases in the weight of
testis, and increases in the weight of liver and lungs of male rats
were observed following exposure to 33 g/m3 for 6 h.
Dermal application of HCFC 132b (2 g/kg) in rats resulted in
clinical signs of CNS effects and swollen livers in some of the
animals. The undiluted compound produced "mild" skin irritation in
guinea-pigs and "mild to moderate" eye irritation in rabbits. No
evidence for skin sensitization in guinea-pigs was obtained. Cardiac
sensitization of dogs to adrenaline by inhaled HCFC 132b occurred at
exposure levels of 27 g/m3 or more.
The predominant consequences of short-term inhalation exposures
of male rats to HCFC 132b, besides CNS depression, were thymic atrophy
and effects on spermatogenesis. Disruption of spermatogenesis was
observed after treatment with 3 g/m3 or more for 13 weeks. Other
effects included bile duct proliferation and increased liver/body
weight ratio in males, even at the lowest exposure level applied (3
g/m3). Female rats appeared to be less sensitive than males to the
liver effects.
HCFC 132b induced embryotoxicity in rats after inhalation
exposure to 3-28 g/m3 during days 6-15 of gestation, this resulting
in increased numbers of resorptions (at 11 and 28 g/m3) and in
decreased fetal weight at all exposure levels. Maternal toxicity was
observed at all dose levels tested.
Based on the limited data available, there is no evidence for
in vitro mutagenicity of HCFC 132b. The carcinogenicity of the
compound has not been studied.
1.6.4 HCFC 133a
No data are available on the acute oral toxicity of HCFC 133a. It
is of low acute toxicity by the inhalation route (the 30-min LC50 in
mice is 738 g/m3), and the principal toxic effects seen are those
associated with anaesthesia. No information is available on cardiac
sensitization, skin or eye irritation or skin sensitization.
Repeated exposures (90 days) of rats to 49 g/m3 produced
chronic inflammation of the nasal passages, pulmonary emphysema and
oedema, bronchitis and pneumonia. Atrophy of the thymus, testis, ovary
and spleen was also observed. No effects were seen in rats or dogs
repeatedly exposed to HCFC 133a for 7 (rats) or 90 (dogs) days at a
concentration of about 25 g/m3, although deaths were observed in
mice exposed for 5 days to 0.5 g/m3 or more (excepting 2.5 g/m3).
Although no conventional studies on the effects of HCFC 133a on
reproduction are available, effects on male fertility and testicular
histopathology were observed in three dominant lethal studies in mice.
Exposures at concentrations of 2.5 g/m3 or more for 5 days resulted
in a reduced number of pregnant females and an increase in the
proportion of abnormal sperm, while exposure at a concentration of 5
g/m3 resulted in histopathological damage to the seminiferous
epithelium.
Studies on rats (treated on days 6-16 of gestation), at exposure
concentrations producing signs of only slight maternal toxicity, have
demonstrated that HCFC 133a is embryotoxic at concentrations of 2
g/m3 or more and embryolethal at 10 g/m3 or more. Progesterone
pretreatment of the pregnant females did not influence the
embryotoxic/lethal effects. Indications of teratogenic effects
(external anomalies of limb and tail) were seen in one study. HCFC
133a produced spontaneous abortions and total embryolethality in
rabbits exposed to 25 g/m3 on days 7-19 of gestation, a
concentration that produced only slight maternal toxicity.
From the studies available, there is no evidence of mutagenic
potential in bacteria. No increase was seen in the proportion of
hamster kidney cells producing transformed colonies in one study.
Dominant lethal effects were observed in two out of three studies
after exposure of male mice to 12 g/m3 or more for 5 days. The
proportion of bone marrow cells with chromosomal aberrations was
unaffected in rats exposed to 98 g/m3 (6 h/day for up to 5 days). In
the single carcinogenicity study, an increase in the incidence of
adenocarcinomas of the uterus and of benign interstitial cell tumours
of the testis was observed in rats that received 300 mg/kg in corn oil
by gavage for 52 weeks (this being followed by an observation period
of 73 weeks).
1.6.5 HCFC 123
HCFC 123 has low acute oral and dermal toxicity. The reported
lowest oral dose of HCFC 123 producing lethality in rats is 9 g/kg. No
mortality was found at a dose level of 2 g/kg in either rats or
rabbits.
The acute inhalation toxicity of HCFC 123 is also low. Effects
seen are similar to those of chlorofluorocarbons, i.e. loss of
coordination and narcosis. The 4-h LC50 is 178 g/m3 in hamsters,
463 g/m3 in mice and ranges from 200 to 329 g/m3 in rats. Cardiac
sensitization after a challenge with injected adrenaline occurred in
dogs at concentrations of 119 g/m3 or more. Liquid HCFC 123 produces
"mild" irritation of the skin and eye in rabbits. It does not cause
skin sensitization in guinea-pigs.
Several short-term toxicity studies have been conducted on HCFC
123 using the inhalation route. Signs of CNS depression are
consistently observed in rats at concentrations of 31 g/m3 or more.
HCFC 123 also caused some liver effects in rats at exposure
concentrations of 31 g/m3 or more. Long-term exposure (4 weeks or
longer) to HCFC 123 also affects lipid and carbohydrate metabolism as
reflected by consistent reduction of serum triglyceride cholesterol
and glucose levels in rats. Interim results from an ongoing chronic
inhalation toxicity/oncogenicity study in rats indicate that HCFC 123
induces effects following long-term exposure to 2, 6 or 31 g/m3. The
no-observed-effect level (NOEL) was not recorded in this study, based
on the effects on lipid metabolism and increased hepatic peroxisomal
activity.
A 2-generation reproduction study in rats exposed by the
inhalation route is currently being conducted on HCFC 123. No evidence
of embryotoxicity was seen in two limited studies in rats at
concentration producing slight maternal toxicity. There is evidence of
embryotoxicity only at high maternally toxic concentrations (more than
62.5 g/m3) in rabbits. Maternal toxicity (lower body weight, CNS
depression) was seen in rats at exposure levels of 31 g/m3 or more,
and in rabbits at 3 g/m3 or more. No evidence of teratogenicity was
seen in either rats or rabbits.
HCFC 123 shows no evidence of mutagenic activity in bacterial and
yeast assays. However, there is evidence of clastogenic activity in
human lymphocytes in vitro, but this finding was not supported by
data from an in vivo mouse micronucleus assay.
A combined chronic inhalation toxicity/carcinogenicity study on
rats is in progress. A preliminary communication indicated that HCFC
123 produces increased incidences of benign tumours of the testis and
exocrine pancreas in male rats. However, an evaluation of the
potential carcinogenicity of HCFC 123 cannot be made until complete
results become available.
1.6.6 HCFC 124
The acute inhalation toxicity of HCFC 124 in animals is low.
Death occurred in rats at 1674 g/m3 (240-min exposure) and in mice
at 2460 g/m3 (10-min exposure). The effects seen are typical of
those of chlorofluorocarbons, i.e. loss of coordination and narcosis.
Cardiac sensitization after a challenge injection of adrenaline
occurred in dogs at concentrations of 140 g/m3 or more. No
information on skin or eye irritation or skin sensitization is
available for this compound.
Short-term inhalation toxicity has been investigated in five
experiments on rats with exposure durations ranging from 14 to 90
days. Histopathological changes in the organs were not observed even
at the highest exposure levels studied (560 g/m3 in a 14-day
experiment, 279 g/m3 in a 90-day study). The NOEL of 28 g/m3 was
reported on the basis of functional observations and blood chemistry
determinations in the 90-day study.
A chronic inhalation toxicity study on HCFC 124 is in progress.
In three limited teratogenicity studies on rats, in which HCFC 124 was
tested at 30 g/m3 or in the range 3-279 g/m3, there was no
evidence of embryotoxicity or teratogenic effects. Maternal toxicity
was demonstrated at 84 g/m3. No information is available on the
effects of HCFC 124 on reproductive potential. Full teratogenicity
studies are in progress.
Available data from several bacterial studies and a single
mammalian cell study, show no evidence of mutagenic potential of HCFC
124. An inhalation carcinogenicity study is in progress.
1.7 Effects on humans
No data are available on the effects of HCFC 141b, HCFC 132b,
HCFC 133a, HCFC 123 or HCFC 124 on humans.
The data from a single study on humans occupationally exposed to
HCFC 142b do not allow the effects of HCFC 142b upon humans to be
evaluated independently of many other exposures.
1.8 Effects on other organisms in the laboratory and field
No information is available on the effects on environmental
organisms of the hydrochlorofluorocarbons reviewed, except for limited
data on HCFC 141b and HCFC 142b. The 96-h LC50 of HCFC 141b for
zebra fish is 126 mg/litre and the 48-h EC50 for the immobilization
of Daphnia magna is 31 mg/litre, both observations having been made
in closed vessels. In the case of HCFC 142b, the 96-h EC50 for
guppies is 220 mg/litre while the 48-h EC50 for the immobilization
of D. magna varies from 160 to > 190 mg/litre. The 96-h LC50 of
HCFC 142b for rainbow trout is 36 mg/litre.
1.9 Evaluation and conclusions
Environmental levels for the six HCFCs reviewed are unknown, but
are considered to be low based on current use patterns.
HCFC 142b has a low toxic potential and is not considered to pose
a significant risk to human health under non-accidental exposure
conditions. The toxicological information on HCFC 141b, HCFC 123 and
HCFC 124 are incomplete and more data are required before an
evaluation of the human health hazard can be made. Both HCFC 133a and
HCFC 132b pose a hazard to human health.
All the six hydrochlorofluorocarbons reviewed either have, or are
expected to have, lower ozone-depleting potentials and have
considerably lower atmospheric residence times than the fully
halogenated chlorofluorocarbons. They should, therefore, pose a lower
indirect health risk. The global-warming potentials are, or are
expected to be, lower than those of the fully halogenated
chlorofluorocarbons and should not contribute significantly to global
warming.
Since the toxicity of HCFC 142b is low and the ozone-depleting
and global-warming potentials are lower than those of the fully
halogenated chlorofluorocarbons, it can be considered as a transient
substitute for the chlorofluorocarbons included in the Montreal
Protocol.
No recommendations can be made for HCFC 141b, HCFC 123 or HCFC
124 until more toxicity data become available. Although HCFC 133a and
HCFC 132b pose low environmental and indirect health risks, they are
not recommended as substitutes for the chlorofluorocarbons included in
the Montreal Protocol because of their toxic potential.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
The hydrochlorofluorocarbons (HCFCs) considered in this monograph
are compounds derived by the partial substitution of the hydrogen
atoms in ethane with both fluorine and chlorine atoms. The chemical
formulae, chemical structures, common names, common synonyms, CAS
registry numbers and conversion factors of the compounds reviewed
(HCFC 141b, HCFC 142b, HCFC 132b, HCFC 133a, HCFC 123 and HCFC 124)
are presented in Table 1.
The individual chemical substances have many different trade
names and are characterized by code numbers which are explained in
Table 1.
2.1.1 Technical products
The HCFCs are being developed in part as substitutes for fully
halogenated chlorofluorocarbons: HCFCs 123 and 141b for CFC-11 and in
admixture for CFC-113; HCFCs 124 and 142b for CFC-12 (Hoffmann, 1990).
HCFCs 133a and 142b are chemical intermediates, and one of the
compounds reviewed, HCFC 132b, is still essentially experimental (see
section 3.3.1). When marketed they are usually available at 99.8% (or
more) purity. Impurities in HCFC 142b have been reported at levels of
0.06% HCFC 141b, very much smaller levels of HCFC-22, CFC-11,
HFC-152a, CFC-113 and traces of other compounds (Hutton & Lieder,
1989a).
2.2 Physical and chemical properties
Some physical and chemical properties of the
hydrochlorofluorocarbons reviewed in this monograph are summarized in
Table 2. Under normal temperatures and pressures, they are flammable
(HCFC 142b) or non-flammable gases (HCFC 133a, HCFC 124) or
non-flammable volatile liquids (HCFC 141b, HCFC 132b and HCFC 123).
They are colourless, and the majority of them are practically
odourless or have a faint ethereal odour (HCFC 141b and HCFC 123).
They are slightly or moderately soluble in water and miscible with
many organic solvents. On heating to decomposition, HCFCs 124, 132a
and 142b produce toxic fumes of fluorine- or chlorine-containing
compounds (Sax, 1984) and this is probably true also of the other
hydrochlorofluorocarbons reviewed. HCFC 142b can react vigorously with
oxidizing materials (Sax, 1984).
Table 1. Identity of hydrochlorofluorocarbonsa
HCFC 141b HCFC 142b HCFC 132b
Chemical structure Cl H F H Cl Cl
' ' ' ' ' '
F - C - C - H Cl - C - C - H F - C - C - H
' ' ' ' ' '
Cl H F H F H
Chemical formula CCl2F-CH3 CClF2CH3 CClF2-CH2Cl
Common names dichlorofluoroethane chlorodifluoroethane dichlorodifluoroethane
Common synonyms 1,1-dichloro-1-fluoroethane; 1-chloro-1,1-difluoroethane; 1,2-dichloro-1,1-difluoroethane;
1-fluoro-1,1-dichloroethane; 1,1-difluoro-1-chloroethane; HCFC 132b
ethane, 1,1-dichloro-1-fluoro; difluoromonochloroethane;
HCFC 141b; Propellant 141b; HCFC 142b;
R-141b
CAS Registry number 1717-00-6 75-68-3 1649-08-7
Conversion factors (20 °C)
ppm -> mg/m3 4.85 4.1 5.5
mg/m3 -> ppm 0.206 0.243 0.181
Table 1 (contd).
HCFC 133a HCFC 123 HCFC 124
Chemical structure H F H F F F
' ' ' ' ' '
H - C - C - F Cl - C - C - F Cl - C - C - F
' ' ' ' ' '
Cl F Cl F H F
Chemical formula CH2Cl-CF3 CHCl2-CF3 CHClF-CF3
Common names chlorotrifluoroethane dichlorotrifluoroethane chlorotetrafluoroethane
Common synonymsb 1-chloro-2,2,2-trifluoroethane; 1,1-dichloro-2,2,2-trifluoroethane; 1-chloro-1,2,2,2-tetrafluoroethane;
1,1,1-trifluoro-2-chloroethane; 2,2-dichloro-1,1,1-trifluoroethane; 1,1,1,2-tetrafluoro-2-chloroethane;
2,2,2-trifluorochloroethane; ethane, dichlorotrifluoro-; Fluoro- Fluorocarbon 124; HCFC 124
1,1,1-trifluoroethyl chloride; carbon 123; HCFC 123; Propellant
CFC 133a; HCFC 133a; R-133a 123; Refrigerant 123; R-123
CAS Registry number 75-88-7 306-83-2 2837-89-0
Conversion factors (20 °C)
ppm -> mg/m3 4.92 6.25 5.58
mg/m3 -> ppm 0.203 0.160 0.179
a Chlorofluorocarbons are numbered as follows: the first digit = number of C atoms minus 1 (for ethane derivatives it is therefore
1); second digit = number of H atoms plus 1; third digit = number of F atoms
b The trade names Arcton, Freon, Genetron and Isotron are used with the corresponding numbers by different manufacturers
Table 2. Physical and chemical properties of the hydrochlorofluorocarbonsa
HCFC 141b HCFC 142b HCFC 132b HCFC 133a HCFC 123 HCFC 124
Physical state liquid gas liquid gas liquid gas
Colour colourless colourless colourless colourless colourless colourless
Relative molecular mass 116.95 100.47 134.92 118.49 152.91 136.48
Boiling point (°C) 32.0 -9.2 46.8 6.93 27.97 -11.0
Freezing point (°C) -103.5 -131.0 -101.2 -105.5 -107.0 -199.0
Liquid density (g/ml) 1.24c 1.123c 1.42c 1.389c 1.46b 1.4d
Vapour pressure
(25 °C, psia) 11.5 49.2 6.1 29.7 14 61
Density of saturated
vapour at boiling
point (g/litre) 4.82 4.72 5.15 5.17 6.38 6.88
Flammability non-flammablee flammable non-flammable non-flammable non-flammable non-flammable
Auto-ignition
temperature (°C) - 632 - - - -
Flammability limits in
air (% vol) - 6.0-14.8 - - - -
Table 2 (contd).
HCFC 141b HCFC 142b HCFC 132b HCFC 133a HCFC 123 HCFC 124
Solubility in water
(g/litre) 4-13b 1.9b 4.9c 8.9b 2.1b 17.1c
Octanol/water partition
coefficient (log Pow) 2.3 1.60f - - - -
a From: Graselli & Ritchey (1975), Hawley (1981), Horrath (1982), Sax (1984), Weast (1985), Solvay et Cie (1989)
b At 25 °C
c At 20 °C
d At 11.3 °C
e No flash point between 21 °C and 33 °C; no explosive properties, but can become flammable as a vapour (personal communication by
Solvay et Cie, 1989). Millischer (1990) lists HCFC 141b as non-flammable.
f Log Kow cited in SRC (personal communication by H. Trochimowicz (1991), C-57 file Haskell Laboratories).
2.3 Conversion factors
Conversion factors for the hydrochlorofluorocarbons reviewed in
this monograph are given in Table 1.
2.4 Analytical methods
Of the analytical procedures described for the determination of
the hydrochlorofluorocarbons reviewed, by far the most frequently
applied methods use gas chromatography with various detection
techniques. For measuring the relatively high chamber concentrations
in toxicology experiments, single beam photometry has been used.
Examples are listed in Table 3.
Table 3. Analytical methods for the determination of hydrochlorofluorocarbons
Hydrochlorofluorocarbons Medium Analytical method Detection limit Reference
HCFC 141b air absorption on silica gel, thermal desorption and - Coombs et al. (1988)
gas chromatography with flame ionization detection
corn oil gas chromatography with electron capture detection - Liggett et al. (1989)
HCFC 142b air gas chromatography with flame ionization detection - Seckar et al. (1986)
water head space analysis using gas chromatography with - Hutton & Lieder (1989)
electron capture detection
HCFC 132b air gas chromatography with thermal conductivity - Hall (1976)
detection
HCFC 133a air gas chromatography with flame ionization detection 0.2 ppm Plummer et al. (1987)
air gas chromatography with flame ionization detection - Kilmartin et al. (1980)
air gas chromatography with thermal conductivity detection - Leuschner et al. (1977)
air gas chromatography with flame ionization detection Hodge et al. (1980)
tissue head space analysis using gas chromatography with - Chapman et al. (1967)
flame ionization detection
tissue head space analysis using gas chromatography with 2.5 pmol/ml blood Maiorino et al. (1979)
flame ionization detection 10 pmol/g liver
HCFC 123 air single beam photometry - Müller & Hofmann (1988)
air gas chromatography with flame ionization detection - Deleba-Crowe (1978)
air gas chromatography with thermal conductivity detection - Trochimowicz & Mullin (1973)
HCFC 124 air gas chromatography with thermal conductivity detection - Hall (1976)
air gas chromatography with dual flame ionization detection - Brewer (1977)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
The hydrochlorofluorocarbons reviewed in this monograph are not
known to occur in nature.
3.2 Anthropogenic sources
3.2.1 Production levels
The manufacturing process for HCFC 124 is still in the
developmental stage, since it is not produced for commercial use but
only in research quantities. HCFC 133a is produced in small quantities
as a chemical intermediate in the manufacture of the anaesthetic
halothane (1-bromo-1-chloro-2,2,2-trifluoroethane) (McNeill, 1979),
and HCFC 142b is produced at the rate of several thousand tonnes per
year as an intermediate in the production of vinylidene fluoride for
the manufacture of fluoropolymers (Seckar et al., 1986). Commercial
quantities of HCFCs 123 and 141b were produced in 1991 (Anon., 1991;
personal communication by R. Millischer, 1991). HCFC 132b does not
appear to be envisaged as a commercial product but it occurs, as do
HCFCs 133a and 141b, as a by-product in the manufacture of other
halogenated ethanes. Others of the hydrochlorofluorocarbons reviewed
probably also occur in this way.
3.2.2 Manufacturing processes
HCFC 133a is produced from trichloroethene and anhydrous hydrogen
fluoride in the presence of an antimony trifluoride catalyst (McNeill,
1979). Similarly, HCFC 142b is produced by hydrofluorination of
methylchloroform or vinylidene chloride in the liquid phase (Seckar et
al., 1986).
3.2.3 Loss during disposal, transport, storage and accidents
Since two of the hydrochlorofluorocarbons reviewed which are
produced in commercial quantities (HCFC 142b and HCFC 133a) are used
as intermediates for subsequent conversions into other fluoro
compounds, the current release into the environment is expected to be
low. There are no published data on losses of any of the
hydrochlorofluorocarbons reviewed.
No information is available on accidental release.
3.3 Use patterns
3.3.1 Major uses
HCFC 133a has a limited use as a chemical intermediate in the
manufacture of the anaesthetic halothane, 1-bromo-1-chloro-2,2,2-
trifluoroethane (McNeill, 1979).
HCFC 123 is used in large industrial chillers.
HCFCs 123 and 141b were developed as substitutes for CFC-11, i.e.
as foam-blowing agents in the plastics industry, aerosol propellants
and, to a lesser degree, refrigerants, but their use requires
equipment changes and they have a slightly poorer performance than the
fully halogenated compounds (Hoffmann, 1990; Prinn & Golombek, 1990;
Ahmadzai & Hedlund, 1990).
A mixture of HCFCs 123 and 141b can substitute for CFC-113, a
washing fluid in the electronic industry (Montague & Perrine, 1990).
HCFCs 124 and 142b have been reported, in admixture with other
compounds, as substitutes for fully halogenated chlorofluorocarbons,
particularly as foam blowing agents and refrigerants. However, the
mixtures developed which contained HCFC 142b were flammable (Hoffmann,
1990; Shankland, 1990). HCFC 142b in admixture with CFC-22 has a small
application as an aerosol propellant.
HCFC 132b appears to be an experimental chemical with no
commercial application at the present time.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Biodegradation and bioaccumulation
Information on biodegradation in the environment is limited to
studies on HCFCs 141b and 142b. Oyama (1990) tested the
biodegradability of HFA 141b by microorganisms (closed bottle method)
at test substance concentrations of 2.0 and 9.0 mg/litre and reported
a biodegradation of 2-10% after 28 days. The biodegradability values
of HCFC 142b at concentrations of 52 and 105 mg/litre were 5.6 and
4.4%, respectively, after 28 days (Matla & Blom, 1991). In both cases
the authors concluded that these compounds are not readily
biodegradable.
The log octanol/water partition coefficient of HCFC 141b is 2.3
and therefore bioaccumulation of this hydrochlorofluorocarbon is
unlikely.
A study on the biodegradation of HCFC 142b is in progress
(personal communication by Ch. de Rooij, Solvay et Cie, 1990).
4.2 Environmental transformation and interaction with other
environmental factors
The scaled atmospheric lifetimes, ozone-depleting potentials and
global-warming potentials of the hydrochlorofluorocarbons reviewed are
shown in Table 4, where they are compared to those of methylchloroform
(1,1,1-trichloroethane). The physical and chemical properties suggest
that these hydrochlorofluorocarbons would be rapidly mixed within the
lower region of the troposphere. Mixing would be expected to be
complete in the hemisphere of the emission (northern or southern)
within months and in the entire troposphere possibly within about
three years. Reaction with naturally occurring hydroxy radicals (OH€)
in the troposphere is expected to be the primary degradation route
(Makide & Rowland, 1981; Prinn & Golombek, 1990).
The hydrochlorofluorocarbons reviewed do not have high
ozone-depleting potentials. This is defined as the calculated
depletion due to the emission of a unit mass of the
hydrochlorofluorocarbon divided by the ozone depletion calculated to
be due to the emission of a unit mass of CFC 11 (the ozone-depleting
potential of CFC 11 is 1.0); calculations are based on steady-state
conditions.
However, if the ozone-depleting potentials listed are compared
with those of methylchloroform (1,1,1-trichloroethane), it can be seen
that those of HCFCs 141b and 142b are of a similar order. The parties
to the Montreal Protocol decided in June 1990 to phase out
methylchloroform manufacture by the year 2005 (Ahmadzai & Hedlund,
1990).
When the global-warming potentials are similarly compared, three
of the compounds reviewed, HCFCs 141b, 142b, and 124, have higher
values than that of methylchloroform and HCFC 123 is only slightly
lower. This effect however, is considered less critical (Montague &
Perrine, 1990).
The possible impact of HCFCs 141b, 142b, 123 and 124 on
tropospheric ozone formation has been estimated to be extremely low
(UNEP/WMO, 1989).
Table 4. Tropospheric lifetime, ozone-depleting potential and global-warming potential
of hydrochlorofluorocarbonsa,b
Hydrochlorofluorocarbon Scaled atmospheric Ozone-depleting potential Global-warming
lifetime potential
(years)c 1-dimensional model 2-dimensional model (1-dimensional model)
HCFC 141b 7.8 0.066-0.092 0.065-0.14 0.087-0.097
HCFC 142b 19.1 0.05-0.06 0.05-0.08 0.34-0.39
HCFC 132b 4.2 0.025 - -
HCFC 133a 4.8 - - -
HCFC 123 1.6 0.013-0.019 0.013-0.027 0.017-0.020
HCFC 124 6.6 0.016-0.021 0.013-0.030 0.092-0.10
Cl3C CH3 6.3 0.092-0.14 0.11-0.20 0.022-0.026
a From: Fisher et al. (1990a,b) and Freemantle (1991). See also Prinn & Golombek (1990).
b The ozone-depleting and global-warming potential values for CFC-11 are defined as 1.0. The values reported
in the table refer to this standard and the ranges are scaled assuming a methylchloroform atmospheric
lifetime of 6.3 years (UNEP/WMO, 1989).
c Other atmospheric lifetimes are: CFC 11 - 75 years, CFC 12 - 110 years and CFC 113 - 90 years.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
HCFCs 141b, 132b, 133a, 123 and 124 are not yet in large-scale
commercial production. HCFC 142b is used as an intermediate and is not
released significantly to the atmosphere. There are, therefore, no
data on environmental levels but these compounds are unlikely to be
present at detectable levels.
5.1.2 Water, food and other edible products
For the reasons cited above (section 5.1.1), no data are
available on the concentrations in environmental water, food or other
edible products of the partially halogenated chlorofluorocarbons
reviewed in this monograph.
5.2 Human exposure
There are no data on human exposure to any of the
hydrochlorofluorocarbons reviewed.
As HCFC 133a is a major metabolite of the anaesthetic halothane,
the use of this anaesthetic may constitute an exposure to this
hydrochlorofluorocarbon.
6. KINETICS AND METABOLISM
6.1 Animal studies
6.1.1 Absorption
No data are available concerning direct measurements of the
absorption of the hydrochlorofluorocarbons reviewed, but it may be
inferred from toxicity studies that absorption occurs (see chapter 7).
The increased urinary fluoride levels observed in inhalation
toxicity studies of HCFC 141b (Doleba-Crowe, 1977), HCFC 132b, HCFC
123 (Doleba-Crowe, 1978; Trochimowicz, 1989; Malley, 1990a) and HCFC
124 (Brewer, 1977; Malley, 1991) also indicate that absorption takes
place (see section 7.1).
6.1.2 Distribution
No data are available on the distribution in animals of the
hydrochlorofluorocarbons reviewed.
6.1.3 Metabolic transformation
6.1.3.1 General considerations
The compounds reviewed in this monograph fit into three
generalized structural classes: 1,1,1-trihaloethanes (HCFC 141b and
142b), 1,1,1-trihalo-2-monohaloethanes (HCFC 132b and 133a) and
1,1,1-trihalo-2,2-dihaloethanes (HCFC 123 and 124). Metabolic studies
have been conducted on a representative from each class and have
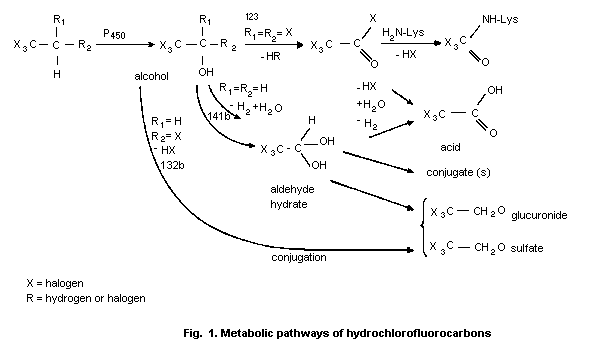
demonstrated similar metabolic pathways for each one as shown in
Fig. 1.
Based on the available literature (Harris & Anders, 1991a,b;
Harris et al., 1991), it is expected that all six chemicals reviewed
would be metabolized by a cytochrome P-450-dependent monooxygenase
liver enzyme to give reactive metabolic products including
1,1,1-trihaloacetic acid and 1,1,1-trihaloethanol. The
1,1,1-trihalo-2,2-dihaloethanes are the only ones that go through the
electrophilic acid chloride in their metabolic pathway. This may
explain why HCFC 123 is the only one, of the three compounds for which
there are data, that shows covalent binding to macromolecules. Salmon
et al. (1981) reported on microsomal dechlorination of chloroethanes
and structure-activity relationships and observed that the
reactivities of the various structural types are markedly different:
RCHCl2 >> RCH2Cl > RCCl3 (in the case of polyhalogenated
ethanes where a less reactive group is linked to the one under
consideration, the contribution of the less reactive group is
ignored). The authors concluded that the dechlorination process shows
the structural specificity commonly seen in enzyme-catalysed
reactions.
 6.1.3.2 HCFC 141b
Harris & Anders (1991a) studied the in vivo metabolism of HCFC
141b. A single fluorinated urinary metabolite, identified as
2,2-dichloro-2-fluoro-ethyl glucuronide, was found in rats exposed to
HCFC 141b (56 g/m3) in air for 2 h. The metabolism was reported to
be similar to that of its chlorinated analogue 1,1,1-trichloroethane,
which is metabolized to 2,2,2-trichloroethanol and excreted as its
glucuronate conjugate (Hake et al., 1960) and as trichloroacetic acid
(Koizumi et al., 1982). It was claimed (although no data were given)
that 2,2-dichlorofluoroacetic acid was also detected in the urine of
rats exposed to a concentration of 194 g/m3 for 4 h, but not to 56
g/m3 for 2 h (Harris & Anders, 1991a).
In a pilot study for absorption and metabolism of HCFC 141b,
seven groups of five male rats were exposed to the vapour by
inhalation in a closed loop exposure system (concentrations ranging
from 0.4 to 12 g/m3). No metabolism was detected but the sensitivity
of the method is such that it will not detect metabolism below 0.15%.
The results suggested that absorption did not take place, but that if
any metabolism occurred it was at a low level (Zwart, 1989).
Evidence of dechlorination was observed when rat hepatic
microsomes were incubated with about 1% HCFC 141b in vitro (Van
Dyke, 1977).
6.1.3.3 HCFC 142b
No in vivo studies on the metabolism of HCFC 142b have been
reported. One in vitro study provided evidence for dechlorination
when rat hepatic microsomes were incubated with 0.6% HCFC 142b (Van
Dyke, 1977).
6.1.3.4 HCFC 132b
Harris & Anders (1991) identified a number of metabolites in the
urine of male Fischer-344 rats given one or four doses of 10 mmol/kg
dissolved in corn oil by intraperitoneal injection. Approximately 1.8%
of the single administered dose was recovered in the urine. The
metabolites excreted in urine during the first 6 h were
2-chloro-2,2-difluoroethyl glucuronide, chlorodifluoroacetic acid and
chlorodifluoroacetaldehyde hydrate (free and conjugated). Repeated
injection of rats with HCFC 132b significantly increased both the rate
of chlorodifluoroacetic acid excretion and the relative fraction of
the HCFC 132b dose excreted as chlorodifluoroacetic acid. The
incubation of HCFC 132b with rat hepatic microsomes yielded
chlorodifluoroacetaldehyde hydrate as the only fluorinated product.
The in vitro metabolism of HCFC 132b was increased in microsomes
from pyridine-treated rats and inhibited by p-nitrophenol. This
inhibition by p-nitrophenol led the authors to suggest an
involvement of cytochrome P-450 IIE1 in the initial hydroxylation of
HCFC 132b.
6.1.3.5 HCFC 133a
Evidence for dechlorination of HCFC 133a was provided by an
in vitro study using a microsomal preparation derived from
Aroclor-1254a-induced rat liver homogenates (Salmon et al., 1981).
6.1.3.6 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 g/m3 or 68 g/m3) or to halothane (2-bromo-2-2
chloro-1,1,1-trifluoroethane) (1.05 g/m3) in air for 2 h. The
pattern of proteins immunoreactive with haptens-specific
anti-trifluoroacetylprotein antibodies was found to be identical in
livers of the rats exposed to HCFC 123 and halothane. Trifluoroacetic
acid was detected in urine of rats exposed to HCFC 123 or halothane by
nuclear magnetic resonance (NMR) and by gas chromatography with mass
spectrometry (GCMS), as had been reported previously (Maiorino et al.,
1980; Trochimowicz, 1989).
6.1.4 Covalent binding to macromolecules
6.1.4.1 HCFC 141b
19F-NMR analysis of microsomal and cytosolic proteins isolated
from the livers of rats killed 15 h after a 2-h exposure to HCFC 141b
(56 g/m3) did not yield any evidence for covalent binding of
fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.2 HCFC 132b
19F-NMR analysis of microsomal and cytosol proteins isolated
from the livers of rats killed 15 h after a single intraperitoneal
dose of HCFC 132b (10 mmol/kg) did not yield evidence for covalent
binding of fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.3 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 or 68 g/m3) or to halothane (105 g/m3) in air for 2 h.
19F-NMR analysis of cytosolic protein and immunoblotting of
microsomal and cytosolic hepatic protein using antibodies against
trifluoroacetylprotein at 15 h after the exposure demonstrated
covalent binding of fluorinated metabolites.
a Aroclor-1254 is a polychlorinated biphenyl mixture.
6.1.5 Elimination
No data are available from animal studies on the elimination of
the hydrochlorofluorocarbons reviewed. However, based on the
information on chlorofluorocarbons, it is likely that the main route
of excretion for hydrochlorofluorocarbons is through the respiratory
tract. Increased urinary inorganic fluoride has been observed in some
inhalation toxicity studies with HCFCs 141b, 132b, 123, and 124
(Doleba-Crowe, 1977, 1978; Brewer, 1977; Trochimowicz, 1989; Malley,
1990a, 1991).
6.2 Human studies
No data are available on the absorption, distribution, metabolic
transformation or elimination of the hydrochlorofluorocarbons
reviewed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute oral toxicity
Available data indicate low toxicity following single oral
exposure associated with four hydrochlorofluorocarbons, i.e. HCFC
141b, 142b, 132b and 123.
7.1.1.1 HCFC 141b
No mortality was observed in rats given a single oral dose of
HCFC 141b (5 g/kg body weight) dissolved in corn oil (Sarver, 1988).
7.1.1.2 HCFC 142b
Other than piloerection, oral dosing with HCFC 142b (up to 5 g/kg
body weight) dissolved in corn oil resulted in no signs of toxicity
(Liggett et al., 1989).
7.1.1.3 HCFC 132b
According to Henry (1975), the lowest dose at which mortality was
observed in rats treated with HCFC 132b was 25 g/kg.
A 2-g/kg dose of HCFC 132b in corn oil was applied by stomach
tubes to five male and five female Wistar rats, and the animals were
observed for 14 days. The clinical signs were indicative of an effect
on the autonomic nervous system (ptosis), on the central nervous
system (diminished alertness and startle response, and positional
passivity), on motor coordination (abnormal body posture and gait, and
loss of righting reflex), on motor activity and on muscle tone
(paralysis). Macroscopic examination showed swollen livers in some
males, and a decrease in absolute and relative liver weights. However,
no treatment-related effects were found during microscopic
examinations (Janssen & Pot, 1989b).
7.1.1.4 HCFC 123
The lowest dose of HCFC 123 producing lethality in rats was
reported to be 9 g/kg when it was administered as a corn oil solution
by intragastric intubation (Henry, 1975a).
7.1.2 Acute inhalation toxicity
7.1.2.1 HCFC 141b
The effects of a single inhalation exposure of rodents to HCFC
141b are shown in Table 5. The main effects at high exposure
concentrations include central nervous system depression, anaesthesia
and death.
7.1.2.2 HCFC 142b
Table 6 summarizes the effects of single inhalation exposure to
HCFC 142b in mice and rats. At high exposure levels, HCFC 142b induces
anaesthesia and death.
7.1.2.3 HCFC 132b
Table 7 summarizes the effects of single inhalation exposures of
mice and rats to HCFC 132b.
7.1.2.4 HCFC 133a
Inhalation of high concentrations of HCFC 133a is characterized
by signs of anaesthesia followed by death, but recovery from nonlethal
exposure is rapid. The effects of inhalation exposure to HCFC 133a are
summarized in Table 8.
7.1.2.5 HCFC 123
The results of studies on the acute inhalation toxicity of HCFC
123 are summarized in Table 9. Anaesthesia and death at higher
exposures were reported for rats and hamsters. No gross morphological
changes were observed in animals that died during exposure. Survivors
recovered within several minutes without showing any observable
clinical signs.
7.1.2.6 HCFC 124
Table 10 summarizes the effects of single inhalation exposure to
high concentrations of HCFC 124. As with other
hydrochlorofluorocarbons, the main effects observed were anaesthesia
and death.
7.1.3 Acute dermal toxicity
There is information only on the hydrochlorofluorocarbons that
are liquid at ambient temperatures, i.e. HCFC 141b, HCFC 132b and HCFC
123.
Table 5. Effects of a single inhalation exposure to HCFC 141b in mice and rats
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3) (h)
Rat 142-366 4 LC50 = 295 g/m3 Hardy et al. (1989a)
(Sprague-Dawley) No deaths were observed at 142 or 217 g/m3. All deaths
at higher concentrations (323 and 366 g/m3) occurred
during exposure, and were preceded by disturbed breathing.
Reduced motor activity, abnormal body carriage, restless
behaviour and exaggerated respiratory movements were seen
at all concentrations during exposure. No treatment-related
macroscopic findings were seen. Focal basophilic staining
was observed in the renal cortical tubules of 4 out of 10
rats at 217 g/m3 and 2 out of 10 rats at 142 g/m3. No
histopathological effects were seen in decedents.
Rat (strain unspecified 6 The lowest concentration producing lethality was 242 g/m3. Doleba-Crowe (1977)
unspecified) range
Mouse 17-388 6 Deaths preceded by signs of narcosis in 6 out of 10) animals Vlachos (1989)
(Crl: CD-1) occurred within 30 min of exposure to 388 g/m3. No deaths
occurred at the next highest (199 g/m3) concentration. Signs
of CNS depression (lethargy, abnormal gait, partially closed
eyes) were seen at 165 and 199 g/m3. No effects were seen at
concentrations of 145 g/m3 or less.
Mouse (strain unspecified 2 LC16 = 115 g/m3; LC50 = 151 g/m3; LC84 = 200 g/m3. Signs of Nikitenko & Tolgskaja
unspecified) range CNS depression and anaesthesia were observed. Death was (1965)
preceded by laboured breathing.
Mouse (Schofield unspecified 0.5 LC50 = 115 g/m3; concentration producing anaesthesia in Davies et al. (1976)
strain) range 50% of animals = 62 g/m3. No other information was given.
Table 6. Effects of single inhalation exposure to HCFC 142b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse "white" up to 2050 2 death; LC50 = 1514 g/m3 Nikitenko & Tolgskaja (1965)
Mouse AP unspecified 0.5 death; LC50 = 1228 g/m3 Davies et al. (1976)
range
Rat "white" 615-3280 0.5 death at 2050 g/m3 and unconsciousness at Lester & Greenberg (1950)
1230 g/m3; postural, righting and corneal
reflexes were lost at 820 g/m3
Rat Sherman 525 4 death (approx 50%) at 525 g/m3 Carpenter et al. (1949)
Table 7. Effects of single inhalation exposure to HCFC 132b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse unspecified range not given 0.5 LC50 = 269 g/m3; AC50 (anaesthesia) = 71 g/m3 Raventós & Lemon (1965)
Rat Wistar derived 55 and 110 4 lethalities at 110 g/m3; rats unsteady, weak and Torkelson (1971)
drowsy at 55 g/m3
Rat Wistar derived 33-72 6 anaesthesia at 82 g/m3; kidney swelling at autopsy; Janssen (1988)
CPB-WU at all dose levels, males showed decreased growth
and testis weight, and increased liver and lung
weights
Rat Wistar derived 55 0.4 decreased respiratory rate with rapid recovery Janssen (1989b)
CPB-WU after exposure
Table 8. Effects of single inhalation exposure to HCFC 133a
Species Exposure Exposure Effectsa Reference
(strain) concentration duration
(g/m3) (min)
Mice, male unspecified 10 anaesthesia and death; convulsions on recovery; AC50 and Robbins (1946)
(white) range LC50 were 394 and 1230 g/m3, respectively
Mice (strain unspecified 30 anaesthesia, convulsions and death; AC50 and LC50 were Raventós & Lemon (1965)
unspecified) range 212 and 738 g/m3, respectively
Mice (strain 123-1230 10 rapid onset of anaesthesia, rapid recovery after cessation of Shulman & Sadove (1965)
unspecified) exposure but no convulsions; AC50 and LC50 were 397 and
1033 g/m3, respectively
Rats, female 2500 - lack of muscular coordination in 3 min, anaesthesia in 4 min Diggle & Gage (1956)
(strain unspecified) and death within 8 min
Dogs unspecified - anaesthesia at 492 g/m3, respiratory depression and arrest Shulman & Sadove (1965)
range occurred at 1131 and 1427 g/m3, and circulatory arrest at
2902 g/m3
a AC50 = calculated concentration expected to produce anaesthesia in 50% of the test group
Table 9. Acute inhalation toxicity of HCFC 123
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3)
Mouse (strain not given 30 min LC50 = 463 g/m3 Raventós & Lemon (1965)
unspecified)
Rat (Charles 129-344 4 h LC50 = 200 g/m3; loss of mobility, lethargy, prostration Hall & Moore (1975)
River CD) at all concentrations; full recovery of survivors within
30 min post exposure
Rat (Charles 49-767 6 h LC50 = 329 g/m3; anaesthesia at 145 g/m3 and higher Coate (1976a)
River CD) concentrations; discoloration of lungs in most animals that
died, discoloration of liver in some of them
Rat (strain 6, 16, 31, 62 15 min unconditioned reflexes, locomotor activity, coordination Trochimowicz (1989)
unspecified) affected at 31 and 62 g/m3; full recovery within 30 min
post exposure
Hamster 63-194 4 h LC50 = 178 g/m3; incoordination, prostration at all Darr (1981)
(Chinese) concentrations; full recovery of survivors after exposure;
0% mortality at 163 g/m3, 100 mortality at 194 g/m3
Table 10. Acute inhalation toxicity of HCFC 124
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (min)
Mouse unspecified 594 10 no effect Wada (1977)a
837 10 narcosis Wada (1977)a
2230 10 no mortality Wada (1977)a
2460 10 death Wada (1977)a
Rat Sprague-Dawley 268 240 no effect Kelly (1990)
Charles River COBS 558 300 reduced activity Coate (1976b)
Sprague-Dawley 893 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1283 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1674 240 death Kelly (1990)
Charles River COBS 2009 300 narcosis, no mortality Coate (1976b)
Dog 2230-3910 10 narcosis Van Poznak & Artusio (1960)
a Attachment to correspondence from H. Wada, Daikon Kogyo Company Ltd. to M.B. Berenbaum, Allied Chemical Corporation,
entitled Anaesthetic activity and fatality (F-123, 123a, 124 and 11)
7.1.3.1 HCFC 141b
No deaths occurred at dermal doses of 2 g/kg body weight either
among rats (Janssen & Pot, 1988; Gardner, 1988) or rabbits (Brock,
1988a).
7.1.3.2 HCFC 132b
When Janssen & Pot (1989a) applied a single dose of HCFC l32b (2
g/kg) under an occluding dressing to the shaved skin of five male and
five female Wistar rats, there were no deaths. The clinical signs
observed were decreased respiratory rate, decreased startle response,
altered locomotor activity, restlessness and vocalization. Three male
and four female rats had swollen or slightly swollen livers on
autopsy.
7.1.3.3 HCFC 123
Several limit tests for dermal toxicity of HCFC 123 were
conducted. No mortality was observed at the limit dose of 2 g/kg body
weight in rats (Brock, 1988d; Trochimowicz, 1989) or rabbits (Brock,
1988e,f; Trochimowicz, 1989). The only clinical signs of toxicity were
red nasal or ocular discharges in one of five male and one of five
female rats, and slight to moderate body weight losses (up to 12% of
initial body weight). No gross pathological abnormality was observed
(Trochimowicz, 1989). In rabbits, only slight to moderate erythema was
observed (Trochimowicz, 1989).
7.2 Short-term inhalation exposure
7.2.1 HCFC 141b
Nikitenko & Tolgskaja (1965) reported a reduction in body weight
gain, a slight decrease in haemoglobin level and moderate
leucocytosis, some "minor changes" in blood parameters related to
liver and kidney function, and histopathological effects in the
respiratory tract of rats and guinea-pigs (number and strains
unspecified) that had been exposed to 40-50 g/m3 (2 h/day, 6
days/week) for 4 weeks. The purity of the substance and the specific
isomer were not indicated.
No adverse clinical signs and only "slight biochemical changes"
(no details given) were reported in rats (number and strain
unspecified) exposed to 48.5 g/m3 (6 h/day, 5 days/week) for 2 weeks
(Pennwalt Corporation, 1987).
In a 2-week inhalation toxicity study, Doleba-Crowe (1977)
exposed groups of 10 male rats to 0 or 48 g/m3 for 6 h/day, 5
days/week. The animals were observed for 14 days after exposure. No
adverse clinical signs were observed, and there were no differences in
body weights between treated and control animals. After the tenth
exposure, elevated red blood cell counts, plasma bilirubin level and
increased urinary fluoride concentrations were found, but all these
parameters returned to normal after 14 days. The treated animals
showed a more severe focal interstitial pneumonitis than controls 14
days after exposure, but no other treatment-related change was
observed.
Coombs et al. (1988) exposed five groups of 10 male and 10 female
Sprague-Dawley rats to 0, 24, 42, 68 and 97 g/m3 (6 h/day for 9
days, i.e. 5 days of exposure followed by 1 day without exposure and
then 4 days with exposure). Signs of central nervous system (CNS)
depression were seen during exposure to concentrations of 42 g/m3 or
more. At 97 g/m3, these signs were accompanied by a decrease in body
weight gain in males and a slightly reduced food intake in both sexes.
Glucose and aspartate serum transaminase (AST) levels were increased
at 97 g/m3, protein, cholesterol and sodium from 68 g/m3,
phosphate from 42 g/m3 and calcium from 24 g/m3. No
treatment-related histopathological changes were observed at any dose
level.
In a 13-week inhalation study (some animals were killed after 4
weeks), four groups each of 15 male and 15 female Fischer-344 rats
were exposed to 0, 10, 39 or 97 g/m3 (6 h/day, 5 days/week) as
described in two reports (Yano et al., 1989; Landry et al., 1989).
Alertness was reduced at 97 g/m3, and body weight gain and food
consumption were slightly reduced in all exposed groups. After both 4
and 13 weeks of exposure, plasma cholesterol, triglycerides and
glucose were slightly elevated in the rats exposed to 97 g/m3. No
changes in haematological or histopathological parameters were found.
7.2.2 HCFC 142b
Rats and guinea-pigs (numbers and strains not specified) were
exposed to a concentration of 448 g/m3 (isomer not specified), 2
h/day, 6 days/week, for 4 weeks (Nikitenko & Tolgskaja, 1965). A
decrease in the rate of body weight gain was observed at the end of
the study, as well as a reduction in haemoglobin concentration and the
number of erythrocytes, and an increase in the number of leucocytes.
Swelling of the alveolar septa and peribronchitis were the
histopathological changes observed in the lungs.
In a study in which 10 adult white rats were exposed to 410
g/m3 for 16 h/day, all animals died within 9 exposures. All of them
showed severe signs of pulmonary irritation at autopsy (consolidation
and hepatization of the lungs). The other organs appeared normal. No
signs of ill health were apparent in five rats exposed to a
concentration of 41 g/m3 (16 h/day for 2 months). Gross examination
of the organs on autopsy revealed no pathological changes, but
microscopic examination of the lungs showed round cell infiltration in
the lung of two animals. The appearance of sections of the livers was
normal (Lester & Greenberg, 1950).
No clinical, haematological, blood chemical, urine analytical or
histopathological evidence of effects attributable to repeated
exposure to HCFC 142b was found in 10 male Charles River CD rats
exposed to a concentration of 82 g/m3 (6 h/day, 5 days/week) for 2
weeks (Moore & Trochimowicz, 1976).
Kelly (1976) did not find any adverse clinical, haematological,
blood chemical, urine analytical or histopathological effects
attributable to HCFC 142b at exposure levels of either 4.1 g/m3 or
41 g/m3 (6 h/day, 5 days/week for 90 days) in groups of male and
female Charles River CD rats (27 of each sex at each treatment level)
or groups of male dogs (4 at each treatment level).
7.2.3 HCFC 132b
When 20 male Crl:CDRBR rats were exposed to 55 g/m3 (6 h/day,
5 days/week) for 2 weeks, reduction in body weight gain, irregular
respiration and CNS depression (lethargy, poor coordination,
occasional tremors and prostration) were seen. The CNS effects
disappeared within 30 min after each exposure. Pathological
examinations of rats sacrificed immediately after the tenth exposure
showed thymic atrophy and spermatogenesis arrest, but these changes
were not present in rats sacrificed 14 days after exposure ceased
(Hall, 1976).
Groups of 20 male and 20 female Crl:CDRBR rats were exposed to
0, 3, 11 and 27 g/m3 (6 h/day, 5 days/week) for 13 weeks (Kelly,
1988). Male rats exposed at all the concentrations of HCFC 132b showed
bile duct proliferation and disruption of spermatogenesis with cell
debris in the epididymides at the two higher concentrations. Other
effects included increases in liver/body weight ratio in males at all
concentrations and in females at the two higher concentrations.
Elevation of serum alkaline phosphatase activity was found in both
sexes exposed to 11 or 27 g/m3. During the study, all groups exposed
to HCFC 132b showed reduced food consumption and body weight gain. In
the two highest exposure groups there were depressions in the absolute
but not relative brain and testes weights. Other organ weight changes
were also seen (slight increases in heart, lung and kidney weights).
The biological significance of these weight changes is not clear since
there were no accompanying histological findings. During exposure to
27 g/m3, rats showed CNS depression as indicated by decreased
activity and low responsiveness to sound.
7.2.4 HCFC 133a
Shulman & Sadove (1965) exposed mice to anaesthetic
concentrations (the actual concentration was not specified) of HCFC
133a for 30 min per day on 12 consecutive days, and the animals were
killed for pathological evaluation after the last exposure by
overdosage of HCFC 133a. None of the mice showed any treatment-related
clinical effects, and no pathological changes were found in the organs
(heart, lung, liver, kidney, adrenal gland, spleen and pancreas)
examined microscopically.
Diggle & Gage (1956) investigated the effects of repeated
exposure (up to 8 days) of groups of 2-3 female rats. Concentrations
of HCFC 133a between 50 and 125 g/m3 caused incoordination and
lethargy, while at 250 or 500 g/m3 rats become comatose. They
recovered between each exposure and no dose-related pathological
changes were found on histological examination. No effect was seen at
25 g/m3 during seven exposures lasting 6 h/day.
In a study by Leuschner et al. (1977), 20 male and 20 female
Sprague-Dawley rats were exposed to 49 g/m3 (6 h/day, every day) for
90 days. Corresponding groups of 20 male and 20 female rats were used
as controls. Observations for overt clinical signs of toxicity and
investigations on body weight, food consumption, haematology, blood
and urine biochemistry, urine sediments, ophthalmology, auditory
reflex, organ weights, and histopathology were performed. There were
no treatment-related deaths. The rats were sedated during each
exposure but appeared normal before and after. Seventeen out of 40
rats developed bloody and inflamed noses; this was associated with
histological evidence of inflammatory changes of the mucosa. Body
weight gain was reduced, so that the terminal average body weights
were approximately 28 and 17% lower than those of male and female
controls, respectively. Food consumption in the treated groups was
also lower than in the controls. Haemoglobin concentration,
haematocrit, red blood cell counts and platelet counts were all
slightly reduced. Reduction in leucocyte counts of approximately 30%
and increase in reticulocyte counts of approximately 40% were seen.
There were reductions in plasma glucose levels of approximately 15%
and in protein levels of approximately 10%. Bromosulfophthalein
retention time was increased by approximately 35% and 62% in males and
females, respectively. There was no change in plasma enzyme
glutamic-pyruvic transaminase (GPT), alkaline phosphatase (AP) or
glutamicoxalacetic transaminase (GOT) activity. The thymus to body
weight ratio was reduced by approximately 50% and the testis and ovary
to body weight ratios by approximately 60 and 35%, respectively.
Histologically, these organs showed atrophy. Thyroid to body weight
ratio was increased by approximately 45% in males only. Atrophy of the
spleen was also observed. The exposure induced emphysema and oedema of
the lungs as well as bronchitis and pneumonia. The testicular atrophy
was consistent with the findings in three dominant lethal studies in
mice (Hodge et al., 1979, 1980; Kilmartin et al., 1980) and a
carcinogenicity study in rats (Longstaff et al., 1984) (see section
7.6 and 7.7).
Six beagle dogs were exposed by Leuschner (1977) to 24 g/m3
(6 h/day, daily) for 3 months and six control dogs were used. No
effects were seen on external appearance, faeces, food and water
consumption, body weight gain, haematology, blood and urine
biochemistry, urine sediments, electrocardiography, blood pressure,
ophthalmology, hearing or dentition. There was no effect on organ
weight at autopsy. No treatment-related histopathological changes were
seen on microscopic examination of a standard range of 24 tissues.
In two dominant lethal studies (for experimental details see
section 7.5.1.4), male mice were exposed to between 0.5 and 49 g/m3,
6 h/day, for 5 days (Hodge et al., 1980; Kilmartin et al., l980). The
mice were subdued at exposure concentrations of 2 g/m3 or more.
Deaths occurred as follows: in the first study 0/60 mice died at 0
g/m3, l7/60 at l2 g/m3, and 28/80 at 49 g/m3; in the second
study 0/80 died at 0 g/m3, 2/59 at 0.5 g/m3, 0/60 at 2.5 g/m3,
5/60 at 5 g/m3, and 20/60 at 12 g/m3.
7.2.5 HCFC 123
In a study by Doleba-Crowe (1978), Sprague-Dawley rats and beagle
dogs were exposed to concentrations of 0, 6 and 62 g/m3 (6 h/day, 5
days/week) for 90 days. At the high exposure level, both species
exhibited lack of motor coordination soon after the start of exposure.
This was followed by reduced motor activity and reduction in
responsiveness to noise. After removal from exposure, coordination and
activity returned to normal within 20 min. Other than final body
weight reductions and increased urinary fluoride level at both
exposure levels, no significant exposure-related effects were observed
in rats. At the high exposure level, dogs exhibited histopathological
changes in the liver and clinical chemistry changes including
increased levels of serum GOT and GPT, which might indicate slight
liver damage. No exposure-related effect was noted at the lower
exposure level.
In a 90-day inhalation study, albino rats obtained from Charles
River Breeding Laboratory were exposed to nominal levels of 0, 3, 6,
and 31 g/m3, 6 h/day, 5 days/week (Rusch, 1985). No
treatment-related deaths occurred in the study and mean body weight
reductions observed in the males at the highest exposure level and in
females at the two highest exposure levels were significant only at
the end of the study. Slight depression was observed in heart weight
in both male and female rats exposed to 31 g/m3. While depressions
of the kidney weights and kidney/brain weight ratio (but not kidney to
body weight ratio) were observed in male rats in all the three
exposure groups, these effects were outside the normal range only at
the highest exposure level. A similar depression in kidney weight and
kidney/body weight ratio (but not in kidney/brain ratio) occurred in
females at the highest exposure level. Increased liver/body weight
ratios (but not liver weight or liver/brain weight ratios) were
observed in all three exposure groups of females, but in males only at
the highest exposure level. No significant difference was found in
organ weights or ratios in animals sacrificed at the end of a 30-day
recovery period. The absence of histopathological findings and of
effects at the end of the recovery period indicates that the
toxicological significance of the organ weight changes in this study
is unclear.
In a 4-week inhalation toxicity study (Kelly, 1989; Trochimowicz,
1989), rats (10 of each sex at each exposure level) were exposed to 0,
6, 31, 62 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Statistically significant body weight depression occurred in all
female groups and in the two highest male groups, but was dose-related
only in the male rats. At concentrations of 31 mg/m3 or more, rats
exhibited dose-related anaesthesia. At 62 and 125 g/m3, rats became
lethargic during exposure but were normal when they were examined
again 16 to 18 h after exposure. A dose-related increase in liver/body
weight ratio was observed in all female groups (a 27% increase at the
highest level) and in the two highest-exposure male groups (an 18%
increase at the highest level). Decreased cytochrome P-450 activity in
the liver was also found in all female exposure groups and in the male
groups exposed at the two highest levels. Histopathological
examination showed no adverse effects attributable to HCFC 123 in
liver or in any other organ at any exposure level. Microscopic
examination revealed that testicular degeneration and hypospermia
occurred in two out of four male exposure groups, but this was
believed to be due to reduced body weight (because of an increase in
relative testicular weight without a corresponding increase in
absolute testicular weight) or may have resulted from spontaneous
lesions. The fact that the incidence of testicular degeneration was
highest in the 31- and 125-g/m3 exposure groups (5 out of 10 and 6
out of 10 animals, respectively), and lower in the 0-, 6-, and
62-g/m3 exposure groups (2 out of 10, 1 out of 10 and 2 out of 10
animals, respectively) is consistent with the sporadic nature of this
lesion in this strain of rat. However, it is not possible to evaluate
the significance of this testicular effect from the information
available.
Another 28-day inhalation toxicity study in rats was conducted to
characterize further the potential effects of HCFC 123 on the liver
(Lewis, 1990). In addition, urine samples were examined to identify
metabolites of HCFC 123. Groups of six male Charles River CD rats were
exposed to 0, 6, 31 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Body weights were statistically significantly reduced in all treated
groups compared to controls, the greatest reduction occurring in the
high-dose group. A concentration-related decrease in serum cholesterol
levels was found in all test groups. This reduction was statistically
significant compared to controls at the medium and high
concentrations. Serum triglyceride levels were significantly reduced
to a similar extent in all treated groups. A statistically significant
increase in absolute and relative liver weights compared to controls
was seen in the high-dose rats. Hepatocyte hypertrophy and mild fatty
vacuolation was found at all concentrations tested but the severity
was greatly reduced at the low exposure level. Electron microscopic
examination revealed a treatment-related induction of peroxisome
proliferation at the medium and high exposure concentrations. A
statistically significant concentration-related increase in relative
testes weight was observed in all treated groups (11-30% above
control), but no compound-related morphological or microscopic changes
were observed. Urine analysis indicated the presence of
trifluoroacetic acid as a major metabolite.
In a study by Malley (1990a), groups of 10 male and 10 female
Crl:CDRBR rats were exposed to concentrations of 0, 2, 6 or 31
g/m3 (6 h/day, 5 days/week) for 90 days. No effect on food
consumption or body weight was observed. At the highest exposure level
the animals exhibited anaesthesia and a decreased response to auditory
stimuli. Serum triglyceride and glucose levels were significantly
decreased at all exposure levels, while serum cholesterol was
significantly lower in females exposed to the two highest
concentrations. The mean lymphocyte and white blood cell counts in
female rats were decreased at the highest exposure level. Male rats
had significantly higher alanine aminotransferase and alkaline
phosphatase activity at the two highest exposure levels. In female
rats alanine aminotransferase activity was elevated at the highest
exposure level. Urine fluoride concentrations were increased in
females at all exposure levels, but in males only at the highest
exposure level. Absolute liver weights were significantly higher in
male rats at the highest exposure level and in female rats at the two
highest exposure levels, while relative liver weights in both male and
female rats were higher at the two highest exposure levels. Hepatic
peroxisomal beta-oxidation activity in both male and female rats was
1.9 to 3.8 times higher than in controls, indicating an induction of
hepatic peroxisome proliferation. No treatment-related gross or
microscopic liver changes were observed.
7.2.6 HCFC 124
When ten male rats were exposed to approximately 560 g/m3 (6
h/day, 5 days/week) for 14 days, no adverse haematology, clinical
chemistry, urine analysis or histopathological changes were observed.
Rats showed irregular respiration, lethargy and poor coordination
(Hall, 1976).
Trochimowicz et al. (1977) reported no adverse effect following
the clinical and histopathological evaluation of rats exposed to
concentrations of 560 g/m3 (6 h/day, 5 days/week) for 2 weeks.
Brewer (1977) exposed groups of 60 Sprague-Dawley rats (35 males and
25 females) to concentrations of 0, 3, 5 or 28 g/m3 (6 h/day, 5
days/week) for 3 months. Ten male and ten female rats were examined
and sacrificed after 45 days and a similar number after 92 days. Ten
male and five female rats of each group were maintained without
further exposure for an additional 30-day period after exposure.
Clinical signs were observed, body and organ weights determined, and
haematological, biochemical and histopathological examinations carried
out on all animals. No statistically significant differences in body
weight gain were noted. Haematology, clinical chemistry, and urine
analysis in treated rats were normal and comparable to findings in
control animals. Urinary fluoride excretion was increased after 45
days of exposure in both males and females (4.0 and 3.5 times,
respectively) at an exposure level of 28 g/m3; at the two lower
levels, determinations were not made. After 95 days of exposure, the
urinary fluoride level was elevated only in males at all three
exposure levels (by 1.5, 1.7 and 1.8 times, respectively), and this
effect persisted throughout the 30-day period after exposure. Gross
and histopathological examinations did not reveal any
treatment-related changes in any group of animals. Statistically
significant difference in organ weights between treated and control
rats were found. Liver weights were increased significantly in males
at 5 and 28 g/m3, while the lung and adrenal gland weights were
significantly decreased in males at all three exposure levels. In the
absence of any histopathological change, the biological significance
of these organ weight changes is unclear.
Malley (1990b) exposed groups of ten male and ten female rats to
HCFC 124 concentrations of 0, 3, 11, 56 or 279 g/m3 (6 h/day, 5
days/week) for 4 weeks. Treatment-related effects on body weight, food
consumption, mortality, clinical laboratory parameters, organ weights,
and tissue morphology changes were not found at any exposure level.
During exposure, rats exposed to 279 g/m3 were lethargic and
uncoordinated. However, no evidence of lethargy and incoordination was
observed shortly after exposure. The author considered the exposure
concentration of 56 g/m3 the no-observed-adverse-effect level
(NOAEL) based on the clinical observation of lethargic and
uncoordinated movement during exposure to 279 g/m3, which was not
observed at 56 g/m3.
Malley (1991) exposed Crl:CDRBR rats (20 rats of each sex at
each exposure level) to HCFC 124 at concentrations of 0, 28, 84 and
279 g/m3 (6 h/day, 5 days/week) for 90 days. As part of this study,
a functional observation battery (FOB) was conducted on 10 rats of
each sex at each exposure level at various intervals during the course
of exposure. There were no compound-related effects relative to body
weight, food consumption, mortality, haematology, organ weights or
histopathology at any exposure concentration. However, male rats, at
a level of 84 and 279 g/m3, had lower serum triglyceride
concentrations and a decreased arousal (4 of 10 and 6 of 10 rats,
respectively). Persistent decrease of forelimb grip strength was found
in high-dose females. However, there was no associated decrease in
hindlimb grip strength or change in gait or footplay. Females at the
highest concentration showed an increase in alkaline phosphatase. Both
sexes at 279 g/m3 were less responsive to auditory stimuli, as
demonstrated by a decreased reaction to a sharp knock on the chamber
wall. Plasma fluoride, urinary fluoride and fractional clearance of
free fluoride were also increased at all exposure levels in both
sexes. The author concluded that the no-observed-effect level (NOEL)
was 28 g/m3 for male rats and 84 g/m3 for female rats.
7.3 Skin and eye irritation; sensitization
7.3.1 Skin and eye irritation
7.3.1.1 HCFC 141b
Treatment of the intact skin of New Zealand albino rabbits with
0.5 ml of undiluted HCFC 141b under occlusive patch (during 4 and 24
h in the two respective studies) did not produce signs of dermal
irritation during a 3-day observation period (Liggett, 1988a; Brock,
1988b).
Two studies were conducted with HCFC 141b on groups of six New
Zealand albino rabbits where the undiluted compound (0.1 ml) was
instilled into the eyes. No signs of irritation occurred within 3 days
in one study (Liggett, 1988b), but the compound was found to be a
"mild" irritant in the other study (Brock, 1988c). The majority of
rabbits in the latter study showed conjunctival redness (5/6), mild
chaemosis (3/8) and blood-tinged discharge (4/6).
7.3.1.2 HCFC 142b
Brittelli (1976a) observed no effects on the cornea or iris but
slight conjunctival swelling with some discharge in an eye irritation
test with HCFC 142b.
7.3.1.3 HCFC 132b
One drop (approximately 0.05 ml) each of 100% HCFC 132b and a 10%
solution in propylene glycol was applied and slightly rubbed into the
shaved intact shoulder skin of 10 male albino guinea-pigs, but the
area was not occluded. The pure compound produced only mild irritation
in one animal only. No irritation was induced by the 10% solution
(Goodman, 1976).
Undiluted HCFC 132b (0.1 ml) was placed into the right
conjunctival sac of two albino rabbits, and after 20 seconds one
treated eye was washed with water for 1 min. Observations of the
cornea, iris and conjunctiva were made after 1 and 4 h, and 1, 2, 3
and 7 days later. Slight corneal opacity and "mild" to "moderate"
conjunctival irritation were seen in both rabbits up to 3 days after
dosing, but had disappeared by 7 days after dosing (Brittelli, 1976b).
7.3.1.4 HCFC 123
Minimal dermal irritation with HCFC 123 was observed in rabbits
(Brock, 1988e,f). HCFC 123 (purity 99.0%) produced no skin irritation
when 0.5 ml/6 cm2 was applied to the clipped intact skin of four
male and two female New Zealand rabbits for 4 h (Trochimowicz, 1989).
Brittelli (1976c) reported HCFC 123 to be a "mild" ocular
irritant causing reversible corneal opacity in rabbits. In another
study by Daly (1979)a, HCFC 123, when instilled undiluted (0.1 ml)
into the conjunctival sac of the rabbit eye without subsequent
washing, produced "mild" to "moderate" conjunctival irritation. With
washing, "mild" transient corneal opacity and "mild" to "moderate"
conjunctival irritation were observed. With or without washing,
complete recovery occurred within 3-7 days.
7.3.2 Skin sensitization
7.3.2.1 HCFC 141b
No delayed contact hypersensitivity was found in any of the 20
Hartley Dunkin guinea-pigs in a Magnusson-Kligman maximisation test
with HCFC 141b (Kynoch & Parcell, 1989).
7.3.2.2 HCFC 132b
A series of four sacral intradermal injections of HCFC 132b was
given (once per week at 7-day intervals) over a 3-week period to
groups of nine male albino guinea-pigs (0.1 ml of a 1% solution in
dimethyl phthalate). Fourteen days after the last application, the
animals were challenged with either 1 drop (0.05 ml) of undiluted
liquid or a 19% solution of test material in propylene glycol on the
shaved skin. No evidence of sensitization was observed (Goodman,
1976).
7.3.2.3 HCFC 123
When applied topically to the back of male guinea-pigs as 10% or
50% solutions in propylene glycol, HCFC 123 produced no sensitization
at challenge (Goodman, 1975; Daly, 1979).
7.4 Long-term exposure
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternative Fluorocarbon Toxicity Testing (Rusch, 1989) sponsored
by an international industry consortium. An interim report after the
first year of the study is available on HCFC 123 (Malley, 1990b).
a Personal communication entitled "Toxicity testing summary for
alternative fluorocarbons" by J.J. Daly to CFTA Interindustry
Safety Committee, Wilmington, Delaware, USA, E.I. Du Pont de
Nemours and Co.
7.4.1 HCFC 142b
Four groups each containing 130 male and 110 female Sprague-
Dawley CD rats were exposed by inhalation to concentrations of 4.1, 41
and 82 g/m3 (6 h/day, 5 days/week) for 104 weeks. No exposure-
related effects were found on mortality, body weight, haematology,
clinical chemistry, urine analysis, histopathological or
ophthalmological findings (Seckar et al., 1986). However, high
mortality (more than 50% by the end of the study) was seen in all
groups including controls.
7.4.2 HCFC 123
Findings associated with the first year of the PAFT-supported
study were provided by Malley (1990c). Following 12 months of
inhalation exposure to 0, 2, 6 or 31 g/m3, 10 rats (Crl:CDRBR) of
each sex at each concentration were sacrificed, selected organs were
weighed, and tissues were examined for gross and microscopic lesions.
Body weight and body weight gain were significantly lower in high-dose
males and in mid- and high-dose females. Food consumption was higher
and food efficiency was lower in high-dose males and females over the
course of the l year of exposure. The lower food efficiency appeared
to be directly related to the lower body weight gain at the high
concentration (31 g/m3). High-dose males and females exhibited
anaesthesia-like behaviour (e.g., less responsiveness to auditory
stimuli compared to control rats). No compound-related effects on
mortality or survival time were found in any treated group. Clinical
chemistry parameters measured at 6 months and 1 year revealed several
compound-related changes. The most noteworthy findings were the effect
on lipid and carbohydrate metabolism. Significant decreases of serum
triglyceride and glucose concentrations were found in males and
females at all dose levels. Serum cholesterol was significantly lower
in females at all dose levels and in high-dose males. Urine analysis
indicated significant increases of urinary fluoride concentration in
both sexes at all dose levels. High-dose (31 g/m3) males and females
had significantly higher mean relative liver weights, but no
compound-related gross or histopathological changes were found in the
livers of exposed animals. Dose-related increases in hepatic
beta-oxidation enzyme activity were found for males (2.3, 3.1 and 4.0
fold increases at 2, 6 and 31 g/m3, respectively) and females (1.7
and 3.1 fold increase at 6 and 31 g/m3). The higher enzyme activity
indicated an induction of hepatic peroxisome proliferation. However,
compound-related differences in the rate of cell proliferation, as
measured by changes in labelling index, were not found at any exposure
concentration. These data indicate that during the first year of
exposure, HCFC 123 did not induce an increase in regenerative repair
of the liver, which is consistent with the absence of morphological or
microscopic changes in the liver of exposed animals. A
no-observed-effect level (NOEL) was not achieved in this study based
on the effects on clinical chemistry parameters and higher hepatic
peroxisomal activity.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
7.5.1.1 HCFC 141b
A 2-generation reproduction study on HCFC 141b is currently in
progress (Rusch, 1989).
