
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 206
METHYL TERTIARY-BUTYL ETHER
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr M. Gillner, National Chemicals
Inspectorate, Solna, Sweden, with contributions from Ms A.-S. Nihlén,
Institute for Working Life, Solna, Sweden
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Methyl tertiary-butyl ether.
(Environmental health criteria ; 206)
1.Methyl ethers 2.Environmental exposure
3.Occupational exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 157206 X (NLM Classification: QD 305.E7)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR METHYL TERTIARY-BUTYL ETHER
PREAMBLE
ABBREVIATIONS
1. SUMMARY
1.1. Identity, physical and chemical properties, analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on laboratory animals and in vitro systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory and field
1.9. Evaluation of human health risks and effects on the
environment
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Procedures
2.4.1.1 Air
2.4.1.2 Soil, water and sediment
2.4.1.3 Gasoline
2.4.1.4 Biological samples
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Uses
3.2.3. Sources and releases to the environment
3.2.3.1 Industrial releases
3.2.3.2 Storage tank release
3.2.3.3 Engine emissions from on-road and off-road
vehicles and recreational boats
3.3. Other pertinent information
4. ENVIRONMENTAL BEHAVIOUR AND FATE
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Multimedia
4.2. Bioconcentration
4.3. Biodegradation and transformation
4.3.1. Aerobic conditions
4.3.2. Anaerobic conditions
4.4. Abiotic degradation
4.4.1. Air
4.4.1.1 Photolysis
4.4.1.2 Hydrolysis
4.4.1.3 Photooxidation
4.4.2. Natural waters
4.4.3. MTBE half-life ranges in environmental compartments
4.5. Ozone-forming potential
4.6. Remediation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Exposure
5.1.1.1 Levels in ambient air and various
microenvironments
5.1.1.2 Dermal exposure
5.1.1.3 Estimation of total personal exposure
5.1.1.4 Other pollutants
5.2. Occupational exposure
5.2.1. Industrial operations - manufacturing and blending
5.2.2. Transportation
5.2.3. Service station attendants and garage mechanics
5.2.4. Occupational exposure limit values
5.3. Exposure via water
5.3.1. Snow and precipitation
5.3.2. Surface water
5.3.3. Groundwater
5.3.4. Drinking-water
5.4. Soil and sediment
5.5. Biota
6. KINETICS AND METABOLISM IN HUMANS AND LABORATORY ANIMALS
6.1. Human data
6.1.1. Controlled human studies
6.1.2. Exposure to oxygenated gasoline
6.2. Animal studies
6.3. In vitro studies
6.4. Physiologically based pharmacokinetic modelling
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
7.1. Single exposure
7.2. Skin, eye and respiratory irritation; skin sensitization
7.2.1. Skin irritation
7.2.2. Eye irritation
7.2.3. Respiratory tract irritation
7.2.4. Skin sensitization
7.3. Neurotoxicity
7.4. Short-term repeated dose studies
7.4.1. Oral studies
7.4.2. Inhalation studies
7.4.3. Intraperitoneal administration
7.5. Neurotoxicity studies
7.6. Reproductive and developmental toxicity
7.6.1. Reproductive toxicity
7.6.2. Developmental toxicity
7.7. Mutagenicity and related end-points
7.8. Carcinogenicity
7.8.1. Initiation-promotion protocol
7.9. Metabolites of MTBE
7.10. Mode of action
7.10.1. Kidney tumours
7.10.2. Liver tumours
8. EFFECTS ON HUMANS
8.1. Population studies
8.2. Controlled studies
8.3. Subpopulations at special risk
8.4. Special studies
8.4.1. Organoleptic properties
8.4.2. Immunological effects
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Algae
9.1.2. Aquatic animal species
9.2. Field experiments
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Human health effects
10.2. Evaluation of effects on the environment
11. RECOMMENDATIONS
REFERENCES
RÉSUMÉ
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary -- a review of the salient facts and the risk evaluation
of the chemical
* Identity -- physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL TERTIARY-
BUTYL ETHER
Members
Dr R. B. Beems, National Institute of Public Health & the Environment,
Bilthoven, The Netherlands
Dr A. Bobra, Environment Canada, Hull, Quebec, Canada
Dr S. Borghoff, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr J.M. Davis, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, North
Carolina, USA (Vice-Chairman)
Dr L. Fishbein, Fairfax, Virginia, USA
Dr M. Gillner, National Chemicals Inspectorate, Solna, Sweden
(Co-Rapporteur)
Mr G. Long, Environmental Health Centre, Health Canada, Ottawa, Canada
(Co-Rapporteur)
Dr M.E. Meek, Environmental Health Centre, Health and Welfare Canada,
Ottawa, Canada (Chairman)
Dr A.A.E. Wibowo, Coronel Institute, University of Amsterdam,
Amsterdam, The Netherlands
Observers
Dr M. Constantini, Health Effects Institute, Cambridge, Massachusetts,
USA (representing the Health Effects Institute (HEI))
Dr J. Del Pup, Texaco Inc., New York, USA (representing the American
Industrial Health Council (AIHC))
Mr R. Hillier, Oil, Chemical and Atomic Workers' International Union
(OCAWIU), Lakewood, Colorado, USA (representing the International
Federation of Chemical, Energy, Mine and General Workers' Unions
(ICEM))
Dr A.K. Mallett, Arco Chemical Europe Inc., Maidenhead, United Kingdom
(representing the European Centre for Ecotoxicology and Toxicology of
Chemicals (ECETOC))
Dr M. Mehlman, Princeton, New Jersey, USA (Technical Adviser to Mr
Hillier, OCAWIU)
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International Union of
Pharmacology (IUPHAR))
Dr J. Zogorski, US Department of the Interior, Rapid City, South
Dakota, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, World Health
Organization, Lyon, France
Assisting the Secretariat
Miss C. Grande, Air Issues Section, Health Canada, Ottawa, Canada
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL
TERTIARY-BUTYL ETHER (MTBE)
A WHO Task Group on Environmental Health Criteria for methyl
tertiary-butyl ether met at the Conference Facility, Lord Elgin
Hotel, Ottawa, Canada from 17 to 21 April 1997. Dr E.M. Smith, IPCS,
welcomed the participants on behalf of Dr M. Mercier, Director of the
IPCS, and the three IPCS cooperating organizations (UNEP/ILO/ WHO).
The Group reviewed and revised the draft and made an evaluation of the
risks for human health and the environment from exposure to methyl
tertiary-butyl ether.
The first draft of the EHC was prepared by Dr M. Gillner,
National Chemicals Inspectorate, Solna, Sweden, with contributions
from Ms A.-S. Nihlén, Institute for Working Life, Solna, Sweden. Dr M.
Gillner and Ms Nihlén also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS contact points for Environmental Health Criteria monographs.
Dr E.M. Smith and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The financial support of the Swedish National Chemicals
Inspectorate in preparing the monograph and the Canadian Health
Protection Branch, Environmental Health Directorate, in funding the
Task Group meeting in Ottawa are gratefully acknowledged.
ABBREVIATIONS
AED atomic emission detector
ALAT alanine aminotransferase
AP alkaline phosphatase
AUC area under the curve
BCF bioconcentration factor
BTEX benzene, toluene, ethyl benzene and xylenes
BUN blood urea nitrogen
bw body weight
CHOL cholesterol
CL total plasma clearance
CNS central nervous system
CO carbon monoxide
DEN diethylnitrosamine
DIPE diisopropyl ether
DMN N-nitrosodimethylamine
EC electron capture
EROD 7-ethoxyresorufin- O-deethylase
ETBE ethyl tertiary-butyl ether
FID flame ionization detector
FOB functional observational battery
FTIR Fourier-transform infrared
GC gas-chromatography
GC-MS gas-chromatography/mass spectrometry
GC-O gas-chromatography using an oxygen-selective detector
Hb haemoglobin
HC hydrocarbon
HPLC high-performance liquid chromatography
HPRT hypoxanthine-guanine phosphoribosyl transferase
IL-1 interleukin-1
IL-4 interleukin-4
ip intraperitoneal
IR infrared
iv intravenous
Koc adsorbtion coefficient to soil organic carbon
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
LGL large granular lymphocyte
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LT50 median lethal time
MCH mean corpuscular haemoglobin
MCHC mean corpuscular haemoglobin concentration
MCS multiple chemical sensitivities
MCV mean corpuscular volume
MTBE methyl tertiary-butyl ether
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIR near infrared
NMOC non-methane organic carbon
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NOx oxides of nitrogen (NO, NO2, N2O4 and N2O3)
PID photoionization detector
ppb parts per billion
ppbv parts per billion (by volume)
ppm parts per million
PROD 7-pentoxyresorufin- O-dealkylase
RBC red blood cell
RFG reformulated gasoline
RID refractive index detector
RPLC reversed-phase liquid chromatography
sc subcutaneous
SCE sister chromatid exchange
SD standard deviation
TBA tertiary-butyl alcohol
TBF tertiary-butyl formate
TWA time-weighted average
UDS unscheduled DNA synthesis
V/F distribution volume
VOC volatile organic compound
1. SUMMARY
Methyl tertiary-butyl ether (MTBE) is one of several ethers
that may be used as fuel additives and is currently by far the
dominant one. Ethyl tertiary-butyl ether (ETBE), tertiary-amyl
methyl ether (TAME), tertiary-amyl ethyl ether (TAEE) and
diisopropyl ether (DIPE), among others, may supplement, or serve as
alternatives to MTBE for oxygenation or octane enhancement purposes
and may be found, therefore, in association with MTBE.
1.1 Identity, physical and chemical properties, analytical methods
MTBE is a volatile, colourless liquid at room temperature with a
terpene-like odour. It has low viscosity and a boiling point of
55.2°C. The freezing point is -109°C. The density is 0.7404 at 20°C.
The vapour pressure is relatively high, 33 500 Pa at 25°C. MTBE is
flammable and can form explosive mixtures with air. It is very soluble
in other ethers and alcohol. It mixes with gasoline (petrol), and is
soluble in water (42 000 g/m3 at 19.8°C). The log n-octanol/water
partition coefficient is 0.94-1.3. It is unstable in acid solution.
MTBE is analysed in all matrices generally by gas chromatography
(GC) using a range of capillary columns and detector systems that are
suited to the specific matrix. Reverse-phase liquid chromatography
(RPLC) has also been used for analysis of petrol samples.
Sorption/desorption, including purge and trap systems, and headspace
procedures have been used to prepare air, water, sediment and
biological samples for analysis.
1.2 Sources of human and environmental exposure
MTBE is not known to occur naturally in the environment.
Industrially, it is derived from the catalytic reaction of methanol
and isobutylene, and has been produced in several countries in
increasing volumes since the late 1970s. MTBE is currently among the
50 highest production volume chemicals. In 1996, the USA capacity for
production was approximately 10.6 million tonnes, and it is
anticipated that the use of MTBE will continue to increase.
Approximately 25% of gasoline in the USA is blended with MTBE. MTBE is
almost exclusively used to provide both octane enhancement and an
increase in the oxygen content of gasoline. MTBE has been added to
gasoline in concentrations up to 17% by volume.
1.3 Environmental transport, distribution and transformation
After discharge into air, MTBE will largely remain in the air,
with smaller amounts entering soil and water. In the atmosphere, MTBE
can partition into rain. However, only a small amount is removed from
the atmosphere in this manner. Atmospheric transformation by hydroxyl
radicals produces a number of products including the photochemically
stable tertiary-butyl formate (TBF) and 2-methoxy-2-methylpropanol,
which is expected to be highly reactive with hydroxyl radicals,
yielding CO2, formaldehyde, acetone and water. When MTBE is
discharged into water, a significant amount is dissolved, with some
partitioning into air. Partitioning into biota and into sediment is
low. Biodegradability in conventional assays is limited. Generally,
biodegradability is believed to be slow in the environment. When MTBE
is released to the soil, it is transported to the air through
volatilization, to surface water through run-off and to groundwater as
a result of leaching. MTBE can persist in groundwater.
1.4 Environmental levels and human exposure
There are few data on environmental levels and human exposure.
In studies of MTBE in urban air of some cities using oxygenated
gasoline with 15% MTBE, ambient concentrations ranged from
non-detectable to 100.9 µg/m3 (0.028 ppm), with several median
concentrations ranging from 0.47 to 14.4 µg/m3 (0.00013 to 0.004
ppm). Concentrations of MTBE in urban air of some cities where MTBE
was used as an octane enhancer at lower concentrations ranged from
non-detectable to 26.4 µg/m3 (0.0073 ppm).
Concentrations at ground level or near refineries ranged from 15
to 281 µg/m3. Median levels in urban air near blending facilities
were 1508 µg/m3 (0.419 ppm), with ranges of 216-35 615 µg/m3 (0.06
to 9.8 ppm).
At service stations in areas where oxygenated gasoline containing
10-15% MTBE is used, concentrations were highest in the breathing zone
during consumer refuelling (range of 300 to 136 000 µg/m3 (0.09 to 38
ppm), with levels rarely exceeding 3600 µg/m3 (10 ppm), slightly
lower at the pump island (non-detectable to 5700 µg/m3 (1.6 ppm) and
lowest at the station perimeter (non-detectable to 500 µg/m3 (0.14
ppm). Levels were generally higher at service stations without vapour
recovery systems.
Levels in the automobile cabin were 7 to 60 µg/m3 (0.002 to
0.017 ppm) during commutes and 20 to 610 µg/m3 (0.006 to 0.172 ppm)
during refuelling.
Based on limited monitoring confined almost exclusively to the
USA, MTBE has been detected in snow, stormwater, surface water
(streams, rivers, and reservoirs), groundwater and drinking-water.
Concentrations of MTBE detected in stormwater ranged from 0.2 to 8.7
µg/litre with a median of less than 1.0 µg/litre. For streams, rivers
and reservoirs, the range of detection was from 0.2 to 30 µg/litre,
and the range of medians for several studies was 0.24 to 7.75
µg/litre.
MTBE has generally not been detected in deeper groundwater or in
shallow groundwater in agricultural areas. When detected, the
concentration is less than 2.0 µg/litre. MTBE is more frequently found
in shallow groundwater (top 5-10 feet of these aquifers) in urban
areas. In this setting, the concentrations range from less than 0.2
µg/litre to 23 mg/litre, with a median value below 0.2 µg/litre.
MTBE is infrequently detected in public drinking-water systems
from groundwater. In all but 3 out of 51 systems in which it was
reported, the concentration was <20 µg/litre. There are inadequate
data to characterize the concentration of MTBE in public
drinking-water systems from surface water. MTBE has been found at high
levels (i.e. >1000 µg/litre) in a few private wells used for
drinking-water. However, it is doubtful that humans would consume
water with concentrations of MTBE greater than about 50-100 µg/litre
because of its low taste and odour threshold.
Workers with potential exposure to MTBE include those involved in
the production and distribution and use of MTBE and MTBE-containing
gasoline, including service station attendants and mechanics.
Short-term exposure (<30 min) in routine manufacturing
operations and maintenance of neat MTBE ranged from 715 to 43 000
µg/m3 (0.2 to 12 ppm), with average median values being about 3400
µg/m3 (0.95 ppm). Longer-term (30 min to 8 h) exposure ranged from
360 to 890 000 µg/m3 (0.01 ppm to 250 ppm), with median levels being
about 540 µg/m3 (0.15 ppm). For workers in blending operations,
short-term values ranged from non-detectable to 360 000 µg/m3 (100
ppm), the average median being about 5700 µg/m3 (1.6 ppm). Long-term
values ranged from non-detectable to 257 000 µg/m3 (72 ppm), the
average median being about 2000 µg/m3 (0.6 ppm).
Exposures were highest during transportation of neat MTBE and
fuel mixtures through pipelines, barges, railroad cars and trucks
(neat MTBE only), short-term values ranging from 4 to 3750 mg/m3
(0.001 to 1050 ppm) with an average median value of 140 mg/m3 (39
ppm). Long-term values ranged from 0.036 to 2540 mg/m3 (0.01 to 712
ppm), the average median value being 2.85 mg/m3 (0.8 ppm). In
distribution (i.e. loading of MTBE fuel mixtures on trucks and
delivering and unloading at service stations), short-term values
ranged from non-detectable to 225 mg/m3 (63 ppm), the average median
values being around 21 mg/m3 (6 ppm). Long-term values ranged from
0.036 to 22 mg/m3 (0.01 to 6.2 ppm), the average median value being
1.79 mg/m3 (0.5 ppm).
Median short-term exposure levels of service station attendants
ranged generally from 1.071 to 21.42 mg/m3 (0.3 to 6 ppm) and rarely
exceeded 35.7 mg/m3 (10 ppm). Median long-term exposure levels of
service station attendants averaged 1.79 mg/m3 (0.5 ppm). Median
exposures of mechanics were below detection levels for one short-term
study; the average median value for long-term exposure was
approximately 360 µg/m3 (0.1 ppm).
1.5 Kinetics and metabolism
Toxicokinetic data on MTBE in humans are mainly derived from
controlled studies in healthy adult volunteers and in a population
exposed to oxygenated gasoline. MTBE is rapidly absorbed into the
circulation following inhalation exposure. In healthy human volunteers
exposed by inhalation, kinetics of MTBE are linear up to
concentrations of 268 mg/m3 (75 ppm). Tertiary-butyl alcohol (TBA),
a metabolite of MTBE, was measured in blood and urine of exposed
humans. The peak blood levels of MTBE and TBA ranged from 17.2 to 1144
µg/litre, and 7.8 to 925 µg/litre, respectively, in humans exposed to
5.0 to 178.5 mg/m3 (1.4 to 50 ppm) MTBE. Based on a monocompartmental
model, rapid (36-90 min) and slower (19 h) components of MTBE
half-life have been identified.
In rodents, MTBE is well absorbed and distributed following oral
administration and inhalation exposure, with lower dermal absorption.
At 400 mg/kg oral and 28 800 mg/m3 (8000 ppm) inhalation exposure,
the percentage of total absorbed dose eliminated in expired air
increased with a corresponding decrease in the percentage eliminated
in urine, indicating a saturation of metabolism. TBA was not
identified in the urine of exposed rats. There is evidence of further
metabolism of TBA, based on the identification of
2-methyl-1,2-propanediol and alpha-hydroxyisobutyric acid excreted in
the urine. In vitro studies provide evidence that MTBE is
metabolized to TBA, formaldehyde and acetone.
1.6 Effects on laboratory animals and in vitro systems
In rats, the acute median oral lethal dose (LD50) is
approximately 3800 mg/kg bw. The acute median lethal concentration
(LC50) value for a 15-min inhalation exposure is about 141 000 mg/m3
air in mice. Signs of intoxication include CNS depression, ataxia and
laboured respiration. When the dose was non-lethal, recovery was
complete. The LD50 for dermal toxicity in rabbits is >10 200 mg/kg
bw.
In a single identified study, MTBE was "moderately" irritating to
skin, causing moderate erythema and oedema following dermal
application to rabbits. It was also irritating to the eyes of rabbits,
causing mild, reversible changes. In the only identified study, MTBE
induced slight to severe respiratory irritation following exposure of
mice to 300 to 30 000 mg/m3, respectively. It did not induce skin
sensitization in studies in guinea-pigs.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Lowest reported effect levels for nephrotoxicity following
ingestion in 90-day studies are 440 mg/kg bw per day (increases in
relative kidney weight and hyaline droplet formation in Sprague-Dawley
rats). With inhalation exposure to 2880 mg/m3 (800 ppm), there were
increases in kidney weight associated at higher concentrations with a
mild increase in hyaline droplets in the proximal tubules in
Fischer-344 rats. In inhalation oncogenicity studies, at 1440 mg/m3
(400 ppm) the incidence and severity of chronic progressive
nephropathy was increased in male rats; in male mice, at this level,
there were increases in absolute liver weight (which correlated with
increases in hepatocellular hypertrophy at higher concentrations) and
an increase in relative kidney weight.
Exposure to MTBE also results in reversible central nervous
system (CNS) effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2880 mg/m3 (800 ppm) produced
reversible dose-related changes in motor activity in single sexes.
These effects were transient and not evident in longer-term studies.
One- and two-generation inhalation reproductive studies in rats
and four developmental studies in rats, mice and rabbits have been
identified. In these studies, specific reproductive effects were not
observed in rats at concentrations up to 28 800 mg/m3. MTBE has not
induced developmental effects at concentrations below those that were
toxic to the mothers. Decreases in uterine weight and increases in
estrogen metabolism in mice have been observed at 28 800 mg/m3.
MTBE has been adequately tested in a broad range of mutagenicity
and other genotoxicity tests. The results from these studies indicate
that MTBE is not genotoxic, although a mouse lymphoma cell tk locus
mutation assay was positive, due to the metabolism of MTBE to
formaldehyde.
Carcinogenicity studies have been conducted involving inhalation
exposure of Fischer-344 rats and CD-1 mice and gavage dosing of
Sprague-Dawley rats. In neither of the inhalation studies were methods
of statistical analysis used that adjusted for survival differences.
There were significant increases in tumour incidence in all three
studies, namely renal tubular cell tumours and Leydig cell tumours in
the male Fischer-344 rats, Leydig cell tumours in male and
leukaemias/lymphomas (combined) in female Sprague-Dawley rats, and
liver cell tumours in female CD-1 mice. The renal tubular cell tumours
and the leukaemia/lymphomas were not observed consistently, therefore,
in the different studies in rats. In addition, the sex-specific kidney
tumours were associated with sex-specific alpha2u-globulin
nephropathy, which was observed in several studies of short duration.
Increases in Leydig cell tumours occurred at the highest dose level
(1000 mg/kg bw) in the Sprague-Dawley rats, but interpretation of the
increases recorded for Fischer-344 rats was complicated by the very
high concurrent and historical control incidences. The mouse liver
tumours occurred at incidences in the control and 28 800 mg/m3 (8000
ppm exposed groups, respectively, of 2/50 and 10/50 in females and
12/49 and 16/49 in males. The increases were modest and were
accompanied by hepatocellular hypertrophy.
1.7 Effects on humans
Following the introduction of two separate fuel programmes in the
USA requiring the use of gasoline oxygenates (not necessarily MTBE),
consumers in some areas have complained about acute health symptoms
such as headache, eye and nose irritation, cough, nausea, dizziness
and disorientation. Epidemiological studies of human populations
exposed under occupational as well as non-occupational conditions, and
experimental studies of human volunteers exposed under controlled
conditions, have not been able to identify a basis for these
complaints. Although results are mixed, community studies conducted in
Alaska, New Jersey, Connecticut, and Wisconsin, USA, have provided
limited or no evidence of an association between MTBE exposure and the
prevalence of health complaints.
In controlled experimental studies on adult volunteers exposed in
inhalation chambers to MTBE at concentrations ranging from 5.0 mg/m3
(1.4 ppm) up to 270 mg/m3 (75 ppm), there were no evident effects in
terms of either subjective reports of symptoms or objective indicators
of irritation or other effects up to 180 mg/m3 (50 ppm) for as long
as 2 h. From this evidence it appears unlikely that MTBE alone induces
adverse acute health effects in the general population under common
conditions of inhalation exposure. However, the potential effects of
mixtures of gasoline and MTBE, and the manner in which most persons
are exposed to MTBE in conjunction with the use of oxygenated fuels,
have not been examined experimentally or through prospective
epidemiological methods. Moreover, the role of factors such as
awareness of MTBE, due in part to its distinctive odour, for example,
have not been investigated.
1.8 Effects on other organisms in the laboratory and field
The experimental acute toxicity (LC50) of MTBE to fish,
amphibians and crustaceans is > 100 mg/litre. There are no data on
chronic or sub-lethal toxicity to aquatic species, or on toxicity to
terrestrial organisms.
1.9 Evaluation of human health risks and effects on the environment
Based on collective evidence, it appears unlikely that MTBE alone
induces adverse acute health effects in the general population under
common exposure conditions.
In studies on animals, MTBE is "moderately" acutely toxic and
induces mild skin and eye irritation but not sensitization. Repeated
exposure affects primarily the kidney of rats and the liver of mice,
with lowest reported adverse effect levels of 440 mg/kg bw per day in
rats following ingestion and 1440 mg/m3 (400 ppm) following
inhalation. MTBE has not induced adverse reproductive or developmental
effects at concentrations less than those that were toxic to the
parents.
MTBE is not genotoxic but has induced tumours in rodents
primarily at high concentrations that also induce other adverse
effects. These data are considered currently inadequate for use in
human carcinogenic risk assessment. The Task Group concluded that, in
order to provide quantitative guidance on relevant limits of exposure
and to estimate risk, acquisition of additional data in several areas
is necessary.
It does not appear that the concentrations of MTBE in ambient
water are toxic to aquatic organisms except during spills. Although
there are no data on the terrestrial toxicity of MTBE, this appears
not to be of concern since concentrations in ambient air are low and
its half-life is relatively short.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C5H12O
Chemical structure: CH3
'
H3C - C - O - CH3
'
CH3
Relative molecular mass: 88.15
Common name: methyl tertiary-butyl ether
IUPAC Chemical name: 2-methoxy-2-methyl propane
CAS registry number: 1634-04-4
Synonyms: 1,1-dimethylethyl methyl ether; ether
tert-butyl methyl; éther methyl
tert-butylique (French); MBE; methyl
1,1-dimethylethyl ether; methyl- t-butyl
ether; methyl tert-butyl ether;
(2-methyl-2-propyl) methyl ether;
metil-terc-butileter (Spanish);
2-methoxy-2-methylpropane; MTBE; propane,
2-methoxy-2-methyl-(CA); t-butyl methyl
ether; tert-butoxymethane; tert-butyl
methyl ether
Major trade names: 3 D Concord
Driveron
HSDB 5487
UN 2398
Constituent components of typical commercial grade:
(ARCO, 1989)
Component Weight %
MTBE 97.5
di-, tri-isobutylene, and
t-butyl alcohol 0.6
Methanol 0.2
C4 hydrocarbons 1
C5 hydrocarbons 0.4
other 0.3
water content <0.05
2.2 Physical and chemical properties
Table 1 lists the physical and chemical properties of MTBE.
2.3 Conversion factors
1 ppm = 3.57 mg/m3 at 25°C (1 atmosphere pressure)
1 mg/m3 = 0.28 ppm at 25°C (1 atmosphere pressure)
2.4 Analytical methods
Analytical methods that have been used for MTBE and for
tertiary-butanol (TBA), which is an intermediate in the aerobic
bacterial degradation of MTBE and in its mammalian metabolism, are
given for various media.
Some commonly used methods are summarized in Table 2.
2.4.1 Procedures
2.4.1.1 Air
Air samples are collected in stainless steel canisters, and the
volatile compounds concentrated in a two-stage trap to sorb the
Table 1. Physical and chemical properties of MTBE organic compounds
and to collect water. Drying is done by purging with dry N2 at 25°C,
and the organic compounds thermally desorbed at 220°C by back-flushing
with helium. The samples can be analysed by gas chromatography/mass
spectrometry (GC-MS) using a capillary column (Kelly et al., 1993).
Harper & Fiore (1995) used a passive diffusion technique to collect
samples.
Automobile exhaust samples are collected in 3-litre bags. Diluted
emissions are concentrated in variable temperature control traps,
operating between -60°C and 180°C (DB 1 column) or between -99°C and
180°C (GS-Q megabore column). Using these twin columns, separation of
all the major components is possible (Hoekman, 1993).
2.4.1.2 Soil, water and sediment
Static headspace analysis can be used for samples of soil and
groundwater. Samples are collected in filled bottles, air is
introduced, and the bottles are shaken and equilibrated before
analysis of the gas phase.
One method is by GC-FID/PID using a megabore DB-1 capillary
column (Roe et al., 1989). Samples of groundwater can be collected
with a cone penetrometer coupled with a porous probe, and analysed by
GC using a photoionization detector (PID) (Chiang et al., 1992).
Table 1. Physical and chemical properties of MTBE
Physical state Liquid
Colour Colourless
Odour Strong, characteristic terpene-like
Freezing point (°C) -109 Windholz, 1983
Boiling point (°C) 53.6-55.2 Mackay et al., 1993
Selected valuea 55.2
Flash point (°C) -28 Budavari et al., 1996
Ignition temperature (°C) 224 Budavari et al., 1996
Spontaneous ignition temperature (°C) 460 Wibowo, 1994
Flammability Flammable/combustible
Flammability limits 1.5-8.5% in air ECETOC, 1997
Vapour pressure (Pa at 25°C) 32 659 to 33 545 Mackay et al., 1993
Selected valuea 33 500 Mackay et al., 1993
Density (g/cm3 at 20°C) 0.7404 to 0.7478 Mackay et al., 1993
Selected valuea 0.7404
Relative vapour density (air=1) 3.1 Wibowo, 1994
Log kow octanol/water partition coefficient 0.94 to 1.30 Mackay et al., 1993
Selected valuea 0.94
Henry's law constant at 25°C (Pa m3/mol) 59.46 to 304.96 Mackay et al., 1993
Selected valuea 70.31
Table 1. (continued)
Physical state Liquid
Dimensionless Henry's law constant (H/RT) at 25°C 0.0239 to 0.1221 Zogorski et al., 1996
Selected valuea 0.018 at 20°C
Water solubility g/m3 at 25°C 32 200 to 54 353 Mackay et al., 1993
Selected valuea 42 000 (at 19.8°C)
Solubility of MTBE in water 48 Budavari et al., 1996
(g/litre) at 25°C
Solubility of water in MTBE (g/litre) at 25°C 15 Budavari et al., 1996
Solubility in organic solvents: - very soluble in other ethers and
alcohols
- mixes with gasoline
Viscosity, g/sec. -cm 0.003 to 0.004 (calculated) Lyman et al., 1990
Other properties Unstable in acid solution pKa = -3.70
at 23°C (measured)
Organoleptic properties
Taste 134 µg/litre (0.134 ppm) TRC, 1993
Odour
- detection threshold 0.19 mg/m3 TRC, 1993
- recognition threshold 0.29 mg/m3 (0.08 ppm) TRC, 1993
a Criteria of selection were based on:
i) the age of the data and acknowledgement of previous conflicting or supporting values;
ii) the method of determination;
iii) the perception of the objectives of the investigators, and their need for quantitative values; and
iv) information derived from Quantitative-Structure-Property-Relationships.
Table 2. Summary of analytical procedures for MTBE
Matrix Procedure Detector Detection limit Reference
Air Sorption/desorption GC-MS 0.72-3.6 µg/m3 Kelly et al., 1993
Vehicle emission Sorption/desorption GC-FID 18-36 µg/m3 Hoekman, 1993
Water Static headspace GC-PID 10.8 µg/m3 (water) Chang et al., 1992
1.08 µg/m3 (air)
Water Purge and trap GC-MS 5 µg/litre Bianchi & Varney, 1989
Water Purge and trap GC-MS 0.52-0.090 µg/litre Munch & Eichelberger, 1992
Water Purge and trap GC-MS 0.06 µg/litre Raese et al., 1995
Sediment Purge and trap GC-MS 10-100 ng/kg Bianchi et al., 1991
Blood Purge and trap GC-MS 0.01 µg/litre Bonin et al., 1994
Gasoline Direct GC-FID 18-36 µg/m3 Johansen, 1984
(5-10 ppbv)
For samples of water and sediment, purge and trap procedures are
widely used to concentrate volatile components before analysis. For
water samples, the analytes are desorbed by open-loop stripping for
60 min at 60°C and collected on a mixture of Tenax TA and
Chromosorb-106. Desorption is then done using helium at 150°C before
analysis.
Analysis can be by GC-MS (Bianchi & Varney 1989). An expanded
procedure for volatile organic compounds developed by the US
Environmental Protection Agency (US EPA) uses a three-trap collection
system (Tenax, silica gel and charcoal) followed by GC-MS
quantification: for MTBE, a detection limit of 0.09 µg/litre was
attained using a DB-624 capillary column and a purging efficiency of
74% (Munch & Eichelberger 1992). An essentially similar procedure has
been used for estuarine sediment samples with an OV-1701 capillary
column (Bianchi et al., 1991).
MTBE in ambient groundwater has been analysed by the US
Geological Survey since 1991 using a purge and trap GC-MS method
(Raese et al., 1995). The estimated detection limit for reagent water
spiked with MTBE at 0.2 µg/litre is 0.06 µg/litre. A method for the
concurrent analysis of MTBE, TBA and tert-butyl formate (TBF) has
been developed (Church et al., 1997). The method employs direct
aqueous injection and GC-MS, and has a detection level of 0.1 µg/litre
for MTBE.
2.4.1.3 Gasoline
Samples of gasoline can be analysed directly by GC using the
following procedures. They have all shown good selectivity for
oxygenates:
- An infrared (IR) detector, using a column of Poropak Q plus
Poropak N, gave a limit of detection of 0.1% (w/v) with the
detector set at 8.3 µm (Cochrane & Hillman 1984).
- A detector system (GC-O-FID), in which oxygenates are
catalytically cracked to CO followed by reduction to methane, has
a selectivity better than 1:107 (Verga et al., 1988).
- FID with a dual column system using Durawax 1 and Durabond-S
gives acceptable accuracy and repeatability at a concentration of
1% (w/w) (Levy & Yancey 1986). An alternative procedure uses
switching (Johansen 1984).
- Atomic emission detection (AED) using 777 nm near infrared (NIR)
emission and a DB-1 capillary column is a sensitive method (Diehl
et al., 1995).
- Reversed-phase liquid chromatography (RPLC) with a Hi-Chrom
"reversible column" packed with Spherisorb ODS-11 and a
refractive index detector (RID) can be used with a mobile phase
of acetonitrile:water (6:4) and back-flushing suited to the
relevant analytes (Pauls 1985). It is important that the analyte
is completely dissolved in the mobile phase.
2.4.1.4 Biological samples
Headspace or purge-and-trap concentrations of MTBE are directly
applicable to blood and urine samples. The purge and trap procedure is
coupled to quantification by GC-MS using 2H-labelled standards.
Direct GC analysis of samples is less commonly used but Schuberth
(1996), using the full headspace technique combined with capillary GC
and ion-trap detection, determined MTBE with a detection limit of
0.4-1 nmol in blood and brain tissue.
a) Blood, urine and tissues
The purge-and-trap system can be used for the analysis of blood
samples. Sorption is done with a Tenax trap and a cryogenic trap
decreasing in temperature to -150°C with desorption at 180°C. GC-MS
analysis uses a DB 624 column. This has been applied to MTBE and to
TBA using [2H12] MTBE and [2H9] TBA as the respective standards
(Bonin et al., 1994).
Headspace analysis has been used for the analysis of both MTBE
and metabolically produced TBA in a range of matrices including blood
and urine. For blood samples, GC with an SE 50 column and FID can be
used (Savolainen et al., 1985). Analysis of TBA produced from MTBE by
hepatic microsomes from rats can be made with a Carbowax B/5% Carbowax
20M packed column and FID (Brady et al., 1990). A procedure applicable
to blood and urine samples uses an SPB-1 column and FID (Streete et
al., 1992). However, this procedure appears not to have been validated
using samples contaminated with MTBE or TBA. The procedure can be
applied to tissue samples after treatment with a proteolytic enzyme
before analysis.
Analysis of MTBE (and TBA) in brain (cerebral hemispheres) and in
perirenal fat from rats dosed with MTBE was made by homogenizing the
samples in dimethyl formamide, centrifuging, and direct GC analysis of
the supernatant using a packed column with Carbowax 20M and FID
(Savolainen et al., 1985).
b) Bacterial cultures
Samples of bacterial cultures that metabolize MTBE have been
analysed for both MTBE and its metabolite TBA by direct GC analysis
using FID and a Quadrex methyl silicone capillary column (Salanitro et
al., 1994). Analysis of MTBE (and TBA) in bacterial cultures that
degraded TBA, though not MTBE, used a GC capillary column coated with
a cross-bound phase (CP-Sil 13, Chrompack) and an FID detector (Allard
et al., 1996).
14C-labelled MTBE has been used in a few investigations. In one
study dealing with aerobic biodegradation, 14CO2 was collected after
incubation as Ba14CO3, and the fraction incorporated into cells was
separated by filtration though 0.45 µm Millipore filters (Salanitro et
al., 1994). In another study on the accumulation of MTBE into plants,
samples were extracted with dimethylformamide for counting (Schroll et
al., 1994).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Natural sources of MTBE have not been reported in the scientific
literature.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
MTBE is an oxygenate (oxygen-containing hydrocarbon) that is
industrially produced in several countries, including Austria,
Belgium, Canada, Finland, France, Germany, Italy, Japan, Mexico, the
Netherlands, Norway, Portugal, Sweden, Taiwan, the United Kingdom, the
USA and Venezuela.
The worldwide annual production of MTBE in 1995 was about 15
million tonnes. In the USA, in 1994 MTBE ranked 18th in terms of
production volume (6 175 000 tonnes (13.61 billion pounds)) and in
1995 there was an increase to 12th position (8 000 000 tonnes (17.62
billion pounds)) (CEN, 1996). During the years 1985-1995, production
of MTBE in the USA showed an annual increase of 25% (Storck et al.,
1996). The potential demand for MTBE is expected to increase to
284 000 barrels/day (12.2 million tonnes per year) in the year 2000.
North America is the largest consumer of MTBE, accounting for
about two-thirds of the world's annual use. In 1996 the USA was the
world's largest consumer of MTBE with a usage of 246 000 barrels/day
(10.6 million tonnes per year). Western Europe, the eastern
Mediterranean area and Asia, and Latin America used progressively
smaller amounts of MTBE in 1995. Most growth in the production
capacity of MTBE is expected to occur in the eastern Mediterranean
area, South America and the USA.
MTBE is prepared principally by reacting isobutylene (contained
in a mixed C4 stream) with methanol over an acidic ion-exchange resin
catalyst such as sulfonated styrene cross-linked with divinyl benzene
in the liquid phase and at 38-93°C and 100-200 psi. It can also be
prepared from methanol, TBA and diazomethane (Budavari et al., 1996).
3.2.2 Uses
The main use of MTBE is as an additive to gasoline. MTBE was
first added to gasoline in the late 1970s on a voluntary basis as an
octane enhancer when the phase-out of tetraethyl lead commenced, and
this use continues. MTBE is also added to gasoline in higher amounts
(up to 15% by volume) as part of national mandated air pollution
abatement programmes to reduce ambient air levels of carbon monoxide
(CO) or ozone, or both, and in reformulated gasoline (RFG) (10-11% by
volume) to reduce the emissions of benzene and other volatile
hydrocarbons. MTBE is also used in the manufacture of isobutene
(Lewis, 1993) and a minor proportion is used as a therapeutic agent
for in vivo dissolution of cholesterol gallstones in humans (Allen
et al., 1985a,b; Di Padova et al., 1986; Murray et al., 1988; Sternal
& Davis, 1992).
In the USA, oxygenated gasolines are required in two national
programmes to improve air quality (the oxygenated fuels programme and
the reformulated gasoline programme) outlined in the 1990 Clean Air
Act Amendments. MTBE is not specifically required in these programmes,
but it is the most widely used oxygenate. The winter oxygenates
programme requires gasoline sold in areas that do not meet federal air
quality standards for CO to contain no less than 2.7% oxygen by
weight, which is equal to 15% MTBE by volume. According to the
reformulated gasoline programme, large metropolitan areas with serious
ozone problems are required to use reformulated gasoline (RFG): this
is a special blend of gasoline that must contain 2% oxygen by weight
and a maximum of 1% benzene and 25% aromatic hydrocarbon by volume. To
meet this requirement, reformulated gasoline would contain 11% MTBE by
volume. About 90% of the MTBE consumed in the USA in 1996 was used in
reformulated gasoline. At the end of 1996, MTBE was used in
approximately 25% of the total gasoline pool.
During the winter driving season, 15% MTBE by volume is added to
gasoline as an oxygenate to reduce CO emissions from motor vehicles.
The extent of CO reductions depends on the fuel metering system and
emissions control technology used on the vehicle (Prakash, 1989). The
addition of oxygenates to gasoline blends generally reduces the
hydrocarbon (HC) emissions to the atmosphere. However, the levels of
exhaust nitrogen oxides (NOx) increase when the oxygenate
concentration exceeds about 2% oxygen by weight (SNV, 1993). It also
increases the aldehyde emissions from automobile exhausts, but has not
been found to have any major influence on the chemical composition of
particulate emissions from vehicles (Watson et al., 1990). The
aldehyde (not specified) emissions are significantly reduced by
three-way catalytic converters (Prakash, 1989).
In a model analysis of changes in the concentrations of eight
volatile organic compounds (VOCs), i.e. acetaldehyde, benzene,
1,3-butadiene, ethylbenzene, formaldehyde, toluene, xylenes, and
particulate organic matter (POM), resulting from the use of
reformulated gasoline and oxyfuel containing MTBE, Spitzer (1997)
concluded that, with the exception of formaldehyde, exhaust emissions
of these VOCs would be decreased. The increased formaldehyde emissions
would, however, be offset by the reduction in the formation in the
atmosphere of formaldehyde from the other VOCs. Erdal et al. (1997)
modelled atmospheric ozone pollution reduction by the use of MTBE in
gasoline. Ozone is formed by the reaction of sunlight with NOx and
VOCs. The use of MTBE reduces VOC and NOx exhaust emissions and also
reduces fuel evaporation. The model estimates a reduction in peak
ambient ozone levels of 3.6-18 µg/m3 (1-5 ppb).
It is estimated that MTBE-blended gasolines account for
approximately 2% of the total unleaded gasoline in Canada (Environment
Canada, 1992). Levels of MTBE in blended gasolines range from 0.04% to
9.09% by volume, depending on the grade of gasoline, season and
geographical area. Since the use of oxygenates is not required in
Canada as part of an air abatement programme, each refiner blends in
the amounts of MTBE that it requires in order to obtain a good
gasoline end-product, depending on the batch of crude oil and the
technology used in the refinery. In 1997, the maximum concentration of
MTBE allowed in Canadian gasoline was 2.7% mass oxygen (approximately
15% by volume).
3.2.3 Sources and releases to the environment
Similar to hydrocarbon components of gasoline, fuel oxygenates
such as MTBE enter the environment during all phases of the petroleum
fuel cycle. Sources include, for example, auto emissions, evaporative
losses from gasoline stations and vehicles, storage tank releases,
pipeline leaks, other accidental spills, and refinery stack releases.
Annual estimates of MTBE mass releases to the environment from all
potential sources have not been reported in the scientific literature.
However, releases from storage tanks, vehicular emissions and
evaporative losses from gasoline stations and vehicles are perceived
to be important sources (Zogorski et al., 1996; US Interagency
Assessment, 1997).
3.2.3.1 Industrial releases
No information on industrial releases of MTBE to the environment
have been found in the scientific literature, except in the case of
the USA and Canada.
Industrial releases of MTBE in the USA have been characterized
for 1993. A total of 136 facilities released MTBE to the environment,
with an estimated total release of 1700 tonnes. Approximately 84% of
the release was by petroleum refineries, and almost all of the MTBE
was released to air (Zogorski et al., 1996).
In 1994, the total Canadian industrial release of MTBE from
refiners and manufacturers was approximately 28.2 tonnes, the bulk of
which was released into the air (98.1%) and a small amount into water
(1.9%) (Environment Canada, 1996a). The highest amounts of MTBE
released were 9.5, 9.1, 8.4 and 1.0 tonnes by industries located in
Sarnia, Burnaby, Edmonton and Saint John, respectively.
3.2.3.2 Storage tank release
Releases of gasoline containing MTBE from storage tanks may
contaminate soil and groundwater. In some cases, MTBE may enter
drinking-water supplies. In 1989 it was estimated that in the USA
there were approximately 14 000 above-ground storage tank facilities
with an estimated 70 000 tanks, of which 30-40% were used for gasoline
storage (API, 1989a). A subsequent survey of 299 storage facilities
showed that 40% had identified subsurface contamination (API, 1994).
Many sites have been identified with soil or groundwater hydrocarbon
contamination that required corrective action. The extent of MTBE
contamination at these sites is largely undocumented because
monitoring of MTBE has not been required. More stringent
release-prevention and -detection standards are now required in the
USA and, when fully implemented by December 1998, these requirements
should considerably decrease the annual volume of gasoline released to
soil and groundwater.
It is important to note that when gasoline containing MTBE enters
groundwater, high concentrations of MTBE (i.e. in excess of 1000
µg/litre) can occur. While comprehensive data on the occurrence of
MTBE in drinking-water provided from groundwater do not exist, there
have been some instances reported in the USA where drinking-water
supplies have been disrupted because of high MTBE levels. For example,
two well fields serving the city of Santa Monica, California, have
been contaminated with MTBE necessitating the purchase of alternative
water for drinking-water.
3.2.3.3 Engine emissions from on-road and off-road vehicles and
recreational boats
The use of gasoline containing MTBE in on-road and off-road
vehicles, boats and small engines will result in MTBE releases to the
environment unless recovery systems are employed. The extent of these
emissions has not been thoroughly studied, and there are few
scientific citations.
Drivas et al. (1991) estimated ambient air concentrations of
evaporative and exhaust emissions of MTBE gasoline blends during two
different situations representing worst-case concentrations: a car
idling in an open garage and a car just stopped and turned off
(hot-soak evaporative emission) in a closed garage. The predicted
maximum exhaust air concentration of MTBE was calculated to be
0.24 mg/m3 (0.07 ppm).
MTBE was not detected in samples from light-duty vehicle
emissions measured in the Caldecott Tunnel, San Francisco Bay Area, in
August 1994, when the average oxygen content of gasoline sold in the
area was 0.3% by weight (Kirchstetter et al., 1996). In October, when
the average oxygen content in MTBE gasoline was 2.0% by weight, the
concentration of MTBE in emissions was 3.3% by weight of total VOCs.
Comparison of emissions from vehicles using a standard fuel and a
reformulated fuel that contained MTBE (11% by volume) showed a
reduction in mass emission rates in the latter (Hoekman, 1992).
Although there was a decrease in the emissions of aromatics and
alkanes, the levels of alkenes and carbonyl compounds increased, and
there was considerable variation among the vehicles that were tested.
A study in California showed that increasing the concentration of MTBE
from 0.3% by weight in August to 1.6 % MTBE plus 0.4% ethanol in
October resulted in lowered emission of aromatics but increased
emissions of isobutene (86%), cisbut-2-ene (150%), formaldehyde (39%),
propionaldehyde (200%), methacrolein (50%) and butyraldehyde (40%)
(Kirchstetter et al., 1996).
Boat motors and small engines used in chain saws, other power
tools, snowmobiles, lawn mowers and garden tillers, for example, may
also release MTBE to the environment via exhaust, evaporative losses
and release of uncombusted fuel. The magnitude and significance of
these releases are not documented. In 1997 MTBE was detected in
several public water supply reservoirs that, in part, provide
drinking-water for Southern California. The predominant source of MTBE
is thought to be associated with small engines used on recreational
boats. Such engines are known to be inefficient, and release
uncombusted gasoline and emissions to water and air.
3.3 Other pertinent information
All aspects of the effectiveness of fuel oxygenates on ambient
air quality, including carbon monoxide, hydrocarbons, oxides of
nitrogen, aromatics, aldehydes and alcohols, and associated
atmospheric degradation products, have been reviewed in a number of
reports (e.g., Prakash, 1989; Environment Canada, 1993; Schuetzle et
al., 1994; HEI, 1996; Kirchstetter et al., 1996; US Interagency
Assessment, 1997).
Overall, these studies indicate that, when compared to other
gasolines, MTBE gasoline blends generally reduce CO and hydrocarbon
exhaust emissions and increase aldehyde and NOx emissions.
4. ENVIRONMENTAL BEHAVIOUR AND FATE
4.1 Transport and distribution between media
A diagram depicting the movement of MTBE in the environment is
shown in Fig. 1.
4.1.1 Air
It can be predictable from its physicochemical properties that,
when MTBE is released into air, the greater part will exist in the
atmosphere, with small amounts entering soil and water (Mackay et al.,
1993). Based on its Henry's law constant, MTBE should partition into
atmospheric water, including rain. The concentration of MTBE in
precipitation would be in direct proportion to its concentration in
air. However, falling precipitation removes only a negligible amount
of the gas-phase compound (Zogorski et al., 1996). Therefore, chemical
degradation of MTBE should be the major removal process from the air
(Mackay et al., 1993).
4.1.2 Water
Transport and distribution of a substance between and within
media in the aquatic environment is dependent upon its solubility,
movement of the water itself, exchanges at the air-water interfaces,
adsorption to sediment and particulate matter, and bioconcentration in
aquatic organisms. The residence time in water is also dependent upon
the type of environmental conditions encountered, such as
temperatures, wind speeds, currents and ice cover (Environment Canada,
1993).
MTBE can volatilize from surface water and be removed by aeration
(Zogorski et al., 1996). According to calculations by Pankow et al.
(1996), no single volatilization half-life (t´) will characterize
the loss process from water. In surface water, the most important
factors for the volatilization rates are the depth and velocity of the
flow. In deep and slow-moving flows, the t´ values at both 5°C and
25°C are 85 and 78 days for calm and windy conditions, respectively.
These rates were shown to be similar to those for benzene, toluene,
ethyl benzene and xylene (BTEX) compounds. In shallow and fast-moving
flows, changing from calm to windy conditions causes a significantly
accelerated volatilization rate. Under these circumstances, MTBE
volatilizes more slowly than benzene, although it was suggested that
this is of no practical significance, as both substances volatilize
quickly in such flows. It was concluded that the t´ values for MTBE
are highly dependent on depth and mean flow velocity. Thus, quite
large as well as very small t´ values are possible.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL TERTIARY-
BUTYL ETHER
Members
Dr R. B. Beems, National Institute of Public Health & the Environment,
Bilthoven, The Netherlands
Dr A. Bobra, Environment Canada, Hull, Quebec, Canada
Dr S. Borghoff, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr J.M. Davis, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, North
Carolina, USA (Vice-Chairman)
Dr L. Fishbein, Fairfax, Virginia, USA
Dr M. Gillner, National Chemicals Inspectorate, Solna, Sweden
(Co-Rapporteur)
Mr G. Long, Environmental Health Centre, Health Canada, Ottawa, Canada
(Co-Rapporteur)
Dr M.E. Meek, Environmental Health Centre, Health and Welfare Canada,
Ottawa, Canada (Chairman)
Dr A.A.E. Wibowo, Coronel Institute, University of Amsterdam,
Amsterdam, The Netherlands
Observers
Dr M. Constantini, Health Effects Institute, Cambridge, Massachusetts,
USA (representing the Health Effects Institute (HEI))
Dr J. Del Pup, Texaco Inc., New York, USA (representing the American
Industrial Health Council (AIHC))
Mr R. Hillier, Oil, Chemical and Atomic Workers' International Union
(OCAWIU), Lakewood, Colorado, USA (representing the International
Federation of Chemical, Energy, Mine and General Workers' Unions
(ICEM))
Dr A.K. Mallett, Arco Chemical Europe Inc., Maidenhead, United Kingdom
(representing the European Centre for Ecotoxicology and Toxicology of
Chemicals (ECETOC))
Dr M. Mehlman, Princeton, New Jersey, USA (Technical Adviser to Mr
Hillier, OCAWIU)
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International Union of
Pharmacology (IUPHAR))
Dr J. Zogorski, US Department of the Interior, Rapid City, South
Dakota, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, World Health
Organization, Lyon, France
Assisting the Secretariat
Miss C. Grande, Air Issues Section, Health Canada, Ottawa, Canada
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL
TERTIARY-BUTYL ETHER (MTBE)
A WHO Task Group on Environmental Health Criteria for methyl
tertiary-butyl ether met at the Conference Facility, Lord Elgin
Hotel, Ottawa, Canada from 17 to 21 April 1997. Dr E.M. Smith, IPCS,
welcomed the participants on behalf of Dr M. Mercier, Director of the
IPCS, and the three IPCS cooperating organizations (UNEP/ILO/ WHO).
The Group reviewed and revised the draft and made an evaluation of the
risks for human health and the environment from exposure to methyl
tertiary-butyl ether.
The first draft of the EHC was prepared by Dr M. Gillner,
National Chemicals Inspectorate, Solna, Sweden, with contributions
from Ms A.-S. Nihlén, Institute for Working Life, Solna, Sweden. Dr M.
Gillner and Ms Nihlén also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS contact points for Environmental Health Criteria monographs.
Dr E.M. Smith and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The financial support of the Swedish National Chemicals
Inspectorate in preparing the monograph and the Canadian Health
Protection Branch, Environmental Health Directorate, in funding the
Task Group meeting in Ottawa are gratefully acknowledged.
ABBREVIATIONS
AED atomic emission detector
ALAT alanine aminotransferase
AP alkaline phosphatase
AUC area under the curve
BCF bioconcentration factor
BTEX benzene, toluene, ethyl benzene and xylenes
BUN blood urea nitrogen
bw body weight
CHOL cholesterol
CL total plasma clearance
CNS central nervous system
CO carbon monoxide
DEN diethylnitrosamine
DIPE diisopropyl ether
DMN N-nitrosodimethylamine
EC electron capture
EROD 7-ethoxyresorufin- O-deethylase
ETBE ethyl tertiary-butyl ether
FID flame ionization detector
FOB functional observational battery
FTIR Fourier-transform infrared
GC gas-chromatography
GC-MS gas-chromatography/mass spectrometry
GC-O gas-chromatography using an oxygen-selective detector
Hb haemoglobin
HC hydrocarbon
HPLC high-performance liquid chromatography
HPRT hypoxanthine-guanine phosphoribosyl transferase
IL-1 interleukin-1
IL-4 interleukin-4
ip intraperitoneal
IR infrared
iv intravenous
Koc adsorbtion coefficient to soil organic carbon
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
LGL large granular lymphocyte
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LT50 median lethal time
MCH mean corpuscular haemoglobin
MCHC mean corpuscular haemoglobin concentration
MCS multiple chemical sensitivities
MCV mean corpuscular volume
MTBE methyl tertiary-butyl ether
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIR near infrared
NMOC non-methane organic carbon
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NOx oxides of nitrogen (NO, NO2, N2O4 and N2O3)
PID photoionization detector
ppb parts per billion
ppbv parts per billion (by volume)
ppm parts per million
PROD 7-pentoxyresorufin- O-dealkylase
RBC red blood cell
RFG reformulated gasoline
RID refractive index detector
RPLC reversed-phase liquid chromatography
sc subcutaneous
SCE sister chromatid exchange
SD standard deviation
TBA tertiary-butyl alcohol
TBF tertiary-butyl formate
TWA time-weighted average
UDS unscheduled DNA synthesis
V/F distribution volume
VOC volatile organic compound
1. SUMMARY
Methyl tertiary-butyl ether (MTBE) is one of several ethers
that may be used as fuel additives and is currently by far the
dominant one. Ethyl tertiary-butyl ether (ETBE), tertiary-amyl
methyl ether (TAME), tertiary-amyl ethyl ether (TAEE) and
diisopropyl ether (DIPE), among others, may supplement, or serve as
alternatives to MTBE for oxygenation or octane enhancement purposes
and may be found, therefore, in association with MTBE.
1.1 Identity, physical and chemical properties, analytical methods
MTBE is a volatile, colourless liquid at room temperature with a
terpene-like odour. It has low viscosity and a boiling point of
55.2°C. The freezing point is -109°C. The density is 0.7404 at 20°C.
The vapour pressure is relatively high, 33 500 Pa at 25°C. MTBE is
flammable and can form explosive mixtures with air. It is very soluble
in other ethers and alcohol. It mixes with gasoline (petrol), and is
soluble in water (42 000 g/m3 at 19.8°C). The log n-octanol/water
partition coefficient is 0.94-1.3. It is unstable in acid solution.
MTBE is analysed in all matrices generally by gas chromatography
(GC) using a range of capillary columns and detector systems that are
suited to the specific matrix. Reverse-phase liquid chromatography
(RPLC) has also been used for analysis of petrol samples.
Sorption/desorption, including purge and trap systems, and headspace
procedures have been used to prepare air, water, sediment and
biological samples for analysis.
1.2 Sources of human and environmental exposure
MTBE is not known to occur naturally in the environment.
Industrially, it is derived from the catalytic reaction of methanol
and isobutylene, and has been produced in several countries in
increasing volumes since the late 1970s. MTBE is currently among the
50 highest production volume chemicals. In 1996, the USA capacity for
production was approximately 10.6 million tonnes, and it is
anticipated that the use of MTBE will continue to increase.
Approximately 25% of gasoline in the USA is blended with MTBE. MTBE is
almost exclusively used to provide both octane enhancement and an
increase in the oxygen content of gasoline. MTBE has been added to
gasoline in concentrations up to 17% by volume.
1.3 Environmental transport, distribution and transformation
After discharge into air, MTBE will largely remain in the air,
with smaller amounts entering soil and water. In the atmosphere, MTBE
can partition into rain. However, only a small amount is removed from
the atmosphere in this manner. Atmospheric transformation by hydroxyl
radicals produces a number of products including the photochemically
stable tertiary-butyl formate (TBF) and 2-methoxy-2-methylpropanol,
which is expected to be highly reactive with hydroxyl radicals,
yielding CO2, formaldehyde, acetone and water. When MTBE is
discharged into water, a significant amount is dissolved, with some
partitioning into air. Partitioning into biota and into sediment is
low. Biodegradability in conventional assays is limited. Generally,
biodegradability is believed to be slow in the environment. When MTBE
is released to the soil, it is transported to the air through
volatilization, to surface water through run-off and to groundwater as
a result of leaching. MTBE can persist in groundwater.
1.4 Environmental levels and human exposure
There are few data on environmental levels and human exposure.
In studies of MTBE in urban air of some cities using oxygenated
gasoline with 15% MTBE, ambient concentrations ranged from
non-detectable to 100.9 µg/m3 (0.028 ppm), with several median
concentrations ranging from 0.47 to 14.4 µg/m3 (0.00013 to 0.004
ppm). Concentrations of MTBE in urban air of some cities where MTBE
was used as an octane enhancer at lower concentrations ranged from
non-detectable to 26.4 µg/m3 (0.0073 ppm).
Concentrations at ground level or near refineries ranged from 15
to 281 µg/m3. Median levels in urban air near blending facilities
were 1508 µg/m3 (0.419 ppm), with ranges of 216-35 615 µg/m3 (0.06
to 9.8 ppm).
At service stations in areas where oxygenated gasoline containing
10-15% MTBE is used, concentrations were highest in the breathing zone
during consumer refuelling (range of 300 to 136 000 µg/m3 (0.09 to 38
ppm), with levels rarely exceeding 3600 µg/m3 (10 ppm), slightly
lower at the pump island (non-detectable to 5700 µg/m3 (1.6 ppm) and
lowest at the station perimeter (non-detectable to 500 µg/m3 (0.14
ppm). Levels were generally higher at service stations without vapour
recovery systems.
Levels in the automobile cabin were 7 to 60 µg/m3 (0.002 to
0.017 ppm) during commutes and 20 to 610 µg/m3 (0.006 to 0.172 ppm)
during refuelling.
Based on limited monitoring confined almost exclusively to the
USA, MTBE has been detected in snow, stormwater, surface water
(streams, rivers, and reservoirs), groundwater and drinking-water.
Concentrations of MTBE detected in stormwater ranged from 0.2 to 8.7
µg/litre with a median of less than 1.0 µg/litre. For streams, rivers
and reservoirs, the range of detection was from 0.2 to 30 µg/litre,
and the range of medians for several studies was 0.24 to 7.75
µg/litre.
MTBE has generally not been detected in deeper groundwater or in
shallow groundwater in agricultural areas. When detected, the
concentration is less than 2.0 µg/litre. MTBE is more frequently found
in shallow groundwater (top 5-10 feet of these aquifers) in urban
areas. In this setting, the concentrations range from less than 0.2
µg/litre to 23 mg/litre, with a median value below 0.2 µg/litre.
MTBE is infrequently detected in public drinking-water systems
from groundwater. In all but 3 out of 51 systems in which it was
reported, the concentration was <20 µg/litre. There are inadequate
data to characterize the concentration of MTBE in public
drinking-water systems from surface water. MTBE has been found at high
levels (i.e. >1000 µg/litre) in a few private wells used for
drinking-water. However, it is doubtful that humans would consume
water with concentrations of MTBE greater than about 50-100 µg/litre
because of its low taste and odour threshold.
Workers with potential exposure to MTBE include those involved in
the production and distribution and use of MTBE and MTBE-containing
gasoline, including service station attendants and mechanics.
Short-term exposure (<30 min) in routine manufacturing
operations and maintenance of neat MTBE ranged from 715 to 43 000
µg/m3 (0.2 to 12 ppm), with average median values being about 3400
µg/m3 (0.95 ppm). Longer-term (30 min to 8 h) exposure ranged from
360 to 890 000 µg/m3 (0.01 ppm to 250 ppm), with median levels being
about 540 µg/m3 (0.15 ppm). For workers in blending operations,
short-term values ranged from non-detectable to 360 000 µg/m3 (100
ppm), the average median being about 5700 µg/m3 (1.6 ppm). Long-term
values ranged from non-detectable to 257 000 µg/m3 (72 ppm), the
average median being about 2000 µg/m3 (0.6 ppm).
Exposures were highest during transportation of neat MTBE and
fuel mixtures through pipelines, barges, railroad cars and trucks
(neat MTBE only), short-term values ranging from 4 to 3750 mg/m3
(0.001 to 1050 ppm) with an average median value of 140 mg/m3 (39
ppm). Long-term values ranged from 0.036 to 2540 mg/m3 (0.01 to 712
ppm), the average median value being 2.85 mg/m3 (0.8 ppm). In
distribution (i.e. loading of MTBE fuel mixtures on trucks and
delivering and unloading at service stations), short-term values
ranged from non-detectable to 225 mg/m3 (63 ppm), the average median
values being around 21 mg/m3 (6 ppm). Long-term values ranged from
0.036 to 22 mg/m3 (0.01 to 6.2 ppm), the average median value being
1.79 mg/m3 (0.5 ppm).
Median short-term exposure levels of service station attendants
ranged generally from 1.071 to 21.42 mg/m3 (0.3 to 6 ppm) and rarely
exceeded 35.7 mg/m3 (10 ppm). Median long-term exposure levels of
service station attendants averaged 1.79 mg/m3 (0.5 ppm). Median
exposures of mechanics were below detection levels for one short-term
study; the average median value for long-term exposure was
approximately 360 µg/m3 (0.1 ppm).
1.5 Kinetics and metabolism
Toxicokinetic data on MTBE in humans are mainly derived from
controlled studies in healthy adult volunteers and in a population
exposed to oxygenated gasoline. MTBE is rapidly absorbed into the
circulation following inhalation exposure. In healthy human volunteers
exposed by inhalation, kinetics of MTBE are linear up to
concentrations of 268 mg/m3 (75 ppm). Tertiary-butyl alcohol (TBA),
a metabolite of MTBE, was measured in blood and urine of exposed
humans. The peak blood levels of MTBE and TBA ranged from 17.2 to 1144
µg/litre, and 7.8 to 925 µg/litre, respectively, in humans exposed to
5.0 to 178.5 mg/m3 (1.4 to 50 ppm) MTBE. Based on a monocompartmental
model, rapid (36-90 min) and slower (19 h) components of MTBE
half-life have been identified.
In rodents, MTBE is well absorbed and distributed following oral
administration and inhalation exposure, with lower dermal absorption.
At 400 mg/kg oral and 28 800 mg/m3 (8000 ppm) inhalation exposure,
the percentage of total absorbed dose eliminated in expired air
increased with a corresponding decrease in the percentage eliminated
in urine, indicating a saturation of metabolism. TBA was not
identified in the urine of exposed rats. There is evidence of further
metabolism of TBA, based on the identification of
2-methyl-1,2-propanediol and alpha-hydroxyisobutyric acid excreted in
the urine. In vitro studies provide evidence that MTBE is
metabolized to TBA, formaldehyde and acetone.
1.6 Effects on laboratory animals and in vitro systems
In rats, the acute median oral lethal dose (LD50) is
approximately 3800 mg/kg bw. The acute median lethal concentration
(LC50) value for a 15-min inhalation exposure is about 141 000 mg/m3
air in mice. Signs of intoxication include CNS depression, ataxia and
laboured respiration. When the dose was non-lethal, recovery was
complete. The LD50 for dermal toxicity in rabbits is >10 200 mg/kg
bw.
In a single identified study, MTBE was "moderately" irritating to
skin, causing moderate erythema and oedema following dermal
application to rabbits. It was also irritating to the eyes of rabbits,
causing mild, reversible changes. In the only identified study, MTBE
induced slight to severe respiratory irritation following exposure of
mice to 300 to 30 000 mg/m3, respectively. It did not induce skin
sensitization in studies in guinea-pigs.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Lowest reported effect levels for nephrotoxicity following
ingestion in 90-day studies are 440 mg/kg bw per day (increases in
relative kidney weight and hyaline droplet formation in Sprague-Dawley
rats). With inhalation exposure to 2880 mg/m3 (800 ppm), there were
increases in kidney weight associated at higher concentrations with a
mild increase in hyaline droplets in the proximal tubules in
Fischer-344 rats. In inhalation oncogenicity studies, at 1440 mg/m3
(400 ppm) the incidence and severity of chronic progressive
nephropathy was increased in male rats; in male mice, at this level,
there were increases in absolute liver weight (which correlated with
increases in hepatocellular hypertrophy at higher concentrations) and
an increase in relative kidney weight.
Exposure to MTBE also results in reversible central nervous
system (CNS) effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2880 mg/m3 (800 ppm) produced
reversible dose-related changes in motor activity in single sexes.
These effects were transient and not evident in longer-term studies.
One- and two-generation inhalation reproductive studies in rats
and four developmental studies in rats, mice and rabbits have been
identified. In these studies, specific reproductive effects were not
observed in rats at concentrations up to 28 800 mg/m3. MTBE has not
induced developmental effects at concentrations below those that were
toxic to the mothers. Decreases in uterine weight and increases in
estrogen metabolism in mice have been observed at 28 800 mg/m3.
MTBE has been adequately tested in a broad range of mutagenicity
and other genotoxicity tests. The results from these studies indicate
that MTBE is not genotoxic, although a mouse lymphoma cell tk locus
mutation assay was positive, due to the metabolism of MTBE to
formaldehyde.
Carcinogenicity studies have been conducted involving inhalation
exposure of Fischer-344 rats and CD-1 mice and gavage dosing of
Sprague-Dawley rats. In neither of the inhalation studies were methods
of statistical analysis used that adjusted for survival differences.
There were significant increases in tumour incidence in all three
studies, namely renal tubular cell tumours and Leydig cell tumours in
the male Fischer-344 rats, Leydig cell tumours in male and
leukaemias/lymphomas (combined) in female Sprague-Dawley rats, and
liver cell tumours in female CD-1 mice. The renal tubular cell tumours
and the leukaemia/lymphomas were not observed consistently, therefore,
in the different studies in rats. In addition, the sex-specific kidney
tumours were associated with sex-specific alpha2u-globulin
nephropathy, which was observed in several studies of short duration.
Increases in Leydig cell tumours occurred at the highest dose level
(1000 mg/kg bw) in the Sprague-Dawley rats, but interpretation of the
increases recorded for Fischer-344 rats was complicated by the very
high concurrent and historical control incidences. The mouse liver
tumours occurred at incidences in the control and 28 800 mg/m3 (8000
ppm exposed groups, respectively, of 2/50 and 10/50 in females and
12/49 and 16/49 in males. The increases were modest and were
accompanied by hepatocellular hypertrophy.
1.7 Effects on humans
Following the introduction of two separate fuel programmes in the
USA requiring the use of gasoline oxygenates (not necessarily MTBE),
consumers in some areas have complained about acute health symptoms
such as headache, eye and nose irritation, cough, nausea, dizziness
and disorientation. Epidemiological studies of human populations
exposed under occupational as well as non-occupational conditions, and
experimental studies of human volunteers exposed under controlled
conditions, have not been able to identify a basis for these
complaints. Although results are mixed, community studies conducted in
Alaska, New Jersey, Connecticut, and Wisconsin, USA, have provided
limited or no evidence of an association between MTBE exposure and the
prevalence of health complaints.
In controlled experimental studies on adult volunteers exposed in
inhalation chambers to MTBE at concentrations ranging from 5.0 mg/m3
(1.4 ppm) up to 270 mg/m3 (75 ppm), there were no evident effects in
terms of either subjective reports of symptoms or objective indicators
of irritation or other effects up to 180 mg/m3 (50 ppm) for as long
as 2 h. From this evidence it appears unlikely that MTBE alone induces
adverse acute health effects in the general population under common
conditions of inhalation exposure. However, the potential effects of
mixtures of gasoline and MTBE, and the manner in which most persons
are exposed to MTBE in conjunction with the use of oxygenated fuels,
have not been examined experimentally or through prospective
epidemiological methods. Moreover, the role of factors such as
awareness of MTBE, due in part to its distinctive odour, for example,
have not been investigated.
1.8 Effects on other organisms in the laboratory and field
The experimental acute toxicity (LC50) of MTBE to fish,
amphibians and crustaceans is > 100 mg/litre. There are no data on
chronic or sub-lethal toxicity to aquatic species, or on toxicity to
terrestrial organisms.
1.9 Evaluation of human health risks and effects on the environment
Based on collective evidence, it appears unlikely that MTBE alone
induces adverse acute health effects in the general population under
common exposure conditions.
In studies on animals, MTBE is "moderately" acutely toxic and
induces mild skin and eye irritation but not sensitization. Repeated
exposure affects primarily the kidney of rats and the liver of mice,
with lowest reported adverse effect levels of 440 mg/kg bw per day in
rats following ingestion and 1440 mg/m3 (400 ppm) following
inhalation. MTBE has not induced adverse reproductive or developmental
effects at concentrations less than those that were toxic to the
parents.
MTBE is not genotoxic but has induced tumours in rodents
primarily at high concentrations that also induce other adverse
effects. These data are considered currently inadequate for use in
human carcinogenic risk assessment. The Task Group concluded that, in
order to provide quantitative guidance on relevant limits of exposure
and to estimate risk, acquisition of additional data in several areas
is necessary.
It does not appear that the concentrations of MTBE in ambient
water are toxic to aquatic organisms except during spills. Although
there are no data on the terrestrial toxicity of MTBE, this appears
not to be of concern since concentrations in ambient air are low and
its half-life is relatively short.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C5H12O
Chemical structure: CH3
'
H3C - C - O - CH3
'
CH3
Relative molecular mass: 88.15
Common name: methyl tertiary-butyl ether
IUPAC Chemical name: 2-methoxy-2-methyl propane
CAS registry number: 1634-04-4
Synonyms: 1,1-dimethylethyl methyl ether; ether
tert-butyl methyl; éther methyl
tert-butylique (French); MBE; methyl
1,1-dimethylethyl ether; methyl- t-butyl
ether; methyl tert-butyl ether;
(2-methyl-2-propyl) methyl ether;
metil-terc-butileter (Spanish);
2-methoxy-2-methylpropane; MTBE; propane,
2-methoxy-2-methyl-(CA); t-butyl methyl
ether; tert-butoxymethane; tert-butyl
methyl ether
Major trade names: 3 D Concord
Driveron
HSDB 5487
UN 2398
Constituent components of typical commercial grade:
(ARCO, 1989)
Component Weight %
MTBE 97.5
di-, tri-isobutylene, and
t-butyl alcohol 0.6
Methanol 0.2
C4 hydrocarbons 1
C5 hydrocarbons 0.4
other 0.3
water content <0.05
2.2 Physical and chemical properties
Table 1 lists the physical and chemical properties of MTBE.
2.3 Conversion factors
1 ppm = 3.57 mg/m3 at 25°C (1 atmosphere pressure)
1 mg/m3 = 0.28 ppm at 25°C (1 atmosphere pressure)
2.4 Analytical methods
Analytical methods that have been used for MTBE and for
tertiary-butanol (TBA), which is an intermediate in the aerobic
bacterial degradation of MTBE and in its mammalian metabolism, are
given for various media.
Some commonly used methods are summarized in Table 2.
2.4.1 Procedures
2.4.1.1 Air
Air samples are collected in stainless steel canisters, and the
volatile compounds concentrated in a two-stage trap to sorb the
Table 1. Physical and chemical properties of MTBE organic compounds
and to collect water. Drying is done by purging with dry N2 at 25°C,
and the organic compounds thermally desorbed at 220°C by back-flushing
with helium. The samples can be analysed by gas chromatography/mass
spectrometry (GC-MS) using a capillary column (Kelly et al., 1993).
Harper & Fiore (1995) used a passive diffusion technique to collect
samples.
Automobile exhaust samples are collected in 3-litre bags. Diluted
emissions are concentrated in variable temperature control traps,
operating between -60°C and 180°C (DB 1 column) or between -99°C and
180°C (GS-Q megabore column). Using these twin columns, separation of
all the major components is possible (Hoekman, 1993).
2.4.1.2 Soil, water and sediment
Static headspace analysis can be used for samples of soil and
groundwater. Samples are collected in filled bottles, air is
introduced, and the bottles are shaken and equilibrated before
analysis of the gas phase.
One method is by GC-FID/PID using a megabore DB-1 capillary
column (Roe et al., 1989). Samples of groundwater can be collected
with a cone penetrometer coupled with a porous probe, and analysed by
GC using a photoionization detector (PID) (Chiang et al., 1992).
Table 1. Physical and chemical properties of MTBE
Physical state Liquid
Colour Colourless
Odour Strong, characteristic terpene-like
Freezing point (°C) -109 Windholz, 1983
Boiling point (°C) 53.6-55.2 Mackay et al., 1993
Selected valuea 55.2
Flash point (°C) -28 Budavari et al., 1996
Ignition temperature (°C) 224 Budavari et al., 1996
Spontaneous ignition temperature (°C) 460 Wibowo, 1994
Flammability Flammable/combustible
Flammability limits 1.5-8.5% in air ECETOC, 1997
Vapour pressure (Pa at 25°C) 32 659 to 33 545 Mackay et al., 1993
Selected valuea 33 500 Mackay et al., 1993
Density (g/cm3 at 20°C) 0.7404 to 0.7478 Mackay et al., 1993
Selected valuea 0.7404
Relative vapour density (air=1) 3.1 Wibowo, 1994
Log kow octanol/water partition coefficient 0.94 to 1.30 Mackay et al., 1993
Selected valuea 0.94
Henry's law constant at 25°C (Pa m3/mol) 59.46 to 304.96 Mackay et al., 1993
Selected valuea 70.31
Table 1. (continued)
Physical state Liquid
Dimensionless Henry's law constant (H/RT) at 25°C 0.0239 to 0.1221 Zogorski et al., 1996
Selected valuea 0.018 at 20°C
Water solubility g/m3 at 25°C 32 200 to 54 353 Mackay et al., 1993
Selected valuea 42 000 (at 19.8°C)
Solubility of MTBE in water 48 Budavari et al., 1996
(g/litre) at 25°C
Solubility of water in MTBE (g/litre) at 25°C 15 Budavari et al., 1996
Solubility in organic solvents: - very soluble in other ethers and
alcohols
- mixes with gasoline
Viscosity, g/sec. -cm 0.003 to 0.004 (calculated) Lyman et al., 1990
Other properties Unstable in acid solution pKa = -3.70
at 23°C (measured)
Organoleptic properties
Taste 134 µg/litre (0.134 ppm) TRC, 1993
Odour
- detection threshold 0.19 mg/m3 TRC, 1993
- recognition threshold 0.29 mg/m3 (0.08 ppm) TRC, 1993
a Criteria of selection were based on:
i) the age of the data and acknowledgement of previous conflicting or supporting values;
ii) the method of determination;
iii) the perception of the objectives of the investigators, and their need for quantitative values; and
iv) information derived from Quantitative-Structure-Property-Relationships.
Table 2. Summary of analytical procedures for MTBE
Matrix Procedure Detector Detection limit Reference
Air Sorption/desorption GC-MS 0.72-3.6 µg/m3 Kelly et al., 1993
Vehicle emission Sorption/desorption GC-FID 18-36 µg/m3 Hoekman, 1993
Water Static headspace GC-PID 10.8 µg/m3 (water) Chang et al., 1992
1.08 µg/m3 (air)
Water Purge and trap GC-MS 5 µg/litre Bianchi & Varney, 1989
Water Purge and trap GC-MS 0.52-0.090 µg/litre Munch & Eichelberger, 1992
Water Purge and trap GC-MS 0.06 µg/litre Raese et al., 1995
Sediment Purge and trap GC-MS 10-100 ng/kg Bianchi et al., 1991
Blood Purge and trap GC-MS 0.01 µg/litre Bonin et al., 1994
Gasoline Direct GC-FID 18-36 µg/m3 Johansen, 1984
(5-10 ppbv)
For samples of water and sediment, purge and trap procedures are
widely used to concentrate volatile components before analysis. For
water samples, the analytes are desorbed by open-loop stripping for
60 min at 60°C and collected on a mixture of Tenax TA and
Chromosorb-106. Desorption is then done using helium at 150°C before
analysis.
Analysis can be by GC-MS (Bianchi & Varney 1989). An expanded
procedure for volatile organic compounds developed by the US
Environmental Protection Agency (US EPA) uses a three-trap collection
system (Tenax, silica gel and charcoal) followed by GC-MS
quantification: for MTBE, a detection limit of 0.09 µg/litre was
attained using a DB-624 capillary column and a purging efficiency of
74% (Munch & Eichelberger 1992). An essentially similar procedure has
been used for estuarine sediment samples with an OV-1701 capillary
column (Bianchi et al., 1991).
MTBE in ambient groundwater has been analysed by the US
Geological Survey since 1991 using a purge and trap GC-MS method
(Raese et al., 1995). The estimated detection limit for reagent water
spiked with MTBE at 0.2 µg/litre is 0.06 µg/litre. A method for the
concurrent analysis of MTBE, TBA and tert-butyl formate (TBF) has
been developed (Church et al., 1997). The method employs direct
aqueous injection and GC-MS, and has a detection level of 0.1 µg/litre
for MTBE.
2.4.1.3 Gasoline
Samples of gasoline can be analysed directly by GC using the
following procedures. They have all shown good selectivity for
oxygenates:
- An infrared (IR) detector, using a column of Poropak Q plus
Poropak N, gave a limit of detection of 0.1% (w/v) with the
detector set at 8.3 µm (Cochrane & Hillman 1984).
- A detector system (GC-O-FID), in which oxygenates are
catalytically cracked to CO followed by reduction to methane, has
a selectivity better than 1:107 (Verga et al., 1988).
- FID with a dual column system using Durawax 1 and Durabond-S
gives acceptable accuracy and repeatability at a concentration of
1% (w/w) (Levy & Yancey 1986). An alternative procedure uses
switching (Johansen 1984).
- Atomic emission detection (AED) using 777 nm near infrared (NIR)
emission and a DB-1 capillary column is a sensitive method (Diehl
et al., 1995).
- Reversed-phase liquid chromatography (RPLC) with a Hi-Chrom
"reversible column" packed with Spherisorb ODS-11 and a
refractive index detector (RID) can be used with a mobile phase
of acetonitrile:water (6:4) and back-flushing suited to the
relevant analytes (Pauls 1985). It is important that the analyte
is completely dissolved in the mobile phase.
2.4.1.4 Biological samples
Headspace or purge-and-trap concentrations of MTBE are directly
applicable to blood and urine samples. The purge and trap procedure is
coupled to quantification by GC-MS using 2H-labelled standards.
Direct GC analysis of samples is less commonly used but Schuberth
(1996), using the full headspace technique combined with capillary GC
and ion-trap detection, determined MTBE with a detection limit of
0.4-1 nmol in blood and brain tissue.
a) Blood, urine and tissues
The purge-and-trap system can be used for the analysis of blood
samples. Sorption is done with a Tenax trap and a cryogenic trap
decreasing in temperature to -150°C with desorption at 180°C. GC-MS
analysis uses a DB 624 column. This has been applied to MTBE and to
TBA using [2H12] MTBE and [2H9] TBA as the respective standards
(Bonin et al., 1994).
Headspace analysis has been used for the analysis of both MTBE
and metabolically produced TBA in a range of matrices including blood
and urine. For blood samples, GC with an SE 50 column and FID can be
used (Savolainen et al., 1985). Analysis of TBA produced from MTBE by
hepatic microsomes from rats can be made with a Carbowax B/5% Carbowax
20M packed column and FID (Brady et al., 1990). A procedure applicable
to blood and urine samples uses an SPB-1 column and FID (Streete et
al., 1992). However, this procedure appears not to have been validated
using samples contaminated with MTBE or TBA. The procedure can be
applied to tissue samples after treatment with a proteolytic enzyme
before analysis.
Analysis of MTBE (and TBA) in brain (cerebral hemispheres) and in
perirenal fat from rats dosed with MTBE was made by homogenizing the
samples in dimethyl formamide, centrifuging, and direct GC analysis of
the supernatant using a packed column with Carbowax 20M and FID
(Savolainen et al., 1985).
b) Bacterial cultures
Samples of bacterial cultures that metabolize MTBE have been
analysed for both MTBE and its metabolite TBA by direct GC analysis
using FID and a Quadrex methyl silicone capillary column (Salanitro et
al., 1994). Analysis of MTBE (and TBA) in bacterial cultures that
degraded TBA, though not MTBE, used a GC capillary column coated with
a cross-bound phase (CP-Sil 13, Chrompack) and an FID detector (Allard
et al., 1996).
14C-labelled MTBE has been used in a few investigations. In one
study dealing with aerobic biodegradation, 14CO2 was collected after
incubation as Ba14CO3, and the fraction incorporated into cells was
separated by filtration though 0.45 µm Millipore filters (Salanitro et
al., 1994). In another study on the accumulation of MTBE into plants,
samples were extracted with dimethylformamide for counting (Schroll et
al., 1994).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Natural sources of MTBE have not been reported in the scientific
literature.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
MTBE is an oxygenate (oxygen-containing hydrocarbon) that is
industrially produced in several countries, including Austria,
Belgium, Canada, Finland, France, Germany, Italy, Japan, Mexico, the
Netherlands, Norway, Portugal, Sweden, Taiwan, the United Kingdom, the
USA and Venezuela.
The worldwide annual production of MTBE in 1995 was about 15
million tonnes. In the USA, in 1994 MTBE ranked 18th in terms of
production volume (6 175 000 tonnes (13.61 billion pounds)) and in
1995 there was an increase to 12th position (8 000 000 tonnes (17.62
billion pounds)) (CEN, 1996). During the years 1985-1995, production
of MTBE in the USA showed an annual increase of 25% (Storck et al.,
1996). The potential demand for MTBE is expected to increase to
284 000 barrels/day (12.2 million tonnes per year) in the year 2000.
North America is the largest consumer of MTBE, accounting for
about two-thirds of the world's annual use. In 1996 the USA was the
world's largest consumer of MTBE with a usage of 246 000 barrels/day
(10.6 million tonnes per year). Western Europe, the eastern
Mediterranean area and Asia, and Latin America used progressively
smaller amounts of MTBE in 1995. Most growth in the production
capacity of MTBE is expected to occur in the eastern Mediterranean
area, South America and the USA.
MTBE is prepared principally by reacting isobutylene (contained
in a mixed C4 stream) with methanol over an acidic ion-exchange resin
catalyst such as sulfonated styrene cross-linked with divinyl benzene
in the liquid phase and at 38-93°C and 100-200 psi. It can also be
prepared from methanol, TBA and diazomethane (Budavari et al., 1996).
3.2.2 Uses
The main use of MTBE is as an additive to gasoline. MTBE was
first added to gasoline in the late 1970s on a voluntary basis as an
octane enhancer when the phase-out of tetraethyl lead commenced, and
this use continues. MTBE is also added to gasoline in higher amounts
(up to 15% by volume) as part of national mandated air pollution
abatement programmes to reduce ambient air levels of carbon monoxide
(CO) or ozone, or both, and in reformulated gasoline (RFG) (10-11% by
volume) to reduce the emissions of benzene and other volatile
hydrocarbons. MTBE is also used in the manufacture of isobutene
(Lewis, 1993) and a minor proportion is used as a therapeutic agent
for in vivo dissolution of cholesterol gallstones in humans (Allen
et al., 1985a,b; Di Padova et al., 1986; Murray et al., 1988; Sternal
& Davis, 1992).
In the USA, oxygenated gasolines are required in two national
programmes to improve air quality (the oxygenated fuels programme and
the reformulated gasoline programme) outlined in the 1990 Clean Air
Act Amendments. MTBE is not specifically required in these programmes,
but it is the most widely used oxygenate. The winter oxygenates
programme requires gasoline sold in areas that do not meet federal air
quality standards for CO to contain no less than 2.7% oxygen by
weight, which is equal to 15% MTBE by volume. According to the
reformulated gasoline programme, large metropolitan areas with serious
ozone problems are required to use reformulated gasoline (RFG): this
is a special blend of gasoline that must contain 2% oxygen by weight
and a maximum of 1% benzene and 25% aromatic hydrocarbon by volume. To
meet this requirement, reformulated gasoline would contain 11% MTBE by
volume. About 90% of the MTBE consumed in the USA in 1996 was used in
reformulated gasoline. At the end of 1996, MTBE was used in
approximately 25% of the total gasoline pool.
During the winter driving season, 15% MTBE by volume is added to
gasoline as an oxygenate to reduce CO emissions from motor vehicles.
The extent of CO reductions depends on the fuel metering system and
emissions control technology used on the vehicle (Prakash, 1989). The
addition of oxygenates to gasoline blends generally reduces the
hydrocarbon (HC) emissions to the atmosphere. However, the levels of
exhaust nitrogen oxides (NOx) increase when the oxygenate
concentration exceeds about 2% oxygen by weight (SNV, 1993). It also
increases the aldehyde emissions from automobile exhausts, but has not
been found to have any major influence on the chemical composition of
particulate emissions from vehicles (Watson et al., 1990). The
aldehyde (not specified) emissions are significantly reduced by
three-way catalytic converters (Prakash, 1989).
In a model analysis of changes in the concentrations of eight
volatile organic compounds (VOCs), i.e. acetaldehyde, benzene,
1,3-butadiene, ethylbenzene, formaldehyde, toluene, xylenes, and
particulate organic matter (POM), resulting from the use of
reformulated gasoline and oxyfuel containing MTBE, Spitzer (1997)
concluded that, with the exception of formaldehyde, exhaust emissions
of these VOCs would be decreased. The increased formaldehyde emissions
would, however, be offset by the reduction in the formation in the
atmosphere of formaldehyde from the other VOCs. Erdal et al. (1997)
modelled atmospheric ozone pollution reduction by the use of MTBE in
gasoline. Ozone is formed by the reaction of sunlight with NOx and
VOCs. The use of MTBE reduces VOC and NOx exhaust emissions and also
reduces fuel evaporation. The model estimates a reduction in peak
ambient ozone levels of 3.6-18 µg/m3 (1-5 ppb).
It is estimated that MTBE-blended gasolines account for
approximately 2% of the total unleaded gasoline in Canada (Environment
Canada, 1992). Levels of MTBE in blended gasolines range from 0.04% to
9.09% by volume, depending on the grade of gasoline, season and
geographical area. Since the use of oxygenates is not required in
Canada as part of an air abatement programme, each refiner blends in
the amounts of MTBE that it requires in order to obtain a good
gasoline end-product, depending on the batch of crude oil and the
technology used in the refinery. In 1997, the maximum concentration of
MTBE allowed in Canadian gasoline was 2.7% mass oxygen (approximately
15% by volume).
3.2.3 Sources and releases to the environment
Similar to hydrocarbon components of gasoline, fuel oxygenates
such as MTBE enter the environment during all phases of the petroleum
fuel cycle. Sources include, for example, auto emissions, evaporative
losses from gasoline stations and vehicles, storage tank releases,
pipeline leaks, other accidental spills, and refinery stack releases.
Annual estimates of MTBE mass releases to the environment from all
potential sources have not been reported in the scientific literature.
However, releases from storage tanks, vehicular emissions and
evaporative losses from gasoline stations and vehicles are perceived
to be important sources (Zogorski et al., 1996; US Interagency
Assessment, 1997).
3.2.3.1 Industrial releases
No information on industrial releases of MTBE to the environment
have been found in the scientific literature, except in the case of
the USA and Canada.
Industrial releases of MTBE in the USA have been characterized
for 1993. A total of 136 facilities released MTBE to the environment,
with an estimated total release of 1700 tonnes. Approximately 84% of
the release was by petroleum refineries, and almost all of the MTBE
was released to air (Zogorski et al., 1996).
In 1994, the total Canadian industrial release of MTBE from
refiners and manufacturers was approximately 28.2 tonnes, the bulk of
which was released into the air (98.1%) and a small amount into water
(1.9%) (Environment Canada, 1996a). The highest amounts of MTBE
released were 9.5, 9.1, 8.4 and 1.0 tonnes by industries located in
Sarnia, Burnaby, Edmonton and Saint John, respectively.
3.2.3.2 Storage tank release
Releases of gasoline containing MTBE from storage tanks may
contaminate soil and groundwater. In some cases, MTBE may enter
drinking-water supplies. In 1989 it was estimated that in the USA
there were approximately 14 000 above-ground storage tank facilities
with an estimated 70 000 tanks, of which 30-40% were used for gasoline
storage (API, 1989a). A subsequent survey of 299 storage facilities
showed that 40% had identified subsurface contamination (API, 1994).
Many sites have been identified with soil or groundwater hydrocarbon
contamination that required corrective action. The extent of MTBE
contamination at these sites is largely undocumented because
monitoring of MTBE has not been required. More stringent
release-prevention and -detection standards are now required in the
USA and, when fully implemented by December 1998, these requirements
should considerably decrease the annual volume of gasoline released to
soil and groundwater.
It is important to note that when gasoline containing MTBE enters
groundwater, high concentrations of MTBE (i.e. in excess of 1000
µg/litre) can occur. While comprehensive data on the occurrence of
MTBE in drinking-water provided from groundwater do not exist, there
have been some instances reported in the USA where drinking-water
supplies have been disrupted because of high MTBE levels. For example,
two well fields serving the city of Santa Monica, California, have
been contaminated with MTBE necessitating the purchase of alternative
water for drinking-water.
3.2.3.3 Engine emissions from on-road and off-road vehicles and
recreational boats
The use of gasoline containing MTBE in on-road and off-road
vehicles, boats and small engines will result in MTBE releases to the
environment unless recovery systems are employed. The extent of these
emissions has not been thoroughly studied, and there are few
scientific citations.
Drivas et al. (1991) estimated ambient air concentrations of
evaporative and exhaust emissions of MTBE gasoline blends during two
different situations representing worst-case concentrations: a car
idling in an open garage and a car just stopped and turned off
(hot-soak evaporative emission) in a closed garage. The predicted
maximum exhaust air concentration of MTBE was calculated to be
0.24 mg/m3 (0.07 ppm).
MTBE was not detected in samples from light-duty vehicle
emissions measured in the Caldecott Tunnel, San Francisco Bay Area, in
August 1994, when the average oxygen content of gasoline sold in the
area was 0.3% by weight (Kirchstetter et al., 1996). In October, when
the average oxygen content in MTBE gasoline was 2.0% by weight, the
concentration of MTBE in emissions was 3.3% by weight of total VOCs.
Comparison of emissions from vehicles using a standard fuel and a
reformulated fuel that contained MTBE (11% by volume) showed a
reduction in mass emission rates in the latter (Hoekman, 1992).
Although there was a decrease in the emissions of aromatics and
alkanes, the levels of alkenes and carbonyl compounds increased, and
there was considerable variation among the vehicles that were tested.
A study in California showed that increasing the concentration of MTBE
from 0.3% by weight in August to 1.6 % MTBE plus 0.4% ethanol in
October resulted in lowered emission of aromatics but increased
emissions of isobutene (86%), cisbut-2-ene (150%), formaldehyde (39%),
propionaldehyde (200%), methacrolein (50%) and butyraldehyde (40%)
(Kirchstetter et al., 1996).
Boat motors and small engines used in chain saws, other power
tools, snowmobiles, lawn mowers and garden tillers, for example, may
also release MTBE to the environment via exhaust, evaporative losses
and release of uncombusted fuel. The magnitude and significance of
these releases are not documented. In 1997 MTBE was detected in
several public water supply reservoirs that, in part, provide
drinking-water for Southern California. The predominant source of MTBE
is thought to be associated with small engines used on recreational
boats. Such engines are known to be inefficient, and release
uncombusted gasoline and emissions to water and air.
3.3 Other pertinent information
All aspects of the effectiveness of fuel oxygenates on ambient
air quality, including carbon monoxide, hydrocarbons, oxides of
nitrogen, aromatics, aldehydes and alcohols, and associated
atmospheric degradation products, have been reviewed in a number of
reports (e.g., Prakash, 1989; Environment Canada, 1993; Schuetzle et
al., 1994; HEI, 1996; Kirchstetter et al., 1996; US Interagency
Assessment, 1997).
Overall, these studies indicate that, when compared to other
gasolines, MTBE gasoline blends generally reduce CO and hydrocarbon
exhaust emissions and increase aldehyde and NOx emissions.
4. ENVIRONMENTAL BEHAVIOUR AND FATE
4.1 Transport and distribution between media
A diagram depicting the movement of MTBE in the environment is
shown in Fig. 1.
4.1.1 Air
It can be predictable from its physicochemical properties that,
when MTBE is released into air, the greater part will exist in the
atmosphere, with small amounts entering soil and water (Mackay et al.,
1993). Based on its Henry's law constant, MTBE should partition into
atmospheric water, including rain. The concentration of MTBE in
precipitation would be in direct proportion to its concentration in
air. However, falling precipitation removes only a negligible amount
of the gas-phase compound (Zogorski et al., 1996). Therefore, chemical
degradation of MTBE should be the major removal process from the air
(Mackay et al., 1993).
4.1.2 Water
Transport and distribution of a substance between and within
media in the aquatic environment is dependent upon its solubility,
movement of the water itself, exchanges at the air-water interfaces,
adsorption to sediment and particulate matter, and bioconcentration in
aquatic organisms. The residence time in water is also dependent upon
the type of environmental conditions encountered, such as
temperatures, wind speeds, currents and ice cover (Environment Canada,
1993).
MTBE can volatilize from surface water and be removed by aeration
(Zogorski et al., 1996). According to calculations by Pankow et al.
(1996), no single volatilization half-life (t´) will characterize
the loss process from water. In surface water, the most important
factors for the volatilization rates are the depth and velocity of the
flow. In deep and slow-moving flows, the t´ values at both 5°C and
25°C are 85 and 78 days for calm and windy conditions, respectively.
These rates were shown to be similar to those for benzene, toluene,
ethyl benzene and xylene (BTEX) compounds. In shallow and fast-moving
flows, changing from calm to windy conditions causes a significantly
accelerated volatilization rate. Under these circumstances, MTBE
volatilizes more slowly than benzene, although it was suggested that
this is of no practical significance, as both substances volatilize
quickly in such flows. It was concluded that the t´ values for MTBE
are highly dependent on depth and mean flow velocity. Thus, quite
large as well as very small t´ values are possible.
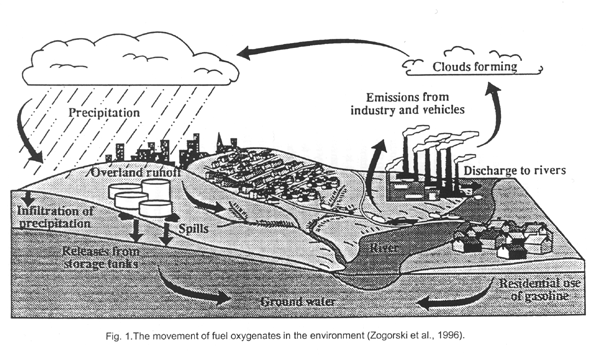 Based on physicochemical properties, it can be predicted that a
release of MTBE into water would result in significant amounts being
dissolved. Most of the MTBE remains in the surface water, with some
partitioning into air and much smaller amounts into sediment and soil
(Mackay et al., 1993). The low Kow of 0.94 suggests that partitioning
from the water to particulates and sediment is not significant. On the
basis of bioconcentration data, MTBE is not subject to bioaccumulation
or biomagnification in aquatic organisms (Environment Canada, 1993).
In the water compartment, the key removal process should be
volatilization. The amount transferred to sediment is negligible
(Mackay et al., 1993; Environment Canada, 1993).
For a gasoline containing 10% MTBE by weight, and assuming that
it does not undergo depletion of the MTBE concentration in the
gasoline due to dissolution into the water, the water solubility of
the MTBE from gasoline will be approximately 5 gm/litre at 25°C. By
comparison, the total hydrocarbon solubility for non-oxygenated fuel
is about 120 mg/litre (Poulsen et al., 1992; Zogorski et al., 1996).
The ability of MTBE to enhance the solubility in water of
monocyclic aromatic gasoline components including BTEX compounds has
been examined in models, and an increase was predicted only at
co-solvent concentrations of greater than 1% (Mihelcic, 1990). In
confirmation of this, the co-solvent effect of MTBE on the aqueous
solubility of hydrocarbons in gasoline was found to be minimal (Cline
et al., 1991). Measurements made in the laboratory in shake-flasks
showed that up to 15% MTBE was unlikely to result in enhanced
concentrations of BTEX in contaminated groundwater (Poulsen et al.,
1992). Such high concentrations of MTBE seem unlikely to be achieved
in groundwater after spillage of gasoline containing MTBE, and
although MTBE is widely distributed in shallow urban groundwater at
low concentrations in the USA, its occurrence in these samples was not
associated with correspondingly increased concentrations of BTEX
(Squillace et al., 1996).
4.1.3 Soil
Based on its physicochemical properties, it can be predicted that
when MTBE is released to the soil, it can be transported to the air
through volatilization, to surface water through run-off, and to
groundwater as a result of leaching. In the first two instances, the
release would have to be at, or near the soil surface. If the release
of MTBE occurs below the soil surface, for example from an underground
storage tank, then the most likely transport mechanism will be
leaching to groundwater. Based on its vapour pressure, volatilization
of MTBE from soil and other surfaces is expected to be significant.
Soil adsorption and mobility are based on the reported and estimated
Koc (organic carbon sorption coefficient) values. Compounds with a
Koc of <100 are considered to be moderately mobile. Thus MTBE, with
a Koc of 91, does not adsorb to soil particles to a great degree and
would be considered mobile. Parameters other than Koc affecting the
leaching of MTBE to groundwater include the soil type (e.g., sandy
versus clay), the amount and frequency of rainfall, the depth of
groundwater, and the extent of degradation of the MTBE (Environment
Canada, 1993).
4.1.4 Multimedia
Several multimedia models using various emission rates and
environmental parameters have been used to predict the distribution
and concentration of MTBE in the environment (Environment Canada,
1993; Mackay et al., 1993; Hsieh & Ouimette, 1994).
4.2 Bioconcentration
Fujiwara et al. (1984) conducted studies on the bioconcentration
of MTBE in Japanese carp (Cyprinus carpio) in a flow-through system
at 25°C. The mean whole-body steady-state bioconcentration factor
(BCF) was 1.5. Further observations indicated that fish exposed for 28
days and then transferred to clean water eliminated almost all MTBE
residues within 3 days. These experimental data support the hypothesis
that MTBE has little tendency to bioaccumulate. Veith & Kosian (1983)
calculated a BCF of 2.74 (r2 = 0.927) for a 28-day exposure of
fathead minnows, based on a Quantitative Structure-Activity
Relationship (QSAR).
Compounds with log Kow values of approximately 5.0 or less do
not have significant food chain build-up. MTBE belongs to this group
(Environment Canada, 1993). Uptake from water is more important than
from food for this group of compounds.
When 14C-labelled MTBE was applied to the soil in a closed
aerated system, the concentrations of MTBE in the roots and the aerial
parts of lettuce and radish showed that transport was dominated by
foliar uptake; subsequently, translocation into the roots took place
(Schroll et al., 1994). Although neither MTBE nor its potential
metabolite TBA was detected in the plants, a considerable fraction of
the 14C label was unaccounted for and was presumed to be associated
with plant constituents.
4.3 Biodegradation and transformation
Only a limited amount of work has been accomplished on the
biodegradability of MTBE. Moreover, the studies are difficult to
compare because they have been performed under a wide variety of
conditions. Aerobic and anaerobic experiments have been conducted. For
most studies, it has been demonstrated that MTBE is difficult to
biodegrade. In contrast, BTEX is more readily biodegraded (Zogorski et
al., 1996). Half-lives for MTBE in various environmental compartments
are shown in Table 3
Table 3. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Air 20.7-265 Based upon measured Howard et al., 1991
photo-oxidation half-life
10-30 Mackay et al., 1993
Soil 672-4320 Estimation based upon US EPA, 1989
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Surface water 672-4320 Estimation based upon Howard et al., 1991
aerobic biodegradation
300-1000 half-life Mackay et al., 1993
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
2688-17 289 Estimation based on Howard et al., 1991
anaerobic degradation
half-life
4.3.1 Aerobic conditions
Results from tests involving biodegradation of MTBE have been
variable.
Pence (1987a) used an acclimated culture containing active
sludge, soil inoculum and raw sewage. The uptake of oxygen was
measured in a mineral medium supplemented with MTBE added to the
acclimated culture at a concentration of 5 mg/litre on days 0, 7 and
11. The results showed that MTBE was poorly biodegradable under these
conditions; only 5.4% biodegradation occurred within 28 days.
No biodegradation of MTBE after 60 days was found in experiments
using aquifer soil material as inoculum; with two types of activated
sludge as inoculum, no degradation of MTBE occurred after 40 days
(Möller Jensen & Arvin, 1990).
With a standard activated sludge, and based on the oxygen uptake
rate, MTBE was biodegraded very slowly (Fujiwara et al., 1984). The
hydrocarbon components of gasoline blended with MTBE were, however,
readily degraded even though the MTBE remained.
A mixed bacterial culture was obtained by enrichment of a
hydrocarbon-contaminated soil in a basal mineral medium containing:
(i) TBA (1 g/litre) as sole carbon source or (ii) methylamine (2
g/litre) as principal carbon source supplemented with TBA. During
incubation of the first culture, the concentration of TBA fell to zero
in 20 days, but incubation of methylamine-grown cells with MTBE showed
no reduction in the concentration of MTBE after 42 days (Allard et
al., 1996). Whereas MTBE was apparently recalcitrant under the
conditions used, TBA, which is one of its putative degradation
products, was biodegradable.
In contrast to these results, a mixed bacterial culture obtained
by continuous aerobic enrichment of a sludge sample from an industrial
bioreactor was able to degrade MTBE at concentrations up to 200
mg/litre (Salanitro et al., 1994). Cell suspensions incubated with
MTBE produced TBA as a transient metabolite. MTBE labelled with 14C
in the methyl group was degraded to 14CO2 and cellular material when
low substrate concentrations (2 mg/litre) were used, although not at a
concentration of 20 mg/litre. This experiment clearly demonstrated
oxidation of the methoxy group but left unresolved the fate of the
carbon atoms of the tertiary-butyl group.
Fifteen pure bacterial strains, with the capacity to degrade MTBE
using it as the sole carbon source, have been isolated from bioreactor
sludges and other sources. Several strains have been identified as
belonging to the genera Rhodococcus, Flavobacterium, Pseudomonas and
Oerskovia. These strains degrade up to 40% of MTBE (200 mg/ litre)
in 1-2 weeks of incubation at 22-25°C. These strains also grow on
tert-butanol, butyl formate, isopropanol, acetone and pyruvate as
sole carbon sources. Cultures of Methylobacterium, Rhodococcus and
Arthrobacter degraded MTBE within 1-2 weeks of incubation at
23-25°C. Growth on MTBE as the sole carbon source was slow compared
with growth on a nutrient-rich medium. When these compounds are mixed
with MTBE, there is a reduction in the degradation of MTBE. However,
when the microbes were initially grown on tert-butanol and then
transferred to medium containing MTBE, there was a greater degradation
of MTBE (Mo et al., 1997).
A mixed culture isolated from biological sludges has been used in
bioreactors utilizing MTBE as a sole carbon source for over a year.
The microbes were able to degrade MTBE at a concentration of 160
mg/litre after 3 days of incubation in batch experiments. Mixed
cultures have greater capacity for degradation of MTBE than pure
cultures. The addition of other ethers causes a reduction in MTBE
degradation. In soil microcosm studies, significant MTBE degradation
by mixed cultures was observed at 24°C and 10°C (Mo et al., 1997).
Howard et al. (1991) estimated, on the basis of screening tests
for aerobic biodegradation with unacclimatized aqueous systems
(Fujiwara et al., 1984), that the half-lives of MTBE in water and soil
under aerobic conditions ranged from 672 to 4320 h.
MTBE was found to be degraded by a number of propane-oxidizing
bacteria. The initial oxidation of MTBE produced nearly stoichiometric
amounts of TBA. The methoxy group of MTBE was further oxidized to
formaldehyde and finally to CO2. At 28°C, rates of MTBE degradation
by these bacteria ranged from 3.9 to 9.2 nmol/min per mg cell protein
weight (Steffan et al., 1997).
4.3.2 Anaerobic conditions
Biodegradability of MTBE to methane under anaerobic conditions
has been determined by measuring the production of CH4 and CO2
during exposure of MTBE to a large population of anaerobic bacteria.
MTBE was biodegraded anaerobically only to a very limited extent
(Pence, 1987b), and an average cumulative theoretical gas production
of only 7.1% was achieve within 56 days. Anaerobic biodegradation to
methane must exceed 50% to meet the validation requirements for
demonstration of anaerobic biodegradability.
The anaerobic degradation of MTBE has been examined in different
soils (unsaturated clay, sandy loam and silty loam) collected from
various depths at three different sites (Novak et al., 1992; Yeh &
Novak, 1994). The experiments were conducted in static small-volume
anaerobic microcosms, and three different oxygen-free conditions were
simulated; with nitrate as electron acceptor (denitrification),
sulfate-reducing conditions, and anaerobic fermentation. Factors
influencing the degradation of MTBE, ETBE and TBA were determined, and
included anaerobic microbial populations, soil anions, soil moisture
content, organic content, nitrogen availability, rate of ammonium
"fixation", and soil pH. The soils were moderately acidic (pH 5.0-6.0)
with the exception of surface soils. The concentration of the added
MTBE was monitored for more than 250 days. Three parameters were
evaluated: degradation rate, lag time and time for 80% of the compound
to be degraded. No anaerobic degradation of MTBE was found in
organic-rich soils over the 250-day study period. The only situation
in which MTBE degradation occurred was in an oligotrophic soil
containing a low level of organic matter and with a pH of 5.0-6.0.
About 10% of the MTBE was lost during the first two months, although
this decrease cannot unambiguously be attributed to biodegradation.
Several conclusions may be drawn from the experiments with TBA and
ETBE:
* Whereas degradation of TBA in soil from the oligotrophic site
could be enhanced by addition of nitrate, the degradation of TBA
was inhibited by adding readily degraded ethanol.
* Biodegradation of ETBE under denitrifying conditions was
extremely sensitive to the presence of readily degraded
substrates.
These results illustrate that care should be exercised in
assessing biodegradability when several readily degraded substrates
are available, a condition that may be encountered in groundwater
contaminated with oxygenate additives.
Suflita & Mormile (1993) used sediment suspensions prepared from
samples collected from an aquifer polluted with leachate from a
municipal landfill. They assessed the formation of methane from a
range of substrates, and after at least 249 days no evidence for
anaerobic degradation of MTBE could be found. Whereas unbranched
alkanols and ketones were readily degraded, ethers in general were
resistant; in addition, oxygenates containing a tertiary or quaternary
carbon atom proved more recalcitrant than their unbranched or
moderately branched chemical analogues to anaerobic degradation.
Comparable experiments using a wider range of sediment samples
(Mormile et al., 1994) showed similar results under sulfate-reducing
or denitrifying conditions, although under methanogenic conditions a
single sample transformed MTBE into TBA. Likewise, the ethers were
unaffected by incubation with cultures of the acetogenic bacteria
Acetobacterium woodii and Eubacaterium limosum that convert
aromatic methoxy groups to acetate.
Based on the above-mentioned studies, MTBE is classed as
recalcitrant under anaerobic conditions.
Howard et al. (1991) estimated that the half-life of MTBE in
water under anaerobic conditions ranges from 2688 to 17 280 h.
4.4 Abiotic degradation
4.4.1 Air
4.4.1.1 Photolysis
Direct photolysis of MTBE is assumed to be environmentally
insignificant since it does not absorb radiation above 230 nm (Calvert
& Pitts, 1966). However, under laboratory conditions MTBE in an
oxygenated slurry system containing TiO2 as catalyst was readily
degraded by UV light from a mercury lamp. MTBE was rapidly
photocatalytically degraded, 76% of the initial concentration being
converted to degradation products, including TBA. After 4 h MTBE was
no longer detectable (Barreto et al., 1995).
4.4.1.2 Hydrolysis
MTBE does not contain hydrolysable functional groups, and,
therefore, it is inert to environmental hydrolysis. Hydrolysis of MTBE
is assumed to be insignificant (Howard et al., 1991).
4.4.1.3 Photooxidation
MTBE is subject to photooxidation in the atmosphere. This will
occur under the influence of various mechanisms, such as the reaction
with hydroxyl radicals, water, alkoxy and peroxy radicals, oxygen
atoms, and ozone. On the basis of the rate constant of each of the
reactions and the concentrations of the reactants, the reaction with
the hydroxyl radical is considered to be the most important removal
process for MTBE in the atmosphere. Several products are generated as
a result. These include tertiary-butyl formate (TBF), the major
product, 2-methoxy-2-methyl propanol, formaldehyde, acetone, NO2, and
the methyl radical. Molar yields of products identified from the
reaction of hydroxyl radicals with MTBE are given in Table 4). TBF is
unreactive to further photo-oxidation, while 2-methoxy-2-methyl
propanol is expected to be highly reactive with hydroxyl radicals,
yielding equimolar amounts of CO2, formaldehyde, acetone and water.
Of these products, formaldehyde is highly reactive with the hydroxyl
radical (Wallington et al., 1988; Japar et al., 1991). Rates of
reaction of oxygenates and their decomposition products with hydroxyl
radicals are given in Table 5.
Factors influencing atmospheric lifetime, such as time of day,
sunlight intensity and temperature, also include those affecting the
availability of hydroxyl radicals. Based upon measured rate constants
for reactions with hydroxyl radicals in air (Cox & Goldstone, 1982;
Atkinson, 1985; Wallington et al., 1988, 1989; Atkinson, 1990; Japar
et al., 1990), the half-life for MTBE has been estimated to be between
20.7 and 265 h (Howard et al., 1991). Hence, MTBE is not considered to
be a greenhouse gas, nor would it contribute to the depletion of the
ozone layer (Environment Canada, 1993).
Table 4. Molar yields of products identified from the reaction of
hydroxyl radicals with MTBE
Product Molar yielda Molar yieldb
TBF 0.68 0.76
Formaldehyde 0.48 0.37
Methyl acetate 0.14 0.17
TBA 0.062 -
Acetone 0.026 0.02
a Smith et al., 1991.
b Tuazon et al., 1991.
Table 5. Rates of reaction of oxygenates and their decomposition
products with hydroxyl radicals at 25°C
Compound Rate Reference
(10-12 cm3
sec-1 molecule-1)
MTBE 3.2 Japar et al., 1991
ETBE 8.5 Japar et al., 1991
TBF 0.74 Smith et al., 1991
TBA 1.1 Japar et al., 1991
Formaldehyde 9.0 Atkinson & Pitts, 1978
2-methoxy-2-methyl propanala 30 Japar et al., 1991
a Estimated from rates for other aldehydes
4.4.2 Natural waters
MTBE is not expected to adsorb significantly to bed sediments of
suspended sediments, hydrolyse, directly photolyse, or photo-oxidize
via reaction with photochemically produced radicals in water. While
MTBE is reported to be chemical unstable in acidic solutions (Budavari
et al., 1996), it is not expected to be hydrolysed in natural waters
under normal pH conditions (Lyman et al., 1990).
4.4.3 MTBE half-life ranges in environmental compartments
The half-life of a chemical in the environment depends not only
on the intrinsic properties of the chemical, but also on the nature of
the surrounding environment, such as sunlight intensity, hydroxyl
radical concentration, the nature of the microbial community and
temperature. Table 6 lists the half-life ranges in various
environmental compartments estimated by Mackay et al. (1993) and
Howard et al. (1991); these estimates are somewhat uncertain, as
implied by the order of magnitude range for some compartments.
4.5 Ozone-forming potential
Photochemical ozone-creation potentials (POCP) ranging from 20.4
to 34.6 have been determined for MTBE using a model that simulates the
formation of photochemical ozone episodes (Derwent et al., 1996). The
POCP values reflect the ability of a substance to form tropospheric
ozone as a result of its atmospheric degradation reactions. The POCP
values are calculated relative to ethylene (a chemical that is thought
to be important in such ozone formation and is given a POCP of 100).
Based on the emissions and the POCP value, MTBE (itself) is likely to
play a minor role in photochemical smog and low-level (tropospheric)
ozone formation near to sources of release.
4.6 Remediation
Examples of remedial methods that can be considered for MTBE are
air stripping, carbon absorption and soil vapour extraction. Intrinsic
bioremediation may be limited due to the variability of rates of
biodegradation of MTBE which have been previously mentioned (Zogorski
et al., 1996).
Table 6. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Soil 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Air 20.7-265 Based upon measured Howard et al., 1991
10-30 photo-oxidation half-life Mackay et al., 1993
Surface water 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The major sources of MTBE to the general population are probably
associated with the distribution, storage and use of oxygenated
gasoline. The main source of non-occupational exposure to MTBE is
evaporative emissions from gasoline. A large portion of the population
is exposed during time spent at service stations, while driving cars,
in public parking garages, and in homes with attached garages. These
exposures generally occur through inhalation. In addition, discharges
into the soil or groundwater are a potential for contaminated water
supply and can lead to exposure when such water is drunk. Dermal
contact with MTBE may occur through accidental spills of MTBE-blended
gasoline or through the use of gasoline as a solvent. In Canada, it
has been estimated that gasolines blended with MTBE account for only
2% of the total annual gasoline consumption. MTBE is used in small
quantities by a few Canadian refiners to boost octane levels in
gasoline. A limited survey of the MTBE content of unleaded regular,
mid-range and premium gasoline across Canada in 1995 showed a range of
0 to 5.2% by volume for winter grade gasoline and 0 to 9% by volume in
summer grade gasoline. In the USA, oxygenated gasoline containing
10-15% MTBE is used in different areas and about 30% of the US
population is exposed to MTBE.
5.1 Environmental levels
5.1.1 Exposure
Ambient air and microenvironment concentrations of MTBE and other
fuel oxygenates have been measured in Canada, the USA and Finland.
When available, air data are presented below in conjunction with data
on MTBE levels in gasoline and with information on the proximity of
the samples to various point sources of MTBE.
Brown (1997) estimated average daily and average lifetime doses
of MTBE from exposure in air and drinking-water for a US population.
Concentration data and several of the population characteristics were
estimated as distributions rather than as point values. Arithmetic
mean occupational doses via air were in the range of 0.1 to
1.0 mg/kg-day, while doses from residential exposures, commuting and
refuelling were in the range of 0.0004 to 0.006 mg/kg-day. Lifetime
doses for workers were in the range of 0.01 to 0.1 mg/kg-day. The
cumulative dose distribution for the entire population of the
MTBE-using regions of the USA was estimated by combining the
distributions of doses and the numbers of people in each exposure
category. In the MTBE-using areas, arithmetic mean doses via air were
estimated to be 0.0053 and 0.00185 mg/kg-day for the chronic and
lifetime cases, respectively. It was found that 1.5% of the population
used water contaminated with MTBE leakage with an estimated geometric
mean concentration of 0.36 µg/litre and a 95th percentile
concentration of 64 µg/litre. Including ingestion, inhalation, and
dermal absorption of contaminated water, the estimated arithmetic mean
does of the population exposed via water was 1.4 × 10-3 mg/kg-day.
5.1.1.1 Levels in ambient air and various microenvironments
a) Canada
The concentrations of MTBE in ambient air at various selected
locations in Canada have been measured as part of the National Air
Pollution Surveillance Programme in 1995 and 1996. This programme is a
joint project of the federal, provincial and municipal levels of
government. Its purpose is to monitor and assess, on a continuing
basis, the quality of the ambient air in the various regions of
Canada. The sites selected for monitoring of MTBE were based on usage
of gasoline with MTBE and/or because of nearby manufacturers of MTBE.
Pollutants from air were collected intermittently using the
canister methodology. Concentrations of MTBE was measured using the
detection principle of gas chromatography furnished with an ion trap
detector. Air samples were first passed through a cryogenic
concentration trap to gather enough analyte before injection into a GC
capillary column to allow compound speciation and quantification.
Approximately 200 ml of the canister sample was concentrated. A
cryogenic trap held at -150°C was used to concentrate the air sample.
Once the sample was concentrated, the trap was heated to 150°C and the
sample was back-flushed onto the column. MTBE and other hydrocarbons
were separated using a fused silica capillary column. The GC oven was
programmed to remain at 60°C for 3 min, then increased to 280°C at a
rate of 8°C/min. Calibration standards were prepared using the static
dilution technique. The detection limits were 0.05 to 0.1 µg/m3.
Table 7 lists the ambient concentration of MTBE in air at various
locations in Canada from 1995 to 1996.
Table 8 shows some MTBE atmospheric concentrations at the fence
line of a petroleum refinery at St John, New Brunswick, Canada, during
a period when there were complaints of odour. The same collection and
analytical methodology was used. The maximum concentration is not
considered representative of the area.
b) USA
In many urban areas in the USA having elevated levels of ozone or
CO, oxygenates such as MTBE are regulated for use in gasoline at
concentrations of 2.0% and 2.7% oxygen by weight (called reformulated
and oxygenated gasoline, respectively). These concentrations are
achieved by adding MTBE at 11% and 15% by volume, respectively. In
other areas, MTBE is used as an octane enhancer in premium gasoline at
concentrations up to 9% by volume, but usually at much lower
concentrations. It is important to note that MTBE is the predominant
oxygenate currently in use in these gasoline mixtures, followed by
ethanol (approximately 65% and 35% of the oxyfuels sold contain MTBE
and ethanol, respectively). Oxygenates used to a minor extent include
ETBE, TAME and DIPE (HEI, 1996). In 1994, oxygenates were added to
more than one-third of the gasoline market in the USA.
Table 7. Concentrations of MTBE in ambient air in Canada (1995-1996)
(Environment Canada, 1996b)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Edmonton(1)c Two petroleum refineries - 1 km. 20/7/95 7.21
Acetic acid plant - 2.5 km
26/7/95 11.39
1/8/95 0.81
7/8/95 2.93
8/8/95 5.50
12/9/95 2.49
27/5/96 3.35
Edmonton(2)d N/A 26/7/95 < DL
1/8/95 < DL
1/9/95 < DL
6/9/95 < DL
24/9/95 < DL
30/9/95 < DL
27/5/96 < DL
Halifaxd N/A 3/4/96 < DL
15/4/96 0.13
21/4/96 0.15
Montreal(1)c Two refineries (BTX, petroleum) 21/8/95 1.54
- 1.6, 2.5 km
21/8/95 0.59
12/9/95 1.06
16/3/96 < DL
15/5/96 0.42
21/5/96 0.28
27/5/96 0.23
Montreal(2)d N/A 16/3/95 0.15
16/5/96 0.18
21/5/96 0.22
27/5/96 0.37
Montreal(3)e N/A 10/3/96 0.16
16/3/96 0.28
9/5/96 0.95
Montreal(4)f N/A 9/5/96 0.22
15/5/96 < DL
21/5/96 0.70
St. Johnc Petroleum refinery - 3 km 9/5/96 1.02
15/5/96 3.73
Stouffvillef N/A 6/10/95 0.19
18/10/95 0.35
Toronto(1)d N/A 29/8/95 < DL
2/9/95 < DL
2/9/95 0.07
Table 7. (continued)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Toronto(2)d N/A 17/8/95 0.03
29/8/95 < DL
2/9/95 < DL
Vancouver(1)f N/A 23/8/95 0.27
29/8/95 0.89
29/8/95 0.16
30/8/95 0.33
Vancouver(2)f N/A 22/3/95 0.14
Vancouver(3)c Two gasoline processing and 1/8/95 2.13
storage plants - 0.5, 3 km
13/8/95 1.82
25/8/95 3.35
2/9/95 1.78
2/9/95 26.43
21/2/96 0.31
10/3/96 1.10
16/3/96 0.48
16/3/96 0.39
22/3/96 1.55
28/3/96 < DL
Vancouver(4)e N/A 10/3/96 1.07
Vancouver(5)f N/A 28/3/96 0.40
Vancouver(6)c Pipeline transfer point 1/9/95 1.79
2/9/95 1.90
22/3/96 0.89
Windsord N/A 2/8/95 0.08
17/8/95 0.05
17/8/95 0.11
21/8/95 0.40
29/8/95 0.27
29/8/95 0.14
16/3/96 < DL
21/4/96 0.15
27/4/96 < DL
Winnipegd N/A 4/3/96 0.02
27/3/96 < DL
a ( ) = different monitoring sites in same city.
b DL = detection limit = 0.1 µg/m3.
c Monitoring site in vicinity of petroleum refinery and/or industrial chemical plant, or
pipeline transfer area.
d Monitoring site in urban area.
e Monitoring site in urban area on busy street.
f Monitoring site in suburban area.
Table 8. MTBE concentrations at petroleum refinery boundary
(St. John, New Brunswick, Canada) during period of odour
complaints
Sampling date MTBE concentration
(µg/m3)
19/7/95 281
2/8/95 15
14/8/95 71
28/8/95 36
MTBE air quality data were collected in the USA as a result of
special studies in six urban centres: Fairbanks (Alaska), Stamford
(Connecticut), Albany (New York), Milwaukee (Wisconsin), Boston
(Massachusetts) and Houston (Texas) (Zogorski et al., 1996). In
addition, collection of MTBE air quality data for selected monitoring
sites in California started in 1996. Although these data cannot be
used to define quantitatively the air quality in these cities and are
not sufficient to provide a national perspective, they can be used to
estimate approximate ranges of MTBE in ambient air in the locations
sampled (Zogorski et al., 1996; US Interagency Assessment, 1997).
Non-occupational and consumer exposure to MTBE is shown in Table 9,
and service station attendants and garage workers in Table 10.
Owing to health complaints (Gordian et al., 1995) following the
introduction of oxygenated fuels (15% MTBE) during the 1992 winter
season in Fairbanks, Alaska, the sale of these fuels was suspended in
mid-December 1992, one month after their introduction. Zweidinger
(1993) analysed air samples for MTBE in ambient air and in various
microenvironments in Fairbanks taken immediately prior to the
suspension (phase I), during the phase-out period (Phase II), and two
months after the suspension, at which time the MTBE fuels were
expected to be at nominal levels (Phase III). Fuel samples collected
from Fairbanks gasoline stations during Phases II and III indicated
that the average percentage by weight of MTBE in unleaded regular
gasoline decreased from 8.5% to 1% while the average for premium
gasoline decreased from 14.7 to 5.6%. For comparison, ambient air
samples were also collected in the spring of 1993 from Stamford,
Connecticut, where 15% MTBE oxygenated gasoline was sold but there
were no consumer health complaints, and Albany, New York, where MTBE
was only present in gasoline at nominal levels to enhance octane and
there were also no health complaints (Zweidinger, 1993).
Ambient air samples in these cities were generally collected over
an 8-h period and were taken from the following areas: (1) outside
city limits, for background levels; (2) in residential areas away from
heavy traffic, and (3) in areas adjacent to major roadways or
intersections. The median and range of MTBE concentrations for the
selected locations and phases are shown in Table 11 (Zweidinger,
1993).
In Fairbanks, MTBE levels in all ambient environments were lower
when the use of MTBE as an oxygenate in gasoline was discontinued.
Overall MTBE ambient air concentrations ranged from non-detectable
levels to a maximum of 100.9 µg/m3 (28.0 ppbv) in the phases prior to
and during the phase-out of MTBE in Alaskan gasoline (Phases I and II)
and ranged from not detectable to 12.3 µg/m3 (3.4 ppbv) when MTBE use
was reduced to nominal levels in fuels (Phase III). Background levels
were low during Phase III, ranging from not detectable to 4.3 µg/m3
(1.2 ppbv) MTBE. Since sampling was limited during Phases I and II,
the levels of MTBE in ambient air outside city limits cannot be
compared for the three sampling periods over which MTBE concentrations
in gasoline were being reduced.
Phase II ambient residential and roadside area air samples
presented the highest levels of MTBE, with concentrations ranging from
6.1 to 100.9 µg/m3 (1.7 to 28.0 ppbv) and 15.1 to 28.5 µg/m3 (4.2 to
17.9 ppbv) respectively, and with medians of 6.1 µg/m3 (4.6 ppbv) and
34.9 µg/m3 (9.7 ppbv), respectively. Owing to lack of samples or
small sample size in Phase I data, comparisons between the phases were
limited to the roadside category. However, in this category, it was
unexpectedly found that, although Phase 1 sampling occurred prior to
suspension of MTBE, the data showed roadside levels of MTBE in ambient
air to be slightly lower than those during the suspension period.
In Stamford, Connecticut, limited measurements of MTBE taken in
the spring of 1993 showed that residential, roadside and gas station
ambient air results were consistently lower than those taken in
Fairbanks during the oxygenates programme, with ranges of not
detectable to 1.1 µg/m3 (0.3 ppbv), not detectable to 1.8 µg/m3
(0.5 ppbv) and 4.3 to 10.1 µg/m3 (1.2 to 2.8 ppbv), respectively.
Limited residential and roadside ambient MTBE air samples taken in
Albany, New York, in May 1993 were lower than levels during a
comparable phase of MTBE use in Fairbanks (Phase III). Ambient
temperature and other meteorological conditions differed significantly
among the cities where samples were collected.
In Fairbanks, Stamford and Albany, roadside ambient air levels
were found to be generally higher than residential area ambient air
levels (Table 11). Consequently, this roadside category may also be
considered to be a microenvironment, especially for those samples
taken in downtown city street canyons. For this particular study, it
was not possible to discern whether this was the case. Table 12
presents information on air samples taken in more clearly defined
microenvironments such as service stations and vehicle interiors
(Zweidinger, 1993).
Table 9. Non-occupational and consumer exposure to MTBE in the USAa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Community air
Milwaukee WI RFG in Yes (in 6/11 0.000025 <0.00413 0.00013 Jan-March 1995; Approximately Allen &
use* some 24-h samples; 50% contained Grande
cases) collected in MTBE, remainder (1995)
evacuated ETBE or ethanol
canisters;
GC(FID)
3/5 0.000025 <0.00106 0.00052 Feb-March 1995;
2-h samples;
collected in
evacuated
canisters;
GC(FID)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 up to (0.002) Feb-March 1995; Approximately Allen
use* 0.0037 2- to 3-h 50% contained & Grande
samples; MTBE, remainder (1995)
collected in ETBE or ethanol
evacuated
canisters;
GC(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Automobile cabin for commuters
New Jersey 15 MTBE NA 20/20 0.002- 0.004 April 1993; Estimated from Lioy et
0.017* approximately graphed data al. (1994)
1-h samples; in the original
Connecticut 20/20 0.003- 0.0056 absorbed onto report
0.009* carboxen 569:
collected in
evacuated
canisters;
GC/MS
Service station refuelling
Phoenix, AZ 12* No 40/40 0.09-38 5.8 Oct-Nov 1990; Samples taken API (1993)
each sample was from one station
collected in which only
Los Angeles, 13 Yes 6/6 1.1-6.5 3.6 during the premium gasoline
CA refuelling of 8 was oxygenated
to 10 vehicles;
each refuelling
was sampled for
1 to 2 min;
absorbed onto
charcoal; GC
(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey,
New York 10-15* Yes 4/4 NS 0.370 April 1993; 5-min Estimated from Lioy et
breathing-zone graphed date in al. (1994)
samples before, the original
Connecticut No 4/4 NS-4.1 0.572 during and after report
refuelling;
absorbed onto
carboxen 569;
GC/MS
Milwaukee WI Jan-March 1995; RFG with MTBE Allen &
Station A 9-10* Yes 6/6 NS (0.39) 15-min used only in Grande (1995)
Station D 9** No 2/2 NS (2.93) breathing-zone higher grades;
samples; adsorbed *ethanol used
onto charcoal; in regular
GC(FID) gasoline; **2%
MTBE used in
regular gasoline
Northeast and 10-17* Yes 8/17 <0.32 <2.1 0.57 Feb-April 1994; Northeast = API (1995c)
southwest 15- to 20-min Connecticut and
areas personal New Jersey
(short-term breathing zone locations;
sample) samples; adsorbed Southwest =
onto charcoal; Arizona
GC(FID) locations
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
In automobile while refuelling
Connecticut, New Jersey and New York Service Stations
Self-serve 10-15* Yes 4/4 0.006- 0.03 April 1993; 5-min Estimated from Lioy et
0.072 breathing-zone graphed data in al. (1994)
samples before, the original
Full-serve Yes 8/8 0.008- 0.034 during and after report
0.172 refuelling;
adsorbed onto
Self-serve No 4/4 NS 0.015 carboxen 589;
GC/MS
Full-serve No 4/4 0.005- 0.041
0.103
Service station pump island
New Jersey: Yes 4/4 0.120- 0.440 April 1995; 4-h API (1995a)
Full-serve 1.600 breathing-zone
samples during
both refuelling
and not
refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 6/6 0.014- 0.048
Self-serve 0.080
Connecticut: No 9/10 0.09 <1.500 0.170
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 15 Yes 3/3 0.08- 0.24 Nov-Dec 1994; 7- Cook &
0.24 to 8-h samples; Kovein (1995)
adsorbed onto
charcoal; GC (FID)
Service station perimeter
Phoenix AZ 12 No 24/24 0.009- 0.02 Oct-Nov 1990; API (1993)
0.09 12-h samples;
4 perimeter
samples plus
samples upwind
and downwind
from the pump
island for each
sample set;
adsorbed onto
charcoal; GC(FID)
New Jersey: Yes 15/16 0.001 <0.036 0.003 April 1995; 4-h API (1995a)
Full-serve samples;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 24/24 0.002- 0.007
Full-serve 0.083
Connecticut: No 38/40 0.001 <0.140 0.014
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Milwaukee WI
Station A 9-10* Yes 2/2 NS (0.0024) Jan-March 1995; *RFG, MTBE used Allen &
Station B 2-9** No 1/1 NS (0.0045) 2-h area samples; only in higher Grande (1995)
collected in grades; ethanol
evacuated in lower grades
canisters; GC(FID) **2% MTBE in
regular gasoline
a Asterisks in any column indicate that further explanation is provided in the comments column.
b Number of samples in which MTBE was detected divided by total number of samples.
c GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
NA = not applicable; NS = not stated.
Table 10. Non-industrial occupational exposures to MTBEa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Service station attendants during refuelling
Phoenix AZ Oct-Nov 1990; each API (1993)
1-2 min 12 No 40/40 0.09-38 5.8 breathing-zone
sample was
collected during
Los Angeles the refuelling of
CA 13 Yes 6/6 1.1-6.5 3.6 8-10 vehicles;
1-2 min each refuelling
was sampled for
1-2 min; adsorbed
onto charcoal;
GC (FID)
New Jersey, 10-15 Yes 4/4 NS 0.37* April 1993; 5-min Estimated from Lioy et
New York, breathing-zone graphed data al. (1994)
samples before, in original
during, and after report
Connecticut: refuelling;
5 min No 4/4 <4.1 0.572 adsorbed onto
carboxen 589;
GC/MS
Milwaukee WI Jan-March 1995; This station used Allen &
Station A 15-min MTBE in middle Grande (1995)
15 min RFG 9-10 Yes NS/6 NS 0.31 breathing-zone and premium grade
samples during gasoline, and
refuelling; ethanol in
collected in regular gasoline
evacuated
canisters
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
15-20 min 10-17 Yes 8/17 0.32 <2.1 0.57 samples; mostly locations;
15- to 20-min Southwest =
personal Arizona
breathing-zone locations
samples during
refuelling;
adsorbed onto
charcoal;
GC (FID)
Various USA 1982-1993: data Service station API (1995b)
locations reported by and retail outlet
American Petroleum personnel
<30 min NS NS 9/11 0.16 0.16- 2.6 Institute member
136.1 companies; higher
frequency of
30 min-6 h 5/5 0.01- 0.34 measurements
2.7 in 1989-1993;
GC(FID) and
other procedures
6-9 h TWA 13/13 0.09- 0.59
34.0
>9 h 11/11 0.01- 1.1
17.20
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 4 h 13-10 Yes 4/4 0.084- 0.245 April 1995; 4-h API (1995a)
(full-serve) 0.52 breathing-zone
samples, during
New York 4 h Yes 5/6 0.077- 0.205 refuelling and
(self-serve) 0.78 not refuelling;
collected in
Connecticut 4 h No 10/10 0.170- 1.5 8-litre evacuated
(self-serve) 2.60 canisters; GC/MS
Phoenix AZ 4 h 14 No 42/42 0.04- 0.55 Oct-Nov 1990; 4-h Hartle (1993)
3.88 (half-shift)
breathing-zone
samples (average
time 224 min);
adsorbed onto
charcoal; GC(FID)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
>8 h 10-17 Yes 18/21 0.03- <0.5 0.27 samples, most locations;
0.11 sampling times Southwest =
were > 6 h; Arizona
adsorbed onto locations
charcoal; GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey Nov-Dec 1994; Cook &
(full shift) 15 Yes 21/21 NS 0.12- 0.48 breathing-zone Kovein (1994)
1.42 samples for 3-8 h;
adsorbed onto
charcoal; GC (FID)
Service station attendants at pump island
New Jersey
4 h 13-16 Yes 4/4 0.12- 0.44 April 1995; 4-h API (1995a)
(full-serve) 1.60 breathing-zone
samples during
refuelling and
not refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York Yes 6/6 0.0005 0.014- 0.048 Cook &
4 h 0.08 Kovein (1994)
(self-serve)
Connecticut 15 No 9/10 NS <1.5 0.17 Nov-Dec 1994; 7-
4 h to 8-h samples;
(self-serve) adsorbed onto
charcoal; GC (FID)
New Jersey Yes 3/3 0.08- 0.24
8 h 0.24
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 0.0023- (0.002) Feb-March 1995; Approximately 50% Allen &
2-3 h use 0.0037 2- to 3-h samples; contained MTBE, Grande (1995)
collected in remainder
evacuated contained ETBE
canisters; or ethanol
GC(FID)
Mechanics Feb-April 1994; Northeast = API (1995c)
Northeast and personal Connecticut and
southwest in breathing-zone New Jersey
winter samples, most locations;
short-term Southwest =
15 min 10-17 Yes 4/13 <0.25- <32 ND sampling times Arizona locations
(<0.26) <0.35 were 15-20 min;
most long-term
>6 h 17/20 <0.02- <2.6 0.09 sampling times
(<1.5) <0.05 were >6 h; adsorbed
onto charcoal;
GC(FID)
Northern New April 1993; 1-h Workers at Mohr et
Jersey breathing-zone service stations al. (1994)
1 h 15 NS NS/13 0.3-6.1 samples (active); and garages
adsorbed onto for State
carboxen; GC/MS vehicles
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Stamford April 1993; Mechanics with Buchta (1993b)
Connecticut full-shift the Department
8-h TWA 13-17 NA 20/28 0.03 <12.04 0.11 samples of Public Works
(approximately and in auto
8 h); adsorbed dealers' garages
onto charcoal;
GC(FID)
Fairbanks AK Dec 1992; Moolenaar
8-h TWA 15 NS NS/10 0.01- 0.10 full-shift et al. (1994)
0.81 samples
(approximately
8 h); collected
in evacuated
canisters in
environment where
workers spent
most of their
day; GC
Other vehicle-related workers
Stamford 13-17 NS 0/7 0.03 <DL <DL April 1993; 8-h Workers who
personal spent time in
breathing-zone traffic
samples; adsorbed
onto charcoal;
GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Connecticut Workers in various Buchta (1993b)
8-h TWA 1/4 <0.15 <DL jobs (mostly
workers in garages
who performed
tasks difference
from the mechanics)
a Number of samples in which MTBE was detected divided by total number of samples.
b GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
c Asterisks in any column indicate that further explanation is provided in the comments column.
TWA = time-weighted average; NA = not applicable; NS = not stated.
Table 11. Concentrations of MTBE in µg/m3 (ppbv) in ambient 8-h air samples taken in Fairbanks, Stamford and Albany, USA,
as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)a
Sampling site Fairbanks Fairbanks Fairbanks Stamford Albany
Phase I Phase II Phase III April 1993 May 1993
early December 1992 late December 1992 Feb/Mar 1993
Background:
number of samples 0 1 5 2 0
median - ND ND 0.72 (0.2) -
range - 1 ND-4.3(1.2) ND-1.1(0.3) -
Residential:
number of samples 2 11 11 2 3
median 14.4(4) 16.6(4.6) 2.5(0.7) 1.1(0.3) ND
range 7.2-21.6 16.1-100.9 ND-9(2.5) ND-1.8(0.5) ND-0.4(0.1)
(2.9-6.0) (1.7-28.0)
Roadside:
number of samples 7 7 10 2 7
median 18(5) 35(9.7) 4.3(1.2) 7.2(2.0) 0.72(0.2)
range 10.8-43.2 15.1-64.5 ND-12.3(3.4) 4.3-10.1 (1.2-2.8) ND-2.5(0.7)
(3.0-12.0) (4.2-17.9)
a For the calculation of a median when n = 2, the two sample values are averaged together; in the case of an ND value,
half the detection limit value is substituted in the calculation, i.e. 0.36 µg/m3 (0.1 ppbv).
ND = non-detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Table 12. Concentrations of MTBE in µg/m3 (ppbv) in various microenvironmental 8-h ambient air samples taken in Fairbanks, Stamford
and Albany, USA, as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Service station pump island:
number of samples 1 1 6 4 4
median 194.6 (54) 134.8 (37.4) 11.5 (3.2) 13.7 (3.8)a 64.2 (17.8)
range - - 6.1-49.7 ND-26.7 (7.4) 23.3-194.6
(1.7-13.8) (6.6-54)
Commercial vehicle interiors:
number of samples 8 - 6 - -
median 126.1 (35) - 31.7 (8.8) - -
range 25.2-1207.3 - 1.4-129 - -
(7-335) (0.4-35.8)
Indoors - commercial garage service areas:
number of samples 5 - 10 8 -
median 1088.4 (302) - 148.5 (41.2) 484 (134.8) -
range 367.6-2922.8 - 21.3-496 4.7-1546.5 -
(102-811) (5.9-137.6) (1.3-429.1)
Indoors - residential area:
number of samples - 3 5 - -
median - 6.5 (1.8) 2.9 (0.8) - -
range - 6.1-15.1 1-4 (0.3-1.1) - -
(1.7-4.2)
Table 12. (continued)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Indoors - public buildings near roadway:
number of samples - 4 5 4 -
median - 32.4 (9) 6.5 (1.8) 1.8 (0.5) -
range - ND-37.1 (10.3) ND-10.5 (2.9) 1.4-1.8 -
(0.4-0.5)
Indoors - home with attached garage:
number of samples - 5 4 - -
median - 27.8 (7.7) 72.1 (20) - -
range - 10.1-75.3 51.5-109.2 - -
(2.8-20.9) (14.3-30.3)
a In Stamford, service station samples were taken 4.6 metres (15 feet) away from the pump.
ND = not detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Kelly et al. (1993) performed 24-h ambient air sampling in the
cities of Boston and Houston where MTBE was used nominally in gasoline
(i.e. approximately < 5% by volume). Sampling took place
approximately every 14 days from August 1990 to April 1991 and from
June to August 1991. The Boston sampling site was categorized as being
in an urban area of mixed industry and office buildings, with high
traffic density, and the sampler was placed on a downtown fire
department rooftop. Conversely, the Houston sampling site was located
in a semi-rural area, on the roof of an air sampling station, and was
expected to receive emissions from industrial and, to a lesser extent,
urban sources. MTBE levels in ambient air at these sites ranged from
< 0.72 to 1.8 µg/m3 (< 0.2 to 0.49 ppbv) and < 0.72 to 10.1 µg/m3
(< 0.2 to 2.8 ppbv), respectively (detection limit = 0.72 µg/m3 (0.2
ppbv). The median level from these data was < 2 ppbv for both Boston
and Houston. MTBE was detected in several samples from Houston (n =
22; in 64 % of samples MTBE was non-detectable), but only at one site
in Boston (n = 22; in 96% of samples MTBE was non-detectable).
Allen & Grande (1995) conducted an ambient air monitoring study
in the city of Milwaukee as a result of public health complaints when
MTBE was first introduced into Milwaukee reformulated gasoline (at
approximately 11% by volume) in 1995. Eleven weekly 24-h samples
collected at the Wisconsin Enhanced Ozone Monitoring Program air
sampling station from January to March 1995 resulted in concentrations
ranging from not detectable to 14.89 µg/m3 (4.13 ppbv) [n = 11; 45%
non-detectable samples; detection limit 0.36 µg/m3 (0.1 ppbv)]. The
median was determined to be 0.47 µg/m3 (0.13 ppbv). A control sample
collected in each of the nearby cities of Madison and Green Bay, where
reformulated gasoline use was not mandated, was found to be below the
detection limit of 0.36 µg/m3 (0.1 ppbv).
In the same study, Allen & Grande (1995) collected 1- to 3-h
roadside air samples at busy intersections and freeway interchanges
where it was expected that there would be high concentrations of
gasoline fumes. Mean levels of MTBE in air samples collected near a
freeway interchange, a busy intersection and a roadway in Milwaukee
were 1.9 µg/m3 (0.53 ppbv) (n = 3), 3.8 µg/m3 (1.06 ppbv) (n = 2)
and 1.8 µg/m3 (0.50 ppbv) (n = 2), respectively. This particular
choice of roadside location is a more clear example of a roadside
microenvironment and it may also be considered an ambient air sample.
The State of California mandated the year-round use of oxygenated
fuels in the South Coast Air Basin in 1995 and throughout California
by June 1996. As a result, MTBE was included in the California's
ambient air monitoring programme in February 1996 for the cities of
Burbank, Long Beach and Los Angeles and in June 1996 for Chico,
Roseville and Fresno. The overall range of 24-h average concentrations
was 1.4-44.7 µg/m3 (0.4-12.4 ppbv) for the selected monitoring sites.
The overall range of averages was closer to the Fairbanks data than to
data from other cities in the continental USA. Averages ranged from
4.7 to 17.3 µg/m3 (1.3 to 4.8 ppbv) with the highest averages
occurring in the cities of Los Angeles and nearby Burbank. The
following are average and ranges for each of the individual cities:
Burbank 17.3, 4.7-31.7 µg/m3 (4.8 ppbv, 1.3-8.8 ppbv), Long Beach
9.4, 3.2-21.6 µg/m3 (2.6 ppbv, 0.9-6.0 ppbv), Los Angeles downtown
13.7, 4-24.1 µg/m3 (3.8 ppbv, 1.1-6.9 ppbv), Chico 8.3, 3.6-27.8
µg/m3 (2.3 ppbv, 1.0-7.7 ppbv), Fresno 9.4, 2.2-44.7 µg/m3 (2.6
ppbv, 0.6-12.4 ppbv) and Roseville 4.7, 2.5-12.3 µg/m3 (1.3 ppbv,
0.7-3.4 ppbv). The number of samples for each city ranged from 18 to
28 and the detection limit for MTBE was 0.72 µg/m3 (0.2 ppbv) (M.
Poore, personal communication).
Measurements of ambient air concentrations of MTBE at service
stations are usually taken at the gasoline pump island and at the
service station perimeter. The air levels of MTBE tend to be higher at
the pump island and lower at the perimeter. In addition, service
stations equipped with vapour recovery systems (Stage II) tend to have
higher MTBE concentrations in both microenvironments. Furthermore,
whereas the pump island data represents a particular microenvironment
due to the presence of gasoline vapour coming from refuelling
activities, the gasoline station perimeter data has been used to
estimate potential community exposures and may be considered
representative of upper end ambient air levels in neighbourhoods.
Short-term peak samples of MTBE were not taken.
MTBE median concentrations generally ranged from 0.32 to 21.4
mg/m3 (0.09-6 ppm) in breathing-zone samples as a result of consumer
refuelling. The values were measured over 2- to 15-min sampling
periods and were highly variable but rarely exceeded 35.7 mg/m3 (10
ppm). The range of median values measured at the pump islands ranged
from 0.18 to 1.57 mg/m3 (0.05-0.44 ppm) over a 4-h sampling period.
The fenceline samples were lower and ranged from 0.004 to 0.5 mg/m3
(0.001 to 0.14 ppm) with a collection period of 4 h. Generally, the
concentrations were higher at service stations that did not have
vapour recovery systems.
Allen & Grande (1995) collected 1- to 3-h MTBE area samples
downwind of the gas pumps on the perimeter of four Wisconsin service
station properties from 21 February 1995 to 9 March 1995. The average
levels of MTBE at two service stations that dispensed reformulated
gasoline and had Stage II vapour recoverya were 8.8 µg/m3 (2.43
ppbv) (n = 2) and 2.7 µg/m3 (0.75 ppbv) (n = 2). The level of MTBE at
a station without vapour recovery was higher and was measured to be
16.5 µg/m3 (4.58 ppbv) (n = 1). Finally, one air sample taken at a
service station where reformulated gasoline was not mandated resulted
in a lower concentration of MTBE i.e. 0.8 µg/m3 (0.25 ppbv).
a Stage II is a vapour recovery system used to trap gasoline vapour
during refuelling of consumer vehicles.
In addition, Zweidinger (1993) analysed a limited number of 8-h
air samples taken in various microenvironments in Fairbanks (Phases I,
II and III), Stamford and Albany for MTBE. Generally,
microenvironmental air concentrations of MTBE decreased from Phase I
or II to Phase III at the following locations: (1) service station
pump island, (2) commercial vehicle interiors, (3) indoor - commercial
garage service areas, (4) indoor - residential area and (5) indoor -
public buildings near roadway (i.e. school, post office), due to the
suspension of the oxyfuel programme. The median and range of MTBE
concentrations for selected locations and phases are shown in Table
12. The air of one home with an attached garage was the exception to
this tendency in that the indoor air samples contained levels of MTBE,
benzene and other compounds associated with gasoline which were
significantly higher than air samples measured outside that home. This
indicated that the residential garage may have had a source of
evaporative emissions after parking the hot car in the garage or from
gasoline stored in the garage.
Huber (1995) used a multizonal mass balance model to predict
indoor air concentrations. Measured evaporative emissions of 0.5 g of
MTBE emitted from an automobile at rest at 23.9°C (a highly
unrealistic estimate for Fairbanks in winter) during 4 h in a garage
attached to a residential house resulted in modelled peak
concentrations of 2.3 mg/m3 (0.65 ppm) in the garage and 0.12 mg/m3
(0.035 ppm) in the residence. Modelled 1-h average concentrations in
the garage ranged from 2.5 to 4.3 mg/m3 (0.7 to 1.22 ppm) while those
in the residence ranged from 0.072 to 0.32 mg/m3 (0.02 to 0.09 ppm).
This was estimated to be a worst-case situation for evaporative
emissions since a newer car or cold winter temperatures would probably
have reduced evaporative emission rates resulting in lower
concentrations (Huber, 1995). However, increased tailpipe vehicle
emissions as a result of cold start (the first few minutes of running
the engine before the catalytic converter starts to function) were not
included in these estimations.
In the case of the Stamford microenvironment samples, the
resulting 8-h average MTBE levels in indoor commercial garage service
areas and indoor locations near a roadway were lower than those taken
in Fairbanks even though the volume of MTBE present in Stamford
gasoline was higher than the volume used in Fairbanks during Phases II
and III. It is important to note that the service station pump island
samples were not strictly comparable since they were taken 4.5 m away
from the pump. When MTBE was only used as an octane enhancer, as was
the case for Albany and Fairbanks Phase III, levels of MTBE in service
station pump island air were found to be much higher in Albany.
Air measurements in the parking garage microenvironment have been
conducted in two studies. MTBE concentrations measured in two 8-h air
samples in a Stamford partially open parking lot (i.e. open-sided with
an office building directly above) in April 1993 were 70.4 µg/m3
(20.1 ppbv) and 177 µg/m3 (49.0 ppbv) with a mean of 124.7 µg/m3
(34.6 ppbv) (RB Zweidinger, personal communication). Allen & Grande
(1995) conducted measurements at an enclosed parking ramp in Milwaukee
in February 1995 in order to show ambient levels during cold starts.
One- to three-hour air samples showed MTBE levels of less than 72.1
µg/m3 (20 ppbv) with mean levels of 7.4 µg/m3 (2.05 ppbv) (n = 8).
The highest level occurred at a point when a large number of vehicles
were making cold starts in a short period of time.
Air samples were taken by Lioy et al. (1994) inside the vehicle
cabin microenvironment for (1) activities surrounding refuelling, and
(2) during suburban commutes before and after refuelling. The study
took place in April 1993 in New Jersey, New York and Connecticut where
gasoline containing 10 to 15% by volume MTBE was sold. The experiment
protocol consisted of a 60-min commuter run that included a 5-min
refuelling stop at full- and self-service stations with or without
Stage II vapour recovery. Resulting in-cabin levels of MTBE taken
immediately before, during and after refuelling ranged from 23.8 to
108 µg/m3 (6.6 to 30 ppbv), 133.4 to 313.5 µg/m3 (37 to 87 ppbv) and
31.4 to 151.4 µg/m3 (8.7 to 42 ppbv), respectively, for three
different cars, with average concentrations of 55.3 µg/m3 (14.8
ppbv), 190.2 µg/m3 (55 ppbv) and 72.1 µg/m3 (20 ppbv). Short-term
peak concentrations occurred during refuelling. In addition,
post-refuelling in-cabin concentrations were slightly higher than
pre-refuelling, although the increase was not statistically
significant. It was noted that the highest of the levels that diffused
into the cabin during refuelling occurred with an older vehicle, which
had an abnormally high evaporative emissions rate. There did not seem
to be a statistically significant difference in in-cabin levels
between the various types of service stations, although the sample
size was too small to be able to make this conclusions.
Microenvironmental in-cabin air concentrations of MTBE measured during
60-min suburban stop/go commutes ranged from 3.6 µg/m3 (1 ppbv) to
576.6 µg/m3 (160 ppbv) with a geometric mean of 21.6 µg/m3 (6 ppbv)
(n = 40). Most values were less than 19.8 µg/m3 (5.5 ppbv). It was
noted that the higher values were associated with the use of the
high-emission vehicle discussed previously.
Area monitoring was conducted in an indoor commercial garage
service area in Fairbanks, Alaska (Buchta 1993a). Three air samples
were taken over a 6- to 7-h period in February 1993 when MTBE use was
limited to purposes of octane enhancement. MTBE was non-detectable in
the three areas (service area, parts department, shop wall) but the
minimum detection limit was high (144.2 µg/m3, 40 ppbv). This is
comparable to the Zweidinger (1993) 8-h indoor commercial garage air
samples that were measured with more sensitive equipment and resulted
in a median concentration for MTBE of 114.4 µg/m3 (31.75 ppbv). The
higher levels in the latter study may have been the result of gasoline
spills.
Ambient air levels of MTBE have been measured near three
refineries in the USA. At one refinery a 24-h MTBE level of 20 µg/m3
was reported for one out of nine downwind samples taken at the
perimeter of a rural refinery, which was stated to release
approximately 33 tonnes of MTBE emissions in air per year. MTBE was
not detected in the 26 other downwind and upwind samples taken at the
refinery during the same period. MTBE was not detected in another 54
24-h samples taken at two other refineries: annual MTBE air emission
release data were not provided for these refineries. It should be
noted that the detection limit for these air samples was high (more
than 20 or 30 µg/m3), but more sensitive canister samples taken for
24 h also resulted in non-detectable concentrations (detection limit =
6 µg/m3) (API, 1989b).
c) Finland
Vainiotalo et al. (1996) reported the concentration of MTBE at
the perimeter and pump island of two self-service stations in Finland
(one urban roadside and one simple roadside) both with Stage Ia
vapour recovery systems where gasoline containing 11% MTBE by volume
was sold. The investigations were conducted during May/June and
October 1995. The average 24-h perimeter concentrations for each of
the 4-day sampling periods were generally higher for the urban
roadside service station samples: 12.4 µg/m3 (June) and 14.1 µg/m3
(October), with 35-36 measurements collected at each side on each
occasion. Several factors such as the volume of gasoline sold, mean
wind speed and number of deliveries of gasoline to the station were
higher for the former and resulted in data with higher variability
during the fall sampling. The overall range of perimeter air samples
for both service stations was from 0.5 to 120.5 µg MTBE/m3. Highest
daily concentrations were usually obtained at the downwind sampling
points. No seasonal influence was discernable. Mean 24-h
concentrations measured in the centre of the pump island ranged from
247 to 1347 µg/m3 (n = 15). The detection limit was not specified in
the study.
5.1.1.2 Dermal exposure
Dermal exposure and absorption may occur from MTBE-blended
gasoline at self-service refuelling or from its use as a solvent. It
may also occur from MTBE-contaminated household water during washing,
bathing or showering. There are, however, no data available to
estimate dermal exposure to MTBE.
5.1.1.3 Estimation of total personal exposure
Exposure is a function of concentration and time. Thus, the time
spent in various activities involving different concentrations and
degrees of contact will affect human exposures to MTBE. Huber (1995)
generated "worst-case scenario" estimates of long-term exposure to
MTBE based on population activity patterns and available
microenvironmental and ambient concentration data (generally rounded
a In the USA, Stage I is a vapour recovery system used during
loading and unloading of gasoline from delivery tankers. Since
this is a Finnish Study, this definition may or may not be
equivalent.
up to the next order of magnitude of 10). His intentionally high
estimates were updated and slightly revised to indicate that an annual
time-weighted average exposure might be as high as about 0.11 mg/m3
(0.03 ppm), assuming that gasoline contained 15% MTBE by volume for 6
months and approximately 10% for the remainder of the year (US
Interagency Assessment, 1997). This upper-end exposure estimate is
highly uncertain, given the lack of adequate data to describe the
distribution of actual personal exposure levels.
5.1.1.4 Other pollutants
There was an increase in indoor air levels of benzene after MTBE
reformulated fuel use was discontinued (Gordian & Guay, 1995). Both
ambient outdoor and specific indoor environment (garages, vehicles,
workplace, school, post office and residence) samples, as well as
blood samples, were collected in Fairbanks during and after the
oxygenated programme. In addition to MTBE, other volatile compounds,
such as benzene and formaldehyde, were evaluated. In indoor samples,
there was a statistically significant increase of benzene in garages
and non-garages after MTBE was discontinued. In garages, the mean
benzene concentration increased from 0.30 mg/m3 (94.02 ppb) in
December to 0.61 mg/m3 (191.62 ppb) in February, and in the school,
post office and residence from 0.02 mg/m3 (5.89 ppb) to 0.06 mg/m3
(20.22 ppb). The NIOSH-recommended exposure limit for benzene is 0.3
mg/m3 (0.1 ppm). In vehicles the data were difficult to interpret
because different vehicles were tested in December and in February.
The same pattern, although not statistically significant, was seen in
the outdoor samples.
5.2 Occupational exposure
5.2.1 Industrial operations - manufacturing and blending
MTBE can be encountered in solution and as vapour during its
manufacturing at chemical plants and refineries, during blending into
gasoline, transportation, distribution, and handling at service
stations. Some industrial hygiene monitoring data are available (Table
13).
At the MTBE unit at the Neches Chemical West Plant in the USA,
the TWA values ranged from less than 0.07 to 120.3 mg/m3 (0.02 to
33.41 ppm) for operations and maintenance personnel (Simer, 1986).
Exposure monitoring in a manufacturing plant showed that all
exposures to MTBE greater than 3.6 mg/m3 occurred during quality
control sampling procedures (ARCO, 1987). Two out of 46 short-term
samples indicated exposures to MTBE at concentrations of 22 mg/m3 and
6.5 mg/m3 (6.1 ppm and 1.8 ppm, respectively); 91% of the full shift
monitoring indicated exposure levels of less than 3.6 mg/m3.
