
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 155
Biomarkers and Risk Assessment:
Concepts and Principles
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Biomarkers and risk assessment : concepts and principles.
(Environmental health criteria ; 155)
1.Biological markers 2.Environmental exposure
3.Hazardous substances 4.Risk factors
I.Series
ISBN 92 4 157155 1 (NLM Classification: QH 541.15.B615)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
BIOMARKERS AND RISK ASSESSMENT: CONCEPTS AND PRINCIPLES
PREFACE
1. INTRODUCTION
1.1. Biomarkers - concepts
1.2. Definitions
1.3. Biomarkers and the risk assessment process
2. USES OF BIOMARKERS
2.1. Use in health risk assessment
2.2. Use for clinical diagnosis
2.3. Use for monitoring purposes
3. SELECTION AND VALIDATION OF BIOMARKERS
3.1. Selection - practical aspects
3.1.1. General laboratory considerations
3.1.2. Quality assurance and control
3.2. Validation and characteristics of biomarkers
4. ETHICS AND SOCIAL CONSIDERATIONS
5. BIOMARKERS OF EXPOSURE
6. BIOMARKERS OF EFFECT
6.1. Haematological biomarkers
6.2. Nephrotoxicity biomarkers
6.3. Liver toxicity biomarkers
6.4. Biomarkers of immunotoxicity
6.5. Biomarkers of pulmonary toxicity
6.6. Biomarkers of reproductive and developmental toxicity
6.7. Biomarkers of neurotoxicity
7. BIOMARKERS AND CHEMICAL CARCINOGENESIS
7.1. Analysis of chemicals and metabolites
7.2. Biomarkers for genotoxic carcinogens
7.2.1. DNA adducts - general considerations
7.2.2. DNA adducts in human samples
7.2.3. Protein adducts
7.2.4. Cytogenetic methods
7.2.5. Chromosome damage
7.2.6. Sister chromatid exchange
7.2.7. Micronuclei
7.2.8. Aneuploidy
7.2.9. Mutation
7.3. Biomarkers for non-genotoxic carcinogenesis
8. BIOMARKERS OF SUSCEPTIBILITY
9. SUMMARY
10. RECOMMENDATIONS
10.1. General
10.2. Research
10.3. Applications
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON BIOMARKERS AND RISK ASSESSMENT:
CONCEPTS AND PRINCIPLES
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
(Chairman)a,b
Dr D. Anderson, British Industrial Biological Research Association,
Carshalton, Surrey, United Kingdom (Rapporteur)a,b
Dr P. Blain, Division of Environmental and Occupational Medicine,
The Medical School, University of Newcastle upon Tyne, United
Kingdomb
Dr J. Bond, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USAb
Dr M. Buratti, Clinica del Lavoro, Instituti Clinici di
Perfezionamento, Milan, Italya
Dr I. Calder, Occupational and Environmental Health, South
Australian Health Commission, Adelaide, South Australia,
Australiab
Dr I. Chahoud, Institute of Toxicology and Embryo-pharmacology, Free
University of Berlin, Berlin, Germanya
Dr J.R. Fowle, Health Effects Research Laboratory, US Environmental
Protection Agency, Research Triangle Park, North Carolina,
USAa
Dr L. Gerhardsson, Department of Occupational and Environmental
Medicine, Lund University Hospital, Lund, Swedenb
Dr. R. Henderson, Lovelace Inhalation Toxicology Research Institute,
Albuquerque, New Mexico (Vice-Chairman)a,b
Dr H.B.W.M. Koëter, TNO-CIVO Institute, AJ Zeist, The Netherlandsa
Dr A. Nishikawa, Division of Pathology, National Institute of
Hygienic Sciences, Tokyo, Japanb
Dr C.L. Thompson, Laboratory of Biochemical Risk Analysis, National
Institute of Environmental Health Sciences, Research Triangle
Park, North Carolina, USAb
Dr H. Zenick, Health Effects Research Laboratory, US Environmental
Protection Agency, Research Triangle Park, North Carolina,
USAb
Observers
Dr J. Lewalter, Institute of Biological Monitoring, Medical
Department, Bayer AG, Leverkusen, Germany (attending on behalf
of CECa and ECETOCb)
Dr D. Howe, Unilever E.S.L., United Kingdom (attending on behalf of
ECETOCb)
Mrs G. Richold, Unilever E.S.L., United Kingdom (attending on behalf
of ECETOCb)
Secretariat
Dr G.C. Becking, International Programme on Chemical Safety,
Interregional Research Unit, World Health Organization,
Research Triangle Park, North Carolina, USAa
Dr J. Hall-Posner, Unit of Mechanisms of Carcinogenesis,
International Agency for Research on Cancer, Lyon, Franceb
Dr F. He, World Health Organization, Division of Health Protection
and Promotion, Occupational Health, Geneva, Switzerlandb
Dr A. Robinson, Ontario Ministry of Labour, Toronto, Ontario, Canada
(Temporary Adviser)b
a Participant in Planning Meeting on Utilization of Biological
Markers in Risk Assessment (Non-Carcinogenic End-Points), 25-27
October 1989, Carshalton, UK
b Participant in Task Group on Biomarkers and Risk Assessment:
Concepts and Principles, 16-20 November 1992, Carshalton, UK
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone No.
9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
BIOMARKERS AND RISK ASSESSMENT: CONCEPTS AND PRINCIPLES
At the Sixth Meeting of the IPCS Programme Advisory Committee
(31 October to 3 November 1989) it was recommended that the IPCS
give priority to work on biomarkers, as outlined at an IPCS Planning
Meeting (25-28 October 1989). One of the recommendations from the
Planning Meeting was for the IPCS to prepare an Environmental Health
Criteria monograph on the concepts and principles supporting the use
of biomarkers in the assessment of human health risks from exposure
to chemicals.
The drafts of this monograph were prepared by Dr A. Robinson,
Toronto, Canada. During the preparation of the monograph many
scientists made constructive suggestions and their assistance is
gratefully acknowledged.
A WHO Task Group on Biomarkers and Risk Assessment: Concepts
and Principles met in Carshalton, United Kingdom, from 16 to 20
November 1992. Dr Robinson opened the meeting on behalf of the heads
of the three cooperating organizations (UNEP/ILO/WHO), and Dr D.
Anderson welcomed the participants on behalf of the British
Industrial Biological Research Association, the host institution.
The Task Group reviewed and revised the draft monograph.
Following the Task Group Meeting, Dr Robinson collated the text
with the assistance of Dr A. Aitio and Dr D. Anderson, Chairman and
Rapporteur, respectively, of the Task Group. The Secretariat wishes
to acknowledge their special contributions in finalizing this
monograph.
Dr A. Robinson was responsible for the overall scientific
content, and Dr P.G. Jenkins (IPCS Central Unit) for the technical
editing.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged. Special
thanks are due to the United Kingdom Department of Health for its
financial support of both the Planning and the Task Group Meetings.
PREFACE
The purpose of this monograph is to examine the concepts and to
identify the principles for the application of biomarkers to
assessment of risk to human health from exposure to chemical agents,
with special attention to criteria for selection and validation.
Information and examples are provided to illustrate and assist
the application of these principles to enhance human health risk
assessment by reducing the uncertainties associated with the
process. Biomarkers may be indicative of exposure, effect(s) or
susceptibility of individuals to chemical agents, but their use must
take account also of ethical and social considerations.
Some guidance is provided for the selection of appropriate
biomarkers to allow identification of individuals and
sub-populations at increased risk, with consequent implications for
administrative intervention, mitigation and health protection.
A review has been made of biomarkers suitable for application
to assessment of the risk of chemicals that are toxic to the
hepatic, renal, haematological, immune, pulmonary, reproductive/
developmental and nervous systems or are associated with
carcinogenic mechanisms. However, greater detail is provided for
biomarkers linked with carcinogenesis, reflecting the volume of
scientific publications resulting from recent intensive studies of
mechanisms, provoked by public attitudes and perceptions associated
with diagnosis of the disease. This section serves to illustrate the
complexity of the interactions and the many factors which will
influence selection and application of biomarkers to improve further
the process of health risk assessment.
1. INTRODUCTION
1.1 Biomarkers - concepts
Analysis of tissues and body fluids for chemicals, metabolites
of chemicals, enzymes and other biochemical substances has been used
to document the interaction of chemicals with biological systems.
Measurements of these substances, now referred to as "biomarkers",
are recognized as providing data linking exposure to a chemical with
internal dose and outcome and as relevant to the process of risk
assessment.
The term "biomarker" is used in this monograph, as it is in the
US National Academy of Sciences report (US NRC, 1989b), in a broad
sense to include almost any measurement reflecting an interaction
between a biological system and a potential hazard, which may be
chemical, physical or biological. The measured response may be
functional and physiological, biochemical at the cellular level, or
a molecular interaction. Various factors will apply in assessing
risks to individuals and population subgroups compared with the
general population.
In the assessment of risk, biomarkers may be used in hazard
identification, exposure assessment and to associate a response with
the probability of a disease outcome. By examining the interactions
between human host and chemical exposure, and comparable data for
experimental studies of mammalian species, criteria for the
selection of biomarkers indicative of exposure, effects,
susceptibility and toxic response(s) to chemicals may be
established.
The reaction to exposure to a chemical depends on inherited and
acquired characteristics and the life-style of the human subject (or
other biological system), the properties and form of the chemical,
and the circumstances of the contact. The outcome may be no effect,
some adverse effect with recovery, or toxicity with morbidity.
Human health is affected by all the activities of an
individual, who is subject to a continuum of chemical exposures in
the external environment, including air, water, soil and food. It
should be noted that distinction of exposure to chemicals on the
basis of context, such as recreational, residential or occupational,
is often made for administrative convenience. The important
considerations for assessment of risk are the dose rate, route,
duration and frequency of exposure.
The application of biomarkers, linked to toxic processes or
mechanisms, to the risk assessment process, and particularly to
quantitative risk assessment, has the potential to provide a more
rational and less judgmental process, particularly when compared
with methods that arbitrarily attach protection factors to doses
assessed to minimize or avoid effects deemed adverse to health.
Selection of appropriate biomarkers is of critical importance
because of the opportunity for greater precision in the assessment
of risk to individuals or population sub-groups, with the consequent
implications for mitigation and health protection. However,
selection will depend upon the state of scientific knowledge and be
influenced by social, ethical and economic factors.
Subject to ethical considerations, the use of validated
biomarkers to monitor exposed populations may provide the basis for
early, health-protective intervention.
Identification of practicable biomarkers associated with
different toxic end-points or outcomes requires interdisciplinary
cooperation and research, and this is evident in relation to
carcinogenesis, neurotoxicity, pulmonary toxicity, immunotoxicity
and human reproduction. While not all of these areas of interest are
equally well developed, use of biomarkers linked with toxicity
should enhance the process and reliability of predictions of risk.
Improved definition of the risk associated with exposure to
chemicals will permit effective preventive intervention to protect
human health both in general and in particular circumstances.
Protective measures may include avoidance of exposure to chemicals
or protection of sensitive individuals.
1.2 Definitions
The term "biomarker" is used in a broad sense to include almost
any measurement reflecting an interaction between a biological
system and an environmental agent, which may be chemical, physical
or biological. However, discussion in this monograph is limited to
chemical agents. Three classes of biomarkers are identified:
* biomarker of exposure: an exogenous substance or its
metabolite or the product of an interaction between a
xenobiotic agent and some target molecule or cell that is
measured in a compartment within an organism;
* biomarker of effect: a measurable biochemical, physiological,
behavioural or other alteration within an organism that,
depending upon the magnitude, can be recognized as associated
with an established or possible health impairment or disease;
* biomarker of susceptibility - an indicator of an inherent or
acquired ability of an organism to respond to the challenge of
exposure to a specific xenobiotic substance.
1.3 Biomarkers and the risk assessment process
For a general discussion of concepts and principles underlying
assessment of risk to human health associated with exposure to
chemicals, the reader is referred to WHO (in press).
The process for assessment of human health risks associated
with exposure to chemicals is multifaceted and incorporates the
following major components:
* hazard identification: to confirm that the chemical is
capable, subject to appropriate circumstances, of causing an
adverse effect in humans;
* dose-response assessment: to establish the quantitative
relationship between dose and effect in humans;
* exposure assessment: to identify and define the exposures
that occur, or are anticipated to occur, in human populations.
Risk characterization is the synthesis of the qualitative and
quantitative information that describes the estimated risk to human
health from the anticipated environmental exposure.
Hazard identification and dose-response assessment make use of
all available data for human and test species and, where
appropriate, for in vitro test systems.
The relevance of biomarkers to the phases of the risk
assessment process is discussed more fully in later sections that
address biomarkers of effects, exposure and susceptibility.
2. USES OF BIOMARKERS
Biomarkers may be used to assess the exposure (absorbed amount
or internal dose) and effect(s) of chemicals and susceptibility of
individuals, and they may be applied whether exposure has been from
dietary, environmental or occupational sources. Biomarkers may be
used to elucidate cause-effect and dose-effect relationships in
health risk assessment, in clinical diagnosis and for monitoring
purposes.
Biomarkers of exposure can be used to confirm and assess the
exposure of individuals or populations to a particular substance,
providing a link between external exposures and internal dosimetry.
Biomarkers of effect can be used to document either preclinical
alterations or adverse health effects elicited by external exposure
and absorption of a chemical. Thus the linkage of biomarkers between
exposure and effect contributes to the definition of dose-response
relationships. Biomarkers of susceptibility help elucidate the
degree of the response to exposure elicited in individuals.
2.1 Use in health risk assessment
Measurements carried out for many years within the context of
"biological monitoring" have been used to assess worker exposure
and, in clinical settings, to evaluate the administration of
therapeutic agents. These measurements, or biomarkers, provide the
critical link between chemical exposure, internal dose and health
impairment, and are of value in assessment of risk. However, there
is a need to identify and validate for each organ system those
characteristic parameter(s) that are indicative of induced
dysfunction, clinical toxicity or pathological change, as well as to
establish the specificity and sensitivity of each biomarker and its
method of measurement.
2.2 Use for clinical diagnosis
Biomarkers may be used to:
* confirm diagnosis of acute or chronic poisoning;
* assess the effectiveness of treatment; and
* evaluate the prognosis of individual cases.
For this purpose, a well-established relationship between
biomarker(s) and outcome must be available. Assessment of exposure
in short-term or long-term exposure situations can be evaluated on a
more meaningful basis where previous exposure has been documented by
consecutive measurements over a period of time. Although this may
not be possible in the circumstances of a major chemical release,
biomarkers of effect may still find useful application to assess
clinical outcome(s).
2.3 Use for monitoring purposes
Biomarkers may be used to confirm the exposure of individuals
in a population to a particular substance, e.g., an organic solvent
in exhaled breath, the cadmium burden of the kidney, lead in bone,
or the fatty tissue storage of chlorinated hydrocarbons (see Table
1, chapter 5). Quantitative measurements may facilitate the
determination of dose-response relationships.
Biomarkers are used for screening and for monitoring (repeated
at timed intervals), and may be determined and applied on an
individual basis or may be related to a population group. Population
groups "at risk" may be identified by deviations from normal of mean
values for biomarkers of exposure or effects; individual variations
will be reflected in statistical terms.
Some public and occupational health surveillance programmes
include the use of biomarkers for screening and monitoring purposes.
Although the terms "biological screening or monitoring" and "health
monitoring" have been applied, there is no agreement that the terms
are appropriate, and repeated measurement of biomarkers may be
cost-effective methodologies to monitor disease development. In
practice, however, ethical and social considerations, rather than
cost, often preclude the widespread use of biomarkers for monitoring
or surveillance purposes.
Biomarkers of exposure or effect may be used to evaluate
compliance with advice for minimizing exposure or for remedial
measures in a public health context, e.g., to confirm reduced
exposure to lead from environmental sources in a population group.
In addition, biomarkers may be used to supplement environmental or
ambient workplace measurements of chemicals with recognized
potential adverse health effects that may be subject to regulatory
controls.
Biomarkers may serve as a basis for assessing individual or
population groups exposed to chemicals from any source, including
life-style activities. In an occupational context, biomarkers will
provide a supplementary means for reviewing the adequacy of
protective measures, including work practices and working
conditions.
When the inter-individual variation of the biomarker is large
in comparison to intra-individual variation, analysis of paired
samples (before, during and after the exposure) may greatly enhance
the power of the biomarker to detect exposure, e.g., serum
acetylcholine esterase measurements in relation to exposure to an
organophosphorus compound.
Use of biomarkers reflecting genetically linked or acquired
susceptibility to specific chemicals or their metabolites provides
an opportunity for the recognition and protection of sensitive
individuals. The classic example of genetically linked
susceptibility is phenylketonuria in newborn infants. An example of
acquired susceptibility is the development of hypersensitivity to
certain inhaled gases or dusts (such as toluene diisocyanate,
trimellitic acid anhydride or cotton dust) in the workplace.
3. SELECTION AND VALIDATION OF BIOMARKERS
The process of selection and validation requires careful
consideration of the specificity and sensitivity of the biomarker as
a measure of the contribution of the exposure to an observed adverse
health outcome. A similar process must be applied also to
establishing the accuracy, precision and quality assurance of the
analytical procedure for measurement of the selected biomarker.
Before discussing the criteria for the selection and validation
of biomarkers of exposure, effect and susceptibility, and their
application to the risk assessment process, it is necessary to
consider key factors that can influence the host reaction to
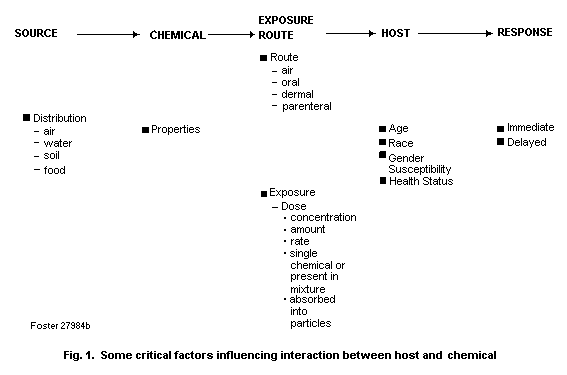
xenobiotic chemicals. Fig. 1 summarizes some of the various factors
that influence the interaction between host and chemical.
These factors may be considered in the context of a
source-chemical-host response where the source of the specific
chemical of concern may be the air, water, soil or food. It is
important to consider the physico-chemical properties of the
chemical (e.g., gas, vapour, particle) and whether the chemical is
present in a complex chemical mixture or adsorbed on a particle. For
example, the initial site of deposition (and perhaps the site of
toxicity) of a chemical in the respiratory tract may be affected by
the strength of the association between the chemical and particulate
matter (which determines bioavailability), as well as by particle
size, in the inhaled atmosphere (e.g., nose versus deep lung).
Several exposure characteristics need also to be considered, such as
the concentration of the chemical and the duration, frequency and
magnitude of exposure. Exposure of the host can be through various
routes including the respiratory tract (inhalation exposure), the
gastrointestinal tract (oral exposure) and the skin (dermal
exposure). Finally, there are a number of host characteristics that
can influence response to chemical exposure, including age, race,
gender, health status, genetic susceptibility, and previous exposure
to the same or other chemicals. Information relating to these
factors can provide clues as to the types of biomarkers that may be
used to assess exposure, effect and susceptibility.
Many factors require consideration in the process for selection
and validation of a biomarker. To select the most appropriate
biomarker requires several steps:
(1) the identification and definition of the end-point of interest;
(2) the assembly of the data base to document the relationship
between the chemical exposure, the possible biomarkers and the
end-point. This will include data from in vitro, mammalian
and human studies, with assessment of the validity of data and
the study protocols;
 (3) selection of biomarker(s) specific to the outcome of interest
with careful consideration of the biomarker to identify what is
being quantified, to assess the sensitivity and specificity of
the marker in relation to exposure, and the significance with
respect to health outcome or pathological change over time;
(4) consideration of specimens potentially available for analysis,
with emphasis on protecting the integrity of the specimen
between collection and analysis, and a preference for
non-invasive techniques;
(5) review of the analytical procedures available for
quantification of biomarkers and their limitations with respect
to detection limit, sensitivity, precision and accuracy;
(6) establishment of an appropriate analytical protocol with
provision for quality assurance and quality control;
(7) evaluation of intra- and inter-individual variation for a
non-exposed population;
(8) analysis of the data base to establish dose-effect and
dose-response relationships and their variation, with special
emphasis on susceptible individuals;
(9) calculation or prediction of risk to human health either for
the general population or a sub-group; and
(10) review of ethical and social considerations.
These issues are discussed more fully in the following
sections.
These steps may need to be carried out in an interactive and
iterative manner before selection of the desired biomarker can be
made.
3.1 Selection - practical aspects
3.1.1 General laboratory considerations
Measurement of biomarkers may range from molecular events to
functional outcomes such as behaviour or pulmonary function; the
same consideration should be applied to all biomarkers.
Analytical considerations include defined and appropriate
precision and accuracy, and quality assurance and control, as well
as the availability of automated instrumentation or alternative
simple, but specific, methodology. Specimen collection, handling and
storage should require the minimum of special precautions to avoid
contamination and/or deterioration. The costs, in terms of skilled
human resources, equipment and reagents should be reasonable.
Sampling and measurements should preferably be:
* non-invasive;
* representative, i.e. the time of the exposure in relation to
measurement should be taken into account; and
* the stability of the analyte in the specimen should be
established.
In general terms, specimens available for analysis will include
blood, urine, sputum, saliva, finger-nails, breath, hair, faeces and
(shed) teeth. The clinic or hospital setting may provide the
opportunity to collect unique fluid samples (e.g., follicular,
amniotic, semen) or tissues accompanying examination of the patient
(e.g., cytological material, pulmonary lavage), tissue biopsies
(e.g., fat) or autopsy specimens.
Specialized techniques for in vivo determination may be
available for some chemicals, e.g., cadmium in kidney or lead in
bone, but such applications require exposure of individuals to
radiation. In such instances, ethical considerations must also be
taken into account.
3.1.2 Quality assurance and control
Critical to the successful and effective application of
biomarkers is a well-documented quality assurance and control
programme. It is beyond the scope of this monograph to discuss such
programmes in detail, but these have been reviewed (Aitio, 1981;
WHO, 1992b). It is important to note that good analytical
performance does not necessarily guarantee accurate results in
biomarker analyses since greater errors may be introduced during
sampling. Thus, the quality assurance protocol has to cover the
entire process. Major impediments to quality assurance of biomarker
analyses include the lack of certified reference materials and
external quality control programmes.
3.2 Validation and characteristics of biomarkers
Validation is a process to establish the qualitative and
quantitative relationship of the biomarker (a) to exposure to a
chemical, and (b) to the selected end-point. Desirable
characteristics of biomarkers include that:
(1) the marker (measurement)
(a) reflects the interaction (qualitative or quantitative) of
the host biological system with the chemical of interest,
(b) has known and appropriate specificity and sensitivity to
the interaction,
(c) is reproducible qualitatively and quantitatively with
respect to time (short- and long-term);
(2) the analytical measurement has defined and appropriate accuracy
and precision;
(3) the marker is common to individuals within a population or
subgroup and is of defined variability within the normal,
non-exposed population or group of interest; and
(4) the marker is common between species.
4. ETHICS AND SOCIAL CONSIDERATIONS
It is important to recognize the ethical and social
implications of the uses of biomarkers, in addition to the
scientific and cost considerations. Ethical concerns may limit the
extent of investigations of chemically exposed human individuals and
populations, particularly those involving the living.
Participation by individuals or groups will be influenced both
by personal and scientific factors. Personal attitudes, ideals and
beliefs will vary geographically with ethnic origin and cultural
practices.
The process leading to participation is critically important
and must respect the dignity, rights and freedom of choice of
individuals; participation must be voluntary and based on full
information.
The freedom of choice of individuals will include the right of
refusal to give blood or other biological samples for analysis of
biomarkers. Personal decisions should be based on full information
with the implications of a refusal being explained and understood.
Before measurement of a biomarker is undertaken, there should
be consideration of how and to whom results should be provided, the
interpretation of the results, and whether this should be on an
individual or group basis, with or without protection of
confidentiality. While practices will vary between countries, it is
particularly important that the role of medical officers be defined
in relation to their responsibility to the individual (patient) and
in relation to administrative (company) management.
It is the ethical responsibility of medical officers to inform
individuals fully of potentially hazardous exposures, recognizing
that remedial action may involve administrative decisions. The
latter may be taken in the context of prevailing economic and social
considerations rather than of individual circumstances.
Investigators need to recognize and accommodate the medical
dilemma created by the conduct of biomarker research in terminally
ill patients, since the data may clarify the use of the biomarker
without contributing to improvement of the health status of the
patient. Thus, careful consideration must be given to the desire to
advance scientific understanding relative to meeting the needs of
the patient.
In addition to the general ethical issues associated with use
of biomarkers, there are particular problems relating to biomarkers
of susceptibility.
Identifying susceptible individuals may help to prevent their
exposure to a specific harmful chemical(s) but may lead to
discrimination in the employment of the susceptible individual when
that chemical is known to be present in the workplace.
It is also an ethical question as to whether individuals should
be given information about their own susceptibility. This knowledge
would allow them to make more informed choices, for example, the
avoidance of exposure to specific substances. It is, however,
important to realize that only for a few biomarkers of
susceptibility is it well established that they are associated with
the development of disease. If the individual does not fully
understand this uncertainty, such information on biomarkers may
cause unnecessary concern and anxiety.
5. BIOMARKERS OF EXPOSURE
The exposure assessment component of the risk assessment
process is an attempt to provide qualitative and quantitative
estimates of human exposure through the use of measurements and
models. In this context, measurements may be made of chemical
concentrations in food, water and air, selected environmental
concentrations (e.g., occupational or residential settings) as well
as measures of the actual exposures experienced by the individual or
population. Exposure biomarkers extend this latter component of
exposure assessment into the realm of data which provide the most
direct evidence of human exposure to a given agent and the absorbed
dose.
Adverse or toxic effects in a biological system are not
produced by chemical agents unless that agent or its
biotransformation products reach appropriate sites in the body at a
concentration and for a length of time sufficient to produce the
toxic manifestation. Thus, to characterize fully the potential
hazard or toxicity of a specific chemical agent in an individual, it
is necessary to identify not only the type of effect and the dose
required to produce the effect but also information about the
duration and frequency of exposure to the agent, and the
susceptibility of the exposed individual.
Methods for assessing exposure to a chemical fall into two
categories:
1. measurement of levels of chemical agents and their metabolites
and/or derivatives in cells, tissue, body fluids or excreta;
and
2. measurement of biological responses such as cytogenetic and
reversible physiological changes in the exposed individuals.
Measurement of covalent adducts formed between chemical agents
and cellular macromolecules (proteins, DNA), or their excretion
products have characteristics of both category one and two above.
In evaluating exposure, distinction is made between the
external dose, defined as the amount of a chemical agent in
environmental contact with the organism, as determined by personal
or area monitoring, and the internal dose, which is the total
amount of a chemical agent absorbed by the organism over a period of
time. Biomarkers of exposure will reflect the distribution of the
chemical or its metabolite throughout the organism. Theoretically,
this distribution can be tracked through various biological levels
(e.g., tissue, cell, etc.) to the ultimate target. The concept of
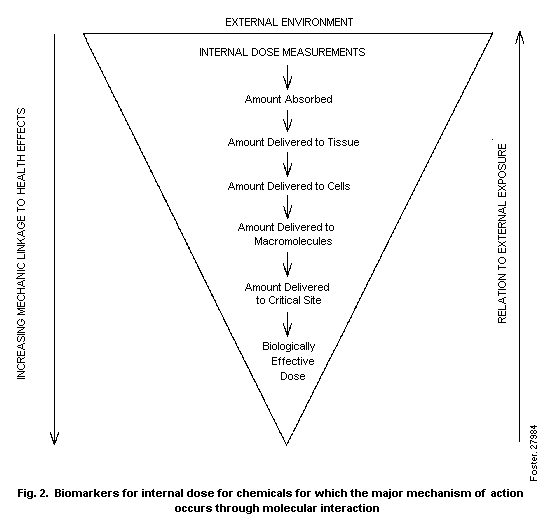
biomarkers of exposure is illustrated in Fig. 2.
This figure illustrates that, of the total amount absorbed,
only a portion will be delivered to a target tissue. A portion will
reach internal macromolecules, and a smaller proportion will reach
the critical site on the macromolecule, with only a fraction of the
latter amount acting as the biologically effective dose. Biomarkers
for each of these forms of internal dose would be useful for
assessment of risk. The decreasing area of the triangle, from the
total absorbed amount to each lower level as distribution and
metabolism occur, illustrates the decreasing mass of the internal
dose that reaches the target tissue, cell, or critical site. In
progressing from biomarkers of total absorbed dose to markers of
biologically effective dose, it becomes increasingly easy to relate
the dose to the mechanism of the induced health effect. In the
reverse process, from the dose for critical sites to the total
amount absorbed, it becomes easier to relate the internal dose to
the external exposure.
The internal dose can be assessed by suitable analyses of
biomarkers in biological samples (urine, faeces, blood and/or its
components, expired air) (Alessio et al., 1983, 1984, 1986, 1987,
1988, 1989; UK HSE, 1991; ACGIH, 1992; DFG, 1992; Bond et al.,
1992). These biomarkers may be the unchanged chemical material, its
known metabolites or biochemical markers affected by absorption of
the chemical. It may be possible to estimate the dose quantitatively
when the toxicokinetics of the chemical is well established and the
sampling is conducted at appropriate points in time. The tissue dose
may be further refined to a specific target dose which may be
defined as the amount of chemical (or its metabolite) which, over a
period of time, reaches the biologically significant site(s) within
the target tissue.
While such measurements may not equate to the biologically
effective dose, the data can provide useful estimates of internal
exposure (dose). Knowledge of the kinetics of formation and removal
from the body of these types of biomarkers provides a link between
exposure and internal dose.
Specific measures of internal dose are the active chemical
species (either parent compound or metabolite) delivered to target
tissues or cells, the reactive chemical species delivered to target
organelles or macromolecules, or the reactive chemical species that
participates in biochemical reactions. For example, quantification
of the generalized covalent binding of reactive species to
macromolecules will provide a measure of absorbed dose delivered to
target tissues or cells, while measurement of total DNA adducts is
indicative of the dose delivered to target organelles or
macromolecules. Finally, specific DNA adducts could be the
biologically effective species that initiate the carcinogenic
process. These issues are discussed in chapter 7.
(3) selection of biomarker(s) specific to the outcome of interest
with careful consideration of the biomarker to identify what is
being quantified, to assess the sensitivity and specificity of
the marker in relation to exposure, and the significance with
respect to health outcome or pathological change over time;
(4) consideration of specimens potentially available for analysis,
with emphasis on protecting the integrity of the specimen
between collection and analysis, and a preference for
non-invasive techniques;
(5) review of the analytical procedures available for
quantification of biomarkers and their limitations with respect
to detection limit, sensitivity, precision and accuracy;
(6) establishment of an appropriate analytical protocol with
provision for quality assurance and quality control;
(7) evaluation of intra- and inter-individual variation for a
non-exposed population;
(8) analysis of the data base to establish dose-effect and
dose-response relationships and their variation, with special
emphasis on susceptible individuals;
(9) calculation or prediction of risk to human health either for
the general population or a sub-group; and
(10) review of ethical and social considerations.
These issues are discussed more fully in the following
sections.
These steps may need to be carried out in an interactive and
iterative manner before selection of the desired biomarker can be
made.
3.1 Selection - practical aspects
3.1.1 General laboratory considerations
Measurement of biomarkers may range from molecular events to
functional outcomes such as behaviour or pulmonary function; the
same consideration should be applied to all biomarkers.
Analytical considerations include defined and appropriate
precision and accuracy, and quality assurance and control, as well
as the availability of automated instrumentation or alternative
simple, but specific, methodology. Specimen collection, handling and
storage should require the minimum of special precautions to avoid
contamination and/or deterioration. The costs, in terms of skilled
human resources, equipment and reagents should be reasonable.
Sampling and measurements should preferably be:
* non-invasive;
* representative, i.e. the time of the exposure in relation to
measurement should be taken into account; and
* the stability of the analyte in the specimen should be
established.
In general terms, specimens available for analysis will include
blood, urine, sputum, saliva, finger-nails, breath, hair, faeces and
(shed) teeth. The clinic or hospital setting may provide the
opportunity to collect unique fluid samples (e.g., follicular,
amniotic, semen) or tissues accompanying examination of the patient
(e.g., cytological material, pulmonary lavage), tissue biopsies
(e.g., fat) or autopsy specimens.
Specialized techniques for in vivo determination may be
available for some chemicals, e.g., cadmium in kidney or lead in
bone, but such applications require exposure of individuals to
radiation. In such instances, ethical considerations must also be
taken into account.
3.1.2 Quality assurance and control
Critical to the successful and effective application of
biomarkers is a well-documented quality assurance and control
programme. It is beyond the scope of this monograph to discuss such
programmes in detail, but these have been reviewed (Aitio, 1981;
WHO, 1992b). It is important to note that good analytical
performance does not necessarily guarantee accurate results in
biomarker analyses since greater errors may be introduced during
sampling. Thus, the quality assurance protocol has to cover the
entire process. Major impediments to quality assurance of biomarker
analyses include the lack of certified reference materials and
external quality control programmes.
3.2 Validation and characteristics of biomarkers
Validation is a process to establish the qualitative and
quantitative relationship of the biomarker (a) to exposure to a
chemical, and (b) to the selected end-point. Desirable
characteristics of biomarkers include that:
(1) the marker (measurement)
(a) reflects the interaction (qualitative or quantitative) of
the host biological system with the chemical of interest,
(b) has known and appropriate specificity and sensitivity to
the interaction,
(c) is reproducible qualitatively and quantitatively with
respect to time (short- and long-term);
(2) the analytical measurement has defined and appropriate accuracy
and precision;
(3) the marker is common to individuals within a population or
subgroup and is of defined variability within the normal,
non-exposed population or group of interest; and
(4) the marker is common between species.
4. ETHICS AND SOCIAL CONSIDERATIONS
It is important to recognize the ethical and social
implications of the uses of biomarkers, in addition to the
scientific and cost considerations. Ethical concerns may limit the
extent of investigations of chemically exposed human individuals and
populations, particularly those involving the living.
Participation by individuals or groups will be influenced both
by personal and scientific factors. Personal attitudes, ideals and
beliefs will vary geographically with ethnic origin and cultural
practices.
The process leading to participation is critically important
and must respect the dignity, rights and freedom of choice of
individuals; participation must be voluntary and based on full
information.
The freedom of choice of individuals will include the right of
refusal to give blood or other biological samples for analysis of
biomarkers. Personal decisions should be based on full information
with the implications of a refusal being explained and understood.
Before measurement of a biomarker is undertaken, there should
be consideration of how and to whom results should be provided, the
interpretation of the results, and whether this should be on an
individual or group basis, with or without protection of
confidentiality. While practices will vary between countries, it is
particularly important that the role of medical officers be defined
in relation to their responsibility to the individual (patient) and
in relation to administrative (company) management.
It is the ethical responsibility of medical officers to inform
individuals fully of potentially hazardous exposures, recognizing
that remedial action may involve administrative decisions. The
latter may be taken in the context of prevailing economic and social
considerations rather than of individual circumstances.
Investigators need to recognize and accommodate the medical
dilemma created by the conduct of biomarker research in terminally
ill patients, since the data may clarify the use of the biomarker
without contributing to improvement of the health status of the
patient. Thus, careful consideration must be given to the desire to
advance scientific understanding relative to meeting the needs of
the patient.
In addition to the general ethical issues associated with use
of biomarkers, there are particular problems relating to biomarkers
of susceptibility.
Identifying susceptible individuals may help to prevent their
exposure to a specific harmful chemical(s) but may lead to
discrimination in the employment of the susceptible individual when
that chemical is known to be present in the workplace.
It is also an ethical question as to whether individuals should
be given information about their own susceptibility. This knowledge
would allow them to make more informed choices, for example, the
avoidance of exposure to specific substances. It is, however,
important to realize that only for a few biomarkers of
susceptibility is it well established that they are associated with
the development of disease. If the individual does not fully
understand this uncertainty, such information on biomarkers may
cause unnecessary concern and anxiety.
5. BIOMARKERS OF EXPOSURE
The exposure assessment component of the risk assessment
process is an attempt to provide qualitative and quantitative
estimates of human exposure through the use of measurements and
models. In this context, measurements may be made of chemical
concentrations in food, water and air, selected environmental
concentrations (e.g., occupational or residential settings) as well
as measures of the actual exposures experienced by the individual or
population. Exposure biomarkers extend this latter component of
exposure assessment into the realm of data which provide the most
direct evidence of human exposure to a given agent and the absorbed
dose.
Adverse or toxic effects in a biological system are not
produced by chemical agents unless that agent or its
biotransformation products reach appropriate sites in the body at a
concentration and for a length of time sufficient to produce the
toxic manifestation. Thus, to characterize fully the potential
hazard or toxicity of a specific chemical agent in an individual, it
is necessary to identify not only the type of effect and the dose
required to produce the effect but also information about the
duration and frequency of exposure to the agent, and the
susceptibility of the exposed individual.
Methods for assessing exposure to a chemical fall into two
categories:
1. measurement of levels of chemical agents and their metabolites
and/or derivatives in cells, tissue, body fluids or excreta;
and
2. measurement of biological responses such as cytogenetic and
reversible physiological changes in the exposed individuals.
Measurement of covalent adducts formed between chemical agents
and cellular macromolecules (proteins, DNA), or their excretion
products have characteristics of both category one and two above.
In evaluating exposure, distinction is made between the
external dose, defined as the amount of a chemical agent in
environmental contact with the organism, as determined by personal
or area monitoring, and the internal dose, which is the total
amount of a chemical agent absorbed by the organism over a period of
time. Biomarkers of exposure will reflect the distribution of the
chemical or its metabolite throughout the organism. Theoretically,
this distribution can be tracked through various biological levels
(e.g., tissue, cell, etc.) to the ultimate target. The concept of
biomarkers of exposure is illustrated in Fig. 2.
This figure illustrates that, of the total amount absorbed,
only a portion will be delivered to a target tissue. A portion will
reach internal macromolecules, and a smaller proportion will reach
the critical site on the macromolecule, with only a fraction of the
latter amount acting as the biologically effective dose. Biomarkers
for each of these forms of internal dose would be useful for
assessment of risk. The decreasing area of the triangle, from the
total absorbed amount to each lower level as distribution and
metabolism occur, illustrates the decreasing mass of the internal
dose that reaches the target tissue, cell, or critical site. In
progressing from biomarkers of total absorbed dose to markers of
biologically effective dose, it becomes increasingly easy to relate
the dose to the mechanism of the induced health effect. In the
reverse process, from the dose for critical sites to the total
amount absorbed, it becomes easier to relate the internal dose to
the external exposure.
The internal dose can be assessed by suitable analyses of
biomarkers in biological samples (urine, faeces, blood and/or its
components, expired air) (Alessio et al., 1983, 1984, 1986, 1987,
1988, 1989; UK HSE, 1991; ACGIH, 1992; DFG, 1992; Bond et al.,
1992). These biomarkers may be the unchanged chemical material, its
known metabolites or biochemical markers affected by absorption of
the chemical. It may be possible to estimate the dose quantitatively
when the toxicokinetics of the chemical is well established and the
sampling is conducted at appropriate points in time. The tissue dose
may be further refined to a specific target dose which may be
defined as the amount of chemical (or its metabolite) which, over a
period of time, reaches the biologically significant site(s) within
the target tissue.
While such measurements may not equate to the biologically
effective dose, the data can provide useful estimates of internal
exposure (dose). Knowledge of the kinetics of formation and removal
from the body of these types of biomarkers provides a link between
exposure and internal dose.
Specific measures of internal dose are the active chemical
species (either parent compound or metabolite) delivered to target
tissues or cells, the reactive chemical species delivered to target
organelles or macromolecules, or the reactive chemical species that
participates in biochemical reactions. For example, quantification
of the generalized covalent binding of reactive species to
macromolecules will provide a measure of absorbed dose delivered to
target tissues or cells, while measurement of total DNA adducts is
indicative of the dose delivered to target organelles or
macromolecules. Finally, specific DNA adducts could be the
biologically effective species that initiate the carcinogenic
process. These issues are discussed in chapter 7.
 The potential impact of target tissue DNA-protein cross-links
as a biomarker of the biologically effective dose is illustrated in
the proposed risk assessment for formaldehyde put forward by US EPA
(1991). The biological, mechanism-based model of formaldehyde
carcinogenesis consists of three submodels (Conolly et al., 1992;
Conolly & Andersen, in press). One of these is a tissue dosimetry
submodel which incorporates formaldehyde-induced cross-links of DNA
with adjacent proteins (Casanova et al., 1989, 1991). Although the
role of DNA-protein cross-links in the mechanism of formaldehyde-
induced nasal cancer is not known, their formation is used only as a
biomarker of the "biologically effective" dose reaching target cells
in the nasal cavity. An earlier proposal (US EPA, 1987) used
external formaldehyde concentration as the measure of dose. The
predicted quantitative human risk at low levels of exposure is lower
when the interspecies extrapolation (from monkeys and rats) is based
on the biomarker, i.e. DNA-protein cross-links, rather than on the
concentration of formaldehyde inhaled. Although, there are several
unresolved issues regarding the use of DNA-protein cross-links as a
biomarker, this example illustrates that mechanistic data and a
biomarker of delivered dose can be used in the risk assessment
process for chemicals.
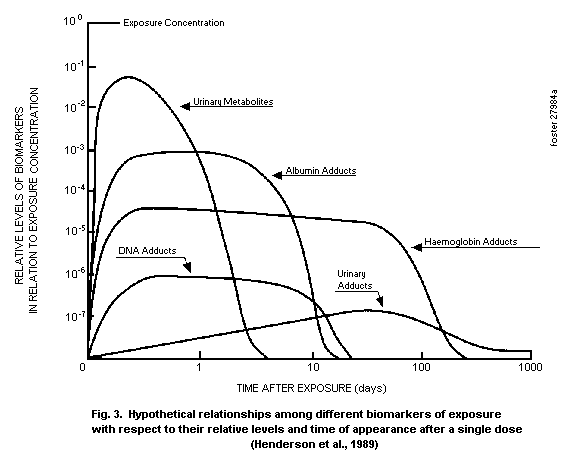
A strategy to help relate biomarkers to prior exposures is to
obtain quantitative information about the kinetics of formation and
breakdown of the biomarker, as shown in Fig. 3. The information
required includes the quantified correlation of the biomarker with a
given exposure scenario and kinetic data for elimination of the
biomarker. Biomarkers for chemicals that are cleared rapidly, such
as vapours in exhaled breath or urinary metabolites, may be present
in large amounts soon, during or immediately following an exposure,
but are not detectable at later times.
Other biomarkers, such as adducts formed with blood proteins,
may represent only a small fraction of the total internal dose but,
because they have a long half-life in the body (relative to exposure
frequency), may accumulate to detectable levels with continued
exposure.
Use can be made of kinetic properties to define prior
exposures. If a person has had only a single, recent exposure to a
chemical, the level of biomarkers with short half-life will be high
relative to those with a longer half-life. With continuing exposure,
the levels of markers with both shorter and longer half-lives should
be high. If a person was exposed in the more distant, rather than
the more recent, past, only the biomarkers with the longer
half-lives will be detectable.
The potential impact of target tissue DNA-protein cross-links
as a biomarker of the biologically effective dose is illustrated in
the proposed risk assessment for formaldehyde put forward by US EPA
(1991). The biological, mechanism-based model of formaldehyde
carcinogenesis consists of three submodels (Conolly et al., 1992;
Conolly & Andersen, in press). One of these is a tissue dosimetry
submodel which incorporates formaldehyde-induced cross-links of DNA
with adjacent proteins (Casanova et al., 1989, 1991). Although the
role of DNA-protein cross-links in the mechanism of formaldehyde-
induced nasal cancer is not known, their formation is used only as a
biomarker of the "biologically effective" dose reaching target cells
in the nasal cavity. An earlier proposal (US EPA, 1987) used
external formaldehyde concentration as the measure of dose. The
predicted quantitative human risk at low levels of exposure is lower
when the interspecies extrapolation (from monkeys and rats) is based
on the biomarker, i.e. DNA-protein cross-links, rather than on the
concentration of formaldehyde inhaled. Although, there are several
unresolved issues regarding the use of DNA-protein cross-links as a
biomarker, this example illustrates that mechanistic data and a
biomarker of delivered dose can be used in the risk assessment
process for chemicals.
A strategy to help relate biomarkers to prior exposures is to
obtain quantitative information about the kinetics of formation and
breakdown of the biomarker, as shown in Fig. 3. The information
required includes the quantified correlation of the biomarker with a
given exposure scenario and kinetic data for elimination of the
biomarker. Biomarkers for chemicals that are cleared rapidly, such
as vapours in exhaled breath or urinary metabolites, may be present
in large amounts soon, during or immediately following an exposure,
but are not detectable at later times.
Other biomarkers, such as adducts formed with blood proteins,
may represent only a small fraction of the total internal dose but,
because they have a long half-life in the body (relative to exposure
frequency), may accumulate to detectable levels with continued
exposure.
Use can be made of kinetic properties to define prior
exposures. If a person has had only a single, recent exposure to a
chemical, the level of biomarkers with short half-life will be high
relative to those with a longer half-life. With continuing exposure,
the levels of markers with both shorter and longer half-lives should
be high. If a person was exposed in the more distant, rather than
the more recent, past, only the biomarkers with the longer
half-lives will be detectable.
 Thus by analysing several biomarkers with different half-lives
(e.g., haemoglobin adducts in the blood, metabolites of the chemical
in urine, parent compound in blood) from a single individual at a
single point in time, more information may be obtained about the
nature of the past exposure than from use of a single biomarker.
Also of value in the interpretation of biomarker data is the
use of mathematical models to describe the kinetics of formation and
elimination of biomarkers of exposure. Absorbed chemicals are
distributed between various compartments in the body, with the
distribution being dependent on the nature of the compartment and
the lipophilicity of the chemical. The most simple models use only
one compartment; however, multicompartmental models are usually
required to describe the disposition of most chemicals in the body
(Gibaldi & Perrier, 1982). Multicompartmental models that
incorporate biomarkers of exposure in relation to toxic end-points
are well established. A biokinetics model for lead has been used to
predict blood lead levels of individuals and communities (DeRosa et
al., 1991).
More recently, physiologically based toxicokinetic (PBTK)
models have been developed that make use of the physico-chemical
properties of a chemical (such as partition coefficients that
indicate how the chemical or its metabolites become partitioned
between different fluids in the body), the kinetics of metabolism of
the chemical (such as Vmax and KM for metabolic pathways), and
physiological parameters of the exposed individual (such as tissue
blood flow, respiratory minute volume, cardiac output) to predict
the actual concentrations of biomarkers that will occur after
specific exposure regimens. These models are adapted for humans by
changing the physiological and metabolic parameters to those
appropriate for humans, and by testing for validity in limited human
studies. These models can then be used to extrapolate between
different exposure situations for the predicted levels of markers
(Ramsay & Andersen, 1984; Droz et al., 1989).
Another use of models is in defining the quantitative
relationship between biomarkers in readily available biological
samples (e.g., blood cells) and in those less readily available
which might be more pertinent biomarkers for the health effect of
concern (e.g., tissue DNA). An example is the use of haemoglobin
adducts to predict the amount of DNA adducts at a critical site,
e.g., after exposure to ethylene oxide (Passingham et al., 1988). To
be able to make such predictions, one must know the kinetics of
formation and breakdown of each of the markers and the factors that
influence those kinetics. Based on such information a model can be
developed to show the quantitative relationships between the markers
under different exposure conditions or at different times after
exposure.
Biomarkers are used extensively in the surveillance of workers
occupationally exposed to metals such as lead, cadmium, mercury,
nickel, chromium and arsenic, and to organic chemicals such as
aniline, benzene, carbon disulfide, styrene, chlorobenzene and
chlorinated aliphatic hydrocarbon solvents (see Table 1). The
examples in Table 1 are given as general information. Before
applying them in specific circumstances, readers must consult the
original references.
These measures are used to indicate the absorbed dose. For a
few chemicals, notably lead, mercury, cadmium and carbon monoxide,
an approximate estimation of the associated health risk may be made.
For other chemicals, exposure may be assessed in quantitative terms.
It is noted that biomarkers are supplementary to environmental
measurements rather than alternative or substitute measures of
exposure.
Considerable efforts are being made to develop biomarkers
associated with exposure to chemical carcinogens and to establish
the relationship between a marker and the future health risk. The
use of animal models may facilitate this process; studies on the DNA
adducts formed by vinyl chloride illustrate the types of strategies
required to make each link (Swenberg et al., 1990). In rats, vinyl
chloride induces liver tumours with a high incidence in young
animals, and the DNA adducts (biomarkers) formed in the liver have
been characterized and the half-lives determined. DNA fidelity
replication assays were used to show one type of adduct that had
both a long half-life and was capable of inducing mutations (Hall et
al., 1981; Barbin et al., 1985; Singer et al., 1987; Swenberg et
al., 1990). The level of this adduct was much higher in the livers
of young rats exposed to vinyl chloride than in adults, and was
characterized as the one most closely related to the health effect.
To extend this animal research to predict human health risks, it
would be necessary to determine if the concentration of the same
adduct is associated with liver tumour formation in human tissue
samples.
Table 1. Some parameters proposed for biological monitoring by different organizations
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase,
inhibitors P-cholinesterase
Aluminium (Al) U-Al U-Al
Aniline U-p-aminophenol,B-Met-Hb U-aniline U-p-aminophenol
Arsenic (As) U-certain volatile U-AsIII+, AsV+ U-AsIII+, AsV+
arsenic compounds MMA+DMA
produced by direct
hydrogenation
Benzene U-phenol B-benzene, U-phenol B-benzene Breath-, B-benzene
p-tert-Butylphenol U-p-tert-butylphenol
Cadmium (Cd) U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd
Carbon disulfide U-TTCA U-TTCA U-TTCA U-TTCA
Carbon monoxide Breath-CO, B-COHb B-COHb B-COHb B-COHb
Chlorobenzene U-4-chlorocatechol U-4-chlorocatechol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Chlorophenols U-tri+tetrapenta-
chlorophenols
Chlorophenoxy acids U-2,4-D+Dichloroprop+
MCPA+Mecoprop
Chromium (Cr) U-Cr U-Cr U-Cr B-Cr, U-Cr
Cobalt (Co) U-Co U-Co U-Co
Dichloromethane B-COHb, B-dichloromethane B-COHb B-COHb, B-dichloromethane
Dieldrin P-dieldrin B-dieldrin
Dimethylformamide U-methylformamide U-methylformamide U-methylformamide
Ethylbenzene U-mandelic acid U-mandelic acid
2-Ethoxyethanol U-ethoxyacetic acid U-ethoxyacetic acid
2-Ethoxyethyl acetate U-ethoxyacetic acid U-ethoxyacetic acid
Ethylene oxide Breath-b, B-ethylene oxide
Fluoride (F) U-F U-F U-F U-F
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Furfural U-furoic acid U-furoic acid
Halothane U-trifluoroacetic acid
Hexachorobenzene P/S-hexachlorobenzene
n-Hexane U-hexanedione, U-hexanedione + U-hexanedione
Breath-n-hexanec dihydroxyhexanone
Hydrazine P-, U-hydrazine
Lead (Pb) B-Pb, U-Pb, B-ZPP B-Pb, U-ALA B-Pb, B-ZPP B-Pb, U-ALA, B-ZPP
Lindane B(P,S)-lindane B-lindane B-lindane
Manganese (Mn) U-Mn B-, U-Mn
Mercury (Hg) B-, U-Hg B-, U-Hg B-, U-Hg
Methanol U-methanol, U-formate U-methanol U-formate
Methylbromide B-Br
Methyl butyl ketone U-hexanedione +
dihyroxyhexanone
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Methylene U-MOCA
bis(2-chloroaniline)
(MOCA)
Methylene dianiline U-MDA
(MDA)
Methyl ethyl ketone U-MEK U-MEK
(MEK)
2-Methoxyethanol U-methoxyacetic acid
2-Methoxyethyl acetate U-methoxyacetic acid
Nickel (Ni) U-Ni U-Ni U-Ni
Nitrobenzene U-p-nitrophenol, B-MetHb
Parathion U-p-nitrophenol, U-p-nitrophenol,
E-cholinesterase E-cholinesterase
Pentachlorophenol U-PCP, P-PCP U-PCP, P-PCP U-PCP
(PCP)
Phenol U-phenol U-phenol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Polychlorinated S-PCB B-PCB
biphenyls (PCB)
2-Propanol U-acetone, B-acetone
Selenium (Se) U-Se
Styrene U-mandelic acid, U-PGA U-mandelic acid, U-mandelic acid+PGA U-mandelic acid
U-mandelic acid+U-PGA
Thallium (Tl) U-Tl
Tetrachloroethylene Breath-, Breath-b, B-tetrachloroethylene
B-tetrachloroethylene B-tetrachloroethylene
Tetrachloromethane Breath-b,
B-tetrachloromethane
Tin (Sn) U-Sn
Toluene U-hippuric acid B-toluene B-toluene B-toluene
1,1,1-Trichloroethane Breath-1,1,1-trichloroethane, Breath-b, B-1,1,1-trichloroethane B-1,1,1-trichloroethane
B-trichloroethanol B-1,1,1-trichloroethane
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Trichloroethylene U-TCA, B-trichloroethanol, U-TCA, B-trichloroethanol U-TCA, U-Trichloroethanol U-TCA
U-TCA+trichloroethanol
Vanadium (V) U-V U-V
Vinyl chloride U-thiodiglycolic acid
Xylenes U-methylhippuric acids U-methylhippuric acids, B- U-methylhippuric acids U-methylhippuric acids
a The following abbreviations have been used: E = erythrocyte; P = plasma; S = serum; U = urine; B = blood; ZPP = erythrocyte zinc
protoporphyrin; ALA = delta-aminolevulinic acid; PGA = phenylglyoxylic acid; MMA = monomethylarsinic acid; DMA = dimethylarsinic acid;
TCA = trichloroacetic acid; MCPA = chloromethylphenoxyacetic acid; 2,4-D = 2,4 dichlorophenoxyacetic acid;
TTCA = 4-thio-4-thiazolidine carboxylic acid.
b DFG (1992) refers to alveolar air
c ACGIH (1992) refers to end-exhaled air
Note: Readers must consult original references before applying the above examples to specific situations
6. BIOMARKERS OF EFFECT
This section focuses on those human biomarkers that can be
applied currently or will be in the near future. Biomarkers of
effect may be used directly in hazard identification and
dose-response assessment components of the risk assessment process.
In hazard identification, biomarkers may facilitate screening and/or
identification of a toxic agent and characterization of the
associated toxicity. Biomarkers that are implicated in toxic
mechanism(s) are preferred for quantitative dose-response
assessments when extrapolating from existing data to a human
situation of concern (e.g., from high to low dose or from test
species to humans).
There are wide inter-individual variations in the response to
equivalent doses of chemicals. While the outcome of a chemical
insult in an individual may be predicted more accurately from
biomarkers of effect(s), such biomarkers may not be specific for a
single causative agent. Many biomarkers of effect are used in
everyday practice to assist in clinical diagnosis, but for
preventive purposes an ideal biomarker of effect is one that
measures change that is still reversible. Nevertheless, certain
biomarkers of nonreversible effects may still be very useful in
epidemiological studies or provide the opportunity for early
clinical intervention.