7.5.1.2 HCFC 142b
In a dominant lethal study on rats, no effect on male
reproduction was found (Seckar et al., 1986). No other studies on
reproductive effects are available.
7.5.1.3 HCFC 132b
No data are available on the effects of HCFC 132b.
7.5.1.4 HCFC 133a
No studies are available in which the effects of HCFC 133a on
reproduction were investigated. Some information is available,
however, on the effects on male fertility from a series of dominant
lethal and "combined dominant lethal fertility" studies in mice (Hodge
et al., 1979, 1980; Kilmartin, 1980). In these studies groups of male
mice were exposed to between 0 and 98 g/m3, 6 h/day, for 5
consecutive days. At the end of the treatment, dominant lethal and
fertility effects were assessed in 15 males (30 controls) from each
treatment group, which were each housed with two virgin females for
four consecutive nights. This 4-nightly mating procedure was continued
for 8-9 consecutive weeks. In the two later studies, satellite group
males (at least two from each group per week) were killed, their
epididymal sperm assessed for abnormalities and their testes examined
histopathologically. A reduction in male fertility (seen as
exposure-related decreases in the proportion of pregnant females:
e.g., 0-60% of pregnant females in treatment groups compared to
90-100% in controls) was seen between weeks 2-8 following exposure.
The severity of the effects were related to time, the maximum
reductions in pregnancies being seen at around 3-6 weeks after
exposure. No effect on fertility was seen at 0.5 g/m3. Reduced
fertility was observed at concentrations of 2.5 g/m3 or more, and
histopathological evidence of degeneration of spermatogenic cells was
seen at concentrations of 5 g/m3 or more. There was a slight and
transient increase in the percentage of abnormal sperm at 2.5 g/m3
or more.
7.5.1.5 HCFC 123
A 2-generation inhalation reproduction study on rats sponsored by
PAFT is currently in progress (Rusch, 1989).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 HCFC 141b
Hughes et al. (1988) exposed three groups of 25 pregnant female
Sprague-Dawley rats to 15, 39 or 97 g/m3 for 6 h/day from days 6 to
15 of pregnancy. Some signs of maternal toxicity (prenarcotic signs,
piloerection and reduced alertness) were observed at all exposure
levels. At the highest level, salivation, hunched posture, and
diaphragmatic breathing, a marked increase in water consumption, a
transient reduction in food intake, and a marginal reduction in body
weight gain were observed. At the highest exposure level, incidences
of subcutaneous oedema and haemorrhaging and embryonal death were
significantly increased. Reduced litter and mean fetal weights and
retarded ossification were observed. However, no teratogenic effect
occurred in any group.
In a further study (Hughes et al., 1989), 16 pregnant female New
Zealand rabbits were exposed to 7, 20 or 61 g/m3 for 6 h/day from
days 7 to 19 of pregnancy. Signs of maternal toxicity (prenarcotic
signs, palpebral ptosis, respiratory disturbances and body weight
loss) were observed at the two highest exposure levels. There was no
indication of any treatment-related effect on embryo or fetal
development or evidence of teratogenicity at any exposure level.
7.5.2.2 HCFC 142b
Groups of 25 pregnant Sprague-Dawley rats were exposed to 4 or 41
g/m3 for 6 h/day from day 3 to day 15 of gestation (Culik & Kelly,
1976). The exposure had no effect on the body weight gain of the
mothers, and no clinical signs of toxicity were observed in any of the
animals. The number of early or late resorptions or number of live
fetuses per litter was not affected by the exposure. Exposure also had
no effect on embryonic development, as measured by the weight and
crown-rump length of the fetuses, and there was no evidence of a
teratogenic effect.
Damske et al. (1978) exposed groups of 20 pregnant female
Sprague-Dawley CD rats to concentrations of 0, 13 and 39 g/m3 for 6
h/day on days 6-15 of gestation. There were no treatment-related
effects in the dams or evidence of exposure-induced terata, variations
in sex ratio, embryotoxicity or inhibition of fetal growth and
development. There was an increase in the incidence of delayed
ossification of the supraoccipital bone in both exposure groups; this
effect was not observed in the control group.
7.5.2.3 HCFC 132b
In a pilot study, groups of seven or eight pregnant rats were
exposed to 3, 11 or 28 g/m3 for 6 h/day on days 6-15 of gestation.
Maternal and fetal body weights were reduced at all exposure levels.
The number of resorptions was increased in the 11- and 27-g/m3
exposure groups (Alvarez, 1988).
In a development screening test using Hydra (Johnson et al.,
1986), embryotoxicity was observed at paternally toxic doses.
7.5.2.4 HCFC 133a
Weigand et al. (1977) investigated the potential embryotoxic or
teratogenic effects of HCFC 133a in Wistar rats and Himalayan rabbits.
They also examined the effects of pre-treatment with progesterone to
determine whether any of the adverse effects of HCFC 133a could be
explained by its interaction with and depletion of progesterone in
early pregnancy, since this hormone is responsible for the electrical
and mechanical quiescence of the myometrium. Twenty-four pregnant
Wistar rats, of which 12 were injected subcutaneously with
progesterone (6 mg/day) prior to HCFC 133a exposure, were exposed to
25 g/m3 for 6 h/day on days 7-16 of gestation (the day on which
sperm was found in the vaginal smear was taken as day 1 of gestation).
Twelve pregnant Himalayan rabbits were exposed to the same
concentration on days 7-19 of gestation (the day of mating being taken
as day 0 of gestation). No controls were used and data were compared
with historical control data. Mild transient sedation, piloerection,
reduced body weight gain and reduced food consumption were observed in
the rats. At autopsy on day 21 of gestation, no macroscopic change was
observed. There was a prenatal mortality of 77% in the dams with no
pre-treatment with progesterone and 82% in the pre-treated dams.
Placental weight, fetal weight and crown-rump length were reduced and
some of the surviving fetuses showed generalized oedema (8/53) and
external anomalies of the limbs and tail (5/53). There was no
significant difference between the rats pre-treated with progesterone
and those without pre-treatment. In rabbits, there were reductions in
body weight gain and food consumption. All animals showed vaginal
bleeding during the last three days of the exposure, and 4 out of 12
aborted. By autopsy on day 29 all fetuses had died.
Culik & Kelly (1979) exposed Charler River CD rats to approximate
concentrations of 0, 2.5, 10, 25 or 98 g/m3 for 6 h/day on days 6-15
of gestation, and the rats were subjected to autopsy on day 21 of
gestation. There was no clear evidence of maternal toxicity. Evidence
of embryotoxicity was observed at all exposure levels. In the control
and the 2.5-g/m3 exposure groups, there was neither fetal death nor
total litter resorption. Fetal weights and crown-rump lengths were
lower in all the groups exposed to HCFC 133a than in controls. At 10
g/m3, 4/21 pregnant females had total resorptions and only 67
fetuses were alive in the other females. At 25 g/m3, 37/41 pregnant
females had total resorptions and only 7 fetuses were alive in the
other females. At 98 g/m3, 22/23 pregnant females had total
resorptions and only one fetus was alive in the other female.
Treatment-related increases in the incidence of runts and delayed
ossification in several bone structures were also observed at all
exposure levels. An increased incidence of hydronephrosis was reported
in all treated groups. Thus, the no-observed-effect level for
embryolethality was 2 g/m3 but, in view of the reduced fetal size
and weight at this exposure level, the no-observed-effect level for
embryotoxicity was not established from this study.
7.5.2.5 HCFC 123
Kelly et al. (1978) exposed 25 pregnant female rats to 62 g/m3
for 6 h/day on days 6-15 of gestation. Dams and fetuses were
sacrificed on day 21 and examined for gross changes. No embryotoxicity
or teratogenic effects were seen.
Two groups of 20 pregnant female rats were exposed to 0 and 31
g/m3 for 6 h/day on days 6-15 of gestation (Rusch, 1985). The
animals were sacrificed on day 20 and all dams and fetuses examined.
The maternal mean body weight in the exposed group was depressed to a
statistically significant degree on days 12 and 15 of the gestation
period. At termination, maternal mean body weights were still
depressed but not to a statistically significant degree. The numbers
of corpora lutea, implantation sites, resorption sites and fetuses
were similar in control and treated dams.
In a range-finding study, groups of six pregnant rabbits were
exposed to HCFC 123 concentrations of 0, 6, 31, 62 and 125 g/m3 for
6 h/day on days 6-18 of gestation (Schroeder, 1989a). All treated
rabbits lost weight during the study and food consumption was markedly
reduced, particularly at the two highest exposure levels. At these two
levels an increased number of resorption was also observed. In the
final study (Schroeder, 1989b; Trochimowicz, 1989), 24 mated females
per exposure group were exposed to 3, 9 or 31 g/m3 for 6 h/day
during days 6-18 of gestation. No mortality was observed in the
control or the low or medium exposure groups. The death of one rabbit
in the high exposure group was not considered by the author to be
treatment-related. There was evidence of maternal toxicity during days
6-18 of gestation at all exposure levels. Statistically significant
treatment-related mean body weight losses were observed in all the
test groups during the exposure period, compared to the control group
which showed a slight mean body weight gain. Mean daily food
consumption was also statistically lower in test groups than in
controls on most exposure days. There was no evidence of embryotoxic,
fetotoxic or teratogenic effects.
7.5.2.6 HCFC 124
Brewer & Smith (1977) exposed a group of 20 pregnant Charles
River CD rats to 30 g/m3 for 6 h/day during days 6-15 of gestation.
Maternal body weight measurements and clinical observations did not
reveal any difference between exposed and control groups, and no
maternal deaths occurred. The incidence of resorption sites in the
treated group was higher than in controls but within the range
commonly experienced with the strain of rats employed. The numbers of
corpora lutea, implantation sites, and fetuses in the treated groups
were similar to those in the controls. Fetal body weights were not
altered by treatment.
In a recent range-findings study, Rickard (1990b) exposed
Sprague-Dawley rats (4-6 per group) to minimal HCFC 124 concentrations
of 0, 3, 11, 56 or 279 g/m3 for 6 h/day on days 6-15 of gestation.
No change in mean body weight gain or evidence of embryotoxic effects
was observed. Internal and skeletal examinations were not conducted;
only external examination of fetuses was performed.
Schroeder (1991), in a range-finding study, exposed New Zealand
white rabbits (7 per group) to nominal HCFC 124 concentrations of 0,
28, 84 and 279 g/m3 for 6 h/day on days 6-18 of gestation. The only
sign of maternal toxicity was decreased activity during exposure at
the two highest concentrations. There was no evidence of
embryotoxicity, fetotoxicity or teratogenicity at any exposure
concentration.
7.6 Mutagenicity
7.6.1 HCFC 141b
The data from in vitro and in vivo studies are summarized in
Table 11.
HCFC 141b did not appear to damage bacterial DNA in a repair
assay, but produced conflicting evidence for mutagenicity in other
bacterial assays. Although sample purities differed in the two assays,
this alone may not account for the difference in response.
No significant response was obtained in the in vitro V79 cell
hprt locus assay.
Chromosomal aberrations were induced in two in vitro studies
with CHO (Chinese hamster ovary) cells, but this activity was not
apparent either in one assay with cultured human lymphocytes or in two
in vivo studies for micronucleus induction in mouse bone marrow
cells.
7.6.2 HCFC 142b
The data from in vitro and in vivo studies are summarized in
Table 12.
There was evidence of induction of base substitution mutations by
HCFC 142b in four of the five bacterial tests conducted. In two of
these studies this effect was apparent only in the presence of
exogenous metabolic activation.
A positive response, but without supporting data, was reported in
a single BHK (baby hamster kidney) 21 cell transformation assay.
No evidence of mutagenic activity of HCFC 142b was seen in a bone
marrow cytogenetic test or a dominant lethal study in rats.
7.6.3 HCFC 132b
The data from in vitro studies are summarized in Table 13.
HCFC 132b was tested in bacterial assays (three studies) and in
an in vitro chromosomal aberration test using a CHO cell line.
There was no evidence of mutagenic potential of HCFC 132b in
these studies.
7.6.4 HCFC 133a
The data from in vitro and in vivo studies are summarized in
Table 14.
HCFC 133a did not induce mutations in bacteria and did not
increase the proportion of BHK 21 cells forming colonies in soft agar.
Chromosomal aberrations were not induced in a single rat bone
marrow test using high concentrations. There were small, statistically
significant increases in the incidences of early deaths in two of
three male mouse dominant lethal assays. These increases occurred in
mating weeks 6-8 in one study and in mating week 7 in the other, in
which the lowest effective dose was 12.3 g/m3. No increases in early
deaths were observed in the third study, in which the highest
concentration tested was 12.3 g/m3.
7.6.5 HCFC 123
The data from in vitro genotoxicity studies are summarized in
Table 15.
HCFC 123 showed no evidence of mutagenic potential when tested in
suspension and plate assays using bacteria or yeasts.
In vitro cytogenetic tests were performed using cultured human
lymphocytes, in which HCFC 123 was tested both in the liquid and
gaseous phase. There was evidence of clastogenic activity in both the
presence and absence of exogenous activation systems, when HCFC 123
was tested in the gaseous phase; in the liquid phase study, an
increase in chromosomal aberrations was seen in the absence of
exogenous activation.
Table 11. Genetic toxicity of HCFC 141b
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Escherichia coli, DNA repair - - 10 mg/ml, Hodson-Walker & May (1988b)
18 hc
Salmonella typhimurium, reverse + + 1455 g/m3, 99.6% purity, positive Hodson-Walker & May (1988a)
mutation with TA98, TA100, TA1535, 48 hc in TA1535, negative
TA1537, TA1538 in other strains
S. typhimurium, reverse mutation with - - 1455 g/m3, 99.95% purity May (1989)
TA98, TA100, TA1535, TA1537, TA1538 48 hc
E. coli, reverse mutation with WP2 uvrA - - 1455 g/m3, 99.95% purity May (1989)
48 hc
Gene mutation in vitro, V79 cells, hprt - - 1430 g/m3, 99.6% purity Bootman et al. (1988a)
locus 3 hc
Chromosomal aberrations in vitro, CHO ± - 1 mg/ml liquid phase; increase Wilmer & De Vogel (1988)
cells in gaps only
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.6% purity Bootman & Hodson-Walker
cells 3 hc (1988)
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.83% purity Hodson-Walker (1990a)
cells 4 hc
Table 11 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations in vitro, human - - 1700 g/m3, 99.83% purity Hodson-Walker (1990b)
lymphocytes 3 h (+ema)c
243 g/m3,
24 h (-ema)
Micronucleus test in vivo, mouse bone - NA 165 g/m3, 99.98% purity; marrow Vlachos (1989)
marrow 6 h sampled at 24, 48 and
72 h
Micronucleus test in vivo, mouse bone - NA 95 g/m3, marrow sampled at 24, Bootman et al. (1988b)
marrow 6 h 48 and 72 h
a NA = not applicable; ± = inconclusive
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
c in vitro assay in an enclosed system
Table 12. Genetic toxicity of HCFC 142b
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 1640 g/m3, negative in all Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hd strains
TA1537
S. typhimurium, reverse mutation with + + 2050 g/m3, weakly positive only Koops (1977)
TA98, TA100, TA1535, TA1537, TA1538 48 hd in TA1535 (2-8 fold
increase at 2050 g/m3)
S. typhimurium, reverse mutation with + + not specifiedd single gaseous conc. Jagannath (1977)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 1-72 h, positive in
TA100 and TA1535
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and McGregor (1976)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
Cell transformation, in vitro BHK 21 ND + not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified, exposure
for 1-24 h, experimental
data not reported
Table 12 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations, in vivo rat - NA 82 g/m3, Seckar et al. (1986)
bone marrow 6 h/day, 5 days/
week for 13 weeks
Dominant lethal assay, male CD-1 rat - NA 82 g/m3, Seckar et al. (1986)
6 h/day, 5 days/
week for 15 weeks
a NA = not applicable; ND = not determined
b LED = lowest effective dose; HED = highest effective dose
c purity not specified for these studies
d in vitro assay in an enclosed system
Table 13. Genetic toxicity of HCFC 132b
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 4 mg/plate liquid phase Russell (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - 550 g/m3b Waskell (1979)
TA98, TA100, TA1535, TA1537
S. typhimurium, reverse mutation with - - 578 g/m3b 99.9% purity Koorn (1988)
TA98, TA100, TA1535, TA1537, TA1538
Chromosomal aberrations, in vitro CHO - - 14.2 mg/ml, liquid phase Wilmer & de Vogel (1988)
cells 3 h (+ema)b
3.0 mg/ml,
21 h (-ema)b
a +ema = with exogenous metabolic activation; -ema = without exogenous metabolic activation; LED = lowest effective dose;
HED = highest effective dose
b in vitro assay in an enclosed system
Table 14. Genetic toxicity of HCFC 133a
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2460 g/m3, McGregor (1976)
mutation with TA98, TA100, TA1535, 24 hd
TA1538
S. typhimurium, reverse mutation with - - 172 g/m3, Waskell (1979)
TA98, TA100 48 hd
S. typhimurium, reverse mutation with - - 49 g/m3, Edmunds et al. (1979)
TA98, TA100 8 hd
Cell transformation, in vitro BHK 21 - - gas, 3 h concentrations not Longstaff et al. (1984)
cell growth in soft agar assay specified, experimental
data not provided
Chromosomal aberrations, in vivo, - NA 98 g/m3, 6 h, marrow sampled 24 h Anderson & Richardson
male AP rat bone marrow and 6 h/day after single and 6 h (1979)
5 days/week after multiple exposure
Dominant lethal assay, male CD-1 + NA 49 g/m3, 6 h/ early deaths increased Hodge et al. (1979)
mouse day, 5 days/week in mating weeks 6-8
Dominant lethal assay, male CD-1 + NA 12 g/m3, 6 h/ early deaths increased Hodge et al. (1980)
mouse day, 5 days/week in mating week 7, no
dose response
Table 14 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Dominant lethal assay, male CD-1 - NA 12 g/m3, 6 h/ Kilmartin et al. (1980)
mouse day, 5 days/week
a NA = not applicable
b HED = highest effective dose; LED = lowest effective dose
c purity not provided for these studies
d in vitro assay in an enclosed system
Table 15. Genetic toxicity of HCFC 123
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 937 g/m3, 6 h Barsky (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - not specifiedc single gaseous conc. Brusick (1976)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 5 or 24 h
S. typhimurium, reverse mutation with - - 625 g/m3, 72 h experimental data not Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 provided
S. typhimurium, reverse mutation with - - not specifiedc gaseous exposure, Callander (1989)
TA98, TA100, TA1535, TA1537, TA1538 concs. not specified
Sacharomyces cerevisiae: D4, forward - - not specified concs. not specified, Brusick (1976)
mutation exposure for 4-72 mins
Chromosomal aberrations, in vitro, + - 75 µg/ml, purity 99.95% Dance (1991)
human lymphocytes 24 h
Chromosomal aberrations, in vitro, + + 1875 g/m3, purity 99.95% Edwards (1991a)
human lymphocytes 3 h (+ema)c
156 g/m3,
24 h (-ema)c
Cell transformation, in vitro, BHK 21 ND - not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified,
experimental data
not provided
Table 15 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Micronucleus test, in vitro, mouse - NA 112 g/m3, 6 h marrow sampled 24, Müller & Hoffman (1988)
bone marrow 48 and 72 h after
exposure
a ND = not determined; NA = not applicable
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous metabolic
activation
c in vitro assay in an enclosed system
A negative response was reported in a BHK transformation assay,
but no supporting data were provided.
There was no evidence of induction of micronuclei in a single
mouse bone marrow assay.
7.6.6 HCFC 124
The data from the small number of in vitro studies are
summarized in Table 16.
HCFC 124 did not induce mutations in bacteria or chromosomal
aberrations in CHO cells.
7.7 Carcinogenicity
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternate Fluorocarbon Toxicity Testing (Rusch, 1989).
7.7.1 HCFC 142b
Seckar et al. (1986) conducted a combined chronic
toxicity/carcinogenicity study of HCFC 142b with Sprague-Dawley CD
rats (details of exposure regimen are given in section 7.4).
Neoplasmic findings in animals that died were similar in the control
and treated animals, and predominantly comprised tumours of the
mammary and subcutaneous tissues in females, and pituitary and adrenal
adenomas in both sexes. A similar pattern existed in surviving
animals.
7.7.2 HCFC 133a
The carcinogenic potential of HCFC 133a has been evaluated by
Longstaff et al. (1984) in a limited study in Alpk/Ap Wistar- derived
rats. The study included one undosed control group of 32 male and 32
female rats and two vehicle-dosed control groups, one consisting of 40
male and 40 female and the other of 36 male and 36 female rats. The
HCFC 133a was dissolved in corn oil to give a 3% solution and dosed by
gavage at 300 mg/kg body weight, 5 days/week until week 52, and the
study was terminated at week 125. Dosed controls were given corn oil
only. Reductions of body weight gain and decreased testicular size
were observed in treated males. Aggressive behaviour occurred,
particularly in males. Body weight gain in females was similar to that
of controls. Mortality was not affected by treatment in either sex.
The treated females had an increased incidence of uterine carcinomas
(15/35) in comparison with controls (1/104). The first of these
carcinomas was seen at week 84. The carcinomas metastasized
transcoelomically to the abdomen in a large proportion of animals. A
small proportion also had lung metastases. Histologically, the
neoplasms were actively infiltrating adenocarcinomas.
Table 16. Genetic toxicity of HCFC 124
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2230 g/m3, Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hb
TA1537, TA1538
S. typhimurium, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
TA98, TA100, TA1535, TA1537, TA1538 48 hb
S. typhimurium, reverse mutation with - - 2790 g/m3, experimental data Longstaff et al. (1984)
TA98, TA100 48 hb not reported
Escherichia coli, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
WP2 uvrA 48 hb
Chromosomal aberrations, in vitro, - - 3348 g/m3, purity > 99% Edwards (1991b)
CHO cells 48 h (-ema)b
3348 g/m3,
4 h (+ema)b
a LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
b In vitro assay in an enclosed system
The treated males had an increased incidence of benign
interstitial cell neoplasms of the testis (29/36) compared with
controls (16/104). In many animals the neoplasms were bilateral. The
first interstitial cell tumour was seen at week 64. The testes of all
treated males, including those with no evidence of neoplasms,
exhibited arrest of spermatogenesis and atrophy of the seminiferous
tubules. These changes were first seen in the first rat to die in this
study, which was in week 37.
7.7.3 HCFC 123
As discussed in section 7.4.2, a 2-year inhalation toxicity/
carcinogenicity study in rats is being conducted. Groups of 80 male
and 80 female Crl:CDRBR rats were exposed to HCFC 123 at 0, 2, 6 or
31 g/m3 (6 h/day, 5 days/week) for 2 years. No histological lesions
were found in 10 rats of each sex in each exposure group, which were
sacrificed at the end of 1 year of exposure (Malley, 1990c).
Preliminary findings from the histopathological examination of tissues
of male rats at the end of the 2-year exposure period indicated an
exposure-related increase in benign tumours of the testis and exocrine
pancreas (US EPA, 1991). A full report is needed before these results
can be evaluated.
7.8 Special studies - cardiovascular and respiratory effects
Chlorofluorocarbons have long been known to sensitize the heart
to adrenaline-induced arrhythmias. Zakhari & Aviado (1982) reviewed
the literature on this subject. Several studies have been conducted to
evaluate the cardiac sensitization and respiratory effect potential of
the alternative HCFC compounds.
7.8.1 HCFC 141b
Mullin (1977) exposed male dogs, which were pretreated with an
intravenous injection of adrenaline (0.008 mg/kg), to HCFC 141b
concentrations of 12, 24, 48 and 97 mg/m3. A challenge dose
(intravenous injection with adrenaline) given during exposure elicited
serious cardiac arrhythmias at the three highest concentrations but no
response was noted at the lowest concentration.
Hardy et al. (1989b) studied cardiac sensitization to adrenaline
in two Cynomolgus monkeys and four beagle dogs and found that the
lowest HCFC 141b concentrations inducing responses were about 24 and
48 g/m3, respectively.
No effect on respiratory rate was observed when three groups of
three male Wistar rats were exposed to approximately 45 g/m3 for 25
min. However, there was a small change in respiratory amplitude
suggesting a decrease in tidal volume (Janssen, 1989a).
7.8.2 HCFC 142b
Adrenaline-induced cardiac arrhythmias were observed in 5 out of
12 dogs exposed to 205 mg/m3. No such effect was noted in monkeys or
mice at concentrations of up to 410 g/m3 (Mullin, 1969). It was also
found that dogs exposed to 3280 g/m3 (80% by volume; 20% oxygen
added) for 30 seconds, followed by a loud noise (to stimulate
endogenous adrenaline), developed cardiac sensitization (Mullin,
1970).
Reinhardt et al. (1971) studied the ability of HCFC 142b to
induce cardiac sensitization to exogenous and endogenous adrenaline in
beagle dogs. No response was noted in six animals exposed to a
concentration of 102.5 g/m3 for 5 min and then challenged with
adrenaline. Five out of 12 dogs exposed to 205 g/m3 showed marked
responses (arrhythmias considered to pose a serious threat to life or
ventricular fibrillation), and all 12 dogs exposed to 410 g/m3
showed such responses. When a group of 12 beagle dogs was exposed to
3280 g/m3 for 30 seconds, without injected adrenaline, one out of 12
showed a marked response. With simultaneous noise stimulation five
dogs showed a marked response. No dogs showed the response when
exposed to noise alone.
No arrhythmia or tachycardia was observed when groups of three
Rhesus monkeys ( Macaca mulatta) were anaesthetised with sodium
pentobarbital and exposed to 205 or 410 g/m3 for 5 min (Belej et
al., 1974). There was no effect on pulmonary resistance or compliance
but respiratory stimulation was observed when three anaesthetised
Rhesus monkeys ( M. mulatta) were exposed to 205 g/m3 and four
monkeys to 410 g/m3 for 5 min (Aviado & Smith, 1975). When dogs were
exposed to four different concentrations between 102.5 and 820 g/m3
for 5 min, hypotension, tachycardia, an increase in pulmonary
resistance, and a decrease in pulmonary compliance were found at the
highest exposure level (Belej & Aviado, 1975).
7.8.3 HCFC 132b
HCFC 132b was reported to produce cardiac sensitization in beagle
dogs in response to an intravenous adrenaline challenge at exposure
levels of 27 g/m3 or more (Mullin, 1976).
7.8.4 HCFC 123
Trochimowicz & Mullin (1973) reported the EC50 (concentration
producing an effect in 50% of the test group) in dogs for cardiac
sensitization to adrenaline challenge to be 119 g/m3 and the NOEL to
be 62 g/m3. At the latter concentration (the lowest one tested) CNS
depression was observed.
7.8.5 HCFC 124
Van Poznak & Artusio (1960) found that HCFC 124 caused
anaesthesia in dogs at concentrations ranging from 2230 to 3910
g/m3; blood pressure was lowered in a dose-dependent fashion.
Although ventilation was adequate even at the highest exposure level,
the femoral arterial systolic pressure fell to as low as 40 mmHg (5.33
kPa). Little or no anaesthetic effect was observed at exposure levels
that did not depress blood pressure. Atropine had little effect on
hypotension. Phenylephrine partially reversed the hypotension and
caused several premature ventricular contractions but no ventricular
fibrillation. Similar effects were produced by intravenously
administered adrenaline. Mullin (1976) reported that the NOEL of HCFC
124 for cardiac sensitization after a challenge injection of
adrenaline in the dog was 56 g/m3, while the next exposure level
tested (140 g/m3) induced sensitization, as did higher
concentrations.
8. EFFECTS ON HUMANS
8.1 General population exposure
No effects on human health of the hydrochlorofluorocarbons
reviewed in this monograph have been reported.
8.2 Occupational exposure
Filicheva (1975) examined 98 male and 98 female workers, 20-40
years of age, reportedly exposed to chlorofluorocarbons 22, 113 and
142 and to some other chlorofluoro and fluoro compounds. The
concentrations were claimed to exceed the threshold limit values but
were not specified. Functional disorders of the nervous system were
reported in 67% of the workers. These included symptoms of
neurovegetative system disturbances, and, in a few cases, polyneuritis
of the upper extremities. Reduced haemoglobin content, moderate
leucocytosis and reduced erythrocyte sedimentation rate were also
reported. Exposure levels were not given, and the workers were exposed
to a number of chemicals in addition to HCFC 142 (isomer not
specified). The information provided in this study could not be used
to evaluate the potential health effects on workers exposed to HCFC
142b alone.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
No information is available on the effects on environmental
organisms of the hydrochlorofluorocarbons reviewed except for limited
data on HCFC 141b and HCFC 142b. However, an ecotoxicology programme
is being developed within the industry-sponsored Programme for
Alternative Fluorocarbon Toxicity Testing (Rusch, 1989).
The 96-h LC50 of HCFC 141b for zebra fish (Brachidario rerio)
was reported to be 126 mg/litre in a static test using a sealed vessel
(Bazzon & Hervouet, 1989). The 48-h EC50 for the immobilization of
Daphnia magna, also using a sealed vessel, was 31.2 mg/litre
(Brinard & Hervouet, 1989).
The 96-h EC50 of HCFC 142b for guppies ( Poecilia
reticulata), tested in a static system in accordance with OECD
Guidelines, was reported to be 220 mg/litre (Groenevald & Kuijpers,
1990a). Groenevald & Kuijpers (1990b) found a 48-h EC50 for Daphnia
magna of 160 mg/litre. No immobilization of Daphnia magna at 190
mg/litre in 48 h was found (Hutton & Lieder, 1989a).
Acute toxicity values of HCFC 142b have also been determined for
rainbow trout. The 96-h LC50 was found to be 36 mg/litre (with a 95%
confidence interval of 28-45 mg/litre). The authors concluded that,
under the conditions of the test, HCFC 142b exhibited moderate acute
toxicity to rainbow trout (Hutton & Lieder, 1989b).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Direct health effects
10.1.1 HCFC 141b
HCFC 141b has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or in the environment. Available information
indicates a low level of metabolism of HCFC 141b. It is of low acute
toxicity. At high sublethal exposure concentrations, it induces signs
of CNS depression and can sensitize the heart to adrenaline, as do
other hydrochlorofluorocarbons. Such effects can appear at
concentrations ranging from 24 to 48 g/m3 in the inhaled air.
HCFC 141b has low irritant potential to the eye and skin. Skin
sensitization has not been demonstrated.
Short-term repeated inhalation exposure (2 to 13 weeks) did not
induce serious toxic effects at concentrations below 97 g/m3.
Effects of HCFC 141b on reproduction cannot be evaluated until
the data from an ongoing two-generation study become available. It has
not demonstrated teratogenic potential in rats or rabbits, although
embryotoxicity was observed, but only at the highest maternally toxic
concentration (97 g/m3) in rats. The no-observed-effect level (NOEL)
for maternal toxicity (excepting minor clinical signs) is 7 g/m3 in
rabbits and 15 g/m3 in rats.
Mutagenicity testing of HCFC 141b yielded conflicting results.
In vitro studies with CHO cells indicated clastogenic potential, but
this was not reflected in a single human lymphocyte test or two
in vivo mouse studies.
A carcinogenicity study in rats is in progress for HCFC 141b.
In summary, based on the toxicological information available at
the time of the Task Group meeting, the toxicity of HCFC 141b is
considered to be low. The present data base does not indicate any
significant direct health effects on humans under non-accidental
exposure conditions. However, studies to evaluate carcinogenicity and
reproduction are still in progress. Consequently, these study results
and any future changes in use pattern may require further evaluation.
10.1.2 HCFC 142b
HCFC 142b is produced in commercial quantities for use as an
intermediate in the synthesis of vinylidene fluoride. Its current
release to the environment has not been quantified and no information
is available on accidental release. No study is available which
permits the assessment of human health responses to HCFC 142b alone.
No information is available from in vivo studies on metabolism
and kinetics, but an in vitro study suggests that dechlorination may
occur.
The acute toxicity is very low after oral or inhalation exposure.
The no-observed-effect level (NOEL) for cardiac sensitization using
exogenous adrenaline in dogs is 102.5 g/m3 for 5 min.
In rat and dog studies, repeated inhalation exposure at
concentrations of up to 41 g/m3 for 90 days caused no adverse
responses. No carcinogenic or other toxicological responses were
reported in a single long-term inhalation study in rats exposed to up
to 82 g/m3 for 104 week.
HCFC 142b has mutagenic potential in bacterial systems. Data from
in vitro mammalian cell systems are lacking. No mutagenic activity
was seen in two in vivo rat studies.
Although no conventional reproductive toxicity studies are
available, two rat teratogenicity tests have been conducted in which
neither teratogenicity nor reproducible signs of embryotoxicity were
observed. In addition, no effect on male fertility was observed in a
dominant lethal study in rats.
In summary, information on human exposures is lacking, but these
are unlikely to approach the sustained high concentrations required to
produce adverse effects in experimental animals. Future usage
patterns, possibly resulting in higher occupational exposure
concentrations and exposure for shorter periods among the general
population, may require further evaluation.
10.1.3 HCFC 132b
HCFC 132b is only produced in small quantities for research
purposes but it occurs as a by-product in the manufacture of some
halogenated ethanes. Release into the environment is expected to be
very low. There appears to be no commercial application for HCFC 132b
at the present time. There are no data on human exposure and on the
effects of HCFC 132b on human health.
Data on the metabolism of HCFC 132b are only available for rats.
They show that the compound can be metabolized to potentially
cytotoxic metabolites and suggest that the compound can induce its own
metabolism. The acute toxicity of the compound is low, the predominant
effects being central nervous system (CNS) depression, anaesthesia
and, at high concentrations, death. The compound has low irritation
potential to the skin and eye. HCFC 132b can sensitize the heart to
adrenaline, as do many other hydrochlorofluorocarbons. Repeated
exposure to HCFC 132b has been shown to cause CNS depression, signs of
hepatotoxicity (at concentrations of 3 g/m3 or more) and effects on
spermatogenesis (at 11 g/m3). HCFC 132b is also embryotoxic and was
maternally toxic at the lowest dose tested (3 g/m3). NOEL values
have not been established for systemic toxicity or for embryotoxicity.
The available mutagenicity studies, which are limited to a few
in vitro experiments, did not suggest that HCFC 132b is genotoxic.
Long-term and carcinogenicity studies have not been performed.
In summary, from the available information, exposure to HCFC 132b
would appear to present a hazard to human health following repeated
exposure. Current use restricts its exposure to research personnel.
Therefore there is no expected health risk to the general population.
10.1.4 HCFC 133a
HCFC 133a is produced in small quantities for use as an
intermediate in the manufacture of the anaesthetic halothane. Although
it has not been quantified, release into the environment is expected
to be low.
No data are available on the toxicokinetics of HCFC 133a,
although absorption can be assumed to occur from the toxic effects it
induces. It is of low acute toxicity, the principal toxic effects
being anaesthesia and death at high concentrations. Although no
information is available, this compound is also expected to induce
cardiac sensitization at high concentrations. In animal studies
repeated exposure to HCFC 133a has induced death (in mice), and nasal
and lung damage changes in clinical chemistry parameters (in rats).
Atrophy of the thymus, testis, ovary and spleen have also been
reported in rats. Reduced fertility and damage to the seminiferous
epithelium have been observed in male mice at exposure concentrations
of 2.5 g/m3 and 5 g/m3, respectively.
HCFC 133a has been clearly demonstrated to be embryotoxic at
exposure concentrations that did not produce clear evidence of
maternal toxicity (2.5 g/m3); there was also indication of a
teratogenic potential in rats. The NOEL for embryolethality was 2.5
g/m3.
HCFC 133a is non-mutagenic in bacterial assays. It gave positive
results in two out of three dominant lethal assays in mice, but these
results are difficult to interpret in view of the negative result in
a single rat bone-marrow cytogenetic assay. From the limited evidence
available, HCFC 133a is a carcinogen in rats inducing adenocarcinomas
of the uterus; it also produces benign interstitial cell tumours of
the testis.
In summary, from the information available, exposure to HCFC 133a
would appear to present a hazard to human health following repeated
exposure and has the potential to cause serious effects in developing
offspring. In the absence of exposure information the potential risk
cannot be determined.
10.1.5 HCFC 123
HCFC 123 has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or the environment.
There is limited information on the kinetics and metabolism of
HCFC 123. It can be absorbed, the inference coming from observed
systemic effects and elevated urinary fluoride levels in toxicity
studies. There is evidence for some metabolism of HCFC 123, based on
the detection of urinary trifluoroacetic acid, elevated urinary
fluoride levels, and the detection of covalent binding to liver
protein.
The acute toxicity of HCFC l23 is low. As with the fully
halogenated chlorofluorocarbons, it is characterized in animals by CNS
depression at high inhalation exposure concentrations. Exposure to
high concentrations of HCFC 123 can sensitize the heart to adrenaline
(the EC50 for dogs is 119 g/m3).
Effects associated with short-term and long-term inhalation
exposure to HCFC 123 in rats include CNS depression, modulation of
lipid and carbohydrate metabolism and mild liver toxicity. The
lowest-observed-effect level (LOEL) for lipid and carbohydrate
metabolism effects and increased hepatic enzyme activity is 2 g/m3
following a 1-year exposure period to HCFC 123.
There is no evidence of teratogenicity in rats and rabbits but
there is evidence of embryotoxicity in rabbits at high inhalation
exposure concentrations (62 g/m3). Maternal toxicity is seen at
exposure concentration of 3 g/m3 or more in rabbits and 31 g/m3 or
more in rats. The potential effect of HCFC 123 on reproduction is
being investigated in a rat study.
There is evidence of clastogenic effects in human lymphocytes
in vitro, although this was not supported by an in vivo mouse
micronucleus study. HCFC 123 is not mutagenic in microorganisms. The
carcinogenicity of HCFC 123 is being investigated in rats and thus
cannot be evaluated at present.
On the basis of available information, HCFC 123 does not appear
to exhibit marked toxicity following short-term or long-term exposure.
However, in the light of some evidence of clastogenicity and the
reported increased incidences of benign tumours of the testes and
exocrine pancreas communicated in an interim report, the potential
health effects of HCFC 123 cannot be fully evaluated until more
information becomes available.
10.1.6 HCFC 124
HCFC 124 is not yet in large scale commercial production.
Therefore, there are no data on environmental levels and human
exposure.
The acute toxicity of HCFC 124 is low and is characterized in
animals by effects on the central nervous system. Subchronic toxicity
studies in rats did not reveal any histopathological changes of
internal organs at concentration of HCFC 124 as high as 279 g/m3.
Based on functional observations and clinical chemistry, the NOEL was
reported to be 28 g/m3 for male rats, and 84 g/m3 for females. In
three limited teratogenicity studies in rats, no indication of
developmental toxicity of HCFC 124 was evident, even at a maternally
toxic concentration. Based on the data available from bacterial and
mammalian cell studies there is no evidence that HCFC 124 has a
mutagenic potential.
The available information indicates that HCFC 124 exhibits low
toxicity, and it is not anticipated to pose significant health risk to
humans at potential environmental or controlled occupational
exposures. However, a firm conclusion on the potential health risk
cannot be made until data from the ongoing teratogenicity and
carcinogenicity studies are available.
10.2 Health effects expected from a depletion of stratospheric
ozone
The possible indirect health effects (e.g., an increase in the
incidence of skin cancer and immunotoxic and ocular effects) of fully
halogenated chlorofluorocarbons, resulting from an increase in UV-B
radiation due to a depletion of the ozone layer, have been discussed
in the Environmental Health Criteria 113: Fully Halogenated
Chlorofluorocarbons (WHO, 1990).
The ozone-depleting potentials of five of the six HCFCs for which
data are available are lower than that of CFC 11 by 33-77 times (HCFCs
123 and 124), 40 times (HCFC 132b), 12-20 times (HCFC 142b), and 7-15
times (HCFC 141b). If the levels of release of the HCFCs reviewed are
adequately controlled their indirect health effects should not be
significant.
10.3 Effects on the environment
Insufficient information is available to evaluate adequately the
direct ecological effects posed by the hydrochlorofluorocarbons
reviewed. With respect to the indirect "greenhouse" effect, the HCFCs
for which data are available have global-warming potentials lower than
that of CFC 11 by about 50 times (HCFC 123), about 10 times (HCFCs 124
and 141b), and about 3 times (HCFC 142b). These compounds are not
expected to contribute significantly to global warming.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Based on the available information the Task Group reached the
following conclusions:
1. All six hydrochlorofluorocarbons reviewed have low acute
toxicity, the main toxic signs being those of CNS depression.
However, accidental overexposure could result in acute poisoning
or even lethality.
2. These chemicals exhibit different toxicity potentials following
repeated exposure.
3. HCFC 141b has a low toxic potential on repeated exposure,
produces no consistent developmental effects and has not been
shown to be mutagenic in animals. The mutagenic status of HCFC
141b tested in vitro is unclear. No information is yet
available to evaluate chronic toxicity/carcinogenicity.
4. HCFC 142b also has a low toxic potential on repeated exposure.
The small data base indicates that HCFC 142b does not produce
developmental effects or affect male fertility. HCFC 142b is
mutagenic in bacteria. It has not been shown to be mutagenic or
carcinogenic in rats.
5. HCFC 132b is toxic following repeated exposure in animals. It
affects development in animals, but has only been tested at
maternally toxic doses. No study on reproduction has been
performed, but damage to spermatogenesis has been noted
histopathologically following repeated exposure. It is not
mutagenic in vitro and no test has been performed in vivo.
There are no data on the carcinogenic potential.
6. HCFC 133a is toxic following repeated exposure in animals,
producing a range of effects. It also induces serious effects on
reproduction and development in animals. In vivo data regarding
mutagenicity are unclear. It is a carcinogen in rats.
7. On repeated exposure, HCFC 123 induces liver toxicity and effects
on lipid and carbohydrate metabolism in rats. Developmental
effects only occur at high maternally toxic exposure
concentrations. No information is available on reproduction
effects. HCFC 123 is clastogenic in vitro, but has not been
shown to be clastogenic in animals. Complete information on the
carcinogenicity in rats is not yet available.
8. HCFC 124 has low toxic potential on repeated exposure. Based on
a small data base, there is no evidence of developmental effects.
No information is available on the reproduction toxicity
potential or carcinogenicity. It is not mutagenic in vitro and
no test has yet been performed in vivo.
9. The hydrochlorofluorocarbons reviewed (with the exception of HCFC
133a for which there is no value but which is expected to have a
value similar to the others reviewed) have a lower
ozone-depleting potential than the fully halogenated
chlorofluorocarbons and should therefore pose a lower indirect
health risk.
10. The global-warming potentials of HCFC 141b, HCFC 142b, HCFC 123
and HCFC 124 are similarly lower than those of the fully
halogenated chlorofluorocarbons. No data are available for HCFC
132b or HCFC 133a, but they would be expected to have similar
values to the other hydrochlorofluorocarbons reviewed. These
compounds are not expected to contribute significantly to global
warming.
11.2 Recommendations for protection of human health and the
environment
1. Since the toxicity of HCFC 142b is low, and the ozone-depleting
and global-warming potentials are considerably lower than those
of the fully halogenated chlorofluorocarbons, HCFC 142b can be
considered as a transient substitute for the chlorofluorocarbons
included in the Montreal Protocol. However, in line with the
conclusions of the 1990 London Meeting of the Montreal Protocol
Parties, efforts should be maintained to develop substitutes that
would pose no risk to the environment and to develop alternative
technologies. Care should be exercised in the use of HCFC 142b
because of its flammability.
2. Since more information regarding the toxicological potential of
HCFC 141b, HCFC 123 and HCFC 124 is required before an evaluation
of their hazard to human health can be made, no recommendation
can be made at present regarding their use as potential transient
substitutes for the fully halogenated chlorofluorocarbons
included in the Montreal Protocol.
3. Although HCFC 133a and HCFC 132b pose low risk to the
environment, they are not recommended as substitutes for the
chlorofluorocarbons included in the Montreal Protocol because of
their toxicity.
4. Since all the hydrochlorofluorocarbons reviewed have some
ozone-depleting potential, their release to the environment
should be minimized.
REFERENCES
Ahmadzai H & Hedlund T (1990) A profile of measures taken in Sweden to
protect the stratospheric ozone layer. Ambio, 19: 341-343.
Alvarez L (1988) Pilot teratogenicity study of FC-132b in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HLR 50-88).
Anderson D & Richardson CR (1979) Arcton 133a: A cytogenetic study in
the rat. Alderley Park, Cheshire, Imperial Chemical Industries Ltd,
Central Toxicology Laboratory (Report No. CTL/P443).
ANON (1991) CFC alternative shows chronic toxicity. Chem Eng News, 8
July: 26.
Aviado DM & Smith DG (1975) Toxicity of aerosol propellants in the
respiratory and circulatory systems. VIII. Respiration and circulation
in primates. Toxicology, 3: 24l-252.
Barsky FC (1976) In vitro microbial mutagenicity studies of
ethane,1-chloro-1,2,2,2-trifluoro. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 349-76).
Bazzon M & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Brachidanio rerio. Vert-le-Petit, Institut national de
Recherche chimique appliquée (IRCHA Study B.7073).
Belej MA & Aviado DM (1975) Cardiopulmonary toxicity of propellants
for aerosols. J Clin Pharmacol, 15: 105-115.
Belej MA, Smith DG, & Aviado DM (1974) Toxicity of aerosol propellants
in the respiratory and circulatory systems. IV. Cardiotoxicity in the
monkey. Toxicology, 2: 381-395.
Bootman J & Hodson-Walker G (1988) In vitro assessment of the
clastogenic activity of CFC141b in cultures of Chinese hamster ovary
(CHO-KI) cells. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV007/214).
Bootman J, Hodson-Walker G, & Lloyd JM (1988a) CFC 141b: investigation
of mutagenic activity at the HGPRT locus in a Chinese hamster V79 cell
mutation system. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV005/257).
Bootman J, Hodson-Walker G, Cracknell S, & Dance CA (1988b) HCFC 141b:
Assessment of clastogenic action on bone marrow erythrocytes in the
micronucleus test. Eye, Suffolk, Life Science Research Ltd (LRS Report
No. 88/PSV029).
Brewer WE (1977) 90-day subacute inhalation toxicity study with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brewer WE & Smith S (1977) Teratogenic study via inhalation with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brinard D & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Daphnia magna. Vert-le-Petit, Institut national de Recherche
chimique appliquée (IRCHA Study B.7072).
Brittelli MR (1976a) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 752-75).
Brittelli MR (1976b) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 13-76).
Brittelli MR (1976c) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 747-75).
Brock WJ (1988a) Acute dermal toxicity study of FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 501-88).
Brock WJ (1988b) Skin irritation test in rabbits of FC-141b. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
312-88).
Brock WJ (1988c) Primary eye irritation study with FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 318-88).
Brock WJ (1988d) Acute dermal toxicity study of HCFC-123 in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 577-88).
Brock WJ (1988e) Acute dermal toxicity of HCFC-123 in rabbits. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
578-88).
Brock WJ (1988f) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 535-88).
Brusick D (1976) Mutagenicity evaluation of Genetron 123. Final
Report. Kensington, Maryland, Litton Bionetics, Inc. (LBI Project No.
254).
Callander RD (1989) HCFC 123 - An evaluation using the Salmonella
mutagenicity assay. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No. CTL/P/2421).
Carpenter CP, Smith HF Jr, & Pozzani UC (1949) The assay of acute
vapour toxicity and the grading and interpretation of results on 96
chemical compounds. J Ind Hyg Toxicol, 31: 343-346.
Chapman J, Hill R, Muir J, Suckling CW, & Viney DJ (1967) Impurities
in halothane: their identities, concentrations and determination. J
Pharm Pharmacol, 19: 231-239.
Coate WB (1976a) LC50 of G123 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. M165-162).
Coate WB (1976b) LC50 of G124 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. 165-163).
Coombs DE, Hardy CJ, Crook D, Mullins PA, & Gopinath C (1988) Two-week
inhalation toxicity study in the rat with FC 141b. Huntington, United
Kingdom, Huntington Research Centre (Report PWT 76/8893).
Culik R & Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled chlorodifluoroethane (FC 142b). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 700-76).
Culik R & Kelly DP (1979) Embryotoxicity and teratogenicity studies in
rats with chlorotrifluoroethane (FC-133a). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 127-79).
Damske DR, Mecler FJ, & Beliles RP (1978) Teratology study in rats;
Isotron 142b. Monochlorodifluoroethane. Final Report. Kensington,
Maryland, Litton Bionetics, Inc. (LBI Project No. 20980).
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC 123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd (Final report No. 91/PFE03/0093).
Darr RW (1981) An acute inhalation toxicity study of Fluorocarbon 123
in the Chinese hamster. Morristown, New Jersey, Corporate Medical
Affairs, Allied Corporation (Report No. MA-25-78-15).
Davies RH, Bagnall RD, Bell W, & Jones WGM (1976) The hydrogen bond
proton donor properties of volatile halogenated hydrocarbons and
ethers and their mode of action in anaesthesia. Int J Quantum Chem
Quantum Biol Symp, 3: 171-185.
Diggle WM & Gage JC (1956) Toxicological report Arctons 9, 33 and 50.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/T/61).
Doleba-Crowe C (1977) Acute inhalation toxicity - 6 hours (HFA 141b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 61-77).
Doleba-Crowe C (1978) Ninety-day inhalation exposure of rats and dogs
to vapors of 2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
229-78).
Edmunds HN, Baden JM, & Simmon VF (1979) Mutagenicity studies with
volatile metabolites of Halothane. Anesthesiology, 51(5): 424-429.
Edwards CN (1991a) HCFC 123 (vapour phase): In vitro assessment of
clastogenic activity in cultured human lymphocytes. Eye, Suffolk, Life
Science Research Ltd (Final Report No. 91/PFE002/0125).
Edwards CN (1991b) In vitro assessment of the clastogenic activity
of HCFC 124 in cultured Chinese hamster ovary (CHO-K1) cells. Eye,
Suffolk, Life Science Research Ltd (Final report No. 91/PAR002/0855a).
Filicheva AP (1975) [Changes in the nervous system following chronic
action of fluorinated aliphatic hydrocarbons.] Gig Tr Prof Zabol, 10:
14-16 (in Russian).
Fisher DA, Hales ChH, Wang WC, Ko MKW, & Dze ND (1990a) Model
calculations of the relative effects of CFCs and their replacements on
global warming. Nature (Lond), 344: 513-516.
Fisher DA, Hales H, Filkin DL, Malcolm KWK, Sze ND, Connell PS,
Wuebbles DJ, Isaksen SA, & Stordal F (1990b) Model calculations of the
relative effects of CFCs and their replacements on stratospheric
ozone. Nature (Lond), 344: 508-512.
Freemantle M (1991) CFCs and their alternatives. Impact Sci Soc, 40:
59-70.
Gardner JR (1988) Acute dermal toxicity to rats of
dichlorofluoroethane. Huntington, United Kingdom, Huntington Research
Centre (Report No. 881114/PWT91/AC).
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 678-75).
Goodman NC (1976) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 14-76).
Graselli JG & Ritchey WM ed. (1975) CRC atlas of spectral data and
physical constants for organic compounds. Cleveland, Ohio, CRC Press,
vol 3.
Groeneveld AHC & Kuijpers LAM (1990a) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to the guppy Poecilia
reticulata. Graveland, The Netherlands, Duphar B.V., Agrobiological
Laboratory (Interim document No. 56635/33/90).
Groeneveld AHC & Kuijpers LAM (1990b) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to Daphnia magna. Graveland,
The Netherlands, Duphar B.V., Agrobiological Laboratory (Interim
Document No. 56635/32/90).
Hake CL, Waggoner TB, Robertson DN, & Rowe VK (1960) The metabolism of
1,1,1-trichloroethane by the rat. Arch Environ Health, 1: 101-105.
Hall GT (1976) Two-week inhalation toxicity study (HFA 132b and HFA
124). Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 727-76).
Hall GT & Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 426-75).
Hardy CJ, Jackson GC, Rao RS, Lewis DJ, & Gopinath C (1989a) HCFC 141b
acute inhalation toxicity study in rats, 4-hour exposure. Huntington,
United Kingdom, Huntington Research Centre (Report No. PWT95/881676).
Hardy CJ, Sharman IJ, & Chanter DO (1989b) Assessment of cardiac
sensitisation potential in dogs and monkeys. Comparison of F-141b and
F11. Huntington, United Kingdom, Huntington Research Centre (Report
No. PWT86/89437).
Harris JW & Anders MW (1991a) In vivo metabolism of the
hydrochlorofluorocarbon 1,1-dichloro-1-fluoroethane (HCFC-141b).
Biochem Pharmacol, 41: R13-R16.
Harris JW & Anders MW (1991b) Metabolism of the
hydrochlorofluorocarbon 1,2-dichloro-1,1-difluoroethane. Chem Res
Toxicol, 4: 180-186.
Harris JW, Pohl LR, Martin JL, & Anders MW (1991) Tissue acylation by
the chlorofluorocarbon substitute 2,2-dichloro-1,1,1-trifluoroethane.
Proc Natl Acad Sci (USA), 88: 1407-1410.
Hawley GG (1981) The condensed chemical dictionary, 10th ed. New York,
Van Nostrand Reinhold Company.
Henry JE (1975a) Acute oral test (132b). Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report No. 680-75).
Henry JE (1975b) Acute oral test on FC-123. Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 638-75).
Hodge MCE, Anderson D, Bennett IP, & Weight TM (1979) Arcton 133a:
dominant lethal study in the mouse. Alderley Park, Cheshire, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report No.
CTL/P/467).
Hodge MCE, Anderson D, Bennett IP, Weight TM, & Wilson J (1980) Arcton
133a: first combined dominant lethal and fertility study in the mouse.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/R/46l).
Hodson-Walker G (1990a) In vitro assessment of the clastogenic
activity of HCFC-141b in cultured Chinese hamster ovary (CHO-K1)
cells. Eye, Suffolk, Life Science Research Ltd (Report No.
89/PFG001/0971a).
Hodson-Walker G (1990b) HCFC 141b: lymphocyte cytogenetic study using
the methodology recommended by the OECD (1983). Eye, Suffolk, Life
Science Research Ltd (Report No. 89/PFG002/1029).
Hodson-Walker G & May K (1988a) CFC 141b: assessment of mutagenic
potential in histidine auxotrophs of Salmonella typhimurium (the
Ames test). Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV004/237).
Hodson-Walker G & May K (1988b) CFC 141b: assessment of its ability to
cause lethal DNA damage in strains of Escherichia coli. Eye,
Suffolk, Life Science Research Ltd (Report No. 88/PSV008/258).
Hoffmann JS (1990) Replacing CFCs; the search for alternatives. Ambio,
19: 329-333.
Horrath AL (1982) Halogenated hydrocarbons. New York, Basel, Marcel
Dekker.
Hughes EW, Homan BA, Kenny TJ, Coombs DW, Hardy CJ, & Tesh SA (1988)
The effect of HCFC 141b on pregnancy of the rat. Huntington, United
Kingdom, Huntington Research Centre (Report No. PWT77/88952).
Hughes EW, Homan BA, John DM, Kenney TJ, Coombs DW, & Hardy CJ (1989)
A study of the effect of PWC 4874-89 on pregnancy of the rabbit.
Huntington, United Kingdom, Huntington Research Centre (Report No. PWT
82/89243).
Hutton DG & Lieder PH (1989a) Static acute 48-hour EC50 of
chlorodifluoroethane to Daphnia magna. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 542-89).
Hutton DG & Lieder PH (1989b) Flow-through acute 96-hour LC50 of
chlorodifluoroethane to rainbow trout ( Oncorhynchus mykiss). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
75-68-3).
Jagannath DR (1977) Mutagenic evaluation of Isotron 142b. Final
Report. Kensington, Maryland, Litton Bionetics, Inc. (LBI Project No.
20838).
Janssen PJM & Pot TE (1988) Acute dermal toxicity study with FC 141b
in rats. Weesp, The Netherlands, Duphar B.V., Department of Toxicology
(Interim Document No. 56645/23/88).
Janssen PJM & Pot TE (1989a) Acute dermal toxicity study with FC 132b
in rats. Weesp, The Netherlands, Duphar B.V. (Report No. S.8807 to
Solvay SA).
Janssen PJM & Pot TE (1989b) Acute oral toxicity study with FC 132b in
rats. Weesp, The Netherlands, Duphar B.V. (Report No. S.8816 to Solvay
SA).
Janssen PJM (1988) Acute inhalation toxicity studies on FC 132b in
rats. Weesp, The Netherlands, Duphar B.V. (Report No. S.8811 to Solvay
SA).
Janssen PJM (1989a) Acute inhalation study to investigate the
respiratory irritating properties of FC 141b in male rats. Weesp, The
Netherlands, Duphar B.V., Department of Toxicology (Interim Document
No. 56645/41/89).
Janssen PTE (1989b) Acute inhalation study to investigate the
respiratory irritating properties of FC 132b in male rats. Weesp, The
Netherlands, Duphar B.V. (Report No. S.8907 to Solvay SA).
Johnson EM, Gabel BEG, Lieder PH, & Staples RE (1986) An in vitro
evaluation of the developmental hazard potential of Dupont compound
H16469. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. HLO-643-86).
Kelly (1976) Ninety-day inhalation exposure of rats and dogs to vapors
of 1-chloro-1,1-difluoroethane (FC-142b). Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 469-76).
Kelly DP (1988) 90-day inhalation toxicity study in rats (HFA 132b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 20-88).
Kelly DP (1989) Four-week inhalation study with HCFC-123 in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 149-76).
Kelly DP (1990) Four-hour inhalation approximate lethal concentration
(ALC) of HCFC-124 in rats. Newark, Delaware, Du Pont de Nemours and
Co., Haskell Laboratory (Report No. 71-90).
Kelly DP, Culik R, Trochimowicz HJ, & Fayerweather WE (1978)
Inhalation teratology studies of three fluorocarbons. Abstract of
paper presented at the 17th Annual Meeting of the Society of
Toxicology. Toxicol Appl Pharmacol, 45: 293.
Kilmartin M, Anderson D, Bennett IP, Richards D, Weight TM, & Wilson
J (1980) Arcton 133a: second combined dominant lethal and fertility
study in the mouse. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No. CT/P/544).
Koizumi A, Kumai M, & Ikeda M (1982) In vivo suppression of
1,1,1-trichloroethane metabolism by co-administered
tetrachloroethylene: an inhalation study. Bull Environ Contam Toxicol,
29: 196-199.
Koops A (1977) Mutagenic activity of ethane, chlorodifluoro- in the
Salmonella/microsome assay. Newark, Delaware, Du Pont de Nemours and
Co., Haskell Laboratory (Report No. 578-77).
Koorn JC (1988) Study to examine the possible mutagenic activity of
the volatile liquid FC 141b in the Salmonella/microsome assay.
Weesp, The Netherlands, Duphar B.V. (Document No. 56645/41/89).
Kynoch SR & Parcell BJ (1989) Delayed contact hypersensitivity in the
guinea-pig with 4874-89. Huntington, United Kingdom, Huntington
Research Centre (Report No. 881236/PWT94SS).
Landry TD, Cieszlak FS, Szabo JR, & Yano BL (1989)
1,1,Dichloro-1-fluoroethane 13-week inhalation toxicity study with
Fisher 344 rats. Midland, Michigan, Dow Chemical Company, Toxicology
Research Laboratory, (Study ID DR-0006-8553-002B).
Lester D & Greenberg LA (1950) Acute and chronic toxicity of some
halogenated derivatives of methane and ethane. Arch Ind Hyg Occup Med,
2: 335-344.
Leuschner F (1977) Summarising report on a subacute toxicity of FKW
133a (90 days) in beagle dogs when administered by inhalation.
Hamburg, Hoechst AG, Laboratory for Pharmacology and Toxicology.
Leuschner F, Leuschner A, Schwertfeger W, Dontenwill W, & Rogulja, P
(1977) Report on the subacute toxicity of chlorofluorocarbon 133a
(CF3CH2Cl) - called FKW 133a - (90 days) in Sprague Dawley rats
administered by inhalation. Hamburg, Hoechst AG, Laboratory for
Pharmacology and Toxicology.
Lewis RW (1990) HCFC 123: 28 day inhalation study to assess changes in
rat liver and plasma. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd., Central Toxicology Laboratory (Report No.
CTL/T/2706).
Liggett MP (1988a) Irritant effects on rabbit skin of 4874-89 (HFA
141b). Huntington, United Kingdom, Huntington Research Centre (Report
No. 881066D/PWT 91/SE).
Liggett MP (1988b) Irritant effects on the rabbit eye of 4874-89 (HFA
141b). Huntington, United Kingdom, Huntington Research Centre (Report
No. 881067D/PWT 93/SE).
Liggett MP, Allan S, & Dawe IS (1989) Acute oral toxicity to rats of
PWC 4874-89 (F 142b). Huntington, United Kingdom, Huntington Research
Centre (Report No. 8913D/PWT 90/AC).
Longstaff E, Robinson M, Bradbrook C, Styles JA, & Purchase IFH (l984)
Genotoxicity and carcinogenicity of fluorocarbons: assessment by
short-term in vitro tests and chronic exposure in rats. Toxicol Appl
Pharmacol, 72: 15-31.
McGregor DB (1976) Mutagenicity testing with Salmonella typhimurium
strains on plates, of gases, liquids and solids. Edinburgh, Inveresk
Research International (Report No. 513).
McNeill WC Jr (1979) Trichloroethylene. In: Grayson MS & Ecroth D ed.
Kirk-Othmer encyclopaedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 5, p 745.
Maiorino RM, Sipes JG, Gandolfi AJ, & Brown BR Jr (1979) Quantitative
analysis of volatile halothane metabolites in biological tissues by
gas chromatography. J Chromatogr, 164: 63-72.
Maiorino RM, Gandolfi AJ, & Sipes JG (1980) Gas-chromatographic method
for the halothane metabolites, trifluoroacetic acid and bromide in
biological fluids. J Anal Toxicol, 4: 250-254.
Makide Y & Rowland FS (1981) Tropospheric concentrations of
methylchloroform, CH3CCl3, in January 1978 and estimates of the
atmospheric residence times for hydrohalocarbons. Proc Natl Acad Sci
(USA), 78: 5933-5937.
Malley LA (1990a) Subchronic inhalation toxicity: 90-day study with
HCFC-123 in rats. Newark, Delaware, Du Pont de Nemours and Co.,
Haskell Laboratory (Report No. 594-89).
Malley LA (1990b) Subchronic inhalation toxicity: four-week study with
HCFC-124. Inhalation study in rats. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 257-90).
Malley LA (1990c) Combined chronic toxicity/oncogenicity study with
HCFC 123. Two-year inhalation study in rats. Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 260-90).
Malley LA (l991) Subchronic inhalation toxicity: 90-day study with
HCFC-124 in rats. Newark, Delaware, Du Pont de Nemours and Co.,
Haskell Laboratory (Report No.79-91).
Matla YA & Blom AJM (1991) Biodegradability of FC 142b according to
OECD 301B (modified Srutm test). Delft (TNO Report No. R 90/307).
May K (1989) HCFC-141b: assessment of mutagenic potential in
amino-acid auxotrophs of Salmonella typhimurium and Escherichia
coli (the Ames test). Eye, Suffolk, Life Science Research Ltd
(LRS-Report No. 89/PFG003/0600).
May K (1991) HCFC 124 in gaseous phase: assessment of mutagenic
potential in amino-acid auxotrophs of Salmonella typhimurium and
Escherichia coli (the Ames test). Eye, Suffolk, Life Science
Research Ltd (Final report No. 91/PAR001/0842).
Millischer R (1990) Joint assessment of commodity chemicals No. 15:
1-Fluoro-1,1-dichloroethane. Brussels, European Chemical Industry
Ecology and Toxicology Centre (ECETOC), 21 pp.
Montague DC & Perrine RL (1990) Preliminary assessment of the
greenhouse warming implications of halocarbon substitutes for CFC-12.
Atmos Environ, 24A: 1331-1339.
Moore BL & Trochimowicz HJ (1976) Subacute (two-week) inhalation
toxicity. Wilmington, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 145-76).
Müller W & Hoffmann T (1988) HCFC 123 micronucleus test in male and
female NMRI mice after inhalation. Frankfurt am Main, Hoechst AG,
Pharma Research Toxicology and Pathology Laboratory (Study No.
88.0372).
Mullin LS (1969) Cardiac sensitization (HFA 142b). Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 354-69).
Mullin LS (1970) Cardiac sensitization - fright exposures (HFA 142b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 81-70).
Mullin LS (1976) Cardiac sensitization. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 113-76).
Mullin LS (1977) Cardiac sensitization (HFA 141b). Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 957-77).
Nikitenko TK & Tolgskaja MS (1965) [Toxicologically induced
pathomorphic changes in animals affected by freons - 141, 142, 143 and
intermediate products arising in the course of their production.] Gig
Tr Prof Zabol, 9: 37-44 (in Russian).
Oyama I (1990) Test on biodegradability of HCFC 141b by
microorganisms. Fukuoka, Kurume Research Laboratory, Chemicals
Inspection and Testing Institute (Test No.11917).
Pennwalt Corporation (1987) Isotron R 141b toxicity comments:
inhalation: Material safety data sheet. Philadelphia, Pennsylvania,
Pennwalt Corporation.
Plummer JL, Steven JM, & Cousins MJ (1987) Metabolism of halothane in
children having repeated halothane anaesthetics. Anaesth Intensive
Care, 15: 136-140.
Prinn RG & Golombek A (1990) Global atmospheric chemistry of CFC-123.
Nature (Lond), 344: 47-49.
Raventós J & Lemon PG (1965) The impurities in Fluothane: their
biological properties. Br J Anaesth, 37: 716-737.
Reinhardt CF, Maxfield ME, Smith PE, & Mullin LS (1971) Cardiac
arrhythmias and aerosol "sniffing". Arch Environ Health, 22: 265-279.
Rickard LB (1990a) Mouse bone marrow micronucleus assay of HCFC-124.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 52-90).
Rickard LB (1990b) Pilot teratogenicity study of HCFC-124 in the rat.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 108-90).
Robbins BH (1946) Preliminary studies of the anesthetic activity of
fluorinated hydrocarbons. Pharmacol Exp Ther, 86: 197-204.
Rusch GM (1985) A ninety-day inhalation toxicology study and an
inhalation teratology study of Genetron 123 in the rat. Morristown,
New Jersey, Allied Corporation, Department of Toxicology (Report No.
MA-127-80-1).
Rusch GM (1989) Overview of PAFT toxicology program. Toulouse,
European Meeting of the Toxicology Forum, p 178-190.
Russell JF (1976) In vitro microbial mutagenicity studies of
ethane,1,2-dichloro-1,1,difluoro-. Wilmington, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 90-76).
Salmon AG, Jones RB, & Mackrodt WC (1981) Microsomal dechlorination of
chloroethanes: structure-reactivity relationships. Xenobiotica, ll:
723-734.
Sarver JW (1988) Acute oral toxicity study with FC-141b in male rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HRL 363-88).
Sax NI (1984) Dangerous properties of industrial materials, 6th ed.
New York, Van Nostrand Reinhold Company.
Schroeder R (1989a) An inhalation range-finding study to evaluate the
toxicity of CFC 123 in the pregnant rabbit. Final report. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Project No.
88-3303).
Schroeder R (1989b) An inhalation developmental toxicity study in
rabbits with HCFC 123. Final report. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Project No. 88-3304).
Schroeder R (1991) An inhalation range-finding developmental toxicity
study in rabbits with HCFC 124. Final report. Newark, Delaware, Du
Pont de Nemours and Co., Haskell Laboratory (Project No. 89-3513).
Seckar JA, Trochimowicz HJ, & Hogan GK (1986) Toxicological evaluation
of hydrochlorofluorocarbon 142b. Food Chem Toxicol, 24(3): 237-240.
Shankland JC (1990) CFC alternatives for thermal insulation foams. Rev
Int Froid, 13: 59-70.
Shulman M & Sadove MS (1965) Safety evaluation and anesthetic
properties of 1,1,1-trifluoroethyl chloride. Toxicol Appl Pharmacol,
7: 473-477.
Solvay (1989) Safety data sheet HCFC 142b (No. 424), Brussels.
Torkelson TR (1971) Single exposures of rats to the vapours of trace
substances in methoxyflurane. Toxicol Appl Pharmacol, 19: 1-9.
Trochimowicz HJ (1989) The toxicology of HCFC 123
(2,2-dichloro-1,1,1-trifluoro-ethane). Presented at the 1989 European
Meeting of the Toxicology Forum, Toulouse, 19-22 September.
Trochimowicz HJ & Mullin LS (1973) Cardiac sensitization potential
(EC50) of trifluorodichloroethane. Wilmington, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 132-73).
Trochimowicz HJ, Moore BL, & Chiu T (1977) Subacute inhalation
toxicity studies on eight fluorocarbons. Toxicol Appl Pharmacol, 41:
198-199.
UNEP/WMO (1989) Scientific assessment of stratospheric ozone: l989,
Section 111 - Chapter 4. Halogenated ozone depletion and global
warming potentials. Reports prepared for the UNEP/WMO Meeting of the
Open-Ended Working Group of the Parties to the Montreal Protocol,
Nairobi, 28 August-5 September 1989. Nairobi, United Nations
Environment Programme.
US EPA (1991) Communications and Public Affairs A-107 on the Test
Program for 3 HCFCs and 2 HFCs: Joint declaration of the US
Environmental Protection Agency, the Commission of the European
Communities, the Ministry of International Trade and Industry and the
Ministry of Health and Welfare in Japan, and the Program for
Alternative Fluorocarbon Toxicity Testing, Washington, DC, US
Environmental Protection Agency.
Van Dyke RA (1977) Dechlorination mechanisms of chlorinated olefins.
Environ Health Perspect, 21: 121-124.
Van Dyke RA & Gandolfi AJ (1974) Studies on irreversible binding of
radioactivity from (14C) halothane to rat hepatic microsomal lipids
and proteins. Drug Metab Dispos, 2: 469-476.
