
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 150
BENZENE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr E.E. McConnell,
Raleigh, North Carolina, USA
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Benzene.
(Environmental health criteria ; 150)
1.Benzene - adverse effects 2.Benzene - toxicity
3.Environmental exposure I.Series
ISBN 92 4 157150 0 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BENZENE
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical
properties, analytical methods
1.2 Sources of human exposure
1.3 Environmental transport, distribution
and transformation
1.4 Environmental levels and human exposure
1.5 Kinetics and metabolism
1.6 Effects on laboratory mammals and
in vitro test systems
1.6.1 Systemic toxicity
1.6.2 Genotoxicity and carcinogenicity
1.6.3 Reproductive toxicity, embryotoxicity
and teratogenicity
1.6.4 Immunotoxicity
1.7 Effects on humans
1.8 Conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES,
ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Conversion factors
2.4 Analytical methods
2.4.1 Environmental samples
2.4.2 Biological materials
3. SOURCES OF HUMAN AND ENVIRONMENTAL
EXPOSURE
3.1 Natural occurrence
3.2 Anthropogenic sources
3.2.1 Production levels and processes
3.2.2 Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION
AND TRANSFORMATION
4.1 Transport and distribution between media
4.2 Environmental degradation
4.2.1 Abiotic degradation
4.2.2 Biodegradation
4.2.3 Bioconcentration
5. ENVIRONMENTAL LEVELS AND HUMAN
EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.2 Water
5.1.3 Soil and sediments
5.1.4 Food
5.2 General population exposure
5.3 Occupational exposure during manufacture,
formulation or use
6. KINETICS AND METABOLISM IN LABORATORY
ANIMALS AND HUMANS
6.1 Absorption
6.1.1 Air
6.1.2 Oral
6.1.3 Dermal
6.2 Distribution
6.2.1 Inhalation exposure
6.2.2 Oral and dermal exposures
6.3 Metabolic transformation
6.4 Elimination and excretion
6.4.1 Inhalation exposure
6.4.2 Oral exposure
6.4.3 Dermal exposure
6.5 Retention and turnover
6.6 Reaction with body components
6.7 Modelling of pharmacokinetic data for benzene
7. EFFECTS ON LABORATORY MAMMALS AND
IN VITRO TEST SYSTEMS
7.1 Single exposure
7.2 Short-term and long-term exposures
7.3 Skin and eye irritation
7.4 Reproductive toxicity, embryotoxicity
and teratogenicity
7.5 Mutagenicity and related end-points
7.5.1 In vitro studies
7.5.2 In vivo studies
7.6 Carcinogenicity
7.6.1 Inhalation studies
7.6.2 Oral and subcutaneous studies
7.7 Special studies
7.7.1 Immunotoxicity
7.7.2 Neurotoxicity
7.8 Factors modifying toxicity
7.9 Mechanism of toxicity
8. EFFECTS ON HUMANS
8.1 General population and occupational exposure
8.1.1 Acute toxicity
8.1.2 Effects of short- and long-term exposures
8.1.2.1 Bone marrow depression; aplastic
anaemia
8.1.2.2 Immunological effects
8.1.2.3 Chromosomal effects
8.1.2.4 Carcinogenic effects
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 General population
9.2 Occupational exposure
9.3 Toxic effects
9.3.1 Short-term and long-term exposures;
organ toxicity
9.3.1.1 Haematotoxicity; bone marrow
depression
9.3.1.2 Mechanism of action and
metabolism
9.3.1.3 Immunotoxicity
9.3.2 Genotoxicity and carcinogenic effects
9.3.2.1 Mechanism of carcinogenicity
9.3.2.2 Human carcinogenesis
9.4 Other toxicological end-points
9.5 Conclusions
10. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
11. FURTHER RESEARCH
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET CONCLUSIONS
RESUMEN Y CONCLUSIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BENZENE
Members
Dr D. Anderson, BIBRA (British Industrial Biological Research
Association), Toxicology International, Carshalton, Surrey, United
Kingdom (Vice-Chairman)
Dr H.A. Greim, Institute of Toxicology, Association for Radiation and
Environmental Research, Neuherberg, Germany (Chairman)
Dr R.F. Henderson, Inhalation Toxicology Research Institute, Lovelace
Biomedical and Environmental Research Institute, Albuquerque, New
Mexico
Dr R. Hertel, Fraunhofer Institute for Toxicology, Hanover, Germany
(now at the Bundesgesundheitsamt, Berlin) Professor A.-A.M. Kamal,
Ain Shams University, Abbassia, Cairo, Egypt
Dr S. Parodi, Istituto Nazionale per la Ricerca sul Cancro, Genoa,
Italy
Dr R.A. Rinsky, Division of Surveillance, Hazard Evaluations and Field
Studies, National Institute of Occupational Safety and Health,
Cincinnati, Ohio, USA
Dr R. Snyder, Department of Pharmacology and Toxicology, Rutgers
University, Piscataway, New Jersey, USA
Dr G.M.H. Swaen, Department of Occupational Medicine, University of
Limburg, Maastricht, The Netherlands
Dr S.-N. Yin, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, China
Observers
Dr M. Bird, Exxon Biomedical Sciences, East Millstone, New Jersey, USA
Dr J. Gamble, Exxon Biomedical Sciences, East Millstone, New Jersey,
USA
Dr J. Kielhorn, Fraunhofer Institute for Toxicology, Hanover, Germany
Dr K. Levsen, Fraunhofer Institute for Toxicology, Hanover, Germany
Dr G. Raabe, Mobil Research, Princeton, New Jersey, USA
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization, Research
Triangle Park, North Carolina, USA (Secretary)
Dr M. Kogevinas, International Agency for Research on Cancer, Lyon,
France
Dr E.E. McConnell, Raleigh, North Carolina, USA (Rapporteur)
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case Postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01 ES02617-14
from the National Institute of Environmental Health Sciences, National
Institutes of Health, USA.
ENVIRONMENTAL HEALTH CRITERIA FOR BENZENE
A WHO Task Group on Environmental Health Criteria for Benzene met
at the Fraunhofer Institute of Toxicology and Aerosol Research,
Hanover, Germany, from 2 to 6 December 1991, the meeting being
sponsored by the German Ministry of the Environment. Dr R.F. Hertel
welcomed the participants on behalf of the host institute. Dr G.C.
Becking, IPCS, welcomed the participants on behalf of Dr M. Mercier,
Director of the IPCS, and the three IPCS Cooperating organizations
(UNEP/ILO/WHO). The Group reviewed and revised the draft document and
made an evaluation of the risks for human health from exposure to
benzene.
The first draft was prepared by Dr E.E. McConnell, Raleigh, North
Carolina, USA. Extensive scientific comments on the first draft were
received from governments, research institutions, and industry; in
particular: Exxon Biomedical Sciences; CONCAWE; Mobil Research;
Health and Welfare Canada; IARC; RIVM, The Netherlands; Fraunhofer
Institute and Ministry of Health, Germany; National Institute of
Environmental Health Sciences, National Institute of Occupational
Safety and Health, and Agency for Toxic Substances and Disease
Registry, USA; Department of Health, United Kingdom; and National
Chemical Inspectorate (KEMI), Sweden. These comments were
incorporated into the second draft by the Secretariat.
Dr H. Greim, Chairman of the Task Group, Dr C. Pohlenz-Michel and
Dr H. Sterzl-Eckert of GSF-Institute of Toxicology deserve special
thanks for the time taken after the Task Group to ensure the
scientific accuracy of the final draft monograph.
Dr G.C. Becking (IPCS Central Unit, Interregional Research Unit)
and Dr P.G. Jenkins (IPCS Central Unit, Geneva) were responsible for
the overall scientific content and technical editing, respectively, of
this monograph. The efforts of all who helped in the preparation and
finalization of this publication are gratefully acknowledged.
ABBREVIATIONS
ALMS Atomic line molecular spectrometry
CHO Chinese hamster ovary
FID flame ionization detection
GC gas chromatography
MS mass spectrometry
SCE sister chromatid exchange
SMR standardized mortality ratio
S-PMA S-phenyl-mercapturic acid
TWA time-weighted average
1. SUMMARY AND CONCLUSIONS
1.1 Identity, physical and chemical properties, analytical methods
Benzene is a stable colourless liquid at room temperature and
normal atmospheric pressure. It has a characteristic aromatic odour,
a relatively low boiling point (80.1 °C) and a high vapour pressure,
which causes it to evaporate rapidly at room temperature, and is
highly flammable. It is slightly soluble in water but miscible with
most other organic solvents.
Analytical methods are available for the detection of benzene in
various media (air, water, organs/tissues). The choice between gas
chromatography (GC) with flame ionization or photoionization detection
and mass spectrometry (MS) depends upon the sensitivity required and
levels of benzene expected. Detection of benzene in the workplace
usually involves collection on charcoal and GC/MS analysis after
desorption. Where sensitivity in the mg/m3 range is sufficient,
portable direct-reading instruments and passive dosimeters are
available. If greater sensitivity is required, methods to detect
benzene at levels as low as 0.01 µg/m3 (air) or 1 ng/kg (soil or
water) have been reported.
1.2 Sources of human exposure
Benzene is a naturally occurring chemical found in crude
petroleum at levels up to 4 g/litre. It is also produced in extremely
large quantities (14.8 million tonnes) worldwide. Emissions arise
during the processing of petroleum products, in the coking of coal,
during the production of toluene, xylene and other aromatic compounds,
and from its use in consumer products, as a chemical intermediate and
as a component of gasoline (petrol).
1.3 Environmental transport, distribution and transformation
Benzene in air exists predominantly in the vapour phase, with
residence times varying between a few hours and a few days, depending
on environment and climate, and on the concentration of hydroxyl
radicals, as well as nitrogen and sulfur dioxides. It can be removed
from air by rain, leading to contamination of surface and ground
water, in which it is soluble at about 1000 mg/litre.
Due primarily to volatilization, the residence time of benzene in
water is a few hours, with little or no adsorption to sediments.
Benzene in soil can be transported to air via volatilization and
to surface waters by run off. If benzene is buried or is released
well below the surface, it will be transported into ground water.
Under aerobic conditions, benzene in water or soil is rapidly
(within hours) degraded by bacteria to lactate and pyruvate through
phenol and catechol intermediates. However, under anaerobic
conditions (for example, in ground water) bacterial degradation is
measured in weeks and months rather than hours. In the absence of
bacterial degradation benzene can be persistent. It has not been
shown to bioconcentrate or bioaccumulate in aquatic or terrestrial
organisms.
1.4 Environmental levels and human exposure
The presence of benzene in gasoline (petrol), and as a widely
used industrial solvent can result in significant and widespread
emissions to the environment. Outdoor environmental levels range from
0.2 µg/m3 in remote rural areas to 349 µg/m3 in industrial centres
with a high density of automobile traffic. During refuelling of
automobiles, levels up to 10 mg/m3 have been measured.
Benzene has been detected at levels as high as 500 µg/m3 in
indoor residential air. Cigarette smoke contributes significant
amounts of benzene to the levels reported in indoor air, with smokers
inhaling approximately 1800 µg benzene/day compared to 50 µg/day by
non-smokers.
In many countries, occupational exposures seldom exceed a
time-weighted average of 15 mg/m3. However, the actual levels
reported depend upon the industry studied and in some industrially
developing countries exposures can be considerably higher.
Water and food-borne benzene contributes only a small percentage
of the total daily intake in non-smoking adults (between about 3 and
24 µg/kg body weight per day).
1.5 Kinetics and metabolism
Benzene is well absorbed in humans and experimental animals after
oral and inhalation exposures, but in humans dermal absorption is
poor. Approximately 50% absorption occurs in humans during continuous
exposures to 163-326 mg/m3 for several hours. After a 4-h exposure
to 170-202 mg/m3, retention in the human body was approximately 30%,
with 16% of the retained dose having been excreted as unchanged
benzene in expired air. Women may retain a greater percentage of
inhaled benzene than men. Benzene tends to accumulate in tissues
containing high amounts of lipids, and it crosses the placenta.
Benzene metabolism occurs mainly in the liver, is mediated
primarily through the cytochrome P-450 IIE1 enzyme system and involves
the formation of a series of unstable reactive metabolites. In rodents
the formation of two putative toxic metabolites, benzoquinone and
muconaldehyde, appears to be saturable. This may have important
implications for dose-response relationships, because a higher
proportion of the benzene will be converted to toxic metabolites at
low doses than at high doses. The metabolic products are excreted
primarily in the urine. Appreciable levels of the known metabolites
phenol, catechol and hydroquinone are found in bone marrow. Phenol is
the predominant urinary metabolite in humans and is mainly found as an
ethereal sulfate conjugate until levels approach 480 mg/litre, at
which time glucuronides are detected. Recent studies suggest that
benzene toxicity is the result of the interactive effects of several
benzene metabolites formed in both the liver and the bone marrow.
Inhaled benzene had been found to bind to rat liver DNA to the
extent of 2.38 µmoles/mole DNA phosphate. Seven deoxyguanosine
adducts and one deoxyadenine adduct have been detected in rabbit bone
marrow mitochondrial DNA.
1.6 Effects on laboratory mammals and in vitro test systems
1.6.1 Systemic toxicity
Benzene appears to be of low acute toxicity in various animal
species, with LD50 values after oral exposure ranging between 3000
and 8100 mg/kg body weight in the rat. Reported LC50 values range
from 15 000 mg/m3 (8 h) in mice to 44 000 mg/m3 (4 h) in rats.
Benzene is a moderate eye irritant and is irritating to rabbit
skin after multiple applications of the undiluted chemical. No
information is available on the skin-sensitizing potential of benzene.
Exposure of mice to benzene by inhalation results in a
significant lowering of blood parameters such as haematocrit,
haemoglobin level, and erythrocyte, leucocyte and platelet counts.
Long-term exposure at high doses results in bone marrow aplasia.
Similar, but less severe, findings were noted in rats.
1.6.2 Genotoxicity and carcinogenicity
Benzene has given negative results in mutagenicity assays
in vitro.
In in vivo studies, benzene or its metabolites cause both
structural and numerical chromosome aberrations in humans and
laboratory animals. In addition, benzene administration results in
the production of sister chromatid exchanges and polychromatic
erythrocytes with micronuclei. Benzene can reach germ cells, after
intraperitoneal dosing, as shown by the production of abnormalities in
sperm head morphology.
Benzene has been reported to cause the production of several
types of neoplasms in both rats and mice after either oral dosing or
inhalation exposures. These include various types of epithelial
neoplasms, e.g., Zymbal gland, liver, mammary tissue and nasal cavity
neoplasms, and a few lymphomas and leukaemias.
In those inhalation studies where a positive carcinogenic
response was reported, exposure levels were between 100 and 960
mg/m3 for 5-7 h/day, 5 days/week. Oral benzene doses of between 25
and 500 mg/kg body weight in mice and rats resulted in the production
of neoplasms. The length of exposure was usually 1-2 years.
1.6.3 Reproductive toxicity, embryotoxicity and teratogenicity
Benzene crosses the placental barrier freely. There are no data
showing that it is teratogenic after numerous experiments in
experimental animals even at maternally toxic doses. However, it has
been shown to be fetotoxic following inhalation exposure in mice (1600
µg/m3, 7 h/day, gestation days 6-15) and in rabbits.
1.6.4 Immunotoxicity
Benzene depresses the proliferative ability of B- and T-cell
lymphocytes. Host resistance to infection in several laboratory
species has been reduced by exposure to benzene.
1.7 Effects on humans
It is known that benzene produces a number of adverse health
effects. The most frequently reported health effect of benzene is
bone marrow depression leading to aplastic anaemia. At high levels of
exposure a high incidence of these diseases is probable.
Benzene is a well-established human carcinogen. Epidemio-logical
studies of benzene-exposed workers have demonstrated a causal
relationship between benzene exposure and the production of
myelogenous leukaemia. A relationship between benzene exposure and
the production of lymphoma and multiple myeloma remains to be
clarified.
The Task Group was of the opinion that the epidemiological
evidence is not capable of distinguishing between a) a small increase
in mortality from leukaemia in workers exposed to low levels of
benzene, and b) a non-risk situation.
1.8 Conclusions
It was concluded that a time-weighted average of 3.2 mg/m3
(1 ppm) over a 40-year working career has not been statistically
associated with any increase in deaths from leukaemia. Because this
is a human carcinogen, however, exposures should be limited to the
lowest level technically feasible. Increases in exposure level to
over 32 mg/m3 (10 ppm) should be avoided. Benzene and
benzene-containing products such as petrol should never be used for
cleaning purposes.
Traditionally, bone marrow depression, i.e. anaemia leucopenia or
thrombocytopenia, in the workplace has been recognized as the first
stage of benzene toxicity and appears to follow a dose-response
relationship. In other words, the higher the dose, the greater the
likelihood of observing decreases in circulating blood cells.
Exposure to high benzene levels (160-320 mg/m3) for one year
would most likely produce bone marrow toxicity in a large percentage
of the workers and aplastic anaemia in some cases, but little effect
would be expected at lower doses. Exposure to both high and low doses
would be expected to produce benzene toxicity after 10 years of
continuous exposure. Thus, a high level of both bone marrow
depression and aplastic anaemia would be seen at the higher doses and
some damage would also be seen at lower doses. The observation of any
of these effects, regardless of the level of exposure, should indicate
the need for improved control over benzene exposures.
There is no evidence of benzene being teratogenic at doses lower
than those that produce maternal toxicity, but fetal toxicity has been
demonstrated.
Neurotoxicity and immunotoxicity of benzene has not been well
studied in experimental animals or humans.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical structure:
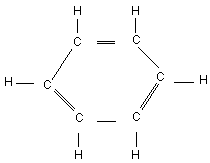 Chemical formula: C6H6
CAS number: 71-43-2
RTECS number: CY1400000
Common name: Benzene
IUPAC name: Benzene
Common synonyms: Annulene, benzine, benzol, benzole, benzol coal
naphtha, cyclohexatriene, mineral naphtha,
motor benzol, phenyl hydride, pyrobenzol,
pyrobenzole
Purity: Nitration grade >99%. Benzol 90 contains
80-85% benzene, 13-15% toluene and 2-3% xylene.
Commercial grades are free of H2S and SO2
and have a maximum of 0.15% non-aromatics
compounds.
2.2 Physical and chemical properties
Benzene is a naturally occurring colourless liquid at room
temperature (20 °C) and ambient pressure (760 mmHg), and has a
characteristic aromatic odour. The principal physical and chemical
properties of benzene are shown in Table 1.
2.3 Conversion factors
1 ppm = 3.2 mg/m3 at 20 °C at normal atmospheric pressure
1 mg/m3 = 0.31 ppm
2.4 Analytical methods
This section does not provide an exhaustive list of the
analytical methods available for detecting and quantifying benzene in
various media. However, those methods that are well established and
have been used in studies of human exposure and in experiments on the
biological effects of benzene will be described briefly.
Table 1. Some physical and chemical properties of benzenea
Physical form (20 °C) clear colourless liquid
Relative molecular mass 78.11
Flash point -11.1 °C
Flammable limits 1.3-7.1%
Melting/freezing point 5.5 °C
Boiling point 80.1 °C at 760 mmHg
Density 0.878
Relative vapour density
(air = 1) 2.7
Vapour pressure (26 °C) 13.3 kPa
Solubilities:
water 1800 mg/litre at 25 °C
non-aqueous solvents miscible with most
Odour threshold 4.8-15.0 mg/m3
Taste threshold (water) 0.5-4.5 mg/litre
Log n-octanol/water partition
coefficient 1.56-2.15
Sorption coefficient (log Koc -
distribution coefficient between
benzene adsorbed to soil organic
carbon and benzene in solution) 1.8-1.9
a Data from: GDCh (1988), RIVM (1988) and ATSDR (1989)
The analytical methods used for the determination of benzene
depend upon the media sampled and the level of sensitivity required.
In all cases proper sampling and sample storage are essential
prerequisites, particularly as microgram and nanogram quantities are
often found in environmental samples.
Some of the commonly used methods for the detection of benzene in
various media are summarized in Table 2.
2.4.1 Environmental samples
Methods are available for the determination of benzene in air,
water sediments, soil, foods, cigarette smoke, and petroleum and
petroleum products. Most involve separation by gas chromatography
(GC) with detection by flame ionization (FID) or photoionization (PID)
or by mass spectrometry (MS).
The measurement of benzene in air (ambient and workplace) usually
involves a preconcentration step in which the sample is passed through
a solid absorbent (Baxter et al., 1980; Pellizzari, 1982; Roberts et
al., 1984; Clark et al., 1984b; Reineke & Bächmann, 1985; Harkov et
al., 1985; Gruenke et al., 1986; OSHA, 1987; Bayer et al., 1988;
Brown, 1988a,b). Commonly used adsorbents are TenaxR resin, silica
gel, and activated carbon. Preconcentration of benzene can also be
accomplished by direct on-column cryogenic trapping (Reineke &
Bächmann, 1985; Holdren et al., 1985; Fung & Wright, 1986), or benzene
can be analysed directly (Clark et al., 1984a; Hadeishi et al., 1985;
Bayer et al., 1988). As noted in Table 2, the limit of detection of
the GC/FID or GC/PID techniques is in the low ppb (µg/m3) to low ppt
(ng/m3) range whereas the GC/MS method has a limit of detection in
the low ppb (µg/m3) range (Gruenke et al., 1986). Although GC/FID
and GC/PID provide greater sensitivity than GC/MS, the latter is
generally considered more reliable for the measurement of benzene in
samples containing multiple components with similar GC elution
characteristics. Atomic line molecular spectrometry (ALMS) has been
developed to monitor benzene and other organic compounds in ambient
air samples (Hadeishi et al., 1985). The detection limit is 800
µg/m3 (250 ppb).
Benzene in the workplace can be measured by portable
direct-reading instruments, real-time continuous monitoring systems
and passive dosimeters (OSHA, 1987) having sensitivities in the ppm
(mg/m3) range. In the USA, the more sensitive procedure of
preconcentration on charcoal followed by GC/MS analysis is generally
preferred (OSHA, 1987).
Benzene in aqueous media is usually isolated by the
purge-and-trap method (Brass et al., 1977; Hammers & Bosman, 1986)
followed by GC/MS, GC/FID or GC/PID analysis (Harland et al., 1985;
Blanchard & Hardy, 1986; Michael et al., 1988). An inert gas such as
nitrogen is used to purge the sample, the benzene is trapped on an
absorbent such as TenaxR or activated charcoal, and this is followed
by thermal desorption. The sensitivity of these methods is in the low
to sub µg/litre range with good recoveries and precision for most
methods.
Table 2. Analytical methods for the determination of benzene
Sample Preparation Analytical methoda Detection limitb Reference
Air silica gel trap indicator tube 4.9 mg/m3 Koljkowsky (1981)
Air charcoal trap, CS2 desorption GC/FID 3.2 µg/m3 Baxter et al. (1980)
Air (ambient) Tenax GC sorbent, thermal desorption capillary GC/MS NR Pellizzari (1982)
computer analysis
Air Tenax GC trap, thermal desorption, C/FID/MS 0.01 µg/m3 Roberts et al. (1984)
cryogenic focusing
Air (ambient) direct injection GC/PID 0.82 µg/m3 Clark et al. (1984a)
Air direct analysis UV Spect. 800 µg/m3 Hadeishi et al. (1985)
Air Tenax or cryogenic trap, thermal desorption GC/FID NR Holdren et al. (1985)
Air near landfills/ Tenax GC trap, thermal decomposition GC/FID/ECD/MS 0.03 µg/m3 Harkov et al. (1985)
waste sites
Air silica gel trap, thermal desorption GC/MS 0.32 µg/m3 Gruenke et al. (1986)
Air (ambient) cryogenic trap, thermal desorption GC/PID 16 ng/m3 Reineke &
GC/FID 77 ng/m3 Bachmann (1985)
Air (ambient) charcoal trap (badge or tube, desorb with GC/FID 0.96 µg/m3 Fung & Wright (1986)
CS2
Air solid sorbent trap, thermal desorption GC/MS NR Bayer et al. (1988)
Air (occupational) activated charcoal sorbent, CS2 desorption GC/FID 0.64 mg/m3 (in Brown (1988a)
12 litres)
Table 2 (contd).
Sample Preparation Analytical methoda Detection limitb Reference
Air (occupational) porous polymeric sorbent, thermal desorption GC/FID 0.83 µg/m3 Brown (1988b)
Water (drinking) purge and trap GC/MS 0.2 µg/litre Brass et al. (1977)
Water (surface or helium purge, Tenax GC trap, thermal GC/MS 0.1 µg/litre Fentiman et al. (1979)
effluents) desorption
Water purge with inert gas, Tenax trap, thermal GC/MS NR Harland et al. (1985)
desorption
Water N2 purge, Tenax GC trap, thermal desorption GC/FID 1 ng/litre Hammers & Bosman (1986)
Water filter through silicone polycarbonate GC/FID 7.2 µg/litre Blanchard & Hardy (1986)
membrane into inert gas stream
Water purge with inert gas, Tenax trap, thermal HRGC/MS 0.1 µg/litre Michael et al. (1988)
desorption to on-column cryogenic trap
Soil N2 purge, Tenax GC trap GC/FID 0.1 µg/kg Fentiman et al. (1979)
Soil N2 purge, Tenax trap, thermal desorption GC/FID 1 ng/kg Hammers & Bosman (1986)
Sediment N2 purge, Tenax trap, thermal desorption GC/MS 0.01 µg/kg Ferrario et al. (1985)
Mainstream filter smoke and direct to GC/MS; for HRGC/MS NR Brunnemann et al. (1989)
cigarette smoke passive smoke collect air in cryogenic
methanol-filled impingers
Jet fuel fumes sample on charcoal, methylene chloride, HPLC/UV 0.29 mg/m3 Dibben et al. (1989)
ethyl acetate desorption; column elution
with acetonitrile
Table 2 (contd).
Sample Preparation Analytical methoda Detection limitb Reference
Blood N2 purge, Tenax GC-silica gel trap GC/MS 0.5 µg/litre Antoine et al. (1986)
Blood extract with toluene, centrifuge; analyse GC/FID 100 µg/litre Jirka & Bourne (1982)
toluene layer
Blood add heparinized sample to isotonic saline HRGC/PID 0.4 µg/litre Pekari et al. (1989)
in headspace via equilibrate with heat
Breath collect on Tenax GC, thermal desorption HRGC/MS 9.8 ng/m3 Pellizzari et al. (1988)
Breath collect on Tenax GC, thermal desorption into GC/MS 5.2 µg/m3 Wallace et al. (1985)
on-column cryogenic trap
Urine extraction GC/MS 2 µmol/litre Stommel et al. (1989)
(as S-PMA)
Urine (phenol enzyme and acid digestion; ethyl ether GC/FID 1 mg/litre Buchet (1988)
and conjugates) extraction
Urine (muconic sample mixed with methanol, centrifuge, HPLC/UV 0.1 mg/litre Inoue et al. (1989)
acids) analyse supernatant, elute with methanol -
acetic acid
Tissues add butyl hydroxytoluene to buffered homo- RID-HPLC/UV 20 pg/g Bechtold et al. (1988)
genate, centrifuge, analyse supernatant
a GC = gas chromatography; FID = flame ionization detection; PID = photoionization detection; MS = mass spectrometry;
HRGC = high resolution (capillary) gas chromatography; RID = reverse isotope dilution; HPLC = high performance liquid chromatography;
UV = ultraviolet detection
b NR = not reported
Benzene in soil, sediment and food samples is usually determined
by purge-and-trap methods (Harland et al., 1985; Ferrario et al.,
1985; Hammers & Bosman, 1986), with headspace analysis (Kiang & Grob,
1986) and liquid extraction (Kozioski, 1985) techniques being used
less frequently. Detection limits as low as 1 ng/kg have been
reported after GC/FID or GC/MS analysis, but recoveries and precision
are frequently low.
Methods have been reported for the analysis of benzene in other
environmental media such as cigarette smoke (Brunnemann et al., 1989,
1990) and in petroleum products such as petrol (gasoline) (Poole et
al., 1988; Dibben et al., 1989).
2.4.2 Biological materials
Benzene levels in exhaled breath, blood, and body tissues have
been analysed by GC/FID, GC/PID or GC/MS, and benzene metabolites in
urine have been measured using GC/FID and high-performance liquid
chromatography (HPLC) with ultraviolet detection.
Prior to analysis, breath samples are usually collected on a
solid sorbent such as activated charcoal, silica gel or TenaxR GC
and thermally desorbed (Wallace et al., 1985; Pellizzari et al.,
1988). Headspace analysis has also been used to analyse levels of
benzene in exhaled breath (Gruenke et al., 1986). Greater selectivity
is achieved if capillary columns are used for high-resolution gas
chromatography (HRGC) (Pellizzari et al., 1988).
Three methods have been used to extract benzene from blood, i.e.
purge-and-trap (Antoine et al., 1986), headspace analysis (Gruenke et
al., 1986; Pekari et al., 1989) and solvent extraction (Jirka &
Bourne, 1982). Sensitivity for the first two procedures is in the sub
to low µg/litre range, whereas solvent extraction is less sensitive
(low to mid µg/litre).
Total phenolic metabolites of benzene have been determined in
urine following hydrolysis, extraction with ethyl ether and GC/FID
analysis (Buchet, 1988). The technique of HPLC/UV has been used to
determine the trans, trans-muconic acid metabolites of benzene in
urine (Inoue et al., 1989). A more sensititive GC/MS method to
monitor muconic acid in the urine of exposed workers has been
developed by Bechtold et al. (1991). Biological monitoring methods
using urine measure concentrations of phenolic conjugates, the major
metabolites of benzene (Buchet, 1988). Such methods, however, lack
adequate specificity and sensitivity for low levels of benzene
exposure. A method based on the determination of the minor metabolite
S-phenyl-mercapturic acid (S-PMA) appears to overcome these
deficiencies (Stommel et al., 1989). Benzene and its organic-soluble
metabolites have been determined quantitatively in rodent tissues
using GC/MS and reverse isotope dilution (RID) combined with
semipreparative HPLC/UV (Bechtold et al., 1988). A method using
ion-pairing HPLC was used to analyse water-soluble metabolites of
benzene in liver and in urine (Sabourin et al., 1988).
Schrenk & Bock (1990) have developed an HPLC method for the
determination of metabolites secreted by isolated hepatocytes.
Brodfuehrer et al. (1990) have reported on the determination of
benzene metabolites in liver slices of rat, mouse and man.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Benzene is released to the environment from both natural and
man-made sources, the latter accounting for the major part of the
emissions.
3.1 Natural occurrence
Benzene is a naturally occurring organic compound. It is a
component of petroleum (1-4%) (IARC, 1989) and can be found in sea
water (0.8 µg/litre) in the vicinity of natural deposits of petroleum
and natural gas (Reynolds & Harrison, 1982).
3.2 Anthropogenic sources
Major anthropogenic sources of benzene include automobile
exhaust, automobile-refuelling operations and industrial emissions.
Automobile exhaust probably accounts for the largest anthropogenic
source in the general environment. Cigarette smoke, off-gassing from
building material and structural fires all lead to increased
atmospheric benzene levels. People are exposed to benzene mainly
through the inhalation of contaminated air, particularly in areas of
heavy automobile traffic and around gasoline (petrol) stations and
other facilities for storage and distribution of petrol, and through
tobacco smoke from both active and passive smoking (ATSDR, 1991).
Other sources of exposure have been reported to include industrial
emissions and consumer products (Wallace et al., 1987). However,
certain individuals may be exposed to potentially high concentrations
of benzene in drinking-water as a result of seepage from underground
petroleum storage tanks, landfills, waste streams, or natural gas
deposits (ATSDR, 1991). Individuals employed in industries that
produce or use benzene or benzene-containing products are probably
exposed to much higher levels than the general population. Industrial
discharge, landfill leachate, and disposal of benzene-containing waste
are also anthropogenic sources.
3.2.1 Production levels and processes
Benzene ranks sixteenth in production volume for chemicals
produced in the USA, with an estimated production of 4.39 x 105
tonnes (1.6 x 109 gallons) in the USA in 1991 (ATSDR, 1991) and 1480
x 103 tonnes in western Europe in 1986 (GDCh, 1988) (Table 3). In
the USA over 90% of the benzene produced is derived from petroleum
sources (ATSDR, 1991), i.e. refinery streams (catalytic reformates),
pyrolysis of gasoline, and toluene hydrodealkylation. In western
Europe 55% of the benzene production is from gasoline pyrolysis, 10%
from coking of coal, and the remaining production is divided
approximately equally between catalytic reformate and the
hydrodealkylation of toluene (GDCh, 1988).
Table 3. World production of benzene in thousands of tonnes
for 1981a
Capacity Production
North & South America (total) 9350 6150
Asia (total) 3550 2460
Western Europe (total) 6950 3800
Eastern Europe (total) 5840 2340
Japan 2880 2060
USA 8030 5190
USSR 3250 1700
Other countries 100 50
World 25 800 14 800
a From: RIVM (1988)
Benzene in petrol is not included.
Given the high production volume, widespread use, and physical
and chemical properties of benzene, there is a high potential for
large amounts to be released to the environment. However, accurate
data on the amounts released are difficult to obtain. The data in
Table 4 are given to show the relative amounts of benzene released to
the air from various industrial sources in several countries. It is
evident that the largest amounts released are from the use of
gasoline. In California (USA), the 1984 benzene emission inventory
totalled 17 500 tonnes (Allen, 1987), with motor vehicle exhaust
accounting for 71% of this amount. Total emissions of benzene from
industrial sources within the USA have been reported to be 33 000 to
34 000 tonnes (US EPA, 1989). Recent emission data related to
automobile use in the USA are difficult to obtain, but in 1980 such
emissions were between 40 000 and 80 000 tonnes (IARC, 1982). In
Germany approximately 80% of the air emissions reported are due to the
use of motor vehicles, whereas coke ovens account for 3.9% of such
emissions. Other sources are gasoline storage and transport (6.2%)
and industrial furnace emissions (4.0%).
Table 4. Major emissions of benzene into the atmosphere in tonnes per yeara
Road traffic Refineries Remaining Total
sources
Belgium/Luxembourg 4950 60 750 5760
Canada 25 895 654 7601 34 150
Denmark 2600 10 390 3000
France 30 000 200 4000 34 200
Germany (FRG) 62 000 200 11 000 73 200
Greece 4700 30 700 5430
Ireland 1650 0 200 1850
Italy 29 000 190 4200 33 390
Netherlands 7300 80 980 8360
United Kingdom 29 000 150 4200 33 350
European Community
(total) 171 200 920 26 420 198 540
a From: RIVM (1988). Calculated using crude oil consumption figures from 1982.
3.2.2 Uses
Benzene has a large number of industrial, commercial and
scientific uses. Approximately, 10% of the total use of benzene is in
gasoline (RIVM, 1988), where levels average < 1% by weight in the USA
(US EPA, 1985) and 2.5-3.0% v/v in western Europe (GDCh, 1988).
Along with other aromatic compounds, benzene is important in the
production of organic chemicals, particularly styrene (Table 5). The
major uses of benzene as a chemical intermediate are summarized in
Table 5. There are no data indicating a major deviation from this
pattern of use, which was reported in 1981.
Table 5. Industrial uses of benzene in 1981. Benzene in petrol
has not been incorporateda
Production of: USA Japan Western Netherlands
Europe
Ethylbenzene/styrene 51.1 50.4 48.6 73
Cumene/phenol 20.6 12.1 19.3 16
Cyclohexane 13.8 25.6 13.4 11
Alkylates 3.0 3.7 5.2 -
Maleic acid anhydride 2.8 2.5 3.3 -
Nitrobenzene/aniline 5.3 - 6.7 -
Chlorinated benzenes 2.6 5.7 2.0 -
Other products 0.8 - 1.5 -
a From: RIVM (1988). Data shown as a percentage of the total benzene
consumed in each area.
In the past, benzene was used widely as a solvent, but this use
is declining in most developed countries; it represents < 2% of
current use. However, it is still used as a solvent in scientific
laboratories, industrial paints, rubber cements, adhesives, paint
removers, degreasing agents, production of artificial leather and of
rubber goods, and in the shoe industry (Mara & Lee, 1978; Windholz et
al., 1983; Gilman et al., 1985). For many solvent uses, benzene has
been replaced by other less toxic organic solvents. However, in the
past significant human exposure occurred when benzene was used as a
paint stripper, a carburettor cleaner, in the production of denatured
alcohol and rubber cements, and in arts and crafts supplies (Young et
al., 1978). It has also been reported that benzene vapours could be
detected from such products as carpet glue, textured carpet, liquid
detergent and furniture wax (Wallace et al., 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Benzene is released into the environment from both natural and
man-made sources, although the latter are the most significant. The
volatility and solubility are the most important properties which
influence its environmental transport (see Table 1). Benzene enters
the atmosphere from direct emissions and volatilization from soil and
water surfaces.
The high volatility of benzene (vapour pressure of 13.3 Kpa at
26 °C), its solubility in water (1800 mg/litre at 25 °C) and a Henry's
law constant of 5.5 x 10-3 atm/m3 per mole at 20 °C suggest that
benzene will partition to the atmosphere from surface water (Mackay &
Leinonen, 1975). These authors have calculated a t´ in water of 4.8
h (1 metre deep at 25 °C). Benzene in air is fairly soluble in water
and is removed from the atmosphere by rain (Ogata & Miyake, 1978).
However, once it has been deposited on soil or water, volatilization
will return a portion back to the atmosphere.
Benzene is not expected to adsorb to bottom sediments for several
reasons: (1) the Koc (soil/organic carbon sorption coefficient)
(Table 1) does not predict adsorption to particles; (2) the solubility
of benzene in water, and (3) the volatility of benzene.
Benzene released to soil can partition to the atmosphere through
volatilization, to surface water through run-off, and to ground water
if released well below the surface. Evaporation from surface soil is
expected to be rapid (Hine & Mookerjee, 1975). With a Koc of 60-83,
benzene is considered fairly mobile in soil (Kenaga, 1980; Karickhoff,
1981). Leaching of benzene into ground water from soil is influenced
by several parameters including type of soil (sand versus clay),
amount of rainfall, depth of ground water and extent of benzene
degradation.
4.2 Environmental degradation
4.2.1 Abiotic degradation
In air benzene exists predominantly in the vapour phase
(Eisenreich et al., 1981). Degradation of benzene in air occurs
mainly by reactions with hydroxy, alkoxy and peroxy radicals, oxygen
atoms and ozone, of which the reaction with hydroxy radicals is the
most important. The rate constant for the reaction has been measured
often (Tully et al., 1981). Assuming an average hydroxy radical
concentration of 1.25 x 106 molecules/cm3 and a rate constant of
1.3 x 10-12 cm3/molecule per second, a t´ of 5.3 days was
calculated for benzene (RIVM, 1988). In areas of high traffic density
where there is a higher concentration of hydroxy radicals (1 x 108
molecules/cm3) and increased levels of NOx, the 24-h average t´
for benzene has been reported as 3-10 days (GDCh, 1988). Under these
conditions phototransformation products may include phenol,
nitrobenzenes, nitrophenol and various ring-opened dicarbonyl
compounds (Bandow et al., 1985). Direct photolysis of benzene in the
troposphere is unlikely since the UV-visible spectrum of benzene shows
no appreciable absorbance at wavelengths longer than 260 nm
(Bryce-Smith & Gilbert, 1976). This hypothesis was supported by Korte
& Klein (1982). No degradation was seen after 6 days irradiation of
benzene in the laboratory with light of wavelength longer than 290 nm.
4.2.2 Biodegradation
Benzene in surface and ground water is biodegradable by a variety
of microorganisms under both aerobic and anaerobic conditions (RIVM,
1988). Under both conditions the mechanism of biodegradation seems to
involve the formation of catechol via cis-1,2-dihydroxy-
1,2-dihydrolbenzene followed by ring cleavage (Högn & Jaenicke, 1972;
Korte & Klein, 1982).
Karlson & Frankenberger (1989) studied the aerobic biodegradation
of benzene in ground water utilizing a mixed bacterial culture
containing petroleum-degrading bacteria from ground water and soil
bacteria capable of using gasoline as a sole carbon source. Under
closed agitated conditions without added nutrients, benzene levels
dropped from 480 µg/litre to 218 µg/litre in 48 h. However, when
nitrogen was added the reaction was much more rapid, with benzene
levels decreasing to 35 µg/litre in 20 h. The biodegradation of
benzene in ground water and river water appears to follow first-order
rate kinetics, with t´ values of 28 and 16 days, respectively,
having been reported for ground water and river water (Vaishnav &
Babeu, 1987).
Korte & Klein (1982) studied the fate of benzene on soil
utilizing composting waste. Of the benzene applied to the waste only
2-2.5% remained in situ whereas 35% volatilized. These authors
concluded that benzene does not usually remain on soil long enough for
biodegradation to play an important role in its removal. A model
developed to predict the environmental fate of benzene following
losses of gasoline from underground tanks indicated that approximately
1% of the benzene would be degraded (Tucker et al., 1986).
Benzene is not usually biodegradable under anaerobic conditions
(GDCh, 1988). However, Wilson et al. (1986) using samples of landfill
leachate showed under methogenic conditions in an anaerobic glove-box
that, although no significant benzene biodegradation occurred during
the first 20 weeks of incubation, after 40 weeks benzene
concentrations were reduced by 72%. Using anaerobic digesting sludge,
Battersby & Wilson (1989) examined the degradation of benzene under
methanotrophic conditions. Benzene, at a level of 50 mg carbon/litre,
remained undegraded after 11 weeks of digestion. Although it is
slowly degraded under anaerobic conditions, benzene levels in sewage
influents up to 6 mg/litre do not affect sewage treatment processes
using activated sludge systems (Bennett, 1989). Jackson & Brown
(1970) reported no toxic effects of benzene on the anaerobic digestion
of sewage sludges until levels of between 50 and 200 mg/litre had been
reached.
4.2.3 Bioconcentration
Benzene is not expected to bioconcentrate to any great extent in
aquatic or terrestrial organisms given the reported values for log
Pow (octanol/water) of 2.13 and for bioconcentration factor (BCF) of
24 (Miller & Wasik, 1985). The BCF for freshwater algae was reported
to be 30 (Geyer et al., 1984), for water fleas ( Daphnia sp.) it was
153-225, depending on the concentration of benzene in their food, and
for goldfish it was 4.3 (Ogata et al., 1984).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Examples of benzene concentrations in urban and rural areas are
given in Table 6. Daily median benzene air concentrations in the USA
have been reported as: remote areas, 0.51 µg/m3 (0.16 ppb); rural
areas, 1.50 µg/m3 (0.47 ppb); and urban/suburban areas, 5.76 µg/m3
(1.8 ppb) (Shah & Singh, 1988).
The concentration appears to depend largely on the density of
automobile traffic and local weather conditions (Wallace, 1989a).
Although the median level in USA urban areas is 5.76 µg/m3 (1.8 ppb)
(Shah & Singh, 1988), levels as high as 112 µg/m3 (35 ppb) have been
observed (US EPA, 1987). Maximum levels of 510 µg/m3 (Wallace et
al., 1985) and 210.6 µg/m3 (Singh et al., 1982) have been reported
in two cities in the USA. In addition to the concentrations of
benzene shown in Table 6, the following levels of benzene have been
reported in the urban air of European cities: London, 10-12 µg/m3
background and 28-31 µg/m3 kerbside (Bailey & Schmidl, 1989);
Hamburg, Elb Tunnel, 80.5-95.3 µg/m3 (Dannecker et al., 1990) and a
residential site 9.3 µg/m3 (Bruckmann et al., 1988); and Stockholm,
average values of 147.7 µg/m3 on a busy street in the city centre
and 7.7 µg/m3 on a quiet street in the city centre (Jonsson et al.,
1985). Country wide averages in Germany have been reported to be 1-10
µg/m3 (0.31-3.1 ppb) (GDCh, 1988) and in three urban areas of Canada
they were 2.9-19.6 µg/m3 (0.9-6.0 ppb) (Government of Canada, in
press). Benzene levels, along with other pollutants, may increase
during periods of still air.
Concentrations of benzene in the atmosphere of cities where
chemical factories use or produce benzene are more variable. In the
USA, benzene concentrations have been shown to vary between 0.4 and 16
µg/m3 (Pellizzari, 1982). Levels of 3.2 mg/m3 (1 ppm) have been
measured in the breathing zone during the refuelling of automobiles
(Bond et al., 1986a).
In Frankfurt, Germany, the highest benzene levels have been
measured in the vicinity of coke ovens (maximum, 166.2 µg/m3;
average, 57.2 µg/m3), near industrial refineries (maximum, 102
µg/m3; average, 13.4 µg/m3), and in congested traffic areas
(maximum, 171.8 µg/m3; average, 16.9 µg/m3) (GDCh, 1988).
It has been reported that people living near petrochemical plants
in New Jersey, USA, have no greater exposure to benzene than the
general population throughout the area (Wallace et al., 1985). Of
particular interest in this study was the observation that in Bayonne,
New Jersey, benzene levels (arithmetic means) in indoor air (29.7
µg/m3) were greater than those reported for outside air (8.6
µg/m3) (Table 6).
Table 6. Examples of the concentrations of benzene measured in air
Concentration (µg/m3)
Location (year) Mean Maximum Reference
Montreal, PQ, Canada (1984-1986) 18.6 81.8 Dann (1987)
Toronto, ONT, Canada (1984-1986) 9.1 37.8 Dann (1987)
Houston, TX, USA (1980) 18.8 122.9 Singh et al. (1982)
Elizabeth & Bayonne, NJ, USA 8.6 91 Wallace et al.
(outdoor air) (1981) (1985)
Elizabeth & Bayonne, NJ, USA 29.7 510 Wallace et al.
(indoor air) (1981) (1985)
Pittsburgh, PA, USA (1981) 16.3 210.6 Singh et al. (1982)
Oslo, Norway (1980) 40 114 Wathne (1983)
Rhine area, Germany (1983) 4.6-22.4 - Bruckmann et al.
(1983)
Black Forest, Germany (1983) 2.0 - Bruckmann et al.
(1983)
London, England (1983) 23 85 Clark et al. (1984a)
England (1983) (45 km from London) 6 16 Clark et al. (1984a)
Bilthoven, Netherlands (1982-1983) 2.8 10.4 RIVM (1988)
The principal source of benzene detected in indoor air appears to
be cigarette smoke, making active smoking and exposure to passive
smoke important sources of exposure to benzene for the general
population. The mainstream cigarette smoke from one cigarette
contains between 6 and 73 µg of benzene (Brunnemann et al., 1989).
Benzene has been found at higher levels in the homes of smokers
(16 µg/m3) than those of nonsmokers (9.2 µg/m3) during the autumn
and winter, whereas levels in the summer were comparable in both
domiciles (4.8 and 4.4 µg/m3, respectively) (Wallace & Pellizzari,
1986). Levels of benzene in a smoke-filled bar in the USA were found
to be 26 to 36 µg/m3 (Brunnemann et al., 1989).
Preliminary studies have indicated the release into indoor air of
low levels of benzene from consumer products such as adhesives,
building materials and paints (Wallace et al., 1987).
5.1.2 Water
Rain water in the United Kingdom has been found to contain
benzene levels as high as 87.2 µg/litre (Colenutt & Thorburn, 1980)
(Table 7).
Concentrations as high as 330 µg/litre have been found in
contaminated well water on the east coast of the USA (Burmaster,
1982). Benzene levels in open ocean samples from the relatively
unpolluted waters of the Gulf of Mexico were found to be 0.005-0.015
µg/litre (Sauer, 1981) and in polluted waters levels were 0.005-0.04
µg/litre (Sauer, 1981).
Representative concentrations of benzene in various sources of
water are given in Table 7.
Benzene concentrations in fresh surface waters are generally less
than 1 µg/litre. In the USA, early studies reported 1-7 µg/litre in
polluted areas (Ewing et al., 1977) whereas McDonald et al. (1988)
reported levels of between 0.004 and 0.91 µg/litre in river water
taken downstream from a chemical plant. Levels between 0.2 and 0.8
µg/litre were reported in the River Rhine in 1976 (Merian & Zander
(1982). In Japan, a survey of 112 water samples revealed benzene in
only 19 of the samples at levels varying from 0.03 to 2.1 µg/litre
(Environment Agency, Japan, 1989).
The limited data available indicate that benzene concentrations
in drinking-water are also in the µg/litre range. Otson (1987)
reported that levels in 10 drinking-water supplies in Canada did not
exceed 1 µg/litre. At a detection limit of 0.1 µg/litre, benzene was
found in 13, 3 and 2 out of 14 samples of treated water in the summer,
winter and spring, respectively. Previously, Otson et al. (1982) had
reported detectable (> 1 µg/litre) levels of benzene in 50 to 60% of
samples taken, the mean concentrations varying between 1 and 3
µg/litre. In the USA, water from contaminated wells contained 30 to
330 µg benzene/litre. In the same area, most samples of
drinking-water taken from surface sources had non-detectable
concentrations of benzene, and a maximum level of 4.4 µg/litre was
detected (Burmaster, 1982).
5.1.3 Soil and sediments
In general, soil contamination does not lead directly to
significant levels of human exposure because of rapid volatilization
to air. Benzene in soil is usually the result of direct contamination
by spillage or leakage. It has been found at levels ranging from
< 2 to 191 µg/kg in soils in the vicinity of five industrial
facilities using or producing benzene in the USA (Fentiman et al.,
1979). Soil concentrations in the Netherlands are low, the measured
concentrations being less than those found in ground water,
i.e. < 0.005 to 0.03 µg/litre (RIVM, 1988).
Benzene was detected in 37 out of 98 bottom sediments in Japan at
levels ranging from 0.5 to 30 µg/kg (Environment Agency, Japan, 1989).
In Lake Pontchartrain, Louisiana, Ferrario et al. (1985) reported
sediment levels of 8 to 21 µg/kg wet weight. Between 1980 and 1982,
benzene was detected in 9% of the sediment samples taken from 335
observation sites in the USA, the median level being < 5 µg/kg
(Staples et al., 1985).
Table 7. Levels of benzene in water
Source Location Concentration (µg/litre) Comments References
Rainwater United Kingdom 87.2 appear high for unknown reason(s) Colenutt & Thorburn (1980)
Germany (Berlin) 0.1-0.5 Lahmann et al. (1977)
Surface water USA (Brazos River, 0.004-0.9 downriver chemical plant outfall McDonald et al. (1988)
TX)
USA (13 sampling 1-13 both upstream and downstream near Fentiman et al. (1979)
locations) industrial outfall
USA (Potomac River) < 2 detection limit, 2 µg/litre Hall et al. (1987)
Switzerland (Lake 0.03 Grob & Grob (1974)
Zurich)
United Kingdom > 7.2 (98.4 maximum) average of 61 of 154 samples above SAC (1989)
(80 water bodies for all samples 0.1 µg/litre detection limit
across UK)
Netherlands < 0.1 sampling in 1979 RIVM (1988)
(Rhine River)
Germany < 0.1-1 occasionally up to 200 µg/litre Reynolds & Harrison (1982)
Table 7 (contd).
Source Location Concentration (µg/litre) Comments References
Sea water Gulf of Mexico 0.005 to 0.015 unpolluted waters; sampling Sauer (1981)
during 1977
USA (Brazos River 0.004-0.2 flows into Gulf of Mexico McDonald et al. (1988)
estuary, TX)
Atlantic Ocean 0.06 x 10-3 open sea OECD (1986)
Baltic Sea 0.1-4.6 x 10-3 open sea OECD (1986)
Drinking-water USA 0.1 to 0.3 US EPA (1980)
Canada (Ontario) < 0.1 to 0.2 10 treatment plants surveyed Otson (1987)
Germany < 0.1-1 occasionally up to 10 µg/litre Reynolds & Harrison (1982)
Ground water USA (Nebraska) 1.6 (median) 63 private wells, 3.2% of samples Goodenhauf & Atkinson (1986)
1.8 (maximum) contained benzene
Germany 0.02-0.05 Korte & Klein (1982)
USA (New York, New 30-300 contaminated well water Burmaster (1982)
Jersey, Connecticut)
Netherlands 0.005-0.03 unpolluted areas RIVM (1988)
5.1.4 Food
Data on the occurrence of benzene in food are limited. However,
early studies reported low levels of benzene in a variety of foods.
Some of the higher levels have been reported in Jamaican rum (120
µg/litre), irradiated beef, (19 µg/kg), heat-treated canned beef
(2 µg/kg) and eggs (500-1900 µg/kg) (IARC, 1982). Other foods where
it has been found but not quantified include haddock fillet, dry red
beans, blue cheese, cheddar cheese, cayenne pepper, pineapple, roasted
filberts, cooked potato peels, cooked chicken, hothouse tomatoes,
strawberries, blackcurrants, roasted peanuts, soybean milk and codfish
(Chang & Peterson, 1977). Benzene was detected at levels of 220 and
260 µg/kg wet weight in one sample of clams and oysters from Lake
Pontchartrain in Louisiana, USA (Ferrario et al., 1985). These
findings were not repeated when a second sample was analysed.
Benzene was detected in 37 out of 114 samples of fish in Japan
within the range of 3-88 µg/kg (Environment Agency, Japan, 1989).
Gossett et al. (1983) reported that livers of marine fish caught in
polluted waters near Los Angeles, USA contained levels of benzene in
the range 15-52 µg/kg.
5.2 General population exposure
Benzene is ubiquitous in the environment. Most of the general
population is exposed to benzene through a variety of sources. The
most important source of exposure for the general population is
through breathing air contaminated from man-made sources (including
cigarette smoking), with inhalation exposures accounting for more than
99% of the general population exposure (Hattemer-Frey et al., 1990).
Inhalation exposures occurring during the refuelling of automobiles
with gasoline can also be important. It has been estimated that a
person is exposed to levels of benzene of about 3.2 mg/m3 while
refuelling a vehicle with regular grade gasoline (Bond et al., 1986a),
which adds about 10 µg of benzene to the average daily intake. Other
sources of inhalation exposure include air near hazardous waste sites
or industrial facilities, and emissions from consumer products,
including off-gassing from particle board (ATSDR, 1991). Based on
extensive studies in the USA, it appears that facilities manufacturing
chemicals, drinking-water, food and beverages, and petroleum refining
operations play only a minimal role in the total exposure of the
general population to benzene (Wallace, 1989b).
Attempts have been made to quantify the level of benzene exposure
in the general population (Wallace, 1989a,b; Government of Canada, in
press). These studies make various assumptions as to the relative
importance and amounts of benzene from various sources, many supported
only in unpublished reports. However, they all agree that personal
sources (use of products emitting benzene, driving or riding in
automobiles), automobile exhaust and smoking (active and passive) are
major sources of benzene to the general population. By far the
greatest source of benzene exposure arises from active smoking (about
1800 µg from about 30 cigarettes/day) (Wallace, 1989b).
In both the USA and Canada, daily intakes from food and water are
minimal (up to about 1.4 µg/day). Intake from ambient and indoor air
is extremely variable depending upon whether one resides in an
industrial or large urban centre or a more rural environment, but it
has been calculated to be about 90 µg/day for a 70-kg adult in Canada
and between 180 and 1300 µg for adults in the USA. Other sources are
passive smoking (50 µg/day) and automobile-related activities (50
µg/day). For an average non-smoking 70-kg Canadian exposed to passive
smoke and various consumer products, the total daily intake of benzene
has been calculated to be approximately 230 µg, with an active smoker
taking in an additional 1800 µg daily (Government of Canada, in
press). Within the USA, daily intakes for non-smokers have been
calculated to range between 430 and 1530 µg/day (Wallace 1989a,b).
The higher levels and wider range of exposures in the USA probably
reflect higher levels of benzene detected in the ambient air of large
cities and the variations from city to city.
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure occurs mainly during the production,
handling and use of benzene and its derivatives. Surveys of
occupational exposure have been reported by Fishbein (1984), UBA
(1982) and Weaver et al. (1983).
Table 8 presents the number of workers in several industrial
sectors exposed to various time-weighted average (TWA) benzene
concentrations. These data are from the USA only and are presented to
show the workers at highest risk within an industrialized country.
Without data to the contrary, it should be assumed that the data in
Table 8 are, in general, representative of other industrialized
countries. The table does not include workers in firms not covered by
the US OSHA regulations, those under other US jurisdictions, those
using chemicals containing low levels of benzene, and tank maintenance
firms. However, these data do show that in seven major industries in
the USA employing 237 812 potentially exposed workers, approximately
95% of the workers were exposed to air levels below 16 mg/m3, i.e.
less than 50% of the 32 mg/m3 TWA. Similarly, most workers in
Sweden are exposed to values less than 16 mg/m3, with occasional
short-term exposures to 32 mg/m3 being reported among workers in
refineries and bulk petrol terminals (Nordlinder & Ramnäs, 1987).
CONCAWE (1986) reported on benzene exposure data measured over
recent years in European countries during the manufacture and
distribution of gasoline. These data represent 8-h TWA exposure
levels in various sectors of the oil industry. The report concluded
that such exposures are normally below 3.2 mg/m3 (1 ppm) for
refinery unit operators, road tanker drivers and service station
attendants. Under some conditions, 8-h TWA exposures may exceed 3.2
Table 8. Percentage of employees in the USA potentially exposed to benzenea
Percentage of observations in each exposure category according to
range of 8-h TWA benzene concentrations (mg/m3)
Industry sector 0.3-0.32 0.33-1.6 1.61-3.2 3.3-16.0 16.1-32 32+ Total number of
employees
Petrochemical plantsb 74.6 23.0 2.4 0.0 4300
Petroleum refineriesc,d 64.6 26.1 4.6 3.8 0.5 0.4 47 547
Coke and coal chemicalse 0.0 39.3 27.6 27.5 4.4 1.3 947
Tyre manufacturersc 53.4 37.5 6.3 2.8 0.0 0.0 65 000
Bulk terminalsc 57.8 32.8 5.3 3.7 0.3 0.1 27 095
Bulk plantsc 57.8 32.8 5.3 3.7 0.3 0.1 45 323
Transportation via tank
truckc 68.4 23.1 5.3 2.9 0.1 0.2 47 600
Total 237 812
a Adapted from: OSHA (1987)
b Percentages represent the proportion of workers whose average exposures are in each category.
c Percentages represent the proportion of sampling results in each exposure category.
d Data do not reflect respirator use and sampling biases.
e Excludes workers employed at the coke ovens.
mg/m3 for operators and supervisors in road tanker filling, in rail
car and marine loading, and in drum filling, but only rarely do these
exceed 32 mg/m3 (10 ppm). Additional information on occupational
exposure levels in these industries is provided in IARC (1989).
Yin et al. (1987) reported benzene concentrations in Chinese
facilities producing paint and manufacturing shoes. While the
majority of the exposures were less than 40 mg/m3, concentrations in
excess of 1000 mg/m3 were found in over 500 workplaces. In
addition, area samples were taken in 50 255 workplaces where benzene
or benzene mixtures were used (Yin et al., 1987). The geometric mean
concentration of benzene in these workplaces was 18.1 mg/m3, and
64.6% of the workplaces had concentrations of less than 40 mg/m3.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
The primary route of benzene exposure and subsequent toxicity is
via inhalation. Dermal and oral exposures are of minimal importance
in terms of total daily intake of the general population.
6.1.1 Air
Studies in rats and mice suggest that the uptake of benzene from
the lungs is nonlinearly related to the exposure concentration, i.e.
the lower the concentration the greater the absorption above
approximately 320 mg/m3 (100 ppm) (Sabourin et al., 1987). The
percentage of inhaled benzene that was retained decreased from 33% to
15% when exposure in rats for 6 h was increased from 32 to 3200
mg/m3) (10-1000 ppm); the values for mice decreased from 50% to 10%
absorption.
Several studies of benzene exposure via inhalation in humans
suggest a lung absorption factor of about 50% for continuous exposure
to 160-320 mg/m3 (50-100 ppm) for several hours (Nomiyama &
Nomiyama, 1974a,b; Snyder et al., 1981). Results from men and women
exposed to benzene concentrations of 170-200 mg/m3 (52-62 ppm) for
4 h showed that retention decreased with the duration of exposure and
reached a constant level after 2 h (Nomiyama & Nomiyama, 1974a,b).
Retention (difference between uptake and elimination) was estimated to
be 30% of the inhaled dose (Nomiyama & Nomiyama, 1974a,b). Absorption
was greatest during the first 5 min and reached a constant level
between 15 min and 3 h of continuous exposure.
6.1.2 Oral
Animal studies support the view that absorption after oral
exposure occurs readily and rapidly. Over 90% of the total
radioactivity of orally administered doses of 14C-benzene to rabbits
(340-500 mg/kg body weight) was absorbed and eliminated in the air and
urine (Parke & Williams, 1953). Similar studies in mice and rats
indicate that > 97% of oral doses (0.5 to 150 mg/kg body weight) was
absorbed in these species (Sabourin et al., 1987).
Definitive studies in humans on the rate of absorption of benzene
after ingestion are not available. However, cases of accidental or
intentional ingestion suggest that it is absorbed readily. Estimated
oral doses from 9 to 30 g have proved fatal (Sandmeyer, 1981).
6.1.3 Dermal
Dermal absorption of benzene has been shown to occur in rhesus
monkeys, minipigs, and hairless mice (Franz, 1984; Susten et al.,
1985). Absorption was less than 1% following one application of
liquid benzene. However, the rate of absorption was high, with the
highest urinary excretion of the absorbed dose being observed in the
first 8 h (Franz, 1984). Maibach & Anjo (1981) measured greater skin
penetration after multiple applications of benzene or after
applications to abraded skin.
It has been shown that benzene is absorbed through the skin of
humans. One study found that on average 0.023% of the benzene applied
to skin was absorbed; the remainder quickly volatilized (Franz, 1984).
Hanke et al. (1961) reported an hourly absorption of 0.4 mg/cm2 when
the forearm was bathed in liquid benzene.
It has been estimated that an adult working in ambient air
containing benzene at a concentration of 32 mg/m3 (10 ppm) would
absorb 7.5 µl/h via inhalation and 1.5 µl/h via whole body (2 m2)
dermal exposure (Blank & McAuliffe, 1985). The authors also estimated
that 100 cm2 of smooth and bare human skin in contact with gasoline
containing 5% benzene would absorb 7.0 µl/h.
6.2 Distribution
6.2.1 Inhalation exposure
In experimental animals, absorbed benzene is distributed
throughout several compartments, with the parent compound being
preferentially stored in fat and fatty tissues.
Steady state benzene concentrations in rats exposed via
inhalation to 1600 mg/m3 (500 ppm) for 6 h were: blood, 11.5 mg/kg;
bone marrow, 37.7 mg/kg; and fat, 164.0 mg/kg (Rickert et al., 1979).
Benzene was also found in the kidney, lung, liver, brain and spleen.
Levels of the benzene metabolites phenol, catechol and hydroquinone
were higher in bone marrow than blood, with phenol being eliminated
more rapidly after exposure than catechol or hydroquinone. Ghantous
& Danielsson (1986) exposed pregnant mice to a benzene concentration
of 6400 mg/m3 (2000 ppm) for 10 min and found benzene and its
metabolites in lipid-rich tissues such as brain and fat, as well as in
perfused tissues such as liver and kidney. Benzene was also found in
the placenta and fetuses immediately following exposure.
Studies on humans exposed to 170-202 mg/m3 (52-62 ppm) for 4 h
showed that 46.9% of the dose was taken up by the subjects; 30.2% was
retained and 16.8% was excreted as unchanged benzene in expired air
(Nomiyama & Nomiyama, 1974a,b). As far as retention is concerned,
there is apparently no difference between men and women. Most data on
distribution of benzene in humans come from case studies. As in
animals, benzene is distributed in several organs, with lipid-rich
tissues containing the highest levels. For example, one autopsy study
of a youth showed 20 mg/litre in blood; 390 mg/kg in brain; 16 mg/kg
in liver; and 22 mg/kg in abdominal fat (Winek & Collom, 1971).
Benzene can cross the human placenta and has been found in cord blood
at amounts equal to or greater than those in the mother (Dowty et al.,
1976).
6.2.2 Oral and dermal exposures
Low et al. (1989) studied the tissue distribution of
radioactivity arising from the administration of 14C-labelled
benzene (0.15, 1.5, 15, 150 or 500 mg/kg body weight) by oral gavage
to Sprague-Dawley rats. At the lowest two dose levels,
radioactivity/kg body weight was highest in the liver and kidney 1 h
after dosing; intermediate levels were found in the blood, and the
lowest levels in the Zymbal gland, nasal cavity and mammary gland.
When doses of 15 mg/kg or more were administered, there were larger
increases in the levels found in mammary glands and bone marrow than
in other tissues. In these studies, it is difficult to differentiate
between benzene distribution and the distribution of metabolites.
After 48 h following dermal application to male rats of
14C-benzene (0.004 mg/cm2) the highest percentage of administered
radioactivity was found in the kidney (0.026%), followed by the liver
(0.013%) and treated skin (0.11%) (Skowronski et al., 1988).
No reports are available regarding the distribution of benzene in
humans after oral or dermal exposures.
6.3 Metabolic transformation
The metabolism of benzene in animals and humans appears to be
qualitatively similar (Snyder, 1987; Snyder et al., 1987). There is
no indication that the route of administration has any marked effect
on the metabolites formed.
Benzene metabolism occurs primarily in the liver through the
cytochrome P-450 IIE1 system (Johansson & Ingelman-Sundberg, 1988;
Koop et al., 1989; Nakajima et al., 1990; Chepiga et al., 1991) and,
to a lesser extent, in such target tissues as the bone marrow (Kalf,
1987). The first step in benzene metabolism is oxidative, yielding
ring-hydroxylated compounds (Fig. 1). There is also a cytochrome
P-450 in bone marrow capable of metabolizing benzene (Gollmer et al.,
1984). The hydroxylated compounds (phenol, catechol, hydroquinone and
1,2,4-trihydroxy-benzene) are excreted in the urine as ethereal
sulfates and glucuronides (Fig. 2). Conjugation with glutathione and
urinary mercapturic acid is considered as an additional detoxification
pathway (Fig. 1). The opening of the benzene ring, presumably at the
epoxide or the dihydrodiol stage, is thought to yield
trans,trans-muconaldehyde (Latriano et al., 1986) which is further
oxidized via a semialdehyde to trans,trans-muconic acid (Kirley et
al., 1989) (Fig. 1 and Fig. 3).
The immediate result of the oxidative metabolism (Fig. 1) is the
formation of a system in equilibrium between benzene oxide and its
oxepin. Although the oxepin is a postulated structure, the strongest
evidence for the formation of the epoxide is the demonstration that
the addition of the enzyme epoxide hydrolase to microsomes used to
metabolize benzene results in the accumulation of benzene dihydrodiol
(Tunek et al., 1978). No other intermediate would yield the
dihydrodiol. Further evidence that the epoxide is an intermediate was
presented by Hinson et al. (1985), who proposed that the NIH shift
should occur if the epoxide was an intermediate. Using deuterated
benzene, he detected the postulated labelled products and concluded
that the epoxide was formed and that cyclohexadienone is a key
intermediate.
On the other hand, Johansson & Ingelman-Sundberg (1988) have
argued that the first step in benzene metabolism is catalysed by a
hydroxy radical generated by cytochrome P-450 LM2 from rabbit liver.
Hydroxy radical attack on the benzene ring was first postulated as a
feasible chemical mechanism by Dorfman et al. (1962) on the basis of
pulse radiolysis studies, and was applied to benzene hydroxylation in
biological systems by Simic et al. (1989) and Karam & Simic (1989).
Gorsky & Coon (1985) were unable to repeat the work of Johansson &
Ingelman-Sundberg (1988) but argued that the essential distinction
between the experiments was that the Swedish group used an extremely
low substrate concentration, far below the Km of the enzyme, and
under these circumstances cytochrome P-450 is uncoupled and is known
to generate hydrogen peroxide. At concentrations of benzene in the
usual substrate range employed, the enzyme is fully coupled, peroxide
is not generated, and the mechanism proceeds via the epoxide
intermediate.
The formation of phenol occurs by the spontaneous, non-enzymatic
rearrangement of the epoxide. Hydroquinone and catechol can then be
formed by hydroxylation of phenol (Sawahata & Neal, 1983; Gilmour et
al., 1986). Catechol can also be formed by a sequential series of
reactions beginning with the hydration of benzene oxide to yield
benzene dihydrodiol, followed by the oxidation of the dihydrodiol by
a dehydrogenase (Jerina & Daly, 1974; Bentley et al., 1976; Vogel et
al., 1980). The latter reaction cannot be observed in microsomal
preparations since the dehydrogenase is a cytoplasmic enzyme. Phenol,
hydroquinone, catechol, and its further hydroxylation product,
1,2,4-trihydroxy-benzene, can be conjugated with ethereal sulfate or
glucuronic acid (Parke & Williams, 1953). In a series of studies on
benzene metabolism and toxicity, performed by Low et al. (1991) it was
found that whereas phenylsulfate, a major conjugated metabolite of
benzene, was found in many tissues after the administration of
14C-benzene, none was found in the Zymbal gland, a significant
target tissue. These authors postulated that phenylsulfate was taken
up by a transport system into the gland, and hydrolysed to yield the
free phenol, which was then further metabolized to form reactive
intermediates responsible for the carcinogenic activity of benzene in
Chemical formula: C6H6
CAS number: 71-43-2
RTECS number: CY1400000
Common name: Benzene
IUPAC name: Benzene
Common synonyms: Annulene, benzine, benzol, benzole, benzol coal
naphtha, cyclohexatriene, mineral naphtha,
motor benzol, phenyl hydride, pyrobenzol,
pyrobenzole
Purity: Nitration grade >99%. Benzol 90 contains
80-85% benzene, 13-15% toluene and 2-3% xylene.
Commercial grades are free of H2S and SO2
and have a maximum of 0.15% non-aromatics
compounds.
2.2 Physical and chemical properties
Benzene is a naturally occurring colourless liquid at room
temperature (20 °C) and ambient pressure (760 mmHg), and has a
characteristic aromatic odour. The principal physical and chemical
properties of benzene are shown in Table 1.
2.3 Conversion factors
1 ppm = 3.2 mg/m3 at 20 °C at normal atmospheric pressure
1 mg/m3 = 0.31 ppm
2.4 Analytical methods
This section does not provide an exhaustive list of the
analytical methods available for detecting and quantifying benzene in
various media. However, those methods that are well established and
have been used in studies of human exposure and in experiments on the
biological effects of benzene will be described briefly.
Table 1. Some physical and chemical properties of benzenea
Physical form (20 °C) clear colourless liquid
Relative molecular mass 78.11
Flash point -11.1 °C
Flammable limits 1.3-7.1%
Melting/freezing point 5.5 °C
Boiling point 80.1 °C at 760 mmHg
Density 0.878
Relative vapour density
(air = 1) 2.7
Vapour pressure (26 °C) 13.3 kPa
Solubilities:
water 1800 mg/litre at 25 °C
non-aqueous solvents miscible with most
Odour threshold 4.8-15.0 mg/m3
Taste threshold (water) 0.5-4.5 mg/litre
Log n-octanol/water partition
coefficient 1.56-2.15
Sorption coefficient (log Koc -
distribution coefficient between
benzene adsorbed to soil organic
carbon and benzene in solution) 1.8-1.9
a Data from: GDCh (1988), RIVM (1988) and ATSDR (1989)
The analytical methods used for the determination of benzene
depend upon the media sampled and the level of sensitivity required.
In all cases proper sampling and sample storage are essential
prerequisites, particularly as microgram and nanogram quantities are
often found in environmental samples.
Some of the commonly used methods for the detection of benzene in
various media are summarized in Table 2.
2.4.1 Environmental samples
Methods are available for the determination of benzene in air,
water sediments, soil, foods, cigarette smoke, and petroleum and
petroleum products. Most involve separation by gas chromatography
(GC) with detection by flame ionization (FID) or photoionization (PID)
or by mass spectrometry (MS).
The measurement of benzene in air (ambient and workplace) usually
involves a preconcentration step in which the sample is passed through
a solid absorbent (Baxter et al., 1980; Pellizzari, 1982; Roberts et
al., 1984; Clark et al., 1984b; Reineke & Bächmann, 1985; Harkov et
al., 1985; Gruenke et al., 1986; OSHA, 1987; Bayer et al., 1988;
Brown, 1988a,b). Commonly used adsorbents are TenaxR resin, silica
gel, and activated carbon. Preconcentration of benzene can also be
accomplished by direct on-column cryogenic trapping (Reineke &
Bächmann, 1985; Holdren et al., 1985; Fung & Wright, 1986), or benzene
can be analysed directly (Clark et al., 1984a; Hadeishi et al., 1985;
Bayer et al., 1988). As noted in Table 2, the limit of detection of
the GC/FID or GC/PID techniques is in the low ppb (µg/m3) to low ppt
(ng/m3) range whereas the GC/MS method has a limit of detection in
the low ppb (µg/m3) range (Gruenke et al., 1986). Although GC/FID
and GC/PID provide greater sensitivity than GC/MS, the latter is
generally considered more reliable for the measurement of benzene in
samples containing multiple components with similar GC elution
characteristics. Atomic line molecular spectrometry (ALMS) has been
developed to monitor benzene and other organic compounds in ambient
air samples (Hadeishi et al., 1985). The detection limit is 800
µg/m3 (250 ppb).
Benzene in the workplace can be measured by portable
direct-reading instruments, real-time continuous monitoring systems
and passive dosimeters (OSHA, 1987) having sensitivities in the ppm
(mg/m3) range. In the USA, the more sensitive procedure of
preconcentration on charcoal followed by GC/MS analysis is generally
preferred (OSHA, 1987).
Benzene in aqueous media is usually isolated by the
purge-and-trap method (Brass et al., 1977; Hammers & Bosman, 1986)
followed by GC/MS, GC/FID or GC/PID analysis (Harland et al., 1985;
Blanchard & Hardy, 1986; Michael et al., 1988). An inert gas such as
nitrogen is used to purge the sample, the benzene is trapped on an
absorbent such as TenaxR or activated charcoal, and this is followed
by thermal desorption. The sensitivity of these methods is in the low
to sub µg/litre range with good recoveries and precision for most
methods.
Table 2. Analytical methods for the determination of benzene
Sample Preparation Analytical methoda Detection limitb Reference
Air silica gel trap indicator tube 4.9 mg/m3 Koljkowsky (1981)
Air charcoal trap, CS2 desorption GC/FID 3.2 µg/m3 Baxter et al. (1980)
Air (ambient) Tenax GC sorbent, thermal desorption capillary GC/MS NR Pellizzari (1982)
computer analysis
Air Tenax GC trap, thermal desorption, C/FID/MS 0.01 µg/m3 Roberts et al. (1984)
cryogenic focusing
Air (ambient) direct injection GC/PID 0.82 µg/m3 Clark et al. (1984a)
Air direct analysis UV Spect. 800 µg/m3 Hadeishi et al. (1985)
Air Tenax or cryogenic trap, thermal desorption GC/FID NR Holdren et al. (1985)
Air near landfills/ Tenax GC trap, thermal decomposition GC/FID/ECD/MS 0.03 µg/m3 Harkov et al. (1985)
waste sites
Air silica gel trap, thermal desorption GC/MS 0.32 µg/m3 Gruenke et al. (1986)
Air (ambient) cryogenic trap, thermal desorption GC/PID 16 ng/m3 Reineke &
GC/FID 77 ng/m3 Bachmann (1985)
Air (ambient) charcoal trap (badge or tube, desorb with GC/FID 0.96 µg/m3 Fung & Wright (1986)
CS2
Air solid sorbent trap, thermal desorption GC/MS NR Bayer et al. (1988)
Air (occupational) activated charcoal sorbent, CS2 desorption GC/FID 0.64 mg/m3 (in Brown (1988a)
12 litres)
Table 2 (contd).
Sample Preparation Analytical methoda Detection limitb Reference
Air (occupational) porous polymeric sorbent, thermal desorption GC/FID 0.83 µg/m3 Brown (1988b)
Water (drinking) purge and trap GC/MS 0.2 µg/litre Brass et al. (1977)
Water (surface or helium purge, Tenax GC trap, thermal GC/MS 0.1 µg/litre Fentiman et al. (1979)
effluents) desorption
Water purge with inert gas, Tenax trap, thermal GC/MS NR Harland et al. (1985)
desorption
Water N2 purge, Tenax GC trap, thermal desorption GC/FID 1 ng/litre Hammers & Bosman (1986)
Water filter through silicone polycarbonate GC/FID 7.2 µg/litre Blanchard & Hardy (1986)
membrane into inert gas stream
Water purge with inert gas, Tenax trap, thermal HRGC/MS 0.1 µg/litre Michael et al. (1988)
desorption to on-column cryogenic trap
Soil N2 purge, Tenax GC trap GC/FID 0.1 µg/kg Fentiman et al. (1979)
Soil N2 purge, Tenax trap, thermal desorption GC/FID 1 ng/kg Hammers & Bosman (1986)
Sediment N2 purge, Tenax trap, thermal desorption GC/MS 0.01 µg/kg Ferrario et al. (1985)
Mainstream filter smoke and direct to GC/MS; for HRGC/MS NR Brunnemann et al. (1989)
cigarette smoke passive smoke collect air in cryogenic
methanol-filled impingers
Jet fuel fumes sample on charcoal, methylene chloride, HPLC/UV 0.29 mg/m3 Dibben et al. (1989)
ethyl acetate desorption; column elution
with acetonitrile
Table 2 (contd).
Sample Preparation Analytical methoda Detection limitb Reference
Blood N2 purge, Tenax GC-silica gel trap GC/MS 0.5 µg/litre Antoine et al. (1986)
Blood extract with toluene, centrifuge; analyse GC/FID 100 µg/litre Jirka & Bourne (1982)
toluene layer
Blood add heparinized sample to isotonic saline HRGC/PID 0.4 µg/litre Pekari et al. (1989)
in headspace via equilibrate with heat
Breath collect on Tenax GC, thermal desorption HRGC/MS 9.8 ng/m3 Pellizzari et al. (1988)
Breath collect on Tenax GC, thermal desorption into GC/MS 5.2 µg/m3 Wallace et al. (1985)
on-column cryogenic trap
Urine extraction GC/MS 2 µmol/litre Stommel et al. (1989)
(as S-PMA)
Urine (phenol enzyme and acid digestion; ethyl ether GC/FID 1 mg/litre Buchet (1988)
and conjugates) extraction
Urine (muconic sample mixed with methanol, centrifuge, HPLC/UV 0.1 mg/litre Inoue et al. (1989)
acids) analyse supernatant, elute with methanol -
acetic acid
Tissues add butyl hydroxytoluene to buffered homo- RID-HPLC/UV 20 pg/g Bechtold et al. (1988)
genate, centrifuge, analyse supernatant
a GC = gas chromatography; FID = flame ionization detection; PID = photoionization detection; MS = mass spectrometry;
HRGC = high resolution (capillary) gas chromatography; RID = reverse isotope dilution; HPLC = high performance liquid chromatography;
UV = ultraviolet detection
b NR = not reported
Benzene in soil, sediment and food samples is usually determined
by purge-and-trap methods (Harland et al., 1985; Ferrario et al.,
1985; Hammers & Bosman, 1986), with headspace analysis (Kiang & Grob,
1986) and liquid extraction (Kozioski, 1985) techniques being used
less frequently. Detection limits as low as 1 ng/kg have been
reported after GC/FID or GC/MS analysis, but recoveries and precision
are frequently low.
Methods have been reported for the analysis of benzene in other
environmental media such as cigarette smoke (Brunnemann et al., 1989,
1990) and in petroleum products such as petrol (gasoline) (Poole et
al., 1988; Dibben et al., 1989).
2.4.2 Biological materials
Benzene levels in exhaled breath, blood, and body tissues have
been analysed by GC/FID, GC/PID or GC/MS, and benzene metabolites in
urine have been measured using GC/FID and high-performance liquid
chromatography (HPLC) with ultraviolet detection.
Prior to analysis, breath samples are usually collected on a
solid sorbent such as activated charcoal, silica gel or TenaxR GC
and thermally desorbed (Wallace et al., 1985; Pellizzari et al.,
1988). Headspace analysis has also been used to analyse levels of
benzene in exhaled breath (Gruenke et al., 1986). Greater selectivity
is achieved if capillary columns are used for high-resolution gas
chromatography (HRGC) (Pellizzari et al., 1988).
Three methods have been used to extract benzene from blood, i.e.
purge-and-trap (Antoine et al., 1986), headspace analysis (Gruenke et
al., 1986; Pekari et al., 1989) and solvent extraction (Jirka &
Bourne, 1982). Sensitivity for the first two procedures is in the sub
to low µg/litre range, whereas solvent extraction is less sensitive
(low to mid µg/litre).
Total phenolic metabolites of benzene have been determined in
urine following hydrolysis, extraction with ethyl ether and GC/FID
analysis (Buchet, 1988). The technique of HPLC/UV has been used to
determine the trans, trans-muconic acid metabolites of benzene in
urine (Inoue et al., 1989). A more sensititive GC/MS method to
monitor muconic acid in the urine of exposed workers has been
developed by Bechtold et al. (1991). Biological monitoring methods
using urine measure concentrations of phenolic conjugates, the major
metabolites of benzene (Buchet, 1988). Such methods, however, lack
adequate specificity and sensitivity for low levels of benzene
exposure. A method based on the determination of the minor metabolite
S-phenyl-mercapturic acid (S-PMA) appears to overcome these
deficiencies (Stommel et al., 1989). Benzene and its organic-soluble
metabolites have been determined quantitatively in rodent tissues
using GC/MS and reverse isotope dilution (RID) combined with
semipreparative HPLC/UV (Bechtold et al., 1988). A method using
ion-pairing HPLC was used to analyse water-soluble metabolites of
benzene in liver and in urine (Sabourin et al., 1988).
Schrenk & Bock (1990) have developed an HPLC method for the
determination of metabolites secreted by isolated hepatocytes.
Brodfuehrer et al. (1990) have reported on the determination of
benzene metabolites in liver slices of rat, mouse and man.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Benzene is released to the environment from both natural and
man-made sources, the latter accounting for the major part of the
emissions.
3.1 Natural occurrence
Benzene is a naturally occurring organic compound. It is a
component of petroleum (1-4%) (IARC, 1989) and can be found in sea
water (0.8 µg/litre) in the vicinity of natural deposits of petroleum
and natural gas (Reynolds & Harrison, 1982).
3.2 Anthropogenic sources
Major anthropogenic sources of benzene include automobile
exhaust, automobile-refuelling operations and industrial emissions.
Automobile exhaust probably accounts for the largest anthropogenic
source in the general environment. Cigarette smoke, off-gassing from
building material and structural fires all lead to increased
atmospheric benzene levels. People are exposed to benzene mainly
through the inhalation of contaminated air, particularly in areas of
heavy automobile traffic and around gasoline (petrol) stations and
other facilities for storage and distribution of petrol, and through
tobacco smoke from both active and passive smoking (ATSDR, 1991).
Other sources of exposure have been reported to include industrial
emissions and consumer products (Wallace et al., 1987). However,
certain individuals may be exposed to potentially high concentrations
of benzene in drinking-water as a result of seepage from underground
petroleum storage tanks, landfills, waste streams, or natural gas
deposits (ATSDR, 1991). Individuals employed in industries that
produce or use benzene or benzene-containing products are probably
exposed to much higher levels than the general population. Industrial
discharge, landfill leachate, and disposal of benzene-containing waste
are also anthropogenic sources.
3.2.1 Production levels and processes
Benzene ranks sixteenth in production volume for chemicals
produced in the USA, with an estimated production of 4.39 x 105
tonnes (1.6 x 109 gallons) in the USA in 1991 (ATSDR, 1991) and 1480
x 103 tonnes in western Europe in 1986 (GDCh, 1988) (Table 3). In
the USA over 90% of the benzene produced is derived from petroleum
sources (ATSDR, 1991), i.e. refinery streams (catalytic reformates),
pyrolysis of gasoline, and toluene hydrodealkylation. In western
Europe 55% of the benzene production is from gasoline pyrolysis, 10%
from coking of coal, and the remaining production is divided
approximately equally between catalytic reformate and the
hydrodealkylation of toluene (GDCh, 1988).
Table 3. World production of benzene in thousands of tonnes
for 1981a
Capacity Production
North & South America (total) 9350 6150
Asia (total) 3550 2460
Western Europe (total) 6950 3800
Eastern Europe (total) 5840 2340
Japan 2880 2060
USA 8030 5190
USSR 3250 1700
Other countries 100 50
World 25 800 14 800
a From: RIVM (1988)
Benzene in petrol is not included.
Given the high production volume, widespread use, and physical
and chemical properties of benzene, there is a high potential for
large amounts to be released to the environment. However, accurate
data on the amounts released are difficult to obtain. The data in
Table 4 are given to show the relative amounts of benzene released to
the air from various industrial sources in several countries. It is
evident that the largest amounts released are from the use of
gasoline. In California (USA), the 1984 benzene emission inventory
totalled 17 500 tonnes (Allen, 1987), with motor vehicle exhaust
accounting for 71% of this amount. Total emissions of benzene from
industrial sources within the USA have been reported to be 33 000 to
34 000 tonnes (US EPA, 1989). Recent emission data related to
automobile use in the USA are difficult to obtain, but in 1980 such
emissions were between 40 000 and 80 000 tonnes (IARC, 1982). In
Germany approximately 80% of the air emissions reported are due to the
use of motor vehicles, whereas coke ovens account for 3.9% of such
emissions. Other sources are gasoline storage and transport (6.2%)
and industrial furnace emissions (4.0%).
Table 4. Major emissions of benzene into the atmosphere in tonnes per yeara
Road traffic Refineries Remaining Total
sources
Belgium/Luxembourg 4950 60 750 5760
Canada 25 895 654 7601 34 150
Denmark 2600 10 390 3000
France 30 000 200 4000 34 200
Germany (FRG) 62 000 200 11 000 73 200
Greece 4700 30 700 5430
Ireland 1650 0 200 1850
Italy 29 000 190 4200 33 390
Netherlands 7300 80 980 8360
United Kingdom 29 000 150 4200 33 350
European Community
(total) 171 200 920 26 420 198 540
a From: RIVM (1988). Calculated using crude oil consumption figures from 1982.
3.2.2 Uses
Benzene has a large number of industrial, commercial and
scientific uses. Approximately, 10% of the total use of benzene is in
gasoline (RIVM, 1988), where levels average < 1% by weight in the USA
(US EPA, 1985) and 2.5-3.0% v/v in western Europe (GDCh, 1988).
Along with other aromatic compounds, benzene is important in the
production of organic chemicals, particularly styrene (Table 5). The
major uses of benzene as a chemical intermediate are summarized in
Table 5. There are no data indicating a major deviation from this
pattern of use, which was reported in 1981.
Table 5. Industrial uses of benzene in 1981. Benzene in petrol
has not been incorporateda
Production of: USA Japan Western Netherlands
Europe
Ethylbenzene/styrene 51.1 50.4 48.6 73
Cumene/phenol 20.6 12.1 19.3 16
Cyclohexane 13.8 25.6 13.4 11
Alkylates 3.0 3.7 5.2 -
Maleic acid anhydride 2.8 2.5 3.3 -
Nitrobenzene/aniline 5.3 - 6.7 -
Chlorinated benzenes 2.6 5.7 2.0 -
Other products 0.8 - 1.5 -
a From: RIVM (1988). Data shown as a percentage of the total benzene
consumed in each area.
In the past, benzene was used widely as a solvent, but this use
is declining in most developed countries; it represents < 2% of
current use. However, it is still used as a solvent in scientific
laboratories, industrial paints, rubber cements, adhesives, paint
removers, degreasing agents, production of artificial leather and of
rubber goods, and in the shoe industry (Mara & Lee, 1978; Windholz et
al., 1983; Gilman et al., 1985). For many solvent uses, benzene has
been replaced by other less toxic organic solvents. However, in the
past significant human exposure occurred when benzene was used as a
paint stripper, a carburettor cleaner, in the production of denatured
alcohol and rubber cements, and in arts and crafts supplies (Young et
al., 1978). It has also been reported that benzene vapours could be
detected from such products as carpet glue, textured carpet, liquid
detergent and furniture wax (Wallace et al., 1987).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Benzene is released into the environment from both natural and
man-made sources, although the latter are the most significant. The
volatility and solubility are the most important properties which
influence its environmental transport (see Table 1). Benzene enters
the atmosphere from direct emissions and volatilization from soil and
water surfaces.
The high volatility of benzene (vapour pressure of 13.3 Kpa at
26 °C), its solubility in water (1800 mg/litre at 25 °C) and a Henry's
law constant of 5.5 x 10-3 atm/m3 per mole at 20 °C suggest that
benzene will partition to the atmosphere from surface water (Mackay &
Leinonen, 1975). These authors have calculated a t´ in water of 4.8
h (1 metre deep at 25 °C). Benzene in air is fairly soluble in water
and is removed from the atmosphere by rain (Ogata & Miyake, 1978).
However, once it has been deposited on soil or water, volatilization
will return a portion back to the atmosphere.
Benzene is not expected to adsorb to bottom sediments for several
reasons: (1) the Koc (soil/organic carbon sorption coefficient)
(Table 1) does not predict adsorption to particles; (2) the solubility
of benzene in water, and (3) the volatility of benzene.
Benzene released to soil can partition to the atmosphere through
volatilization, to surface water through run-off, and to ground water
if released well below the surface. Evaporation from surface soil is
expected to be rapid (Hine & Mookerjee, 1975). With a Koc of 60-83,
benzene is considered fairly mobile in soil (Kenaga, 1980; Karickhoff,
1981). Leaching of benzene into ground water from soil is influenced
by several parameters including type of soil (sand versus clay),
amount of rainfall, depth of ground water and extent of benzene
degradation.
4.2 Environmental degradation
4.2.1 Abiotic degradation
In air benzene exists predominantly in the vapour phase
(Eisenreich et al., 1981). Degradation of benzene in air occurs
mainly by reactions with hydroxy, alkoxy and peroxy radicals, oxygen
atoms and ozone, of which the reaction with hydroxy radicals is the
most important. The rate constant for the reaction has been measured
often (Tully et al., 1981). Assuming an average hydroxy radical
concentration of 1.25 x 106 molecules/cm3 and a rate constant of
1.3 x 10-12 cm3/molecule per second, a t´ of 5.3 days was
calculated for benzene (RIVM, 1988). In areas of high traffic density
where there is a higher concentration of hydroxy radicals (1 x 108
molecules/cm3) and increased levels of NOx, the 24-h average t´
for benzene has been reported as 3-10 days (GDCh, 1988). Under these
conditions phototransformation products may include phenol,
nitrobenzenes, nitrophenol and various ring-opened dicarbonyl
compounds (Bandow et al., 1985). Direct photolysis of benzene in the
troposphere is unlikely since the UV-visible spectrum of benzene shows
no appreciable absorbance at wavelengths longer than 260 nm
(Bryce-Smith & Gilbert, 1976). This hypothesis was supported by Korte
& Klein (1982). No degradation was seen after 6 days irradiation of
benzene in the laboratory with light of wavelength longer than 290 nm.
4.2.2 Biodegradation
Benzene in surface and ground water is biodegradable by a variety
of microorganisms under both aerobic and anaerobic conditions (RIVM,
1988). Under both conditions the mechanism of biodegradation seems to
involve the formation of catechol via cis-1,2-dihydroxy-
1,2-dihydrolbenzene followed by ring cleavage (Högn & Jaenicke, 1972;
Korte & Klein, 1982).
Karlson & Frankenberger (1989) studied the aerobic biodegradation
of benzene in ground water utilizing a mixed bacterial culture
containing petroleum-degrading bacteria from ground water and soil
bacteria capable of using gasoline as a sole carbon source. Under
closed agitated conditions without added nutrients, benzene levels
dropped from 480 µg/litre to 218 µg/litre in 48 h. However, when
nitrogen was added the reaction was much more rapid, with benzene
levels decreasing to 35 µg/litre in 20 h. The biodegradation of
benzene in ground water and river water appears to follow first-order
rate kinetics, with t´ values of 28 and 16 days, respectively,
having been reported for ground water and river water (Vaishnav &
Babeu, 1987).
Korte & Klein (1982) studied the fate of benzene on soil
utilizing composting waste. Of the benzene applied to the waste only
2-2.5% remained in situ whereas 35% volatilized. These authors
concluded that benzene does not usually remain on soil long enough for
biodegradation to play an important role in its removal. A model
developed to predict the environmental fate of benzene following
losses of gasoline from underground tanks indicated that approximately
1% of the benzene would be degraded (Tucker et al., 1986).
Benzene is not usually biodegradable under anaerobic conditions
(GDCh, 1988). However, Wilson et al. (1986) using samples of landfill
leachate showed under methogenic conditions in an anaerobic glove-box
that, although no significant benzene biodegradation occurred during
the first 20 weeks of incubation, after 40 weeks benzene
concentrations were reduced by 72%. Using anaerobic digesting sludge,
Battersby & Wilson (1989) examined the degradation of benzene under
methanotrophic conditions. Benzene, at a level of 50 mg carbon/litre,
remained undegraded after 11 weeks of digestion. Although it is
slowly degraded under anaerobic conditions, benzene levels in sewage
influents up to 6 mg/litre do not affect sewage treatment processes
using activated sludge systems (Bennett, 1989). Jackson & Brown
(1970) reported no toxic effects of benzene on the anaerobic digestion
of sewage sludges until levels of between 50 and 200 mg/litre had been
reached.
4.2.3 Bioconcentration
Benzene is not expected to bioconcentrate to any great extent in
aquatic or terrestrial organisms given the reported values for log
Pow (octanol/water) of 2.13 and for bioconcentration factor (BCF) of
24 (Miller & Wasik, 1985). The BCF for freshwater algae was reported
to be 30 (Geyer et al., 1984), for water fleas ( Daphnia sp.) it was
153-225, depending on the concentration of benzene in their food, and
for goldfish it was 4.3 (Ogata et al., 1984).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Examples of benzene concentrations in urban and rural areas are
given in Table 6. Daily median benzene air concentrations in the USA
have been reported as: remote areas, 0.51 µg/m3 (0.16 ppb); rural
areas, 1.50 µg/m3 (0.47 ppb); and urban/suburban areas, 5.76 µg/m3
(1.8 ppb) (Shah & Singh, 1988).
The concentration appears to depend largely on the density of
automobile traffic and local weather conditions (Wallace, 1989a).
Although the median level in USA urban areas is 5.76 µg/m3 (1.8 ppb)
(Shah & Singh, 1988), levels as high as 112 µg/m3 (35 ppb) have been
observed (US EPA, 1987). Maximum levels of 510 µg/m3 (Wallace et
al., 1985) and 210.6 µg/m3 (Singh et al., 1982) have been reported
in two cities in the USA. In addition to the concentrations of
benzene shown in Table 6, the following levels of benzene have been
reported in the urban air of European cities: London, 10-12 µg/m3
background and 28-31 µg/m3 kerbside (Bailey & Schmidl, 1989);
Hamburg, Elb Tunnel, 80.5-95.3 µg/m3 (Dannecker et al., 1990) and a
residential site 9.3 µg/m3 (Bruckmann et al., 1988); and Stockholm,
average values of 147.7 µg/m3 on a busy street in the city centre
and 7.7 µg/m3 on a quiet street in the city centre (Jonsson et al.,
1985). Country wide averages in Germany have been reported to be 1-10
µg/m3 (0.31-3.1 ppb) (GDCh, 1988) and in three urban areas of Canada
they were 2.9-19.6 µg/m3 (0.9-6.0 ppb) (Government of Canada, in
press). Benzene levels, along with other pollutants, may increase
during periods of still air.
Concentrations of benzene in the atmosphere of cities where
chemical factories use or produce benzene are more variable. In the
USA, benzene concentrations have been shown to vary between 0.4 and 16
µg/m3 (Pellizzari, 1982). Levels of 3.2 mg/m3 (1 ppm) have been
measured in the breathing zone during the refuelling of automobiles
(Bond et al., 1986a).
In Frankfurt, Germany, the highest benzene levels have been
measured in the vicinity of coke ovens (maximum, 166.2 µg/m3;
average, 57.2 µg/m3), near industrial refineries (maximum, 102
µg/m3; average, 13.4 µg/m3), and in congested traffic areas
(maximum, 171.8 µg/m3; average, 16.9 µg/m3) (GDCh, 1988).
It has been reported that people living near petrochemical plants
in New Jersey, USA, have no greater exposure to benzene than the
general population throughout the area (Wallace et al., 1985). Of
particular interest in this study was the observation that in Bayonne,
New Jersey, benzene levels (arithmetic means) in indoor air (29.7
µg/m3) were greater than those reported for outside air (8.6
µg/m3) (Table 6).
Table 6. Examples of the concentrations of benzene measured in air
Concentration (µg/m3)
Location (year) Mean Maximum Reference
Montreal, PQ, Canada (1984-1986) 18.6 81.8 Dann (1987)
Toronto, ONT, Canada (1984-1986) 9.1 37.8 Dann (1987)
Houston, TX, USA (1980) 18.8 122.9 Singh et al. (1982)
Elizabeth & Bayonne, NJ, USA 8.6 91 Wallace et al.
(outdoor air) (1981) (1985)
Elizabeth & Bayonne, NJ, USA 29.7 510 Wallace et al.
(indoor air) (1981) (1985)
Pittsburgh, PA, USA (1981) 16.3 210.6 Singh et al. (1982)
Oslo, Norway (1980) 40 114 Wathne (1983)
Rhine area, Germany (1983) 4.6-22.4 - Bruckmann et al.
(1983)
Black Forest, Germany (1983) 2.0 - Bruckmann et al.
(1983)
London, England (1983) 23 85 Clark et al. (1984a)
England (1983) (45 km from London) 6 16 Clark et al. (1984a)
Bilthoven, Netherlands (1982-1983) 2.8 10.4 RIVM (1988)
The principal source of benzene detected in indoor air appears to
be cigarette smoke, making active smoking and exposure to passive
smoke important sources of exposure to benzene for the general
population. The mainstream cigarette smoke from one cigarette
contains between 6 and 73 µg of benzene (Brunnemann et al., 1989).
Benzene has been found at higher levels in the homes of smokers
(16 µg/m3) than those of nonsmokers (9.2 µg/m3) during the autumn
and winter, whereas levels in the summer were comparable in both
domiciles (4.8 and 4.4 µg/m3, respectively) (Wallace & Pellizzari,
1986). Levels of benzene in a smoke-filled bar in the USA were found
to be 26 to 36 µg/m3 (Brunnemann et al., 1989).
Preliminary studies have indicated the release into indoor air of
low levels of benzene from consumer products such as adhesives,
building materials and paints (Wallace et al., 1987).
5.1.2 Water
Rain water in the United Kingdom has been found to contain
benzene levels as high as 87.2 µg/litre (Colenutt & Thorburn, 1980)
(Table 7).
Concentrations as high as 330 µg/litre have been found in
contaminated well water on the east coast of the USA (Burmaster,
1982). Benzene levels in open ocean samples from the relatively
unpolluted waters of the Gulf of Mexico were found to be 0.005-0.015
µg/litre (Sauer, 1981) and in polluted waters levels were 0.005-0.04
µg/litre (Sauer, 1981).
Representative concentrations of benzene in various sources of
water are given in Table 7.
Benzene concentrations in fresh surface waters are generally less
than 1 µg/litre. In the USA, early studies reported 1-7 µg/litre in
polluted areas (Ewing et al., 1977) whereas McDonald et al. (1988)
reported levels of between 0.004 and 0.91 µg/litre in river water
taken downstream from a chemical plant. Levels between 0.2 and 0.8
µg/litre were reported in the River Rhine in 1976 (Merian & Zander
(1982). In Japan, a survey of 112 water samples revealed benzene in
only 19 of the samples at levels varying from 0.03 to 2.1 µg/litre
(Environment Agency, Japan, 1989).
The limited data available indicate that benzene concentrations
in drinking-water are also in the µg/litre range. Otson (1987)
reported that levels in 10 drinking-water supplies in Canada did not
exceed 1 µg/litre. At a detection limit of 0.1 µg/litre, benzene was
found in 13, 3 and 2 out of 14 samples of treated water in the summer,
winter and spring, respectively. Previously, Otson et al. (1982) had
reported detectable (> 1 µg/litre) levels of benzene in 50 to 60% of
samples taken, the mean concentrations varying between 1 and 3
µg/litre. In the USA, water from contaminated wells contained 30 to
330 µg benzene/litre. In the same area, most samples of
drinking-water taken from surface sources had non-detectable
concentrations of benzene, and a maximum level of 4.4 µg/litre was
detected (Burmaster, 1982).
5.1.3 Soil and sediments
In general, soil contamination does not lead directly to
significant levels of human exposure because of rapid volatilization
to air. Benzene in soil is usually the result of direct contamination
by spillage or leakage. It has been found at levels ranging from
< 2 to 191 µg/kg in soils in the vicinity of five industrial
facilities using or producing benzene in the USA (Fentiman et al.,
1979). Soil concentrations in the Netherlands are low, the measured
concentrations being less than those found in ground water,
i.e. < 0.005 to 0.03 µg/litre (RIVM, 1988).
Benzene was detected in 37 out of 98 bottom sediments in Japan at
levels ranging from 0.5 to 30 µg/kg (Environment Agency, Japan, 1989).
In Lake Pontchartrain, Louisiana, Ferrario et al. (1985) reported
sediment levels of 8 to 21 µg/kg wet weight. Between 1980 and 1982,
benzene was detected in 9% of the sediment samples taken from 335
observation sites in the USA, the median level being < 5 µg/kg
(Staples et al., 1985).
Table 7. Levels of benzene in water
Source Location Concentration (µg/litre) Comments References
Rainwater United Kingdom 87.2 appear high for unknown reason(s) Colenutt & Thorburn (1980)
Germany (Berlin) 0.1-0.5 Lahmann et al. (1977)
Surface water USA (Brazos River, 0.004-0.9 downriver chemical plant outfall McDonald et al. (1988)
TX)
USA (13 sampling 1-13 both upstream and downstream near Fentiman et al. (1979)
locations) industrial outfall
USA (Potomac River) < 2 detection limit, 2 µg/litre Hall et al. (1987)
Switzerland (Lake 0.03 Grob & Grob (1974)
Zurich)
United Kingdom > 7.2 (98.4 maximum) average of 61 of 154 samples above SAC (1989)
(80 water bodies for all samples 0.1 µg/litre detection limit
across UK)
Netherlands < 0.1 sampling in 1979 RIVM (1988)
(Rhine River)
Germany < 0.1-1 occasionally up to 200 µg/litre Reynolds & Harrison (1982)
Table 7 (contd).
Source Location Concentration (µg/litre) Comments References
Sea water Gulf of Mexico 0.005 to 0.015 unpolluted waters; sampling Sauer (1981)
during 1977
USA (Brazos River 0.004-0.2 flows into Gulf of Mexico McDonald et al. (1988)
estuary, TX)
Atlantic Ocean 0.06 x 10-3 open sea OECD (1986)
Baltic Sea 0.1-4.6 x 10-3 open sea OECD (1986)
Drinking-water USA 0.1 to 0.3 US EPA (1980)
Canada (Ontario) < 0.1 to 0.2 10 treatment plants surveyed Otson (1987)
Germany < 0.1-1 occasionally up to 10 µg/litre Reynolds & Harrison (1982)
Ground water USA (Nebraska) 1.6 (median) 63 private wells, 3.2% of samples Goodenhauf & Atkinson (1986)
1.8 (maximum) contained benzene
Germany 0.02-0.05 Korte & Klein (1982)
USA (New York, New 30-300 contaminated well water Burmaster (1982)
Jersey, Connecticut)
Netherlands 0.005-0.03 unpolluted areas RIVM (1988)
5.1.4 Food
Data on the occurrence of benzene in food are limited. However,
early studies reported low levels of benzene in a variety of foods.
Some of the higher levels have been reported in Jamaican rum (120
µg/litre), irradiated beef, (19 µg/kg), heat-treated canned beef
(2 µg/kg) and eggs (500-1900 µg/kg) (IARC, 1982). Other foods where
it has been found but not quantified include haddock fillet, dry red
beans, blue cheese, cheddar cheese, cayenne pepper, pineapple, roasted
filberts, cooked potato peels, cooked chicken, hothouse tomatoes,
strawberries, blackcurrants, roasted peanuts, soybean milk and codfish
(Chang & Peterson, 1977). Benzene was detected at levels of 220 and
260 µg/kg wet weight in one sample of clams and oysters from Lake
Pontchartrain in Louisiana, USA (Ferrario et al., 1985). These
findings were not repeated when a second sample was analysed.
Benzene was detected in 37 out of 114 samples of fish in Japan
within the range of 3-88 µg/kg (Environment Agency, Japan, 1989).
Gossett et al. (1983) reported that livers of marine fish caught in
polluted waters near Los Angeles, USA contained levels of benzene in
the range 15-52 µg/kg.
5.2 General population exposure
Benzene is ubiquitous in the environment. Most of the general
population is exposed to benzene through a variety of sources. The
most important source of exposure for the general population is
through breathing air contaminated from man-made sources (including
cigarette smoking), with inhalation exposures accounting for more than
99% of the general population exposure (Hattemer-Frey et al., 1990).
Inhalation exposures occurring during the refuelling of automobiles
with gasoline can also be important. It has been estimated that a
person is exposed to levels of benzene of about 3.2 mg/m3 while
refuelling a vehicle with regular grade gasoline (Bond et al., 1986a),
which adds about 10 µg of benzene to the average daily intake. Other
sources of inhalation exposure include air near hazardous waste sites
or industrial facilities, and emissions from consumer products,
including off-gassing from particle board (ATSDR, 1991). Based on
extensive studies in the USA, it appears that facilities manufacturing
chemicals, drinking-water, food and beverages, and petroleum refining
operations play only a minimal role in the total exposure of the
general population to benzene (Wallace, 1989b).
Attempts have been made to quantify the level of benzene exposure
in the general population (Wallace, 1989a,b; Government of Canada, in
press). These studies make various assumptions as to the relative
importance and amounts of benzene from various sources, many supported
only in unpublished reports. However, they all agree that personal
sources (use of products emitting benzene, driving or riding in
automobiles), automobile exhaust and smoking (active and passive) are
major sources of benzene to the general population. By far the
greatest source of benzene exposure arises from active smoking (about
1800 µg from about 30 cigarettes/day) (Wallace, 1989b).