Texaco (1993) presented results from a short-term monitoring for
MTBE conducted at a refinery in Guatemala in order to evaluate the
adequacy of current work procedures. No details of ambient temperature
Table 13. Occupational exposure to MTBE in the petroleum industry (adapted from HEI, 1996)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
MTBE manufacturing
Occupational exposure of
oil refinery and chemical
plant personnel handling
neat MTBE during:
Routine operations <30 min 14/27 0.16-1.00 0.16-7.8 1.00 API, 1995b
6-9 h TWA 38/76 0.01-0.03 0.01-248.7 0.03 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.17 0.17 API, 1995b
Routine maintenance <30 min 7/8 0.05 0.05-7.19 0.90 API, 1995b
30 min-6 ha 1/1 Not given 0.20 0.20 API, 1995b
6-9 h TWA 4/4 Not given 0.04-0.7 0.11 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.2 0.18 API, 1995b
Routine operations and
maintenance 8-12 h TWA 8/21 0.02-0.06 <0.02-33.41 1.06 Simer, 1986
20 min 0/1 [1.0] <1.0-1.0 <1.0 ARCO, 1987
4-6 h 1/11 [1.0] <1.0-6.1 <1.0 ARCO, 1987
12 h TWA 2/23 [1.0] 0.8-2.2 Not given ARCO, 1987
QA/QC sampling of MTBE 12-36 min 3/16 [1.0] <1.0-12.2 <1.0 ARCO, 1987
MTBE blending
Occupational exposure of
personnel involved in
fuel-blending activities
involving:
Neat MTBE <30 min 34/35 <0.005 0-97.0 2.90 API, 1995b
30 min-6 h 12/13 0.21 0.21-72.0 1.03 API 1995b
6-9 h TWA 7/12 0.04-1.80 0.04-87.97 2.24 API, 1995b
>9 h TWA 0/9 0.23-0.34 0.23-0.34 0.30 API, 1995b
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
Fuel mixtures <30 min 51/98 0.02-0.23 0.02-100 0.30 API, 1995b
30 min-6 h 5/19 0.03-0.33 0.03-1.98 0.05 API, 1995b
6-9 h TWA 34/112 0.02-0.20 0.02-14 0.04 API, 1995b
>9 h TWA 9/22 <0.005-0.02 0-0.27 0.02 API, 1995b
MTBE transport
Occupational exposure of
marine barge, pipeline and
rail car personnel to:
Neat MTBE <30 min 62/66 0.30-0.60 0.30-1050 13.83 API, 1995b
(trucking personnel included 30 min-6 h 23/27 0.04-0.36 0.04-700 2.20 API, 1995b
only for transport of neat 6-9 h TWA 9/10 0.03 0.03-711.9 0.18 API, 1995b
MTBE) >9 h TWA 1/1 Not given 0.32 0.32 API, 1995b
15 min TWA 4/4 Not given 90-150 110.00 Texaco, 1993
Fuel mixtures <30 min 60/64 0.001-0.14 0.001-507.87 2.44 API, 1995b
30 min-6 h 64/92 0.02-0.04 0.02-59.4 0.42 API, 1995b
6-9 h TWA 28/42 0.007-0.04 0.01-26.24 0.14 API, 1995b
>9 h TWA 8/8 Not given 0.19-4.51 1.49 API, 1995b
MTBE distribution
Occupational exposures of <30 min 93/129 <0.005-0.08 0-14.0 0.75 API, 1995b
marketing terminal and 30 min-6 h 9/10 0.26 0.26-4.05 0.98 API, 1995b
trucking personnel involved 6-9 h TWA 62/87 0.01-0.05 0.01-2.2 0.11 API, 1995b
in the handling of >9 h TWA 46/47 0.06 0.06-6.2 0.71 API, 1995b
Gasoline-MTBE mixtures 15 min TWAe ´ 0.2 <0.2-0.94 0.48 Hebert, 1993
10-12 h TWAe 2/2 Not given 0.08-0.08 0.08 Hebert, 1993
15 min TWAf 4/4 Not given 0.05-0.16 0.14 Gillie, 1993
15 min TWAg 3/3 Not given 1.9-3.6 2.80 Gillie, 1993
12 h TWAg 5/5 Not given 0.24-0.92 0.45 Gillie, 1993
15-40 minf (n=6) 0.2 2.8-42 13 (mean) Hakkola & Saarinen,
1996
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
10-30 minh (n=4) 0.2 20-226 91 (mean) Haakola & Saarinen,
1996
22-44 mini (n=5) 0.2 4.3-27 16 (mean) Haakola & Saarinen,
1996
10-37 minj (n=6) 0.2 10.0-98.0 71 (mean) Haakola & Saarinen,
1996
a Duration was task-related.
b Number of samples in which MTBE was detected divided by total number of samples.
c In the case of "non-detectable" samples the detection limit was used to calculate the median.
d The API (1995b) measurements used different sampling and analytical techniques on both personal breathing zone and area air samples.
e Loading of trucks with vapour recovery.
f Bottom loading of trucks with vapour recovery.
g Truck unloading at service station with vapour recovery.
h Top loading without vapour recovery.
i Truck unloading at service station without vapour recovery - Northern Finland.
j Truck unloading at service station without vapour recovery - Southern Finland.
were given. Air concentrations associated with transfer of neat MTBE
from tank cars to a storage tank ranged from 324 to 540 mg/m3 (90-150
ppm).
Hinton (1993) performed an occupational exposure study of MTBE
employees designed to determine the amount of MTBE exposure during
manufacturing, blending MTBE into gasoline, transportation,
distribution and handling at service stations. The study included 2038
exposure measurements during an 11-year period. Occasionally the TWA
exposure value exceeded 360 mg/m3 (100 ppm) and the short-term (less
than 30 min) exposure 1080 mg/m3 (300 ppm), generally during
non-routine or extraordinary tasks. The maximum short-term value
sampled for less than 30 min, 3780 mg/m3 (1050 ppm), was recorded
during transportation of neat MTBE. The maximum TWA level was 2563
mg/m3 (712 ppm) for the same activity. Usually, the TWA levels were
less than 7.2 mg/m3 (2 ppm) and the short-term levels were less than
36 mg/m3 (10 ppm). Exposures in blending operations were less than
360 mg/m3, and generally less than 36 mg/m3. In distribution, MTBE
levels were less than 3.6 mg/m3, and for service station attendants
levels were less than 10.8 mg/m3 (3 ppm).
5.2.2 Transportation
An exposure assessment for MTBE vapour concentrations conducted
on two gasoline truck drivers in New Jersey showed an average exposure
concentration of 0.29 mg/m3 (0.08 ppm) on a full shift (10-12 h)
basis. The short-term 15-min TWA exposure was 2.05 mg/m3 (0.57 ppm)
(Hebert, 1993).
Monitoring has been made for workplace concentration levels for
seven gasoline truck operators to assess exposure potentials during
loading operations and during full unloading at service stations
(Gillie, 1993). The 12-h TWA ranged from 0.86 to 3.31 mg/m3
(0.24-0.92 ppm). Short-term exposure (5-23 min) during truck loading
operations ranged from 0.18 to 0.58 mg/m3 (0.05-0.16 ppm) and during
fuel unloading from 3.24 to 12.96 mg/m3 (0.9-3.6 ppm).
The occupational exposure of road tanker drivers to gasoline and
some of its components, including MTBE, has been measured in Finland
in two depots and 11 service stations during loading and delivery
(Hakkola & Saarinen, 1996). In Finland, unleaded gasoline contains
10-15% MTBE in liquid phase. The monitoring was made during the
summer, and the temperatures ranged from 4 to 22°C. In the south of
Finland, four measurements were carried out during top loading and six
measurements during delivery at service stations. In the north of
Finland, six measurements were performed during bottom loading and
five measurements at service stations during delivery. The mean
short-term exposures of road tanker drivers to MTBE during loading and
delivery were between 13 and 91 mg/m3. The differences in exposure
during bottom (2.8-42.0 mg/m3, mean 13 mg/m3) and top loading
without vapour recovery (20-226 mg/m3, mean 91 mg/m3) were
statistically significant (p<0.02). There also was a statistically
significant difference (p<0.03) during delivery in northern (4.3-27.0
mg/m3, mean 16.0 mg/m3) and southern Finland (10-98 mg/m3, mean 71
mg/m3). The exposure time for loading was 25-35 min and for
delivering 30-40 min per load.
5.2.3 Service station attendants and garage mechanics
An exposure assessment for MTBE vapour concentrations conducted
on six full-service gas attendants in New Jersey showed an average
exposure of 1.76 mg/m3 (0.49 ppm) on an 8-h TWA basis (Hebert, 1993).
For the short-term 15-min TWA, the average exposure was 2.16 mg/m3
(0.60 ppm).
In an evaluation of exposure among service station attendants and
operators, Hartle (1993) compared the exposure potential at three
categories of service stations. Two facilities in Cincinnati, Ohio,
represented service stations that did not use MTBE or used it only as
an octane enhancer. In Phoenix, Arizona, two high-volume stations were
selected, and in Los Angeles two service stations with advanced vapour
recovery were selected. In Phoenix, where the MTBE content averaged
12.5-13% by liquid volume, the exposure measurements (41 samples)
ranged from 0.14 to 13.97 mg/m3 (0.04-3.88 ppm) with an average of
1.08 mg/m3 (0.3 ppm). The Los Angeles exposure ranged from 0.07 to
2.63 mg/m3 (0.02-0.73 ppm), averaging 0.50 mg/m3 (0.14 ppm). In
Cincinnati, only one of 32 samples was above the analytical limit of
detection, i.e. 0.58 mg/m3 (0.16 ppm).
Giacomello (1996) measured personal exposure of "full service"
attendants to MTBE in 58 Italian service stations. The study included
a number of geographical locations throughout the country and was
conducted in the summer and winter in 1992 and 1995. An overall
geometric mean of 0.71 mg/m3 (1992) or 0.26 mg/m3 (1995) was
recorded in the summer, and 0.37 mg/m3 in winter (latter data only
for 1992).
Three NIOSH studies were performed in workers potentially exposed
to gasoline and exhaust emissions during their work day (Almaguer,
1993; Buchta, 1993a,b). Breathing zone air samples were collected from
workers exposed to MTBE and other gasoline components (benzene,
toluene, and xylene, and, in one study, carbon monoxide) in several
maintenance facilities for motor vehicles located in Fairbanks, Alaska
(Buchta 1993a), in Stamford, Connecticut (Buchta, 1993b), and in
Albany, New York (Almaguer, 1993). In two of the cities (Fairbanks and
Albany), MTBE was only used as an octane enhancer (generally less than
1% of the fuel) during the study period. In Stamford, however, the
MTBE content of the fuel ranged from 13 to 17% with an average of
14.2% by volume. The highest workplace exposure level concentrations
were less than 0.50 mg/m3 (0.14 ppm) in Albany and less than 1.6
mg/m3 (0.45 ppm) in Fairbanks. In Stamford, the MTBE exposure levels
ranged from 0.1 to 44.6 mg/m3 (0.03 to 12.04 ppm). The cause for the
highest value was unknown, and the next highest exposure value was
7.56 mg/m3 (2.1 ppm). In all of the studies, the highest
concentrations were measured on mechanics. The sampling was, however,
conducted in late spring, and dilution ventilation (open windows and
doors) may have affected the results.
5.2.4 Occupational exposure limit values
In the USA, the American Conference of Governmental Industrial
Hygienists (ACGIH, 1994) has recommended a TWA of 144 mg/m3 (40 ppm).
In Sweden, the occupational air exposure TWA limit is 180 mg/m3 (50
ppm) and the 15-min short-term exposure limit (STEL) 250 mg/m3 (75
ppm) (AFS, 1994). The Dutch Expert Committee on Occupational Standards
recommended a health-based occupational 8-h TWA exposure limit of 180
mg/m3 (50 ppm) (DECOS, 1994).
5.3 Exposure via water
MTBE has been found in groundwater, storm water, reservoir water,
and drinking-water in the USA (Garrett et al., 1986; Angle, 1991; Dey
et al., 1991; Post, 1994; Squillace et al., 1995a,b, 1996; Delzer et
al., 1996; Dale et al., 1997; US Interagency Assessment, 1997).
Collectively, these references show that MTBE occurs in water,
especially in areas where MTBE is extensively used, and where releases
of MTBE to air, water and soil occur.
However, while there are some national monitoring data for
ambient groundwater, monitoring data for MTBE in surface water and in
drinking-water in the USA are very limited in scope. In an extensive
review of MTBE in water in the USA, Zogorski et al. (1996) concluded
that sufficient monitoring data were not available to characterize
human exposure to MTBE by the consumption of drinking-water.
The following subsections summarize major findings for MTBE in:
(a) snow and precipitation; (b) surface water; (c) groundwater; and
(d) drinking-water.
5.3.1 Snow and precipitation
MTBE has been detected in snow at ground level in Denver,
Colorado, USA, at very low (water equivalent) concentrations
(Squillace et al., 1995b; Bruce & McMahon, 1996). There is no other
published monitoring information on the presence or concentration of
MTBE in snow or rainfall. Squillace et al. (1996) hypothesized that
concentrations of MTBE in precipitation would be greater during winter
months than warmer summer months due to the temperature effect on
air-water partitioning.
5.3.2 Surface water
Information on MTBE in streams and rivers, in Long Island, New
York and New Jersey, USA, has been reported by Stackelberg et al.
(1997). For Long Island, at a reporting level of 0.5 µg/litre, MTBE
was the second most frequently detected VOC, occurring in 29% of the
samples at concentrations ranging from 0.6 to 20 µg/litre, with an
estimated median of 0.24 µg/litre. MTBE was detected more frequently
in samples collected during winter months (33%) than summer months
(26%). In New Jersey, a limited study was completed in spring 1994
along a ten-mile reach of the Hackensack River. Land use along this
reach is highly urbanized, and numerous industries and municipal
effluents are present. The study involved the collection of a single
water sample at each of 14 sampling points just after a major snow
melt. MTBE was detected in all samples at concentrations ranging from
2.6 to 30 µg/litre, with a median of 7.75 µg/litre (Stackelberg et
al., 1997). Reconnaissance sampling of eight streams elsewhere in New
Jersey in 1996 showed the presence of MTBE in water samples for seven
of eight sites. The concentrations ranged from 0.2 to 4.9 µg/litre
(Stackelberg et al., 1997). MTBE is used in reformulated gasoline at
both Long Island, New York and in New Jersey, as part of a mandatory
air abatement programme.
Measurable but low concentrations of MTBE were found in some of
592 stormwater samples (including samples from culverts, concrete
pipes, lined ditches and channels) collected by the US Geological
Survey in 16 cities and metropolitan areas from 1991 to 1995 (Delzer
et al., 1996). MTBE was found in 6.9% of the samples (41 of 592
samples). When detected, concentrations ranged from 0.2 to 8.7
µg/litre, with a median below 1.0 µg/litre. Eighty-three percent of
the detections occurred during the winter season (October to March)
when oxygenated gasoline to abate CO pollution was expected to be
used. A comparison of MTBE concentrations for samples collected during
the summer and winter periods showed a statistically significant
difference. Twenty-seven out of 148 stormwater samples contained both
MTBE and BTEX compounds, indicating a common source for these samples.
The Metropolitan Water District of Southern California (MWDSC)
relies, in part, on six lake-reservoirs for the storage of raw water
to be used for drinking-water in Southern California. These reservoirs
have varying degrees of recreational use. MWDSC began quarterly
monitoring of these reservoirs for MTBE in the second quarter of 1996.
MTBE was detected at the surface of Lake Perris, with confirmation in
two subsequent samplings, at an average concentration of 15 µg/litre.
The following quarter MTBE was also detected at an average level of 19
µg/litre. No MTBE was detected above the study's reporting level of 1
µg/litre in any of the other reservoirs, which were sampled at outlet
towers, where water is drawn from a lower depth (Dale et al., 1997).
5.3.3 Groundwater
Data from urban and agricultural areas show that MTBE occurs
predominantly in shallow groundwater underlying urban areas, and, when
present, occurs typically at low concentrations. In 1993-1994, the US
Geological Survey measured the concentrations of MTBE and 59 other
VOCs in 210 shallow wells (five drinking-water wells, 12 springs and
193 monitoring wells) in eight urban areas and 549 shallow groundwater
wells from 21 agricultural areas, and deeper groundwater from 412
wells sampled in nine areas throughout the USA (Squillace et al.,
1995a,b, 1996). MTBE occurred in 27% of the shallow urban wells and
springs. Detectable levels of MTBE were found in 86% of the wells in
industrial areas, 31% of the wells in commercial areas, 23% of the
wells in residential areas, and 23% of the wells in areas of mixed
urban land use, parks and recreation areas. MTBE was the second most
frequently detected compound after trichloromethane (chloroform). In
73% of the 210 shallow urban wells, concentrations were less than the
reporting level of 0.2 µg/litre. The estimated median value for urban
areas was below 0.2 µg/litre (Squillace et al., 1996). Three percent
of the wells had concentrations of MTBE above 20 µg/litre. No MTBE was
detected in drinking-water wells in the urban areas. In the
agricultural areas, 1.3% of the 549 shallow agricultural wells sampled
had detectable concentrations of MTBE. MTBE was also detected in four
of the 412 deeper groundwater samples from major aquifers. Three of
these wells were used for domestic or municipal water supply. The
measured maximum concentration of MTBE was 1.3 µg/litre. MTBE in
groundwater was generally not found with BTEX compounds, which
commonly are associated with point source spills of gasoline.
Bruce & McMahon (1996) measured MTBE concentrations in
groundwater in the alluvial aquifer beneath Denver, Colorado, USA, as
part of a survey of groundwater quality examining a range of dissolved
constituents. Thirty randomly selected alluvial wells were sampled.
MTBE was the most frequently detected VOC (23 out of 29 wells). The
maximum concentration was 23 mg/litre.
5.3.4 Drinking-water
Exposure to MTBE via drinking-water may involve more than direct
ingestion of contaminated water. Household uses of water, such as in
cooking, showering, bathing and washing, could result in exposure
through inhalation and dermal absorption, even if ingestion of water
was avoided.
Only limited monitoring data are available for MTBE in
drinking-water sampled at the tap or from a municipal distribution
system. Stern & Tardiff (1997) estimated that about 30% of the US
population lives in areas where MTBE is in regular use; 95% of this
population is unlikely to be exposed to MTBE in tap water at
concentrations exceeding 2 µg/litre, most will be exposed to much
lower or zero concentrations, but 5% could be exposed to higher
concentrations due to fuel tank spills and leaks entering surface and
groundwater. As part of the US Interagency Oxygenated Fuel Assessment
in the USA (US Interagency Assessment, 1997), information on MTBE
levels in drinking-water was sought from state drinking-water agencies
on a voluntary basis by the US Environmental Protection Agency.
Because monitoring for MTBE in drinking-water is not required by the
US Federal Government, only a few states have information on MTBE in
drinking-water. As such, it is not possible to describe levels of MTBE
in drinking-water for the entire USA. Based on information provided by
five states (New Jersey, Iowa, Illinois, Texas and Colorado), MTBE has
been detected in the drinking-water of 51 public water systems.
However, when detected, the concentration of MTBE was generally low
and nearly always below 20 µg/litre (Zogorski et al., 1996). These
data indicated that the consumption of drinking-water was not a major
route of exposure for these few systems (Zogorski et al., 1996). No
data on MTBE in drinking-water are available for other countries.
As noted previously, there have been a few instances in the USA
where groundwater used for drinking-water, both private wells and
public water systems, has become contaminated with levels of MTBE in
excess of 1000 µg/litre (Garrett et al., 1986; State of Connecticut,
1987; Zogorski et al., 1996). Many humans will probably detect MTBE in
drinking-water when the concentration exceeds about 50-100 µg/litre
owing to its low taste and odour threshold (see section 2.1 for taste
and odour values). Some humans will detect MTBE in water at even lower
concentrations. However, Du et al. (1998) considered that an MTBE
concentration below 40 µg/litre in drinking-water would avoid any
unpleasant taste or odour even for the most sensitive members of the
population.
5.4 Soil and sediment
There are very limited data concerning levels of MTBE in the
terrestrial environment. Trace amounts of MTBE have been found in
sediment samples adjacent to motorways and centres of heavy urban road
traffic density in the United Kingdom (Bianchi & Varney, 1989).
5.5 Biota
There are very limited data on MTBE levels in biota. In a study
to detect organic and inorganic contaminants in shellfish in Nova
Scotia, Canada, MTBE was not detected in any of the 21 samples assayed
(detection limit = 0.01 µg/g) (Environment Canada, 1989).
6. KINETICS AND METABOLISM IN HUMANS AND LABORATORY ANIMALS
Kinetic data from human and animal studies are summarized in
Table 14.
6.1 Human data
6.1.1 Controlled human studies
In an inhalation study two healthy young adult male and two
healthy young adult female volunteers were exposed to MTBE at 6 mg/m3
(1.7 ppm) in an environmental chamber for 1 h. The mean blood level
rose from 0.83 ± 0.5 µg/litre (0.009 µmol/litre) preexposure to 17.1 ±
2.0 µg/litre (0.19 µmol/litre) at the end of the 1 h exposure period.
One hour after the end of exposure the mean blood level fell to 6.3 ±
1.6 µg/litre (0.07 µmol/litre) after 1 h (Cain et al., 1996).
In a pharmacokinetics study, two volunteers (one healthy young
adult male and one healthy young adult female) were exposed to
5 mg/m3 (1.394 ppm) for 1 h in an environmental chamber (see also
section 8.2). There was a rapid rise in blood MTBE concentration to
6.1 µg/litre (8.2 ppb) and 10.9 µg/litre (14.7 ppb), respectively, at
1 h from the start of exposure. Following the end of exposure, there
was a rapid decline in blood MTBE concentration in both the male and
female volunteer with half-lives of 36 and 37 min, respectively. By
the end of the 7-h sampling period, blood MTBE concentration had
fallen to 0.149 µg/litre (0.2 ppb) in the male volunteer and 0.447
µg/litre (0.6 ppb) in the female volunteer (Prah et al., 1994).
In another study, the area under the curve (AUC) values of MTBE
and TBA were proportional to the MTBE exposure levels following short-
term inhalation to 18, 90 or 180 mg/m3 (5, 25 and 50 ppm), indicating
linear kinetics up to at least 180 mg/m3 (Johanson et al., 1995).
Following exposure to 180 mg MTBE/m3, the elimination of MTBE and TBA
was complete within 24 and 48 h, respectively (Johanson et al., 1995).
Pekari et al. (1996) measured concentrations of MTBE in blood,
urine and exhaled air from four volunteers exposed to 90 or 270 mg/m3
(25 or 75 ppm) by inhalation for 4 h. A lung retention of around 40%
was recorded, and blood levels of 11 µmol/litre (970 µg/ litre) at 90
mg/m3 or 29 µmol/litre (2556 µg/litre) at 270 mg/m3 were achieved
towards the end of the exposure period. Of the MTBE absorbed, the
majority (about 58%) was excreted unchanged in expired air and small
amounts (1.4%) unchanged in urine. The concentration of TBA in blood
reached a peak of 16 or 34 µmol/litre (1419 or 2997 µg/litre)
(following the low or high exposure, respectively) 15-45 min after
exposure ceased. Trace amounts of TBA (1.2%) were found in urine, but
none was detected in exhaled air. The terminal half-life for MTBE in
blood was determined to be 5 h, while that for TBA was 11.9 h. The
authors concluded that metabolism of MTBE was linear at exposures up
to 268 mg/m3 (75 ppm).
Table 14. Summary of kinetic data for MTBE
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
A. Human
2 male, 2 female rapid absorption mean MTBE blood Cain et
volunteers from 0.003 concentrations rose al. (1996)
Treatment: 6 mg/m3 µg/litre preexposure steeply from 0.83
(1.7 ppm) for 1 h to 0.06 µg/litre ±0.50 µg/litre
in inhalation after 1 h preexposure to a
chamber peak blood level of
17.1±2.01 µg/litre
by the end of
exposure; rapid
elimination, half-life
of 40 min; the rapid
elimination phase
appeared to last
approximately 1 h
1 male, 1 female rapid rise in blood rapid decline observed Prah et
volunteer MTBE to 0.03 µg/litre in blood levels with al. (1994)
Treatment: 5 mg/m3 (8.2 ppb) in the male a half-life of about
(1.39 ppm) as a 1-h and 0.05 mg/m3 35 min and rapid
exposure; blood (14.7 ppb) in the metabolic transformation
samples up to 580 female to TBA; TBA levels
min after start gradually increased and
plateaued at 0.025-0.36
µg/litre (7-10 ppb) and
maintained this
concentration up to 7 h
post-exposure
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult relative MTBE detected in clearance of MTBE Johanson et
male volunteers respiratory uptake expired air; both was 0.5 litre/h per al. (1995)
Treatment: exposed was 32-42%; rapid MTBE and TBA found kg; AUC values of
in chamber to 18, absorption based in blood and urine MTBE and TBA were
90 and 180 mg/m3 upon blood proportional to
(5, 25 and 50 ppm) concentration; by exposure levels,
for 2 h on three 2 h, peak blood suggesting linear
occasions during concentrations of kinetics; elimination
light physical MTBE were 1.3, of MTBE in blood
exercise (50W); 6.3, and 12.2 indicated three
observed up to µmol/litre at 18, phases (6-7 min,
24 h and 48 h 90, and 180 mg/m3, 46-58 min and
(only 180 mg/m3) respectively; 6.2-7.2 h); in
post-exposure TBA 5.7 and 0.7 urine half-lives
µmol/litre at 90 of 16-22 min and
and 180 mg/m3, 3.0-3.1 h were
respectively; steady identified;
state was reached half-life of TBA
for TBA after 3-5 h, in urine was
but was not reached 7.5-8.9 h; by 3.5 h
during the 2 h post-dose, 20-30%
exposure to MTBE of absorbed dose
was eliminated in
expired air; by
24 h, approx. 0.1%
MTBE and 0.5-0.8%
TBA of absorbed
dose was eliminated
in urine
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult respiratory uptake mean concentrations TBA was found in respiratory Nihlén et
male volunteers was 42-49% in blood at the blood and urine exhalation was al. (1998a)
Treatment: exposed three exposure 32-47%; elimination
in chamber to 18, concentrations were in blood was in
90 and 180 mg/m3 1.4, 6.5, and 13 four phases of 1
(5, 25 and 50 ppm) µmol/litre min, 10 min, 1.5 h,
for 2 h on three respectively and and 19 h; kinetics
occasions during were concentration- were linear up to
light physical related the exposure
exercise (50W); concentration of
blood and urine 180 mg/h;
collected during elimination in
exposure and for 3 urine was biphasic
days post-exposure with mean half-lives
of 20 min and 3 h;
excretion was nearly
complete 10 h after
exposure; metabolic
clearance was
0.34-0.52 litre/h
per kg; renal
clearance of TBA
was 0.6-0.7 ml/h
per kg and TBA was
still present after
22 h
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
B. Rodent
I) Oral administration
Species: rapid and extensive apparent volume of in plasma, the dose-related Ferdinandi
Fischer-344 rats absorption of MTBE distribution was major metabolite, differences were et al. (1990a)
Treatment: 40 based upon peak 0.27 to 0.43 litre TBA, peaked at 2 h; observed for the Miller et
rats/sex/dose group plasma concentration male rats had higher plasma elimination al. (1997)
received 40 or 400 within 15 min of plasma concentrations half-time (T´) of
mg/kg as single dosing; lower plasma than female rats; MTBE and TBA:
dose; observed concentrations of dose-unrelated plasma T´ of MTBE:
(sacrificed) for MTBE in female rats concentrations of 0.52-0.62 h (low
various time-points compared to male MTBE and TBA indicate dose) 0.74-0.88 h
until 36 h rats; higher AUC enzyme saturation (high dose) T´ of
post-dose values of MTBE and TBA: 0.95-1.0 h
TBA with oral dosing (low dose)
compared to 1.6-1.9 h (high
intravenous dose) Plasma
administration clearance (CL) of
MTBE:
male rats:
0.36-0.41 litre/h
(both dose-groups)
female rats:
0.48 litre/h (low
dose) 0.29 litre/h
(high-dose)
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: by 48 h, 1.7-3% of higher radioactivity slight sex difference Ferdinandi
Fischer-344 rats administered was recovered in in the route of et al. (1990b)
Treatment: 6 radioactivity was lungs at high oral elimination; by 48 h, Miller et
rats/sex/dose group recovered in dose and lower recovery was 46-54% al. (1997)
received 40 or 400 carcass and tissues; activity in kidneys at low dose and
mg/kg 14C-MTBE as 86 and 81% of suggesting enzyme 65-69% at high dose
a single dose; administered saturation; most of (highest in females);
observed for 48 h radioactivity was the administered 29-36% was recovered
recovered in lungs dose was exhaled as in urine at low dose
and kidneys at low unchanged MTBE and 11-16% at high
and high dose, (predominating) and dose (highest in males)
respectively TBA (2.5-3.1% at low less than 1% was
dose and 1.3-1.4% at recovered in faeces
high dose); in urine,
the major radiolabelled
species (from further
oxidation of TBA) were
2-methyl-1,2-propanediol,
alpha-hydroxyisobutyric
acid, and two minor
unidentified components;
no sex differences in
biotransformation
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
ii) Inhalation
Species: Wistar dose-related MTBE was metabolized Savolainen
rats concentrations of at the ether bond. et al. (1985)
Treatment: 5 male MTBE and TBA in Two weeks of exposure
rats/dose group blood after 2 weeks caused transient
were exposed to of exposure; the increase of the
180, 360 or 1080 concentration of microsomal UDP
mg/m3 (50, 100 or MTBE decreased glucuronosyl-transferase
300 ppm) vapour in after 6 weeks; activities in liver and
exposure chambers the concentrations kidney at all dose
6 h/day, 5 days/week of TBA increased levels. After an initial
for 2, 6, 10 or 15 after 6 weeks of decrease the muscle
weeks exposure and began creatinine kinase
to decrease after activity gradually
10 weeks. increased towards the
Brain levels of MTBE end of the exposure.
and TBA followed a A minor induction of
similar course. MTBE kidney cytochrome
was also found in P-450 was noted. Almost
perirenal fat and at no effect was found on
higher concentrations hepatic cytochrome
than in blood or brain. P-450 concentrations,
Concentrations of MTBE brain succinate
in the blood, brain dehydrogenase, creatine
and perirenal fat were kinase, or
directly related to the acetylcholinesterase
concentrations in the activities
inspired air. The
ratios of TBA/MTBE in
blood increased from
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
week 2 to week 15,
indicating that MTBE
had been oxidized to
TBA, which was
eliminated from the
blood at a slower rate
than MTBE
Species: (T1) rapid absorption (T1) apparent volume (T1) increased AUC Ferdinandi
Fischer-344 rats based upon plasma of distribution: values of MTBE et al.
Treatment: concentration; peak low dose: 35-fold (males) and (1990c)
blood concentration 0.40 (females) and 37-fold (females) Miller et
(T1) 52 rats/sex/ was reached within 0.52 litre (males) while the AUC al. (1997)
dose group received 4-6 h for MTBE and high dose: values of TBA
1440 or 28 800 6.5 h for TBA; low 0.25 (males) and increased by 15-fold
mg/m3 (400 or 8000 but statistically 0.24 litre (females) (males) and 7-fold
ppm) as a single significant (females); quotients
6-h dose. difference in sexes of repeated and
of MTBE AUC values single dose AUC
(T2) 40 rats/sex (low dose); TBA plasma values (T2/T1) for
were exposed 6 h concentration (AUC) MTBE were 0.64
daily to 1440 mg/m3 was lower in female (males) and 0.53
(400 ppm) for 15 rats compared to male (females) and for
days; observed rats, statistically TBA 1.1 (males) and
(sacrificed) at significant at high 1.3 (females); a
various time-points dose significant
until 12 h (T1) and difference in plasma
18 h (T2) post-dose clearance between
single doses 0.53
(males) and 0.57
(females) litre/h
(low dose) and 0.30
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
(males) and 0.32
(females) litre/h
(high dose); the
plasma half-life of
MTBE was 0.52-0.63 h
(T1) and 0.48-0.51 h
(T2); the (T1)
half-lives of TBA
were 2.8 (males) and
3.4 h (females) and
(T2) 1.8 h (males)
and 1.5 h (females)
Species: (T1&T2): rapid and by 48 h, 11-13% (T1&T2): radiolabelled rapid elimination Ferdinandi
Fischer-344 rats extensive absorption (low dose; T1&T2) species in expired air in urine after low et al.(1990d)
Treatment: as indicated by and 4-5% (high dose) were MTBE and TBA; by dose and in expired Miller et
(T1) 6 rats/sex/ marked recovery of of administered 3 h post-dose, 30% air at high dose, al. (1997)
dose group received 14C in urine by 24 h radioactivity (low dose) and 7-10% suggesting enzyme
1440 or 28 800 recovered in skin (high dose) of saturation at high
mg/m3 (400 and of some rats, recovered radioactivity dose; by 48 h, total
8000 ppm) of probably due to were correlated to TBA, recovery of
14C-MTBE as a contamination from while TBA was major radioactivity (14C)
single 6-h urine (lower radioactive component in urine 65-71% (low
exposure radioactivity at 24 h post-dose dose, T1&T2) and
recovered in urine); (>90%, low dose); 35-42% (high dose);
(T2) 40 rats/sex 0.6-1% in skin of major radiolabelled in expired air 17-22%
were pretreated remaining rats, 1-3% species in urine from (low dose) and 54-59%
with 1440 mg/m3 in carcass, and <1% further oxidation of (high dose) and <1%
unlabelled MTBE in tissues TBA were 2-methyl-1, in faeces; no sex
6 h a day for 14 2-propanediol, alpha- difference in rate
days, followed by hydroxyisobutyric acid, and route of
6 h exposure for two minor unidentified radioactivity
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
1440 mg/m3 14C-MTBE components, and minor elimination
on day 15; observed detection of CO2 (1%
for 48 h post-dose of the dose); no sex
differences in
biotransformation
iii) Intravenous administration
Species: a statistically apparent volume of by 2 h, the major half-life of MTBE Ferdinandi
Fischer-344 rats significant distribution 0.27 metabolite TBA was (plasma) 0.45-0.62 h; et al.(1990a)
Treatment: 40 rats/ difference in plasma to 0.31 litre found in blood at half-life of TBA Miller et
sex/group received concentrations (AUC) peak concentration; (plasma) 0.92-1.3 h; al. (1997)
40 mg/kg as a between sexes (lower male rats had higher clearance from plasma
single dose; in females) blood concentration of MTBE 0.36-0.41
observed than female rats litre/h (males) and
(sacrificed) for 0.47 litre/h (females)
various time-points
until 36 h
post-dose
Species: rat, 1.7-3% of administered by 6 h, major by 48 h, 71-73% of Ferdinandi
Fischer-344 radioactivity was radiolabelled species administered et al.(1990b)
Treatment: 6 rats/ recovered in tissues in expired air was radioactivity was Miller et
sex/group received and carcass for each MTBE, with a minor totally recovered: in al. (1997)
40 or 400 mg/kg dose after 48 h elimination of TBA exhaled air (42-46%),
14C-MTBE as a (2.5-3.1% of in urine (26%), and
single dose; administered dose); faeces (<1%); rapid
observed for 48 h the major radiolabelled elimination in lungs,
species in urine (from 94% within 3 h
further oxidation of
TBA) were 2-methyl-1,
2-propanediol, alpha-
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
hydroxyisobutyric acid,
and two minor
unidentified components;
there were no sex
differences in
biotransformation
iv) Intraperitoneal
Species: Charles rapid and extensive the total cumulative methanol and formic 14C activity was mainly Biodynamics
River CD rats absorption based upon 14C activity in tissues acid were found in eliminated in expired (1984)
Treatment: 33 peak blood and plasma averaged 3.39, 1.94 and plasma, liver and air; by 48 h, recovery
animals/sex/group concentrations at 5 1.14% of administered kidney was about 99.86% (approx.
received a single min post-treatment, dose at 15 min, 6 h, 92% as MTBE and approx.
dose of averaging 92.04 ± and 24 h post-treatment, 7.45% as CO2); about 3%
approximately 37.72 and 83.40 ± respectively. At 15 min, in urine and about 0.8%
60µCi 14C-MTBE 20.48 µg 14C-MTBE the majority of the in faeces (male rats);
(about 232 mg equivalents/ml blood 14C-radioactivity was approx. 3% was excreted
MTBE/kg bw) in for male and female found in mesenteric fat, in urine and 0.8% (males)
saline and were rats, respectively. liver and kidney; and 1.25% (females) in
sacrificed at The half-life of however, at 6 h and 24 h faeces. The 14C activity
intervals of 5, 14C-MTBE in blood was no 14C was found in in urine and faeces was
15, 30, and 45 59.8 min for male rats mesenteric fat mainly associated with
min, and 1, 2, 3, and 49 min for female 14C-formic acid (a total
6, 12, 24 and 48 h rats. The half-life of 3.08% of administered
post-treatment in plasma was 2.3 h dose)
for males and 1.3 h
for females
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: mouse, most of the Yoshikawa
ddY administered MTBE et al.(1994)
Treatment: 4 male was eliminated
mice/dose group unchanged in the
were administered exhaled air; >90%
a single dose of of this amount was
50, 100 or 500 eliminated within
mg/kg in corn oil 3 h. The pulmonary
solution; observed elimination showed
for 6 h an initial rapid
decrease of the
elimination ratio
followed by a slow
decrease at 100 and
500 mg/kg. The
calculated half-lives
were 45 and 80 min,
respectively. The
elimination ratios
at the three different
doses were 23.2, 37.6
and 69.0%, respectively
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
C. In vitro
Incubation of 5 or incubation of MTBE with Brady et
14 mM MTBE with liver microsomes from al. (1990)
liver microsomes phenobarbital-pretreated
from phenobarbital- rats resulted in TBA and
or acetone- formaldehyde in
pretreated or equimolar amounts. The
untreated Vmaxa value for
Sprague-Dawley rats demethylation of MTBE
(3-5 males) increased by 4-fold with
acetone-induced and by
5.5-fold with
phenobarbital-induced
microsomes compared with
the control Vmax value.
These results indicate
that cytochrome P450 2B1
(inducible by
phenobarbital) and
cytochrome P450 2E1
(inducible by acetone)
play a role in the
demethylation of MTBE.
Results after inclusion
of monoclonal antibody
against P450 2E1
indicated this enzyme
only partially
contributed to the
demethylation. Microsomes
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
pretreated with MTBE
yielded a 47-fold
induction of
pentoxyresorufin
o-dealkylase, with no
change in
N-nitrosodimethylamine
demethylase activity.
These results are
consistent with an
elevation of P450 2B1
a Vmax : the maximum velocity of the demethylation process.
Nihlén et al. (1998a) studied the uptake, distribution,
metabolism and elimination of MTBE in ten healthy male volunteers. The
subjects were exposed on three different occasions for periods of 2 h
in a chamber to MTBE concentrations of 18, 90 and 180 mg/m3 (5, 25
and 50 ppm) while performing light physical exercise. MTBE (and its
metabolite TBA) were monitored in exhaled air, blood and urine, the
latter being collected up to 3 days after exposure. Respiratory uptake
of MTBE was low (42-49%) and respiratory clearance was high (32-47%).
The metabolic blood clearance was 0.34-0.52 litre/h per kg. The
kinetics of MTBE were linear up to the highest exposure concentration
of 180 mg/m3 (50 ppm). The kinetic profile of MTBE in blood was
described as having four phases, with average half-lives of 1 min, 10
min, 1.5 h and 19 h. In urine the post-exposure decay curve of MTBE
had two linear phases with average half-lives of 20 min and 3 h. The
urinary excretion of MTBE was less than 1% of the absorbed dose.
Biomarkers and partitioning of inhaled MTBE were studied in two
(1 male, 1 female) volunteer subjects exposed for 1 h to a nominal
concentration of 5 µg/m3 (1.39 ppm), followed by clean air exposure
for 7 h (Buckley et al., 1997). MTBE concentrations in expired air,
venous blood and urine were monitored during and after exposure. The
decay of MTBE was assessed by using a 2- or 3-exponential model and
yielded residence times of 2-3 min, 15-50 min, and 3-13 h in alveolar
air, and 5 min, 1 h and 32 h in venous blood. Based on
lower-than-expected blood and expired air MTBE concentrations during
uptake and the decreasing blood-breath ratio during the post-exposure
decay period, the authors hypothesized that the respiratory mucous
membranes acted as a reservoir for MTBE, retaining 6-9% of the MTBE
intake. Compartmental monitoring was used to estimate a blood-breath
partition coefficient of approximately 18. The urinary concentration
of MTBE ranged from 0.37 to 15 µg/litre and bore little relationship
to the exposure: urinary elimination accounted for only a small
fraction (<1%) of total MTBE elimination.
6.1.2 Human exposure to oxygenated gasoline
During an oxyfuels programme in Fairbanks, Alaska, there was a
strong correlation between the workplace air levels ranging from 0.02
to 2.92 mg MTBE/m3 and the difference in blood concentrations of MTBE
between pre-shift and post-shift blood measurements (p=0.0001). The
median pre-shift concentration of MTBE in the blood of 18
occupationally exposed workers was 1.15 µg/litre (range 0.1- 27.8
µg/litre). The median post-shift blood MTBE level was 1.8 µg/litre
(range 0.2-37.0 µg/litre) (Moolenar et al., 1994).
Breath samples were collected from a person pumping gasoline
(containing 15% MTBE by volume) and a nearby observer (within 1 m),
immediately prior to and an hour after refuelling (Lindstrom & Pleil,
1996). The MTBE concentration in the sample collected during
refuelling was 412 µg/m3. The ambient background level was
approximately 25 µg/m3 MTBE. Low concentrations of MTBE (7-10 µg/m3)
were detected in the exhaled breath before the refuelling that took
2 min and 8 seconds; 40 seconds after the exposure the concentrations
had increased by factors of 35 for the observer and 100 for the person
pumping gasoline (see also section 5).
6.2 Animal studies
Repeated exposure (2 to 15 weeks) to MTBE vapour by inhalation (6
h/day, 5 day/week) resulted in dose-dependent increases in MTBE levels
in blood, brain and perirenal fat of Wistar male rats (Savolainen et
al., 1985). Very small differences were observed in blood MTBE levels
following repeated exposure. TBA levels in blood increased with
repeated MTBE exposure when comparing levels at 6, 10 and 15 weeks to
levels following 2 weeks. Perirenal fat/blood MTBE concentration
ratios ranged from 9.1 to 11.6 after 15 weeks of intermittent
exposure. Blood and brain concentrations of TBA, the major main
metabolite, were also dose-dependent. TBA was, however, not found in
quantifiable amounts in the perirenal fat.
A series of studies on the kinetics of MTBE was conducted by
Bio-Research Laboratories (Ferdinandi et al., 1990a-d). These data
have been further published by Miller et al. (1997).
Miller et al. (1997) and Ferdinandi et al. (1990a-d) described
the pharmacokinetics and disposition of MTBE in male and female
Fischer-344 rats following i.v. (40 mg/kg), oral (40 and 400 mg/kg)
and dermal (40 and 400 mg/kg in occluded chambers) administration and
(nose-only) inhalation exposure for 6 h either for a single exposure
or repeated exposure (15 days). The details of these studies are
presented in Table 15. Miller et al. (1997) found that the elimination
of radiolabelled MTBE in rats was rapid and mainly occurred through
lungs and kidneys irrespective of administration route. The
elimination was virtually complete 48 h post-dosing. The renal
elimination was, however, slowest after dermal exposure. Twelve hours
post-dosing, the radiolabelled recovery in urine was 14-26% of the
dermal dose, 41-50% of the oral dose and 25-37% of the intravenously
injected dose. The recovery was 75-94% 36 h post-dosing, irrespective
of dose and administration route. A minor difference in excretion
route was observed between the sexes. Collectively these studies
demonstrated that MTBE is rapidly eliminated from blood (half-life =
0.5 h) by exhalation and metabolism to TBA (see Table 15). The major
metabolites recovered in urine were 2-methyl-1,2-propanediol and
alpha-hydroxyisobutyric acid. Dose-related differences in the AUC for
plasma concentrations of MTBE and TBA were observed in rats exposed to
1440 or 28 800 mg MTBE/m3 (400 or 8000 ppm) by inhalation (Ferdinandi
et al., 1990a-d; Miller et al., 1997). Miller et al. (1997) further
showed that increasing doses of MTBE (by inhalation and, to a lesser
extent, oral administration) to Fischer-344 rats decreased the
recoveries of radioactivity in urine and increased the recovery in
expired air. This indicates a saturation of the oxidative metabolic
pathway of MTBE at inhalation levels above 28 800 mg/m3 (8000 ppm) or
at oral administration levels above 400 mg/kg. There were no
significant sex- or route-dependent differences in the
pharmacokinetics and disposition of MTBE.
Table 15. Pharmacokinetic parameters of MTBE and TBA calculated from mean plasma concentrations of male
F-344 rats exposed to MTBE (Miller et al., 1997)
Exposure route Dose MTBE TBA
AUC Half-life CL V/F or Vssd AUCzero to infinity Half-life
(µg/h per ml) (h) (ml/h) (litre) (µg/h per ml) (h)
Intravenous 40 mg kg 10.7 0.45 413 0.27 26.7 0.92
Oral 40 mg kg 17.0 0.52 392 0.29 39.0 0.95
400 mg kg 230 0.79 358 0.41 304 1.6
Dermal 40 mg kg 7.9 2.3a 389 3.9 26.3 2.1a
400 mg kg 46.9 1.8a 364 1.4 93.9 1.9a
Inhalation low single 84.3 0.52 531 0.40 404 3.3
high single 2960 0.57 299 0.25 6010 3.4
low repeated 6.7b 0.51 c c 127b 1.8
a Calculated from the alpha-phase of a two-compartment model. Half-lives of MTBE from 12 to 45 h post-dose curve at
low and high doses were 92 and 37 h, respectively. Half-lives of TBA from 12 to 45 h post-dose were 170 (low dose)
and 31 h (high dose).
b AUCzero to infinity on 15th day of exposure.
c Values were not calculated because plasma was not collected during exposure.
d Vss = apparent volume of distribution at steady state after repeated inhalation.
Following intraperitoneal administration of 14C-MTBE to rats,
the highest radioactivity was recovered in the expired air
(Biodynamics, 1984). The radioactivity was also distributed throughout
the animal tissues. The amount retained in tissues was less than 2% of
the total dose of MTBE intraperitoneally administered to rats at 6 and
24 h post-treatment. Distribution of the radioactivity was primarily
to the liver and secondarily to the kidney. Approximately 92% of MTBE
was exhaled as the parent compound and approximately 7.5% as CO2 in
48 h. Another 3% was excreted in urine and up to 0.8% in faeces. The
14C-activity in urine and faeces was mainly associated with
14C-formic acid. Peak blood levels were observed in male and female
rats 5 min following intraperitoneal dosing of 14C-MTBE. Peak levels
of 14C activity in the plasma occurred at 5 min post-treatment in
male rats and at 15 min post-treatment in females rats. The
radioactivity decreased sharply during 1 h and thereafter gradually
during the study period (48 h). The half-time of radiolabelled MTBE in
whole blood was 59.8 min for male rats and 49 min for female rats. The
half-life of MTBE in plasma was 2.3 h for male rats and 1.3 h for
female rats (Biodynamics, 1984).
In a study of MTBE distribution in male and female rats following
inhalation exposure to 10 710 mg/m3 (3000 ppm) for 6 h, it was
reported that MTBE levels in the liver and brain were comparable in
males and females but were higher in male kidneys than in those of
females: MTBE was still detectable in male kidneys 18 h after exposure
(Borghoff et al., 1998). The authors considered that this may have
been due to the interaction of MTBE with alpha2u-globulin in the male
rat kidney.
6.3 In vitro studies
In vitro measurement of liquid/air partition coefficients of
MTBE at 37°C, using a vial equilibration technique, showed a human
blood/ air partition coefficient of 17.7 (confidence limits:
17.0-18.4), water/ air 15.2 (CL: 14.9-15.5), and oil/air 120 (CL:
114-125) (Nihlén et al., 1995). There was no significant difference in
partition coefficient for blood/air between the sexes. Liquid/air
partition coefficients for MTBE between different media and air were
also determined by Imbriani et al. (1997). The values were: blood/air
= 20.0; urine/air = 15.6; saline/air =15.3; fat/air = 142.0; and olive
oil/air = 138.0.
MTBE partition coefficients measured in rat tissues demonstrated
a higher solubility of MTBE in fat (115.6) compared to blood (11.5)
and other tissues such as liver (14.5) (Borghoff et al., 1996). The
partition coefficient of MTBE in the male rat kidney was approximately
five times higher than the value measured in female rat kidneys. This
high uptake of MTBE into the male rat kidney was found to be due to an
interaction with the male rat specific protein alpha2u-globulin
(Borghoff et al., 1995; Poet & Borghoff, 1997).
In rats, MTBE is demethylated by hepatic microsomal enzymes to
form TBA and formaldehyde (FA) (Savolainen et al., 1985; Brady et al.,
1990). When using rat liver microsomes, the Vmax for demethylation of
MTBE to FA increased after pretreatment with acetone or phenobarbital
(Savolainen et al., 1985; Brady et al., 1990). The results of Brady et
al. (1990) indicated that both cytochromes P450 2BI and P450 2E1 are
implicated in the metabolism of MTBE. In vitro, TBA has been shown
to be oxidatively demethylated using rat liver microsomes to yield FA
(Cederbaum & Cohen, 1980).
Hong et al. (1997) demonstrated that human liver microsomes
metabolize MTBE to TBA. The activity of 125 ± 11 pmol TBA/min per mg
protein was approximately 50% of the activity in rat and mouse liver
microsomes. The metabolism of MTBE to TBA in human liver microsomes
was NADPH-dependent and was inhibited by carbon monoxide, an inhibitor
of cytochrome P450 (CYP) enzymes, suggesting that CYP enzymes play a
critical role in human metabolism of MTBE. Human CYP2A6 and CYP2E1
cDNAs were each co-expressed with human cytochrome P450 reductase by a
baculovirus expression system and the expressed enzymes used to
metabolize MTBE. CYP2A6 was more active than CYP2E1 (activity 6.1 and
0.7 nmol TBA/min per nmol P450, respectively).
The role of the cytochrome P450 enzyme CYP2E1 in metabolizing
MTBE (and other gasoline ethers) was examined in 2E1 knock-out mice,
which lack demethylation capability. Liver microsomes metabolized MTBE
with an activity level of 0.67 ± 0.1 nmol/min per mg. However, there
were no significant differences in activity levels in microsome
preparations from two 2E1+/+ strains of mice, demonstrating that
CYP2E1 is not important in the metabolism of MTBE in mouse livers
(Hong et al., 1998).
The probable metabolic pathway is presented in Fig. 2.
6.4 Physiologically based pharmacokinetic modelling
A physiologically based pharmacokinetic model (PBPK) to describe
the dosimetry of MTBE in rats has been developed (Borghoff et al.,
1996). Using this PBPK model, MTBE blood levels following different
routes of exposure and various exposure concentrations were predicted.
When human anatomical parameters are used in this model and the
metabolism is scaled allometrically, the model predicts the level of
MTBE in blood (concentrations ranging from 0.03 to 17.1 µg/litre)
during and following exposure of people to 6 mg MTBE/m3 (1.7 ppm)
(Borghoff et al., 1996; Cain et al., 1996). The human PBPK model was
further expanded to include brain as a target tissue and an exposure
model for bathing and showering. Model simulations of a bathing and
showering MTBE exposure scenario in humans at water levels from 0.64
to 1.0 mg/litre and air levels from 5 to 5.7 mg/m3 (1.4 to 1.6 ppm)
predicted maximum brain levels to range from 0.015 to 0.02 mg
MTBE/litre and 0.006 to 0.019 mg TBA/litre (Rao & Ginsberg, 1997).
Based on physicochemical properties, it can be predicted that a
release of MTBE into water would result in significant amounts being
dissolved. Most of the MTBE remains in the surface water, with some
partitioning into air and much smaller amounts into sediment and soil
(Mackay et al., 1993). The low Kow of 0.94 suggests that partitioning
from the water to particulates and sediment is not significant. On the
basis of bioconcentration data, MTBE is not subject to bioaccumulation
or biomagnification in aquatic organisms (Environment Canada, 1993).
In the water compartment, the key removal process should be
volatilization. The amount transferred to sediment is negligible
(Mackay et al., 1993; Environment Canada, 1993).
For a gasoline containing 10% MTBE by weight, and assuming that
it does not undergo depletion of the MTBE concentration in the
gasoline due to dissolution into the water, the water solubility of
the MTBE from gasoline will be approximately 5 gm/litre at 25°C. By
comparison, the total hydrocarbon solubility for non-oxygenated fuel
is about 120 mg/litre (Poulsen et al., 1992; Zogorski et al., 1996).
The ability of MTBE to enhance the solubility in water of
monocyclic aromatic gasoline components including BTEX compounds has
been examined in models, and an increase was predicted only at
co-solvent concentrations of greater than 1% (Mihelcic, 1990). In
confirmation of this, the co-solvent effect of MTBE on the aqueous
solubility of hydrocarbons in gasoline was found to be minimal (Cline
et al., 1991). Measurements made in the laboratory in shake-flasks
showed that up to 15% MTBE was unlikely to result in enhanced
concentrations of BTEX in contaminated groundwater (Poulsen et al.,
1992). Such high concentrations of MTBE seem unlikely to be achieved
in groundwater after spillage of gasoline containing MTBE, and
although MTBE is widely distributed in shallow urban groundwater at
low concentrations in the USA, its occurrence in these samples was not
associated with correspondingly increased concentrations of BTEX
(Squillace et al., 1996).
4.1.3 Soil
Based on its physicochemical properties, it can be predicted that
when MTBE is released to the soil, it can be transported to the air
through volatilization, to surface water through run-off, and to
groundwater as a result of leaching. In the first two instances, the
release would have to be at, or near the soil surface. If the release
of MTBE occurs below the soil surface, for example from an underground
storage tank, then the most likely transport mechanism will be
leaching to groundwater. Based on its vapour pressure, volatilization
of MTBE from soil and other surfaces is expected to be significant.
Soil adsorption and mobility are based on the reported and estimated
Koc (organic carbon sorption coefficient) values. Compounds with a
Koc of <100 are considered to be moderately mobile. Thus MTBE, with
a Koc of 91, does not adsorb to soil particles to a great degree and
would be considered mobile. Parameters other than Koc affecting the
leaching of MTBE to groundwater include the soil type (e.g., sandy
versus clay), the amount and frequency of rainfall, the depth of
groundwater, and the extent of degradation of the MTBE (Environment
Canada, 1993).
4.1.4 Multimedia
Several multimedia models using various emission rates and
environmental parameters have been used to predict the distribution
and concentration of MTBE in the environment (Environment Canada,
1993; Mackay et al., 1993; Hsieh & Ouimette, 1994).
4.2 Bioconcentration
Fujiwara et al. (1984) conducted studies on the bioconcentration
of MTBE in Japanese carp (Cyprinus carpio) in a flow-through system
at 25°C. The mean whole-body steady-state bioconcentration factor
(BCF) was 1.5. Further observations indicated that fish exposed for 28
days and then transferred to clean water eliminated almost all MTBE
residues within 3 days. These experimental data support the hypothesis
that MTBE has little tendency to bioaccumulate. Veith & Kosian (1983)
calculated a BCF of 2.74 (r2 = 0.927) for a 28-day exposure of
fathead minnows, based on a Quantitative Structure-Activity
Relationship (QSAR).
Compounds with log Kow values of approximately 5.0 or less do
not have significant food chain build-up. MTBE belongs to this group
(Environment Canada, 1993). Uptake from water is more important than
from food for this group of compounds.
When 14C-labelled MTBE was applied to the soil in a closed
aerated system, the concentrations of MTBE in the roots and the aerial
parts of lettuce and radish showed that transport was dominated by
foliar uptake; subsequently, translocation into the roots took place
(Schroll et al., 1994). Although neither MTBE nor its potential
metabolite TBA was detected in the plants, a considerable fraction of
the 14C label was unaccounted for and was presumed to be associated
with plant constituents.
4.3 Biodegradation and transformation
Only a limited amount of work has been accomplished on the
biodegradability of MTBE. Moreover, the studies are difficult to
compare because they have been performed under a wide variety of
conditions. Aerobic and anaerobic experiments have been conducted. For
most studies, it has been demonstrated that MTBE is difficult to
biodegrade. In contrast, BTEX is more readily biodegraded (Zogorski et
al., 1996). Half-lives for MTBE in various environmental compartments
are shown in Table 3
Table 3. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Air 20.7-265 Based upon measured Howard et al., 1991
photo-oxidation half-life
10-30 Mackay et al., 1993
Soil 672-4320 Estimation based upon US EPA, 1989
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Surface water 672-4320 Estimation based upon Howard et al., 1991
aerobic biodegradation
300-1000 half-life Mackay et al., 1993
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
2688-17 289 Estimation based on Howard et al., 1991
anaerobic degradation
half-life
4.3.1 Aerobic conditions
Results from tests involving biodegradation of MTBE have been
variable.
Pence (1987a) used an acclimated culture containing active
sludge, soil inoculum and raw sewage. The uptake of oxygen was
measured in a mineral medium supplemented with MTBE added to the
acclimated culture at a concentration of 5 mg/litre on days 0, 7 and
11. The results showed that MTBE was poorly biodegradable under these
conditions; only 5.4% biodegradation occurred within 28 days.
No biodegradation of MTBE after 60 days was found in experiments
using aquifer soil material as inoculum; with two types of activated
sludge as inoculum, no degradation of MTBE occurred after 40 days
(Möller Jensen & Arvin, 1990).
With a standard activated sludge, and based on the oxygen uptake
rate, MTBE was biodegraded very slowly (Fujiwara et al., 1984). The
hydrocarbon components of gasoline blended with MTBE were, however,
readily degraded even though the MTBE remained.
A mixed bacterial culture was obtained by enrichment of a
hydrocarbon-contaminated soil in a basal mineral medium containing:
(i) TBA (1 g/litre) as sole carbon source or (ii) methylamine (2
g/litre) as principal carbon source supplemented with TBA. During
incubation of the first culture, the concentration of TBA fell to zero
in 20 days, but incubation of methylamine-grown cells with MTBE showed
no reduction in the concentration of MTBE after 42 days (Allard et
al., 1996). Whereas MTBE was apparently recalcitrant under the
conditions used, TBA, which is one of its putative degradation
products, was biodegradable.
In contrast to these results, a mixed bacterial culture obtained
by continuous aerobic enrichment of a sludge sample from an industrial
bioreactor was able to degrade MTBE at concentrations up to 200
mg/litre (Salanitro et al., 1994). Cell suspensions incubated with
MTBE produced TBA as a transient metabolite. MTBE labelled with 14C
in the methyl group was degraded to 14CO2 and cellular material when
low substrate concentrations (2 mg/litre) were used, although not at a
concentration of 20 mg/litre. This experiment clearly demonstrated
oxidation of the methoxy group but left unresolved the fate of the
carbon atoms of the tertiary-butyl group.
Fifteen pure bacterial strains, with the capacity to degrade MTBE
using it as the sole carbon source, have been isolated from bioreactor
sludges and other sources. Several strains have been identified as
belonging to the genera Rhodococcus, Flavobacterium, Pseudomonas and
Oerskovia. These strains degrade up to 40% of MTBE (200 mg/ litre)
in 1-2 weeks of incubation at 22-25°C. These strains also grow on
tert-butanol, butyl formate, isopropanol, acetone and pyruvate as
sole carbon sources. Cultures of Methylobacterium, Rhodococcus and
Arthrobacter degraded MTBE within 1-2 weeks of incubation at
23-25°C. Growth on MTBE as the sole carbon source was slow compared
with growth on a nutrient-rich medium. When these compounds are mixed
with MTBE, there is a reduction in the degradation of MTBE. However,
when the microbes were initially grown on tert-butanol and then
transferred to medium containing MTBE, there was a greater degradation
of MTBE (Mo et al., 1997).
A mixed culture isolated from biological sludges has been used in
bioreactors utilizing MTBE as a sole carbon source for over a year.
The microbes were able to degrade MTBE at a concentration of 160
mg/litre after 3 days of incubation in batch experiments. Mixed
cultures have greater capacity for degradation of MTBE than pure
cultures. The addition of other ethers causes a reduction in MTBE
degradation. In soil microcosm studies, significant MTBE degradation
by mixed cultures was observed at 24°C and 10°C (Mo et al., 1997).
Howard et al. (1991) estimated, on the basis of screening tests
for aerobic biodegradation with unacclimatized aqueous systems
(Fujiwara et al., 1984), that the half-lives of MTBE in water and soil
under aerobic conditions ranged from 672 to 4320 h.
MTBE was found to be degraded by a number of propane-oxidizing
bacteria. The initial oxidation of MTBE produced nearly stoichiometric
amounts of TBA. The methoxy group of MTBE was further oxidized to
formaldehyde and finally to CO2. At 28°C, rates of MTBE degradation
by these bacteria ranged from 3.9 to 9.2 nmol/min per mg cell protein
weight (Steffan et al., 1997).
4.3.2 Anaerobic conditions
Biodegradability of MTBE to methane under anaerobic conditions
has been determined by measuring the production of CH4 and CO2
during exposure of MTBE to a large population of anaerobic bacteria.
MTBE was biodegraded anaerobically only to a very limited extent
(Pence, 1987b), and an average cumulative theoretical gas production
of only 7.1% was achieve within 56 days. Anaerobic biodegradation to
methane must exceed 50% to meet the validation requirements for
demonstration of anaerobic biodegradability.
The anaerobic degradation of MTBE has been examined in different
soils (unsaturated clay, sandy loam and silty loam) collected from
various depths at three different sites (Novak et al., 1992; Yeh &
Novak, 1994). The experiments were conducted in static small-volume
anaerobic microcosms, and three different oxygen-free conditions were
simulated; with nitrate as electron acceptor (denitrification),
sulfate-reducing conditions, and anaerobic fermentation. Factors
influencing the degradation of MTBE, ETBE and TBA were determined, and
included anaerobic microbial populations, soil anions, soil moisture
content, organic content, nitrogen availability, rate of ammonium
"fixation", and soil pH. The soils were moderately acidic (pH 5.0-6.0)
with the exception of surface soils. The concentration of the added
MTBE was monitored for more than 250 days. Three parameters were
evaluated: degradation rate, lag time and time for 80% of the compound
to be degraded. No anaerobic degradation of MTBE was found in
organic-rich soils over the 250-day study period. The only situation
in which MTBE degradation occurred was in an oligotrophic soil
containing a low level of organic matter and with a pH of 5.0-6.0.
About 10% of the MTBE was lost during the first two months, although
this decrease cannot unambiguously be attributed to biodegradation.
Several conclusions may be drawn from the experiments with TBA and
ETBE:
* Whereas degradation of TBA in soil from the oligotrophic site
could be enhanced by addition of nitrate, the degradation of TBA
was inhibited by adding readily degraded ethanol.
* Biodegradation of ETBE under denitrifying conditions was
extremely sensitive to the presence of readily degraded
substrates.
These results illustrate that care should be exercised in
assessing biodegradability when several readily degraded substrates
are available, a condition that may be encountered in groundwater
contaminated with oxygenate additives.
Suflita & Mormile (1993) used sediment suspensions prepared from
samples collected from an aquifer polluted with leachate from a
municipal landfill. They assessed the formation of methane from a
range of substrates, and after at least 249 days no evidence for
anaerobic degradation of MTBE could be found. Whereas unbranched
alkanols and ketones were readily degraded, ethers in general were
resistant; in addition, oxygenates containing a tertiary or quaternary
carbon atom proved more recalcitrant than their unbranched or
moderately branched chemical analogues to anaerobic degradation.
Comparable experiments using a wider range of sediment samples
(Mormile et al., 1994) showed similar results under sulfate-reducing
or denitrifying conditions, although under methanogenic conditions a
single sample transformed MTBE into TBA. Likewise, the ethers were
unaffected by incubation with cultures of the acetogenic bacteria
Acetobacterium woodii and Eubacaterium limosum that convert
aromatic methoxy groups to acetate.
Based on the above-mentioned studies, MTBE is classed as
recalcitrant under anaerobic conditions.
Howard et al. (1991) estimated that the half-life of MTBE in
water under anaerobic conditions ranges from 2688 to 17 280 h.
4.4 Abiotic degradation
4.4.1 Air
4.4.1.1 Photolysis
Direct photolysis of MTBE is assumed to be environmentally
insignificant since it does not absorb radiation above 230 nm (Calvert
& Pitts, 1966). However, under laboratory conditions MTBE in an
oxygenated slurry system containing TiO2 as catalyst was readily
degraded by UV light from a mercury lamp. MTBE was rapidly
photocatalytically degraded, 76% of the initial concentration being
converted to degradation products, including TBA. After 4 h MTBE was
no longer detectable (Barreto et al., 1995).
4.4.1.2 Hydrolysis
MTBE does not contain hydrolysable functional groups, and,
therefore, it is inert to environmental hydrolysis. Hydrolysis of MTBE
is assumed to be insignificant (Howard et al., 1991).
4.4.1.3 Photooxidation
MTBE is subject to photooxidation in the atmosphere. This will
occur under the influence of various mechanisms, such as the reaction
with hydroxyl radicals, water, alkoxy and peroxy radicals, oxygen
atoms, and ozone. On the basis of the rate constant of each of the
reactions and the concentrations of the reactants, the reaction with
the hydroxyl radical is considered to be the most important removal
process for MTBE in the atmosphere. Several products are generated as
a result. These include tertiary-butyl formate (TBF), the major
product, 2-methoxy-2-methyl propanol, formaldehyde, acetone, NO2, and
the methyl radical. Molar yields of products identified from the
reaction of hydroxyl radicals with MTBE are given in Table 4). TBF is
unreactive to further photo-oxidation, while 2-methoxy-2-methyl
propanol is expected to be highly reactive with hydroxyl radicals,
yielding equimolar amounts of CO2, formaldehyde, acetone and water.
Of these products, formaldehyde is highly reactive with the hydroxyl
radical (Wallington et al., 1988; Japar et al., 1991). Rates of
reaction of oxygenates and their decomposition products with hydroxyl
radicals are given in Table 5.
Factors influencing atmospheric lifetime, such as time of day,
sunlight intensity and temperature, also include those affecting the
availability of hydroxyl radicals. Based upon measured rate constants
for reactions with hydroxyl radicals in air (Cox & Goldstone, 1982;
Atkinson, 1985; Wallington et al., 1988, 1989; Atkinson, 1990; Japar
et al., 1990), the half-life for MTBE has been estimated to be between
20.7 and 265 h (Howard et al., 1991). Hence, MTBE is not considered to
be a greenhouse gas, nor would it contribute to the depletion of the
ozone layer (Environment Canada, 1993).
Table 4. Molar yields of products identified from the reaction of
hydroxyl radicals with MTBE
Product Molar yielda Molar yieldb
TBF 0.68 0.76
Formaldehyde 0.48 0.37
Methyl acetate 0.14 0.17
TBA 0.062 -
Acetone 0.026 0.02
a Smith et al., 1991.
b Tuazon et al., 1991.
Table 5. Rates of reaction of oxygenates and their decomposition
products with hydroxyl radicals at 25°C
Compound Rate Reference
(10-12 cm3
sec-1 molecule-1)
MTBE 3.2 Japar et al., 1991
ETBE 8.5 Japar et al., 1991
TBF 0.74 Smith et al., 1991
TBA 1.1 Japar et al., 1991
Formaldehyde 9.0 Atkinson & Pitts, 1978
2-methoxy-2-methyl propanala 30 Japar et al., 1991
a Estimated from rates for other aldehydes
4.4.2 Natural waters
MTBE is not expected to adsorb significantly to bed sediments of
suspended sediments, hydrolyse, directly photolyse, or photo-oxidize
via reaction with photochemically produced radicals in water. While
MTBE is reported to be chemical unstable in acidic solutions (Budavari
et al., 1996), it is not expected to be hydrolysed in natural waters
under normal pH conditions (Lyman et al., 1990).
4.4.3 MTBE half-life ranges in environmental compartments
The half-life of a chemical in the environment depends not only
on the intrinsic properties of the chemical, but also on the nature of
the surrounding environment, such as sunlight intensity, hydroxyl
radical concentration, the nature of the microbial community and
temperature. Table 6 lists the half-life ranges in various
environmental compartments estimated by Mackay et al. (1993) and
Howard et al. (1991); these estimates are somewhat uncertain, as
implied by the order of magnitude range for some compartments.
4.5 Ozone-forming potential
Photochemical ozone-creation potentials (POCP) ranging from 20.4
to 34.6 have been determined for MTBE using a model that simulates the
formation of photochemical ozone episodes (Derwent et al., 1996). The
POCP values reflect the ability of a substance to form tropospheric
ozone as a result of its atmospheric degradation reactions. The POCP
values are calculated relative to ethylene (a chemical that is thought
to be important in such ozone formation and is given a POCP of 100).
Based on the emissions and the POCP value, MTBE (itself) is likely to
play a minor role in photochemical smog and low-level (tropospheric)
ozone formation near to sources of release.
4.6 Remediation
Examples of remedial methods that can be considered for MTBE are
air stripping, carbon absorption and soil vapour extraction. Intrinsic
bioremediation may be limited due to the variability of rates of
biodegradation of MTBE which have been previously mentioned (Zogorski
et al., 1996).
Table 6. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Soil 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Air 20.7-265 Based upon measured Howard et al., 1991
10-30 photo-oxidation half-life Mackay et al., 1993
Surface water 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The major sources of MTBE to the general population are probably
associated with the distribution, storage and use of oxygenated
gasoline. The main source of non-occupational exposure to MTBE is
evaporative emissions from gasoline. A large portion of the population
is exposed during time spent at service stations, while driving cars,
in public parking garages, and in homes with attached garages. These
exposures generally occur through inhalation. In addition, discharges
into the soil or groundwater are a potential for contaminated water
supply and can lead to exposure when such water is drunk. Dermal
contact with MTBE may occur through accidental spills of MTBE-blended
gasoline or through the use of gasoline as a solvent. In Canada, it
has been estimated that gasolines blended with MTBE account for only
2% of the total annual gasoline consumption. MTBE is used in small
quantities by a few Canadian refiners to boost octane levels in
gasoline. A limited survey of the MTBE content of unleaded regular,
mid-range and premium gasoline across Canada in 1995 showed a range of
0 to 5.2% by volume for winter grade gasoline and 0 to 9% by volume in
summer grade gasoline. In the USA, oxygenated gasoline containing
10-15% MTBE is used in different areas and about 30% of the US
population is exposed to MTBE.
5.1 Environmental levels
5.1.1 Exposure
Ambient air and microenvironment concentrations of MTBE and other
fuel oxygenates have been measured in Canada, the USA and Finland.
When available, air data are presented below in conjunction with data
on MTBE levels in gasoline and with information on the proximity of
the samples to various point sources of MTBE.
Brown (1997) estimated average daily and average lifetime doses
of MTBE from exposure in air and drinking-water for a US population.
Concentration data and several of the population characteristics were
estimated as distributions rather than as point values. Arithmetic
mean occupational doses via air were in the range of 0.1 to
1.0 mg/kg-day, while doses from residential exposures, commuting and
refuelling were in the range of 0.0004 to 0.006 mg/kg-day. Lifetime
doses for workers were in the range of 0.01 to 0.1 mg/kg-day. The
cumulative dose distribution for the entire population of the
MTBE-using regions of the USA was estimated by combining the
distributions of doses and the numbers of people in each exposure
category. In the MTBE-using areas, arithmetic mean doses via air were
estimated to be 0.0053 and 0.00185 mg/kg-day for the chronic and
lifetime cases, respectively. It was found that 1.5% of the population
used water contaminated with MTBE leakage with an estimated geometric
mean concentration of 0.36 µg/litre and a 95th percentile
concentration of 64 µg/litre. Including ingestion, inhalation, and
dermal absorption of contaminated water, the estimated arithmetic mean
does of the population exposed via water was 1.4 × 10-3 mg/kg-day.
5.1.1.1 Levels in ambient air and various microenvironments
a) Canada
The concentrations of MTBE in ambient air at various selected
locations in Canada have been measured as part of the National Air
Pollution Surveillance Programme in 1995 and 1996. This programme is a
joint project of the federal, provincial and municipal levels of
government. Its purpose is to monitor and assess, on a continuing
basis, the quality of the ambient air in the various regions of
Canada. The sites selected for monitoring of MTBE were based on usage
of gasoline with MTBE and/or because of nearby manufacturers of MTBE.
Pollutants from air were collected intermittently using the
canister methodology. Concentrations of MTBE was measured using the
detection principle of gas chromatography furnished with an ion trap
detector. Air samples were first passed through a cryogenic
concentration trap to gather enough analyte before injection into a GC
capillary column to allow compound speciation and quantification.
Approximately 200 ml of the canister sample was concentrated. A
cryogenic trap held at -150°C was used to concentrate the air sample.
Once the sample was concentrated, the trap was heated to 150°C and the
sample was back-flushed onto the column. MTBE and other hydrocarbons
were separated using a fused silica capillary column. The GC oven was
programmed to remain at 60°C for 3 min, then increased to 280°C at a
rate of 8°C/min. Calibration standards were prepared using the static
dilution technique. The detection limits were 0.05 to 0.1 µg/m3.
Table 7 lists the ambient concentration of MTBE in air at various
locations in Canada from 1995 to 1996.
Table 8 shows some MTBE atmospheric concentrations at the fence
line of a petroleum refinery at St John, New Brunswick, Canada, during
a period when there were complaints of odour. The same collection and
analytical methodology was used. The maximum concentration is not
considered representative of the area.
b) USA
In many urban areas in the USA having elevated levels of ozone or
CO, oxygenates such as MTBE are regulated for use in gasoline at
concentrations of 2.0% and 2.7% oxygen by weight (called reformulated
and oxygenated gasoline, respectively). These concentrations are
achieved by adding MTBE at 11% and 15% by volume, respectively. In
other areas, MTBE is used as an octane enhancer in premium gasoline at
concentrations up to 9% by volume, but usually at much lower
concentrations. It is important to note that MTBE is the predominant
oxygenate currently in use in these gasoline mixtures, followed by
ethanol (approximately 65% and 35% of the oxyfuels sold contain MTBE
and ethanol, respectively). Oxygenates used to a minor extent include
ETBE, TAME and DIPE (HEI, 1996). In 1994, oxygenates were added to
more than one-third of the gasoline market in the USA.
Table 7. Concentrations of MTBE in ambient air in Canada (1995-1996)
(Environment Canada, 1996b)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Edmonton(1)c Two petroleum refineries - 1 km. 20/7/95 7.21
Acetic acid plant - 2.5 km
26/7/95 11.39
1/8/95 0.81
7/8/95 2.93
8/8/95 5.50
12/9/95 2.49
27/5/96 3.35
Edmonton(2)d N/A 26/7/95 < DL
1/8/95 < DL
1/9/95 < DL
6/9/95 < DL
24/9/95 < DL
30/9/95 < DL
27/5/96 < DL
Halifaxd N/A 3/4/96 < DL
15/4/96 0.13
21/4/96 0.15
Montreal(1)c Two refineries (BTX, petroleum) 21/8/95 1.54
- 1.6, 2.5 km
21/8/95 0.59
12/9/95 1.06
16/3/96 < DL
15/5/96 0.42
21/5/96 0.28
27/5/96 0.23
Montreal(2)d N/A 16/3/95 0.15
16/5/96 0.18
21/5/96 0.22
27/5/96 0.37
Montreal(3)e N/A 10/3/96 0.16
16/3/96 0.28
9/5/96 0.95
Montreal(4)f N/A 9/5/96 0.22
15/5/96 < DL
21/5/96 0.70
St. Johnc Petroleum refinery - 3 km 9/5/96 1.02
15/5/96 3.73
Stouffvillef N/A 6/10/95 0.19
18/10/95 0.35
Toronto(1)d N/A 29/8/95 < DL
2/9/95 < DL
2/9/95 0.07
Table 7. (continued)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Toronto(2)d N/A 17/8/95 0.03
29/8/95 < DL
2/9/95 < DL
Vancouver(1)f N/A 23/8/95 0.27
29/8/95 0.89
29/8/95 0.16
30/8/95 0.33
Vancouver(2)f N/A 22/3/95 0.14
Vancouver(3)c Two gasoline processing and 1/8/95 2.13
storage plants - 0.5, 3 km
13/8/95 1.82
25/8/95 3.35
2/9/95 1.78
2/9/95 26.43
21/2/96 0.31
10/3/96 1.10
16/3/96 0.48
16/3/96 0.39
22/3/96 1.55
28/3/96 < DL
Vancouver(4)e N/A 10/3/96 1.07
Vancouver(5)f N/A 28/3/96 0.40
Vancouver(6)c Pipeline transfer point 1/9/95 1.79
2/9/95 1.90
22/3/96 0.89
Windsord N/A 2/8/95 0.08
17/8/95 0.05
17/8/95 0.11
21/8/95 0.40
29/8/95 0.27
29/8/95 0.14
16/3/96 < DL
21/4/96 0.15
27/4/96 < DL
Winnipegd N/A 4/3/96 0.02
27/3/96 < DL
a ( ) = different monitoring sites in same city.
b DL = detection limit = 0.1 µg/m3.
c Monitoring site in vicinity of petroleum refinery and/or industrial chemical plant, or
pipeline transfer area.
d Monitoring site in urban area.
e Monitoring site in urban area on busy street.
f Monitoring site in suburban area.
Table 8. MTBE concentrations at petroleum refinery boundary
(St. John, New Brunswick, Canada) during period of odour
complaints
Sampling date MTBE concentration
(µg/m3)
19/7/95 281
2/8/95 15
14/8/95 71
28/8/95 36
MTBE air quality data were collected in the USA as a result of
special studies in six urban centres: Fairbanks (Alaska), Stamford
(Connecticut), Albany (New York), Milwaukee (Wisconsin), Boston
(Massachusetts) and Houston (Texas) (Zogorski et al., 1996). In
addition, collection of MTBE air quality data for selected monitoring
sites in California started in 1996. Although these data cannot be
used to define quantitatively the air quality in these cities and are
not sufficient to provide a national perspective, they can be used to
estimate approximate ranges of MTBE in ambient air in the locations
sampled (Zogorski et al., 1996; US Interagency Assessment, 1997).
Non-occupational and consumer exposure to MTBE is shown in Table 9,
and service station attendants and garage workers in Table 10.
Owing to health complaints (Gordian et al., 1995) following the
introduction of oxygenated fuels (15% MTBE) during the 1992 winter
season in Fairbanks, Alaska, the sale of these fuels was suspended in
mid-December 1992, one month after their introduction. Zweidinger
(1993) analysed air samples for MTBE in ambient air and in various
microenvironments in Fairbanks taken immediately prior to the
suspension (phase I), during the phase-out period (Phase II), and two
months after the suspension, at which time the MTBE fuels were
expected to be at nominal levels (Phase III). Fuel samples collected
from Fairbanks gasoline stations during Phases II and III indicated
that the average percentage by weight of MTBE in unleaded regular
gasoline decreased from 8.5% to 1% while the average for premium
gasoline decreased from 14.7 to 5.6%. For comparison, ambient air
samples were also collected in the spring of 1993 from Stamford,
Connecticut, where 15% MTBE oxygenated gasoline was sold but there
were no consumer health complaints, and Albany, New York, where MTBE
was only present in gasoline at nominal levels to enhance octane and
there were also no health complaints (Zweidinger, 1993).
Ambient air samples in these cities were generally collected over
an 8-h period and were taken from the following areas: (1) outside
city limits, for background levels; (2) in residential areas away from
heavy traffic, and (3) in areas adjacent to major roadways or
intersections. The median and range of MTBE concentrations for the
selected locations and phases are shown in Table 11 (Zweidinger,
1993).
In Fairbanks, MTBE levels in all ambient environments were lower
when the use of MTBE as an oxygenate in gasoline was discontinued.
Overall MTBE ambient air concentrations ranged from non-detectable
levels to a maximum of 100.9 µg/m3 (28.0 ppbv) in the phases prior to
and during the phase-out of MTBE in Alaskan gasoline (Phases I and II)
and ranged from not detectable to 12.3 µg/m3 (3.4 ppbv) when MTBE use
was reduced to nominal levels in fuels (Phase III). Background levels
were low during Phase III, ranging from not detectable to 4.3 µg/m3
(1.2 ppbv) MTBE. Since sampling was limited during Phases I and II,
the levels of MTBE in ambient air outside city limits cannot be
compared for the three sampling periods over which MTBE concentrations
in gasoline were being reduced.
Phase II ambient residential and roadside area air samples
presented the highest levels of MTBE, with concentrations ranging from
6.1 to 100.9 µg/m3 (1.7 to 28.0 ppbv) and 15.1 to 28.5 µg/m3 (4.2 to
17.9 ppbv) respectively, and with medians of 6.1 µg/m3 (4.6 ppbv) and
34.9 µg/m3 (9.7 ppbv), respectively. Owing to lack of samples or
small sample size in Phase I data, comparisons between the phases were
limited to the roadside category. However, in this category, it was
unexpectedly found that, although Phase 1 sampling occurred prior to
suspension of MTBE, the data showed roadside levels of MTBE in ambient
air to be slightly lower than those during the suspension period.
In Stamford, Connecticut, limited measurements of MTBE taken in
the spring of 1993 showed that residential, roadside and gas station
ambient air results were consistently lower than those taken in
Fairbanks during the oxygenates programme, with ranges of not
detectable to 1.1 µg/m3 (0.3 ppbv), not detectable to 1.8 µg/m3
(0.5 ppbv) and 4.3 to 10.1 µg/m3 (1.2 to 2.8 ppbv), respectively.
Limited residential and roadside ambient MTBE air samples taken in
Albany, New York, in May 1993 were lower than levels during a
comparable phase of MTBE use in Fairbanks (Phase III). Ambient
temperature and other meteorological conditions differed significantly
among the cities where samples were collected.
In Fairbanks, Stamford and Albany, roadside ambient air levels
were found to be generally higher than residential area ambient air
levels (Table 11). Consequently, this roadside category may also be
considered to be a microenvironment, especially for those samples
taken in downtown city street canyons. For this particular study, it
was not possible to discern whether this was the case. Table 12
presents information on air samples taken in more clearly defined
microenvironments such as service stations and vehicle interiors
(Zweidinger, 1993).
Table 9. Non-occupational and consumer exposure to MTBE in the USAa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Community air
Milwaukee WI RFG in Yes (in 6/11 0.000025 <0.00413 0.00013 Jan-March 1995; Approximately Allen &
use* some 24-h samples; 50% contained Grande
cases) collected in MTBE, remainder (1995)
evacuated ETBE or ethanol
canisters;
GC(FID)
3/5 0.000025 <0.00106 0.00052 Feb-March 1995;
2-h samples;
collected in
evacuated
canisters;
GC(FID)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 up to (0.002) Feb-March 1995; Approximately Allen
use* 0.0037 2- to 3-h 50% contained & Grande
samples; MTBE, remainder (1995)
collected in ETBE or ethanol
evacuated
canisters;
GC(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Automobile cabin for commuters
New Jersey 15 MTBE NA 20/20 0.002- 0.004 April 1993; Estimated from Lioy et
0.017* approximately graphed data al. (1994)
1-h samples; in the original
Connecticut 20/20 0.003- 0.0056 absorbed onto report
0.009* carboxen 569:
collected in
evacuated
canisters;
GC/MS
Service station refuelling
Phoenix, AZ 12* No 40/40 0.09-38 5.8 Oct-Nov 1990; Samples taken API (1993)
each sample was from one station
collected in which only
Los Angeles, 13 Yes 6/6 1.1-6.5 3.6 during the premium gasoline
CA refuelling of 8 was oxygenated
to 10 vehicles;
each refuelling
was sampled for
1 to 2 min;
absorbed onto
charcoal; GC
(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey,
New York 10-15* Yes 4/4 NS 0.370 April 1993; 5-min Estimated from Lioy et
breathing-zone graphed date in al. (1994)
samples before, the original
Connecticut No 4/4 NS-4.1 0.572 during and after report
refuelling;
absorbed onto
carboxen 569;
GC/MS
Milwaukee WI Jan-March 1995; RFG with MTBE Allen &
Station A 9-10* Yes 6/6 NS (0.39) 15-min used only in Grande (1995)
Station D 9** No 2/2 NS (2.93) breathing-zone higher grades;
samples; adsorbed *ethanol used
onto charcoal; in regular
GC(FID) gasoline; **2%
MTBE used in
regular gasoline
Northeast and 10-17* Yes 8/17 <0.32 <2.1 0.57 Feb-April 1994; Northeast = API (1995c)
southwest 15- to 20-min Connecticut and
areas personal New Jersey
(short-term breathing zone locations;
sample) samples; adsorbed Southwest =
onto charcoal; Arizona
GC(FID) locations
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
In automobile while refuelling
Connecticut, New Jersey and New York Service Stations
Self-serve 10-15* Yes 4/4 0.006- 0.03 April 1993; 5-min Estimated from Lioy et
0.072 breathing-zone graphed data in al. (1994)
samples before, the original
Full-serve Yes 8/8 0.008- 0.034 during and after report
0.172 refuelling;
adsorbed onto
Self-serve No 4/4 NS 0.015 carboxen 589;
GC/MS
Full-serve No 4/4 0.005- 0.041
0.103
Service station pump island
New Jersey: Yes 4/4 0.120- 0.440 April 1995; 4-h API (1995a)
Full-serve 1.600 breathing-zone
samples during
both refuelling
and not
refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 6/6 0.014- 0.048
Self-serve 0.080
Connecticut: No 9/10 0.09 <1.500 0.170
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 15 Yes 3/3 0.08- 0.24 Nov-Dec 1994; 7- Cook &
0.24 to 8-h samples; Kovein (1995)
adsorbed onto
charcoal; GC (FID)
Service station perimeter
Phoenix AZ 12 No 24/24 0.009- 0.02 Oct-Nov 1990; API (1993)
0.09 12-h samples;
4 perimeter
samples plus
samples upwind
and downwind
from the pump
island for each
sample set;
adsorbed onto
charcoal; GC(FID)
New Jersey: Yes 15/16 0.001 <0.036 0.003 April 1995; 4-h API (1995a)
Full-serve samples;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 24/24 0.002- 0.007
Full-serve 0.083
Connecticut: No 38/40 0.001 <0.140 0.014
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Milwaukee WI
Station A 9-10* Yes 2/2 NS (0.0024) Jan-March 1995; *RFG, MTBE used Allen &
Station B 2-9** No 1/1 NS (0.0045) 2-h area samples; only in higher Grande (1995)
collected in grades; ethanol
evacuated in lower grades
canisters; GC(FID) **2% MTBE in
regular gasoline
a Asterisks in any column indicate that further explanation is provided in the comments column.
b Number of samples in which MTBE was detected divided by total number of samples.
c GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
NA = not applicable; NS = not stated.
Table 10. Non-industrial occupational exposures to MTBEa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Service station attendants during refuelling
Phoenix AZ Oct-Nov 1990; each API (1993)
1-2 min 12 No 40/40 0.09-38 5.8 breathing-zone
sample was
collected during
Los Angeles the refuelling of
CA 13 Yes 6/6 1.1-6.5 3.6 8-10 vehicles;
1-2 min each refuelling
was sampled for
1-2 min; adsorbed
onto charcoal;
GC (FID)
New Jersey, 10-15 Yes 4/4 NS 0.37* April 1993; 5-min Estimated from Lioy et
New York, breathing-zone graphed data al. (1994)
samples before, in original
during, and after report
Connecticut: refuelling;
5 min No 4/4 <4.1 0.572 adsorbed onto
carboxen 589;
GC/MS
Milwaukee WI Jan-March 1995; This station used Allen &
Station A 15-min MTBE in middle Grande (1995)
15 min RFG 9-10 Yes NS/6 NS 0.31 breathing-zone and premium grade
samples during gasoline, and
refuelling; ethanol in
collected in regular gasoline
evacuated
canisters
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
15-20 min 10-17 Yes 8/17 0.32 <2.1 0.57 samples; mostly locations;
15- to 20-min Southwest =
personal Arizona
breathing-zone locations
samples during
refuelling;
adsorbed onto
charcoal;
GC (FID)
Various USA 1982-1993: data Service station API (1995b)
locations reported by and retail outlet
American Petroleum personnel
<30 min NS NS 9/11 0.16 0.16- 2.6 Institute member
136.1 companies; higher
frequency of
30 min-6 h 5/5 0.01- 0.34 measurements
2.7 in 1989-1993;
GC(FID) and
other procedures
6-9 h TWA 13/13 0.09- 0.59
34.0
>9 h 11/11 0.01- 1.1
17.20
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 4 h 13-10 Yes 4/4 0.084- 0.245 April 1995; 4-h API (1995a)
(full-serve) 0.52 breathing-zone
samples, during
New York 4 h Yes 5/6 0.077- 0.205 refuelling and
(self-serve) 0.78 not refuelling;
collected in
Connecticut 4 h No 10/10 0.170- 1.5 8-litre evacuated
(self-serve) 2.60 canisters; GC/MS
Phoenix AZ 4 h 14 No 42/42 0.04- 0.55 Oct-Nov 1990; 4-h Hartle (1993)
3.88 (half-shift)
breathing-zone
samples (average
time 224 min);
adsorbed onto
charcoal; GC(FID)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
>8 h 10-17 Yes 18/21 0.03- <0.5 0.27 samples, most locations;
0.11 sampling times Southwest =
were > 6 h; Arizona
adsorbed onto locations
charcoal; GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey Nov-Dec 1994; Cook &
(full shift) 15 Yes 21/21 NS 0.12- 0.48 breathing-zone Kovein (1994)
1.42 samples for 3-8 h;
adsorbed onto
charcoal; GC (FID)
Service station attendants at pump island
New Jersey
4 h 13-16 Yes 4/4 0.12- 0.44 April 1995; 4-h API (1995a)
(full-serve) 1.60 breathing-zone
samples during
refuelling and
not refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York Yes 6/6 0.0005 0.014- 0.048 Cook &
4 h 0.08 Kovein (1994)
(self-serve)
Connecticut 15 No 9/10 NS <1.5 0.17 Nov-Dec 1994; 7-
4 h to 8-h samples;
(self-serve) adsorbed onto
charcoal; GC (FID)
New Jersey Yes 3/3 0.08- 0.24
8 h 0.24
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 0.0023- (0.002) Feb-March 1995; Approximately 50% Allen &
2-3 h use 0.0037 2- to 3-h samples; contained MTBE, Grande (1995)
collected in remainder
evacuated contained ETBE
canisters; or ethanol
GC(FID)
Mechanics Feb-April 1994; Northeast = API (1995c)
Northeast and personal Connecticut and
southwest in breathing-zone New Jersey
winter samples, most locations;
short-term Southwest =
15 min 10-17 Yes 4/13 <0.25- <32 ND sampling times Arizona locations
(<0.26) <0.35 were 15-20 min;
most long-term
>6 h 17/20 <0.02- <2.6 0.09 sampling times
(<1.5) <0.05 were >6 h; adsorbed
onto charcoal;
GC(FID)
Northern New April 1993; 1-h Workers at Mohr et
Jersey breathing-zone service stations al. (1994)
1 h 15 NS NS/13 0.3-6.1 samples (active); and garages
adsorbed onto for State
carboxen; GC/MS vehicles
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Stamford April 1993; Mechanics with Buchta (1993b)
Connecticut full-shift the Department
8-h TWA 13-17 NA 20/28 0.03 <12.04 0.11 samples of Public Works
(approximately and in auto
8 h); adsorbed dealers' garages
onto charcoal;
GC(FID)
Fairbanks AK Dec 1992; Moolenaar
8-h TWA 15 NS NS/10 0.01- 0.10 full-shift et al. (1994)
0.81 samples
(approximately
8 h); collected
in evacuated
canisters in
environment where
workers spent
most of their
day; GC
Other vehicle-related workers
Stamford 13-17 NS 0/7 0.03 <DL <DL April 1993; 8-h Workers who
personal spent time in
breathing-zone traffic
samples; adsorbed
onto charcoal;
GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Connecticut Workers in various Buchta (1993b)
8-h TWA 1/4 <0.15 <DL jobs (mostly
workers in garages
who performed
tasks difference
from the mechanics)
a Number of samples in which MTBE was detected divided by total number of samples.
b GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
c Asterisks in any column indicate that further explanation is provided in the comments column.
TWA = time-weighted average; NA = not applicable; NS = not stated.
Table 11. Concentrations of MTBE in µg/m3 (ppbv) in ambient 8-h air samples taken in Fairbanks, Stamford and Albany, USA,
as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)a
Sampling site Fairbanks Fairbanks Fairbanks Stamford Albany
Phase I Phase II Phase III April 1993 May 1993
early December 1992 late December 1992 Feb/Mar 1993
Background:
number of samples 0 1 5 2 0
median - ND ND 0.72 (0.2) -
range - 1 ND-4.3(1.2) ND-1.1(0.3) -
Residential:
number of samples 2 11 11 2 3
median 14.4(4) 16.6(4.6) 2.5(0.7) 1.1(0.3) ND
range 7.2-21.6 16.1-100.9 ND-9(2.5) ND-1.8(0.5) ND-0.4(0.1)
(2.9-6.0) (1.7-28.0)
Roadside:
number of samples 7 7 10 2 7
median 18(5) 35(9.7) 4.3(1.2) 7.2(2.0) 0.72(0.2)
range 10.8-43.2 15.1-64.5 ND-12.3(3.4) 4.3-10.1 (1.2-2.8) ND-2.5(0.7)
(3.0-12.0) (4.2-17.9)
a For the calculation of a median when n = 2, the two sample values are averaged together; in the case of an ND value,
half the detection limit value is substituted in the calculation, i.e. 0.36 µg/m3 (0.1 ppbv).
ND = non-detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Table 12. Concentrations of MTBE in µg/m3 (ppbv) in various microenvironmental 8-h ambient air samples taken in Fairbanks, Stamford
and Albany, USA, as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Service station pump island:
number of samples 1 1 6 4 4
median 194.6 (54) 134.8 (37.4) 11.5 (3.2) 13.7 (3.8)a 64.2 (17.8)
range - - 6.1-49.7 ND-26.7 (7.4) 23.3-194.6
(1.7-13.8) (6.6-54)
Commercial vehicle interiors:
number of samples 8 - 6 - -
median 126.1 (35) - 31.7 (8.8) - -
range 25.2-1207.3 - 1.4-129 - -
(7-335) (0.4-35.8)
Indoors - commercial garage service areas:
number of samples 5 - 10 8 -
median 1088.4 (302) - 148.5 (41.2) 484 (134.8) -
range 367.6-2922.8 - 21.3-496 4.7-1546.5 -
(102-811) (5.9-137.6) (1.3-429.1)
Indoors - residential area:
number of samples - 3 5 - -
median - 6.5 (1.8) 2.9 (0.8) - -
range - 6.1-15.1 1-4 (0.3-1.1) - -
(1.7-4.2)
Table 12. (continued)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Indoors - public buildings near roadway:
number of samples - 4 5 4 -
median - 32.4 (9) 6.5 (1.8) 1.8 (0.5) -
range - ND-37.1 (10.3) ND-10.5 (2.9) 1.4-1.8 -
(0.4-0.5)
Indoors - home with attached garage:
number of samples - 5 4 - -
median - 27.8 (7.7) 72.1 (20) - -
range - 10.1-75.3 51.5-109.2 - -
(2.8-20.9) (14.3-30.3)
a In Stamford, service station samples were taken 4.6 metres (15 feet) away from the pump.
ND = not detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Kelly et al. (1993) performed 24-h ambient air sampling in the
cities of Boston and Houston where MTBE was used nominally in gasoline
(i.e. approximately < 5% by volume). Sampling took place
approximately every 14 days from August 1990 to April 1991 and from
June to August 1991. The Boston sampling site was categorized as being
in an urban area of mixed industry and office buildings, with high
traffic density, and the sampler was placed on a downtown fire
department rooftop. Conversely, the Houston sampling site was located
in a semi-rural area, on the roof of an air sampling station, and was
expected to receive emissions from industrial and, to a lesser extent,
urban sources. MTBE levels in ambient air at these sites ranged from
< 0.72 to 1.8 µg/m3 (< 0.2 to 0.49 ppbv) and < 0.72 to 10.1 µg/m3
(< 0.2 to 2.8 ppbv), respectively (detection limit = 0.72 µg/m3 (0.2
ppbv). The median level from these data was < 2 ppbv for both Boston
and Houston. MTBE was detected in several samples from Houston (n =
22; in 64 % of samples MTBE was non-detectable), but only at one site
in Boston (n = 22; in 96% of samples MTBE was non-detectable).
Allen & Grande (1995) conducted an ambient air monitoring study
in the city of Milwaukee as a result of public health complaints when
MTBE was first introduced into Milwaukee reformulated gasoline (at
approximately 11% by volume) in 1995. Eleven weekly 24-h samples
collected at the Wisconsin Enhanced Ozone Monitoring Program air
sampling station from January to March 1995 resulted in concentrations
ranging from not detectable to 14.89 µg/m3 (4.13 ppbv) [n = 11; 45%
non-detectable samples; detection limit 0.36 µg/m3 (0.1 ppbv)]. The
median was determined to be 0.47 µg/m3 (0.13 ppbv). A control sample
collected in each of the nearby cities of Madison and Green Bay, where
reformulated gasoline use was not mandated, was found to be below the
detection limit of 0.36 µg/m3 (0.1 ppbv).
In the same study, Allen & Grande (1995) collected 1- to 3-h
roadside air samples at busy intersections and freeway interchanges
where it was expected that there would be high concentrations of
gasoline fumes. Mean levels of MTBE in air samples collected near a
freeway interchange, a busy intersection and a roadway in Milwaukee
were 1.9 µg/m3 (0.53 ppbv) (n = 3), 3.8 µg/m3 (1.06 ppbv) (n = 2)
and 1.8 µg/m3 (0.50 ppbv) (n = 2), respectively. This particular
choice of roadside location is a more clear example of a roadside
microenvironment and it may also be considered an ambient air sample.
The State of California mandated the year-round use of oxygenated
fuels in the South Coast Air Basin in 1995 and throughout California
by June 1996. As a result, MTBE was included in the California's
ambient air monitoring programme in February 1996 for the cities of
Burbank, Long Beach and Los Angeles and in June 1996 for Chico,
Roseville and Fresno. The overall range of 24-h average concentrations
was 1.4-44.7 µg/m3 (0.4-12.4 ppbv) for the selected monitoring sites.
The overall range of averages was closer to the Fairbanks data than to
data from other cities in the continental USA. Averages ranged from
4.7 to 17.3 µg/m3 (1.3 to 4.8 ppbv) with the highest averages
occurring in the cities of Los Angeles and nearby Burbank. The
following are average and ranges for each of the individual cities:
Burbank 17.3, 4.7-31.7 µg/m3 (4.8 ppbv, 1.3-8.8 ppbv), Long Beach
9.4, 3.2-21.6 µg/m3 (2.6 ppbv, 0.9-6.0 ppbv), Los Angeles downtown
13.7, 4-24.1 µg/m3 (3.8 ppbv, 1.1-6.9 ppbv), Chico 8.3, 3.6-27.8
µg/m3 (2.3 ppbv, 1.0-7.7 ppbv), Fresno 9.4, 2.2-44.7 µg/m3 (2.6
ppbv, 0.6-12.4 ppbv) and Roseville 4.7, 2.5-12.3 µg/m3 (1.3 ppbv,
0.7-3.4 ppbv). The number of samples for each city ranged from 18 to
28 and the detection limit for MTBE was 0.72 µg/m3 (0.2 ppbv) (M.
Poore, personal communication).
Measurements of ambient air concentrations of MTBE at service
stations are usually taken at the gasoline pump island and at the
service station perimeter. The air levels of MTBE tend to be higher at
the pump island and lower at the perimeter. In addition, service
stations equipped with vapour recovery systems (Stage II) tend to have
higher MTBE concentrations in both microenvironments. Furthermore,
whereas the pump island data represents a particular microenvironment
due to the presence of gasoline vapour coming from refuelling
activities, the gasoline station perimeter data has been used to
estimate potential community exposures and may be considered
representative of upper end ambient air levels in neighbourhoods.
Short-term peak samples of MTBE were not taken.
MTBE median concentrations generally ranged from 0.32 to 21.4
mg/m3 (0.09-6 ppm) in breathing-zone samples as a result of consumer
refuelling. The values were measured over 2- to 15-min sampling
periods and were highly variable but rarely exceeded 35.7 mg/m3 (10
ppm). The range of median values measured at the pump islands ranged
from 0.18 to 1.57 mg/m3 (0.05-0.44 ppm) over a 4-h sampling period.
The fenceline samples were lower and ranged from 0.004 to 0.5 mg/m3
(0.001 to 0.14 ppm) with a collection period of 4 h. Generally, the
concentrations were higher at service stations that did not have
vapour recovery systems.
Allen & Grande (1995) collected 1- to 3-h MTBE area samples
downwind of the gas pumps on the perimeter of four Wisconsin service
station properties from 21 February 1995 to 9 March 1995. The average
levels of MTBE at two service stations that dispensed reformulated
gasoline and had Stage II vapour recoverya were 8.8 µg/m3 (2.43
ppbv) (n = 2) and 2.7 µg/m3 (0.75 ppbv) (n = 2). The level of MTBE at
a station without vapour recovery was higher and was measured to be
16.5 µg/m3 (4.58 ppbv) (n = 1). Finally, one air sample taken at a
service station where reformulated gasoline was not mandated resulted
in a lower concentration of MTBE i.e. 0.8 µg/m3 (0.25 ppbv).
a Stage II is a vapour recovery system used to trap gasoline vapour
during refuelling of consumer vehicles.
In addition, Zweidinger (1993) analysed a limited number of 8-h
air samples taken in various microenvironments in Fairbanks (Phases I,
II and III), Stamford and Albany for MTBE. Generally,
microenvironmental air concentrations of MTBE decreased from Phase I
or II to Phase III at the following locations: (1) service station
pump island, (2) commercial vehicle interiors, (3) indoor - commercial
garage service areas, (4) indoor - residential area and (5) indoor -
public buildings near roadway (i.e. school, post office), due to the
suspension of the oxyfuel programme. The median and range of MTBE
concentrations for selected locations and phases are shown in Table
12. The air of one home with an attached garage was the exception to
this tendency in that the indoor air samples contained levels of MTBE,
benzene and other compounds associated with gasoline which were
significantly higher than air samples measured outside that home. This
indicated that the residential garage may have had a source of
evaporative emissions after parking the hot car in the garage or from
gasoline stored in the garage.
Huber (1995) used a multizonal mass balance model to predict
indoor air concentrations. Measured evaporative emissions of 0.5 g of
MTBE emitted from an automobile at rest at 23.9°C (a highly
unrealistic estimate for Fairbanks in winter) during 4 h in a garage
attached to a residential house resulted in modelled peak
concentrations of 2.3 mg/m3 (0.65 ppm) in the garage and 0.12 mg/m3
(0.035 ppm) in the residence. Modelled 1-h average concentrations in
the garage ranged from 2.5 to 4.3 mg/m3 (0.7 to 1.22 ppm) while those
in the residence ranged from 0.072 to 0.32 mg/m3 (0.02 to 0.09 ppm).
This was estimated to be a worst-case situation for evaporative
emissions since a newer car or cold winter temperatures would probably
have reduced evaporative emission rates resulting in lower
concentrations (Huber, 1995). However, increased tailpipe vehicle
emissions as a result of cold start (the first few minutes of running
the engine before the catalytic converter starts to function) were not
included in these estimations.
In the case of the Stamford microenvironment samples, the
resulting 8-h average MTBE levels in indoor commercial garage service
areas and indoor locations near a roadway were lower than those taken
in Fairbanks even though the volume of MTBE present in Stamford
gasoline was higher than the volume used in Fairbanks during Phases II
and III. It is important to note that the service station pump island
samples were not strictly comparable since they were taken 4.5 m away
from the pump. When MTBE was only used as an octane enhancer, as was
the case for Albany and Fairbanks Phase III, levels of MTBE in service
station pump island air were found to be much higher in Albany.
Air measurements in the parking garage microenvironment have been
conducted in two studies. MTBE concentrations measured in two 8-h air
samples in a Stamford partially open parking lot (i.e. open-sided with
an office building directly above) in April 1993 were 70.4 µg/m3
(20.1 ppbv) and 177 µg/m3 (49.0 ppbv) with a mean of 124.7 µg/m3
(34.6 ppbv) (RB Zweidinger, personal communication). Allen & Grande
(1995) conducted measurements at an enclosed parking ramp in Milwaukee
in February 1995 in order to show ambient levels during cold starts.
One- to three-hour air samples showed MTBE levels of less than 72.1
µg/m3 (20 ppbv) with mean levels of 7.4 µg/m3 (2.05 ppbv) (n = 8).
The highest level occurred at a point when a large number of vehicles
were making cold starts in a short period of time.
Air samples were taken by Lioy et al. (1994) inside the vehicle
cabin microenvironment for (1) activities surrounding refuelling, and
(2) during suburban commutes before and after refuelling. The study
took place in April 1993 in New Jersey, New York and Connecticut where
gasoline containing 10 to 15% by volume MTBE was sold. The experiment
protocol consisted of a 60-min commuter run that included a 5-min
refuelling stop at full- and self-service stations with or without
Stage II vapour recovery. Resulting in-cabin levels of MTBE taken
immediately before, during and after refuelling ranged from 23.8 to
108 µg/m3 (6.6 to 30 ppbv), 133.4 to 313.5 µg/m3 (37 to 87 ppbv) and
31.4 to 151.4 µg/m3 (8.7 to 42 ppbv), respectively, for three
different cars, with average concentrations of 55.3 µg/m3 (14.8
ppbv), 190.2 µg/m3 (55 ppbv) and 72.1 µg/m3 (20 ppbv). Short-term
peak concentrations occurred during refuelling. In addition,
post-refuelling in-cabin concentrations were slightly higher than
pre-refuelling, although the increase was not statistically
significant. It was noted that the highest of the levels that diffused
into the cabin during refuelling occurred with an older vehicle, which
had an abnormally high evaporative emissions rate. There did not seem
to be a statistically significant difference in in-cabin levels
between the various types of service stations, although the sample
size was too small to be able to make this conclusions.
Microenvironmental in-cabin air concentrations of MTBE measured during
60-min suburban stop/go commutes ranged from 3.6 µg/m3 (1 ppbv) to
576.6 µg/m3 (160 ppbv) with a geometric mean of 21.6 µg/m3 (6 ppbv)
(n = 40). Most values were less than 19.8 µg/m3 (5.5 ppbv). It was
noted that the higher values were associated with the use of the
high-emission vehicle discussed previously.
Area monitoring was conducted in an indoor commercial garage
service area in Fairbanks, Alaska (Buchta 1993a). Three air samples
were taken over a 6- to 7-h period in February 1993 when MTBE use was
limited to purposes of octane enhancement. MTBE was non-detectable in
the three areas (service area, parts department, shop wall) but the
minimum detection limit was high (144.2 µg/m3, 40 ppbv). This is
comparable to the Zweidinger (1993) 8-h indoor commercial garage air
samples that were measured with more sensitive equipment and resulted
in a median concentration for MTBE of 114.4 µg/m3 (31.75 ppbv). The
higher levels in the latter study may have been the result of gasoline
spills.
Ambient air levels of MTBE have been measured near three
refineries in the USA. At one refinery a 24-h MTBE level of 20 µg/m3
was reported for one out of nine downwind samples taken at the
perimeter of a rural refinery, which was stated to release
approximately 33 tonnes of MTBE emissions in air per year. MTBE was
not detected in the 26 other downwind and upwind samples taken at the
refinery during the same period. MTBE was not detected in another 54
24-h samples taken at two other refineries: annual MTBE air emission
release data were not provided for these refineries. It should be
noted that the detection limit for these air samples was high (more
than 20 or 30 µg/m3), but more sensitive canister samples taken for
24 h also resulted in non-detectable concentrations (detection limit =
6 µg/m3) (API, 1989b).
c) Finland
Vainiotalo et al. (1996) reported the concentration of MTBE at
the perimeter and pump island of two self-service stations in Finland
(one urban roadside and one simple roadside) both with Stage Ia
vapour recovery systems where gasoline containing 11% MTBE by volume
was sold. The investigations were conducted during May/June and
October 1995. The average 24-h perimeter concentrations for each of
the 4-day sampling periods were generally higher for the urban
roadside service station samples: 12.4 µg/m3 (June) and 14.1 µg/m3
(October), with 35-36 measurements collected at each side on each
occasion. Several factors such as the volume of gasoline sold, mean
wind speed and number of deliveries of gasoline to the station were
higher for the former and resulted in data with higher variability
during the fall sampling. The overall range of perimeter air samples
for both service stations was from 0.5 to 120.5 µg MTBE/m3. Highest
daily concentrations were usually obtained at the downwind sampling
points. No seasonal influence was discernable. Mean 24-h
concentrations measured in the centre of the pump island ranged from
247 to 1347 µg/m3 (n = 15). The detection limit was not specified in
the study.
5.1.1.2 Dermal exposure
Dermal exposure and absorption may occur from MTBE-blended
gasoline at self-service refuelling or from its use as a solvent. It
may also occur from MTBE-contaminated household water during washing,
bathing or showering. There are, however, no data available to
estimate dermal exposure to MTBE.
5.1.1.3 Estimation of total personal exposure
Exposure is a function of concentration and time. Thus, the time
spent in various activities involving different concentrations and
degrees of contact will affect human exposures to MTBE. Huber (1995)
generated "worst-case scenario" estimates of long-term exposure to
MTBE based on population activity patterns and available
microenvironmental and ambient concentration data (generally rounded
a In the USA, Stage I is a vapour recovery system used during
loading and unloading of gasoline from delivery tankers. Since
this is a Finnish Study, this definition may or may not be
equivalent.
up to the next order of magnitude of 10). His intentionally high
estimates were updated and slightly revised to indicate that an annual
time-weighted average exposure might be as high as about 0.11 mg/m3
(0.03 ppm), assuming that gasoline contained 15% MTBE by volume for 6
months and approximately 10% for the remainder of the year (US
Interagency Assessment, 1997). This upper-end exposure estimate is
highly uncertain, given the lack of adequate data to describe the
distribution of actual personal exposure levels.
5.1.1.4 Other pollutants
There was an increase in indoor air levels of benzene after MTBE
reformulated fuel use was discontinued (Gordian & Guay, 1995). Both
ambient outdoor and specific indoor environment (garages, vehicles,
workplace, school, post office and residence) samples, as well as
blood samples, were collected in Fairbanks during and after the
oxygenated programme. In addition to MTBE, other volatile compounds,
such as benzene and formaldehyde, were evaluated. In indoor samples,
there was a statistically significant increase of benzene in garages
and non-garages after MTBE was discontinued. In garages, the mean
benzene concentration increased from 0.30 mg/m3 (94.02 ppb) in
December to 0.61 mg/m3 (191.62 ppb) in February, and in the school,
post office and residence from 0.02 mg/m3 (5.89 ppb) to 0.06 mg/m3
(20.22 ppb). The NIOSH-recommended exposure limit for benzene is 0.3
mg/m3 (0.1 ppm). In vehicles the data were difficult to interpret
because different vehicles were tested in December and in February.
The same pattern, although not statistically significant, was seen in
the outdoor samples.
5.2 Occupational exposure
5.2.1 Industrial operations - manufacturing and blending
MTBE can be encountered in solution and as vapour during its
manufacturing at chemical plants and refineries, during blending into
gasoline, transportation, distribution, and handling at service
stations. Some industrial hygiene monitoring data are available (Table
13).
At the MTBE unit at the Neches Chemical West Plant in the USA,
the TWA values ranged from less than 0.07 to 120.3 mg/m3 (0.02 to
33.41 ppm) for operations and maintenance personnel (Simer, 1986).
Exposure monitoring in a manufacturing plant showed that all
exposures to MTBE greater than 3.6 mg/m3 occurred during quality
control sampling procedures (ARCO, 1987). Two out of 46 short-term
samples indicated exposures to MTBE at concentrations of 22 mg/m3 and
6.5 mg/m3 (6.1 ppm and 1.8 ppm, respectively); 91% of the full shift
monitoring indicated exposure levels of less than 3.6 mg/m3.
Texaco (1993) presented results from a short-term monitoring for
MTBE conducted at a refinery in Guatemala in order to evaluate the
adequacy of current work procedures. No details of ambient temperature
Table 13. Occupational exposure to MTBE in the petroleum industry (adapted from HEI, 1996)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
MTBE manufacturing
Occupational exposure of
oil refinery and chemical
plant personnel handling
neat MTBE during:
Routine operations <30 min 14/27 0.16-1.00 0.16-7.8 1.00 API, 1995b
6-9 h TWA 38/76 0.01-0.03 0.01-248.7 0.03 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.17 0.17 API, 1995b
Routine maintenance <30 min 7/8 0.05 0.05-7.19 0.90 API, 1995b
30 min-6 ha 1/1 Not given 0.20 0.20 API, 1995b
6-9 h TWA 4/4 Not given 0.04-0.7 0.11 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.2 0.18 API, 1995b
Routine operations and
maintenance 8-12 h TWA 8/21 0.02-0.06 <0.02-33.41 1.06 Simer, 1986
20 min 0/1 [1.0] <1.0-1.0 <1.0 ARCO, 1987
4-6 h 1/11 [1.0] <1.0-6.1 <1.0 ARCO, 1987
12 h TWA 2/23 [1.0] 0.8-2.2 Not given ARCO, 1987
QA/QC sampling of MTBE 12-36 min 3/16 [1.0] <1.0-12.2 <1.0 ARCO, 1987
MTBE blending
Occupational exposure of
personnel involved in
fuel-blending activities
involving:
Neat MTBE <30 min 34/35 <0.005 0-97.0 2.90 API, 1995b
30 min-6 h 12/13 0.21 0.21-72.0 1.03 API 1995b
6-9 h TWA 7/12 0.04-1.80 0.04-87.97 2.24 API, 1995b
>9 h TWA 0/9 0.23-0.34 0.23-0.34 0.30 API, 1995b
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
Fuel mixtures <30 min 51/98 0.02-0.23 0.02-100 0.30 API, 1995b
30 min-6 h 5/19 0.03-0.33 0.03-1.98 0.05 API, 1995b
6-9 h TWA 34/112 0.02-0.20 0.02-14 0.04 API, 1995b
>9 h TWA 9/22 <0.005-0.02 0-0.27 0.02 API, 1995b
MTBE transport
Occupational exposure of
marine barge, pipeline and
rail car personnel to:
Neat MTBE <30 min 62/66 0.30-0.60 0.30-1050 13.83 API, 1995b
(trucking personnel included 30 min-6 h 23/27 0.04-0.36 0.04-700 2.20 API, 1995b
only for transport of neat 6-9 h TWA 9/10 0.03 0.03-711.9 0.18 API, 1995b
MTBE) >9 h TWA 1/1 Not given 0.32 0.32 API, 1995b
15 min TWA 4/4 Not given 90-150 110.00 Texaco, 1993
Fuel mixtures <30 min 60/64 0.001-0.14 0.001-507.87 2.44 API, 1995b
30 min-6 h 64/92 0.02-0.04 0.02-59.4 0.42 API, 1995b
6-9 h TWA 28/42 0.007-0.04 0.01-26.24 0.14 API, 1995b
>9 h TWA 8/8 Not given 0.19-4.51 1.49 API, 1995b
MTBE distribution
Occupational exposures of <30 min 93/129 <0.005-0.08 0-14.0 0.75 API, 1995b
marketing terminal and 30 min-6 h 9/10 0.26 0.26-4.05 0.98 API, 1995b
trucking personnel involved 6-9 h TWA 62/87 0.01-0.05 0.01-2.2 0.11 API, 1995b
in the handling of >9 h TWA 46/47 0.06 0.06-6.2 0.71 API, 1995b
Gasoline-MTBE mixtures 15 min TWAe ´ 0.2 <0.2-0.94 0.48 Hebert, 1993
10-12 h TWAe 2/2 Not given 0.08-0.08 0.08 Hebert, 1993
15 min TWAf 4/4 Not given 0.05-0.16 0.14 Gillie, 1993
15 min TWAg 3/3 Not given 1.9-3.6 2.80 Gillie, 1993
12 h TWAg 5/5 Not given 0.24-0.92 0.45 Gillie, 1993
15-40 minf (n=6) 0.2 2.8-42 13 (mean) Hakkola & Saarinen,
1996
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
10-30 minh (n=4) 0.2 20-226 91 (mean) Haakola & Saarinen,
1996
22-44 mini (n=5) 0.2 4.3-27 16 (mean) Haakola & Saarinen,
1996
10-37 minj (n=6) 0.2 10.0-98.0 71 (mean) Haakola & Saarinen,
1996
a Duration was task-related.
b Number of samples in which MTBE was detected divided by total number of samples.
c In the case of "non-detectable" samples the detection limit was used to calculate the median.
d The API (1995b) measurements used different sampling and analytical techniques on both personal breathing zone and area air samples.
e Loading of trucks with vapour recovery.
f Bottom loading of trucks with vapour recovery.
g Truck unloading at service station with vapour recovery.
h Top loading without vapour recovery.
i Truck unloading at service station without vapour recovery - Northern Finland.
j Truck unloading at service station without vapour recovery - Southern Finland.
were given. Air concentrations associated with transfer of neat MTBE
from tank cars to a storage tank ranged from 324 to 540 mg/m3 (90-150
ppm).
Hinton (1993) performed an occupational exposure study of MTBE
employees designed to determine the amount of MTBE exposure during
manufacturing, blending MTBE into gasoline, transportation,
distribution and handling at service stations. The study included 2038
exposure measurements during an 11-year period. Occasionally the TWA
exposure value exceeded 360 mg/m3 (100 ppm) and the short-term (less
than 30 min) exposure 1080 mg/m3 (300 ppm), generally during
non-routine or extraordinary tasks. The maximum short-term value
sampled for less than 30 min, 3780 mg/m3 (1050 ppm), was recorded
during transportation of neat MTBE. The maximum TWA level was 2563
mg/m3 (712 ppm) for the same activity. Usually, the TWA levels were
less than 7.2 mg/m3 (2 ppm) and the short-term levels were less than
36 mg/m3 (10 ppm). Exposures in blending operations were less than
360 mg/m3, and generally less than 36 mg/m3. In distribution, MTBE
levels were less than 3.6 mg/m3, and for service station attendants
levels were less than 10.8 mg/m3 (3 ppm).
5.2.2 Transportation
An exposure assessment for MTBE vapour concentrations conducted
on two gasoline truck drivers in New Jersey showed an average exposure
concentration of 0.29 mg/m3 (0.08 ppm) on a full shift (10-12 h)
basis. The short-term 15-min TWA exposure was 2.05 mg/m3 (0.57 ppm)
(Hebert, 1993).
Monitoring has been made for workplace concentration levels for
seven gasoline truck operators to assess exposure potentials during
loading operations and during full unloading at service stations
(Gillie, 1993). The 12-h TWA ranged from 0.86 to 3.31 mg/m3
(0.24-0.92 ppm). Short-term exposure (5-23 min) during truck loading
operations ranged from 0.18 to 0.58 mg/m3 (0.05-0.16 ppm) and during
fuel unloading from 3.24 to 12.96 mg/m3 (0.9-3.6 ppm).
The occupational exposure of road tanker drivers to gasoline and
some of its components, including MTBE, has been measured in Finland
in two depots and 11 service stations during loading and delivery
(Hakkola & Saarinen, 1996). In Finland, unleaded gasoline contains
10-15% MTBE in liquid phase. The monitoring was made during the
summer, and the temperatures ranged from 4 to 22°C. In the south of
Finland, four measurements were carried out during top loading and six
measurements during delivery at service stations. In the north of
Finland, six measurements were performed during bottom loading and
five measurements at service stations during delivery. The mean
short-term exposures of road tanker drivers to MTBE during loading and
delivery were between 13 and 91 mg/m3. The differences in exposure
during bottom (2.8-42.0 mg/m3, mean 13 mg/m3) and top loading
without vapour recovery (20-226 mg/m3, mean 91 mg/m3) were
statistically significant (p<0.02). There also was a statistically
significant difference (p<0.03) during delivery in northern (4.3-27.0
mg/m3, mean 16.0 mg/m3) and southern Finland (10-98 mg/m3, mean 71
mg/m3). The exposure time for loading was 25-35 min and for
delivering 30-40 min per load.
5.2.3 Service station attendants and garage mechanics
An exposure assessment for MTBE vapour concentrations conducted
on six full-service gas attendants in New Jersey showed an average
exposure of 1.76 mg/m3 (0.49 ppm) on an 8-h TWA basis (Hebert, 1993).
For the short-term 15-min TWA, the average exposure was 2.16 mg/m3
(0.60 ppm).
In an evaluation of exposure among service station attendants and
operators, Hartle (1993) compared the exposure potential at three
categories of service stations. Two facilities in Cincinnati, Ohio,
represented service stations that did not use MTBE or used it only as
an octane enhancer. In Phoenix, Arizona, two high-volume stations were
selected, and in Los Angeles two service stations with advanced vapour
recovery were selected. In Phoenix, where the MTBE content averaged
12.5-13% by liquid volume, the exposure measurements (41 samples)
ranged from 0.14 to 13.97 mg/m3 (0.04-3.88 ppm) with an average of
1.08 mg/m3 (0.3 ppm). The Los Angeles exposure ranged from 0.07 to
2.63 mg/m3 (0.02-0.73 ppm), averaging 0.50 mg/m3 (0.14 ppm). In
Cincinnati, only one of 32 samples was above the analytical limit of
detection, i.e. 0.58 mg/m3 (0.16 ppm).
Giacomello (1996) measured personal exposure of "full service"
attendants to MTBE in 58 Italian service stations. The study included
a number of geographical locations throughout the country and was
conducted in the summer and winter in 1992 and 1995. An overall
geometric mean of 0.71 mg/m3 (1992) or 0.26 mg/m3 (1995) was
recorded in the summer, and 0.37 mg/m3 in winter (latter data only
for 1992).
Three NIOSH studies were performed in workers potentially exposed
to gasoline and exhaust emissions during their work day (Almaguer,
1993; Buchta, 1993a,b). Breathing zone air samples were collected from
workers exposed to MTBE and other gasoline components (benzene,
toluene, and xylene, and, in one study, carbon monoxide) in several
maintenance facilities for motor vehicles located in Fairbanks, Alaska
(Buchta 1993a), in Stamford, Connecticut (Buchta, 1993b), and in
Albany, New York (Almaguer, 1993). In two of the cities (Fairbanks and
Albany), MTBE was only used as an octane enhancer (generally less than
1% of the fuel) during the study period. In Stamford, however, the
MTBE content of the fuel ranged from 13 to 17% with an average of
14.2% by volume. The highest workplace exposure level concentrations
were less than 0.50 mg/m3 (0.14 ppm) in Albany and less than 1.6
mg/m3 (0.45 ppm) in Fairbanks. In Stamford, the MTBE exposure levels
ranged from 0.1 to 44.6 mg/m3 (0.03 to 12.04 ppm). The cause for the
highest value was unknown, and the next highest exposure value was
7.56 mg/m3 (2.1 ppm). In all of the studies, the highest
concentrations were measured on mechanics. The sampling was, however,
conducted in late spring, and dilution ventilation (open windows and
doors) may have affected the results.
5.2.4 Occupational exposure limit values
In the USA, the American Conference of Governmental Industrial
Hygienists (ACGIH, 1994) has recommended a TWA of 144 mg/m3 (40 ppm).
In Sweden, the occupational air exposure TWA limit is 180 mg/m3 (50
ppm) and the 15-min short-term exposure limit (STEL) 250 mg/m3 (75
ppm) (AFS, 1994). The Dutch Expert Committee on Occupational Standards
recommended a health-based occupational 8-h TWA exposure limit of 180
mg/m3 (50 ppm) (DECOS, 1994).
5.3 Exposure via water
MTBE has been found in groundwater, storm water, reservoir water,
and drinking-water in the USA (Garrett et al., 1986; Angle, 1991; Dey
et al., 1991; Post, 1994; Squillace et al., 1995a,b, 1996; Delzer et
al., 1996; Dale et al., 1997; US Interagency Assessment, 1997).
Collectively, these references show that MTBE occurs in water,
especially in areas where MTBE is extensively used, and where releases
of MTBE to air, water and soil occur.
However, while there are some national monitoring data for
ambient groundwater, monitoring data for MTBE in surface water and in
drinking-water in the USA are very limited in scope. In an extensive
review of MTBE in water in the USA, Zogorski et al. (1996) concluded
that sufficient monitoring data were not available to characterize
human exposure to MTBE by the consumption of drinking-water.
The following subsections summarize major findings for MTBE in:
(a) snow and precipitation; (b) surface water; (c) groundwater; and
(d) drinking-water.
5.3.1 Snow and precipitation
MTBE has been detected in snow at ground level in Denver,
Colorado, USA, at very low (water equivalent) concentrations
(Squillace et al., 1995b; Bruce & McMahon, 1996). There is no other
published monitoring information on the presence or concentration of
MTBE in snow or rainfall. Squillace et al. (1996) hypothesized that
concentrations of MTBE in precipitation would be greater during winter
months than warmer summer months due to the temperature effect on
air-water partitioning.
5.3.2 Surface water
Information on MTBE in streams and rivers, in Long Island, New
York and New Jersey, USA, has been reported by Stackelberg et al.
(1997). For Long Island, at a reporting level of 0.5 µg/litre, MTBE
was the second most frequently detected VOC, occurring in 29% of the
samples at concentrations ranging from 0.6 to 20 µg/litre, with an
estimated median of 0.24 µg/litre. MTBE was detected more frequently
in samples collected during winter months (33%) than summer months
(26%). In New Jersey, a limited study was completed in spring 1994
along a ten-mile reach of the Hackensack River. Land use along this
reach is highly urbanized, and numerous industries and municipal
effluents are present. The study involved the collection of a single
water sample at each of 14 sampling points just after a major snow
melt. MTBE was detected in all samples at concentrations ranging from
2.6 to 30 µg/litre, with a median of 7.75 µg/litre (Stackelberg et
al., 1997). Reconnaissance sampling of eight streams elsewhere in New
Jersey in 1996 showed the presence of MTBE in water samples for seven
of eight sites. The concentrations ranged from 0.2 to 4.9 µg/litre
(Stackelberg et al., 1997). MTBE is used in reformulated gasoline at
both Long Island, New York and in New Jersey, as part of a mandatory
air abatement programme.
Measurable but low concentrations of MTBE were found in some of
592 stormwater samples (including samples from culverts, concrete
pipes, lined ditches and channels) collected by the US Geological
Survey in 16 cities and metropolitan areas from 1991 to 1995 (Delzer
et al., 1996). MTBE was found in 6.9% of the samples (41 of 592
samples). When detected, concentrations ranged from 0.2 to 8.7
µg/litre, with a median below 1.0 µg/litre. Eighty-three percent of
the detections occurred during the winter season (October to March)
when oxygenated gasoline to abate CO pollution was expected to be
used. A comparison of MTBE concentrations for samples collected during
the summer and winter periods showed a statistically significant
difference. Twenty-seven out of 148 stormwater samples contained both
MTBE and BTEX compounds, indicating a common source for these samples.
The Metropolitan Water District of Southern California (MWDSC)
relies, in part, on six lake-reservoirs for the storage of raw water
to be used for drinking-water in Southern California. These reservoirs
have varying degrees of recreational use. MWDSC began quarterly
monitoring of these reservoirs for MTBE in the second quarter of 1996.
MTBE was detected at the surface of Lake Perris, with confirmation in
two subsequent samplings, at an average concentration of 15 µg/litre.
The following quarter MTBE was also detected at an average level of 19
µg/litre. No MTBE was detected above the study's reporting level of 1
µg/litre in any of the other reservoirs, which were sampled at outlet
towers, where water is drawn from a lower depth (Dale et al., 1997).
5.3.3 Groundwater
Data from urban and agricultural areas show that MTBE occurs
predominantly in shallow groundwater underlying urban areas, and, when
present, occurs typically at low concentrations. In 1993-1994, the US
Geological Survey measured the concentrations of MTBE and 59 other
VOCs in 210 shallow wells (five drinking-water wells, 12 springs and
193 monitoring wells) in eight urban areas and 549 shallow groundwater
wells from 21 agricultural areas, and deeper groundwater from 412
wells sampled in nine areas throughout the USA (Squillace et al.,
1995a,b, 1996). MTBE occurred in 27% of the shallow urban wells and
springs. Detectable levels of MTBE were found in 86% of the wells in
industrial areas, 31% of the wells in commercial areas, 23% of the
wells in residential areas, and 23% of the wells in areas of mixed
urban land use, parks and recreation areas. MTBE was the second most
frequently detected compound after trichloromethane (chloroform). In
73% of the 210 shallow urban wells, concentrations were less than the
reporting level of 0.2 µg/litre. The estimated median value for urban
areas was below 0.2 µg/litre (Squillace et al., 1996). Three percent
of the wells had concentrations of MTBE above 20 µg/litre. No MTBE was
detected in drinking-water wells in the urban areas. In the
agricultural areas, 1.3% of the 549 shallow agricultural wells sampled
had detectable concentrations of MTBE. MTBE was also detected in four
of the 412 deeper groundwater samples from major aquifers. Three of
these wells were used for domestic or municipal water supply. The
measured maximum concentration of MTBE was 1.3 µg/litre. MTBE in
groundwater was generally not found with BTEX compounds, which
commonly are associated with point source spills of gasoline.
Bruce & McMahon (1996) measured MTBE concentrations in
groundwater in the alluvial aquifer beneath Denver, Colorado, USA, as
part of a survey of groundwater quality examining a range of dissolved
constituents. Thirty randomly selected alluvial wells were sampled.
MTBE was the most frequently detected VOC (23 out of 29 wells). The
maximum concentration was 23 mg/litre.
5.3.4 Drinking-water
Exposure to MTBE via drinking-water may involve more than direct
ingestion of contaminated water. Household uses of water, such as in
cooking, showering, bathing and washing, could result in exposure
through inhalation and dermal absorption, even if ingestion of water
was avoided.
Only limited monitoring data are available for MTBE in
drinking-water sampled at the tap or from a municipal distribution
system. Stern & Tardiff (1997) estimated that about 30% of the US
population lives in areas where MTBE is in regular use; 95% of this
population is unlikely to be exposed to MTBE in tap water at
concentrations exceeding 2 µg/litre, most will be exposed to much
lower or zero concentrations, but 5% could be exposed to higher
concentrations due to fuel tank spills and leaks entering surface and
groundwater. As part of the US Interagency Oxygenated Fuel Assessment
in the USA (US Interagency Assessment, 1997), information on MTBE
levels in drinking-water was sought from state drinking-water agencies
on a voluntary basis by the US Environmental Protection Agency.
Because monitoring for MTBE in drinking-water is not required by the
US Federal Government, only a few states have information on MTBE in
drinking-water. As such, it is not possible to describe levels of MTBE
in drinking-water for the entire USA. Based on information provided by
five states (New Jersey, Iowa, Illinois, Texas and Colorado), MTBE has
been detected in the drinking-water of 51 public water systems.
However, when detected, the concentration of MTBE was generally low
and nearly always below 20 µg/litre (Zogorski et al., 1996). These
data indicated that the consumption of drinking-water was not a major
route of exposure for these few systems (Zogorski et al., 1996). No
data on MTBE in drinking-water are available for other countries.
As noted previously, there have been a few instances in the USA
where groundwater used for drinking-water, both private wells and
public water systems, has become contaminated with levels of MTBE in
excess of 1000 µg/litre (Garrett et al., 1986; State of Connecticut,
1987; Zogorski et al., 1996). Many humans will probably detect MTBE in
drinking-water when the concentration exceeds about 50-100 µg/litre
owing to its low taste and odour threshold (see section 2.1 for taste
and odour values). Some humans will detect MTBE in water at even lower
concentrations. However, Du et al. (1998) considered that an MTBE
concentration below 40 µg/litre in drinking-water would avoid any
unpleasant taste or odour even for the most sensitive members of the
population.
5.4 Soil and sediment
There are very limited data concerning levels of MTBE in the
terrestrial environment. Trace amounts of MTBE have been found in
sediment samples adjacent to motorways and centres of heavy urban road
traffic density in the United Kingdom (Bianchi & Varney, 1989).
5.5 Biota
There are very limited data on MTBE levels in biota. In a study
to detect organic and inorganic contaminants in shellfish in Nova
Scotia, Canada, MTBE was not detected in any of the 21 samples assayed
(detection limit = 0.01 µg/g) (Environment Canada, 1989).
6. KINETICS AND METABOLISM IN HUMANS AND LABORATORY ANIMALS
Kinetic data from human and animal studies are summarized in
Table 14.
6.1 Human data
6.1.1 Controlled human studies
In an inhalation study two healthy young adult male and two
healthy young adult female volunteers were exposed to MTBE at 6 mg/m3
(1.7 ppm) in an environmental chamber for 1 h. The mean blood level
rose from 0.83 ± 0.5 µg/litre (0.009 µmol/litre) preexposure to 17.1 ±
2.0 µg/litre (0.19 µmol/litre) at the end of the 1 h exposure period.
One hour after the end of exposure the mean blood level fell to 6.3 ±
1.6 µg/litre (0.07 µmol/litre) after 1 h (Cain et al., 1996).
In a pharmacokinetics study, two volunteers (one healthy young
adult male and one healthy young adult female) were exposed to
5 mg/m3 (1.394 ppm) for 1 h in an environmental chamber (see also
section 8.2). There was a rapid rise in blood MTBE concentration to
6.1 µg/litre (8.2 ppb) and 10.9 µg/litre (14.7 ppb), respectively, at
1 h from the start of exposure. Following the end of exposure, there
was a rapid decline in blood MTBE concentration in both the male and
female volunteer with half-lives of 36 and 37 min, respectively. By
the end of the 7-h sampling period, blood MTBE concentration had
fallen to 0.149 µg/litre (0.2 ppb) in the male volunteer and 0.447
µg/litre (0.6 ppb) in the female volunteer (Prah et al., 1994).
In another study, the area under the curve (AUC) values of MTBE
and TBA were proportional to the MTBE exposure levels following short-
term inhalation to 18, 90 or 180 mg/m3 (5, 25 and 50 ppm), indicating
linear kinetics up to at least 180 mg/m3 (Johanson et al., 1995).
Following exposure to 180 mg MTBE/m3, the elimination of MTBE and TBA
was complete within 24 and 48 h, respectively (Johanson et al., 1995).
Pekari et al. (1996) measured concentrations of MTBE in blood,
urine and exhaled air from four volunteers exposed to 90 or 270 mg/m3
(25 or 75 ppm) by inhalation for 4 h. A lung retention of around 40%
was recorded, and blood levels of 11 µmol/litre (970 µg/ litre) at 90
mg/m3 or 29 µmol/litre (2556 µg/litre) at 270 mg/m3 were achieved
towards the end of the exposure period. Of the MTBE absorbed, the
majority (about 58%) was excreted unchanged in expired air and small
amounts (1.4%) unchanged in urine. The concentration of TBA in blood
reached a peak of 16 or 34 µmol/litre (1419 or 2997 µg/litre)
(following the low or high exposure, respectively) 15-45 min after
exposure ceased. Trace amounts of TBA (1.2%) were found in urine, but
none was detected in exhaled air. The terminal half-life for MTBE in
blood was determined to be 5 h, while that for TBA was 11.9 h. The
authors concluded that metabolism of MTBE was linear at exposures up
to 268 mg/m3 (75 ppm).
Table 14. Summary of kinetic data for MTBE
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
A. Human
2 male, 2 female rapid absorption mean MTBE blood Cain et
volunteers from 0.003 concentrations rose al. (1996)
Treatment: 6 mg/m3 µg/litre preexposure steeply from 0.83
(1.7 ppm) for 1 h to 0.06 µg/litre ±0.50 µg/litre
in inhalation after 1 h preexposure to a
chamber peak blood level of
17.1±2.01 µg/litre
by the end of
exposure; rapid
elimination, half-life
of 40 min; the rapid
elimination phase
appeared to last
approximately 1 h
1 male, 1 female rapid rise in blood rapid decline observed Prah et
volunteer MTBE to 0.03 µg/litre in blood levels with al. (1994)
Treatment: 5 mg/m3 (8.2 ppb) in the male a half-life of about
(1.39 ppm) as a 1-h and 0.05 mg/m3 35 min and rapid
exposure; blood (14.7 ppb) in the metabolic transformation
samples up to 580 female to TBA; TBA levels
min after start gradually increased and
plateaued at 0.025-0.36
µg/litre (7-10 ppb) and
maintained this
concentration up to 7 h
post-exposure
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult relative MTBE detected in clearance of MTBE Johanson et
male volunteers respiratory uptake expired air; both was 0.5 litre/h per al. (1995)
Treatment: exposed was 32-42%; rapid MTBE and TBA found kg; AUC values of
in chamber to 18, absorption based in blood and urine MTBE and TBA were
90 and 180 mg/m3 upon blood proportional to
(5, 25 and 50 ppm) concentration; by exposure levels,
for 2 h on three 2 h, peak blood suggesting linear
occasions during concentrations of kinetics; elimination
light physical MTBE were 1.3, of MTBE in blood
exercise (50W); 6.3, and 12.2 indicated three
observed up to µmol/litre at 18, phases (6-7 min,
24 h and 48 h 90, and 180 mg/m3, 46-58 min and
(only 180 mg/m3) respectively; 6.2-7.2 h); in
post-exposure TBA 5.7 and 0.7 urine half-lives
µmol/litre at 90 of 16-22 min and
and 180 mg/m3, 3.0-3.1 h were
respectively; steady identified;
state was reached half-life of TBA
for TBA after 3-5 h, in urine was
but was not reached 7.5-8.9 h; by 3.5 h
during the 2 h post-dose, 20-30%
exposure to MTBE of absorbed dose
was eliminated in
expired air; by
24 h, approx. 0.1%
MTBE and 0.5-0.8%
TBA of absorbed
dose was eliminated
in urine
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult respiratory uptake mean concentrations TBA was found in respiratory Nihlén et
male volunteers was 42-49% in blood at the blood and urine exhalation was al. (1998a)
Treatment: exposed three exposure 32-47%; elimination
in chamber to 18, concentrations were in blood was in
90 and 180 mg/m3 1.4, 6.5, and 13 four phases of 1
(5, 25 and 50 ppm) µmol/litre min, 10 min, 1.5 h,
for 2 h on three respectively and and 19 h; kinetics
occasions during were concentration- were linear up to
light physical related the exposure
exercise (50W); concentration of
blood and urine 180 mg/h;
collected during elimination in
exposure and for 3 urine was biphasic
days post-exposure with mean half-lives
of 20 min and 3 h;
excretion was nearly
complete 10 h after
exposure; metabolic
clearance was
0.34-0.52 litre/h
per kg; renal
clearance of TBA
was 0.6-0.7 ml/h
per kg and TBA was
still present after
22 h
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
B. Rodent
I) Oral administration
Species: rapid and extensive apparent volume of in plasma, the dose-related Ferdinandi
Fischer-344 rats absorption of MTBE distribution was major metabolite, differences were et al. (1990a)
Treatment: 40 based upon peak 0.27 to 0.43 litre TBA, peaked at 2 h; observed for the Miller et
rats/sex/dose group plasma concentration male rats had higher plasma elimination al. (1997)
received 40 or 400 within 15 min of plasma concentrations half-time (T´) of
mg/kg as single dosing; lower plasma than female rats; MTBE and TBA:
dose; observed concentrations of dose-unrelated plasma T´ of MTBE:
(sacrificed) for MTBE in female rats concentrations of 0.52-0.62 h (low
various time-points compared to male MTBE and TBA indicate dose) 0.74-0.88 h
until 36 h rats; higher AUC enzyme saturation (high dose) T´ of
post-dose values of MTBE and TBA: 0.95-1.0 h
TBA with oral dosing (low dose)
compared to 1.6-1.9 h (high
intravenous dose) Plasma
administration clearance (CL) of
MTBE:
male rats:
0.36-0.41 litre/h
(both dose-groups)
female rats:
0.48 litre/h (low
dose) 0.29 litre/h
(high-dose)
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: by 48 h, 1.7-3% of higher radioactivity slight sex difference Ferdinandi
Fischer-344 rats administered was recovered in in the route of et al. (1990b)
Treatment: 6 radioactivity was lungs at high oral elimination; by 48 h, Miller et
rats/sex/dose group recovered in dose and lower recovery was 46-54% al. (1997)
received 40 or 400 carcass and tissues; activity in kidneys at low dose and
mg/kg 14C-MTBE as 86 and 81% of suggesting enzyme 65-69% at high dose
a single dose; administered saturation; most of (highest in females);
observed for 48 h radioactivity was the administered 29-36% was recovered
recovered in lungs dose was exhaled as in urine at low dose
and kidneys at low unchanged MTBE and 11-16% at high
and high dose, (predominating) and dose (highest in males)
respectively TBA (2.5-3.1% at low less than 1% was
dose and 1.3-1.4% at recovered in faeces
high dose); in urine,
the major radiolabelled
species (from further
oxidation of TBA) were
2-methyl-1,2-propanediol,
alpha-hydroxyisobutyric
acid, and two minor
unidentified components;
no sex differences in
biotransformation
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
ii) Inhalation
Species: Wistar dose-related MTBE was metabolized Savolainen
rats concentrations of at the ether bond. et al. (1985)
Treatment: 5 male MTBE and TBA in Two weeks of exposure
rats/dose group blood after 2 weeks caused transient
were exposed to of exposure; the increase of the
180, 360 or 1080 concentration of microsomal UDP
mg/m3 (50, 100 or MTBE decreased glucuronosyl-transferase
300 ppm) vapour in after 6 weeks; activities in liver and
exposure chambers the concentrations kidney at all dose
6 h/day, 5 days/week of TBA increased levels. After an initial
for 2, 6, 10 or 15 after 6 weeks of decrease the muscle
weeks exposure and began creatinine kinase
to decrease after activity gradually
10 weeks. increased towards the
Brain levels of MTBE end of the exposure.
and TBA followed a A minor induction of
similar course. MTBE kidney cytochrome
was also found in P-450 was noted. Almost
perirenal fat and at no effect was found on
higher concentrations hepatic cytochrome
than in blood or brain. P-450 concentrations,
Concentrations of MTBE brain succinate
in the blood, brain dehydrogenase, creatine
and perirenal fat were kinase, or
directly related to the acetylcholinesterase
concentrations in the activities
inspired air. The
ratios of TBA/MTBE in
blood increased from
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
week 2 to week 15,
indicating that MTBE
had been oxidized to
TBA, which was
eliminated from the
blood at a slower rate
than MTBE
Species: (T1) rapid absorption (T1) apparent volume (T1) increased AUC Ferdinandi
Fischer-344 rats based upon plasma of distribution: values of MTBE et al.
Treatment: concentration; peak low dose: 35-fold (males) and (1990c)
blood concentration 0.40 (females) and 37-fold (females) Miller et
(T1) 52 rats/sex/ was reached within 0.52 litre (males) while the AUC al. (1997)
dose group received 4-6 h for MTBE and high dose: values of TBA
1440 or 28 800 6.5 h for TBA; low 0.25 (males) and increased by 15-fold
mg/m3 (400 or 8000 but statistically 0.24 litre (females) (males) and 7-fold
ppm) as a single significant (females); quotients
6-h dose. difference in sexes of repeated and
of MTBE AUC values single dose AUC
(T2) 40 rats/sex (low dose); TBA plasma values (T2/T1) for
were exposed 6 h concentration (AUC) MTBE were 0.64
daily to 1440 mg/m3 was lower in female (males) and 0.53
(400 ppm) for 15 rats compared to male (females) and for
days; observed rats, statistically TBA 1.1 (males) and
(sacrificed) at significant at high 1.3 (females); a
various time-points dose significant
until 12 h (T1) and difference in plasma
18 h (T2) post-dose clearance between
single doses 0.53
(males) and 0.57
(females) litre/h
(low dose) and 0.30
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
(males) and 0.32
(females) litre/h
(high dose); the
plasma half-life of
MTBE was 0.52-0.63 h
(T1) and 0.48-0.51 h
(T2); the (T1)
half-lives of TBA
were 2.8 (males) and
3.4 h (females) and
(T2) 1.8 h (males)
and 1.5 h (females)
Species: (T1&T2): rapid and by 48 h, 11-13% (T1&T2): radiolabelled rapid elimination Ferdinandi
Fischer-344 rats extensive absorption (low dose; T1&T2) species in expired air in urine after low et al.(1990d)
Treatment: as indicated by and 4-5% (high dose) were MTBE and TBA; by dose and in expired Miller et
(T1) 6 rats/sex/ marked recovery of of administered 3 h post-dose, 30% air at high dose, al. (1997)
dose group received 14C in urine by 24 h radioactivity (low dose) and 7-10% suggesting enzyme
1440 or 28 800 recovered in skin (high dose) of saturation at high
mg/m3 (400 and of some rats, recovered radioactivity dose; by 48 h, total
8000 ppm) of probably due to were correlated to TBA, recovery of
14C-MTBE as a contamination from while TBA was major radioactivity (14C)
single 6-h urine (lower radioactive component in urine 65-71% (low
exposure radioactivity at 24 h post-dose dose, T1&T2) and
recovered in urine); (>90%, low dose); 35-42% (high dose);
(T2) 40 rats/sex 0.6-1% in skin of major radiolabelled in expired air 17-22%
were pretreated remaining rats, 1-3% species in urine from (low dose) and 54-59%
with 1440 mg/m3 in carcass, and <1% further oxidation of (high dose) and <1%
unlabelled MTBE in tissues TBA were 2-methyl-1, in faeces; no sex
6 h a day for 14 2-propanediol, alpha- difference in rate
days, followed by hydroxyisobutyric acid, and route of
6 h exposure for two minor unidentified radioactivity
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
1440 mg/m3 14C-MTBE components, and minor elimination
on day 15; observed detection of CO2 (1%
for 48 h post-dose of the dose); no sex
differences in
biotransformation
iii) Intravenous administration
Species: a statistically apparent volume of by 2 h, the major half-life of MTBE Ferdinandi
Fischer-344 rats significant distribution 0.27 metabolite TBA was (plasma) 0.45-0.62 h; et al.(1990a)
Treatment: 40 rats/ difference in plasma to 0.31 litre found in blood at half-life of TBA Miller et
sex/group received concentrations (AUC) peak concentration; (plasma) 0.92-1.3 h; al. (1997)
40 mg/kg as a between sexes (lower male rats had higher clearance from plasma
single dose; in females) blood concentration of MTBE 0.36-0.41
observed than female rats litre/h (males) and
(sacrificed) for 0.47 litre/h (females)
various time-points
until 36 h
post-dose
Species: rat, 1.7-3% of administered by 6 h, major by 48 h, 71-73% of Ferdinandi
Fischer-344 radioactivity was radiolabelled species administered et al.(1990b)
Treatment: 6 rats/ recovered in tissues in expired air was radioactivity was Miller et
sex/group received and carcass for each MTBE, with a minor totally recovered: in al. (1997)
40 or 400 mg/kg dose after 48 h elimination of TBA exhaled air (42-46%),
14C-MTBE as a (2.5-3.1% of in urine (26%), and
single dose; administered dose); faeces (<1%); rapid
observed for 48 h the major radiolabelled elimination in lungs,
species in urine (from 94% within 3 h
further oxidation of
TBA) were 2-methyl-1,
2-propanediol, alpha-
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
hydroxyisobutyric acid,
and two minor
unidentified components;
there were no sex
differences in
biotransformation
iv) Intraperitoneal
Species: Charles rapid and extensive the total cumulative methanol and formic 14C activity was mainly Biodynamics
River CD rats absorption based upon 14C activity in tissues acid were found in eliminated in expired (1984)
Treatment: 33 peak blood and plasma averaged 3.39, 1.94 and plasma, liver and air; by 48 h, recovery
animals/sex/group concentrations at 5 1.14% of administered kidney was about 99.86% (approx.
received a single min post-treatment, dose at 15 min, 6 h, 92% as MTBE and approx.
dose of averaging 92.04 ± and 24 h post-treatment, 7.45% as CO2); about 3%
approximately 37.72 and 83.40 ± respectively. At 15 min, in urine and about 0.8%
60µCi 14C-MTBE 20.48 µg 14C-MTBE the majority of the in faeces (male rats);
(about 232 mg equivalents/ml blood 14C-radioactivity was approx. 3% was excreted
MTBE/kg bw) in for male and female found in mesenteric fat, in urine and 0.8% (males)
saline and were rats, respectively. liver and kidney; and 1.25% (females) in
sacrificed at The half-life of however, at 6 h and 24 h faeces. The 14C activity
intervals of 5, 14C-MTBE in blood was no 14C was found in in urine and faeces was
15, 30, and 45 59.8 min for male rats mesenteric fat mainly associated with
min, and 1, 2, 3, and 49 min for female 14C-formic acid (a total
6, 12, 24 and 48 h rats. The half-life of 3.08% of administered
post-treatment in plasma was 2.3 h dose)
for males and 1.3 h
for females
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: mouse, most of the Yoshikawa
ddY administered MTBE et al.(1994)
Treatment: 4 male was eliminated
mice/dose group unchanged in the
were administered exhaled air; >90%
a single dose of of this amount was
50, 100 or 500 eliminated within
mg/kg in corn oil 3 h. The pulmonary
solution; observed elimination showed
for 6 h an initial rapid
decrease of the
elimination ratio
followed by a slow
decrease at 100 and
500 mg/kg. The
calculated half-lives
were 45 and 80 min,
respectively. The
elimination ratios
at the three different
doses were 23.2, 37.6
and 69.0%, respectively
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
C. In vitro
Incubation of 5 or incubation of MTBE with Brady et
14 mM MTBE with liver microsomes from al. (1990)
liver microsomes phenobarbital-pretreated
from phenobarbital- rats resulted in TBA and
or acetone- formaldehyde in
pretreated or equimolar amounts. The
untreated Vmaxa value for
Sprague-Dawley rats demethylation of MTBE
(3-5 males) increased by 4-fold with
acetone-induced and by
5.5-fold with
phenobarbital-induced
microsomes compared with
the control Vmax value.
These results indicate
that cytochrome P450 2B1
(inducible by
phenobarbital) and
cytochrome P450 2E1
(inducible by acetone)
play a role in the
demethylation of MTBE.
Results after inclusion
of monoclonal antibody
against P450 2E1
indicated this enzyme
only partially
contributed to the
demethylation. Microsomes
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
pretreated with MTBE
yielded a 47-fold
induction of
pentoxyresorufin
o-dealkylase, with no
change in
N-nitrosodimethylamine
demethylase activity.
These results are
consistent with an
elevation of P450 2B1
a Vmax : the maximum velocity of the demethylation process.
Nihlén et al. (1998a) studied the uptake, distribution,
metabolism and elimination of MTBE in ten healthy male volunteers. The
subjects were exposed on three different occasions for periods of 2 h
in a chamber to MTBE concentrations of 18, 90 and 180 mg/m3 (5, 25
and 50 ppm) while performing light physical exercise. MTBE (and its
metabolite TBA) were monitored in exhaled air, blood and urine, the
latter being collected up to 3 days after exposure. Respiratory uptake
of MTBE was low (42-49%) and respiratory clearance was high (32-47%).
The metabolic blood clearance was 0.34-0.52 litre/h per kg. The
kinetics of MTBE were linear up to the highest exposure concentration
of 180 mg/m3 (50 ppm). The kinetic profile of MTBE in blood was
described as having four phases, with average half-lives of 1 min, 10
min, 1.5 h and 19 h. In urine the post-exposure decay curve of MTBE
had two linear phases with average half-lives of 20 min and 3 h. The
urinary excretion of MTBE was less than 1% of the absorbed dose.
Biomarkers and partitioning of inhaled MTBE were studied in two
(1 male, 1 female) volunteer subjects exposed for 1 h to a nominal
concentration of 5 µg/m3 (1.39 ppm), followed by clean air exposure
for 7 h (Buckley et al., 1997). MTBE concentrations in expired air,
venous blood and urine were monitored during and after exposure. The
decay of MTBE was assessed by using a 2- or 3-exponential model and
yielded residence times of 2-3 min, 15-50 min, and 3-13 h in alveolar
air, and 5 min, 1 h and 32 h in venous blood. Based on
lower-than-expected blood and expired air MTBE concentrations during
uptake and the decreasing blood-breath ratio during the post-exposure
decay period, the authors hypothesized that the respiratory mucous
membranes acted as a reservoir for MTBE, retaining 6-9% of the MTBE
intake. Compartmental monitoring was used to estimate a blood-breath
partition coefficient of approximately 18. The urinary concentration
of MTBE ranged from 0.37 to 15 µg/litre and bore little relationship
to the exposure: urinary elimination accounted for only a small
fraction (<1%) of total MTBE elimination.
6.1.2 Human exposure to oxygenated gasoline
During an oxyfuels programme in Fairbanks, Alaska, there was a
strong correlation between the workplace air levels ranging from 0.02
to 2.92 mg MTBE/m3 and the difference in blood concentrations of MTBE
between pre-shift and post-shift blood measurements (p=0.0001). The
median pre-shift concentration of MTBE in the blood of 18
occupationally exposed workers was 1.15 µg/litre (range 0.1- 27.8
µg/litre). The median post-shift blood MTBE level was 1.8 µg/litre
(range 0.2-37.0 µg/litre) (Moolenar et al., 1994).
Breath samples were collected from a person pumping gasoline
(containing 15% MTBE by volume) and a nearby observer (within 1 m),
immediately prior to and an hour after refuelling (Lindstrom & Pleil,
1996). The MTBE concentration in the sample collected during
refuelling was 412 µg/m3. The ambient background level was
approximately 25 µg/m3 MTBE. Low concentrations of MTBE (7-10 µg/m3)
were detected in the exhaled breath before the refuelling that took
2 min and 8 seconds; 40 seconds after the exposure the concentrations
had increased by factors of 35 for the observer and 100 for the person
pumping gasoline (see also section 5).
6.2 Animal studies
Repeated exposure (2 to 15 weeks) to MTBE vapour by inhalation (6
h/day, 5 day/week) resulted in dose-dependent increases in MTBE levels
in blood, brain and perirenal fat of Wistar male rats (Savolainen et
al., 1985). Very small differences were observed in blood MTBE levels
following repeated exposure. TBA levels in blood increased with
repeated MTBE exposure when comparing levels at 6, 10 and 15 weeks to
levels following 2 weeks. Perirenal fat/blood MTBE concentration
ratios ranged from 9.1 to 11.6 after 15 weeks of intermittent
exposure. Blood and brain concentrations of TBA, the major main
metabolite, were also dose-dependent. TBA was, however, not found in
quantifiable amounts in the perirenal fat.
A series of studies on the kinetics of MTBE was conducted by
Bio-Research Laboratories (Ferdinandi et al., 1990a-d). These data
have been further published by Miller et al. (1997).
Miller et al. (1997) and Ferdinandi et al. (1990a-d) described
the pharmacokinetics and disposition of MTBE in male and female
Fischer-344 rats following i.v. (40 mg/kg), oral (40 and 400 mg/kg)
and dermal (40 and 400 mg/kg in occluded chambers) administration and
(nose-only) inhalation exposure for 6 h either for a single exposure
or repeated exposure (15 days). The details of these studies are
presented in Table 15. Miller et al. (1997) found that the elimination
of radiolabelled MTBE in rats was rapid and mainly occurred through
lungs and kidneys irrespective of administration route. The
elimination was virtually complete 48 h post-dosing. The renal
elimination was, however, slowest after dermal exposure. Twelve hours
post-dosing, the radiolabelled recovery in urine was 14-26% of the
dermal dose, 41-50% of the oral dose and 25-37% of the intravenously
injected dose. The recovery was 75-94% 36 h post-dosing, irrespective
of dose and administration route. A minor difference in excretion
route was observed between the sexes. Collectively these studies
demonstrated that MTBE is rapidly eliminated from blood (half-life =
0.5 h) by exhalation and metabolism to TBA (see Table 15). The major
metabolites recovered in urine were 2-methyl-1,2-propanediol and
alpha-hydroxyisobutyric acid. Dose-related differences in the AUC for
plasma concentrations of MTBE and TBA were observed in rats exposed to
1440 or 28 800 mg MTBE/m3 (400 or 8000 ppm) by inhalation (Ferdinandi
et al., 1990a-d; Miller et al., 1997). Miller et al. (1997) further
showed that increasing doses of MTBE (by inhalation and, to a lesser
extent, oral administration) to Fischer-344 rats decreased the
recoveries of radioactivity in urine and increased the recovery in
expired air. This indicates a saturation of the oxidative metabolic
pathway of MTBE at inhalation levels above 28 800 mg/m3 (8000 ppm) or
at oral administration levels above 400 mg/kg. There were no
significant sex- or route-dependent differences in the
pharmacokinetics and disposition of MTBE.
Table 15. Pharmacokinetic parameters of MTBE and TBA calculated from mean plasma concentrations of male
F-344 rats exposed to MTBE (Miller et al., 1997)
Exposure route Dose MTBE TBA
AUC Half-life CL V/F or Vssd AUCzero to infinity Half-life
(µg/h per ml) (h) (ml/h) (litre) (µg/h per ml) (h)
Intravenous 40 mg kg 10.7 0.45 413 0.27 26.7 0.92
Oral 40 mg kg 17.0 0.52 392 0.29 39.0 0.95
400 mg kg 230 0.79 358 0.41 304 1.6
Dermal 40 mg kg 7.9 2.3a 389 3.9 26.3 2.1a
400 mg kg 46.9 1.8a 364 1.4 93.9 1.9a
Inhalation low single 84.3 0.52 531 0.40 404 3.3
high single 2960 0.57 299 0.25 6010 3.4
low repeated 6.7b 0.51 c c 127b 1.8
a Calculated from the alpha-phase of a two-compartment model. Half-lives of MTBE from 12 to 45 h post-dose curve at
low and high doses were 92 and 37 h, respectively. Half-lives of TBA from 12 to 45 h post-dose were 170 (low dose)
and 31 h (high dose).
b AUCzero to infinity on 15th day of exposure.
c Values were not calculated because plasma was not collected during exposure.
d Vss = apparent volume of distribution at steady state after repeated inhalation.