Van Poznak A & Artusio JF Jr (1960) Anesthetic properties of a series
of fluorinated compounds. I. Fluorinated hydrocarbons. Toxicol Appl
Pharmacol, 2: 363-373.
Vlachos DA (1989) Mouse bone marrow micronucleus assay of FC-141b.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HLR 746-88).
Waskell L (1979) Lack of mutagenicity of two possible metabolites of
Halothane. Anesthesiology, 50: 9-12.
Weast RC ed. (1985) CRC handbook of chemistry and physics, 66th ed.
Boca Raton, Florida, CRC Press.
Weigand W, Leist KH, & Baeder C (1977) Embryotoxic and teratogenic
studies in rats and rabbits with 2-chloro-1,1,1-trifluoroethane (FC
133a). Basel, Hoechst AG, Pharma Research Toxicology and Pathology
Laboratory (Report No. 1104/77).
WHO (1990) Environmental Health Criteria 113: Fully halogenated
chlorofluorocarbons. Geneva, World Health Organization, 164 pp.
WHO (1991) Environmental Health Criteria 126: Partially halogenated
chlorofluorocarbons (methane derivatives). Geneva, World Health
Organization, 97 pp.
Wilmer JWGM & De Vogel N (1988) Chromosome analysis of Chinese hamster
ovary cells treated in vitro with FC-141b. Zeist, TNO-CIVO
Institutes (TNO Report No. V88.156/271236).
Yano BL, Ciezlak FS, & Landry TD (1989) 1,1-Dichloro-1-fluoroethane:
4-week inhalation toxicity study with Fischer 344 rats. Midland,
Michigan, Dow Chemical Company, Toxicology Research Laboratory, Health
and Environmental Sciences (Study DR-0006-8553-002A).
Zakhari S & Aviado DM (1982) Cardiovascular toxicology of aerosol
propellants, refrigerants, and related solvents. In: Van Stee EW ed.
Cardiovascular toxicology. New York, Raven Press, pp 28l-314.
Zwart A (1989) Report of a metabolic elimination study of
1,1,dichloro-1-fluoroethane (HCFC 141b) vapour in male rats. Zeist,
TNO-CIVO Institutes (Report No. V89.214).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
La présente monographie porte sur six hydrochlorofluorocarbures
(HCFC) qui dérivent de l'éthane par substitution partielle des atomes
d'hydrogène par des atomes de fluor et de chlore. Les composés étudiés
dans le présent rapport sont le 1,1-dichloro-1-fluoréthane (HCFC
141b), le 1-chloro-1,1-difluoréthane (HCFC 142b), le
1,2-dichloro-1,1-difluoréthane (HCFC 132b), le 1-chloro-2,2,2-
trifluoréthane (HCFC 133a), le 1,1-dichloro-2,2,2-trifluoréthane (HCFC
123) et enfin le 1-chloro-1,2,2,2-tétrafluoréthane (HCFC 124).
A la température et sous la pression normales ces composés se
présentent sous la forme de gaz inflammable (HCFC 142b),
non-inflammables (HCFC 133a, HCFC 124), ou encore de liquides volatils
ininflammables (HCFC 141b, HCFC 132b, HCFC 123). Ils sont incolores
et, pour la majorité d'entre eux, pratiquement inodores ou dégagent
une odeur éthérée très légère (HCFC 141b et HCFC 123). Ils sont
légèrement ou modérément solubles dans l'eau et miscibles à de
nombreux solvants organiques.
Parmi les méthodes d'analyse utilisables pour le dosage de ces
hydrochlorofluorocarbures, on peut citer la chromatographie en phase
gazeuse avec détection par ionisation en flamme ou capture
d'électrons. La surveillance des HCFC présents dans l'air à fortes
concentrations peut s'effectuer par spectrophotométrie monofaisceau.
2. Sources d'exposition humaine et environnementale
Autant qu'on sache, les hydrochlorofluorocarbures qui font
l'objet de la présente monographie n'existent pas à l'état naturel.
Comme ces composés ne sont pas préparés industriellement pour être
utilisés en tant que tels, il n'y a guère d'exposition humaine ou
d'émissions dans l'environnement. Certains d'entre eux pourraient être
utilisés dans l'avenir pour remplacer les chlorofluorocarbures
totalement halogénés (CFC 11, CFC 12 et CFC 113). Le HCFC 133a et le
HCFC 142b servent d'intermédiaires dans la préparation d'autres
produits fluorés. In vivo, le HCFC 133a est un métabolite de
l'halothane, un anesthésique.
3. Transport, distribution et transformation dans l'environnement
Les données dont on dispose sur la biodégradation de ces composés
dans l'environnement se limitent aux résultats des études consacrées
aux HCFC 141b et 142b qui ne sont pas spontanément dégradés par les
microorganismes. On dispose de peu d'informations sur les coefficients
de partage octanol/eau; toutefois sous sa forme logarithmique, celui
du HCFC 141b est de 2,3 de sorte que la bioaccumulation de cet
hydrochlorofluorocarbure est improbable. Dans la troposphère, ces
composés sont principalement décomposés par réaction avec les radicaux
hydroxyles. Leur durée de séjour dans l'atmosphère (comparée à la
durée de séjour du méthylchloroforme qui est de 6,3 ans) se situe
entre 1,6 an (HCFC 123) et 19,1 ans (HCFC 142b). (La durée de séjour
dans l'atmosphère du CFC 11 est de 74 ans, celle du CFC 12 de 110 ans
et celle du CFC 13 de 90 ans.) A l'exception du HCFC 133a pour lequel
on ne possède pas de chiffres, l'agressivité de ces composés pour la
couche d'ozone et leur effet de serre sont inférieurs ou égaux au
dixième de ceux du CFC 11, le chlorofluorocarbure totalement halogéné
le plus actif à cet égard. (Le HCFC 142b dont l'effet de serre
potentiel est environ égal au tiers de celui du CFC 11 constitue une
exception).
4. Concentrations dans l'environnement et exposition humaine
Comme le HCFC 141b, 132b, 133a, 123 et 124 ne sont pas encore
produits à l'échelle industrielle et que le HCFC 142b n'est utilisé
que comme intermédiaire, ces substances ne sont pas libérées dans
l'environnement en quantité importante. On ne dispose donc d'aucune
donnée sur leur concentration dans l'environnement ni sur l'exposition
humaine.
5. Cinétique et métabolisme chez les animaux de laboratoire
et l'homme
Il n'existe pas de données relatives à la toxicocinétique chez
l'homme de l'un quelconque des HCFCs étudiés.
5.1 HCFC 141b
Les résultats des études toxicologiques incitent à penser que
l'absorbtion du HCFC 141b s'effectue au travers de l'épithélium
respiratoire. Aucune donnée n'est disponible quant à la distribution
de ce composé chez les mammifères. Des études récentes au cours
desquelles des rats ont été soumis à une seule exposition in vivo
ont permis de retrouver dans les urines du 2,2-dichloro-2-fluoréthyl-
glucuronide et de l'acide 2,2,-dichloro-2-fluoracétique.
D'après une étude pilote portant sur l'absorption et le
métabolisme du HCFC 141b chez des rats exposés à des vapeurs de ce
composé, il semblerait que le HCFC 141b ne soit que très faiblement
métabolisé.
Une étude in vitro a montré que le HCFC 141b subissait une
déchloration limitée au niveau des microsomes hépatiques.
5.2 HCFC 142b
On ne dispose d'aucune information sur la toxicocinétique du HCFC
142b. D'après les études toxicologiques qui ont été effectuées sur
l'animal on peut penser que ce composé est effectivement absorbé.
D'après une étude in vitro, il pourrait y avoir déchloration.
5.3 HCFC 132b
Lors d'une étude de métabolisme au cours de laquelle on a
administré du HCFC 132b par voie intrapéritonéale à des rats, on a
retrouvé dans les urines du 2-chloro-2,2-difluoréthylglucuronide, du
chlorodifluoracétaldéhyde (hydraté et conjugué) et de l'acide
chlorodifluoracétique. Lorsqu'on répétait les injections de HCFC 132b
aux animaux, la formation et l'excrétion d'acide chlorodifluoracétique
s'accroissait. Des expériences in vitro sur des microsomes de foie
de rats ont montré qu'il y avait probablement participation du
cytochrome P-450 IIEI à la phase initiale de l'hydroxylation. On n'a
pas observé de signes d'une liaison covalente des métabolites fluorés
aux protéines du foie.
5.4 HCFC 133a
On ne dispose d'aucune donnée sur la toxicocinétique de l'HCFC
133a. On peut néanmoins penser, d'après les effets toxiques observés
lors d'un certain nombre d'études après exposition des animaux, que le
composé est effectivement absorbé. In vitro, on a observé une
déchloration de l'HCFC 133a.
5.5 HCFC 123
On ne dispose d'aucune donnée toxicocinétique sur l'HCFC 123.
Toutefois on peut penser qu'il y a absorption d'après les effets
généraux et les taux élevés de fluorures urinaires observés lors
d'études toxicologiques chez le rat. On a montré que l'HCFC 123 était
métabolisé par l'organisme du rat. On ne sait pas quelle peut être
l'ampleur de cette métabolisation mais, à côté des fluorures, l'acide
trifluoracétique (TFA) apparaît comme un des principaux métabolites
urinaires. On a mis en évidence dans le cas du HCFC 123 une liaison
covalente aux protéines du foie.
5.6 HCFC 124
On ne dispose d'aucune donnée sur la cinétique et le métabolisme
de l'HCFC 124. On peut penser, d'après les résultats des études
toxicologiques par inhalation, qu'il y a absorption de ce composé au
niveau des voies respiratoires.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuves
in vitro
6.1 HCFC 141b
Le HCFC 141b présente une faible toxicité aiguë par voie orale.
Après administration de cette substance à des rats à raison de 5 g/kg,
on n'a observé aucun signe de toxicité.
Des études d'inhalation effectuées sur des rats et des souris ont
montré qu'il y avait dépression du système nerveux central, anesthésie
et mort en cas d'exposition intense. On n'a pas observé d'effets
macroscopiques ou histopathologiques imputables au traitement. Dans
une étude, on fait état d'une CL50 à 4 h chez le rat égale à 295
g/m3 et dans une autre étude, d'une CL50 à 2 h chez la souris
égale à 150 g/m3. Chez le rat, la concentration la plus faible
entraînant la mort serait de 242 g/m3 sur 6 h.
Après exposition cutanée (à raison de 2 g/kg) de rats ou de
lapins on n'a pas observé de mortalité.
Lors d'études d'inhalation à court terme où l'exposition allait
de 10 à 97 g/m3 et durait jusqu'à 90 jours, on n'a pas observé
d'effets toxiques marqués. Les effets observés consistaient en une
réduction du gain de poids, "de légères modifications biochimiques",
et une dépression du système nerveux central. L'étude de 90 jours n'a
pas permis de dégager une valeur pour la dose sans effet observable.
Le HCFC 141b n'a pas produit de signes d'irritation cutanée chez
des lapins ni d'irritation oculaire lors de l'une des deux études
effectuées. Dans la seconde étude, on a observé une légère réaction
d'irritation au niveau de l'oeil. Aucune sensibilisation cutanée n'a
été relevée chez des cobayes.
Une étude de reproduction portant sur deux générations est
actuellement en cours. Les études relatives au développement de
l'embryon font ressortir un accroissement de l'incidence des oedèmes
sous-cutanés et des hémorragies chez les foetus ainsi que des cas de
mort embryonnaire, mais ce, uniquement à la concentration de 97 g/m3
chez le rat, concentration par ailleurs toxique pour la mère. Aucun
effet tératogène n'a été observé. Lors d'une étude sur des lapins,
aucun effet imputable au traitement n'a été observé sur le
développement des embryons ou des foetus.
Le HCFC 141b ne s'est pas révélé mutagène lors d'une épreuve de
réparation de l'ADN bactérien, en revanche il a produit des résultats
contradictoires lors d'autres épreuves de mutation chez des bactéries.
L'épreuve du locus hprt n'a pas permis de relever d'effets sur les
cellules V79. Des aberrations chromosomiques ont été observées après
traitement in vitro de cellules ovariennes d'hamsters chinois, mais
ce phénomène n'a pas été observé lors d'une étude in vitro sur des
lymphocytes humains. Chez la souris, deux épreuves in vivo en vue de
mettre en évidence la formation de micronoyaux se sont révélées
négatives.
Une étude combinée de toxicité et cancérogénicité par inhalation
chronique est en cours sur des rats.
Le HCFC 141b est capable de provoquer une sensibilisation
cardiaque à l'adrénaline exogène chez le chien. Les concentrations de
HCFC 141b les plus faibles qui produisent des réponses sont
respectivement de 24 et de 48 g/m3 chez le chien et le singe.
6.2 HCFC 142b
Après avoir été administré par voie orale, le HCFC 142b n'a
produit que de légers signes de toxicité chez des rats en dose unique
allant jusqu'à 5 g/kg.
Des rats exposés une seule fois par inhalation à 525 g/m3 de
HCFC 142b pendant 4 heures ont présenté une mortalité d'environ 50%.
D'après d'autres études comportant une exposition plus courte, on
évalue la CL50 à plus de 1000 g/m3.
Des études au cours desquelles on a fait respirer à plusieurs
reprises du HCFC 142b à des rats n'ont pas révélé d'effets
indésirables à une concentration de 41 g/m3 (6 heures par jour, 5
jours par semaine pendant 90 jours). En revanche, lorsque la dose
était beaucoup plus élevée, on a observé une très forte irritation
pulmonaire entraînant la mort.
Il ne semble pas que des études aient été consacrées à
l'irritation cutanée ou oculaire ou à la sensibilisation cutanée par
le HCFC 142b. Des études de sensibilisation cardiaque à l'adrénaline
exogène ont été effectuées sur des souris, des chiens et des singes.
Ce sont les chiens qui étaient les plus sensibles; la dose sans effet
observable était de 102,5 g/m3 pour une exposition de 5 minutes et
une dose de 205 g/m3 (également pendant 5 minutes) a provoqué une
arythmie.
Une seule étude à long terme a été rapportée, au cours de
laquelle des rats (130 mâles et 110 femelles par groupe) ont été
exposés à du HCFC 142b aux doses respectives de 4, 41 et 82 g/m3, 6
heures par jour, 5 jours par semaine pendant des périodes pouvant
atteindre 104 semaines. Aucun effet imputable au traitement n'a été
observé en ce qui concerne la NFS, la biochimie du sang et des urines
et l'histopathologie. Aucune modification imputable au traitement n'a
été observée dans l'incidence tumorale.
Aucune étude classique n'a été consacrée aux effets du HCFC 142b
sur la reproduction, toutefois, une étude de létalité dominante n'a
fait ressortir aucun effet sur la fertilité des mâles. Deux études de
tératogénicité ont été effectuées sur des rats. Dans la première, des
rats Sprague-Dawley ont été exposés respectivement à 4 et 41 g/m3 (6
heures par jour du troisième au quinzième jour de la grossesse), alors
que dans l'autre étude, des rats de la même espèce ont été exposés
respectivement à 13 et 39 g/m3 (6 heures par jour du sixième au
quinzième jour de la grossesse). Aucun effet tératogène n'a été noté.
On a observé une réduction de l'ossification chez un petit nombre de
foetus à ces deux doses lors de cette dernière étude mais pas dans la
première.
Le HCFC 142b produit des mutations chez les bactéries mais on ne
dispose pas de données tirées d'épreuves de génotoxicité sur des
cellules mammaliennes en culture. Les tests in vivo qui ont été
pratiqués ne font ressortir aucune augmentation du nombre
d'aberrations chromosomiques dans la moelle osseuse ni d'effets létaux
dominants chez les rats mâles.
6.3 HCFC 132b
Chez le rat, la toxicité aiguë par voie orale du HCFC 132b est
faible. La dose la plus faible à laquelle on ait observé une mortalité
était égale à 25 g/kg. Après administration par voie orale à raison de
2 g/kg, on a observé une dépression du système nerveux autonome et du
système nerveux central, accompagnée d'effets sur la coordination et
l'activité motrice ainsi que sur le tonus musculaire. Chez les mâles,
on a observé une hypertrophie du foie dont le poids était cependant
diminué.
Lorsqu'il est inhalé à fortes doses, le HCFC 132b détermine des
effets aigus caractérisés par une anesthésie. La dose la plus faible
à laquelle on ait observé une mortalité chez des rats exposés de cette
manière pendant 4 heures à du HCFC 132b était égale à 110 g/m3. Chez
la souris, la CL50 à 30 minutes était de 269 g/m3 et l'anesthésie
s'est produite dès 71 g/m3. Dans une étude, on a observé une
réduction du poids des testicules et une augmentation de celui du foie
et des poumons chez les rats mâles après exposition à une dose de 33
g/m3 pendant 6 heures.
L'application cutanée de HCFC 132b à raison de 2 g/kg à des rats
a fait apparaître les signes cliniques d'une action au niveau du
système nerveux central et produit une hypertrophie hépatique chez
certains des animaux. Non dilué, le composé a provoqué une "légère"
irritation de la peau chez des cobayes et une irritation oculaire
"légère à modérée" chez des lapins. On n'a pas pu mettre en évidence
de sensibilisation cutanée chez les cobayes. A partir de 27 g/m3 on
a observé, chez des chiens exposés au HCFC 132b par voie respiratoire,
une sensibilisation cardiaque à l'adrénaline.
A côté de la dépression du système nerveux central, les
principales conséquences d'une inhalation de brève durée de HCFC 132b
par des rats mâles ont été une atrophie du thymus et des effets sur la
spermatogénèse. Après un traitement de 13 semaines à raison de 3
g/m3 on a observé une interruption de la spermatogénèse. Parmi les
autres effets on pouvait noter une prolifération des canaux biliaires
et un accroissement du rapport poids du foie/poids du corps chez les
mâles, même à la dose la plus faible employée (3 g/m3). Les rattes
ont paru moins sensibles que les rats aux effets hépatiques.
Le HCFC 132b a déterminé des réactions d'embryotoxicité chez des
rats après exposition par la voie respiratoire à des doses allant de
3 à 28 g/m3 du sixième au quinzième jour de la gestation. On a
observé un accroissement du nombre de résorptions (aux doses de 11 et
28 g/m3) et une diminution du poids des foetus à toutes les doses.
Toutes les doses utilisées étaient toxiques pour la mère.
En se fondant sur les données limitées dont on dispose, on peut
dire qu'il n'existe aucune preuve d'une mutagénicité in vitro du
HCFC 132b. La cancérogénicité de ce produit n'a pas été étudiée.
6.4 HCFC 133a
Il n'existe pas de données sur la toxicité aiguë par voie orale
du HCFC 133a. Par inhalation, sa toxicité aiguë est faible (la CL50
à 30 minutes chez la souris est de 738 g/m3) et ses principaux
effets toxiques sont ceux d'une anesthésie. On ne dispose d'aucune
donnée sur la sensibilisation cutanée ou cardiaque ni sur l'irritation
de la peau ou des yeux.
L'exposition réitérée (90 jours) de rats à la dose de 49 g/m3
a produit une inflammation chronique des fosses nasales, un emphysème
et un oedème pulmonaire, une bronchite et une pneumopathie. On a
également observé une atrophie du thymus, des testicules, des ovaires
et de la rate. Aucun effet n'a été observé chez des rats et des chiens
exposés à plusieurs reprises à du HCFC 133a pendant 7 jours (rats) ou
90 jours (chiens) à une concentration d'environ 25 g/m3; cependant
une mortalité a été observée chez des souris exposées pendant cinq
jours à une dose de 0,5 g/m3 ou davantage (sauf 2,5 g/m3).
Bien qu'aucune étude de type classique n'ait été consacrée aux
effets du HCFC 133a sur la reproduction, on a observé, au cours de
trois études de létalité dominante chez des souris, un certain nombre
d'effets sur la fertilité des mâles et l'histologie des testicules.
L'exposition à des concentrations de 2,5 g/m3 ou davantage pendant
cinq jours a entraîné une diminution du nombre de souris gravides et
une augmentation de la proportion des spermatozoïdes anormaux, tandis
qu'une exposition à la concentration de 5 g/m3 provoquait des
lésions histopathologiques de l'épithélium des tubes séminifères.
Des études sur des rats (traités du sixième au seizième jour de
la gestation), à des concentrations qui ne produisaient que de légers
signes de toxicité maternelle, ont montré que le HCFC 133a est
embryotoxique aux concentrations supérieures ou égales à 2 g/m3 et
mortel pour l'embryon à partir de 10 g/m3. Une prémédication des
femelles gravides par la progestérone n'a pas eu d'effet sur les
effets embryotoxiques ou létaux. Une autre étude a permis de relever
les indices d'effets tératogènes (anomalies externes des membres et de
la queue). Le HCFC 133a a produit des avortements spontanés et s'est
révélé absolument mortel pour les embryons après exposition de lapines
gravides à la dose de 25 g/m3 du septième au dix-neuvième jour de la
gestation, alors que cette concentration ne produisait que de légers
signes de toxicité maternelle.
D'après les résultats disponibles, rien n'indique que ce composé
soit mutagène chez la bactérie. Une étude, portant sur des cellules de
reins de hamsters n'a pas permis de mettre en évidence d'augmentation
dans la proportion des cellules produisant des colonies transformées.
Sur trois études de mutagénicité, deux ont fait ressortir l'existence
d'effets létaux dominants après exposition de souris mâles à 12 g/m3
ou davantage pendant cinq jours. La proportion des cellules de moelle
osseuse porteuses d'aberrations chromosomiques n'était pas augmentée
chez les rats exposés à 98 g/m3 (6 heures par jour pendant des
durées allant jusqu'à 5 jours). La seule étude de cancérogénicité qui
ait été effectuée a permis de mettre en évidence une augmentation de
l'incidence des adénocarcinomes de l'utérus et des tumeurs du tissu
interstitiel des testicules chez des rats ayant reçu 300 mg/kg de
composé dans de l'huile de maïs par gavage pendant 52 semaines (après
quoi ils ont été placés en observation pendant 73 semaines).
6.5 HCFC 123
Le HCFC 123 présente une faible toxicité aiguë par voie orale et
cutanée. La dose orale la plus faible qui soit mortelle pour le rat
est de 9 g/kg. Administré à raison de 2 g/kg à des rats ou à des
lapins, ce composé n'a entraîné aucune mortalité.
Par la voie respiratoire, le HCFC 123 est également peu toxique.
Ses effets sont analogues à ceux des chlorofluorocarbures,
c'est-à-dire une perte de coordination et une narcose. La CL50 à 4
h est de 178 g/m3 chez le hamster, de 463 g/m3 chez la souris et
varie de 200 à 329 g/m3 chez le rat. Chez le chien, on a obtenu une
sensibilisation cardiaque à des doses supérieures ou égales à 119
g/m3 après une injection d'épreuve d'adrénaline. Le HCFC 123 liquide
provoque une "légère" irritation de la peau et de l'oeil chez le
lapin. Chez le cobaye, il ne provoque pas de sensibilisation cutanée.
Plusieurs études toxicologiques à court terme ont été effectuées
sur le HCFC 123 en utilisant la voie respiratoire. On observe
systématiquement des signes de dépression du système nerveux central
chez le rat à des concentrations de 31 g/m3 ou davantage. On a
également observé certains effets sur le foie de ces animaux. Une
exposition de longue durée (4 semaines ou davantage) au HCFC 123
affecte également le métabolisme des lipides et des glucides comme le
montre la réduction systématique des triglycérides, du cholestérol et
du glucose sériques chez les rats. D'après les résultats provisoires
d'une étude en cours sur la toxicité et l'oncogénicité de ce composé,
il apparaît qu'il exerce un certain nombre d'effets sur des rats
exposés pendant de longues durées à des doses de 2,6 ou de 31 g/m3.
Les effets observés, perturbation du métabolisme lipidique et
accroissement de l'activité des peroxysomes hépatiques, ont servi de
base à l'établissement de la dose sans effet observable, dont la
valeur n'est toutefois pas mentionnée.
Une étude de reproduction portant sur deux générations de rats
exposés par la voie respiratoire à du HCFC 123 est en cours. Deux
autres études de portée limitée n'ont pas permis de mettre en évidence
d'effets embryotoxiques sur des rats à des concentrations qui étaient
légèrement toxiques pour la mère. Les seuls signes d'embryotoxicité
relevés l'ont été chez des lapins et, là encore, seulement lorsque les
concentrations étaient fortement toxiques pour les lapines gravides
(plus de 62,5 g/m3). Cette toxicité maternelle (réduction du poids,
dépression du système nerveux central) s'observe chez des rattes
exposées à des doses supérieures ou égales à 31 g/m3 et chez des
lapines à partir de 3 g/m3. Aucun signe de tératogénicité n'a été
relevé, ni chez les rats ni chez les lapins.
Le HCFC 123 ne paraît avoir aucune activité mutagène sur les
bactéries ou les levures. Toutefois des signes d'une activité
clastogène ont été observés dans des lymphocytes humains in vitro,
encore que cette observation ne soit pas corroborée par les résultats
d'une étude in vivo portant sur la présence de micronoyaux dans des
cellules murines.
Une étude combinée de toxicité et de cancérogénicité par
inhalation chronique de HCFC 123 est en cours sur des rats. Une
communication préliminaire fait état d'une augmentation de l'incidence
des tumeurs bénignes au niveau des testicules et du pancréas exocrine
chez les rats mâles. Toutefois, on ne pourra pas évaluer la
cancérogénicité potentielle du HCFC 123 avant de disposer de
l'ensemble des résultats.
6.6 HCFC 124
Chez l'animal, le HCFC 124 n'a qu'une faible toxicité aiguë par
la voie respiratoire. Une mortalité a été observée chez des rats à la
dose de 1674 g/m3 (exposition de 240 minutes) et chez des souris à
la dose de 2460 g/m3 (exposition de 10 minutes). Les effets observés
sont caractéristiques des chlorofluorocarbures, c'est-à-dire perte de
coordination et narcose. A des doses supérieures ou égales à 140
g/m3, on a observé chez des chiens une sensibilisation cardiaque
après une injection d'épreuve d'adrénaline. On ne dispose d'aucune
donnée sur l'irritation oculaire ni sur l'irritation ou la
sensibilisation cutanée que ce composé pourrait provoquer.
Cinq expériences ont permis d'étudier la toxicité à court terme
de ce composé lorsqu'il est inhalé par des rats pendant des périodes
de 14 à 90 jours. Aux doses les plus fortes étudiées (500 g/m3 sur
14 jours et 279 g/m3 sur 90 jours) on n'a observé aucune
modification histopathologique au niveau des organes. Une dose sans
effet observable de 28 g/m3 a été établie d'après les résultats
concernant les troubles fonctionnels et la biochimie sanguine, fournis
par l'étude de 90 jours.
Une étude toxicologique à long terme utilisant la voie
drespiratoire est en cours sur le HCFC 124.
Lors de trois études de tératogénicité de portée limitée
effectuées sur des rats, au cours desquelles on a fait inhaler du HCFC
124 à raison de 30 g/m3 ou à des doses allant de 3 à 279 g/m3, on
n'a pas relevé de signes d'embryotoxicité ni de tératogénicité. Des
signes d'intoxication maternelle étaient visibles dès 84 g/m3. On ne
dispose d'aucune donnée sur les effets que le HCFC 124 pourrait
exercer sur la fonction de reproduction. Des études de tératogénicité
complètes sont en cours.
Les données fournies par un certain nombre d'études sur des
bactéries ainsi que par une seule et unique étude portant sur des
cellules mammaliennes, n'attribuent aucun pouvoir mutagène au HCFC
124. Une étude de cancérogénicité utilisant la voie respiratoire est
en cours.
7. Effets sur l'homme
On ne dispose d'aucune donnée sur les effets que le HCFC 141b, le
HCFC 132b, le HCFC 133a, le HCFC 123 ou le HCFC 124 pourraient exercer
sur l'homme.
Les données fournies par une seule et unique étude consacrée à
des personnes exposées de par leur profession au HCFC 142b ne
permettent pas d'évaluer les effets de ce composé indépendamment des
autres types d'exposition auxquelles ces personnes avaient été
soumises.
8. Effets sur d'autres êtres vivants au laboratoire et dans leur
milieu naturel
On ne dispose d'aucune donnée sur les effets que les
hydrochlorofluorocarbures en cause pourraient avoir sur les êtres
vivants dans leur milieu naturel, si ce n'est quelques données
concernant le HCFC 141b et le HCFC 142b. La CL50 à 96 h du HCFC 141b
pour les poissons de l'espèce Melambaphes zebra est de 126 mg/litre
et la CL50 à 48 h pour l'immobilisation de la daphnie est de 31
mg/litre. Ces deux observations ont été faites dans des aquariums
clos. Dans le cas du HCFC 142b, la CE50 à 96 h pour le guppy est 220
mg/litre alors que la CL50 à 48 h pour l'immobilisation de la
daphnie varie de 160 à < de 190 mg/litre. La CL50 à 96 h du HCFC
142b pour la truite arc-en-ciel est de 36 mg/litre.
9. Evaluation et conclusions
On ignore qu'elle est la concentration dans l'environnement des
six HCFC étudiés mais, compte tenu de leurs modalités actuelles
d'utilisation, on peut penser qu'elle est faible.
Le HCFC 142b ne présente qu'une faible toxicité potentielle et
l'on estime qu'il ne constitue pas un risque important pour la santé
humaine lorsqu'il n'y a pas d'exposition accidentelle. Les données
toxicologiques concernant le HCFC 141b, le HCFC 123 et le HCFC 124
sont incomplètes et il faudra en obtenir davantage avant qu'on puisse
évaluer les dangers qu'ils représentent pour la santé humaine. En
revanche le HCFC 133a et le HCFC 132b sont dangereux pour la santé.
Par rapport aux chlorofluorocarbures complètement halogénés, ces
six hydrochlorofluorocarbures sont ou devraient être beaucoup moins
agressifs vis-à-vis de la couche d'ozone et leur temps de séjour dans
l'atmosphère est beaucoup plus faible. Ils constituent donc un risque
indirect. Leur contribution potentielle à l'effet de serre est ou
devrait également être plus faible que celle des chlorofluorocarbures
complètement halogénés et ils ne devraient donc pas contribuer de
façon sensible au réchauffement de la planète.
Etant donné que la toxicité du HCFC 142b est faible et qu'il est
moins agressif vis-à-vis de la couche d'ozone et contribue moins à
l'effet de serre que les chlorofluorocarbures complètement halogénés,
on peut considérer qu'il est susceptible d'être provisoirement
substitué aux chlorofluorocarbures visés par le Protocole de Montréal.
Aucune recommandation ne peut être faite concernant le HCFC 141b,
le HCFC 123 ou le HCFC 124 tant qu'on ne disposera pas de données
toxicologiques plus complètes. Bien que le HCFC 133a et le HCFC 132b
ne menacent guère l'environnement et que les risques qu'ils
constituent pour la santé ne soient qu'indirects, il n'est pas
recommandé de les substituer aux chlorofluorocarbures visés par le
Protocole de Montréal en raison de leur toxicité potentielle.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
La presente monografía se ocupa de seis hidroclorofluorocarburos
(HCFCs) derivados de la sustitución parcial de los átomos de hidrógeno
del etano por átomos de flúor y de cloro. En este informe se estudian
los siguientes compuestos: 1,1-dicloro-1-fluoroetano (HCFC 141b),
1-cloro-1,1-difluoroetano (HCFC 142b), 1,2-dicloro-1,1-difluoroetano
(HCFC 132b), 1-cloro-2,2,2-trifluoroetano (HCFC 133a),
1,1-dicloro-2,2,2-trifluoroetano (HCFC 123) y
1-cloro-1,2,2,2-tetrafluoroetano (HCFC 124).
En condiciones normales de presión y temperatura, estos
compuestos son gases inflamables (HCFC 142b) o ininflamables (HCFC
133a, HCFC 124) o líquidos volátiles ininflamables (HCFC 141b, HCFC
132b, HCFC 123). Son incoloros y la mayoría prácticamente inodoros o
con un débil olor a éter (HCFC 141b y HCFC 123). Su solubilidad en
agua es escasa o moderada y son miscibles con muchos disolventes
orgánicos.
Entre los métodos analíticos utilizados para la determinación de
estos hidroclorofluorocarburos figuran la cromatografía de gases con
ionización de llama y la detección con captura de electrones. Se
pueden medir concentraciones relativamente altas en el aire mediante
fotometría de un solo haz.
2. Fuentes de exposición humana y ambiental
Los hidroclorofluorocarburos que se estudian en la presente
monografía no se conocen como productos naturales. Dado que estos
compuestos no se producen comercialmente en gran escala para
utilizarlos como tales, la exposición humana o la liberación al medio
ambiente son muy pequeñas. Algunos de estos compuestos se podrían
utilizar en el futuro como sustitutivos de clorofluorocarburos
completamente halogenados (por ejemplo, CFC 11, CFC 12 y CFC 113). Los
HCFCs 133a y 142b son compuestos intermedios en la fabricación de
otros productos fluorados. El HCFC 133a es un metabolito in vivo del
anestésico halotano.
3. Transporte, distribución y transformación en el medio ambiente
Los datos sobre la biodegradación en el medio ambiente se limitan
a unos estudios sobre los HCFCs 141b y 142b, que han demostrado no ser
fácilmente biodegradables por los microorganismos. Apenas se dispone
de información sobre los logaritmos de los coeficientes de reparto
octanol/agua; en el caso del HCFC 141b es 2,3, por lo que no es
probable que se bioacumule. En la troposfera, estas sustancias se
descomponen principalmente por reacciones con radicales hidroxilo. Su
permanencia en la atmósfera (en relación con los 6,3 años de
permanencia del metilcloroformo) oscila entre 1,6 años (HCFC 123) y
19,1 años (HCFC 142b). (La permanencia en la atmósfera del CFC 11 es
de 75 años, la del CFC 12 de 110 y la del CFC 113 de 90). A excepción
del HCFC 133a, del que no se tienen datos, su contribución potencial
a la destrucción de ozono y al calentamiento del planeta es inferior
o igual al 10 por ciento de la del CFC 11, el clorofluorocarburo
completamente halogenado con la mayor influencia potencial en esos
fenómenos (el HCFC 142b, cuya contribución al calentanierto del
planeta es aproximadamente un tercio de la del CFC 11, es una
excepción).
4. Niveles ambientales y exposición humana
Como los HCFCs 141b, 132b, 133a, 123 y 124 no se producen todavía
comercialmente en gran escala y el HCFC 142b se utiliza sólo como
producto intermedio, no se libera al medio ambiente en cantidades
apreciables. No se dispone, por consiguiente, de datos sobre los
niveles ambientales ni la exposición humana.
5. Cinética y metabolismo en animales de laboratorio y en el
ser humano
No hay datos acerca de la toxicocinética en el ser humano de
ninguno de los HCFCs examinados.
5.1 HCFC 141b
Los resultados obtenidos de los estudios de toxicidad sugieren
que la absorción del HCFC 141b tiene lugar a través del epitelio
respiratorio. No se dispone de información acerca de su distribución
en mamíferos. En estudios recientes de exposición única in vitro
en ratas se detectaron en la orina 2,2-dicloro-2-fluoroetilglucurónido
y ácido 2,2-dicloro-2-fluoroacético. En un estudio piloto sobre la
absorción y el metabolismo del HCFC 141b en ratas expuestas a sus
vapores se puso de manifiesto que sólo se produce transformación
metabólica en muy pequeña medida.
Un estudio in vitro indicó que los microsomas hepáticos
decloran el HCFC 141b en grado limitado.
5.2 HCFC 142b
No se dispone de información sobre la toxicocinética del HCFC
142b. De los estudios de toxicidad en animales se deduce que se
produce absorción. Un estudio in vitro sugirió que puede producirse
decloración.
5.3 HCFC 132b
En un estudio de metabolismo con administración intraperitoneal
de HCFC 132b a ratas se detectaron en la orina 2-cloro-2,2-
difluoroetilglucurónido, clorodifluoroacetaldehído (hidratado y
conjugado) y ácido clorodifluoroacético. La formación y excreción de
este último aumentó al volver a inyectar a los animales HCFC 132b.
Los experimentos in vitro con microsomas hepáticos de rata sugieren
la participación del citocromo P-450 IIEI en el paso inicial de
hidroxilación. No hay pruebas experimentales de la unión covalente de
metabolitos fluorados a las proteínas hepáticas.
5.4 HCFC 133a
Se carece de información sobre la toxicocinética del HCFC 133a.
De los efectos tóxicos observados en diversos estudios puede deducirse
que se produce absorción tras la exposición de animales. Se ha
advertido in vitro la decloración del HCFC 133a.
5.5 HCFC 123
No hay datos acerca de la toxicocinética del HCFC 123. Sin
embargo, de los efectos sistémicos y de los elevados niveles de flúor
en la orina observados en los estudios de toxicidad en ratas se puede
deducir que hay absorción. Se ha demostrado que el HCFC 123
experimenta una transformación metabólica en ratas. No se conoce el
alcance del metabolismo, pero se ha identificado el ácido
trifluoroacético (TFA) como principal metabolito urinario, además del
fluoruro. Se ha demostrado que el HCFC 123 forma enlaces covalentes
con las proteínas hepáticas.
5.6 HCFC 124
Se carece de datos acerca de la cinética y el metabolismo del
HCFC 124. De los estudios de toxicidad por inhalación se puede deducir
que la absorción del HCFC 124 se produce en el tracto respiratorio.
6. Efectos en los animales de laboratorio y en sistemas de prueba
in vitro
6.1 HCFC 141b
La toxicidad aguda del HCFC 141b por vía oral es baja. No se
observaron signos de toxicidad tras suministrar a ratas dosis de 5
g/kg.
En estudios de inhalación aguda en ratas y ratones, con altos
niveles de exposición se observó depresión del sistema nervioso
central, anestesia y muerte. No se advirtieron efectos macroscópicos
o histopatológicos relacionados con el tratamiento. En un estudio, la
CL50 a las 4 h en ratas fue de 295 g/m3, y en otro estudio en
ratones la CL50 a las 2 h fue de 151 g/m3. Se informó que la
concentración letal más baja en ratas era de 242 g/m3 en 6 h.
Tras la exposición cutánea a 2 g/kg no se observó mortalidad en
ratas ni en conejos.
En estudios de inhalación de corta duración con exposiciones que
oscilaron entre 10 y 97 g/m3 y que se prolongaron hasta 90 días no
se advirtió toxicidad pronunciada. Entre otros efectos, se observó
menor aumento del peso corporal, "ligeros cambios bioquímicos" y
depresión del sistema nervioso central. En los 90 días del estudio no
se alcanzó un nivel sin efecto observado.
El HCFC 141b no produjo signos de irritación cutánea en conejos,
ni de irritación ocular en uno de los dos estudios realizados. En el
segundo estudio se observó una respuesta de irritación ocular
"ligera". No se advirtió sensibilización cutánea en cobayos.
Actualmente hay un estudio en curso acerca del efecto del HCFC
141b sobre la reproducción en dos generaciones. En estudios sobre el
desarrollo se observaron frecuencias superiores de edema subcutáneo y
hemorragias en los fetos y de muerte de embriones, pero sólo con la
concentración tóxica para la madre de 97 g/m3 en un estudio en
ratas. No se observaron efectos teratogénicos. En un estudio con
conejos no se advirtieron efectos en el desarrollo embrionario o fetal
a causa del tratamiento.
El HCFC 141b no resultó mutagénico en un ensayo de reparación del
ADN bacteriano y los resultados fueron contradictorios en otras
pruebas de mutación de bacterias. No tuvo ningún efecto sobre las
células V79 en el ensayo sobre el locus hprt. Se detectaron
aberraciones cromosómicas tras el tratamiento in vitro de células de
ovario de hámster chino, pero no aparecieron en un estudio in vitro
con linfocitos humanos. También fueron negativos dos ensayos in vivo
efectuados con micronúcleos de ratones.
Está en marcha un estudio combinado de toxicidad crónica por
inhalación/carcinogenicidad en ratas.
El HCFC 141b muestra en perros un efecto potencial de
sensibilización cardíaca a la adrenalina exógena. Las concentraciones
más bajas de HCFC 141b que indujeron respuesta en perros y monos
fueron respectivamente de 24 y 48 g/m3.
6.2 HCFC 142b
El HCFC 142b administrado por vía oral a dosis únicas de hasta 5
g/kg sólo produjo signos leves de toxicidad en ratas.
La exposición de ratas a una inhalación única de 525 g/m3
durante 4 h produjo la muerte de alrededor del 50% de los animales. En
otros estudios con exposiciones de menor duración se obtuvieron
valores de la CL50 superiores a 1000 g/m3.
En los estudios de exposiciones repetidas por inhalación en ratas
con dosis de 41 g/m3 (6 h/día, 5 días a la semana durante 90 días)
no se observaron respuestos adversas. Con dosis mucho más elevadas se
producía la muerte de las ratas relacionada con una irritación
pulmonar grave.
No se han comunicado estudios sobre el HCFC 142b en relación con
la irritación cutánea u ocular o la sensibilización cutánea. Se
realizaron experimentos de sensibilización cardíaca (utilizando
adrenalina exógena) en ratones, perros y monos. Los perros fueron los
más sensibles; el NOEL fue de 102,5 g/m3 con una exposición de 5
minutos, mientras que 205 g/m3 (también con 5 minutos de exposición)
inducían arritmia cardíaca.
Sólo hay datos de un estudio prolongado, en el que se expusieron
ratas (130 machos y 110 hembras por grupo) a concentraciones de HCFC
142b de 4, 41 y 82 g/m3, 6 h/día, 5 días/semana, hasta un máximo de
104 semanas. No se observaron efectos relacionados con el tratamiento
en ninguno de los parámetros estudiados, que comprendían hematología,
química sanguínea y urinaria e histopatología. No se notificaron
cambios de importancia dependientes del tratamiento en relación con la
aparición de tumores.
No se han hecho estudios convencionales sobre el efecto del HCFC
142b en la reproducción, pero en un estudio de letalidad dominante no
se observaron efectos en la fertilidad de los machos. Se han realizado
dos pruebas de teratogenicidad en ratas. En una de éstas, se expuso a
ratas Sprague-Dawley a concentraciones de 4 y 41 g/m3 (6 h/día desde
el 3 al 15 día de gestación), mientras que en el otro estudio se
expuso a ratas Sprague-Dawley a 13 y 39 g/m3 (6 h/día del 6 al 15
día de gestación). No se observaron efectos teratogénicos. En el
segundo estudio se advirtió para ambas dosis osificación reducida en
un pequeño número de fetos, pero no en el primero.
El HCFC 142b induce mutaciones en bacterias, pero no se dispone
de datos de ensayos de genotoxicidad en cultivos de células de
mamíferos. En los ensayos in vivo no se produjo aumento de las
aberraciones cromosómicas en la médula ósea ni efectos letales
dominantes en las ratas machos.
6.3 HCFC 132b
La toxicidad aguda oral del HCFC 132b en la rata es escasa. La
dosis más baja a la que se ha observado mortalidad es 25 g/kg. Tras la
administración oral de 2 g/kg se advirtió una depresión del sistema
nervioso autónomo y del central, además de otros efectos en la
coordinación motora, la actividad motora y el tono muscular. En los
machos se advirtieron inflamación hepática y disminución del peso del
hígado.
La toxicidad aguda por inhalación del HCFC 132b con niveles de
exposición elevados se caracteriza por un efecto anestésico. La dosis
más baja que produjo mortalidad en ratas tras 4 horas de exposición
fue de 110 g/m3. En ratones, la CL50 en 30 minutos de exposición
fue de 269 g/m3, y se produjo anestesia con 71 g/m3. En un estudio
se advirtió una disminución del peso de los testículos y un aumento
del peso del hígado y los pulmones en ratas macho tras la exposición
a 33 g/m3 durante 6 horas.
La aplicación cutánea de HCFC 132b a ratas se tradujo en algunos
animales en síntomas clínicos de efectos en el SNC e inflamación
hepática. El compuesto sin diluir produjo una "ligera" irritación
cutánea en cobayos y una irritación ocular "de ligera a moderada" en
conejos. No se obtuvieron pruebas de sensibilización cutánea en
cobayos. En los perros se produjo sensibilización cardíaca a la
adrenalina por inhalación de HCFC 132b con niveles de exposición de 27
g/m3 o superiores.
Las consecuencias principales de la exposición de ratas macho a
la inhalación de HCFC 132b durante un breve período fueron, además de
la depresión del SNC, atrofia del timo y efectos en la
espermatogénesis. Se observó alteración de la espermatogénesis tras un
tratamiento con dosis de 3 g/m3 o superiores durante 13 semanas.
Otros efectos que se detectaron fueron una proliferación del conducto
biliar y un aumento de la relación ponderal hígado/cuerpo en machos,
incluso con la aplicación de los niveles más bajos de exposición (3
g/m3). Las ratas hembras resultaron menos sensibles que los machos
a los efectos hepáticos.
El HCFC 132b indujo embriotoxicidad en ratas tras la exposición
por inhalación a 3-28 g/m3 durante los días 6-15 de gestación, dando
lugar a un aumento del número de resorciones (a 11 y 28 g/m3) y a la
disminución del peso fetal en todos los niveles de exposición. Se
observó toxicidad materna con todos los niveles de dosificación que se
probaron.
De acuerdo con los limitados datos disponibles, no hay pruebas de
mutagenicidad in vitro del HCFC 132b. No se ha estudiado la
carcinogenicidad del compuesto.
6.4 HCFC 133a
No se dispone de datos sobre la toxicidad aguda oral del HCFC
133a. Su toxicidad aguda por inhalación es baja (la CL50 a los 30
min en ratones es de 738 g/m3), y los principales efectos observados
están en relación con su acción anestésica. Se carece de información
sobre sensibilización cardíaca, irritación cutánea u ocular o
sensibilización cutánea.
Exposiciones repetidas de ratas (90 días) a 49 g/m3 produjeron
una inflamación crónica del conducto nasal, enfisema y edema pulmonar,
bronquitis y neumonía. También se observaron atrofia del timo, los
testículos, los ovarios y el bazo. No se advirtieron efectos en ratas
ni en perros repetidamente expuestos a concentraciones de HCFC 133a de
unos 25 g/m3 durante siete días (ratas) o 90 días (perros), aunque
se observaron muertes en ratones expuestos a concentraciones iguales
o superiores a 0,5 g/m3 durante 5 días (a excepción de 2,5 g/m3).
Aunque no se conocen estudios convencionales de los efectos del
HCFC 133a en la reproduccción, en tres estudios de letalidad dominante
en ratones se observaron efectos en la fertilidad del macho y la
histopatología testicular. Las exposiciones a concentraciones de 2,5
g/m3 o superiores durante 5 días dieron lugar a una reducción del
número de hembras gestantes y a un aumento de la proporción de esperma
anormal, mientras que la exposición a una concentración de 5 g/m3
produjo lesiones histopatológicas en el epitelio seminífero.
Los estudios en ratas (tratadas del 6 al 16 día de la gestación)
con exposición a concentraciones que producen sólo síntomas de ligera
toxicidad materna han demostrado que el HCFC 133a es embriotóxico en
concentraciones de 2 g/m3 o superiores y embrioletal a 10 g/m3 o
más. El tratamiento previo con progesterona de las hembras preñadas no
tuvo influencia en los efectos embriotóxicos/letales. En un estudio se
observaron síntomas de efectos teratogénicos (anomalías externas de
las extremidades y la cola). El HCFC 133a produjo abortos espontáneos
y embrioletalidad total en conejos expuestos a 25 g/m3 en los días
7 a 19 de la gestación, concentración que produjo sólo una ligera
toxicidad materna.
En los estudios disponibles no hay pruebas de su capacidad
mutagénica en bacterias. En un estudio no se observó aumento en la
proporción de células renales de hámster que producían colonias
transformadas. Se detectaron efectos letales dominantes en dos de tres
estudios tras la exposición de ratones machos a concentraciones de 12
g/m3 o superiores durante 5 días la proporción de células de la
médula ósea con aberraciones cromosómicas se mantuvo inalterada en
ratas expuestas a 98 g/m3 (6 h al día durante 5 días como máximo).
En el único estudio de carcinogenicidad se observó un aumento de la
incidencia de adenocarcinomas del útero y de tumores benignos de las
células intersticiales de los testículos en ratas que recibieron 300
mg/kg de aceite de maíz administrados por sonda durante 52 semanas
(seguidas de un período de observación de 73 semanas).
6.5 HCFC 123
El HCFC 123 tiene una toxicidad aguda oral y cutánea baja. La
dosis oral más baja de HCFC 123 con la que se han observado efectos
letales en ratas es de 9 g/kg. Con dosis de 2 g/kg no se produjo
mortalidad en ratas ni en conejos.
La toxicidad aguda por inhalación de HCFC 123 es también baja.
Los efectos observados son similares a los de los clorofluorocarburos,
es decir, pérdida de coordinación y narcosis. La CL50 a las 4 h es
de 178 g/m3 en el hámster, 463 g/m3 en el ratón y oscila entre 200
y 329 g/m3 en la rata. En el perro, se produjo sensibilización
cardíaca tras la exposición, con inyección de insulina, a
concentraciones de 119 g/m3 o superiores. El HCFC 123 líquido
produce en conejos una irritación "ligera" de la piel y los ojos. No
provoca sensibilización cutánea en los cobayos.
Se han realizado varios estudios de toxicidad de corta duración
con el HCFC 123 por vía respiratoria. En ratas se observan síntomas
invariables de depresión del SNC a concentraciones de 31 g/m3 o
superiores. El HCFC 123 causó también algunos efectos hepáticos en
ratas expuestas a dosis de 31 g/m3 o más. La exposición prolongada
(4 semanas o más) al HCFC 123 afecta también al metabolismo de los
lípidos y los glúcidos, como puso de manifiesto la reducción
invariable de los niveles de triglicéridos, colesterol y glucosa en el
suero. Los resultados provisionales de un estudio en curso de
toxicidad crónica/oncogenicidad por inhalación en ratas indican que el
HCFC 123 induce efectos tras la exposición prolongada a dosis de 2, 6
ó 31 g/m3. En este estudio no se registró el nivel sin efectos
observados (NOEL), basado en los efectos sobre el metabolismo lipídico
y en el aumento de la actividad de los peroxisomas hepáticos.
Actualmente se está llevando a cabo un estudio de reproducción de
dos generaciones de ratas expuestas al HCFC 123 por vía respiratoria.
En dos estudios limitados en ratas, con concentraciones capaces de
producir una ligera toxicidad materna, no se obtuvieron pruebas de
embriotoxicidad. Hay pruebas de toxicidad sólo a concentraciones muy
tóxicas para la madre (superiores a 62,5 g/m3) en conejos. En ratas
expuestas a concentraciones de 31 g/m3 o más y en conejos a niveles
de 3 g/m3 o superiores se observó toxicidad materna (disminución del
peso corporal y depresión del SNC). No aparecieron pruebas de
teratogenicidad en ratas ni en conejos.
El HCFC 123 no demuestra actividad mutagénica en los ensayos
efectuados con bacterias y levaduras. Sin embargo, sí hay pruebas de
actividad clastogénica en linfocitos humanos in vitro, pero los
datos procedentes de un ensayo in vivo en micronúcleos de ratón no
confirmaron ese resultado.
Está en curso un estudio combinado de toxicidad crónica/
carcinogenicidad por inhalación en ratas. En una comunicación
preliminar se ha indicado que el HCFC 123 produce una mayor frecuencia
de tumores benignos en los testículos y en el páncreas exócrino de
ratas macho. Sin embargo, no es posible realizar una evaluación de la
carcinogenicidad potencial del HCFC 123 hasta que no se disponga de
los resultados completos.
6.6 HCFC 124
La toxicidad aguda por inhalación del HCFC 124 en animales es
baja. Se produjo la muerte en ratas expuestas a concentraciones de
1674 g/m3 (durante 240 minutos) y en ratones a 2460 g/m3 (durante
10 minutos). Se observaron los efectos típicos de los
clorofluorocarburos, es decir, pérdida de coordinación y narcosis. Se
produjo sensibilización cardíaca tras la prueba de una inyección de
adrenalina en perros a concentraciones de 140 g/m3 o superiores. Se
carece de información sobre la irritación cutánea u ocular o la
sensibilización cutánea con este compuesto.
Se ha investigado la toxicidad por inhalación durante un breve
período en cinco experimentos en ratas con exposiciones que oscilaron
entre 14 y 90 días. No se observaron cambios histopatológicos en los
órganos, ni siquiera con los niveles de exposición más altos de los
estudiados (560 g/m3 en un experimento de 14 días, 279 g/m3 en un
estudio de 90 días). Se comunicó un NOEL de 28 g/m3 sobre la base de
las observaciones funcionales y los análisis de sangre efectuados en
el estudio de 90 días.
Está en curso un estudio sobre la toxicidad crónica por
inhalación del HCFC 124.
En tres estudios limitados de teratogenicidad en ratas, en los
que se probaron concentraciones de HCFC 124 de 30 g/m3 o
comprendidas entre 3 y 279 g/m3, no se encontraron pruebas de
efectos embriotóxicos o teratogénicos. Con 84 g/m3 apareció
toxicidad materna. No se dispone de información acerca de los efectos
del HCFC 124 en el potencial de reproducción. Se están realizando
estudios completos de teratogenicidad.
Los datos disponibles de varios estudios en bacterias y de un
único estudio en células de mamíferos no demuestran efecto mutagénico
del HCFC 124. Está en marcha un estudio de carcinogenicidad por
inhalación.
7. Efectos en el ser humano
Se carece de datos sobre los efectos del HCFC 151b, el HCFC 132b,
el HCFC 133b, el HCFC 123 y el HCFC 124 en el ser humano.
Los datos de un solo estudio en el ser humano expuesto en el
lugar de trabajo al HCFC 142b no permiten evaluar sus efectos en la
especie humana independientemente de otras muchas exposiciones.
8. Efectos en otros organismos en el laboratorio y en el medio
ambiente
No se dispone de información acerca de los efectos de los
hidroclorofluorocarburos estudiados sobre los organismos presentes en
el medio ambiente, excepto algunos datos limitados de los HCFC 141b y
142b. La CL50 a las 96 h del HCFC 141b para Brachydanio rerio es
de 126 mg/litro y la CE50 a las 48 h para la inmovilización de
Daphnia magna de 31 mg/litro, habiéndose realizado ambas
observaciones en recipientes cerrados. En el caso del HCFC 142b, la
CE50 a las 96 h para Lebistes reticulatus es de 220 mg/litro,
mientras que la CE50 a las 48 h para la inmovilización de Daphnia
magna varía de 160 a > 190 mg/litro. La CL50 a las 96 h del HCFC
142b para la trucha arco irís es de 36 mg/litro.
9. Evaluación y conclusiones
Se desconocen los niveles de los seis HCFCs estudiados presentes
en el medio ambiente, pero se consideran bajos, dado su grado actual
de utilización.
El HCFC 142b tiene un potencial tóxico bajo y se estima que no
supone un riesgo para el ser humano en condiciones de exposición no
debidas a un accidente. La información toxicológica acerca del HCFC
141b, el HCFC 123 y el HCFC 124 es incompleta y se necesitan más datos
para poder evaluar su riesgo para la salud humana. Tanto el HCFC 133a
como el HCFC 132b representan un riesgo para ésta.
Los seis hidroclorofluorocarburos estudiados tienen, o se les
supone, una capacidad de destrucción del ozono más baja y tiempos de
permanencia en la atmósfera considerablemente inferiores a los de los
clorofluorocarburos completamente halogenados. Por consiguiente,
deberían representar un riesgo indirecto menor para la salud. Su
efecto sobre el calentamiento del planeta es, o se supone, inferior al
de los clorofluorocarburos completamente halogenados y no se cree que
contribuyan a él de manera apreciable.
Puesto que la toxicidad del HCFC 142b es baja y su contribución
al agotamiento del ozono y al calentamiento del planeta son inferiores
a los de los clorofluorocarburos completamente halogenados, se lo
puede considerar como un sustitutivo transitorio de los
clorofluorocarburos que figuran en el Protocolo de Montreal.
Hasta que no se disponga de más datos toxicológicos no se pueden
hacer recomendaciones en relación con el HCFC 141b, el HCFC 123 y el
HCFC 124. Debido a su potencial tóxico, no se recomiendan el HCFC 133a
y el HCFC 132b como sustitutivos de los clorofluorocarburos que
figuran en el Protocolo de Montreal, a pesar de representar un riesgo
bajo para el medio ambiente e indirecto para la salud.
6.1.3.2 HCFC 141b
Harris & Anders (1991a) studied the in vivo metabolism of HCFC
141b. A single fluorinated urinary metabolite, identified as
2,2-dichloro-2-fluoro-ethyl glucuronide, was found in rats exposed to
HCFC 141b (56 g/m3) in air for 2 h. The metabolism was reported to
be similar to that of its chlorinated analogue 1,1,1-trichloroethane,
which is metabolized to 2,2,2-trichloroethanol and excreted as its
glucuronate conjugate (Hake et al., 1960) and as trichloroacetic acid
(Koizumi et al., 1982). It was claimed (although no data were given)
that 2,2-dichlorofluoroacetic acid was also detected in the urine of
rats exposed to a concentration of 194 g/m3 for 4 h, but not to 56
g/m3 for 2 h (Harris & Anders, 1991a).
In a pilot study for absorption and metabolism of HCFC 141b,
seven groups of five male rats were exposed to the vapour by
inhalation in a closed loop exposure system (concentrations ranging
from 0.4 to 12 g/m3). No metabolism was detected but the sensitivity
of the method is such that it will not detect metabolism below 0.15%.
The results suggested that absorption did not take place, but that if
any metabolism occurred it was at a low level (Zwart, 1989).
Evidence of dechlorination was observed when rat hepatic
microsomes were incubated with about 1% HCFC 141b in vitro (Van
Dyke, 1977).
6.1.3.3 HCFC 142b
No in vivo studies on the metabolism of HCFC 142b have been
reported. One in vitro study provided evidence for dechlorination
when rat hepatic microsomes were incubated with 0.6% HCFC 142b (Van
Dyke, 1977).
6.1.3.4 HCFC 132b
Harris & Anders (1991) identified a number of metabolites in the
urine of male Fischer-344 rats given one or four doses of 10 mmol/kg
dissolved in corn oil by intraperitoneal injection. Approximately 1.8%
of the single administered dose was recovered in the urine. The
metabolites excreted in urine during the first 6 h were
2-chloro-2,2-difluoroethyl glucuronide, chlorodifluoroacetic acid and
chlorodifluoroacetaldehyde hydrate (free and conjugated). Repeated
injection of rats with HCFC 132b significantly increased both the rate
of chlorodifluoroacetic acid excretion and the relative fraction of
the HCFC 132b dose excreted as chlorodifluoroacetic acid. The
incubation of HCFC 132b with rat hepatic microsomes yielded
chlorodifluoroacetaldehyde hydrate as the only fluorinated product.
The in vitro metabolism of HCFC 132b was increased in microsomes
from pyridine-treated rats and inhibited by p-nitrophenol. This
inhibition by p-nitrophenol led the authors to suggest an
involvement of cytochrome P-450 IIE1 in the initial hydroxylation of
HCFC 132b.
6.1.3.5 HCFC 133a
Evidence for dechlorination of HCFC 133a was provided by an
in vitro study using a microsomal preparation derived from
Aroclor-1254a-induced rat liver homogenates (Salmon et al., 1981).
6.1.3.6 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 g/m3 or 68 g/m3) or to halothane (2-bromo-2-2
chloro-1,1,1-trifluoroethane) (1.05 g/m3) in air for 2 h. The
pattern of proteins immunoreactive with haptens-specific
anti-trifluoroacetylprotein antibodies was found to be identical in
livers of the rats exposed to HCFC 123 and halothane. Trifluoroacetic
acid was detected in urine of rats exposed to HCFC 123 or halothane by
nuclear magnetic resonance (NMR) and by gas chromatography with mass
spectrometry (GCMS), as had been reported previously (Maiorino et al.,
1980; Trochimowicz, 1989).
6.1.4 Covalent binding to macromolecules
6.1.4.1 HCFC 141b
19F-NMR analysis of microsomal and cytosolic proteins isolated
from the livers of rats killed 15 h after a 2-h exposure to HCFC 141b
(56 g/m3) did not yield any evidence for covalent binding of
fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.2 HCFC 132b
19F-NMR analysis of microsomal and cytosol proteins isolated
from the livers of rats killed 15 h after a single intraperitoneal
dose of HCFC 132b (10 mmol/kg) did not yield evidence for covalent
binding of fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.3 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 or 68 g/m3) or to halothane (105 g/m3) in air for 2 h.
19F-NMR analysis of cytosolic protein and immunoblotting of
microsomal and cytosolic hepatic protein using antibodies against
trifluoroacetylprotein at 15 h after the exposure demonstrated
covalent binding of fluorinated metabolites.
a Aroclor-1254 is a polychlorinated biphenyl mixture.
6.1.5 Elimination
No data are available from animal studies on the elimination of
the hydrochlorofluorocarbons reviewed. However, based on the
information on chlorofluorocarbons, it is likely that the main route
of excretion for hydrochlorofluorocarbons is through the respiratory
tract. Increased urinary inorganic fluoride has been observed in some
inhalation toxicity studies with HCFCs 141b, 132b, 123, and 124
(Doleba-Crowe, 1977, 1978; Brewer, 1977; Trochimowicz, 1989; Malley,
1990a, 1991).
6.2 Human studies
No data are available on the absorption, distribution, metabolic
transformation or elimination of the hydrochlorofluorocarbons
reviewed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute oral toxicity
Available data indicate low toxicity following single oral
exposure associated with four hydrochlorofluorocarbons, i.e. HCFC
141b, 142b, 132b and 123.
7.1.1.1 HCFC 141b
No mortality was observed in rats given a single oral dose of
HCFC 141b (5 g/kg body weight) dissolved in corn oil (Sarver, 1988).
7.1.1.2 HCFC 142b
Other than piloerection, oral dosing with HCFC 142b (up to 5 g/kg
body weight) dissolved in corn oil resulted in no signs of toxicity
(Liggett et al., 1989).
7.1.1.3 HCFC 132b
According to Henry (1975), the lowest dose at which mortality was
observed in rats treated with HCFC 132b was 25 g/kg.
A 2-g/kg dose of HCFC 132b in corn oil was applied by stomach
tubes to five male and five female Wistar rats, and the animals were
observed for 14 days. The clinical signs were indicative of an effect
on the autonomic nervous system (ptosis), on the central nervous
system (diminished alertness and startle response, and positional
passivity), on motor coordination (abnormal body posture and gait, and
loss of righting reflex), on motor activity and on muscle tone
(paralysis). Macroscopic examination showed swollen livers in some
males, and a decrease in absolute and relative liver weights. However,
no treatment-related effects were found during microscopic
examinations (Janssen & Pot, 1989b).
7.1.1.4 HCFC 123
The lowest dose of HCFC 123 producing lethality in rats was
reported to be 9 g/kg when it was administered as a corn oil solution
by intragastric intubation (Henry, 1975a).
7.1.2 Acute inhalation toxicity
7.1.2.1 HCFC 141b
The effects of a single inhalation exposure of rodents to HCFC
141b are shown in Table 5. The main effects at high exposure
concentrations include central nervous system depression, anaesthesia
and death.
7.1.2.2 HCFC 142b
Table 6 summarizes the effects of single inhalation exposure to
HCFC 142b in mice and rats. At high exposure levels, HCFC 142b induces
anaesthesia and death.
7.1.2.3 HCFC 132b
Table 7 summarizes the effects of single inhalation exposures of
mice and rats to HCFC 132b.
7.1.2.4 HCFC 133a
Inhalation of high concentrations of HCFC 133a is characterized
by signs of anaesthesia followed by death, but recovery from nonlethal
exposure is rapid. The effects of inhalation exposure to HCFC 133a are
summarized in Table 8.
7.1.2.5 HCFC 123
The results of studies on the acute inhalation toxicity of HCFC
123 are summarized in Table 9. Anaesthesia and death at higher
exposures were reported for rats and hamsters. No gross morphological
changes were observed in animals that died during exposure. Survivors
recovered within several minutes without showing any observable
clinical signs.
7.1.2.6 HCFC 124
Table 10 summarizes the effects of single inhalation exposure to
high concentrations of HCFC 124. As with other
hydrochlorofluorocarbons, the main effects observed were anaesthesia
and death.
7.1.3 Acute dermal toxicity
There is information only on the hydrochlorofluorocarbons that
are liquid at ambient temperatures, i.e. HCFC 141b, HCFC 132b and HCFC
123.
Table 5. Effects of a single inhalation exposure to HCFC 141b in mice and rats
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3) (h)
Rat 142-366 4 LC50 = 295 g/m3 Hardy et al. (1989a)
(Sprague-Dawley) No deaths were observed at 142 or 217 g/m3. All deaths
at higher concentrations (323 and 366 g/m3) occurred
during exposure, and were preceded by disturbed breathing.
Reduced motor activity, abnormal body carriage, restless
behaviour and exaggerated respiratory movements were seen
at all concentrations during exposure. No treatment-related
macroscopic findings were seen. Focal basophilic staining
was observed in the renal cortical tubules of 4 out of 10
rats at 217 g/m3 and 2 out of 10 rats at 142 g/m3. No
histopathological effects were seen in decedents.
Rat (strain unspecified 6 The lowest concentration producing lethality was 242 g/m3. Doleba-Crowe (1977)
unspecified) range
Mouse 17-388 6 Deaths preceded by signs of narcosis in 6 out of 10) animals Vlachos (1989)
(Crl: CD-1) occurred within 30 min of exposure to 388 g/m3. No deaths
occurred at the next highest (199 g/m3) concentration. Signs
of CNS depression (lethargy, abnormal gait, partially closed
eyes) were seen at 165 and 199 g/m3. No effects were seen at
concentrations of 145 g/m3 or less.
Mouse (strain unspecified 2 LC16 = 115 g/m3; LC50 = 151 g/m3; LC84 = 200 g/m3. Signs of Nikitenko & Tolgskaja
unspecified) range CNS depression and anaesthesia were observed. Death was (1965)
preceded by laboured breathing.
Mouse (Schofield unspecified 0.5 LC50 = 115 g/m3; concentration producing anaesthesia in Davies et al. (1976)
strain) range 50% of animals = 62 g/m3. No other information was given.
Table 6. Effects of single inhalation exposure to HCFC 142b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse "white" up to 2050 2 death; LC50 = 1514 g/m3 Nikitenko & Tolgskaja (1965)
Mouse AP unspecified 0.5 death; LC50 = 1228 g/m3 Davies et al. (1976)
range
Rat "white" 615-3280 0.5 death at 2050 g/m3 and unconsciousness at Lester & Greenberg (1950)
1230 g/m3; postural, righting and corneal
reflexes were lost at 820 g/m3
Rat Sherman 525 4 death (approx 50%) at 525 g/m3 Carpenter et al. (1949)
Table 7. Effects of single inhalation exposure to HCFC 132b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse unspecified range not given 0.5 LC50 = 269 g/m3; AC50 (anaesthesia) = 71 g/m3 Raventós & Lemon (1965)
Rat Wistar derived 55 and 110 4 lethalities at 110 g/m3; rats unsteady, weak and Torkelson (1971)
drowsy at 55 g/m3
Rat Wistar derived 33-72 6 anaesthesia at 82 g/m3; kidney swelling at autopsy; Janssen (1988)
CPB-WU at all dose levels, males showed decreased growth
and testis weight, and increased liver and lung
weights
Rat Wistar derived 55 0.4 decreased respiratory rate with rapid recovery Janssen (1989b)
CPB-WU after exposure
Table 8. Effects of single inhalation exposure to HCFC 133a
Species Exposure Exposure Effectsa Reference
(strain) concentration duration
(g/m3) (min)
Mice, male unspecified 10 anaesthesia and death; convulsions on recovery; AC50 and Robbins (1946)
(white) range LC50 were 394 and 1230 g/m3, respectively
Mice (strain unspecified 30 anaesthesia, convulsions and death; AC50 and LC50 were Raventós & Lemon (1965)
unspecified) range 212 and 738 g/m3, respectively
Mice (strain 123-1230 10 rapid onset of anaesthesia, rapid recovery after cessation of Shulman & Sadove (1965)
unspecified) exposure but no convulsions; AC50 and LC50 were 397 and
1033 g/m3, respectively
Rats, female 2500 - lack of muscular coordination in 3 min, anaesthesia in 4 min Diggle & Gage (1956)
(strain unspecified) and death within 8 min
Dogs unspecified - anaesthesia at 492 g/m3, respiratory depression and arrest Shulman & Sadove (1965)
range occurred at 1131 and 1427 g/m3, and circulatory arrest at
2902 g/m3
a AC50 = calculated concentration expected to produce anaesthesia in 50% of the test group
Table 9. Acute inhalation toxicity of HCFC 123
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3)
Mouse (strain not given 30 min LC50 = 463 g/m3 Raventós & Lemon (1965)
unspecified)
Rat (Charles 129-344 4 h LC50 = 200 g/m3; loss of mobility, lethargy, prostration Hall & Moore (1975)
River CD) at all concentrations; full recovery of survivors within
30 min post exposure
Rat (Charles 49-767 6 h LC50 = 329 g/m3; anaesthesia at 145 g/m3 and higher Coate (1976a)
River CD) concentrations; discoloration of lungs in most animals that
died, discoloration of liver in some of them
Rat (strain 6, 16, 31, 62 15 min unconditioned reflexes, locomotor activity, coordination Trochimowicz (1989)
unspecified) affected at 31 and 62 g/m3; full recovery within 30 min
post exposure
Hamster 63-194 4 h LC50 = 178 g/m3; incoordination, prostration at all Darr (1981)
(Chinese) concentrations; full recovery of survivors after exposure;
0% mortality at 163 g/m3, 100 mortality at 194 g/m3
Table 10. Acute inhalation toxicity of HCFC 124
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (min)
Mouse unspecified 594 10 no effect Wada (1977)a
837 10 narcosis Wada (1977)a
2230 10 no mortality Wada (1977)a
2460 10 death Wada (1977)a
Rat Sprague-Dawley 268 240 no effect Kelly (1990)
Charles River COBS 558 300 reduced activity Coate (1976b)
Sprague-Dawley 893 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1283 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1674 240 death Kelly (1990)
Charles River COBS 2009 300 narcosis, no mortality Coate (1976b)
Dog 2230-3910 10 narcosis Van Poznak & Artusio (1960)
a Attachment to correspondence from H. Wada, Daikon Kogyo Company Ltd. to M.B. Berenbaum, Allied Chemical Corporation,
entitled Anaesthetic activity and fatality (F-123, 123a, 124 and 11)
7.1.3.1 HCFC 141b
No deaths occurred at dermal doses of 2 g/kg body weight either
among rats (Janssen & Pot, 1988; Gardner, 1988) or rabbits (Brock,
1988a).