In both the USA and Canada, daily intakes from food and water are
minimal (up to about 1.4 µg/day). Intake from ambient and indoor air
is extremely variable depending upon whether one resides in an
industrial or large urban centre or a more rural environment, but it
has been calculated to be about 90 µg/day for a 70-kg adult in Canada
and between 180 and 1300 µg for adults in the USA. Other sources are
passive smoking (50 µg/day) and automobile-related activities (50
µg/day). For an average non-smoking 70-kg Canadian exposed to passive
smoke and various consumer products, the total daily intake of benzene
has been calculated to be approximately 230 µg, with an active smoker
taking in an additional 1800 µg daily (Government of Canada, in
press). Within the USA, daily intakes for non-smokers have been
calculated to range between 430 and 1530 µg/day (Wallace 1989a,b).
The higher levels and wider range of exposures in the USA probably
reflect higher levels of benzene detected in the ambient air of large
cities and the variations from city to city.
5.3 Occupational exposure during manufacture, formulation or use
Occupational exposure occurs mainly during the production,
handling and use of benzene and its derivatives. Surveys of
occupational exposure have been reported by Fishbein (1984), UBA
(1982) and Weaver et al. (1983).
Table 8 presents the number of workers in several industrial
sectors exposed to various time-weighted average (TWA) benzene
concentrations. These data are from the USA only and are presented to
show the workers at highest risk within an industrialized country.
Without data to the contrary, it should be assumed that the data in
Table 8 are, in general, representative of other industrialized
countries. The table does not include workers in firms not covered by
the US OSHA regulations, those under other US jurisdictions, those
using chemicals containing low levels of benzene, and tank maintenance
firms. However, these data do show that in seven major industries in
the USA employing 237 812 potentially exposed workers, approximately
95% of the workers were exposed to air levels below 16 mg/m3, i.e.
less than 50% of the 32 mg/m3 TWA. Similarly, most workers in
Sweden are exposed to values less than 16 mg/m3, with occasional
short-term exposures to 32 mg/m3 being reported among workers in
refineries and bulk petrol terminals (Nordlinder & Ramnäs, 1987).
CONCAWE (1986) reported on benzene exposure data measured over
recent years in European countries during the manufacture and
distribution of gasoline. These data represent 8-h TWA exposure
levels in various sectors of the oil industry. The report concluded
that such exposures are normally below 3.2 mg/m3 (1 ppm) for
refinery unit operators, road tanker drivers and service station
attendants. Under some conditions, 8-h TWA exposures may exceed 3.2
Table 8. Percentage of employees in the USA potentially exposed to benzenea
Percentage of observations in each exposure category according to
range of 8-h TWA benzene concentrations (mg/m3)
Industry sector 0.3-0.32 0.33-1.6 1.61-3.2 3.3-16.0 16.1-32 32+ Total number of
employees
Petrochemical plantsb 74.6 23.0 2.4 0.0 4300
Petroleum refineriesc,d 64.6 26.1 4.6 3.8 0.5 0.4 47 547
Coke and coal chemicalse 0.0 39.3 27.6 27.5 4.4 1.3 947
Tyre manufacturersc 53.4 37.5 6.3 2.8 0.0 0.0 65 000
Bulk terminalsc 57.8 32.8 5.3 3.7 0.3 0.1 27 095
Bulk plantsc 57.8 32.8 5.3 3.7 0.3 0.1 45 323
Transportation via tank
truckc 68.4 23.1 5.3 2.9 0.1 0.2 47 600
Total 237 812
a Adapted from: OSHA (1987)
b Percentages represent the proportion of workers whose average exposures are in each category.
c Percentages represent the proportion of sampling results in each exposure category.
d Data do not reflect respirator use and sampling biases.
e Excludes workers employed at the coke ovens.
mg/m3 for operators and supervisors in road tanker filling, in rail
car and marine loading, and in drum filling, but only rarely do these
exceed 32 mg/m3 (10 ppm). Additional information on occupational
exposure levels in these industries is provided in IARC (1989).
Yin et al. (1987) reported benzene concentrations in Chinese
facilities producing paint and manufacturing shoes. While the
majority of the exposures were less than 40 mg/m3, concentrations in
excess of 1000 mg/m3 were found in over 500 workplaces. In
addition, area samples were taken in 50 255 workplaces where benzene
or benzene mixtures were used (Yin et al., 1987). The geometric mean
concentration of benzene in these workplaces was 18.1 mg/m3, and
64.6% of the workplaces had concentrations of less than 40 mg/m3.
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
The primary route of benzene exposure and subsequent toxicity is
via inhalation. Dermal and oral exposures are of minimal importance
in terms of total daily intake of the general population.
6.1.1 Air
Studies in rats and mice suggest that the uptake of benzene from
the lungs is nonlinearly related to the exposure concentration, i.e.
the lower the concentration the greater the absorption above
approximately 320 mg/m3 (100 ppm) (Sabourin et al., 1987). The
percentage of inhaled benzene that was retained decreased from 33% to
15% when exposure in rats for 6 h was increased from 32 to 3200
mg/m3) (10-1000 ppm); the values for mice decreased from 50% to 10%
absorption.
Several studies of benzene exposure via inhalation in humans
suggest a lung absorption factor of about 50% for continuous exposure
to 160-320 mg/m3 (50-100 ppm) for several hours (Nomiyama &
Nomiyama, 1974a,b; Snyder et al., 1981). Results from men and women
exposed to benzene concentrations of 170-200 mg/m3 (52-62 ppm) for
4 h showed that retention decreased with the duration of exposure and
reached a constant level after 2 h (Nomiyama & Nomiyama, 1974a,b).
Retention (difference between uptake and elimination) was estimated to
be 30% of the inhaled dose (Nomiyama & Nomiyama, 1974a,b). Absorption
was greatest during the first 5 min and reached a constant level
between 15 min and 3 h of continuous exposure.
6.1.2 Oral
Animal studies support the view that absorption after oral
exposure occurs readily and rapidly. Over 90% of the total
radioactivity of orally administered doses of 14C-benzene to rabbits
(340-500 mg/kg body weight) was absorbed and eliminated in the air and
urine (Parke & Williams, 1953). Similar studies in mice and rats
indicate that > 97% of oral doses (0.5 to 150 mg/kg body weight) was
absorbed in these species (Sabourin et al., 1987).
Definitive studies in humans on the rate of absorption of benzene
after ingestion are not available. However, cases of accidental or
intentional ingestion suggest that it is absorbed readily. Estimated
oral doses from 9 to 30 g have proved fatal (Sandmeyer, 1981).
6.1.3 Dermal
Dermal absorption of benzene has been shown to occur in rhesus
monkeys, minipigs, and hairless mice (Franz, 1984; Susten et al.,
1985). Absorption was less than 1% following one application of
liquid benzene. However, the rate of absorption was high, with the
highest urinary excretion of the absorbed dose being observed in the
first 8 h (Franz, 1984). Maibach & Anjo (1981) measured greater skin
penetration after multiple applications of benzene or after
applications to abraded skin.
It has been shown that benzene is absorbed through the skin of
humans. One study found that on average 0.023% of the benzene applied
to skin was absorbed; the remainder quickly volatilized (Franz, 1984).
Hanke et al. (1961) reported an hourly absorption of 0.4 mg/cm2 when
the forearm was bathed in liquid benzene.
It has been estimated that an adult working in ambient air
containing benzene at a concentration of 32 mg/m3 (10 ppm) would
absorb 7.5 µl/h via inhalation and 1.5 µl/h via whole body (2 m2)
dermal exposure (Blank & McAuliffe, 1985). The authors also estimated
that 100 cm2 of smooth and bare human skin in contact with gasoline
containing 5% benzene would absorb 7.0 µl/h.
6.2 Distribution
6.2.1 Inhalation exposure
In experimental animals, absorbed benzene is distributed
throughout several compartments, with the parent compound being
preferentially stored in fat and fatty tissues.
Steady state benzene concentrations in rats exposed via
inhalation to 1600 mg/m3 (500 ppm) for 6 h were: blood, 11.5 mg/kg;
bone marrow, 37.7 mg/kg; and fat, 164.0 mg/kg (Rickert et al., 1979).
Benzene was also found in the kidney, lung, liver, brain and spleen.
Levels of the benzene metabolites phenol, catechol and hydroquinone
were higher in bone marrow than blood, with phenol being eliminated
more rapidly after exposure than catechol or hydroquinone. Ghantous
& Danielsson (1986) exposed pregnant mice to a benzene concentration
of 6400 mg/m3 (2000 ppm) for 10 min and found benzene and its
metabolites in lipid-rich tissues such as brain and fat, as well as in
perfused tissues such as liver and kidney. Benzene was also found in
the placenta and fetuses immediately following exposure.
Studies on humans exposed to 170-202 mg/m3 (52-62 ppm) for 4 h
showed that 46.9% of the dose was taken up by the subjects; 30.2% was
retained and 16.8% was excreted as unchanged benzene in expired air
(Nomiyama & Nomiyama, 1974a,b). As far as retention is concerned,
there is apparently no difference between men and women. Most data on
distribution of benzene in humans come from case studies. As in
animals, benzene is distributed in several organs, with lipid-rich
tissues containing the highest levels. For example, one autopsy study
of a youth showed 20 mg/litre in blood; 390 mg/kg in brain; 16 mg/kg
in liver; and 22 mg/kg in abdominal fat (Winek & Collom, 1971).
Benzene can cross the human placenta and has been found in cord blood
at amounts equal to or greater than those in the mother (Dowty et al.,
1976).
6.2.2 Oral and dermal exposures
Low et al. (1989) studied the tissue distribution of
radioactivity arising from the administration of 14C-labelled
benzene (0.15, 1.5, 15, 150 or 500 mg/kg body weight) by oral gavage
to Sprague-Dawley rats. At the lowest two dose levels,
radioactivity/kg body weight was highest in the liver and kidney 1 h
after dosing; intermediate levels were found in the blood, and the
lowest levels in the Zymbal gland, nasal cavity and mammary gland.
When doses of 15 mg/kg or more were administered, there were larger
increases in the levels found in mammary glands and bone marrow than
in other tissues. In these studies, it is difficult to differentiate
between benzene distribution and the distribution of metabolites.
After 48 h following dermal application to male rats of
14C-benzene (0.004 mg/cm2) the highest percentage of administered
radioactivity was found in the kidney (0.026%), followed by the liver
(0.013%) and treated skin (0.11%) (Skowronski et al., 1988).
No reports are available regarding the distribution of benzene in
humans after oral or dermal exposures.
6.3 Metabolic transformation
The metabolism of benzene in animals and humans appears to be
qualitatively similar (Snyder, 1987; Snyder et al., 1987). There is
no indication that the route of administration has any marked effect
on the metabolites formed.
Benzene metabolism occurs primarily in the liver through the
cytochrome P-450 IIE1 system (Johansson & Ingelman-Sundberg, 1988;
Koop et al., 1989; Nakajima et al., 1990; Chepiga et al., 1991) and,
to a lesser extent, in such target tissues as the bone marrow (Kalf,
1987). The first step in benzene metabolism is oxidative, yielding
ring-hydroxylated compounds (Fig. 1). There is also a cytochrome
P-450 in bone marrow capable of metabolizing benzene (Gollmer et al.,
1984). The hydroxylated compounds (phenol, catechol, hydroquinone and
1,2,4-trihydroxy-benzene) are excreted in the urine as ethereal
sulfates and glucuronides (Fig. 2). Conjugation with glutathione and
urinary mercapturic acid is considered as an additional detoxification
pathway (Fig. 1). The opening of the benzene ring, presumably at the
epoxide or the dihydrodiol stage, is thought to yield
trans,trans-muconaldehyde (Latriano et al., 1986) which is further
oxidized via a semialdehyde to trans,trans-muconic acid (Kirley et
al., 1989) (Fig. 1 and Fig. 3).
The immediate result of the oxidative metabolism (Fig. 1) is the
formation of a system in equilibrium between benzene oxide and its
oxepin. Although the oxepin is a postulated structure, the strongest
evidence for the formation of the epoxide is the demonstration that
the addition of the enzyme epoxide hydrolase to microsomes used to
metabolize benzene results in the accumulation of benzene dihydrodiol
(Tunek et al., 1978). No other intermediate would yield the
dihydrodiol. Further evidence that the epoxide is an intermediate was
presented by Hinson et al. (1985), who proposed that the NIH shift
should occur if the epoxide was an intermediate. Using deuterated
benzene, he detected the postulated labelled products and concluded
that the epoxide was formed and that cyclohexadienone is a key
intermediate.
On the other hand, Johansson & Ingelman-Sundberg (1988) have
argued that the first step in benzene metabolism is catalysed by a
hydroxy radical generated by cytochrome P-450 LM2 from rabbit liver.
Hydroxy radical attack on the benzene ring was first postulated as a
feasible chemical mechanism by Dorfman et al. (1962) on the basis of
pulse radiolysis studies, and was applied to benzene hydroxylation in
biological systems by Simic et al. (1989) and Karam & Simic (1989).
Gorsky & Coon (1985) were unable to repeat the work of Johansson &
Ingelman-Sundberg (1988) but argued that the essential distinction
between the experiments was that the Swedish group used an extremely
low substrate concentration, far below the Km of the enzyme, and
under these circumstances cytochrome P-450 is uncoupled and is known
to generate hydrogen peroxide. At concentrations of benzene in the
usual substrate range employed, the enzyme is fully coupled, peroxide
is not generated, and the mechanism proceeds via the epoxide
intermediate.
The formation of phenol occurs by the spontaneous, non-enzymatic
rearrangement of the epoxide. Hydroquinone and catechol can then be
formed by hydroxylation of phenol (Sawahata & Neal, 1983; Gilmour et
al., 1986). Catechol can also be formed by a sequential series of
reactions beginning with the hydration of benzene oxide to yield
benzene dihydrodiol, followed by the oxidation of the dihydrodiol by
a dehydrogenase (Jerina & Daly, 1974; Bentley et al., 1976; Vogel et
al., 1980). The latter reaction cannot be observed in microsomal
preparations since the dehydrogenase is a cytoplasmic enzyme. Phenol,
hydroquinone, catechol, and its further hydroxylation product,
1,2,4-trihydroxy-benzene, can be conjugated with ethereal sulfate or
glucuronic acid (Parke & Williams, 1953). In a series of studies on
benzene metabolism and toxicity, performed by Low et al. (1991) it was
found that whereas phenylsulfate, a major conjugated metabolite of
benzene, was found in many tissues after the administration of
14C-benzene, none was found in the Zymbal gland, a significant
target tissue. These authors postulated that phenylsulfate was taken
up by a transport system into the gland, and hydrolysed to yield the
free phenol, which was then further metabolized to form reactive
intermediates responsible for the carcinogenic activity of benzene in
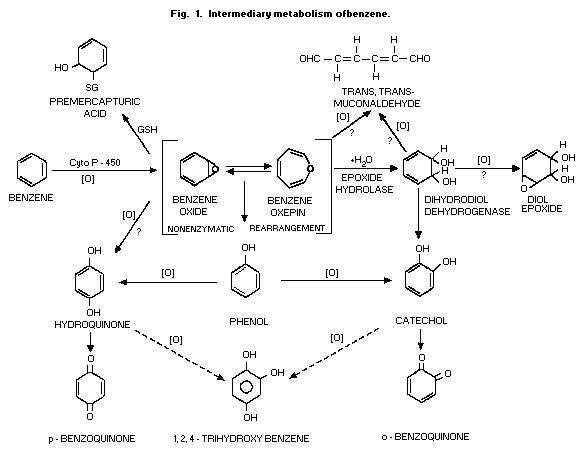
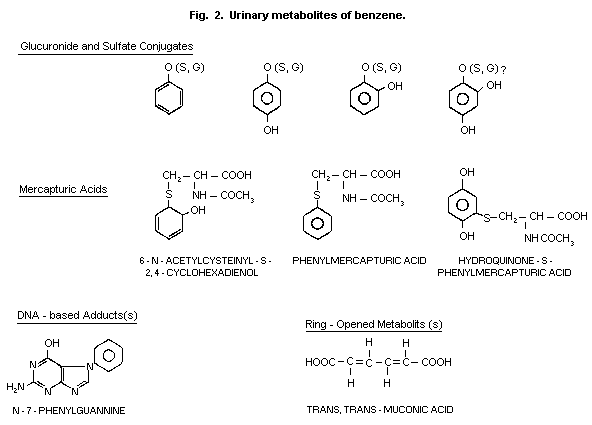
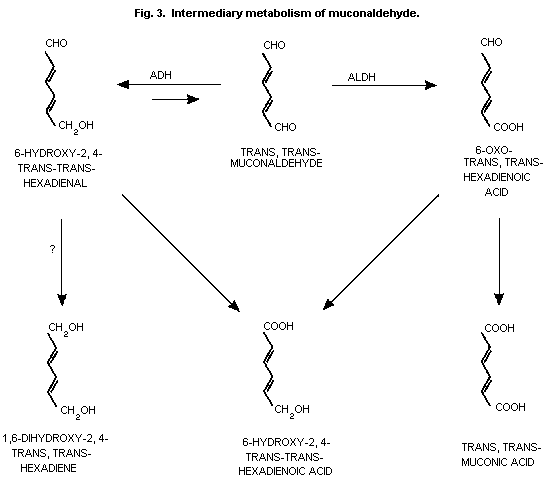 the Zymbal gland. This is the first suggestion that conjugation
products, normally thought of as only a mechanism for urinary
excretion, could also be considered to act as a transporting mechanism
for bringing metabolites from the liver to target tissues.
Parke & Williams (1953) reported that phenyl mercapturic acid was
a urinary end product of benzene metabolism. This observation was
supported by the report of Jerina et al. (1968) who incubated
glutathione with rat liver cytoplasm and benzene oxide and found that
the principal metabolite was S-phenylglutathione. Norpoth (1988) has
developed a method for the determination of phenylmercapturic acid in
human urine as a measure of exposure to benzene based on these
observations. However, Lunte & Kissinger (1983) showed that
p-benzoquinone, an oxidation product of hydroquinone, forms
glutathione conjugates non-enzymatically. Lau et al. (1989) reported
that 1,2,3 or 4 glutathione molecules could conjugate with
p-benzoquinone. Nerland & Pierce (1990) showed the occurrence of
N-acetyl- S-(2,5-dihydroxyphenyl)l-cysteine as a urinary metabolite
of benzene in rats. Stommel et al. (1989) found that the metabolite
phenylmercapturic acid increased proportionally in rats and humans as
the inhaled dose rose to 1600 mg/m3 (500 ppm). Thus, the array of
mercapturic acid metabolites of benzene has expanded and the full
extent of metabolites of this structure may not yet be fully
appreciated.
In summary, the postulated metabolic pathways for benzene are
shown in Figures 1, 2 and 3. The formations of mercapturic acids,
ethereal sulfates and glucuronides are generally considered
detoxification pathways leading to the excretion of benzene
metabolites via the kidney (Henderson et al., 1989). All other
pathways lead to potentially toxic metabolites. This hypothesis is
discussed in more detail in section 7.9.
In both rats and mice the formation of toxic metabolites via the
epoxide pathway appears to be a saturable process, which suggests that
both metabolism and toxicity would be non-linear. In other words, the
proportion of toxic metabolites formed would decrease once the
saturation level is reached, whereas detoxification pathways appear to
be low-affinity high-capacity reactions (Henderson et al., 1989;
Medinsky et al., 1989a). It has been shown that mice metabolize
benzene faster and converted more of the benzene to toxic metabolites
than rats (Henderson et al., 1989). Because of this it has been
suggested that metabolism in mice favours toxification pathways (e.g.,
formation of benzoquinone and muconaldehyde), while in rats metabolism
is primarily detoxification (phenyl conjugates and phenylmercapturic
acids) (Medinsky et al., 1989a). The percentage of benzene or its
metabolites remaining in the body decreased in rats (from 33% to 15%)
and mice (from 50% to 10%) as exposure increased from 32 to 3200
mg/m3 (10 to 1000 ppm) (Sabourin et al., 1987).
Model simulations for total benzene metabolized and for profiles
of benzene metabolites formed after the administration of varying
doses of benzene to rats and mice (Medinsky et al., 1989b,c) have
suggested that the production of hydroquinone and muconic acid
metabolites predominates at lower exposure concentrations, whereas at
high exposure levels the detoxification pathways account for a larger
fraction of benzene metabolized. In addition, these model simulations
have confirmed that mice metabolize more benzene on a µmole/kg body
weight basis than rats after inhalation exposures, whereas rats
metabolize more benzene than mice at oral doses greater than 50 mg/kg
body weight. After either oral or inhalation exposures mice
preferentially form more of the putative toxic metabolites
hydroquinone and muconic acid (Medinsky et al., 1989b). It has also
been reported by Witz et al. (1990b) that DBA/ZN mice (a strain
sensitive to the haematotoxicity of benzene), excrete greater amounts
of trans, trans-muconic acid than the less sensitive C57BL/6 strain
after equivalent exposures to benzene.
6.4 Elimination and excretion
6.4.1 Inhalation exposure
In animals, expired air is the main route of elimination of
unmetabolized benzene, while urine is the major route of excretion of
benzene metabolites (with very little faecal excretion). Rickert et
al. (1979) found a biphasic pattern of excretion of unmetabolized
benzene in rats after a 6-h exposure to 1600 mg/m3 (500 ppm), with
half-times of 0.7 h for the rapid phase and 13.1 h for the slow phase.
The major route of excretion after inhalation exposures of rats and
mice to 32-3200 mg/m3 (10-1000 ppm) appeared to be dependent upon
the concentration inhaled (Sabourin et al., 1987). Under these
conditions mice received 150-200% of the dose given to rats on a per
kg body weight basis. The faecal excretion was < 3.5% in rats and
< 9% in mice. At doses up to 416 mg/m3 (130 ppm), less than 6% of
the radioactivity was eliminated in expired air, whereas at the highest
concentrations 48% of the dose was eliminated as unchanged chemical in
rats and 14% in mice. The total urinary excretion of metabolites at
these high concentrations was 5-37% higher in mice than in rats.
Findings in humans after inhalation exposure to benzene are
similar to those in experimental animals; unmetabolized chemical is
eliminated in expired air whereas metabolites of benzene are excreted
in urine, primarily as the sulfate and glucuronide conjugates of
phenol. Nomiyama & Nomiyama (1974a,b) found similar expiratory
patterns in men and women exposed for 4 h to benzene at concentration
between 166 and 198 mg/m3 (52-62 ppm). The proportion of the
absorbed benzene that was excreted via the lungs was approximately 17%
(Nomiyama & Nomiyama, 1974a,b).
6.4.2. Oral exposure
Parke & Williams (1953) administered radiolabelled benzene
(approximately 340 mg/kg body weight) by oral gavage to rabbits and
reported that 43% of the label was recovered as unmetabolized benzene
in expired air. Urinary excretion accounted for 33% of the dose,
mainly in the form of conjugated phenol (23.5%). Other phenols
excreted were hydroquinone (4.8%), catechol (2.2%), and hydroxyquinol
(1,2,4-trihydroxybenzene) (0.3%). Muconic acid accounted for 1.3% and
L-phenylmercapturic acid for 0.5%, and 5-10% of the radiolabel
remained in the tissues or was excreted in the faeces. The excretion
of benzene and its metabolites in rats and mice at various oral doses
(0.5-300 mg/kg body weight) was studied by Sabourin et al. (1987). In
both species the excretion of urinary metabolites up to a dose of 15
mg/kg accounted for 80% of the administered dose. Above that level
there was an increase in the elimination of 14C in expired air.
Equal amounts of unmetabolized benzene were eliminated in both species
up to dose levels of 50 mg/kg. At dose levels of between 15 and 50
mg/kg body weight, metabolism appears to become saturated in rodents.
In rats, 50% of a 150-mg/kg dose of 14C-benzene was eliminated in
expired air, while in the mouse 69% of this dose was exhaled (Sabourin
et al., 1987).
No studies were found regarding the excretion of benzene in
humans after oral exposures.
6.4.3 Dermal exposure
After the dermal application of 14C-benzene (0.0026 to 0.0036
mg/cm2) to monkeys and minipigs, Franz (1984) collected urine
samples every 5 h for 2-4 days. The rate of excretion was highest
over the first 10 h, the total excretion of radioactivity being higher
in the monkey (0.03 to 0.14% of the applied dose, with an average of
0.06%) than in the minipigs (0.03-0.05%, with an average of 0.04%).
Using a glass cap to minimize volatilization from the skin, Skowronski
et al. (1988) treated male rats dermally with 14C-benzene (0.004
mg/cm2). After 48 h, 86.2% of the initial dose was excreted in the
urine and 12.8% was eliminated in expired air. Phenol was the major
urinary metabolite detected in the 0-12 h sample (37.7% of dose), and
smaller quantities of hydroquinone, catechol and benzenetriol were
also detected.
In a study of four male human subjects, Franz (1984) applied
14C-benzene dermally (0.0024 mg/cm2). A mean of 0.023% (range
0.006-0.054%) of the applied radiolabel was recovered in the urine
over a 36-h period. More than 80% of the excretion occurred within
8 h of application.
6.5 Retention and turnover
Steady state levels of benzene were found within 4 h in blood,
6 h in fat, and 2 h in bone marrow when male rats were exposed to a
benzene concentration of 1600 mg/m3 (500 ppm) by inhalation for 6 h.
After exposure ceased, about 70% of the benzene was eliminated
unchanged in the expired air and about 30% was excreted in urine as
water-soluble metabolites within 15 h. The half-life (t´) for
elimination from these tissues was 0.4-0.8 h, except in the case of
adipose tissue where elimination occurred with a t´ of 1.6 h. The
elimination of unchanged benzene in expired air was biphasic, the t´
being 0.7 h for the first phase and 13.1 h for the slower phase. Free
phenol, catechol and hydroquinone were detected in blood and bone
marrow after exposure ceased. The phenol level declined rapidly over
a 9-h observation period, whereas catechol and hydroquinone levels in
both tissues remained constant over this period (Rickert et al.,
1979).
After intraperitoneal injection, oral gavage, or inhalation
exposures of labelled benzene in rats and mice, over 95% of the
administered radioactivity was excreted within 40 h (Sabourin et al.,
1987; Henderson et al., 1989). Approximately 90% of the metabolites
was excreted in the urine. According to the authors, these studies
indicate that benzene is rapidly metabolized and excreted in the urine
within 40 h of dosing by any route of administration.
6.6 Reaction with body components
3H-benzene metabolites have been shown to bind irreversibly to
proteins in both mouse liver and bone marrow (Snyder et al., 1978a).
Benzene metabolites have also been shown to bind in vivo to mouse
protein in blood (Sun et al., 1990), liver, bone marrow and spleen
(Longacre et al., 1981a,b). Covalent binding increased both with dose
and frequency of dosing. Covalent binding of benzene metabolites to
protein appears to be mediated by microsomal enzymes (Tunek et al.,
1978) and has been suggested to be the result of binding by
hydroquinone and catechol (Wallin et al., 1985). The finding of high
levels of phenylcysteine adducts in the haemoglobin of benzene-exposed
rats suggests that benzene oxide also reacts with proteins to form
adducts (Bechtold et al., 1992). Benzene metabolism and covalent
binding to proteins have been demonstrated in situ in bone marrow
(Irons et al., 1980b).
Lutz (1979) has attempted to quantify the extent to which
chemicals covalently bind to DNA using the concept of the covalent
binding index (CBI). The implication is that the higher the CBI, the
more likely a chemical will be a carcinogen. The calculated value for
benzene, based on binding to liver nuclear DNA, was 1.7. (To put this
value in perspective, CBI values for some common carcinogens were:
aflatoxin B1, > 1000; 2-acetyl-aminifluorene, 100 to several hundred;
polycyclic aromatic hydrocarbons, 10 to 30.) No values were given for
benzene in bone marrow, but Snyder et al. (1978a) compared covalent
binding of benzene residues per g dry weight of liver and bone marrow
and found that, depending upon the dose of benzene, covalent residues
bound to liver ranged from 500 to 800 nmoles/g, whereas binding to
bone marrow ranged from 18 to 96 nmoles/g. Thus, there appeared to be
less covalent binding in the target organ, i.e. bone marrow, than in
the metabolizing organ, i.e. liver.
Inhaled benzene has been found to bind to rat liver DNA to the
extent of 2.38 µmoles/mole DNA phosphate (Lutz & Schlatter, 1977).
Studies of the covalent binding of benzene metabolites to DNA have
resulted in the postulation of several structures for DNA adducts
derived from benzene. Bone marrow mitochondria from rabbits were
incubated sequentially with 3H-deoxyguanosine triphosphate and
14C-benzene to evaluate DNA adducts formed from benzene metabolites
(Snyder et al., 1987b). These authors identified at least seven
deoxyguanosine adducts and one deoxyadenine adduct. Covalent
N-7-phenyl-guanine adducts have been isolated from rat urine after
intraperitoneal dosing with 330-400 mg benzene/kg body weight (Norpoth
et al., 1988). Thus, Jowa et al. (1990) postulated the formation of
an adduct between p-benzoquinone and deoxyguanosine which had the
structure (3'OH)benztheno(1,N2)deoxyguanosine. The structure of an
adduct formed between p-benzoquinone and deoxyadenosine-3'-phos
phate was suggested to be 3'-hydroxy-1,N6-benztheno-2'-deoxy-
adenosine-3'-phosphate (Pongracz & Bodell, 1991). Reddy et al.
(1990), however, reported that they were unable to detect DNA adducts
derived from benzene in the rat in vivo, despite having observed
them in Zymbal gland cells in vitro, using the 32P-post labelling
technique.
6.7 Modelling of pharmacokinetic data for benzene
In order to obtain better insight into the interspecies
variations in the uptake, metabolic fate and excretion of benzene and
its metabolites, both compartmentally (Bailer & Hoel, 1989; Beliles &
Totman, 1989) and physiologically based (Medinsky et al., 1989b,c;
Paxman & Rappaport, 1990; Travis et al., 1990; Bois et al., 1991a,b),
pharmacokinetic models have been developed. These models have been
used as an aid to risk assessment by facilitating extrapolation
between species where various exposure regimens had been utilized.
Also, such models are useful for identifying gaps in knowledge that
have been highlighted by poor fits of the experimental data to the
models developed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Benzene has been shown to produce a number of biological
responses in experimental animals. The acute effects of benzene at
high doses reflect its activity as a general anaesthetic and can lead
to central nervous system (CNS) depression, loss of consciousness and
coincidental sensitization of the myocardium to catecholamines.
Chronic exposure can result in bone marrow depression expressed as
leucopenia, anaemia and/or thrombocytopenia, leading to pancytopenia
and aplastic anaemia. The immunotoxic effects of benzene are probably
related to bone marrow depression. In animal cancer bioassays it is,
primarily, epithelial tumours that have been reported, whereas in
humans the carcinogenic response is leukaemia. A third type of
biological impact is the production of clastogenic responses such as
chromosome aberrations, sister chromatid exchange and micronuclei.
Benzene has also been suggested to produce fetotoxic effects.
7.1 Single exposure
Consistent with many other aromatic hydrocarbons (Patty, 1981),
benzene appears to be of low acute toxicity when administered to
various animal species by various routes of administration (Table 9).
Other reported oral LD50 values for reagent grade benzene in male
rats vary from as low as 930 mg/kg to as high as 5600 mg/kg body
weight (Wolf et al., 1956; Cornish & Ryan, 1965; Kimura et al., 1971;
Withey & Hall, 1975). The LD50 after intraperitoneal injection in
female rats was reported to be 2940 mg/kg (Drew & Fouts, 1974) and in
mice it was 300 mg/kg body weight (Kocsis et al., 1968). Young rats
are more sensitive (in terms of LD50) than older ones (Table 9).
The LC50 in female rats was estimated to be 43 770 mg/m3 (13
700 ppm) after a single 4-h exposure (Drew & Fouts, 1974).
Benzene has a narcotic effect after oral administration in rats
(Withey & Hall, 1975) and after inhalation in mice (Uyeki et al.,
1977). The threshold narcotic effect after inhalation has been
estimated to be approximately 13 000 mg/m3 (Leong, 1977).
Inhalation of air saturated with benzene resulted in ventricular
tachycardia and occasionally ventricular fibrillation and death in
rats, cats, rabbits and primates (Nahum & Hoff, 1934). Respiratory
failure was also observed during narcosis. Pathological findings after
sudden death are congestion of various organs, particularly the lungs
and liver (Jonek et al., 1965).
No information on the acute toxicity/lethality in animals after
dermal exposure has been reported.
Table 9. Toxicity of benzene in animals after acute exposure
Route Species Parameter Value Reference
Oral rat (14 days old) LD50 3000 mg/kg body weight Kimura et al. (1971)
Oral rat (young adult) LD50 3300 mg/kg body weight Kimura et al. (1971)
Oral rat (old adult) LD50 4900 mg/kg body weight HSE (1982); Kimura et al. (1971)
Oral rat LD50 8100 mg/kg body weight Cornish & Ryan (1965)
Inhalation (4 h) rat LC50 44 660 mg/m3 body weight Drew & Fouts (1974)
Inhalation (7 h) rat LC50 32 600 mg/m3 body weight HSE (1982)
Inhalation (2 h) mouse lethal dose 61 125 mg/m3 body weight Jonek et al. (1965)
Intraperitoneal injection rat LD50 2940 mg/kg body weight Drew & Fouts (1974)
Intraperitoneal injection mouse LD50 300 mg/kg body weight Kocsis et al. (1968)
7.2 Short-term and long-term exposures
The studies discussed in this section, some of which are
summarized in Table 10, have a duration of less than one year.
Lifetime (> 1 year) studies are discussed in section 7.6 and
summarized in Tables 15 to 17.
In short-term inhalation studies, three out of eight male rats
died within 24 h after exposure for five periods of 25-35 min to a
benzene concentration of 128 000 mg/m3 (40 000 ppm) and two out of
ten died after exposure for 12.5-30 min daily to 32 000 mg/m3
(10 000 ppm) for 1-12 days (Furnas & Hine, 1958).
Male and female rats and mice exposed to benzene vapour at
concentrations of 3.2, 32, 96 or 960 mg/m3 (1, 10, 30 or 300 ppm)
for 6 h/day, 5 days/week for 13 weeks, and sacrificed at various time
points during the study, showed no haematological effects up to 96
mg/m3 (30 ppm) (Ward et al., 1985). However, at 960 mg/m3 (300
ppm) mice exhibited significant decreases in haematocrit, haemoglobin,
erythrocyte count, leucocyte count, platelet count and the percentage
of lymphocytes. There was an increase in erythrocyte volume and mean
corpuscular haemoglobin. These changes were first observed on days 14
(males) or 28 (females). Most of the haematological effects were also
detected in rats but were of lesser severity. Compound-related
histopathological findings included myeloid hypoplasia, depletion of
the periarteriolar lymphoid sheaths in the spleen, lymphoid depletion
in the mesenteric lymph nodes, and increased extramedullary
haematopoiesis in the spleen. These lesions persisted throughout the
study and increased in severity with time. The only histopathological
lesion observed in rats was slightly reduced cellularity in the bone
marrow of the femur.
A dose-related increase in leucocyte alkaline phosphatase levels
and a decrease in leucocyte levels was observed in female rats exposed
via inhalation to 320, 960, 3200 or 9600 mg/m3 (100, 300, 1000 or
3000 ppm) for 7 or 14 days, but not in those exposed to 64 or 160
mg/m3 (20 or 50 ppm) (Li et al., 1986). In a study on male mice,
with doses of 3.5, 32, 330, 980, 1930, 4080, 7730 and 15 600 mg/m3
(1.1, 9.9, 103, 306, 603, 1276, 2416 and 4862 ppm), granulocytopenia,
lymphocytopenia and reduced bone marrow and splenic cellularity were
observed after exposure to > 330 mg/m3 for 5 h/day for 5 days but
not at lower levels (Green et al., 1981a). These authors found
splenic lesions at levels as low as 32 mg/m3 when exposure was
extended to 10 weeks.
Table 10. Toxicity of benzene in animals after short-term and long-term inhalation exposuresa
Species Dose (mg/m3) Exposure period Effects References
Rat 48 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
28 weeks at term 90 µg/litre
Rat 100 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
7 weeks at term 290 µg/litre
Rat 150 7 h/day; 5 days/week; slight leucopenia, blood benzene level Deichmann et al. (1963)
32 weeks at term 420 µg/litre
Rat 3200 18 h/day; 7 days/week; reversible haemotological effects, Nau et al. (1966)
15 weeks reversible leucopenia within 6 months
Rat 3.2-960 6 h/day; 5 days/week; slight changes in haematological cell counts Ward et al. (1985)
13 weeks and lower cellularity in bone marrow of femur
Rat (female) 64-9600 1-2 weeks dose-related increase in leucocyte alkaline Li et al. (1986)
phosphatase and decreased leucocyte counts
at exposures of 960 mg/m3 or more
Mouse 3.5 to 15 600 6 h/day; 1 week doses > 330 mg/m3 resulted in granulocytopenia, Green et al. (1981a,b)
lymphocytopenia, decreased splenic cellularity
and decreased stem cell production
Mouse (male) 30.7 6 h/day; 5 days/week; some increase in spleen weight and some Green et al. (1981a)
10 weeks increased cellularity
Table 10 (contd).
Route Species Parameter Value Reference
Mouse (male) 960 6 h/day; 5 days/week; increased mortality, marked lymphocytopenia, Green et al. (1981a)
26 weeks reduced red blood cell count, reduced spleen
weight, many morphological abnormalities in
circulating red blood cells and neutrophils
Mouse (male) 32 6 h/day; 5 days/week; depression in the numbers of splenic nucleated Baarson et al. (1984)
25.5 weeks cells and of circulating red cells and lymphocytes
Mouse 3.2-960 6 h/day; 5 days/week; only at 960 mg/m3 decreased blood cell counts, Ward et al. (1985)
13 weeks haematocrit, myeloid hypoplasia, and numbers of
splenic and lymph node lesions
Mouse 32-1280 6 h/day; 5 days/week; reduced bone marrow cellularity and number of Cronkite et al. (1985)
pluripotent stem cells at 320 mg/m3 or more
Guinea-pig 280 7 h/day; 5 days/week; slight leucopenia, increased weight of kidneys Wolf et al. (1956)
4.5 weeks
Pig 64, 320 6 h/day; 5 days/week; leucopenia at 320 and 1600 mg/m3, reversible Johnston et al. (1979)
and 1600 3 weeks 9-16 weeks after exposure
a To avoid duplication, toxicity from life-time exposures (over 1 year) is discussed in section 7.6
(carcinogenicity) and Tables 15 to 17.
Cronkite et al. (1985) exposed male and female mice by inhalation
to benzene at concentrations of 32, 80, 320, 960 or 1280 mg/m3 (10,
25, 100, 300 or 400 ppm) for 2 weeks (6 h/day, 5 days per week). At
320 mg/m3 or more, reduced bone marrow cellularity and a decreased
number of pluripotent stem cells in bone marrow were reported. Under
similar conditions of exposure to 960 mg/m3 for 16 weeks, these
authors reported a lower level of stem cells in bone marrow, which
returned to 92% of control values after 25 weeks post-exposure.
Complete reversibility within 2 weeks was reported after 2 or 4 weeks
of exposure to 960 mg/m3.
Long-term (> 6 months) exposure studies at benzene levels above
approximately 160 mg/m3 (50 ppm) have shown effects on circulating
leucocytes (especially leucopenia). For example, rats exposed via
inhalation to 150 mg/m3 (47 ppm) (7 h/day, 5 days per week for 8
months) showed slight leucopenia, while those exposed to 100 mg/m3
(31 ppm) for 4 months and those exposed to 48 mg/m3 (15 ppm) failed
to show such changes (Deichmann et al., 1963). The lowest reported
exposure in animals that resulted in haematological effects was in
mice (Baarson et al., 1984). These authors reported that male mice
exposed via inhalation to 32 mg/m3 (10 ppm) (6 h/day, 5 days/week)
for 25.5 weeks showed a decrease in the number of circulating
erythrocytes and lymphocytes, a decrease in the number of nucleated
cells in the spleen and a depression of the in vitro colony-forming
ability of erythroid precursor cells (CFU-E).
Uyeki et al. (1977) demonstrated a depression of stem cell
activity in mice using the spleen colony-forming technique (CFU-S)
after exposure to a benzene concentration of 15 000 mg/m3 (4680 ppm)
for 3 days (8 h/day).
Haematotoxicity was also noted after oral exposure in rats and
mice. The animals were dosed by gavage with benzene in corn oil for
120 days at 25, 50, 100, 200, 400 or 600 mg/kg body weight (5
days/week). Five animals in the control, 200- and 600-mg/kg groups
were sacrificed at 60 days (Huff et al., 1989). A dose-related
leucopenia was observed in both male and female rats and lymphoid
depletion in the B-cells of the spleen was observed in both the 200-
and 600-mg/kg groups at 60 days. In mice no compound-related
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females.
No information was found on the haematotoxicity of benzene after
dermal exposure has been reported.
Additional studies on the effects of long-term exposure to
benzene in experimental animals are described in section 7.6
(carcinogenicity) and in Tables 15 to 17.
7.3 Skin and eye irritation
Benzene is considered a moderate eye irritant (as shown in the
rabbit eye test). Two drops of benzene caused moderate conjunctival
irritation and very slight, transient corneal injury (Wolf et al.,
1956).
Undiluted benzene was irritating to the skin (ear) of rabbits
after 10-20 applications (Wolf et al., 1956). Erythema, oedema,
exfoliation, blistering and moderate necrosis were observed after 20
applications.
There is no information available on the skin-sensitizing
potential of benzene. However, no such effect is expected based on
the experience with other aromatic hydrocarbons (GDCh, 1988).
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
Benzene does not appear to be a potent reproductive toxin in
experimental animals. Guinea-pigs and rabbits exposed to benzene by
inhalation (7 h/day, 5 days/week) for up to 6 months showed variable
results; guinea-pigs showed a slight increase in testicular weight at
280 mg/m3 (88 ppm) and rabbits showed slight degeneration of the
seminiferous germinal epithelium at 256 mg/m3 (80 ppm) (Wolf et al.,
1956). Mice, but not rats, exposed to benzene vapour at a
concentration of 960 mg/m3 (300 ppm) (6 h/day, 5 days/week) for 13
weeks showed bilateral cysts in the ovaries and degeneration and
atrophy of the testes (Ward et al., 1985). At concentrations of 3.2,
32 or 96 mg/m3 (1, 10 or 30 ppm) these changes were also seen in
mice, but they were of doubtful biological significance (Ward et al.,
1985). There was a complete absence of litters in female rats exposed
to 670 mg/m3 (210 ppm) for 10-15 days before mating and for 3 weeks
after mating (Gofmekler, 1968). It is not known if this represents a
problem in mating and fertility, or one of maternal or fetal toxicity.
Lower exposures (1 to 64 mg/m3; 0.30 to 20 ppm) produced no such
effects.
Numerous studies on experimental animals have failed to detect
teratogenic effects, even at doses of benzene clearly toxic to the
dam. A few of these studies are summarized in Table 11. However,
benzene has been reported to be fetotoxic in mice, as shown by a
decrease in fetal weight and skeletal variants (missing sternebrae and
extra ribs) in the offspring of dams exposed to 1600 mg/m3 (500 ppm)
for 7 h on gestation days 6-15 (Murray et al., 1979). Similar effects
were seen in rabbits exposed on gestation days 6-18 to the same
levels. However, these effects are not usually considered to be
significant compound-related malformations (Kimmel & Wilson, 1973).
In mice, exposure to 500 or 1000 mg/m3 on gestation days 6-15
resulted in a decrease in fetal weight and an increase in dead or
resorbed fetuses, but no statistically significant increase in
malformations (Ungvary & Tatrai, 1985).
Table 11. Teratogenic effects of benzene in the mouse and rabbita
Animals Route Exposure level Maternal weight Fetal body Resorptions/ Skeletal Malformations References
gain weight fetal death/ variants
abortions
New Zealand inhalation 1600 mg/m3 (-) (-) (-) slightly ** (-) Murray et al.
rabbit (1979)
New Zealand inhalation 500 mg/m3 (-) (-) (-) (-) (-) Ungvary & Tatrai
rabbit 1000 mg/m3 * * **b ** ** (1985)
CF-1 mouse inhalation 1600 mg/m3 (-) * (-) ** (-) Murray et al.
(1979)
CFLP mouse inhalation 500 mg/m3 (-) * **c,d ** (-) Ungvary & Tatrai
1000 mg/m3 (-) * **c,d ** (-) (1985)
CD-1 mouse gavage 0.3 ml/kg body weight (-) * (-) (-) Nawrot &
0.5 ml/kg body weight (-) * **c,d (-) Staples (1979)
1.0 ml/kg body weight (-) * **c,d (-) (-)
a (-) indicates no significant difference from controls; * indicates decrease compared with controls;
** indicates increase compared with controls.
b Fetal death
c Abortions
d Resorptions
Table 12. Teratology studies in rats after inhalation of benzenea
Exposure level Exposure period Maternal weight Resorptions Fetal weight Skeletal variants Malformations Reference
(mg/m3) (h/day) gain
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
32 7 (-) (-) (-) (-) (-) Kuna & Kapp (1981)
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
128 6 (-) (-) (-) (-) (-) Coate et al. (1984)
150 24 * (-) * (-) (-) Tatrai et al. (1980a)
160 7 * (-) * ** (-) Kuna & Kapp (1981)
320 6 (-) (-) (-) (-) (-) Green et al. (1978)
320 6 (-) (-) * (-) (-) Coate et al. (1984)
400 24 * (-) * ** (-) Tatrai et al. (1980b)
450 24 * ** * ** (-) Tatrai et al. (1980a)
960 6 (-) (-) (-) ** (-) Green et al. (1978)
1000 24 * (-) * ** (-) Hudak & Ungvary (1978)
1500 24 * ** * ** (-) Tatrai et al. (1980a)
1600 7 * (-) * ** (-) Kuna & Kapp (1981)
3000 24 * ** * ** (-) Tatrai et al. (1980a)
7000 6 * (-) * ** (-) Green et al. (1978)
a (-) indicates no significant difference compared with controls; * indicates decrease compared with controls;
** indicates increase compared with controls
Several studies in rats show similar results to those in mice and
rabbits (Table 12). Maternal and fetal weight were decreased at
levels > 160 mg/m3 (> 50 ppm) as were the number of skeletal
variants observed. No malformations were noted in any of the studies
even at doses as high as 7100 mg/m3 (2200 ppm).
It is noteworthy that haematopoietic changes were observed in the
fetuses and offspring of mice exposed to 16, 32 or 64 mg/m3 (5, 10
or 20 ppm) for 6 h/day on gestation days 6-15 (Keller & Snyder, 1986).
The changes included a decrease in the number of erythroid
colony-forming cells (at all dose levels) and granulocytic
colony-forming cells at the two highest levels. When the offspring
were re-exposed to benzene as adults the decrease in these progenitor
cells was greater than in adult mice exposed to benzene at the same
levels for the first time.
7.5 Mutagenicity and related end-points
Benzene has been widely studied regarding the production of gene
mutations in in vitro tests, chromosomal effects both in vitro and
in vivo, and effects on DNA (binding, synthesis and damage). An
overview of the testing up to 1985 for the mutagenicity of benzene is
shown in Fig. 4 (IARC, 1987a). Detailed reviews have also been
published (Dean, 1978, 1985a; Huff et al., 1989; ATSDR, 1991),
therefore only some of the many studies are shown in Tables 13
( in vitro) and 14 ( in vivo).
7.5.1 In vitro studies
As shown in Table 13 and Fig. 4, benzene has consistently given
negative results in assays for point mutations in bacteria using
standard test conditions. In such studies, six tester strains have
been used with benzene concentrations ranging from 0.1 to 528 µg per
plate both with and without metabolic activation (HSE, 1982). Other
studies using doses as high as 880 mg/plate failed to cause mutations
in Salmonella typhimurium (Dean, 1978). In some 10 in vitro gene
mutation tests carried out in various human, mouse and Chinese hamster
cells, as part of an international collaborative study, mixed results
were obtained with benzene (Ashby et al., 1985; Venitt, 1985). When
S. typhimurium (strain TA1535) was incubated with benzene in a
desicator to enhance exposure, a doubling of revertants was noted at
10 ppm only in the presence of a post-mitochondrial activating system
(Glatt et al., 1989).
Figure 4. Tabular summary of in vitro and in vivo tests on benzene for mutagenicity and related end-pointsa
Non-mammalian systems Mammalian systems
In vitro In vivo
Prokaryotes Lower Plants Insects Animal cells Human cells Animals Humans
eukaryotes
D G R G A G R G C D G S M C A T D G S C S M C S C
+1 - + + +1 + +1 ? -1 + + - -1 + + + -1 +1 ? +1 + + + -1 +
a Adapted from: IARC (1987a)
A = aneuploidy; C = chromosomal aberrations; D = DNA damage; G = gene mutation; M = micronuclei; R = mitotic recombination and gene
conversion; S = sister chromatid exchange; T = cell transformation; + = considered to be positive for the specific end-point and
level of biological complexity; +1 = considered to be positive, but only one valid study was available to the Working Group;
- = considered to be negative; -1 = considered to be negative, but only one valid study was available to the Working Group; ? =
considered to be equivocal or inconclusive (e.g., there were contradictory results from different laboratories; there were confounding
exposures; the results were equivocal)
Table 13. Some in vitro genotoxicity studies of benzene
End-point Test system Resultsa References
Gene mutations
Ames test Salmonella typhimurium -/- De Flora et al.
-/+ (1984); Venitt (1985);
Glatt et al. (1989)
Azaguanine Salmonella typhimurium -/ Seixas et al.
resistance (1982)
TK test mouse L5178Y cells -/- Oberly et al. (1984)
TK, ouabain, total of 15 studies using mixedb Garner (1985)
HGPRT loci various human, mouse and
Chinese hamster cells
Chromosome abnormalities
Chromosome human lymphocytes mixed Gerner-Smidt &
aberrations Friedrich (1978);
Morimoto (1976)
total of 8 studies using mixed Dean (1985b)
Chinese hamster or human
cells
Sister Chinese hamster ovary and -/- Dean (1985b)
chromatid V79 cells and rat RL4
exchange cells
Table 13 (contd).
End-point Test system Resultsa References
Sister human lymphocytes mixed Morimoto (1983);
chromatid Morimoto et al.
exchange (1983); Erexson
et al. (1985)
Micronuclei Chinese hamster ovary -/- Douglas et al.
cells (1985)
Other effects
DNA breaks Rat hepatocytes ND/- Bradley (1985)
Chinese hamster V79 cells -/- Swenberg et al.
(1976)
Chinese hamster ovary -/- Douglas et al.
cells (1985)
Mouse L5178Y cells -/ Pellack-Walker &
Blumer (1986)
Unscheduled rat hepatocytes ND/- Probst & Hill
DNA synthesis (1985)
HeLa cells -/- Barrett (1985)
DNA synthesis Hela cells -/- Painter & Howard
inhibition (1982)
a Without/with an exogenous metabolic activation system; ND = no data
b The IPCS CSSTT working group disagreed over data analysis and therefore
called the results inconclusive
Whereas the results of in vitro studies for mutations by
benzene have been largely negative, there is some evidence that
treatment of human and animal cells in vitro with benzene can lead
to chromosomal abnormalities. However, as shown in Table 13, mixed
results have been obtained.
The ring-opened metabolite of benzene, trans,trans-muconal-dehyde
(MUC) has been tested for mutagenic and clastogenic activity in CHO
cells and Salmonella typhimurium bacteria, and for its effects on
DNA synthesis in primary rat liver hepatocytes (Witz et al., 1990a).
Only minimal mutational activity in bacteria was reported (in only
S. typhimurium strain TA97 of the five strains tested). However, at
a concentration of 0.4 to 0.8 µg/ml media, MUC resulted in a
dose-related increase in micronuclei in CHO cells. No effect on
unscheduled DNA synthesis or in the HG PRT assay in CHO cells was
reported. Using S. typhimurium (point mutations) and V79 cells
(sister chromatid exchange, acquisition of thioguanine or ouabain
resistance, and induction of micronuclei), 13 other potential benzene
metabolites were examined for genotoxicity. Each metabolite showed a
specific spectrum of activity, the highest genotoxic activity in most
systems being exhibited by quinone, hydroquinone, anti-diol epoxide
and catechol (Glatt et al., 1989).
The negative mutagenic data found in studies where benzene was
added to standard systems in vitro may well have been caused by the
technique used in these studies. Benzene is metabolically activated
to reactive metabolites by cytochrome P-450 IIE1, a natural
constituent of liver microsomes. In many of these studies
insufficient activation of benzene may have occurred. Post & Snyder
(1983) demonstrated that enzyme induction with benzene increased
benzene metabolism by increasing the activity of an enzyme having a
low Km and a high turnover rate for benzene, which is now thought to
be cytochrome P-450 IIE1. These authors also found that phenobarbital
induction reduced benzene metabolism until very high substrate
concentrations were reached. Chepiga et al. (1991), using purified
reconstituted cytochrome P-450, have shown that, whereas the Km
value for benzene as a substrate for cytochrome P-450 IIE1 is quite
low, much higher concentrations are required for benzene to be
metabolized by cytochrome P-450 IIB1, which also has a low affinity
for benzene. Thus, the lack of positive results in mutagenesis tests
involving benzene may have been due to the low activity of
benzene-activating enzymes in these preparations.
7.5.2 In vivo studies
No data are available on the production by benzene of gene
mutations in vivo. Benzene, or its metabolites, cause both
structural and numerical chromosome aberrations in humans (see chapter
8), laboratory animals and cells in culture (see section 7.6.1), as
well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes. Some in vivo studies are summarized in
Table 14.
Chromosomal changes occur after exposure of experimental animals
by the subcutaneous, oral, intraperitoneal or inhalation routes.
Philip & Krogh-Jensen (1970) administered 1750 mg benzene/kg body
weight subcutaneously to rats and noted an increase in chromatid
aberrations 12 and 24 h post-dosing but not after 36 h. This suggests
damage to S and/or G2 phase cells and a rapid elimination of the
alterations. In a study by Kissling & Speck (1972), the subcutaneous
administration of 1750 mg benzene/kg body weight to rabbits 3 times
weekly for 18 weeks led to tetraploidy in one animal as well as a high
percentage (58%) of bone marrow cells having chromosomal aberrations.
Siou et al. (1981) reported an increase in chromosome aberrations in
the bone marrow cells of mice treated orally with doses greater than
56 mg benzene/kg body weight on 2 successive days before sacrifice.
At high levels of benzene administered by inhalation (10 000
mg/m3 for 4 h), a marked increase in SCEs was noted in mouse bone
marrow cells (Tice et al., 1980). In a later experiment a significant
increase was reported in SCEs in mouse bone marrow cells when the
animals were exposed by inhalation to 91 mg/m3 for 4 h (Tice et al.,
1982). Erexson et al. (1986) reported a significant increase in the
levels of SCEs in peripheral lymphocytes after 6 h of exposure to 32
mg/m3 in mice and 9.6 mg/m3 in rats. At these levels, the
frequency of micronuclei in bone marrow smears was also increased.
After 6 weeks of exposure of mice (22 h/day, 7 days/week) at benzene
levels of between 0.128 and 3.2 mg/m3, increased chromosomal
aberrations in lymphocytes from the spleen were reported by Au et al.
(1991). These changes reached a significance of P = 0.05 only in
female mice (in males P = 0.15).
The frequency of micronuclei was increased in the bone marrow of
mice treated orally at doses ranging from 56 to 2200 mg/kg body weight
(Hite et al., 1980; Siou et al., 1981). A dose-related increase in
micronuclei in circulating erythrocytes was seen at 120 days in mice
treated by oral gavage with 25, 50, 100, 200, 400 or 600 mg benzene/kg
body weight. A significant increase was seen at all doses, with male
mice being more sensitive (Choy et al., 1985).
Table 14. Some mammalian in vivo genotoxicity studies on benzene
Route of Test system Results Exposure concentration Reference
administration and duration
Chromosome aberrations
Inhalation mouse bone marrow + 14 to 74 mg/m3, 7 days Zhurkov et al. (1983)
- 10 000 mg/m3, 4 h Tice et al. (1980)
- 9600 mg/m3, 4 h Tice et al. (1982)
+ 9600 mg/m3, 4 h, phenobarbital pretreatment Tice et al. (1982)
rat bone marrow + 3.2-3200 mg/m3, 6 h Styles & Richardson (1984)
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
+ 5-80 mg/kg body weight, daily for 2 days Zhurkov et al. (1983)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
bone marrow
Intraperitoneal rat bone marrow + 1 dose of 878 mg/kg body weight; 24 h prior to Anderson & Richardson
sacrifice (1981)
Micronuclei
Inhalation mouse lymphocytes + 32, 320 and 3200 mg/m3, 6 h Erexson et al. (1986)
mouse lymphocytes + > 67 mg/m3, 4-10 days Toft et al. (1982)
rat lymphocytes + 0.3 to 96 mg/m3, 6 h Erexson et al. (1986)
Table 14 (contd).
Route of Test system Results Exposure concentration Reference
administration and duration
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
mouse bone marrow + 440 mg/kg body weight, 2 doses, 24 h apart Gad El-Karim et al. (1984)
mouse bone marrow + 55 to 1760 mg/kg, daily for 2 days Hite et al. (1980)
mouse circulating + 26 to 440 mg/kg body weight, 5 days Barale et al. (1985)
erythrocytes + 25 to 600 mg/kg, 120 days Choy et al. (1985)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
Sperm head abnormality
Intraperitoneal mouse (spermatogonia + 88 to 880 mg/kg, daily for 5 days Topham (1980)
treated)
There were statistically significant alterations in sperm head
morphology after intraperitoneal doses of 88 to 880 mg/kg body weight
were administered to mice for 5 days and the sperm were examined 5
weeks later (Topham, 1980).
Witz et al. (1990a) reported that administration of
trans,trans-muconaldehyde, a microsomal metabolite of benzene, to
B6C3F1 mice (< 0.1-6.0 mg/kg body weight intraperitoneally)
resulted in the production of SCEs. The lowest dose producing a
significant increase was 3 mg/kg. No increase in the frequency of
micronuclei was reported.
There has been no clear demonstration of dominant lethal effects
in animals following benzene exposure. Fel'dt (1985) found no
significant dominant lethal effect in mice following oral
administration of up to 320 mg benzene/kg body weight. No dominant
lethal effect was reported in rats by Dean (1978) after the
intraperitoneal injection of 440 mg benzene/kg. However, Ciranni et
al. (1988) demonstrated the induction of micronuclei in the bone
marrow cells of pregnant mice and in fetal liver cells after a single
exposure to benzene or its metabolites.
7.6 Carcinogenicity
In several studies benzene has been shown to be carcinogenic in
experimental animals after exposure by inhalation and after oral
(gavage) dosing. These experiments are summarized in Tables 15-18.
As indicated in these Tables, several types of neoplasms have been
reported to be associated with exposure to benzene. Various types of
lymphomas/leukaemias have been found, but the majority of neoplasms
are of epithelial origin, i.e. Zymbal gland, liver, mammary gland and
the oro-nasal cavity. These results support the hypothesis that
benzene exposure in experimental animals can produce cancer at
multiple sites. A review of such studies has been published by Huff
et al. (1989).
7.6.1 Inhalation studies
The experimental design and major effects noted in several
inhalation cancer bioassays on benzene are summarized in Table 15.
Table 15. Inhalation studies on the carcinogenicity of benzene in experimental animals
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse AKR/J, dosed, 60; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 11 weeks; mean lifetime Snyder
males (8 weeks control, 60 5 days/week, lifetime for control group, 39 weeks, death due to aplastic et al.
old) (about 70 weeks) anaemia; bone marrow hypoplasia; no evidence for any (1978b)
tumours at autopsy in test animals
Mouse C57BL, dosed, 40; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 41 weeks; mean lifetime Snyder
males (8 weeks control, 40 5 days/week, lifetime for control group, 75 weeks; anaemia, lymphocytopenia, et al.
old) (about 70 weeks) neutrophilic bone marrow hyperplasia; 6/40 lymphocytic (1980)
lymphoma with thymic involvement (P < 0.01), 1/40 plasma
cytoma, 1/40 haematocytoblastic leukaemia, 2/40 control
mice lymphocytic lymphoma without thymic involvement
Mouse AKR/J, dosed, 50; 320 mg/m3, 6 h/day, mean life-span for dosed group, 39 weeks; for control, Snyder
males (8 weeks control, 50 5 days/week, lifetime 47 weeks; anaemia, lymphocytopenia, neutrophilia, bone et al.
old) (about 70 weeks) marrow hyperplasia 10/49 dosed, 1/50 control (1980)
Mouse Charles number of 320 and 960 mg/m3, two mice of high-dose group developed myelogenous Snyder
river CD-1, animals 6 h/day, 5 days/week, leukaemia et al.
males unknown lifetime (1978b)
Mouse CD-1, dosed, 60; intermittent exposure greater mortality than with continuous exposure to 3480 Snyder
C57BL/6, male control, 60 at 960 mg/m3 for 1 mg/m3 for 10 weeks; elevated incidences of malignant et al.
for each week followed by 2 tumours in both strains; 35% incidence of Zymbal gland (1988)
strain weeks non-exposure; tumours in C57BL (control 0%), lung adenomas in CD-1
6 h/day, 5 days/week (26% versus 7% controls), and no significant increase
for lifetime in incidence of leukaemia or lymphomas
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse CD-1, dosed, 80; short-term exposure to only CD-1 strain showed increased tumour incidence; 46%
C57BL/6, male control, 80 3840 mg/m3, 6 h/day, incidence of lung adenomas (control 24%) in addition to
for each 5 days/week for 10 other malignant and benign tumours; no increase in
strain weeks; observation for incidence of leukaemia or lymphoma; marked haematotoxicity
lifetime after cessation noted (anaemia and lymphocytopenia)
of exposure
Mouse C57BL/6, dosed, 118; control, 960 mg/m3, 6 h/day, 5 48 weeks after treatment survival rates were: dosed (D) Cronkite
female (7-9 116 (groups reduced days/week for 16 weeks; 80/90; control (C) 87/88; of the 10 exposed mice that et al.
weeks old) to 90 and 88 observation period for died, 6 had thymic lymphomas, 2 had unspecified lymphomas; (1985)
respectively for lifetime at end of study tumour incidence reported: leukaemia all
haemopoietic types (D) 20/89, (C) 8/88; lymphomas (thymic) (D) 10/89,
stem cell assays (C) 1/88; lymphomas (non-thymic) (D) 6/89, (C) 2/88
Rat Sprague- dosed, 45; 960 mg/m3, 6 h/day, no evidence of leukaemic response or pre-leukaemic Snyder
Dawley, male control, 25 5 days/week, for 99 effects nor for tumours incidence at any other site et al.
weeks (1978b)
Rat Sprague- dosed, 40; 320 mg/m3; 6 h/day, incidence of total and malignant tumours not significantly Snyder
Dawley, male control, 40 5 days/week, lifetime elevated over controls; several rare tumours in dosed et al.
(6 weeks old) (about 123 weeks) group, not in controls: 4 liver, 2 Zymbal gland and 1 (1984)
chronic myelogenous leukaemia
Rat Sprague- dosed, 70 male, 640 mg/m3; 4 h/day, at end of experiment (150 weeks) no information on survival; Maltoni
Dawley 59 female; control, 5 days/week for 7 weeks at 118 weeks survival rates of dosed (D) and control animals et al.
158 male, 149 female then 7 h/day for 8 (C) comparable; tumour incidence at 150 weeks; Zymbal (1983,
weeks; exposure in gland (D) 4/70 male, 1/59 female with (C) 2/158 male and 1985,
utero from day 12 of 0/149 female; oral cavity (D) 2/70 male, 6/59 female with 1989)
gestation through (C) 0/158 male and 0/149 female; leukaemias (D) 4/70 male,
lactation 4/58 female with (C) 12/158 male and 1/148 female;
hepatomas (D) 2/70 male, 5/59 female with (C) 1/158 male
and 0/149 female
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- Breeders: dosed 640 mg/m3, 4 h/day, at termination (150 weeks) no information on survival; Maltoni
Dawley (breeders (D), 54; control 5 days/week, 7 weeks; at 118 weeks breeder (D) 5/54, (C) 11/60; offspring (D) et al.
13 weeks old) (C), 60 7 h/day, 5 days/week, male 11/75, (D) female 23/65 with (C) males 39/158 and (1983,
12 weeks; and then females 47/149; (C) breeders had 2/60 with malignant 1985,
960 mg/m3, 7 h/day, mammary tumours and (C) offspring had 3/158 and female 1989)
5 days/week, 85 weeks 8/149 malignant mammary tumours; male 1/158 and 0/149
with leukaemias; tumour incidence in (D) breeders was:
Offspring: dosed, Breeders: from day Zymbal gland carcinoma 3/54; oral cavity carcinoma 2/54;
75 male and 65 12 of gestation nasal carcinoma 1/54; malignant mammary tumours 6/54;
female; control: Offspring: in utero, hepatomas 1/54 and leukaemias 0/54; in offspring (D)
158 male and 149 through lactation male 6/75 and female 8/65 Zymbal gland carcinoma; male
female and for 104 weeks 1/75 and female 10/65 oral cavity carcinoma; male 1/75
and female 2/65 nasal cavity carcinoma; male 1/75 and
female 1/65 skin carcinoma; male 0/75 and female 3/65
forestomach carcinomas; male 0/75 and female 9/65
malignant mammary tumours; male 2/75 and female 7/65
hepatomas; and male 6/75 and female 0/65 leukaemias
Snyder et al. (1980) reported the development of malignant
lymphomas in mice after the exposure of male C57BL mice for about 70
weeks to 960 mg benzene/m3. Goldstein et al. (1982) exposed
Sprague-Dawley (SD) rats and three strains of mice (AKR, C57BL and
CD-1) to 320 and 960 mg/m3 for their lifetime and reported a small,
but not statistically significant, increase in the incidence of
granulocytic leukaemia in CD-1 mice (2 cases) and one case of chronic
myelogenous leukaemia in SD rats (these are rare neoplasms in these
strains). Snyder et al. (1984) in a subsequent full report of this
study, also noted increases in the incidence of liver tumours and
Zymbal gland carcinomas. The incidence of malignant lymphoma in male
AKR mice exposed to 320 mg/m3 was not significantly greater than
that in controls (Snyder et al., 1984). At about the same time
Maltoni et al. (1983) reported that Zymbal gland carcinomas were
observed in SD rats exposed to benzene (960 mg/m3) for 86 weeks. At
the end of the observation period (150 weeks), female breeder rats and
their offspring had not developed increased levels of leukaemia but
had an increased incidence of other tumours such as oral and nasal
cavity carcinomas, malignant mammary carcinomas and hepatomas (Maltoni
et al., 1982c, 1983, 1989).
Benzene-induced leukaemia in experimental animals has been
reported (Cronkite et al., 1984, 1985, 1989; Cronkite, 1986). In an
attempt to mimic more closely patterns of human exposure to benzene,
C57BL/6 and CBA/Ca mice were exposed to 960 mg/m3 (300 ppm) (6
h/day, 5 days/week) for 16 weeks, followed by an observation period of
82 weeks. A highly significant increase in leukaemia was noted in
C57BL/6 mice (Cronkite et al., 1984), and a biphasic response was
reported regarding mortality and lymphoma appearance (Cronkite et al.,
1985). The first increase in lymphomas was noted at about 150 days
post-exposure, and there was increased mortality between 330 and 390
days. A second increase in lymphomas as well as solid tumours
occurred at 420 days post-exposure, the mortality again increasing at
570 days post-exposure. Cronkite et al. (1989) reported that benzene
at a concentration of 960 mg/m3 for 76 weeks was leukaemogenic in
both male and female CBA/Ca mice.
Snyder et al. (1988) reported that benzene exposure patterns in
CD-1 and C57BL mice, which were closely related to the occupational
setting (intermittent for lifetime as well as short-term high doses
for a portion of the normal life span), resulted in marked
haematotoxicity as well as being tumorigenic. Neither of the benzene
exposure patterns induced elevated incidences of leukaemia/lymphoma in
either strain. Elevated incidences of malignant tumours (Zymbal gland
and lung) were noted in both strains after intermittent (1 week
exposure, 2 weeks non-exposure) exposure (960 mg/m3, 6 h/day, 5
days/week) over the full lifetime, whereas an increase in lung tumour
incidence was noted in only the CD-1 strain after 10 weeks of exposure
to 3840 mg/m3 (6 h/day, 5 day/week) followed by a lifetime of
non-exposure.
Table 16 presents a summary of the lowest dose levels at which
various authors reported a possible causal relationship between
benzene exposure and the end-point studied.
7.6.2 Oral and subcutaneous studies
Several experiments using oral (gavage) administration of benzene
to experimental animals are summarized in Table 17, and some of the
major effects reported are given. Oral exposure to benzene has
resulted in the induction of neoplasms in 13 different tissue/organs,
namely Zymbal gland, oral and nasal cavities, mammary gland, liver,
forestomach, skin, harderian gland, preputial gland and ovary, and the
haemopoietic and lympho-reticular systems (Maltoni et al., 1983, 1985,
1989; NTP, 1986; Huff et al., 1989). Table 17 indicates the
similarity in protocols used, namely 25-500 mg benzene/kg body weight
per day via gavage, 4 to 5 times weekly for 52-104 weeks and
termination after 103-144 weeks. The lowest dose of benzene that
produced specific neoplasms varied from 25 mg/kg body weight for the
adenomas of the lung, harderian gland and liver of mice to 500 mg/kg
body weight for lymphoreticular neoplasms in rats.
7.7 Special studies
7.7.1 Immunotoxicity
The proliferative ability of B- and T-cell lymphocytes was
depressed in a short-term (6 h/day for 6 days) dose-response study
(32, 96, 320 and 960 mg/m3) on benzene in mice (Rozen et al., 1984).
Liposaccharide-induced B-cell proliferation was depressed at levels as
low as 32 mg/m3 (the range of many occupational exposures), and
phytohaemagglutinin-induced T-cell response was depressed at 96
mg/m3. Peripheral lymphocyte counts were lower at all exposure
levels, but erythrocyte counts were depressed only at 320 and 960
mg/m3. In a subsequent study it was shown that a benzene
concentration of 960 mg/m3 (6 h/day, 5 days/week), administered for
115 days to mice, reduced the number of both B-cells in the spleen and
bone marrow and T-cells in the thymus and spleen and reduced their
response to mitogens (Rozen & Snyder, 1985). Other studies have shown
that polyhydroxylated derivatives of benzene are potent inhibitors of
T- and B-cell function in vitro (Irons et al., 1982).
Table 16. Carcinogenic-related end-points observed in animals exposed to benzene by inhalationa
End-points SD rat C57BL/6J mouse CD-1 mouse CBA/Ca mouse Reference
Lymphocytic lymphoma 960/488 days Snyder et al. (1980)
Myelogenous leukaemia (acute) 960/life Goldstein et al. (1982)
Myelogenous leukaemia (chronic) 320/life 960/life Goldstein et al. (1982)
Zymbal gland carcinoma 320/life Snyder et al. (1984)
960/lifeb 960/lifeb Snyder et al. (1988)
Hepatoma 640-960 Maltoni et al. (1982a)
960/16 Cronkite (1986)
Lung adenoma 3840/lifec Snyder et al. (1988)
Nasal carcinoma 640-960/104 Maltoni et al. (1983)
Thymic lymphoma 960/16 Cronkite et al. (1984)
Lymphoma (unspecified) 960/16 Cronkite et al. (1984)
Liver tumour 320/life Snyder et al. (1984)
Granulocytic leukaemia 320/life Snyder et al. (1984)
Leukaemia 960/16 Cronkite (1986)
a Doses are expressed in mg/m3 given 4-7 h/day, 5 days/week over a number of weeks or lifetime (e.g., 960 mg/m3/16
indicates that 960 mg/m3 was given for 16 weeks); exposures shown are the lowest for which the author claims
possible causal relationship.
b 960 mg/m3 dose intermittent, i.e. 1 week followed by 2 weeks non-exposure.
c Exposed to 3840 mg/m3 for 10 weeks followed by lifetime non-exposure.
Table 17. Long-term toxicity/carcinogenicity of benzene in experimental animals after oral administrationa
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- high dose: 30 m, 50 or 250 mg/kg, only tumours reported in (C) were 4/30 f with malignant Maltoni et
Dawley, males & 35 f; low dose: 4-5 times/week mammary tumours and 1/30 with leukaemia; in dosed animals al. (1982b,
females (13 30 m, 30 f; for 52 weeks; Zymbal gland carcinoma in 2/30 f in low-dose group and 1983, 1985,
weeks old) controls: 30 m, death at 144 8/35 f in high-dose group; oral cavity carcinomas in 2/35 f 1989)
30 f weeks in high-dose group; malignant mammary tumours in 4/30 f in
low-dose group and 7/35 f in high-dose group; hepatomas in
1/35 m in high-dose group and leukaemias in 2/30 f of
low-dose group and 4/35 m and 1/35 f in high-dose group
Rat Sprague- dose: 40 m, 40 f; 500 mg/kg, 4-5 only tumours reported in (C) were 1/50 m with Zymbal gland Maltoni et
Dawley, males & control: 50 m, 50 f days/week for carcinomas; 1/50 skin carcinomas; 7/50 f malignant mammary al. (1982b,
females (6 weeks 104 weeks; tumours; 3/50 m hepatomas; 3/50 m and 1/50 f with 1983, 1985,
old) observed until leukaemias; in (D) following tumours reported: Zymbal
natural death gland carcinomas 18/40 m and 16/40 f; oral cavity
carcinomas 21/40 m and 20/40 f; nasal cavity carcinomas
3/40 m and 1/40 f; skin carcinomas 9/40 m; forestomach
acanthomas and dysplasias 10/40 m and 7/40 f; in situ
carcinomas (forestomach) 6/40 f; invasive carcinomas
(forestomach) 1/40 m; hepatomas 3/40 m and 1/40 f;
angiosarcomas 2/40 m and 3/40 f; leukaemias 1/40 m and
3/40 f
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat F344/N, dose groups: 60 m, all groups dosed number of survivors in control and dosed groups, NTP (1986);
males & females 60 f; control: 5 days/week for respectively; males: 32/50, 29/50, 24/50, 16/50; females: Huff et al.
(7-8 weeks old) 60 m, 60 f 103 weeks; males: 46/50, 38/50, 33/50, 25/50 (1989)
50, 100 or 200
mg/kg per day in control and dosed groups, respectively: Zymbal gland
females: 25, 50 or carcinomas, males: 2/50, 6/50, 10/50, 17/50; females:
100 mg/kg per day 0/50, 5/50, 5/50, 14/50
oral cavity squamous cell papilloma: m 1/50, 60/50, 11/50
and 13/50; f 1/50, 4/50, 8/50, 5/50
oral cavity squamous cell carcinoma: m 0/50, 3/50, 5/50,
70/50; f 0/50, 1/50, 4/50, 5/50
skin squamous cell papilloma: m 0/50, 2/50, 1/50, 5/50
skin squamous cell carcinoma: m 0/50, 5/50, 3/50, 8/50
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Mouse B6C3F1, dose: 60 m, 60 f; males and females: numbers of survivors in control and dosed groups, NTP (1986)
male & females control: 60 m, 60 f 25, 50 or 100 mg/kg respectively: males: 28/50, 22/50, 18/50, 7/50; females:
(6-8 weeks old) per day, 5 days/week 30/50, 25/50, 24/50, 16/50
for 103 weeks
control and dosed groups, respectively: Zymbal gland
carcinomas, males: 0/49, 1/48, 4/50, 21/49; females: 0/49,
0/45, 1/50, 3/49
mammary carcinomas and carcinosarcomas occurred at higher
incidences in the mid- and/or high-dose groups; increased
tumour incidences in dosed mice were also noted elsewhere
including the haemopoietic system, adrenals, ovary, liver,
lung and preputial gland
a m = male animals, f = female animals, (C) = control groups, and (D) = dosed groups
The primary antibody response to fluid tetanus toxoid was reduced
by 74-89% in mice that were exposed to 1280 mg benzene/m3 (6 h/day)
for 5, 7 or 22 days of exposure (Stoner et al., 1981). No effect was
seen at 160 mg/m3. These investigators concluded that the threshold
level for repression of primary antibody response was between 160 and
640 mg/m3.
Host resistance to infection by Listeria monocytogenes in mice
was reduced after exposure to benzene (Rosenthal & Snyder, 1985). The
infection rate, as determined by bacterial counts in the spleen, was
increased by 730% on day 4 post-infection after exposure to 960
mg/m3 for 5 days, but not at lower benzene exposures. In contrast,
increased bacterial counts were seen at all doses of benzene greater
than 32 mg/m3 when benzene exposure was continued after exposure to
L. monocytogenes.
Both the humoral and cellular immune responses in CD-1 mice were
altered by oral administration of benzene at 8, 40 or 180 mg/kg body
weight daily for 4 weeks. A dose-response reduction in peripheral
blood lymphocytes was reported whereas there was no effect on the
levels of neutrophils and other white blood cells (Hsieh et al.,
1988a). A dose-related biphasic splenic lymphocyte proliferative
response to B- and T-cell mitogens was also reported. At the 8 mg/kg
dose the response was enhanced, while at the 40 and 180 mg/kg per day
doses a depression was observed. A similar biphasic response was
reported for cell-mediated immunity.
7.7.2 Neurotoxicity
The neurotoxicity of benzene in experimental animals has not been
well studied. Benzene caused light narcosis after 3 min of exposure
to a level of about 144 000 mg/m3 (45 000 ppm) in rabbits (Carpenter
et al., 1944). At this dose, tremors were noted after 5 min, loss of
pupillary reflex to strong light after 6 min, involuntary blinking
after 15 min, and death after 36 min. Learning defects have been
reported in rats exposed three times intraperitoneally to 550 mg
benzene/kg body weight on days 9, 11 and 13 postpartum (Geist et al.,
1983).
Male adult CD-1 mice received ( ad libitum for 4 weeks)
drinking-water containing 31, 166 and 790 mg benzene/litre (estimated
daily doses of 8, 40 and 180 mg/kg body weight). No treatment-related
behavioural changes were observed in the test animals. However, oral
ingestion of benzene was found to alter the levels of norepinephrine,
serotonin, dopamine and catecholamine in several brain regions (Hsieh
et al., 1988b).
7.8 Factors modifying toxicity
The toxicity of benzene can be modified by several factors,
including species or strain of animal exposed, dose received, and
patterns of exposure.
As shown in Figures 1-3 (section 6.3) benzene metabolism is
complex and involves several detoxification pathways, as well as two
pathways which form the putative metabolites muconaldehyde and
benzoquinone. Henderson et al. (1989) and Sabourin et al. (1987)
demonstrated species differences for the metabolism of benzene, i.e.
mice exhibited a higher rate of metabolism with the production of more
putative toxic metabolites. These authors also reported that
increasing the dose (by oral or inhalation routes) in both rats and
mice resulted in a higher proportion of benzene being metabolized by
detoxification pathways.
The myelotoxicity of benzene in mice is much more pronounced
following a discontinuous dosing regimen than following continuous
exposure (Tice et al., 1989). These effects of exposure route and
regimen suggest that the toxicity of benzene is dependent on cell
cycle kinetics in the bone marrow.
Evidence indicates that benzene must be metabolized prior to
producing adverse effects on the haemopoietic system or leading to
carcinogenic and clastogenic effects (Snyder et al., 1981; Irons,
1985). Therefore, agents or other factors that alter the metabolism
of benzene can also modify its toxicity.
Ethanol and benzene induce formation of cytochrome P-450 IIE1 in
rabbit and rats (Johansson & Ingelman-Sundberg, 1988). The toxicity
of benzene is enhanced by ethanol, increasing the severity of anaemia
induced by benzene, lymphocytopenia and reduction in bone marrow
cellularity (Baarson et al., 1982).
Phenobarbital that induces specific isoenzymes of P-450 increases
the rate of benzene metabolism in vivo in rats resulting in
increased resistance against the leucopenic action of benzene (Ikeda
& Ohtsuji, 1971; Nakajima et al., 1985).
Sabourin et al. (1990) found no evidence of the induction of
benzene metabolism by repeated exposure of rodents to benzene.
7.9 Mechanism of toxicity
It has become increasingly clear that the impact of benzene on
the bone marrow is conferred by a combination of metabolites, rather
than by a single metabolite. Thus, Eastmond et al. (1987) showed that
there was an interaction between phenol and hydroquinone when the two
were co-administered, which resulted in a degree of myelotoxicity
greater than additive. In mice, Snyder et al. (1989) reported that
whereas phenol alone did not decrease erythrocyte production,
hydroquinone, p-benzoquinone, and muconaldehyde were effective
inhibitors of red cell production. These authors also showed that
phenol interacted with hydroquinone to produce greater-than-expected
depression of erythrocyte synthesis. A similar interaction was
observed between phenol and catechol. The most striking interaction
was observed when doses of hydroquinone and muconaldehyde were
selected which were ineffective in inhibiting erythropoiesis when
given alone, but when given together produced cessation of red cell
production. Thus, bone marrow depression appears to be the result of
the combined effects of these metabolites. A further contributing
factor, however, is the finding by Roghani et al. (1987) and Da Silva
et al. (1989) that benzene stimulates the activity of membrane protein
kinase c, an important regulatory enzyme. The effect of its
perturbation may combine with the biological effects of the various
metabolites to yield the disease we term aplastic anaemia.
It is clear that aplastic anaemia requires that benzene be
metabolized to toxic metabolites. It also appears that metabolism is
required for the production of clastogenic responses. While it seems
likely that metabolism is important for the induction of tumours,
there is very little data on this point. One report, however,
suggests a role for benzene metabolites in one type of carcinogenic
response. Busby et al. (1990) explored the ability of several known
benzene metabolites, as well as postulated benzene metabolites, to
induce lung tumours in newborn mice. They examined the effectiveness
of benzene oxide and enantiomers and racemates of benzene dihydrodiols
and diol epoxides given orally using a prescribed regimen. Lung
tumour incidence and multiplicity were increased after treatment with
benzene oxide, racemates of dihydrodiol and by diol epoxide-2.
Benzene and diol epoxide-1 were inactive in this system.
Although it is well known that benzene produces bone marrow
damage resulting from the production of benzene metabolites in liver,
it is also well known that these metabolites do not produce
hepatotoxicity. From the mechanistic point of view, it appears that
the liver is protected against damage from quinone metabolites of
benzene by the enzymes DT-diaphorase (Smart & Zannoni, 1985) and
carbonyl reductase (Wermuth et al., 1986). These enzymes prevent the
metabolic activation of phenolic metabolites to their otherwise toxic
quinones. The bone marrow is relatively deficient in these enzymes,
but partial protection of the marrow has been afforded through the
administration of high doses of ascorbic acid (Smart & Zannoni, 1985).
Metabolic activation of benzene metabolites, once they reach the
bone marrow, may lead to eventual toxicity. For example, in bone
marrow stromal macrophages, phenol (but not benzene) can be
metabolized and in the process inhibit RNA synthesis in macrophages,
thus possibly inhibiting the production of the haemopoietic factor
(Post et al., 1985). It has also been suggested (Kalf et al., 1989)
that the cyclooxygenase component of prostaglandin synthetase plays a
significant role in the metabolism of benzene and/or its metabolites
in bone marrow. Administration of indomethacin or other
cyclooxygenase inhibitors protected against benzene-induced bone
marrow depression and micronucleus formation.
A general mechanism for benzene-induced bone marrow depression
might be that benzene metabolites arising in the liver travel to the
bone marrow where further metabolic activation occurs. The newly
generated metabolites, perhaps acting in concert with unmetabolized
benzene in cell membranes, act upon target cells such as stem cells,
progenitor cells and stromal cells in the marrow to produce bone
marrow depression. Chromosomal damage may ensue, which is reflected
in clastogenesis observed in circulating lymphocytes or bone marrow
cells. The point in this series of events that leads to a
leukaemogenic response requires further examination once an adequate
model for the disease in animals has been established.
8. EFFECTS ON HUMANS
Acute inhalation and oral exposures of humans to high
concentrations of benzene have resulted in central nervous system
depression and death. The most noted effects resulting from
longer-term exposure to lower levels of benzene are haematotoxicity,
immunotoxicity and neoplasia.
8.1 General population and occupational exposure
The human health effects after exposure to benzene are
qualitatively the same for the general population and those exposed in
the workplace. To avoid duplication, the effects on both groups
(general population and workers) will be discussed together, with
emphasis on exposure levels and duration of exposure. The
quantitative response will be determined from such levels of total
daily intake.
8.1.1 Acute toxicity
Exposures in the general population that result in acute toxic
effects are usually related to accidents and misuse or abuse of
benzene. Many deaths and serious health effects have resulted from
benzene exposures after deliberate the "sniffing" of glue and other
products which contain benzene as a solvent (Winek & Collom, 1971).
Blood levels in people who have died as a result of "sniffing" glue
have ranged from 0.94 to 65 mg/litre (Winek et al., 1967; Winek &
Collom, 1971). Autopsy observations in these individuals included
pulmonary haemorrhage and inflammation, renal congestion and cerebral
oedema.
It has been estimated that exposure to benzene concentrations of
about 64 000 mg/m3 (20 000 ppm) for 5-10 min can result in
fatalities, 24 000 mg/m3 (7500 ppm) for 30 min is dangerous to life,
4800 mg/m3 (1500 ppm) for 60 min causes serious symptoms, 1600
mg/m3 (500 ppm) for 60 min leads to symptoms of illness, and 160-480
mg/m3 (50-150 ppm) for 5 h causes headache, lassitude, and weakness,
while 80 mg/m3 (25 ppm) for 8 h is without clinical effect (Gerarde,
1960). The clinical signs of acute toxicity from benzene include CNS
depression, cardiac arrhythmia, and eventually asphyxiation and
respiratory failure if exposures are at the lethal level (Andrews &
Snyder, 1986). Mild CNS symptoms are rapidly reversible following
cessation of exposure and there is no evidence that they result in
neurological brain damage (Marcus, 1990).
The single acute oral lethal dose in humans has been estimated to
be 10 ml of benzene (8.8 g) (Thienes & Haley, 1972). Clinical signs
of toxicity after acute oral exposure include staggering gait,
vomiting, shallow and rapid pulse, somnolence, loss of consciousness,
delirium, pneumonitis, profound CNS depression, and collapse
(Sandmeyer, 1981). High but sublethal oral doses may produce one or
more of the following symptoms: dizziness, visual disturbances,
euphoria, excitation, pallor, flushing, breathlessness and
constriction of the chest, headache, fatigue, sleepiness, and fear of
impending death (Sandmeyer, 1981). In addition to the autopsy
findings noted above, ingestion of benzene has been reported to cause
gastrointestinal ulceration (Appuhn & Goldeck, 1957).
No studies on the acute toxicity of benzene after dermal exposure
are available.
8.1.2 Effects of short- and long-term exposures
The most significant health effects from short- or long-term
exposure to benzene are haematotoxicity, immunotoxicity, neurotoxicity
and carcinogenicity. Three types of bone marrow effects have been
reported in response to benzene exposure; these are bone marrow
depression leading to aplastic anaemia, chromosomal changes and
carcinogenicity.
8.1.2.1 Bone marrow depression; aplastic anaemia
Several types of blood dyscrasias, including pancytopenia,
aplastic anaemia, thrombocytopenia, granulocytopenia, lymphocytopenia
and leukaemia, have been noted after exposure to benzene. These
changes are a continuum and not a discrete disease entity. Which
effect is noted will depend on the dose, length of exposure and the
stage of stem cell development affected (Galton, 1986). As in
experimental animals, the primary target organ of benzene that results
in haematological changes is the bone marrow. It has been suggested
that the cells at highest risk are the rapidly proliferating stem
cells (Marcus, 1990).
A study of 32 patients that were chronically exposed by
inhalation to benzene levels of 480-2100 mg/m3 (150-650 ppm) for 4
months to 15 years revealed pancytopenia with hypoplastic,
hyperplastic or normoblastic bone marrow. Eight of the 32 individuals
showed thrombocytopenia which resulted in haemorrhage and infection
(Aksoy et al., 1972). Haematotoxicity after prolonged exposure has
also been reported in rotogravure workers exposed for 6-60 months at
concentrations of 36-3485 mg/m3 (11-1069 ppm) (Goldwater, 1941) and
77-3400 mg/m3 (24-1060 ppm) (Erf & Rhoads, 1939), shoe factory
workers exposed to 96-670 mg/m3 (30-210 ppm) for 3 months to 17
years (Aksoy et al., 1971), and rubber factory workers exposed to up
to 1600 mg/m3 (500 ppm) (Wilson, 1942). Kipen et al. (1988)
reported on rubber workers who were exposed to benzene during the
1940s. An inverse relationship was found between the mean yearly
white blood cell count and the year that the count was made,
suggesting that exposures to benzene were very high in the early
1940s. As estimated by Crump & Allen (1984), benzene exposures
decreased from 438 mg/m3 (137 ppm) in 1940 to 102 mg/m3 (32 ppm)
in 1948. In a follow-up letter, Hornung et al. (1989) pointed out
that a similar rise in white blood cells counts was seen in
pre-employment physical examinations occurring over the same time
period at the same facility, and that that the trend could not be
attributed soley to benzene exposure. In a study of 1008 male
shoemakers in Florence, excess mortality from aplastic anaemia was
observed (SMR = 1566, 95% CI 547-3264), based on 6 deaths. All cases
of aplastic anaemia occurred among workers first employed before 1964
when the level of exposure to benzene was assumed to be highest (Paci
et al., 1989).
At levels less than 32 mg/m3 (10 ppm) no haematologic effects
have been observed (Collins et al., 1991). These authors found no
haematological effects in 200 benzene-exposed workers (10 year TWA of
0.03-4.5 mg/m3, 0.01-1.4 ppm) or in 268 control workers in the same
plant. In an earlier study of 70 workers in a coke oven by-product
recovery facility, Hancock et al. (1984) measured the levels of red
blood cells, white blood cells and haemoglobin in three groups exposed
to different concentrations of benzene (average, 34 mg/m3, 10.5 ppm;
range, 3.2-534 mg/m3, 1-167 ppm) and one non-exposed control group.
No significant differences between groups were noted in these
haematological parameters.
No data are available regarding haematotoxicity after short-term
or chronic oral or dermal exposure.
8.1.2.2 Immunological effects
As noted in animal studies (section 7.7.1), the immunological
manifestations of benzene toxicity are related to effects on the bone
marrow, resulting in changes to both humoral and cellular acquired
immunity. Workers (76) exposed to benzene (10-22 mg/m3, 3-7 ppm),
as well as to toluene and xylenes, for periods of 1-21 years were
examined for the presence of leucocyte agglutinins and levels of
circulating immunoglobulins. In 10 out of 35 workers where blood was
taken during working hours, the adverse effect of agglutinins reacting
with autoleucocytes was noted (Lange et al., 1973a). In addition, it
was found that the sera from the 35 workers had increased levels of
IgM and decreased levels of IgG and IgA immunoglobulins (Lange et al.,
1973b). The simultaneous exposure of these workers to solvents other
than benzene makes it difficult to interpret these results.
Autoimmunity, as shown by the pressure of antibodies against
leucocytes, platelets, and erythrocytes in the sera of exposed
workers, has been reported (Renova, 1962). Workers have been reported
to have an increased susceptibility to allergies (Aksoy et al., 1971)
when exposed to benzene concentrations as low as 96 mg/m3 (30 ppm).
A loss of leucocytes was observed in several studies of workers
reported to be exposed to benzene levels of 96-2080 mg/m3 (30-650
ppm) (Aksoy et al., 1971, 1974a; Aksoy, 1987). Signs of preleukaemia,
including loss of leucocytes and other blood elements and enlarged
spleens, were reported in one study (Aksoy et al., 1974a). Kipen et
al. (1989) and Yin et al. (1987) also reported decrease in circulating
lymphocytes and other blood elements at benzene exposures ranging from
48 to 240 mg/m3 (15-75 ppm). The number of T-cell lymphocytes were
found to have been reduced in workers exposed chronically to benzene,
toluene and xylene (Moszczynski, 1981).
In a study of workers exposed to low average concentrations of
benzene (< 32 mg/m3), there was no difference in cell cycle
kinetics of phytohaemagglutinin-stimulated lymphocytes in 66 male
workers of a refinery population when compared with 33 control workers
in the same refinery (Yardley-Jones et al., 1988).
No studies are available regarding the immunotoxicity of benzene
in humans after oral or dermal exposures.
8.1.2.3 Chromosomal effects
Both structural and numerical chromosomal aberrations have been
observed fairly consistently in the lymphocytes and bone marrow cells
of individuals occupationally exposed to benzene. It is now generally
accepted that benzene is a human clastogen (IARC, 1987a; Huff et al.,
1989). Increases in the number of both unstable and stable
chromosomal aberrations were observed in men, even 2 years after
cessation of workplace exposure (Tough & Court Brown, 1965). Up to
70% aneuploid lymphocytes were found in five women with benzene
haemopathy (Pollini et al., 1969), the effects still being
demonstrable 5 years post-exposure. Similar effects were observed in
the lymphocytes of workers in a rotogravure plant that had been
exposed to very high levels of benzene 400-1700 mg/m3 (125-532 ppm)
for 1-22 years (Forni et al., 1971a,b).
Recent studies by Yardley-Jones et al. (1988, 1990) revealed much
lower responses in the lymphocytes of workers exposed to low
concentrations of benzene (average < 32 mg/m3). In a study of 66
refinery workers and 33 controls, no alteration in cell cycle kinetics
was noted nor was there any increase in the level of SCEs
(Yardley-Jones et al., 1988). The lymphocytes from 48 of the workers
and 29 of the controls were analysed for chromosomal aberrations.
According to Yardley-Jones et al. (1990), the increase in aberrations
(particularly chromatid deletions and gaps) was of borderline
significance in parametric statistical tests, but was significant
using Fisher's exact test. No lifestyle factors had any consistent
effect on the incidence of chromosomal aberrations.
In an attempt to determine whether benzene and its metabolites
damage certain human chromosomes preferentially, Sasiadek et al.
(1989) examined the karyotypes of 33 workers exposed to less than 99
mg/m3 (31 ppm). At these levels no clinical or haematological
symptoms were noted in 31 workers, but pancytopenia was observed in
two workers. Nonrandom breaks and gaps were observed in the exposed
group; chromosomes two, four and nine were more prone to breaks and
chromosomes one and two more prone to gaps. The results of this study
are of limited value in view of the small number of controls and the
fact that all participants smoked.
Other studies that corroborate the clastogenicity of benzene in
humans have been reviewed by IARC (1982), Dean (1985a) and Kalf
(1987).
8.1.2.4 Carcinogenic effects
The fact that benzene is a human leukaemogen has been well
established by epidemiological and case studies (IARC 1982, 1987b),
most of which have dealt with industrial exposures. The
epidemiological studies reported have been selected because they
contain sufficient quantitative data on exposure and effects to permit
a discussion of the dose-response relationship. Some case reports are
summarized in Table 18, and prospective epidemiological studies are
summarized in Table 19. Of the two major classes of leukaemia
(granulocytic and lymphocytic), the most consistent evidence for a
causal relationship in humans has been found between benzene exposure
and myeloid leukaemia (Goldstein, 1988).
One case study followed the course of 44 patients with
benzene-induced pancytopenia and found that 6 of them later developed
leukaemia (Aksoy & Erdem, 1978).
The first bridge between case reports and a formal
epidemiological investigation was conducted in the early 1970s (Aksoy
et al., 1974b). These investigators reported on a series of cases
from an estimated population of 28 500 Turkish shoe workers exposed
since the 1950s to solvents and adhesives containing high levels of
benzene. Aplastic anaemia was first observed in 1961, and 26 patients
with acute leukaemia were observed by 1967. Peak exposure levels of
benzene were reported to be 96-670 mg/m3 (30-210 ppm), with rare
excursions to 2100 mg/m3 (650 ppm), for periods of 1 to 14 years
(mean 9.7 years). From this group of workers, a leukaemia incidence
rate (number of cases per 100 000 per year) of 13 was estimated
compared with a rate of 6 for the general population (Aksoy et al.,
1974b). It was unclear to the Task Group, from the description of the
authors, which methods were used to ascertain cases and from which
exposed population these cases were derived.
A study showing an excess risk of leukaemia in a cohort of 748
male workers producing "rubber hydrochloride" in three plants in two
locations within the USA during 1940-1949 (a product made from natural
rubber suspended in benzene) was first reported by Infante et al.
(1977). A follow-up study of this cohort was reported by Rinsky et
al. (1981) and this was subsequently updated (Rinsky et al., 1987).
In this most recent follow-up, the cohort definition was expanded to
include workers employed between 1940 and 1965 who had a cumulative
exposure to benzene of 3.2 mg/m3 per day (1 ppm/day) or more. The
Table 18. Case studies of workers occupationally exposed to benzene
Group studied Exposure Condition observed Author's References
conclusiona
44 pancytopenic patients exposed to benzene 480-2100 mg/m3 (150-650 leukaemia PC Aksoy & Erdem
in adhesives ppm); 4 months to 15 myeloid metaplasia * (1978)
years
42 leukaemia patients and 21 patients with not given leukaemia DC Aksoy (1980)
other malignancies; 47 were shoe workers, the multiple myeloma PC
remainder in other occupations using benzene myeloblastic leukaemia *
solvents acute erythroleukaemia *
preleukaemia *
malignant lymphoma PC
paroxymal nocturnal
haematuria *
lung cancer (all heavy
smokers) *
Table 18 (contd).
Group studied Exposure Condition observed Author's References
conclusiona
6 of 94 Hodgkin's patients who had been 480670 mg/m3 (150-210 Hodgekin's disease PC Aksoy et al.
exposed to benzene adhesives ppm); 1-28 years (1974a)
A 35-year-old man who had used benzene 200-1640 mg/m3; 18 months subacute granulocytic DC Sellyei & Kelemen
8 years earlier as a paint solvent leukaemia (1971)
6 leukaemia patients in different occupations levels unknown; 1-20 years haemocytoblastic leukaemia DC Vigliani & Saita
all using benzene solvents (1964)
A 51-year-old chemical worker exposed to 3.2 mg/m3 (< 2 ppm); acute myelogenous PC Ott et 81. (1978)
benzene 15 years earlier 18 months leukemia
a DC = direct correlation; PC = possible correlation; * = no conclusion made
Table 19. Epidemiological studies of workers exposed to benzene
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Incidence of leukaemia in 96-670 mg/m3 aplastic anaemia, 26 200 NR Aksoy et al.
Turkish shoe workers 1950-1965 rarely 2100 mg/m3; acute leukaemia (1974b)
(28 500 shoe, slipper and 1-15 years (mean,
handbag workers) 9.7 years)
Mortality study of rubber within legal limits of malignomas of lymphatic 14 260 NR Infante et
workers exposed to benzene period, i.e. 320 mg/m3 and haemopoietic P < 0.05 al. (1977)
between 1940 and 1949 (100 ppm) down to systems; myeloid and
32 mg/m3 (10 ppm) monocytic leukaemia 7 506 NR
for up to 10 years P < 0,02
Mortality studyb of pliofilm from < 40 ppm-years lymphatic and haemopoietic 15 227 127-376 Rinskyetal.
workers exposed to benzene to > 400 ppm-years neoplasms (1987)
between 1940 and 1965 with
a period at risk from 1950 leukaemia, total 9 337 159-641
to 1981 < 40 ppm-years 2 109 12-394
40-200 ppm-years 2 322 36-1165
205-400 ppm-years 2 1186 133-4289
> 400 ppm-years 3 6637 1334-19 393
multiple myeloma total 4 398 110-1047
< 40 ppm-years 3 458 92-1339
> 40 ppm-years 1 5347 70-29 753
Mortality studyc of 956 > 0.3-114 mg/m3 leukaemia (total) 4 194 52-488 Bond et al.
workers employed at a chemical (> 0.1-35.5 ppm) acute myelogenous 4 444 P < 0.05 (198613)
company between 1940 and estimated TWA for leukaemia
1982 up to 34 years
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective mortality study < 3.2 mg/m3 leukaemia 0 0 Tsai et al.
of workers (454) employed at (< 1 ppm) in 84% (1983)
a Texas refinery between 1952 samples (median,
and 1981 1.6 mg/m3; 0.5 ppm)
in benzene-related
areas
Mortality study of chemical < 15, 15-60 and > 60 lymphatic and haemopoletic, Wong
workers in 7 plants, > 6 ppm-years total: (1987)
months on job at single plant non-exposed 3 39 7-101
between 1946 and 1975 < 15 ppm-years 5 91 30-213
(3636 males) 15-60 ppm-years 5 147 48-343
60 ppm-years 5 179 57-409
leukaemia, total:
non-exposed 0 -
< 15 ppm-years 97 12-349
15-60 ppm-years 78 2-434
60 ppm-years 276 57-806
Retrospective cohort of 259 no benzene levels lymphatic and haemopoietic 4 377 109-1024 Decouflé et
male chemical workers reported; benzene neoplasms (workers with al. (1983)
employed between 1947 and used in large > 1 year of employment)
1960 quantities
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective cohort of shoe exposed for up to 29 aplastic anaemia (males); 4 1566 P < 0.05 Paci et al.
workers (1008 males, 1005 years; levels of leukaemia (males) 6 400 146-870 (1989)
women) employed between benzene not reported
1939 and December 1984 and
still employed in plant in
January 1950
Retrospective cohort study grab samples; means acute and chronic 30 cases 574 P < 0.01 Yin et al.
(28 460 workers in 233 factories); between 10 and 1000 leukaemia 5 deaths (1987)
reference population 28 257 mg/m3
in 83 machine production,
and clothing factories 50-500 mg/m3 found in
most plants
Mortality study of coke plant non-exposed, coke leukaemia (non-exposed) 13 135 NR Swaen et al.
workers (5639) with > 6 months ovens and by-product leukaemia (coke-oven) 6 163 NR (1991)
work between 1945 and 1969 workers; levels of leukaemia (by-product) 7 85 NR
benzene not reported
Retrospective cohort of 391 mean time-weighted leukaemia Hurley et
benzole workers in 2 cohorts average exposure of by-product 1 1 98 2-557 al. (1991)
(cohort 1, 84 workers; coke by-product by product 2 1 76 2-429
cohort 2, 307 workers) workers in Britain
in 1980s 4.2 mg/m3
(1.3 ppm)
a SMR - Standard mortality ratio
b Follow-up of cohort described by Infante et al. (1977)
c Follow-up of cohort described by Ott et al. (1978)
NR = not reported
study included 1165 white males followed from 1950 to 1981. For the
analysis the cohort was divided into four cumulative exposure groups:
< 128 mg/m3-years (< 40 ppm-years), 128-640 mg/m3-years (40-200
ppm-years), 640-1280 mg/m3-years (200-400 ppm-years) and > 1280
mg/m3-years (> 400 ppm-years). A statistically significant excess
risk was observed for all lymphatic and haemopoietic neoplasms (15
observed deaths compared to 6.6 expected; SMR = 227, 95% CI, 127-376).
There were nine deaths from leukaemia compared to an expected 2.66
(SMR = 337, 95% CI, 159-641), and 4 deaths from multiple myeloma (SMR
= 398, 95% CI, 110-1047). A strong positive trend in leukaemia
mortality was obtained with increasing cumulative exposure. Within
the four cumulative exposure groups there were 2,2,2, and 3 deaths
with SMRs of 109, 322, 1186 and 6637, respectively. In order to
investigate further the shape of the exposure-response curve, Rinsky
et al. (1987) conducted a nested case-control study by matching each
of nine deaths due to leukaemia with ten controls. A conditional
logistic regression analysis described a significant positive
association between estimated level and average duration of benzene
exposure and leukaemia that was projected downward to levels of zero
accumulated exposure over a working lifetime. From this model it was
calculated that an exponential relationship existed between benzene
exposure and the developmental leukaemia.
When actual exposure measurements did not exist, Rinsky et al.
(1987) estimated exposures to benzene by averaging historical annual
measured benzene levels from seven existing industrial hygiene survey
sources. The majority of measurements occurred after 1963, but some
data existed as early as 1946. Where no sampling data could be found,
exposure levels were estimated by interpolation from existing
information. Alterative exposure estimates and subsequent reanalyses
have been developed by Crump & Allen (1984) and Paustenbach et al.
(1992). The differences in exposure estimates between Crump & Allen
(1984) and Rinsky et al. (1987) centre primarily on assumptions of
benzene exposure prior to 1946 where no historical data exist.
Paustenbach et al. (1992) gathered additional information and
considered other factors that modified the estimates of exposure over
the entire period during which rubber hydrochloride plants operated
(1936 to 1976) to develop a new set of exposures over time. For the
most part the exposures estimated by Paustenbach et al. (1992) are
higher than those reported by Rinsky et al. (1987) and Crump & Allen
(1984).
In a retrospective study of 594 employees of a chemical company
exposed to levels of benzene between 0.3 and 114 mg/m3 (0.1-35.5
ppm) for up to 34 years, no statistically significant increase in
total mortality was reported (Ott et al., 1978). There were three
cases of myelocytic leukaemia compared to an expected incidence of 0.8
cases (significant at the P < 0.05 level). Some workers in this
cohort were also exposed to vinyl chloride, arsenicals and several
other potentially carcinogenic chemicals. A follow-up study of these
workers expanded the cohort by 362 potentially exposed workers (Bond
et al., 1986b). Four deaths from myelogenous leukaemia were reported
and the SMR for all leukaemias was 194 (95% CI, 52-488). The
difference between observed and expected values was statistically
significant only when myelogenous leukaemia was considered (4
observed, 0.9 expected; P = 0.01).