Following intraperitoneal administration of 14C-MTBE to rats,
the highest radioactivity was recovered in the expired air
(Biodynamics, 1984). The radioactivity was also distributed throughout
the animal tissues. The amount retained in tissues was less than 2% of
the total dose of MTBE intraperitoneally administered to rats at 6 and
24 h post-treatment. Distribution of the radioactivity was primarily
to the liver and secondarily to the kidney. Approximately 92% of MTBE
was exhaled as the parent compound and approximately 7.5% as CO2 in
48 h. Another 3% was excreted in urine and up to 0.8% in faeces. The
14C-activity in urine and faeces was mainly associated with
14C-formic acid. Peak blood levels were observed in male and female
rats 5 min following intraperitoneal dosing of 14C-MTBE. Peak levels
of 14C activity in the plasma occurred at 5 min post-treatment in
male rats and at 15 min post-treatment in females rats. The
radioactivity decreased sharply during 1 h and thereafter gradually
during the study period (48 h). The half-time of radiolabelled MTBE in
whole blood was 59.8 min for male rats and 49 min for female rats. The
half-life of MTBE in plasma was 2.3 h for male rats and 1.3 h for
female rats (Biodynamics, 1984).
In a study of MTBE distribution in male and female rats following
inhalation exposure to 10 710 mg/m3 (3000 ppm) for 6 h, it was
reported that MTBE levels in the liver and brain were comparable in
males and females but were higher in male kidneys than in those of
females: MTBE was still detectable in male kidneys 18 h after exposure
(Borghoff et al., 1998). The authors considered that this may have
been due to the interaction of MTBE with alpha2u-globulin in the male
rat kidney.
6.3 In vitro studies
In vitro measurement of liquid/air partition coefficients of
MTBE at 37°C, using a vial equilibration technique, showed a human
blood/ air partition coefficient of 17.7 (confidence limits:
17.0-18.4), water/ air 15.2 (CL: 14.9-15.5), and oil/air 120 (CL:
114-125) (Nihlén et al., 1995). There was no significant difference in
partition coefficient for blood/air between the sexes. Liquid/air
partition coefficients for MTBE between different media and air were
also determined by Imbriani et al. (1997). The values were: blood/air
= 20.0; urine/air = 15.6; saline/air =15.3; fat/air = 142.0; and olive
oil/air = 138.0.
MTBE partition coefficients measured in rat tissues demonstrated
a higher solubility of MTBE in fat (115.6) compared to blood (11.5)
and other tissues such as liver (14.5) (Borghoff et al., 1996). The
partition coefficient of MTBE in the male rat kidney was approximately
five times higher than the value measured in female rat kidneys. This
high uptake of MTBE into the male rat kidney was found to be due to an
interaction with the male rat specific protein alpha2u-globulin
(Borghoff et al., 1995; Poet & Borghoff, 1997).
In rats, MTBE is demethylated by hepatic microsomal enzymes to
form TBA and formaldehyde (FA) (Savolainen et al., 1985; Brady et al.,
1990). When using rat liver microsomes, the Vmax for demethylation of
MTBE to FA increased after pretreatment with acetone or phenobarbital
(Savolainen et al., 1985; Brady et al., 1990). The results of Brady et
al. (1990) indicated that both cytochromes P450 2BI and P450 2E1 are
implicated in the metabolism of MTBE. In vitro, TBA has been shown
to be oxidatively demethylated using rat liver microsomes to yield FA
(Cederbaum & Cohen, 1980).
Hong et al. (1997) demonstrated that human liver microsomes
metabolize MTBE to TBA. The activity of 125 ± 11 pmol TBA/min per mg
protein was approximately 50% of the activity in rat and mouse liver
microsomes. The metabolism of MTBE to TBA in human liver microsomes
was NADPH-dependent and was inhibited by carbon monoxide, an inhibitor
of cytochrome P450 (CYP) enzymes, suggesting that CYP enzymes play a
critical role in human metabolism of MTBE. Human CYP2A6 and CYP2E1
cDNAs were each co-expressed with human cytochrome P450 reductase by a
baculovirus expression system and the expressed enzymes used to
metabolize MTBE. CYP2A6 was more active than CYP2E1 (activity 6.1 and
0.7 nmol TBA/min per nmol P450, respectively).
The role of the cytochrome P450 enzyme CYP2E1 in metabolizing
MTBE (and other gasoline ethers) was examined in 2E1 knock-out mice,
which lack demethylation capability. Liver microsomes metabolized MTBE
with an activity level of 0.67 ± 0.1 nmol/min per mg. However, there
were no significant differences in activity levels in microsome
preparations from two 2E1+/+ strains of mice, demonstrating that
CYP2E1 is not important in the metabolism of MTBE in mouse livers
(Hong et al., 1998).
The probable metabolic pathway is presented in Fig. 2.
6.4 Physiologically based pharmacokinetic modelling
A physiologically based pharmacokinetic model (PBPK) to describe
the dosimetry of MTBE in rats has been developed (Borghoff et al.,
1996). Using this PBPK model, MTBE blood levels following different
routes of exposure and various exposure concentrations were predicted.
When human anatomical parameters are used in this model and the
metabolism is scaled allometrically, the model predicts the level of
MTBE in blood (concentrations ranging from 0.03 to 17.1 µg/litre)
during and following exposure of people to 6 mg MTBE/m3 (1.7 ppm)
(Borghoff et al., 1996; Cain et al., 1996). The human PBPK model was
further expanded to include brain as a target tissue and an exposure
model for bathing and showering. Model simulations of a bathing and
showering MTBE exposure scenario in humans at water levels from 0.64
to 1.0 mg/litre and air levels from 5 to 5.7 mg/m3 (1.4 to 1.6 ppm)
predicted maximum brain levels to range from 0.015 to 0.02 mg
MTBE/litre and 0.006 to 0.019 mg TBA/litre (Rao & Ginsberg, 1997).
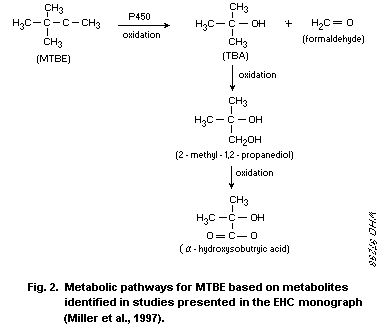 7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
Owing to the limited therapeutic use of MTBE in the dissolution
of cholesterol gallstones in humans, there have been a number of
studies in which effects have been examined following single or
repeated exposure by direct instillation into the gallbladder.
Reported effects at therapeutic doses were mild clinical signs and
inflammatory changes in the gallbladder (Allen et al., 1985a; McGahan
et al., 1988; Adam et al., 1990; Esch et al., l992a,b; Chen et al.,
1995).
7.1 Single exposure
The acute toxicity of MTBE has been studied in several animal
species; the results are summarized in Tables 16 and 17. Studies by
routes most relevant to human exposure are described here. The oral
LD50 value for rats is about 3800 mg/kg bw (ARCO, 1987). Signs of
intoxication after single oral lethal doses consist of CNS depression,
ataxia, laboured respiration and death (ARCO, l987). When the dose was
non-lethal, recovery was complete.
The acute dermal LD50 is >10 200 mg/kg bw for rabbits (ARCO,
1987). Adverse local effects included erythema, oedema, fissuring and
necrosis at dermal application.
In rats, the LC50 value for inhalation exposure to MTBE is about
142 000 mg/m3 air (39 460 ppm) (ARCO, 1987). Reported LC50 values in
mice are 141 000 mg/m3 (1.6 mmol/litre) for 15 min of inhalation
exposure (Marsh & Leake, l950) and 658 000 mg/m3 (18% v/v) for 10 min
of inhalation exposure (Snamprogetti, 1980). Signs noted in rats
following inhalation exposure included eye irritation, incoordination
and loss of righting reflex (ARCO, 1987). Surviving animals appeared
to recover within 24 h.
Results of studies in which neurological effects following single
exposures were examined are reported in section 7.3.
7.2 Skin, eye, and respiratory tract irritation; skin sensitization
7.2.1 Skin irritation
Moderate erythema and oedema were reported by Cuthbert (1979)
following application of 0.5 ml undiluted MTBE to the intact and
abraded skin of six rabbits (applied under occlusion for 24 h). A
primary irritation index of 3.36 was reported. Effects were slightly
more pronounced on abraded skin:
24 h 72 h
intact abraded intact abraded
erythema 1.7 2.2 1.1 2.0
oedema 1.7 2.0 1.0 1.8
MTBE was considered to be a moderate skin irritant.
Table 16. Acute toxicity of MTBE in experimental animals
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Rat: oral (gavage) 2000, 3000, 4600, 3800 hypoactivity, muscular weakness, Industrial Bio-Test
Charles River, 6800, or 10 200 hyperpnoea, lacrimation, Laboratories (1969)
5 male, 5 female/ mg/kg bw prostration and death; the
dose level symptoms were reversible at
sublethal doses.
Inflammation of the stomach
and/or small intestine in
animals that died
Rat: oral (gavage) 3866 CNS depression, ataxia, ARCO (1987)
(strain and number laboured respiration and
not stated) death
Rabbit: dermal 6.8 or 10.2 g/kg >10 200 no deaths; no gross Industrial Bio-Test
New Zealand White bw, occlusive pathological alterations Laboratories (1969)
2 male, 2 female/ dressing, for 24 h; other than necrosis at
dose level 14-day observation application site
Rabbit: dermal 10 000 erythema, oedema, scaling, ARCO (1987)
(strain and number fissuring, and blanching
not stated) hyperaemia in animals that
died
Rat: inhalation LC50 = 142 000 eye irritation, ARCO (1987)
(strain and sex mg/m3 incoordination, tachypnoea,
not stated) (39 460 ppm) loss of righting reflex
and death
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Mouse: inhalation exposure for 15 min LC50 = 141 000 the median concentration Marsh & Leake (1950)
White (strain (whole body mg/m3 for anaesthesia (AC50) =
and sex not exposure) (1.6 mmol/litre) 106 mg/litre (1.2
stated), 20 mmol/litre)
mice/exposure
group
Mouse: inhalation exposure for 5 min; 400 000 mg/m3 median effective concentration Industrial Bio-Test
Swiss, (whole body observation for (260-600 mg/litre) for anaesthesia (EC50) = Laboratories (1969)
4 males/exposure exposure) following 48 h 200 mg/litre air (130-300
group mg/litre); convulsive
hyperventilation,
hyperactivity, ataxia, loss
of righting reflex, clonic,
and sporadic convulsive
seizures
Mouse: inhalation I. 20% v/v in air LT50a = 5.6 min death occurred within 1 h Snamprogetti (1980)
Swiss, (whole body for 3, 4, 5, 6, 9
I. 40 males/ exposure) or 12 min
exposure group II 8, 12, 17, 20, LC50 = 658
II. 20 males per 22 or 26% v/v MTBE mg/litre
group in air for 10 min (18% v/v)
Skin and eye irritancy
Rabbit:
New Zealand skin application 0.5 ml moderate erythema and
White, 3 male, (occluded) oedema
3 female
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
New Zealand conjunctival 0.1 ml moderate conjunctival Cuthbert (1979)
White, 3 male, instillation response; no corneal
3 female or iris involvement
New Zealand
White
I. 6 (sex not conjunctival 0.1 ml conjunctival redness for Hazleton
stated) instillation 48 h, no chemosis or discharge, Laboratories
slightly more marked effects (1979)
II. 3 (sex not conjunctival 0.1 ml with temporary corneal
stated) instillation; opacity for 24 h
washout with
water 30 secs
later
Rabbit conjunctival 0.05 ml or 0.1 ml conjunctival congestion, Snamprogetti (1980)
Both sexes instillation thickening and lacrimation;
(strain and number more marked at high dose;
not stated) reversible
a LT50 =exposure time which causes death in 50% of treated animals.
Table 17. Acute toxicity of MTBE following parenteral injection
Species Injection Dose LD50 Comments Reference
route/site
Rat: subcutaneous 3.0, 4.0, 5.0, 6.0, 6.7 ml/kg bw Snamprogetti (1980)
Wistar, 7.0, 8.0, 9.0 or 10.0 (5.75-7.76 ml/kg)
8 males/dose group mg/kg bw
Mouse: subcutaneous 1.0, 2.0, 3.0, 4.0, 3.6 ml/kg bw Snamprogetti (1980)
Swiss, 4.5, 5.0 or 5.5 ml/kg bw (2.93-3.57 ml/kg)
8 males/dose group
Rat: intravenous 0.1, 0.2, 0.3, 0.5, 0.75, 0.56 ml/kg bw in lethal doses: Snamprogetti (1980)
Wistar, 1.25 or 1.50 mg/kg bw nervous depression,
16 males/dose group sometimes followed
by short clonic
convulsions, autonomic
activity
(hypersalivation,
urination, defaecation),
and respiratory
disorders; death
occurred within
30 min. Surviving
animals: no toxic
symptoms or signs of
nervous depression
lasting more than
15-20 min
Table 17. (continued)
Species Injection Dose LD50 Comments Reference
route/site
Rat: hepatic parenchyma, 0.2 ml/kg bw intracaval injection Akimoto et al.
Sprague-Dawley, inferior vena cava, caused 100% mortality (1992)
7 males dosed tail vein or (pulmonary injury);
intravenously peritoneal cavity intrahepatic injection
caused 59% mortality
10 males dosed and peripheral vein
intraperitoneally injection 17% mortality;
the pulmonary injury
included congestion,
haemorrhage, and
interstitial oedema
Mouse: intraperitoneal 1.5, 2.0, 2.5, 3.0, 3.5, 1.4 ml/kg bw Snamprogetti (1980)
Swiss, 4.0, or 5.0 ml/kg bw
8 males/dose group
Mouse: intraperitoneal 0, 50, 200, or 500 mg/kg 830 mg/kg bw Arashidani et al.
ddy bw (784-878 mg/kg) (1993)
10 males/dose group
7.2.2 Eye irritation
Instillation of 0.05 or 0.1 ml of MTBE into the conjunctival area
of albino rabbits (both sexes) caused reversible eye irritation
(congestion of the conjunctiva, palpebral thickening and lacrimation,
more marked at the high dose) (Snamprogetti, 1980).
Moderate erythema and slight chemosis and discharge, persisting
for 3 days, were noted in another study after instillation of 0.1 ml
MTBE into the conjunctival area of New Zealand white rabbits
(Cuthbert, 1979):
24 h 48 h 72 h 7 days
redness 2.2 1.7 1 0.2
chemosis 1.3 1.2 1 0.1
discharge 0.9 0.6 0.2 0
The reactions had largely resolved by one week post-treatment).
The author concluded that MTBE was irritant to the rabbit eye.
Hazleton Laboratories (1979) conducted a study on nine New
Zealand White rabbits (sex not stated) in which six rabbits had 0.1 ml
MTBE instilled into one eye, the other acting as control, and three
rabbits had the same treatment followed by eye washout with water 30
seconds later. There was slight irritation (mean score for redness 1.5
and 1.0 at 24 and 48 h, negligible chemosis or discharge) in six
rabbits that did not have the eye washout, but slightly more marked
effects, including corneal opacity lasting for 24 h, in the three
rabbits with eye washout. The effects were all reversible.
Exposure to MTBE vapour in inhalation chambers also resulted in
eye irritation. In Fischer-344 rats exposed to MTBE vapour
concentrations of 14 300 or 28 600 mg/m3 (4000 or 8000 ppm) for 6 h,
lacrimation was noted 1 h post-exposure (Gill, 1989).
7.2.3 Respiratory tract irritation
A test of lung irritancy was used by Tepper et al. (1994) to
evaluate the effects of 1-h exposures of Swiss-Webster mice (four
males per dose group) to 300, 1000, 3000, 10 000 and 30 000 mg
MTBE/m3 in inhalation chambers. An almost immediate decrease in
frequency of breathing was observed in all dose groups. The animals
served as their own controls with baseline frequency of breathing and
respiration waveform morphology being obtained by exposure to filtered
air for 15 min. The severity of the irritant response was
dose-related, ranging from "slight" (13% decrease in respiration rate)
to "severe" at 30 000 mg/m3. During the exposures, breathing rate
returned to baseline after about 15 min, except for the highest dose
group, in which the rate gradually decreased for another 40 min. There
was a return to baseline frequency 15 min after end of exposure. Lung
injury could not be confirmed at the 30 000 mg/m3 dose level. Lung
lavage about 20 h post-exposure indicated only a marginal increase of
total protein and lactate dehydrogenase, both measures of lung cell
damage; however, these results were comparable to those of similarly
treated mice, exposed to filtered air, in a previous study.
In a single 6-h exposure vapour neurotoxicity inhalation study
(see also section 7.3) in Fischer-344 rats at target concentrations of
14 400 or 28 800 mg/m3 (4000 and 8000 ppm), survivors killed at the
end of a 14-day observation period had slight to mild lung hyperaemia
(Gill, 1989).
7.2.4 Skin sensitization
The sensitization potential of MTBE was assessed in guinea-pigs
using a Magnusson and Kligman procedure (Cuthbert, 1979). No evidence
of sensitization was reported in any of the 20 test animals following
induction and challenge with 1% MTBE. Dermal sensitization has also
been investigated using a Landsteiner technique (Litton Bionetics,
1980). Guinea-pigs were induced using intradermal injection (initial
treatment 0.5 ml of a 1% aqueous solution, followed by 9 injections of
0.1 ml over 3 weeks). No sensitization reactions were recorded at
challenge 2 weeks later (0.05 ml of a 0.01% solution of MTBE in
water).
7.3 Neurotoxicity
Following a single MTBE vapour exposure, reversible alterations
in central and peripheral nervous system function were observed (Gill,
1989; Daughtrey et al., 1997). Groups of Fischer-344 rats (22 of each
sex per dose level) were exposed for 6 h in inhalation chambers at
target MTBE concentrations of 0, 2860, 14 300 or 28 600 mg/m3 (0,
800, 4000 or 8000 ppm). No mortality or clinical signs of toxicity
were observed at any concentration. Behavioural evaluations performed
1, 6 and 24 h post-exposure included a screen for behavioural function
using a functional observational battery (FOB) and analysis of motor
activity prior to and following exposure. At 1 h post-exposure,
concentration-related increases in the incidence and severity of
ataxia and duck-walk gait appeared in both sexes of the mid- and
high-dose groups. In high-dose males, lacrimation, decreased muscle
tone, decreased rectal temperature, decreased performance time on the
tread mill, and increased hind limb splay were also observed. The
alterations in the FOB were significantly (p<0.01) different from the
control group. An increased incidence of laboured respiration (not
statistically significant) was also found in the high-dose male rats.
Additionally, piloerection was observed for all males in the high-dose
group. However, piloerection was also observed in some male rats in
the control group and in low- and mid-dose males.
Similar concentration-related findings were found in mid- and
high-dose female rats (increased incidence of lacrimation,
piloerection, ataxia and duck-walk gait, decreased rectal temperature,
and decreased hind limb grip strength). Additional exposure-related
findings for females in the high-dose group included a significantly
(p<0.01) increased incidence of laboured respiration and latency to
rotate on the inclined screen. Exposure-related alterations of motor
activity were detected for both male and female rats during the
initial 90 min of the test session. The mean activity was increased
during the entire test period in low-dose males and decreased in
high-dose males compared to the control group. However, in high-dose
males, an initial 10-min decrease in activity was followed by
increased activity during a 20-min interval and decreased thereafter.
Males in the mid-dose group only showed an increased activity
initially. For females, the mean activity for the entire test session
was not different from the control group. No MTBE-related alterations
were observed during the 6-h or 24-h post-exposure evaluation.
7.4 Short-term repeated dose studies
The short-term repeated dose toxicity of MTBE has been studied in
rats, mice and pigs. Typically, effects of irritation and reversible
central nervous system effects, including hypoactivity, ataxia, and
anaesthesia, were noted.
7.4.1 Oral studies
Sprague-Dawley rats (10 males and 10 females per dose group)
administered 0 (corn oil), 357, 714, 1071 or 1428 mg MTBE (99.95%
pure)/kg bw daily by gavage (in corn oil) for 14 days exhibited
transient anaesthesia at 1428 mg/kg and irritation of the pharyngeal
mucosa at the highest dose levels (Robinson et al., 1990). Diarrhoea
and reduced body weight gain were observed in all treatment groups.
Six animals (four from the high-dose group) died during treatment due
to difficulties associated with the gavage procedure, including
pharyngeal irritation in the high-dose animals. Absolute and relative
lung weights were significantly (p<0.001) lower in all exposed
female rats. The mean absolute kidney weights were increased in dosed
male rats and the relative kidney weights were significantly
(p<0.05 and p<0.001) increased over controls in the mid- and
high-dose group, respectively. The cholesterol levels were
significantly (p<0.05) increased in high-dose males and the two
mid-dose groups of females. The blood-urea nitrogen (BUN) and
creatinine were significantly (p<0.05) decreased in high-dose
females. The incidence of renal tubular disease (hyaline droplet
nephropathy) was moderately increased in dosed male rats. Increased
hyaline (protein) droplets within the cytoplasm of proximal tubular
epithelial cells were noted in seven of eight (88%) of the males in
the highest dose group as compared with two of five (40%) of the
controls.
In a 28-day oral study, Sprague-Dawley rats (10/sex/group) were
administered 0, 90, 440 or 1750 mg undiluted MTBE (purity not
specified)/kg bw daily by gavage for a total of 20 h (IITRI, 1992).
Seven rats (one low-dose female, one high-dose male, and five
high-dose females) died accidentally during dosing. This was
attributed to difficulties in dosing the animals owing to the strong
odour, the irritating nature, and high volatility of MTBE. Clinical
observations during the study period included transitory salivation in
all treated groups and transitory hypoactivity and/or ataxia in mid-
and high-dose animals. There were no significant effects on body
weight or body weight gain. The only significant treatment-related
change in haematological or clinical chemistry parameters was an
increase in cholesterol in high-dose males and females.
No treatment-related gross necropsy observations were noted. The
relative liver weights were significantly (p<0.05) increased in males
and females in the high-dose group. In the high-dose males there was
also a significant (p<0.05) increase in relative adrenal weight.
Absolute kidney and relative kidney weights showed a dose-related
increase in both males and females, but achieved statistical
significance (corrected for multiple comparisons) only for relative
weights in males at the mid- and high-doses and in females at the low
and high doses. Histopathology showed hyaline droplet formation in the
proximal convoluted tubules in the kidneys of the mid- and high-dose
males.
In a 90-day study, groups of Sprague-Dawley rats (ten males and
ten females in each test group) were gavaged 0 (corn oil), 100, 300,
900 or 1 200 mg/kg bw of undiluted MTBE (>99.95% pure) daily for 90
days (Robinson et al., 1990). The most pronounced clinical effect of
MTBE was the profound anaesthetic effect at 1200 mg/kg bw; the animals
recovered in about 2 h. In female rats, a significant (p<0.001)
treatment-related decreased BUN level and elevated cholesterol level
were observed at all levels of exposure. In male rats, the mean
absolute kidney weights were significantly (p<0.05) elevated at 900
and 1200 mg/kg bw, respectively. The increase in the relative kidney
weights was statistically significant (p<0.001) at the two highest
dose levels and also the relative liver weights (p<0.05 at 900
mg/kg and p<0.001 at 1200 mg/kg). In females, the relative kidney
weights were significantly (p<0.05) increased at and above 300
mg/kg bw and the relative liver, thymic and cardiac weights showed a
statistically significant (p<0.05) dose-related increase at 900
mg/kg. Microscopic findings included chronic nephropathy in both
control and high-dose male rats, which was more severe in MTBE-treated
rats. At the highest dose level, granular casts were found, and there
was also a slight increase of cytoplasmic hyaline droplets in proximal
tubular epithelial cells.
In a comparison study on the effects of inhalation exposure (see
section 7.3.2), two groups of female B6C3F1 mice (8/group) were given
MTBE by gavage in corn oil (5 ml/kg/bw) at doses of 0 or 1800 mg/kg bw
per day for 3 days and then killed around 18 h after the last dose
(Moser et al., 1996a). Body weight and liver weight were not affected
by treatment. MTBE induced a 37% increase in hepatic cytochrome P450
content (P <0-05), a 9-fold increase in hepatic 7-pentoxy
resorufin- O-dealkylase activity (PROD, a CYP2B marker) and a 2-fold
increase in hepatic 7-ethoxy-resorufin- O-deethylase activity (EROD,
a CAPE marker). MTBE also induced a 2-5-fold increase in the hepatic
cell labelling index (as estimated from the incorporation of
5-bromo-2-deoxyuridine delivered by an implanted osmotic mini pump) in
the absence of hepatotoxicity, judged by the absence of any change in
serum alanine aminotransferase (ALAT) or histological signs of
necrosis (Moser et al., 1996a).
7.4.2 Inhalation studies
Female B6C3F1 mice (five or more/group) were exposed by
inhalation to 0 or 27 900 mg/m3 (7814 ppm) MTBE (>99.95% pure) for 3
or 21 days (6 h per day, 5 days per week) (Moser et al., 1996a). The
exposure resulted in abnormal gait, hypoactivity, decreased muscle
tone and increased lacrimation during exposure and immediately after
termination of exposure to MTBE. The mice recovered quickly following
termination of exposure. There was no significant change in body
weight. The relative liver weights were increased 20% (p<0.05) as
compared to controls at 3 days of exposure but the difference was not
significant at 21 days. MTBE exposure significantly (p<0.05)
decreased relative uterine weight to 48% of the control at 3 days,
with a further significant (p<0.05) decrease to 65% at 21 days. The
relative ovarian weight was decreased to 69% (p<0.05) at 21 days of
exposure. Histopathological examination showed mild centrilobular to
midzonal hepatocyte swelling at 3 days. At 21 days there were no
microscopic exposure-related changes. The total hepatic microsomal
cytochrome P450 content was elevated 40% at 3 days and approximately
200% at 21 days. After 3 days and 21 days of MTBE exposure,
respectively, hepatic PROD activity increased 5-fold and 14-fold,
while hepatic EROD activity increased 1.8-fold and 3.2-fold. There was
a non-significant change in hepatic cell labelling index to a 2.5-fold
higher value at 3 days, but at 21 days the MTBE group showed a
significant reduction to 0.7% in comparison with the control value of
2.3% (p<0.05). There were no histological signs of hepatoxicity and
serum ALAT was unaffected.
Information on the repeated dose toxicity of MTBE (>99.95% pure)
is also available from a study comparing the short-term hepatic
effects on female B6C3F1 and CD-1 mice (Moser et al., 1996b). Groups
of six female mice were exposed in inhalation chambers to
particulate-free control air containing 0 mg/m3 or a target
concentration of 27 900 mg/m3 (7800 ppm) 6 h per day, 5 days per week
for 3 or 21 days. Clinical signs included abnormal gait, hypoactivity,
decreased muscle tone and increased lacrimation during exposure and
immediately after termination of exposure. The animals recovered
quickly after termination of each daily exposure. There was a decrease
of body weight (p<0.05) in CD-1 mice, but not in B6C3F1 mice, after
21 days. Statistically significant (p<0.05) increases were seen in
absolute and relative liver weight in both strains of mice at both
time points. Histopathological examination showed slight centrilobular
hypertrophy in both B6C3F1 mice and CD-1 mice at 3 days as compared
to controls. At 21 days there was no indication of hepatotoxicity or
necrosis. Serum ALAT showed no increased activity at either 3 or 21
days. In the CD-1 mice, after 3 and 21 days, respectively, total
hepatic microsomal cytochrome P450 content increased 2.3-fold
(p<0.05) and 1.8-fold (p<0.05), PROD increased 5-fold (p<0.05) and
5-fold (p<0.05), and EROD increased 2.3-fold (p<0.05) and 3-fold
(p<0.05). Corresponding data for the B6C3F1 mice are described
below. The hepatic cell labelling indices were, in contrast to B6C3F1
mice, increased in CD-1 mice at both time points; the increases were
3-fold and 5-fold, respectively, at 3 and 21 days (Moser et al.,
1996b). A slightly decreased survival relative to control was noted in
female B6C3F1 mice following 16 weeks exposure to 28 450 mg/m3 (7969
ppm) (100% versus 92% survival, respectively). After 32 weeks of
exposure, survival was 96% for controls and 88% for MTBE-exposed mice.
Body weight was significantly decreased (p<0.05) at both time points.
PROD activity was increased 4.9 fold, and EROD activity 1.9 fold,
after 16 weeks treatment. While MTBE exposure produced mild
centrilobular to midzonal hypertrophy, there was no indication of
cytotoxicity or hepatic necrosis, or alteration in serum ALAT (Moser
et al., 1996b).
Sprague-Dawley rats (10/sex/group) showed increasing depth of
anaesthesia with increasing concentrations of MTBE when exposed to
MTBE vapour in exposure chambers at concentrations of 0, 900, 1800 or
3600 mg/m3 (0, 250, 500 and 1000 ppm, respectively) 6 h per day, 5
days per week for 13 weeks (Greenough et al., 1980). The
haematological analyses revealed an increase in haemoglobin levels
during week 13 in male rats exposed to 3600 mg/m3. At autopsy, a
slight reduction in relative and absolute lung weight was detected in
female rats at the same exposure level. There were no other gross or
histopathological effects reported.
In a range-finding study, Fischer-344 rats and CD-1 mice were
exposed to MTBE vapour in inhalation chambers 6 h per day for 13
consecutive days (Dodd & Kintigh, 1989). The target concentrations
were 0, 7150, 14 300 and 28 600 mg/m3 (0, 2000, 4000 and 8000 ppm,
respectively). The measured mean concentrations were 7200, 13 750 and
28 330 mg/m3 (2018, 3850 and 7936 ppm, respectively). No mortality
occurred during the study period. Clinical signs included
hypoactivity, ataxia, and periocular irritation in both rats and mice,
primarily in the high-dose groups during exposure. Reversible
neurobehavioural alterations (ataxia) were observed immediately after
exposure in high-dose rats (mice were not observed). Body weight gain
was depressed in rats (but not in mice) in the mid- and high-dose
groups (statistically significant for male rats). In female mice, both
absolute and relative liver weights were significantly increased at
all levels of exposure. In the mid-dose rats, both absolute and
relative liver weights were significantly increased in females; in
males, there was an increase in relative kidney and liver weights. In
rats at the highest dose, males had an increase in relative weight of
liver, kidney and adrenal. Females had an increase in both absolute
and relative weights of liver and adrenal, and absolute weight of
kidneys was increased.
In a 13-week vapour inhalation study that included neurotoxicity
evaluation, Fischer-344 rats were exposed to target concentrations of
0, 2860, 14 300 and 28 600 mg MTBE/m3 (0, 800, 4000 and 8000 ppm) 6 h
per day, 5 days per week (Dodd & Kintigh, 1989; Lington et al., 1997).
No mortality occurred. Major findings included motor activity changes
and reversible changes in body temperature in mid- and high-dose rats,
ataxia, and depressed body weight gain and food consumption. Only mild
haematological changes (e.g., decreased erythrocyte counts and
increased reticulocyte counts) were observed, primarily in male rats.
The corticosterone levels were significantly increased in both male
and female rats at the highest exposure level. No treatment-related
gross lesions were found at necropsy. The relative weight of liver and
kidney were significantly increased in all male groups and in females
in the two highest exposure groups. Relative weight of adrenal was
significantly increased in males and females at the two highest doses.
There were, however, no treatment-related microscopic changes in these
organs. It is probable that there is an association between the liver
enlargement and the high serum corticosterone levels. (Although this
was reported by the authors, the Task Group considered that it was
more likely that the increase in adrenal weight was associated with
the high serum corticosterone levels). The only treatment-related
microscopic findings were found in males at the highest dose level and
included a statistically significant increase in lymphoid hyperplasia
in the submandibular lymph nodes, an increase (not statistically
significant) in the degree of haemosiderosis in the spleen, and a mild
increase of hyalin droplets in the renal proximal tubules. Proximal
tubule necrosis and protein droplet accumulation were observed in
kidneys from male, but not female, rats exposed to 5400 and 10 760
mg/m3 (1516 and 3013 ppm) for 6 h/day for 10 consecutive days
(Prescott-Mathews et al., 1997). Alpha-2u-globulin immunoreactivity
was present in and confined to protein droplets in male rat kidney. A
mild dose-related increase in alpha-2u concentration in the male rat
kidney correlated with an exposure-related increase in cell
proliferation. No significant differences were observed in female rats
for any of these responses.
7.4.3 Intraperitoneal administration
Katoh et al. (1993) reported that MTBE administered to mice (500
mg/kg bw as a single dose, or 200 mg/kg bw as repeated doses) caused
lipid peroxidation, as demonstrated by increased levels of lipid
peroxide in liver homogenates, and an induction of hepatic microsomal
cytochrome P450 content. Repeated treatment with 200 mg/kg bw for 4
weeks did not affect glutathione content or glutathione-S-transferase
(details on pattern and period of administration were not provided).
7.5 Neurotoxicity studies
A single 6-h inhalation exposure of Fischer-344 rats to MTBE
vapour at target concentrations of 0, 2880, 14 400 or 28 800 mg/m3
(0, 800, 4000 or 8000 ppm) induced reversible alterations in central
and peripheral nervous system function (Gill, 1989). Group of 8 male
and 8 female rats were used at each concentration for behavioural
evaluation and 14 males and 14 females for motor activity
observations. Motor activity changes appeared within 10 min of
exposure to 28 800 mg/m3 (8000 ppm) in both male and female rats. For
male rats, motor activity changes were also observed at 2880 and 14
400 mg/m3). Behavioural evaluation (functional observational battery,
FOB) was performed 1, 6 and 24 h post-exposure. At 1 h post-exposure
there were concentration-related behavioural alterations at 14 400
mg/m3 and 28 800 mg/m3 but these were not found at 6 or 24 h
post-exposure.
In a pilot study for a 13-week exposure study (see also section
7.3), Fischer-344 rats and CD-1 mice (five per sex and species) were
exposed to target MTBE concentrations of 0, 7150, 14 300 and 28 600
mg/m3 (0, 2000, 4000 and 8000 ppm) 6 h per day for 13 consecutive
days (Dodd & Kintigh, 1989). Hypoactivity was observed at all dose
levels and ataxia at the two highest dose levels in both rats and mice
during exposure. In high-dose rats, ataxia, decreased startle and pain
reflexes, and decreased muscular tone were also observed immediately
after exposure. Recovery was complete within 1 h. The NOAEL was
determined to be 7200 mg/m3 in both rats and mice.
The subsequent 13-week study with neurotoxic evaluation included
testing for inhalation toxicity, FOB, motor activity and
neuropathology (Dodd & Kintigh, 1989). Fischer-344 rats (25 rats of
each sex per dose level) were exposed to MTBE vapour at target
concentrations of 0, 2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and
8000 ppm) 6 h per day, 5 days per week (see also section 7.4.2).
Clinical findings included hypoactivity in the mid- and high-dose
groups and ataxia in the high-dose group immediately following the
daily exposure. The exposure resulted in minor changes in the FOB
including elevated body temperature (in high-dose male rats and in
mid- and high-dose female rats) and decreased hind limb grip strength
(in mid-dose males). Decreased motor activity for males in the
high-dose group and increased motor activity for females in the
low-and mid-dose groups were reported. Additional findings are
reported in section 7.3. Necropsy revealed no treatment-related gross
lesions. There were no treatment-related microscopic changes in the
central and peripheral nervous system tissues.
7.6 Reproductive and developmental toxicity
Protocols and results of reproductive/developmental studies are
presented in Table 18 These include one- and two-generation inhalation
studies in rats and four developmental studies (inhalation) in rats,
rabbits and mice. In these investigations, MTBE did not induce
specific adverse reproductive effects; developmental effects were
observed only at dose levels that were maternally toxic. At very high
dose levels (28 000 mg/m3) decreased relative uterine weight was
observed in one study.
Table 18. Reproductive studies with MTBE in laboratory animals
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
One- and two-generation studies
Rat, inhalation 15 males and 0, 1070, 4640 and one generation, two slightly decreased Biles et al.
Sprague-Dawley 30 females/group 12 140 mg/m3 litter study (F1a, F1b) pup viability (1987)
(0, 300, 1300 and (p<0.05) in F1b
3400 ppm) 12 140 mg/m3
Rat, inhalation 25 males and 0, 1430, 10 700, two generation study 1430 mg/m3: no Neeper-Bradley
Sprague-Dawley 25 females/group and 28 600 mg/m3 adverse effects; (1991)
(0, 400, 3000 and >10 700 mg/m3:
8000 ppm) reduced bw, bw
gain and food
consumption in
parental animals
(mainly in males),
clinical signs and
neurotoxic
effects, reduced
pup bw and bw gain
postnatally. NOEL
for general
toxicity 1430
mg/m3; NOEL for
reproductive
effects >28 600
mg/m3; LOEL for
adults and offspring
>10 700 mg/m3
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Developmental studies
Rat, inhalation 25 rats per dose 0, 900, 3600 and gestation days 6-15 reduced food Conaway et
Sprague-Dawley level 9000 mg/m3 consumption in al. (1985)
(0, 250, 1000 and treated groups
2500 ppm) during the day
9-12 interval; no
significant
developmental
toxicity
Mouse, inhalation 25 mice per dose 0, 900, 3600 and gestation days 6-15 a slight (not Conaway et
CD-1 level 9000 mg/m3 statistically al. (1985)
(0, 250, 1000 and significant),
2500 ppm) dose-related
decrease in food
and water
consumption; no
significant
developmental
effects
Mouse, inhalation 30 mice per dose 0, 3600, 14 300 gestation days 6-15 >14 300 mg/m3: Tyl &
CD-1 level and 28 600 mg/m3 clinical signs Neeper-Bradley
(0, 1000, 4000 and of toxicity and (1989)
8000 ppm) reduced fetal bw;
28 600 mg/m3:
reduced bw, bw
gain, and food
consumption;
increased number
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
of non-viable
implantations,
reduced number of
viable
implantations and
% male fetuses,
and increased
incidence of
cleft palate.
NOEL for maternal
and developmental
toxicity 3600 mg/m3
Rabbit, inhalation 8 females per dose 0, 7150, 14 300 and gestation days 6-18 >7150 mg/m3: Tyl (1989)
New Zealand level 28 600 mg/m3 (0, reduced food
White 2000, 4000 and 8000 consumption in
ppm) all dosed groups;
increased
incidence of
lung foci; 28 600
mg/m3: reduced
bw gain, audible
respiration, and
slightly lower
fetal weights
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Rabbit, inhalation 15 females per 0, 3600, 14 300 gestation days 6-18 >14 300 mg/m3: Tyl (1989)
New Zealand dose level and 28 600 mg/m3 reduced bw gain
White (0, 1000, 4000 and food
and 8000 ppm) consumption;
28 600 mg/m3:
hypoactivity,
ataxia, increased
relative liver
weight, decreased
corrected
gestational weight
change and gravid
uterine weight. No
significant
developmental
effects; NOEL for
maternal toxicity
3600 mg/m3; NOEL
for developmental
toxicity >28 600
mg/m3
7.6.1 Reproductive toxicity
Biles et al. (1987) conducted a two-litter, one-generation
inhalation study of reproductive effects in CD Sprague-Dawley rats.
Target concentrations were 0, 890, 3600 and 8925 mg/m3 (0, 250, 1000
and 2500 ppm). Corresponding measured concentrations were,
respectively, 1070, 4430 and 10 640 mg/m3 (300, 1240 and 2980 ppm)
for females and 1030, 4210 and 10 210 mg/m3 (290, 1180 and 2860 ppm)
for males. Fifteen males exposed for 12 weeks were mated to thirty
females exposed for 3 weeks. Exposures continued throughout the mating
period, during gestation and through days 5-21 of lactation. A second
litter (F1b) was produced under the same mating and post-mating
exposure regimen. In the mid- and high-dose groups of the F1b
generation, there was a slight statistically significant (p<0.05)
decrease in pup viability. The authors felt that this was in large
part attributable to the high viability (99%) in the control group.
There were no treatment-related differences between control and
exposed animals based upon examination of clinical signs, gross
post-examination and histopathological examination of the gonads of
exposed adults, mating or fertility indices, pregnancy rates, mean
gestational length and number of pups at birth, litter survival
indices or pup weight. (The NOEL was above 8925 mg/m3 (> 2500 ppm)
in both parents and offspring).
In a two-generation reproductive and fertility inhalation study,
CD Sprague-Dawley rats were exposed to MTBE at concentrations of 0,
1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 or 8000 ppm)
(Neeper-Bradley, 1991; Bevan et al., 1997). There was parental
toxicity at the target concentrations of 28 600 mg/m3 and 10 700
mg/m3. Concomitant perinatal toxicity was also observed at these
concentrations. There were no treatment-related effects on
reproductive indices at any concentration and no adverse effects on
the offspring at concentrations that were not toxic to the parents.
(NOEL = 1430 mg/m3 (400 ppm); hypoactivity, lack of startle reflex
and blepharospasm in parents at 10 700 mg/m3 (3000 ppm); NOEL for
reproductive effects >28 600 mg/m3 (8000 ppm).
Inhalation exposure to 28 800 mg/m3 MTBE (99.95% pure) resulted
in significantly decreased relative uterine weight in B6C3F1 mice at
3 (48%) and 21 days (65%) of exposure as compared to controls (Moser
et al., 1996a) (see also section 7.4.2). The relative ovarian weight
was significantly (p<0.05) decreased at 21 days of exposure. There
were no exposure-related microscopic findings in ovaries, adrenals and
pituitary, and no effects on adrenal and pituitary weight. Moser et
al. (1996a) investigated if the decreased uterine weights were due to
an increased rate of estrogen metabolism by measuring the rate of
conversion of 3H-17B-oestradiol to water-soluble metabolites in
hepatocytes from MTBE-treated female mice. MTBE was found to increase
the oestrogen metabolism by 2.1-fold.
Ward et al. (1994) studied the toxicity of MTBE to germ cells in
CD-1 male and female mice. Groups of 10 mice were given 1, 10, 100 or
1000 mg/kg bw MTBE in corn oil by gavage 5 days per week for 3 weeks;
a negative control group received corn oil only. At the end of
treatment the mice were killed and one testis from each male and both
ovaries from each female were sectioned for cytological evaluation. In
males, sperm number, Sertoli cells, spermatogonia, spermatocytes and
capped spermatids were evaluated, and, in females, oocyte quality.
There were no effects of MTBE on any of the cell types examined.
7.6.2 Developmental toxicity
The results of four inhalation studies on the developmental
toxicity of MTBE are summarized in Table 18.
There was a significant decrease in food consumption on days 9-12
of gestation in pregnant rats exposed to as much as 9000 mg/m3 (2500
ppm) but no other effects that the authors considered to be maternally
toxic, embryotoxic or teratogenic (Conaway et al., 1985). The NOEL for
offspring and parents was >9000 mg/m3 (2500 ppm).
Two different studies were conducted in the same strain of mice
exposed to various concentrations of MTBE for 6 h/day on days 6-15 of
gestation (Conaway et al., 1985; Tyl & Neeper-Bradley, 1989). No
significant maternal toxicity or developmental effects were observed
when groups of 30 pregnant females were exposed to 1000, 3960 or 9675
mg/m3 (280, 1110 or 2710 ppm), though the incidence of lacrimation
was increased in exposed mothers and there was a slight increase in
the incidence of fused sternebrae in the high-dose group (Conaway et
al., 1985). The NOAEL of parents and offspring was 9000 mg/m3 (2500
ppm). Tyl & Neeper-Bradley (1989) concluded that 14 300 mg/m3 (4000
ppm) was maternally toxic, based on observed hypoactivity and ataxia.
There were significant decreases (p<0.01) in body weight, body weight
gain and food consumption at 28 600 mg/m3 (8000 ppm). At 14 300
mg/m3 (4000 ppm) or more, there were significant reductions in fetal
body weight per litter (p<0.01) and increased skeletal variation. The
proportion of male fetuses was significantly reduced (p<0.01) at the
highest dose level in the Tyl & Neeper-Bradley (1989) study, but this
has not been observed in other studies with mice, rats or rabbits. At
28 600 mg/m3 (8000 ppm), the number of non-viable implantations per
litter and incidence of cleft palate was also increased (LOAEL in
parents and offspring = 14 300 mg/m3 (4000 ppm). The NOAEL in this
study was 3570 mg/m3 (1000 ppm).
Maternal toxicity was observed at the two highest concentrations
in rabbits exposed to 3570, 14 300 and 28 600 mg/m3 (1000, 4000 and
8000 ppm) during days 6-18 of gestation (Tyl, 1989). No developmental
effects were observed at any exposure level [NOAEL in offspring = 28
600 mg/m3 (8000 ppm); NOAEL in parents = 3570 mg/m3 (1000 ppm);
LOAEL in parents = 14 300 mg/m3 (4000 ppm)].
7.7 Mutagenicity and related end-points
Genotoxicity study results with MTBE are generally negative.
However, there are indications that MTBE may have some genotoxic
potential in the presence of metabolic activation. Genotoxicity data
for MTBE are compiled in Table 19.
In studies on reverse mutation in Salmonella typhimurium, MTBE
was found to be non-mutagenic in tester strains TA1535, TA1537,
TA1538, TA98 and TA100, with and without S9 (liver enzyme homogenates
from induced Sprague-Dawley male rats) metabolic activation, at doses
up to 10 mg/plate (Cinelli & Seeberg, 1989).
No significant increase in the frequency of recessive lethal
mutations in the X-chromosome could be established after feeding MTBE
(99.14% pure) (0.03, 0.15 or 0.3% MTBE in 5% aqueous sucrose) to adult
Drosophila melanogaster (wild-type Oregon-R males) for 24 h (Sernau,
1989).
In the presence of a liver-derived metabolic system (liver S9
from Arochlor 1254-induced male Sprague-Dawley rats) MTBE (>99% pure;
1.0, 2.0, 3.0 or 4.0 ml/ml) induced forward mutations in vitro at
the thymidine kinase locus of mouse lymphoma cell line L5178Y/ TK+/-
(Mackerer et al., 1996). The observed mutagenicity was dose-dependent.
Other experimental data, using a test system developed to
determine if the mutagenicity of a material is the result of the
presence or release of formaldehyde, had indicated that the
mutagenicity was due to the metabolism of MTBE to formaldehyde
(Blackburn et al., 1991). To establish if formaldehyde, derived from
MTBE in the presence of S9, was responsible for the observed
mutagenicity, Mackerer et al. (1996) used a modified mouse lymphoma
assay. In this assay formaldehyde dehydrogenase and its co-factor
NAD+ were added during the exposure period so that any formaldehyde
produced would be converted to formic acid, which is non-genotoxic. An
MTBE dose-related increase in the frequency of mutant lymphoma cells
occurred without the presence of formaldehyde dehydrogenase and
NAD+, but not when these were present, indicating that formaldehyde
was responsible for the mutations.
In two independent in vitro experiments MTBE (purity not
specified) did not induce unscheduled DNA synthesis (UDS) in primary
rat hepatocytes at concentrations up to 10 mg/ml (Seeberg, 1989).
In an in vivo-in vitro hepatocyte UDS assay, ten male and ten
female CD-1 mice were assigned to each dose group and an air-only
control group and, in addition, five of each sex to a positive control
group (DMN) (Vergnes & Chun, 1994). The animals were exposed to MTBE
(purity not specified) in inhalation chambers 6 h a day for two
consecutive days. The target concentrations were 0, 1440, 10 800 and
Table 19. Genotoxicity studies with MTBE
Species Strain/cells Measured end-point Test conditions Activation Result Reference
Bacterial systems
S. typhimurium TA1535 reverse mutation 625, 1250, 2500, 5000, + - - - Cinelli &
TA 1537 10 000 µg/plate Seeberg (1989)
TA1538
TA98
TA100
S. typhimurium TA98 reverse mutation exhaust particle + nd Clark et al.
TA100 extracts from gasoline + ± (1984)
containing 7% by
volume MTBE; five
concentrations ranging
from 1 to 100 µg/plate;
duplicate plates
Non-mammalian eukaryotic systems
Drosophila wild type Oregon-R, sex-linked recessive Basc test; 0.03, - Sernau (1989)
melanogaster males lethal test 0.15 or 0.3% MTBE;
adult feeding for 24 h
In vitro mammalian systems
Mouse lymphoma cell line forward mutation 1.0, 2.0, 3.0, 4.0 µl/ml + + Mobil Oil
L5178Y/TK Corporation
(1993)
Rat primary hepatocytes UDS 3.16, 10.0, 31.6, 100, - Seeberg (1989)
316, 1000, 3160, 10 000
µg/ml
Table 19. (continued)
Species Strain/cells Measured end-point Test conditions Activation Result Reference
In vivo - in vitro
Mouse, primary hepatocytes UDS vapour exposure: 0, - Vergnes & Chun
CD-1 1440, 10 800, 28 800 (1994)
mg/m3, 6 h/day for 2
consecutive days
In vivo mammalian systems
Rat, bone marrow cells chromosome vapour exposure: 0, - Vergnes & Morabit
Fischer -344 aberrations 2800, 14 400, 28 800 (1989)
mg/m3, 6 h/day for
5 days
Mouse, spleen lymphocytes chromosome oral administration, a slight Ward et al.
CD-1 aberrations 1.0, 10, 100 or 1000 inverse (1994)
mg/kg bw for 3 weeks dose-response
relationship
in male mice
but not in
female mice
Mouse, spleen lymphocytes mutations at hprt oral administration, - Ward et al. (1994)
CD-1 locus 1.0, 10, 100 or 1000
mg/kg bw for 3 weeks
Mouse, bone marrow chromosome vapour exposure: 0, - Vergnes & Kintigh
CD-1 cells damage 1440, 10 800, 28 800 (1993)
mg/m3, 6 h/day for 2
consecutive days
28 800 mg/m3 (0, 400, 3000 8000 ppm). The animals were sacrificed and
hepatocytes were sampled 18 h after the second exposure day (for the
positive control group after approximately 2 h). No dose-related
increase in the DNA repair activity could be established. The UDS
assay is used to indicate primary DNA damage; this is, however,
transient in nature and it is necessary to analyse cells for damage as
soon as possible after cessation of treatment. Since the half-life of
MTBE in the animal body is quite short (1-3 h) and DNA repair is
relatively rapid, the study should have been designed accordingly.
MTBE (purity not specified) was considered nonclastogenic to
Fischer-344 rats in an in vivo test system (Vergnes & Morabit,
1989). No concentration-related or significant increase in the
incidence of chromosome aberrations in rat bone marrow cells was found
in either males or females following whole body exposure to MTBE 6 h
per day for five consecutive days. The target concentrations were 0,
2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and 8000 ppm,
respectively).
MTBE did not induce micronuclei in vivo in mouse bone marrow
cells (Vergnes & Kintigh, 1993). CD-1 mice were exposed to MTBE
(purity not specified) vapour in inhalation chambers (five animals per
sex per dose level) at concentrations of 0, 1430, 10 710 or 28 600
mg/m3 (0, 400, 3000 or 8000 ppm) 6 h a day for two consecutive days.
Bone marrow cells were collected 24 and 48 h after the second exposure
day. No significant, exposure-related increase in the frequency of
micronuclei could be established at any dose level and sampling time
in either sex in this study.
Ward et al. (1994) examined the frequency of somatic cell
mutations in spleen lymphocytes after administration by gavage 5 days
per week for 3 weeks with MTBE (99.8% pure) in corn oil to CD-1 male
and female mice (ten animals per dose group). Ethyl-nitrosourea was
used as a positive control. The doses were 1, 10, 100 and 1000 mg/kg
bw. The frequency of mutations at the hypoxanthine-guanine
phosphoribosyl transferase (hprt) locus was determined 3 weeks after
the cessation of exposure. There was no indication that MTBE produced
a mutagenic effect at the tested dose levels. Ward et al. (1994) also
analysed chromosome aberrations in spleen lymphocytes. This was
performed on the first day after the termination of exposure in 13
male mice and on the second day in the remaining mice. A slight, but
not statistically significant, inverse dose-relationship was seen in
male mice; this was not seen in the females.
7.8 Carcinogenicity
Three bioassays are available on the oncogenicity of MTBE in both
sexes of rats and mice. These include two inhalation studies, one in
Fischer-344 rats and one in CD-1 mice, and one oral study in
Sprague-Dawley rats (Table 20). At high inhalation exposure levels,
MTBE increased the incidence of renal cell carcinomas in male rats and
liver tumours in female mice. Oral administration increased the
incidence of lymphomas and leukaemias in female rats. The studies also
resulted in testicular tumours in both strains of rats following
exposure either by inhalation or oral administration.
Fischer-344 rats (50 of each sex per dose level) were exposed to
MTBE (99% pure) vapour in inhalation chambers at target concentrations
of 0, 1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 and 8000 ppm,
respectively) 6 h per day, 5 days per week (Chun et al., 1992; Bird et
al., 1997). The control group was exposed to filtered air. Increased
mortality and decreased mean survival time were observed for male rats
from all exposure groups. Owing to the high mortality rate, surviving
males, six from the mid-dose group and nine from the high-dose group,
were killed at weeks 97 and 82, respectively. The numbers of surviving
males at the end of the study were 13, 6, 6 and 9 at 0, 1430, 10 700
and 20 600 mg/m3, respectively. Mean survival times were 632, 617
(p<0.05), 587 (p<0.01) and 516 (p<0.01) days, respectively.
Low-dose males and all females were killed during weeks 104 and 105.
Various clinical signs of toxicity (blepharospasm, hypoactivity,
ataxia, lack of startle reflex, swollen periocular tissue and
salivation) were observed in both sexes at the two highest dose
levels. No clinical signs were noticed at the lowest dose level.
Significantly (p<0.01) reduced body weight and body weight gain were
recorded at week 81 for the high-dose rats. For low- and mid-dose male
rats, body weight and body weight gain were slightly to significantly
(p<0.05) increased during the first 70 to 80 weeks. Thereafter, there
were no clear dose-related changes. In females, there was a slight,
but not exposure-related, decrease at the two lower exposure levels.
High-dose male rats also showed a significantly (p<0.05) decreased
corticosterone level at week 81. A trend toward increases in liver and
kidney weights relative to final body weight was recorded for the
mid-dose male rats. In female rats, concentration-related increases in
liver and kidney weight (absolute and relative to body or brain
weight) were observed at the two highest dose levels. There was also a
trend toward an increased adrenal gland weight relative to final body
weight for high-dose males. However, only the organ weight data from
the control and the low-dose group were statistically evaluated due to
the different sacrifice periods for the mid- and high-dose groups.
Non-neoplastic effects of treatment included an increased
incidence and severity of chronic progressive nephropathy in male rats
from all dose groups and in female rats from the mid- and high-dose
groups. Treated males were more severely affected than the females,
which usually showed only slight changes. Chronic progressive
nephropathy was diagnosed as the cause of morbidity or death for 3/37,
16/44, 26/44 and 39/41 male rats and for 0/20, 0/23, 4/27 and 6/25
female rats in the control, low-, mid- and high-dose groups,
respectively. The incidences of nephropathy, with interstitial
fibrosis, in the control, low-, mid- and high-dose groups were 19/37
(51%), 29/44, (66%), 37/44 (84%) and 40/41 (98%), respectively
(significance not specified). Histologically, the chronic progressive
nephropathy included an exposure-related increase in severity for
Table 20. Carcinogenicity studies with MTBE
Species Exposure Tumour Reference
Mouse (CD1) Male: Liver, adenoma: Burleigh-Flayer et al. (1992)
control 11/49 Bird et al. (1997)
1430 mg/m3 (400 ppm) 11/50
10 710 mg/m3 (3000 ppm) 9/50
28 600 mg/m3 (8000 ppm) 12/49
Male: Liver, carcinoma:
control 2/49
1430 mg/m3 (400 ppm) 4/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 8/49
Male: Liver, adenoma and carcinoma:
control 12/49
1430 mg/m3 (400 ppm) 12/50
10 710 mg/m3 (3000 ppm) 12/50
28 600 mg/m3 (8000 ppm) 16/49
Female: Liver, adenoma:
control 2/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 10/50 (p<0.05)
Female: Liver, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 1/50
Table 20. (continued)
Species Exposure Tumour Reference
Female: Liver, adenoma and carcinoma:
control 2/50
1430 mg/m3 (400 ppm) 2/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 11/50
Rat (F344) Male: Killed at: Chun et al. (1992)
control 104 weeks Bird et al. (1997)
1430 mg/m3 (400 ppm) 104 weeks
10 710 mg/m3 (3000 ppm) 97 weeks
28 600 mg/m3 (8000 ppm) 82 weeks
Male: Kidney, adenoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 5/50
28 600 mg/m3 (8000 ppm) 3/50
Male: Kidney, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 0/50
Male: Kidney, adenoma and carcinoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 8/50
28 600 mg/m3 (8000 ppm) 3/50
Table 20. (continued)
Species Exposure Tumour Reference
Male: Testes:
control 32/50
1430 mg/m3 (400 ppm) 35/50
10 710 mg/m3 (3000 ppm) 41/50
28 600 mg/m3 (8000 ppm) 47/50
Male: Pituitary tumours, week 104:
control 6/50
1430 mg/m3 (400 ppm) 5/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 0/50
Female: Kidney, adenoma:
control 0/50
1430 mg/m3 (400 ppm) 0/28
10 710 mg/m3 (3000 ppm) 1/39
28 600 mg/m3 (8000 ppm) 0/50
Rat (Sprague-Dawley) Male: Testicular adenoma Belpoggi et al. (1995)
(denominator is total number
in group):
control 2/60
250 mg/kg 2/60
1000 mg/kg 11/60
Male: Testicular adenoma
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/26
250 mg/kg 2/25
1000 mg/kg 11/32 (p<0.05)
Table 20. (continued)
Species Exposure Tumour Reference
Female: Lymphomas or leukaemia
(denominator is total
number in group):
control 2/60
250 mg/kg 6/60
1000 mg/kg 12/60
Female: Lymphomas or leukaemias
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/58
250 mg/kg 6/51
1000 mg/kg 12/47 (p<0.01)
glomerulosclerosis, tubular proteinosis, interstitial nephritis and
interstitial fibrosis in both male and female rats from the mid- and
high-dose groups. The chronic nephropathy was also associated with
secondary lesions such as fibrous osteodystrophy, hyperplasia within
the parathyroid glands, and mineralization within numerous tissues. An
increased incidence of renal tubular cell adenomas and carcinomas was
noted in mid- and high-dose male rats. The incidence was 8/50 and 3/50
for the mid- and high-dose groups, respectively, and 1/50 for the
control group. The renal tubular cell carcinomas were only noted in
the mid-dose group (3/50). One renal cell adenoma was found in a
mid-dose female.
In mid- and high-dose males, there was also a dose-related
increase of interstitial cell (Leydig cell) adenomas of the testes.
The incidence was 32/50, 35/50, 41/50 and 47/50 (64%, 70%, 82% and 94%
for the control, low-, mid- and high-dose groups, respectively. In
high-dose males, there was also an exposure-related decrease in the
frequency of pituitary adenomas. The incidences were 27/47, 29/48,
27/47 and 2/48 in the control, low-, mid- and high-dose groups of
males, respectively. A treatment-related decrease of large granular
lymphocyte (LGL) leukaemia was also noted. The incidence of LGL in
males was 33/50, 22/50, 20/50 and 3/50 and in females 22/50, 14/50,
15/50 and 16/50 for control, low-, mid- and high-dose rats,
respectively. LGL leukaemia, which is age-dependent and generally does
not appear until 20 months of age, was the main cause of death in the
control and low-dose males. The lymphoid hyperplasia of the
submandibular lymph node observed in a 13-week inhalation study with
Fischer-344 rats at a dose level of 28 600 mg/m3 was not observed in
the present study using the same strain of rats and the same dose
level. No NOEL could be determined for male rats due to a slight
increase of nephropathy at the lowest dose level. For female rats, the
NOEL for toxicity was 1440 mg/m3.
A number of points regarding this study can be made:
a) The tumour incidence values were analysed using methods that are
not appropriate when there are marked inter-group differences in
survival.
b) Testicular adenomas are quite common in untreated aging male
F-344 rats (Haseman et al., 1990), with a spontaneous incidence
in the range 64-98% for animals contemporaneous with those used
here. On this basis, it appears that this tumour type may have
been under-represented in the concurrent controls, influencing
the slope of the dose-response curve. In the Fischer-344 rats
used in this laboratory, the average historical control incidence
of Leydig cell tumours was 88% (Bird et al., 1997). Thus, in
comparison with historical control data, there was no increased
incidence in this tumour in the dosed groups. However, concurrent
control comparisons are always more appropriate, unless it is
known that there had been a particular problem with the study,
e.g., inappropriate randomisation.
c) Neoplasm incidence decreases were observed for pituitary adenomas
in the high-dose males and for large granular lymphocyte
leukaemia in males and females at all dose levels. Decreases in
the high-dose rats might be related to body weight gain
restrictions in this group and to increased mortality rate in
males. Alternatively, the concurrent control values for these
neoplasms may be particularly high in this study.
An oncogenicity study was also carried out on CD-1 mice exposed
to MTBE (99% pure) vapour in inhalation chambers (Burleigh-Flayer et
al., 1992; Bird et al., 1997). Groups of 50 male and 50 female mice
were exposed to target concentrations of 0, 1430, 10 700 and 28 600
mg/m3 (0, 400, 3000 and 8000 ppm, respectively) 6 h per day, five
days per week for 18 months. The control group was exposed to filtered
air. The mortality rates for male mice (including those sacrificed
moribund but excluding procedural and accidental deaths) in the
control, low-, mid- and high-dose groups were 33%, 22%, 35% and 49%,
respectively. The corresponding values for females were 27%, 18%, 23%
and 33%, respectively. The authors reported that increased mortality
rate (significance not reported) and decreased survival time were
observed for male mice from the high-dose group only. This was
considered as a probable result of a slightly increased frequency of
obstructive uropathy (distended urinary bladder and/or obstruction of
the urethra). Clinical signs, i.e. ataxia, blepharospasm,
hypoactivity, prostration, and lack of a startle reflex (in the
high-dose group prostration also and in the mid-dose group stereotypic
behaviour also) were observed in both male and female mice at the two
high-dose levels. Ataxia, observed in most male and female mice from
the highest dose group throughout the study, was the only clinical
finding considered to be exposure related. Body weight and body weight
gain were decreased for both male and female mice from the high-dose
group. At the end of the study, body weight gain was decreased 15%
(p < 0.01) for the males and 24% (p<0.01) for the females from the
high-dose group.
Necropsy showed that the liver was the target organ for toxicity.
There was a dose-related increase in liver weight, both absolute and
relative to body weight, in both sexes (increase in absolute weight in
males significant at all dose levels). A slight (significant),
although not concentration-related, increase in kidney weight was
noted for male mice from all exposure groups and in female mice from
the high-dose group. Decreases, although not statistically
significant, in absolute brain and spleen weight were also noted for
high-dose male and female mice. In addition, an increase in serum
corticosterone levels was observed in both male and female mice from
the high-dose group at week 79. The increase was significant (p<0.05)
only for male mice. A slight decrease in urinary pH and increases in
urine gamma globulin were observed for both male and female mice at
the high-dose level.
For male mice that were found dead in the high-dose group (7/25),
a slightly increased frequency of urinary bladder dilation/ distension
was noted at autopsy as compared to controls (3/18). In addition, the
incidence of the number of liver masses was increased in the high-dose
male mice (13/50 compared to 7/50 for the control group). The only
exposure-related lesion in female mice found at necropsy was an
increased incidence of liver masses in the high-dose group (9/50) when
compared to the controls (0/50). Histopathology showed an
exposure-related increase in hepatocellular hypertrophy in high-dose
male mice (15/49) when compared to controls (5/49). This lesion also
showed an increased, although not statistically significant, incidence
in mid-dose male mice (10/50) and in female mice from the high-dose
group (9/50 as compared to 4/50 in the control group). Exposure to
MTBE did not, however, cause hepatocellular necrosis or degeneration.
In high-dose male and female mice, mineralization within the brain was
decreased. In addition, there was a dose-related decrease in the
incidence of cystic endometrial hyperplasia for female mice.
An increased frequency (not statistically significant) of hepatic
adenomas and carcinomas was observed in male mice at the high-dose
level (16/49 as compared to 12/49 in the control group). The increase
(not statistically significant) was due to a slightly increased
frequency of hepatocellular carcinomas in the high-dose group (8/49)
when compared to the controls (2/49). The analysis for the combined
incidence of hepatocellular adenomas and carcinomas did not, however,
include statistical methods that adjusted for difference in survival
between the control and exposure groups. In female mice, there was a
significant increase in the incidence of hepatocellular adenomas at
the high-dose level (10/50 as compared to 2/50 from the control
group). The induced incidences were modest and occurred in the group
in which hepatocellular hypertrophy also occurred. There was no
exposure-related increase in the incidence of hepatocellular
carcinomas in female mice. The NOEL for toxicity in mice exposed to
MTBE for 18 months was 1440 mg/m3.
In an oral exposure study, male and female Sprague-Dawley rats
(60 per sex and dose group) were administered 0, 250 or 1000 mg/kg bw
MTBE (>99% pure) in 1 ml extra virgin olive oil by gavage four times
a week for 104 weeks on a weekly schedule of 2 days dosing, 1 day
without dosing, 2 days dosing, followed by 2 days without dosing
(Belpoggi et al., 1995). There were no treatment-related differences
in mean body weights of treated groups compared to control groups. The
animals were kept under observation until natural death. High-dose
male rats showed a higher survival than controls at treatment week 80
and thereafter. At week 80, survival was approximately 56% in control
and exposed groups; at 112 weeks, it was approximately 10% in controls
and the low-dose group, but 35% in the high-dose group. In female
rats, a dose-related decrease in survival was observed from
treatment-week 16. It was reported that there were no evident
behavioural changes; at week 72, survival in the high-dose group was
about 65%; at week 120, it was less than 20%; at week 136, it was less
than 5%. The authors reported no evident behavioural effects; however,
the extent of examination of behavioural effects was not specified. No
relevant non-neoplastic changes (including renal) were detected at
autopsy and histopathology. No specific data were, however, reported.
In male rats, there was a dose-related increase in the incidence of
testicular Leydig cell (interstitial cell) tumours (2/60, 2/60 and
11/60), statistically significant at the highest dose (p<0.05). In
female rats, there was a dose-related increase in lymphomas and
leukaemias combined (2/60, 6/60 and 12/60), marginally significant at
the low-dose level and highly significant at the high-dose level
(p<0.01). There also was an increase in dysplastic proliferation of
lymphoreticular tissue in female rats at both dose levels, but the
incidence was higher in the low-dose group. Dose-related decreases in
mammary fibromas and fibroadenomas, and in pituitary adenomas and
tumours of adrenal glands were observed in dosed females. Since these
tumours and the testicular interstitial cell tumours are
age-dependent, these effects were probably at least partly due to the
dose-related early mortality and the prolonged observational period in
the surviving animals.
A number of points regarding this study should be made:
a) there is limited description of the results, particularly the
histopathological findings;
b) diagnostic criteria are not given for the distinction between
Leydig cell tumours and hyperplasia (the latter were not reported
at all, which is unusual for old Sprague-Dawley rats showing
Leydig cell tumours);
c) diagnostic criteria are not given for the distinction between
dysplastic hyperplasia and lymphoma;
d) lymphomas and leukaemias are pooled; specific tumour type and
incidences were not reported;
e) historical control data might aid the evaluation of lymphomas and
leukaemias, particularly if they are available for these rats
within different age ranges;
f) chronic progressive nephropathy was not observed in these
Sprague-Dawley rats, although these lesions might be expected, on
the basis of data from a number of other studies with this strain
of rat.
7.8.1 Initiation-promotion protocol
In a study to investigate if MTBE exhibited hepatic
tumour-promoting activity, 12-day-old female B6C3F1 mice were
initiated with a single intraperitoneal injection of the mutagen
diethylnitrosamine (DEN) or saline and then exposed subsequently to 0
or 28 800 mg/m3 (8000 ppm) MTBE (>99.95% pure) from 8 weeks of age
for 16 or 32 weeks (Moser et al., 1996b). Liver weight was
significantly (p<0.05) increased at both time points in both
saline-treated and DEN-initiated mice after exposure to MTBE, and was
associated with mild centrilobular to midzonal hypertrophy in both
groups. There was no significant difference in the percentage of
microscopic lesions classified as hepatic foci (86%), hepatocellular
adenomas (10%), or hepatocellular carcinomas (4%) in DEN/MTBE mice as
compared to DEN/control mice. However, the absolute number of
microscopic hepatic lesions was 50% less in the DEN/MTBE group than in
DEN/control mice. MTBE appeared inactive in this tumour
initiation-promotion assay.
7.9 Metabolites of MTBE
Animal carcinogenicity experiments have been conducted with the
metabolites tertiary-butyl alcohol (TBA) and formaldehyde (FA). TBA
administered in drinking-water caused increased incidences of renal
tubular adenoma and carcinoma in male Fischer-344 rats and increased
severity of chronic progressive nephropathy (Cirvello et al., 1995).
In female B6C3F1 mice, TBA produced thyroid follicular cell adenoma
and hyperplasia, and, in both male and female mice, inflammation and
hyperplasia of the urinary bladder (Cirvello et al., 1995). FA has
caused nasal squamous cell carcinoma in both Fischer-344 and
Sprague-Dawley rats and in B6C3F1 mice, and also increases of cancers
of the nasopharynx, nasal cavity and sinus in humans (Grindstaff et
al., 1991).
7.10 Mode of action
7.10.1 Kidney tumours
A number of chemicals cause both protein droplet nephropathy and,
with chronic exposure, renal cancer in male rats only (Borghoff et
al., 1990). The proposed mechanism by which these chemicals cause
renal tumours in male rats is based on their ability to cause protein
droplet nephropathy by accumulating alpha2u-globulin, a
male-rat-specific protein of low relative molecular mass. Evidence
suggests that chemical binding to alpha2u-globulin makes the protein
more resistant to hydrolysis, which accounts for its accumulation in
the renal lysosomes in the form of protein droplets. The chemically
induced accumulation of alpha2u-globulin is thought to be responsible
for cytolethality, which in turn stimulates cell division as the
kidney attempts to repair itself. Chronic chemical exposure with
repeated cycles of cytolethality and reparative replication is
probably the cause of the renal tumours in male rats (Borghoff et al.,
1990; Hard et al., 1993). A protein similar to alpha2u-globulin has
not been detected in human kidneys (Borghoff & Lagarde, 1993).
MTBE causes male-rat-specific renal tumours with chronic exposure
(Bird et al., 1997). MTBE also causes the accumulation of protein
droplets in male but not female rats following exposure ranging from
10 days to 13 weeks (Dodd & Kintigh, 1989; Chun & Kintigh, 1993;
Prescott-Mathews et al., 1997). Similar results have been observed
with TBA, a metabolite of MTBE (Lindamood et al., 1992).
alpha2u-Globulin immunoreactivity was present in and confined to
protein droplets in the kidneys of male rats exposed to MTBE in these
studies (Dodd & Kintigh, 1989; Chun & Kintigh, 1993; Prescott-Mathews
et al., 1997). Although a slight increase in alpha2u-globulin-positive
staining was observed in male rats exposed to MTBE, as compared to
controls, a linear exposure-related increase was not observed in any
of these studies. alpha2u-Globulin-positive proteinaceous casts at the
junction of the proximal tubules and the thin limb of Henle were not
observed (Swenberg & Dietrich, 1991). MTBE caused an
exposure-dependent mild increase in the renal concentration of
alpha2u-globulin measured in male rats (Prescott-Mathews et al.,
1997). Immunohistochemical staining of alpha2u-globulin is probably
not as sensitive as actually quantifying the alpha2u-globulin levels
with a mild increase in this protein. Further analysis of the kidney
protein profile from control and MTBE-treated male rats confirmed the
accumulation of alpha2u-globulin with no other protein detected
(Prescott-Mathews et al., 1997).
MTBE-induced kidney lesions, characterized by tubular necrosis
and protein droplet accumulation, were mild, especially when compared
with strong inducers of alpha2u-globulin nephropathy. Additionally,
granular casts, considered characteristic for alpha2u-globulin
nephropathy, were not observed in all studies. In the case of the
10-day MTBE inhalation exposure, however, there was a
concentration-dependent increase in kidney necrosis with minimal
sloughing of epithelial cells in male rat kidney following exposure to
10 710 mg/m3 (3000 ppm) (Prescott-Mathews et al., 1997).
In male and female rats exposed to MTBE vapour for 10 and 28
days, MTBE caused enhanced cell proliferation in male, but not female,
rat kidneys (Chun & Kintigh, 1993; Prescott-Mathews et al., 1997). A
strong positive correlation was observed between the cell
proliferative response and the concentration of alpha2u-globulin in
the kidneys of MTBE-exposed male rats.
With many of the chemicals that cause alpha2u-globulin
nephropathy, either the chemical or a metabolite has been found to
bind reversibly to alpha2u-globulin. In vitro, a high uptake of MTBE
into male rat kidney homogenate could be predicted using a
two-compartment model system when the binding of MTBE to
alpha2u-globulin was described using a dissociation constant of 10-4
M (Poet & Borghoff, 1997). This estimated dissociation constant for
MTBE binding to alpha2u-globulin was found to be similar to the
dissociation constant previously measured for 1,4-dichlorobenzene, a
known inducer of alpha2u-globulin nephropathy and a chemical
identified as bound to alpha2u-globulin following treatment of male
rats with 1,4-dichlorobenzene (Charbonneau et al., 1989). Together
these findings indicate that MTBE interacts, although weakly, with
alpha2u-globulin and induces alpha2u-globulin nephropathy and renal
cell proliferation in male, but not female, rats.
MTBE increases the severity of chronic progressive nephropathy in
both male and female rats, but does not cause renal necrosis, enhanced
cell proliferation or renal cancer in female rats. Chronic progressive
nephropathy alone is not associated with increased incidence of renal
tumours.
7.10.2 Liver tumours
It has been hypothesized that liver tumours in female mice can be
promoted by interfering with the estrogen-mediated suppression of
preneoplastic foci. In this regard, both unleaded gasoline and MTBE
induce cytochrome P450 activity and estrogen metabolism in mouse
hepatocytes, induce a mitogenic response in mouse liver, and decrease
uterine and ovarian weight in exposed mice. Additionally, unleaded
gasoline promotes DEN-initiated female mouse liver tumours, but this
response has not been observed with MTBE (Standeven & Goldsworthy,
1993; Moser et al., 1996b). Therefore, the relevance of this
hypothesis to an interpretation of the MTBE-induced mouse liver
tumours is currently unclear.
8. EFFECTS ON HUMANS
As explained in chapter 3, two separate fuel programmes in the
USA legally require the use of oxygenate in gasoline to address
ambient air quality objectives. Although no specific oxygenate is
required, MTBE has dominated the USA market place and is used at 15%
(by volume) to meet a 2.7% (by weight) oxygen requirement for
oxygenated gasoline in the cold-weather season in areas with excessive
carbon monoxide levels and at 11% (by volume) to meet a 2.0% (by
weight) oxygen requirement for reformulated gasoline sold year-round
in areas with excessive ozone levels. In the fall of 1992, shortly
after the introduction of oxygenated gasoline containing 15% MTBE in
Alaska, consumer complaints were registered about health effects such
as headaches, eye irritation and cough in Fairbanks (Beller &
Middaugh, 1992) and Anchorage (Chandler & Middaugh, 1992).
Subsequently, residents in other places in the USA also reported
health complaints associated with the introduction of cold-season
oxygenated fuel. Somewhat similar public concerns were raised in
Milwaukee, Wisconsin, with the introduction of reformulated gasoline,
some of which contained 11% (by volume) MTBE, in January 1995
(Anderson, 1993; Anderson et al., 1995). Health complaints have also
been registered by some occupationally exposed individuals, such as
tank truck drivers handling bulk MTBE (Gillie, 1993).
These "outbreaks" of health complaints prompted several field and
experimental studies as well as other assessments (e.g., HEI, 1996; US
Interagency Assessment, 1997) of available data and information
generated by these studies. In this section epidemiological studies
will be described first, followed by controlled chamber studies of
human volunteers. Because non-occupationally as well as occupationally
exposed populations were investigated in the epidemiological studies,
these studies will be discussed in one section rather than two
separate sections devoted to general population and occupational
population exposures.
8.1 Population studies
In December 1992, Moolenar et al. (1994) undertook a pilot study
in Fairbanks, Alaska, to investigate the possible relationship between
MTBE exposure and health complaints. In Phase I of the study, exposure
was evaluated by air sampling and by analysing blood samples for MTBE
and TBA in 18 workers heavily exposed to gasoline fumes and exhaust in
their workplace (e.g., service station workers, mechanics, meter
readers). A questionnaire administered to the workers asked about 15
symptoms: seven that had been most frequently reported to a local
telephone hotline the previous month (headache, eye irritation,
burning of the nose or throat, cough, nausea or vomiting, dizziness,
and a sensation of spaciness or disorientation), and eight other
symptoms (fatigue, fever, sweats or chills, diarrhoea, fainting or
black-out spells, skin irritation or redness, muscle aches, and
difficulty breathing). Phase II of the study was conducted in February
1993, after the oxygenated gasoline programme was suspended, and
included 28 (12 of the original) occupationally exposed subjects. Four
workers whose post-shift blood MTBE levels were in the top quartile
(>9.6 µg/litre) all had one or more of the seven key health
complaints, compared with 9 of 14 workers whose levels were in the
lower three quartiles. This finding was not statistically significant,
but the study may not have had adequate statistical power to detect a
relationship, owing to the small sample size. In Phase II, only one
worker reported a health complaint (nausea). Exposures to MTBE, as
well as complaints of both workers and non-occupationally exposed
residents of Fairbanks, declined significantly after the termination
of the oxygenated gasoline programme (CDC, 1993), but interpretation
of these events is possibly confounded by several factors, including a
lack of representative sampling and changes in the cost of gasoline
that may have contributed to public attitudes.
Mohr et al. (1994) conducted a cross-sectional cohort study that
included 237 garage workers exposed to high and low MTBE
concentrations: 115 workers exposed in northern New Jersey during the
wintertime oxyfuel programme and 122 workers in southern New Jersey 10
weeks after the phase-out date for the programme. Both groups of
workers reported feeling significantly worse at the end of the work
day, but there was no difference between the groups across the work
shift. Active air sampling and passive sampling devices confirmed the
higher exposure levels of the workers in northern New Jersey. No
significant differences were found in either the cross-sectional
reporting of symptoms or the pre- and post-shift analyses.
A study performed in Stamford, Connecticut, in April 1993 near
the end of the oxygenated gasoline season there, investigated exposure
to MTBE in oxygenated gasoline and symptom prevalence in
occupationally and non-occupationally exposed subjects (White et al.,
1995). The study included 37 workers and 14 commuters. The prevalence
of symptoms was highest among people who worked in car repair-shops or
around traffic. The eight workers with the highest levels of MTBE in
blood (>3.8 µg/litre) reported one or more key symptoms (OR = 21.0,
95% CI = 1.8-539.0) such as headache, irritated eyes, burning of the
nose and throat, cough, dizziness, spaciousness, disorientation, and
nausea. There were no reports of diarrhoea, difficulty in breathing,
skin irritation, fever, sweats or chills, or fainting.
In response to health concerns raised by the public following the
introduction of the reformulated gasoline (RFG) programme in the
Milwaukee, Wisconsin area, the Wisconsin Department of Health
initiated a random digit-dial telephone survey designed to assess the
prevalence and scope of health complaints (Anderson et al., 1995). A
questionnaire was administered to approximately 1500 persons: 527
residents of the Milwaukee, where RFG was sold and numerous complaints
about the fuel had been registered; 485 residents of Chicago, where
essentially the same fuels were sold but few complaints had been
registered; and 501 residents of the remainder of Wisconsin, where RFG
had not been required. Overall, there was a significantly higher
prevalence rate for "unusual symptoms" in Milwaukee (23%) than in
Chicago and the rest of Wisconsin (6% each). The fact that symptom
prevalence in the Chicago RFG area was so similar to that in non-RFG
areas of Wisconsin suggested that factors other than RFG use
contributed to the difference between Milwaukee and the other two
areas. Although the prevalence of colds and flu was the same in the
three areas, Milwaukee residents were more likely to report unusual
symptoms if they had experienced a cold or the flu, smoked cigarettes,
or were aware that they had purchased RFG. The authors concluded that
many symptoms reported by Milwaukee residents may have actually been
due to colds or flu rather than RFG exposure. Also, individuals who
reported purchasing RFG were more likely to report symptoms than
individuals who said they had not purchased or did not know whether
they had purchased RFG, which suggested that knowledge about RFG may
have increased awareness of an individual's health status and resulted
in the assumption that any health symptoms experienced were unusual
and attributable to gasoline exposure. This study and its conclusions
were reviewed by a panel of independent experts who concluded that
"The study does not support a conclusion that exposure to RFG is
associated with widespread or serious acute adverse health effects."
This evaluation of the study was later endorsed by another,
independent peer review group (HEI, 1996).
Anderson et al. (1995) followed up on 1280 Milwaukee residents
who had initially contacted governmental agencies about their health
complaints. These self-identified individuals were interviewed by
telephone to determine the types of complaints and risk factors that
could be associated with symptoms. Results of the study indicated that
the strongest predictors were age, allergies, and colds or flu since
November 1994. The purchase of RFG, a surrogate for exposure, did not
correlate with self-reported health symptoms.
A cross-sectional study was conducted in Finland to investigate
the occurrence of neurophysiological symptoms in tanker drivers
exposed to gasoline containing approximately 10% MTBE (Hakkola et al.,
1996). A reference group of milk delivery drivers was selected from
the same areas. A total of 201 male drivers participated in the study,
including 101 tanker drivers and 100 milk delivery drivers. The
occurrence of symptoms and Profile on Mood States (POMS) scales showed
an association with age, chronic diseases, and the perceived health of
the drivers in both the exposed and the unexposed group. Although
there were more sensory and motor symptoms in tank drivers, there was
no evidence of any statistically significant difference in the
occurrence of symptoms between the two groups. Duration of work as a
driver, shift schedule and length of the working week had no
statistical connection with symptoms and the modified POMS scales.
8.2 Controlled studies
To determine if 1-h exposures to MTBE at 6 mg/m3 (1.7 ppm) in an
inhalation chamber could result in similar symptoms as those reported
in the field, Cain et al. (1996) performed a controlled study in 22
male and 21 female subjects (ages 18 to 34 years). Both objective and
subjective indices of behavioural and physiological effects were
studied. A control exposure to air and a control exposure to a
17-component mixture of volatile organic compounds (VOCs) (19 mg/m3)
were also included. The selected MTBE concentration and duration were
based upon preliminary results from exposure studies in commuters
(Lioy et al., 1994). The effects of MTBE exposure on discomfort,
symptoms and possible objective correlates of symptoms were
investigated by using questionnaires, various ocular and nasal
inflammation parameters, and neurobehavioural testing. Repeated blood
samples were obtained from a subset of subjects to relate the exposure
to the body burden and toxicokinetics of MTBE (see section 6.1.1). The
exposure produced a mean peak blood MTBE level of 17 µg/litre.
Subjective reactions, such as irritation, fatigue and headache,
typically showed greater sensitivity than objective indices. There was
a differential effect of gender on rated odour, intensity and
pleasantness. Females found odour intensity greater, pleasantness
worse, and air quality worse. Ocular measurements indicated a mild
tendency for eye irritation during exposure; this was, however, not
statistically significant. MTBE caused no objective inflammatory
changes in the nasal mucosa. Aside from odour, no significant
reactions were found.
In another controlled study, conducted by Prah et al. (1994), 20
male and 20 female volunteer subjects (healthy and non-smokers) were
exposed in an inhalation chamber to MTBE at 5 mg/m3 (1.39 ppm) for
1 h. Symptom questionnaires, cognitive testing, and objective measures
of ocular and nasal irritation were obtained before and at the end of
the exposure. In addition, the odour threshold of MTBE and some
pharmacokinetic data were obtained from two additional subjects, one
male and one female (see section 6.1.1). No increase in the reporting
of symptoms such as headache, nasal irritation, cough or eye
irritation was found, apart from a gender effect in reporting of air
quality. Females rated the air quality in the chamber with MTBE
exposure slightly poorer when compared to the clean air exposure.
There were no changes in objective indicators in either the eye or the
nose.
Johanson et al. (1995) and Nihlén et al. (1998b) reported similar
results at much higher concentrations. Ten healthy male volunteers
were exposed to MTBE vapour at 18, 90 or 180 mg/m3 (5, 25 or 50 ppm)
for 2-h periods while performing light physical exercise (see also
section 6.1.1). All the subjects reported the strong smell of MTBE on
entering the chamber but their rating of the smell decreased with
time. On the basis of subjective assessment there was no irritation of
the ocular, nasal or pharyngeal mucosae. The MTBE exposure induced
essentially no effects on eye and mucous membrane irritation.
Riihimaki et al. (1996) assessed a number of subjective (e.g.,
irritant sensations, mood state) and objective (simple reaction time,
postural sway) end-points in 13 male volunteers exposed to 0, 90 or
268 mg/m3 (0, 25 or 75 ppm) MTBE for 1 or 3 h. The authors concluded
that only "mild symptoms, mainly a feeling of heaviness in the head
and, to a smaller extent, of mild mucous membrane irritation, were
reported". The frequency of symptoms was related to the level of MTBE
exposure and reached statistical significance at 268 mg/m3 (75 ppm)
after 3 h of exposure. There was no effect on reaction time or
postural sway.
8.3 Subpopulations at special risk
There are no data by which to identify any subpopulations (e.g.,
the elderly, pregnant women, children or people with allergy or
asthma) who might be at special risk to MTBE exposure.
8.4 Special studies
8.4.1 Organoleptic properties
For many people, MTBE has a quite distinctive odour. Odour
detection thresholds have been reported to average around 0.1 to
0.2 mg/m3 (0.03-0.05 ppm), with average recognition (identification)
thresholds in a range from 0.2 to 0.5 mg/m3 (0.06-0.13 ppm) for neat
MTBE vapour (TRC, 1993; Smith & Duffy, 1995). For gasoline containing
15% (by volume) MTBE in the USA, average odour detection thresholds
ranged from approximately 0.3 to 3 mg/m3 (0.08- 0.9 ppm), with
recognition thresholds ranging from 0.7 to 2.5 mg/m3 (0.2-0.7 ppm).
Odour thresholds for MTBE-oxygenated gasoline may vary considerably,
depending in part on the aromatic and other constituents of the
gasoline and the sensitivity of the individuals who inhale the
vapours. The addition of MTBE to gasoline has been found to reduce the
detection threshold (i.e. increase "detectability") for gasoline by as
much as 80% (HEI, 1996).
8.4.2 Immunological effects
In a study to assess the effects of MTBE on the immune system,
interleukin levels were measured in blood plasma of 22 volunteers at
several different locations around Fairbanks exposed to auto emissions
derived from oxyfuel (Duffy, 1994). The study was performed during a
4-week period in late November and early December 1992. During this
period, the mean daily temperature ranged from about -1.5°C (35°F) to
about -37°C (-38°F). Plasma interleukin 1 ß (IL-1 ß) and interleukin 6
(IL-6) levels were measured at the beginning and at the end of an 8 h
work day. The results showed no difference between the morning mean
levels (2.50 pg/ml ± 2.4 SD and the evening mean levels (2.53 pg/ml ±
2.6 SD). There were, however, 14 out of 22 individuals who showed
slight increases at the end of the work day. IL-1, which was measured
in 10 individuals, was below the detection limit.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Algae
The effect of MTBE on the growth of the unicellular algae
Selenastrum capricornutum (Chlorophyta), Navicula pelliculosa
(Bacillariophyta) and Synechococcus leopoliensis (Cyanobacteria),
representing three taxonomic groups, was investigated under laboratory
conditions (Rousch & Sommerfeld, 1998). The growth of
N. pelliculosa and S. leopoliensis was inhibited at a nominal MTBE
concentration of 2400 mg/litre in the growth medium, whereas
S. capricornutum growth was increased at 600 mg/litre and decreased
at 4800 mg/litre. The authors suggested that the differential
sensitivity of these representative species implies that MTBE could
alter algal community composition in the environment.
9.1.2 Aquatic animal species
The results of aquatic toxicity tests are presented in Table 21.
Experimental data on acute toxicity are available for four
species of invertebrates, four species of fish, and one species of
amphibian. The experimental data ranged from a 96-h LC50 of 553
mg/litre for the crustacean Chaetogammam marinum (Adema, 1982), to a
96-h LC50 of >10 000 mg/litre for a copepod Nitocra spinipes
(Tarkpea & Svanberg, 1982).
Acute toxicities were determined by Tarkpea & Svanberg (1982) for
MTBE alone, MTBE combined with a base fuel at a concentration of 5%,
and the base fuel alone. The acute toxicity tests were conducted on
the harpacticoid copepod Nitocra spinipes under static conditions
for 96 h at 21°C. The test solutions were not aerated. A 96-h LC50
greater than 10 000 mg/litre was reported for MTBE alone. The 96-h
LC50 values were 242 mg/litre for MTBE in a base fuel and 201
mg/litre for the base fuel alone. The results of the tests show that
the acute toxicity of base fuel to aquatic organisms is not increased
by the addition of 5% MTBE.
Tarkpea & Svanberg (1982) also determined the 24-h LC50 of MTBE
on a shoal fish, the bleak Alburnus alburnus in a closed, static
system at 10°C. The LC50 was between 1700 and 1800 mg/litre. When the
bleaks were introduced into the test media, several sublethal effects
were observed, including disturbed balance, surface swimming and
overturning. The sublethal effects were short-lived, as several of the
individual test subjects had recovered when the test was completed.
The environmental significance of these results was not discussed by
the authors.
Table 21. Aquatic toxicity testing results for MTBE
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Invertebrates
Ceriodaphnia LC50 (48-h) 18-21°C 841 THE, 1989
Daphnia magna LC50 (96-h) >1000 Gupta & Lin, 1995
Copepod LC50 (96-h) >10 000 Tarkpea & Svanberg, 1982
(Nitocra spinipes)
Copepod LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
(Nitocra spinipes)
Gammarid LC10 (96-h) 15°C 553 Adema, 1982
(Chaetogammarus marinus)
Fish
Bleak (Alburnus alburnus) LC50 (24-h) 10°C 1700-1800 Tarkpea & Svanberg, 1982
Bleak (Alburnus alburnus) LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
Fathead minnow LC50 (96-h) 25°C 706 Veith et al., 1983
(Pimephales promelas)
Fathead minnow LC50 (96-h) 672 Geiger et al., 1988
(Pimephales promelas)
Rainbow trout LC50 (96-h) 1300 Environment Canada, 1993
(Oncorhynchus mykiss)
Table 21. (continued)
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Rainbow trout LC50 (96-h) 1483 Environment Canada, 1993
(Oncorhynchus mykiss)
Amphibians
European frog tadpole LC0 (48-h) <2000 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC50 (48-h) 2500 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC100 (48-h) <3000 Paulov, 1987
(Rana temporaria)
When European frog tadpoles (Rana temporaria) were exposed to
concentrations of MTBE in water ranging from 100 to 2500 mg/ litre,
various effects were observed. An increase in body weight of frogs and
tadpoles that had undergone metamorphosis was observed at 100
mg/litre, as compared with the controls. At sublethal concentrations
(<2500 mg/m3) in water, accelerated development of the tadpole was
observed and metamorphosis occurred two days earlier than in controls
(Paulov, 1987). The environmental significance of these results was
not discussed by the author.
No data on chronic aquatic toxicity were found in the literature.
Data on the toxicity of MTBE to terrestrial animals, terrestrial
plants or soil biota were not found in the literature, other than
information from mammalian toxicology studies.
9.2 Field experiments
Data on field experiments on MTBE were not found in the
literature.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Total exposure of human populations to MTBE may involve more than
one environmental pathway and route of intake. Populations may be
exposed to MTBE in air in areas where it is used in gasoline, though
available data on environmental levels and human exposure are limited.
In several studies, median concentrations of MTBE in ambient air
ranged from 0.47 to 14.4 µg/m3 (0.00013 to 0.004 ppm) where MTBE is
used in oxygenated gasoline, and non-detectable to 26.4 µg/m3 (0.0073
ppm) in urban air of cities where MTBE is used as an octane enhancer.
Concentrations near industrial facilities range up to 35.7 mg/m3 (10
ppm). Median 1- to 2-min exposure levels gathered in the breathing
zone of service station attendants and consumers while refuelling were
highly variable, ranging from 1.0 to 21.4 mg/m3 (0.03 to 6 ppm) and
occasionally exceeding 35.7 mg/m3 (10 ppm).
Monitoring data for MTBE are too limited to characterize
adequately its occurrence in drinking-water. The intake of MTBE in
drinking-water is generally expected to be negligible, although
drinking-water may be polluted from point sources such as accidental
spills of large amounts of MTBE in gasoline. Exposure could also occur
through dermal absorption or inhalation of MTBE vapour from household
water used for bathing, cooking and laundering.
Potentially exposed workers include those involved in the
production, handling and use of MTBE and MTBE-containing gasoline,
including mechanics and service station attendants. Occupational
exposure of workers transporting MTBE is highest, with an average
short-term median concentration of 140 mg/m3 (39 ppm). Long-term
average median levels for this group of workers were about 2.85 mg/m3
(0.8 ppm). Median long-term exposure of service station attendants
averaged 1.79 mg/m3 (0.5 ppm). The long-term median value for
mechanics was 0.36 mg/m3 (0.1 ppm).
10.1.2 Human health effects
Consumers in some areas of the USA have complained about acute
health symptoms such as headache, eye and nose irritation, cough,
nausea, dizziness and disorientation associated with the use of
oxygenated fuels such as gasoline containing MTBE. Epidemiological
studies of human populations exposed under occupational and
non-occupational conditions, as well as experimental studies of human
volunteers exposed under controlled conditions, have not been able to
identify a basis for these complaints. Results of community studies
conducted in Alaska, New Jersey, Connecticut and Wisconsin, USA, have
been mixed and provided limited or no evidence of an association
between MTBE exposure and the prevalence of health complaints. In
addition, independently conducted experimental studies of volunteers
exposed in inhalation chambers to MTBE concentrations ranging from 5.0
mg/m3 (1.4 ppm) in one study to 180 mg/m3 (50 ppm) for 2 h in
another study have shown no evident effects in terms of either
subjective reports of symptoms or objective indicators of irritation
or other effects. Based on the collective evidence, it appears
unlikely that MTBE alone induces adverse acute health effects in the
general population under common inhalation exposure conditions.
However, the potential effects of mixtures of gasoline and MTBE, as
well as the manner in which most people are exposed to MTBE in
conjunction with the use of oxygenated fuels, have not been examined
experimentally or through prospective epidemiological methods.
Moreover, the role of factors such as awareness of MTBE, due in part
to its distinctive odour, for example, has not been investigated.
In studies on animals, MTBE is "moderately" acutely toxic, with
an oral LD50 in rats of approximately 3800 mg/kg bw and LC50 value
(15 min) of about 141 000 mg/m3 air in mice. Signs of intoxication
include CNS depression, ataxia and laboured respiration. The LD50 for
dermal toxicity in rabbits is >10 200 mg/kg bw.
MTBE is considered to be a mild skin and eye irritant but does
not induce skin sensitization.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Effect levels are compiled in Tables 22 and 23. Concentrations
or doses that induced significant increases in organ weights, for
which histopathological effects were observed at higher levels, were
considered LOELs. Doses at which histopathological effects were
observed were considered LOAELs (Tables 22 and 23).
Lowest reported effect levels for nephrotoxicity following
ingestion in subchronic studies were 440 mg/kg bw per day (increases
in relative kidney weight and hyaline droplet formation in
Sprague-Dawley rats). At 2860 mg/m3 (800 ppm), in a 90-day inhalation
study, there were increases in kidney weight associated at higher
concentrations with a mild increase in hyaline droplets in the
proximal tubules in Fischer-344 rats. At 1430 mg/m3 (400 ppm), in
inhalation oncogenicity studies, there was an increase in absolute
liver weight, which correlated with increased severity of
hepatocellular hypertrophy at higher concentrations and an increase in
relative kidney weight in male mice; in rats, incidence and severity
of chronic progressive nephropathy were increased at this level.
Exposure to MTBE also results in reversible central nervous
system effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations, and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2860 mg/m3 (800 ppm) produced
reversible, non-monotonically dose-related changes in motor activity.
These effects were transient and not observed in longer-term studies.
Table 22. MTBE levels for non-neoplastic effects following oral exposure
Species Protocol Effect Level Basis of Effect Level Reference
(mg/kg bw/day)
Sprague-Dawley rats 28-day gavage LOAEL 440 males: increase in relative IITRI (1992)
(undiluted) kidney weight; hyaline droplet
formation in convoluted
NOAEL 90 tubules
Sprague-Dawley rats 90-day gavage in LOEL 900 increase in male absolute and Robinson et al. (1990)
corn oil relative kidney weight; chronic
nephropathy and increase in
NOAEL 300 hyaline droplets in proximal
tubular cells at the next
higher dose (1200 mg/kg bw/day)
Sprague-Dawley rats 104 weeks gavage NOEL 1000 no effects at any dose Belpoggi et al. (1995)
in olive oil;
maintained until
death
Table 23. MTBE levels for non-neoplastic effects following inhalation exposure
Species Protocol Effect level Basis for effect level Reference
Fischer-344 rats single exposure LOEL for reversible biphasic changes in motor Gill (1989);
neurological effects = activity in females Daughtrey et al.
2860 mg/m3 (800 ppm) (1997)
Fischer-344 rats 13 week study LOEL 2860 mg/m3 males: increase in relative Dodd & Kintigh (1989);
(800 ppm) weight of liver and kidney; Daughtrey et al. (1997)
no NOAEL males: at 28 600 mg/m3, increase
in lymphoid hyperplasia in
submandibular lymph nodes; mild
increase in hyaline droplets in
renal proximal tubules
Fischer-344 rats up to 104 weeks LOAEL 10 700 mg/m3 males: increased mortality and Chun et al. (1992);
(3000 ppm) decreased survival time at 10 700 Bird et al. (1997)
LOEL 1430 mg/m3 mg/m3; chronic progressive
(400 ppm) nephropathy was the major cause
of death at 10 700 mg/m3; increased
incidence and severity of chronic
progressive nephropathy at all
doses (significance not specified)
CD1 mice up to 18 months LOEL 1430 mg/m3 increase in absolute liver Chun et al. (1992)
(400 ppm) weight in male mice which Bird et al. (1997)
correlated with increased
severity of hepatocellular
hypertrophy at higher
concentrations; increase in
relative kidney weight in males
Specific adverse effects on reproduction have not been observed
in rats at concentrations up to 28 600 mg/m3 (8000 ppm). MTBE has not
induced developmental effects in rats, mice or rabbits at
concentrations less than those that were toxic to the mothers.
Decreases in uterine weight and increases in estrogen metabolism have
been observed at 28 600 mg/m3.
The weight of evidence indicates that MTBE is not genotoxic.
Identified oncogenicity studies include an inhalation study in rats
and mice and an oral study (gavage) in rats. In these investigations,
MTBE induced testicular (Leydig cell) tumours in male rats
(Fischer-344 and Sprague-Dawley), renal tumours in male rats
(Fischer-344) liver tumours in female mice (CD-1) and lymphomas and
leukaemias (combined) in female (Sprague-Dawley) rats.
All investigations on nephrotoxicity are consistent with the
renal tumours observed in Fischer-344 rats being related to
alpha2u-globulin nephropathy. alpha2u-Globulin nephropathy is
considered an effect specific to male rats and, therefore, these
tumours are of questionable relevance to humans.
Leydig cell tumours have been induced by MTBE in two strains of
rats. This type of tumour has been reported to be induced by
non-genotoxic carcinogens that disturb the hormonal balance of
testosterone, luteinizing hormone and luteinizing hormone releasing
factor in rats. Owing to differences between rats and humans in the
regulation of gonadotropins, it is questionable that a similar effect
will occur in humans. Although such a mechanism may be relevant, this
is not substantiated by experimental evidence, since these hormones
were not determined in any of the studies with MTBE.
Liver tumours have been induced by MTBE in female mice and
possibly in male mice (the data on male mice were not corrected for
increased mortality). The effect was modest and occurred only at
28 600 mg/m3 (8000 ppm) and in association with hepatocellular
hypertrophy (indicating enzyme induction) and altered estrogen
metabolism. The relevance of these mouse liver tumours for human risk
estimation is considered to be questionable.
In a single oral study in SD rats, the frequency of lymphomas and
leukaemias (combined) were increased in the high-dose group. This
observation was not supported by any indications of relevant
(preneoplastic) effects on the lymphoid system in other studies.
Moreover, the description of the study made it difficult to evaluate
adequately the results. However, since the effect observed appears to
be rather pronounced, it is not justified to neglect this finding,
based on presumed experimental deficiencies. For a proper evaluation,
additional information is required.
On the basis of these data, MTBE should be considered a rodent
carcinogen. MTBE is not genotoxic and the carcinogenic response is
only evident at high levels of exposure that also induce other adverse
effects. The available data are inconclusive and prohibit their use
for human carcinogenic risk assessment until outstanding complications
in their interpretation have been addressed.a
10.2 Evaluation of effects on the environment
MTBE emissions and leakages can be widespread in the environment
in areas where MTBE is used as an octane improver and oxygenate in
oxygenated gasoline.
MTBE is predominately emitted into air; however, it can be
released into the water and soil compartments. Ambient concentrations
in air are low. There are no terrestrial toxicity data for exposure to
MTBE in air; however, this appears not to be of concern to an
environmental evaluation since ambient air concentrations are low and
its half-life is relatively short.
Owing to its physical and chemical properties, MTBE can persist
longer in water and soil than in air. There are very limited data on
concentrations in ambient surface water. The biodegradation of MTBE in
water and soil is not well understood but is believed to be relatively
slow. MTBE in soil can leach into groundwater and persist there, due
to its lack of removal. MTBE has not been generally detected in deeper
groundwater or in shallow groundwater in agricultural areas. It is
more frequently found in shallower groundwater in urban areas where
MTBE is most extensively used.
Data available for ecotoxicological assessment refer almost
exclusively to MTBE in water. It can be classified as relatively
non-toxic for aquatic biota, with a lowest acute effect for several
aquatic organisms of more than 100 mg/litre. No long-term aquatic
toxicity tests at low concentrations have been identified. However,
the limited data on concentrations of MTBE in ambient surface water
have shown that concentrations range from non-detectable to 30
µg/litre. The maximum concentration is several orders of magnitude
below the effect level of the most sensitive organism tested to date.
It does not appear that the concentrations of MTBE in ambient water
are toxic to aquatic organisms, except during spills when very high
levels of MTBE may be found.
There are no data on concentrations of MTBE in soil or on
terrestrial toxicity. However, concentrations in this medium are
expected to be low except in the case of spills.
a MTBE was reviewed by an International Agency for Research on
Cancer (IARC) Working Group in October 1998. The conclusions were that
there was inadequate evidence for the carcinogenicity in humans of
MTBE, limited evidence for its carcinogenicity in experimental
animals, and the overall evaluation was that MTBE was not classifiable
as to its carcinogenicity for humans (Group 3).
11. RECOMMENDATIONS
To provide quantitative guidance on relevant limits of exposure
and to estimate risk, it is recommended that additional data be
acquired in the following areas:
a) additional information to evaluate the induction of
lymphomas/leukaemias in Sprague-Dawley rats;
b) mechanistic data on the induction of Leydig cell tumours and sex
specificity of liver tumours in mice;
c) controlled exposure studies to characterize the dose-response in
humans for MTBE and MTBE-containing mixtures;
d) monitoring data for better characterization of human exposure,
with particular attention to microenvironments;
e) potentiation studies of MTBE with BTX components of gasoline;
f) monitoring of environmental concentrations of MTBE in soil and
biota in areas adjacent to major sources and ambient areas in
order to verify theoretical values;
g) long-term toxicity tests in aquatic and possibly terrestrial
organisms;
h) field degradation tests to determine how persistent MTBE can be
in soil and groundwater under a range of redox conditions.
REFERENCES
ACGIH (1994) 1994-1995 threshold limit values for chemical substances
and physical agents and indices. Cincinnati, Ohio, American Conference
of Governmental Industrial Hygienists, p 27.
Adam G, Knuechel R, Vorwerk D, Held C, & Guenther RW (1990) Tissue
response of the biliary and digestive system of rabbits after MTBE
infusion into the gallbladder. Invest Radiol, 25(1): 58-61.
Adema DMM (1982) Tests and desk studies carried out by MT-TNO during
1980-1981 for Annex II of MARPOL 1973. Delft, The Netherlands,
Organization for Applied Scientific Research, 51 pp (Report CL
82/1499).
AFS (1994) Statute book of the Swedish National Board of Occupational
Safety and Health, Ordinance AFS 1993: 9: Occupational exposure limit
values. Solna, The Swedish National Board of Occupational Safety and
Health, pp 46, 52.
Akimoto R, Rieger E, Moossa AR, Hofmann AF, & Wahlstrom HE (1992)
Systemic and local toxicity in the rat of methyl tert-butyl ether: a
gallstone dissolution agent. J Surg Res, 53: 572-577.
Allard A-S, Remberger M, & Neilson AH (1996) The aerobic
biodegradation of tert-butyl methyl ether and tert-butanol: an
initiatory study. Stockholm, The Swedish Environmental Research
Institute, 9 pp (IVL Report No. B 1197).
Allen MK & Grande D (1995) Reformulated gasoline air monitoring study.
Madison, Wisconsin, Department of Natural Resources, Bureau of Air
Management, 50 pp (Publication No. AM-175-95).
Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985a) Rapid dissolution of gallstones by methyl tert-butyl ether.
N Engl J Med, 312(4): 217-220.
Allen MJ, Barody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985b) Cholelitholysis using methyl tertiary butyl ether.
Gastroenterology, 88: 122-125.
Almaguer D (1993) Health hazard evaluation report HETA 93-0884-2344:
National Center for Environmental Health, Albany, New York.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 33 pp (NTIS/PB94-129020).
Anderson EV (1993) Health studies indicate MTBE is safe gasoline
additive. Chem Eng News, September 20: 9-18.
Anderson HA, Hanrahan L, Goldring J, & Delaney B (1995) An
investigation of health concerns attributed to reformulated gasoline
use in southwest Wisconsin - Final Report. Madison, Wisconsin,
Department of Health and Social Services, 50 pp.
Angle CR (1991) If the tap water smells foul, think MTBE. J Am Med
Assoc, 266: 2985-2986.
API (1989a) Aboveground storage tank survey. Washington, DC, American
Petroleum Institute, Health and Environment Affairs Department, 44 pp
(Publication No. 301).
API (1989b) Monitoring near refineries for airborne chemicals - Volume
1: Validated ambient air concentrations around three refineries.
Washington, DC, American Petroleum Institute, Health and Environment
Affairs Department, 83 pp (Publication No. 4484).
API (1993) Gasoline vapor exposure assessment at service stations.
Washington, DC, American Petroleum Institute, 8 pp (Publication No.
4553).
API (1994) A survey of API members' aboveground storage tank
facilities. Washington, DC, American Petroleum Institute, 65 pp
(Publication No. 330).
API (1995a) Executive summary of a study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 5 pp (Publication No. 4619).
API (1995b) Petroleum Institute data characterizing occupational
exposures to methyl tertiary butyl ether (MTBE) 1983-1993. Washington,
DC, American Petroleum Institute, 21 pp (Publication No. 4622).
API (1995c) Service station personnel exposures to oxygenated fuel
components - 1994: Executive summary. Washington, DC, American
Petroleum Institute, 3 pp (Publication No. 4625).
Arashidani K, Katoh T, Yoshikawa M, Kikuchi M, Kawamoto J, & Kodama Y
(1993) LD50 and weight change in organs of mice following
intraperitoneal administration of methyl tertiary-butyl ether. Jpn J
Ind Health, 35: 404-405.
ARCO (1987) Methyl tert-butyl ether exposure monitoring study.
Channelview, Texas, ARCO Chemical Company, 39 pp.
Atkinson R (1990) Gas-phase tropospheric chemistry of organic
compounds : a review. Atmos Environ, 24A: 1-41.
Atkinson R & Pitts JN Jr (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-246°K. J
Chem Phys, 68(8): 3581-3584.
Barreto RD, Gray KA, & Anders K (1995) Photocatalytic degradation of
methyl- tert-butyl ether in TiO2 slurries: a proposed reaction
scheme. Water Res, 29(5): 1243-1248.
Beller M & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska. Anchorage, Alaska, Department
of Health and Social Services, Section of Epidemiology, 5 pp.
Belpoggi F, Soffritti M, & Maltoni C (1995) Methyl-tertiary-butyl
ether (MTBE) - a gasoline additive - causes testicular and
lympho-haematopoietic cancers in rats. Toxicol Ind Health, 11(2):
119-149.
Bengtsson EB & Tarkpea M (1983) The acute aquatic toxicity of some
substances carried by ships. Mar Pollut Bull, 14(6): 213-214.
Bevan C, Neeper-Bradley TL, Tyl RW, Fisher LC, Panson RD, Kneiss JJ, &
Andrews LS (1997) Two-generation reproductive toxicity study of methyl
tertiary-butyl ether (MTBE) in rats. J Appl Toxicol, 17(suppl 1):
S13-S19.
Bianchi A & Varney MS (1989) Analysis of methyl tert-butyl ether and
1,2-dihaloethanes in estuarine water and sediments using
purge-and-trap/gas chromatography. J High Res Chromatogr, 12(3):
184-186.
Bianchi A, Varney MS, & Phillips J (1991) Analysis of volatile organic
compounds in estuarine sediments using dynamic headspace and gas
chromatography-mass spectrometry. J Chromatogr, 542: 413-450.
Biles RW, Schroeder RE, & Holdsworth CE (1987) Methyl tertiary butyl
ether inhalation in rats: a single generation reproduction study.
Toxicol Ind Health, 3(4): 519-534.
Biodynamics (1984) The metabolic fate of methyl-t-butyl ether (MTBE)
following an acute intraperitoneal injection (Project No. 80089).
Washington, DC, American Petroleum Institute, 97 pp.
Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, & Andrews
LS (1997) Oncogenicity studies of inhaled methyl tertiary-butyl ether
in CD1 mice and F-344 rats. J Appl Toxicol, 17(suppl 1): S45-S55.
Blackburn GR, Dooley JF III, Schreiner CA, & Mackerer CR (1991)
Specific identification of formaldehyde-mediated mutagenicity using
the mouse lymphoma L5178Y TK+/- assay supplemented with formaldehyde
dehydrogenase. In Vitro Toxicol, 4(2): 121-132.
Bonin MA, Ashley DL, Cardinali FL, McGraw JM, & Wooten JV (1994)
Measurement of methyl tert-butyl ether and tert-butyl alcohol in
human blood and urine by purge-and-trap gas chromatography-mass
spectrometry using an isotope dilution method. In: 208th National
Meeting of the American Chemical Society, 1994. Washington, DC,
American Chemical Society, 1 p (Abstract P102-ENVR).
Borghoff SJ & Lagarde WH (1993) Assessment of binding of
2,4,4-trimethyl-2-pentanol to low-molecular-weight proteins isolated
from kidneys of male rats and humans. Toxicol Appl Pharmacol, 119:
228-235.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha2u-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 264-275.
Borghoff SJ, Jayjock E, & Murphy JE (1995) Methyl tert-butyl ether
(MTBE) vs ethyl tert-butyl ether (ETBE): A comparison of blood and
tissue partition coefficients in male and female F344 rats.
Toxicologist, 30: 34.
Borghoff SJ, Murphy JA, & Medinsky MA (1996) Development of a
physiologically based pharmacokinetic model for methyl
tertiary-butyl ether and tertiary-butanol in male Fischer-344
rats. Fundam Appl Toxicol, 30: 264-275.
Borghoff SJ, Murphy JE, Turner M, & James AR (1998) Dosimetry of
methyl t-butyl ether (MTBE) and t-butyl alcohol in male and female
rats following inhalation exposure to MTBE. Toxicologist, 42(1-S):
A1049.
Brady JF, Xiao F, Ning SM, & Yang CS (1990) Metabolism of methyl
tertiary-butyl ether by rat hepatic microsomes. Arch Toxicol, 64:
157-160.
Brown SL (1997) Atmospheric and potable water exposures to methyl
tert-butyl ether (MTBE). Regul Toxicol Pharmacol, 25: 256-276.
Bruce BW & McMahon PB (1996) Shallow ground-water quality beneath a
major urban center: Denver, Colorado, USA. J Hydrol, 186: 129-151.
Buckley TJ, Prah JD, Ashley D, Zweidinger RA, & Wallace LA (1997) Body
burden measurements and models to assess inhalation exposure to methyl
tertiary butyl ether (MTBE). J Air Waste Manage Assoc, 47: 739-752.
Buchta TM (1993a) Health hazard evaluation report HETA 93-606-2336:
National Centers for Environmental Health, Fairbanks, Alaska.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 37 pp (NTIS/PB94-133667).
Buchta TM (1993b) Health hazard evaluation report HETA 93-802-2338:
National Centers for Environmental Health, Stamford Connecticut.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 39 pp (NTIS/PB94-134343).
Budavari S, O'Heil MJ, Smith A, Heckelman PE, & Kinneary JF ed. (1996)
Methyl- tert-butyl ether. In: The Merck index, 12th ed. Whitehouse
Station, New Jersey, Merck & Co., Inc., p 1611.
Burleigh-Flayer HD, Chun JS, & Kintigh WJ (1992) Methyl tertiary butyl
ether: vapor inhalation oncogenicity study in CD-1 mice (Laboratory
project ID 91N0013A). Export, Pennsylvania, Bushy Run Research Center,
1068 pp (Report to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Cain WS, Leaderer BP, Ginsberg GL, Andrews LS, Cometto-Muniz JE, Gent
JF, Buck M, Berglund LG, Mohsenin V, Monahan E, & Kjaergaard S. (1996)
Acute exposure to low-level methyl tertiary-butyl ether (MTBE): human
reactions and pharmacokinetic response. Inhal Toxicol, 8: 21-48.
Calvert JG & Pitts JN Jr (1966) Photochemistry. New York, John Wiley &
Sons, Inc., pp 441-442.
CDC (1993) An investigation of exposure to methyl tertiary butyl ether
among motorists and exposed workers in Stamford, Connecticut.
Presented at the EPA Workshop on MTBE and other Oxygenates, Falls
Church, Virginia, 27 July 1993. Atlanta, Georgia, Centers for Disease
Control and Prevention, 35 pp.
Cederbaum AI & Cohen G (1980) Oxidative demethylation of t-butyl
alcohol by rat liver microsomes. Biochem Biophys Res Commun, 97:
730-736.
CEN (1996) Growth of top 50 chemicals slowed in 1995 from very high
1994 rate. Chem Eng News, April 8:16-20.
Chandler B & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska: Preliminary results. Anchorage,
Alaska, Department of Health and Social Services, 5 pp.
Charbonneau M, Strasser J Jr, Lock EA, Turner MJ Jr, & Swenberg JA
(1989) Involvement of reversible binding to alpha2u-globulin in
1,4-dichlorobenzene-induced nephrotoxicity. Toxicol Appl Pharmacol,
99: 122-132.
Chen CY, Chang KK, Chow NH, Leow TC, Chou TC, & Lin XZ (1995) Toxic
effects of cholelitholytic solvents on gallbladder and liver. Dig Dis
Sci, 40(2): 419-426.
Chiang CY, Loos KR, & Klopp RA (1992) Field determination of
geological/chemical properties of an aquifer by cone penetrometry and
headspace analysis. Ground Water, 30: 428-436.
Chun JS & Kintigh WJ (1993) Methyl tertiary-butyl ether: twenty-eight
day vapor inhalation study in rats and mice. Export, Pennsylvania,
Bushy Run Research Center, 387 pp (BRRC Report 93N1241).
Chun JS, Burleigh-Flayer HD, & Kintigh WJ (1992) Methyl tertiary butyl
ether (MTBE): vapor inhalation oncogenicity study in Fischer 344 rats
(Laboratory project ID 91N0013B). Export, Pennsylvania, Bushy Run
Research Center, 1463 pp (Report for the Methyl Tertiary Butyl Ether
Committee, Washington, DC).
Church CD, Isabelle LM, Pankow JF, Tratnyek PG, & Rose DL (1997)
Assessing the in situ degradation of methyl tert-butyl ether
(MTBE) byproduct identification at the sub-PPB level using direct
aqueous injection GC/MS: Extended abstract - Proceedings of ACS
National Meeting, San Francisco, California, 16-17 April 1997.
Washington, DC, American Chemical Society, 4 pp.
Cinelli S & Seeberg AH (1989) Reverse mutation in Salmonella
typhimurium. Test substance: MTBE. Rome, Life Science Research,
Toxicology Centre, 50 pp (LSR-RTC Report No. 216001-M-03489).
Cirvello JD, Radovsky A, Heath JE, Farnell DR, & Lindamood C (1995)
Toxicity and carcinogenicity of T-butyl alcohol in rats and mice
following chronic exposure in drinking water. Toxicol Ind Health, 11:
151-165.
Clark CR, Dutcher JS, Henderson TR, McClellan RO, Marshall WF, Naman
TM, & Seizinger DE (1984) Mutagenicity of automotive particulate
exhaust: influence of fuel extenders, additives, and aromatic content.
In: MacFarland HN, Holdsworth CE, MacGregor JA, Call RW, & Lane ML ed.
Applied toxicology of petroleum hydrocarbons. Princeton, New Jersey,
Princeton Scientific Publishers, pp 109-122.
Clayton Environmental Consultants (1991) Gasoline vapor exposure
assessment for the American Petroleum Institute (API) (Project No.
31774.00). Cypress, California, Clayton Environmental Consultants, 139
pp.
Cline PV, Delfino JJ, & Rao SC (1991) Partitioning of aromatic
constituents into water from gasoline and other complex solvent
mixtures. Environ Sci Technol, 25: 914-920.
Cochrane RA & Hillman DE (1984) Direct gas chromatographic
determination of alcohols and methyl tert-butyl ether in gasolines
using infrared detection. J Chromatogr, 287: 197-201.
Conaway CC, Schroeder RE, & Snyder NK (1985) Teratology evaluation of
methyl tertiary butyl ether in rats and mice. J Toxicol Environ
Health, 16: 797-809.
Cook CK & Kovein RJ (1995) NIOSH health hazard evaluation report No.
94-0220-2526: Exxon Company, Houston, Texas, USA. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 28 pp.
Cox RA & Goldstone A (1982) Atmospheric reactivity of oxygenated motor
fuel additives. In: Proceedings of the 2nd European Symposium on the
Physico-chemical Behaviour of Atmospheric Pollutants, Varese, Italy,
29 September-1 October 1981. Dordrecht, D Riedel Publishing Co, pp
112-119.
Cuthbert JA (1979) Safety tests on methyl- tert-butyl-ether.
Edinburgh, Inveresk Research International, 18 pp (IRI Report No.
1300).
Dale MS, Losee RF, Crofts EW, & Davis MK (1997) MTBE: Occurrence and
fate in source-water supplies. J Am Chem Soc, 37: 376-377.
Daughtrey WC, Gill MW, Pritts IM, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Neurotoxicological evaluation of methyl tertiary-butyl ether in
rats. J Appl Toxicol, 17(suppl 1): S57-S64.
DECOS (Dutch Expert Committee on Occupational Standards) (1994)
Methyl-t-butylether: Health based recommended occupational exposure
limit. The Hague, Health Council of the Netherlands, 75 pp
(Publication No. 1994/23).
Delzer GC, Zogorski JS, Lopes TJ, & Bosshart RL (1996) Occurrence of
the gasoline oxygenate MTBE and BTEX compounds in urban stormwater in
the United States, 1991-95: US Geological Survey. Rapid City, South
Dakota, US Geological Survey, 6 pp (Water-Resources Investigations
Report No. 96-4145).
Derwent RG, Jenkin ME, & Sandow SM (1996) Photochemical ozone creation
potentials for a large number of reactive hydrocarbons under European
conditions. Atmos Environ, 30(2): 181-199.
Dey JC, Brown RA, & McFarland WE (1991) Methyl tert-butyl ether.
Hazard Mater Control, 4: 32-39.
Diehl JW, Finkbeiner JW, & Di Sanzo FP (1995) Determination of ethers
and alcohols in reformulated gasolines by gas chromatography/atomic
emission detection. J High Res Chromatogr, 18: 108-110.
Di Padova C, Di Padova F, Montorsi W, & Tritapepe R (1986) Methyl
tert-butyl ether fails to dissolve retained radiolucent common bile
duct stones. Gastroenterology, 91: 1296-1300.
Dodd DE & Kintigh WJ (1989) Methyl tertiary butyl ether (MTBE):
repeated (13-week) vapor inhalation study in rats with neurotoxicity
evaluation. Export, Pennsylvania, Bushy Run Research Center, 502 pp
(Report No. 52-507 to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Drivas PJ, Valberg PA, & Gauthier TD (1991) Health assessment of air
toxics emissions from alternative fuels. Paper presented at the 84th
Annual Meeting of the Air and Waste Management Association, Vancouver,
British Columbia, 16-21 June 1991. Washington, DC, Air and Waste
Management Association, 17 pp.
Du JT, Abernathy CO, Donohue J, Mahfouz A, & Khanna K (1998) A
drinking water advisory: consumer acceptability and advice and health
effects analysis on methyl tertiary-butyl ether (MTBE). Toxicologist,
42(1-S): A 1123.
Duffy LK (1994) Oxyfuel in Alaska: use of interleukins to monitor
effects on the immune system. Sci Total Environ, 151: 253-256.
ECETOC (1997) Methyl tert-butyl ether (MTBE): Health risk
characterization. Brussels, European Centre for Ecotoxicology and
Toxicology of Chemicals, 126 pp (Technical Report No. 72).
Environment Canada (1989) Analysis of shellfish for organic and
inorganic contaminants. Dartmouth, Nova Scotia, Environment Canada
Conservation and Protection, 10 pp (Report No. AN 893118).
Environment Canada (1992) Canadian Environmental Protection Act -
Priority substances list: Methyl tertiary-butyl ether. Ottawa,
Minister of Supply and Services, 19 pp (Assessment Report No. 5).
Environment Canada (1993) Canadian Environmental Protection Act -
Priority substances list: Supporting document methyl tertiary butyl
ether. Hull, Quebec, Environment Canada, 49 pp.
Environment Canada (1996a) Canadian Environmental Protection Act -
National pollutant release inventory: Summary report 1994. Hull,
Quebec, Environment Canada, 143 pp.
Environment Canada (1996b) MTBE concentrations at selected locations:
National air pollution surveillance programme 1995-1996. Hull, Quebec,
Environment Canada, 21 pp.
Erdal S, Gong H Jr, Linn WS, & Rykowski R (1997) Projection of health
benefits from ambient ozone reduction related to the use of methyl
tertiary butyl ether (MTBE) in the reformulated gasoline program.
Risk Anal, 17: 693-704.
Esch O, Spinosa JC, Hamilton RL, Crombie DL, Schteingart CD, Rondinone
JF, DAgostino HB, Lillienau J, & Hofmann AF (1992a) Acute effects of
topical methyl tert-butyl ether or ethyl propionate on gallbladder
histology in animals: a comparison of two solvents for contact
dissolution of cholesterol gallstones. Hepatology, 16(4): 984-991.
Esch O, Schteingart CD, Pappert D, Kirby D, Streich R, & Hofmann AF
(1992b) Increased blood levels of methyl tert-butyl ether but not of
ethyl propionate during instillation with contact gallstone
dissolution agents in the pig. Hepatology, 18(2): 373-379.
Ferdinandi ES, Houle J-M, Lalande M, Provencher A, & St-Onge Brault G
(1990a) Pharmacokinetics of methyl tert-butyl ether (MTBE) and
tert-butyl alcohol (TBA) in male and female Fischer-344 rats after
administration of MTBE by the intravenous, oral and dermal routes.
Montreal, Bio-Research Laboratories Ltd, 98 pp (Report No. 38842 to
the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lalande M, Houle J-M, Provencher A, & Ducharme S
(1990b) Mass balance of radioactivity and metabolism of methyl
ter-butyl ether (MTBE) in male and female Fischer 344 rats after
intravenous, oral and dermal administration of 14C-MTBE. Montreal,
Bio-Research Laboratories Ltd, 127 pp (Report No. 38843 to the MTBE
Task Force, Washington, DC).
Ferdinandi ES, Houle J-M, Lalande M, Lulham G, Pilon D, Provencher A,
Labbé R, & St-Onge Brault G (1990c) Pharmacokinetics of methyl
tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) in male and
female Fischer-344 rats after single and repeat inhalation nose-only
exposures to MTBE. Montreal, Bio-Research Laboratories Ltd, 172 pp
(Report No. 38844 to the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lulham G, Pilon D, Labbé R, Lalande M, Provencher A,
Houle J-M, & Ducharme S (1990d) Disposition of radioactivity and
metabolism of methyl tert-butyl ether (MTBE) in male and female
Fischer 344 rats after nose-only inhalation exposure to 14C-MTBE.
Montreal, Bio-Research Laboratories Ltd, 89 pp (Report No. 38845 to
the MTBE Task Force, Washington, DC).
Fujiwara Y, Kinoshita T, Sato H, & Kojima I (1984) [Biodegradation and
bioconcentration of alkyl ethers.] Yukagatu, 33(2): 111-114 (in
Japanese with tables and abstract in English).
Garrett P, Moreau M, & Lowry JD (1986) MTBE as a groundwater
contaminant. In: Proceedings of the 1986 National Water Well
Association and American Petroleum Institute Conference on Petroleum
and Organic Chemicals in Groundwater, Houston, Texas, 12-14 November
1986. Dublin, Ohio, National Water Well Association, pp 227-238.
Geiger DL, Call DJ, & Brooks LT (1988) Acute toxicities of organic
chemicals to Fathead Minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 4, pp 75-76.
Giacomello P (1996) MTBE exposure in service stations: Italian study.
Paper presented at the 7th European Fuel Oxygenates Association
Conference, Sodehotel, 24-25 October 1996. Brussels, European Chemical
Industries Federation, 8 pp.
Gill MW (1989) Methyl tertiary butyl ether single exposure vapor
inhalation neurotoxicity study in rats. Export, Pennsylvania, Bushy
Run Research Center, 116 pp (Report No. 52-533 to the Methyl Tertiary
Butyl Ether Committee, Washington, DC).
Gillie AD (1993) Industrial hygiene monitoring methyl tertiary butyl
ether, benzene and gasoline. San Diego, California, Texaco Inc.,
Environmental Health and Safety Division, Industrial Hygiene, 31 pp.
Gordian ME & Guay G (1995) Benzene in Alaska. Alsk Med, 37: 25-28, 36.
Gordian ME, Huelsman MD, Brecht M-L, & Fisher DG (1995) Health effects
of methyl tertiary butyl ether (MTBE) in gasoline in Alaska. Alsk Med,
37: 101-103, 119.
Greenough RJ, McDonald P, Robinson P, Cowie JR, Maule W, Macnaughton
F, & Rushton A (1980) Methyl tertiary-butyl ether (Driveron): three
month inhalation toxicity in rats (Project No. 413038). Edinburgh,
Scotland, Inveresk Research International, 230 pp.
Grindstaff G, Henry M, Hernandez O, Hogan K, Lai D, & Siegel-Scott C
(1991) Formaldehyde risk assessment update. Washington DC, US
Environmental Protection Agency, Office of Toxic Substances, 140 pp.
Gupta G & & Lin YJ (1995) Toxicity of methyl tertiary butyl ether to
Daphnia magna and Photobacterium phosphoreum. Bull Environ Contam
Toxicol, 55: 618-620.
Hakkola M & Saarinen L (1996) Exposure of tanker drivers to gasoline
and some of its components. Ann Occup Hyg, 40: 1-10.
Hakkola M, Honkasalo ML, & Pulkkinen P (1996) Neuropsychological
symptoms among tanker drivers exposed to gasoline. J Occup Med, 46:
124-130.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, & Valcovic
LR (1993) Hazard evaluation of chemicals that cause accumulation of
alpha-globulin, hyaline droplet nephropathy, and tubule neoplasia in
the kidneys of male rats. Environ Health Perspect, 99: 313-349.
Harper M & Fiore AA (1995) Determination of methyl tert-butyl ether
in air using a diffusive sampler. Appl Occup Environ Hyg, 10: 616-619.
Hartle R (1993) Exposure to methyl tert-butyl ether and benzene
among service station attendants and operators. Environ Health
Perspect, 101(suppl 6): 23-26.
Haseman JK, Arnold J, & Eustis SL (1990) Tumor incidences in Fischer
344 rats: NTP historical data. In: Pathology of the Fischer rat:
Reference and atlas. McLean, Virginia, Academic Press, chapter 35, pp
555-564.
Hazleton Laboratories (1979) Acute eye irritation study in rabbits:
tert-butyl methyl ether (95% pure). Leesburg, Virginia, Hazleton
Laboratories America, Inc., 14 pp (Report No. 2024-132).
Hebert RS (1993) Industrial hygiene exposure assessment for methyl
tert-butyl ether (MTBE), benzene and gasoline. Houston, Texas, Star
Enterprise Environment, Health and Safety Department, 36 pp.
HEI (1996) The potential health effects of oxygenates added to
gasoline: a review of the current literature. A special report of the
Institute's oxygenates evaluation committee. Cambridge, Massachusetts,
Health Effects Institute, 158 pp.
Hinton J (1993) Occupational exposures. Presented at the MTBE
Conference in Washington, DC, 26-27 July 1993. Beacon, New York,
Texaco Inc., 37 pp.
Hoekman SK (1992) Speciated measurements and calculated reactivities
of vehicle exhaust emissions from conventional and reformulated
gasolines. Environ Sci Technol, 26: 1206-1216.
Hoekman SK (1993) Improved gas chromatography procedure for speciated
hydrocarbon measurements of vehicle emissions. J Chromatogr, 639:
239-253.
Hong J-Y, Yang CS, Lee M, Wang Y-Y, Huang WQ, Tan y, Patten CJ, &
Bondoc FY (1997) Role of cytochromes P450 in the metabolism of methyl
tert-butyl ether in human livers. Arch Toxicol, 71: 266-269.
Hong J-Y, Yang CS, Wang Y-Y, Bondoc FY, Pan J, Cokonis C, & Bao ZP
(1998) Use of CYP2E1 knock-out mice to assess the role of CYP2E1 in
metabolizing methyl tert-butyl ether (MTBE) and other gasoline ethers.
Toxicologist, 42(1-S): A87.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, Michigan, Lewis
Publishers, Inc., pp 653-654.
Hsieh CR & Ouimette JR (1994) Comparative study of multimedia modeling
for dynamic partitioning of fossil fuels-related pollutants. J Hazard
Mater, 37: 489-505.
Huber AH (1995) Human exposure estimates of methyl tertiary butyl
ether (MTBE). Presentation at Conference on MTBE and Other Oxygenates:
A Research Update, Falls Church, Virginia, 26-28 July 1993.
Washington, DC, National Technical Information Service, 32 pp (EPA
/600/A-94/255; NTIS/PB95-177150).
IITRI (1992) 28-day oral (gavage) toxicity of methyl tert-butyl
ether (MTBE) in rats (Project No. L08100). Chicago, Illinois, Illinois
Institute of Technology Research, 48 pp.
Imbriani M, Ghittori S, & Pezzagno G (1997) Partition coefficients of
methyl tert-butyl ether. G Ital Med Lav Ergon, 19: 63-65.
Industrial Bio-Test Laboratories (1969) Acute toxicity studies on
X-801-25. Northbrook, Illinois, Industrial Bio-Test Laboratories, 27
pp (Report No. A6809 to Sun Oil Company).
Japar SM, Wallington TJ, Richert JFO, & Ball JC (1990) The atmospheric
chemistry of oxygenated fuel additives: t-butyl alcohol, dimethyl
ether, and methyl t-butyl ether. Int J Chem Kinet, 22: 1257-1269.
Japar SM, Wallington TJ, Rudy SJ, & Chong TY (1991) Ozone-forming
potential of a series of oxygenated organic compounds. Environ Sci
Technol, 25: 415-420.
Johansen NG (1984) The analysis of C1-C4 alcohols, MTBE, and DIPE in
motor gasolines by multi-dimensional capillary column gas
chromatography. J High Res Chromatogr Chromatogr Commun, 7: 487-489.
Johanson G, Nihlén A, & Löf, A (1995) Toxicokinetics and acute effects
of MTBE and ETBE in male volunteers. Toxicol Lett, 82/83: 713-718.
Johnson T, McCoy M, & Wisbith T (1995) A study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 108 pp (API Publication No. 4619).
Katoh T, Arashidani K, Kikuchi M, Yoshikawa M, & Kodama Y (1993)
Effects of methyl tertiary butyl ether on hepatic lipid peroxidation
in mice. Jpn J Hyg, 48: 373-378.
Kelly TJ, Callahan PJ, Piell J, & Evans GF (1993) Method development
and field measurements for polar volatile organic compounds in ambient
air. Environ Sci Technol, 27: 1146-1153.
Kirchstetter TW, Singer BC, Harley RA, Kendall GR, & Chan W (1996)
Impact of oxygenated gasoline use on California light-duty vehicle
emissions. Environ Sci Technol, 30: 661-670.
Levy JM & Yancey JA (1986) Dual capillary gas chromatographic analysis
of alcohols and methyl tert-butyl ether in gasolines. J High Res
Chromatogr Chromatogr Commun, 9: 383-387.
Lewis RJ (1993) Methyl -tert-butyl ether (MTBE). In: Hawley's
condensed chemical dictionary, 12th ed. New York, Van Nostrand
Reinhold Co., p 760.
Lindamood C, Farnell DR, Giles HD, Prejean JD, Collins JJ, Takahashi
K, & Maronpot RR (1992) Subchronic toxicity studies to t-butyl
alcohol in rats and mice. Fundam Appl Toxicol, 19: 91-100.
Lindstrom AB & Pleil JD (1996) Alveolar breath sampling and analysis
to assess exposure to methyl tertiary butyl ether (MTBE) during motor
vehicle refuelling. J Air Waste Manage Assoc, 46: 676-682.
Lington AW, Dodd DE, Ridlon SA, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Evaluation of 13-week inhalation toxicity study on methyl
t-butyl ether (MTBE) in Fischer 344 rats. J Appl Toxicol, 17 (suppl
1): S37-S44.
Lioy PJ, Weisel CP, Jo WK, Pellizzari E, & Raymer JH (1994)
Microenvironmental and personal measurements of methyl-tertiary butyl
ether (MTBE) associated with automobile use activities. J Expo Anal
Environ Epidemiol, 4(4): 427-444.
Litton Bionetics (1980) Guinea pig sensitization study (TBME-99).
Kensington, Maryland, Litton Bionetics, Inc., 30 pp (Report No.
22011).
Lyman WJ, Reehl WF, & Rosenblatt DH (1990) Handbook of organic
chemical property estimation methods: Environmental behavior of
organic compounds. Washington, DC, American Chemical Society, 960 pp.
McGahan JP, Tesluk H, Brock JM, Johnston R, & Shaul DB (1988)
Dissolution of gallstones using methyl tertiary-butyl ether in an
animal model. Invest Radiol, 23: 599-603.
Mackay D, Shiu WY, & Ma KC (1993) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals - volume 3: Volatile organic chemicals. Boca Raton, Florida,
Lewis Publishers, 916 pp.
Mackerer CR, Angelosanto FA, Blackburn GR, & Schreiner CA (1996)
Identification of formaldehyde as the metabolite responsible for the
mutagenicity of methyl tertiary-butyl ether in the activated mouse
lymphoma assay. Proc Soc Exp Biol Med, 212: 338-341.
Marsh DF & Leake CD (1950) The comparative anesthetic activity of the
aliphatic ethers. Anesthesiology, 11: 455-463.
Mihelcic JR (1990) Modeling the potential effect of additives on
enhancing the solubility of aromatic solutes contained in gasoline.
Ground Water Monit Rev, 10: 132-137.
Miller MJ, Ferdinandi ES, Andrews LS, Douglas JF, & Kneiss JJ (1997)
Pharmacokinetics and disposition of methyl t-butyl ether in
Fischer-344 rats. J Appl Toxicol, 17(suppl 1): S3-S12.
Mo K, Lora CO, Javanmardian M, Yang X, & Kulpa CF (1997)
Biodegradation of methyl t-butyl ether by pure bacterial cultures.
Appl Microbiol Biotechnol, 47: 69-72.
Mobil Oil Corporation (1993) Activated mouse lymphoma (L5178Y/TK/+/-)
mutagenicity assay supplemented with formaldehyde dehydrogenase for
methyl tertiary butyl ether. Princeton, New Jersey, Mobil Oil
Corporation, 17 pp (Status report No. 65579).
Mohr SN, Fiedler N, Weisel C, & Kelly-McNeil K (1994) Health effects
of MTBE among New Jersey garage workers. Inhal Toxicol, 6(6): 553-562.
Möller Jensen H & Arvin E (1990) Solubility and degradability of the
gasoline additive MTBE, methyl tert-butyl ether, and gasoline
compounds in water. In: Arendt F, Hinsewald M, & van der Brink WJ ed.
Contaminated soil 90. Dodrecht, Kluwer Academic Publishers, pp
445-448.
Moolenaar RL, Hefflin BJ, Ashley DL, Middaugh JP, & Etzel RA (1994)
Methyl tertiary butyl ether in human blood after exposure to
oxygenated fuel in Faibanks, Alaska. Arch Environ Health, 49(5):
402-409.
Mormile MR, Liu S, & Suflita JM (1994) Anaerobic biodegradation of
gasoline oxygenates: Extrapolation of information to multiple sites
and redox conditions. Environ Sci Technol, 28: 1727-1732.
Moser GJ, Wong BA, Wolf DC, Moss OR, & Goldsworthy TL (1996a)
Comparative short-term effects of methyl tertiary butyl ether and
unleaded gasoline vapor in female B6C3P1 mice. Fundam Appl Toxicol,
31: 173-183.
Moser GJ, Wong BA, Wolf DG, Fransson-Steen RL, & Goldsworthy TL
(1996b) Methyl tertiary butyl ether lacks tumor-promoting activity on
N-nitosodiethylamine-initiated B6C3F1 female mouse liver.
Carcinogenesis, 17: 2753-2761.
Munch JW & Eichelberger JW (1992) Evaluation of 48 compounds for
possible inclusion in U.S. EPA Method 524.2, Revision 3.0: expansion
of the method analyte list to a total of 83 compounds. J Chromatogr
Sci, 30: 471-477.
Murray WR, Laferla G, & Fullarton GM (1988) Choledolithiasis -
in vivo stone dissolution using methyl tertiary butyl ether (MTBE).
Gut, 29: 143-145.
Neeper-Bradley TL (1991) Two-generation reproduction study of inhaled
methyl tertiary butyl ether in CD (Sprague-Dawley) rats. Export,
Pennsylvania, Bushy Run Research Center, 499 pp (Report No. 53-594 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
Nihlén A, Löf A, & Johanson G (1995) Liquid/air partition coefficients
of methyl and ethyl t-butyl ethers, t-amyl methyl ether, and t-butyl
alcohol. J Expo Anal Environ Epidemiol, 5(4): 573-582.
Nihlén A, Löf A, & Johanson G (1998a) Experimental exposure to methyl
tertiary-butyl ether: I. Toxicokinetics in humans. Toxicol Appl
Pharmacol, 148: 281-287.
Nihlén A, Wålinder R, Löf A, & Johanson G (1998b) Experimental
exposure to methyl tertiary-butyl ether: II. Acute effects in humans.
Toxicol Appl Pharmacol, 148: 281-287.
Novak JT, Yeh C, Gullic D, Eichenberger J, & Benoit RE (1992). The
influence of microbial ecology on subsurface degradation of petroleum
contaminants. Blacksburg, Virginia, Virginia Polytechnic Institute and
State University, Water Resources Research Center, 86 pp (Bulletin No.
177).
Pankow JF, Rathbun RE, & Zogorski JS (1996) Calculated volatilization
rates of fuel oxygenate compounds and other gasoline-related compounds
from rivers and streams. Chemosphere, 33: 921-937.
Paulov S (1987) [Action of the anti-detonation preparation tert-butyl
methyl ether on the model species Rana temporaria L.] Biologia, 42:
185-189 (in Slovak with English abstract).
Pauls RE (1985) Determination of high octane components: methyl
t-butyl ether, benzene, toluene, and ethanol in gasoline by liquid
chromatography. J Chromatogr Sci, 23: 437-441.
Pekari K, Riihimaki V, Vainiotalo S, Teräväinen E, & Aitio A (1996)
Experimental exposure to methyl- tert-butyl ether (MTBE) and
methyl- tert-amyl ether (MTAE). In: Proceedings of the International
Symposium on Biological Monitoring in Occupational and Environmental
Health, Espoo, Finland, 11-13 September 1996. Helsinki, Institute of
Occupational Health, pp 27-28.
Pence VH (1987a) The evaluation of the biodegradation of 606610 using
a modified closed bottle method. Miamiville, Ohio, Hill Top Biolabs,
Inc., 92 pp (Report No. 87-0479-11, prepared for ARCO Chemical
Company).
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Poet TS & Borghoff SJ (1997) In vitro uptake of methyl tert-butyl
ether in male rat kidney: Use of a two-compartment model to describe
protein interactions. Toxicol Appl Pharmacol, 145: 340-348.
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Post G (1994) Methyl tertiary butyl ether: health-based maximum
contaminant level document. Trenton, New Jersey, New Jersey Department
of Environmental Protection, Division of Sciences and Research.
Poulsen M, Lemon L, & Barker JF (1992) Dissolution of monoaromatic
hydrocarbons into groundwater from gasoline-oxygenate mixtures.
Environ Sci Technol, 26: 2483-2489.
Prah JD, Goldstein GM, Devlin R, Otto D, Ashley D, House D, Cohen KL,
& Gerrity T (1994) Sensory, symptomatic, inflammatory, and ocular
responses to and the metabolism of methyl tertiary butyl ether in a
controlled human exposure experiment. Inhal Toxicol, 6(6): 521-538.
Prakash CB (1989) Motor vehicle emissions from gasoline containing
MTBE. Ottawa, Ontario, Environment Canada, Environmental Protection
Directorate, Conservation and Protection, Industrial Programs Branch,
25 pp (Report No. IP-97).
Prescott-Mathews JS, Wolf DC, Wong BA, & Borghoff SJ (1997) Methyl
tert-butyl ether causes alpha2u-globulin nephropathy in male
Fischer-344 rats. Toxicol Appl Pharmacol, 143: 301-314.
Raese JW, Rose DL, & Sandstrom MW (1995) US Geological Survey
laboratory method for methyl tert-butyl ether and other fuel
oxygenates. Rapid City, South Dakota, US Geological Survey, 4 pp (US
Geological Survey Fact Sheet FS-219-95).
Rao HV & Ginsberg GL (1997) Physiologically-based pharmacokinetic
(PBPK) model assessment of methyl t-butyl ether (MTBE) in groundwater
for a bathing and showering determination. Risk Anal, 17: 583-598.
Riihimaki V, Matikainen E, Akila R, Teräväinen E, Mutanen P, Pekari K,
Vainiotalo S, Vilhunen R, & Aitio A (1996) Central nervous system
effects of the gasoline additive methyl- tert-butyl ether (MTBE). In:
Proceedings of the Third International Symposium on Biological
Monitoring in Occupational and Environmental Health, Espoo, Finland,
11-13 September 1996. Helsinki, Institute of Occupational Health, p
75.
Robinson M, Bruner RH, & Olson GR (1990) Fourteen- and ninety-day oral
toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley
rats. J Am Coll Toxicol, 9(5): 525-540.
Roe VD, Lacy MJ, Stuart JD, & Robbins GA (1989) Manual headspace
method to analyze for the volatile aromatics of gasoline in
groundwater and soil samples. Anal Chem, 61: 2584-2585.
Rousch JM & Sommerfeld MR (1998) Liquid-gas partitioning of the
gasoline oxygenate methyl tert-butyl ether (MTBE) under laboratory
conditions and its effect on growth of selected algae. Arch Environ
Contam Toxicol, 34: 6-11.
Salanitro JP, Diaz LA, Williams MP, & Wisniewski HL (1994) Isolation
of a bacterial culture that degrades methyl t-butyl ether. Appl
Environ Microbiol, 60: 2593-2596.
Savolainen H, Pfäffli P, & Elovaara E (1985) Biochemical effects of
methyl tertiary-butyl ether in extended vapour exposure of rats.
Arch Toxicol, 57: 285-288.
Schroll R, Bierling B, Cao G, Dörfler U, Lahaniati M, Langenbach T,
Scheunert I, & Winkler R (1994) Uptake pathways of organic chemicals
from soil by agricultural plants. Chemosphere, 28: 297-303.
Schuberth J (1996) A full evaporation headspace technique with
capillary GC and ITD: a means for quantitating volatile organic
compounds in biological samples. J Chromatogr Soc, 34: 314-319.
Schuetzle D, Siegl WO, Trescott EJ, Jensen TE, Dearth MA, Kaiser EW,
Gorse R, Kreucher W, & Kulik E (1994) The relationship between
gasoline composition and vehicle hydrocarbon emissions: A review of
current studies and future research needs. Environ Health Perspect,
102(suppl 4): 3-12.
Seeberg AH (1989) Unscheduled DNA synthesis (UDS) in primary rat
hepatocytes (autoradiographic method). Test substance: MTBE. Rome,
Life Science Research, Toxicology Centre, 79 pp (LSR-RTC Report No.
216003-M-03689).
Sernau RC (1989) Mutagenicity test on methyl tertiary butyl ether
Drosophila melanogaster sex-linked recessive test (Study No.
10484-0-461). Kensington, Maryland, Hazleton Laboratories America,
Inc., 23 pp (Report to the Methyl Tertiary Butyl Ether Toxicology
Committee, Washington, DC).
Simer MM (1986) Texaco Chemical Corporation - Safety (SAF) studies,
surveys and reports: methanol, tertiary butyl alcohol, 1,3-butadiene,
methyl tertiary butyl ether, total other hydrocarbons. Port Neches,
Texas, Texaco Chemical Corporation, 15 pp.
Smith Sl & Duffy LK (1995) Odor and health complaints with Alaskan
gasolines. Chem Health Saf, 2: 32-38.
Smith DF, Kleindienst TE, Hudgens EE, McIver CD & Bufalini JJ (1991)
The photooxidation of methyl tertiary butyl ether. Int J Chem Kinet,
23: 907-924.
Snamprogetti (1980) MTBE toxicological data book. Rome, Snamprogetti
Engineering Company S.p.A., 202 pp (Report No. LICE/0086/AA/1g).
SNV (1993) [Better environmental qualities in petrol - a proposal for
environmental classification.] Stockholm, National Environmental
Protection Agency, 69 pp (in Swedish).
Spitzer HL (1997) An analysis of health benefits associated with the
use of MTBE reformulated gasoline and oxygenated fuels in reducing
atmospheric concentrations of selected volatile organic compounds.
Risk Anal, 17: 683-691.
Squillace PJ, Pope DA, & Price CV (1995a) Occurrence of the gasoline
additive MTBE in shallow ground water in urban and agricultural areas.
Rapid City, South Dakota, US Geological Survey, 4 pp (US Geological
Survey Fact Sheet FS-114-95).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1995b) A preliminary
assessment of the occurrence and possible sources of MTBE in ground
water of the United States, 1993-94. Rapid City, South Dakota, US
Geological Survey, 29 pp (File Report No. 95-456).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1996) Preliminary
assessment of occurrence and possible sources of MTBE in groundwater
in the United States, 1993-1994. Environ Sci Technol, 30: 1721-1730.
Stackelberg PE, O'Brien AK, & Terracciano SA (1997) Occurrence of MTBE
in surface and ground water, Long Island, New York and New Jersey.
Proceedings of American Chemical Society meeting, San Francisco,
California, 16-17 April 1996. Washington, DC, American Chemical
Society, 4 pp.
Standeven AM & Goldsworthy TL (1993) Promotion of preneoplastic
lesions and induction of CYP2B by unleaded gasoline vapor in female
B6C3F1 mouse liver. Carcinogenesis, 14: 2137-2141.
State of Connecticut (1987) The incidence of MTBE in Connecticut.
Hertford, Connecticut, Department of Health Services, 3 pp (Document
No. FYI-OTS 0987-0574).
Steffan RJ, McClay K, Vainberg S, Condee CW, & Zhang D (1997)
Biodegradation of the gasoline oxygenates methyl tert-butyl ether,
ethyl tert-butyl ether, and tert-amyl methyl ether by propane
oxidising bacteria. Appl Environ Microbiol, 63: 4216-4222.
Stern BR & Tardiff RG (1997) Risk characterization of methyl
tertiary butyl ether (MTBE) in tap water. Risk Anal, 17: 727-743.
Sternal RS & Davis MA (1992) In vitro dissolution of cholesterol
gallstones. Invest Radiol, 27(12): 1040-1043.
Storck WJ, Layman PL, Reisch MS, Thayer AM, Kirschner EM, Peaff G, &
Tremblay J-F (1996) Facts and figures for the chemical industry. Chem
Eng News, June 24: 38-46.
Streete PJ, Ruprah M, Ramsey JD, & Flanagan RJ (1992) Detection and
identification of volatile substances by headspace capillary gas
chromatography to aid diagnosis of acute poisoning. Analyst, 117:
1111-1127.
Suflita JM & Mormile MR (1993) Anaerobic biodegradation of known and
potential gasoline oxygenates in the terrestrial subsurface. Environ
Sci Technol, 27: 976-978.
Swenberg JA & Dietrich DR (1991) Immunohistochemical localization of
alpha 2µ-globulin in kidneys of treated and control rats of a 13-week
vapor inhalation study undertaken with methyl tertiary butyl ether.
Washington, DC, Synthetic Organic Chemical Manufacturers Association,
5 pp (Report to the MTBE Health Effects Testing Task Force,
Washington, DC).
Tarkpea M & Svanberg O (1982) The acute toxicity of motor fuels to
brackish water organisms. Mar Pollut Bull, 13(4): 125-127.
Tepper JS, Jackson MC, McGee JK, Costa DL, & Graham JA (1994)
Estimation of respiratory irritancy from inhaled methyl tertiary butyl
ether in mice. Inhal Toxicol, 6(6): 563-569.
Texaco (1993) Industrial hygiene air sampling results: Guatemala
Refinery - methyl tert-butyl ether (MTBE). Beacon, New York, Texaco
Inc., Environment Health and Safety Division, 3 pp.
THE (1989) Biomonitoring results using MTBE. Wheat Ridge, Colorado,
THE Consultants, 8 pp.
TRC (1993) Final report to ARCO Chemical Company on the odor and taste
threshold studies performed with methyl tertiary-butyl ether (MTBE)
and ethyl tertiary-butyl ether (ETBE). Windsor, Connecticut, TRC
Environmental Corporation, 37 pp.
Tuazon EC, Carter WPL, Aschmann SM, & Atkinson R (1991) Products of
the gas-phase reaction of methyl tert-butyl ether with the OH
radical in the presence of NOx. Int J Chem Kinet, 23: 1003-1015.
Tyl RW (1989) Developmental toxicity study of inhaled methyl tertiary
butyl ether in New Zealand White rabbits. Export, Pennsylvania, Bushy
Run Research Center, 260 pp (Report No. 51-628 to the Methyl Tertiary
butyl Ether Toxicology Committee, Washington, DC).
Tyl RW & Neeper-Bradley TL (1989) Developmental toxicity study of
inhaled methyl tertiary butyl ether in CD-1 mice. Export,
Pennsylvania, Bushy Run Research Center, 342 pp (Report No. 52-526 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
US EPA (1989) Chemical rate constants for superfund health evaluation
manual chemicals. Washington, DC, US Environmental Protection Agency,
pp 645-646 (Report No. 68-02-4254).
US Interagency Assessment (1997) Interagency assessment of oxygenated
fuels. Washington, DC, National Science and Technology Council, 256
pp.
Vainiotalo S, Peltonen Y, & Pfäffli P (1996) MTBE exposure in service
stations. Paper presented at the 7th European Fuel Oxygenates
Association Conference, Sodehotel, 24-25 October 1996. Brussels,
European Chemical Industries Federation, 7 pp.
Veith GD & Kosian P (1983) Estimating bioconcentration potential from
octanol/water partition coefficients. In: Mackay D, Paterson S,
Eisenreich SJ, & Simmons MS ed. Physical behavior of PCBs in the Great
Lakes. Ann Arbor, Michigan, Ann Arbor Science Publishers, Chapter 15,
pp 269-282.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: narcotic industrial
chemicals. Can J Fish Aquat Sci, 40(6): 743-748.
Verga GR, Sironi A, Schneider W, & Frohne JCh (1988) Selective
determination of oxygenates in complex samples with the O-FID analyze.
J High Res Chromatogr Chromatogr Commun, 11: 248-252.
Vergnes JS & Chun JS (1994) Methyl tertiary butyl ether: in vivo-
in vitro hepatocyte unscheduled DNA synthesis assay in mice
(Laboratory project ID 93N1316). Export, Pennsylvania, Bushy Run
Research Center, 67 pp (Report to the MTBE Effects Testing Task Force,
Washington, DC).
Vergnes JS & Kintigh WJ (1993) Methyl tertiary butyl ether: bone
marrow micronucleus test in mice (Laboratory project ID 93N1244).
Export, Pennsylvania, Bushy Run Research Center, 99 pp (Report to the
MTBE Effects Testing Task Force, Washington, DC).
Vergnes JS & Morabit ER (1989) Methyl tertiary butyl ether repeated
exposure vapor inhalation study in rats: in vivo cytogenetic
evaluation. Export, Pennsylvania, Bushy Run Research Center, 40 pp
(Report No. 51-635 to the MTBE Effects Testing Task Force, Washington,
DC).
Wallington TJ, Dagaut P, Liu R, & Kurylo MJ (1988) Gas-phase reactions
of hydroxyl radicals with the fuel additives methyl tert-butyl ether
and tert-butyl alcohol over the temperature range 240-440 K. Environ
Sci Technol, 22(7): 842-844.
Wallington TJ, Andino JM, Skewes LM, Siegl WO, & Japar SM (1989)
Kinetics of the reaction of OH radicals with a series of ethers under
simulated atmospheric conditions at 295 K. Int J Chem Kinet, 21:
993-1001.
Ward JB, Au WW, Whorton EB, & Legator MS (1994) Genetic toxicology of
methyl tertiary butyl ether. Galveston, Texas, University of Texas, pp
57-134 (Final Report to the Agency for Toxic Substances and Disease
Registry).
Watson JG, Chow JC, Pritchett LC, Houck JA, Ragazzi RA, & Burns S
(1990) Chemical source profiles for particulate motor vehicle exhaust
under cold and high altitude operationg conditions. Sci Total Environ,
93: 183-190.
White MC, Johnson CA, Ashley DL, Buchta TM, & Pelletier DJ (1995)
Exposure to methyl tertiary-butyl ether from oxygenated gasoline in
Stamford, Connecticut. Arch Environ Health, 50: 183-189.
Wibowo AAE (1994) DEFOS (Dutch Expert Committee for Occupational
Standards) and NEG (Nordic Expert Group for Criteria Documentation of
Health Risks from Chemicals) basis for an occupational standard:
methyl-tert-butyl-ether. Solna, Sweden, National Institute of
Occupational Health, 21 pp.
Windholz M ed. (1983) The Merck index: an encyclopedia of chemicals,
drugs, and biologicals, 10th ed. Rahway, New Jersey, Merck and Co.,
Inc., p 865.
Yeh CK & Novak JT (1994) Anaerobic biodegradation of gasoline
oxygenates in soils. Water Environ Res, 66: 744-752.
Yoshikawa M, Arashidani K, Katoh T, Kawamoto T, & Kodama Y (1994)
Pulmonary elimination of methyl tertiary-butyl ether after
intraperitoneal administration in mice. Arch Toxicol, 68(8): 517-519.
Zogorski JS, Morduchowitz A, Baehr AL, Bauman BJ, Drew RT, Korte NE,
Lepham WW, Pankow JF, & Washington ER (1996) Fuel oxygenates and water
quality: Current understanding of sources, occurrence in natural
waters, environmental behavior, fate, and significance. Washington,
DC, Office of Science and Technology Policy, 91 pp (Final report
prepared for the Interagency Oxygenated Fuel Assessment).
Zweidinger RB (1993) Air quality measurements in Fairbanks, Stamford,
and Albany. Research Triangle Park, North Carolina, United States
Environmental Protection Agency, Atmospheric Research and
Environmental Assessment Laboratory, 19 pp (Report ACC-1539).
RÉSUMÉ
Le méthyltertiobutyléther (MTBE) est actuellement le plus utilisé
des éthers que l'on peut employer comme additifs de l'essence.
L'éthyltertiobutyléther (ETBE), le tertioamylméthyléther (TAME), le
tertioamyléthyléther (TAEE) et le diisopropyléther (DIPE), entre
autres, peuvent être ajoutés ou substitués au MTBE afin d'améliorer
l'oxygénation et l'indice d'octane, aussi peut-on en trouver à côté du
MTBE.