7.1.3.2 HCFC 132b
When Janssen & Pot (1989a) applied a single dose of HCFC l32b (2
g/kg) under an occluding dressing to the shaved skin of five male and
five female Wistar rats, there were no deaths. The clinical signs
observed were decreased respiratory rate, decreased startle response,
altered locomotor activity, restlessness and vocalization. Three male
and four female rats had swollen or slightly swollen livers on
autopsy.
7.1.3.3 HCFC 123
Several limit tests for dermal toxicity of HCFC 123 were
conducted. No mortality was observed at the limit dose of 2 g/kg body
weight in rats (Brock, 1988d; Trochimowicz, 1989) or rabbits (Brock,
1988e,f; Trochimowicz, 1989). The only clinical signs of toxicity were
red nasal or ocular discharges in one of five male and one of five
female rats, and slight to moderate body weight losses (up to 12% of
initial body weight). No gross pathological abnormality was observed
(Trochimowicz, 1989). In rabbits, only slight to moderate erythema was
observed (Trochimowicz, 1989).
7.2 Short-term inhalation exposure
7.2.1 HCFC 141b
Nikitenko & Tolgskaja (1965) reported a reduction in body weight
gain, a slight decrease in haemoglobin level and moderate
leucocytosis, some "minor changes" in blood parameters related to
liver and kidney function, and histopathological effects in the
respiratory tract of rats and guinea-pigs (number and strains
unspecified) that had been exposed to 40-50 g/m3 (2 h/day, 6
days/week) for 4 weeks. The purity of the substance and the specific
isomer were not indicated.
No adverse clinical signs and only "slight biochemical changes"
(no details given) were reported in rats (number and strain
unspecified) exposed to 48.5 g/m3 (6 h/day, 5 days/week) for 2 weeks
(Pennwalt Corporation, 1987).
In a 2-week inhalation toxicity study, Doleba-Crowe (1977)
exposed groups of 10 male rats to 0 or 48 g/m3 for 6 h/day, 5
days/week. The animals were observed for 14 days after exposure. No
adverse clinical signs were observed, and there were no differences in
body weights between treated and control animals. After the tenth
exposure, elevated red blood cell counts, plasma bilirubin level and
increased urinary fluoride concentrations were found, but all these
parameters returned to normal after 14 days. The treated animals
showed a more severe focal interstitial pneumonitis than controls 14
days after exposure, but no other treatment-related change was
observed.
Coombs et al. (1988) exposed five groups of 10 male and 10 female
Sprague-Dawley rats to 0, 24, 42, 68 and 97 g/m3 (6 h/day for 9
days, i.e. 5 days of exposure followed by 1 day without exposure and
then 4 days with exposure). Signs of central nervous system (CNS)
depression were seen during exposure to concentrations of 42 g/m3 or
more. At 97 g/m3, these signs were accompanied by a decrease in body
weight gain in males and a slightly reduced food intake in both sexes.
Glucose and aspartate serum transaminase (AST) levels were increased
at 97 g/m3, protein, cholesterol and sodium from 68 g/m3,
phosphate from 42 g/m3 and calcium from 24 g/m3. No
treatment-related histopathological changes were observed at any dose
level.
In a 13-week inhalation study (some animals were killed after 4
weeks), four groups each of 15 male and 15 female Fischer-344 rats
were exposed to 0, 10, 39 or 97 g/m3 (6 h/day, 5 days/week) as
described in two reports (Yano et al., 1989; Landry et al., 1989).
Alertness was reduced at 97 g/m3, and body weight gain and food
consumption were slightly reduced in all exposed groups. After both 4
and 13 weeks of exposure, plasma cholesterol, triglycerides and
glucose were slightly elevated in the rats exposed to 97 g/m3. No
changes in haematological or histopathological parameters were found.
7.2.2 HCFC 142b
Rats and guinea-pigs (numbers and strains not specified) were
exposed to a concentration of 448 g/m3 (isomer not specified), 2
h/day, 6 days/week, for 4 weeks (Nikitenko & Tolgskaja, 1965). A
decrease in the rate of body weight gain was observed at the end of
the study, as well as a reduction in haemoglobin concentration and the
number of erythrocytes, and an increase in the number of leucocytes.
Swelling of the alveolar septa and peribronchitis were the
histopathological changes observed in the lungs.
In a study in which 10 adult white rats were exposed to 410
g/m3 for 16 h/day, all animals died within 9 exposures. All of them
showed severe signs of pulmonary irritation at autopsy (consolidation
and hepatization of the lungs). The other organs appeared normal. No
signs of ill health were apparent in five rats exposed to a
concentration of 41 g/m3 (16 h/day for 2 months). Gross examination
of the organs on autopsy revealed no pathological changes, but
microscopic examination of the lungs showed round cell infiltration in
the lung of two animals. The appearance of sections of the livers was
normal (Lester & Greenberg, 1950).
No clinical, haematological, blood chemical, urine analytical or
histopathological evidence of effects attributable to repeated
exposure to HCFC 142b was found in 10 male Charles River CD rats
exposed to a concentration of 82 g/m3 (6 h/day, 5 days/week) for 2
weeks (Moore & Trochimowicz, 1976).
Kelly (1976) did not find any adverse clinical, haematological,
blood chemical, urine analytical or histopathological effects
attributable to HCFC 142b at exposure levels of either 4.1 g/m3 or
41 g/m3 (6 h/day, 5 days/week for 90 days) in groups of male and
female Charles River CD rats (27 of each sex at each treatment level)
or groups of male dogs (4 at each treatment level).
7.2.3 HCFC 132b
When 20 male Crl:CDRBR rats were exposed to 55 g/m3 (6 h/day,
5 days/week) for 2 weeks, reduction in body weight gain, irregular
respiration and CNS depression (lethargy, poor coordination,
occasional tremors and prostration) were seen. The CNS effects
disappeared within 30 min after each exposure. Pathological
examinations of rats sacrificed immediately after the tenth exposure
showed thymic atrophy and spermatogenesis arrest, but these changes
were not present in rats sacrificed 14 days after exposure ceased
(Hall, 1976).
Groups of 20 male and 20 female Crl:CDRBR rats were exposed to
0, 3, 11 and 27 g/m3 (6 h/day, 5 days/week) for 13 weeks (Kelly,
1988). Male rats exposed at all the concentrations of HCFC 132b showed
bile duct proliferation and disruption of spermatogenesis with cell
debris in the epididymides at the two higher concentrations. Other
effects included increases in liver/body weight ratio in males at all
concentrations and in females at the two higher concentrations.
Elevation of serum alkaline phosphatase activity was found in both
sexes exposed to 11 or 27 g/m3. During the study, all groups exposed
to HCFC 132b showed reduced food consumption and body weight gain. In
the two highest exposure groups there were depressions in the absolute
but not relative brain and testes weights. Other organ weight changes
were also seen (slight increases in heart, lung and kidney weights).
The biological significance of these weight changes is not clear since
there were no accompanying histological findings. During exposure to
27 g/m3, rats showed CNS depression as indicated by decreased
activity and low responsiveness to sound.
7.2.4 HCFC 133a
Shulman & Sadove (1965) exposed mice to anaesthetic
concentrations (the actual concentration was not specified) of HCFC
133a for 30 min per day on 12 consecutive days, and the animals were
killed for pathological evaluation after the last exposure by
overdosage of HCFC 133a. None of the mice showed any treatment-related
clinical effects, and no pathological changes were found in the organs
(heart, lung, liver, kidney, adrenal gland, spleen and pancreas)
examined microscopically.
Diggle & Gage (1956) investigated the effects of repeated
exposure (up to 8 days) of groups of 2-3 female rats. Concentrations
of HCFC 133a between 50 and 125 g/m3 caused incoordination and
lethargy, while at 250 or 500 g/m3 rats become comatose. They
recovered between each exposure and no dose-related pathological
changes were found on histological examination. No effect was seen at
25 g/m3 during seven exposures lasting 6 h/day.
In a study by Leuschner et al. (1977), 20 male and 20 female
Sprague-Dawley rats were exposed to 49 g/m3 (6 h/day, every day) for
90 days. Corresponding groups of 20 male and 20 female rats were used
as controls. Observations for overt clinical signs of toxicity and
investigations on body weight, food consumption, haematology, blood
and urine biochemistry, urine sediments, ophthalmology, auditory
reflex, organ weights, and histopathology were performed. There were
no treatment-related deaths. The rats were sedated during each
exposure but appeared normal before and after. Seventeen out of 40
rats developed bloody and inflamed noses; this was associated with
histological evidence of inflammatory changes of the mucosa. Body
weight gain was reduced, so that the terminal average body weights
were approximately 28 and 17% lower than those of male and female
controls, respectively. Food consumption in the treated groups was
also lower than in the controls. Haemoglobin concentration,
haematocrit, red blood cell counts and platelet counts were all
slightly reduced. Reduction in leucocyte counts of approximately 30%
and increase in reticulocyte counts of approximately 40% were seen.
There were reductions in plasma glucose levels of approximately 15%
and in protein levels of approximately 10%. Bromosulfophthalein
retention time was increased by approximately 35% and 62% in males and
females, respectively. There was no change in plasma enzyme
glutamic-pyruvic transaminase (GPT), alkaline phosphatase (AP) or
glutamicoxalacetic transaminase (GOT) activity. The thymus to body
weight ratio was reduced by approximately 50% and the testis and ovary
to body weight ratios by approximately 60 and 35%, respectively.
Histologically, these organs showed atrophy. Thyroid to body weight
ratio was increased by approximately 45% in males only. Atrophy of the
spleen was also observed. The exposure induced emphysema and oedema of
the lungs as well as bronchitis and pneumonia. The testicular atrophy
was consistent with the findings in three dominant lethal studies in
mice (Hodge et al., 1979, 1980; Kilmartin et al., 1980) and a
carcinogenicity study in rats (Longstaff et al., 1984) (see section
7.6 and 7.7).
Six beagle dogs were exposed by Leuschner (1977) to 24 g/m3
(6 h/day, daily) for 3 months and six control dogs were used. No
effects were seen on external appearance, faeces, food and water
consumption, body weight gain, haematology, blood and urine
biochemistry, urine sediments, electrocardiography, blood pressure,
ophthalmology, hearing or dentition. There was no effect on organ
weight at autopsy. No treatment-related histopathological changes were
seen on microscopic examination of a standard range of 24 tissues.
In two dominant lethal studies (for experimental details see
section 7.5.1.4), male mice were exposed to between 0.5 and 49 g/m3,
6 h/day, for 5 days (Hodge et al., 1980; Kilmartin et al., l980). The
mice were subdued at exposure concentrations of 2 g/m3 or more.
Deaths occurred as follows: in the first study 0/60 mice died at 0
g/m3, l7/60 at l2 g/m3, and 28/80 at 49 g/m3; in the second
study 0/80 died at 0 g/m3, 2/59 at 0.5 g/m3, 0/60 at 2.5 g/m3,
5/60 at 5 g/m3, and 20/60 at 12 g/m3.
7.2.5 HCFC 123
In a study by Doleba-Crowe (1978), Sprague-Dawley rats and beagle
dogs were exposed to concentrations of 0, 6 and 62 g/m3 (6 h/day, 5
days/week) for 90 days. At the high exposure level, both species
exhibited lack of motor coordination soon after the start of exposure.
This was followed by reduced motor activity and reduction in
responsiveness to noise. After removal from exposure, coordination and
activity returned to normal within 20 min. Other than final body
weight reductions and increased urinary fluoride level at both
exposure levels, no significant exposure-related effects were observed
in rats. At the high exposure level, dogs exhibited histopathological
changes in the liver and clinical chemistry changes including
increased levels of serum GOT and GPT, which might indicate slight
liver damage. No exposure-related effect was noted at the lower
exposure level.
In a 90-day inhalation study, albino rats obtained from Charles
River Breeding Laboratory were exposed to nominal levels of 0, 3, 6,
and 31 g/m3, 6 h/day, 5 days/week (Rusch, 1985). No
treatment-related deaths occurred in the study and mean body weight
reductions observed in the males at the highest exposure level and in
females at the two highest exposure levels were significant only at
the end of the study. Slight depression was observed in heart weight
in both male and female rats exposed to 31 g/m3. While depressions
of the kidney weights and kidney/brain weight ratio (but not kidney to
body weight ratio) were observed in male rats in all the three
exposure groups, these effects were outside the normal range only at
the highest exposure level. A similar depression in kidney weight and
kidney/body weight ratio (but not in kidney/brain ratio) occurred in
females at the highest exposure level. Increased liver/body weight
ratios (but not liver weight or liver/brain weight ratios) were
observed in all three exposure groups of females, but in males only at
the highest exposure level. No significant difference was found in
organ weights or ratios in animals sacrificed at the end of a 30-day
recovery period. The absence of histopathological findings and of
effects at the end of the recovery period indicates that the
toxicological significance of the organ weight changes in this study
is unclear.
In a 4-week inhalation toxicity study (Kelly, 1989; Trochimowicz,
1989), rats (10 of each sex at each exposure level) were exposed to 0,
6, 31, 62 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Statistically significant body weight depression occurred in all
female groups and in the two highest male groups, but was dose-related
only in the male rats. At concentrations of 31 mg/m3 or more, rats
exhibited dose-related anaesthesia. At 62 and 125 g/m3, rats became
lethargic during exposure but were normal when they were examined
again 16 to 18 h after exposure. A dose-related increase in liver/body
weight ratio was observed in all female groups (a 27% increase at the
highest level) and in the two highest-exposure male groups (an 18%
increase at the highest level). Decreased cytochrome P-450 activity in
the liver was also found in all female exposure groups and in the male
groups exposed at the two highest levels. Histopathological
examination showed no adverse effects attributable to HCFC 123 in
liver or in any other organ at any exposure level. Microscopic
examination revealed that testicular degeneration and hypospermia
occurred in two out of four male exposure groups, but this was
believed to be due to reduced body weight (because of an increase in
relative testicular weight without a corresponding increase in
absolute testicular weight) or may have resulted from spontaneous
lesions. The fact that the incidence of testicular degeneration was
highest in the 31- and 125-g/m3 exposure groups (5 out of 10 and 6
out of 10 animals, respectively), and lower in the 0-, 6-, and
62-g/m3 exposure groups (2 out of 10, 1 out of 10 and 2 out of 10
animals, respectively) is consistent with the sporadic nature of this
lesion in this strain of rat. However, it is not possible to evaluate
the significance of this testicular effect from the information
available.
Another 28-day inhalation toxicity study in rats was conducted to
characterize further the potential effects of HCFC 123 on the liver
(Lewis, 1990). In addition, urine samples were examined to identify
metabolites of HCFC 123. Groups of six male Charles River CD rats were
exposed to 0, 6, 31 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Body weights were statistically significantly reduced in all treated
groups compared to controls, the greatest reduction occurring in the
high-dose group. A concentration-related decrease in serum cholesterol
levels was found in all test groups. This reduction was statistically
significant compared to controls at the medium and high
concentrations. Serum triglyceride levels were significantly reduced
to a similar extent in all treated groups. A statistically significant
increase in absolute and relative liver weights compared to controls
was seen in the high-dose rats. Hepatocyte hypertrophy and mild fatty
vacuolation was found at all concentrations tested but the severity
was greatly reduced at the low exposure level. Electron microscopic
examination revealed a treatment-related induction of peroxisome
proliferation at the medium and high exposure concentrations. A
statistically significant concentration-related increase in relative
testes weight was observed in all treated groups (11-30% above
control), but no compound-related morphological or microscopic changes
were observed. Urine analysis indicated the presence of
trifluoroacetic acid as a major metabolite.
In a study by Malley (1990a), groups of 10 male and 10 female
Crl:CDRBR rats were exposed to concentrations of 0, 2, 6 or 31
g/m3 (6 h/day, 5 days/week) for 90 days. No effect on food
consumption or body weight was observed. At the highest exposure level
the animals exhibited anaesthesia and a decreased response to auditory
stimuli. Serum triglyceride and glucose levels were significantly
decreased at all exposure levels, while serum cholesterol was
significantly lower in females exposed to the two highest
concentrations. The mean lymphocyte and white blood cell counts in
female rats were decreased at the highest exposure level. Male rats
had significantly higher alanine aminotransferase and alkaline
phosphatase activity at the two highest exposure levels. In female
rats alanine aminotransferase activity was elevated at the highest
exposure level. Urine fluoride concentrations were increased in
females at all exposure levels, but in males only at the highest
exposure level. Absolute liver weights were significantly higher in
male rats at the highest exposure level and in female rats at the two
highest exposure levels, while relative liver weights in both male and
female rats were higher at the two highest exposure levels. Hepatic
peroxisomal beta-oxidation activity in both male and female rats was
1.9 to 3.8 times higher than in controls, indicating an induction of
hepatic peroxisome proliferation. No treatment-related gross or
microscopic liver changes were observed.
7.2.6 HCFC 124
When ten male rats were exposed to approximately 560 g/m3 (6
h/day, 5 days/week) for 14 days, no adverse haematology, clinical
chemistry, urine analysis or histopathological changes were observed.
Rats showed irregular respiration, lethargy and poor coordination
(Hall, 1976).
Trochimowicz et al. (1977) reported no adverse effect following
the clinical and histopathological evaluation of rats exposed to
concentrations of 560 g/m3 (6 h/day, 5 days/week) for 2 weeks.
Brewer (1977) exposed groups of 60 Sprague-Dawley rats (35 males and
25 females) to concentrations of 0, 3, 5 or 28 g/m3 (6 h/day, 5
days/week) for 3 months. Ten male and ten female rats were examined
and sacrificed after 45 days and a similar number after 92 days. Ten
male and five female rats of each group were maintained without
further exposure for an additional 30-day period after exposure.
Clinical signs were observed, body and organ weights determined, and
haematological, biochemical and histopathological examinations carried
out on all animals. No statistically significant differences in body
weight gain were noted. Haematology, clinical chemistry, and urine
analysis in treated rats were normal and comparable to findings in
control animals. Urinary fluoride excretion was increased after 45
days of exposure in both males and females (4.0 and 3.5 times,
respectively) at an exposure level of 28 g/m3; at the two lower
levels, determinations were not made. After 95 days of exposure, the
urinary fluoride level was elevated only in males at all three
exposure levels (by 1.5, 1.7 and 1.8 times, respectively), and this
effect persisted throughout the 30-day period after exposure. Gross
and histopathological examinations did not reveal any
treatment-related changes in any group of animals. Statistically
significant difference in organ weights between treated and control
rats were found. Liver weights were increased significantly in males
at 5 and 28 g/m3, while the lung and adrenal gland weights were
significantly decreased in males at all three exposure levels. In the
absence of any histopathological change, the biological significance
of these organ weight changes is unclear.
Malley (1990b) exposed groups of ten male and ten female rats to
HCFC 124 concentrations of 0, 3, 11, 56 or 279 g/m3 (6 h/day, 5
days/week) for 4 weeks. Treatment-related effects on body weight, food
consumption, mortality, clinical laboratory parameters, organ weights,
and tissue morphology changes were not found at any exposure level.
During exposure, rats exposed to 279 g/m3 were lethargic and
uncoordinated. However, no evidence of lethargy and incoordination was
observed shortly after exposure. The author considered the exposure
concentration of 56 g/m3 the no-observed-adverse-effect level
(NOAEL) based on the clinical observation of lethargic and
uncoordinated movement during exposure to 279 g/m3, which was not
observed at 56 g/m3.
Malley (1991) exposed Crl:CDRBR rats (20 rats of each sex at
each exposure level) to HCFC 124 at concentrations of 0, 28, 84 and
279 g/m3 (6 h/day, 5 days/week) for 90 days. As part of this study,
a functional observation battery (FOB) was conducted on 10 rats of
each sex at each exposure level at various intervals during the course
of exposure. There were no compound-related effects relative to body
weight, food consumption, mortality, haematology, organ weights or
histopathology at any exposure concentration. However, male rats, at
a level of 84 and 279 g/m3, had lower serum triglyceride
concentrations and a decreased arousal (4 of 10 and 6 of 10 rats,
respectively). Persistent decrease of forelimb grip strength was found
in high-dose females. However, there was no associated decrease in
hindlimb grip strength or change in gait or footplay. Females at the
highest concentration showed an increase in alkaline phosphatase. Both
sexes at 279 g/m3 were less responsive to auditory stimuli, as
demonstrated by a decreased reaction to a sharp knock on the chamber
wall. Plasma fluoride, urinary fluoride and fractional clearance of
free fluoride were also increased at all exposure levels in both
sexes. The author concluded that the no-observed-effect level (NOEL)
was 28 g/m3 for male rats and 84 g/m3 for female rats.
7.3 Skin and eye irritation; sensitization
7.3.1 Skin and eye irritation
7.3.1.1 HCFC 141b
Treatment of the intact skin of New Zealand albino rabbits with
0.5 ml of undiluted HCFC 141b under occlusive patch (during 4 and 24
h in the two respective studies) did not produce signs of dermal
irritation during a 3-day observation period (Liggett, 1988a; Brock,
1988b).
Two studies were conducted with HCFC 141b on groups of six New
Zealand albino rabbits where the undiluted compound (0.1 ml) was
instilled into the eyes. No signs of irritation occurred within 3 days
in one study (Liggett, 1988b), but the compound was found to be a
"mild" irritant in the other study (Brock, 1988c). The majority of
rabbits in the latter study showed conjunctival redness (5/6), mild
chaemosis (3/8) and blood-tinged discharge (4/6).
7.3.1.2 HCFC 142b
Brittelli (1976a) observed no effects on the cornea or iris but
slight conjunctival swelling with some discharge in an eye irritation
test with HCFC 142b.
7.3.1.3 HCFC 132b
One drop (approximately 0.05 ml) each of 100% HCFC 132b and a 10%
solution in propylene glycol was applied and slightly rubbed into the
shaved intact shoulder skin of 10 male albino guinea-pigs, but the
area was not occluded. The pure compound produced only mild irritation
in one animal only. No irritation was induced by the 10% solution
(Goodman, 1976).
Undiluted HCFC 132b (0.1 ml) was placed into the right
conjunctival sac of two albino rabbits, and after 20 seconds one
treated eye was washed with water for 1 min. Observations of the
cornea, iris and conjunctiva were made after 1 and 4 h, and 1, 2, 3
and 7 days later. Slight corneal opacity and "mild" to "moderate"
conjunctival irritation were seen in both rabbits up to 3 days after
dosing, but had disappeared by 7 days after dosing (Brittelli, 1976b).
7.3.1.4 HCFC 123
Minimal dermal irritation with HCFC 123 was observed in rabbits
(Brock, 1988e,f). HCFC 123 (purity 99.0%) produced no skin irritation
when 0.5 ml/6 cm2 was applied to the clipped intact skin of four
male and two female New Zealand rabbits for 4 h (Trochimowicz, 1989).
Brittelli (1976c) reported HCFC 123 to be a "mild" ocular
irritant causing reversible corneal opacity in rabbits. In another
study by Daly (1979)a, HCFC 123, when instilled undiluted (0.1 ml)
into the conjunctival sac of the rabbit eye without subsequent
washing, produced "mild" to "moderate" conjunctival irritation. With
washing, "mild" transient corneal opacity and "mild" to "moderate"
conjunctival irritation were observed. With or without washing,
complete recovery occurred within 3-7 days.
7.3.2 Skin sensitization
7.3.2.1 HCFC 141b
No delayed contact hypersensitivity was found in any of the 20
Hartley Dunkin guinea-pigs in a Magnusson-Kligman maximisation test
with HCFC 141b (Kynoch & Parcell, 1989).
7.3.2.2 HCFC 132b
A series of four sacral intradermal injections of HCFC 132b was
given (once per week at 7-day intervals) over a 3-week period to
groups of nine male albino guinea-pigs (0.1 ml of a 1% solution in
dimethyl phthalate). Fourteen days after the last application, the
animals were challenged with either 1 drop (0.05 ml) of undiluted
liquid or a 19% solution of test material in propylene glycol on the
shaved skin. No evidence of sensitization was observed (Goodman,
1976).
7.3.2.3 HCFC 123
When applied topically to the back of male guinea-pigs as 10% or
50% solutions in propylene glycol, HCFC 123 produced no sensitization
at challenge (Goodman, 1975; Daly, 1979).
7.4 Long-term exposure
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternative Fluorocarbon Toxicity Testing (Rusch, 1989) sponsored
by an international industry consortium. An interim report after the
first year of the study is available on HCFC 123 (Malley, 1990b).
a Personal communication entitled "Toxicity testing summary for
alternative fluorocarbons" by J.J. Daly to CFTA Interindustry
Safety Committee, Wilmington, Delaware, USA, E.I. Du Pont de
Nemours and Co.
7.4.1 HCFC 142b
Four groups each containing 130 male and 110 female Sprague-
Dawley CD rats were exposed by inhalation to concentrations of 4.1, 41
and 82 g/m3 (6 h/day, 5 days/week) for 104 weeks. No exposure-
related effects were found on mortality, body weight, haematology,
clinical chemistry, urine analysis, histopathological or
ophthalmological findings (Seckar et al., 1986). However, high
mortality (more than 50% by the end of the study) was seen in all
groups including controls.
7.4.2 HCFC 123
Findings associated with the first year of the PAFT-supported
study were provided by Malley (1990c). Following 12 months of
inhalation exposure to 0, 2, 6 or 31 g/m3, 10 rats (Crl:CDRBR) of
each sex at each concentration were sacrificed, selected organs were
weighed, and tissues were examined for gross and microscopic lesions.
Body weight and body weight gain were significantly lower in high-dose
males and in mid- and high-dose females. Food consumption was higher
and food efficiency was lower in high-dose males and females over the
course of the l year of exposure. The lower food efficiency appeared
to be directly related to the lower body weight gain at the high
concentration (31 g/m3). High-dose males and females exhibited
anaesthesia-like behaviour (e.g., less responsiveness to auditory
stimuli compared to control rats). No compound-related effects on
mortality or survival time were found in any treated group. Clinical
chemistry parameters measured at 6 months and 1 year revealed several
compound-related changes. The most noteworthy findings were the effect
on lipid and carbohydrate metabolism. Significant decreases of serum
triglyceride and glucose concentrations were found in males and
females at all dose levels. Serum cholesterol was significantly lower
in females at all dose levels and in high-dose males. Urine analysis
indicated significant increases of urinary fluoride concentration in
both sexes at all dose levels. High-dose (31 g/m3) males and females
had significantly higher mean relative liver weights, but no
compound-related gross or histopathological changes were found in the
livers of exposed animals. Dose-related increases in hepatic
beta-oxidation enzyme activity were found for males (2.3, 3.1 and 4.0
fold increases at 2, 6 and 31 g/m3, respectively) and females (1.7
and 3.1 fold increase at 6 and 31 g/m3). The higher enzyme activity
indicated an induction of hepatic peroxisome proliferation. However,
compound-related differences in the rate of cell proliferation, as
measured by changes in labelling index, were not found at any exposure
concentration. These data indicate that during the first year of
exposure, HCFC 123 did not induce an increase in regenerative repair
of the liver, which is consistent with the absence of morphological or
microscopic changes in the liver of exposed animals. A
no-observed-effect level (NOEL) was not achieved in this study based
on the effects on clinical chemistry parameters and higher hepatic
peroxisomal activity.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
7.5.1.1 HCFC 141b
A 2-generation reproduction study on HCFC 141b is currently in
progress (Rusch, 1989).
7.5.1.2 HCFC 142b
In a dominant lethal study on rats, no effect on male
reproduction was found (Seckar et al., 1986). No other studies on
reproductive effects are available.
7.5.1.3 HCFC 132b
No data are available on the effects of HCFC 132b.
7.5.1.4 HCFC 133a
No studies are available in which the effects of HCFC 133a on
reproduction were investigated. Some information is available,
however, on the effects on male fertility from a series of dominant
lethal and "combined dominant lethal fertility" studies in mice (Hodge
et al., 1979, 1980; Kilmartin, 1980). In these studies groups of male
mice were exposed to between 0 and 98 g/m3, 6 h/day, for 5
consecutive days. At the end of the treatment, dominant lethal and
fertility effects were assessed in 15 males (30 controls) from each
treatment group, which were each housed with two virgin females for
four consecutive nights. This 4-nightly mating procedure was continued
for 8-9 consecutive weeks. In the two later studies, satellite group
males (at least two from each group per week) were killed, their
epididymal sperm assessed for abnormalities and their testes examined
histopathologically. A reduction in male fertility (seen as
exposure-related decreases in the proportion of pregnant females:
e.g., 0-60% of pregnant females in treatment groups compared to
90-100% in controls) was seen between weeks 2-8 following exposure.
The severity of the effects were related to time, the maximum
reductions in pregnancies being seen at around 3-6 weeks after
exposure. No effect on fertility was seen at 0.5 g/m3. Reduced
fertility was observed at concentrations of 2.5 g/m3 or more, and
histopathological evidence of degeneration of spermatogenic cells was
seen at concentrations of 5 g/m3 or more. There was a slight and
transient increase in the percentage of abnormal sperm at 2.5 g/m3
or more.
7.5.1.5 HCFC 123
A 2-generation inhalation reproduction study on rats sponsored by
PAFT is currently in progress (Rusch, 1989).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 HCFC 141b
Hughes et al. (1988) exposed three groups of 25 pregnant female
Sprague-Dawley rats to 15, 39 or 97 g/m3 for 6 h/day from days 6 to
15 of pregnancy. Some signs of maternal toxicity (prenarcotic signs,
piloerection and reduced alertness) were observed at all exposure
levels. At the highest level, salivation, hunched posture, and
diaphragmatic breathing, a marked increase in water consumption, a
transient reduction in food intake, and a marginal reduction in body
weight gain were observed. At the highest exposure level, incidences
of subcutaneous oedema and haemorrhaging and embryonal death were
significantly increased. Reduced litter and mean fetal weights and
retarded ossification were observed. However, no teratogenic effect
occurred in any group.
In a further study (Hughes et al., 1989), 16 pregnant female New
Zealand rabbits were exposed to 7, 20 or 61 g/m3 for 6 h/day from
days 7 to 19 of pregnancy. Signs of maternal toxicity (prenarcotic
signs, palpebral ptosis, respiratory disturbances and body weight
loss) were observed at the two highest exposure levels. There was no
indication of any treatment-related effect on embryo or fetal
development or evidence of teratogenicity at any exposure level.
7.5.2.2 HCFC 142b
Groups of 25 pregnant Sprague-Dawley rats were exposed to 4 or 41
g/m3 for 6 h/day from day 3 to day 15 of gestation (Culik & Kelly,
1976). The exposure had no effect on the body weight gain of the
mothers, and no clinical signs of toxicity were observed in any of the
animals. The number of early or late resorptions or number of live
fetuses per litter was not affected by the exposure. Exposure also had
no effect on embryonic development, as measured by the weight and
crown-rump length of the fetuses, and there was no evidence of a
teratogenic effect.
Damske et al. (1978) exposed groups of 20 pregnant female
Sprague-Dawley CD rats to concentrations of 0, 13 and 39 g/m3 for 6
h/day on days 6-15 of gestation. There were no treatment-related
effects in the dams or evidence of exposure-induced terata, variations
in sex ratio, embryotoxicity or inhibition of fetal growth and
development. There was an increase in the incidence of delayed
ossification of the supraoccipital bone in both exposure groups; this
effect was not observed in the control group.
7.5.2.3 HCFC 132b
In a pilot study, groups of seven or eight pregnant rats were
exposed to 3, 11 or 28 g/m3 for 6 h/day on days 6-15 of gestation.
Maternal and fetal body weights were reduced at all exposure levels.
The number of resorptions was increased in the 11- and 27-g/m3
exposure groups (Alvarez, 1988).
In a development screening test using Hydra (Johnson et al.,
1986), embryotoxicity was observed at paternally toxic doses.
7.5.2.4 HCFC 133a
Weigand et al. (1977) investigated the potential embryotoxic or
teratogenic effects of HCFC 133a in Wistar rats and Himalayan rabbits.
They also examined the effects of pre-treatment with progesterone to
determine whether any of the adverse effects of HCFC 133a could be
explained by its interaction with and depletion of progesterone in
early pregnancy, since this hormone is responsible for the electrical
and mechanical quiescence of the myometrium. Twenty-four pregnant
Wistar rats, of which 12 were injected subcutaneously with
progesterone (6 mg/day) prior to HCFC 133a exposure, were exposed to
25 g/m3 for 6 h/day on days 7-16 of gestation (the day on which
sperm was found in the vaginal smear was taken as day 1 of gestation).
Twelve pregnant Himalayan rabbits were exposed to the same
concentration on days 7-19 of gestation (the day of mating being taken
as day 0 of gestation). No controls were used and data were compared
with historical control data. Mild transient sedation, piloerection,
reduced body weight gain and reduced food consumption were observed in
the rats. At autopsy on day 21 of gestation, no macroscopic change was
observed. There was a prenatal mortality of 77% in the dams with no
pre-treatment with progesterone and 82% in the pre-treated dams.
Placental weight, fetal weight and crown-rump length were reduced and
some of the surviving fetuses showed generalized oedema (8/53) and
external anomalies of the limbs and tail (5/53). There was no
significant difference between the rats pre-treated with progesterone
and those without pre-treatment. In rabbits, there were reductions in
body weight gain and food consumption. All animals showed vaginal
bleeding during the last three days of the exposure, and 4 out of 12
aborted. By autopsy on day 29 all fetuses had died.
Culik & Kelly (1979) exposed Charler River CD rats to approximate
concentrations of 0, 2.5, 10, 25 or 98 g/m3 for 6 h/day on days 6-15
of gestation, and the rats were subjected to autopsy on day 21 of
gestation. There was no clear evidence of maternal toxicity. Evidence
of embryotoxicity was observed at all exposure levels. In the control
and the 2.5-g/m3 exposure groups, there was neither fetal death nor
total litter resorption. Fetal weights and crown-rump lengths were
lower in all the groups exposed to HCFC 133a than in controls. At 10
g/m3, 4/21 pregnant females had total resorptions and only 67
fetuses were alive in the other females. At 25 g/m3, 37/41 pregnant
females had total resorptions and only 7 fetuses were alive in the
other females. At 98 g/m3, 22/23 pregnant females had total
resorptions and only one fetus was alive in the other female.
Treatment-related increases in the incidence of runts and delayed
ossification in several bone structures were also observed at all
exposure levels. An increased incidence of hydronephrosis was reported
in all treated groups. Thus, the no-observed-effect level for
embryolethality was 2 g/m3 but, in view of the reduced fetal size
and weight at this exposure level, the no-observed-effect level for
embryotoxicity was not established from this study.
7.5.2.5 HCFC 123
Kelly et al. (1978) exposed 25 pregnant female rats to 62 g/m3
for 6 h/day on days 6-15 of gestation. Dams and fetuses were
sacrificed on day 21 and examined for gross changes. No embryotoxicity
or teratogenic effects were seen.
Two groups of 20 pregnant female rats were exposed to 0 and 31
g/m3 for 6 h/day on days 6-15 of gestation (Rusch, 1985). The
animals were sacrificed on day 20 and all dams and fetuses examined.
The maternal mean body weight in the exposed group was depressed to a
statistically significant degree on days 12 and 15 of the gestation
period. At termination, maternal mean body weights were still
depressed but not to a statistically significant degree. The numbers
of corpora lutea, implantation sites, resorption sites and fetuses
were similar in control and treated dams.
In a range-finding study, groups of six pregnant rabbits were
exposed to HCFC 123 concentrations of 0, 6, 31, 62 and 125 g/m3 for
6 h/day on days 6-18 of gestation (Schroeder, 1989a). All treated
rabbits lost weight during the study and food consumption was markedly
reduced, particularly at the two highest exposure levels. At these two
levels an increased number of resorption was also observed. In the
final study (Schroeder, 1989b; Trochimowicz, 1989), 24 mated females
per exposure group were exposed to 3, 9 or 31 g/m3 for 6 h/day
during days 6-18 of gestation. No mortality was observed in the
control or the low or medium exposure groups. The death of one rabbit
in the high exposure group was not considered by the author to be
treatment-related. There was evidence of maternal toxicity during days
6-18 of gestation at all exposure levels. Statistically significant
treatment-related mean body weight losses were observed in all the
test groups during the exposure period, compared to the control group
which showed a slight mean body weight gain. Mean daily food
consumption was also statistically lower in test groups than in
controls on most exposure days. There was no evidence of embryotoxic,
fetotoxic or teratogenic effects.
7.5.2.6 HCFC 124
Brewer & Smith (1977) exposed a group of 20 pregnant Charles
River CD rats to 30 g/m3 for 6 h/day during days 6-15 of gestation.
Maternal body weight measurements and clinical observations did not
reveal any difference between exposed and control groups, and no
maternal deaths occurred. The incidence of resorption sites in the
treated group was higher than in controls but within the range
commonly experienced with the strain of rats employed. The numbers of
corpora lutea, implantation sites, and fetuses in the treated groups
were similar to those in the controls. Fetal body weights were not
altered by treatment.
In a recent range-findings study, Rickard (1990b) exposed
Sprague-Dawley rats (4-6 per group) to minimal HCFC 124 concentrations
of 0, 3, 11, 56 or 279 g/m3 for 6 h/day on days 6-15 of gestation.
No change in mean body weight gain or evidence of embryotoxic effects
was observed. Internal and skeletal examinations were not conducted;
only external examination of fetuses was performed.
Schroeder (1991), in a range-finding study, exposed New Zealand
white rabbits (7 per group) to nominal HCFC 124 concentrations of 0,
28, 84 and 279 g/m3 for 6 h/day on days 6-18 of gestation. The only
sign of maternal toxicity was decreased activity during exposure at
the two highest concentrations. There was no evidence of
embryotoxicity, fetotoxicity or teratogenicity at any exposure
concentration.
7.6 Mutagenicity
7.6.1 HCFC 141b
The data from in vitro and in vivo studies are summarized in
Table 11.
HCFC 141b did not appear to damage bacterial DNA in a repair
assay, but produced conflicting evidence for mutagenicity in other
bacterial assays. Although sample purities differed in the two assays,
this alone may not account for the difference in response.
No significant response was obtained in the in vitro V79 cell
hprt locus assay.
Chromosomal aberrations were induced in two in vitro studies
with CHO (Chinese hamster ovary) cells, but this activity was not
apparent either in one assay with cultured human lymphocytes or in two
in vivo studies for micronucleus induction in mouse bone marrow
cells.
7.6.2 HCFC 142b
The data from in vitro and in vivo studies are summarized in
Table 12.
There was evidence of induction of base substitution mutations by
HCFC 142b in four of the five bacterial tests conducted. In two of
these studies this effect was apparent only in the presence of
exogenous metabolic activation.
A positive response, but without supporting data, was reported in
a single BHK (baby hamster kidney) 21 cell transformation assay.
No evidence of mutagenic activity of HCFC 142b was seen in a bone
marrow cytogenetic test or a dominant lethal study in rats.
7.6.3 HCFC 132b
The data from in vitro studies are summarized in Table 13.
HCFC 132b was tested in bacterial assays (three studies) and in
an in vitro chromosomal aberration test using a CHO cell line.
There was no evidence of mutagenic potential of HCFC 132b in
these studies.
7.6.4 HCFC 133a
The data from in vitro and in vivo studies are summarized in
Table 14.
HCFC 133a did not induce mutations in bacteria and did not
increase the proportion of BHK 21 cells forming colonies in soft agar.
Chromosomal aberrations were not induced in a single rat bone
marrow test using high concentrations. There were small, statistically
significant increases in the incidences of early deaths in two of
three male mouse dominant lethal assays. These increases occurred in
mating weeks 6-8 in one study and in mating week 7 in the other, in
which the lowest effective dose was 12.3 g/m3. No increases in early
deaths were observed in the third study, in which the highest
concentration tested was 12.3 g/m3.
7.6.5 HCFC 123
The data from in vitro genotoxicity studies are summarized in
Table 15.
HCFC 123 showed no evidence of mutagenic potential when tested in
suspension and plate assays using bacteria or yeasts.
In vitro cytogenetic tests were performed using cultured human
lymphocytes, in which HCFC 123 was tested both in the liquid and
gaseous phase. There was evidence of clastogenic activity in both the
presence and absence of exogenous activation systems, when HCFC 123
was tested in the gaseous phase; in the liquid phase study, an
increase in chromosomal aberrations was seen in the absence of
exogenous activation.
Table 11. Genetic toxicity of HCFC 141b
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Escherichia coli, DNA repair - - 10 mg/ml, Hodson-Walker & May (1988b)
18 hc
Salmonella typhimurium, reverse + + 1455 g/m3, 99.6% purity, positive Hodson-Walker & May (1988a)
mutation with TA98, TA100, TA1535, 48 hc in TA1535, negative
TA1537, TA1538 in other strains
S. typhimurium, reverse mutation with - - 1455 g/m3, 99.95% purity May (1989)
TA98, TA100, TA1535, TA1537, TA1538 48 hc
E. coli, reverse mutation with WP2 uvrA - - 1455 g/m3, 99.95% purity May (1989)
48 hc
Gene mutation in vitro, V79 cells, hprt - - 1430 g/m3, 99.6% purity Bootman et al. (1988a)
locus 3 hc
Chromosomal aberrations in vitro, CHO ± - 1 mg/ml liquid phase; increase Wilmer & De Vogel (1988)
cells in gaps only
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.6% purity Bootman & Hodson-Walker
cells 3 hc (1988)
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.83% purity Hodson-Walker (1990a)
cells 4 hc
Table 11 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations in vitro, human - - 1700 g/m3, 99.83% purity Hodson-Walker (1990b)
lymphocytes 3 h (+ema)c
243 g/m3,
24 h (-ema)
Micronucleus test in vivo, mouse bone - NA 165 g/m3, 99.98% purity; marrow Vlachos (1989)
marrow 6 h sampled at 24, 48 and
72 h
Micronucleus test in vivo, mouse bone - NA 95 g/m3, marrow sampled at 24, Bootman et al. (1988b)
marrow 6 h 48 and 72 h
a NA = not applicable; ± = inconclusive
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
c in vitro assay in an enclosed system
Table 12. Genetic toxicity of HCFC 142b
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 1640 g/m3, negative in all Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hd strains
TA1537
S. typhimurium, reverse mutation with + + 2050 g/m3, weakly positive only Koops (1977)
TA98, TA100, TA1535, TA1537, TA1538 48 hd in TA1535 (2-8 fold
increase at 2050 g/m3)
S. typhimurium, reverse mutation with + + not specifiedd single gaseous conc. Jagannath (1977)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 1-72 h, positive in
TA100 and TA1535
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and McGregor (1976)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
Cell transformation, in vitro BHK 21 ND + not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified, exposure
for 1-24 h, experimental
data not reported
Table 12 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations, in vivo rat - NA 82 g/m3, Seckar et al. (1986)
bone marrow 6 h/day, 5 days/
week for 13 weeks
Dominant lethal assay, male CD-1 rat - NA 82 g/m3, Seckar et al. (1986)
6 h/day, 5 days/
week for 15 weeks
a NA = not applicable; ND = not determined
b LED = lowest effective dose; HED = highest effective dose
c purity not specified for these studies
d in vitro assay in an enclosed system
Table 13. Genetic toxicity of HCFC 132b
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 4 mg/plate liquid phase Russell (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - 550 g/m3b Waskell (1979)
TA98, TA100, TA1535, TA1537
S. typhimurium, reverse mutation with - - 578 g/m3b 99.9% purity Koorn (1988)
TA98, TA100, TA1535, TA1537, TA1538
Chromosomal aberrations, in vitro CHO - - 14.2 mg/ml, liquid phase Wilmer & de Vogel (1988)
cells 3 h (+ema)b
3.0 mg/ml,
21 h (-ema)b
a +ema = with exogenous metabolic activation; -ema = without exogenous metabolic activation; LED = lowest effective dose;
HED = highest effective dose
b in vitro assay in an enclosed system
Table 14. Genetic toxicity of HCFC 133a
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2460 g/m3, McGregor (1976)
mutation with TA98, TA100, TA1535, 24 hd
TA1538
S. typhimurium, reverse mutation with - - 172 g/m3, Waskell (1979)
TA98, TA100 48 hd
S. typhimurium, reverse mutation with - - 49 g/m3, Edmunds et al. (1979)
TA98, TA100 8 hd
Cell transformation, in vitro BHK 21 - - gas, 3 h concentrations not Longstaff et al. (1984)
cell growth in soft agar assay specified, experimental
data not provided
Chromosomal aberrations, in vivo, - NA 98 g/m3, 6 h, marrow sampled 24 h Anderson & Richardson
male AP rat bone marrow and 6 h/day after single and 6 h (1979)
5 days/week after multiple exposure
Dominant lethal assay, male CD-1 + NA 49 g/m3, 6 h/ early deaths increased Hodge et al. (1979)
mouse day, 5 days/week in mating weeks 6-8
Dominant lethal assay, male CD-1 + NA 12 g/m3, 6 h/ early deaths increased Hodge et al. (1980)
mouse day, 5 days/week in mating week 7, no
dose response
Table 14 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Dominant lethal assay, male CD-1 - NA 12 g/m3, 6 h/ Kilmartin et al. (1980)
mouse day, 5 days/week
a NA = not applicable
b HED = highest effective dose; LED = lowest effective dose
c purity not provided for these studies
d in vitro assay in an enclosed system
Table 15. Genetic toxicity of HCFC 123
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 937 g/m3, 6 h Barsky (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - not specifiedc single gaseous conc. Brusick (1976)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 5 or 24 h
S. typhimurium, reverse mutation with - - 625 g/m3, 72 h experimental data not Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 provided
S. typhimurium, reverse mutation with - - not specifiedc gaseous exposure, Callander (1989)
TA98, TA100, TA1535, TA1537, TA1538 concs. not specified
Sacharomyces cerevisiae: D4, forward - - not specified concs. not specified, Brusick (1976)
mutation exposure for 4-72 mins
Chromosomal aberrations, in vitro, + - 75 µg/ml, purity 99.95% Dance (1991)
human lymphocytes 24 h
Chromosomal aberrations, in vitro, + + 1875 g/m3, purity 99.95% Edwards (1991a)
human lymphocytes 3 h (+ema)c
156 g/m3,
24 h (-ema)c
Cell transformation, in vitro, BHK 21 ND - not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified,
experimental data
not provided
Table 15 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Micronucleus test, in vitro, mouse - NA 112 g/m3, 6 h marrow sampled 24, Müller & Hoffman (1988)
bone marrow 48 and 72 h after
exposure
a ND = not determined; NA = not applicable
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous metabolic
activation
c in vitro assay in an enclosed system
A negative response was reported in a BHK transformation assay,
but no supporting data were provided.
There was no evidence of induction of micronuclei in a single
mouse bone marrow assay.
7.6.6 HCFC 124
The data from the small number of in vitro studies are
summarized in Table 16.
HCFC 124 did not induce mutations in bacteria or chromosomal
aberrations in CHO cells.
7.7 Carcinogenicity
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternate Fluorocarbon Toxicity Testing (Rusch, 1989).
7.7.1 HCFC 142b
Seckar et al. (1986) conducted a combined chronic
toxicity/carcinogenicity study of HCFC 142b with Sprague-Dawley CD
rats (details of exposure regimen are given in section 7.4).
Neoplasmic findings in animals that died were similar in the control
and treated animals, and predominantly comprised tumours of the
mammary and subcutaneous tissues in females, and pituitary and adrenal
adenomas in both sexes. A similar pattern existed in surviving
animals.
7.7.2 HCFC 133a
The carcinogenic potential of HCFC 133a has been evaluated by
Longstaff et al. (1984) in a limited study in Alpk/Ap Wistar- derived
rats. The study included one undosed control group of 32 male and 32
female rats and two vehicle-dosed control groups, one consisting of 40
male and 40 female and the other of 36 male and 36 female rats. The
HCFC 133a was dissolved in corn oil to give a 3% solution and dosed by
gavage at 300 mg/kg body weight, 5 days/week until week 52, and the
study was terminated at week 125. Dosed controls were given corn oil
only. Reductions of body weight gain and decreased testicular size
were observed in treated males. Aggressive behaviour occurred,
particularly in males. Body weight gain in females was similar to that
of controls. Mortality was not affected by treatment in either sex.
The treated females had an increased incidence of uterine carcinomas
(15/35) in comparison with controls (1/104). The first of these
carcinomas was seen at week 84. The carcinomas metastasized
transcoelomically to the abdomen in a large proportion of animals. A
small proportion also had lung metastases. Histologically, the
neoplasms were actively infiltrating adenocarcinomas.
Table 16. Genetic toxicity of HCFC 124
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2230 g/m3, Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hb
TA1537, TA1538
S. typhimurium, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
TA98, TA100, TA1535, TA1537, TA1538 48 hb
S. typhimurium, reverse mutation with - - 2790 g/m3, experimental data Longstaff et al. (1984)
TA98, TA100 48 hb not reported
Escherichia coli, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
WP2 uvrA 48 hb
Chromosomal aberrations, in vitro, - - 3348 g/m3, purity > 99% Edwards (1991b)
CHO cells 48 h (-ema)b
3348 g/m3,
4 h (+ema)b
a LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
b In vitro assay in an enclosed system
The treated males had an increased incidence of benign
interstitial cell neoplasms of the testis (29/36) compared with
controls (16/104). In many animals the neoplasms were bilateral. The
first interstitial cell tumour was seen at week 64. The testes of all
treated males, including those with no evidence of neoplasms,
exhibited arrest of spermatogenesis and atrophy of the seminiferous
tubules. These changes were first seen in the first rat to die in this
study, which was in week 37.
7.7.3 HCFC 123
As discussed in section 7.4.2, a 2-year inhalation toxicity/
carcinogenicity study in rats is being conducted. Groups of 80 male
and 80 female Crl:CDRBR rats were exposed to HCFC 123 at 0, 2, 6 or
31 g/m3 (6 h/day, 5 days/week) for 2 years. No histological lesions
were found in 10 rats of each sex in each exposure group, which were
sacrificed at the end of 1 year of exposure (Malley, 1990c).
Preliminary findings from the histopathological examination of tissues
of male rats at the end of the 2-year exposure period indicated an
exposure-related increase in benign tumours of the testis and exocrine
pancreas (US EPA, 1991). A full report is needed before these results
can be evaluated.
7.8 Special studies - cardiovascular and respiratory effects
Chlorofluorocarbons have long been known to sensitize the heart
to adrenaline-induced arrhythmias. Zakhari & Aviado (1982) reviewed
the literature on this subject. Several studies have been conducted to
evaluate the cardiac sensitization and respiratory effect potential of
the alternative HCFC compounds.
7.8.1 HCFC 141b
Mullin (1977) exposed male dogs, which were pretreated with an
intravenous injection of adrenaline (0.008 mg/kg), to HCFC 141b
concentrations of 12, 24, 48 and 97 mg/m3. A challenge dose
(intravenous injection with adrenaline) given during exposure elicited
serious cardiac arrhythmias at the three highest concentrations but no
response was noted at the lowest concentration.
Hardy et al. (1989b) studied cardiac sensitization to adrenaline
in two Cynomolgus monkeys and four beagle dogs and found that the
lowest HCFC 141b concentrations inducing responses were about 24 and
48 g/m3, respectively.
No effect on respiratory rate was observed when three groups of
three male Wistar rats were exposed to approximately 45 g/m3 for 25
min. However, there was a small change in respiratory amplitude
suggesting a decrease in tidal volume (Janssen, 1989a).
7.8.2 HCFC 142b
Adrenaline-induced cardiac arrhythmias were observed in 5 out of
12 dogs exposed to 205 mg/m3. No such effect was noted in monkeys or
mice at concentrations of up to 410 g/m3 (Mullin, 1969). It was also
found that dogs exposed to 3280 g/m3 (80% by volume; 20% oxygen
added) for 30 seconds, followed by a loud noise (to stimulate
endogenous adrenaline), developed cardiac sensitization (Mullin,
1970).
Reinhardt et al. (1971) studied the ability of HCFC 142b to
induce cardiac sensitization to exogenous and endogenous adrenaline in
beagle dogs. No response was noted in six animals exposed to a
concentration of 102.5 g/m3 for 5 min and then challenged with
adrenaline. Five out of 12 dogs exposed to 205 g/m3 showed marked
responses (arrhythmias considered to pose a serious threat to life or
ventricular fibrillation), and all 12 dogs exposed to 410 g/m3
showed such responses. When a group of 12 beagle dogs was exposed to
3280 g/m3 for 30 seconds, without injected adrenaline, one out of 12
showed a marked response. With simultaneous noise stimulation five
dogs showed a marked response. No dogs showed the response when
exposed to noise alone.
No arrhythmia or tachycardia was observed when groups of three
Rhesus monkeys ( Macaca mulatta) were anaesthetised with sodium
pentobarbital and exposed to 205 or 410 g/m3 for 5 min (Belej et
al., 1974). There was no effect on pulmonary resistance or compliance
but respiratory stimulation was observed when three anaesthetised
Rhesus monkeys ( M. mulatta) were exposed to 205 g/m3 and four
monkeys to 410 g/m3 for 5 min (Aviado & Smith, 1975). When dogs were
exposed to four different concentrations between 102.5 and 820 g/m3
for 5 min, hypotension, tachycardia, an increase in pulmonary
resistance, and a decrease in pulmonary compliance were found at the
highest exposure level (Belej & Aviado, 1975).
7.8.3 HCFC 132b
HCFC 132b was reported to produce cardiac sensitization in beagle
dogs in response to an intravenous adrenaline challenge at exposure
levels of 27 g/m3 or more (Mullin, 1976).
7.8.4 HCFC 123
Trochimowicz & Mullin (1973) reported the EC50 (concentration
producing an effect in 50% of the test group) in dogs for cardiac
sensitization to adrenaline challenge to be 119 g/m3 and the NOEL to
be 62 g/m3. At the latter concentration (the lowest one tested) CNS
depression was observed.
7.8.5 HCFC 124
Van Poznak & Artusio (1960) found that HCFC 124 caused
anaesthesia in dogs at concentrations ranging from 2230 to 3910
g/m3; blood pressure was lowered in a dose-dependent fashion.
Although ventilation was adequate even at the highest exposure level,
the femoral arterial systolic pressure fell to as low as 40 mmHg (5.33
kPa). Little or no anaesthetic effect was observed at exposure levels
that did not depress blood pressure. Atropine had little effect on
hypotension. Phenylephrine partially reversed the hypotension and
caused several premature ventricular contractions but no ventricular
fibrillation. Similar effects were produced by intravenously
administered adrenaline. Mullin (1976) reported that the NOEL of HCFC
124 for cardiac sensitization after a challenge injection of
adrenaline in the dog was 56 g/m3, while the next exposure level
tested (140 g/m3) induced sensitization, as did higher
concentrations.
8. EFFECTS ON HUMANS
8.1 General population exposure
No effects on human health of the hydrochlorofluorocarbons
reviewed in this monograph have been reported.
8.2 Occupational exposure
Filicheva (1975) examined 98 male and 98 female workers, 20-40
years of age, reportedly exposed to chlorofluorocarbons 22, 113 and
142 and to some other chlorofluoro and fluoro compounds. The
concentrations were claimed to exceed the threshold limit values but
were not specified. Functional disorders of the nervous system were
reported in 67% of the workers. These included symptoms of
neurovegetative system disturbances, and, in a few cases, polyneuritis
of the upper extremities. Reduced haemoglobin content, moderate
leucocytosis and reduced erythrocyte sedimentation rate were also
reported. Exposure levels were not given, and the workers were exposed
to a number of chemicals in addition to HCFC 142 (isomer not
specified). The information provided in this study could not be used
to evaluate the potential health effects on workers exposed to HCFC
142b alone.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
No information is available on the effects on environmental
organisms of the hydrochlorofluorocarbons reviewed except for limited
data on HCFC 141b and HCFC 142b. However, an ecotoxicology programme
is being developed within the industry-sponsored Programme for
Alternative Fluorocarbon Toxicity Testing (Rusch, 1989).
The 96-h LC50 of HCFC 141b for zebra fish (Brachidario rerio)
was reported to be 126 mg/litre in a static test using a sealed vessel
(Bazzon & Hervouet, 1989). The 48-h EC50 for the immobilization of
Daphnia magna, also using a sealed vessel, was 31.2 mg/litre
(Brinard & Hervouet, 1989).
The 96-h EC50 of HCFC 142b for guppies ( Poecilia
reticulata), tested in a static system in accordance with OECD
Guidelines, was reported to be 220 mg/litre (Groenevald & Kuijpers,
1990a). Groenevald & Kuijpers (1990b) found a 48-h EC50 for Daphnia
magna of 160 mg/litre. No immobilization of Daphnia magna at 190
mg/litre in 48 h was found (Hutton & Lieder, 1989a).
Acute toxicity values of HCFC 142b have also been determined for
rainbow trout. The 96-h LC50 was found to be 36 mg/litre (with a 95%
confidence interval of 28-45 mg/litre). The authors concluded that,
under the conditions of the test, HCFC 142b exhibited moderate acute
toxicity to rainbow trout (Hutton & Lieder, 1989b).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Direct health effects
10.1.1 HCFC 141b
HCFC 141b has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or in the environment. Available information
indicates a low level of metabolism of HCFC 141b. It is of low acute
toxicity. At high sublethal exposure concentrations, it induces signs
of CNS depression and can sensitize the heart to adrenaline, as do
other hydrochlorofluorocarbons. Such effects can appear at
concentrations ranging from 24 to 48 g/m3 in the inhaled air.
HCFC 141b has low irritant potential to the eye and skin. Skin
sensitization has not been demonstrated.
Short-term repeated inhalation exposure (2 to 13 weeks) did not
induce serious toxic effects at concentrations below 97 g/m3.
Effects of HCFC 141b on reproduction cannot be evaluated until
the data from an ongoing two-generation study become available. It has
not demonstrated teratogenic potential in rats or rabbits, although
embryotoxicity was observed, but only at the highest maternally toxic
concentration (97 g/m3) in rats. The no-observed-effect level (NOEL)
for maternal toxicity (excepting minor clinical signs) is 7 g/m3 in
rabbits and 15 g/m3 in rats.
Mutagenicity testing of HCFC 141b yielded conflicting results.
In vitro studies with CHO cells indicated clastogenic potential, but
this was not reflected in a single human lymphocyte test or two
in vivo mouse studies.
A carcinogenicity study in rats is in progress for HCFC 141b.
In summary, based on the toxicological information available at
the time of the Task Group meeting, the toxicity of HCFC 141b is
considered to be low. The present data base does not indicate any
significant direct health effects on humans under non-accidental
exposure conditions. However, studies to evaluate carcinogenicity and
reproduction are still in progress. Consequently, these study results
and any future changes in use pattern may require further evaluation.
10.1.2 HCFC 142b
HCFC 142b is produced in commercial quantities for use as an
intermediate in the synthesis of vinylidene fluoride. Its current
release to the environment has not been quantified and no information
is available on accidental release. No study is available which
permits the assessment of human health responses to HCFC 142b alone.
No information is available from in vivo studies on metabolism
and kinetics, but an in vitro study suggests that dechlorination may
occur.
The acute toxicity is very low after oral or inhalation exposure.
The no-observed-effect level (NOEL) for cardiac sensitization using
exogenous adrenaline in dogs is 102.5 g/m3 for 5 min.
In rat and dog studies, repeated inhalation exposure at
concentrations of up to 41 g/m3 for 90 days caused no adverse
responses. No carcinogenic or other toxicological responses were
reported in a single long-term inhalation study in rats exposed to up
to 82 g/m3 for 104 week.
HCFC 142b has mutagenic potential in bacterial systems. Data from
in vitro mammalian cell systems are lacking. No mutagenic activity
was seen in two in vivo rat studies.
Although no conventional reproductive toxicity studies are
available, two rat teratogenicity tests have been conducted in which
neither teratogenicity nor reproducible signs of embryotoxicity were
observed. In addition, no effect on male fertility was observed in a
dominant lethal study in rats.
In summary, information on human exposures is lacking, but these
are unlikely to approach the sustained high concentrations required to
produce adverse effects in experimental animals. Future usage
patterns, possibly resulting in higher occupational exposure
concentrations and exposure for shorter periods among the general
population, may require further evaluation.
10.1.3 HCFC 132b
HCFC 132b is only produced in small quantities for research
purposes but it occurs as a by-product in the manufacture of some
halogenated ethanes. Release into the environment is expected to be
very low. There appears to be no commercial application for HCFC 132b
at the present time. There are no data on human exposure and on the
effects of HCFC 132b on human health.
Data on the metabolism of HCFC 132b are only available for rats.
They show that the compound can be metabolized to potentially
cytotoxic metabolites and suggest that the compound can induce its own
metabolism. The acute toxicity of the compound is low, the predominant
effects being central nervous system (CNS) depression, anaesthesia
and, at high concentrations, death. The compound has low irritation
potential to the skin and eye. HCFC 132b can sensitize the heart to
adrenaline, as do many other hydrochlorofluorocarbons. Repeated
exposure to HCFC 132b has been shown to cause CNS depression, signs of
hepatotoxicity (at concentrations of 3 g/m3 or more) and effects on
spermatogenesis (at 11 g/m3). HCFC 132b is also embryotoxic and was
maternally toxic at the lowest dose tested (3 g/m3). NOEL values
have not been established for systemic toxicity or for embryotoxicity.
The available mutagenicity studies, which are limited to a few
in vitro experiments, did not suggest that HCFC 132b is genotoxic.
Long-term and carcinogenicity studies have not been performed.
In summary, from the available information, exposure to HCFC 132b
would appear to present a hazard to human health following repeated
exposure. Current use restricts its exposure to research personnel.
Therefore there is no expected health risk to the general population.
10.1.4 HCFC 133a
HCFC 133a is produced in small quantities for use as an
intermediate in the manufacture of the anaesthetic halothane. Although
it has not been quantified, release into the environment is expected
to be low.
No data are available on the toxicokinetics of HCFC 133a,
although absorption can be assumed to occur from the toxic effects it
induces. It is of low acute toxicity, the principal toxic effects
being anaesthesia and death at high concentrations. Although no
information is available, this compound is also expected to induce
cardiac sensitization at high concentrations. In animal studies
repeated exposure to HCFC 133a has induced death (in mice), and nasal
and lung damage changes in clinical chemistry parameters (in rats).
Atrophy of the thymus, testis, ovary and spleen have also been
reported in rats. Reduced fertility and damage to the seminiferous
epithelium have been observed in male mice at exposure concentrations
of 2.5 g/m3 and 5 g/m3, respectively.
HCFC 133a has been clearly demonstrated to be embryotoxic at
exposure concentrations that did not produce clear evidence of
maternal toxicity (2.5 g/m3); there was also indication of a
teratogenic potential in rats. The NOEL for embryolethality was 2.5
g/m3.
HCFC 133a is non-mutagenic in bacterial assays. It gave positive
results in two out of three dominant lethal assays in mice, but these
results are difficult to interpret in view of the negative result in
a single rat bone-marrow cytogenetic assay. From the limited evidence
available, HCFC 133a is a carcinogen in rats inducing adenocarcinomas
of the uterus; it also produces benign interstitial cell tumours of
the testis.
In summary, from the information available, exposure to HCFC 133a
would appear to present a hazard to human health following repeated
exposure and has the potential to cause serious effects in developing
offspring. In the absence of exposure information the potential risk
cannot be determined.
10.1.5 HCFC 123
HCFC 123 has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or the environment.
There is limited information on the kinetics and metabolism of
HCFC 123. It can be absorbed, the inference coming from observed
systemic effects and elevated urinary fluoride levels in toxicity
studies. There is evidence for some metabolism of HCFC 123, based on
the detection of urinary trifluoroacetic acid, elevated urinary
fluoride levels, and the detection of covalent binding to liver
protein.
The acute toxicity of HCFC l23 is low. As with the fully
halogenated chlorofluorocarbons, it is characterized in animals by CNS
depression at high inhalation exposure concentrations. Exposure to
high concentrations of HCFC 123 can sensitize the heart to adrenaline
(the EC50 for dogs is 119 g/m3).
Effects associated with short-term and long-term inhalation
exposure to HCFC 123 in rats include CNS depression, modulation of
lipid and carbohydrate metabolism and mild liver toxicity. The
lowest-observed-effect level (LOEL) for lipid and carbohydrate
metabolism effects and increased hepatic enzyme activity is 2 g/m3
following a 1-year exposure period to HCFC 123.
There is no evidence of teratogenicity in rats and rabbits but
there is evidence of embryotoxicity in rabbits at high inhalation
exposure concentrations (62 g/m3). Maternal toxicity is seen at
exposure concentration of 3 g/m3 or more in rabbits and 31 g/m3 or
more in rats. The potential effect of HCFC 123 on reproduction is
being investigated in a rat study.
There is evidence of clastogenic effects in human lymphocytes
in vitro, although this was not supported by an in vivo mouse
micronucleus study. HCFC 123 is not mutagenic in microorganisms. The
carcinogenicity of HCFC 123 is being investigated in rats and thus
cannot be evaluated at present.
On the basis of available information, HCFC 123 does not appear
to exhibit marked toxicity following short-term or long-term exposure.
However, in the light of some evidence of clastogenicity and the
reported increased incidences of benign tumours of the testes and
exocrine pancreas communicated in an interim report, the potential
health effects of HCFC 123 cannot be fully evaluated until more
information becomes available.
10.1.6 HCFC 124
HCFC 124 is not yet in large scale commercial production.
Therefore, there are no data on environmental levels and human
exposure.
The acute toxicity of HCFC 124 is low and is characterized in
animals by effects on the central nervous system. Subchronic toxicity
studies in rats did not reveal any histopathological changes of
internal organs at concentration of HCFC 124 as high as 279 g/m3.
Based on functional observations and clinical chemistry, the NOEL was
reported to be 28 g/m3 for male rats, and 84 g/m3 for females. In
three limited teratogenicity studies in rats, no indication of
developmental toxicity of HCFC 124 was evident, even at a maternally
toxic concentration. Based on the data available from bacterial and
mammalian cell studies there is no evidence that HCFC 124 has a
mutagenic potential.
The available information indicates that HCFC 124 exhibits low
toxicity, and it is not anticipated to pose significant health risk to
humans at potential environmental or controlled occupational
exposures. However, a firm conclusion on the potential health risk
cannot be made until data from the ongoing teratogenicity and
carcinogenicity studies are available.
10.2 Health effects expected from a depletion of stratospheric
ozone
The possible indirect health effects (e.g., an increase in the
incidence of skin cancer and immunotoxic and ocular effects) of fully
halogenated chlorofluorocarbons, resulting from an increase in UV-B
radiation due to a depletion of the ozone layer, have been discussed
in the Environmental Health Criteria 113: Fully Halogenated
Chlorofluorocarbons (WHO, 1990).
The ozone-depleting potentials of five of the six HCFCs for which
data are available are lower than that of CFC 11 by 33-77 times (HCFCs
123 and 124), 40 times (HCFC 132b), 12-20 times (HCFC 142b), and 7-15
times (HCFC 141b). If the levels of release of the HCFCs reviewed are
adequately controlled their indirect health effects should not be
significant.
10.3 Effects on the environment
Insufficient information is available to evaluate adequately the
direct ecological effects posed by the hydrochlorofluorocarbons
reviewed. With respect to the indirect "greenhouse" effect, the HCFCs
for which data are available have global-warming potentials lower than
that of CFC 11 by about 50 times (HCFC 123), about 10 times (HCFCs 124
and 141b), and about 3 times (HCFC 142b). These compounds are not
expected to contribute significantly to global warming.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Based on the available information the Task Group reached the
following conclusions:
1. All six hydrochlorofluorocarbons reviewed have low acute
toxicity, the main toxic signs being those of CNS depression.
However, accidental overexposure could result in acute poisoning
or even lethality.
2. These chemicals exhibit different toxicity potentials following
repeated exposure.
3. HCFC 141b has a low toxic potential on repeated exposure,
produces no consistent developmental effects and has not been
shown to be mutagenic in animals. The mutagenic status of HCFC
141b tested in vitro is unclear. No information is yet
available to evaluate chronic toxicity/carcinogenicity.
4. HCFC 142b also has a low toxic potential on repeated exposure.
The small data base indicates that HCFC 142b does not produce
developmental effects or affect male fertility. HCFC 142b is
mutagenic in bacteria. It has not been shown to be mutagenic or
carcinogenic in rats.
5. HCFC 132b is toxic following repeated exposure in animals. It
affects development in animals, but has only been tested at
maternally toxic doses. No study on reproduction has been
performed, but damage to spermatogenesis has been noted
histopathologically following repeated exposure. It is not
mutagenic in vitro and no test has been performed in vivo.
There are no data on the carcinogenic potential.
6. HCFC 133a is toxic following repeated exposure in animals,
producing a range of effects. It also induces serious effects on
reproduction and development in animals. In vivo data regarding
mutagenicity are unclear. It is a carcinogen in rats.
7. On repeated exposure, HCFC 123 induces liver toxicity and effects
on lipid and carbohydrate metabolism in rats. Developmental
effects only occur at high maternally toxic exposure
concentrations. No information is available on reproduction
effects. HCFC 123 is clastogenic in vitro, but has not been
shown to be clastogenic in animals. Complete information on the
carcinogenicity in rats is not yet available.
8. HCFC 124 has low toxic potential on repeated exposure. Based on
a small data base, there is no evidence of developmental effects.
No information is available on the reproduction toxicity
potential or carcinogenicity. It is not mutagenic in vitro and
no test has yet been performed in vivo.
9. The hydrochlorofluorocarbons reviewed (with the exception of HCFC
133a for which there is no value but which is expected to have a
value similar to the others reviewed) have a lower
ozone-depleting potential than the fully halogenated
chlorofluorocarbons and should therefore pose a lower indirect
health risk.
10. The global-warming potentials of HCFC 141b, HCFC 142b, HCFC 123
and HCFC 124 are similarly lower than those of the fully
halogenated chlorofluorocarbons. No data are available for HCFC
132b or HCFC 133a, but they would be expected to have similar
values to the other hydrochlorofluorocarbons reviewed. These
compounds are not expected to contribute significantly to global
warming.