A limited range of tissues is available for routine analysis of
biomarkers. The more accessible tissues are therefore used as
surrogates for the known or putative target tissues. In some
instances biomarkers of effect are not mechanistically related to
chemically induced lesions, but may represent concomitant,
independent changes. Therefore, although an effect (e.g., sister
chromatid exchange) is being analysed, the use is conceptually close
to assessment of exposure.
6.1 Haematological biomarkers
Inhibition of the enzymes in the haem synthesis pathway (e.g.,
ferrochelatase, levulinate dehydratase) has been used as a marker of
effect of exposure to lead. This effect is reflected also in the
levels of free erythrocyte protoporphyrin (FEP) and
delta-aminolevulinate in the urine. Elevated levels of urinary
delta-amino-levulinate are observed at higher lead exposures than
changes in FEP, for example, while basophilic stippling of
erythrocytes is an even less sensitive biomarker for the effects of
lead. However, the effects on haem synthesis are not specific to
lead as a causative agent; iron deficiency has a similar effect on
FEP. The relationship of these effect biomarkers to toxicity
requires further elucidation.
Routine leucocyte, erythrocyte and thrombocyte counts have been
used in the surveillance of patients treated with cytostatic drugs
and in the monitoring of benzene-exposed workers. The predictive
power in relation to benzene-induced aplastic anaemia or leukaemia
is limited (Townsend et. al., 1978; Hancock et al., 1984; Lamm et
al., 1989). Ferrokinetic measurements, such as plasma iron
disappearance half-time, erythrocyte utilization of iron, plasma
iron transport rate, or erythrocyte iron turnover rate, have been
suggested as biomarkers of myelotoxicity (Rajamaki, 1984).
6.2 Nephrotoxicity biomarkers
Several different types of measures have been tested and used
as biomarkers of renal damage. These have been classified as
functional markers (e.g., serum creatinine and ß2-microglobulin),
urinary proteins of low or high molecular weight (e.g., albumin,
transferrin, retinol-binding globulin, rheumatoid factor,
immunoglobulin G), cytotoxicity markers (tubular antigens, e.g.,
BB50, BBA, HF5), enzymes (e.g., N-acetylglucosaminidase,
ß-galactosidase) in urine, and biochemical markers (eicosanoids,
e.g., 6-keto PGF2alpha, PGE2, PGF2alpha and TXB2, fibronectin,
kallikrein activity, sialic acid and glycosaminoglycans in urine,
and red blood cell negative charges) (Cardenas et al., 1993a,b;
Roels et al., 1993).
Biomarkers for nephrotoxicity were reviewed in WHO (1991) and
are well validated in relation to exposure to cadmium (WHO, 1992a;
Roels et al., 1993) but not in relation to exposure to mercury or
lead (Cardenas et al., 1993a,b).
6.3 Liver toxicity biomarkers
Effects of chemicals on the liver have been estimated
traditionally by measuring the activities of, for example,
aminotransferase (most often aspartate or alanine aminotransferase)
in the serum, where they are found when liver cells have been
damaged and have leaked their contents. Many other enzymes have also
been analysed for this purpose; they include 5-nucleotidase, alcohol
dehydrogenase, lactate dehydrogenase, isocitrate dehydrogenase,
leucine aminopeptidase, glutathione S-transferase, ornithine
carbamoyl transferase). Since tissues other than liver also contain
these enzymes, their activities may be elevated in serum not only
after liver damage but also when non-hepatic tissues have been
damaged. To overcome this lack of specificity, analysis of specific
isoenzymes has been used. Serum activities of enzymes such as
alkaline phosphatase and gamma-glutamyl transpeptidase may be used
as biomarkers of hepatic damage mainly involving biliary excretion.
Several liver function tests can also be used as biomarkers of
effects; these include the concentrations of serum proteins
synthesized in the liver, e.g., albumin and clotting factors, or
serum concentrations of bile acids, also synthesized in the liver,
as well as tests for the hepatic excretory function such as
bromsulfphthalein half-time. These parameters lack specificity since
hepatic viral infections, alcohol and drug use affect these enzymes.
Indirect measures of chemically induced change(s) in the cytochrome
P-450 enzyme system, using provocation tests, have been proposed as
sensitive indicators. However, the relationship to liver damage and
disease is not established and the requirement for the
administration of a drug limits the use of such tests.
Hepatotoxicity is caused by a number of chemicals that are
metabolized by the cytochrome P-450-dependent mixed-function oxidase
system to reactive intermediates. For example, carbon tetrachloride
has been studied extensively; it is metabolized to a reactive
intermediate which initially depletes intracellular glutathione to a
level that is no longer protective when the metabolite reacts with
critical macromolecules leading to cell death and hepatoxicity. In
this example, biomarkers of effect could include glutathione levels,
lipid peroxidation or the number of necrotic cells.
6.4 Biomarkers of immunotoxicity
The immune system protects the organism against infectious
microorganisms and the growth of at least some neoplasms. Reactions
of the immune system are influenced by genetic factors, age,
nutrition, life-style and health status. Xenobiotics may stimulate
or suppress the immune system. After initial sensitization, even a
minimal new exposure may lead to an anaphylactic reaction. The
immune system may be more sensitive to chemical challenge than any
other body system.
Hypersensitivity reactions following inhalation exposure
include asthma, rhinitis, pneumonitis and granulomatous pulmonary
reactions (see section 6.5). Hypersensitive dermal reactions induced
by chemicals include a wide variety of acute, subchronic and chronic
changes. Patch testing has been used traditionally as a biomarker
for identification of the causative agent of an allergic skin
reaction. However, the possibility of inducing hypersensitivity by
patch testing has been well documented and should not be overlooked
(Adams & Fisher, 1990).
Elevated levels of specific antibodies, usually of the IgE
type, may indicate existing sensitization. However, not all
individuals with elevated levels are symptomatic and not all
symptomatic individuals exhibit elevated IgE levels (Horak, 1985;
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology, 1989; Nielsen et al., 1992).
Suppression of the immune system increases susceptibility to
infections and neoplasia. Changes in the relative abundance of
different lymphocyte subpopulations (suppressor and helper T-cells)
have been used as biomarkers for the immune suppression (Jennings et
al., 1988; Sullivan, 1989; Holsapple, et al., 1991). Individuals
with asbestos-induced pleural or pulmonary changes, or
asbestos-induced cancer, as well as those heavily exposed to
asbestos but without apparent disease, have been reported to exhibit
an altered immunological status (e.g., reductions in T-lymphocyte
subsets) (Bekes et al., 1987).
In view of the growing incidence of hypersensitivity reactions
to chemicals, development and application of biomarkers for
immunotoxic effects is important. However, this is made difficult by
the current limited understanding of basic immunological mechanisms
and the effects of chemicals thereon (US NRC 1992).
6.5 Biomarkers of pulmonary toxicity
The most frequently used markers of pulmonary toxicity measure
gross effects on pulmonary function (e.g., peak expiratory flow,
forced expiratory volume, transfer factors) rather than effects on
cells or biochemical processes (US NRC, 1989a). These measures tend
to be nonspecific with respect to the causative agent and may
overlook effects specific to a certain cell type. Peak expiratory
flow measurements can be performed by the exposed individuals
themselves at the workplace, at home or elsewhere, and they provide
information on the underlying causes of air-way obstruction,
allowing a closer association between exposure, atmosphere and
response.
Air-way hyperactivity can be assessed by challenge tests using
inhalation exposure. Although such tests may assist in identifying
the factors causing hypersensitive pulmonary reactions, there is a
clear risk of acute reactions, and testing should be carried out by
qualified personnel in carefully controlled environments.
Recently, analysis of bronchoalveolar lavage fluid (BALF) has
been used to detect lung injury or to follow the progress of
pulmonary disease or the efficacy of therapeutic treatment
(Reynolds, 1987; Henderson, 1988).
The use of cellular elements as markers of pulmonary disease
state has been emphasized in human BALF analysis (Reynolds, 1987).
Total cell counts and differential counts, including use of
monoclonal antibody staining to distinguish T-cell subtypes, are
used to detect alveolitis and to aid in the diagnosis of
interstitial lung disease. High percentages of lymphocytes are
indicators of granulomatous processes, such as sarcoidosis, or
hypersensitivity pneumonitis. High percentages of neutrophils with
some eosinophils indicate possible idiopathic pulmonary fibrosis.
Other extracellular components, such as cytokines and other
mediators of inflammation, have been used on an experimental basis
to answer specific research questions.
The analysis of BALF has been used to define the dose-response
characteristics of inhaled or instilled toxins in animal toxicity
studies (Henderson, 1988). The most sensitive biomarker of an
inflammatory response in the bronchoalveolar region is the number of
neutrophils in BALF. The levels of protein and of extracellular
enzymatic activity are also useful markers of pulmonary toxicity.
Increases in protein concentrations in BALF indicate increased
permeability of the alveolar/capillary barrier. Lactate
dehydrogenase (LDH) is a cytoplasmic enzyme that is found
extracellularly only in the presence of lysed or damaged cells.
Beta-glucuronidase or similar lysosomal hydrolytic enzymes are
excellent markers for the toxicity of inhaled particles. These
particles are phagocytosed by macrophages, and the enzymes are
released from activated or lysed macrophages.
The secretion of cytokines from pulmonary macrophages obtained
by bronchoalveolar lavage provides markers of developing fibrosis.
Recent studies by Piguet et al. (1990) demonstrate that the level of
secretion of tumour necrosis factor (TNF) by pulmonary macrophages
is associated with quartz-induced fibrotic processes. Lassalle et
al. (1990) found elevated secretion of TNF by macrophages obtained
from individuals with coal-workers pneumoconioses compared with
macrophages from controls. The secretion of platelet-derived growth
factor from pulmonary macrophage was elevated in patients with
idiopathic pulmonary fibrosis (IPF).
Glutathione (GSH), a tripeptide protective against oxidative
stress, is present in BALF, and a decrease in GSH in BALF is a
potential marker for oxidative stress. Decreased levels of GSH have
been observed in patient with IPF (Cantin et al., 1989) and in
animals exposed chronically to diesel exhaust, resulting in
pulmonary fibrosis (Henderson, 1988).
Nasal lavage fluid (NLF) also provides markers of response to
inhaled toxins. The work of Graham et al. (1988) demonstrates the
potential use of NLF analysis to document the influx of neutrophils
into the nasal cavity in humans in response to inhaled ozone.
Biomarkers in blood related to lung injury have not been
validated. However, the work of Cavalleri et al. (1991) indicates
that serum aminoterminal propeptide of type III procollagen (PIIINP)
may become useful as an early marker for developing fibrosis. A
dose-dependant increase in serum PIIINP was found in individuals
exposed to low or high levels of asbestos.
Finally, urinary levels of amino acids associated with the
connective tissue of the lung (hydroxyproline, hydroxylysine,
desmosine and isodesmosine) have been used as markers of lung injury
(Harel et al., 1980; Yanagisawa et al., 1986; Stone et al., 1991).
However, such assays are not specific for lung injury and merely
indicate the breakdown of connective tissue in any organ in the
body.
6.6 Biomarkers of reproductive and developmental toxicity
Markers associated with an adverse outcome in reproduction may
reflect toxic effects in the male or female or be associated with
development during the embryonic, fetal, perinatal or neonatal
period (US NRC, 1989b; Mattison, 1991).
Biomarkers for the male reproductive system may include
physiological indicators of impaired testicular function, or sperm
number or characteristics (including cytogenetics). Measures of
hormonal status (i.e. FSH, LH and testosterone) can also be readily
obtained from blood and, in the case of testosterone, from urine and
saliva. However, these levels are greatly influenced by circadian
rhythms and demonstrate large inter- and intra-individual
variability. A clearer picture of hormonal status can be obtained by
administering GnRH or LH and examining the hormone response to these
challenges. Biomarkers for the male reproductive system are rather
easily accessible and some even reasonably well validated; such
markers are less well developed for the female reproductive system.
Biomarkers indicative of developmental toxicity should also be
considered. As is the case for many biological markers of effects,
it is often difficult to identify the causative agent in the absence
of any specific exposure history. Biomarkers could include
measurements of detrimental effects produced by chemical or other
exposures during embryonic or fetal stages of development.
Irreversible lesions can be embryolethal or result in functional
anomalies in the offspring. Examples of biomarkers of developmental
toxicity include low birth weight, chromosome anomalies, delayed
growth of specific organ systems, mental retardation, and subtle
behavioural changes. The changes associated with F1 male-mediated
abnormalities have been discussed by Anderson (1990). Some of these
biomarkers of developmental effects (malformations, mental
retardation) are not biomarkers of effect as far as the individual
is concerned, but rather represent the adverse health outcome
itself. However, from the point of view of the exposed population,
they may be considered as biomarkers since they show that within a
population a harmful exposure has taken place.
Several biomarkers have been proposed for use during pregnancy,
e.g., early pregnancy loss and assays for genetic defects of the
conceptus. The latter comprise both classical cytogenetic studies,
as well as specific DNA probes (US NRC, 1989b). The use of urinary
human chorionic gonadotrophin (HCG) has been well documented as a
biomarker for early fetal loss (US NRC, 1989b). Many different
biomarkers have been used to follow the development of the pregnancy
and the well-being of the conceptus, but they have not yet been
applied to studies of effects of chemicals on pregnancy.
6.7 Biomarkers of neurotoxicity
The functions of the nervous system are complex and biomarkers
may range from effects of chemicals on neural cellular and molecular
processes to neurophysiological and neuro-behavioural measurements
of complex functional entities.
Inhibition of plasma and erythrocyte acetylcholine esterase
(AchE) provides biomarkers of exposure to organophosphorus compounds
and other cholinesterase inhibitors. While erythrocyte
cholinesterase is similar to brain cholinesterase, and is therefore
an effect biomarker, plasma nonspecific pseudocholinesterase only
reflects exposure and is not a marker of CNS effects.
Measures of the function of the peripheral nervous system
(e.g., electroneuromyography, nerve conduction velocities, vibration
sensitivity) are well defined. Assessment of peripheral nervous
system dysfunction associated with exposure to chemicals can be
carried out using electroneuromyography at the preclinical stage
(Seppalainen et al., 1979).
Some well-established neurophysiological (e.g., evoked
potentials, electroencephalography) and neurobehavioural (e.g., the
WHO Neurobehavioural Core Test Battery, Cassito et al., 1990)
measures may be used as biomarkers to evaluate CNS dysfunction
induced by neurotoxicants. These tests must be carried out under
well-controlled conditions.
Methods for assessing changes in higher cognitive function
(e.g., learning and memory) have been used extensively, e.g., in
workers exposed to solvents or heavy metals, but require further
refinement.
Available neuroimaging procedures, e.g., computed axial
tomography (CAT), magnetic resonance imaging (MRI), nuclear magnetic
resonance spectroscopy (MRS) and positron-emission tomography (PET),
are considered non-invasive, but some of them require exposure to
ionizing radiation. CAT and MRI can be carried out with current
clinical techniques to assess chemically induced changes in the
brain. The use of MRS and PET can provide a more detailed evaluation
of the biochemical status (e.g., rate of energy generation, blood
flow, L-glucose metabolism) in the central nervous system. They can
be used as biomarkers for assessing exposure to neurotoxicants
inducing brain alterations. However, they are expensive, of little
use in assessing spinal cord, nerve and muscle changes, and there is
only minimal data validating their use in neurotoxicology (US NRC,
1992).
Other promising biomarkers for neurotoxicity in animal studies
include glial fibrillary acidic protein (localized in the
astrocytes), which increases in localized areas within the brain
where injury due to toxicants occurs (O'Callaghan, 1991).
7. BIOMARKERS AND CHEMICAL CARCINOGENESIS
As new information about the multistep process of
carcinogenesis unfolds, it is instructive to consider the various
mechanisms by which chemicals induce cancer. Knowledge of mechanisms
will enable the selection of appropriate biomarkers for use in risk
assessment of carcinogens. Some chemicals are direct acting and
others require metabolic activation. Once absorbed most chemicals
undergo enzyme-mediated reactions that either detoxify them or
activate them to reactive species. The balance between activating
and detoxifying enzyme systems governs the rate of delivery of
bioactive metabolites to the macromolecular site (Harris, 1991). The
resulting macromolecular interaction could be a DNA adduct for
carcinogens that are initiating agents or receptor occupancy for
chemicals that are tumour promotors. Certain of the DNA adducts
produced by such interactions are pro-mutagenic, and replication of
the damaged DNA could lead to DNA sequence changes which may result
in altered gene expression or mutated gene products. Weisburger &
Williams (1981) have suggested that chemical carcinogens be
classified as those that interact with DNA (genotoxic) and those
that do not (epigenetic or non-genotoxic). The importance of mitotic
activity in the latter group has recently been elaborated further
(Cohen & Ellwein, 1990).
The implications for invoking the "mechanistic" approach to the
selection of appropriate biomarkers are significant. For example,
chemicals that stimulate cell proliferation via mitogenesis or
cytotoxicity (and subsequent proliferation) might require different
biomarkers than chemicals whose major mechanism of action is based
on DNA reactivity. In the latter case, measurements of DNA adducts
or chromosome alterations may serve as suitable biomarkers, whereas
in the former case alternate biomarkers (e.g., cell turnover
measurements) may be more appropriate.
7.1 Analysis of chemicals and metabolites
Urinary or blood concentrations of several chemicals shown or
suspected to be carcinogenic to humans (e.g., arsenic, cadmium,
chromium, nickel, benzene, MOCA, polychlorinated biphenyls, styrene,
tetrachloroethylene) have for long been used as biomarkers of
exposure. Among people exposed to arsenic in a copper smelter, a
dose relationship has been observed between the cumulative urinary
excretion of arsenic and the risk of lung cancer (Enterline & Marsh,
1982). For other carcinogenic chemicals, such data are not
available, and the measured concentrations may only be interpreted
in terms of exposure.
Sensitive techniques based on physicochemical or immunochemical
methods for the detection of a variety of carcinogen-modified DNA
bases have been developed (Shuker & Farmer, 1992). These include the
alkylated purines, aflatoxin-guanine adducts, cis-platinum adducts,
thymine glycol, 8-hydroxydeoxyguanosine and PAH-derived adducts.
The aflatoxin marker has been extensively used in both animal
and human studies on the relationship between exposure and liver
cancer induction. In an ongoing prospective study in Shanghai,
China, Ross et al. (1992) reported that subjects with liver cancer
were more likely than controls to have detectable concentrations of
any of the known aflatoxin metabolites in their urine. Groopman et
al. (1991) recently explored the relationship between dietary
aflatoxin and excretion in the urine of aflatoxin metabolites and an
aflatoxin-DNA adduct. This study was conducted on people living in
the Guangxi Autonomous Region, China. These investigators found a
positive correlation between aflatoxin N7-guanine and specific
metabolites excreted in urine and aflatoxin B1 intake from the
previous day.
Exposure to chemical compounds capable of interacting with
cellular macromolecules can originate from both exogenous and
endogenous sources. Nitrite, nitrate and nitrosating agents can be
synthesized endogenously in reactions mediated by bacteria and
activated macrophage. In this way endogenous formation of
N-nitroso compounds can occur at various sites in the body.
Endogenously formed N-nitroso compounds may be considered as
biomarkers of susceptibility; they have been associated in humans
with increased risk of cancer of the stomach, oesophagus and urinary
bladder, although unequivocal epidemiological data are lacking
(Bartsch & Montesano, 1984). The quantitative estimation of
endogenous nitrosation in humans can be measured using the
N-nitroso-proline test. L-proline is utilized as a probe for
nitrosatable amines and N-nitroso-proline excreted in the urine is
determined as a marker. This assay has been applied in some
population studies (Bartsch et al. 1991).
7.2 Biomarkers for genotoxic carcinogens
7.2.1 DNA adducts - general considerations
DNA adducts are being used both as molecular dosimeters
(biomarker of exposure) and to assess the genotoxic potential of
chemicals (biomarker of effect). The biological significance of such
adducts must be assessed on the basis of adduct heterogeneity and of
cell and tissue specificity for adduct formation, persistence and
repair. Some DNA adducts result in mutation whereas others do not.
Mutational specificity in the p53 gene produced by a variety of
chemical carcinogens provides evidence that DNA adduct location
influences site-specific mutations (Hollstein et al., 1991). Some
DNA sequence changes may lead to phenotypic alterations that can be
selected, whereas others may not (Compton et al., 1991).
Most tissues are comprised of multiple cell types, and cell
types vary considerably in their capacity to convert chemicals to
DNA reactive species. For example, lung is composed of multiple cell
types in which the relative concentrations of various P-450
isoenzymes and enzymes depends on the cell type. Thus, one compound
may produce high concentrations of pro-mutagenic adducts in one cell
type, but not in another, whereas the opposite might occur for a
compound which is activated by a different P-450 isozyme. DNA adduct
concentrations derived from a whole tissue homogenate may grossly
overestimate or underestimate adduct concentrations in a given cell
type.
Some DNA adducts are repaired quickly, others hardly at all,
the adduct loss correlating with cell turnover. Therefore, the
concentration and gene location of DNA adducts will change with time
after exposure to a genotoxic chemical. Furthermore, the existence
of non-random repair in the genome makes it difficult to utilize
total DNA repair capacity as an indicator of cell susceptibility to
carcinogens (Hanawalt, 1987). It is especially important in human
studies to know the duration and timing of exposure for proper
evaluation of the biological significance of a given adduct
concentration.
Many of the human studies described below have involved
measuring metabolism, DNA adduct formation and repair in whole
tissues. Techniques need to be refined in order that
cell-type-specific variations can be monitored in human tissues, as
well as experimental studies in animal models using
immunohistochemical techniques for the cell type. Specific
localization of DNA adducts has clearly demonstrated that such
variations occur. Treatment of rats with the tobacco-specific
nitrosamine, 4-( N-methyl- N-nitrosamine)-1-(3-pyridyl)-1-butanone
(NNK), results in the induction of tumours in the nasal cavity,
lung, liver and pancreas (Hoffman et al., 1984; Rivenson et al.,
1988). At low doses of NNK, the prevalence of malignant lung tumours
was higher than that observed in other tissues. Cell-type-specific
differences have been observed within the lung, the highest
concentration of O6-methylguanine having been found in the Clara
cells. These cells have the highest levels of the P-450 metabolizing
enzymes for NNK and low levels of the O6-methylguanine DNA
methyltransferase repair enzymes (Belinsky et al., 1987). Pulmonary
tumours are also induced in mice and hamsters following either
short- or long-term exposure to this carcinogen (Hecht et al.,
1983).
As animals age, DNA adducts are detected in increasing amounts,
and, although the relationship of these adducts to tumour
development is unclear, they are believed to be derived from dietary
constituents or endogenous chemicals such as hormones (Randerath &
Randerath, 1991).
7.2.2 DNA adducts in human samples
In human studies, it is difficult to obtain non-tumourous
target tissue for the quantification of DNA adducts. Lymphocytes are
a readily accessible source of human cells that are known to contain
DNA adducts. However, there is little information on the reliability
of using lymphocyte adduct concentrations for the estimation of
target cell or tissue adduct concentrations (Lucier & Thompson,
1987).
Evaluation of dose-response relationships for chemical
carcinogens in humans is more complex than in animal models.
Radio-labelled carcinogens cannot be administered and the
accessibility of tissues and cells is limited. Several approaches to
detect DNA adducts in human samples have been evaluated (Wogan &
Gorelick, 1985; Santella, 1988). The most frequently used methods
are immunoassays and 32P-postlabelling. Other analytical
techniques such as GC-MS and synchronous fluorescence spectroscopy
are being used to measure DNA adducts (Weston & Bowman, 1991). In
general, immunoassays are both specific and sensitive for alkylated
adducts and aflatoxin adducts (Wild & Montesano, 1991; Groopman et
al., 1991). However, these methods are not easily applied to
quantification of adducts for bulky aromatic hydrocarbons such as
benzo [a]pyrene-derived adducts. The main problem is the lack of
specificity of the antibodies used in the assay which cross-react
with a number of PAH-related adducts (Santella et al., 1985).
The second assay frequently used to quantify DNA adducts in
humans is the 32P postlabelling technique. For a complete
description of this assay, see Randerath & Randerath (1991), Beach &
Gupta (1992), IARC (1992). The assay is extraordinarily sensitive,
being capable of detecting 1 adduct in 1010 normal nucleotides
when appropriate modifications are made to the procedure. The assay
is particularly useful for detecting adducts of non-polar polycyclic
aromatic hydrocarbons such as 7,8-diol-9,10-oxide-benzo [a]pyrene
deoxyguanosine (BPDE). Some DNA modifications such as alkylated DNA
adducts, which cannot be easily detected by this assay due to the
limitations of the chromatographic systems, can be quantified using
a combined 32P immunochemical precipitation technique (Kang et
al., 1993). Studies using the 32P labelling or immunological
methods have been reviewed by Beach & Gupta (1992) and Wild &
Montesano (1991). The groups of chemicals studied include alkylating
agents, polycyclic aromatic hydrocarbons (PAHs), heterocyclic PAHs,
nitro PAHs, cyclopenta-fused PAHs, aromatic amines, alkylbenzenes,
quinones, mycotoxins, chemotherapeutic agents, pesticides and
aldehydes.
7.2.3 Protein adducts
Ehrenberg and his associates pioneered the use of protein
adducts as dose monitors for carcinogen exposure in humans, and this
work has been reviewed by Hsia (1991). To date the class of proteins
that have been most extensively studied are those found in
circulating blood, i.e. haemoglobin and albumin. This is mainly
because these proteins are relatively abundant and can be easily
isolated for analysis.
Haemoglobin has the unique biological property of having a life
span equivalent to that of the erythrocyte, which in humans is
approximately 120 days, and therefore adduct levels reflect
exposures over several months. In contrast, albumin adducts can only
be used for assessing recent exposure because of the faster turnover
of albumin (half-life of 20-25 days). Protein adducts can be
quantified using chemical methods, e.g., aromatic amine release by
acid or basic hydrolysis from haemoglobin followed by derivatization
and GC-MS analysis (Farmer, 1991), or immunological techniques
(e.g., aflatoxin-albumin adducts, Wild et al., 1990).
During the past few years, several human monitoring studies
have demonstrated the usefulness of protein adducts as biomarkers of
exposure. Examples of chemicals that have been detected as protein
adducts in human studies include ethylene and propylene oxide,
aniline, cigarette smoke, aromatic amines such as 4-amino-biphenyl,
and aflatoxin (Wogan, 1989; Farmer, 1991). Albumin adducts of
aflatoxin B1 have also been used in epidemiological studies of
their role in the etiology of hepatocellular carcinoma in man. A
significant correlation was observed, at the individual level,
between dietary intake and the level of albumin-bound aflatoxin in a
chronically exposed population in the Gambia (Wild et al., 1992).
7.2.4 Cytogenetic methods
Cytogenetic methods are used as biomarkers of exposure to
DNA-damaging agents.
Many studies relating to cytogenetic changes in exposed human
populations have been reviewed in a special issue of Mutation
Research (Anderson, 1988). A second comprehensive review of more
recent studies has also been published (Anderson, 1990), and a
further review is in press (Anderson, in press).
All human monitoring studies suffer from variability of
baseline frequencies (Carrano & Natarajan, 1988) due to the presence
of endogenous (gender, age, medical history, etc.) and exogenous
factors (life-style, smoking, drinking, eating habits, etc.).
Anderson et al. (1991) have investigated the effect of these changes
on baseline variability eight times over a two-year period.
In contrast to studies on radiation, for which a marker
(dicentric chromosome) has been identified, studies with chemicals
have not yet identified a specific marker chromosome. For radiation
a dicentric is a quantitative dosimeter. Therefore, after radiation
exposure results can be used on an individual basis and a highly
exposed individual removed from the radiation source. Results from
chemical exposure studies, however, can only be used on a group
basis, due to the lack of a specific marker chromosome.
7.2.5 Chromosome damage
Both chromosome and chromatid aberrations are induced in
individuals exposed to chemical mutagens. The chromosome aberrations
are thought to arise from misrepair of lesions in the G0 stage of
circulating lymphocytes as well as from precursor cells in bone
marrow and thymus (Carrano & Natarajan, 1988). Chromatid aberrations
include chromatid breaks, intrachanges and exchanges, while
chromosome aberrations include acentric fragments, dicentric
chromosomes and ring chromosomes. Balanced translocation and
inversions can also arise and are difficult to quantify without
banding analysis. Structural aberrations can be classified as
unstable and stable depending on their ability to persist in
dividing cell populations. Unstable aberrations consist of rings,
acentric fragments and other asymmetrical rearrangements, and will
lead to the death of the cell. Stable aberrations consist of
balanced translocation inversions and other symmetrical
rearrangements which can be transmitted to progeny cells at
division. Therefore stable aberrations are more biologically
significant than unstable ones and could be involved in the cancer
process. Many human carcinogens have been shown to produce
chromosome damage in populations exposed to them, although no causal
relationship has been demonstrated (Sorsa et al., 1992). Proven
human carcinogens for which cytogenetic endpoints have been measured
in humans and corresponding animal data are available are listed in
Table 2.
In a preliminary report of a prospective study among people
whose lymphocytes were assayed for chromosome aberrations and SCE,
high rates of chromosome aberrations were observed and appeared to
be linked to cancer risk, but the finding was of borderline
statistical significance (Sorsa et al., 1990).
7.2.6 Sister chromatid exchange
Sister chromatid exchange (SCE) is considered to be a more
sensitive, rapid and simple cytogenetic end-point than chromosome
aberrations for evaluating the genotoxic potential of a variety of
mutagenic and carcinogenic agents. It is also used to detect and
differentiate many chromosome fragility diseases that predispose to
neoplasia. SCE is a DNA-replication-dependent phenomenon. Cellular
Table 2. Proven human carcinogens for which cytogenetic end-points have been measured in humans
and corresponding data are available for experimental animalsa
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Human carcinogens (Group 1)
Alcoholic beverages + + _ + ?
Aluminium production _ _
Arsenic and arsenic compounds ? ? + +
Asbestos ? _ _
Azathioprine ? _ + _ +
Benzene + + + +
Betel quid with tobacco + + +
Bis(chloromethyl)ether and (+) _
chloromethyl methyl ether
(technical grade)
1,4-Butanediol dimethanesulfonate (+) + + +
(Myleran)
Chlorambucil ? + +
Cyclosporin (+) _ _
Coal-tars +
Coke production +
Combined oral contraceptives _ _
Cyclophosphamide + + + + +
Hexavalent chromium compounds + + + + +
Melphalan + + +
8-Methoxypsoralen plus _ _ +
ultraviolet A radiation
Mineral oils, untreated and +
mildly treated
Nickel compounds + _ ? _
Table 2 (contd)
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Painter, occupational exposures as _
Radon + _
Rubber industry ? ?
Tobacco products, smokeless + +
Tobacco smoke + + + +
Tris(1-aziridinyl)phosphine sulfide (+) + + +
(Thiotepa)
Vinyl chloride + ? + + +
a From: Sorsa et al. (1992); CA = chromosome aberrations; SCE = sister chromatid exchange;
MN = micronuclei + = positive result; - = negative result; (+) = equivocal result;
? = doubtful result
factors such as nucleotide pools, repair and replication enzymes,
and biorhythms can play an important role in its formation. A major
source of variation can be attributed to the concentration of
bromodeoxyuridine relative to the number of lymphocytes in the
culture (Das, 1988; Morris, 1991; Morris et al., 1992). In a
prospective cancer study (Sorsa et al., 1990), no relationship was
observed between the frequency of SCE and the risk of cancer.
7.2.7 Micronuclei
Micronuclei are formed by condensation of acentric chromosomal
fragments or by whole chromosomes that are left behind during
anaphase movements (lagging chromosomes). The presence of
micronuclei can therefore be taken as an indication of the previous
existence of chromosomal aberrations. To visualize micronuclei,
cells have to undergo mitosis. In peripheral lymphocyte cultures it
is not easy to distinguish interface nuclei that have undergone a
division from those that have not. This makes it difficult to
quantify the frequencies of micronuclei for comparative purposes. A
method using cytochalasin B can distinguish nuclei that have divided
once (French & Morley, 1985).
7.2.8 Aneuploidy
Aneuploidy is a condition in which the number of chromosomes in
cells of individuals is not an exact multiple of the typical haploid
set for the species. Trisomy results when a single extra chromosome
is added to a pair of homologous chromosomes. If one chromosome of a
pair is missing, the result is monosomy. Absence of the pair is
nullisomy. Two or more copies of a homologue result in tetrasomy or
polysomy. Cells of individuals with missing or extra chromosomes are
hypoploid or hyperploid (UK DH, 1989). The best-known numerical
abnormalities result in the syndromes of Down (trisomy of chromosome
21), Klinefelter (sex chromosome genotype is XXY), and Turner (sex
chromosome genotype is X0). Aneuploidy is almost always found in
human cancers (Dellarco et al., 1985).
7.2.9 Mutation
Current somatic gene mutation assays used as biomarkers in
human studies select for a change or loss of a normal protein
produced by specific genes. Mutations at the X-linked hypoxanthine
guanine phosphoribosyl transferase gene in cloned T-lymphocytes and
in the autosomal locus for human leucocyte antigen-A (HLA-A) have
provided information on frequency of mutation and molecular spectra
of mutants. Detection of haemoglobin variants and loss of the cell
surface glycoprotein glycophorin A measured in red blood cells have
such limitations that analysis of the DNA mutations induced cannot
be made (Compton et al., 1991). The background frequency of each of
these specific locus assays varies greatly (Lambert, 1992) and is
dependent on numerous confounding factors (e.g., age and smoking).
Information on the mutation spectra at a particular locus will be
extremely useful in elucidating the mechanisms by which mutations
occur in human cells in vivo. By comparing spontaneous and
chemically induced mutational spectra in different populations, the
etiological contributions of both exogenous and endogenous factors
to human carcinogenesis could be assessed.
An alternative approach for the measurement of induced base
changes which does not require prior selection of a mutant
population uses the restriction site mutation technique. This is
based on the detection of DNA sequences resistant to the cutting
action of specific restriction enzymes, and the amplification of
these resistant sequences using the polymerase chain reaction. It
may theoretically be applied to the study of DNA base changes in any
gene for which the sequence has been determined (Parry et al.,
1990).
More relevant biomarkers for chemically induced cancers would,
however, preferably measure changes in genes thought to be important
for cancer. Mutations that activate proto-oncogenes, which stimulate
growth or inactivate suppressor genes to liberate cells from growth
constraints, could lead to unregulated proliferation of cancer cells
(Weinberg, 1991). For the most part, mutations in oncogenes and
tumour suppressor genes have been characterized in tumour tissue. It
remains to be determined whether the detection of mutant cells
against a background of normal cells can be achieved prior to
clinical diagnosis of cancer. Activated oncogenes have already been
identified in many human cancers, and considerable progress has been
made in elucidating the potential role of chemical carcinogens in
the activation of oncogenes and the contribution of the latter to
tumorigenesis in animal models (Balmain & Brown, 1988). Brandt-Rauf
(1991) presented data from pilot studies that demonstrated the
presence of the p21 protein product of the ras oncogene in the serum
of 15 out of 18 lung cancer patients who were all current or former
smokers. The protein was not found in the serum of any of the 18
healthy non-smoking controls, but was present in 2 out of 8
clinically healthy smoking controls. However, in another study, p21
protein was not detected in 20 smokers in a normal population or in
20 male healthy non-smokers (Brinkworth et al., 1992). The
mutational spectrum for the tumour suppressor gene p53 in human
tumours has been reviewed by Hollstein et al. (1991). Mutations of
the p53 gene are the most common cancer-related genetic changes at
the gene level and are widespread over the conserved codons of the
p53 gene. Hence, mutational spectra could be compared for tumours at
different sites and arising from different etiological backgrounds.
The mutational spectrum appears to differ among cancers of the
colon, lung, oesophagus, breast, liver, brain, reticuloendothelial
tissues and haemopoietic tissues. In two populations where aflatoxin
B1 exposure was implicated as one of the etiological factors in
hepatocellular carcinomas, the same mutational hotspot (i.e. G-T
transversion at codon 249) in the p53 gene has been identified (Hsu
et al., 1991).
7.3 Biomarkers for non-genotoxic carcinogenesis
Although only a few non-genotoxic human carcinogens have been
recognized (e.g., cyclosporin, diethylstilbestrol and estrogenic
hormones), many non-genotoxic carcinogens have been identified in
rodents. A compilation of NTP rodent data, designed to test the
concordance between short-term tests and in vivo carcinogenicity
assays, showed that more than 30% of rodent carcinogens do not test
positively for genotoxicity (Ashby & Tennant, 1991).
The mechanisms of action for non-genotoxic carcinogens need to
be considered in predicting human risk from chemical exposures.
Although the modes of action of non-genotoxic carcinogens are poorly
understood, several have been proposed, including immunosuppression,
hormonal effects, promotion, inorganic carcinogenesis,
co-carcinogenic effects and solid-state carcinogenesis (Weisburger &
Williams, 1981). Recently some of these mechanisms were grouped
under the headings of cytotoxicity and mitogenic growth stimulation
(Butterworth et al., 1992). Non-genotoxic carcinogens are believed
to exert their carcinogenic effects through mechanisms that do not
involve direct binding of the chemical or its metabolites to DNA (UK
DH, 1989). The key mechanism of non-genotoxic chemicals is to
increase cell proliferation, either by mitogenesis of the target
cells or by cytotoxicity, which is followed by regenerative cell
proliferation (Ramel, 1992). Cohen & Ellwein (1990) suggested that
non-genotoxic chemicals can be further categorized as to whether or
not their main mechanism of action is mediated via receptor-binding
(e.g., dioxin).
Cell replication and proliferation are potential biomarkers of
effect. Cell replication is the production of daughter cells by the
process of replicative DNA synthesis, while cell proliferation is
the enhanced replication of a selected cell population as observed
in regenerating tissues. Cells undergoing replicative DNA synthesis
(S-phase) are the most commonly used markers. The detection of cell
proliferation involves the incorporation of DNA precursors like
3H-thymidine or the base analogue 5-bromo-2'-deoxyuridine (BrdU)
into cellular DNA during S-phase. This is accomplished by
administering these precursors to animals by injection or through
implanted osmotic pumps. These S-phase cells can be identified
histoautotoradiographically or immunohistochemically (Goldsworthy et
al., 1991). The invasiveness of these techniques currently limits
their use to animal studies.
The identification of biomarkers of effect for non-genotoxic
carcinogens, whose major mechanism of action is via receptor
occupancy, may be difficult. This is primarily because carcinogens
of this type activate a variety of genes, some of which may not be
involved in the carcinogenic process. However, a good example of a
non-genotoxic carcinogen for which there is a good biomarker of
effect is 2,3,7,8-tetrachlorinated dibenzo- p-dioxin (TCDD). TCDD
interacts with a cytosolic receptor that is specific for it and its
structural analogues (Poland et al., 1976). In addition to
activating a number of growth factor and growth factor receptor
genes, TCDD induces a number of enzymes, one of which is cytochrome
P-450 1A1. Although the induction of this enzyme is probably not
directly related to the biological mechanism leading to cancer from
TCDD exposure, it is a sensitive marker for exposure (Tritscher et
al., 1992).
8. BIOMARKERS OF SUSCEPTIBILITY
This chapter focuses on the genetic predisposition of an
individual as it affects susceptibility to chemical materials. There
are a number of external factors, such as age, diet and health
status, that can also influence the susceptibility of an individual
exposed to chemicals. Some discussion will be directed towards the
effects of previous exposure on subsequent susceptibility, such as
to sensitization and enzyme induction/inhibition by previous
exposure. Table 3 lists some genetic and acquired factors affecting
susceptibility (Calabrese, 1986).
Although individuals may experience similar environmental
exposures, genetic differences in metabolism may produce markedly
different doses at the target site and thus a different level of
response. Even when target doses are similar, markedly different
responses may be noted in individuals due to varying degrees of
inherent biological responsiveness. Biomarkers of susceptibility may
reflect the acquired or genetic factors that influence the response
to exposure. These are pre-existing factors and are independent of
the exposure. They are predominantly genetic in origin, although
disease, physiological changes, medication and exposure to other
environmental agents may also alter individual susceptibility.
Biomarkers of susceptibility identify those individuals in a
population who have an acquired or genetic difference in
susceptibility to the effects of chemical exposure.
Biomarkers of susceptibility indicate which factors may
increase or decrease an individual's risk of developing a toxic
response following exposure to an environmental agent. Polymorphism
is present for some metabolic activation/deactivation enzymes,
including cytochrome P-450 isozymes (Nebert, 1988a, 1988b) and at
least one form of glutathione transferase (Seidegard et al., 1990).
Differing rates of enzyme activity controlling the activation or
detoxification of xenobiotics lead to differences in susceptibility
by increasing or decreasing the biologically effective dose of the
environmental agent.
The effect may vary between ethnic groups. For instance, there
are approximately equal numbers of fast and slow acetylator
phenotypes in a Caucasian population, whereas in a Japanese
population the distribution is 10% slow acetylators and 90% fast.
Genetic polymorphisms for drug metabolism have been widely studied
using phenotypic assays which involves measuring drug clearance in
individuals. Differential rates of metabolism will affect the
distribution and persistence of metabolites, which may have
implications for the site of toxicity. Epidemiological studies
suggest that, with respect to aromatic amines, slow acetylators are
more likely to contract bladder cancer but are at decreased risk
for colo-rectal cancer (Guengerich, 1991; Kadlubar et al., 1992).
Table 3. Some examples of established and suspected biomarkers of susceptibilitya
Biomarker of susceptibility Environmental agent Disease
Genetic
Debrisoquine hydroxylation phenotype cigarette smoke lung cancer
Acetylator phenotype aflatoxin, liver cancer,
aromatic amines bladder cancer
Ataxia telangiectasia genotype bleomycin, epoxides cancer at various sites
Xeroderma pigmentosum genotype agents that cause oxidative damage to skin cancer,
DNA, PAH, aromatic amines and aflatoxin B1 other cancers
Arylhydrocarbon hydroxylase inducibility polycyclic aromatic hydrocarbons lung cancer
alpha-1-antitrypsin cigarette smoke pulmonary emphysema
Franconi's anaemia phenotype cross-linking agents acute leukaemia
Glucose-6P-dehydrogenese deficiency phenotype oxidative agents, aromatic amines, poor resistance to oxidative
nitro-aromatic compounds stress,
aromatic amines
Sickle cell phenotype aromatic amino and nitro compounds, anaemia
carbon monoxide, cyanide
Thalassemia phenotype lead, benzene anaemia
Erythrocyte porphyria chloroquine, hexachlorobenzene, lead, anaemia
various drugs including barbituates,
sulfonamides, others
Sulfite oxidase deficiency heterozygotes sulfite, bisulfite, sulfur dioxide pulmonary disease
Alcohol dehydrogenase variant metabolize alcohols (e.g., ethanol)
more quickly than normal
GSTµ phenotype cigarette smoke lung cancer
Pseudocholinesterase variants organophosphate and carbamate insecticides, neurotoxicity
muscle relaxant drugs
IgA deficiency respiratory irritants irritation of respiratory tract
phenyl ketones in urine precursors of phenyl ketones phenylketonuria
Table 3 (contd)
Biomarker of susceptibility Environmental agent Disease
Acquired
Deficient diet chemical decreased resistance to
effects of many chemicals
Induced P-45O IIE1 alcohol consumption cancer at various sites
Antigen-specific antibodies chemicals, dusts decreased pulmonary
functions, skin rashes
a Modified from Calabrese (1986)
Polymorphism of N-oxidation has been linked to susceptibility to
colonic cancer (Kadlubar et al., 1992) and polymorphism in
glutathione S-transferase to increased lung cancer, particularly
adenocarcinoma (Seidegard et al., 1990).
The methodology for determining the phenotypes of individuals
for polymorphisms in metabolizing genes requires the administration
of a relevant test drug to the person and the subsequent measurement
of its clearance from the body. More recently, techniques based on
polymerase chain reactions, using DNA isolated from lymphocytes and
other cells, have been developed which allow the detection of
genetypes of known polymorphisms involving a variety of
xenobiotic-metabolizing enzymes, including GST1
(gluthione- S-transferase µ) and NAT2( N-acetyl transferase), as
well as two cytochrome P-450 isoenzymes: CYP1A1 and CYP2D6 (Bell,
1991; Blum et al., 1991; Wolf et al., 1992; Hirvonen et al., 1992;
Hollstein et al., 1992).
Cigarette smoking provides another example that illustrates the
effect of genetic polymorphisms on the response to chemicals.
Cigarette smoking is associated with the development of lung cancer
but not all smokers get lung cancer. This appears to be due in part
to genetic variations in arylhydrocarbon hydroxylase activity, which
results in a large variability in the binding of benz [a]pyrene to
DNA in cigarette smokers. A genetically based low level of
alpha-1-anti-trypsin activity greatly increases the risk of
emphysema from cigarette smoking.
In some situations a genetic trait may make an individual more
susceptible to one environmental agent but less so to another. For
example, the sickle cell trait predisposes to anaemia and altitude
sickness but offers some protection to the individual from infection
by the malaria parasite. Inherent differences in susceptibility
depend upon variations in the function of genes controlling enzyme
activity or the production of other proteins. Although a genotoxic
agent may reach the target tissue, the significance of any
chromosomal break will depend on the efficiency of the DNA repair
mechanisms. In xeroderma pigmentosum the individual is at an
increased risk of skin cancer after exposure to UV light because of
an inherited defect in DNA repair proteins (Cleaver, 1969).
Heterozygotes also have an increased risk of cancer and so the
frequency of the gene may affect the incidence of this cancer. Other
inherited diseases (e.g., ataxia-telangiectasia) that affect the
efficiency of DNA replication or repair may affect susceptibility to
carcinogenic agents (Swift et al., 1992). UV-DNA repair capacity has
been found to be lower in the lymphocytes isolated from individuals
with basal cell and squamous cell carcinoma than in the case of
their age-matched controls, and this repair capacity has been found
to decrease with increasing age in both groups (Wei et al., 1993).
Another form of susceptibility has an immunological basis.
Prior exposure to a chemical may induce an immune response that
sensitizes the individual to subsequent exposures. Such responses
occur in only a small fraction of the exposed population; an example
is the development of pulmonary hypersensitivity to industrial
agents such as toluene diisocyanate or cotton dust. The biomarkers
of susceptibility are the antigen-specific antibodies developed
against the chemical.
9. SUMMARY
The Task Group considered biomarkers in three categories,
biomarkers of exposure, of effect, and of susceptibility, while
recognising that clear distinction of category is often not
possible.
The Task Group agreed that the use of biomarkers can improve,
and should be used in, the process for the assessment of human
health risks caused by exposure to chemicals. Biomarkers may be
applied to the estimation of exposure and internal dose in
individuals and in groups and may allow identification of those at
greater or lesser risk than average.
Biomarkers must be validated before application in the risk
assessment process, i.e. the relationship between the biomarker, the
exposure, and the health outcome must be established. The selection,
validation and application of any biomarker is a complicated
process, which will vary for different markers. Examples were
selected to illustrate the concepts and principles.
Research and use of biomarkers involves complex ethical, social
and legal issues, which may vary in different countries. These
issues may impose constraints on research and use of biomarkers in
risk assessment and risk management decisions. The ethical, social
and legal aspects of biomarkers require careful consideration prior
to any application.
10. RECOMMENDATIONS
In making the following recommendations, the Task Group
recognized the role given IPCS to facilitate and increase
coordination of international activities in order to promote the
further work needed to define human health effects associated with
exposure to chemicals and to provide the basis for priority-setting
actions in order to protect public health.
10.1 General
* To promote the wider use of validated biomarkers in the
risk-assessment process
* To promote interdisciplinary cooperation and communication in
order to facilitate application of research findings
* To examine the feasibility of developing a data bank of
information on biomarkers applied to the process of risk and
their applications
10.2 Research
* To develop, refine and validate models to relate biomarkers of
exposure and of effect, qualitatively and quantitatively, to
exposure and to health outcome, particularly for end-points
other than cancer
* To identify and validate biomarkers of susceptibility in
relation to the chemical, and to inter-individual variation in
response, and investigate genetic polymorphism as a basis for
individual hypersensitivity
* To assess the use of information on individual susceptibility
in relation to protection of health with due respect to the
ethical, social and legal issues
* To develop strategies to link exposure and internal dose with
human health outcome by integration of mechanistically
validated biomarkers of exposure, effect and susceptibility
10.3 Applications
* To develop a practical protocol for use of biomarkers in human
studies, taking into account scientific, emotional, ethical,
legal and social aspects and including guidelines for risk
communication, with emphasis on the right of participants to
non-biased, intelligible information
* To encourage the production of certified reference materials
for biomarker analyses and support the functioning of
international quality assurance programmes
* To include consideration of biomarkers of exposure, effect, and
susceptibility in future Environmental Health Criteria
monographs
* To consider the need to update this monograph on principles and
concepts of biomarkers at an early date
REFERENCES
ACGIH (1992) 1992-1993 Threshold limit values for chemical
substances and physical agents and biological exposure indices.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
Adams RM & Fisher T (1990) Diagnostic patch testing.In: Adams R ed.
Occupational skin disease, 2nd ed. Philadelphia, Pennsylvania, W.B.
Saunders Company, pp 223-253.
Aitio A (1981) Laboratory quality control. Copenhagen, World Health
Organization, Regional Office for Europe, 74 pp (Health Aspects of
Chemical Safety - Interim Document 4).
Alessio L, Berlin A, & Roi R (1983) Human biological monitoring of
industrial chemicals series. Luxembourg, Office for Official
Publications of the Commission of European Communities (Industrial
Health and Safety Series).
Alessio L, Berlin A, & Roi R (1984) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 8903 EN).
Alessio L, Berlin A, & Roi R (1986) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 10704 EN).
Alessio L, Berlin A, & Roi R (1987) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11135 EN).
Alessio L, Berlin A, & Roi R (1988) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11478 EN).
Alessio L, Berlin A, & Roi R (1989) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 12174 EN).
Anderson D ed. (1988) Genetic toxicology testing and biomonitoring
of environmental or occupational exposure: human monitoring. Mutat
Res, 204(Spec Issue 3): 353-551.
Anderson D ed. (1990) Fundamental and molecular mechanisms of
mutagenesis. Mutat Res, 229(Spec Issue 2): 103-247.
Anderson D ed. (in press) Human monitoring II. Mutat Res (Spec
Issue).
Anderson D, Francis AJ, Godbert P, Jenkinson PC, & Butterworth KR
(1991) Chromosomal aberrations (CA), sister-chromatid exchanges
(SCE) and mitogen-induced blastogenesis in cultures of peripheral
lymphocytes from 48 control individuals sampled 8 times over 2
years. Mutat Res, 250: 467-476.
Ashby J & Tennant RW (1991) Definitive relationships among chemical
structure, carcinogenicity and mutagenicity for 301 chemicals tested
by the US National Toxicology Program. Mutat Res, 257: 229-306.
Balmain A & Brown K (1988) Oncogene activation in chemical
carcinogenesis. Adv Cancer Res, 51: 147-182.
Barbin A, Laib RJ, & Bartsch H (1985) Lack of miscoding properties
of 7-(2-oxoethyl)guanine, the major vinyl chloride-DNA adduct.
Cancer Res, 45: 2440-2444.
Bartsch H & Montesano R (1984) Commentary: relevance of nitrosamines
to human cancer. Carcinogenesis, 5: 1381-1393.
Bartsch H, Ohshima H, & Shuker DEG (1991) Noninvasive methods for
measuring exposure to alkylating agents: recent studies on human
subjects. In: Groopman JD & Skipper PL ed. Molecular dosimetry and
human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 281-301.
Beach AC & Gupta RC (1992) Human biomonitoring and the
32P-postlabeling assay. Carcinogenesis, 13: 1053-1074.
Bekes JG, Roboz JP, Fischbein A, & Selikoff IJ (1987) Clinical
immunology studies in individuals exposed to environmental
chemicals. In: Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F
ed. Immunotoxicology. Proceedings of the International Seminar on
the Immunological System as a Target for Toxic Damage: Present
Status, Open Problems and Future Perspectives. Dordrecht, Boston,
Lancaster, Martinus Nijhoff Publishers, pp 347-361 (IPCS Joint
Seminar 10; EUR 10041 EN).
Belinsky SA, White CM, Devereux TR, Swenberg JA, & Anderson MW
(1987) Cell selective alkylation of DNA in rat lung following low
dose exposure to the tobacco specific carcinogen
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res, 47:
1143-1148.
Bell DA (1991) Detection of DNA sequence polymorphisms in carcinogen
metabolism genes by polymerase chain reaction. Environ Mol Mutagen,
18: 245-248.
Blum M, Demierre A, Grant DM, Helm UA, & Meuer UA (1991) Molecular
mechanisms of slow acetylation of drugs and carcinogens in humans.
Proc Natl Acad Sci (USA), 88: 5237-5241.
Bond JA, Wallace LA, Osterman-Golkar S, Lucier GW, Buckpitt A, &
Henderson RF (1992) Assessment of exposure to pulmonary toxicants:
use of biological markers. Fundam Appl Toxicol, 18: 161-174.
Brandt-Rauf PW (1991) Advances in cancer biomarkers as applied to
chemical exposures: the ras oncogene and p21 protein and pulmonary
carcinogenesis. J Occup Med, 33: 951-955.
Brinkworth MH, Yardley-Jones A, Edwards AJ, Hughes JA, & Anderson D
(1992) A comparison of smokers with respect to oncogene products and
cytogenetic parameters. J Occup Med, 34: 1181-1189.
Butterworth BE, Popp JA, Conolly RB, & Goldsworthy TL (1992)
Chemically induced cell proliferation in carcinogenesis. In: Vainio
H, Magee PN, McGregor DB, & McMichael AJ ed. Mechanisms of
carcinogenesis in risk identification. Lyon, International Agency
for Research on Cancer, pp 279-305 (IARC Scientific Publications No.
116).
Calabrese EJ (1986) Ecogenetics: historical foundation and current
status (genetic factors). J Occup Med, 28: 1096-1102.
Cantin AM, Hubbard RC, & Crystal RG (1989) Glutathione deficiency in
the epithelial lining fluid of the lower respiratory tract in
idiopathic pulmonary fibrosis. Am Rev Respir Dis, 139: 370-372.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Hotter G, Mutti A, Franchini I, Fels LM, Stolte H, de
Broe ME, Nuyts GD, Taylor SA, & Price RG (1993a) Markers of early
renal changes induced by industrial pollutants. 1 Application to
workers exposed to mercury vapour. Br J Ind Med, 50: 17-27.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Ramis I, Mutti A, Franchini I, Fels LM, Stolte H, de Broe
ME, Nuyts GD, Taylor SA, & Price RG (1993b) Markers of early renal
changes induced by industrial pollutants. 2 Application to workers
exposed to lead. Br J Ind Med, 50: 28-36.
Carrano AV & Natarajan AT (1988) Considerations for population
monitoring using cytogenetic techniques. Mutat Res, 204: 379-406.
Casanova M, Deyo DF, & Heck HD'A (1989) Covalent binding of inhaled
formaldehyde to DNA in the nasal mucosa of Fischer 344 rats:
analysis of formaldehyde and DNA by high-performance liquid
chromatography and provisional pharmacokinetic interpretation.
Fundam Appl Toxicol, 12: 397-417.
Casanova M, Morgan KT, Steinhagen WH, Everitt JI, Popp JA, & Heck
HD'A (1991) Covalent binding of inhaled formaldehyde to DNA in the
respiratory tract of rhesus monkeys: pharmacokinetics, rat-to-monkey
interspecies scaling, and extrapolation to man. Fundam Appl Toxicol,
17: 409-428.
Cassito MG, Camerino D, Hanninen H, & Anger KW (1990) International
collaboration to evaluate the WHO neurobehavioural core test
battery. In: Johnson BL, Anger WK, Durao A, & Xinteras C ed.