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le MTBE est un composé volatil et incolore, à l'odeur terpénique,
qui est liquide à la température ambiante. Sa viscosité est faible et
son point d'ébullition est de 55,2°C. Son point de congélation est de
-109°C. Sa densité est de 0,7404 à 20°C. Sa tension de vapeur est
relativement élevée: 33 500 Pa à 25°C. C'est une substance inflammable
qui peut en outre former des mélanges explosifs avec l'air. Il est
très soluble dans les autres éthers et dans l'alcool. Miscible à
l'essence, il est également soluble dans l'eau (42 000 g/m3 à
19,8°C). Son coefficient de partage entre l'octanol et l'eau (log
Kow) est de 0,94-1,3. Il est instable en solution acide.
La recherche et le dosage du MTBE se font dans tous types de
matrices par chromatographie en phase gazeuse au moyen de détecteurs
et de colonnes capillaires adaptés à la matrice en cause. On a
également recours à la chromatographie liquide à phases inversées pour
l'analyse des échantillons d'essence. On utilise aussi, pour la
préparation des échantillons d'air, d'eau et de sédiments ou encore
des échantillons biologiques, divers systèmes de purge et de piégeage,
la sorption/désorption et des méthodes basées sur l'espace de tête.
2. Sources d'exposition humaine et environnementale
Autant qu'on sache, le MTBE n'existe pas à l'état naturel. Dans
l'industrie, on l'obtient par l'action du méthanol sur l'isobutylène
en présence d'un catalyseur. Un certain nombre de pays le produisent
en quantités croissantes depuis la fin des années 70. Il compte
actuellement parmi les 50 produits chimiques dont le volume de
production est le plus élevé. En 1996, la capacité de production des
Etats-Unis était de 10,6 millions de tonnes et on estime que la
demande de MTBE va encore augmenter. Environ 25% de l'essence vendue
aux Etats-Unis est additionnée de MTBE. On l'utilise presque
exclusivement pour améliorer l'indice d'octane et accroître la teneur
de l'essence en oxygène. On en ajoute à l'essence jusqu'à 17% en
volume.
3. Transport, distribution et transformation dans l'environnement
Une fois libéré dans l'air, le MTBE y reste en majeure partie
avec seulement de petites quantités qui passent dans le sol et dans
l'eau. Le MTBE présent dans l'atmosphère peut passer en partie dans
l'eau de pluie, mais la proportion qui s'élimine ainsi reste faible.
Dans l'atmosphère, l'action des radicaux hydroxyle entraîne la
formation d'un certain nombre de composés et en particulier de
formiate de tertiobutyle, photochimiquement stable, et de
2-méthoxy-2-méthylpropanol, qui doit réagir énergiquement avec les
radicaux hydroxyles pour donner du CO2, du formaldéhyde, de l'acétone
et de l'eau. Lorsque du MTBE est libéré dans l'eau, il se dissout
partiellement, une partie passant dans l'air. Les quantités qui
passent dans les biotes et les sédiments sont faibles. Les épreuves
classiques indiquent une faible biodégradabilité. On pense que d'une
façon générale, la biodégradation est lente dans l'environnement.
Lorsque du MTBE est libéré dans le sol, il passe dans l'air par
volatilisation, dans les eaux de surface par entraînement et dans les
eaux souterraines par lessivage. Le MTBE peut persister dans les eaux
souterraines.
4. Concentrations dans l'environnement et exposition humaine
Les données relatives aux concentrations dans l'environnement et
à l'exposition humaine sont peu nombreuses.
Dans des études portant sur l'air de certaines villes où les
véhicules utilisaient de l'essence oxygénée contenant 15% de MTBE, on
a relevé des concentrations ambiantes allant de "non décelable" à
100,9 µg/m3 (0,028 ppm), avec plusieurs concentrations médianes
allant de 0,47 à 14,4 µg/m3 (0,00013 à 0,004 ppm). Dans l'air de
quelques villes où le MBTE était utilisé à plus faible teneur pour
augmenter l'indice d'octane, la concentration de ce composé allait de
non décelable à 26,4 µg/m3 (0,0073).
Au niveau du sol ou à proximité de raffineries de pétrole, la
concentration allait de 15 à 281 µg/m3. Dans l'air urbain, à
proximité d'ateliers où l'on procédait au mélange de cet additif à
l'essence, la concentration était de 1508 µg/m3 (0,419 ppm), avec des
valeurs extrêmes de 216-35 615 µg/m3 (0,06-9,8 ppm).
Dans les stations service situées dans des zones où l'on
utilisait de l'essence oxygénée à 10-15% de MTBE, c'est dans la zone
de respiration des consommateurs, au moment des pleins, que la
concentration de l'additif était la plus forte (300 à 136 000 µg/m3,
soit 0,09 à 38 ppm), les valeurs dépassant toutefois rarement 3600
µg/m3 (10 ppm) et tombant un peu plus bas au niveau des pompes (de
non décelable à 5700 µg/m3, soit 1,6 ppm). Les valeurs les plus
faibles ont été relevées sur le périmètre de la station (de non
décelable à 500 µg/m3, soit 0,14 ppm). Les concentrations relevées
dans les stations services dépourvues de système de récupération des
vapeurs étaient généralement plus élevées.
A l'intérieur d'une automobile, on a relevé des valeurs de 7 à 60
µg/m3 (0,002 à 0,017 ppm) au cours de navettes et de 20 610 µg/m3
(0,006 à 0,172 ppm) lors des pleins.
D'après des données de surveillance qui se limitent
presqu'exclusivement aux Etats-Unis, la présence de MTBE a été décelée
dans de la neige, des eaux d'orage, des eaux de surface (ruisseaux,
rivières et retenues), des eaux souterraines et dans de l'eau de
boisson. Les valeurs relevées dans les eaux d'orage allaient de 0,2 à
8,7 µg/litre avec une valeur médiane inférieure à 1,0 µg/litre. Dans
le cas des ruisseaux, rivières et retenues, les concentrations
s'étageaient entre 0,2 et 30 µg/litre, les valeurs médianes obtenues
dans diverses études allant de 0,24 à 7,75 µg/litre.
La présence de MTBE n'a généralement pas été décelée dans les
eaux souterraines profondes ou non des zones agricoles. Lorsqu'on en a
trouvé, la concentration était inférieure à 2,0 µg/litre. La présence
de MTBE est plus fréquente dans eaux souterraines de faible profondeur
des régions urbanisées (dans les premiers 1,5 à 3 m des nappes
phréatiques). On trouve alors des concentrations de moins de 0,2
µg/litre à 23 µg/litre, avec une valeur médiane de moins de 0,2
µg/litre.
On trouve rarement du MTBE dans l'eau des réseaux d'adduction qui
est pompée dans les nappes souterraines. Sur 51 réseaux contrôlés sauf
3, la concentration était inférieure ou égale à 20 µg/litre. Il est
difficile de donner des valeurs caractéristiques pour l'eau
d'adduction captée en surface car les données sont insuffisantes. On a
trouvé de fortes concentrations de MTBE dans quelques puits privés
utilisés comme source d'eau potable (soit >1000 µg/m3). On peut
toutefois douter que de l'eau contenant plus de 50 à 100 µg/litre de
MTBE soit encore buvable, car le seuil organoleptique du MTBE est bas.
Parmi les travailleurs exposés au MTBE, on peut citer ceux qui
produisent, distribuent ou utilisent ce composé ou de l'essence qui en
contient, y compris les pompistes et les mécaniciens des stations
service.
En ce qui concerne l'exposition de courte durée (<30 min) lors
d'opérations habituelles de production ou de stockage de MTBE pur, les
chiffres vont de 715 à 43 000 µg/m3 (0,2 à 12 ppm), avec une valeur
médiane moyenne de 3400 µg/m3 (0,95 ppm). Pour l'exposition de plus
longue durée (30 min à 8 h), les valeurs vont de 360 à 890 000 µg/m3
(0,01 à 250 ppm), avec une valeur médiane d'environ 540 µg/m3 (0,15
ppm). Chez les ouvriers qui mélangent l'additif à l'essence, les
valeurs de l'exposition de courte durée vont de non décelable à
360 000 µg/m3 (100 ppm), la médiane se situant à environ 5700 µg/m3
(1,6 ppm). Dans le cas d'une exposition de plus longue durée, les
valeurs obtenues vont de non décelable à 257 000 µg/m3 (72 ppm), avec
une valeur médiane moyenne d'environ 2000 µg/m3 (0,6 ppm).
C'est lors du transport de MTBE pur ou en mélange avec des
carburants, dans des canalisations, sur des péniches, des wagons de
chemin de fer ou des camions (MTBE pur seulement) que l'on a
enregistré les expositions les plus fortes, avec des valeurs à court
terme allant de 3750 mg/m3 (0,001 à 1050 ppm) et une valeur médiane
moyenne de 140 mg/m3 (39 ppm). Dans le cas d'expositions à long
terme, les valeurs allaient de 0,036 à 2540 mg/m3 (0,01 à 712 ppm),
la valeur médiane moyenne se situant à 2,85 mg/m3 (0,8 ppm). Lors de
la distribution (c'est-à-dire du chargement de mélanges carburant-MTBE
sur des camions et de leur livraison et déchargement dans des stations
service), on a relevé des valeurs à court terme allant de non
décelable à 225 mg/m3 (63 ppm), les valeurs médianes moyennes se
situant autour de 21 mg/m3 (6 ppm). Les valeurs à long terme allaient
de 0,036 à 22 mg/m3 (0,01 à 6,2 ppm), avec une valeur médiane moyenne
de 1,79 mg/m3 (0,5 ppm).
L'exposition médiane moyenne à court terme des pompistes de
stations service allait, selon certaines mesures, généralement de
1,071 à 21,42 mg/m3 (0,3 à 6 ppm) et dépassait rarement 35,7 mg/m3
(10 ppm). Dans le cas de l'exposition médiane à long terme, on a
obtenu la valeur de 1,79 mg/m3 (0,5 ppm). L'exposition médiane des
mécaniciens est restée inférieure au seuil de détection dans une étude
à court terme; dans le cas de l'exposition à long terme, la valeur
était d'environ 360 µg/m3 (0,1 ppm).
5. Cinétique et métabolisme
Les données toxicocinétiques relatives aux effets du MTBE sur
l'Homme proviennent essentiellement d'études contrôlées pratiquées sur
des volontaires adultes ou sur une population exposée à de l'essence
oxygénée. Après inhalation, le MTBE passe rapidement dans le courant
sanguin. Chez des volontaires humains en bonne santé exposés par voie
respiratoire, on constate que la cinétique est linéaire jusqu'à la
concentration de 268 mg/m3 (75 ppm). On a procédé au dosage de
l'alcool tertiobutylique (en abrégé TBA, un métabolite du MTBE) dans
le sang et les urines. Chez des sujets humains exposés à des
concentrations de MTBE allant de 5,0 à 178,5 mg/m3 (1,4 à 50 ppm), la
concentration maximale de MTBE et de TBA allait respectivement de 17,2
à 1144 µg/litre et de 7,8 à 925 µg/litre. En utilisant un modèle
monocompartimental, on a pu constater qu'intervenaient dans la
demi-vie globale du MTBE des constituants à demi-vie brève (36-90 min)
et des constituants à demi-vie longue (19 h).
Chez les rongeurs, le MTBE est bien résorbé et réparti après
administration per os ou exposition par la voie respiratoire.
L'absorption est moindre par voie percutanée. A la dose de 400 mg/kg
per os et de 28 800 mg/m3 (8000 ppm) par inhalation, la proportion
de la dose totale absorbée qui était éliminée dans l'air expiré
augmentait à mesure que diminuait la proportion éliminée dans les
urines, ce qui est le signe d'une saturation du métabolisme. On n'a
pas mis en évidence de TBA dans l'urine des rats exposés. La présence
de 2-méthyl-1,2-propanediol et d'acide alpha-hydroxyisobutyrique dans
l'urine indique que le TBA est également métabolisé. Les études
in vitro montrent que le MTBE est métabolisé en TBA, formaldéhyde et
acétone.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat, la dose létale médiane aiguë par voie buccale
(DL50) est égale à environ 3 800 mg/kg de poids corporel. La
concentration létale médiane aiguë (CL50) pour une exposition de 15
minutes par inhalation se situe aux environs de 141 000 mg/m3 d'air
chez la souris. Parmi les signes d'intoxication on peut citer une
dépression du SNC, une ataxie et des difficultés respiratoires. Aux
doses non létales, la récupération a été complète. Par voie
percutanée, la DL50 est >10 200 mg/kg de poids corporel chez le
lapin.
On n'a trouvé qu'une seule étude où il soit question d'un effet
"modérément" irritant pour la peau, l'irritation consistant en un
érythème et un oedème modérés après application sur la peau de lapins.
Chez ce même animal, le MTBE s'est également révélé irritant pour la
muqueuse oculaire, les effets produits étant bénins et réversibles.
Dans la seule étude retrouvée, le MTBE a provoqué une irritation
légère à forte des voies respiratoires lors de l'exposition de souris
à des doses de 300 à 30 000 mg/m3. Il n'a pas produit de
sensibilisation cutanée chez le cobaye.
L'expérimentation sur des rats et des souris montre que des
expositions réitérées conduisent principalement à une augmentation du
poids des organes et ont des effets histopathologiques sur le rein
(rat) et sur le foie (souris). Une étude d'ingestion de 90 jours a
montré que la limite inférieure d'apparition d'effets néphrotoxiques
se situe à 440 mg/kg p.c. par jour (augmentation du poids des reins et
dégénérescence hyaline chez des rats Sprague-Dawley). En exposant des
rats Fischer-344 par inhalation à une concentration de 2880 mg/m3
(800 ppm) de MTBE, on a obtenu une augmentation du poids rénal
accompagnée, lorsqu'on accroissait la concentration, d'une
augmentation modérée de la dégénérescence hyaline au niveau des
tubules proximaux. Lors d'études d'oncogénicité comportant
l'exposition des animaux par inhalation, on a observé à la dose de
1440 mg/m3 (400 ppm), un accroissement de la fréquence et de la
gravité des néphropathies progressives chroniques chez les rats mâles,
alors que chez les souris mâles, il y avait à cette même dose
augmentation du poids absolu du foie (corrélée avec une augmentation
des hypertrophies hépatocellulaires à plus forte concentration) et du
poids relatif des reins.
L'exposition au MTBE provoque également des effets irréversibles
sur le système nerveux central (SNC) consistant notamment en sédation,
diminution de l'activité, ataxie et anesthésie à forte concentration.
Des effets biphasiques s'observent également sur l'activité motrice à
plus faible concentration. Lors d'une étude sur des rats comportant
une exposition de 6 h par inhalation, on a constaté qu'à la dose de
2880 mg/m3 (800 ppm), il se produisait, chez les animaux d'un des
deux sexes, des modifications réversibles et liées à la dose de
l'activité motrice. Ces effets étaient passagers et n'apparaissaient
plus guère lors des études à long terme.
On a pu retrouver des études de reproduction portant sur une ou
deux générations de rats ainsi que quatre études relatives au
développement de rats, de souris et de lapins exposés à du MTBE. Ces
études n'ont pas permis de mettre en évidence d'effets spécifiques sur
la reproduction des rats à des concentrations allant jusqu'à 28 800
mg/m3. En outre, aux concentrations inférieures à celles qui se
révélaient toxiques pour les mères, le MTBE n'a pas eu non plus
d'effets sur le développement de la progéniture. A la dose de 28 800
mg/m3, on a constaté, chez la souris, une augmentation du poids de
l'utérus et un accroissement du métabolisme des estrogènes.
Le MTBE a fait l'objet d'un grand nombre d'épreuves valables de
mutagénicité et autres études de génotoxicité. Les résultats obtenus
montrent que le composé n'est pas génotoxique, même si un résultat
positif a été obtenu dans l'épreuve de mutation portant sur le locus
tk des cellules lymphomateuses. Ce résultat s'explique en effet par la
métabolisation du MTBE en formaldéhyde.
Pour les études de cancérogénicité, on a exposé par inhalation
des rats Fischer-344 et des souris CD-1 ou gavé des rats
Sprague-Dawley avec une nourriture contenant du MTBE. Dans aucune des
études d'exposition par inhalation on a procédé à une correction
statistique pour tenir compte des différences de survie. Dans les
trois études, on a constaté une augmentation sensible de l'incidence
des tumeurs, localisées, chez les rats mâles Fischer-344, au niveau
des tubules rénaux et des cellules de Leydig, chez les rats mâles
Sprague-Dawley, au niveau des cellules de Leydig (lymphomes et
leucémies chez les femelles) et chez les souris femelles CD-1, au
niveau du foie. Les tumeurs des tubules rénaux et les
leucémies/lymphomes n'ont donc pas été observées systématiquement chez
le rat lors des différentes études. En outre, les tumeurs rénales
sexospécifiques étaient associées à une néphropathie également
sexospécifique mettant en jeu l'alpha2u-globuline, qui a été observée
dans plusieurs études de courte durée. L'augmentation des tumeurs des
cellules de Leydig a été observée à la dose la plus élevée chez les
rats Sprague-Dawley (1000 mg/kg p.c.), mais chez les rats Fischer-344,
l'interprétation de cet accroissement est rendu délicate par la très
forte incidence tumorale également observée chez les témoins
concomitants et les témoins historiques. Les tumeurs hépatiques ont
été observées dans les groupes témoins et à la dose de 28 800 mg/m3
(8000 ppm) dans les groupes exposés avec des incidences respectives de
2/50 et 10/50 chez les femelles et de 12/49 et 16/49 chez les mâles.
L'accroissement d'incidence était modeste et s'accompagnait d'une
hypertrophie hépatocellulaire.
7. Effets sur l'Homme
Après la mise sur le marché, aux Etats-Unis, de deux types
d'essence nécessitant l'utilisation d'additifs d'oxygénation (pas
obligatoirement du MTBE), on a constaté que les usagers se plaignaient
de symptômes aigus tels que maux de tête, irritation des yeux et du
nez, toux, nausées, vertiges et désorientation. Les études
épidémiologiques effectuées sur des populations humaines en milieu
professionnel ou non, de même que les études expérimentales sur
volontaires humains exposés dans des conditions contrôlées, n'ont pas
permis de découvrir si ces plaintes étaient fondées. Des études
intracommunautaires menées en Alaska, au New Jersey, dans le
Connecticut et dans le Wisconsin ont, avec des résultats divers il est
vrai, montré qu'il n'y avait guère de relation entre l'exposition au
MTBE et les symptômes dont la population se plaignait.
Des volontaires adultes ont été placés, dans le cadre d'études
expérimentales contrôlées, dans des chambres d'inhalation où on leur a
fait respirer du MTBE à des concentrations allant de 5,0 mg/m3
(1,4 ppm) à 270 mg/m3 (75 ppm). Aucun effet patent n'a été relevé,
qu'il s'agisse de la relation subjective de symptômes ou d'indicateurs
objectifs tels qu'une irritation ou d'autres signes, à des
concentrations allant jusqu'à 180 mg/m3 (50 ppm) et pendant une durée
pouvant atteindre 2 h. A en juger d'après ces résultats, il est peu
probable que le MTBE puisse à lui seul exercer des effets toxiques
aigus sur la population générale dans les conditions habituelles
d'exposition par la voie respiratoire. Il est cependant à noter que
les effets potentiels d'essences additionnées de MTBE, dans les
conditions où la plupart des gens sont exposés à cet additif
lorsqu'ils utilisent des carburants oxygénés, n'ont été étudiés ni
expérimentalement, ni par le biais de méthodes épidémiologiques
prospectives. Par ailleurs, le rôle de facteurs tels que la perception
de la présence de MTBE, explicable en partie par l'odeur particulière
de ce composé, n'a pas été étudié non plus.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Expérimentalement, la toxicité aiguë (exprimée par la CL50) du
MTBE pour les poissons, les amphibiens et les crustacés se révèle
supérieure à 100 mg/litre. On ne possède pas de données sur la
toxicité chronique ou subléthale de ce composé pour les organismes
aquatiques, ni sur sa toxicité pour les organismes terrestres.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
A en juger par les données collectives, il semble peu probable
que le MTBE puisse à lui seul et dans les conditions usuelles
d'exposition, provoquer des effets toxiques aigus dans la population
générale.
D'après les études effectuées sur l'animal, le MTBE possède une
toxicité aiguë "modérée" et il provoque une légère irritation cutanée
et oculaire, mais pas de sensibilisation. Des expositions répétées
entraînent des effets essentiellement localisés au rein chez le rat et
au foie chez la souris, la dose nocive la plus faible étant de 440
mg/kg p.c. par jour chez le rat après ingestion et de 1440 mg/m3
(400 ppm) après inhalation. Aux concentrations inférieures au seuil de
toxicité parentale, le MTBE n'a pas eu d'effets nocifs sur la
reproduction ou le développement.
Le MTBE n'est pas génotoxique mais il peut provoquer la formation
de tumeurs chez les rongeurs, surtout aux concentrations suffisamment
élevées pour avoir d'autres effets toxiques. On considère actuellement
que ces données ne sont pas suffisantes pour que l'on puisse en tirer
une évaluation du risque cancérogène chez l'Homme. Le Groupe spécial a
conclu que, pour être en mesure de donner des indications
quantitatives concernant les limites d'exposition et d'évaluer le
risque, il fallait obtenir des données supplémentaires sur un certain
nombre de points.
Il ne semble pas que le MTBE, aux concentrations auxquelles il se
trouve dans l'eau, puisse être toxique pour les organismes aquatiques,
sauf en cas de déversement. On ne possède pas de données sur la
toxicité du MTBE pour les organismes terrestres mais il n'y a
vraisemblablement pas lieu de s'alarmer, étant donné que sa
concentration est faible dans l'air ambiant et que sa demi-vie est
relativement brève.
RESUMEN
El éter metil- terciario-butílico (MTBE) es uno de los distintos
éteres que pueden utilizarse como aditivos de combustibles y en la
actualidad es con gran diferencia el más usado. El éter
etil- terciario-butílico (ETBE), el éter terciario-amil-metílico
(TAME), el éter terciario-amil-etílico (TAEE) y el éter
diisopropílico (DIPE), entre otros, pueden ser suplementos del MTBE o
sustituirlo para fines de oxigenación o mejora de los octanos y, en
consecuencia, pueden hallarse en asociación con el MTBE.
1. Identidad, propiedades físicas y químicas y métodos analíticos
El MTBE es un líquido volátil e incoloro a la temperatura
ambiente, de olor parecido al terpeno. Su viscosidad es baja y tiene
un punto de ebullición de 55,2°C. El punto de congelación es de -
109°C. La densidad es de 0,7404 a 20°C. La presión de vapor es
relativamente alta: 33 500 Pa a 25°C. El MTBE es inflamable y puede
formar mezclas explosivas con el aire. Es muy soluble en otros éteres
y alcohol. Se mezcla con la gasolina y es soluble en agua (42 000
g/m3 a 19,8°C). El coeficiente de partición log n-octanol/agua es
de 0,94-1,3. Es inestable en solución ácida.
El MTBE se analiza en todas las matrices en general por
cromatografía de gases, utilizando una gama de columnas capilares y
sistemas detectores que son apropiados para la matriz específica.
También se ha utilizado la cromatografía inversa en fase líquida para
el análisis de las muestras de gasolina. Se han empleado sistemas de
sorción-desorción, incluidos sistemas de purga y captación, así como
procedimientos de recámara, a fin de preparar muestras de aire, agua,
sedimento y biológicas para el análisis.
2. Fuentes de exposición humana y ambiental
No se conoce la presencia natural de MTBE en el medio ambiente.
En la industria deriva de la reacción catalítica del metanol y el
isobutileno, y en varios países se ha producido en volúmenes
crecientes desde los últimos años setenta. El MTBE figura actualmente
entre los 50 productos químicos de mayor producción en volumen. En
1996, la capacidad estadounidense de producción fue aproximadamente de
10,6 millones de toneladas, previéndose un constante aumento del uso
de MTBE. El 25% aproximadamente de la gasolina en los EE.UU está
mezclada con MTBE. El MTBE se utiliza casi exclusivamente para el
refuerzo de los octanos y para aumentar el contenido de la gasolina en
oxígeno. El MTBE se ha añadido a la gasolina en concentraciones de
hasta el 17% en volumen.
3. Transporte, distribución y transformación en el medio ambiente
Tras su eliminación en el aire, el MTBE permanecerá en gran parte
en este medio, penetrando cantidades menores en el suelo y el agua. En
la atmósfera, el MTBE puede ser arrastrado por la lluvia. Sin embargo,
sólo una pequeña cantidad es eliminada de la atmósfera de este modo.
La transformación atmosférica por radicales hidroxilos produce varios
productos, entre los que figuran el formato terciario-butílico (TBF)
estable y el 2-metoxi-2-metilpropanol, que se supone que son muy
reactivos con los radicales hidroxilos, dando CO2, formaldehido,
acetona y agua. Cuando el MTBE pasa al agua se disuelve una cantidad
significativa, con cierta proporción en el aire. La proporción que
pasa a los biota y el sedimento es escasa. La biodegradabilidad en
ensayos convencionales es limitada. Se cree que por lo general es
lenta en el medio ambiente. Cuando el MTBE pasa al suelo, es
transportado al aire por volatilización, al agua superficial por
escurrimiento y al agua subterránea como resultado de la lixiviación.
El MTBE puede persistir en el agua subterránea.
4. Niveles medioambientales y exposición humana
Se dispone de escasos datos sobre los niveles medioambientales y
la exposición humana.
En los estudios sobre el MTBE en el aire de algunas ciudades que
utilizan gasolina oxigenada con MTBE al 15%, las concentraciones
ambientales iban del nivel indetectable a 100,9 µg/m3 (0,028 ppm),
con varias concentraciones medianas de 0,47 a 14,4 µg/m3 (0,00013 a
0,004 ppm). Las concentraciones de MTBE en el aire de algunas ciudades
en donde se utiliza MTBE como reforzador de octanos en concentraciones
inferiores van del nivel no detectable a 26,4 µg/m3 (0,0073 ppm).
Las concentraciones a nivel del suelo o cerca de las refinerías
eran de 15 a 281 µg/m3. Los niveles medianos en el aire urbano cerca
de instalaciones de mezclado eran de 1508 µg/m3 (0,419 ppm), con
gamas de 216-35 615 µg/m3 (0,06 a 9,8 ppm).
En las estaciones de servicio situadas en zonas en donde la
gasolina oxigenada contiene el 10-15% de MTBE, las concentraciones
alcanzaban el nivel máximo en la zona de respiración durante el
llenado de los depósitos por los consumidores (gama de 300 a
136 000 µg/m3 (0,09 a 38 ppm)), con niveles que rara vez pasaban de
3600 µg/m3 (10 ppm), siendo ligeramente inferiores en la zona de
bombas (indetectables a 5700 µg/m3 (1,6 ppm)) y mínimos en el
perímetro de la estación (indetectables a 550 µg/m3 (0,14 ppm)). En
general las concentraciones eran superiores en las estaciones de
servicio sin sistemas de recuperación de vapores.
En la cabina del automóvil, las concentraciones eran de 7 a
60 µg/m3 (0,002 a 0,017 ppm) durante la conducción y de 20 a
610 µg/m3 (0,006 a 0,172 ppm) al llenar el depósito.
Basándose en operaciones limitadas de vigilancia realizadas casi
exclusivamente en los EE.UU., se ha detectado el MTBE en la nieve, el
agua de tormenta, las aguas superficiales (riachuelos, ríos y
embalses), las aguas subterráneas y el agua de beber. Las
concentraciones de MTBE detectadas en el agua de tormenta iban de 0,02
a 8,7 µg/litro, con un valor mediano de menos de 1,0 µg/litro. En los
riachuelos, ríos y embalses, la gama de detección era de 0,2 a
30 µg/litro y la gama de valores medianos en varios estudios era de
0,24 a 7,75 µg/litro.
En general no se ha detectado el MTBE en las aguas subterráneas
profundas o cercanas a la superficie en zonas agrícolas. Cuando se ha
detectado, la concentración era inferior a 2,0 µg/litro. El MTBE se
halla con más frecuencia en las aguas subterráneas cercanas a la
superficie (1,6 a 3,2 metros de estos acuíferos) de las zonas urbanas.
En este entorno, las concentraciones van de menos de 0,2 µg/litro a 2
mg/litro, con un valor mediano inferior a 0,2 µg/litro.
El MTBE se halla poco frecuentemente en sistemas de
abastecimiento público de agua procedente de capas freáticas. Entre 51
sistemas estudiados, en 48 la concentración era de <20 µg/litro.
Son insuficientes los datos disponibles para caracterizar la
concentración de MTBE en los sistemas de abastecimiento público de
agua procedentes de aguas superficiales. El MTBE se ha hallado en
concentraciones altas (esto es, >1000 µg/litro) en algunos pozos
privados utilizados para obtener agua de beber. Sin embargo, es dudoso
que las personas puedan consumir agua con concentraciones de MTBE
superiores a unos 50-100 µg/litro debido al bajo umbral de su gusto y
olor.
Entre los trabajadores con posible exposición al MTBE figuran los
ocupados en la producción, distribución y uso de MTBE y de gasolina
con MTBE, que incluye el personal de estaciones de servicio y los
mecánicos.
La exposición a corto plazo (<30 min) en operaciones corrientes
de fabricación y mantenimiento de MTBE puro iba de 715 a 43 000 µg/m3
(0,2 a 12 ppm), siendo el promedio de los valores medianos de
3400 µg/m3 aproximadamente (0,95 ppm). La exposición a largo plazo
(30 min a 8 h) era de 360 a 890 000 µg/m3 (0,01 ppm a 250 ppm), con
valores medianos de aproximadamente 540 µg/m3 (0,15 ppm). En el caso
de los trabajadores de operaciones de mezclado, los valores a corto
plazo oscilaban entre niveles indetectables y 360 000 µg/m3
(100 ppm), siendo el promedio de los valores medianos de 5700 µg/m3
aproximadamente (1,6 ppm). Los valores a largo plazo comprendían desde
niveles indetectables hasta 257 000 µg/m3 (72 ppm), siendo el
promedio de los valores medianos de 2000 µg/m3 aproximadamente
(0,6 ppm).
La exposición alcanzó el nivel máximo durante el transporte de
MTBE puro y de mezclas de combustible en oleoductos, barcazas, vagones
de ferrocarril y camiones (sólo MTBE puro), variando los valores a
corto plazo entre 4 y 3750 mg/m3 (0,001 a 1050 ppm), con un promedio
de los valores medianos de 140 mg/m3 (39 ppm). Los valores a largo
plazo fueron de 0,036 a 2540 mg/m3 (0,01 a 712 ppm), con un promedio
de los valores medianos de 2,85 mg/m3 (0,8 ppm). En las operaciones
de distribución (esto es, carga de mezclas de combustible y MTBE en
camiones y entrega y descarga en las estaciones de servicio), los
valores a corto plazo oscilaron entre niveles indetectables y
225 mg/m3 (63 ppm), siendo el promedio de los valores medianos de
21 mg/m3 aproximadamente (6 ppm). Los valores a largo plazo fueron de
0,036 a 22 mg/m3 (0,01 a 6,2 ppm), siendo el promedio de los valores
medianos de 1,79 mg/m3 (0,5 ppm).
Los valores medianos de la exposición a corto plazo de operarios
de estaciones de servicio fueron en general de 1,071 a 21,42 mg/m3
(0,3 a 6 ppm), excediendo rara vez de 35,7 mg/m3 (10 ppm). Los
valores medianos de la exposición a largo plazo en operarios de
estaciones de servicio presentaron un promedio de 1,79 mg/m3
(0,5 ppm). Los valores medianos de la exposición de mecánicos estaban
por debajo de los niveles de detección en un estudio a corto plazo; el
promedio de los valores medianos para la exposición a largo plazo fue
aproximadamente de 360 µg/m3 (0,1 ppm).
5. Cinética y metabolismo
Los datos toxicocinéticos sobre el MTBE en personas proceden
principalmente de estudios controlados en voluntarios adultos sanos y
en una población expuesta a la gasolina oxigenada. El MTBE pasa
rápidamente a la circulación después de la exposición por inhalación.
En voluntarios sanos expuestos a la inhalación, la cinética del MTBE
era lineal hasta concentraciones de 268 mg/m3 (75 ppm). Se midió en
la sangre y orina de personas expuestas el alcohol
terciario-butílico, metabolito del MTBE. Las concentraciones
sanguíneas máximas del MTBE y el alcohol terciario-butílico fueron
de 17,2 a 1144 µg/m3 y de 7,8 a 925 µg/m3, respectivamente, en
personas expuestas a 5,0 a 178,5 mg/m3 (1,4 a 50 ppm) de MTBE.
Basándose en un modelo de monocompartimiento se identificaron
componentes rápidos (36- 90 min) y lentos (19 h) de la semivida del
MTBE.
En los roedores, el MTBE se absorbe y distribuye bien después de
la administración oral y la exposición por inhalación, con menor
absorción cutánea. En la administración oral de 400 mg/kg y en la
inhalación de 28 800 mg/m3 (8000 ppm) aumentó el porcentaje de la
dosis absorbida total eliminado en el aire espirado, con un descenso
correspondiente del porcentaje eliminado por la orina, indicando la
saturación metabólica. No se identificó la presencia de alcohol
terciario-butílico (TBA) en la orina de ratas expuestas. Hubo
indicios de un metabolismo adicional del TBA, basados en la
identificación de 2-metil-1,2-propanodiol y de ácido
alpha-hidroxiisobutírico eliminado por la orina. Los estudios
in vitro prueban que el MTBE se metaboliza hasta TBA, formaldehido y
acetona.
6. Efectos en los animales de laboratorio y en los sistemas
in vitro
En las ratas, la dosis letal oral mediana aguda (DL50) es
aproximadamente de 3800 mg/kg de peso corporal. La concentración letal
mediana aguda (CL50) para una exposición por inhalación de 15 minutos
es de aproximadamente 141 000 mg/m3 de aire en ratones. Entre los
signos de intoxicación figuran la depresión del SNC, la ataxia y la
respiración laboriosa. Si la dosis no es letal, la recuperación es
completa. La DL50 para la toxicidad cutánea en conejos es de
>10 200 mg/kg de peso corporal.
En un solo estudio identificado, el MTBE resultó "moderadamente"
irritante para la piel, produciendo eritema moderado y edema después
de la aplicación cutánea en conejos. También resultó irritante para
los ojos de los conejos, produciendo lesiones leves y reversibles. En
el único estudio identificado, el MTBE produjo irritación respiratoria
ligera a intensa después de la exposición de ratones a 300 y
30 000 mg/m3, respectivamente. No causó sensibilización cutánea en
estudios en cobayos.
La exposición repetida produce fundamentalmente aumentos del peso
de los órganos y lesiones histopatológicas en el riñón de ratas y en
el hígado de ratones. Los niveles de mínimo efecto señalado de
nefrotoxicidad tras la ingestión en estudios de 90 días fueron de
400 mg/kg de peso corporal por día (aumentos del peso renal relativo y
formación de gotas hialinas en ratas Sprague-Dawley). En la exposición
por inhalación a 2880 mg/m3 (800 ppm) se produjeron aumentos del peso
del riñón asociados a las concentraciones más altas, con moderado
aumento de las gotas hialinas en los túbulos proximales en ratas
Fischer-344. En estudios de oncogenicidad por inhalación en dosis de
1440 mg/m3 (400 ppm), la incidencia y la gravedad de la nefropatía
progresiva crónica aumentó en ratas machos; en esta concentración, en
ratones machos se observó un aumento del peso absoluto del hígado (que
guardaba correlación con el aumento de la hipertrofia hepatocelular en
concentraciones superiores) y un aumento del peso renal relativo.
La exposición al MTBE también produjo lesiones reversibles del
sistema nervioso central (SNC), incluidas sedación, hipoactividad,
ataxia y anestesia en concentraciones superiores y efectos bifásicos
sobre la actividad motriz en concentraciones inferiores. En un estudio
de una sola exposición de 6 horas en ratas, las concentraciones de
2880 mg/m3 (800 ppm) produjeron cambios reversibles de la actividad
motriz relacionados con la dosis en sexos separados. Esos efectos
fueron transitorios y no se pusieron de manifiesto en estudios a largo
plazo.
Se han efectuado estudios reproductivos por inhalación de una y
dos generaciones y estudios de cuatro generaciones en ratas, ratones y
conejos. En esos estudios no se hallaron efectos reproductivos
específicos en ratas en concentraciones de hasta 28 800 mg/m3. El
MTBE no ha producido efectos en el desarrollo en concentraciones
inferiores a las que resultaron tóxicas en las madres. Se han
observado disminuciones del peso del útero y aumentos del metabolismo
estrogénico en ratonas con dosis de 28 000 mg/m3.
El MTBE ha sido sometido a pruebas apropiadas en una amplia gama
de ensayos de mutagenicidad y de genotoxicidad. Los resultados
muestran que el MTBE no es genotóxico, aunque resultó positiva una
prueba de mutación del locus tk en células linfomatosas de ratón
debido al paso metabólico de MTBE a formaldehido.
Se han realizado estudios de carcinogenicidad que comprendieron
la exposición por inhalación de ratas Fischer-344 y de ratones CD-1 y
el cebado de ratas Sprague-Dawley. En ninguno de los dos estudios de
inhalación se utilizaron métodos de análisis estadístico que
efectuaran el reajuste de las diferencias de supervivencia. En los
tres estudios se produjeron aumentos significativos de la incidencia
de tumores, esto es, tumores de células tubulares renales y tumores de
células de Leydig en ratas Fischer-344 machos, tumores de células de
Leydig en ratas Sprague-Dawley machos y linfomas-leucemias
(combinadas) en ratas hembras de la misma especie, y tumores de
células hepáticas en ratones CD-1 hembras. Así pues, no se observaron
constantemente tumores de células tubulares renales ni
leucemias-linfomas en los distintos estudios en ratas. Además, los
tumores renales específicos del sexo se asociaron a la nefropatía de
la alpha2u-globulina específica del sexo, observada en varios estudios
de breve duración. Se observaron aumentos de los tumores de células de
Leydig con la dosis más alta (1000 mg/kg de peso corporal) en la ratas
Sprague-Dawley, pero la interpretación de los aumentos registrados en
las ratas Fischer-344 resultó compleja por las incidencias muy altas
concurrentes y de los testigos históricos. En los ratones, las
incidencias de los tumores hepáticos fueron en los testigos y en los
grupos expuestos a 28 800 mg/m3 (8000 ppm), respectivamente, de 2/50
y 10/50 en las hembras y de 12/49 y 16/49 en los machos. Los aumentos
fueron moderados y acompañados de hipertrofia hepatocelular.
7. Efectos en el ser humano
Tras la introducción de dos programas separados relativos a los
combustibles en los EE.UU., que requieren el empleo de productos de
oxigenación de la gasolina (no necesariamente MTBE), los consumidores
de algunas zonas se han quejado de trastornos agudos de la salud, como
dolor de cabeza, irritación de los ojos y la nariz, tos, náuseas,
mareos y desorientación. Los estudios epidemiológicos de poblaciones
humanas expuestas en condiciones profesionales o no profesionales, así
como los estudios experimentales de voluntarios expuestos en
condiciones controladas, no han podido identificar la base de esos
trastornos. Aunque los resultados son variados, los estudios
comunitarios efectuados en Alaska, New Jersey, Connecticut y Wisconsin
(EE.UU.) no han proporcionado indicios, o éstos han sido limitados, de
la asociación entre la exposición al MTBE y la prevalencia de
trastornos de la salud.
En estudios experimentales controlados en voluntarios humanos
expuestos en cámaras de inhalación al MTBE en concentraciones de 5,0
mg/m3 (1,4 ppm) a 270 mg/m3 (75 ppm) no hubo efectos manifiestos en
términos de presencia subjetiva de síntomas o de indicadores objetivos
de irritación u otros efectos en concentraciones de hasta 180 mg/m3
(50 ppm) durante dos horas. Partiendo de esos datos parece improbable
que el MTBE por sí solo produzca efectos agudos adversos en la salud
en la población general en las condiciones corrientes de exposición
por inhalación. Sin embargo, los posibles efectos de las mezclas de
gasolina y MTBE y el modo de exposición de la mayor parte de las
personas al MTBE en asociación con el empleo de combustibles
oxigenados, no se han examinado experimentalmente ni por métodos
epidemiológicos prospectivos. Por otra parte, no se ha investigado,
por ejemplo, la función de factores tales como la percepción del MTBE,
debida en parte a su olor distintivo.
8. Efectos en otros organismos en el laboratorio y sobre el
terreno
La toxicidad aguda experimental (CL50) del MTBE en los peces,
los anfibios y los crustáceos es >100 mg/litro. No existen datos
sobre la toxicidad crónica o subletal para las especies acuáticas ni
la toxicidad para los organismos terrestres.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
Basándose en datos de observación colectiva, parece improbable
que el MTBE por sí solo induzca efectos agudos adversos en la salud de
la población general en las condiciones corrientes de exposición.
En estudios en animales, el MTBE es "moderadamente" tóxico en
forma aguda y produce irritación cutánea y ocular moderada, pero no
sensibilización. La exposición repetida afecta fundamentalmente al
riñón de ratas y al hígado de ratones, observándose los efectos
adversos mínimos con concentraciones de 440 mg/kg de peso corporal por
día en ratas después de la ingestión y de 1400 mg/m3 (400 ppm)
después de la inhalación. El MTBE no ha inducido efectos adversos en
la reproducción o el desarrollo en concentraciones inferiores a las
que eran tóxicas para los padres.
El MTBE no es genotóxico, pero ha producido tumores en roedores,
principalmente con concentraciones altas, que también inducen otros
efectos adversos. Esos datos se consideran en la actualidad
insuficientes para la evaluación del riesgo carcinogénico en seres
humanos. El Grupo Especial llegó a la conclusión de que para
proporcionar orientación cuantitativa sobre los límites pertinentes de
exposición y para estimar el riesgo se necesita adquirir datos
adicionales en distintos sectores.
No parece que las concentraciones de MTBE en el agua ambiental
sean tóxicas para los organismos acuáticos, excepto en caso de
escapes. Aunque no hay datos sobre la toxicidad terrestre del MTBE,
parece que no es preocupante ya que las concentraciones en el aire
ambiental son bajas y la semivida del MTBE es relativamente breve.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
Owing to the limited therapeutic use of MTBE in the dissolution
of cholesterol gallstones in humans, there have been a number of
studies in which effects have been examined following single or
repeated exposure by direct instillation into the gallbladder.
Reported effects at therapeutic doses were mild clinical signs and
inflammatory changes in the gallbladder (Allen et al., 1985a; McGahan
et al., 1988; Adam et al., 1990; Esch et al., l992a,b; Chen et al.,
1995).
7.1 Single exposure
The acute toxicity of MTBE has been studied in several animal
species; the results are summarized in Tables 16 and 17. Studies by
routes most relevant to human exposure are described here. The oral
LD50 value for rats is about 3800 mg/kg bw (ARCO, 1987). Signs of
intoxication after single oral lethal doses consist of CNS depression,
ataxia, laboured respiration and death (ARCO, l987). When the dose was
non-lethal, recovery was complete.
The acute dermal LD50 is >10 200 mg/kg bw for rabbits (ARCO,
1987). Adverse local effects included erythema, oedema, fissuring and
necrosis at dermal application.
In rats, the LC50 value for inhalation exposure to MTBE is about
142 000 mg/m3 air (39 460 ppm) (ARCO, 1987). Reported LC50 values in
mice are 141 000 mg/m3 (1.6 mmol/litre) for 15 min of inhalation
exposure (Marsh & Leake, l950) and 658 000 mg/m3 (18% v/v) for 10 min
of inhalation exposure (Snamprogetti, 1980). Signs noted in rats
following inhalation exposure included eye irritation, incoordination
and loss of righting reflex (ARCO, 1987). Surviving animals appeared
to recover within 24 h.
Results of studies in which neurological effects following single
exposures were examined are reported in section 7.3.
7.2 Skin, eye, and respiratory tract irritation; skin sensitization
7.2.1 Skin irritation
Moderate erythema and oedema were reported by Cuthbert (1979)
following application of 0.5 ml undiluted MTBE to the intact and
abraded skin of six rabbits (applied under occlusion for 24 h). A
primary irritation index of 3.36 was reported. Effects were slightly
more pronounced on abraded skin:
24 h 72 h
intact abraded intact abraded
erythema 1.7 2.2 1.1 2.0
oedema 1.7 2.0 1.0 1.8
MTBE was considered to be a moderate skin irritant.
Table 16. Acute toxicity of MTBE in experimental animals
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Rat: oral (gavage) 2000, 3000, 4600, 3800 hypoactivity, muscular weakness, Industrial Bio-Test
Charles River, 6800, or 10 200 hyperpnoea, lacrimation, Laboratories (1969)
5 male, 5 female/ mg/kg bw prostration and death; the
dose level symptoms were reversible at
sublethal doses.
Inflammation of the stomach
and/or small intestine in
animals that died
Rat: oral (gavage) 3866 CNS depression, ataxia, ARCO (1987)
(strain and number laboured respiration and
not stated) death
Rabbit: dermal 6.8 or 10.2 g/kg >10 200 no deaths; no gross Industrial Bio-Test
New Zealand White bw, occlusive pathological alterations Laboratories (1969)
2 male, 2 female/ dressing, for 24 h; other than necrosis at
dose level 14-day observation application site
Rabbit: dermal 10 000 erythema, oedema, scaling, ARCO (1987)
(strain and number fissuring, and blanching
not stated) hyperaemia in animals that
died
Rat: inhalation LC50 = 142 000 eye irritation, ARCO (1987)
(strain and sex mg/m3 incoordination, tachypnoea,
not stated) (39 460 ppm) loss of righting reflex
and death
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Mouse: inhalation exposure for 15 min LC50 = 141 000 the median concentration Marsh & Leake (1950)
White (strain (whole body mg/m3 for anaesthesia (AC50) =
and sex not exposure) (1.6 mmol/litre) 106 mg/litre (1.2
stated), 20 mmol/litre)
mice/exposure
group
Mouse: inhalation exposure for 5 min; 400 000 mg/m3 median effective concentration Industrial Bio-Test
Swiss, (whole body observation for (260-600 mg/litre) for anaesthesia (EC50) = Laboratories (1969)
4 males/exposure exposure) following 48 h 200 mg/litre air (130-300
group mg/litre); convulsive
hyperventilation,
hyperactivity, ataxia, loss
of righting reflex, clonic,
and sporadic convulsive
seizures
Mouse: inhalation I. 20% v/v in air LT50a = 5.6 min death occurred within 1 h Snamprogetti (1980)
Swiss, (whole body for 3, 4, 5, 6, 9
I. 40 males/ exposure) or 12 min
exposure group II 8, 12, 17, 20, LC50 = 658
II. 20 males per 22 or 26% v/v MTBE mg/litre
group in air for 10 min (18% v/v)
Skin and eye irritancy
Rabbit:
New Zealand skin application 0.5 ml moderate erythema and
White, 3 male, (occluded) oedema
3 female
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
New Zealand conjunctival 0.1 ml moderate conjunctival Cuthbert (1979)
White, 3 male, instillation response; no corneal
3 female or iris involvement
New Zealand
White
I. 6 (sex not conjunctival 0.1 ml conjunctival redness for Hazleton
stated) instillation 48 h, no chemosis or discharge, Laboratories
slightly more marked effects (1979)
II. 3 (sex not conjunctival 0.1 ml with temporary corneal
stated) instillation; opacity for 24 h
washout with
water 30 secs
later
Rabbit conjunctival 0.05 ml or 0.1 ml conjunctival congestion, Snamprogetti (1980)
Both sexes instillation thickening and lacrimation;
(strain and number more marked at high dose;
not stated) reversible
a LT50 =exposure time which causes death in 50% of treated animals.
Table 17. Acute toxicity of MTBE following parenteral injection
Species Injection Dose LD50 Comments Reference
route/site
Rat: subcutaneous 3.0, 4.0, 5.0, 6.0, 6.7 ml/kg bw Snamprogetti (1980)
Wistar, 7.0, 8.0, 9.0 or 10.0 (5.75-7.76 ml/kg)
8 males/dose group mg/kg bw
Mouse: subcutaneous 1.0, 2.0, 3.0, 4.0, 3.6 ml/kg bw Snamprogetti (1980)
Swiss, 4.5, 5.0 or 5.5 ml/kg bw (2.93-3.57 ml/kg)
8 males/dose group
Rat: intravenous 0.1, 0.2, 0.3, 0.5, 0.75, 0.56 ml/kg bw in lethal doses: Snamprogetti (1980)
Wistar, 1.25 or 1.50 mg/kg bw nervous depression,
16 males/dose group sometimes followed
by short clonic
convulsions, autonomic
activity
(hypersalivation,
urination, defaecation),
and respiratory
disorders; death
occurred within
30 min. Surviving
animals: no toxic
symptoms or signs of
nervous depression
lasting more than
15-20 min
Table 17. (continued)
Species Injection Dose LD50 Comments Reference
route/site
Rat: hepatic parenchyma, 0.2 ml/kg bw intracaval injection Akimoto et al.
Sprague-Dawley, inferior vena cava, caused 100% mortality (1992)
7 males dosed tail vein or (pulmonary injury);
intravenously peritoneal cavity intrahepatic injection
caused 59% mortality
10 males dosed and peripheral vein
intraperitoneally injection 17% mortality;
the pulmonary injury
included congestion,
haemorrhage, and
interstitial oedema
Mouse: intraperitoneal 1.5, 2.0, 2.5, 3.0, 3.5, 1.4 ml/kg bw Snamprogetti (1980)
Swiss, 4.0, or 5.0 ml/kg bw
8 males/dose group
Mouse: intraperitoneal 0, 50, 200, or 500 mg/kg 830 mg/kg bw Arashidani et al.
ddy bw (784-878 mg/kg) (1993)
10 males/dose group
7.2.2 Eye irritation
Instillation of 0.05 or 0.1 ml of MTBE into the conjunctival area
of albino rabbits (both sexes) caused reversible eye irritation
(congestion of the conjunctiva, palpebral thickening and lacrimation,
more marked at the high dose) (Snamprogetti, 1980).
Moderate erythema and slight chemosis and discharge, persisting
for 3 days, were noted in another study after instillation of 0.1 ml
MTBE into the conjunctival area of New Zealand white rabbits
(Cuthbert, 1979):
24 h 48 h 72 h 7 days
redness 2.2 1.7 1 0.2
chemosis 1.3 1.2 1 0.1
discharge 0.9 0.6 0.2 0
The reactions had largely resolved by one week post-treatment).
The author concluded that MTBE was irritant to the rabbit eye.
Hazleton Laboratories (1979) conducted a study on nine New
Zealand White rabbits (sex not stated) in which six rabbits had 0.1 ml
MTBE instilled into one eye, the other acting as control, and three
rabbits had the same treatment followed by eye washout with water 30
seconds later. There was slight irritation (mean score for redness 1.5
and 1.0 at 24 and 48 h, negligible chemosis or discharge) in six
rabbits that did not have the eye washout, but slightly more marked
effects, including corneal opacity lasting for 24 h, in the three
rabbits with eye washout. The effects were all reversible.
Exposure to MTBE vapour in inhalation chambers also resulted in
eye irritation. In Fischer-344 rats exposed to MTBE vapour
concentrations of 14 300 or 28 600 mg/m3 (4000 or 8000 ppm) for 6 h,
lacrimation was noted 1 h post-exposure (Gill, 1989).
7.2.3 Respiratory tract irritation
A test of lung irritancy was used by Tepper et al. (1994) to
evaluate the effects of 1-h exposures of Swiss-Webster mice (four
males per dose group) to 300, 1000, 3000, 10 000 and 30 000 mg
MTBE/m3 in inhalation chambers. An almost immediate decrease in
frequency of breathing was observed in all dose groups. The animals
served as their own controls with baseline frequency of breathing and
respiration waveform morphology being obtained by exposure to filtered
air for 15 min. The severity of the irritant response was
dose-related, ranging from "slight" (13% decrease in respiration rate)
to "severe" at 30 000 mg/m3. During the exposures, breathing rate
returned to baseline after about 15 min, except for the highest dose
group, in which the rate gradually decreased for another 40 min. There
was a return to baseline frequency 15 min after end of exposure. Lung
injury could not be confirmed at the 30 000 mg/m3 dose level. Lung
lavage about 20 h post-exposure indicated only a marginal increase of
total protein and lactate dehydrogenase, both measures of lung cell
damage; however, these results were comparable to those of similarly
treated mice, exposed to filtered air, in a previous study.
In a single 6-h exposure vapour neurotoxicity inhalation study
(see also section 7.3) in Fischer-344 rats at target concentrations of
14 400 or 28 800 mg/m3 (4000 and 8000 ppm), survivors killed at the
end of a 14-day observation period had slight to mild lung hyperaemia
(Gill, 1989).
7.2.4 Skin sensitization
The sensitization potential of MTBE was assessed in guinea-pigs
using a Magnusson and Kligman procedure (Cuthbert, 1979). No evidence
of sensitization was reported in any of the 20 test animals following
induction and challenge with 1% MTBE. Dermal sensitization has also
been investigated using a Landsteiner technique (Litton Bionetics,
1980). Guinea-pigs were induced using intradermal injection (initial
treatment 0.5 ml of a 1% aqueous solution, followed by 9 injections of
0.1 ml over 3 weeks). No sensitization reactions were recorded at
challenge 2 weeks later (0.05 ml of a 0.01% solution of MTBE in
water).
7.3 Neurotoxicity
Following a single MTBE vapour exposure, reversible alterations
in central and peripheral nervous system function were observed (Gill,
1989; Daughtrey et al., 1997). Groups of Fischer-344 rats (22 of each
sex per dose level) were exposed for 6 h in inhalation chambers at
target MTBE concentrations of 0, 2860, 14 300 or 28 600 mg/m3 (0,
800, 4000 or 8000 ppm). No mortality or clinical signs of toxicity
were observed at any concentration. Behavioural evaluations performed
1, 6 and 24 h post-exposure included a screen for behavioural function
using a functional observational battery (FOB) and analysis of motor
activity prior to and following exposure. At 1 h post-exposure,
concentration-related increases in the incidence and severity of
ataxia and duck-walk gait appeared in both sexes of the mid- and
high-dose groups. In high-dose males, lacrimation, decreased muscle
tone, decreased rectal temperature, decreased performance time on the
tread mill, and increased hind limb splay were also observed. The
alterations in the FOB were significantly (p<0.01) different from the
control group. An increased incidence of laboured respiration (not
statistically significant) was also found in the high-dose male rats.
Additionally, piloerection was observed for all males in the high-dose
group. However, piloerection was also observed in some male rats in
the control group and in low- and mid-dose males.
Similar concentration-related findings were found in mid- and
high-dose female rats (increased incidence of lacrimation,
piloerection, ataxia and duck-walk gait, decreased rectal temperature,
and decreased hind limb grip strength). Additional exposure-related
findings for females in the high-dose group included a significantly
(p<0.01) increased incidence of laboured respiration and latency to
rotate on the inclined screen. Exposure-related alterations of motor
activity were detected for both male and female rats during the
initial 90 min of the test session. The mean activity was increased
during the entire test period in low-dose males and decreased in
high-dose males compared to the control group. However, in high-dose
males, an initial 10-min decrease in activity was followed by
increased activity during a 20-min interval and decreased thereafter.
Males in the mid-dose group only showed an increased activity
initially. For females, the mean activity for the entire test session
was not different from the control group. No MTBE-related alterations
were observed during the 6-h or 24-h post-exposure evaluation.
7.4 Short-term repeated dose studies
The short-term repeated dose toxicity of MTBE has been studied in
rats, mice and pigs. Typically, effects of irritation and reversible
central nervous system effects, including hypoactivity, ataxia, and
anaesthesia, were noted.
7.4.1 Oral studies
Sprague-Dawley rats (10 males and 10 females per dose group)
administered 0 (corn oil), 357, 714, 1071 or 1428 mg MTBE (99.95%
pure)/kg bw daily by gavage (in corn oil) for 14 days exhibited
transient anaesthesia at 1428 mg/kg and irritation of the pharyngeal
mucosa at the highest dose levels (Robinson et al., 1990). Diarrhoea
and reduced body weight gain were observed in all treatment groups.
Six animals (four from the high-dose group) died during treatment due
to difficulties associated with the gavage procedure, including
pharyngeal irritation in the high-dose animals. Absolute and relative
lung weights were significantly (p<0.001) lower in all exposed
female rats. The mean absolute kidney weights were increased in dosed
male rats and the relative kidney weights were significantly
(p<0.05 and p<0.001) increased over controls in the mid- and
high-dose group, respectively. The cholesterol levels were
significantly (p<0.05) increased in high-dose males and the two
mid-dose groups of females. The blood-urea nitrogen (BUN) and
creatinine were significantly (p<0.05) decreased in high-dose
females. The incidence of renal tubular disease (hyaline droplet
nephropathy) was moderately increased in dosed male rats. Increased
hyaline (protein) droplets within the cytoplasm of proximal tubular
epithelial cells were noted in seven of eight (88%) of the males in
the highest dose group as compared with two of five (40%) of the
controls.
In a 28-day oral study, Sprague-Dawley rats (10/sex/group) were
administered 0, 90, 440 or 1750 mg undiluted MTBE (purity not
specified)/kg bw daily by gavage for a total of 20 h (IITRI, 1992).
Seven rats (one low-dose female, one high-dose male, and five
high-dose females) died accidentally during dosing. This was
attributed to difficulties in dosing the animals owing to the strong
odour, the irritating nature, and high volatility of MTBE. Clinical
observations during the study period included transitory salivation in
all treated groups and transitory hypoactivity and/or ataxia in mid-
and high-dose animals. There were no significant effects on body
weight or body weight gain. The only significant treatment-related
change in haematological or clinical chemistry parameters was an
increase in cholesterol in high-dose males and females.
No treatment-related gross necropsy observations were noted. The
relative liver weights were significantly (p<0.05) increased in males
and females in the high-dose group. In the high-dose males there was
also a significant (p<0.05) increase in relative adrenal weight.
Absolute kidney and relative kidney weights showed a dose-related
increase in both males and females, but achieved statistical
significance (corrected for multiple comparisons) only for relative
weights in males at the mid- and high-doses and in females at the low
and high doses. Histopathology showed hyaline droplet formation in the
proximal convoluted tubules in the kidneys of the mid- and high-dose
males.
In a 90-day study, groups of Sprague-Dawley rats (ten males and
ten females in each test group) were gavaged 0 (corn oil), 100, 300,
900 or 1 200 mg/kg bw of undiluted MTBE (>99.95% pure) daily for 90
days (Robinson et al., 1990). The most pronounced clinical effect of
MTBE was the profound anaesthetic effect at 1200 mg/kg bw; the animals
recovered in about 2 h. In female rats, a significant (p<0.001)
treatment-related decreased BUN level and elevated cholesterol level
were observed at all levels of exposure. In male rats, the mean
absolute kidney weights were significantly (p<0.05) elevated at 900
and 1200 mg/kg bw, respectively. The increase in the relative kidney
weights was statistically significant (p<0.001) at the two highest
dose levels and also the relative liver weights (p<0.05 at 900
mg/kg and p<0.001 at 1200 mg/kg). In females, the relative kidney
weights were significantly (p<0.05) increased at and above 300
mg/kg bw and the relative liver, thymic and cardiac weights showed a
statistically significant (p<0.05) dose-related increase at 900
mg/kg. Microscopic findings included chronic nephropathy in both
control and high-dose male rats, which was more severe in MTBE-treated
rats. At the highest dose level, granular casts were found, and there
was also a slight increase of cytoplasmic hyaline droplets in proximal
tubular epithelial cells.
In a comparison study on the effects of inhalation exposure (see
section 7.3.2), two groups of female B6C3F1 mice (8/group) were given
MTBE by gavage in corn oil (5 ml/kg/bw) at doses of 0 or 1800 mg/kg bw
per day for 3 days and then killed around 18 h after the last dose
(Moser et al., 1996a). Body weight and liver weight were not affected
by treatment. MTBE induced a 37% increase in hepatic cytochrome P450
content (P <0-05), a 9-fold increase in hepatic 7-pentoxy
resorufin- O-dealkylase activity (PROD, a CYP2B marker) and a 2-fold
increase in hepatic 7-ethoxy-resorufin- O-deethylase activity (EROD,
a CAPE marker). MTBE also induced a 2-5-fold increase in the hepatic
cell labelling index (as estimated from the incorporation of
5-bromo-2-deoxyuridine delivered by an implanted osmotic mini pump) in
the absence of hepatotoxicity, judged by the absence of any change in
serum alanine aminotransferase (ALAT) or histological signs of
necrosis (Moser et al., 1996a).
7.4.2 Inhalation studies
Female B6C3F1 mice (five or more/group) were exposed by
inhalation to 0 or 27 900 mg/m3 (7814 ppm) MTBE (>99.95% pure) for 3
or 21 days (6 h per day, 5 days per week) (Moser et al., 1996a). The
exposure resulted in abnormal gait, hypoactivity, decreased muscle
tone and increased lacrimation during exposure and immediately after
termination of exposure to MTBE. The mice recovered quickly following
termination of exposure. There was no significant change in body
weight. The relative liver weights were increased 20% (p<0.05) as
compared to controls at 3 days of exposure but the difference was not
significant at 21 days. MTBE exposure significantly (p<0.05)
decreased relative uterine weight to 48% of the control at 3 days,
with a further significant (p<0.05) decrease to 65% at 21 days. The
relative ovarian weight was decreased to 69% (p<0.05) at 21 days of
exposure. Histopathological examination showed mild centrilobular to
midzonal hepatocyte swelling at 3 days. At 21 days there were no
microscopic exposure-related changes. The total hepatic microsomal
cytochrome P450 content was elevated 40% at 3 days and approximately
200% at 21 days. After 3 days and 21 days of MTBE exposure,
respectively, hepatic PROD activity increased 5-fold and 14-fold,
while hepatic EROD activity increased 1.8-fold and 3.2-fold. There was
a non-significant change in hepatic cell labelling index to a 2.5-fold
higher value at 3 days, but at 21 days the MTBE group showed a
significant reduction to 0.7% in comparison with the control value of
2.3% (p<0.05). There were no histological signs of hepatoxicity and
serum ALAT was unaffected.
Information on the repeated dose toxicity of MTBE (>99.95% pure)
is also available from a study comparing the short-term hepatic
effects on female B6C3F1 and CD-1 mice (Moser et al., 1996b). Groups
of six female mice were exposed in inhalation chambers to
particulate-free control air containing 0 mg/m3 or a target
concentration of 27 900 mg/m3 (7800 ppm) 6 h per day, 5 days per week
for 3 or 21 days. Clinical signs included abnormal gait, hypoactivity,
decreased muscle tone and increased lacrimation during exposure and
immediately after termination of exposure. The animals recovered
quickly after termination of each daily exposure. There was a decrease
of body weight (p<0.05) in CD-1 mice, but not in B6C3F1 mice, after
21 days. Statistically significant (p<0.05) increases were seen in
absolute and relative liver weight in both strains of mice at both
time points. Histopathological examination showed slight centrilobular
hypertrophy in both B6C3F1 mice and CD-1 mice at 3 days as compared
to controls. At 21 days there was no indication of hepatotoxicity or
necrosis. Serum ALAT showed no increased activity at either 3 or 21
days. In the CD-1 mice, after 3 and 21 days, respectively, total
hepatic microsomal cytochrome P450 content increased 2.3-fold
(p<0.05) and 1.8-fold (p<0.05), PROD increased 5-fold (p<0.05) and
5-fold (p<0.05), and EROD increased 2.3-fold (p<0.05) and 3-fold
(p<0.05). Corresponding data for the B6C3F1 mice are described
below. The hepatic cell labelling indices were, in contrast to B6C3F1
mice, increased in CD-1 mice at both time points; the increases were
3-fold and 5-fold, respectively, at 3 and 21 days (Moser et al.,
1996b). A slightly decreased survival relative to control was noted in
female B6C3F1 mice following 16 weeks exposure to 28 450 mg/m3 (7969
ppm) (100% versus 92% survival, respectively). After 32 weeks of
exposure, survival was 96% for controls and 88% for MTBE-exposed mice.
Body weight was significantly decreased (p<0.05) at both time points.
PROD activity was increased 4.9 fold, and EROD activity 1.9 fold,
after 16 weeks treatment. While MTBE exposure produced mild
centrilobular to midzonal hypertrophy, there was no indication of
cytotoxicity or hepatic necrosis, or alteration in serum ALAT (Moser
et al., 1996b).
Sprague-Dawley rats (10/sex/group) showed increasing depth of
anaesthesia with increasing concentrations of MTBE when exposed to
MTBE vapour in exposure chambers at concentrations of 0, 900, 1800 or
3600 mg/m3 (0, 250, 500 and 1000 ppm, respectively) 6 h per day, 5
days per week for 13 weeks (Greenough et al., 1980). The
haematological analyses revealed an increase in haemoglobin levels
during week 13 in male rats exposed to 3600 mg/m3. At autopsy, a
slight reduction in relative and absolute lung weight was detected in
female rats at the same exposure level. There were no other gross or
histopathological effects reported.
In a range-finding study, Fischer-344 rats and CD-1 mice were
exposed to MTBE vapour in inhalation chambers 6 h per day for 13
consecutive days (Dodd & Kintigh, 1989). The target concentrations
were 0, 7150, 14 300 and 28 600 mg/m3 (0, 2000, 4000 and 8000 ppm,
respectively). The measured mean concentrations were 7200, 13 750 and
28 330 mg/m3 (2018, 3850 and 7936 ppm, respectively). No mortality
occurred during the study period. Clinical signs included
hypoactivity, ataxia, and periocular irritation in both rats and mice,
primarily in the high-dose groups during exposure. Reversible
neurobehavioural alterations (ataxia) were observed immediately after
exposure in high-dose rats (mice were not observed). Body weight gain
was depressed in rats (but not in mice) in the mid- and high-dose
groups (statistically significant for male rats). In female mice, both
absolute and relative liver weights were significantly increased at
all levels of exposure. In the mid-dose rats, both absolute and
relative liver weights were significantly increased in females; in
males, there was an increase in relative kidney and liver weights. In
rats at the highest dose, males had an increase in relative weight of
liver, kidney and adrenal. Females had an increase in both absolute
and relative weights of liver and adrenal, and absolute weight of
kidneys was increased.
In a 13-week vapour inhalation study that included neurotoxicity
evaluation, Fischer-344 rats were exposed to target concentrations of
0, 2860, 14 300 and 28 600 mg MTBE/m3 (0, 800, 4000 and 8000 ppm) 6 h
per day, 5 days per week (Dodd & Kintigh, 1989; Lington et al., 1997).
No mortality occurred. Major findings included motor activity changes
and reversible changes in body temperature in mid- and high-dose rats,
ataxia, and depressed body weight gain and food consumption. Only mild
haematological changes (e.g., decreased erythrocyte counts and
increased reticulocyte counts) were observed, primarily in male rats.
The corticosterone levels were significantly increased in both male
and female rats at the highest exposure level. No treatment-related
gross lesions were found at necropsy. The relative weight of liver and
kidney were significantly increased in all male groups and in females
in the two highest exposure groups. Relative weight of adrenal was
significantly increased in males and females at the two highest doses.
There were, however, no treatment-related microscopic changes in these
organs. It is probable that there is an association between the liver
enlargement and the high serum corticosterone levels. (Although this
was reported by the authors, the Task Group considered that it was
more likely that the increase in adrenal weight was associated with
the high serum corticosterone levels). The only treatment-related
microscopic findings were found in males at the highest dose level and
included a statistically significant increase in lymphoid hyperplasia
in the submandibular lymph nodes, an increase (not statistically
significant) in the degree of haemosiderosis in the spleen, and a mild
increase of hyalin droplets in the renal proximal tubules. Proximal
tubule necrosis and protein droplet accumulation were observed in
kidneys from male, but not female, rats exposed to 5400 and 10 760
mg/m3 (1516 and 3013 ppm) for 6 h/day for 10 consecutive days
(Prescott-Mathews et al., 1997). Alpha-2u-globulin immunoreactivity
was present in and confined to protein droplets in male rat kidney. A
mild dose-related increase in alpha-2u concentration in the male rat
kidney correlated with an exposure-related increase in cell
proliferation. No significant differences were observed in female rats
for any of these responses.
7.4.3 Intraperitoneal administration
Katoh et al. (1993) reported that MTBE administered to mice (500
mg/kg bw as a single dose, or 200 mg/kg bw as repeated doses) caused
lipid peroxidation, as demonstrated by increased levels of lipid
peroxide in liver homogenates, and an induction of hepatic microsomal
cytochrome P450 content. Repeated treatment with 200 mg/kg bw for 4
weeks did not affect glutathione content or glutathione-S-transferase
(details on pattern and period of administration were not provided).
7.5 Neurotoxicity studies
A single 6-h inhalation exposure of Fischer-344 rats to MTBE
vapour at target concentrations of 0, 2880, 14 400 or 28 800 mg/m3
(0, 800, 4000 or 8000 ppm) induced reversible alterations in central
and peripheral nervous system function (Gill, 1989). Group of 8 male
and 8 female rats were used at each concentration for behavioural
evaluation and 14 males and 14 females for motor activity
observations. Motor activity changes appeared within 10 min of
exposure to 28 800 mg/m3 (8000 ppm) in both male and female rats. For
male rats, motor activity changes were also observed at 2880 and 14
400 mg/m3). Behavioural evaluation (functional observational battery,
FOB) was performed 1, 6 and 24 h post-exposure. At 1 h post-exposure
there were concentration-related behavioural alterations at 14 400
mg/m3 and 28 800 mg/m3 but these were not found at 6 or 24 h
post-exposure.
In a pilot study for a 13-week exposure study (see also section
7.3), Fischer-344 rats and CD-1 mice (five per sex and species) were
exposed to target MTBE concentrations of 0, 7150, 14 300 and 28 600
mg/m3 (0, 2000, 4000 and 8000 ppm) 6 h per day for 13 consecutive
days (Dodd & Kintigh, 1989). Hypoactivity was observed at all dose
levels and ataxia at the two highest dose levels in both rats and mice
during exposure. In high-dose rats, ataxia, decreased startle and pain
reflexes, and decreased muscular tone were also observed immediately
after exposure. Recovery was complete within 1 h. The NOAEL was
determined to be 7200 mg/m3 in both rats and mice.
The subsequent 13-week study with neurotoxic evaluation included
testing for inhalation toxicity, FOB, motor activity and
neuropathology (Dodd & Kintigh, 1989). Fischer-344 rats (25 rats of
each sex per dose level) were exposed to MTBE vapour at target
concentrations of 0, 2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and
8000 ppm) 6 h per day, 5 days per week (see also section 7.4.2).
Clinical findings included hypoactivity in the mid- and high-dose
groups and ataxia in the high-dose group immediately following the
daily exposure. The exposure resulted in minor changes in the FOB
including elevated body temperature (in high-dose male rats and in
mid- and high-dose female rats) and decreased hind limb grip strength
(in mid-dose males). Decreased motor activity for males in the
high-dose group and increased motor activity for females in the
low-and mid-dose groups were reported. Additional findings are
reported in section 7.3. Necropsy revealed no treatment-related gross
lesions. There were no treatment-related microscopic changes in the
central and peripheral nervous system tissues.
7.6 Reproductive and developmental toxicity
Protocols and results of reproductive/developmental studies are
presented in Table 18 These include one- and two-generation inhalation
studies in rats and four developmental studies (inhalation) in rats,
rabbits and mice. In these investigations, MTBE did not induce
specific adverse reproductive effects; developmental effects were
observed only at dose levels that were maternally toxic. At very high
dose levels (28 000 mg/m3) decreased relative uterine weight was
observed in one study.
Table 18. Reproductive studies with MTBE in laboratory animals
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
One- and two-generation studies
Rat, inhalation 15 males and 0, 1070, 4640 and one generation, two slightly decreased Biles et al.
Sprague-Dawley 30 females/group 12 140 mg/m3 litter study (F1a, F1b) pup viability (1987)
(0, 300, 1300 and (p<0.05) in F1b
3400 ppm) 12 140 mg/m3
Rat, inhalation 25 males and 0, 1430, 10 700, two generation study 1430 mg/m3: no Neeper-Bradley
Sprague-Dawley 25 females/group and 28 600 mg/m3 adverse effects; (1991)
(0, 400, 3000 and >10 700 mg/m3:
8000 ppm) reduced bw, bw
gain and food
consumption in
parental animals
(mainly in males),
clinical signs and
neurotoxic
effects, reduced
pup bw and bw gain
postnatally. NOEL
for general
toxicity 1430
mg/m3; NOEL for
reproductive
effects >28 600
mg/m3; LOEL for
adults and offspring
>10 700 mg/m3
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Developmental studies
Rat, inhalation 25 rats per dose 0, 900, 3600 and gestation days 6-15 reduced food Conaway et
Sprague-Dawley level 9000 mg/m3 consumption in al. (1985)
(0, 250, 1000 and treated groups
2500 ppm) during the day
9-12 interval; no
significant
developmental
toxicity
Mouse, inhalation 25 mice per dose 0, 900, 3600 and gestation days 6-15 a slight (not Conaway et
CD-1 level 9000 mg/m3 statistically al. (1985)
(0, 250, 1000 and significant),
2500 ppm) dose-related
decrease in food
and water
consumption; no
significant
developmental
effects
Mouse, inhalation 30 mice per dose 0, 3600, 14 300 gestation days 6-15 >14 300 mg/m3: Tyl &
CD-1 level and 28 600 mg/m3 clinical signs Neeper-Bradley
(0, 1000, 4000 and of toxicity and (1989)
8000 ppm) reduced fetal bw;
28 600 mg/m3:
reduced bw, bw
gain, and food
consumption;
increased number
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
of non-viable
implantations,
reduced number of
viable
implantations and
% male fetuses,
and increased
incidence of
cleft palate.
NOEL for maternal
and developmental
toxicity 3600 mg/m3
Rabbit, inhalation 8 females per dose 0, 7150, 14 300 and gestation days 6-18 >7150 mg/m3: Tyl (1989)
New Zealand level 28 600 mg/m3 (0, reduced food
White 2000, 4000 and 8000 consumption in
ppm) all dosed groups;
increased
incidence of
lung foci; 28 600
mg/m3: reduced
bw gain, audible
respiration, and
slightly lower
fetal weights
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Rabbit, inhalation 15 females per 0, 3600, 14 300 gestation days 6-18 >14 300 mg/m3: Tyl (1989)
New Zealand dose level and 28 600 mg/m3 reduced bw gain
White (0, 1000, 4000 and food
and 8000 ppm) consumption;
28 600 mg/m3:
hypoactivity,
ataxia, increased
relative liver
weight, decreased
corrected
gestational weight
change and gravid
uterine weight. No
significant
developmental
effects; NOEL for
maternal toxicity
3600 mg/m3; NOEL
for developmental
toxicity >28 600
mg/m3
7.6.1 Reproductive toxicity
Biles et al. (1987) conducted a two-litter, one-generation
inhalation study of reproductive effects in CD Sprague-Dawley rats.
Target concentrations were 0, 890, 3600 and 8925 mg/m3 (0, 250, 1000
and 2500 ppm). Corresponding measured concentrations were,
respectively, 1070, 4430 and 10 640 mg/m3 (300, 1240 and 2980 ppm)
for females and 1030, 4210 and 10 210 mg/m3 (290, 1180 and 2860 ppm)
for males. Fifteen males exposed for 12 weeks were mated to thirty
females exposed for 3 weeks. Exposures continued throughout the mating
period, during gestation and through days 5-21 of lactation. A second
litter (F1b) was produced under the same mating and post-mating
exposure regimen. In the mid- and high-dose groups of the F1b
generation, there was a slight statistically significant (p<0.05)
decrease in pup viability. The authors felt that this was in large
part attributable to the high viability (99%) in the control group.
There were no treatment-related differences between control and
exposed animals based upon examination of clinical signs, gross
post-examination and histopathological examination of the gonads of
exposed adults, mating or fertility indices, pregnancy rates, mean
gestational length and number of pups at birth, litter survival
indices or pup weight. (The NOEL was above 8925 mg/m3 (> 2500 ppm)
in both parents and offspring).
In a two-generation reproductive and fertility inhalation study,
CD Sprague-Dawley rats were exposed to MTBE at concentrations of 0,
1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 or 8000 ppm)
(Neeper-Bradley, 1991; Bevan et al., 1997). There was parental
toxicity at the target concentrations of 28 600 mg/m3 and 10 700
mg/m3. Concomitant perinatal toxicity was also observed at these
concentrations. There were no treatment-related effects on
reproductive indices at any concentration and no adverse effects on
the offspring at concentrations that were not toxic to the parents.
(NOEL = 1430 mg/m3 (400 ppm); hypoactivity, lack of startle reflex
and blepharospasm in parents at 10 700 mg/m3 (3000 ppm); NOEL for
reproductive effects >28 600 mg/m3 (8000 ppm).
Inhalation exposure to 28 800 mg/m3 MTBE (99.95% pure) resulted
in significantly decreased relative uterine weight in B6C3F1 mice at
3 (48%) and 21 days (65%) of exposure as compared to controls (Moser
et al., 1996a) (see also section 7.4.2). The relative ovarian weight
was significantly (p<0.05) decreased at 21 days of exposure. There
were no exposure-related microscopic findings in ovaries, adrenals and
pituitary, and no effects on adrenal and pituitary weight. Moser et
al. (1996a) investigated if the decreased uterine weights were due to
an increased rate of estrogen metabolism by measuring the rate of
conversion of 3H-17B-oestradiol to water-soluble metabolites in
hepatocytes from MTBE-treated female mice. MTBE was found to increase
the oestrogen metabolism by 2.1-fold.
Ward et al. (1994) studied the toxicity of MTBE to germ cells in
CD-1 male and female mice. Groups of 10 mice were given 1, 10, 100 or
1000 mg/kg bw MTBE in corn oil by gavage 5 days per week for 3 weeks;
a negative control group received corn oil only. At the end of
treatment the mice were killed and one testis from each male and both
ovaries from each female were sectioned for cytological evaluation. In
males, sperm number, Sertoli cells, spermatogonia, spermatocytes and
capped spermatids were evaluated, and, in females, oocyte quality.
There were no effects of MTBE on any of the cell types examined.
7.6.2 Developmental toxicity
The results of four inhalation studies on the developmental
toxicity of MTBE are summarized in Table 18.
There was a significant decrease in food consumption on days 9-12
of gestation in pregnant rats exposed to as much as 9000 mg/m3 (2500
ppm) but no other effects that the authors considered to be maternally
toxic, embryotoxic or teratogenic (Conaway et al., 1985). The NOEL for
offspring and parents was >9000 mg/m3 (2500 ppm).
Two different studies were conducted in the same strain of mice
exposed to various concentrations of MTBE for 6 h/day on days 6-15 of
gestation (Conaway et al., 1985; Tyl & Neeper-Bradley, 1989). No
significant maternal toxicity or developmental effects were observed
when groups of 30 pregnant females were exposed to 1000, 3960 or 9675
mg/m3 (280, 1110 or 2710 ppm), though the incidence of lacrimation
was increased in exposed mothers and there was a slight increase in
the incidence of fused sternebrae in the high-dose group (Conaway et
al., 1985). The NOAEL of parents and offspring was 9000 mg/m3 (2500
ppm). Tyl & Neeper-Bradley (1989) concluded that 14 300 mg/m3 (4000
ppm) was maternally toxic, based on observed hypoactivity and ataxia.
There were significant decreases (p<0.01) in body weight, body weight
gain and food consumption at 28 600 mg/m3 (8000 ppm). At 14 300
mg/m3 (4000 ppm) or more, there were significant reductions in fetal
body weight per litter (p<0.01) and increased skeletal variation. The
proportion of male fetuses was significantly reduced (p<0.01) at the
highest dose level in the Tyl & Neeper-Bradley (1989) study, but this
has not been observed in other studies with mice, rats or rabbits. At
28 600 mg/m3 (8000 ppm), the number of non-viable implantations per
litter and incidence of cleft palate was also increased (LOAEL in
parents and offspring = 14 300 mg/m3 (4000 ppm). The NOAEL in this
study was 3570 mg/m3 (1000 ppm).
Maternal toxicity was observed at the two highest concentrations
in rabbits exposed to 3570, 14 300 and 28 600 mg/m3 (1000, 4000 and
8000 ppm) during days 6-18 of gestation (Tyl, 1989). No developmental
effects were observed at any exposure level [NOAEL in offspring = 28
600 mg/m3 (8000 ppm); NOAEL in parents = 3570 mg/m3 (1000 ppm);
LOAEL in parents = 14 300 mg/m3 (4000 ppm)].
7.7 Mutagenicity and related end-points
Genotoxicity study results with MTBE are generally negative.
However, there are indications that MTBE may have some genotoxic
potential in the presence of metabolic activation. Genotoxicity data
for MTBE are compiled in Table 19.
In studies on reverse mutation in Salmonella typhimurium, MTBE
was found to be non-mutagenic in tester strains TA1535, TA1537,
TA1538, TA98 and TA100, with and without S9 (liver enzyme homogenates
from induced Sprague-Dawley male rats) metabolic activation, at doses
up to 10 mg/plate (Cinelli & Seeberg, 1989).
No significant increase in the frequency of recessive lethal
mutations in the X-chromosome could be established after feeding MTBE
(99.14% pure) (0.03, 0.15 or 0.3% MTBE in 5% aqueous sucrose) to adult
Drosophila melanogaster (wild-type Oregon-R males) for 24 h (Sernau,
1989).
In the presence of a liver-derived metabolic system (liver S9
from Arochlor 1254-induced male Sprague-Dawley rats) MTBE (>99% pure;
1.0, 2.0, 3.0 or 4.0 ml/ml) induced forward mutations in vitro at
the thymidine kinase locus of mouse lymphoma cell line L5178Y/ TK+/-
(Mackerer et al., 1996). The observed mutagenicity was dose-dependent.
Other experimental data, using a test system developed to
determine if the mutagenicity of a material is the result of the
presence or release of formaldehyde, had indicated that the
mutagenicity was due to the metabolism of MTBE to formaldehyde
(Blackburn et al., 1991). To establish if formaldehyde, derived from
MTBE in the presence of S9, was responsible for the observed
mutagenicity, Mackerer et al. (1996) used a modified mouse lymphoma
assay. In this assay formaldehyde dehydrogenase and its co-factor
NAD+ were added during the exposure period so that any formaldehyde
produced would be converted to formic acid, which is non-genotoxic. An
MTBE dose-related increase in the frequency of mutant lymphoma cells
occurred without the presence of formaldehyde dehydrogenase and
NAD+, but not when these were present, indicating that formaldehyde
was responsible for the mutations.
In two independent in vitro experiments MTBE (purity not
specified) did not induce unscheduled DNA synthesis (UDS) in primary
rat hepatocytes at concentrations up to 10 mg/ml (Seeberg, 1989).
In an in vivo-in vitro hepatocyte UDS assay, ten male and ten
female CD-1 mice were assigned to each dose group and an air-only
control group and, in addition, five of each sex to a positive control
group (DMN) (Vergnes & Chun, 1994). The animals were exposed to MTBE
(purity not specified) in inhalation chambers 6 h a day for two
consecutive days. The target concentrations were 0, 1440, 10 800 and
Table 19. Genotoxicity studies with MTBE
Species Strain/cells Measured end-point Test conditions Activation Result Reference
Bacterial systems
S. typhimurium TA1535 reverse mutation 625, 1250, 2500, 5000, + - - - Cinelli &
TA 1537 10 000 µg/plate Seeberg (1989)
TA1538
TA98
TA100
S. typhimurium TA98 reverse mutation exhaust particle + nd Clark et al.
TA100 extracts from gasoline + ± (1984)
containing 7% by
volume MTBE; five
concentrations ranging
from 1 to 100 µg/plate;
duplicate plates
Non-mammalian eukaryotic systems
Drosophila wild type Oregon-R, sex-linked recessive Basc test; 0.03, - Sernau (1989)
melanogaster males lethal test 0.15 or 0.3% MTBE;
adult feeding for 24 h
In vitro mammalian systems
Mouse lymphoma cell line forward mutation 1.0, 2.0, 3.0, 4.0 µl/ml + + Mobil Oil
L5178Y/TK Corporation
(1993)
Rat primary hepatocytes UDS 3.16, 10.0, 31.6, 100, - Seeberg (1989)
316, 1000, 3160, 10 000
µg/ml
Table 19. (continued)
Species Strain/cells Measured end-point Test conditions Activation Result Reference
In vivo - in vitro
Mouse, primary hepatocytes UDS vapour exposure: 0, - Vergnes & Chun
CD-1 1440, 10 800, 28 800 (1994)
mg/m3, 6 h/day for 2
consecutive days
In vivo mammalian systems
Rat, bone marrow cells chromosome vapour exposure: 0, - Vergnes & Morabit
Fischer -344 aberrations 2800, 14 400, 28 800 (1989)
mg/m3, 6 h/day for
5 days
Mouse, spleen lymphocytes chromosome oral administration, a slight Ward et al.
CD-1 aberrations 1.0, 10, 100 or 1000 inverse (1994)
mg/kg bw for 3 weeks dose-response
relationship
in male mice
but not in
female mice
Mouse, spleen lymphocytes mutations at hprt oral administration, - Ward et al. (1994)
CD-1 locus 1.0, 10, 100 or 1000
mg/kg bw for 3 weeks
Mouse, bone marrow chromosome vapour exposure: 0, - Vergnes & Kintigh
CD-1 cells damage 1440, 10 800, 28 800 (1993)
mg/m3, 6 h/day for 2
consecutive days
28 800 mg/m3 (0, 400, 3000 8000 ppm). The animals were sacrificed and
hepatocytes were sampled 18 h after the second exposure day (for the
positive control group after approximately 2 h). No dose-related
increase in the DNA repair activity could be established. The UDS
assay is used to indicate primary DNA damage; this is, however,
transient in nature and it is necessary to analyse cells for damage as
soon as possible after cessation of treatment. Since the half-life of
MTBE in the animal body is quite short (1-3 h) and DNA repair is
relatively rapid, the study should have been designed accordingly.
MTBE (purity not specified) was considered nonclastogenic to
Fischer-344 rats in an in vivo test system (Vergnes & Morabit,
1989). No concentration-related or significant increase in the
incidence of chromosome aberrations in rat bone marrow cells was found
in either males or females following whole body exposure to MTBE 6 h
per day for five consecutive days. The target concentrations were 0,
2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and 8000 ppm,
respectively).
MTBE did not induce micronuclei in vivo in mouse bone marrow
cells (Vergnes & Kintigh, 1993). CD-1 mice were exposed to MTBE
(purity not specified) vapour in inhalation chambers (five animals per
sex per dose level) at concentrations of 0, 1430, 10 710 or 28 600
mg/m3 (0, 400, 3000 or 8000 ppm) 6 h a day for two consecutive days.
Bone marrow cells were collected 24 and 48 h after the second exposure
day. No significant, exposure-related increase in the frequency of
micronuclei could be established at any dose level and sampling time
in either sex in this study.
Ward et al. (1994) examined the frequency of somatic cell
mutations in spleen lymphocytes after administration by gavage 5 days
per week for 3 weeks with MTBE (99.8% pure) in corn oil to CD-1 male
and female mice (ten animals per dose group). Ethyl-nitrosourea was
used as a positive control. The doses were 1, 10, 100 and 1000 mg/kg
bw. The frequency of mutations at the hypoxanthine-guanine
phosphoribosyl transferase (hprt) locus was determined 3 weeks after
the cessation of exposure. There was no indication that MTBE produced
a mutagenic effect at the tested dose levels. Ward et al. (1994) also
analysed chromosome aberrations in spleen lymphocytes. This was
performed on the first day after the termination of exposure in 13
male mice and on the second day in the remaining mice. A slight, but
not statistically significant, inverse dose-relationship was seen in
male mice; this was not seen in the females.
7.8 Carcinogenicity
Three bioassays are available on the oncogenicity of MTBE in both
sexes of rats and mice. These include two inhalation studies, one in
Fischer-344 rats and one in CD-1 mice, and one oral study in
Sprague-Dawley rats (Table 20). At high inhalation exposure levels,
MTBE increased the incidence of renal cell carcinomas in male rats and
liver tumours in female mice. Oral administration increased the
incidence of lymphomas and leukaemias in female rats. The studies also
resulted in testicular tumours in both strains of rats following
exposure either by inhalation or oral administration.
Fischer-344 rats (50 of each sex per dose level) were exposed to
MTBE (99% pure) vapour in inhalation chambers at target concentrations
of 0, 1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 and 8000 ppm,
respectively) 6 h per day, 5 days per week (Chun et al., 1992; Bird et
al., 1997). The control group was exposed to filtered air. Increased
mortality and decreased mean survival time were observed for male rats
from all exposure groups. Owing to the high mortality rate, surviving
males, six from the mid-dose group and nine from the high-dose group,
were killed at weeks 97 and 82, respectively. The numbers of surviving
males at the end of the study were 13, 6, 6 and 9 at 0, 1430, 10 700
and 20 600 mg/m3, respectively. Mean survival times were 632, 617
(p<0.05), 587 (p<0.01) and 516 (p<0.01) days, respectively.
Low-dose males and all females were killed during weeks 104 and 105.
Various clinical signs of toxicity (blepharospasm, hypoactivity,
ataxia, lack of startle reflex, swollen periocular tissue and
salivation) were observed in both sexes at the two highest dose
levels. No clinical signs were noticed at the lowest dose level.
Significantly (p<0.01) reduced body weight and body weight gain were
recorded at week 81 for the high-dose rats. For low- and mid-dose male
rats, body weight and body weight gain were slightly to significantly
(p<0.05) increased during the first 70 to 80 weeks. Thereafter, there
were no clear dose-related changes. In females, there was a slight,
but not exposure-related, decrease at the two lower exposure levels.
High-dose male rats also showed a significantly (p<0.05) decreased
corticosterone level at week 81. A trend toward increases in liver and
kidney weights relative to final body weight was recorded for the
mid-dose male rats. In female rats, concentration-related increases in
liver and kidney weight (absolute and relative to body or brain
weight) were observed at the two highest dose levels. There was also a
trend toward an increased adrenal gland weight relative to final body
weight for high-dose males. However, only the organ weight data from
the control and the low-dose group were statistically evaluated due to
the different sacrifice periods for the mid- and high-dose groups.
Non-neoplastic effects of treatment included an increased
incidence and severity of chronic progressive nephropathy in male rats
from all dose groups and in female rats from the mid- and high-dose
groups. Treated males were more severely affected than the females,
which usually showed only slight changes. Chronic progressive
nephropathy was diagnosed as the cause of morbidity or death for 3/37,
16/44, 26/44 and 39/41 male rats and for 0/20, 0/23, 4/27 and 6/25
female rats in the control, low-, mid- and high-dose groups,
respectively. The incidences of nephropathy, with interstitial
fibrosis, in the control, low-, mid- and high-dose groups were 19/37
(51%), 29/44, (66%), 37/44 (84%) and 40/41 (98%), respectively
(significance not specified). Histologically, the chronic progressive
nephropathy included an exposure-related increase in severity for
Table 20. Carcinogenicity studies with MTBE
Species Exposure Tumour Reference
Mouse (CD1) Male: Liver, adenoma: Burleigh-Flayer et al. (1992)
control 11/49 Bird et al. (1997)
1430 mg/m3 (400 ppm) 11/50
10 710 mg/m3 (3000 ppm) 9/50
28 600 mg/m3 (8000 ppm) 12/49
Male: Liver, carcinoma:
control 2/49
1430 mg/m3 (400 ppm) 4/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 8/49
Male: Liver, adenoma and carcinoma:
control 12/49
1430 mg/m3 (400 ppm) 12/50
10 710 mg/m3 (3000 ppm) 12/50
28 600 mg/m3 (8000 ppm) 16/49
Female: Liver, adenoma:
control 2/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 10/50 (p<0.05)
Female: Liver, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 1/50
Table 20. (continued)
Species Exposure Tumour Reference
Female: Liver, adenoma and carcinoma:
control 2/50
1430 mg/m3 (400 ppm) 2/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 11/50
Rat (F344) Male: Killed at: Chun et al. (1992)
control 104 weeks Bird et al. (1997)
1430 mg/m3 (400 ppm) 104 weeks
10 710 mg/m3 (3000 ppm) 97 weeks
28 600 mg/m3 (8000 ppm) 82 weeks
Male: Kidney, adenoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 5/50
28 600 mg/m3 (8000 ppm) 3/50
Male: Kidney, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 0/50
Male: Kidney, adenoma and carcinoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 8/50
28 600 mg/m3 (8000 ppm) 3/50
Table 20. (continued)
Species Exposure Tumour Reference
Male: Testes:
control 32/50
1430 mg/m3 (400 ppm) 35/50
10 710 mg/m3 (3000 ppm) 41/50
28 600 mg/m3 (8000 ppm) 47/50
Male: Pituitary tumours, week 104:
control 6/50
1430 mg/m3 (400 ppm) 5/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 0/50
Female: Kidney, adenoma:
control 0/50
1430 mg/m3 (400 ppm) 0/28
10 710 mg/m3 (3000 ppm) 1/39
28 600 mg/m3 (8000 ppm) 0/50
Rat (Sprague-Dawley) Male: Testicular adenoma Belpoggi et al. (1995)
(denominator is total number
in group):
control 2/60
250 mg/kg 2/60
1000 mg/kg 11/60
Male: Testicular adenoma
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/26
250 mg/kg 2/25
1000 mg/kg 11/32 (p<0.05)
Table 20. (continued)
Species Exposure Tumour Reference
Female: Lymphomas or leukaemia
(denominator is total
number in group):
control 2/60
250 mg/kg 6/60
1000 mg/kg 12/60
Female: Lymphomas or leukaemias
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/58
250 mg/kg 6/51
1000 mg/kg 12/47 (p<0.01)
glomerulosclerosis, tubular proteinosis, interstitial nephritis and
interstitial fibrosis in both male and female rats from the mid- and
high-dose groups. The chronic nephropathy was also associated with
secondary lesions such as fibrous osteodystrophy, hyperplasia within
the parathyroid glands, and mineralization within numerous tissues. An
increased incidence of renal tubular cell adenomas and carcinomas was
noted in mid- and high-dose male rats. The incidence was 8/50 and 3/50
for the mid- and high-dose groups, respectively, and 1/50 for the
control group. The renal tubular cell carcinomas were only noted in
the mid-dose group (3/50). One renal cell adenoma was found in a
mid-dose female.
In mid- and high-dose males, there was also a dose-related
increase of interstitial cell (Leydig cell) adenomas of the testes.
The incidence was 32/50, 35/50, 41/50 and 47/50 (64%, 70%, 82% and 94%
for the control, low-, mid- and high-dose groups, respectively. In
high-dose males, there was also an exposure-related decrease in the
frequency of pituitary adenomas. The incidences were 27/47, 29/48,
27/47 and 2/48 in the control, low-, mid- and high-dose groups of
males, respectively. A treatment-related decrease of large granular
lymphocyte (LGL) leukaemia was also noted. The incidence of LGL in
males was 33/50, 22/50, 20/50 and 3/50 and in females 22/50, 14/50,
15/50 and 16/50 for control, low-, mid- and high-dose rats,
respectively. LGL leukaemia, which is age-dependent and generally does
not appear until 20 months of age, was the main cause of death in the
control and low-dose males. The lymphoid hyperplasia of the
submandibular lymph node observed in a 13-week inhalation study with
Fischer-344 rats at a dose level of 28 600 mg/m3 was not observed in
the present study using the same strain of rats and the same dose
level. No NOEL could be determined for male rats due to a slight
increase of nephropathy at the lowest dose level. For female rats, the
NOEL for toxicity was 1440 mg/m3.
A number of points regarding this study can be made:
a) The tumour incidence values were analysed using methods that are
not appropriate when there are marked inter-group differences in
survival.
b) Testicular adenomas are quite common in untreated aging male
F-344 rats (Haseman et al., 1990), with a spontaneous incidence
in the range 64-98% for animals contemporaneous with those used
here. On this basis, it appears that this tumour type may have
been under-represented in the concurrent controls, influencing
the slope of the dose-response curve. In the Fischer-344 rats
used in this laboratory, the average historical control incidence
of Leydig cell tumours was 88% (Bird et al., 1997). Thus, in
comparison with historical control data, there was no increased
incidence in this tumour in the dosed groups. However, concurrent
control comparisons are always more appropriate, unless it is
known that there had been a particular problem with the study,
e.g., inappropriate randomisation.
c) Neoplasm incidence decreases were observed for pituitary adenomas
in the high-dose males and for large granular lymphocyte
leukaemia in males and females at all dose levels. Decreases in
the high-dose rats might be related to body weight gain
restrictions in this group and to increased mortality rate in
males. Alternatively, the concurrent control values for these
neoplasms may be particularly high in this study.
An oncogenicity study was also carried out on CD-1 mice exposed
to MTBE (99% pure) vapour in inhalation chambers (Burleigh-Flayer et
al., 1992; Bird et al., 1997). Groups of 50 male and 50 female mice
were exposed to target concentrations of 0, 1430, 10 700 and 28 600
mg/m3 (0, 400, 3000 and 8000 ppm, respectively) 6 h per day, five
days per week for 18 months. The control group was exposed to filtered
air. The mortality rates for male mice (including those sacrificed
moribund but excluding procedural and accidental deaths) in the
control, low-, mid- and high-dose groups were 33%, 22%, 35% and 49%,
respectively. The corresponding values for females were 27%, 18%, 23%
and 33%, respectively. The authors reported that increased mortality
rate (significance not reported) and decreased survival time were
observed for male mice from the high-dose group only. This was
considered as a probable result of a slightly increased frequency of
obstructive uropathy (distended urinary bladder and/or obstruction of
the urethra). Clinical signs, i.e. ataxia, blepharospasm,
hypoactivity, prostration, and lack of a startle reflex (in the
high-dose group prostration also and in the mid-dose group stereotypic
behaviour also) were observed in both male and female mice at the two
high-dose levels. Ataxia, observed in most male and female mice from
the highest dose group throughout the study, was the only clinical
finding considered to be exposure related. Body weight and body weight
gain were decreased for both male and female mice from the high-dose
group. At the end of the study, body weight gain was decreased 15%
(p < 0.01) for the males and 24% (p<0.01) for the females from the
high-dose group.
Necropsy showed that the liver was the target organ for toxicity.
There was a dose-related increase in liver weight, both absolute and
relative to body weight, in both sexes (increase in absolute weight in
males significant at all dose levels). A slight (significant),
although not concentration-related, increase in kidney weight was
noted for male mice from all exposure groups and in female mice from
the high-dose group. Decreases, although not statistically
significant, in absolute brain and spleen weight were also noted for
high-dose male and female mice. In addition, an increase in serum
corticosterone levels was observed in both male and female mice from
the high-dose group at week 79. The increase was significant (p<0.05)
only for male mice. A slight decrease in urinary pH and increases in
urine gamma globulin were observed for both male and female mice at
the high-dose level.
For male mice that were found dead in the high-dose group (7/25),
a slightly increased frequency of urinary bladder dilation/ distension
was noted at autopsy as compared to controls (3/18). In addition, the
incidence of the number of liver masses was increased in the high-dose
male mice (13/50 compared to 7/50 for the control group). The only
exposure-related lesion in female mice found at necropsy was an
increased incidence of liver masses in the high-dose group (9/50) when
compared to the controls (0/50). Histopathology showed an
exposure-related increase in hepatocellular hypertrophy in high-dose
male mice (15/49) when compared to controls (5/49). This lesion also
showed an increased, although not statistically significant, incidence
in mid-dose male mice (10/50) and in female mice from the high-dose
group (9/50 as compared to 4/50 in the control group). Exposure to
MTBE did not, however, cause hepatocellular necrosis or degeneration.
In high-dose male and female mice, mineralization within the brain was
decreased. In addition, there was a dose-related decrease in the
incidence of cystic endometrial hyperplasia for female mice.
An increased frequency (not statistically significant) of hepatic
adenomas and carcinomas was observed in male mice at the high-dose
level (16/49 as compared to 12/49 in the control group). The increase
(not statistically significant) was due to a slightly increased
frequency of hepatocellular carcinomas in the high-dose group (8/49)
when compared to the controls (2/49). The analysis for the combined
incidence of hepatocellular adenomas and carcinomas did not, however,
include statistical methods that adjusted for difference in survival
between the control and exposure groups. In female mice, there was a
significant increase in the incidence of hepatocellular adenomas at
the high-dose level (10/50 as compared to 2/50 from the control
group). The induced incidences were modest and occurred in the group
in which hepatocellular hypertrophy also occurred. There was no
exposure-related increase in the incidence of hepatocellular
carcinomas in female mice. The NOEL for toxicity in mice exposed to
MTBE for 18 months was 1440 mg/m3.
In an oral exposure study, male and female Sprague-Dawley rats
(60 per sex and dose group) were administered 0, 250 or 1000 mg/kg bw
MTBE (>99% pure) in 1 ml extra virgin olive oil by gavage four times
a week for 104 weeks on a weekly schedule of 2 days dosing, 1 day
without dosing, 2 days dosing, followed by 2 days without dosing
(Belpoggi et al., 1995). There were no treatment-related differences
in mean body weights of treated groups compared to control groups. The
animals were kept under observation until natural death. High-dose
male rats showed a higher survival than controls at treatment week 80
and thereafter. At week 80, survival was approximately 56% in control
and exposed groups; at 112 weeks, it was approximately 10% in controls
and the low-dose group, but 35% in the high-dose group. In female
rats, a dose-related decrease in survival was observed from
treatment-week 16. It was reported that there were no evident
behavioural changes; at week 72, survival in the high-dose group was
about 65%; at week 120, it was less than 20%; at week 136, it was less
than 5%. The authors reported no evident behavioural effects; however,
the extent of examination of behavioural effects was not specified. No
relevant non-neoplastic changes (including renal) were detected at
autopsy and histopathology. No specific data were, however, reported.
In male rats, there was a dose-related increase in the incidence of
testicular Leydig cell (interstitial cell) tumours (2/60, 2/60 and
11/60), statistically significant at the highest dose (p<0.05). In
female rats, there was a dose-related increase in lymphomas and
leukaemias combined (2/60, 6/60 and 12/60), marginally significant at
the low-dose level and highly significant at the high-dose level
(p<0.01). There also was an increase in dysplastic proliferation of
lymphoreticular tissue in female rats at both dose levels, but the
incidence was higher in the low-dose group. Dose-related decreases in
mammary fibromas and fibroadenomas, and in pituitary adenomas and
tumours of adrenal glands were observed in dosed females. Since these
tumours and the testicular interstitial cell tumours are
age-dependent, these effects were probably at least partly due to the
dose-related early mortality and the prolonged observational period in
the surviving animals.
A number of points regarding this study should be made:
a) there is limited description of the results, particularly the
histopathological findings;
b) diagnostic criteria are not given for the distinction between
Leydig cell tumours and hyperplasia (the latter were not reported
at all, which is unusual for old Sprague-Dawley rats showing
Leydig cell tumours);
c) diagnostic criteria are not given for the distinction between
dysplastic hyperplasia and lymphoma;
d) lymphomas and leukaemias are pooled; specific tumour type and
incidences were not reported;
e) historical control data might aid the evaluation of lymphomas and
leukaemias, particularly if they are available for these rats
within different age ranges;
f) chronic progressive nephropathy was not observed in these
Sprague-Dawley rats, although these lesions might be expected, on
the basis of data from a number of other studies with this strain
of rat.
7.8.1 Initiation-promotion protocol
In a study to investigate if MTBE exhibited hepatic
tumour-promoting activity, 12-day-old female B6C3F1 mice were
initiated with a single intraperitoneal injection of the mutagen
diethylnitrosamine (DEN) or saline and then exposed subsequently to 0
or 28 800 mg/m3 (8000 ppm) MTBE (>99.95% pure) from 8 weeks of age
for 16 or 32 weeks (Moser et al., 1996b). Liver weight was
significantly (p<0.05) increased at both time points in both
saline-treated and DEN-initiated mice after exposure to MTBE, and was
associated with mild centrilobular to midzonal hypertrophy in both
groups. There was no significant difference in the percentage of
microscopic lesions classified as hepatic foci (86%), hepatocellular
adenomas (10%), or hepatocellular carcinomas (4%) in DEN/MTBE mice as
compared to DEN/control mice. However, the absolute number of
microscopic hepatic lesions was 50% less in the DEN/MTBE group than in
DEN/control mice. MTBE appeared inactive in this tumour
initiation-promotion assay.
7.9 Metabolites of MTBE
Animal carcinogenicity experiments have been conducted with the
metabolites tertiary-butyl alcohol (TBA) and formaldehyde (FA). TBA
administered in drinking-water caused increased incidences of renal
tubular adenoma and carcinoma in male Fischer-344 rats and increased
severity of chronic progressive nephropathy (Cirvello et al., 1995).
In female B6C3F1 mice, TBA produced thyroid follicular cell adenoma
and hyperplasia, and, in both male and female mice, inflammation and
hyperplasia of the urinary bladder (Cirvello et al., 1995). FA has
caused nasal squamous cell carcinoma in both Fischer-344 and
Sprague-Dawley rats and in B6C3F1 mice, and also increases of cancers
of the nasopharynx, nasal cavity and sinus in humans (Grindstaff et
al., 1991).
7.10 Mode of action
7.10.1 Kidney tumours
A number of chemicals cause both protein droplet nephropathy and,
with chronic exposure, renal cancer in male rats only (Borghoff et
al., 1990). The proposed mechanism by which these chemicals cause
renal tumours in male rats is based on their ability to cause protein
droplet nephropathy by accumulating alpha2u-globulin, a
male-rat-specific protein of low relative molecular mass. Evidence
suggests that chemical binding to alpha2u-globulin makes the protein
more resistant to hydrolysis, which accounts for its accumulation in
the renal lysosomes in the form of protein droplets. The chemically
induced accumulation of alpha2u-globulin is thought to be responsible
for cytolethality, which in turn stimulates cell division as the
kidney attempts to repair itself. Chronic chemical exposure with
repeated cycles of cytolethality and reparative replication is
probably the cause of the renal tumours in male rats (Borghoff et al.,
1990; Hard et al., 1993). A protein similar to alpha2u-globulin has
not been detected in human kidneys (Borghoff & Lagarde, 1993).
MTBE causes male-rat-specific renal tumours with chronic exposure
(Bird et al., 1997). MTBE also causes the accumulation of protein
droplets in male but not female rats following exposure ranging from
10 days to 13 weeks (Dodd & Kintigh, 1989; Chun & Kintigh, 1993;
Prescott-Mathews et al., 1997). Similar results have been observed
with TBA, a metabolite of MTBE (Lindamood et al., 1992).
alpha2u-Globulin immunoreactivity was present in and confined to
protein droplets in the kidneys of male rats exposed to MTBE in these
studies (Dodd & Kintigh, 1989; Chun & Kintigh, 1993; Prescott-Mathews
et al., 1997). Although a slight increase in alpha2u-globulin-positive
staining was observed in male rats exposed to MTBE, as compared to
controls, a linear exposure-related increase was not observed in any
of these studies. alpha2u-Globulin-positive proteinaceous casts at the
junction of the proximal tubules and the thin limb of Henle were not
observed (Swenberg & Dietrich, 1991). MTBE caused an
exposure-dependent mild increase in the renal concentration of
alpha2u-globulin measured in male rats (Prescott-Mathews et al.,
1997). Immunohistochemical staining of alpha2u-globulin is probably
not as sensitive as actually quantifying the alpha2u-globulin levels
with a mild increase in this protein. Further analysis of the kidney
protein profile from control and MTBE-treated male rats confirmed the
accumulation of alpha2u-globulin with no other protein detected
(Prescott-Mathews et al., 1997).
MTBE-induced kidney lesions, characterized by tubular necrosis
and protein droplet accumulation, were mild, especially when compared
with strong inducers of alpha2u-globulin nephropathy. Additionally,
granular casts, considered characteristic for alpha2u-globulin
nephropathy, were not observed in all studies. In the case of the
10-day MTBE inhalation exposure, however, there was a
concentration-dependent increase in kidney necrosis with minimal
sloughing of epithelial cells in male rat kidney following exposure to
10 710 mg/m3 (3000 ppm) (Prescott-Mathews et al., 1997).
In male and female rats exposed to MTBE vapour for 10 and 28
days, MTBE caused enhanced cell proliferation in male, but not female,
rat kidneys (Chun & Kintigh, 1993; Prescott-Mathews et al., 1997). A
strong positive correlation was observed between the cell
proliferative response and the concentration of alpha2u-globulin in
the kidneys of MTBE-exposed male rats.
With many of the chemicals that cause alpha2u-globulin
nephropathy, either the chemical or a metabolite has been found to
bind reversibly to alpha2u-globulin. In vitro, a high uptake of MTBE
into male rat kidney homogenate could be predicted using a
two-compartment model system when the binding of MTBE to
alpha2u-globulin was described using a dissociation constant of 10-4
M (Poet & Borghoff, 1997). This estimated dissociation constant for
MTBE binding to alpha2u-globulin was found to be similar to the
dissociation constant previously measured for 1,4-dichlorobenzene, a
known inducer of alpha2u-globulin nephropathy and a chemical
identified as bound to alpha2u-globulin following treatment of male
rats with 1,4-dichlorobenzene (Charbonneau et al., 1989). Together
these findings indicate that MTBE interacts, although weakly, with
alpha2u-globulin and induces alpha2u-globulin nephropathy and renal
cell proliferation in male, but not female, rats.
MTBE increases the severity of chronic progressive nephropathy in
both male and female rats, but does not cause renal necrosis, enhanced
cell proliferation or renal cancer in female rats. Chronic progressive
nephropathy alone is not associated with increased incidence of renal
tumours.
7.10.2 Liver tumours
It has been hypothesized that liver tumours in female mice can be
promoted by interfering with the estrogen-mediated suppression of
preneoplastic foci. In this regard, both unleaded gasoline and MTBE
induce cytochrome P450 activity and estrogen metabolism in mouse
hepatocytes, induce a mitogenic response in mouse liver, and decrease
uterine and ovarian weight in exposed mice. Additionally, unleaded
gasoline promotes DEN-initiated female mouse liver tumours, but this
response has not been observed with MTBE (Standeven & Goldsworthy,
1993; Moser et al., 1996b). Therefore, the relevance of this
hypothesis to an interpretation of the MTBE-induced mouse liver
tumours is currently unclear.
8. EFFECTS ON HUMANS
As explained in chapter 3, two separate fuel programmes in the
USA legally require the use of oxygenate in gasoline to address
ambient air quality objectives. Although no specific oxygenate is
required, MTBE has dominated the USA market place and is used at 15%
(by volume) to meet a 2.7% (by weight) oxygen requirement for
oxygenated gasoline in the cold-weather season in areas with excessive
carbon monoxide levels and at 11% (by volume) to meet a 2.0% (by
weight) oxygen requirement for reformulated gasoline sold year-round
in areas with excessive ozone levels. In the fall of 1992, shortly
after the introduction of oxygenated gasoline containing 15% MTBE in
Alaska, consumer complaints were registered about health effects such
as headaches, eye irritation and cough in Fairbanks (Beller &
Middaugh, 1992) and Anchorage (Chandler & Middaugh, 1992).
Subsequently, residents in other places in the USA also reported
health complaints associated with the introduction of cold-season
oxygenated fuel. Somewhat similar public concerns were raised in
Milwaukee, Wisconsin, with the introduction of reformulated gasoline,
some of which contained 11% (by volume) MTBE, in January 1995
(Anderson, 1993; Anderson et al., 1995). Health complaints have also
been registered by some occupationally exposed individuals, such as
tank truck drivers handling bulk MTBE (Gillie, 1993).
These "outbreaks" of health complaints prompted several field and
experimental studies as well as other assessments (e.g., HEI, 1996; US
Interagency Assessment, 1997) of available data and information
generated by these studies. In this section epidemiological studies
will be described first, followed by controlled chamber studies of
human volunteers. Because non-occupationally as well as occupationally
exposed populations were investigated in the epidemiological studies,
these studies will be discussed in one section rather than two
separate sections devoted to general population and occupational
population exposures.
8.1 Population studies
In December 1992, Moolenar et al. (1994) undertook a pilot study
in Fairbanks, Alaska, to investigate the possible relationship between
MTBE exposure and health complaints. In Phase I of the study, exposure
was evaluated by air sampling and by analysing blood samples for MTBE
and TBA in 18 workers heavily exposed to gasoline fumes and exhaust in
their workplace (e.g., service station workers, mechanics, meter
readers). A questionnaire administered to the workers asked about 15
symptoms: seven that had been most frequently reported to a local
telephone hotline the previous month (headache, eye irritation,
burning of the nose or throat, cough, nausea or vomiting, dizziness,
and a sensation of spaciness or disorientation), and eight other
symptoms (fatigue, fever, sweats or chills, diarrhoea, fainting or
black-out spells, skin irritation or redness, muscle aches, and
difficulty breathing). Phase II of the study was conducted in February
1993, after the oxygenated gasoline programme was suspended, and
included 28 (12 of the original) occupationally exposed subjects. Four
workers whose post-shift blood MTBE levels were in the top quartile
(>9.6 µg/litre) all had one or more of the seven key health
complaints, compared with 9 of 14 workers whose levels were in the
lower three quartiles. This finding was not statistically significant,
but the study may not have had adequate statistical power to detect a
relationship, owing to the small sample size. In Phase II, only one
worker reported a health complaint (nausea). Exposures to MTBE, as
well as complaints of both workers and non-occupationally exposed
residents of Fairbanks, declined significantly after the termination
of the oxygenated gasoline programme (CDC, 1993), but interpretation
of these events is possibly confounded by several factors, including a
lack of representative sampling and changes in the cost of gasoline
that may have contributed to public attitudes.
Mohr et al. (1994) conducted a cross-sectional cohort study that
included 237 garage workers exposed to high and low MTBE
concentrations: 115 workers exposed in northern New Jersey during the
wintertime oxyfuel programme and 122 workers in southern New Jersey 10
weeks after the phase-out date for the programme. Both groups of
workers reported feeling significantly worse at the end of the work
day, but there was no difference between the groups across the work
shift. Active air sampling and passive sampling devices confirmed the
higher exposure levels of the workers in northern New Jersey. No
significant differences were found in either the cross-sectional
reporting of symptoms or the pre- and post-shift analyses.
A study performed in Stamford, Connecticut, in April 1993 near
the end of the oxygenated gasoline season there, investigated exposure
to MTBE in oxygenated gasoline and symptom prevalence in
occupationally and non-occupationally exposed subjects (White et al.,
1995). The study included 37 workers and 14 commuters. The prevalence
of symptoms was highest among people who worked in car repair-shops or
around traffic. The eight workers with the highest levels of MTBE in
blood (>3.8 µg/litre) reported one or more key symptoms (OR = 21.0,
95% CI = 1.8-539.0) such as headache, irritated eyes, burning of the
nose and throat, cough, dizziness, spaciousness, disorientation, and
nausea. There were no reports of diarrhoea, difficulty in breathing,
skin irritation, fever, sweats or chills, or fainting.
In response to health concerns raised by the public following the
introduction of the reformulated gasoline (RFG) programme in the
Milwaukee, Wisconsin area, the Wisconsin Department of Health
initiated a random digit-dial telephone survey designed to assess the
prevalence and scope of health complaints (Anderson et al., 1995). A
questionnaire was administered to approximately 1500 persons: 527
residents of the Milwaukee, where RFG was sold and numerous complaints
about the fuel had been registered; 485 residents of Chicago, where
essentially the same fuels were sold but few complaints had been
registered; and 501 residents of the remainder of Wisconsin, where RFG
had not been required. Overall, there was a significantly higher
prevalence rate for "unusual symptoms" in Milwaukee (23%) than in
Chicago and the rest of Wisconsin (6% each). The fact that symptom
prevalence in the Chicago RFG area was so similar to that in non-RFG
areas of Wisconsin suggested that factors other than RFG use
contributed to the difference between Milwaukee and the other two
areas. Although the prevalence of colds and flu was the same in the
three areas, Milwaukee residents were more likely to report unusual
symptoms if they had experienced a cold or the flu, smoked cigarettes,
or were aware that they had purchased RFG. The authors concluded that
many symptoms reported by Milwaukee residents may have actually been
due to colds or flu rather than RFG exposure. Also, individuals who
reported purchasing RFG were more likely to report symptoms than
individuals who said they had not purchased or did not know whether
they had purchased RFG, which suggested that knowledge about RFG may
have increased awareness of an individual's health status and resulted
in the assumption that any health symptoms experienced were unusual
and attributable to gasoline exposure. This study and its conclusions
were reviewed by a panel of independent experts who concluded that
"The study does not support a conclusion that exposure to RFG is
associated with widespread or serious acute adverse health effects."
This evaluation of the study was later endorsed by another,
independent peer review group (HEI, 1996).
Anderson et al. (1995) followed up on 1280 Milwaukee residents
who had initially contacted governmental agencies about their health
complaints. These self-identified individuals were interviewed by
telephone to determine the types of complaints and risk factors that
could be associated with symptoms. Results of the study indicated that
the strongest predictors were age, allergies, and colds or flu since
November 1994. The purchase of RFG, a surrogate for exposure, did not
correlate with self-reported health symptoms.
A cross-sectional study was conducted in Finland to investigate
the occurrence of neurophysiological symptoms in tanker drivers
exposed to gasoline containing approximately 10% MTBE (Hakkola et al.,
1996). A reference group of milk delivery drivers was selected from
the same areas. A total of 201 male drivers participated in the study,
including 101 tanker drivers and 100 milk delivery drivers. The
occurrence of symptoms and Profile on Mood States (POMS) scales showed
an association with age, chronic diseases, and the perceived health of
the drivers in both the exposed and the unexposed group. Although
there were more sensory and motor symptoms in tank drivers, there was
no evidence of any statistically significant difference in the
occurrence of symptoms between the two groups. Duration of work as a
driver, shift schedule and length of the working week had no
statistical connection with symptoms and the modified POMS scales.
8.2 Controlled studies
To determine if 1-h exposures to MTBE at 6 mg/m3 (1.7 ppm) in an
inhalation chamber could result in similar symptoms as those reported
in the field, Cain et al. (1996) performed a controlled study in 22
male and 21 female subjects (ages 18 to 34 years). Both objective and
subjective indices of behavioural and physiological effects were
studied. A control exposure to air and a control exposure to a
17-component mixture of volatile organic compounds (VOCs) (19 mg/m3)
were also included. The selected MTBE concentration and duration were
based upon preliminary results from exposure studies in commuters
(Lioy et al., 1994). The effects of MTBE exposure on discomfort,
symptoms and possible objective correlates of symptoms were
investigated by using questionnaires, various ocular and nasal
inflammation parameters, and neurobehavioural testing. Repeated blood
samples were obtained from a subset of subjects to relate the exposure
to the body burden and toxicokinetics of MTBE (see section 6.1.1). The
exposure produced a mean peak blood MTBE level of 17 µg/litre.
Subjective reactions, such as irritation, fatigue and headache,
typically showed greater sensitivity than objective indices. There was
a differential effect of gender on rated odour, intensity and
pleasantness. Females found odour intensity greater, pleasantness
worse, and air quality worse. Ocular measurements indicated a mild
tendency for eye irritation during exposure; this was, however, not
statistically significant. MTBE caused no objective inflammatory
changes in the nasal mucosa. Aside from odour, no significant
reactions were found.
In another controlled study, conducted by Prah et al. (1994), 20
male and 20 female volunteer subjects (healthy and non-smokers) were
exposed in an inhalation chamber to MTBE at 5 mg/m3 (1.39 ppm) for
1 h. Symptom questionnaires, cognitive testing, and objective measures
of ocular and nasal irritation were obtained before and at the end of
the exposure. In addition, the odour threshold of MTBE and some
pharmacokinetic data were obtained from two additional subjects, one
male and one female (see section 6.1.1). No increase in the reporting
of symptoms such as headache, nasal irritation, cough or eye
irritation was found, apart from a gender effect in reporting of air
quality. Females rated the air quality in the chamber with MTBE
exposure slightly poorer when compared to the clean air exposure.
There were no changes in objective indicators in either the eye or the
nose.
Johanson et al. (1995) and Nihlén et al. (1998b) reported similar
results at much higher concentrations. Ten healthy male volunteers
were exposed to MTBE vapour at 18, 90 or 180 mg/m3 (5, 25 or 50 ppm)
for 2-h periods while performing light physical exercise (see also
section 6.1.1). All the subjects reported the strong smell of MTBE on
entering the chamber but their rating of the smell decreased with
time. On the basis of subjective assessment there was no irritation of
the ocular, nasal or pharyngeal mucosae. The MTBE exposure induced
essentially no effects on eye and mucous membrane irritation.
Riihimaki et al. (1996) assessed a number of subjective (e.g.,
irritant sensations, mood state) and objective (simple reaction time,
postural sway) end-points in 13 male volunteers exposed to 0, 90 or
268 mg/m3 (0, 25 or 75 ppm) MTBE for 1 or 3 h. The authors concluded
that only "mild symptoms, mainly a feeling of heaviness in the head
and, to a smaller extent, of mild mucous membrane irritation, were
reported". The frequency of symptoms was related to the level of MTBE
exposure and reached statistical significance at 268 mg/m3 (75 ppm)
after 3 h of exposure. There was no effect on reaction time or
postural sway.
8.3 Subpopulations at special risk
There are no data by which to identify any subpopulations (e.g.,
the elderly, pregnant women, children or people with allergy or
asthma) who might be at special risk to MTBE exposure.
8.4 Special studies
8.4.1 Organoleptic properties
For many people, MTBE has a quite distinctive odour. Odour
detection thresholds have been reported to average around 0.1 to
0.2 mg/m3 (0.03-0.05 ppm), with average recognition (identification)
thresholds in a range from 0.2 to 0.5 mg/m3 (0.06-0.13 ppm) for neat
MTBE vapour (TRC, 1993; Smith & Duffy, 1995). For gasoline containing
15% (by volume) MTBE in the USA, average odour detection thresholds
ranged from approximately 0.3 to 3 mg/m3 (0.08- 0.9 ppm), with
recognition thresholds ranging from 0.7 to 2.5 mg/m3 (0.2-0.7 ppm).
Odour thresholds for MTBE-oxygenated gasoline may vary considerably,
depending in part on the aromatic and other constituents of the
gasoline and the sensitivity of the individuals who inhale the
vapours. The addition of MTBE to gasoline has been found to reduce the
detection threshold (i.e. increase "detectability") for gasoline by as
much as 80% (HEI, 1996).
8.4.2 Immunological effects
In a study to assess the effects of MTBE on the immune system,
interleukin levels were measured in blood plasma of 22 volunteers at
several different locations around Fairbanks exposed to auto emissions
derived from oxyfuel (Duffy, 1994). The study was performed during a
4-week period in late November and early December 1992. During this
period, the mean daily temperature ranged from about -1.5°C (35°F) to
about -37°C (-38°F). Plasma interleukin 1 ß (IL-1 ß) and interleukin 6
(IL-6) levels were measured at the beginning and at the end of an 8 h
work day. The results showed no difference between the morning mean
levels (2.50 pg/ml ± 2.4 SD and the evening mean levels (2.53 pg/ml ±
2.6 SD). There were, however, 14 out of 22 individuals who showed
slight increases at the end of the work day. IL-1, which was measured
in 10 individuals, was below the detection limit.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Algae
The effect of MTBE on the growth of the unicellular algae
Selenastrum capricornutum (Chlorophyta), Navicula pelliculosa
(Bacillariophyta) and Synechococcus leopoliensis (Cyanobacteria),
representing three taxonomic groups, was investigated under laboratory
conditions (Rousch & Sommerfeld, 1998). The growth of
N. pelliculosa and S. leopoliensis was inhibited at a nominal MTBE
concentration of 2400 mg/litre in the growth medium, whereas
S. capricornutum growth was increased at 600 mg/litre and decreased
at 4800 mg/litre. The authors suggested that the differential
sensitivity of these representative species implies that MTBE could
alter algal community composition in the environment.
9.1.2 Aquatic animal species
The results of aquatic toxicity tests are presented in Table 21.
Experimental data on acute toxicity are available for four
species of invertebrates, four species of fish, and one species of
amphibian. The experimental data ranged from a 96-h LC50 of 553
mg/litre for the crustacean Chaetogammam marinum (Adema, 1982), to a
96-h LC50 of >10 000 mg/litre for a copepod Nitocra spinipes
(Tarkpea & Svanberg, 1982).
Acute toxicities were determined by Tarkpea & Svanberg (1982) for
MTBE alone, MTBE combined with a base fuel at a concentration of 5%,
and the base fuel alone. The acute toxicity tests were conducted on
the harpacticoid copepod Nitocra spinipes under static conditions
for 96 h at 21°C. The test solutions were not aerated. A 96-h LC50
greater than 10 000 mg/litre was reported for MTBE alone. The 96-h
LC50 values were 242 mg/litre for MTBE in a base fuel and 201
mg/litre for the base fuel alone. The results of the tests show that
the acute toxicity of base fuel to aquatic organisms is not increased
by the addition of 5% MTBE.
Tarkpea & Svanberg (1982) also determined the 24-h LC50 of MTBE
on a shoal fish, the bleak Alburnus alburnus in a closed, static
system at 10°C. The LC50 was between 1700 and 1800 mg/litre. When the
bleaks were introduced into the test media, several sublethal effects
were observed, including disturbed balance, surface swimming and
overturning. The sublethal effects were short-lived, as several of the
individual test subjects had recovered when the test was completed.
The environmental significance of these results was not discussed by
the authors.
Table 21. Aquatic toxicity testing results for MTBE
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Invertebrates
Ceriodaphnia LC50 (48-h) 18-21°C 841 THE, 1989
Daphnia magna LC50 (96-h) >1000 Gupta & Lin, 1995
Copepod LC50 (96-h) >10 000 Tarkpea & Svanberg, 1982
(Nitocra spinipes)
Copepod LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
(Nitocra spinipes)
Gammarid LC10 (96-h) 15°C 553 Adema, 1982
(Chaetogammarus marinus)
Fish
Bleak (Alburnus alburnus) LC50 (24-h) 10°C 1700-1800 Tarkpea & Svanberg, 1982
Bleak (Alburnus alburnus) LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
Fathead minnow LC50 (96-h) 25°C 706 Veith et al., 1983
(Pimephales promelas)
Fathead minnow LC50 (96-h) 672 Geiger et al., 1988
(Pimephales promelas)
Rainbow trout LC50 (96-h) 1300 Environment Canada, 1993
(Oncorhynchus mykiss)
Table 21. (continued)
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Rainbow trout LC50 (96-h) 1483 Environment Canada, 1993
(Oncorhynchus mykiss)
Amphibians
European frog tadpole LC0 (48-h) <2000 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC50 (48-h) 2500 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC100 (48-h) <3000 Paulov, 1987
(Rana temporaria)
When European frog tadpoles (Rana temporaria) were exposed to
concentrations of MTBE in water ranging from 100 to 2500 mg/ litre,
various effects were observed. An increase in body weight of frogs and
tadpoles that had undergone metamorphosis was observed at 100
mg/litre, as compared with the controls. At sublethal concentrations
(<2500 mg/m3) in water, accelerated development of the tadpole was
observed and metamorphosis occurred two days earlier than in controls
(Paulov, 1987). The environmental significance of these results was
not discussed by the author.
No data on chronic aquatic toxicity were found in the literature.
Data on the toxicity of MTBE to terrestrial animals, terrestrial
plants or soil biota were not found in the literature, other than
information from mammalian toxicology studies.
9.2 Field experiments
Data on field experiments on MTBE were not found in the
literature.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Total exposure of human populations to MTBE may involve more than
one environmental pathway and route of intake. Populations may be
exposed to MTBE in air in areas where it is used in gasoline, though
available data on environmental levels and human exposure are limited.
In several studies, median concentrations of MTBE in ambient air
ranged from 0.47 to 14.4 µg/m3 (0.00013 to 0.004 ppm) where MTBE is
used in oxygenated gasoline, and non-detectable to 26.4 µg/m3 (0.0073
ppm) in urban air of cities where MTBE is used as an octane enhancer.
Concentrations near industrial facilities range up to 35.7 mg/m3 (10
ppm). Median 1- to 2-min exposure levels gathered in the breathing
zone of service station attendants and consumers while refuelling were
highly variable, ranging from 1.0 to 21.4 mg/m3 (0.03 to 6 ppm) and
occasionally exceeding 35.7 mg/m3 (10 ppm).
Monitoring data for MTBE are too limited to characterize
adequately its occurrence in drinking-water. The intake of MTBE in
drinking-water is generally expected to be negligible, although
drinking-water may be polluted from point sources such as accidental
spills of large amounts of MTBE in gasoline. Exposure could also occur
through dermal absorption or inhalation of MTBE vapour from household
water used for bathing, cooking and laundering.
Potentially exposed workers include those involved in the
production, handling and use of MTBE and MTBE-containing gasoline,
including mechanics and service station attendants. Occupational
exposure of workers transporting MTBE is highest, with an average
short-term median concentration of 140 mg/m3 (39 ppm). Long-term
average median levels for this group of workers were about 2.85 mg/m3
(0.8 ppm). Median long-term exposure of service station attendants
averaged 1.79 mg/m3 (0.5 ppm). The long-term median value for
mechanics was 0.36 mg/m3 (0.1 ppm).
10.1.2 Human health effects
Consumers in some areas of the USA have complained about acute
health symptoms such as headache, eye and nose irritation, cough,
nausea, dizziness and disorientation associated with the use of
oxygenated fuels such as gasoline containing MTBE. Epidemiological
studies of human populations exposed under occupational and
non-occupational conditions, as well as experimental studies of human
volunteers exposed under controlled conditions, have not been able to
identify a basis for these complaints. Results of community studies
conducted in Alaska, New Jersey, Connecticut and Wisconsin, USA, have
been mixed and provided limited or no evidence of an association
between MTBE exposure and the prevalence of health complaints. In
addition, independently conducted experimental studies of volunteers
exposed in inhalation chambers to MTBE concentrations ranging from 5.0
mg/m3 (1.4 ppm) in one study to 180 mg/m3 (50 ppm) for 2 h in
another study have shown no evident effects in terms of either
subjective reports of symptoms or objective indicators of irritation
or other effects. Based on the collective evidence, it appears
unlikely that MTBE alone induces adverse acute health effects in the
general population under common inhalation exposure conditions.
However, the potential effects of mixtures of gasoline and MTBE, as
well as the manner in which most people are exposed to MTBE in
conjunction with the use of oxygenated fuels, have not been examined
experimentally or through prospective epidemiological methods.
Moreover, the role of factors such as awareness of MTBE, due in part
to its distinctive odour, for example, has not been investigated.
In studies on animals, MTBE is "moderately" acutely toxic, with
an oral LD50 in rats of approximately 3800 mg/kg bw and LC50 value
(15 min) of about 141 000 mg/m3 air in mice. Signs of intoxication
include CNS depression, ataxia and laboured respiration. The LD50 for
dermal toxicity in rabbits is >10 200 mg/kg bw.
MTBE is considered to be a mild skin and eye irritant but does
not induce skin sensitization.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Effect levels are compiled in Tables 22 and 23. Concentrations
or doses that induced significant increases in organ weights, for
which histopathological effects were observed at higher levels, were
considered LOELs. Doses at which histopathological effects were
observed were considered LOAELs (Tables 22 and 23).
Lowest reported effect levels for nephrotoxicity following
ingestion in subchronic studies were 440 mg/kg bw per day (increases
in relative kidney weight and hyaline droplet formation in
Sprague-Dawley rats). At 2860 mg/m3 (800 ppm), in a 90-day inhalation
study, there were increases in kidney weight associated at higher
concentrations with a mild increase in hyaline droplets in the
proximal tubules in Fischer-344 rats. At 1430 mg/m3 (400 ppm), in
inhalation oncogenicity studies, there was an increase in absolute
liver weight, which correlated with increased severity of
hepatocellular hypertrophy at higher concentrations and an increase in
relative kidney weight in male mice; in rats, incidence and severity
of chronic progressive nephropathy were increased at this level.
Exposure to MTBE also results in reversible central nervous
system effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations, and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2860 mg/m3 (800 ppm) produced
reversible, non-monotonically dose-related changes in motor activity.
These effects were transient and not observed in longer-term studies.
Table 22. MTBE levels for non-neoplastic effects following oral exposure
Species Protocol Effect Level Basis of Effect Level Reference
(mg/kg bw/day)
Sprague-Dawley rats 28-day gavage LOAEL 440 males: increase in relative IITRI (1992)
(undiluted) kidney weight; hyaline droplet
formation in convoluted
NOAEL 90 tubules
Sprague-Dawley rats 90-day gavage in LOEL 900 increase in male absolute and Robinson et al. (1990)
corn oil relative kidney weight; chronic
nephropathy and increase in
NOAEL 300 hyaline droplets in proximal
tubular cells at the next
higher dose (1200 mg/kg bw/day)
Sprague-Dawley rats 104 weeks gavage NOEL 1000 no effects at any dose Belpoggi et al. (1995)
in olive oil;
maintained until
death
Table 23. MTBE levels for non-neoplastic effects following inhalation exposure
Species Protocol Effect level Basis for effect level Reference
Fischer-344 rats single exposure LOEL for reversible biphasic changes in motor Gill (1989);
neurological effects = activity in females Daughtrey et al.
2860 mg/m3 (800 ppm) (1997)
Fischer-344 rats 13 week study LOEL 2860 mg/m3 males: increase in relative Dodd & Kintigh (1989);
(800 ppm) weight of liver and kidney; Daughtrey et al. (1997)
no NOAEL males: at 28 600 mg/m3, increase
in lymphoid hyperplasia in
submandibular lymph nodes; mild
increase in hyaline droplets in
renal proximal tubules
Fischer-344 rats up to 104 weeks LOAEL 10 700 mg/m3 males: increased mortality and Chun et al. (1992);
(3000 ppm) decreased survival time at 10 700 Bird et al. (1997)
LOEL 1430 mg/m3 mg/m3; chronic progressive
(400 ppm) nephropathy was the major cause
of death at 10 700 mg/m3; increased
incidence and severity of chronic
progressive nephropathy at all
doses (significance not specified)
CD1 mice up to 18 months LOEL 1430 mg/m3 increase in absolute liver Chun et al. (1992)
(400 ppm) weight in male mice which Bird et al. (1997)
correlated with increased
severity of hepatocellular
hypertrophy at higher
concentrations; increase in
relative kidney weight in males
Specific adverse effects on reproduction have not been observed
in rats at concentrations up to 28 600 mg/m3 (8000 ppm). MTBE has not
induced developmental effects in rats, mice or rabbits at
concentrations less than those that were toxic to the mothers.
Decreases in uterine weight and increases in estrogen metabolism have
been observed at 28 600 mg/m3.
The weight of evidence indicates that MTBE is not genotoxic.
Identified oncogenicity studies include an inhalation study in rats
and mice and an oral study (gavage) in rats. In these investigations,
MTBE induced testicular (Leydig cell) tumours in male rats
(Fischer-344 and Sprague-Dawley), renal tumours in male rats
(Fischer-344) liver tumours in female mice (CD-1) and lymphomas and
leukaemias (combined) in female (Sprague-Dawley) rats.
All investigations on nephrotoxicity are consistent with the
renal tumours observed in Fischer-344 rats being related to
alpha2u-globulin nephropathy. alpha2u-Globulin nephropathy is
considered an effect specific to male rats and, therefore, these
tumours are of questionable relevance to humans.
Leydig cell tumours have been induced by MTBE in two strains of
rats. This type of tumour has been reported to be induced by
non-genotoxic carcinogens that disturb the hormonal balance of
testosterone, luteinizing hormone and luteinizing hormone releasing
factor in rats. Owing to differences between rats and humans in the
regulation of gonadotropins, it is questionable that a similar effect
will occur in humans. Although such a mechanism may be relevant, this
is not substantiated by experimental evidence, since these hormones
were not determined in any of the studies with MTBE.
Liver tumours have been induced by MTBE in female mice and
possibly in male mice (the data on male mice were not corrected for
increased mortality). The effect was modest and occurred only at
28 600 mg/m3 (8000 ppm) and in association with hepatocellular
hypertrophy (indicating enzyme induction) and altered estrogen
metabolism. The relevance of these mouse liver tumours for human risk
estimation is considered to be questionable.
In a single oral study in SD rats, the frequency of lymphomas and
leukaemias (combined) were increased in the high-dose group. This
observation was not supported by any indications of relevant
(preneoplastic) effects on the lymphoid system in other studies.
Moreover, the description of the study made it difficult to evaluate
adequately the results. However, since the effect observed appears to
be rather pronounced, it is not justified to neglect this finding,
based on presumed experimental deficiencies. For a proper evaluation,
additional information is required.
On the basis of these data, MTBE should be considered a rodent
carcinogen. MTBE is not genotoxic and the carcinogenic response is
only evident at high levels of exposure that also induce other adverse
effects. The available data are inconclusive and prohibit their use
for human carcinogenic risk assessment until outstanding complications
in their interpretation have been addressed.a
10.2 Evaluation of effects on the environment
MTBE emissions and leakages can be widespread in the environment
in areas where MTBE is used as an octane improver and oxygenate in
oxygenated gasoline.
MTBE is predominately emitted into air; however, it can be
released into the water and soil compartments. Ambient concentrations
in air are low. There are no terrestrial toxicity data for exposure to
MTBE in air; however, this appears not to be of concern to an
environmental evaluation since ambient air concentrations are low and
its half-life is relatively short.
Owing to its physical and chemical properties, MTBE can persist
longer in water and soil than in air. There are very limited data on
concentrations in ambient surface water. The biodegradation of MTBE in
water and soil is not well understood but is believed to be relatively
slow. MTBE in soil can leach into groundwater and persist there, due
to its lack of removal. MTBE has not been generally detected in deeper
groundwater or in shallow groundwater in agricultural areas. It is
more frequently found in shallower groundwater in urban areas where
MTBE is most extensively used.
Data available for ecotoxicological assessment refer almost
exclusively to MTBE in water. It can be classified as relatively
non-toxic for aquatic biota, with a lowest acute effect for several
aquatic organisms of more than 100 mg/litre. No long-term aquatic
toxicity tests at low concentrations have been identified. However,
the limited data on concentrations of MTBE in ambient surface water
have shown that concentrations range from non-detectable to 30
µg/litre. The maximum concentration is several orders of magnitude
below the effect level of the most sensitive organism tested to date.
It does not appear that the concentrations of MTBE in ambient water
are toxic to aquatic organisms, except during spills when very high
levels of MTBE may be found.
There are no data on concentrations of MTBE in soil or on
terrestrial toxicity. However, concentrations in this medium are
expected to be low except in the case of spills.
a MTBE was reviewed by an International Agency for Research on
Cancer (IARC) Working Group in October 1998. The conclusions were that
there was inadequate evidence for the carcinogenicity in humans of
MTBE, limited evidence for its carcinogenicity in experimental
animals, and the overall evaluation was that MTBE was not classifiable
as to its carcinogenicity for humans (Group 3).
11. RECOMMENDATIONS
To provide quantitative guidance on relevant limits of exposure
and to estimate risk, it is recommended that additional data be
acquired in the following areas:
a) additional information to evaluate the induction of
lymphomas/leukaemias in Sprague-Dawley rats;
b) mechanistic data on the induction of Leydig cell tumours and sex
specificity of liver tumours in mice;
c) controlled exposure studies to characterize the dose-response in
humans for MTBE and MTBE-containing mixtures;
d) monitoring data for better characterization of human exposure,
with particular attention to microenvironments;
e) potentiation studies of MTBE with BTX components of gasoline;
f) monitoring of environmental concentrations of MTBE in soil and
biota in areas adjacent to major sources and ambient areas in
order to verify theoretical values;
g) long-term toxicity tests in aquatic and possibly terrestrial
organisms;
h) field degradation tests to determine how persistent MTBE can be
in soil and groundwater under a range of redox conditions.
REFERENCES
ACGIH (1994) 1994-1995 threshold limit values for chemical substances
and physical agents and indices. Cincinnati, Ohio, American Conference
of Governmental Industrial Hygienists, p 27.
Adam G, Knuechel R, Vorwerk D, Held C, & Guenther RW (1990) Tissue
response of the biliary and digestive system of rabbits after MTBE
infusion into the gallbladder. Invest Radiol, 25(1): 58-61.
Adema DMM (1982) Tests and desk studies carried out by MT-TNO during
1980-1981 for Annex II of MARPOL 1973. Delft, The Netherlands,
Organization for Applied Scientific Research, 51 pp (Report CL
82/1499).
AFS (1994) Statute book of the Swedish National Board of Occupational
Safety and Health, Ordinance AFS 1993: 9: Occupational exposure limit
values. Solna, The Swedish National Board of Occupational Safety and
Health, pp 46, 52.
Akimoto R, Rieger E, Moossa AR, Hofmann AF, & Wahlstrom HE (1992)
Systemic and local toxicity in the rat of methyl tert-butyl ether: a
gallstone dissolution agent. J Surg Res, 53: 572-577.
Allard A-S, Remberger M, & Neilson AH (1996) The aerobic
biodegradation of tert-butyl methyl ether and tert-butanol: an
initiatory study. Stockholm, The Swedish Environmental Research
Institute, 9 pp (IVL Report No. B 1197).
Allen MK & Grande D (1995) Reformulated gasoline air monitoring study.
Madison, Wisconsin, Department of Natural Resources, Bureau of Air
Management, 50 pp (Publication No. AM-175-95).
Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985a) Rapid dissolution of gallstones by methyl tert-butyl ether.
N Engl J Med, 312(4): 217-220.
Allen MJ, Barody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985b) Cholelitholysis using methyl tertiary butyl ether.
Gastroenterology, 88: 122-125.
Almaguer D (1993) Health hazard evaluation report HETA 93-0884-2344:
National Center for Environmental Health, Albany, New York.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 33 pp (NTIS/PB94-129020).
Anderson EV (1993) Health studies indicate MTBE is safe gasoline
additive. Chem Eng News, September 20: 9-18.
Anderson HA, Hanrahan L, Goldring J, & Delaney B (1995) An
investigation of health concerns attributed to reformulated gasoline
use in southwest Wisconsin - Final Report. Madison, Wisconsin,
Department of Health and Social Services, 50 pp.
Angle CR (1991) If the tap water smells foul, think MTBE. J Am Med
Assoc, 266: 2985-2986.
API (1989a) Aboveground storage tank survey. Washington, DC, American
Petroleum Institute, Health and Environment Affairs Department, 44 pp
(Publication No. 301).
API (1989b) Monitoring near refineries for airborne chemicals - Volume
1: Validated ambient air concentrations around three refineries.
Washington, DC, American Petroleum Institute, Health and Environment
Affairs Department, 83 pp (Publication No. 4484).
API (1993) Gasoline vapor exposure assessment at service stations.
Washington, DC, American Petroleum Institute, 8 pp (Publication No.
4553).
API (1994) A survey of API members' aboveground storage tank
facilities. Washington, DC, American Petroleum Institute, 65 pp
(Publication No. 330).
API (1995a) Executive summary of a study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 5 pp (Publication No. 4619).
API (1995b) Petroleum Institute data characterizing occupational
exposures to methyl tertiary butyl ether (MTBE) 1983-1993. Washington,
DC, American Petroleum Institute, 21 pp (Publication No. 4622).
API (1995c) Service station personnel exposures to oxygenated fuel
components - 1994: Executive summary. Washington, DC, American
Petroleum Institute, 3 pp (Publication No. 4625).
Arashidani K, Katoh T, Yoshikawa M, Kikuchi M, Kawamoto J, & Kodama Y
(1993) LD50 and weight change in organs of mice following
intraperitoneal administration of methyl tertiary-butyl ether. Jpn J
Ind Health, 35: 404-405.
ARCO (1987) Methyl tert-butyl ether exposure monitoring study.
Channelview, Texas, ARCO Chemical Company, 39 pp.
Atkinson R (1990) Gas-phase tropospheric chemistry of organic
compounds : a review. Atmos Environ, 24A: 1-41.
Atkinson R & Pitts JN Jr (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-246°K. J
Chem Phys, 68(8): 3581-3584.
Barreto RD, Gray KA, & Anders K (1995) Photocatalytic degradation of
methyl- tert-butyl ether in TiO2 slurries: a proposed reaction
scheme. Water Res, 29(5): 1243-1248.
Beller M & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska. Anchorage, Alaska, Department
of Health and Social Services, Section of Epidemiology, 5 pp.
Belpoggi F, Soffritti M, & Maltoni C (1995) Methyl-tertiary-butyl
ether (MTBE) - a gasoline additive - causes testicular and
lympho-haematopoietic cancers in rats. Toxicol Ind Health, 11(2):
119-149.
Bengtsson EB & Tarkpea M (1983) The acute aquatic toxicity of some
substances carried by ships. Mar Pollut Bull, 14(6): 213-214.
Bevan C, Neeper-Bradley TL, Tyl RW, Fisher LC, Panson RD, Kneiss JJ, &
Andrews LS (1997) Two-generation reproductive toxicity study of methyl
tertiary-butyl ether (MTBE) in rats. J Appl Toxicol, 17(suppl 1):
S13-S19.
Bianchi A & Varney MS (1989) Analysis of methyl tert-butyl ether and
1,2-dihaloethanes in estuarine water and sediments using
purge-and-trap/gas chromatography. J High Res Chromatogr, 12(3):
184-186.
Bianchi A, Varney MS, & Phillips J (1991) Analysis of volatile organic
compounds in estuarine sediments using dynamic headspace and gas
chromatography-mass spectrometry. J Chromatogr, 542: 413-450.
Biles RW, Schroeder RE, & Holdsworth CE (1987) Methyl tertiary butyl
ether inhalation in rats: a single generation reproduction study.
Toxicol Ind Health, 3(4): 519-534.
Biodynamics (1984) The metabolic fate of methyl-t-butyl ether (MTBE)
following an acute intraperitoneal injection (Project No. 80089).
Washington, DC, American Petroleum Institute, 97 pp.
Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, & Andrews
LS (1997) Oncogenicity studies of inhaled methyl tertiary-butyl ether
in CD1 mice and F-344 rats. J Appl Toxicol, 17(suppl 1): S45-S55.
Blackburn GR, Dooley JF III, Schreiner CA, & Mackerer CR (1991)
Specific identification of formaldehyde-mediated mutagenicity using
the mouse lymphoma L5178Y TK+/- assay supplemented with formaldehyde
dehydrogenase. In Vitro Toxicol, 4(2): 121-132.
Bonin MA, Ashley DL, Cardinali FL, McGraw JM, & Wooten JV (1994)
Measurement of methyl tert-butyl ether and tert-butyl alcohol in
human blood and urine by purge-and-trap gas chromatography-mass
spectrometry using an isotope dilution method. In: 208th National
Meeting of the American Chemical Society, 1994. Washington, DC,
American Chemical Society, 1 p (Abstract P102-ENVR).
Borghoff SJ & Lagarde WH (1993) Assessment of binding of
2,4,4-trimethyl-2-pentanol to low-molecular-weight proteins isolated
from kidneys of male rats and humans. Toxicol Appl Pharmacol, 119:
228-235.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha2u-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 264-275.
Borghoff SJ, Jayjock E, & Murphy JE (1995) Methyl tert-butyl ether
(MTBE) vs ethyl tert-butyl ether (ETBE): A comparison of blood and
tissue partition coefficients in male and female F344 rats.
Toxicologist, 30: 34.
Borghoff SJ, Murphy JA, & Medinsky MA (1996) Development of a
physiologically based pharmacokinetic model for methyl
tertiary-butyl ether and tertiary-butanol in male Fischer-344
rats. Fundam Appl Toxicol, 30: 264-275.
Borghoff SJ, Murphy JE, Turner M, & James AR (1998) Dosimetry of
methyl t-butyl ether (MTBE) and t-butyl alcohol in male and female
rats following inhalation exposure to MTBE. Toxicologist, 42(1-S):
A1049.
Brady JF, Xiao F, Ning SM, & Yang CS (1990) Metabolism of methyl
tertiary-butyl ether by rat hepatic microsomes. Arch Toxicol, 64:
157-160.
Brown SL (1997) Atmospheric and potable water exposures to methyl
tert-butyl ether (MTBE). Regul Toxicol Pharmacol, 25: 256-276.
Bruce BW & McMahon PB (1996) Shallow ground-water quality beneath a
major urban center: Denver, Colorado, USA. J Hydrol, 186: 129-151.
Buckley TJ, Prah JD, Ashley D, Zweidinger RA, & Wallace LA (1997) Body
burden measurements and models to assess inhalation exposure to methyl
tertiary butyl ether (MTBE). J Air Waste Manage Assoc, 47: 739-752.
Buchta TM (1993a) Health hazard evaluation report HETA 93-606-2336:
National Centers for Environmental Health, Fairbanks, Alaska.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 37 pp (NTIS/PB94-133667).
Buchta TM (1993b) Health hazard evaluation report HETA 93-802-2338:
National Centers for Environmental Health, Stamford Connecticut.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 39 pp (NTIS/PB94-134343).
Budavari S, O'Heil MJ, Smith A, Heckelman PE, & Kinneary JF ed. (1996)
Methyl- tert-butyl ether. In: The Merck index, 12th ed. Whitehouse
Station, New Jersey, Merck & Co., Inc., p 1611.
Burleigh-Flayer HD, Chun JS, & Kintigh WJ (1992) Methyl tertiary butyl
ether: vapor inhalation oncogenicity study in CD-1 mice (Laboratory
project ID 91N0013A). Export, Pennsylvania, Bushy Run Research Center,
1068 pp (Report to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Cain WS, Leaderer BP, Ginsberg GL, Andrews LS, Cometto-Muniz JE, Gent
JF, Buck M, Berglund LG, Mohsenin V, Monahan E, & Kjaergaard S. (1996)
Acute exposure to low-level methyl tertiary-butyl ether (MTBE): human
reactions and pharmacokinetic response. Inhal Toxicol, 8: 21-48.
Calvert JG & Pitts JN Jr (1966) Photochemistry. New York, John Wiley &
Sons, Inc., pp 441-442.
CDC (1993) An investigation of exposure to methyl tertiary butyl ether
among motorists and exposed workers in Stamford, Connecticut.
Presented at the EPA Workshop on MTBE and other Oxygenates, Falls
Church, Virginia, 27 July 1993. Atlanta, Georgia, Centers for Disease
Control and Prevention, 35 pp.
Cederbaum AI & Cohen G (1980) Oxidative demethylation of t-butyl
alcohol by rat liver microsomes. Biochem Biophys Res Commun, 97:
730-736.
CEN (1996) Growth of top 50 chemicals slowed in 1995 from very high
1994 rate. Chem Eng News, April 8:16-20.
Chandler B & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska: Preliminary results. Anchorage,
Alaska, Department of Health and Social Services, 5 pp.
Charbonneau M, Strasser J Jr, Lock EA, Turner MJ Jr, & Swenberg JA
(1989) Involvement of reversible binding to alpha2u-globulin in
1,4-dichlorobenzene-induced nephrotoxicity. Toxicol Appl Pharmacol,
99: 122-132.
Chen CY, Chang KK, Chow NH, Leow TC, Chou TC, & Lin XZ (1995) Toxic
effects of cholelitholytic solvents on gallbladder and liver. Dig Dis
Sci, 40(2): 419-426.
Chiang CY, Loos KR, & Klopp RA (1992) Field determination of
geological/chemical properties of an aquifer by cone penetrometry and
headspace analysis. Ground Water, 30: 428-436.
Chun JS & Kintigh WJ (1993) Methyl tertiary-butyl ether: twenty-eight
day vapor inhalation study in rats and mice. Export, Pennsylvania,
Bushy Run Research Center, 387 pp (BRRC Report 93N1241).
Chun JS, Burleigh-Flayer HD, & Kintigh WJ (1992) Methyl tertiary butyl
ether (MTBE): vapor inhalation oncogenicity study in Fischer 344 rats
(Laboratory project ID 91N0013B). Export, Pennsylvania, Bushy Run
Research Center, 1463 pp (Report for the Methyl Tertiary Butyl Ether
Committee, Washington, DC).
Church CD, Isabelle LM, Pankow JF, Tratnyek PG, & Rose DL (1997)
Assessing the in situ degradation of methyl tert-butyl ether
(MTBE) byproduct identification at the sub-PPB level using direct
aqueous injection GC/MS: Extended abstract - Proceedings of ACS
National Meeting, San Francisco, California, 16-17 April 1997.
Washington, DC, American Chemical Society, 4 pp.
Cinelli S & Seeberg AH (1989) Reverse mutation in Salmonella
typhimurium. Test substance: MTBE. Rome, Life Science Research,
Toxicology Centre, 50 pp (LSR-RTC Report No. 216001-M-03489).
Cirvello JD, Radovsky A, Heath JE, Farnell DR, & Lindamood C (1995)
Toxicity and carcinogenicity of T-butyl alcohol in rats and mice
following chronic exposure in drinking water. Toxicol Ind Health, 11:
151-165.
Clark CR, Dutcher JS, Henderson TR, McClellan RO, Marshall WF, Naman
TM, & Seizinger DE (1984) Mutagenicity of automotive particulate
exhaust: influence of fuel extenders, additives, and aromatic content.
In: MacFarland HN, Holdsworth CE, MacGregor JA, Call RW, & Lane ML ed.
Applied toxicology of petroleum hydrocarbons. Princeton, New Jersey,
Princeton Scientific Publishers, pp 109-122.
Clayton Environmental Consultants (1991) Gasoline vapor exposure
assessment for the American Petroleum Institute (API) (Project No.
31774.00). Cypress, California, Clayton Environmental Consultants, 139
pp.
Cline PV, Delfino JJ, & Rao SC (1991) Partitioning of aromatic
constituents into water from gasoline and other complex solvent
mixtures. Environ Sci Technol, 25: 914-920.
Cochrane RA & Hillman DE (1984) Direct gas chromatographic
determination of alcohols and methyl tert-butyl ether in gasolines
using infrared detection. J Chromatogr, 287: 197-201.
Conaway CC, Schroeder RE, & Snyder NK (1985) Teratology evaluation of
methyl tertiary butyl ether in rats and mice. J Toxicol Environ
Health, 16: 797-809.
Cook CK & Kovein RJ (1995) NIOSH health hazard evaluation report No.
94-0220-2526: Exxon Company, Houston, Texas, USA. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 28 pp.
Cox RA & Goldstone A (1982) Atmospheric reactivity of oxygenated motor
fuel additives. In: Proceedings of the 2nd European Symposium on the
Physico-chemical Behaviour of Atmospheric Pollutants, Varese, Italy,
29 September-1 October 1981. Dordrecht, D Riedel Publishing Co, pp
112-119.
Cuthbert JA (1979) Safety tests on methyl- tert-butyl-ether.
Edinburgh, Inveresk Research International, 18 pp (IRI Report No.
1300).
Dale MS, Losee RF, Crofts EW, & Davis MK (1997) MTBE: Occurrence and
fate in source-water supplies. J Am Chem Soc, 37: 376-377.
Daughtrey WC, Gill MW, Pritts IM, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Neurotoxicological evaluation of methyl tertiary-butyl ether in
rats. J Appl Toxicol, 17(suppl 1): S57-S64.
DECOS (Dutch Expert Committee on Occupational Standards) (1994)
Methyl-t-butylether: Health based recommended occupational exposure
limit. The Hague, Health Council of the Netherlands, 75 pp
(Publication No. 1994/23).
Delzer GC, Zogorski JS, Lopes TJ, & Bosshart RL (1996) Occurrence of
the gasoline oxygenate MTBE and BTEX compounds in urban stormwater in
the United States, 1991-95: US Geological Survey. Rapid City, South
Dakota, US Geological Survey, 6 pp (Water-Resources Investigations
Report No. 96-4145).
Derwent RG, Jenkin ME, & Sandow SM (1996) Photochemical ozone creation
potentials for a large number of reactive hydrocarbons under European
conditions. Atmos Environ, 30(2): 181-199.
Dey JC, Brown RA, & McFarland WE (1991) Methyl tert-butyl ether.
Hazard Mater Control, 4: 32-39.
Diehl JW, Finkbeiner JW, & Di Sanzo FP (1995) Determination of ethers
and alcohols in reformulated gasolines by gas chromatography/atomic
emission detection. J High Res Chromatogr, 18: 108-110.
Di Padova C, Di Padova F, Montorsi W, & Tritapepe R (1986) Methyl
tert-butyl ether fails to dissolve retained radiolucent common bile
duct stones. Gastroenterology, 91: 1296-1300.
Dodd DE & Kintigh WJ (1989) Methyl tertiary butyl ether (MTBE):
repeated (13-week) vapor inhalation study in rats with neurotoxicity
evaluation. Export, Pennsylvania, Bushy Run Research Center, 502 pp
(Report No. 52-507 to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Drivas PJ, Valberg PA, & Gauthier TD (1991) Health assessment of air
toxics emissions from alternative fuels. Paper presented at the 84th
Annual Meeting of the Air and Waste Management Association, Vancouver,
British Columbia, 16-21 June 1991. Washington, DC, Air and Waste
Management Association, 17 pp.