11.2 Recommendations for protection of human health and the
environment
1. Since the toxicity of HCFC 142b is low, and the ozone-depleting
and global-warming potentials are considerably lower than those
of the fully halogenated chlorofluorocarbons, HCFC 142b can be
considered as a transient substitute for the chlorofluorocarbons
included in the Montreal Protocol. However, in line with the
conclusions of the 1990 London Meeting of the Montreal Protocol
Parties, efforts should be maintained to develop substitutes that
would pose no risk to the environment and to develop alternative
technologies. Care should be exercised in the use of HCFC 142b
because of its flammability.
2. Since more information regarding the toxicological potential of
HCFC 141b, HCFC 123 and HCFC 124 is required before an evaluation
of their hazard to human health can be made, no recommendation
can be made at present regarding their use as potential transient
substitutes for the fully halogenated chlorofluorocarbons
included in the Montreal Protocol.
3. Although HCFC 133a and HCFC 132b pose low risk to the
environment, they are not recommended as substitutes for the
chlorofluorocarbons included in the Montreal Protocol because of
their toxicity.
4. Since all the hydrochlorofluorocarbons reviewed have some
ozone-depleting potential, their release to the environment
should be minimized.
REFERENCES
Ahmadzai H & Hedlund T (1990) A profile of measures taken in Sweden to
protect the stratospheric ozone layer. Ambio, 19: 341-343.
Alvarez L (1988) Pilot teratogenicity study of FC-132b in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HLR 50-88).
Anderson D & Richardson CR (1979) Arcton 133a: A cytogenetic study in
the rat. Alderley Park, Cheshire, Imperial Chemical Industries Ltd,
Central Toxicology Laboratory (Report No. CTL/P443).
ANON (1991) CFC alternative shows chronic toxicity. Chem Eng News, 8
July: 26.
Aviado DM & Smith DG (1975) Toxicity of aerosol propellants in the
respiratory and circulatory systems. VIII. Respiration and circulation
in primates. Toxicology, 3: 24l-252.
Barsky FC (1976) In vitro microbial mutagenicity studies of
ethane,1-chloro-1,2,2,2-trifluoro. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 349-76).
Bazzon M & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Brachidanio rerio. Vert-le-Petit, Institut national de
Recherche chimique appliquée (IRCHA Study B.7073).
Belej MA & Aviado DM (1975) Cardiopulmonary toxicity of propellants
for aerosols. J Clin Pharmacol, 15: 105-115.
Belej MA, Smith DG, & Aviado DM (1974) Toxicity of aerosol propellants
in the respiratory and circulatory systems. IV. Cardiotoxicity in the
monkey. Toxicology, 2: 381-395.
Bootman J & Hodson-Walker G (1988) In vitro assessment of the
clastogenic activity of CFC141b in cultures of Chinese hamster ovary
(CHO-KI) cells. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV007/214).
Bootman J, Hodson-Walker G, & Lloyd JM (1988a) CFC 141b: investigation
of mutagenic activity at the HGPRT locus in a Chinese hamster V79 cell
mutation system. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV005/257).
Bootman J, Hodson-Walker G, Cracknell S, & Dance CA (1988b) HCFC 141b:
Assessment of clastogenic action on bone marrow erythrocytes in the
micronucleus test. Eye, Suffolk, Life Science Research Ltd (LRS Report
No. 88/PSV029).
Brewer WE (1977) 90-day subacute inhalation toxicity study with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brewer WE & Smith S (1977) Teratogenic study via inhalation with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brinard D & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Daphnia magna. Vert-le-Petit, Institut national de Recherche
chimique appliquée (IRCHA Study B.7072).
Brittelli MR (1976a) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 752-75).
Brittelli MR (1976b) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 13-76).
Brittelli MR (1976c) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 747-75).
Brock WJ (1988a) Acute dermal toxicity study of FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 501-88).
Brock WJ (1988b) Skin irritation test in rabbits of FC-141b. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
312-88).
Brock WJ (1988c) Primary eye irritation study with FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 318-88).
Brock WJ (1988d) Acute dermal toxicity study of HCFC-123 in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 577-88).
Brock WJ (1988e) Acute dermal toxicity of HCFC-123 in rabbits. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
578-88).
Brock WJ (1988f) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 535-88).
Brusick D (1976) Mutagenicity evaluation of Genetron 123. Final
Report. Kensington, Maryland, Litton Bionetics, Inc. (LBI Project No.
254).
Callander RD (1989) HCFC 123 - An evaluation using the Salmonella
mutagenicity assay. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No. CTL/P/2421).
Carpenter CP, Smith HF Jr, & Pozzani UC (1949) The assay of acute
vapour toxicity and the grading and interpretation of results on 96
chemical compounds. J Ind Hyg Toxicol, 31: 343-346.
Chapman J, Hill R, Muir J, Suckling CW, & Viney DJ (1967) Impurities
in halothane: their identities, concentrations and determination. J
Pharm Pharmacol, 19: 231-239.
Coate WB (1976a) LC50 of G123 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. M165-162).
Coate WB (1976b) LC50 of G124 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. 165-163).
Coombs DE, Hardy CJ, Crook D, Mullins PA, & Gopinath C (1988) Two-week
inhalation toxicity study in the rat with FC 141b. Huntington, United
Kingdom, Huntington Research Centre (Report PWT 76/8893).
Culik R & Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled chlorodifluoroethane (FC 142b). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 700-76).
Culik R & Kelly DP (1979) Embryotoxicity and teratogenicity studies in
rats with chlorotrifluoroethane (FC-133a). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 127-79).
Damske DR, Mecler FJ, & Beliles RP (1978) Teratology study in rats;
Isotron 142b. Monochlorodifluoroethane. Final Report. Kensington,
Maryland, Litton Bionetics, Inc. (LBI Project No. 20980).
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC 123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd (Final report No. 91/PFE03/0093).
Darr RW (1981) An acute inhalation toxicity study of Fluorocarbon 123
in the Chinese hamster. Morristown, New Jersey, Corporate Medical
Affairs, Allied Corporation (Report No. MA-25-78-15).
Davies RH, Bagnall RD, Bell W, & Jones WGM (1976) The hydrogen bond
proton donor properties of volatile halogenated hydrocarbons and
ethers and their mode of action in anaesthesia. Int J Quantum Chem
Quantum Biol Symp, 3: 171-185.
Diggle WM & Gage JC (1956) Toxicological report Arctons 9, 33 and 50.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/T/61).
Doleba-Crowe C (1977) Acute inhalation toxicity - 6 hours (HFA 141b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 61-77).
Doleba-Crowe C (1978) Ninety-day inhalation exposure of rats and dogs
to vapors of 2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
229-78).
Edmunds HN, Baden JM, & Simmon VF (1979) Mutagenicity studies with
volatile metabolites of Halothane. Anesthesiology, 51(5): 424-429.
Edwards CN (1991a) HCFC 123 (vapour phase): In vitro assessment of
clastogenic activity in cultured human lymphocytes. Eye, Suffolk, Life
Science Research Ltd (Final Report No. 91/PFE002/0125).
Edwards CN (1991b) In vitro assessment of the clastogenic activity
of HCFC 124 in cultured Chinese hamster ovary (CHO-K1) cells. Eye,
Suffolk, Life Science Research Ltd (Final report No. 91/PAR002/0855a).
Filicheva AP (1975) [Changes in the nervous system following chronic
action of fluorinated aliphatic hydrocarbons.] Gig Tr Prof Zabol, 10:
14-16 (in Russian).
Fisher DA, Hales ChH, Wang WC, Ko MKW, & Dze ND (1990a) Model
calculations of the relative effects of CFCs and their replacements on
global warming. Nature (Lond), 344: 513-516.
Fisher DA, Hales H, Filkin DL, Malcolm KWK, Sze ND, Connell PS,
Wuebbles DJ, Isaksen SA, & Stordal F (1990b) Model calculations of the
relative effects of CFCs and their replacements on stratospheric
ozone. Nature (Lond), 344: 508-512.
Freemantle M (1991) CFCs and their alternatives. Impact Sci Soc, 40:
59-70.
Gardner JR (1988) Acute dermal toxicity to rats of
dichlorofluoroethane. Huntington, United Kingdom, Huntington Research
Centre (Report No. 881114/PWT91/AC).
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 678-75).
Goodman NC (1976) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 14-76).
Graselli JG & Ritchey WM ed. (1975) CRC atlas of spectral data and
physical constants for organic compounds. Cleveland, Ohio, CRC Press,
vol 3.
Groeneveld AHC & Kuijpers LAM (1990a) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to the guppy Poecilia
reticulata. Graveland, The Netherlands, Duphar B.V., Agrobiological
Laboratory (Interim document No. 56635/33/90).
Groeneveld AHC & Kuijpers LAM (1990b) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to Daphnia magna. Graveland,
The Netherlands, Duphar B.V., Agrobiological Laboratory (Interim
Document No. 56635/32/90).
Hake CL, Waggoner TB, Robertson DN, & Rowe VK (1960) The metabolism of
1,1,1-trichloroethane by the rat. Arch Environ Health, 1: 101-105.
Hall GT (1976) Two-week inhalation toxicity study (HFA 132b and HFA
124). Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 727-76).
Hall GT & Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 426-75).
Hardy CJ, Jackson GC, Rao RS, Lewis DJ, & Gopinath C (1989a) HCFC 141b
acute inhalation toxicity study in rats, 4-hour exposure. Huntington,
United Kingdom, Huntington Research Centre (Report No. PWT95/881676).
Hardy CJ, Sharman IJ, & Chanter DO (1989b) Assessment of cardiac
sensitisation potential in dogs and monkeys. Comparison of F-141b and
F11. Huntington, United Kingdom, Huntington Research Centre (Report
No. PWT86/89437).
Harris JW & Anders MW (1991a) In vivo metabolism of the
hydrochlorofluorocarbon 1,1-dichloro-1-fluoroethane (HCFC-141b).
Biochem Pharmacol, 41: R13-R16.
Harris JW & Anders MW (1991b) Metabolism of the
hydrochlorofluorocarbon 1,2-dichloro-1,1-difluoroethane. Chem Res
Toxicol, 4: 180-186.
Harris JW, Pohl LR, Martin JL, & Anders MW (1991) Tissue acylation by
the chlorofluorocarbon substitute 2,2-dichloro-1,1,1-trifluoroethane.
Proc Natl Acad Sci (USA), 88: 1407-1410.
Hawley GG (1981) The condensed chemical dictionary, 10th ed. New York,
Van Nostrand Reinhold Company.
Henry JE (1975a) Acute oral test (132b). Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report No. 680-75).
Henry JE (1975b) Acute oral test on FC-123. Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 638-75).
Hodge MCE, Anderson D, Bennett IP, & Weight TM (1979) Arcton 133a:
dominant lethal study in the mouse. Alderley Park, Cheshire, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report No.
CTL/P/467).
Hodge MCE, Anderson D, Bennett IP, Weight TM, & Wilson J (1980) Arcton
133a: first combined dominant lethal and fertility study in the mouse.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/R/46l).
Hodson-Walker G (1990a) In vitro assessment of the clastogenic
activity of HCFC-141b in cultured Chinese hamster ovary (CHO-K1)
cells. Eye, Suffolk, Life Science Research Ltd (Report No.
89/PFG001/0971a).
Hodson-Walker G (1990b) HCFC 141b: lymphocyte cytogenetic study using
the methodology recommended by the OECD (1983). Eye, Suffolk, Life
Science Research Ltd (Report No. 89/PFG002/1029).
Hodson-Walker G & May K (1988a) CFC 141b: assessment of mutagenic
potential in histidine auxotrophs of Salmonella typhimurium (the
Ames test). Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV004/237).
Hodson-Walker G & May K (1988b) CFC 141b: assessment of its ability to
cause lethal DNA damage in strains of Escherichia coli. Eye,
Suffolk, Life Science Research Ltd (Report No. 88/PSV008/258).
Hoffmann JS (1990) Replacing CFCs; the search for alternatives. Ambio,
19: 329-333.
Horrath AL (1982) Halogenated hydrocarbons. New York, Basel, Marcel
Dekker.
Hughes EW, Homan BA, Kenny TJ, Coombs DW, Hardy CJ, & Tesh SA (1988)
The effect of HCFC 141b on pregnancy of the rat. Huntington, United
Kingdom, Huntington Research Centre (Report No. PWT77/88952).
Hughes EW, Homan BA, John DM, Kenney TJ, Coombs DW, & Hardy CJ (1989)
A study of the effect of PWC 4874-89 on pregnancy of the rabbit.
Huntington, United Kingdom, Huntington Research Centre (Report No. PWT
82/89243).
Hutton DG & Lieder PH (1989a) Static acute 48-hour EC50 of
chlorodifluoroethane to Daphnia magna. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 542-89).
Hutton DG & Lieder PH (1989b) Flow-through acute 96-hour LC50 of
chlorodifluoroethane to rainbow trout ( Oncorhynchus mykiss). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
75-68-3).
Jagannath DR (1977) Mutagenic evaluation of Isotron 142b. Final
Report. Kensington, Maryland, Litton Bionetics, Inc. (LBI Project No.
20838).
Janssen PJM & Pot TE (1988) Acute dermal toxicity study with FC 141b
in rats. Weesp, The Netherlands, Duphar B.V., Department of Toxicology
(Interim Document No. 56645/23/88).
Janssen PJM & Pot TE (1989a) Acute dermal toxicity study with FC 132b
in rats. Weesp, The Netherlands, Duphar B.V. (Report No. S.8807 to
Solvay SA).
Janssen PJM & Pot TE (1989b) Acute oral toxicity study with FC 132b in
rats. Weesp, The Netherlands, Duphar B.V. (Report No. S.8816 to Solvay
SA).
Janssen PJM (1988) Acute inhalation toxicity studies on FC 132b in
rats. Weesp, The Netherlands, Duphar B.V. (Report No. S.8811 to Solvay
SA).
Janssen PJM (1989a) Acute inhalation study to investigate the
respiratory irritating properties of FC 141b in male rats. Weesp, The
Netherlands, Duphar B.V., Department of Toxicology (Interim Document
No. 56645/41/89).
Janssen PTE (1989b) Acute inhalation study to investigate the
respiratory irritating properties of FC 132b in male rats. Weesp, The
Netherlands, Duphar B.V. (Report No. S.8907 to Solvay SA).
Johnson EM, Gabel BEG, Lieder PH, & Staples RE (1986) An in vitro
evaluation of the developmental hazard potential of Dupont compound
H16469. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. HLO-643-86).
Kelly (1976) Ninety-day inhalation exposure of rats and dogs to vapors
of 1-chloro-1,1-difluoroethane (FC-142b). Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 469-76).
Kelly DP (1988) 90-day inhalation toxicity study in rats (HFA 132b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 20-88).
Kelly DP (1989) Four-week inhalation study with HCFC-123 in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 149-76).
Kelly DP (1990) Four-hour inhalation approximate lethal concentration
(ALC) of HCFC-124 in rats. Newark, Delaware, Du Pont de Nemours and
Co., Haskell Laboratory (Report No. 71-90).
Kelly DP, Culik R, Trochimowicz HJ, & Fayerweather WE (1978)
Inhalation teratology studies of three fluorocarbons. Abstract of
paper presented at the 17th Annual Meeting of the Society of
Toxicology. Toxicol Appl Pharmacol, 45: 293.
Kilmartin M, Anderson D, Bennett IP, Richards D, Weight TM, & Wilson
J (1980) Arcton 133a: second combined dominant lethal and fertility
study in the mouse. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No. CT/P/544).
Koizumi A, Kumai M, & Ikeda M (1982) In vivo suppression of
1,1,1-trichloroethane metabolism by co-administered
tetrachloroethylene: an inhalation study. Bull Environ Contam Toxicol,
29: 196-199.
Koops A (1977) Mutagenic activity of ethane, chlorodifluoro- in the
Salmonella/microsome assay. Newark, Delaware, Du Pont de Nemours and
Co., Haskell Laboratory (Report No. 578-77).
Koorn JC (1988) Study to examine the possible mutagenic activity of
the volatile liquid FC 141b in the Salmonella/microsome assay.
Weesp, The Netherlands, Duphar B.V. (Document No. 56645/41/89).
Kynoch SR & Parcell BJ (1989) Delayed contact hypersensitivity in the
guinea-pig with 4874-89. Huntington, United Kingdom, Huntington
Research Centre (Report No. 881236/PWT94SS).
Landry TD, Cieszlak FS, Szabo JR, & Yano BL (1989)
1,1,Dichloro-1-fluoroethane 13-week inhalation toxicity study with
Fisher 344 rats. Midland, Michigan, Dow Chemical Company, Toxicology
Research Laboratory, (Study ID DR-0006-8553-002B).
Lester D & Greenberg LA (1950) Acute and chronic toxicity of some
halogenated derivatives of methane and ethane. Arch Ind Hyg Occup Med,
2: 335-344.
Leuschner F (1977) Summarising report on a subacute toxicity of FKW
133a (90 days) in beagle dogs when administered by inhalation.
Hamburg, Hoechst AG, Laboratory for Pharmacology and Toxicology.
Leuschner F, Leuschner A, Schwertfeger W, Dontenwill W, & Rogulja, P
(1977) Report on the subacute toxicity of chlorofluorocarbon 133a
(CF3CH2Cl) - called FKW 133a - (90 days) in Sprague Dawley rats
administered by inhalation. Hamburg, Hoechst AG, Laboratory for
Pharmacology and Toxicology.
Lewis RW (1990) HCFC 123: 28 day inhalation study to assess changes in
rat liver and plasma. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd., Central Toxicology Laboratory (Report No.
CTL/T/2706).
Liggett MP (1988a) Irritant effects on rabbit skin of 4874-89 (HFA
141b). Huntington, United Kingdom, Huntington Research Centre (Report
No. 881066D/PWT 91/SE).
Liggett MP (1988b) Irritant effects on the rabbit eye of 4874-89 (HFA
141b). Huntington, United Kingdom, Huntington Research Centre (Report
No. 881067D/PWT 93/SE).
Liggett MP, Allan S, & Dawe IS (1989) Acute oral toxicity to rats of
PWC 4874-89 (F 142b). Huntington, United Kingdom, Huntington Research
Centre (Report No. 8913D/PWT 90/AC).
Longstaff E, Robinson M, Bradbrook C, Styles JA, & Purchase IFH (l984)
Genotoxicity and carcinogenicity of fluorocarbons: assessment by
short-term in vitro tests and chronic exposure in rats. Toxicol Appl
Pharmacol, 72: 15-31.
McGregor DB (1976) Mutagenicity testing with Salmonella typhimurium
strains on plates, of gases, liquids and solids. Edinburgh, Inveresk
Research International (Report No. 513).
McNeill WC Jr (1979) Trichloroethylene. In: Grayson MS & Ecroth D ed.
Kirk-Othmer encyclopaedia of chemical technology, 3rd ed. New York,
John Wiley & Sons, vol 5, p 745.
Maiorino RM, Sipes JG, Gandolfi AJ, & Brown BR Jr (1979) Quantitative
analysis of volatile halothane metabolites in biological tissues by
gas chromatography. J Chromatogr, 164: 63-72.
Maiorino RM, Gandolfi AJ, & Sipes JG (1980) Gas-chromatographic method
for the halothane metabolites, trifluoroacetic acid and bromide in
biological fluids. J Anal Toxicol, 4: 250-254.
Makide Y & Rowland FS (1981) Tropospheric concentrations of
methylchloroform, CH3CCl3, in January 1978 and estimates of the
atmospheric residence times for hydrohalocarbons. Proc Natl Acad Sci
(USA), 78: 5933-5937.
Malley LA (1990a) Subchronic inhalation toxicity: 90-day study with
HCFC-123 in rats. Newark, Delaware, Du Pont de Nemours and Co.,
Haskell Laboratory (Report No. 594-89).
Malley LA (1990b) Subchronic inhalation toxicity: four-week study with
HCFC-124. Inhalation study in rats. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 257-90).
Malley LA (1990c) Combined chronic toxicity/oncogenicity study with
HCFC 123. Two-year inhalation study in rats. Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 260-90).
Malley LA (l991) Subchronic inhalation toxicity: 90-day study with
HCFC-124 in rats. Newark, Delaware, Du Pont de Nemours and Co.,
Haskell Laboratory (Report No.79-91).
Matla YA & Blom AJM (1991) Biodegradability of FC 142b according to
OECD 301B (modified Srutm test). Delft (TNO Report No. R 90/307).
May K (1989) HCFC-141b: assessment of mutagenic potential in
amino-acid auxotrophs of Salmonella typhimurium and Escherichia
coli (the Ames test). Eye, Suffolk, Life Science Research Ltd
(LRS-Report No. 89/PFG003/0600).
May K (1991) HCFC 124 in gaseous phase: assessment of mutagenic
potential in amino-acid auxotrophs of Salmonella typhimurium and
Escherichia coli (the Ames test). Eye, Suffolk, Life Science
Research Ltd (Final report No. 91/PAR001/0842).
Millischer R (1990) Joint assessment of commodity chemicals No. 15:
1-Fluoro-1,1-dichloroethane. Brussels, European Chemical Industry
Ecology and Toxicology Centre (ECETOC), 21 pp.
Montague DC & Perrine RL (1990) Preliminary assessment of the
greenhouse warming implications of halocarbon substitutes for CFC-12.
Atmos Environ, 24A: 1331-1339.
Moore BL & Trochimowicz HJ (1976) Subacute (two-week) inhalation
toxicity. Wilmington, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 145-76).
Müller W & Hoffmann T (1988) HCFC 123 micronucleus test in male and
female NMRI mice after inhalation. Frankfurt am Main, Hoechst AG,
Pharma Research Toxicology and Pathology Laboratory (Study No.
88.0372).
Mullin LS (1969) Cardiac sensitization (HFA 142b). Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 354-69).
Mullin LS (1970) Cardiac sensitization - fright exposures (HFA 142b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 81-70).
Mullin LS (1976) Cardiac sensitization. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 113-76).
Mullin LS (1977) Cardiac sensitization (HFA 141b). Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 957-77).
Nikitenko TK & Tolgskaja MS (1965) [Toxicologically induced
pathomorphic changes in animals affected by freons - 141, 142, 143 and
intermediate products arising in the course of their production.] Gig
Tr Prof Zabol, 9: 37-44 (in Russian).
Oyama I (1990) Test on biodegradability of HCFC 141b by
microorganisms. Fukuoka, Kurume Research Laboratory, Chemicals
Inspection and Testing Institute (Test No.11917).
Pennwalt Corporation (1987) Isotron R 141b toxicity comments:
inhalation: Material safety data sheet. Philadelphia, Pennsylvania,
Pennwalt Corporation.
Plummer JL, Steven JM, & Cousins MJ (1987) Metabolism of halothane in
children having repeated halothane anaesthetics. Anaesth Intensive
Care, 15: 136-140.
Prinn RG & Golombek A (1990) Global atmospheric chemistry of CFC-123.
Nature (Lond), 344: 47-49.
Raventós J & Lemon PG (1965) The impurities in Fluothane: their
biological properties. Br J Anaesth, 37: 716-737.
Reinhardt CF, Maxfield ME, Smith PE, & Mullin LS (1971) Cardiac
arrhythmias and aerosol "sniffing". Arch Environ Health, 22: 265-279.
Rickard LB (1990a) Mouse bone marrow micronucleus assay of HCFC-124.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 52-90).
Rickard LB (1990b) Pilot teratogenicity study of HCFC-124 in the rat.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 108-90).
Robbins BH (1946) Preliminary studies of the anesthetic activity of
fluorinated hydrocarbons. Pharmacol Exp Ther, 86: 197-204.
Rusch GM (1985) A ninety-day inhalation toxicology study and an
inhalation teratology study of Genetron 123 in the rat. Morristown,
New Jersey, Allied Corporation, Department of Toxicology (Report No.
MA-127-80-1).
Rusch GM (1989) Overview of PAFT toxicology program. Toulouse,
European Meeting of the Toxicology Forum, p 178-190.
Russell JF (1976) In vitro microbial mutagenicity studies of
ethane,1,2-dichloro-1,1,difluoro-. Wilmington, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 90-76).
Salmon AG, Jones RB, & Mackrodt WC (1981) Microsomal dechlorination of
chloroethanes: structure-reactivity relationships. Xenobiotica, ll:
723-734.
Sarver JW (1988) Acute oral toxicity study with FC-141b in male rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HRL 363-88).
Sax NI (1984) Dangerous properties of industrial materials, 6th ed.
New York, Van Nostrand Reinhold Company.
Schroeder R (1989a) An inhalation range-finding study to evaluate the
toxicity of CFC 123 in the pregnant rabbit. Final report. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Project No.
88-3303).
Schroeder R (1989b) An inhalation developmental toxicity study in
rabbits with HCFC 123. Final report. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Project No. 88-3304).
Schroeder R (1991) An inhalation range-finding developmental toxicity
study in rabbits with HCFC 124. Final report. Newark, Delaware, Du
Pont de Nemours and Co., Haskell Laboratory (Project No. 89-3513).
Seckar JA, Trochimowicz HJ, & Hogan GK (1986) Toxicological evaluation
of hydrochlorofluorocarbon 142b. Food Chem Toxicol, 24(3): 237-240.
Shankland JC (1990) CFC alternatives for thermal insulation foams. Rev
Int Froid, 13: 59-70.
Shulman M & Sadove MS (1965) Safety evaluation and anesthetic
properties of 1,1,1-trifluoroethyl chloride. Toxicol Appl Pharmacol,
7: 473-477.
Solvay (1989) Safety data sheet HCFC 142b (No. 424), Brussels.
Torkelson TR (1971) Single exposures of rats to the vapours of trace
substances in methoxyflurane. Toxicol Appl Pharmacol, 19: 1-9.
Trochimowicz HJ (1989) The toxicology of HCFC 123
(2,2-dichloro-1,1,1-trifluoro-ethane). Presented at the 1989 European
Meeting of the Toxicology Forum, Toulouse, 19-22 September.
Trochimowicz HJ & Mullin LS (1973) Cardiac sensitization potential
(EC50) of trifluorodichloroethane. Wilmington, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 132-73).
Trochimowicz HJ, Moore BL, & Chiu T (1977) Subacute inhalation
toxicity studies on eight fluorocarbons. Toxicol Appl Pharmacol, 41:
198-199.
UNEP/WMO (1989) Scientific assessment of stratospheric ozone: l989,
Section 111 - Chapter 4. Halogenated ozone depletion and global
warming potentials. Reports prepared for the UNEP/WMO Meeting of the
Open-Ended Working Group of the Parties to the Montreal Protocol,
Nairobi, 28 August-5 September 1989. Nairobi, United Nations
Environment Programme.
US EPA (1991) Communications and Public Affairs A-107 on the Test
Program for 3 HCFCs and 2 HFCs: Joint declaration of the US
Environmental Protection Agency, the Commission of the European
Communities, the Ministry of International Trade and Industry and the
Ministry of Health and Welfare in Japan, and the Program for
Alternative Fluorocarbon Toxicity Testing, Washington, DC, US
Environmental Protection Agency.
Van Dyke RA (1977) Dechlorination mechanisms of chlorinated olefins.
Environ Health Perspect, 21: 121-124.
Van Dyke RA & Gandolfi AJ (1974) Studies on irreversible binding of
radioactivity from (14C) halothane to rat hepatic microsomal lipids
and proteins. Drug Metab Dispos, 2: 469-476.
Van Poznak A & Artusio JF Jr (1960) Anesthetic properties of a series
of fluorinated compounds. I. Fluorinated hydrocarbons. Toxicol Appl
Pharmacol, 2: 363-373.
Vlachos DA (1989) Mouse bone marrow micronucleus assay of FC-141b.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HLR 746-88).
Waskell L (1979) Lack of mutagenicity of two possible metabolites of
Halothane. Anesthesiology, 50: 9-12.
Weast RC ed. (1985) CRC handbook of chemistry and physics, 66th ed.
Boca Raton, Florida, CRC Press.
Weigand W, Leist KH, & Baeder C (1977) Embryotoxic and teratogenic
studies in rats and rabbits with 2-chloro-1,1,1-trifluoroethane (FC
133a). Basel, Hoechst AG, Pharma Research Toxicology and Pathology
Laboratory (Report No. 1104/77).
WHO (1990) Environmental Health Criteria 113: Fully halogenated
chlorofluorocarbons. Geneva, World Health Organization, 164 pp.
WHO (1991) Environmental Health Criteria 126: Partially halogenated
chlorofluorocarbons (methane derivatives). Geneva, World Health
Organization, 97 pp.
Wilmer JWGM & De Vogel N (1988) Chromosome analysis of Chinese hamster
ovary cells treated in vitro with FC-141b. Zeist, TNO-CIVO
Institutes (TNO Report No. V88.156/271236).
Yano BL, Ciezlak FS, & Landry TD (1989) 1,1-Dichloro-1-fluoroethane:
4-week inhalation toxicity study with Fischer 344 rats. Midland,
Michigan, Dow Chemical Company, Toxicology Research Laboratory, Health
and Environmental Sciences (Study DR-0006-8553-002A).
Zakhari S & Aviado DM (1982) Cardiovascular toxicology of aerosol
propellants, refrigerants, and related solvents. In: Van Stee EW ed.
Cardiovascular toxicology. New York, Raven Press, pp 28l-314.
Zwart A (1989) Report of a metabolic elimination study of
1,1,dichloro-1-fluoroethane (HCFC 141b) vapour in male rats. Zeist,
TNO-CIVO Institutes (Report No. V89.214).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
La présente monographie porte sur six hydrochlorofluorocarbures
(HCFC) qui dérivent de l'éthane par substitution partielle des atomes
d'hydrogène par des atomes de fluor et de chlore. Les composés étudiés
dans le présent rapport sont le 1,1-dichloro-1-fluoréthane (HCFC
141b), le 1-chloro-1,1-difluoréthane (HCFC 142b), le
1,2-dichloro-1,1-difluoréthane (HCFC 132b), le 1-chloro-2,2,2-
trifluoréthane (HCFC 133a), le 1,1-dichloro-2,2,2-trifluoréthane (HCFC
123) et enfin le 1-chloro-1,2,2,2-tétrafluoréthane (HCFC 124).
A la température et sous la pression normales ces composés se
présentent sous la forme de gaz inflammable (HCFC 142b),
non-inflammables (HCFC 133a, HCFC 124), ou encore de liquides volatils
ininflammables (HCFC 141b, HCFC 132b, HCFC 123). Ils sont incolores
et, pour la majorité d'entre eux, pratiquement inodores ou dégagent
une odeur éthérée très légère (HCFC 141b et HCFC 123). Ils sont
légèrement ou modérément solubles dans l'eau et miscibles à de
nombreux solvants organiques.
Parmi les méthodes d'analyse utilisables pour le dosage de ces
hydrochlorofluorocarbures, on peut citer la chromatographie en phase
gazeuse avec détection par ionisation en flamme ou capture
d'électrons. La surveillance des HCFC présents dans l'air à fortes
concentrations peut s'effectuer par spectrophotométrie monofaisceau.
2. Sources d'exposition humaine et environnementale
Autant qu'on sache, les hydrochlorofluorocarbures qui font
l'objet de la présente monographie n'existent pas à l'état naturel.
Comme ces composés ne sont pas préparés industriellement pour être
utilisés en tant que tels, il n'y a guère d'exposition humaine ou
d'émissions dans l'environnement. Certains d'entre eux pourraient être
utilisés dans l'avenir pour remplacer les chlorofluorocarbures
totalement halogénés (CFC 11, CFC 12 et CFC 113). Le HCFC 133a et le
HCFC 142b servent d'intermédiaires dans la préparation d'autres
produits fluorés. In vivo, le HCFC 133a est un métabolite de
l'halothane, un anesthésique.
3. Transport, distribution et transformation dans l'environnement
Les données dont on dispose sur la biodégradation de ces composés
dans l'environnement se limitent aux résultats des études consacrées
aux HCFC 141b et 142b qui ne sont pas spontanément dégradés par les
microorganismes. On dispose de peu d'informations sur les coefficients
de partage octanol/eau; toutefois sous sa forme logarithmique, celui
du HCFC 141b est de 2,3 de sorte que la bioaccumulation de cet
hydrochlorofluorocarbure est improbable. Dans la troposphère, ces
composés sont principalement décomposés par réaction avec les radicaux
hydroxyles. Leur durée de séjour dans l'atmosphère (comparée à la
durée de séjour du méthylchloroforme qui est de 6,3 ans) se situe
entre 1,6 an (HCFC 123) et 19,1 ans (HCFC 142b). (La durée de séjour
dans l'atmosphère du CFC 11 est de 74 ans, celle du CFC 12 de 110 ans
et celle du CFC 13 de 90 ans.) A l'exception du HCFC 133a pour lequel
on ne possède pas de chiffres, l'agressivité de ces composés pour la
couche d'ozone et leur effet de serre sont inférieurs ou égaux au
dixième de ceux du CFC 11, le chlorofluorocarbure totalement halogéné
le plus actif à cet égard. (Le HCFC 142b dont l'effet de serre
potentiel est environ égal au tiers de celui du CFC 11 constitue une
exception).
4. Concentrations dans l'environnement et exposition humaine
Comme le HCFC 141b, 132b, 133a, 123 et 124 ne sont pas encore
produits à l'échelle industrielle et que le HCFC 142b n'est utilisé
que comme intermédiaire, ces substances ne sont pas libérées dans
l'environnement en quantité importante. On ne dispose donc d'aucune
donnée sur leur concentration dans l'environnement ni sur l'exposition
humaine.
5. Cinétique et métabolisme chez les animaux de laboratoire
et l'homme
Il n'existe pas de données relatives à la toxicocinétique chez
l'homme de l'un quelconque des HCFCs étudiés.
5.1 HCFC 141b
Les résultats des études toxicologiques incitent à penser que
l'absorbtion du HCFC 141b s'effectue au travers de l'épithélium
respiratoire. Aucune donnée n'est disponible quant à la distribution
de ce composé chez les mammifères. Des études récentes au cours
desquelles des rats ont été soumis à une seule exposition in vivo
ont permis de retrouver dans les urines du 2,2-dichloro-2-fluoréthyl-
glucuronide et de l'acide 2,2,-dichloro-2-fluoracétique.
D'après une étude pilote portant sur l'absorption et le
métabolisme du HCFC 141b chez des rats exposés à des vapeurs de ce
composé, il semblerait que le HCFC 141b ne soit que très faiblement
métabolisé.
Une étude in vitro a montré que le HCFC 141b subissait une
déchloration limitée au niveau des microsomes hépatiques.
5.2 HCFC 142b
On ne dispose d'aucune information sur la toxicocinétique du HCFC
142b. D'après les études toxicologiques qui ont été effectuées sur
l'animal on peut penser que ce composé est effectivement absorbé.
D'après une étude in vitro, il pourrait y avoir déchloration.
5.3 HCFC 132b
Lors d'une étude de métabolisme au cours de laquelle on a
administré du HCFC 132b par voie intrapéritonéale à des rats, on a
retrouvé dans les urines du 2-chloro-2,2-difluoréthylglucuronide, du
chlorodifluoracétaldéhyde (hydraté et conjugué) et de l'acide
chlorodifluoracétique. Lorsqu'on répétait les injections de HCFC 132b
aux animaux, la formation et l'excrétion d'acide chlorodifluoracétique
s'accroissait. Des expériences in vitro sur des microsomes de foie
de rats ont montré qu'il y avait probablement participation du
cytochrome P-450 IIEI à la phase initiale de l'hydroxylation. On n'a
pas observé de signes d'une liaison covalente des métabolites fluorés
aux protéines du foie.
5.4 HCFC 133a
On ne dispose d'aucune donnée sur la toxicocinétique de l'HCFC
133a. On peut néanmoins penser, d'après les effets toxiques observés
lors d'un certain nombre d'études après exposition des animaux, que le
composé est effectivement absorbé. In vitro, on a observé une
déchloration de l'HCFC 133a.
5.5 HCFC 123
On ne dispose d'aucune donnée toxicocinétique sur l'HCFC 123.
Toutefois on peut penser qu'il y a absorption d'après les effets
généraux et les taux élevés de fluorures urinaires observés lors
d'études toxicologiques chez le rat. On a montré que l'HCFC 123 était
métabolisé par l'organisme du rat. On ne sait pas quelle peut être
l'ampleur de cette métabolisation mais, à côté des fluorures, l'acide
trifluoracétique (TFA) apparaît comme un des principaux métabolites
urinaires. On a mis en évidence dans le cas du HCFC 123 une liaison
covalente aux protéines du foie.
5.6 HCFC 124
On ne dispose d'aucune donnée sur la cinétique et le métabolisme
de l'HCFC 124. On peut penser, d'après les résultats des études
toxicologiques par inhalation, qu'il y a absorption de ce composé au
niveau des voies respiratoires.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuves
in vitro
6.1 HCFC 141b
Le HCFC 141b présente une faible toxicité aiguë par voie orale.
Après administration de cette substance à des rats à raison de 5 g/kg,
on n'a observé aucun signe de toxicité.
Des études d'inhalation effectuées sur des rats et des souris ont
montré qu'il y avait dépression du système nerveux central, anesthésie
et mort en cas d'exposition intense. On n'a pas observé d'effets
macroscopiques ou histopathologiques imputables au traitement. Dans
une étude, on fait état d'une CL50 à 4 h chez le rat égale à 295
g/m3 et dans une autre étude, d'une CL50 à 2 h chez la souris
égale à 150 g/m3. Chez le rat, la concentration la plus faible
entraînant la mort serait de 242 g/m3 sur 6 h.
Après exposition cutanée (à raison de 2 g/kg) de rats ou de
lapins on n'a pas observé de mortalité.
Lors d'études d'inhalation à court terme où l'exposition allait
de 10 à 97 g/m3 et durait jusqu'à 90 jours, on n'a pas observé
d'effets toxiques marqués. Les effets observés consistaient en une
réduction du gain de poids, "de légères modifications biochimiques",
et une dépression du système nerveux central. L'étude de 90 jours n'a
pas permis de dégager une valeur pour la dose sans effet observable.
Le HCFC 141b n'a pas produit de signes d'irritation cutanée chez
des lapins ni d'irritation oculaire lors de l'une des deux études
effectuées. Dans la seconde étude, on a observé une légère réaction
d'irritation au niveau de l'oeil. Aucune sensibilisation cutanée n'a
été relevée chez des cobayes.
Une étude de reproduction portant sur deux générations est
actuellement en cours. Les études relatives au développement de
l'embryon font ressortir un accroissement de l'incidence des oedèmes
sous-cutanés et des hémorragies chez les foetus ainsi que des cas de
mort embryonnaire, mais ce, uniquement à la concentration de 97 g/m3
chez le rat, concentration par ailleurs toxique pour la mère. Aucun
effet tératogène n'a été observé. Lors d'une étude sur des lapins,
aucun effet imputable au traitement n'a été observé sur le
développement des embryons ou des foetus.
Le HCFC 141b ne s'est pas révélé mutagène lors d'une épreuve de
réparation de l'ADN bactérien, en revanche il a produit des résultats
contradictoires lors d'autres épreuves de mutation chez des bactéries.
L'épreuve du locus hprt n'a pas permis de relever d'effets sur les
cellules V79. Des aberrations chromosomiques ont été observées après
traitement in vitro de cellules ovariennes d'hamsters chinois, mais
ce phénomène n'a pas été observé lors d'une étude in vitro sur des
lymphocytes humains. Chez la souris, deux épreuves in vivo en vue de
mettre en évidence la formation de micronoyaux se sont révélées
négatives.
Une étude combinée de toxicité et cancérogénicité par inhalation
chronique est en cours sur des rats.
Le HCFC 141b est capable de provoquer une sensibilisation
cardiaque à l'adrénaline exogène chez le chien. Les concentrations de
HCFC 141b les plus faibles qui produisent des réponses sont
respectivement de 24 et de 48 g/m3 chez le chien et le singe.
6.2 HCFC 142b
Après avoir été administré par voie orale, le HCFC 142b n'a
produit que de légers signes de toxicité chez des rats en dose unique
allant jusqu'à 5 g/kg.
Des rats exposés une seule fois par inhalation à 525 g/m3 de
HCFC 142b pendant 4 heures ont présenté une mortalité d'environ 50%.
D'après d'autres études comportant une exposition plus courte, on
évalue la CL50 à plus de 1000 g/m3.
Des études au cours desquelles on a fait respirer à plusieurs
reprises du HCFC 142b à des rats n'ont pas révélé d'effets
indésirables à une concentration de 41 g/m3 (6 heures par jour, 5
jours par semaine pendant 90 jours). En revanche, lorsque la dose
était beaucoup plus élevée, on a observé une très forte irritation
pulmonaire entraînant la mort.
Il ne semble pas que des études aient été consacrées à
l'irritation cutanée ou oculaire ou à la sensibilisation cutanée par
le HCFC 142b. Des études de sensibilisation cardiaque à l'adrénaline
exogène ont été effectuées sur des souris, des chiens et des singes.
Ce sont les chiens qui étaient les plus sensibles; la dose sans effet
observable était de 102,5 g/m3 pour une exposition de 5 minutes et
une dose de 205 g/m3 (également pendant 5 minutes) a provoqué une
arythmie.
Une seule étude à long terme a été rapportée, au cours de
laquelle des rats (130 mâles et 110 femelles par groupe) ont été
exposés à du HCFC 142b aux doses respectives de 4, 41 et 82 g/m3, 6
heures par jour, 5 jours par semaine pendant des périodes pouvant
atteindre 104 semaines. Aucun effet imputable au traitement n'a été
observé en ce qui concerne la NFS, la biochimie du sang et des urines
et l'histopathologie. Aucune modification imputable au traitement n'a
été observée dans l'incidence tumorale.
Aucune étude classique n'a été consacrée aux effets du HCFC 142b
sur la reproduction, toutefois, une étude de létalité dominante n'a
fait ressortir aucun effet sur la fertilité des mâles. Deux études de
tératogénicité ont été effectuées sur des rats. Dans la première, des
rats Sprague-Dawley ont été exposés respectivement à 4 et 41 g/m3 (6
heures par jour du troisième au quinzième jour de la grossesse), alors
que dans l'autre étude, des rats de la même espèce ont été exposés
respectivement à 13 et 39 g/m3 (6 heures par jour du sixième au
quinzième jour de la grossesse). Aucun effet tératogène n'a été noté.
On a observé une réduction de l'ossification chez un petit nombre de
foetus à ces deux doses lors de cette dernière étude mais pas dans la
première.
Le HCFC 142b produit des mutations chez les bactéries mais on ne
dispose pas de données tirées d'épreuves de génotoxicité sur des
cellules mammaliennes en culture. Les tests in vivo qui ont été
pratiqués ne font ressortir aucune augmentation du nombre
d'aberrations chromosomiques dans la moelle osseuse ni d'effets létaux
dominants chez les rats mâles.
6.3 HCFC 132b
Chez le rat, la toxicité aiguë par voie orale du HCFC 132b est
faible. La dose la plus faible à laquelle on ait observé une mortalité
était égale à 25 g/kg. Après administration par voie orale à raison de
2 g/kg, on a observé une dépression du système nerveux autonome et du
système nerveux central, accompagnée d'effets sur la coordination et
l'activité motrice ainsi que sur le tonus musculaire. Chez les mâles,
on a observé une hypertrophie du foie dont le poids était cependant
diminué.
Lorsqu'il est inhalé à fortes doses, le HCFC 132b détermine des
effets aigus caractérisés par une anesthésie. La dose la plus faible
à laquelle on ait observé une mortalité chez des rats exposés de cette
manière pendant 4 heures à du HCFC 132b était égale à 110 g/m3. Chez
la souris, la CL50 à 30 minutes était de 269 g/m3 et l'anesthésie
s'est produite dès 71 g/m3. Dans une étude, on a observé une
réduction du poids des testicules et une augmentation de celui du foie
et des poumons chez les rats mâles après exposition à une dose de 33
g/m3 pendant 6 heures.
L'application cutanée de HCFC 132b à raison de 2 g/kg à des rats
a fait apparaître les signes cliniques d'une action au niveau du
système nerveux central et produit une hypertrophie hépatique chez
certains des animaux. Non dilué, le composé a provoqué une "légère"
irritation de la peau chez des cobayes et une irritation oculaire
"légère à modérée" chez des lapins. On n'a pas pu mettre en évidence
de sensibilisation cutanée chez les cobayes. A partir de 27 g/m3 on
a observé, chez des chiens exposés au HCFC 132b par voie respiratoire,
une sensibilisation cardiaque à l'adrénaline.
A côté de la dépression du système nerveux central, les
principales conséquences d'une inhalation de brève durée de HCFC 132b
par des rats mâles ont été une atrophie du thymus et des effets sur la
spermatogénèse. Après un traitement de 13 semaines à raison de 3
g/m3 on a observé une interruption de la spermatogénèse. Parmi les
autres effets on pouvait noter une prolifération des canaux biliaires
et un accroissement du rapport poids du foie/poids du corps chez les
mâles, même à la dose la plus faible employée (3 g/m3). Les rattes
ont paru moins sensibles que les rats aux effets hépatiques.
Le HCFC 132b a déterminé des réactions d'embryotoxicité chez des
rats après exposition par la voie respiratoire à des doses allant de
3 à 28 g/m3 du sixième au quinzième jour de la gestation. On a
observé un accroissement du nombre de résorptions (aux doses de 11 et
28 g/m3) et une diminution du poids des foetus à toutes les doses.
Toutes les doses utilisées étaient toxiques pour la mère.
En se fondant sur les données limitées dont on dispose, on peut
dire qu'il n'existe aucune preuve d'une mutagénicité in vitro du
HCFC 132b. La cancérogénicité de ce produit n'a pas été étudiée.
6.4 HCFC 133a
Il n'existe pas de données sur la toxicité aiguë par voie orale
du HCFC 133a. Par inhalation, sa toxicité aiguë est faible (la CL50
à 30 minutes chez la souris est de 738 g/m3) et ses principaux
effets toxiques sont ceux d'une anesthésie. On ne dispose d'aucune
donnée sur la sensibilisation cutanée ou cardiaque ni sur l'irritation
de la peau ou des yeux.
L'exposition réitérée (90 jours) de rats à la dose de 49 g/m3
a produit une inflammation chronique des fosses nasales, un emphysème
et un oedème pulmonaire, une bronchite et une pneumopathie. On a
également observé une atrophie du thymus, des testicules, des ovaires
et de la rate. Aucun effet n'a été observé chez des rats et des chiens
exposés à plusieurs reprises à du HCFC 133a pendant 7 jours (rats) ou
90 jours (chiens) à une concentration d'environ 25 g/m3; cependant
une mortalité a été observée chez des souris exposées pendant cinq
jours à une dose de 0,5 g/m3 ou davantage (sauf 2,5 g/m3).
Bien qu'aucune étude de type classique n'ait été consacrée aux
effets du HCFC 133a sur la reproduction, on a observé, au cours de
trois études de létalité dominante chez des souris, un certain nombre
d'effets sur la fertilité des mâles et l'histologie des testicules.
L'exposition à des concentrations de 2,5 g/m3 ou davantage pendant
cinq jours a entraîné une diminution du nombre de souris gravides et
une augmentation de la proportion des spermatozoïdes anormaux, tandis
qu'une exposition à la concentration de 5 g/m3 provoquait des
lésions histopathologiques de l'épithélium des tubes séminifères.
Des études sur des rats (traités du sixième au seizième jour de
la gestation), à des concentrations qui ne produisaient que de légers
signes de toxicité maternelle, ont montré que le HCFC 133a est
embryotoxique aux concentrations supérieures ou égales à 2 g/m3 et
mortel pour l'embryon à partir de 10 g/m3. Une prémédication des
femelles gravides par la progestérone n'a pas eu d'effet sur les
effets embryotoxiques ou létaux. Une autre étude a permis de relever
les indices d'effets tératogènes (anomalies externes des membres et de
la queue). Le HCFC 133a a produit des avortements spontanés et s'est
révélé absolument mortel pour les embryons après exposition de lapines
gravides à la dose de 25 g/m3 du septième au dix-neuvième jour de la
gestation, alors que cette concentration ne produisait que de légers
signes de toxicité maternelle.
D'après les résultats disponibles, rien n'indique que ce composé
soit mutagène chez la bactérie. Une étude, portant sur des cellules de
reins de hamsters n'a pas permis de mettre en évidence d'augmentation
dans la proportion des cellules produisant des colonies transformées.
Sur trois études de mutagénicité, deux ont fait ressortir l'existence
d'effets létaux dominants après exposition de souris mâles à 12 g/m3
ou davantage pendant cinq jours. La proportion des cellules de moelle
osseuse porteuses d'aberrations chromosomiques n'était pas augmentée
chez les rats exposés à 98 g/m3 (6 heures par jour pendant des
durées allant jusqu'à 5 jours). La seule étude de cancérogénicité qui
ait été effectuée a permis de mettre en évidence une augmentation de
l'incidence des adénocarcinomes de l'utérus et des tumeurs du tissu
interstitiel des testicules chez des rats ayant reçu 300 mg/kg de
composé dans de l'huile de maïs par gavage pendant 52 semaines (après
quoi ils ont été placés en observation pendant 73 semaines).
6.5 HCFC 123
Le HCFC 123 présente une faible toxicité aiguë par voie orale et
cutanée. La dose orale la plus faible qui soit mortelle pour le rat
est de 9 g/kg. Administré à raison de 2 g/kg à des rats ou à des
lapins, ce composé n'a entraîné aucune mortalité.
Par la voie respiratoire, le HCFC 123 est également peu toxique.
Ses effets sont analogues à ceux des chlorofluorocarbures,
c'est-à-dire une perte de coordination et une narcose. La CL50 à 4
h est de 178 g/m3 chez le hamster, de 463 g/m3 chez la souris et
varie de 200 à 329 g/m3 chez le rat. Chez le chien, on a obtenu une
sensibilisation cardiaque à des doses supérieures ou égales à 119
g/m3 après une injection d'épreuve d'adrénaline. Le HCFC 123 liquide
provoque une "légère" irritation de la peau et de l'oeil chez le
lapin. Chez le cobaye, il ne provoque pas de sensibilisation cutanée.
Plusieurs études toxicologiques à court terme ont été effectuées
sur le HCFC 123 en utilisant la voie respiratoire. On observe
systématiquement des signes de dépression du système nerveux central
chez le rat à des concentrations de 31 g/m3 ou davantage. On a
également observé certains effets sur le foie de ces animaux. Une
exposition de longue durée (4 semaines ou davantage) au HCFC 123
affecte également le métabolisme des lipides et des glucides comme le
montre la réduction systématique des triglycérides, du cholestérol et
du glucose sériques chez les rats. D'après les résultats provisoires
d'une étude en cours sur la toxicité et l'oncogénicité de ce composé,
il apparaît qu'il exerce un certain nombre d'effets sur des rats
exposés pendant de longues durées à des doses de 2,6 ou de 31 g/m3.
Les effets observés, perturbation du métabolisme lipidique et
accroissement de l'activité des peroxysomes hépatiques, ont servi de
base à l'établissement de la dose sans effet observable, dont la
valeur n'est toutefois pas mentionnée.
Une étude de reproduction portant sur deux générations de rats
exposés par la voie respiratoire à du HCFC 123 est en cours. Deux
autres études de portée limitée n'ont pas permis de mettre en évidence
d'effets embryotoxiques sur des rats à des concentrations qui étaient
légèrement toxiques pour la mère. Les seuls signes d'embryotoxicité
relevés l'ont été chez des lapins et, là encore, seulement lorsque les
concentrations étaient fortement toxiques pour les lapines gravides
(plus de 62,5 g/m3). Cette toxicité maternelle (réduction du poids,
dépression du système nerveux central) s'observe chez des rattes
exposées à des doses supérieures ou égales à 31 g/m3 et chez des
lapines à partir de 3 g/m3. Aucun signe de tératogénicité n'a été
relevé, ni chez les rats ni chez les lapins.
Le HCFC 123 ne paraît avoir aucune activité mutagène sur les
bactéries ou les levures. Toutefois des signes d'une activité
clastogène ont été observés dans des lymphocytes humains in vitro,
encore que cette observation ne soit pas corroborée par les résultats
d'une étude in vivo portant sur la présence de micronoyaux dans des
cellules murines.
Une étude combinée de toxicité et de cancérogénicité par
inhalation chronique de HCFC 123 est en cours sur des rats. Une
communication préliminaire fait état d'une augmentation de l'incidence
des tumeurs bénignes au niveau des testicules et du pancréas exocrine
chez les rats mâles. Toutefois, on ne pourra pas évaluer la
cancérogénicité potentielle du HCFC 123 avant de disposer de
l'ensemble des résultats.
6.6 HCFC 124
Chez l'animal, le HCFC 124 n'a qu'une faible toxicité aiguë par
la voie respiratoire. Une mortalité a été observée chez des rats à la
dose de 1674 g/m3 (exposition de 240 minutes) et chez des souris à
la dose de 2460 g/m3 (exposition de 10 minutes). Les effets observés
sont caractéristiques des chlorofluorocarbures, c'est-à-dire perte de
coordination et narcose. A des doses supérieures ou égales à 140
g/m3, on a observé chez des chiens une sensibilisation cardiaque
après une injection d'épreuve d'adrénaline. On ne dispose d'aucune
donnée sur l'irritation oculaire ni sur l'irritation ou la
sensibilisation cutanée que ce composé pourrait provoquer.
Cinq expériences ont permis d'étudier la toxicité à court terme
de ce composé lorsqu'il est inhalé par des rats pendant des périodes
de 14 à 90 jours. Aux doses les plus fortes étudiées (500 g/m3 sur
14 jours et 279 g/m3 sur 90 jours) on n'a observé aucune
modification histopathologique au niveau des organes. Une dose sans
effet observable de 28 g/m3 a été établie d'après les résultats
concernant les troubles fonctionnels et la biochimie sanguine, fournis
par l'étude de 90 jours.
Une étude toxicologique à long terme utilisant la voie
drespiratoire est en cours sur le HCFC 124.
Lors de trois études de tératogénicité de portée limitée
effectuées sur des rats, au cours desquelles on a fait inhaler du HCFC
124 à raison de 30 g/m3 ou à des doses allant de 3 à 279 g/m3, on
n'a pas relevé de signes d'embryotoxicité ni de tératogénicité. Des
signes d'intoxication maternelle étaient visibles dès 84 g/m3. On ne
dispose d'aucune donnée sur les effets que le HCFC 124 pourrait
exercer sur la fonction de reproduction. Des études de tératogénicité
complètes sont en cours.
Les données fournies par un certain nombre d'études sur des
bactéries ainsi que par une seule et unique étude portant sur des
cellules mammaliennes, n'attribuent aucun pouvoir mutagène au HCFC
124. Une étude de cancérogénicité utilisant la voie respiratoire est
en cours.
7. Effets sur l'homme
On ne dispose d'aucune donnée sur les effets que le HCFC 141b, le
HCFC 132b, le HCFC 133a, le HCFC 123 ou le HCFC 124 pourraient exercer
sur l'homme.
Les données fournies par une seule et unique étude consacrée à
des personnes exposées de par leur profession au HCFC 142b ne
permettent pas d'évaluer les effets de ce composé indépendamment des
autres types d'exposition auxquelles ces personnes avaient été
soumises.
8. Effets sur d'autres êtres vivants au laboratoire et dans leur
milieu naturel
On ne dispose d'aucune donnée sur les effets que les
hydrochlorofluorocarbures en cause pourraient avoir sur les êtres
vivants dans leur milieu naturel, si ce n'est quelques données
concernant le HCFC 141b et le HCFC 142b. La CL50 à 96 h du HCFC 141b
pour les poissons de l'espèce Melambaphes zebra est de 126 mg/litre
et la CL50 à 48 h pour l'immobilisation de la daphnie est de 31
mg/litre. Ces deux observations ont été faites dans des aquariums
clos. Dans le cas du HCFC 142b, la CE50 à 96 h pour le guppy est 220
mg/litre alors que la CL50 à 48 h pour l'immobilisation de la
daphnie varie de 160 à < de 190 mg/litre. La CL50 à 96 h du HCFC
142b pour la truite arc-en-ciel est de 36 mg/litre.
9. Evaluation et conclusions
On ignore qu'elle est la concentration dans l'environnement des
six HCFC étudiés mais, compte tenu de leurs modalités actuelles
d'utilisation, on peut penser qu'elle est faible.
Le HCFC 142b ne présente qu'une faible toxicité potentielle et
l'on estime qu'il ne constitue pas un risque important pour la santé
humaine lorsqu'il n'y a pas d'exposition accidentelle. Les données
toxicologiques concernant le HCFC 141b, le HCFC 123 et le HCFC 124
sont incomplètes et il faudra en obtenir davantage avant qu'on puisse
évaluer les dangers qu'ils représentent pour la santé humaine. En
revanche le HCFC 133a et le HCFC 132b sont dangereux pour la santé.
Par rapport aux chlorofluorocarbures complètement halogénés, ces
six hydrochlorofluorocarbures sont ou devraient être beaucoup moins
agressifs vis-à-vis de la couche d'ozone et leur temps de séjour dans
l'atmosphère est beaucoup plus faible. Ils constituent donc un risque
indirect. Leur contribution potentielle à l'effet de serre est ou
devrait également être plus faible que celle des chlorofluorocarbures
complètement halogénés et ils ne devraient donc pas contribuer de
façon sensible au réchauffement de la planète.
Etant donné que la toxicité du HCFC 142b est faible et qu'il est
moins agressif vis-à-vis de la couche d'ozone et contribue moins à
l'effet de serre que les chlorofluorocarbures complètement halogénés,
on peut considérer qu'il est susceptible d'être provisoirement
substitué aux chlorofluorocarbures visés par le Protocole de Montréal.
Aucune recommandation ne peut être faite concernant le HCFC 141b,
le HCFC 123 ou le HCFC 124 tant qu'on ne disposera pas de données
toxicologiques plus complètes. Bien que le HCFC 133a et le HCFC 132b
ne menacent guère l'environnement et que les risques qu'ils
constituent pour la santé ne soient qu'indirects, il n'est pas
recommandé de les substituer aux chlorofluorocarbures visés par le
Protocole de Montréal en raison de leur toxicité potentielle.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
La presente monografía se ocupa de seis hidroclorofluorocarburos
(HCFCs) derivados de la sustitución parcial de los átomos de hidrógeno
del etano por átomos de flúor y de cloro. En este informe se estudian
los siguientes compuestos: 1,1-dicloro-1-fluoroetano (HCFC 141b),
1-cloro-1,1-difluoroetano (HCFC 142b), 1,2-dicloro-1,1-difluoroetano
(HCFC 132b), 1-cloro-2,2,2-trifluoroetano (HCFC 133a),
1,1-dicloro-2,2,2-trifluoroetano (HCFC 123) y
1-cloro-1,2,2,2-tetrafluoroetano (HCFC 124).
En condiciones normales de presión y temperatura, estos
compuestos son gases inflamables (HCFC 142b) o ininflamables (HCFC
133a, HCFC 124) o líquidos volátiles ininflamables (HCFC 141b, HCFC
132b, HCFC 123). Son incoloros y la mayoría prácticamente inodoros o
con un débil olor a éter (HCFC 141b y HCFC 123). Su solubilidad en
agua es escasa o moderada y son miscibles con muchos disolventes
orgánicos.
Entre los métodos analíticos utilizados para la determinación de
estos hidroclorofluorocarburos figuran la cromatografía de gases con
ionización de llama y la detección con captura de electrones. Se
pueden medir concentraciones relativamente altas en el aire mediante
fotometría de un solo haz.
2. Fuentes de exposición humana y ambiental
Los hidroclorofluorocarburos que se estudian en la presente
monografía no se conocen como productos naturales. Dado que estos
compuestos no se producen comercialmente en gran escala para
utilizarlos como tales, la exposición humana o la liberación al medio
ambiente son muy pequeñas. Algunos de estos compuestos se podrían
utilizar en el futuro como sustitutivos de clorofluorocarburos
completamente halogenados (por ejemplo, CFC 11, CFC 12 y CFC 113). Los
HCFCs 133a y 142b son compuestos intermedios en la fabricación de
otros productos fluorados. El HCFC 133a es un metabolito in vivo del
anestésico halotano.
3. Transporte, distribución y transformación en el medio ambiente
Los datos sobre la biodegradación en el medio ambiente se limitan
a unos estudios sobre los HCFCs 141b y 142b, que han demostrado no ser
fácilmente biodegradables por los microorganismos. Apenas se dispone
de información sobre los logaritmos de los coeficientes de reparto
octanol/agua; en el caso del HCFC 141b es 2,3, por lo que no es
probable que se bioacumule. En la troposfera, estas sustancias se
descomponen principalmente por reacciones con radicales hidroxilo. Su
permanencia en la atmósfera (en relación con los 6,3 años de
permanencia del metilcloroformo) oscila entre 1,6 años (HCFC 123) y
19,1 años (HCFC 142b). (La permanencia en la atmósfera del CFC 11 es
de 75 años, la del CFC 12 de 110 y la del CFC 113 de 90). A excepción
del HCFC 133a, del que no se tienen datos, su contribución potencial
a la destrucción de ozono y al calentamiento del planeta es inferior
o igual al 10 por ciento de la del CFC 11, el clorofluorocarburo
completamente halogenado con la mayor influencia potencial en esos
fenómenos (el HCFC 142b, cuya contribución al calentanierto del
planeta es aproximadamente un tercio de la del CFC 11, es una
excepción).
4. Niveles ambientales y exposición humana
Como los HCFCs 141b, 132b, 133a, 123 y 124 no se producen todavía
comercialmente en gran escala y el HCFC 142b se utiliza sólo como
producto intermedio, no se libera al medio ambiente en cantidades
apreciables. No se dispone, por consiguiente, de datos sobre los
niveles ambientales ni la exposición humana.
5. Cinética y metabolismo en animales de laboratorio y en el
ser humano
No hay datos acerca de la toxicocinética en el ser humano de
ninguno de los HCFCs examinados.
5.1 HCFC 141b
Los resultados obtenidos de los estudios de toxicidad sugieren
que la absorción del HCFC 141b tiene lugar a través del epitelio
respiratorio. No se dispone de información acerca de su distribución
en mamíferos. En estudios recientes de exposición única in vitro
en ratas se detectaron en la orina 2,2-dicloro-2-fluoroetilglucurónido
y ácido 2,2-dicloro-2-fluoroacético. En un estudio piloto sobre la
absorción y el metabolismo del HCFC 141b en ratas expuestas a sus
vapores se puso de manifiesto que sólo se produce transformación
metabólica en muy pequeña medida.
Un estudio in vitro indicó que los microsomas hepáticos
decloran el HCFC 141b en grado limitado.
5.2 HCFC 142b
No se dispone de información sobre la toxicocinética del HCFC
142b. De los estudios de toxicidad en animales se deduce que se
produce absorción. Un estudio in vitro sugirió que puede producirse
decloración.
5.3 HCFC 132b
En un estudio de metabolismo con administración intraperitoneal
de HCFC 132b a ratas se detectaron en la orina 2-cloro-2,2-
difluoroetilglucurónido, clorodifluoroacetaldehído (hidratado y
conjugado) y ácido clorodifluoroacético. La formación y excreción de
este último aumentó al volver a inyectar a los animales HCFC 132b.
Los experimentos in vitro con microsomas hepáticos de rata sugieren
la participación del citocromo P-450 IIEI en el paso inicial de
hidroxilación. No hay pruebas experimentales de la unión covalente de
metabolitos fluorados a las proteínas hepáticas.
5.4 HCFC 133a
Se carece de información sobre la toxicocinética del HCFC 133a.
De los efectos tóxicos observados en diversos estudios puede deducirse
que se produce absorción tras la exposición de animales. Se ha
advertido in vitro la decloración del HCFC 133a.
5.5 HCFC 123
No hay datos acerca de la toxicocinética del HCFC 123. Sin
embargo, de los efectos sistémicos y de los elevados niveles de flúor
en la orina observados en los estudios de toxicidad en ratas se puede
deducir que hay absorción. Se ha demostrado que el HCFC 123
experimenta una transformación metabólica en ratas. No se conoce el
alcance del metabolismo, pero se ha identificado el ácido
trifluoroacético (TFA) como principal metabolito urinario, además del
fluoruro. Se ha demostrado que el HCFC 123 forma enlaces covalentes
con las proteínas hepáticas.
5.6 HCFC 124
Se carece de datos acerca de la cinética y el metabolismo del
HCFC 124. De los estudios de toxicidad por inhalación se puede deducir
que la absorción del HCFC 124 se produce en el tracto respiratorio.
6. Efectos en los animales de laboratorio y en sistemas de prueba
in vitro
6.1 HCFC 141b
La toxicidad aguda del HCFC 141b por vía oral es baja. No se
observaron signos de toxicidad tras suministrar a ratas dosis de 5
g/kg.
En estudios de inhalación aguda en ratas y ratones, con altos
niveles de exposición se observó depresión del sistema nervioso
central, anestesia y muerte. No se advirtieron efectos macroscópicos
o histopatológicos relacionados con el tratamiento. En un estudio, la
CL50 a las 4 h en ratas fue de 295 g/m3, y en otro estudio en
ratones la CL50 a las 2 h fue de 151 g/m3. Se informó que la
concentración letal más baja en ratas era de 242 g/m3 en 6 h.
Tras la exposición cutánea a 2 g/kg no se observó mortalidad en
ratas ni en conejos.
En estudios de inhalación de corta duración con exposiciones que
oscilaron entre 10 y 97 g/m3 y que se prolongaron hasta 90 días no
se advirtió toxicidad pronunciada. Entre otros efectos, se observó
menor aumento del peso corporal, "ligeros cambios bioquímicos" y
depresión del sistema nervioso central. En los 90 días del estudio no
se alcanzó un nivel sin efecto observado.
El HCFC 141b no produjo signos de irritación cutánea en conejos,
ni de irritación ocular en uno de los dos estudios realizados. En el
segundo estudio se observó una respuesta de irritación ocular
"ligera". No se advirtió sensibilización cutánea en cobayos.
Actualmente hay un estudio en curso acerca del efecto del HCFC
141b sobre la reproducción en dos generaciones. En estudios sobre el
desarrollo se observaron frecuencias superiores de edema subcutáneo y
hemorragias en los fetos y de muerte de embriones, pero sólo con la
concentración tóxica para la madre de 97 g/m3 en un estudio en
ratas. No se observaron efectos teratogénicos. En un estudio con
conejos no se advirtieron efectos en el desarrollo embrionario o fetal
a causa del tratamiento.
El HCFC 141b no resultó mutagénico en un ensayo de reparación del
ADN bacteriano y los resultados fueron contradictorios en otras
pruebas de mutación de bacterias. No tuvo ningún efecto sobre las
células V79 en el ensayo sobre el locus hprt. Se detectaron
aberraciones cromosómicas tras el tratamiento in vitro de células de
ovario de hámster chino, pero no aparecieron en un estudio in vitro
con linfocitos humanos. También fueron negativos dos ensayos in vivo
efectuados con micronúcleos de ratones.
Está en marcha un estudio combinado de toxicidad crónica por
inhalación/carcinogenicidad en ratas.
El HCFC 141b muestra en perros un efecto potencial de
sensibilización cardíaca a la adrenalina exógena. Las concentraciones
más bajas de HCFC 141b que indujeron respuesta en perros y monos
fueron respectivamente de 24 y 48 g/m3.
6.2 HCFC 142b
El HCFC 142b administrado por vía oral a dosis únicas de hasta 5
g/kg sólo produjo signos leves de toxicidad en ratas.
La exposición de ratas a una inhalación única de 525 g/m3
durante 4 h produjo la muerte de alrededor del 50% de los animales. En
otros estudios con exposiciones de menor duración se obtuvieron
valores de la CL50 superiores a 1000 g/m3.
En los estudios de exposiciones repetidas por inhalación en ratas
con dosis de 41 g/m3 (6 h/día, 5 días a la semana durante 90 días)
no se observaron respuestos adversas. Con dosis mucho más elevadas se
producía la muerte de las ratas relacionada con una irritación
pulmonar grave.
No se han comunicado estudios sobre el HCFC 142b en relación con
la irritación cutánea u ocular o la sensibilización cutánea. Se
realizaron experimentos de sensibilización cardíaca (utilizando
adrenalina exógena) en ratones, perros y monos. Los perros fueron los
más sensibles; el NOEL fue de 102,5 g/m3 con una exposición de 5
minutos, mientras que 205 g/m3 (también con 5 minutos de exposición)
inducían arritmia cardíaca.
Sólo hay datos de un estudio prolongado, en el que se expusieron
ratas (130 machos y 110 hembras por grupo) a concentraciones de HCFC
142b de 4, 41 y 82 g/m3, 6 h/día, 5 días/semana, hasta un máximo de
104 semanas. No se observaron efectos relacionados con el tratamiento
en ninguno de los parámetros estudiados, que comprendían hematología,
química sanguínea y urinaria e histopatología. No se notificaron
cambios de importancia dependientes del tratamiento en relación con la
aparición de tumores.
No se han hecho estudios convencionales sobre el efecto del HCFC
142b en la reproducción, pero en un estudio de letalidad dominante no
se observaron efectos en la fertilidad de los machos. Se han realizado
dos pruebas de teratogenicidad en ratas. En una de éstas, se expuso a
ratas Sprague-Dawley a concentraciones de 4 y 41 g/m3 (6 h/día desde
el 3 al 15 día de gestación), mientras que en el otro estudio se
expuso a ratas Sprague-Dawley a 13 y 39 g/m3 (6 h/día del 6 al 15
día de gestación). No se observaron efectos teratogénicos. En el
segundo estudio se advirtió para ambas dosis osificación reducida en
un pequeño número de fetos, pero no en el primero.
El HCFC 142b induce mutaciones en bacterias, pero no se dispone
de datos de ensayos de genotoxicidad en cultivos de células de
mamíferos. En los ensayos in vivo no se produjo aumento de las
aberraciones cromosómicas en la médula ósea ni efectos letales
dominantes en las ratas machos.
6.3 HCFC 132b
La toxicidad aguda oral del HCFC 132b en la rata es escasa. La
dosis más baja a la que se ha observado mortalidad es 25 g/kg. Tras la
administración oral de 2 g/kg se advirtió una depresión del sistema
nervioso autónomo y del central, además de otros efectos en la
coordinación motora, la actividad motora y el tono muscular. En los
machos se advirtieron inflamación hepática y disminución del peso del
hígado.