Wong (1987) reported a significant dose-response relationship
between cumulative exposure to benzene and mortality from leukaemia
and all lymphopoietic cancers combined. The mortality experience of
3536 workers who had continuous exposure to benzene was compared to
that of an internal comparison group of 3074 workers not exposed to
benzene but who had worked at the same plant. The 3536 exposed
workers were categorized into cumulative exposure categories of < 48
mg/m3-years (< 15 ppm-years), 48 to 192 mg/m3-years (15 to 60
ppm-years), and > 192 mg/m3-years (> 60 ppm-years). There was an
increasing trend in the SMRs for lymphatic and haemopoietic cancers as
exposure increased (SMR = 35, 91, 147 and 175 for the non-exposed and
the 3 exposure categories, respectively (P = 0.02). The respective
SMRs for leukaemia were 0, 97, 78 and 275 (P = 0.01). It should be
noted that none of the six leukaemia deaths was from acute myeloid
leukaemia. In addition, the highest category of exposure started at
only 192 mg/m3-years (60 ppm-years), the equivalent of 32 mg/m3
(10 ppm) annually for only a six-year working career. The
exposure-response relationship between cumulative benzene exposure and
non-Hodgkin's lymphoma was of marginal statistical significance.
A retrospective cohort mortality study was conducted on 259 male
employees at a chemical plant in the USA where benzene had been used
in large quantities (Decouflé et al., 1983). The study group included
workers employed between 1947 and 1960, and workers were followed
until 1977. Among workers with more than one year of employment, a
statistically significant excess risk was observed for neoplasms of
the lymphatic and haemopoietic systems (SMR=377; 95% CI 109-1024, 4
deaths). No SMR was given specifically for mortalities to leukaemia
and multiple myeloma. Three of these deaths were leukaemias and the
fourth a multiple myeloma.
Coke-oven workers and some workers at coke by-product plants are
exposed to low levels of benzene. A study in the Netherlands examined
5639 workers in a coke plant and a comparison group of 5740 workers in
a nitrogen-fixation plant (Swaen et al., 1991), employed for at least
six months between 1945 and 1965. The SMR for leukaemia in by-product
benzene plant workers was 85 (7 deaths), in coke-oven workers 163 (6
deaths), and in non-exposed workers 135 (13 deaths). Among the 222
workers in the benzene plant, no indication of an increased leukaemia
risk was found, but the expected number was small.
Hurley et al. (1991) have reported preliminary results on the
mortality of 6520 male coke plant workers from 27 plants in the United
Kingdom. Personal air samples were taken from 84 benzole workers from
14 plants in one cohort with levels of < 0.6-22 mg/m3 (< 0.19-7
ppm) and 307 benzole workers from 13 plants in the second cohort with
levels of < 0.6-48 mg/m3 (< 0.19-14.99 ppm). Mean time-weighted
average concentrations for benzole house workers in the United Kingdom
in the 1980s was considered to be about 4.2 mg/m3 (1.3 ppm). No
increased risk of mortality from leukaemia was reported in either
cohort (cohort 1 SMR = 98, 95% CI 2-557, 1 death; and cohort 2 SMR =
76, 95% CI 2-429, 1 death). These SMRs have been calculated utilizing
data from all 1293 by-product workers. Only a proportion of the
by-product workers had worked in the benzene plants where the greatest
exposure to benzene occurred. The SMR for leukaemia among 2349
coke-oven workers was 34 for cohort 1 (95% CI 0-186, 1 death) and 35
for cohort 2 (95% CI 1-192, 1 death).
In a retrospective cohort study from China encompassing 28 460
workers exposed to benzene in 233 factories, 30 cases of leukaemia
(23 acute, 7 chronic) were found, as compared to four cases in a
reference cohort of 28 257 workers in 83 machine production, textile
and cloth factories (Yin et al., 1987, 1989). The mortality rate (per
100 000 person-years) from leukaemia was 14 among the exposed and 2
among the unexposed (SMR, 574; P < 0.01). Mortality was especially
high for workers engaged in organic synthesis, painting and rubber
production. The mortality from leukaemia for cases that had
previously experienced benzene poisoning was 701/100 000 person-years.
Grab-samples of benzene in air were taken during the time of the
survey in workplaces where cases of leukaemia were observed; the mean
concentrations varied over a wide range (from 10 to 1000 mg/m3) but
the range was 50-500 mg/m3 in most locations. The mortality
incidence from all malignant neoplasms was higher in the exposed group
(123 per 100 000) than in controls (55 per 100 000). In addition, a
statistically significant excess risk for lung cancer (SMR = 231) was
observed in male workers exposed to benzene (Yin et al., 1989).
However, little description was given of the methods used to eliminate
the possible confounding effects of smoking.
A statistically significant excess risk for aplastic anaemia
(SMR = 1566, 4 deaths) and for leukaemia (SMR = 400, 95% CI, 146-870,
6 deaths) was reported for 1008 male workers in a shoe factory in
Florence, Italy. Included were workers employed on or after January
1950 and vital status of cohort members was ascertained through 1984.
No workplace monitoring was reported. The period of maximum benzene
exposure was considered to be 1953-1962, given the amount of benzene
used per day (about 30 kg) (Paci et al., 1989), and all cohort members
who developed aplastic anaemia and leukaemia were employed during this
period.
The results of a cohort study of 34 781 workers in eight oil
refineries in the United Kingdom which examined workers employed for
1 year between 1950-1975 gave no indication of increased mortality
from leukaemia (Rushton & Alderson, 1981a). Within this cohort a
nested case-control study was conducted to investigate the association
between exposure to benzene and leukaemia (Rushton & Alderson, 1981b).
The 36 leukaemia cases and their controls were allocated to "low",
"medium", and "high" benzene exposure categories based on their job
history. The authors reported a significant (P = 0.05) association in
the combined medium and highly exposed workers compared to those in
the low exposure group (RR = 3.0, 95% CI, 1.2-7.2).
A study (Tsai et al., 1983) of 454 petroleum refinery workers in
the USA employed between 1952 and 1978 in the petrochemical units
showed no deaths from leukaemia (0.4 expected). However, the median
exposure to benzene throughout the refinery was 0.45 mg/m3 (0.14
ppm), and only 16% of 1394 personal samples, taken between 1973 and
1982 (inclusive), contained more than 3.2 mg/m3 (1 ppm). The median
exposure intensity in "benzene-related units: petrochemical units" was
1.7 mg/m3 (0.53 ppm). No significant changes in blood indices
(counts of white and of red blood cells, haemoglobin, haematocrit,
platelets, clotting and bleeding times) were reported.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 General population
Benzene is ubiquitous in the environment, resulting in the
exposure of most humans to trace levels (or more) of this chemical.
Exposure in the general population is primarily to air-borne benzene
and derives from active and passive tobacco smoke, industrial
activity, and use of the automobile (gasoline fumes from refilling,
etc., and exhaust emissions). Estimates of the daily amounts of
benzene consumed in drinking-water and food-stuffs vary considerably
and are of the order of µg/day. Depending upon the assumptions made
with respect to levels of benzene from tobacco products and
foodstuffs, estimates for the exposure of the general smoking
population in industrialized countries range from 2000 to 3500 µg
benzene/day. Adult (70 kg) non-smokers are considered to be exposed
to about 200 to 1700 µg benzene/day (about 3 to 25 µg/kg body weight
per day). It would be helpful to have more information on total human
exposure, particularly in developing countries.
9.2 Occupational exposure
The major factors controlling industrial exposure to benzene are
process technology, worker practices and the efficiency and
sophistication of engineering controls. When appropriate engineering
controls are in place, available monitoring data indicate that
exposures of workers involved in the production, handling and use of
benzene and benzene-containing materials vary from non-detectable
levels to approximately 15 mg/m3 (8-h TWA), in addition to the
amounts estimated for the general population. In developing countries
the exposure can be several times higher. Due to the nature of the
processes involved, a small percentage of workers may be exposed to
more than 320 mg benzene/shift. In some developing countries, benzene
exposure may be sufficiently high to cause acute toxicity. Dermal
exposure to benzene has generally not been included in these
estimates. Validated benzene-specific biological markers of exposure
to low levels are not available.
9.3 Toxic effects
Acute lethal doses of benzene in experimental animals cause
narcosis, ventricular tachycardia and respiratory failure. The
threshold for narcotic effects in rats is about 13 000 mg/m3.
Reported oral LD50 values in rats vary from 3000 to 8100 mg
benzene/kg body weight and the 4-h LC50 in rats has been reported to
range between 32 600 and 44 600. However, although the clinical
pathological observations in animals are relevant to humans, the
latter would not be expected to be exposed to such high levels for
such long periods of time.
In humans, exposure to high concentrations of benzene (e.g.,
65 200 mg/m3 for 5-10 min) can result in central nervous system
depression, cardiac arrhythmia, respiratory failure and death, while
exposure to levels of benzene between 163 and 489 mg/m3 for 5 h
leads to headaches, lassitude and general weakness. The single acute
oral dose that has been reported to be lethal to humans is 8800 mg/kg
body weight.
9.3.1 Short-term and long-term exposures; organ toxicity
The most significant adverse effects from short- or long-term
exposure to benzene are haematotoxicity, i.e. bone marrow suppression,
immunotoxicity, genotoxicity and carcinogenicity.
9.3.1.1 Haematotoxicity; bone marrow depression
The level, timing and pattern of exposure are extremely important
factors in determining the incidence and severity of haematological
and bone marrow changes. A significant species difference (rats and
mice) in effects has also been reported, probably a reflection of the
more rapid metabolism of benzene by mice and the production of a
higher proportion of putative toxic metabolites than in rats. In rats
and mice, decreases in haematological cell counts (leucopenia and
haematocrit) and in bone marrow cellularity generally occur only after
several weeks of exposure to levels of benzene between 320 and 978
mg/m3, the mouse being more sensitive than rats. The lowest
reported exposure in experimental animals leading to haematological
effects was 32 mg/m3 (6 h/day, 5 days/week for 178 days) in male
mice. After oral administration of benzene to rats and mice for 120
days, leucopenia was observed in both male and female rats, and
lymphoid depletion in the B-cells of the spleen was observed in both
male and females at doses of > 200 mg/kg body weight. In mice, no
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females given 25, 50, 100, 200, 400 or
600 mg/kg body weight (5 days/week).
9.3.1.2 Mechanism of action and metabolism
In humans, a spectrum of blood dyscrasias, including
pancytopenia, aplastic anaemia, thrombocytopenia, granulocytopenia,
lymphocytopenia, myeloid leukaemia and acute leukaemia, can result
from benzene exposure. The dose, length of exposure and the stage of
stem cell development affected will determine which effect is
observed. Pancytopenia was noted in 32 patients exposed to 489-2119
mg benzene/m3 for periods of between 4 months and 15 years. At
levels of benzene between 102 and 438 mg/m3, haematotoxicity in
rubber factory workers was reported, the correlation being better
between benzene exposure and WBC counts than in the case of RBC
counts. However, at levels of benzene less than 32 mg/m3, there is
only weak evidence for a leukaemogenic effect; no haematological
effects were noted in 200 workers exposed to 10 year TWA benzene
levels of 0.03-4.5 mg/m3.
The hepatic metabolism of benzene is responsible for
detoxification of benzene via the formation of etheral sulfate,
glucuronides and glutathione conjugates. It also leads to the
production of metabolites, such as hydroquinone, p-benzoquinone,
muconaldehyde and perhaps others, which appear to be required for the
production of benzene toxicity in bone marrow. Current concepts of
the mechanism of benzene toxicity suggest that it is the result of the
combined effects of several metabolites, perhaps acting in concert
with unmodified benzene, to adversely alter the functions of stem
cells, progenitor cells and stromal cells in the bone marrow. It has
been postulated that the specific intracellular targets are proteins
and nucleic acids. The terminal result of these biochemical insults
is the development of aplastic anaemia. It is likely that benzene
metabolites damage chromosomes by causing DNA or protein adduct
formation or generating oxidative damage to DNA, which may contribute
to the chromosomal changes associated with benzene exposure. Animal
models exist for studying the mechanism of benzene-induced aplastic
anaemia and chromosome damage. However, no acceptable model for
benzene-induced leukaemia has been developed.
Since bone marrow depression and the production of leukaemia both
involve the bone marrow, it is important to ascertain the relationship
between these two biological effects of benzene. The specific
question in need of an answer is: Is it necessary for aplastic
anaemia to develop before leukaemia can be exhibited? In humans, the
continuum of effects of benzene on bone marrow involves first the
production of either anaemia, leucopenia or thrombocytopenia, and this
is followed by pancytopenia. Aplastic anaemia is the ultimate form of
bone marrow depression. Leukaemia has also been associated with
benzene exposure. In addition to studying the relationship between
these two phenomena, it is important to study the role of metabolites
in each process.
Several authors have recently used pharmacokinetic models to
extrapolate from animal experiments to expected human dosimetry. Some
models require the use of physiological parameters, partition
coefficients and metabolic data. Of these, the data on such metabolic
parameters in humans are least well known. With increasing
information on appropriate human metabolic data, such models will be
useful in extrapolating from animals to humans and from high- to
low-dose exposure.
9.3.1.3 Immunotoxicity
Benzene-induced immunological effects are probably a reflection
of bone marrow toxicity. Immunological function was depressed in mice
exposed to benzene levels between 32 and 96 mg/m3, 6 h/day, for 6
days. In mice, polyhydroxylated metabolites of benzene have been
shown to depress B- and T-cell functions. Although the relevance of
the animal data to human immunological functions has not been
established, human immunological alterations have been observed after
exposure to benzene.
In workers exposed to, but not seriously intoxicated by, benzene
(10-22 mg/m3), the serum complement levels of IgA and IgG were
decreased but the levels of IgM were slightly increased. An increased
susceptibility to allergies was found in workers exposed to benzene
levels as low as 96 mg/m3. A loss of leucocytes and other blood
elements has been noted at benzene levels ranging between 48 and 240
mg/m3. A study of low exposures to benzene (TWA: 32 mg/m3, 10
ppm) showed no differences in mitogen-induced blastogenesis in exposed
workers and controls.
9.3.2 Genotoxicity and carcinogenic effects
In vitro tests indicate that benzene is not mutagenic.
However, benzene, or its metabolites have been shown to cause both
structural and numerical chromosomal aberrations in experimental
animals as well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes.
Humans with benzene haemopathy have been found to have a high
percentage of aneuploid lymphocytes. Increases in the number of both
stable and unstable chromosomal aberrations were observed in workers
exposed to high levels (408-1734 mg/m3) of benzene for 1 to 22
years. Therefore, benzene should be considered a clastogen in both
animals and humans.
Recent studies of workers exposed to lower concentrations of
benzene (TWA: < 3.2-32 mg/m3, < 1-10 ppm) revealed no alteration
in cell-cycle kinetics and no increase in SCEs. Only a marginally
significant increase in chromosomal aberrations (chromatid deletions
and gaps) was noted at these exposure levels.
9.3.2.1 Mechanism of carcinogenicity
It is broadly accepted today, as a result of several studies in
the field of molecular biology, that chromosomal rearrangements
generating gene translocations, gene deletions and gene amplifications
are relevant steps in the carcinogenic process.
There is at present no adequate animal model for benzene-induced
leukaemia in humans. However, benzene has been shown to be
carcinogenic in experimental animals after inhalation or oral
exposure. While several types of neoplasms have been reported to be
associated with benzene exposure in rats and mice, these are primarily
of epithelial origin, i.e. zymbal gland, liver, mammary gland and
nasal cavity. Lymphomas/leukaemias have been observed with lesser
frequency.
One study showed an increased incidence of lymphoma in the CBA/CA
mouse. No statistically significant increase was seen for lymphoid
tumour incidence in the Sprague-Dawley rat, Wistar rat or Swiss mouse.
In the B6C3F1 mouse the incidence was not dose-related.
Dose-related (possibly linear) increases in epithelial tumours were
observed in mice and rats. The results in animals indicate that
benzene is an experimental multipotential carcinogen, although there
is not the leukaemogenic response seen in humans. The lowest dose
resulting in increased incidence of tumours was demonstrated in
B6C3F1 mice (25 mg/kg body weight, 5 days per week for 103 weeks).
No useful information is available for doses below this level.
Attempts to understand the mechanism of benzene-induced
carcinogenesis can be based on basic principles of chemical
carcinogenesis. Thus, one mechanism of cancer has been postulated to
be the result of autosomal mutations due to the formation of DNA
adducts, which result in alteration of cell function and control of
replication. Secondly, cancer appears to be induced by a combination
of multiple genetic and epigenetic events.
Metabolic data suggest that several reactive metabolites of
benzene are formed and these can potentially form adducts both with
DNA and protein. Binding of benzene metabolites to the protein
components of the spindle apparatus has been suggested to inhibit
mitosis. The data for the formation of DNA adducts by reactive
metabolites of benzene suggests that, compared with other carcinogens,
benzene does not form large amounts of DNA adducts. Furthermore,
although there is evidence for DNA-adduct formation in liver,
DNA-adduct formation in bone marrow has been a subject of some
controversy. Assuming that adduct formation is an initiating event,
there is the possibility of a protective event if DNA repair or immune
surveillance intervenes. A promotional event might lead to a
carcinogenic response. It is possible that benzene acts as a promoter
of its own initiation.
While it is clear that a greater percentage of benzene
metabolites are converted to reactive metabolites at low doses than at
high doses, the total amounts of reactive metabolites increases with
increasing dose. Hence, it is clear that the severity of benzene
toxicity increases with increasing dose. By analogy, it is expected
that increasing doses of benzene should yield more leukaemia in
humans.
The negative results obtained with in vitro mutagenicity tests
could be related to an inadequate production of mutagenic metabolites
in the system. The failure to produce leukaemia in animals may be due
to lack of adequate formation of leukaemogenic metabolites or the need
to produce bone marrow damage prior to the induction of leukaemia. If
the bone marrow must undergo a stage of aplastic anaemia prior to the
development of leukaemia, one would have to estimate higher exposure
to benzene and to define a threshold. Alternatively, at doses lower
than those producing aplastic anaemia, sufficient damage may occur to
induce tumour promotion.
Both animal and human studies show that benzene exposure produces
bone marrow and chromosomal damage. However, the various stages in
carcinogenesis cited above have not been observed with benzene either
because they do not occur in these species or because of technical
problems. The results of both animal and human studies suggest that
benzene is a weak carcinogen on a molar basis.
9.3.2.2 Human carcinogenesis
Benzene is a well-established human leukaemogen. There have been
numerous epidemiological studies on the effects of benzene, most of
which have dealt with chronic industrial exposures. Increased
leukaemia risk was identified in studies of shoemakers, chemical
workers and workers in oil refineries, and in a nationwide study of
benzene-exposed workers in different industries. The most consistent
evidence for a causal association in humans has been found between
benzene exposure and myeloid leukaemia. An exposure-response
relationship was identified in some studies, the response being
influenced both by exposure levels and duration of exposure. In the
study where estimated past exposures were based on the most extensive
exposure measurements, a three-fold increased leukaemia risk was
identified in workers exposed to benzene levels of 128-640
mg/m3-years (40-200 ppm-years) (ppm-years is the average
concentration times the duration of exposure in years, e.g., 4 ppm for
10 years is equivalent to 40 ppm-years) and a statistically
significant 12-fold risk for workers exposed to benzene levels between
of 640-1280 mg/m3-years (200-400 ppm-years). The scientific
assessment of alternative exposure estimates for the rubber
hydrochloride cohort has not yet been fully explored. Of the two
alternative exposures estimates so far postulated for this study, they
both suggest a lower estimate of risk than that described above.
Other leukaemia types, multiple myeloma and other lymphomas were also
reported.
A statistically significant excess risk for multiple myeloma was
found in the rubber hydrochloride study. A marginally significant
exposure-response relationship for malignant lymphomas was reported in
a study on chemical workers. Increased risk for skin, stomach and
lung cancers has been reported in some studies, but these findings
have not been consistent and may possibly be attributed to concomitant
exposures to other chemicals or statistical artifact.
Studies examining leukaemia risk in coke-oven workers, assumed to
have been exposed to fairly low levels of benzene, have not identified
excess leukaemia risk. In these studies no attempt was made to
examine leukaemia subtypes. These studies do not provide enough
evidence to prove that there is no risk of leukaemia as a result of
exposure to these low concentrations of benzene.
The Task Group is of the opinion that the epidemiological
evidence presented so far is not capable of distinguishing between
(a) a small increase in leukaemia mortality in workers exposed to low
benzene levels, and (b) a no-risk situation.
9.4 Other toxicological end-points
Benzene does cross the placenta of experimental animals, since
haemopoietic changes have been observed in the fetuses and offspring
of mice exposed to 16, 33 or 65 mg benzene/m3, 6 h/day during days
6-15 of gestation. Following inhalation exposure to high doses (500
to 1600 mg/m3) in rabbits and mice, an increase in fetal resorptions
or fetal death was observed. However, benzene does not appear to be
teratogenic, although it is fetotoxic, in experimental animals, and no
evidence is available to permit the conclusion that it causes adverse
reproductive effects in humans.
The neurotoxicity of benzene in animals and humans has not been
well studied. An early study showed subtle changes such as reduced
food intake and decreased hind-limb grip strength following exposures
to 3300 and 9900 mg benzene/m3, and learning defects were observed
in rats dosed orally with 550 mg benzene/kg body weight.
9.5 Conclusions
To assist Member States in the development of standards for
benzene exposure, the Task Group concludes that a TWA of 3.2 mg/m3
(1 ppm) over a 40-year working career has not been statistically
associated with any increase in deaths from leukaemia. However, since
benzene is a human carcinogen, exposures should be limited to the
lowest possible technically feasible level. Increases in exposure to
over 32 mg/m3 (10 ppm) should be avoided. Benzene and
benzene-containing products such as gasoline should never be used for
cleaning purposes.
Traditionally, bone marrow depression, i.e. anaemia, leucopenia
or thrombocytopenia, in the workplace has been recognized as the first
stage of benzene toxicity and appears to follow a dose-response
relationship, i.e. the higher the dose, the greater the likelihood of
observing decreases in circulating blood cell counts.
Table 20 shows some "rough" estimates of the percentages of
workers that might exhibit either bone marrow depression or frank
aplastic anaemia after exposure to benzene for either 1 year or 10
years at concentrations of 3.2, 32, 160 or 320 mg/m3 (1, 10, 50 or
100 ppm). These estimations are an interpretation of the literature
using the experience of the Task Group. The speculative nature of
this table precludes its use in regulatory standard setting. Exposure
at high doses (160-320 mg/m3; 50-100 ppm) for one year would most
likely produce bone marrow toxicity in a large percentage of the
workers, and in some cases aplastic anaemia, but little effect would
be expected at the lower doses. Exposure to both high and low doses
would be expected to produce benzene toxicity after 10 years. Thus,
a high level of both bone marrow depression and aplastic anaemia would
be seen at the higher doses and some damage would also be seen at the
lower doses. The observation of any of these effects, regardless of
the dose or period of exposure, should indicate the need for improved
control of benzene exposure.
There is no evidence of benzene being teratogenic at doses lower
than those that produce maternal toxicity, but fetal toxicity has been
demonstrated.
The neurotoxicity and immunotoxicity of benzene have not been
well studied in either experimental animals or humans.
Table 20. Estimated percentage of worker populations that might
develop bone marrow depression or aplastic anaemia after
chronic exposure to benzene ( Before using table note
cautionary footnotea)
Duration Exposure Bone marrow Aplastic
depression anaemia
1 year 320 mg/m3 (100 ppm) 90 10
160 mg/m3 (50 ppm) 50 5
32 mg/m3 (10 ppm) 1 0b
3.2 mg/m3 (1 ppm) 0b 0b
10 years 320 mg/m3 (100 ppm) 99 50
160 mg/m3 (50 ppm) 75 10
32 mg/m3 (10 ppm) 5 0b
3.2 mg/m3 (1 ppm) < 1 0b
a This estimation is an interpretation of the literature and is
based on the experience of the Task Group. The speculative
nature of this table precludes its use in regulatory standard
setting.
b Occasional cases may be observed.
10. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Benzene and benzene-containing products, including gasoline
(petrol), should never be used for cleaning purposes.
b) Systematic information on occupational and non-occupational
exposure should be collected using the total human exposure
approach where possible.
c) The health risk of low-level benzene exposure is not clearly
established. Exposure should, therefore, be avoided as much as
possible.
d) The occurrence of benzene in environmental media such as air and
water where there exists potential human exposure should be
evaluated.
e) A search for less toxic solvents to replace benzene in industrial
processes should be encouraged.
11. FURTHER RESEARCH
a) Epidemiological studies of the risks of haematological
malignancies, blood changes (red and white blood cells) and
genotoxic effects at low and high exposure concentrations should
have high priority.
b) Information on the mechanisms by which benzene induces neoplasms
is required. In particular, there is a need for animal models of
benzene-induced haemopoietic malignancies similar to those seen
in humans and a better understanding of the role of reactive
intermediate.
c) Further studies are needed to elucidate the potential link
between bone marrow suppression and the eventual occurrence of
leukaemia.
d) Biological markers of benzene exposure, especially urinary
muconic acid and macromolecular adducts, should be validated.
e) Individual susceptibility factors in benzene-induced toxicities
should be investigated.
f) Animals and human studies are required to assist in validating
physiologically based pharmacokinetic models using all routes of
exposure.
g) The multigenerational effects of benzene exposure should be
investigated.
h) Studies on immunotoxicological effects following benzene exposure
should be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of benzene has been evaluated by the
International Agency for Research on Cancer (IARC, 1982, 1987b). It
was concluded that there was sufficient evidence for the
carcinogenicity of benzene in both animals and humans.
A guideline value of 10 µg/litre was recommended by WHO (WHO,
1984) for benzene in drinking-water based on data for the production
of leukaemia after inhalation exposures in humans and using a linear
multistage extrapolation model and a life-time risk level of 1 in
100 000. This guideline remained unchanged during the revisions
recently completed (WHO, 1993).
A Task Group convened by the WHO Regional Office for Europe
concluded that an air quality guideline value could not be set for
benzene in view of its carcinogenic activity in humans (WHO, 1987).
Assuming no threshold and an average relative risk model, it was
calculated that at an air concentration of 1 µg benzene/m3, the
estimated lifetime risk of leukaemia would be 4 x 10-6.
Regulatory standards for benzene established by national bodies
in some countries are summarized in the Legal File of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
Recently the Commission of the European Communities has proposed an
occupational exposure limit for benzene of 1.6 mg/m3 (0.5 ppm) (CEC,
1993).
References
Aksoy M (1980) Different types of malignancies due to occupational
exposure to benzene. A review of recent observations in Turkey.
Environ Res, 23: 181-190.
Aksoy M & Erdem S (1978) Follow-up study on the mortality and the
development of leukaemia in 44 pancytopaenic patients with chronic
exposure to benzene. Blood, 52: 285-292.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1971) Haematological
effects of chronic benzene poisoning in 217 workers. Br J Ind Med, 28:
296-302.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1972) Details of
blood changes in 32 patients with pancytopaenia associated with
long-term exposure to benzene. Br J Ind Med, 29: 56-64.
Aksoy M, Erdem S, Hepyüksel T, & Dincol G (1974a) Chronic exposure to
benzene as a possible contributory etiologic factor in Hodgkin's
Disease. Blood, 38: 93-298.
Aksoy M, Erdem S, & Dincol G (1974b) Leukemia in shoe-workers exposed
chronically to benzene. Blood, 44: 837-841.
Allen PD (1987) Residential population exposure to ambient benzene in
California. Sacramento, California, California Air Resources Board.
Anderson D & Richardson CR (1981) Issues relevant to the assessment of
chemically induced chromosome damage in vivo and their relationship
to chemical mutagenesis. Mutat Res, 90: 261-272.
Andrews LS & Snyder R (1986) Toxic effects of solvents and vapors. In:
Casarett LJ & Doull J ed. Toxicology: The basic science of poisons,
3rd ed. New York, McMillan Publishing Co., chapter 20, p 641.
Antoine SR, Delon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36(3): 364-371.
Appuhn E & Goldeck H (1957) [Early and late disorders of
haematopoiesis caused by benzene and its homologues.] Arch
Gewerbepathol Gewerbehyg, 15: 399-428 (in German).
Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin BH, Matter
BE, & Shelby MD ed. (1985) Evaluation of short-term tests for
carcinogens. Report of the International Programme on Chemical
Safety's collaborative study on in vitro assays. Amsterdam, Oxford,
New York, Elsevier Science Publishers (Progress in Mutation Research,
Volume 5).
ATSDR (1989) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 173 pp
(ATSDR/TP-88/03; PB/89/209464/AS).
ATSDR (1991) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 193 pp.
Au WW, Ramanujam VMS, Ward JB Jr, & Legator MS (1991) Chromosome
aberrations in lymphocytes of mice after sub-acute low-level
inhalation exposure to benzene. Mutat Res, 260: 219-224.
Baarson K, Snyder CA, & Green J (1982) The hematotoxic effects of
inhaled benzene on peripheral blood, bone marrow, and spleen cells are
increased by ingested ethanol. Toxicol Appl Pharmacol, 64: 393-404.
Baarson K, Snyder CA, & Albert RE (1984) Repeated exposures of C57B1
mice to 10 ppm inhaled benzene markedly depressed erythropoietic
colony formation. Toxicol Lett, 20: 337-342.
Bailer AJ & Hoel DG (1989) Metabolite-based internal doses used in a
risk assessment of benzene. Environ Health Perspect, 82: 177-184.
Bailey JC & Schmidl B (1989) A survey of hydrocarbons emitted in
vehicle exhaust gases, over a range of driving speeds and conditions
from a representative sample of the 1986-87 UK vehicle fleet.
Stevenage, Herts, Warren Spring Laboratory (DTI) (Report No. LR 673).
Bandow H, Washida N, & Akimoto H (1985) Ring-cleavage reactions of
aromatic hydrocarbons studies by FT-IR spectroscopy. I. Photooxidation
of toluene and benzene in the NOx-air system. Bull Chem Soc Jpn, 58:
2531-2540.
Barale R, Giorgelli F, Migliore L, Ciranno R, Casini D, Zucconi D, &
Loprieno N (1985) Benzene induces micronuclei in circulating
erythrocytes of chronically treated mice. Mutat Res, 144: 193-196.
Barrett RH (1985) Assays for unscheduled DNA synthesis in HeLa S3
cells. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin
BH, Matter BE, & Shelby MD ed. Short-term tests for carcinogens:
Report of the International Programme on Chemical Safety's
collaborative study on in vitro assays. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 347-352 (Progress in Mutation
Research, Volume 5).
Battersby NS & Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Appl Environ
Microbiol, 52(2): 433-439.
Baxter HG, Blakemore R, Moore JP, & Coker DT (1980) The measurement of
airborne benzene vapour. Ann Occup Hyg, 23: 117-132.
Bayer CW, Black MS, & Galloway LM (1988) Sampling and analysis
techniques for trace volatile organic emissions from consumer
products. J Chromatogr Sci, 26: 168-173.
Bechtold WE, Sabourin PJ, & Henderson RF (1988) A reverse isotope
dilution method for determining benzene and metabolites in tissues.
J Anal Toxicol, 12(7/8): 176-179.
Bechtold WE, Lucier G, Birnbaum LS, Yin SN, Li GL, & Henderson RF
(1991) Muconic acid determinations in urine as a biological exposure
index for workers occupationally exposed to benzene. Am Ind Hyg Assoc
J, 52(11): 473-478.
Bechtold WE, Sun JD, Birnbaum LS, Yin SN, Li GL, Kasicki S, Lucier G,
& Henderson RF (1992) S-Phenylcysteine formation in haemoglobin as a
biological exposure index to benzene. Arch Toxicol, 66: 303-309.
Beliles RP & Totman LC (1989) Pharmacokinetically based risk
assessment of workplace exposure to benzene. Regul Toxicol Pharmacol,
9: 186-195.
Bennett GF (1989) Impact of toxic chemicals on local wastewater
treatment plant and the environment. Environ Geol Water Sci, 13(3):
201-212.
Bentley P, Sschassmann H, Sims P, & Oesch F (1976) Epoxides derived
from various polycyclic hydrocarbons as substrates of homogeneous and
microsome-bound epoxide hydratase. A general assay and kinetic
properties. Eur J Biochem, 69: 97-103.
Blanchard RD & Hardy JK (1986) Continuous monitoring device for the
collection of 23 volatile organic priority pollutants. Anal Chem, 58:
1529-1532.
Blank IH & McAuliffe DJ (1985) Penetration of benzene through human
skin. J Invest Dermatol, 85: 522-526.
Bois FY, Woodruff TJ, & Spear RC (1991a) Comparison of three
physiologically based pharmacokinetic models of benzene disposition.
Toxicol Appl Pharmacol, 110: 79-88.
Bois FY, Smith MT, & Spear RC (1991b) Mechanisms of benzene
carcinogenesis: application of a physiological model of benzene
pharmacokinetics and metabolism. Toxicol Lett, 56: 283-298.
Bond AE, Thompson VL, & Ortman GC (1986) Self service station vehicle
refueling exposure study. In: Proceedings of the 1986 EPA/APCA
Symposium on Measurement of Toxic Air Pollutants. Pittsburgh,
Pennsylvania, Air Pollution Control Association, pp 458-466.
Bond GG, McLaren EA, Baldwin CL, & Cook RR (1986) An update of
mortality among chemical workers exposed to benzene. Br J Ind Med, 43:
685-691.
Bradley MO (1985) Measurement of DNA single-strand breaks by alkaline
elution in rat hepatocytes. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 353-357 (Progress in Mutation Research, Volume 5).
Brodfuehrer JI, Chapman DE, Wilke TJ, & Powis G (1990) Comparative
studies of the in vitro metabolism and covalent binding of
14C-benzene by liver slices and microsomal fraction of mouse, rat
and human. Drug Metab Dispos, 18: 20-27.
Brown, RH (1988a) Determination of benzene, toluene, and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 225-233.
Brown RH (1988b) Determination of benzene, toluene, and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 235-242.
Bruckmann P, Beier R, & Krautscheid S (1983) [Measurements of
emissions of hydrocarbons in areas of high exposure in the Rhine-Ruhr
district.] Staub-Reinhalt-Luft, 43: 404 (in German).
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1989) Determination of
benzene, toluene and 1,3-butadiene in cigarette smoke by GC-MSD. Exp
Pathol, 37: 108-113.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1990) Analysis of
1,3-butadiene and other selected gas-phase components in cigarette
mainstream and sidestream smoke by gas chromatography - mass selective
detection. Carcinogenesis, 11(10): 1863-1868.
Bryce-Smith D & Gilbert A (1976) The organic photochemistry of
benzene. I. Tetrahedron, 32: 1309-1326.
Buchet JP (1988) Determination of phenol and its glucurono- and
sulfoconjugates in urine by gas chromatography. In: Fishbein L &
O'Neill JK ed. Environmental carcinogens methods of analysis and
exposure measurement - Volume 10: Benzene and alkylated benzenes. New
York, London, Oxford University Press, pp 281-286.
Burmaster DE (1982) The new pollution. Groundwater contamination.
Environment, 24: 33-36.
Busby WF Jr, Wang J-S, Stevens EK, Padykula RE, Aleksejczyk RA, &
Berchtold GA (1990) Lung tumourigenicity of benzene oxide, benzene
dihydrodiols and diolepoxides in the BLV: Ha newborn mouse assay.
Carcinogenesis, 11: 1473-1478.
Carpenter C, Shaffer C, Weil C, & Smyth H (1944) Studies on the
inhalation of 1,3-butadiene; with comparison of its narcotic effect
with benzol, toluol, and styrene and a note on the elimination of
styrene by the human. J Ind Hyg Toxicol, 26: 69-78.
CEC (1993) Occupational exposure limits - Criteria document for
benzene. Luxembourg, Commission of the European Communities,
Directorate-General Employment, Industrial Relations and Social
Affairs (EUR 14491 EN) (Health and Safety Series).
Chang SS & Peterson RJ (1977) Symposium: The basis of quality in
muscle foods. Recent developments in the flavor of meat. J Food Sci,
42: 298-306.
Chepiga TA, Yang CS, & Snyder R (1991) Benzene metabolism in two
purified, reconstituted rat hepatic mixed function oxidase systems.
In: Witmer CM, Snyder R, Jollow DJ, Kalf GF, Kocsis JJ, & Sipes IG ed.
Biological reactive intermediates: IV. Molecular and cellular effects
and their impact on human health. New York, London, Plenum Press,
pp 261-265.
Choy WN, MacGregor JT, & Shelby MD (1985) Induction of micronuclei by
benzene in B6C3F1 mice: Retrospective analysis of peripheral blood
smears from the NTP carcinogenesis bioassay. Mutat Res, 143: 55-59.
Ciranni R, Barale R, Marrazzini A, & Loprieno N (1988) Benzene and the
genotoxicity of its metabolites. I. Transplacental activity in mouse
fetuses and in their dams. Mutat Res, 208: 61-67.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, & Lester JN (1984b) A comparison of
photoionization detection gas chromatography with a Tenax GC sampling
tube procedure for the measurement of aromatic hydrocarbons in ambient
air. J Environ Anal Chem, 17: 315-326.
Coate WB, Hoberman AM, & Durloo RS (1984) Inhalation teratology study
of benzene in rats. Adv Mod Environ Toxicol, 6: 187-198.
Colenutt BA & Thornburn S (1980) Gas-chromatographic analysis of trace
hydrocarbon pollutants in water samples. Int J Environ Stud, 15:
25-32.
Collins JJ, Conner P, Friedlander BR, Easterday PA, Nair RS, Rashmi S,
& Braun J (1991) A study of the hematological effects of chronic
low-level exposure to benzene. J Occup Med, 33(5): 619-626.
CONCAWE (1986) Review of European oil industry benzene exposure data.
The Hague, European Oil Companies Organization for Environmental and
Health Protection (Report No. 3/86).
Cornish HH & Ryan RC (1965) Metabolism of benzene in non-fasted,
fasted, and arylhydroxylase inhibited rats. Toxicol Appl Pharmacol,
7: 767-771.
Cronkite EP (1986) Benzene hematotoxicity and leukemogenesis. Blood
Cells, 12: 129-137.
Cronkite EP, Bullis J, Inoue T, & Drew RT (1984) Benzene inhalation
produces leukemia in mice. Toxicol Appl Pharmacol, 75: 358.
Cronkite EP, Drew RT, Inoue T, & Bullis JE (1985) Benzene
hematotoxicity and leukemogenesis. Am J Ind Med, 7: 447-456.
Cronkite EP, Drew RT, Inoue T, Hirabayashi Y, & Bullis JE (1989)
Hematotoxicity and carcinogenicity of inhaled benzene. Environ Health
Perspect, 82: 97-108.
Crump K & Allen B (1984) Quantitative estimates of risk of leukemia
from occupational exposure to benzene. Washington, DC, US Department
of Labour (OSHA Docket H-059b, Exhibit 152, Annex B).
Dann T (1987) Ambient benzene levels in urban areas of Canada. Report
of the Technology Development and Technical Services Branch,
Conservation and Protection. Ottawa, Environment Canada.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Da Silva C, Fan X, & Castagna M (1989) Benzene-mediated protein kinase
c activation. Environ Health Perspect, 82: 91-95.
Dean BJ (1978) Genetic toxicology of benzene, toluene, xylenes and
phenols. Mutat Res, 47: 75-97.
Dean BJ (1985a) Recent findings on the genetic toxicology of benzene,
toluene, xylenes and phenols. Mutat Res, 154: 153-181.
Dean BJ (1985b) Summary report on the performance of cytogenetic
assays in cultured mammalian cells. In: Ashby J, de Serres FJ, Draper
M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation
of short-term tests for carcinogens. Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 69-83 (Progress in Mutation Research, Volume 5).
Decouflé P, Blattner WA, & Blair A (1983) Mortality among chemical
workers exposed to benzene and other agents. Environ. Res., 30: 16-25.
De Flora S, Zanacchi P, Camoirano A, Bennicelli C, & Badolati GS
(1984) Genotoxic activity and potency of 135 compounds in the Ames
reversion test and in a bacterial DNA-repair test. Mutat Res, 133:
161-198.
Deichmann WB, MacDonald WE, & Bernal E (1963) The hemopoietic tissue
toxicity of benzene vapors. Toxicol Appl Pharmacol, 5: 201-224.
Dibben MJ, Thomas TC, & Anderson MP (1989) Is benzene a problem in
fuel operations: A new analytical method to overcome potential
interferences in the present methods. Liq Chromatogr-Gas Chromatogr
Int, 2: 54, 56, 58.
Dorfman LM, Taub IA, & Buhler RE (1962) Pulse radiolysis studies. I.
Transient spectra and reaction-rate constants in irradiated aqueous
solutions of benzene. J Chem Phys, 36: 3051-3061.
Douglas GR, Blakely DH, Liu-Lee VW, Bell RDL, & Bayley JM (1985)
Alkaline sucrose sedimentation, sister-chromatid exchange and
micronucleus assays in CHO cells. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 359-366 (Progress in Mutation Research, Volume 5).
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Drew RT & Fouts JR (1974) The lack of effects of pretreatment with
phenobarbital and chlorpromazine on the acute toxicity of benzene in
rats. Toxicol Appl Pharmacol, 27: 183-193.
Eastmond DA, Smith MT, & Irons RD (1987) An interaction of benzene
metabolites reproduces the myelotoxicity observed with benzene
exposure. Toxicol Appl Pharmacol, 91: 85-95.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol, 15:
30-38.
Environment Agency, Japan (1989) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1986 and 1987. Tokyo, Environment Agency, Department of Environmental
Health, Office of Health Studies.
Erexson GL, Wilmer JL, & Kligerman AD (1985) Sister chromatid exchange
in human lymphocytes exposed to benzene and its metabolites
in vitro. Cancer Res, 45: 2471-2477.
Erexson GL, Wilmer JL, Steinhagen WH, & Kligerman AD (1986) Induction
of cytogenetic damage in rodents after short-term inhalation of
benzene. Environ Mutagen, 8: 29-40.
Erf LA & Rhoads CP (1939) The hematological effects of benzene
(benzol) poisoning. J Ind Hyg Toxicol, 20(8): 421.
Ewing BB, Chain ESK, Cook JC, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Washington, DC, US Environmental Protection Agency
(EPA-560/6/77/015A).
Fel'dt EG (1985) [Evaluation of the mutagenic hazards of benzene and
some of its derivatives.] Gig i Sanit, 7: 21-23 (in Russian with
English summary).
Fentiman AF, Neher MB, Kinzer GW, Sticksel PR, & Coutant RW (1979)
Environmental monitoring benzene. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/679/006)
(Prepared by Battelle Columbus for US EPA).
Ferrario JB, Lawler GC, Delon IR, & Laseter JL (1985) Volatile organic
pollutants in biota and sediments of Lake Pontchartrain. Bull Environ
Contam Toxicol, 34(2): 246-255.
Fishbein L (1984) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. I. Benzene. Sci Total Environ, 40:
189-218.
Forni A, Pacifico E, & Limonta A (1971a) Chromosome studies in workers
exposed to benzene or toluene or both. Arch Environ Health, 22:
373-378.
Forni AM, Cappellini A, Pacifico E, & Vigliani EC (1971b) Chromosome
changes and their evolution in subjects with past exposure to benzene.
Arch Environ Health, 23: 385-391.
Franz TJ (1984) Percutaneous absorption of benzene. In: Applied
toxicology of hydrocarbons. Princeton, New Jersey, Princeton
Scientific Publishers, Inc., pp 61-70 (Advances in Modern
Environmental Toxicology, Volume VI).
Fung KK & Wright BJ (1986) Monitoring of benzene in ambient air with
organic vapor badges. J Air Pollut Control Assoc, 36: 819-821.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected
hydrocarbons. Arch Ind Health, 18: 9-15.
Gad-El-Karim MM, Harper BL, & Legator MS (1984) Modifications in the
myeloclastogenic effect of benzene in mice with toluene,
phenobarbital, 3-methylcholanthrene, Aroclor 1254 and SKF-525A. Mutat
Res, 135: 225-243.
Galton DAG (1986) The myelodysplastic syndromes. Scand J Haematol, 36:
11-20.
Garner RC (1985) Summary report on the performance of gene mutation
assays in mammalian cells in culture. In: Ashby J, de Serres FJ,
Draper M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed.
Evaluation of short-term tests for carcinogens. Report of the
International Programme on Chemical Safety's collaborative study on
in vitro assays. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 85-94 (Progress in Mutation Research, Volume 5).
GDCh (Society of German Chemists) (1988) [Advisory Committee on
Existing Chemicals of Environmental Relevance. Benzene.] Weinheim, VCH
Verlagsgesellschaft, 82 pp (BUA Report 24) (in German with English
summary).
Geist CR, Kelly LD, Schoenheit CM, & Praed JE (1983) Learning
impairments following postnatal exposures to benzene. Percept Motor
Skills, 57: 1083-1086.
Gerarde HW (1960) Toxicology and biochemistry of aromatic
hydrocarbons. Amsterdam, Oxford, New York, Elsevier Science
Publishers.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res, 58:
313-316.
Geyer H, Politzki G, & Freitag D (1984) Prediction of ecotoxicological
behaviour of chemicals: relationship between n-octanol/water partition
coefficient and bioaccumulation of organic chemicals by the alga
Chlorella. Chemosphere, 13: 269-284.
Ghantous H & Danielsson BRG (1986) Placental transfer and distribution
of toluene, xylene, and benzene, and their metabolites during
gestation in mice. Biol Res Pregnancy, 7: 98-105.
Gilman AG, Goodman LA, & Gilman A (1985) The Goodman and Gilman's
pharmacological basis of therapeutics, 7th ed. New York, MacMillan
Publishing Co.
Gilmour SK, Kalf GF, & Snyder R (1986) Comparison of the metabolism of
benzene and its metabolite phenol in rat liver microsomes. In: Kocsis
JJ, Jollow DJ, Witmer CM, Nelson J, & Snyder R ed. Biological reactive
intermediates. III - Mechanisms of action in animal models and human
disease. New York, Plenum Publishing Co., pp 223-235.
Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, &
Oesch F (1989) Multiple activation pathways of benzene leading to
products with varying genotoxic characteristics. Environ Health
Perspect, 82: 81-89.
Gofmekler VA (1968) [The embryotropic action of benzene and
formaldehyde in experimental administration by inhalation.] Gig i
Sanit, 33: 12-16 (in Russian with English summary).
Goldstein BD (1988) Benzene toxicity: occupational medicine. State Art
Rev, 3: 541-554.
Goldstein BD, Snyder CA, Laskin S, Brombert I, Albert RE, & Nelson N
(1982) Myelogenous leukemia in rodents inhaling benzene. Toxicol Lett,
13: 169-173.
Goldwater LJ (1941) Disturbances in the blood following exposure to
benzol. J Lab Clin Med, 26: 957.
Gollmer L, Graf H, & Ulrich V (1984) Characterization of the benzene
monooxygenase system in rabbit bone marrow. Biochem Pharmacol, 33:
3597-3602.
Goodenkaug O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24(2): 231-233.
Gorsky LD & Coon MJ (1985) Evaluation of the role of free hydroxyl
radicals in the cytochrome P-450 catalyzed oxidation of benzene in
microsomes and reconstituted enzyme systems from rabbit liver. J Biol
Chem, 258: 7311.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Government of Canada (in press) Canadian environmental protection act
- Priority assessment report on benzene. Ottawa, Environment
Canada/Health and Welfare Canada.
Green JD, Leong BKJ, Lobue J, Goldstein BD, & Albert RE (1978)
Inhaled benzene fetotoxicity in rats. Toxicol Appl Pharmacol, 46:
9-18.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981a) Acute
and chronic dose/response effect of benzene inhalation on the
peripheral blood, bone marrow, and spleen cells of CD-1 male mice.
Toxicol Appl Pharmacol, 59: 204-214.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981b) Acute
and chronic dose/response effects of inhaled benzene on multipotential
hematopoietic stem (CFU-S) and granulocyte/macrophase progenitor
(GM-CFU-C) cells in CD-1 mice. Toxicol Appl Pharmacol, 58: 492-503.
Grob K & Grob G (1974) Organic substances in potable water and its
precursor. Part II. Applications in the area of Zurich. J Chromatogr,
90: 303-313.
Gruenke LD, Craig JC, Wester RC, & Maibach HI (1986) Quantitative
analysis of benzene by selected ion monitoring/gas chromatography/mass
spectrometry. J Anal Toxicol, 10(11/12): 225-232.
Hadeishi T, Pollard M, McLaughlin R, & Koga M (1985) Development of an
optical monitor for toxic organic compounds in air. Research Triangle
Park, North Carolina, US Environmental Protection Agency
(EPA/600/4-85-043).
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hammers WE & Bosman HFPM (1986) Quantitative evaluation of a simple
dynamic headspace analysis technique for non-polar pollutants in
aqueous samples at the ng/kg level. J Chromatogr, 360: 425-432.
Hancock DG, Moffitt AE Jr, & Hay EB (1984) Hematological findings
among workers exposed to benzene at a coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Hanke J, Dutkiewicz T, & Piotrowski J (1961) [The absorption of
benzene through the skin in men.] Med Pracy, 12: 413-426 (in Polish).
Harkov R, Gianti SJ, Bozzelli JW, & Laregina JE (1985) Monitoring
volatile organic compounds at hazardous and sanitary landfills in New
Jersey. J Environ Sci Health, A20(5): 491-501.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-311.
Hattemer-Frey HA, Travis CC, & Land ML (1990) Environmental
partitioning and human exposure. Environ Res, 53: 221-232.
Henderson RF, Sabourin PJ, Bechtold WE, Griffith WC, Medinsky MA,
Birnbaum LS, & Lucier GW (1989) The effect of dose, dose rate, route
of administration, and species on tissue and blood levels of benzene
metabolites. Environ Health Perspect, 82: 9-17.
Hine J & Mookerjee PK (1975) The intrinsic hydrophilic character of
organic compounds. Correlations in terms of structural contributions.
J Org Chem, 40: 292-298.
Hinson JA, Freeman JP, Potter DW, Mitchum RK, & Evans FE (1985)
Mechanism of microsomal metabolism of benzene to phenol. Mol
Pharmacol, 27: 574-577.
Hite M, Pecharo M, Smith I, & Thornton S (1980) The effect of benzene
in the micronucleus test. Mutat Res, 77: 149-155.
Högn T & Jaenicke L (1972) Benzene metabolism in Moraxella species.
Eur J Biochem, 30: 369-375.
Holdren MW, Smith DL, & Smith RN (1985) Comparison of ambient air
sampling techniques for volatile organic compounds. Washington, DC, US
Environmental Protection Agency (EPA/600/S4-85-067; PB86-120953/GAR).
Hornung RW, Ward E, Morris JA, & Rinsky RA (1989) Letter to the
editor. Haematologic effects of benzene: a thirty-five year
longitudinal study of rubber workers. Toxicol Ind Health, 5(6):
1153-1155.
HSE (1982) Toxicity review No. 4: Benzene. London, Health and Safety
Executive, Her Majesty's Stationery Office, 36 pp.
Hsieh GC, Sharma RP, & Parker RDR (1988a) Subclinical effects of
groundwater contaminants. I. Alteration of humoral and cellular
immunity by benzene in CD-1 mice. Arch Environ Contam Toxicol, 17:
151-158.
Hsieh GC, Parker RDR, & Sharma RP (1988b) Subclinical effects of
groundwater contaminants. II. Alteration of regional brain monoamine
neurotransmitters by benzene in CD-1 mice. Arch Environ Contam
Toxicol, 17: 799-805.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: toluene and xylene. Toxicology, 11: 55-63.
Huff JE, Haseman JK, Demarini DM, Eustis S, Maronpot RR, Peters AC,
Persing RL, Chrisp CE, & Jacobs AC (1989) Multiple-site
carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice.
Environ Health Perspect, 82: 125-163.
Hurley JF, Cherrie JW, & MacLaren W (1991) Exposure to benzene and
mortality from leukaemia: results from coke oven and other coal
product workers. Br J Ind Med, 48: 502-504.
IARC (1982) Benzene. In: Some industrial chemicals and dyestuffs.
Lyon, International Agency for Research on Cancer, pp 93-148
(Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 29).
IARC (1987a) Benzene. In: Genetic and related effects: an updating of
selected IARC Monographs from Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 91-95 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Formaldehyde. In: Overall evaluations of carcinogenicity:
An updating of IARC Monographs Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 211-216 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 7).
IARC (1989) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, 322 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
Ikeda M & Ohtsuji H (1971) Phenobarbital-induced protection against
toxicity of toluene and benzene in the rat. Toxicol Appl Pharmacol,
20: 30-43.
Infante PF, Wagoner JK, Rinsky RA, & Young RJ (1977) Leukaemia in
benzene workers. Lancet, 2: 76-78.
Inoue O, Seiji K, & Nakatsuka H (1989) Urinary t,t-muconic acid as an
indicator of exposure to benzene. Br J Ind Med, 46: 122-127.
Irons RD (1985) Quinones as toxic metabolites of benzene. J Toxicol
Environ Health, 16: 673-678.
Irons RD, Dent JG, Baker TS, & Rickert DE (1980) Benzene is
metabolized and covalently bound in bone marrow in situ. Chem-Biol
Interact, 30: 241-245.
Irons RD, Neptun DA, & Pfeifer RW (1981) Inhibition of lymphocyte
transformation and microtubule assembly by quinone metabolites of
benzene: Evidence for a common mechanism. J Reticuloendothel Soc, 30:
359-372.
IRPTC (1987) IRPTC Legal file 1986 on benzene - Volume 1. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
Jackson S & Brown VM (1970) Effect of toxic wastes on treatment
processes and watercourses. Water Pollut Control, 69(3): 292-313.
Jerina DM & Daly JW (1974) Arene oxides: a new aspect of drug
metabolism. Science, 185: 573-582.
Jerina D, Daly J, Witkop B, Zaltzman-Norenberg P, & Udenfriend S
(1968) Role of the arene oxide-oxepin system in the metabolism of
aromatic substrates. I. In vitro conversion of benzene oxide to a
premercapturic acid and a dihydrodiol. Arch Biochem Biophys, 128:
176-183.
Jirka AM & Bourne S (1982) Gas-chromatographic analysis for benzene in
blood. Clin Chem, 28: 1492-1494.
Johansson I & Ingelman-Sundberg M (1988) Benzene metabolism by
ethanol-, acetone-, and benzene-inducible cytochrome P-450(IIE1) in
rat and rabbit liver microsomes. Cancer Res, 48: 5387-5390.
Johnston RV, Pinkerton MN, & Mensik DC (1979) Haematological and
myelogenous effects of inhaled benzene in the pig and rat. J Toxicol
Environ Health, 5: 1025-1035.
Jonek J, Olkowski Z, & Zieleznik B (1965) Histochemical studies on the
spinal cord of mice poisoned with benzene. Acta Histochem, 20:
286-296.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular-weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jowa L, Witz G, Snyder R, Winkle S, & Kalf GF (1990) Synthesis and
characterization of deoxyguanosine-benzoquinone adduct. J Appl
Toxicol, 10(1): 47-54.
Kalf GF (1987) Recent advances in the metabolism and toxicity of
benzene. CRC Crit Rev Toxicol, 18: 141-159.
Kalf GF, Schlosser MJ, Renz JF, & Priozzi SJ (1989) Prevention of
benzene-induced myelotoxicity by nonsteroidal anti-inflammatory drugs.
Environ Health Perspect, 82: 57-64.
Karam LR & Simic MG (1989) Mechanisms of free radical chemistry and
biochemistry of benzene. Environ Health Perspect, 82: 37-41.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soils. Chemosphere,
10(8): 833-846.
Karlson U & Frankenberger WT Jr (1989) Microbial degradation of
benzene and toluene in groundwater. Bull Environ Contam Toxicol, 43:
505-510.
Keller KA & Snyder CA (1986) Mice exposed in utero to low
concentrations of benzene exhibit enduring changes in their colony
forming hematopoietic cells. Toxicology, 42: 171.
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol Environ
Saf, 4: 26-38.
Kiang PH & Grob RL (1986) A headspace technique for the determination
of volatile compounds in soil. J Environ Sci Health, A21: 71-100.
Kimmel CA & Wilson JG (1973) Skeletal deviations in rats:
malformations or variations? Teratology, 8: 309-316.
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Kipen HM, Cody RP, Crump KS, Allen BC, & Goldstein BD (1988)
Hematologic effects of benzene: a thirty-five year longitudinal study
of rubber workers. Toxicol Ind Health, 4(4): 411.
Kipen HM, Cody RP, & Goldstein BD (1989) Use of longitudinal analysis
of peripheral blood counts to validate historical reconstructions of
benzene exposure. Environ Health Perspect, 82: 199-206.
Kirley TA, Goldstein BD, Maniara WM, & Witz G (1989) Metabolism of
trans,trans-muconaldehyde, a microsomal hematotoxic metabolite of
benzene by purified yeast aldehyde dehydrogenase and a mouse liver
soluble fraction. Toxicol Appl Pharmacol, 100: 360-367.
Kissling M & Speck B (1972) Further studies on experimental benzene
induced aplastic anemia. Blut, 25: 97-103.
Kocsis JJ, Harkaway S, Santoyo MC, & Snyder R (1968) Dimethyl
sulfoxide: interactions with aromatic hydrocarbons. Science, 160:
427-428.
Koljkowsky P (1981) Indicator-tube method for the determination of
benzene in air. Analyst, 94: 918-920.
Korte F & Klein W (1982) Degradation of benzene in the environment.
Ecotoxicol Environ Saf, 6(4): 311-327.
Kozioski RP (1985) Determination of benzene and toluene in soils and
plant material by azeotropic distillation. Bull Environ Contam
Toxicol, 34: 10-16.
Kuna RA & Kapp RW (1981) Embyrotoxic/teratogenic potential of benzene
vapor in rats. Toxicol Appl Pharmacol, 57: 1-7.
Lahmann E, Seifert B, & Ullrich D (1977) The pollution of ambient air
and rain water by organic components of motor vehicle exhaust-gases.
In: Kasuga S ed. Proceedings of the 4th International Clean Air
Congress. Tokyo, Japanese Union of Air Pollution and Prevention
Association, pp 595-597.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973a) Leukocyte
agglutinins in workers exposed to benzene, toluene and xylene. Int
Arch Arbeitsmed, 31: 45-50.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973b) Serum
immunoglobulin levels in workers exposed to benzene, toluene and
xylene. Int Arch Arbeitsmed, 31: 37-44.
Latriano L, Goldstein BD, & Witz G (1986) Formation of muconaldehyde,
an open-ring metabolite of benzene, in mouse liver microsomes: an
additional pathway for toxic metabolites. Proc Natl Acad Sci (USA),
83: 8356-8360.
Leong B (1977) Experimental benzene intoxication. In: Laskin S &
Goldstein B ed. Benzene toxicity: A critical revaluation. J Toxicol
Environ Health, 3(2): 45-61.
Li G-L, Yin N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda M
(1986) Benzene-specific increase in leukocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581.
Longacre S, Kocsis J, & Snyder R (1981a) Influence of strain
differences in mice on the metabolism and toxicity of benzene. Toxicol
Appl Pharmacol, 60: 398-409.
Longacre S, Kocsis J, Witmer CM, Lee EW, Sammett D, & Snyder R (1981b)
Toxicological and biochemical effects of repeated administration of
benzene in mice. J Toxicol Environ Health, 7: 223-237.
Low LK, Meeks JR, Norris KJ, Mehlman MA, & Macherer CR (1989)
Pharmacokinetics and metabolism of benzene in Zymbal gland and other
key target tissues after oral administration in rats. Environ Health
Perspect, 82: 215-222.
Low LK, Reddy MV, Blackburn GR, & Mackerer CR (1991) Benzene
mechanistic research. Final report of collaborative study by Mobil,
Amoco Ashland, Standard Oil and Sun Company. Princeton, New Jersey,
Mobil Oil Corporation, 76 pp.
Lunte SM & Kissinger PT (1983) Detections and identification of
sulfhydryl conjugates of p-benzoquinone in microsomal incubations of
benzene and phenol. Chem-Biol Interact, 47: 195-212.
Lutz WK (1979) In vivo covalent binding of organic chemicals to DNA
as a quantitative indicator in the process of chemical carcinogenesis.
Mutat Res, 65: 289-356.
Lutz WK & Schlatter C (1977) Mechanisms of the carcinogenic action of
benzene: irreversible binding to rat liver DNA. Chem-Biol Interact,
18(2): 241-246.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal Gulf of Mexico site. Chemosphere, 17(1):
123-136.
MacKay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol, 9:
1178-1180.
Maibach HI & Anjo DM (1981) Percutaneous penetration of benzene and
benzene contained in solvents in the rubber industry. Arch Environ
Health, 36: 256-260.
Maltoni C, Ciliberti A, & Carretti D (1982a) Experimental
contributions in identifying brain potential carcinogens in the
petrochemical industry. Ann NY Acad Sci, 381: 216-249.
Maltoni C, Conti B, & Scarnato C (1982b) Squamous cell carcinomas of
the oral cavity in Sprague-Dawley rats following exposure to benzene
by ingestion. Med. Lav, 4: 441-445.
Maltoni C, Cotti G, Valgimigli L, & Mandrioli A (1982c)
Hepatocarcinomas in Sprague-Dawley rats following exposure to benzene
by inhalation. Med Lav, 4: 446-450.
Maltoni C, Conti B, & Cotti G (1983) Benzene: a multipotential
carcinogen. Results of long-term bioassays performed at the Bologna
Institute of Oncology. Am J Ind Med, 4: 589-630.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
current results and ongoing research. Am J Ind Med, 7: 415-446.
Maltoni C, Ciliberti A, Cotti G, Conti B, & Belpoggi F (1989) Benzene,
an experimental multipotential carcinogen: results of the long-term
bioassays performed at the Bologna Institute of Oncology. Environ
Health Perspect, 82: 109-124.
Mara SJ & Lee SS (1978) Assessment of human exposures to atmospheric
benzene. Research Triangle Park, North Carolina, US Environmental
Protection Agency (EPA/450/378-031).
Marcus WL (1990) Chemical of current interest: benzene. Cancer risk
from benzene. Adv Mod Environ Toxicol, 17: 127-188.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989a) Differences in the pathways for metabolism of benzene in rats
and mice simulated by a physiological model. Environ Health Perspect,
82: 43-49.
Medinsky MA, Sabourin PJ, Lucier G, Birnbaum LS, & Henderson RF
(1989b) A toxicological model for simulation of benzene metabolism.
Exp Pathol, 37: 150-154.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989c) A physiological model for simulation of benzene metabolism by
rats and mice. Toxicol Appl Pharmacol, 99: 193-206.
Merian E & Zander M (1982) Volatile aromatics. In: Hutzinger O ed. The
handbook of environmental chemistry, vol 3, Part B: Anthropogenic
compounds. Berlin, Heidelberg, New York, Springer-Verlag, pp 117-161.
Michael LC, Pellizzari ED, & Wiseman RW (1988) Development and
evaluation of a procedure for determining volatile organics in water.
Environ Sci Technol, 22: 565-570.
Miller MM & Wasik SP (1985) Relationships between octanol-water
partition coefficient and aqueous solubility. Environ Sci Technol,
19(6): 522-529.
Morimoto K (1976) Analysis of combined effects of benzene with
radiation on chromosomes in cultured human leukocytes. Jpn J Ind
Health, 18: 23-24.
Morimoto K (1983) Induction of sister chromatid exchanges and cell
division delays in human lymphocytes by microsomal activation of
benzene. Cancer Res, 43: 1330-1334.
Morimoto K, Wolff S, & Koizumi A (1983) Induction of sister-chromatid
exchanges in human lymphocytes by microsomal activation of benzene
metabolites. Mutat Res, 119: 355-360.
Moszczynski P (1981) Organic solvents and T-lymphocytes. Lancet, 1:
438.
Murray FJ, John JA, Rampy LW, Kuna RA, & Schweta BA (1979)
Embryotoxicity of inhaled benzene in mice and rabbits. Am Ind Hyg
Assoc J, 40: 993-998.
Nahum LH & Hoff HE (1934) The mechanism of sudden death in
experimental benzol poisoning. J Pharmacol Exp Ther, 50: 336.
Nakajima T, Okuyama S, & Yonekura I (1985) Effects of ethanol and
phenobarbital administration on the metabolism and toxicity of
benzene. Chem-Biol Interact, 55: 23-38.
Nakajima T, Elovaara E, Park SS, Gelbain HV, Hietanen E, & Vainio H
(1990) Monoclonal antibody-directed characterization of benzene,
ethoxy-resorufin and pentoxy-resorufin metabolism in rat liver
microsomes. Biochem Pharmacol, 40: 1255-1261.
Nau CA, Neal J, & Thornton M (1966) C9-C12 fractions obtained from
petroleum distillates. Arch Environ Health, 12: 382-393.
Nawrot PS & Staples RE (1979) Embryo-fetal toxicity and teratogenicity
of benzene and toluene in the mouse. Teratology, 19: 41A.
Nerland DE & Pierce WM (1990) Identification of N-acetyl-
S-(2,5-dihydroxyphenyl)-L-cysteine as a urinary metabolite of benzene,
phenol and hydroquinone. Drug Metab Dispos, 18: 958-961.
Nomiyama K & Nomiyama H (1974a) Respiratory retention, uptake and
excretion of organic solvents in man. Benzene, toluene, n-hexane,
trichloroethylene, acetone, ethyl acetate and ethyl alcohol. Int Arch
Arbeitsmed, 32: 75-83.
Nomiyama K & Nomiyama H (1974b) Respiratory elimination of organic
solvents in man. Benzene, toluene, n-hexane, trichloroethylene,
acetone, ethyl acetate and ethyl alcohol. Int Arch Arbeitsmed, 32:
85-91.
Nordlinder R & Ramnäs O (1987) Exposure to benzene at different work
places in Sweden. Ann Occup Hyg, 31: 345-355.
Norpoth K, Stücker W, Krewer E, & Müller G (1988) Biomonitoring of
benzene exposure by trace analyses of phenylguanine. Arch Occup
Environ Health, 60: 163-168.
NTP (1986) Toxicology and carcinogenesis studies of benzene (CAS No.
71-43-2) in F344/N rats and B6C3F1 mice (gavage studies). Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (NTP TR 289; NIH Publication
86-2545).
Oberly TJ, Bewsey BJ, & Probst GS (1984) An evaluation of the L5178Y
TK+/- mouse lymphoma forward mutation assay using 42 chemicals. Mutat
Res, 125: 291-306.
OECD (1986) Control of toxic substances in the atmosphere: Benzene.
Paris, Organisation for Economic Cooperation and Development
(Environment Monographs No. 5).
Ogata M & Miyake Y (1978) Disappearance of aromatic hydrocarbons and
organic sulphur compounds from fish flesh reared in crude oil
suspension. Water Res, 12: 1041-1044.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
OSHA (Occupational Safety and Health Administration) (1987)
Occupational exposure to benzene. Fed Reg, 52(176): 34460-34578.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. Int J Environ Anal Chem, 31: 41-53.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 54: 1370-1374.
Ott MG, Townsend JC, Fishbeck WA, & Langner RA (1978) Mortality among
workers occupationally exposed to benzene. Arch Environ Health, 33:
3-10.
Paci E, Buiatti E, Costantini AS, Miligi L, Pucci N, Scarpelli A,
Petriolo G, Simonata L, Winkelmann R, & Kaldor JM (1989) Aplastic
anemia, leukemia and other cancer mortality in a cohort of shoe
workers exposed to benzene. Scand J Work Environ Health, 15: 313-318.
Painter RB & Howard R (1982) The HeLa-DNA synthesis inhibition test as
a rapid screen for mutagenic carcinogens. Mutat Res, 92: 427-437.
Parke DV & Williams RT (1953) Studies in detoxication. 49. The
metabolism of benzene containing [14C1]benzene. Biochem J, 54:
231-238.
Patty F (1981) Industrial hygiene and toxicology, 3rd revis ed. New
York, Interscience Publishers.
Paustembach DJ, Price PS, Ollison W, Blank C, Jernigan JD, Bass RD, &
Peterson HD (1992) Reevaluation of benzene exposure for the pliofilm
(rubberworker). J Toxicol Environ Health, 36: 177-231.
Paxman D & Rappaport SM (1990) Analysis of OSHA's short-term-exposure
limit for benzene. Regul Toxicol Pharmacol, 11: 275-287.
Pekari K, Riellola M-L, & Aitio A (1989) Simultaneous determination of
benzene and toluene in blood using head-space gas chromatography. J
Chromatogr, 491: 309-320.
Pellack-Walker P & Blumer JL (1986) DNA damage in L5178YS cells
following exposure to benzene metabolites. Mol Pharmacol, 30: 42-47.
Pellizzari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16(11): 781-785.
Pellizzari ED, Zweidinger RA, & Sheldon LS (1988) Determination of
benzene, toluene and xylene in breath samples by gas
chromatography/mass spectrometry. In: Fishbein L & O'Neill JK ed.
Environmental carcinogens method of analysis and exposure measurement
- Volume 10: Benzene and alkylated benzenes. New York, London, Oxford
University Press, pp 267-279.
Philip P & Krogh-Jensen M (1970) Benzene induced chromosome
abnormalities in rat bone marrow cells. Acta Pathol Microbiol Scand,
A78: 489-490.
Pollini G, Biscaldi GP, & Robustelli della Cuna G (1969) [Chromosome
changes in lymphocytes five years after benzene haemopathy.] Med Lav,
60: 743-758 (in Italian).
Pongracz K & Bodell WJ (1991) Detection of 3@-hydroxy-1,
N6-benzetheno-2'-deoxyadenosine 3'-phosphate by 32P postlabelling
of DNA reacted with p-benzoquinone. Chem Res Toxicol, 4: 199-202.
Poole SK, Furton KG, & Poole CF (1988) Determination of benzene and
toluene in gasoline by gas chromatography using a liquid organic salt
column. J Chromatogr Sci, 26: 67-73.
Post GB & Snyder R (1983) Effects of enzyme induction on microsomal
benzene metabolism. J Toxicol Environ Health, 11: 811.
Post GB, Snyder R, & Kalf GF (1985) Inhibition of RNA synthesis and
interleukin-2 production in lymphocytes in vitro by benzene and its
metabolites, hydroquinone and p-benzoquinone. Toxicol Lett, 29:
161-167.
Probst GS & Hill LE (1985) Tests for the induction of DNA-repair
synthesis in primary cultures of adult rat hepatocytes. In: Ashby J,
de Serres FJ, Draper M, Ishidata M Jr, Margolin BH, Matter BE, &
Shelby MD ed. Evaluation of short-term tests for carcinogens: Report
of the International Programme on Chemical Safety's collaborative
study on in vitro assays. Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp 381-386 (Progress in Mutation Research,
Volume 5).
Reddy MV, Bleicher WT, Blackburn GR, & Mackerer CR (1990) DNA
adduction by phenol hydroquinone, or benzoquinone in vitro but not
in vivo: nuclease P1-enhanced 32P-postlabelling of adducts as
labeled nucleoside biphosphates, dinucleotides and nucleoside
monophosphates. Carcinogenesis, 11: 1349-1357.
Reineke FJ & Bächmann K (1985) Gas chromatographic determination of
C2-C8 hydrocarbons and halocarbons in ambient air by simultaneous
use of three detectors. J Chromatogr, 323: 323-329.
Renova NW (1962) [Concerning auto-immunity shifts in chronic
occupational benzol poisoning.] Gig Tr Prof Zabol, 7: 38-42 (in
Russian).
Reynolds LF & Harrison DW (1982) Study of discharges of: benzene,
chloroform and carbon tetrachloride into the aquatic environment and
the best technical means for the reduction of water pollution from
such discharges. Final Report. Brussels, Commission of the European
Communities (BL/A/2198).
Rickert DE, Baker TS, Bus JS, Barrow CS, & Irons RD (1979) Benzene
disposition in the rat after exposure by inhalation. Toxicol Appl
Pharmacol, 49: 417-423.
Rinsky RA, Alexander B, Smith MD, Hornung R, Filloon TG, Young RJ,
Okun AH, & Landrigan PJ (1987) Benzene and leukemia: an
epidemiological risk assessment. New Engl J Med, 316: 1044-1050.
RIVM (1988) Integrated criteria document on benzene. Bilthoven, The
Netherlands, National Institute for Public Health and Environmental
Protection, 138 pp (Report No. 758476003).
Roberts JM, Rehsenfeld FC, & Liu SC (1984) Measurements of aromatic
hydrocarbon ratios and nitrogen oxide concentrations in the rural
troposphere: observation of air mass photochemical aging and nitrogen
oxides removal. Atmos Environ, 18(11): 2421-2432.
Roghani M, Da Silva C, Guvelli D, & Castagna M (1987) Benzene and
toluene activate protein kinase C. Carcinogenesis, 8: 1105-1107.
Rosenthal GJ & Snyder CA (1985) Modulation of the immune response to
Listeria monocytogenes by benzene inhalation. Toxicol Appl
Pharmacol, 80: 502.
Rozen MG & Snyder CA (1985) Protracted exposure of C57B1/6 mice to 300
ppm benzene depresses B- and T-lymphocyte numbers and mitogen
responses. Evidence for thymic and bone marrow proliferation in
response to the exposures. Toxicology, 37(1-2): 13.
Rozen MG, Snyder CA, & Albert RE (1984) Depressions in B- and
T-lymphocyte mitogen-induced blastogenesis in mice exposed to low
concentrations of benzene. Toxicol Lett, 20: 343.
Rushton L & Alderson M (1981a) An epidemiological survey of eight oil
refineries in Britain. Br J Ind Med, 38: 225-234.
Rushton L & Alderson M (1981b) Case control study to investigate the
association between exposure to benzene and deaths from leukemia in
oil refinery workers. Br J Cancer, 43: 77-84.
Sabourin PJ, Chen BT, Lucier G, Birnbaum LS, Fisher E, & Henderson RF
(1987) Effect of dose on the absorption and excretion of [14C]
benzene administered orally or by inhalation in rats and mice. Toxicol
Appl Pharmacol, 87: 325-336.
Sabourin PJ, Bechtold WE, & Henderson RF (1988) A high pressure liquid
chromatographic method for the separation and quantitation of
water-soluble radiolabeled benzene metabolites. Anal Biochem, 170:
316-327.
Sabourin PJ, Sun JD, MacGregor JT, Wehr CM, Birnbaum LS, Lucier G, &
Henderson RF (1990) Effect of repeated benzene inhalation exposures on
benzene metabolism, binding to hemoglobin, and induction of
micronuclei. Toxicol Appl Pharmacol, 103: 452-462.
SAC (1989) Analysis of potentially dangerous substances in UK water
(Prepared under contract to DOE). London, SAC Scientific Ltd (DOE
Reference No. PECD 7/7/306).
Sandmeyer EE (1981) Aromatic hydrocarbons. In: Clayton GD & Clayton FE
ed. Patty's Industrial hygiene and toxicology, 3rd revis ed. New York,
Interscience Publishers, vol 2, pp 3253-3283.
Sasiadek M, Jagielski J, & Smolik R (1989) Localization of breakpoints
in the karyotype of workers professionally exposed to benzene. Mutat
Res, 224: 235-240.
Sauer TC Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Org Geochem, 3: 91-101.
Sawahata T & Neal RA (1983) Biotransformation of phenol to
hydroquinone and catechol by rat liver microsomes. Mol Pharmacol, 23:
453-460.
Schrenk D & Bock KW (1990) Metabolism of benzene in rat hepatocytes.
Influence of inducers on phenol glucuronidation. Drug Metab Dispos,
18(5): 720-725.
Seixas G, Andon BM, Hollingshead PG, & Thilly WG (1982) The aza-arenes
as mutagens for Salmonella typhimurium. Mutat Res, 102: 201-212.
Sellyei M & Kelemen E (1971) Chromosome study in a case of
granulocytic leukemia with "pelgerisation" seven years after benzene
pancytopenia. Eur J Cancer, 7: 83-85.
Shah JJ & Singh HB (1988) Distribution of volatile organic chemicals
in outdoor and indoor air. A national VOCs data base. Environ Sci
Technol, 22(12): 1381-1388.
Simic MG, Bergtold DS, & Karam LR (1989) Generation of oxy radicals in
biosystems. Mutat Res, 214: 3-12.
Singh HB, Salas L, & Stiles RE (1982) Distribution of selected gaseous
organic mutagens and suspected carcinogens in ambient air. Environ Sci
Technol, 16: 872.
Siou G, Conan L, & El Haitem M (1981) Evaluation of the clastogenic
action of benzene by oral administration with 2 cytogenetic techniques
in mouse and Chinese hamster. Mutat Res, 90: 273-278.
Skowronski GA, Turkall RM, & Abdel-Rahman MS (1988) Soil adsorption
alters bioavailability of benzene in dermally exposed male rats. Am
Ind Hyg Assoc J, 49(10): 506-511.
Smart RC & Zannoni VG (1985) Effect of ascorbate on covalent binding
of benzene and metabolites to isolated tissue preparations. Toxicol
Appl Pharmacol, 77: 334-343.
Snyder CA (1987) Benzene in Ethel Browning's toxicity and metabolism
of industrial solvents. Volume 1: Hydrocarbons, 2nd ed. Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp 3-37.
Snyder R, Lee EW, & Kocsis JJ (1978a) Binding of labelled metabolites
to mouse liver and bone marrow. Res Commun Chem Pathol Pharmacol, 20:
191-194.
Snyder CA, Goldstein BD, Sellakumar A, Wolman S, Bromberg I, Erlichman
MN, & Laskin S (1978b) Hematotoxicity of inhaled benzene to Sprague
Dawley rats and AKR mice at 300 ppm. J Toxicol Environ Health, 4:
605-618.
Snyder CA, Goldstein BD, Sellakumar A, Bromberg I, Laskin S, & Albert
RE (1980) The inhalation toxicology of benzene: incidence of
hematopoietic neoplasms and hematotoxicity in AKR/J and C57BL/6J mice.
Toxicol Appl Pharmacol, 54: 323-331.
Snyder R, Longacre SL, Witmer CM, & Kocsis JJ (1981) Biochemical
toxicology of benzene. In: Hodgson E, Bend JR, & Philpot RM ed.
Reviews in biochemical toxicology 3. Amsterdam, Oxford, New York,
Elsevier/North Holland, pp 123-153.
Snyder CA, Goldstein BD, Sellakumar AR, & Albert RE (1984) Evidence
for hematotoxicity and tumorigenesis in rats exposed to 100 ppm
benzene. Am J Ind Med, 5: 429-434.
Snyder R, Jowa L, Witz G, Jakf G, & Rushmore T (1987) Formation of
reactive metabolites from benzene. Arch Toxicol, 60(1-3): 61-64.
Snyder CA, Sellakumar AR, James DJ, & Albert RE (1988) The
carcinogenicity of discontinuous inhaled benzene exposures in CD-1 and
C57B1/6 mice. Arch Toxicol, 62: 331-335.
Snyder R, Dimitriadis E, Guy R, Hu P, Cooper K, Bauer H, Witz G, &
Goldstein BD (1989) Studies on the mechanism of benzene toxicity.
Environ Health Perspect, 82: 31-35.
Staples CA, Werner AF, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4: 131-142.
Stommel P, Müller G, Stücker W, Verkoyen C, Schöbel S, & Norpoth K
(1989) Determination of S-phenylmercapturic acid in the urine - an
improvement in the biological monitoring of benzene exposure.
Carcinogenesis, 10: 279-282.
Stoner RD, Drew RT, & Bernstein DM (1981) Benzene inhalation effect
upon tetanus antitoxin responses and leukemogenesis in mice. In:
Mahlum DD, Gray RH, & Felix WD ed. Coal conversion and the
environment. Oak Ridge, Tennessee, US Department of Energy, Technical
Information Center, pp 445-461.
Styles JA & Richardson CR (1984) Cytogenetic effects of benzene:
dosimetric studies on rats exposed to benzene vapor. Mutat Res, 135:
203-209.
Sun JD, Medinsky MA, & Birnbaum LS (1990) Benzene hemoglobin adducts
in mice and rats: Characterization of formation and physiological
modeling. Fundam Appl Toxicol, 15: 468-475.
Susten A, Dames B, & Burg J (1985) Percutaneous penetration of benzene
in hairless mice: An estimate of dermal absorption during
tire-building operations. Am J Ind Med, 7: 323-335.
Swaen GMH, Slangen JJM, Volovics A, Hayes RB, Scheffers T, & Sturmans
F (1991) Mortality of coke plant workers in the Netherlands. Br J Ind
Med, 48: 130-135.
Swenberg JA, Petzold GL, & Harbach PR (1976) In vitro DNA
damage/alkaline elution assay for predicting carcinogenic potential.
Biochem Biophys Res Commun, 72: 732-738.
Tatrai E, Ungvary GY, Hudak A, Rodics K, Loerincz M, & Barcza GY
(1980a) Concentration of dependence of the embryotoxic effects of
benzene inhalation in CFY rats. J Hyg Epidemiol Microbiol Immunol, 24:
363-371.
Tatrai E, Rodics K, & Ungvary GY (1980b) Embryotoxic effects of
simultaneously applied exposure of benzene and toluene. Folia Morphol
(Praha), 28: 286-289.
Thienes H & Haley TJ (1972) Clinical toxicology, 5th ed. Philadelphia,
Pennsylvania, Lea & Febiger, pp 124-127.
Tice RR, Costa DL, & Drew RT (1980) Cytogenetic effects of inhaled
benzene in murine bone marrow: Induction of sister chromatid
exchanges, chromosomal aberrations and cellular proliferation
inhibition in DBA/2 mice. Proc Natl Acad Sci (USA), 77: 2148-2152.
Tice RR, Vogt T, & Costa D (1982) Cytogenetic effects of inhaled
benzene in murine bone marrow. In: Genotoxic effects of airborne
agents. New York, London, Plenum Press, pp 257-275.
Tice RR, Luke CA, & Drew RT (1989) Effect of exposure route, regimen,
and duration on benzene-induced genotoxic and cytotoxic bone marrow
damage in mice. Environ Health Perspect, 82: 65-74.
Toft K, Olofsson T, Tunek A, & Berlin M (1982) Toxic effects on mouse
bone marrow caused by inhalation of benzene. Arch Toxicol, 51:
295-302.
Topham JC (1980) Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutat Res, 74: 379-387.
Tough IM & Court Brown WM (1965) Chromosome aberrations and exposure
to ambient benzene. Lancet, 1: 684.
Travis CC, Quillen JL, & Arms AD (1990) Pharmacokinetics of benzene.
Toxicol Appl Pharmacol, 102: 400-420.
Tsai SP, Wen CP, Weiss NS, Wong O, McClellan WA, & Gibson RL (1983)
Retrospective mortality and medical surveillance studies of workers in
benzene areas of refineries. J Occup Med, 25: 685-692.
Tucker WA, Huang C, & Bral JM (1986) Validation of transport model.
In: Benzene in Florida groundwater: an assessment of the significance
to human health. Tallahassee, Florida, Florida Petroleum Council,
American Petroleum Institute, pp 93-108.
Tully FP, Ravishankara AR, Thopmson RL, Nicovich JM, Shah RC, Kreutter
NM, & Wine PH (1981) Kinetics of the reactions of hydroxyl radicals
with benzene and toluene. J Phys Chem, 85: 2262.
Tunek A, Platt KL, Bentley P, & Oesch F (1978) Microsomal metabolism
of benzene to species irreversibly binding to microsomal protein and
effects of modification of this metabolism. Mol Pharmacol, 14:
920-929.
UBA (1982) Air quality criteria for benzene. Berlin, Federal Office
for the Environment (Report 6/82).
Ungvary G & Tatrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats and rabbits. Arch Toxicol, 8:
425-430.
US EPA (1985) Drinking water criteria document on benzene. Washington,
DC, US Environmental Protection Agency, Office of Drinking Water
(PB86-118122).
US EPA (1987) June-September, 6-9 AM, ambient air benzene
concentrations in 39 U.S. cities, 1984-1986. Research Triangle Park,
North Carolina, US Environmental Protection Agency, Atmospheric
Sciences Research Laboratory (EPA/600/D-87/160).
US EPA (1989) National emissions standards for hazardous air
pollutants: Benzene emissions from maleic anhydride plants,
ethylbenzene styrene plants, benzene storage vessels, benzene
equipment leaks, and coke by-product recovery plants. Fed Reg, 54:
38044-38139.
Uyeki EM, Ashkar AE, Shoeman DW, & Bisel TU (1977) Acute toxicity of
benzene inhalation to hemopoietic precursor cells. Toxicol Appl
Pharmacol, 40: 49-57.
Vaishnav DD & Babeu L (1987) Comparison of occurrence and rates of
chemical biodegradation in natural waters. Bull Environ Contam
Toxicol, 39: 237-244.
Venitt S (1985) Summary report on the performance of the bacterial
mutation assays. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr,
Margolin BH, Matter BE, & Shelby MD ed. Evaluation of short-term tests
for carcinogens. Report of the International Programme on Chemical
Safety's collaborative study on in vitro assays. Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp 11-23 (Progress in Mutation
Research, Volume 5).
Vigliani EC & Saita G (1964) Benzene and leukemia. New Engl J Med,
271(17): 872-876.
Vogel K, Bentley P, Platt KL, & Oesch F (1980) Rat liver cytoplasmic
dihydrodiol dehydrogenase. J Biol Chem, 255: 9621-9625.
Wallace LA (1989a) The exposure of the general population to benzene.
Cell Biol Toxicol, 5(3): 197.
Wallace LA (1989b) Major sources of benzene exposure. Environ Health
Perspect, 82: 165-169.
Wallace LA & Pellizzari ED (1986) Personal air exposures and breath
concentrations of benzene and other volatile hydrocarbons for smokers
and nonsmokers. Toxicol Lett, 35: 113-116.
Wallace LA, Pellizzari E, & Hartwell TD (1985) Personal exposures,
indoor-outdoor relationships, and breath levels of toxic air
pollutants measured for 355 persons in New Jersey. Atmos Environ, 19:
1651-1661.
Wallace LA, Pellizzari E, Leader B, Zelon H, & Sheldon L (1987)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Wallin H, Melin P, Schelin C, & Jergil B (1985) Evidence that covalent
binding of metabolically activated phenol to microsomal proteins is
caused by oxidised products of hydroquinone and catechol. Chem-Biol
Interact, 55: 335-346.
Ward CO, Kuna RA, Snyder NK, Alsaker RD, Coate WB, & Craig PH (1985)
Subchronic inhalation toxicity of benzene in rats and mice. Am J Ind
Med, 7: 457.
Wathne BM (1983) Measurements of benzene, toluene and xylenes in urban
air. Atmos Environ, 17: 1713.
Weaver NK, Gibson RL, & Smith CW (1983) Occupational exposure to
benzene in the petroleum and petrochemical industries. Adv Mod Environ
Toxicol, 4: 63-75.
Wermuth B, Platts K, Seidel A, & Oesch F (1986) Carbonyl reductase
provides the enzymatic basis of quinone reduction in man. Biochem
Pharmacol, 35: 1277.
WHO (1984) Guidelines for drinking-water quality. Volume 1:
Recommendations. Geneva, World Health Organization, 130 pp.
WHO (1987) Benzene. In: Air quality guidelines for Europe. Copenhagen,
World Health Organization, Regional Office for Europe, pp 45-58 (WHO
Regional Publications, European Series No. 23).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations. Geneva, World Health Organization.
Wilson RH (1942) Benzene poisoning in industry. J Lab Clin Med, 27:
1517-1521.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformation of selected
alkylbenzenes and halogenated hydrocarbons in methanogenic aquifer
material: A microcosm study. Environ Sci Technol, 20: 997-1002.
Windholz M, Budavari S, Blumetti RF, & Otterbein ES (1983) The Merck
Index: An encyclopedia of chemicals, drugs, and biologicals, 10th ed.
Rahway, New Jersey, Merck and Co., Inc., p 151.
Winek CL, Collom WD, & Wecht CH (1967) Fatal benzene exposure by glue
sniffing. Lancet, March 25: 683
Winek CL & Collom WD (1971) Benzene and toluene fatalities. J Occup
Med, 13: 259-261.
Withey RJ & Hall JW (1975) The joint action of perchloroethylene with
benzene or toluene in rats. Toxicology, 4: 5-15.
Witz G, Gad SC, Tice RR, Oshiro Y, Piper CE, & Goldstein BD (1990a)
Genetic toxicity of the benzene metabolite trans,trans-muconaldehyde
in mammalian and bacterial cells. Mutat Res, 240: 295-306.
Witz G, Maniara W, Mylavarapu V, & Goldstein BD (1990b) Comparative
metabolism of benzene and trans,trans-muconaldehyde to
trans,trans-muconic acid in DBA/2N and C57Bl/6 mice. Biochem
Pharmacol, 40: 1275-1280.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene. Arch
Ind Health, 14: 387-398.
Wong O (1987) An industry wide mortality study of chemical workers
occupationally exposed to benzene: II. Dose response analyses. Br J
Ind Med, 44: 382-395.
Yardley-Jones A, Anderson D, Jenkinson PC, Lovell DP, Blowers SD, &
Davies MJ (1988) Genotoxic effects in peripheral blood and urine of
workers exposed to low level benzene. Br J Ind Med, 45: 694-700.
Yardley-Jones A, Anderson D, Lovell DP, & Jenkinson PC (1990)
Analysis of chromosomal aberrations in workers exposed to low level
benzene. Br J Ind Med, 47: 48-51.
Yin S-N, Li G-L, Tain F-D, Fu Z-I, Jin C, Chen Y-J, Luo S-J, Ye P-Z,
Zhang J-Z, Wang G-C, Zhang X-C, Wu H-N, & Zhong Q-C (1987) Leukaemia
in benzene workers: a retrospective cohort study. Br J Ind Med, 44:
124-128.
Yin S-N, Li G-L, Tain F-D, Fu Z-I, Jin C, Chen Y-J, Luo S-J, Ye P-Z,
Zhang J-Z, Wang G-C, Zhang X-C, Wu H-N, & Zhong Q-C (1989) A
retrospective cohort study of leukemia and other cancers in benzene
workers. Environ Health Perspect, 82: 207-213.
Young RJ, Rinsky RA, Infante PF, & Wagoner JK (1978) Benzene in
consumer products. Science, 199: 248.
Zhurkov VS, Fel'dt EG, & Kosyakov VV (1983) Dependence of the
frequency of chromosomal aberrations in mouse bone marrow cells on
concentration (dose) and mode of administration of benzene. Bull Exp
Biol Med, 96: 1741-1743.
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le benzène est un liquide incolore, stable à la température
ambiante et sous la pression atmosphérique normale. Il possède une
odeur aromatique caractéristique et du fait de son bas point
d'ébullition (80,1 °C) et de sa forte tension de vapeur, il s'évapore
rapidement et il est très inflammable. Il est légèrement soluble dans
l'eau mais miscible à la plupart des solvants organiques.
Il existe des méthodes qui permettent de rechercher la présence
de benzène dans différents milieux (air, eau, organes/tissus). On
peut utiliser à cette fin la chromatographie en phase gazeuse avec, au
choix, une détection par ionisation de flamme, par photoionisation ou
par spectrométrie de masse, en fonction de la sensibilité nécessaire
et des concentrations attendues. La recherche du benzène sur le lieu
de travail s'effectue généralement par adsorption sur charbon actif
puis analyse par chromatographie en phase gazeuse couplée à la
spectrométrie de masse après désorption. Lorsqu'on peut se contenter
d'une sensibilité de l'ordre du mg/m3, on peut utiliser des
instruments à lecture directe et des dosimètres passifs. Si l'on
désire une meilleure sensibilité, il existe des méthodes qui
permettent de déceler la présence de benzène à des concentrations ne
dépassant pas 0,01 µg/m3 d'air ou 1 ng/kg de terre ou d'eau.
2. Sources d'exposition humaine
Le benzène existe à l'état naturel dans le pétrole brut à des
concentrations pouvant atteindre 4 g/litre. Il est également produit
partout dans le monde en quantités extrêmement importantes (14,8
millions de tonnes). Des émissions peuvent se produire lors du
traitement des produits pétroliers, de la cokéfaction du charbon, de
la production de toluène, de xylène et autres dérivés aromatiques
ainsi que lorsqu'il est utilisé dans certains produits de
consommation, comme intermédiaire ou comme constituant de l'essence.
3. Transport, distribution et transformation dans l'environnement
Dans l'air, le benzène est présent essentiellement en phase
gazeuse, et sa durée de séjour varie de quelques heures à quelques
jours, en fonction de l'environnement et du climat et aussi de la
concentration des radicaux hydroxyles, des oxydes d'azote et de
soufre. Lorsqu'il est éliminé de l'air par la pluie, il peut
contaminer les eaux de surface et les eaux souterraines dans
lesquelles il est soluble à raison d'environ 1000 mg/litre.
En raison principalement de sa volatilisation dans l'atmosphère,
le temps de séjour du benzène dans l'eau est limité à quelques heures
et il est peu ou pas adsorbé par les sédiments.
Le benzène présent dans le sol peut passer dans l'air par
volatilisation et dans les eaux de surface par ruissellement. En cas
d'enfouissement ou de libération à profondeur importante, il passe
dans les eaux souterraines.
En aérobiose, le benzène présent dans l'eau ou dans le sol est
rapidement dégradé (quelques heures) par les bactéries en lactate et
en pyruvate avec formation intermédiaire de phénol et de catéchol.
Cependant, en anaérobiose (par exemple dans les eaux souterraines) la
dégradation bactérienne prend des semaines, voire des mois et non plus
des heures. Il y a persistance du benzène lorsque la dégradation
bactérienne ne se produit pas. Il ne semble pas subir de
bioconcentration ou de bioaccumulation dans les organismes aquatiques
ou terrestres.
4. Concentrations dans l'environnement et exposition humaine
La présence de benzène dans l'essence et sa large utilisation
comme solvant industriel peuvent conduire à des émissions importantes
un peu partout dans l'environnement. A l'extérieur, les
concentrations vont de 0,2 µg/m3 dans les régions rurales écartées
à 349 µg/m3 dans les zones industrielles où la circulation
automobile est dense. Lors du remplissage du réservoir d'un véhicule
à moteur, les concentrations peuvent atteindre 10 mg/m3.
On a décelé la présence de benzène à des concentrations
atteignant 500 µg/m3 dans l'air intérieur des pièces de séjour. La
fumée de cigarette contribue de façon importante à la présence de
benzène dans l'air intérieur, les fumeurs inhalant environ 1800 µg de
benzène par jour contre 50 µg pour les non fumeurs.
Dans de nombreux pays, l'exposition professionnelle dépasse
rarement 15 mg/m3 en moyenne pondérée par rapport au temps.
Toutefois, les concentrations effectives rapportées dépendent du type
d'industries étudiées et elles peuvent être beaucoup plus élevées dans
les pays en voie de développement industriel.
L'eau et les aliments ne contribuent que pour une faible part à
l'apport journalier de benzène chez les adultes non fumeurs (entre 3
et 24 µg/kg de poids corporel et par jour).
5. Cinétique et métabolisme
Le benzène est bien absorbé chez l'homme et les animaux de
laboratoire après exposition par voie orale ou respiratoire, mais chez
l'homme, le benzène n'est que faiblement absorbé par voie percutanée.
L'absorption se produit chez l'homme à hauteur d'environ 50% lors
d'expositions continues pendant plusieurs heures à des concentrations
de 163 à 326 mg/m3. On a constaté qu'après quatre heures
d'exposition à 170-202 mg/m3, le benzène était retenu par
l'organisme humain dans la proportion d'environ 30%, 16% de cette dose
étant rejetés tels quels dans l'air expiré. Le benzène inhalé est
davantage retenu par l'organisme féminin que par l'organisme masculin.
Le benzène à tendance à s'accumuler dans les tissus à forte teneur en
lipides et il traverse la barrière placentaire.
Le métabolisme du benzène s'effectue principalement dans le foie,
essentiellement par l'intermédiaire du système enzymatique du
cytochrome P-450 IIE1 et il comporte la formation d'une série de
métabolites réactifs instables. Chez les rongeurs, les processus de
formation de deux métabolites toxiques supposés, la benzoquinone et le
muconaldéhyde, se révèlent être saturables. Ce phénomène peut avoir
des conséquences importantes en ce qui concerne les relations
dose-réponse car la proportion de benzène transformée en métabolites
toxiques sera plus forte à faibles doses qu'à doses élevées. Les
produits du métabolisme sont principalement excrétés dans les urines.
Les métabolites reconnus du benzène: phénol, catéchol et hydroquinone
- se retrouvent en quantité appréciable dans la moelle osseuse. Le
phénol est le principal métabolite urinaire chez l'homme et on le
retrouve dans l'urine, essentiellement sous forme de sulfoconjugué
jusqu'à ce que les concentrations atteignent 480 mg/litre, après quoi
on observe la formation de glucuronides. D'après des études récentes,
la toxicité du benzène serait due à l'interaction des différents
métabolites de ce composé qui se forment tant dans le foie que dans la
moelle osseuse.
Une fois inhalé, le benzène se fixe à l'ADN du foie chez le rat
à raison de 2,38 µmol/mol d'ester phosphorique. On a décelé dix
adduits de désoxyguanosine et un adduit de désoxyadénine dans l'ADN
mitochondrial de la moelle osseuse du lapin.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
6.1 Toxicité générale
Le benzène ne présente qu'une faible toxicité aiguë chez diverses
espèces animales, les valeurs de la DL50 après exposition orale
allant de 3000 à 8100 mg/kg de poids corporel chez le rat, par
exemple. On a fait état de valeurs de la CL50 allant de 15 000
mg/m3 (8 h) chez la souris à 44 000 mg/m3 (4 h) chez le rat.
Le benzène est modérément irritant pour la muqueuse oculaire et
provoque une irritation dermique chez le lapin après plusieurs
applications de produit non dilué. On ne dispose d'aucune donnée sur
le pouvoir de sensibilisation cutanée du benzène.
L'exposition de souris à du benzène par la voie respiratoire
entraîne une baisse sensible de certains paramètres hématologiques tel
que l'hématocrite, le taux d'hémoglobine ainsi que le nombre
d'érythrocytes, de leucocytes et de plaquettes. Une exposition de
longue durée à de fortes doses provoque une aplasie médullaire. Chez
le rat les effets sont analogues mais moins graves.
6.2 Génotoxicité et cancérogénicité
Les tests de mutagénicité in vitro ont donné des résultats
négatifs.
En ce qui concerne les études in vivo, on observe que le
benzène et ses métabolites entraînent des aberrations dans la
structure et le nombre des chromosomes chez l'homme et les animaux de
laboratoire. En outre, l'administration de benzène provoque des
échanges entre chromatides soeurs et la formation d'érythrocytes
polychromatiques avec micronoyaux. Après administration par la voie
intrapéritonéale, le benzène peut atteindre les cellules germinales
comme le montrent les anomalies observées dans la morphologie de la
tête des spermatozoïdes.
On a fait état de la formation de plusieurs types de cancers dus
au benzène chez le rat et la souris après administration par voie
orale ou exposition par la voie respiratoire. Il s'agit de divers
types de tumeurs malignes épithéliales concernant par exemple la
glande de Zymbal, le foie, le tissu mammaire et les fosses nasales,
avec en outre quelques lymphomes et leucémies.
Dans les études comportant une exposition par inhalation et au
cours desquelles on a relevé effectivement une action cancérogène, les
doses allaient de 100 à 960 mg/m3, cinq à sept heures par jour et
cinq jours par semaine. L'administration par voie orale de benzène à
des doses allant de 25 à 500 mg/kg de poids corporel à des souris et
à des rats, a entraîné la formation de néoplasmes. La durée
d'exposition était généralement de un à deux ans.
6.3 Effets toxiques sur la reproduction; embryotoxicité et tératogénicité
Le benzène traverse facilement la barrière placentaire. De
nombreuses expériences au cours desquelles des animaux de laboratoire
ont été soumis à des doses atteignant même les valeurs toxiques pour
la mère n'ont pas permis de recueillir de données indicatives d'un
effet tératogène. Toutefois, on a montré que le benzène était
foetotoxique après exposition par la voie respiratoire, chez la souris
(1600 µg/m3, sept heures par jour, du sixième au quinzième jours de
la gestation) et le lapin.
6.4 Immunotoxicité
Le benzène réduit l'aptitude des lymphocytes B et T à proliférer.
Chez plusieurs espèces d'animaux de laboratoire exposés au benzène, on
a noté une diminution de la résistance de l'hôte aux infections.
7. Effets sur l'homme
On sait que le benzène produit un certain nombre d'effets nocifs
pour la santé humaine. Le plus fréquemment cité de ces effets est une
dépression médullaire qui conduit à une anémie aplasique.
L'exposition à de fortes doses de benzène entraîne probablement une
forte incidence de ces maladies.
Il est bien établi que le benzène est cancérogène pour l'homme.
Des études épidémiologiques effectuées sur les travailleurs exposés au
benzène ont montré qu'il existait une relation causale entre
l'exposition à cette substance et l'apparition d'une leucémie
myéloïde. La relation qui a été observée entre l'exposition au
benzène et l'apparition de lymphomes ou de myélomes multiples reste à
élucider.
Le Groupe de travail a estimé que les données épidémiologiques ne
permettent pas de distinguer entre (a) une faible augmentation de la
mortalité par leucémie chez les travailleurs exposés à de faibles
doses de benzène et (b) une situation où le risque n'existe pas.
8. Conclusions
Le Groupe a conclu qu'une exposition moyenne pondérée par rapport
au temps de l'ordre de 3,2 mg/m3 (1 ppm) au cours d'une carrière de
40 ans, n'entraîne pas, statistiquement parlant, de surmortalité par
leucémie. Toutefois, comme le benzène est cancérogène pour l'homme,
il convient de limiter l'exposition à la dose la plus faible
compatible avec les exigences techniques. Il convient d'éviter
également tout accroissement de l'exposition au-delà de la valeur de
32 mg/m3 (10 ppm). Le benzène et les produits qui en contiennent,
comme l'essence, ne doivent jamais être utilisés comme agents de
nettoyage.
On admet traditionnellement que la dépression médullaire,
c'est-à-dire une anémie, une leucopénie ou une thrombocytopénie,
observées sur les lieux de travail, constituent le premier stade d'une
intoxication par le benzène et que ces affections sont liées à la
dose. En d'autres termes, plus la dose est élevée, plus la
probabilité d'observer une réduction des éléments figurés du sang est
élevée.
L'exposition à de fortes doses de benzène (160 à 320 mg/m3)
pendant un an entraînerait selon toute probabilité une toxicité
médullaire chez une proportion importante des travailleurs, voire une
anémie aplasique chez certains d'entre eux, mais les effets ne
seraient guère marqués à plus faibles doses. Une exposition à de
faibles et fortes doses devrait entraîner une intoxication benzénique
au bout de dix années d'exposition continue. On peut donc dire qu'à
fortes doses, on observerait un nombre élevé de cas de dépression
médullaire et d'anémie aplasique avec également quelques lésions à
faibles doses. Au cas où l'on observerait l'un quelconque de ces
effets quel que soit le niveau d'exposition, il faudrait prendre des
mesures pour améliorer le contrôle de cette exposition.
Rien d'indique que le benzène soit tératogène à des doses plus
faibles que celles qui sont toxiques pour la mère, toutefois on a
observé une toxicité foetale.
La neurotoxicité et l'immunotoxicité du benzène n'ont pas été
bien étudiées, ni chez l'animal de laboratoire ni chez l'homme.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El benceno es un líquido incoloro y estable a temperatura
ambiente y presión atmosférica normal. Posee un olor aromático
característico, un punto de ebullición relativamente bajo (80,1 °C) y
una elevada presión de vapor, lo que hace que se evapore rápidamente
a temperatura ambiente, y es altamente inflamable. Es ligeramente
soluble en agua, pero también es miscible en la mayoría de los otros
disolventes orgánicos.
Se dispone de métodos analíticos para detectar benceno en
diversos medios (aire, agua, órganos/tejidos). La elección entre
cromatografía de gases (CG), con detección mediante ionización de
llama o fotoionización, o espectrometría de masas (EM) depende de la
sensibilidad requerida y de los niveles de benceno previstos. La
detección de benceno en el lugar de trabajo se realiza normalmente
mediante captación con carbón vegetal, desorción, y análisis por CG o
EM. Si es suficiente una sensibilidad del orden de mg/m3, pueden
emplearse instrumentos portátiles de lectura directa y dosímetros
pasivos. Para los casos en que hace falta una mayor sensibilidad, se
han notificado métodos válidos para detectar benceno a niveles de sólo
0,01 µg/m3 (aire) o 1 ng/kg (suelo o agua).
2. Fuentes de exposición humana
El benceno es un producto químico natural, que se halla en el
petróleo crudo a niveles de hasta 4 g/litro. Además, es producido en
muy grandes cantidades (14,8 millones de toneladas) en todo el mundo.
Se producen emisiones de benceno durante el procesamiento de los
productos petroleros, durante la producción de coque a partir de
carbón, durante la producción de tolueno, xileno y otros compuestos
aromáticos, y como consecuencia de su empleo en productos de consumo,
como compuesto intermedio y como componente de la gasolina.
3. Transporte, distribución y transformación en el medio ambiente
El benceno presente en el aire se halla predominantemente en la
fase de vapor, y su tiempo de persistencia varía entre unas horas y
unos días, según el entorno y el clima, y en función de la
concentración de radicales hidroxilo, así como de dióxidos de azufre
y de nitrógeno. El benceno presente en el aire es eliminado por la
lluvia, con la consiguiente contaminación de las aguas superficiales
y subterráneas, en las que es soluble a razón de aproximadamente 1000
mg/litro.
Debido fundamentalmente a la volatilización, el tiempo de
persistencia del benceno en el agua es de unas cuantas horas, y su
adsorción por los sedimentos es escasa o nula.
El benceno presente en el suelo puede pasar al aire por
volatilización, y a las aguas superficiales por la escorrentía. Si es
enterrado o liberado muy por debajo de la superficie, será
transportado hasta las aguas subterráneas.
En condiciones aerobias el benceno presente en el agua o el suelo
es rápidamente (en cuestión de horas) degradado por las bacterias a
lactato y piruvato, previa transformación en los productos intermedios
fenol y catecol. En cambio, en condiciones anaerobias (por ejemplo en
las aguas subterráneas) la degradación bacteriana requiere semanas o
meses en lugar de horas. Si no hay degradación bacteriana el benceno
puede persistir. No hay pruebas de una bioconcentración o
bioacumulación de benceno en organismos acuáticos o terrestres.