Du JT, Abernathy CO, Donohue J, Mahfouz A, & Khanna K (1998) A
drinking water advisory: consumer acceptability and advice and health
effects analysis on methyl tertiary-butyl ether (MTBE). Toxicologist,
42(1-S): A 1123.
Duffy LK (1994) Oxyfuel in Alaska: use of interleukins to monitor
effects on the immune system. Sci Total Environ, 151: 253-256.
ECETOC (1997) Methyl tert-butyl ether (MTBE): Health risk
characterization. Brussels, European Centre for Ecotoxicology and
Toxicology of Chemicals, 126 pp (Technical Report No. 72).
Environment Canada (1989) Analysis of shellfish for organic and
inorganic contaminants. Dartmouth, Nova Scotia, Environment Canada
Conservation and Protection, 10 pp (Report No. AN 893118).
Environment Canada (1992) Canadian Environmental Protection Act -
Priority substances list: Methyl tertiary-butyl ether. Ottawa,
Minister of Supply and Services, 19 pp (Assessment Report No. 5).
Environment Canada (1993) Canadian Environmental Protection Act -
Priority substances list: Supporting document methyl tertiary butyl
ether. Hull, Quebec, Environment Canada, 49 pp.
Environment Canada (1996a) Canadian Environmental Protection Act -
National pollutant release inventory: Summary report 1994. Hull,
Quebec, Environment Canada, 143 pp.
Environment Canada (1996b) MTBE concentrations at selected locations:
National air pollution surveillance programme 1995-1996. Hull, Quebec,
Environment Canada, 21 pp.
Erdal S, Gong H Jr, Linn WS, & Rykowski R (1997) Projection of health
benefits from ambient ozone reduction related to the use of methyl
tertiary butyl ether (MTBE) in the reformulated gasoline program.
Risk Anal, 17: 693-704.
Esch O, Spinosa JC, Hamilton RL, Crombie DL, Schteingart CD, Rondinone
JF, DAgostino HB, Lillienau J, & Hofmann AF (1992a) Acute effects of
topical methyl tert-butyl ether or ethyl propionate on gallbladder
histology in animals: a comparison of two solvents for contact
dissolution of cholesterol gallstones. Hepatology, 16(4): 984-991.
Esch O, Schteingart CD, Pappert D, Kirby D, Streich R, & Hofmann AF
(1992b) Increased blood levels of methyl tert-butyl ether but not of
ethyl propionate during instillation with contact gallstone
dissolution agents in the pig. Hepatology, 18(2): 373-379.
Ferdinandi ES, Houle J-M, Lalande M, Provencher A, & St-Onge Brault G
(1990a) Pharmacokinetics of methyl tert-butyl ether (MTBE) and
tert-butyl alcohol (TBA) in male and female Fischer-344 rats after
administration of MTBE by the intravenous, oral and dermal routes.
Montreal, Bio-Research Laboratories Ltd, 98 pp (Report No. 38842 to
the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lalande M, Houle J-M, Provencher A, & Ducharme S
(1990b) Mass balance of radioactivity and metabolism of methyl
ter-butyl ether (MTBE) in male and female Fischer 344 rats after
intravenous, oral and dermal administration of 14C-MTBE. Montreal,
Bio-Research Laboratories Ltd, 127 pp (Report No. 38843 to the MTBE
Task Force, Washington, DC).
Ferdinandi ES, Houle J-M, Lalande M, Lulham G, Pilon D, Provencher A,
Labbé R, & St-Onge Brault G (1990c) Pharmacokinetics of methyl
tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) in male and
female Fischer-344 rats after single and repeat inhalation nose-only
exposures to MTBE. Montreal, Bio-Research Laboratories Ltd, 172 pp
(Report No. 38844 to the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lulham G, Pilon D, Labbé R, Lalande M, Provencher A,
Houle J-M, & Ducharme S (1990d) Disposition of radioactivity and
metabolism of methyl tert-butyl ether (MTBE) in male and female
Fischer 344 rats after nose-only inhalation exposure to 14C-MTBE.
Montreal, Bio-Research Laboratories Ltd, 89 pp (Report No. 38845 to
the MTBE Task Force, Washington, DC).
Fujiwara Y, Kinoshita T, Sato H, & Kojima I (1984) [Biodegradation and
bioconcentration of alkyl ethers.] Yukagatu, 33(2): 111-114 (in
Japanese with tables and abstract in English).
Garrett P, Moreau M, & Lowry JD (1986) MTBE as a groundwater
contaminant. In: Proceedings of the 1986 National Water Well
Association and American Petroleum Institute Conference on Petroleum
and Organic Chemicals in Groundwater, Houston, Texas, 12-14 November
1986. Dublin, Ohio, National Water Well Association, pp 227-238.
Geiger DL, Call DJ, & Brooks LT (1988) Acute toxicities of organic
chemicals to Fathead Minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 4, pp 75-76.
Giacomello P (1996) MTBE exposure in service stations: Italian study.
Paper presented at the 7th European Fuel Oxygenates Association
Conference, Sodehotel, 24-25 October 1996. Brussels, European Chemical
Industries Federation, 8 pp.
Gill MW (1989) Methyl tertiary butyl ether single exposure vapor
inhalation neurotoxicity study in rats. Export, Pennsylvania, Bushy
Run Research Center, 116 pp (Report No. 52-533 to the Methyl Tertiary
Butyl Ether Committee, Washington, DC).
Gillie AD (1993) Industrial hygiene monitoring methyl tertiary butyl
ether, benzene and gasoline. San Diego, California, Texaco Inc.,
Environmental Health and Safety Division, Industrial Hygiene, 31 pp.
Gordian ME & Guay G (1995) Benzene in Alaska. Alsk Med, 37: 25-28, 36.
Gordian ME, Huelsman MD, Brecht M-L, & Fisher DG (1995) Health effects
of methyl tertiary butyl ether (MTBE) in gasoline in Alaska. Alsk Med,
37: 101-103, 119.
Greenough RJ, McDonald P, Robinson P, Cowie JR, Maule W, Macnaughton
F, & Rushton A (1980) Methyl tertiary-butyl ether (Driveron): three
month inhalation toxicity in rats (Project No. 413038). Edinburgh,
Scotland, Inveresk Research International, 230 pp.
Grindstaff G, Henry M, Hernandez O, Hogan K, Lai D, & Siegel-Scott C
(1991) Formaldehyde risk assessment update. Washington DC, US
Environmental Protection Agency, Office of Toxic Substances, 140 pp.
Gupta G & & Lin YJ (1995) Toxicity of methyl tertiary butyl ether to
Daphnia magna and Photobacterium phosphoreum. Bull Environ Contam
Toxicol, 55: 618-620.
Hakkola M & Saarinen L (1996) Exposure of tanker drivers to gasoline
and some of its components. Ann Occup Hyg, 40: 1-10.
Hakkola M, Honkasalo ML, & Pulkkinen P (1996) Neuropsychological
symptoms among tanker drivers exposed to gasoline. J Occup Med, 46:
124-130.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, & Valcovic
LR (1993) Hazard evaluation of chemicals that cause accumulation of
alpha-globulin, hyaline droplet nephropathy, and tubule neoplasia in
the kidneys of male rats. Environ Health Perspect, 99: 313-349.
Harper M & Fiore AA (1995) Determination of methyl tert-butyl ether
in air using a diffusive sampler. Appl Occup Environ Hyg, 10: 616-619.
Hartle R (1993) Exposure to methyl tert-butyl ether and benzene
among service station attendants and operators. Environ Health
Perspect, 101(suppl 6): 23-26.
Haseman JK, Arnold J, & Eustis SL (1990) Tumor incidences in Fischer
344 rats: NTP historical data. In: Pathology of the Fischer rat:
Reference and atlas. McLean, Virginia, Academic Press, chapter 35, pp
555-564.
Hazleton Laboratories (1979) Acute eye irritation study in rabbits:
tert-butyl methyl ether (95% pure). Leesburg, Virginia, Hazleton
Laboratories America, Inc., 14 pp (Report No. 2024-132).
Hebert RS (1993) Industrial hygiene exposure assessment for methyl
tert-butyl ether (MTBE), benzene and gasoline. Houston, Texas, Star
Enterprise Environment, Health and Safety Department, 36 pp.
HEI (1996) The potential health effects of oxygenates added to
gasoline: a review of the current literature. A special report of the
Institute's oxygenates evaluation committee. Cambridge, Massachusetts,
Health Effects Institute, 158 pp.
Hinton J (1993) Occupational exposures. Presented at the MTBE
Conference in Washington, DC, 26-27 July 1993. Beacon, New York,
Texaco Inc., 37 pp.
Hoekman SK (1992) Speciated measurements and calculated reactivities
of vehicle exhaust emissions from conventional and reformulated
gasolines. Environ Sci Technol, 26: 1206-1216.
Hoekman SK (1993) Improved gas chromatography procedure for speciated
hydrocarbon measurements of vehicle emissions. J Chromatogr, 639:
239-253.
Hong J-Y, Yang CS, Lee M, Wang Y-Y, Huang WQ, Tan y, Patten CJ, &
Bondoc FY (1997) Role of cytochromes P450 in the metabolism of methyl
tert-butyl ether in human livers. Arch Toxicol, 71: 266-269.
Hong J-Y, Yang CS, Wang Y-Y, Bondoc FY, Pan J, Cokonis C, & Bao ZP
(1998) Use of CYP2E1 knock-out mice to assess the role of CYP2E1 in
metabolizing methyl tert-butyl ether (MTBE) and other gasoline ethers.
Toxicologist, 42(1-S): A87.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, Michigan, Lewis
Publishers, Inc., pp 653-654.
Hsieh CR & Ouimette JR (1994) Comparative study of multimedia modeling
for dynamic partitioning of fossil fuels-related pollutants. J Hazard
Mater, 37: 489-505.
Huber AH (1995) Human exposure estimates of methyl tertiary butyl
ether (MTBE). Presentation at Conference on MTBE and Other Oxygenates:
A Research Update, Falls Church, Virginia, 26-28 July 1993.
Washington, DC, National Technical Information Service, 32 pp (EPA
/600/A-94/255; NTIS/PB95-177150).
IITRI (1992) 28-day oral (gavage) toxicity of methyl tert-butyl
ether (MTBE) in rats (Project No. L08100). Chicago, Illinois, Illinois
Institute of Technology Research, 48 pp.
Imbriani M, Ghittori S, & Pezzagno G (1997) Partition coefficients of
methyl tert-butyl ether. G Ital Med Lav Ergon, 19: 63-65.
Industrial Bio-Test Laboratories (1969) Acute toxicity studies on
X-801-25. Northbrook, Illinois, Industrial Bio-Test Laboratories, 27
pp (Report No. A6809 to Sun Oil Company).
Japar SM, Wallington TJ, Richert JFO, & Ball JC (1990) The atmospheric
chemistry of oxygenated fuel additives: t-butyl alcohol, dimethyl
ether, and methyl t-butyl ether. Int J Chem Kinet, 22: 1257-1269.
Japar SM, Wallington TJ, Rudy SJ, & Chong TY (1991) Ozone-forming
potential of a series of oxygenated organic compounds. Environ Sci
Technol, 25: 415-420.
Johansen NG (1984) The analysis of C1-C4 alcohols, MTBE, and DIPE in
motor gasolines by multi-dimensional capillary column gas
chromatography. J High Res Chromatogr Chromatogr Commun, 7: 487-489.
Johanson G, Nihlén A, & Löf, A (1995) Toxicokinetics and acute effects
of MTBE and ETBE in male volunteers. Toxicol Lett, 82/83: 713-718.
Johnson T, McCoy M, & Wisbith T (1995) A study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 108 pp (API Publication No. 4619).
Katoh T, Arashidani K, Kikuchi M, Yoshikawa M, & Kodama Y (1993)
Effects of methyl tertiary butyl ether on hepatic lipid peroxidation
in mice. Jpn J Hyg, 48: 373-378.
Kelly TJ, Callahan PJ, Piell J, & Evans GF (1993) Method development
and field measurements for polar volatile organic compounds in ambient
air. Environ Sci Technol, 27: 1146-1153.
Kirchstetter TW, Singer BC, Harley RA, Kendall GR, & Chan W (1996)
Impact of oxygenated gasoline use on California light-duty vehicle
emissions. Environ Sci Technol, 30: 661-670.
Levy JM & Yancey JA (1986) Dual capillary gas chromatographic analysis
of alcohols and methyl tert-butyl ether in gasolines. J High Res
Chromatogr Chromatogr Commun, 9: 383-387.
Lewis RJ (1993) Methyl -tert-butyl ether (MTBE). In: Hawley's
condensed chemical dictionary, 12th ed. New York, Van Nostrand
Reinhold Co., p 760.
Lindamood C, Farnell DR, Giles HD, Prejean JD, Collins JJ, Takahashi
K, & Maronpot RR (1992) Subchronic toxicity studies to t-butyl
alcohol in rats and mice. Fundam Appl Toxicol, 19: 91-100.
Lindstrom AB & Pleil JD (1996) Alveolar breath sampling and analysis
to assess exposure to methyl tertiary butyl ether (MTBE) during motor
vehicle refuelling. J Air Waste Manage Assoc, 46: 676-682.
Lington AW, Dodd DE, Ridlon SA, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Evaluation of 13-week inhalation toxicity study on methyl
t-butyl ether (MTBE) in Fischer 344 rats. J Appl Toxicol, 17 (suppl
1): S37-S44.
Lioy PJ, Weisel CP, Jo WK, Pellizzari E, & Raymer JH (1994)
Microenvironmental and personal measurements of methyl-tertiary butyl
ether (MTBE) associated with automobile use activities. J Expo Anal
Environ Epidemiol, 4(4): 427-444.
Litton Bionetics (1980) Guinea pig sensitization study (TBME-99).
Kensington, Maryland, Litton Bionetics, Inc., 30 pp (Report No.
22011).
Lyman WJ, Reehl WF, & Rosenblatt DH (1990) Handbook of organic
chemical property estimation methods: Environmental behavior of
organic compounds. Washington, DC, American Chemical Society, 960 pp.
McGahan JP, Tesluk H, Brock JM, Johnston R, & Shaul DB (1988)
Dissolution of gallstones using methyl tertiary-butyl ether in an
animal model. Invest Radiol, 23: 599-603.
Mackay D, Shiu WY, & Ma KC (1993) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals - volume 3: Volatile organic chemicals. Boca Raton, Florida,
Lewis Publishers, 916 pp.
Mackerer CR, Angelosanto FA, Blackburn GR, & Schreiner CA (1996)
Identification of formaldehyde as the metabolite responsible for the
mutagenicity of methyl tertiary-butyl ether in the activated mouse
lymphoma assay. Proc Soc Exp Biol Med, 212: 338-341.
Marsh DF & Leake CD (1950) The comparative anesthetic activity of the
aliphatic ethers. Anesthesiology, 11: 455-463.
Mihelcic JR (1990) Modeling the potential effect of additives on
enhancing the solubility of aromatic solutes contained in gasoline.
Ground Water Monit Rev, 10: 132-137.
Miller MJ, Ferdinandi ES, Andrews LS, Douglas JF, & Kneiss JJ (1997)
Pharmacokinetics and disposition of methyl t-butyl ether in
Fischer-344 rats. J Appl Toxicol, 17(suppl 1): S3-S12.
Mo K, Lora CO, Javanmardian M, Yang X, & Kulpa CF (1997)
Biodegradation of methyl t-butyl ether by pure bacterial cultures.
Appl Microbiol Biotechnol, 47: 69-72.
Mobil Oil Corporation (1993) Activated mouse lymphoma (L5178Y/TK/+/-)
mutagenicity assay supplemented with formaldehyde dehydrogenase for
methyl tertiary butyl ether. Princeton, New Jersey, Mobil Oil
Corporation, 17 pp (Status report No. 65579).
Mohr SN, Fiedler N, Weisel C, & Kelly-McNeil K (1994) Health effects
of MTBE among New Jersey garage workers. Inhal Toxicol, 6(6): 553-562.
Möller Jensen H & Arvin E (1990) Solubility and degradability of the
gasoline additive MTBE, methyl tert-butyl ether, and gasoline
compounds in water. In: Arendt F, Hinsewald M, & van der Brink WJ ed.
Contaminated soil 90. Dodrecht, Kluwer Academic Publishers, pp
445-448.
Moolenaar RL, Hefflin BJ, Ashley DL, Middaugh JP, & Etzel RA (1994)
Methyl tertiary butyl ether in human blood after exposure to
oxygenated fuel in Faibanks, Alaska. Arch Environ Health, 49(5):
402-409.
Mormile MR, Liu S, & Suflita JM (1994) Anaerobic biodegradation of
gasoline oxygenates: Extrapolation of information to multiple sites
and redox conditions. Environ Sci Technol, 28: 1727-1732.
Moser GJ, Wong BA, Wolf DC, Moss OR, & Goldsworthy TL (1996a)
Comparative short-term effects of methyl tertiary butyl ether and
unleaded gasoline vapor in female B6C3P1 mice. Fundam Appl Toxicol,
31: 173-183.
Moser GJ, Wong BA, Wolf DG, Fransson-Steen RL, & Goldsworthy TL
(1996b) Methyl tertiary butyl ether lacks tumor-promoting activity on
N-nitosodiethylamine-initiated B6C3F1 female mouse liver.
Carcinogenesis, 17: 2753-2761.
Munch JW & Eichelberger JW (1992) Evaluation of 48 compounds for
possible inclusion in U.S. EPA Method 524.2, Revision 3.0: expansion
of the method analyte list to a total of 83 compounds. J Chromatogr
Sci, 30: 471-477.
Murray WR, Laferla G, & Fullarton GM (1988) Choledolithiasis -
in vivo stone dissolution using methyl tertiary butyl ether (MTBE).
Gut, 29: 143-145.
Neeper-Bradley TL (1991) Two-generation reproduction study of inhaled
methyl tertiary butyl ether in CD (Sprague-Dawley) rats. Export,
Pennsylvania, Bushy Run Research Center, 499 pp (Report No. 53-594 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
Nihlén A, Löf A, & Johanson G (1995) Liquid/air partition coefficients
of methyl and ethyl t-butyl ethers, t-amyl methyl ether, and t-butyl
alcohol. J Expo Anal Environ Epidemiol, 5(4): 573-582.
Nihlén A, Löf A, & Johanson G (1998a) Experimental exposure to methyl
tertiary-butyl ether: I. Toxicokinetics in humans. Toxicol Appl
Pharmacol, 148: 281-287.
Nihlén A, Wålinder R, Löf A, & Johanson G (1998b) Experimental
exposure to methyl tertiary-butyl ether: II. Acute effects in humans.
Toxicol Appl Pharmacol, 148: 281-287.
Novak JT, Yeh C, Gullic D, Eichenberger J, & Benoit RE (1992). The
influence of microbial ecology on subsurface degradation of petroleum
contaminants. Blacksburg, Virginia, Virginia Polytechnic Institute and
State University, Water Resources Research Center, 86 pp (Bulletin No.
177).
Pankow JF, Rathbun RE, & Zogorski JS (1996) Calculated volatilization
rates of fuel oxygenate compounds and other gasoline-related compounds
from rivers and streams. Chemosphere, 33: 921-937.
Paulov S (1987) [Action of the anti-detonation preparation tert-butyl
methyl ether on the model species Rana temporaria L.] Biologia, 42:
185-189 (in Slovak with English abstract).
Pauls RE (1985) Determination of high octane components: methyl
t-butyl ether, benzene, toluene, and ethanol in gasoline by liquid
chromatography. J Chromatogr Sci, 23: 437-441.
Pekari K, Riihimaki V, Vainiotalo S, Teräväinen E, & Aitio A (1996)
Experimental exposure to methyl- tert-butyl ether (MTBE) and
methyl- tert-amyl ether (MTAE). In: Proceedings of the International
Symposium on Biological Monitoring in Occupational and Environmental
Health, Espoo, Finland, 11-13 September 1996. Helsinki, Institute of
Occupational Health, pp 27-28.
Pence VH (1987a) The evaluation of the biodegradation of 606610 using
a modified closed bottle method. Miamiville, Ohio, Hill Top Biolabs,
Inc., 92 pp (Report No. 87-0479-11, prepared for ARCO Chemical
Company).
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Poet TS & Borghoff SJ (1997) In vitro uptake of methyl tert-butyl
ether in male rat kidney: Use of a two-compartment model to describe
protein interactions. Toxicol Appl Pharmacol, 145: 340-348.
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Post G (1994) Methyl tertiary butyl ether: health-based maximum
contaminant level document. Trenton, New Jersey, New Jersey Department
of Environmental Protection, Division of Sciences and Research.
Poulsen M, Lemon L, & Barker JF (1992) Dissolution of monoaromatic
hydrocarbons into groundwater from gasoline-oxygenate mixtures.
Environ Sci Technol, 26: 2483-2489.
Prah JD, Goldstein GM, Devlin R, Otto D, Ashley D, House D, Cohen KL,
& Gerrity T (1994) Sensory, symptomatic, inflammatory, and ocular
responses to and the metabolism of methyl tertiary butyl ether in a
controlled human exposure experiment. Inhal Toxicol, 6(6): 521-538.
Prakash CB (1989) Motor vehicle emissions from gasoline containing
MTBE. Ottawa, Ontario, Environment Canada, Environmental Protection
Directorate, Conservation and Protection, Industrial Programs Branch,
25 pp (Report No. IP-97).
Prescott-Mathews JS, Wolf DC, Wong BA, & Borghoff SJ (1997) Methyl
tert-butyl ether causes alpha2u-globulin nephropathy in male
Fischer-344 rats. Toxicol Appl Pharmacol, 143: 301-314.
Raese JW, Rose DL, & Sandstrom MW (1995) US Geological Survey
laboratory method for methyl tert-butyl ether and other fuel
oxygenates. Rapid City, South Dakota, US Geological Survey, 4 pp (US
Geological Survey Fact Sheet FS-219-95).
Rao HV & Ginsberg GL (1997) Physiologically-based pharmacokinetic
(PBPK) model assessment of methyl t-butyl ether (MTBE) in groundwater
for a bathing and showering determination. Risk Anal, 17: 583-598.
Riihimaki V, Matikainen E, Akila R, Teräväinen E, Mutanen P, Pekari K,
Vainiotalo S, Vilhunen R, & Aitio A (1996) Central nervous system
effects of the gasoline additive methyl- tert-butyl ether (MTBE). In:
Proceedings of the Third International Symposium on Biological
Monitoring in Occupational and Environmental Health, Espoo, Finland,
11-13 September 1996. Helsinki, Institute of Occupational Health, p
75.
Robinson M, Bruner RH, & Olson GR (1990) Fourteen- and ninety-day oral
toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley
rats. J Am Coll Toxicol, 9(5): 525-540.
Roe VD, Lacy MJ, Stuart JD, & Robbins GA (1989) Manual headspace
method to analyze for the volatile aromatics of gasoline in
groundwater and soil samples. Anal Chem, 61: 2584-2585.
Rousch JM & Sommerfeld MR (1998) Liquid-gas partitioning of the
gasoline oxygenate methyl tert-butyl ether (MTBE) under laboratory
conditions and its effect on growth of selected algae. Arch Environ
Contam Toxicol, 34: 6-11.
Salanitro JP, Diaz LA, Williams MP, & Wisniewski HL (1994) Isolation
of a bacterial culture that degrades methyl t-butyl ether. Appl
Environ Microbiol, 60: 2593-2596.
Savolainen H, Pfäffli P, & Elovaara E (1985) Biochemical effects of
methyl tertiary-butyl ether in extended vapour exposure of rats.
Arch Toxicol, 57: 285-288.
Schroll R, Bierling B, Cao G, Dörfler U, Lahaniati M, Langenbach T,
Scheunert I, & Winkler R (1994) Uptake pathways of organic chemicals
from soil by agricultural plants. Chemosphere, 28: 297-303.
Schuberth J (1996) A full evaporation headspace technique with
capillary GC and ITD: a means for quantitating volatile organic
compounds in biological samples. J Chromatogr Soc, 34: 314-319.
Schuetzle D, Siegl WO, Trescott EJ, Jensen TE, Dearth MA, Kaiser EW,
Gorse R, Kreucher W, & Kulik E (1994) The relationship between
gasoline composition and vehicle hydrocarbon emissions: A review of
current studies and future research needs. Environ Health Perspect,
102(suppl 4): 3-12.
Seeberg AH (1989) Unscheduled DNA synthesis (UDS) in primary rat
hepatocytes (autoradiographic method). Test substance: MTBE. Rome,
Life Science Research, Toxicology Centre, 79 pp (LSR-RTC Report No.
216003-M-03689).
Sernau RC (1989) Mutagenicity test on methyl tertiary butyl ether
Drosophila melanogaster sex-linked recessive test (Study No.
10484-0-461). Kensington, Maryland, Hazleton Laboratories America,
Inc., 23 pp (Report to the Methyl Tertiary Butyl Ether Toxicology
Committee, Washington, DC).
Simer MM (1986) Texaco Chemical Corporation - Safety (SAF) studies,
surveys and reports: methanol, tertiary butyl alcohol, 1,3-butadiene,
methyl tertiary butyl ether, total other hydrocarbons. Port Neches,
Texas, Texaco Chemical Corporation, 15 pp.
Smith Sl & Duffy LK (1995) Odor and health complaints with Alaskan
gasolines. Chem Health Saf, 2: 32-38.
Smith DF, Kleindienst TE, Hudgens EE, McIver CD & Bufalini JJ (1991)
The photooxidation of methyl tertiary butyl ether. Int J Chem Kinet,
23: 907-924.
Snamprogetti (1980) MTBE toxicological data book. Rome, Snamprogetti
Engineering Company S.p.A., 202 pp (Report No. LICE/0086/AA/1g).
SNV (1993) [Better environmental qualities in petrol - a proposal for
environmental classification.] Stockholm, National Environmental
Protection Agency, 69 pp (in Swedish).
Spitzer HL (1997) An analysis of health benefits associated with the
use of MTBE reformulated gasoline and oxygenated fuels in reducing
atmospheric concentrations of selected volatile organic compounds.
Risk Anal, 17: 683-691.
Squillace PJ, Pope DA, & Price CV (1995a) Occurrence of the gasoline
additive MTBE in shallow ground water in urban and agricultural areas.
Rapid City, South Dakota, US Geological Survey, 4 pp (US Geological
Survey Fact Sheet FS-114-95).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1995b) A preliminary
assessment of the occurrence and possible sources of MTBE in ground
water of the United States, 1993-94. Rapid City, South Dakota, US
Geological Survey, 29 pp (File Report No. 95-456).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1996) Preliminary
assessment of occurrence and possible sources of MTBE in groundwater
in the United States, 1993-1994. Environ Sci Technol, 30: 1721-1730.
Stackelberg PE, O'Brien AK, & Terracciano SA (1997) Occurrence of MTBE
in surface and ground water, Long Island, New York and New Jersey.
Proceedings of American Chemical Society meeting, San Francisco,
California, 16-17 April 1996. Washington, DC, American Chemical
Society, 4 pp.
Standeven AM & Goldsworthy TL (1993) Promotion of preneoplastic
lesions and induction of CYP2B by unleaded gasoline vapor in female
B6C3F1 mouse liver. Carcinogenesis, 14: 2137-2141.
State of Connecticut (1987) The incidence of MTBE in Connecticut.
Hertford, Connecticut, Department of Health Services, 3 pp (Document
No. FYI-OTS 0987-0574).
Steffan RJ, McClay K, Vainberg S, Condee CW, & Zhang D (1997)
Biodegradation of the gasoline oxygenates methyl tert-butyl ether,
ethyl tert-butyl ether, and tert-amyl methyl ether by propane
oxidising bacteria. Appl Environ Microbiol, 63: 4216-4222.
Stern BR & Tardiff RG (1997) Risk characterization of methyl
tertiary butyl ether (MTBE) in tap water. Risk Anal, 17: 727-743.
Sternal RS & Davis MA (1992) In vitro dissolution of cholesterol
gallstones. Invest Radiol, 27(12): 1040-1043.
Storck WJ, Layman PL, Reisch MS, Thayer AM, Kirschner EM, Peaff G, &
Tremblay J-F (1996) Facts and figures for the chemical industry. Chem
Eng News, June 24: 38-46.
Streete PJ, Ruprah M, Ramsey JD, & Flanagan RJ (1992) Detection and
identification of volatile substances by headspace capillary gas
chromatography to aid diagnosis of acute poisoning. Analyst, 117:
1111-1127.
Suflita JM & Mormile MR (1993) Anaerobic biodegradation of known and
potential gasoline oxygenates in the terrestrial subsurface. Environ
Sci Technol, 27: 976-978.
Swenberg JA & Dietrich DR (1991) Immunohistochemical localization of
alpha 2µ-globulin in kidneys of treated and control rats of a 13-week
vapor inhalation study undertaken with methyl tertiary butyl ether.
Washington, DC, Synthetic Organic Chemical Manufacturers Association,
5 pp (Report to the MTBE Health Effects Testing Task Force,
Washington, DC).
Tarkpea M & Svanberg O (1982) The acute toxicity of motor fuels to
brackish water organisms. Mar Pollut Bull, 13(4): 125-127.
Tepper JS, Jackson MC, McGee JK, Costa DL, & Graham JA (1994)
Estimation of respiratory irritancy from inhaled methyl tertiary butyl
ether in mice. Inhal Toxicol, 6(6): 563-569.
Texaco (1993) Industrial hygiene air sampling results: Guatemala
Refinery - methyl tert-butyl ether (MTBE). Beacon, New York, Texaco
Inc., Environment Health and Safety Division, 3 pp.
THE (1989) Biomonitoring results using MTBE. Wheat Ridge, Colorado,
THE Consultants, 8 pp.
TRC (1993) Final report to ARCO Chemical Company on the odor and taste
threshold studies performed with methyl tertiary-butyl ether (MTBE)
and ethyl tertiary-butyl ether (ETBE). Windsor, Connecticut, TRC
Environmental Corporation, 37 pp.
Tuazon EC, Carter WPL, Aschmann SM, & Atkinson R (1991) Products of
the gas-phase reaction of methyl tert-butyl ether with the OH
radical in the presence of NOx. Int J Chem Kinet, 23: 1003-1015.
Tyl RW (1989) Developmental toxicity study of inhaled methyl tertiary
butyl ether in New Zealand White rabbits. Export, Pennsylvania, Bushy
Run Research Center, 260 pp (Report No. 51-628 to the Methyl Tertiary
butyl Ether Toxicology Committee, Washington, DC).
Tyl RW & Neeper-Bradley TL (1989) Developmental toxicity study of
inhaled methyl tertiary butyl ether in CD-1 mice. Export,
Pennsylvania, Bushy Run Research Center, 342 pp (Report No. 52-526 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
US EPA (1989) Chemical rate constants for superfund health evaluation
manual chemicals. Washington, DC, US Environmental Protection Agency,
pp 645-646 (Report No. 68-02-4254).
US Interagency Assessment (1997) Interagency assessment of oxygenated
fuels. Washington, DC, National Science and Technology Council, 256
pp.
Vainiotalo S, Peltonen Y, & Pfäffli P (1996) MTBE exposure in service
stations. Paper presented at the 7th European Fuel Oxygenates
Association Conference, Sodehotel, 24-25 October 1996. Brussels,
European Chemical Industries Federation, 7 pp.
Veith GD & Kosian P (1983) Estimating bioconcentration potential from
octanol/water partition coefficients. In: Mackay D, Paterson S,
Eisenreich SJ, & Simmons MS ed. Physical behavior of PCBs in the Great
Lakes. Ann Arbor, Michigan, Ann Arbor Science Publishers, Chapter 15,
pp 269-282.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: narcotic industrial
chemicals. Can J Fish Aquat Sci, 40(6): 743-748.
Verga GR, Sironi A, Schneider W, & Frohne JCh (1988) Selective
determination of oxygenates in complex samples with the O-FID analyze.
J High Res Chromatogr Chromatogr Commun, 11: 248-252.
Vergnes JS & Chun JS (1994) Methyl tertiary butyl ether: in vivo-
in vitro hepatocyte unscheduled DNA synthesis assay in mice
(Laboratory project ID 93N1316). Export, Pennsylvania, Bushy Run
Research Center, 67 pp (Report to the MTBE Effects Testing Task Force,
Washington, DC).
Vergnes JS & Kintigh WJ (1993) Methyl tertiary butyl ether: bone
marrow micronucleus test in mice (Laboratory project ID 93N1244).
Export, Pennsylvania, Bushy Run Research Center, 99 pp (Report to the
MTBE Effects Testing Task Force, Washington, DC).
Vergnes JS & Morabit ER (1989) Methyl tertiary butyl ether repeated
exposure vapor inhalation study in rats: in vivo cytogenetic
evaluation. Export, Pennsylvania, Bushy Run Research Center, 40 pp
(Report No. 51-635 to the MTBE Effects Testing Task Force, Washington,
DC).
Wallington TJ, Dagaut P, Liu R, & Kurylo MJ (1988) Gas-phase reactions
of hydroxyl radicals with the fuel additives methyl tert-butyl ether
and tert-butyl alcohol over the temperature range 240-440 K. Environ
Sci Technol, 22(7): 842-844.
Wallington TJ, Andino JM, Skewes LM, Siegl WO, & Japar SM (1989)
Kinetics of the reaction of OH radicals with a series of ethers under
simulated atmospheric conditions at 295 K. Int J Chem Kinet, 21:
993-1001.
Ward JB, Au WW, Whorton EB, & Legator MS (1994) Genetic toxicology of
methyl tertiary butyl ether. Galveston, Texas, University of Texas, pp
57-134 (Final Report to the Agency for Toxic Substances and Disease
Registry).
Watson JG, Chow JC, Pritchett LC, Houck JA, Ragazzi RA, & Burns S
(1990) Chemical source profiles for particulate motor vehicle exhaust
under cold and high altitude operationg conditions. Sci Total Environ,
93: 183-190.
White MC, Johnson CA, Ashley DL, Buchta TM, & Pelletier DJ (1995)
Exposure to methyl tertiary-butyl ether from oxygenated gasoline in
Stamford, Connecticut. Arch Environ Health, 50: 183-189.
Wibowo AAE (1994) DEFOS (Dutch Expert Committee for Occupational
Standards) and NEG (Nordic Expert Group for Criteria Documentation of
Health Risks from Chemicals) basis for an occupational standard:
methyl-tert-butyl-ether. Solna, Sweden, National Institute of
Occupational Health, 21 pp.
Windholz M ed. (1983) The Merck index: an encyclopedia of chemicals,
drugs, and biologicals, 10th ed. Rahway, New Jersey, Merck and Co.,
Inc., p 865.
Yeh CK & Novak JT (1994) Anaerobic biodegradation of gasoline
oxygenates in soils. Water Environ Res, 66: 744-752.
Yoshikawa M, Arashidani K, Katoh T, Kawamoto T, & Kodama Y (1994)
Pulmonary elimination of methyl tertiary-butyl ether after
intraperitoneal administration in mice. Arch Toxicol, 68(8): 517-519.
Zogorski JS, Morduchowitz A, Baehr AL, Bauman BJ, Drew RT, Korte NE,
Lepham WW, Pankow JF, & Washington ER (1996) Fuel oxygenates and water
quality: Current understanding of sources, occurrence in natural
waters, environmental behavior, fate, and significance. Washington,
DC, Office of Science and Technology Policy, 91 pp (Final report
prepared for the Interagency Oxygenated Fuel Assessment).
Zweidinger RB (1993) Air quality measurements in Fairbanks, Stamford,
and Albany. Research Triangle Park, North Carolina, United States
Environmental Protection Agency, Atmospheric Research and
Environmental Assessment Laboratory, 19 pp (Report ACC-1539).
RÉSUMÉ
Le méthyltertiobutyléther (MTBE) est actuellement le plus utilisé
des éthers que l'on peut employer comme additifs de l'essence.
L'éthyltertiobutyléther (ETBE), le tertioamylméthyléther (TAME), le
tertioamyléthyléther (TAEE) et le diisopropyléther (DIPE), entre
autres, peuvent être ajoutés ou substitués au MTBE afin d'améliorer
l'oxygénation et l'indice d'octane, aussi peut-on en trouver à côté du
MTBE.
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le MTBE est un composé volatil et incolore, à l'odeur terpénique,
qui est liquide à la température ambiante. Sa viscosité est faible et
son point d'ébullition est de 55,2°C. Son point de congélation est de
-109°C. Sa densité est de 0,7404 à 20°C. Sa tension de vapeur est
relativement élevée: 33 500 Pa à 25°C. C'est une substance inflammable
qui peut en outre former des mélanges explosifs avec l'air. Il est
très soluble dans les autres éthers et dans l'alcool. Miscible à
l'essence, il est également soluble dans l'eau (42 000 g/m3 à
19,8°C). Son coefficient de partage entre l'octanol et l'eau (log
Kow) est de 0,94-1,3. Il est instable en solution acide.
La recherche et le dosage du MTBE se font dans tous types de
matrices par chromatographie en phase gazeuse au moyen de détecteurs
et de colonnes capillaires adaptés à la matrice en cause. On a
également recours à la chromatographie liquide à phases inversées pour
l'analyse des échantillons d'essence. On utilise aussi, pour la
préparation des échantillons d'air, d'eau et de sédiments ou encore
des échantillons biologiques, divers systèmes de purge et de piégeage,
la sorption/désorption et des méthodes basées sur l'espace de tête.
2. Sources d'exposition humaine et environnementale
Autant qu'on sache, le MTBE n'existe pas à l'état naturel. Dans
l'industrie, on l'obtient par l'action du méthanol sur l'isobutylène
en présence d'un catalyseur. Un certain nombre de pays le produisent
en quantités croissantes depuis la fin des années 70. Il compte
actuellement parmi les 50 produits chimiques dont le volume de
production est le plus élevé. En 1996, la capacité de production des
Etats-Unis était de 10,6 millions de tonnes et on estime que la
demande de MTBE va encore augmenter. Environ 25% de l'essence vendue
aux Etats-Unis est additionnée de MTBE. On l'utilise presque
exclusivement pour améliorer l'indice d'octane et accroître la teneur
de l'essence en oxygène. On en ajoute à l'essence jusqu'à 17% en
volume.
3. Transport, distribution et transformation dans l'environnement
Une fois libéré dans l'air, le MTBE y reste en majeure partie
avec seulement de petites quantités qui passent dans le sol et dans
l'eau. Le MTBE présent dans l'atmosphère peut passer en partie dans
l'eau de pluie, mais la proportion qui s'élimine ainsi reste faible.
Dans l'atmosphère, l'action des radicaux hydroxyle entraîne la
formation d'un certain nombre de composés et en particulier de
formiate de tertiobutyle, photochimiquement stable, et de
2-méthoxy-2-méthylpropanol, qui doit réagir énergiquement avec les
radicaux hydroxyles pour donner du CO2, du formaldéhyde, de l'acétone
et de l'eau. Lorsque du MTBE est libéré dans l'eau, il se dissout
partiellement, une partie passant dans l'air. Les quantités qui
passent dans les biotes et les sédiments sont faibles. Les épreuves
classiques indiquent une faible biodégradabilité. On pense que d'une
façon générale, la biodégradation est lente dans l'environnement.
Lorsque du MTBE est libéré dans le sol, il passe dans l'air par
volatilisation, dans les eaux de surface par entraînement et dans les
eaux souterraines par lessivage. Le MTBE peut persister dans les eaux
souterraines.
4. Concentrations dans l'environnement et exposition humaine
Les données relatives aux concentrations dans l'environnement et
à l'exposition humaine sont peu nombreuses.
Dans des études portant sur l'air de certaines villes où les
véhicules utilisaient de l'essence oxygénée contenant 15% de MTBE, on
a relevé des concentrations ambiantes allant de "non décelable" à
100,9 µg/m3 (0,028 ppm), avec plusieurs concentrations médianes
allant de 0,47 à 14,4 µg/m3 (0,00013 à 0,004 ppm). Dans l'air de
quelques villes où le MBTE était utilisé à plus faible teneur pour
augmenter l'indice d'octane, la concentration de ce composé allait de
non décelable à 26,4 µg/m3 (0,0073).
Au niveau du sol ou à proximité de raffineries de pétrole, la
concentration allait de 15 à 281 µg/m3. Dans l'air urbain, à
proximité d'ateliers où l'on procédait au mélange de cet additif à
l'essence, la concentration était de 1508 µg/m3 (0,419 ppm), avec des
valeurs extrêmes de 216-35 615 µg/m3 (0,06-9,8 ppm).
Dans les stations service situées dans des zones où l'on
utilisait de l'essence oxygénée à 10-15% de MTBE, c'est dans la zone
de respiration des consommateurs, au moment des pleins, que la
concentration de l'additif était la plus forte (300 à 136 000 µg/m3,
soit 0,09 à 38 ppm), les valeurs dépassant toutefois rarement 3600
µg/m3 (10 ppm) et tombant un peu plus bas au niveau des pompes (de
non décelable à 5700 µg/m3, soit 1,6 ppm). Les valeurs les plus
faibles ont été relevées sur le périmètre de la station (de non
décelable à 500 µg/m3, soit 0,14 ppm). Les concentrations relevées
dans les stations services dépourvues de système de récupération des
vapeurs étaient généralement plus élevées.
A l'intérieur d'une automobile, on a relevé des valeurs de 7 à 60
µg/m3 (0,002 à 0,017 ppm) au cours de navettes et de 20 610 µg/m3
(0,006 à 0,172 ppm) lors des pleins.
D'après des données de surveillance qui se limitent
presqu'exclusivement aux Etats-Unis, la présence de MTBE a été décelée
dans de la neige, des eaux d'orage, des eaux de surface (ruisseaux,
rivières et retenues), des eaux souterraines et dans de l'eau de
boisson. Les valeurs relevées dans les eaux d'orage allaient de 0,2 à
8,7 µg/litre avec une valeur médiane inférieure à 1,0 µg/litre. Dans
le cas des ruisseaux, rivières et retenues, les concentrations
s'étageaient entre 0,2 et 30 µg/litre, les valeurs médianes obtenues
dans diverses études allant de 0,24 à 7,75 µg/litre.
La présence de MTBE n'a généralement pas été décelée dans les
eaux souterraines profondes ou non des zones agricoles. Lorsqu'on en a
trouvé, la concentration était inférieure à 2,0 µg/litre. La présence
de MTBE est plus fréquente dans eaux souterraines de faible profondeur
des régions urbanisées (dans les premiers 1,5 à 3 m des nappes
phréatiques). On trouve alors des concentrations de moins de 0,2
µg/litre à 23 µg/litre, avec une valeur médiane de moins de 0,2
µg/litre.
On trouve rarement du MTBE dans l'eau des réseaux d'adduction qui
est pompée dans les nappes souterraines. Sur 51 réseaux contrôlés sauf
3, la concentration était inférieure ou égale à 20 µg/litre. Il est
difficile de donner des valeurs caractéristiques pour l'eau
d'adduction captée en surface car les données sont insuffisantes. On a
trouvé de fortes concentrations de MTBE dans quelques puits privés
utilisés comme source d'eau potable (soit >1000 µg/m3). On peut
toutefois douter que de l'eau contenant plus de 50 à 100 µg/litre de
MTBE soit encore buvable, car le seuil organoleptique du MTBE est bas.
Parmi les travailleurs exposés au MTBE, on peut citer ceux qui
produisent, distribuent ou utilisent ce composé ou de l'essence qui en
contient, y compris les pompistes et les mécaniciens des stations
service.
En ce qui concerne l'exposition de courte durée (<30 min) lors
d'opérations habituelles de production ou de stockage de MTBE pur, les
chiffres vont de 715 à 43 000 µg/m3 (0,2 à 12 ppm), avec une valeur
médiane moyenne de 3400 µg/m3 (0,95 ppm). Pour l'exposition de plus
longue durée (30 min à 8 h), les valeurs vont de 360 à 890 000 µg/m3
(0,01 à 250 ppm), avec une valeur médiane d'environ 540 µg/m3 (0,15
ppm). Chez les ouvriers qui mélangent l'additif à l'essence, les
valeurs de l'exposition de courte durée vont de non décelable à
360 000 µg/m3 (100 ppm), la médiane se situant à environ 5700 µg/m3
(1,6 ppm). Dans le cas d'une exposition de plus longue durée, les
valeurs obtenues vont de non décelable à 257 000 µg/m3 (72 ppm), avec
une valeur médiane moyenne d'environ 2000 µg/m3 (0,6 ppm).
C'est lors du transport de MTBE pur ou en mélange avec des
carburants, dans des canalisations, sur des péniches, des wagons de
chemin de fer ou des camions (MTBE pur seulement) que l'on a
enregistré les expositions les plus fortes, avec des valeurs à court
terme allant de 3750 mg/m3 (0,001 à 1050 ppm) et une valeur médiane
moyenne de 140 mg/m3 (39 ppm). Dans le cas d'expositions à long
terme, les valeurs allaient de 0,036 à 2540 mg/m3 (0,01 à 712 ppm),
la valeur médiane moyenne se situant à 2,85 mg/m3 (0,8 ppm). Lors de
la distribution (c'est-à-dire du chargement de mélanges carburant-MTBE
sur des camions et de leur livraison et déchargement dans des stations
service), on a relevé des valeurs à court terme allant de non
décelable à 225 mg/m3 (63 ppm), les valeurs médianes moyennes se
situant autour de 21 mg/m3 (6 ppm). Les valeurs à long terme allaient
de 0,036 à 22 mg/m3 (0,01 à 6,2 ppm), avec une valeur médiane moyenne
de 1,79 mg/m3 (0,5 ppm).
L'exposition médiane moyenne à court terme des pompistes de
stations service allait, selon certaines mesures, généralement de
1,071 à 21,42 mg/m3 (0,3 à 6 ppm) et dépassait rarement 35,7 mg/m3
(10 ppm). Dans le cas de l'exposition médiane à long terme, on a
obtenu la valeur de 1,79 mg/m3 (0,5 ppm). L'exposition médiane des
mécaniciens est restée inférieure au seuil de détection dans une étude
à court terme; dans le cas de l'exposition à long terme, la valeur
était d'environ 360 µg/m3 (0,1 ppm).
5. Cinétique et métabolisme
Les données toxicocinétiques relatives aux effets du MTBE sur
l'Homme proviennent essentiellement d'études contrôlées pratiquées sur
des volontaires adultes ou sur une population exposée à de l'essence
oxygénée. Après inhalation, le MTBE passe rapidement dans le courant
sanguin. Chez des volontaires humains en bonne santé exposés par voie
respiratoire, on constate que la cinétique est linéaire jusqu'à la
concentration de 268 mg/m3 (75 ppm). On a procédé au dosage de
l'alcool tertiobutylique (en abrégé TBA, un métabolite du MTBE) dans
le sang et les urines. Chez des sujets humains exposés à des
concentrations de MTBE allant de 5,0 à 178,5 mg/m3 (1,4 à 50 ppm), la
concentration maximale de MTBE et de TBA allait respectivement de 17,2
à 1144 µg/litre et de 7,8 à 925 µg/litre. En utilisant un modèle
monocompartimental, on a pu constater qu'intervenaient dans la
demi-vie globale du MTBE des constituants à demi-vie brève (36-90 min)
et des constituants à demi-vie longue (19 h).
Chez les rongeurs, le MTBE est bien résorbé et réparti après
administration per os ou exposition par la voie respiratoire.
L'absorption est moindre par voie percutanée. A la dose de 400 mg/kg
per os et de 28 800 mg/m3 (8000 ppm) par inhalation, la proportion
de la dose totale absorbée qui était éliminée dans l'air expiré
augmentait à mesure que diminuait la proportion éliminée dans les
urines, ce qui est le signe d'une saturation du métabolisme. On n'a
pas mis en évidence de TBA dans l'urine des rats exposés. La présence
de 2-méthyl-1,2-propanediol et d'acide alpha-hydroxyisobutyrique dans
l'urine indique que le TBA est également métabolisé. Les études
in vitro montrent que le MTBE est métabolisé en TBA, formaldéhyde et
acétone.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat, la dose létale médiane aiguë par voie buccale
(DL50) est égale à environ 3 800 mg/kg de poids corporel. La
concentration létale médiane aiguë (CL50) pour une exposition de 15
minutes par inhalation se situe aux environs de 141 000 mg/m3 d'air
chez la souris. Parmi les signes d'intoxication on peut citer une
dépression du SNC, une ataxie et des difficultés respiratoires. Aux
doses non létales, la récupération a été complète. Par voie
percutanée, la DL50 est >10 200 mg/kg de poids corporel chez le
lapin.
On n'a trouvé qu'une seule étude où il soit question d'un effet
"modérément" irritant pour la peau, l'irritation consistant en un
érythème et un oedème modérés après application sur la peau de lapins.
Chez ce même animal, le MTBE s'est également révélé irritant pour la
muqueuse oculaire, les effets produits étant bénins et réversibles.
Dans la seule étude retrouvée, le MTBE a provoqué une irritation
légère à forte des voies respiratoires lors de l'exposition de souris
à des doses de 300 à 30 000 mg/m3. Il n'a pas produit de
sensibilisation cutanée chez le cobaye.
L'expérimentation sur des rats et des souris montre que des
expositions réitérées conduisent principalement à une augmentation du
poids des organes et ont des effets histopathologiques sur le rein
(rat) et sur le foie (souris). Une étude d'ingestion de 90 jours a
montré que la limite inférieure d'apparition d'effets néphrotoxiques
se situe à 440 mg/kg p.c. par jour (augmentation du poids des reins et
dégénérescence hyaline chez des rats Sprague-Dawley). En exposant des
rats Fischer-344 par inhalation à une concentration de 2880 mg/m3
(800 ppm) de MTBE, on a obtenu une augmentation du poids rénal
accompagnée, lorsqu'on accroissait la concentration, d'une
augmentation modérée de la dégénérescence hyaline au niveau des
tubules proximaux. Lors d'études d'oncogénicité comportant
l'exposition des animaux par inhalation, on a observé à la dose de
1440 mg/m3 (400 ppm), un accroissement de la fréquence et de la
gravité des néphropathies progressives chroniques chez les rats mâles,
alors que chez les souris mâles, il y avait à cette même dose
augmentation du poids absolu du foie (corrélée avec une augmentation
des hypertrophies hépatocellulaires à plus forte concentration) et du
poids relatif des reins.
L'exposition au MTBE provoque également des effets irréversibles
sur le système nerveux central (SNC) consistant notamment en sédation,
diminution de l'activité, ataxie et anesthésie à forte concentration.
Des effets biphasiques s'observent également sur l'activité motrice à
plus faible concentration. Lors d'une étude sur des rats comportant
une exposition de 6 h par inhalation, on a constaté qu'à la dose de
2880 mg/m3 (800 ppm), il se produisait, chez les animaux d'un des
deux sexes, des modifications réversibles et liées à la dose de
l'activité motrice. Ces effets étaient passagers et n'apparaissaient
plus guère lors des études à long terme.
On a pu retrouver des études de reproduction portant sur une ou
deux générations de rats ainsi que quatre études relatives au
développement de rats, de souris et de lapins exposés à du MTBE. Ces
études n'ont pas permis de mettre en évidence d'effets spécifiques sur
la reproduction des rats à des concentrations allant jusqu'à 28 800
mg/m3. En outre, aux concentrations inférieures à celles qui se
révélaient toxiques pour les mères, le MTBE n'a pas eu non plus
d'effets sur le développement de la progéniture. A la dose de 28 800
mg/m3, on a constaté, chez la souris, une augmentation du poids de
l'utérus et un accroissement du métabolisme des estrogènes.
Le MTBE a fait l'objet d'un grand nombre d'épreuves valables de
mutagénicité et autres études de génotoxicité. Les résultats obtenus
montrent que le composé n'est pas génotoxique, même si un résultat
positif a été obtenu dans l'épreuve de mutation portant sur le locus
tk des cellules lymphomateuses. Ce résultat s'explique en effet par la
métabolisation du MTBE en formaldéhyde.
Pour les études de cancérogénicité, on a exposé par inhalation
des rats Fischer-344 et des souris CD-1 ou gavé des rats
Sprague-Dawley avec une nourriture contenant du MTBE. Dans aucune des
études d'exposition par inhalation on a procédé à une correction
statistique pour tenir compte des différences de survie. Dans les
trois études, on a constaté une augmentation sensible de l'incidence
des tumeurs, localisées, chez les rats mâles Fischer-344, au niveau
des tubules rénaux et des cellules de Leydig, chez les rats mâles
Sprague-Dawley, au niveau des cellules de Leydig (lymphomes et
leucémies chez les femelles) et chez les souris femelles CD-1, au
niveau du foie. Les tumeurs des tubules rénaux et les
leucémies/lymphomes n'ont donc pas été observées systématiquement chez
le rat lors des différentes études. En outre, les tumeurs rénales
sexospécifiques étaient associées à une néphropathie également
sexospécifique mettant en jeu l'alpha2u-globuline, qui a été observée
dans plusieurs études de courte durée. L'augmentation des tumeurs des
cellules de Leydig a été observée à la dose la plus élevée chez les
rats Sprague-Dawley (1000 mg/kg p.c.), mais chez les rats Fischer-344,
l'interprétation de cet accroissement est rendu délicate par la très
forte incidence tumorale également observée chez les témoins
concomitants et les témoins historiques. Les tumeurs hépatiques ont
été observées dans les groupes témoins et à la dose de 28 800 mg/m3
(8000 ppm) dans les groupes exposés avec des incidences respectives de
2/50 et 10/50 chez les femelles et de 12/49 et 16/49 chez les mâles.
L'accroissement d'incidence était modeste et s'accompagnait d'une
hypertrophie hépatocellulaire.
7. Effets sur l'Homme
Après la mise sur le marché, aux Etats-Unis, de deux types
d'essence nécessitant l'utilisation d'additifs d'oxygénation (pas
obligatoirement du MTBE), on a constaté que les usagers se plaignaient
de symptômes aigus tels que maux de tête, irritation des yeux et du
nez, toux, nausées, vertiges et désorientation. Les études
épidémiologiques effectuées sur des populations humaines en milieu
professionnel ou non, de même que les études expérimentales sur
volontaires humains exposés dans des conditions contrôlées, n'ont pas
permis de découvrir si ces plaintes étaient fondées. Des études
intracommunautaires menées en Alaska, au New Jersey, dans le
Connecticut et dans le Wisconsin ont, avec des résultats divers il est
vrai, montré qu'il n'y avait guère de relation entre l'exposition au
MTBE et les symptômes dont la population se plaignait.
Des volontaires adultes ont été placés, dans le cadre d'études
expérimentales contrôlées, dans des chambres d'inhalation où on leur a
fait respirer du MTBE à des concentrations allant de 5,0 mg/m3
(1,4 ppm) à 270 mg/m3 (75 ppm). Aucun effet patent n'a été relevé,
qu'il s'agisse de la relation subjective de symptômes ou d'indicateurs
objectifs tels qu'une irritation ou d'autres signes, à des
concentrations allant jusqu'à 180 mg/m3 (50 ppm) et pendant une durée
pouvant atteindre 2 h. A en juger d'après ces résultats, il est peu
probable que le MTBE puisse à lui seul exercer des effets toxiques
aigus sur la population générale dans les conditions habituelles
d'exposition par la voie respiratoire. Il est cependant à noter que
les effets potentiels d'essences additionnées de MTBE, dans les
conditions où la plupart des gens sont exposés à cet additif
lorsqu'ils utilisent des carburants oxygénés, n'ont été étudiés ni
expérimentalement, ni par le biais de méthodes épidémiologiques
prospectives. Par ailleurs, le rôle de facteurs tels que la perception
de la présence de MTBE, explicable en partie par l'odeur particulière
de ce composé, n'a pas été étudié non plus.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Expérimentalement, la toxicité aiguë (exprimée par la CL50) du
MTBE pour les poissons, les amphibiens et les crustacés se révèle
supérieure à 100 mg/litre. On ne possède pas de données sur la
toxicité chronique ou subléthale de ce composé pour les organismes
aquatiques, ni sur sa toxicité pour les organismes terrestres.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
A en juger par les données collectives, il semble peu probable
que le MTBE puisse à lui seul et dans les conditions usuelles
d'exposition, provoquer des effets toxiques aigus dans la population
générale.
D'après les études effectuées sur l'animal, le MTBE possède une
toxicité aiguë "modérée" et il provoque une légère irritation cutanée
et oculaire, mais pas de sensibilisation. Des expositions répétées
entraînent des effets essentiellement localisés au rein chez le rat et
au foie chez la souris, la dose nocive la plus faible étant de 440
mg/kg p.c. par jour chez le rat après ingestion et de 1440 mg/m3
(400 ppm) après inhalation. Aux concentrations inférieures au seuil de
toxicité parentale, le MTBE n'a pas eu d'effets nocifs sur la
reproduction ou le développement.
Le MTBE n'est pas génotoxique mais il peut provoquer la formation
de tumeurs chez les rongeurs, surtout aux concentrations suffisamment
élevées pour avoir d'autres effets toxiques. On considère actuellement
que ces données ne sont pas suffisantes pour que l'on puisse en tirer
une évaluation du risque cancérogène chez l'Homme. Le Groupe spécial a
conclu que, pour être en mesure de donner des indications
quantitatives concernant les limites d'exposition et d'évaluer le
risque, il fallait obtenir des données supplémentaires sur un certain
nombre de points.
Il ne semble pas que le MTBE, aux concentrations auxquelles il se
trouve dans l'eau, puisse être toxique pour les organismes aquatiques,
sauf en cas de déversement. On ne possède pas de données sur la
toxicité du MTBE pour les organismes terrestres mais il n'y a
vraisemblablement pas lieu de s'alarmer, étant donné que sa
concentration est faible dans l'air ambiant et que sa demi-vie est
relativement brève.
RESUMEN
El éter metil- terciario-butílico (MTBE) es uno de los distintos
éteres que pueden utilizarse como aditivos de combustibles y en la
actualidad es con gran diferencia el más usado. El éter
etil- terciario-butílico (ETBE), el éter terciario-amil-metílico
(TAME), el éter terciario-amil-etílico (TAEE) y el éter
diisopropílico (DIPE), entre otros, pueden ser suplementos del MTBE o
sustituirlo para fines de oxigenación o mejora de los octanos y, en
consecuencia, pueden hallarse en asociación con el MTBE.
1. Identidad, propiedades físicas y químicas y métodos analíticos
El MTBE es un líquido volátil e incoloro a la temperatura
ambiente, de olor parecido al terpeno. Su viscosidad es baja y tiene
un punto de ebullición de 55,2°C. El punto de congelación es de -
109°C. La densidad es de 0,7404 a 20°C. La presión de vapor es
relativamente alta: 33 500 Pa a 25°C. El MTBE es inflamable y puede
formar mezclas explosivas con el aire. Es muy soluble en otros éteres
y alcohol. Se mezcla con la gasolina y es soluble en agua (42 000
g/m3 a 19,8°C). El coeficiente de partición log n-octanol/agua es
de 0,94-1,3. Es inestable en solución ácida.
El MTBE se analiza en todas las matrices en general por
cromatografía de gases, utilizando una gama de columnas capilares y
sistemas detectores que son apropiados para la matriz específica.
También se ha utilizado la cromatografía inversa en fase líquida para
el análisis de las muestras de gasolina. Se han empleado sistemas de
sorción-desorción, incluidos sistemas de purga y captación, así como
procedimientos de recámara, a fin de preparar muestras de aire, agua,
sedimento y biológicas para el análisis.
2. Fuentes de exposición humana y ambiental
No se conoce la presencia natural de MTBE en el medio ambiente.
En la industria deriva de la reacción catalítica del metanol y el
isobutileno, y en varios países se ha producido en volúmenes
crecientes desde los últimos años setenta. El MTBE figura actualmente
entre los 50 productos químicos de mayor producción en volumen. En
1996, la capacidad estadounidense de producción fue aproximadamente de
10,6 millones de toneladas, previéndose un constante aumento del uso
de MTBE. El 25% aproximadamente de la gasolina en los EE.UU está
mezclada con MTBE. El MTBE se utiliza casi exclusivamente para el
refuerzo de los octanos y para aumentar el contenido de la gasolina en
oxígeno. El MTBE se ha añadido a la gasolina en concentraciones de
hasta el 17% en volumen.
3. Transporte, distribución y transformación en el medio ambiente
Tras su eliminación en el aire, el MTBE permanecerá en gran parte
en este medio, penetrando cantidades menores en el suelo y el agua. En
la atmósfera, el MTBE puede ser arrastrado por la lluvia. Sin embargo,
sólo una pequeña cantidad es eliminada de la atmósfera de este modo.
La transformación atmosférica por radicales hidroxilos produce varios
productos, entre los que figuran el formato terciario-butílico (TBF)
estable y el 2-metoxi-2-metilpropanol, que se supone que son muy
reactivos con los radicales hidroxilos, dando CO2, formaldehido,
acetona y agua. Cuando el MTBE pasa al agua se disuelve una cantidad
significativa, con cierta proporción en el aire. La proporción que
pasa a los biota y el sedimento es escasa. La biodegradabilidad en
ensayos convencionales es limitada. Se cree que por lo general es
lenta en el medio ambiente. Cuando el MTBE pasa al suelo, es
transportado al aire por volatilización, al agua superficial por
escurrimiento y al agua subterránea como resultado de la lixiviación.
El MTBE puede persistir en el agua subterránea.
4. Niveles medioambientales y exposición humana
Se dispone de escasos datos sobre los niveles medioambientales y
la exposición humana.
En los estudios sobre el MTBE en el aire de algunas ciudades que
utilizan gasolina oxigenada con MTBE al 15%, las concentraciones
ambientales iban del nivel indetectable a 100,9 µg/m3 (0,028 ppm),
con varias concentraciones medianas de 0,47 a 14,4 µg/m3 (0,00013 a
0,004 ppm). Las concentraciones de MTBE en el aire de algunas ciudades
en donde se utiliza MTBE como reforzador de octanos en concentraciones
inferiores van del nivel no detectable a 26,4 µg/m3 (0,0073 ppm).
Las concentraciones a nivel del suelo o cerca de las refinerías
eran de 15 a 281 µg/m3. Los niveles medianos en el aire urbano cerca
de instalaciones de mezclado eran de 1508 µg/m3 (0,419 ppm), con
gamas de 216-35 615 µg/m3 (0,06 a 9,8 ppm).
En las estaciones de servicio situadas en zonas en donde la
gasolina oxigenada contiene el 10-15% de MTBE, las concentraciones
alcanzaban el nivel máximo en la zona de respiración durante el
llenado de los depósitos por los consumidores (gama de 300 a
136 000 µg/m3 (0,09 a 38 ppm)), con niveles que rara vez pasaban de
3600 µg/m3 (10 ppm), siendo ligeramente inferiores en la zona de
bombas (indetectables a 5700 µg/m3 (1,6 ppm)) y mínimos en el
perímetro de la estación (indetectables a 550 µg/m3 (0,14 ppm)). En
general las concentraciones eran superiores en las estaciones de
servicio sin sistemas de recuperación de vapores.
En la cabina del automóvil, las concentraciones eran de 7 a
60 µg/m3 (0,002 a 0,017 ppm) durante la conducción y de 20 a
610 µg/m3 (0,006 a 0,172 ppm) al llenar el depósito.
Basándose en operaciones limitadas de vigilancia realizadas casi
exclusivamente en los EE.UU., se ha detectado el MTBE en la nieve, el
agua de tormenta, las aguas superficiales (riachuelos, ríos y
embalses), las aguas subterráneas y el agua de beber. Las
concentraciones de MTBE detectadas en el agua de tormenta iban de 0,02
a 8,7 µg/litro, con un valor mediano de menos de 1,0 µg/litro. En los
riachuelos, ríos y embalses, la gama de detección era de 0,2 a
30 µg/litro y la gama de valores medianos en varios estudios era de
0,24 a 7,75 µg/litro.
En general no se ha detectado el MTBE en las aguas subterráneas
profundas o cercanas a la superficie en zonas agrícolas. Cuando se ha
detectado, la concentración era inferior a 2,0 µg/litro. El MTBE se
halla con más frecuencia en las aguas subterráneas cercanas a la
superficie (1,6 a 3,2 metros de estos acuíferos) de las zonas urbanas.
En este entorno, las concentraciones van de menos de 0,2 µg/litro a 2
mg/litro, con un valor mediano inferior a 0,2 µg/litro.
El MTBE se halla poco frecuentemente en sistemas de
abastecimiento público de agua procedente de capas freáticas. Entre 51
sistemas estudiados, en 48 la concentración era de <20 µg/litro.
Son insuficientes los datos disponibles para caracterizar la
concentración de MTBE en los sistemas de abastecimiento público de
agua procedentes de aguas superficiales. El MTBE se ha hallado en
concentraciones altas (esto es, >1000 µg/litro) en algunos pozos
privados utilizados para obtener agua de beber. Sin embargo, es dudoso
que las personas puedan consumir agua con concentraciones de MTBE
superiores a unos 50-100 µg/litro debido al bajo umbral de su gusto y
olor.
Entre los trabajadores con posible exposición al MTBE figuran los
ocupados en la producción, distribución y uso de MTBE y de gasolina
con MTBE, que incluye el personal de estaciones de servicio y los
mecánicos.
La exposición a corto plazo (<30 min) en operaciones corrientes
de fabricación y mantenimiento de MTBE puro iba de 715 a 43 000 µg/m3
(0,2 a 12 ppm), siendo el promedio de los valores medianos de
3400 µg/m3 aproximadamente (0,95 ppm). La exposición a largo plazo
(30 min a 8 h) era de 360 a 890 000 µg/m3 (0,01 ppm a 250 ppm), con
valores medianos de aproximadamente 540 µg/m3 (0,15 ppm). En el caso
de los trabajadores de operaciones de mezclado, los valores a corto
plazo oscilaban entre niveles indetectables y 360 000 µg/m3
(100 ppm), siendo el promedio de los valores medianos de 5700 µg/m3
aproximadamente (1,6 ppm). Los valores a largo plazo comprendían desde
niveles indetectables hasta 257 000 µg/m3 (72 ppm), siendo el
promedio de los valores medianos de 2000 µg/m3 aproximadamente
(0,6 ppm).
La exposición alcanzó el nivel máximo durante el transporte de
MTBE puro y de mezclas de combustible en oleoductos, barcazas, vagones
de ferrocarril y camiones (sólo MTBE puro), variando los valores a
corto plazo entre 4 y 3750 mg/m3 (0,001 a 1050 ppm), con un promedio
de los valores medianos de 140 mg/m3 (39 ppm). Los valores a largo
plazo fueron de 0,036 a 2540 mg/m3 (0,01 a 712 ppm), con un promedio
de los valores medianos de 2,85 mg/m3 (0,8 ppm). En las operaciones
de distribución (esto es, carga de mezclas de combustible y MTBE en
camiones y entrega y descarga en las estaciones de servicio), los
valores a corto plazo oscilaron entre niveles indetectables y
225 mg/m3 (63 ppm), siendo el promedio de los valores medianos de
21 mg/m3 aproximadamente (6 ppm). Los valores a largo plazo fueron de
0,036 a 22 mg/m3 (0,01 a 6,2 ppm), siendo el promedio de los valores
medianos de 1,79 mg/m3 (0,5 ppm).
Los valores medianos de la exposición a corto plazo de operarios
de estaciones de servicio fueron en general de 1,071 a 21,42 mg/m3
(0,3 a 6 ppm), excediendo rara vez de 35,7 mg/m3 (10 ppm). Los
valores medianos de la exposición a largo plazo en operarios de
estaciones de servicio presentaron un promedio de 1,79 mg/m3
(0,5 ppm). Los valores medianos de la exposición de mecánicos estaban
por debajo de los niveles de detección en un estudio a corto plazo; el
promedio de los valores medianos para la exposición a largo plazo fue
aproximadamente de 360 µg/m3 (0,1 ppm).
5. Cinética y metabolismo
Los datos toxicocinéticos sobre el MTBE en personas proceden
principalmente de estudios controlados en voluntarios adultos sanos y
en una población expuesta a la gasolina oxigenada. El MTBE pasa
rápidamente a la circulación después de la exposición por inhalación.
En voluntarios sanos expuestos a la inhalación, la cinética del MTBE
era lineal hasta concentraciones de 268 mg/m3 (75 ppm). Se midió en
la sangre y orina de personas expuestas el alcohol
terciario-butílico, metabolito del MTBE. Las concentraciones
sanguíneas máximas del MTBE y el alcohol terciario-butílico fueron
de 17,2 a 1144 µg/m3 y de 7,8 a 925 µg/m3, respectivamente, en
personas expuestas a 5,0 a 178,5 mg/m3 (1,4 a 50 ppm) de MTBE.
Basándose en un modelo de monocompartimiento se identificaron
componentes rápidos (36- 90 min) y lentos (19 h) de la semivida del
MTBE.
En los roedores, el MTBE se absorbe y distribuye bien después de
la administración oral y la exposición por inhalación, con menor
absorción cutánea. En la administración oral de 400 mg/kg y en la
inhalación de 28 800 mg/m3 (8000 ppm) aumentó el porcentaje de la
dosis absorbida total eliminado en el aire espirado, con un descenso
correspondiente del porcentaje eliminado por la orina, indicando la
saturación metabólica. No se identificó la presencia de alcohol
terciario-butílico (TBA) en la orina de ratas expuestas. Hubo
indicios de un metabolismo adicional del TBA, basados en la
identificación de 2-metil-1,2-propanodiol y de ácido
alpha-hidroxiisobutírico eliminado por la orina. Los estudios
in vitro prueban que el MTBE se metaboliza hasta TBA, formaldehido y
acetona.
6. Efectos en los animales de laboratorio y en los sistemas
in vitro
En las ratas, la dosis letal oral mediana aguda (DL50) es
aproximadamente de 3800 mg/kg de peso corporal. La concentración letal
mediana aguda (CL50) para una exposición por inhalación de 15 minutos
es de aproximadamente 141 000 mg/m3 de aire en ratones. Entre los
signos de intoxicación figuran la depresión del SNC, la ataxia y la
respiración laboriosa. Si la dosis no es letal, la recuperación es
completa. La DL50 para la toxicidad cutánea en conejos es de
>10 200 mg/kg de peso corporal.
En un solo estudio identificado, el MTBE resultó "moderadamente"
irritante para la piel, produciendo eritema moderado y edema después
de la aplicación cutánea en conejos. También resultó irritante para
los ojos de los conejos, produciendo lesiones leves y reversibles. En
el único estudio identificado, el MTBE produjo irritación respiratoria
ligera a intensa después de la exposición de ratones a 300 y
30 000 mg/m3, respectivamente. No causó sensibilización cutánea en
estudios en cobayos.
La exposición repetida produce fundamentalmente aumentos del peso
de los órganos y lesiones histopatológicas en el riñón de ratas y en
el hígado de ratones. Los niveles de mínimo efecto señalado de
nefrotoxicidad tras la ingestión en estudios de 90 días fueron de
400 mg/kg de peso corporal por día (aumentos del peso renal relativo y
formación de gotas hialinas en ratas Sprague-Dawley). En la exposición
por inhalación a 2880 mg/m3 (800 ppm) se produjeron aumentos del peso
del riñón asociados a las concentraciones más altas, con moderado
aumento de las gotas hialinas en los túbulos proximales en ratas
Fischer-344. En estudios de oncogenicidad por inhalación en dosis de
1440 mg/m3 (400 ppm), la incidencia y la gravedad de la nefropatía
progresiva crónica aumentó en ratas machos; en esta concentración, en
ratones machos se observó un aumento del peso absoluto del hígado (que
guardaba correlación con el aumento de la hipertrofia hepatocelular en
concentraciones superiores) y un aumento del peso renal relativo.
La exposición al MTBE también produjo lesiones reversibles del
sistema nervioso central (SNC), incluidas sedación, hipoactividad,
ataxia y anestesia en concentraciones superiores y efectos bifásicos
sobre la actividad motriz en concentraciones inferiores. En un estudio
de una sola exposición de 6 horas en ratas, las concentraciones de
2880 mg/m3 (800 ppm) produjeron cambios reversibles de la actividad
motriz relacionados con la dosis en sexos separados. Esos efectos
fueron transitorios y no se pusieron de manifiesto en estudios a largo
plazo.
Se han efectuado estudios reproductivos por inhalación de una y
dos generaciones y estudios de cuatro generaciones en ratas, ratones y
conejos. En esos estudios no se hallaron efectos reproductivos
específicos en ratas en concentraciones de hasta 28 800 mg/m3. El
MTBE no ha producido efectos en el desarrollo en concentraciones
inferiores a las que resultaron tóxicas en las madres. Se han
observado disminuciones del peso del útero y aumentos del metabolismo
estrogénico en ratonas con dosis de 28 000 mg/m3.
El MTBE ha sido sometido a pruebas apropiadas en una amplia gama
de ensayos de mutagenicidad y de genotoxicidad. Los resultados
muestran que el MTBE no es genotóxico, aunque resultó positiva una
prueba de mutación del locus tk en células linfomatosas de ratón
debido al paso metabólico de MTBE a formaldehido.
Se han realizado estudios de carcinogenicidad que comprendieron
la exposición por inhalación de ratas Fischer-344 y de ratones CD-1 y
el cebado de ratas Sprague-Dawley. En ninguno de los dos estudios de
inhalación se utilizaron métodos de análisis estadístico que
efectuaran el reajuste de las diferencias de supervivencia. En los
tres estudios se produjeron aumentos significativos de la incidencia
de tumores, esto es, tumores de células tubulares renales y tumores de
células de Leydig en ratas Fischer-344 machos, tumores de células de
Leydig en ratas Sprague-Dawley machos y linfomas-leucemias
(combinadas) en ratas hembras de la misma especie, y tumores de
células hepáticas en ratones CD-1 hembras. Así pues, no se observaron
constantemente tumores de células tubulares renales ni
leucemias-linfomas en los distintos estudios en ratas. Además, los
tumores renales específicos del sexo se asociaron a la nefropatía de
la alpha2u-globulina específica del sexo, observada en varios estudios
de breve duración. Se observaron aumentos de los tumores de células de
Leydig con la dosis más alta (1000 mg/kg de peso corporal) en la ratas
Sprague-Dawley, pero la interpretación de los aumentos registrados en
las ratas Fischer-344 resultó compleja por las incidencias muy altas
concurrentes y de los testigos históricos. En los ratones, las
incidencias de los tumores hepáticos fueron en los testigos y en los
grupos expuestos a 28 800 mg/m3 (8000 ppm), respectivamente, de 2/50
y 10/50 en las hembras y de 12/49 y 16/49 en los machos. Los aumentos
fueron moderados y acompañados de hipertrofia hepatocelular.
7. Efectos en el ser humano
Tras la introducción de dos programas separados relativos a los
combustibles en los EE.UU., que requieren el empleo de productos de
oxigenación de la gasolina (no necesariamente MTBE), los consumidores
de algunas zonas se han quejado de trastornos agudos de la salud, como
dolor de cabeza, irritación de los ojos y la nariz, tos, náuseas,
mareos y desorientación. Los estudios epidemiológicos de poblaciones
humanas expuestas en condiciones profesionales o no profesionales, así
como los estudios experimentales de voluntarios expuestos en
condiciones controladas, no han podido identificar la base de esos
trastornos. Aunque los resultados son variados, los estudios
comunitarios efectuados en Alaska, New Jersey, Connecticut y Wisconsin
(EE.UU.) no han proporcionado indicios, o éstos han sido limitados, de
la asociación entre la exposición al MTBE y la prevalencia de
trastornos de la salud.
En estudios experimentales controlados en voluntarios humanos
expuestos en cámaras de inhalación al MTBE en concentraciones de 5,0
mg/m3 (1,4 ppm) a 270 mg/m3 (75 ppm) no hubo efectos manifiestos en
términos de presencia subjetiva de síntomas o de indicadores objetivos
de irritación u otros efectos en concentraciones de hasta 180 mg/m3
(50 ppm) durante dos horas. Partiendo de esos datos parece improbable
que el MTBE por sí solo produzca efectos agudos adversos en la salud
en la población general en las condiciones corrientes de exposición
por inhalación. Sin embargo, los posibles efectos de las mezclas de
gasolina y MTBE y el modo de exposición de la mayor parte de las
personas al MTBE en asociación con el empleo de combustibles
oxigenados, no se han examinado experimentalmente ni por métodos
epidemiológicos prospectivos. Por otra parte, no se ha investigado,
por ejemplo, la función de factores tales como la percepción del MTBE,
debida en parte a su olor distintivo.
8. Efectos en otros organismos en el laboratorio y sobre el
terreno
La toxicidad aguda experimental (CL50) del MTBE en los peces,
los anfibios y los crustáceos es >100 mg/litro. No existen datos
sobre la toxicidad crónica o subletal para las especies acuáticas ni
la toxicidad para los organismos terrestres.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
Basándose en datos de observación colectiva, parece improbable
que el MTBE por sí solo induzca efectos agudos adversos en la salud de
la población general en las condiciones corrientes de exposición.
En estudios en animales, el MTBE es "moderadamente" tóxico en
forma aguda y produce irritación cutánea y ocular moderada, pero no
sensibilización. La exposición repetida afecta fundamentalmente al
riñón de ratas y al hígado de ratones, observándose los efectos
adversos mínimos con concentraciones de 440 mg/kg de peso corporal por
día en ratas después de la ingestión y de 1400 mg/m3 (400 ppm)
después de la inhalación. El MTBE no ha inducido efectos adversos en
la reproducción o el desarrollo en concentraciones inferiores a las
que eran tóxicas para los padres.
El MTBE no es genotóxico, pero ha producido tumores en roedores,
principalmente con concentraciones altas, que también inducen otros
efectos adversos. Esos datos se consideran en la actualidad
insuficientes para la evaluación del riesgo carcinogénico en seres
humanos. El Grupo Especial llegó a la conclusión de que para
proporcionar orientación cuantitativa sobre los límites pertinentes de
exposición y para estimar el riesgo se necesita adquirir datos
adicionales en distintos sectores.
No parece que las concentraciones de MTBE en el agua ambiental
sean tóxicas para los organismos acuáticos, excepto en caso de
escapes. Aunque no hay datos sobre la toxicidad terrestre del MTBE,
parece que no es preocupante ya que las concentraciones en el aire
ambiental son bajas y la semivida del MTBE es relativamente breve.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL TERTIARY-
BUTYL ETHER
Members
Dr R. B. Beems, National Institute of Public Health & the Environment,
Bilthoven, The Netherlands
Dr A. Bobra, Environment Canada, Hull, Quebec, Canada
Dr S. Borghoff, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr J.M. Davis, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, North
Carolina, USA (Vice-Chairman)
Dr L. Fishbein, Fairfax, Virginia, USA
Dr M. Gillner, National Chemicals Inspectorate, Solna, Sweden
(Co-Rapporteur)
Mr G. Long, Environmental Health Centre, Health Canada, Ottawa, Canada
(Co-Rapporteur)
Dr M.E. Meek, Environmental Health Centre, Health and Welfare Canada,
Ottawa, Canada (Chairman)
Dr A.A.E. Wibowo, Coronel Institute, University of Amsterdam,
Amsterdam, The Netherlands
Observers
Dr M. Constantini, Health Effects Institute, Cambridge, Massachusetts,
USA (representing the Health Effects Institute (HEI))
Dr J. Del Pup, Texaco Inc., New York, USA (representing the American
Industrial Health Council (AIHC))
Mr R. Hillier, Oil, Chemical and Atomic Workers' International Union
(OCAWIU), Lakewood, Colorado, USA (representing the International
Federation of Chemical, Energy, Mine and General Workers' Unions
(ICEM))
Dr A.K. Mallett, Arco Chemical Europe Inc., Maidenhead, United Kingdom
(representing the European Centre for Ecotoxicology and Toxicology of
Chemicals (ECETOC))
Dr M. Mehlman, Princeton, New Jersey, USA (Technical Adviser to Mr
Hillier, OCAWIU)
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International Union of
Pharmacology (IUPHAR))
Dr J. Zogorski, US Department of the Interior, Rapid City, South
Dakota, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, World Health
Organization, Lyon, France
Assisting the Secretariat
Miss C. Grande, Air Issues Section, Health Canada, Ottawa, Canada
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL
TERTIARY-BUTYL ETHER (MTBE)
A WHO Task Group on Environmental Health Criteria for methyl
tertiary-butyl ether met at the Conference Facility, Lord Elgin
Hotel, Ottawa, Canada from 17 to 21 April 1997. Dr E.M. Smith, IPCS,
welcomed the participants on behalf of Dr M. Mercier, Director of the
IPCS, and the three IPCS cooperating organizations (UNEP/ILO/ WHO).
The Group reviewed and revised the draft and made an evaluation of the
risks for human health and the environment from exposure to methyl
tertiary-butyl ether.
The first draft of the EHC was prepared by Dr M. Gillner,
National Chemicals Inspectorate, Solna, Sweden, with contributions
from Ms A.-S. Nihlén, Institute for Working Life, Solna, Sweden. Dr M.
Gillner and Ms Nihlén also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS contact points for Environmental Health Criteria monographs.
Dr E.M. Smith and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The financial support of the Swedish National Chemicals
Inspectorate in preparing the monograph and the Canadian Health
Protection Branch, Environmental Health Directorate, in funding the
Task Group meeting in Ottawa are gratefully acknowledged.
ABBREVIATIONS
AED atomic emission detector
ALAT alanine aminotransferase
AP alkaline phosphatase
AUC area under the curve
BCF bioconcentration factor
BTEX benzene, toluene, ethyl benzene and xylenes
BUN blood urea nitrogen
bw body weight
CHOL cholesterol
CL total plasma clearance
CNS central nervous system
CO carbon monoxide
DEN diethylnitrosamine
DIPE diisopropyl ether
DMN N-nitrosodimethylamine
EC electron capture
EROD 7-ethoxyresorufin- O-deethylase
ETBE ethyl tertiary-butyl ether
FID flame ionization detector
FOB functional observational battery
FTIR Fourier-transform infrared
GC gas-chromatography
GC-MS gas-chromatography/mass spectrometry
GC-O gas-chromatography using an oxygen-selective detector
Hb haemoglobin
HC hydrocarbon
HPLC high-performance liquid chromatography
HPRT hypoxanthine-guanine phosphoribosyl transferase
IL-1 interleukin-1
IL-4 interleukin-4
ip intraperitoneal
IR infrared
iv intravenous
Koc adsorbtion coefficient to soil organic carbon
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
LGL large granular lymphocyte
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LT50 median lethal time
MCH mean corpuscular haemoglobin
MCHC mean corpuscular haemoglobin concentration
MCS multiple chemical sensitivities
MCV mean corpuscular volume
MTBE methyl tertiary-butyl ether
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIR near infrared
NMOC non-methane organic carbon
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NOx oxides of nitrogen (NO, NO2, N2O4 and N2O3)
PID photoionization detector
ppb parts per billion
ppbv parts per billion (by volume)
ppm parts per million
PROD 7-pentoxyresorufin- O-dealkylase
RBC red blood cell
RFG reformulated gasoline
RID refractive index detector
RPLC reversed-phase liquid chromatography
sc subcutaneous
SCE sister chromatid exchange
SD standard deviation
TBA tertiary-butyl alcohol
TBF tertiary-butyl formate
TWA time-weighted average
UDS unscheduled DNA synthesis
V/F distribution volume
VOC volatile organic compound
1. SUMMARY
Methyl tertiary-butyl ether (MTBE) is one of several ethers
that may be used as fuel additives and is currently by far the
dominant one. Ethyl tertiary-butyl ether (ETBE), tertiary-amyl
methyl ether (TAME), tertiary-amyl ethyl ether (TAEE) and
diisopropyl ether (DIPE), among others, may supplement, or serve as
alternatives to MTBE for oxygenation or octane enhancement purposes
and may be found, therefore, in association with MTBE.
1.1 Identity, physical and chemical properties, analytical methods
MTBE is a volatile, colourless liquid at room temperature with a
terpene-like odour. It has low viscosity and a boiling point of
55.2°C. The freezing point is -109°C. The density is 0.7404 at 20°C.
The vapour pressure is relatively high, 33 500 Pa at 25°C. MTBE is
flammable and can form explosive mixtures with air. It is very soluble
in other ethers and alcohol. It mixes with gasoline (petrol), and is
soluble in water (42 000 g/m3 at 19.8°C). The log n-octanol/water
partition coefficient is 0.94-1.3. It is unstable in acid solution.
MTBE is analysed in all matrices generally by gas chromatography
(GC) using a range of capillary columns and detector systems that are
suited to the specific matrix. Reverse-phase liquid chromatography
(RPLC) has also been used for analysis of petrol samples.
Sorption/desorption, including purge and trap systems, and headspace
procedures have been used to prepare air, water, sediment and
biological samples for analysis.
1.2 Sources of human and environmental exposure
MTBE is not known to occur naturally in the environment.
Industrially, it is derived from the catalytic reaction of methanol
and isobutylene, and has been produced in several countries in
increasing volumes since the late 1970s. MTBE is currently among the
50 highest production volume chemicals. In 1996, the USA capacity for
production was approximately 10.6 million tonnes, and it is
anticipated that the use of MTBE will continue to increase.
Approximately 25% of gasoline in the USA is blended with MTBE. MTBE is
almost exclusively used to provide both octane enhancement and an
increase in the oxygen content of gasoline. MTBE has been added to
gasoline in concentrations up to 17% by volume.
1.3 Environmental transport, distribution and transformation
After discharge into air, MTBE will largely remain in the air,
with smaller amounts entering soil and water. In the atmosphere, MTBE
can partition into rain. However, only a small amount is removed from
the atmosphere in this manner. Atmospheric transformation by hydroxyl
radicals produces a number of products including the photochemically
stable tertiary-butyl formate (TBF) and 2-methoxy-2-methylpropanol,
which is expected to be highly reactive with hydroxyl radicals,
yielding CO2, formaldehyde, acetone and water. When MTBE is
discharged into water, a significant amount is dissolved, with some
partitioning into air. Partitioning into biota and into sediment is
low. Biodegradability in conventional assays is limited. Generally,
biodegradability is believed to be slow in the environment. When MTBE
is released to the soil, it is transported to the air through
volatilization, to surface water through run-off and to groundwater as
a result of leaching. MTBE can persist in groundwater.
1.4 Environmental levels and human exposure
There are few data on environmental levels and human exposure.
In studies of MTBE in urban air of some cities using oxygenated
gasoline with 15% MTBE, ambient concentrations ranged from
non-detectable to 100.9 µg/m3 (0.028 ppm), with several median
concentrations ranging from 0.47 to 14.4 µg/m3 (0.00013 to 0.004
ppm). Concentrations of MTBE in urban air of some cities where MTBE
was used as an octane enhancer at lower concentrations ranged from
non-detectable to 26.4 µg/m3 (0.0073 ppm).
Concentrations at ground level or near refineries ranged from 15
to 281 µg/m3. Median levels in urban air near blending facilities
were 1508 µg/m3 (0.419 ppm), with ranges of 216-35 615 µg/m3 (0.06
to 9.8 ppm).
At service stations in areas where oxygenated gasoline containing
10-15% MTBE is used, concentrations were highest in the breathing zone
during consumer refuelling (range of 300 to 136 000 µg/m3 (0.09 to 38
ppm), with levels rarely exceeding 3600 µg/m3 (10 ppm), slightly
lower at the pump island (non-detectable to 5700 µg/m3 (1.6 ppm) and
lowest at the station perimeter (non-detectable to 500 µg/m3 (0.14
ppm). Levels were generally higher at service stations without vapour
recovery systems.
Levels in the automobile cabin were 7 to 60 µg/m3 (0.002 to
0.017 ppm) during commutes and 20 to 610 µg/m3 (0.006 to 0.172 ppm)
during refuelling.
Based on limited monitoring confined almost exclusively to the
USA, MTBE has been detected in snow, stormwater, surface water
(streams, rivers, and reservoirs), groundwater and drinking-water.
Concentrations of MTBE detected in stormwater ranged from 0.2 to 8.7
µg/litre with a median of less than 1.0 µg/litre. For streams, rivers
and reservoirs, the range of detection was from 0.2 to 30 µg/litre,
and the range of medians for several studies was 0.24 to 7.75
µg/litre.
MTBE has generally not been detected in deeper groundwater or in
shallow groundwater in agricultural areas. When detected, the
concentration is less than 2.0 µg/litre. MTBE is more frequently found
in shallow groundwater (top 5-10 feet of these aquifers) in urban
areas. In this setting, the concentrations range from less than 0.2
µg/litre to 23 mg/litre, with a median value below 0.2 µg/litre.
MTBE is infrequently detected in public drinking-water systems
from groundwater. In all but 3 out of 51 systems in which it was
reported, the concentration was <20 µg/litre. There are inadequate
data to characterize the concentration of MTBE in public
drinking-water systems from surface water. MTBE has been found at high
levels (i.e. >1000 µg/litre) in a few private wells used for
drinking-water. However, it is doubtful that humans would consume
water with concentrations of MTBE greater than about 50-100 µg/litre
because of its low taste and odour threshold.
Workers with potential exposure to MTBE include those involved in
the production and distribution and use of MTBE and MTBE-containing
gasoline, including service station attendants and mechanics.
Short-term exposure (<30 min) in routine manufacturing
operations and maintenance of neat MTBE ranged from 715 to 43 000
µg/m3 (0.2 to 12 ppm), with average median values being about 3400
µg/m3 (0.95 ppm). Longer-term (30 min to 8 h) exposure ranged from
360 to 890 000 µg/m3 (0.01 ppm to 250 ppm), with median levels being
about 540 µg/m3 (0.15 ppm). For workers in blending operations,
short-term values ranged from non-detectable to 360 000 µg/m3 (100
ppm), the average median being about 5700 µg/m3 (1.6 ppm). Long-term
values ranged from non-detectable to 257 000 µg/m3 (72 ppm), the
average median being about 2000 µg/m3 (0.6 ppm).
Exposures were highest during transportation of neat MTBE and
fuel mixtures through pipelines, barges, railroad cars and trucks
(neat MTBE only), short-term values ranging from 4 to 3750 mg/m3
(0.001 to 1050 ppm) with an average median value of 140 mg/m3 (39
ppm). Long-term values ranged from 0.036 to 2540 mg/m3 (0.01 to 712
ppm), the average median value being 2.85 mg/m3 (0.8 ppm). In
distribution (i.e. loading of MTBE fuel mixtures on trucks and
delivering and unloading at service stations), short-term values
ranged from non-detectable to 225 mg/m3 (63 ppm), the average median
values being around 21 mg/m3 (6 ppm). Long-term values ranged from
0.036 to 22 mg/m3 (0.01 to 6.2 ppm), the average median value being
1.79 mg/m3 (0.5 ppm).
Median short-term exposure levels of service station attendants
ranged generally from 1.071 to 21.42 mg/m3 (0.3 to 6 ppm) and rarely
exceeded 35.7 mg/m3 (10 ppm). Median long-term exposure levels of
service station attendants averaged 1.79 mg/m3 (0.5 ppm). Median
exposures of mechanics were below detection levels for one short-term
study; the average median value for long-term exposure was
approximately 360 µg/m3 (0.1 ppm).
1.5 Kinetics and metabolism
Toxicokinetic data on MTBE in humans are mainly derived from
controlled studies in healthy adult volunteers and in a population
exposed to oxygenated gasoline. MTBE is rapidly absorbed into the
circulation following inhalation exposure. In healthy human volunteers
exposed by inhalation, kinetics of MTBE are linear up to
concentrations of 268 mg/m3 (75 ppm). Tertiary-butyl alcohol (TBA),
a metabolite of MTBE, was measured in blood and urine of exposed
humans. The peak blood levels of MTBE and TBA ranged from 17.2 to 1144
µg/litre, and 7.8 to 925 µg/litre, respectively, in humans exposed to
5.0 to 178.5 mg/m3 (1.4 to 50 ppm) MTBE. Based on a monocompartmental
model, rapid (36-90 min) and slower (19 h) components of MTBE
half-life have been identified.
In rodents, MTBE is well absorbed and distributed following oral
administration and inhalation exposure, with lower dermal absorption.
At 400 mg/kg oral and 28 800 mg/m3 (8000 ppm) inhalation exposure,
the percentage of total absorbed dose eliminated in expired air
increased with a corresponding decrease in the percentage eliminated
in urine, indicating a saturation of metabolism. TBA was not
identified in the urine of exposed rats. There is evidence of further
metabolism of TBA, based on the identification of
2-methyl-1,2-propanediol and alpha-hydroxyisobutyric acid excreted in
the urine. In vitro studies provide evidence that MTBE is
metabolized to TBA, formaldehyde and acetone.
1.6 Effects on laboratory animals and in vitro systems
In rats, the acute median oral lethal dose (LD50) is
approximately 3800 mg/kg bw. The acute median lethal concentration
(LC50) value for a 15-min inhalation exposure is about 141 000 mg/m3
air in mice. Signs of intoxication include CNS depression, ataxia and
laboured respiration. When the dose was non-lethal, recovery was
complete. The LD50 for dermal toxicity in rabbits is >10 200 mg/kg
bw.
In a single identified study, MTBE was "moderately" irritating to
skin, causing moderate erythema and oedema following dermal
application to rabbits. It was also irritating to the eyes of rabbits,
causing mild, reversible changes. In the only identified study, MTBE
induced slight to severe respiratory irritation following exposure of
mice to 300 to 30 000 mg/m3, respectively. It did not induce skin
sensitization in studies in guinea-pigs.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Lowest reported effect levels for nephrotoxicity following
ingestion in 90-day studies are 440 mg/kg bw per day (increases in
relative kidney weight and hyaline droplet formation in Sprague-Dawley
rats). With inhalation exposure to 2880 mg/m3 (800 ppm), there were
increases in kidney weight associated at higher concentrations with a
mild increase in hyaline droplets in the proximal tubules in
Fischer-344 rats. In inhalation oncogenicity studies, at 1440 mg/m3
(400 ppm) the incidence and severity of chronic progressive
nephropathy was increased in male rats; in male mice, at this level,
there were increases in absolute liver weight (which correlated with
increases in hepatocellular hypertrophy at higher concentrations) and
an increase in relative kidney weight.
Exposure to MTBE also results in reversible central nervous
system (CNS) effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2880 mg/m3 (800 ppm) produced
reversible dose-related changes in motor activity in single sexes.
These effects were transient and not evident in longer-term studies.
One- and two-generation inhalation reproductive studies in rats
and four developmental studies in rats, mice and rabbits have been
identified. In these studies, specific reproductive effects were not
observed in rats at concentrations up to 28 800 mg/m3. MTBE has not
induced developmental effects at concentrations below those that were
toxic to the mothers. Decreases in uterine weight and increases in
estrogen metabolism in mice have been observed at 28 800 mg/m3.
MTBE has been adequately tested in a broad range of mutagenicity
and other genotoxicity tests. The results from these studies indicate
that MTBE is not genotoxic, although a mouse lymphoma cell tk locus
mutation assay was positive, due to the metabolism of MTBE to
formaldehyde.
Carcinogenicity studies have been conducted involving inhalation
exposure of Fischer-344 rats and CD-1 mice and gavage dosing of
Sprague-Dawley rats. In neither of the inhalation studies were methods
of statistical analysis used that adjusted for survival differences.
There were significant increases in tumour incidence in all three
studies, namely renal tubular cell tumours and Leydig cell tumours in
the male Fischer-344 rats, Leydig cell tumours in male and
leukaemias/lymphomas (combined) in female Sprague-Dawley rats, and
liver cell tumours in female CD-1 mice. The renal tubular cell tumours
and the leukaemia/lymphomas were not observed consistently, therefore,
in the different studies in rats. In addition, the sex-specific kidney
tumours were associated with sex-specific alpha2u-globulin
nephropathy, which was observed in several studies of short duration.
Increases in Leydig cell tumours occurred at the highest dose level
(1000 mg/kg bw) in the Sprague-Dawley rats, but interpretation of the
increases recorded for Fischer-344 rats was complicated by the very
high concurrent and historical control incidences. The mouse liver
tumours occurred at incidences in the control and 28 800 mg/m3 (8000
ppm exposed groups, respectively, of 2/50 and 10/50 in females and
12/49 and 16/49 in males. The increases were modest and were
accompanied by hepatocellular hypertrophy.
1.7 Effects on humans
Following the introduction of two separate fuel programmes in the
USA requiring the use of gasoline oxygenates (not necessarily MTBE),
consumers in some areas have complained about acute health symptoms
such as headache, eye and nose irritation, cough, nausea, dizziness
and disorientation. Epidemiological studies of human populations
exposed under occupational as well as non-occupational conditions, and
experimental studies of human volunteers exposed under controlled
conditions, have not been able to identify a basis for these
complaints. Although results are mixed, community studies conducted in
Alaska, New Jersey, Connecticut, and Wisconsin, USA, have provided
limited or no evidence of an association between MTBE exposure and the
prevalence of health complaints.
In controlled experimental studies on adult volunteers exposed in
inhalation chambers to MTBE at concentrations ranging from 5.0 mg/m3
(1.4 ppm) up to 270 mg/m3 (75 ppm), there were no evident effects in
terms of either subjective reports of symptoms or objective indicators
of irritation or other effects up to 180 mg/m3 (50 ppm) for as long
as 2 h. From this evidence it appears unlikely that MTBE alone induces
adverse acute health effects in the general population under common
conditions of inhalation exposure. However, the potential effects of
mixtures of gasoline and MTBE, and the manner in which most persons
are exposed to MTBE in conjunction with the use of oxygenated fuels,
have not been examined experimentally or through prospective
epidemiological methods. Moreover, the role of factors such as
awareness of MTBE, due in part to its distinctive odour, for example,
have not been investigated.
1.8 Effects on other organisms in the laboratory and field
The experimental acute toxicity (LC50) of MTBE to fish,
amphibians and crustaceans is > 100 mg/litre. There are no data on
chronic or sub-lethal toxicity to aquatic species, or on toxicity to
terrestrial organisms.
1.9 Evaluation of human health risks and effects on the environment
Based on collective evidence, it appears unlikely that MTBE alone
induces adverse acute health effects in the general population under
common exposure conditions.
In studies on animals, MTBE is "moderately" acutely toxic and
induces mild skin and eye irritation but not sensitization. Repeated
exposure affects primarily the kidney of rats and the liver of mice,
with lowest reported adverse effect levels of 440 mg/kg bw per day in
rats following ingestion and 1440 mg/m3 (400 ppm) following
inhalation. MTBE has not induced adverse reproductive or developmental
effects at concentrations less than those that were toxic to the
parents.
MTBE is not genotoxic but has induced tumours in rodents
primarily at high concentrations that also induce other adverse
effects. These data are considered currently inadequate for use in
human carcinogenic risk assessment. The Task Group concluded that, in
order to provide quantitative guidance on relevant limits of exposure
and to estimate risk, acquisition of additional data in several areas
is necessary.
It does not appear that the concentrations of MTBE in ambient
water are toxic to aquatic organisms except during spills. Although
there are no data on the terrestrial toxicity of MTBE, this appears
not to be of concern since concentrations in ambient air are low and
its half-life is relatively short.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C5H12O
Chemical structure: CH3
'
H3C - C - O - CH3
'
CH3
Relative molecular mass: 88.15
Common name: methyl tertiary-butyl ether
IUPAC Chemical name: 2-methoxy-2-methyl propane
CAS registry number:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL TERTIARY-
BUTYL ETHER
Members
Dr R. B. Beems, National Institute of Public Health & the Environment,
Bilthoven, The Netherlands
Dr A. Bobra, Environment Canada, Hull, Quebec, Canada
Dr S. Borghoff, Chemical Industry Institute of Toxicology, Research
Triangle Park, North Carolina, USA
Dr J.M. Davis, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, North
Carolina, USA (Vice-Chairman)
Dr L. Fishbein, Fairfax, Virginia, USA
Dr M. Gillner, National Chemicals Inspectorate, Solna, Sweden
(Co-Rapporteur)
Mr G. Long, Environmental Health Centre, Health Canada, Ottawa, Canada
(Co-Rapporteur)
Dr M.E. Meek, Environmental Health Centre, Health and Welfare Canada,
Ottawa, Canada (Chairman)
Dr A.A.E. Wibowo, Coronel Institute, University of Amsterdam,
Amsterdam, The Netherlands
Observers
Dr M. Constantini, Health Effects Institute, Cambridge, Massachusetts,
USA (representing the Health Effects Institute (HEI))
Dr J. Del Pup, Texaco Inc., New York, USA (representing the American
Industrial Health Council (AIHC))
Mr R. Hillier, Oil, Chemical and Atomic Workers' International Union
(OCAWIU), Lakewood, Colorado, USA (representing the International
Federation of Chemical, Energy, Mine and General Workers' Unions
(ICEM))
Dr A.K. Mallett, Arco Chemical Europe Inc., Maidenhead, United Kingdom
(representing the European Centre for Ecotoxicology and Toxicology of
Chemicals (ECETOC))
Dr M. Mehlman, Princeton, New Jersey, USA (Technical Adviser to Mr
Hillier, OCAWIU)
Dr P. Montuschi, Department of Pharmacology, Catholic University of
the Sacred Heart, Rome, Italy (representing the International Union of
Pharmacology (IUPHAR))
Dr J. Zogorski, US Department of the Interior, Rapid City, South
Dakota, USA
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr D. McGregor, Unit of Carcinogen Identification and Evaluation,
International Agency for Research on Cancer, World Health
Organization, Lyon, France
Assisting the Secretariat
Miss C. Grande, Air Issues Section, Health Canada, Ottawa, Canada
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL
TERTIARY-BUTYL ETHER (MTBE)
A WHO Task Group on Environmental Health Criteria for methyl
tertiary-butyl ether met at the Conference Facility, Lord Elgin
Hotel, Ottawa, Canada from 17 to 21 April 1997. Dr E.M. Smith, IPCS,
welcomed the participants on behalf of Dr M. Mercier, Director of the
IPCS, and the three IPCS cooperating organizations (UNEP/ILO/ WHO).
The Group reviewed and revised the draft and made an evaluation of the
risks for human health and the environment from exposure to methyl
tertiary-butyl ether.
The first draft of the EHC was prepared by Dr M. Gillner,
National Chemicals Inspectorate, Solna, Sweden, with contributions
from Ms A.-S. Nihlén, Institute for Working Life, Solna, Sweden. Dr M.
Gillner and Ms Nihlén also prepared the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS contact points for Environmental Health Criteria monographs.
Dr E.M. Smith and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific aspects of the monograph and for
the technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
The financial support of the Swedish National Chemicals
Inspectorate in preparing the monograph and the Canadian Health
Protection Branch, Environmental Health Directorate, in funding the
Task Group meeting in Ottawa are gratefully acknowledged.
ABBREVIATIONS
AED atomic emission detector
ALAT alanine aminotransferase
AP alkaline phosphatase
AUC area under the curve
BCF bioconcentration factor
BTEX benzene, toluene, ethyl benzene and xylenes
BUN blood urea nitrogen
bw body weight
CHOL cholesterol
CL total plasma clearance
CNS central nervous system
CO carbon monoxide
DEN diethylnitrosamine
DIPE diisopropyl ether
DMN N-nitrosodimethylamine
EC electron capture
EROD 7-ethoxyresorufin- O-deethylase
ETBE ethyl tertiary-butyl ether
FID flame ionization detector
FOB functional observational battery
FTIR Fourier-transform infrared
GC gas-chromatography
GC-MS gas-chromatography/mass spectrometry
GC-O gas-chromatography using an oxygen-selective detector
Hb haemoglobin
HC hydrocarbon
HPLC high-performance liquid chromatography
HPRT hypoxanthine-guanine phosphoribosyl transferase
IL-1 interleukin-1
IL-4 interleukin-4
ip intraperitoneal
IR infrared
iv intravenous
Koc adsorbtion coefficient to soil organic carbon
Kow octanol/water partition coefficient
LC50 median lethal concentration
LD50 median lethal dose
LGL large granular lymphocyte
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
LT50 median lethal time
MCH mean corpuscular haemoglobin
MCHC mean corpuscular haemoglobin concentration
MCS multiple chemical sensitivities
MCV mean corpuscular volume
MTBE methyl tertiary-butyl ether
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIR near infrared
NMOC non-methane organic carbon
NOAEL no-observed-adverse-effect level
NOEL no-observed-effect level
NOx oxides of nitrogen (NO, NO2, N2O4 and N2O3)
PID photoionization detector
ppb parts per billion
ppbv parts per billion (by volume)
ppm parts per million
PROD 7-pentoxyresorufin- O-dealkylase
RBC red blood cell
RFG reformulated gasoline
RID refractive index detector
RPLC reversed-phase liquid chromatography
sc subcutaneous
SCE sister chromatid exchange
SD standard deviation
TBA tertiary-butyl alcohol
TBF tertiary-butyl formate
TWA time-weighted average
UDS unscheduled DNA synthesis
V/F distribution volume
VOC volatile organic compound
1. SUMMARY
Methyl tertiary-butyl ether (MTBE) is one of several ethers
that may be used as fuel additives and is currently by far the
dominant one. Ethyl tertiary-butyl ether (ETBE), tertiary-amyl
methyl ether (TAME), tertiary-amyl ethyl ether (TAEE) and
diisopropyl ether (DIPE), among others, may supplement, or serve as
alternatives to MTBE for oxygenation or octane enhancement purposes
and may be found, therefore, in association with MTBE.
1.1 Identity, physical and chemical properties, analytical methods
MTBE is a volatile, colourless liquid at room temperature with a
terpene-like odour. It has low viscosity and a boiling point of
55.2°C. The freezing point is -109°C. The density is 0.7404 at 20°C.
The vapour pressure is relatively high, 33 500 Pa at 25°C. MTBE is
flammable and can form explosive mixtures with air. It is very soluble
in other ethers and alcohol. It mixes with gasoline (petrol), and is
soluble in water (42 000 g/m3 at 19.8°C). The log n-octanol/water
partition coefficient is 0.94-1.3. It is unstable in acid solution.
MTBE is analysed in all matrices generally by gas chromatography
(GC) using a range of capillary columns and detector systems that are
suited to the specific matrix. Reverse-phase liquid chromatography
(RPLC) has also been used for analysis of petrol samples.
Sorption/desorption, including purge and trap systems, and headspace
procedures have been used to prepare air, water, sediment and
biological samples for analysis.
1.2 Sources of human and environmental exposure
MTBE is not known to occur naturally in the environment.
Industrially, it is derived from the catalytic reaction of methanol
and isobutylene, and has been produced in several countries in
increasing volumes since the late 1970s. MTBE is currently among the
50 highest production volume chemicals. In 1996, the USA capacity for
production was approximately 10.6 million tonnes, and it is
anticipated that the use of MTBE will continue to increase.
Approximately 25% of gasoline in the USA is blended with MTBE. MTBE is
almost exclusively used to provide both octane enhancement and an
increase in the oxygen content of gasoline. MTBE has been added to
gasoline in concentrations up to 17% by volume.
1.3 Environmental transport, distribution and transformation
After discharge into air, MTBE will largely remain in the air,
with smaller amounts entering soil and water. In the atmosphere, MTBE
can partition into rain. However, only a small amount is removed from
the atmosphere in this manner. Atmospheric transformation by hydroxyl
radicals produces a number of products including the photochemically
stable tertiary-butyl formate (TBF) and 2-methoxy-2-methylpropanol,
which is expected to be highly reactive with hydroxyl radicals,
yielding CO2, formaldehyde, acetone and water. When MTBE is
discharged into water, a significant amount is dissolved, with some
partitioning into air. Partitioning into biota and into sediment is
low. Biodegradability in conventional assays is limited. Generally,
biodegradability is believed to be slow in the environment. When MTBE
is released to the soil, it is transported to the air through
volatilization, to surface water through run-off and to groundwater as
a result of leaching. MTBE can persist in groundwater.
1.4 Environmental levels and human exposure
There are few data on environmental levels and human exposure.
In studies of MTBE in urban air of some cities using oxygenated
gasoline with 15% MTBE, ambient concentrations ranged from
non-detectable to 100.9 µg/m3 (0.028 ppm), with several median
concentrations ranging from 0.47 to 14.4 µg/m3 (0.00013 to 0.004
ppm). Concentrations of MTBE in urban air of some cities where MTBE
was used as an octane enhancer at lower concentrations ranged from
non-detectable to 26.4 µg/m3 (0.0073 ppm).
Concentrations at ground level or near refineries ranged from 15
to 281 µg/m3. Median levels in urban air near blending facilities
were 1508 µg/m3 (0.419 ppm), with ranges of 216-35 615 µg/m3 (0.06
to 9.8 ppm).
At service stations in areas where oxygenated gasoline containing
10-15% MTBE is used, concentrations were highest in the breathing zone
during consumer refuelling (range of 300 to 136 000 µg/m3 (0.09 to 38
ppm), with levels rarely exceeding 3600 µg/m3 (10 ppm), slightly
lower at the pump island (non-detectable to 5700 µg/m3 (1.6 ppm) and
lowest at the station perimeter (non-detectable to 500 µg/m3 (0.14
ppm). Levels were generally higher at service stations without vapour
recovery systems.
Levels in the automobile cabin were 7 to 60 µg/m3 (0.002 to
0.017 ppm) during commutes and 20 to 610 µg/m3 (0.006 to 0.172 ppm)
during refuelling.
Based on limited monitoring confined almost exclusively to the
USA, MTBE has been detected in snow, stormwater, surface water
(streams, rivers, and reservoirs), groundwater and drinking-water.
Concentrations of MTBE detected in stormwater ranged from 0.2 to 8.7
µg/litre with a median of less than 1.0 µg/litre. For streams, rivers
and reservoirs, the range of detection was from 0.2 to 30 µg/litre,
and the range of medians for several studies was 0.24 to 7.75
µg/litre.
MTBE has generally not been detected in deeper groundwater or in
shallow groundwater in agricultural areas. When detected, the
concentration is less than 2.0 µg/litre. MTBE is more frequently found
in shallow groundwater (top 5-10 feet of these aquifers) in urban
areas. In this setting, the concentrations range from less than 0.2
µg/litre to 23 mg/litre, with a median value below 0.2 µg/litre.
MTBE is infrequently detected in public drinking-water systems
from groundwater. In all but 3 out of 51 systems in which it was
reported, the concentration was <20 µg/litre. There are inadequate
data to characterize the concentration of MTBE in public
drinking-water systems from surface water. MTBE has been found at high
levels (i.e. >1000 µg/litre) in a few private wells used for
drinking-water. However, it is doubtful that humans would consume
water with concentrations of MTBE greater than about 50-100 µg/litre
because of its low taste and odour threshold.
Workers with potential exposure to MTBE include those involved in
the production and distribution and use of MTBE and MTBE-containing
gasoline, including service station attendants and mechanics.
Short-term exposure (<30 min) in routine manufacturing
operations and maintenance of neat MTBE ranged from 715 to 43 000
µg/m3 (0.2 to 12 ppm), with average median values being about 3400
µg/m3 (0.95 ppm). Longer-term (30 min to 8 h) exposure ranged from
360 to 890 000 µg/m3 (0.01 ppm to 250 ppm), with median levels being
about 540 µg/m3 (0.15 ppm). For workers in blending operations,
short-term values ranged from non-detectable to 360 000 µg/m3 (100
ppm), the average median being about 5700 µg/m3 (1.6 ppm). Long-term
values ranged from non-detectable to 257 000 µg/m3 (72 ppm), the
average median being about 2000 µg/m3 (0.6 ppm).
Exposures were highest during transportation of neat MTBE and
fuel mixtures through pipelines, barges, railroad cars and trucks
(neat MTBE only), short-term values ranging from 4 to 3750 mg/m3
(0.001 to 1050 ppm) with an average median value of 140 mg/m3 (39
ppm). Long-term values ranged from 0.036 to 2540 mg/m3 (0.01 to 712
ppm), the average median value being 2.85 mg/m3 (0.8 ppm). In
distribution (i.e. loading of MTBE fuel mixtures on trucks and
delivering and unloading at service stations), short-term values
ranged from non-detectable to 225 mg/m3 (63 ppm), the average median
values being around 21 mg/m3 (6 ppm). Long-term values ranged from
0.036 to 22 mg/m3 (0.01 to 6.2 ppm), the average median value being
1.79 mg/m3 (0.5 ppm).
Median short-term exposure levels of service station attendants
ranged generally from 1.071 to 21.42 mg/m3 (0.3 to 6 ppm) and rarely
exceeded 35.7 mg/m3 (10 ppm). Median long-term exposure levels of
service station attendants averaged 1.79 mg/m3 (0.5 ppm). Median
exposures of mechanics were below detection levels for one short-term
study; the average median value for long-term exposure was
approximately 360 µg/m3 (0.1 ppm).
1.5 Kinetics and metabolism
Toxicokinetic data on MTBE in humans are mainly derived from
controlled studies in healthy adult volunteers and in a population
exposed to oxygenated gasoline. MTBE is rapidly absorbed into the
circulation following inhalation exposure. In healthy human volunteers
exposed by inhalation, kinetics of MTBE are linear up to
concentrations of 268 mg/m3 (75 ppm). Tertiary-butyl alcohol (TBA),
a metabolite of MTBE, was measured in blood and urine of exposed
humans. The peak blood levels of MTBE and TBA ranged from 17.2 to 1144
µg/litre, and 7.8 to 925 µg/litre, respectively, in humans exposed to
5.0 to 178.5 mg/m3 (1.4 to 50 ppm) MTBE. Based on a monocompartmental
model, rapid (36-90 min) and slower (19 h) components of MTBE
half-life have been identified.
In rodents, MTBE is well absorbed and distributed following oral
administration and inhalation exposure, with lower dermal absorption.
At 400 mg/kg oral and 28 800 mg/m3 (8000 ppm) inhalation exposure,
the percentage of total absorbed dose eliminated in expired air
increased with a corresponding decrease in the percentage eliminated
in urine, indicating a saturation of metabolism. TBA was not
identified in the urine of exposed rats. There is evidence of further
metabolism of TBA, based on the identification of
2-methyl-1,2-propanediol and alpha-hydroxyisobutyric acid excreted in
the urine. In vitro studies provide evidence that MTBE is
metabolized to TBA, formaldehyde and acetone.
1.6 Effects on laboratory animals and in vitro systems
In rats, the acute median oral lethal dose (LD50) is
approximately 3800 mg/kg bw. The acute median lethal concentration
(LC50) value for a 15-min inhalation exposure is about 141 000 mg/m3
air in mice. Signs of intoxication include CNS depression, ataxia and
laboured respiration. When the dose was non-lethal, recovery was
complete. The LD50 for dermal toxicity in rabbits is >10 200 mg/kg
bw.
In a single identified study, MTBE was "moderately" irritating to
skin, causing moderate erythema and oedema following dermal
application to rabbits. It was also irritating to the eyes of rabbits,
causing mild, reversible changes. In the only identified study, MTBE
induced slight to severe respiratory irritation following exposure of
mice to 300 to 30 000 mg/m3, respectively. It did not induce skin
sensitization in studies in guinea-pigs.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Lowest reported effect levels for nephrotoxicity following
ingestion in 90-day studies are 440 mg/kg bw per day (increases in
relative kidney weight and hyaline droplet formation in Sprague-Dawley
rats). With inhalation exposure to 2880 mg/m3 (800 ppm), there were
increases in kidney weight associated at higher concentrations with a
mild increase in hyaline droplets in the proximal tubules in
Fischer-344 rats. In inhalation oncogenicity studies, at 1440 mg/m3
(400 ppm) the incidence and severity of chronic progressive
nephropathy was increased in male rats; in male mice, at this level,
there were increases in absolute liver weight (which correlated with
increases in hepatocellular hypertrophy at higher concentrations) and
an increase in relative kidney weight.
Exposure to MTBE also results in reversible central nervous
system (CNS) effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2880 mg/m3 (800 ppm) produced
reversible dose-related changes in motor activity in single sexes.
These effects were transient and not evident in longer-term studies.
One- and two-generation inhalation reproductive studies in rats
and four developmental studies in rats, mice and rabbits have been
identified. In these studies, specific reproductive effects were not
observed in rats at concentrations up to 28 800 mg/m3. MTBE has not
induced developmental effects at concentrations below those that were
toxic to the mothers. Decreases in uterine weight and increases in
estrogen metabolism in mice have been observed at 28 800 mg/m3.
MTBE has been adequately tested in a broad range of mutagenicity
and other genotoxicity tests. The results from these studies indicate
that MTBE is not genotoxic, although a mouse lymphoma cell tk locus
mutation assay was positive, due to the metabolism of MTBE to
formaldehyde.
Carcinogenicity studies have been conducted involving inhalation
exposure of Fischer-344 rats and CD-1 mice and gavage dosing of
Sprague-Dawley rats. In neither of the inhalation studies were methods
of statistical analysis used that adjusted for survival differences.
There were significant increases in tumour incidence in all three
studies, namely renal tubular cell tumours and Leydig cell tumours in
the male Fischer-344 rats, Leydig cell tumours in male and
leukaemias/lymphomas (combined) in female Sprague-Dawley rats, and
liver cell tumours in female CD-1 mice. The renal tubular cell tumours
and the leukaemia/lymphomas were not observed consistently, therefore,
in the different studies in rats. In addition, the sex-specific kidney
tumours were associated with sex-specific alpha2u-globulin
nephropathy, which was observed in several studies of short duration.
Increases in Leydig cell tumours occurred at the highest dose level
(1000 mg/kg bw) in the Sprague-Dawley rats, but interpretation of the
increases recorded for Fischer-344 rats was complicated by the very
high concurrent and historical control incidences. The mouse liver
tumours occurred at incidences in the control and 28 800 mg/m3 (8000
ppm exposed groups, respectively, of 2/50 and 10/50 in females and
12/49 and 16/49 in males. The increases were modest and were
accompanied by hepatocellular hypertrophy.
1.7 Effects on humans
Following the introduction of two separate fuel programmes in the
USA requiring the use of gasoline oxygenates (not necessarily MTBE),
consumers in some areas have complained about acute health symptoms
such as headache, eye and nose irritation, cough, nausea, dizziness
and disorientation. Epidemiological studies of human populations
exposed under occupational as well as non-occupational conditions, and
experimental studies of human volunteers exposed under controlled
conditions, have not been able to identify a basis for these
complaints. Although results are mixed, community studies conducted in
Alaska, New Jersey, Connecticut, and Wisconsin, USA, have provided
limited or no evidence of an association between MTBE exposure and the
prevalence of health complaints.
In controlled experimental studies on adult volunteers exposed in
inhalation chambers to MTBE at concentrations ranging from 5.0 mg/m3
(1.4 ppm) up to 270 mg/m3 (75 ppm), there were no evident effects in
terms of either subjective reports of symptoms or objective indicators
of irritation or other effects up to 180 mg/m3 (50 ppm) for as long
as 2 h. From this evidence it appears unlikely that MTBE alone induces
adverse acute health effects in the general population under common
conditions of inhalation exposure. However, the potential effects of
mixtures of gasoline and MTBE, and the manner in which most persons
are exposed to MTBE in conjunction with the use of oxygenated fuels,
have not been examined experimentally or through prospective
epidemiological methods. Moreover, the role of factors such as
awareness of MTBE, due in part to its distinctive odour, for example,
have not been investigated.
1.8 Effects on other organisms in the laboratory and field
The experimental acute toxicity (LC50) of MTBE to fish,
amphibians and crustaceans is > 100 mg/litre. There are no data on
chronic or sub-lethal toxicity to aquatic species, or on toxicity to
terrestrial organisms.
1.9 Evaluation of human health risks and effects on the environment
Based on collective evidence, it appears unlikely that MTBE alone
induces adverse acute health effects in the general population under
common exposure conditions.
In studies on animals, MTBE is "moderately" acutely toxic and
induces mild skin and eye irritation but not sensitization. Repeated
exposure affects primarily the kidney of rats and the liver of mice,
with lowest reported adverse effect levels of 440 mg/kg bw per day in
rats following ingestion and 1440 mg/m3 (400 ppm) following
inhalation. MTBE has not induced adverse reproductive or developmental
effects at concentrations less than those that were toxic to the
parents.
MTBE is not genotoxic but has induced tumours in rodents
primarily at high concentrations that also induce other adverse
effects. These data are considered currently inadequate for use in
human carcinogenic risk assessment. The Task Group concluded that, in
order to provide quantitative guidance on relevant limits of exposure
and to estimate risk, acquisition of additional data in several areas
is necessary.
It does not appear that the concentrations of MTBE in ambient
water are toxic to aquatic organisms except during spills. Although
there are no data on the terrestrial toxicity of MTBE, this appears
not to be of concern since concentrations in ambient air are low and
its half-life is relatively short.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Chemical formula: C5H12O
Chemical structure: CH3
'
H3C - C - O - CH3
'
CH3
Relative molecular mass: 88.15
Common name: methyl tertiary-butyl ether
IUPAC Chemical name: 2-methoxy-2-methyl propane
CAS registry number:  Based on physicochemical properties, it can be predicted that a
release of MTBE into water would result in significant amounts being
dissolved. Most of the MTBE remains in the surface water, with some
partitioning into air and much smaller amounts into sediment and soil
(Mackay et al., 1993). The low Kow of 0.94 suggests that partitioning
from the water to particulates and sediment is not significant. On the
basis of bioconcentration data, MTBE is not subject to bioaccumulation
or biomagnification in aquatic organisms (Environment Canada, 1993).
In the water compartment, the key removal process should be
volatilization. The amount transferred to sediment is negligible
(Mackay et al., 1993; Environment Canada, 1993).
For a gasoline containing 10% MTBE by weight, and assuming that
it does not undergo depletion of the MTBE concentration in the
gasoline due to dissolution into the water, the water solubility of
the MTBE from gasoline will be approximately 5 gm/litre at 25°C. By
comparison, the total hydrocarbon solubility for non-oxygenated fuel
is about 120 mg/litre (Poulsen et al., 1992; Zogorski et al., 1996).
The ability of MTBE to enhance the solubility in water of
monocyclic aromatic gasoline components including BTEX compounds has
been examined in models, and an increase was predicted only at
co-solvent concentrations of greater than 1% (Mihelcic, 1990). In
confirmation of this, the co-solvent effect of MTBE on the aqueous
solubility of hydrocarbons in gasoline was found to be minimal (Cline
et al., 1991). Measurements made in the laboratory in shake-flasks
showed that up to 15% MTBE was unlikely to result in enhanced
concentrations of BTEX in contaminated groundwater (Poulsen et al.,
1992). Such high concentrations of MTBE seem unlikely to be achieved
in groundwater after spillage of gasoline containing MTBE, and
although MTBE is widely distributed in shallow urban groundwater at
low concentrations in the USA, its occurrence in these samples was not
associated with correspondingly increased concentrations of BTEX
(Squillace et al., 1996).
4.1.3 Soil
Based on its physicochemical properties, it can be predicted that
when MTBE is released to the soil, it can be transported to the air
through volatilization, to surface water through run-off, and to
groundwater as a result of leaching. In the first two instances, the
release would have to be at, or near the soil surface. If the release
of MTBE occurs below the soil surface, for example from an underground
storage tank, then the most likely transport mechanism will be
leaching to groundwater. Based on its vapour pressure, volatilization
of MTBE from soil and other surfaces is expected to be significant.
Soil adsorption and mobility are based on the reported and estimated
Koc (organic carbon sorption coefficient) values. Compounds with a
Koc of <100 are considered to be moderately mobile. Thus MTBE, with
a Koc of 91, does not adsorb to soil particles to a great degree and
would be considered mobile. Parameters other than Koc affecting the
leaching of MTBE to groundwater include the soil type (e.g., sandy
versus clay), the amount and frequency of rainfall, the depth of
groundwater, and the extent of degradation of the MTBE (Environment
Canada, 1993).
4.1.4 Multimedia
Several multimedia models using various emission rates and
environmental parameters have been used to predict the distribution
and concentration of MTBE in the environment (Environment Canada,
1993; Mackay et al., 1993; Hsieh & Ouimette, 1994).
4.2 Bioconcentration
Fujiwara et al. (1984) conducted studies on the bioconcentration
of MTBE in Japanese carp (Cyprinus carpio) in a flow-through system
at 25°C. The mean whole-body steady-state bioconcentration factor
(BCF) was 1.5. Further observations indicated that fish exposed for 28
days and then transferred to clean water eliminated almost all MTBE
residues within 3 days. These experimental data support the hypothesis
that MTBE has little tendency to bioaccumulate. Veith & Kosian (1983)
calculated a BCF of 2.74 (r2 = 0.927) for a 28-day exposure of
fathead minnows, based on a Quantitative Structure-Activity
Relationship (QSAR).
Compounds with log Kow values of approximately 5.0 or less do
not have significant food chain build-up. MTBE belongs to this group
(Environment Canada, 1993). Uptake from water is more important than
from food for this group of compounds.
When 14C-labelled MTBE was applied to the soil in a closed
aerated system, the concentrations of MTBE in the roots and the aerial
parts of lettuce and radish showed that transport was dominated by
foliar uptake; subsequently, translocation into the roots took place
(Schroll et al., 1994). Although neither MTBE nor its potential
metabolite TBA was detected in the plants, a considerable fraction of
the 14C label was unaccounted for and was presumed to be associated
with plant constituents.
4.3 Biodegradation and transformation
Only a limited amount of work has been accomplished on the
biodegradability of MTBE. Moreover, the studies are difficult to
compare because they have been performed under a wide variety of
conditions. Aerobic and anaerobic experiments have been conducted. For
most studies, it has been demonstrated that MTBE is difficult to
biodegrade. In contrast, BTEX is more readily biodegraded (Zogorski et
al., 1996). Half-lives for MTBE in various environmental compartments
are shown in Table 3
Table 3. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Air 20.7-265 Based upon measured Howard et al., 1991
photo-oxidation half-life
10-30 Mackay et al., 1993
Soil 672-4320 Estimation based upon US EPA, 1989
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Surface water 672-4320 Estimation based upon Howard et al., 1991
aerobic biodegradation
300-1000 half-life Mackay et al., 1993
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
2688-17 289 Estimation based on Howard et al., 1991
anaerobic degradation
half-life
4.3.1 Aerobic conditions
Results from tests involving biodegradation of MTBE have been
variable.
Pence (1987a) used an acclimated culture containing active
sludge, soil inoculum and raw sewage. The uptake of oxygen was
measured in a mineral medium supplemented with MTBE added to the
acclimated culture at a concentration of 5 mg/litre on days 0, 7 and
11. The results showed that MTBE was poorly biodegradable under these
conditions; only 5.4% biodegradation occurred within 28 days.
No biodegradation of MTBE after 60 days was found in experiments
using aquifer soil material as inoculum; with two types of activated
sludge as inoculum, no degradation of MTBE occurred after 40 days
(Möller Jensen & Arvin, 1990).
With a standard activated sludge, and based on the oxygen uptake
rate, MTBE was biodegraded very slowly (Fujiwara et al., 1984). The
hydrocarbon components of gasoline blended with MTBE were, however,
readily degraded even though the MTBE remained.
A mixed bacterial culture was obtained by enrichment of a
hydrocarbon-contaminated soil in a basal mineral medium containing:
(i) TBA (1 g/litre) as sole carbon source or (ii) methylamine (2
g/litre) as principal carbon source supplemented with TBA. During
incubation of the first culture, the concentration of TBA fell to zero
in 20 days, but incubation of methylamine-grown cells with MTBE showed
no reduction in the concentration of MTBE after 42 days (Allard et
al., 1996). Whereas MTBE was apparently recalcitrant under the
conditions used, TBA, which is one of its putative degradation
products, was biodegradable.
In contrast to these results, a mixed bacterial culture obtained
by continuous aerobic enrichment of a sludge sample from an industrial
bioreactor was able to degrade MTBE at concentrations up to 200
mg/litre (Salanitro et al., 1994). Cell suspensions incubated with
MTBE produced TBA as a transient metabolite. MTBE labelled with 14C
in the methyl group was degraded to 14CO2 and cellular material when
low substrate concentrations (2 mg/litre) were used, although not at a
concentration of 20 mg/litre. This experiment clearly demonstrated
oxidation of the methoxy group but left unresolved the fate of the
carbon atoms of the tertiary-butyl group.
Fifteen pure bacterial strains, with the capacity to degrade MTBE
using it as the sole carbon source, have been isolated from bioreactor
sludges and other sources. Several strains have been identified as
belonging to the genera Rhodococcus, Flavobacterium, Pseudomonas and
Oerskovia. These strains degrade up to 40% of MTBE (200 mg/ litre)
in 1-2 weeks of incubation at 22-25°C. These strains also grow on
tert-butanol, butyl formate, isopropanol, acetone and pyruvate as
sole carbon sources. Cultures of Methylobacterium, Rhodococcus and
Arthrobacter degraded MTBE within 1-2 weeks of incubation at
23-25°C. Growth on MTBE as the sole carbon source was slow compared
with growth on a nutrient-rich medium. When these compounds are mixed
with MTBE, there is a reduction in the degradation of MTBE. However,
when the microbes were initially grown on tert-butanol and then
transferred to medium containing MTBE, there was a greater degradation
of MTBE (Mo et al., 1997).
A mixed culture isolated from biological sludges has been used in
bioreactors utilizing MTBE as a sole carbon source for over a year.
The microbes were able to degrade MTBE at a concentration of 160
mg/litre after 3 days of incubation in batch experiments. Mixed
cultures have greater capacity for degradation of MTBE than pure
cultures. The addition of other ethers causes a reduction in MTBE
degradation. In soil microcosm studies, significant MTBE degradation
by mixed cultures was observed at 24°C and 10°C (Mo et al., 1997).
Howard et al. (1991) estimated, on the basis of screening tests
for aerobic biodegradation with unacclimatized aqueous systems
(Fujiwara et al., 1984), that the half-lives of MTBE in water and soil
under aerobic conditions ranged from 672 to 4320 h.
MTBE was found to be degraded by a number of propane-oxidizing
bacteria. The initial oxidation of MTBE produced nearly stoichiometric
amounts of TBA. The methoxy group of MTBE was further oxidized to
formaldehyde and finally to CO2. At 28°C, rates of MTBE degradation
by these bacteria ranged from 3.9 to 9.2 nmol/min per mg cell protein
weight (Steffan et al., 1997).
4.3.2 Anaerobic conditions
Biodegradability of MTBE to methane under anaerobic conditions
has been determined by measuring the production of CH4 and CO2
during exposure of MTBE to a large population of anaerobic bacteria.
MTBE was biodegraded anaerobically only to a very limited extent
(Pence, 1987b), and an average cumulative theoretical gas production
of only 7.1% was achieve within 56 days. Anaerobic biodegradation to
methane must exceed 50% to meet the validation requirements for
demonstration of anaerobic biodegradability.
The anaerobic degradation of MTBE has been examined in different
soils (unsaturated clay, sandy loam and silty loam) collected from
various depths at three different sites (Novak et al., 1992; Yeh &
Novak, 1994). The experiments were conducted in static small-volume
anaerobic microcosms, and three different oxygen-free conditions were
simulated; with nitrate as electron acceptor (denitrification),
sulfate-reducing conditions, and anaerobic fermentation. Factors
influencing the degradation of MTBE, ETBE and TBA were determined, and
included anaerobic microbial populations, soil anions, soil moisture
content, organic content, nitrogen availability, rate of ammonium
"fixation", and soil pH. The soils were moderately acidic (pH 5.0-6.0)
with the exception of surface soils. The concentration of the added
MTBE was monitored for more than 250 days. Three parameters were
evaluated: degradation rate, lag time and time for 80% of the compound
to be degraded. No anaerobic degradation of MTBE was found in
organic-rich soils over the 250-day study period. The only situation
in which MTBE degradation occurred was in an oligotrophic soil
containing a low level of organic matter and with a pH of 5.0-6.0.
About 10% of the MTBE was lost during the first two months, although
this decrease cannot unambiguously be attributed to biodegradation.
Several conclusions may be drawn from the experiments with TBA and
ETBE:
* Whereas degradation of TBA in soil from the oligotrophic site
could be enhanced by addition of nitrate, the degradation of TBA
was inhibited by adding readily degraded ethanol.
* Biodegradation of ETBE under denitrifying conditions was
extremely sensitive to the presence of readily degraded
substrates.
These results illustrate that care should be exercised in
assessing biodegradability when several readily degraded substrates
are available, a condition that may be encountered in groundwater
contaminated with oxygenate additives.
Suflita & Mormile (1993) used sediment suspensions prepared from
samples collected from an aquifer polluted with leachate from a
municipal landfill. They assessed the formation of methane from a
range of substrates, and after at least 249 days no evidence for
anaerobic degradation of MTBE could be found. Whereas unbranched
alkanols and ketones were readily degraded, ethers in general were
resistant; in addition, oxygenates containing a tertiary or quaternary
carbon atom proved more recalcitrant than their unbranched or
moderately branched chemical analogues to anaerobic degradation.
Comparable experiments using a wider range of sediment samples
(Mormile et al., 1994) showed similar results under sulfate-reducing
or denitrifying conditions, although under methanogenic conditions a
single sample transformed MTBE into TBA. Likewise, the ethers were
unaffected by incubation with cultures of the acetogenic bacteria
Acetobacterium woodii and Eubacaterium limosum that convert
aromatic methoxy groups to acetate.
Based on the above-mentioned studies, MTBE is classed as
recalcitrant under anaerobic conditions.
Howard et al. (1991) estimated that the half-life of MTBE in
water under anaerobic conditions ranges from 2688 to 17 280 h.
4.4 Abiotic degradation
4.4.1 Air
4.4.1.1 Photolysis
Direct photolysis of MTBE is assumed to be environmentally
insignificant since it does not absorb radiation above 230 nm (Calvert
& Pitts, 1966). However, under laboratory conditions MTBE in an
oxygenated slurry system containing TiO2 as catalyst was readily
degraded by UV light from a mercury lamp. MTBE was rapidly
photocatalytically degraded, 76% of the initial concentration being
converted to degradation products, including TBA. After 4 h MTBE was
no longer detectable (Barreto et al., 1995).
4.4.1.2 Hydrolysis
MTBE does not contain hydrolysable functional groups, and,
therefore, it is inert to environmental hydrolysis. Hydrolysis of MTBE
is assumed to be insignificant (Howard et al., 1991).
4.4.1.3 Photooxidation
MTBE is subject to photooxidation in the atmosphere. This will
occur under the influence of various mechanisms, such as the reaction
with hydroxyl radicals, water, alkoxy and peroxy radicals, oxygen
atoms, and ozone. On the basis of the rate constant of each of the
reactions and the concentrations of the reactants, the reaction with
the hydroxyl radical is considered to be the most important removal
process for MTBE in the atmosphere. Several products are generated as
a result. These include tertiary-butyl formate (TBF), the major
product, 2-methoxy-2-methyl propanol, formaldehyde, acetone, NO2, and
the methyl radical. Molar yields of products identified from the
reaction of hydroxyl radicals with MTBE are given in Table 4). TBF is
unreactive to further photo-oxidation, while 2-methoxy-2-methyl
propanol is expected to be highly reactive with hydroxyl radicals,
yielding equimolar amounts of CO2, formaldehyde, acetone and water.
Of these products, formaldehyde is highly reactive with the hydroxyl
radical (Wallington et al., 1988; Japar et al., 1991). Rates of
reaction of oxygenates and their decomposition products with hydroxyl
radicals are given in Table 5.
Factors influencing atmospheric lifetime, such as time of day,
sunlight intensity and temperature, also include those affecting the
availability of hydroxyl radicals. Based upon measured rate constants
for reactions with hydroxyl radicals in air (Cox & Goldstone, 1982;
Atkinson, 1985; Wallington et al., 1988, 1989; Atkinson, 1990; Japar
et al., 1990), the half-life for MTBE has been estimated to be between
20.7 and 265 h (Howard et al., 1991). Hence, MTBE is not considered to
be a greenhouse gas, nor would it contribute to the depletion of the
ozone layer (Environment Canada, 1993).
Table 4. Molar yields of products identified from the reaction of
hydroxyl radicals with MTBE
Product Molar yielda Molar yieldb
TBF 0.68 0.76
Formaldehyde 0.48 0.37
Methyl acetate 0.14 0.17
TBA 0.062 -
Acetone 0.026 0.02
a Smith et al., 1991.
b Tuazon et al., 1991.
Table 5. Rates of reaction of oxygenates and their decomposition
products with hydroxyl radicals at 25°C
Compound Rate Reference
(10-12 cm3
sec-1 molecule-1)
MTBE 3.2 Japar et al., 1991
ETBE 8.5 Japar et al., 1991
TBF 0.74 Smith et al., 1991
TBA 1.1 Japar et al., 1991
Formaldehyde 9.0 Atkinson & Pitts, 1978
2-methoxy-2-methyl propanala 30 Japar et al., 1991
a Estimated from rates for other aldehydes
4.4.2 Natural waters
MTBE is not expected to adsorb significantly to bed sediments of
suspended sediments, hydrolyse, directly photolyse, or photo-oxidize
via reaction with photochemically produced radicals in water. While
MTBE is reported to be chemical unstable in acidic solutions (Budavari
et al., 1996), it is not expected to be hydrolysed in natural waters
under normal pH conditions (Lyman et al., 1990).
4.4.3 MTBE half-life ranges in environmental compartments
The half-life of a chemical in the environment depends not only
on the intrinsic properties of the chemical, but also on the nature of
the surrounding environment, such as sunlight intensity, hydroxyl
radical concentration, the nature of the microbial community and
temperature. Table 6 lists the half-life ranges in various
environmental compartments estimated by Mackay et al. (1993) and
Howard et al. (1991); these estimates are somewhat uncertain, as
implied by the order of magnitude range for some compartments.
4.5 Ozone-forming potential
Photochemical ozone-creation potentials (POCP) ranging from 20.4
to 34.6 have been determined for MTBE using a model that simulates the
formation of photochemical ozone episodes (Derwent et al., 1996). The
POCP values reflect the ability of a substance to form tropospheric
ozone as a result of its atmospheric degradation reactions. The POCP
values are calculated relative to ethylene (a chemical that is thought
to be important in such ozone formation and is given a POCP of 100).
Based on the emissions and the POCP value, MTBE (itself) is likely to
play a minor role in photochemical smog and low-level (tropospheric)
ozone formation near to sources of release.
4.6 Remediation
Examples of remedial methods that can be considered for MTBE are
air stripping, carbon absorption and soil vapour extraction. Intrinsic
bioremediation may be limited due to the variability of rates of
biodegradation of MTBE which have been previously mentioned (Zogorski
et al., 1996).
Table 6. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Soil 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Air 20.7-265 Based upon measured Howard et al., 1991
10-30 photo-oxidation half-life Mackay et al., 1993
Surface water 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The major sources of MTBE to the general population are probably
associated with the distribution, storage and use of oxygenated
gasoline. The main source of non-occupational exposure to MTBE is
evaporative emissions from gasoline. A large portion of the population
is exposed during time spent at service stations, while driving cars,
in public parking garages, and in homes with attached garages. These
exposures generally occur through inhalation. In addition, discharges
into the soil or groundwater are a potential for contaminated water
supply and can lead to exposure when such water is drunk. Dermal
contact with MTBE may occur through accidental spills of MTBE-blended
gasoline or through the use of gasoline as a solvent. In Canada, it
has been estimated that gasolines blended with MTBE account for only
2% of the total annual gasoline consumption. MTBE is used in small
quantities by a few Canadian refiners to boost octane levels in
gasoline. A limited survey of the MTBE content of unleaded regular,
mid-range and premium gasoline across Canada in 1995 showed a range of
0 to 5.2% by volume for winter grade gasoline and 0 to 9% by volume in
summer grade gasoline. In the USA, oxygenated gasoline containing
10-15% MTBE is used in different areas and about 30% of the US
population is exposed to MTBE.
5.1 Environmental levels
5.1.1 Exposure
Ambient air and microenvironment concentrations of MTBE and other
fuel oxygenates have been measured in Canada, the USA and Finland.
When available, air data are presented below in conjunction with data
on MTBE levels in gasoline and with information on the proximity of
the samples to various point sources of MTBE.
Brown (1997) estimated average daily and average lifetime doses
of MTBE from exposure in air and drinking-water for a US population.
Concentration data and several of the population characteristics were
estimated as distributions rather than as point values. Arithmetic
mean occupational doses via air were in the range of 0.1 to
1.0 mg/kg-day, while doses from residential exposures, commuting and
refuelling were in the range of 0.0004 to 0.006 mg/kg-day. Lifetime
doses for workers were in the range of 0.01 to 0.1 mg/kg-day. The
cumulative dose distribution for the entire population of the
MTBE-using regions of the USA was estimated by combining the
distributions of doses and the numbers of people in each exposure
category. In the MTBE-using areas, arithmetic mean doses via air were
estimated to be 0.0053 and 0.00185 mg/kg-day for the chronic and
lifetime cases, respectively. It was found that 1.5% of the population
used water contaminated with MTBE leakage with an estimated geometric
mean concentration of 0.36 µg/litre and a 95th percentile
concentration of 64 µg/litre. Including ingestion, inhalation, and
dermal absorption of contaminated water, the estimated arithmetic mean
does of the population exposed via water was 1.4 × 10-3 mg/kg-day.
5.1.1.1 Levels in ambient air and various microenvironments
a) Canada
The concentrations of MTBE in ambient air at various selected
locations in Canada have been measured as part of the National Air
Pollution Surveillance Programme in 1995 and 1996. This programme is a
joint project of the federal, provincial and municipal levels of
government. Its purpose is to monitor and assess, on a continuing
basis, the quality of the ambient air in the various regions of
Canada. The sites selected for monitoring of MTBE were based on usage
of gasoline with MTBE and/or because of nearby manufacturers of MTBE.
Pollutants from air were collected intermittently using the
canister methodology. Concentrations of MTBE was measured using the
detection principle of gas chromatography furnished with an ion trap
detector. Air samples were first passed through a cryogenic
concentration trap to gather enough analyte before injection into a GC
capillary column to allow compound speciation and quantification.
Approximately 200 ml of the canister sample was concentrated. A
cryogenic trap held at -150°C was used to concentrate the air sample.
Once the sample was concentrated, the trap was heated to 150°C and the
sample was back-flushed onto the column. MTBE and other hydrocarbons
were separated using a fused silica capillary column. The GC oven was
programmed to remain at 60°C for 3 min, then increased to 280°C at a
rate of 8°C/min. Calibration standards were prepared using the static
dilution technique. The detection limits were 0.05 to 0.1 µg/m3.
Table 7 lists the ambient concentration of MTBE in air at various
locations in Canada from 1995 to 1996.
Table 8 shows some MTBE atmospheric concentrations at the fence
line of a petroleum refinery at St John, New Brunswick, Canada, during
a period when there were complaints of odour. The same collection and
analytical methodology was used. The maximum concentration is not
considered representative of the area.
b) USA
In many urban areas in the USA having elevated levels of ozone or
CO, oxygenates such as MTBE are regulated for use in gasoline at
concentrations of 2.0% and 2.7% oxygen by weight (called reformulated
and oxygenated gasoline, respectively). These concentrations are
achieved by adding MTBE at 11% and 15% by volume, respectively. In
other areas, MTBE is used as an octane enhancer in premium gasoline at
concentrations up to 9% by volume, but usually at much lower
concentrations. It is important to note that MTBE is the predominant
oxygenate currently in use in these gasoline mixtures, followed by
ethanol (approximately 65% and 35% of the oxyfuels sold contain MTBE
and ethanol, respectively). Oxygenates used to a minor extent include
ETBE, TAME and DIPE (HEI, 1996). In 1994, oxygenates were added to
more than one-third of the gasoline market in the USA.
Table 7. Concentrations of MTBE in ambient air in Canada (1995-1996)
(Environment Canada, 1996b)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Edmonton(1)c Two petroleum refineries - 1 km. 20/7/95 7.21
Acetic acid plant - 2.5 km
26/7/95 11.39
1/8/95 0.81
7/8/95 2.93
8/8/95 5.50
12/9/95 2.49
27/5/96 3.35
Edmonton(2)d N/A 26/7/95 < DL
1/8/95 < DL
1/9/95 < DL
6/9/95 < DL
24/9/95 < DL
30/9/95 < DL
27/5/96 < DL
Halifaxd N/A 3/4/96 < DL
15/4/96 0.13
21/4/96 0.15
Montreal(1)c Two refineries (BTX, petroleum) 21/8/95 1.54
- 1.6, 2.5 km
21/8/95 0.59
12/9/95 1.06
16/3/96 < DL
15/5/96 0.42
21/5/96 0.28
27/5/96 0.23
Montreal(2)d N/A 16/3/95 0.15
16/5/96 0.18
21/5/96 0.22
27/5/96 0.37
Montreal(3)e N/A 10/3/96 0.16
16/3/96 0.28
9/5/96 0.95
Montreal(4)f N/A 9/5/96 0.22
15/5/96 < DL
21/5/96 0.70
St. Johnc Petroleum refinery - 3 km 9/5/96 1.02
15/5/96 3.73
Stouffvillef N/A 6/10/95 0.19
18/10/95 0.35
Toronto(1)d N/A 29/8/95 < DL
2/9/95 < DL
2/9/95 0.07
Table 7. (continued)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Toronto(2)d N/A 17/8/95 0.03
29/8/95 < DL
2/9/95 < DL
Vancouver(1)f N/A 23/8/95 0.27
29/8/95 0.89
29/8/95 0.16
30/8/95 0.33
Vancouver(2)f N/A 22/3/95 0.14
Vancouver(3)c Two gasoline processing and 1/8/95 2.13
storage plants - 0.5, 3 km
13/8/95 1.82
25/8/95 3.35
2/9/95 1.78
2/9/95 26.43
21/2/96 0.31
10/3/96 1.10
16/3/96 0.48
16/3/96 0.39
22/3/96 1.55
28/3/96 < DL
Vancouver(4)e N/A 10/3/96 1.07
Vancouver(5)f N/A 28/3/96 0.40
Vancouver(6)c Pipeline transfer point 1/9/95 1.79
2/9/95 1.90
22/3/96 0.89
Windsord N/A 2/8/95 0.08
17/8/95 0.05
17/8/95 0.11
21/8/95 0.40
29/8/95 0.27
29/8/95 0.14
16/3/96 < DL
21/4/96 0.15
27/4/96 < DL
Winnipegd N/A 4/3/96 0.02
27/3/96 < DL
a ( ) = different monitoring sites in same city.
b DL = detection limit = 0.1 µg/m3.
c Monitoring site in vicinity of petroleum refinery and/or industrial chemical plant, or
pipeline transfer area.
d Monitoring site in urban area.
e Monitoring site in urban area on busy street.
f Monitoring site in suburban area.
Table 8. MTBE concentrations at petroleum refinery boundary
(St. John, New Brunswick, Canada) during period of odour
complaints
Sampling date MTBE concentration
(µg/m3)
19/7/95 281
2/8/95 15
14/8/95 71
28/8/95 36
MTBE air quality data were collected in the USA as a result of
special studies in six urban centres: Fairbanks (Alaska), Stamford
(Connecticut), Albany (New York), Milwaukee (Wisconsin), Boston
(Massachusetts) and Houston (Texas) (Zogorski et al., 1996). In
addition, collection of MTBE air quality data for selected monitoring
sites in California started in 1996. Although these data cannot be
used to define quantitatively the air quality in these cities and are
not sufficient to provide a national perspective, they can be used to
estimate approximate ranges of MTBE in ambient air in the locations
sampled (Zogorski et al., 1996; US Interagency Assessment, 1997).
Non-occupational and consumer exposure to MTBE is shown in Table 9,
and service station attendants and garage workers in Table 10.
Owing to health complaints (Gordian et al., 1995) following the
introduction of oxygenated fuels (15% MTBE) during the 1992 winter
season in Fairbanks, Alaska, the sale of these fuels was suspended in
mid-December 1992, one month after their introduction. Zweidinger
(1993) analysed air samples for MTBE in ambient air and in various
microenvironments in Fairbanks taken immediately prior to the
suspension (phase I), during the phase-out period (Phase II), and two
months after the suspension, at which time the MTBE fuels were
expected to be at nominal levels (Phase III). Fuel samples collected
from Fairbanks gasoline stations during Phases II and III indicated
that the average percentage by weight of MTBE in unleaded regular
gasoline decreased from 8.5% to 1% while the average for premium
gasoline decreased from 14.7 to 5.6%. For comparison, ambient air
samples were also collected in the spring of 1993 from Stamford,
Connecticut, where 15% MTBE oxygenated gasoline was sold but there
were no consumer health complaints, and Albany, New York, where MTBE
was only present in gasoline at nominal levels to enhance octane and
there were also no health complaints (Zweidinger, 1993).
Ambient air samples in these cities were generally collected over
an 8-h period and were taken from the following areas: (1) outside
city limits, for background levels; (2) in residential areas away from
heavy traffic, and (3) in areas adjacent to major roadways or
intersections. The median and range of MTBE concentrations for the
selected locations and phases are shown in Table 11 (Zweidinger,
1993).
In Fairbanks, MTBE levels in all ambient environments were lower
when the use of MTBE as an oxygenate in gasoline was discontinued.
Overall MTBE ambient air concentrations ranged from non-detectable
levels to a maximum of 100.9 µg/m3 (28.0 ppbv) in the phases prior to
and during the phase-out of MTBE in Alaskan gasoline (Phases I and II)
and ranged from not detectable to 12.3 µg/m3 (3.4 ppbv) when MTBE use
was reduced to nominal levels in fuels (Phase III). Background levels
were low during Phase III, ranging from not detectable to 4.3 µg/m3
(1.2 ppbv) MTBE. Since sampling was limited during Phases I and II,
the levels of MTBE in ambient air outside city limits cannot be
compared for the three sampling periods over which MTBE concentrations
in gasoline were being reduced.
Phase II ambient residential and roadside area air samples
presented the highest levels of MTBE, with concentrations ranging from
6.1 to 100.9 µg/m3 (1.7 to 28.0 ppbv) and 15.1 to 28.5 µg/m3 (4.2 to
17.9 ppbv) respectively, and with medians of 6.1 µg/m3 (4.6 ppbv) and
34.9 µg/m3 (9.7 ppbv), respectively. Owing to lack of samples or
small sample size in Phase I data, comparisons between the phases were
limited to the roadside category. However, in this category, it was
unexpectedly found that, although Phase 1 sampling occurred prior to
suspension of MTBE, the data showed roadside levels of MTBE in ambient
air to be slightly lower than those during the suspension period.
In Stamford, Connecticut, limited measurements of MTBE taken in
the spring of 1993 showed that residential, roadside and gas station
ambient air results were consistently lower than those taken in
Fairbanks during the oxygenates programme, with ranges of not
detectable to 1.1 µg/m3 (0.3 ppbv), not detectable to 1.8 µg/m3
(0.5 ppbv) and 4.3 to 10.1 µg/m3 (1.2 to 2.8 ppbv), respectively.
Limited residential and roadside ambient MTBE air samples taken in
Albany, New York, in May 1993 were lower than levels during a
comparable phase of MTBE use in Fairbanks (Phase III). Ambient
temperature and other meteorological conditions differed significantly
among the cities where samples were collected.
In Fairbanks, Stamford and Albany, roadside ambient air levels
were found to be generally higher than residential area ambient air
levels (Table 11). Consequently, this roadside category may also be
considered to be a microenvironment, especially for those samples
taken in downtown city street canyons. For this particular study, it
was not possible to discern whether this was the case. Table 12
presents information on air samples taken in more clearly defined
microenvironments such as service stations and vehicle interiors
(Zweidinger, 1993).
Table 9. Non-occupational and consumer exposure to MTBE in the USAa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Community air
Milwaukee WI RFG in Yes (in 6/11 0.000025 <0.00413 0.00013 Jan-March 1995; Approximately Allen &
use* some 24-h samples; 50% contained Grande
cases) collected in MTBE, remainder (1995)
evacuated ETBE or ethanol
canisters;
GC(FID)
3/5 0.000025 <0.00106 0.00052 Feb-March 1995;
2-h samples;
collected in
evacuated
canisters;
GC(FID)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 up to (0.002) Feb-March 1995; Approximately Allen
use* 0.0037 2- to 3-h 50% contained & Grande
samples; MTBE, remainder (1995)
collected in ETBE or ethanol
evacuated
canisters;
GC(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Automobile cabin for commuters
New Jersey 15 MTBE NA 20/20 0.002- 0.004 April 1993; Estimated from Lioy et
0.017* approximately graphed data al. (1994)
1-h samples; in the original
Connecticut 20/20 0.003- 0.0056 absorbed onto report
0.009* carboxen 569:
collected in
evacuated
canisters;
GC/MS
Service station refuelling
Phoenix, AZ 12* No 40/40 0.09-38 5.8 Oct-Nov 1990; Samples taken API (1993)
each sample was from one station
collected in which only
Los Angeles, 13 Yes 6/6 1.1-6.5 3.6 during the premium gasoline
CA refuelling of 8 was oxygenated
to 10 vehicles;
each refuelling
was sampled for
1 to 2 min;
absorbed onto
charcoal; GC
(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey,
New York 10-15* Yes 4/4 NS 0.370 April 1993; 5-min Estimated from Lioy et
breathing-zone graphed date in al. (1994)
samples before, the original
Connecticut No 4/4 NS-4.1 0.572 during and after report
refuelling;
absorbed onto
carboxen 569;
GC/MS
Milwaukee WI Jan-March 1995; RFG with MTBE Allen &
Station A 9-10* Yes 6/6 NS (0.39) 15-min used only in Grande (1995)
Station D 9** No 2/2 NS (2.93) breathing-zone higher grades;
samples; adsorbed *ethanol used
onto charcoal; in regular
GC(FID) gasoline; **2%
MTBE used in
regular gasoline
Northeast and 10-17* Yes 8/17 <0.32 <2.1 0.57 Feb-April 1994; Northeast = API (1995c)
southwest 15- to 20-min Connecticut and
areas personal New Jersey
(short-term breathing zone locations;
sample) samples; adsorbed Southwest =
onto charcoal; Arizona
GC(FID) locations
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
In automobile while refuelling
Connecticut, New Jersey and New York Service Stations
Self-serve 10-15* Yes 4/4 0.006- 0.03 April 1993; 5-min Estimated from Lioy et
0.072 breathing-zone graphed data in al. (1994)
samples before, the original
Full-serve Yes 8/8 0.008- 0.034 during and after report
0.172 refuelling;
adsorbed onto
Self-serve No 4/4 NS 0.015 carboxen 589;
GC/MS
Full-serve No 4/4 0.005- 0.041
0.103
Service station pump island
New Jersey: Yes 4/4 0.120- 0.440 April 1995; 4-h API (1995a)
Full-serve 1.600 breathing-zone
samples during
both refuelling
and not
refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 6/6 0.014- 0.048
Self-serve 0.080
Connecticut: No 9/10 0.09 <1.500 0.170
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 15 Yes 3/3 0.08- 0.24 Nov-Dec 1994; 7- Cook &
0.24 to 8-h samples; Kovein (1995)
adsorbed onto
charcoal; GC (FID)
Service station perimeter
Phoenix AZ 12 No 24/24 0.009- 0.02 Oct-Nov 1990; API (1993)
0.09 12-h samples;
4 perimeter
samples plus
samples upwind
and downwind
from the pump
island for each
sample set;
adsorbed onto
charcoal; GC(FID)
New Jersey: Yes 15/16 0.001 <0.036 0.003 April 1995; 4-h API (1995a)
Full-serve samples;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 24/24 0.002- 0.007
Full-serve 0.083
Connecticut: No 38/40 0.001 <0.140 0.014
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Milwaukee WI
Station A 9-10* Yes 2/2 NS (0.0024) Jan-March 1995; *RFG, MTBE used Allen &
Station B 2-9** No 1/1 NS (0.0045) 2-h area samples; only in higher Grande (1995)
collected in grades; ethanol
evacuated in lower grades
canisters; GC(FID) **2% MTBE in
regular gasoline
a Asterisks in any column indicate that further explanation is provided in the comments column.
b Number of samples in which MTBE was detected divided by total number of samples.
c GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
NA = not applicable; NS = not stated.