La toxicidad aguda por inhalación del HCFC 132b con niveles de
exposición elevados se caracteriza por un efecto anestésico. La dosis
más baja que produjo mortalidad en ratas tras 4 horas de exposición
fue de 110 g/m3. En ratones, la CL50 en 30 minutos de exposición
fue de 269 g/m3, y se produjo anestesia con 71 g/m3. En un estudio
se advirtió una disminución del peso de los testículos y un aumento
del peso del hígado y los pulmones en ratas macho tras la exposición
a 33 g/m3 durante 6 horas.
La aplicación cutánea de HCFC 132b a ratas se tradujo en algunos
animales en síntomas clínicos de efectos en el SNC e inflamación
hepática. El compuesto sin diluir produjo una "ligera" irritación
cutánea en cobayos y una irritación ocular "de ligera a moderada" en
conejos. No se obtuvieron pruebas de sensibilización cutánea en
cobayos. En los perros se produjo sensibilización cardíaca a la
adrenalina por inhalación de HCFC 132b con niveles de exposición de 27
g/m3 o superiores.
Las consecuencias principales de la exposición de ratas macho a
la inhalación de HCFC 132b durante un breve período fueron, además de
la depresión del SNC, atrofia del timo y efectos en la
espermatogénesis. Se observó alteración de la espermatogénesis tras un
tratamiento con dosis de 3 g/m3 o superiores durante 13 semanas.
Otros efectos que se detectaron fueron una proliferación del conducto
biliar y un aumento de la relación ponderal hígado/cuerpo en machos,
incluso con la aplicación de los niveles más bajos de exposición (3
g/m3). Las ratas hembras resultaron menos sensibles que los machos
a los efectos hepáticos.
El HCFC 132b indujo embriotoxicidad en ratas tras la exposición
por inhalación a 3-28 g/m3 durante los días 6-15 de gestación, dando
lugar a un aumento del número de resorciones (a 11 y 28 g/m3) y a la
disminución del peso fetal en todos los niveles de exposición. Se
observó toxicidad materna con todos los niveles de dosificación que se
probaron.
De acuerdo con los limitados datos disponibles, no hay pruebas de
mutagenicidad in vitro del HCFC 132b. No se ha estudiado la
carcinogenicidad del compuesto.
6.4 HCFC 133a
No se dispone de datos sobre la toxicidad aguda oral del HCFC
133a. Su toxicidad aguda por inhalación es baja (la CL50 a los 30
min en ratones es de 738 g/m3), y los principales efectos observados
están en relación con su acción anestésica. Se carece de información
sobre sensibilización cardíaca, irritación cutánea u ocular o
sensibilización cutánea.
Exposiciones repetidas de ratas (90 días) a 49 g/m3 produjeron
una inflamación crónica del conducto nasal, enfisema y edema pulmonar,
bronquitis y neumonía. También se observaron atrofia del timo, los
testículos, los ovarios y el bazo. No se advirtieron efectos en ratas
ni en perros repetidamente expuestos a concentraciones de HCFC 133a de
unos 25 g/m3 durante siete días (ratas) o 90 días (perros), aunque
se observaron muertes en ratones expuestos a concentraciones iguales
o superiores a 0,5 g/m3 durante 5 días (a excepción de 2,5 g/m3).
Aunque no se conocen estudios convencionales de los efectos del
HCFC 133a en la reproduccción, en tres estudios de letalidad dominante
en ratones se observaron efectos en la fertilidad del macho y la
histopatología testicular. Las exposiciones a concentraciones de 2,5
g/m3 o superiores durante 5 días dieron lugar a una reducción del
número de hembras gestantes y a un aumento de la proporción de esperma
anormal, mientras que la exposición a una concentración de 5 g/m3
produjo lesiones histopatológicas en el epitelio seminífero.
Los estudios en ratas (tratadas del 6 al 16 día de la gestación)
con exposición a concentraciones que producen sólo síntomas de ligera
toxicidad materna han demostrado que el HCFC 133a es embriotóxico en
concentraciones de 2 g/m3 o superiores y embrioletal a 10 g/m3 o
más. El tratamiento previo con progesterona de las hembras preñadas no
tuvo influencia en los efectos embriotóxicos/letales. En un estudio se
observaron síntomas de efectos teratogénicos (anomalías externas de
las extremidades y la cola). El HCFC 133a produjo abortos espontáneos
y embrioletalidad total en conejos expuestos a 25 g/m3 en los días
7 a 19 de la gestación, concentración que produjo sólo una ligera
toxicidad materna.
En los estudios disponibles no hay pruebas de su capacidad
mutagénica en bacterias. En un estudio no se observó aumento en la
proporción de células renales de hámster que producían colonias
transformadas. Se detectaron efectos letales dominantes en dos de tres
estudios tras la exposición de ratones machos a concentraciones de 12
g/m3 o superiores durante 5 días la proporción de células de la
médula ósea con aberraciones cromosómicas se mantuvo inalterada en
ratas expuestas a 98 g/m3 (6 h al día durante 5 días como máximo).
En el único estudio de carcinogenicidad se observó un aumento de la
incidencia de adenocarcinomas del útero y de tumores benignos de las
células intersticiales de los testículos en ratas que recibieron 300
mg/kg de aceite de maíz administrados por sonda durante 52 semanas
(seguidas de un período de observación de 73 semanas).
6.5 HCFC 123
El HCFC 123 tiene una toxicidad aguda oral y cutánea baja. La
dosis oral más baja de HCFC 123 con la que se han observado efectos
letales en ratas es de 9 g/kg. Con dosis de 2 g/kg no se produjo
mortalidad en ratas ni en conejos.
La toxicidad aguda por inhalación de HCFC 123 es también baja.
Los efectos observados son similares a los de los clorofluorocarburos,
es decir, pérdida de coordinación y narcosis. La CL50 a las 4 h es
de 178 g/m3 en el hámster, 463 g/m3 en el ratón y oscila entre 200
y 329 g/m3 en la rata. En el perro, se produjo sensibilización
cardíaca tras la exposición, con inyección de insulina, a
concentraciones de 119 g/m3 o superiores. El HCFC 123 líquido
produce en conejos una irritación "ligera" de la piel y los ojos. No
provoca sensibilización cutánea en los cobayos.
Se han realizado varios estudios de toxicidad de corta duración
con el HCFC 123 por vía respiratoria. En ratas se observan síntomas
invariables de depresión del SNC a concentraciones de 31 g/m3 o
superiores. El HCFC 123 causó también algunos efectos hepáticos en
ratas expuestas a dosis de 31 g/m3 o más. La exposición prolongada
(4 semanas o más) al HCFC 123 afecta también al metabolismo de los
lípidos y los glúcidos, como puso de manifiesto la reducción
invariable de los niveles de triglicéridos, colesterol y glucosa en el
suero. Los resultados provisionales de un estudio en curso de
toxicidad crónica/oncogenicidad por inhalación en ratas indican que el
HCFC 123 induce efectos tras la exposición prolongada a dosis de 2, 6
ó 31 g/m3. En este estudio no se registró el nivel sin efectos
observados (NOEL), basado en los efectos sobre el metabolismo lipídico
y en el aumento de la actividad de los peroxisomas hepáticos.
Actualmente se está llevando a cabo un estudio de reproducción de
dos generaciones de ratas expuestas al HCFC 123 por vía respiratoria.
En dos estudios limitados en ratas, con concentraciones capaces de
producir una ligera toxicidad materna, no se obtuvieron pruebas de
embriotoxicidad. Hay pruebas de toxicidad sólo a concentraciones muy
tóxicas para la madre (superiores a 62,5 g/m3) en conejos. En ratas
expuestas a concentraciones de 31 g/m3 o más y en conejos a niveles
de 3 g/m3 o superiores se observó toxicidad materna (disminución del
peso corporal y depresión del SNC). No aparecieron pruebas de
teratogenicidad en ratas ni en conejos.
El HCFC 123 no demuestra actividad mutagénica en los ensayos
efectuados con bacterias y levaduras. Sin embargo, sí hay pruebas de
actividad clastogénica en linfocitos humanos in vitro, pero los
datos procedentes de un ensayo in vivo en micronúcleos de ratón no
confirmaron ese resultado.
Está en curso un estudio combinado de toxicidad crónica/
carcinogenicidad por inhalación en ratas. En una comunicación
preliminar se ha indicado que el HCFC 123 produce una mayor frecuencia
de tumores benignos en los testículos y en el páncreas exócrino de
ratas macho. Sin embargo, no es posible realizar una evaluación de la
carcinogenicidad potencial del HCFC 123 hasta que no se disponga de
los resultados completos.
6.6 HCFC 124
La toxicidad aguda por inhalación del HCFC 124 en animales es
baja. Se produjo la muerte en ratas expuestas a concentraciones de
1674 g/m3 (durante 240 minutos) y en ratones a 2460 g/m3 (durante
10 minutos). Se observaron los efectos típicos de los
clorofluorocarburos, es decir, pérdida de coordinación y narcosis. Se
produjo sensibilización cardíaca tras la prueba de una inyección de
adrenalina en perros a concentraciones de 140 g/m3 o superiores. Se
carece de información sobre la irritación cutánea u ocular o la
sensibilización cutánea con este compuesto.
Se ha investigado la toxicidad por inhalación durante un breve
período en cinco experimentos en ratas con exposiciones que oscilaron
entre 14 y 90 días. No se observaron cambios histopatológicos en los
órganos, ni siquiera con los niveles de exposición más altos de los
estudiados (560 g/m3 en un experimento de 14 días, 279 g/m3 en un
estudio de 90 días). Se comunicó un NOEL de 28 g/m3 sobre la base de
las observaciones funcionales y los análisis de sangre efectuados en
el estudio de 90 días.
Está en curso un estudio sobre la toxicidad crónica por
inhalación del HCFC 124.
En tres estudios limitados de teratogenicidad en ratas, en los
que se probaron concentraciones de HCFC 124 de 30 g/m3 o
comprendidas entre 3 y 279 g/m3, no se encontraron pruebas de
efectos embriotóxicos o teratogénicos. Con 84 g/m3 apareció
toxicidad materna. No se dispone de información acerca de los efectos
del HCFC 124 en el potencial de reproducción. Se están realizando
estudios completos de teratogenicidad.
Los datos disponibles de varios estudios en bacterias y de un
único estudio en células de mamíferos no demuestran efecto mutagénico
del HCFC 124. Está en marcha un estudio de carcinogenicidad por
inhalación.
7. Efectos en el ser humano
Se carece de datos sobre los efectos del HCFC 151b, el HCFC 132b,
el HCFC 133b, el HCFC 123 y el HCFC 124 en el ser humano.
Los datos de un solo estudio en el ser humano expuesto en el
lugar de trabajo al HCFC 142b no permiten evaluar sus efectos en la
especie humana independientemente de otras muchas exposiciones.
8. Efectos en otros organismos en el laboratorio y en el medio
ambiente
No se dispone de información acerca de los efectos de los
hidroclorofluorocarburos estudiados sobre los organismos presentes en
el medio ambiente, excepto algunos datos limitados de los HCFC 141b y
142b. La CL50 a las 96 h del HCFC 141b para Brachydanio rerio es
de 126 mg/litro y la CE50 a las 48 h para la inmovilización de
Daphnia magna de 31 mg/litro, habiéndose realizado ambas
observaciones en recipientes cerrados. En el caso del HCFC 142b, la
CE50 a las 96 h para Lebistes reticulatus es de 220 mg/litro,
mientras que la CE50 a las 48 h para la inmovilización de Daphnia
magna varía de 160 a > 190 mg/litro. La CL50 a las 96 h del HCFC
142b para la trucha arco irís es de 36 mg/litro.
9. Evaluación y conclusiones
Se desconocen los niveles de los seis HCFCs estudiados presentes
en el medio ambiente, pero se consideran bajos, dado su grado actual
de utilización.
El HCFC 142b tiene un potencial tóxico bajo y se estima que no
supone un riesgo para el ser humano en condiciones de exposición no
debidas a un accidente. La información toxicológica acerca del HCFC
141b, el HCFC 123 y el HCFC 124 es incompleta y se necesitan más datos
para poder evaluar su riesgo para la salud humana. Tanto el HCFC 133a
como el HCFC 132b representan un riesgo para ésta.
Los seis hidroclorofluorocarburos estudiados tienen, o se les
supone, una capacidad de destrucción del ozono más baja y tiempos de
permanencia en la atmósfera considerablemente inferiores a los de los
clorofluorocarburos completamente halogenados. Por consiguiente,
deberían representar un riesgo indirecto menor para la salud. Su
efecto sobre el calentamiento del planeta es, o se supone, inferior al
de los clorofluorocarburos completamente halogenados y no se cree que
contribuyan a él de manera apreciable.
Puesto que la toxicidad del HCFC 142b es baja y su contribución
al agotamiento del ozono y al calentamiento del planeta son inferiores
a los de los clorofluorocarburos completamente halogenados, se lo
puede considerar como un sustitutivo transitorio de los
clorofluorocarburos que figuran en el Protocolo de Montreal.
Hasta que no se disponga de más datos toxicológicos no se pueden
hacer recomendaciones en relación con el HCFC 141b, el HCFC 123 y el
HCFC 124. Debido a su potencial tóxico, no se recomiendan el HCFC 133a
y el HCFC 132b como sustitutivos de los clorofluorocarburos que
figuran en el Protocolo de Montreal, a pesar de representar un riesgo
bajo para el medio ambiente e indirecto para la salud.
 6.1.3.2 HCFC 141b
Harris & Anders (1991a) studied the in vivo metabolism of HCFC
141b. A single fluorinated urinary metabolite, identified as
2,2-dichloro-2-fluoro-ethyl glucuronide, was found in rats exposed to
HCFC 141b (56 g/m3) in air for 2 h. The metabolism was reported to
be similar to that of its chlorinated analogue 1,1,1-trichloroethane,
which is metabolized to 2,2,2-trichloroethanol and excreted as its
glucuronate conjugate (Hake et al., 1960) and as trichloroacetic acid
(Koizumi et al., 1982). It was claimed (although no data were given)
that 2,2-dichlorofluoroacetic acid was also detected in the urine of
rats exposed to a concentration of 194 g/m3 for 4 h, but not to 56
g/m3 for 2 h (Harris & Anders, 1991a).
In a pilot study for absorption and metabolism of HCFC 141b,
seven groups of five male rats were exposed to the vapour by
inhalation in a closed loop exposure system (concentrations ranging
from 0.4 to 12 g/m3). No metabolism was detected but the sensitivity
of the method is such that it will not detect metabolism below 0.15%.
The results suggested that absorption did not take place, but that if
any metabolism occurred it was at a low level (Zwart, 1989).
Evidence of dechlorination was observed when rat hepatic
microsomes were incubated with about 1% HCFC 141b in vitro (Van
Dyke, 1977).
6.1.3.3 HCFC 142b
No in vivo studies on the metabolism of HCFC 142b have been
reported. One in vitro study provided evidence for dechlorination
when rat hepatic microsomes were incubated with 0.6% HCFC 142b (Van
Dyke, 1977).
6.1.3.4 HCFC 132b
Harris & Anders (1991) identified a number of metabolites in the
urine of male Fischer-344 rats given one or four doses of 10 mmol/kg
dissolved in corn oil by intraperitoneal injection. Approximately 1.8%
of the single administered dose was recovered in the urine. The
metabolites excreted in urine during the first 6 h were
2-chloro-2,2-difluoroethyl glucuronide, chlorodifluoroacetic acid and
chlorodifluoroacetaldehyde hydrate (free and conjugated). Repeated
injection of rats with HCFC 132b significantly increased both the rate
of chlorodifluoroacetic acid excretion and the relative fraction of
the HCFC 132b dose excreted as chlorodifluoroacetic acid. The
incubation of HCFC 132b with rat hepatic microsomes yielded
chlorodifluoroacetaldehyde hydrate as the only fluorinated product.
The in vitro metabolism of HCFC 132b was increased in microsomes
from pyridine-treated rats and inhibited by p-nitrophenol. This
inhibition by p-nitrophenol led the authors to suggest an
involvement of cytochrome P-450 IIE1 in the initial hydroxylation of
HCFC 132b.
6.1.3.5 HCFC 133a
Evidence for dechlorination of HCFC 133a was provided by an
in vitro study using a microsomal preparation derived from
Aroclor-1254a-induced rat liver homogenates (Salmon et al., 1981).
6.1.3.6 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 g/m3 or 68 g/m3) or to halothane (2-bromo-2-2
chloro-1,1,1-trifluoroethane) (1.05 g/m3) in air for 2 h. The
pattern of proteins immunoreactive with haptens-specific
anti-trifluoroacetylprotein antibodies was found to be identical in
livers of the rats exposed to HCFC 123 and halothane. Trifluoroacetic
acid was detected in urine of rats exposed to HCFC 123 or halothane by
nuclear magnetic resonance (NMR) and by gas chromatography with mass
spectrometry (GCMS), as had been reported previously (Maiorino et al.,
1980; Trochimowicz, 1989).
6.1.4 Covalent binding to macromolecules
6.1.4.1 HCFC 141b
19F-NMR analysis of microsomal and cytosolic proteins isolated
from the livers of rats killed 15 h after a 2-h exposure to HCFC 141b
(56 g/m3) did not yield any evidence for covalent binding of
fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.2 HCFC 132b
19F-NMR analysis of microsomal and cytosol proteins isolated
from the livers of rats killed 15 h after a single intraperitoneal
dose of HCFC 132b (10 mmol/kg) did not yield evidence for covalent
binding of fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.3 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 or 68 g/m3) or to halothane (105 g/m3) in air for 2 h.
19F-NMR analysis of cytosolic protein and immunoblotting of
microsomal and cytosolic hepatic protein using antibodies against
trifluoroacetylprotein at 15 h after the exposure demonstrated
covalent binding of fluorinated metabolites.
a Aroclor-1254 is a polychlorinated biphenyl mixture.
6.1.5 Elimination
No data are available from animal studies on the elimination of
the hydrochlorofluorocarbons reviewed. However, based on the
information on chlorofluorocarbons, it is likely that the main route
of excretion for hydrochlorofluorocarbons is through the respiratory
tract. Increased urinary inorganic fluoride has been observed in some
inhalation toxicity studies with HCFCs 141b, 132b, 123, and 124
(Doleba-Crowe, 1977, 1978; Brewer, 1977; Trochimowicz, 1989; Malley,
1990a, 1991).
6.2 Human studies
No data are available on the absorption, distribution, metabolic
transformation or elimination of the hydrochlorofluorocarbons
reviewed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute oral toxicity
Available data indicate low toxicity following single oral
exposure associated with four hydrochlorofluorocarbons, i.e. HCFC
141b, 142b, 132b and 123.
7.1.1.1 HCFC 141b
No mortality was observed in rats given a single oral dose of
HCFC 141b (5 g/kg body weight) dissolved in corn oil (Sarver, 1988).
7.1.1.2 HCFC 142b
Other than piloerection, oral dosing with HCFC 142b (up to 5 g/kg
body weight) dissolved in corn oil resulted in no signs of toxicity
(Liggett et al., 1989).
7.1.1.3 HCFC 132b
According to Henry (1975), the lowest dose at which mortality was
observed in rats treated with HCFC 132b was 25 g/kg.
A 2-g/kg dose of HCFC 132b in corn oil was applied by stomach
tubes to five male and five female Wistar rats, and the animals were
observed for 14 days. The clinical signs were indicative of an effect
on the autonomic nervous system (ptosis), on the central nervous
system (diminished alertness and startle response, and positional
passivity), on motor coordination (abnormal body posture and gait, and
loss of righting reflex), on motor activity and on muscle tone
(paralysis). Macroscopic examination showed swollen livers in some
males, and a decrease in absolute and relative liver weights. However,
no treatment-related effects were found during microscopic
examinations (Janssen & Pot, 1989b).
7.1.1.4 HCFC 123
The lowest dose of HCFC 123 producing lethality in rats was
reported to be 9 g/kg when it was administered as a corn oil solution
by intragastric intubation (Henry, 1975a).
7.1.2 Acute inhalation toxicity
7.1.2.1 HCFC 141b
The effects of a single inhalation exposure of rodents to HCFC
141b are shown in Table 5. The main effects at high exposure
concentrations include central nervous system depression, anaesthesia
and death.
7.1.2.2 HCFC 142b
Table 6 summarizes the effects of single inhalation exposure to
HCFC 142b in mice and rats. At high exposure levels, HCFC 142b induces
anaesthesia and death.
7.1.2.3 HCFC 132b
Table 7 summarizes the effects of single inhalation exposures of
mice and rats to HCFC 132b.
7.1.2.4 HCFC 133a
Inhalation of high concentrations of HCFC 133a is characterized
by signs of anaesthesia followed by death, but recovery from nonlethal
exposure is rapid. The effects of inhalation exposure to HCFC 133a are
summarized in Table 8.
7.1.2.5 HCFC 123
The results of studies on the acute inhalation toxicity of HCFC
123 are summarized in Table 9. Anaesthesia and death at higher
exposures were reported for rats and hamsters. No gross morphological
changes were observed in animals that died during exposure. Survivors
recovered within several minutes without showing any observable
clinical signs.
7.1.2.6 HCFC 124
Table 10 summarizes the effects of single inhalation exposure to
high concentrations of HCFC 124. As with other
hydrochlorofluorocarbons, the main effects observed were anaesthesia
and death.
7.1.3 Acute dermal toxicity
There is information only on the hydrochlorofluorocarbons that
are liquid at ambient temperatures, i.e. HCFC 141b, HCFC 132b and HCFC
123.
Table 5. Effects of a single inhalation exposure to HCFC 141b in mice and rats
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3) (h)
Rat 142-366 4 LC50 = 295 g/m3 Hardy et al. (1989a)
(Sprague-Dawley) No deaths were observed at 142 or 217 g/m3. All deaths
at higher concentrations (323 and 366 g/m3) occurred
during exposure, and were preceded by disturbed breathing.
Reduced motor activity, abnormal body carriage, restless
behaviour and exaggerated respiratory movements were seen
at all concentrations during exposure. No treatment-related
macroscopic findings were seen. Focal basophilic staining
was observed in the renal cortical tubules of 4 out of 10
rats at 217 g/m3 and 2 out of 10 rats at 142 g/m3. No
histopathological effects were seen in decedents.
Rat (strain unspecified 6 The lowest concentration producing lethality was 242 g/m3. Doleba-Crowe (1977)
unspecified) range
Mouse 17-388 6 Deaths preceded by signs of narcosis in 6 out of 10) animals Vlachos (1989)
(Crl: CD-1) occurred within 30 min of exposure to 388 g/m3. No deaths
occurred at the next highest (199 g/m3) concentration. Signs
of CNS depression (lethargy, abnormal gait, partially closed
eyes) were seen at 165 and 199 g/m3. No effects were seen at
concentrations of 145 g/m3 or less.
Mouse (strain unspecified 2 LC16 = 115 g/m3; LC50 = 151 g/m3; LC84 = 200 g/m3. Signs of Nikitenko & Tolgskaja
unspecified) range CNS depression and anaesthesia were observed. Death was (1965)
preceded by laboured breathing.
Mouse (Schofield unspecified 0.5 LC50 = 115 g/m3; concentration producing anaesthesia in Davies et al. (1976)
strain) range 50% of animals = 62 g/m3. No other information was given.
Table 6. Effects of single inhalation exposure to HCFC 142b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse "white" up to 2050 2 death; LC50 = 1514 g/m3 Nikitenko & Tolgskaja (1965)
Mouse AP unspecified 0.5 death; LC50 = 1228 g/m3 Davies et al. (1976)
range
Rat "white" 615-3280 0.5 death at 2050 g/m3 and unconsciousness at Lester & Greenberg (1950)
1230 g/m3; postural, righting and corneal
reflexes were lost at 820 g/m3
Rat Sherman 525 4 death (approx 50%) at 525 g/m3 Carpenter et al. (1949)
Table 7. Effects of single inhalation exposure to HCFC 132b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse unspecified range not given 0.5 LC50 = 269 g/m3; AC50 (anaesthesia) = 71 g/m3 Raventós & Lemon (1965)
Rat Wistar derived 55 and 110 4 lethalities at 110 g/m3; rats unsteady, weak and Torkelson (1971)
drowsy at 55 g/m3
Rat Wistar derived 33-72 6 anaesthesia at 82 g/m3; kidney swelling at autopsy; Janssen (1988)
CPB-WU at all dose levels, males showed decreased growth
and testis weight, and increased liver and lung
weights
Rat Wistar derived 55 0.4 decreased respiratory rate with rapid recovery Janssen (1989b)
CPB-WU after exposure
Table 8. Effects of single inhalation exposure to HCFC 133a
Species Exposure Exposure Effectsa Reference
(strain) concentration duration
(g/m3) (min)
Mice, male unspecified 10 anaesthesia and death; convulsions on recovery; AC50 and Robbins (1946)
(white) range LC50 were 394 and 1230 g/m3, respectively
Mice (strain unspecified 30 anaesthesia, convulsions and death; AC50 and LC50 were Raventós & Lemon (1965)
unspecified) range 212 and 738 g/m3, respectively
Mice (strain 123-1230 10 rapid onset of anaesthesia, rapid recovery after cessation of Shulman & Sadove (1965)
unspecified) exposure but no convulsions; AC50 and LC50 were 397 and
1033 g/m3, respectively
Rats, female 2500 - lack of muscular coordination in 3 min, anaesthesia in 4 min Diggle & Gage (1956)
(strain unspecified) and death within 8 min
Dogs unspecified - anaesthesia at 492 g/m3, respiratory depression and arrest Shulman & Sadove (1965)
range occurred at 1131 and 1427 g/m3, and circulatory arrest at
2902 g/m3
a AC50 = calculated concentration expected to produce anaesthesia in 50% of the test group
Table 9. Acute inhalation toxicity of HCFC 123
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3)
Mouse (strain not given 30 min LC50 = 463 g/m3 Raventós & Lemon (1965)
unspecified)
Rat (Charles 129-344 4 h LC50 = 200 g/m3; loss of mobility, lethargy, prostration Hall & Moore (1975)
River CD) at all concentrations; full recovery of survivors within
30 min post exposure
Rat (Charles 49-767 6 h LC50 = 329 g/m3; anaesthesia at 145 g/m3 and higher Coate (1976a)
River CD) concentrations; discoloration of lungs in most animals that
died, discoloration of liver in some of them
Rat (strain 6, 16, 31, 62 15 min unconditioned reflexes, locomotor activity, coordination Trochimowicz (1989)
unspecified) affected at 31 and 62 g/m3; full recovery within 30 min
post exposure
Hamster 63-194 4 h LC50 = 178 g/m3; incoordination, prostration at all Darr (1981)
(Chinese) concentrations; full recovery of survivors after exposure;
0% mortality at 163 g/m3, 100 mortality at 194 g/m3
Table 10. Acute inhalation toxicity of HCFC 124
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (min)
Mouse unspecified 594 10 no effect Wada (1977)a
837 10 narcosis Wada (1977)a
2230 10 no mortality Wada (1977)a
2460 10 death Wada (1977)a
Rat Sprague-Dawley 268 240 no effect Kelly (1990)
Charles River COBS 558 300 reduced activity Coate (1976b)
Sprague-Dawley 893 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1283 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1674 240 death Kelly (1990)
Charles River COBS 2009 300 narcosis, no mortality Coate (1976b)
Dog 2230-3910 10 narcosis Van Poznak & Artusio (1960)
a Attachment to correspondence from H. Wada, Daikon Kogyo Company Ltd. to M.B. Berenbaum, Allied Chemical Corporation,
entitled Anaesthetic activity and fatality (F-123, 123a, 124 and 11)
7.1.3.1 HCFC 141b
No deaths occurred at dermal doses of 2 g/kg body weight either
among rats (Janssen & Pot, 1988; Gardner, 1988) or rabbits (Brock,
1988a).
7.1.3.2 HCFC 132b
When Janssen & Pot (1989a) applied a single dose of HCFC l32b (2
g/kg) under an occluding dressing to the shaved skin of five male and
five female Wistar rats, there were no deaths. The clinical signs
observed were decreased respiratory rate, decreased startle response,
altered locomotor activity, restlessness and vocalization. Three male
and four female rats had swollen or slightly swollen livers on
autopsy.
7.1.3.3 HCFC 123
Several limit tests for dermal toxicity of HCFC 123 were
conducted. No mortality was observed at the limit dose of 2 g/kg body
weight in rats (Brock, 1988d; Trochimowicz, 1989) or rabbits (Brock,
1988e,f; Trochimowicz, 1989). The only clinical signs of toxicity were
red nasal or ocular discharges in one of five male and one of five
female rats, and slight to moderate body weight losses (up to 12% of
initial body weight). No gross pathological abnormality was observed
(Trochimowicz, 1989). In rabbits, only slight to moderate erythema was
observed (Trochimowicz, 1989).
7.2 Short-term inhalation exposure
7.2.1 HCFC 141b
Nikitenko & Tolgskaja (1965) reported a reduction in body weight
gain, a slight decrease in haemoglobin level and moderate
leucocytosis, some "minor changes" in blood parameters related to
liver and kidney function, and histopathological effects in the
respiratory tract of rats and guinea-pigs (number and strains
unspecified) that had been exposed to 40-50 g/m3 (2 h/day, 6
days/week) for 4 weeks. The purity of the substance and the specific
isomer were not indicated.
No adverse clinical signs and only "slight biochemical changes"
(no details given) were reported in rats (number and strain
unspecified) exposed to 48.5 g/m3 (6 h/day, 5 days/week) for 2 weeks
(Pennwalt Corporation, 1987).
In a 2-week inhalation toxicity study, Doleba-Crowe (1977)
exposed groups of 10 male rats to 0 or 48 g/m3 for 6 h/day, 5
days/week. The animals were observed for 14 days after exposure. No
adverse clinical signs were observed, and there were no differences in
body weights between treated and control animals. After the tenth
exposure, elevated red blood cell counts, plasma bilirubin level and
increased urinary fluoride concentrations were found, but all these
parameters returned to normal after 14 days. The treated animals
showed a more severe focal interstitial pneumonitis than controls 14
days after exposure, but no other treatment-related change was
observed.
Coombs et al. (1988) exposed five groups of 10 male and 10 female
Sprague-Dawley rats to 0, 24, 42, 68 and 97 g/m3 (6 h/day for 9
days, i.e. 5 days of exposure followed by 1 day without exposure and
then 4 days with exposure). Signs of central nervous system (CNS)
depression were seen during exposure to concentrations of 42 g/m3 or
more. At 97 g/m3, these signs were accompanied by a decrease in body
weight gain in males and a slightly reduced food intake in both sexes.
Glucose and aspartate serum transaminase (AST) levels were increased
at 97 g/m3, protein, cholesterol and sodium from 68 g/m3,
phosphate from 42 g/m3 and calcium from 24 g/m3. No
treatment-related histopathological changes were observed at any dose
level.
In a 13-week inhalation study (some animals were killed after 4
weeks), four groups each of 15 male and 15 female Fischer-344 rats
were exposed to 0, 10, 39 or 97 g/m3 (6 h/day, 5 days/week) as
described in two reports (Yano et al., 1989; Landry et al., 1989).
Alertness was reduced at 97 g/m3, and body weight gain and food
consumption were slightly reduced in all exposed groups. After both 4
and 13 weeks of exposure, plasma cholesterol, triglycerides and
glucose were slightly elevated in the rats exposed to 97 g/m3. No
changes in haematological or histopathological parameters were found.
7.2.2 HCFC 142b
Rats and guinea-pigs (numbers and strains not specified) were
exposed to a concentration of 448 g/m3 (isomer not specified), 2
h/day, 6 days/week, for 4 weeks (Nikitenko & Tolgskaja, 1965). A
decrease in the rate of body weight gain was observed at the end of
the study, as well as a reduction in haemoglobin concentration and the
number of erythrocytes, and an increase in the number of leucocytes.
Swelling of the alveolar septa and peribronchitis were the
histopathological changes observed in the lungs.
In a study in which 10 adult white rats were exposed to 410
g/m3 for 16 h/day, all animals died within 9 exposures. All of them
showed severe signs of pulmonary irritation at autopsy (consolidation
and hepatization of the lungs). The other organs appeared normal. No
signs of ill health were apparent in five rats exposed to a
concentration of 41 g/m3 (16 h/day for 2 months). Gross examination
of the organs on autopsy revealed no pathological changes, but
microscopic examination of the lungs showed round cell infiltration in
the lung of two animals. The appearance of sections of the livers was
normal (Lester & Greenberg, 1950).
No clinical, haematological, blood chemical, urine analytical or
histopathological evidence of effects attributable to repeated
exposure to HCFC 142b was found in 10 male Charles River CD rats
exposed to a concentration of 82 g/m3 (6 h/day, 5 days/week) for 2
weeks (Moore & Trochimowicz, 1976).
Kelly (1976) did not find any adverse clinical, haematological,
blood chemical, urine analytical or histopathological effects
attributable to HCFC 142b at exposure levels of either 4.1 g/m3 or
41 g/m3 (6 h/day, 5 days/week for 90 days) in groups of male and
female Charles River CD rats (27 of each sex at each treatment level)
or groups of male dogs (4 at each treatment level).
7.2.3 HCFC 132b
When 20 male Crl:CDRBR rats were exposed to 55 g/m3 (6 h/day,
5 days/week) for 2 weeks, reduction in body weight gain, irregular
respiration and CNS depression (lethargy, poor coordination,
occasional tremors and prostration) were seen. The CNS effects
disappeared within 30 min after each exposure. Pathological
examinations of rats sacrificed immediately after the tenth exposure
showed thymic atrophy and spermatogenesis arrest, but these changes
were not present in rats sacrificed 14 days after exposure ceased
(Hall, 1976).
Groups of 20 male and 20 female Crl:CDRBR rats were exposed to
0, 3, 11 and 27 g/m3 (6 h/day, 5 days/week) for 13 weeks (Kelly,
1988). Male rats exposed at all the concentrations of HCFC 132b showed
bile duct proliferation and disruption of spermatogenesis with cell
debris in the epididymides at the two higher concentrations. Other
effects included increases in liver/body weight ratio in males at all
concentrations and in females at the two higher concentrations.
Elevation of serum alkaline phosphatase activity was found in both
sexes exposed to 11 or 27 g/m3. During the study, all groups exposed
to HCFC 132b showed reduced food consumption and body weight gain. In
the two highest exposure groups there were depressions in the absolute
but not relative brain and testes weights. Other organ weight changes
were also seen (slight increases in heart, lung and kidney weights).
The biological significance of these weight changes is not clear since
there were no accompanying histological findings. During exposure to
27 g/m3, rats showed CNS depression as indicated by decreased
activity and low responsiveness to sound.
7.2.4 HCFC 133a
Shulman & Sadove (1965) exposed mice to anaesthetic
concentrations (the actual concentration was not specified) of HCFC
133a for 30 min per day on 12 consecutive days, and the animals were
killed for pathological evaluation after the last exposure by
overdosage of HCFC 133a. None of the mice showed any treatment-related
clinical effects, and no pathological changes were found in the organs
(heart, lung, liver, kidney, adrenal gland, spleen and pancreas)
examined microscopically.
Diggle & Gage (1956) investigated the effects of repeated
exposure (up to 8 days) of groups of 2-3 female rats. Concentrations
of HCFC 133a between 50 and 125 g/m3 caused incoordination and
lethargy, while at 250 or 500 g/m3 rats become comatose. They
recovered between each exposure and no dose-related pathological
changes were found on histological examination. No effect was seen at
25 g/m3 during seven exposures lasting 6 h/day.
In a study by Leuschner et al. (1977), 20 male and 20 female
Sprague-Dawley rats were exposed to 49 g/m3 (6 h/day, every day) for
90 days. Corresponding groups of 20 male and 20 female rats were used
as controls. Observations for overt clinical signs of toxicity and
investigations on body weight, food consumption, haematology, blood
and urine biochemistry, urine sediments, ophthalmology, auditory
reflex, organ weights, and histopathology were performed. There were
no treatment-related deaths. The rats were sedated during each
exposure but appeared normal before and after. Seventeen out of 40
rats developed bloody and inflamed noses; this was associated with
histological evidence of inflammatory changes of the mucosa. Body
weight gain was reduced, so that the terminal average body weights
were approximately 28 and 17% lower than those of male and female
controls, respectively. Food consumption in the treated groups was
also lower than in the controls. Haemoglobin concentration,
haematocrit, red blood cell counts and platelet counts were all
slightly reduced. Reduction in leucocyte counts of approximately 30%
and increase in reticulocyte counts of approximately 40% were seen.
There were reductions in plasma glucose levels of approximately 15%
and in protein levels of approximately 10%. Bromosulfophthalein
retention time was increased by approximately 35% and 62% in males and
females, respectively. There was no change in plasma enzyme
glutamic-pyruvic transaminase (GPT), alkaline phosphatase (AP) or
glutamicoxalacetic transaminase (GOT) activity. The thymus to body
weight ratio was reduced by approximately 50% and the testis and ovary
to body weight ratios by approximately 60 and 35%, respectively.
Histologically, these organs showed atrophy. Thyroid to body weight
ratio was increased by approximately 45% in males only. Atrophy of the
spleen was also observed. The exposure induced emphysema and oedema of
the lungs as well as bronchitis and pneumonia. The testicular atrophy
was consistent with the findings in three dominant lethal studies in
mice (Hodge et al., 1979, 1980; Kilmartin et al., 1980) and a
carcinogenicity study in rats (Longstaff et al., 1984) (see section
7.6 and 7.7).
Six beagle dogs were exposed by Leuschner (1977) to 24 g/m3
(6 h/day, daily) for 3 months and six control dogs were used. No
effects were seen on external appearance, faeces, food and water
consumption, body weight gain, haematology, blood and urine
biochemistry, urine sediments, electrocardiography, blood pressure,
ophthalmology, hearing or dentition. There was no effect on organ
weight at autopsy. No treatment-related histopathological changes were
seen on microscopic examination of a standard range of 24 tissues.
In two dominant lethal studies (for experimental details see
section 7.5.1.4), male mice were exposed to between 0.5 and 49 g/m3,
6 h/day, for 5 days (Hodge et al., 1980; Kilmartin et al., l980). The
mice were subdued at exposure concentrations of 2 g/m3 or more.
Deaths occurred as follows: in the first study 0/60 mice died at 0
g/m3, l7/60 at l2 g/m3, and 28/80 at 49 g/m3; in the second
study 0/80 died at 0 g/m3, 2/59 at 0.5 g/m3, 0/60 at 2.5 g/m3,
5/60 at 5 g/m3, and 20/60 at 12 g/m3.
7.2.5 HCFC 123
In a study by Doleba-Crowe (1978), Sprague-Dawley rats and beagle
dogs were exposed to concentrations of 0, 6 and 62 g/m3 (6 h/day, 5
days/week) for 90 days. At the high exposure level, both species
exhibited lack of motor coordination soon after the start of exposure.
This was followed by reduced motor activity and reduction in
responsiveness to noise. After removal from exposure, coordination and
activity returned to normal within 20 min. Other than final body
weight reductions and increased urinary fluoride level at both
exposure levels, no significant exposure-related effects were observed
in rats. At the high exposure level, dogs exhibited histopathological
changes in the liver and clinical chemistry changes including
increased levels of serum GOT and GPT, which might indicate slight
liver damage. No exposure-related effect was noted at the lower
exposure level.
In a 90-day inhalation study, albino rats obtained from Charles
River Breeding Laboratory were exposed to nominal levels of 0, 3, 6,
and 31 g/m3, 6 h/day, 5 days/week (Rusch, 1985). No
treatment-related deaths occurred in the study and mean body weight
reductions observed in the males at the highest exposure level and in
females at the two highest exposure levels were significant only at
the end of the study. Slight depression was observed in heart weight
in both male and female rats exposed to 31 g/m3. While depressions
of the kidney weights and kidney/brain weight ratio (but not kidney to
body weight ratio) were observed in male rats in all the three
exposure groups, these effects were outside the normal range only at
the highest exposure level. A similar depression in kidney weight and
kidney/body weight ratio (but not in kidney/brain ratio) occurred in
females at the highest exposure level. Increased liver/body weight
ratios (but not liver weight or liver/brain weight ratios) were
observed in all three exposure groups of females, but in males only at
the highest exposure level. No significant difference was found in
organ weights or ratios in animals sacrificed at the end of a 30-day
recovery period. The absence of histopathological findings and of
effects at the end of the recovery period indicates that the
toxicological significance of the organ weight changes in this study
is unclear.
In a 4-week inhalation toxicity study (Kelly, 1989; Trochimowicz,
1989), rats (10 of each sex at each exposure level) were exposed to 0,
6, 31, 62 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Statistically significant body weight depression occurred in all
female groups and in the two highest male groups, but was dose-related
only in the male rats. At concentrations of 31 mg/m3 or more, rats
exhibited dose-related anaesthesia. At 62 and 125 g/m3, rats became
lethargic during exposure but were normal when they were examined
again 16 to 18 h after exposure. A dose-related increase in liver/body
weight ratio was observed in all female groups (a 27% increase at the
highest level) and in the two highest-exposure male groups (an 18%
increase at the highest level). Decreased cytochrome P-450 activity in
the liver was also found in all female exposure groups and in the male
groups exposed at the two highest levels. Histopathological
examination showed no adverse effects attributable to HCFC 123 in
liver or in any other organ at any exposure level. Microscopic
examination revealed that testicular degeneration and hypospermia
occurred in two out of four male exposure groups, but this was
believed to be due to reduced body weight (because of an increase in
relative testicular weight without a corresponding increase in
absolute testicular weight) or may have resulted from spontaneous
lesions. The fact that the incidence of testicular degeneration was
highest in the 31- and 125-g/m3 exposure groups (5 out of 10 and 6
out of 10 animals, respectively), and lower in the 0-, 6-, and
62-g/m3 exposure groups (2 out of 10, 1 out of 10 and 2 out of 10
animals, respectively) is consistent with the sporadic nature of this
lesion in this strain of rat. However, it is not possible to evaluate
the significance of this testicular effect from the information
available.
Another 28-day inhalation toxicity study in rats was conducted to
characterize further the potential effects of HCFC 123 on the liver
(Lewis, 1990). In addition, urine samples were examined to identify
metabolites of HCFC 123. Groups of six male Charles River CD rats were
exposed to 0, 6, 31 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Body weights were statistically significantly reduced in all treated
groups compared to controls, the greatest reduction occurring in the
high-dose group. A concentration-related decrease in serum cholesterol
levels was found in all test groups. This reduction was statistically
significant compared to controls at the medium and high
concentrations. Serum triglyceride levels were significantly reduced
to a similar extent in all treated groups. A statistically significant
increase in absolute and relative liver weights compared to controls
was seen in the high-dose rats. Hepatocyte hypertrophy and mild fatty
vacuolation was found at all concentrations tested but the severity
was greatly reduced at the low exposure level. Electron microscopic
examination revealed a treatment-related induction of peroxisome
proliferation at the medium and high exposure concentrations. A
statistically significant concentration-related increase in relative
testes weight was observed in all treated groups (11-30% above
control), but no compound-related morphological or microscopic changes
were observed. Urine analysis indicated the presence of
trifluoroacetic acid as a major metabolite.
In a study by Malley (1990a), groups of 10 male and 10 female
Crl:CDRBR rats were exposed to concentrations of 0, 2, 6 or 31
g/m3 (6 h/day, 5 days/week) for 90 days. No effect on food
consumption or body weight was observed. At the highest exposure level
the animals exhibited anaesthesia and a decreased response to auditory
stimuli. Serum triglyceride and glucose levels were significantly
decreased at all exposure levels, while serum cholesterol was
significantly lower in females exposed to the two highest
concentrations. The mean lymphocyte and white blood cell counts in
female rats were decreased at the highest exposure level. Male rats
had significantly higher alanine aminotransferase and alkaline
phosphatase activity at the two highest exposure levels. In female
rats alanine aminotransferase activity was elevated at the highest
exposure level. Urine fluoride concentrations were increased in
females at all exposure levels, but in males only at the highest
exposure level. Absolute liver weights were significantly higher in
male rats at the highest exposure level and in female rats at the two
highest exposure levels, while relative liver weights in both male and
female rats were higher at the two highest exposure levels. Hepatic
peroxisomal beta-oxidation activity in both male and female rats was
1.9 to 3.8 times higher than in controls, indicating an induction of
hepatic peroxisome proliferation. No treatment-related gross or
microscopic liver changes were observed.
7.2.6 HCFC 124
When ten male rats were exposed to approximately 560 g/m3 (6
h/day, 5 days/week) for 14 days, no adverse haematology, clinical
chemistry, urine analysis or histopathological changes were observed.
Rats showed irregular respiration, lethargy and poor coordination
(Hall, 1976).
Trochimowicz et al. (1977) reported no adverse effect following
the clinical and histopathological evaluation of rats exposed to
concentrations of 560 g/m3 (6 h/day, 5 days/week) for 2 weeks.
Brewer (1977) exposed groups of 60 Sprague-Dawley rats (35 males and
25 females) to concentrations of 0, 3, 5 or 28 g/m3 (6 h/day, 5
days/week) for 3 months. Ten male and ten female rats were examined
and sacrificed after 45 days and a similar number after 92 days. Ten
male and five female rats of each group were maintained without
further exposure for an additional 30-day period after exposure.
Clinical signs were observed, body and organ weights determined, and
haematological, biochemical and histopathological examinations carried
out on all animals. No statistically significant differences in body
weight gain were noted. Haematology, clinical chemistry, and urine
analysis in treated rats were normal and comparable to findings in
control animals. Urinary fluoride excretion was increased after 45
days of exposure in both males and females (4.0 and 3.5 times,
respectively) at an exposure level of 28 g/m3; at the two lower
levels, determinations were not made. After 95 days of exposure, the
urinary fluoride level was elevated only in males at all three
exposure levels (by 1.5, 1.7 and 1.8 times, respectively), and this
effect persisted throughout the 30-day period after exposure. Gross
and histopathological examinations did not reveal any
treatment-related changes in any group of animals. Statistically
significant difference in organ weights between treated and control
rats were found. Liver weights were increased significantly in males
at 5 and 28 g/m3, while the lung and adrenal gland weights were
significantly decreased in males at all three exposure levels. In the
absence of any histopathological change, the biological significance
of these organ weight changes is unclear.
Malley (1990b) exposed groups of ten male and ten female rats to
HCFC 124 concentrations of 0, 3, 11, 56 or 279 g/m3 (6 h/day, 5
days/week) for 4 weeks. Treatment-related effects on body weight, food
consumption, mortality, clinical laboratory parameters, organ weights,
and tissue morphology changes were not found at any exposure level.
During exposure, rats exposed to 279 g/m3 were lethargic and
uncoordinated. However, no evidence of lethargy and incoordination was
observed shortly after exposure. The author considered the exposure
concentration of 56 g/m3 the no-observed-adverse-effect level
(NOAEL) based on the clinical observation of lethargic and
uncoordinated movement during exposure to 279 g/m3, which was not
observed at 56 g/m3.
Malley (1991) exposed Crl:CDRBR rats (20 rats of each sex at
each exposure level) to HCFC 124 at concentrations of 0, 28, 84 and
279 g/m3 (6 h/day, 5 days/week) for 90 days. As part of this study,
a functional observation battery (FOB) was conducted on 10 rats of
each sex at each exposure level at various intervals during the course
of exposure. There were no compound-related effects relative to body
weight, food consumption, mortality, haematology, organ weights or
histopathology at any exposure concentration. However, male rats, at
a level of 84 and 279 g/m3, had lower serum triglyceride
concentrations and a decreased arousal (4 of 10 and 6 of 10 rats,
respectively). Persistent decrease of forelimb grip strength was found
in high-dose females. However, there was no associated decrease in
hindlimb grip strength or change in gait or footplay. Females at the
highest concentration showed an increase in alkaline phosphatase. Both
sexes at 279 g/m3 were less responsive to auditory stimuli, as
demonstrated by a decreased reaction to a sharp knock on the chamber
wall. Plasma fluoride, urinary fluoride and fractional clearance of
free fluoride were also increased at all exposure levels in both
sexes. The author concluded that the no-observed-effect level (NOEL)
was 28 g/m3 for male rats and 84 g/m3 for female rats.
7.3 Skin and eye irritation; sensitization
7.3.1 Skin and eye irritation
7.3.1.1 HCFC 141b
Treatment of the intact skin of New Zealand albino rabbits with
0.5 ml of undiluted HCFC 141b under occlusive patch (during 4 and 24
h in the two respective studies) did not produce signs of dermal
irritation during a 3-day observation period (Liggett, 1988a; Brock,
1988b).
Two studies were conducted with HCFC 141b on groups of six New
Zealand albino rabbits where the undiluted compound (0.1 ml) was
instilled into the eyes. No signs of irritation occurred within 3 days
in one study (Liggett, 1988b), but the compound was found to be a
"mild" irritant in the other study (Brock, 1988c). The majority of
rabbits in the latter study showed conjunctival redness (5/6), mild
chaemosis (3/8) and blood-tinged discharge (4/6).
7.3.1.2 HCFC 142b
Brittelli (1976a) observed no effects on the cornea or iris but
slight conjunctival swelling with some discharge in an eye irritation
test with HCFC 142b.
7.3.1.3 HCFC 132b
One drop (approximately 0.05 ml) each of 100% HCFC 132b and a 10%
solution in propylene glycol was applied and slightly rubbed into the
shaved intact shoulder skin of 10 male albino guinea-pigs, but the
area was not occluded. The pure compound produced only mild irritation
in one animal only. No irritation was induced by the 10% solution
(Goodman, 1976).
Undiluted HCFC 132b (0.1 ml) was placed into the right
conjunctival sac of two albino rabbits, and after 20 seconds one
treated eye was washed with water for 1 min. Observations of the
cornea, iris and conjunctiva were made after 1 and 4 h, and 1, 2, 3
and 7 days later. Slight corneal opacity and "mild" to "moderate"
conjunctival irritation were seen in both rabbits up to 3 days after
dosing, but had disappeared by 7 days after dosing (Brittelli, 1976b).
7.3.1.4 HCFC 123
Minimal dermal irritation with HCFC 123 was observed in rabbits
(Brock, 1988e,f). HCFC 123 (purity 99.0%) produced no skin irritation
when 0.5 ml/6 cm2 was applied to the clipped intact skin of four
male and two female New Zealand rabbits for 4 h (Trochimowicz, 1989).
Brittelli (1976c) reported HCFC 123 to be a "mild" ocular
irritant causing reversible corneal opacity in rabbits. In another
study by Daly (1979)a, HCFC 123, when instilled undiluted (0.1 ml)
into the conjunctival sac of the rabbit eye without subsequent
washing, produced "mild" to "moderate" conjunctival irritation. With
washing, "mild" transient corneal opacity and "mild" to "moderate"
conjunctival irritation were observed. With or without washing,
complete recovery occurred within 3-7 days.
7.3.2 Skin sensitization
7.3.2.1 HCFC 141b
No delayed contact hypersensitivity was found in any of the 20
Hartley Dunkin guinea-pigs in a Magnusson-Kligman maximisation test
with HCFC 141b (Kynoch & Parcell, 1989).
7.3.2.2 HCFC 132b
A series of four sacral intradermal injections of HCFC 132b was
given (once per week at 7-day intervals) over a 3-week period to
groups of nine male albino guinea-pigs (0.1 ml of a 1% solution in
dimethyl phthalate). Fourteen days after the last application, the
animals were challenged with either 1 drop (0.05 ml) of undiluted
liquid or a 19% solution of test material in propylene glycol on the
shaved skin. No evidence of sensitization was observed (Goodman,
1976).
7.3.2.3 HCFC 123
When applied topically to the back of male guinea-pigs as 10% or
50% solutions in propylene glycol, HCFC 123 produced no sensitization
at challenge (Goodman, 1975; Daly, 1979).
7.4 Long-term exposure
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternative Fluorocarbon Toxicity Testing (Rusch, 1989) sponsored
by an international industry consortium. An interim report after the
first year of the study is available on HCFC 123 (Malley, 1990b).
a Personal communication entitled "Toxicity testing summary for
alternative fluorocarbons" by J.J. Daly to CFTA Interindustry
Safety Committee, Wilmington, Delaware, USA, E.I. Du Pont de
Nemours and Co.
7.4.1 HCFC 142b
Four groups each containing 130 male and 110 female Sprague-
Dawley CD rats were exposed by inhalation to concentrations of 4.1, 41
and 82 g/m3 (6 h/day, 5 days/week) for 104 weeks. No exposure-
related effects were found on mortality, body weight, haematology,
clinical chemistry, urine analysis, histopathological or
ophthalmological findings (Seckar et al., 1986). However, high
mortality (more than 50% by the end of the study) was seen in all
groups including controls.
7.4.2 HCFC 123
Findings associated with the first year of the PAFT-supported
study were provided by Malley (1990c). Following 12 months of
inhalation exposure to 0, 2, 6 or 31 g/m3, 10 rats (Crl:CDRBR) of
each sex at each concentration were sacrificed, selected organs were
weighed, and tissues were examined for gross and microscopic lesions.
Body weight and body weight gain were significantly lower in high-dose
males and in mid- and high-dose females. Food consumption was higher
and food efficiency was lower in high-dose males and females over the
course of the l year of exposure. The lower food efficiency appeared
to be directly related to the lower body weight gain at the high
concentration (31 g/m3). High-dose males and females exhibited
anaesthesia-like behaviour (e.g., less responsiveness to auditory
stimuli compared to control rats). No compound-related effects on
mortality or survival time were found in any treated group. Clinical
chemistry parameters measured at 6 months and 1 year revealed several
compound-related changes. The most noteworthy findings were the effect
on lipid and carbohydrate metabolism. Significant decreases of serum
triglyceride and glucose concentrations were found in males and
females at all dose levels. Serum cholesterol was significantly lower
in females at all dose levels and in high-dose males. Urine analysis
indicated significant increases of urinary fluoride concentration in
both sexes at all dose levels. High-dose (31 g/m3) males and females
had significantly higher mean relative liver weights, but no
compound-related gross or histopathological changes were found in the
livers of exposed animals. Dose-related increases in hepatic
beta-oxidation enzyme activity were found for males (2.3, 3.1 and 4.0
fold increases at 2, 6 and 31 g/m3, respectively) and females (1.7
and 3.1 fold increase at 6 and 31 g/m3). The higher enzyme activity
indicated an induction of hepatic peroxisome proliferation. However,
compound-related differences in the rate of cell proliferation, as
measured by changes in labelling index, were not found at any exposure
concentration. These data indicate that during the first year of
exposure, HCFC 123 did not induce an increase in regenerative repair
of the liver, which is consistent with the absence of morphological or
microscopic changes in the liver of exposed animals. A
no-observed-effect level (NOEL) was not achieved in this study based
on the effects on clinical chemistry parameters and higher hepatic
peroxisomal activity.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
7.5.1.1 HCFC 141b
A 2-generation reproduction study on HCFC 141b is currently in
progress (Rusch, 1989).
7.5.1.2 HCFC 142b
In a dominant lethal study on rats, no effect on male
reproduction was found (Seckar et al., 1986). No other studies on
reproductive effects are available.
7.5.1.3 HCFC 132b
No data are available on the effects of HCFC 132b.
7.5.1.4 HCFC 133a
No studies are available in which the effects of HCFC 133a on
reproduction were investigated. Some information is available,
however, on the effects on male fertility from a series of dominant
lethal and "combined dominant lethal fertility" studies in mice (Hodge
et al., 1979, 1980; Kilmartin, 1980). In these studies groups of male
mice were exposed to between 0 and 98 g/m3, 6 h/day, for 5
consecutive days. At the end of the treatment, dominant lethal and
fertility effects were assessed in 15 males (30 controls) from each
treatment group, which were each housed with two virgin females for
four consecutive nights. This 4-nightly mating procedure was continued
for 8-9 consecutive weeks. In the two later studies, satellite group
males (at least two from each group per week) were killed, their
epididymal sperm assessed for abnormalities and their testes examined
histopathologically. A reduction in male fertility (seen as
exposure-related decreases in the proportion of pregnant females:
e.g., 0-60% of pregnant females in treatment groups compared to
90-100% in controls) was seen between weeks 2-8 following exposure.
The severity of the effects were related to time, the maximum
reductions in pregnancies being seen at around 3-6 weeks after
exposure. No effect on fertility was seen at 0.5 g/m3. Reduced
fertility was observed at concentrations of 2.5 g/m3 or more, and
histopathological evidence of degeneration of spermatogenic cells was
seen at concentrations of 5 g/m3 or more. There was a slight and
transient increase in the percentage of abnormal sperm at 2.5 g/m3
or more.
7.5.1.5 HCFC 123
A 2-generation inhalation reproduction study on rats sponsored by
PAFT is currently in progress (Rusch, 1989).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 HCFC 141b
Hughes et al. (1988) exposed three groups of 25 pregnant female
Sprague-Dawley rats to 15, 39 or 97 g/m3 for 6 h/day from days 6 to
15 of pregnancy. Some signs of maternal toxicity (prenarcotic signs,
piloerection and reduced alertness) were observed at all exposure
levels. At the highest level, salivation, hunched posture, and
diaphragmatic breathing, a marked increase in water consumption, a
transient reduction in food intake, and a marginal reduction in body
weight gain were observed. At the highest exposure level, incidences
of subcutaneous oedema and haemorrhaging and embryonal death were
significantly increased. Reduced litter and mean fetal weights and
retarded ossification were observed. However, no teratogenic effect
occurred in any group.
In a further study (Hughes et al., 1989), 16 pregnant female New
Zealand rabbits were exposed to 7, 20 or 61 g/m3 for 6 h/day from
days 7 to 19 of pregnancy. Signs of maternal toxicity (prenarcotic
signs, palpebral ptosis, respiratory disturbances and body weight
loss) were observed at the two highest exposure levels. There was no
indication of any treatment-related effect on embryo or fetal
development or evidence of teratogenicity at any exposure level.
7.5.2.2 HCFC 142b
Groups of 25 pregnant Sprague-Dawley rats were exposed to 4 or 41
g/m3 for 6 h/day from day 3 to day 15 of gestation (Culik & Kelly,
1976). The exposure had no effect on the body weight gain of the
mothers, and no clinical signs of toxicity were observed in any of the
animals. The number of early or late resorptions or number of live
fetuses per litter was not affected by the exposure. Exposure also had
no effect on embryonic development, as measured by the weight and
crown-rump length of the fetuses, and there was no evidence of a
teratogenic effect.
Damske et al. (1978) exposed groups of 20 pregnant female
Sprague-Dawley CD rats to concentrations of 0, 13 and 39 g/m3 for 6
h/day on days 6-15 of gestation. There were no treatment-related
effects in the dams or evidence of exposure-induced terata, variations
in sex ratio, embryotoxicity or inhibition of fetal growth and
development. There was an increase in the incidence of delayed
ossification of the supraoccipital bone in both exposure groups; this
effect was not observed in the control group.
7.5.2.3 HCFC 132b
In a pilot study, groups of seven or eight pregnant rats were
exposed to 3, 11 or 28 g/m3 for 6 h/day on days 6-15 of gestation.
Maternal and fetal body weights were reduced at all exposure levels.
The number of resorptions was increased in the 11- and 27-g/m3
exposure groups (Alvarez, 1988).
In a development screening test using Hydra (Johnson et al.,
1986), embryotoxicity was observed at paternally toxic doses.
7.5.2.4 HCFC 133a
Weigand et al. (1977) investigated the potential embryotoxic or
teratogenic effects of HCFC 133a in Wistar rats and Himalayan rabbits.
They also examined the effects of pre-treatment with progesterone to
determine whether any of the adverse effects of HCFC 133a could be
explained by its interaction with and depletion of progesterone in
early pregnancy, since this hormone is responsible for the electrical
and mechanical quiescence of the myometrium. Twenty-four pregnant
Wistar rats, of which 12 were injected subcutaneously with
progesterone (6 mg/day) prior to HCFC 133a exposure, were exposed to
25 g/m3 for 6 h/day on days 7-16 of gestation (the day on which
sperm was found in the vaginal smear was taken as day 1 of gestation).
Twelve pregnant Himalayan rabbits were exposed to the same
concentration on days 7-19 of gestation (the day of mating being taken
as day 0 of gestation). No controls were used and data were compared
with historical control data. Mild transient sedation, piloerection,
reduced body weight gain and reduced food consumption were observed in
the rats. At autopsy on day 21 of gestation, no macroscopic change was
observed. There was a prenatal mortality of 77% in the dams with no
pre-treatment with progesterone and 82% in the pre-treated dams.
Placental weight, fetal weight and crown-rump length were reduced and
some of the surviving fetuses showed generalized oedema (8/53) and
external anomalies of the limbs and tail (5/53). There was no
significant difference between the rats pre-treated with progesterone
and those without pre-treatment. In rabbits, there were reductions in
body weight gain and food consumption. All animals showed vaginal
bleeding during the last three days of the exposure, and 4 out of 12
aborted. By autopsy on day 29 all fetuses had died.
Culik & Kelly (1979) exposed Charler River CD rats to approximate
concentrations of 0, 2.5, 10, 25 or 98 g/m3 for 6 h/day on days 6-15
of gestation, and the rats were subjected to autopsy on day 21 of
gestation. There was no clear evidence of maternal toxicity. Evidence
of embryotoxicity was observed at all exposure levels. In the control
and the 2.5-g/m3 exposure groups, there was neither fetal death nor
total litter resorption. Fetal weights and crown-rump lengths were
lower in all the groups exposed to HCFC 133a than in controls. At 10
g/m3, 4/21 pregnant females had total resorptions and only 67
fetuses were alive in the other females. At 25 g/m3, 37/41 pregnant
females had total resorptions and only 7 fetuses were alive in the
other females. At 98 g/m3, 22/23 pregnant females had total
resorptions and only one fetus was alive in the other female.
Treatment-related increases in the incidence of runts and delayed
ossification in several bone structures were also observed at all
exposure levels. An increased incidence of hydronephrosis was reported
in all treated groups. Thus, the no-observed-effect level for
embryolethality was 2 g/m3 but, in view of the reduced fetal size
and weight at this exposure level, the no-observed-effect level for
embryotoxicity was not established from this study.
7.5.2.5 HCFC 123
Kelly et al. (1978) exposed 25 pregnant female rats to 62 g/m3
for 6 h/day on days 6-15 of gestation. Dams and fetuses were
sacrificed on day 21 and examined for gross changes. No embryotoxicity
or teratogenic effects were seen.
Two groups of 20 pregnant female rats were exposed to 0 and 31
g/m3 for 6 h/day on days 6-15 of gestation (Rusch, 1985). The
animals were sacrificed on day 20 and all dams and fetuses examined.
The maternal mean body weight in the exposed group was depressed to a
statistically significant degree on days 12 and 15 of the gestation
period. At termination, maternal mean body weights were still
depressed but not to a statistically significant degree. The numbers
of corpora lutea, implantation sites, resorption sites and fetuses
were similar in control and treated dams.
In a range-finding study, groups of six pregnant rabbits were
exposed to HCFC 123 concentrations of 0, 6, 31, 62 and 125 g/m3 for
6 h/day on days 6-18 of gestation (Schroeder, 1989a). All treated
rabbits lost weight during the study and food consumption was markedly
reduced, particularly at the two highest exposure levels. At these two
levels an increased number of resorption was also observed. In the
final study (Schroeder, 1989b; Trochimowicz, 1989), 24 mated females
per exposure group were exposed to 3, 9 or 31 g/m3 for 6 h/day
during days 6-18 of gestation. No mortality was observed in the
control or the low or medium exposure groups. The death of one rabbit
in the high exposure group was not considered by the author to be
treatment-related. There was evidence of maternal toxicity during days
6-18 of gestation at all exposure levels. Statistically significant
treatment-related mean body weight losses were observed in all the
test groups during the exposure period, compared to the control group
which showed a slight mean body weight gain. Mean daily food
consumption was also statistically lower in test groups than in
controls on most exposure days. There was no evidence of embryotoxic,
fetotoxic or teratogenic effects.
7.5.2.6 HCFC 124
Brewer & Smith (1977) exposed a group of 20 pregnant Charles
River CD rats to 30 g/m3 for 6 h/day during days 6-15 of gestation.
Maternal body weight measurements and clinical observations did not
reveal any difference between exposed and control groups, and no
maternal deaths occurred. The incidence of resorption sites in the
treated group was higher than in controls but within the range
commonly experienced with the strain of rats employed. The numbers of
corpora lutea, implantation sites, and fetuses in the treated groups
were similar to those in the controls. Fetal body weights were not
altered by treatment.
In a recent range-findings study, Rickard (1990b) exposed
Sprague-Dawley rats (4-6 per group) to minimal HCFC 124 concentrations
of 0, 3, 11, 56 or 279 g/m3 for 6 h/day on days 6-15 of gestation.
No change in mean body weight gain or evidence of embryotoxic effects
was observed. Internal and skeletal examinations were not conducted;
only external examination of fetuses was performed.
Schroeder (1991), in a range-finding study, exposed New Zealand
white rabbits (7 per group) to nominal HCFC 124 concentrations of 0,
28, 84 and 279 g/m3 for 6 h/day on days 6-18 of gestation. The only
sign of maternal toxicity was decreased activity during exposure at
the two highest concentrations. There was no evidence of
embryotoxicity, fetotoxicity or teratogenicity at any exposure
concentration.
7.6 Mutagenicity
7.6.1 HCFC 141b
The data from in vitro and in vivo studies are summarized in
Table 11.
HCFC 141b did not appear to damage bacterial DNA in a repair
assay, but produced conflicting evidence for mutagenicity in other
bacterial assays. Although sample purities differed in the two assays,
this alone may not account for the difference in response.
No significant response was obtained in the in vitro V79 cell
hprt locus assay.
Chromosomal aberrations were induced in two in vitro studies
with CHO (Chinese hamster ovary) cells, but this activity was not
apparent either in one assay with cultured human lymphocytes or in two
in vivo studies for micronucleus induction in mouse bone marrow
cells.
7.6.2 HCFC 142b
The data from in vitro and in vivo studies are summarized in
Table 12.
There was evidence of induction of base substitution mutations by
HCFC 142b in four of the five bacterial tests conducted. In two of
these studies this effect was apparent only in the presence of
exogenous metabolic activation.
A positive response, but without supporting data, was reported in
a single BHK (baby hamster kidney) 21 cell transformation assay.
No evidence of mutagenic activity of HCFC 142b was seen in a bone
marrow cytogenetic test or a dominant lethal study in rats.
7.6.3 HCFC 132b
The data from in vitro studies are summarized in Table 13.
HCFC 132b was tested in bacterial assays (three studies) and in
an in vitro chromosomal aberration test using a CHO cell line.
There was no evidence of mutagenic potential of HCFC 132b in
these studies.
7.6.4 HCFC 133a
The data from in vitro and in vivo studies are summarized in
Table 14.
HCFC 133a did not induce mutations in bacteria and did not
increase the proportion of BHK 21 cells forming colonies in soft agar.
Chromosomal aberrations were not induced in a single rat bone
marrow test using high concentrations. There were small, statistically
significant increases in the incidences of early deaths in two of
three male mouse dominant lethal assays. These increases occurred in
mating weeks 6-8 in one study and in mating week 7 in the other, in
which the lowest effective dose was 12.3 g/m3. No increases in early
deaths were observed in the third study, in which the highest
concentration tested was 12.3 g/m3.
7.6.5 HCFC 123
The data from in vitro genotoxicity studies are summarized in
Table 15.
HCFC 123 showed no evidence of mutagenic potential when tested in
suspension and plate assays using bacteria or yeasts.
In vitro cytogenetic tests were performed using cultured human
lymphocytes, in which HCFC 123 was tested both in the liquid and
gaseous phase. There was evidence of clastogenic activity in both the
presence and absence of exogenous activation systems, when HCFC 123
was tested in the gaseous phase; in the liquid phase study, an
increase in chromosomal aberrations was seen in the absence of
exogenous activation.