4. Niveles ambientales y exposición humana
La presencia de benceno en la gasolina y su amplio uso como
disolvente industrial puede dar lugar a emisiones importantes y
generalizadas al medio ambiente. Sus niveles ambientales al aire
libre oscilan entre los 0,2 µg/m3 hallados en zonas rurales aisladas
y los 349 µg/m3 detectados en centros industriales con alta densidad
de tráfico de automóviles. Durante las operaciones de
reabastecimiento de combustible de los automóviles se han llegado a
registrar niveles de hasta 10 mg/m3.
En el aire del interior de las viviendas se han detectado niveles
de benceno de hasta 500 µg/m3. El humo del tabaco contribuye con
importantes cantidades de benceno a los niveles registrados en el aire
de los espacios interiores, cifrándose las cantidades inhaladas por
los fumadores en aproximadamente 1800 µg de benceno al día, frente a
50 µg/día los no fumadores.
En numerosos países la exposición ocupacional rara vez supera una
media ponderada respecto al tiempo de 15 mg/m3. Sin embargo, los
niveles reales notificados dependen de la industria estudiada, y en
algunos países en fase de desarrollo industrial las exposiciones
pueden ser considerablemente superiores.
El benceno transmitido por el agua y los alimentos representa
sólo un pequeño porcentaje de la ingesta diaria total de los adultos
no fumadores (entre unos 3 y 24 µg/kg de peso corporal al día).
5. Cinética y metabolismo
El benceno es fácilmente absorbido por el hombre y los animales
experimentales que entran en contacto con el producto por exposición
oral o inhalación, pero en la especie humana la absorción cutánea es
escasa. Con una exposición continua a niveles de 163-326 mg/m3
durante varias horas, la absorción en el hombre es de aproximadamente
un 50%. Tras una exposición de 4 horas a niveles de 170-202 mg/m3,
la retención en el organismo humano fue de aproximadamente un 30%,
habiéndose excretado un 16% de la dosis retenida en forma de benceno
inalterado a través del aire expirado. Las mujeres suelen retener un
mayor porcentaje del benceno inhalado que los hombres. El benceno
tiende a acumularse en los tejidos que contienen gran cantidad de
lípidos y atraviesa la placenta.
El metabolismo del benceno se produce principalmente en el
hígado, depende básicamente del sistema enzimático del citocromo P-450
IIE1 y conlleva la formación de una serie de metabolitos reactivos
inestables. En los roedores la formación de dos presuntos metabolitos
tóxicos, la benzoquinona y el muconaldehído, parece ser saturable, lo
que puede tener gran importancia desde el punto de vista de la
relación dosis-respuesta, pues significa que a dosis bajas la
proporción de benceno transformada en metabolitos tóxicos será mayor
que a dosis altas. Los productos metabólicos son excretados
principalmente por la orina. En la médula ósea se observan niveles
importantes de los conocidos metabolitos fenol, catecol e
hidroquinona. En el hombre el metabolito urinario predominante es el
fenol, que aparece sobre todo conjugado con sulfato como éter a
niveles inferiores a 480 mg/litro, concentración a la cual se empiezan
a detectar glucurónidos. Estudios recientes llevan a pensar que la
toxicidad del benceno se debe a la interacción de varios metabolitos
bencénicos formados tanto en el hígado como en la médula ósea.
Se ha observado que el benceno inhalado se une al ADN hepático de
la rata a razón de 2,38 µmoles por mol de fosfato de ADN. En el ADN
mitocondrial de la médula ósea de conejo se han detectado siete
aductos de desoxiguanosina y un aducto de desoxiadenina.
6. Efectos en los mamíferos de laboratorio y en las pruebas in vitro
6.1 Toxicidad sistémica
El benceno tiene al parecer una toxicidad aguda baja en diversas
especies animales, oscilando las DL50 por exposición oral en la rata
entre 3000 y 8100 mg/kg de peso corporal. Las CL50 notificadas
oscilan entre los 15 000 mg/m3 (8 h) del ratón y los 44 000 mg/m3
(4 h) de la rata.
El benceno tiene un efecto irritante moderado sobre los ojos, y
aplicado reiteradamente y sin diluir también es irritante para la piel
del conejo. No se dispone de información sobre el potencial de
sensibilización cutánea del benceno.
Los ratones expuestos a benceno por inhalación presentan una
disminución importante del valor de parámetros hemáticos tales como el
hematocrito, la hemoglobina y el número de eritrocitos, leucocitos y
plaquetas. La exposición prolongada a altas dosis provoca aplasia de
la médula ósea. También en la rata se han observado efectos
similares, aunque menos graves.
6.2 Genotoxicidad y carcinogenicidad
Las pruebas de mutagenicidad del benceno in vitro han arrojado
resultados negativos.
En los estudios in vivo el benceno o sus metabolitos causan
aberraciones cromosómicas tanto estructurales como numéricas en el
hombre y en los animales de laboratorio. La administración de
benceno, además, da lugar a intercambios entre cromatidios hermanos y
a la producción de eritrocitos policromáticos con micronúcleos.
Administrado interperitonealmente el benceno puede alcanzar las
células germinales, como demuestra la aparición de alteraciones
morfológicas de la cabeza de los espermatozoides.
Se ha notificado que la administración oral o la inhalación de
benceno provocan tanto en la rata como en el ratón varios tipos de
neoplasma, entre ellos diversos tipos de neoplasma epitelial, por
ejemplo de la glándula de Zymbal, hígado, tejido mamario y cavidades
nasales, y algunos linfomas y leucemias.
En los estudios en que se observó una respuesta carcinógena
positiva a la inhalación, los niveles de exposición oscilaban entre
100 y 960 mg/m3 durante 5 a 7 h/día, cinco días por semana. En el
ratón y la rata, la administración oral de benceno a dosis de 25-500
mg/kg de peso corporal provocó la aparición de neoplasmas; la duración
de la exposición fue por lo general de 1 a 2 años.
6.3 Toxicidad en la reproducción, embriotoxicidad y teratogenicidad
El benceno atraviesa libremente la barrera placentaria. Tras
numerosos experimentos realizados con animales a dosis incluso tóxicas
para la madre, no se ha obtenido ningún dato que demuestre que tenga
efectos teratógenos. No obstante, se ha demostrado que su inhalación
tiene efectos fetotóxicos en el ratón (1600 µg/m3, 7 h/día, días 6
a 15 de gestación) y en el conejo.
6.4 Inmunotoxicidad
El benceno deprime la capacidad de proliferación de los
linfocitos B y T. Se ha observado una menor resistencia a las
infecciones en varias especies de laboratorio expuestas al benceno.
7. Efectos en el ser humano
Es sabido que el benceno tiene varios efectos perjudiciales para
la salud, entre los que destaca por su frecuencia la depresión de la
médula ósea, que conduce a la anemia aplásica. Unos niveles altos de
exposición hacen probable una elevada incidencia de esas enfermedades.
Está demostrado que el benceno tiene un efecto carcinógeno en el
ser humano. Los estudios epidemiológicos realizados sobre
trabajadores expuestos al benceno han demostrado la existencia de una
relación causal entre la exposición al benceno y la incidencia de
leucemia mielógena. Resta por aclarar si existe también una relación
entre la exposición al benceno y la aparición de linfoma y mieloma
múltiple.
El Grupo de Estudio era de la opinión de que los datos
epidemiológicos no permiten distinguir entre a) un ligero aumento de
la mortalidad por leucemia entre los trabajadores expuestos a niveles
bajos de benceno, y b) una situación sin riesgo.
8. Conclusiones
Se llegó a la conclusión de que una media ponderada respecto al
tiempo de 3,2 mg/m3 (1 ppm) a lo largo de 40 años de trabajo no
determina un aumento estadísticamente significativo del número de
defunciones por leucemia. Debido a sus efectos carcinógenos sobre el
hombre, sin embargo, las exposiciones se deberán limitar al nivel
técnicamente más bajo posible. Deberán evitarse las exposiciones
superiores a 32 mg/m3 (10 ppm). El benceno y los productos que lo
contienen, como la gasolina, no se deberían emplear nunca en
operaciones de limpieza.
Tradicionalmente se ha considerado que la aparición de depresión
de médula ósea - esto es, de anemia, leucopenia o trombocitopenia - en
el lugar de trabajo representa la primera fase de toxicidad del
benceno. Esa manifestación obedece al parecer a una relación
dosis-respuesta; en otras palabras, cuanto mayor la dosis, mayor
también la probabilidad de observar una disminución del número de
células sanguíneas circulantes.
La exposición a altos niveles de benceno (160-320 mg/m3)
durante un año tendría con toda probabilidad efectos tóxicos sobre la
médula ósea en un elevado porcentaje de los trabajadores, y provocaría
anemia aplásica en algunos casos, pero dosis menores apenas tendrían
efectos. En cambio, cabe prever que la exposición continua durante
diez años a dosis altas o bajas tendría efectos tóxicos. Así, con las
dosis elevadas se observaría una alta incidencia tanto de depresión de
la médula ósea como de anemia aplásica, y con las dosis más bajas se
observarían también algunas lesiones. La observación de cualquiera de
esos efectos, con independencia del nivel de exposición, será
reveladora de la necesidad de mejorar la vigilancia de la exposición
al benceno.
No hay indicios de que el benceno tenga efectos teratógenos a
dosis inferiores a las que resultan tóxicas para la madre, pero sí se
ha demostrado que tiene efectos tóxicos para el feto.
La neurotoxicidad y la inmunotoxicidad del benceno no han sido
suficientemente estudiadas ni en animales experimentales ni en el ser
humano.
the Zymbal gland. This is the first suggestion that conjugation
products, normally thought of as only a mechanism for urinary
excretion, could also be considered to act as a transporting mechanism
for bringing metabolites from the liver to target tissues.
Parke & Williams (1953) reported that phenyl mercapturic acid was
a urinary end product of benzene metabolism. This observation was
supported by the report of Jerina et al. (1968) who incubated
glutathione with rat liver cytoplasm and benzene oxide and found that
the principal metabolite was S-phenylglutathione. Norpoth (1988) has
developed a method for the determination of phenylmercapturic acid in
human urine as a measure of exposure to benzene based on these
observations. However, Lunte & Kissinger (1983) showed that
p-benzoquinone, an oxidation product of hydroquinone, forms
glutathione conjugates non-enzymatically. Lau et al. (1989) reported
that 1,2,3 or 4 glutathione molecules could conjugate with
p-benzoquinone. Nerland & Pierce (1990) showed the occurrence of
N-acetyl- S-(2,5-dihydroxyphenyl)l-cysteine as a urinary metabolite
of benzene in rats. Stommel et al. (1989) found that the metabolite
phenylmercapturic acid increased proportionally in rats and humans as
the inhaled dose rose to 1600 mg/m3 (500 ppm). Thus, the array of
mercapturic acid metabolites of benzene has expanded and the full
extent of metabolites of this structure may not yet be fully
appreciated.
In summary, the postulated metabolic pathways for benzene are
shown in Figures 1, 2 and 3. The formations of mercapturic acids,
ethereal sulfates and glucuronides are generally considered
detoxification pathways leading to the excretion of benzene
metabolites via the kidney (Henderson et al., 1989). All other
pathways lead to potentially toxic metabolites. This hypothesis is
discussed in more detail in section 7.9.
In both rats and mice the formation of toxic metabolites via the
epoxide pathway appears to be a saturable process, which suggests that
both metabolism and toxicity would be non-linear. In other words, the
proportion of toxic metabolites formed would decrease once the
saturation level is reached, whereas detoxification pathways appear to
be low-affinity high-capacity reactions (Henderson et al., 1989;
Medinsky et al., 1989a). It has been shown that mice metabolize
benzene faster and converted more of the benzene to toxic metabolites
than rats (Henderson et al., 1989). Because of this it has been
suggested that metabolism in mice favours toxification pathways (e.g.,
formation of benzoquinone and muconaldehyde), while in rats metabolism
is primarily detoxification (phenyl conjugates and phenylmercapturic
acids) (Medinsky et al., 1989a). The percentage of benzene or its
metabolites remaining in the body decreased in rats (from 33% to 15%)
and mice (from 50% to 10%) as exposure increased from 32 to 3200
mg/m3 (10 to 1000 ppm) (Sabourin et al., 1987).
Model simulations for total benzene metabolized and for profiles
of benzene metabolites formed after the administration of varying
doses of benzene to rats and mice (Medinsky et al., 1989b,c) have
suggested that the production of hydroquinone and muconic acid
metabolites predominates at lower exposure concentrations, whereas at
high exposure levels the detoxification pathways account for a larger
fraction of benzene metabolized. In addition, these model simulations
have confirmed that mice metabolize more benzene on a µmole/kg body
weight basis than rats after inhalation exposures, whereas rats
metabolize more benzene than mice at oral doses greater than 50 mg/kg
body weight. After either oral or inhalation exposures mice
preferentially form more of the putative toxic metabolites
hydroquinone and muconic acid (Medinsky et al., 1989b). It has also
been reported by Witz et al. (1990b) that DBA/ZN mice (a strain
sensitive to the haematotoxicity of benzene), excrete greater amounts
of trans, trans-muconic acid than the less sensitive C57BL/6 strain
after equivalent exposures to benzene.
6.4 Elimination and excretion
6.4.1 Inhalation exposure
In animals, expired air is the main route of elimination of
unmetabolized benzene, while urine is the major route of excretion of
benzene metabolites (with very little faecal excretion). Rickert et
al. (1979) found a biphasic pattern of excretion of unmetabolized
benzene in rats after a 6-h exposure to 1600 mg/m3 (500 ppm), with
half-times of 0.7 h for the rapid phase and 13.1 h for the slow phase.
The major route of excretion after inhalation exposures of rats and
mice to 32-3200 mg/m3 (10-1000 ppm) appeared to be dependent upon
the concentration inhaled (Sabourin et al., 1987). Under these
conditions mice received 150-200% of the dose given to rats on a per
kg body weight basis. The faecal excretion was < 3.5% in rats and
< 9% in mice. At doses up to 416 mg/m3 (130 ppm), less than 6% of
the radioactivity was eliminated in expired air, whereas at the highest
concentrations 48% of the dose was eliminated as unchanged chemical in
rats and 14% in mice. The total urinary excretion of metabolites at
these high concentrations was 5-37% higher in mice than in rats.
Findings in humans after inhalation exposure to benzene are
similar to those in experimental animals; unmetabolized chemical is
eliminated in expired air whereas metabolites of benzene are excreted
in urine, primarily as the sulfate and glucuronide conjugates of
phenol. Nomiyama & Nomiyama (1974a,b) found similar expiratory
patterns in men and women exposed for 4 h to benzene at concentration
between 166 and 198 mg/m3 (52-62 ppm). The proportion of the
absorbed benzene that was excreted via the lungs was approximately 17%
(Nomiyama & Nomiyama, 1974a,b).
6.4.2. Oral exposure
Parke & Williams (1953) administered radiolabelled benzene
(approximately 340 mg/kg body weight) by oral gavage to rabbits and
reported that 43% of the label was recovered as unmetabolized benzene
in expired air. Urinary excretion accounted for 33% of the dose,
mainly in the form of conjugated phenol (23.5%). Other phenols
excreted were hydroquinone (4.8%), catechol (2.2%), and hydroxyquinol
(1,2,4-trihydroxybenzene) (0.3%). Muconic acid accounted for 1.3% and
L-phenylmercapturic acid for 0.5%, and 5-10% of the radiolabel
remained in the tissues or was excreted in the faeces. The excretion
of benzene and its metabolites in rats and mice at various oral doses
(0.5-300 mg/kg body weight) was studied by Sabourin et al. (1987). In
both species the excretion of urinary metabolites up to a dose of 15
mg/kg accounted for 80% of the administered dose. Above that level
there was an increase in the elimination of 14C in expired air.
Equal amounts of unmetabolized benzene were eliminated in both species
up to dose levels of 50 mg/kg. At dose levels of between 15 and 50
mg/kg body weight, metabolism appears to become saturated in rodents.
In rats, 50% of a 150-mg/kg dose of 14C-benzene was eliminated in
expired air, while in the mouse 69% of this dose was exhaled (Sabourin
et al., 1987).
No studies were found regarding the excretion of benzene in
humans after oral exposures.
6.4.3 Dermal exposure
After the dermal application of 14C-benzene (0.0026 to 0.0036
mg/cm2) to monkeys and minipigs, Franz (1984) collected urine
samples every 5 h for 2-4 days. The rate of excretion was highest
over the first 10 h, the total excretion of radioactivity being higher
in the monkey (0.03 to 0.14% of the applied dose, with an average of
0.06%) than in the minipigs (0.03-0.05%, with an average of 0.04%).
Using a glass cap to minimize volatilization from the skin, Skowronski
et al. (1988) treated male rats dermally with 14C-benzene (0.004
mg/cm2). After 48 h, 86.2% of the initial dose was excreted in the
urine and 12.8% was eliminated in expired air. Phenol was the major
urinary metabolite detected in the 0-12 h sample (37.7% of dose), and
smaller quantities of hydroquinone, catechol and benzenetriol were
also detected.
In a study of four male human subjects, Franz (1984) applied
14C-benzene dermally (0.0024 mg/cm2). A mean of 0.023% (range
0.006-0.054%) of the applied radiolabel was recovered in the urine
over a 36-h period. More than 80% of the excretion occurred within
8 h of application.
6.5 Retention and turnover
Steady state levels of benzene were found within 4 h in blood,
6 h in fat, and 2 h in bone marrow when male rats were exposed to a
benzene concentration of 1600 mg/m3 (500 ppm) by inhalation for 6 h.
After exposure ceased, about 70% of the benzene was eliminated
unchanged in the expired air and about 30% was excreted in urine as
water-soluble metabolites within 15 h. The half-life (t´) for
elimination from these tissues was 0.4-0.8 h, except in the case of
adipose tissue where elimination occurred with a t´ of 1.6 h. The
elimination of unchanged benzene in expired air was biphasic, the t´
being 0.7 h for the first phase and 13.1 h for the slower phase. Free
phenol, catechol and hydroquinone were detected in blood and bone
marrow after exposure ceased. The phenol level declined rapidly over
a 9-h observation period, whereas catechol and hydroquinone levels in
both tissues remained constant over this period (Rickert et al.,
1979).
After intraperitoneal injection, oral gavage, or inhalation
exposures of labelled benzene in rats and mice, over 95% of the
administered radioactivity was excreted within 40 h (Sabourin et al.,
1987; Henderson et al., 1989). Approximately 90% of the metabolites
was excreted in the urine. According to the authors, these studies
indicate that benzene is rapidly metabolized and excreted in the urine
within 40 h of dosing by any route of administration.
6.6 Reaction with body components
3H-benzene metabolites have been shown to bind irreversibly to
proteins in both mouse liver and bone marrow (Snyder et al., 1978a).
Benzene metabolites have also been shown to bind in vivo to mouse
protein in blood (Sun et al., 1990), liver, bone marrow and spleen
(Longacre et al., 1981a,b). Covalent binding increased both with dose
and frequency of dosing. Covalent binding of benzene metabolites to
protein appears to be mediated by microsomal enzymes (Tunek et al.,
1978) and has been suggested to be the result of binding by
hydroquinone and catechol (Wallin et al., 1985). The finding of high
levels of phenylcysteine adducts in the haemoglobin of benzene-exposed
rats suggests that benzene oxide also reacts with proteins to form
adducts (Bechtold et al., 1992). Benzene metabolism and covalent
binding to proteins have been demonstrated in situ in bone marrow
(Irons et al., 1980b).
Lutz (1979) has attempted to quantify the extent to which
chemicals covalently bind to DNA using the concept of the covalent
binding index (CBI). The implication is that the higher the CBI, the
more likely a chemical will be a carcinogen. The calculated value for
benzene, based on binding to liver nuclear DNA, was 1.7. (To put this
value in perspective, CBI values for some common carcinogens were:
aflatoxin B1, > 1000; 2-acetyl-aminifluorene, 100 to several hundred;
polycyclic aromatic hydrocarbons, 10 to 30.) No values were given for
benzene in bone marrow, but Snyder et al. (1978a) compared covalent
binding of benzene residues per g dry weight of liver and bone marrow
and found that, depending upon the dose of benzene, covalent residues
bound to liver ranged from 500 to 800 nmoles/g, whereas binding to
bone marrow ranged from 18 to 96 nmoles/g. Thus, there appeared to be
less covalent binding in the target organ, i.e. bone marrow, than in
the metabolizing organ, i.e. liver.
Inhaled benzene has been found to bind to rat liver DNA to the
extent of 2.38 µmoles/mole DNA phosphate (Lutz & Schlatter, 1977).
Studies of the covalent binding of benzene metabolites to DNA have
resulted in the postulation of several structures for DNA adducts
derived from benzene. Bone marrow mitochondria from rabbits were
incubated sequentially with 3H-deoxyguanosine triphosphate and
14C-benzene to evaluate DNA adducts formed from benzene metabolites
(Snyder et al., 1987b). These authors identified at least seven
deoxyguanosine adducts and one deoxyadenine adduct. Covalent
N-7-phenyl-guanine adducts have been isolated from rat urine after
intraperitoneal dosing with 330-400 mg benzene/kg body weight (Norpoth
et al., 1988). Thus, Jowa et al. (1990) postulated the formation of
an adduct between p-benzoquinone and deoxyguanosine which had the
structure (3'OH)benztheno(1,N2)deoxyguanosine. The structure of an
adduct formed between p-benzoquinone and deoxyadenosine-3'-phos
phate was suggested to be 3'-hydroxy-1,N6-benztheno-2'-deoxy-
adenosine-3'-phosphate (Pongracz & Bodell, 1991). Reddy et al.
(1990), however, reported that they were unable to detect DNA adducts
derived from benzene in the rat in vivo, despite having observed
them in Zymbal gland cells in vitro, using the 32P-post labelling
technique.
6.7 Modelling of pharmacokinetic data for benzene
In order to obtain better insight into the interspecies
variations in the uptake, metabolic fate and excretion of benzene and
its metabolites, both compartmentally (Bailer & Hoel, 1989; Beliles &
Totman, 1989) and physiologically based (Medinsky et al., 1989b,c;
Paxman & Rappaport, 1990; Travis et al., 1990; Bois et al., 1991a,b),
pharmacokinetic models have been developed. These models have been
used as an aid to risk assessment by facilitating extrapolation
between species where various exposure regimens had been utilized.
Also, such models are useful for identifying gaps in knowledge that
have been highlighted by poor fits of the experimental data to the
models developed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Benzene has been shown to produce a number of biological
responses in experimental animals. The acute effects of benzene at
high doses reflect its activity as a general anaesthetic and can lead
to central nervous system (CNS) depression, loss of consciousness and
coincidental sensitization of the myocardium to catecholamines.
Chronic exposure can result in bone marrow depression expressed as
leucopenia, anaemia and/or thrombocytopenia, leading to pancytopenia
and aplastic anaemia. The immunotoxic effects of benzene are probably
related to bone marrow depression. In animal cancer bioassays it is,
primarily, epithelial tumours that have been reported, whereas in
humans the carcinogenic response is leukaemia. A third type of
biological impact is the production of clastogenic responses such as
chromosome aberrations, sister chromatid exchange and micronuclei.
Benzene has also been suggested to produce fetotoxic effects.
7.1 Single exposure
Consistent with many other aromatic hydrocarbons (Patty, 1981),
benzene appears to be of low acute toxicity when administered to
various animal species by various routes of administration (Table 9).
Other reported oral LD50 values for reagent grade benzene in male
rats vary from as low as 930 mg/kg to as high as 5600 mg/kg body
weight (Wolf et al., 1956; Cornish & Ryan, 1965; Kimura et al., 1971;
Withey & Hall, 1975). The LD50 after intraperitoneal injection in
female rats was reported to be 2940 mg/kg (Drew & Fouts, 1974) and in
mice it was 300 mg/kg body weight (Kocsis et al., 1968). Young rats
are more sensitive (in terms of LD50) than older ones (Table 9).
The LC50 in female rats was estimated to be 43 770 mg/m3 (13
700 ppm) after a single 4-h exposure (Drew & Fouts, 1974).
Benzene has a narcotic effect after oral administration in rats
(Withey & Hall, 1975) and after inhalation in mice (Uyeki et al.,
1977). The threshold narcotic effect after inhalation has been
estimated to be approximately 13 000 mg/m3 (Leong, 1977).
Inhalation of air saturated with benzene resulted in ventricular
tachycardia and occasionally ventricular fibrillation and death in
rats, cats, rabbits and primates (Nahum & Hoff, 1934). Respiratory
failure was also observed during narcosis. Pathological findings after
sudden death are congestion of various organs, particularly the lungs
and liver (Jonek et al., 1965).
No information on the acute toxicity/lethality in animals after
dermal exposure has been reported.
Table 9. Toxicity of benzene in animals after acute exposure
Route Species Parameter Value Reference
Oral rat (14 days old) LD50 3000 mg/kg body weight Kimura et al. (1971)
Oral rat (young adult) LD50 3300 mg/kg body weight Kimura et al. (1971)
Oral rat (old adult) LD50 4900 mg/kg body weight HSE (1982); Kimura et al. (1971)
Oral rat LD50 8100 mg/kg body weight Cornish & Ryan (1965)
Inhalation (4 h) rat LC50 44 660 mg/m3 body weight Drew & Fouts (1974)
Inhalation (7 h) rat LC50 32 600 mg/m3 body weight HSE (1982)
Inhalation (2 h) mouse lethal dose 61 125 mg/m3 body weight Jonek et al. (1965)
Intraperitoneal injection rat LD50 2940 mg/kg body weight Drew & Fouts (1974)
Intraperitoneal injection mouse LD50 300 mg/kg body weight Kocsis et al. (1968)
7.2 Short-term and long-term exposures
The studies discussed in this section, some of which are
summarized in Table 10, have a duration of less than one year.
Lifetime (> 1 year) studies are discussed in section 7.6 and
summarized in Tables 15 to 17.
In short-term inhalation studies, three out of eight male rats
died within 24 h after exposure for five periods of 25-35 min to a
benzene concentration of 128 000 mg/m3 (40 000 ppm) and two out of
ten died after exposure for 12.5-30 min daily to 32 000 mg/m3
(10 000 ppm) for 1-12 days (Furnas & Hine, 1958).
Male and female rats and mice exposed to benzene vapour at
concentrations of 3.2, 32, 96 or 960 mg/m3 (1, 10, 30 or 300 ppm)
for 6 h/day, 5 days/week for 13 weeks, and sacrificed at various time
points during the study, showed no haematological effects up to 96
mg/m3 (30 ppm) (Ward et al., 1985). However, at 960 mg/m3 (300
ppm) mice exhibited significant decreases in haematocrit, haemoglobin,
erythrocyte count, leucocyte count, platelet count and the percentage
of lymphocytes. There was an increase in erythrocyte volume and mean
corpuscular haemoglobin. These changes were first observed on days 14
(males) or 28 (females). Most of the haematological effects were also
detected in rats but were of lesser severity. Compound-related
histopathological findings included myeloid hypoplasia, depletion of
the periarteriolar lymphoid sheaths in the spleen, lymphoid depletion
in the mesenteric lymph nodes, and increased extramedullary
haematopoiesis in the spleen. These lesions persisted throughout the
study and increased in severity with time. The only histopathological
lesion observed in rats was slightly reduced cellularity in the bone
marrow of the femur.
A dose-related increase in leucocyte alkaline phosphatase levels
and a decrease in leucocyte levels was observed in female rats exposed
via inhalation to 320, 960, 3200 or 9600 mg/m3 (100, 300, 1000 or
3000 ppm) for 7 or 14 days, but not in those exposed to 64 or 160
mg/m3 (20 or 50 ppm) (Li et al., 1986). In a study on male mice,
with doses of 3.5, 32, 330, 980, 1930, 4080, 7730 and 15 600 mg/m3
(1.1, 9.9, 103, 306, 603, 1276, 2416 and 4862 ppm), granulocytopenia,
lymphocytopenia and reduced bone marrow and splenic cellularity were
observed after exposure to > 330 mg/m3 for 5 h/day for 5 days but
not at lower levels (Green et al., 1981a). These authors found
splenic lesions at levels as low as 32 mg/m3 when exposure was
extended to 10 weeks.
Table 10. Toxicity of benzene in animals after short-term and long-term inhalation exposuresa
Species Dose (mg/m3) Exposure period Effects References
Rat 48 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
28 weeks at term 90 µg/litre
Rat 100 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
7 weeks at term 290 µg/litre
Rat 150 7 h/day; 5 days/week; slight leucopenia, blood benzene level Deichmann et al. (1963)
32 weeks at term 420 µg/litre
Rat 3200 18 h/day; 7 days/week; reversible haemotological effects, Nau et al. (1966)
15 weeks reversible leucopenia within 6 months
Rat 3.2-960 6 h/day; 5 days/week; slight changes in haematological cell counts Ward et al. (1985)
13 weeks and lower cellularity in bone marrow of femur
Rat (female) 64-9600 1-2 weeks dose-related increase in leucocyte alkaline Li et al. (1986)
phosphatase and decreased leucocyte counts
at exposures of 960 mg/m3 or more
Mouse 3.5 to 15 600 6 h/day; 1 week doses > 330 mg/m3 resulted in granulocytopenia, Green et al. (1981a,b)
lymphocytopenia, decreased splenic cellularity
and decreased stem cell production
Mouse (male) 30.7 6 h/day; 5 days/week; some increase in spleen weight and some Green et al. (1981a)
10 weeks increased cellularity
Table 10 (contd).
Route Species Parameter Value Reference
Mouse (male) 960 6 h/day; 5 days/week; increased mortality, marked lymphocytopenia, Green et al. (1981a)
26 weeks reduced red blood cell count, reduced spleen
weight, many morphological abnormalities in
circulating red blood cells and neutrophils
Mouse (male) 32 6 h/day; 5 days/week; depression in the numbers of splenic nucleated Baarson et al. (1984)
25.5 weeks cells and of circulating red cells and lymphocytes
Mouse 3.2-960 6 h/day; 5 days/week; only at 960 mg/m3 decreased blood cell counts, Ward et al. (1985)
13 weeks haematocrit, myeloid hypoplasia, and numbers of
splenic and lymph node lesions
Mouse 32-1280 6 h/day; 5 days/week; reduced bone marrow cellularity and number of Cronkite et al. (1985)
pluripotent stem cells at 320 mg/m3 or more
Guinea-pig 280 7 h/day; 5 days/week; slight leucopenia, increased weight of kidneys Wolf et al. (1956)
4.5 weeks
Pig 64, 320 6 h/day; 5 days/week; leucopenia at 320 and 1600 mg/m3, reversible Johnston et al. (1979)
and 1600 3 weeks 9-16 weeks after exposure
a To avoid duplication, toxicity from life-time exposures (over 1 year) is discussed in section 7.6
(carcinogenicity) and Tables 15 to 17.
Cronkite et al. (1985) exposed male and female mice by inhalation
to benzene at concentrations of 32, 80, 320, 960 or 1280 mg/m3 (10,
25, 100, 300 or 400 ppm) for 2 weeks (6 h/day, 5 days per week). At
320 mg/m3 or more, reduced bone marrow cellularity and a decreased
number of pluripotent stem cells in bone marrow were reported. Under
similar conditions of exposure to 960 mg/m3 for 16 weeks, these
authors reported a lower level of stem cells in bone marrow, which
returned to 92% of control values after 25 weeks post-exposure.
Complete reversibility within 2 weeks was reported after 2 or 4 weeks
of exposure to 960 mg/m3.
Long-term (> 6 months) exposure studies at benzene levels above
approximately 160 mg/m3 (50 ppm) have shown effects on circulating
leucocytes (especially leucopenia). For example, rats exposed via
inhalation to 150 mg/m3 (47 ppm) (7 h/day, 5 days per week for 8
months) showed slight leucopenia, while those exposed to 100 mg/m3
(31 ppm) for 4 months and those exposed to 48 mg/m3 (15 ppm) failed
to show such changes (Deichmann et al., 1963). The lowest reported
exposure in animals that resulted in haematological effects was in
mice (Baarson et al., 1984). These authors reported that male mice
exposed via inhalation to 32 mg/m3 (10 ppm) (6 h/day, 5 days/week)
for 25.5 weeks showed a decrease in the number of circulating
erythrocytes and lymphocytes, a decrease in the number of nucleated
cells in the spleen and a depression of the in vitro colony-forming
ability of erythroid precursor cells (CFU-E).
Uyeki et al. (1977) demonstrated a depression of stem cell
activity in mice using the spleen colony-forming technique (CFU-S)
after exposure to a benzene concentration of 15 000 mg/m3 (4680 ppm)
for 3 days (8 h/day).
Haematotoxicity was also noted after oral exposure in rats and
mice. The animals were dosed by gavage with benzene in corn oil for
120 days at 25, 50, 100, 200, 400 or 600 mg/kg body weight (5
days/week). Five animals in the control, 200- and 600-mg/kg groups
were sacrificed at 60 days (Huff et al., 1989). A dose-related
leucopenia was observed in both male and female rats and lymphoid
depletion in the B-cells of the spleen was observed in both the 200-
and 600-mg/kg groups at 60 days. In mice no compound-related
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females.
No information was found on the haematotoxicity of benzene after
dermal exposure has been reported.
Additional studies on the effects of long-term exposure to
benzene in experimental animals are described in section 7.6
(carcinogenicity) and in Tables 15 to 17.
7.3 Skin and eye irritation
Benzene is considered a moderate eye irritant (as shown in the
rabbit eye test). Two drops of benzene caused moderate conjunctival
irritation and very slight, transient corneal injury (Wolf et al.,
1956).
Undiluted benzene was irritating to the skin (ear) of rabbits
after 10-20 applications (Wolf et al., 1956). Erythema, oedema,
exfoliation, blistering and moderate necrosis were observed after 20
applications.
There is no information available on the skin-sensitizing
potential of benzene. However, no such effect is expected based on
the experience with other aromatic hydrocarbons (GDCh, 1988).
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
Benzene does not appear to be a potent reproductive toxin in
experimental animals. Guinea-pigs and rabbits exposed to benzene by
inhalation (7 h/day, 5 days/week) for up to 6 months showed variable
results; guinea-pigs showed a slight increase in testicular weight at
280 mg/m3 (88 ppm) and rabbits showed slight degeneration of the
seminiferous germinal epithelium at 256 mg/m3 (80 ppm) (Wolf et al.,
1956). Mice, but not rats, exposed to benzene vapour at a
concentration of 960 mg/m3 (300 ppm) (6 h/day, 5 days/week) for 13
weeks showed bilateral cysts in the ovaries and degeneration and
atrophy of the testes (Ward et al., 1985). At concentrations of 3.2,
32 or 96 mg/m3 (1, 10 or 30 ppm) these changes were also seen in
mice, but they were of doubtful biological significance (Ward et al.,
1985). There was a complete absence of litters in female rats exposed
to 670 mg/m3 (210 ppm) for 10-15 days before mating and for 3 weeks
after mating (Gofmekler, 1968). It is not known if this represents a
problem in mating and fertility, or one of maternal or fetal toxicity.
Lower exposures (1 to 64 mg/m3; 0.30 to 20 ppm) produced no such
effects.
Numerous studies on experimental animals have failed to detect
teratogenic effects, even at doses of benzene clearly toxic to the
dam. A few of these studies are summarized in Table 11. However,
benzene has been reported to be fetotoxic in mice, as shown by a
decrease in fetal weight and skeletal variants (missing sternebrae and
extra ribs) in the offspring of dams exposed to 1600 mg/m3 (500 ppm)
for 7 h on gestation days 6-15 (Murray et al., 1979). Similar effects
were seen in rabbits exposed on gestation days 6-18 to the same
levels. However, these effects are not usually considered to be
significant compound-related malformations (Kimmel & Wilson, 1973).
In mice, exposure to 500 or 1000 mg/m3 on gestation days 6-15
resulted in a decrease in fetal weight and an increase in dead or
resorbed fetuses, but no statistically significant increase in
malformations (Ungvary & Tatrai, 1985).
Table 11. Teratogenic effects of benzene in the mouse and rabbita
Animals Route Exposure level Maternal weight Fetal body Resorptions/ Skeletal Malformations References
gain weight fetal death/ variants
abortions
New Zealand inhalation 1600 mg/m3 (-) (-) (-) slightly ** (-) Murray et al.
rabbit (1979)
New Zealand inhalation 500 mg/m3 (-) (-) (-) (-) (-) Ungvary & Tatrai
rabbit 1000 mg/m3 * * **b ** ** (1985)
CF-1 mouse inhalation 1600 mg/m3 (-) * (-) ** (-) Murray et al.
(1979)
CFLP mouse inhalation 500 mg/m3 (-) * **c,d ** (-) Ungvary & Tatrai
1000 mg/m3 (-) * **c,d ** (-) (1985)
CD-1 mouse gavage 0.3 ml/kg body weight (-) * (-) (-) Nawrot &
0.5 ml/kg body weight (-) * **c,d (-) Staples (1979)
1.0 ml/kg body weight (-) * **c,d (-) (-)
a (-) indicates no significant difference from controls; * indicates decrease compared with controls;
** indicates increase compared with controls.
b Fetal death
c Abortions
d Resorptions
Table 12. Teratology studies in rats after inhalation of benzenea
Exposure level Exposure period Maternal weight Resorptions Fetal weight Skeletal variants Malformations Reference
(mg/m3) (h/day) gain
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
32 7 (-) (-) (-) (-) (-) Kuna & Kapp (1981)
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
128 6 (-) (-) (-) (-) (-) Coate et al. (1984)
150 24 * (-) * (-) (-) Tatrai et al. (1980a)
160 7 * (-) * ** (-) Kuna & Kapp (1981)
320 6 (-) (-) (-) (-) (-) Green et al. (1978)
320 6 (-) (-) * (-) (-) Coate et al. (1984)
400 24 * (-) * ** (-) Tatrai et al. (1980b)
450 24 * ** * ** (-) Tatrai et al. (1980a)
960 6 (-) (-) (-) ** (-) Green et al. (1978)
1000 24 * (-) * ** (-) Hudak & Ungvary (1978)
1500 24 * ** * ** (-) Tatrai et al. (1980a)
1600 7 * (-) * ** (-) Kuna & Kapp (1981)
3000 24 * ** * ** (-) Tatrai et al. (1980a)
7000 6 * (-) * ** (-) Green et al. (1978)
a (-) indicates no significant difference compared with controls; * indicates decrease compared with controls;
** indicates increase compared with controls
Several studies in rats show similar results to those in mice and
rabbits (Table 12). Maternal and fetal weight were decreased at
levels > 160 mg/m3 (> 50 ppm) as were the number of skeletal
variants observed. No malformations were noted in any of the studies
even at doses as high as 7100 mg/m3 (2200 ppm).
It is noteworthy that haematopoietic changes were observed in the
fetuses and offspring of mice exposed to 16, 32 or 64 mg/m3 (5, 10
or 20 ppm) for 6 h/day on gestation days 6-15 (Keller & Snyder, 1986).
The changes included a decrease in the number of erythroid
colony-forming cells (at all dose levels) and granulocytic
colony-forming cells at the two highest levels. When the offspring
were re-exposed to benzene as adults the decrease in these progenitor
cells was greater than in adult mice exposed to benzene at the same
levels for the first time.
7.5 Mutagenicity and related end-points
Benzene has been widely studied regarding the production of gene
mutations in in vitro tests, chromosomal effects both in vitro and
in vivo, and effects on DNA (binding, synthesis and damage). An
overview of the testing up to 1985 for the mutagenicity of benzene is
shown in Fig. 4 (IARC, 1987a). Detailed reviews have also been
published (Dean, 1978, 1985a; Huff et al., 1989; ATSDR, 1991),
therefore only some of the many studies are shown in Tables 13
( in vitro) and 14 ( in vivo).
7.5.1 In vitro studies
As shown in Table 13 and Fig. 4, benzene has consistently given
negative results in assays for point mutations in bacteria using
standard test conditions. In such studies, six tester strains have
been used with benzene concentrations ranging from 0.1 to 528 µg per
plate both with and without metabolic activation (HSE, 1982). Other
studies using doses as high as 880 mg/plate failed to cause mutations
in Salmonella typhimurium (Dean, 1978). In some 10 in vitro gene
mutation tests carried out in various human, mouse and Chinese hamster
cells, as part of an international collaborative study, mixed results
were obtained with benzene (Ashby et al., 1985; Venitt, 1985). When
S. typhimurium (strain TA1535) was incubated with benzene in a
desicator to enhance exposure, a doubling of revertants was noted at
10 ppm only in the presence of a post-mitochondrial activating system
(Glatt et al., 1989).
Figure 4. Tabular summary of in vitro and in vivo tests on benzene for mutagenicity and related end-pointsa
Non-mammalian systems Mammalian systems
In vitro In vivo
Prokaryotes Lower Plants Insects Animal cells Human cells Animals Humans
eukaryotes
D G R G A G R G C D G S M C A T D G S C S M C S C
+1 - + + +1 + +1 ? -1 + + - -1 + + + -1 +1 ? +1 + + + -1 +
a Adapted from: IARC (1987a)
A = aneuploidy; C = chromosomal aberrations; D = DNA damage; G = gene mutation; M = micronuclei; R = mitotic recombination and gene
conversion; S = sister chromatid exchange; T = cell transformation; + = considered to be positive for the specific end-point and
level of biological complexity; +1 = considered to be positive, but only one valid study was available to the Working Group;
- = considered to be negative; -1 = considered to be negative, but only one valid study was available to the Working Group; ? =
considered to be equivocal or inconclusive (e.g., there were contradictory results from different laboratories; there were confounding
exposures; the results were equivocal)
Table 13. Some in vitro genotoxicity studies of benzene
End-point Test system Resultsa References
Gene mutations
Ames test Salmonella typhimurium -/- De Flora et al.
-/+ (1984); Venitt (1985);
Glatt et al. (1989)
Azaguanine Salmonella typhimurium -/ Seixas et al.
resistance (1982)
TK test mouse L5178Y cells -/- Oberly et al. (1984)
TK, ouabain, total of 15 studies using mixedb Garner (1985)
HGPRT loci various human, mouse and
Chinese hamster cells
Chromosome abnormalities
Chromosome human lymphocytes mixed Gerner-Smidt &
aberrations Friedrich (1978);
Morimoto (1976)
total of 8 studies using mixed Dean (1985b)
Chinese hamster or human
cells
Sister Chinese hamster ovary and -/- Dean (1985b)
chromatid V79 cells and rat RL4
exchange cells
Table 13 (contd).
End-point Test system Resultsa References
Sister human lymphocytes mixed Morimoto (1983);
chromatid Morimoto et al.
exchange (1983); Erexson
et al. (1985)
Micronuclei Chinese hamster ovary -/- Douglas et al.
cells (1985)
Other effects
DNA breaks Rat hepatocytes ND/- Bradley (1985)
Chinese hamster V79 cells -/- Swenberg et al.
(1976)
Chinese hamster ovary -/- Douglas et al.
cells (1985)
Mouse L5178Y cells -/ Pellack-Walker &
Blumer (1986)
Unscheduled rat hepatocytes ND/- Probst & Hill
DNA synthesis (1985)
HeLa cells -/- Barrett (1985)
DNA synthesis Hela cells -/- Painter & Howard
inhibition (1982)
a Without/with an exogenous metabolic activation system; ND = no data
b The IPCS CSSTT working group disagreed over data analysis and therefore
called the results inconclusive
Whereas the results of in vitro studies for mutations by
benzene have been largely negative, there is some evidence that
treatment of human and animal cells in vitro with benzene can lead
to chromosomal abnormalities. However, as shown in Table 13, mixed
results have been obtained.
The ring-opened metabolite of benzene, trans,trans-muconal-dehyde
(MUC) has been tested for mutagenic and clastogenic activity in CHO
cells and Salmonella typhimurium bacteria, and for its effects on
DNA synthesis in primary rat liver hepatocytes (Witz et al., 1990a).
Only minimal mutational activity in bacteria was reported (in only
S. typhimurium strain TA97 of the five strains tested). However, at
a concentration of 0.4 to 0.8 µg/ml media, MUC resulted in a
dose-related increase in micronuclei in CHO cells. No effect on
unscheduled DNA synthesis or in the HG PRT assay in CHO cells was
reported. Using S. typhimurium (point mutations) and V79 cells
(sister chromatid exchange, acquisition of thioguanine or ouabain
resistance, and induction of micronuclei), 13 other potential benzene
metabolites were examined for genotoxicity. Each metabolite showed a
specific spectrum of activity, the highest genotoxic activity in most
systems being exhibited by quinone, hydroquinone, anti-diol epoxide
and catechol (Glatt et al., 1989).
The negative mutagenic data found in studies where benzene was
added to standard systems in vitro may well have been caused by the
technique used in these studies. Benzene is metabolically activated
to reactive metabolites by cytochrome P-450 IIE1, a natural
constituent of liver microsomes. In many of these studies
insufficient activation of benzene may have occurred. Post & Snyder
(1983) demonstrated that enzyme induction with benzene increased
benzene metabolism by increasing the activity of an enzyme having a
low Km and a high turnover rate for benzene, which is now thought to
be cytochrome P-450 IIE1. These authors also found that phenobarbital
induction reduced benzene metabolism until very high substrate
concentrations were reached. Chepiga et al. (1991), using purified
reconstituted cytochrome P-450, have shown that, whereas the Km
value for benzene as a substrate for cytochrome P-450 IIE1 is quite
low, much higher concentrations are required for benzene to be
metabolized by cytochrome P-450 IIB1, which also has a low affinity
for benzene. Thus, the lack of positive results in mutagenesis tests
involving benzene may have been due to the low activity of
benzene-activating enzymes in these preparations.
7.5.2 In vivo studies
No data are available on the production by benzene of gene
mutations in vivo. Benzene, or its metabolites, cause both
structural and numerical chromosome aberrations in humans (see chapter
8), laboratory animals and cells in culture (see section 7.6.1), as
well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes. Some in vivo studies are summarized in
Table 14.
Chromosomal changes occur after exposure of experimental animals
by the subcutaneous, oral, intraperitoneal or inhalation routes.
Philip & Krogh-Jensen (1970) administered 1750 mg benzene/kg body
weight subcutaneously to rats and noted an increase in chromatid
aberrations 12 and 24 h post-dosing but not after 36 h. This suggests
damage to S and/or G2 phase cells and a rapid elimination of the
alterations. In a study by Kissling & Speck (1972), the subcutaneous
administration of 1750 mg benzene/kg body weight to rabbits 3 times
weekly for 18 weeks led to tetraploidy in one animal as well as a high
percentage (58%) of bone marrow cells having chromosomal aberrations.
Siou et al. (1981) reported an increase in chromosome aberrations in
the bone marrow cells of mice treated orally with doses greater than
56 mg benzene/kg body weight on 2 successive days before sacrifice.
At high levels of benzene administered by inhalation (10 000
mg/m3 for 4 h), a marked increase in SCEs was noted in mouse bone
marrow cells (Tice et al., 1980). In a later experiment a significant
increase was reported in SCEs in mouse bone marrow cells when the
animals were exposed by inhalation to 91 mg/m3 for 4 h (Tice et al.,
1982). Erexson et al. (1986) reported a significant increase in the
levels of SCEs in peripheral lymphocytes after 6 h of exposure to 32
mg/m3 in mice and 9.6 mg/m3 in rats. At these levels, the
frequency of micronuclei in bone marrow smears was also increased.
After 6 weeks of exposure of mice (22 h/day, 7 days/week) at benzene
levels of between 0.128 and 3.2 mg/m3, increased chromosomal
aberrations in lymphocytes from the spleen were reported by Au et al.
(1991). These changes reached a significance of P = 0.05 only in
female mice (in males P = 0.15).
The frequency of micronuclei was increased in the bone marrow of
mice treated orally at doses ranging from 56 to 2200 mg/kg body weight
(Hite et al., 1980; Siou et al., 1981). A dose-related increase in
micronuclei in circulating erythrocytes was seen at 120 days in mice
treated by oral gavage with 25, 50, 100, 200, 400 or 600 mg benzene/kg
body weight. A significant increase was seen at all doses, with male
mice being more sensitive (Choy et al., 1985).
Table 14. Some mammalian in vivo genotoxicity studies on benzene
Route of Test system Results Exposure concentration Reference
administration and duration
Chromosome aberrations
Inhalation mouse bone marrow + 14 to 74 mg/m3, 7 days Zhurkov et al. (1983)
- 10 000 mg/m3, 4 h Tice et al. (1980)
- 9600 mg/m3, 4 h Tice et al. (1982)
+ 9600 mg/m3, 4 h, phenobarbital pretreatment Tice et al. (1982)
rat bone marrow + 3.2-3200 mg/m3, 6 h Styles & Richardson (1984)
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
+ 5-80 mg/kg body weight, daily for 2 days Zhurkov et al. (1983)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
bone marrow
Intraperitoneal rat bone marrow + 1 dose of 878 mg/kg body weight; 24 h prior to Anderson & Richardson
sacrifice (1981)
Micronuclei
Inhalation mouse lymphocytes + 32, 320 and 3200 mg/m3, 6 h Erexson et al. (1986)
mouse lymphocytes + > 67 mg/m3, 4-10 days Toft et al. (1982)
rat lymphocytes + 0.3 to 96 mg/m3, 6 h Erexson et al. (1986)
Table 14 (contd).
Route of Test system Results Exposure concentration Reference
administration and duration
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
mouse bone marrow + 440 mg/kg body weight, 2 doses, 24 h apart Gad El-Karim et al. (1984)
mouse bone marrow + 55 to 1760 mg/kg, daily for 2 days Hite et al. (1980)
mouse circulating + 26 to 440 mg/kg body weight, 5 days Barale et al. (1985)
erythrocytes + 25 to 600 mg/kg, 120 days Choy et al. (1985)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
Sperm head abnormality
Intraperitoneal mouse (spermatogonia + 88 to 880 mg/kg, daily for 5 days Topham (1980)
treated)
There were statistically significant alterations in sperm head
morphology after intraperitoneal doses of 88 to 880 mg/kg body weight
were administered to mice for 5 days and the sperm were examined 5
weeks later (Topham, 1980).
Witz et al. (1990a) reported that administration of
trans,trans-muconaldehyde, a microsomal metabolite of benzene, to
B6C3F1 mice (< 0.1-6.0 mg/kg body weight intraperitoneally)
resulted in the production of SCEs. The lowest dose producing a
significant increase was 3 mg/kg. No increase in the frequency of
micronuclei was reported.
There has been no clear demonstration of dominant lethal effects
in animals following benzene exposure. Fel'dt (1985) found no
significant dominant lethal effect in mice following oral
administration of up to 320 mg benzene/kg body weight. No dominant
lethal effect was reported in rats by Dean (1978) after the
intraperitoneal injection of 440 mg benzene/kg. However, Ciranni et
al. (1988) demonstrated the induction of micronuclei in the bone
marrow cells of pregnant mice and in fetal liver cells after a single
exposure to benzene or its metabolites.
7.6 Carcinogenicity
In several studies benzene has been shown to be carcinogenic in
experimental animals after exposure by inhalation and after oral
(gavage) dosing. These experiments are summarized in Tables 15-18.
As indicated in these Tables, several types of neoplasms have been
reported to be associated with exposure to benzene. Various types of
lymphomas/leukaemias have been found, but the majority of neoplasms
are of epithelial origin, i.e. Zymbal gland, liver, mammary gland and
the oro-nasal cavity. These results support the hypothesis that
benzene exposure in experimental animals can produce cancer at
multiple sites. A review of such studies has been published by Huff
et al. (1989).
7.6.1 Inhalation studies
The experimental design and major effects noted in several
inhalation cancer bioassays on benzene are summarized in Table 15.
Table 15. Inhalation studies on the carcinogenicity of benzene in experimental animals
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse AKR/J, dosed, 60; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 11 weeks; mean lifetime Snyder
males (8 weeks control, 60 5 days/week, lifetime for control group, 39 weeks, death due to aplastic et al.
old) (about 70 weeks) anaemia; bone marrow hypoplasia; no evidence for any (1978b)
tumours at autopsy in test animals
Mouse C57BL, dosed, 40; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 41 weeks; mean lifetime Snyder
males (8 weeks control, 40 5 days/week, lifetime for control group, 75 weeks; anaemia, lymphocytopenia, et al.
old) (about 70 weeks) neutrophilic bone marrow hyperplasia; 6/40 lymphocytic (1980)
lymphoma with thymic involvement (P < 0.01), 1/40 plasma
cytoma, 1/40 haematocytoblastic leukaemia, 2/40 control
mice lymphocytic lymphoma without thymic involvement
Mouse AKR/J, dosed, 50; 320 mg/m3, 6 h/day, mean life-span for dosed group, 39 weeks; for control, Snyder
males (8 weeks control, 50 5 days/week, lifetime 47 weeks; anaemia, lymphocytopenia, neutrophilia, bone et al.
old) (about 70 weeks) marrow hyperplasia 10/49 dosed, 1/50 control (1980)
Mouse Charles number of 320 and 960 mg/m3, two mice of high-dose group developed myelogenous Snyder
river CD-1, animals 6 h/day, 5 days/week, leukaemia et al.
males unknown lifetime (1978b)
Mouse CD-1, dosed, 60; intermittent exposure greater mortality than with continuous exposure to 3480 Snyder
C57BL/6, male control, 60 at 960 mg/m3 for 1 mg/m3 for 10 weeks; elevated incidences of malignant et al.
for each week followed by 2 tumours in both strains; 35% incidence of Zymbal gland (1988)
strain weeks non-exposure; tumours in C57BL (control 0%), lung adenomas in CD-1
6 h/day, 5 days/week (26% versus 7% controls), and no significant increase
for lifetime in incidence of leukaemia or lymphomas
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse CD-1, dosed, 80; short-term exposure to only CD-1 strain showed increased tumour incidence; 46%
C57BL/6, male control, 80 3840 mg/m3, 6 h/day, incidence of lung adenomas (control 24%) in addition to
for each 5 days/week for 10 other malignant and benign tumours; no increase in
strain weeks; observation for incidence of leukaemia or lymphoma; marked haematotoxicity
lifetime after cessation noted (anaemia and lymphocytopenia)
of exposure
Mouse C57BL/6, dosed, 118; control, 960 mg/m3, 6 h/day, 5 48 weeks after treatment survival rates were: dosed (D) Cronkite
female (7-9 116 (groups reduced days/week for 16 weeks; 80/90; control (C) 87/88; of the 10 exposed mice that et al.
weeks old) to 90 and 88 observation period for died, 6 had thymic lymphomas, 2 had unspecified lymphomas; (1985)
respectively for lifetime at end of study tumour incidence reported: leukaemia all
haemopoietic types (D) 20/89, (C) 8/88; lymphomas (thymic) (D) 10/89,
stem cell assays (C) 1/88; lymphomas (non-thymic) (D) 6/89, (C) 2/88
Rat Sprague- dosed, 45; 960 mg/m3, 6 h/day, no evidence of leukaemic response or pre-leukaemic Snyder
Dawley, male control, 25 5 days/week, for 99 effects nor for tumours incidence at any other site et al.
weeks (1978b)
Rat Sprague- dosed, 40; 320 mg/m3; 6 h/day, incidence of total and malignant tumours not significantly Snyder
Dawley, male control, 40 5 days/week, lifetime elevated over controls; several rare tumours in dosed et al.
(6 weeks old) (about 123 weeks) group, not in controls: 4 liver, 2 Zymbal gland and 1 (1984)
chronic myelogenous leukaemia
Rat Sprague- dosed, 70 male, 640 mg/m3; 4 h/day, at end of experiment (150 weeks) no information on survival; Maltoni
Dawley 59 female; control, 5 days/week for 7 weeks at 118 weeks survival rates of dosed (D) and control animals et al.
158 male, 149 female then 7 h/day for 8 (C) comparable; tumour incidence at 150 weeks; Zymbal (1983,
weeks; exposure in gland (D) 4/70 male, 1/59 female with (C) 2/158 male and 1985,
utero from day 12 of 0/149 female; oral cavity (D) 2/70 male, 6/59 female with 1989)
gestation through (C) 0/158 male and 0/149 female; leukaemias (D) 4/70 male,
lactation 4/58 female with (C) 12/158 male and 1/148 female;
hepatomas (D) 2/70 male, 5/59 female with (C) 1/158 male
and 0/149 female
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- Breeders: dosed 640 mg/m3, 4 h/day, at termination (150 weeks) no information on survival; Maltoni
Dawley (breeders (D), 54; control 5 days/week, 7 weeks; at 118 weeks breeder (D) 5/54, (C) 11/60; offspring (D) et al.
13 weeks old) (C), 60 7 h/day, 5 days/week, male 11/75, (D) female 23/65 with (C) males 39/158 and (1983,
12 weeks; and then females 47/149; (C) breeders had 2/60 with malignant 1985,
960 mg/m3, 7 h/day, mammary tumours and (C) offspring had 3/158 and female 1989)
5 days/week, 85 weeks 8/149 malignant mammary tumours; male 1/158 and 0/149
with leukaemias; tumour incidence in (D) breeders was:
Offspring: dosed, Breeders: from day Zymbal gland carcinoma 3/54; oral cavity carcinoma 2/54;
75 male and 65 12 of gestation nasal carcinoma 1/54; malignant mammary tumours 6/54;
female; control: Offspring: in utero, hepatomas 1/54 and leukaemias 0/54; in offspring (D)
158 male and 149 through lactation male 6/75 and female 8/65 Zymbal gland carcinoma; male
female and for 104 weeks 1/75 and female 10/65 oral cavity carcinoma; male 1/75
and female 2/65 nasal cavity carcinoma; male 1/75 and
female 1/65 skin carcinoma; male 0/75 and female 3/65
forestomach carcinomas; male 0/75 and female 9/65
malignant mammary tumours; male 2/75 and female 7/65
hepatomas; and male 6/75 and female 0/65 leukaemias
Snyder et al. (1980) reported the development of malignant
lymphomas in mice after the exposure of male C57BL mice for about 70
weeks to 960 mg benzene/m3. Goldstein et al. (1982) exposed
Sprague-Dawley (SD) rats and three strains of mice (AKR, C57BL and
CD-1) to 320 and 960 mg/m3 for their lifetime and reported a small,
but not statistically significant, increase in the incidence of
granulocytic leukaemia in CD-1 mice (2 cases) and one case of chronic
myelogenous leukaemia in SD rats (these are rare neoplasms in these
strains). Snyder et al. (1984) in a subsequent full report of this
study, also noted increases in the incidence of liver tumours and
Zymbal gland carcinomas. The incidence of malignant lymphoma in male
AKR mice exposed to 320 mg/m3 was not significantly greater than
that in controls (Snyder et al., 1984). At about the same time
Maltoni et al. (1983) reported that Zymbal gland carcinomas were
observed in SD rats exposed to benzene (960 mg/m3) for 86 weeks. At
the end of the observation period (150 weeks), female breeder rats and
their offspring had not developed increased levels of leukaemia but
had an increased incidence of other tumours such as oral and nasal
cavity carcinomas, malignant mammary carcinomas and hepatomas (Maltoni
et al., 1982c, 1983, 1989).
Benzene-induced leukaemia in experimental animals has been
reported (Cronkite et al., 1984, 1985, 1989; Cronkite, 1986). In an
attempt to mimic more closely patterns of human exposure to benzene,
C57BL/6 and CBA/Ca mice were exposed to 960 mg/m3 (300 ppm) (6
h/day, 5 days/week) for 16 weeks, followed by an observation period of
82 weeks. A highly significant increase in leukaemia was noted in
C57BL/6 mice (Cronkite et al., 1984), and a biphasic response was
reported regarding mortality and lymphoma appearance (Cronkite et al.,
1985). The first increase in lymphomas was noted at about 150 days
post-exposure, and there was increased mortality between 330 and 390
days. A second increase in lymphomas as well as solid tumours
occurred at 420 days post-exposure, the mortality again increasing at
570 days post-exposure. Cronkite et al. (1989) reported that benzene
at a concentration of 960 mg/m3 for 76 weeks was leukaemogenic in
both male and female CBA/Ca mice.
Snyder et al. (1988) reported that benzene exposure patterns in
CD-1 and C57BL mice, which were closely related to the occupational
setting (intermittent for lifetime as well as short-term high doses
for a portion of the normal life span), resulted in marked
haematotoxicity as well as being tumorigenic. Neither of the benzene
exposure patterns induced elevated incidences of leukaemia/lymphoma in
either strain. Elevated incidences of malignant tumours (Zymbal gland
and lung) were noted in both strains after intermittent (1 week
exposure, 2 weeks non-exposure) exposure (960 mg/m3, 6 h/day, 5
days/week) over the full lifetime, whereas an increase in lung tumour
incidence was noted in only the CD-1 strain after 10 weeks of exposure
to 3840 mg/m3 (6 h/day, 5 day/week) followed by a lifetime of
non-exposure.
Table 16 presents a summary of the lowest dose levels at which
various authors reported a possible causal relationship between
benzene exposure and the end-point studied.
7.6.2 Oral and subcutaneous studies
Several experiments using oral (gavage) administration of benzene
to experimental animals are summarized in Table 17, and some of the
major effects reported are given. Oral exposure to benzene has
resulted in the induction of neoplasms in 13 different tissue/organs,
namely Zymbal gland, oral and nasal cavities, mammary gland, liver,
forestomach, skin, harderian gland, preputial gland and ovary, and the
haemopoietic and lympho-reticular systems (Maltoni et al., 1983, 1985,
1989; NTP, 1986; Huff et al., 1989). Table 17 indicates the
similarity in protocols used, namely 25-500 mg benzene/kg body weight
per day via gavage, 4 to 5 times weekly for 52-104 weeks and
termination after 103-144 weeks. The lowest dose of benzene that
produced specific neoplasms varied from 25 mg/kg body weight for the
adenomas of the lung, harderian gland and liver of mice to 500 mg/kg
body weight for lymphoreticular neoplasms in rats.
7.7 Special studies
7.7.1 Immunotoxicity
The proliferative ability of B- and T-cell lymphocytes was
depressed in a short-term (6 h/day for 6 days) dose-response study
(32, 96, 320 and 960 mg/m3) on benzene in mice (Rozen et al., 1984).
Liposaccharide-induced B-cell proliferation was depressed at levels as
low as 32 mg/m3 (the range of many occupational exposures), and
phytohaemagglutinin-induced T-cell response was depressed at 96
mg/m3. Peripheral lymphocyte counts were lower at all exposure
levels, but erythrocyte counts were depressed only at 320 and 960
mg/m3. In a subsequent study it was shown that a benzene
concentration of 960 mg/m3 (6 h/day, 5 days/week), administered for
115 days to mice, reduced the number of both B-cells in the spleen and
bone marrow and T-cells in the thymus and spleen and reduced their
response to mitogens (Rozen & Snyder, 1985). Other studies have shown
that polyhydroxylated derivatives of benzene are potent inhibitors of
T- and B-cell function in vitro (Irons et al., 1982).
Table 16. Carcinogenic-related end-points observed in animals exposed to benzene by inhalationa
End-points SD rat C57BL/6J mouse CD-1 mouse CBA/Ca mouse Reference
Lymphocytic lymphoma 960/488 days Snyder et al. (1980)
Myelogenous leukaemia (acute) 960/life Goldstein et al. (1982)
Myelogenous leukaemia (chronic) 320/life 960/life Goldstein et al. (1982)
Zymbal gland carcinoma 320/life Snyder et al. (1984)
960/lifeb 960/lifeb Snyder et al. (1988)
Hepatoma 640-960 Maltoni et al. (1982a)
960/16 Cronkite (1986)
Lung adenoma 3840/lifec Snyder et al. (1988)
Nasal carcinoma 640-960/104 Maltoni et al. (1983)
Thymic lymphoma 960/16 Cronkite et al. (1984)
Lymphoma (unspecified) 960/16 Cronkite et al. (1984)
Liver tumour 320/life Snyder et al. (1984)
Granulocytic leukaemia 320/life Snyder et al. (1984)
Leukaemia 960/16 Cronkite (1986)
a Doses are expressed in mg/m3 given 4-7 h/day, 5 days/week over a number of weeks or lifetime (e.g., 960 mg/m3/16
indicates that 960 mg/m3 was given for 16 weeks); exposures shown are the lowest for which the author claims
possible causal relationship.
b 960 mg/m3 dose intermittent, i.e. 1 week followed by 2 weeks non-exposure.
c Exposed to 3840 mg/m3 for 10 weeks followed by lifetime non-exposure.
Table 17. Long-term toxicity/carcinogenicity of benzene in experimental animals after oral administrationa
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- high dose: 30 m, 50 or 250 mg/kg, only tumours reported in (C) were 4/30 f with malignant Maltoni et
Dawley, males & 35 f; low dose: 4-5 times/week mammary tumours and 1/30 with leukaemia; in dosed animals al. (1982b,
females (13 30 m, 30 f; for 52 weeks; Zymbal gland carcinoma in 2/30 f in low-dose group and 1983, 1985,
weeks old) controls: 30 m, death at 144 8/35 f in high-dose group; oral cavity carcinomas in 2/35 f 1989)
30 f weeks in high-dose group; malignant mammary tumours in 4/30 f in
low-dose group and 7/35 f in high-dose group; hepatomas in
1/35 m in high-dose group and leukaemias in 2/30 f of
low-dose group and 4/35 m and 1/35 f in high-dose group
Rat Sprague- dose: 40 m, 40 f; 500 mg/kg, 4-5 only tumours reported in (C) were 1/50 m with Zymbal gland Maltoni et
Dawley, males & control: 50 m, 50 f days/week for carcinomas; 1/50 skin carcinomas; 7/50 f malignant mammary al. (1982b,
females (6 weeks 104 weeks; tumours; 3/50 m hepatomas; 3/50 m and 1/50 f with 1983, 1985,
old) observed until leukaemias; in (D) following tumours reported: Zymbal
natural death gland carcinomas 18/40 m and 16/40 f; oral cavity
carcinomas 21/40 m and 20/40 f; nasal cavity carcinomas
3/40 m and 1/40 f; skin carcinomas 9/40 m; forestomach
acanthomas and dysplasias 10/40 m and 7/40 f; in situ
carcinomas (forestomach) 6/40 f; invasive carcinomas
(forestomach) 1/40 m; hepatomas 3/40 m and 1/40 f;
angiosarcomas 2/40 m and 3/40 f; leukaemias 1/40 m and
3/40 f
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat F344/N, dose groups: 60 m, all groups dosed number of survivors in control and dosed groups, NTP (1986);
males & females 60 f; control: 5 days/week for respectively; males: 32/50, 29/50, 24/50, 16/50; females: Huff et al.
(7-8 weeks old) 60 m, 60 f 103 weeks; males: 46/50, 38/50, 33/50, 25/50 (1989)
50, 100 or 200
mg/kg per day in control and dosed groups, respectively: Zymbal gland
females: 25, 50 or carcinomas, males: 2/50, 6/50, 10/50, 17/50; females:
100 mg/kg per day 0/50, 5/50, 5/50, 14/50
oral cavity squamous cell papilloma: m 1/50, 60/50, 11/50
and 13/50; f 1/50, 4/50, 8/50, 5/50
oral cavity squamous cell carcinoma: m 0/50, 3/50, 5/50,
70/50; f 0/50, 1/50, 4/50, 5/50
skin squamous cell papilloma: m 0/50, 2/50, 1/50, 5/50
skin squamous cell carcinoma: m 0/50, 5/50, 3/50, 8/50
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Mouse B6C3F1, dose: 60 m, 60 f; males and females: numbers of survivors in control and dosed groups, NTP (1986)
male & females control: 60 m, 60 f 25, 50 or 100 mg/kg respectively: males: 28/50, 22/50, 18/50, 7/50; females:
(6-8 weeks old) per day, 5 days/week 30/50, 25/50, 24/50, 16/50
for 103 weeks
control and dosed groups, respectively: Zymbal gland
carcinomas, males: 0/49, 1/48, 4/50, 21/49; females: 0/49,
0/45, 1/50, 3/49
mammary carcinomas and carcinosarcomas occurred at higher
incidences in the mid- and/or high-dose groups; increased
tumour incidences in dosed mice were also noted elsewhere
including the haemopoietic system, adrenals, ovary, liver,
lung and preputial gland
a m = male animals, f = female animals, (C) = control groups, and (D) = dosed groups
The primary antibody response to fluid tetanus toxoid was reduced
by 74-89% in mice that were exposed to 1280 mg benzene/m3 (6 h/day)
for 5, 7 or 22 days of exposure (Stoner et al., 1981). No effect was
seen at 160 mg/m3. These investigators concluded that the threshold
level for repression of primary antibody response was between 160 and
640 mg/m3.
Host resistance to infection by Listeria monocytogenes in mice
was reduced after exposure to benzene (Rosenthal & Snyder, 1985). The
infection rate, as determined by bacterial counts in the spleen, was
increased by 730% on day 4 post-infection after exposure to 960
mg/m3 for 5 days, but not at lower benzene exposures. In contrast,
increased bacterial counts were seen at all doses of benzene greater
than 32 mg/m3 when benzene exposure was continued after exposure to
L. monocytogenes.
Both the humoral and cellular immune responses in CD-1 mice were
altered by oral administration of benzene at 8, 40 or 180 mg/kg body
weight daily for 4 weeks. A dose-response reduction in peripheral
blood lymphocytes was reported whereas there was no effect on the
levels of neutrophils and other white blood cells (Hsieh et al.,
1988a). A dose-related biphasic splenic lymphocyte proliferative
response to B- and T-cell mitogens was also reported. At the 8 mg/kg
dose the response was enhanced, while at the 40 and 180 mg/kg per day
doses a depression was observed. A similar biphasic response was
reported for cell-mediated immunity.
7.7.2 Neurotoxicity
The neurotoxicity of benzene in experimental animals has not been
well studied. Benzene caused light narcosis after 3 min of exposure
to a level of about 144 000 mg/m3 (45 000 ppm) in rabbits (Carpenter
et al., 1944). At this dose, tremors were noted after 5 min, loss of
pupillary reflex to strong light after 6 min, involuntary blinking
after 15 min, and death after 36 min. Learning defects have been
reported in rats exposed three times intraperitoneally to 550 mg
benzene/kg body weight on days 9, 11 and 13 postpartum (Geist et al.,
1983).
Male adult CD-1 mice received ( ad libitum for 4 weeks)
drinking-water containing 31, 166 and 790 mg benzene/litre (estimated
daily doses of 8, 40 and 180 mg/kg body weight). No treatment-related
behavioural changes were observed in the test animals. However, oral
ingestion of benzene was found to alter the levels of norepinephrine,
serotonin, dopamine and catecholamine in several brain regions (Hsieh
et al., 1988b).
7.8 Factors modifying toxicity
The toxicity of benzene can be modified by several factors,
including species or strain of animal exposed, dose received, and
patterns of exposure.
As shown in Figures 1-3 (section 6.3) benzene metabolism is
complex and involves several detoxification pathways, as well as two
pathways which form the putative metabolites muconaldehyde and
benzoquinone. Henderson et al. (1989) and Sabourin et al. (1987)
demonstrated species differences for the metabolism of benzene, i.e.
mice exhibited a higher rate of metabolism with the production of more
putative toxic metabolites. These authors also reported that
increasing the dose (by oral or inhalation routes) in both rats and
mice resulted in a higher proportion of benzene being metabolized by
detoxification pathways.
The myelotoxicity of benzene in mice is much more pronounced
following a discontinuous dosing regimen than following continuous
exposure (Tice et al., 1989). These effects of exposure route and
regimen suggest that the toxicity of benzene is dependent on cell
cycle kinetics in the bone marrow.
Evidence indicates that benzene must be metabolized prior to
producing adverse effects on the haemopoietic system or leading to
carcinogenic and clastogenic effects (Snyder et al., 1981; Irons,
1985). Therefore, agents or other factors that alter the metabolism
of benzene can also modify its toxicity.
Ethanol and benzene induce formation of cytochrome P-450 IIE1 in
rabbit and rats (Johansson & Ingelman-Sundberg, 1988). The toxicity
of benzene is enhanced by ethanol, increasing the severity of anaemia
induced by benzene, lymphocytopenia and reduction in bone marrow
cellularity (Baarson et al., 1982).
Phenobarbital that induces specific isoenzymes of P-450 increases
the rate of benzene metabolism in vivo in rats resulting in
increased resistance against the leucopenic action of benzene (Ikeda
& Ohtsuji, 1971; Nakajima et al., 1985).
Sabourin et al. (1990) found no evidence of the induction of
benzene metabolism by repeated exposure of rodents to benzene.
7.9 Mechanism of toxicity
It has become increasingly clear that the impact of benzene on
the bone marrow is conferred by a combination of metabolites, rather
than by a single metabolite. Thus, Eastmond et al. (1987) showed that
there was an interaction between phenol and hydroquinone when the two
were co-administered, which resulted in a degree of myelotoxicity
greater than additive. In mice, Snyder et al. (1989) reported that
whereas phenol alone did not decrease erythrocyte production,
hydroquinone, p-benzoquinone, and muconaldehyde were effective
inhibitors of red cell production. These authors also showed that
phenol interacted with hydroquinone to produce greater-than-expected
depression of erythrocyte synthesis. A similar interaction was
observed between phenol and catechol. The most striking interaction
was observed when doses of hydroquinone and muconaldehyde were
selected which were ineffective in inhibiting erythropoiesis when
given alone, but when given together produced cessation of red cell
production. Thus, bone marrow depression appears to be the result of
the combined effects of these metabolites. A further contributing
factor, however, is the finding by Roghani et al. (1987) and Da Silva
et al. (1989) that benzene stimulates the activity of membrane protein
kinase c, an important regulatory enzyme. The effect of its
perturbation may combine with the biological effects of the various
metabolites to yield the disease we term aplastic anaemia.
It is clear that aplastic anaemia requires that benzene be
metabolized to toxic metabolites. It also appears that metabolism is
required for the production of clastogenic responses. While it seems
likely that metabolism is important for the induction of tumours,
there is very little data on this point. One report, however,
suggests a role for benzene metabolites in one type of carcinogenic
response. Busby et al. (1990) explored the ability of several known
benzene metabolites, as well as postulated benzene metabolites, to
induce lung tumours in newborn mice. They examined the effectiveness
of benzene oxide and enantiomers and racemates of benzene dihydrodiols
and diol epoxides given orally using a prescribed regimen. Lung
tumour incidence and multiplicity were increased after treatment with
benzene oxide, racemates of dihydrodiol and by diol epoxide-2.
Benzene and diol epoxide-1 were inactive in this system.
Although it is well known that benzene produces bone marrow
damage resulting from the production of benzene metabolites in liver,
it is also well known that these metabolites do not produce
hepatotoxicity. From the mechanistic point of view, it appears that
the liver is protected against damage from quinone metabolites of
benzene by the enzymes DT-diaphorase (Smart & Zannoni, 1985) and
carbonyl reductase (Wermuth et al., 1986). These enzymes prevent the
metabolic activation of phenolic metabolites to their otherwise toxic
quinones. The bone marrow is relatively deficient in these enzymes,
but partial protection of the marrow has been afforded through the
administration of high doses of ascorbic acid (Smart & Zannoni, 1985).
Metabolic activation of benzene metabolites, once they reach the
bone marrow, may lead to eventual toxicity. For example, in bone
marrow stromal macrophages, phenol (but not benzene) can be
metabolized and in the process inhibit RNA synthesis in macrophages,
thus possibly inhibiting the production of the haemopoietic factor
(Post et al., 1985). It has also been suggested (Kalf et al., 1989)
that the cyclooxygenase component of prostaglandin synthetase plays a
significant role in the metabolism of benzene and/or its metabolites
in bone marrow. Administration of indomethacin or other
cyclooxygenase inhibitors protected against benzene-induced bone
marrow depression and micronucleus formation.
A general mechanism for benzene-induced bone marrow depression
might be that benzene metabolites arising in the liver travel to the
bone marrow where further metabolic activation occurs. The newly
generated metabolites, perhaps acting in concert with unmetabolized
benzene in cell membranes, act upon target cells such as stem cells,
progenitor cells and stromal cells in the marrow to produce bone
marrow depression. Chromosomal damage may ensue, which is reflected
in clastogenesis observed in circulating lymphocytes or bone marrow
cells. The point in this series of events that leads to a
leukaemogenic response requires further examination once an adequate
model for the disease in animals has been established.
8. EFFECTS ON HUMANS
Acute inhalation and oral exposures of humans to high
concentrations of benzene have resulted in central nervous system
depression and death. The most noted effects resulting from
longer-term exposure to lower levels of benzene are haematotoxicity,
immunotoxicity and neoplasia.
8.1 General population and occupational exposure
The human health effects after exposure to benzene are
qualitatively the same for the general population and those exposed in
the workplace. To avoid duplication, the effects on both groups
(general population and workers) will be discussed together, with
emphasis on exposure levels and duration of exposure. The
quantitative response will be determined from such levels of total
daily intake.
8.1.1 Acute toxicity
Exposures in the general population that result in acute toxic
effects are usually related to accidents and misuse or abuse of
benzene. Many deaths and serious health effects have resulted from
benzene exposures after deliberate the "sniffing" of glue and other
products which contain benzene as a solvent (Winek & Collom, 1971).
Blood levels in people who have died as a result of "sniffing" glue
have ranged from 0.94 to 65 mg/litre (Winek et al., 1967; Winek &
Collom, 1971). Autopsy observations in these individuals included
pulmonary haemorrhage and inflammation, renal congestion and cerebral
oedema.
It has been estimated that exposure to benzene concentrations of
about 64 000 mg/m3 (20 000 ppm) for 5-10 min can result in
fatalities, 24 000 mg/m3 (7500 ppm) for 30 min is dangerous to life,
4800 mg/m3 (1500 ppm) for 60 min causes serious symptoms, 1600
mg/m3 (500 ppm) for 60 min leads to symptoms of illness, and 160-480
mg/m3 (50-150 ppm) for 5 h causes headache, lassitude, and weakness,
while 80 mg/m3 (25 ppm) for 8 h is without clinical effect (Gerarde,
1960). The clinical signs of acute toxicity from benzene include CNS
depression, cardiac arrhythmia, and eventually asphyxiation and
respiratory failure if exposures are at the lethal level (Andrews &
Snyder, 1986). Mild CNS symptoms are rapidly reversible following
cessation of exposure and there is no evidence that they result in
neurological brain damage (Marcus, 1990).
The single acute oral lethal dose in humans has been estimated to
be 10 ml of benzene (8.8 g) (Thienes & Haley, 1972). Clinical signs
of toxicity after acute oral exposure include staggering gait,
vomiting, shallow and rapid pulse, somnolence, loss of consciousness,
delirium, pneumonitis, profound CNS depression, and collapse
(Sandmeyer, 1981). High but sublethal oral doses may produce one or
more of the following symptoms: dizziness, visual disturbances,
euphoria, excitation, pallor, flushing, breathlessness and
constriction of the chest, headache, fatigue, sleepiness, and fear of
impending death (Sandmeyer, 1981). In addition to the autopsy
findings noted above, ingestion of benzene has been reported to cause
gastrointestinal ulceration (Appuhn & Goldeck, 1957).
No studies on the acute toxicity of benzene after dermal exposure
are available.
8.1.2 Effects of short- and long-term exposures
The most significant health effects from short- or long-term
exposure to benzene are haematotoxicity, immunotoxicity, neurotoxicity
and carcinogenicity. Three types of bone marrow effects have been
reported in response to benzene exposure; these are bone marrow
depression leading to aplastic anaemia, chromosomal changes and
carcinogenicity.
8.1.2.1 Bone marrow depression; aplastic anaemia
Several types of blood dyscrasias, including pancytopenia,
aplastic anaemia, thrombocytopenia, granulocytopenia, lymphocytopenia
and leukaemia, have been noted after exposure to benzene. These
changes are a continuum and not a discrete disease entity. Which
effect is noted will depend on the dose, length of exposure and the
stage of stem cell development affected (Galton, 1986). As in
experimental animals, the primary target organ of benzene that results
in haematological changes is the bone marrow. It has been suggested
that the cells at highest risk are the rapidly proliferating stem
cells (Marcus, 1990).
A study of 32 patients that were chronically exposed by
inhalation to benzene levels of 480-2100 mg/m3 (150-650 ppm) for 4
months to 15 years revealed pancytopenia with hypoplastic,
hyperplastic or normoblastic bone marrow. Eight of the 32 individuals
showed thrombocytopenia which resulted in haemorrhage and infection
(Aksoy et al., 1972). Haematotoxicity after prolonged exposure has
also been reported in rotogravure workers exposed for 6-60 months at
concentrations of 36-3485 mg/m3 (11-1069 ppm) (Goldwater, 1941) and
77-3400 mg/m3 (24-1060 ppm) (Erf & Rhoads, 1939), shoe factory
workers exposed to 96-670 mg/m3 (30-210 ppm) for 3 months to 17
years (Aksoy et al., 1971), and rubber factory workers exposed to up
to 1600 mg/m3 (500 ppm) (Wilson, 1942). Kipen et al. (1988)
reported on rubber workers who were exposed to benzene during the
1940s. An inverse relationship was found between the mean yearly
white blood cell count and the year that the count was made,
suggesting that exposures to benzene were very high in the early
1940s. As estimated by Crump & Allen (1984), benzene exposures
decreased from 438 mg/m3 (137 ppm) in 1940 to 102 mg/m3 (32 ppm)
in 1948. In a follow-up letter, Hornung et al. (1989) pointed out
that a similar rise in white blood cells counts was seen in
pre-employment physical examinations occurring over the same time
period at the same facility, and that that the trend could not be
attributed soley to benzene exposure. In a study of 1008 male
shoemakers in Florence, excess mortality from aplastic anaemia was
observed (SMR = 1566, 95% CI 547-3264), based on 6 deaths. All cases
of aplastic anaemia occurred among workers first employed before 1964
when the level of exposure to benzene was assumed to be highest (Paci
et al., 1989).
At levels less than 32 mg/m3 (10 ppm) no haematologic effects
have been observed (Collins et al., 1991). These authors found no
haematological effects in 200 benzene-exposed workers (10 year TWA of
0.03-4.5 mg/m3, 0.01-1.4 ppm) or in 268 control workers in the same
plant. In an earlier study of 70 workers in a coke oven by-product
recovery facility, Hancock et al. (1984) measured the levels of red
blood cells, white blood cells and haemoglobin in three groups exposed
to different concentrations of benzene (average, 34 mg/m3, 10.5 ppm;
range, 3.2-534 mg/m3, 1-167 ppm) and one non-exposed control group.
No significant differences between groups were noted in these
haematological parameters.
No data are available regarding haematotoxicity after short-term
or chronic oral or dermal exposure.
8.1.2.2 Immunological effects
As noted in animal studies (section 7.7.1), the immunological
manifestations of benzene toxicity are related to effects on the bone
marrow, resulting in changes to both humoral and cellular acquired
immunity. Workers (76) exposed to benzene (10-22 mg/m3, 3-7 ppm),
as well as to toluene and xylenes, for periods of 1-21 years were
examined for the presence of leucocyte agglutinins and levels of
circulating immunoglobulins. In 10 out of 35 workers where blood was
taken during working hours, the adverse effect of agglutinins reacting
with autoleucocytes was noted (Lange et al., 1973a). In addition, it
was found that the sera from the 35 workers had increased levels of
IgM and decreased levels of IgG and IgA immunoglobulins (Lange et al.,
1973b). The simultaneous exposure of these workers to solvents other
than benzene makes it difficult to interpret these results.
Autoimmunity, as shown by the pressure of antibodies against
leucocytes, platelets, and erythrocytes in the sera of exposed
workers, has been reported (Renova, 1962). Workers have been reported
to have an increased susceptibility to allergies (Aksoy et al., 1971)
when exposed to benzene concentrations as low as 96 mg/m3 (30 ppm).
A loss of leucocytes was observed in several studies of workers
reported to be exposed to benzene levels of 96-2080 mg/m3 (30-650
ppm) (Aksoy et al., 1971, 1974a; Aksoy, 1987). Signs of preleukaemia,
including loss of leucocytes and other blood elements and enlarged
spleens, were reported in one study (Aksoy et al., 1974a). Kipen et
al. (1989) and Yin et al. (1987) also reported decrease in circulating
lymphocytes and other blood elements at benzene exposures ranging from
48 to 240 mg/m3 (15-75 ppm). The number of T-cell lymphocytes were
found to have been reduced in workers exposed chronically to benzene,
toluene and xylene (Moszczynski, 1981).
In a study of workers exposed to low average concentrations of
benzene (< 32 mg/m3), there was no difference in cell cycle
kinetics of phytohaemagglutinin-stimulated lymphocytes in 66 male
workers of a refinery population when compared with 33 control workers
in the same refinery (Yardley-Jones et al., 1988).
No studies are available regarding the immunotoxicity of benzene
in humans after oral or dermal exposures.
8.1.2.3 Chromosomal effects
Both structural and numerical chromosomal aberrations have been
observed fairly consistently in the lymphocytes and bone marrow cells
of individuals occupationally exposed to benzene. It is now generally
accepted that benzene is a human clastogen (IARC, 1987a; Huff et al.,
1989). Increases in the number of both unstable and stable
chromosomal aberrations were observed in men, even 2 years after
cessation of workplace exposure (Tough & Court Brown, 1965). Up to
70% aneuploid lymphocytes were found in five women with benzene
haemopathy (Pollini et al., 1969), the effects still being
demonstrable 5 years post-exposure. Similar effects were observed in
the lymphocytes of workers in a rotogravure plant that had been
exposed to very high levels of benzene 400-1700 mg/m3 (125-532 ppm)
for 1-22 years (Forni et al., 1971a,b).
Recent studies by Yardley-Jones et al. (1988, 1990) revealed much
lower responses in the lymphocytes of workers exposed to low
concentrations of benzene (average < 32 mg/m3). In a study of 66
refinery workers and 33 controls, no alteration in cell cycle kinetics
was noted nor was there any increase in the level of SCEs
(Yardley-Jones et al., 1988). The lymphocytes from 48 of the workers
and 29 of the controls were analysed for chromosomal aberrations.
According to Yardley-Jones et al. (1990), the increase in aberrations
(particularly chromatid deletions and gaps) was of borderline
significance in parametric statistical tests, but was significant
using Fisher's exact test. No lifestyle factors had any consistent
effect on the incidence of chromosomal aberrations.
In an attempt to determine whether benzene and its metabolites
damage certain human chromosomes preferentially, Sasiadek et al.
(1989) examined the karyotypes of 33 workers exposed to less than 99
mg/m3 (31 ppm). At these levels no clinical or haematological
symptoms were noted in 31 workers, but pancytopenia was observed in
two workers. Nonrandom breaks and gaps were observed in the exposed
group; chromosomes two, four and nine were more prone to breaks and
chromosomes one and two more prone to gaps. The results of this study
are of limited value in view of the small number of controls and the
fact that all participants smoked.
Other studies that corroborate the clastogenicity of benzene in
humans have been reviewed by IARC (1982), Dean (1985a) and Kalf
(1987).
8.1.2.4 Carcinogenic effects
The fact that benzene is a human leukaemogen has been well
established by epidemiological and case studies (IARC 1982, 1987b),
most of which have dealt with industrial exposures. The
epidemiological studies reported have been selected because they
contain sufficient quantitative data on exposure and effects to permit
a discussion of the dose-response relationship. Some case reports are
summarized in Table 18, and prospective epidemiological studies are
summarized in Table 19. Of the two major classes of leukaemia
(granulocytic and lymphocytic), the most consistent evidence for a
causal relationship in humans has been found between benzene exposure
and myeloid leukaemia (Goldstein, 1988).
One case study followed the course of 44 patients with
benzene-induced pancytopenia and found that 6 of them later developed
leukaemia (Aksoy & Erdem, 1978).
The first bridge between case reports and a formal
epidemiological investigation was conducted in the early 1970s (Aksoy
et al., 1974b). These investigators reported on a series of cases
from an estimated population of 28 500 Turkish shoe workers exposed
since the 1950s to solvents and adhesives containing high levels of
benzene. Aplastic anaemia was first observed in 1961, and 26 patients
with acute leukaemia were observed by 1967. Peak exposure levels of
benzene were reported to be 96-670 mg/m3 (30-210 ppm), with rare
excursions to 2100 mg/m3 (650 ppm), for periods of 1 to 14 years
(mean 9.7 years). From this group of workers, a leukaemia incidence
rate (number of cases per 100 000 per year) of 13 was estimated
compared with a rate of 6 for the general population (Aksoy et al.,
1974b). It was unclear to the Task Group, from the description of the
authors, which methods were used to ascertain cases and from which
exposed population these cases were derived.
A study showing an excess risk of leukaemia in a cohort of 748
male workers producing "rubber hydrochloride" in three plants in two
locations within the USA during 1940-1949 (a product made from natural
rubber suspended in benzene) was first reported by Infante et al.
(1977). A follow-up study of this cohort was reported by Rinsky et
al. (1981) and this was subsequently updated (Rinsky et al., 1987).
In this most recent follow-up, the cohort definition was expanded to
include workers employed between 1940 and 1965 who had a cumulative
exposure to benzene of 3.2 mg/m3 per day (1 ppm/day) or more. The
Table 18. Case studies of workers occupationally exposed to benzene
Group studied Exposure Condition observed Author's References
conclusiona
44 pancytopenic patients exposed to benzene 480-2100 mg/m3 (150-650 leukaemia PC Aksoy & Erdem
in adhesives ppm); 4 months to 15 myeloid metaplasia * (1978)
years
42 leukaemia patients and 21 patients with not given leukaemia DC Aksoy (1980)
other malignancies; 47 were shoe workers, the multiple myeloma PC
remainder in other occupations using benzene myeloblastic leukaemia *
solvents acute erythroleukaemia *
preleukaemia *
malignant lymphoma PC
paroxymal nocturnal
haematuria *
lung cancer (all heavy
smokers) *
Table 18 (contd).
Group studied Exposure Condition observed Author's References
conclusiona
6 of 94 Hodgkin's patients who had been 480670 mg/m3 (150-210 Hodgekin's disease PC Aksoy et al.
exposed to benzene adhesives ppm); 1-28 years (1974a)
A 35-year-old man who had used benzene 200-1640 mg/m3; 18 months subacute granulocytic DC Sellyei & Kelemen
8 years earlier as a paint solvent leukaemia (1971)
6 leukaemia patients in different occupations levels unknown; 1-20 years haemocytoblastic leukaemia DC Vigliani & Saita
all using benzene solvents (1964)
A 51-year-old chemical worker exposed to 3.2 mg/m3 (< 2 ppm); acute myelogenous PC Ott et 81. (1978)
benzene 15 years earlier 18 months leukemia
a DC = direct correlation; PC = possible correlation; * = no conclusion made
Table 19. Epidemiological studies of workers exposed to benzene
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Incidence of leukaemia in 96-670 mg/m3 aplastic anaemia, 26 200 NR Aksoy et al.
Turkish shoe workers 1950-1965 rarely 2100 mg/m3; acute leukaemia (1974b)
(28 500 shoe, slipper and 1-15 years (mean,
handbag workers) 9.7 years)
Mortality study of rubber within legal limits of malignomas of lymphatic 14 260 NR Infante et
workers exposed to benzene period, i.e. 320 mg/m3 and haemopoietic P < 0.05 al. (1977)
between 1940 and 1949 (100 ppm) down to systems; myeloid and
32 mg/m3 (10 ppm) monocytic leukaemia 7 506 NR
for up to 10 years P < 0,02
Mortality studyb of pliofilm from < 40 ppm-years lymphatic and haemopoietic 15 227 127-376 Rinskyetal.
workers exposed to benzene to > 400 ppm-years neoplasms (1987)
between 1940 and 1965 with
a period at risk from 1950 leukaemia, total 9 337 159-641
to 1981 < 40 ppm-years 2 109 12-394
40-200 ppm-years 2 322 36-1165
205-400 ppm-years 2 1186 133-4289
> 400 ppm-years 3 6637 1334-19 393
multiple myeloma total 4 398 110-1047
< 40 ppm-years 3 458 92-1339
> 40 ppm-years 1 5347 70-29 753
Mortality studyc of 956 > 0.3-114 mg/m3 leukaemia (total) 4 194 52-488 Bond et al.
workers employed at a chemical (> 0.1-35.5 ppm) acute myelogenous 4 444 P < 0.05 (198613)
company between 1940 and estimated TWA for leukaemia
1982 up to 34 years
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective mortality study < 3.2 mg/m3 leukaemia 0 0 Tsai et al.
of workers (454) employed at (< 1 ppm) in 84% (1983)
a Texas refinery between 1952 samples (median,
and 1981 1.6 mg/m3; 0.5 ppm)
in benzene-related
areas
Mortality study of chemical < 15, 15-60 and > 60 lymphatic and haemopoletic, Wong
workers in 7 plants, > 6 ppm-years total: (1987)
months on job at single plant non-exposed 3 39 7-101
between 1946 and 1975 < 15 ppm-years 5 91 30-213
(3636 males) 15-60 ppm-years 5 147 48-343
60 ppm-years 5 179 57-409
leukaemia, total:
non-exposed 0 -
< 15 ppm-years 97 12-349
15-60 ppm-years 78 2-434
60 ppm-years 276 57-806
Retrospective cohort of 259 no benzene levels lymphatic and haemopoietic 4 377 109-1024 Decouflé et
male chemical workers reported; benzene neoplasms (workers with al. (1983)
employed between 1947 and used in large > 1 year of employment)
1960 quantities
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective cohort of shoe exposed for up to 29 aplastic anaemia (males); 4 1566 P < 0.05 Paci et al.
workers (1008 males, 1005 years; levels of leukaemia (males) 6 400 146-870 (1989)
women) employed between benzene not reported
1939 and December 1984 and
still employed in plant in
January 1950
Retrospective cohort study grab samples; means acute and chronic 30 cases 574 P < 0.01 Yin et al.
(28 460 workers in 233 factories); between 10 and 1000 leukaemia 5 deaths (1987)
reference population 28 257 mg/m3
in 83 machine production,
and clothing factories 50-500 mg/m3 found in
most plants
Mortality study of coke plant non-exposed, coke leukaemia (non-exposed) 13 135 NR Swaen et al.
workers (5639) with > 6 months ovens and by-product leukaemia (coke-oven) 6 163 NR (1991)
work between 1945 and 1969 workers; levels of leukaemia (by-product) 7 85 NR
benzene not reported
Retrospective cohort of 391 mean time-weighted leukaemia Hurley et
benzole workers in 2 cohorts average exposure of by-product 1 1 98 2-557 al. (1991)
(cohort 1, 84 workers; coke by-product by product 2 1 76 2-429
cohort 2, 307 workers) workers in Britain
in 1980s 4.2 mg/m3
(1.3 ppm)
a SMR - Standard mortality ratio
b Follow-up of cohort described by Infante et al. (1977)
c Follow-up of cohort described by Ott et al. (1978)
NR = not reported
study included 1165 white males followed from 1950 to 1981. For the
analysis the cohort was divided into four cumulative exposure groups:
< 128 mg/m3-years (< 40 ppm-years), 128-640 mg/m3-years (40-200
ppm-years), 640-1280 mg/m3-years (200-400 ppm-years) and > 1280
mg/m3-years (> 400 ppm-years). A statistically significant excess
risk was observed for all lymphatic and haemopoietic neoplasms (15
observed deaths compared to 6.6 expected; SMR = 227, 95% CI, 127-376).
There were nine deaths from leukaemia compared to an expected 2.66
(SMR = 337, 95% CI, 159-641), and 4 deaths from multiple myeloma (SMR
= 398, 95% CI, 110-1047). A strong positive trend in leukaemia
mortality was obtained with increasing cumulative exposure. Within
the four cumulative exposure groups there were 2,2,2, and 3 deaths
with SMRs of 109, 322, 1186 and 6637, respectively. In order to
investigate further the shape of the exposure-response curve, Rinsky
et al. (1987) conducted a nested case-control study by matching each
of nine deaths due to leukaemia with ten controls. A conditional
logistic regression analysis described a significant positive
association between estimated level and average duration of benzene
exposure and leukaemia that was projected downward to levels of zero
accumulated exposure over a working lifetime. From this model it was
calculated that an exponential relationship existed between benzene
exposure and the developmental leukaemia.
When actual exposure measurements did not exist, Rinsky et al.
(1987) estimated exposures to benzene by averaging historical annual
measured benzene levels from seven existing industrial hygiene survey
sources. The majority of measurements occurred after 1963, but some
data existed as early as 1946. Where no sampling data could be found,
exposure levels were estimated by interpolation from existing
information. Alterative exposure estimates and subsequent reanalyses
have been developed by Crump & Allen (1984) and Paustenbach et al.
(1992). The differences in exposure estimates between Crump & Allen
(1984) and Rinsky et al. (1987) centre primarily on assumptions of
benzene exposure prior to 1946 where no historical data exist.
Paustenbach et al. (1992) gathered additional information and
considered other factors that modified the estimates of exposure over
the entire period during which rubber hydrochloride plants operated
(1936 to 1976) to develop a new set of exposures over time. For the
most part the exposures estimated by Paustenbach et al. (1992) are
higher than those reported by Rinsky et al. (1987) and Crump & Allen
(1984).
In a retrospective study of 594 employees of a chemical company
exposed to levels of benzene between 0.3 and 114 mg/m3 (0.1-35.5
ppm) for up to 34 years, no statistically significant increase in
total mortality was reported (Ott et al., 1978). There were three
cases of myelocytic leukaemia compared to an expected incidence of 0.8
cases (significant at the P < 0.05 level). Some workers in this
cohort were also exposed to vinyl chloride, arsenicals and several
other potentially carcinogenic chemicals. A follow-up study of these
workers expanded the cohort by 362 potentially exposed workers (Bond
et al., 1986b). Four deaths from myelogenous leukaemia were reported
and the SMR for all leukaemias was 194 (95% CI, 52-488). The
difference between observed and expected values was statistically
significant only when myelogenous leukaemia was considered (4
observed, 0.9 expected; P = 0.01).
Wong (1987) reported a significant dose-response relationship
between cumulative exposure to benzene and mortality from leukaemia
and all lymphopoietic cancers combined. The mortality experience of
3536 workers who had continuous exposure to benzene was compared to
that of an internal comparison group of 3074 workers not exposed to
benzene but who had worked at the same plant. The 3536 exposed
workers were categorized into cumulative exposure categories of < 48
mg/m3-years (< 15 ppm-years), 48 to 192 mg/m3-years (15 to 60
ppm-years), and > 192 mg/m3-years (> 60 ppm-years). There was an
increasing trend in the SMRs for lymphatic and haemopoietic cancers as
exposure increased (SMR = 35, 91, 147 and 175 for the non-exposed and
the 3 exposure categories, respectively (P = 0.02). The respective
SMRs for leukaemia were 0, 97, 78 and 275 (P = 0.01). It should be
noted that none of the six leukaemia deaths was from acute myeloid
leukaemia. In addition, the highest category of exposure started at
only 192 mg/m3-years (60 ppm-years), the equivalent of 32 mg/m3
(10 ppm) annually for only a six-year working career. The
exposure-response relationship between cumulative benzene exposure and
non-Hodgkin's lymphoma was of marginal statistical significance.
A retrospective cohort mortality study was conducted on 259 male
employees at a chemical plant in the USA where benzene had been used
in large quantities (Decouflé et al., 1983). The study group included
workers employed between 1947 and 1960, and workers were followed
until 1977. Among workers with more than one year of employment, a
statistically significant excess risk was observed for neoplasms of
the lymphatic and haemopoietic systems (SMR=377; 95% CI 109-1024, 4
deaths). No SMR was given specifically for mortalities to leukaemia
and multiple myeloma. Three of these deaths were leukaemias and the
fourth a multiple myeloma.
Coke-oven workers and some workers at coke by-product plants are
exposed to low levels of benzene. A study in the Netherlands examined
5639 workers in a coke plant and a comparison group of 5740 workers in
a nitrogen-fixation plant (Swaen et al., 1991), employed for at least
six months between 1945 and 1965. The SMR for leukaemia in by-product
benzene plant workers was 85 (7 deaths), in coke-oven workers 163 (6
deaths), and in non-exposed workers 135 (13 deaths). Among the 222
workers in the benzene plant, no indication of an increased leukaemia
risk was found, but the expected number was small.
Hurley et al. (1991) have reported preliminary results on the
mortality of 6520 male coke plant workers from 27 plants in the United
Kingdom. Personal air samples were taken from 84 benzole workers from
14 plants in one cohort with levels of < 0.6-22 mg/m3 (< 0.19-7
ppm) and 307 benzole workers from 13 plants in the second cohort with
levels of < 0.6-48 mg/m3 (< 0.19-14.99 ppm). Mean time-weighted
average concentrations for benzole house workers in the United Kingdom
in the 1980s was considered to be about 4.2 mg/m3 (1.3 ppm). No
increased risk of mortality from leukaemia was reported in either
cohort (cohort 1 SMR = 98, 95% CI 2-557, 1 death; and cohort 2 SMR =
76, 95% CI 2-429, 1 death). These SMRs have been calculated utilizing
data from all 1293 by-product workers. Only a proportion of the
by-product workers had worked in the benzene plants where the greatest
exposure to benzene occurred. The SMR for leukaemia among 2349
coke-oven workers was 34 for cohort 1 (95% CI 0-186, 1 death) and 35
for cohort 2 (95% CI 1-192, 1 death).
In a retrospective cohort study from China encompassing 28 460
workers exposed to benzene in 233 factories, 30 cases of leukaemia
(23 acute, 7 chronic) were found, as compared to four cases in a
reference cohort of 28 257 workers in 83 machine production, textile
and cloth factories (Yin et al., 1987, 1989). The mortality rate (per
100 000 person-years) from leukaemia was 14 among the exposed and 2
among the unexposed (SMR, 574; P < 0.01). Mortality was especially
high for workers engaged in organic synthesis, painting and rubber
production. The mortality from leukaemia for cases that had
previously experienced benzene poisoning was 701/100 000 person-years.
Grab-samples of benzene in air were taken during the time of the
survey in workplaces where cases of leukaemia were observed; the mean
concentrations varied over a wide range (from 10 to 1000 mg/m3) but
the range was 50-500 mg/m3 in most locations. The mortality
incidence from all malignant neoplasms was higher in the exposed group
(123 per 100 000) than in controls (55 per 100 000). In addition, a
statistically significant excess risk for lung cancer (SMR = 231) was
observed in male workers exposed to benzene (Yin et al., 1989).
However, little description was given of the methods used to eliminate
the possible confounding effects of smoking.
A statistically significant excess risk for aplastic anaemia
(SMR = 1566, 4 deaths) and for leukaemia (SMR = 400, 95% CI, 146-870,
6 deaths) was reported for 1008 male workers in a shoe factory in
Florence, Italy. Included were workers employed on or after January
1950 and vital status of cohort members was ascertained through 1984.
No workplace monitoring was reported. The period of maximum benzene
exposure was considered to be 1953-1962, given the amount of benzene
used per day (about 30 kg) (Paci et al., 1989), and all cohort members
who developed aplastic anaemia and leukaemia were employed during this
period.
The results of a cohort study of 34 781 workers in eight oil
refineries in the United Kingdom which examined workers employed for
1 year between 1950-1975 gave no indication of increased mortality
from leukaemia (Rushton & Alderson, 1981a). Within this cohort a
nested case-control study was conducted to investigate the association
between exposure to benzene and leukaemia (Rushton & Alderson, 1981b).
The 36 leukaemia cases and their controls were allocated to "low",
"medium", and "high" benzene exposure categories based on their job
history. The authors reported a significant (P = 0.05) association in
the combined medium and highly exposed workers compared to those in
the low exposure group (RR = 3.0, 95% CI, 1.2-7.2).
A study (Tsai et al., 1983) of 454 petroleum refinery workers in
the USA employed between 1952 and 1978 in the petrochemical units
showed no deaths from leukaemia (0.4 expected). However, the median
exposure to benzene throughout the refinery was 0.45 mg/m3 (0.14
ppm), and only 16% of 1394 personal samples, taken between 1973 and
1982 (inclusive), contained more than 3.2 mg/m3 (1 ppm). The median
exposure intensity in "benzene-related units: petrochemical units" was
1.7 mg/m3 (0.53 ppm). No significant changes in blood indices
(counts of white and of red blood cells, haemoglobin, haematocrit,
platelets, clotting and bleeding times) were reported.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 General population
Benzene is ubiquitous in the environment, resulting in the
exposure of most humans to trace levels (or more) of this chemical.
Exposure in the general population is primarily to air-borne benzene
and derives from active and passive tobacco smoke, industrial
activity, and use of the automobile (gasoline fumes from refilling,
etc., and exhaust emissions). Estimates of the daily amounts of
benzene consumed in drinking-water and food-stuffs vary considerably
and are of the order of µg/day. Depending upon the assumptions made
with respect to levels of benzene from tobacco products and
foodstuffs, estimates for the exposure of the general smoking
population in industrialized countries range from 2000 to 3500 µg
benzene/day. Adult (70 kg) non-smokers are considered to be exposed
to about 200 to 1700 µg benzene/day (about 3 to 25 µg/kg body weight
per day). It would be helpful to have more information on total human
exposure, particularly in developing countries.
9.2 Occupational exposure
The major factors controlling industrial exposure to benzene are
process technology, worker practices and the efficiency and
sophistication of engineering controls. When appropriate engineering
controls are in place, available monitoring data indicate that
exposures of workers involved in the production, handling and use of
benzene and benzene-containing materials vary from non-detectable
levels to approximately 15 mg/m3 (8-h TWA), in addition to the
amounts estimated for the general population. In developing countries
the exposure can be several times higher. Due to the nature of the
processes involved, a small percentage of workers may be exposed to
more than 320 mg benzene/shift. In some developing countries, benzene
exposure may be sufficiently high to cause acute toxicity. Dermal
exposure to benzene has generally not been included in these
estimates. Validated benzene-specific biological markers of exposure
to low levels are not available.