Table 10. Non-industrial occupational exposures to MTBEa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Service station attendants during refuelling
Phoenix AZ Oct-Nov 1990; each API (1993)
1-2 min 12 No 40/40 0.09-38 5.8 breathing-zone
sample was
collected during
Los Angeles the refuelling of
CA 13 Yes 6/6 1.1-6.5 3.6 8-10 vehicles;
1-2 min each refuelling
was sampled for
1-2 min; adsorbed
onto charcoal;
GC (FID)
New Jersey, 10-15 Yes 4/4 NS 0.37* April 1993; 5-min Estimated from Lioy et
New York, breathing-zone graphed data al. (1994)
samples before, in original
during, and after report
Connecticut: refuelling;
5 min No 4/4 <4.1 0.572 adsorbed onto
carboxen 589;
GC/MS
Milwaukee WI Jan-March 1995; This station used Allen &
Station A 15-min MTBE in middle Grande (1995)
15 min RFG 9-10 Yes NS/6 NS 0.31 breathing-zone and premium grade
samples during gasoline, and
refuelling; ethanol in
collected in regular gasoline
evacuated
canisters
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
15-20 min 10-17 Yes 8/17 0.32 <2.1 0.57 samples; mostly locations;
15- to 20-min Southwest =
personal Arizona
breathing-zone locations
samples during
refuelling;
adsorbed onto
charcoal;
GC (FID)
Various USA 1982-1993: data Service station API (1995b)
locations reported by and retail outlet
American Petroleum personnel
<30 min NS NS 9/11 0.16 0.16- 2.6 Institute member
136.1 companies; higher
frequency of
30 min-6 h 5/5 0.01- 0.34 measurements
2.7 in 1989-1993;
GC(FID) and
other procedures
6-9 h TWA 13/13 0.09- 0.59
34.0
>9 h 11/11 0.01- 1.1
17.20
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 4 h 13-10 Yes 4/4 0.084- 0.245 April 1995; 4-h API (1995a)
(full-serve) 0.52 breathing-zone
samples, during
New York 4 h Yes 5/6 0.077- 0.205 refuelling and
(self-serve) 0.78 not refuelling;
collected in
Connecticut 4 h No 10/10 0.170- 1.5 8-litre evacuated
(self-serve) 2.60 canisters; GC/MS
Phoenix AZ 4 h 14 No 42/42 0.04- 0.55 Oct-Nov 1990; 4-h Hartle (1993)
3.88 (half-shift)
breathing-zone
samples (average
time 224 min);
adsorbed onto
charcoal; GC(FID)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
>8 h 10-17 Yes 18/21 0.03- <0.5 0.27 samples, most locations;
0.11 sampling times Southwest =
were > 6 h; Arizona
adsorbed onto locations
charcoal; GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey Nov-Dec 1994; Cook &
(full shift) 15 Yes 21/21 NS 0.12- 0.48 breathing-zone Kovein (1994)
1.42 samples for 3-8 h;
adsorbed onto
charcoal; GC (FID)
Service station attendants at pump island
New Jersey
4 h 13-16 Yes 4/4 0.12- 0.44 April 1995; 4-h API (1995a)
(full-serve) 1.60 breathing-zone
samples during
refuelling and
not refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York Yes 6/6 0.0005 0.014- 0.048 Cook &
4 h 0.08 Kovein (1994)
(self-serve)
Connecticut 15 No 9/10 NS <1.5 0.17 Nov-Dec 1994; 7-
4 h to 8-h samples;
(self-serve) adsorbed onto
charcoal; GC (FID)
New Jersey Yes 3/3 0.08- 0.24
8 h 0.24
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 0.0023- (0.002) Feb-March 1995; Approximately 50% Allen &
2-3 h use 0.0037 2- to 3-h samples; contained MTBE, Grande (1995)
collected in remainder
evacuated contained ETBE
canisters; or ethanol
GC(FID)
Mechanics Feb-April 1994; Northeast = API (1995c)
Northeast and personal Connecticut and
southwest in breathing-zone New Jersey
winter samples, most locations;
short-term Southwest =
15 min 10-17 Yes 4/13 <0.25- <32 ND sampling times Arizona locations
(<0.26) <0.35 were 15-20 min;
most long-term
>6 h 17/20 <0.02- <2.6 0.09 sampling times
(<1.5) <0.05 were >6 h; adsorbed
onto charcoal;
GC(FID)
Northern New April 1993; 1-h Workers at Mohr et
Jersey breathing-zone service stations al. (1994)
1 h 15 NS NS/13 0.3-6.1 samples (active); and garages
adsorbed onto for State
carboxen; GC/MS vehicles
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Stamford April 1993; Mechanics with Buchta (1993b)
Connecticut full-shift the Department
8-h TWA 13-17 NA 20/28 0.03 <12.04 0.11 samples of Public Works
(approximately and in auto
8 h); adsorbed dealers' garages
onto charcoal;
GC(FID)
Fairbanks AK Dec 1992; Moolenaar
8-h TWA 15 NS NS/10 0.01- 0.10 full-shift et al. (1994)
0.81 samples
(approximately
8 h); collected
in evacuated
canisters in
environment where
workers spent
most of their
day; GC
Other vehicle-related workers
Stamford 13-17 NS 0/7 0.03 <DL <DL April 1993; 8-h Workers who
personal spent time in
breathing-zone traffic
samples; adsorbed
onto charcoal;
GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Connecticut Workers in various Buchta (1993b)
8-h TWA 1/4 <0.15 <DL jobs (mostly
workers in garages
who performed
tasks difference
from the mechanics)
a Number of samples in which MTBE was detected divided by total number of samples.
b GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
c Asterisks in any column indicate that further explanation is provided in the comments column.
TWA = time-weighted average; NA = not applicable; NS = not stated.
Table 11. Concentrations of MTBE in µg/m3 (ppbv) in ambient 8-h air samples taken in Fairbanks, Stamford and Albany, USA,
as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)a
Sampling site Fairbanks Fairbanks Fairbanks Stamford Albany
Phase I Phase II Phase III April 1993 May 1993
early December 1992 late December 1992 Feb/Mar 1993
Background:
number of samples 0 1 5 2 0
median - ND ND 0.72 (0.2) -
range - 1 ND-4.3(1.2) ND-1.1(0.3) -
Residential:
number of samples 2 11 11 2 3
median 14.4(4) 16.6(4.6) 2.5(0.7) 1.1(0.3) ND
range 7.2-21.6 16.1-100.9 ND-9(2.5) ND-1.8(0.5) ND-0.4(0.1)
(2.9-6.0) (1.7-28.0)
Roadside:
number of samples 7 7 10 2 7
median 18(5) 35(9.7) 4.3(1.2) 7.2(2.0) 0.72(0.2)
range 10.8-43.2 15.1-64.5 ND-12.3(3.4) 4.3-10.1 (1.2-2.8) ND-2.5(0.7)
(3.0-12.0) (4.2-17.9)
a For the calculation of a median when n = 2, the two sample values are averaged together; in the case of an ND value,
half the detection limit value is substituted in the calculation, i.e. 0.36 µg/m3 (0.1 ppbv).
ND = non-detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Table 12. Concentrations of MTBE in µg/m3 (ppbv) in various microenvironmental 8-h ambient air samples taken in Fairbanks, Stamford
and Albany, USA, as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Service station pump island:
number of samples 1 1 6 4 4
median 194.6 (54) 134.8 (37.4) 11.5 (3.2) 13.7 (3.8)a 64.2 (17.8)
range - - 6.1-49.7 ND-26.7 (7.4) 23.3-194.6
(1.7-13.8) (6.6-54)
Commercial vehicle interiors:
number of samples 8 - 6 - -
median 126.1 (35) - 31.7 (8.8) - -
range 25.2-1207.3 - 1.4-129 - -
(7-335) (0.4-35.8)
Indoors - commercial garage service areas:
number of samples 5 - 10 8 -
median 1088.4 (302) - 148.5 (41.2) 484 (134.8) -
range 367.6-2922.8 - 21.3-496 4.7-1546.5 -
(102-811) (5.9-137.6) (1.3-429.1)
Indoors - residential area:
number of samples - 3 5 - -
median - 6.5 (1.8) 2.9 (0.8) - -
range - 6.1-15.1 1-4 (0.3-1.1) - -
(1.7-4.2)
Table 12. (continued)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Indoors - public buildings near roadway:
number of samples - 4 5 4 -
median - 32.4 (9) 6.5 (1.8) 1.8 (0.5) -
range - ND-37.1 (10.3) ND-10.5 (2.9) 1.4-1.8 -
(0.4-0.5)
Indoors - home with attached garage:
number of samples - 5 4 - -
median - 27.8 (7.7) 72.1 (20) - -
range - 10.1-75.3 51.5-109.2 - -
(2.8-20.9) (14.3-30.3)
a In Stamford, service station samples were taken 4.6 metres (15 feet) away from the pump.
ND = not detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Kelly et al. (1993) performed 24-h ambient air sampling in the
cities of Boston and Houston where MTBE was used nominally in gasoline
(i.e. approximately < 5% by volume). Sampling took place
approximately every 14 days from August 1990 to April 1991 and from
June to August 1991. The Boston sampling site was categorized as being
in an urban area of mixed industry and office buildings, with high
traffic density, and the sampler was placed on a downtown fire
department rooftop. Conversely, the Houston sampling site was located
in a semi-rural area, on the roof of an air sampling station, and was
expected to receive emissions from industrial and, to a lesser extent,
urban sources. MTBE levels in ambient air at these sites ranged from
< 0.72 to 1.8 µg/m3 (< 0.2 to 0.49 ppbv) and < 0.72 to 10.1 µg/m3
(< 0.2 to 2.8 ppbv), respectively (detection limit = 0.72 µg/m3 (0.2
ppbv). The median level from these data was < 2 ppbv for both Boston
and Houston. MTBE was detected in several samples from Houston (n =
22; in 64 % of samples MTBE was non-detectable), but only at one site
in Boston (n = 22; in 96% of samples MTBE was non-detectable).
Allen & Grande (1995) conducted an ambient air monitoring study
in the city of Milwaukee as a result of public health complaints when
MTBE was first introduced into Milwaukee reformulated gasoline (at
approximately 11% by volume) in 1995. Eleven weekly 24-h samples
collected at the Wisconsin Enhanced Ozone Monitoring Program air
sampling station from January to March 1995 resulted in concentrations
ranging from not detectable to 14.89 µg/m3 (4.13 ppbv) [n = 11; 45%
non-detectable samples; detection limit 0.36 µg/m3 (0.1 ppbv)]. The
median was determined to be 0.47 µg/m3 (0.13 ppbv). A control sample
collected in each of the nearby cities of Madison and Green Bay, where
reformulated gasoline use was not mandated, was found to be below the
detection limit of 0.36 µg/m3 (0.1 ppbv).
In the same study, Allen & Grande (1995) collected 1- to 3-h
roadside air samples at busy intersections and freeway interchanges
where it was expected that there would be high concentrations of
gasoline fumes. Mean levels of MTBE in air samples collected near a
freeway interchange, a busy intersection and a roadway in Milwaukee
were 1.9 µg/m3 (0.53 ppbv) (n = 3), 3.8 µg/m3 (1.06 ppbv) (n = 2)
and 1.8 µg/m3 (0.50 ppbv) (n = 2), respectively. This particular
choice of roadside location is a more clear example of a roadside
microenvironment and it may also be considered an ambient air sample.
The State of California mandated the year-round use of oxygenated
fuels in the South Coast Air Basin in 1995 and throughout California
by June 1996. As a result, MTBE was included in the California's
ambient air monitoring programme in February 1996 for the cities of
Burbank, Long Beach and Los Angeles and in June 1996 for Chico,
Roseville and Fresno. The overall range of 24-h average concentrations
was 1.4-44.7 µg/m3 (0.4-12.4 ppbv) for the selected monitoring sites.
The overall range of averages was closer to the Fairbanks data than to
data from other cities in the continental USA. Averages ranged from
4.7 to 17.3 µg/m3 (1.3 to 4.8 ppbv) with the highest averages
occurring in the cities of Los Angeles and nearby Burbank. The
following are average and ranges for each of the individual cities:
Burbank 17.3, 4.7-31.7 µg/m3 (4.8 ppbv, 1.3-8.8 ppbv), Long Beach
9.4, 3.2-21.6 µg/m3 (2.6 ppbv, 0.9-6.0 ppbv), Los Angeles downtown
13.7, 4-24.1 µg/m3 (3.8 ppbv, 1.1-6.9 ppbv), Chico 8.3, 3.6-27.8
µg/m3 (2.3 ppbv, 1.0-7.7 ppbv), Fresno 9.4, 2.2-44.7 µg/m3 (2.6
ppbv, 0.6-12.4 ppbv) and Roseville 4.7, 2.5-12.3 µg/m3 (1.3 ppbv,
0.7-3.4 ppbv). The number of samples for each city ranged from 18 to
28 and the detection limit for MTBE was 0.72 µg/m3 (0.2 ppbv) (M.
Poore, personal communication).
Measurements of ambient air concentrations of MTBE at service
stations are usually taken at the gasoline pump island and at the
service station perimeter. The air levels of MTBE tend to be higher at
the pump island and lower at the perimeter. In addition, service
stations equipped with vapour recovery systems (Stage II) tend to have
higher MTBE concentrations in both microenvironments. Furthermore,
whereas the pump island data represents a particular microenvironment
due to the presence of gasoline vapour coming from refuelling
activities, the gasoline station perimeter data has been used to
estimate potential community exposures and may be considered
representative of upper end ambient air levels in neighbourhoods.
Short-term peak samples of MTBE were not taken.
MTBE median concentrations generally ranged from 0.32 to 21.4
mg/m3 (0.09-6 ppm) in breathing-zone samples as a result of consumer
refuelling. The values were measured over 2- to 15-min sampling
periods and were highly variable but rarely exceeded 35.7 mg/m3 (10
ppm). The range of median values measured at the pump islands ranged
from 0.18 to 1.57 mg/m3 (0.05-0.44 ppm) over a 4-h sampling period.
The fenceline samples were lower and ranged from 0.004 to 0.5 mg/m3
(0.001 to 0.14 ppm) with a collection period of 4 h. Generally, the
concentrations were higher at service stations that did not have
vapour recovery systems.
Allen & Grande (1995) collected 1- to 3-h MTBE area samples
downwind of the gas pumps on the perimeter of four Wisconsin service
station properties from 21 February 1995 to 9 March 1995. The average
levels of MTBE at two service stations that dispensed reformulated
gasoline and had Stage II vapour recoverya were 8.8 µg/m3 (2.43
ppbv) (n = 2) and 2.7 µg/m3 (0.75 ppbv) (n = 2). The level of MTBE at
a station without vapour recovery was higher and was measured to be
16.5 µg/m3 (4.58 ppbv) (n = 1). Finally, one air sample taken at a
service station where reformulated gasoline was not mandated resulted
in a lower concentration of MTBE i.e. 0.8 µg/m3 (0.25 ppbv).
a Stage II is a vapour recovery system used to trap gasoline vapour
during refuelling of consumer vehicles.
In addition, Zweidinger (1993) analysed a limited number of 8-h
air samples taken in various microenvironments in Fairbanks (Phases I,
II and III), Stamford and Albany for MTBE. Generally,
microenvironmental air concentrations of MTBE decreased from Phase I
or II to Phase III at the following locations: (1) service station
pump island, (2) commercial vehicle interiors, (3) indoor - commercial
garage service areas, (4) indoor - residential area and (5) indoor -
public buildings near roadway (i.e. school, post office), due to the
suspension of the oxyfuel programme. The median and range of MTBE
concentrations for selected locations and phases are shown in Table
12. The air of one home with an attached garage was the exception to
this tendency in that the indoor air samples contained levels of MTBE,
benzene and other compounds associated with gasoline which were
significantly higher than air samples measured outside that home. This
indicated that the residential garage may have had a source of
evaporative emissions after parking the hot car in the garage or from
gasoline stored in the garage.
Huber (1995) used a multizonal mass balance model to predict
indoor air concentrations. Measured evaporative emissions of 0.5 g of
MTBE emitted from an automobile at rest at 23.9°C (a highly
unrealistic estimate for Fairbanks in winter) during 4 h in a garage
attached to a residential house resulted in modelled peak
concentrations of 2.3 mg/m3 (0.65 ppm) in the garage and 0.12 mg/m3
(0.035 ppm) in the residence. Modelled 1-h average concentrations in
the garage ranged from 2.5 to 4.3 mg/m3 (0.7 to 1.22 ppm) while those
in the residence ranged from 0.072 to 0.32 mg/m3 (0.02 to 0.09 ppm).
This was estimated to be a worst-case situation for evaporative
emissions since a newer car or cold winter temperatures would probably
have reduced evaporative emission rates resulting in lower
concentrations (Huber, 1995). However, increased tailpipe vehicle
emissions as a result of cold start (the first few minutes of running
the engine before the catalytic converter starts to function) were not
included in these estimations.
In the case of the Stamford microenvironment samples, the
resulting 8-h average MTBE levels in indoor commercial garage service
areas and indoor locations near a roadway were lower than those taken
in Fairbanks even though the volume of MTBE present in Stamford
gasoline was higher than the volume used in Fairbanks during Phases II
and III. It is important to note that the service station pump island
samples were not strictly comparable since they were taken 4.5 m away
from the pump. When MTBE was only used as an octane enhancer, as was
the case for Albany and Fairbanks Phase III, levels of MTBE in service
station pump island air were found to be much higher in Albany.
Air measurements in the parking garage microenvironment have been
conducted in two studies. MTBE concentrations measured in two 8-h air
samples in a Stamford partially open parking lot (i.e. open-sided with
an office building directly above) in April 1993 were 70.4 µg/m3
(20.1 ppbv) and 177 µg/m3 (49.0 ppbv) with a mean of 124.7 µg/m3
(34.6 ppbv) (RB Zweidinger, personal communication). Allen & Grande
(1995) conducted measurements at an enclosed parking ramp in Milwaukee
in February 1995 in order to show ambient levels during cold starts.
One- to three-hour air samples showed MTBE levels of less than 72.1
µg/m3 (20 ppbv) with mean levels of 7.4 µg/m3 (2.05 ppbv) (n = 8).
The highest level occurred at a point when a large number of vehicles
were making cold starts in a short period of time.
Air samples were taken by Lioy et al. (1994) inside the vehicle
cabin microenvironment for (1) activities surrounding refuelling, and
(2) during suburban commutes before and after refuelling. The study
took place in April 1993 in New Jersey, New York and Connecticut where
gasoline containing 10 to 15% by volume MTBE was sold. The experiment
protocol consisted of a 60-min commuter run that included a 5-min
refuelling stop at full- and self-service stations with or without
Stage II vapour recovery. Resulting in-cabin levels of MTBE taken
immediately before, during and after refuelling ranged from 23.8 to
108 µg/m3 (6.6 to 30 ppbv), 133.4 to 313.5 µg/m3 (37 to 87 ppbv) and
31.4 to 151.4 µg/m3 (8.7 to 42 ppbv), respectively, for three
different cars, with average concentrations of 55.3 µg/m3 (14.8
ppbv), 190.2 µg/m3 (55 ppbv) and 72.1 µg/m3 (20 ppbv). Short-term
peak concentrations occurred during refuelling. In addition,
post-refuelling in-cabin concentrations were slightly higher than
pre-refuelling, although the increase was not statistically
significant. It was noted that the highest of the levels that diffused
into the cabin during refuelling occurred with an older vehicle, which
had an abnormally high evaporative emissions rate. There did not seem
to be a statistically significant difference in in-cabin levels
between the various types of service stations, although the sample
size was too small to be able to make this conclusions.
Microenvironmental in-cabin air concentrations of MTBE measured during
60-min suburban stop/go commutes ranged from 3.6 µg/m3 (1 ppbv) to
576.6 µg/m3 (160 ppbv) with a geometric mean of 21.6 µg/m3 (6 ppbv)
(n = 40). Most values were less than 19.8 µg/m3 (5.5 ppbv). It was
noted that the higher values were associated with the use of the
high-emission vehicle discussed previously.
Area monitoring was conducted in an indoor commercial garage
service area in Fairbanks, Alaska (Buchta 1993a). Three air samples
were taken over a 6- to 7-h period in February 1993 when MTBE use was
limited to purposes of octane enhancement. MTBE was non-detectable in
the three areas (service area, parts department, shop wall) but the
minimum detection limit was high (144.2 µg/m3, 40 ppbv). This is
comparable to the Zweidinger (1993) 8-h indoor commercial garage air
samples that were measured with more sensitive equipment and resulted
in a median concentration for MTBE of 114.4 µg/m3 (31.75 ppbv). The
higher levels in the latter study may have been the result of gasoline
spills.
Ambient air levels of MTBE have been measured near three
refineries in the USA. At one refinery a 24-h MTBE level of 20 µg/m3
was reported for one out of nine downwind samples taken at the
perimeter of a rural refinery, which was stated to release
approximately 33 tonnes of MTBE emissions in air per year. MTBE was
not detected in the 26 other downwind and upwind samples taken at the
refinery during the same period. MTBE was not detected in another 54
24-h samples taken at two other refineries: annual MTBE air emission
release data were not provided for these refineries. It should be
noted that the detection limit for these air samples was high (more
than 20 or 30 µg/m3), but more sensitive canister samples taken for
24 h also resulted in non-detectable concentrations (detection limit =
6 µg/m3) (API, 1989b).
c) Finland
Vainiotalo et al. (1996) reported the concentration of MTBE at
the perimeter and pump island of two self-service stations in Finland
(one urban roadside and one simple roadside) both with Stage Ia
vapour recovery systems where gasoline containing 11% MTBE by volume
was sold. The investigations were conducted during May/June and
October 1995. The average 24-h perimeter concentrations for each of
the 4-day sampling periods were generally higher for the urban
roadside service station samples: 12.4 µg/m3 (June) and 14.1 µg/m3
(October), with 35-36 measurements collected at each side on each
occasion. Several factors such as the volume of gasoline sold, mean
wind speed and number of deliveries of gasoline to the station were
higher for the former and resulted in data with higher variability
during the fall sampling. The overall range of perimeter air samples
for both service stations was from 0.5 to 120.5 µg MTBE/m3. Highest
daily concentrations were usually obtained at the downwind sampling
points. No seasonal influence was discernable. Mean 24-h
concentrations measured in the centre of the pump island ranged from
247 to 1347 µg/m3 (n = 15). The detection limit was not specified in
the study.
5.1.1.2 Dermal exposure
Dermal exposure and absorption may occur from MTBE-blended
gasoline at self-service refuelling or from its use as a solvent. It
may also occur from MTBE-contaminated household water during washing,
bathing or showering. There are, however, no data available to
estimate dermal exposure to MTBE.
5.1.1.3 Estimation of total personal exposure
Exposure is a function of concentration and time. Thus, the time
spent in various activities involving different concentrations and
degrees of contact will affect human exposures to MTBE. Huber (1995)
generated "worst-case scenario" estimates of long-term exposure to
MTBE based on population activity patterns and available
microenvironmental and ambient concentration data (generally rounded
a In the USA, Stage I is a vapour recovery system used during
loading and unloading of gasoline from delivery tankers. Since
this is a Finnish Study, this definition may or may not be
equivalent.
up to the next order of magnitude of 10). His intentionally high
estimates were updated and slightly revised to indicate that an annual
time-weighted average exposure might be as high as about 0.11 mg/m3
(0.03 ppm), assuming that gasoline contained 15% MTBE by volume for 6
months and approximately 10% for the remainder of the year (US
Interagency Assessment, 1997). This upper-end exposure estimate is
highly uncertain, given the lack of adequate data to describe the
distribution of actual personal exposure levels.
5.1.1.4 Other pollutants
There was an increase in indoor air levels of benzene after MTBE
reformulated fuel use was discontinued (Gordian & Guay, 1995). Both
ambient outdoor and specific indoor environment (garages, vehicles,
workplace, school, post office and residence) samples, as well as
blood samples, were collected in Fairbanks during and after the
oxygenated programme. In addition to MTBE, other volatile compounds,
such as benzene and formaldehyde, were evaluated. In indoor samples,
there was a statistically significant increase of benzene in garages
and non-garages after MTBE was discontinued. In garages, the mean
benzene concentration increased from 0.30 mg/m3 (94.02 ppb) in
December to 0.61 mg/m3 (191.62 ppb) in February, and in the school,
post office and residence from 0.02 mg/m3 (5.89 ppb) to 0.06 mg/m3
(20.22 ppb). The NIOSH-recommended exposure limit for benzene is 0.3
mg/m3 (0.1 ppm). In vehicles the data were difficult to interpret
because different vehicles were tested in December and in February.
The same pattern, although not statistically significant, was seen in
the outdoor samples.
5.2 Occupational exposure
5.2.1 Industrial operations - manufacturing and blending
MTBE can be encountered in solution and as vapour during its
manufacturing at chemical plants and refineries, during blending into
gasoline, transportation, distribution, and handling at service
stations. Some industrial hygiene monitoring data are available (Table
13).
At the MTBE unit at the Neches Chemical West Plant in the USA,
the TWA values ranged from less than 0.07 to 120.3 mg/m3 (0.02 to
33.41 ppm) for operations and maintenance personnel (Simer, 1986).
Exposure monitoring in a manufacturing plant showed that all
exposures to MTBE greater than 3.6 mg/m3 occurred during quality
control sampling procedures (ARCO, 1987). Two out of 46 short-term
samples indicated exposures to MTBE at concentrations of 22 mg/m3 and
6.5 mg/m3 (6.1 ppm and 1.8 ppm, respectively); 91% of the full shift
monitoring indicated exposure levels of less than 3.6 mg/m3.
Texaco (1993) presented results from a short-term monitoring for
MTBE conducted at a refinery in Guatemala in order to evaluate the
adequacy of current work procedures. No details of ambient temperature
Table 13. Occupational exposure to MTBE in the petroleum industry (adapted from HEI, 1996)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
MTBE manufacturing
Occupational exposure of
oil refinery and chemical
plant personnel handling
neat MTBE during:
Routine operations <30 min 14/27 0.16-1.00 0.16-7.8 1.00 API, 1995b
6-9 h TWA 38/76 0.01-0.03 0.01-248.7 0.03 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.17 0.17 API, 1995b
Routine maintenance <30 min 7/8 0.05 0.05-7.19 0.90 API, 1995b
30 min-6 ha 1/1 Not given 0.20 0.20 API, 1995b
6-9 h TWA 4/4 Not given 0.04-0.7 0.11 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.2 0.18 API, 1995b
Routine operations and
maintenance 8-12 h TWA 8/21 0.02-0.06 <0.02-33.41 1.06 Simer, 1986
20 min 0/1 [1.0] <1.0-1.0 <1.0 ARCO, 1987
4-6 h 1/11 [1.0] <1.0-6.1 <1.0 ARCO, 1987
12 h TWA 2/23 [1.0] 0.8-2.2 Not given ARCO, 1987
QA/QC sampling of MTBE 12-36 min 3/16 [1.0] <1.0-12.2 <1.0 ARCO, 1987
MTBE blending
Occupational exposure of
personnel involved in
fuel-blending activities
involving:
Neat MTBE <30 min 34/35 <0.005 0-97.0 2.90 API, 1995b
30 min-6 h 12/13 0.21 0.21-72.0 1.03 API 1995b
6-9 h TWA 7/12 0.04-1.80 0.04-87.97 2.24 API, 1995b
>9 h TWA 0/9 0.23-0.34 0.23-0.34 0.30 API, 1995b
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
Fuel mixtures <30 min 51/98 0.02-0.23 0.02-100 0.30 API, 1995b
30 min-6 h 5/19 0.03-0.33 0.03-1.98 0.05 API, 1995b
6-9 h TWA 34/112 0.02-0.20 0.02-14 0.04 API, 1995b
>9 h TWA 9/22 <0.005-0.02 0-0.27 0.02 API, 1995b
MTBE transport
Occupational exposure of
marine barge, pipeline and
rail car personnel to:
Neat MTBE <30 min 62/66 0.30-0.60 0.30-1050 13.83 API, 1995b
(trucking personnel included 30 min-6 h 23/27 0.04-0.36 0.04-700 2.20 API, 1995b
only for transport of neat 6-9 h TWA 9/10 0.03 0.03-711.9 0.18 API, 1995b
MTBE) >9 h TWA 1/1 Not given 0.32 0.32 API, 1995b
15 min TWA 4/4 Not given 90-150 110.00 Texaco, 1993
Fuel mixtures <30 min 60/64 0.001-0.14 0.001-507.87 2.44 API, 1995b
30 min-6 h 64/92 0.02-0.04 0.02-59.4 0.42 API, 1995b
6-9 h TWA 28/42 0.007-0.04 0.01-26.24 0.14 API, 1995b
>9 h TWA 8/8 Not given 0.19-4.51 1.49 API, 1995b
MTBE distribution
Occupational exposures of <30 min 93/129 <0.005-0.08 0-14.0 0.75 API, 1995b
marketing terminal and 30 min-6 h 9/10 0.26 0.26-4.05 0.98 API, 1995b
trucking personnel involved 6-9 h TWA 62/87 0.01-0.05 0.01-2.2 0.11 API, 1995b
in the handling of >9 h TWA 46/47 0.06 0.06-6.2 0.71 API, 1995b
Gasoline-MTBE mixtures 15 min TWAe ´ 0.2 <0.2-0.94 0.48 Hebert, 1993
10-12 h TWAe 2/2 Not given 0.08-0.08 0.08 Hebert, 1993
15 min TWAf 4/4 Not given 0.05-0.16 0.14 Gillie, 1993
15 min TWAg 3/3 Not given 1.9-3.6 2.80 Gillie, 1993
12 h TWAg 5/5 Not given 0.24-0.92 0.45 Gillie, 1993
15-40 minf (n=6) 0.2 2.8-42 13 (mean) Hakkola & Saarinen,
1996
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
10-30 minh (n=4) 0.2 20-226 91 (mean) Haakola & Saarinen,
1996
22-44 mini (n=5) 0.2 4.3-27 16 (mean) Haakola & Saarinen,
1996
10-37 minj (n=6) 0.2 10.0-98.0 71 (mean) Haakola & Saarinen,
1996
a Duration was task-related.
b Number of samples in which MTBE was detected divided by total number of samples.
c In the case of "non-detectable" samples the detection limit was used to calculate the median.
d The API (1995b) measurements used different sampling and analytical techniques on both personal breathing zone and area air samples.
e Loading of trucks with vapour recovery.
f Bottom loading of trucks with vapour recovery.
g Truck unloading at service station with vapour recovery.
h Top loading without vapour recovery.
i Truck unloading at service station without vapour recovery - Northern Finland.
j Truck unloading at service station without vapour recovery - Southern Finland.
were given. Air concentrations associated with transfer of neat MTBE
from tank cars to a storage tank ranged from 324 to 540 mg/m3 (90-150
ppm).
Hinton (1993) performed an occupational exposure study of MTBE
employees designed to determine the amount of MTBE exposure during
manufacturing, blending MTBE into gasoline, transportation,
distribution and handling at service stations. The study included 2038
exposure measurements during an 11-year period. Occasionally the TWA
exposure value exceeded 360 mg/m3 (100 ppm) and the short-term (less
than 30 min) exposure 1080 mg/m3 (300 ppm), generally during
non-routine or extraordinary tasks. The maximum short-term value
sampled for less than 30 min, 3780 mg/m3 (1050 ppm), was recorded
during transportation of neat MTBE. The maximum TWA level was 2563
mg/m3 (712 ppm) for the same activity. Usually, the TWA levels were
less than 7.2 mg/m3 (2 ppm) and the short-term levels were less than
36 mg/m3 (10 ppm). Exposures in blending operations were less than
360 mg/m3, and generally less than 36 mg/m3. In distribution, MTBE
levels were less than 3.6 mg/m3, and for service station attendants
levels were less than 10.8 mg/m3 (3 ppm).
5.2.2 Transportation
An exposure assessment for MTBE vapour concentrations conducted
on two gasoline truck drivers in New Jersey showed an average exposure
concentration of 0.29 mg/m3 (0.08 ppm) on a full shift (10-12 h)
basis. The short-term 15-min TWA exposure was 2.05 mg/m3 (0.57 ppm)
(Hebert, 1993).
Monitoring has been made for workplace concentration levels for
seven gasoline truck operators to assess exposure potentials during
loading operations and during full unloading at service stations
(Gillie, 1993). The 12-h TWA ranged from 0.86 to 3.31 mg/m3
(0.24-0.92 ppm). Short-term exposure (5-23 min) during truck loading
operations ranged from 0.18 to 0.58 mg/m3 (0.05-0.16 ppm) and during
fuel unloading from 3.24 to 12.96 mg/m3 (0.9-3.6 ppm).
The occupational exposure of road tanker drivers to gasoline and
some of its components, including MTBE, has been measured in Finland
in two depots and 11 service stations during loading and delivery
(Hakkola & Saarinen, 1996). In Finland, unleaded gasoline contains
10-15% MTBE in liquid phase. The monitoring was made during the
summer, and the temperatures ranged from 4 to 22°C. In the south of
Finland, four measurements were carried out during top loading and six
measurements during delivery at service stations. In the north of
Finland, six measurements were performed during bottom loading and
five measurements at service stations during delivery. The mean
short-term exposures of road tanker drivers to MTBE during loading and
delivery were between 13 and 91 mg/m3. The differences in exposure
during bottom (2.8-42.0 mg/m3, mean 13 mg/m3) and top loading
without vapour recovery (20-226 mg/m3, mean 91 mg/m3) were
statistically significant (p<0.02). There also was a statistically
significant difference (p<0.03) during delivery in northern (4.3-27.0
mg/m3, mean 16.0 mg/m3) and southern Finland (10-98 mg/m3, mean 71
mg/m3). The exposure time for loading was 25-35 min and for
delivering 30-40 min per load.
5.2.3 Service station attendants and garage mechanics
An exposure assessment for MTBE vapour concentrations conducted
on six full-service gas attendants in New Jersey showed an average
exposure of 1.76 mg/m3 (0.49 ppm) on an 8-h TWA basis (Hebert, 1993).
For the short-term 15-min TWA, the average exposure was 2.16 mg/m3
(0.60 ppm).
In an evaluation of exposure among service station attendants and
operators, Hartle (1993) compared the exposure potential at three
categories of service stations. Two facilities in Cincinnati, Ohio,
represented service stations that did not use MTBE or used it only as
an octane enhancer. In Phoenix, Arizona, two high-volume stations were
selected, and in Los Angeles two service stations with advanced vapour
recovery were selected. In Phoenix, where the MTBE content averaged
12.5-13% by liquid volume, the exposure measurements (41 samples)
ranged from 0.14 to 13.97 mg/m3 (0.04-3.88 ppm) with an average of
1.08 mg/m3 (0.3 ppm). The Los Angeles exposure ranged from 0.07 to
2.63 mg/m3 (0.02-0.73 ppm), averaging 0.50 mg/m3 (0.14 ppm). In
Cincinnati, only one of 32 samples was above the analytical limit of
detection, i.e. 0.58 mg/m3 (0.16 ppm).
Giacomello (1996) measured personal exposure of "full service"
attendants to MTBE in 58 Italian service stations. The study included
a number of geographical locations throughout the country and was
conducted in the summer and winter in 1992 and 1995. An overall
geometric mean of 0.71 mg/m3 (1992) or 0.26 mg/m3 (1995) was
recorded in the summer, and 0.37 mg/m3 in winter (latter data only
for 1992).
Three NIOSH studies were performed in workers potentially exposed
to gasoline and exhaust emissions during their work day (Almaguer,
1993; Buchta, 1993a,b). Breathing zone air samples were collected from
workers exposed to MTBE and other gasoline components (benzene,
toluene, and xylene, and, in one study, carbon monoxide) in several
maintenance facilities for motor vehicles located in Fairbanks, Alaska
(Buchta 1993a), in Stamford, Connecticut (Buchta, 1993b), and in
Albany, New York (Almaguer, 1993). In two of the cities (Fairbanks and
Albany), MTBE was only used as an octane enhancer (generally less than
1% of the fuel) during the study period. In Stamford, however, the
MTBE content of the fuel ranged from 13 to 17% with an average of
14.2% by volume. The highest workplace exposure level concentrations
were less than 0.50 mg/m3 (0.14 ppm) in Albany and less than 1.6
mg/m3 (0.45 ppm) in Fairbanks. In Stamford, the MTBE exposure levels
ranged from 0.1 to 44.6 mg/m3 (0.03 to 12.04 ppm). The cause for the
highest value was unknown, and the next highest exposure value was
7.56 mg/m3 (2.1 ppm). In all of the studies, the highest
concentrations were measured on mechanics. The sampling was, however,
conducted in late spring, and dilution ventilation (open windows and
doors) may have affected the results.
5.2.4 Occupational exposure limit values
In the USA, the American Conference of Governmental Industrial
Hygienists (ACGIH, 1994) has recommended a TWA of 144 mg/m3 (40 ppm).
In Sweden, the occupational air exposure TWA limit is 180 mg/m3 (50
ppm) and the 15-min short-term exposure limit (STEL) 250 mg/m3 (75
ppm) (AFS, 1994). The Dutch Expert Committee on Occupational Standards
recommended a health-based occupational 8-h TWA exposure limit of 180
mg/m3 (50 ppm) (DECOS, 1994).
5.3 Exposure via water
MTBE has been found in groundwater, storm water, reservoir water,
and drinking-water in the USA (Garrett et al., 1986; Angle, 1991; Dey
et al., 1991; Post, 1994; Squillace et al., 1995a,b, 1996; Delzer et
al., 1996; Dale et al., 1997; US Interagency Assessment, 1997).
Collectively, these references show that MTBE occurs in water,
especially in areas where MTBE is extensively used, and where releases
of MTBE to air, water and soil occur.
However, while there are some national monitoring data for
ambient groundwater, monitoring data for MTBE in surface water and in
drinking-water in the USA are very limited in scope. In an extensive
review of MTBE in water in the USA, Zogorski et al. (1996) concluded
that sufficient monitoring data were not available to characterize
human exposure to MTBE by the consumption of drinking-water.
The following subsections summarize major findings for MTBE in:
(a) snow and precipitation; (b) surface water; (c) groundwater; and
(d) drinking-water.
5.3.1 Snow and precipitation
MTBE has been detected in snow at ground level in Denver,
Colorado, USA, at very low (water equivalent) concentrations
(Squillace et al., 1995b; Bruce & McMahon, 1996). There is no other
published monitoring information on the presence or concentration of
MTBE in snow or rainfall. Squillace et al. (1996) hypothesized that
concentrations of MTBE in precipitation would be greater during winter
months than warmer summer months due to the temperature effect on
air-water partitioning.
5.3.2 Surface water
Information on MTBE in streams and rivers, in Long Island, New
York and New Jersey, USA, has been reported by Stackelberg et al.
(1997). For Long Island, at a reporting level of 0.5 µg/litre, MTBE
was the second most frequently detected VOC, occurring in 29% of the
samples at concentrations ranging from 0.6 to 20 µg/litre, with an
estimated median of 0.24 µg/litre. MTBE was detected more frequently
in samples collected during winter months (33%) than summer months
(26%). In New Jersey, a limited study was completed in spring 1994
along a ten-mile reach of the Hackensack River. Land use along this
reach is highly urbanized, and numerous industries and municipal
effluents are present. The study involved the collection of a single
water sample at each of 14 sampling points just after a major snow
melt. MTBE was detected in all samples at concentrations ranging from
2.6 to 30 µg/litre, with a median of 7.75 µg/litre (Stackelberg et
al., 1997). Reconnaissance sampling of eight streams elsewhere in New
Jersey in 1996 showed the presence of MTBE in water samples for seven
of eight sites. The concentrations ranged from 0.2 to 4.9 µg/litre
(Stackelberg et al., 1997). MTBE is used in reformulated gasoline at
both Long Island, New York and in New Jersey, as part of a mandatory
air abatement programme.
Measurable but low concentrations of MTBE were found in some of
592 stormwater samples (including samples from culverts, concrete
pipes, lined ditches and channels) collected by the US Geological
Survey in 16 cities and metropolitan areas from 1991 to 1995 (Delzer
et al., 1996). MTBE was found in 6.9% of the samples (41 of 592
samples). When detected, concentrations ranged from 0.2 to 8.7
µg/litre, with a median below 1.0 µg/litre. Eighty-three percent of
the detections occurred during the winter season (October to March)
when oxygenated gasoline to abate CO pollution was expected to be
used. A comparison of MTBE concentrations for samples collected during
the summer and winter periods showed a statistically significant
difference. Twenty-seven out of 148 stormwater samples contained both
MTBE and BTEX compounds, indicating a common source for these samples.
The Metropolitan Water District of Southern California (MWDSC)
relies, in part, on six lake-reservoirs for the storage of raw water
to be used for drinking-water in Southern California. These reservoirs
have varying degrees of recreational use. MWDSC began quarterly
monitoring of these reservoirs for MTBE in the second quarter of 1996.
MTBE was detected at the surface of Lake Perris, with confirmation in
two subsequent samplings, at an average concentration of 15 µg/litre.
The following quarter MTBE was also detected at an average level of 19
µg/litre. No MTBE was detected above the study's reporting level of 1
µg/litre in any of the other reservoirs, which were sampled at outlet
towers, where water is drawn from a lower depth (Dale et al., 1997).
5.3.3 Groundwater
Data from urban and agricultural areas show that MTBE occurs
predominantly in shallow groundwater underlying urban areas, and, when
present, occurs typically at low concentrations. In 1993-1994, the US
Geological Survey measured the concentrations of MTBE and 59 other
VOCs in 210 shallow wells (five drinking-water wells, 12 springs and
193 monitoring wells) in eight urban areas and 549 shallow groundwater
wells from 21 agricultural areas, and deeper groundwater from 412
wells sampled in nine areas throughout the USA (Squillace et al.,
1995a,b, 1996). MTBE occurred in 27% of the shallow urban wells and
springs. Detectable levels of MTBE were found in 86% of the wells in
industrial areas, 31% of the wells in commercial areas, 23% of the
wells in residential areas, and 23% of the wells in areas of mixed
urban land use, parks and recreation areas. MTBE was the second most
frequently detected compound after trichloromethane (chloroform). In
73% of the 210 shallow urban wells, concentrations were less than the
reporting level of 0.2 µg/litre. The estimated median value for urban
areas was below 0.2 µg/litre (Squillace et al., 1996). Three percent
of the wells had concentrations of MTBE above 20 µg/litre. No MTBE was
detected in drinking-water wells in the urban areas. In the
agricultural areas, 1.3% of the 549 shallow agricultural wells sampled
had detectable concentrations of MTBE. MTBE was also detected in four
of the 412 deeper groundwater samples from major aquifers. Three of
these wells were used for domestic or municipal water supply. The
measured maximum concentration of MTBE was 1.3 µg/litre. MTBE in
groundwater was generally not found with BTEX compounds, which
commonly are associated with point source spills of gasoline.
Bruce & McMahon (1996) measured MTBE concentrations in
groundwater in the alluvial aquifer beneath Denver, Colorado, USA, as
part of a survey of groundwater quality examining a range of dissolved
constituents. Thirty randomly selected alluvial wells were sampled.
MTBE was the most frequently detected VOC (23 out of 29 wells). The
maximum concentration was 23 mg/litre.
5.3.4 Drinking-water
Exposure to MTBE via drinking-water may involve more than direct
ingestion of contaminated water. Household uses of water, such as in
cooking, showering, bathing and washing, could result in exposure
through inhalation and dermal absorption, even if ingestion of water
was avoided.
Only limited monitoring data are available for MTBE in
drinking-water sampled at the tap or from a municipal distribution
system. Stern & Tardiff (1997) estimated that about 30% of the US
population lives in areas where MTBE is in regular use; 95% of this
population is unlikely to be exposed to MTBE in tap water at
concentrations exceeding 2 µg/litre, most will be exposed to much
lower or zero concentrations, but 5% could be exposed to higher
concentrations due to fuel tank spills and leaks entering surface and
groundwater. As part of the US Interagency Oxygenated Fuel Assessment
in the USA (US Interagency Assessment, 1997), information on MTBE
levels in drinking-water was sought from state drinking-water agencies
on a voluntary basis by the US Environmental Protection Agency.
Because monitoring for MTBE in drinking-water is not required by the
US Federal Government, only a few states have information on MTBE in
drinking-water. As such, it is not possible to describe levels of MTBE
in drinking-water for the entire USA. Based on information provided by
five states (New Jersey, Iowa, Illinois, Texas and Colorado), MTBE has
been detected in the drinking-water of 51 public water systems.
However, when detected, the concentration of MTBE was generally low
and nearly always below 20 µg/litre (Zogorski et al., 1996). These
data indicated that the consumption of drinking-water was not a major
route of exposure for these few systems (Zogorski et al., 1996). No
data on MTBE in drinking-water are available for other countries.
As noted previously, there have been a few instances in the USA
where groundwater used for drinking-water, both private wells and
public water systems, has become contaminated with levels of MTBE in
excess of 1000 µg/litre (Garrett et al., 1986; State of Connecticut,
1987; Zogorski et al., 1996). Many humans will probably detect MTBE in
drinking-water when the concentration exceeds about 50-100 µg/litre
owing to its low taste and odour threshold (see section 2.1 for taste
and odour values). Some humans will detect MTBE in water at even lower
concentrations. However, Du et al. (1998) considered that an MTBE
concentration below 40 µg/litre in drinking-water would avoid any
unpleasant taste or odour even for the most sensitive members of the
population.
5.4 Soil and sediment
There are very limited data concerning levels of MTBE in the
terrestrial environment. Trace amounts of MTBE have been found in
sediment samples adjacent to motorways and centres of heavy urban road
traffic density in the United Kingdom (Bianchi & Varney, 1989).
5.5 Biota
There are very limited data on MTBE levels in biota. In a study
to detect organic and inorganic contaminants in shellfish in Nova
Scotia, Canada, MTBE was not detected in any of the 21 samples assayed
(detection limit = 0.01 µg/g) (Environment Canada, 1989).
6. KINETICS AND METABOLISM IN HUMANS AND LABORATORY ANIMALS
Kinetic data from human and animal studies are summarized in
Table 14.
6.1 Human data
6.1.1 Controlled human studies
In an inhalation study two healthy young adult male and two
healthy young adult female volunteers were exposed to MTBE at 6 mg/m3
(1.7 ppm) in an environmental chamber for 1 h. The mean blood level
rose from 0.83 ± 0.5 µg/litre (0.009 µmol/litre) preexposure to 17.1 ±
2.0 µg/litre (0.19 µmol/litre) at the end of the 1 h exposure period.
One hour after the end of exposure the mean blood level fell to 6.3 ±
1.6 µg/litre (0.07 µmol/litre) after 1 h (Cain et al., 1996).
In a pharmacokinetics study, two volunteers (one healthy young
adult male and one healthy young adult female) were exposed to
5 mg/m3 (1.394 ppm) for 1 h in an environmental chamber (see also
section 8.2). There was a rapid rise in blood MTBE concentration to
6.1 µg/litre (8.2 ppb) and 10.9 µg/litre (14.7 ppb), respectively, at
1 h from the start of exposure. Following the end of exposure, there
was a rapid decline in blood MTBE concentration in both the male and
female volunteer with half-lives of 36 and 37 min, respectively. By
the end of the 7-h sampling period, blood MTBE concentration had
fallen to 0.149 µg/litre (0.2 ppb) in the male volunteer and 0.447
µg/litre (0.6 ppb) in the female volunteer (Prah et al., 1994).
In another study, the area under the curve (AUC) values of MTBE
and TBA were proportional to the MTBE exposure levels following short-
term inhalation to 18, 90 or 180 mg/m3 (5, 25 and 50 ppm), indicating
linear kinetics up to at least 180 mg/m3 (Johanson et al., 1995).
Following exposure to 180 mg MTBE/m3, the elimination of MTBE and TBA
was complete within 24 and 48 h, respectively (Johanson et al., 1995).
Pekari et al. (1996) measured concentrations of MTBE in blood,
urine and exhaled air from four volunteers exposed to 90 or 270 mg/m3
(25 or 75 ppm) by inhalation for 4 h. A lung retention of around 40%
was recorded, and blood levels of 11 µmol/litre (970 µg/ litre) at 90
mg/m3 or 29 µmol/litre (2556 µg/litre) at 270 mg/m3 were achieved
towards the end of the exposure period. Of the MTBE absorbed, the
majority (about 58%) was excreted unchanged in expired air and small
amounts (1.4%) unchanged in urine. The concentration of TBA in blood
reached a peak of 16 or 34 µmol/litre (1419 or 2997 µg/litre)
(following the low or high exposure, respectively) 15-45 min after
exposure ceased. Trace amounts of TBA (1.2%) were found in urine, but
none was detected in exhaled air. The terminal half-life for MTBE in
blood was determined to be 5 h, while that for TBA was 11.9 h. The
authors concluded that metabolism of MTBE was linear at exposures up
to 268 mg/m3 (75 ppm).
Table 14. Summary of kinetic data for MTBE
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
A. Human
2 male, 2 female rapid absorption mean MTBE blood Cain et
volunteers from 0.003 concentrations rose al. (1996)
Treatment: 6 mg/m3 µg/litre preexposure steeply from 0.83
(1.7 ppm) for 1 h to 0.06 µg/litre ±0.50 µg/litre
in inhalation after 1 h preexposure to a
chamber peak blood level of
17.1±2.01 µg/litre
by the end of
exposure; rapid
elimination, half-life
of 40 min; the rapid
elimination phase
appeared to last
approximately 1 h
1 male, 1 female rapid rise in blood rapid decline observed Prah et
volunteer MTBE to 0.03 µg/litre in blood levels with al. (1994)
Treatment: 5 mg/m3 (8.2 ppb) in the male a half-life of about
(1.39 ppm) as a 1-h and 0.05 mg/m3 35 min and rapid
exposure; blood (14.7 ppb) in the metabolic transformation
samples up to 580 female to TBA; TBA levels
min after start gradually increased and
plateaued at 0.025-0.36
µg/litre (7-10 ppb) and
maintained this
concentration up to 7 h
post-exposure
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult relative MTBE detected in clearance of MTBE Johanson et
male volunteers respiratory uptake expired air; both was 0.5 litre/h per al. (1995)
Treatment: exposed was 32-42%; rapid MTBE and TBA found kg; AUC values of
in chamber to 18, absorption based in blood and urine MTBE and TBA were
90 and 180 mg/m3 upon blood proportional to
(5, 25 and 50 ppm) concentration; by exposure levels,
for 2 h on three 2 h, peak blood suggesting linear
occasions during concentrations of kinetics; elimination
light physical MTBE were 1.3, of MTBE in blood
exercise (50W); 6.3, and 12.2 indicated three
observed up to µmol/litre at 18, phases (6-7 min,
24 h and 48 h 90, and 180 mg/m3, 46-58 min and
(only 180 mg/m3) respectively; 6.2-7.2 h); in
post-exposure TBA 5.7 and 0.7 urine half-lives
µmol/litre at 90 of 16-22 min and
and 180 mg/m3, 3.0-3.1 h were
respectively; steady identified;
state was reached half-life of TBA
for TBA after 3-5 h, in urine was
but was not reached 7.5-8.9 h; by 3.5 h
during the 2 h post-dose, 20-30%
exposure to MTBE of absorbed dose
was eliminated in
expired air; by
24 h, approx. 0.1%
MTBE and 0.5-0.8%
TBA of absorbed
dose was eliminated
in urine
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult respiratory uptake mean concentrations TBA was found in respiratory Nihlén et
male volunteers was 42-49% in blood at the blood and urine exhalation was al. (1998a)
Treatment: exposed three exposure 32-47%; elimination
in chamber to 18, concentrations were in blood was in
90 and 180 mg/m3 1.4, 6.5, and 13 four phases of 1
(5, 25 and 50 ppm) µmol/litre min, 10 min, 1.5 h,
for 2 h on three respectively and and 19 h; kinetics
occasions during were concentration- were linear up to
light physical related the exposure
exercise (50W); concentration of
blood and urine 180 mg/h;
collected during elimination in
exposure and for 3 urine was biphasic
days post-exposure with mean half-lives
of 20 min and 3 h;
excretion was nearly
complete 10 h after
exposure; metabolic
clearance was
0.34-0.52 litre/h
per kg; renal
clearance of TBA
was 0.6-0.7 ml/h
per kg and TBA was
still present after
22 h
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
B. Rodent
I) Oral administration
Species: rapid and extensive apparent volume of in plasma, the dose-related Ferdinandi
Fischer-344 rats absorption of MTBE distribution was major metabolite, differences were et al. (1990a)
Treatment: 40 based upon peak 0.27 to 0.43 litre TBA, peaked at 2 h; observed for the Miller et
rats/sex/dose group plasma concentration male rats had higher plasma elimination al. (1997)
received 40 or 400 within 15 min of plasma concentrations half-time (T´) of
mg/kg as single dosing; lower plasma than female rats; MTBE and TBA:
dose; observed concentrations of dose-unrelated plasma T´ of MTBE:
(sacrificed) for MTBE in female rats concentrations of 0.52-0.62 h (low
various time-points compared to male MTBE and TBA indicate dose) 0.74-0.88 h
until 36 h rats; higher AUC enzyme saturation (high dose) T´ of
post-dose values of MTBE and TBA: 0.95-1.0 h
TBA with oral dosing (low dose)
compared to 1.6-1.9 h (high
intravenous dose) Plasma
administration clearance (CL) of
MTBE:
male rats:
0.36-0.41 litre/h
(both dose-groups)
female rats:
0.48 litre/h (low
dose) 0.29 litre/h
(high-dose)
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: by 48 h, 1.7-3% of higher radioactivity slight sex difference Ferdinandi
Fischer-344 rats administered was recovered in in the route of et al. (1990b)
Treatment: 6 radioactivity was lungs at high oral elimination; by 48 h, Miller et
rats/sex/dose group recovered in dose and lower recovery was 46-54% al. (1997)
received 40 or 400 carcass and tissues; activity in kidneys at low dose and
mg/kg 14C-MTBE as 86 and 81% of suggesting enzyme 65-69% at high dose
a single dose; administered saturation; most of (highest in females);
observed for 48 h radioactivity was the administered 29-36% was recovered
recovered in lungs dose was exhaled as in urine at low dose
and kidneys at low unchanged MTBE and 11-16% at high
and high dose, (predominating) and dose (highest in males)
respectively TBA (2.5-3.1% at low less than 1% was
dose and 1.3-1.4% at recovered in faeces
high dose); in urine,
the major radiolabelled
species (from further
oxidation of TBA) were
2-methyl-1,2-propanediol,
alpha-hydroxyisobutyric
acid, and two minor
unidentified components;
no sex differences in
biotransformation
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
ii) Inhalation
Species: Wistar dose-related MTBE was metabolized Savolainen
rats concentrations of at the ether bond. et al. (1985)
Treatment: 5 male MTBE and TBA in Two weeks of exposure
rats/dose group blood after 2 weeks caused transient
were exposed to of exposure; the increase of the
180, 360 or 1080 concentration of microsomal UDP
mg/m3 (50, 100 or MTBE decreased glucuronosyl-transferase
300 ppm) vapour in after 6 weeks; activities in liver and
exposure chambers the concentrations kidney at all dose
6 h/day, 5 days/week of TBA increased levels. After an initial
for 2, 6, 10 or 15 after 6 weeks of decrease the muscle
weeks exposure and began creatinine kinase
to decrease after activity gradually
10 weeks. increased towards the
Brain levels of MTBE end of the exposure.
and TBA followed a A minor induction of
similar course. MTBE kidney cytochrome
was also found in P-450 was noted. Almost
perirenal fat and at no effect was found on
higher concentrations hepatic cytochrome
than in blood or brain. P-450 concentrations,
Concentrations of MTBE brain succinate
in the blood, brain dehydrogenase, creatine
and perirenal fat were kinase, or
directly related to the acetylcholinesterase
concentrations in the activities
inspired air. The
ratios of TBA/MTBE in
blood increased from
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
week 2 to week 15,
indicating that MTBE
had been oxidized to
TBA, which was
eliminated from the
blood at a slower rate
than MTBE
Species: (T1) rapid absorption (T1) apparent volume (T1) increased AUC Ferdinandi
Fischer-344 rats based upon plasma of distribution: values of MTBE et al.
Treatment: concentration; peak low dose: 35-fold (males) and (1990c)
blood concentration 0.40 (females) and 37-fold (females) Miller et
(T1) 52 rats/sex/ was reached within 0.52 litre (males) while the AUC al. (1997)
dose group received 4-6 h for MTBE and high dose: values of TBA
1440 or 28 800 6.5 h for TBA; low 0.25 (males) and increased by 15-fold
mg/m3 (400 or 8000 but statistically 0.24 litre (females) (males) and 7-fold
ppm) as a single significant (females); quotients
6-h dose. difference in sexes of repeated and
of MTBE AUC values single dose AUC
(T2) 40 rats/sex (low dose); TBA plasma values (T2/T1) for
were exposed 6 h concentration (AUC) MTBE were 0.64
daily to 1440 mg/m3 was lower in female (males) and 0.53
(400 ppm) for 15 rats compared to male (females) and for
days; observed rats, statistically TBA 1.1 (males) and
(sacrificed) at significant at high 1.3 (females); a
various time-points dose significant
until 12 h (T1) and difference in plasma
18 h (T2) post-dose clearance between
single doses 0.53
(males) and 0.57
(females) litre/h
(low dose) and 0.30
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
(males) and 0.32
(females) litre/h
(high dose); the
plasma half-life of
MTBE was 0.52-0.63 h
(T1) and 0.48-0.51 h
(T2); the (T1)
half-lives of TBA
were 2.8 (males) and
3.4 h (females) and
(T2) 1.8 h (males)
and 1.5 h (females)
Species: (T1&T2): rapid and by 48 h, 11-13% (T1&T2): radiolabelled rapid elimination Ferdinandi
Fischer-344 rats extensive absorption (low dose; T1&T2) species in expired air in urine after low et al.(1990d)
Treatment: as indicated by and 4-5% (high dose) were MTBE and TBA; by dose and in expired Miller et
(T1) 6 rats/sex/ marked recovery of of administered 3 h post-dose, 30% air at high dose, al. (1997)
dose group received 14C in urine by 24 h radioactivity (low dose) and 7-10% suggesting enzyme
1440 or 28 800 recovered in skin (high dose) of saturation at high
mg/m3 (400 and of some rats, recovered radioactivity dose; by 48 h, total
8000 ppm) of probably due to were correlated to TBA, recovery of
14C-MTBE as a contamination from while TBA was major radioactivity (14C)
single 6-h urine (lower radioactive component in urine 65-71% (low
exposure radioactivity at 24 h post-dose dose, T1&T2) and
recovered in urine); (>90%, low dose); 35-42% (high dose);
(T2) 40 rats/sex 0.6-1% in skin of major radiolabelled in expired air 17-22%
were pretreated remaining rats, 1-3% species in urine from (low dose) and 54-59%
with 1440 mg/m3 in carcass, and <1% further oxidation of (high dose) and <1%
unlabelled MTBE in tissues TBA were 2-methyl-1, in faeces; no sex
6 h a day for 14 2-propanediol, alpha- difference in rate
days, followed by hydroxyisobutyric acid, and route of
6 h exposure for two minor unidentified radioactivity
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
1440 mg/m3 14C-MTBE components, and minor elimination
on day 15; observed detection of CO2 (1%
for 48 h post-dose of the dose); no sex
differences in
biotransformation
iii) Intravenous administration
Species: a statistically apparent volume of by 2 h, the major half-life of MTBE Ferdinandi
Fischer-344 rats significant distribution 0.27 metabolite TBA was (plasma) 0.45-0.62 h; et al.(1990a)
Treatment: 40 rats/ difference in plasma to 0.31 litre found in blood at half-life of TBA Miller et
sex/group received concentrations (AUC) peak concentration; (plasma) 0.92-1.3 h; al. (1997)
40 mg/kg as a between sexes (lower male rats had higher clearance from plasma
single dose; in females) blood concentration of MTBE 0.36-0.41
observed than female rats litre/h (males) and
(sacrificed) for 0.47 litre/h (females)
various time-points
until 36 h
post-dose
Species: rat, 1.7-3% of administered by 6 h, major by 48 h, 71-73% of Ferdinandi
Fischer-344 radioactivity was radiolabelled species administered et al.(1990b)
Treatment: 6 rats/ recovered in tissues in expired air was radioactivity was Miller et
sex/group received and carcass for each MTBE, with a minor totally recovered: in al. (1997)
40 or 400 mg/kg dose after 48 h elimination of TBA exhaled air (42-46%),
14C-MTBE as a (2.5-3.1% of in urine (26%), and
single dose; administered dose); faeces (<1%); rapid
observed for 48 h the major radiolabelled elimination in lungs,
species in urine (from 94% within 3 h
further oxidation of
TBA) were 2-methyl-1,
2-propanediol, alpha-
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
hydroxyisobutyric acid,
and two minor
unidentified components;
there were no sex
differences in
biotransformation
iv) Intraperitoneal
Species: Charles rapid and extensive the total cumulative methanol and formic 14C activity was mainly Biodynamics
River CD rats absorption based upon 14C activity in tissues acid were found in eliminated in expired (1984)
Treatment: 33 peak blood and plasma averaged 3.39, 1.94 and plasma, liver and air; by 48 h, recovery
animals/sex/group concentrations at 5 1.14% of administered kidney was about 99.86% (approx.
received a single min post-treatment, dose at 15 min, 6 h, 92% as MTBE and approx.
dose of averaging 92.04 ± and 24 h post-treatment, 7.45% as CO2); about 3%
approximately 37.72 and 83.40 ± respectively. At 15 min, in urine and about 0.8%
60µCi 14C-MTBE 20.48 µg 14C-MTBE the majority of the in faeces (male rats);
(about 232 mg equivalents/ml blood 14C-radioactivity was approx. 3% was excreted
MTBE/kg bw) in for male and female found in mesenteric fat, in urine and 0.8% (males)
saline and were rats, respectively. liver and kidney; and 1.25% (females) in
sacrificed at The half-life of however, at 6 h and 24 h faeces. The 14C activity
intervals of 5, 14C-MTBE in blood was no 14C was found in in urine and faeces was
15, 30, and 45 59.8 min for male rats mesenteric fat mainly associated with
min, and 1, 2, 3, and 49 min for female 14C-formic acid (a total
6, 12, 24 and 48 h rats. The half-life of 3.08% of administered
post-treatment in plasma was 2.3 h dose)
for males and 1.3 h
for females
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: mouse, most of the Yoshikawa
ddY administered MTBE et al.(1994)
Treatment: 4 male was eliminated
mice/dose group unchanged in the
were administered exhaled air; >90%
a single dose of of this amount was
50, 100 or 500 eliminated within
mg/kg in corn oil 3 h. The pulmonary
solution; observed elimination showed
for 6 h an initial rapid
decrease of the
elimination ratio
followed by a slow
decrease at 100 and
500 mg/kg. The
calculated half-lives
were 45 and 80 min,
respectively. The
elimination ratios
at the three different
doses were 23.2, 37.6
and 69.0%, respectively
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
C. In vitro
Incubation of 5 or incubation of MTBE with Brady et
14 mM MTBE with liver microsomes from al. (1990)
liver microsomes phenobarbital-pretreated
from phenobarbital- rats resulted in TBA and
or acetone- formaldehyde in
pretreated or equimolar amounts. The
untreated Vmaxa value for
Sprague-Dawley rats demethylation of MTBE
(3-5 males) increased by 4-fold with
acetone-induced and by
5.5-fold with
phenobarbital-induced
microsomes compared with
the control Vmax value.
These results indicate
that cytochrome P450 2B1
(inducible by
phenobarbital) and
cytochrome P450 2E1
(inducible by acetone)
play a role in the
demethylation of MTBE.
Results after inclusion
of monoclonal antibody
against P450 2E1
indicated this enzyme
only partially
contributed to the
demethylation. Microsomes
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
pretreated with MTBE
yielded a 47-fold
induction of
pentoxyresorufin
o-dealkylase, with no
change in
N-nitrosodimethylamine
demethylase activity.
These results are
consistent with an
elevation of P450 2B1
a Vmax : the maximum velocity of the demethylation process.
Nihlén et al. (1998a) studied the uptake, distribution,
metabolism and elimination of MTBE in ten healthy male volunteers. The
subjects were exposed on three different occasions for periods of 2 h
in a chamber to MTBE concentrations of 18, 90 and 180 mg/m3 (5, 25
and 50 ppm) while performing light physical exercise. MTBE (and its
metabolite TBA) were monitored in exhaled air, blood and urine, the
latter being collected up to 3 days after exposure. Respiratory uptake
of MTBE was low (42-49%) and respiratory clearance was high (32-47%).
The metabolic blood clearance was 0.34-0.52 litre/h per kg. The
kinetics of MTBE were linear up to the highest exposure concentration
of 180 mg/m3 (50 ppm). The kinetic profile of MTBE in blood was
described as having four phases, with average half-lives of 1 min, 10
min, 1.5 h and 19 h. In urine the post-exposure decay curve of MTBE
had two linear phases with average half-lives of 20 min and 3 h. The
urinary excretion of MTBE was less than 1% of the absorbed dose.
Biomarkers and partitioning of inhaled MTBE were studied in two
(1 male, 1 female) volunteer subjects exposed for 1 h to a nominal
concentration of 5 µg/m3 (1.39 ppm), followed by clean air exposure
for 7 h (Buckley et al., 1997). MTBE concentrations in expired air,
venous blood and urine were monitored during and after exposure. The
decay of MTBE was assessed by using a 2- or 3-exponential model and
yielded residence times of 2-3 min, 15-50 min, and 3-13 h in alveolar
air, and 5 min, 1 h and 32 h in venous blood. Based on
lower-than-expected blood and expired air MTBE concentrations during
uptake and the decreasing blood-breath ratio during the post-exposure
decay period, the authors hypothesized that the respiratory mucous
membranes acted as a reservoir for MTBE, retaining 6-9% of the MTBE
intake. Compartmental monitoring was used to estimate a blood-breath
partition coefficient of approximately 18. The urinary concentration
of MTBE ranged from 0.37 to 15 µg/litre and bore little relationship
to the exposure: urinary elimination accounted for only a small
fraction (<1%) of total MTBE elimination.
6.1.2 Human exposure to oxygenated gasoline
During an oxyfuels programme in Fairbanks, Alaska, there was a
strong correlation between the workplace air levels ranging from 0.02
to 2.92 mg MTBE/m3 and the difference in blood concentrations of MTBE
between pre-shift and post-shift blood measurements (p=0.0001). The
median pre-shift concentration of MTBE in the blood of 18
occupationally exposed workers was 1.15 µg/litre (range 0.1- 27.8
µg/litre). The median post-shift blood MTBE level was 1.8 µg/litre
(range 0.2-37.0 µg/litre) (Moolenar et al., 1994).
Breath samples were collected from a person pumping gasoline
(containing 15% MTBE by volume) and a nearby observer (within 1 m),
immediately prior to and an hour after refuelling (Lindstrom & Pleil,
1996). The MTBE concentration in the sample collected during
refuelling was 412 µg/m3. The ambient background level was
approximately 25 µg/m3 MTBE. Low concentrations of MTBE (7-10 µg/m3)
were detected in the exhaled breath before the refuelling that took
2 min and 8 seconds; 40 seconds after the exposure the concentrations
had increased by factors of 35 for the observer and 100 for the person
pumping gasoline (see also section 5).
6.2 Animal studies
Repeated exposure (2 to 15 weeks) to MTBE vapour by inhalation (6
h/day, 5 day/week) resulted in dose-dependent increases in MTBE levels
in blood, brain and perirenal fat of Wistar male rats (Savolainen et
al., 1985). Very small differences were observed in blood MTBE levels
following repeated exposure. TBA levels in blood increased with
repeated MTBE exposure when comparing levels at 6, 10 and 15 weeks to
levels following 2 weeks. Perirenal fat/blood MTBE concentration
ratios ranged from 9.1 to 11.6 after 15 weeks of intermittent
exposure. Blood and brain concentrations of TBA, the major main
metabolite, were also dose-dependent. TBA was, however, not found in
quantifiable amounts in the perirenal fat.
A series of studies on the kinetics of MTBE was conducted by
Bio-Research Laboratories (Ferdinandi et al., 1990a-d). These data
have been further published by Miller et al. (1997).
Miller et al. (1997) and Ferdinandi et al. (1990a-d) described
the pharmacokinetics and disposition of MTBE in male and female
Fischer-344 rats following i.v. (40 mg/kg), oral (40 and 400 mg/kg)
and dermal (40 and 400 mg/kg in occluded chambers) administration and
(nose-only) inhalation exposure for 6 h either for a single exposure
or repeated exposure (15 days). The details of these studies are
presented in Table 15. Miller et al. (1997) found that the elimination
of radiolabelled MTBE in rats was rapid and mainly occurred through
lungs and kidneys irrespective of administration route. The
elimination was virtually complete 48 h post-dosing. The renal
elimination was, however, slowest after dermal exposure. Twelve hours
post-dosing, the radiolabelled recovery in urine was 14-26% of the
dermal dose, 41-50% of the oral dose and 25-37% of the intravenously
injected dose. The recovery was 75-94% 36 h post-dosing, irrespective
of dose and administration route. A minor difference in excretion
route was observed between the sexes. Collectively these studies
demonstrated that MTBE is rapidly eliminated from blood (half-life =
0.5 h) by exhalation and metabolism to TBA (see Table 15). The major
metabolites recovered in urine were 2-methyl-1,2-propanediol and
alpha-hydroxyisobutyric acid. Dose-related differences in the AUC for
plasma concentrations of MTBE and TBA were observed in rats exposed to
1440 or 28 800 mg MTBE/m3 (400 or 8000 ppm) by inhalation (Ferdinandi
et al., 1990a-d; Miller et al., 1997). Miller et al. (1997) further
showed that increasing doses of MTBE (by inhalation and, to a lesser
extent, oral administration) to Fischer-344 rats decreased the
recoveries of radioactivity in urine and increased the recovery in
expired air. This indicates a saturation of the oxidative metabolic
pathway of MTBE at inhalation levels above 28 800 mg/m3 (8000 ppm) or
at oral administration levels above 400 mg/kg. There were no
significant sex- or route-dependent differences in the
pharmacokinetics and disposition of MTBE.
Table 15. Pharmacokinetic parameters of MTBE and TBA calculated from mean plasma concentrations of male
F-344 rats exposed to MTBE (Miller et al., 1997)
Exposure route Dose MTBE TBA
AUC Half-life CL V/F or Vssd AUCzero to infinity Half-life
(µg/h per ml) (h) (ml/h) (litre) (µg/h per ml) (h)
Intravenous 40 mg kg 10.7 0.45 413 0.27 26.7 0.92
Oral 40 mg kg 17.0 0.52 392 0.29 39.0 0.95
400 mg kg 230 0.79 358 0.41 304 1.6
Dermal 40 mg kg 7.9 2.3a 389 3.9 26.3 2.1a
400 mg kg 46.9 1.8a 364 1.4 93.9 1.9a
Inhalation low single 84.3 0.52 531 0.40 404 3.3
high single 2960 0.57 299 0.25 6010 3.4
low repeated 6.7b 0.51 c c 127b 1.8
a Calculated from the alpha-phase of a two-compartment model. Half-lives of MTBE from 12 to 45 h post-dose curve at
low and high doses were 92 and 37 h, respectively. Half-lives of TBA from 12 to 45 h post-dose were 170 (low dose)
and 31 h (high dose).
b AUCzero to infinity on 15th day of exposure.
c Values were not calculated because plasma was not collected during exposure.
d Vss = apparent volume of distribution at steady state after repeated inhalation.
Following intraperitoneal administration of 14C-MTBE to rats,
the highest radioactivity was recovered in the expired air
(Biodynamics, 1984). The radioactivity was also distributed throughout
the animal tissues. The amount retained in tissues was less than 2% of
the total dose of MTBE intraperitoneally administered to rats at 6 and
24 h post-treatment. Distribution of the radioactivity was primarily
to the liver and secondarily to the kidney. Approximately 92% of MTBE
was exhaled as the parent compound and approximately 7.5% as CO2 in
48 h. Another 3% was excreted in urine and up to 0.8% in faeces. The
14C-activity in urine and faeces was mainly associated with
14C-formic acid. Peak blood levels were observed in male and female
rats 5 min following intraperitoneal dosing of 14C-MTBE. Peak levels
of 14C activity in the plasma occurred at 5 min post-treatment in
male rats and at 15 min post-treatment in females rats. The
radioactivity decreased sharply during 1 h and thereafter gradually
during the study period (48 h). The half-time of radiolabelled MTBE in
whole blood was 59.8 min for male rats and 49 min for female rats. The
half-life of MTBE in plasma was 2.3 h for male rats and 1.3 h for
female rats (Biodynamics, 1984).
In a study of MTBE distribution in male and female rats following
inhalation exposure to 10 710 mg/m3 (3000 ppm) for 6 h, it was
reported that MTBE levels in the liver and brain were comparable in
males and females but were higher in male kidneys than in those of
females: MTBE was still detectable in male kidneys 18 h after exposure
(Borghoff et al., 1998). The authors considered that this may have
been due to the interaction of MTBE with alpha2u-globulin in the male
rat kidney.
6.3 In vitro studies
In vitro measurement of liquid/air partition coefficients of
MTBE at 37°C, using a vial equilibration technique, showed a human
blood/ air partition coefficient of 17.7 (confidence limits:
17.0-18.4), water/ air 15.2 (CL: 14.9-15.5), and oil/air 120 (CL:
114-125) (Nihlén et al., 1995). There was no significant difference in
partition coefficient for blood/air between the sexes. Liquid/air
partition coefficients for MTBE between different media and air were
also determined by Imbriani et al. (1997). The values were: blood/air
= 20.0; urine/air = 15.6; saline/air =15.3; fat/air = 142.0; and olive
oil/air = 138.0.
MTBE partition coefficients measured in rat tissues demonstrated
a higher solubility of MTBE in fat (115.6) compared to blood (11.5)
and other tissues such as liver (14.5) (Borghoff et al., 1996). The
partition coefficient of MTBE in the male rat kidney was approximately
five times higher than the value measured in female rat kidneys. This
high uptake of MTBE into the male rat kidney was found to be due to an
interaction with the male rat specific protein alpha2u-globulin
(Borghoff et al., 1995; Poet & Borghoff, 1997).
In rats, MTBE is demethylated by hepatic microsomal enzymes to
form TBA and formaldehyde (FA) (Savolainen et al., 1985; Brady et al.,
1990). When using rat liver microsomes, the Vmax for demethylation of
MTBE to FA increased after pretreatment with acetone or phenobarbital
(Savolainen et al., 1985; Brady et al., 1990). The results of Brady et
al. (1990) indicated that both cytochromes P450 2BI and P450 2E1 are
implicated in the metabolism of MTBE. In vitro, TBA has been shown
to be oxidatively demethylated using rat liver microsomes to yield FA
(Cederbaum & Cohen, 1980).
Hong et al. (1997) demonstrated that human liver microsomes
metabolize MTBE to TBA. The activity of 125 ± 11 pmol TBA/min per mg
protein was approximately 50% of the activity in rat and mouse liver
microsomes. The metabolism of MTBE to TBA in human liver microsomes
was NADPH-dependent and was inhibited by carbon monoxide, an inhibitor
of cytochrome P450 (CYP) enzymes, suggesting that CYP enzymes play a
critical role in human metabolism of MTBE. Human CYP2A6 and CYP2E1
cDNAs were each co-expressed with human cytochrome P450 reductase by a
baculovirus expression system and the expressed enzymes used to
metabolize MTBE. CYP2A6 was more active than CYP2E1 (activity 6.1 and
0.7 nmol TBA/min per nmol P450, respectively).
The role of the cytochrome P450 enzyme CYP2E1 in metabolizing
MTBE (and other gasoline ethers) was examined in 2E1 knock-out mice,
which lack demethylation capability. Liver microsomes metabolized MTBE
with an activity level of 0.67 ± 0.1 nmol/min per mg. However, there
were no significant differences in activity levels in microsome
preparations from two 2E1+/+ strains of mice, demonstrating that
CYP2E1 is not important in the metabolism of MTBE in mouse livers
(Hong et al., 1998).
The probable metabolic pathway is presented in Fig. 2.
6.4 Physiologically based pharmacokinetic modelling
A physiologically based pharmacokinetic model (PBPK) to describe
the dosimetry of MTBE in rats has been developed (Borghoff et al.,
1996). Using this PBPK model, MTBE blood levels following different
routes of exposure and various exposure concentrations were predicted.
When human anatomical parameters are used in this model and the
metabolism is scaled allometrically, the model predicts the level of
MTBE in blood (concentrations ranging from 0.03 to 17.1 µg/litre)
during and following exposure of people to 6 mg MTBE/m3 (1.7 ppm)
(Borghoff et al., 1996; Cain et al., 1996). The human PBPK model was
further expanded to include brain as a target tissue and an exposure
model for bathing and showering. Model simulations of a bathing and
showering MTBE exposure scenario in humans at water levels from 0.64
to 1.0 mg/litre and air levels from 5 to 5.7 mg/m3 (1.4 to 1.6 ppm)
predicted maximum brain levels to range from 0.015 to 0.02 mg
MTBE/litre and 0.006 to 0.019 mg TBA/litre (Rao & Ginsberg, 1997).
Based on physicochemical properties, it can be predicted that a
release of MTBE into water would result in significant amounts being
dissolved. Most of the MTBE remains in the surface water, with some
partitioning into air and much smaller amounts into sediment and soil
(Mackay et al., 1993). The low Kow of 0.94 suggests that partitioning
from the water to particulates and sediment is not significant. On the
basis of bioconcentration data, MTBE is not subject to bioaccumulation
or biomagnification in aquatic organisms (Environment Canada, 1993).
In the water compartment, the key removal process should be
volatilization. The amount transferred to sediment is negligible
(Mackay et al., 1993; Environment Canada, 1993).
For a gasoline containing 10% MTBE by weight, and assuming that
it does not undergo depletion of the MTBE concentration in the
gasoline due to dissolution into the water, the water solubility of
the MTBE from gasoline will be approximately 5 gm/litre at 25°C. By
comparison, the total hydrocarbon solubility for non-oxygenated fuel
is about 120 mg/litre (Poulsen et al., 1992; Zogorski et al., 1996).
The ability of MTBE to enhance the solubility in water of
monocyclic aromatic gasoline components including BTEX compounds has
been examined in models, and an increase was predicted only at
co-solvent concentrations of greater than 1% (Mihelcic, 1990). In
confirmation of this, the co-solvent effect of MTBE on the aqueous
solubility of hydrocarbons in gasoline was found to be minimal (Cline
et al., 1991). Measurements made in the laboratory in shake-flasks
showed that up to 15% MTBE was unlikely to result in enhanced
concentrations of BTEX in contaminated groundwater (Poulsen et al.,
1992). Such high concentrations of MTBE seem unlikely to be achieved
in groundwater after spillage of gasoline containing MTBE, and
although MTBE is widely distributed in shallow urban groundwater at
low concentrations in the USA, its occurrence in these samples was not
associated with correspondingly increased concentrations of BTEX
(Squillace et al., 1996).
4.1.3 Soil
Based on its physicochemical properties, it can be predicted that
when MTBE is released to the soil, it can be transported to the air
through volatilization, to surface water through run-off, and to
groundwater as a result of leaching. In the first two instances, the
release would have to be at, or near the soil surface. If the release
of MTBE occurs below the soil surface, for example from an underground
storage tank, then the most likely transport mechanism will be
leaching to groundwater. Based on its vapour pressure, volatilization
of MTBE from soil and other surfaces is expected to be significant.
Soil adsorption and mobility are based on the reported and estimated
Koc (organic carbon sorption coefficient) values. Compounds with a
Koc of <100 are considered to be moderately mobile. Thus MTBE, with
a Koc of 91, does not adsorb to soil particles to a great degree and
would be considered mobile. Parameters other than Koc affecting the
leaching of MTBE to groundwater include the soil type (e.g., sandy
versus clay), the amount and frequency of rainfall, the depth of
groundwater, and the extent of degradation of the MTBE (Environment
Canada, 1993).
4.1.4 Multimedia
Several multimedia models using various emission rates and
environmental parameters have been used to predict the distribution
and concentration of MTBE in the environment (Environment Canada,
1993; Mackay et al., 1993; Hsieh & Ouimette, 1994).
4.2 Bioconcentration
Fujiwara et al. (1984) conducted studies on the bioconcentration
of MTBE in Japanese carp (Cyprinus carpio) in a flow-through system
at 25°C. The mean whole-body steady-state bioconcentration factor
(BCF) was 1.5. Further observations indicated that fish exposed for 28
days and then transferred to clean water eliminated almost all MTBE
residues within 3 days. These experimental data support the hypothesis
that MTBE has little tendency to bioaccumulate. Veith & Kosian (1983)
calculated a BCF of 2.74 (r2 = 0.927) for a 28-day exposure of
fathead minnows, based on a Quantitative Structure-Activity
Relationship (QSAR).
Compounds with log Kow values of approximately 5.0 or less do
not have significant food chain build-up. MTBE belongs to this group
(Environment Canada, 1993). Uptake from water is more important than
from food for this group of compounds.
When 14C-labelled MTBE was applied to the soil in a closed
aerated system, the concentrations of MTBE in the roots and the aerial
parts of lettuce and radish showed that transport was dominated by
foliar uptake; subsequently, translocation into the roots took place
(Schroll et al., 1994). Although neither MTBE nor its potential
metabolite TBA was detected in the plants, a considerable fraction of
the 14C label was unaccounted for and was presumed to be associated
with plant constituents.
4.3 Biodegradation and transformation
Only a limited amount of work has been accomplished on the
biodegradability of MTBE. Moreover, the studies are difficult to
compare because they have been performed under a wide variety of
conditions. Aerobic and anaerobic experiments have been conducted. For
most studies, it has been demonstrated that MTBE is difficult to
biodegrade. In contrast, BTEX is more readily biodegraded (Zogorski et
al., 1996). Half-lives for MTBE in various environmental compartments
are shown in Table 3
Table 3. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Air 20.7-265 Based upon measured Howard et al., 1991
photo-oxidation half-life
10-30 Mackay et al., 1993
Soil 672-4320 Estimation based upon US EPA, 1989
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Surface water 672-4320 Estimation based upon Howard et al., 1991
aerobic biodegradation
300-1000 half-life Mackay et al., 1993
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
2688-17 289 Estimation based on Howard et al., 1991
anaerobic degradation
half-life
4.3.1 Aerobic conditions
Results from tests involving biodegradation of MTBE have been
variable.
Pence (1987a) used an acclimated culture containing active
sludge, soil inoculum and raw sewage. The uptake of oxygen was
measured in a mineral medium supplemented with MTBE added to the
acclimated culture at a concentration of 5 mg/litre on days 0, 7 and
11. The results showed that MTBE was poorly biodegradable under these
conditions; only 5.4% biodegradation occurred within 28 days.
No biodegradation of MTBE after 60 days was found in experiments
using aquifer soil material as inoculum; with two types of activated
sludge as inoculum, no degradation of MTBE occurred after 40 days
(Möller Jensen & Arvin, 1990).
With a standard activated sludge, and based on the oxygen uptake
rate, MTBE was biodegraded very slowly (Fujiwara et al., 1984). The
hydrocarbon components of gasoline blended with MTBE were, however,
readily degraded even though the MTBE remained.
A mixed bacterial culture was obtained by enrichment of a
hydrocarbon-contaminated soil in a basal mineral medium containing:
(i) TBA (1 g/litre) as sole carbon source or (ii) methylamine (2
g/litre) as principal carbon source supplemented with TBA. During
incubation of the first culture, the concentration of TBA fell to zero
in 20 days, but incubation of methylamine-grown cells with MTBE showed
no reduction in the concentration of MTBE after 42 days (Allard et
al., 1996). Whereas MTBE was apparently recalcitrant under the
conditions used, TBA, which is one of its putative degradation
products, was biodegradable.
In contrast to these results, a mixed bacterial culture obtained
by continuous aerobic enrichment of a sludge sample from an industrial
bioreactor was able to degrade MTBE at concentrations up to 200
mg/litre (Salanitro et al., 1994). Cell suspensions incubated with
MTBE produced TBA as a transient metabolite. MTBE labelled with 14C
in the methyl group was degraded to 14CO2 and cellular material when
low substrate concentrations (2 mg/litre) were used, although not at a
concentration of 20 mg/litre. This experiment clearly demonstrated
oxidation of the methoxy group but left unresolved the fate of the
carbon atoms of the tertiary-butyl group.
Fifteen pure bacterial strains, with the capacity to degrade MTBE
using it as the sole carbon source, have been isolated from bioreactor
sludges and other sources. Several strains have been identified as
belonging to the genera Rhodococcus, Flavobacterium, Pseudomonas and
Oerskovia. These strains degrade up to 40% of MTBE (200 mg/ litre)
in 1-2 weeks of incubation at 22-25°C. These strains also grow on
tert-butanol, butyl formate, isopropanol, acetone and pyruvate as
sole carbon sources. Cultures of Methylobacterium, Rhodococcus and
Arthrobacter degraded MTBE within 1-2 weeks of incubation at
23-25°C. Growth on MTBE as the sole carbon source was slow compared
with growth on a nutrient-rich medium. When these compounds are mixed
with MTBE, there is a reduction in the degradation of MTBE. However,
when the microbes were initially grown on tert-butanol and then
transferred to medium containing MTBE, there was a greater degradation
of MTBE (Mo et al., 1997).
A mixed culture isolated from biological sludges has been used in
bioreactors utilizing MTBE as a sole carbon source for over a year.
The microbes were able to degrade MTBE at a concentration of 160
mg/litre after 3 days of incubation in batch experiments. Mixed
cultures have greater capacity for degradation of MTBE than pure
cultures. The addition of other ethers causes a reduction in MTBE
degradation. In soil microcosm studies, significant MTBE degradation
by mixed cultures was observed at 24°C and 10°C (Mo et al., 1997).
Howard et al. (1991) estimated, on the basis of screening tests
for aerobic biodegradation with unacclimatized aqueous systems
(Fujiwara et al., 1984), that the half-lives of MTBE in water and soil
under aerobic conditions ranged from 672 to 4320 h.
MTBE was found to be degraded by a number of propane-oxidizing
bacteria. The initial oxidation of MTBE produced nearly stoichiometric
amounts of TBA. The methoxy group of MTBE was further oxidized to
formaldehyde and finally to CO2. At 28°C, rates of MTBE degradation
by these bacteria ranged from 3.9 to 9.2 nmol/min per mg cell protein
weight (Steffan et al., 1997).
4.3.2 Anaerobic conditions
Biodegradability of MTBE to methane under anaerobic conditions
has been determined by measuring the production of CH4 and CO2
during exposure of MTBE to a large population of anaerobic bacteria.
MTBE was biodegraded anaerobically only to a very limited extent
(Pence, 1987b), and an average cumulative theoretical gas production
of only 7.1% was achieve within 56 days. Anaerobic biodegradation to
methane must exceed 50% to meet the validation requirements for
demonstration of anaerobic biodegradability.
The anaerobic degradation of MTBE has been examined in different
soils (unsaturated clay, sandy loam and silty loam) collected from
various depths at three different sites (Novak et al., 1992; Yeh &
Novak, 1994). The experiments were conducted in static small-volume
anaerobic microcosms, and three different oxygen-free conditions were
simulated; with nitrate as electron acceptor (denitrification),
sulfate-reducing conditions, and anaerobic fermentation. Factors
influencing the degradation of MTBE, ETBE and TBA were determined, and
included anaerobic microbial populations, soil anions, soil moisture
content, organic content, nitrogen availability, rate of ammonium
"fixation", and soil pH. The soils were moderately acidic (pH 5.0-6.0)
with the exception of surface soils. The concentration of the added
MTBE was monitored for more than 250 days. Three parameters were
evaluated: degradation rate, lag time and time for 80% of the compound
to be degraded. No anaerobic degradation of MTBE was found in
organic-rich soils over the 250-day study period. The only situation
in which MTBE degradation occurred was in an oligotrophic soil
containing a low level of organic matter and with a pH of 5.0-6.0.
About 10% of the MTBE was lost during the first two months, although
this decrease cannot unambiguously be attributed to biodegradation.
Several conclusions may be drawn from the experiments with TBA and
ETBE:
* Whereas degradation of TBA in soil from the oligotrophic site
could be enhanced by addition of nitrate, the degradation of TBA
was inhibited by adding readily degraded ethanol.
* Biodegradation of ETBE under denitrifying conditions was
extremely sensitive to the presence of readily degraded
substrates.
These results illustrate that care should be exercised in
assessing biodegradability when several readily degraded substrates
are available, a condition that may be encountered in groundwater
contaminated with oxygenate additives.
Suflita & Mormile (1993) used sediment suspensions prepared from
samples collected from an aquifer polluted with leachate from a
municipal landfill. They assessed the formation of methane from a
range of substrates, and after at least 249 days no evidence for
anaerobic degradation of MTBE could be found. Whereas unbranched
alkanols and ketones were readily degraded, ethers in general were
resistant; in addition, oxygenates containing a tertiary or quaternary
carbon atom proved more recalcitrant than their unbranched or
moderately branched chemical analogues to anaerobic degradation.
Comparable experiments using a wider range of sediment samples
(Mormile et al., 1994) showed similar results under sulfate-reducing
or denitrifying conditions, although under methanogenic conditions a
single sample transformed MTBE into TBA. Likewise, the ethers were
unaffected by incubation with cultures of the acetogenic bacteria
Acetobacterium woodii and Eubacaterium limosum that convert
aromatic methoxy groups to acetate.
Based on the above-mentioned studies, MTBE is classed as
recalcitrant under anaerobic conditions.
Howard et al. (1991) estimated that the half-life of MTBE in
water under anaerobic conditions ranges from 2688 to 17 280 h.
4.4 Abiotic degradation
4.4.1 Air
4.4.1.1 Photolysis
Direct photolysis of MTBE is assumed to be environmentally
insignificant since it does not absorb radiation above 230 nm (Calvert
& Pitts, 1966). However, under laboratory conditions MTBE in an
oxygenated slurry system containing TiO2 as catalyst was readily
degraded by UV light from a mercury lamp. MTBE was rapidly
photocatalytically degraded, 76% of the initial concentration being
converted to degradation products, including TBA. After 4 h MTBE was
no longer detectable (Barreto et al., 1995).
4.4.1.2 Hydrolysis
MTBE does not contain hydrolysable functional groups, and,
therefore, it is inert to environmental hydrolysis. Hydrolysis of MTBE
is assumed to be insignificant (Howard et al., 1991).
4.4.1.3 Photooxidation
MTBE is subject to photooxidation in the atmosphere. This will
occur under the influence of various mechanisms, such as the reaction
with hydroxyl radicals, water, alkoxy and peroxy radicals, oxygen
atoms, and ozone. On the basis of the rate constant of each of the
reactions and the concentrations of the reactants, the reaction with
the hydroxyl radical is considered to be the most important removal
process for MTBE in the atmosphere. Several products are generated as
a result. These include tertiary-butyl formate (TBF), the major
product, 2-methoxy-2-methyl propanol, formaldehyde, acetone, NO2, and
the methyl radical. Molar yields of products identified from the
reaction of hydroxyl radicals with MTBE are given in Table 4). TBF is
unreactive to further photo-oxidation, while 2-methoxy-2-methyl
propanol is expected to be highly reactive with hydroxyl radicals,
yielding equimolar amounts of CO2, formaldehyde, acetone and water.
Of these products, formaldehyde is highly reactive with the hydroxyl
radical (Wallington et al., 1988; Japar et al., 1991). Rates of
reaction of oxygenates and their decomposition products with hydroxyl
radicals are given in Table 5.
Factors influencing atmospheric lifetime, such as time of day,
sunlight intensity and temperature, also include those affecting the
availability of hydroxyl radicals. Based upon measured rate constants
for reactions with hydroxyl radicals in air (Cox & Goldstone, 1982;
Atkinson, 1985; Wallington et al., 1988, 1989; Atkinson, 1990; Japar
et al., 1990), the half-life for MTBE has been estimated to be between
20.7 and 265 h (Howard et al., 1991). Hence, MTBE is not considered to
be a greenhouse gas, nor would it contribute to the depletion of the
ozone layer (Environment Canada, 1993).
Table 4. Molar yields of products identified from the reaction of
hydroxyl radicals with MTBE
Product Molar yielda Molar yieldb
TBF 0.68 0.76
Formaldehyde 0.48 0.37
Methyl acetate 0.14 0.17
TBA 0.062 -
Acetone 0.026 0.02
a Smith et al., 1991.
b Tuazon et al., 1991.
Table 5. Rates of reaction of oxygenates and their decomposition
products with hydroxyl radicals at 25°C
Compound Rate Reference
(10-12 cm3
sec-1 molecule-1)
MTBE 3.2 Japar et al., 1991
ETBE 8.5 Japar et al., 1991
TBF 0.74 Smith et al., 1991
TBA 1.1 Japar et al., 1991
Formaldehyde 9.0 Atkinson & Pitts, 1978
2-methoxy-2-methyl propanala 30 Japar et al., 1991
a Estimated from rates for other aldehydes
4.4.2 Natural waters
MTBE is not expected to adsorb significantly to bed sediments of
suspended sediments, hydrolyse, directly photolyse, or photo-oxidize
via reaction with photochemically produced radicals in water. While
MTBE is reported to be chemical unstable in acidic solutions (Budavari
et al., 1996), it is not expected to be hydrolysed in natural waters
under normal pH conditions (Lyman et al., 1990).
4.4.3 MTBE half-life ranges in environmental compartments
The half-life of a chemical in the environment depends not only
on the intrinsic properties of the chemical, but also on the nature of
the surrounding environment, such as sunlight intensity, hydroxyl
radical concentration, the nature of the microbial community and
temperature. Table 6 lists the half-life ranges in various
environmental compartments estimated by Mackay et al. (1993) and
Howard et al. (1991); these estimates are somewhat uncertain, as
implied by the order of magnitude range for some compartments.
4.5 Ozone-forming potential
Photochemical ozone-creation potentials (POCP) ranging from 20.4
to 34.6 have been determined for MTBE using a model that simulates the
formation of photochemical ozone episodes (Derwent et al., 1996). The
POCP values reflect the ability of a substance to form tropospheric
ozone as a result of its atmospheric degradation reactions. The POCP
values are calculated relative to ethylene (a chemical that is thought
to be important in such ozone formation and is given a POCP of 100).
Based on the emissions and the POCP value, MTBE (itself) is likely to
play a minor role in photochemical smog and low-level (tropospheric)
ozone formation near to sources of release.
4.6 Remediation
Examples of remedial methods that can be considered for MTBE are
air stripping, carbon absorption and soil vapour extraction. Intrinsic
bioremediation may be limited due to the variability of rates of
biodegradation of MTBE which have been previously mentioned (Zogorski
et al., 1996).
Table 6. Half-life ranges of MTBE in various compartments
Environmental Half-life ranges Comments Reference
compartment (h)
Soil 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Air 20.7-265 Based upon measured Howard et al., 1991
10-30 photo-oxidation half-life Mackay et al., 1993
Surface water 672-4320 Estimation based upon Howard et al., 1991
300-1000 aerobic biodegradation Mackay et al., 1993
half-life
Sediment 1000-3000 Mackay et al., 1993
Groundwater 1344-8640 Estimation based upon Howard et al., 1991
aerobic biodegradation
half-life
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The major sources of MTBE to the general population are probably
associated with the distribution, storage and use of oxygenated
gasoline. The main source of non-occupational exposure to MTBE is
evaporative emissions from gasoline. A large portion of the population
is exposed during time spent at service stations, while driving cars,
in public parking garages, and in homes with attached garages. These
exposures generally occur through inhalation. In addition, discharges
into the soil or groundwater are a potential for contaminated water
supply and can lead to exposure when such water is drunk. Dermal
contact with MTBE may occur through accidental spills of MTBE-blended
gasoline or through the use of gasoline as a solvent. In Canada, it
has been estimated that gasolines blended with MTBE account for only
2% of the total annual gasoline consumption. MTBE is used in small
quantities by a few Canadian refiners to boost octane levels in
gasoline. A limited survey of the MTBE content of unleaded regular,
mid-range and premium gasoline across Canada in 1995 showed a range of
0 to 5.2% by volume for winter grade gasoline and 0 to 9% by volume in
summer grade gasoline. In the USA, oxygenated gasoline containing
10-15% MTBE is used in different areas and about 30% of the US
population is exposed to MTBE.
5.1 Environmental levels
5.1.1 Exposure
Ambient air and microenvironment concentrations of MTBE and other
fuel oxygenates have been measured in Canada, the USA and Finland.
When available, air data are presented below in conjunction with data
on MTBE levels in gasoline and with information on the proximity of
the samples to various point sources of MTBE.
Brown (1997) estimated average daily and average lifetime doses
of MTBE from exposure in air and drinking-water for a US population.
Concentration data and several of the population characteristics were
estimated as distributions rather than as point values. Arithmetic
mean occupational doses via air were in the range of 0.1 to
1.0 mg/kg-day, while doses from residential exposures, commuting and
refuelling were in the range of 0.0004 to 0.006 mg/kg-day. Lifetime
doses for workers were in the range of 0.01 to 0.1 mg/kg-day. The
cumulative dose distribution for the entire population of the
MTBE-using regions of the USA was estimated by combining the
distributions of doses and the numbers of people in each exposure
category. In the MTBE-using areas, arithmetic mean doses via air were
estimated to be 0.0053 and 0.00185 mg/kg-day for the chronic and
lifetime cases, respectively. It was found that 1.5% of the population
used water contaminated with MTBE leakage with an estimated geometric
mean concentration of 0.36 µg/litre and a 95th percentile
concentration of 64 µg/litre. Including ingestion, inhalation, and
dermal absorption of contaminated water, the estimated arithmetic mean
does of the population exposed via water was 1.4 × 10-3 mg/kg-day.
5.1.1.1 Levels in ambient air and various microenvironments
a) Canada
The concentrations of MTBE in ambient air at various selected
locations in Canada have been measured as part of the National Air
Pollution Surveillance Programme in 1995 and 1996. This programme is a
joint project of the federal, provincial and municipal levels of
government. Its purpose is to monitor and assess, on a continuing
basis, the quality of the ambient air in the various regions of
Canada. The sites selected for monitoring of MTBE were based on usage
of gasoline with MTBE and/or because of nearby manufacturers of MTBE.
Pollutants from air were collected intermittently using the
canister methodology. Concentrations of MTBE was measured using the
detection principle of gas chromatography furnished with an ion trap
detector. Air samples were first passed through a cryogenic
concentration trap to gather enough analyte before injection into a GC
capillary column to allow compound speciation and quantification.
Approximately 200 ml of the canister sample was concentrated. A
cryogenic trap held at -150°C was used to concentrate the air sample.
Once the sample was concentrated, the trap was heated to 150°C and the
sample was back-flushed onto the column. MTBE and other hydrocarbons
were separated using a fused silica capillary column. The GC oven was
programmed to remain at 60°C for 3 min, then increased to 280°C at a
rate of 8°C/min. Calibration standards were prepared using the static
dilution technique. The detection limits were 0.05 to 0.1 µg/m3.
Table 7 lists the ambient concentration of MTBE in air at various
locations in Canada from 1995 to 1996.
Table 8 shows some MTBE atmospheric concentrations at the fence
line of a petroleum refinery at St John, New Brunswick, Canada, during
a period when there were complaints of odour. The same collection and
analytical methodology was used. The maximum concentration is not
considered representative of the area.
b) USA
In many urban areas in the USA having elevated levels of ozone or
CO, oxygenates such as MTBE are regulated for use in gasoline at
concentrations of 2.0% and 2.7% oxygen by weight (called reformulated
and oxygenated gasoline, respectively). These concentrations are
achieved by adding MTBE at 11% and 15% by volume, respectively. In
other areas, MTBE is used as an octane enhancer in premium gasoline at
concentrations up to 9% by volume, but usually at much lower
concentrations. It is important to note that MTBE is the predominant
oxygenate currently in use in these gasoline mixtures, followed by
ethanol (approximately 65% and 35% of the oxyfuels sold contain MTBE
and ethanol, respectively). Oxygenates used to a minor extent include
ETBE, TAME and DIPE (HEI, 1996). In 1994, oxygenates were added to
more than one-third of the gasoline market in the USA.
Table 7. Concentrations of MTBE in ambient air in Canada (1995-1996)
(Environment Canada, 1996b)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Edmonton(1)c Two petroleum refineries - 1 km. 20/7/95 7.21
Acetic acid plant - 2.5 km
26/7/95 11.39
1/8/95 0.81
7/8/95 2.93
8/8/95 5.50
12/9/95 2.49
27/5/96 3.35
Edmonton(2)d N/A 26/7/95 < DL
1/8/95 < DL
1/9/95 < DL
6/9/95 < DL
24/9/95 < DL
30/9/95 < DL
27/5/96 < DL
Halifaxd N/A 3/4/96 < DL
15/4/96 0.13
21/4/96 0.15
Montreal(1)c Two refineries (BTX, petroleum) 21/8/95 1.54
- 1.6, 2.5 km
21/8/95 0.59
12/9/95 1.06
16/3/96 < DL
15/5/96 0.42
21/5/96 0.28
27/5/96 0.23
Montreal(2)d N/A 16/3/95 0.15
16/5/96 0.18
21/5/96 0.22
27/5/96 0.37
Montreal(3)e N/A 10/3/96 0.16
16/3/96 0.28
9/5/96 0.95
Montreal(4)f N/A 9/5/96 0.22
15/5/96 < DL
21/5/96 0.70
St. Johnc Petroleum refinery - 3 km 9/5/96 1.02
15/5/96 3.73
Stouffvillef N/A 6/10/95 0.19
18/10/95 0.35
Toronto(1)d N/A 29/8/95 < DL
2/9/95 < DL
2/9/95 0.07
Table 7. (continued)
Citya Industrial site(s) and distance(s) Sample date MTBE
to monitoring site (where applicable) concentration
(µg/m3)b
Toronto(2)d N/A 17/8/95 0.03
29/8/95 < DL
2/9/95 < DL
Vancouver(1)f N/A 23/8/95 0.27
29/8/95 0.89
29/8/95 0.16
30/8/95 0.33
Vancouver(2)f N/A 22/3/95 0.14
Vancouver(3)c Two gasoline processing and 1/8/95 2.13
storage plants - 0.5, 3 km
13/8/95 1.82
25/8/95 3.35
2/9/95 1.78
2/9/95 26.43
21/2/96 0.31
10/3/96 1.10
16/3/96 0.48
16/3/96 0.39
22/3/96 1.55
28/3/96 < DL
Vancouver(4)e N/A 10/3/96 1.07
Vancouver(5)f N/A 28/3/96 0.40
Vancouver(6)c Pipeline transfer point 1/9/95 1.79
2/9/95 1.90
22/3/96 0.89
Windsord N/A 2/8/95 0.08
17/8/95 0.05
17/8/95 0.11
21/8/95 0.40
29/8/95 0.27
29/8/95 0.14
16/3/96 < DL
21/4/96 0.15
27/4/96 < DL
Winnipegd N/A 4/3/96 0.02
27/3/96 < DL
a ( ) = different monitoring sites in same city.
b DL = detection limit = 0.1 µg/m3.
c Monitoring site in vicinity of petroleum refinery and/or industrial chemical plant, or
pipeline transfer area.
d Monitoring site in urban area.
e Monitoring site in urban area on busy street.
f Monitoring site in suburban area.
Table 8. MTBE concentrations at petroleum refinery boundary
(St. John, New Brunswick, Canada) during period of odour
complaints
Sampling date MTBE concentration
(µg/m3)
19/7/95 281
2/8/95 15
14/8/95 71
28/8/95 36
MTBE air quality data were collected in the USA as a result of
special studies in six urban centres: Fairbanks (Alaska), Stamford
(Connecticut), Albany (New York), Milwaukee (Wisconsin), Boston
(Massachusetts) and Houston (Texas) (Zogorski et al., 1996). In
addition, collection of MTBE air quality data for selected monitoring
sites in California started in 1996. Although these data cannot be
used to define quantitatively the air quality in these cities and are
not sufficient to provide a national perspective, they can be used to
estimate approximate ranges of MTBE in ambient air in the locations
sampled (Zogorski et al., 1996; US Interagency Assessment, 1997).
Non-occupational and consumer exposure to MTBE is shown in Table 9,
and service station attendants and garage workers in Table 10.
Owing to health complaints (Gordian et al., 1995) following the
introduction of oxygenated fuels (15% MTBE) during the 1992 winter
season in Fairbanks, Alaska, the sale of these fuels was suspended in
mid-December 1992, one month after their introduction. Zweidinger
(1993) analysed air samples for MTBE in ambient air and in various
microenvironments in Fairbanks taken immediately prior to the
suspension (phase I), during the phase-out period (Phase II), and two
months after the suspension, at which time the MTBE fuels were
expected to be at nominal levels (Phase III). Fuel samples collected
from Fairbanks gasoline stations during Phases II and III indicated
that the average percentage by weight of MTBE in unleaded regular
gasoline decreased from 8.5% to 1% while the average for premium
gasoline decreased from 14.7 to 5.6%. For comparison, ambient air
samples were also collected in the spring of 1993 from Stamford,
Connecticut, where 15% MTBE oxygenated gasoline was sold but there
were no consumer health complaints, and Albany, New York, where MTBE
was only present in gasoline at nominal levels to enhance octane and
there were also no health complaints (Zweidinger, 1993).
Ambient air samples in these cities were generally collected over
an 8-h period and were taken from the following areas: (1) outside
city limits, for background levels; (2) in residential areas away from
heavy traffic, and (3) in areas adjacent to major roadways or
intersections. The median and range of MTBE concentrations for the
selected locations and phases are shown in Table 11 (Zweidinger,
1993).
In Fairbanks, MTBE levels in all ambient environments were lower
when the use of MTBE as an oxygenate in gasoline was discontinued.
Overall MTBE ambient air concentrations ranged from non-detectable
levels to a maximum of 100.9 µg/m3 (28.0 ppbv) in the phases prior to
and during the phase-out of MTBE in Alaskan gasoline (Phases I and II)
and ranged from not detectable to 12.3 µg/m3 (3.4 ppbv) when MTBE use
was reduced to nominal levels in fuels (Phase III). Background levels
were low during Phase III, ranging from not detectable to 4.3 µg/m3
(1.2 ppbv) MTBE. Since sampling was limited during Phases I and II,
the levels of MTBE in ambient air outside city limits cannot be
compared for the three sampling periods over which MTBE concentrations
in gasoline were being reduced.
Phase II ambient residential and roadside area air samples
presented the highest levels of MTBE, with concentrations ranging from
6.1 to 100.9 µg/m3 (1.7 to 28.0 ppbv) and 15.1 to 28.5 µg/m3 (4.2 to
17.9 ppbv) respectively, and with medians of 6.1 µg/m3 (4.6 ppbv) and
34.9 µg/m3 (9.7 ppbv), respectively. Owing to lack of samples or
small sample size in Phase I data, comparisons between the phases were
limited to the roadside category. However, in this category, it was
unexpectedly found that, although Phase 1 sampling occurred prior to
suspension of MTBE, the data showed roadside levels of MTBE in ambient
air to be slightly lower than those during the suspension period.
In Stamford, Connecticut, limited measurements of MTBE taken in
the spring of 1993 showed that residential, roadside and gas station
ambient air results were consistently lower than those taken in
Fairbanks during the oxygenates programme, with ranges of not
detectable to 1.1 µg/m3 (0.3 ppbv), not detectable to 1.8 µg/m3
(0.5 ppbv) and 4.3 to 10.1 µg/m3 (1.2 to 2.8 ppbv), respectively.
Limited residential and roadside ambient MTBE air samples taken in
Albany, New York, in May 1993 were lower than levels during a
comparable phase of MTBE use in Fairbanks (Phase III). Ambient
temperature and other meteorological conditions differed significantly
among the cities where samples were collected.
In Fairbanks, Stamford and Albany, roadside ambient air levels
were found to be generally higher than residential area ambient air
levels (Table 11). Consequently, this roadside category may also be
considered to be a microenvironment, especially for those samples
taken in downtown city street canyons. For this particular study, it
was not possible to discern whether this was the case. Table 12
presents information on air samples taken in more clearly defined
microenvironments such as service stations and vehicle interiors
(Zweidinger, 1993).
Table 9. Non-occupational and consumer exposure to MTBE in the USAa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Community air
Milwaukee WI RFG in Yes (in 6/11 0.000025 <0.00413 0.00013 Jan-March 1995; Approximately Allen &
use* some 24-h samples; 50% contained Grande
cases) collected in MTBE, remainder (1995)
evacuated ETBE or ethanol
canisters;
GC(FID)
3/5 0.000025 <0.00106 0.00052 Feb-March 1995;
2-h samples;
collected in
evacuated
canisters;
GC(FID)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 up to (0.002) Feb-March 1995; Approximately Allen
use* 0.0037 2- to 3-h 50% contained & Grande
samples; MTBE, remainder (1995)
collected in ETBE or ethanol
evacuated
canisters;
GC(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Automobile cabin for commuters
New Jersey 15 MTBE NA 20/20 0.002- 0.004 April 1993; Estimated from Lioy et
0.017* approximately graphed data al. (1994)
1-h samples; in the original
Connecticut 20/20 0.003- 0.0056 absorbed onto report
0.009* carboxen 569:
collected in
evacuated
canisters;
GC/MS
Service station refuelling
Phoenix, AZ 12* No 40/40 0.09-38 5.8 Oct-Nov 1990; Samples taken API (1993)
each sample was from one station
collected in which only
Los Angeles, 13 Yes 6/6 1.1-6.5 3.6 during the premium gasoline
CA refuelling of 8 was oxygenated
to 10 vehicles;
each refuelling
was sampled for
1 to 2 min;
absorbed onto
charcoal; GC
(FID)
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey,
New York 10-15* Yes 4/4 NS 0.370 April 1993; 5-min Estimated from Lioy et
breathing-zone graphed date in al. (1994)
samples before, the original
Connecticut No 4/4 NS-4.1 0.572 during and after report
refuelling;
absorbed onto
carboxen 569;
GC/MS
Milwaukee WI Jan-March 1995; RFG with MTBE Allen &
Station A 9-10* Yes 6/6 NS (0.39) 15-min used only in Grande (1995)
Station D 9** No 2/2 NS (2.93) breathing-zone higher grades;
samples; adsorbed *ethanol used
onto charcoal; in regular
GC(FID) gasoline; **2%
MTBE used in
regular gasoline
Northeast and 10-17* Yes 8/17 <0.32 <2.1 0.57 Feb-April 1994; Northeast = API (1995c)
southwest 15- to 20-min Connecticut and
areas personal New Jersey
(short-term breathing zone locations;
sample) samples; adsorbed Southwest =
onto charcoal; Arizona
GC(FID) locations
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
In automobile while refuelling
Connecticut, New Jersey and New York Service Stations
Self-serve 10-15* Yes 4/4 0.006- 0.03 April 1993; 5-min Estimated from Lioy et
0.072 breathing-zone graphed data in al. (1994)
samples before, the original
Full-serve Yes 8/8 0.008- 0.034 during and after report
0.172 refuelling;
adsorbed onto
Self-serve No 4/4 NS 0.015 carboxen 589;
GC/MS
Full-serve No 4/4 0.005- 0.041
0.103
Service station pump island
New Jersey: Yes 4/4 0.120- 0.440 April 1995; 4-h API (1995a)
Full-serve 1.600 breathing-zone
samples during
both refuelling
and not
refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 6/6 0.014- 0.048
Self-serve 0.080
Connecticut: No 9/10 0.09 <1.500 0.170
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 15 Yes 3/3 0.08- 0.24 Nov-Dec 1994; 7- Cook &
0.24 to 8-h samples; Kovein (1995)
adsorbed onto
charcoal; GC (FID)
Service station perimeter
Phoenix AZ 12 No 24/24 0.009- 0.02 Oct-Nov 1990; API (1993)
0.09 12-h samples;
4 perimeter
samples plus
samples upwind
and downwind
from the pump
island for each
sample set;
adsorbed onto
charcoal; GC(FID)
New Jersey: Yes 15/16 0.001 <0.036 0.003 April 1995; 4-h API (1995a)
Full-serve samples;
collected in
6-litre evacuated
canisters; GC/MS
New York: Yes 24/24 0.002- 0.007
Full-serve 0.083
Connecticut: No 38/40 0.001 <0.140 0.014
Self-serve
Table 9. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Milwaukee WI
Station A 9-10* Yes 2/2 NS (0.0024) Jan-March 1995; *RFG, MTBE used Allen &
Station B 2-9** No 1/1 NS (0.0045) 2-h area samples; only in higher Grande (1995)
collected in grades; ethanol
evacuated in lower grades
canisters; GC(FID) **2% MTBE in
regular gasoline
a Asterisks in any column indicate that further explanation is provided in the comments column.
b Number of samples in which MTBE was detected divided by total number of samples.
c GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
NA = not applicable; NS = not stated.
Table 10. Non-industrial occupational exposures to MTBEa (adapted from HEI, 1996)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Service station attendants during refuelling
Phoenix AZ Oct-Nov 1990; each API (1993)
1-2 min 12 No 40/40 0.09-38 5.8 breathing-zone
sample was
collected during
Los Angeles the refuelling of
CA 13 Yes 6/6 1.1-6.5 3.6 8-10 vehicles;
1-2 min each refuelling
was sampled for
1-2 min; adsorbed
onto charcoal;
GC (FID)
New Jersey, 10-15 Yes 4/4 NS 0.37* April 1993; 5-min Estimated from Lioy et
New York, breathing-zone graphed data al. (1994)
samples before, in original
during, and after report
Connecticut: refuelling;
5 min No 4/4 <4.1 0.572 adsorbed onto
carboxen 589;
GC/MS
Milwaukee WI Jan-March 1995; This station used Allen &
Station A 15-min MTBE in middle Grande (1995)
15 min RFG 9-10 Yes NS/6 NS 0.31 breathing-zone and premium grade
samples during gasoline, and
refuelling; ethanol in
collected in regular gasoline
evacuated
canisters
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
15-20 min 10-17 Yes 8/17 0.32 <2.1 0.57 samples; mostly locations;
15- to 20-min Southwest =
personal Arizona
breathing-zone locations
samples during
refuelling;
adsorbed onto
charcoal;
GC (FID)
Various USA 1982-1993: data Service station API (1995b)
locations reported by and retail outlet
American Petroleum personnel
<30 min NS NS 9/11 0.16 0.16- 2.6 Institute member
136.1 companies; higher
frequency of
30 min-6 h 5/5 0.01- 0.34 measurements
2.7 in 1989-1993;
GC(FID) and
other procedures
6-9 h TWA 13/13 0.09- 0.59
34.0
>9 h 11/11 0.01- 1.1
17.20
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey 4 h 13-10 Yes 4/4 0.084- 0.245 April 1995; 4-h API (1995a)
(full-serve) 0.52 breathing-zone
samples, during
New York 4 h Yes 5/6 0.077- 0.205 refuelling and
(self-serve) 0.78 not refuelling;
collected in
Connecticut 4 h No 10/10 0.170- 1.5 8-litre evacuated
(self-serve) 2.60 canisters; GC/MS
Phoenix AZ 4 h 14 No 42/42 0.04- 0.55 Oct-Nov 1990; 4-h Hartle (1993)
3.88 (half-shift)
breathing-zone
samples (average
time 224 min);
adsorbed onto
charcoal; GC(FID)
Northeast and Feb-April 1994; Northeast = API (1995c)
southwest personal Connecticut and
in winter breathing-zone New Jersey
>8 h 10-17 Yes 18/21 0.03- <0.5 0.27 samples, most locations;
0.11 sampling times Southwest =
were > 6 h; Arizona
adsorbed onto locations
charcoal; GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
New Jersey Nov-Dec 1994; Cook &
(full shift) 15 Yes 21/21 NS 0.12- 0.48 breathing-zone Kovein (1994)
1.42 samples for 3-8 h;
adsorbed onto
charcoal; GC (FID)
Service station attendants at pump island
New Jersey
4 h 13-16 Yes 4/4 0.12- 0.44 April 1995; 4-h API (1995a)
(full-serve) 1.60 breathing-zone
samples during
refuelling and
not refuelling;
collected in
6-litre evacuated
canisters; GC/MS
New York Yes 6/6 0.0005 0.014- 0.048 Cook &
4 h 0.08 Kovein (1994)
(self-serve)
Connecticut 15 No 9/10 NS <1.5 0.17 Nov-Dec 1994; 7-
4 h to 8-h samples;
(self-serve) adsorbed onto
charcoal; GC (FID)
New Jersey Yes 3/3 0.08- 0.24
8 h 0.24
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Parking garage ramp
Milwaukee WI RFG in NA 8/8 0.0023- (0.002) Feb-March 1995; Approximately 50% Allen &
2-3 h use 0.0037 2- to 3-h samples; contained MTBE, Grande (1995)
collected in remainder
evacuated contained ETBE
canisters; or ethanol
GC(FID)
Mechanics Feb-April 1994; Northeast = API (1995c)
Northeast and personal Connecticut and
southwest in breathing-zone New Jersey
winter samples, most locations;
short-term Southwest =
15 min 10-17 Yes 4/13 <0.25- <32 ND sampling times Arizona locations
(<0.26) <0.35 were 15-20 min;
most long-term
>6 h 17/20 <0.02- <2.6 0.09 sampling times
(<1.5) <0.05 were >6 h; adsorbed
onto charcoal;
GC(FID)
Northern New April 1993; 1-h Workers at Mohr et
Jersey breathing-zone service stations al. (1994)
1 h 15 NS NS/13 0.3-6.1 samples (active); and garages
adsorbed onto for State
carboxen; GC/MS vehicles
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Stamford April 1993; Mechanics with Buchta (1993b)
Connecticut full-shift the Department
8-h TWA 13-17 NA 20/28 0.03 <12.04 0.11 samples of Public Works
(approximately and in auto
8 h); adsorbed dealers' garages
onto charcoal;
GC(FID)
Fairbanks AK Dec 1992; Moolenaar
8-h TWA 15 NS NS/10 0.01- 0.10 full-shift et al. (1994)
0.81 samples
(approximately
8 h); collected
in evacuated
canisters in
environment where
workers spent
most of their
day; GC
Other vehicle-related workers
Stamford 13-17 NS 0/7 0.03 <DL <DL April 1993; 8-h Workers who
personal spent time in
breathing-zone traffic
samples; adsorbed
onto charcoal;
GC(FID)
Table 10. (continued)
Sampling Oxygenate Vapour Detection Detection MTBE (ppm) Sampling, Commentsa Reference
site content recovery frequencyb limit collection
(vol %) system (ppm) Range Median and analysisc
(Mean)
Connecticut Workers in various Buchta (1993b)
8-h TWA 1/4 <0.15 <DL jobs (mostly
workers in garages
who performed
tasks difference
from the mechanics)
a Number of samples in which MTBE was detected divided by total number of samples.
b GC/MS = gas chromatography with verification by mass spectrometry; GC(FID) = gas chromatography with flame ionization detection.
c Asterisks in any column indicate that further explanation is provided in the comments column.
TWA = time-weighted average; NA = not applicable; NS = not stated.
Table 11. Concentrations of MTBE in µg/m3 (ppbv) in ambient 8-h air samples taken in Fairbanks, Stamford and Albany, USA,
as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)a
Sampling site Fairbanks Fairbanks Fairbanks Stamford Albany
Phase I Phase II Phase III April 1993 May 1993
early December 1992 late December 1992 Feb/Mar 1993
Background:
number of samples 0 1 5 2 0
median - ND ND 0.72 (0.2) -
range - 1 ND-4.3(1.2) ND-1.1(0.3) -
Residential:
number of samples 2 11 11 2 3
median 14.4(4) 16.6(4.6) 2.5(0.7) 1.1(0.3) ND
range 7.2-21.6 16.1-100.9 ND-9(2.5) ND-1.8(0.5) ND-0.4(0.1)
(2.9-6.0) (1.7-28.0)
Roadside:
number of samples 7 7 10 2 7
median 18(5) 35(9.7) 4.3(1.2) 7.2(2.0) 0.72(0.2)
range 10.8-43.2 15.1-64.5 ND-12.3(3.4) 4.3-10.1 (1.2-2.8) ND-2.5(0.7)
(3.0-12.0) (4.2-17.9)
a For the calculation of a median when n = 2, the two sample values are averaged together; in the case of an ND value,
half the detection limit value is substituted in the calculation, i.e. 0.36 µg/m3 (0.1 ppbv).
ND = non-detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Table 12. Concentrations of MTBE in µg/m3 (ppbv) in various microenvironmental 8-h ambient air samples taken in Fairbanks, Stamford
and Albany, USA, as a result of the oxyfuels programme (Zweidinger, 1993; Zweidinger, personal communication)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Service station pump island:
number of samples 1 1 6 4 4
median 194.6 (54) 134.8 (37.4) 11.5 (3.2) 13.7 (3.8)a 64.2 (17.8)
range - - 6.1-49.7 ND-26.7 (7.4) 23.3-194.6
(1.7-13.8) (6.6-54)
Commercial vehicle interiors:
number of samples 8 - 6 - -
median 126.1 (35) - 31.7 (8.8) - -
range 25.2-1207.3 - 1.4-129 - -
(7-335) (0.4-35.8)
Indoors - commercial garage service areas:
number of samples 5 - 10 8 -
median 1088.4 (302) - 148.5 (41.2) 484 (134.8) -
range 367.6-2922.8 - 21.3-496 4.7-1546.5 -
(102-811) (5.9-137.6) (1.3-429.1)
Indoors - residential area:
number of samples - 3 5 - -
median - 6.5 (1.8) 2.9 (0.8) - -
range - 6.1-15.1 1-4 (0.3-1.1) - -
(1.7-4.2)
Table 12. (continued)
Sampling site Fairbanks Phase I, Fairbanks Phase II, Fairbanks Phase III, Stamford Albany
early Dec 1992 late Dec 1992 Feb/Mar 1993 April 1993 May 1993
Indoors - public buildings near roadway:
number of samples - 4 5 4 -
median - 32.4 (9) 6.5 (1.8) 1.8 (0.5) -
range - ND-37.1 (10.3) ND-10.5 (2.9) 1.4-1.8 -
(0.4-0.5)
Indoors - home with attached garage:
number of samples - 5 4 - -
median - 27.8 (7.7) 72.1 (20) - -
range - 10.1-75.3 51.5-109.2 - -
(2.8-20.9) (14.3-30.3)
a In Stamford, service station samples were taken 4.6 metres (15 feet) away from the pump.
ND = not detectable; detection limit = 0.72 µg/m3 (0.2 ppbv).
Kelly et al. (1993) performed 24-h ambient air sampling in the
cities of Boston and Houston where MTBE was used nominally in gasoline
(i.e. approximately < 5% by volume). Sampling took place
approximately every 14 days from August 1990 to April 1991 and from
June to August 1991. The Boston sampling site was categorized as being
in an urban area of mixed industry and office buildings, with high
traffic density, and the sampler was placed on a downtown fire
department rooftop. Conversely, the Houston sampling site was located
in a semi-rural area, on the roof of an air sampling station, and was
expected to receive emissions from industrial and, to a lesser extent,
urban sources. MTBE levels in ambient air at these sites ranged from
< 0.72 to 1.8 µg/m3 (< 0.2 to 0.49 ppbv) and < 0.72 to 10.1 µg/m3
(< 0.2 to 2.8 ppbv), respectively (detection limit = 0.72 µg/m3 (0.2
ppbv). The median level from these data was < 2 ppbv for both Boston
and Houston. MTBE was detected in several samples from Houston (n =
22; in 64 % of samples MTBE was non-detectable), but only at one site
in Boston (n = 22; in 96% of samples MTBE was non-detectable).
Allen & Grande (1995) conducted an ambient air monitoring study
in the city of Milwaukee as a result of public health complaints when
MTBE was first introduced into Milwaukee reformulated gasoline (at
approximately 11% by volume) in 1995. Eleven weekly 24-h samples
collected at the Wisconsin Enhanced Ozone Monitoring Program air
sampling station from January to March 1995 resulted in concentrations
ranging from not detectable to 14.89 µg/m3 (4.13 ppbv) [n = 11; 45%
non-detectable samples; detection limit 0.36 µg/m3 (0.1 ppbv)]. The
median was determined to be 0.47 µg/m3 (0.13 ppbv). A control sample
collected in each of the nearby cities of Madison and Green Bay, where
reformulated gasoline use was not mandated, was found to be below the
detection limit of 0.36 µg/m3 (0.1 ppbv).
In the same study, Allen & Grande (1995) collected 1- to 3-h
roadside air samples at busy intersections and freeway interchanges
where it was expected that there would be high concentrations of
gasoline fumes. Mean levels of MTBE in air samples collected near a
freeway interchange, a busy intersection and a roadway in Milwaukee
were 1.9 µg/m3 (0.53 ppbv) (n = 3), 3.8 µg/m3 (1.06 ppbv) (n = 2)
and 1.8 µg/m3 (0.50 ppbv) (n = 2), respectively. This particular
choice of roadside location is a more clear example of a roadside
microenvironment and it may also be considered an ambient air sample.
The State of California mandated the year-round use of oxygenated
fuels in the South Coast Air Basin in 1995 and throughout California
by June 1996. As a result, MTBE was included in the California's
ambient air monitoring programme in February 1996 for the cities of
Burbank, Long Beach and Los Angeles and in June 1996 for Chico,
Roseville and Fresno. The overall range of 24-h average concentrations
was 1.4-44.7 µg/m3 (0.4-12.4 ppbv) for the selected monitoring sites.
The overall range of averages was closer to the Fairbanks data than to
data from other cities in the continental USA. Averages ranged from
4.7 to 17.3 µg/m3 (1.3 to 4.8 ppbv) with the highest averages
occurring in the cities of Los Angeles and nearby Burbank. The
following are average and ranges for each of the individual cities:
Burbank 17.3, 4.7-31.7 µg/m3 (4.8 ppbv, 1.3-8.8 ppbv), Long Beach
9.4, 3.2-21.6 µg/m3 (2.6 ppbv, 0.9-6.0 ppbv), Los Angeles downtown
13.7, 4-24.1 µg/m3 (3.8 ppbv, 1.1-6.9 ppbv), Chico 8.3, 3.6-27.8
µg/m3 (2.3 ppbv, 1.0-7.7 ppbv), Fresno 9.4, 2.2-44.7 µg/m3 (2.6
ppbv, 0.6-12.4 ppbv) and Roseville 4.7, 2.5-12.3 µg/m3 (1.3 ppbv,
0.7-3.4 ppbv). The number of samples for each city ranged from 18 to
28 and the detection limit for MTBE was 0.72 µg/m3 (0.2 ppbv) (M.
Poore, personal communication).
Measurements of ambient air concentrations of MTBE at service
stations are usually taken at the gasoline pump island and at the
service station perimeter. The air levels of MTBE tend to be higher at
the pump island and lower at the perimeter. In addition, service
stations equipped with vapour recovery systems (Stage II) tend to have
higher MTBE concentrations in both microenvironments. Furthermore,
whereas the pump island data represents a particular microenvironment
due to the presence of gasoline vapour coming from refuelling
activities, the gasoline station perimeter data has been used to
estimate potential community exposures and may be considered
representative of upper end ambient air levels in neighbourhoods.
Short-term peak samples of MTBE were not taken.
MTBE median concentrations generally ranged from 0.32 to 21.4
mg/m3 (0.09-6 ppm) in breathing-zone samples as a result of consumer
refuelling. The values were measured over 2- to 15-min sampling
periods and were highly variable but rarely exceeded 35.7 mg/m3 (10
ppm). The range of median values measured at the pump islands ranged
from 0.18 to 1.57 mg/m3 (0.05-0.44 ppm) over a 4-h sampling period.
The fenceline samples were lower and ranged from 0.004 to 0.5 mg/m3
(0.001 to 0.14 ppm) with a collection period of 4 h. Generally, the
concentrations were higher at service stations that did not have
vapour recovery systems.
Allen & Grande (1995) collected 1- to 3-h MTBE area samples
downwind of the gas pumps on the perimeter of four Wisconsin service
station properties from 21 February 1995 to 9 March 1995. The average
levels of MTBE at two service stations that dispensed reformulated
gasoline and had Stage II vapour recoverya were 8.8 µg/m3 (2.43
ppbv) (n = 2) and 2.7 µg/m3 (0.75 ppbv) (n = 2). The level of MTBE at
a station without vapour recovery was higher and was measured to be
16.5 µg/m3 (4.58 ppbv) (n = 1). Finally, one air sample taken at a
service station where reformulated gasoline was not mandated resulted
in a lower concentration of MTBE i.e. 0.8 µg/m3 (0.25 ppbv).
a Stage II is a vapour recovery system used to trap gasoline vapour
during refuelling of consumer vehicles.
In addition, Zweidinger (1993) analysed a limited number of 8-h
air samples taken in various microenvironments in Fairbanks (Phases I,
II and III), Stamford and Albany for MTBE. Generally,
microenvironmental air concentrations of MTBE decreased from Phase I
or II to Phase III at the following locations: (1) service station
pump island, (2) commercial vehicle interiors, (3) indoor - commercial
garage service areas, (4) indoor - residential area and (5) indoor -
public buildings near roadway (i.e. school, post office), due to the
suspension of the oxyfuel programme. The median and range of MTBE
concentrations for selected locations and phases are shown in Table
12. The air of one home with an attached garage was the exception to
this tendency in that the indoor air samples contained levels of MTBE,
benzene and other compounds associated with gasoline which were
significantly higher than air samples measured outside that home. This
indicated that the residential garage may have had a source of
evaporative emissions after parking the hot car in the garage or from
gasoline stored in the garage.
Huber (1995) used a multizonal mass balance model to predict
indoor air concentrations. Measured evaporative emissions of 0.5 g of
MTBE emitted from an automobile at rest at 23.9°C (a highly
unrealistic estimate for Fairbanks in winter) during 4 h in a garage
attached to a residential house resulted in modelled peak
concentrations of 2.3 mg/m3 (0.65 ppm) in the garage and 0.12 mg/m3
(0.035 ppm) in the residence. Modelled 1-h average concentrations in
the garage ranged from 2.5 to 4.3 mg/m3 (0.7 to 1.22 ppm) while those
in the residence ranged from 0.072 to 0.32 mg/m3 (0.02 to 0.09 ppm).
This was estimated to be a worst-case situation for evaporative
emissions since a newer car or cold winter temperatures would probably
have reduced evaporative emission rates resulting in lower
concentrations (Huber, 1995). However, increased tailpipe vehicle
emissions as a result of cold start (the first few minutes of running
the engine before the catalytic converter starts to function) were not
included in these estimations.
In the case of the Stamford microenvironment samples, the
resulting 8-h average MTBE levels in indoor commercial garage service
areas and indoor locations near a roadway were lower than those taken
in Fairbanks even though the volume of MTBE present in Stamford
gasoline was higher than the volume used in Fairbanks during Phases II
and III. It is important to note that the service station pump island
samples were not strictly comparable since they were taken 4.5 m away
from the pump. When MTBE was only used as an octane enhancer, as was
the case for Albany and Fairbanks Phase III, levels of MTBE in service
station pump island air were found to be much higher in Albany.
Air measurements in the parking garage microenvironment have been
conducted in two studies. MTBE concentrations measured in two 8-h air
samples in a Stamford partially open parking lot (i.e. open-sided with
an office building directly above) in April 1993 were 70.4 µg/m3
(20.1 ppbv) and 177 µg/m3 (49.0 ppbv) with a mean of 124.7 µg/m3
(34.6 ppbv) (RB Zweidinger, personal communication). Allen & Grande
(1995) conducted measurements at an enclosed parking ramp in Milwaukee
in February 1995 in order to show ambient levels during cold starts.
One- to three-hour air samples showed MTBE levels of less than 72.1
µg/m3 (20 ppbv) with mean levels of 7.4 µg/m3 (2.05 ppbv) (n = 8).
The highest level occurred at a point when a large number of vehicles
were making cold starts in a short period of time.
Air samples were taken by Lioy et al. (1994) inside the vehicle
cabin microenvironment for (1) activities surrounding refuelling, and
(2) during suburban commutes before and after refuelling. The study
took place in April 1993 in New Jersey, New York and Connecticut where
gasoline containing 10 to 15% by volume MTBE was sold. The experiment
protocol consisted of a 60-min commuter run that included a 5-min
refuelling stop at full- and self-service stations with or without
Stage II vapour recovery. Resulting in-cabin levels of MTBE taken
immediately before, during and after refuelling ranged from 23.8 to
108 µg/m3 (6.6 to 30 ppbv), 133.4 to 313.5 µg/m3 (37 to 87 ppbv) and
31.4 to 151.4 µg/m3 (8.7 to 42 ppbv), respectively, for three
different cars, with average concentrations of 55.3 µg/m3 (14.8
ppbv), 190.2 µg/m3 (55 ppbv) and 72.1 µg/m3 (20 ppbv). Short-term
peak concentrations occurred during refuelling. In addition,
post-refuelling in-cabin concentrations were slightly higher than
pre-refuelling, although the increase was not statistically
significant. It was noted that the highest of the levels that diffused
into the cabin during refuelling occurred with an older vehicle, which
had an abnormally high evaporative emissions rate. There did not seem
to be a statistically significant difference in in-cabin levels
between the various types of service stations, although the sample
size was too small to be able to make this conclusions.
Microenvironmental in-cabin air concentrations of MTBE measured during
60-min suburban stop/go commutes ranged from 3.6 µg/m3 (1 ppbv) to
576.6 µg/m3 (160 ppbv) with a geometric mean of 21.6 µg/m3 (6 ppbv)
(n = 40). Most values were less than 19.8 µg/m3 (5.5 ppbv). It was
noted that the higher values were associated with the use of the
high-emission vehicle discussed previously.
Area monitoring was conducted in an indoor commercial garage
service area in Fairbanks, Alaska (Buchta 1993a). Three air samples
were taken over a 6- to 7-h period in February 1993 when MTBE use was
limited to purposes of octane enhancement. MTBE was non-detectable in
the three areas (service area, parts department, shop wall) but the
minimum detection limit was high (144.2 µg/m3, 40 ppbv). This is
comparable to the Zweidinger (1993) 8-h indoor commercial garage air
samples that were measured with more sensitive equipment and resulted
in a median concentration for MTBE of 114.4 µg/m3 (31.75 ppbv). The
higher levels in the latter study may have been the result of gasoline
spills.
Ambient air levels of MTBE have been measured near three
refineries in the USA. At one refinery a 24-h MTBE level of 20 µg/m3
was reported for one out of nine downwind samples taken at the
perimeter of a rural refinery, which was stated to release
approximately 33 tonnes of MTBE emissions in air per year. MTBE was
not detected in the 26 other downwind and upwind samples taken at the
refinery during the same period. MTBE was not detected in another 54
24-h samples taken at two other refineries: annual MTBE air emission
release data were not provided for these refineries. It should be
noted that the detection limit for these air samples was high (more
than 20 or 30 µg/m3), but more sensitive canister samples taken for
24 h also resulted in non-detectable concentrations (detection limit =
6 µg/m3) (API, 1989b).
c) Finland
Vainiotalo et al. (1996) reported the concentration of MTBE at
the perimeter and pump island of two self-service stations in Finland
(one urban roadside and one simple roadside) both with Stage Ia
vapour recovery systems where gasoline containing 11% MTBE by volume
was sold. The investigations were conducted during May/June and
October 1995. The average 24-h perimeter concentrations for each of
the 4-day sampling periods were generally higher for the urban
roadside service station samples: 12.4 µg/m3 (June) and 14.1 µg/m3
(October), with 35-36 measurements collected at each side on each
occasion. Several factors such as the volume of gasoline sold, mean
wind speed and number of deliveries of gasoline to the station were
higher for the former and resulted in data with higher variability
during the fall sampling. The overall range of perimeter air samples
for both service stations was from 0.5 to 120.5 µg MTBE/m3. Highest
daily concentrations were usually obtained at the downwind sampling
points. No seasonal influence was discernable. Mean 24-h
concentrations measured in the centre of the pump island ranged from
247 to 1347 µg/m3 (n = 15). The detection limit was not specified in
the study.
5.1.1.2 Dermal exposure
Dermal exposure and absorption may occur from MTBE-blended
gasoline at self-service refuelling or from its use as a solvent. It
may also occur from MTBE-contaminated household water during washing,
bathing or showering. There are, however, no data available to
estimate dermal exposure to MTBE.
5.1.1.3 Estimation of total personal exposure
Exposure is a function of concentration and time. Thus, the time
spent in various activities involving different concentrations and
degrees of contact will affect human exposures to MTBE. Huber (1995)
generated "worst-case scenario" estimates of long-term exposure to
MTBE based on population activity patterns and available
microenvironmental and ambient concentration data (generally rounded
a In the USA, Stage I is a vapour recovery system used during
loading and unloading of gasoline from delivery tankers. Since
this is a Finnish Study, this definition may or may not be
equivalent.
up to the next order of magnitude of 10). His intentionally high
estimates were updated and slightly revised to indicate that an annual
time-weighted average exposure might be as high as about 0.11 mg/m3
(0.03 ppm), assuming that gasoline contained 15% MTBE by volume for 6
months and approximately 10% for the remainder of the year (US
Interagency Assessment, 1997). This upper-end exposure estimate is
highly uncertain, given the lack of adequate data to describe the
distribution of actual personal exposure levels.
5.1.1.4 Other pollutants
There was an increase in indoor air levels of benzene after MTBE
reformulated fuel use was discontinued (Gordian & Guay, 1995). Both
ambient outdoor and specific indoor environment (garages, vehicles,
workplace, school, post office and residence) samples, as well as
blood samples, were collected in Fairbanks during and after the
oxygenated programme. In addition to MTBE, other volatile compounds,
such as benzene and formaldehyde, were evaluated. In indoor samples,
there was a statistically significant increase of benzene in garages
and non-garages after MTBE was discontinued. In garages, the mean
benzene concentration increased from 0.30 mg/m3 (94.02 ppb) in
December to 0.61 mg/m3 (191.62 ppb) in February, and in the school,
post office and residence from 0.02 mg/m3 (5.89 ppb) to 0.06 mg/m3
(20.22 ppb). The NIOSH-recommended exposure limit for benzene is 0.3
mg/m3 (0.1 ppm). In vehicles the data were difficult to interpret
because different vehicles were tested in December and in February.
The same pattern, although not statistically significant, was seen in
the outdoor samples.
5.2 Occupational exposure
5.2.1 Industrial operations - manufacturing and blending
MTBE can be encountered in solution and as vapour during its
manufacturing at chemical plants and refineries, during blending into
gasoline, transportation, distribution, and handling at service
stations. Some industrial hygiene monitoring data are available (Table
13).
At the MTBE unit at the Neches Chemical West Plant in the USA,
the TWA values ranged from less than 0.07 to 120.3 mg/m3 (0.02 to
33.41 ppm) for operations and maintenance personnel (Simer, 1986).
Exposure monitoring in a manufacturing plant showed that all
exposures to MTBE greater than 3.6 mg/m3 occurred during quality
control sampling procedures (ARCO, 1987). Two out of 46 short-term
samples indicated exposures to MTBE at concentrations of 22 mg/m3 and
6.5 mg/m3 (6.1 ppm and 1.8 ppm, respectively); 91% of the full shift
monitoring indicated exposure levels of less than 3.6 mg/m3.
Texaco (1993) presented results from a short-term monitoring for
MTBE conducted at a refinery in Guatemala in order to evaluate the
adequacy of current work procedures. No details of ambient temperature
Table 13. Occupational exposure to MTBE in the petroleum industry (adapted from HEI, 1996)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
MTBE manufacturing
Occupational exposure of
oil refinery and chemical
plant personnel handling
neat MTBE during:
Routine operations <30 min 14/27 0.16-1.00 0.16-7.8 1.00 API, 1995b
6-9 h TWA 38/76 0.01-0.03 0.01-248.7 0.03 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.17 0.17 API, 1995b
Routine maintenance <30 min 7/8 0.05 0.05-7.19 0.90 API, 1995b
30 min-6 ha 1/1 Not given 0.20 0.20 API, 1995b
6-9 h TWA 4/4 Not given 0.04-0.7 0.11 API, 1995b
>9 h TWA 2/2 Not given 0.16-0.2 0.18 API, 1995b
Routine operations and
maintenance 8-12 h TWA 8/21 0.02-0.06 <0.02-33.41 1.06 Simer, 1986
20 min 0/1 [1.0] <1.0-1.0 <1.0 ARCO, 1987
4-6 h 1/11 [1.0] <1.0-6.1 <1.0 ARCO, 1987
12 h TWA 2/23 [1.0] 0.8-2.2 Not given ARCO, 1987
QA/QC sampling of MTBE 12-36 min 3/16 [1.0] <1.0-12.2 <1.0 ARCO, 1987
MTBE blending
Occupational exposure of
personnel involved in
fuel-blending activities
involving:
Neat MTBE <30 min 34/35 <0.005 0-97.0 2.90 API, 1995b
30 min-6 h 12/13 0.21 0.21-72.0 1.03 API 1995b
6-9 h TWA 7/12 0.04-1.80 0.04-87.97 2.24 API, 1995b
>9 h TWA 0/9 0.23-0.34 0.23-0.34 0.30 API, 1995b
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
Fuel mixtures <30 min 51/98 0.02-0.23 0.02-100 0.30 API, 1995b
30 min-6 h 5/19 0.03-0.33 0.03-1.98 0.05 API, 1995b
6-9 h TWA 34/112 0.02-0.20 0.02-14 0.04 API, 1995b
>9 h TWA 9/22 <0.005-0.02 0-0.27 0.02 API, 1995b
MTBE transport
Occupational exposure of
marine barge, pipeline and
rail car personnel to:
Neat MTBE <30 min 62/66 0.30-0.60 0.30-1050 13.83 API, 1995b
(trucking personnel included 30 min-6 h 23/27 0.04-0.36 0.04-700 2.20 API, 1995b
only for transport of neat 6-9 h TWA 9/10 0.03 0.03-711.9 0.18 API, 1995b
MTBE) >9 h TWA 1/1 Not given 0.32 0.32 API, 1995b
15 min TWA 4/4 Not given 90-150 110.00 Texaco, 1993
Fuel mixtures <30 min 60/64 0.001-0.14 0.001-507.87 2.44 API, 1995b
30 min-6 h 64/92 0.02-0.04 0.02-59.4 0.42 API, 1995b
6-9 h TWA 28/42 0.007-0.04 0.01-26.24 0.14 API, 1995b
>9 h TWA 8/8 Not given 0.19-4.51 1.49 API, 1995b
MTBE distribution
Occupational exposures of <30 min 93/129 <0.005-0.08 0-14.0 0.75 API, 1995b
marketing terminal and 30 min-6 h 9/10 0.26 0.26-4.05 0.98 API, 1995b
trucking personnel involved 6-9 h TWA 62/87 0.01-0.05 0.01-2.2 0.11 API, 1995b
in the handling of >9 h TWA 46/47 0.06 0.06-6.2 0.71 API, 1995b
Gasoline-MTBE mixtures 15 min TWAe ´ 0.2 <0.2-0.94 0.48 Hebert, 1993
10-12 h TWAe 2/2 Not given 0.08-0.08 0.08 Hebert, 1993
15 min TWAf 4/4 Not given 0.05-0.16 0.14 Gillie, 1993
15 min TWAg 3/3 Not given 1.9-3.6 2.80 Gillie, 1993
12 h TWAg 5/5 Not given 0.24-0.92 0.45 Gillie, 1993
15-40 minf (n=6) 0.2 2.8-42 13 (mean) Hakkola & Saarinen,
1996
Table 13. (continued)
Occupational exposure Sampling Detection Detection Range of MTBE Median MTBEc Referencesd
category timea frequencyb limit (ppm) concentrations concentration
(ppm)
10-30 minh (n=4) 0.2 20-226 91 (mean) Haakola & Saarinen,
1996
22-44 mini (n=5) 0.2 4.3-27 16 (mean) Haakola & Saarinen,
1996
10-37 minj (n=6) 0.2 10.0-98.0 71 (mean) Haakola & Saarinen,
1996
a Duration was task-related.
b Number of samples in which MTBE was detected divided by total number of samples.
c In the case of "non-detectable" samples the detection limit was used to calculate the median.
d The API (1995b) measurements used different sampling and analytical techniques on both personal breathing zone and area air samples.
e Loading of trucks with vapour recovery.
f Bottom loading of trucks with vapour recovery.
g Truck unloading at service station with vapour recovery.
h Top loading without vapour recovery.
i Truck unloading at service station without vapour recovery - Northern Finland.
j Truck unloading at service station without vapour recovery - Southern Finland.
were given. Air concentrations associated with transfer of neat MTBE
from tank cars to a storage tank ranged from 324 to 540 mg/m3 (90-150
ppm).
Hinton (1993) performed an occupational exposure study of MTBE
employees designed to determine the amount of MTBE exposure during
manufacturing, blending MTBE into gasoline, transportation,
distribution and handling at service stations. The study included 2038
exposure measurements during an 11-year period. Occasionally the TWA
exposure value exceeded 360 mg/m3 (100 ppm) and the short-term (less
than 30 min) exposure 1080 mg/m3 (300 ppm), generally during
non-routine or extraordinary tasks. The maximum short-term value
sampled for less than 30 min, 3780 mg/m3 (1050 ppm), was recorded
during transportation of neat MTBE. The maximum TWA level was 2563
mg/m3 (712 ppm) for the same activity. Usually, the TWA levels were
less than 7.2 mg/m3 (2 ppm) and the short-term levels were less than
36 mg/m3 (10 ppm). Exposures in blending operations were less than
360 mg/m3, and generally less than 36 mg/m3. In distribution, MTBE
levels were less than 3.6 mg/m3, and for service station attendants
levels were less than 10.8 mg/m3 (3 ppm).
5.2.2 Transportation
An exposure assessment for MTBE vapour concentrations conducted
on two gasoline truck drivers in New Jersey showed an average exposure
concentration of 0.29 mg/m3 (0.08 ppm) on a full shift (10-12 h)
basis. The short-term 15-min TWA exposure was 2.05 mg/m3 (0.57 ppm)
(Hebert, 1993).
Monitoring has been made for workplace concentration levels for
seven gasoline truck operators to assess exposure potentials during
loading operations and during full unloading at service stations
(Gillie, 1993). The 12-h TWA ranged from 0.86 to 3.31 mg/m3
(0.24-0.92 ppm). Short-term exposure (5-23 min) during truck loading
operations ranged from 0.18 to 0.58 mg/m3 (0.05-0.16 ppm) and during
fuel unloading from 3.24 to 12.96 mg/m3 (0.9-3.6 ppm).
The occupational exposure of road tanker drivers to gasoline and
some of its components, including MTBE, has been measured in Finland
in two depots and 11 service stations during loading and delivery
(Hakkola & Saarinen, 1996). In Finland, unleaded gasoline contains
10-15% MTBE in liquid phase. The monitoring was made during the
summer, and the temperatures ranged from 4 to 22°C. In the south of
Finland, four measurements were carried out during top loading and six
measurements during delivery at service stations. In the north of
Finland, six measurements were performed during bottom loading and
five measurements at service stations during delivery. The mean
short-term exposures of road tanker drivers to MTBE during loading and
delivery were between 13 and 91 mg/m3. The differences in exposure
during bottom (2.8-42.0 mg/m3, mean 13 mg/m3) and top loading
without vapour recovery (20-226 mg/m3, mean 91 mg/m3) were
statistically significant (p<0.02). There also was a statistically
significant difference (p<0.03) during delivery in northern (4.3-27.0
mg/m3, mean 16.0 mg/m3) and southern Finland (10-98 mg/m3, mean 71
mg/m3). The exposure time for loading was 25-35 min and for
delivering 30-40 min per load.
5.2.3 Service station attendants and garage mechanics
An exposure assessment for MTBE vapour concentrations conducted
on six full-service gas attendants in New Jersey showed an average
exposure of 1.76 mg/m3 (0.49 ppm) on an 8-h TWA basis (Hebert, 1993).
For the short-term 15-min TWA, the average exposure was 2.16 mg/m3
(0.60 ppm).
In an evaluation of exposure among service station attendants and
operators, Hartle (1993) compared the exposure potential at three
categories of service stations. Two facilities in Cincinnati, Ohio,
represented service stations that did not use MTBE or used it only as
an octane enhancer. In Phoenix, Arizona, two high-volume stations were
selected, and in Los Angeles two service stations with advanced vapour
recovery were selected. In Phoenix, where the MTBE content averaged
12.5-13% by liquid volume, the exposure measurements (41 samples)
ranged from 0.14 to 13.97 mg/m3 (0.04-3.88 ppm) with an average of
1.08 mg/m3 (0.3 ppm). The Los Angeles exposure ranged from 0.07 to
2.63 mg/m3 (0.02-0.73 ppm), averaging 0.50 mg/m3 (0.14 ppm). In
Cincinnati, only one of 32 samples was above the analytical limit of
detection, i.e. 0.58 mg/m3 (0.16 ppm).
Giacomello (1996) measured personal exposure of "full service"
attendants to MTBE in 58 Italian service stations. The study included
a number of geographical locations throughout the country and was
conducted in the summer and winter in 1992 and 1995. An overall
geometric mean of 0.71 mg/m3 (1992) or 0.26 mg/m3 (1995) was
recorded in the summer, and 0.37 mg/m3 in winter (latter data only
for 1992).
Three NIOSH studies were performed in workers potentially exposed
to gasoline and exhaust emissions during their work day (Almaguer,
1993; Buchta, 1993a,b). Breathing zone air samples were collected from
workers exposed to MTBE and other gasoline components (benzene,
toluene, and xylene, and, in one study, carbon monoxide) in several
maintenance facilities for motor vehicles located in Fairbanks, Alaska
(Buchta 1993a), in Stamford, Connecticut (Buchta, 1993b), and in
Albany, New York (Almaguer, 1993). In two of the cities (Fairbanks and
Albany), MTBE was only used as an octane enhancer (generally less than
1% of the fuel) during the study period. In Stamford, however, the
MTBE content of the fuel ranged from 13 to 17% with an average of
14.2% by volume. The highest workplace exposure level concentrations
were less than 0.50 mg/m3 (0.14 ppm) in Albany and less than 1.6
mg/m3 (0.45 ppm) in Fairbanks. In Stamford, the MTBE exposure levels
ranged from 0.1 to 44.6 mg/m3 (0.03 to 12.04 ppm). The cause for the
highest value was unknown, and the next highest exposure value was
7.56 mg/m3 (2.1 ppm). In all of the studies, the highest
concentrations were measured on mechanics. The sampling was, however,
conducted in late spring, and dilution ventilation (open windows and
doors) may have affected the results.
5.2.4 Occupational exposure limit values
In the USA, the American Conference of Governmental Industrial
Hygienists (ACGIH, 1994) has recommended a TWA of 144 mg/m3 (40 ppm).
In Sweden, the occupational air exposure TWA limit is 180 mg/m3 (50
ppm) and the 15-min short-term exposure limit (STEL) 250 mg/m3 (75
ppm) (AFS, 1994). The Dutch Expert Committee on Occupational Standards
recommended a health-based occupational 8-h TWA exposure limit of 180
mg/m3 (50 ppm) (DECOS, 1994).
5.3 Exposure via water
MTBE has been found in groundwater, storm water, reservoir water,
and drinking-water in the USA (Garrett et al., 1986; Angle, 1991; Dey
et al., 1991; Post, 1994; Squillace et al., 1995a,b, 1996; Delzer et
al., 1996; Dale et al., 1997; US Interagency Assessment, 1997).
Collectively, these references show that MTBE occurs in water,
especially in areas where MTBE is extensively used, and where releases
of MTBE to air, water and soil occur.
However, while there are some national monitoring data for
ambient groundwater, monitoring data for MTBE in surface water and in
drinking-water in the USA are very limited in scope. In an extensive
review of MTBE in water in the USA, Zogorski et al. (1996) concluded
that sufficient monitoring data were not available to characterize
human exposure to MTBE by the consumption of drinking-water.
The following subsections summarize major findings for MTBE in:
(a) snow and precipitation; (b) surface water; (c) groundwater; and
(d) drinking-water.
5.3.1 Snow and precipitation
MTBE has been detected in snow at ground level in Denver,
Colorado, USA, at very low (water equivalent) concentrations
(Squillace et al., 1995b; Bruce & McMahon, 1996). There is no other
published monitoring information on the presence or concentration of
MTBE in snow or rainfall. Squillace et al. (1996) hypothesized that
concentrations of MTBE in precipitation would be greater during winter
months than warmer summer months due to the temperature effect on
air-water partitioning.
5.3.2 Surface water
Information on MTBE in streams and rivers, in Long Island, New
York and New Jersey, USA, has been reported by Stackelberg et al.
(1997). For Long Island, at a reporting level of 0.5 µg/litre, MTBE
was the second most frequently detected VOC, occurring in 29% of the
samples at concentrations ranging from 0.6 to 20 µg/litre, with an
estimated median of 0.24 µg/litre. MTBE was detected more frequently
in samples collected during winter months (33%) than summer months
(26%). In New Jersey, a limited study was completed in spring 1994
along a ten-mile reach of the Hackensack River. Land use along this
reach is highly urbanized, and numerous industries and municipal
effluents are present. The study involved the collection of a single
water sample at each of 14 sampling points just after a major snow
melt. MTBE was detected in all samples at concentrations ranging from
2.6 to 30 µg/litre, with a median of 7.75 µg/litre (Stackelberg et
al., 1997). Reconnaissance sampling of eight streams elsewhere in New
Jersey in 1996 showed the presence of MTBE in water samples for seven
of eight sites. The concentrations ranged from 0.2 to 4.9 µg/litre
(Stackelberg et al., 1997). MTBE is used in reformulated gasoline at
both Long Island, New York and in New Jersey, as part of a mandatory
air abatement programme.
Measurable but low concentrations of MTBE were found in some of
592 stormwater samples (including samples from culverts, concrete
pipes, lined ditches and channels) collected by the US Geological
Survey in 16 cities and metropolitan areas from 1991 to 1995 (Delzer
et al., 1996). MTBE was found in 6.9% of the samples (41 of 592
samples). When detected, concentrations ranged from 0.2 to 8.7
µg/litre, with a median below 1.0 µg/litre. Eighty-three percent of
the detections occurred during the winter season (October to March)
when oxygenated gasoline to abate CO pollution was expected to be
used. A comparison of MTBE concentrations for samples collected during
the summer and winter periods showed a statistically significant
difference. Twenty-seven out of 148 stormwater samples contained both
MTBE and BTEX compounds, indicating a common source for these samples.
The Metropolitan Water District of Southern California (MWDSC)
relies, in part, on six lake-reservoirs for the storage of raw water
to be used for drinking-water in Southern California. These reservoirs
have varying degrees of recreational use. MWDSC began quarterly
monitoring of these reservoirs for MTBE in the second quarter of 1996.
MTBE was detected at the surface of Lake Perris, with confirmation in
two subsequent samplings, at an average concentration of 15 µg/litre.
The following quarter MTBE was also detected at an average level of 19
µg/litre. No MTBE was detected above the study's reporting level of 1
µg/litre in any of the other reservoirs, which were sampled at outlet
towers, where water is drawn from a lower depth (Dale et al., 1997).
5.3.3 Groundwater
Data from urban and agricultural areas show that MTBE occurs
predominantly in shallow groundwater underlying urban areas, and, when
present, occurs typically at low concentrations. In 1993-1994, the US
Geological Survey measured the concentrations of MTBE and 59 other
VOCs in 210 shallow wells (five drinking-water wells, 12 springs and
193 monitoring wells) in eight urban areas and 549 shallow groundwater
wells from 21 agricultural areas, and deeper groundwater from 412
wells sampled in nine areas throughout the USA (Squillace et al.,
1995a,b, 1996). MTBE occurred in 27% of the shallow urban wells and
springs. Detectable levels of MTBE were found in 86% of the wells in
industrial areas, 31% of the wells in commercial areas, 23% of the
wells in residential areas, and 23% of the wells in areas of mixed
urban land use, parks and recreation areas. MTBE was the second most
frequently detected compound after trichloromethane (chloroform). In
73% of the 210 shallow urban wells, concentrations were less than the
reporting level of 0.2 µg/litre. The estimated median value for urban
areas was below 0.2 µg/litre (Squillace et al., 1996). Three percent
of the wells had concentrations of MTBE above 20 µg/litre. No MTBE was
detected in drinking-water wells in the urban areas. In the
agricultural areas, 1.3% of the 549 shallow agricultural wells sampled
had detectable concentrations of MTBE. MTBE was also detected in four
of the 412 deeper groundwater samples from major aquifers. Three of
these wells were used for domestic or municipal water supply. The
measured maximum concentration of MTBE was 1.3 µg/litre. MTBE in
groundwater was generally not found with BTEX compounds, which
commonly are associated with point source spills of gasoline.
Bruce & McMahon (1996) measured MTBE concentrations in
groundwater in the alluvial aquifer beneath Denver, Colorado, USA, as
part of a survey of groundwater quality examining a range of dissolved
constituents. Thirty randomly selected alluvial wells were sampled.
MTBE was the most frequently detected VOC (23 out of 29 wells). The
maximum concentration was 23 mg/litre.
5.3.4 Drinking-water
Exposure to MTBE via drinking-water may involve more than direct
ingestion of contaminated water. Household uses of water, such as in
cooking, showering, bathing and washing, could result in exposure
through inhalation and dermal absorption, even if ingestion of water
was avoided.
Only limited monitoring data are available for MTBE in
drinking-water sampled at the tap or from a municipal distribution
system. Stern & Tardiff (1997) estimated that about 30% of the US
population lives in areas where MTBE is in regular use; 95% of this
population is unlikely to be exposed to MTBE in tap water at
concentrations exceeding 2 µg/litre, most will be exposed to much
lower or zero concentrations, but 5% could be exposed to higher
concentrations due to fuel tank spills and leaks entering surface and
groundwater. As part of the US Interagency Oxygenated Fuel Assessment
in the USA (US Interagency Assessment, 1997), information on MTBE
levels in drinking-water was sought from state drinking-water agencies
on a voluntary basis by the US Environmental Protection Agency.
Because monitoring for MTBE in drinking-water is not required by the
US Federal Government, only a few states have information on MTBE in
drinking-water. As such, it is not possible to describe levels of MTBE
in drinking-water for the entire USA. Based on information provided by
five states (New Jersey, Iowa, Illinois, Texas and Colorado), MTBE has
been detected in the drinking-water of 51 public water systems.