Advances in neurobehavioural toxicology: applications in
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., pp 203-223.
Costa LG (1992) Effect of neurotoxicants on brain neurochemistry.
In: Tilson H & Mitchell C ed. Neurotoxicology. New York, Raven Press
Ltd, pp 101-123.
Cavalleri A, Gobba F, Bacchella L, & Ferrari D (1991) Evaluation of
serum aminoterminal propeptide of type III procollagen as an early
marker of the active fibrotic process in asbestos-exposed workers.
Scand J Work Environ Health, 17: 139-144.
Cleaver JE (1969) Xeroderma pigmentosum: a human disease in which an
initial stage of DNA repair is defective. Proc Natl Acad Sci (USA),
63: 428-435.
Cohen SM & Ellwein LB (1990) Cell proliferation in carcinogenesis.
Science, 249: 1007-1011.
Compton PJE, Hooper K, & Smith MT (1991) Human somatic mutation
assays as biomarkers of carcinogenesis. Environ Health Perspect, 94:
135-141.
Conolly RB & Andersen ME (in press) An approach to mechanism-based
cancer risk assessment: formaldehyde. Environ Health Perspect, 101.
Conolly RB, Monticello TM, Morgan KT, Clewell HJ, & Andersen ME
(1992) A biologically-based risk assessment strategy for inhaled
formaldehyde. Comments Toxicol, 4: 269-293.
Das BC (1988) Factors that influence formation of sister chromatid
exchanges in human blood lymphocytes. Crit Rev Toxicol, 19: 43-86.
Dellarco V, Voytek P, & Hollaender A (1985) Aneuploidy: Etiology and
mechanisms. New York, London, Plenum Press.
Derosa CT, Choudhury H, & Peirano WB (1991) An integrated
exposure/pharmacokinetic based approach to the assessment of complex
exposures. Toxicol Ind Health, 7(4): 231-248.
DFG (German Association for the Encouragement of Research) (1992)
Commission for the Investigation of Health Hazards of Chemical
Compounds in the Work Area: MAK- and BAT-values 1992. Weinheim, VCH.
Droz PO, Wu MM, & Cumberland WG (1989) Variability in biological
monitoring of organic solvent exposure. 2 Application of a
population physiological model. Br J Ind Med, 46: 547-558.
Enterline PE & Marsh GM (1982) Cancer among workers exposed to
arsenic and other substances in a copper smelter. Am J Epidemiol,
116: 895-911.
Farmer PB (1991) Analytical approaches for the determination of
protein-carcinogen adducts using mass spectrometry. In: Groopman JD
& Skipper PL ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 189-210.
FIOH (Työterveyslaitos) (1993) [Biological monitoring. Guide for
sample collection 1993.] Helsinki, Finnish Institute of Health, 115
pp (in Finnish).
French M & Morley AA (1985) Measurement of micronuclei in
lymphocytes. Mutat Res, 147: 29-36.
Gibaldi M & Perrier D (1982) Pharmacokinetics. New York, Basel,
Marcel Dekker Inc.
Goldsworthy TL, Morgan KT, Popp JA, & Butterworth BE (1991)
Guidelines for measuring chemically-induced cell proliferation in
specific rat organs. In: Butterworth BE & Sloga TJ ed. Chemically
induced cell proliferation: Implications for risk assessment. New
York, Wiley-Liss, pp 253-284.
Graham D, Henderson FW, & House D (1988) Neutrophil influx measured
in nasal lavages of humans exposed to ozone. Arch Environ Health,
43: 228-233.
Groopman JD, Sabbioni G, & Wild CP (1991) Molecular dosimetry of
human aflatoxin exposures. In: Groopman JD & Skipper PL ed.
Molecular dosimetry and human cancer -Analytical, epidemiological
and social considerations. Boca Raton, Florida, CRC Press, pp
303-324.
Guengerich FP (1991) Interindividual variation in biotransformation
of carcinogens: basis and relevance. In: Groopman JD & Skipper PL
ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 27-52.
Hall JA, Saffhill R, Green T, & Hathway DE (1981) The induction of
errors during in vitro DNA synthesis following
chloroacetaldehyde-treatment of poly(dA-dT) and poly(dC-dG)
templates. Carcinogenesis, 2: 141-146.
Hanawalt PC (1987) Preferential DNA repair in expressed genes.
Environ Health Perspect, 76: 9-14.
Hancock DG, Moffitt AE, & Hay EB (1984) Hematological findings among
workers exposed to benzene at coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Harel S, Janoff A, Yu SY, Hurewitz A, & Bergofsky EH (1980)
Desmosine radioimmunoassay for measuring elastin degradation in
vivo. Am Rev Respir Dis, 122: 769-773.
Harris CC (1991) Molecular epidemiology: overview of molecular and
biochemical basis. In: Groopman JD & Skipper PL ed. Molecular
dosimetry and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 15-26.
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumours in Syrian golden hamsters by a single dose
of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and the
effect of smoke inhalation. Carcinogenesis, 4(10): 1287-1290.
Henderson RF (1988) Use of bronchoalveolar lavage to detect lung
damage. In: Target organ toxicology: Lung. New York, Raven Press, pp
239-268.
Henderson RF, Bechtold WE, Bond JA, & Sun JD (1989) The use of
biological markers in toxicology. Crit Rev Toxicol, 20: 65-82.
Hirvonen A, Husgafvel-Pursiainen K, Karjalainen A, Anttila S, &
Vainio H (1992) Point-mutational Msp1 and lle-Val polymorphisms
closely linked in the CYP1A1 Gene: Lack of association with
susceptibility to lung cancer in a Finnish study population. Cancer
Epidemiol Biomarkers Prev, 1: 485-489.
Hoffman D, Rivenson A, Amin S, & Hecht SS (1984) Dose-response study
of the carcinogenicity of tobacco-specific N-nitrosamines in F344
Rats. J Cancer Res Clin Oncol, 108: 81-86.
Hollstein M, Sidransky D, Vogelstein B, & Harris CC (1991) p53
Mutations in human cancers. Science, 253: 49-53.
Hollstein MC, Wild CP, Bleicher F, Chutimataewin S, Harris CC,
Srivatanakul P, & Montesano R (1992) p53 Mutations and aflatoxin
B1h exposure in hepatocellular carcinoma patients from Thailand.
Int J Cancer, 53: 1-5.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-255.
Horak F (1985) Manifestation of allergic rhinitis in
latent-sensitized patients. A prospective study. Arch
Otorhinolaryngol, 242: 224-239.
Hsia MTS (1991) Carcinogen-macromolecular adducts as biomarkers in
human cancer risk assessment. Biomed Environ Sci, 4: 104-112.
Hsu IC, Metcalf RA, Sun T, Welsh J, Wang NJ, & Harris CC (1991)
Mutational hotspot in the p53 gene in human hepatocellular
carcinomas. Nature (Lond), 350: 427-428.
IARC (1992) In: Vainio H, Magee PN, McGregor D, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer (IARC Scientific
Publications No. 116).
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunologic
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin. Br J Ind Med, 45: 701-704.
Kadlubar FF, Butler MA, Kaderlik KR, Chou H-S, & Lang NP (1992)
Polymorphisms for aromatic amine metabolism in humans: relevance for
human carcinogenesis. Environ Health Perspect, 98: 69-74.
Kang H-I, Konishi C, Kuroko T, & Huh N-K (1993) A highly sensitive
and specific method for quantitation of O-alkylated DNA adducts and
its application to the analysis of human tissue. Environ Health
Perspect, 99: 269-271.
Lambert B (1992) Biological markers in exposed humans: gene
mutation. In: Vainio H, Magee PN, McGregor DB, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer, pp 535-542 (IARC
Scientific Publications No. 116).
Lamm SH, Walters AS, Wilson R, Byrd DM, & Grunwalt H (1989)
Consistencies and inconsistencies underlying the quantitative
assessment of leukemia risk from benzene exposure. Environ Health
Perspect, 82: 289-297.
Lassalle P, Cosset P, & Aerts C (1990) Abnormal secretion of
interleukin-1 and tumor necrosis factor alpha by alveolar
macrophages in coal worker's pneumoconiosis: comparison between
simple pneumoconiosis and progressive massive fibrosis. Exp Lung
Res, 16: 73-80.
Lucier G & Thompson CL (1987) Issues in biochemical applications to
risk assessment: when can lymphocytes be used as surrogate markers?
Environ Health Perspect, 76: 187-191.
Mattison DR (1991) An overview on biological markers in reproductive
and development toxicology: concepts and definitions, and use in
risk assessment. Biomed Environ Sci, 4: 8-34.
Morris SM (1991) The genetic toxicology of 5-bromodeoxyuridine in
mammalian cells. Mutat Res, 258: 161-188.
Morris SM, Domon OE, McGarrity LJ, Kodell RL, & Casciano DA (1992)
Effect of bromodeoxyuridine on the proliferation and growth of ethyl
methanesulfonate-exposed P3 cells: relationship to the induction of
sister chromatid exchanges. Cell Biol Toxicol, 8: 75-87.
Nebert DW (1988a) The 1986 Bernard B. Brodie award lecture. The
genetic regulation of drug-metabolizing enzymes. Drug Metab Dispos,
16: 1-8.
Nebert DW (1988b) Genes encoding drug metabolizing enzymes: possible
role in human disease. Basic Life Sci, 43: 45-64.
Nielsen J, Welinder H, Horstmann V, & Skerfving S (1992) Allergy to
methyltetrahydrophthalic anhydride in epoxy resin workers. Br J Ind
Med, 49: 769-775.
O'Callaghan JP (1991) Quantification of glial fibrillary acidic
protein: Comparison of slot-immunobinding assays with a novel
sandwich ELISA. Neurotoxicol Teratol, 13: 275-281.
Parry JM, Shamsher M, & Skibinski DOF (1990) Restriction site
mutation analysis, a proposed methodology for the detection and
study of DNA base changes following mutation exposure. Mutagenesis,
5: 209-212.
Passingham BJ, Farmer FB, Bailey E, Brookes AGF, & Yates DW (1988)
2-Hydroxyethylation of haemoglobin in man. In: Methods for detecting
DNA and damaging agents in humans: applications in cancer
epidemiology and prevention. Lyon, International Agency for Research
on Cancer, pp 279-285 (IARC Scientific Publications No. 89).
Piguet PF, Collart MA, Grau GE, Sappino AP, & Vassalli P (1990)
Requirement of tumor necrosis factor for development of
silica-induced pulmonary fibrosis. Nature (Lond), 344: 245-247.
Poland A, Glover E, & Kende AS (1976) Stereospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol.
Evidence that the binding species is receptor for induction of aryl
hydrocarbon hydroxylase. J Biol Chem, 251: 4936-4946.
Rajamaki A (1984) Assessment of early myelotoxicity. In: Aitio A,
Riihimaki V, & Vainio H ed. Biological monitoring and surveillance
of workers exposed to chemicals. Washington, New York, London,
Hemisphere Publishing Corporation, pp 303-308.
Ramel C (1992) Genotoxic and nongenotoxic carcinogens: mechanisms of
action and testing strategies. In: Vainio H, Magee PN, McGregor DB,
& McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 195-209 (IARC Scientific Publications No. 116).
Ramsay JC & Andersen ME (1984) A physiologically based description
of the inhalation pharmacokinetics of styrene monomer in rats and
humans. Toxicol Appl Pharmacol, 73: 159-175.
Randerath K & Randerath E (1991) 32P-postlabeling analysis of
mutagen- and carcinogen-adducts and age-related DNA modifications
(I-compounds). In: Groopman JD & Skipper PL ed. Molecular dosimetry
and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 131-150.
Reynolds HY (1987) Bronchoalveolar lavage. Am Rev Respir Dis, 135:
250-263.
Rivenson A, Hoffman D, Prokopszyk B, Amin S, & Hecht SS (1988)
Induction of lung and pancreas tumours in F344 rats by
tobacco-specific and areca derived N-nitrosamines. Cancer Res, 48:
6912-6917.
Roels H, Bernard AM, Cardenas AR, Buchet JP, Lauwerys RR, Hotter G,
Ramis I, Mutti A, Frandhini I, Bundschuh I, Stolte H, de Broe ME,
Nuyts GD, Taylor SA, & Price RG (1993) Markers of early renal
changes induced by industrial pollutants. 3 Application to workers
exposed to cadmium. Br J Ind Med, 50: 37-48.
Ross RK, Yuan J-M, Wogan GN, Qian G-S, Tu J-T, Groopman JD, Gao Y-T,
& Henderson BE (1992) Urinary aflatoxin biomarkers and risk of
hepatocellular carcinoma. Lancet, 339: 943-946.
Santella RM (1988) Application of new techniques for the detection
of carcinogen adducts to human population monitoring. Mutat Res,
205: 271-282.
Santella RM, Gaspara F, & Hsieh L (1985) Quantitation of
carcinogen-DNA adducts with monoclonal antibodies. Prog Exp Tumour
Res, 31: 63-75.
Seidegard J, Pero RW, Markowitz MM, Rousch G, Miller DG, & Beattle
EJ (1990) Isoenzyme(s) of glutathione transferase (class mu) as a
marker for the susceptibility to lung cancer: A follow-up study.
Carcinogenesis, 11: 33-36.
Seppalainen A-M, Hernberg S, & Kock B (1979) Relationship between
blood lead levels and nerve conduction velocities. Neurotoxicology,
1: 313-332.
Shuker DEG & Farmer PB (1992) Relevance of urinary DNA adducts as
markers of carcinogen exposure. Chem Res Toxicol, 5: 450-460.
Singer B, Chavez SJ, & Kusmierek JT (1987) The vinyl
chloride-derived nucleoside N2,3-ethenoguanosine, is a highly
efficient mutagen in transcription. Carcinogenesis, 8: 745-747.
Sorsa M, Ojajarvi A, & Salomaa S (1990) Cytogenetic surveillance of
workers exposed to genotoxic chemicals: preliminary experiences from
a prospective cancer study in a cytogenetic cohort. Teratog Carcinog
Mutagen, 10: 215-221.
Sorsa M, Wilbourn J, & Vanio H (1992) Human cytogenetic damage as a
predictor of cancer risk. In: Vanio H, Magee PN, McGregor D, &
McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 543-554 (IARC Scientific Publications No. 116).
Stone PJ, Bryan-Rhadfi J, & Lucey EC (1991) Measurement of urinary
desmosine by isotope dilution and high performance liquid
chromatography. Am Rev Respir Dis, 144: 284-290.
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology (1989) In: Dreborg S ed. Skin tests for
diagnosis of IgE-mediated allergy. Position paper. Allergy, 44(Suppl
10): 31-37.
Sullivan JB (1989) Immunological alterations and chemical exposure.
Clin Toxicol, 27: 311-343.
Swenberg JA, Fedtke N, Fennell TR, & Walker VE (1990) Relationships
between carcinogen exposure, DNA adducts and carcinogenesis. In:
Clayson DB, Munro IC, Shubik P, & Swenberg JA ed. Progress in
predictive toxicology. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 161-184.
Swift M, Morrel D, Massey RB, & Chase CL (1992) Incidence of cancer
in 161 families affected by ataxia-telangiectasia. New Engl J Med,
325: 1831-1836.
Townsend JC, Ott MG, & Fishbeck WA (1978) Health exam findings among
individuals occupationally exposed to benzene. J Occup Med, 20:
543-548.
Tritscher AM, Goldstein JA, Portier CJ, McCoy ZM, Clark GC, & Lucier
GW (1992) Dose-response relationships for chronic exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin in a rat tumour promotion model:
quantification and immunolocalization of CYP1A1 and CYP1A2 in the
liver. Cancer Res, 52: 3436-3442.
UK DH (1989) Committee on Mutagenicity of Chemicals in Food.
Guidelines for the testing of chemicals for mutagenicity - Consumer
Products and the Environment. London, Department of Health, Her
Majesty's Stationery Office (HMSO), 99 pp (Report No. 35).
UK HSE (1991) Guidance on laboratory techniques in occupational
medicine, 5th ed. London, Health and Safety Executive, Library and
Information Services, 117 pp.
US EPA (1991) Formaldehyde risk assessment update. Washington, DC,
US Environmental Protection Office, Office of Toxic Substances.
US EPA (1987) Assessment of health risks to garment workers and
certain home residents from exposure to formaldehyde. Washington,
DC, US Environmental Protection Office, Office of Toxic Substances.
US NRC (US National Research Council) (1989a) Biologic markers in
pulmonary toxicology. Washington, DC, National Academy Press, 179
pp.
US NRC (US National Research Council) (1989b) Biologic markers in
reproductive toxicology. Washington, DC, National Academy Press, 395
pp.
US NRC (US National Research Council) (1992a) Biologic markers in
immunotoxicology. Washington, DC, National Academy Press, 206 pp.
US NRC (US National Research Council) (1992) Environmental
neurotoxicology. Report of Committee on Neurotoxicology and Models
for Assessing Risk. Washington, DC, National Academy Press, 154 pp.
Wei Q, Matanoski GM, Farmer ER, Hedayati MA, & Grossman L (1993) DNA
repair and aging in basal cell carcinoma: a molecular epidemiology
study. Proc Natl Acad Sci (USA), 90: 1614-1618.
Weinberg RA (1991) Tumour suppressor genes. Science, 254: 1138-1146.
Weisburger JH & Williams GM (1981) Carcinogen testing: current
problems and new approaches. Science, 214: 401-407.
Weston A & Bowman ED (1991) Fluorescence detection of
benzo(a)pyrene-DNA adducts in human lung. Carcinogenesis, 12:
1445-1449.
WHO (1991) IPCS Environmental Health Criteria 119: Principles and
methods for the assessment of nephrotoxicity associated with
exposure to chemicals. Geneva, World Health Organization, 266 pp.
WHO (1992a) IPCS Environmental Health Criteria 134: Cadmium. Geneva,
World Health Organization, 280 pp.
WHO (1992b) IPCS Environmental Health Criteria 141: Quality
management for chemical safety testing. Geneva, World Health
Organization, 112 pp.
WHO (in press) Principles for assessment of risks from exposure to
chemicals (Part A).
Wild CP & Montesano R (1991) Immunological quantitation of human
exposure to aflatoxins and N-nitrosamines. In: Vanderlaan M, Stanker
LH, Watkins BE, & Roberts DW ed. Immunoassays for trace chemical
analysis. Washington, DC, American Chemical Society, pp 215-228 (ACS
Symposium Series No. 451).
Wild CP, Jiang YZ, Sabbioni G, Chapot B, & Montesano R (1990)
Evaluation of methods for quantitation of aflatoxin-albumin adducts
and their application to human exposure assessment. Cancer Res, 50:
245-251.
Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle
H, Montesano R, & Groopman JD (1992) Dietary intake of aflatoxins
and the level of albumin-bound aflatoxin in peripheral blood in The
Gambia, West Africa. Cancer Epidemiol Biomarkers Prev, 1: 229-234.
Wogan GN (1989) Markers of exposure to carcinogens: methods for
human monitoring. J Am Coll Toxicol, 8: 871-881.
Wogan GN & Gorelick NJ (1985) Chemical and biochemical dosimetry of
exposure to genotoxic chemicals. Environ Health Perspect, 62: 5-18.
Wolf CR, Dale Smith CA, Gough AC, Moss JE, Vallis KA, Howard G,
Carey FJ, Mills K, McNee W, Carmichael J, & Spurr NK (1992)
Relationship between the debrisoquine hydroxylase polymorphism and
cancer susceptibility. Carcinogenesis, 13: 1035-1038.
Yanagisawa Y, Nishimura H, Matsuki H, Osaka F, & Kasuga H (1986)
Personal exposure and health effect relationship for NO2 with
urinary hydroxyproline to creatinine ratio as indicator. Arch
Environ Health, 41: 41-48.
RESUME
Le groupe spécial a divisé les marqueurs biologiques en trois
catégories, les marqueurs d'exposition, les marqueurs d'effet et les
marqueurs de réceptivité, tout en admettant que bien souvent il
n'était pas possible d'établir une distinction nette entre ces
diverses catégories.
Le groupe spécial a admis que le recours aux marqueurs
biologiques pouvait améliorer l'évaluation des risques pour la santé
humaine découlant d'une exposition à des produits chimiques et qu'il
fallait donc en faire usage. Les marqueurs biologiques peuvent être
utilisés pour évaluer l'exposition et la dose interne chez des
individus et des groupes et peuvent faciliter l'identification de
ceux de ces individus ou de ces groupes qui sont plus ou moins
exposés aux risques que la moyenne.
Avant d'utiliser les marqueurs biologiques pour l'évaluation du
risque il faut les valider, c'est-à-dire établir la relation qui
existe entre le marqueur, l'exposition et ses conséquences pour la
santé. Le choix, la validation et l'utilisation de tout marqueur
biologique constituent des processus complexes qui varient d'un
marqueur à l'autre. Pour mettre en lumière ces notions et ces
principes on a choisi un certain nombre d'exemples.
La recherche et l'utilisation des marqueurs biologiques
soulèvent des questions complexes sur le plan éthique, social et
juridique, qui peuvent d'ailleurs varier d'un pays à l'autre. Il
peut s'en suivre un certain nombre de contraintes imposées à la
recherche et à l'utilisation des marqueurs biologiques dans
l'évaluation des risques et dans les décisions relatives à la
gestion de ces risques. Avant toute application il importe d'étudier
avec soin les aspects éthiques, sociaux et juridiques des marqueurs
biologiques.
RECOMMANDATIONS
En formulant les recommandations ci-après, le groupe de travail
a pris en considération le rôle dévolu au PISC, à savoir de
faciliter et de développer la coordination des activités
internationales afin d'encourager les travaux à poursuivre pour
définir les effets sur la santé humaine qu'entraîne l'exposition aux
substances chimiques et de jeter les bases des actions prioritaires
à entreprendre pour protéger la santé publique.
1. Généralités
* Encourager un plus large recours aux marqueurs biologiques dans
l'évaluation des risques
* Favoriser la coopération et la communication
inter-disciplinaires afin de faciliter l'application des
résultats de la recherche
* Etudier la faisabilité d'une banque de données sur les
marqueurs biologiques dans l'évaluation des risques et ses
applications
2. Recherche
* Mettre au point, affiner et valider des modèles qui permettent
de corréler les marqueurs biologiques de l'exposition et des
effets tant qualitativement que quantitativement, à
l'exposition et aux conséquences biologiques, en particulier
aux conséquences autres que le cancer.
* Recenser et valider des marqueurs biologiques de réceptivité,
par rapport à la réaction aux produits chimiques et aux
variations interindividuelles à cette réaction et étudier le
polymorphisme génétique en tant que base de l'hypersensibilité
individuelle.
* Voir dans quelle mesure il est possible d'utiliser certains
renseignements sur la réceptivité individuelle pour la
protection de la santé, dans le respect des impératifs
éthiques, sociaux et juridiques.
* Mettre au point des stratégies afin de relier l'exposition et
la dose interne aux conséquences biologiques pour l'homme
grâceà une synthèse de biomarqueurs de l'exposition, de l'effet
et de la réceptivité, mécanistiquement validés.
3. Applications
* Mettre au point un protocole pratique pour l'utilisation des
marqueurs biologiques dans les études sur l'homme, qui prenne
en considération les aspects scientifiques, émotionnels,
éthiques, juridiques et sociaux et qui comporte des directives
en matière de communication, en insistant sur le droit des
participants à disposer d'informations intelligibles et non
biaisées.
* Encourager la production de substances de référence homologuées
pour l'analyse des marqueurs biologiques et aider les
programmes internationaux d'assurance de la qualité à
fonctionner.
* Faire figurer des considérations sur les biomarqueurs
d'exposition, d'effet et de réceptivité dans les futures
monographies de la série Critères d'hygiène de l'environnement.
* Examiner s'il est nécessaire de mettre à jour à bref délai la
présente monographie consacrée aux principes et conceptions en
matière de marqueurs biologiques.
RESUMEN
El Grupo Especial distinguió tres clases de biomarcadores: de
exposición, de efecto y de susceptibilidad, reconociendo sin embargo
que a menudo resulta imposible establecer claramente la pertenencia
a una de esas clases.
El Grupo Especial coincidió en que los biomarcadores permiten
mejorar, y deben emplearse a ese efecto, el proceso de evaluación de
los riesgos que para la salud humana conlleva la exposición a
productos químicos. Los biomarcadores se pueden emplear para
calcular la exposición y la dosis interna recibida por individuos y
grupos, con la consiguiente identificación de quienes sufren un
mayor o menor riesgo que la media.
Los biomarcadores deben ser validados antes de aplicarlos a la
evaluación del riesgo, lo que significa que debe determinarse la
relación entre el biomarcador, la exposición y el estado de salud.
La selección, validación y empleo de cualquier biomarcador es un
proceso complicado, distinto para cada marcador. Se eligieron
algunos ejemplos para ilustrar los conceptos y principios
relacionados.
La investigación y el empleo de los biomarcadores plantea
complejas cuestiones éticas, sociales y jurídicas, que pueden
diferir de un país a otro. Algunos de esos problemas limitan el
alcance de las investigaciones sobre los biomarcadores y de su
aplicación a la evaluación de riesgos y la adopción de decisiones
relacionadas con la gestión de riesgos. Los problemas éticos,
sociales y jurídicos que plantean los biomarcadores deben ser objeto
de un detenido análisis antes de su eventual uso.
RECOMENDACIONES
Al formular las siguientes recomendaciones, el Grupo Especial
reconoció la función asignada al IPCS de facilitar e intensificar la
coordinación de las actividades internacionales con miras a fomentar
los trabajos que aún será necesario realizar para determinar los
efectos sobre la salud relacionados con la exposición a productos
químicos, así como para establecer las bases que permitan señalar
las prioridades a que haya que atenerse para proteger la salud
pública.
1. Recomendaciones generales
* Promover un mayor uso de los biomarcadores validados en el
proceso de evaluación de riesgos.
* Fomentar la cooperación y la comunicación interdisciplinarias
para facilitar la aplicación de los resultados de las
investigaciones.
* Estudiar la posibilidad de crear un banco de datos sobre
biomarcadores aplicados a la evaluación de riesgos y sus
posibles usos.
2. Investigaciones
* Desarrollar, perfeccionar y validar modelos aptos para
relacionar los biomarcadores de exposición y de efecto,
cualitativa y cuantitativamente, con la exposición y con el
estado de salud, sobre todo para puntos finales distintos del
cáncer.
* Identificar y validar biomarcadores de susceptibilidad en
relación con el producto químico y con la variación
interindividual de la respuesta, e investigar la influencia del
polimorfismo genético en la hipersensibilidad individual.
* Evaluar el uso de la información referente a la susceptibilidad
individual desde la perspectiva de una protección de la salud
atenta a los aspectos éticos, sociales y jurídicos.
* Formular estrategias para relacionar la exposición y la dosis
interna con el estado de salud mediante la integración de
biomarcadores de exposición, efecto y susceptibilidad validados
sistemáticamente.
3. Aplicaciones
* Elaborar un protocolo práctico para el uso de biomarcadores en
los estudios realizados en el hombre, teniendo en cuenta los
aspectos científicos, psicológicos, éticos, jurídicos y
sociales, con inclusión de directrices para la comunicación del
riesgo y haciendo hincapié en el derecho de los participantes a
una información inteligible e imparcial.
* Fomentar la producción de material de referencia certificado
para el análisis de biomarcadores y respaldar la aplicación de
programas internacionales de garantía de la calidad.
* Incluir la consideración de los biomarcadores de exposición,
efecto y susceptibilidad en las futuras monografías de la serie
Criterios de Salud Ambiental.
* Tener presente la necesidad de actualizar con prontitud la
presente monografía sobre los principios y nociones relativos a
los biomarcadores.
Thus by analysing several biomarkers with different half-lives
(e.g., haemoglobin adducts in the blood, metabolites of the chemical
in urine, parent compound in blood) from a single individual at a
single point in time, more information may be obtained about the
nature of the past exposure than from use of a single biomarker.
Also of value in the interpretation of biomarker data is the
use of mathematical models to describe the kinetics of formation and
elimination of biomarkers of exposure. Absorbed chemicals are
distributed between various compartments in the body, with the
distribution being dependent on the nature of the compartment and
the lipophilicity of the chemical. The most simple models use only
one compartment; however, multicompartmental models are usually
required to describe the disposition of most chemicals in the body
(Gibaldi & Perrier, 1982). Multicompartmental models that
incorporate biomarkers of exposure in relation to toxic end-points
are well established. A biokinetics model for lead has been used to
predict blood lead levels of individuals and communities (DeRosa et
al., 1991).
More recently, physiologically based toxicokinetic (PBTK)
models have been developed that make use of the physico-chemical
properties of a chemical (such as partition coefficients that
indicate how the chemical or its metabolites become partitioned
between different fluids in the body), the kinetics of metabolism of
the chemical (such as Vmax and KM for metabolic pathways), and
physiological parameters of the exposed individual (such as tissue
blood flow, respiratory minute volume, cardiac output) to predict
the actual concentrations of biomarkers that will occur after
specific exposure regimens. These models are adapted for humans by
changing the physiological and metabolic parameters to those
appropriate for humans, and by testing for validity in limited human
studies. These models can then be used to extrapolate between
different exposure situations for the predicted levels of markers
(Ramsay & Andersen, 1984; Droz et al., 1989).
Another use of models is in defining the quantitative
relationship between biomarkers in readily available biological
samples (e.g., blood cells) and in those less readily available
which might be more pertinent biomarkers for the health effect of
concern (e.g., tissue DNA). An example is the use of haemoglobin
adducts to predict the amount of DNA adducts at a critical site,
e.g., after exposure to ethylene oxide (Passingham et al., 1988). To
be able to make such predictions, one must know the kinetics of
formation and breakdown of each of the markers and the factors that
influence those kinetics. Based on such information a model can be
developed to show the quantitative relationships between the markers
under different exposure conditions or at different times after
exposure.
Biomarkers are used extensively in the surveillance of workers
occupationally exposed to metals such as lead, cadmium, mercury,
nickel, chromium and arsenic, and to organic chemicals such as
aniline, benzene, carbon disulfide, styrene, chlorobenzene and
chlorinated aliphatic hydrocarbon solvents (see Table 1). The
examples in Table 1 are given as general information. Before
applying them in specific circumstances, readers must consult the
original references.
These measures are used to indicate the absorbed dose. For a
few chemicals, notably lead, mercury, cadmium and carbon monoxide,
an approximate estimation of the associated health risk may be made.
For other chemicals, exposure may be assessed in quantitative terms.
It is noted that biomarkers are supplementary to environmental
measurements rather than alternative or substitute measures of
exposure.
Considerable efforts are being made to develop biomarkers
associated with exposure to chemical carcinogens and to establish
the relationship between a marker and the future health risk. The
use of animal models may facilitate this process; studies on the DNA
adducts formed by vinyl chloride illustrate the types of strategies
required to make each link (Swenberg et al., 1990). In rats, vinyl
chloride induces liver tumours with a high incidence in young
animals, and the DNA adducts (biomarkers) formed in the liver have
been characterized and the half-lives determined. DNA fidelity
replication assays were used to show one type of adduct that had
both a long half-life and was capable of inducing mutations (Hall et
al., 1981; Barbin et al., 1985; Singer et al., 1987; Swenberg et
al., 1990). The level of this adduct was much higher in the livers
of young rats exposed to vinyl chloride than in adults, and was
characterized as the one most closely related to the health effect.
To extend this animal research to predict human health risks, it
would be necessary to determine if the concentration of the same
adduct is associated with liver tumour formation in human tissue
samples.
Table 1. Some parameters proposed for biological monitoring by different organizations
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase,
inhibitors P-cholinesterase
Aluminium (Al) U-Al U-Al
Aniline U-p-aminophenol,B-Met-Hb U-aniline U-p-aminophenol
Arsenic (As) U-certain volatile U-AsIII+, AsV+ U-AsIII+, AsV+
arsenic compounds MMA+DMA
produced by direct
hydrogenation
Benzene U-phenol B-benzene, U-phenol B-benzene Breath-, B-benzene
p-tert-Butylphenol U-p-tert-butylphenol
Cadmium (Cd) U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd
Carbon disulfide U-TTCA U-TTCA U-TTCA U-TTCA
Carbon monoxide Breath-CO, B-COHb B-COHb B-COHb B-COHb
Chlorobenzene U-4-chlorocatechol U-4-chlorocatechol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Chlorophenols U-tri+tetrapenta-
chlorophenols
Chlorophenoxy acids U-2,4-D+Dichloroprop+
MCPA+Mecoprop
Chromium (Cr) U-Cr U-Cr U-Cr B-Cr, U-Cr
Cobalt (Co) U-Co U-Co U-Co
Dichloromethane B-COHb, B-dichloromethane B-COHb B-COHb, B-dichloromethane
Dieldrin P-dieldrin B-dieldrin
Dimethylformamide U-methylformamide U-methylformamide U-methylformamide
Ethylbenzene U-mandelic acid U-mandelic acid
2-Ethoxyethanol U-ethoxyacetic acid U-ethoxyacetic acid
2-Ethoxyethyl acetate U-ethoxyacetic acid U-ethoxyacetic acid
Ethylene oxide Breath-b, B-ethylene oxide
Fluoride (F) U-F U-F U-F U-F
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Furfural U-furoic acid U-furoic acid
Halothane U-trifluoroacetic acid
Hexachorobenzene P/S-hexachlorobenzene
n-Hexane U-hexanedione, U-hexanedione + U-hexanedione
Breath-n-hexanec dihydroxyhexanone
Hydrazine P-, U-hydrazine
Lead (Pb) B-Pb, U-Pb, B-ZPP B-Pb, U-ALA B-Pb, B-ZPP B-Pb, U-ALA, B-ZPP
Lindane B(P,S)-lindane B-lindane B-lindane
Manganese (Mn) U-Mn B-, U-Mn
Mercury (Hg) B-, U-Hg B-, U-Hg B-, U-Hg
Methanol U-methanol, U-formate U-methanol U-formate
Methylbromide B-Br
Methyl butyl ketone U-hexanedione +
dihyroxyhexanone
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Methylene U-MOCA
bis(2-chloroaniline)
(MOCA)
Methylene dianiline U-MDA
(MDA)
Methyl ethyl ketone U-MEK U-MEK
(MEK)
2-Methoxyethanol U-methoxyacetic acid
2-Methoxyethyl acetate U-methoxyacetic acid
Nickel (Ni) U-Ni U-Ni U-Ni
Nitrobenzene U-p-nitrophenol, B-MetHb
Parathion U-p-nitrophenol, U-p-nitrophenol,
E-cholinesterase E-cholinesterase
Pentachlorophenol U-PCP, P-PCP U-PCP, P-PCP U-PCP
(PCP)
Phenol U-phenol U-phenol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Polychlorinated S-PCB B-PCB
biphenyls (PCB)
2-Propanol U-acetone, B-acetone
Selenium (Se) U-Se
Styrene U-mandelic acid, U-PGA U-mandelic acid, U-mandelic acid+PGA U-mandelic acid
U-mandelic acid+U-PGA
Thallium (Tl) U-Tl
Tetrachloroethylene Breath-, Breath-b, B-tetrachloroethylene
B-tetrachloroethylene B-tetrachloroethylene
Tetrachloromethane Breath-b,
B-tetrachloromethane
Tin (Sn) U-Sn
Toluene U-hippuric acid B-toluene B-toluene B-toluene
1,1,1-Trichloroethane Breath-1,1,1-trichloroethane, Breath-b, B-1,1,1-trichloroethane B-1,1,1-trichloroethane
B-trichloroethanol B-1,1,1-trichloroethane
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Trichloroethylene U-TCA, B-trichloroethanol, U-TCA, B-trichloroethanol U-TCA, U-Trichloroethanol U-TCA
U-TCA+trichloroethanol
Vanadium (V) U-V U-V
Vinyl chloride U-thiodiglycolic acid
Xylenes U-methylhippuric acids U-methylhippuric acids, B- U-methylhippuric acids U-methylhippuric acids
a The following abbreviations have been used: E = erythrocyte; P = plasma; S = serum; U = urine; B = blood; ZPP = erythrocyte zinc
protoporphyrin; ALA = delta-aminolevulinic acid; PGA = phenylglyoxylic acid; MMA = monomethylarsinic acid; DMA = dimethylarsinic acid;
TCA = trichloroacetic acid; MCPA = chloromethylphenoxyacetic acid; 2,4-D = 2,4 dichlorophenoxyacetic acid;
TTCA = 4-thio-4-thiazolidine carboxylic acid.
b DFG (1992) refers to alveolar air
c ACGIH (1992) refers to end-exhaled air
Note: Readers must consult original references before applying the above examples to specific situations
6. BIOMARKERS OF EFFECT
This section focuses on those human biomarkers that can be
applied currently or will be in the near future. Biomarkers of
effect may be used directly in hazard identification and
dose-response assessment components of the risk assessment process.
In hazard identification, biomarkers may facilitate screening and/or
identification of a toxic agent and characterization of the
associated toxicity. Biomarkers that are implicated in toxic
mechanism(s) are preferred for quantitative dose-response
assessments when extrapolating from existing data to a human
situation of concern (e.g., from high to low dose or from test
species to humans).
There are wide inter-individual variations in the response to
equivalent doses of chemicals. While the outcome of a chemical
insult in an individual may be predicted more accurately from
biomarkers of effect(s), such biomarkers may not be specific for a
single causative agent. Many biomarkers of effect are used in
everyday practice to assist in clinical diagnosis, but for
preventive purposes an ideal biomarker of effect is one that
measures change that is still reversible. Nevertheless, certain
biomarkers of nonreversible effects may still be very useful in
epidemiological studies or provide the opportunity for early
clinical intervention.
A limited range of tissues is available for routine analysis of
biomarkers. The more accessible tissues are therefore used as
surrogates for the known or putative target tissues. In some
instances biomarkers of effect are not mechanistically related to
chemically induced lesions, but may represent concomitant,
independent changes. Therefore, although an effect (e.g., sister
chromatid exchange) is being analysed, the use is conceptually close
to assessment of exposure.
6.1 Haematological biomarkers
Inhibition of the enzymes in the haem synthesis pathway (e.g.,
ferrochelatase, levulinate dehydratase) has been used as a marker of
effect of exposure to lead. This effect is reflected also in the
levels of free erythrocyte protoporphyrin (FEP) and
delta-aminolevulinate in the urine. Elevated levels of urinary
delta-amino-levulinate are observed at higher lead exposures than
changes in FEP, for example, while basophilic stippling of
erythrocytes is an even less sensitive biomarker for the effects of
lead. However, the effects on haem synthesis are not specific to
lead as a causative agent; iron deficiency has a similar effect on
FEP. The relationship of these effect biomarkers to toxicity
requires further elucidation.
Routine leucocyte, erythrocyte and thrombocyte counts have been
used in the surveillance of patients treated with cytostatic drugs
and in the monitoring of benzene-exposed workers. The predictive
power in relation to benzene-induced aplastic anaemia or leukaemia
is limited (Townsend et. al., 1978; Hancock et al., 1984; Lamm et
al., 1989). Ferrokinetic measurements, such as plasma iron
disappearance half-time, erythrocyte utilization of iron, plasma
iron transport rate, or erythrocyte iron turnover rate, have been
suggested as biomarkers of myelotoxicity (Rajamaki, 1984).
6.2 Nephrotoxicity biomarkers
Several different types of measures have been tested and used
as biomarkers of renal damage. These have been classified as
functional markers (e.g., serum creatinine and ß2-microglobulin),
urinary proteins of low or high molecular weight (e.g., albumin,
transferrin, retinol-binding globulin, rheumatoid factor,
immunoglobulin G), cytotoxicity markers (tubular antigens, e.g.,
BB50, BBA, HF5), enzymes (e.g., N-acetylglucosaminidase,
ß-galactosidase) in urine, and biochemical markers (eicosanoids,
e.g., 6-keto PGF2alpha, PGE2, PGF2alpha and TXB2, fibronectin,
kallikrein activity, sialic acid and glycosaminoglycans in urine,
and red blood cell negative charges) (Cardenas et al., 1993a,b;
Roels et al., 1993).
Biomarkers for nephrotoxicity were reviewed in WHO (1991) and
are well validated in relation to exposure to cadmium (WHO, 1992a;
Roels et al., 1993) but not in relation to exposure to mercury or
lead (Cardenas et al., 1993a,b).
6.3 Liver toxicity biomarkers
Effects of chemicals on the liver have been estimated
traditionally by measuring the activities of, for example,
aminotransferase (most often aspartate or alanine aminotransferase)
in the serum, where they are found when liver cells have been
damaged and have leaked their contents. Many other enzymes have also
been analysed for this purpose; they include 5-nucleotidase, alcohol
dehydrogenase, lactate dehydrogenase, isocitrate dehydrogenase,
leucine aminopeptidase, glutathione S-transferase, ornithine
carbamoyl transferase). Since tissues other than liver also contain
these enzymes, their activities may be elevated in serum not only
after liver damage but also when non-hepatic tissues have been
damaged. To overcome this lack of specificity, analysis of specific
isoenzymes has been used. Serum activities of enzymes such as
alkaline phosphatase and gamma-glutamyl transpeptidase may be used
as biomarkers of hepatic damage mainly involving biliary excretion.
Several liver function tests can also be used as biomarkers of
effects; these include the concentrations of serum proteins
synthesized in the liver, e.g., albumin and clotting factors, or
serum concentrations of bile acids, also synthesized in the liver,
as well as tests for the hepatic excretory function such as
bromsulfphthalein half-time. These parameters lack specificity since
hepatic viral infections, alcohol and drug use affect these enzymes.
Indirect measures of chemically induced change(s) in the cytochrome
P-450 enzyme system, using provocation tests, have been proposed as
sensitive indicators. However, the relationship to liver damage and
disease is not established and the requirement for the
administration of a drug limits the use of such tests.
Hepatotoxicity is caused by a number of chemicals that are
metabolized by the cytochrome P-450-dependent mixed-function oxidase
system to reactive intermediates. For example, carbon tetrachloride
has been studied extensively; it is metabolized to a reactive
intermediate which initially depletes intracellular glutathione to a
level that is no longer protective when the metabolite reacts with
critical macromolecules leading to cell death and hepatoxicity. In
this example, biomarkers of effect could include glutathione levels,
lipid peroxidation or the number of necrotic cells.
6.4 Biomarkers of immunotoxicity
The immune system protects the organism against infectious
microorganisms and the growth of at least some neoplasms. Reactions
of the immune system are influenced by genetic factors, age,
nutrition, life-style and health status. Xenobiotics may stimulate
or suppress the immune system. After initial sensitization, even a
minimal new exposure may lead to an anaphylactic reaction. The
immune system may be more sensitive to chemical challenge than any
other body system.
Hypersensitivity reactions following inhalation exposure
include asthma, rhinitis, pneumonitis and granulomatous pulmonary
reactions (see section 6.5). Hypersensitive dermal reactions induced
by chemicals include a wide variety of acute, subchronic and chronic
changes. Patch testing has been used traditionally as a biomarker
for identification of the causative agent of an allergic skin
reaction. However, the possibility of inducing hypersensitivity by
patch testing has been well documented and should not be overlooked
(Adams & Fisher, 1990).
Elevated levels of specific antibodies, usually of the IgE
type, may indicate existing sensitization. However, not all
individuals with elevated levels are symptomatic and not all
symptomatic individuals exhibit elevated IgE levels (Horak, 1985;
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology, 1989; Nielsen et al., 1992).
Suppression of the immune system increases susceptibility to
infections and neoplasia. Changes in the relative abundance of
different lymphocyte subpopulations (suppressor and helper T-cells)
have been used as biomarkers for the immune suppression (Jennings et
al., 1988; Sullivan, 1989; Holsapple, et al., 1991). Individuals
with asbestos-induced pleural or pulmonary changes, or
asbestos-induced cancer, as well as those heavily exposed to
asbestos but without apparent disease, have been reported to exhibit
an altered immunological status (e.g., reductions in T-lymphocyte
subsets) (Bekes et al., 1987).
In view of the growing incidence of hypersensitivity reactions
to chemicals, development and application of biomarkers for
immunotoxic effects is important. However, this is made difficult by
the current limited understanding of basic immunological mechanisms
and the effects of chemicals thereon (US NRC 1992).
6.5 Biomarkers of pulmonary toxicity
The most frequently used markers of pulmonary toxicity measure
gross effects on pulmonary function (e.g., peak expiratory flow,
forced expiratory volume, transfer factors) rather than effects on
cells or biochemical processes (US NRC, 1989a). These measures tend
to be nonspecific with respect to the causative agent and may
overlook effects specific to a certain cell type. Peak expiratory
flow measurements can be performed by the exposed individuals
themselves at the workplace, at home or elsewhere, and they provide
information on the underlying causes of air-way obstruction,
allowing a closer association between exposure, atmosphere and
response.
Air-way hyperactivity can be assessed by challenge tests using
inhalation exposure. Although such tests may assist in identifying
the factors causing hypersensitive pulmonary reactions, there is a
clear risk of acute reactions, and testing should be carried out by
qualified personnel in carefully controlled environments.
Recently, analysis of bronchoalveolar lavage fluid (BALF) has
been used to detect lung injury or to follow the progress of
pulmonary disease or the efficacy of therapeutic treatment
(Reynolds, 1987; Henderson, 1988).
The use of cellular elements as markers of pulmonary disease
state has been emphasized in human BALF analysis (Reynolds, 1987).
Total cell counts and differential counts, including use of
monoclonal antibody staining to distinguish T-cell subtypes, are
used to detect alveolitis and to aid in the diagnosis of
interstitial lung disease. High percentages of lymphocytes are
indicators of granulomatous processes, such as sarcoidosis, or
hypersensitivity pneumonitis. High percentages of neutrophils with
some eosinophils indicate possible idiopathic pulmonary fibrosis.
Other extracellular components, such as cytokines and other
mediators of inflammation, have been used on an experimental basis
to answer specific research questions.
The analysis of BALF has been used to define the dose-response
characteristics of inhaled or instilled toxins in animal toxicity
studies (Henderson, 1988). The most sensitive biomarker of an
inflammatory response in the bronchoalveolar region is the number of
neutrophils in BALF. The levels of protein and of extracellular
enzymatic activity are also useful markers of pulmonary toxicity.
Increases in protein concentrations in BALF indicate increased
permeability of the alveolar/capillary barrier. Lactate
dehydrogenase (LDH) is a cytoplasmic enzyme that is found
extracellularly only in the presence of lysed or damaged cells.
Beta-glucuronidase or similar lysosomal hydrolytic enzymes are
excellent markers for the toxicity of inhaled particles. These
particles are phagocytosed by macrophages, and the enzymes are
released from activated or lysed macrophages.
The secretion of cytokines from pulmonary macrophages obtained
by bronchoalveolar lavage provides markers of developing fibrosis.
Recent studies by Piguet et al. (1990) demonstrate that the level of
secretion of tumour necrosis factor (TNF) by pulmonary macrophages
is associated with quartz-induced fibrotic processes. Lassalle et
al. (1990) found elevated secretion of TNF by macrophages obtained
from individuals with coal-workers pneumoconioses compared with
macrophages from controls. The secretion of platelet-derived growth
factor from pulmonary macrophage was elevated in patients with
idiopathic pulmonary fibrosis (IPF).
Glutathione (GSH), a tripeptide protective against oxidative
stress, is present in BALF, and a decrease in GSH in BALF is a
potential marker for oxidative stress. Decreased levels of GSH have
been observed in patient with IPF (Cantin et al., 1989) and in
animals exposed chronically to diesel exhaust, resulting in
pulmonary fibrosis (Henderson, 1988).
Nasal lavage fluid (NLF) also provides markers of response to
inhaled toxins. The work of Graham et al. (1988) demonstrates the
potential use of NLF analysis to document the influx of neutrophils
into the nasal cavity in humans in response to inhaled ozone.
Biomarkers in blood related to lung injury have not been
validated. However, the work of Cavalleri et al. (1991) indicates
that serum aminoterminal propeptide of type III procollagen (PIIINP)
may become useful as an early marker for developing fibrosis. A
dose-dependant increase in serum PIIINP was found in individuals
exposed to low or high levels of asbestos.
Finally, urinary levels of amino acids associated with the
connective tissue of the lung (hydroxyproline, hydroxylysine,
desmosine and isodesmosine) have been used as markers of lung injury
(Harel et al., 1980; Yanagisawa et al., 1986; Stone et al., 1991).
However, such assays are not specific for lung injury and merely
indicate the breakdown of connective tissue in any organ in the
body.
6.6 Biomarkers of reproductive and developmental toxicity
Markers associated with an adverse outcome in reproduction may
reflect toxic effects in the male or female or be associated with
development during the embryonic, fetal, perinatal or neonatal
period (US NRC, 1989b; Mattison, 1991).
Biomarkers for the male reproductive system may include
physiological indicators of impaired testicular function, or sperm
number or characteristics (including cytogenetics). Measures of
hormonal status (i.e. FSH, LH and testosterone) can also be readily
obtained from blood and, in the case of testosterone, from urine and
saliva. However, these levels are greatly influenced by circadian
rhythms and demonstrate large inter- and intra-individual
variability. A clearer picture of hormonal status can be obtained by
administering GnRH or LH and examining the hormone response to these
challenges. Biomarkers for the male reproductive system are rather
easily accessible and some even reasonably well validated; such
markers are less well developed for the female reproductive system.
Biomarkers indicative of developmental toxicity should also be
considered. As is the case for many biological markers of effects,
it is often difficult to identify the causative agent in the absence
of any specific exposure history. Biomarkers could include
measurements of detrimental effects produced by chemical or other
exposures during embryonic or fetal stages of development.
Irreversible lesions can be embryolethal or result in functional
anomalies in the offspring. Examples of biomarkers of developmental
toxicity include low birth weight, chromosome anomalies, delayed
growth of specific organ systems, mental retardation, and subtle
behavioural changes. The changes associated with F1 male-mediated
abnormalities have been discussed by Anderson (1990). Some of these
biomarkers of developmental effects (malformations, mental
retardation) are not biomarkers of effect as far as the individual
is concerned, but rather represent the adverse health outcome
itself. However, from the point of view of the exposed population,
they may be considered as biomarkers since they show that within a
population a harmful exposure has taken place.
Several biomarkers have been proposed for use during pregnancy,
e.g., early pregnancy loss and assays for genetic defects of the
conceptus. The latter comprise both classical cytogenetic studies,
as well as specific DNA probes (US NRC, 1989b). The use of urinary
human chorionic gonadotrophin (HCG) has been well documented as a
biomarker for early fetal loss (US NRC, 1989b). Many different
biomarkers have been used to follow the development of the pregnancy
and the well-being of the conceptus, but they have not yet been
applied to studies of effects of chemicals on pregnancy.
6.7 Biomarkers of neurotoxicity
The functions of the nervous system are complex and biomarkers
may range from effects of chemicals on neural cellular and molecular
processes to neurophysiological and neuro-behavioural measurements
of complex functional entities.
Inhibition of plasma and erythrocyte acetylcholine esterase
(AchE) provides biomarkers of exposure to organophosphorus compounds
and other cholinesterase inhibitors. While erythrocyte
cholinesterase is similar to brain cholinesterase, and is therefore
an effect biomarker, plasma nonspecific pseudocholinesterase only
reflects exposure and is not a marker of CNS effects.
Measures of the function of the peripheral nervous system
(e.g., electroneuromyography, nerve conduction velocities, vibration
sensitivity) are well defined. Assessment of peripheral nervous
system dysfunction associated with exposure to chemicals can be
carried out using electroneuromyography at the preclinical stage
(Seppalainen et al., 1979).
Some well-established neurophysiological (e.g., evoked
potentials, electroencephalography) and neurobehavioural (e.g., the
WHO Neurobehavioural Core Test Battery, Cassito et al., 1990)
measures may be used as biomarkers to evaluate CNS dysfunction
induced by neurotoxicants. These tests must be carried out under
well-controlled conditions.
Methods for assessing changes in higher cognitive function
(e.g., learning and memory) have been used extensively, e.g., in
workers exposed to solvents or heavy metals, but require further
refinement.
Available neuroimaging procedures, e.g., computed axial
tomography (CAT), magnetic resonance imaging (MRI), nuclear magnetic
resonance spectroscopy (MRS) and positron-emission tomography (PET),
are considered non-invasive, but some of them require exposure to
ionizing radiation. CAT and MRI can be carried out with current
clinical techniques to assess chemically induced changes in the
brain. The use of MRS and PET can provide a more detailed evaluation
of the biochemical status (e.g., rate of energy generation, blood
flow, L-glucose metabolism) in the central nervous system. They can
be used as biomarkers for assessing exposure to neurotoxicants
inducing brain alterations. However, they are expensive, of little
use in assessing spinal cord, nerve and muscle changes, and there is
only minimal data validating their use in neurotoxicology (US NRC,
1992).
Other promising biomarkers for neurotoxicity in animal studies
include glial fibrillary acidic protein (localized in the
astrocytes), which increases in localized areas within the brain
where injury due to toxicants occurs (O'Callaghan, 1991).
7. BIOMARKERS AND CHEMICAL CARCINOGENESIS
As new information about the multistep process of
carcinogenesis unfolds, it is instructive to consider the various
mechanisms by which chemicals induce cancer. Knowledge of mechanisms
will enable the selection of appropriate biomarkers for use in risk
assessment of carcinogens. Some chemicals are direct acting and
others require metabolic activation. Once absorbed most chemicals
undergo enzyme-mediated reactions that either detoxify them or
activate them to reactive species. The balance between activating
and detoxifying enzyme systems governs the rate of delivery of
bioactive metabolites to the macromolecular site (Harris, 1991). The
resulting macromolecular interaction could be a DNA adduct for
carcinogens that are initiating agents or receptor occupancy for
chemicals that are tumour promotors. Certain of the DNA adducts
produced by such interactions are pro-mutagenic, and replication of
the damaged DNA could lead to DNA sequence changes which may result
in altered gene expression or mutated gene products. Weisburger &
Williams (1981) have suggested that chemical carcinogens be
classified as those that interact with DNA (genotoxic) and those
that do not (epigenetic or non-genotoxic). The importance of mitotic
activity in the latter group has recently been elaborated further
(Cohen & Ellwein, 1990).
The implications for invoking the "mechanistic" approach to the
selection of appropriate biomarkers are significant. For example,
chemicals that stimulate cell proliferation via mitogenesis or
cytotoxicity (and subsequent proliferation) might require different
biomarkers than chemicals whose major mechanism of action is based
on DNA reactivity. In the latter case, measurements of DNA adducts
or chromosome alterations may serve as suitable biomarkers, whereas
in the former case alternate biomarkers (e.g., cell turnover
measurements) may be more appropriate.
7.1 Analysis of chemicals and metabolites
Urinary or blood concentrations of several chemicals shown or
suspected to be carcinogenic to humans (e.g., arsenic, cadmium,
chromium, nickel, benzene, MOCA, polychlorinated biphenyls, styrene,
tetrachloroethylene) have for long been used as biomarkers of
exposure. Among people exposed to arsenic in a copper smelter, a
dose relationship has been observed between the cumulative urinary
excretion of arsenic and the risk of lung cancer (Enterline & Marsh,
1982). For other carcinogenic chemicals, such data are not
available, and the measured concentrations may only be interpreted
in terms of exposure.
Sensitive techniques based on physicochemical or immunochemical
methods for the detection of a variety of carcinogen-modified DNA
bases have been developed (Shuker & Farmer, 1992). These include the
alkylated purines, aflatoxin-guanine adducts, cis-platinum adducts,
thymine glycol, 8-hydroxydeoxyguanosine and PAH-derived adducts.