Table 11. Genetic toxicity of HCFC 141b
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Escherichia coli, DNA repair - - 10 mg/ml, Hodson-Walker & May (1988b)
18 hc
Salmonella typhimurium, reverse + + 1455 g/m3, 99.6% purity, positive Hodson-Walker & May (1988a)
mutation with TA98, TA100, TA1535, 48 hc in TA1535, negative
TA1537, TA1538 in other strains
S. typhimurium, reverse mutation with - - 1455 g/m3, 99.95% purity May (1989)
TA98, TA100, TA1535, TA1537, TA1538 48 hc
E. coli, reverse mutation with WP2 uvrA - - 1455 g/m3, 99.95% purity May (1989)
48 hc
Gene mutation in vitro, V79 cells, hprt - - 1430 g/m3, 99.6% purity Bootman et al. (1988a)
locus 3 hc
Chromosomal aberrations in vitro, CHO ± - 1 mg/ml liquid phase; increase Wilmer & De Vogel (1988)
cells in gaps only
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.6% purity Bootman & Hodson-Walker
cells 3 hc (1988)
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.83% purity Hodson-Walker (1990a)
cells 4 hc
Table 11 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations in vitro, human - - 1700 g/m3, 99.83% purity Hodson-Walker (1990b)
lymphocytes 3 h (+ema)c
243 g/m3,
24 h (-ema)
Micronucleus test in vivo, mouse bone - NA 165 g/m3, 99.98% purity; marrow Vlachos (1989)
marrow 6 h sampled at 24, 48 and
72 h
Micronucleus test in vivo, mouse bone - NA 95 g/m3, marrow sampled at 24, Bootman et al. (1988b)
marrow 6 h 48 and 72 h
a NA = not applicable; ± = inconclusive
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
c in vitro assay in an enclosed system
Table 12. Genetic toxicity of HCFC 142b
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 1640 g/m3, negative in all Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hd strains
TA1537
S. typhimurium, reverse mutation with + + 2050 g/m3, weakly positive only Koops (1977)
TA98, TA100, TA1535, TA1537, TA1538 48 hd in TA1535 (2-8 fold
increase at 2050 g/m3)
S. typhimurium, reverse mutation with + + not specifiedd single gaseous conc. Jagannath (1977)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 1-72 h, positive in
TA100 and TA1535
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and McGregor (1976)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
Cell transformation, in vitro BHK 21 ND + not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified, exposure
for 1-24 h, experimental
data not reported
Table 12 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations, in vivo rat - NA 82 g/m3, Seckar et al. (1986)
bone marrow 6 h/day, 5 days/
week for 13 weeks
Dominant lethal assay, male CD-1 rat - NA 82 g/m3, Seckar et al. (1986)
6 h/day, 5 days/
week for 15 weeks
a NA = not applicable; ND = not determined
b LED = lowest effective dose; HED = highest effective dose
c purity not specified for these studies
d in vitro assay in an enclosed system
Table 13. Genetic toxicity of HCFC 132b
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 4 mg/plate liquid phase Russell (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - 550 g/m3b Waskell (1979)
TA98, TA100, TA1535, TA1537
S. typhimurium, reverse mutation with - - 578 g/m3b 99.9% purity Koorn (1988)
TA98, TA100, TA1535, TA1537, TA1538
Chromosomal aberrations, in vitro CHO - - 14.2 mg/ml, liquid phase Wilmer & de Vogel (1988)
cells 3 h (+ema)b
3.0 mg/ml,
21 h (-ema)b
a +ema = with exogenous metabolic activation; -ema = without exogenous metabolic activation; LED = lowest effective dose;
HED = highest effective dose
b in vitro assay in an enclosed system
Table 14. Genetic toxicity of HCFC 133a
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2460 g/m3, McGregor (1976)
mutation with TA98, TA100, TA1535, 24 hd
TA1538
S. typhimurium, reverse mutation with - - 172 g/m3, Waskell (1979)
TA98, TA100 48 hd
S. typhimurium, reverse mutation with - - 49 g/m3, Edmunds et al. (1979)
TA98, TA100 8 hd
Cell transformation, in vitro BHK 21 - - gas, 3 h concentrations not Longstaff et al. (1984)
cell growth in soft agar assay specified, experimental
data not provided
Chromosomal aberrations, in vivo, - NA 98 g/m3, 6 h, marrow sampled 24 h Anderson & Richardson
male AP rat bone marrow and 6 h/day after single and 6 h (1979)
5 days/week after multiple exposure
Dominant lethal assay, male CD-1 + NA 49 g/m3, 6 h/ early deaths increased Hodge et al. (1979)
mouse day, 5 days/week in mating weeks 6-8
Dominant lethal assay, male CD-1 + NA 12 g/m3, 6 h/ early deaths increased Hodge et al. (1980)
mouse day, 5 days/week in mating week 7, no
dose response
Table 14 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Dominant lethal assay, male CD-1 - NA 12 g/m3, 6 h/ Kilmartin et al. (1980)
mouse day, 5 days/week
a NA = not applicable
b HED = highest effective dose; LED = lowest effective dose
c purity not provided for these studies
d in vitro assay in an enclosed system
Table 15. Genetic toxicity of HCFC 123
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 937 g/m3, 6 h Barsky (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - not specifiedc single gaseous conc. Brusick (1976)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 5 or 24 h
S. typhimurium, reverse mutation with - - 625 g/m3, 72 h experimental data not Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 provided
S. typhimurium, reverse mutation with - - not specifiedc gaseous exposure, Callander (1989)
TA98, TA100, TA1535, TA1537, TA1538 concs. not specified
Sacharomyces cerevisiae: D4, forward - - not specified concs. not specified, Brusick (1976)
mutation exposure for 4-72 mins
Chromosomal aberrations, in vitro, + - 75 µg/ml, purity 99.95% Dance (1991)
human lymphocytes 24 h
Chromosomal aberrations, in vitro, + + 1875 g/m3, purity 99.95% Edwards (1991a)
human lymphocytes 3 h (+ema)c
156 g/m3,
24 h (-ema)c
Cell transformation, in vitro, BHK 21 ND - not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified,
experimental data
not provided
Table 15 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Micronucleus test, in vitro, mouse - NA 112 g/m3, 6 h marrow sampled 24, Müller & Hoffman (1988)
bone marrow 48 and 72 h after
exposure
a ND = not determined; NA = not applicable
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous metabolic
activation
c in vitro assay in an enclosed system
A negative response was reported in a BHK transformation assay,
but no supporting data were provided.
There was no evidence of induction of micronuclei in a single
mouse bone marrow assay.
7.6.6 HCFC 124
The data from the small number of in vitro studies are
summarized in Table 16.
HCFC 124 did not induce mutations in bacteria or chromosomal
aberrations in CHO cells.
7.7 Carcinogenicity
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternate Fluorocarbon Toxicity Testing (Rusch, 1989).
7.7.1 HCFC 142b
Seckar et al. (1986) conducted a combined chronic
toxicity/carcinogenicity study of HCFC 142b with Sprague-Dawley CD
rats (details of exposure regimen are given in section 7.4).
Neoplasmic findings in animals that died were similar in the control
and treated animals, and predominantly comprised tumours of the
mammary and subcutaneous tissues in females, and pituitary and adrenal
adenomas in both sexes. A similar pattern existed in surviving
animals.
7.7.2 HCFC 133a
The carcinogenic potential of HCFC 133a has been evaluated by
Longstaff et al. (1984) in a limited study in Alpk/Ap Wistar- derived
rats. The study included one undosed control group of 32 male and 32
female rats and two vehicle-dosed control groups, one consisting of 40
male and 40 female and the other of 36 male and 36 female rats. The
HCFC 133a was dissolved in corn oil to give a 3% solution and dosed by
gavage at 300 mg/kg body weight, 5 days/week until week 52, and the
study was terminated at week 125. Dosed controls were given corn oil
only. Reductions of body weight gain and decreased testicular size
were observed in treated males. Aggressive behaviour occurred,
particularly in males. Body weight gain in females was similar to that
of controls. Mortality was not affected by treatment in either sex.
The treated females had an increased incidence of uterine carcinomas
(15/35) in comparison with controls (1/104). The first of these
carcinomas was seen at week 84. The carcinomas metastasized
transcoelomically to the abdomen in a large proportion of animals. A
small proportion also had lung metastases. Histologically, the
neoplasms were actively infiltrating adenocarcinomas.
Table 16. Genetic toxicity of HCFC 124
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2230 g/m3, Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hb
TA1537, TA1538
S. typhimurium, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
TA98, TA100, TA1535, TA1537, TA1538 48 hb
S. typhimurium, reverse mutation with - - 2790 g/m3, experimental data Longstaff et al. (1984)
TA98, TA100 48 hb not reported
Escherichia coli, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
WP2 uvrA 48 hb
Chromosomal aberrations, in vitro, - - 3348 g/m3, purity > 99% Edwards (1991b)
CHO cells 48 h (-ema)b
3348 g/m3,
4 h (+ema)b
a LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
b In vitro assay in an enclosed system
The treated males had an increased incidence of benign
interstitial cell neoplasms of the testis (29/36) compared with
controls (16/104). In many animals the neoplasms were bilateral. The
first interstitial cell tumour was seen at week 64. The testes of all
treated males, including those with no evidence of neoplasms,
exhibited arrest of spermatogenesis and atrophy of the seminiferous
tubules. These changes were first seen in the first rat to die in this
study, which was in week 37.
7.7.3 HCFC 123
As discussed in section 7.4.2, a 2-year inhalation toxicity/
carcinogenicity study in rats is being conducted. Groups of 80 male
and 80 female Crl:CDRBR rats were exposed to HCFC 123 at 0, 2, 6 or
31 g/m3 (6 h/day, 5 days/week) for 2 years. No histological lesions
were found in 10 rats of each sex in each exposure group, which were
sacrificed at the end of 1 year of exposure (Malley, 1990c).
Preliminary findings from the histopathological examination of tissues
of male rats at the end of the 2-year exposure period indicated an
exposure-related increase in benign tumours of the testis and exocrine
pancreas (US EPA, 1991). A full report is needed before these results
can be evaluated.
7.8 Special studies - cardiovascular and respiratory effects
Chlorofluorocarbons have long been known to sensitize the heart
to adrenaline-induced arrhythmias. Zakhari & Aviado (1982) reviewed
the literature on this subject. Several studies have been conducted to
evaluate the cardiac sensitization and respiratory effect potential of
the alternative HCFC compounds.
7.8.1 HCFC 141b
Mullin (1977) exposed male dogs, which were pretreated with an
intravenous injection of adrenaline (0.008 mg/kg), to HCFC 141b
concentrations of 12, 24, 48 and 97 mg/m3. A challenge dose
(intravenous injection with adrenaline) given during exposure elicited
serious cardiac arrhythmias at the three highest concentrations but no
response was noted at the lowest concentration.
Hardy et al. (1989b) studied cardiac sensitization to adrenaline
in two Cynomolgus monkeys and four beagle dogs and found that the
lowest HCFC 141b concentrations inducing responses were about 24 and
48 g/m3, respectively.
No effect on respiratory rate was observed when three groups of
three male Wistar rats were exposed to approximately 45 g/m3 for 25
min. However, there was a small change in respiratory amplitude
suggesting a decrease in tidal volume (Janssen, 1989a).
7.8.2 HCFC 142b
Adrenaline-induced cardiac arrhythmias were observed in 5 out of
12 dogs exposed to 205 mg/m3. No such effect was noted in monkeys or
mice at concentrations of up to 410 g/m3 (Mullin, 1969). It was also
found that dogs exposed to 3280 g/m3 (80% by volume; 20% oxygen
added) for 30 seconds, followed by a loud noise (to stimulate
endogenous adrenaline), developed cardiac sensitization (Mullin,
1970).
Reinhardt et al. (1971) studied the ability of HCFC 142b to
induce cardiac sensitization to exogenous and endogenous adrenaline in
beagle dogs. No response was noted in six animals exposed to a
concentration of 102.5 g/m3 for 5 min and then challenged with
adrenaline. Five out of 12 dogs exposed to 205 g/m3 showed marked
responses (arrhythmias considered to pose a serious threat to life or
ventricular fibrillation), and all 12 dogs exposed to 410 g/m3
showed such responses. When a group of 12 beagle dogs was exposed to
3280 g/m3 for 30 seconds, without injected adrenaline, one out of 12
showed a marked response. With simultaneous noise stimulation five
dogs showed a marked response. No dogs showed the response when
exposed to noise alone.
No arrhythmia or tachycardia was observed when groups of three
Rhesus monkeys ( Macaca mulatta) were anaesthetised with sodium
pentobarbital and exposed to 205 or 410 g/m3 for 5 min (Belej et
al., 1974). There was no effect on pulmonary resistance or compliance
but respiratory stimulation was observed when three anaesthetised
Rhesus monkeys ( M. mulatta) were exposed to 205 g/m3 and four
monkeys to 410 g/m3 for 5 min (Aviado & Smith, 1975). When dogs were
exposed to four different concentrations between 102.5 and 820 g/m3
for 5 min, hypotension, tachycardia, an increase in pulmonary
resistance, and a decrease in pulmonary compliance were found at the
highest exposure level (Belej & Aviado, 1975).
7.8.3 HCFC 132b
HCFC 132b was reported to produce cardiac sensitization in beagle
dogs in response to an intravenous adrenaline challenge at exposure
levels of 27 g/m3 or more (Mullin, 1976).
7.8.4 HCFC 123
Trochimowicz & Mullin (1973) reported the EC50 (concentration
producing an effect in 50% of the test group) in dogs for cardiac
sensitization to adrenaline challenge to be 119 g/m3 and the NOEL to
be 62 g/m3. At the latter concentration (the lowest one tested) CNS
depression was observed.
7.8.5 HCFC 124
Van Poznak & Artusio (1960) found that HCFC 124 caused
anaesthesia in dogs at concentrations ranging from 2230 to 3910
g/m3; blood pressure was lowered in a dose-dependent fashion.
Although ventilation was adequate even at the highest exposure level,
the femoral arterial systolic pressure fell to as low as 40 mmHg (5.33
kPa). Little or no anaesthetic effect was observed at exposure levels
that did not depress blood pressure. Atropine had little effect on
hypotension. Phenylephrine partially reversed the hypotension and
caused several premature ventricular contractions but no ventricular
fibrillation. Similar effects were produced by intravenously
administered adrenaline. Mullin (1976) reported that the NOEL of HCFC
124 for cardiac sensitization after a challenge injection of
adrenaline in the dog was 56 g/m3, while the next exposure level
tested (140 g/m3) induced sensitization, as did higher
concentrations.
8. EFFECTS ON HUMANS
8.1 General population exposure
No effects on human health of the hydrochlorofluorocarbons
reviewed in this monograph have been reported.
8.2 Occupational exposure
Filicheva (1975) examined 98 male and 98 female workers, 20-40
years of age, reportedly exposed to chlorofluorocarbons 22, 113 and
142 and to some other chlorofluoro and fluoro compounds. The
concentrations were claimed to exceed the threshold limit values but
were not specified. Functional disorders of the nervous system were
reported in 67% of the workers. These included symptoms of
neurovegetative system disturbances, and, in a few cases, polyneuritis
of the upper extremities. Reduced haemoglobin content, moderate
leucocytosis and reduced erythrocyte sedimentation rate were also
reported. Exposure levels were not given, and the workers were exposed
to a number of chemicals in addition to HCFC 142 (isomer not
specified). The information provided in this study could not be used
to evaluate the potential health effects on workers exposed to HCFC
142b alone.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
No information is available on the effects on environmental
organisms of the hydrochlorofluorocarbons reviewed except for limited
data on HCFC 141b and HCFC 142b. However, an ecotoxicology programme
is being developed within the industry-sponsored Programme for
Alternative Fluorocarbon Toxicity Testing (Rusch, 1989).
The 96-h LC50 of HCFC 141b for zebra fish (Brachidario rerio)
was reported to be 126 mg/litre in a static test using a sealed vessel
(Bazzon & Hervouet, 1989). The 48-h EC50 for the immobilization of
Daphnia magna, also using a sealed vessel, was 31.2 mg/litre
(Brinard & Hervouet, 1989).
The 96-h EC50 of HCFC 142b for guppies ( Poecilia
reticulata), tested in a static system in accordance with OECD
Guidelines, was reported to be 220 mg/litre (Groenevald & Kuijpers,
1990a). Groenevald & Kuijpers (1990b) found a 48-h EC50 for Daphnia
magna of 160 mg/litre. No immobilization of Daphnia magna at 190
mg/litre in 48 h was found (Hutton & Lieder, 1989a).
Acute toxicity values of HCFC 142b have also been determined for
rainbow trout. The 96-h LC50 was found to be 36 mg/litre (with a 95%
confidence interval of 28-45 mg/litre). The authors concluded that,
under the conditions of the test, HCFC 142b exhibited moderate acute
toxicity to rainbow trout (Hutton & Lieder, 1989b).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Direct health effects
10.1.1 HCFC 141b
HCFC 141b has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or in the environment. Available information
indicates a low level of metabolism of HCFC 141b. It is of low acute
toxicity. At high sublethal exposure concentrations, it induces signs
of CNS depression and can sensitize the heart to adrenaline, as do
other hydrochlorofluorocarbons. Such effects can appear at
concentrations ranging from 24 to 48 g/m3 in the inhaled air.
HCFC 141b has low irritant potential to the eye and skin. Skin
sensitization has not been demonstrated.
Short-term repeated inhalation exposure (2 to 13 weeks) did not
induce serious toxic effects at concentrations below 97 g/m3.
Effects of HCFC 141b on reproduction cannot be evaluated until
the data from an ongoing two-generation study become available. It has
not demonstrated teratogenic potential in rats or rabbits, although
embryotoxicity was observed, but only at the highest maternally toxic
concentration (97 g/m3) in rats. The no-observed-effect level (NOEL)
for maternal toxicity (excepting minor clinical signs) is 7 g/m3 in
rabbits and 15 g/m3 in rats.
Mutagenicity testing of HCFC 141b yielded conflicting results.
In vitro studies with CHO cells indicated clastogenic potential, but
this was not reflected in a single human lymphocyte test or two
in vivo mouse studies.
A carcinogenicity study in rats is in progress for HCFC 141b.
In summary, based on the toxicological information available at
the time of the Task Group meeting, the toxicity of HCFC 141b is
considered to be low. The present data base does not indicate any
significant direct health effects on humans under non-accidental
exposure conditions. However, studies to evaluate carcinogenicity and
reproduction are still in progress. Consequently, these study results
and any future changes in use pattern may require further evaluation.
10.1.2 HCFC 142b
HCFC 142b is produced in commercial quantities for use as an
intermediate in the synthesis of vinylidene fluoride. Its current
release to the environment has not been quantified and no information
is available on accidental release. No study is available which
permits the assessment of human health responses to HCFC 142b alone.
No information is available from in vivo studies on metabolism
and kinetics, but an in vitro study suggests that dechlorination may
occur.
The acute toxicity is very low after oral or inhalation exposure.
The no-observed-effect level (NOEL) for cardiac sensitization using
exogenous adrenaline in dogs is 102.5 g/m3 for 5 min.
In rat and dog studies, repeated inhalation exposure at
concentrations of up to 41 g/m3 for 90 days caused no adverse
responses. No carcinogenic or other toxicological responses were
reported in a single long-term inhalation study in rats exposed to up
to 82 g/m3 for 104 week.
HCFC 142b has mutagenic potential in bacterial systems. Data from
in vitro mammalian cell systems are lacking. No mutagenic activity
was seen in two in vivo rat studies.
Although no conventional reproductive toxicity studies are
available, two rat teratogenicity tests have been conducted in which
neither teratogenicity nor reproducible signs of embryotoxicity were
observed. In addition, no effect on male fertility was observed in a
dominant lethal study in rats.
In summary, information on human exposures is lacking, but these
are unlikely to approach the sustained high concentrations required to
produce adverse effects in experimental animals. Future usage
patterns, possibly resulting in higher occupational exposure
concentrations and exposure for shorter periods among the general
population, may require further evaluation.
10.1.3 HCFC 132b
HCFC 132b is only produced in small quantities for research
purposes but it occurs as a by-product in the manufacture of some
halogenated ethanes. Release into the environment is expected to be
very low. There appears to be no commercial application for HCFC 132b
at the present time. There are no data on human exposure and on the
effects of HCFC 132b on human health.
Data on the metabolism of HCFC 132b are only available for rats.
They show that the compound can be metabolized to potentially
cytotoxic metabolites and suggest that the compound can induce its own
metabolism. The acute toxicity of the compound is low, the predominant
effects being central nervous system (CNS) depression, anaesthesia
and, at high concentrations, death. The compound has low irritation
potential to the skin and eye. HCFC 132b can sensitize the heart to
adrenaline, as do many other hydrochlorofluorocarbons. Repeated
exposure to HCFC 132b has been shown to cause CNS depression, signs of
hepatotoxicity (at concentrations of 3 g/m3 or more) and effects on
spermatogenesis (at 11 g/m3). HCFC 132b is also embryotoxic and was
maternally toxic at the lowest dose tested (3 g/m3). NOEL values
have not been established for systemic toxicity or for embryotoxicity.
The available mutagenicity studies, which are limited to a few
in vitro experiments, did not suggest that HCFC 132b is genotoxic.
Long-term and carcinogenicity studies have not been performed.
In summary, from the available information, exposure to HCFC 132b
would appear to present a hazard to human health following repeated
exposure. Current use restricts its exposure to research personnel.
Therefore there is no expected health risk to the general population.
10.1.4 HCFC 133a
HCFC 133a is produced in small quantities for use as an
intermediate in the manufacture of the anaesthetic halothane. Although
it has not been quantified, release into the environment is expected
to be low.
No data are available on the toxicokinetics of HCFC 133a,
although absorption can be assumed to occur from the toxic effects it
induces. It is of low acute toxicity, the principal toxic effects
being anaesthesia and death at high concentrations. Although no
information is available, this compound is also expected to induce
cardiac sensitization at high concentrations. In animal studies
repeated exposure to HCFC 133a has induced death (in mice), and nasal
and lung damage changes in clinical chemistry parameters (in rats).
Atrophy of the thymus, testis, ovary and spleen have also been
reported in rats. Reduced fertility and damage to the seminiferous
epithelium have been observed in male mice at exposure concentrations
of 2.5 g/m3 and 5 g/m3, respectively.
HCFC 133a has been clearly demonstrated to be embryotoxic at
exposure concentrations that did not produce clear evidence of
maternal toxicity (2.5 g/m3); there was also indication of a
teratogenic potential in rats. The NOEL for embryolethality was 2.5
g/m3.
HCFC 133a is non-mutagenic in bacterial assays. It gave positive
results in two out of three dominant lethal assays in mice, but these
results are difficult to interpret in view of the negative result in
a single rat bone-marrow cytogenetic assay. From the limited evidence
available, HCFC 133a is a carcinogen in rats inducing adenocarcinomas
of the uterus; it also produces benign interstitial cell tumours of
the testis.
In summary, from the information available, exposure to HCFC 133a
would appear to present a hazard to human health following repeated
exposure and has the potential to cause serious effects in developing
offspring. In the absence of exposure information the potential risk
cannot be determined.
10.1.5 HCFC 123
HCFC 123 has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or the environment.
There is limited information on the kinetics and metabolism of
HCFC 123. It can be absorbed, the inference coming from observed
systemic effects and elevated urinary fluoride levels in toxicity
studies. There is evidence for some metabolism of HCFC 123, based on
the detection of urinary trifluoroacetic acid, elevated urinary
fluoride levels, and the detection of covalent binding to liver
protein.
The acute toxicity of HCFC l23 is low. As with the fully
halogenated chlorofluorocarbons, it is characterized in animals by CNS
depression at high inhalation exposure concentrations. Exposure to
high concentrations of HCFC 123 can sensitize the heart to adrenaline
(the EC50 for dogs is 119 g/m3).
Effects associated with short-term and long-term inhalation
exposure to HCFC 123 in rats include CNS depression, modulation of
lipid and carbohydrate metabolism and mild liver toxicity. The
lowest-observed-effect level (LOEL) for lipid and carbohydrate
metabolism effects and increased hepatic enzyme activity is 2 g/m3
following a 1-year exposure period to HCFC 123.
There is no evidence of teratogenicity in rats and rabbits but
there is evidence of embryotoxicity in rabbits at high inhalation
exposure concentrations (62 g/m3). Maternal toxicity is seen at
exposure concentration of 3 g/m3 or more in rabbits and 31 g/m3 or
more in rats. The potential effect of HCFC 123 on reproduction is
being investigated in a rat study.
There is evidence of clastogenic effects in human lymphocytes
in vitro, although this was not supported by an in vivo mouse
micronucleus study. HCFC 123 is not mutagenic in microorganisms. The
carcinogenicity of HCFC 123 is being investigated in rats and thus
cannot be evaluated at present.
On the basis of available information, HCFC 123 does not appear
to exhibit marked toxicity following short-term or long-term exposure.
However, in the light of some evidence of clastogenicity and the
reported increased incidences of benign tumours of the testes and
exocrine pancreas communicated in an interim report, the potential
health effects of HCFC 123 cannot be fully evaluated until more
information becomes available.
10.1.6 HCFC 124
HCFC 124 is not yet in large scale commercial production.
Therefore, there are no data on environmental levels and human
exposure.
The acute toxicity of HCFC 124 is low and is characterized in
animals by effects on the central nervous system. Subchronic toxicity
studies in rats did not reveal any histopathological changes of
internal organs at concentration of HCFC 124 as high as 279 g/m3.
Based on functional observations and clinical chemistry, the NOEL was
reported to be 28 g/m3 for male rats, and 84 g/m3 for females. In
three limited teratogenicity studies in rats, no indication of
developmental toxicity of HCFC 124 was evident, even at a maternally
toxic concentration. Based on the data available from bacterial and
mammalian cell studies there is no evidence that HCFC 124 has a
mutagenic potential.
The available information indicates that HCFC 124 exhibits low
toxicity, and it is not anticipated to pose significant health risk to
humans at potential environmental or controlled occupational
exposures. However, a firm conclusion on the potential health risk
cannot be made until data from the ongoing teratogenicity and
carcinogenicity studies are available.
10.2 Health effects expected from a depletion of stratospheric
ozone
The possible indirect health effects (e.g., an increase in the
incidence of skin cancer and immunotoxic and ocular effects) of fully
halogenated chlorofluorocarbons, resulting from an increase in UV-B
radiation due to a depletion of the ozone layer, have been discussed
in the Environmental Health Criteria 113: Fully Halogenated
Chlorofluorocarbons (WHO, 1990).
The ozone-depleting potentials of five of the six HCFCs for which
data are available are lower than that of CFC 11 by 33-77 times (HCFCs
123 and 124), 40 times (HCFC 132b), 12-20 times (HCFC 142b), and 7-15
times (HCFC 141b). If the levels of release of the HCFCs reviewed are
adequately controlled their indirect health effects should not be
significant.
10.3 Effects on the environment
Insufficient information is available to evaluate adequately the
direct ecological effects posed by the hydrochlorofluorocarbons
reviewed. With respect to the indirect "greenhouse" effect, the HCFCs
for which data are available have global-warming potentials lower than
that of CFC 11 by about 50 times (HCFC 123), about 10 times (HCFCs 124
and 141b), and about 3 times (HCFC 142b). These compounds are not
expected to contribute significantly to global warming.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Based on the available information the Task Group reached the
following conclusions:
1. All six hydrochlorofluorocarbons reviewed have low acute
toxicity, the main toxic signs being those of CNS depression.
However, accidental overexposure could result in acute poisoning
or even lethality.
2. These chemicals exhibit different toxicity potentials following
repeated exposure.
3. HCFC 141b has a low toxic potential on repeated exposure,
produces no consistent developmental effects and has not been
shown to be mutagenic in animals. The mutagenic status of HCFC
141b tested in vitro is unclear. No information is yet
available to evaluate chronic toxicity/carcinogenicity.
4. HCFC 142b also has a low toxic potential on repeated exposure.
The small data base indicates that HCFC 142b does not produce
developmental effects or affect male fertility. HCFC 142b is
mutagenic in bacteria. It has not been shown to be mutagenic or
carcinogenic in rats.
5. HCFC 132b is toxic following repeated exposure in animals. It
affects development in animals, but has only been tested at
maternally toxic doses. No study on reproduction has been
performed, but damage to spermatogenesis has been noted
histopathologically following repeated exposure. It is not
mutagenic in vitro and no test has been performed in vivo.
There are no data on the carcinogenic potential.
6. HCFC 133a is toxic following repeated exposure in animals,
producing a range of effects. It also induces serious effects on
reproduction and development in animals. In vivo data regarding
mutagenicity are unclear. It is a carcinogen in rats.
7. On repeated exposure, HCFC 123 induces liver toxicity and effects
on lipid and carbohydrate metabolism in rats. Developmental
effects only occur at high maternally toxic exposure
concentrations. No information is available on reproduction
effects. HCFC 123 is clastogenic in vitro, but has not been
shown to be clastogenic in animals. Complete information on the
carcinogenicity in rats is not yet available.
8. HCFC 124 has low toxic potential on repeated exposure. Based on
a small data base, there is no evidence of developmental effects.
No information is available on the reproduction toxicity
potential or carcinogenicity. It is not mutagenic in vitro and
no test has yet been performed in vivo.
9. The hydrochlorofluorocarbons reviewed (with the exception of HCFC
133a for which there is no value but which is expected to have a
value similar to the others reviewed) have a lower
ozone-depleting potential than the fully halogenated
chlorofluorocarbons and should therefore pose a lower indirect
health risk.
10. The global-warming potentials of HCFC 141b, HCFC 142b, HCFC 123
and HCFC 124 are similarly lower than those of the fully
halogenated chlorofluorocarbons. No data are available for HCFC
132b or HCFC 133a, but they would be expected to have similar
values to the other hydrochlorofluorocarbons reviewed. These
compounds are not expected to contribute significantly to global
warming.
11.2 Recommendations for protection of human health and the
environment
1. Since the toxicity of HCFC 142b is low, and the ozone-depleting
and global-warming potentials are considerably lower than those
of the fully halogenated chlorofluorocarbons, HCFC 142b can be
considered as a transient substitute for the chlorofluorocarbons
included in the Montreal Protocol. However, in line with the
conclusions of the 1990 London Meeting of the Montreal Protocol
Parties, efforts should be maintained to develop substitutes that
would pose no risk to the environment and to develop alternative
technologies. Care should be exercised in the use of HCFC 142b
because of its flammability.
2. Since more information regarding the toxicological potential of
HCFC 141b, HCFC 123 and HCFC 124 is required before an evaluation
of their hazard to human health can be made, no recommendation
can be made at present regarding their use as potential transient
substitutes for the fully halogenated chlorofluorocarbons
included in the Montreal Protocol.
3. Although HCFC 133a and HCFC 132b pose low risk to the
environment, they are not recommended as substitutes for the
chlorofluorocarbons included in the Montreal Protocol because of
their toxicity.
4. Since all the hydrochlorofluorocarbons reviewed have some
ozone-depleting potential, their release to the environment
should be minimized.
REFERENCES
Ahmadzai H & Hedlund T (1990) A profile of measures taken in Sweden to
protect the stratospheric ozone layer. Ambio, 19: 341-343.
Alvarez L (1988) Pilot teratogenicity study of FC-132b in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HLR 50-88).
Anderson D & Richardson CR (1979) Arcton 133a: A cytogenetic study in
the rat. Alderley Park, Cheshire, Imperial Chemical Industries Ltd,
Central Toxicology Laboratory (Report No. CTL/P443).
ANON (1991) CFC alternative shows chronic toxicity. Chem Eng News, 8
July: 26.
Aviado DM & Smith DG (1975) Toxicity of aerosol propellants in the
respiratory and circulatory systems. VIII. Respiration and circulation
in primates. Toxicology, 3: 24l-252.
Barsky FC (1976) In vitro microbial mutagenicity studies of
ethane,1-chloro-1,2,2,2-trifluoro. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 349-76).
Bazzon M & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Brachidanio rerio. Vert-le-Petit, Institut national de
Recherche chimique appliquée (IRCHA Study B.7073).
Belej MA & Aviado DM (1975) Cardiopulmonary toxicity of propellants
for aerosols. J Clin Pharmacol, 15: 105-115.
Belej MA, Smith DG, & Aviado DM (1974) Toxicity of aerosol propellants
in the respiratory and circulatory systems. IV. Cardiotoxicity in the
monkey. Toxicology, 2: 381-395.
Bootman J & Hodson-Walker G (1988) In vitro assessment of the
clastogenic activity of CFC141b in cultures of Chinese hamster ovary
(CHO-KI) cells. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV007/214).
Bootman J, Hodson-Walker G, & Lloyd JM (1988a) CFC 141b: investigation
of mutagenic activity at the HGPRT locus in a Chinese hamster V79 cell
mutation system. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV005/257).
Bootman J, Hodson-Walker G, Cracknell S, & Dance CA (1988b) HCFC 141b:
Assessment of clastogenic action on bone marrow erythrocytes in the
micronucleus test. Eye, Suffolk, Life Science Research Ltd (LRS Report
No. 88/PSV029).
Brewer WE (1977) 90-day subacute inhalation toxicity study with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brewer WE & Smith S (1977) Teratogenic study via inhalation with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brinard D & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Daphnia magna. Vert-le-Petit, Institut national de Recherche
chimique appliquée (IRCHA Study B.7072).
Brittelli MR (1976a) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 752-75).
Brittelli MR (1976b) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 13-76).
Brittelli MR (1976c) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 747-75).
Brock WJ (1988a) Acute dermal toxicity study of FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 501-88).
Brock WJ (1988b) Skin irritation test in rabbits of FC-141b. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
312-88).
Brock WJ (1988c) Primary eye irritation study with FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 318-88).
Brock WJ (1988d) Acute dermal toxicity study of HCFC-123 in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 577-88).
Brock WJ (1988e) Acute dermal toxicity of HCFC-123 in rabbits. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
578-88).
Brock WJ (1988f) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 535-88).
Brusick D (1976) Mutagenicity evaluation of Genetron 123. Final
Report. Kensington, Maryland, Litton Bionetics, Inc. (LBI Project No.
254).
Callander RD (1989) HCFC 123 - An evaluation using the Salmonella
mutagenicity assay. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No. CTL/P/2421).
Carpenter CP, Smith HF Jr, & Pozzani UC (1949) The assay of acute
vapour toxicity and the grading and interpretation of results on 96
chemical compounds. J Ind Hyg Toxicol, 31: 343-346.
Chapman J, Hill R, Muir J, Suckling CW, & Viney DJ (1967) Impurities
in halothane: their identities, concentrations and determination. J
Pharm Pharmacol, 19: 231-239.
Coate WB (1976a) LC50 of G123 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. M165-162).
Coate WB (1976b) LC50 of G124 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. 165-163).
Coombs DE, Hardy CJ, Crook D, Mullins PA, & Gopinath C (1988) Two-week
inhalation toxicity study in the rat with FC 141b. Huntington, United
Kingdom, Huntington Research Centre (Report PWT 76/8893).
Culik R & Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled chlorodifluoroethane (FC 142b). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 700-76).
Culik R & Kelly DP (1979) Embryotoxicity and teratogenicity studies in
rats with chlorotrifluoroethane (FC-133a). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 127-79).
Damske DR, Mecler FJ, & Beliles RP (1978) Teratology study in rats;
Isotron 142b. Monochlorodifluoroethane. Final Report. Kensington,
Maryland, Litton Bionetics, Inc. (LBI Project No. 20980).
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC 123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd (Final report No. 91/PFE03/0093).
Darr RW (1981) An acute inhalation toxicity study of Fluorocarbon 123
in the Chinese hamster. Morristown, New Jersey, Corporate Medical
Affairs, Allied Corporation (Report No. MA-25-78-15).
Davies RH, Bagnall RD, Bell W, & Jones WGM (1976) The hydrogen bond
proton donor properties of volatile halogenated hydrocarbons and
ethers and their mode of action in anaesthesia. Int J Quantum Chem
Quantum Biol Symp, 3: 171-185.
Diggle WM & Gage JC (1956) Toxicological report Arctons 9, 33 and 50.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/T/61).
Doleba-Crowe C (1977) Acute inhalation toxicity - 6 hours (HFA 141b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 61-77).
Doleba-Crowe C (1978) Ninety-day inhalation exposure of rats and dogs
to vapors of 2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
229-78).
Edmunds HN, Baden JM, & Simmon VF (1979) Mutagenicity studies with
volatile metabolites of Halothane. Anesthesiology, 51(5): 424-429.
Edwards CN (1991a) HCFC 123 (vapour phase): In vitro assessment of
clastogenic activity in cultured human lymphocytes. Eye, Suffolk, Life
Science Research Ltd (Final Report No. 91/PFE002/0125).
Edwards CN (1991b) In vitro assessment of the clastogenic activity
of HCFC 124 in cultured Chinese hamster ovary (CHO-K1) cells. Eye,
Suffolk, Life Science Research Ltd (Final report No. 91/PAR002/0855a).
Filicheva AP (1975) [Changes in the nervous system following chronic
action of fluorinated aliphatic hydrocarbons.] Gig Tr Prof Zabol, 10:
14-16 (in Russian).
Fisher DA, Hales ChH, Wang WC, Ko MKW, & Dze ND (1990a) Model
calculations of the relative effects of CFCs and their replacements on
global warming. Nature (Lond), 344: 513-516.
Fisher DA, Hales H, Filkin DL, Malcolm KWK, Sze ND, Connell PS,
Wuebbles DJ, Isaksen SA, & Stordal F (1990b) Model calculations of the
relative effects of CFCs and their replacements on stratospheric
ozone. Nature (Lond), 344: 508-512.
Freemantle M (1991) CFCs and their alternatives. Impact Sci Soc, 40:
59-70.
Gardner JR (1988) Acute dermal toxicity to rats of
dichlorofluoroethane. Huntington, United Kingdom, Huntington Research
Centre (Report No. 881114/PWT91/AC).
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 678-75).
Goodman NC (1976) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 14-76).
Graselli JG & Ritchey WM ed. (1975) CRC atlas of spectral data and
physical constants for organic compounds. Cleveland, Ohio, CRC Press,
vol 3.
Groeneveld AHC & Kuijpers LAM (1990a) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to the guppy Poecilia
reticulata. Graveland, The Netherlands, Duphar B.V., Agrobiological
Laboratory (Interim document No. 56635/33/90).
Groeneveld AHC & Kuijpers LAM (1990b) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to Daphnia magna. Graveland,
The Netherlands, Duphar B.V., Agrobiological Laboratory (Interim
Document No. 56635/32/90).
Hake CL, Waggoner TB, Robertson DN, & Rowe VK (1960) The metabolism of
1,1,1-trichloroethane by the rat. Arch Environ Health, 1: 101-105.
Hall GT (1976) Two-week inhalation toxicity study (HFA 132b and HFA
124). Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 727-76).
Hall GT & Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 426-75).
Hardy CJ, Jackson GC, Rao RS, Lewis DJ, & Gopinath C (1989a) HCFC 141b
acute inhalation toxicity study in rats, 4-hour exposure. Huntington,
United Kingdom, Huntington Research Centre (Report No. PWT95/881676).
Hardy CJ, Sharman IJ, & Chanter DO (1989b) Assessment of cardiac
sensitisation potential in dogs and monkeys. Comparison of F-141b and
F11. Huntington, United Kingdom, Huntington Research Centre (Report
No. PWT86/89437).
Harris JW & Anders MW (1991a) In vivo metabolism of the
hydrochlorofluorocarbon 1,1-dichloro-1-fluoroethane (HCFC-141b).
Biochem Pharmacol, 41: R13-R16.
Harris JW & Anders MW (1991b) Metabolism of the
hydrochlorofluorocarbon 1,2-dichloro-1,1-difluoroethane. Chem Res
Toxicol, 4: 180-186.
Harris JW, Pohl LR, Martin JL, & Anders MW (1991) Tissue acylation by
the chlorofluorocarbon substitute 2,2-dichloro-1,1,1-trifluoroethane.
Proc Natl Acad Sci (USA), 88: 1407-1410.
Hawley GG (1981) The condensed chemical dictionary, 10th ed. New York,
Van Nostrand Reinhold Company.
Henry JE (1975a) Acute oral test (132b). Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report No. 680-75).
Henry JE (1975b) Acute oral test on FC-123. Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 638-75).
Hodge MCE, Anderson D, Bennett IP, & Weight TM (1979) Arcton 133a:
dominant lethal study in the mouse. Alderley Park, Cheshire, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report No.
CTL/P/467).
Hodge MCE, Anderson D, Bennett IP, Weight TM, & Wilson J (1980) Arcton
133a: first combined dominant lethal and fertility study in the mouse.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/R/46l).
Hodson-Walker G (1990a) In vitro assessment of the clastogenic
activity of HCFC-141b in cultured Chinese hamster ovary (CHO-K1)
cells. Eye, Suffolk, Life Science Research Ltd (Report No.
89/PFG001/0971a).
Hodson-Walker G (1990b) HCFC 141b: lymphocyte cytogenetic study using
the methodology recommended by the OECD (1983). Eye, Suffolk, Life
Science Research Ltd (Report No. 89/PFG002/1029).
Hodson-Walker G & May K (1988a) CFC 141b: assessment of mutagenic
potential in histidine auxotrophs of Salmonella typhimurium (the
Ames test). Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV004/237).
Hodson-Walker G & May K (1988b) CFC 141b: assessment of its ability to
cause lethal DNA damage in strains of Escherichia coli. Eye,
Suffolk, Life Science Research Ltd (Report No. 88/PSV008/258).
Hoffmann JS (1990) Replacing CFCs; the search for alternatives. Ambio,
19: 329-333.
Horrath AL (1982) Halogenated hydrocarbons. New York, Basel, Marcel
Dekker.
Hughes EW, Homan BA, Kenny TJ, Coombs DW, Hardy CJ, & Tesh SA (1988)
The effect of HCFC 141b on pregnancy of the rat. Huntington, United
Kingdom, Huntington Research Centre (Report No. PWT77/88952).
Hughes EW, Homan BA, John DM, Kenney TJ, Coombs DW, & Hardy CJ (1989)
A study of the effect of PWC 4874-89 on pregnancy of the rabbit.
Huntington, United Kingdom, Huntington Research Centre (Report No. PWT
82/89243).
Hutton DG & Lieder PH (1989a) Static acute 48-hour EC50 of
chlorodifluoroethane to Daphnia magna. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 542-89).
Hutton DG & Lieder PH (1989b) Flow-through acute 96-hour LC50 of
chlorodifluoroethane to rainbow trout ( Oncorhynchus mykiss). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
6.1.3.2 HCFC 141b
Harris & Anders (1991a) studied the in vivo metabolism of HCFC
141b. A single fluorinated urinary metabolite, identified as
2,2-dichloro-2-fluoro-ethyl glucuronide, was found in rats exposed to
HCFC 141b (56 g/m3) in air for 2 h. The metabolism was reported to
be similar to that of its chlorinated analogue 1,1,1-trichloroethane,
which is metabolized to 2,2,2-trichloroethanol and excreted as its
glucuronate conjugate (Hake et al., 1960) and as trichloroacetic acid
(Koizumi et al., 1982). It was claimed (although no data were given)
that 2,2-dichlorofluoroacetic acid was also detected in the urine of
rats exposed to a concentration of 194 g/m3 for 4 h, but not to 56
g/m3 for 2 h (Harris & Anders, 1991a).
In a pilot study for absorption and metabolism of HCFC 141b,
seven groups of five male rats were exposed to the vapour by
inhalation in a closed loop exposure system (concentrations ranging
from 0.4 to 12 g/m3). No metabolism was detected but the sensitivity
of the method is such that it will not detect metabolism below 0.15%.
The results suggested that absorption did not take place, but that if
any metabolism occurred it was at a low level (Zwart, 1989).
Evidence of dechlorination was observed when rat hepatic
microsomes were incubated with about 1% HCFC 141b in vitro (Van
Dyke, 1977).
6.1.3.3 HCFC 142b
No in vivo studies on the metabolism of HCFC 142b have been
reported. One in vitro study provided evidence for dechlorination
when rat hepatic microsomes were incubated with 0.6% HCFC 142b (Van
Dyke, 1977).
6.1.3.4 HCFC 132b
Harris & Anders (1991) identified a number of metabolites in the
urine of male Fischer-344 rats given one or four doses of 10 mmol/kg
dissolved in corn oil by intraperitoneal injection. Approximately 1.8%
of the single administered dose was recovered in the urine. The
metabolites excreted in urine during the first 6 h were
2-chloro-2,2-difluoroethyl glucuronide, chlorodifluoroacetic acid and
chlorodifluoroacetaldehyde hydrate (free and conjugated). Repeated
injection of rats with HCFC 132b significantly increased both the rate
of chlorodifluoroacetic acid excretion and the relative fraction of
the HCFC 132b dose excreted as chlorodifluoroacetic acid. The
incubation of HCFC 132b with rat hepatic microsomes yielded
chlorodifluoroacetaldehyde hydrate as the only fluorinated product.
The in vitro metabolism of HCFC 132b was increased in microsomes
from pyridine-treated rats and inhibited by p-nitrophenol. This
inhibition by p-nitrophenol led the authors to suggest an
involvement of cytochrome P-450 IIE1 in the initial hydroxylation of
HCFC 132b.
6.1.3.5 HCFC 133a
Evidence for dechlorination of HCFC 133a was provided by an
in vitro study using a microsomal preparation derived from
Aroclor-1254a-induced rat liver homogenates (Salmon et al., 1981).
6.1.3.6 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 g/m3 or 68 g/m3) or to halothane (2-bromo-2-2
chloro-1,1,1-trifluoroethane) (1.05 g/m3) in air for 2 h. The
pattern of proteins immunoreactive with haptens-specific
anti-trifluoroacetylprotein antibodies was found to be identical in
livers of the rats exposed to HCFC 123 and halothane. Trifluoroacetic
acid was detected in urine of rats exposed to HCFC 123 or halothane by
nuclear magnetic resonance (NMR) and by gas chromatography with mass
spectrometry (GCMS), as had been reported previously (Maiorino et al.,
1980; Trochimowicz, 1989).
6.1.4 Covalent binding to macromolecules
6.1.4.1 HCFC 141b
19F-NMR analysis of microsomal and cytosolic proteins isolated
from the livers of rats killed 15 h after a 2-h exposure to HCFC 141b
(56 g/m3) did not yield any evidence for covalent binding of
fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.2 HCFC 132b
19F-NMR analysis of microsomal and cytosol proteins isolated
from the livers of rats killed 15 h after a single intraperitoneal
dose of HCFC 132b (10 mmol/kg) did not yield evidence for covalent
binding of fluorinated metabolites (Harris & Anders, 1991a).
6.1.4.3 HCFC 123
Harris et al. (1991) exposed adult male Fischer-344 rats to HCFC
123 (43 or 68 g/m3) or to halothane (105 g/m3) in air for 2 h.
19F-NMR analysis of cytosolic protein and immunoblotting of
microsomal and cytosolic hepatic protein using antibodies against
trifluoroacetylprotein at 15 h after the exposure demonstrated
covalent binding of fluorinated metabolites.
a Aroclor-1254 is a polychlorinated biphenyl mixture.
6.1.5 Elimination
No data are available from animal studies on the elimination of
the hydrochlorofluorocarbons reviewed. However, based on the
information on chlorofluorocarbons, it is likely that the main route
of excretion for hydrochlorofluorocarbons is through the respiratory
tract. Increased urinary inorganic fluoride has been observed in some
inhalation toxicity studies with HCFCs 141b, 132b, 123, and 124
(Doleba-Crowe, 1977, 1978; Brewer, 1977; Trochimowicz, 1989; Malley,
1990a, 1991).
6.2 Human studies
No data are available on the absorption, distribution, metabolic
transformation or elimination of the hydrochlorofluorocarbons
reviewed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Acute oral toxicity
Available data indicate low toxicity following single oral
exposure associated with four hydrochlorofluorocarbons, i.e. HCFC
141b, 142b, 132b and 123.
7.1.1.1 HCFC 141b
No mortality was observed in rats given a single oral dose of
HCFC 141b (5 g/kg body weight) dissolved in corn oil (Sarver, 1988).
7.1.1.2 HCFC 142b
Other than piloerection, oral dosing with HCFC 142b (up to 5 g/kg
body weight) dissolved in corn oil resulted in no signs of toxicity
(Liggett et al., 1989).
7.1.1.3 HCFC 132b
According to Henry (1975), the lowest dose at which mortality was
observed in rats treated with HCFC 132b was 25 g/kg.
A 2-g/kg dose of HCFC 132b in corn oil was applied by stomach
tubes to five male and five female Wistar rats, and the animals were
observed for 14 days. The clinical signs were indicative of an effect
on the autonomic nervous system (ptosis), on the central nervous
system (diminished alertness and startle response, and positional
passivity), on motor coordination (abnormal body posture and gait, and
loss of righting reflex), on motor activity and on muscle tone
(paralysis). Macroscopic examination showed swollen livers in some
males, and a decrease in absolute and relative liver weights. However,
no treatment-related effects were found during microscopic
examinations (Janssen & Pot, 1989b).
7.1.1.4 HCFC 123
The lowest dose of HCFC 123 producing lethality in rats was
reported to be 9 g/kg when it was administered as a corn oil solution
by intragastric intubation (Henry, 1975a).
7.1.2 Acute inhalation toxicity
7.1.2.1 HCFC 141b
The effects of a single inhalation exposure of rodents to HCFC
141b are shown in Table 5. The main effects at high exposure
concentrations include central nervous system depression, anaesthesia
and death.
7.1.2.2 HCFC 142b
Table 6 summarizes the effects of single inhalation exposure to
HCFC 142b in mice and rats. At high exposure levels, HCFC 142b induces
anaesthesia and death.
7.1.2.3 HCFC 132b
Table 7 summarizes the effects of single inhalation exposures of
mice and rats to HCFC 132b.
7.1.2.4 HCFC 133a
Inhalation of high concentrations of HCFC 133a is characterized
by signs of anaesthesia followed by death, but recovery from nonlethal
exposure is rapid. The effects of inhalation exposure to HCFC 133a are
summarized in Table 8.
7.1.2.5 HCFC 123
The results of studies on the acute inhalation toxicity of HCFC
123 are summarized in Table 9. Anaesthesia and death at higher
exposures were reported for rats and hamsters. No gross morphological
changes were observed in animals that died during exposure. Survivors
recovered within several minutes without showing any observable
clinical signs.
7.1.2.6 HCFC 124
Table 10 summarizes the effects of single inhalation exposure to
high concentrations of HCFC 124. As with other
hydrochlorofluorocarbons, the main effects observed were anaesthesia
and death.
7.1.3 Acute dermal toxicity
There is information only on the hydrochlorofluorocarbons that
are liquid at ambient temperatures, i.e. HCFC 141b, HCFC 132b and HCFC
123.
Table 5. Effects of a single inhalation exposure to HCFC 141b in mice and rats
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3) (h)
Rat 142-366 4 LC50 = 295 g/m3 Hardy et al. (1989a)
(Sprague-Dawley) No deaths were observed at 142 or 217 g/m3. All deaths
at higher concentrations (323 and 366 g/m3) occurred
during exposure, and were preceded by disturbed breathing.
Reduced motor activity, abnormal body carriage, restless
behaviour and exaggerated respiratory movements were seen
at all concentrations during exposure. No treatment-related
macroscopic findings were seen. Focal basophilic staining
was observed in the renal cortical tubules of 4 out of 10
rats at 217 g/m3 and 2 out of 10 rats at 142 g/m3. No
histopathological effects were seen in decedents.
Rat (strain unspecified 6 The lowest concentration producing lethality was 242 g/m3. Doleba-Crowe (1977)
unspecified) range
Mouse 17-388 6 Deaths preceded by signs of narcosis in 6 out of 10) animals Vlachos (1989)
(Crl: CD-1) occurred within 30 min of exposure to 388 g/m3. No deaths
occurred at the next highest (199 g/m3) concentration. Signs
of CNS depression (lethargy, abnormal gait, partially closed
eyes) were seen at 165 and 199 g/m3. No effects were seen at
concentrations of 145 g/m3 or less.
Mouse (strain unspecified 2 LC16 = 115 g/m3; LC50 = 151 g/m3; LC84 = 200 g/m3. Signs of Nikitenko & Tolgskaja
unspecified) range CNS depression and anaesthesia were observed. Death was (1965)
preceded by laboured breathing.
Mouse (Schofield unspecified 0.5 LC50 = 115 g/m3; concentration producing anaesthesia in Davies et al. (1976)
strain) range 50% of animals = 62 g/m3. No other information was given.
Table 6. Effects of single inhalation exposure to HCFC 142b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse "white" up to 2050 2 death; LC50 = 1514 g/m3 Nikitenko & Tolgskaja (1965)
Mouse AP unspecified 0.5 death; LC50 = 1228 g/m3 Davies et al. (1976)
range
Rat "white" 615-3280 0.5 death at 2050 g/m3 and unconsciousness at Lester & Greenberg (1950)
1230 g/m3; postural, righting and corneal
reflexes were lost at 820 g/m3
Rat Sherman 525 4 death (approx 50%) at 525 g/m3 Carpenter et al. (1949)
Table 7. Effects of single inhalation exposure to HCFC 132b in mice and rats
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (h)
Mouse unspecified range not given 0.5 LC50 = 269 g/m3; AC50 (anaesthesia) = 71 g/m3 Raventós & Lemon (1965)
Rat Wistar derived 55 and 110 4 lethalities at 110 g/m3; rats unsteady, weak and Torkelson (1971)
drowsy at 55 g/m3
Rat Wistar derived 33-72 6 anaesthesia at 82 g/m3; kidney swelling at autopsy; Janssen (1988)
CPB-WU at all dose levels, males showed decreased growth
and testis weight, and increased liver and lung
weights
Rat Wistar derived 55 0.4 decreased respiratory rate with rapid recovery Janssen (1989b)
CPB-WU after exposure
Table 8. Effects of single inhalation exposure to HCFC 133a
Species Exposure Exposure Effectsa Reference
(strain) concentration duration
(g/m3) (min)
Mice, male unspecified 10 anaesthesia and death; convulsions on recovery; AC50 and Robbins (1946)
(white) range LC50 were 394 and 1230 g/m3, respectively
Mice (strain unspecified 30 anaesthesia, convulsions and death; AC50 and LC50 were Raventós & Lemon (1965)
unspecified) range 212 and 738 g/m3, respectively
Mice (strain 123-1230 10 rapid onset of anaesthesia, rapid recovery after cessation of Shulman & Sadove (1965)
unspecified) exposure but no convulsions; AC50 and LC50 were 397 and
1033 g/m3, respectively
Rats, female 2500 - lack of muscular coordination in 3 min, anaesthesia in 4 min Diggle & Gage (1956)
(strain unspecified) and death within 8 min
Dogs unspecified - anaesthesia at 492 g/m3, respiratory depression and arrest Shulman & Sadove (1965)
range occurred at 1131 and 1427 g/m3, and circulatory arrest at
2902 g/m3
a AC50 = calculated concentration expected to produce anaesthesia in 50% of the test group
Table 9. Acute inhalation toxicity of HCFC 123
Species Exposure Exposure Effects Reference
(strain) concentration duration
(g/m3)
Mouse (strain not given 30 min LC50 = 463 g/m3 Raventós & Lemon (1965)
unspecified)
Rat (Charles 129-344 4 h LC50 = 200 g/m3; loss of mobility, lethargy, prostration Hall & Moore (1975)
River CD) at all concentrations; full recovery of survivors within
30 min post exposure
Rat (Charles 49-767 6 h LC50 = 329 g/m3; anaesthesia at 145 g/m3 and higher Coate (1976a)
River CD) concentrations; discoloration of lungs in most animals that
died, discoloration of liver in some of them
Rat (strain 6, 16, 31, 62 15 min unconditioned reflexes, locomotor activity, coordination Trochimowicz (1989)
unspecified) affected at 31 and 62 g/m3; full recovery within 30 min
post exposure
Hamster 63-194 4 h LC50 = 178 g/m3; incoordination, prostration at all Darr (1981)
(Chinese) concentrations; full recovery of survivors after exposure;
0% mortality at 163 g/m3, 100 mortality at 194 g/m3
Table 10. Acute inhalation toxicity of HCFC 124
Species Strain Exposure Exposure Effects Reference
concentration duration
(g/m3) (min)
Mouse unspecified 594 10 no effect Wada (1977)a
837 10 narcosis Wada (1977)a
2230 10 no mortality Wada (1977)a
2460 10 death Wada (1977)a
Rat Sprague-Dawley 268 240 no effect Kelly (1990)
Charles River COBS 558 300 reduced activity Coate (1976b)
Sprague-Dawley 893 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1283 240 prostration, lethargy, incoordination Kelly (1990)
Sprague-Dawley 1674 240 death Kelly (1990)
Charles River COBS 2009 300 narcosis, no mortality Coate (1976b)
Dog 2230-3910 10 narcosis Van Poznak & Artusio (1960)
a Attachment to correspondence from H. Wada, Daikon Kogyo Company Ltd. to M.B. Berenbaum, Allied Chemical Corporation,
entitled Anaesthetic activity and fatality (F-123, 123a, 124 and 11)
7.1.3.1 HCFC 141b
No deaths occurred at dermal doses of 2 g/kg body weight either
among rats (Janssen & Pot, 1988; Gardner, 1988) or rabbits (Brock,
1988a).
7.1.3.2 HCFC 132b
When Janssen & Pot (1989a) applied a single dose of HCFC l32b (2
g/kg) under an occluding dressing to the shaved skin of five male and
five female Wistar rats, there were no deaths. The clinical signs
observed were decreased respiratory rate, decreased startle response,
altered locomotor activity, restlessness and vocalization. Three male
and four female rats had swollen or slightly swollen livers on
autopsy.
7.1.3.3 HCFC 123
Several limit tests for dermal toxicity of HCFC 123 were
conducted. No mortality was observed at the limit dose of 2 g/kg body
weight in rats (Brock, 1988d; Trochimowicz, 1989) or rabbits (Brock,
1988e,f; Trochimowicz, 1989). The only clinical signs of toxicity were
red nasal or ocular discharges in one of five male and one of five
female rats, and slight to moderate body weight losses (up to 12% of
initial body weight). No gross pathological abnormality was observed
(Trochimowicz, 1989). In rabbits, only slight to moderate erythema was
observed (Trochimowicz, 1989).
7.2 Short-term inhalation exposure
7.2.1 HCFC 141b
Nikitenko & Tolgskaja (1965) reported a reduction in body weight
gain, a slight decrease in haemoglobin level and moderate
leucocytosis, some "minor changes" in blood parameters related to
liver and kidney function, and histopathological effects in the
respiratory tract of rats and guinea-pigs (number and strains
unspecified) that had been exposed to 40-50 g/m3 (2 h/day, 6
days/week) for 4 weeks. The purity of the substance and the specific
isomer were not indicated.
No adverse clinical signs and only "slight biochemical changes"
(no details given) were reported in rats (number and strain
unspecified) exposed to 48.5 g/m3 (6 h/day, 5 days/week) for 2 weeks
(Pennwalt Corporation, 1987).
In a 2-week inhalation toxicity study, Doleba-Crowe (1977)
exposed groups of 10 male rats to 0 or 48 g/m3 for 6 h/day, 5
days/week. The animals were observed for 14 days after exposure. No
adverse clinical signs were observed, and there were no differences in
body weights between treated and control animals. After the tenth
exposure, elevated red blood cell counts, plasma bilirubin level and
increased urinary fluoride concentrations were found, but all these
parameters returned to normal after 14 days. The treated animals
showed a more severe focal interstitial pneumonitis than controls 14
days after exposure, but no other treatment-related change was
observed.
Coombs et al. (1988) exposed five groups of 10 male and 10 female
Sprague-Dawley rats to 0, 24, 42, 68 and 97 g/m3 (6 h/day for 9
days, i.e. 5 days of exposure followed by 1 day without exposure and
then 4 days with exposure). Signs of central nervous system (CNS)
depression were seen during exposure to concentrations of 42 g/m3 or
more. At 97 g/m3, these signs were accompanied by a decrease in body
weight gain in males and a slightly reduced food intake in both sexes.
Glucose and aspartate serum transaminase (AST) levels were increased
at 97 g/m3, protein, cholesterol and sodium from 68 g/m3,
phosphate from 42 g/m3 and calcium from 24 g/m3. No
treatment-related histopathological changes were observed at any dose
level.
In a 13-week inhalation study (some animals were killed after 4
weeks), four groups each of 15 male and 15 female Fischer-344 rats
were exposed to 0, 10, 39 or 97 g/m3 (6 h/day, 5 days/week) as
described in two reports (Yano et al., 1989; Landry et al., 1989).
Alertness was reduced at 97 g/m3, and body weight gain and food
consumption were slightly reduced in all exposed groups. After both 4
and 13 weeks of exposure, plasma cholesterol, triglycerides and
glucose were slightly elevated in the rats exposed to 97 g/m3. No
changes in haematological or histopathological parameters were found.
7.2.2 HCFC 142b
Rats and guinea-pigs (numbers and strains not specified) were
exposed to a concentration of 448 g/m3 (isomer not specified), 2
h/day, 6 days/week, for 4 weeks (Nikitenko & Tolgskaja, 1965). A
decrease in the rate of body weight gain was observed at the end of
the study, as well as a reduction in haemoglobin concentration and the
number of erythrocytes, and an increase in the number of leucocytes.
Swelling of the alveolar septa and peribronchitis were the
histopathological changes observed in the lungs.
In a study in which 10 adult white rats were exposed to 410
g/m3 for 16 h/day, all animals died within 9 exposures. All of them
showed severe signs of pulmonary irritation at autopsy (consolidation
and hepatization of the lungs). The other organs appeared normal. No
signs of ill health were apparent in five rats exposed to a
concentration of 41 g/m3 (16 h/day for 2 months). Gross examination
of the organs on autopsy revealed no pathological changes, but
microscopic examination of the lungs showed round cell infiltration in
the lung of two animals. The appearance of sections of the livers was
normal (Lester & Greenberg, 1950).
No clinical, haematological, blood chemical, urine analytical or
histopathological evidence of effects attributable to repeated
exposure to HCFC 142b was found in 10 male Charles River CD rats
exposed to a concentration of 82 g/m3 (6 h/day, 5 days/week) for 2
weeks (Moore & Trochimowicz, 1976).
Kelly (1976) did not find any adverse clinical, haematological,
blood chemical, urine analytical or histopathological effects
attributable to HCFC 142b at exposure levels of either 4.1 g/m3 or
41 g/m3 (6 h/day, 5 days/week for 90 days) in groups of male and
female Charles River CD rats (27 of each sex at each treatment level)
or groups of male dogs (4 at each treatment level).
7.2.3 HCFC 132b
When 20 male Crl:CDRBR rats were exposed to 55 g/m3 (6 h/day,
5 days/week) for 2 weeks, reduction in body weight gain, irregular
respiration and CNS depression (lethargy, poor coordination,
occasional tremors and prostration) were seen. The CNS effects
disappeared within 30 min after each exposure. Pathological
examinations of rats sacrificed immediately after the tenth exposure
showed thymic atrophy and spermatogenesis arrest, but these changes
were not present in rats sacrificed 14 days after exposure ceased
(Hall, 1976).
Groups of 20 male and 20 female Crl:CDRBR rats were exposed to
0, 3, 11 and 27 g/m3 (6 h/day, 5 days/week) for 13 weeks (Kelly,
1988). Male rats exposed at all the concentrations of HCFC 132b showed
bile duct proliferation and disruption of spermatogenesis with cell
debris in the epididymides at the two higher concentrations. Other
effects included increases in liver/body weight ratio in males at all
concentrations and in females at the two higher concentrations.
Elevation of serum alkaline phosphatase activity was found in both
sexes exposed to 11 or 27 g/m3. During the study, all groups exposed
to HCFC 132b showed reduced food consumption and body weight gain. In
the two highest exposure groups there were depressions in the absolute
but not relative brain and testes weights. Other organ weight changes
were also seen (slight increases in heart, lung and kidney weights).
The biological significance of these weight changes is not clear since
there were no accompanying histological findings. During exposure to
27 g/m3, rats showed CNS depression as indicated by decreased
activity and low responsiveness to sound.
7.2.4 HCFC 133a
Shulman & Sadove (1965) exposed mice to anaesthetic
concentrations (the actual concentration was not specified) of HCFC
133a for 30 min per day on 12 consecutive days, and the animals were
killed for pathological evaluation after the last exposure by
overdosage of HCFC 133a. None of the mice showed any treatment-related
clinical effects, and no pathological changes were found in the organs
(heart, lung, liver, kidney, adrenal gland, spleen and pancreas)
examined microscopically.
Diggle & Gage (1956) investigated the effects of repeated
exposure (up to 8 days) of groups of 2-3 female rats. Concentrations
of HCFC 133a between 50 and 125 g/m3 caused incoordination and
lethargy, while at 250 or 500 g/m3 rats become comatose. They
recovered between each exposure and no dose-related pathological
changes were found on histological examination. No effect was seen at
25 g/m3 during seven exposures lasting 6 h/day.
In a study by Leuschner et al. (1977), 20 male and 20 female
Sprague-Dawley rats were exposed to 49 g/m3 (6 h/day, every day) for
90 days. Corresponding groups of 20 male and 20 female rats were used
as controls. Observations for overt clinical signs of toxicity and
investigations on body weight, food consumption, haematology, blood
and urine biochemistry, urine sediments, ophthalmology, auditory
reflex, organ weights, and histopathology were performed. There were
no treatment-related deaths. The rats were sedated during each
exposure but appeared normal before and after. Seventeen out of 40
rats developed bloody and inflamed noses; this was associated with
histological evidence of inflammatory changes of the mucosa. Body
weight gain was reduced, so that the terminal average body weights
were approximately 28 and 17% lower than those of male and female
controls, respectively. Food consumption in the treated groups was
also lower than in the controls. Haemoglobin concentration,
haematocrit, red blood cell counts and platelet counts were all
slightly reduced. Reduction in leucocyte counts of approximately 30%
and increase in reticulocyte counts of approximately 40% were seen.
There were reductions in plasma glucose levels of approximately 15%
and in protein levels of approximately 10%. Bromosulfophthalein
retention time was increased by approximately 35% and 62% in males and
females, respectively. There was no change in plasma enzyme
glutamic-pyruvic transaminase (GPT), alkaline phosphatase (AP) or
glutamicoxalacetic transaminase (GOT) activity. The thymus to body
weight ratio was reduced by approximately 50% and the testis and ovary
to body weight ratios by approximately 60 and 35%, respectively.
Histologically, these organs showed atrophy. Thyroid to body weight
ratio was increased by approximately 45% in males only. Atrophy of the
spleen was also observed. The exposure induced emphysema and oedema of
the lungs as well as bronchitis and pneumonia. The testicular atrophy
was consistent with the findings in three dominant lethal studies in
mice (Hodge et al., 1979, 1980; Kilmartin et al., 1980) and a
carcinogenicity study in rats (Longstaff et al., 1984) (see section
7.6 and 7.7).
Six beagle dogs were exposed by Leuschner (1977) to 24 g/m3
(6 h/day, daily) for 3 months and six control dogs were used. No
effects were seen on external appearance, faeces, food and water
consumption, body weight gain, haematology, blood and urine
biochemistry, urine sediments, electrocardiography, blood pressure,
ophthalmology, hearing or dentition. There was no effect on organ
weight at autopsy. No treatment-related histopathological changes were
seen on microscopic examination of a standard range of 24 tissues.
In two dominant lethal studies (for experimental details see
section 7.5.1.4), male mice were exposed to between 0.5 and 49 g/m3,
6 h/day, for 5 days (Hodge et al., 1980; Kilmartin et al., l980). The
mice were subdued at exposure concentrations of 2 g/m3 or more.
Deaths occurred as follows: in the first study 0/60 mice died at 0
g/m3, l7/60 at l2 g/m3, and 28/80 at 49 g/m3; in the second
study 0/80 died at 0 g/m3, 2/59 at 0.5 g/m3, 0/60 at 2.5 g/m3,
5/60 at 5 g/m3, and 20/60 at 12 g/m3.
7.2.5 HCFC 123
In a study by Doleba-Crowe (1978), Sprague-Dawley rats and beagle
dogs were exposed to concentrations of 0, 6 and 62 g/m3 (6 h/day, 5
days/week) for 90 days. At the high exposure level, both species
exhibited lack of motor coordination soon after the start of exposure.
This was followed by reduced motor activity and reduction in
responsiveness to noise. After removal from exposure, coordination and
activity returned to normal within 20 min. Other than final body
weight reductions and increased urinary fluoride level at both
exposure levels, no significant exposure-related effects were observed
in rats. At the high exposure level, dogs exhibited histopathological
changes in the liver and clinical chemistry changes including
increased levels of serum GOT and GPT, which might indicate slight
liver damage. No exposure-related effect was noted at the lower
exposure level.
In a 90-day inhalation study, albino rats obtained from Charles
River Breeding Laboratory were exposed to nominal levels of 0, 3, 6,
and 31 g/m3, 6 h/day, 5 days/week (Rusch, 1985). No
treatment-related deaths occurred in the study and mean body weight
reductions observed in the males at the highest exposure level and in
females at the two highest exposure levels were significant only at
the end of the study. Slight depression was observed in heart weight
in both male and female rats exposed to 31 g/m3. While depressions
of the kidney weights and kidney/brain weight ratio (but not kidney to
body weight ratio) were observed in male rats in all the three
exposure groups, these effects were outside the normal range only at
the highest exposure level. A similar depression in kidney weight and
kidney/body weight ratio (but not in kidney/brain ratio) occurred in
females at the highest exposure level. Increased liver/body weight
ratios (but not liver weight or liver/brain weight ratios) were
observed in all three exposure groups of females, but in males only at
the highest exposure level. No significant difference was found in
organ weights or ratios in animals sacrificed at the end of a 30-day
recovery period. The absence of histopathological findings and of
effects at the end of the recovery period indicates that the
toxicological significance of the organ weight changes in this study
is unclear.
In a 4-week inhalation toxicity study (Kelly, 1989; Trochimowicz,
1989), rats (10 of each sex at each exposure level) were exposed to 0,
6, 31, 62 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Statistically significant body weight depression occurred in all
female groups and in the two highest male groups, but was dose-related
only in the male rats. At concentrations of 31 mg/m3 or more, rats
exhibited dose-related anaesthesia. At 62 and 125 g/m3, rats became
lethargic during exposure but were normal when they were examined
again 16 to 18 h after exposure. A dose-related increase in liver/body
weight ratio was observed in all female groups (a 27% increase at the
highest level) and in the two highest-exposure male groups (an 18%
increase at the highest level). Decreased cytochrome P-450 activity in
the liver was also found in all female exposure groups and in the male
groups exposed at the two highest levels. Histopathological
examination showed no adverse effects attributable to HCFC 123 in
liver or in any other organ at any exposure level. Microscopic
examination revealed that testicular degeneration and hypospermia
occurred in two out of four male exposure groups, but this was
believed to be due to reduced body weight (because of an increase in
relative testicular weight without a corresponding increase in
absolute testicular weight) or may have resulted from spontaneous
lesions. The fact that the incidence of testicular degeneration was
highest in the 31- and 125-g/m3 exposure groups (5 out of 10 and 6
out of 10 animals, respectively), and lower in the 0-, 6-, and
62-g/m3 exposure groups (2 out of 10, 1 out of 10 and 2 out of 10
animals, respectively) is consistent with the sporadic nature of this
lesion in this strain of rat. However, it is not possible to evaluate
the significance of this testicular effect from the information
available.
Another 28-day inhalation toxicity study in rats was conducted to
characterize further the potential effects of HCFC 123 on the liver
(Lewis, 1990). In addition, urine samples were examined to identify
metabolites of HCFC 123. Groups of six male Charles River CD rats were
exposed to 0, 6, 31 or 125 g/m3 (6 h/day, 5 days/week) for 4 weeks.
Body weights were statistically significantly reduced in all treated
groups compared to controls, the greatest reduction occurring in the
high-dose group. A concentration-related decrease in serum cholesterol
levels was found in all test groups. This reduction was statistically
significant compared to controls at the medium and high
concentrations. Serum triglyceride levels were significantly reduced
to a similar extent in all treated groups. A statistically significant
increase in absolute and relative liver weights compared to controls
was seen in the high-dose rats. Hepatocyte hypertrophy and mild fatty
vacuolation was found at all concentrations tested but the severity
was greatly reduced at the low exposure level. Electron microscopic
examination revealed a treatment-related induction of peroxisome
proliferation at the medium and high exposure concentrations. A
statistically significant concentration-related increase in relative
testes weight was observed in all treated groups (11-30% above
control), but no compound-related morphological or microscopic changes
were observed. Urine analysis indicated the presence of
trifluoroacetic acid as a major metabolite.