However, when detected, the concentration of MTBE was generally low
and nearly always below 20 µg/litre (Zogorski et al., 1996). These
data indicated that the consumption of drinking-water was not a major
route of exposure for these few systems (Zogorski et al., 1996). No
data on MTBE in drinking-water are available for other countries.
As noted previously, there have been a few instances in the USA
where groundwater used for drinking-water, both private wells and
public water systems, has become contaminated with levels of MTBE in
excess of 1000 µg/litre (Garrett et al., 1986; State of Connecticut,
1987; Zogorski et al., 1996). Many humans will probably detect MTBE in
drinking-water when the concentration exceeds about 50-100 µg/litre
owing to its low taste and odour threshold (see section 2.1 for taste
and odour values). Some humans will detect MTBE in water at even lower
concentrations. However, Du et al. (1998) considered that an MTBE
concentration below 40 µg/litre in drinking-water would avoid any
unpleasant taste or odour even for the most sensitive members of the
population.
5.4 Soil and sediment
There are very limited data concerning levels of MTBE in the
terrestrial environment. Trace amounts of MTBE have been found in
sediment samples adjacent to motorways and centres of heavy urban road
traffic density in the United Kingdom (Bianchi & Varney, 1989).
5.5 Biota
There are very limited data on MTBE levels in biota. In a study
to detect organic and inorganic contaminants in shellfish in Nova
Scotia, Canada, MTBE was not detected in any of the 21 samples assayed
(detection limit = 0.01 µg/g) (Environment Canada, 1989).
6. KINETICS AND METABOLISM IN HUMANS AND LABORATORY ANIMALS
Kinetic data from human and animal studies are summarized in
Table 14.
6.1 Human data
6.1.1 Controlled human studies
In an inhalation study two healthy young adult male and two
healthy young adult female volunteers were exposed to MTBE at 6 mg/m3
(1.7 ppm) in an environmental chamber for 1 h. The mean blood level
rose from 0.83 ± 0.5 µg/litre (0.009 µmol/litre) preexposure to 17.1 ±
2.0 µg/litre (0.19 µmol/litre) at the end of the 1 h exposure period.
One hour after the end of exposure the mean blood level fell to 6.3 ±
1.6 µg/litre (0.07 µmol/litre) after 1 h (Cain et al., 1996).
In a pharmacokinetics study, two volunteers (one healthy young
adult male and one healthy young adult female) were exposed to
5 mg/m3 (1.394 ppm) for 1 h in an environmental chamber (see also
section 8.2). There was a rapid rise in blood MTBE concentration to
6.1 µg/litre (8.2 ppb) and 10.9 µg/litre (14.7 ppb), respectively, at
1 h from the start of exposure. Following the end of exposure, there
was a rapid decline in blood MTBE concentration in both the male and
female volunteer with half-lives of 36 and 37 min, respectively. By
the end of the 7-h sampling period, blood MTBE concentration had
fallen to 0.149 µg/litre (0.2 ppb) in the male volunteer and 0.447
µg/litre (0.6 ppb) in the female volunteer (Prah et al., 1994).
In another study, the area under the curve (AUC) values of MTBE
and TBA were proportional to the MTBE exposure levels following short-
term inhalation to 18, 90 or 180 mg/m3 (5, 25 and 50 ppm), indicating
linear kinetics up to at least 180 mg/m3 (Johanson et al., 1995).
Following exposure to 180 mg MTBE/m3, the elimination of MTBE and TBA
was complete within 24 and 48 h, respectively (Johanson et al., 1995).
Pekari et al. (1996) measured concentrations of MTBE in blood,
urine and exhaled air from four volunteers exposed to 90 or 270 mg/m3
(25 or 75 ppm) by inhalation for 4 h. A lung retention of around 40%
was recorded, and blood levels of 11 µmol/litre (970 µg/ litre) at 90
mg/m3 or 29 µmol/litre (2556 µg/litre) at 270 mg/m3 were achieved
towards the end of the exposure period. Of the MTBE absorbed, the
majority (about 58%) was excreted unchanged in expired air and small
amounts (1.4%) unchanged in urine. The concentration of TBA in blood
reached a peak of 16 or 34 µmol/litre (1419 or 2997 µg/litre)
(following the low or high exposure, respectively) 15-45 min after
exposure ceased. Trace amounts of TBA (1.2%) were found in urine, but
none was detected in exhaled air. The terminal half-life for MTBE in
blood was determined to be 5 h, while that for TBA was 11.9 h. The
authors concluded that metabolism of MTBE was linear at exposures up
to 268 mg/m3 (75 ppm).
Table 14. Summary of kinetic data for MTBE
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
A. Human
2 male, 2 female rapid absorption mean MTBE blood Cain et
volunteers from 0.003 concentrations rose al. (1996)
Treatment: 6 mg/m3 µg/litre preexposure steeply from 0.83
(1.7 ppm) for 1 h to 0.06 µg/litre ±0.50 µg/litre
in inhalation after 1 h preexposure to a
chamber peak blood level of
17.1±2.01 µg/litre
by the end of
exposure; rapid
elimination, half-life
of 40 min; the rapid
elimination phase
appeared to last
approximately 1 h
1 male, 1 female rapid rise in blood rapid decline observed Prah et
volunteer MTBE to 0.03 µg/litre in blood levels with al. (1994)
Treatment: 5 mg/m3 (8.2 ppb) in the male a half-life of about
(1.39 ppm) as a 1-h and 0.05 mg/m3 35 min and rapid
exposure; blood (14.7 ppb) in the metabolic transformation
samples up to 580 female to TBA; TBA levels
min after start gradually increased and
plateaued at 0.025-0.36
µg/litre (7-10 ppb) and
maintained this
concentration up to 7 h
post-exposure
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult relative MTBE detected in clearance of MTBE Johanson et
male volunteers respiratory uptake expired air; both was 0.5 litre/h per al. (1995)
Treatment: exposed was 32-42%; rapid MTBE and TBA found kg; AUC values of
in chamber to 18, absorption based in blood and urine MTBE and TBA were
90 and 180 mg/m3 upon blood proportional to
(5, 25 and 50 ppm) concentration; by exposure levels,
for 2 h on three 2 h, peak blood suggesting linear
occasions during concentrations of kinetics; elimination
light physical MTBE were 1.3, of MTBE in blood
exercise (50W); 6.3, and 12.2 indicated three
observed up to µmol/litre at 18, phases (6-7 min,
24 h and 48 h 90, and 180 mg/m3, 46-58 min and
(only 180 mg/m3) respectively; 6.2-7.2 h); in
post-exposure TBA 5.7 and 0.7 urine half-lives
µmol/litre at 90 of 16-22 min and
and 180 mg/m3, 3.0-3.1 h were
respectively; steady identified;
state was reached half-life of TBA
for TBA after 3-5 h, in urine was
but was not reached 7.5-8.9 h; by 3.5 h
during the 2 h post-dose, 20-30%
exposure to MTBE of absorbed dose
was eliminated in
expired air; by
24 h, approx. 0.1%
MTBE and 0.5-0.8%
TBA of absorbed
dose was eliminated
in urine
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
10 healthy adult respiratory uptake mean concentrations TBA was found in respiratory Nihlén et
male volunteers was 42-49% in blood at the blood and urine exhalation was al. (1998a)
Treatment: exposed three exposure 32-47%; elimination
in chamber to 18, concentrations were in blood was in
90 and 180 mg/m3 1.4, 6.5, and 13 four phases of 1
(5, 25 and 50 ppm) µmol/litre min, 10 min, 1.5 h,
for 2 h on three respectively and and 19 h; kinetics
occasions during were concentration- were linear up to
light physical related the exposure
exercise (50W); concentration of
blood and urine 180 mg/h;
collected during elimination in
exposure and for 3 urine was biphasic
days post-exposure with mean half-lives
of 20 min and 3 h;
excretion was nearly
complete 10 h after
exposure; metabolic
clearance was
0.34-0.52 litre/h
per kg; renal
clearance of TBA
was 0.6-0.7 ml/h
per kg and TBA was
still present after
22 h
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
B. Rodent
I) Oral administration
Species: rapid and extensive apparent volume of in plasma, the dose-related Ferdinandi
Fischer-344 rats absorption of MTBE distribution was major metabolite, differences were et al. (1990a)
Treatment: 40 based upon peak 0.27 to 0.43 litre TBA, peaked at 2 h; observed for the Miller et
rats/sex/dose group plasma concentration male rats had higher plasma elimination al. (1997)
received 40 or 400 within 15 min of plasma concentrations half-time (T´) of
mg/kg as single dosing; lower plasma than female rats; MTBE and TBA:
dose; observed concentrations of dose-unrelated plasma T´ of MTBE:
(sacrificed) for MTBE in female rats concentrations of 0.52-0.62 h (low
various time-points compared to male MTBE and TBA indicate dose) 0.74-0.88 h
until 36 h rats; higher AUC enzyme saturation (high dose) T´ of
post-dose values of MTBE and TBA: 0.95-1.0 h
TBA with oral dosing (low dose)
compared to 1.6-1.9 h (high
intravenous dose) Plasma
administration clearance (CL) of
MTBE:
male rats:
0.36-0.41 litre/h
(both dose-groups)
female rats:
0.48 litre/h (low
dose) 0.29 litre/h
(high-dose)
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: by 48 h, 1.7-3% of higher radioactivity slight sex difference Ferdinandi
Fischer-344 rats administered was recovered in in the route of et al. (1990b)
Treatment: 6 radioactivity was lungs at high oral elimination; by 48 h, Miller et
rats/sex/dose group recovered in dose and lower recovery was 46-54% al. (1997)
received 40 or 400 carcass and tissues; activity in kidneys at low dose and
mg/kg 14C-MTBE as 86 and 81% of suggesting enzyme 65-69% at high dose
a single dose; administered saturation; most of (highest in females);
observed for 48 h radioactivity was the administered 29-36% was recovered
recovered in lungs dose was exhaled as in urine at low dose
and kidneys at low unchanged MTBE and 11-16% at high
and high dose, (predominating) and dose (highest in males)
respectively TBA (2.5-3.1% at low less than 1% was
dose and 1.3-1.4% at recovered in faeces
high dose); in urine,
the major radiolabelled
species (from further
oxidation of TBA) were
2-methyl-1,2-propanediol,
alpha-hydroxyisobutyric
acid, and two minor
unidentified components;
no sex differences in
biotransformation
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
ii) Inhalation
Species: Wistar dose-related MTBE was metabolized Savolainen
rats concentrations of at the ether bond. et al. (1985)
Treatment: 5 male MTBE and TBA in Two weeks of exposure
rats/dose group blood after 2 weeks caused transient
were exposed to of exposure; the increase of the
180, 360 or 1080 concentration of microsomal UDP
mg/m3 (50, 100 or MTBE decreased glucuronosyl-transferase
300 ppm) vapour in after 6 weeks; activities in liver and
exposure chambers the concentrations kidney at all dose
6 h/day, 5 days/week of TBA increased levels. After an initial
for 2, 6, 10 or 15 after 6 weeks of decrease the muscle
weeks exposure and began creatinine kinase
to decrease after activity gradually
10 weeks. increased towards the
Brain levels of MTBE end of the exposure.
and TBA followed a A minor induction of
similar course. MTBE kidney cytochrome
was also found in P-450 was noted. Almost
perirenal fat and at no effect was found on
higher concentrations hepatic cytochrome
than in blood or brain. P-450 concentrations,
Concentrations of MTBE brain succinate
in the blood, brain dehydrogenase, creatine
and perirenal fat were kinase, or
directly related to the acetylcholinesterase
concentrations in the activities
inspired air. The
ratios of TBA/MTBE in
blood increased from
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
week 2 to week 15,
indicating that MTBE
had been oxidized to
TBA, which was
eliminated from the
blood at a slower rate
than MTBE
Species: (T1) rapid absorption (T1) apparent volume (T1) increased AUC Ferdinandi
Fischer-344 rats based upon plasma of distribution: values of MTBE et al.
Treatment: concentration; peak low dose: 35-fold (males) and (1990c)
blood concentration 0.40 (females) and 37-fold (females) Miller et
(T1) 52 rats/sex/ was reached within 0.52 litre (males) while the AUC al. (1997)
dose group received 4-6 h for MTBE and high dose: values of TBA
1440 or 28 800 6.5 h for TBA; low 0.25 (males) and increased by 15-fold
mg/m3 (400 or 8000 but statistically 0.24 litre (females) (males) and 7-fold
ppm) as a single significant (females); quotients
6-h dose. difference in sexes of repeated and
of MTBE AUC values single dose AUC
(T2) 40 rats/sex (low dose); TBA plasma values (T2/T1) for
were exposed 6 h concentration (AUC) MTBE were 0.64
daily to 1440 mg/m3 was lower in female (males) and 0.53
(400 ppm) for 15 rats compared to male (females) and for
days; observed rats, statistically TBA 1.1 (males) and
(sacrificed) at significant at high 1.3 (females); a
various time-points dose significant
until 12 h (T1) and difference in plasma
18 h (T2) post-dose clearance between
single doses 0.53
(males) and 0.57
(females) litre/h
(low dose) and 0.30
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
(males) and 0.32
(females) litre/h
(high dose); the
plasma half-life of
MTBE was 0.52-0.63 h
(T1) and 0.48-0.51 h
(T2); the (T1)
half-lives of TBA
were 2.8 (males) and
3.4 h (females) and
(T2) 1.8 h (males)
and 1.5 h (females)
Species: (T1&T2): rapid and by 48 h, 11-13% (T1&T2): radiolabelled rapid elimination Ferdinandi
Fischer-344 rats extensive absorption (low dose; T1&T2) species in expired air in urine after low et al.(1990d)
Treatment: as indicated by and 4-5% (high dose) were MTBE and TBA; by dose and in expired Miller et
(T1) 6 rats/sex/ marked recovery of of administered 3 h post-dose, 30% air at high dose, al. (1997)
dose group received 14C in urine by 24 h radioactivity (low dose) and 7-10% suggesting enzyme
1440 or 28 800 recovered in skin (high dose) of saturation at high
mg/m3 (400 and of some rats, recovered radioactivity dose; by 48 h, total
8000 ppm) of probably due to were correlated to TBA, recovery of
14C-MTBE as a contamination from while TBA was major radioactivity (14C)
single 6-h urine (lower radioactive component in urine 65-71% (low
exposure radioactivity at 24 h post-dose dose, T1&T2) and
recovered in urine); (>90%, low dose); 35-42% (high dose);
(T2) 40 rats/sex 0.6-1% in skin of major radiolabelled in expired air 17-22%
were pretreated remaining rats, 1-3% species in urine from (low dose) and 54-59%
with 1440 mg/m3 in carcass, and <1% further oxidation of (high dose) and <1%
unlabelled MTBE in tissues TBA were 2-methyl-1, in faeces; no sex
6 h a day for 14 2-propanediol, alpha- difference in rate
days, followed by hydroxyisobutyric acid, and route of
6 h exposure for two minor unidentified radioactivity
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
1440 mg/m3 14C-MTBE components, and minor elimination
on day 15; observed detection of CO2 (1%
for 48 h post-dose of the dose); no sex
differences in
biotransformation
iii) Intravenous administration
Species: a statistically apparent volume of by 2 h, the major half-life of MTBE Ferdinandi
Fischer-344 rats significant distribution 0.27 metabolite TBA was (plasma) 0.45-0.62 h; et al.(1990a)
Treatment: 40 rats/ difference in plasma to 0.31 litre found in blood at half-life of TBA Miller et
sex/group received concentrations (AUC) peak concentration; (plasma) 0.92-1.3 h; al. (1997)
40 mg/kg as a between sexes (lower male rats had higher clearance from plasma
single dose; in females) blood concentration of MTBE 0.36-0.41
observed than female rats litre/h (males) and
(sacrificed) for 0.47 litre/h (females)
various time-points
until 36 h
post-dose
Species: rat, 1.7-3% of administered by 6 h, major by 48 h, 71-73% of Ferdinandi
Fischer-344 radioactivity was radiolabelled species administered et al.(1990b)
Treatment: 6 rats/ recovered in tissues in expired air was radioactivity was Miller et
sex/group received and carcass for each MTBE, with a minor totally recovered: in al. (1997)
40 or 400 mg/kg dose after 48 h elimination of TBA exhaled air (42-46%),
14C-MTBE as a (2.5-3.1% of in urine (26%), and
single dose; administered dose); faeces (<1%); rapid
observed for 48 h the major radiolabelled elimination in lungs,
species in urine (from 94% within 3 h
further oxidation of
TBA) were 2-methyl-1,
2-propanediol, alpha-
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
hydroxyisobutyric acid,
and two minor
unidentified components;
there were no sex
differences in
biotransformation
iv) Intraperitoneal
Species: Charles rapid and extensive the total cumulative methanol and formic 14C activity was mainly Biodynamics
River CD rats absorption based upon 14C activity in tissues acid were found in eliminated in expired (1984)
Treatment: 33 peak blood and plasma averaged 3.39, 1.94 and plasma, liver and air; by 48 h, recovery
animals/sex/group concentrations at 5 1.14% of administered kidney was about 99.86% (approx.
received a single min post-treatment, dose at 15 min, 6 h, 92% as MTBE and approx.
dose of averaging 92.04 ± and 24 h post-treatment, 7.45% as CO2); about 3%
approximately 37.72 and 83.40 ± respectively. At 15 min, in urine and about 0.8%
60µCi 14C-MTBE 20.48 µg 14C-MTBE the majority of the in faeces (male rats);
(about 232 mg equivalents/ml blood 14C-radioactivity was approx. 3% was excreted
MTBE/kg bw) in for male and female found in mesenteric fat, in urine and 0.8% (males)
saline and were rats, respectively. liver and kidney; and 1.25% (females) in
sacrificed at The half-life of however, at 6 h and 24 h faeces. The 14C activity
intervals of 5, 14C-MTBE in blood was no 14C was found in in urine and faeces was
15, 30, and 45 59.8 min for male rats mesenteric fat mainly associated with
min, and 1, 2, 3, and 49 min for female 14C-formic acid (a total
6, 12, 24 and 48 h rats. The half-life of 3.08% of administered
post-treatment in plasma was 2.3 h dose)
for males and 1.3 h
for females
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
Species: mouse, most of the Yoshikawa
ddY administered MTBE et al.(1994)
Treatment: 4 male was eliminated
mice/dose group unchanged in the
were administered exhaled air; >90%
a single dose of of this amount was
50, 100 or 500 eliminated within
mg/kg in corn oil 3 h. The pulmonary
solution; observed elimination showed
for 6 h an initial rapid
decrease of the
elimination ratio
followed by a slow
decrease at 100 and
500 mg/kg. The
calculated half-lives
were 45 and 80 min,
respectively. The
elimination ratios
at the three different
doses were 23.2, 37.6
and 69.0%, respectively
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
C. In vitro
Incubation of 5 or incubation of MTBE with Brady et
14 mM MTBE with liver microsomes from al. (1990)
liver microsomes phenobarbital-pretreated
from phenobarbital- rats resulted in TBA and
or acetone- formaldehyde in
pretreated or equimolar amounts. The
untreated Vmaxa value for
Sprague-Dawley rats demethylation of MTBE
(3-5 males) increased by 4-fold with
acetone-induced and by
5.5-fold with
phenobarbital-induced
microsomes compared with
the control Vmax value.
These results indicate
that cytochrome P450 2B1
(inducible by
phenobarbital) and
cytochrome P450 2E1
(inducible by acetone)
play a role in the
demethylation of MTBE.
Results after inclusion
of monoclonal antibody
against P450 2E1
indicated this enzyme
only partially
contributed to the
demethylation. Microsomes
Table 14. (continued)
Species and Absorption Distribution Metabolic Elimination Reference
treatment transformation and excretion
pretreated with MTBE
yielded a 47-fold
induction of
pentoxyresorufin
o-dealkylase, with no
change in
N-nitrosodimethylamine
demethylase activity.
These results are
consistent with an
elevation of P450 2B1
a Vmax : the maximum velocity of the demethylation process.
Nihlén et al. (1998a) studied the uptake, distribution,
metabolism and elimination of MTBE in ten healthy male volunteers. The
subjects were exposed on three different occasions for periods of 2 h
in a chamber to MTBE concentrations of 18, 90 and 180 mg/m3 (5, 25
and 50 ppm) while performing light physical exercise. MTBE (and its
metabolite TBA) were monitored in exhaled air, blood and urine, the
latter being collected up to 3 days after exposure. Respiratory uptake
of MTBE was low (42-49%) and respiratory clearance was high (32-47%).
The metabolic blood clearance was 0.34-0.52 litre/h per kg. The
kinetics of MTBE were linear up to the highest exposure concentration
of 180 mg/m3 (50 ppm). The kinetic profile of MTBE in blood was
described as having four phases, with average half-lives of 1 min, 10
min, 1.5 h and 19 h. In urine the post-exposure decay curve of MTBE
had two linear phases with average half-lives of 20 min and 3 h. The
urinary excretion of MTBE was less than 1% of the absorbed dose.
Biomarkers and partitioning of inhaled MTBE were studied in two
(1 male, 1 female) volunteer subjects exposed for 1 h to a nominal
concentration of 5 µg/m3 (1.39 ppm), followed by clean air exposure
for 7 h (Buckley et al., 1997). MTBE concentrations in expired air,
venous blood and urine were monitored during and after exposure. The
decay of MTBE was assessed by using a 2- or 3-exponential model and
yielded residence times of 2-3 min, 15-50 min, and 3-13 h in alveolar
air, and 5 min, 1 h and 32 h in venous blood. Based on
lower-than-expected blood and expired air MTBE concentrations during
uptake and the decreasing blood-breath ratio during the post-exposure
decay period, the authors hypothesized that the respiratory mucous
membranes acted as a reservoir for MTBE, retaining 6-9% of the MTBE
intake. Compartmental monitoring was used to estimate a blood-breath
partition coefficient of approximately 18. The urinary concentration
of MTBE ranged from 0.37 to 15 µg/litre and bore little relationship
to the exposure: urinary elimination accounted for only a small
fraction (<1%) of total MTBE elimination.
6.1.2 Human exposure to oxygenated gasoline
During an oxyfuels programme in Fairbanks, Alaska, there was a
strong correlation between the workplace air levels ranging from 0.02
to 2.92 mg MTBE/m3 and the difference in blood concentrations of MTBE
between pre-shift and post-shift blood measurements (p=0.0001). The
median pre-shift concentration of MTBE in the blood of 18
occupationally exposed workers was 1.15 µg/litre (range 0.1- 27.8
µg/litre). The median post-shift blood MTBE level was 1.8 µg/litre
(range 0.2-37.0 µg/litre) (Moolenar et al., 1994).
Breath samples were collected from a person pumping gasoline
(containing 15% MTBE by volume) and a nearby observer (within 1 m),
immediately prior to and an hour after refuelling (Lindstrom & Pleil,
1996). The MTBE concentration in the sample collected during
refuelling was 412 µg/m3. The ambient background level was
approximately 25 µg/m3 MTBE. Low concentrations of MTBE (7-10 µg/m3)
were detected in the exhaled breath before the refuelling that took
2 min and 8 seconds; 40 seconds after the exposure the concentrations
had increased by factors of 35 for the observer and 100 for the person
pumping gasoline (see also section 5).
6.2 Animal studies
Repeated exposure (2 to 15 weeks) to MTBE vapour by inhalation (6
h/day, 5 day/week) resulted in dose-dependent increases in MTBE levels
in blood, brain and perirenal fat of Wistar male rats (Savolainen et
al., 1985). Very small differences were observed in blood MTBE levels
following repeated exposure. TBA levels in blood increased with
repeated MTBE exposure when comparing levels at 6, 10 and 15 weeks to
levels following 2 weeks. Perirenal fat/blood MTBE concentration
ratios ranged from 9.1 to 11.6 after 15 weeks of intermittent
exposure. Blood and brain concentrations of TBA, the major main
metabolite, were also dose-dependent. TBA was, however, not found in
quantifiable amounts in the perirenal fat.
A series of studies on the kinetics of MTBE was conducted by
Bio-Research Laboratories (Ferdinandi et al., 1990a-d). These data
have been further published by Miller et al. (1997).
Miller et al. (1997) and Ferdinandi et al. (1990a-d) described
the pharmacokinetics and disposition of MTBE in male and female
Fischer-344 rats following i.v. (40 mg/kg), oral (40 and 400 mg/kg)
and dermal (40 and 400 mg/kg in occluded chambers) administration and
(nose-only) inhalation exposure for 6 h either for a single exposure
or repeated exposure (15 days). The details of these studies are
presented in Table 15. Miller et al. (1997) found that the elimination
of radiolabelled MTBE in rats was rapid and mainly occurred through
lungs and kidneys irrespective of administration route. The
elimination was virtually complete 48 h post-dosing. The renal
elimination was, however, slowest after dermal exposure. Twelve hours
post-dosing, the radiolabelled recovery in urine was 14-26% of the
dermal dose, 41-50% of the oral dose and 25-37% of the intravenously
injected dose. The recovery was 75-94% 36 h post-dosing, irrespective
of dose and administration route. A minor difference in excretion
route was observed between the sexes. Collectively these studies
demonstrated that MTBE is rapidly eliminated from blood (half-life =
0.5 h) by exhalation and metabolism to TBA (see Table 15). The major
metabolites recovered in urine were 2-methyl-1,2-propanediol and
alpha-hydroxyisobutyric acid. Dose-related differences in the AUC for
plasma concentrations of MTBE and TBA were observed in rats exposed to
1440 or 28 800 mg MTBE/m3 (400 or 8000 ppm) by inhalation (Ferdinandi
et al., 1990a-d; Miller et al., 1997). Miller et al. (1997) further
showed that increasing doses of MTBE (by inhalation and, to a lesser
extent, oral administration) to Fischer-344 rats decreased the
recoveries of radioactivity in urine and increased the recovery in
expired air. This indicates a saturation of the oxidative metabolic
pathway of MTBE at inhalation levels above 28 800 mg/m3 (8000 ppm) or
at oral administration levels above 400 mg/kg. There were no
significant sex- or route-dependent differences in the
pharmacokinetics and disposition of MTBE.
Table 15. Pharmacokinetic parameters of MTBE and TBA calculated from mean plasma concentrations of male
F-344 rats exposed to MTBE (Miller et al., 1997)
Exposure route Dose MTBE TBA
AUC Half-life CL V/F or Vssd AUCzero to infinity Half-life
(µg/h per ml) (h) (ml/h) (litre) (µg/h per ml) (h)
Intravenous 40 mg kg 10.7 0.45 413 0.27 26.7 0.92
Oral 40 mg kg 17.0 0.52 392 0.29 39.0 0.95
400 mg kg 230 0.79 358 0.41 304 1.6
Dermal 40 mg kg 7.9 2.3a 389 3.9 26.3 2.1a
400 mg kg 46.9 1.8a 364 1.4 93.9 1.9a
Inhalation low single 84.3 0.52 531 0.40 404 3.3
high single 2960 0.57 299 0.25 6010 3.4
low repeated 6.7b 0.51 c c 127b 1.8
a Calculated from the alpha-phase of a two-compartment model. Half-lives of MTBE from 12 to 45 h post-dose curve at
low and high doses were 92 and 37 h, respectively. Half-lives of TBA from 12 to 45 h post-dose were 170 (low dose)
and 31 h (high dose).
b AUCzero to infinity on 15th day of exposure.
c Values were not calculated because plasma was not collected during exposure.
d Vss = apparent volume of distribution at steady state after repeated inhalation.
Following intraperitoneal administration of 14C-MTBE to rats,
the highest radioactivity was recovered in the expired air
(Biodynamics, 1984). The radioactivity was also distributed throughout
the animal tissues. The amount retained in tissues was less than 2% of
the total dose of MTBE intraperitoneally administered to rats at 6 and
24 h post-treatment. Distribution of the radioactivity was primarily
to the liver and secondarily to the kidney. Approximately 92% of MTBE
was exhaled as the parent compound and approximately 7.5% as CO2 in
48 h. Another 3% was excreted in urine and up to 0.8% in faeces. The
14C-activity in urine and faeces was mainly associated with
14C-formic acid. Peak blood levels were observed in male and female
rats 5 min following intraperitoneal dosing of 14C-MTBE. Peak levels
of 14C activity in the plasma occurred at 5 min post-treatment in
male rats and at 15 min post-treatment in females rats. The
radioactivity decreased sharply during 1 h and thereafter gradually
during the study period (48 h). The half-time of radiolabelled MTBE in
whole blood was 59.8 min for male rats and 49 min for female rats. The
half-life of MTBE in plasma was 2.3 h for male rats and 1.3 h for
female rats (Biodynamics, 1984).
In a study of MTBE distribution in male and female rats following
inhalation exposure to 10 710 mg/m3 (3000 ppm) for 6 h, it was
reported that MTBE levels in the liver and brain were comparable in
males and females but were higher in male kidneys than in those of
females: MTBE was still detectable in male kidneys 18 h after exposure
(Borghoff et al., 1998). The authors considered that this may have
been due to the interaction of MTBE with alpha2u-globulin in the male
rat kidney.
6.3 In vitro studies
In vitro measurement of liquid/air partition coefficients of
MTBE at 37°C, using a vial equilibration technique, showed a human
blood/ air partition coefficient of 17.7 (confidence limits:
17.0-18.4), water/ air 15.2 (CL: 14.9-15.5), and oil/air 120 (CL:
114-125) (Nihlén et al., 1995). There was no significant difference in
partition coefficient for blood/air between the sexes. Liquid/air
partition coefficients for MTBE between different media and air were
also determined by Imbriani et al. (1997). The values were: blood/air
= 20.0; urine/air = 15.6; saline/air =15.3; fat/air = 142.0; and olive
oil/air = 138.0.
MTBE partition coefficients measured in rat tissues demonstrated
a higher solubility of MTBE in fat (115.6) compared to blood (11.5)
and other tissues such as liver (14.5) (Borghoff et al., 1996). The
partition coefficient of MTBE in the male rat kidney was approximately
five times higher than the value measured in female rat kidneys. This
high uptake of MTBE into the male rat kidney was found to be due to an
interaction with the male rat specific protein alpha2u-globulin
(Borghoff et al., 1995; Poet & Borghoff, 1997).
In rats, MTBE is demethylated by hepatic microsomal enzymes to
form TBA and formaldehyde (FA) (Savolainen et al., 1985; Brady et al.,
1990). When using rat liver microsomes, the Vmax for demethylation of
MTBE to FA increased after pretreatment with acetone or phenobarbital
(Savolainen et al., 1985; Brady et al., 1990). The results of Brady et
al. (1990) indicated that both cytochromes P450 2BI and P450 2E1 are
implicated in the metabolism of MTBE. In vitro, TBA has been shown
to be oxidatively demethylated using rat liver microsomes to yield FA
(Cederbaum & Cohen, 1980).
Hong et al. (1997) demonstrated that human liver microsomes
metabolize MTBE to TBA. The activity of 125 ± 11 pmol TBA/min per mg
protein was approximately 50% of the activity in rat and mouse liver
microsomes. The metabolism of MTBE to TBA in human liver microsomes
was NADPH-dependent and was inhibited by carbon monoxide, an inhibitor
of cytochrome P450 (CYP) enzymes, suggesting that CYP enzymes play a
critical role in human metabolism of MTBE. Human CYP2A6 and CYP2E1
cDNAs were each co-expressed with human cytochrome P450 reductase by a
baculovirus expression system and the expressed enzymes used to
metabolize MTBE. CYP2A6 was more active than CYP2E1 (activity 6.1 and
0.7 nmol TBA/min per nmol P450, respectively).
The role of the cytochrome P450 enzyme CYP2E1 in metabolizing
MTBE (and other gasoline ethers) was examined in 2E1 knock-out mice,
which lack demethylation capability. Liver microsomes metabolized MTBE
with an activity level of 0.67 ± 0.1 nmol/min per mg. However, there
were no significant differences in activity levels in microsome
preparations from two 2E1+/+ strains of mice, demonstrating that
CYP2E1 is not important in the metabolism of MTBE in mouse livers
(Hong et al., 1998).
The probable metabolic pathway is presented in Fig. 2.
6.4 Physiologically based pharmacokinetic modelling
A physiologically based pharmacokinetic model (PBPK) to describe
the dosimetry of MTBE in rats has been developed (Borghoff et al.,
1996). Using this PBPK model, MTBE blood levels following different
routes of exposure and various exposure concentrations were predicted.
When human anatomical parameters are used in this model and the
metabolism is scaled allometrically, the model predicts the level of
MTBE in blood (concentrations ranging from 0.03 to 17.1 µg/litre)
during and following exposure of people to 6 mg MTBE/m3 (1.7 ppm)
(Borghoff et al., 1996; Cain et al., 1996). The human PBPK model was
further expanded to include brain as a target tissue and an exposure
model for bathing and showering. Model simulations of a bathing and
showering MTBE exposure scenario in humans at water levels from 0.64
to 1.0 mg/litre and air levels from 5 to 5.7 mg/m3 (1.4 to 1.6 ppm)
predicted maximum brain levels to range from 0.015 to 0.02 mg
MTBE/litre and 0.006 to 0.019 mg TBA/litre (Rao & Ginsberg, 1997).
 7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
Owing to the limited therapeutic use of MTBE in the dissolution
of cholesterol gallstones in humans, there have been a number of
studies in which effects have been examined following single or
repeated exposure by direct instillation into the gallbladder.
Reported effects at therapeutic doses were mild clinical signs and
inflammatory changes in the gallbladder (Allen et al., 1985a; McGahan
et al., 1988; Adam et al., 1990; Esch et al., l992a,b; Chen et al.,
1995).
7.1 Single exposure
The acute toxicity of MTBE has been studied in several animal
species; the results are summarized in Tables 16 and 17. Studies by
routes most relevant to human exposure are described here. The oral
LD50 value for rats is about 3800 mg/kg bw (ARCO, 1987). Signs of
intoxication after single oral lethal doses consist of CNS depression,
ataxia, laboured respiration and death (ARCO, l987). When the dose was
non-lethal, recovery was complete.
The acute dermal LD50 is >10 200 mg/kg bw for rabbits (ARCO,
1987). Adverse local effects included erythema, oedema, fissuring and
necrosis at dermal application.
In rats, the LC50 value for inhalation exposure to MTBE is about
142 000 mg/m3 air (39 460 ppm) (ARCO, 1987). Reported LC50 values in
mice are 141 000 mg/m3 (1.6 mmol/litre) for 15 min of inhalation
exposure (Marsh & Leake, l950) and 658 000 mg/m3 (18% v/v) for 10 min
of inhalation exposure (Snamprogetti, 1980). Signs noted in rats
following inhalation exposure included eye irritation, incoordination
and loss of righting reflex (ARCO, 1987). Surviving animals appeared
to recover within 24 h.
Results of studies in which neurological effects following single
exposures were examined are reported in section 7.3.
7.2 Skin, eye, and respiratory tract irritation; skin sensitization
7.2.1 Skin irritation
Moderate erythema and oedema were reported by Cuthbert (1979)
following application of 0.5 ml undiluted MTBE to the intact and
abraded skin of six rabbits (applied under occlusion for 24 h). A
primary irritation index of 3.36 was reported. Effects were slightly
more pronounced on abraded skin:
24 h 72 h
intact abraded intact abraded
erythema 1.7 2.2 1.1 2.0
oedema 1.7 2.0 1.0 1.8
MTBE was considered to be a moderate skin irritant.
Table 16. Acute toxicity of MTBE in experimental animals
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Rat: oral (gavage) 2000, 3000, 4600, 3800 hypoactivity, muscular weakness, Industrial Bio-Test
Charles River, 6800, or 10 200 hyperpnoea, lacrimation, Laboratories (1969)
5 male, 5 female/ mg/kg bw prostration and death; the
dose level symptoms were reversible at
sublethal doses.
Inflammation of the stomach
and/or small intestine in
animals that died
Rat: oral (gavage) 3866 CNS depression, ataxia, ARCO (1987)
(strain and number laboured respiration and
not stated) death
Rabbit: dermal 6.8 or 10.2 g/kg >10 200 no deaths; no gross Industrial Bio-Test
New Zealand White bw, occlusive pathological alterations Laboratories (1969)
2 male, 2 female/ dressing, for 24 h; other than necrosis at
dose level 14-day observation application site
Rabbit: dermal 10 000 erythema, oedema, scaling, ARCO (1987)
(strain and number fissuring, and blanching
not stated) hyperaemia in animals that
died
Rat: inhalation LC50 = 142 000 eye irritation, ARCO (1987)
(strain and sex mg/m3 incoordination, tachypnoea,
not stated) (39 460 ppm) loss of righting reflex
and death
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Mouse: inhalation exposure for 15 min LC50 = 141 000 the median concentration Marsh & Leake (1950)
White (strain (whole body mg/m3 for anaesthesia (AC50) =
and sex not exposure) (1.6 mmol/litre) 106 mg/litre (1.2
stated), 20 mmol/litre)
mice/exposure
group
Mouse: inhalation exposure for 5 min; 400 000 mg/m3 median effective concentration Industrial Bio-Test
Swiss, (whole body observation for (260-600 mg/litre) for anaesthesia (EC50) = Laboratories (1969)
4 males/exposure exposure) following 48 h 200 mg/litre air (130-300
group mg/litre); convulsive
hyperventilation,
hyperactivity, ataxia, loss
of righting reflex, clonic,
and sporadic convulsive
seizures
Mouse: inhalation I. 20% v/v in air LT50a = 5.6 min death occurred within 1 h Snamprogetti (1980)
Swiss, (whole body for 3, 4, 5, 6, 9
I. 40 males/ exposure) or 12 min
exposure group II 8, 12, 17, 20, LC50 = 658
II. 20 males per 22 or 26% v/v MTBE mg/litre
group in air for 10 min (18% v/v)
Skin and eye irritancy
Rabbit:
New Zealand skin application 0.5 ml moderate erythema and
White, 3 male, (occluded) oedema
3 female
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
New Zealand conjunctival 0.1 ml moderate conjunctival Cuthbert (1979)
White, 3 male, instillation response; no corneal
3 female or iris involvement
New Zealand
White
I. 6 (sex not conjunctival 0.1 ml conjunctival redness for Hazleton
stated) instillation 48 h, no chemosis or discharge, Laboratories
slightly more marked effects (1979)
II. 3 (sex not conjunctival 0.1 ml with temporary corneal
stated) instillation; opacity for 24 h
washout with
water 30 secs
later
Rabbit conjunctival 0.05 ml or 0.1 ml conjunctival congestion, Snamprogetti (1980)
Both sexes instillation thickening and lacrimation;
(strain and number more marked at high dose;
not stated) reversible
a LT50 =exposure time which causes death in 50% of treated animals.
Table 17. Acute toxicity of MTBE following parenteral injection
Species Injection Dose LD50 Comments Reference
route/site
Rat: subcutaneous 3.0, 4.0, 5.0, 6.0, 6.7 ml/kg bw Snamprogetti (1980)
Wistar, 7.0, 8.0, 9.0 or 10.0 (5.75-7.76 ml/kg)
8 males/dose group mg/kg bw
Mouse: subcutaneous 1.0, 2.0, 3.0, 4.0, 3.6 ml/kg bw Snamprogetti (1980)
Swiss, 4.5, 5.0 or 5.5 ml/kg bw (2.93-3.57 ml/kg)
8 males/dose group
Rat: intravenous 0.1, 0.2, 0.3, 0.5, 0.75, 0.56 ml/kg bw in lethal doses: Snamprogetti (1980)
Wistar, 1.25 or 1.50 mg/kg bw nervous depression,
16 males/dose group sometimes followed
by short clonic
convulsions, autonomic
activity
(hypersalivation,
urination, defaecation),
and respiratory
disorders; death
occurred within
30 min. Surviving
animals: no toxic
symptoms or signs of
nervous depression
lasting more than
15-20 min
Table 17. (continued)
Species Injection Dose LD50 Comments Reference
route/site
Rat: hepatic parenchyma, 0.2 ml/kg bw intracaval injection Akimoto et al.
Sprague-Dawley, inferior vena cava, caused 100% mortality (1992)
7 males dosed tail vein or (pulmonary injury);
intravenously peritoneal cavity intrahepatic injection
caused 59% mortality
10 males dosed and peripheral vein
intraperitoneally injection 17% mortality;
the pulmonary injury
included congestion,
haemorrhage, and
interstitial oedema
Mouse: intraperitoneal 1.5, 2.0, 2.5, 3.0, 3.5, 1.4 ml/kg bw Snamprogetti (1980)
Swiss, 4.0, or 5.0 ml/kg bw
8 males/dose group
Mouse: intraperitoneal 0, 50, 200, or 500 mg/kg 830 mg/kg bw Arashidani et al.
ddy bw (784-878 mg/kg) (1993)
10 males/dose group
7.2.2 Eye irritation
Instillation of 0.05 or 0.1 ml of MTBE into the conjunctival area
of albino rabbits (both sexes) caused reversible eye irritation
(congestion of the conjunctiva, palpebral thickening and lacrimation,
more marked at the high dose) (Snamprogetti, 1980).
Moderate erythema and slight chemosis and discharge, persisting
for 3 days, were noted in another study after instillation of 0.1 ml
MTBE into the conjunctival area of New Zealand white rabbits
(Cuthbert, 1979):
24 h 48 h 72 h 7 days
redness 2.2 1.7 1 0.2
chemosis 1.3 1.2 1 0.1
discharge 0.9 0.6 0.2 0
The reactions had largely resolved by one week post-treatment).
The author concluded that MTBE was irritant to the rabbit eye.
Hazleton Laboratories (1979) conducted a study on nine New
Zealand White rabbits (sex not stated) in which six rabbits had 0.1 ml
MTBE instilled into one eye, the other acting as control, and three
rabbits had the same treatment followed by eye washout with water 30
seconds later. There was slight irritation (mean score for redness 1.5
and 1.0 at 24 and 48 h, negligible chemosis or discharge) in six
rabbits that did not have the eye washout, but slightly more marked
effects, including corneal opacity lasting for 24 h, in the three
rabbits with eye washout. The effects were all reversible.
Exposure to MTBE vapour in inhalation chambers also resulted in
eye irritation. In Fischer-344 rats exposed to MTBE vapour
concentrations of 14 300 or 28 600 mg/m3 (4000 or 8000 ppm) for 6 h,
lacrimation was noted 1 h post-exposure (Gill, 1989).
7.2.3 Respiratory tract irritation
A test of lung irritancy was used by Tepper et al. (1994) to
evaluate the effects of 1-h exposures of Swiss-Webster mice (four
males per dose group) to 300, 1000, 3000, 10 000 and 30 000 mg
MTBE/m3 in inhalation chambers. An almost immediate decrease in
frequency of breathing was observed in all dose groups. The animals
served as their own controls with baseline frequency of breathing and
respiration waveform morphology being obtained by exposure to filtered
air for 15 min. The severity of the irritant response was
dose-related, ranging from "slight" (13% decrease in respiration rate)
to "severe" at 30 000 mg/m3. During the exposures, breathing rate
returned to baseline after about 15 min, except for the highest dose
group, in which the rate gradually decreased for another 40 min. There
was a return to baseline frequency 15 min after end of exposure. Lung
injury could not be confirmed at the 30 000 mg/m3 dose level. Lung
lavage about 20 h post-exposure indicated only a marginal increase of
total protein and lactate dehydrogenase, both measures of lung cell
damage; however, these results were comparable to those of similarly
treated mice, exposed to filtered air, in a previous study.
In a single 6-h exposure vapour neurotoxicity inhalation study
(see also section 7.3) in Fischer-344 rats at target concentrations of
14 400 or 28 800 mg/m3 (4000 and 8000 ppm), survivors killed at the
end of a 14-day observation period had slight to mild lung hyperaemia
(Gill, 1989).
7.2.4 Skin sensitization
The sensitization potential of MTBE was assessed in guinea-pigs
using a Magnusson and Kligman procedure (Cuthbert, 1979). No evidence
of sensitization was reported in any of the 20 test animals following
induction and challenge with 1% MTBE. Dermal sensitization has also
been investigated using a Landsteiner technique (Litton Bionetics,
1980). Guinea-pigs were induced using intradermal injection (initial
treatment 0.5 ml of a 1% aqueous solution, followed by 9 injections of
0.1 ml over 3 weeks). No sensitization reactions were recorded at
challenge 2 weeks later (0.05 ml of a 0.01% solution of MTBE in
water).
7.3 Neurotoxicity
Following a single MTBE vapour exposure, reversible alterations
in central and peripheral nervous system function were observed (Gill,
1989; Daughtrey et al., 1997). Groups of Fischer-344 rats (22 of each
sex per dose level) were exposed for 6 h in inhalation chambers at
target MTBE concentrations of 0, 2860, 14 300 or 28 600 mg/m3 (0,
800, 4000 or 8000 ppm). No mortality or clinical signs of toxicity
were observed at any concentration. Behavioural evaluations performed
1, 6 and 24 h post-exposure included a screen for behavioural function
using a functional observational battery (FOB) and analysis of motor
activity prior to and following exposure. At 1 h post-exposure,
concentration-related increases in the incidence and severity of
ataxia and duck-walk gait appeared in both sexes of the mid- and
high-dose groups. In high-dose males, lacrimation, decreased muscle
tone, decreased rectal temperature, decreased performance time on the
tread mill, and increased hind limb splay were also observed. The
alterations in the FOB were significantly (p<0.01) different from the
control group. An increased incidence of laboured respiration (not
statistically significant) was also found in the high-dose male rats.
Additionally, piloerection was observed for all males in the high-dose
group. However, piloerection was also observed in some male rats in
the control group and in low- and mid-dose males.
Similar concentration-related findings were found in mid- and
high-dose female rats (increased incidence of lacrimation,
piloerection, ataxia and duck-walk gait, decreased rectal temperature,
and decreased hind limb grip strength). Additional exposure-related
findings for females in the high-dose group included a significantly
(p<0.01) increased incidence of laboured respiration and latency to
rotate on the inclined screen. Exposure-related alterations of motor
activity were detected for both male and female rats during the
initial 90 min of the test session. The mean activity was increased
during the entire test period in low-dose males and decreased in
high-dose males compared to the control group. However, in high-dose
males, an initial 10-min decrease in activity was followed by
increased activity during a 20-min interval and decreased thereafter.
Males in the mid-dose group only showed an increased activity
initially. For females, the mean activity for the entire test session
was not different from the control group. No MTBE-related alterations
were observed during the 6-h or 24-h post-exposure evaluation.
7.4 Short-term repeated dose studies
The short-term repeated dose toxicity of MTBE has been studied in
rats, mice and pigs. Typically, effects of irritation and reversible
central nervous system effects, including hypoactivity, ataxia, and
anaesthesia, were noted.
7.4.1 Oral studies
Sprague-Dawley rats (10 males and 10 females per dose group)
administered 0 (corn oil), 357, 714, 1071 or 1428 mg MTBE (99.95%
pure)/kg bw daily by gavage (in corn oil) for 14 days exhibited
transient anaesthesia at 1428 mg/kg and irritation of the pharyngeal
mucosa at the highest dose levels (Robinson et al., 1990). Diarrhoea
and reduced body weight gain were observed in all treatment groups.
Six animals (four from the high-dose group) died during treatment due
to difficulties associated with the gavage procedure, including
pharyngeal irritation in the high-dose animals. Absolute and relative
lung weights were significantly (p<0.001) lower in all exposed
female rats. The mean absolute kidney weights were increased in dosed
male rats and the relative kidney weights were significantly
(p<0.05 and p<0.001) increased over controls in the mid- and
high-dose group, respectively. The cholesterol levels were
significantly (p<0.05) increased in high-dose males and the two
mid-dose groups of females. The blood-urea nitrogen (BUN) and
creatinine were significantly (p<0.05) decreased in high-dose
females. The incidence of renal tubular disease (hyaline droplet
nephropathy) was moderately increased in dosed male rats. Increased
hyaline (protein) droplets within the cytoplasm of proximal tubular
epithelial cells were noted in seven of eight (88%) of the males in
the highest dose group as compared with two of five (40%) of the
controls.
In a 28-day oral study, Sprague-Dawley rats (10/sex/group) were
administered 0, 90, 440 or 1750 mg undiluted MTBE (purity not
specified)/kg bw daily by gavage for a total of 20 h (IITRI, 1992).
Seven rats (one low-dose female, one high-dose male, and five
high-dose females) died accidentally during dosing. This was
attributed to difficulties in dosing the animals owing to the strong
odour, the irritating nature, and high volatility of MTBE. Clinical
observations during the study period included transitory salivation in
all treated groups and transitory hypoactivity and/or ataxia in mid-
and high-dose animals. There were no significant effects on body
weight or body weight gain. The only significant treatment-related
change in haematological or clinical chemistry parameters was an
increase in cholesterol in high-dose males and females.
No treatment-related gross necropsy observations were noted. The
relative liver weights were significantly (p<0.05) increased in males
and females in the high-dose group. In the high-dose males there was
also a significant (p<0.05) increase in relative adrenal weight.
Absolute kidney and relative kidney weights showed a dose-related
increase in both males and females, but achieved statistical
significance (corrected for multiple comparisons) only for relative
weights in males at the mid- and high-doses and in females at the low
and high doses. Histopathology showed hyaline droplet formation in the
proximal convoluted tubules in the kidneys of the mid- and high-dose
males.
In a 90-day study, groups of Sprague-Dawley rats (ten males and
ten females in each test group) were gavaged 0 (corn oil), 100, 300,
900 or 1 200 mg/kg bw of undiluted MTBE (>99.95% pure) daily for 90
days (Robinson et al., 1990). The most pronounced clinical effect of
MTBE was the profound anaesthetic effect at 1200 mg/kg bw; the animals
recovered in about 2 h. In female rats, a significant (p<0.001)
treatment-related decreased BUN level and elevated cholesterol level
were observed at all levels of exposure. In male rats, the mean
absolute kidney weights were significantly (p<0.05) elevated at 900
and 1200 mg/kg bw, respectively. The increase in the relative kidney
weights was statistically significant (p<0.001) at the two highest
dose levels and also the relative liver weights (p<0.05 at 900
mg/kg and p<0.001 at 1200 mg/kg). In females, the relative kidney
weights were significantly (p<0.05) increased at and above 300
mg/kg bw and the relative liver, thymic and cardiac weights showed a
statistically significant (p<0.05) dose-related increase at 900
mg/kg. Microscopic findings included chronic nephropathy in both
control and high-dose male rats, which was more severe in MTBE-treated
rats. At the highest dose level, granular casts were found, and there
was also a slight increase of cytoplasmic hyaline droplets in proximal
tubular epithelial cells.
In a comparison study on the effects of inhalation exposure (see
section 7.3.2), two groups of female B6C3F1 mice (8/group) were given
MTBE by gavage in corn oil (5 ml/kg/bw) at doses of 0 or 1800 mg/kg bw
per day for 3 days and then killed around 18 h after the last dose
(Moser et al., 1996a). Body weight and liver weight were not affected
by treatment. MTBE induced a 37% increase in hepatic cytochrome P450
content (P <0-05), a 9-fold increase in hepatic 7-pentoxy
resorufin- O-dealkylase activity (PROD, a CYP2B marker) and a 2-fold
increase in hepatic 7-ethoxy-resorufin- O-deethylase activity (EROD,
a CAPE marker). MTBE also induced a 2-5-fold increase in the hepatic
cell labelling index (as estimated from the incorporation of
5-bromo-2-deoxyuridine delivered by an implanted osmotic mini pump) in
the absence of hepatotoxicity, judged by the absence of any change in
serum alanine aminotransferase (ALAT) or histological signs of
necrosis (Moser et al., 1996a).
7.4.2 Inhalation studies
Female B6C3F1 mice (five or more/group) were exposed by
inhalation to 0 or 27 900 mg/m3 (7814 ppm) MTBE (>99.95% pure) for 3
or 21 days (6 h per day, 5 days per week) (Moser et al., 1996a). The
exposure resulted in abnormal gait, hypoactivity, decreased muscle
tone and increased lacrimation during exposure and immediately after
termination of exposure to MTBE. The mice recovered quickly following
termination of exposure. There was no significant change in body
weight. The relative liver weights were increased 20% (p<0.05) as
compared to controls at 3 days of exposure but the difference was not
significant at 21 days. MTBE exposure significantly (p<0.05)
decreased relative uterine weight to 48% of the control at 3 days,
with a further significant (p<0.05) decrease to 65% at 21 days. The
relative ovarian weight was decreased to 69% (p<0.05) at 21 days of
exposure. Histopathological examination showed mild centrilobular to
midzonal hepatocyte swelling at 3 days. At 21 days there were no
microscopic exposure-related changes. The total hepatic microsomal
cytochrome P450 content was elevated 40% at 3 days and approximately
200% at 21 days. After 3 days and 21 days of MTBE exposure,
respectively, hepatic PROD activity increased 5-fold and 14-fold,
while hepatic EROD activity increased 1.8-fold and 3.2-fold. There was
a non-significant change in hepatic cell labelling index to a 2.5-fold
higher value at 3 days, but at 21 days the MTBE group showed a
significant reduction to 0.7% in comparison with the control value of
2.3% (p<0.05). There were no histological signs of hepatoxicity and
serum ALAT was unaffected.
Information on the repeated dose toxicity of MTBE (>99.95% pure)
is also available from a study comparing the short-term hepatic
effects on female B6C3F1 and CD-1 mice (Moser et al., 1996b). Groups
of six female mice were exposed in inhalation chambers to
particulate-free control air containing 0 mg/m3 or a target
concentration of 27 900 mg/m3 (7800 ppm) 6 h per day, 5 days per week
for 3 or 21 days. Clinical signs included abnormal gait, hypoactivity,
decreased muscle tone and increased lacrimation during exposure and
immediately after termination of exposure. The animals recovered
quickly after termination of each daily exposure. There was a decrease
of body weight (p<0.05) in CD-1 mice, but not in B6C3F1 mice, after
21 days. Statistically significant (p<0.05) increases were seen in
absolute and relative liver weight in both strains of mice at both
time points. Histopathological examination showed slight centrilobular
hypertrophy in both B6C3F1 mice and CD-1 mice at 3 days as compared
to controls. At 21 days there was no indication of hepatotoxicity or
necrosis. Serum ALAT showed no increased activity at either 3 or 21
days. In the CD-1 mice, after 3 and 21 days, respectively, total
hepatic microsomal cytochrome P450 content increased 2.3-fold
(p<0.05) and 1.8-fold (p<0.05), PROD increased 5-fold (p<0.05) and
5-fold (p<0.05), and EROD increased 2.3-fold (p<0.05) and 3-fold
(p<0.05). Corresponding data for the B6C3F1 mice are described
below. The hepatic cell labelling indices were, in contrast to B6C3F1
mice, increased in CD-1 mice at both time points; the increases were
3-fold and 5-fold, respectively, at 3 and 21 days (Moser et al.,
1996b). A slightly decreased survival relative to control was noted in
female B6C3F1 mice following 16 weeks exposure to 28 450 mg/m3 (7969
ppm) (100% versus 92% survival, respectively). After 32 weeks of
exposure, survival was 96% for controls and 88% for MTBE-exposed mice.
Body weight was significantly decreased (p<0.05) at both time points.
PROD activity was increased 4.9 fold, and EROD activity 1.9 fold,
after 16 weeks treatment. While MTBE exposure produced mild
centrilobular to midzonal hypertrophy, there was no indication of
cytotoxicity or hepatic necrosis, or alteration in serum ALAT (Moser
et al., 1996b).
Sprague-Dawley rats (10/sex/group) showed increasing depth of
anaesthesia with increasing concentrations of MTBE when exposed to
MTBE vapour in exposure chambers at concentrations of 0, 900, 1800 or
3600 mg/m3 (0, 250, 500 and 1000 ppm, respectively) 6 h per day, 5
days per week for 13 weeks (Greenough et al., 1980). The
haematological analyses revealed an increase in haemoglobin levels
during week 13 in male rats exposed to 3600 mg/m3. At autopsy, a
slight reduction in relative and absolute lung weight was detected in
female rats at the same exposure level. There were no other gross or
histopathological effects reported.
In a range-finding study, Fischer-344 rats and CD-1 mice were
exposed to MTBE vapour in inhalation chambers 6 h per day for 13
consecutive days (Dodd & Kintigh, 1989). The target concentrations
were 0, 7150, 14 300 and 28 600 mg/m3 (0, 2000, 4000 and 8000 ppm,
respectively). The measured mean concentrations were 7200, 13 750 and
28 330 mg/m3 (2018, 3850 and 7936 ppm, respectively). No mortality
occurred during the study period. Clinical signs included
hypoactivity, ataxia, and periocular irritation in both rats and mice,
primarily in the high-dose groups during exposure. Reversible
neurobehavioural alterations (ataxia) were observed immediately after
exposure in high-dose rats (mice were not observed). Body weight gain
was depressed in rats (but not in mice) in the mid- and high-dose
groups (statistically significant for male rats). In female mice, both
absolute and relative liver weights were significantly increased at
all levels of exposure. In the mid-dose rats, both absolute and
relative liver weights were significantly increased in females; in
males, there was an increase in relative kidney and liver weights. In
rats at the highest dose, males had an increase in relative weight of
liver, kidney and adrenal. Females had an increase in both absolute
and relative weights of liver and adrenal, and absolute weight of
kidneys was increased.
In a 13-week vapour inhalation study that included neurotoxicity
evaluation, Fischer-344 rats were exposed to target concentrations of
0, 2860, 14 300 and 28 600 mg MTBE/m3 (0, 800, 4000 and 8000 ppm) 6 h
per day, 5 days per week (Dodd & Kintigh, 1989; Lington et al., 1997).
No mortality occurred. Major findings included motor activity changes
and reversible changes in body temperature in mid- and high-dose rats,
ataxia, and depressed body weight gain and food consumption. Only mild
haematological changes (e.g., decreased erythrocyte counts and
increased reticulocyte counts) were observed, primarily in male rats.
The corticosterone levels were significantly increased in both male
and female rats at the highest exposure level. No treatment-related
gross lesions were found at necropsy. The relative weight of liver and
kidney were significantly increased in all male groups and in females
in the two highest exposure groups. Relative weight of adrenal was
significantly increased in males and females at the two highest doses.
There were, however, no treatment-related microscopic changes in these
organs. It is probable that there is an association between the liver
enlargement and the high serum corticosterone levels. (Although this
was reported by the authors, the Task Group considered that it was
more likely that the increase in adrenal weight was associated with
the high serum corticosterone levels). The only treatment-related
microscopic findings were found in males at the highest dose level and
included a statistically significant increase in lymphoid hyperplasia
in the submandibular lymph nodes, an increase (not statistically
significant) in the degree of haemosiderosis in the spleen, and a mild
increase of hyalin droplets in the renal proximal tubules. Proximal
tubule necrosis and protein droplet accumulation were observed in
kidneys from male, but not female, rats exposed to 5400 and 10 760
mg/m3 (1516 and 3013 ppm) for 6 h/day for 10 consecutive days
(Prescott-Mathews et al., 1997). Alpha-2u-globulin immunoreactivity
was present in and confined to protein droplets in male rat kidney. A
mild dose-related increase in alpha-2u concentration in the male rat
kidney correlated with an exposure-related increase in cell
proliferation. No significant differences were observed in female rats
for any of these responses.
7.4.3 Intraperitoneal administration
Katoh et al. (1993) reported that MTBE administered to mice (500
mg/kg bw as a single dose, or 200 mg/kg bw as repeated doses) caused
lipid peroxidation, as demonstrated by increased levels of lipid
peroxide in liver homogenates, and an induction of hepatic microsomal
cytochrome P450 content. Repeated treatment with 200 mg/kg bw for 4
weeks did not affect glutathione content or glutathione-S-transferase
(details on pattern and period of administration were not provided).
7.5 Neurotoxicity studies
A single 6-h inhalation exposure of Fischer-344 rats to MTBE
vapour at target concentrations of 0, 2880, 14 400 or 28 800 mg/m3
(0, 800, 4000 or 8000 ppm) induced reversible alterations in central
and peripheral nervous system function (Gill, 1989). Group of 8 male
and 8 female rats were used at each concentration for behavioural
evaluation and 14 males and 14 females for motor activity
observations. Motor activity changes appeared within 10 min of
exposure to 28 800 mg/m3 (8000 ppm) in both male and female rats. For
male rats, motor activity changes were also observed at 2880 and 14
400 mg/m3). Behavioural evaluation (functional observational battery,
FOB) was performed 1, 6 and 24 h post-exposure. At 1 h post-exposure
there were concentration-related behavioural alterations at 14 400
mg/m3 and 28 800 mg/m3 but these were not found at 6 or 24 h
post-exposure.
In a pilot study for a 13-week exposure study (see also section
7.3), Fischer-344 rats and CD-1 mice (five per sex and species) were
exposed to target MTBE concentrations of 0, 7150, 14 300 and 28 600
mg/m3 (0, 2000, 4000 and 8000 ppm) 6 h per day for 13 consecutive
days (Dodd & Kintigh, 1989). Hypoactivity was observed at all dose
levels and ataxia at the two highest dose levels in both rats and mice
during exposure. In high-dose rats, ataxia, decreased startle and pain
reflexes, and decreased muscular tone were also observed immediately
after exposure. Recovery was complete within 1 h. The NOAEL was
determined to be 7200 mg/m3 in both rats and mice.
The subsequent 13-week study with neurotoxic evaluation included
testing for inhalation toxicity, FOB, motor activity and
neuropathology (Dodd & Kintigh, 1989). Fischer-344 rats (25 rats of
each sex per dose level) were exposed to MTBE vapour at target
concentrations of 0, 2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and
8000 ppm) 6 h per day, 5 days per week (see also section 7.4.2).
Clinical findings included hypoactivity in the mid- and high-dose
groups and ataxia in the high-dose group immediately following the
daily exposure. The exposure resulted in minor changes in the FOB
including elevated body temperature (in high-dose male rats and in
mid- and high-dose female rats) and decreased hind limb grip strength
(in mid-dose males). Decreased motor activity for males in the
high-dose group and increased motor activity for females in the
low-and mid-dose groups were reported. Additional findings are
reported in section 7.3. Necropsy revealed no treatment-related gross
lesions. There were no treatment-related microscopic changes in the
central and peripheral nervous system tissues.
7.6 Reproductive and developmental toxicity
Protocols and results of reproductive/developmental studies are
presented in Table 18 These include one- and two-generation inhalation
studies in rats and four developmental studies (inhalation) in rats,
rabbits and mice. In these investigations, MTBE did not induce
specific adverse reproductive effects; developmental effects were
observed only at dose levels that were maternally toxic. At very high
dose levels (28 000 mg/m3) decreased relative uterine weight was
observed in one study.
Table 18. Reproductive studies with MTBE in laboratory animals
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
One- and two-generation studies
Rat, inhalation 15 males and 0, 1070, 4640 and one generation, two slightly decreased Biles et al.
Sprague-Dawley 30 females/group 12 140 mg/m3 litter study (F1a, F1b) pup viability (1987)
(0, 300, 1300 and (p<0.05) in F1b
3400 ppm) 12 140 mg/m3
Rat, inhalation 25 males and 0, 1430, 10 700, two generation study 1430 mg/m3: no Neeper-Bradley
Sprague-Dawley 25 females/group and 28 600 mg/m3 adverse effects; (1991)
(0, 400, 3000 and >10 700 mg/m3:
8000 ppm) reduced bw, bw
gain and food
consumption in
parental animals
(mainly in males),
clinical signs and
neurotoxic
effects, reduced
pup bw and bw gain
postnatally. NOEL
for general
toxicity 1430
mg/m3; NOEL for
reproductive
effects >28 600
mg/m3; LOEL for
adults and offspring
>10 700 mg/m3
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Developmental studies
Rat, inhalation 25 rats per dose 0, 900, 3600 and gestation days 6-15 reduced food Conaway et
Sprague-Dawley level 9000 mg/m3 consumption in al. (1985)
(0, 250, 1000 and treated groups
2500 ppm) during the day
9-12 interval; no
significant
developmental
toxicity
Mouse, inhalation 25 mice per dose 0, 900, 3600 and gestation days 6-15 a slight (not Conaway et
CD-1 level 9000 mg/m3 statistically al. (1985)
(0, 250, 1000 and significant),
2500 ppm) dose-related
decrease in food
and water
consumption; no
significant
developmental
effects
Mouse, inhalation 30 mice per dose 0, 3600, 14 300 gestation days 6-15 >14 300 mg/m3: Tyl &
CD-1 level and 28 600 mg/m3 clinical signs Neeper-Bradley
(0, 1000, 4000 and of toxicity and (1989)
8000 ppm) reduced fetal bw;
28 600 mg/m3:
reduced bw, bw
gain, and food
consumption;
increased number
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
of non-viable
implantations,
reduced number of
viable
implantations and
% male fetuses,
and increased
incidence of
cleft palate.
NOEL for maternal
and developmental
toxicity 3600 mg/m3
Rabbit, inhalation 8 females per dose 0, 7150, 14 300 and gestation days 6-18 >7150 mg/m3: Tyl (1989)
New Zealand level 28 600 mg/m3 (0, reduced food
White 2000, 4000 and 8000 consumption in
ppm) all dosed groups;
increased
incidence of
lung foci; 28 600
mg/m3: reduced
bw gain, audible
respiration, and
slightly lower
fetal weights
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Rabbit, inhalation 15 females per 0, 3600, 14 300 gestation days 6-18 >14 300 mg/m3: Tyl (1989)
New Zealand dose level and 28 600 mg/m3 reduced bw gain
White (0, 1000, 4000 and food
and 8000 ppm) consumption;
28 600 mg/m3:
hypoactivity,
ataxia, increased
relative liver
weight, decreased
corrected
gestational weight
change and gravid
uterine weight. No
significant
developmental
effects; NOEL for
maternal toxicity
3600 mg/m3; NOEL
for developmental
toxicity >28 600
mg/m3
7.6.1 Reproductive toxicity
Biles et al. (1987) conducted a two-litter, one-generation
inhalation study of reproductive effects in CD Sprague-Dawley rats.
Target concentrations were 0, 890, 3600 and 8925 mg/m3 (0, 250, 1000
and 2500 ppm). Corresponding measured concentrations were,
respectively, 1070, 4430 and 10 640 mg/m3 (300, 1240 and 2980 ppm)
for females and 1030, 4210 and 10 210 mg/m3 (290, 1180 and 2860 ppm)
for males. Fifteen males exposed for 12 weeks were mated to thirty
females exposed for 3 weeks. Exposures continued throughout the mating
period, during gestation and through days 5-21 of lactation. A second
litter (F1b) was produced under the same mating and post-mating
exposure regimen. In the mid- and high-dose groups of the F1b
generation, there was a slight statistically significant (p<0.05)
decrease in pup viability. The authors felt that this was in large
part attributable to the high viability (99%) in the control group.
There were no treatment-related differences between control and
exposed animals based upon examination of clinical signs, gross
post-examination and histopathological examination of the gonads of
exposed adults, mating or fertility indices, pregnancy rates, mean
gestational length and number of pups at birth, litter survival
indices or pup weight. (The NOEL was above 8925 mg/m3 (> 2500 ppm)
in both parents and offspring).
In a two-generation reproductive and fertility inhalation study,
CD Sprague-Dawley rats were exposed to MTBE at concentrations of 0,
1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 or 8000 ppm)
(Neeper-Bradley, 1991; Bevan et al., 1997). There was parental
toxicity at the target concentrations of 28 600 mg/m3 and 10 700
mg/m3. Concomitant perinatal toxicity was also observed at these
concentrations. There were no treatment-related effects on
reproductive indices at any concentration and no adverse effects on
the offspring at concentrations that were not toxic to the parents.
(NOEL = 1430 mg/m3 (400 ppm); hypoactivity, lack of startle reflex
and blepharospasm in parents at 10 700 mg/m3 (3000 ppm); NOEL for
reproductive effects >28 600 mg/m3 (8000 ppm).
Inhalation exposure to 28 800 mg/m3 MTBE (99.95% pure) resulted
in significantly decreased relative uterine weight in B6C3F1 mice at
3 (48%) and 21 days (65%) of exposure as compared to controls (Moser
et al., 1996a) (see also section 7.4.2). The relative ovarian weight
was significantly (p<0.05) decreased at 21 days of exposure. There
were no exposure-related microscopic findings in ovaries, adrenals and
pituitary, and no effects on adrenal and pituitary weight. Moser et
al. (1996a) investigated if the decreased uterine weights were due to
an increased rate of estrogen metabolism by measuring the rate of
conversion of 3H-17B-oestradiol to water-soluble metabolites in
hepatocytes from MTBE-treated female mice. MTBE was found to increase
the oestrogen metabolism by 2.1-fold.
Ward et al. (1994) studied the toxicity of MTBE to germ cells in
CD-1 male and female mice. Groups of 10 mice were given 1, 10, 100 or
1000 mg/kg bw MTBE in corn oil by gavage 5 days per week for 3 weeks;
a negative control group received corn oil only. At the end of
treatment the mice were killed and one testis from each male and both
ovaries from each female were sectioned for cytological evaluation. In
males, sperm number, Sertoli cells, spermatogonia, spermatocytes and
capped spermatids were evaluated, and, in females, oocyte quality.
There were no effects of MTBE on any of the cell types examined.
7.6.2 Developmental toxicity
The results of four inhalation studies on the developmental
toxicity of MTBE are summarized in Table 18.
There was a significant decrease in food consumption on days 9-12
of gestation in pregnant rats exposed to as much as 9000 mg/m3 (2500
ppm) but no other effects that the authors considered to be maternally
toxic, embryotoxic or teratogenic (Conaway et al., 1985). The NOEL for
offspring and parents was >9000 mg/m3 (2500 ppm).
Two different studies were conducted in the same strain of mice
exposed to various concentrations of MTBE for 6 h/day on days 6-15 of
gestation (Conaway et al., 1985; Tyl & Neeper-Bradley, 1989). No
significant maternal toxicity or developmental effects were observed
when groups of 30 pregnant females were exposed to 1000, 3960 or 9675
mg/m3 (280, 1110 or 2710 ppm), though the incidence of lacrimation
was increased in exposed mothers and there was a slight increase in
the incidence of fused sternebrae in the high-dose group (Conaway et
al., 1985). The NOAEL of parents and offspring was 9000 mg/m3 (2500
ppm). Tyl & Neeper-Bradley (1989) concluded that 14 300 mg/m3 (4000
ppm) was maternally toxic, based on observed hypoactivity and ataxia.
There were significant decreases (p<0.01) in body weight, body weight
gain and food consumption at 28 600 mg/m3 (8000 ppm). At 14 300
mg/m3 (4000 ppm) or more, there were significant reductions in fetal
body weight per litter (p<0.01) and increased skeletal variation. The
proportion of male fetuses was significantly reduced (p<0.01) at the
highest dose level in the Tyl & Neeper-Bradley (1989) study, but this
has not been observed in other studies with mice, rats or rabbits. At
28 600 mg/m3 (8000 ppm), the number of non-viable implantations per
litter and incidence of cleft palate was also increased (LOAEL in
parents and offspring = 14 300 mg/m3 (4000 ppm). The NOAEL in this
study was 3570 mg/m3 (1000 ppm).
Maternal toxicity was observed at the two highest concentrations
in rabbits exposed to 3570, 14 300 and 28 600 mg/m3 (1000, 4000 and
8000 ppm) during days 6-18 of gestation (Tyl, 1989). No developmental
effects were observed at any exposure level [NOAEL in offspring = 28
600 mg/m3 (8000 ppm); NOAEL in parents = 3570 mg/m3 (1000 ppm);
LOAEL in parents = 14 300 mg/m3 (4000 ppm)].
7.7 Mutagenicity and related end-points
Genotoxicity study results with MTBE are generally negative.
However, there are indications that MTBE may have some genotoxic
potential in the presence of metabolic activation. Genotoxicity data
for MTBE are compiled in Table 19.
In studies on reverse mutation in Salmonella typhimurium, MTBE
was found to be non-mutagenic in tester strains TA1535, TA1537,
TA1538, TA98 and TA100, with and without S9 (liver enzyme homogenates
from induced Sprague-Dawley male rats) metabolic activation, at doses
up to 10 mg/plate (Cinelli & Seeberg, 1989).
No significant increase in the frequency of recessive lethal
mutations in the X-chromosome could be established after feeding MTBE
(99.14% pure) (0.03, 0.15 or 0.3% MTBE in 5% aqueous sucrose) to adult
Drosophila melanogaster (wild-type Oregon-R males) for 24 h (Sernau,
1989).
In the presence of a liver-derived metabolic system (liver S9
from Arochlor 1254-induced male Sprague-Dawley rats) MTBE (>99% pure;
1.0, 2.0, 3.0 or 4.0 ml/ml) induced forward mutations in vitro at
the thymidine kinase locus of mouse lymphoma cell line L5178Y/ TK+/-
(Mackerer et al., 1996). The observed mutagenicity was dose-dependent.
Other experimental data, using a test system developed to
determine if the mutagenicity of a material is the result of the
presence or release of formaldehyde, had indicated that the
mutagenicity was due to the metabolism of MTBE to formaldehyde
(Blackburn et al., 1991). To establish if formaldehyde, derived from
MTBE in the presence of S9, was responsible for the observed
mutagenicity, Mackerer et al. (1996) used a modified mouse lymphoma
assay. In this assay formaldehyde dehydrogenase and its co-factor
NAD+ were added during the exposure period so that any formaldehyde
produced would be converted to formic acid, which is non-genotoxic. An
MTBE dose-related increase in the frequency of mutant lymphoma cells
occurred without the presence of formaldehyde dehydrogenase and
NAD+, but not when these were present, indicating that formaldehyde
was responsible for the mutations.
In two independent in vitro experiments MTBE (purity not
specified) did not induce unscheduled DNA synthesis (UDS) in primary
rat hepatocytes at concentrations up to 10 mg/ml (Seeberg, 1989).
In an in vivo-in vitro hepatocyte UDS assay, ten male and ten
female CD-1 mice were assigned to each dose group and an air-only
control group and, in addition, five of each sex to a positive control
group (DMN) (Vergnes & Chun, 1994). The animals were exposed to MTBE
(purity not specified) in inhalation chambers 6 h a day for two
consecutive days. The target concentrations were 0, 1440, 10 800 and
Table 19. Genotoxicity studies with MTBE
Species Strain/cells Measured end-point Test conditions Activation Result Reference
Bacterial systems
S. typhimurium TA1535 reverse mutation 625, 1250, 2500, 5000, + - - - Cinelli &
TA 1537 10 000 µg/plate Seeberg (1989)
TA1538
TA98
TA100
S. typhimurium TA98 reverse mutation exhaust particle + nd Clark et al.
TA100 extracts from gasoline + ± (1984)
containing 7% by
volume MTBE; five
concentrations ranging
from 1 to 100 µg/plate;
duplicate plates
Non-mammalian eukaryotic systems
Drosophila wild type Oregon-R, sex-linked recessive Basc test; 0.03, - Sernau (1989)
melanogaster males lethal test 0.15 or 0.3% MTBE;
adult feeding for 24 h
In vitro mammalian systems
Mouse lymphoma cell line forward mutation 1.0, 2.0, 3.0, 4.0 µl/ml + + Mobil Oil
L5178Y/TK Corporation
(1993)
Rat primary hepatocytes UDS 3.16, 10.0, 31.6, 100, - Seeberg (1989)
316, 1000, 3160, 10 000
µg/ml
Table 19. (continued)
Species Strain/cells Measured end-point Test conditions Activation Result Reference
In vivo - in vitro
Mouse, primary hepatocytes UDS vapour exposure: 0, - Vergnes & Chun
CD-1 1440, 10 800, 28 800 (1994)
mg/m3, 6 h/day for 2
consecutive days
In vivo mammalian systems
Rat, bone marrow cells chromosome vapour exposure: 0, - Vergnes & Morabit
Fischer -344 aberrations 2800, 14 400, 28 800 (1989)
mg/m3, 6 h/day for
5 days
Mouse, spleen lymphocytes chromosome oral administration, a slight Ward et al.
CD-1 aberrations 1.0, 10, 100 or 1000 inverse (1994)
mg/kg bw for 3 weeks dose-response
relationship
in male mice
but not in
female mice
Mouse, spleen lymphocytes mutations at hprt oral administration, - Ward et al. (1994)
CD-1 locus 1.0, 10, 100 or 1000
mg/kg bw for 3 weeks
Mouse, bone marrow chromosome vapour exposure: 0, - Vergnes & Kintigh
CD-1 cells damage 1440, 10 800, 28 800 (1993)
mg/m3, 6 h/day for 2
consecutive days
28 800 mg/m3 (0, 400, 3000 8000 ppm). The animals were sacrificed and
hepatocytes were sampled 18 h after the second exposure day (for the
positive control group after approximately 2 h). No dose-related
increase in the DNA repair activity could be established. The UDS
assay is used to indicate primary DNA damage; this is, however,
transient in nature and it is necessary to analyse cells for damage as
soon as possible after cessation of treatment. Since the half-life of
MTBE in the animal body is quite short (1-3 h) and DNA repair is
relatively rapid, the study should have been designed accordingly.
MTBE (purity not specified) was considered nonclastogenic to
Fischer-344 rats in an in vivo test system (Vergnes & Morabit,
1989). No concentration-related or significant increase in the
incidence of chromosome aberrations in rat bone marrow cells was found
in either males or females following whole body exposure to MTBE 6 h
per day for five consecutive days. The target concentrations were 0,
2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and 8000 ppm,
respectively).
MTBE did not induce micronuclei in vivo in mouse bone marrow
cells (Vergnes & Kintigh, 1993). CD-1 mice were exposed to MTBE
(purity not specified) vapour in inhalation chambers (five animals per
sex per dose level) at concentrations of 0, 1430, 10 710 or 28 600
mg/m3 (0, 400, 3000 or 8000 ppm) 6 h a day for two consecutive days.
Bone marrow cells were collected 24 and 48 h after the second exposure
day. No significant, exposure-related increase in the frequency of
micronuclei could be established at any dose level and sampling time
in either sex in this study.
Ward et al. (1994) examined the frequency of somatic cell
mutations in spleen lymphocytes after administration by gavage 5 days
per week for 3 weeks with MTBE (99.8% pure) in corn oil to CD-1 male
and female mice (ten animals per dose group). Ethyl-nitrosourea was
used as a positive control. The doses were 1, 10, 100 and 1000 mg/kg
bw. The frequency of mutations at the hypoxanthine-guanine
phosphoribosyl transferase (hprt) locus was determined 3 weeks after
the cessation of exposure. There was no indication that MTBE produced
a mutagenic effect at the tested dose levels. Ward et al. (1994) also
analysed chromosome aberrations in spleen lymphocytes. This was
performed on the first day after the termination of exposure in 13
male mice and on the second day in the remaining mice. A slight, but
not statistically significant, inverse dose-relationship was seen in
male mice; this was not seen in the females.
7.8 Carcinogenicity
Three bioassays are available on the oncogenicity of MTBE in both
sexes of rats and mice. These include two inhalation studies, one in
Fischer-344 rats and one in CD-1 mice, and one oral study in
Sprague-Dawley rats (Table 20). At high inhalation exposure levels,
MTBE increased the incidence of renal cell carcinomas in male rats and
liver tumours in female mice. Oral administration increased the
incidence of lymphomas and leukaemias in female rats. The studies also
resulted in testicular tumours in both strains of rats following
exposure either by inhalation or oral administration.
Fischer-344 rats (50 of each sex per dose level) were exposed to
MTBE (99% pure) vapour in inhalation chambers at target concentrations
of 0, 1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 and 8000 ppm,
respectively) 6 h per day, 5 days per week (Chun et al., 1992; Bird et
al., 1997). The control group was exposed to filtered air. Increased
mortality and decreased mean survival time were observed for male rats
from all exposure groups. Owing to the high mortality rate, surviving
males, six from the mid-dose group and nine from the high-dose group,
were killed at weeks 97 and 82, respectively. The numbers of surviving
males at the end of the study were 13, 6, 6 and 9 at 0, 1430, 10 700
and 20 600 mg/m3, respectively. Mean survival times were 632, 617
(p<0.05), 587 (p<0.01) and 516 (p<0.01) days, respectively.
Low-dose males and all females were killed during weeks 104 and 105.
Various clinical signs of toxicity (blepharospasm, hypoactivity,
ataxia, lack of startle reflex, swollen periocular tissue and
salivation) were observed in both sexes at the two highest dose
levels. No clinical signs were noticed at the lowest dose level.
Significantly (p<0.01) reduced body weight and body weight gain were
recorded at week 81 for the high-dose rats. For low- and mid-dose male
rats, body weight and body weight gain were slightly to significantly
(p<0.05) increased during the first 70 to 80 weeks. Thereafter, there
were no clear dose-related changes. In females, there was a slight,
but not exposure-related, decrease at the two lower exposure levels.
High-dose male rats also showed a significantly (p<0.05) decreased
corticosterone level at week 81. A trend toward increases in liver and
kidney weights relative to final body weight was recorded for the
mid-dose male rats. In female rats, concentration-related increases in
liver and kidney weight (absolute and relative to body or brain
weight) were observed at the two highest dose levels. There was also a
trend toward an increased adrenal gland weight relative to final body
weight for high-dose males. However, only the organ weight data from
the control and the low-dose group were statistically evaluated due to
the different sacrifice periods for the mid- and high-dose groups.
Non-neoplastic effects of treatment included an increased
incidence and severity of chronic progressive nephropathy in male rats
from all dose groups and in female rats from the mid- and high-dose
groups. Treated males were more severely affected than the females,
which usually showed only slight changes. Chronic progressive
nephropathy was diagnosed as the cause of morbidity or death for 3/37,
16/44, 26/44 and 39/41 male rats and for 0/20, 0/23, 4/27 and 6/25
female rats in the control, low-, mid- and high-dose groups,
respectively. The incidences of nephropathy, with interstitial
fibrosis, in the control, low-, mid- and high-dose groups were 19/37
(51%), 29/44, (66%), 37/44 (84%) and 40/41 (98%), respectively
(significance not specified). Histologically, the chronic progressive
nephropathy included an exposure-related increase in severity for
Table 20. Carcinogenicity studies with MTBE
Species Exposure Tumour Reference
Mouse (CD1) Male: Liver, adenoma: Burleigh-Flayer et al. (1992)
control 11/49 Bird et al. (1997)
1430 mg/m3 (400 ppm) 11/50
10 710 mg/m3 (3000 ppm) 9/50
28 600 mg/m3 (8000 ppm) 12/49
Male: Liver, carcinoma:
control 2/49
1430 mg/m3 (400 ppm) 4/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 8/49
Male: Liver, adenoma and carcinoma:
control 12/49
1430 mg/m3 (400 ppm) 12/50
10 710 mg/m3 (3000 ppm) 12/50
28 600 mg/m3 (8000 ppm) 16/49
Female: Liver, adenoma:
control 2/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 10/50 (p<0.05)
Female: Liver, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 1/50
Table 20. (continued)
Species Exposure Tumour Reference
Female: Liver, adenoma and carcinoma:
control 2/50
1430 mg/m3 (400 ppm) 2/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 11/50
Rat (F344) Male: Killed at: Chun et al. (1992)
control 104 weeks Bird et al. (1997)
1430 mg/m3 (400 ppm) 104 weeks
10 710 mg/m3 (3000 ppm) 97 weeks
28 600 mg/m3 (8000 ppm) 82 weeks
Male: Kidney, adenoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 5/50
28 600 mg/m3 (8000 ppm) 3/50
Male: Kidney, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 0/50
Male: Kidney, adenoma and carcinoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 8/50
28 600 mg/m3 (8000 ppm) 3/50
Table 20. (continued)
Species Exposure Tumour Reference
Male: Testes:
control 32/50
1430 mg/m3 (400 ppm) 35/50
10 710 mg/m3 (3000 ppm) 41/50
28 600 mg/m3 (8000 ppm) 47/50
Male: Pituitary tumours, week 104:
control 6/50
1430 mg/m3 (400 ppm) 5/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 0/50
Female: Kidney, adenoma:
control 0/50
1430 mg/m3 (400 ppm) 0/28
10 710 mg/m3 (3000 ppm) 1/39
28 600 mg/m3 (8000 ppm) 0/50
Rat (Sprague-Dawley) Male: Testicular adenoma Belpoggi et al. (1995)
(denominator is total number
in group):
control 2/60
250 mg/kg 2/60
1000 mg/kg 11/60
Male: Testicular adenoma
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/26
250 mg/kg 2/25
1000 mg/kg 11/32 (p<0.05)
Table 20. (continued)
Species Exposure Tumour Reference
Female: Lymphomas or leukaemia
(denominator is total
number in group):
control 2/60
250 mg/kg 6/60
1000 mg/kg 12/60
Female: Lymphomas or leukaemias
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/58
250 mg/kg 6/51
1000 mg/kg 12/47 (p<0.01)
glomerulosclerosis, tubular proteinosis, interstitial nephritis and
interstitial fibrosis in both male and female rats from the mid- and
high-dose groups. The chronic nephropathy was also associated with
secondary lesions such as fibrous osteodystrophy, hyperplasia within
the parathyroid glands, and mineralization within numerous tissues. An
increased incidence of renal tubular cell adenomas and carcinomas was
noted in mid- and high-dose male rats. The incidence was 8/50 and 3/50
for the mid- and high-dose groups, respectively, and 1/50 for the
control group. The renal tubular cell carcinomas were only noted in
the mid-dose group (3/50). One renal cell adenoma was found in a
mid-dose female.
In mid- and high-dose males, there was also a dose-related
increase of interstitial cell (Leydig cell) adenomas of the testes.
The incidence was 32/50, 35/50, 41/50 and 47/50 (64%, 70%, 82% and 94%
for the control, low-, mid- and high-dose groups, respectively. In
high-dose males, there was also an exposure-related decrease in the
frequency of pituitary adenomas. The incidences were 27/47, 29/48,
27/47 and 2/48 in the control, low-, mid- and high-dose groups of
males, respectively. A treatment-related decrease of large granular
lymphocyte (LGL) leukaemia was also noted. The incidence of LGL in
males was 33/50, 22/50, 20/50 and 3/50 and in females 22/50, 14/50,
15/50 and 16/50 for control, low-, mid- and high-dose rats,
respectively. LGL leukaemia, which is age-dependent and generally does
not appear until 20 months of age, was the main cause of death in the
control and low-dose males. The lymphoid hyperplasia of the
submandibular lymph node observed in a 13-week inhalation study with
Fischer-344 rats at a dose level of 28 600 mg/m3 was not observed in
the present study using the same strain of rats and the same dose
level. No NOEL could be determined for male rats due to a slight
increase of nephropathy at the lowest dose level. For female rats, the
NOEL for toxicity was 1440 mg/m3.
A number of points regarding this study can be made:
a) The tumour incidence values were analysed using methods that are
not appropriate when there are marked inter-group differences in
survival.
b) Testicular adenomas are quite common in untreated aging male
F-344 rats (Haseman et al., 1990), with a spontaneous incidence
in the range 64-98% for animals contemporaneous with those used
here. On this basis, it appears that this tumour type may have
been under-represented in the concurrent controls, influencing
the slope of the dose-response curve. In the Fischer-344 rats
used in this laboratory, the average historical control incidence
of Leydig cell tumours was 88% (Bird et al., 1997). Thus, in
comparison with historical control data, there was no increased
incidence in this tumour in the dosed groups. However, concurrent
control comparisons are always more appropriate, unless it is
known that there had been a particular problem with the study,
e.g., inappropriate randomisation.
c) Neoplasm incidence decreases were observed for pituitary adenomas
in the high-dose males and for large granular lymphocyte
leukaemia in males and females at all dose levels. Decreases in
the high-dose rats might be related to body weight gain
restrictions in this group and to increased mortality rate in
males. Alternatively, the concurrent control values for these
neoplasms may be particularly high in this study.
An oncogenicity study was also carried out on CD-1 mice exposed
to MTBE (99% pure) vapour in inhalation chambers (Burleigh-Flayer et
al., 1992; Bird et al., 1997). Groups of 50 male and 50 female mice
were exposed to target concentrations of 0, 1430, 10 700 and 28 600
mg/m3 (0, 400, 3000 and 8000 ppm, respectively) 6 h per day, five
days per week for 18 months. The control group was exposed to filtered
air. The mortality rates for male mice (including those sacrificed
moribund but excluding procedural and accidental deaths) in the
control, low-, mid- and high-dose groups were 33%, 22%, 35% and 49%,
respectively. The corresponding values for females were 27%, 18%, 23%
and 33%, respectively. The authors reported that increased mortality
rate (significance not reported) and decreased survival time were
observed for male mice from the high-dose group only. This was
considered as a probable result of a slightly increased frequency of
obstructive uropathy (distended urinary bladder and/or obstruction of
the urethra). Clinical signs, i.e. ataxia, blepharospasm,
hypoactivity, prostration, and lack of a startle reflex (in the
high-dose group prostration also and in the mid-dose group stereotypic
behaviour also) were observed in both male and female mice at the two
high-dose levels. Ataxia, observed in most male and female mice from
the highest dose group throughout the study, was the only clinical
finding considered to be exposure related. Body weight and body weight
gain were decreased for both male and female mice from the high-dose
group. At the end of the study, body weight gain was decreased 15%
(p < 0.01) for the males and 24% (p<0.01) for the females from the
high-dose group.
Necropsy showed that the liver was the target organ for toxicity.
There was a dose-related increase in liver weight, both absolute and
relative to body weight, in both sexes (increase in absolute weight in
males significant at all dose levels). A slight (significant),
although not concentration-related, increase in kidney weight was
noted for male mice from all exposure groups and in female mice from
the high-dose group. Decreases, although not statistically
significant, in absolute brain and spleen weight were also noted for
high-dose male and female mice. In addition, an increase in serum
corticosterone levels was observed in both male and female mice from
the high-dose group at week 79. The increase was significant (p<0.05)
only for male mice. A slight decrease in urinary pH and increases in
urine gamma globulin were observed for both male and female mice at
the high-dose level.
For male mice that were found dead in the high-dose group (7/25),
a slightly increased frequency of urinary bladder dilation/ distension
was noted at autopsy as compared to controls (3/18). In addition, the
incidence of the number of liver masses was increased in the high-dose
male mice (13/50 compared to 7/50 for the control group). The only
exposure-related lesion in female mice found at necropsy was an
increased incidence of liver masses in the high-dose group (9/50) when
compared to the controls (0/50). Histopathology showed an
exposure-related increase in hepatocellular hypertrophy in high-dose
male mice (15/49) when compared to controls (5/49). This lesion also
showed an increased, although not statistically significant, incidence
in mid-dose male mice (10/50) and in female mice from the high-dose
group (9/50 as compared to 4/50 in the control group). Exposure to
MTBE did not, however, cause hepatocellular necrosis or degeneration.
In high-dose male and female mice, mineralization within the brain was
decreased. In addition, there was a dose-related decrease in the
incidence of cystic endometrial hyperplasia for female mice.
An increased frequency (not statistically significant) of hepatic
adenomas and carcinomas was observed in male mice at the high-dose
level (16/49 as compared to 12/49 in the control group). The increase
(not statistically significant) was due to a slightly increased
frequency of hepatocellular carcinomas in the high-dose group (8/49)
when compared to the controls (2/49). The analysis for the combined
incidence of hepatocellular adenomas and carcinomas did not, however,
include statistical methods that adjusted for difference in survival
between the control and exposure groups. In female mice, there was a
significant increase in the incidence of hepatocellular adenomas at
the high-dose level (10/50 as compared to 2/50 from the control
group). The induced incidences were modest and occurred in the group
in which hepatocellular hypertrophy also occurred. There was no
exposure-related increase in the incidence of hepatocellular
carcinomas in female mice. The NOEL for toxicity in mice exposed to
MTBE for 18 months was 1440 mg/m3.
In an oral exposure study, male and female Sprague-Dawley rats
(60 per sex and dose group) were administered 0, 250 or 1000 mg/kg bw
MTBE (>99% pure) in 1 ml extra virgin olive oil by gavage four times
a week for 104 weeks on a weekly schedule of 2 days dosing, 1 day
without dosing, 2 days dosing, followed by 2 days without dosing
(Belpoggi et al., 1995). There were no treatment-related differences
in mean body weights of treated groups compared to control groups. The
animals were kept under observation until natural death. High-dose
male rats showed a higher survival than controls at treatment week 80
and thereafter. At week 80, survival was approximately 56% in control
and exposed groups; at 112 weeks, it was approximately 10% in controls
and the low-dose group, but 35% in the high-dose group. In female
rats, a dose-related decrease in survival was observed from
treatment-week 16. It was reported that there were no evident
behavioural changes; at week 72, survival in the high-dose group was
about 65%; at week 120, it was less than 20%; at week 136, it was less
than 5%. The authors reported no evident behavioural effects; however,
the extent of examination of behavioural effects was not specified. No
relevant non-neoplastic changes (including renal) were detected at
autopsy and histopathology. No specific data were, however, reported.
In male rats, there was a dose-related increase in the incidence of
testicular Leydig cell (interstitial cell) tumours (2/60, 2/60 and
11/60), statistically significant at the highest dose (p<0.05). In
female rats, there was a dose-related increase in lymphomas and
leukaemias combined (2/60, 6/60 and 12/60), marginally significant at
the low-dose level and highly significant at the high-dose level
(p<0.01). There also was an increase in dysplastic proliferation of
lymphoreticular tissue in female rats at both dose levels, but the
incidence was higher in the low-dose group. Dose-related decreases in
mammary fibromas and fibroadenomas, and in pituitary adenomas and
tumours of adrenal glands were observed in dosed females. Since these
tumours and the testicular interstitial cell tumours are
age-dependent, these effects were probably at least partly due to the
dose-related early mortality and the prolonged observational period in
the surviving animals.
A number of points regarding this study should be made:
a) there is limited description of the results, particularly the
histopathological findings;
b) diagnostic criteria are not given for the distinction between
Leydig cell tumours and hyperplasia (the latter were not reported
at all, which is unusual for old Sprague-Dawley rats showing
Leydig cell tumours);
c) diagnostic criteria are not given for the distinction between
dysplastic hyperplasia and lymphoma;
d) lymphomas and leukaemias are pooled; specific tumour type and
incidences were not reported;
e) historical control data might aid the evaluation of lymphomas and
leukaemias, particularly if they are available for these rats
within different age ranges;
f) chronic progressive nephropathy was not observed in these
Sprague-Dawley rats, although these lesions might be expected, on
the basis of data from a number of other studies with this strain
of rat.
7.8.1 Initiation-promotion protocol
In a study to investigate if MTBE exhibited hepatic
tumour-promoting activity, 12-day-old female B6C3F1 mice were
initiated with a single intraperitoneal injection of the mutagen
diethylnitrosamine (DEN) or saline and then exposed subsequently to 0
or 28 800 mg/m3 (8000 ppm) MTBE (>99.95% pure) from 8 weeks of age
for 16 or 32 weeks (Moser et al., 1996b). Liver weight was
significantly (p<0.05) increased at both time points in both
saline-treated and DEN-initiated mice after exposure to MTBE, and was
associated with mild centrilobular to midzonal hypertrophy in both
groups. There was no significant difference in the percentage of
microscopic lesions classified as hepatic foci (86%), hepatocellular
adenomas (10%), or hepatocellular carcinomas (4%) in DEN/MTBE mice as
compared to DEN/control mice. However, the absolute number of
microscopic hepatic lesions was 50% less in the DEN/MTBE group than in
DEN/control mice. MTBE appeared inactive in this tumour
initiation-promotion assay.
7.9 Metabolites of MTBE
Animal carcinogenicity experiments have been conducted with the
metabolites tertiary-butyl alcohol (TBA) and formaldehyde (FA). TBA
administered in drinking-water caused increased incidences of renal
tubular adenoma and carcinoma in male Fischer-344 rats and increased
severity of chronic progressive nephropathy (Cirvello et al., 1995).
In female B6C3F1 mice, TBA produced thyroid follicular cell adenoma
and hyperplasia, and, in both male and female mice, inflammation and
hyperplasia of the urinary bladder (Cirvello et al., 1995). FA has
caused nasal squamous cell carcinoma in both Fischer-344 and
Sprague-Dawley rats and in B6C3F1 mice, and also increases of cancers
of the nasopharynx, nasal cavity and sinus in humans (Grindstaff et
al., 1991).
7.10 Mode of action
7.10.1 Kidney tumours
A number of chemicals cause both protein droplet nephropathy and,
with chronic exposure, renal cancer in male rats only (Borghoff et
al., 1990). The proposed mechanism by which these chemicals cause
renal tumours in male rats is based on their ability to cause protein
droplet nephropathy by accumulating alpha2u-globulin, a
male-rat-specific protein of low relative molecular mass. Evidence
suggests that chemical binding to alpha2u-globulin makes the protein
more resistant to hydrolysis, which accounts for its accumulation in
the renal lysosomes in the form of protein droplets. The chemically
induced accumulation of alpha2u-globulin is thought to be responsible
for cytolethality, which in turn stimulates cell division as the
kidney attempts to repair itself. Chronic chemical exposure with
repeated cycles of cytolethality and reparative replication is
probably the cause of the renal tumours in male rats (Borghoff et al.,
1990; Hard et al., 1993). A protein similar to alpha2u-globulin has
not been detected in human kidneys (Borghoff & Lagarde, 1993).
MTBE causes male-rat-specific renal tumours with chronic exposure
(Bird et al., 1997). MTBE also causes the accumulation of protein
droplets in male but not female rats following exposure ranging from
10 days to 13 weeks (Dodd & Kintigh, 1989; Chun & Kintigh, 1993;
Prescott-Mathews et al., 1997). Similar results have been observed
with TBA, a metabolite of MTBE (Lindamood et al., 1992).
alpha2u-Globulin immunoreactivity was present in and confined to
protein droplets in the kidneys of male rats exposed to MTBE in these
studies (Dodd & Kintigh, 1989; Chun & Kintigh, 1993; Prescott-Mathews
et al., 1997). Although a slight increase in alpha2u-globulin-positive
staining was observed in male rats exposed to MTBE, as compared to
controls, a linear exposure-related increase was not observed in any
of these studies. alpha2u-Globulin-positive proteinaceous casts at the
junction of the proximal tubules and the thin limb of Henle were not
observed (Swenberg & Dietrich, 1991). MTBE caused an
exposure-dependent mild increase in the renal concentration of
alpha2u-globulin measured in male rats (Prescott-Mathews et al.,
1997). Immunohistochemical staining of alpha2u-globulin is probably
not as sensitive as actually quantifying the alpha2u-globulin levels
with a mild increase in this protein. Further analysis of the kidney
protein profile from control and MTBE-treated male rats confirmed the
accumulation of alpha2u-globulin with no other protein detected
(Prescott-Mathews et al., 1997).
MTBE-induced kidney lesions, characterized by tubular necrosis
and protein droplet accumulation, were mild, especially when compared
with strong inducers of alpha2u-globulin nephropathy. Additionally,
granular casts, considered characteristic for alpha2u-globulin
nephropathy, were not observed in all studies. In the case of the
10-day MTBE inhalation exposure, however, there was a
concentration-dependent increase in kidney necrosis with minimal
sloughing of epithelial cells in male rat kidney following exposure to
10 710 mg/m3 (3000 ppm) (Prescott-Mathews et al., 1997).
In male and female rats exposed to MTBE vapour for 10 and 28
days, MTBE caused enhanced cell proliferation in male, but not female,
rat kidneys (Chun & Kintigh, 1993; Prescott-Mathews et al., 1997). A
strong positive correlation was observed between the cell
proliferative response and the concentration of alpha2u-globulin in
the kidneys of MTBE-exposed male rats.
With many of the chemicals that cause alpha2u-globulin
nephropathy, either the chemical or a metabolite has been found to
bind reversibly to alpha2u-globulin. In vitro, a high uptake of MTBE
into male rat kidney homogenate could be predicted using a
two-compartment model system when the binding of MTBE to
alpha2u-globulin was described using a dissociation constant of 10-4
M (Poet & Borghoff, 1997). This estimated dissociation constant for
MTBE binding to alpha2u-globulin was found to be similar to the
dissociation constant previously measured for 1,4-dichlorobenzene, a
known inducer of alpha2u-globulin nephropathy and a chemical
identified as bound to alpha2u-globulin following treatment of male
rats with 1,4-dichlorobenzene (Charbonneau et al., 1989). Together
these findings indicate that MTBE interacts, although weakly, with
alpha2u-globulin and induces alpha2u-globulin nephropathy and renal
cell proliferation in male, but not female, rats.
MTBE increases the severity of chronic progressive nephropathy in
both male and female rats, but does not cause renal necrosis, enhanced
cell proliferation or renal cancer in female rats. Chronic progressive
nephropathy alone is not associated with increased incidence of renal
tumours.
7.10.2 Liver tumours
It has been hypothesized that liver tumours in female mice can be
promoted by interfering with the estrogen-mediated suppression of
preneoplastic foci. In this regard, both unleaded gasoline and MTBE
induce cytochrome P450 activity and estrogen metabolism in mouse
hepatocytes, induce a mitogenic response in mouse liver, and decrease
uterine and ovarian weight in exposed mice. Additionally, unleaded
gasoline promotes DEN-initiated female mouse liver tumours, but this
response has not been observed with MTBE (Standeven & Goldsworthy,
1993; Moser et al., 1996b). Therefore, the relevance of this
hypothesis to an interpretation of the MTBE-induced mouse liver
tumours is currently unclear.
8. EFFECTS ON HUMANS
As explained in chapter 3, two separate fuel programmes in the
USA legally require the use of oxygenate in gasoline to address
ambient air quality objectives. Although no specific oxygenate is
required, MTBE has dominated the USA market place and is used at 15%
(by volume) to meet a 2.7% (by weight) oxygen requirement for
oxygenated gasoline in the cold-weather season in areas with excessive
carbon monoxide levels and at 11% (by volume) to meet a 2.0% (by
weight) oxygen requirement for reformulated gasoline sold year-round
in areas with excessive ozone levels. In the fall of 1992, shortly
after the introduction of oxygenated gasoline containing 15% MTBE in
Alaska, consumer complaints were registered about health effects such
as headaches, eye irritation and cough in Fairbanks (Beller &
Middaugh, 1992) and Anchorage (Chandler & Middaugh, 1992).
Subsequently, residents in other places in the USA also reported
health complaints associated with the introduction of cold-season
oxygenated fuel. Somewhat similar public concerns were raised in
Milwaukee, Wisconsin, with the introduction of reformulated gasoline,
some of which contained 11% (by volume) MTBE, in January 1995
(Anderson, 1993; Anderson et al., 1995). Health complaints have also
been registered by some occupationally exposed individuals, such as
tank truck drivers handling bulk MTBE (Gillie, 1993).
These "outbreaks" of health complaints prompted several field and
experimental studies as well as other assessments (e.g., HEI, 1996; US
Interagency Assessment, 1997) of available data and information
generated by these studies. In this section epidemiological studies
will be described first, followed by controlled chamber studies of
human volunteers. Because non-occupationally as well as occupationally
exposed populations were investigated in the epidemiological studies,
these studies will be discussed in one section rather than two
separate sections devoted to general population and occupational
population exposures.
8.1 Population studies
In December 1992, Moolenar et al. (1994) undertook a pilot study
in Fairbanks, Alaska, to investigate the possible relationship between
MTBE exposure and health complaints. In Phase I of the study, exposure
was evaluated by air sampling and by analysing blood samples for MTBE
and TBA in 18 workers heavily exposed to gasoline fumes and exhaust in
their workplace (e.g., service station workers, mechanics, meter
readers). A questionnaire administered to the workers asked about 15
symptoms: seven that had been most frequently reported to a local
telephone hotline the previous month (headache, eye irritation,
burning of the nose or throat, cough, nausea or vomiting, dizziness,
and a sensation of spaciness or disorientation), and eight other
symptoms (fatigue, fever, sweats or chills, diarrhoea, fainting or
black-out spells, skin irritation or redness, muscle aches, and
difficulty breathing). Phase II of the study was conducted in February
1993, after the oxygenated gasoline programme was suspended, and
included 28 (12 of the original) occupationally exposed subjects. Four
workers whose post-shift blood MTBE levels were in the top quartile
(>9.6 µg/litre) all had one or more of the seven key health
complaints, compared with 9 of 14 workers whose levels were in the
lower three quartiles. This finding was not statistically significant,
but the study may not have had adequate statistical power to detect a
relationship, owing to the small sample size. In Phase II, only one
worker reported a health complaint (nausea). Exposures to MTBE, as
well as complaints of both workers and non-occupationally exposed
residents of Fairbanks, declined significantly after the termination
of the oxygenated gasoline programme (CDC, 1993), but interpretation
of these events is possibly confounded by several factors, including a
lack of representative sampling and changes in the cost of gasoline
that may have contributed to public attitudes.
Mohr et al. (1994) conducted a cross-sectional cohort study that
included 237 garage workers exposed to high and low MTBE
concentrations: 115 workers exposed in northern New Jersey during the
wintertime oxyfuel programme and 122 workers in southern New Jersey 10
weeks after the phase-out date for the programme. Both groups of
workers reported feeling significantly worse at the end of the work
day, but there was no difference between the groups across the work
shift. Active air sampling and passive sampling devices confirmed the
higher exposure levels of the workers in northern New Jersey. No
significant differences were found in either the cross-sectional
reporting of symptoms or the pre- and post-shift analyses.
A study performed in Stamford, Connecticut, in April 1993 near
the end of the oxygenated gasoline season there, investigated exposure
to MTBE in oxygenated gasoline and symptom prevalence in
occupationally and non-occupationally exposed subjects (White et al.,
1995). The study included 37 workers and 14 commuters. The prevalence
of symptoms was highest among people who worked in car repair-shops or
around traffic. The eight workers with the highest levels of MTBE in
blood (>3.8 µg/litre) reported one or more key symptoms (OR = 21.0,
95% CI = 1.8-539.0) such as headache, irritated eyes, burning of the
nose and throat, cough, dizziness, spaciousness, disorientation, and
nausea. There were no reports of diarrhoea, difficulty in breathing,
skin irritation, fever, sweats or chills, or fainting.
In response to health concerns raised by the public following the
introduction of the reformulated gasoline (RFG) programme in the
Milwaukee, Wisconsin area, the Wisconsin Department of Health
initiated a random digit-dial telephone survey designed to assess the
prevalence and scope of health complaints (Anderson et al., 1995). A
questionnaire was administered to approximately 1500 persons: 527
residents of the Milwaukee, where RFG was sold and numerous complaints
about the fuel had been registered; 485 residents of Chicago, where
essentially the same fuels were sold but few complaints had been
registered; and 501 residents of the remainder of Wisconsin, where RFG
had not been required. Overall, there was a significantly higher
prevalence rate for "unusual symptoms" in Milwaukee (23%) than in
Chicago and the rest of Wisconsin (6% each). The fact that symptom
prevalence in the Chicago RFG area was so similar to that in non-RFG
areas of Wisconsin suggested that factors other than RFG use
contributed to the difference between Milwaukee and the other two
areas. Although the prevalence of colds and flu was the same in the
three areas, Milwaukee residents were more likely to report unusual
symptoms if they had experienced a cold or the flu, smoked cigarettes,
or were aware that they had purchased RFG. The authors concluded that
many symptoms reported by Milwaukee residents may have actually been
due to colds or flu rather than RFG exposure. Also, individuals who
reported purchasing RFG were more likely to report symptoms than
individuals who said they had not purchased or did not know whether
they had purchased RFG, which suggested that knowledge about RFG may
have increased awareness of an individual's health status and resulted
in the assumption that any health symptoms experienced were unusual
and attributable to gasoline exposure. This study and its conclusions
were reviewed by a panel of independent experts who concluded that
"The study does not support a conclusion that exposure to RFG is
associated with widespread or serious acute adverse health effects."
This evaluation of the study was later endorsed by another,
independent peer review group (HEI, 1996).
Anderson et al. (1995) followed up on 1280 Milwaukee residents
who had initially contacted governmental agencies about their health
complaints. These self-identified individuals were interviewed by
telephone to determine the types of complaints and risk factors that
could be associated with symptoms. Results of the study indicated that
the strongest predictors were age, allergies, and colds or flu since
November 1994. The purchase of RFG, a surrogate for exposure, did not
correlate with self-reported health symptoms.
A cross-sectional study was conducted in Finland to investigate
the occurrence of neurophysiological symptoms in tanker drivers
exposed to gasoline containing approximately 10% MTBE (Hakkola et al.,
1996). A reference group of milk delivery drivers was selected from
the same areas. A total of 201 male drivers participated in the study,
including 101 tanker drivers and 100 milk delivery drivers. The
occurrence of symptoms and Profile on Mood States (POMS) scales showed
an association with age, chronic diseases, and the perceived health of
the drivers in both the exposed and the unexposed group. Although
there were more sensory and motor symptoms in tank drivers, there was
no evidence of any statistically significant difference in the
occurrence of symptoms between the two groups. Duration of work as a
driver, shift schedule and length of the working week had no
statistical connection with symptoms and the modified POMS scales.
8.2 Controlled studies
To determine if 1-h exposures to MTBE at 6 mg/m3 (1.7 ppm) in an
inhalation chamber could result in similar symptoms as those reported
in the field, Cain et al. (1996) performed a controlled study in 22
male and 21 female subjects (ages 18 to 34 years). Both objective and
subjective indices of behavioural and physiological effects were
studied. A control exposure to air and a control exposure to a
17-component mixture of volatile organic compounds (VOCs) (19 mg/m3)
were also included. The selected MTBE concentration and duration were
based upon preliminary results from exposure studies in commuters
(Lioy et al., 1994). The effects of MTBE exposure on discomfort,
symptoms and possible objective correlates of symptoms were
investigated by using questionnaires, various ocular and nasal
inflammation parameters, and neurobehavioural testing. Repeated blood
samples were obtained from a subset of subjects to relate the exposure
to the body burden and toxicokinetics of MTBE (see section 6.1.1). The
exposure produced a mean peak blood MTBE level of 17 µg/litre.
Subjective reactions, such as irritation, fatigue and headache,
typically showed greater sensitivity than objective indices. There was
a differential effect of gender on rated odour, intensity and
pleasantness. Females found odour intensity greater, pleasantness
worse, and air quality worse. Ocular measurements indicated a mild
tendency for eye irritation during exposure; this was, however, not
statistically significant. MTBE caused no objective inflammatory
changes in the nasal mucosa. Aside from odour, no significant
reactions were found.
In another controlled study, conducted by Prah et al. (1994), 20
male and 20 female volunteer subjects (healthy and non-smokers) were
exposed in an inhalation chamber to MTBE at 5 mg/m3 (1.39 ppm) for
1 h. Symptom questionnaires, cognitive testing, and objective measures
of ocular and nasal irritation were obtained before and at the end of
the exposure. In addition, the odour threshold of MTBE and some
pharmacokinetic data were obtained from two additional subjects, one
male and one female (see section 6.1.1). No increase in the reporting
of symptoms such as headache, nasal irritation, cough or eye
irritation was found, apart from a gender effect in reporting of air
quality. Females rated the air quality in the chamber with MTBE
exposure slightly poorer when compared to the clean air exposure.
There were no changes in objective indicators in either the eye or the
nose.
Johanson et al. (1995) and Nihlén et al. (1998b) reported similar
results at much higher concentrations. Ten healthy male volunteers
were exposed to MTBE vapour at 18, 90 or 180 mg/m3 (5, 25 or 50 ppm)
for 2-h periods while performing light physical exercise (see also
section 6.1.1). All the subjects reported the strong smell of MTBE on
entering the chamber but their rating of the smell decreased with
time. On the basis of subjective assessment there was no irritation of
the ocular, nasal or pharyngeal mucosae. The MTBE exposure induced
essentially no effects on eye and mucous membrane irritation.
Riihimaki et al. (1996) assessed a number of subjective (e.g.,
irritant sensations, mood state) and objective (simple reaction time,
postural sway) end-points in 13 male volunteers exposed to 0, 90 or
268 mg/m3 (0, 25 or 75 ppm) MTBE for 1 or 3 h. The authors concluded
that only "mild symptoms, mainly a feeling of heaviness in the head
and, to a smaller extent, of mild mucous membrane irritation, were
reported". The frequency of symptoms was related to the level of MTBE
exposure and reached statistical significance at 268 mg/m3 (75 ppm)
after 3 h of exposure. There was no effect on reaction time or
postural sway.
8.3 Subpopulations at special risk
There are no data by which to identify any subpopulations (e.g.,
the elderly, pregnant women, children or people with allergy or
asthma) who might be at special risk to MTBE exposure.
8.4 Special studies
8.4.1 Organoleptic properties
For many people, MTBE has a quite distinctive odour. Odour
detection thresholds have been reported to average around 0.1 to
0.2 mg/m3 (0.03-0.05 ppm), with average recognition (identification)
thresholds in a range from 0.2 to 0.5 mg/m3 (0.06-0.13 ppm) for neat
MTBE vapour (TRC, 1993; Smith & Duffy, 1995). For gasoline containing
15% (by volume) MTBE in the USA, average odour detection thresholds
ranged from approximately 0.3 to 3 mg/m3 (0.08- 0.9 ppm), with
recognition thresholds ranging from 0.7 to 2.5 mg/m3 (0.2-0.7 ppm).
Odour thresholds for MTBE-oxygenated gasoline may vary considerably,
depending in part on the aromatic and other constituents of the
gasoline and the sensitivity of the individuals who inhale the
vapours. The addition of MTBE to gasoline has been found to reduce the
detection threshold (i.e. increase "detectability") for gasoline by as
much as 80% (HEI, 1996).
8.4.2 Immunological effects
In a study to assess the effects of MTBE on the immune system,
interleukin levels were measured in blood plasma of 22 volunteers at
several different locations around Fairbanks exposed to auto emissions
derived from oxyfuel (Duffy, 1994). The study was performed during a
4-week period in late November and early December 1992. During this
period, the mean daily temperature ranged from about -1.5°C (35°F) to
about -37°C (-38°F). Plasma interleukin 1 ß (IL-1 ß) and interleukin 6
(IL-6) levels were measured at the beginning and at the end of an 8 h
work day. The results showed no difference between the morning mean
levels (2.50 pg/ml ± 2.4 SD and the evening mean levels (2.53 pg/ml ±
2.6 SD). There were, however, 14 out of 22 individuals who showed
slight increases at the end of the work day. IL-1, which was measured
in 10 individuals, was below the detection limit.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Algae
The effect of MTBE on the growth of the unicellular algae
Selenastrum capricornutum (Chlorophyta), Navicula pelliculosa
(Bacillariophyta) and Synechococcus leopoliensis (Cyanobacteria),
representing three taxonomic groups, was investigated under laboratory
conditions (Rousch & Sommerfeld, 1998). The growth of
N. pelliculosa and S. leopoliensis was inhibited at a nominal MTBE
concentration of 2400 mg/litre in the growth medium, whereas
S. capricornutum growth was increased at 600 mg/litre and decreased
at 4800 mg/litre. The authors suggested that the differential
sensitivity of these representative species implies that MTBE could
alter algal community composition in the environment.
9.1.2 Aquatic animal species
The results of aquatic toxicity tests are presented in Table 21.
Experimental data on acute toxicity are available for four
species of invertebrates, four species of fish, and one species of
amphibian. The experimental data ranged from a 96-h LC50 of 553
mg/litre for the crustacean Chaetogammam marinum (Adema, 1982), to a
96-h LC50 of >10 000 mg/litre for a copepod Nitocra spinipes
(Tarkpea & Svanberg, 1982).
Acute toxicities were determined by Tarkpea & Svanberg (1982) for
MTBE alone, MTBE combined with a base fuel at a concentration of 5%,
and the base fuel alone. The acute toxicity tests were conducted on
the harpacticoid copepod Nitocra spinipes under static conditions
for 96 h at 21°C. The test solutions were not aerated. A 96-h LC50
greater than 10 000 mg/litre was reported for MTBE alone. The 96-h
LC50 values were 242 mg/litre for MTBE in a base fuel and 201
mg/litre for the base fuel alone. The results of the tests show that
the acute toxicity of base fuel to aquatic organisms is not increased
by the addition of 5% MTBE.
Tarkpea & Svanberg (1982) also determined the 24-h LC50 of MTBE
on a shoal fish, the bleak Alburnus alburnus in a closed, static
system at 10°C. The LC50 was between 1700 and 1800 mg/litre. When the
bleaks were introduced into the test media, several sublethal effects
were observed, including disturbed balance, surface swimming and
overturning. The sublethal effects were short-lived, as several of the
individual test subjects had recovered when the test was completed.
The environmental significance of these results was not discussed by
the authors.
Table 21. Aquatic toxicity testing results for MTBE
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Invertebrates
Ceriodaphnia LC50 (48-h) 18-21°C 841 THE, 1989
Daphnia magna LC50 (96-h) >1000 Gupta & Lin, 1995
Copepod LC50 (96-h) >10 000 Tarkpea & Svanberg, 1982
(Nitocra spinipes)
Copepod LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
(Nitocra spinipes)
Gammarid LC10 (96-h) 15°C 553 Adema, 1982
(Chaetogammarus marinus)
Fish
Bleak (Alburnus alburnus) LC50 (24-h) 10°C 1700-1800 Tarkpea & Svanberg, 1982
Bleak (Alburnus alburnus) LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
Fathead minnow LC50 (96-h) 25°C 706 Veith et al., 1983
(Pimephales promelas)
Fathead minnow LC50 (96-h) 672 Geiger et al., 1988
(Pimephales promelas)
Rainbow trout LC50 (96-h) 1300 Environment Canada, 1993
(Oncorhynchus mykiss)
Table 21. (continued)
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Rainbow trout LC50 (96-h) 1483 Environment Canada, 1993
(Oncorhynchus mykiss)
Amphibians
European frog tadpole LC0 (48-h) <2000 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC50 (48-h) 2500 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC100 (48-h) <3000 Paulov, 1987
(Rana temporaria)
When European frog tadpoles (Rana temporaria) were exposed to
concentrations of MTBE in water ranging from 100 to 2500 mg/ litre,
various effects were observed. An increase in body weight of frogs and
tadpoles that had undergone metamorphosis was observed at 100
mg/litre, as compared with the controls. At sublethal concentrations
(<2500 mg/m3) in water, accelerated development of the tadpole was
observed and metamorphosis occurred two days earlier than in controls
(Paulov, 1987). The environmental significance of these results was
not discussed by the author.
No data on chronic aquatic toxicity were found in the literature.
Data on the toxicity of MTBE to terrestrial animals, terrestrial
plants or soil biota were not found in the literature, other than
information from mammalian toxicology studies.
9.2 Field experiments
Data on field experiments on MTBE were not found in the
literature.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Total exposure of human populations to MTBE may involve more than
one environmental pathway and route of intake. Populations may be
exposed to MTBE in air in areas where it is used in gasoline, though
available data on environmental levels and human exposure are limited.
In several studies, median concentrations of MTBE in ambient air
ranged from 0.47 to 14.4 µg/m3 (0.00013 to 0.004 ppm) where MTBE is
used in oxygenated gasoline, and non-detectable to 26.4 µg/m3 (0.0073
ppm) in urban air of cities where MTBE is used as an octane enhancer.
Concentrations near industrial facilities range up to 35.7 mg/m3 (10
ppm). Median 1- to 2-min exposure levels gathered in the breathing
zone of service station attendants and consumers while refuelling were
highly variable, ranging from 1.0 to 21.4 mg/m3 (0.03 to 6 ppm) and
occasionally exceeding 35.7 mg/m3 (10 ppm).
Monitoring data for MTBE are too limited to characterize
adequately its occurrence in drinking-water. The intake of MTBE in
drinking-water is generally expected to be negligible, although
drinking-water may be polluted from point sources such as accidental
spills of large amounts of MTBE in gasoline. Exposure could also occur
through dermal absorption or inhalation of MTBE vapour from household
water used for bathing, cooking and laundering.
Potentially exposed workers include those involved in the
production, handling and use of MTBE and MTBE-containing gasoline,
including mechanics and service station attendants. Occupational
exposure of workers transporting MTBE is highest, with an average
short-term median concentration of 140 mg/m3 (39 ppm). Long-term
average median levels for this group of workers were about 2.85 mg/m3
(0.8 ppm). Median long-term exposure of service station attendants
averaged 1.79 mg/m3 (0.5 ppm). The long-term median value for
mechanics was 0.36 mg/m3 (0.1 ppm).
10.1.2 Human health effects
Consumers in some areas of the USA have complained about acute
health symptoms such as headache, eye and nose irritation, cough,
nausea, dizziness and disorientation associated with the use of
oxygenated fuels such as gasoline containing MTBE. Epidemiological
studies of human populations exposed under occupational and
non-occupational conditions, as well as experimental studies of human
volunteers exposed under controlled conditions, have not been able to
identify a basis for these complaints. Results of community studies
conducted in Alaska, New Jersey, Connecticut and Wisconsin, USA, have
been mixed and provided limited or no evidence of an association
between MTBE exposure and the prevalence of health complaints. In
addition, independently conducted experimental studies of volunteers
exposed in inhalation chambers to MTBE concentrations ranging from 5.0
mg/m3 (1.4 ppm) in one study to 180 mg/m3 (50 ppm) for 2 h in
another study have shown no evident effects in terms of either
subjective reports of symptoms or objective indicators of irritation
or other effects. Based on the collective evidence, it appears
unlikely that MTBE alone induces adverse acute health effects in the
general population under common inhalation exposure conditions.
However, the potential effects of mixtures of gasoline and MTBE, as
well as the manner in which most people are exposed to MTBE in
conjunction with the use of oxygenated fuels, have not been examined
experimentally or through prospective epidemiological methods.
Moreover, the role of factors such as awareness of MTBE, due in part
to its distinctive odour, for example, has not been investigated.
In studies on animals, MTBE is "moderately" acutely toxic, with
an oral LD50 in rats of approximately 3800 mg/kg bw and LC50 value
(15 min) of about 141 000 mg/m3 air in mice. Signs of intoxication
include CNS depression, ataxia and laboured respiration. The LD50 for
dermal toxicity in rabbits is >10 200 mg/kg bw.
MTBE is considered to be a mild skin and eye irritant but does
not induce skin sensitization.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Effect levels are compiled in Tables 22 and 23. Concentrations
or doses that induced significant increases in organ weights, for
which histopathological effects were observed at higher levels, were
considered LOELs. Doses at which histopathological effects were
observed were considered LOAELs (Tables 22 and 23).
Lowest reported effect levels for nephrotoxicity following
ingestion in subchronic studies were 440 mg/kg bw per day (increases
in relative kidney weight and hyaline droplet formation in
Sprague-Dawley rats). At 2860 mg/m3 (800 ppm), in a 90-day inhalation
study, there were increases in kidney weight associated at higher
concentrations with a mild increase in hyaline droplets in the
proximal tubules in Fischer-344 rats. At 1430 mg/m3 (400 ppm), in
inhalation oncogenicity studies, there was an increase in absolute
liver weight, which correlated with increased severity of
hepatocellular hypertrophy at higher concentrations and an increase in
relative kidney weight in male mice; in rats, incidence and severity
of chronic progressive nephropathy were increased at this level.
Exposure to MTBE also results in reversible central nervous
system effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations, and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2860 mg/m3 (800 ppm) produced
reversible, non-monotonically dose-related changes in motor activity.
These effects were transient and not observed in longer-term studies.
Table 22. MTBE levels for non-neoplastic effects following oral exposure
Species Protocol Effect Level Basis of Effect Level Reference
(mg/kg bw/day)
Sprague-Dawley rats 28-day gavage LOAEL 440 males: increase in relative IITRI (1992)
(undiluted) kidney weight; hyaline droplet
formation in convoluted
NOAEL 90 tubules
Sprague-Dawley rats 90-day gavage in LOEL 900 increase in male absolute and Robinson et al. (1990)
corn oil relative kidney weight; chronic
nephropathy and increase in
NOAEL 300 hyaline droplets in proximal
tubular cells at the next
higher dose (1200 mg/kg bw/day)
Sprague-Dawley rats 104 weeks gavage NOEL 1000 no effects at any dose Belpoggi et al. (1995)
in olive oil;
maintained until
death
Table 23. MTBE levels for non-neoplastic effects following inhalation exposure
Species Protocol Effect level Basis for effect level Reference
Fischer-344 rats single exposure LOEL for reversible biphasic changes in motor Gill (1989);
neurological effects = activity in females Daughtrey et al.
2860 mg/m3 (800 ppm) (1997)
Fischer-344 rats 13 week study LOEL 2860 mg/m3 males: increase in relative Dodd & Kintigh (1989);
(800 ppm) weight of liver and kidney; Daughtrey et al. (1997)
no NOAEL males: at 28 600 mg/m3, increase
in lymphoid hyperplasia in
submandibular lymph nodes; mild
increase in hyaline droplets in
renal proximal tubules
Fischer-344 rats up to 104 weeks LOAEL 10 700 mg/m3 males: increased mortality and Chun et al. (1992);
(3000 ppm) decreased survival time at 10 700 Bird et al. (1997)
LOEL 1430 mg/m3 mg/m3; chronic progressive
(400 ppm) nephropathy was the major cause
of death at 10 700 mg/m3; increased
incidence and severity of chronic
progressive nephropathy at all
doses (significance not specified)
CD1 mice up to 18 months LOEL 1430 mg/m3 increase in absolute liver Chun et al. (1992)
(400 ppm) weight in male mice which Bird et al. (1997)
correlated with increased
severity of hepatocellular
hypertrophy at higher
concentrations; increase in
relative kidney weight in males
Specific adverse effects on reproduction have not been observed
in rats at concentrations up to 28 600 mg/m3 (8000 ppm). MTBE has not
induced developmental effects in rats, mice or rabbits at
concentrations less than those that were toxic to the mothers.
Decreases in uterine weight and increases in estrogen metabolism have
been observed at 28 600 mg/m3.
The weight of evidence indicates that MTBE is not genotoxic.
Identified oncogenicity studies include an inhalation study in rats
and mice and an oral study (gavage) in rats. In these investigations,
MTBE induced testicular (Leydig cell) tumours in male rats
(Fischer-344 and Sprague-Dawley), renal tumours in male rats
(Fischer-344) liver tumours in female mice (CD-1) and lymphomas and
leukaemias (combined) in female (Sprague-Dawley) rats.
All investigations on nephrotoxicity are consistent with the
renal tumours observed in Fischer-344 rats being related to
alpha2u-globulin nephropathy. alpha2u-Globulin nephropathy is
considered an effect specific to male rats and, therefore, these
tumours are of questionable relevance to humans.
Leydig cell tumours have been induced by MTBE in two strains of
rats. This type of tumour has been reported to be induced by
non-genotoxic carcinogens that disturb the hormonal balance of
testosterone, luteinizing hormone and luteinizing hormone releasing
factor in rats. Owing to differences between rats and humans in the
regulation of gonadotropins, it is questionable that a similar effect
will occur in humans. Although such a mechanism may be relevant, this
is not substantiated by experimental evidence, since these hormones
were not determined in any of the studies with MTBE.
Liver tumours have been induced by MTBE in female mice and
possibly in male mice (the data on male mice were not corrected for
increased mortality). The effect was modest and occurred only at
28 600 mg/m3 (8000 ppm) and in association with hepatocellular
hypertrophy (indicating enzyme induction) and altered estrogen
metabolism. The relevance of these mouse liver tumours for human risk
estimation is considered to be questionable.
In a single oral study in SD rats, the frequency of lymphomas and
leukaemias (combined) were increased in the high-dose group. This
observation was not supported by any indications of relevant
(preneoplastic) effects on the lymphoid system in other studies.
Moreover, the description of the study made it difficult to evaluate
adequately the results. However, since the effect observed appears to
be rather pronounced, it is not justified to neglect this finding,
based on presumed experimental deficiencies. For a proper evaluation,
additional information is required.
On the basis of these data, MTBE should be considered a rodent
carcinogen. MTBE is not genotoxic and the carcinogenic response is
only evident at high levels of exposure that also induce other adverse
effects. The available data are inconclusive and prohibit their use
for human carcinogenic risk assessment until outstanding complications
in their interpretation have been addressed.a
10.2 Evaluation of effects on the environment
MTBE emissions and leakages can be widespread in the environment
in areas where MTBE is used as an octane improver and oxygenate in
oxygenated gasoline.
MTBE is predominately emitted into air; however, it can be
released into the water and soil compartments. Ambient concentrations
in air are low. There are no terrestrial toxicity data for exposure to
MTBE in air; however, this appears not to be of concern to an
environmental evaluation since ambient air concentrations are low and
its half-life is relatively short.
Owing to its physical and chemical properties, MTBE can persist
longer in water and soil than in air. There are very limited data on
concentrations in ambient surface water. The biodegradation of MTBE in
water and soil is not well understood but is believed to be relatively
slow. MTBE in soil can leach into groundwater and persist there, due
to its lack of removal. MTBE has not been generally detected in deeper
groundwater or in shallow groundwater in agricultural areas. It is
more frequently found in shallower groundwater in urban areas where
MTBE is most extensively used.
Data available for ecotoxicological assessment refer almost
exclusively to MTBE in water. It can be classified as relatively
non-toxic for aquatic biota, with a lowest acute effect for several
aquatic organisms of more than 100 mg/litre. No long-term aquatic
toxicity tests at low concentrations have been identified. However,
the limited data on concentrations of MTBE in ambient surface water
have shown that concentrations range from non-detectable to 30
µg/litre. The maximum concentration is several orders of magnitude
below the effect level of the most sensitive organism tested to date.
It does not appear that the concentrations of MTBE in ambient water
are toxic to aquatic organisms, except during spills when very high
levels of MTBE may be found.
There are no data on concentrations of MTBE in soil or on
terrestrial toxicity. However, concentrations in this medium are
expected to be low except in the case of spills.
a MTBE was reviewed by an International Agency for Research on
Cancer (IARC) Working Group in October 1998. The conclusions were that
there was inadequate evidence for the carcinogenicity in humans of
MTBE, limited evidence for its carcinogenicity in experimental
animals, and the overall evaluation was that MTBE was not classifiable
as to its carcinogenicity for humans (Group 3).
11. RECOMMENDATIONS
To provide quantitative guidance on relevant limits of exposure
and to estimate risk, it is recommended that additional data be
acquired in the following areas:
a) additional information to evaluate the induction of
lymphomas/leukaemias in Sprague-Dawley rats;
b) mechanistic data on the induction of Leydig cell tumours and sex
specificity of liver tumours in mice;
c) controlled exposure studies to characterize the dose-response in
humans for MTBE and MTBE-containing mixtures;
d) monitoring data for better characterization of human exposure,
with particular attention to microenvironments;
e) potentiation studies of MTBE with BTX components of gasoline;
f) monitoring of environmental concentrations of MTBE in soil and
biota in areas adjacent to major sources and ambient areas in
order to verify theoretical values;
g) long-term toxicity tests in aquatic and possibly terrestrial
organisms;
h) field degradation tests to determine how persistent MTBE can be
in soil and groundwater under a range of redox conditions.
REFERENCES
ACGIH (1994) 1994-1995 threshold limit values for chemical substances
and physical agents and indices. Cincinnati, Ohio, American Conference
of Governmental Industrial Hygienists, p 27.
Adam G, Knuechel R, Vorwerk D, Held C, & Guenther RW (1990) Tissue
response of the biliary and digestive system of rabbits after MTBE
infusion into the gallbladder. Invest Radiol, 25(1): 58-61.
Adema DMM (1982) Tests and desk studies carried out by MT-TNO during
1980-1981 for Annex II of MARPOL 1973. Delft, The Netherlands,
Organization for Applied Scientific Research, 51 pp (Report CL
82/1499).
AFS (1994) Statute book of the Swedish National Board of Occupational
Safety and Health, Ordinance AFS 1993: 9: Occupational exposure limit
values. Solna, The Swedish National Board of Occupational Safety and
Health, pp 46, 52.
Akimoto R, Rieger E, Moossa AR, Hofmann AF, & Wahlstrom HE (1992)
Systemic and local toxicity in the rat of methyl tert-butyl ether: a
gallstone dissolution agent. J Surg Res, 53: 572-577.
Allard A-S, Remberger M, & Neilson AH (1996) The aerobic
biodegradation of tert-butyl methyl ether and tert-butanol: an
initiatory study. Stockholm, The Swedish Environmental Research
Institute, 9 pp (IVL Report No. B 1197).
Allen MK & Grande D (1995) Reformulated gasoline air monitoring study.
Madison, Wisconsin, Department of Natural Resources, Bureau of Air
Management, 50 pp (Publication No. AM-175-95).
Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985a) Rapid dissolution of gallstones by methyl tert-butyl ether.
N Engl J Med, 312(4): 217-220.
Allen MJ, Barody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985b) Cholelitholysis using methyl tertiary butyl ether.
Gastroenterology, 88: 122-125.
Almaguer D (1993) Health hazard evaluation report HETA 93-0884-2344:
National Center for Environmental Health, Albany, New York.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 33 pp (NTIS/PB94-129020).
Anderson EV (1993) Health studies indicate MTBE is safe gasoline
additive. Chem Eng News, September 20: 9-18.
Anderson HA, Hanrahan L, Goldring J, & Delaney B (1995) An
investigation of health concerns attributed to reformulated gasoline
use in southwest Wisconsin - Final Report. Madison, Wisconsin,
Department of Health and Social Services, 50 pp.
Angle CR (1991) If the tap water smells foul, think MTBE. J Am Med
Assoc, 266: 2985-2986.
API (1989a) Aboveground storage tank survey. Washington, DC, American
Petroleum Institute, Health and Environment Affairs Department, 44 pp
(Publication No. 301).
API (1989b) Monitoring near refineries for airborne chemicals - Volume
1: Validated ambient air concentrations around three refineries.
Washington, DC, American Petroleum Institute, Health and Environment
Affairs Department, 83 pp (Publication No. 4484).
API (1993) Gasoline vapor exposure assessment at service stations.
Washington, DC, American Petroleum Institute, 8 pp (Publication No.
4553).
API (1994) A survey of API members' aboveground storage tank
facilities. Washington, DC, American Petroleum Institute, 65 pp
(Publication No. 330).
API (1995a) Executive summary of a study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 5 pp (Publication No. 4619).
API (1995b) Petroleum Institute data characterizing occupational
exposures to methyl tertiary butyl ether (MTBE) 1983-1993. Washington,
DC, American Petroleum Institute, 21 pp (Publication No. 4622).
API (1995c) Service station personnel exposures to oxygenated fuel
components - 1994: Executive summary. Washington, DC, American
Petroleum Institute, 3 pp (Publication No. 4625).
Arashidani K, Katoh T, Yoshikawa M, Kikuchi M, Kawamoto J, & Kodama Y
(1993) LD50 and weight change in organs of mice following
intraperitoneal administration of methyl tertiary-butyl ether. Jpn J
Ind Health, 35: 404-405.
ARCO (1987) Methyl tert-butyl ether exposure monitoring study.
Channelview, Texas, ARCO Chemical Company, 39 pp.
Atkinson R (1990) Gas-phase tropospheric chemistry of organic
compounds : a review. Atmos Environ, 24A: 1-41.
Atkinson R & Pitts JN Jr (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-246°K. J
Chem Phys, 68(8): 3581-3584.
Barreto RD, Gray KA, & Anders K (1995) Photocatalytic degradation of
methyl- tert-butyl ether in TiO2 slurries: a proposed reaction
scheme. Water Res, 29(5): 1243-1248.
Beller M & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska. Anchorage, Alaska, Department
of Health and Social Services, Section of Epidemiology, 5 pp.
Belpoggi F, Soffritti M, & Maltoni C (1995) Methyl-tertiary-butyl
ether (MTBE) - a gasoline additive - causes testicular and
lympho-haematopoietic cancers in rats. Toxicol Ind Health, 11(2):
119-149.
Bengtsson EB & Tarkpea M (1983) The acute aquatic toxicity of some
substances carried by ships. Mar Pollut Bull, 14(6): 213-214.
Bevan C, Neeper-Bradley TL, Tyl RW, Fisher LC, Panson RD, Kneiss JJ, &
Andrews LS (1997) Two-generation reproductive toxicity study of methyl
tertiary-butyl ether (MTBE) in rats. J Appl Toxicol, 17(suppl 1):
S13-S19.
Bianchi A & Varney MS (1989) Analysis of methyl tert-butyl ether and
1,2-dihaloethanes in estuarine water and sediments using
purge-and-trap/gas chromatography. J High Res Chromatogr, 12(3):
184-186.
Bianchi A, Varney MS, & Phillips J (1991) Analysis of volatile organic
compounds in estuarine sediments using dynamic headspace and gas
chromatography-mass spectrometry. J Chromatogr, 542: 413-450.
Biles RW, Schroeder RE, & Holdsworth CE (1987) Methyl tertiary butyl
ether inhalation in rats: a single generation reproduction study.
Toxicol Ind Health, 3(4): 519-534.
Biodynamics (1984) The metabolic fate of methyl-t-butyl ether (MTBE)
following an acute intraperitoneal injection (Project No. 80089).
Washington, DC, American Petroleum Institute, 97 pp.
Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, & Andrews
LS (1997) Oncogenicity studies of inhaled methyl tertiary-butyl ether
in CD1 mice and F-344 rats. J Appl Toxicol, 17(suppl 1): S45-S55.
Blackburn GR, Dooley JF III, Schreiner CA, & Mackerer CR (1991)
Specific identification of formaldehyde-mediated mutagenicity using
the mouse lymphoma L5178Y TK+/- assay supplemented with formaldehyde
dehydrogenase. In Vitro Toxicol, 4(2): 121-132.
Bonin MA, Ashley DL, Cardinali FL, McGraw JM, & Wooten JV (1994)
Measurement of methyl tert-butyl ether and tert-butyl alcohol in
human blood and urine by purge-and-trap gas chromatography-mass
spectrometry using an isotope dilution method. In: 208th National
Meeting of the American Chemical Society, 1994. Washington, DC,
American Chemical Society, 1 p (Abstract P102-ENVR).
Borghoff SJ & Lagarde WH (1993) Assessment of binding of
2,4,4-trimethyl-2-pentanol to low-molecular-weight proteins isolated
from kidneys of male rats and humans. Toxicol Appl Pharmacol, 119:
228-235.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha2u-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 264-275.
Borghoff SJ, Jayjock E, & Murphy JE (1995) Methyl tert-butyl ether
(MTBE) vs ethyl tert-butyl ether (ETBE): A comparison of blood and
tissue partition coefficients in male and female F344 rats.
Toxicologist, 30: 34.
Borghoff SJ, Murphy JA, & Medinsky MA (1996) Development of a
physiologically based pharmacokinetic model for methyl
tertiary-butyl ether and tertiary-butanol in male Fischer-344
rats. Fundam Appl Toxicol, 30: 264-275.
Borghoff SJ, Murphy JE, Turner M, & James AR (1998) Dosimetry of
methyl t-butyl ether (MTBE) and t-butyl alcohol in male and female
rats following inhalation exposure to MTBE. Toxicologist, 42(1-S):
A1049.
Brady JF, Xiao F, Ning SM, & Yang CS (1990) Metabolism of methyl
tertiary-butyl ether by rat hepatic microsomes. Arch Toxicol, 64:
157-160.
Brown SL (1997) Atmospheric and potable water exposures to methyl
tert-butyl ether (MTBE). Regul Toxicol Pharmacol, 25: 256-276.
Bruce BW & McMahon PB (1996) Shallow ground-water quality beneath a
major urban center: Denver, Colorado, USA. J Hydrol, 186: 129-151.
Buckley TJ, Prah JD, Ashley D, Zweidinger RA, & Wallace LA (1997) Body
burden measurements and models to assess inhalation exposure to methyl
tertiary butyl ether (MTBE). J Air Waste Manage Assoc, 47: 739-752.
Buchta TM (1993a) Health hazard evaluation report HETA 93-606-2336:
National Centers for Environmental Health, Fairbanks, Alaska.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 37 pp (NTIS/PB94-133667).
Buchta TM (1993b) Health hazard evaluation report HETA 93-802-2338:
National Centers for Environmental Health, Stamford Connecticut.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 39 pp (NTIS/PB94-134343).
Budavari S, O'Heil MJ, Smith A, Heckelman PE, & Kinneary JF ed. (1996)
Methyl- tert-butyl ether. In: The Merck index, 12th ed. Whitehouse
Station, New Jersey, Merck & Co., Inc., p 1611.
Burleigh-Flayer HD, Chun JS, & Kintigh WJ (1992) Methyl tertiary butyl
ether: vapor inhalation oncogenicity study in CD-1 mice (Laboratory
project ID 91N0013A). Export, Pennsylvania, Bushy Run Research Center,
1068 pp (Report to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Cain WS, Leaderer BP, Ginsberg GL, Andrews LS, Cometto-Muniz JE, Gent
JF, Buck M, Berglund LG, Mohsenin V, Monahan E, & Kjaergaard S. (1996)
Acute exposure to low-level methyl tertiary-butyl ether (MTBE): human
reactions and pharmacokinetic response. Inhal Toxicol, 8: 21-48.
Calvert JG & Pitts JN Jr (1966) Photochemistry. New York, John Wiley &
Sons, Inc., pp 441-442.
CDC (1993) An investigation of exposure to methyl tertiary butyl ether
among motorists and exposed workers in Stamford, Connecticut.
Presented at the EPA Workshop on MTBE and other Oxygenates, Falls
Church, Virginia, 27 July 1993. Atlanta, Georgia, Centers for Disease
Control and Prevention, 35 pp.
Cederbaum AI & Cohen G (1980) Oxidative demethylation of t-butyl
alcohol by rat liver microsomes. Biochem Biophys Res Commun, 97:
730-736.
CEN (1996) Growth of top 50 chemicals slowed in 1995 from very high
1994 rate. Chem Eng News, April 8:16-20.
Chandler B & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska: Preliminary results. Anchorage,
Alaska, Department of Health and Social Services, 5 pp.
Charbonneau M, Strasser J Jr, Lock EA, Turner MJ Jr, & Swenberg JA
(1989) Involvement of reversible binding to alpha2u-globulin in
1,4-dichlorobenzene-induced nephrotoxicity. Toxicol Appl Pharmacol,
99: 122-132.
Chen CY, Chang KK, Chow NH, Leow TC, Chou TC, & Lin XZ (1995) Toxic
effects of cholelitholytic solvents on gallbladder and liver. Dig Dis
Sci, 40(2): 419-426.
Chiang CY, Loos KR, & Klopp RA (1992) Field determination of
geological/chemical properties of an aquifer by cone penetrometry and
headspace analysis. Ground Water, 30: 428-436.
Chun JS & Kintigh WJ (1993) Methyl tertiary-butyl ether: twenty-eight
day vapor inhalation study in rats and mice. Export, Pennsylvania,
Bushy Run Research Center, 387 pp (BRRC Report 93N1241).
Chun JS, Burleigh-Flayer HD, & Kintigh WJ (1992) Methyl tertiary butyl
ether (MTBE): vapor inhalation oncogenicity study in Fischer 344 rats
(Laboratory project ID 91N0013B). Export, Pennsylvania, Bushy Run
Research Center, 1463 pp (Report for the Methyl Tertiary Butyl Ether
Committee, Washington, DC).
Church CD, Isabelle LM, Pankow JF, Tratnyek PG, & Rose DL (1997)
Assessing the in situ degradation of methyl tert-butyl ether
(MTBE) byproduct identification at the sub-PPB level using direct
aqueous injection GC/MS: Extended abstract - Proceedings of ACS
National Meeting, San Francisco, California, 16-17 April 1997.
Washington, DC, American Chemical Society, 4 pp.
Cinelli S & Seeberg AH (1989) Reverse mutation in Salmonella
typhimurium. Test substance: MTBE. Rome, Life Science Research,
Toxicology Centre, 50 pp (LSR-RTC Report No. 216001-M-03489).
Cirvello JD, Radovsky A, Heath JE, Farnell DR, & Lindamood C (1995)
Toxicity and carcinogenicity of T-butyl alcohol in rats and mice
following chronic exposure in drinking water. Toxicol Ind Health, 11:
151-165.
Clark CR, Dutcher JS, Henderson TR, McClellan RO, Marshall WF, Naman
TM, & Seizinger DE (1984) Mutagenicity of automotive particulate
exhaust: influence of fuel extenders, additives, and aromatic content.
In: MacFarland HN, Holdsworth CE, MacGregor JA, Call RW, & Lane ML ed.
Applied toxicology of petroleum hydrocarbons. Princeton, New Jersey,
Princeton Scientific Publishers, pp 109-122.
Clayton Environmental Consultants (1991) Gasoline vapor exposure
assessment for the American Petroleum Institute (API) (Project No.
31774.00). Cypress, California, Clayton Environmental Consultants, 139
pp.
Cline PV, Delfino JJ, & Rao SC (1991) Partitioning of aromatic
constituents into water from gasoline and other complex solvent
mixtures. Environ Sci Technol, 25: 914-920.
Cochrane RA & Hillman DE (1984) Direct gas chromatographic
determination of alcohols and methyl tert-butyl ether in gasolines
using infrared detection. J Chromatogr, 287: 197-201.
Conaway CC, Schroeder RE, & Snyder NK (1985) Teratology evaluation of
methyl tertiary butyl ether in rats and mice. J Toxicol Environ
Health, 16: 797-809.
Cook CK & Kovein RJ (1995) NIOSH health hazard evaluation report No.
94-0220-2526: Exxon Company, Houston, Texas, USA. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 28 pp.
Cox RA & Goldstone A (1982) Atmospheric reactivity of oxygenated motor
fuel additives. In: Proceedings of the 2nd European Symposium on the
Physico-chemical Behaviour of Atmospheric Pollutants, Varese, Italy,
29 September-1 October 1981. Dordrecht, D Riedel Publishing Co, pp
112-119.
Cuthbert JA (1979) Safety tests on methyl- tert-butyl-ether.
Edinburgh, Inveresk Research International, 18 pp (IRI Report No.
1300).
Dale MS, Losee RF, Crofts EW, & Davis MK (1997) MTBE: Occurrence and
fate in source-water supplies. J Am Chem Soc, 37: 376-377.
Daughtrey WC, Gill MW, Pritts IM, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Neurotoxicological evaluation of methyl tertiary-butyl ether in
rats. J Appl Toxicol, 17(suppl 1): S57-S64.
DECOS (Dutch Expert Committee on Occupational Standards) (1994)
Methyl-t-butylether: Health based recommended occupational exposure
limit. The Hague, Health Council of the Netherlands, 75 pp
(Publication No. 1994/23).
Delzer GC, Zogorski JS, Lopes TJ, & Bosshart RL (1996) Occurrence of
the gasoline oxygenate MTBE and BTEX compounds in urban stormwater in
the United States, 1991-95: US Geological Survey. Rapid City, South
Dakota, US Geological Survey, 6 pp (Water-Resources Investigations
Report No. 96-4145).
Derwent RG, Jenkin ME, & Sandow SM (1996) Photochemical ozone creation
potentials for a large number of reactive hydrocarbons under European
conditions. Atmos Environ, 30(2): 181-199.
Dey JC, Brown RA, & McFarland WE (1991) Methyl tert-butyl ether.
Hazard Mater Control, 4: 32-39.
Diehl JW, Finkbeiner JW, & Di Sanzo FP (1995) Determination of ethers
and alcohols in reformulated gasolines by gas chromatography/atomic
emission detection. J High Res Chromatogr, 18: 108-110.
Di Padova C, Di Padova F, Montorsi W, & Tritapepe R (1986) Methyl
tert-butyl ether fails to dissolve retained radiolucent common bile
duct stones. Gastroenterology, 91: 1296-1300.
Dodd DE & Kintigh WJ (1989) Methyl tertiary butyl ether (MTBE):
repeated (13-week) vapor inhalation study in rats with neurotoxicity
evaluation. Export, Pennsylvania, Bushy Run Research Center, 502 pp
(Report No. 52-507 to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Drivas PJ, Valberg PA, & Gauthier TD (1991) Health assessment of air
toxics emissions from alternative fuels. Paper presented at the 84th
Annual Meeting of the Air and Waste Management Association, Vancouver,
British Columbia, 16-21 June 1991. Washington, DC, Air and Waste
Management Association, 17 pp.
Du JT, Abernathy CO, Donohue J, Mahfouz A, & Khanna K (1998) A
drinking water advisory: consumer acceptability and advice and health
effects analysis on methyl tertiary-butyl ether (MTBE). Toxicologist,
42(1-S): A 1123.
Duffy LK (1994) Oxyfuel in Alaska: use of interleukins to monitor
effects on the immune system. Sci Total Environ, 151: 253-256.
ECETOC (1997) Methyl tert-butyl ether (MTBE): Health risk
characterization. Brussels, European Centre for Ecotoxicology and
Toxicology of Chemicals, 126 pp (Technical Report No. 72).
Environment Canada (1989) Analysis of shellfish for organic and
inorganic contaminants. Dartmouth, Nova Scotia, Environment Canada
Conservation and Protection, 10 pp (Report No. AN 893118).
Environment Canada (1992) Canadian Environmental Protection Act -
Priority substances list: Methyl tertiary-butyl ether. Ottawa,
Minister of Supply and Services, 19 pp (Assessment Report No. 5).
Environment Canada (1993) Canadian Environmental Protection Act -
Priority substances list: Supporting document methyl tertiary butyl
ether. Hull, Quebec, Environment Canada, 49 pp.
Environment Canada (1996a) Canadian Environmental Protection Act -
National pollutant release inventory: Summary report 1994. Hull,
Quebec, Environment Canada, 143 pp.
Environment Canada (1996b) MTBE concentrations at selected locations:
National air pollution surveillance programme 1995-1996. Hull, Quebec,
Environment Canada, 21 pp.
Erdal S, Gong H Jr, Linn WS, & Rykowski R (1997) Projection of health
benefits from ambient ozone reduction related to the use of methyl
tertiary butyl ether (MTBE) in the reformulated gasoline program.
Risk Anal, 17: 693-704.
Esch O, Spinosa JC, Hamilton RL, Crombie DL, Schteingart CD, Rondinone
JF, DAgostino HB, Lillienau J, & Hofmann AF (1992a) Acute effects of
topical methyl tert-butyl ether or ethyl propionate on gallbladder
histology in animals: a comparison of two solvents for contact
dissolution of cholesterol gallstones. Hepatology, 16(4): 984-991.
Esch O, Schteingart CD, Pappert D, Kirby D, Streich R, & Hofmann AF
(1992b) Increased blood levels of methyl tert-butyl ether but not of
ethyl propionate during instillation with contact gallstone
dissolution agents in the pig. Hepatology, 18(2): 373-379.
Ferdinandi ES, Houle J-M, Lalande M, Provencher A, & St-Onge Brault G
(1990a) Pharmacokinetics of methyl tert-butyl ether (MTBE) and
tert-butyl alcohol (TBA) in male and female Fischer-344 rats after
administration of MTBE by the intravenous, oral and dermal routes.
Montreal, Bio-Research Laboratories Ltd, 98 pp (Report No. 38842 to
the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lalande M, Houle J-M, Provencher A, & Ducharme S
(1990b) Mass balance of radioactivity and metabolism of methyl
ter-butyl ether (MTBE) in male and female Fischer 344 rats after
intravenous, oral and dermal administration of 14C-MTBE. Montreal,
Bio-Research Laboratories Ltd, 127 pp (Report No. 38843 to the MTBE
Task Force, Washington, DC).
Ferdinandi ES, Houle J-M, Lalande M, Lulham G, Pilon D, Provencher A,
Labbé R, & St-Onge Brault G (1990c) Pharmacokinetics of methyl
tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) in male and
female Fischer-344 rats after single and repeat inhalation nose-only
exposures to MTBE. Montreal, Bio-Research Laboratories Ltd, 172 pp
(Report No. 38844 to the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lulham G, Pilon D, Labbé R, Lalande M, Provencher A,
Houle J-M, & Ducharme S (1990d) Disposition of radioactivity and
metabolism of methyl tert-butyl ether (MTBE) in male and female
Fischer 344 rats after nose-only inhalation exposure to 14C-MTBE.
Montreal, Bio-Research Laboratories Ltd, 89 pp (Report No. 38845 to
the MTBE Task Force, Washington, DC).
Fujiwara Y, Kinoshita T, Sato H, & Kojima I (1984) [Biodegradation and
bioconcentration of alkyl ethers.] Yukagatu, 33(2): 111-114 (in
Japanese with tables and abstract in English).
Garrett P, Moreau M, & Lowry JD (1986) MTBE as a groundwater
contaminant. In: Proceedings of the 1986 National Water Well
Association and American Petroleum Institute Conference on Petroleum
and Organic Chemicals in Groundwater, Houston, Texas, 12-14 November
1986. Dublin, Ohio, National Water Well Association, pp 227-238.
Geiger DL, Call DJ, & Brooks LT (1988) Acute toxicities of organic
chemicals to Fathead Minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 4, pp 75-76.
Giacomello P (1996) MTBE exposure in service stations: Italian study.
Paper presented at the 7th European Fuel Oxygenates Association
Conference, Sodehotel, 24-25 October 1996. Brussels, European Chemical
Industries Federation, 8 pp.
Gill MW (1989) Methyl tertiary butyl ether single exposure vapor
inhalation neurotoxicity study in rats. Export, Pennsylvania, Bushy
Run Research Center, 116 pp (Report No. 52-533 to the Methyl Tertiary
Butyl Ether Committee, Washington, DC).
Gillie AD (1993) Industrial hygiene monitoring methyl tertiary butyl
ether, benzene and gasoline. San Diego, California, Texaco Inc.,
Environmental Health and Safety Division, Industrial Hygiene, 31 pp.
Gordian ME & Guay G (1995) Benzene in Alaska. Alsk Med, 37: 25-28, 36.
Gordian ME, Huelsman MD, Brecht M-L, & Fisher DG (1995) Health effects
of methyl tertiary butyl ether (MTBE) in gasoline in Alaska. Alsk Med,
37: 101-103, 119.
Greenough RJ, McDonald P, Robinson P, Cowie JR, Maule W, Macnaughton
F, & Rushton A (1980) Methyl tertiary-butyl ether (Driveron): three
month inhalation toxicity in rats (Project No. 413038). Edinburgh,
Scotland, Inveresk Research International, 230 pp.
Grindstaff G, Henry M, Hernandez O, Hogan K, Lai D, & Siegel-Scott C
(1991) Formaldehyde risk assessment update. Washington DC, US
Environmental Protection Agency, Office of Toxic Substances, 140 pp.
Gupta G & & Lin YJ (1995) Toxicity of methyl tertiary butyl ether to
Daphnia magna and Photobacterium phosphoreum. Bull Environ Contam
Toxicol, 55: 618-620.
Hakkola M & Saarinen L (1996) Exposure of tanker drivers to gasoline
and some of its components. Ann Occup Hyg, 40: 1-10.
Hakkola M, Honkasalo ML, & Pulkkinen P (1996) Neuropsychological
symptoms among tanker drivers exposed to gasoline. J Occup Med, 46:
124-130.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, & Valcovic
LR (1993) Hazard evaluation of chemicals that cause accumulation of
alpha-globulin, hyaline droplet nephropathy, and tubule neoplasia in
the kidneys of male rats. Environ Health Perspect, 99: 313-349.
Harper M & Fiore AA (1995) Determination of methyl tert-butyl ether
in air using a diffusive sampler. Appl Occup Environ Hyg, 10: 616-619.
Hartle R (1993) Exposure to methyl tert-butyl ether and benzene
among service station attendants and operators. Environ Health
Perspect, 101(suppl 6): 23-26.
Haseman JK, Arnold J, & Eustis SL (1990) Tumor incidences in Fischer
344 rats: NTP historical data. In: Pathology of the Fischer rat:
Reference and atlas. McLean, Virginia, Academic Press, chapter 35, pp
555-564.
Hazleton Laboratories (1979) Acute eye irritation study in rabbits:
tert-butyl methyl ether (95% pure). Leesburg, Virginia, Hazleton
Laboratories America, Inc., 14 pp (Report No. 2024-132).
Hebert RS (1993) Industrial hygiene exposure assessment for methyl
tert-butyl ether (MTBE), benzene and gasoline. Houston, Texas, Star
Enterprise Environment, Health and Safety Department, 36 pp.
HEI (1996) The potential health effects of oxygenates added to
gasoline: a review of the current literature. A special report of the
Institute's oxygenates evaluation committee. Cambridge, Massachusetts,
Health Effects Institute, 158 pp.
Hinton J (1993) Occupational exposures. Presented at the MTBE
Conference in Washington, DC, 26-27 July 1993. Beacon, New York,
Texaco Inc., 37 pp.
Hoekman SK (1992) Speciated measurements and calculated reactivities
of vehicle exhaust emissions from conventional and reformulated
gasolines. Environ Sci Technol, 26: 1206-1216.
Hoekman SK (1993) Improved gas chromatography procedure for speciated
hydrocarbon measurements of vehicle emissions. J Chromatogr, 639:
239-253.
Hong J-Y, Yang CS, Lee M, Wang Y-Y, Huang WQ, Tan y, Patten CJ, &
Bondoc FY (1997) Role of cytochromes P450 in the metabolism of methyl
tert-butyl ether in human livers. Arch Toxicol, 71: 266-269.