The aflatoxin marker has been extensively used in both animal
and human studies on the relationship between exposure and liver
cancer induction. In an ongoing prospective study in Shanghai,
China, Ross et al. (1992) reported that subjects with liver cancer
were more likely than controls to have detectable concentrations of
any of the known aflatoxin metabolites in their urine. Groopman et
al. (1991) recently explored the relationship between dietary
aflatoxin and excretion in the urine of aflatoxin metabolites and an
aflatoxin-DNA adduct. This study was conducted on people living in
the Guangxi Autonomous Region, China. These investigators found a
positive correlation between aflatoxin N7-guanine and specific
metabolites excreted in urine and aflatoxin B1 intake from the
previous day.
Exposure to chemical compounds capable of interacting with
cellular macromolecules can originate from both exogenous and
endogenous sources. Nitrite, nitrate and nitrosating agents can be
synthesized endogenously in reactions mediated by bacteria and
activated macrophage. In this way endogenous formation of
N-nitroso compounds can occur at various sites in the body.
Endogenously formed N-nitroso compounds may be considered as
biomarkers of susceptibility; they have been associated in humans
with increased risk of cancer of the stomach, oesophagus and urinary
bladder, although unequivocal epidemiological data are lacking
(Bartsch & Montesano, 1984). The quantitative estimation of
endogenous nitrosation in humans can be measured using the
N-nitroso-proline test. L-proline is utilized as a probe for
nitrosatable amines and N-nitroso-proline excreted in the urine is
determined as a marker. This assay has been applied in some
population studies (Bartsch et al. 1991).
7.2 Biomarkers for genotoxic carcinogens
7.2.1 DNA adducts - general considerations
DNA adducts are being used both as molecular dosimeters
(biomarker of exposure) and to assess the genotoxic potential of
chemicals (biomarker of effect). The biological significance of such
adducts must be assessed on the basis of adduct heterogeneity and of
cell and tissue specificity for adduct formation, persistence and
repair. Some DNA adducts result in mutation whereas others do not.
Mutational specificity in the p53 gene produced by a variety of
chemical carcinogens provides evidence that DNA adduct location
influences site-specific mutations (Hollstein et al., 1991). Some
DNA sequence changes may lead to phenotypic alterations that can be
selected, whereas others may not (Compton et al., 1991).
Most tissues are comprised of multiple cell types, and cell
types vary considerably in their capacity to convert chemicals to
DNA reactive species. For example, lung is composed of multiple cell
types in which the relative concentrations of various P-450
isoenzymes and enzymes depends on the cell type. Thus, one compound
may produce high concentrations of pro-mutagenic adducts in one cell
type, but not in another, whereas the opposite might occur for a
compound which is activated by a different P-450 isozyme. DNA adduct
concentrations derived from a whole tissue homogenate may grossly
overestimate or underestimate adduct concentrations in a given cell
type.
Some DNA adducts are repaired quickly, others hardly at all,
the adduct loss correlating with cell turnover. Therefore, the
concentration and gene location of DNA adducts will change with time
after exposure to a genotoxic chemical. Furthermore, the existence
of non-random repair in the genome makes it difficult to utilize
total DNA repair capacity as an indicator of cell susceptibility to
carcinogens (Hanawalt, 1987). It is especially important in human
studies to know the duration and timing of exposure for proper
evaluation of the biological significance of a given adduct
concentration.
Many of the human studies described below have involved
measuring metabolism, DNA adduct formation and repair in whole
tissues. Techniques need to be refined in order that
cell-type-specific variations can be monitored in human tissues, as
well as experimental studies in animal models using
immunohistochemical techniques for the cell type. Specific
localization of DNA adducts has clearly demonstrated that such
variations occur. Treatment of rats with the tobacco-specific
nitrosamine, 4-( N-methyl- N-nitrosamine)-1-(3-pyridyl)-1-butanone
(NNK), results in the induction of tumours in the nasal cavity,
lung, liver and pancreas (Hoffman et al., 1984; Rivenson et al.,
1988). At low doses of NNK, the prevalence of malignant lung tumours
was higher than that observed in other tissues. Cell-type-specific
differences have been observed within the lung, the highest
concentration of O6-methylguanine having been found in the Clara
cells. These cells have the highest levels of the P-450 metabolizing
enzymes for NNK and low levels of the O6-methylguanine DNA
methyltransferase repair enzymes (Belinsky et al., 1987). Pulmonary
tumours are also induced in mice and hamsters following either
short- or long-term exposure to this carcinogen (Hecht et al.,
1983).
As animals age, DNA adducts are detected in increasing amounts,
and, although the relationship of these adducts to tumour
development is unclear, they are believed to be derived from dietary
constituents or endogenous chemicals such as hormones (Randerath &
Randerath, 1991).
7.2.2 DNA adducts in human samples
In human studies, it is difficult to obtain non-tumourous
target tissue for the quantification of DNA adducts. Lymphocytes are
a readily accessible source of human cells that are known to contain
DNA adducts. However, there is little information on the reliability
of using lymphocyte adduct concentrations for the estimation of
target cell or tissue adduct concentrations (Lucier & Thompson,
1987).
Evaluation of dose-response relationships for chemical
carcinogens in humans is more complex than in animal models.
Radio-labelled carcinogens cannot be administered and the
accessibility of tissues and cells is limited. Several approaches to
detect DNA adducts in human samples have been evaluated (Wogan &
Gorelick, 1985; Santella, 1988). The most frequently used methods
are immunoassays and 32P-postlabelling. Other analytical
techniques such as GC-MS and synchronous fluorescence spectroscopy
are being used to measure DNA adducts (Weston & Bowman, 1991). In
general, immunoassays are both specific and sensitive for alkylated
adducts and aflatoxin adducts (Wild & Montesano, 1991; Groopman et
al., 1991). However, these methods are not easily applied to
quantification of adducts for bulky aromatic hydrocarbons such as
benzo [a]pyrene-derived adducts. The main problem is the lack of
specificity of the antibodies used in the assay which cross-react
with a number of PAH-related adducts (Santella et al., 1985).
The second assay frequently used to quantify DNA adducts in
humans is the 32P postlabelling technique. For a complete
description of this assay, see Randerath & Randerath (1991), Beach &
Gupta (1992), IARC (1992). The assay is extraordinarily sensitive,
being capable of detecting 1 adduct in 1010 normal nucleotides
when appropriate modifications are made to the procedure. The assay
is particularly useful for detecting adducts of non-polar polycyclic
aromatic hydrocarbons such as 7,8-diol-9,10-oxide-benzo [a]pyrene
deoxyguanosine (BPDE). Some DNA modifications such as alkylated DNA
adducts, which cannot be easily detected by this assay due to the
limitations of the chromatographic systems, can be quantified using
a combined 32P immunochemical precipitation technique (Kang et
al., 1993). Studies using the 32P labelling or immunological
methods have been reviewed by Beach & Gupta (1992) and Wild &
Montesano (1991). The groups of chemicals studied include alkylating
agents, polycyclic aromatic hydrocarbons (PAHs), heterocyclic PAHs,
nitro PAHs, cyclopenta-fused PAHs, aromatic amines, alkylbenzenes,
quinones, mycotoxins, chemotherapeutic agents, pesticides and
aldehydes.
7.2.3 Protein adducts
Ehrenberg and his associates pioneered the use of protein
adducts as dose monitors for carcinogen exposure in humans, and this
work has been reviewed by Hsia (1991). To date the class of proteins
that have been most extensively studied are those found in
circulating blood, i.e. haemoglobin and albumin. This is mainly
because these proteins are relatively abundant and can be easily
isolated for analysis.
Haemoglobin has the unique biological property of having a life
span equivalent to that of the erythrocyte, which in humans is
approximately 120 days, and therefore adduct levels reflect
exposures over several months. In contrast, albumin adducts can only
be used for assessing recent exposure because of the faster turnover
of albumin (half-life of 20-25 days). Protein adducts can be
quantified using chemical methods, e.g., aromatic amine release by
acid or basic hydrolysis from haemoglobin followed by derivatization
and GC-MS analysis (Farmer, 1991), or immunological techniques
(e.g., aflatoxin-albumin adducts, Wild et al., 1990).
During the past few years, several human monitoring studies
have demonstrated the usefulness of protein adducts as biomarkers of
exposure. Examples of chemicals that have been detected as protein
adducts in human studies include ethylene and propylene oxide,
aniline, cigarette smoke, aromatic amines such as 4-amino-biphenyl,
and aflatoxin (Wogan, 1989; Farmer, 1991). Albumin adducts of
aflatoxin B1 have also been used in epidemiological studies of
their role in the etiology of hepatocellular carcinoma in man. A
significant correlation was observed, at the individual level,
between dietary intake and the level of albumin-bound aflatoxin in a
chronically exposed population in the Gambia (Wild et al., 1992).
7.2.4 Cytogenetic methods
Cytogenetic methods are used as biomarkers of exposure to
DNA-damaging agents.
Many studies relating to cytogenetic changes in exposed human
populations have been reviewed in a special issue of Mutation
Research (Anderson, 1988). A second comprehensive review of more
recent studies has also been published (Anderson, 1990), and a
further review is in press (Anderson, in press).
All human monitoring studies suffer from variability of
baseline frequencies (Carrano & Natarajan, 1988) due to the presence
of endogenous (gender, age, medical history, etc.) and exogenous
factors (life-style, smoking, drinking, eating habits, etc.).
Anderson et al. (1991) have investigated the effect of these changes
on baseline variability eight times over a two-year period.
In contrast to studies on radiation, for which a marker
(dicentric chromosome) has been identified, studies with chemicals
have not yet identified a specific marker chromosome. For radiation
a dicentric is a quantitative dosimeter. Therefore, after radiation
exposure results can be used on an individual basis and a highly
exposed individual removed from the radiation source. Results from
chemical exposure studies, however, can only be used on a group
basis, due to the lack of a specific marker chromosome.
7.2.5 Chromosome damage
Both chromosome and chromatid aberrations are induced in
individuals exposed to chemical mutagens. The chromosome aberrations
are thought to arise from misrepair of lesions in the G0 stage of
circulating lymphocytes as well as from precursor cells in bone
marrow and thymus (Carrano & Natarajan, 1988). Chromatid aberrations
include chromatid breaks, intrachanges and exchanges, while
chromosome aberrations include acentric fragments, dicentric
chromosomes and ring chromosomes. Balanced translocation and
inversions can also arise and are difficult to quantify without
banding analysis. Structural aberrations can be classified as
unstable and stable depending on their ability to persist in
dividing cell populations. Unstable aberrations consist of rings,
acentric fragments and other asymmetrical rearrangements, and will
lead to the death of the cell. Stable aberrations consist of
balanced translocation inversions and other symmetrical
rearrangements which can be transmitted to progeny cells at
division. Therefore stable aberrations are more biologically
significant than unstable ones and could be involved in the cancer
process. Many human carcinogens have been shown to produce
chromosome damage in populations exposed to them, although no causal
relationship has been demonstrated (Sorsa et al., 1992). Proven
human carcinogens for which cytogenetic endpoints have been measured
in humans and corresponding animal data are available are listed in
Table 2.
In a preliminary report of a prospective study among people
whose lymphocytes were assayed for chromosome aberrations and SCE,
high rates of chromosome aberrations were observed and appeared to
be linked to cancer risk, but the finding was of borderline
statistical significance (Sorsa et al., 1990).
7.2.6 Sister chromatid exchange
Sister chromatid exchange (SCE) is considered to be a more
sensitive, rapid and simple cytogenetic end-point than chromosome
aberrations for evaluating the genotoxic potential of a variety of
mutagenic and carcinogenic agents. It is also used to detect and
differentiate many chromosome fragility diseases that predispose to
neoplasia. SCE is a DNA-replication-dependent phenomenon. Cellular
Table 2. Proven human carcinogens for which cytogenetic end-points have been measured in humans
and corresponding data are available for experimental animalsa
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Human carcinogens (Group 1)
Alcoholic beverages + + _ + ?
Aluminium production _ _
Arsenic and arsenic compounds ? ? + +
Asbestos ? _ _
Azathioprine ? _ + _ +
Benzene + + + +
Betel quid with tobacco + + +
Bis(chloromethyl)ether and (+) _
chloromethyl methyl ether
(technical grade)
1,4-Butanediol dimethanesulfonate (+) + + +
(Myleran)
Chlorambucil ? + +
Cyclosporin (+) _ _
Coal-tars +
Coke production +
Combined oral contraceptives _ _
Cyclophosphamide + + + + +
Hexavalent chromium compounds + + + + +
Melphalan + + +
8-Methoxypsoralen plus _ _ +
ultraviolet A radiation
Mineral oils, untreated and +
mildly treated
Nickel compounds + _ ? _
Table 2 (contd)
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Painter, occupational exposures as _
Radon + _
Rubber industry ? ?
Tobacco products, smokeless + +
Tobacco smoke + + + +
Tris(1-aziridinyl)phosphine sulfide (+) + + +
(Thiotepa)
Vinyl chloride + ? + + +
a From: Sorsa et al. (1992); CA = chromosome aberrations; SCE = sister chromatid exchange;
MN = micronuclei + = positive result; - = negative result; (+) = equivocal result;
? = doubtful result
factors such as nucleotide pools, repair and replication enzymes,
and biorhythms can play an important role in its formation. A major
source of variation can be attributed to the concentration of
bromodeoxyuridine relative to the number of lymphocytes in the
culture (Das, 1988; Morris, 1991; Morris et al., 1992). In a
prospective cancer study (Sorsa et al., 1990), no relationship was
observed between the frequency of SCE and the risk of cancer.
7.2.7 Micronuclei
Micronuclei are formed by condensation of acentric chromosomal
fragments or by whole chromosomes that are left behind during
anaphase movements (lagging chromosomes). The presence of
micronuclei can therefore be taken as an indication of the previous
existence of chromosomal aberrations. To visualize micronuclei,
cells have to undergo mitosis. In peripheral lymphocyte cultures it
is not easy to distinguish interface nuclei that have undergone a
division from those that have not. This makes it difficult to
quantify the frequencies of micronuclei for comparative purposes. A
method using cytochalasin B can distinguish nuclei that have divided
once (French & Morley, 1985).
7.2.8 Aneuploidy
Aneuploidy is a condition in which the number of chromosomes in
cells of individuals is not an exact multiple of the typical haploid
set for the species. Trisomy results when a single extra chromosome
is added to a pair of homologous chromosomes. If one chromosome of a
pair is missing, the result is monosomy. Absence of the pair is
nullisomy. Two or more copies of a homologue result in tetrasomy or
polysomy. Cells of individuals with missing or extra chromosomes are
hypoploid or hyperploid (UK DH, 1989). The best-known numerical
abnormalities result in the syndromes of Down (trisomy of chromosome
21), Klinefelter (sex chromosome genotype is XXY), and Turner (sex
chromosome genotype is X0). Aneuploidy is almost always found in
human cancers (Dellarco et al., 1985).
7.2.9 Mutation
Current somatic gene mutation assays used as biomarkers in
human studies select for a change or loss of a normal protein
produced by specific genes. Mutations at the X-linked hypoxanthine
guanine phosphoribosyl transferase gene in cloned T-lymphocytes and
in the autosomal locus for human leucocyte antigen-A (HLA-A) have
provided information on frequency of mutation and molecular spectra
of mutants. Detection of haemoglobin variants and loss of the cell
surface glycoprotein glycophorin A measured in red blood cells have
such limitations that analysis of the DNA mutations induced cannot
be made (Compton et al., 1991). The background frequency of each of
these specific locus assays varies greatly (Lambert, 1992) and is
dependent on numerous confounding factors (e.g., age and smoking).
Information on the mutation spectra at a particular locus will be
extremely useful in elucidating the mechanisms by which mutations
occur in human cells in vivo. By comparing spontaneous and
chemically induced mutational spectra in different populations, the
etiological contributions of both exogenous and endogenous factors
to human carcinogenesis could be assessed.
An alternative approach for the measurement of induced base
changes which does not require prior selection of a mutant
population uses the restriction site mutation technique. This is
based on the detection of DNA sequences resistant to the cutting
action of specific restriction enzymes, and the amplification of
these resistant sequences using the polymerase chain reaction. It
may theoretically be applied to the study of DNA base changes in any
gene for which the sequence has been determined (Parry et al.,
1990).
More relevant biomarkers for chemically induced cancers would,
however, preferably measure changes in genes thought to be important
for cancer. Mutations that activate proto-oncogenes, which stimulate
growth or inactivate suppressor genes to liberate cells from growth
constraints, could lead to unregulated proliferation of cancer cells
(Weinberg, 1991). For the most part, mutations in oncogenes and
tumour suppressor genes have been characterized in tumour tissue. It
remains to be determined whether the detection of mutant cells
against a background of normal cells can be achieved prior to
clinical diagnosis of cancer. Activated oncogenes have already been
identified in many human cancers, and considerable progress has been
made in elucidating the potential role of chemical carcinogens in
the activation of oncogenes and the contribution of the latter to
tumorigenesis in animal models (Balmain & Brown, 1988). Brandt-Rauf
(1991) presented data from pilot studies that demonstrated the
presence of the p21 protein product of the ras oncogene in the serum
of 15 out of 18 lung cancer patients who were all current or former
smokers. The protein was not found in the serum of any of the 18
healthy non-smoking controls, but was present in 2 out of 8
clinically healthy smoking controls. However, in another study, p21
protein was not detected in 20 smokers in a normal population or in
20 male healthy non-smokers (Brinkworth et al., 1992). The
mutational spectrum for the tumour suppressor gene p53 in human
tumours has been reviewed by Hollstein et al. (1991). Mutations of
the p53 gene are the most common cancer-related genetic changes at
the gene level and are widespread over the conserved codons of the
p53 gene. Hence, mutational spectra could be compared for tumours at
different sites and arising from different etiological backgrounds.
The mutational spectrum appears to differ among cancers of the
colon, lung, oesophagus, breast, liver, brain, reticuloendothelial
tissues and haemopoietic tissues. In two populations where aflatoxin
B1 exposure was implicated as one of the etiological factors in
hepatocellular carcinomas, the same mutational hotspot (i.e. G-T
transversion at codon 249) in the p53 gene has been identified (Hsu
et al., 1991).
7.3 Biomarkers for non-genotoxic carcinogenesis
Although only a few non-genotoxic human carcinogens have been
recognized (e.g., cyclosporin, diethylstilbestrol and estrogenic
hormones), many non-genotoxic carcinogens have been identified in
rodents. A compilation of NTP rodent data, designed to test the
concordance between short-term tests and in vivo carcinogenicity
assays, showed that more than 30% of rodent carcinogens do not test
positively for genotoxicity (Ashby & Tennant, 1991).
The mechanisms of action for non-genotoxic carcinogens need to
be considered in predicting human risk from chemical exposures.
Although the modes of action of non-genotoxic carcinogens are poorly
understood, several have been proposed, including immunosuppression,
hormonal effects, promotion, inorganic carcinogenesis,
co-carcinogenic effects and solid-state carcinogenesis (Weisburger &
Williams, 1981). Recently some of these mechanisms were grouped
under the headings of cytotoxicity and mitogenic growth stimulation
(Butterworth et al., 1992). Non-genotoxic carcinogens are believed
to exert their carcinogenic effects through mechanisms that do not
involve direct binding of the chemical or its metabolites to DNA (UK
DH, 1989). The key mechanism of non-genotoxic chemicals is to
increase cell proliferation, either by mitogenesis of the target
cells or by cytotoxicity, which is followed by regenerative cell
proliferation (Ramel, 1992). Cohen & Ellwein (1990) suggested that
non-genotoxic chemicals can be further categorized as to whether or
not their main mechanism of action is mediated via receptor-binding
(e.g., dioxin).
Cell replication and proliferation are potential biomarkers of
effect. Cell replication is the production of daughter cells by the
process of replicative DNA synthesis, while cell proliferation is
the enhanced replication of a selected cell population as observed
in regenerating tissues. Cells undergoing replicative DNA synthesis
(S-phase) are the most commonly used markers. The detection of cell
proliferation involves the incorporation of DNA precursors like
3H-thymidine or the base analogue 5-bromo-2'-deoxyuridine (BrdU)
into cellular DNA during S-phase. This is accomplished by
administering these precursors to animals by injection or through
implanted osmotic pumps. These S-phase cells can be identified
histoautotoradiographically or immunohistochemically (Goldsworthy et
al., 1991). The invasiveness of these techniques currently limits
their use to animal studies.
The identification of biomarkers of effect for non-genotoxic
carcinogens, whose major mechanism of action is via receptor
occupancy, may be difficult. This is primarily because carcinogens
of this type activate a variety of genes, some of which may not be
involved in the carcinogenic process. However, a good example of a
non-genotoxic carcinogen for which there is a good biomarker of
effect is 2,3,7,8-tetrachlorinated dibenzo- p-dioxin (TCDD). TCDD
interacts with a cytosolic receptor that is specific for it and its
structural analogues (Poland et al., 1976). In addition to
activating a number of growth factor and growth factor receptor
genes, TCDD induces a number of enzymes, one of which is cytochrome
P-450 1A1. Although the induction of this enzyme is probably not
directly related to the biological mechanism leading to cancer from
TCDD exposure, it is a sensitive marker for exposure (Tritscher et
al., 1992).
8. BIOMARKERS OF SUSCEPTIBILITY
This chapter focuses on the genetic predisposition of an
individual as it affects susceptibility to chemical materials. There
are a number of external factors, such as age, diet and health
status, that can also influence the susceptibility of an individual
exposed to chemicals. Some discussion will be directed towards the
effects of previous exposure on subsequent susceptibility, such as
to sensitization and enzyme induction/inhibition by previous
exposure. Table 3 lists some genetic and acquired factors affecting
susceptibility (Calabrese, 1986).
Although individuals may experience similar environmental
exposures, genetic differences in metabolism may produce markedly
different doses at the target site and thus a different level of
response. Even when target doses are similar, markedly different
responses may be noted in individuals due to varying degrees of
inherent biological responsiveness. Biomarkers of susceptibility may
reflect the acquired or genetic factors that influence the response
to exposure. These are pre-existing factors and are independent of
the exposure. They are predominantly genetic in origin, although
disease, physiological changes, medication and exposure to other
environmental agents may also alter individual susceptibility.
Biomarkers of susceptibility identify those individuals in a
population who have an acquired or genetic difference in
susceptibility to the effects of chemical exposure.
Biomarkers of susceptibility indicate which factors may
increase or decrease an individual's risk of developing a toxic
response following exposure to an environmental agent. Polymorphism
is present for some metabolic activation/deactivation enzymes,
including cytochrome P-450 isozymes (Nebert, 1988a, 1988b) and at
least one form of glutathione transferase (Seidegard et al., 1990).
Differing rates of enzyme activity controlling the activation or
detoxification of xenobiotics lead to differences in susceptibility
by increasing or decreasing the biologically effective dose of the
environmental agent.
The effect may vary between ethnic groups. For instance, there
are approximately equal numbers of fast and slow acetylator
phenotypes in a Caucasian population, whereas in a Japanese
population the distribution is 10% slow acetylators and 90% fast.
Genetic polymorphisms for drug metabolism have been widely studied
using phenotypic assays which involves measuring drug clearance in
individuals. Differential rates of metabolism will affect the
distribution and persistence of metabolites, which may have
implications for the site of toxicity. Epidemiological studies
suggest that, with respect to aromatic amines, slow acetylators are
more likely to contract bladder cancer but are at decreased risk
for colo-rectal cancer (Guengerich, 1991; Kadlubar et al., 1992).
Table 3. Some examples of established and suspected biomarkers of susceptibilitya
Biomarker of susceptibility Environmental agent Disease
Genetic
Debrisoquine hydroxylation phenotype cigarette smoke lung cancer
Acetylator phenotype aflatoxin, liver cancer,
aromatic amines bladder cancer
Ataxia telangiectasia genotype bleomycin, epoxides cancer at various sites
Xeroderma pigmentosum genotype agents that cause oxidative damage to skin cancer,
DNA, PAH, aromatic amines and aflatoxin B1 other cancers
Arylhydrocarbon hydroxylase inducibility polycyclic aromatic hydrocarbons lung cancer
alpha-1-antitrypsin cigarette smoke pulmonary emphysema
Franconi's anaemia phenotype cross-linking agents acute leukaemia
Glucose-6P-dehydrogenese deficiency phenotype oxidative agents, aromatic amines, poor resistance to oxidative
nitro-aromatic compounds stress,
aromatic amines
Sickle cell phenotype aromatic amino and nitro compounds, anaemia
carbon monoxide, cyanide
Thalassemia phenotype lead, benzene anaemia
Erythrocyte porphyria chloroquine, hexachlorobenzene, lead, anaemia
various drugs including barbituates,
sulfonamides, others
Sulfite oxidase deficiency heterozygotes sulfite, bisulfite, sulfur dioxide pulmonary disease
Alcohol dehydrogenase variant metabolize alcohols (e.g., ethanol)
more quickly than normal
GSTµ phenotype cigarette smoke lung cancer
Pseudocholinesterase variants organophosphate and carbamate insecticides, neurotoxicity
muscle relaxant drugs
IgA deficiency respiratory irritants irritation of respiratory tract
phenyl ketones in urine precursors of phenyl ketones phenylketonuria
Table 3 (contd)
Biomarker of susceptibility Environmental agent Disease
Acquired
Deficient diet chemical decreased resistance to
effects of many chemicals
Induced P-45O IIE1 alcohol consumption cancer at various sites
Antigen-specific antibodies chemicals, dusts decreased pulmonary
functions, skin rashes
a Modified from Calabrese (1986)
Polymorphism of N-oxidation has been linked to susceptibility to
colonic cancer (Kadlubar et al., 1992) and polymorphism in
glutathione S-transferase to increased lung cancer, particularly
adenocarcinoma (Seidegard et al., 1990).
The methodology for determining the phenotypes of individuals
for polymorphisms in metabolizing genes requires the administration
of a relevant test drug to the person and the subsequent measurement
of its clearance from the body. More recently, techniques based on
polymerase chain reactions, using DNA isolated from lymphocytes and
other cells, have been developed which allow the detection of
genetypes of known polymorphisms involving a variety of
xenobiotic-metabolizing enzymes, including GST1
(gluthione- S-transferase µ) and NAT2( N-acetyl transferase), as
well as two cytochrome P-450 isoenzymes: CYP1A1 and CYP2D6 (Bell,
1991; Blum et al., 1991; Wolf et al., 1992; Hirvonen et al., 1992;
Hollstein et al., 1992).
Cigarette smoking provides another example that illustrates the
effect of genetic polymorphisms on the response to chemicals.
Cigarette smoking is associated with the development of lung cancer
but not all smokers get lung cancer. This appears to be due in part
to genetic variations in arylhydrocarbon hydroxylase activity, which
results in a large variability in the binding of benz [a]pyrene to
DNA in cigarette smokers. A genetically based low level of
alpha-1-anti-trypsin activity greatly increases the risk of
emphysema from cigarette smoking.
In some situations a genetic trait may make an individual more
susceptible to one environmental agent but less so to another. For
example, the sickle cell trait predisposes to anaemia and altitude
sickness but offers some protection to the individual from infection
by the malaria parasite. Inherent differences in susceptibility
depend upon variations in the function of genes controlling enzyme
activity or the production of other proteins. Although a genotoxic
agent may reach the target tissue, the significance of any
chromosomal break will depend on the efficiency of the DNA repair
mechanisms. In xeroderma pigmentosum the individual is at an
increased risk of skin cancer after exposure to UV light because of
an inherited defect in DNA repair proteins (Cleaver, 1969).
Heterozygotes also have an increased risk of cancer and so the
frequency of the gene may affect the incidence of this cancer. Other
inherited diseases (e.g., ataxia-telangiectasia) that affect the
efficiency of DNA replication or repair may affect susceptibility to
carcinogenic agents (Swift et al., 1992). UV-DNA repair capacity has
been found to be lower in the lymphocytes isolated from individuals
with basal cell and squamous cell carcinoma than in the case of
their age-matched controls, and this repair capacity has been found
to decrease with increasing age in both groups (Wei et al., 1993).
Another form of susceptibility has an immunological basis.
Prior exposure to a chemical may induce an immune response that
sensitizes the individual to subsequent exposures. Such responses
occur in only a small fraction of the exposed population; an example
is the development of pulmonary hypersensitivity to industrial
agents such as toluene diisocyanate or cotton dust. The biomarkers
of susceptibility are the antigen-specific antibodies developed
against the chemical.
9. SUMMARY
The Task Group considered biomarkers in three categories,
biomarkers of exposure, of effect, and of susceptibility, while
recognising that clear distinction of category is often not
possible.
The Task Group agreed that the use of biomarkers can improve,
and should be used in, the process for the assessment of human
health risks caused by exposure to chemicals. Biomarkers may be
applied to the estimation of exposure and internal dose in
individuals and in groups and may allow identification of those at
greater or lesser risk than average.
Biomarkers must be validated before application in the risk
assessment process, i.e. the relationship between the biomarker, the
exposure, and the health outcome must be established. The selection,
validation and application of any biomarker is a complicated
process, which will vary for different markers. Examples were
selected to illustrate the concepts and principles.
Research and use of biomarkers involves complex ethical, social
and legal issues, which may vary in different countries. These
issues may impose constraints on research and use of biomarkers in
risk assessment and risk management decisions. The ethical, social
and legal aspects of biomarkers require careful consideration prior
to any application.
10. RECOMMENDATIONS
In making the following recommendations, the Task Group
recognized the role given IPCS to facilitate and increase
coordination of international activities in order to promote the
further work needed to define human health effects associated with
exposure to chemicals and to provide the basis for priority-setting
actions in order to protect public health.
10.1 General
* To promote the wider use of validated biomarkers in the
risk-assessment process
* To promote interdisciplinary cooperation and communication in
order to facilitate application of research findings
* To examine the feasibility of developing a data bank of
information on biomarkers applied to the process of risk and
their applications
10.2 Research
* To develop, refine and validate models to relate biomarkers of
exposure and of effect, qualitatively and quantitatively, to
exposure and to health outcome, particularly for end-points
other than cancer
* To identify and validate biomarkers of susceptibility in
relation to the chemical, and to inter-individual variation in
response, and investigate genetic polymorphism as a basis for
individual hypersensitivity
* To assess the use of information on individual susceptibility
in relation to protection of health with due respect to the
ethical, social and legal issues
* To develop strategies to link exposure and internal dose with
human health outcome by integration of mechanistically
validated biomarkers of exposure, effect and susceptibility
10.3 Applications
* To develop a practical protocol for use of biomarkers in human
studies, taking into account scientific, emotional, ethical,
legal and social aspects and including guidelines for risk
communication, with emphasis on the right of participants to
non-biased, intelligible information
* To encourage the production of certified reference materials
for biomarker analyses and support the functioning of
international quality assurance programmes
* To include consideration of biomarkers of exposure, effect, and
susceptibility in future Environmental Health Criteria
monographs
* To consider the need to update this monograph on principles and
concepts of biomarkers at an early date
REFERENCES
ACGIH (1992) 1992-1993 Threshold limit values for chemical
substances and physical agents and biological exposure indices.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
Adams RM & Fisher T (1990) Diagnostic patch testing.In: Adams R ed.
Occupational skin disease, 2nd ed. Philadelphia, Pennsylvania, W.B.
Saunders Company, pp 223-253.
Aitio A (1981) Laboratory quality control. Copenhagen, World Health
Organization, Regional Office for Europe, 74 pp (Health Aspects of
Chemical Safety - Interim Document 4).
Alessio L, Berlin A, & Roi R (1983) Human biological monitoring of
industrial chemicals series. Luxembourg, Office for Official
Publications of the Commission of European Communities (Industrial
Health and Safety Series).
Alessio L, Berlin A, & Roi R (1984) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 8903 EN).
Alessio L, Berlin A, & Roi R (1986) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 10704 EN).
Alessio L, Berlin A, & Roi R (1987) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11135 EN).
Alessio L, Berlin A, & Roi R (1988) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11478 EN).
Alessio L, Berlin A, & Roi R (1989) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 12174 EN).
Anderson D ed. (1988) Genetic toxicology testing and biomonitoring
of environmental or occupational exposure: human monitoring. Mutat
Res, 204(Spec Issue 3): 353-551.
Anderson D ed. (1990) Fundamental and molecular mechanisms of
mutagenesis. Mutat Res, 229(Spec Issue 2): 103-247.
Anderson D ed. (in press) Human monitoring II. Mutat Res (Spec
Issue).
Anderson D, Francis AJ, Godbert P, Jenkinson PC, & Butterworth KR
(1991) Chromosomal aberrations (CA), sister-chromatid exchanges
(SCE) and mitogen-induced blastogenesis in cultures of peripheral
lymphocytes from 48 control individuals sampled 8 times over 2
years. Mutat Res, 250: 467-476.
Ashby J & Tennant RW (1991) Definitive relationships among chemical
structure, carcinogenicity and mutagenicity for 301 chemicals tested
by the US National Toxicology Program. Mutat Res, 257: 229-306.
Balmain A & Brown K (1988) Oncogene activation in chemical
carcinogenesis. Adv Cancer Res, 51: 147-182.
Barbin A, Laib RJ, & Bartsch H (1985) Lack of miscoding properties
of 7-(2-oxoethyl)guanine, the major vinyl chloride-DNA adduct.
Cancer Res, 45: 2440-2444.
Bartsch H & Montesano R (1984) Commentary: relevance of nitrosamines
to human cancer. Carcinogenesis, 5: 1381-1393.
Bartsch H, Ohshima H, & Shuker DEG (1991) Noninvasive methods for
measuring exposure to alkylating agents: recent studies on human
subjects. In: Groopman JD & Skipper PL ed. Molecular dosimetry and
human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 281-301.
Beach AC & Gupta RC (1992) Human biomonitoring and the
32P-postlabeling assay. Carcinogenesis, 13: 1053-1074.
Bekes JG, Roboz JP, Fischbein A, & Selikoff IJ (1987) Clinical
immunology studies in individuals exposed to environmental
chemicals. In: Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F
ed. Immunotoxicology. Proceedings of the International Seminar on
the Immunological System as a Target for Toxic Damage: Present
Status, Open Problems and Future Perspectives. Dordrecht, Boston,
Lancaster, Martinus Nijhoff Publishers, pp 347-361 (IPCS Joint
Seminar 10; EUR 10041 EN).
Belinsky SA, White CM, Devereux TR, Swenberg JA, & Anderson MW
(1987) Cell selective alkylation of DNA in rat lung following low
dose exposure to the tobacco specific carcinogen
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res, 47:
1143-1148.
Bell DA (1991) Detection of DNA sequence polymorphisms in carcinogen
metabolism genes by polymerase chain reaction. Environ Mol Mutagen,
18: 245-248.
Blum M, Demierre A, Grant DM, Helm UA, & Meuer UA (1991) Molecular
mechanisms of slow acetylation of drugs and carcinogens in humans.
Proc Natl Acad Sci (USA), 88: 5237-5241.
Bond JA, Wallace LA, Osterman-Golkar S, Lucier GW, Buckpitt A, &
Henderson RF (1992) Assessment of exposure to pulmonary toxicants:
use of biological markers. Fundam Appl Toxicol, 18: 161-174.
Brandt-Rauf PW (1991) Advances in cancer biomarkers as applied to
chemical exposures: the ras oncogene and p21 protein and pulmonary
carcinogenesis. J Occup Med, 33: 951-955.
Brinkworth MH, Yardley-Jones A, Edwards AJ, Hughes JA, & Anderson D
(1992) A comparison of smokers with respect to oncogene products and
cytogenetic parameters. J Occup Med, 34: 1181-1189.
Butterworth BE, Popp JA, Conolly RB, & Goldsworthy TL (1992)
Chemically induced cell proliferation in carcinogenesis. In: Vainio
H, Magee PN, McGregor DB, & McMichael AJ ed. Mechanisms of
carcinogenesis in risk identification. Lyon, International Agency
for Research on Cancer, pp 279-305 (IARC Scientific Publications No.
116).
Calabrese EJ (1986) Ecogenetics: historical foundation and current
status (genetic factors). J Occup Med, 28: 1096-1102.
Cantin AM, Hubbard RC, & Crystal RG (1989) Glutathione deficiency in
the epithelial lining fluid of the lower respiratory tract in
idiopathic pulmonary fibrosis. Am Rev Respir Dis, 139: 370-372.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Hotter G, Mutti A, Franchini I, Fels LM, Stolte H, de
Broe ME, Nuyts GD, Taylor SA, & Price RG (1993a) Markers of early
renal changes induced by industrial pollutants. 1 Application to
workers exposed to mercury vapour. Br J Ind Med, 50: 17-27.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Ramis I, Mutti A, Franchini I, Fels LM, Stolte H, de Broe
ME, Nuyts GD, Taylor SA, & Price RG (1993b) Markers of early renal
changes induced by industrial pollutants. 2 Application to workers
exposed to lead. Br J Ind Med, 50: 28-36.
Carrano AV & Natarajan AT (1988) Considerations for population
monitoring using cytogenetic techniques. Mutat Res, 204: 379-406.
Casanova M, Deyo DF, & Heck HD'A (1989) Covalent binding of inhaled
formaldehyde to DNA in the nasal mucosa of Fischer 344 rats:
analysis of formaldehyde and DNA by high-performance liquid
chromatography and provisional pharmacokinetic interpretation.
Fundam Appl Toxicol, 12: 397-417.
Casanova M, Morgan KT, Steinhagen WH, Everitt JI, Popp JA, & Heck
HD'A (1991) Covalent binding of inhaled formaldehyde to DNA in the
respiratory tract of rhesus monkeys: pharmacokinetics, rat-to-monkey
interspecies scaling, and extrapolation to man. Fundam Appl Toxicol,
17: 409-428.
Cassito MG, Camerino D, Hanninen H, & Anger KW (1990) International
collaboration to evaluate the WHO neurobehavioural core test
battery. In: Johnson BL, Anger WK, Durao A, & Xinteras C ed.
Advances in neurobehavioural toxicology: applications in
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., pp 203-223.
Costa LG (1992) Effect of neurotoxicants on brain neurochemistry.
In: Tilson H & Mitchell C ed. Neurotoxicology. New York, Raven Press
Ltd, pp 101-123.
Cavalleri A, Gobba F, Bacchella L, & Ferrari D (1991) Evaluation of
serum aminoterminal propeptide of type III procollagen as an early
marker of the active fibrotic process in asbestos-exposed workers.
Scand J Work Environ Health, 17: 139-144.
Cleaver JE (1969) Xeroderma pigmentosum: a human disease in which an
initial stage of DNA repair is defective. Proc Natl Acad Sci (USA),
63: 428-435.
Cohen SM & Ellwein LB (1990) Cell proliferation in carcinogenesis.
Science, 249: 1007-1011.
Compton PJE, Hooper K, & Smith MT (1991) Human somatic mutation
assays as biomarkers of carcinogenesis. Environ Health Perspect, 94:
135-141.
Conolly RB & Andersen ME (in press) An approach to mechanism-based
cancer risk assessment: formaldehyde. Environ Health Perspect, 101.
Conolly RB, Monticello TM, Morgan KT, Clewell HJ, & Andersen ME
(1992) A biologically-based risk assessment strategy for inhaled
formaldehyde. Comments Toxicol, 4: 269-293.
Das BC (1988) Factors that influence formation of sister chromatid
exchanges in human blood lymphocytes. Crit Rev Toxicol, 19: 43-86.
Dellarco V, Voytek P, & Hollaender A (1985) Aneuploidy: Etiology and
mechanisms. New York, London, Plenum Press.
Derosa CT, Choudhury H, & Peirano WB (1991) An integrated
exposure/pharmacokinetic based approach to the assessment of complex
exposures. Toxicol Ind Health, 7(4): 231-248.
DFG (German Association for the Encouragement of Research) (1992)
Commission for the Investigation of Health Hazards of Chemical
Compounds in the Work Area: MAK- and BAT-values 1992. Weinheim, VCH.
Droz PO, Wu MM, & Cumberland WG (1989) Variability in biological
monitoring of organic solvent exposure. 2 Application of a
population physiological model. Br J Ind Med, 46: 547-558.
Enterline PE & Marsh GM (1982) Cancer among workers exposed to
arsenic and other substances in a copper smelter. Am J Epidemiol,
116: 895-911.
Farmer PB (1991) Analytical approaches for the determination of
protein-carcinogen adducts using mass spectrometry. In: Groopman JD
& Skipper PL ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 189-210.
FIOH (Työterveyslaitos) (1993) [Biological monitoring. Guide for
sample collection 1993.] Helsinki, Finnish Institute of Health, 115
pp (in Finnish).
French M & Morley AA (1985) Measurement of micronuclei in
lymphocytes. Mutat Res, 147: 29-36.
Gibaldi M & Perrier D (1982) Pharmacokinetics. New York, Basel,
Marcel Dekker Inc.
Goldsworthy TL, Morgan KT, Popp JA, & Butterworth BE (1991)
Guidelines for measuring chemically-induced cell proliferation in
specific rat organs. In: Butterworth BE & Sloga TJ ed. Chemically
induced cell proliferation: Implications for risk assessment. New
York, Wiley-Liss, pp 253-284.
Graham D, Henderson FW, & House D (1988) Neutrophil influx measured
in nasal lavages of humans exposed to ozone. Arch Environ Health,
43: 228-233.
Groopman JD, Sabbioni G, & Wild CP (1991) Molecular dosimetry of
human aflatoxin exposures. In: Groopman JD & Skipper PL ed.
Molecular dosimetry and human cancer -Analytical, epidemiological
and social considerations. Boca Raton, Florida, CRC Press, pp
303-324.
Guengerich FP (1991) Interindividual variation in biotransformation
of carcinogens: basis and relevance. In: Groopman JD & Skipper PL
ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 27-52.
Hall JA, Saffhill R, Green T, & Hathway DE (1981) The induction of
errors during in vitro DNA synthesis following
chloroacetaldehyde-treatment of poly(dA-dT) and poly(dC-dG)
templates. Carcinogenesis, 2: 141-146.
Hanawalt PC (1987) Preferential DNA repair in expressed genes.
Environ Health Perspect, 76: 9-14.
Hancock DG, Moffitt AE, & Hay EB (1984) Hematological findings among
workers exposed to benzene at coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Harel S, Janoff A, Yu SY, Hurewitz A, & Bergofsky EH (1980)
Desmosine radioimmunoassay for measuring elastin degradation in
vivo. Am Rev Respir Dis, 122: 769-773.
Harris CC (1991) Molecular epidemiology: overview of molecular and
biochemical basis. In: Groopman JD & Skipper PL ed. Molecular
dosimetry and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 15-26.
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumours in Syrian golden hamsters by a single dose
of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and the
effect of smoke inhalation. Carcinogenesis, 4(10): 1287-1290.
Henderson RF (1988) Use of bronchoalveolar lavage to detect lung
damage. In: Target organ toxicology: Lung. New York, Raven Press, pp
239-268.
Henderson RF, Bechtold WE, Bond JA, & Sun JD (1989) The use of
biological markers in toxicology. Crit Rev Toxicol, 20: 65-82.
Hirvonen A, Husgafvel-Pursiainen K, Karjalainen A, Anttila S, &
Vainio H (1992) Point-mutational Msp1 and lle-Val polymorphisms
closely linked in the CYP1A1 Gene: Lack of association with
susceptibility to lung cancer in a Finnish study population. Cancer
Epidemiol Biomarkers Prev, 1: 485-489.
Hoffman D, Rivenson A, Amin S, & Hecht SS (1984) Dose-response study
of the carcinogenicity of tobacco-specific N-nitrosamines in F344
Rats. J Cancer Res Clin Oncol, 108: 81-86.
Hollstein M, Sidransky D, Vogelstein B, & Harris CC (1991) p53
Mutations in human cancers. Science, 253: 49-53.
Hollstein MC, Wild CP, Bleicher F, Chutimataewin S, Harris CC,
Srivatanakul P, & Montesano R (1992) p53 Mutations and aflatoxin
B1h exposure in hepatocellular carcinoma patients from Thailand.
Int J Cancer, 53: 1-5.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-255.
Horak F (1985) Manifestation of allergic rhinitis in
latent-sensitized patients. A prospective study. Arch
Otorhinolaryngol, 242: 224-239.
Hsia MTS (1991) Carcinogen-macromolecular adducts as biomarkers in
human cancer risk assessment. Biomed Environ Sci, 4: 104-112.
Hsu IC, Metcalf RA, Sun T, Welsh J, Wang NJ, & Harris CC (1991)
Mutational hotspot in the p53 gene in human hepatocellular
carcinomas. Nature (Lond), 350: 427-428.
IARC (1992) In: Vainio H, Magee PN, McGregor D, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer (IARC Scientific
Publications No. 116).
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunologic
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin. Br J Ind Med, 45: 701-704.
Kadlubar FF, Butler MA, Kaderlik KR, Chou H-S, & Lang NP (1992)
Polymorphisms for aromatic amine metabolism in humans: relevance for
human carcinogenesis. Environ Health Perspect, 98: 69-74.
Kang H-I, Konishi C, Kuroko T, & Huh N-K (1993) A highly sensitive
and specific method for quantitation of O-alkylated DNA adducts and
its application to the analysis of human tissue. Environ Health
Perspect, 99: 269-271.
Lambert B (1992) Biological markers in exposed humans: gene
mutation. In: Vainio H, Magee PN, McGregor DB, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer, pp 535-542 (IARC
Scientific Publications No. 116).
Lamm SH, Walters AS, Wilson R, Byrd DM, & Grunwalt H (1989)
Consistencies and inconsistencies underlying the quantitative
assessment of leukemia risk from benzene exposure. Environ Health
Perspect, 82: 289-297.
Lassalle P, Cosset P, & Aerts C (1990) Abnormal secretion of
interleukin-1 and tumor necrosis factor alpha by alveolar
macrophages in coal worker's pneumoconiosis: comparison between
simple pneumoconiosis and progressive massive fibrosis. Exp Lung
Res, 16: 73-80.
Lucier G & Thompson CL (1987) Issues in biochemical applications to
risk assessment: when can lymphocytes be used as surrogate markers?
Environ Health Perspect, 76: 187-191.
Mattison DR (1991) An overview on biological markers in reproductive
and development toxicology: concepts and definitions, and use in
risk assessment. Biomed Environ Sci, 4: 8-34.
Morris SM (1991) The genetic toxicology of 5-bromodeoxyuridine in
mammalian cells. Mutat Res, 258: 161-188.
Morris SM, Domon OE, McGarrity LJ, Kodell RL, & Casciano DA (1992)
Effect of bromodeoxyuridine on the proliferation and growth of ethyl
methanesulfonate-exposed P3 cells: relationship to the induction of
sister chromatid exchanges. Cell Biol Toxicol, 8: 75-87.
Nebert DW (1988a) The 1986 Bernard B. Brodie award lecture. The
genetic regulation of drug-metabolizing enzymes. Drug Metab Dispos,
16: 1-8.
Nebert DW (1988b) Genes encoding drug metabolizing enzymes: possible
role in human disease. Basic Life Sci, 43: 45-64.
Nielsen J, Welinder H, Horstmann V, & Skerfving S (1992) Allergy to
methyltetrahydrophthalic anhydride in epoxy resin workers. Br J Ind
Med, 49: 769-775.
O'Callaghan JP (1991) Quantification of glial fibrillary acidic
protein: Comparison of slot-immunobinding assays with a novel
sandwich ELISA. Neurotoxicol Teratol, 13: 275-281.
Parry JM, Shamsher M, & Skibinski DOF (1990) Restriction site
mutation analysis, a proposed methodology for the detection and
study of DNA base changes following mutation exposure. Mutagenesis,
5: 209-212.
Passingham BJ, Farmer FB, Bailey E, Brookes AGF, & Yates DW (1988)
2-Hydroxyethylation of haemoglobin in man. In: Methods for detecting
DNA and damaging agents in humans: applications in cancer
epidemiology and prevention. Lyon, International Agency for Research
on Cancer, pp 279-285 (IARC Scientific Publications No. 89).
Piguet PF, Collart MA, Grau GE, Sappino AP, & Vassalli P (1990)
Requirement of tumor necrosis factor for development of
silica-induced pulmonary fibrosis. Nature (Lond), 344: 245-247.
Poland A, Glover E, & Kende AS (1976) Stereospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol.
Evidence that the binding species is receptor for induction of aryl
hydrocarbon hydroxylase. J Biol Chem, 251: 4936-4946.
Rajamaki A (1984) Assessment of early myelotoxicity. In: Aitio A,
Riihimaki V, & Vainio H ed. Biological monitoring and surveillance
of workers exposed to chemicals. Washington, New York, London,
Hemisphere Publishing Corporation, pp 303-308.
Ramel C (1992) Genotoxic and nongenotoxic carcinogens: mechanisms of
action and testing strategies. In: Vainio H, Magee PN, McGregor DB,
& McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 195-209 (IARC Scientific Publications No. 116).
Ramsay JC & Andersen ME (1984) A physiologically based description
of the inhalation pharmacokinetics of styrene monomer in rats and
humans. Toxicol Appl Pharmacol, 73: 159-175.
Randerath K & Randerath E (1991) 32P-postlabeling analysis of
mutagen- and carcinogen-adducts and age-related DNA modifications
(I-compounds). In: Groopman JD & Skipper PL ed. Molecular dosimetry
and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 131-150.
Reynolds HY (1987) Bronchoalveolar lavage. Am Rev Respir Dis, 135:
250-263.
Rivenson A, Hoffman D, Prokopszyk B, Amin S, & Hecht SS (1988)
Induction of lung and pancreas tumours in F344 rats by
tobacco-specific and areca derived N-nitrosamines. Cancer Res, 48:
6912-6917.
Roels H, Bernard AM, Cardenas AR, Buchet JP, Lauwerys RR, Hotter G,
Ramis I, Mutti A, Frandhini I, Bundschuh I, Stolte H, de Broe ME,
Nuyts GD, Taylor SA, & Price RG (1993) Markers of early renal
changes induced by industrial pollutants. 3 Application to workers
exposed to cadmium. Br J Ind Med, 50: 37-48.
Ross RK, Yuan J-M, Wogan GN, Qian G-S, Tu J-T, Groopman JD, Gao Y-T,
& Henderson BE (1992) Urinary aflatoxin biomarkers and risk of
hepatocellular carcinoma. Lancet, 339: 943-946.
Santella RM (1988) Application of new techniques for the detection
of carcinogen adducts to human population monitoring. Mutat Res,
205: 271-282.
Santella RM, Gaspara F, & Hsieh L (1985) Quantitation of
carcinogen-DNA adducts with monoclonal antibodies. Prog Exp Tumour
Res, 31: 63-75.
Seidegard J, Pero RW, Markowitz MM, Rousch G, Miller DG, & Beattle
EJ (1990) Isoenzyme(s) of glutathione transferase (class mu) as a
marker for the susceptibility to lung cancer: A follow-up study.
Carcinogenesis, 11: 33-36.
Seppalainen A-M, Hernberg S, & Kock B (1979) Relationship between
blood lead levels and nerve conduction velocities. Neurotoxicology,
1: 313-332.
Shuker DEG & Farmer PB (1992) Relevance of urinary DNA adducts as
markers of carcinogen exposure. Chem Res Toxicol, 5: 450-460.
Singer B, Chavez SJ, & Kusmierek JT (1987) The vinyl
chloride-derived nucleoside N2,3-ethenoguanosine, is a highly
efficient mutagen in transcription. Carcinogenesis, 8: 745-747.
Sorsa M, Ojajarvi A, & Salomaa S (1990) Cytogenetic surveillance of
workers exposed to genotoxic chemicals: preliminary experiences from
a prospective cancer study in a cytogenetic cohort. Teratog Carcinog
Mutagen, 10: 215-221.
Sorsa M, Wilbourn J, & Vanio H (1992) Human cytogenetic damage as a
predictor of cancer risk. In: Vanio H, Magee PN, McGregor D, &
McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 543-554 (IARC Scientific Publications No. 116).
Stone PJ, Bryan-Rhadfi J, & Lucey EC (1991) Measurement of urinary
desmosine by isotope dilution and high performance liquid
chromatography. Am Rev Respir Dis, 144: 284-290.
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology (1989) In: Dreborg S ed. Skin tests for
diagnosis of IgE-mediated allergy. Position paper. Allergy, 44(Suppl
10): 31-37.
Sullivan JB (1989) Immunological alterations and chemical exposure.
Clin Toxicol, 27: 311-343.
Swenberg JA, Fedtke N, Fennell TR, & Walker VE (1990) Relationships
between carcinogen exposure, DNA adducts and carcinogenesis. In:
Clayson DB, Munro IC, Shubik P, & Swenberg JA ed. Progress in
predictive toxicology. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 161-184.
Swift M, Morrel D, Massey RB, & Chase CL (1992) Incidence of cancer
in 161 families affected by ataxia-telangiectasia. New Engl J Med,
325: 1831-1836.
Townsend JC, Ott MG, & Fishbeck WA (1978) Health exam findings among
individuals occupationally exposed to benzene. J Occup Med, 20:
543-548.
Tritscher AM, Goldstein JA, Portier CJ, McCoy ZM, Clark GC, & Lucier
GW (1992) Dose-response relationships for chronic exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin in a rat tumour promotion model:
quantification and immunolocalization of CYP1A1 and CYP1A2 in the
liver. Cancer Res, 52: 3436-3442.
UK DH (1989) Committee on Mutagenicity of Chemicals in Food.
Guidelines for the testing of chemicals for mutagenicity - Consumer
Products and the Environment. London, Department of Health, Her
Majesty's Stationery Office (HMSO), 99 pp (Report No. 35).
UK HSE (1991) Guidance on laboratory techniques in occupational
medicine, 5th ed. London, Health and Safety Executive, Library and
Information Services, 117 pp.
US EPA (1991) Formaldehyde risk assessment update. Washington, DC,
US Environmental Protection Office, Office of Toxic Substances.
US EPA (1987) Assessment of health risks to garment workers and
certain home residents from exposure to formaldehyde. Washington,
DC, US Environmental Protection Office, Office of Toxic Substances.
US NRC (US National Research Council) (1989a) Biologic markers in
pulmonary toxicology. Washington, DC, National Academy Press, 179
pp.
US NRC (US National Research Council) (1989b) Biologic markers in
reproductive toxicology. Washington, DC, National Academy Press, 395
pp.
US NRC (US National Research Council) (1992a) Biologic markers in
immunotoxicology. Washington, DC, National Academy Press, 206 pp.
US NRC (US National Research Council) (1992) Environmental
neurotoxicology. Report of Committee on Neurotoxicology and Models
for Assessing Risk. Washington, DC, National Academy Press, 154 pp.
Wei Q, Matanoski GM, Farmer ER, Hedayati MA, & Grossman L (1993) DNA
repair and aging in basal cell carcinoma: a molecular epidemiology
study. Proc Natl Acad Sci (USA), 90: 1614-1618.
Weinberg RA (1991) Tumour suppressor genes. Science, 254: 1138-1146.
Weisburger JH & Williams GM (1981) Carcinogen testing: current
problems and new approaches. Science, 214: 401-407.
Weston A & Bowman ED (1991) Fluorescence detection of
benzo(a)pyrene-DNA adducts in human lung. Carcinogenesis, 12:
1445-1449.
WHO (1991) IPCS Environmental Health Criteria 119: Principles and
methods for the assessment of nephrotoxicity associated with
exposure to chemicals. Geneva, World Health Organization, 266 pp.
WHO (1992a) IPCS Environmental Health Criteria 134: Cadmium. Geneva,
World Health Organization, 280 pp.
WHO (1992b) IPCS Environmental Health Criteria 141: Quality
management for chemical safety testing. Geneva, World Health
Organization, 112 pp.
WHO (in press) Principles for assessment of risks from exposure to
chemicals (Part A).