9.3 Toxic effects
Acute lethal doses of benzene in experimental animals cause
narcosis, ventricular tachycardia and respiratory failure. The
threshold for narcotic effects in rats is about 13 000 mg/m3.
Reported oral LD50 values in rats vary from 3000 to 8100 mg
benzene/kg body weight and the 4-h LC50 in rats has been reported to
range between 32 600 and 44 600. However, although the clinical
pathological observations in animals are relevant to humans, the
latter would not be expected to be exposed to such high levels for
such long periods of time.
In humans, exposure to high concentrations of benzene (e.g.,
65 200 mg/m3 for 5-10 min) can result in central nervous system
depression, cardiac arrhythmia, respiratory failure and death, while
exposure to levels of benzene between 163 and 489 mg/m3 for 5 h
leads to headaches, lassitude and general weakness. The single acute
oral dose that has been reported to be lethal to humans is 8800 mg/kg
body weight.
9.3.1 Short-term and long-term exposures; organ toxicity
The most significant adverse effects from short- or long-term
exposure to benzene are haematotoxicity, i.e. bone marrow suppression,
immunotoxicity, genotoxicity and carcinogenicity.
9.3.1.1 Haematotoxicity; bone marrow depression
The level, timing and pattern of exposure are extremely important
factors in determining the incidence and severity of haematological
and bone marrow changes. A significant species difference (rats and
mice) in effects has also been reported, probably a reflection of the
more rapid metabolism of benzene by mice and the production of a
higher proportion of putative toxic metabolites than in rats. In rats
and mice, decreases in haematological cell counts (leucopenia and
haematocrit) and in bone marrow cellularity generally occur only after
several weeks of exposure to levels of benzene between 320 and 978
mg/m3, the mouse being more sensitive than rats. The lowest
reported exposure in experimental animals leading to haematological
effects was 32 mg/m3 (6 h/day, 5 days/week for 178 days) in male
mice. After oral administration of benzene to rats and mice for 120
days, leucopenia was observed in both male and female rats, and
lymphoid depletion in the B-cells of the spleen was observed in both
male and females at doses of > 200 mg/kg body weight. In mice, no
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females given 25, 50, 100, 200, 400 or
600 mg/kg body weight (5 days/week).
9.3.1.2 Mechanism of action and metabolism
In humans, a spectrum of blood dyscrasias, including
pancytopenia, aplastic anaemia, thrombocytopenia, granulocytopenia,
lymphocytopenia, myeloid leukaemia and acute leukaemia, can result
from benzene exposure. The dose, length of exposure and the stage of
stem cell development affected will determine which effect is
observed. Pancytopenia was noted in 32 patients exposed to 489-2119
mg benzene/m3 for periods of between 4 months and 15 years. At
levels of benzene between 102 and 438 mg/m3, haematotoxicity in
rubber factory workers was reported, the correlation being better
between benzene exposure and WBC counts than in the case of RBC
counts. However, at levels of benzene less than 32 mg/m3, there is
only weak evidence for a leukaemogenic effect; no haematological
effects were noted in 200 workers exposed to 10 year TWA benzene
levels of 0.03-4.5 mg/m3.
The hepatic metabolism of benzene is responsible for
detoxification of benzene via the formation of etheral sulfate,
glucuronides and glutathione conjugates. It also leads to the
production of metabolites, such as hydroquinone, p-benzoquinone,
muconaldehyde and perhaps others, which appear to be required for the
production of benzene toxicity in bone marrow. Current concepts of
the mechanism of benzene toxicity suggest that it is the result of the
combined effects of several metabolites, perhaps acting in concert
with unmodified benzene, to adversely alter the functions of stem
cells, progenitor cells and stromal cells in the bone marrow. It has
been postulated that the specific intracellular targets are proteins
and nucleic acids. The terminal result of these biochemical insults
is the development of aplastic anaemia. It is likely that benzene
metabolites damage chromosomes by causing DNA or protein adduct
formation or generating oxidative damage to DNA, which may contribute
to the chromosomal changes associated with benzene exposure. Animal
models exist for studying the mechanism of benzene-induced aplastic
anaemia and chromosome damage. However, no acceptable model for
benzene-induced leukaemia has been developed.
Since bone marrow depression and the production of leukaemia both
involve the bone marrow, it is important to ascertain the relationship
between these two biological effects of benzene. The specific
question in need of an answer is: Is it necessary for aplastic
anaemia to develop before leukaemia can be exhibited? In humans, the
continuum of effects of benzene on bone marrow involves first the
production of either anaemia, leucopenia or thrombocytopenia, and this
is followed by pancytopenia. Aplastic anaemia is the ultimate form of
bone marrow depression. Leukaemia has also been associated with
benzene exposure. In addition to studying the relationship between
these two phenomena, it is important to study the role of metabolites
in each process.
Several authors have recently used pharmacokinetic models to
extrapolate from animal experiments to expected human dosimetry. Some
models require the use of physiological parameters, partition
coefficients and metabolic data. Of these, the data on such metabolic
parameters in humans are least well known. With increasing
information on appropriate human metabolic data, such models will be
useful in extrapolating from animals to humans and from high- to
low-dose exposure.
9.3.1.3 Immunotoxicity
Benzene-induced immunological effects are probably a reflection
of bone marrow toxicity. Immunological function was depressed in mice
exposed to benzene levels between 32 and 96 mg/m3, 6 h/day, for 6
days. In mice, polyhydroxylated metabolites of benzene have been
shown to depress B- and T-cell functions. Although the relevance of
the animal data to human immunological functions has not been
established, human immunological alterations have been observed after
exposure to benzene.
In workers exposed to, but not seriously intoxicated by, benzene
(10-22 mg/m3), the serum complement levels of IgA and IgG were
decreased but the levels of IgM were slightly increased. An increased
susceptibility to allergies was found in workers exposed to benzene
levels as low as 96 mg/m3. A loss of leucocytes and other blood
elements has been noted at benzene levels ranging between 48 and 240
mg/m3. A study of low exposures to benzene (TWA: 32 mg/m3, 10
ppm) showed no differences in mitogen-induced blastogenesis in exposed
workers and controls.
9.3.2 Genotoxicity and carcinogenic effects
In vitro tests indicate that benzene is not mutagenic.
However, benzene, or its metabolites have been shown to cause both
structural and numerical chromosomal aberrations in experimental
animals as well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes.
Humans with benzene haemopathy have been found to have a high
percentage of aneuploid lymphocytes. Increases in the number of both
stable and unstable chromosomal aberrations were observed in workers
exposed to high levels (408-1734 mg/m3) of benzene for 1 to 22
years. Therefore, benzene should be considered a clastogen in both
animals and humans.
Recent studies of workers exposed to lower concentrations of
benzene (TWA: < 3.2-32 mg/m3, < 1-10 ppm) revealed no alteration
in cell-cycle kinetics and no increase in SCEs. Only a marginally
significant increase in chromosomal aberrations (chromatid deletions
and gaps) was noted at these exposure levels.
9.3.2.1 Mechanism of carcinogenicity
It is broadly accepted today, as a result of several studies in
the field of molecular biology, that chromosomal rearrangements
generating gene translocations, gene deletions and gene amplifications
are relevant steps in the carcinogenic process.
There is at present no adequate animal model for benzene-induced
leukaemia in humans. However, benzene has been shown to be
carcinogenic in experimental animals after inhalation or oral
exposure. While several types of neoplasms have been reported to be
associated with benzene exposure in rats and mice, these are primarily
of epithelial origin, i.e. zymbal gland, liver, mammary gland and
nasal cavity. Lymphomas/leukaemias have been observed with lesser
frequency.
One study showed an increased incidence of lymphoma in the CBA/CA
mouse. No statistically significant increase was seen for lymphoid
tumour incidence in the Sprague-Dawley rat, Wistar rat or Swiss mouse.
In the B6C3F1 mouse the incidence was not dose-related.
Dose-related (possibly linear) increases in epithelial tumours were
observed in mice and rats. The results in animals indicate that
benzene is an experimental multipotential carcinogen, although there
is not the leukaemogenic response seen in humans. The lowest dose
resulting in increased incidence of tumours was demonstrated in
B6C3F1 mice (25 mg/kg body weight, 5 days per week for 103 weeks).
No useful information is available for doses below this level.
Attempts to understand the mechanism of benzene-induced
carcinogenesis can be based on basic principles of chemical
carcinogenesis. Thus, one mechanism of cancer has been postulated to
be the result of autosomal mutations due to the formation of DNA
adducts, which result in alteration of cell function and control of
replication. Secondly, cancer appears to be induced by a combination
of multiple genetic and epigenetic events.
Metabolic data suggest that several reactive metabolites of
benzene are formed and these can potentially form adducts both with
DNA and protein. Binding of benzene metabolites to the protein
components of the spindle apparatus has been suggested to inhibit
mitosis. The data for the formation of DNA adducts by reactive
metabolites of benzene suggests that, compared with other carcinogens,
benzene does not form large amounts of DNA adducts. Furthermore,
although there is evidence for DNA-adduct formation in liver,
DNA-adduct formation in bone marrow has been a subject of some
controversy. Assuming that adduct formation is an initiating event,
there is the possibility of a protective event if DNA repair or immune
surveillance intervenes. A promotional event might lead to a
carcinogenic response. It is possible that benzene acts as a promoter
of its own initiation.
While it is clear that a greater percentage of benzene
metabolites are converted to reactive metabolites at low doses than at
high doses, the total amounts of reactive metabolites increases with
increasing dose. Hence, it is clear that the severity of benzene
toxicity increases with increasing dose. By analogy, it is expected
that increasing doses of benzene should yield more leukaemia in
humans.
The negative results obtained with in vitro mutagenicity tests
could be related to an inadequate production of mutagenic metabolites
in the system. The failure to produce leukaemia in animals may be due
to lack of adequate formation of leukaemogenic metabolites or the need
to produce bone marrow damage prior to the induction of leukaemia. If
the bone marrow must undergo a stage of aplastic anaemia prior to the
development of leukaemia, one would have to estimate higher exposure
to benzene and to define a threshold. Alternatively, at doses lower
than those producing aplastic anaemia, sufficient damage may occur to
induce tumour promotion.
Both animal and human studies show that benzene exposure produces
bone marrow and chromosomal damage. However, the various stages in
carcinogenesis cited above have not been observed with benzene either
because they do not occur in these species or because of technical
problems. The results of both animal and human studies suggest that
benzene is a weak carcinogen on a molar basis.
9.3.2.2 Human carcinogenesis
Benzene is a well-established human leukaemogen. There have been
numerous epidemiological studies on the effects of benzene, most of
which have dealt with chronic industrial exposures. Increased
leukaemia risk was identified in studies of shoemakers, chemical
workers and workers in oil refineries, and in a nationwide study of
benzene-exposed workers in different industries. The most consistent
evidence for a causal association in humans has been found between
benzene exposure and myeloid leukaemia. An exposure-response
relationship was identified in some studies, the response being
influenced both by exposure levels and duration of exposure. In the
study where estimated past exposures were based on the most extensive
exposure measurements, a three-fold increased leukaemia risk was
identified in workers exposed to benzene levels of 128-640
mg/m3-years (40-200 ppm-years) (ppm-years is the average
concentration times the duration of exposure in years, e.g., 4 ppm for
10 years is equivalent to 40 ppm-years) and a statistically
significant 12-fold risk for workers exposed to benzene levels between
of 640-1280 mg/m3-years (200-400 ppm-years). The scientific
assessment of alternative exposure estimates for the rubber
hydrochloride cohort has not yet been fully explored. Of the two
alternative exposures estimates so far postulated for this study, they
both suggest a lower estimate of risk than that described above.
Other leukaemia types, multiple myeloma and other lymphomas were also
reported.
A statistically significant excess risk for multiple myeloma was
found in the rubber hydrochloride study. A marginally significant
exposure-response relationship for malignant lymphomas was reported in
a study on chemical workers. Increased risk for skin, stomach and
lung cancers has been reported in some studies, but these findings
have not been consistent and may possibly be attributed to concomitant
exposures to other chemicals or statistical artifact.
Studies examining leukaemia risk in coke-oven workers, assumed to
have been exposed to fairly low levels of benzene, have not identified
excess leukaemia risk. In these studies no attempt was made to
examine leukaemia subtypes. These studies do not provide enough
evidence to prove that there is no risk of leukaemia as a result of
exposure to these low concentrations of benzene.
The Task Group is of the opinion that the epidemiological
evidence presented so far is not capable of distinguishing between
(a) a small increase in leukaemia mortality in workers exposed to low
benzene levels, and (b) a no-risk situation.
9.4 Other toxicological end-points
Benzene does cross the placenta of experimental animals, since
haemopoietic changes have been observed in the fetuses and offspring
of mice exposed to 16, 33 or 65 mg benzene/m3, 6 h/day during days
6-15 of gestation. Following inhalation exposure to high doses (500
to 1600 mg/m3) in rabbits and mice, an increase in fetal resorptions
or fetal death was observed. However, benzene does not appear to be
teratogenic, although it is fetotoxic, in experimental animals, and no
evidence is available to permit the conclusion that it causes adverse
reproductive effects in humans.
The neurotoxicity of benzene in animals and humans has not been
well studied. An early study showed subtle changes such as reduced
food intake and decreased hind-limb grip strength following exposures
to 3300 and 9900 mg benzene/m3, and learning defects were observed
in rats dosed orally with 550 mg benzene/kg body weight.
9.5 Conclusions
To assist Member States in the development of standards for
benzene exposure, the Task Group concludes that a TWA of 3.2 mg/m3
(1 ppm) over a 40-year working career has not been statistically
associated with any increase in deaths from leukaemia. However, since
benzene is a human carcinogen, exposures should be limited to the
lowest possible technically feasible level. Increases in exposure to
over 32 mg/m3 (10 ppm) should be avoided. Benzene and
benzene-containing products such as gasoline should never be used for
cleaning purposes.
Traditionally, bone marrow depression, i.e. anaemia, leucopenia
or thrombocytopenia, in the workplace has been recognized as the first
stage of benzene toxicity and appears to follow a dose-response
relationship, i.e. the higher the dose, the greater the likelihood of
observing decreases in circulating blood cell counts.
Table 20 shows some "rough" estimates of the percentages of
workers that might exhibit either bone marrow depression or frank
aplastic anaemia after exposure to benzene for either 1 year or 10
years at concentrations of 3.2, 32, 160 or 320 mg/m3 (1, 10, 50 or
100 ppm). These estimations are an interpretation of the literature
using the experience of the Task Group. The speculative nature of
this table precludes its use in regulatory standard setting. Exposure
at high doses (160-320 mg/m3; 50-100 ppm) for one year would most
likely produce bone marrow toxicity in a large percentage of the
workers, and in some cases aplastic anaemia, but little effect would
be expected at the lower doses. Exposure to both high and low doses
would be expected to produce benzene toxicity after 10 years. Thus,
a high level of both bone marrow depression and aplastic anaemia would
be seen at the higher doses and some damage would also be seen at the
lower doses. The observation of any of these effects, regardless of
the dose or period of exposure, should indicate the need for improved
control of benzene exposure.
There is no evidence of benzene being teratogenic at doses lower
than those that produce maternal toxicity, but fetal toxicity has been
demonstrated.
The neurotoxicity and immunotoxicity of benzene have not been
well studied in either experimental animals or humans.
Table 20. Estimated percentage of worker populations that might
develop bone marrow depression or aplastic anaemia after
chronic exposure to benzene ( Before using table note
cautionary footnotea)
Duration Exposure Bone marrow Aplastic
depression anaemia
1 year 320 mg/m3 (100 ppm) 90 10
160 mg/m3 (50 ppm) 50 5
32 mg/m3 (10 ppm) 1 0b
3.2 mg/m3 (1 ppm) 0b 0b
10 years 320 mg/m3 (100 ppm) 99 50
160 mg/m3 (50 ppm) 75 10
32 mg/m3 (10 ppm) 5 0b
3.2 mg/m3 (1 ppm) < 1 0b
a This estimation is an interpretation of the literature and is
based on the experience of the Task Group. The speculative
nature of this table precludes its use in regulatory standard
setting.
b Occasional cases may be observed.
10. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Benzene and benzene-containing products, including gasoline
(petrol), should never be used for cleaning purposes.
b) Systematic information on occupational and non-occupational
exposure should be collected using the total human exposure
approach where possible.
c) The health risk of low-level benzene exposure is not clearly
established. Exposure should, therefore, be avoided as much as
possible.
d) The occurrence of benzene in environmental media such as air and
water where there exists potential human exposure should be
evaluated.
e) A search for less toxic solvents to replace benzene in industrial
processes should be encouraged.
11. FURTHER RESEARCH
a) Epidemiological studies of the risks of haematological
malignancies, blood changes (red and white blood cells) and
genotoxic effects at low and high exposure concentrations should
have high priority.
b) Information on the mechanisms by which benzene induces neoplasms
is required. In particular, there is a need for animal models of
benzene-induced haemopoietic malignancies similar to those seen
in humans and a better understanding of the role of reactive
intermediate.
c) Further studies are needed to elucidate the potential link
between bone marrow suppression and the eventual occurrence of
leukaemia.
d) Biological markers of benzene exposure, especially urinary
muconic acid and macromolecular adducts, should be validated.
e) Individual susceptibility factors in benzene-induced toxicities
should be investigated.
f) Animals and human studies are required to assist in validating
physiologically based pharmacokinetic models using all routes of
exposure.
g) The multigenerational effects of benzene exposure should be
investigated.
h) Studies on immunotoxicological effects following benzene exposure
should be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of benzene has been evaluated by the
International Agency for Research on Cancer (IARC, 1982, 1987b). It
was concluded that there was sufficient evidence for the
carcinogenicity of benzene in both animals and humans.
A guideline value of 10 µg/litre was recommended by WHO (WHO,
1984) for benzene in drinking-water based on data for the production
of leukaemia after inhalation exposures in humans and using a linear
multistage extrapolation model and a life-time risk level of 1 in
100 000. This guideline remained unchanged during the revisions
recently completed (WHO, 1993).
A Task Group convened by the WHO Regional Office for Europe
concluded that an air quality guideline value could not be set for
benzene in view of its carcinogenic activity in humans (WHO, 1987).
Assuming no threshold and an average relative risk model, it was
calculated that at an air concentration of 1 µg benzene/m3, the
estimated lifetime risk of leukaemia would be 4 x 10-6.
Regulatory standards for benzene established by national bodies
in some countries are summarized in the Legal File of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
Recently the Commission of the European Communities has proposed an
occupational exposure limit for benzene of 1.6 mg/m3 (0.5 ppm) (CEC,
1993).
References
Aksoy M (1980) Different types of malignancies due to occupational
exposure to benzene. A review of recent observations in Turkey.
Environ Res, 23: 181-190.
Aksoy M & Erdem S (1978) Follow-up study on the mortality and the
development of leukaemia in 44 pancytopaenic patients with chronic
exposure to benzene. Blood, 52: 285-292.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1971) Haematological
effects of chronic benzene poisoning in 217 workers. Br J Ind Med, 28:
296-302.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1972) Details of
blood changes in 32 patients with pancytopaenia associated with
long-term exposure to benzene. Br J Ind Med, 29: 56-64.
Aksoy M, Erdem S, Hepyüksel T, & Dincol G (1974a) Chronic exposure to
benzene as a possible contributory etiologic factor in Hodgkin's
Disease. Blood, 38: 93-298.
Aksoy M, Erdem S, & Dincol G (1974b) Leukemia in shoe-workers exposed
chronically to benzene. Blood, 44: 837-841.
Allen PD (1987) Residential population exposure to ambient benzene in
California. Sacramento, California, California Air Resources Board.
Anderson D & Richardson CR (1981) Issues relevant to the assessment of
chemically induced chromosome damage in vivo and their relationship
to chemical mutagenesis. Mutat Res, 90: 261-272.
Andrews LS & Snyder R (1986) Toxic effects of solvents and vapors. In:
Casarett LJ & Doull J ed. Toxicology: The basic science of poisons,
3rd ed. New York, McMillan Publishing Co., chapter 20, p 641.
Antoine SR, Delon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36(3): 364-371.
Appuhn E & Goldeck H (1957) [Early and late disorders of
haematopoiesis caused by benzene and its homologues.] Arch
Gewerbepathol Gewerbehyg, 15: 399-428 (in German).
Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin BH, Matter
BE, & Shelby MD ed. (1985) Evaluation of short-term tests for
carcinogens. Report of the International Programme on Chemical
Safety's collaborative study on in vitro assays. Amsterdam, Oxford,
New York, Elsevier Science Publishers (Progress in Mutation Research,
Volume 5).
ATSDR (1989) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 173 pp
(ATSDR/TP-88/03; PB/89/209464/AS).
ATSDR (1991) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 193 pp.
Au WW, Ramanujam VMS, Ward JB Jr, & Legator MS (1991) Chromosome
aberrations in lymphocytes of mice after sub-acute low-level
inhalation exposure to benzene. Mutat Res, 260: 219-224.
Baarson K, Snyder CA, & Green J (1982) The hematotoxic effects of
inhaled benzene on peripheral blood, bone marrow, and spleen cells are
increased by ingested ethanol. Toxicol Appl Pharmacol, 64: 393-404.
Baarson K, Snyder CA, & Albert RE (1984) Repeated exposures of C57B1
mice to 10 ppm inhaled benzene markedly depressed erythropoietic
colony formation. Toxicol Lett, 20: 337-342.
Bailer AJ & Hoel DG (1989) Metabolite-based internal doses used in a
risk assessment of benzene. Environ Health Perspect, 82: 177-184.
Bailey JC & Schmidl B (1989) A survey of hydrocarbons emitted in
vehicle exhaust gases, over a range of driving speeds and conditions
from a representative sample of the 1986-87 UK vehicle fleet.
Stevenage, Herts, Warren Spring Laboratory (DTI) (Report No. LR 673).
Bandow H, Washida N, & Akimoto H (1985) Ring-cleavage reactions of
aromatic hydrocarbons studies by FT-IR spectroscopy. I. Photooxidation
of toluene and benzene in the NOx-air system. Bull Chem Soc Jpn, 58:
2531-2540.
Barale R, Giorgelli F, Migliore L, Ciranno R, Casini D, Zucconi D, &
Loprieno N (1985) Benzene induces micronuclei in circulating
erythrocytes of chronically treated mice. Mutat Res, 144: 193-196.
Barrett RH (1985) Assays for unscheduled DNA synthesis in HeLa S3
cells. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin
BH, Matter BE, & Shelby MD ed. Short-term tests for carcinogens:
Report of the International Programme on Chemical Safety's
collaborative study on in vitro assays. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 347-352 (Progress in Mutation
Research, Volume 5).
Battersby NS & Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Appl Environ
Microbiol, 52(2): 433-439.
Baxter HG, Blakemore R, Moore JP, & Coker DT (1980) The measurement of
airborne benzene vapour. Ann Occup Hyg, 23: 117-132.
Bayer CW, Black MS, & Galloway LM (1988) Sampling and analysis
techniques for trace volatile organic emissions from consumer
products. J Chromatogr Sci, 26: 168-173.
Bechtold WE, Sabourin PJ, & Henderson RF (1988) A reverse isotope
dilution method for determining benzene and metabolites in tissues.
J Anal Toxicol, 12(7/8): 176-179.
Bechtold WE, Lucier G, Birnbaum LS, Yin SN, Li GL, & Henderson RF
(1991) Muconic acid determinations in urine as a biological exposure
index for workers occupationally exposed to benzene. Am Ind Hyg Assoc
J, 52(11): 473-478.
Bechtold WE, Sun JD, Birnbaum LS, Yin SN, Li GL, Kasicki S, Lucier G,
& Henderson RF (1992) S-Phenylcysteine formation in haemoglobin as a
biological exposure index to benzene. Arch Toxicol, 66: 303-309.
Beliles RP & Totman LC (1989) Pharmacokinetically based risk
assessment of workplace exposure to benzene. Regul Toxicol Pharmacol,
9: 186-195.
Bennett GF (1989) Impact of toxic chemicals on local wastewater
treatment plant and the environment. Environ Geol Water Sci, 13(3):
201-212.
Bentley P, Sschassmann H, Sims P, & Oesch F (1976) Epoxides derived
from various polycyclic hydrocarbons as substrates of homogeneous and
microsome-bound epoxide hydratase. A general assay and kinetic
properties. Eur J Biochem, 69: 97-103.
Blanchard RD & Hardy JK (1986) Continuous monitoring device for the
collection of 23 volatile organic priority pollutants. Anal Chem, 58:
1529-1532.
Blank IH & McAuliffe DJ (1985) Penetration of benzene through human
skin. J Invest Dermatol, 85: 522-526.
Bois FY, Woodruff TJ, & Spear RC (1991a) Comparison of three
physiologically based pharmacokinetic models of benzene disposition.
Toxicol Appl Pharmacol, 110: 79-88.
Bois FY, Smith MT, & Spear RC (1991b) Mechanisms of benzene
carcinogenesis: application of a physiological model of benzene
pharmacokinetics and metabolism. Toxicol Lett, 56: 283-298.
Bond AE, Thompson VL, & Ortman GC (1986) Self service station vehicle
refueling exposure study. In: Proceedings of the 1986 EPA/APCA
Symposium on Measurement of Toxic Air Pollutants. Pittsburgh,
Pennsylvania, Air Pollution Control Association, pp 458-466.
Bond GG, McLaren EA, Baldwin CL, & Cook RR (1986) An update of
mortality among chemical workers exposed to benzene. Br J Ind Med, 43:
685-691.
Bradley MO (1985) Measurement of DNA single-strand breaks by alkaline
elution in rat hepatocytes. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 353-357 (Progress in Mutation Research, Volume 5).
Brodfuehrer JI, Chapman DE, Wilke TJ, & Powis G (1990) Comparative
studies of the in vitro metabolism and covalent binding of
14C-benzene by liver slices and microsomal fraction of mouse, rat
and human. Drug Metab Dispos, 18: 20-27.
Brown, RH (1988a) Determination of benzene, toluene, and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 225-233.
Brown RH (1988b) Determination of benzene, toluene, and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 235-242.
Bruckmann P, Beier R, & Krautscheid S (1983) [Measurements of
emissions of hydrocarbons in areas of high exposure in the Rhine-Ruhr
district.] Staub-Reinhalt-Luft, 43: 404 (in German).
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1989) Determination of
benzene, toluene and 1,3-butadiene in cigarette smoke by GC-MSD. Exp
Pathol, 37: 108-113.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1990) Analysis of
1,3-butadiene and other selected gas-phase components in cigarette
mainstream and sidestream smoke by gas chromatography - mass selective
detection. Carcinogenesis, 11(10): 1863-1868.
Bryce-Smith D & Gilbert A (1976) The organic photochemistry of
benzene. I. Tetrahedron, 32: 1309-1326.
Buchet JP (1988) Determination of phenol and its glucurono- and
sulfoconjugates in urine by gas chromatography. In: Fishbein L &
O'Neill JK ed. Environmental carcinogens methods of analysis and
exposure measurement - Volume 10: Benzene and alkylated benzenes. New
York, London, Oxford University Press, pp 281-286.
Burmaster DE (1982) The new pollution. Groundwater contamination.
Environment, 24: 33-36.
Busby WF Jr, Wang J-S, Stevens EK, Padykula RE, Aleksejczyk RA, &
Berchtold GA (1990) Lung tumourigenicity of benzene oxide, benzene
dihydrodiols and diolepoxides in the BLV: Ha newborn mouse assay.
Carcinogenesis, 11: 1473-1478.
Carpenter C, Shaffer C, Weil C, & Smyth H (1944) Studies on the
inhalation of 1,3-butadiene; with comparison of its narcotic effect
with benzol, toluol, and styrene and a note on the elimination of
styrene by the human. J Ind Hyg Toxicol, 26: 69-78.
CEC (1993) Occupational exposure limits - Criteria document for
benzene. Luxembourg, Commission of the European Communities,
Directorate-General Employment, Industrial Relations and Social
Affairs (EUR 14491 EN) (Health and Safety Series).
Chang SS & Peterson RJ (1977) Symposium: The basis of quality in
muscle foods. Recent developments in the flavor of meat. J Food Sci,
42: 298-306.
Chepiga TA, Yang CS, & Snyder R (1991) Benzene metabolism in two
purified, reconstituted rat hepatic mixed function oxidase systems.
In: Witmer CM, Snyder R, Jollow DJ, Kalf GF, Kocsis JJ, & Sipes IG ed.
Biological reactive intermediates: IV. Molecular and cellular effects
and their impact on human health. New York, London, Plenum Press,
pp 261-265.
Choy WN, MacGregor JT, & Shelby MD (1985) Induction of micronuclei by
benzene in B6C3F1 mice: Retrospective analysis of peripheral blood
smears from the NTP carcinogenesis bioassay. Mutat Res, 143: 55-59.
Ciranni R, Barale R, Marrazzini A, & Loprieno N (1988) Benzene and the
genotoxicity of its metabolites. I. Transplacental activity in mouse
fetuses and in their dams. Mutat Res, 208: 61-67.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, & Lester JN (1984b) A comparison of
photoionization detection gas chromatography with a Tenax GC sampling
tube procedure for the measurement of aromatic hydrocarbons in ambient
air. J Environ Anal Chem, 17: 315-326.
Coate WB, Hoberman AM, & Durloo RS (1984) Inhalation teratology study
of benzene in rats. Adv Mod Environ Toxicol, 6: 187-198.
Colenutt BA & Thornburn S (1980) Gas-chromatographic analysis of trace
hydrocarbon pollutants in water samples. Int J Environ Stud, 15:
25-32.
Collins JJ, Conner P, Friedlander BR, Easterday PA, Nair RS, Rashmi S,
& Braun J (1991) A study of the hematological effects of chronic
low-level exposure to benzene. J Occup Med, 33(5): 619-626.
CONCAWE (1986) Review of European oil industry benzene exposure data.
The Hague, European Oil Companies Organization for Environmental and
Health Protection (Report No. 3/86).
Cornish HH & Ryan RC (1965) Metabolism of benzene in non-fasted,
fasted, and arylhydroxylase inhibited rats. Toxicol Appl Pharmacol,
7: 767-771.
Cronkite EP (1986) Benzene hematotoxicity and leukemogenesis. Blood
Cells, 12: 129-137.
Cronkite EP, Bullis J, Inoue T, & Drew RT (1984) Benzene inhalation
produces leukemia in mice. Toxicol Appl Pharmacol, 75: 358.
Cronkite EP, Drew RT, Inoue T, & Bullis JE (1985) Benzene
hematotoxicity and leukemogenesis. Am J Ind Med, 7: 447-456.
Cronkite EP, Drew RT, Inoue T, Hirabayashi Y, & Bullis JE (1989)
Hematotoxicity and carcinogenicity of inhaled benzene. Environ Health
Perspect, 82: 97-108.
Crump K & Allen B (1984) Quantitative estimates of risk of leukemia
from occupational exposure to benzene. Washington, DC, US Department
of Labour (OSHA Docket H-059b, Exhibit 152, Annex B).
Dann T (1987) Ambient benzene levels in urban areas of Canada. Report
of the Technology Development and Technical Services Branch,
Conservation and Protection. Ottawa, Environment Canada.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Da Silva C, Fan X, & Castagna M (1989) Benzene-mediated protein kinase
c activation. Environ Health Perspect, 82: 91-95.
Dean BJ (1978) Genetic toxicology of benzene, toluene, xylenes and
phenols. Mutat Res, 47: 75-97.
Dean BJ (1985a) Recent findings on the genetic toxicology of benzene,
toluene, xylenes and phenols. Mutat Res, 154: 153-181.
Dean BJ (1985b) Summary report on the performance of cytogenetic
assays in cultured mammalian cells. In: Ashby J, de Serres FJ, Draper
M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation
of short-term tests for carcinogens. Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 69-83 (Progress in Mutation Research, Volume 5).
Decouflé P, Blattner WA, & Blair A (1983) Mortality among chemical
workers exposed to benzene and other agents. Environ. Res., 30: 16-25.
De Flora S, Zanacchi P, Camoirano A, Bennicelli C, & Badolati GS
(1984) Genotoxic activity and potency of 135 compounds in the Ames
reversion test and in a bacterial DNA-repair test. Mutat Res, 133:
161-198.
Deichmann WB, MacDonald WE, & Bernal E (1963) The hemopoietic tissue
toxicity of benzene vapors. Toxicol Appl Pharmacol, 5: 201-224.
Dibben MJ, Thomas TC, & Anderson MP (1989) Is benzene a problem in
fuel operations: A new analytical method to overcome potential
interferences in the present methods. Liq Chromatogr-Gas Chromatogr
Int, 2: 54, 56, 58.
Dorfman LM, Taub IA, & Buhler RE (1962) Pulse radiolysis studies. I.
Transient spectra and reaction-rate constants in irradiated aqueous
solutions of benzene. J Chem Phys, 36: 3051-3061.
Douglas GR, Blakely DH, Liu-Lee VW, Bell RDL, & Bayley JM (1985)
Alkaline sucrose sedimentation, sister-chromatid exchange and
micronucleus assays in CHO cells. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 359-366 (Progress in Mutation Research, Volume 5).
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Drew RT & Fouts JR (1974) The lack of effects of pretreatment with
phenobarbital and chlorpromazine on the acute toxicity of benzene in
rats. Toxicol Appl Pharmacol, 27: 183-193.
Eastmond DA, Smith MT, & Irons RD (1987) An interaction of benzene
metabolites reproduces the myelotoxicity observed with benzene
exposure. Toxicol Appl Pharmacol, 91: 85-95.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol, 15:
30-38.
Environment Agency, Japan (1989) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1986 and 1987. Tokyo, Environment Agency, Department of Environmental
Health, Office of Health Studies.
Erexson GL, Wilmer JL, & Kligerman AD (1985) Sister chromatid exchange
in human lymphocytes exposed to benzene and its metabolites
in vitro. Cancer Res, 45: 2471-2477.
Erexson GL, Wilmer JL, Steinhagen WH, & Kligerman AD (1986) Induction
of cytogenetic damage in rodents after short-term inhalation of
benzene. Environ Mutagen, 8: 29-40.
Erf LA & Rhoads CP (1939) The hematological effects of benzene
(benzol) poisoning. J Ind Hyg Toxicol, 20(8): 421.
Ewing BB, Chain ESK, Cook JC, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Washington, DC, US Environmental Protection Agency
(EPA-560/6/77/015A).
Fel'dt EG (1985) [Evaluation of the mutagenic hazards of benzene and
some of its derivatives.] Gig i Sanit, 7: 21-23 (in Russian with
English summary).
Fentiman AF, Neher MB, Kinzer GW, Sticksel PR, & Coutant RW (1979)
Environmental monitoring benzene. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/679/006)
(Prepared by Battelle Columbus for US EPA).
Ferrario JB, Lawler GC, Delon IR, & Laseter JL (1985) Volatile organic
pollutants in biota and sediments of Lake Pontchartrain. Bull Environ
Contam Toxicol, 34(2): 246-255.
Fishbein L (1984) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. I. Benzene. Sci Total Environ, 40:
189-218.
Forni A, Pacifico E, & Limonta A (1971a) Chromosome studies in workers
exposed to benzene or toluene or both. Arch Environ Health, 22:
373-378.
Forni AM, Cappellini A, Pacifico E, & Vigliani EC (1971b) Chromosome
changes and their evolution in subjects with past exposure to benzene.
Arch Environ Health, 23: 385-391.
Franz TJ (1984) Percutaneous absorption of benzene. In: Applied
toxicology of hydrocarbons. Princeton, New Jersey, Princeton
Scientific Publishers, Inc., pp 61-70 (Advances in Modern
Environmental Toxicology, Volume VI).
Fung KK & Wright BJ (1986) Monitoring of benzene in ambient air with
organic vapor badges. J Air Pollut Control Assoc, 36: 819-821.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected
hydrocarbons. Arch Ind Health, 18: 9-15.
Gad-El-Karim MM, Harper BL, & Legator MS (1984) Modifications in the
myeloclastogenic effect of benzene in mice with toluene,
phenobarbital, 3-methylcholanthrene, Aroclor 1254 and SKF-525A. Mutat
Res, 135: 225-243.
Galton DAG (1986) The myelodysplastic syndromes. Scand J Haematol, 36:
11-20.
Garner RC (1985) Summary report on the performance of gene mutation
assays in mammalian cells in culture. In: Ashby J, de Serres FJ,
Draper M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed.
Evaluation of short-term tests for carcinogens. Report of the
International Programme on Chemical Safety's collaborative study on
in vitro assays. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 85-94 (Progress in Mutation Research, Volume 5).
GDCh (Society of German Chemists) (1988) [Advisory Committee on
Existing Chemicals of Environmental Relevance. Benzene.] Weinheim, VCH
Verlagsgesellschaft, 82 pp (BUA Report 24) (in German with English
summary).
Geist CR, Kelly LD, Schoenheit CM, & Praed JE (1983) Learning
impairments following postnatal exposures to benzene. Percept Motor
Skills, 57: 1083-1086.
Gerarde HW (1960) Toxicology and biochemistry of aromatic
hydrocarbons. Amsterdam, Oxford, New York, Elsevier Science
Publishers.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res, 58:
313-316.
Geyer H, Politzki G, & Freitag D (1984) Prediction of ecotoxicological
behaviour of chemicals: relationship between n-octanol/water partition
coefficient and bioaccumulation of organic chemicals by the alga
Chlorella. Chemosphere, 13: 269-284.
Ghantous H & Danielsson BRG (1986) Placental transfer and distribution
of toluene, xylene, and benzene, and their metabolites during
gestation in mice. Biol Res Pregnancy, 7: 98-105.
Gilman AG, Goodman LA, & Gilman A (1985) The Goodman and Gilman's
pharmacological basis of therapeutics, 7th ed. New York, MacMillan
Publishing Co.
Gilmour SK, Kalf GF, & Snyder R (1986) Comparison of the metabolism of
benzene and its metabolite phenol in rat liver microsomes. In: Kocsis
JJ, Jollow DJ, Witmer CM, Nelson J, & Snyder R ed. Biological reactive
intermediates. III - Mechanisms of action in animal models and human
disease. New York, Plenum Publishing Co., pp 223-235.
Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, &
Oesch F (1989) Multiple activation pathways of benzene leading to
products with varying genotoxic characteristics. Environ Health
Perspect, 82: 81-89.
Gofmekler VA (1968) [The embryotropic action of benzene and
formaldehyde in experimental administration by inhalation.] Gig i
Sanit, 33: 12-16 (in Russian with English summary).
Goldstein BD (1988) Benzene toxicity: occupational medicine. State Art
Rev, 3: 541-554.
Goldstein BD, Snyder CA, Laskin S, Brombert I, Albert RE, & Nelson N
(1982) Myelogenous leukemia in rodents inhaling benzene. Toxicol Lett,
13: 169-173.
Goldwater LJ (1941) Disturbances in the blood following exposure to
benzol. J Lab Clin Med, 26: 957.
Gollmer L, Graf H, & Ulrich V (1984) Characterization of the benzene
monooxygenase system in rabbit bone marrow. Biochem Pharmacol, 33:
3597-3602.
Goodenkaug O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24(2): 231-233.
Gorsky LD & Coon MJ (1985) Evaluation of the role of free hydroxyl
radicals in the cytochrome P-450 catalyzed oxidation of benzene in
microsomes and reconstituted enzyme systems from rabbit liver. J Biol
Chem, 258: 7311.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Government of Canada (in press) Canadian environmental protection act
- Priority assessment report on benzene. Ottawa, Environment
Canada/Health and Welfare Canada.
Green JD, Leong BKJ, Lobue J, Goldstein BD, & Albert RE (1978)
Inhaled benzene fetotoxicity in rats. Toxicol Appl Pharmacol, 46:
9-18.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981a) Acute
and chronic dose/response effect of benzene inhalation on the
peripheral blood, bone marrow, and spleen cells of CD-1 male mice.
Toxicol Appl Pharmacol, 59: 204-214.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981b) Acute
and chronic dose/response effects of inhaled benzene on multipotential
hematopoietic stem (CFU-S) and granulocyte/macrophase progenitor
(GM-CFU-C) cells in CD-1 mice. Toxicol Appl Pharmacol, 58: 492-503.
Grob K & Grob G (1974) Organic substances in potable water and its
precursor. Part II. Applications in the area of Zurich. J Chromatogr,
90: 303-313.
Gruenke LD, Craig JC, Wester RC, & Maibach HI (1986) Quantitative
analysis of benzene by selected ion monitoring/gas chromatography/mass
spectrometry. J Anal Toxicol, 10(11/12): 225-232.
Hadeishi T, Pollard M, McLaughlin R, & Koga M (1985) Development of an
optical monitor for toxic organic compounds in air. Research Triangle
Park, North Carolina, US Environmental Protection Agency
(EPA/600/4-85-043).
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hammers WE & Bosman HFPM (1986) Quantitative evaluation of a simple
dynamic headspace analysis technique for non-polar pollutants in
aqueous samples at the ng/kg level. J Chromatogr, 360: 425-432.
Hancock DG, Moffitt AE Jr, & Hay EB (1984) Hematological findings
among workers exposed to benzene at a coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Hanke J, Dutkiewicz T, & Piotrowski J (1961) [The absorption of
benzene through the skin in men.] Med Pracy, 12: 413-426 (in Polish).
Harkov R, Gianti SJ, Bozzelli JW, & Laregina JE (1985) Monitoring
volatile organic compounds at hazardous and sanitary landfills in New
Jersey. J Environ Sci Health, A20(5): 491-501.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-311.
Hattemer-Frey HA, Travis CC, & Land ML (1990) Environmental
partitioning and human exposure. Environ Res, 53: 221-232.
Henderson RF, Sabourin PJ, Bechtold WE, Griffith WC, Medinsky MA,
Birnbaum LS, & Lucier GW (1989) The effect of dose, dose rate, route
of administration, and species on tissue and blood levels of benzene
metabolites. Environ Health Perspect, 82: 9-17.
Hine J & Mookerjee PK (1975) The intrinsic hydrophilic character of
organic compounds. Correlations in terms of structural contributions.
J Org Chem, 40: 292-298.
Hinson JA, Freeman JP, Potter DW, Mitchum RK, & Evans FE (1985)
Mechanism of microsomal metabolism of benzene to phenol. Mol
Pharmacol, 27: 574-577.
Hite M, Pecharo M, Smith I, & Thornton S (1980) The effect of benzene
in the micronucleus test. Mutat Res, 77: 149-155.
Högn T & Jaenicke L (1972) Benzene metabolism in Moraxella species.
Eur J Biochem, 30: 369-375.
Holdren MW, Smith DL, & Smith RN (1985) Comparison of ambient air
sampling techniques for volatile organic compounds. Washington, DC, US
Environmental Protection Agency (EPA/600/S4-85-067; PB86-120953/GAR).
Hornung RW, Ward E, Morris JA, & Rinsky RA (1989) Letter to the
editor. Haematologic effects of benzene: a thirty-five year
longitudinal study of rubber workers. Toxicol Ind Health, 5(6):
1153-1155.
HSE (1982) Toxicity review No. 4: Benzene. London, Health and Safety
Executive, Her Majesty's Stationery Office, 36 pp.
Hsieh GC, Sharma RP, & Parker RDR (1988a) Subclinical effects of
groundwater contaminants. I. Alteration of humoral and cellular
immunity by benzene in CD-1 mice. Arch Environ Contam Toxicol, 17:
151-158.
Hsieh GC, Parker RDR, & Sharma RP (1988b) Subclinical effects of
groundwater contaminants. II. Alteration of regional brain monoamine
neurotransmitters by benzene in CD-1 mice. Arch Environ Contam
Toxicol, 17: 799-805.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: toluene and xylene. Toxicology, 11: 55-63.
Huff JE, Haseman JK, Demarini DM, Eustis S, Maronpot RR, Peters AC,
Persing RL, Chrisp CE, & Jacobs AC (1989) Multiple-site
carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice.
Environ Health Perspect, 82: 125-163.
Hurley JF, Cherrie JW, & MacLaren W (1991) Exposure to benzene and
mortality from leukaemia: results from coke oven and other coal
product workers. Br J Ind Med, 48: 502-504.
IARC (1982) Benzene. In: Some industrial chemicals and dyestuffs.
Lyon, International Agency for Research on Cancer, pp 93-148
(Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 29).
IARC (1987a) Benzene. In: Genetic and related effects: an updating of
selected IARC Monographs from Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 91-95 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Formaldehyde. In: Overall evaluations of carcinogenicity:
An updating of IARC Monographs Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 211-216 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 7).
IARC (1989) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, 322 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
Ikeda M & Ohtsuji H (1971) Phenobarbital-induced protection against
toxicity of toluene and benzene in the rat. Toxicol Appl Pharmacol,
20: 30-43.
Infante PF, Wagoner JK, Rinsky RA, & Young RJ (1977) Leukaemia in
benzene workers. Lancet, 2: 76-78.
Inoue O, Seiji K, & Nakatsuka H (1989) Urinary t,t-muconic acid as an
indicator of exposure to benzene. Br J Ind Med, 46: 122-127.
Irons RD (1985) Quinones as toxic metabolites of benzene. J Toxicol
Environ Health, 16: 673-678.
Irons RD, Dent JG, Baker TS, & Rickert DE (1980) Benzene is
metabolized and covalently bound in bone marrow in situ. Chem-Biol
Interact, 30: 241-245.
Irons RD, Neptun DA, & Pfeifer RW (1981) Inhibition of lymphocyte
transformation and microtubule assembly by quinone metabolites of
benzene: Evidence for a common mechanism. J Reticuloendothel Soc, 30:
359-372.
IRPTC (1987) IRPTC Legal file 1986 on benzene - Volume 1. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
Jackson S & Brown VM (1970) Effect of toxic wastes on treatment
processes and watercourses. Water Pollut Control, 69(3): 292-313.
Jerina DM & Daly JW (1974) Arene oxides: a new aspect of drug
metabolism. Science, 185: 573-582.
Jerina D, Daly J, Witkop B, Zaltzman-Norenberg P, & Udenfriend S
(1968) Role of the arene oxide-oxepin system in the metabolism of
aromatic substrates. I. In vitro conversion of benzene oxide to a
premercapturic acid and a dihydrodiol. Arch Biochem Biophys, 128:
176-183.
Jirka AM & Bourne S (1982) Gas-chromatographic analysis for benzene in
blood. Clin Chem, 28: 1492-1494.
Johansson I & Ingelman-Sundberg M (1988) Benzene metabolism by
ethanol-, acetone-, and benzene-inducible cytochrome P-450(IIE1) in
rat and rabbit liver microsomes. Cancer Res, 48: 5387-5390.
Johnston RV, Pinkerton MN, & Mensik DC (1979) Haematological and
myelogenous effects of inhaled benzene in the pig and rat. J Toxicol
Environ Health, 5: 1025-1035.
Jonek J, Olkowski Z, & Zieleznik B (1965) Histochemical studies on the
spinal cord of mice poisoned with benzene. Acta Histochem, 20:
286-296.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular-weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jowa L, Witz G, Snyder R, Winkle S, & Kalf GF (1990) Synthesis and
characterization of deoxyguanosine-benzoquinone adduct. J Appl
Toxicol, 10(1): 47-54.
Kalf GF (1987) Recent advances in the metabolism and toxicity of
benzene. CRC Crit Rev Toxicol, 18: 141-159.
Kalf GF, Schlosser MJ, Renz JF, & Priozzi SJ (1989) Prevention of
benzene-induced myelotoxicity by nonsteroidal anti-inflammatory drugs.
Environ Health Perspect, 82: 57-64.
Karam LR & Simic MG (1989) Mechanisms of free radical chemistry and
biochemistry of benzene. Environ Health Perspect, 82: 37-41.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soils. Chemosphere,
10(8): 833-846.
Karlson U & Frankenberger WT Jr (1989) Microbial degradation of
benzene and toluene in groundwater. Bull Environ Contam Toxicol, 43:
505-510.
Keller KA & Snyder CA (1986) Mice exposed in utero to low
concentrations of benzene exhibit enduring changes in their colony
forming hematopoietic cells. Toxicology, 42: 171.
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol Environ
Saf, 4: 26-38.
Kiang PH & Grob RL (1986) A headspace technique for the determination
of volatile compounds in soil. J Environ Sci Health, A21: 71-100.
Kimmel CA & Wilson JG (1973) Skeletal deviations in rats:
malformations or variations? Teratology, 8: 309-316.
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Kipen HM, Cody RP, Crump KS, Allen BC, & Goldstein BD (1988)
Hematologic effects of benzene: a thirty-five year longitudinal study
of rubber workers. Toxicol Ind Health, 4(4): 411.
Kipen HM, Cody RP, & Goldstein BD (1989) Use of longitudinal analysis
of peripheral blood counts to validate historical reconstructions of
benzene exposure. Environ Health Perspect, 82: 199-206.
Kirley TA, Goldstein BD, Maniara WM, & Witz G (1989) Metabolism of
trans,trans-muconaldehyde, a microsomal hematotoxic metabolite of
benzene by purified yeast aldehyde dehydrogenase and a mouse liver
soluble fraction. Toxicol Appl Pharmacol, 100: 360-367.
Kissling M & Speck B (1972) Further studies on experimental benzene
induced aplastic anemia. Blut, 25: 97-103.
Kocsis JJ, Harkaway S, Santoyo MC, & Snyder R (1968) Dimethyl
sulfoxide: interactions with aromatic hydrocarbons. Science, 160:
427-428.
Koljkowsky P (1981) Indicator-tube method for the determination of
benzene in air. Analyst, 94: 918-920.
Korte F & Klein W (1982) Degradation of benzene in the environment.
Ecotoxicol Environ Saf, 6(4): 311-327.
Kozioski RP (1985) Determination of benzene and toluene in soils and
plant material by azeotropic distillation. Bull Environ Contam
Toxicol, 34: 10-16.
Kuna RA & Kapp RW (1981) Embyrotoxic/teratogenic potential of benzene
vapor in rats. Toxicol Appl Pharmacol, 57: 1-7.
Lahmann E, Seifert B, & Ullrich D (1977) The pollution of ambient air
and rain water by organic components of motor vehicle exhaust-gases.
In: Kasuga S ed. Proceedings of the 4th International Clean Air
Congress. Tokyo, Japanese Union of Air Pollution and Prevention
Association, pp 595-597.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973a) Leukocyte
agglutinins in workers exposed to benzene, toluene and xylene. Int
Arch Arbeitsmed, 31: 45-50.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973b) Serum
immunoglobulin levels in workers exposed to benzene, toluene and
xylene. Int Arch Arbeitsmed, 31: 37-44.
Latriano L, Goldstein BD, & Witz G (1986) Formation of muconaldehyde,
an open-ring metabolite of benzene, in mouse liver microsomes: an
additional pathway for toxic metabolites. Proc Natl Acad Sci (USA),
83: 8356-8360.
Leong B (1977) Experimental benzene intoxication. In: Laskin S &
Goldstein B ed. Benzene toxicity: A critical revaluation. J Toxicol
Environ Health, 3(2): 45-61.
Li G-L, Yin N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda M
(1986) Benzene-specific increase in leukocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581.
Longacre S, Kocsis J, & Snyder R (1981a) Influence of strain
differences in mice on the metabolism and toxicity of benzene. Toxicol
Appl Pharmacol, 60: 398-409.
Longacre S, Kocsis J, Witmer CM, Lee EW, Sammett D, & Snyder R (1981b)
Toxicological and biochemical effects of repeated administration of
benzene in mice. J Toxicol Environ Health, 7: 223-237.
Low LK, Meeks JR, Norris KJ, Mehlman MA, & Macherer CR (1989)
Pharmacokinetics and metabolism of benzene in Zymbal gland and other
key target tissues after oral administration in rats. Environ Health
Perspect, 82: 215-222.
Low LK, Reddy MV, Blackburn GR, & Mackerer CR (1991) Benzene
mechanistic research. Final report of collaborative study by Mobil,
Amoco Ashland, Standard Oil and Sun Company. Princeton, New Jersey,
Mobil Oil Corporation, 76 pp.
Lunte SM & Kissinger PT (1983) Detections and identification of
sulfhydryl conjugates of p-benzoquinone in microsomal incubations of
benzene and phenol. Chem-Biol Interact, 47: 195-212.
Lutz WK (1979) In vivo covalent binding of organic chemicals to DNA
as a quantitative indicator in the process of chemical carcinogenesis.
Mutat Res, 65: 289-356.
Lutz WK & Schlatter C (1977) Mechanisms of the carcinogenic action of
benzene: irreversible binding to rat liver DNA. Chem-Biol Interact,
18(2): 241-246.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal Gulf of Mexico site. Chemosphere, 17(1):
123-136.
MacKay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol, 9:
1178-1180.
Maibach HI & Anjo DM (1981) Percutaneous penetration of benzene and
benzene contained in solvents in the rubber industry. Arch Environ
Health, 36: 256-260.
Maltoni C, Ciliberti A, & Carretti D (1982a) Experimental
contributions in identifying brain potential carcinogens in the
petrochemical industry. Ann NY Acad Sci, 381: 216-249.
Maltoni C, Conti B, & Scarnato C (1982b) Squamous cell carcinomas of
the oral cavity in Sprague-Dawley rats following exposure to benzene
by ingestion. Med. Lav, 4: 441-445.
Maltoni C, Cotti G, Valgimigli L, & Mandrioli A (1982c)
Hepatocarcinomas in Sprague-Dawley rats following exposure to benzene
by inhalation. Med Lav, 4: 446-450.
Maltoni C, Conti B, & Cotti G (1983) Benzene: a multipotential
carcinogen. Results of long-term bioassays performed at the Bologna
Institute of Oncology. Am J Ind Med, 4: 589-630.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
current results and ongoing research. Am J Ind Med, 7: 415-446.
Maltoni C, Ciliberti A, Cotti G, Conti B, & Belpoggi F (1989) Benzene,
an experimental multipotential carcinogen: results of the long-term
bioassays performed at the Bologna Institute of Oncology. Environ
Health Perspect, 82: 109-124.
Mara SJ & Lee SS (1978) Assessment of human exposures to atmospheric
benzene. Research Triangle Park, North Carolina, US Environmental
Protection Agency (EPA/450/378-031).
Marcus WL (1990) Chemical of current interest: benzene. Cancer risk
from benzene. Adv Mod Environ Toxicol, 17: 127-188.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989a) Differences in the pathways for metabolism of benzene in rats
and mice simulated by a physiological model. Environ Health Perspect,
82: 43-49.
Medinsky MA, Sabourin PJ, Lucier G, Birnbaum LS, & Henderson RF
(1989b) A toxicological model for simulation of benzene metabolism.
Exp Pathol, 37: 150-154.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989c) A physiological model for simulation of benzene metabolism by
rats and mice. Toxicol Appl Pharmacol, 99: 193-206.
Merian E & Zander M (1982) Volatile aromatics. In: Hutzinger O ed. The
handbook of environmental chemistry, vol 3, Part B: Anthropogenic
compounds. Berlin, Heidelberg, New York, Springer-Verlag, pp 117-161.
Michael LC, Pellizzari ED, & Wiseman RW (1988) Development and
evaluation of a procedure for determining volatile organics in water.
Environ Sci Technol, 22: 565-570.
Miller MM & Wasik SP (1985) Relationships between octanol-water
partition coefficient and aqueous solubility. Environ Sci Technol,
19(6): 522-529.
Morimoto K (1976) Analysis of combined effects of benzene with
radiation on chromosomes in cultured human leukocytes. Jpn J Ind
Health, 18: 23-24.
Morimoto K (1983) Induction of sister chromatid exchanges and cell
division delays in human lymphocytes by microsomal activation of
benzene. Cancer Res, 43: 1330-1334.
Morimoto K, Wolff S, & Koizumi A (1983) Induction of sister-chromatid
exchanges in human lymphocytes by microsomal activation of benzene
metabolites. Mutat Res, 119: 355-360.
Moszczynski P (1981) Organic solvents and T-lymphocytes. Lancet, 1:
438.
Murray FJ, John JA, Rampy LW, Kuna RA, & Schweta BA (1979)
Embryotoxicity of inhaled benzene in mice and rabbits. Am Ind Hyg
Assoc J, 40: 993-998.
Nahum LH & Hoff HE (1934) The mechanism of sudden death in
experimental benzol poisoning. J Pharmacol Exp Ther, 50: 336.
Nakajima T, Okuyama S, & Yonekura I (1985) Effects of ethanol and
phenobarbital administration on the metabolism and toxicity of
benzene. Chem-Biol Interact, 55: 23-38.
Nakajima T, Elovaara E, Park SS, Gelbain HV, Hietanen E, & Vainio H
(1990) Monoclonal antibody-directed characterization of benzene,
ethoxy-resorufin and pentoxy-resorufin metabolism in rat liver
microsomes. Biochem Pharmacol, 40: 1255-1261.
Nau CA, Neal J, & Thornton M (1966) C9-C12 fractions obtained from
petroleum distillates. Arch Environ Health, 12: 382-393.
Nawrot PS & Staples RE (1979) Embryo-fetal toxicity and teratogenicity
of benzene and toluene in the mouse. Teratology, 19: 41A.
Nerland DE & Pierce WM (1990) Identification of N-acetyl-
S-(2,5-dihydroxyphenyl)-L-cysteine as a urinary metabolite of benzene,
phenol and hydroquinone. Drug Metab Dispos, 18: 958-961.
Nomiyama K & Nomiyama H (1974a) Respiratory retention, uptake and
excretion of organic solvents in man. Benzene, toluene, n-hexane,
trichloroethylene, acetone, ethyl acetate and ethyl alcohol. Int Arch
Arbeitsmed, 32: 75-83.
Nomiyama K & Nomiyama H (1974b) Respiratory elimination of organic
solvents in man. Benzene, toluene, n-hexane, trichloroethylene,
acetone, ethyl acetate and ethyl alcohol. Int Arch Arbeitsmed, 32:
85-91.
Nordlinder R & Ramnäs O (1987) Exposure to benzene at different work
places in Sweden. Ann Occup Hyg, 31: 345-355.
Norpoth K, Stücker W, Krewer E, & Müller G (1988) Biomonitoring of
benzene exposure by trace analyses of phenylguanine. Arch Occup
Environ Health, 60: 163-168.
NTP (1986) Toxicology and carcinogenesis studies of benzene (CAS No.
71-43-2) in F344/N rats and B6C3F1 mice (gavage studies). Research
Triangle Park, North Carolina, US Department of Health and Human
Services, National Toxicology Program (NTP TR 289; NIH Publication
86-2545).
Oberly TJ, Bewsey BJ, & Probst GS (1984) An evaluation of the L5178Y
TK+/- mouse lymphoma forward mutation assay using 42 chemicals. Mutat
Res, 125: 291-306.
OECD (1986) Control of toxic substances in the atmosphere: Benzene.
Paris, Organisation for Economic Cooperation and Development
(Environment Monographs No. 5).
Ogata M & Miyake Y (1978) Disappearance of aromatic hydrocarbons and
organic sulphur compounds from fish flesh reared in crude oil
suspension. Water Res, 12: 1041-1044.
Ogata M, Fujisawa K, Ogino Y, & Mano E (1984) Partition coefficients
as a measure of bioconcentration potential of crude oil compounds in
fish and shellfish. Bull Environ Contam Toxicol, 33: 561-567.
OSHA (Occupational Safety and Health Administration) (1987)
Occupational exposure to benzene. Fed Reg, 52(176): 34460-34578.
Otson R (1987) Purgeable organics in Great Lakes raw and treated
water. Int J Environ Anal Chem, 31: 41-53.
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 54: 1370-1374.
Ott MG, Townsend JC, Fishbeck WA, & Langner RA (1978) Mortality among
workers occupationally exposed to benzene. Arch Environ Health, 33:
3-10.
Paci E, Buiatti E, Costantini AS, Miligi L, Pucci N, Scarpelli A,
Petriolo G, Simonata L, Winkelmann R, & Kaldor JM (1989) Aplastic
anemia, leukemia and other cancer mortality in a cohort of shoe
workers exposed to benzene. Scand J Work Environ Health, 15: 313-318.
Painter RB & Howard R (1982) The HeLa-DNA synthesis inhibition test as
a rapid screen for mutagenic carcinogens. Mutat Res, 92: 427-437.
Parke DV & Williams RT (1953) Studies in detoxication. 49. The
metabolism of benzene containing [14C1]benzene. Biochem J, 54:
231-238.
Patty F (1981) Industrial hygiene and toxicology, 3rd revis ed. New
York, Interscience Publishers.
Paustembach DJ, Price PS, Ollison W, Blank C, Jernigan JD, Bass RD, &
Peterson HD (1992) Reevaluation of benzene exposure for the pliofilm
(rubberworker). J Toxicol Environ Health, 36: 177-231.
Paxman D & Rappaport SM (1990) Analysis of OSHA's short-term-exposure
limit for benzene. Regul Toxicol Pharmacol, 11: 275-287.
Pekari K, Riellola M-L, & Aitio A (1989) Simultaneous determination of
benzene and toluene in blood using head-space gas chromatography. J
Chromatogr, 491: 309-320.
Pellack-Walker P & Blumer JL (1986) DNA damage in L5178YS cells
following exposure to benzene metabolites. Mol Pharmacol, 30: 42-47.
Pellizzari ED (1982) Analysis for organic vapor emissions near
industrial and chemical waste disposal sites. Environ Sci Technol,
16(11): 781-785.
Pellizzari ED, Zweidinger RA, & Sheldon LS (1988) Determination of
benzene, toluene and xylene in breath samples by gas
chromatography/mass spectrometry. In: Fishbein L & O'Neill JK ed.
Environmental carcinogens method of analysis and exposure measurement
- Volume 10: Benzene and alkylated benzenes. New York, London, Oxford
University Press, pp 267-279.
Philip P & Krogh-Jensen M (1970) Benzene induced chromosome
abnormalities in rat bone marrow cells. Acta Pathol Microbiol Scand,
A78: 489-490.
Pollini G, Biscaldi GP, & Robustelli della Cuna G (1969) [Chromosome
changes in lymphocytes five years after benzene haemopathy.] Med Lav,
60: 743-758 (in Italian).
Pongracz K & Bodell WJ (1991) Detection of 3@-hydroxy-1,
N6-benzetheno-2'-deoxyadenosine 3'-phosphate by 32P postlabelling
of DNA reacted with p-benzoquinone. Chem Res Toxicol, 4: 199-202.
Poole SK, Furton KG, & Poole CF (1988) Determination of benzene and
toluene in gasoline by gas chromatography using a liquid organic salt
column. J Chromatogr Sci, 26: 67-73.
Post GB & Snyder R (1983) Effects of enzyme induction on microsomal
benzene metabolism. J Toxicol Environ Health, 11: 811.
Post GB, Snyder R, & Kalf GF (1985) Inhibition of RNA synthesis and
interleukin-2 production in lymphocytes in vitro by benzene and its
metabolites, hydroquinone and p-benzoquinone. Toxicol Lett, 29:
161-167.
Probst GS & Hill LE (1985) Tests for the induction of DNA-repair
synthesis in primary cultures of adult rat hepatocytes. In: Ashby J,
de Serres FJ, Draper M, Ishidata M Jr, Margolin BH, Matter BE, &
Shelby MD ed. Evaluation of short-term tests for carcinogens: Report
of the International Programme on Chemical Safety's collaborative
study on in vitro assays. Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp 381-386 (Progress in Mutation Research,
Volume 5).
Reddy MV, Bleicher WT, Blackburn GR, & Mackerer CR (1990) DNA
adduction by phenol hydroquinone, or benzoquinone in vitro but not
in vivo: nuclease P1-enhanced 32P-postlabelling of adducts as
labeled nucleoside biphosphates, dinucleotides and nucleoside
monophosphates. Carcinogenesis, 11: 1349-1357.
Reineke FJ & Bächmann K (1985) Gas chromatographic determination of
C2-C8 hydrocarbons and halocarbons in ambient air by simultaneous
use of three detectors. J Chromatogr, 323: 323-329.
Renova NW (1962) [Concerning auto-immunity shifts in chronic
occupational benzol poisoning.] Gig Tr Prof Zabol, 7: 38-42 (in
Russian).
Reynolds LF & Harrison DW (1982) Study of discharges of: benzene,
chloroform and carbon tetrachloride into the aquatic environment and
the best technical means for the reduction of water pollution from
such discharges. Final Report. Brussels, Commission of the European
Communities (BL/A/2198).
Rickert DE, Baker TS, Bus JS, Barrow CS, & Irons RD (1979) Benzene
disposition in the rat after exposure by inhalation. Toxicol Appl
Pharmacol, 49: 417-423.
Rinsky RA, Alexander B, Smith MD, Hornung R, Filloon TG, Young RJ,
Okun AH, & Landrigan PJ (1987) Benzene and leukemia: an
epidemiological risk assessment. New Engl J Med, 316: 1044-1050.
RIVM (1988) Integrated criteria document on benzene. Bilthoven, The
Netherlands, National Institute for Public Health and Environmental
Protection, 138 pp (Report No. 758476003).
Roberts JM, Rehsenfeld FC, & Liu SC (1984) Measurements of aromatic
hydrocarbon ratios and nitrogen oxide concentrations in the rural
troposphere: observation of air mass photochemical aging and nitrogen
oxides removal. Atmos Environ, 18(11): 2421-2432.
Roghani M, Da Silva C, Guvelli D, & Castagna M (1987) Benzene and
toluene activate protein kinase C. Carcinogenesis, 8: 1105-1107.
Rosenthal GJ & Snyder CA (1985) Modulation of the immune response to
Listeria monocytogenes by benzene inhalation. Toxicol Appl
Pharmacol, 80: 502.
Rozen MG & Snyder CA (1985) Protracted exposure of C57B1/6 mice to 300
ppm benzene depresses B- and T-lymphocyte numbers and mitogen
responses. Evidence for thymic and bone marrow proliferation in
response to the exposures. Toxicology, 37(1-2): 13.
Rozen MG, Snyder CA, & Albert RE (1984) Depressions in B- and
T-lymphocyte mitogen-induced blastogenesis in mice exposed to low
concentrations of benzene. Toxicol Lett, 20: 343.
Rushton L & Alderson M (1981a) An epidemiological survey of eight oil
refineries in Britain. Br J Ind Med, 38: 225-234.
Rushton L & Alderson M (1981b) Case control study to investigate the
association between exposure to benzene and deaths from leukemia in
oil refinery workers. Br J Cancer, 43: 77-84.
Sabourin PJ, Chen BT, Lucier G, Birnbaum LS, Fisher E, & Henderson RF
(1987) Effect of dose on the absorption and excretion of [14C]
benzene administered orally or by inhalation in rats and mice. Toxicol
Appl Pharmacol, 87: 325-336.
Sabourin PJ, Bechtold WE, & Henderson RF (1988) A high pressure liquid
chromatographic method for the separation and quantitation of
water-soluble radiolabeled benzene metabolites. Anal Biochem, 170:
316-327.
Sabourin PJ, Sun JD, MacGregor JT, Wehr CM, Birnbaum LS, Lucier G, &
Henderson RF (1990) Effect of repeated benzene inhalation exposures on
benzene metabolism, binding to hemoglobin, and induction of
micronuclei. Toxicol Appl Pharmacol, 103: 452-462.
SAC (1989) Analysis of potentially dangerous substances in UK water
(Prepared under contract to DOE). London, SAC Scientific Ltd (DOE
Reference No. PECD 7/7/306).
Sandmeyer EE (1981) Aromatic hydrocarbons. In: Clayton GD & Clayton FE
ed. Patty's Industrial hygiene and toxicology, 3rd revis ed. New York,
Interscience Publishers, vol 2, pp 3253-3283.
Sasiadek M, Jagielski J, & Smolik R (1989) Localization of breakpoints
in the karyotype of workers professionally exposed to benzene. Mutat
Res, 224: 235-240.
Sauer TC Jr (1981) Volatile organic compounds in open ocean and
coastal surface waters. Org Geochem, 3: 91-101.
Sawahata T & Neal RA (1983) Biotransformation of phenol to
hydroquinone and catechol by rat liver microsomes. Mol Pharmacol, 23:
453-460.
Schrenk D & Bock KW (1990) Metabolism of benzene in rat hepatocytes.
Influence of inducers on phenol glucuronidation. Drug Metab Dispos,
18(5): 720-725.
Seixas G, Andon BM, Hollingshead PG, & Thilly WG (1982) The aza-arenes
as mutagens for Salmonella typhimurium. Mutat Res, 102: 201-212.
Sellyei M & Kelemen E (1971) Chromosome study in a case of
granulocytic leukemia with "pelgerisation" seven years after benzene
pancytopenia. Eur J Cancer, 7: 83-85.
Shah JJ & Singh HB (1988) Distribution of volatile organic chemicals
in outdoor and indoor air. A national VOCs data base. Environ Sci
Technol, 22(12): 1381-1388.
Simic MG, Bergtold DS, & Karam LR (1989) Generation of oxy radicals in
biosystems. Mutat Res, 214: 3-12.
Singh HB, Salas L, & Stiles RE (1982) Distribution of selected gaseous
organic mutagens and suspected carcinogens in ambient air. Environ Sci
Technol, 16: 872.
Siou G, Conan L, & El Haitem M (1981) Evaluation of the clastogenic
action of benzene by oral administration with 2 cytogenetic techniques
in mouse and Chinese hamster. Mutat Res, 90: 273-278.
Skowronski GA, Turkall RM, & Abdel-Rahman MS (1988) Soil adsorption
alters bioavailability of benzene in dermally exposed male rats. Am
Ind Hyg Assoc J, 49(10): 506-511.
Smart RC & Zannoni VG (1985) Effect of ascorbate on covalent binding
of benzene and metabolites to isolated tissue preparations. Toxicol
Appl Pharmacol, 77: 334-343.
Snyder CA (1987) Benzene in Ethel Browning's toxicity and metabolism
of industrial solvents. Volume 1: Hydrocarbons, 2nd ed. Amsterdam,
Oxford, New York, Elsevier Science Publishers, pp 3-37.
Snyder R, Lee EW, & Kocsis JJ (1978a) Binding of labelled metabolites
to mouse liver and bone marrow. Res Commun Chem Pathol Pharmacol, 20:
191-194.
Snyder CA, Goldstein BD, Sellakumar A, Wolman S, Bromberg I, Erlichman
MN, & Laskin S (1978b) Hematotoxicity of inhaled benzene to Sprague
Dawley rats and AKR mice at 300 ppm. J Toxicol Environ Health, 4:
605-618.
Snyder CA, Goldstein BD, Sellakumar A, Bromberg I, Laskin S, & Albert
RE (1980) The inhalation toxicology of benzene: incidence of
hematopoietic neoplasms and hematotoxicity in AKR/J and C57BL/6J mice.
Toxicol Appl Pharmacol, 54: 323-331.
Snyder R, Longacre SL, Witmer CM, & Kocsis JJ (1981) Biochemical
toxicology of benzene. In: Hodgson E, Bend JR, & Philpot RM ed.
Reviews in biochemical toxicology 3. Amsterdam, Oxford, New York,
Elsevier/North Holland, pp 123-153.
Snyder CA, Goldstein BD, Sellakumar AR, & Albert RE (1984) Evidence
for hematotoxicity and tumorigenesis in rats exposed to 100 ppm
benzene. Am J Ind Med, 5: 429-434.
Snyder R, Jowa L, Witz G, Jakf G, & Rushmore T (1987) Formation of
reactive metabolites from benzene. Arch Toxicol, 60(1-3): 61-64.
Snyder CA, Sellakumar AR, James DJ, & Albert RE (1988) The
carcinogenicity of discontinuous inhaled benzene exposures in CD-1 and
C57B1/6 mice. Arch Toxicol, 62: 331-335.
Snyder R, Dimitriadis E, Guy R, Hu P, Cooper K, Bauer H, Witz G, &
Goldstein BD (1989) Studies on the mechanism of benzene toxicity.
Environ Health Perspect, 82: 31-35.
Staples CA, Werner AF, & Hoogheem TJ (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environ Toxicol Chem, 4: 131-142.
Stommel P, Müller G, Stücker W, Verkoyen C, Schöbel S, & Norpoth K
(1989) Determination of S-phenylmercapturic acid in the urine - an
improvement in the biological monitoring of benzene exposure.
Carcinogenesis, 10: 279-282.
Stoner RD, Drew RT, & Bernstein DM (1981) Benzene inhalation effect
upon tetanus antitoxin responses and leukemogenesis in mice. In:
Mahlum DD, Gray RH, & Felix WD ed. Coal conversion and the
environment. Oak Ridge, Tennessee, US Department of Energy, Technical
Information Center, pp 445-461.
Styles JA & Richardson CR (1984) Cytogenetic effects of benzene:
dosimetric studies on rats exposed to benzene vapor. Mutat Res, 135:
203-209.
Sun JD, Medinsky MA, & Birnbaum LS (1990) Benzene hemoglobin adducts
in mice and rats: Characterization of formation and physiological
modeling. Fundam Appl Toxicol, 15: 468-475.
Susten A, Dames B, & Burg J (1985) Percutaneous penetration of benzene
in hairless mice: An estimate of dermal absorption during
tire-building operations. Am J Ind Med, 7: 323-335.
Swaen GMH, Slangen JJM, Volovics A, Hayes RB, Scheffers T, & Sturmans
F (1991) Mortality of coke plant workers in the Netherlands. Br J Ind
Med, 48: 130-135.
Swenberg JA, Petzold GL, & Harbach PR (1976) In vitro DNA
damage/alkaline elution assay for predicting carcinogenic potential.
Biochem Biophys Res Commun, 72: 732-738.
Tatrai E, Ungvary GY, Hudak A, Rodics K, Loerincz M, & Barcza GY
(1980a) Concentration of dependence of the embryotoxic effects of
benzene inhalation in CFY rats. J Hyg Epidemiol Microbiol Immunol, 24:
363-371.
Tatrai E, Rodics K, & Ungvary GY (1980b) Embryotoxic effects of
simultaneously applied exposure of benzene and toluene. Folia Morphol
(Praha), 28: 286-289.
Thienes H & Haley TJ (1972) Clinical toxicology, 5th ed. Philadelphia,
Pennsylvania, Lea & Febiger, pp 124-127.
Tice RR, Costa DL, & Drew RT (1980) Cytogenetic effects of inhaled
benzene in murine bone marrow: Induction of sister chromatid
exchanges, chromosomal aberrations and cellular proliferation
inhibition in DBA/2 mice. Proc Natl Acad Sci (USA), 77: 2148-2152.
Tice RR, Vogt T, & Costa D (1982) Cytogenetic effects of inhaled
benzene in murine bone marrow. In: Genotoxic effects of airborne
agents. New York, London, Plenum Press, pp 257-275.
Tice RR, Luke CA, & Drew RT (1989) Effect of exposure route, regimen,
and duration on benzene-induced genotoxic and cytotoxic bone marrow
damage in mice. Environ Health Perspect, 82: 65-74.
Toft K, Olofsson T, Tunek A, & Berlin M (1982) Toxic effects on mouse
bone marrow caused by inhalation of benzene. Arch Toxicol, 51:
295-302.
Topham JC (1980) Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutat Res, 74: 379-387.
Tough IM & Court Brown WM (1965) Chromosome aberrations and exposure
to ambient benzene. Lancet, 1: 684.
Travis CC, Quillen JL, & Arms AD (1990) Pharmacokinetics of benzene.
Toxicol Appl Pharmacol, 102: 400-420.
Tsai SP, Wen CP, Weiss NS, Wong O, McClellan WA, & Gibson RL (1983)
Retrospective mortality and medical surveillance studies of workers in
benzene areas of refineries. J Occup Med, 25: 685-692.
Tucker WA, Huang C, & Bral JM (1986) Validation of transport model.
In: Benzene in Florida groundwater: an assessment of the significance
to human health. Tallahassee, Florida, Florida Petroleum Council,
American Petroleum Institute, pp 93-108.
Tully FP, Ravishankara AR, Thopmson RL, Nicovich JM, Shah RC, Kreutter
NM, & Wine PH (1981) Kinetics of the reactions of hydroxyl radicals
with benzene and toluene. J Phys Chem, 85: 2262.
Tunek A, Platt KL, Bentley P, & Oesch F (1978) Microsomal metabolism
of benzene to species irreversibly binding to microsomal protein and
effects of modification of this metabolism. Mol Pharmacol, 14:
920-929.
UBA (1982) Air quality criteria for benzene. Berlin, Federal Office
for the Environment (Report 6/82).
Ungvary G & Tatrai E (1985) On the embryotoxic effects of benzene and
its alkyl derivatives in mice, rats and rabbits. Arch Toxicol, 8:
425-430.
US EPA (1985) Drinking water criteria document on benzene. Washington,
DC, US Environmental Protection Agency, Office of Drinking Water
(PB86-118122).
US EPA (1987) June-September, 6-9 AM, ambient air benzene
concentrations in 39 U.S. cities, 1984-1986. Research Triangle Park,
North Carolina, US Environmental Protection Agency, Atmospheric
Sciences Research Laboratory (EPA/600/D-87/160).
US EPA (1989) National emissions standards for hazardous air
pollutants: Benzene emissions from maleic anhydride plants,
ethylbenzene styrene plants, benzene storage vessels, benzene
equipment leaks, and coke by-product recovery plants. Fed Reg, 54:
38044-38139.
Uyeki EM, Ashkar AE, Shoeman DW, & Bisel TU (1977) Acute toxicity of
benzene inhalation to hemopoietic precursor cells. Toxicol Appl
Pharmacol, 40: 49-57.
Vaishnav DD & Babeu L (1987) Comparison of occurrence and rates of
chemical biodegradation in natural waters. Bull Environ Contam
Toxicol, 39: 237-244.
Venitt S (1985) Summary report on the performance of the bacterial
mutation assays. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr,
Margolin BH, Matter BE, & Shelby MD ed. Evaluation of short-term tests
for carcinogens. Report of the International Programme on Chemical
Safety's collaborative study on in vitro assays. Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp 11-23 (Progress in Mutation
Research, Volume 5).
Vigliani EC & Saita G (1964) Benzene and leukemia. New Engl J Med,
271(17): 872-876.
Vogel K, Bentley P, Platt KL, & Oesch F (1980) Rat liver cytoplasmic
dihydrodiol dehydrogenase. J Biol Chem, 255: 9621-9625.
Wallace LA (1989a) The exposure of the general population to benzene.
Cell Biol Toxicol, 5(3): 197.
Wallace LA (1989b) Major sources of benzene exposure. Environ Health
Perspect, 82: 165-169.
Wallace LA & Pellizzari ED (1986) Personal air exposures and breath
concentrations of benzene and other volatile hydrocarbons for smokers
and nonsmokers. Toxicol Lett, 35: 113-116.
Wallace LA, Pellizzari E, & Hartwell TD (1985) Personal exposures,
indoor-outdoor relationships, and breath levels of toxic air
pollutants measured for 355 persons in New Jersey. Atmos Environ, 19:
1651-1661.
Wallace LA, Pellizzari E, Leader B, Zelon H, & Sheldon L (1987)
Emissions of volatile organic compounds from building materials and
consumer products. Atmos Environ, 21(2): 385-393.
Wallin H, Melin P, Schelin C, & Jergil B (1985) Evidence that covalent
binding of metabolically activated phenol to microsomal proteins is
caused by oxidised products of hydroquinone and catechol. Chem-Biol
Interact, 55: 335-346.
Ward CO, Kuna RA, Snyder NK, Alsaker RD, Coate WB, & Craig PH (1985)
Subchronic inhalation toxicity of benzene in rats and mice. Am J Ind
Med, 7: 457.
Wathne BM (1983) Measurements of benzene, toluene and xylenes in urban
air. Atmos Environ, 17: 1713.
Weaver NK, Gibson RL, & Smith CW (1983) Occupational exposure to
benzene in the petroleum and petrochemical industries. Adv Mod Environ
Toxicol, 4: 63-75.
Wermuth B, Platts K, Seidel A, & Oesch F (1986) Carbonyl reductase
provides the enzymatic basis of quinone reduction in man. Biochem
Pharmacol, 35: 1277.
WHO (1984) Guidelines for drinking-water quality. Volume 1:
Recommendations. Geneva, World Health Organization, 130 pp.
WHO (1987) Benzene. In: Air quality guidelines for Europe. Copenhagen,
World Health Organization, Regional Office for Europe, pp 45-58 (WHO
Regional Publications, European Series No. 23).
WHO (1993) Guidelines for drinking-water quality. Volume 1:
Recommendations. Geneva, World Health Organization.
Wilson RH (1942) Benzene poisoning in industry. J Lab Clin Med, 27:
1517-1521.
Wilson BH, Smith GB, & Rees JF (1986) Biotransformation of selected
alkylbenzenes and halogenated hydrocarbons in methanogenic aquifer
material: A microcosm study. Environ Sci Technol, 20: 997-1002.
Windholz M, Budavari S, Blumetti RF, & Otterbein ES (1983) The Merck
Index: An encyclopedia of chemicals, drugs, and biologicals, 10th ed.
Rahway, New Jersey, Merck and Co., Inc., p 151.
Winek CL, Collom WD, & Wecht CH (1967) Fatal benzene exposure by glue
sniffing. Lancet, March 25: 683
Winek CL & Collom WD (1971) Benzene and toluene fatalities. J Occup
Med, 13: 259-261.
Withey RJ & Hall JW (1975) The joint action of perchloroethylene with
benzene or toluene in rats. Toxicology, 4: 5-15.
Witz G, Gad SC, Tice RR, Oshiro Y, Piper CE, & Goldstein BD (1990a)
Genetic toxicity of the benzene metabolite trans,trans-muconaldehyde
in mammalian and bacterial cells. Mutat Res, 240: 295-306.
Witz G, Maniara W, Mylavarapu V, & Goldstein BD (1990b) Comparative
metabolism of benzene and trans,trans-muconaldehyde to
trans,trans-muconic acid in DBA/2N and C57Bl/6 mice. Biochem
Pharmacol, 40: 1275-1280.
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, & Oyen F (1956)
Toxicological studies of certain alkylated benzenes and benzene. Arch
Ind Health, 14: 387-398.
Wong O (1987) An industry wide mortality study of chemical workers
occupationally exposed to benzene: II. Dose response analyses. Br J
Ind Med, 44: 382-395.
Yardley-Jones A, Anderson D, Jenkinson PC, Lovell DP, Blowers SD, &
Davies MJ (1988) Genotoxic effects in peripheral blood and urine of
workers exposed to low level benzene. Br J Ind Med, 45: 694-700.
Yardley-Jones A, Anderson D, Lovell DP, & Jenkinson PC (1990)
Analysis of chromosomal aberrations in workers exposed to low level
benzene. Br J Ind Med, 47: 48-51.
Yin S-N, Li G-L, Tain F-D, Fu Z-I, Jin C, Chen Y-J, Luo S-J, Ye P-Z,
Zhang J-Z, Wang G-C, Zhang X-C, Wu H-N, & Zhong Q-C (1987) Leukaemia
in benzene workers: a retrospective cohort study. Br J Ind Med, 44:
124-128.
Yin S-N, Li G-L, Tain F-D, Fu Z-I, Jin C, Chen Y-J, Luo S-J, Ye P-Z,
Zhang J-Z, Wang G-C, Zhang X-C, Wu H-N, & Zhong Q-C (1989) A
retrospective cohort study of leukemia and other cancers in benzene
workers. Environ Health Perspect, 82: 207-213.
Young RJ, Rinsky RA, Infante PF, & Wagoner JK (1978) Benzene in
consumer products. Science, 199: 248.
Zhurkov VS, Fel'dt EG, & Kosyakov VV (1983) Dependence of the
frequency of chromosomal aberrations in mouse bone marrow cells on
concentration (dose) and mode of administration of benzene. Bull Exp
Biol Med, 96: 1741-1743.
RESUME ET CONCLUSIONS
1. Identité, propriétés physiques et chimiques, méthodes d'analyse
Le benzène est un liquide incolore, stable à la température
ambiante et sous la pression atmosphérique normale. Il possède une
odeur aromatique caractéristique et du fait de son bas point
d'ébullition (80,1 °C) et de sa forte tension de vapeur, il s'évapore
rapidement et il est très inflammable. Il est légèrement soluble dans
l'eau mais miscible à la plupart des solvants organiques.
Il existe des méthodes qui permettent de rechercher la présence
de benzène dans différents milieux (air, eau, organes/tissus). On
peut utiliser à cette fin la chromatographie en phase gazeuse avec, au
choix, une détection par ionisation de flamme, par photoionisation ou
par spectrométrie de masse, en fonction de la sensibilité nécessaire
et des concentrations attendues. La recherche du benzène sur le lieu
de travail s'effectue généralement par adsorption sur charbon actif
puis analyse par chromatographie en phase gazeuse couplée à la
spectrométrie de masse après désorption. Lorsqu'on peut se contenter
d'une sensibilité de l'ordre du mg/m3, on peut utiliser des
instruments à lecture directe et des dosimètres passifs. Si l'on
désire une meilleure sensibilité, il existe des méthodes qui
permettent de déceler la présence de benzène à des concentrations ne
dépassant pas 0,01 µg/m3 d'air ou 1 ng/kg de terre ou d'eau.
2. Sources d'exposition humaine
Le benzène existe à l'état naturel dans le pétrole brut à des
concentrations pouvant atteindre 4 g/litre. Il est également produit
partout dans le monde en quantités extrêmement importantes (14,8
millions de tonnes). Des émissions peuvent se produire lors du
traitement des produits pétroliers, de la cokéfaction du charbon, de
la production de toluène, de xylène et autres dérivés aromatiques
ainsi que lorsqu'il est utilisé dans certains produits de
consommation, comme intermédiaire ou comme constituant de l'essence.
3. Transport, distribution et transformation dans l'environnement
Dans l'air, le benzène est présent essentiellement en phase
gazeuse, et sa durée de séjour varie de quelques heures à quelques
jours, en fonction de l'environnement et du climat et aussi de la
concentration des radicaux hydroxyles, des oxydes d'azote et de
soufre. Lorsqu'il est éliminé de l'air par la pluie, il peut
contaminer les eaux de surface et les eaux souterraines dans
lesquelles il est soluble à raison d'environ 1000 mg/litre.
En raison principalement de sa volatilisation dans l'atmosphère,
le temps de séjour du benzène dans l'eau est limité à quelques heures
et il est peu ou pas adsorbé par les sédiments.
Le benzène présent dans le sol peut passer dans l'air par
volatilisation et dans les eaux de surface par ruissellement. En cas
d'enfouissement ou de libération à profondeur importante, il passe
dans les eaux souterraines.
En aérobiose, le benzène présent dans l'eau ou dans le sol est
rapidement dégradé (quelques heures) par les bactéries en lactate et
en pyruvate avec formation intermédiaire de phénol et de catéchol.
Cependant, en anaérobiose (par exemple dans les eaux souterraines) la
dégradation bactérienne prend des semaines, voire des mois et non plus
des heures. Il y a persistance du benzène lorsque la dégradation
bactérienne ne se produit pas. Il ne semble pas subir de
bioconcentration ou de bioaccumulation dans les organismes aquatiques
ou terrestres.
4. Concentrations dans l'environnement et exposition humaine
La présence de benzène dans l'essence et sa large utilisation
comme solvant industriel peuvent conduire à des émissions importantes
un peu partout dans l'environnement. A l'extérieur, les
concentrations vont de 0,2 µg/m3 dans les régions rurales écartées
à 349 µg/m3 dans les zones industrielles où la circulation
automobile est dense. Lors du remplissage du réservoir d'un véhicule
à moteur, les concentrations peuvent atteindre 10 mg/m3.
On a décelé la présence de benzène à des concentrations
atteignant 500 µg/m3 dans l'air intérieur des pièces de séjour. La
fumée de cigarette contribue de façon importante à la présence de
benzène dans l'air intérieur, les fumeurs inhalant environ 1800 µg de
benzène par jour contre 50 µg pour les non fumeurs.
Dans de nombreux pays, l'exposition professionnelle dépasse
rarement 15 mg/m3 en moyenne pondérée par rapport au temps.
Toutefois, les concentrations effectives rapportées dépendent du type
d'industries étudiées et elles peuvent être beaucoup plus élevées dans
les pays en voie de développement industriel.
L'eau et les aliments ne contribuent que pour une faible part à
l'apport journalier de benzène chez les adultes non fumeurs (entre 3
et 24 µg/kg de poids corporel et par jour).
5. Cinétique et métabolisme
Le benzène est bien absorbé chez l'homme et les animaux de
laboratoire après exposition par voie orale ou respiratoire, mais chez
l'homme, le benzène n'est que faiblement absorbé par voie percutanée.
L'absorption se produit chez l'homme à hauteur d'environ 50% lors
d'expositions continues pendant plusieurs heures à des concentrations
de 163 à 326 mg/m3. On a constaté qu'après quatre heures
d'exposition à 170-202 mg/m3, le benzène était retenu par
l'organisme humain dans la proportion d'environ 30%, 16% de cette dose
étant rejetés tels quels dans l'air expiré. Le benzène inhalé est
davantage retenu par l'organisme féminin que par l'organisme masculin.
Le benzène à tendance à s'accumuler dans les tissus à forte teneur en
lipides et il traverse la barrière placentaire.
Le métabolisme du benzène s'effectue principalement dans le foie,
essentiellement par l'intermédiaire du système enzymatique du
cytochrome P-450 IIE1 et il comporte la formation d'une série de
métabolites réactifs instables. Chez les rongeurs, les processus de
formation de deux métabolites toxiques supposés, la benzoquinone et le
muconaldéhyde, se révèlent être saturables. Ce phénomène peut avoir
des conséquences importantes en ce qui concerne les relations
dose-réponse car la proportion de benzène transformée en métabolites
toxiques sera plus forte à faibles doses qu'à doses élevées. Les
produits du métabolisme sont principalement excrétés dans les urines.
Les métabolites reconnus du benzène: phénol, catéchol et hydroquinone
- se retrouvent en quantité appréciable dans la moelle osseuse. Le
phénol est le principal métabolite urinaire chez l'homme et on le
retrouve dans l'urine, essentiellement sous forme de sulfoconjugué
jusqu'à ce que les concentrations atteignent 480 mg/litre, après quoi
on observe la formation de glucuronides. D'après des études récentes,
la toxicité du benzène serait due à l'interaction des différents
métabolites de ce composé qui se forment tant dans le foie que dans la
moelle osseuse.
Une fois inhalé, le benzène se fixe à l'ADN du foie chez le rat
à raison de 2,38 µmol/mol d'ester phosphorique. On a décelé dix
adduits de désoxyguanosine et un adduit de désoxyadénine dans l'ADN
mitochondrial de la moelle osseuse du lapin.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuves in vitro
6.1 Toxicité générale
Le benzène ne présente qu'une faible toxicité aiguë chez diverses
espèces animales, les valeurs de la DL50 après exposition orale
allant de 3000 à 8100 mg/kg de poids corporel chez le rat, par
exemple. On a fait état de valeurs de la CL50 allant de 15 000
mg/m3 (8 h) chez la souris à 44 000 mg/m3 (4 h) chez le rat.
Le benzène est modérément irritant pour la muqueuse oculaire et
provoque une irritation dermique chez le lapin après plusieurs
applications de produit non dilué. On ne dispose d'aucune donnée sur
le pouvoir de sensibilisation cutanée du benzène.
L'exposition de souris à du benzène par la voie respiratoire
entraîne une baisse sensible de certains paramètres hématologiques tel
que l'hématocrite, le taux d'hémoglobine ainsi que le nombre
d'érythrocytes, de leucocytes et de plaquettes. Une exposition de
longue durée à de fortes doses provoque une aplasie médullaire. Chez
le rat les effets sont analogues mais moins graves.
6.2 Génotoxicité et cancérogénicité
Les tests de mutagénicité in vitro ont donné des résultats
négatifs.
En ce qui concerne les études in vivo, on observe que le
benzène et ses métabolites entraînent des aberrations dans la
structure et le nombre des chromosomes chez l'homme et les animaux de
laboratoire. En outre, l'administration de benzène provoque des
échanges entre chromatides soeurs et la formation d'érythrocytes
polychromatiques avec micronoyaux. Après administration par la voie
intrapéritonéale, le benzène peut atteindre les cellules germinales
comme le montrent les anomalies observées dans la morphologie de la
tête des spermatozoïdes.
On a fait état de la formation de plusieurs types de cancers dus
au benzène chez le rat et la souris après administration par voie
orale ou exposition par la voie respiratoire. Il s'agit de divers
types de tumeurs malignes épithéliales concernant par exemple la
glande de Zymbal, le foie, le tissu mammaire et les fosses nasales,
avec en outre quelques lymphomes et leucémies.
Dans les études comportant une exposition par inhalation et au
cours desquelles on a relevé effectivement une action cancérogène, les
doses allaient de 100 à 960 mg/m3, cinq à sept heures par jour et
cinq jours par semaine. L'administration par voie orale de benzène à
des doses allant de 25 à 500 mg/kg de poids corporel à des souris et
à des rats, a entraîné la formation de néoplasmes. La durée
d'exposition était généralement de un à deux ans.
6.3 Effets toxiques sur la reproduction; embryotoxicité et tératogénicité
Le benzène traverse facilement la barrière placentaire. De
nombreuses expériences au cours desquelles des animaux de laboratoire
ont été soumis à des doses atteignant même les valeurs toxiques pour
la mère n'ont pas permis de recueillir de données indicatives d'un
effet tératogène. Toutefois, on a montré que le benzène était
foetotoxique après exposition par la voie respiratoire, chez la souris
(1600 µg/m3, sept heures par jour, du sixième au quinzième jours de
la gestation) et le lapin.
6.4 Immunotoxicité
Le benzène réduit l'aptitude des lymphocytes B et T à proliférer.
Chez plusieurs espèces d'animaux de laboratoire exposés au benzène, on
a noté une diminution de la résistance de l'hôte aux infections.
7. Effets sur l'homme
On sait que le benzène produit un certain nombre d'effets nocifs
pour la santé humaine. Le plus fréquemment cité de ces effets est une
dépression médullaire qui conduit à une anémie aplasique.
L'exposition à de fortes doses de benzène entraîne probablement une
forte incidence de ces maladies.
Il est bien établi que le benzène est cancérogène pour l'homme.
Des études épidémiologiques effectuées sur les travailleurs exposés au
benzène ont montré qu'il existait une relation causale entre
l'exposition à cette substance et l'apparition d'une leucémie
myéloïde. La relation qui a été observée entre l'exposition au
benzène et l'apparition de lymphomes ou de myélomes multiples reste à
élucider.
Le Groupe de travail a estimé que les données épidémiologiques ne
permettent pas de distinguer entre (a) une faible augmentation de la
mortalité par leucémie chez les travailleurs exposés à de faibles
doses de benzène et (b) une situation où le risque n'existe pas.
8. Conclusions
Le Groupe a conclu qu'une exposition moyenne pondérée par rapport
au temps de l'ordre de 3,2 mg/m3 (1 ppm) au cours d'une carrière de
40 ans, n'entraîne pas, statistiquement parlant, de surmortalité par
leucémie. Toutefois, comme le benzène est cancérogène pour l'homme,
il convient de limiter l'exposition à la dose la plus faible
compatible avec les exigences techniques. Il convient d'éviter
également tout accroissement de l'exposition au-delà de la valeur de
32 mg/m3 (10 ppm). Le benzène et les produits qui en contiennent,
comme l'essence, ne doivent jamais être utilisés comme agents de
nettoyage.
On admet traditionnellement que la dépression médullaire,
c'est-à-dire une anémie, une leucopénie ou une thrombocytopénie,
observées sur les lieux de travail, constituent le premier stade d'une
intoxication par le benzène et que ces affections sont liées à la
dose. En d'autres termes, plus la dose est élevée, plus la
probabilité d'observer une réduction des éléments figurés du sang est
élevée.
L'exposition à de fortes doses de benzène (160 à 320 mg/m3)
pendant un an entraînerait selon toute probabilité une toxicité
médullaire chez une proportion importante des travailleurs, voire une
anémie aplasique chez certains d'entre eux, mais les effets ne
seraient guère marqués à plus faibles doses. Une exposition à de
faibles et fortes doses devrait entraîner une intoxication benzénique
au bout de dix années d'exposition continue. On peut donc dire qu'à
fortes doses, on observerait un nombre élevé de cas de dépression
médullaire et d'anémie aplasique avec également quelques lésions à
faibles doses. Au cas où l'on observerait l'un quelconque de ces
effets quel que soit le niveau d'exposition, il faudrait prendre des
mesures pour améliorer le contrôle de cette exposition.
Rien d'indique que le benzène soit tératogène à des doses plus
faibles que celles qui sont toxiques pour la mère, toutefois on a
observé une toxicité foetale.
La neurotoxicité et l'immunotoxicité du benzène n'ont pas été
bien étudiées, ni chez l'animal de laboratoire ni chez l'homme.
RESUMEN Y CONCLUSIONES
1. Identidad, propiedades físicas y químicas y métodos analíticos
El benceno es un líquido incoloro y estable a temperatura
ambiente y presión atmosférica normal. Posee un olor aromático
característico, un punto de ebullición relativamente bajo (80,1 °C) y
una elevada presión de vapor, lo que hace que se evapore rápidamente
a temperatura ambiente, y es altamente inflamable. Es ligeramente
soluble en agua, pero también es miscible en la mayoría de los otros
disolventes orgánicos.
Se dispone de métodos analíticos para detectar benceno en
diversos medios (aire, agua, órganos/tejidos). La elección entre
cromatografía de gases (CG), con detección mediante ionización de
llama o fotoionización, o espectrometría de masas (EM) depende de la
sensibilidad requerida y de los niveles de benceno previstos. La
detección de benceno en el lugar de trabajo se realiza normalmente
mediante captación con carbón vegetal, desorción, y análisis por CG o
EM. Si es suficiente una sensibilidad del orden de mg/m3, pueden
emplearse instrumentos portátiles de lectura directa y dosímetros
pasivos. Para los casos en que hace falta una mayor sensibilidad, se
han notificado métodos válidos para detectar benceno a niveles de sólo
0,01 µg/m3 (aire) o 1 ng/kg (suelo o agua).
2. Fuentes de exposición humana
El benceno es un producto químico natural, que se halla en el
petróleo crudo a niveles de hasta 4 g/litro. Además, es producido en
muy grandes cantidades (14,8 millones de toneladas) en todo el mundo.
Se producen emisiones de benceno durante el procesamiento de los
productos petroleros, durante la producción de coque a partir de
carbón, durante la producción de tolueno, xileno y otros compuestos
aromáticos, y como consecuencia de su empleo en productos de consumo,
como compuesto intermedio y como componente de la gasolina.
3. Transporte, distribución y transformación en el medio ambiente
El benceno presente en el aire se halla predominantemente en la
fase de vapor, y su tiempo de persistencia varía entre unas horas y
unos días, según el entorno y el clima, y en función de la
concentración de radicales hidroxilo, así como de dióxidos de azufre
y de nitrógeno. El benceno presente en el aire es eliminado por la
lluvia, con la consiguiente contaminación de las aguas superficiales
y subterráneas, en las que es soluble a razón de aproximadamente 1000
mg/litro.
Debido fundamentalmente a la volatilización, el tiempo de
persistencia del benceno en el agua es de unas cuantas horas, y su
adsorción por los sedimentos es escasa o nula.
El benceno presente en el suelo puede pasar al aire por
volatilización, y a las aguas superficiales por la escorrentía. Si es
enterrado o liberado muy por debajo de la superficie, será
transportado hasta las aguas subterráneas.
En condiciones aerobias el benceno presente en el agua o el suelo
es rápidamente (en cuestión de horas) degradado por las bacterias a
lactato y piruvato, previa transformación en los productos intermedios
fenol y catecol. En cambio, en condiciones anaerobias (por ejemplo en
las aguas subterráneas) la degradación bacteriana requiere semanas o
meses en lugar de horas. Si no hay degradación bacteriana el benceno
puede persistir. No hay pruebas de una bioconcentración o
bioacumulación de benceno en organismos acuáticos o terrestres.
4. Niveles ambientales y exposición humana
La presencia de benceno en la gasolina y su amplio uso como
disolvente industrial puede dar lugar a emisiones importantes y
generalizadas al medio ambiente. Sus niveles ambientales al aire
libre oscilan entre los 0,2 µg/m3 hallados en zonas rurales aisladas
y los 349 µg/m3 detectados en centros industriales con alta densidad
de tráfico de automóviles. Durante las operaciones de
reabastecimiento de combustible de los automóviles se han llegado a
registrar niveles de hasta 10 mg/m3.
En el aire del interior de las viviendas se han detectado niveles
de benceno de hasta 500 µg/m3. El humo del tabaco contribuye con
importantes cantidades de benceno a los niveles registrados en el aire
de los espacios interiores, cifrándose las cantidades inhaladas por
los fumadores en aproximadamente 1800 µg de benceno al día, frente a
50 µg/día los no fumadores.
En numerosos países la exposición ocupacional rara vez supera una
media ponderada respecto al tiempo de 15 mg/m3. Sin embargo, los
niveles reales notificados dependen de la industria estudiada, y en
algunos países en fase de desarrollo industrial las exposiciones
pueden ser considerablemente superiores.
El benceno transmitido por el agua y los alimentos representa
sólo un pequeño porcentaje de la ingesta diaria total de los adultos
no fumadores (entre unos 3 y 24 µg/kg de peso corporal al día).
5. Cinética y metabolismo
El benceno es fácilmente absorbido por el hombre y los animales
experimentales que entran en contacto con el producto por exposición
oral o inhalación, pero en la especie humana la absorción cutánea es
escasa. Con una exposición continua a niveles de 163-326 mg/m3
durante varias horas, la absorción en el hombre es de aproximadamente
un 50%. Tras una exposición de 4 horas a niveles de 170-202 mg/m3,
la retención en el organismo humano fue de aproximadamente un 30%,
habiéndose excretado un 16% de la dosis retenida en forma de benceno
inalterado a través del aire expirado. Las mujeres suelen retener un
mayor porcentaje del benceno inhalado que los hombres. El benceno
tiende a acumularse en los tejidos que contienen gran cantidad de
lípidos y atraviesa la placenta.
El metabolismo del benceno se produce principalmente en el
hígado, depende básicamente del sistema enzimático del citocromo P-450
IIE1 y conlleva la formación de una serie de metabolitos reactivos
inestables. En los roedores la formación de dos presuntos metabolitos
tóxicos, la benzoquinona y el muconaldehído, parece ser saturable, lo
que puede tener gran importancia desde el punto de vista de la
relación dosis-respuesta, pues significa que a dosis bajas la
proporción de benceno transformada en metabolitos tóxicos será mayor
que a dosis altas. Los productos metabólicos son excretados
principalmente por la orina. En la médula ósea se observan niveles
importantes de los conocidos metabolitos fenol, catecol e
hidroquinona. En el hombre el metabolito urinario predominante es el
fenol, que aparece sobre todo conjugado con sulfato como éter a
niveles inferiores a 480 mg/litro, concentración a la cual se empiezan
a detectar glucurónidos. Estudios recientes llevan a pensar que la
toxicidad del benceno se debe a la interacción de varios metabolitos
bencénicos formados tanto en el hígado como en la médula ósea.
Se ha observado que el benceno inhalado se une al ADN hepático de
la rata a razón de 2,38 µmoles por mol de fosfato de ADN. En el ADN
mitocondrial de la médula ósea de conejo se han detectado siete
aductos de desoxiguanosina y un aducto de desoxiadenina.
6. Efectos en los mamíferos de laboratorio y en las pruebas in vitro
6.1 Toxicidad sistémica
El benceno tiene al parecer una toxicidad aguda baja en diversas
especies animales, oscilando las DL50 por exposición oral en la rata
entre 3000 y 8100 mg/kg de peso corporal. Las CL50 notificadas
oscilan entre los 15 000 mg/m3 (8 h) del ratón y los 44 000 mg/m3
(4 h) de la rata.
El benceno tiene un efecto irritante moderado sobre los ojos, y
aplicado reiteradamente y sin diluir también es irritante para la piel
del conejo. No se dispone de información sobre el potencial de
sensibilización cutánea del benceno.
Los ratones expuestos a benceno por inhalación presentan una
disminución importante del valor de parámetros hemáticos tales como el
hematocrito, la hemoglobina y el número de eritrocitos, leucocitos y
plaquetas. La exposición prolongada a altas dosis provoca aplasia de
la médula ósea. También en la rata se han observado efectos
similares, aunque menos graves.
6.2 Genotoxicidad y carcinogenicidad
Las pruebas de mutagenicidad del benceno in vitro han arrojado
resultados negativos.
En los estudios in vivo el benceno o sus metabolitos causan
aberraciones cromosómicas tanto estructurales como numéricas en el
hombre y en los animales de laboratorio. La administración de
benceno, además, da lugar a intercambios entre cromatidios hermanos y
a la producción de eritrocitos policromáticos con micronúcleos.
Administrado interperitonealmente el benceno puede alcanzar las
células germinales, como demuestra la aparición de alteraciones
morfológicas de la cabeza de los espermatozoides.
Se ha notificado que la administración oral o la inhalación de
benceno provocan tanto en la rata como en el ratón varios tipos de
neoplasma, entre ellos diversos tipos de neoplasma epitelial, por
ejemplo de la glándula de Zymbal, hígado, tejido mamario y cavidades
nasales, y algunos linfomas y leucemias.
En los estudios en que se observó una respuesta carcinógena
positiva a la inhalación, los niveles de exposición oscilaban entre
100 y 960 mg/m3 durante 5 a 7 h/día, cinco días por semana. En el
ratón y la rata, la administración oral de benceno a dosis de 25-500
mg/kg de peso corporal provocó la aparición de neoplasmas; la duración
de la exposición fue por lo general de 1 a 2 años.
6.3 Toxicidad en la reproducción, embriotoxicidad y teratogenicidad
El benceno atraviesa libremente la barrera placentaria. Tras
numerosos experimentos realizados con animales a dosis incluso tóxicas
para la madre, no se ha obtenido ningún dato que demuestre que tenga
efectos teratógenos. No obstante, se ha demostrado que su inhalación
tiene efectos fetotóxicos en el ratón (1600 µg/m3, 7 h/día, días 6
a 15 de gestación) y en el conejo.