In a study by Malley (1990a), groups of 10 male and 10 female
Crl:CDRBR rats were exposed to concentrations of 0, 2, 6 or 31
g/m3 (6 h/day, 5 days/week) for 90 days. No effect on food
consumption or body weight was observed. At the highest exposure level
the animals exhibited anaesthesia and a decreased response to auditory
stimuli. Serum triglyceride and glucose levels were significantly
decreased at all exposure levels, while serum cholesterol was
significantly lower in females exposed to the two highest
concentrations. The mean lymphocyte and white blood cell counts in
female rats were decreased at the highest exposure level. Male rats
had significantly higher alanine aminotransferase and alkaline
phosphatase activity at the two highest exposure levels. In female
rats alanine aminotransferase activity was elevated at the highest
exposure level. Urine fluoride concentrations were increased in
females at all exposure levels, but in males only at the highest
exposure level. Absolute liver weights were significantly higher in
male rats at the highest exposure level and in female rats at the two
highest exposure levels, while relative liver weights in both male and
female rats were higher at the two highest exposure levels. Hepatic
peroxisomal beta-oxidation activity in both male and female rats was
1.9 to 3.8 times higher than in controls, indicating an induction of
hepatic peroxisome proliferation. No treatment-related gross or
microscopic liver changes were observed.
7.2.6 HCFC 124
When ten male rats were exposed to approximately 560 g/m3 (6
h/day, 5 days/week) for 14 days, no adverse haematology, clinical
chemistry, urine analysis or histopathological changes were observed.
Rats showed irregular respiration, lethargy and poor coordination
(Hall, 1976).
Trochimowicz et al. (1977) reported no adverse effect following
the clinical and histopathological evaluation of rats exposed to
concentrations of 560 g/m3 (6 h/day, 5 days/week) for 2 weeks.
Brewer (1977) exposed groups of 60 Sprague-Dawley rats (35 males and
25 females) to concentrations of 0, 3, 5 or 28 g/m3 (6 h/day, 5
days/week) for 3 months. Ten male and ten female rats were examined
and sacrificed after 45 days and a similar number after 92 days. Ten
male and five female rats of each group were maintained without
further exposure for an additional 30-day period after exposure.
Clinical signs were observed, body and organ weights determined, and
haematological, biochemical and histopathological examinations carried
out on all animals. No statistically significant differences in body
weight gain were noted. Haematology, clinical chemistry, and urine
analysis in treated rats were normal and comparable to findings in
control animals. Urinary fluoride excretion was increased after 45
days of exposure in both males and females (4.0 and 3.5 times,
respectively) at an exposure level of 28 g/m3; at the two lower
levels, determinations were not made. After 95 days of exposure, the
urinary fluoride level was elevated only in males at all three
exposure levels (by 1.5, 1.7 and 1.8 times, respectively), and this
effect persisted throughout the 30-day period after exposure. Gross
and histopathological examinations did not reveal any
treatment-related changes in any group of animals. Statistically
significant difference in organ weights between treated and control
rats were found. Liver weights were increased significantly in males
at 5 and 28 g/m3, while the lung and adrenal gland weights were
significantly decreased in males at all three exposure levels. In the
absence of any histopathological change, the biological significance
of these organ weight changes is unclear.
Malley (1990b) exposed groups of ten male and ten female rats to
HCFC 124 concentrations of 0, 3, 11, 56 or 279 g/m3 (6 h/day, 5
days/week) for 4 weeks. Treatment-related effects on body weight, food
consumption, mortality, clinical laboratory parameters, organ weights,
and tissue morphology changes were not found at any exposure level.
During exposure, rats exposed to 279 g/m3 were lethargic and
uncoordinated. However, no evidence of lethargy and incoordination was
observed shortly after exposure. The author considered the exposure
concentration of 56 g/m3 the no-observed-adverse-effect level
(NOAEL) based on the clinical observation of lethargic and
uncoordinated movement during exposure to 279 g/m3, which was not
observed at 56 g/m3.
Malley (1991) exposed Crl:CDRBR rats (20 rats of each sex at
each exposure level) to HCFC 124 at concentrations of 0, 28, 84 and
279 g/m3 (6 h/day, 5 days/week) for 90 days. As part of this study,
a functional observation battery (FOB) was conducted on 10 rats of
each sex at each exposure level at various intervals during the course
of exposure. There were no compound-related effects relative to body
weight, food consumption, mortality, haematology, organ weights or
histopathology at any exposure concentration. However, male rats, at
a level of 84 and 279 g/m3, had lower serum triglyceride
concentrations and a decreased arousal (4 of 10 and 6 of 10 rats,
respectively). Persistent decrease of forelimb grip strength was found
in high-dose females. However, there was no associated decrease in
hindlimb grip strength or change in gait or footplay. Females at the
highest concentration showed an increase in alkaline phosphatase. Both
sexes at 279 g/m3 were less responsive to auditory stimuli, as
demonstrated by a decreased reaction to a sharp knock on the chamber
wall. Plasma fluoride, urinary fluoride and fractional clearance of
free fluoride were also increased at all exposure levels in both
sexes. The author concluded that the no-observed-effect level (NOEL)
was 28 g/m3 for male rats and 84 g/m3 for female rats.
7.3 Skin and eye irritation; sensitization
7.3.1 Skin and eye irritation
7.3.1.1 HCFC 141b
Treatment of the intact skin of New Zealand albino rabbits with
0.5 ml of undiluted HCFC 141b under occlusive patch (during 4 and 24
h in the two respective studies) did not produce signs of dermal
irritation during a 3-day observation period (Liggett, 1988a; Brock,
1988b).
Two studies were conducted with HCFC 141b on groups of six New
Zealand albino rabbits where the undiluted compound (0.1 ml) was
instilled into the eyes. No signs of irritation occurred within 3 days
in one study (Liggett, 1988b), but the compound was found to be a
"mild" irritant in the other study (Brock, 1988c). The majority of
rabbits in the latter study showed conjunctival redness (5/6), mild
chaemosis (3/8) and blood-tinged discharge (4/6).
7.3.1.2 HCFC 142b
Brittelli (1976a) observed no effects on the cornea or iris but
slight conjunctival swelling with some discharge in an eye irritation
test with HCFC 142b.
7.3.1.3 HCFC 132b
One drop (approximately 0.05 ml) each of 100% HCFC 132b and a 10%
solution in propylene glycol was applied and slightly rubbed into the
shaved intact shoulder skin of 10 male albino guinea-pigs, but the
area was not occluded. The pure compound produced only mild irritation
in one animal only. No irritation was induced by the 10% solution
(Goodman, 1976).
Undiluted HCFC 132b (0.1 ml) was placed into the right
conjunctival sac of two albino rabbits, and after 20 seconds one
treated eye was washed with water for 1 min. Observations of the
cornea, iris and conjunctiva were made after 1 and 4 h, and 1, 2, 3
and 7 days later. Slight corneal opacity and "mild" to "moderate"
conjunctival irritation were seen in both rabbits up to 3 days after
dosing, but had disappeared by 7 days after dosing (Brittelli, 1976b).
7.3.1.4 HCFC 123
Minimal dermal irritation with HCFC 123 was observed in rabbits
(Brock, 1988e,f). HCFC 123 (purity 99.0%) produced no skin irritation
when 0.5 ml/6 cm2 was applied to the clipped intact skin of four
male and two female New Zealand rabbits for 4 h (Trochimowicz, 1989).
Brittelli (1976c) reported HCFC 123 to be a "mild" ocular
irritant causing reversible corneal opacity in rabbits. In another
study by Daly (1979)a, HCFC 123, when instilled undiluted (0.1 ml)
into the conjunctival sac of the rabbit eye without subsequent
washing, produced "mild" to "moderate" conjunctival irritation. With
washing, "mild" transient corneal opacity and "mild" to "moderate"
conjunctival irritation were observed. With or without washing,
complete recovery occurred within 3-7 days.
7.3.2 Skin sensitization
7.3.2.1 HCFC 141b
No delayed contact hypersensitivity was found in any of the 20
Hartley Dunkin guinea-pigs in a Magnusson-Kligman maximisation test
with HCFC 141b (Kynoch & Parcell, 1989).
7.3.2.2 HCFC 132b
A series of four sacral intradermal injections of HCFC 132b was
given (once per week at 7-day intervals) over a 3-week period to
groups of nine male albino guinea-pigs (0.1 ml of a 1% solution in
dimethyl phthalate). Fourteen days after the last application, the
animals were challenged with either 1 drop (0.05 ml) of undiluted
liquid or a 19% solution of test material in propylene glycol on the
shaved skin. No evidence of sensitization was observed (Goodman,
1976).
7.3.2.3 HCFC 123
When applied topically to the back of male guinea-pigs as 10% or
50% solutions in propylene glycol, HCFC 123 produced no sensitization
at challenge (Goodman, 1975; Daly, 1979).
7.4 Long-term exposure
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternative Fluorocarbon Toxicity Testing (Rusch, 1989) sponsored
by an international industry consortium. An interim report after the
first year of the study is available on HCFC 123 (Malley, 1990b).
a Personal communication entitled "Toxicity testing summary for
alternative fluorocarbons" by J.J. Daly to CFTA Interindustry
Safety Committee, Wilmington, Delaware, USA, E.I. Du Pont de
Nemours and Co.
7.4.1 HCFC 142b
Four groups each containing 130 male and 110 female Sprague-
Dawley CD rats were exposed by inhalation to concentrations of 4.1, 41
and 82 g/m3 (6 h/day, 5 days/week) for 104 weeks. No exposure-
related effects were found on mortality, body weight, haematology,
clinical chemistry, urine analysis, histopathological or
ophthalmological findings (Seckar et al., 1986). However, high
mortality (more than 50% by the end of the study) was seen in all
groups including controls.
7.4.2 HCFC 123
Findings associated with the first year of the PAFT-supported
study were provided by Malley (1990c). Following 12 months of
inhalation exposure to 0, 2, 6 or 31 g/m3, 10 rats (Crl:CDRBR) of
each sex at each concentration were sacrificed, selected organs were
weighed, and tissues were examined for gross and microscopic lesions.
Body weight and body weight gain were significantly lower in high-dose
males and in mid- and high-dose females. Food consumption was higher
and food efficiency was lower in high-dose males and females over the
course of the l year of exposure. The lower food efficiency appeared
to be directly related to the lower body weight gain at the high
concentration (31 g/m3). High-dose males and females exhibited
anaesthesia-like behaviour (e.g., less responsiveness to auditory
stimuli compared to control rats). No compound-related effects on
mortality or survival time were found in any treated group. Clinical
chemistry parameters measured at 6 months and 1 year revealed several
compound-related changes. The most noteworthy findings were the effect
on lipid and carbohydrate metabolism. Significant decreases of serum
triglyceride and glucose concentrations were found in males and
females at all dose levels. Serum cholesterol was significantly lower
in females at all dose levels and in high-dose males. Urine analysis
indicated significant increases of urinary fluoride concentration in
both sexes at all dose levels. High-dose (31 g/m3) males and females
had significantly higher mean relative liver weights, but no
compound-related gross or histopathological changes were found in the
livers of exposed animals. Dose-related increases in hepatic
beta-oxidation enzyme activity were found for males (2.3, 3.1 and 4.0
fold increases at 2, 6 and 31 g/m3, respectively) and females (1.7
and 3.1 fold increase at 6 and 31 g/m3). The higher enzyme activity
indicated an induction of hepatic peroxisome proliferation. However,
compound-related differences in the rate of cell proliferation, as
measured by changes in labelling index, were not found at any exposure
concentration. These data indicate that during the first year of
exposure, HCFC 123 did not induce an increase in regenerative repair
of the liver, which is consistent with the absence of morphological or
microscopic changes in the liver of exposed animals. A
no-observed-effect level (NOEL) was not achieved in this study based
on the effects on clinical chemistry parameters and higher hepatic
peroxisomal activity.
7.5 Reproduction, embryotoxicity, and teratogenicity
7.5.1 Reproduction
7.5.1.1 HCFC 141b
A 2-generation reproduction study on HCFC 141b is currently in
progress (Rusch, 1989).
7.5.1.2 HCFC 142b
In a dominant lethal study on rats, no effect on male
reproduction was found (Seckar et al., 1986). No other studies on
reproductive effects are available.
7.5.1.3 HCFC 132b
No data are available on the effects of HCFC 132b.
7.5.1.4 HCFC 133a
No studies are available in which the effects of HCFC 133a on
reproduction were investigated. Some information is available,
however, on the effects on male fertility from a series of dominant
lethal and "combined dominant lethal fertility" studies in mice (Hodge
et al., 1979, 1980; Kilmartin, 1980). In these studies groups of male
mice were exposed to between 0 and 98 g/m3, 6 h/day, for 5
consecutive days. At the end of the treatment, dominant lethal and
fertility effects were assessed in 15 males (30 controls) from each
treatment group, which were each housed with two virgin females for
four consecutive nights. This 4-nightly mating procedure was continued
for 8-9 consecutive weeks. In the two later studies, satellite group
males (at least two from each group per week) were killed, their
epididymal sperm assessed for abnormalities and their testes examined
histopathologically. A reduction in male fertility (seen as
exposure-related decreases in the proportion of pregnant females:
e.g., 0-60% of pregnant females in treatment groups compared to
90-100% in controls) was seen between weeks 2-8 following exposure.
The severity of the effects were related to time, the maximum
reductions in pregnancies being seen at around 3-6 weeks after
exposure. No effect on fertility was seen at 0.5 g/m3. Reduced
fertility was observed at concentrations of 2.5 g/m3 or more, and
histopathological evidence of degeneration of spermatogenic cells was
seen at concentrations of 5 g/m3 or more. There was a slight and
transient increase in the percentage of abnormal sperm at 2.5 g/m3
or more.
7.5.1.5 HCFC 123
A 2-generation inhalation reproduction study on rats sponsored by
PAFT is currently in progress (Rusch, 1989).
7.5.2 Embryotoxicity and teratogenicity
7.5.2.1 HCFC 141b
Hughes et al. (1988) exposed three groups of 25 pregnant female
Sprague-Dawley rats to 15, 39 or 97 g/m3 for 6 h/day from days 6 to
15 of pregnancy. Some signs of maternal toxicity (prenarcotic signs,
piloerection and reduced alertness) were observed at all exposure
levels. At the highest level, salivation, hunched posture, and
diaphragmatic breathing, a marked increase in water consumption, a
transient reduction in food intake, and a marginal reduction in body
weight gain were observed. At the highest exposure level, incidences
of subcutaneous oedema and haemorrhaging and embryonal death were
significantly increased. Reduced litter and mean fetal weights and
retarded ossification were observed. However, no teratogenic effect
occurred in any group.
In a further study (Hughes et al., 1989), 16 pregnant female New
Zealand rabbits were exposed to 7, 20 or 61 g/m3 for 6 h/day from
days 7 to 19 of pregnancy. Signs of maternal toxicity (prenarcotic
signs, palpebral ptosis, respiratory disturbances and body weight
loss) were observed at the two highest exposure levels. There was no
indication of any treatment-related effect on embryo or fetal
development or evidence of teratogenicity at any exposure level.
7.5.2.2 HCFC 142b
Groups of 25 pregnant Sprague-Dawley rats were exposed to 4 or 41
g/m3 for 6 h/day from day 3 to day 15 of gestation (Culik & Kelly,
1976). The exposure had no effect on the body weight gain of the
mothers, and no clinical signs of toxicity were observed in any of the
animals. The number of early or late resorptions or number of live
fetuses per litter was not affected by the exposure. Exposure also had
no effect on embryonic development, as measured by the weight and
crown-rump length of the fetuses, and there was no evidence of a
teratogenic effect.
Damske et al. (1978) exposed groups of 20 pregnant female
Sprague-Dawley CD rats to concentrations of 0, 13 and 39 g/m3 for 6
h/day on days 6-15 of gestation. There were no treatment-related
effects in the dams or evidence of exposure-induced terata, variations
in sex ratio, embryotoxicity or inhibition of fetal growth and
development. There was an increase in the incidence of delayed
ossification of the supraoccipital bone in both exposure groups; this
effect was not observed in the control group.
7.5.2.3 HCFC 132b
In a pilot study, groups of seven or eight pregnant rats were
exposed to 3, 11 or 28 g/m3 for 6 h/day on days 6-15 of gestation.
Maternal and fetal body weights were reduced at all exposure levels.
The number of resorptions was increased in the 11- and 27-g/m3
exposure groups (Alvarez, 1988).
In a development screening test using Hydra (Johnson et al.,
1986), embryotoxicity was observed at paternally toxic doses.
7.5.2.4 HCFC 133a
Weigand et al. (1977) investigated the potential embryotoxic or
teratogenic effects of HCFC 133a in Wistar rats and Himalayan rabbits.
They also examined the effects of pre-treatment with progesterone to
determine whether any of the adverse effects of HCFC 133a could be
explained by its interaction with and depletion of progesterone in
early pregnancy, since this hormone is responsible for the electrical
and mechanical quiescence of the myometrium. Twenty-four pregnant
Wistar rats, of which 12 were injected subcutaneously with
progesterone (6 mg/day) prior to HCFC 133a exposure, were exposed to
25 g/m3 for 6 h/day on days 7-16 of gestation (the day on which
sperm was found in the vaginal smear was taken as day 1 of gestation).
Twelve pregnant Himalayan rabbits were exposed to the same
concentration on days 7-19 of gestation (the day of mating being taken
as day 0 of gestation). No controls were used and data were compared
with historical control data. Mild transient sedation, piloerection,
reduced body weight gain and reduced food consumption were observed in
the rats. At autopsy on day 21 of gestation, no macroscopic change was
observed. There was a prenatal mortality of 77% in the dams with no
pre-treatment with progesterone and 82% in the pre-treated dams.
Placental weight, fetal weight and crown-rump length were reduced and
some of the surviving fetuses showed generalized oedema (8/53) and
external anomalies of the limbs and tail (5/53). There was no
significant difference between the rats pre-treated with progesterone
and those without pre-treatment. In rabbits, there were reductions in
body weight gain and food consumption. All animals showed vaginal
bleeding during the last three days of the exposure, and 4 out of 12
aborted. By autopsy on day 29 all fetuses had died.
Culik & Kelly (1979) exposed Charler River CD rats to approximate
concentrations of 0, 2.5, 10, 25 or 98 g/m3 for 6 h/day on days 6-15
of gestation, and the rats were subjected to autopsy on day 21 of
gestation. There was no clear evidence of maternal toxicity. Evidence
of embryotoxicity was observed at all exposure levels. In the control
and the 2.5-g/m3 exposure groups, there was neither fetal death nor
total litter resorption. Fetal weights and crown-rump lengths were
lower in all the groups exposed to HCFC 133a than in controls. At 10
g/m3, 4/21 pregnant females had total resorptions and only 67
fetuses were alive in the other females. At 25 g/m3, 37/41 pregnant
females had total resorptions and only 7 fetuses were alive in the
other females. At 98 g/m3, 22/23 pregnant females had total
resorptions and only one fetus was alive in the other female.
Treatment-related increases in the incidence of runts and delayed
ossification in several bone structures were also observed at all
exposure levels. An increased incidence of hydronephrosis was reported
in all treated groups. Thus, the no-observed-effect level for
embryolethality was 2 g/m3 but, in view of the reduced fetal size
and weight at this exposure level, the no-observed-effect level for
embryotoxicity was not established from this study.
7.5.2.5 HCFC 123
Kelly et al. (1978) exposed 25 pregnant female rats to 62 g/m3
for 6 h/day on days 6-15 of gestation. Dams and fetuses were
sacrificed on day 21 and examined for gross changes. No embryotoxicity
or teratogenic effects were seen.
Two groups of 20 pregnant female rats were exposed to 0 and 31
g/m3 for 6 h/day on days 6-15 of gestation (Rusch, 1985). The
animals were sacrificed on day 20 and all dams and fetuses examined.
The maternal mean body weight in the exposed group was depressed to a
statistically significant degree on days 12 and 15 of the gestation
period. At termination, maternal mean body weights were still
depressed but not to a statistically significant degree. The numbers
of corpora lutea, implantation sites, resorption sites and fetuses
were similar in control and treated dams.
In a range-finding study, groups of six pregnant rabbits were
exposed to HCFC 123 concentrations of 0, 6, 31, 62 and 125 g/m3 for
6 h/day on days 6-18 of gestation (Schroeder, 1989a). All treated
rabbits lost weight during the study and food consumption was markedly
reduced, particularly at the two highest exposure levels. At these two
levels an increased number of resorption was also observed. In the
final study (Schroeder, 1989b; Trochimowicz, 1989), 24 mated females
per exposure group were exposed to 3, 9 or 31 g/m3 for 6 h/day
during days 6-18 of gestation. No mortality was observed in the
control or the low or medium exposure groups. The death of one rabbit
in the high exposure group was not considered by the author to be
treatment-related. There was evidence of maternal toxicity during days
6-18 of gestation at all exposure levels. Statistically significant
treatment-related mean body weight losses were observed in all the
test groups during the exposure period, compared to the control group
which showed a slight mean body weight gain. Mean daily food
consumption was also statistically lower in test groups than in
controls on most exposure days. There was no evidence of embryotoxic,
fetotoxic or teratogenic effects.
7.5.2.6 HCFC 124
Brewer & Smith (1977) exposed a group of 20 pregnant Charles
River CD rats to 30 g/m3 for 6 h/day during days 6-15 of gestation.
Maternal body weight measurements and clinical observations did not
reveal any difference between exposed and control groups, and no
maternal deaths occurred. The incidence of resorption sites in the
treated group was higher than in controls but within the range
commonly experienced with the strain of rats employed. The numbers of
corpora lutea, implantation sites, and fetuses in the treated groups
were similar to those in the controls. Fetal body weights were not
altered by treatment.
In a recent range-findings study, Rickard (1990b) exposed
Sprague-Dawley rats (4-6 per group) to minimal HCFC 124 concentrations
of 0, 3, 11, 56 or 279 g/m3 for 6 h/day on days 6-15 of gestation.
No change in mean body weight gain or evidence of embryotoxic effects
was observed. Internal and skeletal examinations were not conducted;
only external examination of fetuses was performed.
Schroeder (1991), in a range-finding study, exposed New Zealand
white rabbits (7 per group) to nominal HCFC 124 concentrations of 0,
28, 84 and 279 g/m3 for 6 h/day on days 6-18 of gestation. The only
sign of maternal toxicity was decreased activity during exposure at
the two highest concentrations. There was no evidence of
embryotoxicity, fetotoxicity or teratogenicity at any exposure
concentration.
7.6 Mutagenicity
7.6.1 HCFC 141b
The data from in vitro and in vivo studies are summarized in
Table 11.
HCFC 141b did not appear to damage bacterial DNA in a repair
assay, but produced conflicting evidence for mutagenicity in other
bacterial assays. Although sample purities differed in the two assays,
this alone may not account for the difference in response.
No significant response was obtained in the in vitro V79 cell
hprt locus assay.
Chromosomal aberrations were induced in two in vitro studies
with CHO (Chinese hamster ovary) cells, but this activity was not
apparent either in one assay with cultured human lymphocytes or in two
in vivo studies for micronucleus induction in mouse bone marrow
cells.
7.6.2 HCFC 142b
The data from in vitro and in vivo studies are summarized in
Table 12.
There was evidence of induction of base substitution mutations by
HCFC 142b in four of the five bacterial tests conducted. In two of
these studies this effect was apparent only in the presence of
exogenous metabolic activation.
A positive response, but without supporting data, was reported in
a single BHK (baby hamster kidney) 21 cell transformation assay.
No evidence of mutagenic activity of HCFC 142b was seen in a bone
marrow cytogenetic test or a dominant lethal study in rats.
7.6.3 HCFC 132b
The data from in vitro studies are summarized in Table 13.
HCFC 132b was tested in bacterial assays (three studies) and in
an in vitro chromosomal aberration test using a CHO cell line.
There was no evidence of mutagenic potential of HCFC 132b in
these studies.
7.6.4 HCFC 133a
The data from in vitro and in vivo studies are summarized in
Table 14.
HCFC 133a did not induce mutations in bacteria and did not
increase the proportion of BHK 21 cells forming colonies in soft agar.
Chromosomal aberrations were not induced in a single rat bone
marrow test using high concentrations. There were small, statistically
significant increases in the incidences of early deaths in two of
three male mouse dominant lethal assays. These increases occurred in
mating weeks 6-8 in one study and in mating week 7 in the other, in
which the lowest effective dose was 12.3 g/m3. No increases in early
deaths were observed in the third study, in which the highest
concentration tested was 12.3 g/m3.
7.6.5 HCFC 123
The data from in vitro genotoxicity studies are summarized in
Table 15.
HCFC 123 showed no evidence of mutagenic potential when tested in
suspension and plate assays using bacteria or yeasts.
In vitro cytogenetic tests were performed using cultured human
lymphocytes, in which HCFC 123 was tested both in the liquid and
gaseous phase. There was evidence of clastogenic activity in both the
presence and absence of exogenous activation systems, when HCFC 123
was tested in the gaseous phase; in the liquid phase study, an
increase in chromosomal aberrations was seen in the absence of
exogenous activation.
Table 11. Genetic toxicity of HCFC 141b
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Escherichia coli, DNA repair - - 10 mg/ml, Hodson-Walker & May (1988b)
18 hc
Salmonella typhimurium, reverse + + 1455 g/m3, 99.6% purity, positive Hodson-Walker & May (1988a)
mutation with TA98, TA100, TA1535, 48 hc in TA1535, negative
TA1537, TA1538 in other strains
S. typhimurium, reverse mutation with - - 1455 g/m3, 99.95% purity May (1989)
TA98, TA100, TA1535, TA1537, TA1538 48 hc
E. coli, reverse mutation with WP2 uvrA - - 1455 g/m3, 99.95% purity May (1989)
48 hc
Gene mutation in vitro, V79 cells, hprt - - 1430 g/m3, 99.6% purity Bootman et al. (1988a)
locus 3 hc
Chromosomal aberrations in vitro, CHO ± - 1 mg/ml liquid phase; increase Wilmer & De Vogel (1988)
cells in gaps only
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.6% purity Bootman & Hodson-Walker
cells 3 hc (1988)
Chromosomal aberrations in vitro, CHO + + 485 g/m3, 99.83% purity Hodson-Walker (1990a)
cells 4 hc
Table 11 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations in vitro, human - - 1700 g/m3, 99.83% purity Hodson-Walker (1990b)
lymphocytes 3 h (+ema)c
243 g/m3,
24 h (-ema)
Micronucleus test in vivo, mouse bone - NA 165 g/m3, 99.98% purity; marrow Vlachos (1989)
marrow 6 h sampled at 24, 48 and
72 h
Micronucleus test in vivo, mouse bone - NA 95 g/m3, marrow sampled at 24, Bootman et al. (1988b)
marrow 6 h 48 and 72 h
a NA = not applicable; ± = inconclusive
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
c in vitro assay in an enclosed system
Table 12. Genetic toxicity of HCFC 142b
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 1640 g/m3, negative in all Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hd strains
TA1537
S. typhimurium, reverse mutation with + + 2050 g/m3, weakly positive only Koops (1977)
TA98, TA100, TA1535, TA1537, TA1538 48 hd in TA1535 (2-8 fold
increase at 2050 g/m3)
S. typhimurium, reverse mutation with + + not specifiedd single gaseous conc. Jagannath (1977)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 1-72 h, positive in
TA100 and TA1535
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and McGregor (1976)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
S. typhimurium, reverse mutation with - + 2050 g/m3, positive in TA100 and Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 48 hd TA1535, experimental
data not reported
Cell transformation, in vitro BHK 21 ND + not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified, exposure
for 1-24 h, experimental
data not reported
Table 12 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Chromosomal aberrations, in vivo rat - NA 82 g/m3, Seckar et al. (1986)
bone marrow 6 h/day, 5 days/
week for 13 weeks
Dominant lethal assay, male CD-1 rat - NA 82 g/m3, Seckar et al. (1986)
6 h/day, 5 days/
week for 15 weeks
a NA = not applicable; ND = not determined
b LED = lowest effective dose; HED = highest effective dose
c purity not specified for these studies
d in vitro assay in an enclosed system
Table 13. Genetic toxicity of HCFC 132b
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 4 mg/plate liquid phase Russell (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - 550 g/m3b Waskell (1979)
TA98, TA100, TA1535, TA1537
S. typhimurium, reverse mutation with - - 578 g/m3b 99.9% purity Koorn (1988)
TA98, TA100, TA1535, TA1537, TA1538
Chromosomal aberrations, in vitro CHO - - 14.2 mg/ml, liquid phase Wilmer & de Vogel (1988)
cells 3 h (+ema)b
3.0 mg/ml,
21 h (-ema)b
a +ema = with exogenous metabolic activation; -ema = without exogenous metabolic activation; LED = lowest effective dose;
HED = highest effective dose
b in vitro assay in an enclosed system
Table 14. Genetic toxicity of HCFC 133a
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2460 g/m3, McGregor (1976)
mutation with TA98, TA100, TA1535, 24 hd
TA1538
S. typhimurium, reverse mutation with - - 172 g/m3, Waskell (1979)
TA98, TA100 48 hd
S. typhimurium, reverse mutation with - - 49 g/m3, Edmunds et al. (1979)
TA98, TA100 8 hd
Cell transformation, in vitro BHK 21 - - gas, 3 h concentrations not Longstaff et al. (1984)
cell growth in soft agar assay specified, experimental
data not provided
Chromosomal aberrations, in vivo, - NA 98 g/m3, 6 h, marrow sampled 24 h Anderson & Richardson
male AP rat bone marrow and 6 h/day after single and 6 h (1979)
5 days/week after multiple exposure
Dominant lethal assay, male CD-1 + NA 49 g/m3, 6 h/ early deaths increased Hodge et al. (1979)
mouse day, 5 days/week in mating weeks 6-8
Dominant lethal assay, male CD-1 + NA 12 g/m3, 6 h/ early deaths increased Hodge et al. (1980)
mouse day, 5 days/week in mating week 7, no
dose response
Table 14 (contd).
Test system Resultsa Dose Remarksc References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Dominant lethal assay, male CD-1 - NA 12 g/m3, 6 h/ Kilmartin et al. (1980)
mouse day, 5 days/week
a NA = not applicable
b HED = highest effective dose; LED = lowest effective dose
c purity not provided for these studies
d in vitro assay in an enclosed system
Table 15. Genetic toxicity of HCFC 123
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 937 g/m3, 6 h Barsky (1976)
mutation with TA98, TA100, TA1535,
TA1537, TA1538
S. typhimurium, reverse mutation with - - not specifiedc single gaseous conc. Brusick (1976)
TA98, TA100, TA1535, TA1537, TA1538 (not specified), exposure
for 5 or 24 h
S. typhimurium, reverse mutation with - - 625 g/m3, 72 h experimental data not Longstaff et al. (1984)
TA98, TA100, TA1535, TA1538 provided
S. typhimurium, reverse mutation with - - not specifiedc gaseous exposure, Callander (1989)
TA98, TA100, TA1535, TA1537, TA1538 concs. not specified
Sacharomyces cerevisiae: D4, forward - - not specified concs. not specified, Brusick (1976)
mutation exposure for 4-72 mins
Chromosomal aberrations, in vitro, + - 75 µg/ml, purity 99.95% Dance (1991)
human lymphocytes 24 h
Chromosomal aberrations, in vitro, + + 1875 g/m3, purity 99.95% Edwards (1991a)
human lymphocytes 3 h (+ema)c
156 g/m3,
24 h (-ema)c
Cell transformation, in vitro, BHK 21 ND - not specified liquid phase, concs. Longstaff et al. (1984)
cell growth in soft agar assay not specified,
experimental data
not provided
Table 15 (contd).
Test system Resultsa Dose Remarks References
LED/HEDb
Without With
exogenous exogenous
metabolic metabolic
activation activation
Micronucleus test, in vitro, mouse - NA 112 g/m3, 6 h marrow sampled 24, Müller & Hoffman (1988)
bone marrow 48 and 72 h after
exposure
a ND = not determined; NA = not applicable
b LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous metabolic
activation
c in vitro assay in an enclosed system
A negative response was reported in a BHK transformation assay,
but no supporting data were provided.
There was no evidence of induction of micronuclei in a single
mouse bone marrow assay.
7.6.6 HCFC 124
The data from the small number of in vitro studies are
summarized in Table 16.
HCFC 124 did not induce mutations in bacteria or chromosomal
aberrations in CHO cells.
7.7 Carcinogenicity
Combined chronic inhalation toxicity/carcinogenicity studies on
HCFC 141b, HCFC 123 and HCFC 124 are in progress within the Programme
for Alternate Fluorocarbon Toxicity Testing (Rusch, 1989).
7.7.1 HCFC 142b
Seckar et al. (1986) conducted a combined chronic
toxicity/carcinogenicity study of HCFC 142b with Sprague-Dawley CD
rats (details of exposure regimen are given in section 7.4).
Neoplasmic findings in animals that died were similar in the control
and treated animals, and predominantly comprised tumours of the
mammary and subcutaneous tissues in females, and pituitary and adrenal
adenomas in both sexes. A similar pattern existed in surviving
animals.
7.7.2 HCFC 133a
The carcinogenic potential of HCFC 133a has been evaluated by
Longstaff et al. (1984) in a limited study in Alpk/Ap Wistar- derived
rats. The study included one undosed control group of 32 male and 32
female rats and two vehicle-dosed control groups, one consisting of 40
male and 40 female and the other of 36 male and 36 female rats. The
HCFC 133a was dissolved in corn oil to give a 3% solution and dosed by
gavage at 300 mg/kg body weight, 5 days/week until week 52, and the
study was terminated at week 125. Dosed controls were given corn oil
only. Reductions of body weight gain and decreased testicular size
were observed in treated males. Aggressive behaviour occurred,
particularly in males. Body weight gain in females was similar to that
of controls. Mortality was not affected by treatment in either sex.
The treated females had an increased incidence of uterine carcinomas
(15/35) in comparison with controls (1/104). The first of these
carcinomas was seen at week 84. The carcinomas metastasized
transcoelomically to the abdomen in a large proportion of animals. A
small proportion also had lung metastases. Histologically, the
neoplasms were actively infiltrating adenocarcinomas.
Table 16. Genetic toxicity of HCFC 124
Test system Results Dose Remarks References
LED/HEDa
Without With
exogenous exogenous
metabolic metabolic
activation activation
Salmonella typhimurium, reverse - - 2230 g/m3, Barsky (1976)
mutation with TA98, TA100, TA1535, 6 hb
TA1537, TA1538
S. typhimurium, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
TA98, TA100, TA1535, TA1537, TA1538 48 hb
S. typhimurium, reverse mutation with - - 2790 g/m3, experimental data Longstaff et al. (1984)
TA98, TA100 48 hb not reported
Escherichia coli, reverse mutation with - - 2790 g/m3, purity > 99% May (1991)
WP2 uvrA 48 hb
Chromosomal aberrations, in vitro, - - 3348 g/m3, purity > 99% Edwards (1991b)
CHO cells 48 h (-ema)b
3348 g/m3,
4 h (+ema)b
a LED = lowest effective dose; HED = highest effective dose; +ema = with exogenous metabolic activation; -ema = without exogenous
metabolic activation
b In vitro assay in an enclosed system
The treated males had an increased incidence of benign
interstitial cell neoplasms of the testis (29/36) compared with
controls (16/104). In many animals the neoplasms were bilateral. The
first interstitial cell tumour was seen at week 64. The testes of all
treated males, including those with no evidence of neoplasms,
exhibited arrest of spermatogenesis and atrophy of the seminiferous
tubules. These changes were first seen in the first rat to die in this
study, which was in week 37.
7.7.3 HCFC 123
As discussed in section 7.4.2, a 2-year inhalation toxicity/
carcinogenicity study in rats is being conducted. Groups of 80 male
and 80 female Crl:CDRBR rats were exposed to HCFC 123 at 0, 2, 6 or
31 g/m3 (6 h/day, 5 days/week) for 2 years. No histological lesions
were found in 10 rats of each sex in each exposure group, which were
sacrificed at the end of 1 year of exposure (Malley, 1990c).
Preliminary findings from the histopathological examination of tissues
of male rats at the end of the 2-year exposure period indicated an
exposure-related increase in benign tumours of the testis and exocrine
pancreas (US EPA, 1991). A full report is needed before these results
can be evaluated.
7.8 Special studies - cardiovascular and respiratory effects
Chlorofluorocarbons have long been known to sensitize the heart
to adrenaline-induced arrhythmias. Zakhari & Aviado (1982) reviewed
the literature on this subject. Several studies have been conducted to
evaluate the cardiac sensitization and respiratory effect potential of
the alternative HCFC compounds.
7.8.1 HCFC 141b
Mullin (1977) exposed male dogs, which were pretreated with an
intravenous injection of adrenaline (0.008 mg/kg), to HCFC 141b
concentrations of 12, 24, 48 and 97 mg/m3. A challenge dose
(intravenous injection with adrenaline) given during exposure elicited
serious cardiac arrhythmias at the three highest concentrations but no
response was noted at the lowest concentration.
Hardy et al. (1989b) studied cardiac sensitization to adrenaline
in two Cynomolgus monkeys and four beagle dogs and found that the
lowest HCFC 141b concentrations inducing responses were about 24 and
48 g/m3, respectively.
No effect on respiratory rate was observed when three groups of
three male Wistar rats were exposed to approximately 45 g/m3 for 25
min. However, there was a small change in respiratory amplitude
suggesting a decrease in tidal volume (Janssen, 1989a).
7.8.2 HCFC 142b
Adrenaline-induced cardiac arrhythmias were observed in 5 out of
12 dogs exposed to 205 mg/m3. No such effect was noted in monkeys or
mice at concentrations of up to 410 g/m3 (Mullin, 1969). It was also
found that dogs exposed to 3280 g/m3 (80% by volume; 20% oxygen
added) for 30 seconds, followed by a loud noise (to stimulate
endogenous adrenaline), developed cardiac sensitization (Mullin,
1970).
Reinhardt et al. (1971) studied the ability of HCFC 142b to
induce cardiac sensitization to exogenous and endogenous adrenaline in
beagle dogs. No response was noted in six animals exposed to a
concentration of 102.5 g/m3 for 5 min and then challenged with
adrenaline. Five out of 12 dogs exposed to 205 g/m3 showed marked
responses (arrhythmias considered to pose a serious threat to life or
ventricular fibrillation), and all 12 dogs exposed to 410 g/m3
showed such responses. When a group of 12 beagle dogs was exposed to
3280 g/m3 for 30 seconds, without injected adrenaline, one out of 12
showed a marked response. With simultaneous noise stimulation five
dogs showed a marked response. No dogs showed the response when
exposed to noise alone.
No arrhythmia or tachycardia was observed when groups of three
Rhesus monkeys ( Macaca mulatta) were anaesthetised with sodium
pentobarbital and exposed to 205 or 410 g/m3 for 5 min (Belej et
al., 1974). There was no effect on pulmonary resistance or compliance
but respiratory stimulation was observed when three anaesthetised
Rhesus monkeys ( M. mulatta) were exposed to 205 g/m3 and four
monkeys to 410 g/m3 for 5 min (Aviado & Smith, 1975). When dogs were
exposed to four different concentrations between 102.5 and 820 g/m3
for 5 min, hypotension, tachycardia, an increase in pulmonary
resistance, and a decrease in pulmonary compliance were found at the
highest exposure level (Belej & Aviado, 1975).
7.8.3 HCFC 132b
HCFC 132b was reported to produce cardiac sensitization in beagle
dogs in response to an intravenous adrenaline challenge at exposure
levels of 27 g/m3 or more (Mullin, 1976).
7.8.4 HCFC 123
Trochimowicz & Mullin (1973) reported the EC50 (concentration
producing an effect in 50% of the test group) in dogs for cardiac
sensitization to adrenaline challenge to be 119 g/m3 and the NOEL to
be 62 g/m3. At the latter concentration (the lowest one tested) CNS
depression was observed.
7.8.5 HCFC 124
Van Poznak & Artusio (1960) found that HCFC 124 caused
anaesthesia in dogs at concentrations ranging from 2230 to 3910
g/m3; blood pressure was lowered in a dose-dependent fashion.
Although ventilation was adequate even at the highest exposure level,
the femoral arterial systolic pressure fell to as low as 40 mmHg (5.33
kPa). Little or no anaesthetic effect was observed at exposure levels
that did not depress blood pressure. Atropine had little effect on
hypotension. Phenylephrine partially reversed the hypotension and
caused several premature ventricular contractions but no ventricular
fibrillation. Similar effects were produced by intravenously
administered adrenaline. Mullin (1976) reported that the NOEL of HCFC
124 for cardiac sensitization after a challenge injection of
adrenaline in the dog was 56 g/m3, while the next exposure level
tested (140 g/m3) induced sensitization, as did higher
concentrations.
8. EFFECTS ON HUMANS
8.1 General population exposure
No effects on human health of the hydrochlorofluorocarbons
reviewed in this monograph have been reported.
8.2 Occupational exposure
Filicheva (1975) examined 98 male and 98 female workers, 20-40
years of age, reportedly exposed to chlorofluorocarbons 22, 113 and
142 and to some other chlorofluoro and fluoro compounds. The
concentrations were claimed to exceed the threshold limit values but
were not specified. Functional disorders of the nervous system were
reported in 67% of the workers. These included symptoms of
neurovegetative system disturbances, and, in a few cases, polyneuritis
of the upper extremities. Reduced haemoglobin content, moderate
leucocytosis and reduced erythrocyte sedimentation rate were also
reported. Exposure levels were not given, and the workers were exposed
to a number of chemicals in addition to HCFC 142 (isomer not
specified). The information provided in this study could not be used
to evaluate the potential health effects on workers exposed to HCFC
142b alone.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
No information is available on the effects on environmental
organisms of the hydrochlorofluorocarbons reviewed except for limited
data on HCFC 141b and HCFC 142b. However, an ecotoxicology programme
is being developed within the industry-sponsored Programme for
Alternative Fluorocarbon Toxicity Testing (Rusch, 1989).
The 96-h LC50 of HCFC 141b for zebra fish (Brachidario rerio)
was reported to be 126 mg/litre in a static test using a sealed vessel
(Bazzon & Hervouet, 1989). The 48-h EC50 for the immobilization of
Daphnia magna, also using a sealed vessel, was 31.2 mg/litre
(Brinard & Hervouet, 1989).
The 96-h EC50 of HCFC 142b for guppies ( Poecilia
reticulata), tested in a static system in accordance with OECD
Guidelines, was reported to be 220 mg/litre (Groenevald & Kuijpers,
1990a). Groenevald & Kuijpers (1990b) found a 48-h EC50 for Daphnia
magna of 160 mg/litre. No immobilization of Daphnia magna at 190
mg/litre in 48 h was found (Hutton & Lieder, 1989a).
Acute toxicity values of HCFC 142b have also been determined for
rainbow trout. The 96-h LC50 was found to be 36 mg/litre (with a 95%
confidence interval of 28-45 mg/litre). The authors concluded that,
under the conditions of the test, HCFC 142b exhibited moderate acute
toxicity to rainbow trout (Hutton & Lieder, 1989b).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Direct health effects
10.1.1 HCFC 141b
HCFC 141b has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or in the environment. Available information
indicates a low level of metabolism of HCFC 141b. It is of low acute
toxicity. At high sublethal exposure concentrations, it induces signs
of CNS depression and can sensitize the heart to adrenaline, as do
other hydrochlorofluorocarbons. Such effects can appear at
concentrations ranging from 24 to 48 g/m3 in the inhaled air.
HCFC 141b has low irritant potential to the eye and skin. Skin
sensitization has not been demonstrated.
Short-term repeated inhalation exposure (2 to 13 weeks) did not
induce serious toxic effects at concentrations below 97 g/m3.
Effects of HCFC 141b on reproduction cannot be evaluated until
the data from an ongoing two-generation study become available. It has
not demonstrated teratogenic potential in rats or rabbits, although
embryotoxicity was observed, but only at the highest maternally toxic
concentration (97 g/m3) in rats. The no-observed-effect level (NOEL)
for maternal toxicity (excepting minor clinical signs) is 7 g/m3 in
rabbits and 15 g/m3 in rats.
Mutagenicity testing of HCFC 141b yielded conflicting results.
In vitro studies with CHO cells indicated clastogenic potential, but
this was not reflected in a single human lymphocyte test or two
in vivo mouse studies.
A carcinogenicity study in rats is in progress for HCFC 141b.
In summary, based on the toxicological information available at
the time of the Task Group meeting, the toxicity of HCFC 141b is
considered to be low. The present data base does not indicate any
significant direct health effects on humans under non-accidental
exposure conditions. However, studies to evaluate carcinogenicity and
reproduction are still in progress. Consequently, these study results
and any future changes in use pattern may require further evaluation.
10.1.2 HCFC 142b
HCFC 142b is produced in commercial quantities for use as an
intermediate in the synthesis of vinylidene fluoride. Its current
release to the environment has not been quantified and no information
is available on accidental release. No study is available which
permits the assessment of human health responses to HCFC 142b alone.
No information is available from in vivo studies on metabolism
and kinetics, but an in vitro study suggests that dechlorination may
occur.
The acute toxicity is very low after oral or inhalation exposure.
The no-observed-effect level (NOEL) for cardiac sensitization using
exogenous adrenaline in dogs is 102.5 g/m3 for 5 min.
In rat and dog studies, repeated inhalation exposure at
concentrations of up to 41 g/m3 for 90 days caused no adverse
responses. No carcinogenic or other toxicological responses were
reported in a single long-term inhalation study in rats exposed to up
to 82 g/m3 for 104 week.
HCFC 142b has mutagenic potential in bacterial systems. Data from
in vitro mammalian cell systems are lacking. No mutagenic activity
was seen in two in vivo rat studies.
Although no conventional reproductive toxicity studies are
available, two rat teratogenicity tests have been conducted in which
neither teratogenicity nor reproducible signs of embryotoxicity were
observed. In addition, no effect on male fertility was observed in a
dominant lethal study in rats.
In summary, information on human exposures is lacking, but these
are unlikely to approach the sustained high concentrations required to
produce adverse effects in experimental animals. Future usage
patterns, possibly resulting in higher occupational exposure
concentrations and exposure for shorter periods among the general
population, may require further evaluation.
10.1.3 HCFC 132b
HCFC 132b is only produced in small quantities for research
purposes but it occurs as a by-product in the manufacture of some
halogenated ethanes. Release into the environment is expected to be
very low. There appears to be no commercial application for HCFC 132b
at the present time. There are no data on human exposure and on the
effects of HCFC 132b on human health.
Data on the metabolism of HCFC 132b are only available for rats.
They show that the compound can be metabolized to potentially
cytotoxic metabolites and suggest that the compound can induce its own
metabolism. The acute toxicity of the compound is low, the predominant
effects being central nervous system (CNS) depression, anaesthesia
and, at high concentrations, death. The compound has low irritation
potential to the skin and eye. HCFC 132b can sensitize the heart to
adrenaline, as do many other hydrochlorofluorocarbons. Repeated
exposure to HCFC 132b has been shown to cause CNS depression, signs of
hepatotoxicity (at concentrations of 3 g/m3 or more) and effects on
spermatogenesis (at 11 g/m3). HCFC 132b is also embryotoxic and was
maternally toxic at the lowest dose tested (3 g/m3). NOEL values
have not been established for systemic toxicity or for embryotoxicity.
The available mutagenicity studies, which are limited to a few
in vitro experiments, did not suggest that HCFC 132b is genotoxic.
Long-term and carcinogenicity studies have not been performed.
In summary, from the available information, exposure to HCFC 132b
would appear to present a hazard to human health following repeated
exposure. Current use restricts its exposure to research personnel.
Therefore there is no expected health risk to the general population.
10.1.4 HCFC 133a
HCFC 133a is produced in small quantities for use as an
intermediate in the manufacture of the anaesthetic halothane. Although
it has not been quantified, release into the environment is expected
to be low.
No data are available on the toxicokinetics of HCFC 133a,
although absorption can be assumed to occur from the toxic effects it
induces. It is of low acute toxicity, the principal toxic effects
being anaesthesia and death at high concentrations. Although no
information is available, this compound is also expected to induce
cardiac sensitization at high concentrations. In animal studies
repeated exposure to HCFC 133a has induced death (in mice), and nasal
and lung damage changes in clinical chemistry parameters (in rats).
Atrophy of the thymus, testis, ovary and spleen have also been
reported in rats. Reduced fertility and damage to the seminiferous
epithelium have been observed in male mice at exposure concentrations
of 2.5 g/m3 and 5 g/m3, respectively.
HCFC 133a has been clearly demonstrated to be embryotoxic at
exposure concentrations that did not produce clear evidence of
maternal toxicity (2.5 g/m3); there was also indication of a
teratogenic potential in rats. The NOEL for embryolethality was 2.5
g/m3.
HCFC 133a is non-mutagenic in bacterial assays. It gave positive
results in two out of three dominant lethal assays in mice, but these
results are difficult to interpret in view of the negative result in
a single rat bone-marrow cytogenetic assay. From the limited evidence
available, HCFC 133a is a carcinogen in rats inducing adenocarcinomas
of the uterus; it also produces benign interstitial cell tumours of
the testis.
In summary, from the information available, exposure to HCFC 133a
would appear to present a hazard to human health following repeated
exposure and has the potential to cause serious effects in developing
offspring. In the absence of exposure information the potential risk
cannot be determined.
10.1.5 HCFC 123
HCFC 123 has only recently become available for commercial and
industrial use. There is no information on exposure levels for the
general population or the environment.
There is limited information on the kinetics and metabolism of
HCFC 123. It can be absorbed, the inference coming from observed
systemic effects and elevated urinary fluoride levels in toxicity
studies. There is evidence for some metabolism of HCFC 123, based on
the detection of urinary trifluoroacetic acid, elevated urinary
fluoride levels, and the detection of covalent binding to liver
protein.
The acute toxicity of HCFC l23 is low. As with the fully
halogenated chlorofluorocarbons, it is characterized in animals by CNS
depression at high inhalation exposure concentrations. Exposure to
high concentrations of HCFC 123 can sensitize the heart to adrenaline
(the EC50 for dogs is 119 g/m3).
Effects associated with short-term and long-term inhalation
exposure to HCFC 123 in rats include CNS depression, modulation of
lipid and carbohydrate metabolism and mild liver toxicity. The
lowest-observed-effect level (LOEL) for lipid and carbohydrate
metabolism effects and increased hepatic enzyme activity is 2 g/m3
following a 1-year exposure period to HCFC 123.
There is no evidence of teratogenicity in rats and rabbits but
there is evidence of embryotoxicity in rabbits at high inhalation
exposure concentrations (62 g/m3). Maternal toxicity is seen at
exposure concentration of 3 g/m3 or more in rabbits and 31 g/m3 or
more in rats. The potential effect of HCFC 123 on reproduction is
being investigated in a rat study.
There is evidence of clastogenic effects in human lymphocytes
in vitro, although this was not supported by an in vivo mouse
micronucleus study. HCFC 123 is not mutagenic in microorganisms. The
carcinogenicity of HCFC 123 is being investigated in rats and thus
cannot be evaluated at present.
On the basis of available information, HCFC 123 does not appear
to exhibit marked toxicity following short-term or long-term exposure.
However, in the light of some evidence of clastogenicity and the
reported increased incidences of benign tumours of the testes and
exocrine pancreas communicated in an interim report, the potential
health effects of HCFC 123 cannot be fully evaluated until more
information becomes available.
10.1.6 HCFC 124
HCFC 124 is not yet in large scale commercial production.
Therefore, there are no data on environmental levels and human
exposure.
The acute toxicity of HCFC 124 is low and is characterized in
animals by effects on the central nervous system. Subchronic toxicity
studies in rats did not reveal any histopathological changes of
internal organs at concentration of HCFC 124 as high as 279 g/m3.
Based on functional observations and clinical chemistry, the NOEL was
reported to be 28 g/m3 for male rats, and 84 g/m3 for females. In
three limited teratogenicity studies in rats, no indication of
developmental toxicity of HCFC 124 was evident, even at a maternally
toxic concentration. Based on the data available from bacterial and
mammalian cell studies there is no evidence that HCFC 124 has a
mutagenic potential.
The available information indicates that HCFC 124 exhibits low
toxicity, and it is not anticipated to pose significant health risk to
humans at potential environmental or controlled occupational
exposures. However, a firm conclusion on the potential health risk
cannot be made until data from the ongoing teratogenicity and
carcinogenicity studies are available.
10.2 Health effects expected from a depletion of stratospheric
ozone
The possible indirect health effects (e.g., an increase in the
incidence of skin cancer and immunotoxic and ocular effects) of fully
halogenated chlorofluorocarbons, resulting from an increase in UV-B
radiation due to a depletion of the ozone layer, have been discussed
in the Environmental Health Criteria 113: Fully Halogenated
Chlorofluorocarbons (WHO, 1990).
The ozone-depleting potentials of five of the six HCFCs for which
data are available are lower than that of CFC 11 by 33-77 times (HCFCs
123 and 124), 40 times (HCFC 132b), 12-20 times (HCFC 142b), and 7-15
times (HCFC 141b). If the levels of release of the HCFCs reviewed are
adequately controlled their indirect health effects should not be
significant.
10.3 Effects on the environment
Insufficient information is available to evaluate adequately the
direct ecological effects posed by the hydrochlorofluorocarbons
reviewed. With respect to the indirect "greenhouse" effect, the HCFCs
for which data are available have global-warming potentials lower than
that of CFC 11 by about 50 times (HCFC 123), about 10 times (HCFCs 124
and 141b), and about 3 times (HCFC 142b). These compounds are not
expected to contribute significantly to global warming.
11. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
11.1 Conclusions
Based on the available information the Task Group reached the
following conclusions:
1. All six hydrochlorofluorocarbons reviewed have low acute
toxicity, the main toxic signs being those of CNS depression.
However, accidental overexposure could result in acute poisoning
or even lethality.
2. These chemicals exhibit different toxicity potentials following
repeated exposure.
3. HCFC 141b has a low toxic potential on repeated exposure,
produces no consistent developmental effects and has not been
shown to be mutagenic in animals. The mutagenic status of HCFC
141b tested in vitro is unclear. No information is yet
available to evaluate chronic toxicity/carcinogenicity.
4. HCFC 142b also has a low toxic potential on repeated exposure.
The small data base indicates that HCFC 142b does not produce
developmental effects or affect male fertility. HCFC 142b is
mutagenic in bacteria. It has not been shown to be mutagenic or
carcinogenic in rats.
5. HCFC 132b is toxic following repeated exposure in animals. It
affects development in animals, but has only been tested at
maternally toxic doses. No study on reproduction has been
performed, but damage to spermatogenesis has been noted
histopathologically following repeated exposure. It is not
mutagenic in vitro and no test has been performed in vivo.
There are no data on the carcinogenic potential.
6. HCFC 133a is toxic following repeated exposure in animals,
producing a range of effects. It also induces serious effects on
reproduction and development in animals. In vivo data regarding
mutagenicity are unclear. It is a carcinogen in rats.
7. On repeated exposure, HCFC 123 induces liver toxicity and effects
on lipid and carbohydrate metabolism in rats. Developmental
effects only occur at high maternally toxic exposure
concentrations. No information is available on reproduction
effects. HCFC 123 is clastogenic in vitro, but has not been
shown to be clastogenic in animals. Complete information on the
carcinogenicity in rats is not yet available.
8. HCFC 124 has low toxic potential on repeated exposure. Based on
a small data base, there is no evidence of developmental effects.
No information is available on the reproduction toxicity
potential or carcinogenicity. It is not mutagenic in vitro and
no test has yet been performed in vivo.
9. The hydrochlorofluorocarbons reviewed (with the exception of HCFC
133a for which there is no value but which is expected to have a
value similar to the others reviewed) have a lower
ozone-depleting potential than the fully halogenated
chlorofluorocarbons and should therefore pose a lower indirect
health risk.
10. The global-warming potentials of HCFC 141b, HCFC 142b, HCFC 123
and HCFC 124 are similarly lower than those of the fully
halogenated chlorofluorocarbons. No data are available for HCFC
132b or HCFC 133a, but they would be expected to have similar
values to the other hydrochlorofluorocarbons reviewed. These
compounds are not expected to contribute significantly to global
warming.
11.2 Recommendations for protection of human health and the
environment
1. Since the toxicity of HCFC 142b is low, and the ozone-depleting
and global-warming potentials are considerably lower than those
of the fully halogenated chlorofluorocarbons, HCFC 142b can be
considered as a transient substitute for the chlorofluorocarbons
included in the Montreal Protocol. However, in line with the
conclusions of the 1990 London Meeting of the Montreal Protocol
Parties, efforts should be maintained to develop substitutes that
would pose no risk to the environment and to develop alternative
technologies. Care should be exercised in the use of HCFC 142b
because of its flammability.
2. Since more information regarding the toxicological potential of
HCFC 141b, HCFC 123 and HCFC 124 is required before an evaluation
of their hazard to human health can be made, no recommendation
can be made at present regarding their use as potential transient
substitutes for the fully halogenated chlorofluorocarbons
included in the Montreal Protocol.
3. Although HCFC 133a and HCFC 132b pose low risk to the
environment, they are not recommended as substitutes for the
chlorofluorocarbons included in the Montreal Protocol because of
their toxicity.
4. Since all the hydrochlorofluorocarbons reviewed have some
ozone-depleting potential, their release to the environment
should be minimized.
REFERENCES
Ahmadzai H & Hedlund T (1990) A profile of measures taken in Sweden to
protect the stratospheric ozone layer. Ambio, 19: 341-343.
Alvarez L (1988) Pilot teratogenicity study of FC-132b in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. HLR 50-88).
Anderson D & Richardson CR (1979) Arcton 133a: A cytogenetic study in
the rat. Alderley Park, Cheshire, Imperial Chemical Industries Ltd,
Central Toxicology Laboratory (Report No. CTL/P443).
ANON (1991) CFC alternative shows chronic toxicity. Chem Eng News, 8
July: 26.
Aviado DM & Smith DG (1975) Toxicity of aerosol propellants in the
respiratory and circulatory systems. VIII. Respiration and circulation
in primates. Toxicology, 3: 24l-252.
Barsky FC (1976) In vitro microbial mutagenicity studies of
ethane,1-chloro-1,2,2,2-trifluoro. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 349-76).
Bazzon M & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Brachidanio rerio. Vert-le-Petit, Institut national de
Recherche chimique appliquée (IRCHA Study B.7073).
Belej MA & Aviado DM (1975) Cardiopulmonary toxicity of propellants
for aerosols. J Clin Pharmacol, 15: 105-115.
Belej MA, Smith DG, & Aviado DM (1974) Toxicity of aerosol propellants
in the respiratory and circulatory systems. IV. Cardiotoxicity in the
monkey. Toxicology, 2: 381-395.
Bootman J & Hodson-Walker G (1988) In vitro assessment of the
clastogenic activity of CFC141b in cultures of Chinese hamster ovary
(CHO-KI) cells. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV007/214).
Bootman J, Hodson-Walker G, & Lloyd JM (1988a) CFC 141b: investigation
of mutagenic activity at the HGPRT locus in a Chinese hamster V79 cell
mutation system. Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV005/257).
Bootman J, Hodson-Walker G, Cracknell S, & Dance CA (1988b) HCFC 141b:
Assessment of clastogenic action on bone marrow erythrocytes in the
micronucleus test. Eye, Suffolk, Life Science Research Ltd (LRS Report
No. 88/PSV029).
Brewer WE (1977) 90-day subacute inhalation toxicity study with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brewer WE & Smith S (1977) Teratogenic study via inhalation with
Genetron 124 in albino rats. Northbrook, Illinois, Industrial Bio-Test
Laboratories, Inc. (Report IBT No. 8562-09345).
Brinard D & Hervouet G (1989) Determination of acute toxicity of HCFC
141b to Daphnia magna. Vert-le-Petit, Institut national de Recherche
chimique appliquée (IRCHA Study B.7072).
Brittelli MR (1976a) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 752-75).
Brittelli MR (1976b) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 13-76).
Brittelli MR (1976c) Eye irritation test in rabbits. Newark, Delaware,
Du Pont de Nemours and Co., Haskell Laboratory (Report No. 747-75).
Brock WJ (1988a) Acute dermal toxicity study of FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 501-88).
Brock WJ (1988b) Skin irritation test in rabbits of FC-141b. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
312-88).
Brock WJ (1988c) Primary eye irritation study with FC-141b in rabbits.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 318-88).
Brock WJ (1988d) Acute dermal toxicity study of HCFC-123 in rats.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 577-88).
Brock WJ (1988e) Acute dermal toxicity of HCFC-123 in rabbits. Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
578-88).
Brock WJ (1988f) Primary dermal irritation study with HCFC-123 in
rabbits. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 535-88).
Brusick D (1976) Mutagenicity evaluation of Genetron 123. Final
Report. Kensington, Maryland, Litton Bionetics, Inc. (LBI Project No.
254).
Callander RD (1989) HCFC 123 - An evaluation using the Salmonella
mutagenicity assay. Alderley Park, Cheshire, Imperial Chemical
Industries Ltd, Central Toxicology Laboratory (Report No. CTL/P/2421).
Carpenter CP, Smith HF Jr, & Pozzani UC (1949) The assay of acute
vapour toxicity and the grading and interpretation of results on 96
chemical compounds. J Ind Hyg Toxicol, 31: 343-346.
Chapman J, Hill R, Muir J, Suckling CW, & Viney DJ (1967) Impurities
in halothane: their identities, concentrations and determination. J
Pharm Pharmacol, 19: 231-239.
Coate WB (1976a) LC50 of G123 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. M165-162).
Coate WB (1976b) LC50 of G124 in rats. Final Report. Vienna,
Virginia, Hazleton Laboratories America, Inc. (Project No. 165-163).
Coombs DE, Hardy CJ, Crook D, Mullins PA, & Gopinath C (1988) Two-week
inhalation toxicity study in the rat with FC 141b. Huntington, United
Kingdom, Huntington Research Centre (Report PWT 76/8893).
Culik R & Kelly DP (1976) Embryotoxic and teratogenic studies in rats
with inhaled chlorodifluoroethane (FC 142b). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 700-76).
Culik R & Kelly DP (1979) Embryotoxicity and teratogenicity studies in
rats with chlorotrifluoroethane (FC-133a). Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 127-79).
Damske DR, Mecler FJ, & Beliles RP (1978) Teratology study in rats;
Isotron 142b. Monochlorodifluoroethane. Final Report. Kensington,
Maryland, Litton Bionetics, Inc. (LBI Project No. 20980).
Dance CA (1991) In vitro assessment of the clastogenic activity of
HCFC 123 in cultured human lymphocytes. Eye, Suffolk, Life Science
Research Ltd (Final report No. 91/PFE03/0093).
Darr RW (1981) An acute inhalation toxicity study of Fluorocarbon 123
in the Chinese hamster. Morristown, New Jersey, Corporate Medical
Affairs, Allied Corporation (Report No. MA-25-78-15).
Davies RH, Bagnall RD, Bell W, & Jones WGM (1976) The hydrogen bond
proton donor properties of volatile halogenated hydrocarbons and
ethers and their mode of action in anaesthesia. Int J Quantum Chem
Quantum Biol Symp, 3: 171-185.
Diggle WM & Gage JC (1956) Toxicological report Arctons 9, 33 and 50.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/T/61).
Doleba-Crowe C (1977) Acute inhalation toxicity - 6 hours (HFA 141b).
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 61-77).
Doleba-Crowe C (1978) Ninety-day inhalation exposure of rats and dogs
to vapors of 2,2-dichloro-1,1,1-trifluoroethane (FC-123). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
229-78).
Edmunds HN, Baden JM, & Simmon VF (1979) Mutagenicity studies with
volatile metabolites of Halothane. Anesthesiology, 51(5): 424-429.
Edwards CN (1991a) HCFC 123 (vapour phase): In vitro assessment of
clastogenic activity in cultured human lymphocytes. Eye, Suffolk, Life
Science Research Ltd (Final Report No. 91/PFE002/0125).
Edwards CN (1991b) In vitro assessment of the clastogenic activity
of HCFC 124 in cultured Chinese hamster ovary (CHO-K1) cells. Eye,
Suffolk, Life Science Research Ltd (Final report No. 91/PAR002/0855a).
Filicheva AP (1975) [Changes in the nervous system following chronic
action of fluorinated aliphatic hydrocarbons.] Gig Tr Prof Zabol, 10:
14-16 (in Russian).
Fisher DA, Hales ChH, Wang WC, Ko MKW, & Dze ND (1990a) Model
calculations of the relative effects of CFCs and their replacements on
global warming. Nature (Lond), 344: 513-516.
Fisher DA, Hales H, Filkin DL, Malcolm KWK, Sze ND, Connell PS,
Wuebbles DJ, Isaksen SA, & Stordal F (1990b) Model calculations of the
relative effects of CFCs and their replacements on stratospheric
ozone. Nature (Lond), 344: 508-512.
Freemantle M (1991) CFCs and their alternatives. Impact Sci Soc, 40:
59-70.
Gardner JR (1988) Acute dermal toxicity to rats of
dichlorofluoroethane. Huntington, United Kingdom, Huntington Research
Centre (Report No. 881114/PWT91/AC).
Goodman NC (1975) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 678-75).
Goodman NC (1976) Primary skin irritation and sensitization tests on
guinea-pigs. Newark, Delaware, Du Pont de Nemours and Co., Haskell
Laboratory (Report No. 14-76).
Graselli JG & Ritchey WM ed. (1975) CRC atlas of spectral data and
physical constants for organic compounds. Cleveland, Ohio, CRC Press,
vol 3.
Groeneveld AHC & Kuijpers LAM (1990a) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to the guppy Poecilia
reticulata. Graveland, The Netherlands, Duphar B.V., Agrobiological
Laboratory (Interim document No. 56635/33/90).
Groeneveld AHC & Kuijpers LAM (1990b) The acute toxicity of
1-chloro-1,1-difluoroethane (HCFC142b) to Daphnia magna. Graveland,
The Netherlands, Duphar B.V., Agrobiological Laboratory (Interim
Document No. 56635/32/90).
Hake CL, Waggoner TB, Robertson DN, & Rowe VK (1960) The metabolism of
1,1,1-trichloroethane by the rat. Arch Environ Health, 1: 101-105.
Hall GT (1976) Two-week inhalation toxicity study (HFA 132b and HFA
124). Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 727-76).
Hall GT & Moore BL (1975) Acute inhalation toxicity on Freon 123.
Newark, Delaware, Du Pont de Nemours and Co., Haskell Laboratory
(Report No. 426-75).
Hardy CJ, Jackson GC, Rao RS, Lewis DJ, & Gopinath C (1989a) HCFC 141b
acute inhalation toxicity study in rats, 4-hour exposure. Huntington,
United Kingdom, Huntington Research Centre (Report No. PWT95/881676).
Hardy CJ, Sharman IJ, & Chanter DO (1989b) Assessment of cardiac
sensitisation potential in dogs and monkeys. Comparison of F-141b and
F11. Huntington, United Kingdom, Huntington Research Centre (Report
No. PWT86/89437).
Harris JW & Anders MW (1991a) In vivo metabolism of the
hydrochlorofluorocarbon 1,1-dichloro-1-fluoroethane (HCFC-141b).
Biochem Pharmacol, 41: R13-R16.
Harris JW & Anders MW (1991b) Metabolism of the
hydrochlorofluorocarbon 1,2-dichloro-1,1-difluoroethane. Chem Res
Toxicol, 4: 180-186.
Harris JW, Pohl LR, Martin JL, & Anders MW (1991) Tissue acylation by
the chlorofluorocarbon substitute 2,2-dichloro-1,1,1-trifluoroethane.
Proc Natl Acad Sci (USA), 88: 1407-1410.
Hawley GG (1981) The condensed chemical dictionary, 10th ed. New York,
Van Nostrand Reinhold Company.
Henry JE (1975a) Acute oral test (132b). Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Unpublished report No. 680-75).
Henry JE (1975b) Acute oral test on FC-123. Newark, Delaware, Du Pont
de Nemours and Co., Haskell Laboratory (Report No. 638-75).
Hodge MCE, Anderson D, Bennett IP, & Weight TM (1979) Arcton 133a:
dominant lethal study in the mouse. Alderley Park, Cheshire, Imperial
Chemical Industries Ltd, Central Toxicology Laboratory (Report No.
CTL/P/467).
Hodge MCE, Anderson D, Bennett IP, Weight TM, & Wilson J (1980) Arcton
133a: first combined dominant lethal and fertility study in the mouse.
Alderley Park, Cheshire, Imperial Chemical Industries Ltd, Central
Toxicology Laboratory (Report No. CTL/R/46l).
Hodson-Walker G (1990a) In vitro assessment of the clastogenic
activity of HCFC-141b in cultured Chinese hamster ovary (CHO-K1)
cells. Eye, Suffolk, Life Science Research Ltd (Report No.
89/PFG001/0971a).
Hodson-Walker G (1990b) HCFC 141b: lymphocyte cytogenetic study using
the methodology recommended by the OECD (1983). Eye, Suffolk, Life
Science Research Ltd (Report No. 89/PFG002/1029).
Hodson-Walker G & May K (1988a) CFC 141b: assessment of mutagenic
potential in histidine auxotrophs of Salmonella typhimurium (the
Ames test). Eye, Suffolk, Life Science Research Ltd (Report No.
88/PSV004/237).
Hodson-Walker G & May K (1988b) CFC 141b: assessment of its ability to
cause lethal DNA damage in strains of Escherichia coli. Eye,
Suffolk, Life Science Research Ltd (Report No. 88/PSV008/258).
Hoffmann JS (1990) Replacing CFCs; the search for alternatives. Ambio,
19: 329-333.
Horrath AL (1982) Halogenated hydrocarbons. New York, Basel, Marcel
Dekker.
Hughes EW, Homan BA, Kenny TJ, Coombs DW, Hardy CJ, & Tesh SA (1988)
The effect of HCFC 141b on pregnancy of the rat. Huntington, United
Kingdom, Huntington Research Centre (Report No. PWT77/88952).
Hughes EW, Homan BA, John DM, Kenney TJ, Coombs DW, & Hardy CJ (1989)
A study of the effect of PWC 4874-89 on pregnancy of the rabbit.
Huntington, United Kingdom, Huntington Research Centre (Report No. PWT
82/89243).
Hutton DG & Lieder PH (1989a) Static acute 48-hour EC50 of
chlorodifluoroethane to Daphnia magna. Newark, Delaware, Du Pont de
Nemours and Co., Haskell Laboratory (Report No. 542-89).
Hutton DG & Lieder PH (1989b) Flow-through acute 96-hour LC50 of
chlorodifluoroethane to rainbow trout ( Oncorhynchus mykiss). Newark,
Delaware, Du Pont de Nemours and Co., Haskell Laboratory (Report No.