Wild CP & Montesano R (1991) Immunological quantitation of human
exposure to aflatoxins and N-nitrosamines. In: Vanderlaan M, Stanker
LH, Watkins BE, & Roberts DW ed. Immunoassays for trace chemical
analysis. Washington, DC, American Chemical Society, pp 215-228 (ACS
Symposium Series No. 451).
Wild CP, Jiang YZ, Sabbioni G, Chapot B, & Montesano R (1990)
Evaluation of methods for quantitation of aflatoxin-albumin adducts
and their application to human exposure assessment. Cancer Res, 50:
245-251.
Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle
H, Montesano R, & Groopman JD (1992) Dietary intake of aflatoxins
and the level of albumin-bound aflatoxin in peripheral blood in The
Gambia, West Africa. Cancer Epidemiol Biomarkers Prev, 1: 229-234.
Wogan GN (1989) Markers of exposure to carcinogens: methods for
human monitoring. J Am Coll Toxicol, 8: 871-881.
Wogan GN & Gorelick NJ (1985) Chemical and biochemical dosimetry of
exposure to genotoxic chemicals. Environ Health Perspect, 62: 5-18.
Wolf CR, Dale Smith CA, Gough AC, Moss JE, Vallis KA, Howard G,
Carey FJ, Mills K, McNee W, Carmichael J, & Spurr NK (1992)
Relationship between the debrisoquine hydroxylase polymorphism and
cancer susceptibility. Carcinogenesis, 13: 1035-1038.
Yanagisawa Y, Nishimura H, Matsuki H, Osaka F, & Kasuga H (1986)
Personal exposure and health effect relationship for NO2 with
urinary hydroxyproline to creatinine ratio as indicator. Arch
Environ Health, 41: 41-48.
RESUME
Le groupe spécial a divisé les marqueurs biologiques en trois
catégories, les marqueurs d'exposition, les marqueurs d'effet et les
marqueurs de réceptivité, tout en admettant que bien souvent il
n'était pas possible d'établir une distinction nette entre ces
diverses catégories.
Le groupe spécial a admis que le recours aux marqueurs
biologiques pouvait améliorer l'évaluation des risques pour la santé
humaine découlant d'une exposition à des produits chimiques et qu'il
fallait donc en faire usage. Les marqueurs biologiques peuvent être
utilisés pour évaluer l'exposition et la dose interne chez des
individus et des groupes et peuvent faciliter l'identification de
ceux de ces individus ou de ces groupes qui sont plus ou moins
exposés aux risques que la moyenne.
Avant d'utiliser les marqueurs biologiques pour l'évaluation du
risque il faut les valider, c'est-à-dire établir la relation qui
existe entre le marqueur, l'exposition et ses conséquences pour la
santé. Le choix, la validation et l'utilisation de tout marqueur
biologique constituent des processus complexes qui varient d'un
marqueur à l'autre. Pour mettre en lumière ces notions et ces
principes on a choisi un certain nombre d'exemples.
La recherche et l'utilisation des marqueurs biologiques
soulèvent des questions complexes sur le plan éthique, social et
juridique, qui peuvent d'ailleurs varier d'un pays à l'autre. Il
peut s'en suivre un certain nombre de contraintes imposées à la
recherche et à l'utilisation des marqueurs biologiques dans
l'évaluation des risques et dans les décisions relatives à la
gestion de ces risques. Avant toute application il importe d'étudier
avec soin les aspects éthiques, sociaux et juridiques des marqueurs
biologiques.
RECOMMANDATIONS
En formulant les recommandations ci-après, le groupe de travail
a pris en considération le rôle dévolu au PISC, à savoir de
faciliter et de développer la coordination des activités
internationales afin d'encourager les travaux à poursuivre pour
définir les effets sur la santé humaine qu'entraîne l'exposition aux
substances chimiques et de jeter les bases des actions prioritaires
à entreprendre pour protéger la santé publique.
1. Généralités
* Encourager un plus large recours aux marqueurs biologiques dans
l'évaluation des risques
* Favoriser la coopération et la communication
inter-disciplinaires afin de faciliter l'application des
résultats de la recherche
* Etudier la faisabilité d'une banque de données sur les
marqueurs biologiques dans l'évaluation des risques et ses
applications
2. Recherche
* Mettre au point, affiner et valider des modèles qui permettent
de corréler les marqueurs biologiques de l'exposition et des
effets tant qualitativement que quantitativement, à
l'exposition et aux conséquences biologiques, en particulier
aux conséquences autres que le cancer.
* Recenser et valider des marqueurs biologiques de réceptivité,
par rapport à la réaction aux produits chimiques et aux
variations interindividuelles à cette réaction et étudier le
polymorphisme génétique en tant que base de l'hypersensibilité
individuelle.
* Voir dans quelle mesure il est possible d'utiliser certains
renseignements sur la réceptivité individuelle pour la
protection de la santé, dans le respect des impératifs
éthiques, sociaux et juridiques.
* Mettre au point des stratégies afin de relier l'exposition et
la dose interne aux conséquences biologiques pour l'homme
grâceà une synthèse de biomarqueurs de l'exposition, de l'effet
et de la réceptivité, mécanistiquement validés.
3. Applications
* Mettre au point un protocole pratique pour l'utilisation des
marqueurs biologiques dans les études sur l'homme, qui prenne
en considération les aspects scientifiques, émotionnels,
éthiques, juridiques et sociaux et qui comporte des directives
en matière de communication, en insistant sur le droit des
participants à disposer d'informations intelligibles et non
biaisées.
* Encourager la production de substances de référence homologuées
pour l'analyse des marqueurs biologiques et aider les
programmes internationaux d'assurance de la qualité à
fonctionner.
* Faire figurer des considérations sur les biomarqueurs
d'exposition, d'effet et de réceptivité dans les futures
monographies de la série Critères d'hygiène de l'environnement.
* Examiner s'il est nécessaire de mettre à jour à bref délai la
présente monographie consacrée aux principes et conceptions en
matière de marqueurs biologiques.
RESUMEN
El Grupo Especial distinguió tres clases de biomarcadores: de
exposición, de efecto y de susceptibilidad, reconociendo sin embargo
que a menudo resulta imposible establecer claramente la pertenencia
a una de esas clases.
El Grupo Especial coincidió en que los biomarcadores permiten
mejorar, y deben emplearse a ese efecto, el proceso de evaluación de
los riesgos que para la salud humana conlleva la exposición a
productos químicos. Los biomarcadores se pueden emplear para
calcular la exposición y la dosis interna recibida por individuos y
grupos, con la consiguiente identificación de quienes sufren un
mayor o menor riesgo que la media.
Los biomarcadores deben ser validados antes de aplicarlos a la
evaluación del riesgo, lo que significa que debe determinarse la
relación entre el biomarcador, la exposición y el estado de salud.
La selección, validación y empleo de cualquier biomarcador es un
proceso complicado, distinto para cada marcador. Se eligieron
algunos ejemplos para ilustrar los conceptos y principios
relacionados.
La investigación y el empleo de los biomarcadores plantea
complejas cuestiones éticas, sociales y jurídicas, que pueden
diferir de un país a otro. Algunos de esos problemas limitan el
alcance de las investigaciones sobre los biomarcadores y de su
aplicación a la evaluación de riesgos y la adopción de decisiones
relacionadas con la gestión de riesgos. Los problemas éticos,
sociales y jurídicos que plantean los biomarcadores deben ser objeto
de un detenido análisis antes de su eventual uso.
RECOMENDACIONES
Al formular las siguientes recomendaciones, el Grupo Especial
reconoció la función asignada al IPCS de facilitar e intensificar la
coordinación de las actividades internacionales con miras a fomentar
los trabajos que aún será necesario realizar para determinar los
efectos sobre la salud relacionados con la exposición a productos
químicos, así como para establecer las bases que permitan señalar
las prioridades a que haya que atenerse para proteger la salud
pública.
1. Recomendaciones generales
* Promover un mayor uso de los biomarcadores validados en el
proceso de evaluación de riesgos.
* Fomentar la cooperación y la comunicación interdisciplinarias
para facilitar la aplicación de los resultados de las
investigaciones.
* Estudiar la posibilidad de crear un banco de datos sobre
biomarcadores aplicados a la evaluación de riesgos y sus
posibles usos.
2. Investigaciones
* Desarrollar, perfeccionar y validar modelos aptos para
relacionar los biomarcadores de exposición y de efecto,
cualitativa y cuantitativamente, con la exposición y con el
estado de salud, sobre todo para puntos finales distintos del
cáncer.
* Identificar y validar biomarcadores de susceptibilidad en
relación con el producto químico y con la variación
interindividual de la respuesta, e investigar la influencia del
polimorfismo genético en la hipersensibilidad individual.
* Evaluar el uso de la información referente a la susceptibilidad
individual desde la perspectiva de una protección de la salud
atenta a los aspectos éticos, sociales y jurídicos.
* Formular estrategias para relacionar la exposición y la dosis
interna con el estado de salud mediante la integración de
biomarcadores de exposición, efecto y susceptibilidad validados
sistemáticamente.
3. Aplicaciones
* Elaborar un protocolo práctico para el uso de biomarcadores en
los estudios realizados en el hombre, teniendo en cuenta los
aspectos científicos, psicológicos, éticos, jurídicos y
sociales, con inclusión de directrices para la comunicación del
riesgo y haciendo hincapié en el derecho de los participantes a
una información inteligible e imparcial.
* Fomentar la producción de material de referencia certificado
para el análisis de biomarcadores y respaldar la aplicación de
programas internacionales de garantía de la calidad.
* Incluir la consideración de los biomarcadores de exposición,
efecto y susceptibilidad en las futuras monografías de la serie
Criterios de Salud Ambiental.
* Tener presente la necesidad de actualizar con prontitud la
presente monografía sobre los principios y nociones relativos a
los biomarcadores.
 (3) selection of biomarker(s) specific to the outcome of interest
with careful consideration of the biomarker to identify what is
being quantified, to assess the sensitivity and specificity of
the marker in relation to exposure, and the significance with
respect to health outcome or pathological change over time;
(4) consideration of specimens potentially available for analysis,
with emphasis on protecting the integrity of the specimen
between collection and analysis, and a preference for
non-invasive techniques;
(5) review of the analytical procedures available for
quantification of biomarkers and their limitations with respect
to detection limit, sensitivity, precision and accuracy;
(6) establishment of an appropriate analytical protocol with
provision for quality assurance and quality control;
(7) evaluation of intra- and inter-individual variation for a
non-exposed population;
(8) analysis of the data base to establish dose-effect and
dose-response relationships and their variation, with special
emphasis on susceptible individuals;
(9) calculation or prediction of risk to human health either for
the general population or a sub-group; and
(10) review of ethical and social considerations.
These issues are discussed more fully in the following
sections.
These steps may need to be carried out in an interactive and
iterative manner before selection of the desired biomarker can be
made.
3.1 Selection - practical aspects
3.1.1 General laboratory considerations
Measurement of biomarkers may range from molecular events to
functional outcomes such as behaviour or pulmonary function; the
same consideration should be applied to all biomarkers.
Analytical considerations include defined and appropriate
precision and accuracy, and quality assurance and control, as well
as the availability of automated instrumentation or alternative
simple, but specific, methodology. Specimen collection, handling and
storage should require the minimum of special precautions to avoid
contamination and/or deterioration. The costs, in terms of skilled
human resources, equipment and reagents should be reasonable.
Sampling and measurements should preferably be:
* non-invasive;
* representative, i.e. the time of the exposure in relation to
measurement should be taken into account; and
* the stability of the analyte in the specimen should be
established.
In general terms, specimens available for analysis will include
blood, urine, sputum, saliva, finger-nails, breath, hair, faeces and
(shed) teeth. The clinic or hospital setting may provide the
opportunity to collect unique fluid samples (e.g., follicular,
amniotic, semen) or tissues accompanying examination of the patient
(e.g., cytological material, pulmonary lavage), tissue biopsies
(e.g., fat) or autopsy specimens.
Specialized techniques for in vivo determination may be
available for some chemicals, e.g., cadmium in kidney or lead in
bone, but such applications require exposure of individuals to
radiation. In such instances, ethical considerations must also be
taken into account.
3.1.2 Quality assurance and control
Critical to the successful and effective application of
biomarkers is a well-documented quality assurance and control
programme. It is beyond the scope of this monograph to discuss such
programmes in detail, but these have been reviewed (Aitio, 1981;
WHO, 1992b). It is important to note that good analytical
performance does not necessarily guarantee accurate results in
biomarker analyses since greater errors may be introduced during
sampling. Thus, the quality assurance protocol has to cover the
entire process. Major impediments to quality assurance of biomarker
analyses include the lack of certified reference materials and
external quality control programmes.
3.2 Validation and characteristics of biomarkers
Validation is a process to establish the qualitative and
quantitative relationship of the biomarker (a) to exposure to a
chemical, and (b) to the selected end-point. Desirable
characteristics of biomarkers include that:
(1) the marker (measurement)
(a) reflects the interaction (qualitative or quantitative) of
the host biological system with the chemical of interest,
(b) has known and appropriate specificity and sensitivity to
the interaction,
(c) is reproducible qualitatively and quantitatively with
respect to time (short- and long-term);
(2) the analytical measurement has defined and appropriate accuracy
and precision;
(3) the marker is common to individuals within a population or
subgroup and is of defined variability within the normal,
non-exposed population or group of interest; and
(4) the marker is common between species.
4. ETHICS AND SOCIAL CONSIDERATIONS
It is important to recognize the ethical and social
implications of the uses of biomarkers, in addition to the
scientific and cost considerations. Ethical concerns may limit the
extent of investigations of chemically exposed human individuals and
populations, particularly those involving the living.
Participation by individuals or groups will be influenced both
by personal and scientific factors. Personal attitudes, ideals and
beliefs will vary geographically with ethnic origin and cultural
practices.
The process leading to participation is critically important
and must respect the dignity, rights and freedom of choice of
individuals; participation must be voluntary and based on full
information.
The freedom of choice of individuals will include the right of
refusal to give blood or other biological samples for analysis of
biomarkers. Personal decisions should be based on full information
with the implications of a refusal being explained and understood.
Before measurement of a biomarker is undertaken, there should
be consideration of how and to whom results should be provided, the
interpretation of the results, and whether this should be on an
individual or group basis, with or without protection of
confidentiality. While practices will vary between countries, it is
particularly important that the role of medical officers be defined
in relation to their responsibility to the individual (patient) and
in relation to administrative (company) management.
It is the ethical responsibility of medical officers to inform
individuals fully of potentially hazardous exposures, recognizing
that remedial action may involve administrative decisions. The
latter may be taken in the context of prevailing economic and social
considerations rather than of individual circumstances.
Investigators need to recognize and accommodate the medical
dilemma created by the conduct of biomarker research in terminally
ill patients, since the data may clarify the use of the biomarker
without contributing to improvement of the health status of the
patient. Thus, careful consideration must be given to the desire to
advance scientific understanding relative to meeting the needs of
the patient.
In addition to the general ethical issues associated with use
of biomarkers, there are particular problems relating to biomarkers
of susceptibility.
Identifying susceptible individuals may help to prevent their
exposure to a specific harmful chemical(s) but may lead to
discrimination in the employment of the susceptible individual when
that chemical is known to be present in the workplace.
It is also an ethical question as to whether individuals should
be given information about their own susceptibility. This knowledge
would allow them to make more informed choices, for example, the
avoidance of exposure to specific substances. It is, however,
important to realize that only for a few biomarkers of
susceptibility is it well established that they are associated with
the development of disease. If the individual does not fully
understand this uncertainty, such information on biomarkers may
cause unnecessary concern and anxiety.
5. BIOMARKERS OF EXPOSURE
The exposure assessment component of the risk assessment
process is an attempt to provide qualitative and quantitative
estimates of human exposure through the use of measurements and
models. In this context, measurements may be made of chemical
concentrations in food, water and air, selected environmental
concentrations (e.g., occupational or residential settings) as well
as measures of the actual exposures experienced by the individual or
population. Exposure biomarkers extend this latter component of
exposure assessment into the realm of data which provide the most
direct evidence of human exposure to a given agent and the absorbed
dose.
Adverse or toxic effects in a biological system are not
produced by chemical agents unless that agent or its
biotransformation products reach appropriate sites in the body at a
concentration and for a length of time sufficient to produce the
toxic manifestation. Thus, to characterize fully the potential
hazard or toxicity of a specific chemical agent in an individual, it
is necessary to identify not only the type of effect and the dose
required to produce the effect but also information about the
duration and frequency of exposure to the agent, and the
susceptibility of the exposed individual.
Methods for assessing exposure to a chemical fall into two
categories:
1. measurement of levels of chemical agents and their metabolites
and/or derivatives in cells, tissue, body fluids or excreta;
and
2. measurement of biological responses such as cytogenetic and
reversible physiological changes in the exposed individuals.
Measurement of covalent adducts formed between chemical agents
and cellular macromolecules (proteins, DNA), or their excretion
products have characteristics of both category one and two above.
In evaluating exposure, distinction is made between the
external dose, defined as the amount of a chemical agent in
environmental contact with the organism, as determined by personal
or area monitoring, and the internal dose, which is the total
amount of a chemical agent absorbed by the organism over a period of
time. Biomarkers of exposure will reflect the distribution of the
chemical or its metabolite throughout the organism. Theoretically,
this distribution can be tracked through various biological levels
(e.g., tissue, cell, etc.) to the ultimate target. The concept of
biomarkers of exposure is illustrated in Fig. 2.
This figure illustrates that, of the total amount absorbed,
only a portion will be delivered to a target tissue. A portion will
reach internal macromolecules, and a smaller proportion will reach
the critical site on the macromolecule, with only a fraction of the
latter amount acting as the biologically effective dose. Biomarkers
for each of these forms of internal dose would be useful for
assessment of risk. The decreasing area of the triangle, from the
total absorbed amount to each lower level as distribution and
metabolism occur, illustrates the decreasing mass of the internal
dose that reaches the target tissue, cell, or critical site. In
progressing from biomarkers of total absorbed dose to markers of
biologically effective dose, it becomes increasingly easy to relate
the dose to the mechanism of the induced health effect. In the
reverse process, from the dose for critical sites to the total
amount absorbed, it becomes easier to relate the internal dose to
the external exposure.
The internal dose can be assessed by suitable analyses of
biomarkers in biological samples (urine, faeces, blood and/or its
components, expired air) (Alessio et al., 1983, 1984, 1986, 1987,
1988, 1989; UK HSE, 1991; ACGIH, 1992; DFG, 1992; Bond et al.,
1992). These biomarkers may be the unchanged chemical material, its
known metabolites or biochemical markers affected by absorption of
the chemical. It may be possible to estimate the dose quantitatively
when the toxicokinetics of the chemical is well established and the
sampling is conducted at appropriate points in time. The tissue dose
may be further refined to a specific target dose which may be
defined as the amount of chemical (or its metabolite) which, over a
period of time, reaches the biologically significant site(s) within
the target tissue.
While such measurements may not equate to the biologically
effective dose, the data can provide useful estimates of internal
exposure (dose). Knowledge of the kinetics of formation and removal
from the body of these types of biomarkers provides a link between
exposure and internal dose.
Specific measures of internal dose are the active chemical
species (either parent compound or metabolite) delivered to target
tissues or cells, the reactive chemical species delivered to target
organelles or macromolecules, or the reactive chemical species that
participates in biochemical reactions. For example, quantification
of the generalized covalent binding of reactive species to
macromolecules will provide a measure of absorbed dose delivered to
target tissues or cells, while measurement of total DNA adducts is
indicative of the dose delivered to target organelles or
macromolecules. Finally, specific DNA adducts could be the
biologically effective species that initiate the carcinogenic
process. These issues are discussed in chapter 7.
(3) selection of biomarker(s) specific to the outcome of interest
with careful consideration of the biomarker to identify what is
being quantified, to assess the sensitivity and specificity of
the marker in relation to exposure, and the significance with
respect to health outcome or pathological change over time;
(4) consideration of specimens potentially available for analysis,
with emphasis on protecting the integrity of the specimen
between collection and analysis, and a preference for
non-invasive techniques;
(5) review of the analytical procedures available for
quantification of biomarkers and their limitations with respect
to detection limit, sensitivity, precision and accuracy;
(6) establishment of an appropriate analytical protocol with
provision for quality assurance and quality control;
(7) evaluation of intra- and inter-individual variation for a
non-exposed population;
(8) analysis of the data base to establish dose-effect and
dose-response relationships and their variation, with special
emphasis on susceptible individuals;
(9) calculation or prediction of risk to human health either for
the general population or a sub-group; and
(10) review of ethical and social considerations.
These issues are discussed more fully in the following
sections.
These steps may need to be carried out in an interactive and
iterative manner before selection of the desired biomarker can be
made.
3.1 Selection - practical aspects
3.1.1 General laboratory considerations
Measurement of biomarkers may range from molecular events to
functional outcomes such as behaviour or pulmonary function; the
same consideration should be applied to all biomarkers.
Analytical considerations include defined and appropriate
precision and accuracy, and quality assurance and control, as well
as the availability of automated instrumentation or alternative
simple, but specific, methodology. Specimen collection, handling and
storage should require the minimum of special precautions to avoid
contamination and/or deterioration. The costs, in terms of skilled
human resources, equipment and reagents should be reasonable.
Sampling and measurements should preferably be:
* non-invasive;
* representative, i.e. the time of the exposure in relation to
measurement should be taken into account; and
* the stability of the analyte in the specimen should be
established.
In general terms, specimens available for analysis will include
blood, urine, sputum, saliva, finger-nails, breath, hair, faeces and
(shed) teeth. The clinic or hospital setting may provide the
opportunity to collect unique fluid samples (e.g., follicular,
amniotic, semen) or tissues accompanying examination of the patient
(e.g., cytological material, pulmonary lavage), tissue biopsies
(e.g., fat) or autopsy specimens.
Specialized techniques for in vivo determination may be
available for some chemicals, e.g., cadmium in kidney or lead in
bone, but such applications require exposure of individuals to
radiation. In such instances, ethical considerations must also be
taken into account.
3.1.2 Quality assurance and control
Critical to the successful and effective application of
biomarkers is a well-documented quality assurance and control
programme. It is beyond the scope of this monograph to discuss such
programmes in detail, but these have been reviewed (Aitio, 1981;
WHO, 1992b). It is important to note that good analytical
performance does not necessarily guarantee accurate results in
biomarker analyses since greater errors may be introduced during
sampling. Thus, the quality assurance protocol has to cover the
entire process. Major impediments to quality assurance of biomarker
analyses include the lack of certified reference materials and
external quality control programmes.
3.2 Validation and characteristics of biomarkers
Validation is a process to establish the qualitative and
quantitative relationship of the biomarker (a) to exposure to a
chemical, and (b) to the selected end-point. Desirable
characteristics of biomarkers include that:
(1) the marker (measurement)
(a) reflects the interaction (qualitative or quantitative) of
the host biological system with the chemical of interest,
(b) has known and appropriate specificity and sensitivity to
the interaction,
(c) is reproducible qualitatively and quantitatively with
respect to time (short- and long-term);
(2) the analytical measurement has defined and appropriate accuracy
and precision;
(3) the marker is common to individuals within a population or
subgroup and is of defined variability within the normal,
non-exposed population or group of interest; and
(4) the marker is common between species.
4. ETHICS AND SOCIAL CONSIDERATIONS
It is important to recognize the ethical and social
implications of the uses of biomarkers, in addition to the
scientific and cost considerations. Ethical concerns may limit the
extent of investigations of chemically exposed human individuals and
populations, particularly those involving the living.
Participation by individuals or groups will be influenced both
by personal and scientific factors. Personal attitudes, ideals and
beliefs will vary geographically with ethnic origin and cultural
practices.
The process leading to participation is critically important
and must respect the dignity, rights and freedom of choice of
individuals; participation must be voluntary and based on full
information.
The freedom of choice of individuals will include the right of
refusal to give blood or other biological samples for analysis of
biomarkers. Personal decisions should be based on full information
with the implications of a refusal being explained and understood.
Before measurement of a biomarker is undertaken, there should
be consideration of how and to whom results should be provided, the
interpretation of the results, and whether this should be on an
individual or group basis, with or without protection of
confidentiality. While practices will vary between countries, it is
particularly important that the role of medical officers be defined
in relation to their responsibility to the individual (patient) and
in relation to administrative (company) management.
It is the ethical responsibility of medical officers to inform
individuals fully of potentially hazardous exposures, recognizing
that remedial action may involve administrative decisions. The
latter may be taken in the context of prevailing economic and social
considerations rather than of individual circumstances.
Investigators need to recognize and accommodate the medical
dilemma created by the conduct of biomarker research in terminally
ill patients, since the data may clarify the use of the biomarker
without contributing to improvement of the health status of the
patient. Thus, careful consideration must be given to the desire to
advance scientific understanding relative to meeting the needs of
the patient.
In addition to the general ethical issues associated with use
of biomarkers, there are particular problems relating to biomarkers
of susceptibility.
Identifying susceptible individuals may help to prevent their
exposure to a specific harmful chemical(s) but may lead to
discrimination in the employment of the susceptible individual when
that chemical is known to be present in the workplace.
It is also an ethical question as to whether individuals should
be given information about their own susceptibility. This knowledge
would allow them to make more informed choices, for example, the
avoidance of exposure to specific substances. It is, however,
important to realize that only for a few biomarkers of
susceptibility is it well established that they are associated with
the development of disease. If the individual does not fully
understand this uncertainty, such information on biomarkers may
cause unnecessary concern and anxiety.
5. BIOMARKERS OF EXPOSURE
The exposure assessment component of the risk assessment
process is an attempt to provide qualitative and quantitative
estimates of human exposure through the use of measurements and
models. In this context, measurements may be made of chemical
concentrations in food, water and air, selected environmental
concentrations (e.g., occupational or residential settings) as well
as measures of the actual exposures experienced by the individual or
population. Exposure biomarkers extend this latter component of
exposure assessment into the realm of data which provide the most
direct evidence of human exposure to a given agent and the absorbed
dose.
Adverse or toxic effects in a biological system are not
produced by chemical agents unless that agent or its
biotransformation products reach appropriate sites in the body at a
concentration and for a length of time sufficient to produce the
toxic manifestation. Thus, to characterize fully the potential
hazard or toxicity of a specific chemical agent in an individual, it
is necessary to identify not only the type of effect and the dose
required to produce the effect but also information about the
duration and frequency of exposure to the agent, and the
susceptibility of the exposed individual.
Methods for assessing exposure to a chemical fall into two
categories:
1. measurement of levels of chemical agents and their metabolites
and/or derivatives in cells, tissue, body fluids or excreta;
and
2. measurement of biological responses such as cytogenetic and
reversible physiological changes in the exposed individuals.
Measurement of covalent adducts formed between chemical agents
and cellular macromolecules (proteins, DNA), or their excretion
products have characteristics of both category one and two above.
In evaluating exposure, distinction is made between the
external dose, defined as the amount of a chemical agent in
environmental contact with the organism, as determined by personal
or area monitoring, and the internal dose, which is the total
amount of a chemical agent absorbed by the organism over a period of
time. Biomarkers of exposure will reflect the distribution of the
chemical or its metabolite throughout the organism. Theoretically,
this distribution can be tracked through various biological levels
(e.g., tissue, cell, etc.) to the ultimate target. The concept of
biomarkers of exposure is illustrated in Fig. 2.
This figure illustrates that, of the total amount absorbed,
only a portion will be delivered to a target tissue. A portion will
reach internal macromolecules, and a smaller proportion will reach
the critical site on the macromolecule, with only a fraction of the
latter amount acting as the biologically effective dose. Biomarkers
for each of these forms of internal dose would be useful for
assessment of risk. The decreasing area of the triangle, from the
total absorbed amount to each lower level as distribution and
metabolism occur, illustrates the decreasing mass of the internal
dose that reaches the target tissue, cell, or critical site. In
progressing from biomarkers of total absorbed dose to markers of
biologically effective dose, it becomes increasingly easy to relate
the dose to the mechanism of the induced health effect. In the
reverse process, from the dose for critical sites to the total
amount absorbed, it becomes easier to relate the internal dose to
the external exposure.
The internal dose can be assessed by suitable analyses of
biomarkers in biological samples (urine, faeces, blood and/or its
components, expired air) (Alessio et al., 1983, 1984, 1986, 1987,
1988, 1989; UK HSE, 1991; ACGIH, 1992; DFG, 1992; Bond et al.,
1992). These biomarkers may be the unchanged chemical material, its
known metabolites or biochemical markers affected by absorption of
the chemical. It may be possible to estimate the dose quantitatively
when the toxicokinetics of the chemical is well established and the
sampling is conducted at appropriate points in time. The tissue dose
may be further refined to a specific target dose which may be
defined as the amount of chemical (or its metabolite) which, over a
period of time, reaches the biologically significant site(s) within
the target tissue.
While such measurements may not equate to the biologically
effective dose, the data can provide useful estimates of internal
exposure (dose). Knowledge of the kinetics of formation and removal
from the body of these types of biomarkers provides a link between
exposure and internal dose.
Specific measures of internal dose are the active chemical
species (either parent compound or metabolite) delivered to target
tissues or cells, the reactive chemical species delivered to target
organelles or macromolecules, or the reactive chemical species that
participates in biochemical reactions. For example, quantification
of the generalized covalent binding of reactive species to
macromolecules will provide a measure of absorbed dose delivered to
target tissues or cells, while measurement of total DNA adducts is
indicative of the dose delivered to target organelles or
macromolecules. Finally, specific DNA adducts could be the
biologically effective species that initiate the carcinogenic
process. These issues are discussed in chapter 7.
 The potential impact of target tissue DNA-protein cross-links
as a biomarker of the biologically effective dose is illustrated in
the proposed risk assessment for formaldehyde put forward by US EPA
(1991). The biological, mechanism-based model of formaldehyde
carcinogenesis consists of three submodels (Conolly et al., 1992;
Conolly & Andersen, in press). One of these is a tissue dosimetry
submodel which incorporates formaldehyde-induced cross-links of DNA
with adjacent proteins (Casanova et al., 1989, 1991). Although the
role of DNA-protein cross-links in the mechanism of formaldehyde-
induced nasal cancer is not known, their formation is used only as a
biomarker of the "biologically effective" dose reaching target cells
in the nasal cavity. An earlier proposal (US EPA, 1987) used
external formaldehyde concentration as the measure of dose. The
predicted quantitative human risk at low levels of exposure is lower
when the interspecies extrapolation (from monkeys and rats) is based
on the biomarker, i.e. DNA-protein cross-links, rather than on the
concentration of formaldehyde inhaled. Although, there are several
unresolved issues regarding the use of DNA-protein cross-links as a
biomarker, this example illustrates that mechanistic data and a
biomarker of delivered dose can be used in the risk assessment
process for chemicals.
A strategy to help relate biomarkers to prior exposures is to
obtain quantitative information about the kinetics of formation and
breakdown of the biomarker, as shown in Fig. 3. The information
required includes the quantified correlation of the biomarker with a
given exposure scenario and kinetic data for elimination of the
biomarker. Biomarkers for chemicals that are cleared rapidly, such
as vapours in exhaled breath or urinary metabolites, may be present
in large amounts soon, during or immediately following an exposure,
but are not detectable at later times.
Other biomarkers, such as adducts formed with blood proteins,
may represent only a small fraction of the total internal dose but,
because they have a long half-life in the body (relative to exposure
frequency), may accumulate to detectable levels with continued
exposure.
Use can be made of kinetic properties to define prior
exposures. If a person has had only a single, recent exposure to a
chemical, the level of biomarkers with short half-life will be high
relative to those with a longer half-life. With continuing exposure,
the levels of markers with both shorter and longer half-lives should
be high. If a person was exposed in the more distant, rather than
the more recent, past, only the biomarkers with the longer
half-lives will be detectable.
The potential impact of target tissue DNA-protein cross-links
as a biomarker of the biologically effective dose is illustrated in
the proposed risk assessment for formaldehyde put forward by US EPA
(1991). The biological, mechanism-based model of formaldehyde
carcinogenesis consists of three submodels (Conolly et al., 1992;
Conolly & Andersen, in press). One of these is a tissue dosimetry
submodel which incorporates formaldehyde-induced cross-links of DNA
with adjacent proteins (Casanova et al., 1989, 1991). Although the
role of DNA-protein cross-links in the mechanism of formaldehyde-
induced nasal cancer is not known, their formation is used only as a
biomarker of the "biologically effective" dose reaching target cells
in the nasal cavity. An earlier proposal (US EPA, 1987) used
external formaldehyde concentration as the measure of dose. The
predicted quantitative human risk at low levels of exposure is lower
when the interspecies extrapolation (from monkeys and rats) is based
on the biomarker, i.e. DNA-protein cross-links, rather than on the
concentration of formaldehyde inhaled. Although, there are several
unresolved issues regarding the use of DNA-protein cross-links as a
biomarker, this example illustrates that mechanistic data and a
biomarker of delivered dose can be used in the risk assessment
process for chemicals.
A strategy to help relate biomarkers to prior exposures is to
obtain quantitative information about the kinetics of formation and
breakdown of the biomarker, as shown in Fig. 3. The information
required includes the quantified correlation of the biomarker with a
given exposure scenario and kinetic data for elimination of the
biomarker. Biomarkers for chemicals that are cleared rapidly, such
as vapours in exhaled breath or urinary metabolites, may be present
in large amounts soon, during or immediately following an exposure,
but are not detectable at later times.
Other biomarkers, such as adducts formed with blood proteins,
may represent only a small fraction of the total internal dose but,
because they have a long half-life in the body (relative to exposure
frequency), may accumulate to detectable levels with continued
exposure.
Use can be made of kinetic properties to define prior
exposures. If a person has had only a single, recent exposure to a
chemical, the level of biomarkers with short half-life will be high
relative to those with a longer half-life. With continuing exposure,
the levels of markers with both shorter and longer half-lives should
be high. If a person was exposed in the more distant, rather than
the more recent, past, only the biomarkers with the longer
half-lives will be detectable.
 Thus by analysing several biomarkers with different half-lives
(e.g., haemoglobin adducts in the blood, metabolites of the chemical
in urine, parent compound in blood) from a single individual at a
single point in time, more information may be obtained about the
nature of the past exposure than from use of a single biomarker.
Also of value in the interpretation of biomarker data is the
use of mathematical models to describe the kinetics of formation and
elimination of biomarkers of exposure. Absorbed chemicals are
distributed between various compartments in the body, with the
distribution being dependent on the nature of the compartment and
the lipophilicity of the chemical. The most simple models use only
one compartment; however, multicompartmental models are usually
required to describe the disposition of most chemicals in the body
(Gibaldi & Perrier, 1982). Multicompartmental models that
incorporate biomarkers of exposure in relation to toxic end-points
are well established. A biokinetics model for lead has been used to
predict blood lead levels of individuals and communities (DeRosa et
al., 1991).
More recently, physiologically based toxicokinetic (PBTK)
models have been developed that make use of the physico-chemical
properties of a chemical (such as partition coefficients that
indicate how the chemical or its metabolites become partitioned
between different fluids in the body), the kinetics of metabolism of
the chemical (such as Vmax and KM for metabolic pathways), and
physiological parameters of the exposed individual (such as tissue
blood flow, respiratory minute volume, cardiac output) to predict
the actual concentrations of biomarkers that will occur after
specific exposure regimens. These models are adapted for humans by
changing the physiological and metabolic parameters to those
appropriate for humans, and by testing for validity in limited human
studies. These models can then be used to extrapolate between
different exposure situations for the predicted levels of markers
(Ramsay & Andersen, 1984; Droz et al., 1989).
Another use of models is in defining the quantitative
relationship between biomarkers in readily available biological
samples (e.g., blood cells) and in those less readily available
which might be more pertinent biomarkers for the health effect of
concern (e.g., tissue DNA). An example is the use of haemoglobin
adducts to predict the amount of DNA adducts at a critical site,
e.g., after exposure to ethylene oxide (Passingham et al., 1988). To
be able to make such predictions, one must know the kinetics of
formation and breakdown of each of the markers and the factors that
influence those kinetics. Based on such information a model can be
developed to show the quantitative relationships between the markers
under different exposure conditions or at different times after
exposure.
Biomarkers are used extensively in the surveillance of workers
occupationally exposed to metals such as lead, cadmium, mercury,
nickel, chromium and arsenic, and to organic chemicals such as
aniline, benzene, carbon disulfide, styrene, chlorobenzene and
chlorinated aliphatic hydrocarbon solvents (see Table 1). The
examples in Table 1 are given as general information. Before
applying them in specific circumstances, readers must consult the
original references.
These measures are used to indicate the absorbed dose. For a
few chemicals, notably lead, mercury, cadmium and carbon monoxide,
an approximate estimation of the associated health risk may be made.
For other chemicals, exposure may be assessed in quantitative terms.
It is noted that biomarkers are supplementary to environmental
measurements rather than alternative or substitute measures of
exposure.
Considerable efforts are being made to develop biomarkers
associated with exposure to chemical carcinogens and to establish
the relationship between a marker and the future health risk. The
use of animal models may facilitate this process; studies on the DNA
adducts formed by vinyl chloride illustrate the types of strategies
required to make each link (Swenberg et al., 1990). In rats, vinyl
chloride induces liver tumours with a high incidence in young
animals, and the DNA adducts (biomarkers) formed in the liver have
been characterized and the half-lives determined. DNA fidelity
replication assays were used to show one type of adduct that had
both a long half-life and was capable of inducing mutations (Hall et
al., 1981; Barbin et al., 1985; Singer et al., 1987; Swenberg et
al., 1990). The level of this adduct was much higher in the livers
of young rats exposed to vinyl chloride than in adults, and was
characterized as the one most closely related to the health effect.
To extend this animal research to predict human health risks, it
would be necessary to determine if the concentration of the same
adduct is associated with liver tumour formation in human tissue
samples.
Table 1. Some parameters proposed for biological monitoring by different organizations
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase,
inhibitors P-cholinesterase
Aluminium (Al) U-Al U-Al
Aniline U-p-aminophenol,B-Met-Hb U-aniline U-p-aminophenol
Arsenic (As) U-certain volatile U-AsIII+, AsV+ U-AsIII+, AsV+
arsenic compounds MMA+DMA
produced by direct
hydrogenation
Benzene U-phenol B-benzene, U-phenol B-benzene Breath-, B-benzene
p-tert-Butylphenol U-p-tert-butylphenol
Cadmium (Cd) U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd
Carbon disulfide U-TTCA U-TTCA U-TTCA U-TTCA
Carbon monoxide Breath-CO, B-COHb B-COHb B-COHb B-COHb
Chlorobenzene U-4-chlorocatechol U-4-chlorocatechol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Chlorophenols U-tri+tetrapenta-
chlorophenols
Chlorophenoxy acids U-2,4-D+Dichloroprop+
MCPA+Mecoprop
Chromium (Cr) U-Cr U-Cr U-Cr B-Cr, U-Cr
Cobalt (Co) U-Co U-Co U-Co
Dichloromethane B-COHb, B-dichloromethane B-COHb B-COHb, B-dichloromethane
Dieldrin P-dieldrin B-dieldrin
Dimethylformamide U-methylformamide U-methylformamide U-methylformamide
Ethylbenzene U-mandelic acid U-mandelic acid
2-Ethoxyethanol U-ethoxyacetic acid U-ethoxyacetic acid
2-Ethoxyethyl acetate U-ethoxyacetic acid U-ethoxyacetic acid
Ethylene oxide Breath-b, B-ethylene oxide
Fluoride (F) U-F U-F U-F U-F
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Furfural U-furoic acid U-furoic acid
Halothane U-trifluoroacetic acid
Hexachorobenzene P/S-hexachlorobenzene
n-Hexane U-hexanedione, U-hexanedione + U-hexanedione
Breath-n-hexanec dihydroxyhexanone
Hydrazine P-, U-hydrazine
Lead (Pb) B-Pb, U-Pb, B-ZPP B-Pb, U-ALA B-Pb, B-ZPP B-Pb, U-ALA, B-ZPP
Lindane B(P,S)-lindane B-lindane B-lindane
Manganese (Mn) U-Mn B-, U-Mn
Mercury (Hg) B-, U-Hg B-, U-Hg B-, U-Hg
Methanol U-methanol, U-formate U-methanol U-formate
Methylbromide B-Br
Methyl butyl ketone U-hexanedione +
dihyroxyhexanone
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Methylene U-MOCA
bis(2-chloroaniline)
(MOCA)
Methylene dianiline U-MDA
(MDA)
Methyl ethyl ketone U-MEK U-MEK
(MEK)
2-Methoxyethanol U-methoxyacetic acid
2-Methoxyethyl acetate U-methoxyacetic acid
Nickel (Ni) U-Ni U-Ni U-Ni
Nitrobenzene U-p-nitrophenol, B-MetHb
Parathion U-p-nitrophenol, U-p-nitrophenol,
E-cholinesterase E-cholinesterase
Pentachlorophenol U-PCP, P-PCP U-PCP, P-PCP U-PCP
(PCP)
Phenol U-phenol U-phenol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Polychlorinated S-PCB B-PCB
biphenyls (PCB)
2-Propanol U-acetone, B-acetone
Selenium (Se) U-Se
Styrene U-mandelic acid, U-PGA U-mandelic acid, U-mandelic acid+PGA U-mandelic acid
U-mandelic acid+U-PGA
Thallium (Tl) U-Tl
Tetrachloroethylene Breath-, Breath-b, B-tetrachloroethylene
B-tetrachloroethylene B-tetrachloroethylene
Tetrachloromethane Breath-b,
B-tetrachloromethane
Tin (Sn) U-Sn
Toluene U-hippuric acid B-toluene B-toluene B-toluene
1,1,1-Trichloroethane Breath-1,1,1-trichloroethane, Breath-b, B-1,1,1-trichloroethane B-1,1,1-trichloroethane
B-trichloroethanol B-1,1,1-trichloroethane
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Trichloroethylene U-TCA, B-trichloroethanol, U-TCA, B-trichloroethanol U-TCA, U-Trichloroethanol U-TCA
U-TCA+trichloroethanol
Vanadium (V) U-V U-V
Vinyl chloride U-thiodiglycolic acid
Xylenes U-methylhippuric acids U-methylhippuric acids, B- U-methylhippuric acids U-methylhippuric acids
a The following abbreviations have been used: E = erythrocyte; P = plasma; S = serum; U = urine; B = blood; ZPP = erythrocyte zinc
protoporphyrin; ALA = delta-aminolevulinic acid; PGA = phenylglyoxylic acid; MMA = monomethylarsinic acid; DMA = dimethylarsinic acid;
TCA = trichloroacetic acid; MCPA = chloromethylphenoxyacetic acid; 2,4-D = 2,4 dichlorophenoxyacetic acid;
TTCA = 4-thio-4-thiazolidine carboxylic acid.
b DFG (1992) refers to alveolar air
c ACGIH (1992) refers to end-exhaled air
Note: Readers must consult original references before applying the above examples to specific situations
6. BIOMARKERS OF EFFECT
This section focuses on those human biomarkers that can be
applied currently or will be in the near future. Biomarkers of
effect may be used directly in hazard identification and
dose-response assessment components of the risk assessment process.
In hazard identification, biomarkers may facilitate screening and/or
identification of a toxic agent and characterization of the
associated toxicity. Biomarkers that are implicated in toxic
mechanism(s) are preferred for quantitative dose-response
assessments when extrapolating from existing data to a human
situation of concern (e.g., from high to low dose or from test
species to humans).
There are wide inter-individual variations in the response to
equivalent doses of chemicals. While the outcome of a chemical
insult in an individual may be predicted more accurately from
biomarkers of effect(s), such biomarkers may not be specific for a
single causative agent. Many biomarkers of effect are used in
everyday practice to assist in clinical diagnosis, but for
preventive purposes an ideal biomarker of effect is one that
measures change that is still reversible. Nevertheless, certain
biomarkers of nonreversible effects may still be very useful in
epidemiological studies or provide the opportunity for early
clinical intervention.
A limited range of tissues is available for routine analysis of
biomarkers. The more accessible tissues are therefore used as
surrogates for the known or putative target tissues. In some
instances biomarkers of effect are not mechanistically related to
chemically induced lesions, but may represent concomitant,
independent changes. Therefore, although an effect (e.g., sister
chromatid exchange) is being analysed, the use is conceptually close
to assessment of exposure.
6.1 Haematological biomarkers
Inhibition of the enzymes in the haem synthesis pathway (e.g.,
ferrochelatase, levulinate dehydratase) has been used as a marker of
effect of exposure to lead. This effect is reflected also in the
levels of free erythrocyte protoporphyrin (FEP) and
delta-aminolevulinate in the urine. Elevated levels of urinary
delta-amino-levulinate are observed at higher lead exposures than
changes in FEP, for example, while basophilic stippling of
erythrocytes is an even less sensitive biomarker for the effects of
lead. However, the effects on haem synthesis are not specific to
lead as a causative agent; iron deficiency has a similar effect on
FEP. The relationship of these effect biomarkers to toxicity
requires further elucidation.
Routine leucocyte, erythrocyte and thrombocyte counts have been
used in the surveillance of patients treated with cytostatic drugs
and in the monitoring of benzene-exposed workers. The predictive
power in relation to benzene-induced aplastic anaemia or leukaemia
is limited (Townsend et. al., 1978; Hancock et al., 1984; Lamm et
al., 1989). Ferrokinetic measurements, such as plasma iron
disappearance half-time, erythrocyte utilization of iron, plasma
iron transport rate, or erythrocyte iron turnover rate, have been
suggested as biomarkers of myelotoxicity (Rajamaki, 1984).
6.2 Nephrotoxicity biomarkers
Several different types of measures have been tested and used
as biomarkers of renal damage. These have been classified as
functional markers (e.g., serum creatinine and ß2-microglobulin),
urinary proteins of low or high molecular weight (e.g., albumin,
transferrin, retinol-binding globulin, rheumatoid factor,
immunoglobulin G), cytotoxicity markers (tubular antigens, e.g.,
BB50, BBA, HF5), enzymes (e.g., N-acetylglucosaminidase,
ß-galactosidase) in urine, and biochemical markers (eicosanoids,
e.g., 6-keto PGF2alpha, PGE2, PGF2alpha and TXB2, fibronectin,
kallikrein activity, sialic acid and glycosaminoglycans in urine,
and red blood cell negative charges) (Cardenas et al., 1993a,b;
Roels et al., 1993).
Biomarkers for nephrotoxicity were reviewed in WHO (1991) and
are well validated in relation to exposure to cadmium (WHO, 1992a;
Roels et al., 1993) but not in relation to exposure to mercury or
lead (Cardenas et al., 1993a,b).
6.3 Liver toxicity biomarkers
Effects of chemicals on the liver have been estimated
traditionally by measuring the activities of, for example,
aminotransferase (most often aspartate or alanine aminotransferase)
in the serum, where they are found when liver cells have been
damaged and have leaked their contents. Many other enzymes have also
been analysed for this purpose; they include 5-nucleotidase, alcohol
dehydrogenase, lactate dehydrogenase, isocitrate dehydrogenase,
leucine aminopeptidase, glutathione S-transferase, ornithine
carbamoyl transferase). Since tissues other than liver also contain
these enzymes, their activities may be elevated in serum not only
after liver damage but also when non-hepatic tissues have been
damaged. To overcome this lack of specificity, analysis of specific
isoenzymes has been used. Serum activities of enzymes such as
alkaline phosphatase and gamma-glutamyl transpeptidase may be used
as biomarkers of hepatic damage mainly involving biliary excretion.
Several liver function tests can also be used as biomarkers of
effects; these include the concentrations of serum proteins
synthesized in the liver, e.g., albumin and clotting factors, or
serum concentrations of bile acids, also synthesized in the liver,
as well as tests for the hepatic excretory function such as
bromsulfphthalein half-time. These parameters lack specificity since
hepatic viral infections, alcohol and drug use affect these enzymes.
Indirect measures of chemically induced change(s) in the cytochrome
P-450 enzyme system, using provocation tests, have been proposed as
sensitive indicators. However, the relationship to liver damage and
disease is not established and the requirement for the
administration of a drug limits the use of such tests.
Hepatotoxicity is caused by a number of chemicals that are
metabolized by the cytochrome P-450-dependent mixed-function oxidase
system to reactive intermediates. For example, carbon tetrachloride
has been studied extensively; it is metabolized to a reactive
intermediate which initially depletes intracellular glutathione to a
level that is no longer protective when the metabolite reacts with
critical macromolecules leading to cell death and hepatoxicity. In
this example, biomarkers of effect could include glutathione levels,
lipid peroxidation or the number of necrotic cells.
6.4 Biomarkers of immunotoxicity
The immune system protects the organism against infectious
microorganisms and the growth of at least some neoplasms. Reactions
of the immune system are influenced by genetic factors, age,
nutrition, life-style and health status. Xenobiotics may stimulate
or suppress the immune system. After initial sensitization, even a
minimal new exposure may lead to an anaphylactic reaction. The
immune system may be more sensitive to chemical challenge than any
other body system.
Hypersensitivity reactions following inhalation exposure
include asthma, rhinitis, pneumonitis and granulomatous pulmonary
reactions (see section 6.5). Hypersensitive dermal reactions induced
by chemicals include a wide variety of acute, subchronic and chronic
changes. Patch testing has been used traditionally as a biomarker
for identification of the causative agent of an allergic skin
reaction. However, the possibility of inducing hypersensitivity by
patch testing has been well documented and should not be overlooked
(Adams & Fisher, 1990).
Elevated levels of specific antibodies, usually of the IgE
type, may indicate existing sensitization. However, not all
individuals with elevated levels are symptomatic and not all
symptomatic individuals exhibit elevated IgE levels (Horak, 1985;
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology, 1989; Nielsen et al., 1992).
Suppression of the immune system increases susceptibility to
infections and neoplasia. Changes in the relative abundance of
different lymphocyte subpopulations (suppressor and helper T-cells)
have been used as biomarkers for the immune suppression (Jennings et
al., 1988; Sullivan, 1989; Holsapple, et al., 1991). Individuals
with asbestos-induced pleural or pulmonary changes, or
asbestos-induced cancer, as well as those heavily exposed to
asbestos but without apparent disease, have been reported to exhibit
an altered immunological status (e.g., reductions in T-lymphocyte
subsets) (Bekes et al., 1987).
In view of the growing incidence of hypersensitivity reactions
to chemicals, development and application of biomarkers for
immunotoxic effects is important. However, this is made difficult by
the current limited understanding of basic immunological mechanisms
and the effects of chemicals thereon (US NRC 1992).
6.5 Biomarkers of pulmonary toxicity
The most frequently used markers of pulmonary toxicity measure
gross effects on pulmonary function (e.g., peak expiratory flow,
forced expiratory volume, transfer factors) rather than effects on
cells or biochemical processes (US NRC, 1989a). These measures tend
to be nonspecific with respect to the causative agent and may
overlook effects specific to a certain cell type. Peak expiratory
flow measurements can be performed by the exposed individuals
themselves at the workplace, at home or elsewhere, and they provide
information on the underlying causes of air-way obstruction,
allowing a closer association between exposure, atmosphere and
response.
Air-way hyperactivity can be assessed by challenge tests using
inhalation exposure. Although such tests may assist in identifying
the factors causing hypersensitive pulmonary reactions, there is a
clear risk of acute reactions, and testing should be carried out by
qualified personnel in carefully controlled environments.
Recently, analysis of bronchoalveolar lavage fluid (BALF) has
been used to detect lung injury or to follow the progress of
pulmonary disease or the efficacy of therapeutic treatment
(Reynolds, 1987; Henderson, 1988).
The use of cellular elements as markers of pulmonary disease
state has been emphasized in human BALF analysis (Reynolds, 1987).
Total cell counts and differential counts, including use of
monoclonal antibody staining to distinguish T-cell subtypes, are
used to detect alveolitis and to aid in the diagnosis of
interstitial lung disease. High percentages of lymphocytes are
indicators of granulomatous processes, such as sarcoidosis, or
hypersensitivity pneumonitis. High percentages of neutrophils with
some eosinophils indicate possible idiopathic pulmonary fibrosis.
Other extracellular components, such as cytokines and other
mediators of inflammation, have been used on an experimental basis
to answer specific research questions.
The analysis of BALF has been used to define the dose-response
characteristics of inhaled or instilled toxins in animal toxicity
studies (Henderson, 1988). The most sensitive biomarker of an
inflammatory response in the bronchoalveolar region is the number of
neutrophils in BALF. The levels of protein and of extracellular
enzymatic activity are also useful markers of pulmonary toxicity.
Increases in protein concentrations in BALF indicate increased
permeability of the alveolar/capillary barrier. Lactate
dehydrogenase (LDH) is a cytoplasmic enzyme that is found
extracellularly only in the presence of lysed or damaged cells.
Beta-glucuronidase or similar lysosomal hydrolytic enzymes are
excellent markers for the toxicity of inhaled particles. These
particles are phagocytosed by macrophages, and the enzymes are
released from activated or lysed macrophages.
The secretion of cytokines from pulmonary macrophages obtained
by bronchoalveolar lavage provides markers of developing fibrosis.
Recent studies by Piguet et al. (1990) demonstrate that the level of
secretion of tumour necrosis factor (TNF) by pulmonary macrophages
is associated with quartz-induced fibrotic processes. Lassalle et
al. (1990) found elevated secretion of TNF by macrophages obtained
from individuals with coal-workers pneumoconioses compared with
macrophages from controls. The secretion of platelet-derived growth
factor from pulmonary macrophage was elevated in patients with
idiopathic pulmonary fibrosis (IPF).
Glutathione (GSH), a tripeptide protective against oxidative
stress, is present in BALF, and a decrease in GSH in BALF is a
potential marker for oxidative stress. Decreased levels of GSH have
been observed in patient with IPF (Cantin et al., 1989) and in
animals exposed chronically to diesel exhaust, resulting in
pulmonary fibrosis (Henderson, 1988).
Nasal lavage fluid (NLF) also provides markers of response to
inhaled toxins. The work of Graham et al. (1988) demonstrates the
potential use of NLF analysis to document the influx of neutrophils
into the nasal cavity in humans in response to inhaled ozone.
Biomarkers in blood related to lung injury have not been
validated. However, the work of Cavalleri et al. (1991) indicates
that serum aminoterminal propeptide of type III procollagen (PIIINP)
may become useful as an early marker for developing fibrosis. A
dose-dependant increase in serum PIIINP was found in individuals
exposed to low or high levels of asbestos.
Finally, urinary levels of amino acids associated with the
connective tissue of the lung (hydroxyproline, hydroxylysine,
desmosine and isodesmosine) have been used as markers of lung injury
(Harel et al., 1980; Yanagisawa et al., 1986; Stone et al., 1991).
However, such assays are not specific for lung injury and merely
indicate the breakdown of connective tissue in any organ in the
body.
6.6 Biomarkers of reproductive and developmental toxicity
Markers associated with an adverse outcome in reproduction may
reflect toxic effects in the male or female or be associated with
development during the embryonic, fetal, perinatal or neonatal
period (US NRC, 1989b; Mattison, 1991).
Biomarkers for the male reproductive system may include
physiological indicators of impaired testicular function, or sperm
number or characteristics (including cytogenetics). Measures of
hormonal status (i.e. FSH, LH and testosterone) can also be readily
obtained from blood and, in the case of testosterone, from urine and
saliva. However, these levels are greatly influenced by circadian
rhythms and demonstrate large inter- and intra-individual
variability. A clearer picture of hormonal status can be obtained by
administering GnRH or LH and examining the hormone response to these
challenges. Biomarkers for the male reproductive system are rather
easily accessible and some even reasonably well validated; such
markers are less well developed for the female reproductive system.