6.4 Inmunotoxicidad
El benceno deprime la capacidad de proliferación de los
linfocitos B y T. Se ha observado una menor resistencia a las
infecciones en varias especies de laboratorio expuestas al benceno.
7. Efectos en el ser humano
Es sabido que el benceno tiene varios efectos perjudiciales para
la salud, entre los que destaca por su frecuencia la depresión de la
médula ósea, que conduce a la anemia aplásica. Unos niveles altos de
exposición hacen probable una elevada incidencia de esas enfermedades.
Está demostrado que el benceno tiene un efecto carcinógeno en el
ser humano. Los estudios epidemiológicos realizados sobre
trabajadores expuestos al benceno han demostrado la existencia de una
relación causal entre la exposición al benceno y la incidencia de
leucemia mielógena. Resta por aclarar si existe también una relación
entre la exposición al benceno y la aparición de linfoma y mieloma
múltiple.
El Grupo de Estudio era de la opinión de que los datos
epidemiológicos no permiten distinguir entre a) un ligero aumento de
la mortalidad por leucemia entre los trabajadores expuestos a niveles
bajos de benceno, y b) una situación sin riesgo.
8. Conclusiones
Se llegó a la conclusión de que una media ponderada respecto al
tiempo de 3,2 mg/m3 (1 ppm) a lo largo de 40 años de trabajo no
determina un aumento estadísticamente significativo del número de
defunciones por leucemia. Debido a sus efectos carcinógenos sobre el
hombre, sin embargo, las exposiciones se deberán limitar al nivel
técnicamente más bajo posible. Deberán evitarse las exposiciones
superiores a 32 mg/m3 (10 ppm). El benceno y los productos que lo
contienen, como la gasolina, no se deberían emplear nunca en
operaciones de limpieza.
Tradicionalmente se ha considerado que la aparición de depresión
de médula ósea - esto es, de anemia, leucopenia o trombocitopenia - en
el lugar de trabajo representa la primera fase de toxicidad del
benceno. Esa manifestación obedece al parecer a una relación
dosis-respuesta; en otras palabras, cuanto mayor la dosis, mayor
también la probabilidad de observar una disminución del número de
células sanguíneas circulantes.
La exposición a altos niveles de benceno (160-320 mg/m3)
durante un año tendría con toda probabilidad efectos tóxicos sobre la
médula ósea en un elevado porcentaje de los trabajadores, y provocaría
anemia aplásica en algunos casos, pero dosis menores apenas tendrían
efectos. En cambio, cabe prever que la exposición continua durante
diez años a dosis altas o bajas tendría efectos tóxicos. Así, con las
dosis elevadas se observaría una alta incidencia tanto de depresión de
la médula ósea como de anemia aplásica, y con las dosis más bajas se
observarían también algunas lesiones. La observación de cualquiera de
esos efectos, con independencia del nivel de exposición, será
reveladora de la necesidad de mejorar la vigilancia de la exposición
al benceno.
No hay indicios de que el benceno tenga efectos teratógenos a
dosis inferiores a las que resultan tóxicas para la madre, pero sí se
ha demostrado que tiene efectos tóxicos para el feto.
La neurotoxicidad y la inmunotoxicidad del benceno no han sido
suficientemente estudiadas ni en animales experimentales ni en el ser
humano.
 Chemical formula: C6H6
CAS number:
Chemical formula: C6H6
CAS number: 

 the Zymbal gland. This is the first suggestion that conjugation
products, normally thought of as only a mechanism for urinary
excretion, could also be considered to act as a transporting mechanism
for bringing metabolites from the liver to target tissues.
Parke & Williams (1953) reported that phenyl mercapturic acid was
a urinary end product of benzene metabolism. This observation was
supported by the report of Jerina et al. (1968) who incubated
glutathione with rat liver cytoplasm and benzene oxide and found that
the principal metabolite was S-phenylglutathione. Norpoth (1988) has
developed a method for the determination of phenylmercapturic acid in
human urine as a measure of exposure to benzene based on these
observations. However, Lunte & Kissinger (1983) showed that
p-benzoquinone, an oxidation product of hydroquinone, forms
glutathione conjugates non-enzymatically. Lau et al. (1989) reported
that 1,2,3 or 4 glutathione molecules could conjugate with
p-benzoquinone. Nerland & Pierce (1990) showed the occurrence of
N-acetyl- S-(2,5-dihydroxyphenyl)l-cysteine as a urinary metabolite
of benzene in rats. Stommel et al. (1989) found that the metabolite
phenylmercapturic acid increased proportionally in rats and humans as
the inhaled dose rose to 1600 mg/m3 (500 ppm). Thus, the array of
mercapturic acid metabolites of benzene has expanded and the full
extent of metabolites of this structure may not yet be fully
appreciated.
In summary, the postulated metabolic pathways for benzene are
shown in Figures 1, 2 and 3. The formations of mercapturic acids,
ethereal sulfates and glucuronides are generally considered
detoxification pathways leading to the excretion of benzene
metabolites via the kidney (Henderson et al., 1989). All other
pathways lead to potentially toxic metabolites. This hypothesis is
discussed in more detail in section 7.9.
In both rats and mice the formation of toxic metabolites via the
epoxide pathway appears to be a saturable process, which suggests that
both metabolism and toxicity would be non-linear. In other words, the
proportion of toxic metabolites formed would decrease once the
saturation level is reached, whereas detoxification pathways appear to
be low-affinity high-capacity reactions (Henderson et al., 1989;
Medinsky et al., 1989a). It has been shown that mice metabolize
benzene faster and converted more of the benzene to toxic metabolites
than rats (Henderson et al., 1989). Because of this it has been
suggested that metabolism in mice favours toxification pathways (e.g.,
formation of benzoquinone and muconaldehyde), while in rats metabolism
is primarily detoxification (phenyl conjugates and phenylmercapturic
acids) (Medinsky et al., 1989a). The percentage of benzene or its
metabolites remaining in the body decreased in rats (from 33% to 15%)
and mice (from 50% to 10%) as exposure increased from 32 to 3200
mg/m3 (10 to 1000 ppm) (Sabourin et al., 1987).
Model simulations for total benzene metabolized and for profiles
of benzene metabolites formed after the administration of varying
doses of benzene to rats and mice (Medinsky et al., 1989b,c) have
suggested that the production of hydroquinone and muconic acid
metabolites predominates at lower exposure concentrations, whereas at
high exposure levels the detoxification pathways account for a larger
fraction of benzene metabolized. In addition, these model simulations
have confirmed that mice metabolize more benzene on a µmole/kg body
weight basis than rats after inhalation exposures, whereas rats
metabolize more benzene than mice at oral doses greater than 50 mg/kg
body weight. After either oral or inhalation exposures mice
preferentially form more of the putative toxic metabolites
hydroquinone and muconic acid (Medinsky et al., 1989b). It has also
been reported by Witz et al. (1990b) that DBA/ZN mice (a strain
sensitive to the haematotoxicity of benzene), excrete greater amounts
of trans, trans-muconic acid than the less sensitive C57BL/6 strain
after equivalent exposures to benzene.
6.4 Elimination and excretion
6.4.1 Inhalation exposure
In animals, expired air is the main route of elimination of
unmetabolized benzene, while urine is the major route of excretion of
benzene metabolites (with very little faecal excretion). Rickert et
al. (1979) found a biphasic pattern of excretion of unmetabolized
benzene in rats after a 6-h exposure to 1600 mg/m3 (500 ppm), with
half-times of 0.7 h for the rapid phase and 13.1 h for the slow phase.
The major route of excretion after inhalation exposures of rats and
mice to 32-3200 mg/m3 (10-1000 ppm) appeared to be dependent upon
the concentration inhaled (Sabourin et al., 1987). Under these
conditions mice received 150-200% of the dose given to rats on a per
kg body weight basis. The faecal excretion was < 3.5% in rats and
< 9% in mice. At doses up to 416 mg/m3 (130 ppm), less than 6% of
the radioactivity was eliminated in expired air, whereas at the highest
concentrations 48% of the dose was eliminated as unchanged chemical in
rats and 14% in mice. The total urinary excretion of metabolites at
these high concentrations was 5-37% higher in mice than in rats.
Findings in humans after inhalation exposure to benzene are
similar to those in experimental animals; unmetabolized chemical is
eliminated in expired air whereas metabolites of benzene are excreted
in urine, primarily as the sulfate and glucuronide conjugates of
phenol. Nomiyama & Nomiyama (1974a,b) found similar expiratory
patterns in men and women exposed for 4 h to benzene at concentration
between 166 and 198 mg/m3 (52-62 ppm). The proportion of the
absorbed benzene that was excreted via the lungs was approximately 17%
(Nomiyama & Nomiyama, 1974a,b).
6.4.2. Oral exposure
Parke & Williams (1953) administered radiolabelled benzene
(approximately 340 mg/kg body weight) by oral gavage to rabbits and
reported that 43% of the label was recovered as unmetabolized benzene
in expired air. Urinary excretion accounted for 33% of the dose,
mainly in the form of conjugated phenol (23.5%). Other phenols
excreted were hydroquinone (4.8%), catechol (2.2%), and hydroxyquinol
(1,2,4-trihydroxybenzene) (0.3%). Muconic acid accounted for 1.3% and
L-phenylmercapturic acid for 0.5%, and 5-10% of the radiolabel
remained in the tissues or was excreted in the faeces. The excretion
of benzene and its metabolites in rats and mice at various oral doses
(0.5-300 mg/kg body weight) was studied by Sabourin et al. (1987). In
both species the excretion of urinary metabolites up to a dose of 15
mg/kg accounted for 80% of the administered dose. Above that level
there was an increase in the elimination of 14C in expired air.
Equal amounts of unmetabolized benzene were eliminated in both species
up to dose levels of 50 mg/kg. At dose levels of between 15 and 50
mg/kg body weight, metabolism appears to become saturated in rodents.
In rats, 50% of a 150-mg/kg dose of 14C-benzene was eliminated in
expired air, while in the mouse 69% of this dose was exhaled (Sabourin
et al., 1987).
No studies were found regarding the excretion of benzene in
humans after oral exposures.
6.4.3 Dermal exposure
After the dermal application of 14C-benzene (0.0026 to 0.0036
mg/cm2) to monkeys and minipigs, Franz (1984) collected urine
samples every 5 h for 2-4 days. The rate of excretion was highest
over the first 10 h, the total excretion of radioactivity being higher
in the monkey (0.03 to 0.14% of the applied dose, with an average of
0.06%) than in the minipigs (0.03-0.05%, with an average of 0.04%).
Using a glass cap to minimize volatilization from the skin, Skowronski
et al. (1988) treated male rats dermally with 14C-benzene (0.004
mg/cm2). After 48 h, 86.2% of the initial dose was excreted in the
urine and 12.8% was eliminated in expired air. Phenol was the major
urinary metabolite detected in the 0-12 h sample (37.7% of dose), and
smaller quantities of hydroquinone, catechol and benzenetriol were
also detected.
In a study of four male human subjects, Franz (1984) applied
14C-benzene dermally (0.0024 mg/cm2). A mean of 0.023% (range
0.006-0.054%) of the applied radiolabel was recovered in the urine
over a 36-h period. More than 80% of the excretion occurred within
8 h of application.
6.5 Retention and turnover
Steady state levels of benzene were found within 4 h in blood,
6 h in fat, and 2 h in bone marrow when male rats were exposed to a
benzene concentration of 1600 mg/m3 (500 ppm) by inhalation for 6 h.
After exposure ceased, about 70% of the benzene was eliminated
unchanged in the expired air and about 30% was excreted in urine as
water-soluble metabolites within 15 h. The half-life (t´) for
elimination from these tissues was 0.4-0.8 h, except in the case of
adipose tissue where elimination occurred with a t´ of 1.6 h. The
elimination of unchanged benzene in expired air was biphasic, the t´
being 0.7 h for the first phase and 13.1 h for the slower phase. Free
phenol, catechol and hydroquinone were detected in blood and bone
marrow after exposure ceased. The phenol level declined rapidly over
a 9-h observation period, whereas catechol and hydroquinone levels in
both tissues remained constant over this period (Rickert et al.,
1979).
After intraperitoneal injection, oral gavage, or inhalation
exposures of labelled benzene in rats and mice, over 95% of the
administered radioactivity was excreted within 40 h (Sabourin et al.,
1987; Henderson et al., 1989). Approximately 90% of the metabolites
was excreted in the urine. According to the authors, these studies
indicate that benzene is rapidly metabolized and excreted in the urine
within 40 h of dosing by any route of administration.
6.6 Reaction with body components
3H-benzene metabolites have been shown to bind irreversibly to
proteins in both mouse liver and bone marrow (Snyder et al., 1978a).
Benzene metabolites have also been shown to bind in vivo to mouse
protein in blood (Sun et al., 1990), liver, bone marrow and spleen
(Longacre et al., 1981a,b). Covalent binding increased both with dose
and frequency of dosing. Covalent binding of benzene metabolites to
protein appears to be mediated by microsomal enzymes (Tunek et al.,
1978) and has been suggested to be the result of binding by
hydroquinone and catechol (Wallin et al., 1985). The finding of high
levels of phenylcysteine adducts in the haemoglobin of benzene-exposed
rats suggests that benzene oxide also reacts with proteins to form
adducts (Bechtold et al., 1992). Benzene metabolism and covalent
binding to proteins have been demonstrated in situ in bone marrow
(Irons et al., 1980b).
Lutz (1979) has attempted to quantify the extent to which
chemicals covalently bind to DNA using the concept of the covalent
binding index (CBI). The implication is that the higher the CBI, the
more likely a chemical will be a carcinogen. The calculated value for
benzene, based on binding to liver nuclear DNA, was 1.7. (To put this
value in perspective, CBI values for some common carcinogens were:
aflatoxin B1, > 1000; 2-acetyl-aminifluorene, 100 to several hundred;
polycyclic aromatic hydrocarbons, 10 to 30.) No values were given for
benzene in bone marrow, but Snyder et al. (1978a) compared covalent
binding of benzene residues per g dry weight of liver and bone marrow
and found that, depending upon the dose of benzene, covalent residues
bound to liver ranged from 500 to 800 nmoles/g, whereas binding to
bone marrow ranged from 18 to 96 nmoles/g. Thus, there appeared to be
less covalent binding in the target organ, i.e. bone marrow, than in
the metabolizing organ, i.e. liver.
Inhaled benzene has been found to bind to rat liver DNA to the
extent of 2.38 µmoles/mole DNA phosphate (Lutz & Schlatter, 1977).
Studies of the covalent binding of benzene metabolites to DNA have
resulted in the postulation of several structures for DNA adducts
derived from benzene. Bone marrow mitochondria from rabbits were
incubated sequentially with 3H-deoxyguanosine triphosphate and
14C-benzene to evaluate DNA adducts formed from benzene metabolites
(Snyder et al., 1987b). These authors identified at least seven
deoxyguanosine adducts and one deoxyadenine adduct. Covalent
N-7-phenyl-guanine adducts have been isolated from rat urine after
intraperitoneal dosing with 330-400 mg benzene/kg body weight (Norpoth
et al., 1988). Thus, Jowa et al. (1990) postulated the formation of
an adduct between p-benzoquinone and deoxyguanosine which had the
structure (3'OH)benztheno(1,N2)deoxyguanosine. The structure of an
adduct formed between p-benzoquinone and deoxyadenosine-3'-phos
phate was suggested to be 3'-hydroxy-1,N6-benztheno-2'-deoxy-
adenosine-3'-phosphate (Pongracz & Bodell, 1991). Reddy et al.
(1990), however, reported that they were unable to detect DNA adducts
derived from benzene in the rat in vivo, despite having observed
them in Zymbal gland cells in vitro, using the 32P-post labelling
technique.
6.7 Modelling of pharmacokinetic data for benzene
In order to obtain better insight into the interspecies
variations in the uptake, metabolic fate and excretion of benzene and
its metabolites, both compartmentally (Bailer & Hoel, 1989; Beliles &
Totman, 1989) and physiologically based (Medinsky et al., 1989b,c;
Paxman & Rappaport, 1990; Travis et al., 1990; Bois et al., 1991a,b),
pharmacokinetic models have been developed. These models have been
used as an aid to risk assessment by facilitating extrapolation
between species where various exposure regimens had been utilized.
Also, such models are useful for identifying gaps in knowledge that
have been highlighted by poor fits of the experimental data to the
models developed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Benzene has been shown to produce a number of biological
responses in experimental animals. The acute effects of benzene at
high doses reflect its activity as a general anaesthetic and can lead
to central nervous system (CNS) depression, loss of consciousness and
coincidental sensitization of the myocardium to catecholamines.
Chronic exposure can result in bone marrow depression expressed as
leucopenia, anaemia and/or thrombocytopenia, leading to pancytopenia
and aplastic anaemia. The immunotoxic effects of benzene are probably
related to bone marrow depression. In animal cancer bioassays it is,
primarily, epithelial tumours that have been reported, whereas in
humans the carcinogenic response is leukaemia. A third type of
biological impact is the production of clastogenic responses such as
chromosome aberrations, sister chromatid exchange and micronuclei.
Benzene has also been suggested to produce fetotoxic effects.
7.1 Single exposure
Consistent with many other aromatic hydrocarbons (Patty, 1981),
benzene appears to be of low acute toxicity when administered to
various animal species by various routes of administration (Table 9).
Other reported oral LD50 values for reagent grade benzene in male
rats vary from as low as 930 mg/kg to as high as 5600 mg/kg body
weight (Wolf et al., 1956; Cornish & Ryan, 1965; Kimura et al., 1971;
Withey & Hall, 1975). The LD50 after intraperitoneal injection in
female rats was reported to be 2940 mg/kg (Drew & Fouts, 1974) and in
mice it was 300 mg/kg body weight (Kocsis et al., 1968). Young rats
are more sensitive (in terms of LD50) than older ones (Table 9).
The LC50 in female rats was estimated to be 43 770 mg/m3 (13
700 ppm) after a single 4-h exposure (Drew & Fouts, 1974).
Benzene has a narcotic effect after oral administration in rats
(Withey & Hall, 1975) and after inhalation in mice (Uyeki et al.,
1977). The threshold narcotic effect after inhalation has been
estimated to be approximately 13 000 mg/m3 (Leong, 1977).
Inhalation of air saturated with benzene resulted in ventricular
tachycardia and occasionally ventricular fibrillation and death in
rats, cats, rabbits and primates (Nahum & Hoff, 1934). Respiratory
failure was also observed during narcosis. Pathological findings after
sudden death are congestion of various organs, particularly the lungs
and liver (Jonek et al., 1965).
No information on the acute toxicity/lethality in animals after
dermal exposure has been reported.
Table 9. Toxicity of benzene in animals after acute exposure
Route Species Parameter Value Reference
Oral rat (14 days old) LD50 3000 mg/kg body weight Kimura et al. (1971)
Oral rat (young adult) LD50 3300 mg/kg body weight Kimura et al. (1971)
Oral rat (old adult) LD50 4900 mg/kg body weight HSE (1982); Kimura et al. (1971)
Oral rat LD50 8100 mg/kg body weight Cornish & Ryan (1965)
Inhalation (4 h) rat LC50 44 660 mg/m3 body weight Drew & Fouts (1974)
Inhalation (7 h) rat LC50 32 600 mg/m3 body weight HSE (1982)
Inhalation (2 h) mouse lethal dose 61 125 mg/m3 body weight Jonek et al. (1965)
Intraperitoneal injection rat LD50 2940 mg/kg body weight Drew & Fouts (1974)
Intraperitoneal injection mouse LD50 300 mg/kg body weight Kocsis et al. (1968)
7.2 Short-term and long-term exposures
The studies discussed in this section, some of which are
summarized in Table 10, have a duration of less than one year.
Lifetime (> 1 year) studies are discussed in section 7.6 and
summarized in Tables 15 to 17.
In short-term inhalation studies, three out of eight male rats
died within 24 h after exposure for five periods of 25-35 min to a
benzene concentration of 128 000 mg/m3 (40 000 ppm) and two out of
ten died after exposure for 12.5-30 min daily to 32 000 mg/m3
(10 000 ppm) for 1-12 days (Furnas & Hine, 1958).
Male and female rats and mice exposed to benzene vapour at
concentrations of 3.2, 32, 96 or 960 mg/m3 (1, 10, 30 or 300 ppm)
for 6 h/day, 5 days/week for 13 weeks, and sacrificed at various time
points during the study, showed no haematological effects up to 96
mg/m3 (30 ppm) (Ward et al., 1985). However, at 960 mg/m3 (300
ppm) mice exhibited significant decreases in haematocrit, haemoglobin,
erythrocyte count, leucocyte count, platelet count and the percentage
of lymphocytes. There was an increase in erythrocyte volume and mean
corpuscular haemoglobin. These changes were first observed on days 14
(males) or 28 (females). Most of the haematological effects were also
detected in rats but were of lesser severity. Compound-related
histopathological findings included myeloid hypoplasia, depletion of
the periarteriolar lymphoid sheaths in the spleen, lymphoid depletion
in the mesenteric lymph nodes, and increased extramedullary
haematopoiesis in the spleen. These lesions persisted throughout the
study and increased in severity with time. The only histopathological
lesion observed in rats was slightly reduced cellularity in the bone
marrow of the femur.
A dose-related increase in leucocyte alkaline phosphatase levels
and a decrease in leucocyte levels was observed in female rats exposed
via inhalation to 320, 960, 3200 or 9600 mg/m3 (100, 300, 1000 or
3000 ppm) for 7 or 14 days, but not in those exposed to 64 or 160
mg/m3 (20 or 50 ppm) (Li et al., 1986). In a study on male mice,
with doses of 3.5, 32, 330, 980, 1930, 4080, 7730 and 15 600 mg/m3
(1.1, 9.9, 103, 306, 603, 1276, 2416 and 4862 ppm), granulocytopenia,
lymphocytopenia and reduced bone marrow and splenic cellularity were
observed after exposure to > 330 mg/m3 for 5 h/day for 5 days but
not at lower levels (Green et al., 1981a). These authors found
splenic lesions at levels as low as 32 mg/m3 when exposure was
extended to 10 weeks.
Table 10. Toxicity of benzene in animals after short-term and long-term inhalation exposuresa
Species Dose (mg/m3) Exposure period Effects References
Rat 48 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
28 weeks at term 90 µg/litre
Rat 100 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
7 weeks at term 290 µg/litre
Rat 150 7 h/day; 5 days/week; slight leucopenia, blood benzene level Deichmann et al. (1963)
32 weeks at term 420 µg/litre
Rat 3200 18 h/day; 7 days/week; reversible haemotological effects, Nau et al. (1966)
15 weeks reversible leucopenia within 6 months
Rat 3.2-960 6 h/day; 5 days/week; slight changes in haematological cell counts Ward et al. (1985)
13 weeks and lower cellularity in bone marrow of femur
Rat (female) 64-9600 1-2 weeks dose-related increase in leucocyte alkaline Li et al. (1986)
phosphatase and decreased leucocyte counts
at exposures of 960 mg/m3 or more
Mouse 3.5 to 15 600 6 h/day; 1 week doses > 330 mg/m3 resulted in granulocytopenia, Green et al. (1981a,b)
lymphocytopenia, decreased splenic cellularity
and decreased stem cell production
Mouse (male) 30.7 6 h/day; 5 days/week; some increase in spleen weight and some Green et al. (1981a)
10 weeks increased cellularity
Table 10 (contd).
Route Species Parameter Value Reference
Mouse (male) 960 6 h/day; 5 days/week; increased mortality, marked lymphocytopenia, Green et al. (1981a)
26 weeks reduced red blood cell count, reduced spleen
weight, many morphological abnormalities in
circulating red blood cells and neutrophils
Mouse (male) 32 6 h/day; 5 days/week; depression in the numbers of splenic nucleated Baarson et al. (1984)
25.5 weeks cells and of circulating red cells and lymphocytes
Mouse 3.2-960 6 h/day; 5 days/week; only at 960 mg/m3 decreased blood cell counts, Ward et al. (1985)
13 weeks haematocrit, myeloid hypoplasia, and numbers of
splenic and lymph node lesions
Mouse 32-1280 6 h/day; 5 days/week; reduced bone marrow cellularity and number of Cronkite et al. (1985)
pluripotent stem cells at 320 mg/m3 or more
Guinea-pig 280 7 h/day; 5 days/week; slight leucopenia, increased weight of kidneys Wolf et al. (1956)
4.5 weeks
Pig 64, 320 6 h/day; 5 days/week; leucopenia at 320 and 1600 mg/m3, reversible Johnston et al. (1979)
and 1600 3 weeks 9-16 weeks after exposure
a To avoid duplication, toxicity from life-time exposures (over 1 year) is discussed in section 7.6
(carcinogenicity) and Tables 15 to 17.
Cronkite et al. (1985) exposed male and female mice by inhalation
to benzene at concentrations of 32, 80, 320, 960 or 1280 mg/m3 (10,
25, 100, 300 or 400 ppm) for 2 weeks (6 h/day, 5 days per week). At
320 mg/m3 or more, reduced bone marrow cellularity and a decreased
number of pluripotent stem cells in bone marrow were reported. Under
similar conditions of exposure to 960 mg/m3 for 16 weeks, these
authors reported a lower level of stem cells in bone marrow, which
returned to 92% of control values after 25 weeks post-exposure.
Complete reversibility within 2 weeks was reported after 2 or 4 weeks
of exposure to 960 mg/m3.
Long-term (> 6 months) exposure studies at benzene levels above
approximately 160 mg/m3 (50 ppm) have shown effects on circulating
leucocytes (especially leucopenia). For example, rats exposed via
inhalation to 150 mg/m3 (47 ppm) (7 h/day, 5 days per week for 8
months) showed slight leucopenia, while those exposed to 100 mg/m3
(31 ppm) for 4 months and those exposed to 48 mg/m3 (15 ppm) failed
to show such changes (Deichmann et al., 1963). The lowest reported
exposure in animals that resulted in haematological effects was in
mice (Baarson et al., 1984). These authors reported that male mice
exposed via inhalation to 32 mg/m3 (10 ppm) (6 h/day, 5 days/week)
for 25.5 weeks showed a decrease in the number of circulating
erythrocytes and lymphocytes, a decrease in the number of nucleated
cells in the spleen and a depression of the in vitro colony-forming
ability of erythroid precursor cells (CFU-E).
Uyeki et al. (1977) demonstrated a depression of stem cell
activity in mice using the spleen colony-forming technique (CFU-S)
after exposure to a benzene concentration of 15 000 mg/m3 (4680 ppm)
for 3 days (8 h/day).
Haematotoxicity was also noted after oral exposure in rats and
mice. The animals were dosed by gavage with benzene in corn oil for
120 days at 25, 50, 100, 200, 400 or 600 mg/kg body weight (5
days/week). Five animals in the control, 200- and 600-mg/kg groups
were sacrificed at 60 days (Huff et al., 1989). A dose-related
leucopenia was observed in both male and female rats and lymphoid
depletion in the B-cells of the spleen was observed in both the 200-
and 600-mg/kg groups at 60 days. In mice no compound-related
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females.
No information was found on the haematotoxicity of benzene after
dermal exposure has been reported.
Additional studies on the effects of long-term exposure to
benzene in experimental animals are described in section 7.6
(carcinogenicity) and in Tables 15 to 17.
7.3 Skin and eye irritation
Benzene is considered a moderate eye irritant (as shown in the
rabbit eye test). Two drops of benzene caused moderate conjunctival
irritation and very slight, transient corneal injury (Wolf et al.,
1956).
Undiluted benzene was irritating to the skin (ear) of rabbits
after 10-20 applications (Wolf et al., 1956). Erythema, oedema,
exfoliation, blistering and moderate necrosis were observed after 20
applications.
There is no information available on the skin-sensitizing
potential of benzene. However, no such effect is expected based on
the experience with other aromatic hydrocarbons (GDCh, 1988).
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
Benzene does not appear to be a potent reproductive toxin in
experimental animals. Guinea-pigs and rabbits exposed to benzene by
inhalation (7 h/day, 5 days/week) for up to 6 months showed variable
results; guinea-pigs showed a slight increase in testicular weight at
280 mg/m3 (88 ppm) and rabbits showed slight degeneration of the
seminiferous germinal epithelium at 256 mg/m3 (80 ppm) (Wolf et al.,
1956). Mice, but not rats, exposed to benzene vapour at a
concentration of 960 mg/m3 (300 ppm) (6 h/day, 5 days/week) for 13
weeks showed bilateral cysts in the ovaries and degeneration and
atrophy of the testes (Ward et al., 1985). At concentrations of 3.2,
32 or 96 mg/m3 (1, 10 or 30 ppm) these changes were also seen in
mice, but they were of doubtful biological significance (Ward et al.,
1985). There was a complete absence of litters in female rats exposed
to 670 mg/m3 (210 ppm) for 10-15 days before mating and for 3 weeks
after mating (Gofmekler, 1968). It is not known if this represents a
problem in mating and fertility, or one of maternal or fetal toxicity.
Lower exposures (1 to 64 mg/m3; 0.30 to 20 ppm) produced no such
effects.
Numerous studies on experimental animals have failed to detect
teratogenic effects, even at doses of benzene clearly toxic to the
dam. A few of these studies are summarized in Table 11. However,
benzene has been reported to be fetotoxic in mice, as shown by a
decrease in fetal weight and skeletal variants (missing sternebrae and
extra ribs) in the offspring of dams exposed to 1600 mg/m3 (500 ppm)
for 7 h on gestation days 6-15 (Murray et al., 1979). Similar effects
were seen in rabbits exposed on gestation days 6-18 to the same
levels. However, these effects are not usually considered to be
significant compound-related malformations (Kimmel & Wilson, 1973).
In mice, exposure to 500 or 1000 mg/m3 on gestation days 6-15
resulted in a decrease in fetal weight and an increase in dead or
resorbed fetuses, but no statistically significant increase in
malformations (Ungvary & Tatrai, 1985).
Table 11. Teratogenic effects of benzene in the mouse and rabbita
Animals Route Exposure level Maternal weight Fetal body Resorptions/ Skeletal Malformations References
gain weight fetal death/ variants
abortions
New Zealand inhalation 1600 mg/m3 (-) (-) (-) slightly ** (-) Murray et al.
rabbit (1979)
New Zealand inhalation 500 mg/m3 (-) (-) (-) (-) (-) Ungvary & Tatrai
rabbit 1000 mg/m3 * * **b ** ** (1985)
CF-1 mouse inhalation 1600 mg/m3 (-) * (-) ** (-) Murray et al.
(1979)
CFLP mouse inhalation 500 mg/m3 (-) * **c,d ** (-) Ungvary & Tatrai
1000 mg/m3 (-) * **c,d ** (-) (1985)
CD-1 mouse gavage 0.3 ml/kg body weight (-) * (-) (-) Nawrot &
0.5 ml/kg body weight (-) * **c,d (-) Staples (1979)
1.0 ml/kg body weight (-) * **c,d (-) (-)
a (-) indicates no significant difference from controls; * indicates decrease compared with controls;
** indicates increase compared with controls.
b Fetal death
c Abortions
d Resorptions
Table 12. Teratology studies in rats after inhalation of benzenea
Exposure level Exposure period Maternal weight Resorptions Fetal weight Skeletal variants Malformations Reference
(mg/m3) (h/day) gain
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
32 7 (-) (-) (-) (-) (-) Kuna & Kapp (1981)
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
128 6 (-) (-) (-) (-) (-) Coate et al. (1984)
150 24 * (-) * (-) (-) Tatrai et al. (1980a)
160 7 * (-) * ** (-) Kuna & Kapp (1981)
320 6 (-) (-) (-) (-) (-) Green et al. (1978)
320 6 (-) (-) * (-) (-) Coate et al. (1984)
400 24 * (-) * ** (-) Tatrai et al. (1980b)
450 24 * ** * ** (-) Tatrai et al. (1980a)
960 6 (-) (-) (-) ** (-) Green et al. (1978)
1000 24 * (-) * ** (-) Hudak & Ungvary (1978)
1500 24 * ** * ** (-) Tatrai et al. (1980a)
1600 7 * (-) * ** (-) Kuna & Kapp (1981)
3000 24 * ** * ** (-) Tatrai et al. (1980a)
7000 6 * (-) * ** (-) Green et al. (1978)
a (-) indicates no significant difference compared with controls; * indicates decrease compared with controls;
** indicates increase compared with controls
Several studies in rats show similar results to those in mice and
rabbits (Table 12). Maternal and fetal weight were decreased at
levels > 160 mg/m3 (> 50 ppm) as were the number of skeletal
variants observed. No malformations were noted in any of the studies
even at doses as high as 7100 mg/m3 (2200 ppm).
It is noteworthy that haematopoietic changes were observed in the
fetuses and offspring of mice exposed to 16, 32 or 64 mg/m3 (5, 10
or 20 ppm) for 6 h/day on gestation days 6-15 (Keller & Snyder, 1986).
The changes included a decrease in the number of erythroid
colony-forming cells (at all dose levels) and granulocytic
colony-forming cells at the two highest levels. When the offspring
were re-exposed to benzene as adults the decrease in these progenitor
cells was greater than in adult mice exposed to benzene at the same
levels for the first time.
7.5 Mutagenicity and related end-points
Benzene has been widely studied regarding the production of gene
mutations in in vitro tests, chromosomal effects both in vitro and
in vivo, and effects on DNA (binding, synthesis and damage). An
overview of the testing up to 1985 for the mutagenicity of benzene is
shown in Fig. 4 (IARC, 1987a). Detailed reviews have also been
published (Dean, 1978, 1985a; Huff et al., 1989; ATSDR, 1991),
therefore only some of the many studies are shown in Tables 13
( in vitro) and 14 ( in vivo).
7.5.1 In vitro studies
As shown in Table 13 and Fig. 4, benzene has consistently given
negative results in assays for point mutations in bacteria using
standard test conditions. In such studies, six tester strains have
been used with benzene concentrations ranging from 0.1 to 528 µg per
plate both with and without metabolic activation (HSE, 1982). Other
studies using doses as high as 880 mg/plate failed to cause mutations
in Salmonella typhimurium (Dean, 1978). In some 10 in vitro gene
mutation tests carried out in various human, mouse and Chinese hamster
cells, as part of an international collaborative study, mixed results
were obtained with benzene (Ashby et al., 1985; Venitt, 1985). When
S. typhimurium (strain TA1535) was incubated with benzene in a
desicator to enhance exposure, a doubling of revertants was noted at
10 ppm only in the presence of a post-mitochondrial activating system
(Glatt et al., 1989).
Figure 4. Tabular summary of in vitro and in vivo tests on benzene for mutagenicity and related end-pointsa
Non-mammalian systems Mammalian systems
In vitro In vivo
Prokaryotes Lower Plants Insects Animal cells Human cells Animals Humans
eukaryotes
D G R G A G R G C D G S M C A T D G S C S M C S C
+1 - + + +1 + +1 ? -1 + + - -1 + + + -1 +1 ? +1 + + + -1 +
a Adapted from: IARC (1987a)
A = aneuploidy; C = chromosomal aberrations; D = DNA damage; G = gene mutation; M = micronuclei; R = mitotic recombination and gene
conversion; S = sister chromatid exchange; T = cell transformation; + = considered to be positive for the specific end-point and
level of biological complexity; +1 = considered to be positive, but only one valid study was available to the Working Group;
- = considered to be negative; -1 = considered to be negative, but only one valid study was available to the Working Group; ? =
considered to be equivocal or inconclusive (e.g., there were contradictory results from different laboratories; there were confounding
exposures; the results were equivocal)
Table 13. Some in vitro genotoxicity studies of benzene
End-point Test system Resultsa References
Gene mutations
Ames test Salmonella typhimurium -/- De Flora et al.
-/+ (1984); Venitt (1985);
Glatt et al. (1989)
Azaguanine Salmonella typhimurium -/ Seixas et al.
resistance (1982)
TK test mouse L5178Y cells -/- Oberly et al. (1984)
TK, ouabain, total of 15 studies using mixedb Garner (1985)
HGPRT loci various human, mouse and
Chinese hamster cells
Chromosome abnormalities
Chromosome human lymphocytes mixed Gerner-Smidt &
aberrations Friedrich (1978);
Morimoto (1976)
total of 8 studies using mixed Dean (1985b)
Chinese hamster or human
cells
Sister Chinese hamster ovary and -/- Dean (1985b)
chromatid V79 cells and rat RL4
exchange cells
Table 13 (contd).
End-point Test system Resultsa References
Sister human lymphocytes mixed Morimoto (1983);
chromatid Morimoto et al.
exchange (1983); Erexson
et al. (1985)
Micronuclei Chinese hamster ovary -/- Douglas et al.
cells (1985)
Other effects
DNA breaks Rat hepatocytes ND/- Bradley (1985)
Chinese hamster V79 cells -/- Swenberg et al.
(1976)
Chinese hamster ovary -/- Douglas et al.
cells (1985)
Mouse L5178Y cells -/ Pellack-Walker &
Blumer (1986)
Unscheduled rat hepatocytes ND/- Probst & Hill
DNA synthesis (1985)
HeLa cells -/- Barrett (1985)
DNA synthesis Hela cells -/- Painter & Howard
inhibition (1982)
a Without/with an exogenous metabolic activation system; ND = no data
b The IPCS CSSTT working group disagreed over data analysis and therefore
called the results inconclusive
Whereas the results of in vitro studies for mutations by
benzene have been largely negative, there is some evidence that
treatment of human and animal cells in vitro with benzene can lead
to chromosomal abnormalities. However, as shown in Table 13, mixed
results have been obtained.
The ring-opened metabolite of benzene, trans,trans-muconal-dehyde
(MUC) has been tested for mutagenic and clastogenic activity in CHO
cells and Salmonella typhimurium bacteria, and for its effects on
DNA synthesis in primary rat liver hepatocytes (Witz et al., 1990a).
Only minimal mutational activity in bacteria was reported (in only
S. typhimurium strain TA97 of the five strains tested). However, at
a concentration of 0.4 to 0.8 µg/ml media, MUC resulted in a
dose-related increase in micronuclei in CHO cells. No effect on
unscheduled DNA synthesis or in the HG PRT assay in CHO cells was
reported. Using S. typhimurium (point mutations) and V79 cells
(sister chromatid exchange, acquisition of thioguanine or ouabain
resistance, and induction of micronuclei), 13 other potential benzene
metabolites were examined for genotoxicity. Each metabolite showed a
specific spectrum of activity, the highest genotoxic activity in most
systems being exhibited by quinone, hydroquinone, anti-diol epoxide
and catechol (Glatt et al., 1989).
The negative mutagenic data found in studies where benzene was
added to standard systems in vitro may well have been caused by the
technique used in these studies. Benzene is metabolically activated
to reactive metabolites by cytochrome P-450 IIE1, a natural
constituent of liver microsomes. In many of these studies
insufficient activation of benzene may have occurred. Post & Snyder
(1983) demonstrated that enzyme induction with benzene increased
benzene metabolism by increasing the activity of an enzyme having a
low Km and a high turnover rate for benzene, which is now thought to
be cytochrome P-450 IIE1. These authors also found that phenobarbital
induction reduced benzene metabolism until very high substrate
concentrations were reached. Chepiga et al. (1991), using purified
reconstituted cytochrome P-450, have shown that, whereas the Km
value for benzene as a substrate for cytochrome P-450 IIE1 is quite
low, much higher concentrations are required for benzene to be
metabolized by cytochrome P-450 IIB1, which also has a low affinity
for benzene. Thus, the lack of positive results in mutagenesis tests
involving benzene may have been due to the low activity of
benzene-activating enzymes in these preparations.
7.5.2 In vivo studies
No data are available on the production by benzene of gene
mutations in vivo. Benzene, or its metabolites, cause both
structural and numerical chromosome aberrations in humans (see chapter
8), laboratory animals and cells in culture (see section 7.6.1), as
well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes. Some in vivo studies are summarized in
Table 14.
Chromosomal changes occur after exposure of experimental animals
by the subcutaneous, oral, intraperitoneal or inhalation routes.
Philip & Krogh-Jensen (1970) administered 1750 mg benzene/kg body
weight subcutaneously to rats and noted an increase in chromatid
aberrations 12 and 24 h post-dosing but not after 36 h. This suggests
damage to S and/or G2 phase cells and a rapid elimination of the
alterations. In a study by Kissling & Speck (1972), the subcutaneous
administration of 1750 mg benzene/kg body weight to rabbits 3 times
weekly for 18 weeks led to tetraploidy in one animal as well as a high
percentage (58%) of bone marrow cells having chromosomal aberrations.
Siou et al. (1981) reported an increase in chromosome aberrations in
the bone marrow cells of mice treated orally with doses greater than
56 mg benzene/kg body weight on 2 successive days before sacrifice.
At high levels of benzene administered by inhalation (10 000
mg/m3 for 4 h), a marked increase in SCEs was noted in mouse bone
marrow cells (Tice et al., 1980). In a later experiment a significant
increase was reported in SCEs in mouse bone marrow cells when the
animals were exposed by inhalation to 91 mg/m3 for 4 h (Tice et al.,
1982). Erexson et al. (1986) reported a significant increase in the
levels of SCEs in peripheral lymphocytes after 6 h of exposure to 32
mg/m3 in mice and 9.6 mg/m3 in rats. At these levels, the
frequency of micronuclei in bone marrow smears was also increased.
After 6 weeks of exposure of mice (22 h/day, 7 days/week) at benzene
levels of between 0.128 and 3.2 mg/m3, increased chromosomal
aberrations in lymphocytes from the spleen were reported by Au et al.
(1991). These changes reached a significance of P = 0.05 only in
female mice (in males P = 0.15).
The frequency of micronuclei was increased in the bone marrow of
mice treated orally at doses ranging from 56 to 2200 mg/kg body weight
(Hite et al., 1980; Siou et al., 1981). A dose-related increase in
micronuclei in circulating erythrocytes was seen at 120 days in mice
treated by oral gavage with 25, 50, 100, 200, 400 or 600 mg benzene/kg
body weight. A significant increase was seen at all doses, with male
mice being more sensitive (Choy et al., 1985).
Table 14. Some mammalian in vivo genotoxicity studies on benzene
Route of Test system Results Exposure concentration Reference
administration and duration
Chromosome aberrations
Inhalation mouse bone marrow + 14 to 74 mg/m3, 7 days Zhurkov et al. (1983)
- 10 000 mg/m3, 4 h Tice et al. (1980)
- 9600 mg/m3, 4 h Tice et al. (1982)
+ 9600 mg/m3, 4 h, phenobarbital pretreatment Tice et al. (1982)
rat bone marrow + 3.2-3200 mg/m3, 6 h Styles & Richardson (1984)
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
+ 5-80 mg/kg body weight, daily for 2 days Zhurkov et al. (1983)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
bone marrow
Intraperitoneal rat bone marrow + 1 dose of 878 mg/kg body weight; 24 h prior to Anderson & Richardson
sacrifice (1981)
Micronuclei
Inhalation mouse lymphocytes + 32, 320 and 3200 mg/m3, 6 h Erexson et al. (1986)
mouse lymphocytes + > 67 mg/m3, 4-10 days Toft et al. (1982)
rat lymphocytes + 0.3 to 96 mg/m3, 6 h Erexson et al. (1986)
Table 14 (contd).
Route of Test system Results Exposure concentration Reference
administration and duration
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
mouse bone marrow + 440 mg/kg body weight, 2 doses, 24 h apart Gad El-Karim et al. (1984)
mouse bone marrow + 55 to 1760 mg/kg, daily for 2 days Hite et al. (1980)
mouse circulating + 26 to 440 mg/kg body weight, 5 days Barale et al. (1985)
erythrocytes + 25 to 600 mg/kg, 120 days Choy et al. (1985)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
Sperm head abnormality
Intraperitoneal mouse (spermatogonia + 88 to 880 mg/kg, daily for 5 days Topham (1980)
treated)
There were statistically significant alterations in sperm head
morphology after intraperitoneal doses of 88 to 880 mg/kg body weight
were administered to mice for 5 days and the sperm were examined 5
weeks later (Topham, 1980).
Witz et al. (1990a) reported that administration of
trans,trans-muconaldehyde, a microsomal metabolite of benzene, to
B6C3F1 mice (< 0.1-6.0 mg/kg body weight intraperitoneally)
resulted in the production of SCEs. The lowest dose producing a
significant increase was 3 mg/kg. No increase in the frequency of
micronuclei was reported.
There has been no clear demonstration of dominant lethal effects
in animals following benzene exposure. Fel'dt (1985) found no
significant dominant lethal effect in mice following oral
administration of up to 320 mg benzene/kg body weight. No dominant
lethal effect was reported in rats by Dean (1978) after the
intraperitoneal injection of 440 mg benzene/kg. However, Ciranni et
al. (1988) demonstrated the induction of micronuclei in the bone
marrow cells of pregnant mice and in fetal liver cells after a single
exposure to benzene or its metabolites.
7.6 Carcinogenicity
In several studies benzene has been shown to be carcinogenic in
experimental animals after exposure by inhalation and after oral
(gavage) dosing. These experiments are summarized in Tables 15-18.
As indicated in these Tables, several types of neoplasms have been
reported to be associated with exposure to benzene. Various types of
lymphomas/leukaemias have been found, but the majority of neoplasms
are of epithelial origin, i.e. Zymbal gland, liver, mammary gland and
the oro-nasal cavity. These results support the hypothesis that
benzene exposure in experimental animals can produce cancer at
multiple sites. A review of such studies has been published by Huff
et al. (1989).
7.6.1 Inhalation studies
The experimental design and major effects noted in several
inhalation cancer bioassays on benzene are summarized in Table 15.
Table 15. Inhalation studies on the carcinogenicity of benzene in experimental animals
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse AKR/J, dosed, 60; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 11 weeks; mean lifetime Snyder
males (8 weeks control, 60 5 days/week, lifetime for control group, 39 weeks, death due to aplastic et al.
old) (about 70 weeks) anaemia; bone marrow hypoplasia; no evidence for any (1978b)
tumours at autopsy in test animals
Mouse C57BL, dosed, 40; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 41 weeks; mean lifetime Snyder
males (8 weeks control, 40 5 days/week, lifetime for control group, 75 weeks; anaemia, lymphocytopenia, et al.
old) (about 70 weeks) neutrophilic bone marrow hyperplasia; 6/40 lymphocytic (1980)
lymphoma with thymic involvement (P < 0.01), 1/40 plasma
cytoma, 1/40 haematocytoblastic leukaemia, 2/40 control
mice lymphocytic lymphoma without thymic involvement
Mouse AKR/J, dosed, 50; 320 mg/m3, 6 h/day, mean life-span for dosed group, 39 weeks; for control, Snyder
males (8 weeks control, 50 5 days/week, lifetime 47 weeks; anaemia, lymphocytopenia, neutrophilia, bone et al.
old) (about 70 weeks) marrow hyperplasia 10/49 dosed, 1/50 control (1980)
Mouse Charles number of 320 and 960 mg/m3, two mice of high-dose group developed myelogenous Snyder
river CD-1, animals 6 h/day, 5 days/week, leukaemia et al.
males unknown lifetime (1978b)
Mouse CD-1, dosed, 60; intermittent exposure greater mortality than with continuous exposure to 3480 Snyder
C57BL/6, male control, 60 at 960 mg/m3 for 1 mg/m3 for 10 weeks; elevated incidences of malignant et al.
for each week followed by 2 tumours in both strains; 35% incidence of Zymbal gland (1988)
strain weeks non-exposure; tumours in C57BL (control 0%), lung adenomas in CD-1
6 h/day, 5 days/week (26% versus 7% controls), and no significant increase
for lifetime in incidence of leukaemia or lymphomas
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse CD-1, dosed, 80; short-term exposure to only CD-1 strain showed increased tumour incidence; 46%
C57BL/6, male control, 80 3840 mg/m3, 6 h/day, incidence of lung adenomas (control 24%) in addition to
for each 5 days/week for 10 other malignant and benign tumours; no increase in
strain weeks; observation for incidence of leukaemia or lymphoma; marked haematotoxicity
lifetime after cessation noted (anaemia and lymphocytopenia)
of exposure
Mouse C57BL/6, dosed, 118; control, 960 mg/m3, 6 h/day, 5 48 weeks after treatment survival rates were: dosed (D) Cronkite
female (7-9 116 (groups reduced days/week for 16 weeks; 80/90; control (C) 87/88; of the 10 exposed mice that et al.
weeks old) to 90 and 88 observation period for died, 6 had thymic lymphomas, 2 had unspecified lymphomas; (1985)
respectively for lifetime at end of study tumour incidence reported: leukaemia all
haemopoietic types (D) 20/89, (C) 8/88; lymphomas (thymic) (D) 10/89,
stem cell assays (C) 1/88; lymphomas (non-thymic) (D) 6/89, (C) 2/88
Rat Sprague- dosed, 45; 960 mg/m3, 6 h/day, no evidence of leukaemic response or pre-leukaemic Snyder
Dawley, male control, 25 5 days/week, for 99 effects nor for tumours incidence at any other site et al.
weeks (1978b)
Rat Sprague- dosed, 40; 320 mg/m3; 6 h/day, incidence of total and malignant tumours not significantly Snyder
Dawley, male control, 40 5 days/week, lifetime elevated over controls; several rare tumours in dosed et al.
(6 weeks old) (about 123 weeks) group, not in controls: 4 liver, 2 Zymbal gland and 1 (1984)
chronic myelogenous leukaemia
Rat Sprague- dosed, 70 male, 640 mg/m3; 4 h/day, at end of experiment (150 weeks) no information on survival; Maltoni
Dawley 59 female; control, 5 days/week for 7 weeks at 118 weeks survival rates of dosed (D) and control animals et al.
158 male, 149 female then 7 h/day for 8 (C) comparable; tumour incidence at 150 weeks; Zymbal (1983,
weeks; exposure in gland (D) 4/70 male, 1/59 female with (C) 2/158 male and 1985,
utero from day 12 of 0/149 female; oral cavity (D) 2/70 male, 6/59 female with 1989)
gestation through (C) 0/158 male and 0/149 female; leukaemias (D) 4/70 male,
lactation 4/58 female with (C) 12/158 male and 1/148 female;
hepatomas (D) 2/70 male, 5/59 female with (C) 1/158 male
and 0/149 female
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- Breeders: dosed 640 mg/m3, 4 h/day, at termination (150 weeks) no information on survival; Maltoni
Dawley (breeders (D), 54; control 5 days/week, 7 weeks; at 118 weeks breeder (D) 5/54, (C) 11/60; offspring (D) et al.
13 weeks old) (C), 60 7 h/day, 5 days/week, male 11/75, (D) female 23/65 with (C) males 39/158 and (1983,
12 weeks; and then females 47/149; (C) breeders had 2/60 with malignant 1985,
960 mg/m3, 7 h/day, mammary tumours and (C) offspring had 3/158 and female 1989)
5 days/week, 85 weeks 8/149 malignant mammary tumours; male 1/158 and 0/149
with leukaemias; tumour incidence in (D) breeders was:
Offspring: dosed, Breeders: from day Zymbal gland carcinoma 3/54; oral cavity carcinoma 2/54;
75 male and 65 12 of gestation nasal carcinoma 1/54; malignant mammary tumours 6/54;
female; control: Offspring: in utero, hepatomas 1/54 and leukaemias 0/54; in offspring (D)
158 male and 149 through lactation male 6/75 and female 8/65 Zymbal gland carcinoma; male
female and for 104 weeks 1/75 and female 10/65 oral cavity carcinoma; male 1/75
and female 2/65 nasal cavity carcinoma; male 1/75 and
female 1/65 skin carcinoma; male 0/75 and female 3/65
forestomach carcinomas; male 0/75 and female 9/65
malignant mammary tumours; male 2/75 and female 7/65
hepatomas; and male 6/75 and female 0/65 leukaemias
Snyder et al. (1980) reported the development of malignant
lymphomas in mice after the exposure of male C57BL mice for about 70
weeks to 960 mg benzene/m3. Goldstein et al. (1982) exposed
Sprague-Dawley (SD) rats and three strains of mice (AKR, C57BL and
CD-1) to 320 and 960 mg/m3 for their lifetime and reported a small,
but not statistically significant, increase in the incidence of
granulocytic leukaemia in CD-1 mice (2 cases) and one case of chronic
myelogenous leukaemia in SD rats (these are rare neoplasms in these
strains). Snyder et al. (1984) in a subsequent full report of this
study, also noted increases in the incidence of liver tumours and
Zymbal gland carcinomas. The incidence of malignant lymphoma in male
AKR mice exposed to 320 mg/m3 was not significantly greater than
that in controls (Snyder et al., 1984). At about the same time
Maltoni et al. (1983) reported that Zymbal gland carcinomas were
observed in SD rats exposed to benzene (960 mg/m3) for 86 weeks. At
the end of the observation period (150 weeks), female breeder rats and
their offspring had not developed increased levels of leukaemia but
had an increased incidence of other tumours such as oral and nasal
cavity carcinomas, malignant mammary carcinomas and hepatomas (Maltoni
et al., 1982c, 1983, 1989).
Benzene-induced leukaemia in experimental animals has been
reported (Cronkite et al., 1984, 1985, 1989; Cronkite, 1986). In an
attempt to mimic more closely patterns of human exposure to benzene,
C57BL/6 and CBA/Ca mice were exposed to 960 mg/m3 (300 ppm) (6
h/day, 5 days/week) for 16 weeks, followed by an observation period of
82 weeks. A highly significant increase in leukaemia was noted in
C57BL/6 mice (Cronkite et al., 1984), and a biphasic response was
reported regarding mortality and lymphoma appearance (Cronkite et al.,
1985). The first increase in lymphomas was noted at about 150 days
post-exposure, and there was increased mortality between 330 and 390
days. A second increase in lymphomas as well as solid tumours
occurred at 420 days post-exposure, the mortality again increasing at
570 days post-exposure. Cronkite et al. (1989) reported that benzene
at a concentration of 960 mg/m3 for 76 weeks was leukaemogenic in
both male and female CBA/Ca mice.
Snyder et al. (1988) reported that benzene exposure patterns in
CD-1 and C57BL mice, which were closely related to the occupational
setting (intermittent for lifetime as well as short-term high doses
for a portion of the normal life span), resulted in marked
haematotoxicity as well as being tumorigenic. Neither of the benzene
exposure patterns induced elevated incidences of leukaemia/lymphoma in
either strain. Elevated incidences of malignant tumours (Zymbal gland
and lung) were noted in both strains after intermittent (1 week
exposure, 2 weeks non-exposure) exposure (960 mg/m3, 6 h/day, 5
days/week) over the full lifetime, whereas an increase in lung tumour
incidence was noted in only the CD-1 strain after 10 weeks of exposure
to 3840 mg/m3 (6 h/day, 5 day/week) followed by a lifetime of
non-exposure.
Table 16 presents a summary of the lowest dose levels at which
various authors reported a possible causal relationship between
benzene exposure and the end-point studied.
7.6.2 Oral and subcutaneous studies
Several experiments using oral (gavage) administration of benzene
to experimental animals are summarized in Table 17, and some of the
major effects reported are given. Oral exposure to benzene has
resulted in the induction of neoplasms in 13 different tissue/organs,
namely Zymbal gland, oral and nasal cavities, mammary gland, liver,
forestomach, skin, harderian gland, preputial gland and ovary, and the
haemopoietic and lympho-reticular systems (Maltoni et al., 1983, 1985,
1989; NTP, 1986; Huff et al., 1989). Table 17 indicates the
similarity in protocols used, namely 25-500 mg benzene/kg body weight
per day via gavage, 4 to 5 times weekly for 52-104 weeks and
termination after 103-144 weeks. The lowest dose of benzene that
produced specific neoplasms varied from 25 mg/kg body weight for the
adenomas of the lung, harderian gland and liver of mice to 500 mg/kg
body weight for lymphoreticular neoplasms in rats.
7.7 Special studies
7.7.1 Immunotoxicity
The proliferative ability of B- and T-cell lymphocytes was
depressed in a short-term (6 h/day for 6 days) dose-response study
(32, 96, 320 and 960 mg/m3) on benzene in mice (Rozen et al., 1984).
Liposaccharide-induced B-cell proliferation was depressed at levels as
low as 32 mg/m3 (the range of many occupational exposures), and
phytohaemagglutinin-induced T-cell response was depressed at 96
mg/m3. Peripheral lymphocyte counts were lower at all exposure
levels, but erythrocyte counts were depressed only at 320 and 960
mg/m3. In a subsequent study it was shown that a benzene
concentration of 960 mg/m3 (6 h/day, 5 days/week), administered for
115 days to mice, reduced the number of both B-cells in the spleen and
bone marrow and T-cells in the thymus and spleen and reduced their
response to mitogens (Rozen & Snyder, 1985). Other studies have shown
that polyhydroxylated derivatives of benzene are potent inhibitors of
T- and B-cell function in vitro (Irons et al., 1982).
Table 16. Carcinogenic-related end-points observed in animals exposed to benzene by inhalationa
End-points SD rat C57BL/6J mouse CD-1 mouse CBA/Ca mouse Reference
Lymphocytic lymphoma 960/488 days Snyder et al. (1980)
Myelogenous leukaemia (acute) 960/life Goldstein et al. (1982)
Myelogenous leukaemia (chronic) 320/life 960/life Goldstein et al. (1982)
Zymbal gland carcinoma 320/life Snyder et al. (1984)
960/lifeb 960/lifeb Snyder et al. (1988)
Hepatoma 640-960 Maltoni et al. (1982a)
960/16 Cronkite (1986)
Lung adenoma 3840/lifec Snyder et al. (1988)
Nasal carcinoma 640-960/104 Maltoni et al. (1983)
Thymic lymphoma 960/16 Cronkite et al. (1984)
Lymphoma (unspecified) 960/16 Cronkite et al. (1984)
Liver tumour 320/life Snyder et al. (1984)
Granulocytic leukaemia 320/life Snyder et al. (1984)
Leukaemia 960/16 Cronkite (1986)
a Doses are expressed in mg/m3 given 4-7 h/day, 5 days/week over a number of weeks or lifetime (e.g., 960 mg/m3/16
indicates that 960 mg/m3 was given for 16 weeks); exposures shown are the lowest for which the author claims
possible causal relationship.
b 960 mg/m3 dose intermittent, i.e. 1 week followed by 2 weeks non-exposure.
c Exposed to 3840 mg/m3 for 10 weeks followed by lifetime non-exposure.
Table 17. Long-term toxicity/carcinogenicity of benzene in experimental animals after oral administrationa
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- high dose: 30 m, 50 or 250 mg/kg, only tumours reported in (C) were 4/30 f with malignant Maltoni et
Dawley, males & 35 f; low dose: 4-5 times/week mammary tumours and 1/30 with leukaemia; in dosed animals al. (1982b,
females (13 30 m, 30 f; for 52 weeks; Zymbal gland carcinoma in 2/30 f in low-dose group and 1983, 1985,
weeks old) controls: 30 m, death at 144 8/35 f in high-dose group; oral cavity carcinomas in 2/35 f 1989)
30 f weeks in high-dose group; malignant mammary tumours in 4/30 f in
low-dose group and 7/35 f in high-dose group; hepatomas in
1/35 m in high-dose group and leukaemias in 2/30 f of
low-dose group and 4/35 m and 1/35 f in high-dose group
Rat Sprague- dose: 40 m, 40 f; 500 mg/kg, 4-5 only tumours reported in (C) were 1/50 m with Zymbal gland Maltoni et
Dawley, males & control: 50 m, 50 f days/week for carcinomas; 1/50 skin carcinomas; 7/50 f malignant mammary al. (1982b,
females (6 weeks 104 weeks; tumours; 3/50 m hepatomas; 3/50 m and 1/50 f with 1983, 1985,
old) observed until leukaemias; in (D) following tumours reported: Zymbal
natural death gland carcinomas 18/40 m and 16/40 f; oral cavity
carcinomas 21/40 m and 20/40 f; nasal cavity carcinomas
3/40 m and 1/40 f; skin carcinomas 9/40 m; forestomach
acanthomas and dysplasias 10/40 m and 7/40 f; in situ
carcinomas (forestomach) 6/40 f; invasive carcinomas
(forestomach) 1/40 m; hepatomas 3/40 m and 1/40 f;
angiosarcomas 2/40 m and 3/40 f; leukaemias 1/40 m and
3/40 f
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat F344/N, dose groups: 60 m, all groups dosed number of survivors in control and dosed groups, NTP (1986);
males & females 60 f; control: 5 days/week for respectively; males: 32/50, 29/50, 24/50, 16/50; females: Huff et al.
(7-8 weeks old) 60 m, 60 f 103 weeks; males: 46/50, 38/50, 33/50, 25/50 (1989)
50, 100 or 200
mg/kg per day in control and dosed groups, respectively: Zymbal gland
females: 25, 50 or carcinomas, males: 2/50, 6/50, 10/50, 17/50; females:
100 mg/kg per day 0/50, 5/50, 5/50, 14/50
oral cavity squamous cell papilloma: m 1/50, 60/50, 11/50
and 13/50; f 1/50, 4/50, 8/50, 5/50
oral cavity squamous cell carcinoma: m 0/50, 3/50, 5/50,
70/50; f 0/50, 1/50, 4/50, 5/50
skin squamous cell papilloma: m 0/50, 2/50, 1/50, 5/50
skin squamous cell carcinoma: m 0/50, 5/50, 3/50, 8/50
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Mouse B6C3F1, dose: 60 m, 60 f; males and females: numbers of survivors in control and dosed groups, NTP (1986)
male & females control: 60 m, 60 f 25, 50 or 100 mg/kg respectively: males: 28/50, 22/50, 18/50, 7/50; females:
(6-8 weeks old) per day, 5 days/week 30/50, 25/50, 24/50, 16/50
for 103 weeks
control and dosed groups, respectively: Zymbal gland
carcinomas, males: 0/49, 1/48, 4/50, 21/49; females: 0/49,
0/45, 1/50, 3/49
mammary carcinomas and carcinosarcomas occurred at higher
incidences in the mid- and/or high-dose groups; increased
tumour incidences in dosed mice were also noted elsewhere
including the haemopoietic system, adrenals, ovary, liver,
lung and preputial gland
a m = male animals, f = female animals, (C) = control groups, and (D) = dosed groups
The primary antibody response to fluid tetanus toxoid was reduced
by 74-89% in mice that were exposed to 1280 mg benzene/m3 (6 h/day)
for 5, 7 or 22 days of exposure (Stoner et al., 1981). No effect was
seen at 160 mg/m3. These investigators concluded that the threshold
level for repression of primary antibody response was between 160 and
640 mg/m3.
Host resistance to infection by Listeria monocytogenes in mice
was reduced after exposure to benzene (Rosenthal & Snyder, 1985). The
infection rate, as determined by bacterial counts in the spleen, was
increased by 730% on day 4 post-infection after exposure to 960
mg/m3 for 5 days, but not at lower benzene exposures. In contrast,
increased bacterial counts were seen at all doses of benzene greater
than 32 mg/m3 when benzene exposure was continued after exposure to
L. monocytogenes.
Both the humoral and cellular immune responses in CD-1 mice were
altered by oral administration of benzene at 8, 40 or 180 mg/kg body
weight daily for 4 weeks. A dose-response reduction in peripheral
blood lymphocytes was reported whereas there was no effect on the
levels of neutrophils and other white blood cells (Hsieh et al.,
1988a). A dose-related biphasic splenic lymphocyte proliferative
response to B- and T-cell mitogens was also reported. At the 8 mg/kg
dose the response was enhanced, while at the 40 and 180 mg/kg per day
doses a depression was observed. A similar biphasic response was
reported for cell-mediated immunity.
7.7.2 Neurotoxicity
The neurotoxicity of benzene in experimental animals has not been
well studied. Benzene caused light narcosis after 3 min of exposure
to a level of about 144 000 mg/m3 (45 000 ppm) in rabbits (Carpenter
et al., 1944). At this dose, tremors were noted after 5 min, loss of
pupillary reflex to strong light after 6 min, involuntary blinking
after 15 min, and death after 36 min. Learning defects have been
reported in rats exposed three times intraperitoneally to 550 mg
benzene/kg body weight on days 9, 11 and 13 postpartum (Geist et al.,
1983).
Male adult CD-1 mice received ( ad libitum for 4 weeks)
drinking-water containing 31, 166 and 790 mg benzene/litre (estimated
daily doses of 8, 40 and 180 mg/kg body weight). No treatment-related
behavioural changes were observed in the test animals. However, oral
ingestion of benzene was found to alter the levels of norepinephrine,
serotonin, dopamine and catecholamine in several brain regions (Hsieh
et al., 1988b).
7.8 Factors modifying toxicity
The toxicity of benzene can be modified by several factors,
including species or strain of animal exposed, dose received, and
patterns of exposure.
As shown in Figures 1-3 (section 6.3) benzene metabolism is
complex and involves several detoxification pathways, as well as two
pathways which form the putative metabolites muconaldehyde and
benzoquinone. Henderson et al. (1989) and Sabourin et al. (1987)
demonstrated species differences for the metabolism of benzene, i.e.
mice exhibited a higher rate of metabolism with the production of more
putative toxic metabolites. These authors also reported that
increasing the dose (by oral or inhalation routes) in both rats and
mice resulted in a higher proportion of benzene being metabolized by
detoxification pathways.
The myelotoxicity of benzene in mice is much more pronounced
following a discontinuous dosing regimen than following continuous
exposure (Tice et al., 1989). These effects of exposure route and
regimen suggest that the toxicity of benzene is dependent on cell
cycle kinetics in the bone marrow.
Evidence indicates that benzene must be metabolized prior to
producing adverse effects on the haemopoietic system or leading to
carcinogenic and clastogenic effects (Snyder et al., 1981; Irons,
1985). Therefore, agents or other factors that alter the metabolism
of benzene can also modify its toxicity.
Ethanol and benzene induce formation of cytochrome P-450 IIE1 in
rabbit and rats (Johansson & Ingelman-Sundberg, 1988). The toxicity
of benzene is enhanced by ethanol, increasing the severity of anaemia
induced by benzene, lymphocytopenia and reduction in bone marrow
cellularity (Baarson et al., 1982).
Phenobarbital that induces specific isoenzymes of P-450 increases
the rate of benzene metabolism in vivo in rats resulting in
increased resistance against the leucopenic action of benzene (Ikeda
& Ohtsuji, 1971; Nakajima et al., 1985).
Sabourin et al. (1990) found no evidence of the induction of
benzene metabolism by repeated exposure of rodents to benzene.
7.9 Mechanism of toxicity
It has become increasingly clear that the impact of benzene on
the bone marrow is conferred by a combination of metabolites, rather
than by a single metabolite. Thus, Eastmond et al. (1987) showed that
there was an interaction between phenol and hydroquinone when the two
were co-administered, which resulted in a degree of myelotoxicity
greater than additive. In mice, Snyder et al. (1989) reported that
whereas phenol alone did not decrease erythrocyte production,
hydroquinone, p-benzoquinone, and muconaldehyde were effective
inhibitors of red cell production. These authors also showed that
phenol interacted with hydroquinone to produce greater-than-expected
depression of erythrocyte synthesis. A similar interaction was
observed between phenol and catechol. The most striking interaction
was observed when doses of hydroquinone and muconaldehyde were
selected which were ineffective in inhibiting erythropoiesis when
given alone, but when given together produced cessation of red cell
production. Thus, bone marrow depression appears to be the result of
the combined effects of these metabolites. A further contributing
factor, however, is the finding by Roghani et al. (1987) and Da Silva
et al. (1989) that benzene stimulates the activity of membrane protein
kinase c, an important regulatory enzyme. The effect of its
perturbation may combine with the biological effects of the various
metabolites to yield the disease we term aplastic anaemia.
It is clear that aplastic anaemia requires that benzene be
metabolized to toxic metabolites. It also appears that metabolism is
required for the production of clastogenic responses. While it seems
likely that metabolism is important for the induction of tumours,
there is very little data on this point. One report, however,
suggests a role for benzene metabolites in one type of carcinogenic
response. Busby et al. (1990) explored the ability of several known
benzene metabolites, as well as postulated benzene metabolites, to
induce lung tumours in newborn mice. They examined the effectiveness
of benzene oxide and enantiomers and racemates of benzene dihydrodiols
and diol epoxides given orally using a prescribed regimen. Lung
tumour incidence and multiplicity were increased after treatment with
benzene oxide, racemates of dihydrodiol and by diol epoxide-2.
Benzene and diol epoxide-1 were inactive in this system.
Although it is well known that benzene produces bone marrow
damage resulting from the production of benzene metabolites in liver,
it is also well known that these metabolites do not produce
hepatotoxicity. From the mechanistic point of view, it appears that
the liver is protected against damage from quinone metabolites of
benzene by the enzymes DT-diaphorase (Smart & Zannoni, 1985) and
carbonyl reductase (Wermuth et al., 1986). These enzymes prevent the
metabolic activation of phenolic metabolites to their otherwise toxic
quinones. The bone marrow is relatively deficient in these enzymes,
but partial protection of the marrow has been afforded through the
administration of high doses of ascorbic acid (Smart & Zannoni, 1985).
Metabolic activation of benzene metabolites, once they reach the
bone marrow, may lead to eventual toxicity. For example, in bone
marrow stromal macrophages, phenol (but not benzene) can be
metabolized and in the process inhibit RNA synthesis in macrophages,
thus possibly inhibiting the production of the haemopoietic factor
(Post et al., 1985). It has also been suggested (Kalf et al., 1989)
that the cyclooxygenase component of prostaglandin synthetase plays a
significant role in the metabolism of benzene and/or its metabolites
in bone marrow. Administration of indomethacin or other
cyclooxygenase inhibitors protected against benzene-induced bone
marrow depression and micronucleus formation.
A general mechanism for benzene-induced bone marrow depression
might be that benzene metabolites arising in the liver travel to the
bone marrow where further metabolic activation occurs. The newly
generated metabolites, perhaps acting in concert with unmetabolized
benzene in cell membranes, act upon target cells such as stem cells,
progenitor cells and stromal cells in the marrow to produce bone
marrow depression. Chromosomal damage may ensue, which is reflected
in clastogenesis observed in circulating lymphocytes or bone marrow
cells. The point in this series of events that leads to a
leukaemogenic response requires further examination once an adequate
model for the disease in animals has been established.
8. EFFECTS ON HUMANS
Acute inhalation and oral exposures of humans to high
concentrations of benzene have resulted in central nervous system
depression and death. The most noted effects resulting from
longer-term exposure to lower levels of benzene are haematotoxicity,
immunotoxicity and neoplasia.
8.1 General population and occupational exposure
The human health effects after exposure to benzene are
qualitatively the same for the general population and those exposed in
the workplace. To avoid duplication, the effects on both groups
(general population and workers) will be discussed together, with
emphasis on exposure levels and duration of exposure. The
quantitative response will be determined from such levels of total
daily intake.
8.1.1 Acute toxicity
Exposures in the general population that result in acute toxic
effects are usually related to accidents and misuse or abuse of
benzene. Many deaths and serious health effects have resulted from
benzene exposures after deliberate the "sniffing" of glue and other
products which contain benzene as a solvent (Winek & Collom, 1971).
Blood levels in people who have died as a result of "sniffing" glue
have ranged from 0.94 to 65 mg/litre (Winek et al., 1967; Winek &
Collom, 1971). Autopsy observations in these individuals included
pulmonary haemorrhage and inflammation, renal congestion and cerebral
oedema.
It has been estimated that exposure to benzene concentrations of
about 64 000 mg/m3 (20 000 ppm) for 5-10 min can result in
fatalities, 24 000 mg/m3 (7500 ppm) for 30 min is dangerous to life,
4800 mg/m3 (1500 ppm) for 60 min causes serious symptoms, 1600
mg/m3 (500 ppm) for 60 min leads to symptoms of illness, and 160-480
mg/m3 (50-150 ppm) for 5 h causes headache, lassitude, and weakness,
while 80 mg/m3 (25 ppm) for 8 h is without clinical effect (Gerarde,
1960). The clinical signs of acute toxicity from benzene include CNS
depression, cardiac arrhythmia, and eventually asphyxiation and
respiratory failure if exposures are at the lethal level (Andrews &
Snyder, 1986). Mild CNS symptoms are rapidly reversible following
cessation of exposure and there is no evidence that they result in
neurological brain damage (Marcus, 1990).
The single acute oral lethal dose in humans has been estimated to
be 10 ml of benzene (8.8 g) (Thienes & Haley, 1972). Clinical signs
of toxicity after acute oral exposure include staggering gait,
vomiting, shallow and rapid pulse, somnolence, loss of consciousness,
delirium, pneumonitis, profound CNS depression, and collapse
(Sandmeyer, 1981). High but sublethal oral doses may produce one or
more of the following symptoms: dizziness, visual disturbances,
euphoria, excitation, pallor, flushing, breathlessness and
constriction of the chest, headache, fatigue, sleepiness, and fear of
impending death (Sandmeyer, 1981). In addition to the autopsy
findings noted above, ingestion of benzene has been reported to cause
gastrointestinal ulceration (Appuhn & Goldeck, 1957).
No studies on the acute toxicity of benzene after dermal exposure
are available.
8.1.2 Effects of short- and long-term exposures
The most significant health effects from short- or long-term
exposure to benzene are haematotoxicity, immunotoxicity, neurotoxicity
and carcinogenicity. Three types of bone marrow effects have been
reported in response to benzene exposure; these are bone marrow
depression leading to aplastic anaemia, chromosomal changes and
carcinogenicity.
8.1.2.1 Bone marrow depression; aplastic anaemia
Several types of blood dyscrasias, including pancytopenia,
aplastic anaemia, thrombocytopenia, granulocytopenia, lymphocytopenia
and leukaemia, have been noted after exposure to benzene. These
changes are a continuum and not a discrete disease entity. Which
effect is noted will depend on the dose, length of exposure and the
stage of stem cell development affected (Galton, 1986). As in
experimental animals, the primary target organ of benzene that results
in haematological changes is the bone marrow. It has been suggested
that the cells at highest risk are the rapidly proliferating stem
cells (Marcus, 1990).
A study of 32 patients that were chronically exposed by
inhalation to benzene levels of 480-2100 mg/m3 (150-650 ppm) for 4
months to 15 years revealed pancytopenia with hypoplastic,
hyperplastic or normoblastic bone marrow. Eight of the 32 individuals
showed thrombocytopenia which resulted in haemorrhage and infection
(Aksoy et al., 1972). Haematotoxicity after prolonged exposure has
also been reported in rotogravure workers exposed for 6-60 months at
concentrations of 36-3485 mg/m3 (11-1069 ppm) (Goldwater, 1941) and
77-3400 mg/m3 (24-1060 ppm) (Erf & Rhoads, 1939), shoe factory
workers exposed to 96-670 mg/m3 (30-210 ppm) for 3 months to 17
years (Aksoy et al., 1971), and rubber factory workers exposed to up
to 1600 mg/m3 (500 ppm) (Wilson, 1942). Kipen et al. (1988)
reported on rubber workers who were exposed to benzene during the
1940s. An inverse relationship was found between the mean yearly
white blood cell count and the year that the count was made,
suggesting that exposures to benzene were very high in the early
1940s. As estimated by Crump & Allen (1984), benzene exposures
decreased from 438 mg/m3 (137 ppm) in 1940 to 102 mg/m3 (32 ppm)
in 1948. In a follow-up letter, Hornung et al. (1989) pointed out
that a similar rise in white blood cells counts was seen in
pre-employment physical examinations occurring over the same time
period at the same facility, and that that the trend could not be
attributed soley to benzene exposure. In a study of 1008 male
shoemakers in Florence, excess mortality from aplastic anaemia was
observed (SMR = 1566, 95% CI 547-3264), based on 6 deaths. All cases
of aplastic anaemia occurred among workers first employed before 1964
when the level of exposure to benzene was assumed to be highest (Paci
et al., 1989).
At levels less than 32 mg/m3 (10 ppm) no haematologic effects
have been observed (Collins et al., 1991). These authors found no
haematological effects in 200 benzene-exposed workers (10 year TWA of
0.03-4.5 mg/m3, 0.01-1.4 ppm) or in 268 control workers in the same
plant. In an earlier study of 70 workers in a coke oven by-product
recovery facility, Hancock et al. (1984) measured the levels of red
blood cells, white blood cells and haemoglobin in three groups exposed
to different concentrations of benzene (average, 34 mg/m3, 10.5 ppm;
range, 3.2-534 mg/m3, 1-167 ppm) and one non-exposed control group.
No significant differences between groups were noted in these
haematological parameters.
No data are available regarding haematotoxicity after short-term
or chronic oral or dermal exposure.
8.1.2.2 Immunological effects
As noted in animal studies (section 7.7.1), the immunological
manifestations of benzene toxicity are related to effects on the bone
marrow, resulting in changes to both humoral and cellular acquired
immunity. Workers (76) exposed to benzene (10-22 mg/m3, 3-7 ppm),
as well as to toluene and xylenes, for periods of 1-21 years were
examined for the presence of leucocyte agglutinins and levels of
circulating immunoglobulins. In 10 out of 35 workers where blood was
taken during working hours, the adverse effect of agglutinins reacting
with autoleucocytes was noted (Lange et al., 1973a). In addition, it
was found that the sera from the 35 workers had increased levels of
IgM and decreased levels of IgG and IgA immunoglobulins (Lange et al.,
1973b). The simultaneous exposure of these workers to solvents other
than benzene makes it difficult to interpret these results.
Autoimmunity, as shown by the pressure of antibodies against
leucocytes, platelets, and erythrocytes in the sera of exposed
workers, has been reported (Renova, 1962). Workers have been reported
to have an increased susceptibility to allergies (Aksoy et al., 1971)
when exposed to benzene concentrations as low as 96 mg/m3 (30 ppm).
A loss of leucocytes was observed in several studies of workers
reported to be exposed to benzene levels of 96-2080 mg/m3 (30-650
ppm) (Aksoy et al., 1971, 1974a; Aksoy, 1987). Signs of preleukaemia,
including loss of leucocytes and other blood elements and enlarged
spleens, were reported in one study (Aksoy et al., 1974a). Kipen et
al. (1989) and Yin et al. (1987) also reported decrease in circulating
lymphocytes and other blood elements at benzene exposures ranging from
48 to 240 mg/m3 (15-75 ppm). The number of T-cell lymphocytes were
found to have been reduced in workers exposed chronically to benzene,
toluene and xylene (Moszczynski, 1981).
In a study of workers exposed to low average concentrations of
benzene (< 32 mg/m3), there was no difference in cell cycle
kinetics of phytohaemagglutinin-stimulated lymphocytes in 66 male
workers of a refinery population when compared with 33 control workers
in the same refinery (Yardley-Jones et al., 1988).
No studies are available regarding the immunotoxicity of benzene
in humans after oral or dermal exposures.
8.1.2.3 Chromosomal effects
Both structural and numerical chromosomal aberrations have been
observed fairly consistently in the lymphocytes and bone marrow cells
of individuals occupationally exposed to benzene. It is now generally
accepted that benzene is a human clastogen (IARC, 1987a; Huff et al.,
1989). Increases in the number of both unstable and stable
chromosomal aberrations were observed in men, even 2 years after
cessation of workplace exposure (Tough & Court Brown, 1965). Up to
70% aneuploid lymphocytes were found in five women with benzene
haemopathy (Pollini et al., 1969), the effects still being
demonstrable 5 years post-exposure. Similar effects were observed in
the lymphocytes of workers in a rotogravure plant that had been
exposed to very high levels of benzene 400-1700 mg/m3 (125-532 ppm)
for 1-22 years (Forni et al., 1971a,b).
Recent studies by Yardley-Jones et al. (1988, 1990) revealed much
lower responses in the lymphocytes of workers exposed to low
concentrations of benzene (average < 32 mg/m3). In a study of 66
refinery workers and 33 controls, no alteration in cell cycle kinetics
was noted nor was there any increase in the level of SCEs
(Yardley-Jones et al., 1988). The lymphocytes from 48 of the workers
and 29 of the controls were analysed for chromosomal aberrations.
According to Yardley-Jones et al. (1990), the increase in aberrations
(particularly chromatid deletions and gaps) was of borderline
significance in parametric statistical tests, but was significant
using Fisher's exact test. No lifestyle factors had any consistent
effect on the incidence of chromosomal aberrations.
In an attempt to determine whether benzene and its metabolites
damage certain human chromosomes preferentially, Sasiadek et al.
(1989) examined the karyotypes of 33 workers exposed to less than 99
mg/m3 (31 ppm). At these levels no clinical or haematological
symptoms were noted in 31 workers, but pancytopenia was observed in
two workers. Nonrandom breaks and gaps were observed in the exposed
group; chromosomes two, four and nine were more prone to breaks and
chromosomes one and two more prone to gaps. The results of this study
are of limited value in view of the small number of controls and the
fact that all participants smoked.
Other studies that corroborate the clastogenicity of benzene in
humans have been reviewed by IARC (1982), Dean (1985a) and Kalf
(1987).
8.1.2.4 Carcinogenic effects
The fact that benzene is a human leukaemogen has been well
established by epidemiological and case studies (IARC 1982, 1987b),
most of which have dealt with industrial exposures. The
epidemiological studies reported have been selected because they
contain sufficient quantitative data on exposure and effects to permit
a discussion of the dose-response relationship. Some case reports are
summarized in Table 18, and prospective epidemiological studies are
summarized in Table 19. Of the two major classes of leukaemia
(granulocytic and lymphocytic), the most consistent evidence for a
causal relationship in humans has been found between benzene exposure
and myeloid leukaemia (Goldstein, 1988).
One case study followed the course of 44 patients with
benzene-induced pancytopenia and found that 6 of them later developed
leukaemia (Aksoy & Erdem, 1978).
The first bridge between case reports and a formal
epidemiological investigation was conducted in the early 1970s (Aksoy
et al., 1974b). These investigators reported on a series of cases
from an estimated population of 28 500 Turkish shoe workers exposed
since the 1950s to solvents and adhesives containing high levels of
benzene. Aplastic anaemia was first observed in 1961, and 26 patients
with acute leukaemia were observed by 1967. Peak exposure levels of
benzene were reported to be 96-670 mg/m3 (30-210 ppm), with rare
excursions to 2100 mg/m3 (650 ppm), for periods of 1 to 14 years
(mean 9.7 years). From this group of workers, a leukaemia incidence
rate (number of cases per 100 000 per year) of 13 was estimated
compared with a rate of 6 for the general population (Aksoy et al.,
1974b). It was unclear to the Task Group, from the description of the
authors, which methods were used to ascertain cases and from which
exposed population these cases were derived.
A study showing an excess risk of leukaemia in a cohort of 748
male workers producing "rubber hydrochloride" in three plants in two
locations within the USA during 1940-1949 (a product made from natural
rubber suspended in benzene) was first reported by Infante et al.
(1977). A follow-up study of this cohort was reported by Rinsky et
al. (1981) and this was subsequently updated (Rinsky et al., 1987).
In this most recent follow-up, the cohort definition was expanded to
include workers employed between 1940 and 1965 who had a cumulative
exposure to benzene of 3.2 mg/m3 per day (1 ppm/day) or more. The
Table 18. Case studies of workers occupationally exposed to benzene
Group studied Exposure Condition observed Author's References
conclusiona
44 pancytopenic patients exposed to benzene 480-2100 mg/m3 (150-650 leukaemia PC Aksoy & Erdem
in adhesives ppm); 4 months to 15 myeloid metaplasia * (1978)
years
42 leukaemia patients and 21 patients with not given leukaemia DC Aksoy (1980)
other malignancies; 47 were shoe workers, the multiple myeloma PC
remainder in other occupations using benzene myeloblastic leukaemia *
solvents acute erythroleukaemia *
preleukaemia *
malignant lymphoma PC
paroxymal nocturnal
haematuria *
lung cancer (all heavy
smokers) *
Table 18 (contd).
Group studied Exposure Condition observed Author's References
conclusiona
6 of 94 Hodgkin's patients who had been 480670 mg/m3 (150-210 Hodgekin's disease PC Aksoy et al.
exposed to benzene adhesives ppm); 1-28 years (1974a)
A 35-year-old man who had used benzene 200-1640 mg/m3; 18 months subacute granulocytic DC Sellyei & Kelemen
8 years earlier as a paint solvent leukaemia (1971)
6 leukaemia patients in different occupations levels unknown; 1-20 years haemocytoblastic leukaemia DC Vigliani & Saita
all using benzene solvents (1964)
A 51-year-old chemical worker exposed to 3.2 mg/m3 (< 2 ppm); acute myelogenous PC Ott et 81. (1978)
benzene 15 years earlier 18 months leukemia
a DC = direct correlation; PC = possible correlation; * = no conclusion made
Table 19. Epidemiological studies of workers exposed to benzene
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Incidence of leukaemia in 96-670 mg/m3 aplastic anaemia, 26 200 NR Aksoy et al.
Turkish shoe workers 1950-1965 rarely 2100 mg/m3; acute leukaemia (1974b)
(28 500 shoe, slipper and 1-15 years (mean,
handbag workers) 9.7 years)
Mortality study of rubber within legal limits of malignomas of lymphatic 14 260 NR Infante et
workers exposed to benzene period, i.e. 320 mg/m3 and haemopoietic P < 0.05 al. (1977)
between 1940 and 1949 (100 ppm) down to systems; myeloid and
32 mg/m3 (10 ppm) monocytic leukaemia 7 506 NR
for up to 10 years P < 0,02
Mortality studyb of pliofilm from < 40 ppm-years lymphatic and haemopoietic 15 227 127-376 Rinskyetal.
workers exposed to benzene to > 400 ppm-years neoplasms (1987)
between 1940 and 1965 with
a period at risk from 1950 leukaemia, total 9 337 159-641
to 1981 < 40 ppm-years 2 109 12-394
40-200 ppm-years 2 322 36-1165
205-400 ppm-years 2 1186 133-4289
> 400 ppm-years 3 6637 1334-19 393
multiple myeloma total 4 398 110-1047
< 40 ppm-years 3 458 92-1339
> 40 ppm-years 1 5347 70-29 753
Mortality studyc of 956 > 0.3-114 mg/m3 leukaemia (total) 4 194 52-488 Bond et al.
workers employed at a chemical (> 0.1-35.5 ppm) acute myelogenous 4 444 P < 0.05 (198613)
company between 1940 and estimated TWA for leukaemia
1982 up to 34 years
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective mortality study < 3.2 mg/m3 leukaemia 0 0 Tsai et al.
of workers (454) employed at (< 1 ppm) in 84% (1983)
a Texas refinery between 1952 samples (median,
and 1981 1.6 mg/m3; 0.5 ppm)
in benzene-related
areas
Mortality study of chemical < 15, 15-60 and > 60 lymphatic and haemopoletic, Wong
workers in 7 plants, > 6 ppm-years total: (1987)
months on job at single plant non-exposed 3 39 7-101
between 1946 and 1975 < 15 ppm-years 5 91 30-213
(3636 males) 15-60 ppm-years 5 147 48-343
60 ppm-years 5 179 57-409
leukaemia, total:
non-exposed 0 -
< 15 ppm-years 97 12-349
15-60 ppm-years 78 2-434
60 ppm-years 276 57-806
Retrospective cohort of 259 no benzene levels lymphatic and haemopoietic 4 377 109-1024 Decouflé et
male chemical workers reported; benzene neoplasms (workers with al. (1983)
employed between 1947 and used in large > 1 year of employment)
1960 quantities
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective cohort of shoe exposed for up to 29 aplastic anaemia (males); 4 1566 P < 0.05 Paci et al.
workers (1008 males, 1005 years; levels of leukaemia (males) 6 400 146-870 (1989)
women) employed between benzene not reported
1939 and December 1984 and
still employed in plant in
January 1950
Retrospective cohort study grab samples; means acute and chronic 30 cases 574 P < 0.01 Yin et al.
(28 460 workers in 233 factories); between 10 and 1000 leukaemia 5 deaths (1987)
reference population 28 257 mg/m3
in 83 machine production,
and clothing factories 50-500 mg/m3 found in
most plants
Mortality study of coke plant non-exposed, coke leukaemia (non-exposed) 13 135 NR Swaen et al.
workers (5639) with > 6 months ovens and by-product leukaemia (coke-oven) 6 163 NR (1991)
work between 1945 and 1969 workers; levels of leukaemia (by-product) 7 85 NR
benzene not reported
Retrospective cohort of 391 mean time-weighted leukaemia Hurley et
benzole workers in 2 cohorts average exposure of by-product 1 1 98 2-557 al. (1991)
(cohort 1, 84 workers; coke by-product by product 2 1 76 2-429
cohort 2, 307 workers) workers in Britain
in 1980s 4.2 mg/m3
(1.3 ppm)
a SMR - Standard mortality ratio
b Follow-up of cohort described by Infante et al. (1977)
c Follow-up of cohort described by Ott et al. (1978)
NR = not reported
study included 1165 white males followed from 1950 to 1981. For the
analysis the cohort was divided into four cumulative exposure groups:
< 128 mg/m3-years (< 40 ppm-years), 128-640 mg/m3-years (40-200
ppm-years), 640-1280 mg/m3-years (200-400 ppm-years) and > 1280
mg/m3-years (> 400 ppm-years). A statistically significant excess
risk was observed for all lymphatic and haemopoietic neoplasms (15
observed deaths compared to 6.6 expected; SMR = 227, 95% CI, 127-376).
There were nine deaths from leukaemia compared to an expected 2.66
(SMR = 337, 95% CI, 159-641), and 4 deaths from multiple myeloma (SMR
= 398, 95% CI, 110-1047). A strong positive trend in leukaemia
mortality was obtained with increasing cumulative exposure. Within
the four cumulative exposure groups there were 2,2,2, and 3 deaths
with SMRs of 109, 322, 1186 and 6637, respectively. In order to
investigate further the shape of the exposure-response curve, Rinsky
et al. (1987) conducted a nested case-control study by matching each
of nine deaths due to leukaemia with ten controls. A conditional
logistic regression analysis described a significant positive
association between estimated level and average duration of benzene
exposure and leukaemia that was projected downward to levels of zero
accumulated exposure over a working lifetime. From this model it was
calculated that an exponential relationship existed between benzene
exposure and the developmental leukaemia.
When actual exposure measurements did not exist, Rinsky et al.
(1987) estimated exposures to benzene by averaging historical annual
measured benzene levels from seven existing industrial hygiene survey
sources. The majority of measurements occurred after 1963, but some
data existed as early as 1946. Where no sampling data could be found,
exposure levels were estimated by interpolation from existing
information. Alterative exposure estimates and subsequent reanalyses
have been developed by Crump & Allen (1984) and Paustenbach et al.
(1992). The differences in exposure estimates between Crump & Allen
(1984) and Rinsky et al. (1987) centre primarily on assumptions of
benzene exposure prior to 1946 where no historical data exist.
Paustenbach et al. (1992) gathered additional information and
considered other factors that modified the estimates of exposure over
the entire period during which rubber hydrochloride plants operated
(1936 to 1976) to develop a new set of exposures over time. For the
most part the exposures estimated by Paustenbach et al. (1992) are
higher than those reported by Rinsky et al. (1987) and Crump & Allen
(1984).
In a retrospective study of 594 employees of a chemical company
exposed to levels of benzene between 0.3 and 114 mg/m3 (0.1-35.5
ppm) for up to 34 years, no statistically significant increase in
total mortality was reported (Ott et al., 1978). There were three
cases of myelocytic leukaemia compared to an expected incidence of 0.8
cases (significant at the P < 0.05 level). Some workers in this
cohort were also exposed to vinyl chloride, arsenicals and several
other potentially carcinogenic chemicals. A follow-up study of these
workers expanded the cohort by 362 potentially exposed workers (Bond
et al., 1986b). Four deaths from myelogenous leukaemia were reported
and the SMR for all leukaemias was 194 (95% CI, 52-488). The
difference between observed and expected values was statistically
significant only when myelogenous leukaemia was considered (4
observed, 0.9 expected; P = 0.01).
Wong (1987) reported a significant dose-response relationship
between cumulative exposure to benzene and mortality from leukaemia
and all lymphopoietic cancers combined. The mortality experience of
3536 workers who had continuous exposure to benzene was compared to
that of an internal comparison group of 3074 workers not exposed to
benzene but who had worked at the same plant. The 3536 exposed
workers were categorized into cumulative exposure categories of < 48
mg/m3-years (< 15 ppm-years), 48 to 192 mg/m3-years (15 to 60
ppm-years), and > 192 mg/m3-years (> 60 ppm-years). There was an
increasing trend in the SMRs for lymphatic and haemopoietic cancers as
exposure increased (SMR = 35, 91, 147 and 175 for the non-exposed and
the 3 exposure categories, respectively (P = 0.02). The respective
SMRs for leukaemia were 0, 97, 78 and 275 (P = 0.01). It should be
noted that none of the six leukaemia deaths was from acute myeloid
leukaemia. In addition, the highest category of exposure started at
only 192 mg/m3-years (60 ppm-years), the equivalent of 32 mg/m3
(10 ppm) annually for only a six-year working career. The
exposure-response relationship between cumulative benzene exposure and
non-Hodgkin's lymphoma was of marginal statistical significance.
A retrospective cohort mortality study was conducted on 259 male
employees at a chemical plant in the USA where benzene had been used
in large quantities (Decouflé et al., 1983). The study group included
workers employed between 1947 and 1960, and workers were followed
until 1977. Among workers with more than one year of employment, a
statistically significant excess risk was observed for neoplasms of
the lymphatic and haemopoietic systems (SMR=377; 95% CI 109-1024, 4
deaths). No SMR was given specifically for mortalities to leukaemia
and multiple myeloma. Three of these deaths were leukaemias and the
fourth a multiple myeloma.
Coke-oven workers and some workers at coke by-product plants are
exposed to low levels of benzene. A study in the Netherlands examined
5639 workers in a coke plant and a comparison group of 5740 workers in
a nitrogen-fixation plant (Swaen et al., 1991), employed for at least
six months between 1945 and 1965. The SMR for leukaemia in by-product
benzene plant workers was 85 (7 deaths), in coke-oven workers 163 (6
deaths), and in non-exposed workers 135 (13 deaths). Among the 222
workers in the benzene plant, no indication of an increased leukaemia
risk was found, but the expected number was small.
Hurley et al. (1991) have reported preliminary results on the
mortality of 6520 male coke plant workers from 27 plants in the United
Kingdom. Personal air samples were taken from 84 benzole workers from
14 plants in one cohort with levels of < 0.6-22 mg/m3 (< 0.19-7
ppm) and 307 benzole workers from 13 plants in the second cohort with
levels of < 0.6-48 mg/m3 (< 0.19-14.99 ppm). Mean time-weighted
average concentrations for benzole house workers in the United Kingdom
in the 1980s was considered to be about 4.2 mg/m3 (1.3 ppm). No
increased risk of mortality from leukaemia was reported in either
cohort (cohort 1 SMR = 98, 95% CI 2-557, 1 death; and cohort 2 SMR =
76, 95% CI 2-429, 1 death). These SMRs have been calculated utilizing
data from all 1293 by-product workers. Only a proportion of the
by-product workers had worked in the benzene plants where the greatest
exposure to benzene occurred. The SMR for leukaemia among 2349
coke-oven workers was 34 for cohort 1 (95% CI 0-186, 1 death) and 35
for cohort 2 (95% CI 1-192, 1 death).
In a retrospective cohort study from China encompassing 28 460
workers exposed to benzene in 233 factories, 30 cases of leukaemia
(23 acute, 7 chronic) were found, as compared to four cases in a
reference cohort of 28 257 workers in 83 machine production, textile
and cloth factories (Yin et al., 1987, 1989). The mortality rate (per
100 000 person-years) from leukaemia was 14 among the exposed and 2
among the unexposed (SMR, 574; P < 0.01). Mortality was especially
high for workers engaged in organic synthesis, painting and rubber
production. The mortality from leukaemia for cases that had
previously experienced benzene poisoning was 701/100 000 person-years.
Grab-samples of benzene in air were taken during the time of the
survey in workplaces where cases of leukaemia were observed; the mean
concentrations varied over a wide range (from 10 to 1000 mg/m3) but
the range was 50-500 mg/m3 in most locations. The mortality
incidence from all malignant neoplasms was higher in the exposed group
(123 per 100 000) than in controls (55 per 100 000). In addition, a
statistically significant excess risk for lung cancer (SMR = 231) was
observed in male workers exposed to benzene (Yin et al., 1989).
However, little description was given of the methods used to eliminate
the possible confounding effects of smoking.
A statistically significant excess risk for aplastic anaemia
(SMR = 1566, 4 deaths) and for leukaemia (SMR = 400, 95% CI, 146-870,
6 deaths) was reported for 1008 male workers in a shoe factory in
Florence, Italy. Included were workers employed on or after January
1950 and vital status of cohort members was ascertained through 1984.
No workplace monitoring was reported. The period of maximum benzene
exposure was considered to be 1953-1962, given the amount of benzene
used per day (about 30 kg) (Paci et al., 1989), and all cohort members
who developed aplastic anaemia and leukaemia were employed during this
period.
The results of a cohort study of 34 781 workers in eight oil
refineries in the United Kingdom which examined workers employed for
1 year between 1950-1975 gave no indication of increased mortality
from leukaemia (Rushton & Alderson, 1981a). Within this cohort a
nested case-control study was conducted to investigate the association
between exposure to benzene and leukaemia (Rushton & Alderson, 1981b).
The 36 leukaemia cases and their controls were allocated to "low",
"medium", and "high" benzene exposure categories based on their job
history. The authors reported a significant (P = 0.05) association in
the combined medium and highly exposed workers compared to those in
the low exposure group (RR = 3.0, 95% CI, 1.2-7.2).
A study (Tsai et al., 1983) of 454 petroleum refinery workers in
the USA employed between 1952 and 1978 in the petrochemical units
showed no deaths from leukaemia (0.4 expected). However, the median
exposure to benzene throughout the refinery was 0.45 mg/m3 (0.14
ppm), and only 16% of 1394 personal samples, taken between 1973 and
1982 (inclusive), contained more than 3.2 mg/m3 (1 ppm). The median
exposure intensity in "benzene-related units: petrochemical units" was
1.7 mg/m3 (0.53 ppm). No significant changes in blood indices
(counts of white and of red blood cells, haemoglobin, haematocrit,
platelets, clotting and bleeding times) were reported.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 General population
Benzene is ubiquitous in the environment, resulting in the
exposure of most humans to trace levels (or more) of this chemical.
Exposure in the general population is primarily to air-borne benzene
and derives from active and passive tobacco smoke, industrial
activity, and use of the automobile (gasoline fumes from refilling,
etc., and exhaust emissions). Estimates of the daily amounts of
benzene consumed in drinking-water and food-stuffs vary considerably
and are of the order of µg/day. Depending upon the assumptions made
with respect to levels of benzene from tobacco products and
foodstuffs, estimates for the exposure of the general smoking
population in industrialized countries range from 2000 to 3500 µg
benzene/day. Adult (70 kg) non-smokers are considered to be exposed
to about 200 to 1700 µg benzene/day (about 3 to 25 µg/kg body weight
per day). It would be helpful to have more information on total human
exposure, particularly in developing countries.
9.2 Occupational exposure
The major factors controlling industrial exposure to benzene are
process technology, worker practices and the efficiency and
sophistication of engineering controls. When appropriate engineering
controls are in place, available monitoring data indicate that
exposures of workers involved in the production, handling and use of
benzene and benzene-containing materials vary from non-detectable
levels to approximately 15 mg/m3 (8-h TWA), in addition to the
amounts estimated for the general population. In developing countries
the exposure can be several times higher. Due to the nature of the
processes involved, a small percentage of workers may be exposed to
more than 320 mg benzene/shift. In some developing countries, benzene
exposure may be sufficiently high to cause acute toxicity. Dermal
exposure to benzene has generally not been included in these
estimates. Validated benzene-specific biological markers of exposure
to low levels are not available.
9.3 Toxic effects
Acute lethal doses of benzene in experimental animals cause
narcosis, ventricular tachycardia and respiratory failure. The
threshold for narcotic effects in rats is about 13 000 mg/m3.
Reported oral LD50 values in rats vary from 3000 to 8100 mg
benzene/kg body weight and the 4-h LC50 in rats has been reported to
range between 32 600 and 44 600. However, although the clinical
pathological observations in animals are relevant to humans, the
latter would not be expected to be exposed to such high levels for
such long periods of time.
In humans, exposure to high concentrations of benzene (e.g.,
65 200 mg/m3 for 5-10 min) can result in central nervous system
depression, cardiac arrhythmia, respiratory failure and death, while
exposure to levels of benzene between 163 and 489 mg/m3 for 5 h
leads to headaches, lassitude and general weakness. The single acute
oral dose that has been reported to be lethal to humans is 8800 mg/kg
body weight.
9.3.1 Short-term and long-term exposures; organ toxicity
The most significant adverse effects from short- or long-term
exposure to benzene are haematotoxicity, i.e. bone marrow suppression,
immunotoxicity, genotoxicity and carcinogenicity.
9.3.1.1 Haematotoxicity; bone marrow depression
The level, timing and pattern of exposure are extremely important
factors in determining the incidence and severity of haematological
and bone marrow changes. A significant species difference (rats and
mice) in effects has also been reported, probably a reflection of the
more rapid metabolism of benzene by mice and the production of a
higher proportion of putative toxic metabolites than in rats. In rats
and mice, decreases in haematological cell counts (leucopenia and
haematocrit) and in bone marrow cellularity generally occur only after
several weeks of exposure to levels of benzene between 320 and 978
mg/m3, the mouse being more sensitive than rats. The lowest
reported exposure in experimental animals leading to haematological
effects was 32 mg/m3 (6 h/day, 5 days/week for 178 days) in male
mice. After oral administration of benzene to rats and mice for 120
days, leucopenia was observed in both male and female rats, and
lymphoid depletion in the B-cells of the spleen was observed in both
male and females at doses of > 200 mg/kg body weight. In mice, no
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females given 25, 50, 100, 200, 400 or
600 mg/kg body weight (5 days/week).
9.3.1.2 Mechanism of action and metabolism
In humans, a spectrum of blood dyscrasias, including
pancytopenia, aplastic anaemia, thrombocytopenia, granulocytopenia,
lymphocytopenia, myeloid leukaemia and acute leukaemia, can result
from benzene exposure. The dose, length of exposure and the stage of
stem cell development affected will determine which effect is
observed. Pancytopenia was noted in 32 patients exposed to 489-2119
mg benzene/m3 for periods of between 4 months and 15 years. At
levels of benzene between 102 and 438 mg/m3, haematotoxicity in
rubber factory workers was reported, the correlation being better
between benzene exposure and WBC counts than in the case of RBC
counts. However, at levels of benzene less than 32 mg/m3, there is
only weak evidence for a leukaemogenic effect; no haematological
effects were noted in 200 workers exposed to 10 year TWA benzene
levels of 0.03-4.5 mg/m3.
The hepatic metabolism of benzene is responsible for
detoxification of benzene via the formation of etheral sulfate,
glucuronides and glutathione conjugates. It also leads to the
production of metabolites, such as hydroquinone, p-benzoquinone,
muconaldehyde and perhaps others, which appear to be required for the
production of benzene toxicity in bone marrow. Current concepts of
the mechanism of benzene toxicity suggest that it is the result of the
combined effects of several metabolites, perhaps acting in concert
with unmodified benzene, to adversely alter the functions of stem
cells, progenitor cells and stromal cells in the bone marrow. It has
been postulated that the specific intracellular targets are proteins
and nucleic acids. The terminal result of these biochemical insults
is the development of aplastic anaemia. It is likely that benzene
metabolites damage chromosomes by causing DNA or protein adduct
formation or generating oxidative damage to DNA, which may contribute
to the chromosomal changes associated with benzene exposure. Animal
models exist for studying the mechanism of benzene-induced aplastic
anaemia and chromosome damage. However, no acceptable model for
benzene-induced leukaemia has been developed.
Since bone marrow depression and the production of leukaemia both
involve the bone marrow, it is important to ascertain the relationship
between these two biological effects of benzene. The specific
question in need of an answer is: Is it necessary for aplastic
anaemia to develop before leukaemia can be exhibited? In humans, the
continuum of effects of benzene on bone marrow involves first the
production of either anaemia, leucopenia or thrombocytopenia, and this
is followed by pancytopenia. Aplastic anaemia is the ultimate form of
bone marrow depression. Leukaemia has also been associated with
benzene exposure. In addition to studying the relationship between
these two phenomena, it is important to study the role of metabolites
in each process.
Several authors have recently used pharmacokinetic models to
extrapolate from animal experiments to expected human dosimetry. Some
models require the use of physiological parameters, partition
coefficients and metabolic data. Of these, the data on such metabolic
parameters in humans are least well known. With increasing
information on appropriate human metabolic data, such models will be
useful in extrapolating from animals to humans and from high- to
low-dose exposure.
9.3.1.3 Immunotoxicity
Benzene-induced immunological effects are probably a reflection
of bone marrow toxicity. Immunological function was depressed in mice
exposed to benzene levels between 32 and 96 mg/m3, 6 h/day, for 6
days. In mice, polyhydroxylated metabolites of benzene have been
shown to depress B- and T-cell functions. Although the relevance of
the animal data to human immunological functions has not been
established, human immunological alterations have been observed after
exposure to benzene.
In workers exposed to, but not seriously intoxicated by, benzene
(10-22 mg/m3), the serum complement levels of IgA and IgG were
decreased but the levels of IgM were slightly increased. An increased
susceptibility to allergies was found in workers exposed to benzene
levels as low as 96 mg/m3. A loss of leucocytes and other blood
elements has been noted at benzene levels ranging between 48 and 240
mg/m3. A study of low exposures to benzene (TWA: 32 mg/m3, 10
ppm) showed no differences in mitogen-induced blastogenesis in exposed
workers and controls.
9.3.2 Genotoxicity and carcinogenic effects
In vitro tests indicate that benzene is not mutagenic.
However, benzene, or its metabolites have been shown to cause both
structural and numerical chromosomal aberrations in experimental
animals as well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes.
Humans with benzene haemopathy have been found to have a high
percentage of aneuploid lymphocytes. Increases in the number of both
stable and unstable chromosomal aberrations were observed in workers
exposed to high levels (408-1734 mg/m3) of benzene for 1 to 22
years. Therefore, benzene should be considered a clastogen in both
animals and humans.