Biomarkers indicative of developmental toxicity should also be
considered. As is the case for many biological markers of effects,
it is often difficult to identify the causative agent in the absence
of any specific exposure history. Biomarkers could include
measurements of detrimental effects produced by chemical or other
exposures during embryonic or fetal stages of development.
Irreversible lesions can be embryolethal or result in functional
anomalies in the offspring. Examples of biomarkers of developmental
toxicity include low birth weight, chromosome anomalies, delayed
growth of specific organ systems, mental retardation, and subtle
behavioural changes. The changes associated with F1 male-mediated
abnormalities have been discussed by Anderson (1990). Some of these
biomarkers of developmental effects (malformations, mental
retardation) are not biomarkers of effect as far as the individual
is concerned, but rather represent the adverse health outcome
itself. However, from the point of view of the exposed population,
they may be considered as biomarkers since they show that within a
population a harmful exposure has taken place.
Several biomarkers have been proposed for use during pregnancy,
e.g., early pregnancy loss and assays for genetic defects of the
conceptus. The latter comprise both classical cytogenetic studies,
as well as specific DNA probes (US NRC, 1989b). The use of urinary
human chorionic gonadotrophin (HCG) has been well documented as a
biomarker for early fetal loss (US NRC, 1989b). Many different
biomarkers have been used to follow the development of the pregnancy
and the well-being of the conceptus, but they have not yet been
applied to studies of effects of chemicals on pregnancy.
6.7 Biomarkers of neurotoxicity
The functions of the nervous system are complex and biomarkers
may range from effects of chemicals on neural cellular and molecular
processes to neurophysiological and neuro-behavioural measurements
of complex functional entities.
Inhibition of plasma and erythrocyte acetylcholine esterase
(AchE) provides biomarkers of exposure to organophosphorus compounds
and other cholinesterase inhibitors. While erythrocyte
cholinesterase is similar to brain cholinesterase, and is therefore
an effect biomarker, plasma nonspecific pseudocholinesterase only
reflects exposure and is not a marker of CNS effects.
Measures of the function of the peripheral nervous system
(e.g., electroneuromyography, nerve conduction velocities, vibration
sensitivity) are well defined. Assessment of peripheral nervous
system dysfunction associated with exposure to chemicals can be
carried out using electroneuromyography at the preclinical stage
(Seppalainen et al., 1979).
Some well-established neurophysiological (e.g., evoked
potentials, electroencephalography) and neurobehavioural (e.g., the
WHO Neurobehavioural Core Test Battery, Cassito et al., 1990)
measures may be used as biomarkers to evaluate CNS dysfunction
induced by neurotoxicants. These tests must be carried out under
well-controlled conditions.
Methods for assessing changes in higher cognitive function
(e.g., learning and memory) have been used extensively, e.g., in
workers exposed to solvents or heavy metals, but require further
refinement.
Available neuroimaging procedures, e.g., computed axial
tomography (CAT), magnetic resonance imaging (MRI), nuclear magnetic
resonance spectroscopy (MRS) and positron-emission tomography (PET),
are considered non-invasive, but some of them require exposure to
ionizing radiation. CAT and MRI can be carried out with current
clinical techniques to assess chemically induced changes in the
brain. The use of MRS and PET can provide a more detailed evaluation
of the biochemical status (e.g., rate of energy generation, blood
flow, L-glucose metabolism) in the central nervous system. They can
be used as biomarkers for assessing exposure to neurotoxicants
inducing brain alterations. However, they are expensive, of little
use in assessing spinal cord, nerve and muscle changes, and there is
only minimal data validating their use in neurotoxicology (US NRC,
1992).
Other promising biomarkers for neurotoxicity in animal studies
include glial fibrillary acidic protein (localized in the
astrocytes), which increases in localized areas within the brain
where injury due to toxicants occurs (O'Callaghan, 1991).
7. BIOMARKERS AND CHEMICAL CARCINOGENESIS
As new information about the multistep process of
carcinogenesis unfolds, it is instructive to consider the various
mechanisms by which chemicals induce cancer. Knowledge of mechanisms
will enable the selection of appropriate biomarkers for use in risk
assessment of carcinogens. Some chemicals are direct acting and
others require metabolic activation. Once absorbed most chemicals
undergo enzyme-mediated reactions that either detoxify them or
activate them to reactive species. The balance between activating
and detoxifying enzyme systems governs the rate of delivery of
bioactive metabolites to the macromolecular site (Harris, 1991). The
resulting macromolecular interaction could be a DNA adduct for
carcinogens that are initiating agents or receptor occupancy for
chemicals that are tumour promotors. Certain of the DNA adducts
produced by such interactions are pro-mutagenic, and replication of
the damaged DNA could lead to DNA sequence changes which may result
in altered gene expression or mutated gene products. Weisburger &
Williams (1981) have suggested that chemical carcinogens be
classified as those that interact with DNA (genotoxic) and those
that do not (epigenetic or non-genotoxic). The importance of mitotic
activity in the latter group has recently been elaborated further
(Cohen & Ellwein, 1990).
The implications for invoking the "mechanistic" approach to the
selection of appropriate biomarkers are significant. For example,
chemicals that stimulate cell proliferation via mitogenesis or
cytotoxicity (and subsequent proliferation) might require different
biomarkers than chemicals whose major mechanism of action is based
on DNA reactivity. In the latter case, measurements of DNA adducts
or chromosome alterations may serve as suitable biomarkers, whereas
in the former case alternate biomarkers (e.g., cell turnover
measurements) may be more appropriate.
7.1 Analysis of chemicals and metabolites
Urinary or blood concentrations of several chemicals shown or
suspected to be carcinogenic to humans (e.g., arsenic, cadmium,
chromium, nickel, benzene, MOCA, polychlorinated biphenyls, styrene,
tetrachloroethylene) have for long been used as biomarkers of
exposure. Among people exposed to arsenic in a copper smelter, a
dose relationship has been observed between the cumulative urinary
excretion of arsenic and the risk of lung cancer (Enterline & Marsh,
1982). For other carcinogenic chemicals, such data are not
available, and the measured concentrations may only be interpreted
in terms of exposure.
Sensitive techniques based on physicochemical or immunochemical
methods for the detection of a variety of carcinogen-modified DNA
bases have been developed (Shuker & Farmer, 1992). These include the
alkylated purines, aflatoxin-guanine adducts, cis-platinum adducts,
thymine glycol, 8-hydroxydeoxyguanosine and PAH-derived adducts.
The aflatoxin marker has been extensively used in both animal
and human studies on the relationship between exposure and liver
cancer induction. In an ongoing prospective study in Shanghai,
China, Ross et al. (1992) reported that subjects with liver cancer
were more likely than controls to have detectable concentrations of
any of the known aflatoxin metabolites in their urine. Groopman et
al. (1991) recently explored the relationship between dietary
aflatoxin and excretion in the urine of aflatoxin metabolites and an
aflatoxin-DNA adduct. This study was conducted on people living in
the Guangxi Autonomous Region, China. These investigators found a
positive correlation between aflatoxin N7-guanine and specific
metabolites excreted in urine and aflatoxin B1 intake from the
previous day.
Exposure to chemical compounds capable of interacting with
cellular macromolecules can originate from both exogenous and
endogenous sources. Nitrite, nitrate and nitrosating agents can be
synthesized endogenously in reactions mediated by bacteria and
activated macrophage. In this way endogenous formation of
N-nitroso compounds can occur at various sites in the body.
Endogenously formed N-nitroso compounds may be considered as
biomarkers of susceptibility; they have been associated in humans
with increased risk of cancer of the stomach, oesophagus and urinary
bladder, although unequivocal epidemiological data are lacking
(Bartsch & Montesano, 1984). The quantitative estimation of
endogenous nitrosation in humans can be measured using the
N-nitroso-proline test. L-proline is utilized as a probe for
nitrosatable amines and N-nitroso-proline excreted in the urine is
determined as a marker. This assay has been applied in some
population studies (Bartsch et al. 1991).
7.2 Biomarkers for genotoxic carcinogens
7.2.1 DNA adducts - general considerations
DNA adducts are being used both as molecular dosimeters
(biomarker of exposure) and to assess the genotoxic potential of
chemicals (biomarker of effect). The biological significance of such
adducts must be assessed on the basis of adduct heterogeneity and of
cell and tissue specificity for adduct formation, persistence and
repair. Some DNA adducts result in mutation whereas others do not.
Mutational specificity in the p53 gene produced by a variety of
chemical carcinogens provides evidence that DNA adduct location
influences site-specific mutations (Hollstein et al., 1991). Some
DNA sequence changes may lead to phenotypic alterations that can be
selected, whereas others may not (Compton et al., 1991).
Most tissues are comprised of multiple cell types, and cell
types vary considerably in their capacity to convert chemicals to
DNA reactive species. For example, lung is composed of multiple cell
types in which the relative concentrations of various P-450
isoenzymes and enzymes depends on the cell type. Thus, one compound
may produce high concentrations of pro-mutagenic adducts in one cell
type, but not in another, whereas the opposite might occur for a
compound which is activated by a different P-450 isozyme. DNA adduct
concentrations derived from a whole tissue homogenate may grossly
overestimate or underestimate adduct concentrations in a given cell
type.
Some DNA adducts are repaired quickly, others hardly at all,
the adduct loss correlating with cell turnover. Therefore, the
concentration and gene location of DNA adducts will change with time
after exposure to a genotoxic chemical. Furthermore, the existence
of non-random repair in the genome makes it difficult to utilize
total DNA repair capacity as an indicator of cell susceptibility to
carcinogens (Hanawalt, 1987). It is especially important in human
studies to know the duration and timing of exposure for proper
evaluation of the biological significance of a given adduct
concentration.
Many of the human studies described below have involved
measuring metabolism, DNA adduct formation and repair in whole
tissues. Techniques need to be refined in order that
cell-type-specific variations can be monitored in human tissues, as
well as experimental studies in animal models using
immunohistochemical techniques for the cell type. Specific
localization of DNA adducts has clearly demonstrated that such
variations occur. Treatment of rats with the tobacco-specific
nitrosamine, 4-( N-methyl- N-nitrosamine)-1-(3-pyridyl)-1-butanone
(NNK), results in the induction of tumours in the nasal cavity,
lung, liver and pancreas (Hoffman et al., 1984; Rivenson et al.,
1988). At low doses of NNK, the prevalence of malignant lung tumours
was higher than that observed in other tissues. Cell-type-specific
differences have been observed within the lung, the highest
concentration of O6-methylguanine having been found in the Clara
cells. These cells have the highest levels of the P-450 metabolizing
enzymes for NNK and low levels of the O6-methylguanine DNA
methyltransferase repair enzymes (Belinsky et al., 1987). Pulmonary
tumours are also induced in mice and hamsters following either
short- or long-term exposure to this carcinogen (Hecht et al.,
1983).
As animals age, DNA adducts are detected in increasing amounts,
and, although the relationship of these adducts to tumour
development is unclear, they are believed to be derived from dietary
constituents or endogenous chemicals such as hormones (Randerath &
Randerath, 1991).
7.2.2 DNA adducts in human samples
In human studies, it is difficult to obtain non-tumourous
target tissue for the quantification of DNA adducts. Lymphocytes are
a readily accessible source of human cells that are known to contain
DNA adducts. However, there is little information on the reliability
of using lymphocyte adduct concentrations for the estimation of
target cell or tissue adduct concentrations (Lucier & Thompson,
1987).
Evaluation of dose-response relationships for chemical
carcinogens in humans is more complex than in animal models.
Radio-labelled carcinogens cannot be administered and the
accessibility of tissues and cells is limited. Several approaches to
detect DNA adducts in human samples have been evaluated (Wogan &
Gorelick, 1985; Santella, 1988). The most frequently used methods
are immunoassays and 32P-postlabelling. Other analytical
techniques such as GC-MS and synchronous fluorescence spectroscopy
are being used to measure DNA adducts (Weston & Bowman, 1991). In
general, immunoassays are both specific and sensitive for alkylated
adducts and aflatoxin adducts (Wild & Montesano, 1991; Groopman et
al., 1991). However, these methods are not easily applied to
quantification of adducts for bulky aromatic hydrocarbons such as
benzo [a]pyrene-derived adducts. The main problem is the lack of
specificity of the antibodies used in the assay which cross-react
with a number of PAH-related adducts (Santella et al., 1985).
The second assay frequently used to quantify DNA adducts in
humans is the 32P postlabelling technique. For a complete
description of this assay, see Randerath & Randerath (1991), Beach &
Gupta (1992), IARC (1992). The assay is extraordinarily sensitive,
being capable of detecting 1 adduct in 1010 normal nucleotides
when appropriate modifications are made to the procedure. The assay
is particularly useful for detecting adducts of non-polar polycyclic
aromatic hydrocarbons such as 7,8-diol-9,10-oxide-benzo [a]pyrene
deoxyguanosine (BPDE). Some DNA modifications such as alkylated DNA
adducts, which cannot be easily detected by this assay due to the
limitations of the chromatographic systems, can be quantified using
a combined 32P immunochemical precipitation technique (Kang et
al., 1993). Studies using the 32P labelling or immunological
methods have been reviewed by Beach & Gupta (1992) and Wild &
Montesano (1991). The groups of chemicals studied include alkylating
agents, polycyclic aromatic hydrocarbons (PAHs), heterocyclic PAHs,
nitro PAHs, cyclopenta-fused PAHs, aromatic amines, alkylbenzenes,
quinones, mycotoxins, chemotherapeutic agents, pesticides and
aldehydes.
7.2.3 Protein adducts
Ehrenberg and his associates pioneered the use of protein
adducts as dose monitors for carcinogen exposure in humans, and this
work has been reviewed by Hsia (1991). To date the class of proteins
that have been most extensively studied are those found in
circulating blood, i.e. haemoglobin and albumin. This is mainly
because these proteins are relatively abundant and can be easily
isolated for analysis.
Haemoglobin has the unique biological property of having a life
span equivalent to that of the erythrocyte, which in humans is
approximately 120 days, and therefore adduct levels reflect
exposures over several months. In contrast, albumin adducts can only
be used for assessing recent exposure because of the faster turnover
of albumin (half-life of 20-25 days). Protein adducts can be
quantified using chemical methods, e.g., aromatic amine release by
acid or basic hydrolysis from haemoglobin followed by derivatization
and GC-MS analysis (Farmer, 1991), or immunological techniques
(e.g., aflatoxin-albumin adducts, Wild et al., 1990).
During the past few years, several human monitoring studies
have demonstrated the usefulness of protein adducts as biomarkers of
exposure. Examples of chemicals that have been detected as protein
adducts in human studies include ethylene and propylene oxide,
aniline, cigarette smoke, aromatic amines such as 4-amino-biphenyl,
and aflatoxin (Wogan, 1989; Farmer, 1991). Albumin adducts of
aflatoxin B1 have also been used in epidemiological studies of
their role in the etiology of hepatocellular carcinoma in man. A
significant correlation was observed, at the individual level,
between dietary intake and the level of albumin-bound aflatoxin in a
chronically exposed population in the Gambia (Wild et al., 1992).
7.2.4 Cytogenetic methods
Cytogenetic methods are used as biomarkers of exposure to
DNA-damaging agents.
Many studies relating to cytogenetic changes in exposed human
populations have been reviewed in a special issue of Mutation
Research (Anderson, 1988). A second comprehensive review of more
recent studies has also been published (Anderson, 1990), and a
further review is in press (Anderson, in press).
All human monitoring studies suffer from variability of
baseline frequencies (Carrano & Natarajan, 1988) due to the presence
of endogenous (gender, age, medical history, etc.) and exogenous
factors (life-style, smoking, drinking, eating habits, etc.).
Anderson et al. (1991) have investigated the effect of these changes
on baseline variability eight times over a two-year period.
In contrast to studies on radiation, for which a marker
(dicentric chromosome) has been identified, studies with chemicals
have not yet identified a specific marker chromosome. For radiation
a dicentric is a quantitative dosimeter. Therefore, after radiation
exposure results can be used on an individual basis and a highly
exposed individual removed from the radiation source. Results from
chemical exposure studies, however, can only be used on a group
basis, due to the lack of a specific marker chromosome.
7.2.5 Chromosome damage
Both chromosome and chromatid aberrations are induced in
individuals exposed to chemical mutagens. The chromosome aberrations
are thought to arise from misrepair of lesions in the G0 stage of
circulating lymphocytes as well as from precursor cells in bone
marrow and thymus (Carrano & Natarajan, 1988). Chromatid aberrations
include chromatid breaks, intrachanges and exchanges, while
chromosome aberrations include acentric fragments, dicentric
chromosomes and ring chromosomes. Balanced translocation and
inversions can also arise and are difficult to quantify without
banding analysis. Structural aberrations can be classified as
unstable and stable depending on their ability to persist in
dividing cell populations. Unstable aberrations consist of rings,
acentric fragments and other asymmetrical rearrangements, and will
lead to the death of the cell. Stable aberrations consist of
balanced translocation inversions and other symmetrical
rearrangements which can be transmitted to progeny cells at
division. Therefore stable aberrations are more biologically
significant than unstable ones and could be involved in the cancer
process. Many human carcinogens have been shown to produce
chromosome damage in populations exposed to them, although no causal
relationship has been demonstrated (Sorsa et al., 1992). Proven
human carcinogens for which cytogenetic endpoints have been measured
in humans and corresponding animal data are available are listed in
Table 2.
In a preliminary report of a prospective study among people
whose lymphocytes were assayed for chromosome aberrations and SCE,
high rates of chromosome aberrations were observed and appeared to
be linked to cancer risk, but the finding was of borderline
statistical significance (Sorsa et al., 1990).
7.2.6 Sister chromatid exchange
Sister chromatid exchange (SCE) is considered to be a more
sensitive, rapid and simple cytogenetic end-point than chromosome
aberrations for evaluating the genotoxic potential of a variety of
mutagenic and carcinogenic agents. It is also used to detect and
differentiate many chromosome fragility diseases that predispose to
neoplasia. SCE is a DNA-replication-dependent phenomenon. Cellular
Table 2. Proven human carcinogens for which cytogenetic end-points have been measured in humans
and corresponding data are available for experimental animalsa
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Human carcinogens (Group 1)
Alcoholic beverages + + _ + ?
Aluminium production _ _
Arsenic and arsenic compounds ? ? + +
Asbestos ? _ _
Azathioprine ? _ + _ +
Benzene + + + +
Betel quid with tobacco + + +
Bis(chloromethyl)ether and (+) _
chloromethyl methyl ether
(technical grade)
1,4-Butanediol dimethanesulfonate (+) + + +
(Myleran)
Chlorambucil ? + +
Cyclosporin (+) _ _
Coal-tars +
Coke production +
Combined oral contraceptives _ _
Cyclophosphamide + + + + +
Hexavalent chromium compounds + + + + +
Melphalan + + +
8-Methoxypsoralen plus _ _ +
ultraviolet A radiation
Mineral oils, untreated and +
mildly treated
Nickel compounds + _ ? _
Table 2 (contd)
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Painter, occupational exposures as _
Radon + _
Rubber industry ? ?
Tobacco products, smokeless + +
Tobacco smoke + + + +
Tris(1-aziridinyl)phosphine sulfide (+) + + +
(Thiotepa)
Vinyl chloride + ? + + +
a From: Sorsa et al. (1992); CA = chromosome aberrations; SCE = sister chromatid exchange;
MN = micronuclei + = positive result; - = negative result; (+) = equivocal result;
? = doubtful result
factors such as nucleotide pools, repair and replication enzymes,
and biorhythms can play an important role in its formation. A major
source of variation can be attributed to the concentration of
bromodeoxyuridine relative to the number of lymphocytes in the
culture (Das, 1988; Morris, 1991; Morris et al., 1992). In a
prospective cancer study (Sorsa et al., 1990), no relationship was
observed between the frequency of SCE and the risk of cancer.
7.2.7 Micronuclei
Micronuclei are formed by condensation of acentric chromosomal
fragments or by whole chromosomes that are left behind during
anaphase movements (lagging chromosomes). The presence of
micronuclei can therefore be taken as an indication of the previous
existence of chromosomal aberrations. To visualize micronuclei,
cells have to undergo mitosis. In peripheral lymphocyte cultures it
is not easy to distinguish interface nuclei that have undergone a
division from those that have not. This makes it difficult to
quantify the frequencies of micronuclei for comparative purposes. A
method using cytochalasin B can distinguish nuclei that have divided
once (French & Morley, 1985).
7.2.8 Aneuploidy
Aneuploidy is a condition in which the number of chromosomes in
cells of individuals is not an exact multiple of the typical haploid
set for the species. Trisomy results when a single extra chromosome
is added to a pair of homologous chromosomes. If one chromosome of a
pair is missing, the result is monosomy. Absence of the pair is
nullisomy. Two or more copies of a homologue result in tetrasomy or
polysomy. Cells of individuals with missing or extra chromosomes are
hypoploid or hyperploid (UK DH, 1989). The best-known numerical
abnormalities result in the syndromes of Down (trisomy of chromosome
21), Klinefelter (sex chromosome genotype is XXY), and Turner (sex
chromosome genotype is X0). Aneuploidy is almost always found in
human cancers (Dellarco et al., 1985).
7.2.9 Mutation
Current somatic gene mutation assays used as biomarkers in
human studies select for a change or loss of a normal protein
produced by specific genes. Mutations at the X-linked hypoxanthine
guanine phosphoribosyl transferase gene in cloned T-lymphocytes and
in the autosomal locus for human leucocyte antigen-A (HLA-A) have
provided information on frequency of mutation and molecular spectra
of mutants. Detection of haemoglobin variants and loss of the cell
surface glycoprotein glycophorin A measured in red blood cells have
such limitations that analysis of the DNA mutations induced cannot
be made (Compton et al., 1991). The background frequency of each of
these specific locus assays varies greatly (Lambert, 1992) and is
dependent on numerous confounding factors (e.g., age and smoking).
Information on the mutation spectra at a particular locus will be
extremely useful in elucidating the mechanisms by which mutations
occur in human cells in vivo. By comparing spontaneous and
chemically induced mutational spectra in different populations, the
etiological contributions of both exogenous and endogenous factors
to human carcinogenesis could be assessed.
An alternative approach for the measurement of induced base
changes which does not require prior selection of a mutant
population uses the restriction site mutation technique. This is
based on the detection of DNA sequences resistant to the cutting
action of specific restriction enzymes, and the amplification of
these resistant sequences using the polymerase chain reaction. It
may theoretically be applied to the study of DNA base changes in any
gene for which the sequence has been determined (Parry et al.,
1990).
More relevant biomarkers for chemically induced cancers would,
however, preferably measure changes in genes thought to be important
for cancer. Mutations that activate proto-oncogenes, which stimulate
growth or inactivate suppressor genes to liberate cells from growth
constraints, could lead to unregulated proliferation of cancer cells
(Weinberg, 1991). For the most part, mutations in oncogenes and
tumour suppressor genes have been characterized in tumour tissue. It
remains to be determined whether the detection of mutant cells
against a background of normal cells can be achieved prior to
clinical diagnosis of cancer. Activated oncogenes have already been
identified in many human cancers, and considerable progress has been
made in elucidating the potential role of chemical carcinogens in
the activation of oncogenes and the contribution of the latter to
tumorigenesis in animal models (Balmain & Brown, 1988). Brandt-Rauf
(1991) presented data from pilot studies that demonstrated the
presence of the p21 protein product of the ras oncogene in the serum
of 15 out of 18 lung cancer patients who were all current or former
smokers. The protein was not found in the serum of any of the 18
healthy non-smoking controls, but was present in 2 out of 8
clinically healthy smoking controls. However, in another study, p21
protein was not detected in 20 smokers in a normal population or in
20 male healthy non-smokers (Brinkworth et al., 1992). The
mutational spectrum for the tumour suppressor gene p53 in human
tumours has been reviewed by Hollstein et al. (1991). Mutations of
the p53 gene are the most common cancer-related genetic changes at
the gene level and are widespread over the conserved codons of the
p53 gene. Hence, mutational spectra could be compared for tumours at
different sites and arising from different etiological backgrounds.
The mutational spectrum appears to differ among cancers of the
colon, lung, oesophagus, breast, liver, brain, reticuloendothelial
tissues and haemopoietic tissues. In two populations where aflatoxin
B1 exposure was implicated as one of the etiological factors in
hepatocellular carcinomas, the same mutational hotspot (i.e. G-T
transversion at codon 249) in the p53 gene has been identified (Hsu
et al., 1991).
7.3 Biomarkers for non-genotoxic carcinogenesis
Although only a few non-genotoxic human carcinogens have been
recognized (e.g., cyclosporin, diethylstilbestrol and estrogenic
hormones), many non-genotoxic carcinogens have been identified in
rodents. A compilation of NTP rodent data, designed to test the
concordance between short-term tests and in vivo carcinogenicity
assays, showed that more than 30% of rodent carcinogens do not test
positively for genotoxicity (Ashby & Tennant, 1991).
The mechanisms of action for non-genotoxic carcinogens need to
be considered in predicting human risk from chemical exposures.
Although the modes of action of non-genotoxic carcinogens are poorly
understood, several have been proposed, including immunosuppression,
hormonal effects, promotion, inorganic carcinogenesis,
co-carcinogenic effects and solid-state carcinogenesis (Weisburger &
Williams, 1981). Recently some of these mechanisms were grouped
under the headings of cytotoxicity and mitogenic growth stimulation
(Butterworth et al., 1992). Non-genotoxic carcinogens are believed
to exert their carcinogenic effects through mechanisms that do not
involve direct binding of the chemical or its metabolites to DNA (UK
DH, 1989). The key mechanism of non-genotoxic chemicals is to
increase cell proliferation, either by mitogenesis of the target
cells or by cytotoxicity, which is followed by regenerative cell
proliferation (Ramel, 1992). Cohen & Ellwein (1990) suggested that
non-genotoxic chemicals can be further categorized as to whether or
not their main mechanism of action is mediated via receptor-binding
(e.g., dioxin).
Cell replication and proliferation are potential biomarkers of
effect. Cell replication is the production of daughter cells by the
process of replicative DNA synthesis, while cell proliferation is
the enhanced replication of a selected cell population as observed
in regenerating tissues. Cells undergoing replicative DNA synthesis
(S-phase) are the most commonly used markers. The detection of cell
proliferation involves the incorporation of DNA precursors like
3H-thymidine or the base analogue 5-bromo-2'-deoxyuridine (BrdU)
into cellular DNA during S-phase. This is accomplished by
administering these precursors to animals by injection or through
implanted osmotic pumps. These S-phase cells can be identified
histoautotoradiographically or immunohistochemically (Goldsworthy et
al., 1991). The invasiveness of these techniques currently limits
their use to animal studies.
The identification of biomarkers of effect for non-genotoxic
carcinogens, whose major mechanism of action is via receptor
occupancy, may be difficult. This is primarily because carcinogens
of this type activate a variety of genes, some of which may not be
involved in the carcinogenic process. However, a good example of a
non-genotoxic carcinogen for which there is a good biomarker of
effect is 2,3,7,8-tetrachlorinated dibenzo- p-dioxin (TCDD). TCDD
interacts with a cytosolic receptor that is specific for it and its
structural analogues (Poland et al., 1976). In addition to
activating a number of growth factor and growth factor receptor
genes, TCDD induces a number of enzymes, one of which is cytochrome
P-450 1A1. Although the induction of this enzyme is probably not
directly related to the biological mechanism leading to cancer from
TCDD exposure, it is a sensitive marker for exposure (Tritscher et
al., 1992).
8. BIOMARKERS OF SUSCEPTIBILITY
This chapter focuses on the genetic predisposition of an
individual as it affects susceptibility to chemical materials. There
are a number of external factors, such as age, diet and health
status, that can also influence the susceptibility of an individual
exposed to chemicals. Some discussion will be directed towards the
effects of previous exposure on subsequent susceptibility, such as
to sensitization and enzyme induction/inhibition by previous
exposure. Table 3 lists some genetic and acquired factors affecting
susceptibility (Calabrese, 1986).
Although individuals may experience similar environmental
exposures, genetic differences in metabolism may produce markedly
different doses at the target site and thus a different level of
response. Even when target doses are similar, markedly different
responses may be noted in individuals due to varying degrees of
inherent biological responsiveness. Biomarkers of susceptibility may
reflect the acquired or genetic factors that influence the response
to exposure. These are pre-existing factors and are independent of
the exposure. They are predominantly genetic in origin, although
disease, physiological changes, medication and exposure to other
environmental agents may also alter individual susceptibility.
Biomarkers of susceptibility identify those individuals in a
population who have an acquired or genetic difference in
susceptibility to the effects of chemical exposure.
Biomarkers of susceptibility indicate which factors may
increase or decrease an individual's risk of developing a toxic
response following exposure to an environmental agent. Polymorphism
is present for some metabolic activation/deactivation enzymes,
including cytochrome P-450 isozymes (Nebert, 1988a, 1988b) and at
least one form of glutathione transferase (Seidegard et al., 1990).
Differing rates of enzyme activity controlling the activation or
detoxification of xenobiotics lead to differences in susceptibility
by increasing or decreasing the biologically effective dose of the
environmental agent.
The effect may vary between ethnic groups. For instance, there
are approximately equal numbers of fast and slow acetylator
phenotypes in a Caucasian population, whereas in a Japanese
population the distribution is 10% slow acetylators and 90% fast.
Genetic polymorphisms for drug metabolism have been widely studied
using phenotypic assays which involves measuring drug clearance in
individuals. Differential rates of metabolism will affect the
distribution and persistence of metabolites, which may have
implications for the site of toxicity. Epidemiological studies
suggest that, with respect to aromatic amines, slow acetylators are
more likely to contract bladder cancer but are at decreased risk
for colo-rectal cancer (Guengerich, 1991; Kadlubar et al., 1992).
Table 3. Some examples of established and suspected biomarkers of susceptibilitya
Biomarker of susceptibility Environmental agent Disease
Genetic
Debrisoquine hydroxylation phenotype cigarette smoke lung cancer
Acetylator phenotype aflatoxin, liver cancer,
aromatic amines bladder cancer
Ataxia telangiectasia genotype bleomycin, epoxides cancer at various sites
Xeroderma pigmentosum genotype agents that cause oxidative damage to skin cancer,
DNA, PAH, aromatic amines and aflatoxin B1 other cancers
Arylhydrocarbon hydroxylase inducibility polycyclic aromatic hydrocarbons lung cancer
alpha-1-antitrypsin cigarette smoke pulmonary emphysema
Franconi's anaemia phenotype cross-linking agents acute leukaemia
Glucose-6P-dehydrogenese deficiency phenotype oxidative agents, aromatic amines, poor resistance to oxidative
nitro-aromatic compounds stress,
aromatic amines
Sickle cell phenotype aromatic amino and nitro compounds, anaemia
carbon monoxide, cyanide
Thalassemia phenotype lead, benzene anaemia
Erythrocyte porphyria chloroquine, hexachlorobenzene, lead, anaemia
various drugs including barbituates,
sulfonamides, others
Sulfite oxidase deficiency heterozygotes sulfite, bisulfite, sulfur dioxide pulmonary disease
Alcohol dehydrogenase variant metabolize alcohols (e.g., ethanol)
more quickly than normal
GSTµ phenotype cigarette smoke lung cancer
Pseudocholinesterase variants organophosphate and carbamate insecticides, neurotoxicity
muscle relaxant drugs
IgA deficiency respiratory irritants irritation of respiratory tract
phenyl ketones in urine precursors of phenyl ketones phenylketonuria
Table 3 (contd)
Biomarker of susceptibility Environmental agent Disease
Acquired
Deficient diet chemical decreased resistance to
effects of many chemicals
Induced P-45O IIE1 alcohol consumption cancer at various sites
Antigen-specific antibodies chemicals, dusts decreased pulmonary
functions, skin rashes
a Modified from Calabrese (1986)
Polymorphism of N-oxidation has been linked to susceptibility to
colonic cancer (Kadlubar et al., 1992) and polymorphism in
glutathione S-transferase to increased lung cancer, particularly
adenocarcinoma (Seidegard et al., 1990).
The methodology for determining the phenotypes of individuals
for polymorphisms in metabolizing genes requires the administration
of a relevant test drug to the person and the subsequent measurement
of its clearance from the body. More recently, techniques based on
polymerase chain reactions, using DNA isolated from lymphocytes and
other cells, have been developed which allow the detection of
genetypes of known polymorphisms involving a variety of
xenobiotic-metabolizing enzymes, including GST1
(gluthione- S-transferase µ) and NAT2( N-acetyl transferase), as
well as two cytochrome P-450 isoenzymes: CYP1A1 and CYP2D6 (Bell,
1991; Blum et al., 1991; Wolf et al., 1992; Hirvonen et al., 1992;
Hollstein et al., 1992).
Cigarette smoking provides another example that illustrates the
effect of genetic polymorphisms on the response to chemicals.
Cigarette smoking is associated with the development of lung cancer
but not all smokers get lung cancer. This appears to be due in part
to genetic variations in arylhydrocarbon hydroxylase activity, which
results in a large variability in the binding of benz [a]pyrene to
DNA in cigarette smokers. A genetically based low level of
alpha-1-anti-trypsin activity greatly increases the risk of
emphysema from cigarette smoking.
In some situations a genetic trait may make an individual more
susceptible to one environmental agent but less so to another. For
example, the sickle cell trait predisposes to anaemia and altitude
sickness but offers some protection to the individual from infection
by the malaria parasite. Inherent differences in susceptibility
depend upon variations in the function of genes controlling enzyme
activity or the production of other proteins. Although a genotoxic
agent may reach the target tissue, the significance of any
chromosomal break will depend on the efficiency of the DNA repair
mechanisms. In xeroderma pigmentosum the individual is at an
increased risk of skin cancer after exposure to UV light because of
an inherited defect in DNA repair proteins (Cleaver, 1969).
Heterozygotes also have an increased risk of cancer and so the
frequency of the gene may affect the incidence of this cancer. Other
inherited diseases (e.g., ataxia-telangiectasia) that affect the
efficiency of DNA replication or repair may affect susceptibility to
carcinogenic agents (Swift et al., 1992). UV-DNA repair capacity has
been found to be lower in the lymphocytes isolated from individuals
with basal cell and squamous cell carcinoma than in the case of
their age-matched controls, and this repair capacity has been found
to decrease with increasing age in both groups (Wei et al., 1993).
Another form of susceptibility has an immunological basis.
Prior exposure to a chemical may induce an immune response that
sensitizes the individual to subsequent exposures. Such responses
occur in only a small fraction of the exposed population; an example
is the development of pulmonary hypersensitivity to industrial
agents such as toluene diisocyanate or cotton dust. The biomarkers
of susceptibility are the antigen-specific antibodies developed
against the chemical.
9. SUMMARY
The Task Group considered biomarkers in three categories,
biomarkers of exposure, of effect, and of susceptibility, while
recognising that clear distinction of category is often not
possible.
The Task Group agreed that the use of biomarkers can improve,
and should be used in, the process for the assessment of human
health risks caused by exposure to chemicals. Biomarkers may be
applied to the estimation of exposure and internal dose in
individuals and in groups and may allow identification of those at
greater or lesser risk than average.
Biomarkers must be validated before application in the risk
assessment process, i.e. the relationship between the biomarker, the
exposure, and the health outcome must be established. The selection,
validation and application of any biomarker is a complicated
process, which will vary for different markers. Examples were
selected to illustrate the concepts and principles.
Research and use of biomarkers involves complex ethical, social
and legal issues, which may vary in different countries. These
issues may impose constraints on research and use of biomarkers in
risk assessment and risk management decisions. The ethical, social
and legal aspects of biomarkers require careful consideration prior
to any application.
10. RECOMMENDATIONS
In making the following recommendations, the Task Group
recognized the role given IPCS to facilitate and increase
coordination of international activities in order to promote the
further work needed to define human health effects associated with
exposure to chemicals and to provide the basis for priority-setting
actions in order to protect public health.
10.1 General
* To promote the wider use of validated biomarkers in the
risk-assessment process
* To promote interdisciplinary cooperation and communication in
order to facilitate application of research findings
* To examine the feasibility of developing a data bank of
information on biomarkers applied to the process of risk and
their applications
10.2 Research
* To develop, refine and validate models to relate biomarkers of
exposure and of effect, qualitatively and quantitatively, to
exposure and to health outcome, particularly for end-points
other than cancer
* To identify and validate biomarkers of susceptibility in
relation to the chemical, and to inter-individual variation in
response, and investigate genetic polymorphism as a basis for
individual hypersensitivity
* To assess the use of information on individual susceptibility
in relation to protection of health with due respect to the
ethical, social and legal issues
* To develop strategies to link exposure and internal dose with
human health outcome by integration of mechanistically
validated biomarkers of exposure, effect and susceptibility
10.3 Applications
* To develop a practical protocol for use of biomarkers in human
studies, taking into account scientific, emotional, ethical,
legal and social aspects and including guidelines for risk
communication, with emphasis on the right of participants to
non-biased, intelligible information
* To encourage the production of certified reference materials
for biomarker analyses and support the functioning of
international quality assurance programmes
* To include consideration of biomarkers of exposure, effect, and
susceptibility in future Environmental Health Criteria
monographs
* To consider the need to update this monograph on principles and
concepts of biomarkers at an early date
REFERENCES
ACGIH (1992) 1992-1993 Threshold limit values for chemical
substances and physical agents and biological exposure indices.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
Adams RM & Fisher T (1990) Diagnostic patch testing.In: Adams R ed.
Occupational skin disease, 2nd ed. Philadelphia, Pennsylvania, W.B.
Saunders Company, pp 223-253.
Aitio A (1981) Laboratory quality control. Copenhagen, World Health
Organization, Regional Office for Europe, 74 pp (Health Aspects of
Chemical Safety - Interim Document 4).
Alessio L, Berlin A, & Roi R (1983) Human biological monitoring of
industrial chemicals series. Luxembourg, Office for Official
Publications of the Commission of European Communities (Industrial
Health and Safety Series).
Alessio L, Berlin A, & Roi R (1984) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 8903 EN).
Alessio L, Berlin A, & Roi R (1986) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 10704 EN).
Alessio L, Berlin A, & Roi R (1987) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11135 EN).
Alessio L, Berlin A, & Roi R (1988) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11478 EN).
Alessio L, Berlin A, & Roi R (1989) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 12174 EN).
Anderson D ed. (1988) Genetic toxicology testing and biomonitoring
of environmental or occupational exposure: human monitoring. Mutat
Res, 204(Spec Issue 3): 353-551.
Anderson D ed. (1990) Fundamental and molecular mechanisms of
mutagenesis. Mutat Res, 229(Spec Issue 2): 103-247.
Anderson D ed. (in press) Human monitoring II. Mutat Res (Spec
Issue).
Anderson D, Francis AJ, Godbert P, Jenkinson PC, & Butterworth KR
(1991) Chromosomal aberrations (CA), sister-chromatid exchanges
(SCE) and mitogen-induced blastogenesis in cultures of peripheral
lymphocytes from 48 control individuals sampled 8 times over 2
years. Mutat Res, 250: 467-476.
Ashby J & Tennant RW (1991) Definitive relationships among chemical
structure, carcinogenicity and mutagenicity for 301 chemicals tested
by the US National Toxicology Program. Mutat Res, 257: 229-306.
Balmain A & Brown K (1988) Oncogene activation in chemical
carcinogenesis. Adv Cancer Res, 51: 147-182.
Barbin A, Laib RJ, & Bartsch H (1985) Lack of miscoding properties
of 7-(2-oxoethyl)guanine, the major vinyl chloride-DNA adduct.
Cancer Res, 45: 2440-2444.
Bartsch H & Montesano R (1984) Commentary: relevance of nitrosamines
to human cancer. Carcinogenesis, 5: 1381-1393.
Bartsch H, Ohshima H, & Shuker DEG (1991) Noninvasive methods for
measuring exposure to alkylating agents: recent studies on human
subjects. In: Groopman JD & Skipper PL ed. Molecular dosimetry and
human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 281-301.
Beach AC & Gupta RC (1992) Human biomonitoring and the
32P-postlabeling assay. Carcinogenesis, 13: 1053-1074.
Bekes JG, Roboz JP, Fischbein A, & Selikoff IJ (1987) Clinical
immunology studies in individuals exposed to environmental
chemicals. In: Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F
ed. Immunotoxicology. Proceedings of the International Seminar on
the Immunological System as a Target for Toxic Damage: Present
Status, Open Problems and Future Perspectives. Dordrecht, Boston,
Lancaster, Martinus Nijhoff Publishers, pp 347-361 (IPCS Joint
Seminar 10; EUR 10041 EN).
Belinsky SA, White CM, Devereux TR, Swenberg JA, & Anderson MW
(1987) Cell selective alkylation of DNA in rat lung following low
dose exposure to the tobacco specific carcinogen
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res, 47:
1143-1148.
Bell DA (1991) Detection of DNA sequence polymorphisms in carcinogen
metabolism genes by polymerase chain reaction. Environ Mol Mutagen,
18: 245-248.
Blum M, Demierre A, Grant DM, Helm UA, & Meuer UA (1991) Molecular
mechanisms of slow acetylation of drugs and carcinogens in humans.
Proc Natl Acad Sci (USA), 88: 5237-5241.
Bond JA, Wallace LA, Osterman-Golkar S, Lucier GW, Buckpitt A, &
Henderson RF (1992) Assessment of exposure to pulmonary toxicants:
use of biological markers. Fundam Appl Toxicol, 18: 161-174.
Brandt-Rauf PW (1991) Advances in cancer biomarkers as applied to
chemical exposures: the ras oncogene and p21 protein and pulmonary
carcinogenesis. J Occup Med, 33: 951-955.
Brinkworth MH, Yardley-Jones A, Edwards AJ, Hughes JA, & Anderson D
(1992) A comparison of smokers with respect to oncogene products and
cytogenetic parameters. J Occup Med, 34: 1181-1189.
Butterworth BE, Popp JA, Conolly RB, & Goldsworthy TL (1992)
Chemically induced cell proliferation in carcinogenesis. In: Vainio
H, Magee PN, McGregor DB, & McMichael AJ ed. Mechanisms of
carcinogenesis in risk identification. Lyon, International Agency
for Research on Cancer, pp 279-305 (IARC Scientific Publications No.
116).
Calabrese EJ (1986) Ecogenetics: historical foundation and current
status (genetic factors). J Occup Med, 28: 1096-1102.
Cantin AM, Hubbard RC, & Crystal RG (1989) Glutathione deficiency in
the epithelial lining fluid of the lower respiratory tract in
idiopathic pulmonary fibrosis. Am Rev Respir Dis, 139: 370-372.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Hotter G, Mutti A, Franchini I, Fels LM, Stolte H, de
Broe ME, Nuyts GD, Taylor SA, & Price RG (1993a) Markers of early
renal changes induced by industrial pollutants. 1 Application to
workers exposed to mercury vapour. Br J Ind Med, 50: 17-27.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Ramis I, Mutti A, Franchini I, Fels LM, Stolte H, de Broe
ME, Nuyts GD, Taylor SA, & Price RG (1993b) Markers of early renal
changes induced by industrial pollutants. 2 Application to workers
exposed to lead. Br J Ind Med, 50: 28-36.
Carrano AV & Natarajan AT (1988) Considerations for population
monitoring using cytogenetic techniques. Mutat Res, 204: 379-406.
Casanova M, Deyo DF, & Heck HD'A (1989) Covalent binding of inhaled
formaldehyde to DNA in the nasal mucosa of Fischer 344 rats:
analysis of formaldehyde and DNA by high-performance liquid
chromatography and provisional pharmacokinetic interpretation.
Fundam Appl Toxicol, 12: 397-417.
Casanova M, Morgan KT, Steinhagen WH, Everitt JI, Popp JA, & Heck
HD'A (1991) Covalent binding of inhaled formaldehyde to DNA in the
respiratory tract of rhesus monkeys: pharmacokinetics, rat-to-monkey
interspecies scaling, and extrapolation to man. Fundam Appl Toxicol,
17: 409-428.
Cassito MG, Camerino D, Hanninen H, & Anger KW (1990) International
collaboration to evaluate the WHO neurobehavioural core test
battery. In: Johnson BL, Anger WK, Durao A, & Xinteras C ed.
Advances in neurobehavioural toxicology: applications in
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., pp 203-223.
Costa LG (1992) Effect of neurotoxicants on brain neurochemistry.
In: Tilson H & Mitchell C ed. Neurotoxicology. New York, Raven Press
Ltd, pp 101-123.
Cavalleri A, Gobba F, Bacchella L, & Ferrari D (1991) Evaluation of
serum aminoterminal propeptide of type III procollagen as an early
marker of the active fibrotic process in asbestos-exposed workers.
Scand J Work Environ Health, 17: 139-144.
Cleaver JE (1969) Xeroderma pigmentosum: a human disease in which an
initial stage of DNA repair is defective. Proc Natl Acad Sci (USA),
63: 428-435.
Cohen SM & Ellwein LB (1990) Cell proliferation in carcinogenesis.
Science, 249: 1007-1011.
Compton PJE, Hooper K, & Smith MT (1991) Human somatic mutation
assays as biomarkers of carcinogenesis. Environ Health Perspect, 94:
135-141.
Conolly RB & Andersen ME (in press) An approach to mechanism-based
cancer risk assessment: formaldehyde. Environ Health Perspect, 101.
Conolly RB, Monticello TM, Morgan KT, Clewell HJ, & Andersen ME
(1992) A biologically-based risk assessment strategy for inhaled
formaldehyde. Comments Toxicol, 4: 269-293.
Das BC (1988) Factors that influence formation of sister chromatid
exchanges in human blood lymphocytes. Crit Rev Toxicol, 19: 43-86.
Dellarco V, Voytek P, & Hollaender A (1985) Aneuploidy: Etiology and
mechanisms. New York, London, Plenum Press.
Derosa CT, Choudhury H, & Peirano WB (1991) An integrated
exposure/pharmacokinetic based approach to the assessment of complex
exposures. Toxicol Ind Health, 7(4): 231-248.
DFG (German Association for the Encouragement of Research) (1992)
Commission for the Investigation of Health Hazards of Chemical
Compounds in the Work Area: MAK- and BAT-values 1992. Weinheim, VCH.
Droz PO, Wu MM, & Cumberland WG (1989) Variability in biological
monitoring of organic solvent exposure. 2 Application of a
population physiological model. Br J Ind Med, 46: 547-558.
Enterline PE & Marsh GM (1982) Cancer among workers exposed to
arsenic and other substances in a copper smelter. Am J Epidemiol,
116: 895-911.
Farmer PB (1991) Analytical approaches for the determination of
protein-carcinogen adducts using mass spectrometry. In: Groopman JD
& Skipper PL ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 189-210.
FIOH (Työterveyslaitos) (1993) [Biological monitoring. Guide for
sample collection 1993.] Helsinki, Finnish Institute of Health, 115
pp (in Finnish).
French M & Morley AA (1985) Measurement of micronuclei in
lymphocytes. Mutat Res, 147: 29-36.
Gibaldi M & Perrier D (1982) Pharmacokinetics. New York, Basel,
Marcel Dekker Inc.
Goldsworthy TL, Morgan KT, Popp JA, & Butterworth BE (1991)
Guidelines for measuring chemically-induced cell proliferation in
specific rat organs. In: Butterworth BE & Sloga TJ ed. Chemically
induced cell proliferation: Implications for risk assessment. New
York, Wiley-Liss, pp 253-284.
Graham D, Henderson FW, & House D (1988) Neutrophil influx measured
in nasal lavages of humans exposed to ozone. Arch Environ Health,
43: 228-233.
Groopman JD, Sabbioni G, & Wild CP (1991) Molecular dosimetry of
human aflatoxin exposures. In: Groopman JD & Skipper PL ed.
Molecular dosimetry and human cancer -Analytical, epidemiological
and social considerations. Boca Raton, Florida, CRC Press, pp
303-324.
Guengerich FP (1991) Interindividual variation in biotransformation
of carcinogens: basis and relevance. In: Groopman JD & Skipper PL
ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 27-52.
Hall JA, Saffhill R, Green T, & Hathway DE (1981) The induction of
errors during in vitro DNA synthesis following
chloroacetaldehyde-treatment of poly(dA-dT) and poly(dC-dG)
templates. Carcinogenesis, 2: 141-146.
Hanawalt PC (1987) Preferential DNA repair in expressed genes.
Environ Health Perspect, 76: 9-14.
Hancock DG, Moffitt AE, & Hay EB (1984) Hematological findings among
workers exposed to benzene at coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Harel S, Janoff A, Yu SY, Hurewitz A, & Bergofsky EH (1980)
Desmosine radioimmunoassay for measuring elastin degradation in
vivo. Am Rev Respir Dis, 122: 769-773.
Harris CC (1991) Molecular epidemiology: overview of molecular and
biochemical basis. In: Groopman JD & Skipper PL ed. Molecular
dosimetry and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 15-26.
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumours in Syrian golden hamsters by a single dose
of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and the
effect of smoke inhalation. Carcinogenesis, 4(10): 1287-1290.
Henderson RF (1988) Use of bronchoalveolar lavage to detect lung
damage. In: Target organ toxicology: Lung. New York, Raven Press, pp
239-268.
Henderson RF, Bechtold WE, Bond JA, & Sun JD (1989) The use of
biological markers in toxicology. Crit Rev Toxicol, 20: 65-82.
Hirvonen A, Husgafvel-Pursiainen K, Karjalainen A, Anttila S, &
Vainio H (1992) Point-mutational Msp1 and lle-Val polymorphisms
closely linked in the CYP1A1 Gene: Lack of association with
susceptibility to lung cancer in a Finnish study population. Cancer
Epidemiol Biomarkers Prev, 1: 485-489.
Hoffman D, Rivenson A, Amin S, & Hecht SS (1984) Dose-response study
of the carcinogenicity of tobacco-specific N-nitrosamines in F344
Rats. J Cancer Res Clin Oncol, 108: 81-86.
Hollstein M, Sidransky D, Vogelstein B, & Harris CC (1991) p53
Mutations in human cancers. Science, 253: 49-53.
Hollstein MC, Wild CP, Bleicher F, Chutimataewin S, Harris CC,
Srivatanakul P, & Montesano R (1992) p53 Mutations and aflatoxin
B1h exposure in hepatocellular carcinoma patients from Thailand.
Int J Cancer, 53: 1-5.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-255.
Horak F (1985) Manifestation of allergic rhinitis in
latent-sensitized patients. A prospective study. Arch
Otorhinolaryngol, 242: 224-239.
Hsia MTS (1991) Carcinogen-macromolecular adducts as biomarkers in
human cancer risk assessment. Biomed Environ Sci, 4: 104-112.
Hsu IC, Metcalf RA, Sun T, Welsh J, Wang NJ, & Harris CC (1991)
Mutational hotspot in the p53 gene in human hepatocellular
carcinomas. Nature (Lond), 350: 427-428.
IARC (1992) In: Vainio H, Magee PN, McGregor D, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer (IARC Scientific
Publications No. 116).
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunologic
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin. Br J Ind Med, 45: 701-704.
Kadlubar FF, Butler MA, Kaderlik KR, Chou H-S, & Lang NP (1992)
Polymorphisms for aromatic amine metabolism in humans: relevance for
human carcinogenesis. Environ Health Perspect, 98: 69-74.
Kang H-I, Konishi C, Kuroko T, & Huh N-K (1993) A highly sensitive
and specific method for quantitation of O-alkylated DNA adducts and
its application to the analysis of human tissue. Environ Health
Perspect, 99: 269-271.
Lambert B (1992) Biological markers in exposed humans: gene
mutation. In: Vainio H, Magee PN, McGregor DB, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer, pp 535-542 (IARC
Scientific Publications No. 116).
Lamm SH, Walters AS, Wilson R, Byrd DM, & Grunwalt H (1989)
Consistencies and inconsistencies underlying the quantitative
assessment of leukemia risk from benzene exposure. Environ Health
Perspect, 82: 289-297.
Lassalle P, Cosset P, & Aerts C (1990) Abnormal secretion of
interleukin-1 and tumor necrosis factor alpha by alveolar
macrophages in coal worker's pneumoconiosis: comparison between
simple pneumoconiosis and progressive massive fibrosis. Exp Lung
Res, 16: 73-80.
Lucier G & Thompson CL (1987) Issues in biochemical applications to
risk assessment: when can lymphocytes be used as surrogate markers?
Environ Health Perspect, 76: 187-191.
Mattison DR (1991) An overview on biological markers in reproductive
and development toxicology: concepts and definitions, and use in
risk assessment. Biomed Environ Sci, 4: 8-34.
Morris SM (1991) The genetic toxicology of 5-bromodeoxyuridine in
mammalian cells. Mutat Res, 258: 161-188.
Morris SM, Domon OE, McGarrity LJ, Kodell RL, & Casciano DA (1992)
Effect of bromodeoxyuridine on the proliferation and growth of ethyl
methanesulfonate-exposed P3 cells: relationship to the induction of
sister chromatid exchanges. Cell Biol Toxicol, 8: 75-87.
Nebert DW (1988a) The 1986 Bernard B. Brodie award lecture. The
genetic regulation of drug-metabolizing enzymes. Drug Metab Dispos,
16: 1-8.
Nebert DW (1988b) Genes encoding drug metabolizing enzymes: possible
role in human disease. Basic Life Sci, 43: 45-64.
Nielsen J, Welinder H, Horstmann V, & Skerfving S (1992) Allergy to
methyltetrahydrophthalic anhydride in epoxy resin workers. Br J Ind
Med, 49: 769-775.
O'Callaghan JP (1991) Quantification of glial fibrillary acidic
protein: Comparison of slot-immunobinding assays with a novel
sandwich ELISA. Neurotoxicol Teratol, 13: 275-281.
Parry JM, Shamsher M, & Skibinski DOF (1990) Restriction site
mutation analysis, a proposed methodology for the detection and
study of DNA base changes following mutation exposure. Mutagenesis,
5: 209-212.
Passingham BJ, Farmer FB, Bailey E, Brookes AGF, & Yates DW (1988)
2-Hydroxyethylation of haemoglobin in man. In: Methods for detecting
DNA and damaging agents in humans: applications in cancer
epidemiology and prevention. Lyon, International Agency for Research
on Cancer, pp 279-285 (IARC Scientific Publications No. 89).
Piguet PF, Collart MA, Grau GE, Sappino AP, & Vassalli P (1990)
Requirement of tumor necrosis factor for development of
silica-induced pulmonary fibrosis. Nature (Lond), 344: 245-247.
Poland A, Glover E, & Kende AS (1976) Stereospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol.
Evidence that the binding species is receptor for induction of aryl
hydrocarbon hydroxylase. J Biol Chem, 251: 4936-4946.
Rajamaki A (1984) Assessment of early myelotoxicity. In: Aitio A,
Riihimaki V, & Vainio H ed. Biological monitoring and surveillance
of workers exposed to chemicals. Washington, New York, London,
Hemisphere Publishing Corporation, pp 303-308.
Ramel C (1992) Genotoxic and nongenotoxic carcinogens: mechanisms of
action and testing strategies. In: Vainio H, Magee PN, McGregor DB,
& McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 195-209 (IARC Scientific Publications No. 116).
Ramsay JC & Andersen ME (1984) A physiologically based description
of the inhalation pharmacokinetics of styrene monomer in rats and
humans. Toxicol Appl Pharmacol, 73: 159-175.
Randerath K & Randerath E (1991) 32P-postlabeling analysis of
mutagen- and carcinogen-adducts and age-related DNA modifications
(I-compounds). In: Groopman JD & Skipper PL ed. Molecular dosimetry
and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 131-150.
Reynolds HY (1987) Bronchoalveolar lavage. Am Rev Respir Dis, 135:
250-263.
Rivenson A, Hoffman D, Prokopszyk B, Amin S, & Hecht SS (1988)
Induction of lung and pancreas tumours in F344 rats by
tobacco-specific and areca derived N-nitrosamines. Cancer Res, 48:
6912-6917.
Roels H, Bernard AM, Cardenas AR, Buchet JP, Lauwerys RR, Hotter G,
Ramis I, Mutti A, Frandhini I, Bundschuh I, Stolte H, de Broe ME,
Nuyts GD, Taylor SA, & Price RG (1993) Markers of early renal
changes induced by industrial pollutants. 3 Application to workers
exposed to cadmium. Br J Ind Med, 50: 37-48.
Ross RK, Yuan J-M, Wogan GN, Qian G-S, Tu J-T, Groopman JD, Gao Y-T,
& Henderson BE (1992) Urinary aflatoxin biomarkers and risk of
hepatocellular carcinoma. Lancet, 339: 943-946.
Santella RM (1988) Application of new techniques for the detection
of carcinogen adducts to human population monitoring. Mutat Res,
205: 271-282.
Santella RM, Gaspara F, & Hsieh L (1985) Quantitation of
carcinogen-DNA adducts with monoclonal antibodies. Prog Exp Tumour
Res, 31: 63-75.
Seidegard J, Pero RW, Markowitz MM, Rousch G, Miller DG, & Beattle
EJ (1990) Isoenzyme(s) of glutathione transferase (class mu) as a
marker for the susceptibility to lung cancer: A follow-up study.
Carcinogenesis, 11: 33-36.
Seppalainen A-M, Hernberg S, & Kock B (1979) Relationship between
blood lead levels and nerve conduction velocities. Neurotoxicology,
1: 313-332.
Shuker DEG & Farmer PB (1992) Relevance of urinary DNA adducts as
markers of carcinogen exposure. Chem Res Toxicol, 5: 450-460.
Singer B, Chavez SJ, & Kusmierek JT (1987) The vinyl
chloride-derived nucleoside N2,3-ethenoguanosine, is a highly
efficient mutagen in transcription. Carcinogenesis, 8: 745-747.
Sorsa M, Ojajarvi A, & Salomaa S (1990) Cytogenetic surveillance of
workers exposed to genotoxic chemicals: preliminary experiences from
a prospective cancer study in a cytogenetic cohort. Teratog Carcinog
Mutagen, 10: 215-221.
Sorsa M, Wilbourn J, & Vanio H (1992) Human cytogenetic damage as a
predictor of cancer risk. In: Vanio H, Magee PN, McGregor D, &
McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 543-554 (IARC Scientific Publications No. 116).
Stone PJ, Bryan-Rhadfi J, & Lucey EC (1991) Measurement of urinary
desmosine by isotope dilution and high performance liquid
chromatography. Am Rev Respir Dis, 144: 284-290.
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology (1989) In: Dreborg S ed. Skin tests for
diagnosis of IgE-mediated allergy. Position paper. Allergy, 44(Suppl
10): 31-37.
Sullivan JB (1989) Immunological alterations and chemical exposure.
Clin Toxicol, 27: 311-343.
Swenberg JA, Fedtke N, Fennell TR, & Walker VE (1990) Relationships
between carcinogen exposure, DNA adducts and carcinogenesis. In:
Clayson DB, Munro IC, Shubik P, & Swenberg JA ed. Progress in
predictive toxicology. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 161-184.
Swift M, Morrel D, Massey RB, & Chase CL (1992) Incidence of cancer
in 161 families affected by ataxia-telangiectasia. New Engl J Med,
325: 1831-1836.
Townsend JC, Ott MG, & Fishbeck WA (1978) Health exam findings among
individuals occupationally exposed to benzene. J Occup Med, 20:
543-548.
Tritscher AM, Goldstein JA, Portier CJ, McCoy ZM, Clark GC, & Lucier
GW (1992) Dose-response relationships for chronic exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin in a rat tumour promotion model:
quantification and immunolocalization of CYP1A1 and CYP1A2 in the
liver. Cancer Res, 52: 3436-3442.
UK DH (1989) Committee on Mutagenicity of Chemicals in Food.
Guidelines for the testing of chemicals for mutagenicity - Consumer
Products and the Environment. London, Department of Health, Her
Majesty's Stationery Office (HMSO), 99 pp (Report No. 35).
UK HSE (1991) Guidance on laboratory techniques in occupational
medicine, 5th ed. London, Health and Safety Executive, Library and
Information Services, 117 pp.
US EPA (1991) Formaldehyde risk assessment update. Washington, DC,
US Environmental Protection Office, Office of Toxic Substances.
US EPA (1987) Assessment of health risks to garment workers and
certain home residents from exposure to formaldehyde. Washington,
DC, US Environmental Protection Office, Office of Toxic Substances.
US NRC (US National Research Council) (1989a) Biologic markers in
pulmonary toxicology. Washington, DC, National Academy Press, 179
pp.
US NRC (US National Research Council) (1989b) Biologic markers in
reproductive toxicology. Washington, DC, National Academy Press, 395
pp.
US NRC (US National Research Council) (1992a) Biologic markers in
immunotoxicology. Washington, DC, National Academy Press, 206 pp.
US NRC (US National Research Council) (1992) Environmental
neurotoxicology. Report of Committee on Neurotoxicology and Models
for Assessing Risk. Washington, DC, National Academy Press, 154 pp.
Wei Q, Matanoski GM, Farmer ER, Hedayati MA, & Grossman L (1993) DNA
repair and aging in basal cell carcinoma: a molecular epidemiology
study. Proc Natl Acad Sci (USA), 90: 1614-1618.
Weinberg RA (1991) Tumour suppressor genes. Science, 254: 1138-1146.
Weisburger JH & Williams GM (1981) Carcinogen testing: current
problems and new approaches. Science, 214: 401-407.
Weston A & Bowman ED (1991) Fluorescence detection of
benzo(a)pyrene-DNA adducts in human lung. Carcinogenesis, 12:
1445-1449.
WHO (1991) IPCS Environmental Health Criteria 119: Principles and
methods for the assessment of nephrotoxicity associated with
exposure to chemicals. Geneva, World Health Organization, 266 pp.
WHO (1992a) IPCS Environmental Health Criteria 134: Cadmium. Geneva,
World Health Organization, 280 pp.
WHO (1992b) IPCS Environmental Health Criteria 141: Quality
management for chemical safety testing. Geneva, World Health
Organization, 112 pp.
WHO (in press) Principles for assessment of risks from exposure to
chemicals (Part A).
Wild CP & Montesano R (1991) Immunological quantitation of human
exposure to aflatoxins and N-nitrosamines. In: Vanderlaan M, Stanker
LH, Watkins BE, & Roberts DW ed. Immunoassays for trace chemical
analysis. Washington, DC, American Chemical Society, pp 215-228 (ACS
Symposium Series No. 451).
Wild CP, Jiang YZ, Sabbioni G, Chapot B, & Montesano R (1990)
Evaluation of methods for quantitation of aflatoxin-albumin adducts
and their application to human exposure assessment. Cancer Res, 50:
245-251.
Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle
H, Montesano R, & Groopman JD (1992) Dietary intake of aflatoxins
and the level of albumin-bound aflatoxin in peripheral blood in The
Gambia, West Africa. Cancer Epidemiol Biomarkers Prev, 1: 229-234.
Wogan GN (1989) Markers of exposure to carcinogens: methods for
human monitoring. J Am Coll Toxicol, 8: 871-881.
Wogan GN & Gorelick NJ (1985) Chemical and biochemical dosimetry of
exposure to genotoxic chemicals. Environ Health Perspect, 62: 5-18.
Wolf CR, Dale Smith CA, Gough AC, Moss JE, Vallis KA, Howard G,
Carey FJ, Mills K, McNee W, Carmichael J, & Spurr NK (1992)
Relationship between the debrisoquine hydroxylase polymorphism and
cancer susceptibility. Carcinogenesis, 13: 1035-1038.
Yanagisawa Y, Nishimura H, Matsuki H, Osaka F, & Kasuga H (1986)
Personal exposure and health effect relationship for NO2 with
urinary hydroxyproline to creatinine ratio as indicator. Arch
Environ Health, 41: 41-48.
RESUME
Le groupe spécial a divisé les marqueurs biologiques en trois
catégories, les marqueurs d'exposition, les marqueurs d'effet et les
marqueurs de réceptivité, tout en admettant que bien souvent il
n'était pas possible d'établir une distinction nette entre ces
diverses catégories.
Le groupe spécial a admis que le recours aux marqueurs
biologiques pouvait améliorer l'évaluation des risques pour la santé
humaine découlant d'une exposition à des produits chimiques et qu'il
fallait donc en faire usage. Les marqueurs biologiques peuvent être
utilisés pour évaluer l'exposition et la dose interne chez des
individus et des groupes et peuvent faciliter l'identification de
ceux de ces individus ou de ces groupes qui sont plus ou moins
exposés aux risques que la moyenne.
Avant d'utiliser les marqueurs biologiques pour l'évaluation du
risque il faut les valider, c'est-à-dire établir la relation qui
existe entre le marqueur, l'exposition et ses conséquences pour la
santé. Le choix, la validation et l'utilisation de tout marqueur
biologique constituent des processus complexes qui varient d'un
marqueur à l'autre. Pour mettre en lumière ces notions et ces
principes on a choisi un certain nombre d'exemples.
La recherche et l'utilisation des marqueurs biologiques
soulèvent des questions complexes sur le plan éthique, social et
juridique, qui peuvent d'ailleurs varier d'un pays à l'autre. Il
peut s'en suivre un certain nombre de contraintes imposées à la
recherche et à l'utilisation des marqueurs biologiques dans
l'évaluation des risques et dans les décisions relatives à la
gestion de ces risques. Avant toute application il importe d'étudier
avec soin les aspects éthiques, sociaux et juridiques des marqueurs
biologiques.
RECOMMANDATIONS
En formulant les recommandations ci-après, le groupe de travail
a pris en considération le rôle dévolu au PISC, à savoir de
faciliter et de développer la coordination des activités
internationales afin d'encourager les travaux à poursuivre pour
définir les effets sur la santé humaine qu'entraîne l'exposition aux
substances chimiques et de jeter les bases des actions prioritaires
à entreprendre pour protéger la santé publique.
1. Généralités
* Encourager un plus large recours aux marqueurs biologiques dans
l'évaluation des risques
* Favoriser la coopération et la communication
inter-disciplinaires afin de faciliter l'application des
résultats de la recherche
* Etudier la faisabilité d'une banque de données sur les
marqueurs biologiques dans l'évaluation des risques et ses
applications
2. Recherche
* Mettre au point, affiner et valider des modèles qui permettent
de corréler les marqueurs biologiques de l'exposition et des
effets tant qualitativement que quantitativement, à
l'exposition et aux conséquences biologiques, en particulier
aux conséquences autres que le cancer.
* Recenser et valider des marqueurs biologiques de réceptivité,
par rapport à la réaction aux produits chimiques et aux
variations interindividuelles à cette réaction et étudier le
polymorphisme génétique en tant que base de l'hypersensibilité
individuelle.
* Voir dans quelle mesure il est possible d'utiliser certains
renseignements sur la réceptivité individuelle pour la
protection de la santé, dans le respect des impératifs
éthiques, sociaux et juridiques.
* Mettre au point des stratégies afin de relier l'exposition et
la dose interne aux conséquences biologiques pour l'homme
grâceà une synthèse de biomarqueurs de l'exposition, de l'effet
et de la réceptivité, mécanistiquement validés.
3. Applications
* Mettre au point un protocole pratique pour l'utilisation des
marqueurs biologiques dans les études sur l'homme, qui prenne
en considération les aspects scientifiques, émotionnels,
éthiques, juridiques et sociaux et qui comporte des directives
en matière de communication, en insistant sur le droit des
participants à disposer d'informations intelligibles et non
biaisées.
* Encourager la production de substances de référence homologuées
pour l'analyse des marqueurs biologiques et aider les
programmes internationaux d'assurance de la qualité à
fonctionner.
* Faire figurer des considérations sur les biomarqueurs
d'exposition, d'effet et de réceptivité dans les futures
monographies de la série Critères d'hygiène de l'environnement.
* Examiner s'il est nécessaire de mettre à jour à bref délai la
présente monographie consacrée aux principes et conceptions en
matière de marqueurs biologiques.
RESUMEN
El Grupo Especial distinguió tres clases de biomarcadores: de
exposición, de efecto y de susceptibilidad, reconociendo sin embargo
que a menudo resulta imposible establecer claramente la pertenencia
a una de esas clases.
El Grupo Especial coincidió en que los biomarcadores permiten
mejorar, y deben emplearse a ese efecto, el proceso de evaluación de
los riesgos que para la salud humana conlleva la exposición a
productos químicos. Los biomarcadores se pueden emplear para
calcular la exposición y la dosis interna recibida por individuos y
grupos, con la consiguiente identificación de quienes sufren un
mayor o menor riesgo que la media.
Los biomarcadores deben ser validados antes de aplicarlos a la
evaluación del riesgo, lo que significa que debe determinarse la
relación entre el biomarcador, la exposición y el estado de salud.
La selección, validación y empleo de cualquier biomarcador es un
proceso complicado, distinto para cada marcador. Se eligieron
algunos ejemplos para ilustrar los conceptos y principios
relacionados.
La investigación y el empleo de los biomarcadores plantea
complejas cuestiones éticas, sociales y jurídicas, que pueden
diferir de un país a otro. Algunos de esos problemas limitan el
alcance de las investigaciones sobre los biomarcadores y de su
aplicación a la evaluación de riesgos y la adopción de decisiones
relacionadas con la gestión de riesgos. Los problemas éticos,
sociales y jurídicos que plantean los biomarcadores deben ser objeto
de un detenido análisis antes de su eventual uso.
RECOMENDACIONES
Al formular las siguientes recomendaciones, el Grupo Especial
reconoció la función asignada al IPCS de facilitar e intensificar la
coordinación de las actividades internacionales con miras a fomentar
los trabajos que aún será necesario realizar para determinar los
efectos sobre la salud relacionados con la exposición a productos
químicos, así como para establecer las bases que permitan señalar
las prioridades a que haya que atenerse para proteger la salud
pública.
1. Recomendaciones generales
* Promover un mayor uso de los biomarcadores validados en el
proceso de evaluación de riesgos.
* Fomentar la cooperación y la comunicación interdisciplinarias
para facilitar la aplicación de los resultados de las
investigaciones.
* Estudiar la posibilidad de crear un banco de datos sobre
biomarcadores aplicados a la evaluación de riesgos y sus
posibles usos.
2. Investigaciones
* Desarrollar, perfeccionar y validar modelos aptos para
relacionar los biomarcadores de exposición y de efecto,
cualitativa y cuantitativamente, con la exposición y con el
estado de salud, sobre todo para puntos finales distintos del
cáncer.
* Identificar y validar biomarcadores de susceptibilidad en
relación con el producto químico y con la variación
interindividual de la respuesta, e investigar la influencia del
polimorfismo genético en la hipersensibilidad individual.
* Evaluar el uso de la información referente a la susceptibilidad
individual desde la perspectiva de una protección de la salud
atenta a los aspectos éticos, sociales y jurídicos.
* Formular estrategias para relacionar la exposición y la dosis
interna con el estado de salud mediante la integración de
biomarcadores de exposición, efecto y susceptibilidad validados
sistemáticamente.
3. Aplicaciones
* Elaborar un protocolo práctico para el uso de biomarcadores en
los estudios realizados en el hombre, teniendo en cuenta los
aspectos científicos, psicológicos, éticos, jurídicos y
sociales, con inclusión de directrices para la comunicación del
riesgo y haciendo hincapié en el derecho de los participantes a
una información inteligible e imparcial.
* Fomentar la producción de material de referencia certificado
para el análisis de biomarcadores y respaldar la aplicación de
programas internacionales de garantía de la calidad.
* Incluir la consideración de los biomarcadores de exposición,
efecto y susceptibilidad en las futuras monografías de la serie
Criterios de Salud Ambiental.
* Tener presente la necesidad de actualizar con prontitud la
presente monografía sobre los principios y nociones relativos a
los biomarcadores.
Thus by analysing several biomarkers with different half-lives
(e.g., haemoglobin adducts in the blood, metabolites of the chemical
in urine, parent compound in blood) from a single individual at a
single point in time, more information may be obtained about the
nature of the past exposure than from use of a single biomarker.
Also of value in the interpretation of biomarker data is the
use of mathematical models to describe the kinetics of formation and
elimination of biomarkers of exposure. Absorbed chemicals are
distributed between various compartments in the body, with the
distribution being dependent on the nature of the compartment and
the lipophilicity of the chemical. The most simple models use only
one compartment; however, multicompartmental models are usually
required to describe the disposition of most chemicals in the body
(Gibaldi & Perrier, 1982). Multicompartmental models that
incorporate biomarkers of exposure in relation to toxic end-points
are well established. A biokinetics model for lead has been used to
predict blood lead levels of individuals and communities (DeRosa et
al., 1991).
More recently, physiologically based toxicokinetic (PBTK)
models have been developed that make use of the physico-chemical
properties of a chemical (such as partition coefficients that
indicate how the chemical or its metabolites become partitioned
between different fluids in the body), the kinetics of metabolism of
the chemical (such as Vmax and KM for metabolic pathways), and
physiological parameters of the exposed individual (such as tissue
blood flow, respiratory minute volume, cardiac output) to predict
the actual concentrations of biomarkers that will occur after
specific exposure regimens. These models are adapted for humans by
changing the physiological and metabolic parameters to those
appropriate for humans, and by testing for validity in limited human
studies. These models can then be used to extrapolate between
different exposure situations for the predicted levels of markers
(Ramsay & Andersen, 1984; Droz et al., 1989).
Another use of models is in defining the quantitative
relationship between biomarkers in readily available biological
samples (e.g., blood cells) and in those less readily available
which might be more pertinent biomarkers for the health effect of
concern (e.g., tissue DNA). An example is the use of haemoglobin
adducts to predict the amount of DNA adducts at a critical site,
e.g., after exposure to ethylene oxide (Passingham et al., 1988). To
be able to make such predictions, one must know the kinetics of
formation and breakdown of each of the markers and the factors that
influence those kinetics. Based on such information a model can be
developed to show the quantitative relationships between the markers
under different exposure conditions or at different times after
exposure.
Biomarkers are used extensively in the surveillance of workers
occupationally exposed to metals such as lead, cadmium, mercury,
nickel, chromium and arsenic, and to organic chemicals such as
aniline, benzene, carbon disulfide, styrene, chlorobenzene and
chlorinated aliphatic hydrocarbon solvents (see Table 1). The
examples in Table 1 are given as general information. Before
applying them in specific circumstances, readers must consult the
original references.
These measures are used to indicate the absorbed dose. For a
few chemicals, notably lead, mercury, cadmium and carbon monoxide,
an approximate estimation of the associated health risk may be made.
For other chemicals, exposure may be assessed in quantitative terms.
It is noted that biomarkers are supplementary to environmental
measurements rather than alternative or substitute measures of
exposure.
Considerable efforts are being made to develop biomarkers
associated with exposure to chemical carcinogens and to establish
the relationship between a marker and the future health risk. The
use of animal models may facilitate this process; studies on the DNA
adducts formed by vinyl chloride illustrate the types of strategies
required to make each link (Swenberg et al., 1990). In rats, vinyl
chloride induces liver tumours with a high incidence in young
animals, and the DNA adducts (biomarkers) formed in the liver have
been characterized and the half-lives determined. DNA fidelity
replication assays were used to show one type of adduct that had
both a long half-life and was capable of inducing mutations (Hall et
al., 1981; Barbin et al., 1985; Singer et al., 1987; Swenberg et
al., 1990). The level of this adduct was much higher in the livers
of young rats exposed to vinyl chloride than in adults, and was
characterized as the one most closely related to the health effect.
To extend this animal research to predict human health risks, it
would be necessary to determine if the concentration of the same
adduct is associated with liver tumour formation in human tissue
samples.
Table 1. Some parameters proposed for biological monitoring by different organizations
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase E-acetylcholinesterase,
inhibitors P-cholinesterase
Aluminium (Al) U-Al U-Al
Aniline U-p-aminophenol,B-Met-Hb U-aniline U-p-aminophenol
Arsenic (As) U-certain volatile U-AsIII+, AsV+ U-AsIII+, AsV+
arsenic compounds MMA+DMA
produced by direct
hydrogenation
Benzene U-phenol B-benzene, U-phenol B-benzene Breath-, B-benzene
p-tert-Butylphenol U-p-tert-butylphenol
Cadmium (Cd) U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd U-Cd, B-Cd
Carbon disulfide U-TTCA U-TTCA U-TTCA U-TTCA
Carbon monoxide Breath-CO, B-COHb B-COHb B-COHb B-COHb
Chlorobenzene U-4-chlorocatechol U-4-chlorocatechol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Chlorophenols U-tri+tetrapenta-
chlorophenols
Chlorophenoxy acids U-2,4-D+Dichloroprop+
MCPA+Mecoprop
Chromium (Cr) U-Cr U-Cr U-Cr B-Cr, U-Cr
Cobalt (Co) U-Co U-Co U-Co
Dichloromethane B-COHb, B-dichloromethane B-COHb B-COHb, B-dichloromethane
Dieldrin P-dieldrin B-dieldrin
Dimethylformamide U-methylformamide U-methylformamide U-methylformamide
Ethylbenzene U-mandelic acid U-mandelic acid
2-Ethoxyethanol U-ethoxyacetic acid U-ethoxyacetic acid
2-Ethoxyethyl acetate U-ethoxyacetic acid U-ethoxyacetic acid
Ethylene oxide Breath-b, B-ethylene oxide
Fluoride (F) U-F U-F U-F U-F
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Furfural U-furoic acid U-furoic acid
Halothane U-trifluoroacetic acid
Hexachorobenzene P/S-hexachlorobenzene
n-Hexane U-hexanedione, U-hexanedione + U-hexanedione
Breath-n-hexanec dihydroxyhexanone
Hydrazine P-, U-hydrazine
Lead (Pb) B-Pb, U-Pb, B-ZPP B-Pb, U-ALA B-Pb, B-ZPP B-Pb, U-ALA, B-ZPP
Lindane B(P,S)-lindane B-lindane B-lindane
Manganese (Mn) U-Mn B-, U-Mn
Mercury (Hg) B-, U-Hg B-, U-Hg B-, U-Hg
Methanol U-methanol, U-formate U-methanol U-formate
Methylbromide B-Br
Methyl butyl ketone U-hexanedione +
dihyroxyhexanone
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Methylene U-MOCA
bis(2-chloroaniline)
(MOCA)
Methylene dianiline U-MDA
(MDA)
Methyl ethyl ketone U-MEK U-MEK
(MEK)
2-Methoxyethanol U-methoxyacetic acid
2-Methoxyethyl acetate U-methoxyacetic acid
Nickel (Ni) U-Ni U-Ni U-Ni
Nitrobenzene U-p-nitrophenol, B-MetHb
Parathion U-p-nitrophenol, U-p-nitrophenol,
E-cholinesterase E-cholinesterase
Pentachlorophenol U-PCP, P-PCP U-PCP, P-PCP U-PCP
(PCP)
Phenol U-phenol U-phenol
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Polychlorinated S-PCB B-PCB
biphenyls (PCB)
2-Propanol U-acetone, B-acetone
Selenium (Se) U-Se
Styrene U-mandelic acid, U-PGA U-mandelic acid, U-mandelic acid+PGA U-mandelic acid
U-mandelic acid+U-PGA
Thallium (Tl) U-Tl
Tetrachloroethylene Breath-, Breath-b, B-tetrachloroethylene
B-tetrachloroethylene B-tetrachloroethylene
Tetrachloromethane Breath-b,
B-tetrachloromethane
Tin (Sn) U-Sn
Toluene U-hippuric acid B-toluene B-toluene B-toluene
1,1,1-Trichloroethane Breath-1,1,1-trichloroethane, Breath-b, B-1,1,1-trichloroethane B-1,1,1-trichloroethane
B-trichloroethanol B-1,1,1-trichloroethane
Table 1 (contd)
Exposure Measured parametera
American Conference of Deutsche Finnish Institute of United Kingdom Health
Governmental Industrial Forschungsgemeinschaft Occupational Health and Safety Executive
Hygienists (ACGIH, 1992) (DFG, 1992) (FIOH, 1993) (UK HSE, 1991)
Trichloroethylene U-TCA, B-trichloroethanol, U-TCA, B-trichloroethanol U-TCA, U-Trichloroethanol U-TCA
U-TCA+trichloroethanol
Vanadium (V) U-V U-V
Vinyl chloride U-thiodiglycolic acid
Xylenes U-methylhippuric acids U-methylhippuric acids, B- U-methylhippuric acids U-methylhippuric acids
a The following abbreviations have been used: E = erythrocyte; P = plasma; S = serum; U = urine; B = blood; ZPP = erythrocyte zinc
protoporphyrin; ALA = delta-aminolevulinic acid; PGA = phenylglyoxylic acid; MMA = monomethylarsinic acid; DMA = dimethylarsinic acid;
TCA = trichloroacetic acid; MCPA = chloromethylphenoxyacetic acid; 2,4-D = 2,4 dichlorophenoxyacetic acid;
TTCA = 4-thio-4-thiazolidine carboxylic acid.
b DFG (1992) refers to alveolar air
c ACGIH (1992) refers to end-exhaled air
Note: Readers must consult original references before applying the above examples to specific situations
6. BIOMARKERS OF EFFECT
This section focuses on those human biomarkers that can be
applied currently or will be in the near future. Biomarkers of
effect may be used directly in hazard identification and
dose-response assessment components of the risk assessment process.
In hazard identification, biomarkers may facilitate screening and/or
identification of a toxic agent and characterization of the
associated toxicity. Biomarkers that are implicated in toxic
mechanism(s) are preferred for quantitative dose-response
assessments when extrapolating from existing data to a human
situation of concern (e.g., from high to low dose or from test
species to humans).
There are wide inter-individual variations in the response to
equivalent doses of chemicals. While the outcome of a chemical
insult in an individual may be predicted more accurately from
biomarkers of effect(s), such biomarkers may not be specific for a
single causative agent. Many biomarkers of effect are used in
everyday practice to assist in clinical diagnosis, but for
preventive purposes an ideal biomarker of effect is one that
measures change that is still reversible. Nevertheless, certain
biomarkers of nonreversible effects may still be very useful in
epidemiological studies or provide the opportunity for early
clinical intervention.
A limited range of tissues is available for routine analysis of
biomarkers. The more accessible tissues are therefore used as
surrogates for the known or putative target tissues. In some
instances biomarkers of effect are not mechanistically related to
chemically induced lesions, but may represent concomitant,
independent changes. Therefore, although an effect (e.g., sister
chromatid exchange) is being analysed, the use is conceptually close
to assessment of exposure.
6.1 Haematological biomarkers
Inhibition of the enzymes in the haem synthesis pathway (e.g.,
ferrochelatase, levulinate dehydratase) has been used as a marker of
effect of exposure to lead. This effect is reflected also in the
levels of free erythrocyte protoporphyrin (FEP) and
delta-aminolevulinate in the urine. Elevated levels of urinary
delta-amino-levulinate are observed at higher lead exposures than
changes in FEP, for example, while basophilic stippling of
erythrocytes is an even less sensitive biomarker for the effects of
lead. However, the effects on haem synthesis are not specific to
lead as a causative agent; iron deficiency has a similar effect on
FEP. The relationship of these effect biomarkers to toxicity
requires further elucidation.
Routine leucocyte, erythrocyte and thrombocyte counts have been
used in the surveillance of patients treated with cytostatic drugs
and in the monitoring of benzene-exposed workers. The predictive
power in relation to benzene-induced aplastic anaemia or leukaemia
is limited (Townsend et. al., 1978; Hancock et al., 1984; Lamm et
al., 1989). Ferrokinetic measurements, such as plasma iron
disappearance half-time, erythrocyte utilization of iron, plasma
iron transport rate, or erythrocyte iron turnover rate, have been
suggested as biomarkers of myelotoxicity (Rajamaki, 1984).
6.2 Nephrotoxicity biomarkers
Several different types of measures have been tested and used
as biomarkers of renal damage. These have been classified as
functional markers (e.g., serum creatinine and ß2-microglobulin),
urinary proteins of low or high molecular weight (e.g., albumin,
transferrin, retinol-binding globulin, rheumatoid factor,
immunoglobulin G), cytotoxicity markers (tubular antigens, e.g.,
BB50, BBA, HF5), enzymes (e.g., N-acetylglucosaminidase,
ß-galactosidase) in urine, and biochemical markers (eicosanoids,
e.g., 6-keto PGF2alpha, PGE2, PGF2alpha and TXB2, fibronectin,
kallikrein activity, sialic acid and glycosaminoglycans in urine,
and red blood cell negative charges) (Cardenas et al., 1993a,b;
Roels et al., 1993).
Biomarkers for nephrotoxicity were reviewed in WHO (1991) and
are well validated in relation to exposure to cadmium (WHO, 1992a;
Roels et al., 1993) but not in relation to exposure to mercury or
lead (Cardenas et al., 1993a,b).
6.3 Liver toxicity biomarkers
Effects of chemicals on the liver have been estimated
traditionally by measuring the activities of, for example,
aminotransferase (most often aspartate or alanine aminotransferase)
in the serum, where they are found when liver cells have been
damaged and have leaked their contents. Many other enzymes have also
been analysed for this purpose; they include 5-nucleotidase, alcohol
dehydrogenase, lactate dehydrogenase, isocitrate dehydrogenase,
leucine aminopeptidase, glutathione S-transferase, ornithine
carbamoyl transferase). Since tissues other than liver also contain
these enzymes, their activities may be elevated in serum not only
after liver damage but also when non-hepatic tissues have been
damaged. To overcome this lack of specificity, analysis of specific
isoenzymes has been used. Serum activities of enzymes such as
alkaline phosphatase and gamma-glutamyl transpeptidase may be used
as biomarkers of hepatic damage mainly involving biliary excretion.
Several liver function tests can also be used as biomarkers of
effects; these include the concentrations of serum proteins
synthesized in the liver, e.g., albumin and clotting factors, or
serum concentrations of bile acids, also synthesized in the liver,
as well as tests for the hepatic excretory function such as
bromsulfphthalein half-time. These parameters lack specificity since
hepatic viral infections, alcohol and drug use affect these enzymes.
Indirect measures of chemically induced change(s) in the cytochrome
P-450 enzyme system, using provocation tests, have been proposed as
sensitive indicators. However, the relationship to liver damage and
disease is not established and the requirement for the
administration of a drug limits the use of such tests.
Hepatotoxicity is caused by a number of chemicals that are
metabolized by the cytochrome P-450-dependent mixed-function oxidase
system to reactive intermediates. For example, carbon tetrachloride
has been studied extensively; it is metabolized to a reactive
intermediate which initially depletes intracellular glutathione to a
level that is no longer protective when the metabolite reacts with
critical macromolecules leading to cell death and hepatoxicity. In
this example, biomarkers of effect could include glutathione levels,
lipid peroxidation or the number of necrotic cells.
6.4 Biomarkers of immunotoxicity
The immune system protects the organism against infectious
microorganisms and the growth of at least some neoplasms. Reactions
of the immune system are influenced by genetic factors, age,
nutrition, life-style and health status. Xenobiotics may stimulate
or suppress the immune system. After initial sensitization, even a
minimal new exposure may lead to an anaphylactic reaction. The
immune system may be more sensitive to chemical challenge than any
other body system.
Hypersensitivity reactions following inhalation exposure
include asthma, rhinitis, pneumonitis and granulomatous pulmonary
reactions (see section 6.5). Hypersensitive dermal reactions induced
by chemicals include a wide variety of acute, subchronic and chronic
changes. Patch testing has been used traditionally as a biomarker
for identification of the causative agent of an allergic skin
reaction. However, the possibility of inducing hypersensitivity by
patch testing has been well documented and should not be overlooked
(Adams & Fisher, 1990).
Elevated levels of specific antibodies, usually of the IgE
type, may indicate existing sensitization. However, not all
individuals with elevated levels are symptomatic and not all
symptomatic individuals exhibit elevated IgE levels (Horak, 1985;
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology, 1989; Nielsen et al., 1992).
Suppression of the immune system increases susceptibility to
infections and neoplasia. Changes in the relative abundance of
different lymphocyte subpopulations (suppressor and helper T-cells)
have been used as biomarkers for the immune suppression (Jennings et
al., 1988; Sullivan, 1989; Holsapple, et al., 1991). Individuals
with asbestos-induced pleural or pulmonary changes, or
asbestos-induced cancer, as well as those heavily exposed to
asbestos but without apparent disease, have been reported to exhibit
an altered immunological status (e.g., reductions in T-lymphocyte
subsets) (Bekes et al., 1987).
In view of the growing incidence of hypersensitivity reactions
to chemicals, development and application of biomarkers for
immunotoxic effects is important. However, this is made difficult by
the current limited understanding of basic immunological mechanisms
and the effects of chemicals thereon (US NRC 1992).
6.5 Biomarkers of pulmonary toxicity
The most frequently used markers of pulmonary toxicity measure
gross effects on pulmonary function (e.g., peak expiratory flow,
forced expiratory volume, transfer factors) rather than effects on
cells or biochemical processes (US NRC, 1989a). These measures tend
to be nonspecific with respect to the causative agent and may
overlook effects specific to a certain cell type. Peak expiratory
flow measurements can be performed by the exposed individuals
themselves at the workplace, at home or elsewhere, and they provide
information on the underlying causes of air-way obstruction,
allowing a closer association between exposure, atmosphere and
response.
Air-way hyperactivity can be assessed by challenge tests using
inhalation exposure. Although such tests may assist in identifying
the factors causing hypersensitive pulmonary reactions, there is a
clear risk of acute reactions, and testing should be carried out by
qualified personnel in carefully controlled environments.
Recently, analysis of bronchoalveolar lavage fluid (BALF) has
been used to detect lung injury or to follow the progress of
pulmonary disease or the efficacy of therapeutic treatment
(Reynolds, 1987; Henderson, 1988).
The use of cellular elements as markers of pulmonary disease
state has been emphasized in human BALF analysis (Reynolds, 1987).
Total cell counts and differential counts, including use of
monoclonal antibody staining to distinguish T-cell subtypes, are
used to detect alveolitis and to aid in the diagnosis of
interstitial lung disease. High percentages of lymphocytes are
indicators of granulomatous processes, such as sarcoidosis, or
hypersensitivity pneumonitis. High percentages of neutrophils with
some eosinophils indicate possible idiopathic pulmonary fibrosis.
Other extracellular components, such as cytokines and other
mediators of inflammation, have been used on an experimental basis
to answer specific research questions.
The analysis of BALF has been used to define the dose-response
characteristics of inhaled or instilled toxins in animal toxicity
studies (Henderson, 1988). The most sensitive biomarker of an
inflammatory response in the bronchoalveolar region is the number of
neutrophils in BALF. The levels of protein and of extracellular
enzymatic activity are also useful markers of pulmonary toxicity.
Increases in protein concentrations in BALF indicate increased
permeability of the alveolar/capillary barrier. Lactate
dehydrogenase (LDH) is a cytoplasmic enzyme that is found
extracellularly only in the presence of lysed or damaged cells.
Beta-glucuronidase or similar lysosomal hydrolytic enzymes are
excellent markers for the toxicity of inhaled particles. These
particles are phagocytosed by macrophages, and the enzymes are
released from activated or lysed macrophages.
The secretion of cytokines from pulmonary macrophages obtained
by bronchoalveolar lavage provides markers of developing fibrosis.
Recent studies by Piguet et al. (1990) demonstrate that the level of
secretion of tumour necrosis factor (TNF) by pulmonary macrophages
is associated with quartz-induced fibrotic processes. Lassalle et
al. (1990) found elevated secretion of TNF by macrophages obtained
from individuals with coal-workers pneumoconioses compared with
macrophages from controls. The secretion of platelet-derived growth
factor from pulmonary macrophage was elevated in patients with
idiopathic pulmonary fibrosis (IPF).
Glutathione (GSH), a tripeptide protective against oxidative
stress, is present in BALF, and a decrease in GSH in BALF is a
potential marker for oxidative stress. Decreased levels of GSH have
been observed in patient with IPF (Cantin et al., 1989) and in
animals exposed chronically to diesel exhaust, resulting in
pulmonary fibrosis (Henderson, 1988).
Nasal lavage fluid (NLF) also provides markers of response to
inhaled toxins. The work of Graham et al. (1988) demonstrates the
potential use of NLF analysis to document the influx of neutrophils
into the nasal cavity in humans in response to inhaled ozone.
Biomarkers in blood related to lung injury have not been
validated. However, the work of Cavalleri et al. (1991) indicates
that serum aminoterminal propeptide of type III procollagen (PIIINP)
may become useful as an early marker for developing fibrosis. A
dose-dependant increase in serum PIIINP was found in individuals
exposed to low or high levels of asbestos.
Finally, urinary levels of amino acids associated with the
connective tissue of the lung (hydroxyproline, hydroxylysine,
desmosine and isodesmosine) have been used as markers of lung injury
(Harel et al., 1980; Yanagisawa et al., 1986; Stone et al., 1991).
However, such assays are not specific for lung injury and merely
indicate the breakdown of connective tissue in any organ in the
body.
6.6 Biomarkers of reproductive and developmental toxicity
Markers associated with an adverse outcome in reproduction may
reflect toxic effects in the male or female or be associated with
development during the embryonic, fetal, perinatal or neonatal
period (US NRC, 1989b; Mattison, 1991).
Biomarkers for the male reproductive system may include
physiological indicators of impaired testicular function, or sperm
number or characteristics (including cytogenetics). Measures of
hormonal status (i.e. FSH, LH and testosterone) can also be readily
obtained from blood and, in the case of testosterone, from urine and
saliva. However, these levels are greatly influenced by circadian
rhythms and demonstrate large inter- and intra-individual
variability. A clearer picture of hormonal status can be obtained by
administering GnRH or LH and examining the hormone response to these
challenges. Biomarkers for the male reproductive system are rather
easily accessible and some even reasonably well validated; such
markers are less well developed for the female reproductive system.
Biomarkers indicative of developmental toxicity should also be
considered. As is the case for many biological markers of effects,
it is often difficult to identify the causative agent in the absence
of any specific exposure history. Biomarkers could include
measurements of detrimental effects produced by chemical or other
exposures during embryonic or fetal stages of development.
Irreversible lesions can be embryolethal or result in functional
anomalies in the offspring. Examples of biomarkers of developmental
toxicity include low birth weight, chromosome anomalies, delayed
growth of specific organ systems, mental retardation, and subtle
behavioural changes. The changes associated with F1 male-mediated
abnormalities have been discussed by Anderson (1990). Some of these
biomarkers of developmental effects (malformations, mental
retardation) are not biomarkers of effect as far as the individual
is concerned, but rather represent the adverse health outcome
itself. However, from the point of view of the exposed population,
they may be considered as biomarkers since they show that within a
population a harmful exposure has taken place.
Several biomarkers have been proposed for use during pregnancy,
e.g., early pregnancy loss and assays for genetic defects of the
conceptus. The latter comprise both classical cytogenetic studies,
as well as specific DNA probes (US NRC, 1989b). The use of urinary
human chorionic gonadotrophin (HCG) has been well documented as a
biomarker for early fetal loss (US NRC, 1989b). Many different
biomarkers have been used to follow the development of the pregnancy
and the well-being of the conceptus, but they have not yet been
applied to studies of effects of chemicals on pregnancy.
6.7 Biomarkers of neurotoxicity
The functions of the nervous system are complex and biomarkers
may range from effects of chemicals on neural cellular and molecular
processes to neurophysiological and neuro-behavioural measurements
of complex functional entities.
Inhibition of plasma and erythrocyte acetylcholine esterase
(AchE) provides biomarkers of exposure to organophosphorus compounds
and other cholinesterase inhibitors. While erythrocyte
cholinesterase is similar to brain cholinesterase, and is therefore
an effect biomarker, plasma nonspecific pseudocholinesterase only
reflects exposure and is not a marker of CNS effects.
Measures of the function of the peripheral nervous system
(e.g., electroneuromyography, nerve conduction velocities, vibration
sensitivity) are well defined. Assessment of peripheral nervous
system dysfunction associated with exposure to chemicals can be
carried out using electroneuromyography at the preclinical stage
(Seppalainen et al., 1979).
Some well-established neurophysiological (e.g., evoked
potentials, electroencephalography) and neurobehavioural (e.g., the
WHO Neurobehavioural Core Test Battery, Cassito et al., 1990)
measures may be used as biomarkers to evaluate CNS dysfunction
induced by neurotoxicants. These tests must be carried out under
well-controlled conditions.
Methods for assessing changes in higher cognitive function
(e.g., learning and memory) have been used extensively, e.g., in
workers exposed to solvents or heavy metals, but require further
refinement.
Available neuroimaging procedures, e.g., computed axial
tomography (CAT), magnetic resonance imaging (MRI), nuclear magnetic
resonance spectroscopy (MRS) and positron-emission tomography (PET),
are considered non-invasive, but some of them require exposure to
ionizing radiation. CAT and MRI can be carried out with current
clinical techniques to assess chemically induced changes in the
brain. The use of MRS and PET can provide a more detailed evaluation
of the biochemical status (e.g., rate of energy generation, blood
flow, L-glucose metabolism) in the central nervous system. They can
be used as biomarkers for assessing exposure to neurotoxicants
inducing brain alterations. However, they are expensive, of little
use in assessing spinal cord, nerve and muscle changes, and there is
only minimal data validating their use in neurotoxicology (US NRC,
1992).
Other promising biomarkers for neurotoxicity in animal studies
include glial fibrillary acidic protein (localized in the
astrocytes), which increases in localized areas within the brain
where injury due to toxicants occurs (O'Callaghan, 1991).
7. BIOMARKERS AND CHEMICAL CARCINOGENESIS
As new information about the multistep process of
carcinogenesis unfolds, it is instructive to consider the various
mechanisms by which chemicals induce cancer. Knowledge of mechanisms
will enable the selection of appropriate biomarkers for use in risk
assessment of carcinogens. Some chemicals are direct acting and
others require metabolic activation. Once absorbed most chemicals
undergo enzyme-mediated reactions that either detoxify them or
activate them to reactive species. The balance between activating
and detoxifying enzyme systems governs the rate of delivery of
bioactive metabolites to the macromolecular site (Harris, 1991). The
resulting macromolecular interaction could be a DNA adduct for
carcinogens that are initiating agents or receptor occupancy for
chemicals that are tumour promotors. Certain of the DNA adducts
produced by such interactions are pro-mutagenic, and replication of
the damaged DNA could lead to DNA sequence changes which may result
in altered gene expression or mutated gene products. Weisburger &
Williams (1981) have suggested that chemical carcinogens be
classified as those that interact with DNA (genotoxic) and those
that do not (epigenetic or non-genotoxic). The importance of mitotic
activity in the latter group has recently been elaborated further
(Cohen & Ellwein, 1990).
The implications for invoking the "mechanistic" approach to the
selection of appropriate biomarkers are significant. For example,
chemicals that stimulate cell proliferation via mitogenesis or
cytotoxicity (and subsequent proliferation) might require different
biomarkers than chemicals whose major mechanism of action is based
on DNA reactivity. In the latter case, measurements of DNA adducts
or chromosome alterations may serve as suitable biomarkers, whereas
in the former case alternate biomarkers (e.g., cell turnover
measurements) may be more appropriate.
7.1 Analysis of chemicals and metabolites
Urinary or blood concentrations of several chemicals shown or
suspected to be carcinogenic to humans (e.g., arsenic, cadmium,
chromium, nickel, benzene, MOCA, polychlorinated biphenyls, styrene,
tetrachloroethylene) have for long been used as biomarkers of
exposure. Among people exposed to arsenic in a copper smelter, a
dose relationship has been observed between the cumulative urinary
excretion of arsenic and the risk of lung cancer (Enterline & Marsh,
1982). For other carcinogenic chemicals, such data are not
available, and the measured concentrations may only be interpreted
in terms of exposure.
Sensitive techniques based on physicochemical or immunochemical
methods for the detection of a variety of carcinogen-modified DNA
bases have been developed (Shuker & Farmer, 1992). These include the
alkylated purines, aflatoxin-guanine adducts, cis-platinum adducts,
thymine glycol, 8-hydroxydeoxyguanosine and PAH-derived adducts.
The aflatoxin marker has been extensively used in both animal
and human studies on the relationship between exposure and liver
cancer induction. In an ongoing prospective study in Shanghai,
China, Ross et al. (1992) reported that subjects with liver cancer
were more likely than controls to have detectable concentrations of
any of the known aflatoxin metabolites in their urine. Groopman et
al. (1991) recently explored the relationship between dietary
aflatoxin and excretion in the urine of aflatoxin metabolites and an
aflatoxin-DNA adduct. This study was conducted on people living in
the Guangxi Autonomous Region, China. These investigators found a
positive correlation between aflatoxin N7-guanine and specific
metabolites excreted in urine and aflatoxin B1 intake from the
previous day.
Exposure to chemical compounds capable of interacting with
cellular macromolecules can originate from both exogenous and
endogenous sources. Nitrite, nitrate and nitrosating agents can be
synthesized endogenously in reactions mediated by bacteria and
activated macrophage. In this way endogenous formation of
N-nitroso compounds can occur at various sites in the body.
Endogenously formed N-nitroso compounds may be considered as
biomarkers of susceptibility; they have been associated in humans
with increased risk of cancer of the stomach, oesophagus and urinary
bladder, although unequivocal epidemiological data are lacking
(Bartsch & Montesano, 1984). The quantitative estimation of
endogenous nitrosation in humans can be measured using the
N-nitroso-proline test. L-proline is utilized as a probe for
nitrosatable amines and N-nitroso-proline excreted in the urine is
determined as a marker. This assay has been applied in some
population studies (Bartsch et al. 1991).
7.2 Biomarkers for genotoxic carcinogens
7.2.1 DNA adducts - general considerations
DNA adducts are being used both as molecular dosimeters
(biomarker of exposure) and to assess the genotoxic potential of
chemicals (biomarker of effect). The biological significance of such
adducts must be assessed on the basis of adduct heterogeneity and of
cell and tissue specificity for adduct formation, persistence and
repair. Some DNA adducts result in mutation whereas others do not.
Mutational specificity in the p53 gene produced by a variety of
chemical carcinogens provides evidence that DNA adduct location
influences site-specific mutations (Hollstein et al., 1991). Some
DNA sequence changes may lead to phenotypic alterations that can be
selected, whereas others may not (Compton et al., 1991).
Most tissues are comprised of multiple cell types, and cell
types vary considerably in their capacity to convert chemicals to
DNA reactive species. For example, lung is composed of multiple cell
types in which the relative concentrations of various P-450
isoenzymes and enzymes depends on the cell type. Thus, one compound
may produce high concentrations of pro-mutagenic adducts in one cell
type, but not in another, whereas the opposite might occur for a
compound which is activated by a different P-450 isozyme. DNA adduct
concentrations derived from a whole tissue homogenate may grossly
overestimate or underestimate adduct concentrations in a given cell
type.
Some DNA adducts are repaired quickly, others hardly at all,
the adduct loss correlating with cell turnover. Therefore, the
concentration and gene location of DNA adducts will change with time
after exposure to a genotoxic chemical. Furthermore, the existence
of non-random repair in the genome makes it difficult to utilize
total DNA repair capacity as an indicator of cell susceptibility to
carcinogens (Hanawalt, 1987). It is especially important in human
studies to know the duration and timing of exposure for proper
evaluation of the biological significance of a given adduct
concentration.
Many of the human studies described below have involved
measuring metabolism, DNA adduct formation and repair in whole
tissues. Techniques need to be refined in order that
cell-type-specific variations can be monitored in human tissues, as
well as experimental studies in animal models using
immunohistochemical techniques for the cell type. Specific
localization of DNA adducts has clearly demonstrated that such
variations occur. Treatment of rats with the tobacco-specific
nitrosamine, 4-( N-methyl- N-nitrosamine)-1-(3-pyridyl)-1-butanone
(NNK), results in the induction of tumours in the nasal cavity,
lung, liver and pancreas (Hoffman et al., 1984; Rivenson et al.,
1988). At low doses of NNK, the prevalence of malignant lung tumours
was higher than that observed in other tissues. Cell-type-specific
differences have been observed within the lung, the highest
concentration of O6-methylguanine having been found in the Clara
cells. These cells have the highest levels of the P-450 metabolizing
enzymes for NNK and low levels of the O6-methylguanine DNA
methyltransferase repair enzymes (Belinsky et al., 1987). Pulmonary
tumours are also induced in mice and hamsters following either
short- or long-term exposure to this carcinogen (Hecht et al.,
1983).
As animals age, DNA adducts are detected in increasing amounts,
and, although the relationship of these adducts to tumour
development is unclear, they are believed to be derived from dietary
constituents or endogenous chemicals such as hormones (Randerath &
Randerath, 1991).
7.2.2 DNA adducts in human samples
In human studies, it is difficult to obtain non-tumourous
target tissue for the quantification of DNA adducts. Lymphocytes are
a readily accessible source of human cells that are known to contain
DNA adducts. However, there is little information on the reliability
of using lymphocyte adduct concentrations for the estimation of
target cell or tissue adduct concentrations (Lucier & Thompson,
1987).
Evaluation of dose-response relationships for chemical
carcinogens in humans is more complex than in animal models.
Radio-labelled carcinogens cannot be administered and the
accessibility of tissues and cells is limited. Several approaches to
detect DNA adducts in human samples have been evaluated (Wogan &
Gorelick, 1985; Santella, 1988). The most frequently used methods
are immunoassays and 32P-postlabelling. Other analytical
techniques such as GC-MS and synchronous fluorescence spectroscopy
are being used to measure DNA adducts (Weston & Bowman, 1991). In
general, immunoassays are both specific and sensitive for alkylated
adducts and aflatoxin adducts (Wild & Montesano, 1991; Groopman et
al., 1991). However, these methods are not easily applied to
quantification of adducts for bulky aromatic hydrocarbons such as
benzo [a]pyrene-derived adducts. The main problem is the lack of
specificity of the antibodies used in the assay which cross-react
with a number of PAH-related adducts (Santella et al., 1985).
The second assay frequently used to quantify DNA adducts in
humans is the 32P postlabelling technique. For a complete
description of this assay, see Randerath & Randerath (1991), Beach &
Gupta (1992), IARC (1992). The assay is extraordinarily sensitive,
being capable of detecting 1 adduct in 1010 normal nucleotides
when appropriate modifications are made to the procedure. The assay
is particularly useful for detecting adducts of non-polar polycyclic
aromatic hydrocarbons such as 7,8-diol-9,10-oxide-benzo [a]pyrene
deoxyguanosine (BPDE). Some DNA modifications such as alkylated DNA
adducts, which cannot be easily detected by this assay due to the
limitations of the chromatographic systems, can be quantified using
a combined 32P immunochemical precipitation technique (Kang et
al., 1993). Studies using the 32P labelling or immunological
methods have been reviewed by Beach & Gupta (1992) and Wild &
Montesano (1991). The groups of chemicals studied include alkylating
agents, polycyclic aromatic hydrocarbons (PAHs), heterocyclic PAHs,
nitro PAHs, cyclopenta-fused PAHs, aromatic amines, alkylbenzenes,
quinones, mycotoxins, chemotherapeutic agents, pesticides and
aldehydes.
7.2.3 Protein adducts
Ehrenberg and his associates pioneered the use of protein
adducts as dose monitors for carcinogen exposure in humans, and this
work has been reviewed by Hsia (1991). To date the class of proteins
that have been most extensively studied are those found in
circulating blood, i.e. haemoglobin and albumin. This is mainly
because these proteins are relatively abundant and can be easily
isolated for analysis.
Haemoglobin has the unique biological property of having a life
span equivalent to that of the erythrocyte, which in humans is
approximately 120 days, and therefore adduct levels reflect
exposures over several months. In contrast, albumin adducts can only
be used for assessing recent exposure because of the faster turnover
of albumin (half-life of 20-25 days). Protein adducts can be
quantified using chemical methods, e.g., aromatic amine release by
acid or basic hydrolysis from haemoglobin followed by derivatization
and GC-MS analysis (Farmer, 1991), or immunological techniques
(e.g., aflatoxin-albumin adducts, Wild et al., 1990).
During the past few years, several human monitoring studies
have demonstrated the usefulness of protein adducts as biomarkers of
exposure. Examples of chemicals that have been detected as protein
adducts in human studies include ethylene and propylene oxide,
aniline, cigarette smoke, aromatic amines such as 4-amino-biphenyl,
and aflatoxin (Wogan, 1989; Farmer, 1991). Albumin adducts of
aflatoxin B1 have also been used in epidemiological studies of
their role in the etiology of hepatocellular carcinoma in man. A
significant correlation was observed, at the individual level,
between dietary intake and the level of albumin-bound aflatoxin in a
chronically exposed population in the Gambia (Wild et al., 1992).
7.2.4 Cytogenetic methods
Cytogenetic methods are used as biomarkers of exposure to
DNA-damaging agents.
Many studies relating to cytogenetic changes in exposed human
populations have been reviewed in a special issue of Mutation
Research (Anderson, 1988). A second comprehensive review of more
recent studies has also been published (Anderson, 1990), and a
further review is in press (Anderson, in press).
All human monitoring studies suffer from variability of
baseline frequencies (Carrano & Natarajan, 1988) due to the presence
of endogenous (gender, age, medical history, etc.) and exogenous
factors (life-style, smoking, drinking, eating habits, etc.).
Anderson et al. (1991) have investigated the effect of these changes
on baseline variability eight times over a two-year period.
In contrast to studies on radiation, for which a marker
(dicentric chromosome) has been identified, studies with chemicals
have not yet identified a specific marker chromosome. For radiation
a dicentric is a quantitative dosimeter. Therefore, after radiation
exposure results can be used on an individual basis and a highly
exposed individual removed from the radiation source. Results from
chemical exposure studies, however, can only be used on a group
basis, due to the lack of a specific marker chromosome.
7.2.5 Chromosome damage
Both chromosome and chromatid aberrations are induced in
individuals exposed to chemical mutagens. The chromosome aberrations
are thought to arise from misrepair of lesions in the G0 stage of
circulating lymphocytes as well as from precursor cells in bone
marrow and thymus (Carrano & Natarajan, 1988). Chromatid aberrations
include chromatid breaks, intrachanges and exchanges, while
chromosome aberrations include acentric fragments, dicentric
chromosomes and ring chromosomes. Balanced translocation and
inversions can also arise and are difficult to quantify without
banding analysis. Structural aberrations can be classified as
unstable and stable depending on their ability to persist in
dividing cell populations. Unstable aberrations consist of rings,
acentric fragments and other asymmetrical rearrangements, and will
lead to the death of the cell. Stable aberrations consist of
balanced translocation inversions and other symmetrical
rearrangements which can be transmitted to progeny cells at
division. Therefore stable aberrations are more biologically
significant than unstable ones and could be involved in the cancer
process. Many human carcinogens have been shown to produce
chromosome damage in populations exposed to them, although no causal
relationship has been demonstrated (Sorsa et al., 1992). Proven
human carcinogens for which cytogenetic endpoints have been measured
in humans and corresponding animal data are available are listed in
Table 2.
In a preliminary report of a prospective study among people
whose lymphocytes were assayed for chromosome aberrations and SCE,
high rates of chromosome aberrations were observed and appeared to
be linked to cancer risk, but the finding was of borderline
statistical significance (Sorsa et al., 1990).