Recent studies of workers exposed to lower concentrations of
benzene (TWA: < 3.2-32 mg/m3, < 1-10 ppm) revealed no alteration
in cell-cycle kinetics and no increase in SCEs. Only a marginally
significant increase in chromosomal aberrations (chromatid deletions
and gaps) was noted at these exposure levels.
9.3.2.1 Mechanism of carcinogenicity
It is broadly accepted today, as a result of several studies in
the field of molecular biology, that chromosomal rearrangements
generating gene translocations, gene deletions and gene amplifications
are relevant steps in the carcinogenic process.
There is at present no adequate animal model for benzene-induced
leukaemia in humans. However, benzene has been shown to be
carcinogenic in experimental animals after inhalation or oral
exposure. While several types of neoplasms have been reported to be
associated with benzene exposure in rats and mice, these are primarily
of epithelial origin, i.e. zymbal gland, liver, mammary gland and
nasal cavity. Lymphomas/leukaemias have been observed with lesser
frequency.
One study showed an increased incidence of lymphoma in the CBA/CA
mouse. No statistically significant increase was seen for lymphoid
tumour incidence in the Sprague-Dawley rat, Wistar rat or Swiss mouse.
In the B6C3F1 mouse the incidence was not dose-related.
Dose-related (possibly linear) increases in epithelial tumours were
observed in mice and rats. The results in animals indicate that
benzene is an experimental multipotential carcinogen, although there
is not the leukaemogenic response seen in humans. The lowest dose
resulting in increased incidence of tumours was demonstrated in
B6C3F1 mice (25 mg/kg body weight, 5 days per week for 103 weeks).
No useful information is available for doses below this level.
Attempts to understand the mechanism of benzene-induced
carcinogenesis can be based on basic principles of chemical
carcinogenesis. Thus, one mechanism of cancer has been postulated to
be the result of autosomal mutations due to the formation of DNA
adducts, which result in alteration of cell function and control of
replication. Secondly, cancer appears to be induced by a combination
of multiple genetic and epigenetic events.
Metabolic data suggest that several reactive metabolites of
benzene are formed and these can potentially form adducts both with
DNA and protein. Binding of benzene metabolites to the protein
components of the spindle apparatus has been suggested to inhibit
mitosis. The data for the formation of DNA adducts by reactive
metabolites of benzene suggests that, compared with other carcinogens,
benzene does not form large amounts of DNA adducts. Furthermore,
although there is evidence for DNA-adduct formation in liver,
DNA-adduct formation in bone marrow has been a subject of some
controversy. Assuming that adduct formation is an initiating event,
there is the possibility of a protective event if DNA repair or immune
surveillance intervenes. A promotional event might lead to a
carcinogenic response. It is possible that benzene acts as a promoter
of its own initiation.
While it is clear that a greater percentage of benzene
metabolites are converted to reactive metabolites at low doses than at
high doses, the total amounts of reactive metabolites increases with
increasing dose. Hence, it is clear that the severity of benzene
toxicity increases with increasing dose. By analogy, it is expected
that increasing doses of benzene should yield more leukaemia in
humans.
The negative results obtained with in vitro mutagenicity tests
could be related to an inadequate production of mutagenic metabolites
in the system. The failure to produce leukaemia in animals may be due
to lack of adequate formation of leukaemogenic metabolites or the need
to produce bone marrow damage prior to the induction of leukaemia. If
the bone marrow must undergo a stage of aplastic anaemia prior to the
development of leukaemia, one would have to estimate higher exposure
to benzene and to define a threshold. Alternatively, at doses lower
than those producing aplastic anaemia, sufficient damage may occur to
induce tumour promotion.
Both animal and human studies show that benzene exposure produces
bone marrow and chromosomal damage. However, the various stages in
carcinogenesis cited above have not been observed with benzene either
because they do not occur in these species or because of technical
problems. The results of both animal and human studies suggest that
benzene is a weak carcinogen on a molar basis.
9.3.2.2 Human carcinogenesis
Benzene is a well-established human leukaemogen. There have been
numerous epidemiological studies on the effects of benzene, most of
which have dealt with chronic industrial exposures. Increased
leukaemia risk was identified in studies of shoemakers, chemical
workers and workers in oil refineries, and in a nationwide study of
benzene-exposed workers in different industries. The most consistent
evidence for a causal association in humans has been found between
benzene exposure and myeloid leukaemia. An exposure-response
relationship was identified in some studies, the response being
influenced both by exposure levels and duration of exposure. In the
study where estimated past exposures were based on the most extensive
exposure measurements, a three-fold increased leukaemia risk was
identified in workers exposed to benzene levels of 128-640
mg/m3-years (40-200 ppm-years) (ppm-years is the average
concentration times the duration of exposure in years, e.g., 4 ppm for
10 years is equivalent to 40 ppm-years) and a statistically
significant 12-fold risk for workers exposed to benzene levels between
of 640-1280 mg/m3-years (200-400 ppm-years). The scientific
assessment of alternative exposure estimates for the rubber
hydrochloride cohort has not yet been fully explored. Of the two
alternative exposures estimates so far postulated for this study, they
both suggest a lower estimate of risk than that described above.
Other leukaemia types, multiple myeloma and other lymphomas were also
reported.
A statistically significant excess risk for multiple myeloma was
found in the rubber hydrochloride study. A marginally significant
exposure-response relationship for malignant lymphomas was reported in
a study on chemical workers. Increased risk for skin, stomach and
lung cancers has been reported in some studies, but these findings
have not been consistent and may possibly be attributed to concomitant
exposures to other chemicals or statistical artifact.
Studies examining leukaemia risk in coke-oven workers, assumed to
have been exposed to fairly low levels of benzene, have not identified
excess leukaemia risk. In these studies no attempt was made to
examine leukaemia subtypes. These studies do not provide enough
evidence to prove that there is no risk of leukaemia as a result of
exposure to these low concentrations of benzene.
The Task Group is of the opinion that the epidemiological
evidence presented so far is not capable of distinguishing between
(a) a small increase in leukaemia mortality in workers exposed to low
benzene levels, and (b) a no-risk situation.
9.4 Other toxicological end-points
Benzene does cross the placenta of experimental animals, since
haemopoietic changes have been observed in the fetuses and offspring
of mice exposed to 16, 33 or 65 mg benzene/m3, 6 h/day during days
6-15 of gestation. Following inhalation exposure to high doses (500
to 1600 mg/m3) in rabbits and mice, an increase in fetal resorptions
or fetal death was observed. However, benzene does not appear to be
teratogenic, although it is fetotoxic, in experimental animals, and no
evidence is available to permit the conclusion that it causes adverse
reproductive effects in humans.
The neurotoxicity of benzene in animals and humans has not been
well studied. An early study showed subtle changes such as reduced
food intake and decreased hind-limb grip strength following exposures
to 3300 and 9900 mg benzene/m3, and learning defects were observed
in rats dosed orally with 550 mg benzene/kg body weight.
9.5 Conclusions
To assist Member States in the development of standards for
benzene exposure, the Task Group concludes that a TWA of 3.2 mg/m3
(1 ppm) over a 40-year working career has not been statistically
associated with any increase in deaths from leukaemia. However, since
benzene is a human carcinogen, exposures should be limited to the
lowest possible technically feasible level. Increases in exposure to
over 32 mg/m3 (10 ppm) should be avoided. Benzene and
benzene-containing products such as gasoline should never be used for
cleaning purposes.
Traditionally, bone marrow depression, i.e. anaemia, leucopenia
or thrombocytopenia, in the workplace has been recognized as the first
stage of benzene toxicity and appears to follow a dose-response
relationship, i.e. the higher the dose, the greater the likelihood of
observing decreases in circulating blood cell counts.
Table 20 shows some "rough" estimates of the percentages of
workers that might exhibit either bone marrow depression or frank
aplastic anaemia after exposure to benzene for either 1 year or 10
years at concentrations of 3.2, 32, 160 or 320 mg/m3 (1, 10, 50 or
100 ppm). These estimations are an interpretation of the literature
using the experience of the Task Group. The speculative nature of
this table precludes its use in regulatory standard setting. Exposure
at high doses (160-320 mg/m3; 50-100 ppm) for one year would most
likely produce bone marrow toxicity in a large percentage of the
workers, and in some cases aplastic anaemia, but little effect would
be expected at the lower doses. Exposure to both high and low doses
would be expected to produce benzene toxicity after 10 years. Thus,
a high level of both bone marrow depression and aplastic anaemia would
be seen at the higher doses and some damage would also be seen at the
lower doses. The observation of any of these effects, regardless of
the dose or period of exposure, should indicate the need for improved
control of benzene exposure.
There is no evidence of benzene being teratogenic at doses lower
than those that produce maternal toxicity, but fetal toxicity has been
demonstrated.
The neurotoxicity and immunotoxicity of benzene have not been
well studied in either experimental animals or humans.
Table 20. Estimated percentage of worker populations that might
develop bone marrow depression or aplastic anaemia after
chronic exposure to benzene ( Before using table note
cautionary footnotea)
Duration Exposure Bone marrow Aplastic
depression anaemia
1 year 320 mg/m3 (100 ppm) 90 10
160 mg/m3 (50 ppm) 50 5
32 mg/m3 (10 ppm) 1 0b
3.2 mg/m3 (1 ppm) 0b 0b
10 years 320 mg/m3 (100 ppm) 99 50
160 mg/m3 (50 ppm) 75 10
32 mg/m3 (10 ppm) 5 0b
3.2 mg/m3 (1 ppm) < 1 0b
a This estimation is an interpretation of the literature and is
based on the experience of the Task Group. The speculative
nature of this table precludes its use in regulatory standard
setting.
b Occasional cases may be observed.
10. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Benzene and benzene-containing products, including gasoline
(petrol), should never be used for cleaning purposes.
b) Systematic information on occupational and non-occupational
exposure should be collected using the total human exposure
approach where possible.
c) The health risk of low-level benzene exposure is not clearly
established. Exposure should, therefore, be avoided as much as
possible.
d) The occurrence of benzene in environmental media such as air and
water where there exists potential human exposure should be
evaluated.
e) A search for less toxic solvents to replace benzene in industrial
processes should be encouraged.
11. FURTHER RESEARCH
a) Epidemiological studies of the risks of haematological
malignancies, blood changes (red and white blood cells) and
genotoxic effects at low and high exposure concentrations should
have high priority.
b) Information on the mechanisms by which benzene induces neoplasms
is required. In particular, there is a need for animal models of
benzene-induced haemopoietic malignancies similar to those seen
in humans and a better understanding of the role of reactive
intermediate.
c) Further studies are needed to elucidate the potential link
between bone marrow suppression and the eventual occurrence of
leukaemia.
d) Biological markers of benzene exposure, especially urinary
muconic acid and macromolecular adducts, should be validated.
e) Individual susceptibility factors in benzene-induced toxicities
should be investigated.
f) Animals and human studies are required to assist in validating
physiologically based pharmacokinetic models using all routes of
exposure.
g) The multigenerational effects of benzene exposure should be
investigated.
h) Studies on immunotoxicological effects following benzene exposure
should be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of benzene has been evaluated by the
International Agency for Research on Cancer (IARC, 1982, 1987b). It
was concluded that there was sufficient evidence for the
carcinogenicity of benzene in both animals and humans.
A guideline value of 10 µg/litre was recommended by WHO (WHO,
1984) for benzene in drinking-water based on data for the production
of leukaemia after inhalation exposures in humans and using a linear
multistage extrapolation model and a life-time risk level of 1 in
100 000. This guideline remained unchanged during the revisions
recently completed (WHO, 1993).
A Task Group convened by the WHO Regional Office for Europe
concluded that an air quality guideline value could not be set for
benzene in view of its carcinogenic activity in humans (WHO, 1987).
Assuming no threshold and an average relative risk model, it was
calculated that at an air concentration of 1 µg benzene/m3, the
estimated lifetime risk of leukaemia would be 4 x 10-6.
Regulatory standards for benzene established by national bodies
in some countries are summarized in the Legal File of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
Recently the Commission of the European Communities has proposed an
occupational exposure limit for benzene of 1.6 mg/m3 (0.5 ppm) (CEC,
1993).
References
Aksoy M (1980) Different types of malignancies due to occupational
exposure to benzene. A review of recent observations in Turkey.
Environ Res, 23: 181-190.
Aksoy M & Erdem S (1978) Follow-up study on the mortality and the
development of leukaemia in 44 pancytopaenic patients with chronic
exposure to benzene. Blood, 52: 285-292.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1971) Haematological
effects of chronic benzene poisoning in 217 workers. Br J Ind Med, 28:
296-302.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1972) Details of
blood changes in 32 patients with pancytopaenia associated with
long-term exposure to benzene. Br J Ind Med, 29: 56-64.
Aksoy M, Erdem S, Hepyüksel T, & Dincol G (1974a) Chronic exposure to
benzene as a possible contributory etiologic factor in Hodgkin's
Disease. Blood, 38: 93-298.
Aksoy M, Erdem S, & Dincol G (1974b) Leukemia in shoe-workers exposed
chronically to benzene. Blood, 44: 837-841.
Allen PD (1987) Residential population exposure to ambient benzene in
California. Sacramento, California, California Air Resources Board.
Anderson D & Richardson CR (1981) Issues relevant to the assessment of
chemically induced chromosome damage in vivo and their relationship
to chemical mutagenesis. Mutat Res, 90: 261-272.
Andrews LS & Snyder R (1986) Toxic effects of solvents and vapors. In:
Casarett LJ & Doull J ed. Toxicology: The basic science of poisons,
3rd ed. New York, McMillan Publishing Co., chapter 20, p 641.
Antoine SR, Delon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36(3): 364-371.
Appuhn E & Goldeck H (1957) [Early and late disorders of
haematopoiesis caused by benzene and its homologues.] Arch
Gewerbepathol Gewerbehyg, 15: 399-428 (in German).
Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin BH, Matter
BE, & Shelby MD ed. (1985) Evaluation of short-term tests for
carcinogens. Report of the International Programme on Chemical
Safety's collaborative study on in vitro assays. Amsterdam, Oxford,
New York, Elsevier Science Publishers (Progress in Mutation Research,
Volume 5).
ATSDR (1989) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 173 pp
(ATSDR/TP-88/03; PB/89/209464/AS).
ATSDR (1991) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 193 pp.
Au WW, Ramanujam VMS, Ward JB Jr, & Legator MS (1991) Chromosome
aberrations in lymphocytes of mice after sub-acute low-level
inhalation exposure to benzene. Mutat Res, 260: 219-224.
Baarson K, Snyder CA, & Green J (1982) The hematotoxic effects of
inhaled benzene on peripheral blood, bone marrow, and spleen cells are
increased by ingested ethanol. Toxicol Appl Pharmacol, 64: 393-404.
Baarson K, Snyder CA, & Albert RE (1984) Repeated exposures of C57B1
mice to 10 ppm inhaled benzene markedly depressed erythropoietic
colony formation. Toxicol Lett, 20: 337-342.
Bailer AJ & Hoel DG (1989) Metabolite-based internal doses used in a
risk assessment of benzene. Environ Health Perspect, 82: 177-184.
Bailey JC & Schmidl B (1989) A survey of hydrocarbons emitted in
vehicle exhaust gases, over a range of driving speeds and conditions
from a representative sample of the 1986-87 UK vehicle fleet.
Stevenage, Herts, Warren Spring Laboratory (DTI) (Report No. LR 673).
Bandow H, Washida N, & Akimoto H (1985) Ring-cleavage reactions of
aromatic hydrocarbons studies by FT-IR spectroscopy. I. Photooxidation
of toluene and benzene in the NOx-air system. Bull Chem Soc Jpn, 58:
2531-2540.
Barale R, Giorgelli F, Migliore L, Ciranno R, Casini D, Zucconi D, &
Loprieno N (1985) Benzene induces micronuclei in circulating
erythrocytes of chronically treated mice. Mutat Res, 144: 193-196.
Barrett RH (1985) Assays for unscheduled DNA synthesis in HeLa S3
cells. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin
BH, Matter BE, & Shelby MD ed. Short-term tests for carcinogens:
Report of the International Programme on Chemical Safety's
collaborative study on in vitro assays. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 347-352 (Progress in Mutation
Research, Volume 5).
Battersby NS & Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Appl Environ
Microbiol, 52(2): 433-439.
Baxter HG, Blakemore R, Moore JP, & Coker DT (1980) The measurement of
airborne benzene vapour. Ann Occup Hyg, 23: 117-132.
Bayer CW, Black MS, & Galloway LM (1988) Sampling and analysis
techniques for trace volatile organic emissions from consumer
products. J Chromatogr Sci, 26: 168-173.
Bechtold WE, Sabourin PJ, & Henderson RF (1988) A reverse isotope
dilution method for determining benzene and metabolites in tissues.
J Anal Toxicol, 12(7/8): 176-179.
Bechtold WE, Lucier G, Birnbaum LS, Yin SN, Li GL, & Henderson RF
(1991) Muconic acid determinations in urine as a biological exposure
index for workers occupationally exposed to benzene. Am Ind Hyg Assoc
J, 52(11): 473-478.
Bechtold WE, Sun JD, Birnbaum LS, Yin SN, Li GL, Kasicki S, Lucier G,
& Henderson RF (1992) S-Phenylcysteine formation in haemoglobin as a
biological exposure index to benzene. Arch Toxicol, 66: 303-309.
Beliles RP & Totman LC (1989) Pharmacokinetically based risk
assessment of workplace exposure to benzene. Regul Toxicol Pharmacol,
9: 186-195.
Bennett GF (1989) Impact of toxic chemicals on local wastewater
treatment plant and the environment. Environ Geol Water Sci, 13(3):
201-212.
Bentley P, Sschassmann H, Sims P, & Oesch F (1976) Epoxides derived
from various polycyclic hydrocarbons as substrates of homogeneous and
microsome-bound epoxide hydratase. A general assay and kinetic
properties. Eur J Biochem, 69: 97-103.
Blanchard RD & Hardy JK (1986) Continuous monitoring device for the
collection of 23 volatile organic priority pollutants. Anal Chem, 58:
1529-1532.
Blank IH & McAuliffe DJ (1985) Penetration of benzene through human
skin. J Invest Dermatol, 85: 522-526.
Bois FY, Woodruff TJ, & Spear RC (1991a) Comparison of three
physiologically based pharmacokinetic models of benzene disposition.
Toxicol Appl Pharmacol, 110: 79-88.
Bois FY, Smith MT, & Spear RC (1991b) Mechanisms of benzene
carcinogenesis: application of a physiological model of benzene
pharmacokinetics and metabolism. Toxicol Lett, 56: 283-298.
Bond AE, Thompson VL, & Ortman GC (1986) Self service station vehicle
refueling exposure study. In: Proceedings of the 1986 EPA/APCA
Symposium on Measurement of Toxic Air Pollutants. Pittsburgh,
Pennsylvania, Air Pollution Control Association, pp 458-466.
Bond GG, McLaren EA, Baldwin CL, & Cook RR (1986) An update of
mortality among chemical workers exposed to benzene. Br J Ind Med, 43:
685-691.
Bradley MO (1985) Measurement of DNA single-strand breaks by alkaline
elution in rat hepatocytes. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 353-357 (Progress in Mutation Research, Volume 5).
Brodfuehrer JI, Chapman DE, Wilke TJ, & Powis G (1990) Comparative
studies of the in vitro metabolism and covalent binding of
14C-benzene by liver slices and microsomal fraction of mouse, rat
and human. Drug Metab Dispos, 18: 20-27.
Brown, RH (1988a) Determination of benzene, toluene, and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 225-233.
Brown RH (1988b) Determination of benzene, toluene, and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 235-242.
Bruckmann P, Beier R, & Krautscheid S (1983) [Measurements of
emissions of hydrocarbons in areas of high exposure in the Rhine-Ruhr
district.] Staub-Reinhalt-Luft, 43: 404 (in German).
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1989) Determination of
benzene, toluene and 1,3-butadiene in cigarette smoke by GC-MSD. Exp
Pathol, 37: 108-113.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1990) Analysis of
1,3-butadiene and other selected gas-phase components in cigarette
mainstream and sidestream smoke by gas chromatography - mass selective
detection. Carcinogenesis, 11(10): 1863-1868.
Bryce-Smith D & Gilbert A (1976) The organic photochemistry of
benzene. I. Tetrahedron, 32: 1309-1326.
Buchet JP (1988) Determination of phenol and its glucurono- and
sulfoconjugates in urine by gas chromatography. In: Fishbein L &
O'Neill JK ed. Environmental carcinogens methods of analysis and
exposure measurement - Volume 10: Benzene and alkylated benzenes. New
York, London, Oxford University Press, pp 281-286.
Burmaster DE (1982) The new pollution. Groundwater contamination.
Environment, 24: 33-36.
Busby WF Jr, Wang J-S, Stevens EK, Padykula RE, Aleksejczyk RA, &
Berchtold GA (1990) Lung tumourigenicity of benzene oxide, benzene
dihydrodiols and diolepoxides in the BLV: Ha newborn mouse assay.
Carcinogenesis, 11: 1473-1478.
Carpenter C, Shaffer C, Weil C, & Smyth H (1944) Studies on the
inhalation of 1,3-butadiene; with comparison of its narcotic effect
with benzol, toluol, and styrene and a note on the elimination of
styrene by the human. J Ind Hyg Toxicol, 26: 69-78.
CEC (1993) Occupational exposure limits - Criteria document for
benzene. Luxembourg, Commission of the European Communities,
Directorate-General Employment, Industrial Relations and Social
Affairs (EUR 14491 EN) (Health and Safety Series).
Chang SS & Peterson RJ (1977) Symposium: The basis of quality in
muscle foods. Recent developments in the flavor of meat. J Food Sci,
42: 298-306.
Chepiga TA, Yang CS, & Snyder R (1991) Benzene metabolism in two
purified, reconstituted rat hepatic mixed function oxidase systems.
In: Witmer CM, Snyder R, Jollow DJ, Kalf GF, Kocsis JJ, & Sipes IG ed.
Biological reactive intermediates: IV. Molecular and cellular effects
and their impact on human health. New York, London, Plenum Press,
pp 261-265.
Choy WN, MacGregor JT, & Shelby MD (1985) Induction of micronuclei by
benzene in B6C3F1 mice: Retrospective analysis of peripheral blood
smears from the NTP carcinogenesis bioassay. Mutat Res, 143: 55-59.
Ciranni R, Barale R, Marrazzini A, & Loprieno N (1988) Benzene and the
genotoxicity of its metabolites. I. Transplacental activity in mouse
fetuses and in their dams. Mutat Res, 208: 61-67.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, & Lester JN (1984b) A comparison of
photoionization detection gas chromatography with a Tenax GC sampling
tube procedure for the measurement of aromatic hydrocarbons in ambient
air. J Environ Anal Chem, 17: 315-326.
Coate WB, Hoberman AM, & Durloo RS (1984) Inhalation teratology study
of benzene in rats. Adv Mod Environ Toxicol, 6: 187-198.
Colenutt BA & Thornburn S (1980) Gas-chromatographic analysis of trace
hydrocarbon pollutants in water samples. Int J Environ Stud, 15:
25-32.
Collins JJ, Conner P, Friedlander BR, Easterday PA, Nair RS, Rashmi S,
& Braun J (1991) A study of the hematological effects of chronic
low-level exposure to benzene. J Occup Med, 33(5): 619-626.
CONCAWE (1986) Review of European oil industry benzene exposure data.
The Hague, European Oil Companies Organization for Environmental and
Health Protection (Report No. 3/86).
Cornish HH & Ryan RC (1965) Metabolism of benzene in non-fasted,
fasted, and arylhydroxylase inhibited rats. Toxicol Appl Pharmacol,
7: 767-771.
Cronkite EP (1986) Benzene hematotoxicity and leukemogenesis. Blood
Cells, 12: 129-137.
Cronkite EP, Bullis J, Inoue T, & Drew RT (1984) Benzene inhalation
produces leukemia in mice. Toxicol Appl Pharmacol, 75: 358.
Cronkite EP, Drew RT, Inoue T, & Bullis JE (1985) Benzene
hematotoxicity and leukemogenesis. Am J Ind Med, 7: 447-456.
Cronkite EP, Drew RT, Inoue T, Hirabayashi Y, & Bullis JE (1989)
Hematotoxicity and carcinogenicity of inhaled benzene. Environ Health
Perspect, 82: 97-108.
Crump K & Allen B (1984) Quantitative estimates of risk of leukemia
from occupational exposure to benzene. Washington, DC, US Department
of Labour (OSHA Docket H-059b, Exhibit 152, Annex B).
Dann T (1987) Ambient benzene levels in urban areas of Canada. Report
of the Technology Development and Technical Services Branch,
Conservation and Protection. Ottawa, Environment Canada.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Da Silva C, Fan X, & Castagna M (1989) Benzene-mediated protein kinase
c activation. Environ Health Perspect, 82: 91-95.
Dean BJ (1978) Genetic toxicology of benzene, toluene, xylenes and
phenols. Mutat Res, 47: 75-97.
Dean BJ (1985a) Recent findings on the genetic toxicology of benzene,
toluene, xylenes and phenols. Mutat Res, 154: 153-181.
Dean BJ (1985b) Summary report on the performance of cytogenetic
assays in cultured mammalian cells. In: Ashby J, de Serres FJ, Draper
M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation
of short-term tests for carcinogens. Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 69-83 (Progress in Mutation Research, Volume 5).
Decouflé P, Blattner WA, & Blair A (1983) Mortality among chemical
workers exposed to benzene and other agents. Environ. Res., 30: 16-25.
De Flora S, Zanacchi P, Camoirano A, Bennicelli C, & Badolati GS
(1984) Genotoxic activity and potency of 135 compounds in the Ames
reversion test and in a bacterial DNA-repair test. Mutat Res, 133:
161-198.
Deichmann WB, MacDonald WE, & Bernal E (1963) The hemopoietic tissue
toxicity of benzene vapors. Toxicol Appl Pharmacol, 5: 201-224.
Dibben MJ, Thomas TC, & Anderson MP (1989) Is benzene a problem in
fuel operations: A new analytical method to overcome potential
interferences in the present methods. Liq Chromatogr-Gas Chromatogr
Int, 2: 54, 56, 58.
Dorfman LM, Taub IA, & Buhler RE (1962) Pulse radiolysis studies. I.
Transient spectra and reaction-rate constants in irradiated aqueous
solutions of benzene. J Chem Phys, 36: 3051-3061.
Douglas GR, Blakely DH, Liu-Lee VW, Bell RDL, & Bayley JM (1985)
Alkaline sucrose sedimentation, sister-chromatid exchange and
micronucleus assays in CHO cells. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 359-366 (Progress in Mutation Research, Volume 5).
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Drew RT & Fouts JR (1974) The lack of effects of pretreatment with
phenobarbital and chlorpromazine on the acute toxicity of benzene in
rats. Toxicol Appl Pharmacol, 27: 183-193.
Eastmond DA, Smith MT, & Irons RD (1987) An interaction of benzene
metabolites reproduces the myelotoxicity observed with benzene
exposure. Toxicol Appl Pharmacol, 91: 85-95.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol, 15:
30-38.
Environment Agency, Japan (1989) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1986 and 1987. Tokyo, Environment Agency, Department of Environmental
Health, Office of Health Studies.
Erexson GL, Wilmer JL, & Kligerman AD (1985) Sister chromatid exchange
in human lymphocytes exposed to benzene and its metabolites
in vitro. Cancer Res, 45: 2471-2477.
Erexson GL, Wilmer JL, Steinhagen WH, & Kligerman AD (1986) Induction
of cytogenetic damage in rodents after short-term inhalation of
benzene. Environ Mutagen, 8: 29-40.
Erf LA & Rhoads CP (1939) The hematological effects of benzene
(benzol) poisoning. J Ind Hyg Toxicol, 20(8): 421.
Ewing BB, Chain ESK, Cook JC, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Washington, DC, US Environmental Protection Agency
(EPA-560/6/77/015A).
Fel'dt EG (1985) [Evaluation of the mutagenic hazards of benzene and
some of its derivatives.] Gig i Sanit, 7: 21-23 (in Russian with
English summary).
Fentiman AF, Neher MB, Kinzer GW, Sticksel PR, & Coutant RW (1979)
Environmental monitoring benzene. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/679/006)
(Prepared by Battelle Columbus for US EPA).
Ferrario JB, Lawler GC, Delon IR, & Laseter JL (1985) Volatile organic
pollutants in biota and sediments of Lake Pontchartrain. Bull Environ
Contam Toxicol, 34(2): 246-255.
Fishbein L (1984) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. I. Benzene. Sci Total Environ, 40:
189-218.
Forni A, Pacifico E, & Limonta A (1971a) Chromosome studies in workers
exposed to benzene or toluene or both. Arch Environ Health, 22:
373-378.
Forni AM, Cappellini A, Pacifico E, & Vigliani EC (1971b) Chromosome
changes and their evolution in subjects with past exposure to benzene.
Arch Environ Health, 23: 385-391.
Franz TJ (1984) Percutaneous absorption of benzene. In: Applied
toxicology of hydrocarbons. Princeton, New Jersey, Princeton
Scientific Publishers, Inc., pp 61-70 (Advances in Modern
Environmental Toxicology, Volume VI).
Fung KK & Wright BJ (1986) Monitoring of benzene in ambient air with
organic vapor badges. J Air Pollut Control Assoc, 36: 819-821.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected
hydrocarbons. Arch Ind Health, 18: 9-15.
Gad-El-Karim MM, Harper BL, & Legator MS (1984) Modifications in the
myeloclastogenic effect of benzene in mice with toluene,
phenobarbital, 3-methylcholanthrene, Aroclor 1254 and SKF-525A. Mutat
Res, 135: 225-243.
Galton DAG (1986) The myelodysplastic syndromes. Scand J Haematol, 36:
11-20.
Garner RC (1985) Summary report on the performance of gene mutation
assays in mammalian cells in culture. In: Ashby J, de Serres FJ,
Draper M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed.
Evaluation of short-term tests for carcinogens. Report of the
International Programme on Chemical Safety's collaborative study on
in vitro assays. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 85-94 (Progress in Mutation Research, Volume 5).
GDCh (Society of German Chemists) (1988) [Advisory Committee on
Existing Chemicals of Environmental Relevance. Benzene.] Weinheim, VCH
Verlagsgesellschaft, 82 pp (BUA Report 24) (in German with English
summary).
Geist CR, Kelly LD, Schoenheit CM, & Praed JE (1983) Learning
impairments following postnatal exposures to benzene. Percept Motor
Skills, 57: 1083-1086.
Gerarde HW (1960) Toxicology and biochemistry of aromatic
hydrocarbons. Amsterdam, Oxford, New York, Elsevier Science
Publishers.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res, 58:
313-316.
Geyer H, Politzki G, & Freitag D (1984) Prediction of ecotoxicological
behaviour of chemicals: relationship between n-octanol/water partition
coefficient and bioaccumulation of organic chemicals by the alga
Chlorella. Chemosphere, 13: 269-284.
Ghantous H & Danielsson BRG (1986) Placental transfer and distribution
of toluene, xylene, and benzene, and their metabolites during
gestation in mice. Biol Res Pregnancy, 7: 98-105.
Gilman AG, Goodman LA, & Gilman A (1985) The Goodman and Gilman's
pharmacological basis of therapeutics, 7th ed. New York, MacMillan
Publishing Co.
Gilmour SK, Kalf GF, & Snyder R (1986) Comparison of the metabolism of
benzene and its metabolite phenol in rat liver microsomes. In: Kocsis
JJ, Jollow DJ, Witmer CM, Nelson J, & Snyder R ed. Biological reactive
intermediates. III - Mechanisms of action in animal models and human
disease. New York, Plenum Publishing Co., pp 223-235.
Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, &
Oesch F (1989) Multiple activation pathways of benzene leading to
products with varying genotoxic characteristics. Environ Health
Perspect, 82: 81-89.
Gofmekler VA (1968) [The embryotropic action of benzene and
formaldehyde in experimental administration by inhalation.] Gig i
Sanit, 33: 12-16 (in Russian with English summary).
Goldstein BD (1988) Benzene toxicity: occupational medicine. State Art
Rev, 3: 541-554.
Goldstein BD, Snyder CA, Laskin S, Brombert I, Albert RE, & Nelson N
(1982) Myelogenous leukemia in rodents inhaling benzene. Toxicol Lett,
13: 169-173.
Goldwater LJ (1941) Disturbances in the blood following exposure to
benzol. J Lab Clin Med, 26: 957.
Gollmer L, Graf H, & Ulrich V (1984) Characterization of the benzene
monooxygenase system in rabbit bone marrow. Biochem Pharmacol, 33:
3597-3602.
Goodenkaug O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24(2): 231-233.
Gorsky LD & Coon MJ (1985) Evaluation of the role of free hydroxyl
radicals in the cytochrome P-450 catalyzed oxidation of benzene in
microsomes and reconstituted enzyme systems from rabbit liver. J Biol
Chem, 258: 7311.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Government of Canada (in press) Canadian environmental protection act
- Priority assessment report on benzene. Ottawa, Environment
Canada/Health and Welfare Canada.
Green JD, Leong BKJ, Lobue J, Goldstein BD, & Albert RE (1978)
Inhaled benzene fetotoxicity in rats. Toxicol Appl Pharmacol, 46:
9-18.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981a) Acute
and chronic dose/response effect of benzene inhalation on the
peripheral blood, bone marrow, and spleen cells of CD-1 male mice.
Toxicol Appl Pharmacol, 59: 204-214.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981b) Acute
and chronic dose/response effects of inhaled benzene on multipotential
hematopoietic stem (CFU-S) and granulocyte/macrophase progenitor
(GM-CFU-C) cells in CD-1 mice. Toxicol Appl Pharmacol, 58: 492-503.
Grob K & Grob G (1974) Organic substances in potable water and its
precursor. Part II. Applications in the area of Zurich. J Chromatogr,
90: 303-313.
Gruenke LD, Craig JC, Wester RC, & Maibach HI (1986) Quantitative
analysis of benzene by selected ion monitoring/gas chromatography/mass
spectrometry. J Anal Toxicol, 10(11/12): 225-232.
Hadeishi T, Pollard M, McLaughlin R, & Koga M (1985) Development of an
optical monitor for toxic organic compounds in air. Research Triangle
Park, North Carolina, US Environmental Protection Agency
(EPA/600/4-85-043).
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hammers WE & Bosman HFPM (1986) Quantitative evaluation of a simple
dynamic headspace analysis technique for non-polar pollutants in
aqueous samples at the ng/kg level. J Chromatogr, 360: 425-432.
Hancock DG, Moffitt AE Jr, & Hay EB (1984) Hematological findings
among workers exposed to benzene at a coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Hanke J, Dutkiewicz T, & Piotrowski J (1961) [The absorption of
benzene through the skin in men.] Med Pracy, 12: 413-426 (in Polish).
Harkov R, Gianti SJ, Bozzelli JW, & Laregina JE (1985) Monitoring
volatile organic compounds at hazardous and sanitary landfills in New
Jersey. J Environ Sci Health, A20(5): 491-501.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-311.
Hattemer-Frey HA, Travis CC, & Land ML (1990) Environmental
partitioning and human exposure. Environ Res, 53: 221-232.
Henderson RF, Sabourin PJ, Bechtold WE, Griffith WC, Medinsky MA,
Birnbaum LS, & Lucier GW (1989) The effect of dose, dose rate, route
of administration, and species on tissue and blood levels of benzene
metabolites. Environ Health Perspect, 82: 9-17.
Hine J & Mookerjee PK (1975) The intrinsic hydrophilic character of
organic compounds. Correlations in terms of structural contributions.
J Org Chem, 40: 292-298.
Hinson JA, Freeman JP, Potter DW, Mitchum RK, & Evans FE (1985)
Mechanism of microsomal metabolism of benzene to phenol. Mol
Pharmacol, 27: 574-577.
Hite M, Pecharo M, Smith I, & Thornton S (1980) The effect of benzene
in the micronucleus test. Mutat Res, 77: 149-155.
Högn T & Jaenicke L (1972) Benzene metabolism in Moraxella species.
Eur J Biochem, 30: 369-375.
Holdren MW, Smith DL, & Smith RN (1985) Comparison of ambient air
sampling techniques for volatile organic compounds. Washington, DC, US
Environmental Protection Agency (EPA/600/S4-85-067; PB86-120953/GAR).
Hornung RW, Ward E, Morris JA, & Rinsky RA (1989) Letter to the
editor. Haematologic effects of benzene: a thirty-five year
longitudinal study of rubber workers. Toxicol Ind Health, 5(6):
1153-1155.
HSE (1982) Toxicity review No. 4: Benzene. London, Health and Safety
Executive, Her Majesty's Stationery Office, 36 pp.
Hsieh GC, Sharma RP, & Parker RDR (1988a) Subclinical effects of
groundwater contaminants. I. Alteration of humoral and cellular
immunity by benzene in CD-1 mice. Arch Environ Contam Toxicol, 17:
151-158.
Hsieh GC, Parker RDR, & Sharma RP (1988b) Subclinical effects of
groundwater contaminants. II. Alteration of regional brain monoamine
neurotransmitters by benzene in CD-1 mice. Arch Environ Contam
Toxicol, 17: 799-805.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: toluene and xylene. Toxicology, 11: 55-63.
Huff JE, Haseman JK, Demarini DM, Eustis S, Maronpot RR, Peters AC,
Persing RL, Chrisp CE, & Jacobs AC (1989) Multiple-site
carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice.
Environ Health Perspect, 82: 125-163.
Hurley JF, Cherrie JW, & MacLaren W (1991) Exposure to benzene and
mortality from leukaemia: results from coke oven and other coal
product workers. Br J Ind Med, 48: 502-504.
IARC (1982) Benzene. In: Some industrial chemicals and dyestuffs.
Lyon, International Agency for Research on Cancer, pp 93-148
(Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 29).
IARC (1987a) Benzene. In: Genetic and related effects: an updating of
selected IARC Monographs from Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 91-95 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Formaldehyde. In: Overall evaluations of carcinogenicity:
An updating of IARC Monographs Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 211-216 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 7).
IARC (1989) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, 322 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
Ikeda M & Ohtsuji H (1971) Phenobarbital-induced protection against
toxicity of toluene and benzene in the rat. Toxicol Appl Pharmacol,
20: 30-43.
Infante PF, Wagoner JK, Rinsky RA, & Young RJ (1977) Leukaemia in
benzene workers. Lancet, 2: 76-78.
Inoue O, Seiji K, & Nakatsuka H (1989) Urinary t,t-muconic acid as an
indicator of exposure to benzene. Br J Ind Med, 46: 122-127.
Irons RD (1985) Quinones as toxic metabolites of benzene. J Toxicol
Environ Health, 16: 673-678.
Irons RD, Dent JG, Baker TS, & Rickert DE (1980) Benzene is
metabolized and covalently bound in bone marrow in situ. Chem-Biol
Interact, 30: 241-245.
Irons RD, Neptun DA, & Pfeifer RW (1981) Inhibition of lymphocyte
transformation and microtubule assembly by quinone metabolites of
benzene: Evidence for a common mechanism. J Reticuloendothel Soc, 30:
359-372.
IRPTC (1987) IRPTC Legal file 1986 on benzene - Volume 1. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
Jackson S & Brown VM (1970) Effect of toxic wastes on treatment
processes and watercourses. Water Pollut Control, 69(3): 292-313.
Jerina DM & Daly JW (1974) Arene oxides: a new aspect of drug
metabolism. Science, 185: 573-582.
Jerina D, Daly J, Witkop B, Zaltzman-Norenberg P, & Udenfriend S
(1968) Role of the arene oxide-oxepin system in the metabolism of
aromatic substrates. I. In vitro conversion of benzene oxide to a
premercapturic acid and a dihydrodiol. Arch Biochem Biophys, 128:
176-183.
Jirka AM & Bourne S (1982) Gas-chromatographic analysis for benzene in
blood. Clin Chem, 28: 1492-1494.
Johansson I & Ingelman-Sundberg M (1988) Benzene metabolism by
ethanol-, acetone-, and benzene-inducible cytochrome P-450(IIE1) in
rat and rabbit liver microsomes. Cancer Res, 48: 5387-5390.
Johnston RV, Pinkerton MN, & Mensik DC (1979) Haematological and
myelogenous effects of inhaled benzene in the pig and rat. J Toxicol
Environ Health, 5: 1025-1035.
Jonek J, Olkowski Z, & Zieleznik B (1965) Histochemical studies on the
spinal cord of mice poisoned with benzene. Acta Histochem, 20:
286-296.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular-weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jowa L, Witz G, Snyder R, Winkle S, & Kalf GF (1990) Synthesis and
characterization of deoxyguanosine-benzoquinone adduct. J Appl
Toxicol, 10(1): 47-54.
Kalf GF (1987) Recent advances in the metabolism and toxicity of
benzene. CRC Crit Rev Toxicol, 18: 141-159.
Kalf GF, Schlosser MJ, Renz JF, & Priozzi SJ (1989) Prevention of
benzene-induced myelotoxicity by nonsteroidal anti-inflammatory drugs.
Environ Health Perspect, 82: 57-64.
Karam LR & Simic MG (1989) Mechanisms of free radical chemistry and
biochemistry of benzene. Environ Health Perspect, 82: 37-41.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soils. Chemosphere,
10(8): 833-846.
Karlson U & Frankenberger WT Jr (1989) Microbial degradation of
benzene and toluene in groundwater. Bull Environ Contam Toxicol, 43:
505-510.
Keller KA & Snyder CA (1986) Mice exposed in utero to low
concentrations of benzene exhibit enduring changes in their colony
forming hematopoietic cells. Toxicology, 42: 171.
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol Environ
Saf, 4: 26-38.
Kiang PH & Grob RL (1986) A headspace technique for the determination
of volatile compounds in soil. J Environ Sci Health, A21: 71-100.
Kimmel CA & Wilson JG (1973) Skeletal deviations in rats:
malformations or variations? Teratology, 8: 309-316.
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Kipen HM, Cody RP, Crump KS, Allen BC, & Goldstein BD (1988)
Hematologic effects of benzene: a thirty-five year longitudinal study
of rubber workers. Toxicol Ind Health, 4(4): 411.
Kipen HM, Cody RP, & Goldstein BD (1989) Use of longitudinal analysis
of peripheral blood counts to validate historical reconstructions of
benzene exposure. Environ Health Perspect, 82: 199-206.
Kirley TA, Goldstein BD, Maniara WM, & Witz G (1989) Metabolism of
trans,trans-muconaldehyde, a microsomal hematotoxic metabolite of
benzene by purified yeast aldehyde dehydrogenase and a mouse liver
soluble fraction. Toxicol Appl Pharmacol, 100: 360-367.
Kissling M & Speck B (1972) Further studies on experimental benzene
induced aplastic anemia. Blut, 25: 97-103.
Kocsis JJ, Harkaway S, Santoyo MC, & Snyder R (1968) Dimethyl
sulfoxide: interactions with aromatic hydrocarbons. Science, 160:
427-428.
Koljkowsky P (1981) Indicator-tube method for the determination of
benzene in air. Analyst, 94: 918-920.
Korte F & Klein W (1982) Degradation of benzene in the environment.
Ecotoxicol Environ Saf, 6(4): 311-327.
Kozioski RP (1985) Determination of benzene and toluene in soils and
plant material by azeotropic distillation. Bull Environ Contam
Toxicol, 34: 10-16.
Kuna RA & Kapp RW (1981) Embyrotoxic/teratogenic potential of benzene
vapor in rats. Toxicol Appl Pharmacol, 57: 1-7.
Lahmann E, Seifert B, & Ullrich D (1977) The pollution of ambient air
and rain water by organic components of motor vehicle exhaust-gases.
In: Kasuga S ed. Proceedings of the 4th International Clean Air
Congress. Tokyo, Japanese Union of Air Pollution and Prevention
Association, pp 595-597.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973a) Leukocyte
agglutinins in workers exposed to benzene, toluene and xylene. Int
Arch Arbeitsmed, 31: 45-50.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973b) Serum
immunoglobulin levels in workers exposed to benzene, toluene and
xylene. Int Arch Arbeitsmed, 31: 37-44.
Latriano L, Goldstein BD, & Witz G (1986) Formation of muconaldehyde,
an open-ring metabolite of benzene, in mouse liver microsomes: an
additional pathway for toxic metabolites. Proc Natl Acad Sci (USA),
83: 8356-8360.
Leong B (1977) Experimental benzene intoxication. In: Laskin S &
Goldstein B ed. Benzene toxicity: A critical revaluation. J Toxicol
Environ Health, 3(2): 45-61.
Li G-L, Yin N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda M
(1986) Benzene-specific increase in leukocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581.
Longacre S, Kocsis J, & Snyder R (1981a) Influence of strain
differences in mice on the metabolism and toxicity of benzene. Toxicol
Appl Pharmacol, 60: 398-409.
Longacre S, Kocsis J, Witmer CM, Lee EW, Sammett D, & Snyder R (1981b)
Toxicological and biochemical effects of repeated administration of
benzene in mice. J Toxicol Environ Health, 7: 223-237.
Low LK, Meeks JR, Norris KJ, Mehlman MA, & Macherer CR (1989)
Pharmacokinetics and metabolism of benzene in Zymbal gland and other
key target tissues after oral administration in rats. Environ Health
Perspect, 82: 215-222.
Low LK, Reddy MV, Blackburn GR, & Mackerer CR (1991) Benzene
mechanistic research. Final report of collaborative study by Mobil,
Amoco Ashland, Standard Oil and Sun Company. Princeton, New Jersey,
Mobil Oil Corporation, 76 pp.
Lunte SM & Kissinger PT (1983) Detections and identification of
sulfhydryl conjugates of p-benzoquinone in microsomal incubations of
benzene and phenol. Chem-Biol Interact, 47: 195-212.
Lutz WK (1979) In vivo covalent binding of organic chemicals to DNA
as a quantitative indicator in the process of chemical carcinogenesis.
Mutat Res, 65: 289-356.
Lutz WK & Schlatter C (1977) Mechanisms of the carcinogenic action of
benzene: irreversible binding to rat liver DNA. Chem-Biol Interact,
18(2): 241-246.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal Gulf of Mexico site. Chemosphere, 17(1):
123-136.
MacKay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol, 9:
1178-1180.
Maibach HI & Anjo DM (1981) Percutaneous penetration of benzene and
benzene contained in solvents in the rubber industry. Arch Environ
Health, 36: 256-260.
Maltoni C, Ciliberti A, & Carretti D (1982a) Experimental
contributions in identifying brain potential carcinogens in the
petrochemical industry. Ann NY Acad Sci, 381: 216-249.
Maltoni C, Conti B, & Scarnato C (1982b) Squamous cell carcinomas of
the oral cavity in Sprague-Dawley rats following exposure to benzene
by ingestion. Med. Lav, 4: 441-445.
Maltoni C, Cotti G, Valgimigli L, & Mandrioli A (1982c)
Hepatocarcinomas in Sprague-Dawley rats following exposure to benzene
by inhalation. Med Lav, 4: 446-450.
Maltoni C, Conti B, & Cotti G (1983) Benzene: a multipotential
carcinogen. Results of long-term bioassays performed at the Bologna
Institute of Oncology. Am J Ind Med, 4: 589-630.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
current results and ongoing research. Am J Ind Med, 7: 415-446.
Maltoni C, Ciliberti A, Cotti G, Conti B, & Belpoggi F (1989) Benzene,
an experimental multipotential carcinogen: results of the long-term
bioassays performed at the Bologna Institute of Oncology. Environ
Health Perspect, 82: 109-124.
Mara SJ & Lee SS (1978) Assessment of human exposures to atmospheric
benzene. Research Triangle Park, North Carolina, US Environmental
Protection Agency (EPA/450/378-031).
Marcus WL (1990) Chemical of current interest: benzene. Cancer risk
from benzene. Adv Mod Environ Toxicol, 17: 127-188.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989a) Differences in the pathways for metabolism of benzene in rats
and mice simulated by a physiological model. Environ Health Perspect,
82: 43-49.
Medinsky MA, Sabourin PJ, Lucier G, Birnbaum LS, & Henderson RF
(1989b) A toxicological model for simulation of benzene metabolism.
Exp Pathol, 37: 150-154.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989c) A physiological model for simulation of benzene metabolism by
rats and mice. Toxicol Appl Pharmacol, 99: 193-206.
Merian E & Zander M (1982) Volatile aromatics. In: Hutzinger O ed. The
handbook of environmental chemistry, vol 3, Part B: Anthropogenic
compounds. Berlin, Heidelberg, New York, Springer-Verlag, pp 117-161.
Michael LC, Pellizzari ED, & Wiseman RW (1988) Development and
evaluation of a procedure for determining volatile organics in water.
Environ Sci Technol, 22: 565-570.
Miller MM & Wasik SP (1985) Relationships between octanol-water
partition coefficient and aqueous solubility. Environ Sci Technol,
19(6): 522-529.
Morimoto K (1976) Analysis of combined effects of benzene with
radiation on chromosomes in cultured human leukocytes. Jpn J Ind
Health, 18: 23-24.
Morimoto K (1983) Induction of sister chromatid exchanges and cell
division delays in human lymphocytes by microsomal activation of
benzene. Cancer Res, 43: 1330-1334.
Morimoto K, Wolff S, & Koizumi A (1983) Induction of sister-chromatid
exchanges in human lymphocytes by microsomal activation of benzene
metabolites. Mutat Res, 119: 355-360.
Moszczynski P (1981) Organic solvents and T-lymphocytes. Lancet, 1:
438.
Murray FJ, John JA, Rampy LW, Kuna RA, & Schweta BA (1979)
Embryotoxicity of inhaled benzene in mice and rabbits. Am Ind Hyg
Assoc J, 40: 993-998.
Nahum LH & Hoff HE (1934) The mechanism of sudden death in
experimental benzol poisoning. J Pharmacol Exp Ther, 50: 336.
Nakajima T, Okuyama S, & Yonekura I (1985) Effects of ethanol and
phenobarbital administration on the metabolism and toxicity of
benzene. Chem-Biol Interact, 55: 23-38.
Nakajima T, Elovaara E, Park SS, Gelbain HV, Hietanen E, & Vainio H
(1990) Monoclonal antibody-directed characterization of benzene,
ethoxy-resorufin and pentoxy-resorufin metabolism in rat liver
microsomes. Biochem Pharmacol, 40: 1255-1261.
Nau CA, Neal J, & Thornton M (1966) C9-C12 fractions obtained from
petroleum distillates. Arch Environ Health, 12: 382-393.
Nawrot PS & Staples RE (1979) Embryo-fetal toxicity and teratogenicity
of benzene and toluene in the mouse. Teratology, 19: 41A.
Nerland DE & Pierce WM (1990) Identification of N-acetyl-
S-(2,5-dihydroxyphenyl)-L-cysteine as a urinary metabolite of benzene,
phenol and hydroquinone. Drug Metab Dispos, 18: 958-961.
Nomiyama K & Nomiyama H (1974a) Respiratory retention, uptake and
excretion of organic solvents in man. Benzene, toluene, n-hexane,
trichloroethylene, acetone, ethyl acetate and ethyl alcohol. Int Arch
Arbeitsmed, 32: 75-83.
Nomiyama K & Nomiyama H (1974b) Respiratory elimination of organic
solvents in man. Benzene, toluene, n-hexane, trichloroethylene,
acetone, ethyl acetate and ethyl alcohol. Int Arch Arbeitsmed, 32:
85-91.
Nordlinder R & Ramnäs O (1987) Exposure to benzene at different work
places in Sweden. Ann Occup Hyg, 31: 345-355.
Norpoth K, Stücker W, Krewer E, & Müller G (1988) Biomonitoring of
benzene exposure by trace analyses of phenylguanine. Arch Occup
Environ Health, 60: 163-168.
NTP (1986) Toxicology and carcinogenesis studies of benzene (CAS No.
the Zymbal gland. This is the first suggestion that conjugation
products, normally thought of as only a mechanism for urinary
excretion, could also be considered to act as a transporting mechanism
for bringing metabolites from the liver to target tissues.
Parke & Williams (1953) reported that phenyl mercapturic acid was
a urinary end product of benzene metabolism. This observation was
supported by the report of Jerina et al. (1968) who incubated
glutathione with rat liver cytoplasm and benzene oxide and found that
the principal metabolite was S-phenylglutathione. Norpoth (1988) has
developed a method for the determination of phenylmercapturic acid in
human urine as a measure of exposure to benzene based on these
observations. However, Lunte & Kissinger (1983) showed that
p-benzoquinone, an oxidation product of hydroquinone, forms
glutathione conjugates non-enzymatically. Lau et al. (1989) reported
that 1,2,3 or 4 glutathione molecules could conjugate with
p-benzoquinone. Nerland & Pierce (1990) showed the occurrence of
N-acetyl- S-(2,5-dihydroxyphenyl)l-cysteine as a urinary metabolite
of benzene in rats. Stommel et al. (1989) found that the metabolite
phenylmercapturic acid increased proportionally in rats and humans as
the inhaled dose rose to 1600 mg/m3 (500 ppm). Thus, the array of
mercapturic acid metabolites of benzene has expanded and the full
extent of metabolites of this structure may not yet be fully
appreciated.
In summary, the postulated metabolic pathways for benzene are
shown in Figures 1, 2 and 3. The formations of mercapturic acids,
ethereal sulfates and glucuronides are generally considered
detoxification pathways leading to the excretion of benzene
metabolites via the kidney (Henderson et al., 1989). All other
pathways lead to potentially toxic metabolites. This hypothesis is
discussed in more detail in section 7.9.
In both rats and mice the formation of toxic metabolites via the
epoxide pathway appears to be a saturable process, which suggests that
both metabolism and toxicity would be non-linear. In other words, the
proportion of toxic metabolites formed would decrease once the
saturation level is reached, whereas detoxification pathways appear to
be low-affinity high-capacity reactions (Henderson et al., 1989;
Medinsky et al., 1989a). It has been shown that mice metabolize
benzene faster and converted more of the benzene to toxic metabolites
than rats (Henderson et al., 1989). Because of this it has been
suggested that metabolism in mice favours toxification pathways (e.g.,
formation of benzoquinone and muconaldehyde), while in rats metabolism
is primarily detoxification (phenyl conjugates and phenylmercapturic
acids) (Medinsky et al., 1989a). The percentage of benzene or its
metabolites remaining in the body decreased in rats (from 33% to 15%)
and mice (from 50% to 10%) as exposure increased from 32 to 3200
mg/m3 (10 to 1000 ppm) (Sabourin et al., 1987).
Model simulations for total benzene metabolized and for profiles
of benzene metabolites formed after the administration of varying
doses of benzene to rats and mice (Medinsky et al., 1989b,c) have
suggested that the production of hydroquinone and muconic acid
metabolites predominates at lower exposure concentrations, whereas at
high exposure levels the detoxification pathways account for a larger
fraction of benzene metabolized. In addition, these model simulations
have confirmed that mice metabolize more benzene on a µmole/kg body
weight basis than rats after inhalation exposures, whereas rats
metabolize more benzene than mice at oral doses greater than 50 mg/kg
body weight. After either oral or inhalation exposures mice
preferentially form more of the putative toxic metabolites
hydroquinone and muconic acid (Medinsky et al., 1989b). It has also
been reported by Witz et al. (1990b) that DBA/ZN mice (a strain
sensitive to the haematotoxicity of benzene), excrete greater amounts
of trans, trans-muconic acid than the less sensitive C57BL/6 strain
after equivalent exposures to benzene.
6.4 Elimination and excretion
6.4.1 Inhalation exposure
In animals, expired air is the main route of elimination of
unmetabolized benzene, while urine is the major route of excretion of
benzene metabolites (with very little faecal excretion). Rickert et
al. (1979) found a biphasic pattern of excretion of unmetabolized
benzene in rats after a 6-h exposure to 1600 mg/m3 (500 ppm), with
half-times of 0.7 h for the rapid phase and 13.1 h for the slow phase.
The major route of excretion after inhalation exposures of rats and
mice to 32-3200 mg/m3 (10-1000 ppm) appeared to be dependent upon
the concentration inhaled (Sabourin et al., 1987). Under these
conditions mice received 150-200% of the dose given to rats on a per
kg body weight basis. The faecal excretion was < 3.5% in rats and
< 9% in mice. At doses up to 416 mg/m3 (130 ppm), less than 6% of
the radioactivity was eliminated in expired air, whereas at the highest
concentrations 48% of the dose was eliminated as unchanged chemical in
rats and 14% in mice. The total urinary excretion of metabolites at
these high concentrations was 5-37% higher in mice than in rats.
Findings in humans after inhalation exposure to benzene are
similar to those in experimental animals; unmetabolized chemical is
eliminated in expired air whereas metabolites of benzene are excreted
in urine, primarily as the sulfate and glucuronide conjugates of
phenol. Nomiyama & Nomiyama (1974a,b) found similar expiratory
patterns in men and women exposed for 4 h to benzene at concentration
between 166 and 198 mg/m3 (52-62 ppm). The proportion of the
absorbed benzene that was excreted via the lungs was approximately 17%
(Nomiyama & Nomiyama, 1974a,b).
6.4.2. Oral exposure
Parke & Williams (1953) administered radiolabelled benzene
(approximately 340 mg/kg body weight) by oral gavage to rabbits and
reported that 43% of the label was recovered as unmetabolized benzene
in expired air. Urinary excretion accounted for 33% of the dose,
mainly in the form of conjugated phenol (23.5%). Other phenols
excreted were hydroquinone (4.8%), catechol (2.2%), and hydroxyquinol
(1,2,4-trihydroxybenzene) (0.3%). Muconic acid accounted for 1.3% and
L-phenylmercapturic acid for 0.5%, and 5-10% of the radiolabel
remained in the tissues or was excreted in the faeces. The excretion
of benzene and its metabolites in rats and mice at various oral doses
(0.5-300 mg/kg body weight) was studied by Sabourin et al. (1987). In
both species the excretion of urinary metabolites up to a dose of 15
mg/kg accounted for 80% of the administered dose. Above that level
there was an increase in the elimination of 14C in expired air.
Equal amounts of unmetabolized benzene were eliminated in both species
up to dose levels of 50 mg/kg. At dose levels of between 15 and 50
mg/kg body weight, metabolism appears to become saturated in rodents.
In rats, 50% of a 150-mg/kg dose of 14C-benzene was eliminated in
expired air, while in the mouse 69% of this dose was exhaled (Sabourin
et al., 1987).
No studies were found regarding the excretion of benzene in
humans after oral exposures.
6.4.3 Dermal exposure
After the dermal application of 14C-benzene (0.0026 to 0.0036
mg/cm2) to monkeys and minipigs, Franz (1984) collected urine
samples every 5 h for 2-4 days. The rate of excretion was highest
over the first 10 h, the total excretion of radioactivity being higher
in the monkey (0.03 to 0.14% of the applied dose, with an average of
0.06%) than in the minipigs (0.03-0.05%, with an average of 0.04%).
Using a glass cap to minimize volatilization from the skin, Skowronski
et al. (1988) treated male rats dermally with 14C-benzene (0.004
mg/cm2). After 48 h, 86.2% of the initial dose was excreted in the
urine and 12.8% was eliminated in expired air. Phenol was the major
urinary metabolite detected in the 0-12 h sample (37.7% of dose), and
smaller quantities of hydroquinone, catechol and benzenetriol were
also detected.
In a study of four male human subjects, Franz (1984) applied
14C-benzene dermally (0.0024 mg/cm2). A mean of 0.023% (range
0.006-0.054%) of the applied radiolabel was recovered in the urine
over a 36-h period. More than 80% of the excretion occurred within
8 h of application.
6.5 Retention and turnover
Steady state levels of benzene were found within 4 h in blood,
6 h in fat, and 2 h in bone marrow when male rats were exposed to a
benzene concentration of 1600 mg/m3 (500 ppm) by inhalation for 6 h.
After exposure ceased, about 70% of the benzene was eliminated
unchanged in the expired air and about 30% was excreted in urine as
water-soluble metabolites within 15 h. The half-life (t´) for
elimination from these tissues was 0.4-0.8 h, except in the case of
adipose tissue where elimination occurred with a t´ of 1.6 h. The
elimination of unchanged benzene in expired air was biphasic, the t´
being 0.7 h for the first phase and 13.1 h for the slower phase. Free
phenol, catechol and hydroquinone were detected in blood and bone
marrow after exposure ceased. The phenol level declined rapidly over
a 9-h observation period, whereas catechol and hydroquinone levels in
both tissues remained constant over this period (Rickert et al.,
1979).
After intraperitoneal injection, oral gavage, or inhalation
exposures of labelled benzene in rats and mice, over 95% of the
administered radioactivity was excreted within 40 h (Sabourin et al.,
1987; Henderson et al., 1989). Approximately 90% of the metabolites
was excreted in the urine. According to the authors, these studies
indicate that benzene is rapidly metabolized and excreted in the urine
within 40 h of dosing by any route of administration.
6.6 Reaction with body components
3H-benzene metabolites have been shown to bind irreversibly to
proteins in both mouse liver and bone marrow (Snyder et al., 1978a).
Benzene metabolites have also been shown to bind in vivo to mouse
protein in blood (Sun et al., 1990), liver, bone marrow and spleen
(Longacre et al., 1981a,b). Covalent binding increased both with dose
and frequency of dosing. Covalent binding of benzene metabolites to
protein appears to be mediated by microsomal enzymes (Tunek et al.,
1978) and has been suggested to be the result of binding by
hydroquinone and catechol (Wallin et al., 1985). The finding of high
levels of phenylcysteine adducts in the haemoglobin of benzene-exposed
rats suggests that benzene oxide also reacts with proteins to form
adducts (Bechtold et al., 1992). Benzene metabolism and covalent
binding to proteins have been demonstrated in situ in bone marrow
(Irons et al., 1980b).
Lutz (1979) has attempted to quantify the extent to which
chemicals covalently bind to DNA using the concept of the covalent
binding index (CBI). The implication is that the higher the CBI, the
more likely a chemical will be a carcinogen. The calculated value for
benzene, based on binding to liver nuclear DNA, was 1.7. (To put this
value in perspective, CBI values for some common carcinogens were:
aflatoxin B1, > 1000; 2-acetyl-aminifluorene, 100 to several hundred;
polycyclic aromatic hydrocarbons, 10 to 30.) No values were given for
benzene in bone marrow, but Snyder et al. (1978a) compared covalent
binding of benzene residues per g dry weight of liver and bone marrow
and found that, depending upon the dose of benzene, covalent residues
bound to liver ranged from 500 to 800 nmoles/g, whereas binding to
bone marrow ranged from 18 to 96 nmoles/g. Thus, there appeared to be
less covalent binding in the target organ, i.e. bone marrow, than in
the metabolizing organ, i.e. liver.
Inhaled benzene has been found to bind to rat liver DNA to the
extent of 2.38 µmoles/mole DNA phosphate (Lutz & Schlatter, 1977).
Studies of the covalent binding of benzene metabolites to DNA have
resulted in the postulation of several structures for DNA adducts
derived from benzene. Bone marrow mitochondria from rabbits were
incubated sequentially with 3H-deoxyguanosine triphosphate and
14C-benzene to evaluate DNA adducts formed from benzene metabolites
(Snyder et al., 1987b). These authors identified at least seven
deoxyguanosine adducts and one deoxyadenine adduct. Covalent
N-7-phenyl-guanine adducts have been isolated from rat urine after
intraperitoneal dosing with 330-400 mg benzene/kg body weight (Norpoth
et al., 1988). Thus, Jowa et al. (1990) postulated the formation of
an adduct between p-benzoquinone and deoxyguanosine which had the
structure (3'OH)benztheno(1,N2)deoxyguanosine. The structure of an
adduct formed between p-benzoquinone and deoxyadenosine-3'-phos
phate was suggested to be 3'-hydroxy-1,N6-benztheno-2'-deoxy-
adenosine-3'-phosphate (Pongracz & Bodell, 1991). Reddy et al.
(1990), however, reported that they were unable to detect DNA adducts
derived from benzene in the rat in vivo, despite having observed
them in Zymbal gland cells in vitro, using the 32P-post labelling
technique.
6.7 Modelling of pharmacokinetic data for benzene
In order to obtain better insight into the interspecies
variations in the uptake, metabolic fate and excretion of benzene and
its metabolites, both compartmentally (Bailer & Hoel, 1989; Beliles &
Totman, 1989) and physiologically based (Medinsky et al., 1989b,c;
Paxman & Rappaport, 1990; Travis et al., 1990; Bois et al., 1991a,b),
pharmacokinetic models have been developed. These models have been
used as an aid to risk assessment by facilitating extrapolation
between species where various exposure regimens had been utilized.
Also, such models are useful for identifying gaps in knowledge that
have been highlighted by poor fits of the experimental data to the
models developed.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
Benzene has been shown to produce a number of biological
responses in experimental animals. The acute effects of benzene at
high doses reflect its activity as a general anaesthetic and can lead
to central nervous system (CNS) depression, loss of consciousness and
coincidental sensitization of the myocardium to catecholamines.
Chronic exposure can result in bone marrow depression expressed as
leucopenia, anaemia and/or thrombocytopenia, leading to pancytopenia
and aplastic anaemia. The immunotoxic effects of benzene are probably
related to bone marrow depression. In animal cancer bioassays it is,
primarily, epithelial tumours that have been reported, whereas in
humans the carcinogenic response is leukaemia. A third type of
biological impact is the production of clastogenic responses such as
chromosome aberrations, sister chromatid exchange and micronuclei.
Benzene has also been suggested to produce fetotoxic effects.
7.1 Single exposure
Consistent with many other aromatic hydrocarbons (Patty, 1981),
benzene appears to be of low acute toxicity when administered to
various animal species by various routes of administration (Table 9).
Other reported oral LD50 values for reagent grade benzene in male
rats vary from as low as 930 mg/kg to as high as 5600 mg/kg body
weight (Wolf et al., 1956; Cornish & Ryan, 1965; Kimura et al., 1971;
Withey & Hall, 1975). The LD50 after intraperitoneal injection in
female rats was reported to be 2940 mg/kg (Drew & Fouts, 1974) and in
mice it was 300 mg/kg body weight (Kocsis et al., 1968). Young rats
are more sensitive (in terms of LD50) than older ones (Table 9).
The LC50 in female rats was estimated to be 43 770 mg/m3 (13
700 ppm) after a single 4-h exposure (Drew & Fouts, 1974).
Benzene has a narcotic effect after oral administration in rats
(Withey & Hall, 1975) and after inhalation in mice (Uyeki et al.,
1977). The threshold narcotic effect after inhalation has been
estimated to be approximately 13 000 mg/m3 (Leong, 1977).
Inhalation of air saturated with benzene resulted in ventricular
tachycardia and occasionally ventricular fibrillation and death in
rats, cats, rabbits and primates (Nahum & Hoff, 1934). Respiratory
failure was also observed during narcosis. Pathological findings after
sudden death are congestion of various organs, particularly the lungs
and liver (Jonek et al., 1965).
No information on the acute toxicity/lethality in animals after
dermal exposure has been reported.
Table 9. Toxicity of benzene in animals after acute exposure
Route Species Parameter Value Reference
Oral rat (14 days old) LD50 3000 mg/kg body weight Kimura et al. (1971)
Oral rat (young adult) LD50 3300 mg/kg body weight Kimura et al. (1971)
Oral rat (old adult) LD50 4900 mg/kg body weight HSE (1982); Kimura et al. (1971)
Oral rat LD50 8100 mg/kg body weight Cornish & Ryan (1965)
Inhalation (4 h) rat LC50 44 660 mg/m3 body weight Drew & Fouts (1974)
Inhalation (7 h) rat LC50 32 600 mg/m3 body weight HSE (1982)
Inhalation (2 h) mouse lethal dose 61 125 mg/m3 body weight Jonek et al. (1965)
Intraperitoneal injection rat LD50 2940 mg/kg body weight Drew & Fouts (1974)
Intraperitoneal injection mouse LD50 300 mg/kg body weight Kocsis et al. (1968)
7.2 Short-term and long-term exposures
The studies discussed in this section, some of which are
summarized in Table 10, have a duration of less than one year.
Lifetime (> 1 year) studies are discussed in section 7.6 and
summarized in Tables 15 to 17.
In short-term inhalation studies, three out of eight male rats
died within 24 h after exposure for five periods of 25-35 min to a
benzene concentration of 128 000 mg/m3 (40 000 ppm) and two out of
ten died after exposure for 12.5-30 min daily to 32 000 mg/m3
(10 000 ppm) for 1-12 days (Furnas & Hine, 1958).
Male and female rats and mice exposed to benzene vapour at
concentrations of 3.2, 32, 96 or 960 mg/m3 (1, 10, 30 or 300 ppm)
for 6 h/day, 5 days/week for 13 weeks, and sacrificed at various time
points during the study, showed no haematological effects up to 96
mg/m3 (30 ppm) (Ward et al., 1985). However, at 960 mg/m3 (300
ppm) mice exhibited significant decreases in haematocrit, haemoglobin,
erythrocyte count, leucocyte count, platelet count and the percentage
of lymphocytes. There was an increase in erythrocyte volume and mean
corpuscular haemoglobin. These changes were first observed on days 14
(males) or 28 (females). Most of the haematological effects were also
detected in rats but were of lesser severity. Compound-related
histopathological findings included myeloid hypoplasia, depletion of
the periarteriolar lymphoid sheaths in the spleen, lymphoid depletion
in the mesenteric lymph nodes, and increased extramedullary
haematopoiesis in the spleen. These lesions persisted throughout the
study and increased in severity with time. The only histopathological
lesion observed in rats was slightly reduced cellularity in the bone
marrow of the femur.
A dose-related increase in leucocyte alkaline phosphatase levels
and a decrease in leucocyte levels was observed in female rats exposed
via inhalation to 320, 960, 3200 or 9600 mg/m3 (100, 300, 1000 or
3000 ppm) for 7 or 14 days, but not in those exposed to 64 or 160
mg/m3 (20 or 50 ppm) (Li et al., 1986). In a study on male mice,
with doses of 3.5, 32, 330, 980, 1930, 4080, 7730 and 15 600 mg/m3
(1.1, 9.9, 103, 306, 603, 1276, 2416 and 4862 ppm), granulocytopenia,
lymphocytopenia and reduced bone marrow and splenic cellularity were
observed after exposure to > 330 mg/m3 for 5 h/day for 5 days but
not at lower levels (Green et al., 1981a). These authors found
splenic lesions at levels as low as 32 mg/m3 when exposure was
extended to 10 weeks.
Table 10. Toxicity of benzene in animals after short-term and long-term inhalation exposuresa
Species Dose (mg/m3) Exposure period Effects References
Rat 48 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
28 weeks at term 90 µg/litre
Rat 100 7 h/day; 5 days/week; no adverse effects, blood benzene level Deichmann et al. (1963)
7 weeks at term 290 µg/litre
Rat 150 7 h/day; 5 days/week; slight leucopenia, blood benzene level Deichmann et al. (1963)
32 weeks at term 420 µg/litre
Rat 3200 18 h/day; 7 days/week; reversible haemotological effects, Nau et al. (1966)
15 weeks reversible leucopenia within 6 months
Rat 3.2-960 6 h/day; 5 days/week; slight changes in haematological cell counts Ward et al. (1985)
13 weeks and lower cellularity in bone marrow of femur
Rat (female) 64-9600 1-2 weeks dose-related increase in leucocyte alkaline Li et al. (1986)
phosphatase and decreased leucocyte counts
at exposures of 960 mg/m3 or more
Mouse 3.5 to 15 600 6 h/day; 1 week doses > 330 mg/m3 resulted in granulocytopenia, Green et al. (1981a,b)
lymphocytopenia, decreased splenic cellularity
and decreased stem cell production
Mouse (male) 30.7 6 h/day; 5 days/week; some increase in spleen weight and some Green et al. (1981a)
10 weeks increased cellularity
Table 10 (contd).
Route Species Parameter Value Reference
Mouse (male) 960 6 h/day; 5 days/week; increased mortality, marked lymphocytopenia, Green et al. (1981a)
26 weeks reduced red blood cell count, reduced spleen
weight, many morphological abnormalities in
circulating red blood cells and neutrophils
Mouse (male) 32 6 h/day; 5 days/week; depression in the numbers of splenic nucleated Baarson et al. (1984)
25.5 weeks cells and of circulating red cells and lymphocytes
Mouse 3.2-960 6 h/day; 5 days/week; only at 960 mg/m3 decreased blood cell counts, Ward et al. (1985)
13 weeks haematocrit, myeloid hypoplasia, and numbers of
splenic and lymph node lesions
Mouse 32-1280 6 h/day; 5 days/week; reduced bone marrow cellularity and number of Cronkite et al. (1985)
pluripotent stem cells at 320 mg/m3 or more
Guinea-pig 280 7 h/day; 5 days/week; slight leucopenia, increased weight of kidneys Wolf et al. (1956)
4.5 weeks
Pig 64, 320 6 h/day; 5 days/week; leucopenia at 320 and 1600 mg/m3, reversible Johnston et al. (1979)
and 1600 3 weeks 9-16 weeks after exposure
a To avoid duplication, toxicity from life-time exposures (over 1 year) is discussed in section 7.6
(carcinogenicity) and Tables 15 to 17.
Cronkite et al. (1985) exposed male and female mice by inhalation
to benzene at concentrations of 32, 80, 320, 960 or 1280 mg/m3 (10,
25, 100, 300 or 400 ppm) for 2 weeks (6 h/day, 5 days per week). At
320 mg/m3 or more, reduced bone marrow cellularity and a decreased
number of pluripotent stem cells in bone marrow were reported. Under
similar conditions of exposure to 960 mg/m3 for 16 weeks, these
authors reported a lower level of stem cells in bone marrow, which
returned to 92% of control values after 25 weeks post-exposure.
Complete reversibility within 2 weeks was reported after 2 or 4 weeks
of exposure to 960 mg/m3.
Long-term (> 6 months) exposure studies at benzene levels above
approximately 160 mg/m3 (50 ppm) have shown effects on circulating
leucocytes (especially leucopenia). For example, rats exposed via
inhalation to 150 mg/m3 (47 ppm) (7 h/day, 5 days per week for 8
months) showed slight leucopenia, while those exposed to 100 mg/m3
(31 ppm) for 4 months and those exposed to 48 mg/m3 (15 ppm) failed
to show such changes (Deichmann et al., 1963). The lowest reported
exposure in animals that resulted in haematological effects was in
mice (Baarson et al., 1984). These authors reported that male mice
exposed via inhalation to 32 mg/m3 (10 ppm) (6 h/day, 5 days/week)
for 25.5 weeks showed a decrease in the number of circulating
erythrocytes and lymphocytes, a decrease in the number of nucleated
cells in the spleen and a depression of the in vitro colony-forming
ability of erythroid precursor cells (CFU-E).
Uyeki et al. (1977) demonstrated a depression of stem cell
activity in mice using the spleen colony-forming technique (CFU-S)
after exposure to a benzene concentration of 15 000 mg/m3 (4680 ppm)
for 3 days (8 h/day).
Haematotoxicity was also noted after oral exposure in rats and
mice. The animals were dosed by gavage with benzene in corn oil for
120 days at 25, 50, 100, 200, 400 or 600 mg/kg body weight (5
days/week). Five animals in the control, 200- and 600-mg/kg groups
were sacrificed at 60 days (Huff et al., 1989). A dose-related
leucopenia was observed in both male and female rats and lymphoid
depletion in the B-cells of the spleen was observed in both the 200-
and 600-mg/kg groups at 60 days. In mice no compound-related
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females.
No information was found on the haematotoxicity of benzene after
dermal exposure has been reported.
Additional studies on the effects of long-term exposure to
benzene in experimental animals are described in section 7.6
(carcinogenicity) and in Tables 15 to 17.
7.3 Skin and eye irritation
Benzene is considered a moderate eye irritant (as shown in the
rabbit eye test). Two drops of benzene caused moderate conjunctival
irritation and very slight, transient corneal injury (Wolf et al.,
1956).
Undiluted benzene was irritating to the skin (ear) of rabbits
after 10-20 applications (Wolf et al., 1956). Erythema, oedema,
exfoliation, blistering and moderate necrosis were observed after 20
applications.
There is no information available on the skin-sensitizing
potential of benzene. However, no such effect is expected based on
the experience with other aromatic hydrocarbons (GDCh, 1988).
7.4 Reproductive toxicity, embryotoxicity and teratogenicity
Benzene does not appear to be a potent reproductive toxin in
experimental animals. Guinea-pigs and rabbits exposed to benzene by
inhalation (7 h/day, 5 days/week) for up to 6 months showed variable
results; guinea-pigs showed a slight increase in testicular weight at
280 mg/m3 (88 ppm) and rabbits showed slight degeneration of the
seminiferous germinal epithelium at 256 mg/m3 (80 ppm) (Wolf et al.,
1956). Mice, but not rats, exposed to benzene vapour at a
concentration of 960 mg/m3 (300 ppm) (6 h/day, 5 days/week) for 13
weeks showed bilateral cysts in the ovaries and degeneration and
atrophy of the testes (Ward et al., 1985). At concentrations of 3.2,
32 or 96 mg/m3 (1, 10 or 30 ppm) these changes were also seen in
mice, but they were of doubtful biological significance (Ward et al.,
1985). There was a complete absence of litters in female rats exposed
to 670 mg/m3 (210 ppm) for 10-15 days before mating and for 3 weeks
after mating (Gofmekler, 1968). It is not known if this represents a
problem in mating and fertility, or one of maternal or fetal toxicity.
Lower exposures (1 to 64 mg/m3; 0.30 to 20 ppm) produced no such
effects.
Numerous studies on experimental animals have failed to detect
teratogenic effects, even at doses of benzene clearly toxic to the
dam. A few of these studies are summarized in Table 11. However,
benzene has been reported to be fetotoxic in mice, as shown by a
decrease in fetal weight and skeletal variants (missing sternebrae and
extra ribs) in the offspring of dams exposed to 1600 mg/m3 (500 ppm)
for 7 h on gestation days 6-15 (Murray et al., 1979). Similar effects
were seen in rabbits exposed on gestation days 6-18 to the same
levels. However, these effects are not usually considered to be
significant compound-related malformations (Kimmel & Wilson, 1973).
In mice, exposure to 500 or 1000 mg/m3 on gestation days 6-15
resulted in a decrease in fetal weight and an increase in dead or
resorbed fetuses, but no statistically significant increase in
malformations (Ungvary & Tatrai, 1985).
Table 11. Teratogenic effects of benzene in the mouse and rabbita
Animals Route Exposure level Maternal weight Fetal body Resorptions/ Skeletal Malformations References
gain weight fetal death/ variants
abortions
New Zealand inhalation 1600 mg/m3 (-) (-) (-) slightly ** (-) Murray et al.
rabbit (1979)
New Zealand inhalation 500 mg/m3 (-) (-) (-) (-) (-) Ungvary & Tatrai
rabbit 1000 mg/m3 * * **b ** ** (1985)
CF-1 mouse inhalation 1600 mg/m3 (-) * (-) ** (-) Murray et al.
(1979)
CFLP mouse inhalation 500 mg/m3 (-) * **c,d ** (-) Ungvary & Tatrai
1000 mg/m3 (-) * **c,d ** (-) (1985)
CD-1 mouse gavage 0.3 ml/kg body weight (-) * (-) (-) Nawrot &
0.5 ml/kg body weight (-) * **c,d (-) Staples (1979)
1.0 ml/kg body weight (-) * **c,d (-) (-)
a (-) indicates no significant difference from controls; * indicates decrease compared with controls;
** indicates increase compared with controls.
b Fetal death
c Abortions
d Resorptions
Table 12. Teratology studies in rats after inhalation of benzenea
Exposure level Exposure period Maternal weight Resorptions Fetal weight Skeletal variants Malformations Reference
(mg/m3) (h/day) gain
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
32 7 (-) (-) (-) (-) (-) Kuna & Kapp (1981)
32 6 (-) (-) (-) (-) (-) Coate et al. (1984)
128 6 (-) (-) (-) (-) (-) Coate et al. (1984)
150 24 * (-) * (-) (-) Tatrai et al. (1980a)
160 7 * (-) * ** (-) Kuna & Kapp (1981)
320 6 (-) (-) (-) (-) (-) Green et al. (1978)
320 6 (-) (-) * (-) (-) Coate et al. (1984)
400 24 * (-) * ** (-) Tatrai et al. (1980b)
450 24 * ** * ** (-) Tatrai et al. (1980a)
960 6 (-) (-) (-) ** (-) Green et al. (1978)
1000 24 * (-) * ** (-) Hudak & Ungvary (1978)
1500 24 * ** * ** (-) Tatrai et al. (1980a)
1600 7 * (-) * ** (-) Kuna & Kapp (1981)
3000 24 * ** * ** (-) Tatrai et al. (1980a)
7000 6 * (-) * ** (-) Green et al. (1978)
a (-) indicates no significant difference compared with controls; * indicates decrease compared with controls;
** indicates increase compared with controls
Several studies in rats show similar results to those in mice and
rabbits (Table 12). Maternal and fetal weight were decreased at
levels > 160 mg/m3 (> 50 ppm) as were the number of skeletal
variants observed. No malformations were noted in any of the studies
even at doses as high as 7100 mg/m3 (2200 ppm).
It is noteworthy that haematopoietic changes were observed in the
fetuses and offspring of mice exposed to 16, 32 or 64 mg/m3 (5, 10
or 20 ppm) for 6 h/day on gestation days 6-15 (Keller & Snyder, 1986).
The changes included a decrease in the number of erythroid
colony-forming cells (at all dose levels) and granulocytic
colony-forming cells at the two highest levels. When the offspring
were re-exposed to benzene as adults the decrease in these progenitor
cells was greater than in adult mice exposed to benzene at the same
levels for the first time.
7.5 Mutagenicity and related end-points
Benzene has been widely studied regarding the production of gene
mutations in in vitro tests, chromosomal effects both in vitro and
in vivo, and effects on DNA (binding, synthesis and damage). An
overview of the testing up to 1985 for the mutagenicity of benzene is
shown in Fig. 4 (IARC, 1987a). Detailed reviews have also been
published (Dean, 1978, 1985a; Huff et al., 1989; ATSDR, 1991),
therefore only some of the many studies are shown in Tables 13
( in vitro) and 14 ( in vivo).
7.5.1 In vitro studies
As shown in Table 13 and Fig. 4, benzene has consistently given
negative results in assays for point mutations in bacteria using
standard test conditions. In such studies, six tester strains have
been used with benzene concentrations ranging from 0.1 to 528 µg per
plate both with and without metabolic activation (HSE, 1982). Other
studies using doses as high as 880 mg/plate failed to cause mutations
in Salmonella typhimurium (Dean, 1978). In some 10 in vitro gene
mutation tests carried out in various human, mouse and Chinese hamster
cells, as part of an international collaborative study, mixed results
were obtained with benzene (Ashby et al., 1985; Venitt, 1985). When
S. typhimurium (strain TA1535) was incubated with benzene in a
desicator to enhance exposure, a doubling of revertants was noted at
10 ppm only in the presence of a post-mitochondrial activating system
(Glatt et al., 1989).
Figure 4. Tabular summary of in vitro and in vivo tests on benzene for mutagenicity and related end-pointsa
Non-mammalian systems Mammalian systems
In vitro In vivo
Prokaryotes Lower Plants Insects Animal cells Human cells Animals Humans
eukaryotes
D G R G A G R G C D G S M C A T D G S C S M C S C
+1 - + + +1 + +1 ? -1 + + - -1 + + + -1 +1 ? +1 + + + -1 +
a Adapted from: IARC (1987a)
A = aneuploidy; C = chromosomal aberrations; D = DNA damage; G = gene mutation; M = micronuclei; R = mitotic recombination and gene
conversion; S = sister chromatid exchange; T = cell transformation; + = considered to be positive for the specific end-point and
level of biological complexity; +1 = considered to be positive, but only one valid study was available to the Working Group;
- = considered to be negative; -1 = considered to be negative, but only one valid study was available to the Working Group; ? =
considered to be equivocal or inconclusive (e.g., there were contradictory results from different laboratories; there were confounding
exposures; the results were equivocal)
Table 13. Some in vitro genotoxicity studies of benzene
End-point Test system Resultsa References
Gene mutations
Ames test Salmonella typhimurium -/- De Flora et al.
-/+ (1984); Venitt (1985);
Glatt et al. (1989)
Azaguanine Salmonella typhimurium -/ Seixas et al.
resistance (1982)
TK test mouse L5178Y cells -/- Oberly et al. (1984)
TK, ouabain, total of 15 studies using mixedb Garner (1985)
HGPRT loci various human, mouse and
Chinese hamster cells
Chromosome abnormalities
Chromosome human lymphocytes mixed Gerner-Smidt &
aberrations Friedrich (1978);
Morimoto (1976)
total of 8 studies using mixed Dean (1985b)
Chinese hamster or human
cells
Sister Chinese hamster ovary and -/- Dean (1985b)
chromatid V79 cells and rat RL4
exchange cells
Table 13 (contd).
End-point Test system Resultsa References
Sister human lymphocytes mixed Morimoto (1983);
chromatid Morimoto et al.
exchange (1983); Erexson
et al. (1985)
Micronuclei Chinese hamster ovary -/- Douglas et al.
cells (1985)
Other effects
DNA breaks Rat hepatocytes ND/- Bradley (1985)
Chinese hamster V79 cells -/- Swenberg et al.
(1976)
Chinese hamster ovary -/- Douglas et al.
cells (1985)
Mouse L5178Y cells -/ Pellack-Walker &
Blumer (1986)
Unscheduled rat hepatocytes ND/- Probst & Hill
DNA synthesis (1985)
HeLa cells -/- Barrett (1985)
DNA synthesis Hela cells -/- Painter & Howard
inhibition (1982)
a Without/with an exogenous metabolic activation system; ND = no data
b The IPCS CSSTT working group disagreed over data analysis and therefore
called the results inconclusive
Whereas the results of in vitro studies for mutations by
benzene have been largely negative, there is some evidence that
treatment of human and animal cells in vitro with benzene can lead
to chromosomal abnormalities. However, as shown in Table 13, mixed
results have been obtained.
The ring-opened metabolite of benzene, trans,trans-muconal-dehyde
(MUC) has been tested for mutagenic and clastogenic activity in CHO
cells and Salmonella typhimurium bacteria, and for its effects on
DNA synthesis in primary rat liver hepatocytes (Witz et al., 1990a).
Only minimal mutational activity in bacteria was reported (in only
S. typhimurium strain TA97 of the five strains tested). However, at
a concentration of 0.4 to 0.8 µg/ml media, MUC resulted in a
dose-related increase in micronuclei in CHO cells. No effect on
unscheduled DNA synthesis or in the HG PRT assay in CHO cells was
reported. Using S. typhimurium (point mutations) and V79 cells
(sister chromatid exchange, acquisition of thioguanine or ouabain
resistance, and induction of micronuclei), 13 other potential benzene
metabolites were examined for genotoxicity. Each metabolite showed a
specific spectrum of activity, the highest genotoxic activity in most
systems being exhibited by quinone, hydroquinone, anti-diol epoxide
and catechol (Glatt et al., 1989).
The negative mutagenic data found in studies where benzene was
added to standard systems in vitro may well have been caused by the
technique used in these studies. Benzene is metabolically activated
to reactive metabolites by cytochrome P-450 IIE1, a natural
constituent of liver microsomes. In many of these studies
insufficient activation of benzene may have occurred. Post & Snyder
(1983) demonstrated that enzyme induction with benzene increased
benzene metabolism by increasing the activity of an enzyme having a
low Km and a high turnover rate for benzene, which is now thought to
be cytochrome P-450 IIE1. These authors also found that phenobarbital
induction reduced benzene metabolism until very high substrate
concentrations were reached. Chepiga et al. (1991), using purified
reconstituted cytochrome P-450, have shown that, whereas the Km
value for benzene as a substrate for cytochrome P-450 IIE1 is quite
low, much higher concentrations are required for benzene to be
metabolized by cytochrome P-450 IIB1, which also has a low affinity
for benzene. Thus, the lack of positive results in mutagenesis tests
involving benzene may have been due to the low activity of
benzene-activating enzymes in these preparations.
7.5.2 In vivo studies
No data are available on the production by benzene of gene
mutations in vivo. Benzene, or its metabolites, cause both
structural and numerical chromosome aberrations in humans (see chapter
8), laboratory animals and cells in culture (see section 7.6.1), as
well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes. Some in vivo studies are summarized in
Table 14.
Chromosomal changes occur after exposure of experimental animals
by the subcutaneous, oral, intraperitoneal or inhalation routes.
Philip & Krogh-Jensen (1970) administered 1750 mg benzene/kg body
weight subcutaneously to rats and noted an increase in chromatid
aberrations 12 and 24 h post-dosing but not after 36 h. This suggests
damage to S and/or G2 phase cells and a rapid elimination of the
alterations. In a study by Kissling & Speck (1972), the subcutaneous
administration of 1750 mg benzene/kg body weight to rabbits 3 times
weekly for 18 weeks led to tetraploidy in one animal as well as a high
percentage (58%) of bone marrow cells having chromosomal aberrations.
Siou et al. (1981) reported an increase in chromosome aberrations in
the bone marrow cells of mice treated orally with doses greater than
56 mg benzene/kg body weight on 2 successive days before sacrifice.
At high levels of benzene administered by inhalation (10 000
mg/m3 for 4 h), a marked increase in SCEs was noted in mouse bone
marrow cells (Tice et al., 1980). In a later experiment a significant
increase was reported in SCEs in mouse bone marrow cells when the
animals were exposed by inhalation to 91 mg/m3 for 4 h (Tice et al.,
1982). Erexson et al. (1986) reported a significant increase in the
levels of SCEs in peripheral lymphocytes after 6 h of exposure to 32
mg/m3 in mice and 9.6 mg/m3 in rats. At these levels, the
frequency of micronuclei in bone marrow smears was also increased.
After 6 weeks of exposure of mice (22 h/day, 7 days/week) at benzene
levels of between 0.128 and 3.2 mg/m3, increased chromosomal
aberrations in lymphocytes from the spleen were reported by Au et al.
(1991). These changes reached a significance of P = 0.05 only in
female mice (in males P = 0.15).
The frequency of micronuclei was increased in the bone marrow of
mice treated orally at doses ranging from 56 to 2200 mg/kg body weight
(Hite et al., 1980; Siou et al., 1981). A dose-related increase in
micronuclei in circulating erythrocytes was seen at 120 days in mice
treated by oral gavage with 25, 50, 100, 200, 400 or 600 mg benzene/kg
body weight. A significant increase was seen at all doses, with male
mice being more sensitive (Choy et al., 1985).
Table 14. Some mammalian in vivo genotoxicity studies on benzene
Route of Test system Results Exposure concentration Reference
administration and duration
Chromosome aberrations
Inhalation mouse bone marrow + 14 to 74 mg/m3, 7 days Zhurkov et al. (1983)
- 10 000 mg/m3, 4 h Tice et al. (1980)
- 9600 mg/m3, 4 h Tice et al. (1982)
+ 9600 mg/m3, 4 h, phenobarbital pretreatment Tice et al. (1982)
rat bone marrow + 3.2-3200 mg/m3, 6 h Styles & Richardson (1984)
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
+ 5-80 mg/kg body weight, daily for 2 days Zhurkov et al. (1983)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
bone marrow
Intraperitoneal rat bone marrow + 1 dose of 878 mg/kg body weight; 24 h prior to Anderson & Richardson
sacrifice (1981)
Micronuclei
Inhalation mouse lymphocytes + 32, 320 and 3200 mg/m3, 6 h Erexson et al. (1986)
mouse lymphocytes + > 67 mg/m3, 4-10 days Toft et al. (1982)
rat lymphocytes + 0.3 to 96 mg/m3, 6 h Erexson et al. (1986)
Table 14 (contd).
Route of Test system Results Exposure concentration Reference
administration and duration
Oral mouse bone marrow + 6 doses of between 9 and 2200 mg/kg, daily Siou et al. (1981)
for 2 days
mouse bone marrow + 440 mg/kg body weight, 2 doses, 24 h apart Gad El-Karim et al. (1984)
mouse bone marrow + 55 to 1760 mg/kg, daily for 2 days Hite et al. (1980)
mouse circulating + 26 to 440 mg/kg body weight, 5 days Barale et al. (1985)
erythrocytes + 25 to 600 mg/kg, 120 days Choy et al. (1985)
Chinese hamster - 2 doses of 2200 and 8800 mg/kg, daily for 2 days Siou et al. (1981)
Sperm head abnormality
Intraperitoneal mouse (spermatogonia + 88 to 880 mg/kg, daily for 5 days Topham (1980)
treated)
There were statistically significant alterations in sperm head
morphology after intraperitoneal doses of 88 to 880 mg/kg body weight
were administered to mice for 5 days and the sperm were examined 5
weeks later (Topham, 1980).
Witz et al. (1990a) reported that administration of
trans,trans-muconaldehyde, a microsomal metabolite of benzene, to
B6C3F1 mice (< 0.1-6.0 mg/kg body weight intraperitoneally)
resulted in the production of SCEs. The lowest dose producing a
significant increase was 3 mg/kg. No increase in the frequency of
micronuclei was reported.
There has been no clear demonstration of dominant lethal effects
in animals following benzene exposure. Fel'dt (1985) found no
significant dominant lethal effect in mice following oral
administration of up to 320 mg benzene/kg body weight. No dominant
lethal effect was reported in rats by Dean (1978) after the
intraperitoneal injection of 440 mg benzene/kg. However, Ciranni et
al. (1988) demonstrated the induction of micronuclei in the bone
marrow cells of pregnant mice and in fetal liver cells after a single
exposure to benzene or its metabolites.
7.6 Carcinogenicity
In several studies benzene has been shown to be carcinogenic in
experimental animals after exposure by inhalation and after oral
(gavage) dosing. These experiments are summarized in Tables 15-18.
As indicated in these Tables, several types of neoplasms have been
reported to be associated with exposure to benzene. Various types of
lymphomas/leukaemias have been found, but the majority of neoplasms
are of epithelial origin, i.e. Zymbal gland, liver, mammary gland and
the oro-nasal cavity. These results support the hypothesis that
benzene exposure in experimental animals can produce cancer at
multiple sites. A review of such studies has been published by Huff
et al. (1989).
7.6.1 Inhalation studies
The experimental design and major effects noted in several
inhalation cancer bioassays on benzene are summarized in Table 15.
Table 15. Inhalation studies on the carcinogenicity of benzene in experimental animals
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse AKR/J, dosed, 60; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 11 weeks; mean lifetime Snyder
males (8 weeks control, 60 5 days/week, lifetime for control group, 39 weeks, death due to aplastic et al.
old) (about 70 weeks) anaemia; bone marrow hypoplasia; no evidence for any (1978b)
tumours at autopsy in test animals
Mouse C57BL, dosed, 40; 960 mg/m3, 6 h/day, mean lifetime for dosed group, 41 weeks; mean lifetime Snyder
males (8 weeks control, 40 5 days/week, lifetime for control group, 75 weeks; anaemia, lymphocytopenia, et al.
old) (about 70 weeks) neutrophilic bone marrow hyperplasia; 6/40 lymphocytic (1980)
lymphoma with thymic involvement (P < 0.01), 1/40 plasma
cytoma, 1/40 haematocytoblastic leukaemia, 2/40 control
mice lymphocytic lymphoma without thymic involvement
Mouse AKR/J, dosed, 50; 320 mg/m3, 6 h/day, mean life-span for dosed group, 39 weeks; for control, Snyder
males (8 weeks control, 50 5 days/week, lifetime 47 weeks; anaemia, lymphocytopenia, neutrophilia, bone et al.
old) (about 70 weeks) marrow hyperplasia 10/49 dosed, 1/50 control (1980)
Mouse Charles number of 320 and 960 mg/m3, two mice of high-dose group developed myelogenous Snyder
river CD-1, animals 6 h/day, 5 days/week, leukaemia et al.
males unknown lifetime (1978b)
Mouse CD-1, dosed, 60; intermittent exposure greater mortality than with continuous exposure to 3480 Snyder
C57BL/6, male control, 60 at 960 mg/m3 for 1 mg/m3 for 10 weeks; elevated incidences of malignant et al.
for each week followed by 2 tumours in both strains; 35% incidence of Zymbal gland (1988)
strain weeks non-exposure; tumours in C57BL (control 0%), lung adenomas in CD-1
6 h/day, 5 days/week (26% versus 7% controls), and no significant increase
for lifetime in incidence of leukaemia or lymphomas
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Mouse CD-1, dosed, 80; short-term exposure to only CD-1 strain showed increased tumour incidence; 46%
C57BL/6, male control, 80 3840 mg/m3, 6 h/day, incidence of lung adenomas (control 24%) in addition to
for each 5 days/week for 10 other malignant and benign tumours; no increase in
strain weeks; observation for incidence of leukaemia or lymphoma; marked haematotoxicity
lifetime after cessation noted (anaemia and lymphocytopenia)
of exposure
Mouse C57BL/6, dosed, 118; control, 960 mg/m3, 6 h/day, 5 48 weeks after treatment survival rates were: dosed (D) Cronkite
female (7-9 116 (groups reduced days/week for 16 weeks; 80/90; control (C) 87/88; of the 10 exposed mice that et al.
weeks old) to 90 and 88 observation period for died, 6 had thymic lymphomas, 2 had unspecified lymphomas; (1985)
respectively for lifetime at end of study tumour incidence reported: leukaemia all
haemopoietic types (D) 20/89, (C) 8/88; lymphomas (thymic) (D) 10/89,
stem cell assays (C) 1/88; lymphomas (non-thymic) (D) 6/89, (C) 2/88
Rat Sprague- dosed, 45; 960 mg/m3, 6 h/day, no evidence of leukaemic response or pre-leukaemic Snyder
Dawley, male control, 25 5 days/week, for 99 effects nor for tumours incidence at any other site et al.
weeks (1978b)
Rat Sprague- dosed, 40; 320 mg/m3; 6 h/day, incidence of total and malignant tumours not significantly Snyder
Dawley, male control, 40 5 days/week, lifetime elevated over controls; several rare tumours in dosed et al.
(6 weeks old) (about 123 weeks) group, not in controls: 4 liver, 2 Zymbal gland and 1 (1984)
chronic myelogenous leukaemia
Rat Sprague- dosed, 70 male, 640 mg/m3; 4 h/day, at end of experiment (150 weeks) no information on survival; Maltoni
Dawley 59 female; control, 5 days/week for 7 weeks at 118 weeks survival rates of dosed (D) and control animals et al.
158 male, 149 female then 7 h/day for 8 (C) comparable; tumour incidence at 150 weeks; Zymbal (1983,
weeks; exposure in gland (D) 4/70 male, 1/59 female with (C) 2/158 male and 1985,
utero from day 12 of 0/149 female; oral cavity (D) 2/70 male, 6/59 female with 1989)
gestation through (C) 0/158 male and 0/149 female; leukaemias (D) 4/70 male,
lactation 4/58 female with (C) 12/158 male and 1/148 female;
hepatomas (D) 2/70 male, 5/59 female with (C) 1/158 male
and 0/149 female
Table 15 (contd).
Species Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- Breeders: dosed 640 mg/m3, 4 h/day, at termination (150 weeks) no information on survival; Maltoni
Dawley (breeders (D), 54; control 5 days/week, 7 weeks; at 118 weeks breeder (D) 5/54, (C) 11/60; offspring (D) et al.
13 weeks old) (C), 60 7 h/day, 5 days/week, male 11/75, (D) female 23/65 with (C) males 39/158 and (1983,
12 weeks; and then females 47/149; (C) breeders had 2/60 with malignant 1985,
960 mg/m3, 7 h/day, mammary tumours and (C) offspring had 3/158 and female 1989)
5 days/week, 85 weeks 8/149 malignant mammary tumours; male 1/158 and 0/149
with leukaemias; tumour incidence in (D) breeders was:
Offspring: dosed, Breeders: from day Zymbal gland carcinoma 3/54; oral cavity carcinoma 2/54;
75 male and 65 12 of gestation nasal carcinoma 1/54; malignant mammary tumours 6/54;
female; control: Offspring: in utero, hepatomas 1/54 and leukaemias 0/54; in offspring (D)
158 male and 149 through lactation male 6/75 and female 8/65 Zymbal gland carcinoma; male
female and for 104 weeks 1/75 and female 10/65 oral cavity carcinoma; male 1/75
and female 2/65 nasal cavity carcinoma; male 1/75 and
female 1/65 skin carcinoma; male 0/75 and female 3/65
forestomach carcinomas; male 0/75 and female 9/65
malignant mammary tumours; male 2/75 and female 7/65
hepatomas; and male 6/75 and female 0/65 leukaemias
Snyder et al. (1980) reported the development of malignant
lymphomas in mice after the exposure of male C57BL mice for about 70
weeks to 960 mg benzene/m3. Goldstein et al. (1982) exposed
Sprague-Dawley (SD) rats and three strains of mice (AKR, C57BL and
CD-1) to 320 and 960 mg/m3 for their lifetime and reported a small,
but not statistically significant, increase in the incidence of
granulocytic leukaemia in CD-1 mice (2 cases) and one case of chronic
myelogenous leukaemia in SD rats (these are rare neoplasms in these
strains). Snyder et al. (1984) in a subsequent full report of this
study, also noted increases in the incidence of liver tumours and
Zymbal gland carcinomas. The incidence of malignant lymphoma in male
AKR mice exposed to 320 mg/m3 was not significantly greater than
that in controls (Snyder et al., 1984). At about the same time
Maltoni et al. (1983) reported that Zymbal gland carcinomas were
observed in SD rats exposed to benzene (960 mg/m3) for 86 weeks. At
the end of the observation period (150 weeks), female breeder rats and
their offspring had not developed increased levels of leukaemia but
had an increased incidence of other tumours such as oral and nasal
cavity carcinomas, malignant mammary carcinomas and hepatomas (Maltoni
et al., 1982c, 1983, 1989).
Benzene-induced leukaemia in experimental animals has been
reported (Cronkite et al., 1984, 1985, 1989; Cronkite, 1986). In an
attempt to mimic more closely patterns of human exposure to benzene,
C57BL/6 and CBA/Ca mice were exposed to 960 mg/m3 (300 ppm) (6
h/day, 5 days/week) for 16 weeks, followed by an observation period of
82 weeks. A highly significant increase in leukaemia was noted in
C57BL/6 mice (Cronkite et al., 1984), and a biphasic response was
reported regarding mortality and lymphoma appearance (Cronkite et al.,
1985). The first increase in lymphomas was noted at about 150 days
post-exposure, and there was increased mortality between 330 and 390
days. A second increase in lymphomas as well as solid tumours
occurred at 420 days post-exposure, the mortality again increasing at
570 days post-exposure. Cronkite et al. (1989) reported that benzene
at a concentration of 960 mg/m3 for 76 weeks was leukaemogenic in
both male and female CBA/Ca mice.
Snyder et al. (1988) reported that benzene exposure patterns in
CD-1 and C57BL mice, which were closely related to the occupational
setting (intermittent for lifetime as well as short-term high doses
for a portion of the normal life span), resulted in marked
haematotoxicity as well as being tumorigenic. Neither of the benzene
exposure patterns induced elevated incidences of leukaemia/lymphoma in
either strain. Elevated incidences of malignant tumours (Zymbal gland
and lung) were noted in both strains after intermittent (1 week
exposure, 2 weeks non-exposure) exposure (960 mg/m3, 6 h/day, 5
days/week) over the full lifetime, whereas an increase in lung tumour
incidence was noted in only the CD-1 strain after 10 weeks of exposure
to 3840 mg/m3 (6 h/day, 5 day/week) followed by a lifetime of
non-exposure.
Table 16 presents a summary of the lowest dose levels at which
various authors reported a possible causal relationship between
benzene exposure and the end-point studied.
7.6.2 Oral and subcutaneous studies
Several experiments using oral (gavage) administration of benzene
to experimental animals are summarized in Table 17, and some of the
major effects reported are given. Oral exposure to benzene has
resulted in the induction of neoplasms in 13 different tissue/organs,
namely Zymbal gland, oral and nasal cavities, mammary gland, liver,
forestomach, skin, harderian gland, preputial gland and ovary, and the
haemopoietic and lympho-reticular systems (Maltoni et al., 1983, 1985,
1989; NTP, 1986; Huff et al., 1989). Table 17 indicates the
similarity in protocols used, namely 25-500 mg benzene/kg body weight
per day via gavage, 4 to 5 times weekly for 52-104 weeks and
termination after 103-144 weeks. The lowest dose of benzene that
produced specific neoplasms varied from 25 mg/kg body weight for the
adenomas of the lung, harderian gland and liver of mice to 500 mg/kg
body weight for lymphoreticular neoplasms in rats.
7.7 Special studies
7.7.1 Immunotoxicity
The proliferative ability of B- and T-cell lymphocytes was
depressed in a short-term (6 h/day for 6 days) dose-response study
(32, 96, 320 and 960 mg/m3) on benzene in mice (Rozen et al., 1984).
Liposaccharide-induced B-cell proliferation was depressed at levels as
low as 32 mg/m3 (the range of many occupational exposures), and
phytohaemagglutinin-induced T-cell response was depressed at 96
mg/m3. Peripheral lymphocyte counts were lower at all exposure
levels, but erythrocyte counts were depressed only at 320 and 960
mg/m3. In a subsequent study it was shown that a benzene
concentration of 960 mg/m3 (6 h/day, 5 days/week), administered for
115 days to mice, reduced the number of both B-cells in the spleen and
bone marrow and T-cells in the thymus and spleen and reduced their
response to mitogens (Rozen & Snyder, 1985). Other studies have shown
that polyhydroxylated derivatives of benzene are potent inhibitors of
T- and B-cell function in vitro (Irons et al., 1982).
Table 16. Carcinogenic-related end-points observed in animals exposed to benzene by inhalationa
End-points SD rat C57BL/6J mouse CD-1 mouse CBA/Ca mouse Reference
Lymphocytic lymphoma 960/488 days Snyder et al. (1980)
Myelogenous leukaemia (acute) 960/life Goldstein et al. (1982)
Myelogenous leukaemia (chronic) 320/life 960/life Goldstein et al. (1982)
Zymbal gland carcinoma 320/life Snyder et al. (1984)
960/lifeb 960/lifeb Snyder et al. (1988)
Hepatoma 640-960 Maltoni et al. (1982a)
960/16 Cronkite (1986)
Lung adenoma 3840/lifec Snyder et al. (1988)
Nasal carcinoma 640-960/104 Maltoni et al. (1983)
Thymic lymphoma 960/16 Cronkite et al. (1984)
Lymphoma (unspecified) 960/16 Cronkite et al. (1984)
Liver tumour 320/life Snyder et al. (1984)
Granulocytic leukaemia 320/life Snyder et al. (1984)
Leukaemia 960/16 Cronkite (1986)
a Doses are expressed in mg/m3 given 4-7 h/day, 5 days/week over a number of weeks or lifetime (e.g., 960 mg/m3/16
indicates that 960 mg/m3 was given for 16 weeks); exposures shown are the lowest for which the author claims
possible causal relationship.
b 960 mg/m3 dose intermittent, i.e. 1 week followed by 2 weeks non-exposure.
c Exposed to 3840 mg/m3 for 10 weeks followed by lifetime non-exposure.
Table 17. Long-term toxicity/carcinogenicity of benzene in experimental animals after oral administrationa
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat Sprague- high dose: 30 m, 50 or 250 mg/kg, only tumours reported in (C) were 4/30 f with malignant Maltoni et
Dawley, males & 35 f; low dose: 4-5 times/week mammary tumours and 1/30 with leukaemia; in dosed animals al. (1982b,
females (13 30 m, 30 f; for 52 weeks; Zymbal gland carcinoma in 2/30 f in low-dose group and 1983, 1985,
weeks old) controls: 30 m, death at 144 8/35 f in high-dose group; oral cavity carcinomas in 2/35 f 1989)
30 f weeks in high-dose group; malignant mammary tumours in 4/30 f in
low-dose group and 7/35 f in high-dose group; hepatomas in
1/35 m in high-dose group and leukaemias in 2/30 f of
low-dose group and 4/35 m and 1/35 f in high-dose group
Rat Sprague- dose: 40 m, 40 f; 500 mg/kg, 4-5 only tumours reported in (C) were 1/50 m with Zymbal gland Maltoni et
Dawley, males & control: 50 m, 50 f days/week for carcinomas; 1/50 skin carcinomas; 7/50 f malignant mammary al. (1982b,
females (6 weeks 104 weeks; tumours; 3/50 m hepatomas; 3/50 m and 1/50 f with 1983, 1985,
old) observed until leukaemias; in (D) following tumours reported: Zymbal
natural death gland carcinomas 18/40 m and 16/40 f; oral cavity
carcinomas 21/40 m and 20/40 f; nasal cavity carcinomas
3/40 m and 1/40 f; skin carcinomas 9/40 m; forestomach
acanthomas and dysplasias 10/40 m and 7/40 f; in situ
carcinomas (forestomach) 6/40 f; invasive carcinomas
(forestomach) 1/40 m; hepatomas 3/40 m and 1/40 f;
angiosarcomas 2/40 m and 3/40 f; leukaemias 1/40 m and
3/40 f
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Rat F344/N, dose groups: 60 m, all groups dosed number of survivors in control and dosed groups, NTP (1986);
males & females 60 f; control: 5 days/week for respectively; males: 32/50, 29/50, 24/50, 16/50; females: Huff et al.
(7-8 weeks old) 60 m, 60 f 103 weeks; males: 46/50, 38/50, 33/50, 25/50 (1989)
50, 100 or 200
mg/kg per day in control and dosed groups, respectively: Zymbal gland
females: 25, 50 or carcinomas, males: 2/50, 6/50, 10/50, 17/50; females:
100 mg/kg per day 0/50, 5/50, 5/50, 14/50
oral cavity squamous cell papilloma: m 1/50, 60/50, 11/50
and 13/50; f 1/50, 4/50, 8/50, 5/50
oral cavity squamous cell carcinoma: m 0/50, 3/50, 5/50,
70/50; f 0/50, 1/50, 4/50, 5/50
skin squamous cell papilloma: m 0/50, 2/50, 1/50, 5/50
skin squamous cell carcinoma: m 0/50, 5/50, 3/50, 8/50
Table 17 (contd).
Species/groups Number of animals Exposure concentration Effects Reference
and duration
Mouse B6C3F1, dose: 60 m, 60 f; males and females: numbers of survivors in control and dosed groups, NTP (1986)
male & females control: 60 m, 60 f 25, 50 or 100 mg/kg respectively: males: 28/50, 22/50, 18/50, 7/50; females:
(6-8 weeks old) per day, 5 days/week 30/50, 25/50, 24/50, 16/50
for 103 weeks
control and dosed groups, respectively: Zymbal gland
carcinomas, males: 0/49, 1/48, 4/50, 21/49; females: 0/49,
0/45, 1/50, 3/49
mammary carcinomas and carcinosarcomas occurred at higher
incidences in the mid- and/or high-dose groups; increased
tumour incidences in dosed mice were also noted elsewhere
including the haemopoietic system, adrenals, ovary, liver,
lung and preputial gland
a m = male animals, f = female animals, (C) = control groups, and (D) = dosed groups
The primary antibody response to fluid tetanus toxoid was reduced
by 74-89% in mice that were exposed to 1280 mg benzene/m3 (6 h/day)
for 5, 7 or 22 days of exposure (Stoner et al., 1981). No effect was
seen at 160 mg/m3. These investigators concluded that the threshold
level for repression of primary antibody response was between 160 and
640 mg/m3.
Host resistance to infection by Listeria monocytogenes in mice
was reduced after exposure to benzene (Rosenthal & Snyder, 1985). The
infection rate, as determined by bacterial counts in the spleen, was
increased by 730% on day 4 post-infection after exposure to 960
mg/m3 for 5 days, but not at lower benzene exposures. In contrast,
increased bacterial counts were seen at all doses of benzene greater
than 32 mg/m3 when benzene exposure was continued after exposure to
L. monocytogenes.
Both the humoral and cellular immune responses in CD-1 mice were
altered by oral administration of benzene at 8, 40 or 180 mg/kg body
weight daily for 4 weeks. A dose-response reduction in peripheral
blood lymphocytes was reported whereas there was no effect on the
levels of neutrophils and other white blood cells (Hsieh et al.,
1988a). A dose-related biphasic splenic lymphocyte proliferative
response to B- and T-cell mitogens was also reported. At the 8 mg/kg
dose the response was enhanced, while at the 40 and 180 mg/kg per day
doses a depression was observed. A similar biphasic response was
reported for cell-mediated immunity.
7.7.2 Neurotoxicity
The neurotoxicity of benzene in experimental animals has not been
well studied. Benzene caused light narcosis after 3 min of exposure
to a level of about 144 000 mg/m3 (45 000 ppm) in rabbits (Carpenter
et al., 1944). At this dose, tremors were noted after 5 min, loss of
pupillary reflex to strong light after 6 min, involuntary blinking
after 15 min, and death after 36 min. Learning defects have been
reported in rats exposed three times intraperitoneally to 550 mg
benzene/kg body weight on days 9, 11 and 13 postpartum (Geist et al.,
1983).
Male adult CD-1 mice received ( ad libitum for 4 weeks)
drinking-water containing 31, 166 and 790 mg benzene/litre (estimated
daily doses of 8, 40 and 180 mg/kg body weight). No treatment-related
behavioural changes were observed in the test animals. However, oral
ingestion of benzene was found to alter the levels of norepinephrine,
serotonin, dopamine and catecholamine in several brain regions (Hsieh
et al., 1988b).
7.8 Factors modifying toxicity
The toxicity of benzene can be modified by several factors,
including species or strain of animal exposed, dose received, and
patterns of exposure.
As shown in Figures 1-3 (section 6.3) benzene metabolism is
complex and involves several detoxification pathways, as well as two
pathways which form the putative metabolites muconaldehyde and
benzoquinone. Henderson et al. (1989) and Sabourin et al. (1987)
demonstrated species differences for the metabolism of benzene, i.e.
mice exhibited a higher rate of metabolism with the production of more
putative toxic metabolites. These authors also reported that
increasing the dose (by oral or inhalation routes) in both rats and
mice resulted in a higher proportion of benzene being metabolized by
detoxification pathways.
The myelotoxicity of benzene in mice is much more pronounced
following a discontinuous dosing regimen than following continuous
exposure (Tice et al., 1989). These effects of exposure route and
regimen suggest that the toxicity of benzene is dependent on cell
cycle kinetics in the bone marrow.
Evidence indicates that benzene must be metabolized prior to
producing adverse effects on the haemopoietic system or leading to
carcinogenic and clastogenic effects (Snyder et al., 1981; Irons,
1985). Therefore, agents or other factors that alter the metabolism
of benzene can also modify its toxicity.
Ethanol and benzene induce formation of cytochrome P-450 IIE1 in
rabbit and rats (Johansson & Ingelman-Sundberg, 1988). The toxicity
of benzene is enhanced by ethanol, increasing the severity of anaemia
induced by benzene, lymphocytopenia and reduction in bone marrow
cellularity (Baarson et al., 1982).
Phenobarbital that induces specific isoenzymes of P-450 increases
the rate of benzene metabolism in vivo in rats resulting in
increased resistance against the leucopenic action of benzene (Ikeda
& Ohtsuji, 1971; Nakajima et al., 1985).
Sabourin et al. (1990) found no evidence of the induction of
benzene metabolism by repeated exposure of rodents to benzene.
7.9 Mechanism of toxicity
It has become increasingly clear that the impact of benzene on
the bone marrow is conferred by a combination of metabolites, rather
than by a single metabolite. Thus, Eastmond et al. (1987) showed that
there was an interaction between phenol and hydroquinone when the two
were co-administered, which resulted in a degree of myelotoxicity
greater than additive. In mice, Snyder et al. (1989) reported that
whereas phenol alone did not decrease erythrocyte production,
hydroquinone, p-benzoquinone, and muconaldehyde were effective
inhibitors of red cell production. These authors also showed that
phenol interacted with hydroquinone to produce greater-than-expected
depression of erythrocyte synthesis. A similar interaction was
observed between phenol and catechol. The most striking interaction
was observed when doses of hydroquinone and muconaldehyde were
selected which were ineffective in inhibiting erythropoiesis when
given alone, but when given together produced cessation of red cell
production. Thus, bone marrow depression appears to be the result of
the combined effects of these metabolites. A further contributing
factor, however, is the finding by Roghani et al. (1987) and Da Silva
et al. (1989) that benzene stimulates the activity of membrane protein
kinase c, an important regulatory enzyme. The effect of its
perturbation may combine with the biological effects of the various
metabolites to yield the disease we term aplastic anaemia.
It is clear that aplastic anaemia requires that benzene be
metabolized to toxic metabolites. It also appears that metabolism is
required for the production of clastogenic responses. While it seems
likely that metabolism is important for the induction of tumours,
there is very little data on this point. One report, however,
suggests a role for benzene metabolites in one type of carcinogenic
response. Busby et al. (1990) explored the ability of several known
benzene metabolites, as well as postulated benzene metabolites, to
induce lung tumours in newborn mice. They examined the effectiveness
of benzene oxide and enantiomers and racemates of benzene dihydrodiols
and diol epoxides given orally using a prescribed regimen. Lung
tumour incidence and multiplicity were increased after treatment with
benzene oxide, racemates of dihydrodiol and by diol epoxide-2.
Benzene and diol epoxide-1 were inactive in this system.
Although it is well known that benzene produces bone marrow
damage resulting from the production of benzene metabolites in liver,
it is also well known that these metabolites do not produce
hepatotoxicity. From the mechanistic point of view, it appears that
the liver is protected against damage from quinone metabolites of
benzene by the enzymes DT-diaphorase (Smart & Zannoni, 1985) and
carbonyl reductase (Wermuth et al., 1986). These enzymes prevent the
metabolic activation of phenolic metabolites to their otherwise toxic
quinones. The bone marrow is relatively deficient in these enzymes,
but partial protection of the marrow has been afforded through the
administration of high doses of ascorbic acid (Smart & Zannoni, 1985).
Metabolic activation of benzene metabolites, once they reach the
bone marrow, may lead to eventual toxicity. For example, in bone
marrow stromal macrophages, phenol (but not benzene) can be
metabolized and in the process inhibit RNA synthesis in macrophages,
thus possibly inhibiting the production of the haemopoietic factor
(Post et al., 1985). It has also been suggested (Kalf et al., 1989)
that the cyclooxygenase component of prostaglandin synthetase plays a
significant role in the metabolism of benzene and/or its metabolites
in bone marrow. Administration of indomethacin or other
cyclooxygenase inhibitors protected against benzene-induced bone
marrow depression and micronucleus formation.
A general mechanism for benzene-induced bone marrow depression
might be that benzene metabolites arising in the liver travel to the
bone marrow where further metabolic activation occurs. The newly
generated metabolites, perhaps acting in concert with unmetabolized
benzene in cell membranes, act upon target cells such as stem cells,
progenitor cells and stromal cells in the marrow to produce bone
marrow depression. Chromosomal damage may ensue, which is reflected
in clastogenesis observed in circulating lymphocytes or bone marrow
cells. The point in this series of events that leads to a
leukaemogenic response requires further examination once an adequate
model for the disease in animals has been established.
8. EFFECTS ON HUMANS
Acute inhalation and oral exposures of humans to high
concentrations of benzene have resulted in central nervous system
depression and death. The most noted effects resulting from
longer-term exposure to lower levels of benzene are haematotoxicity,
immunotoxicity and neoplasia.
8.1 General population and occupational exposure
The human health effects after exposure to benzene are
qualitatively the same for the general population and those exposed in
the workplace. To avoid duplication, the effects on both groups
(general population and workers) will be discussed together, with
emphasis on exposure levels and duration of exposure. The
quantitative response will be determined from such levels of total
daily intake.
8.1.1 Acute toxicity
Exposures in the general population that result in acute toxic
effects are usually related to accidents and misuse or abuse of
benzene. Many deaths and serious health effects have resulted from
benzene exposures after deliberate the "sniffing" of glue and other
products which contain benzene as a solvent (Winek & Collom, 1971).
Blood levels in people who have died as a result of "sniffing" glue
have ranged from 0.94 to 65 mg/litre (Winek et al., 1967; Winek &
Collom, 1971). Autopsy observations in these individuals included
pulmonary haemorrhage and inflammation, renal congestion and cerebral
oedema.
It has been estimated that exposure to benzene concentrations of
about 64 000 mg/m3 (20 000 ppm) for 5-10 min can result in
fatalities, 24 000 mg/m3 (7500 ppm) for 30 min is dangerous to life,
4800 mg/m3 (1500 ppm) for 60 min causes serious symptoms, 1600
mg/m3 (500 ppm) for 60 min leads to symptoms of illness, and 160-480
mg/m3 (50-150 ppm) for 5 h causes headache, lassitude, and weakness,
while 80 mg/m3 (25 ppm) for 8 h is without clinical effect (Gerarde,
1960). The clinical signs of acute toxicity from benzene include CNS
depression, cardiac arrhythmia, and eventually asphyxiation and
respiratory failure if exposures are at the lethal level (Andrews &
Snyder, 1986). Mild CNS symptoms are rapidly reversible following
cessation of exposure and there is no evidence that they result in
neurological brain damage (Marcus, 1990).
The single acute oral lethal dose in humans has been estimated to
be 10 ml of benzene (8.8 g) (Thienes & Haley, 1972). Clinical signs
of toxicity after acute oral exposure include staggering gait,
vomiting, shallow and rapid pulse, somnolence, loss of consciousness,
delirium, pneumonitis, profound CNS depression, and collapse
(Sandmeyer, 1981). High but sublethal oral doses may produce one or
more of the following symptoms: dizziness, visual disturbances,
euphoria, excitation, pallor, flushing, breathlessness and
constriction of the chest, headache, fatigue, sleepiness, and fear of
impending death (Sandmeyer, 1981). In addition to the autopsy
findings noted above, ingestion of benzene has been reported to cause
gastrointestinal ulceration (Appuhn & Goldeck, 1957).
No studies on the acute toxicity of benzene after dermal exposure
are available.
8.1.2 Effects of short- and long-term exposures
The most significant health effects from short- or long-term
exposure to benzene are haematotoxicity, immunotoxicity, neurotoxicity
and carcinogenicity. Three types of bone marrow effects have been
reported in response to benzene exposure; these are bone marrow
depression leading to aplastic anaemia, chromosomal changes and
carcinogenicity.
8.1.2.1 Bone marrow depression; aplastic anaemia
Several types of blood dyscrasias, including pancytopenia,
aplastic anaemia, thrombocytopenia, granulocytopenia, lymphocytopenia
and leukaemia, have been noted after exposure to benzene. These
changes are a continuum and not a discrete disease entity. Which
effect is noted will depend on the dose, length of exposure and the
stage of stem cell development affected (Galton, 1986). As in
experimental animals, the primary target organ of benzene that results
in haematological changes is the bone marrow. It has been suggested
that the cells at highest risk are the rapidly proliferating stem
cells (Marcus, 1990).
A study of 32 patients that were chronically exposed by
inhalation to benzene levels of 480-2100 mg/m3 (150-650 ppm) for 4
months to 15 years revealed pancytopenia with hypoplastic,
hyperplastic or normoblastic bone marrow. Eight of the 32 individuals
showed thrombocytopenia which resulted in haemorrhage and infection
(Aksoy et al., 1972). Haematotoxicity after prolonged exposure has
also been reported in rotogravure workers exposed for 6-60 months at
concentrations of 36-3485 mg/m3 (11-1069 ppm) (Goldwater, 1941) and
77-3400 mg/m3 (24-1060 ppm) (Erf & Rhoads, 1939), shoe factory
workers exposed to 96-670 mg/m3 (30-210 ppm) for 3 months to 17
years (Aksoy et al., 1971), and rubber factory workers exposed to up
to 1600 mg/m3 (500 ppm) (Wilson, 1942). Kipen et al. (1988)
reported on rubber workers who were exposed to benzene during the
1940s. An inverse relationship was found between the mean yearly
white blood cell count and the year that the count was made,
suggesting that exposures to benzene were very high in the early
1940s. As estimated by Crump & Allen (1984), benzene exposures
decreased from 438 mg/m3 (137 ppm) in 1940 to 102 mg/m3 (32 ppm)
in 1948. In a follow-up letter, Hornung et al. (1989) pointed out
that a similar rise in white blood cells counts was seen in
pre-employment physical examinations occurring over the same time
period at the same facility, and that that the trend could not be
attributed soley to benzene exposure. In a study of 1008 male
shoemakers in Florence, excess mortality from aplastic anaemia was
observed (SMR = 1566, 95% CI 547-3264), based on 6 deaths. All cases
of aplastic anaemia occurred among workers first employed before 1964
when the level of exposure to benzene was assumed to be highest (Paci
et al., 1989).
At levels less than 32 mg/m3 (10 ppm) no haematologic effects
have been observed (Collins et al., 1991). These authors found no
haematological effects in 200 benzene-exposed workers (10 year TWA of
0.03-4.5 mg/m3, 0.01-1.4 ppm) or in 268 control workers in the same
plant. In an earlier study of 70 workers in a coke oven by-product
recovery facility, Hancock et al. (1984) measured the levels of red
blood cells, white blood cells and haemoglobin in three groups exposed
to different concentrations of benzene (average, 34 mg/m3, 10.5 ppm;
range, 3.2-534 mg/m3, 1-167 ppm) and one non-exposed control group.
No significant differences between groups were noted in these
haematological parameters.
No data are available regarding haematotoxicity after short-term
or chronic oral or dermal exposure.
8.1.2.2 Immunological effects
As noted in animal studies (section 7.7.1), the immunological
manifestations of benzene toxicity are related to effects on the bone
marrow, resulting in changes to both humoral and cellular acquired
immunity. Workers (76) exposed to benzene (10-22 mg/m3, 3-7 ppm),
as well as to toluene and xylenes, for periods of 1-21 years were
examined for the presence of leucocyte agglutinins and levels of
circulating immunoglobulins. In 10 out of 35 workers where blood was
taken during working hours, the adverse effect of agglutinins reacting
with autoleucocytes was noted (Lange et al., 1973a). In addition, it
was found that the sera from the 35 workers had increased levels of
IgM and decreased levels of IgG and IgA immunoglobulins (Lange et al.,
1973b). The simultaneous exposure of these workers to solvents other
than benzene makes it difficult to interpret these results.
Autoimmunity, as shown by the pressure of antibodies against
leucocytes, platelets, and erythrocytes in the sera of exposed
workers, has been reported (Renova, 1962). Workers have been reported
to have an increased susceptibility to allergies (Aksoy et al., 1971)
when exposed to benzene concentrations as low as 96 mg/m3 (30 ppm).
A loss of leucocytes was observed in several studies of workers
reported to be exposed to benzene levels of 96-2080 mg/m3 (30-650
ppm) (Aksoy et al., 1971, 1974a; Aksoy, 1987). Signs of preleukaemia,
including loss of leucocytes and other blood elements and enlarged
spleens, were reported in one study (Aksoy et al., 1974a). Kipen et
al. (1989) and Yin et al. (1987) also reported decrease in circulating
lymphocytes and other blood elements at benzene exposures ranging from
48 to 240 mg/m3 (15-75 ppm). The number of T-cell lymphocytes were
found to have been reduced in workers exposed chronically to benzene,
toluene and xylene (Moszczynski, 1981).
In a study of workers exposed to low average concentrations of
benzene (< 32 mg/m3), there was no difference in cell cycle
kinetics of phytohaemagglutinin-stimulated lymphocytes in 66 male
workers of a refinery population when compared with 33 control workers
in the same refinery (Yardley-Jones et al., 1988).
No studies are available regarding the immunotoxicity of benzene
in humans after oral or dermal exposures.
8.1.2.3 Chromosomal effects
Both structural and numerical chromosomal aberrations have been
observed fairly consistently in the lymphocytes and bone marrow cells
of individuals occupationally exposed to benzene. It is now generally
accepted that benzene is a human clastogen (IARC, 1987a; Huff et al.,
1989). Increases in the number of both unstable and stable
chromosomal aberrations were observed in men, even 2 years after
cessation of workplace exposure (Tough & Court Brown, 1965). Up to
70% aneuploid lymphocytes were found in five women with benzene
haemopathy (Pollini et al., 1969), the effects still being
demonstrable 5 years post-exposure. Similar effects were observed in
the lymphocytes of workers in a rotogravure plant that had been
exposed to very high levels of benzene 400-1700 mg/m3 (125-532 ppm)
for 1-22 years (Forni et al., 1971a,b).
Recent studies by Yardley-Jones et al. (1988, 1990) revealed much
lower responses in the lymphocytes of workers exposed to low
concentrations of benzene (average < 32 mg/m3). In a study of 66
refinery workers and 33 controls, no alteration in cell cycle kinetics
was noted nor was there any increase in the level of SCEs
(Yardley-Jones et al., 1988). The lymphocytes from 48 of the workers
and 29 of the controls were analysed for chromosomal aberrations.
According to Yardley-Jones et al. (1990), the increase in aberrations
(particularly chromatid deletions and gaps) was of borderline
significance in parametric statistical tests, but was significant
using Fisher's exact test. No lifestyle factors had any consistent
effect on the incidence of chromosomal aberrations.
In an attempt to determine whether benzene and its metabolites
damage certain human chromosomes preferentially, Sasiadek et al.
(1989) examined the karyotypes of 33 workers exposed to less than 99
mg/m3 (31 ppm). At these levels no clinical or haematological
symptoms were noted in 31 workers, but pancytopenia was observed in
two workers. Nonrandom breaks and gaps were observed in the exposed
group; chromosomes two, four and nine were more prone to breaks and
chromosomes one and two more prone to gaps. The results of this study
are of limited value in view of the small number of controls and the
fact that all participants smoked.
Other studies that corroborate the clastogenicity of benzene in
humans have been reviewed by IARC (1982), Dean (1985a) and Kalf
(1987).
8.1.2.4 Carcinogenic effects
The fact that benzene is a human leukaemogen has been well
established by epidemiological and case studies (IARC 1982, 1987b),
most of which have dealt with industrial exposures. The
epidemiological studies reported have been selected because they
contain sufficient quantitative data on exposure and effects to permit
a discussion of the dose-response relationship. Some case reports are
summarized in Table 18, and prospective epidemiological studies are
summarized in Table 19. Of the two major classes of leukaemia
(granulocytic and lymphocytic), the most consistent evidence for a
causal relationship in humans has been found between benzene exposure
and myeloid leukaemia (Goldstein, 1988).
One case study followed the course of 44 patients with
benzene-induced pancytopenia and found that 6 of them later developed
leukaemia (Aksoy & Erdem, 1978).
The first bridge between case reports and a formal
epidemiological investigation was conducted in the early 1970s (Aksoy
et al., 1974b). These investigators reported on a series of cases
from an estimated population of 28 500 Turkish shoe workers exposed
since the 1950s to solvents and adhesives containing high levels of
benzene. Aplastic anaemia was first observed in 1961, and 26 patients
with acute leukaemia were observed by 1967. Peak exposure levels of
benzene were reported to be 96-670 mg/m3 (30-210 ppm), with rare
excursions to 2100 mg/m3 (650 ppm), for periods of 1 to 14 years
(mean 9.7 years). From this group of workers, a leukaemia incidence
rate (number of cases per 100 000 per year) of 13 was estimated
compared with a rate of 6 for the general population (Aksoy et al.,
1974b). It was unclear to the Task Group, from the description of the
authors, which methods were used to ascertain cases and from which
exposed population these cases were derived.
A study showing an excess risk of leukaemia in a cohort of 748
male workers producing "rubber hydrochloride" in three plants in two
locations within the USA during 1940-1949 (a product made from natural
rubber suspended in benzene) was first reported by Infante et al.
(1977). A follow-up study of this cohort was reported by Rinsky et
al. (1981) and this was subsequently updated (Rinsky et al., 1987).
In this most recent follow-up, the cohort definition was expanded to
include workers employed between 1940 and 1965 who had a cumulative
exposure to benzene of 3.2 mg/m3 per day (1 ppm/day) or more. The
Table 18. Case studies of workers occupationally exposed to benzene
Group studied Exposure Condition observed Author's References
conclusiona
44 pancytopenic patients exposed to benzene 480-2100 mg/m3 (150-650 leukaemia PC Aksoy & Erdem
in adhesives ppm); 4 months to 15 myeloid metaplasia * (1978)
years
42 leukaemia patients and 21 patients with not given leukaemia DC Aksoy (1980)
other malignancies; 47 were shoe workers, the multiple myeloma PC
remainder in other occupations using benzene myeloblastic leukaemia *
solvents acute erythroleukaemia *
preleukaemia *
malignant lymphoma PC
paroxymal nocturnal
haematuria *
lung cancer (all heavy
smokers) *
Table 18 (contd).
Group studied Exposure Condition observed Author's References
conclusiona
6 of 94 Hodgkin's patients who had been 480670 mg/m3 (150-210 Hodgekin's disease PC Aksoy et al.
exposed to benzene adhesives ppm); 1-28 years (1974a)
A 35-year-old man who had used benzene 200-1640 mg/m3; 18 months subacute granulocytic DC Sellyei & Kelemen
8 years earlier as a paint solvent leukaemia (1971)
6 leukaemia patients in different occupations levels unknown; 1-20 years haemocytoblastic leukaemia DC Vigliani & Saita
all using benzene solvents (1964)
A 51-year-old chemical worker exposed to 3.2 mg/m3 (< 2 ppm); acute myelogenous PC Ott et 81. (1978)
benzene 15 years earlier 18 months leukemia
a DC = direct correlation; PC = possible correlation; * = no conclusion made
Table 19. Epidemiological studies of workers exposed to benzene
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Incidence of leukaemia in 96-670 mg/m3 aplastic anaemia, 26 200 NR Aksoy et al.
Turkish shoe workers 1950-1965 rarely 2100 mg/m3; acute leukaemia (1974b)
(28 500 shoe, slipper and 1-15 years (mean,
handbag workers) 9.7 years)
Mortality study of rubber within legal limits of malignomas of lymphatic 14 260 NR Infante et
workers exposed to benzene period, i.e. 320 mg/m3 and haemopoietic P < 0.05 al. (1977)
between 1940 and 1949 (100 ppm) down to systems; myeloid and
32 mg/m3 (10 ppm) monocytic leukaemia 7 506 NR
for up to 10 years P < 0,02
Mortality studyb of pliofilm from < 40 ppm-years lymphatic and haemopoietic 15 227 127-376 Rinskyetal.
workers exposed to benzene to > 400 ppm-years neoplasms (1987)
between 1940 and 1965 with
a period at risk from 1950 leukaemia, total 9 337 159-641
to 1981 < 40 ppm-years 2 109 12-394
40-200 ppm-years 2 322 36-1165
205-400 ppm-years 2 1186 133-4289
> 400 ppm-years 3 6637 1334-19 393
multiple myeloma total 4 398 110-1047
< 40 ppm-years 3 458 92-1339
> 40 ppm-years 1 5347 70-29 753
Mortality studyc of 956 > 0.3-114 mg/m3 leukaemia (total) 4 194 52-488 Bond et al.
workers employed at a chemical (> 0.1-35.5 ppm) acute myelogenous 4 444 P < 0.05 (198613)
company between 1940 and estimated TWA for leukaemia
1982 up to 34 years
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective mortality study < 3.2 mg/m3 leukaemia 0 0 Tsai et al.
of workers (454) employed at (< 1 ppm) in 84% (1983)
a Texas refinery between 1952 samples (median,
and 1981 1.6 mg/m3; 0.5 ppm)
in benzene-related
areas
Mortality study of chemical < 15, 15-60 and > 60 lymphatic and haemopoletic, Wong
workers in 7 plants, > 6 ppm-years total: (1987)
months on job at single plant non-exposed 3 39 7-101
between 1946 and 1975 < 15 ppm-years 5 91 30-213
(3636 males) 15-60 ppm-years 5 147 48-343
60 ppm-years 5 179 57-409
leukaemia, total:
non-exposed 0 -
< 15 ppm-years 97 12-349
15-60 ppm-years 78 2-434
60 ppm-years 276 57-806
Retrospective cohort of 259 no benzene levels lymphatic and haemopoietic 4 377 109-1024 Decouflé et
male chemical workers reported; benzene neoplasms (workers with al. (1983)
employed between 1947 and used in large > 1 year of employment)
1960 quantities
Table 19 (contd).
Group studied Exposure Condition observed Number of SMRa 95% CI References
observed deaths
Retrospective cohort of shoe exposed for up to 29 aplastic anaemia (males); 4 1566 P < 0.05 Paci et al.
workers (1008 males, 1005 years; levels of leukaemia (males) 6 400 146-870 (1989)
women) employed between benzene not reported
1939 and December 1984 and
still employed in plant in
January 1950
Retrospective cohort study grab samples; means acute and chronic 30 cases 574 P < 0.01 Yin et al.
(28 460 workers in 233 factories); between 10 and 1000 leukaemia 5 deaths (1987)
reference population 28 257 mg/m3
in 83 machine production,
and clothing factories 50-500 mg/m3 found in
most plants
Mortality study of coke plant non-exposed, coke leukaemia (non-exposed) 13 135 NR Swaen et al.
workers (5639) with > 6 months ovens and by-product leukaemia (coke-oven) 6 163 NR (1991)
work between 1945 and 1969 workers; levels of leukaemia (by-product) 7 85 NR
benzene not reported
Retrospective cohort of 391 mean time-weighted leukaemia Hurley et
benzole workers in 2 cohorts average exposure of by-product 1 1 98 2-557 al. (1991)
(cohort 1, 84 workers; coke by-product by product 2 1 76 2-429
cohort 2, 307 workers) workers in Britain
in 1980s 4.2 mg/m3
(1.3 ppm)
a SMR - Standard mortality ratio
b Follow-up of cohort described by Infante et al. (1977)
c Follow-up of cohort described by Ott et al. (1978)
NR = not reported
study included 1165 white males followed from 1950 to 1981. For the
analysis the cohort was divided into four cumulative exposure groups:
< 128 mg/m3-years (< 40 ppm-years), 128-640 mg/m3-years (40-200
ppm-years), 640-1280 mg/m3-years (200-400 ppm-years) and > 1280
mg/m3-years (> 400 ppm-years). A statistically significant excess
risk was observed for all lymphatic and haemopoietic neoplasms (15
observed deaths compared to 6.6 expected; SMR = 227, 95% CI, 127-376).
There were nine deaths from leukaemia compared to an expected 2.66
(SMR = 337, 95% CI, 159-641), and 4 deaths from multiple myeloma (SMR
= 398, 95% CI, 110-1047). A strong positive trend in leukaemia
mortality was obtained with increasing cumulative exposure. Within
the four cumulative exposure groups there were 2,2,2, and 3 deaths
with SMRs of 109, 322, 1186 and 6637, respectively. In order to
investigate further the shape of the exposure-response curve, Rinsky
et al. (1987) conducted a nested case-control study by matching each
of nine deaths due to leukaemia with ten controls. A conditional
logistic regression analysis described a significant positive
association between estimated level and average duration of benzene
exposure and leukaemia that was projected downward to levels of zero
accumulated exposure over a working lifetime. From this model it was
calculated that an exponential relationship existed between benzene
exposure and the developmental leukaemia.
When actual exposure measurements did not exist, Rinsky et al.
(1987) estimated exposures to benzene by averaging historical annual
measured benzene levels from seven existing industrial hygiene survey
sources. The majority of measurements occurred after 1963, but some
data existed as early as 1946. Where no sampling data could be found,
exposure levels were estimated by interpolation from existing
information. Alterative exposure estimates and subsequent reanalyses
have been developed by Crump & Allen (1984) and Paustenbach et al.
(1992). The differences in exposure estimates between Crump & Allen
(1984) and Rinsky et al. (1987) centre primarily on assumptions of
benzene exposure prior to 1946 where no historical data exist.
Paustenbach et al. (1992) gathered additional information and
considered other factors that modified the estimates of exposure over
the entire period during which rubber hydrochloride plants operated
(1936 to 1976) to develop a new set of exposures over time. For the
most part the exposures estimated by Paustenbach et al. (1992) are
higher than those reported by Rinsky et al. (1987) and Crump & Allen
(1984).
In a retrospective study of 594 employees of a chemical company
exposed to levels of benzene between 0.3 and 114 mg/m3 (0.1-35.5
ppm) for up to 34 years, no statistically significant increase in
total mortality was reported (Ott et al., 1978). There were three
cases of myelocytic leukaemia compared to an expected incidence of 0.8
cases (significant at the P < 0.05 level). Some workers in this
cohort were also exposed to vinyl chloride, arsenicals and several
other potentially carcinogenic chemicals. A follow-up study of these
workers expanded the cohort by 362 potentially exposed workers (Bond
et al., 1986b). Four deaths from myelogenous leukaemia were reported
and the SMR for all leukaemias was 194 (95% CI, 52-488). The
difference between observed and expected values was statistically
significant only when myelogenous leukaemia was considered (4
observed, 0.9 expected; P = 0.01).
Wong (1987) reported a significant dose-response relationship
between cumulative exposure to benzene and mortality from leukaemia
and all lymphopoietic cancers combined. The mortality experience of
3536 workers who had continuous exposure to benzene was compared to
that of an internal comparison group of 3074 workers not exposed to
benzene but who had worked at the same plant. The 3536 exposed
workers were categorized into cumulative exposure categories of < 48
mg/m3-years (< 15 ppm-years), 48 to 192 mg/m3-years (15 to 60
ppm-years), and > 192 mg/m3-years (> 60 ppm-years). There was an
increasing trend in the SMRs for lymphatic and haemopoietic cancers as
exposure increased (SMR = 35, 91, 147 and 175 for the non-exposed and
the 3 exposure categories, respectively (P = 0.02). The respective
SMRs for leukaemia were 0, 97, 78 and 275 (P = 0.01). It should be
noted that none of the six leukaemia deaths was from acute myeloid
leukaemia. In addition, the highest category of exposure started at
only 192 mg/m3-years (60 ppm-years), the equivalent of 32 mg/m3
(10 ppm) annually for only a six-year working career. The
exposure-response relationship between cumulative benzene exposure and
non-Hodgkin's lymphoma was of marginal statistical significance.
A retrospective cohort mortality study was conducted on 259 male
employees at a chemical plant in the USA where benzene had been used
in large quantities (Decouflé et al., 1983). The study group included
workers employed between 1947 and 1960, and workers were followed
until 1977. Among workers with more than one year of employment, a
statistically significant excess risk was observed for neoplasms of
the lymphatic and haemopoietic systems (SMR=377; 95% CI 109-1024, 4
deaths). No SMR was given specifically for mortalities to leukaemia
and multiple myeloma. Three of these deaths were leukaemias and the
fourth a multiple myeloma.
Coke-oven workers and some workers at coke by-product plants are
exposed to low levels of benzene. A study in the Netherlands examined
5639 workers in a coke plant and a comparison group of 5740 workers in
a nitrogen-fixation plant (Swaen et al., 1991), employed for at least
six months between 1945 and 1965. The SMR for leukaemia in by-product
benzene plant workers was 85 (7 deaths), in coke-oven workers 163 (6
deaths), and in non-exposed workers 135 (13 deaths). Among the 222
workers in the benzene plant, no indication of an increased leukaemia
risk was found, but the expected number was small.
Hurley et al. (1991) have reported preliminary results on the
mortality of 6520 male coke plant workers from 27 plants in the United
Kingdom. Personal air samples were taken from 84 benzole workers from
14 plants in one cohort with levels of < 0.6-22 mg/m3 (< 0.19-7
ppm) and 307 benzole workers from 13 plants in the second cohort with
levels of < 0.6-48 mg/m3 (< 0.19-14.99 ppm). Mean time-weighted
average concentrations for benzole house workers in the United Kingdom
in the 1980s was considered to be about 4.2 mg/m3 (1.3 ppm). No
increased risk of mortality from leukaemia was reported in either
cohort (cohort 1 SMR = 98, 95% CI 2-557, 1 death; and cohort 2 SMR =
76, 95% CI 2-429, 1 death). These SMRs have been calculated utilizing
data from all 1293 by-product workers. Only a proportion of the
by-product workers had worked in the benzene plants where the greatest
exposure to benzene occurred. The SMR for leukaemia among 2349
coke-oven workers was 34 for cohort 1 (95% CI 0-186, 1 death) and 35
for cohort 2 (95% CI 1-192, 1 death).
In a retrospective cohort study from China encompassing 28 460
workers exposed to benzene in 233 factories, 30 cases of leukaemia
(23 acute, 7 chronic) were found, as compared to four cases in a
reference cohort of 28 257 workers in 83 machine production, textile
and cloth factories (Yin et al., 1987, 1989). The mortality rate (per
100 000 person-years) from leukaemia was 14 among the exposed and 2
among the unexposed (SMR, 574; P < 0.01). Mortality was especially
high for workers engaged in organic synthesis, painting and rubber
production. The mortality from leukaemia for cases that had
previously experienced benzene poisoning was 701/100 000 person-years.
Grab-samples of benzene in air were taken during the time of the
survey in workplaces where cases of leukaemia were observed; the mean
concentrations varied over a wide range (from 10 to 1000 mg/m3) but
the range was 50-500 mg/m3 in most locations. The mortality
incidence from all malignant neoplasms was higher in the exposed group
(123 per 100 000) than in controls (55 per 100 000). In addition, a
statistically significant excess risk for lung cancer (SMR = 231) was
observed in male workers exposed to benzene (Yin et al., 1989).
However, little description was given of the methods used to eliminate
the possible confounding effects of smoking.
A statistically significant excess risk for aplastic anaemia
(SMR = 1566, 4 deaths) and for leukaemia (SMR = 400, 95% CI, 146-870,
6 deaths) was reported for 1008 male workers in a shoe factory in
Florence, Italy. Included were workers employed on or after January
1950 and vital status of cohort members was ascertained through 1984.
No workplace monitoring was reported. The period of maximum benzene
exposure was considered to be 1953-1962, given the amount of benzene
used per day (about 30 kg) (Paci et al., 1989), and all cohort members
who developed aplastic anaemia and leukaemia were employed during this
period.
The results of a cohort study of 34 781 workers in eight oil
refineries in the United Kingdom which examined workers employed for
1 year between 1950-1975 gave no indication of increased mortality
from leukaemia (Rushton & Alderson, 1981a). Within this cohort a
nested case-control study was conducted to investigate the association
between exposure to benzene and leukaemia (Rushton & Alderson, 1981b).
The 36 leukaemia cases and their controls were allocated to "low",
"medium", and "high" benzene exposure categories based on their job
history. The authors reported a significant (P = 0.05) association in
the combined medium and highly exposed workers compared to those in
the low exposure group (RR = 3.0, 95% CI, 1.2-7.2).
A study (Tsai et al., 1983) of 454 petroleum refinery workers in
the USA employed between 1952 and 1978 in the petrochemical units
showed no deaths from leukaemia (0.4 expected). However, the median
exposure to benzene throughout the refinery was 0.45 mg/m3 (0.14
ppm), and only 16% of 1394 personal samples, taken between 1973 and
1982 (inclusive), contained more than 3.2 mg/m3 (1 ppm). The median
exposure intensity in "benzene-related units: petrochemical units" was
1.7 mg/m3 (0.53 ppm). No significant changes in blood indices
(counts of white and of red blood cells, haemoglobin, haematocrit,
platelets, clotting and bleeding times) were reported.
9. EVALUATION OF HUMAN HEALTH RISKS
9.1 General population
Benzene is ubiquitous in the environment, resulting in the
exposure of most humans to trace levels (or more) of this chemical.
Exposure in the general population is primarily to air-borne benzene
and derives from active and passive tobacco smoke, industrial
activity, and use of the automobile (gasoline fumes from refilling,
etc., and exhaust emissions). Estimates of the daily amounts of
benzene consumed in drinking-water and food-stuffs vary considerably
and are of the order of µg/day. Depending upon the assumptions made
with respect to levels of benzene from tobacco products and
foodstuffs, estimates for the exposure of the general smoking
population in industrialized countries range from 2000 to 3500 µg
benzene/day. Adult (70 kg) non-smokers are considered to be exposed
to about 200 to 1700 µg benzene/day (about 3 to 25 µg/kg body weight
per day). It would be helpful to have more information on total human
exposure, particularly in developing countries.
9.2 Occupational exposure
The major factors controlling industrial exposure to benzene are
process technology, worker practices and the efficiency and
sophistication of engineering controls. When appropriate engineering
controls are in place, available monitoring data indicate that
exposures of workers involved in the production, handling and use of
benzene and benzene-containing materials vary from non-detectable
levels to approximately 15 mg/m3 (8-h TWA), in addition to the
amounts estimated for the general population. In developing countries
the exposure can be several times higher. Due to the nature of the
processes involved, a small percentage of workers may be exposed to
more than 320 mg benzene/shift. In some developing countries, benzene
exposure may be sufficiently high to cause acute toxicity. Dermal
exposure to benzene has generally not been included in these
estimates. Validated benzene-specific biological markers of exposure
to low levels are not available.
9.3 Toxic effects
Acute lethal doses of benzene in experimental animals cause
narcosis, ventricular tachycardia and respiratory failure. The
threshold for narcotic effects in rats is about 13 000 mg/m3.
Reported oral LD50 values in rats vary from 3000 to 8100 mg
benzene/kg body weight and the 4-h LC50 in rats has been reported to
range between 32 600 and 44 600. However, although the clinical
pathological observations in animals are relevant to humans, the
latter would not be expected to be exposed to such high levels for
such long periods of time.
In humans, exposure to high concentrations of benzene (e.g.,
65 200 mg/m3 for 5-10 min) can result in central nervous system
depression, cardiac arrhythmia, respiratory failure and death, while
exposure to levels of benzene between 163 and 489 mg/m3 for 5 h
leads to headaches, lassitude and general weakness. The single acute
oral dose that has been reported to be lethal to humans is 8800 mg/kg
body weight.
9.3.1 Short-term and long-term exposures; organ toxicity
The most significant adverse effects from short- or long-term
exposure to benzene are haematotoxicity, i.e. bone marrow suppression,
immunotoxicity, genotoxicity and carcinogenicity.
9.3.1.1 Haematotoxicity; bone marrow depression
The level, timing and pattern of exposure are extremely important
factors in determining the incidence and severity of haematological
and bone marrow changes. A significant species difference (rats and
mice) in effects has also been reported, probably a reflection of the
more rapid metabolism of benzene by mice and the production of a
higher proportion of putative toxic metabolites than in rats. In rats
and mice, decreases in haematological cell counts (leucopenia and
haematocrit) and in bone marrow cellularity generally occur only after
several weeks of exposure to levels of benzene between 320 and 978
mg/m3, the mouse being more sensitive than rats. The lowest
reported exposure in experimental animals leading to haematological
effects was 32 mg/m3 (6 h/day, 5 days/week for 178 days) in male
mice. After oral administration of benzene to rats and mice for 120
days, leucopenia was observed in both male and female rats, and
lymphoid depletion in the B-cells of the spleen was observed in both
male and females at doses of > 200 mg/kg body weight. In mice, no
histopathological effects were observed, but a dose-related leucopenia
was observed in both males and females given 25, 50, 100, 200, 400 or
600 mg/kg body weight (5 days/week).
9.3.1.2 Mechanism of action and metabolism
In humans, a spectrum of blood dyscrasias, including
pancytopenia, aplastic anaemia, thrombocytopenia, granulocytopenia,
lymphocytopenia, myeloid leukaemia and acute leukaemia, can result
from benzene exposure. The dose, length of exposure and the stage of
stem cell development affected will determine which effect is
observed. Pancytopenia was noted in 32 patients exposed to 489-2119
mg benzene/m3 for periods of between 4 months and 15 years. At
levels of benzene between 102 and 438 mg/m3, haematotoxicity in
rubber factory workers was reported, the correlation being better
between benzene exposure and WBC counts than in the case of RBC
counts. However, at levels of benzene less than 32 mg/m3, there is
only weak evidence for a leukaemogenic effect; no haematological
effects were noted in 200 workers exposed to 10 year TWA benzene
levels of 0.03-4.5 mg/m3.
The hepatic metabolism of benzene is responsible for
detoxification of benzene via the formation of etheral sulfate,
glucuronides and glutathione conjugates. It also leads to the
production of metabolites, such as hydroquinone, p-benzoquinone,
muconaldehyde and perhaps others, which appear to be required for the
production of benzene toxicity in bone marrow. Current concepts of
the mechanism of benzene toxicity suggest that it is the result of the
combined effects of several metabolites, perhaps acting in concert
with unmodified benzene, to adversely alter the functions of stem
cells, progenitor cells and stromal cells in the bone marrow. It has
been postulated that the specific intracellular targets are proteins
and nucleic acids. The terminal result of these biochemical insults
is the development of aplastic anaemia. It is likely that benzene
metabolites damage chromosomes by causing DNA or protein adduct
formation or generating oxidative damage to DNA, which may contribute
to the chromosomal changes associated with benzene exposure. Animal
models exist for studying the mechanism of benzene-induced aplastic
anaemia and chromosome damage. However, no acceptable model for
benzene-induced leukaemia has been developed.
Since bone marrow depression and the production of leukaemia both
involve the bone marrow, it is important to ascertain the relationship
between these two biological effects of benzene. The specific
question in need of an answer is: Is it necessary for aplastic
anaemia to develop before leukaemia can be exhibited? In humans, the
continuum of effects of benzene on bone marrow involves first the
production of either anaemia, leucopenia or thrombocytopenia, and this
is followed by pancytopenia. Aplastic anaemia is the ultimate form of
bone marrow depression. Leukaemia has also been associated with
benzene exposure. In addition to studying the relationship between
these two phenomena, it is important to study the role of metabolites
in each process.
Several authors have recently used pharmacokinetic models to
extrapolate from animal experiments to expected human dosimetry. Some
models require the use of physiological parameters, partition
coefficients and metabolic data. Of these, the data on such metabolic
parameters in humans are least well known. With increasing
information on appropriate human metabolic data, such models will be
useful in extrapolating from animals to humans and from high- to
low-dose exposure.
9.3.1.3 Immunotoxicity
Benzene-induced immunological effects are probably a reflection
of bone marrow toxicity. Immunological function was depressed in mice
exposed to benzene levels between 32 and 96 mg/m3, 6 h/day, for 6
days. In mice, polyhydroxylated metabolites of benzene have been
shown to depress B- and T-cell functions. Although the relevance of
the animal data to human immunological functions has not been
established, human immunological alterations have been observed after
exposure to benzene.
In workers exposed to, but not seriously intoxicated by, benzene
(10-22 mg/m3), the serum complement levels of IgA and IgG were
decreased but the levels of IgM were slightly increased. An increased
susceptibility to allergies was found in workers exposed to benzene
levels as low as 96 mg/m3. A loss of leucocytes and other blood
elements has been noted at benzene levels ranging between 48 and 240
mg/m3. A study of low exposures to benzene (TWA: 32 mg/m3, 10
ppm) showed no differences in mitogen-induced blastogenesis in exposed
workers and controls.
9.3.2 Genotoxicity and carcinogenic effects
In vitro tests indicate that benzene is not mutagenic.
However, benzene, or its metabolites have been shown to cause both
structural and numerical chromosomal aberrations in experimental
animals as well as sister chromatid exchanges (SCE) and micronuclei in
polychromatic erythrocytes.
Humans with benzene haemopathy have been found to have a high
percentage of aneuploid lymphocytes. Increases in the number of both
stable and unstable chromosomal aberrations were observed in workers
exposed to high levels (408-1734 mg/m3) of benzene for 1 to 22
years. Therefore, benzene should be considered a clastogen in both
animals and humans.
Recent studies of workers exposed to lower concentrations of
benzene (TWA: < 3.2-32 mg/m3, < 1-10 ppm) revealed no alteration
in cell-cycle kinetics and no increase in SCEs. Only a marginally
significant increase in chromosomal aberrations (chromatid deletions
and gaps) was noted at these exposure levels.
9.3.2.1 Mechanism of carcinogenicity
It is broadly accepted today, as a result of several studies in
the field of molecular biology, that chromosomal rearrangements
generating gene translocations, gene deletions and gene amplifications
are relevant steps in the carcinogenic process.
There is at present no adequate animal model for benzene-induced
leukaemia in humans. However, benzene has been shown to be
carcinogenic in experimental animals after inhalation or oral
exposure. While several types of neoplasms have been reported to be
associated with benzene exposure in rats and mice, these are primarily
of epithelial origin, i.e. zymbal gland, liver, mammary gland and
nasal cavity. Lymphomas/leukaemias have been observed with lesser
frequency.
One study showed an increased incidence of lymphoma in the CBA/CA
mouse. No statistically significant increase was seen for lymphoid
tumour incidence in the Sprague-Dawley rat, Wistar rat or Swiss mouse.
In the B6C3F1 mouse the incidence was not dose-related.
Dose-related (possibly linear) increases in epithelial tumours were
observed in mice and rats. The results in animals indicate that
benzene is an experimental multipotential carcinogen, although there
is not the leukaemogenic response seen in humans. The lowest dose
resulting in increased incidence of tumours was demonstrated in
B6C3F1 mice (25 mg/kg body weight, 5 days per week for 103 weeks).
No useful information is available for doses below this level.
Attempts to understand the mechanism of benzene-induced
carcinogenesis can be based on basic principles of chemical
carcinogenesis. Thus, one mechanism of cancer has been postulated to
be the result of autosomal mutations due to the formation of DNA
adducts, which result in alteration of cell function and control of
replication. Secondly, cancer appears to be induced by a combination
of multiple genetic and epigenetic events.
Metabolic data suggest that several reactive metabolites of
benzene are formed and these can potentially form adducts both with
DNA and protein. Binding of benzene metabolites to the protein
components of the spindle apparatus has been suggested to inhibit
mitosis. The data for the formation of DNA adducts by reactive
metabolites of benzene suggests that, compared with other carcinogens,
benzene does not form large amounts of DNA adducts. Furthermore,
although there is evidence for DNA-adduct formation in liver,
DNA-adduct formation in bone marrow has been a subject of some
controversy. Assuming that adduct formation is an initiating event,
there is the possibility of a protective event if DNA repair or immune
surveillance intervenes. A promotional event might lead to a
carcinogenic response. It is possible that benzene acts as a promoter
of its own initiation.
While it is clear that a greater percentage of benzene
metabolites are converted to reactive metabolites at low doses than at
high doses, the total amounts of reactive metabolites increases with
increasing dose. Hence, it is clear that the severity of benzene
toxicity increases with increasing dose. By analogy, it is expected
that increasing doses of benzene should yield more leukaemia in
humans.
The negative results obtained with in vitro mutagenicity tests
could be related to an inadequate production of mutagenic metabolites
in the system. The failure to produce leukaemia in animals may be due
to lack of adequate formation of leukaemogenic metabolites or the need
to produce bone marrow damage prior to the induction of leukaemia. If
the bone marrow must undergo a stage of aplastic anaemia prior to the
development of leukaemia, one would have to estimate higher exposure
to benzene and to define a threshold. Alternatively, at doses lower
than those producing aplastic anaemia, sufficient damage may occur to
induce tumour promotion.
Both animal and human studies show that benzene exposure produces
bone marrow and chromosomal damage. However, the various stages in
carcinogenesis cited above have not been observed with benzene either
because they do not occur in these species or because of technical
problems. The results of both animal and human studies suggest that
benzene is a weak carcinogen on a molar basis.
9.3.2.2 Human carcinogenesis
Benzene is a well-established human leukaemogen. There have been
numerous epidemiological studies on the effects of benzene, most of
which have dealt with chronic industrial exposures. Increased
leukaemia risk was identified in studies of shoemakers, chemical
workers and workers in oil refineries, and in a nationwide study of
benzene-exposed workers in different industries. The most consistent
evidence for a causal association in humans has been found between
benzene exposure and myeloid leukaemia. An exposure-response
relationship was identified in some studies, the response being
influenced both by exposure levels and duration of exposure. In the
study where estimated past exposures were based on the most extensive
exposure measurements, a three-fold increased leukaemia risk was
identified in workers exposed to benzene levels of 128-640
mg/m3-years (40-200 ppm-years) (ppm-years is the average
concentration times the duration of exposure in years, e.g., 4 ppm for
10 years is equivalent to 40 ppm-years) and a statistically
significant 12-fold risk for workers exposed to benzene levels between
of 640-1280 mg/m3-years (200-400 ppm-years). The scientific
assessment of alternative exposure estimates for the rubber
hydrochloride cohort has not yet been fully explored. Of the two
alternative exposures estimates so far postulated for this study, they
both suggest a lower estimate of risk than that described above.
Other leukaemia types, multiple myeloma and other lymphomas were also
reported.
A statistically significant excess risk for multiple myeloma was
found in the rubber hydrochloride study. A marginally significant
exposure-response relationship for malignant lymphomas was reported in
a study on chemical workers. Increased risk for skin, stomach and
lung cancers has been reported in some studies, but these findings
have not been consistent and may possibly be attributed to concomitant
exposures to other chemicals or statistical artifact.
Studies examining leukaemia risk in coke-oven workers, assumed to
have been exposed to fairly low levels of benzene, have not identified
excess leukaemia risk. In these studies no attempt was made to
examine leukaemia subtypes. These studies do not provide enough
evidence to prove that there is no risk of leukaemia as a result of
exposure to these low concentrations of benzene.
The Task Group is of the opinion that the epidemiological
evidence presented so far is not capable of distinguishing between
(a) a small increase in leukaemia mortality in workers exposed to low
benzene levels, and (b) a no-risk situation.
9.4 Other toxicological end-points
Benzene does cross the placenta of experimental animals, since
haemopoietic changes have been observed in the fetuses and offspring
of mice exposed to 16, 33 or 65 mg benzene/m3, 6 h/day during days
6-15 of gestation. Following inhalation exposure to high doses (500
to 1600 mg/m3) in rabbits and mice, an increase in fetal resorptions
or fetal death was observed. However, benzene does not appear to be
teratogenic, although it is fetotoxic, in experimental animals, and no
evidence is available to permit the conclusion that it causes adverse
reproductive effects in humans.
The neurotoxicity of benzene in animals and humans has not been
well studied. An early study showed subtle changes such as reduced
food intake and decreased hind-limb grip strength following exposures
to 3300 and 9900 mg benzene/m3, and learning defects were observed
in rats dosed orally with 550 mg benzene/kg body weight.
9.5 Conclusions
To assist Member States in the development of standards for
benzene exposure, the Task Group concludes that a TWA of 3.2 mg/m3
(1 ppm) over a 40-year working career has not been statistically
associated with any increase in deaths from leukaemia. However, since
benzene is a human carcinogen, exposures should be limited to the
lowest possible technically feasible level. Increases in exposure to
over 32 mg/m3 (10 ppm) should be avoided. Benzene and
benzene-containing products such as gasoline should never be used for
cleaning purposes.
Traditionally, bone marrow depression, i.e. anaemia, leucopenia
or thrombocytopenia, in the workplace has been recognized as the first
stage of benzene toxicity and appears to follow a dose-response
relationship, i.e. the higher the dose, the greater the likelihood of
observing decreases in circulating blood cell counts.
Table 20 shows some "rough" estimates of the percentages of
workers that might exhibit either bone marrow depression or frank
aplastic anaemia after exposure to benzene for either 1 year or 10
years at concentrations of 3.2, 32, 160 or 320 mg/m3 (1, 10, 50 or
100 ppm). These estimations are an interpretation of the literature
using the experience of the Task Group. The speculative nature of
this table precludes its use in regulatory standard setting. Exposure
at high doses (160-320 mg/m3; 50-100 ppm) for one year would most
likely produce bone marrow toxicity in a large percentage of the
workers, and in some cases aplastic anaemia, but little effect would
be expected at the lower doses. Exposure to both high and low doses
would be expected to produce benzene toxicity after 10 years. Thus,
a high level of both bone marrow depression and aplastic anaemia would
be seen at the higher doses and some damage would also be seen at the
lower doses. The observation of any of these effects, regardless of
the dose or period of exposure, should indicate the need for improved
control of benzene exposure.
There is no evidence of benzene being teratogenic at doses lower
than those that produce maternal toxicity, but fetal toxicity has been
demonstrated.
The neurotoxicity and immunotoxicity of benzene have not been
well studied in either experimental animals or humans.
Table 20. Estimated percentage of worker populations that might
develop bone marrow depression or aplastic anaemia after
chronic exposure to benzene ( Before using table note
cautionary footnotea)
Duration Exposure Bone marrow Aplastic
depression anaemia
1 year 320 mg/m3 (100 ppm) 90 10
160 mg/m3 (50 ppm) 50 5
32 mg/m3 (10 ppm) 1 0b
3.2 mg/m3 (1 ppm) 0b 0b
10 years 320 mg/m3 (100 ppm) 99 50
160 mg/m3 (50 ppm) 75 10
32 mg/m3 (10 ppm) 5 0b
3.2 mg/m3 (1 ppm) < 1 0b
a This estimation is an interpretation of the literature and is
based on the experience of the Task Group. The speculative
nature of this table precludes its use in regulatory standard
setting.
b Occasional cases may be observed.
10. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Benzene and benzene-containing products, including gasoline
(petrol), should never be used for cleaning purposes.
b) Systematic information on occupational and non-occupational
exposure should be collected using the total human exposure
approach where possible.
c) The health risk of low-level benzene exposure is not clearly
established. Exposure should, therefore, be avoided as much as
possible.
d) The occurrence of benzene in environmental media such as air and
water where there exists potential human exposure should be
evaluated.
e) A search for less toxic solvents to replace benzene in industrial
processes should be encouraged.
11. FURTHER RESEARCH
a) Epidemiological studies of the risks of haematological
malignancies, blood changes (red and white blood cells) and
genotoxic effects at low and high exposure concentrations should
have high priority.
b) Information on the mechanisms by which benzene induces neoplasms
is required. In particular, there is a need for animal models of
benzene-induced haemopoietic malignancies similar to those seen
in humans and a better understanding of the role of reactive
intermediate.
c) Further studies are needed to elucidate the potential link
between bone marrow suppression and the eventual occurrence of
leukaemia.
d) Biological markers of benzene exposure, especially urinary
muconic acid and macromolecular adducts, should be validated.
e) Individual susceptibility factors in benzene-induced toxicities
should be investigated.
f) Animals and human studies are required to assist in validating
physiologically based pharmacokinetic models using all routes of
exposure.
g) The multigenerational effects of benzene exposure should be
investigated.
h) Studies on immunotoxicological effects following benzene exposure
should be performed.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The carcinogenicity of benzene has been evaluated by the
International Agency for Research on Cancer (IARC, 1982, 1987b). It
was concluded that there was sufficient evidence for the
carcinogenicity of benzene in both animals and humans.
A guideline value of 10 µg/litre was recommended by WHO (WHO,
1984) for benzene in drinking-water based on data for the production
of leukaemia after inhalation exposures in humans and using a linear
multistage extrapolation model and a life-time risk level of 1 in
100 000. This guideline remained unchanged during the revisions
recently completed (WHO, 1993).
A Task Group convened by the WHO Regional Office for Europe
concluded that an air quality guideline value could not be set for
benzene in view of its carcinogenic activity in humans (WHO, 1987).
Assuming no threshold and an average relative risk model, it was
calculated that at an air concentration of 1 µg benzene/m3, the
estimated lifetime risk of leukaemia would be 4 x 10-6.
Regulatory standards for benzene established by national bodies
in some countries are summarized in the Legal File of the
International Register of Potentially Toxic Chemicals (IRPTC, 1987).
Recently the Commission of the European Communities has proposed an
occupational exposure limit for benzene of 1.6 mg/m3 (0.5 ppm) (CEC,
1993).
References
Aksoy M (1980) Different types of malignancies due to occupational
exposure to benzene. A review of recent observations in Turkey.
Environ Res, 23: 181-190.
Aksoy M & Erdem S (1978) Follow-up study on the mortality and the
development of leukaemia in 44 pancytopaenic patients with chronic
exposure to benzene. Blood, 52: 285-292.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1971) Haematological
effects of chronic benzene poisoning in 217 workers. Br J Ind Med, 28:
296-302.
Aksoy M, Dincol K, Akgun T, Erdem S, & Dincol G (1972) Details of
blood changes in 32 patients with pancytopaenia associated with
long-term exposure to benzene. Br J Ind Med, 29: 56-64.
Aksoy M, Erdem S, Hepyüksel T, & Dincol G (1974a) Chronic exposure to
benzene as a possible contributory etiologic factor in Hodgkin's
Disease. Blood, 38: 93-298.
Aksoy M, Erdem S, & Dincol G (1974b) Leukemia in shoe-workers exposed
chronically to benzene. Blood, 44: 837-841.
Allen PD (1987) Residential population exposure to ambient benzene in
California. Sacramento, California, California Air Resources Board.
Anderson D & Richardson CR (1981) Issues relevant to the assessment of
chemically induced chromosome damage in vivo and their relationship
to chemical mutagenesis. Mutat Res, 90: 261-272.
Andrews LS & Snyder R (1986) Toxic effects of solvents and vapors. In:
Casarett LJ & Doull J ed. Toxicology: The basic science of poisons,
3rd ed. New York, McMillan Publishing Co., chapter 20, p 641.
Antoine SR, Delon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36(3): 364-371.
Appuhn E & Goldeck H (1957) [Early and late disorders of
haematopoiesis caused by benzene and its homologues.] Arch
Gewerbepathol Gewerbehyg, 15: 399-428 (in German).
Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin BH, Matter
BE, & Shelby MD ed. (1985) Evaluation of short-term tests for
carcinogens. Report of the International Programme on Chemical
Safety's collaborative study on in vitro assays. Amsterdam, Oxford,
New York, Elsevier Science Publishers (Progress in Mutation Research,
Volume 5).
ATSDR (1989) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 173 pp
(ATSDR/TP-88/03; PB/89/209464/AS).
ATSDR (1991) Toxicological profile for benzene. Atlanta, Georgia,
Agency for Toxic Substances and Disease Registry, 193 pp.
Au WW, Ramanujam VMS, Ward JB Jr, & Legator MS (1991) Chromosome
aberrations in lymphocytes of mice after sub-acute low-level
inhalation exposure to benzene. Mutat Res, 260: 219-224.
Baarson K, Snyder CA, & Green J (1982) The hematotoxic effects of
inhaled benzene on peripheral blood, bone marrow, and spleen cells are
increased by ingested ethanol. Toxicol Appl Pharmacol, 64: 393-404.
Baarson K, Snyder CA, & Albert RE (1984) Repeated exposures of C57B1
mice to 10 ppm inhaled benzene markedly depressed erythropoietic
colony formation. Toxicol Lett, 20: 337-342.
Bailer AJ & Hoel DG (1989) Metabolite-based internal doses used in a
risk assessment of benzene. Environ Health Perspect, 82: 177-184.
Bailey JC & Schmidl B (1989) A survey of hydrocarbons emitted in
vehicle exhaust gases, over a range of driving speeds and conditions
from a representative sample of the 1986-87 UK vehicle fleet.
Stevenage, Herts, Warren Spring Laboratory (DTI) (Report No. LR 673).
Bandow H, Washida N, & Akimoto H (1985) Ring-cleavage reactions of
aromatic hydrocarbons studies by FT-IR spectroscopy. I. Photooxidation
of toluene and benzene in the NOx-air system. Bull Chem Soc Jpn, 58:
2531-2540.
Barale R, Giorgelli F, Migliore L, Ciranno R, Casini D, Zucconi D, &
Loprieno N (1985) Benzene induces micronuclei in circulating
erythrocytes of chronically treated mice. Mutat Res, 144: 193-196.
Barrett RH (1985) Assays for unscheduled DNA synthesis in HeLa S3
cells. In: Ashby J, de Serres FJ, Draper M, Ishidate M Jr, Margolin
BH, Matter BE, & Shelby MD ed. Short-term tests for carcinogens:
Report of the International Programme on Chemical Safety's
collaborative study on in vitro assays. Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp 347-352 (Progress in Mutation
Research, Volume 5).
Battersby NS & Wilson V (1989) Survey of the anaerobic biodegradation
potential of organic chemicals in digesting sludge. Appl Environ
Microbiol, 52(2): 433-439.
Baxter HG, Blakemore R, Moore JP, & Coker DT (1980) The measurement of
airborne benzene vapour. Ann Occup Hyg, 23: 117-132.
Bayer CW, Black MS, & Galloway LM (1988) Sampling and analysis
techniques for trace volatile organic emissions from consumer
products. J Chromatogr Sci, 26: 168-173.
Bechtold WE, Sabourin PJ, & Henderson RF (1988) A reverse isotope
dilution method for determining benzene and metabolites in tissues.
J Anal Toxicol, 12(7/8): 176-179.
Bechtold WE, Lucier G, Birnbaum LS, Yin SN, Li GL, & Henderson RF
(1991) Muconic acid determinations in urine as a biological exposure
index for workers occupationally exposed to benzene. Am Ind Hyg Assoc
J, 52(11): 473-478.
Bechtold WE, Sun JD, Birnbaum LS, Yin SN, Li GL, Kasicki S, Lucier G,
& Henderson RF (1992) S-Phenylcysteine formation in haemoglobin as a
biological exposure index to benzene. Arch Toxicol, 66: 303-309.
Beliles RP & Totman LC (1989) Pharmacokinetically based risk
assessment of workplace exposure to benzene. Regul Toxicol Pharmacol,
9: 186-195.
Bennett GF (1989) Impact of toxic chemicals on local wastewater
treatment plant and the environment. Environ Geol Water Sci, 13(3):
201-212.
Bentley P, Sschassmann H, Sims P, & Oesch F (1976) Epoxides derived
from various polycyclic hydrocarbons as substrates of homogeneous and
microsome-bound epoxide hydratase. A general assay and kinetic
properties. Eur J Biochem, 69: 97-103.
Blanchard RD & Hardy JK (1986) Continuous monitoring device for the
collection of 23 volatile organic priority pollutants. Anal Chem, 58:
1529-1532.
Blank IH & McAuliffe DJ (1985) Penetration of benzene through human
skin. J Invest Dermatol, 85: 522-526.
Bois FY, Woodruff TJ, & Spear RC (1991a) Comparison of three
physiologically based pharmacokinetic models of benzene disposition.
Toxicol Appl Pharmacol, 110: 79-88.
Bois FY, Smith MT, & Spear RC (1991b) Mechanisms of benzene
carcinogenesis: application of a physiological model of benzene
pharmacokinetics and metabolism. Toxicol Lett, 56: 283-298.
Bond AE, Thompson VL, & Ortman GC (1986) Self service station vehicle
refueling exposure study. In: Proceedings of the 1986 EPA/APCA
Symposium on Measurement of Toxic Air Pollutants. Pittsburgh,
Pennsylvania, Air Pollution Control Association, pp 458-466.
Bond GG, McLaren EA, Baldwin CL, & Cook RR (1986) An update of
mortality among chemical workers exposed to benzene. Br J Ind Med, 43:
685-691.
Bradley MO (1985) Measurement of DNA single-strand breaks by alkaline
elution in rat hepatocytes. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 353-357 (Progress in Mutation Research, Volume 5).
Brodfuehrer JI, Chapman DE, Wilke TJ, & Powis G (1990) Comparative
studies of the in vitro metabolism and covalent binding of
14C-benzene by liver slices and microsomal fraction of mouse, rat
and human. Drug Metab Dispos, 18: 20-27.
Brown, RH (1988a) Determination of benzene, toluene, and xylene in
industrial air by charcoal tube, solvent desorption and gas
chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 225-233.
Brown RH (1988b) Determination of benzene, toluene, and xylene in
industrial air by porous polymer adsorption tube, thermal desorption
and gas chromatography. In: Fishbein L & O'Neill JK ed. Environmental
carcinogens method of analysis and exposure measurement - Volume 10:
Benzene and alkylated benzenes. New York, London, Oxford University
Press, pp 235-242.
Bruckmann P, Beier R, & Krautscheid S (1983) [Measurements of
emissions of hydrocarbons in areas of high exposure in the Rhine-Ruhr
district.] Staub-Reinhalt-Luft, 43: 404 (in German).
Bruckmann P, Kersten W, Funcke W, Balfanz E, König J, Theisen J, Ball
M, & Päpke O (1988) The occurrence of chlorinated and other organic
trace compounds in urban air. Chemosphere, 17(12): 2363-2380.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1989) Determination of
benzene, toluene and 1,3-butadiene in cigarette smoke by GC-MSD. Exp
Pathol, 37: 108-113.
Brunnemann KD, Kagan MR, Cox JE, & Hoffmann D (1990) Analysis of
1,3-butadiene and other selected gas-phase components in cigarette
mainstream and sidestream smoke by gas chromatography - mass selective
detection. Carcinogenesis, 11(10): 1863-1868.
Bryce-Smith D & Gilbert A (1976) The organic photochemistry of
benzene. I. Tetrahedron, 32: 1309-1326.
Buchet JP (1988) Determination of phenol and its glucurono- and
sulfoconjugates in urine by gas chromatography. In: Fishbein L &
O'Neill JK ed. Environmental carcinogens methods of analysis and
exposure measurement - Volume 10: Benzene and alkylated benzenes. New
York, London, Oxford University Press, pp 281-286.
Burmaster DE (1982) The new pollution. Groundwater contamination.
Environment, 24: 33-36.
Busby WF Jr, Wang J-S, Stevens EK, Padykula RE, Aleksejczyk RA, &
Berchtold GA (1990) Lung tumourigenicity of benzene oxide, benzene
dihydrodiols and diolepoxides in the BLV: Ha newborn mouse assay.
Carcinogenesis, 11: 1473-1478.
Carpenter C, Shaffer C, Weil C, & Smyth H (1944) Studies on the
inhalation of 1,3-butadiene; with comparison of its narcotic effect
with benzol, toluol, and styrene and a note on the elimination of
styrene by the human. J Ind Hyg Toxicol, 26: 69-78.
CEC (1993) Occupational exposure limits - Criteria document for
benzene. Luxembourg, Commission of the European Communities,
Directorate-General Employment, Industrial Relations and Social
Affairs (EUR 14491 EN) (Health and Safety Series).
Chang SS & Peterson RJ (1977) Symposium: The basis of quality in
muscle foods. Recent developments in the flavor of meat. J Food Sci,
42: 298-306.
Chepiga TA, Yang CS, & Snyder R (1991) Benzene metabolism in two
purified, reconstituted rat hepatic mixed function oxidase systems.
In: Witmer CM, Snyder R, Jollow DJ, Kalf GF, Kocsis JJ, & Sipes IG ed.
Biological reactive intermediates: IV. Molecular and cellular effects
and their impact on human health. New York, London, Plenum Press,
pp 261-265.
Choy WN, MacGregor JT, & Shelby MD (1985) Induction of micronuclei by
benzene in B6C3F1 mice: Retrospective analysis of peripheral blood
smears from the NTP carcinogenesis bioassay. Mutat Res, 143: 55-59.
Ciranni R, Barale R, Marrazzini A, & Loprieno N (1988) Benzene and the
genotoxicity of its metabolites. I. Transplacental activity in mouse
fetuses and in their dams. Mutat Res, 208: 61-67.
Clark AI, McIntyre AE, Lester JN, & Perry R (1984a) Ambient air
measurements of aromatic and halogenated hydrocarbons at urban, rural
and motorway locations. Sci Total Environ, 39: 265-279.
Clark AI, McIntyre AE, & Lester JN (1984b) A comparison of
photoionization detection gas chromatography with a Tenax GC sampling
tube procedure for the measurement of aromatic hydrocarbons in ambient
air. J Environ Anal Chem, 17: 315-326.
Coate WB, Hoberman AM, & Durloo RS (1984) Inhalation teratology study
of benzene in rats. Adv Mod Environ Toxicol, 6: 187-198.
Colenutt BA & Thornburn S (1980) Gas-chromatographic analysis of trace
hydrocarbon pollutants in water samples. Int J Environ Stud, 15:
25-32.
Collins JJ, Conner P, Friedlander BR, Easterday PA, Nair RS, Rashmi S,
& Braun J (1991) A study of the hematological effects of chronic
low-level exposure to benzene. J Occup Med, 33(5): 619-626.
CONCAWE (1986) Review of European oil industry benzene exposure data.
The Hague, European Oil Companies Organization for Environmental and
Health Protection (Report No. 3/86).
Cornish HH & Ryan RC (1965) Metabolism of benzene in non-fasted,
fasted, and arylhydroxylase inhibited rats. Toxicol Appl Pharmacol,
7: 767-771.
Cronkite EP (1986) Benzene hematotoxicity and leukemogenesis. Blood
Cells, 12: 129-137.
Cronkite EP, Bullis J, Inoue T, & Drew RT (1984) Benzene inhalation
produces leukemia in mice. Toxicol Appl Pharmacol, 75: 358.
Cronkite EP, Drew RT, Inoue T, & Bullis JE (1985) Benzene
hematotoxicity and leukemogenesis. Am J Ind Med, 7: 447-456.
Cronkite EP, Drew RT, Inoue T, Hirabayashi Y, & Bullis JE (1989)
Hematotoxicity and carcinogenicity of inhaled benzene. Environ Health
Perspect, 82: 97-108.
Crump K & Allen B (1984) Quantitative estimates of risk of leukemia
from occupational exposure to benzene. Washington, DC, US Department
of Labour (OSHA Docket H-059b, Exhibit 152, Annex B).
Dann T (1987) Ambient benzene levels in urban areas of Canada. Report
of the Technology Development and Technical Services Branch,
Conservation and Protection. Ottawa, Environment Canada.
Dannecker W, Schröder B, & Stechmann H (1990) Organic and inorganic
substances in highway tunnel exhaust air. Sci Total Environ, 93:
293-300.
Da Silva C, Fan X, & Castagna M (1989) Benzene-mediated protein kinase
c activation. Environ Health Perspect, 82: 91-95.
Dean BJ (1978) Genetic toxicology of benzene, toluene, xylenes and
phenols. Mutat Res, 47: 75-97.
Dean BJ (1985a) Recent findings on the genetic toxicology of benzene,
toluene, xylenes and phenols. Mutat Res, 154: 153-181.
Dean BJ (1985b) Summary report on the performance of cytogenetic
assays in cultured mammalian cells. In: Ashby J, de Serres FJ, Draper
M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation
of short-term tests for carcinogens. Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 69-83 (Progress in Mutation Research, Volume 5).
Decouflé P, Blattner WA, & Blair A (1983) Mortality among chemical
workers exposed to benzene and other agents. Environ. Res., 30: 16-25.
De Flora S, Zanacchi P, Camoirano A, Bennicelli C, & Badolati GS
(1984) Genotoxic activity and potency of 135 compounds in the Ames
reversion test and in a bacterial DNA-repair test. Mutat Res, 133:
161-198.
Deichmann WB, MacDonald WE, & Bernal E (1963) The hemopoietic tissue
toxicity of benzene vapors. Toxicol Appl Pharmacol, 5: 201-224.
Dibben MJ, Thomas TC, & Anderson MP (1989) Is benzene a problem in
fuel operations: A new analytical method to overcome potential
interferences in the present methods. Liq Chromatogr-Gas Chromatogr
Int, 2: 54, 56, 58.
Dorfman LM, Taub IA, & Buhler RE (1962) Pulse radiolysis studies. I.
Transient spectra and reaction-rate constants in irradiated aqueous
solutions of benzene. J Chem Phys, 36: 3051-3061.
Douglas GR, Blakely DH, Liu-Lee VW, Bell RDL, & Bayley JM (1985)
Alkaline sucrose sedimentation, sister-chromatid exchange and
micronucleus assays in CHO cells. In: Ashby J, de Serres FJ, Draper M,
Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed. Evaluation of
short-term tests for carcinogens: Report of the International
Programme on Chemical Safety's collaborative study on in vitro
assays. Amsterdam, Oxford, New York, Elsevier Science Publishers,
pp 359-366 (Progress in Mutation Research, Volume 5).
Dowty BJ, Laseter JL, & Storer J (1976) The transplacental migration
and accumulation in blood of volatile organic constituents. Pediatr
Res, 10: 696-701.
Drew RT & Fouts JR (1974) The lack of effects of pretreatment with
phenobarbital and chlorpromazine on the acute toxicity of benzene in
rats. Toxicol Appl Pharmacol, 27: 183-193.
Eastmond DA, Smith MT, & Irons RD (1987) An interaction of benzene
metabolites reproduces the myelotoxicity observed with benzene
exposure. Toxicol Appl Pharmacol, 91: 85-95.
Eisenreich SJ, Looney BB, & Thornton JD (1981) Airborne organic
contaminants in the Great Lakes ecosystem. Environ Sci Technol, 15:
30-38.
Environment Agency, Japan (1989) Chemicals in the environment. Report
on environmental survey and wildlife monitoring of chemicals in F.Y.
1986 and 1987. Tokyo, Environment Agency, Department of Environmental
Health, Office of Health Studies.
Erexson GL, Wilmer JL, & Kligerman AD (1985) Sister chromatid exchange
in human lymphocytes exposed to benzene and its metabolites
in vitro. Cancer Res, 45: 2471-2477.
Erexson GL, Wilmer JL, Steinhagen WH, & Kligerman AD (1986) Induction
of cytogenetic damage in rodents after short-term inhalation of
benzene. Environ Mutagen, 8: 29-40.
Erf LA & Rhoads CP (1939) The hematological effects of benzene
(benzol) poisoning. J Ind Hyg Toxicol, 20(8): 421.
Ewing BB, Chain ESK, Cook JC, Evans CA, Hopke PK, & Perkins EG (1977)
Monitoring to detect previously unrecognized pollutants in surface
waters. Washington, DC, US Environmental Protection Agency
(EPA-560/6/77/015A).
Fel'dt EG (1985) [Evaluation of the mutagenic hazards of benzene and
some of its derivatives.] Gig i Sanit, 7: 21-23 (in Russian with
English summary).
Fentiman AF, Neher MB, Kinzer GW, Sticksel PR, & Coutant RW (1979)
Environmental monitoring benzene. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (EPA-560/679/006)
(Prepared by Battelle Columbus for US EPA).
Ferrario JB, Lawler GC, Delon IR, & Laseter JL (1985) Volatile organic
pollutants in biota and sediments of Lake Pontchartrain. Bull Environ
Contam Toxicol, 34(2): 246-255.
Fishbein L (1984) An overview of environmental and toxicological
aspects of aromatic hydrocarbons. I. Benzene. Sci Total Environ, 40:
189-218.
Forni A, Pacifico E, & Limonta A (1971a) Chromosome studies in workers
exposed to benzene or toluene or both. Arch Environ Health, 22:
373-378.
Forni AM, Cappellini A, Pacifico E, & Vigliani EC (1971b) Chromosome
changes and their evolution in subjects with past exposure to benzene.
Arch Environ Health, 23: 385-391.
Franz TJ (1984) Percutaneous absorption of benzene. In: Applied
toxicology of hydrocarbons. Princeton, New Jersey, Princeton
Scientific Publishers, Inc., pp 61-70 (Advances in Modern
Environmental Toxicology, Volume VI).
Fung KK & Wright BJ (1986) Monitoring of benzene in ambient air with
organic vapor badges. J Air Pollut Control Assoc, 36: 819-821.
Furnas DW & Hine CH (1958) Neurotoxicity of some selected
hydrocarbons. Arch Ind Health, 18: 9-15.
Gad-El-Karim MM, Harper BL, & Legator MS (1984) Modifications in the
myeloclastogenic effect of benzene in mice with toluene,
phenobarbital, 3-methylcholanthrene, Aroclor 1254 and SKF-525A. Mutat
Res, 135: 225-243.
Galton DAG (1986) The myelodysplastic syndromes. Scand J Haematol, 36:
11-20.
Garner RC (1985) Summary report on the performance of gene mutation
assays in mammalian cells in culture. In: Ashby J, de Serres FJ,
Draper M, Ishidate M Jr, Margolin BH, Matter BE, & Shelby MD ed.
Evaluation of short-term tests for carcinogens. Report of the
International Programme on Chemical Safety's collaborative study on
in vitro assays. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 85-94 (Progress in Mutation Research, Volume 5).
GDCh (Society of German Chemists) (1988) [Advisory Committee on
Existing Chemicals of Environmental Relevance. Benzene.] Weinheim, VCH
Verlagsgesellschaft, 82 pp (BUA Report 24) (in German with English
summary).
Geist CR, Kelly LD, Schoenheit CM, & Praed JE (1983) Learning
impairments following postnatal exposures to benzene. Percept Motor
Skills, 57: 1083-1086.
Gerarde HW (1960) Toxicology and biochemistry of aromatic
hydrocarbons. Amsterdam, Oxford, New York, Elsevier Science
Publishers.
Gerner-Smidt P & Friedrich U (1978) The mutagenic effect of benzene,
toluene and xylene studied by the SCE technique. Mutat Res, 58:
313-316.
Geyer H, Politzki G, & Freitag D (1984) Prediction of ecotoxicological
behaviour of chemicals: relationship between n-octanol/water partition
coefficient and bioaccumulation of organic chemicals by the alga
Chlorella. Chemosphere, 13: 269-284.
Ghantous H & Danielsson BRG (1986) Placental transfer and distribution
of toluene, xylene, and benzene, and their metabolites during
gestation in mice. Biol Res Pregnancy, 7: 98-105.
Gilman AG, Goodman LA, & Gilman A (1985) The Goodman and Gilman's
pharmacological basis of therapeutics, 7th ed. New York, MacMillan
Publishing Co.
Gilmour SK, Kalf GF, & Snyder R (1986) Comparison of the metabolism of
benzene and its metabolite phenol in rat liver microsomes. In: Kocsis
JJ, Jollow DJ, Witmer CM, Nelson J, & Snyder R ed. Biological reactive
intermediates. III - Mechanisms of action in animal models and human
disease. New York, Plenum Publishing Co., pp 223-235.
Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, &
Oesch F (1989) Multiple activation pathways of benzene leading to
products with varying genotoxic characteristics. Environ Health
Perspect, 82: 81-89.
Gofmekler VA (1968) [The embryotropic action of benzene and
formaldehyde in experimental administration by inhalation.] Gig i
Sanit, 33: 12-16 (in Russian with English summary).
Goldstein BD (1988) Benzene toxicity: occupational medicine. State Art
Rev, 3: 541-554.
Goldstein BD, Snyder CA, Laskin S, Brombert I, Albert RE, & Nelson N
(1982) Myelogenous leukemia in rodents inhaling benzene. Toxicol Lett,
13: 169-173.
Goldwater LJ (1941) Disturbances in the blood following exposure to
benzol. J Lab Clin Med, 26: 957.
Gollmer L, Graf H, & Ulrich V (1984) Characterization of the benzene
monooxygenase system in rabbit bone marrow. Biochem Pharmacol, 33:
3597-3602.
Goodenkaug O & Atkinson JC (1986) Occurrence of volatile organic
chemicals in Nebraska ground water. Ground Water, 24(2): 231-233.
Gorsky LD & Coon MJ (1985) Evaluation of the role of free hydroxyl
radicals in the cytochrome P-450 catalyzed oxidation of benzene in
microsomes and reconstituted enzyme systems from rabbit liver. J Biol
Chem, 258: 7311.
Gossett RW, Brown DA, & Young DR (1983) Predicting the bioaccumulation
of organic compounds in marine organisms using octanol/water partition
coefficients. Mar Pollut Bull, 14: 387-392.
Government of Canada (in press) Canadian environmental protection act
- Priority assessment report on benzene. Ottawa, Environment
Canada/Health and Welfare Canada.
Green JD, Leong BKJ, Lobue J, Goldstein BD, & Albert RE (1978)
Inhaled benzene fetotoxicity in rats. Toxicol Appl Pharmacol, 46:
9-18.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981a) Acute
and chronic dose/response effect of benzene inhalation on the
peripheral blood, bone marrow, and spleen cells of CD-1 male mice.
Toxicol Appl Pharmacol, 59: 204-214.
Green JD, Snyder CA, Lobue J, Goldstein BD, & Albert RE (1981b) Acute
and chronic dose/response effects of inhaled benzene on multipotential
hematopoietic stem (CFU-S) and granulocyte/macrophase progenitor
(GM-CFU-C) cells in CD-1 mice. Toxicol Appl Pharmacol, 58: 492-503.
Grob K & Grob G (1974) Organic substances in potable water and its
precursor. Part II. Applications in the area of Zurich. J Chromatogr,
90: 303-313.
Gruenke LD, Craig JC, Wester RC, & Maibach HI (1986) Quantitative
analysis of benzene by selected ion monitoring/gas chromatography/mass
spectrometry. J Anal Toxicol, 10(11/12): 225-232.
Hadeishi T, Pollard M, McLaughlin R, & Koga M (1985) Development of an
optical monitor for toxic organic compounds in air. Research Triangle
Park, North Carolina, US Environmental Protection Agency
(EPA/600/4-85-043).
Hall LW Jr, Hall WS, Bushong SJ, & Herman RL (1987) In situ striped
bass (Morone saxatilis) contaminant and water quality studies in the
Potomac River. Aquat Toxicol, 10: 73-99.
Hammers WE & Bosman HFPM (1986) Quantitative evaluation of a simple
dynamic headspace analysis technique for non-polar pollutants in
aqueous samples at the ng/kg level. J Chromatogr, 360: 425-432.
Hancock DG, Moffitt AE Jr, & Hay EB (1984) Hematological findings
among workers exposed to benzene at a coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Hanke J, Dutkiewicz T, & Piotrowski J (1961) [The absorption of
benzene through the skin in men.] Med Pracy, 12: 413-426 (in Polish).
Harkov R, Gianti SJ, Bozzelli JW, & Laregina JE (1985) Monitoring
volatile organic compounds at hazardous and sanitary landfills in New
Jersey. J Environ Sci Health, A20(5): 491-501.
Harland BJ, Whitby FJ, & Comber MHI (1985) Measurement of volatile
aromatic hydrocarbons in an industrial estuary. Int J Environ Anal
Chem, 20: 295-311.
Hattemer-Frey HA, Travis CC, & Land ML (1990) Environmental
partitioning and human exposure. Environ Res, 53: 221-232.
Henderson RF, Sabourin PJ, Bechtold WE, Griffith WC, Medinsky MA,
Birnbaum LS, & Lucier GW (1989) The effect of dose, dose rate, route
of administration, and species on tissue and blood levels of benzene
metabolites. Environ Health Perspect, 82: 9-17.
Hine J & Mookerjee PK (1975) The intrinsic hydrophilic character of
organic compounds. Correlations in terms of structural contributions.
J Org Chem, 40: 292-298.
Hinson JA, Freeman JP, Potter DW, Mitchum RK, & Evans FE (1985)
Mechanism of microsomal metabolism of benzene to phenol. Mol
Pharmacol, 27: 574-577.
Hite M, Pecharo M, Smith I, & Thornton S (1980) The effect of benzene
in the micronucleus test. Mutat Res, 77: 149-155.
Högn T & Jaenicke L (1972) Benzene metabolism in Moraxella species.
Eur J Biochem, 30: 369-375.
Holdren MW, Smith DL, & Smith RN (1985) Comparison of ambient air
sampling techniques for volatile organic compounds. Washington, DC, US
Environmental Protection Agency (EPA/600/S4-85-067; PB86-120953/GAR).
Hornung RW, Ward E, Morris JA, & Rinsky RA (1989) Letter to the
editor. Haematologic effects of benzene: a thirty-five year
longitudinal study of rubber workers. Toxicol Ind Health, 5(6):
1153-1155.
HSE (1982) Toxicity review No. 4: Benzene. London, Health and Safety
Executive, Her Majesty's Stationery Office, 36 pp.
Hsieh GC, Sharma RP, & Parker RDR (1988a) Subclinical effects of
groundwater contaminants. I. Alteration of humoral and cellular
immunity by benzene in CD-1 mice. Arch Environ Contam Toxicol, 17:
151-158.
Hsieh GC, Parker RDR, & Sharma RP (1988b) Subclinical effects of
groundwater contaminants. II. Alteration of regional brain monoamine
neurotransmitters by benzene in CD-1 mice. Arch Environ Contam
Toxicol, 17: 799-805.
Hudak A & Ungvary G (1978) Embryotoxic effects of benzene and its
methyl derivatives: toluene and xylene. Toxicology, 11: 55-63.
Huff JE, Haseman JK, Demarini DM, Eustis S, Maronpot RR, Peters AC,
Persing RL, Chrisp CE, & Jacobs AC (1989) Multiple-site
carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice.
Environ Health Perspect, 82: 125-163.
Hurley JF, Cherrie JW, & MacLaren W (1991) Exposure to benzene and
mortality from leukaemia: results from coke oven and other coal
product workers. Br J Ind Med, 48: 502-504.
IARC (1982) Benzene. In: Some industrial chemicals and dyestuffs.
Lyon, International Agency for Research on Cancer, pp 93-148
(Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to
Humans, Volume 29).
IARC (1987a) Benzene. In: Genetic and related effects: an updating of
selected IARC Monographs from Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 91-95 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 6).
IARC (1987b) Formaldehyde. In: Overall evaluations of carcinogenicity:
An updating of IARC Monographs Volumes 1 to 42. Lyon, International
Agency for Research on Cancer, pp 211-216 (IARC Monographs on the
Evaluation of Carcinogenic Risks to Humans, Supplement 7).
IARC (1989) Occupational exposures in petroleum refining; crude oil
and major petroleum fuels. Lyon, International Agency for Research on
Cancer, 322 pp (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 45).
Ikeda M & Ohtsuji H (1971) Phenobarbital-induced protection against
toxicity of toluene and benzene in the rat. Toxicol Appl Pharmacol,
20: 30-43.
Infante PF, Wagoner JK, Rinsky RA, & Young RJ (1977) Leukaemia in
benzene workers. Lancet, 2: 76-78.
Inoue O, Seiji K, & Nakatsuka H (1989) Urinary t,t-muconic acid as an
indicator of exposure to benzene. Br J Ind Med, 46: 122-127.
Irons RD (1985) Quinones as toxic metabolites of benzene. J Toxicol
Environ Health, 16: 673-678.
Irons RD, Dent JG, Baker TS, & Rickert DE (1980) Benzene is
metabolized and covalently bound in bone marrow in situ. Chem-Biol
Interact, 30: 241-245.
Irons RD, Neptun DA, & Pfeifer RW (1981) Inhibition of lymphocyte
transformation and microtubule assembly by quinone metabolites of
benzene: Evidence for a common mechanism. J Reticuloendothel Soc, 30:
359-372.
IRPTC (1987) IRPTC Legal file 1986 on benzene - Volume 1. Geneva,
International Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
Jackson S & Brown VM (1970) Effect of toxic wastes on treatment
processes and watercourses. Water Pollut Control, 69(3): 292-313.
Jerina DM & Daly JW (1974) Arene oxides: a new aspect of drug
metabolism. Science, 185: 573-582.
Jerina D, Daly J, Witkop B, Zaltzman-Norenberg P, & Udenfriend S
(1968) Role of the arene oxide-oxepin system in the metabolism of
aromatic substrates. I. In vitro conversion of benzene oxide to a
premercapturic acid and a dihydrodiol. Arch Biochem Biophys, 128:
176-183.
Jirka AM & Bourne S (1982) Gas-chromatographic analysis for benzene in
blood. Clin Chem, 28: 1492-1494.
Johansson I & Ingelman-Sundberg M (1988) Benzene metabolism by
ethanol-, acetone-, and benzene-inducible cytochrome P-450(IIE1) in
rat and rabbit liver microsomes. Cancer Res, 48: 5387-5390.
Johnston RV, Pinkerton MN, & Mensik DC (1979) Haematological and
myelogenous effects of inhaled benzene in the pig and rat. J Toxicol
Environ Health, 5: 1025-1035.
Jonek J, Olkowski Z, & Zieleznik B (1965) Histochemical studies on the
spinal cord of mice poisoned with benzene. Acta Histochem, 20:
286-296.
Jonsson A, Persson KA, & Grigoriadis V (1985) Measurements of some low
molecular-weight oxygenated, aromatic and chlorinated hydrocarbons in
ambient air and in vehicle emissions. Environ Int, 11: 383-392.
Jowa L, Witz G, Snyder R, Winkle S, & Kalf GF (1990) Synthesis and
characterization of deoxyguanosine-benzoquinone adduct. J Appl
Toxicol, 10(1): 47-54.
Kalf GF (1987) Recent advances in the metabolism and toxicity of
benzene. CRC Crit Rev Toxicol, 18: 141-159.
Kalf GF, Schlosser MJ, Renz JF, & Priozzi SJ (1989) Prevention of
benzene-induced myelotoxicity by nonsteroidal anti-inflammatory drugs.
Environ Health Perspect, 82: 57-64.
Karam LR & Simic MG (1989) Mechanisms of free radical chemistry and
biochemistry of benzene. Environ Health Perspect, 82: 37-41.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soils. Chemosphere,
10(8): 833-846.
Karlson U & Frankenberger WT Jr (1989) Microbial degradation of
benzene and toluene in groundwater. Bull Environ Contam Toxicol, 43:
505-510.
Keller KA & Snyder CA (1986) Mice exposed in utero to low
concentrations of benzene exhibit enduring changes in their colony
forming hematopoietic cells. Toxicology, 42: 171.
Kenaga EE (1980) Predicted bioconcentration factors and soil sorption
coefficients of pesticides and other chemicals. Ecotoxicol Environ
Saf, 4: 26-38.
Kiang PH & Grob RL (1986) A headspace technique for the determination
of volatile compounds in soil. J Environ Sci Health, A21: 71-100.
Kimmel CA & Wilson JG (1973) Skeletal deviations in rats:
malformations or variations? Teratology, 8: 309-316.
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Kipen HM, Cody RP, Crump KS, Allen BC, & Goldstein BD (1988)
Hematologic effects of benzene: a thirty-five year longitudinal study
of rubber workers. Toxicol Ind Health, 4(4): 411.
Kipen HM, Cody RP, & Goldstein BD (1989) Use of longitudinal analysis
of peripheral blood counts to validate historical reconstructions of
benzene exposure. Environ Health Perspect, 82: 199-206.
Kirley TA, Goldstein BD, Maniara WM, & Witz G (1989) Metabolism of
trans,trans-muconaldehyde, a microsomal hematotoxic metabolite of
benzene by purified yeast aldehyde dehydrogenase and a mouse liver
soluble fraction. Toxicol Appl Pharmacol, 100: 360-367.
Kissling M & Speck B (1972) Further studies on experimental benzene
induced aplastic anemia. Blut, 25: 97-103.
Kocsis JJ, Harkaway S, Santoyo MC, & Snyder R (1968) Dimethyl
sulfoxide: interactions with aromatic hydrocarbons. Science, 160:
427-428.
Koljkowsky P (1981) Indicator-tube method for the determination of
benzene in air. Analyst, 94: 918-920.
Korte F & Klein W (1982) Degradation of benzene in the environment.
Ecotoxicol Environ Saf, 6(4): 311-327.
Kozioski RP (1985) Determination of benzene and toluene in soils and
plant material by azeotropic distillation. Bull Environ Contam
Toxicol, 34: 10-16.
Kuna RA & Kapp RW (1981) Embyrotoxic/teratogenic potential of benzene
vapor in rats. Toxicol Appl Pharmacol, 57: 1-7.
Lahmann E, Seifert B, & Ullrich D (1977) The pollution of ambient air
and rain water by organic components of motor vehicle exhaust-gases.
In: Kasuga S ed. Proceedings of the 4th International Clean Air
Congress. Tokyo, Japanese Union of Air Pollution and Prevention
Association, pp 595-597.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973a) Leukocyte
agglutinins in workers exposed to benzene, toluene and xylene. Int
Arch Arbeitsmed, 31: 45-50.
Lange A, Smolik R, Zatonski W, & Szymanska J (1973b) Serum
immunoglobulin levels in workers exposed to benzene, toluene and
xylene. Int Arch Arbeitsmed, 31: 37-44.
Latriano L, Goldstein BD, & Witz G (1986) Formation of muconaldehyde,
an open-ring metabolite of benzene, in mouse liver microsomes: an
additional pathway for toxic metabolites. Proc Natl Acad Sci (USA),
83: 8356-8360.
Leong B (1977) Experimental benzene intoxication. In: Laskin S &
Goldstein B ed. Benzene toxicity: A critical revaluation. J Toxicol
Environ Health, 3(2): 45-61.
Li G-L, Yin N, Watanabe T, Nakatsuka H, Kasahara M, Abe H, & Ikeda M
(1986) Benzene-specific increase in leukocyte alkaline phosphatase
activity in rats exposed to vapors of various organic solvents. J
Toxicol Environ Health, 19: 581.
Longacre S, Kocsis J, & Snyder R (1981a) Influence of strain
differences in mice on the metabolism and toxicity of benzene. Toxicol
Appl Pharmacol, 60: 398-409.
Longacre S, Kocsis J, Witmer CM, Lee EW, Sammett D, & Snyder R (1981b)
Toxicological and biochemical effects of repeated administration of
benzene in mice. J Toxicol Environ Health, 7: 223-237.
Low LK, Meeks JR, Norris KJ, Mehlman MA, & Macherer CR (1989)
Pharmacokinetics and metabolism of benzene in Zymbal gland and other
key target tissues after oral administration in rats. Environ Health
Perspect, 82: 215-222.
Low LK, Reddy MV, Blackburn GR, & Mackerer CR (1991) Benzene
mechanistic research. Final report of collaborative study by Mobil,
Amoco Ashland, Standard Oil and Sun Company. Princeton, New Jersey,
Mobil Oil Corporation, 76 pp.
Lunte SM & Kissinger PT (1983) Detections and identification of
sulfhydryl conjugates of p-benzoquinone in microsomal incubations of
benzene and phenol. Chem-Biol Interact, 47: 195-212.
Lutz WK (1979) In vivo covalent binding of organic chemicals to DNA
as a quantitative indicator in the process of chemical carcinogenesis.
Mutat Res, 65: 289-356.
Lutz WK & Schlatter C (1977) Mechanisms of the carcinogenic action of
benzene: irreversible binding to rat liver DNA. Chem-Biol Interact,
18(2): 241-246.
McDonald TJ, Kennicutt MC II, & Brooks JM (1988) Volatile organic
compounds at a coastal Gulf of Mexico site. Chemosphere, 17(1):
123-136.
MacKay D & Leinonen PJ (1975) Rate of evaporation of low-solubility
contaminants from water bodies to atmosphere. Environ Sci Technol, 9:
1178-1180.
Maibach HI & Anjo DM (1981) Percutaneous penetration of benzene and
benzene contained in solvents in the rubber industry. Arch Environ
Health, 36: 256-260.
Maltoni C, Ciliberti A, & Carretti D (1982a) Experimental
contributions in identifying brain potential carcinogens in the
petrochemical industry. Ann NY Acad Sci, 381: 216-249.
Maltoni C, Conti B, & Scarnato C (1982b) Squamous cell carcinomas of
the oral cavity in Sprague-Dawley rats following exposure to benzene
by ingestion. Med. Lav, 4: 441-445.
Maltoni C, Cotti G, Valgimigli L, & Mandrioli A (1982c)
Hepatocarcinomas in Sprague-Dawley rats following exposure to benzene
by inhalation. Med Lav, 4: 446-450.
Maltoni C, Conti B, & Cotti G (1983) Benzene: a multipotential
carcinogen. Results of long-term bioassays performed at the Bologna
Institute of Oncology. Am J Ind Med, 4: 589-630.
Maltoni C, Conti B, Cotti G, & Belpoggi F (1985) Experimental studies
on benzene carcinogenicity at the Bologna Institute of Oncology:
current results and ongoing research. Am J Ind Med, 7: 415-446.
Maltoni C, Ciliberti A, Cotti G, Conti B, & Belpoggi F (1989) Benzene,
an experimental multipotential carcinogen: results of the long-term
bioassays performed at the Bologna Institute of Oncology. Environ
Health Perspect, 82: 109-124.
Mara SJ & Lee SS (1978) Assessment of human exposures to atmospheric
benzene. Research Triangle Park, North Carolina, US Environmental
Protection Agency (EPA/450/378-031).
Marcus WL (1990) Chemical of current interest: benzene. Cancer risk
from benzene. Adv Mod Environ Toxicol, 17: 127-188.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989a) Differences in the pathways for metabolism of benzene in rats
and mice simulated by a physiological model. Environ Health Perspect,
82: 43-49.
Medinsky MA, Sabourin PJ, Lucier G, Birnbaum LS, & Henderson RF
(1989b) A toxicological model for simulation of benzene metabolism.
Exp Pathol, 37: 150-154.
Medinsky MA, Sabourin PJ, Henderson RF, Lucier GW, & Birnbaum LS
(1989c) A physiological model for simulation of benzene metabolism by
rats and mice. Toxicol Appl Pharmacol, 99: 193-206.
Merian E & Zander M (1982) Volatile aromatics. In: Hutzinger O ed. The
handbook of environmental chemistry, vol 3, Part B: Anthropogenic
compounds. Berlin, Heidelberg, New York, Springer-Verlag, pp 117-161.
Michael LC, Pellizzari ED, & Wiseman RW (1988) Development and
evaluation of a procedure for determining volatile organics in water.
Environ Sci Technol, 22: 565-570.
Miller MM & Wasik SP (1985) Relationships between octanol-water
partition coefficient and aqueous solubility. Environ Sci Technol,
19(6): 522-529.
Morimoto K (1976) Analysis of combined effects of benzene with
radiation on chromosomes in cultured human leukocytes. Jpn J Ind
Health, 18: 23-24.
Morimoto K (1983) Induction of sister chromatid exchanges and cell
division delays in human lymphocytes by microsomal activation of
benzene. Cancer Res, 43: 1330-1334.
Morimoto K, Wolff S, & Koizumi A (1983) Induction of sister-chromatid
exchanges in human lymphocytes by microsomal activation of benzene
metabolites. Mutat Res, 119: 355-360.
Moszczynski P (1981) Organic solvents and T-lymphocytes. Lancet, 1:
438.
Murray FJ, John JA, Rampy LW, Kuna RA, & Schweta BA (1979)
Embryotoxicity of inhaled benzene in mice and rabbits. Am Ind Hyg
Assoc J, 40: 993-998.
Nahum LH & Hoff HE (1934) The mechanism of sudden death in
experimental benzol poisoning. J Pharmacol Exp Ther, 50: 336.
Nakajima T, Okuyama S, & Yonekura I (1985) Effects of ethanol and
phenobarbital administration on the metabolism and toxicity of
benzene. Chem-Biol Interact, 55: 23-38.
Nakajima T, Elovaara E, Park SS, Gelbain HV, Hietanen E, & Vainio H
(1990) Monoclonal antibody-directed characterization of benzene,
ethoxy-resorufin and pentoxy-resorufin metabolism in rat liver
microsomes. Biochem Pharmacol, 40: 1255-1261.
Nau CA, Neal J, & Thornton M (1966) C9-C12 fractions obtained from
petroleum distillates. Arch Environ Health, 12: 382-393.
Nawrot PS & Staples RE (1979) Embryo-fetal toxicity and teratogenicity
of benzene and toluene in the mouse. Teratology, 19: 41A.
Nerland DE & Pierce WM (1990) Identification of N-acetyl-
S-(2,5-dihydroxyphenyl)-L-cysteine as a urinary metabolite of benzene,
phenol and hydroquinone. Drug Metab Dispos, 18: 958-961.
Nomiyama K & Nomiyama H (1974a) Respiratory retention, uptake and
excretion of organic solvents in man. Benzene, toluene, n-hexane,
trichloroethylene, acetone, ethyl acetate and ethyl alcohol. Int Arch
Arbeitsmed, 32: 75-83.
Nomiyama K & Nomiyama H (1974b) Respiratory elimination of organic
solvents in man. Benzene, toluene, n-hexane, trichloroethylene,
acetone, ethyl acetate and ethyl alcohol. Int Arch Arbeitsmed, 32:
85-91.
Nordlinder R & Ramnäs O (1987) Exposure to benzene at different work
places in Sweden. Ann Occup Hyg, 31: 345-355.
Norpoth K, Stücker W, Krewer E, & Müller G (1988) Biomonitoring of
benzene exposure by trace analyses of phenylguanine. Arch Occup
Environ Health, 60: 163-168.
NTP (1986) Toxicology and carcinogenesis studies of benzene (CAS No.