7.2.6 Sister chromatid exchange
Sister chromatid exchange (SCE) is considered to be a more
sensitive, rapid and simple cytogenetic end-point than chromosome
aberrations for evaluating the genotoxic potential of a variety of
mutagenic and carcinogenic agents. It is also used to detect and
differentiate many chromosome fragility diseases that predispose to
neoplasia. SCE is a DNA-replication-dependent phenomenon. Cellular
Table 2. Proven human carcinogens for which cytogenetic end-points have been measured in humans
and corresponding data are available for experimental animalsa
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Human carcinogens (Group 1)
Alcoholic beverages + + _ + ?
Aluminium production _ _
Arsenic and arsenic compounds ? ? + +
Asbestos ? _ _
Azathioprine ? _ + _ +
Benzene + + + +
Betel quid with tobacco + + +
Bis(chloromethyl)ether and (+) _
chloromethyl methyl ether
(technical grade)
1,4-Butanediol dimethanesulfonate (+) + + +
(Myleran)
Chlorambucil ? + +
Cyclosporin (+) _ _
Coal-tars +
Coke production +
Combined oral contraceptives _ _
Cyclophosphamide + + + + +
Hexavalent chromium compounds + + + + +
Melphalan + + +
8-Methoxypsoralen plus _ _ +
ultraviolet A radiation
Mineral oils, untreated and +
mildly treated
Nickel compounds + _ ? _
Table 2 (contd)
Agent/exposure Cytogenetic findingsa
Humans Animals
CA SCE MN CA SCE MN
Painter, occupational exposures as _
Radon + _
Rubber industry ? ?
Tobacco products, smokeless + +
Tobacco smoke + + + +
Tris(1-aziridinyl)phosphine sulfide (+) + + +
(Thiotepa)
Vinyl chloride + ? + + +
a From: Sorsa et al. (1992); CA = chromosome aberrations; SCE = sister chromatid exchange;
MN = micronuclei + = positive result; - = negative result; (+) = equivocal result;
? = doubtful result
factors such as nucleotide pools, repair and replication enzymes,
and biorhythms can play an important role in its formation. A major
source of variation can be attributed to the concentration of
bromodeoxyuridine relative to the number of lymphocytes in the
culture (Das, 1988; Morris, 1991; Morris et al., 1992). In a
prospective cancer study (Sorsa et al., 1990), no relationship was
observed between the frequency of SCE and the risk of cancer.
7.2.7 Micronuclei
Micronuclei are formed by condensation of acentric chromosomal
fragments or by whole chromosomes that are left behind during
anaphase movements (lagging chromosomes). The presence of
micronuclei can therefore be taken as an indication of the previous
existence of chromosomal aberrations. To visualize micronuclei,
cells have to undergo mitosis. In peripheral lymphocyte cultures it
is not easy to distinguish interface nuclei that have undergone a
division from those that have not. This makes it difficult to
quantify the frequencies of micronuclei for comparative purposes. A
method using cytochalasin B can distinguish nuclei that have divided
once (French & Morley, 1985).
7.2.8 Aneuploidy
Aneuploidy is a condition in which the number of chromosomes in
cells of individuals is not an exact multiple of the typical haploid
set for the species. Trisomy results when a single extra chromosome
is added to a pair of homologous chromosomes. If one chromosome of a
pair is missing, the result is monosomy. Absence of the pair is
nullisomy. Two or more copies of a homologue result in tetrasomy or
polysomy. Cells of individuals with missing or extra chromosomes are
hypoploid or hyperploid (UK DH, 1989). The best-known numerical
abnormalities result in the syndromes of Down (trisomy of chromosome
21), Klinefelter (sex chromosome genotype is XXY), and Turner (sex
chromosome genotype is X0). Aneuploidy is almost always found in
human cancers (Dellarco et al., 1985).
7.2.9 Mutation
Current somatic gene mutation assays used as biomarkers in
human studies select for a change or loss of a normal protein
produced by specific genes. Mutations at the X-linked hypoxanthine
guanine phosphoribosyl transferase gene in cloned T-lymphocytes and
in the autosomal locus for human leucocyte antigen-A (HLA-A) have
provided information on frequency of mutation and molecular spectra
of mutants. Detection of haemoglobin variants and loss of the cell
surface glycoprotein glycophorin A measured in red blood cells have
such limitations that analysis of the DNA mutations induced cannot
be made (Compton et al., 1991). The background frequency of each of
these specific locus assays varies greatly (Lambert, 1992) and is
dependent on numerous confounding factors (e.g., age and smoking).
Information on the mutation spectra at a particular locus will be
extremely useful in elucidating the mechanisms by which mutations
occur in human cells in vivo. By comparing spontaneous and
chemically induced mutational spectra in different populations, the
etiological contributions of both exogenous and endogenous factors
to human carcinogenesis could be assessed.
An alternative approach for the measurement of induced base
changes which does not require prior selection of a mutant
population uses the restriction site mutation technique. This is
based on the detection of DNA sequences resistant to the cutting
action of specific restriction enzymes, and the amplification of
these resistant sequences using the polymerase chain reaction. It
may theoretically be applied to the study of DNA base changes in any
gene for which the sequence has been determined (Parry et al.,
1990).
More relevant biomarkers for chemically induced cancers would,
however, preferably measure changes in genes thought to be important
for cancer. Mutations that activate proto-oncogenes, which stimulate
growth or inactivate suppressor genes to liberate cells from growth
constraints, could lead to unregulated proliferation of cancer cells
(Weinberg, 1991). For the most part, mutations in oncogenes and
tumour suppressor genes have been characterized in tumour tissue. It
remains to be determined whether the detection of mutant cells
against a background of normal cells can be achieved prior to
clinical diagnosis of cancer. Activated oncogenes have already been
identified in many human cancers, and considerable progress has been
made in elucidating the potential role of chemical carcinogens in
the activation of oncogenes and the contribution of the latter to
tumorigenesis in animal models (Balmain & Brown, 1988). Brandt-Rauf
(1991) presented data from pilot studies that demonstrated the
presence of the p21 protein product of the ras oncogene in the serum
of 15 out of 18 lung cancer patients who were all current or former
smokers. The protein was not found in the serum of any of the 18
healthy non-smoking controls, but was present in 2 out of 8
clinically healthy smoking controls. However, in another study, p21
protein was not detected in 20 smokers in a normal population or in
20 male healthy non-smokers (Brinkworth et al., 1992). The
mutational spectrum for the tumour suppressor gene p53 in human
tumours has been reviewed by Hollstein et al. (1991). Mutations of
the p53 gene are the most common cancer-related genetic changes at
the gene level and are widespread over the conserved codons of the
p53 gene. Hence, mutational spectra could be compared for tumours at
different sites and arising from different etiological backgrounds.
The mutational spectrum appears to differ among cancers of the
colon, lung, oesophagus, breast, liver, brain, reticuloendothelial
tissues and haemopoietic tissues. In two populations where aflatoxin
B1 exposure was implicated as one of the etiological factors in
hepatocellular carcinomas, the same mutational hotspot (i.e. G-T
transversion at codon 249) in the p53 gene has been identified (Hsu
et al., 1991).
7.3 Biomarkers for non-genotoxic carcinogenesis
Although only a few non-genotoxic human carcinogens have been
recognized (e.g., cyclosporin, diethylstilbestrol and estrogenic
hormones), many non-genotoxic carcinogens have been identified in
rodents. A compilation of NTP rodent data, designed to test the
concordance between short-term tests and in vivo carcinogenicity
assays, showed that more than 30% of rodent carcinogens do not test
positively for genotoxicity (Ashby & Tennant, 1991).
The mechanisms of action for non-genotoxic carcinogens need to
be considered in predicting human risk from chemical exposures.
Although the modes of action of non-genotoxic carcinogens are poorly
understood, several have been proposed, including immunosuppression,
hormonal effects, promotion, inorganic carcinogenesis,
co-carcinogenic effects and solid-state carcinogenesis (Weisburger &
Williams, 1981). Recently some of these mechanisms were grouped
under the headings of cytotoxicity and mitogenic growth stimulation
(Butterworth et al., 1992). Non-genotoxic carcinogens are believed
to exert their carcinogenic effects through mechanisms that do not
involve direct binding of the chemical or its metabolites to DNA (UK
DH, 1989). The key mechanism of non-genotoxic chemicals is to
increase cell proliferation, either by mitogenesis of the target
cells or by cytotoxicity, which is followed by regenerative cell
proliferation (Ramel, 1992). Cohen & Ellwein (1990) suggested that
non-genotoxic chemicals can be further categorized as to whether or
not their main mechanism of action is mediated via receptor-binding
(e.g., dioxin).
Cell replication and proliferation are potential biomarkers of
effect. Cell replication is the production of daughter cells by the
process of replicative DNA synthesis, while cell proliferation is
the enhanced replication of a selected cell population as observed
in regenerating tissues. Cells undergoing replicative DNA synthesis
(S-phase) are the most commonly used markers. The detection of cell
proliferation involves the incorporation of DNA precursors like
3H-thymidine or the base analogue 5-bromo-2'-deoxyuridine (BrdU)
into cellular DNA during S-phase. This is accomplished by
administering these precursors to animals by injection or through
implanted osmotic pumps. These S-phase cells can be identified
histoautotoradiographically or immunohistochemically (Goldsworthy et
al., 1991). The invasiveness of these techniques currently limits
their use to animal studies.
The identification of biomarkers of effect for non-genotoxic
carcinogens, whose major mechanism of action is via receptor
occupancy, may be difficult. This is primarily because carcinogens
of this type activate a variety of genes, some of which may not be
involved in the carcinogenic process. However, a good example of a
non-genotoxic carcinogen for which there is a good biomarker of
effect is 2,3,7,8-tetrachlorinated dibenzo- p-dioxin (TCDD). TCDD
interacts with a cytosolic receptor that is specific for it and its
structural analogues (Poland et al., 1976). In addition to
activating a number of growth factor and growth factor receptor
genes, TCDD induces a number of enzymes, one of which is cytochrome
P-450 1A1. Although the induction of this enzyme is probably not
directly related to the biological mechanism leading to cancer from
TCDD exposure, it is a sensitive marker for exposure (Tritscher et
al., 1992).
8. BIOMARKERS OF SUSCEPTIBILITY
This chapter focuses on the genetic predisposition of an
individual as it affects susceptibility to chemical materials. There
are a number of external factors, such as age, diet and health
status, that can also influence the susceptibility of an individual
exposed to chemicals. Some discussion will be directed towards the
effects of previous exposure on subsequent susceptibility, such as
to sensitization and enzyme induction/inhibition by previous
exposure. Table 3 lists some genetic and acquired factors affecting
susceptibility (Calabrese, 1986).
Although individuals may experience similar environmental
exposures, genetic differences in metabolism may produce markedly
different doses at the target site and thus a different level of
response. Even when target doses are similar, markedly different
responses may be noted in individuals due to varying degrees of
inherent biological responsiveness. Biomarkers of susceptibility may
reflect the acquired or genetic factors that influence the response
to exposure. These are pre-existing factors and are independent of
the exposure. They are predominantly genetic in origin, although
disease, physiological changes, medication and exposure to other
environmental agents may also alter individual susceptibility.
Biomarkers of susceptibility identify those individuals in a
population who have an acquired or genetic difference in
susceptibility to the effects of chemical exposure.
Biomarkers of susceptibility indicate which factors may
increase or decrease an individual's risk of developing a toxic
response following exposure to an environmental agent. Polymorphism
is present for some metabolic activation/deactivation enzymes,
including cytochrome P-450 isozymes (Nebert, 1988a, 1988b) and at
least one form of glutathione transferase (Seidegard et al., 1990).
Differing rates of enzyme activity controlling the activation or
detoxification of xenobiotics lead to differences in susceptibility
by increasing or decreasing the biologically effective dose of the
environmental agent.
The effect may vary between ethnic groups. For instance, there
are approximately equal numbers of fast and slow acetylator
phenotypes in a Caucasian population, whereas in a Japanese
population the distribution is 10% slow acetylators and 90% fast.
Genetic polymorphisms for drug metabolism have been widely studied
using phenotypic assays which involves measuring drug clearance in
individuals. Differential rates of metabolism will affect the
distribution and persistence of metabolites, which may have
implications for the site of toxicity. Epidemiological studies
suggest that, with respect to aromatic amines, slow acetylators are
more likely to contract bladder cancer but are at decreased risk
for colo-rectal cancer (Guengerich, 1991; Kadlubar et al., 1992).
Table 3. Some examples of established and suspected biomarkers of susceptibilitya
Biomarker of susceptibility Environmental agent Disease
Genetic
Debrisoquine hydroxylation phenotype cigarette smoke lung cancer
Acetylator phenotype aflatoxin, liver cancer,
aromatic amines bladder cancer
Ataxia telangiectasia genotype bleomycin, epoxides cancer at various sites
Xeroderma pigmentosum genotype agents that cause oxidative damage to skin cancer,
DNA, PAH, aromatic amines and aflatoxin B1 other cancers
Arylhydrocarbon hydroxylase inducibility polycyclic aromatic hydrocarbons lung cancer
alpha-1-antitrypsin cigarette smoke pulmonary emphysema
Franconi's anaemia phenotype cross-linking agents acute leukaemia
Glucose-6P-dehydrogenese deficiency phenotype oxidative agents, aromatic amines, poor resistance to oxidative
nitro-aromatic compounds stress,
aromatic amines
Sickle cell phenotype aromatic amino and nitro compounds, anaemia
carbon monoxide, cyanide
Thalassemia phenotype lead, benzene anaemia
Erythrocyte porphyria chloroquine, hexachlorobenzene, lead, anaemia
various drugs including barbituates,
sulfonamides, others
Sulfite oxidase deficiency heterozygotes sulfite, bisulfite, sulfur dioxide pulmonary disease
Alcohol dehydrogenase variant metabolize alcohols (e.g., ethanol)
more quickly than normal
GSTµ phenotype cigarette smoke lung cancer
Pseudocholinesterase variants organophosphate and carbamate insecticides, neurotoxicity
muscle relaxant drugs
IgA deficiency respiratory irritants irritation of respiratory tract
phenyl ketones in urine precursors of phenyl ketones phenylketonuria
Table 3 (contd)
Biomarker of susceptibility Environmental agent Disease
Acquired
Deficient diet chemical decreased resistance to
effects of many chemicals
Induced P-45O IIE1 alcohol consumption cancer at various sites
Antigen-specific antibodies chemicals, dusts decreased pulmonary
functions, skin rashes
a Modified from Calabrese (1986)
Polymorphism of N-oxidation has been linked to susceptibility to
colonic cancer (Kadlubar et al., 1992) and polymorphism in
glutathione S-transferase to increased lung cancer, particularly
adenocarcinoma (Seidegard et al., 1990).
The methodology for determining the phenotypes of individuals
for polymorphisms in metabolizing genes requires the administration
of a relevant test drug to the person and the subsequent measurement
of its clearance from the body. More recently, techniques based on
polymerase chain reactions, using DNA isolated from lymphocytes and
other cells, have been developed which allow the detection of
genetypes of known polymorphisms involving a variety of
xenobiotic-metabolizing enzymes, including GST1
(gluthione- S-transferase µ) and NAT2( N-acetyl transferase), as
well as two cytochrome P-450 isoenzymes: CYP1A1 and CYP2D6 (Bell,
1991; Blum et al., 1991; Wolf et al., 1992; Hirvonen et al., 1992;
Hollstein et al., 1992).
Cigarette smoking provides another example that illustrates the
effect of genetic polymorphisms on the response to chemicals.
Cigarette smoking is associated with the development of lung cancer
but not all smokers get lung cancer. This appears to be due in part
to genetic variations in arylhydrocarbon hydroxylase activity, which
results in a large variability in the binding of benz [a]pyrene to
DNA in cigarette smokers. A genetically based low level of
alpha-1-anti-trypsin activity greatly increases the risk of
emphysema from cigarette smoking.
In some situations a genetic trait may make an individual more
susceptible to one environmental agent but less so to another. For
example, the sickle cell trait predisposes to anaemia and altitude
sickness but offers some protection to the individual from infection
by the malaria parasite. Inherent differences in susceptibility
depend upon variations in the function of genes controlling enzyme
activity or the production of other proteins. Although a genotoxic
agent may reach the target tissue, the significance of any
chromosomal break will depend on the efficiency of the DNA repair
mechanisms. In xeroderma pigmentosum the individual is at an
increased risk of skin cancer after exposure to UV light because of
an inherited defect in DNA repair proteins (Cleaver, 1969).
Heterozygotes also have an increased risk of cancer and so the
frequency of the gene may affect the incidence of this cancer. Other
inherited diseases (e.g., ataxia-telangiectasia) that affect the
efficiency of DNA replication or repair may affect susceptibility to
carcinogenic agents (Swift et al., 1992). UV-DNA repair capacity has
been found to be lower in the lymphocytes isolated from individuals
with basal cell and squamous cell carcinoma than in the case of
their age-matched controls, and this repair capacity has been found
to decrease with increasing age in both groups (Wei et al., 1993).
Another form of susceptibility has an immunological basis.
Prior exposure to a chemical may induce an immune response that
sensitizes the individual to subsequent exposures. Such responses
occur in only a small fraction of the exposed population; an example
is the development of pulmonary hypersensitivity to industrial
agents such as toluene diisocyanate or cotton dust. The biomarkers
of susceptibility are the antigen-specific antibodies developed
against the chemical.
9. SUMMARY
The Task Group considered biomarkers in three categories,
biomarkers of exposure, of effect, and of susceptibility, while
recognising that clear distinction of category is often not
possible.
The Task Group agreed that the use of biomarkers can improve,
and should be used in, the process for the assessment of human
health risks caused by exposure to chemicals. Biomarkers may be
applied to the estimation of exposure and internal dose in
individuals and in groups and may allow identification of those at
greater or lesser risk than average.
Biomarkers must be validated before application in the risk
assessment process, i.e. the relationship between the biomarker, the
exposure, and the health outcome must be established. The selection,
validation and application of any biomarker is a complicated
process, which will vary for different markers. Examples were
selected to illustrate the concepts and principles.
Research and use of biomarkers involves complex ethical, social
and legal issues, which may vary in different countries. These
issues may impose constraints on research and use of biomarkers in
risk assessment and risk management decisions. The ethical, social
and legal aspects of biomarkers require careful consideration prior
to any application.
10. RECOMMENDATIONS
In making the following recommendations, the Task Group
recognized the role given IPCS to facilitate and increase
coordination of international activities in order to promote the
further work needed to define human health effects associated with
exposure to chemicals and to provide the basis for priority-setting
actions in order to protect public health.
10.1 General
* To promote the wider use of validated biomarkers in the
risk-assessment process
* To promote interdisciplinary cooperation and communication in
order to facilitate application of research findings
* To examine the feasibility of developing a data bank of
information on biomarkers applied to the process of risk and
their applications
10.2 Research
* To develop, refine and validate models to relate biomarkers of
exposure and of effect, qualitatively and quantitatively, to
exposure and to health outcome, particularly for end-points
other than cancer
* To identify and validate biomarkers of susceptibility in
relation to the chemical, and to inter-individual variation in
response, and investigate genetic polymorphism as a basis for
individual hypersensitivity
* To assess the use of information on individual susceptibility
in relation to protection of health with due respect to the
ethical, social and legal issues
* To develop strategies to link exposure and internal dose with
human health outcome by integration of mechanistically
validated biomarkers of exposure, effect and susceptibility
10.3 Applications
* To develop a practical protocol for use of biomarkers in human
studies, taking into account scientific, emotional, ethical,
legal and social aspects and including guidelines for risk
communication, with emphasis on the right of participants to
non-biased, intelligible information
* To encourage the production of certified reference materials
for biomarker analyses and support the functioning of
international quality assurance programmes
* To include consideration of biomarkers of exposure, effect, and
susceptibility in future Environmental Health Criteria
monographs
* To consider the need to update this monograph on principles and
concepts of biomarkers at an early date
REFERENCES
ACGIH (1992) 1992-1993 Threshold limit values for chemical
substances and physical agents and biological exposure indices.
Cincinnati, Ohio, American Conference of Governmental Industrial
Hygienists.
Adams RM & Fisher T (1990) Diagnostic patch testing.In: Adams R ed.
Occupational skin disease, 2nd ed. Philadelphia, Pennsylvania, W.B.
Saunders Company, pp 223-253.
Aitio A (1981) Laboratory quality control. Copenhagen, World Health
Organization, Regional Office for Europe, 74 pp (Health Aspects of
Chemical Safety - Interim Document 4).
Alessio L, Berlin A, & Roi R (1983) Human biological monitoring of
industrial chemicals series. Luxembourg, Office for Official
Publications of the Commission of European Communities (Industrial
Health and Safety Series).
Alessio L, Berlin A, & Roi R (1984) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 8903 EN).
Alessio L, Berlin A, & Roi R (1986) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 10704 EN).
Alessio L, Berlin A, & Roi R (1987) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11135 EN).
Alessio L, Berlin A, & Roi R (1988) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 11478 EN).
Alessio L, Berlin A, & Roi R (1989) Biological indicators for the
assessment of human exposure to industrial chemicals. Luxembourg,
Office for Official Publications of the Commission of the European
Communities (Industrial Health and Safety Series - EUR 12174 EN).
Anderson D ed. (1988) Genetic toxicology testing and biomonitoring
of environmental or occupational exposure: human monitoring. Mutat
Res, 204(Spec Issue 3): 353-551.
Anderson D ed. (1990) Fundamental and molecular mechanisms of
mutagenesis. Mutat Res, 229(Spec Issue 2): 103-247.
Anderson D ed. (in press) Human monitoring II. Mutat Res (Spec
Issue).
Anderson D, Francis AJ, Godbert P, Jenkinson PC, & Butterworth KR
(1991) Chromosomal aberrations (CA), sister-chromatid exchanges
(SCE) and mitogen-induced blastogenesis in cultures of peripheral
lymphocytes from 48 control individuals sampled 8 times over 2
years. Mutat Res, 250: 467-476.
Ashby J & Tennant RW (1991) Definitive relationships among chemical
structure, carcinogenicity and mutagenicity for 301 chemicals tested
by the US National Toxicology Program. Mutat Res, 257: 229-306.
Balmain A & Brown K (1988) Oncogene activation in chemical
carcinogenesis. Adv Cancer Res, 51: 147-182.
Barbin A, Laib RJ, & Bartsch H (1985) Lack of miscoding properties
of 7-(2-oxoethyl)guanine, the major vinyl chloride-DNA adduct.
Cancer Res, 45: 2440-2444.
Bartsch H & Montesano R (1984) Commentary: relevance of nitrosamines
to human cancer. Carcinogenesis, 5: 1381-1393.
Bartsch H, Ohshima H, & Shuker DEG (1991) Noninvasive methods for
measuring exposure to alkylating agents: recent studies on human
subjects. In: Groopman JD & Skipper PL ed. Molecular dosimetry and
human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 281-301.
Beach AC & Gupta RC (1992) Human biomonitoring and the
32P-postlabeling assay. Carcinogenesis, 13: 1053-1074.
Bekes JG, Roboz JP, Fischbein A, & Selikoff IJ (1987) Clinical
immunology studies in individuals exposed to environmental
chemicals. In: Berlin A, Dean J, Draper MH, Smith EMB, & Spreafico F
ed. Immunotoxicology. Proceedings of the International Seminar on
the Immunological System as a Target for Toxic Damage: Present
Status, Open Problems and Future Perspectives. Dordrecht, Boston,
Lancaster, Martinus Nijhoff Publishers, pp 347-361 (IPCS Joint
Seminar 10; EUR 10041 EN).
Belinsky SA, White CM, Devereux TR, Swenberg JA, & Anderson MW
(1987) Cell selective alkylation of DNA in rat lung following low
dose exposure to the tobacco specific carcinogen
4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res, 47:
1143-1148.
Bell DA (1991) Detection of DNA sequence polymorphisms in carcinogen
metabolism genes by polymerase chain reaction. Environ Mol Mutagen,
18: 245-248.
Blum M, Demierre A, Grant DM, Helm UA, & Meuer UA (1991) Molecular
mechanisms of slow acetylation of drugs and carcinogens in humans.
Proc Natl Acad Sci (USA), 88: 5237-5241.
Bond JA, Wallace LA, Osterman-Golkar S, Lucier GW, Buckpitt A, &
Henderson RF (1992) Assessment of exposure to pulmonary toxicants:
use of biological markers. Fundam Appl Toxicol, 18: 161-174.
Brandt-Rauf PW (1991) Advances in cancer biomarkers as applied to
chemical exposures: the ras oncogene and p21 protein and pulmonary
carcinogenesis. J Occup Med, 33: 951-955.
Brinkworth MH, Yardley-Jones A, Edwards AJ, Hughes JA, & Anderson D
(1992) A comparison of smokers with respect to oncogene products and
cytogenetic parameters. J Occup Med, 34: 1181-1189.
Butterworth BE, Popp JA, Conolly RB, & Goldsworthy TL (1992)
Chemically induced cell proliferation in carcinogenesis. In: Vainio
H, Magee PN, McGregor DB, & McMichael AJ ed. Mechanisms of
carcinogenesis in risk identification. Lyon, International Agency
for Research on Cancer, pp 279-305 (IARC Scientific Publications No.
116).
Calabrese EJ (1986) Ecogenetics: historical foundation and current
status (genetic factors). J Occup Med, 28: 1096-1102.
Cantin AM, Hubbard RC, & Crystal RG (1989) Glutathione deficiency in
the epithelial lining fluid of the lower respiratory tract in
idiopathic pulmonary fibrosis. Am Rev Respir Dis, 139: 370-372.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Hotter G, Mutti A, Franchini I, Fels LM, Stolte H, de
Broe ME, Nuyts GD, Taylor SA, & Price RG (1993a) Markers of early
renal changes induced by industrial pollutants. 1 Application to
workers exposed to mercury vapour. Br J Ind Med, 50: 17-27.
Cardenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR,
Rosello J, Ramis I, Mutti A, Franchini I, Fels LM, Stolte H, de Broe
ME, Nuyts GD, Taylor SA, & Price RG (1993b) Markers of early renal
changes induced by industrial pollutants. 2 Application to workers
exposed to lead. Br J Ind Med, 50: 28-36.
Carrano AV & Natarajan AT (1988) Considerations for population
monitoring using cytogenetic techniques. Mutat Res, 204: 379-406.
Casanova M, Deyo DF, & Heck HD'A (1989) Covalent binding of inhaled
formaldehyde to DNA in the nasal mucosa of Fischer 344 rats:
analysis of formaldehyde and DNA by high-performance liquid
chromatography and provisional pharmacokinetic interpretation.
Fundam Appl Toxicol, 12: 397-417.
Casanova M, Morgan KT, Steinhagen WH, Everitt JI, Popp JA, & Heck
HD'A (1991) Covalent binding of inhaled formaldehyde to DNA in the
respiratory tract of rhesus monkeys: pharmacokinetics, rat-to-monkey
interspecies scaling, and extrapolation to man. Fundam Appl Toxicol,
17: 409-428.
Cassito MG, Camerino D, Hanninen H, & Anger KW (1990) International
collaboration to evaluate the WHO neurobehavioural core test
battery. In: Johnson BL, Anger WK, Durao A, & Xinteras C ed.
Advances in neurobehavioural toxicology: applications in
environmental and occupational health. Chelsea, Michigan, Lewis
Publishers, Inc., pp 203-223.
Costa LG (1992) Effect of neurotoxicants on brain neurochemistry.
In: Tilson H & Mitchell C ed. Neurotoxicology. New York, Raven Press
Ltd, pp 101-123.
Cavalleri A, Gobba F, Bacchella L, & Ferrari D (1991) Evaluation of
serum aminoterminal propeptide of type III procollagen as an early
marker of the active fibrotic process in asbestos-exposed workers.
Scand J Work Environ Health, 17: 139-144.
Cleaver JE (1969) Xeroderma pigmentosum: a human disease in which an
initial stage of DNA repair is defective. Proc Natl Acad Sci (USA),
63: 428-435.
Cohen SM & Ellwein LB (1990) Cell proliferation in carcinogenesis.
Science, 249: 1007-1011.
Compton PJE, Hooper K, & Smith MT (1991) Human somatic mutation
assays as biomarkers of carcinogenesis. Environ Health Perspect, 94:
135-141.
Conolly RB & Andersen ME (in press) An approach to mechanism-based
cancer risk assessment: formaldehyde. Environ Health Perspect, 101.
Conolly RB, Monticello TM, Morgan KT, Clewell HJ, & Andersen ME
(1992) A biologically-based risk assessment strategy for inhaled
formaldehyde. Comments Toxicol, 4: 269-293.
Das BC (1988) Factors that influence formation of sister chromatid
exchanges in human blood lymphocytes. Crit Rev Toxicol, 19: 43-86.
Dellarco V, Voytek P, & Hollaender A (1985) Aneuploidy: Etiology and
mechanisms. New York, London, Plenum Press.
Derosa CT, Choudhury H, & Peirano WB (1991) An integrated
exposure/pharmacokinetic based approach to the assessment of complex
exposures. Toxicol Ind Health, 7(4): 231-248.
DFG (German Association for the Encouragement of Research) (1992)
Commission for the Investigation of Health Hazards of Chemical
Compounds in the Work Area: MAK- and BAT-values 1992. Weinheim, VCH.
Droz PO, Wu MM, & Cumberland WG (1989) Variability in biological
monitoring of organic solvent exposure. 2 Application of a
population physiological model. Br J Ind Med, 46: 547-558.
Enterline PE & Marsh GM (1982) Cancer among workers exposed to
arsenic and other substances in a copper smelter. Am J Epidemiol,
116: 895-911.
Farmer PB (1991) Analytical approaches for the determination of
protein-carcinogen adducts using mass spectrometry. In: Groopman JD
& Skipper PL ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 189-210.
FIOH (Työterveyslaitos) (1993) [Biological monitoring. Guide for
sample collection 1993.] Helsinki, Finnish Institute of Health, 115
pp (in Finnish).
French M & Morley AA (1985) Measurement of micronuclei in
lymphocytes. Mutat Res, 147: 29-36.
Gibaldi M & Perrier D (1982) Pharmacokinetics. New York, Basel,
Marcel Dekker Inc.
Goldsworthy TL, Morgan KT, Popp JA, & Butterworth BE (1991)
Guidelines for measuring chemically-induced cell proliferation in
specific rat organs. In: Butterworth BE & Sloga TJ ed. Chemically
induced cell proliferation: Implications for risk assessment. New
York, Wiley-Liss, pp 253-284.
Graham D, Henderson FW, & House D (1988) Neutrophil influx measured
in nasal lavages of humans exposed to ozone. Arch Environ Health,
43: 228-233.
Groopman JD, Sabbioni G, & Wild CP (1991) Molecular dosimetry of
human aflatoxin exposures. In: Groopman JD & Skipper PL ed.
Molecular dosimetry and human cancer -Analytical, epidemiological
and social considerations. Boca Raton, Florida, CRC Press, pp
303-324.
Guengerich FP (1991) Interindividual variation in biotransformation
of carcinogens: basis and relevance. In: Groopman JD & Skipper PL
ed. Molecular dosimetry and human cancer - Analytical,
epidemiological and social considerations. Boca Raton, Florida, CRC
Press, pp 27-52.
Hall JA, Saffhill R, Green T, & Hathway DE (1981) The induction of
errors during in vitro DNA synthesis following
chloroacetaldehyde-treatment of poly(dA-dT) and poly(dC-dG)
templates. Carcinogenesis, 2: 141-146.
Hanawalt PC (1987) Preferential DNA repair in expressed genes.
Environ Health Perspect, 76: 9-14.
Hancock DG, Moffitt AE, & Hay EB (1984) Hematological findings among
workers exposed to benzene at coke oven by-product recovery
facility. Arch Environ Health, 39: 414-418.
Harel S, Janoff A, Yu SY, Hurewitz A, & Bergofsky EH (1980)
Desmosine radioimmunoassay for measuring elastin degradation in
vivo. Am Rev Respir Dis, 122: 769-773.
Harris CC (1991) Molecular epidemiology: overview of molecular and
biochemical basis. In: Groopman JD & Skipper PL ed. Molecular
dosimetry and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 15-26.
Hecht SS, Adams JD, Numoto S, & Hoffmann D (1983) Induction of
respiratory tract tumours in Syrian golden hamsters by a single dose
of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and the
effect of smoke inhalation. Carcinogenesis, 4(10): 1287-1290.
Henderson RF (1988) Use of bronchoalveolar lavage to detect lung
damage. In: Target organ toxicology: Lung. New York, Raven Press, pp
239-268.
Henderson RF, Bechtold WE, Bond JA, & Sun JD (1989) The use of
biological markers in toxicology. Crit Rev Toxicol, 20: 65-82.
Hirvonen A, Husgafvel-Pursiainen K, Karjalainen A, Anttila S, &
Vainio H (1992) Point-mutational Msp1 and lle-Val polymorphisms
closely linked in the CYP1A1 Gene: Lack of association with
susceptibility to lung cancer in a Finnish study population. Cancer
Epidemiol Biomarkers Prev, 1: 485-489.
Hoffman D, Rivenson A, Amin S, & Hecht SS (1984) Dose-response study
of the carcinogenicity of tobacco-specific N-nitrosamines in F344
Rats. J Cancer Res Clin Oncol, 108: 81-86.
Hollstein M, Sidransky D, Vogelstein B, & Harris CC (1991) p53
Mutations in human cancers. Science, 253: 49-53.
Hollstein MC, Wild CP, Bleicher F, Chutimataewin S, Harris CC,
Srivatanakul P, & Montesano R (1992) p53 Mutations and aflatoxin
B1h exposure in hepatocellular carcinoma patients from Thailand.
Int J Cancer, 53: 1-5.
Holsapple MP, Snyder NK, Wood SC, & Morris DL (1991) A review of
2,3,7,8-tetrachlorodibenzo-p-dioxin-induced changes in
immunocompetence: 1991 update. Toxicology, 69: 219-255.
Horak F (1985) Manifestation of allergic rhinitis in
latent-sensitized patients. A prospective study. Arch
Otorhinolaryngol, 242: 224-239.
Hsia MTS (1991) Carcinogen-macromolecular adducts as biomarkers in
human cancer risk assessment. Biomed Environ Sci, 4: 104-112.
Hsu IC, Metcalf RA, Sun T, Welsh J, Wang NJ, & Harris CC (1991)
Mutational hotspot in the p53 gene in human hepatocellular
carcinomas. Nature (Lond), 350: 427-428.
IARC (1992) In: Vainio H, Magee PN, McGregor D, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer (IARC Scientific
Publications No. 116).
Jennings AM, Wild G, Ward JD, & Milford Ward A (1988) Immunologic
abnormalities 17 years after accidental exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin. Br J Ind Med, 45: 701-704.
Kadlubar FF, Butler MA, Kaderlik KR, Chou H-S, & Lang NP (1992)
Polymorphisms for aromatic amine metabolism in humans: relevance for
human carcinogenesis. Environ Health Perspect, 98: 69-74.
Kang H-I, Konishi C, Kuroko T, & Huh N-K (1993) A highly sensitive
and specific method for quantitation of O-alkylated DNA adducts and
its application to the analysis of human tissue. Environ Health
Perspect, 99: 269-271.
Lambert B (1992) Biological markers in exposed humans: gene
mutation. In: Vainio H, Magee PN, McGregor DB, & McMichael AJ ed.
Mechanisms of carcinogenesis in risk identification. Lyon,
International Agency for Research on Cancer, pp 535-542 (IARC
Scientific Publications No. 116).
Lamm SH, Walters AS, Wilson R, Byrd DM, & Grunwalt H (1989)
Consistencies and inconsistencies underlying the quantitative
assessment of leukemia risk from benzene exposure. Environ Health
Perspect, 82: 289-297.
Lassalle P, Cosset P, & Aerts C (1990) Abnormal secretion of
interleukin-1 and tumor necrosis factor alpha by alveolar
macrophages in coal worker's pneumoconiosis: comparison between
simple pneumoconiosis and progressive massive fibrosis. Exp Lung
Res, 16: 73-80.
Lucier G & Thompson CL (1987) Issues in biochemical applications to
risk assessment: when can lymphocytes be used as surrogate markers?
Environ Health Perspect, 76: 187-191.
Mattison DR (1991) An overview on biological markers in reproductive
and development toxicology: concepts and definitions, and use in
risk assessment. Biomed Environ Sci, 4: 8-34.
Morris SM (1991) The genetic toxicology of 5-bromodeoxyuridine in
mammalian cells. Mutat Res, 258: 161-188.
Morris SM, Domon OE, McGarrity LJ, Kodell RL, & Casciano DA (1992)
Effect of bromodeoxyuridine on the proliferation and growth of ethyl
methanesulfonate-exposed P3 cells: relationship to the induction of
sister chromatid exchanges. Cell Biol Toxicol, 8: 75-87.
Nebert DW (1988a) The 1986 Bernard B. Brodie award lecture. The
genetic regulation of drug-metabolizing enzymes. Drug Metab Dispos,
16: 1-8.
Nebert DW (1988b) Genes encoding drug metabolizing enzymes: possible
role in human disease. Basic Life Sci, 43: 45-64.
Nielsen J, Welinder H, Horstmann V, & Skerfving S (1992) Allergy to
methyltetrahydrophthalic anhydride in epoxy resin workers. Br J Ind
Med, 49: 769-775.
O'Callaghan JP (1991) Quantification of glial fibrillary acidic
protein: Comparison of slot-immunobinding assays with a novel
sandwich ELISA. Neurotoxicol Teratol, 13: 275-281.
Parry JM, Shamsher M, & Skibinski DOF (1990) Restriction site
mutation analysis, a proposed methodology for the detection and
study of DNA base changes following mutation exposure. Mutagenesis,
5: 209-212.
Passingham BJ, Farmer FB, Bailey E, Brookes AGF, & Yates DW (1988)
2-Hydroxyethylation of haemoglobin in man. In: Methods for detecting
DNA and damaging agents in humans: applications in cancer
epidemiology and prevention. Lyon, International Agency for Research
on Cancer, pp 279-285 (IARC Scientific Publications No. 89).
Piguet PF, Collart MA, Grau GE, Sappino AP, & Vassalli P (1990)
Requirement of tumor necrosis factor for development of
silica-induced pulmonary fibrosis. Nature (Lond), 344: 245-247.
Poland A, Glover E, & Kende AS (1976) Stereospecific, high affinity
binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol.
Evidence that the binding species is receptor for induction of aryl
hydrocarbon hydroxylase. J Biol Chem, 251: 4936-4946.
Rajamaki A (1984) Assessment of early myelotoxicity. In: Aitio A,
Riihimaki V, & Vainio H ed. Biological monitoring and surveillance
of workers exposed to chemicals. Washington, New York, London,
Hemisphere Publishing Corporation, pp 303-308.
Ramel C (1992) Genotoxic and nongenotoxic carcinogens: mechanisms of
action and testing strategies. In: Vainio H, Magee PN, McGregor DB,
& McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 195-209 (IARC Scientific Publications No. 116).
Ramsay JC & Andersen ME (1984) A physiologically based description
of the inhalation pharmacokinetics of styrene monomer in rats and
humans. Toxicol Appl Pharmacol, 73: 159-175.
Randerath K & Randerath E (1991) 32P-postlabeling analysis of
mutagen- and carcinogen-adducts and age-related DNA modifications
(I-compounds). In: Groopman JD & Skipper PL ed. Molecular dosimetry
and human cancer - Analytical, epidemiological and social
considerations. Boca Raton, Florida, CRC Press, pp 131-150.
Reynolds HY (1987) Bronchoalveolar lavage. Am Rev Respir Dis, 135:
250-263.
Rivenson A, Hoffman D, Prokopszyk B, Amin S, & Hecht SS (1988)
Induction of lung and pancreas tumours in F344 rats by
tobacco-specific and areca derived N-nitrosamines. Cancer Res, 48:
6912-6917.
Roels H, Bernard AM, Cardenas AR, Buchet JP, Lauwerys RR, Hotter G,
Ramis I, Mutti A, Frandhini I, Bundschuh I, Stolte H, de Broe ME,
Nuyts GD, Taylor SA, & Price RG (1993) Markers of early renal
changes induced by industrial pollutants. 3 Application to workers
exposed to cadmium. Br J Ind Med, 50: 37-48.
Ross RK, Yuan J-M, Wogan GN, Qian G-S, Tu J-T, Groopman JD, Gao Y-T,
& Henderson BE (1992) Urinary aflatoxin biomarkers and risk of
hepatocellular carcinoma. Lancet, 339: 943-946.
Santella RM (1988) Application of new techniques for the detection
of carcinogen adducts to human population monitoring. Mutat Res,
205: 271-282.
Santella RM, Gaspara F, & Hsieh L (1985) Quantitation of
carcinogen-DNA adducts with monoclonal antibodies. Prog Exp Tumour
Res, 31: 63-75.
Seidegard J, Pero RW, Markowitz MM, Rousch G, Miller DG, & Beattle
EJ (1990) Isoenzyme(s) of glutathione transferase (class mu) as a
marker for the susceptibility to lung cancer: A follow-up study.
Carcinogenesis, 11: 33-36.
Seppalainen A-M, Hernberg S, & Kock B (1979) Relationship between
blood lead levels and nerve conduction velocities. Neurotoxicology,
1: 313-332.
Shuker DEG & Farmer PB (1992) Relevance of urinary DNA adducts as
markers of carcinogen exposure. Chem Res Toxicol, 5: 450-460.
Singer B, Chavez SJ, & Kusmierek JT (1987) The vinyl
chloride-derived nucleoside N2,3-ethenoguanosine, is a highly
efficient mutagen in transcription. Carcinogenesis, 8: 745-747.
Sorsa M, Ojajarvi A, & Salomaa S (1990) Cytogenetic surveillance of
workers exposed to genotoxic chemicals: preliminary experiences from
a prospective cancer study in a cytogenetic cohort. Teratog Carcinog
Mutagen, 10: 215-221.
Sorsa M, Wilbourn J, & Vanio H (1992) Human cytogenetic damage as a
predictor of cancer risk. In: Vanio H, Magee PN, McGregor D, &
McMichael AJ ed. Mechanisms of carcinogenesis in risk
identification. Lyon, International Agency for Research on Cancer,
pp 543-554 (IARC Scientific Publications No. 116).
Stone PJ, Bryan-Rhadfi J, & Lucey EC (1991) Measurement of urinary
desmosine by isotope dilution and high performance liquid
chromatography. Am Rev Respir Dis, 144: 284-290.
Sub-Committee on Skin Tests of the European Academy of Allergology
and Clinical Immunology (1989) In: Dreborg S ed. Skin tests for
diagnosis of IgE-mediated allergy. Position paper. Allergy, 44(Suppl
10): 31-37.
Sullivan JB (1989) Immunological alterations and chemical exposure.
Clin Toxicol, 27: 311-343.
Swenberg JA, Fedtke N, Fennell TR, & Walker VE (1990) Relationships
between carcinogen exposure, DNA adducts and carcinogenesis. In:
Clayson DB, Munro IC, Shubik P, & Swenberg JA ed. Progress in
predictive toxicology. Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp 161-184.
Swift M, Morrel D, Massey RB, & Chase CL (1992) Incidence of cancer
in 161 families affected by ataxia-telangiectasia. New Engl J Med,
325: 1831-1836.
Townsend JC, Ott MG, & Fishbeck WA (1978) Health exam findings among
individuals occupationally exposed to benzene. J Occup Med, 20:
543-548.
Tritscher AM, Goldstein JA, Portier CJ, McCoy ZM, Clark GC, & Lucier
GW (1992) Dose-response relationships for chronic exposure to
2,3,7,8-tetrachlorodibenzo-p-dioxin in a rat tumour promotion model:
quantification and immunolocalization of CYP1A1 and CYP1A2 in the
liver. Cancer Res, 52: 3436-3442.
UK DH (1989) Committee on Mutagenicity of Chemicals in Food.
Guidelines for the testing of chemicals for mutagenicity - Consumer
Products and the Environment. London, Department of Health, Her
Majesty's Stationery Office (HMSO), 99 pp (Report No. 35).
UK HSE (1991) Guidance on laboratory techniques in occupational
medicine, 5th ed. London, Health and Safety Executive, Library and
Information Services, 117 pp.
US EPA (1991) Formaldehyde risk assessment update. Washington, DC,
US Environmental Protection Office, Office of Toxic Substances.
US EPA (1987) Assessment of health risks to garment workers and
certain home residents from exposure to formaldehyde. Washington,
DC, US Environmental Protection Office, Office of Toxic Substances.
US NRC (US National Research Council) (1989a) Biologic markers in
pulmonary toxicology. Washington, DC, National Academy Press, 179
pp.
US NRC (US National Research Council) (1989b) Biologic markers in
reproductive toxicology. Washington, DC, National Academy Press, 395
pp.
US NRC (US National Research Council) (1992a) Biologic markers in
immunotoxicology. Washington, DC, National Academy Press, 206 pp.
US NRC (US National Research Council) (1992) Environmental
neurotoxicology. Report of Committee on Neurotoxicology and Models
for Assessing Risk. Washington, DC, National Academy Press, 154 pp.
Wei Q, Matanoski GM, Farmer ER, Hedayati MA, & Grossman L (1993) DNA
repair and aging in basal cell carcinoma: a molecular epidemiology
study. Proc Natl Acad Sci (USA), 90: 1614-1618.
Weinberg RA (1991) Tumour suppressor genes. Science, 254: 1138-1146.
Weisburger JH & Williams GM (1981) Carcinogen testing: current
problems and new approaches. Science, 214: 401-407.
Weston A & Bowman ED (1991) Fluorescence detection of
benzo(a)pyrene-DNA adducts in human lung. Carcinogenesis, 12:
1445-1449.
WHO (1991) IPCS Environmental Health Criteria 119: Principles and
methods for the assessment of nephrotoxicity associated with
exposure to chemicals. Geneva, World Health Organization, 266 pp.
WHO (1992a) IPCS Environmental Health Criteria 134: Cadmium. Geneva,
World Health Organization, 280 pp.
WHO (1992b) IPCS Environmental Health Criteria 141: Quality
management for chemical safety testing. Geneva, World Health
Organization, 112 pp.
WHO (in press) Principles for assessment of risks from exposure to
chemicals (Part A).
Wild CP & Montesano R (1991) Immunological quantitation of human
exposure to aflatoxins and N-nitrosamines. In: Vanderlaan M, Stanker
LH, Watkins BE, & Roberts DW ed. Immunoassays for trace chemical
analysis. Washington, DC, American Chemical Society, pp 215-228 (ACS
Symposium Series No. 451).
Wild CP, Jiang YZ, Sabbioni G, Chapot B, & Montesano R (1990)
Evaluation of methods for quantitation of aflatoxin-albumin adducts
and their application to human exposure assessment. Cancer Res, 50:
245-251.
Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle
H, Montesano R, & Groopman JD (1992) Dietary intake of aflatoxins
and the level of albumin-bound aflatoxin in peripheral blood in The
Gambia, West Africa. Cancer Epidemiol Biomarkers Prev, 1: 229-234.
Wogan GN (1989) Markers of exposure to carcinogens: methods for
human monitoring. J Am Coll Toxicol, 8: 871-881.
Wogan GN & Gorelick NJ (1985) Chemical and biochemical dosimetry of
exposure to genotoxic chemicals. Environ Health Perspect, 62: 5-18.
Wolf CR, Dale Smith CA, Gough AC, Moss JE, Vallis KA, Howard G,
Carey FJ, Mills K, McNee W, Carmichael J, & Spurr NK (1992)
Relationship between the debrisoquine hydroxylase polymorphism and
cancer susceptibility. Carcinogenesis, 13: 1035-1038.
Yanagisawa Y, Nishimura H, Matsuki H, Osaka F, & Kasuga H (1986)
Personal exposure and health effect relationship for NO2 with
urinary hydroxyproline to creatinine ratio as indicator. Arch
Environ Health, 41: 41-48.
RESUME
Le groupe spécial a divisé les marqueurs biologiques en trois
catégories, les marqueurs d'exposition, les marqueurs d'effet et les
marqueurs de réceptivité, tout en admettant que bien souvent il
n'était pas possible d'établir une distinction nette entre ces
diverses catégories.
Le groupe spécial a admis que le recours aux marqueurs
biologiques pouvait améliorer l'évaluation des risques pour la santé
humaine découlant d'une exposition à des produits chimiques et qu'il
fallait donc en faire usage. Les marqueurs biologiques peuvent être
utilisés pour évaluer l'exposition et la dose interne chez des
individus et des groupes et peuvent faciliter l'identification de
ceux de ces individus ou de ces groupes qui sont plus ou moins
exposés aux risques que la moyenne.
Avant d'utiliser les marqueurs biologiques pour l'évaluation du
risque il faut les valider, c'est-à-dire établir la relation qui
existe entre le marqueur, l'exposition et ses conséquences pour la
santé. Le choix, la validation et l'utilisation de tout marqueur
biologique constituent des processus complexes qui varient d'un
marqueur à l'autre. Pour mettre en lumière ces notions et ces
principes on a choisi un certain nombre d'exemples.
La recherche et l'utilisation des marqueurs biologiques
soulèvent des questions complexes sur le plan éthique, social et
juridique, qui peuvent d'ailleurs varier d'un pays à l'autre. Il
peut s'en suivre un certain nombre de contraintes imposées à la
recherche et à l'utilisation des marqueurs biologiques dans
l'évaluation des risques et dans les décisions relatives à la
gestion de ces risques. Avant toute application il importe d'étudier
avec soin les aspects éthiques, sociaux et juridiques des marqueurs
biologiques.
RECOMMANDATIONS
En formulant les recommandations ci-après, le groupe de travail
a pris en considération le rôle dévolu au PISC, à savoir de
faciliter et de développer la coordination des activités
internationales afin d'encourager les travaux à poursuivre pour
définir les effets sur la santé humaine qu'entraîne l'exposition aux
substances chimiques et de jeter les bases des actions prioritaires
à entreprendre pour protéger la santé publique.
1. Généralités
* Encourager un plus large recours aux marqueurs biologiques dans
l'évaluation des risques
* Favoriser la coopération et la communication
inter-disciplinaires afin de faciliter l'application des
résultats de la recherche
* Etudier la faisabilité d'une banque de données sur les
marqueurs biologiques dans l'évaluation des risques et ses
applications
2. Recherche
* Mettre au point, affiner et valider des modèles qui permettent
de corréler les marqueurs biologiques de l'exposition et des
effets tant qualitativement que quantitativement, à
l'exposition et aux conséquences biologiques, en particulier
aux conséquences autres que le cancer.
* Recenser et valider des marqueurs biologiques de réceptivité,
par rapport à la réaction aux produits chimiques et aux
variations interindividuelles à cette réaction et étudier le
polymorphisme génétique en tant que base de l'hypersensibilité
individuelle.
* Voir dans quelle mesure il est possible d'utiliser certains
renseignements sur la réceptivité individuelle pour la
protection de la santé, dans le respect des impératifs
éthiques, sociaux et juridiques.
* Mettre au point des stratégies afin de relier l'exposition et
la dose interne aux conséquences biologiques pour l'homme
grâceà une synthèse de biomarqueurs de l'exposition, de l'effet
et de la réceptivité, mécanistiquement validés.
3. Applications
* Mettre au point un protocole pratique pour l'utilisation des
marqueurs biologiques dans les études sur l'homme, qui prenne
en considération les aspects scientifiques, émotionnels,
éthiques, juridiques et sociaux et qui comporte des directives
en matière de communication, en insistant sur le droit des
participants à disposer d'informations intelligibles et non
biaisées.
* Encourager la production de substances de référence homologuées
pour l'analyse des marqueurs biologiques et aider les
programmes internationaux d'assurance de la qualité à
fonctionner.
* Faire figurer des considérations sur les biomarqueurs
d'exposition, d'effet et de réceptivité dans les futures
monographies de la série Critères d'hygiène de l'environnement.
* Examiner s'il est nécessaire de mettre à jour à bref délai la
présente monographie consacrée aux principes et conceptions en
matière de marqueurs biologiques.
RESUMEN
El Grupo Especial distinguió tres clases de biomarcadores: de
exposición, de efecto y de susceptibilidad, reconociendo sin embargo
que a menudo resulta imposible establecer claramente la pertenencia
a una de esas clases.
El Grupo Especial coincidió en que los biomarcadores permiten
mejorar, y deben emplearse a ese efecto, el proceso de evaluación de
los riesgos que para la salud humana conlleva la exposición a
productos químicos. Los biomarcadores se pueden emplear para
calcular la exposición y la dosis interna recibida por individuos y
grupos, con la consiguiente identificación de quienes sufren un
mayor o menor riesgo que la media.
Los biomarcadores deben ser validados antes de aplicarlos a la
evaluación del riesgo, lo que significa que debe determinarse la
relación entre el biomarcador, la exposición y el estado de salud.
La selección, validación y empleo de cualquier biomarcador es un
proceso complicado, distinto para cada marcador. Se eligieron
algunos ejemplos para ilustrar los conceptos y principios
relacionados.
La investigación y el empleo de los biomarcadores plantea
complejas cuestiones éticas, sociales y jurídicas, que pueden
diferir de un país a otro. Algunos de esos problemas limitan el
alcance de las investigaciones sobre los biomarcadores y de su
aplicación a la evaluación de riesgos y la adopción de decisiones
relacionadas con la gestión de riesgos. Los problemas éticos,
sociales y jurídicos que plantean los biomarcadores deben ser objeto
de un detenido análisis antes de su eventual uso.
RECOMENDACIONES
Al formular las siguientes recomendaciones, el Grupo Especial
reconoció la función asignada al IPCS de facilitar e intensificar la
coordinación de las actividades internacionales con miras a fomentar
los trabajos que aún será necesario realizar para determinar los
efectos sobre la salud relacionados con la exposición a productos
químicos, así como para establecer las bases que permitan señalar
las prioridades a que haya que atenerse para proteger la salud
pública.
1. Recomendaciones generales
* Promover un mayor uso de los biomarcadores validados en el
proceso de evaluación de riesgos.
* Fomentar la cooperación y la comunicación interdisciplinarias
para facilitar la aplicación de los resultados de las
investigaciones.
* Estudiar la posibilidad de crear un banco de datos sobre
biomarcadores aplicados a la evaluación de riesgos y sus
posibles usos.
2. Investigaciones
* Desarrollar, perfeccionar y validar modelos aptos para
relacionar los biomarcadores de exposición y de efecto,
cualitativa y cuantitativamente, con la exposición y con el
estado de salud, sobre todo para puntos finales distintos del
cáncer.
* Identificar y validar biomarcadores de susceptibilidad en
relación con el producto químico y con la variación
interindividual de la respuesta, e investigar la influencia del
polimorfismo genético en la hipersensibilidad individual.
* Evaluar el uso de la información referente a la susceptibilidad
individual desde la perspectiva de una protección de la salud
atenta a los aspectos éticos, sociales y jurídicos.
* Formular estrategias para relacionar la exposición y la dosis
interna con el estado de salud mediante la integración de
biomarcadores de exposición, efecto y susceptibilidad validados
sistemáticamente.
3. Aplicaciones
* Elaborar un protocolo práctico para el uso de biomarcadores en
los estudios realizados en el hombre, teniendo en cuenta los
aspectos científicos, psicológicos, éticos, jurídicos y
sociales, con inclusión de directrices para la comunicación del
riesgo y haciendo hincapié en el derecho de los participantes a
una información inteligible e imparcial.
* Fomentar la producción de material de referencia certificado
para el análisis de biomarcadores y respaldar la aplicación de
programas internacionales de garantía de la calidad.
* Incluir la consideración de los biomarcadores de exposición,
efecto y susceptibilidad en las futuras monografías de la serie
Criterios de Salud Ambiental.
* Tener presente la necesidad de actualizar con prontitud la
presente monografía sobre los principios y nociones relativos a
los biomarcadores.