Hong J-Y, Yang CS, Wang Y-Y, Bondoc FY, Pan J, Cokonis C, & Bao ZP
(1998) Use of CYP2E1 knock-out mice to assess the role of CYP2E1 in
metabolizing methyl tert-butyl ether (MTBE) and other gasoline ethers.
Toxicologist, 42(1-S): A87.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, Michigan, Lewis
Publishers, Inc., pp 653-654.
Hsieh CR & Ouimette JR (1994) Comparative study of multimedia modeling
for dynamic partitioning of fossil fuels-related pollutants. J Hazard
Mater, 37: 489-505.
Huber AH (1995) Human exposure estimates of methyl tertiary butyl
ether (MTBE). Presentation at Conference on MTBE and Other Oxygenates:
A Research Update, Falls Church, Virginia, 26-28 July 1993.
Washington, DC, National Technical Information Service, 32 pp (EPA
/600/A-94/255; NTIS/PB95-177150).
IITRI (1992) 28-day oral (gavage) toxicity of methyl tert-butyl
ether (MTBE) in rats (Project No. L08100). Chicago, Illinois, Illinois
Institute of Technology Research, 48 pp.
Imbriani M, Ghittori S, & Pezzagno G (1997) Partition coefficients of
methyl tert-butyl ether. G Ital Med Lav Ergon, 19: 63-65.
Industrial Bio-Test Laboratories (1969) Acute toxicity studies on
X-801-25. Northbrook, Illinois, Industrial Bio-Test Laboratories, 27
pp (Report No. A6809 to Sun Oil Company).
Japar SM, Wallington TJ, Richert JFO, & Ball JC (1990) The atmospheric
chemistry of oxygenated fuel additives: t-butyl alcohol, dimethyl
ether, and methyl t-butyl ether. Int J Chem Kinet, 22: 1257-1269.
Japar SM, Wallington TJ, Rudy SJ, & Chong TY (1991) Ozone-forming
potential of a series of oxygenated organic compounds. Environ Sci
Technol, 25: 415-420.
Johansen NG (1984) The analysis of C1-C4 alcohols, MTBE, and DIPE in
motor gasolines by multi-dimensional capillary column gas
chromatography. J High Res Chromatogr Chromatogr Commun, 7: 487-489.
Johanson G, Nihlén A, & Löf, A (1995) Toxicokinetics and acute effects
of MTBE and ETBE in male volunteers. Toxicol Lett, 82/83: 713-718.
Johnson T, McCoy M, & Wisbith T (1995) A study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 108 pp (API Publication No. 4619).
Katoh T, Arashidani K, Kikuchi M, Yoshikawa M, & Kodama Y (1993)
Effects of methyl tertiary butyl ether on hepatic lipid peroxidation
in mice. Jpn J Hyg, 48: 373-378.
Kelly TJ, Callahan PJ, Piell J, & Evans GF (1993) Method development
and field measurements for polar volatile organic compounds in ambient
air. Environ Sci Technol, 27: 1146-1153.
Kirchstetter TW, Singer BC, Harley RA, Kendall GR, & Chan W (1996)
Impact of oxygenated gasoline use on California light-duty vehicle
emissions. Environ Sci Technol, 30: 661-670.
Levy JM & Yancey JA (1986) Dual capillary gas chromatographic analysis
of alcohols and methyl tert-butyl ether in gasolines. J High Res
Chromatogr Chromatogr Commun, 9: 383-387.
Lewis RJ (1993) Methyl -tert-butyl ether (MTBE). In: Hawley's
condensed chemical dictionary, 12th ed. New York, Van Nostrand
Reinhold Co., p 760.
Lindamood C, Farnell DR, Giles HD, Prejean JD, Collins JJ, Takahashi
K, & Maronpot RR (1992) Subchronic toxicity studies to t-butyl
alcohol in rats and mice. Fundam Appl Toxicol, 19: 91-100.
Lindstrom AB & Pleil JD (1996) Alveolar breath sampling and analysis
to assess exposure to methyl tertiary butyl ether (MTBE) during motor
vehicle refuelling. J Air Waste Manage Assoc, 46: 676-682.
Lington AW, Dodd DE, Ridlon SA, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Evaluation of 13-week inhalation toxicity study on methyl
t-butyl ether (MTBE) in Fischer 344 rats. J Appl Toxicol, 17 (suppl
1): S37-S44.
Lioy PJ, Weisel CP, Jo WK, Pellizzari E, & Raymer JH (1994)
Microenvironmental and personal measurements of methyl-tertiary butyl
ether (MTBE) associated with automobile use activities. J Expo Anal
Environ Epidemiol, 4(4): 427-444.
Litton Bionetics (1980) Guinea pig sensitization study (TBME-99).
Kensington, Maryland, Litton Bionetics, Inc., 30 pp (Report No.
22011).
Lyman WJ, Reehl WF, & Rosenblatt DH (1990) Handbook of organic
chemical property estimation methods: Environmental behavior of
organic compounds. Washington, DC, American Chemical Society, 960 pp.
McGahan JP, Tesluk H, Brock JM, Johnston R, & Shaul DB (1988)
Dissolution of gallstones using methyl tertiary-butyl ether in an
animal model. Invest Radiol, 23: 599-603.
Mackay D, Shiu WY, & Ma KC (1993) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals - volume 3: Volatile organic chemicals. Boca Raton, Florida,
Lewis Publishers, 916 pp.
Mackerer CR, Angelosanto FA, Blackburn GR, & Schreiner CA (1996)
Identification of formaldehyde as the metabolite responsible for the
mutagenicity of methyl tertiary-butyl ether in the activated mouse
lymphoma assay. Proc Soc Exp Biol Med, 212: 338-341.
Marsh DF & Leake CD (1950) The comparative anesthetic activity of the
aliphatic ethers. Anesthesiology, 11: 455-463.
Mihelcic JR (1990) Modeling the potential effect of additives on
enhancing the solubility of aromatic solutes contained in gasoline.
Ground Water Monit Rev, 10: 132-137.
Miller MJ, Ferdinandi ES, Andrews LS, Douglas JF, & Kneiss JJ (1997)
Pharmacokinetics and disposition of methyl t-butyl ether in
Fischer-344 rats. J Appl Toxicol, 17(suppl 1): S3-S12.
Mo K, Lora CO, Javanmardian M, Yang X, & Kulpa CF (1997)
Biodegradation of methyl t-butyl ether by pure bacterial cultures.
Appl Microbiol Biotechnol, 47: 69-72.
Mobil Oil Corporation (1993) Activated mouse lymphoma (L5178Y/TK/+/-)
mutagenicity assay supplemented with formaldehyde dehydrogenase for
methyl tertiary butyl ether. Princeton, New Jersey, Mobil Oil
Corporation, 17 pp (Status report No. 65579).
Mohr SN, Fiedler N, Weisel C, & Kelly-McNeil K (1994) Health effects
of MTBE among New Jersey garage workers. Inhal Toxicol, 6(6): 553-562.
Möller Jensen H & Arvin E (1990) Solubility and degradability of the
gasoline additive MTBE, methyl tert-butyl ether, and gasoline
compounds in water. In: Arendt F, Hinsewald M, & van der Brink WJ ed.
Contaminated soil 90. Dodrecht, Kluwer Academic Publishers, pp
445-448.
Moolenaar RL, Hefflin BJ, Ashley DL, Middaugh JP, & Etzel RA (1994)
Methyl tertiary butyl ether in human blood after exposure to
oxygenated fuel in Faibanks, Alaska. Arch Environ Health, 49(5):
402-409.
Mormile MR, Liu S, & Suflita JM (1994) Anaerobic biodegradation of
gasoline oxygenates: Extrapolation of information to multiple sites
and redox conditions. Environ Sci Technol, 28: 1727-1732.
Moser GJ, Wong BA, Wolf DC, Moss OR, & Goldsworthy TL (1996a)
Comparative short-term effects of methyl tertiary butyl ether and
unleaded gasoline vapor in female B6C3P1 mice. Fundam Appl Toxicol,
31: 173-183.
Moser GJ, Wong BA, Wolf DG, Fransson-Steen RL, & Goldsworthy TL
(1996b) Methyl tertiary butyl ether lacks tumor-promoting activity on
N-nitosodiethylamine-initiated B6C3F1 female mouse liver.
Carcinogenesis, 17: 2753-2761.
Munch JW & Eichelberger JW (1992) Evaluation of 48 compounds for
possible inclusion in U.S. EPA Method 524.2, Revision 3.0: expansion
of the method analyte list to a total of 83 compounds. J Chromatogr
Sci, 30: 471-477.
Murray WR, Laferla G, & Fullarton GM (1988) Choledolithiasis -
in vivo stone dissolution using methyl tertiary butyl ether (MTBE).
Gut, 29: 143-145.
Neeper-Bradley TL (1991) Two-generation reproduction study of inhaled
methyl tertiary butyl ether in CD (Sprague-Dawley) rats. Export,
Pennsylvania, Bushy Run Research Center, 499 pp (Report No. 53-594 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
Nihlén A, Löf A, & Johanson G (1995) Liquid/air partition coefficients
of methyl and ethyl t-butyl ethers, t-amyl methyl ether, and t-butyl
alcohol. J Expo Anal Environ Epidemiol, 5(4): 573-582.
Nihlén A, Löf A, & Johanson G (1998a) Experimental exposure to methyl
tertiary-butyl ether: I. Toxicokinetics in humans. Toxicol Appl
Pharmacol, 148: 281-287.
Nihlén A, Wålinder R, Löf A, & Johanson G (1998b) Experimental
exposure to methyl tertiary-butyl ether: II. Acute effects in humans.
Toxicol Appl Pharmacol, 148: 281-287.
Novak JT, Yeh C, Gullic D, Eichenberger J, & Benoit RE (1992). The
influence of microbial ecology on subsurface degradation of petroleum
contaminants. Blacksburg, Virginia, Virginia Polytechnic Institute and
State University, Water Resources Research Center, 86 pp (Bulletin No.
177).
Pankow JF, Rathbun RE, & Zogorski JS (1996) Calculated volatilization
rates of fuel oxygenate compounds and other gasoline-related compounds
from rivers and streams. Chemosphere, 33: 921-937.
Paulov S (1987) [Action of the anti-detonation preparation tert-butyl
methyl ether on the model species Rana temporaria L.] Biologia, 42:
185-189 (in Slovak with English abstract).
Pauls RE (1985) Determination of high octane components: methyl
t-butyl ether, benzene, toluene, and ethanol in gasoline by liquid
chromatography. J Chromatogr Sci, 23: 437-441.
Pekari K, Riihimaki V, Vainiotalo S, Teräväinen E, & Aitio A (1996)
Experimental exposure to methyl- tert-butyl ether (MTBE) and
methyl- tert-amyl ether (MTAE). In: Proceedings of the International
Symposium on Biological Monitoring in Occupational and Environmental
Health, Espoo, Finland, 11-13 September 1996. Helsinki, Institute of
Occupational Health, pp 27-28.
Pence VH (1987a) The evaluation of the biodegradation of 606610 using
a modified closed bottle method. Miamiville, Ohio, Hill Top Biolabs,
Inc., 92 pp (Report No. 87-0479-11, prepared for ARCO Chemical
Company).
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Poet TS & Borghoff SJ (1997) In vitro uptake of methyl tert-butyl
ether in male rat kidney: Use of a two-compartment model to describe
protein interactions. Toxicol Appl Pharmacol, 145: 340-348.
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Post G (1994) Methyl tertiary butyl ether: health-based maximum
contaminant level document. Trenton, New Jersey, New Jersey Department
of Environmental Protection, Division of Sciences and Research.
Poulsen M, Lemon L, & Barker JF (1992) Dissolution of monoaromatic
hydrocarbons into groundwater from gasoline-oxygenate mixtures.
Environ Sci Technol, 26: 2483-2489.
Prah JD, Goldstein GM, Devlin R, Otto D, Ashley D, House D, Cohen KL,
& Gerrity T (1994) Sensory, symptomatic, inflammatory, and ocular
responses to and the metabolism of methyl tertiary butyl ether in a
controlled human exposure experiment. Inhal Toxicol, 6(6): 521-538.
Prakash CB (1989) Motor vehicle emissions from gasoline containing
MTBE. Ottawa, Ontario, Environment Canada, Environmental Protection
Directorate, Conservation and Protection, Industrial Programs Branch,
25 pp (Report No. IP-97).
Prescott-Mathews JS, Wolf DC, Wong BA, & Borghoff SJ (1997) Methyl
tert-butyl ether causes alpha2u-globulin nephropathy in male
Fischer-344 rats. Toxicol Appl Pharmacol, 143: 301-314.
Raese JW, Rose DL, & Sandstrom MW (1995) US Geological Survey
laboratory method for methyl tert-butyl ether and other fuel
oxygenates. Rapid City, South Dakota, US Geological Survey, 4 pp (US
Geological Survey Fact Sheet FS-219-95).
Rao HV & Ginsberg GL (1997) Physiologically-based pharmacokinetic
(PBPK) model assessment of methyl t-butyl ether (MTBE) in groundwater
for a bathing and showering determination. Risk Anal, 17: 583-598.
Riihimaki V, Matikainen E, Akila R, Teräväinen E, Mutanen P, Pekari K,
Vainiotalo S, Vilhunen R, & Aitio A (1996) Central nervous system
effects of the gasoline additive methyl- tert-butyl ether (MTBE). In:
Proceedings of the Third International Symposium on Biological
Monitoring in Occupational and Environmental Health, Espoo, Finland,
11-13 September 1996. Helsinki, Institute of Occupational Health, p
75.
Robinson M, Bruner RH, & Olson GR (1990) Fourteen- and ninety-day oral
toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley
rats. J Am Coll Toxicol, 9(5): 525-540.
Roe VD, Lacy MJ, Stuart JD, & Robbins GA (1989) Manual headspace
method to analyze for the volatile aromatics of gasoline in
groundwater and soil samples. Anal Chem, 61: 2584-2585.
Rousch JM & Sommerfeld MR (1998) Liquid-gas partitioning of the
gasoline oxygenate methyl tert-butyl ether (MTBE) under laboratory
conditions and its effect on growth of selected algae. Arch Environ
Contam Toxicol, 34: 6-11.
Salanitro JP, Diaz LA, Williams MP, & Wisniewski HL (1994) Isolation
of a bacterial culture that degrades methyl t-butyl ether. Appl
Environ Microbiol, 60: 2593-2596.
Savolainen H, Pfäffli P, & Elovaara E (1985) Biochemical effects of
methyl tertiary-butyl ether in extended vapour exposure of rats.
Arch Toxicol, 57: 285-288.
Schroll R, Bierling B, Cao G, Dörfler U, Lahaniati M, Langenbach T,
Scheunert I, & Winkler R (1994) Uptake pathways of organic chemicals
from soil by agricultural plants. Chemosphere, 28: 297-303.
Schuberth J (1996) A full evaporation headspace technique with
capillary GC and ITD: a means for quantitating volatile organic
compounds in biological samples. J Chromatogr Soc, 34: 314-319.
Schuetzle D, Siegl WO, Trescott EJ, Jensen TE, Dearth MA, Kaiser EW,
Gorse R, Kreucher W, & Kulik E (1994) The relationship between
gasoline composition and vehicle hydrocarbon emissions: A review of
current studies and future research needs. Environ Health Perspect,
102(suppl 4): 3-12.
Seeberg AH (1989) Unscheduled DNA synthesis (UDS) in primary rat
hepatocytes (autoradiographic method). Test substance: MTBE. Rome,
Life Science Research, Toxicology Centre, 79 pp (LSR-RTC Report No.
216003-M-03689).
Sernau RC (1989) Mutagenicity test on methyl tertiary butyl ether
Drosophila melanogaster sex-linked recessive test (Study No.
10484-0-461). Kensington, Maryland, Hazleton Laboratories America,
Inc., 23 pp (Report to the Methyl Tertiary Butyl Ether Toxicology
Committee, Washington, DC).
Simer MM (1986) Texaco Chemical Corporation - Safety (SAF) studies,
surveys and reports: methanol, tertiary butyl alcohol, 1,3-butadiene,
methyl tertiary butyl ether, total other hydrocarbons. Port Neches,
Texas, Texaco Chemical Corporation, 15 pp.
Smith Sl & Duffy LK (1995) Odor and health complaints with Alaskan
gasolines. Chem Health Saf, 2: 32-38.
Smith DF, Kleindienst TE, Hudgens EE, McIver CD & Bufalini JJ (1991)
The photooxidation of methyl tertiary butyl ether. Int J Chem Kinet,
23: 907-924.
Snamprogetti (1980) MTBE toxicological data book. Rome, Snamprogetti
Engineering Company S.p.A., 202 pp (Report No. LICE/0086/AA/1g).
SNV (1993) [Better environmental qualities in petrol - a proposal for
environmental classification.] Stockholm, National Environmental
Protection Agency, 69 pp (in Swedish).
Spitzer HL (1997) An analysis of health benefits associated with the
use of MTBE reformulated gasoline and oxygenated fuels in reducing
atmospheric concentrations of selected volatile organic compounds.
Risk Anal, 17: 683-691.
Squillace PJ, Pope DA, & Price CV (1995a) Occurrence of the gasoline
additive MTBE in shallow ground water in urban and agricultural areas.
Rapid City, South Dakota, US Geological Survey, 4 pp (US Geological
Survey Fact Sheet FS-114-95).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1995b) A preliminary
assessment of the occurrence and possible sources of MTBE in ground
water of the United States, 1993-94. Rapid City, South Dakota, US
Geological Survey, 29 pp (File Report No. 95-456).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1996) Preliminary
assessment of occurrence and possible sources of MTBE in groundwater
in the United States, 1993-1994. Environ Sci Technol, 30: 1721-1730.
Stackelberg PE, O'Brien AK, & Terracciano SA (1997) Occurrence of MTBE
in surface and ground water, Long Island, New York and New Jersey.
Proceedings of American Chemical Society meeting, San Francisco,
California, 16-17 April 1996. Washington, DC, American Chemical
Society, 4 pp.
Standeven AM & Goldsworthy TL (1993) Promotion of preneoplastic
lesions and induction of CYP2B by unleaded gasoline vapor in female
B6C3F1 mouse liver. Carcinogenesis, 14: 2137-2141.
State of Connecticut (1987) The incidence of MTBE in Connecticut.
Hertford, Connecticut, Department of Health Services, 3 pp (Document
No. FYI-OTS 0987-0574).
Steffan RJ, McClay K, Vainberg S, Condee CW, & Zhang D (1997)
Biodegradation of the gasoline oxygenates methyl tert-butyl ether,
ethyl tert-butyl ether, and tert-amyl methyl ether by propane
oxidising bacteria. Appl Environ Microbiol, 63: 4216-4222.
Stern BR & Tardiff RG (1997) Risk characterization of methyl
tertiary butyl ether (MTBE) in tap water. Risk Anal, 17: 727-743.
Sternal RS & Davis MA (1992) In vitro dissolution of cholesterol
gallstones. Invest Radiol, 27(12): 1040-1043.
Storck WJ, Layman PL, Reisch MS, Thayer AM, Kirschner EM, Peaff G, &
Tremblay J-F (1996) Facts and figures for the chemical industry. Chem
Eng News, June 24: 38-46.
Streete PJ, Ruprah M, Ramsey JD, & Flanagan RJ (1992) Detection and
identification of volatile substances by headspace capillary gas
chromatography to aid diagnosis of acute poisoning. Analyst, 117:
1111-1127.
Suflita JM & Mormile MR (1993) Anaerobic biodegradation of known and
potential gasoline oxygenates in the terrestrial subsurface. Environ
Sci Technol, 27: 976-978.
Swenberg JA & Dietrich DR (1991) Immunohistochemical localization of
alpha 2µ-globulin in kidneys of treated and control rats of a 13-week
vapor inhalation study undertaken with methyl tertiary butyl ether.
Washington, DC, Synthetic Organic Chemical Manufacturers Association,
5 pp (Report to the MTBE Health Effects Testing Task Force,
Washington, DC).
Tarkpea M & Svanberg O (1982) The acute toxicity of motor fuels to
brackish water organisms. Mar Pollut Bull, 13(4): 125-127.
Tepper JS, Jackson MC, McGee JK, Costa DL, & Graham JA (1994)
Estimation of respiratory irritancy from inhaled methyl tertiary butyl
ether in mice. Inhal Toxicol, 6(6): 563-569.
Texaco (1993) Industrial hygiene air sampling results: Guatemala
Refinery - methyl tert-butyl ether (MTBE). Beacon, New York, Texaco
Inc., Environment Health and Safety Division, 3 pp.
THE (1989) Biomonitoring results using MTBE. Wheat Ridge, Colorado,
THE Consultants, 8 pp.
TRC (1993) Final report to ARCO Chemical Company on the odor and taste
threshold studies performed with methyl tertiary-butyl ether (MTBE)
and ethyl tertiary-butyl ether (ETBE). Windsor, Connecticut, TRC
Environmental Corporation, 37 pp.
Tuazon EC, Carter WPL, Aschmann SM, & Atkinson R (1991) Products of
the gas-phase reaction of methyl tert-butyl ether with the OH
radical in the presence of NOx. Int J Chem Kinet, 23: 1003-1015.
Tyl RW (1989) Developmental toxicity study of inhaled methyl tertiary
butyl ether in New Zealand White rabbits. Export, Pennsylvania, Bushy
Run Research Center, 260 pp (Report No. 51-628 to the Methyl Tertiary
butyl Ether Toxicology Committee, Washington, DC).
Tyl RW & Neeper-Bradley TL (1989) Developmental toxicity study of
inhaled methyl tertiary butyl ether in CD-1 mice. Export,
Pennsylvania, Bushy Run Research Center, 342 pp (Report No. 52-526 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
US EPA (1989) Chemical rate constants for superfund health evaluation
manual chemicals. Washington, DC, US Environmental Protection Agency,
pp 645-646 (Report No. 68-02-4254).
US Interagency Assessment (1997) Interagency assessment of oxygenated
fuels. Washington, DC, National Science and Technology Council, 256
pp.
Vainiotalo S, Peltonen Y, & Pfäffli P (1996) MTBE exposure in service
stations. Paper presented at the 7th European Fuel Oxygenates
Association Conference, Sodehotel, 24-25 October 1996. Brussels,
European Chemical Industries Federation, 7 pp.
Veith GD & Kosian P (1983) Estimating bioconcentration potential from
octanol/water partition coefficients. In: Mackay D, Paterson S,
Eisenreich SJ, & Simmons MS ed. Physical behavior of PCBs in the Great
Lakes. Ann Arbor, Michigan, Ann Arbor Science Publishers, Chapter 15,
pp 269-282.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: narcotic industrial
chemicals. Can J Fish Aquat Sci, 40(6): 743-748.
Verga GR, Sironi A, Schneider W, & Frohne JCh (1988) Selective
determination of oxygenates in complex samples with the O-FID analyze.
J High Res Chromatogr Chromatogr Commun, 11: 248-252.
Vergnes JS & Chun JS (1994) Methyl tertiary butyl ether: in vivo-
in vitro hepatocyte unscheduled DNA synthesis assay in mice
(Laboratory project ID 93N1316). Export, Pennsylvania, Bushy Run
Research Center, 67 pp (Report to the MTBE Effects Testing Task Force,
Washington, DC).
Vergnes JS & Kintigh WJ (1993) Methyl tertiary butyl ether: bone
marrow micronucleus test in mice (Laboratory project ID 93N1244).
Export, Pennsylvania, Bushy Run Research Center, 99 pp (Report to the
MTBE Effects Testing Task Force, Washington, DC).
Vergnes JS & Morabit ER (1989) Methyl tertiary butyl ether repeated
exposure vapor inhalation study in rats: in vivo cytogenetic
evaluation. Export, Pennsylvania, Bushy Run Research Center, 40 pp
(Report No. 51-635 to the MTBE Effects Testing Task Force, Washington,
DC).
Wallington TJ, Dagaut P, Liu R, & Kurylo MJ (1988) Gas-phase reactions
of hydroxyl radicals with the fuel additives methyl tert-butyl ether
and tert-butyl alcohol over the temperature range 240-440 K. Environ
Sci Technol, 22(7): 842-844.
Wallington TJ, Andino JM, Skewes LM, Siegl WO, & Japar SM (1989)
Kinetics of the reaction of OH radicals with a series of ethers under
simulated atmospheric conditions at 295 K. Int J Chem Kinet, 21:
993-1001.
Ward JB, Au WW, Whorton EB, & Legator MS (1994) Genetic toxicology of
methyl tertiary butyl ether. Galveston, Texas, University of Texas, pp
57-134 (Final Report to the Agency for Toxic Substances and Disease
Registry).
Watson JG, Chow JC, Pritchett LC, Houck JA, Ragazzi RA, & Burns S
(1990) Chemical source profiles for particulate motor vehicle exhaust
under cold and high altitude operationg conditions. Sci Total Environ,
93: 183-190.
White MC, Johnson CA, Ashley DL, Buchta TM, & Pelletier DJ (1995)
Exposure to methyl tertiary-butyl ether from oxygenated gasoline in
Stamford, Connecticut. Arch Environ Health, 50: 183-189.
Wibowo AAE (1994) DEFOS (Dutch Expert Committee for Occupational
Standards) and NEG (Nordic Expert Group for Criteria Documentation of
Health Risks from Chemicals) basis for an occupational standard:
methyl-tert-butyl-ether. Solna, Sweden, National Institute of
Occupational Health, 21 pp.
Windholz M ed. (1983) The Merck index: an encyclopedia of chemicals,
drugs, and biologicals, 10th ed. Rahway, New Jersey, Merck and Co.,
Inc., p 865.
Yeh CK & Novak JT (1994) Anaerobic biodegradation of gasoline
oxygenates in soils. Water Environ Res, 66: 744-752.
Yoshikawa M, Arashidani K, Katoh T, Kawamoto T, & Kodama Y (1994)
Pulmonary elimination of methyl tertiary-butyl ether after
intraperitoneal administration in mice. Arch Toxicol, 68(8): 517-519.
Zogorski JS, Morduchowitz A, Baehr AL, Bauman BJ, Drew RT, Korte NE,
Lepham WW, Pankow JF, & Washington ER (1996) Fuel oxygenates and water
quality: Current understanding of sources, occurrence in natural
waters, environmental behavior, fate, and significance. Washington,
DC, Office of Science and Technology Policy, 91 pp (Final report
prepared for the Interagency Oxygenated Fuel Assessment).
Zweidinger RB (1993) Air quality measurements in Fairbanks, Stamford,
and Albany. Research Triangle Park, North Carolina, United States
Environmental Protection Agency, Atmospheric Research and
Environmental Assessment Laboratory, 19 pp (Report ACC-1539).
RÉSUMÉ
Le méthyltertiobutyléther (MTBE) est actuellement le plus utilisé
des éthers que l'on peut employer comme additifs de l'essence.
L'éthyltertiobutyléther (ETBE), le tertioamylméthyléther (TAME), le
tertioamyléthyléther (TAEE) et le diisopropyléther (DIPE), entre
autres, peuvent être ajoutés ou substitués au MTBE afin d'améliorer
l'oxygénation et l'indice d'octane, aussi peut-on en trouver à côté du
MTBE.
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le MTBE est un composé volatil et incolore, à l'odeur terpénique,
qui est liquide à la température ambiante. Sa viscosité est faible et
son point d'ébullition est de 55,2°C. Son point de congélation est de
-109°C. Sa densité est de 0,7404 à 20°C. Sa tension de vapeur est
relativement élevée: 33 500 Pa à 25°C. C'est une substance inflammable
qui peut en outre former des mélanges explosifs avec l'air. Il est
très soluble dans les autres éthers et dans l'alcool. Miscible à
l'essence, il est également soluble dans l'eau (42 000 g/m3 à
19,8°C). Son coefficient de partage entre l'octanol et l'eau (log
Kow) est de 0,94-1,3. Il est instable en solution acide.
La recherche et le dosage du MTBE se font dans tous types de
matrices par chromatographie en phase gazeuse au moyen de détecteurs
et de colonnes capillaires adaptés à la matrice en cause. On a
également recours à la chromatographie liquide à phases inversées pour
l'analyse des échantillons d'essence. On utilise aussi, pour la
préparation des échantillons d'air, d'eau et de sédiments ou encore
des échantillons biologiques, divers systèmes de purge et de piégeage,
la sorption/désorption et des méthodes basées sur l'espace de tête.
2. Sources d'exposition humaine et environnementale
Autant qu'on sache, le MTBE n'existe pas à l'état naturel. Dans
l'industrie, on l'obtient par l'action du méthanol sur l'isobutylène
en présence d'un catalyseur. Un certain nombre de pays le produisent
en quantités croissantes depuis la fin des années 70. Il compte
actuellement parmi les 50 produits chimiques dont le volume de
production est le plus élevé. En 1996, la capacité de production des
Etats-Unis était de 10,6 millions de tonnes et on estime que la
demande de MTBE va encore augmenter. Environ 25% de l'essence vendue
aux Etats-Unis est additionnée de MTBE. On l'utilise presque
exclusivement pour améliorer l'indice d'octane et accroître la teneur
de l'essence en oxygène. On en ajoute à l'essence jusqu'à 17% en
volume.
3. Transport, distribution et transformation dans l'environnement
Une fois libéré dans l'air, le MTBE y reste en majeure partie
avec seulement de petites quantités qui passent dans le sol et dans
l'eau. Le MTBE présent dans l'atmosphère peut passer en partie dans
l'eau de pluie, mais la proportion qui s'élimine ainsi reste faible.
Dans l'atmosphère, l'action des radicaux hydroxyle entraîne la
formation d'un certain nombre de composés et en particulier de
formiate de tertiobutyle, photochimiquement stable, et de
2-méthoxy-2-méthylpropanol, qui doit réagir énergiquement avec les
radicaux hydroxyles pour donner du CO2, du formaldéhyde, de l'acétone
et de l'eau. Lorsque du MTBE est libéré dans l'eau, il se dissout
partiellement, une partie passant dans l'air. Les quantités qui
passent dans les biotes et les sédiments sont faibles. Les épreuves
classiques indiquent une faible biodégradabilité. On pense que d'une
façon générale, la biodégradation est lente dans l'environnement.
Lorsque du MTBE est libéré dans le sol, il passe dans l'air par
volatilisation, dans les eaux de surface par entraînement et dans les
eaux souterraines par lessivage. Le MTBE peut persister dans les eaux
souterraines.
4. Concentrations dans l'environnement et exposition humaine
Les données relatives aux concentrations dans l'environnement et
à l'exposition humaine sont peu nombreuses.
Dans des études portant sur l'air de certaines villes où les
véhicules utilisaient de l'essence oxygénée contenant 15% de MTBE, on
a relevé des concentrations ambiantes allant de "non décelable" à
100,9 µg/m3 (0,028 ppm), avec plusieurs concentrations médianes
allant de 0,47 à 14,4 µg/m3 (0,00013 à 0,004 ppm). Dans l'air de
quelques villes où le MBTE était utilisé à plus faible teneur pour
augmenter l'indice d'octane, la concentration de ce composé allait de
non décelable à 26,4 µg/m3 (0,0073).
Au niveau du sol ou à proximité de raffineries de pétrole, la
concentration allait de 15 à 281 µg/m3. Dans l'air urbain, à
proximité d'ateliers où l'on procédait au mélange de cet additif à
l'essence, la concentration était de 1508 µg/m3 (0,419 ppm), avec des
valeurs extrêmes de 216-35 615 µg/m3 (0,06-9,8 ppm).
Dans les stations service situées dans des zones où l'on
utilisait de l'essence oxygénée à 10-15% de MTBE, c'est dans la zone
de respiration des consommateurs, au moment des pleins, que la
concentration de l'additif était la plus forte (300 à 136 000 µg/m3,
soit 0,09 à 38 ppm), les valeurs dépassant toutefois rarement 3600
µg/m3 (10 ppm) et tombant un peu plus bas au niveau des pompes (de
non décelable à 5700 µg/m3, soit 1,6 ppm). Les valeurs les plus
faibles ont été relevées sur le périmètre de la station (de non
décelable à 500 µg/m3, soit 0,14 ppm). Les concentrations relevées
dans les stations services dépourvues de système de récupération des
vapeurs étaient généralement plus élevées.
A l'intérieur d'une automobile, on a relevé des valeurs de 7 à 60
µg/m3 (0,002 à 0,017 ppm) au cours de navettes et de 20 610 µg/m3
(0,006 à 0,172 ppm) lors des pleins.
D'après des données de surveillance qui se limitent
presqu'exclusivement aux Etats-Unis, la présence de MTBE a été décelée
dans de la neige, des eaux d'orage, des eaux de surface (ruisseaux,
rivières et retenues), des eaux souterraines et dans de l'eau de
boisson. Les valeurs relevées dans les eaux d'orage allaient de 0,2 à
8,7 µg/litre avec une valeur médiane inférieure à 1,0 µg/litre. Dans
le cas des ruisseaux, rivières et retenues, les concentrations
s'étageaient entre 0,2 et 30 µg/litre, les valeurs médianes obtenues
dans diverses études allant de 0,24 à 7,75 µg/litre.
La présence de MTBE n'a généralement pas été décelée dans les
eaux souterraines profondes ou non des zones agricoles. Lorsqu'on en a
trouvé, la concentration était inférieure à 2,0 µg/litre. La présence
de MTBE est plus fréquente dans eaux souterraines de faible profondeur
des régions urbanisées (dans les premiers 1,5 à 3 m des nappes
phréatiques). On trouve alors des concentrations de moins de 0,2
µg/litre à 23 µg/litre, avec une valeur médiane de moins de 0,2
µg/litre.
On trouve rarement du MTBE dans l'eau des réseaux d'adduction qui
est pompée dans les nappes souterraines. Sur 51 réseaux contrôlés sauf
3, la concentration était inférieure ou égale à 20 µg/litre. Il est
difficile de donner des valeurs caractéristiques pour l'eau
d'adduction captée en surface car les données sont insuffisantes. On a
trouvé de fortes concentrations de MTBE dans quelques puits privés
utilisés comme source d'eau potable (soit >1000 µg/m3). On peut
toutefois douter que de l'eau contenant plus de 50 à 100 µg/litre de
MTBE soit encore buvable, car le seuil organoleptique du MTBE est bas.
Parmi les travailleurs exposés au MTBE, on peut citer ceux qui
produisent, distribuent ou utilisent ce composé ou de l'essence qui en
contient, y compris les pompistes et les mécaniciens des stations
service.
En ce qui concerne l'exposition de courte durée (<30 min) lors
d'opérations habituelles de production ou de stockage de MTBE pur, les
chiffres vont de 715 à 43 000 µg/m3 (0,2 à 12 ppm), avec une valeur
médiane moyenne de 3400 µg/m3 (0,95 ppm). Pour l'exposition de plus
longue durée (30 min à 8 h), les valeurs vont de 360 à 890 000 µg/m3
(0,01 à 250 ppm), avec une valeur médiane d'environ 540 µg/m3 (0,15
ppm). Chez les ouvriers qui mélangent l'additif à l'essence, les
valeurs de l'exposition de courte durée vont de non décelable à
360 000 µg/m3 (100 ppm), la médiane se situant à environ 5700 µg/m3
(1,6 ppm). Dans le cas d'une exposition de plus longue durée, les
valeurs obtenues vont de non décelable à 257 000 µg/m3 (72 ppm), avec
une valeur médiane moyenne d'environ 2000 µg/m3 (0,6 ppm).
C'est lors du transport de MTBE pur ou en mélange avec des
carburants, dans des canalisations, sur des péniches, des wagons de
chemin de fer ou des camions (MTBE pur seulement) que l'on a
enregistré les expositions les plus fortes, avec des valeurs à court
terme allant de 3750 mg/m3 (0,001 à 1050 ppm) et une valeur médiane
moyenne de 140 mg/m3 (39 ppm). Dans le cas d'expositions à long
terme, les valeurs allaient de 0,036 à 2540 mg/m3 (0,01 à 712 ppm),
la valeur médiane moyenne se situant à 2,85 mg/m3 (0,8 ppm). Lors de
la distribution (c'est-à-dire du chargement de mélanges carburant-MTBE
sur des camions et de leur livraison et déchargement dans des stations
service), on a relevé des valeurs à court terme allant de non
décelable à 225 mg/m3 (63 ppm), les valeurs médianes moyennes se
situant autour de 21 mg/m3 (6 ppm). Les valeurs à long terme allaient
de 0,036 à 22 mg/m3 (0,01 à 6,2 ppm), avec une valeur médiane moyenne
de 1,79 mg/m3 (0,5 ppm).
L'exposition médiane moyenne à court terme des pompistes de
stations service allait, selon certaines mesures, généralement de
1,071 à 21,42 mg/m3 (0,3 à 6 ppm) et dépassait rarement 35,7 mg/m3
(10 ppm). Dans le cas de l'exposition médiane à long terme, on a
obtenu la valeur de 1,79 mg/m3 (0,5 ppm). L'exposition médiane des
mécaniciens est restée inférieure au seuil de détection dans une étude
à court terme; dans le cas de l'exposition à long terme, la valeur
était d'environ 360 µg/m3 (0,1 ppm).
5. Cinétique et métabolisme
Les données toxicocinétiques relatives aux effets du MTBE sur
l'Homme proviennent essentiellement d'études contrôlées pratiquées sur
des volontaires adultes ou sur une population exposée à de l'essence
oxygénée. Après inhalation, le MTBE passe rapidement dans le courant
sanguin. Chez des volontaires humains en bonne santé exposés par voie
respiratoire, on constate que la cinétique est linéaire jusqu'à la
concentration de 268 mg/m3 (75 ppm). On a procédé au dosage de
l'alcool tertiobutylique (en abrégé TBA, un métabolite du MTBE) dans
le sang et les urines. Chez des sujets humains exposés à des
concentrations de MTBE allant de 5,0 à 178,5 mg/m3 (1,4 à 50 ppm), la
concentration maximale de MTBE et de TBA allait respectivement de 17,2
à 1144 µg/litre et de 7,8 à 925 µg/litre. En utilisant un modèle
monocompartimental, on a pu constater qu'intervenaient dans la
demi-vie globale du MTBE des constituants à demi-vie brève (36-90 min)
et des constituants à demi-vie longue (19 h).
Chez les rongeurs, le MTBE est bien résorbé et réparti après
administration per os ou exposition par la voie respiratoire.
L'absorption est moindre par voie percutanée. A la dose de 400 mg/kg
per os et de 28 800 mg/m3 (8000 ppm) par inhalation, la proportion
de la dose totale absorbée qui était éliminée dans l'air expiré
augmentait à mesure que diminuait la proportion éliminée dans les
urines, ce qui est le signe d'une saturation du métabolisme. On n'a
pas mis en évidence de TBA dans l'urine des rats exposés. La présence
de 2-méthyl-1,2-propanediol et d'acide alpha-hydroxyisobutyrique dans
l'urine indique que le TBA est également métabolisé. Les études
in vitro montrent que le MTBE est métabolisé en TBA, formaldéhyde et
acétone.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat, la dose létale médiane aiguë par voie buccale
(DL50) est égale à environ 3 800 mg/kg de poids corporel. La
concentration létale médiane aiguë (CL50) pour une exposition de 15
minutes par inhalation se situe aux environs de 141 000 mg/m3 d'air
chez la souris. Parmi les signes d'intoxication on peut citer une
dépression du SNC, une ataxie et des difficultés respiratoires. Aux
doses non létales, la récupération a été complète. Par voie
percutanée, la DL50 est >10 200 mg/kg de poids corporel chez le
lapin.
On n'a trouvé qu'une seule étude où il soit question d'un effet
"modérément" irritant pour la peau, l'irritation consistant en un
érythème et un oedème modérés après application sur la peau de lapins.
Chez ce même animal, le MTBE s'est également révélé irritant pour la
muqueuse oculaire, les effets produits étant bénins et réversibles.
Dans la seule étude retrouvée, le MTBE a provoqué une irritation
légère à forte des voies respiratoires lors de l'exposition de souris
à des doses de 300 à 30 000 mg/m3. Il n'a pas produit de
sensibilisation cutanée chez le cobaye.
L'expérimentation sur des rats et des souris montre que des
expositions réitérées conduisent principalement à une augmentation du
poids des organes et ont des effets histopathologiques sur le rein
(rat) et sur le foie (souris). Une étude d'ingestion de 90 jours a
montré que la limite inférieure d'apparition d'effets néphrotoxiques
se situe à 440 mg/kg p.c. par jour (augmentation du poids des reins et
dégénérescence hyaline chez des rats Sprague-Dawley). En exposant des
rats Fischer-344 par inhalation à une concentration de 2880 mg/m3
(800 ppm) de MTBE, on a obtenu une augmentation du poids rénal
accompagnée, lorsqu'on accroissait la concentration, d'une
augmentation modérée de la dégénérescence hyaline au niveau des
tubules proximaux. Lors d'études d'oncogénicité comportant
l'exposition des animaux par inhalation, on a observé à la dose de
1440 mg/m3 (400 ppm), un accroissement de la fréquence et de la
gravité des néphropathies progressives chroniques chez les rats mâles,
alors que chez les souris mâles, il y avait à cette même dose
augmentation du poids absolu du foie (corrélée avec une augmentation
des hypertrophies hépatocellulaires à plus forte concentration) et du
poids relatif des reins.
L'exposition au MTBE provoque également des effets irréversibles
sur le système nerveux central (SNC) consistant notamment en sédation,
diminution de l'activité, ataxie et anesthésie à forte concentration.
Des effets biphasiques s'observent également sur l'activité motrice à
plus faible concentration. Lors d'une étude sur des rats comportant
une exposition de 6 h par inhalation, on a constaté qu'à la dose de
2880 mg/m3 (800 ppm), il se produisait, chez les animaux d'un des
deux sexes, des modifications réversibles et liées à la dose de
l'activité motrice. Ces effets étaient passagers et n'apparaissaient
plus guère lors des études à long terme.
On a pu retrouver des études de reproduction portant sur une ou
deux générations de rats ainsi que quatre études relatives au
développement de rats, de souris et de lapins exposés à du MTBE. Ces
études n'ont pas permis de mettre en évidence d'effets spécifiques sur
la reproduction des rats à des concentrations allant jusqu'à 28 800
mg/m3. En outre, aux concentrations inférieures à celles qui se
révélaient toxiques pour les mères, le MTBE n'a pas eu non plus
d'effets sur le développement de la progéniture. A la dose de 28 800
mg/m3, on a constaté, chez la souris, une augmentation du poids de
l'utérus et un accroissement du métabolisme des estrogènes.
Le MTBE a fait l'objet d'un grand nombre d'épreuves valables de
mutagénicité et autres études de génotoxicité. Les résultats obtenus
montrent que le composé n'est pas génotoxique, même si un résultat
positif a été obtenu dans l'épreuve de mutation portant sur le locus
tk des cellules lymphomateuses. Ce résultat s'explique en effet par la
métabolisation du MTBE en formaldéhyde.
Pour les études de cancérogénicité, on a exposé par inhalation
des rats Fischer-344 et des souris CD-1 ou gavé des rats
Sprague-Dawley avec une nourriture contenant du MTBE. Dans aucune des
études d'exposition par inhalation on a procédé à une correction
statistique pour tenir compte des différences de survie. Dans les
trois études, on a constaté une augmentation sensible de l'incidence
des tumeurs, localisées, chez les rats mâles Fischer-344, au niveau
des tubules rénaux et des cellules de Leydig, chez les rats mâles
Sprague-Dawley, au niveau des cellules de Leydig (lymphomes et
leucémies chez les femelles) et chez les souris femelles CD-1, au
niveau du foie. Les tumeurs des tubules rénaux et les
leucémies/lymphomes n'ont donc pas été observées systématiquement chez
le rat lors des différentes études. En outre, les tumeurs rénales
sexospécifiques étaient associées à une néphropathie également
sexospécifique mettant en jeu l'alpha2u-globuline, qui a été observée
dans plusieurs études de courte durée. L'augmentation des tumeurs des
cellules de Leydig a été observée à la dose la plus élevée chez les
rats Sprague-Dawley (1000 mg/kg p.c.), mais chez les rats Fischer-344,
l'interprétation de cet accroissement est rendu délicate par la très
forte incidence tumorale également observée chez les témoins
concomitants et les témoins historiques. Les tumeurs hépatiques ont
été observées dans les groupes témoins et à la dose de 28 800 mg/m3
(8000 ppm) dans les groupes exposés avec des incidences respectives de
2/50 et 10/50 chez les femelles et de 12/49 et 16/49 chez les mâles.
L'accroissement d'incidence était modeste et s'accompagnait d'une
hypertrophie hépatocellulaire.
7. Effets sur l'Homme
Après la mise sur le marché, aux Etats-Unis, de deux types
d'essence nécessitant l'utilisation d'additifs d'oxygénation (pas
obligatoirement du MTBE), on a constaté que les usagers se plaignaient
de symptômes aigus tels que maux de tête, irritation des yeux et du
nez, toux, nausées, vertiges et désorientation. Les études
épidémiologiques effectuées sur des populations humaines en milieu
professionnel ou non, de même que les études expérimentales sur
volontaires humains exposés dans des conditions contrôlées, n'ont pas
permis de découvrir si ces plaintes étaient fondées. Des études
intracommunautaires menées en Alaska, au New Jersey, dans le
Connecticut et dans le Wisconsin ont, avec des résultats divers il est
vrai, montré qu'il n'y avait guère de relation entre l'exposition au
MTBE et les symptômes dont la population se plaignait.
Des volontaires adultes ont été placés, dans le cadre d'études
expérimentales contrôlées, dans des chambres d'inhalation où on leur a
fait respirer du MTBE à des concentrations allant de 5,0 mg/m3
(1,4 ppm) à 270 mg/m3 (75 ppm). Aucun effet patent n'a été relevé,
qu'il s'agisse de la relation subjective de symptômes ou d'indicateurs
objectifs tels qu'une irritation ou d'autres signes, à des
concentrations allant jusqu'à 180 mg/m3 (50 ppm) et pendant une durée
pouvant atteindre 2 h. A en juger d'après ces résultats, il est peu
probable que le MTBE puisse à lui seul exercer des effets toxiques
aigus sur la population générale dans les conditions habituelles
d'exposition par la voie respiratoire. Il est cependant à noter que
les effets potentiels d'essences additionnées de MTBE, dans les
conditions où la plupart des gens sont exposés à cet additif
lorsqu'ils utilisent des carburants oxygénés, n'ont été étudiés ni
expérimentalement, ni par le biais de méthodes épidémiologiques
prospectives. Par ailleurs, le rôle de facteurs tels que la perception
de la présence de MTBE, explicable en partie par l'odeur particulière
de ce composé, n'a pas été étudié non plus.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Expérimentalement, la toxicité aiguë (exprimée par la CL50) du
MTBE pour les poissons, les amphibiens et les crustacés se révèle
supérieure à 100 mg/litre. On ne possède pas de données sur la
toxicité chronique ou subléthale de ce composé pour les organismes
aquatiques, ni sur sa toxicité pour les organismes terrestres.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
A en juger par les données collectives, il semble peu probable
que le MTBE puisse à lui seul et dans les conditions usuelles
d'exposition, provoquer des effets toxiques aigus dans la population
générale.
D'après les études effectuées sur l'animal, le MTBE possède une
toxicité aiguë "modérée" et il provoque une légère irritation cutanée
et oculaire, mais pas de sensibilisation. Des expositions répétées
entraînent des effets essentiellement localisés au rein chez le rat et
au foie chez la souris, la dose nocive la plus faible étant de 440
mg/kg p.c. par jour chez le rat après ingestion et de 1440 mg/m3
(400 ppm) après inhalation. Aux concentrations inférieures au seuil de
toxicité parentale, le MTBE n'a pas eu d'effets nocifs sur la
reproduction ou le développement.
Le MTBE n'est pas génotoxique mais il peut provoquer la formation
de tumeurs chez les rongeurs, surtout aux concentrations suffisamment
élevées pour avoir d'autres effets toxiques. On considère actuellement
que ces données ne sont pas suffisantes pour que l'on puisse en tirer
une évaluation du risque cancérogène chez l'Homme. Le Groupe spécial a
conclu que, pour être en mesure de donner des indications
quantitatives concernant les limites d'exposition et d'évaluer le
risque, il fallait obtenir des données supplémentaires sur un certain
nombre de points.
Il ne semble pas que le MTBE, aux concentrations auxquelles il se
trouve dans l'eau, puisse être toxique pour les organismes aquatiques,
sauf en cas de déversement. On ne possède pas de données sur la
toxicité du MTBE pour les organismes terrestres mais il n'y a
vraisemblablement pas lieu de s'alarmer, étant donné que sa
concentration est faible dans l'air ambiant et que sa demi-vie est
relativement brève.
RESUMEN
El éter metil- terciario-butílico (MTBE) es uno de los distintos
éteres que pueden utilizarse como aditivos de combustibles y en la
actualidad es con gran diferencia el más usado. El éter
etil- terciario-butílico (ETBE), el éter terciario-amil-metílico
(TAME), el éter terciario-amil-etílico (TAEE) y el éter
diisopropílico (DIPE), entre otros, pueden ser suplementos del MTBE o
sustituirlo para fines de oxigenación o mejora de los octanos y, en
consecuencia, pueden hallarse en asociación con el MTBE.
1. Identidad, propiedades físicas y químicas y métodos analíticos
El MTBE es un líquido volátil e incoloro a la temperatura
ambiente, de olor parecido al terpeno. Su viscosidad es baja y tiene
un punto de ebullición de 55,2°C. El punto de congelación es de -
109°C. La densidad es de 0,7404 a 20°C. La presión de vapor es
relativamente alta: 33 500 Pa a 25°C. El MTBE es inflamable y puede
formar mezclas explosivas con el aire. Es muy soluble en otros éteres
y alcohol. Se mezcla con la gasolina y es soluble en agua (42 000
g/m3 a 19,8°C). El coeficiente de partición log n-octanol/agua es
de 0,94-1,3. Es inestable en solución ácida.
El MTBE se analiza en todas las matrices en general por
cromatografía de gases, utilizando una gama de columnas capilares y
sistemas detectores que son apropiados para la matriz específica.
También se ha utilizado la cromatografía inversa en fase líquida para
el análisis de las muestras de gasolina. Se han empleado sistemas de
sorción-desorción, incluidos sistemas de purga y captación, así como
procedimientos de recámara, a fin de preparar muestras de aire, agua,
sedimento y biológicas para el análisis.
2. Fuentes de exposición humana y ambiental
No se conoce la presencia natural de MTBE en el medio ambiente.
En la industria deriva de la reacción catalítica del metanol y el
isobutileno, y en varios países se ha producido en volúmenes
crecientes desde los últimos años setenta. El MTBE figura actualmente
entre los 50 productos químicos de mayor producción en volumen. En
1996, la capacidad estadounidense de producción fue aproximadamente de
10,6 millones de toneladas, previéndose un constante aumento del uso
de MTBE. El 25% aproximadamente de la gasolina en los EE.UU está
mezclada con MTBE. El MTBE se utiliza casi exclusivamente para el
refuerzo de los octanos y para aumentar el contenido de la gasolina en
oxígeno. El MTBE se ha añadido a la gasolina en concentraciones de
hasta el 17% en volumen.
3. Transporte, distribución y transformación en el medio ambiente
Tras su eliminación en el aire, el MTBE permanecerá en gran parte
en este medio, penetrando cantidades menores en el suelo y el agua. En
la atmósfera, el MTBE puede ser arrastrado por la lluvia. Sin embargo,
sólo una pequeña cantidad es eliminada de la atmósfera de este modo.
La transformación atmosférica por radicales hidroxilos produce varios
productos, entre los que figuran el formato terciario-butílico (TBF)
estable y el 2-metoxi-2-metilpropanol, que se supone que son muy
reactivos con los radicales hidroxilos, dando CO2, formaldehido,
acetona y agua. Cuando el MTBE pasa al agua se disuelve una cantidad
significativa, con cierta proporción en el aire. La proporción que
pasa a los biota y el sedimento es escasa. La biodegradabilidad en
ensayos convencionales es limitada. Se cree que por lo general es
lenta en el medio ambiente. Cuando el MTBE pasa al suelo, es
transportado al aire por volatilización, al agua superficial por
escurrimiento y al agua subterránea como resultado de la lixiviación.
El MTBE puede persistir en el agua subterránea.
4. Niveles medioambientales y exposición humana
Se dispone de escasos datos sobre los niveles medioambientales y
la exposición humana.
En los estudios sobre el MTBE en el aire de algunas ciudades que
utilizan gasolina oxigenada con MTBE al 15%, las concentraciones
ambientales iban del nivel indetectable a 100,9 µg/m3 (0,028 ppm),
con varias concentraciones medianas de 0,47 a 14,4 µg/m3 (0,00013 a
0,004 ppm). Las concentraciones de MTBE en el aire de algunas ciudades
en donde se utiliza MTBE como reforzador de octanos en concentraciones
inferiores van del nivel no detectable a 26,4 µg/m3 (0,0073 ppm).
Las concentraciones a nivel del suelo o cerca de las refinerías
eran de 15 a 281 µg/m3. Los niveles medianos en el aire urbano cerca
de instalaciones de mezclado eran de 1508 µg/m3 (0,419 ppm), con
gamas de 216-35 615 µg/m3 (0,06 a 9,8 ppm).
En las estaciones de servicio situadas en zonas en donde la
gasolina oxigenada contiene el 10-15% de MTBE, las concentraciones
alcanzaban el nivel máximo en la zona de respiración durante el
llenado de los depósitos por los consumidores (gama de 300 a
136 000 µg/m3 (0,09 a 38 ppm)), con niveles que rara vez pasaban de
3600 µg/m3 (10 ppm), siendo ligeramente inferiores en la zona de
bombas (indetectables a 5700 µg/m3 (1,6 ppm)) y mínimos en el
perímetro de la estación (indetectables a 550 µg/m3 (0,14 ppm)). En
general las concentraciones eran superiores en las estaciones de
servicio sin sistemas de recuperación de vapores.
En la cabina del automóvil, las concentraciones eran de 7 a
60 µg/m3 (0,002 a 0,017 ppm) durante la conducción y de 20 a
610 µg/m3 (0,006 a 0,172 ppm) al llenar el depósito.
Basándose en operaciones limitadas de vigilancia realizadas casi
exclusivamente en los EE.UU., se ha detectado el MTBE en la nieve, el
agua de tormenta, las aguas superficiales (riachuelos, ríos y
embalses), las aguas subterráneas y el agua de beber. Las
concentraciones de MTBE detectadas en el agua de tormenta iban de 0,02
a 8,7 µg/litro, con un valor mediano de menos de 1,0 µg/litro. En los
riachuelos, ríos y embalses, la gama de detección era de 0,2 a
30 µg/litro y la gama de valores medianos en varios estudios era de
0,24 a 7,75 µg/litro.
En general no se ha detectado el MTBE en las aguas subterráneas
profundas o cercanas a la superficie en zonas agrícolas. Cuando se ha
detectado, la concentración era inferior a 2,0 µg/litro. El MTBE se
halla con más frecuencia en las aguas subterráneas cercanas a la
superficie (1,6 a 3,2 metros de estos acuíferos) de las zonas urbanas.
En este entorno, las concentraciones van de menos de 0,2 µg/litro a 2
mg/litro, con un valor mediano inferior a 0,2 µg/litro.
El MTBE se halla poco frecuentemente en sistemas de
abastecimiento público de agua procedente de capas freáticas. Entre 51
sistemas estudiados, en 48 la concentración era de <20 µg/litro.
Son insuficientes los datos disponibles para caracterizar la
concentración de MTBE en los sistemas de abastecimiento público de
agua procedentes de aguas superficiales. El MTBE se ha hallado en
concentraciones altas (esto es, >1000 µg/litro) en algunos pozos
privados utilizados para obtener agua de beber. Sin embargo, es dudoso
que las personas puedan consumir agua con concentraciones de MTBE
superiores a unos 50-100 µg/litro debido al bajo umbral de su gusto y
olor.
Entre los trabajadores con posible exposición al MTBE figuran los
ocupados en la producción, distribución y uso de MTBE y de gasolina
con MTBE, que incluye el personal de estaciones de servicio y los
mecánicos.
La exposición a corto plazo (<30 min) en operaciones corrientes
de fabricación y mantenimiento de MTBE puro iba de 715 a 43 000 µg/m3
(0,2 a 12 ppm), siendo el promedio de los valores medianos de
3400 µg/m3 aproximadamente (0,95 ppm). La exposición a largo plazo
(30 min a 8 h) era de 360 a 890 000 µg/m3 (0,01 ppm a 250 ppm), con
valores medianos de aproximadamente 540 µg/m3 (0,15 ppm). En el caso
de los trabajadores de operaciones de mezclado, los valores a corto
plazo oscilaban entre niveles indetectables y 360 000 µg/m3
(100 ppm), siendo el promedio de los valores medianos de 5700 µg/m3
aproximadamente (1,6 ppm). Los valores a largo plazo comprendían desde
niveles indetectables hasta 257 000 µg/m3 (72 ppm), siendo el
promedio de los valores medianos de 2000 µg/m3 aproximadamente
(0,6 ppm).
La exposición alcanzó el nivel máximo durante el transporte de
MTBE puro y de mezclas de combustible en oleoductos, barcazas, vagones
de ferrocarril y camiones (sólo MTBE puro), variando los valores a
corto plazo entre 4 y 3750 mg/m3 (0,001 a 1050 ppm), con un promedio
de los valores medianos de 140 mg/m3 (39 ppm). Los valores a largo
plazo fueron de 0,036 a 2540 mg/m3 (0,01 a 712 ppm), con un promedio
de los valores medianos de 2,85 mg/m3 (0,8 ppm). En las operaciones
de distribución (esto es, carga de mezclas de combustible y MTBE en
camiones y entrega y descarga en las estaciones de servicio), los
valores a corto plazo oscilaron entre niveles indetectables y
225 mg/m3 (63 ppm), siendo el promedio de los valores medianos de
21 mg/m3 aproximadamente (6 ppm). Los valores a largo plazo fueron de
0,036 a 22 mg/m3 (0,01 a 6,2 ppm), siendo el promedio de los valores
medianos de 1,79 mg/m3 (0,5 ppm).
Los valores medianos de la exposición a corto plazo de operarios
de estaciones de servicio fueron en general de 1,071 a 21,42 mg/m3
(0,3 a 6 ppm), excediendo rara vez de 35,7 mg/m3 (10 ppm). Los
valores medianos de la exposición a largo plazo en operarios de
estaciones de servicio presentaron un promedio de 1,79 mg/m3
(0,5 ppm). Los valores medianos de la exposición de mecánicos estaban
por debajo de los niveles de detección en un estudio a corto plazo; el
promedio de los valores medianos para la exposición a largo plazo fue
aproximadamente de 360 µg/m3 (0,1 ppm).
5. Cinética y metabolismo
Los datos toxicocinéticos sobre el MTBE en personas proceden
principalmente de estudios controlados en voluntarios adultos sanos y
en una población expuesta a la gasolina oxigenada. El MTBE pasa
rápidamente a la circulación después de la exposición por inhalación.
En voluntarios sanos expuestos a la inhalación, la cinética del MTBE
era lineal hasta concentraciones de 268 mg/m3 (75 ppm). Se midió en
la sangre y orina de personas expuestas el alcohol
terciario-butílico, metabolito del MTBE. Las concentraciones
sanguíneas máximas del MTBE y el alcohol terciario-butílico fueron
de 17,2 a 1144 µg/m3 y de 7,8 a 925 µg/m3, respectivamente, en
personas expuestas a 5,0 a 178,5 mg/m3 (1,4 a 50 ppm) de MTBE.
Basándose en un modelo de monocompartimiento se identificaron
componentes rápidos (36- 90 min) y lentos (19 h) de la semivida del
MTBE.
En los roedores, el MTBE se absorbe y distribuye bien después de
la administración oral y la exposición por inhalación, con menor
absorción cutánea. En la administración oral de 400 mg/kg y en la
inhalación de 28 800 mg/m3 (8000 ppm) aumentó el porcentaje de la
dosis absorbida total eliminado en el aire espirado, con un descenso
correspondiente del porcentaje eliminado por la orina, indicando la
saturación metabólica. No se identificó la presencia de alcohol
terciario-butílico (TBA) en la orina de ratas expuestas. Hubo
indicios de un metabolismo adicional del TBA, basados en la
identificación de 2-metil-1,2-propanodiol y de ácido
alpha-hidroxiisobutírico eliminado por la orina. Los estudios
in vitro prueban que el MTBE se metaboliza hasta TBA, formaldehido y
acetona.
6. Efectos en los animales de laboratorio y en los sistemas
in vitro
En las ratas, la dosis letal oral mediana aguda (DL50) es
aproximadamente de 3800 mg/kg de peso corporal. La concentración letal
mediana aguda (CL50) para una exposición por inhalación de 15 minutos
es de aproximadamente 141 000 mg/m3 de aire en ratones. Entre los
signos de intoxicación figuran la depresión del SNC, la ataxia y la
respiración laboriosa. Si la dosis no es letal, la recuperación es
completa. La DL50 para la toxicidad cutánea en conejos es de
>10 200 mg/kg de peso corporal.
En un solo estudio identificado, el MTBE resultó "moderadamente"
irritante para la piel, produciendo eritema moderado y edema después
de la aplicación cutánea en conejos. También resultó irritante para
los ojos de los conejos, produciendo lesiones leves y reversibles. En
el único estudio identificado, el MTBE produjo irritación respiratoria
ligera a intensa después de la exposición de ratones a 300 y
30 000 mg/m3, respectivamente. No causó sensibilización cutánea en
estudios en cobayos.
La exposición repetida produce fundamentalmente aumentos del peso
de los órganos y lesiones histopatológicas en el riñón de ratas y en
el hígado de ratones. Los niveles de mínimo efecto señalado de
nefrotoxicidad tras la ingestión en estudios de 90 días fueron de
400 mg/kg de peso corporal por día (aumentos del peso renal relativo y
formación de gotas hialinas en ratas Sprague-Dawley). En la exposición
por inhalación a 2880 mg/m3 (800 ppm) se produjeron aumentos del peso
del riñón asociados a las concentraciones más altas, con moderado
aumento de las gotas hialinas en los túbulos proximales en ratas
Fischer-344. En estudios de oncogenicidad por inhalación en dosis de
1440 mg/m3 (400 ppm), la incidencia y la gravedad de la nefropatía
progresiva crónica aumentó en ratas machos; en esta concentración, en
ratones machos se observó un aumento del peso absoluto del hígado (que
guardaba correlación con el aumento de la hipertrofia hepatocelular en
concentraciones superiores) y un aumento del peso renal relativo.
La exposición al MTBE también produjo lesiones reversibles del
sistema nervioso central (SNC), incluidas sedación, hipoactividad,
ataxia y anestesia en concentraciones superiores y efectos bifásicos
sobre la actividad motriz en concentraciones inferiores. En un estudio
de una sola exposición de 6 horas en ratas, las concentraciones de
2880 mg/m3 (800 ppm) produjeron cambios reversibles de la actividad
motriz relacionados con la dosis en sexos separados. Esos efectos
fueron transitorios y no se pusieron de manifiesto en estudios a largo
plazo.
Se han efectuado estudios reproductivos por inhalación de una y
dos generaciones y estudios de cuatro generaciones en ratas, ratones y
conejos. En esos estudios no se hallaron efectos reproductivos
específicos en ratas en concentraciones de hasta 28 800 mg/m3. El
MTBE no ha producido efectos en el desarrollo en concentraciones
inferiores a las que resultaron tóxicas en las madres. Se han
observado disminuciones del peso del útero y aumentos del metabolismo
estrogénico en ratonas con dosis de 28 000 mg/m3.
El MTBE ha sido sometido a pruebas apropiadas en una amplia gama
de ensayos de mutagenicidad y de genotoxicidad. Los resultados
muestran que el MTBE no es genotóxico, aunque resultó positiva una
prueba de mutación del locus tk en células linfomatosas de ratón
debido al paso metabólico de MTBE a formaldehido.
Se han realizado estudios de carcinogenicidad que comprendieron
la exposición por inhalación de ratas Fischer-344 y de ratones CD-1 y
el cebado de ratas Sprague-Dawley. En ninguno de los dos estudios de
inhalación se utilizaron métodos de análisis estadístico que
efectuaran el reajuste de las diferencias de supervivencia. En los
tres estudios se produjeron aumentos significativos de la incidencia
de tumores, esto es, tumores de células tubulares renales y tumores de
células de Leydig en ratas Fischer-344 machos, tumores de células de
Leydig en ratas Sprague-Dawley machos y linfomas-leucemias
(combinadas) en ratas hembras de la misma especie, y tumores de
células hepáticas en ratones CD-1 hembras. Así pues, no se observaron
constantemente tumores de células tubulares renales ni
leucemias-linfomas en los distintos estudios en ratas. Además, los
tumores renales específicos del sexo se asociaron a la nefropatía de
la alpha2u-globulina específica del sexo, observada en varios estudios
de breve duración. Se observaron aumentos de los tumores de células de
Leydig con la dosis más alta (1000 mg/kg de peso corporal) en la ratas
Sprague-Dawley, pero la interpretación de los aumentos registrados en
las ratas Fischer-344 resultó compleja por las incidencias muy altas
concurrentes y de los testigos históricos. En los ratones, las
incidencias de los tumores hepáticos fueron en los testigos y en los
grupos expuestos a 28 800 mg/m3 (8000 ppm), respectivamente, de 2/50
y 10/50 en las hembras y de 12/49 y 16/49 en los machos. Los aumentos
fueron moderados y acompañados de hipertrofia hepatocelular.
7. Efectos en el ser humano
Tras la introducción de dos programas separados relativos a los
combustibles en los EE.UU., que requieren el empleo de productos de
oxigenación de la gasolina (no necesariamente MTBE), los consumidores
de algunas zonas se han quejado de trastornos agudos de la salud, como
dolor de cabeza, irritación de los ojos y la nariz, tos, náuseas,
mareos y desorientación. Los estudios epidemiológicos de poblaciones
humanas expuestas en condiciones profesionales o no profesionales, así
como los estudios experimentales de voluntarios expuestos en
condiciones controladas, no han podido identificar la base de esos
trastornos. Aunque los resultados son variados, los estudios
comunitarios efectuados en Alaska, New Jersey, Connecticut y Wisconsin
(EE.UU.) no han proporcionado indicios, o éstos han sido limitados, de
la asociación entre la exposición al MTBE y la prevalencia de
trastornos de la salud.
En estudios experimentales controlados en voluntarios humanos
expuestos en cámaras de inhalación al MTBE en concentraciones de 5,0
mg/m3 (1,4 ppm) a 270 mg/m3 (75 ppm) no hubo efectos manifiestos en
términos de presencia subjetiva de síntomas o de indicadores objetivos
de irritación u otros efectos en concentraciones de hasta 180 mg/m3
(50 ppm) durante dos horas. Partiendo de esos datos parece improbable
que el MTBE por sí solo produzca efectos agudos adversos en la salud
en la población general en las condiciones corrientes de exposición
por inhalación. Sin embargo, los posibles efectos de las mezclas de
gasolina y MTBE y el modo de exposición de la mayor parte de las
personas al MTBE en asociación con el empleo de combustibles
oxigenados, no se han examinado experimentalmente ni por métodos
epidemiológicos prospectivos. Por otra parte, no se ha investigado,
por ejemplo, la función de factores tales como la percepción del MTBE,
debida en parte a su olor distintivo.
8. Efectos en otros organismos en el laboratorio y sobre el
terreno
La toxicidad aguda experimental (CL50) del MTBE en los peces,
los anfibios y los crustáceos es >100 mg/litro. No existen datos
sobre la toxicidad crónica o subletal para las especies acuáticas ni
la toxicidad para los organismos terrestres.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
Basándose en datos de observación colectiva, parece improbable
que el MTBE por sí solo induzca efectos agudos adversos en la salud de
la población general en las condiciones corrientes de exposición.
En estudios en animales, el MTBE es "moderadamente" tóxico en
forma aguda y produce irritación cutánea y ocular moderada, pero no
sensibilización. La exposición repetida afecta fundamentalmente al
riñón de ratas y al hígado de ratones, observándose los efectos
adversos mínimos con concentraciones de 440 mg/kg de peso corporal por
día en ratas después de la ingestión y de 1400 mg/m3 (400 ppm)
después de la inhalación. El MTBE no ha inducido efectos adversos en
la reproducción o el desarrollo en concentraciones inferiores a las
que eran tóxicas para los padres.
El MTBE no es genotóxico, pero ha producido tumores en roedores,
principalmente con concentraciones altas, que también inducen otros
efectos adversos. Esos datos se consideran en la actualidad
insuficientes para la evaluación del riesgo carcinogénico en seres
humanos. El Grupo Especial llegó a la conclusión de que para
proporcionar orientación cuantitativa sobre los límites pertinentes de
exposición y para estimar el riesgo se necesita adquirir datos
adicionales en distintos sectores.
No parece que las concentraciones de MTBE en el agua ambiental
sean tóxicas para los organismos acuáticos, excepto en caso de
escapes. Aunque no hay datos sobre la toxicidad terrestre del MTBE,
parece que no es preocupante ya que las concentraciones en el aire
ambiental son bajas y la semivida del MTBE es relativamente breve.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO SYSTEMS
Owing to the limited therapeutic use of MTBE in the dissolution
of cholesterol gallstones in humans, there have been a number of
studies in which effects have been examined following single or
repeated exposure by direct instillation into the gallbladder.
Reported effects at therapeutic doses were mild clinical signs and
inflammatory changes in the gallbladder (Allen et al., 1985a; McGahan
et al., 1988; Adam et al., 1990; Esch et al., l992a,b; Chen et al.,
1995).
7.1 Single exposure
The acute toxicity of MTBE has been studied in several animal
species; the results are summarized in Tables 16 and 17. Studies by
routes most relevant to human exposure are described here. The oral
LD50 value for rats is about 3800 mg/kg bw (ARCO, 1987). Signs of
intoxication after single oral lethal doses consist of CNS depression,
ataxia, laboured respiration and death (ARCO, l987). When the dose was
non-lethal, recovery was complete.
The acute dermal LD50 is >10 200 mg/kg bw for rabbits (ARCO,
1987). Adverse local effects included erythema, oedema, fissuring and
necrosis at dermal application.
In rats, the LC50 value for inhalation exposure to MTBE is about
142 000 mg/m3 air (39 460 ppm) (ARCO, 1987). Reported LC50 values in
mice are 141 000 mg/m3 (1.6 mmol/litre) for 15 min of inhalation
exposure (Marsh & Leake, l950) and 658 000 mg/m3 (18% v/v) for 10 min
of inhalation exposure (Snamprogetti, 1980). Signs noted in rats
following inhalation exposure included eye irritation, incoordination
and loss of righting reflex (ARCO, 1987). Surviving animals appeared
to recover within 24 h.
Results of studies in which neurological effects following single
exposures were examined are reported in section 7.3.
7.2 Skin, eye, and respiratory tract irritation; skin sensitization
7.2.1 Skin irritation
Moderate erythema and oedema were reported by Cuthbert (1979)
following application of 0.5 ml undiluted MTBE to the intact and
abraded skin of six rabbits (applied under occlusion for 24 h). A
primary irritation index of 3.36 was reported. Effects were slightly
more pronounced on abraded skin:
24 h 72 h
intact abraded intact abraded
erythema 1.7 2.2 1.1 2.0
oedema 1.7 2.0 1.0 1.8
MTBE was considered to be a moderate skin irritant.
Table 16. Acute toxicity of MTBE in experimental animals
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Rat: oral (gavage) 2000, 3000, 4600, 3800 hypoactivity, muscular weakness, Industrial Bio-Test
Charles River, 6800, or 10 200 hyperpnoea, lacrimation, Laboratories (1969)
5 male, 5 female/ mg/kg bw prostration and death; the
dose level symptoms were reversible at
sublethal doses.
Inflammation of the stomach
and/or small intestine in
animals that died
Rat: oral (gavage) 3866 CNS depression, ataxia, ARCO (1987)
(strain and number laboured respiration and
not stated) death
Rabbit: dermal 6.8 or 10.2 g/kg >10 200 no deaths; no gross Industrial Bio-Test
New Zealand White bw, occlusive pathological alterations Laboratories (1969)
2 male, 2 female/ dressing, for 24 h; other than necrosis at
dose level 14-day observation application site
Rabbit: dermal 10 000 erythema, oedema, scaling, ARCO (1987)
(strain and number fissuring, and blanching
not stated) hyperaemia in animals that
died
Rat: inhalation LC50 = 142 000 eye irritation, ARCO (1987)
(strain and sex mg/m3 incoordination, tachypnoea,
not stated) (39 460 ppm) loss of righting reflex
and death
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
Mouse: inhalation exposure for 15 min LC50 = 141 000 the median concentration Marsh & Leake (1950)
White (strain (whole body mg/m3 for anaesthesia (AC50) =
and sex not exposure) (1.6 mmol/litre) 106 mg/litre (1.2
stated), 20 mmol/litre)
mice/exposure
group
Mouse: inhalation exposure for 5 min; 400 000 mg/m3 median effective concentration Industrial Bio-Test
Swiss, (whole body observation for (260-600 mg/litre) for anaesthesia (EC50) = Laboratories (1969)
4 males/exposure exposure) following 48 h 200 mg/litre air (130-300
group mg/litre); convulsive
hyperventilation,
hyperactivity, ataxia, loss
of righting reflex, clonic,
and sporadic convulsive
seizures
Mouse: inhalation I. 20% v/v in air LT50a = 5.6 min death occurred within 1 h Snamprogetti (1980)
Swiss, (whole body for 3, 4, 5, 6, 9
I. 40 males/ exposure) or 12 min
exposure group II 8, 12, 17, 20, LC50 = 658
II. 20 males per 22 or 26% v/v MTBE mg/litre
group in air for 10 min (18% v/v)
Skin and eye irritancy
Rabbit:
New Zealand skin application 0.5 ml moderate erythema and
White, 3 male, (occluded) oedema
3 female
Table 16. (continued)
Species Administration Dose LD50 (mg/kg bw) Observation Reference
route (unless stated
otherwise)
New Zealand conjunctival 0.1 ml moderate conjunctival Cuthbert (1979)
White, 3 male, instillation response; no corneal
3 female or iris involvement
New Zealand
White
I. 6 (sex not conjunctival 0.1 ml conjunctival redness for Hazleton
stated) instillation 48 h, no chemosis or discharge, Laboratories
slightly more marked effects (1979)
II. 3 (sex not conjunctival 0.1 ml with temporary corneal
stated) instillation; opacity for 24 h
washout with
water 30 secs
later
Rabbit conjunctival 0.05 ml or 0.1 ml conjunctival congestion, Snamprogetti (1980)
Both sexes instillation thickening and lacrimation;
(strain and number more marked at high dose;
not stated) reversible
a LT50 =exposure time which causes death in 50% of treated animals.
Table 17. Acute toxicity of MTBE following parenteral injection
Species Injection Dose LD50 Comments Reference
route/site
Rat: subcutaneous 3.0, 4.0, 5.0, 6.0, 6.7 ml/kg bw Snamprogetti (1980)
Wistar, 7.0, 8.0, 9.0 or 10.0 (5.75-7.76 ml/kg)
8 males/dose group mg/kg bw
Mouse: subcutaneous 1.0, 2.0, 3.0, 4.0, 3.6 ml/kg bw Snamprogetti (1980)
Swiss, 4.5, 5.0 or 5.5 ml/kg bw (2.93-3.57 ml/kg)
8 males/dose group
Rat: intravenous 0.1, 0.2, 0.3, 0.5, 0.75, 0.56 ml/kg bw in lethal doses: Snamprogetti (1980)
Wistar, 1.25 or 1.50 mg/kg bw nervous depression,
16 males/dose group sometimes followed
by short clonic
convulsions, autonomic
activity
(hypersalivation,
urination, defaecation),
and respiratory
disorders; death
occurred within
30 min. Surviving
animals: no toxic
symptoms or signs of
nervous depression
lasting more than
15-20 min
Table 17. (continued)
Species Injection Dose LD50 Comments Reference
route/site
Rat: hepatic parenchyma, 0.2 ml/kg bw intracaval injection Akimoto et al.
Sprague-Dawley, inferior vena cava, caused 100% mortality (1992)
7 males dosed tail vein or (pulmonary injury);
intravenously peritoneal cavity intrahepatic injection
caused 59% mortality
10 males dosed and peripheral vein
intraperitoneally injection 17% mortality;
the pulmonary injury
included congestion,
haemorrhage, and
interstitial oedema
Mouse: intraperitoneal 1.5, 2.0, 2.5, 3.0, 3.5, 1.4 ml/kg bw Snamprogetti (1980)
Swiss, 4.0, or 5.0 ml/kg bw
8 males/dose group
Mouse: intraperitoneal 0, 50, 200, or 500 mg/kg 830 mg/kg bw Arashidani et al.
ddy bw (784-878 mg/kg) (1993)
10 males/dose group
7.2.2 Eye irritation
Instillation of 0.05 or 0.1 ml of MTBE into the conjunctival area
of albino rabbits (both sexes) caused reversible eye irritation
(congestion of the conjunctiva, palpebral thickening and lacrimation,
more marked at the high dose) (Snamprogetti, 1980).
Moderate erythema and slight chemosis and discharge, persisting
for 3 days, were noted in another study after instillation of 0.1 ml
MTBE into the conjunctival area of New Zealand white rabbits
(Cuthbert, 1979):
24 h 48 h 72 h 7 days
redness 2.2 1.7 1 0.2
chemosis 1.3 1.2 1 0.1
discharge 0.9 0.6 0.2 0
The reactions had largely resolved by one week post-treatment).
The author concluded that MTBE was irritant to the rabbit eye.
Hazleton Laboratories (1979) conducted a study on nine New
Zealand White rabbits (sex not stated) in which six rabbits had 0.1 ml
MTBE instilled into one eye, the other acting as control, and three
rabbits had the same treatment followed by eye washout with water 30
seconds later. There was slight irritation (mean score for redness 1.5
and 1.0 at 24 and 48 h, negligible chemosis or discharge) in six
rabbits that did not have the eye washout, but slightly more marked
effects, including corneal opacity lasting for 24 h, in the three
rabbits with eye washout. The effects were all reversible.
Exposure to MTBE vapour in inhalation chambers also resulted in
eye irritation. In Fischer-344 rats exposed to MTBE vapour
concentrations of 14 300 or 28 600 mg/m3 (4000 or 8000 ppm) for 6 h,
lacrimation was noted 1 h post-exposure (Gill, 1989).
7.2.3 Respiratory tract irritation
A test of lung irritancy was used by Tepper et al. (1994) to
evaluate the effects of 1-h exposures of Swiss-Webster mice (four
males per dose group) to 300, 1000, 3000, 10 000 and 30 000 mg
MTBE/m3 in inhalation chambers. An almost immediate decrease in
frequency of breathing was observed in all dose groups. The animals
served as their own controls with baseline frequency of breathing and
respiration waveform morphology being obtained by exposure to filtered
air for 15 min. The severity of the irritant response was
dose-related, ranging from "slight" (13% decrease in respiration rate)
to "severe" at 30 000 mg/m3. During the exposures, breathing rate
returned to baseline after about 15 min, except for the highest dose
group, in which the rate gradually decreased for another 40 min. There
was a return to baseline frequency 15 min after end of exposure. Lung
injury could not be confirmed at the 30 000 mg/m3 dose level. Lung
lavage about 20 h post-exposure indicated only a marginal increase of
total protein and lactate dehydrogenase, both measures of lung cell
damage; however, these results were comparable to those of similarly
treated mice, exposed to filtered air, in a previous study.
In a single 6-h exposure vapour neurotoxicity inhalation study
(see also section 7.3) in Fischer-344 rats at target concentrations of
14 400 or 28 800 mg/m3 (4000 and 8000 ppm), survivors killed at the
end of a 14-day observation period had slight to mild lung hyperaemia
(Gill, 1989).
7.2.4 Skin sensitization
The sensitization potential of MTBE was assessed in guinea-pigs
using a Magnusson and Kligman procedure (Cuthbert, 1979). No evidence
of sensitization was reported in any of the 20 test animals following
induction and challenge with 1% MTBE. Dermal sensitization has also
been investigated using a Landsteiner technique (Litton Bionetics,
1980). Guinea-pigs were induced using intradermal injection (initial
treatment 0.5 ml of a 1% aqueous solution, followed by 9 injections of
0.1 ml over 3 weeks). No sensitization reactions were recorded at
challenge 2 weeks later (0.05 ml of a 0.01% solution of MTBE in
water).
7.3 Neurotoxicity
Following a single MTBE vapour exposure, reversible alterations
in central and peripheral nervous system function were observed (Gill,
1989; Daughtrey et al., 1997). Groups of Fischer-344 rats (22 of each
sex per dose level) were exposed for 6 h in inhalation chambers at
target MTBE concentrations of 0, 2860, 14 300 or 28 600 mg/m3 (0,
800, 4000 or 8000 ppm). No mortality or clinical signs of toxicity
were observed at any concentration. Behavioural evaluations performed
1, 6 and 24 h post-exposure included a screen for behavioural function
using a functional observational battery (FOB) and analysis of motor
activity prior to and following exposure. At 1 h post-exposure,
concentration-related increases in the incidence and severity of
ataxia and duck-walk gait appeared in both sexes of the mid- and
high-dose groups. In high-dose males, lacrimation, decreased muscle
tone, decreased rectal temperature, decreased performance time on the
tread mill, and increased hind limb splay were also observed. The
alterations in the FOB were significantly (p<0.01) different from the
control group. An increased incidence of laboured respiration (not
statistically significant) was also found in the high-dose male rats.
Additionally, piloerection was observed for all males in the high-dose
group. However, piloerection was also observed in some male rats in
the control group and in low- and mid-dose males.
Similar concentration-related findings were found in mid- and
high-dose female rats (increased incidence of lacrimation,
piloerection, ataxia and duck-walk gait, decreased rectal temperature,
and decreased hind limb grip strength). Additional exposure-related
findings for females in the high-dose group included a significantly
(p<0.01) increased incidence of laboured respiration and latency to
rotate on the inclined screen. Exposure-related alterations of motor
activity were detected for both male and female rats during the
initial 90 min of the test session. The mean activity was increased
during the entire test period in low-dose males and decreased in
high-dose males compared to the control group. However, in high-dose
males, an initial 10-min decrease in activity was followed by
increased activity during a 20-min interval and decreased thereafter.
Males in the mid-dose group only showed an increased activity
initially. For females, the mean activity for the entire test session
was not different from the control group. No MTBE-related alterations
were observed during the 6-h or 24-h post-exposure evaluation.
7.4 Short-term repeated dose studies
The short-term repeated dose toxicity of MTBE has been studied in
rats, mice and pigs. Typically, effects of irritation and reversible
central nervous system effects, including hypoactivity, ataxia, and
anaesthesia, were noted.
7.4.1 Oral studies
Sprague-Dawley rats (10 males and 10 females per dose group)
administered 0 (corn oil), 357, 714, 1071 or 1428 mg MTBE (99.95%
pure)/kg bw daily by gavage (in corn oil) for 14 days exhibited
transient anaesthesia at 1428 mg/kg and irritation of the pharyngeal
mucosa at the highest dose levels (Robinson et al., 1990). Diarrhoea
and reduced body weight gain were observed in all treatment groups.
Six animals (four from the high-dose group) died during treatment due
to difficulties associated with the gavage procedure, including
pharyngeal irritation in the high-dose animals. Absolute and relative
lung weights were significantly (p<0.001) lower in all exposed
female rats. The mean absolute kidney weights were increased in dosed
male rats and the relative kidney weights were significantly
(p<0.05 and p<0.001) increased over controls in the mid- and
high-dose group, respectively. The cholesterol levels were
significantly (p<0.05) increased in high-dose males and the two
mid-dose groups of females. The blood-urea nitrogen (BUN) and
creatinine were significantly (p<0.05) decreased in high-dose
females. The incidence of renal tubular disease (hyaline droplet
nephropathy) was moderately increased in dosed male rats. Increased
hyaline (protein) droplets within the cytoplasm of proximal tubular
epithelial cells were noted in seven of eight (88%) of the males in
the highest dose group as compared with two of five (40%) of the
controls.
In a 28-day oral study, Sprague-Dawley rats (10/sex/group) were
administered 0, 90, 440 or 1750 mg undiluted MTBE (purity not
specified)/kg bw daily by gavage for a total of 20 h (IITRI, 1992).
Seven rats (one low-dose female, one high-dose male, and five
high-dose females) died accidentally during dosing. This was
attributed to difficulties in dosing the animals owing to the strong
odour, the irritating nature, and high volatility of MTBE. Clinical
observations during the study period included transitory salivation in
all treated groups and transitory hypoactivity and/or ataxia in mid-
and high-dose animals. There were no significant effects on body
weight or body weight gain. The only significant treatment-related
change in haematological or clinical chemistry parameters was an
increase in cholesterol in high-dose males and females.
No treatment-related gross necropsy observations were noted. The
relative liver weights were significantly (p<0.05) increased in males
and females in the high-dose group. In the high-dose males there was
also a significant (p<0.05) increase in relative adrenal weight.
Absolute kidney and relative kidney weights showed a dose-related
increase in both males and females, but achieved statistical
significance (corrected for multiple comparisons) only for relative
weights in males at the mid- and high-doses and in females at the low
and high doses. Histopathology showed hyaline droplet formation in the
proximal convoluted tubules in the kidneys of the mid- and high-dose
males.
In a 90-day study, groups of Sprague-Dawley rats (ten males and
ten females in each test group) were gavaged 0 (corn oil), 100, 300,
900 or 1 200 mg/kg bw of undiluted MTBE (>99.95% pure) daily for 90
days (Robinson et al., 1990). The most pronounced clinical effect of
MTBE was the profound anaesthetic effect at 1200 mg/kg bw; the animals
recovered in about 2 h. In female rats, a significant (p<0.001)
treatment-related decreased BUN level and elevated cholesterol level
were observed at all levels of exposure. In male rats, the mean
absolute kidney weights were significantly (p<0.05) elevated at 900
and 1200 mg/kg bw, respectively. The increase in the relative kidney
weights was statistically significant (p<0.001) at the two highest
dose levels and also the relative liver weights (p<0.05 at 900
mg/kg and p<0.001 at 1200 mg/kg). In females, the relative kidney
weights were significantly (p<0.05) increased at and above 300
mg/kg bw and the relative liver, thymic and cardiac weights showed a
statistically significant (p<0.05) dose-related increase at 900
mg/kg. Microscopic findings included chronic nephropathy in both
control and high-dose male rats, which was more severe in MTBE-treated
rats. At the highest dose level, granular casts were found, and there
was also a slight increase of cytoplasmic hyaline droplets in proximal
tubular epithelial cells.
In a comparison study on the effects of inhalation exposure (see
section 7.3.2), two groups of female B6C3F1 mice (8/group) were given
MTBE by gavage in corn oil (5 ml/kg/bw) at doses of 0 or 1800 mg/kg bw
per day for 3 days and then killed around 18 h after the last dose
(Moser et al., 1996a). Body weight and liver weight were not affected
by treatment. MTBE induced a 37% increase in hepatic cytochrome P450
content (P <0-05), a 9-fold increase in hepatic 7-pentoxy
resorufin- O-dealkylase activity (PROD, a CYP2B marker) and a 2-fold
increase in hepatic 7-ethoxy-resorufin- O-deethylase activity (EROD,
a CAPE marker). MTBE also induced a 2-5-fold increase in the hepatic
cell labelling index (as estimated from the incorporation of
5-bromo-2-deoxyuridine delivered by an implanted osmotic mini pump) in
the absence of hepatotoxicity, judged by the absence of any change in
serum alanine aminotransferase (ALAT) or histological signs of
necrosis (Moser et al., 1996a).
7.4.2 Inhalation studies
Female B6C3F1 mice (five or more/group) were exposed by
inhalation to 0 or 27 900 mg/m3 (7814 ppm) MTBE (>99.95% pure) for 3
or 21 days (6 h per day, 5 days per week) (Moser et al., 1996a). The
exposure resulted in abnormal gait, hypoactivity, decreased muscle
tone and increased lacrimation during exposure and immediately after
termination of exposure to MTBE. The mice recovered quickly following
termination of exposure. There was no significant change in body
weight. The relative liver weights were increased 20% (p<0.05) as
compared to controls at 3 days of exposure but the difference was not
significant at 21 days. MTBE exposure significantly (p<0.05)
decreased relative uterine weight to 48% of the control at 3 days,
with a further significant (p<0.05) decrease to 65% at 21 days. The
relative ovarian weight was decreased to 69% (p<0.05) at 21 days of
exposure. Histopathological examination showed mild centrilobular to
midzonal hepatocyte swelling at 3 days. At 21 days there were no
microscopic exposure-related changes. The total hepatic microsomal
cytochrome P450 content was elevated 40% at 3 days and approximately
200% at 21 days. After 3 days and 21 days of MTBE exposure,
respectively, hepatic PROD activity increased 5-fold and 14-fold,
while hepatic EROD activity increased 1.8-fold and 3.2-fold. There was
a non-significant change in hepatic cell labelling index to a 2.5-fold
higher value at 3 days, but at 21 days the MTBE group showed a
significant reduction to 0.7% in comparison with the control value of
2.3% (p<0.05). There were no histological signs of hepatoxicity and
serum ALAT was unaffected.
Information on the repeated dose toxicity of MTBE (>99.95% pure)
is also available from a study comparing the short-term hepatic
effects on female B6C3F1 and CD-1 mice (Moser et al., 1996b). Groups
of six female mice were exposed in inhalation chambers to
particulate-free control air containing 0 mg/m3 or a target
concentration of 27 900 mg/m3 (7800 ppm) 6 h per day, 5 days per week
for 3 or 21 days. Clinical signs included abnormal gait, hypoactivity,
decreased muscle tone and increased lacrimation during exposure and
immediately after termination of exposure. The animals recovered
quickly after termination of each daily exposure. There was a decrease
of body weight (p<0.05) in CD-1 mice, but not in B6C3F1 mice, after
21 days. Statistically significant (p<0.05) increases were seen in
absolute and relative liver weight in both strains of mice at both
time points. Histopathological examination showed slight centrilobular
hypertrophy in both B6C3F1 mice and CD-1 mice at 3 days as compared
to controls. At 21 days there was no indication of hepatotoxicity or
necrosis. Serum ALAT showed no increased activity at either 3 or 21
days. In the CD-1 mice, after 3 and 21 days, respectively, total
hepatic microsomal cytochrome P450 content increased 2.3-fold
(p<0.05) and 1.8-fold (p<0.05), PROD increased 5-fold (p<0.05) and
5-fold (p<0.05), and EROD increased 2.3-fold (p<0.05) and 3-fold
(p<0.05). Corresponding data for the B6C3F1 mice are described
below. The hepatic cell labelling indices were, in contrast to B6C3F1
mice, increased in CD-1 mice at both time points; the increases were
3-fold and 5-fold, respectively, at 3 and 21 days (Moser et al.,
1996b). A slightly decreased survival relative to control was noted in
female B6C3F1 mice following 16 weeks exposure to 28 450 mg/m3 (7969
ppm) (100% versus 92% survival, respectively). After 32 weeks of
exposure, survival was 96% for controls and 88% for MTBE-exposed mice.
Body weight was significantly decreased (p<0.05) at both time points.
PROD activity was increased 4.9 fold, and EROD activity 1.9 fold,
after 16 weeks treatment. While MTBE exposure produced mild
centrilobular to midzonal hypertrophy, there was no indication of
cytotoxicity or hepatic necrosis, or alteration in serum ALAT (Moser
et al., 1996b).
Sprague-Dawley rats (10/sex/group) showed increasing depth of
anaesthesia with increasing concentrations of MTBE when exposed to
MTBE vapour in exposure chambers at concentrations of 0, 900, 1800 or
3600 mg/m3 (0, 250, 500 and 1000 ppm, respectively) 6 h per day, 5
days per week for 13 weeks (Greenough et al., 1980). The
haematological analyses revealed an increase in haemoglobin levels
during week 13 in male rats exposed to 3600 mg/m3. At autopsy, a
slight reduction in relative and absolute lung weight was detected in
female rats at the same exposure level. There were no other gross or
histopathological effects reported.
In a range-finding study, Fischer-344 rats and CD-1 mice were
exposed to MTBE vapour in inhalation chambers 6 h per day for 13
consecutive days (Dodd & Kintigh, 1989). The target concentrations
were 0, 7150, 14 300 and 28 600 mg/m3 (0, 2000, 4000 and 8000 ppm,
respectively). The measured mean concentrations were 7200, 13 750 and
28 330 mg/m3 (2018, 3850 and 7936 ppm, respectively). No mortality
occurred during the study period. Clinical signs included
hypoactivity, ataxia, and periocular irritation in both rats and mice,
primarily in the high-dose groups during exposure. Reversible
neurobehavioural alterations (ataxia) were observed immediately after
exposure in high-dose rats (mice were not observed). Body weight gain
was depressed in rats (but not in mice) in the mid- and high-dose
groups (statistically significant for male rats). In female mice, both
absolute and relative liver weights were significantly increased at
all levels of exposure. In the mid-dose rats, both absolute and
relative liver weights were significantly increased in females; in
males, there was an increase in relative kidney and liver weights. In
rats at the highest dose, males had an increase in relative weight of
liver, kidney and adrenal. Females had an increase in both absolute
and relative weights of liver and adrenal, and absolute weight of
kidneys was increased.
In a 13-week vapour inhalation study that included neurotoxicity
evaluation, Fischer-344 rats were exposed to target concentrations of
0, 2860, 14 300 and 28 600 mg MTBE/m3 (0, 800, 4000 and 8000 ppm) 6 h
per day, 5 days per week (Dodd & Kintigh, 1989; Lington et al., 1997).
No mortality occurred. Major findings included motor activity changes
and reversible changes in body temperature in mid- and high-dose rats,
ataxia, and depressed body weight gain and food consumption. Only mild
haematological changes (e.g., decreased erythrocyte counts and
increased reticulocyte counts) were observed, primarily in male rats.
The corticosterone levels were significantly increased in both male
and female rats at the highest exposure level. No treatment-related
gross lesions were found at necropsy. The relative weight of liver and
kidney were significantly increased in all male groups and in females
in the two highest exposure groups. Relative weight of adrenal was
significantly increased in males and females at the two highest doses.
There were, however, no treatment-related microscopic changes in these
organs. It is probable that there is an association between the liver
enlargement and the high serum corticosterone levels. (Although this
was reported by the authors, the Task Group considered that it was
more likely that the increase in adrenal weight was associated with
the high serum corticosterone levels). The only treatment-related
microscopic findings were found in males at the highest dose level and
included a statistically significant increase in lymphoid hyperplasia
in the submandibular lymph nodes, an increase (not statistically
significant) in the degree of haemosiderosis in the spleen, and a mild
increase of hyalin droplets in the renal proximal tubules. Proximal
tubule necrosis and protein droplet accumulation were observed in
kidneys from male, but not female, rats exposed to 5400 and 10 760
mg/m3 (1516 and 3013 ppm) for 6 h/day for 10 consecutive days
(Prescott-Mathews et al., 1997). Alpha-2u-globulin immunoreactivity
was present in and confined to protein droplets in male rat kidney. A
mild dose-related increase in alpha-2u concentration in the male rat
kidney correlated with an exposure-related increase in cell
proliferation. No significant differences were observed in female rats
for any of these responses.
7.4.3 Intraperitoneal administration
Katoh et al. (1993) reported that MTBE administered to mice (500
mg/kg bw as a single dose, or 200 mg/kg bw as repeated doses) caused
lipid peroxidation, as demonstrated by increased levels of lipid
peroxide in liver homogenates, and an induction of hepatic microsomal
cytochrome P450 content. Repeated treatment with 200 mg/kg bw for 4
weeks did not affect glutathione content or glutathione-S-transferase
(details on pattern and period of administration were not provided).
7.5 Neurotoxicity studies
A single 6-h inhalation exposure of Fischer-344 rats to MTBE
vapour at target concentrations of 0, 2880, 14 400 or 28 800 mg/m3
(0, 800, 4000 or 8000 ppm) induced reversible alterations in central
and peripheral nervous system function (Gill, 1989). Group of 8 male
and 8 female rats were used at each concentration for behavioural
evaluation and 14 males and 14 females for motor activity
observations. Motor activity changes appeared within 10 min of
exposure to 28 800 mg/m3 (8000 ppm) in both male and female rats. For
male rats, motor activity changes were also observed at 2880 and 14
400 mg/m3). Behavioural evaluation (functional observational battery,
FOB) was performed 1, 6 and 24 h post-exposure. At 1 h post-exposure
there were concentration-related behavioural alterations at 14 400
mg/m3 and 28 800 mg/m3 but these were not found at 6 or 24 h
post-exposure.
In a pilot study for a 13-week exposure study (see also section
7.3), Fischer-344 rats and CD-1 mice (five per sex and species) were
exposed to target MTBE concentrations of 0, 7150, 14 300 and 28 600
mg/m3 (0, 2000, 4000 and 8000 ppm) 6 h per day for 13 consecutive
days (Dodd & Kintigh, 1989). Hypoactivity was observed at all dose
levels and ataxia at the two highest dose levels in both rats and mice
during exposure. In high-dose rats, ataxia, decreased startle and pain
reflexes, and decreased muscular tone were also observed immediately
after exposure. Recovery was complete within 1 h. The NOAEL was
determined to be 7200 mg/m3 in both rats and mice.
The subsequent 13-week study with neurotoxic evaluation included
testing for inhalation toxicity, FOB, motor activity and
neuropathology (Dodd & Kintigh, 1989). Fischer-344 rats (25 rats of
each sex per dose level) were exposed to MTBE vapour at target
concentrations of 0, 2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and
8000 ppm) 6 h per day, 5 days per week (see also section 7.4.2).
Clinical findings included hypoactivity in the mid- and high-dose
groups and ataxia in the high-dose group immediately following the
daily exposure. The exposure resulted in minor changes in the FOB
including elevated body temperature (in high-dose male rats and in
mid- and high-dose female rats) and decreased hind limb grip strength
(in mid-dose males). Decreased motor activity for males in the
high-dose group and increased motor activity for females in the
low-and mid-dose groups were reported. Additional findings are
reported in section 7.3. Necropsy revealed no treatment-related gross
lesions. There were no treatment-related microscopic changes in the
central and peripheral nervous system tissues.
7.6 Reproductive and developmental toxicity
Protocols and results of reproductive/developmental studies are
presented in Table 18 These include one- and two-generation inhalation
studies in rats and four developmental studies (inhalation) in rats,
rabbits and mice. In these investigations, MTBE did not induce
specific adverse reproductive effects; developmental effects were
observed only at dose levels that were maternally toxic. At very high
dose levels (28 000 mg/m3) decreased relative uterine weight was
observed in one study.
Table 18. Reproductive studies with MTBE in laboratory animals
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
One- and two-generation studies
Rat, inhalation 15 males and 0, 1070, 4640 and one generation, two slightly decreased Biles et al.
Sprague-Dawley 30 females/group 12 140 mg/m3 litter study (F1a, F1b) pup viability (1987)
(0, 300, 1300 and (p<0.05) in F1b
3400 ppm) 12 140 mg/m3
Rat, inhalation 25 males and 0, 1430, 10 700, two generation study 1430 mg/m3: no Neeper-Bradley
Sprague-Dawley 25 females/group and 28 600 mg/m3 adverse effects; (1991)
(0, 400, 3000 and >10 700 mg/m3:
8000 ppm) reduced bw, bw
gain and food
consumption in
parental animals
(mainly in males),
clinical signs and
neurotoxic
effects, reduced
pup bw and bw gain
postnatally. NOEL
for general
toxicity 1430
mg/m3; NOEL for
reproductive
effects >28 600
mg/m3; LOEL for
adults and offspring
>10 700 mg/m3
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Developmental studies
Rat, inhalation 25 rats per dose 0, 900, 3600 and gestation days 6-15 reduced food Conaway et
Sprague-Dawley level 9000 mg/m3 consumption in al. (1985)
(0, 250, 1000 and treated groups
2500 ppm) during the day
9-12 interval; no
significant
developmental
toxicity
Mouse, inhalation 25 mice per dose 0, 900, 3600 and gestation days 6-15 a slight (not Conaway et
CD-1 level 9000 mg/m3 statistically al. (1985)
(0, 250, 1000 and significant),
2500 ppm) dose-related
decrease in food
and water
consumption; no
significant
developmental
effects
Mouse, inhalation 30 mice per dose 0, 3600, 14 300 gestation days 6-15 >14 300 mg/m3: Tyl &
CD-1 level and 28 600 mg/m3 clinical signs Neeper-Bradley
(0, 1000, 4000 and of toxicity and (1989)
8000 ppm) reduced fetal bw;
28 600 mg/m3:
reduced bw, bw
gain, and food
consumption;
increased number
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
of non-viable
implantations,
reduced number of
viable
implantations and
% male fetuses,
and increased
incidence of
cleft palate.
NOEL for maternal
and developmental
toxicity 3600 mg/m3
Rabbit, inhalation 8 females per dose 0, 7150, 14 300 and gestation days 6-18 >7150 mg/m3: Tyl (1989)
New Zealand level 28 600 mg/m3 (0, reduced food
White 2000, 4000 and 8000 consumption in
ppm) all dosed groups;
increased
incidence of
lung foci; 28 600
mg/m3: reduced
bw gain, audible
respiration, and
slightly lower
fetal weights
Table 18. (continued)
Species Route of Number of animals Dosage Time of treatment Results Reference
exposure
Rabbit, inhalation 15 females per 0, 3600, 14 300 gestation days 6-18 >14 300 mg/m3: Tyl (1989)
New Zealand dose level and 28 600 mg/m3 reduced bw gain
White (0, 1000, 4000 and food
and 8000 ppm) consumption;
28 600 mg/m3:
hypoactivity,
ataxia, increased
relative liver
weight, decreased
corrected
gestational weight
change and gravid
uterine weight. No
significant
developmental
effects; NOEL for
maternal toxicity
3600 mg/m3; NOEL
for developmental
toxicity >28 600
mg/m3
7.6.1 Reproductive toxicity
Biles et al. (1987) conducted a two-litter, one-generation
inhalation study of reproductive effects in CD Sprague-Dawley rats.
Target concentrations were 0, 890, 3600 and 8925 mg/m3 (0, 250, 1000
and 2500 ppm). Corresponding measured concentrations were,
respectively, 1070, 4430 and 10 640 mg/m3 (300, 1240 and 2980 ppm)
for females and 1030, 4210 and 10 210 mg/m3 (290, 1180 and 2860 ppm)
for males. Fifteen males exposed for 12 weeks were mated to thirty
females exposed for 3 weeks. Exposures continued throughout the mating
period, during gestation and through days 5-21 of lactation. A second
litter (F1b) was produced under the same mating and post-mating
exposure regimen. In the mid- and high-dose groups of the F1b
generation, there was a slight statistically significant (p<0.05)
decrease in pup viability. The authors felt that this was in large
part attributable to the high viability (99%) in the control group.
There were no treatment-related differences between control and
exposed animals based upon examination of clinical signs, gross
post-examination and histopathological examination of the gonads of
exposed adults, mating or fertility indices, pregnancy rates, mean
gestational length and number of pups at birth, litter survival
indices or pup weight. (The NOEL was above 8925 mg/m3 (> 2500 ppm)
in both parents and offspring).
In a two-generation reproductive and fertility inhalation study,
CD Sprague-Dawley rats were exposed to MTBE at concentrations of 0,
1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 or 8000 ppm)
(Neeper-Bradley, 1991; Bevan et al., 1997). There was parental
toxicity at the target concentrations of 28 600 mg/m3 and 10 700
mg/m3. Concomitant perinatal toxicity was also observed at these
concentrations. There were no treatment-related effects on
reproductive indices at any concentration and no adverse effects on
the offspring at concentrations that were not toxic to the parents.
(NOEL = 1430 mg/m3 (400 ppm); hypoactivity, lack of startle reflex
and blepharospasm in parents at 10 700 mg/m3 (3000 ppm); NOEL for
reproductive effects >28 600 mg/m3 (8000 ppm).
Inhalation exposure to 28 800 mg/m3 MTBE (99.95% pure) resulted
in significantly decreased relative uterine weight in B6C3F1 mice at
3 (48%) and 21 days (65%) of exposure as compared to controls (Moser
et al., 1996a) (see also section 7.4.2). The relative ovarian weight
was significantly (p<0.05) decreased at 21 days of exposure. There
were no exposure-related microscopic findings in ovaries, adrenals and
pituitary, and no effects on adrenal and pituitary weight. Moser et
al. (1996a) investigated if the decreased uterine weights were due to
an increased rate of estrogen metabolism by measuring the rate of
conversion of 3H-17B-oestradiol to water-soluble metabolites in
hepatocytes from MTBE-treated female mice. MTBE was found to increase
the oestrogen metabolism by 2.1-fold.
Ward et al. (1994) studied the toxicity of MTBE to germ cells in
CD-1 male and female mice. Groups of 10 mice were given 1, 10, 100 or
1000 mg/kg bw MTBE in corn oil by gavage 5 days per week for 3 weeks;
a negative control group received corn oil only. At the end of
treatment the mice were killed and one testis from each male and both
ovaries from each female were sectioned for cytological evaluation. In
males, sperm number, Sertoli cells, spermatogonia, spermatocytes and
capped spermatids were evaluated, and, in females, oocyte quality.
There were no effects of MTBE on any of the cell types examined.
7.6.2 Developmental toxicity
The results of four inhalation studies on the developmental
toxicity of MTBE are summarized in Table 18.
There was a significant decrease in food consumption on days 9-12
of gestation in pregnant rats exposed to as much as 9000 mg/m3 (2500
ppm) but no other effects that the authors considered to be maternally
toxic, embryotoxic or teratogenic (Conaway et al., 1985). The NOEL for
offspring and parents was >9000 mg/m3 (2500 ppm).
Two different studies were conducted in the same strain of mice
exposed to various concentrations of MTBE for 6 h/day on days 6-15 of
gestation (Conaway et al., 1985; Tyl & Neeper-Bradley, 1989). No
significant maternal toxicity or developmental effects were observed
when groups of 30 pregnant females were exposed to 1000, 3960 or 9675
mg/m3 (280, 1110 or 2710 ppm), though the incidence of lacrimation
was increased in exposed mothers and there was a slight increase in
the incidence of fused sternebrae in the high-dose group (Conaway et
al., 1985). The NOAEL of parents and offspring was 9000 mg/m3 (2500
ppm). Tyl & Neeper-Bradley (1989) concluded that 14 300 mg/m3 (4000
ppm) was maternally toxic, based on observed hypoactivity and ataxia.
There were significant decreases (p<0.01) in body weight, body weight
gain and food consumption at 28 600 mg/m3 (8000 ppm). At 14 300
mg/m3 (4000 ppm) or more, there were significant reductions in fetal
body weight per litter (p<0.01) and increased skeletal variation. The
proportion of male fetuses was significantly reduced (p<0.01) at the
highest dose level in the Tyl & Neeper-Bradley (1989) study, but this
has not been observed in other studies with mice, rats or rabbits. At
28 600 mg/m3 (8000 ppm), the number of non-viable implantations per
litter and incidence of cleft palate was also increased (LOAEL in
parents and offspring = 14 300 mg/m3 (4000 ppm). The NOAEL in this
study was 3570 mg/m3 (1000 ppm).
Maternal toxicity was observed at the two highest concentrations
in rabbits exposed to 3570, 14 300 and 28 600 mg/m3 (1000, 4000 and
8000 ppm) during days 6-18 of gestation (Tyl, 1989). No developmental
effects were observed at any exposure level [NOAEL in offspring = 28
600 mg/m3 (8000 ppm); NOAEL in parents = 3570 mg/m3 (1000 ppm);
LOAEL in parents = 14 300 mg/m3 (4000 ppm)].
7.7 Mutagenicity and related end-points
Genotoxicity study results with MTBE are generally negative.
However, there are indications that MTBE may have some genotoxic
potential in the presence of metabolic activation. Genotoxicity data
for MTBE are compiled in Table 19.
In studies on reverse mutation in Salmonella typhimurium, MTBE
was found to be non-mutagenic in tester strains TA1535, TA1537,
TA1538, TA98 and TA100, with and without S9 (liver enzyme homogenates
from induced Sprague-Dawley male rats) metabolic activation, at doses
up to 10 mg/plate (Cinelli & Seeberg, 1989).
No significant increase in the frequency of recessive lethal
mutations in the X-chromosome could be established after feeding MTBE
(99.14% pure) (0.03, 0.15 or 0.3% MTBE in 5% aqueous sucrose) to adult
Drosophila melanogaster (wild-type Oregon-R males) for 24 h (Sernau,
1989).
In the presence of a liver-derived metabolic system (liver S9
from Arochlor 1254-induced male Sprague-Dawley rats) MTBE (>99% pure;
1.0, 2.0, 3.0 or 4.0 ml/ml) induced forward mutations in vitro at
the thymidine kinase locus of mouse lymphoma cell line L5178Y/ TK+/-
(Mackerer et al., 1996). The observed mutagenicity was dose-dependent.
Other experimental data, using a test system developed to
determine if the mutagenicity of a material is the result of the
presence or release of formaldehyde, had indicated that the
mutagenicity was due to the metabolism of MTBE to formaldehyde
(Blackburn et al., 1991). To establish if formaldehyde, derived from
MTBE in the presence of S9, was responsible for the observed
mutagenicity, Mackerer et al. (1996) used a modified mouse lymphoma
assay. In this assay formaldehyde dehydrogenase and its co-factor
NAD+ were added during the exposure period so that any formaldehyde
produced would be converted to formic acid, which is non-genotoxic. An
MTBE dose-related increase in the frequency of mutant lymphoma cells
occurred without the presence of formaldehyde dehydrogenase and
NAD+, but not when these were present, indicating that formaldehyde
was responsible for the mutations.
In two independent in vitro experiments MTBE (purity not
specified) did not induce unscheduled DNA synthesis (UDS) in primary
rat hepatocytes at concentrations up to 10 mg/ml (Seeberg, 1989).
In an in vivo-in vitro hepatocyte UDS assay, ten male and ten
female CD-1 mice were assigned to each dose group and an air-only
control group and, in addition, five of each sex to a positive control
group (DMN) (Vergnes & Chun, 1994). The animals were exposed to MTBE
(purity not specified) in inhalation chambers 6 h a day for two
consecutive days. The target concentrations were 0, 1440, 10 800 and
Table 19. Genotoxicity studies with MTBE
Species Strain/cells Measured end-point Test conditions Activation Result Reference
Bacterial systems
S. typhimurium TA1535 reverse mutation 625, 1250, 2500, 5000, + - - - Cinelli &
TA 1537 10 000 µg/plate Seeberg (1989)
TA1538
TA98
TA100
S. typhimurium TA98 reverse mutation exhaust particle + nd Clark et al.
TA100 extracts from gasoline + ± (1984)
containing 7% by
volume MTBE; five
concentrations ranging
from 1 to 100 µg/plate;
duplicate plates
Non-mammalian eukaryotic systems
Drosophila wild type Oregon-R, sex-linked recessive Basc test; 0.03, - Sernau (1989)
melanogaster males lethal test 0.15 or 0.3% MTBE;
adult feeding for 24 h
In vitro mammalian systems
Mouse lymphoma cell line forward mutation 1.0, 2.0, 3.0, 4.0 µl/ml + + Mobil Oil
L5178Y/TK Corporation
(1993)
Rat primary hepatocytes UDS 3.16, 10.0, 31.6, 100, - Seeberg (1989)
316, 1000, 3160, 10 000
µg/ml
Table 19. (continued)
Species Strain/cells Measured end-point Test conditions Activation Result Reference
In vivo - in vitro
Mouse, primary hepatocytes UDS vapour exposure: 0, - Vergnes & Chun
CD-1 1440, 10 800, 28 800 (1994)
mg/m3, 6 h/day for 2
consecutive days
In vivo mammalian systems
Rat, bone marrow cells chromosome vapour exposure: 0, - Vergnes & Morabit
Fischer -344 aberrations 2800, 14 400, 28 800 (1989)
mg/m3, 6 h/day for
5 days
Mouse, spleen lymphocytes chromosome oral administration, a slight Ward et al.
CD-1 aberrations 1.0, 10, 100 or 1000 inverse (1994)
mg/kg bw for 3 weeks dose-response
relationship
in male mice
but not in
female mice
Mouse, spleen lymphocytes mutations at hprt oral administration, - Ward et al. (1994)
CD-1 locus 1.0, 10, 100 or 1000
mg/kg bw for 3 weeks
Mouse, bone marrow chromosome vapour exposure: 0, - Vergnes & Kintigh
CD-1 cells damage 1440, 10 800, 28 800 (1993)
mg/m3, 6 h/day for 2
consecutive days
28 800 mg/m3 (0, 400, 3000 8000 ppm). The animals were sacrificed and
hepatocytes were sampled 18 h after the second exposure day (for the
positive control group after approximately 2 h). No dose-related
increase in the DNA repair activity could be established. The UDS
assay is used to indicate primary DNA damage; this is, however,
transient in nature and it is necessary to analyse cells for damage as
soon as possible after cessation of treatment. Since the half-life of
MTBE in the animal body is quite short (1-3 h) and DNA repair is
relatively rapid, the study should have been designed accordingly.
MTBE (purity not specified) was considered nonclastogenic to
Fischer-344 rats in an in vivo test system (Vergnes & Morabit,
1989). No concentration-related or significant increase in the
incidence of chromosome aberrations in rat bone marrow cells was found
in either males or females following whole body exposure to MTBE 6 h
per day for five consecutive days. The target concentrations were 0,
2860, 14 300 and 28 600 mg/m3 (0, 800, 4000 and 8000 ppm,
respectively).
MTBE did not induce micronuclei in vivo in mouse bone marrow
cells (Vergnes & Kintigh, 1993). CD-1 mice were exposed to MTBE
(purity not specified) vapour in inhalation chambers (five animals per
sex per dose level) at concentrations of 0, 1430, 10 710 or 28 600
mg/m3 (0, 400, 3000 or 8000 ppm) 6 h a day for two consecutive days.
Bone marrow cells were collected 24 and 48 h after the second exposure
day. No significant, exposure-related increase in the frequency of
micronuclei could be established at any dose level and sampling time
in either sex in this study.
Ward et al. (1994) examined the frequency of somatic cell
mutations in spleen lymphocytes after administration by gavage 5 days
per week for 3 weeks with MTBE (99.8% pure) in corn oil to CD-1 male
and female mice (ten animals per dose group). Ethyl-nitrosourea was
used as a positive control. The doses were 1, 10, 100 and 1000 mg/kg
bw. The frequency of mutations at the hypoxanthine-guanine
phosphoribosyl transferase (hprt) locus was determined 3 weeks after
the cessation of exposure. There was no indication that MTBE produced
a mutagenic effect at the tested dose levels. Ward et al. (1994) also
analysed chromosome aberrations in spleen lymphocytes. This was
performed on the first day after the termination of exposure in 13
male mice and on the second day in the remaining mice. A slight, but
not statistically significant, inverse dose-relationship was seen in
male mice; this was not seen in the females.
7.8 Carcinogenicity
Three bioassays are available on the oncogenicity of MTBE in both
sexes of rats and mice. These include two inhalation studies, one in
Fischer-344 rats and one in CD-1 mice, and one oral study in
Sprague-Dawley rats (Table 20). At high inhalation exposure levels,
MTBE increased the incidence of renal cell carcinomas in male rats and
liver tumours in female mice. Oral administration increased the
incidence of lymphomas and leukaemias in female rats. The studies also
resulted in testicular tumours in both strains of rats following
exposure either by inhalation or oral administration.
Fischer-344 rats (50 of each sex per dose level) were exposed to
MTBE (99% pure) vapour in inhalation chambers at target concentrations
of 0, 1430, 10 700 or 28 600 mg/m3 (0, 400, 3000 and 8000 ppm,
respectively) 6 h per day, 5 days per week (Chun et al., 1992; Bird et
al., 1997). The control group was exposed to filtered air. Increased
mortality and decreased mean survival time were observed for male rats
from all exposure groups. Owing to the high mortality rate, surviving
males, six from the mid-dose group and nine from the high-dose group,
were killed at weeks 97 and 82, respectively. The numbers of surviving
males at the end of the study were 13, 6, 6 and 9 at 0, 1430, 10 700
and 20 600 mg/m3, respectively. Mean survival times were 632, 617
(p<0.05), 587 (p<0.01) and 516 (p<0.01) days, respectively.
Low-dose males and all females were killed during weeks 104 and 105.
Various clinical signs of toxicity (blepharospasm, hypoactivity,
ataxia, lack of startle reflex, swollen periocular tissue and
salivation) were observed in both sexes at the two highest dose
levels. No clinical signs were noticed at the lowest dose level.
Significantly (p<0.01) reduced body weight and body weight gain were
recorded at week 81 for the high-dose rats. For low- and mid-dose male
rats, body weight and body weight gain were slightly to significantly
(p<0.05) increased during the first 70 to 80 weeks. Thereafter, there
were no clear dose-related changes. In females, there was a slight,
but not exposure-related, decrease at the two lower exposure levels.
High-dose male rats also showed a significantly (p<0.05) decreased
corticosterone level at week 81. A trend toward increases in liver and
kidney weights relative to final body weight was recorded for the
mid-dose male rats. In female rats, concentration-related increases in
liver and kidney weight (absolute and relative to body or brain
weight) were observed at the two highest dose levels. There was also a
trend toward an increased adrenal gland weight relative to final body
weight for high-dose males. However, only the organ weight data from
the control and the low-dose group were statistically evaluated due to
the different sacrifice periods for the mid- and high-dose groups.
Non-neoplastic effects of treatment included an increased
incidence and severity of chronic progressive nephropathy in male rats
from all dose groups and in female rats from the mid- and high-dose
groups. Treated males were more severely affected than the females,
which usually showed only slight changes. Chronic progressive
nephropathy was diagnosed as the cause of morbidity or death for 3/37,
16/44, 26/44 and 39/41 male rats and for 0/20, 0/23, 4/27 and 6/25
female rats in the control, low-, mid- and high-dose groups,
respectively. The incidences of nephropathy, with interstitial
fibrosis, in the control, low-, mid- and high-dose groups were 19/37
(51%), 29/44, (66%), 37/44 (84%) and 40/41 (98%), respectively
(significance not specified). Histologically, the chronic progressive
nephropathy included an exposure-related increase in severity for
Table 20. Carcinogenicity studies with MTBE
Species Exposure Tumour Reference
Mouse (CD1) Male: Liver, adenoma: Burleigh-Flayer et al. (1992)
control 11/49 Bird et al. (1997)
1430 mg/m3 (400 ppm) 11/50
10 710 mg/m3 (3000 ppm) 9/50
28 600 mg/m3 (8000 ppm) 12/49
Male: Liver, carcinoma:
control 2/49
1430 mg/m3 (400 ppm) 4/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 8/49
Male: Liver, adenoma and carcinoma:
control 12/49
1430 mg/m3 (400 ppm) 12/50
10 710 mg/m3 (3000 ppm) 12/50
28 600 mg/m3 (8000 ppm) 16/49
Female: Liver, adenoma:
control 2/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 10/50 (p<0.05)
Female: Liver, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 1/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 1/50
Table 20. (continued)
Species Exposure Tumour Reference
Female: Liver, adenoma and carcinoma:
control 2/50
1430 mg/m3 (400 ppm) 2/50
10 710 mg/m3 (3000 ppm) 2/50
28 600 mg/m3 (8000 ppm) 11/50
Rat (F344) Male: Killed at: Chun et al. (1992)
control 104 weeks Bird et al. (1997)
1430 mg/m3 (400 ppm) 104 weeks
10 710 mg/m3 (3000 ppm) 97 weeks
28 600 mg/m3 (8000 ppm) 82 weeks
Male: Kidney, adenoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 5/50
28 600 mg/m3 (8000 ppm) 3/50
Male: Kidney, carcinoma:
control 0/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 3/50
28 600 mg/m3 (8000 ppm) 0/50
Male: Kidney, adenoma and carcinoma:
control 1/50
1430 mg/m3 (400 ppm) 0/50
10 710 mg/m3 (3000 ppm) 8/50
28 600 mg/m3 (8000 ppm) 3/50
Table 20. (continued)
Species Exposure Tumour Reference
Male: Testes:
control 32/50
1430 mg/m3 (400 ppm) 35/50
10 710 mg/m3 (3000 ppm) 41/50
28 600 mg/m3 (8000 ppm) 47/50
Male: Pituitary tumours, week 104:
control 6/50
1430 mg/m3 (400 ppm) 5/50
10 710 mg/m3 (3000 ppm) 0/50
28 600 mg/m3 (8000 ppm) 0/50
Female: Kidney, adenoma:
control 0/50
1430 mg/m3 (400 ppm) 0/28
10 710 mg/m3 (3000 ppm) 1/39
28 600 mg/m3 (8000 ppm) 0/50
Rat (Sprague-Dawley) Male: Testicular adenoma Belpoggi et al. (1995)
(denominator is total number
in group):
control 2/60
250 mg/kg 2/60
1000 mg/kg 11/60
Male: Testicular adenoma
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/26
250 mg/kg 2/25
1000 mg/kg 11/32 (p<0.05)
Table 20. (continued)
Species Exposure Tumour Reference
Female: Lymphomas or leukaemia
(denominator is total
number in group):
control 2/60
250 mg/kg 6/60
1000 mg/kg 12/60
Female: Lymphomas or leukaemias
(denominator is number
of animals surviving at
the time this tumour
first appeared):
control 2/58
250 mg/kg 6/51
1000 mg/kg 12/47 (p<0.01)
glomerulosclerosis, tubular proteinosis, interstitial nephritis and
interstitial fibrosis in both male and female rats from the mid- and
high-dose groups. The chronic nephropathy was also associated with
secondary lesions such as fibrous osteodystrophy, hyperplasia within
the parathyroid glands, and mineralization within numerous tissues. An
increased incidence of renal tubular cell adenomas and carcinomas was
noted in mid- and high-dose male rats. The incidence was 8/50 and 3/50
for the mid- and high-dose groups, respectively, and 1/50 for the
control group. The renal tubular cell carcinomas were only noted in
the mid-dose group (3/50). One renal cell adenoma was found in a
mid-dose female.
In mid- and high-dose males, there was also a dose-related
increase of interstitial cell (Leydig cell) adenomas of the testes.
The incidence was 32/50, 35/50, 41/50 and 47/50 (64%, 70%, 82% and 94%
for the control, low-, mid- and high-dose groups, respectively. In
high-dose males, there was also an exposure-related decrease in the
frequency of pituitary adenomas. The incidences were 27/47, 29/48,
27/47 and 2/48 in the control, low-, mid- and high-dose groups of
males, respectively. A treatment-related decrease of large granular
lymphocyte (LGL) leukaemia was also noted. The incidence of LGL in
males was 33/50, 22/50, 20/50 and 3/50 and in females 22/50, 14/50,
15/50 and 16/50 for control, low-, mid- and high-dose rats,
respectively. LGL leukaemia, which is age-dependent and generally does
not appear until 20 months of age, was the main cause of death in the
control and low-dose males. The lymphoid hyperplasia of the
submandibular lymph node observed in a 13-week inhalation study with
Fischer-344 rats at a dose level of 28 600 mg/m3 was not observed in
the present study using the same strain of rats and the same dose
level. No NOEL could be determined for male rats due to a slight
increase of nephropathy at the lowest dose level. For female rats, the
NOEL for toxicity was 1440 mg/m3.
A number of points regarding this study can be made:
a) The tumour incidence values were analysed using methods that are
not appropriate when there are marked inter-group differences in
survival.
b) Testicular adenomas are quite common in untreated aging male
F-344 rats (Haseman et al., 1990), with a spontaneous incidence
in the range 64-98% for animals contemporaneous with those used
here. On this basis, it appears that this tumour type may have
been under-represented in the concurrent controls, influencing
the slope of the dose-response curve. In the Fischer-344 rats
used in this laboratory, the average historical control incidence
of Leydig cell tumours was 88% (Bird et al., 1997). Thus, in
comparison with historical control data, there was no increased
incidence in this tumour in the dosed groups. However, concurrent
control comparisons are always more appropriate, unless it is
known that there had been a particular problem with the study,
e.g., inappropriate randomisation.
c) Neoplasm incidence decreases were observed for pituitary adenomas
in the high-dose males and for large granular lymphocyte
leukaemia in males and females at all dose levels. Decreases in
the high-dose rats might be related to body weight gain
restrictions in this group and to increased mortality rate in
males. Alternatively, the concurrent control values for these
neoplasms may be particularly high in this study.
An oncogenicity study was also carried out on CD-1 mice exposed
to MTBE (99% pure) vapour in inhalation chambers (Burleigh-Flayer et
al., 1992; Bird et al., 1997). Groups of 50 male and 50 female mice
were exposed to target concentrations of 0, 1430, 10 700 and 28 600
mg/m3 (0, 400, 3000 and 8000 ppm, respectively) 6 h per day, five
days per week for 18 months. The control group was exposed to filtered
air. The mortality rates for male mice (including those sacrificed
moribund but excluding procedural and accidental deaths) in the
control, low-, mid- and high-dose groups were 33%, 22%, 35% and 49%,
respectively. The corresponding values for females were 27%, 18%, 23%
and 33%, respectively. The authors reported that increased mortality
rate (significance not reported) and decreased survival time were
observed for male mice from the high-dose group only. This was
considered as a probable result of a slightly increased frequency of
obstructive uropathy (distended urinary bladder and/or obstruction of
the urethra). Clinical signs, i.e. ataxia, blepharospasm,
hypoactivity, prostration, and lack of a startle reflex (in the
high-dose group prostration also and in the mid-dose group stereotypic
behaviour also) were observed in both male and female mice at the two
high-dose levels. Ataxia, observed in most male and female mice from
the highest dose group throughout the study, was the only clinical
finding considered to be exposure related. Body weight and body weight
gain were decreased for both male and female mice from the high-dose
group. At the end of the study, body weight gain was decreased 15%
(p < 0.01) for the males and 24% (p<0.01) for the females from the
high-dose group.
Necropsy showed that the liver was the target organ for toxicity.
There was a dose-related increase in liver weight, both absolute and
relative to body weight, in both sexes (increase in absolute weight in
males significant at all dose levels). A slight (significant),
although not concentration-related, increase in kidney weight was
noted for male mice from all exposure groups and in female mice from
the high-dose group. Decreases, although not statistically
significant, in absolute brain and spleen weight were also noted for
high-dose male and female mice. In addition, an increase in serum
corticosterone levels was observed in both male and female mice from
the high-dose group at week 79. The increase was significant (p<0.05)
only for male mice. A slight decrease in urinary pH and increases in
urine gamma globulin were observed for both male and female mice at
the high-dose level.
For male mice that were found dead in the high-dose group (7/25),
a slightly increased frequency of urinary bladder dilation/ distension
was noted at autopsy as compared to controls (3/18). In addition, the
incidence of the number of liver masses was increased in the high-dose
male mice (13/50 compared to 7/50 for the control group). The only
exposure-related lesion in female mice found at necropsy was an
increased incidence of liver masses in the high-dose group (9/50) when
compared to the controls (0/50). Histopathology showed an
exposure-related increase in hepatocellular hypertrophy in high-dose
male mice (15/49) when compared to controls (5/49). This lesion also
showed an increased, although not statistically significant, incidence
in mid-dose male mice (10/50) and in female mice from the high-dose
group (9/50 as compared to 4/50 in the control group). Exposure to
MTBE did not, however, cause hepatocellular necrosis or degeneration.
In high-dose male and female mice, mineralization within the brain was
decreased. In addition, there was a dose-related decrease in the
incidence of cystic endometrial hyperplasia for female mice.
An increased frequency (not statistically significant) of hepatic
adenomas and carcinomas was observed in male mice at the high-dose
level (16/49 as compared to 12/49 in the control group). The increase
(not statistically significant) was due to a slightly increased
frequency of hepatocellular carcinomas in the high-dose group (8/49)
when compared to the controls (2/49). The analysis for the combined
incidence of hepatocellular adenomas and carcinomas did not, however,
include statistical methods that adjusted for difference in survival
between the control and exposure groups. In female mice, there was a
significant increase in the incidence of hepatocellular adenomas at
the high-dose level (10/50 as compared to 2/50 from the control
group). The induced incidences were modest and occurred in the group
in which hepatocellular hypertrophy also occurred. There was no
exposure-related increase in the incidence of hepatocellular
carcinomas in female mice. The NOEL for toxicity in mice exposed to
MTBE for 18 months was 1440 mg/m3.
In an oral exposure study, male and female Sprague-Dawley rats
(60 per sex and dose group) were administered 0, 250 or 1000 mg/kg bw
MTBE (>99% pure) in 1 ml extra virgin olive oil by gavage four times
a week for 104 weeks on a weekly schedule of 2 days dosing, 1 day
without dosing, 2 days dosing, followed by 2 days without dosing
(Belpoggi et al., 1995). There were no treatment-related differences
in mean body weights of treated groups compared to control groups. The
animals were kept under observation until natural death. High-dose
male rats showed a higher survival than controls at treatment week 80
and thereafter. At week 80, survival was approximately 56% in control
and exposed groups; at 112 weeks, it was approximately 10% in controls
and the low-dose group, but 35% in the high-dose group. In female
rats, a dose-related decrease in survival was observed from
treatment-week 16. It was reported that there were no evident
behavioural changes; at week 72, survival in the high-dose group was
about 65%; at week 120, it was less than 20%; at week 136, it was less
than 5%. The authors reported no evident behavioural effects; however,
the extent of examination of behavioural effects was not specified. No
relevant non-neoplastic changes (including renal) were detected at
autopsy and histopathology. No specific data were, however, reported.
In male rats, there was a dose-related increase in the incidence of
testicular Leydig cell (interstitial cell) tumours (2/60, 2/60 and
11/60), statistically significant at the highest dose (p<0.05). In
female rats, there was a dose-related increase in lymphomas and
leukaemias combined (2/60, 6/60 and 12/60), marginally significant at
the low-dose level and highly significant at the high-dose level
(p<0.01). There also was an increase in dysplastic proliferation of
lymphoreticular tissue in female rats at both dose levels, but the
incidence was higher in the low-dose group. Dose-related decreases in
mammary fibromas and fibroadenomas, and in pituitary adenomas and
tumours of adrenal glands were observed in dosed females. Since these
tumours and the testicular interstitial cell tumours are
age-dependent, these effects were probably at least partly due to the
dose-related early mortality and the prolonged observational period in
the surviving animals.
A number of points regarding this study should be made:
a) there is limited description of the results, particularly the
histopathological findings;
b) diagnostic criteria are not given for the distinction between
Leydig cell tumours and hyperplasia (the latter were not reported
at all, which is unusual for old Sprague-Dawley rats showing
Leydig cell tumours);
c) diagnostic criteria are not given for the distinction between
dysplastic hyperplasia and lymphoma;
d) lymphomas and leukaemias are pooled; specific tumour type and
incidences were not reported;
e) historical control data might aid the evaluation of lymphomas and
leukaemias, particularly if they are available for these rats
within different age ranges;
f) chronic progressive nephropathy was not observed in these
Sprague-Dawley rats, although these lesions might be expected, on
the basis of data from a number of other studies with this strain
of rat.
7.8.1 Initiation-promotion protocol
In a study to investigate if MTBE exhibited hepatic
tumour-promoting activity, 12-day-old female B6C3F1 mice were
initiated with a single intraperitoneal injection of the mutagen
diethylnitrosamine (DEN) or saline and then exposed subsequently to 0
or 28 800 mg/m3 (8000 ppm) MTBE (>99.95% pure) from 8 weeks of age
for 16 or 32 weeks (Moser et al., 1996b). Liver weight was
significantly (p<0.05) increased at both time points in both
saline-treated and DEN-initiated mice after exposure to MTBE, and was
associated with mild centrilobular to midzonal hypertrophy in both
groups. There was no significant difference in the percentage of
microscopic lesions classified as hepatic foci (86%), hepatocellular
adenomas (10%), or hepatocellular carcinomas (4%) in DEN/MTBE mice as
compared to DEN/control mice. However, the absolute number of
microscopic hepatic lesions was 50% less in the DEN/MTBE group than in
DEN/control mice. MTBE appeared inactive in this tumour
initiation-promotion assay.
7.9 Metabolites of MTBE
Animal carcinogenicity experiments have been conducted with the
metabolites tertiary-butyl alcohol (TBA) and formaldehyde (FA). TBA
administered in drinking-water caused increased incidences of renal
tubular adenoma and carcinoma in male Fischer-344 rats and increased
severity of chronic progressive nephropathy (Cirvello et al., 1995).
In female B6C3F1 mice, TBA produced thyroid follicular cell adenoma
and hyperplasia, and, in both male and female mice, inflammation and
hyperplasia of the urinary bladder (Cirvello et al., 1995). FA has
caused nasal squamous cell carcinoma in both Fischer-344 and
Sprague-Dawley rats and in B6C3F1 mice, and also increases of cancers
of the nasopharynx, nasal cavity and sinus in humans (Grindstaff et
al., 1991).
7.10 Mode of action
7.10.1 Kidney tumours
A number of chemicals cause both protein droplet nephropathy and,
with chronic exposure, renal cancer in male rats only (Borghoff et
al., 1990). The proposed mechanism by which these chemicals cause
renal tumours in male rats is based on their ability to cause protein
droplet nephropathy by accumulating alpha2u-globulin, a
male-rat-specific protein of low relative molecular mass. Evidence
suggests that chemical binding to alpha2u-globulin makes the protein
more resistant to hydrolysis, which accounts for its accumulation in
the renal lysosomes in the form of protein droplets. The chemically
induced accumulation of alpha2u-globulin is thought to be responsible
for cytolethality, which in turn stimulates cell division as the
kidney attempts to repair itself. Chronic chemical exposure with
repeated cycles of cytolethality and reparative replication is
probably the cause of the renal tumours in male rats (Borghoff et al.,
1990; Hard et al., 1993). A protein similar to alpha2u-globulin has
not been detected in human kidneys (Borghoff & Lagarde, 1993).
MTBE causes male-rat-specific renal tumours with chronic exposure
(Bird et al., 1997). MTBE also causes the accumulation of protein
droplets in male but not female rats following exposure ranging from
10 days to 13 weeks (Dodd & Kintigh, 1989; Chun & Kintigh, 1993;
Prescott-Mathews et al., 1997). Similar results have been observed
with TBA, a metabolite of MTBE (Lindamood et al., 1992).
alpha2u-Globulin immunoreactivity was present in and confined to
protein droplets in the kidneys of male rats exposed to MTBE in these
studies (Dodd & Kintigh, 1989; Chun & Kintigh, 1993; Prescott-Mathews
et al., 1997). Although a slight increase in alpha2u-globulin-positive
staining was observed in male rats exposed to MTBE, as compared to
controls, a linear exposure-related increase was not observed in any
of these studies. alpha2u-Globulin-positive proteinaceous casts at the
junction of the proximal tubules and the thin limb of Henle were not
observed (Swenberg & Dietrich, 1991). MTBE caused an
exposure-dependent mild increase in the renal concentration of
alpha2u-globulin measured in male rats (Prescott-Mathews et al.,
1997). Immunohistochemical staining of alpha2u-globulin is probably
not as sensitive as actually quantifying the alpha2u-globulin levels
with a mild increase in this protein. Further analysis of the kidney
protein profile from control and MTBE-treated male rats confirmed the
accumulation of alpha2u-globulin with no other protein detected
(Prescott-Mathews et al., 1997).
MTBE-induced kidney lesions, characterized by tubular necrosis
and protein droplet accumulation, were mild, especially when compared
with strong inducers of alpha2u-globulin nephropathy. Additionally,
granular casts, considered characteristic for alpha2u-globulin
nephropathy, were not observed in all studies. In the case of the
10-day MTBE inhalation exposure, however, there was a
concentration-dependent increase in kidney necrosis with minimal
sloughing of epithelial cells in male rat kidney following exposure to
10 710 mg/m3 (3000 ppm) (Prescott-Mathews et al., 1997).
In male and female rats exposed to MTBE vapour for 10 and 28
days, MTBE caused enhanced cell proliferation in male, but not female,
rat kidneys (Chun & Kintigh, 1993; Prescott-Mathews et al., 1997). A
strong positive correlation was observed between the cell
proliferative response and the concentration of alpha2u-globulin in
the kidneys of MTBE-exposed male rats.
With many of the chemicals that cause alpha2u-globulin
nephropathy, either the chemical or a metabolite has been found to
bind reversibly to alpha2u-globulin. In vitro, a high uptake of MTBE
into male rat kidney homogenate could be predicted using a
two-compartment model system when the binding of MTBE to
alpha2u-globulin was described using a dissociation constant of 10-4
M (Poet & Borghoff, 1997). This estimated dissociation constant for
MTBE binding to alpha2u-globulin was found to be similar to the
dissociation constant previously measured for 1,4-dichlorobenzene, a
known inducer of alpha2u-globulin nephropathy and a chemical
identified as bound to alpha2u-globulin following treatment of male
rats with 1,4-dichlorobenzene (Charbonneau et al., 1989). Together
these findings indicate that MTBE interacts, although weakly, with
alpha2u-globulin and induces alpha2u-globulin nephropathy and renal
cell proliferation in male, but not female, rats.
MTBE increases the severity of chronic progressive nephropathy in
both male and female rats, but does not cause renal necrosis, enhanced
cell proliferation or renal cancer in female rats. Chronic progressive
nephropathy alone is not associated with increased incidence of renal
tumours.
7.10.2 Liver tumours
It has been hypothesized that liver tumours in female mice can be
promoted by interfering with the estrogen-mediated suppression of
preneoplastic foci. In this regard, both unleaded gasoline and MTBE
induce cytochrome P450 activity and estrogen metabolism in mouse
hepatocytes, induce a mitogenic response in mouse liver, and decrease
uterine and ovarian weight in exposed mice. Additionally, unleaded
gasoline promotes DEN-initiated female mouse liver tumours, but this
response has not been observed with MTBE (Standeven & Goldsworthy,
1993; Moser et al., 1996b). Therefore, the relevance of this
hypothesis to an interpretation of the MTBE-induced mouse liver
tumours is currently unclear.
8. EFFECTS ON HUMANS
As explained in chapter 3, two separate fuel programmes in the
USA legally require the use of oxygenate in gasoline to address
ambient air quality objectives. Although no specific oxygenate is
required, MTBE has dominated the USA market place and is used at 15%
(by volume) to meet a 2.7% (by weight) oxygen requirement for
oxygenated gasoline in the cold-weather season in areas with excessive
carbon monoxide levels and at 11% (by volume) to meet a 2.0% (by
weight) oxygen requirement for reformulated gasoline sold year-round
in areas with excessive ozone levels. In the fall of 1992, shortly
after the introduction of oxygenated gasoline containing 15% MTBE in
Alaska, consumer complaints were registered about health effects such
as headaches, eye irritation and cough in Fairbanks (Beller &
Middaugh, 1992) and Anchorage (Chandler & Middaugh, 1992).
Subsequently, residents in other places in the USA also reported
health complaints associated with the introduction of cold-season
oxygenated fuel. Somewhat similar public concerns were raised in
Milwaukee, Wisconsin, with the introduction of reformulated gasoline,
some of which contained 11% (by volume) MTBE, in January 1995
(Anderson, 1993; Anderson et al., 1995). Health complaints have also
been registered by some occupationally exposed individuals, such as
tank truck drivers handling bulk MTBE (Gillie, 1993).
These "outbreaks" of health complaints prompted several field and
experimental studies as well as other assessments (e.g., HEI, 1996; US
Interagency Assessment, 1997) of available data and information
generated by these studies. In this section epidemiological studies
will be described first, followed by controlled chamber studies of
human volunteers. Because non-occupationally as well as occupationally
exposed populations were investigated in the epidemiological studies,
these studies will be discussed in one section rather than two
separate sections devoted to general population and occupational
population exposures.
8.1 Population studies
In December 1992, Moolenar et al. (1994) undertook a pilot study
in Fairbanks, Alaska, to investigate the possible relationship between
MTBE exposure and health complaints. In Phase I of the study, exposure
was evaluated by air sampling and by analysing blood samples for MTBE
and TBA in 18 workers heavily exposed to gasoline fumes and exhaust in
their workplace (e.g., service station workers, mechanics, meter
readers). A questionnaire administered to the workers asked about 15
symptoms: seven that had been most frequently reported to a local
telephone hotline the previous month (headache, eye irritation,
burning of the nose or throat, cough, nausea or vomiting, dizziness,
and a sensation of spaciness or disorientation), and eight other
symptoms (fatigue, fever, sweats or chills, diarrhoea, fainting or
black-out spells, skin irritation or redness, muscle aches, and
difficulty breathing). Phase II of the study was conducted in February
1993, after the oxygenated gasoline programme was suspended, and
included 28 (12 of the original) occupationally exposed subjects. Four
workers whose post-shift blood MTBE levels were in the top quartile
(>9.6 µg/litre) all had one or more of the seven key health
complaints, compared with 9 of 14 workers whose levels were in the
lower three quartiles. This finding was not statistically significant,
but the study may not have had adequate statistical power to detect a
relationship, owing to the small sample size. In Phase II, only one
worker reported a health complaint (nausea). Exposures to MTBE, as
well as complaints of both workers and non-occupationally exposed
residents of Fairbanks, declined significantly after the termination
of the oxygenated gasoline programme (CDC, 1993), but interpretation
of these events is possibly confounded by several factors, including a
lack of representative sampling and changes in the cost of gasoline
that may have contributed to public attitudes.
Mohr et al. (1994) conducted a cross-sectional cohort study that
included 237 garage workers exposed to high and low MTBE
concentrations: 115 workers exposed in northern New Jersey during the
wintertime oxyfuel programme and 122 workers in southern New Jersey 10
weeks after the phase-out date for the programme. Both groups of
workers reported feeling significantly worse at the end of the work
day, but there was no difference between the groups across the work
shift. Active air sampling and passive sampling devices confirmed the
higher exposure levels of the workers in northern New Jersey. No
significant differences were found in either the cross-sectional
reporting of symptoms or the pre- and post-shift analyses.
A study performed in Stamford, Connecticut, in April 1993 near
the end of the oxygenated gasoline season there, investigated exposure
to MTBE in oxygenated gasoline and symptom prevalence in
occupationally and non-occupationally exposed subjects (White et al.,
1995). The study included 37 workers and 14 commuters. The prevalence
of symptoms was highest among people who worked in car repair-shops or
around traffic. The eight workers with the highest levels of MTBE in
blood (>3.8 µg/litre) reported one or more key symptoms (OR = 21.0,
95% CI = 1.8-539.0) such as headache, irritated eyes, burning of the
nose and throat, cough, dizziness, spaciousness, disorientation, and
nausea. There were no reports of diarrhoea, difficulty in breathing,
skin irritation, fever, sweats or chills, or fainting.
In response to health concerns raised by the public following the
introduction of the reformulated gasoline (RFG) programme in the
Milwaukee, Wisconsin area, the Wisconsin Department of Health
initiated a random digit-dial telephone survey designed to assess the
prevalence and scope of health complaints (Anderson et al., 1995). A
questionnaire was administered to approximately 1500 persons: 527
residents of the Milwaukee, where RFG was sold and numerous complaints
about the fuel had been registered; 485 residents of Chicago, where
essentially the same fuels were sold but few complaints had been
registered; and 501 residents of the remainder of Wisconsin, where RFG
had not been required. Overall, there was a significantly higher
prevalence rate for "unusual symptoms" in Milwaukee (23%) than in
Chicago and the rest of Wisconsin (6% each). The fact that symptom
prevalence in the Chicago RFG area was so similar to that in non-RFG
areas of Wisconsin suggested that factors other than RFG use
contributed to the difference between Milwaukee and the other two
areas. Although the prevalence of colds and flu was the same in the
three areas, Milwaukee residents were more likely to report unusual
symptoms if they had experienced a cold or the flu, smoked cigarettes,
or were aware that they had purchased RFG. The authors concluded that
many symptoms reported by Milwaukee residents may have actually been
due to colds or flu rather than RFG exposure. Also, individuals who
reported purchasing RFG were more likely to report symptoms than
individuals who said they had not purchased or did not know whether
they had purchased RFG, which suggested that knowledge about RFG may
have increased awareness of an individual's health status and resulted
in the assumption that any health symptoms experienced were unusual
and attributable to gasoline exposure. This study and its conclusions
were reviewed by a panel of independent experts who concluded that
"The study does not support a conclusion that exposure to RFG is
associated with widespread or serious acute adverse health effects."
This evaluation of the study was later endorsed by another,
independent peer review group (HEI, 1996).
Anderson et al. (1995) followed up on 1280 Milwaukee residents
who had initially contacted governmental agencies about their health
complaints. These self-identified individuals were interviewed by
telephone to determine the types of complaints and risk factors that
could be associated with symptoms. Results of the study indicated that
the strongest predictors were age, allergies, and colds or flu since
November 1994. The purchase of RFG, a surrogate for exposure, did not
correlate with self-reported health symptoms.
A cross-sectional study was conducted in Finland to investigate
the occurrence of neurophysiological symptoms in tanker drivers
exposed to gasoline containing approximately 10% MTBE (Hakkola et al.,
1996). A reference group of milk delivery drivers was selected from
the same areas. A total of 201 male drivers participated in the study,
including 101 tanker drivers and 100 milk delivery drivers. The
occurrence of symptoms and Profile on Mood States (POMS) scales showed
an association with age, chronic diseases, and the perceived health of
the drivers in both the exposed and the unexposed group. Although
there were more sensory and motor symptoms in tank drivers, there was
no evidence of any statistically significant difference in the
occurrence of symptoms between the two groups. Duration of work as a
driver, shift schedule and length of the working week had no
statistical connection with symptoms and the modified POMS scales.
8.2 Controlled studies
To determine if 1-h exposures to MTBE at 6 mg/m3 (1.7 ppm) in an
inhalation chamber could result in similar symptoms as those reported
in the field, Cain et al. (1996) performed a controlled study in 22
male and 21 female subjects (ages 18 to 34 years). Both objective and
subjective indices of behavioural and physiological effects were
studied. A control exposure to air and a control exposure to a
17-component mixture of volatile organic compounds (VOCs) (19 mg/m3)
were also included. The selected MTBE concentration and duration were
based upon preliminary results from exposure studies in commuters
(Lioy et al., 1994). The effects of MTBE exposure on discomfort,
symptoms and possible objective correlates of symptoms were
investigated by using questionnaires, various ocular and nasal
inflammation parameters, and neurobehavioural testing. Repeated blood
samples were obtained from a subset of subjects to relate the exposure
to the body burden and toxicokinetics of MTBE (see section 6.1.1). The
exposure produced a mean peak blood MTBE level of 17 µg/litre.
Subjective reactions, such as irritation, fatigue and headache,
typically showed greater sensitivity than objective indices. There was
a differential effect of gender on rated odour, intensity and
pleasantness. Females found odour intensity greater, pleasantness
worse, and air quality worse. Ocular measurements indicated a mild
tendency for eye irritation during exposure; this was, however, not
statistically significant. MTBE caused no objective inflammatory
changes in the nasal mucosa. Aside from odour, no significant
reactions were found.
In another controlled study, conducted by Prah et al. (1994), 20
male and 20 female volunteer subjects (healthy and non-smokers) were
exposed in an inhalation chamber to MTBE at 5 mg/m3 (1.39 ppm) for
1 h. Symptom questionnaires, cognitive testing, and objective measures
of ocular and nasal irritation were obtained before and at the end of
the exposure. In addition, the odour threshold of MTBE and some
pharmacokinetic data were obtained from two additional subjects, one
male and one female (see section 6.1.1). No increase in the reporting
of symptoms such as headache, nasal irritation, cough or eye
irritation was found, apart from a gender effect in reporting of air
quality. Females rated the air quality in the chamber with MTBE
exposure slightly poorer when compared to the clean air exposure.
There were no changes in objective indicators in either the eye or the
nose.
Johanson et al. (1995) and Nihlén et al. (1998b) reported similar
results at much higher concentrations. Ten healthy male volunteers
were exposed to MTBE vapour at 18, 90 or 180 mg/m3 (5, 25 or 50 ppm)
for 2-h periods while performing light physical exercise (see also
section 6.1.1). All the subjects reported the strong smell of MTBE on
entering the chamber but their rating of the smell decreased with
time. On the basis of subjective assessment there was no irritation of
the ocular, nasal or pharyngeal mucosae. The MTBE exposure induced
essentially no effects on eye and mucous membrane irritation.
Riihimaki et al. (1996) assessed a number of subjective (e.g.,
irritant sensations, mood state) and objective (simple reaction time,
postural sway) end-points in 13 male volunteers exposed to 0, 90 or
268 mg/m3 (0, 25 or 75 ppm) MTBE for 1 or 3 h. The authors concluded
that only "mild symptoms, mainly a feeling of heaviness in the head
and, to a smaller extent, of mild mucous membrane irritation, were
reported". The frequency of symptoms was related to the level of MTBE
exposure and reached statistical significance at 268 mg/m3 (75 ppm)
after 3 h of exposure. There was no effect on reaction time or
postural sway.
8.3 Subpopulations at special risk
There are no data by which to identify any subpopulations (e.g.,
the elderly, pregnant women, children or people with allergy or
asthma) who might be at special risk to MTBE exposure.
8.4 Special studies
8.4.1 Organoleptic properties
For many people, MTBE has a quite distinctive odour. Odour
detection thresholds have been reported to average around 0.1 to
0.2 mg/m3 (0.03-0.05 ppm), with average recognition (identification)
thresholds in a range from 0.2 to 0.5 mg/m3 (0.06-0.13 ppm) for neat
MTBE vapour (TRC, 1993; Smith & Duffy, 1995). For gasoline containing
15% (by volume) MTBE in the USA, average odour detection thresholds
ranged from approximately 0.3 to 3 mg/m3 (0.08- 0.9 ppm), with
recognition thresholds ranging from 0.7 to 2.5 mg/m3 (0.2-0.7 ppm).
Odour thresholds for MTBE-oxygenated gasoline may vary considerably,
depending in part on the aromatic and other constituents of the
gasoline and the sensitivity of the individuals who inhale the
vapours. The addition of MTBE to gasoline has been found to reduce the
detection threshold (i.e. increase "detectability") for gasoline by as
much as 80% (HEI, 1996).
8.4.2 Immunological effects
In a study to assess the effects of MTBE on the immune system,
interleukin levels were measured in blood plasma of 22 volunteers at
several different locations around Fairbanks exposed to auto emissions
derived from oxyfuel (Duffy, 1994). The study was performed during a
4-week period in late November and early December 1992. During this
period, the mean daily temperature ranged from about -1.5°C (35°F) to
about -37°C (-38°F). Plasma interleukin 1 ß (IL-1 ß) and interleukin 6
(IL-6) levels were measured at the beginning and at the end of an 8 h
work day. The results showed no difference between the morning mean
levels (2.50 pg/ml ± 2.4 SD and the evening mean levels (2.53 pg/ml ±
2.6 SD). There were, however, 14 out of 22 individuals who showed
slight increases at the end of the work day. IL-1, which was measured
in 10 individuals, was below the detection limit.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Algae
The effect of MTBE on the growth of the unicellular algae
Selenastrum capricornutum (Chlorophyta), Navicula pelliculosa
(Bacillariophyta) and Synechococcus leopoliensis (Cyanobacteria),
representing three taxonomic groups, was investigated under laboratory
conditions (Rousch & Sommerfeld, 1998). The growth of
N. pelliculosa and S. leopoliensis was inhibited at a nominal MTBE
concentration of 2400 mg/litre in the growth medium, whereas
S. capricornutum growth was increased at 600 mg/litre and decreased
at 4800 mg/litre. The authors suggested that the differential
sensitivity of these representative species implies that MTBE could
alter algal community composition in the environment.
9.1.2 Aquatic animal species
The results of aquatic toxicity tests are presented in Table 21.
Experimental data on acute toxicity are available for four
species of invertebrates, four species of fish, and one species of
amphibian. The experimental data ranged from a 96-h LC50 of 553
mg/litre for the crustacean Chaetogammam marinum (Adema, 1982), to a
96-h LC50 of >10 000 mg/litre for a copepod Nitocra spinipes
(Tarkpea & Svanberg, 1982).
Acute toxicities were determined by Tarkpea & Svanberg (1982) for
MTBE alone, MTBE combined with a base fuel at a concentration of 5%,
and the base fuel alone. The acute toxicity tests were conducted on
the harpacticoid copepod Nitocra spinipes under static conditions
for 96 h at 21°C. The test solutions were not aerated. A 96-h LC50
greater than 10 000 mg/litre was reported for MTBE alone. The 96-h
LC50 values were 242 mg/litre for MTBE in a base fuel and 201
mg/litre for the base fuel alone. The results of the tests show that
the acute toxicity of base fuel to aquatic organisms is not increased
by the addition of 5% MTBE.
Tarkpea & Svanberg (1982) also determined the 24-h LC50 of MTBE
on a shoal fish, the bleak Alburnus alburnus in a closed, static
system at 10°C. The LC50 was between 1700 and 1800 mg/litre. When the
bleaks were introduced into the test media, several sublethal effects
were observed, including disturbed balance, surface swimming and
overturning. The sublethal effects were short-lived, as several of the
individual test subjects had recovered when the test was completed.
The environmental significance of these results was not discussed by
the authors.
Table 21. Aquatic toxicity testing results for MTBE
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Invertebrates
Ceriodaphnia LC50 (48-h) 18-21°C 841 THE, 1989
Daphnia magna LC50 (96-h) >1000 Gupta & Lin, 1995
Copepod LC50 (96-h) >10 000 Tarkpea & Svanberg, 1982
(Nitocra spinipes)
Copepod LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
(Nitocra spinipes)
Gammarid LC10 (96-h) 15°C 553 Adema, 1982
(Chaetogammarus marinus)
Fish
Bleak (Alburnus alburnus) LC50 (24-h) 10°C 1700-1800 Tarkpea & Svanberg, 1982
Bleak (Alburnus alburnus) LC50 (96-h) >1000 Bengtsson & Tarkpea, 1983
Fathead minnow LC50 (96-h) 25°C 706 Veith et al., 1983
(Pimephales promelas)
Fathead minnow LC50 (96-h) 672 Geiger et al., 1988
(Pimephales promelas)
Rainbow trout LC50 (96-h) 1300 Environment Canada, 1993
(Oncorhynchus mykiss)
Table 21. (continued)
Species Parameter Temperature Concentration Reference
(°C) (mg/litre)
Rainbow trout LC50 (96-h) 1483 Environment Canada, 1993
(Oncorhynchus mykiss)
Amphibians
European frog tadpole LC0 (48-h) <2000 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC50 (48-h) 2500 Paulov, 1987
(Rana temporaria)
European frog-tadpole LC100 (48-h) <3000 Paulov, 1987
(Rana temporaria)
When European frog tadpoles (Rana temporaria) were exposed to
concentrations of MTBE in water ranging from 100 to 2500 mg/ litre,
various effects were observed. An increase in body weight of frogs and
tadpoles that had undergone metamorphosis was observed at 100
mg/litre, as compared with the controls. At sublethal concentrations
(<2500 mg/m3) in water, accelerated development of the tadpole was
observed and metamorphosis occurred two days earlier than in controls
(Paulov, 1987). The environmental significance of these results was
not discussed by the author.
No data on chronic aquatic toxicity were found in the literature.
Data on the toxicity of MTBE to terrestrial animals, terrestrial
plants or soil biota were not found in the literature, other than
information from mammalian toxicology studies.
9.2 Field experiments
Data on field experiments on MTBE were not found in the
literature.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Total exposure of human populations to MTBE may involve more than
one environmental pathway and route of intake. Populations may be
exposed to MTBE in air in areas where it is used in gasoline, though
available data on environmental levels and human exposure are limited.
In several studies, median concentrations of MTBE in ambient air
ranged from 0.47 to 14.4 µg/m3 (0.00013 to 0.004 ppm) where MTBE is
used in oxygenated gasoline, and non-detectable to 26.4 µg/m3 (0.0073
ppm) in urban air of cities where MTBE is used as an octane enhancer.
Concentrations near industrial facilities range up to 35.7 mg/m3 (10
ppm). Median 1- to 2-min exposure levels gathered in the breathing
zone of service station attendants and consumers while refuelling were
highly variable, ranging from 1.0 to 21.4 mg/m3 (0.03 to 6 ppm) and
occasionally exceeding 35.7 mg/m3 (10 ppm).
Monitoring data for MTBE are too limited to characterize
adequately its occurrence in drinking-water. The intake of MTBE in
drinking-water is generally expected to be negligible, although
drinking-water may be polluted from point sources such as accidental
spills of large amounts of MTBE in gasoline. Exposure could also occur
through dermal absorption or inhalation of MTBE vapour from household
water used for bathing, cooking and laundering.
Potentially exposed workers include those involved in the
production, handling and use of MTBE and MTBE-containing gasoline,
including mechanics and service station attendants. Occupational
exposure of workers transporting MTBE is highest, with an average
short-term median concentration of 140 mg/m3 (39 ppm). Long-term
average median levels for this group of workers were about 2.85 mg/m3
(0.8 ppm). Median long-term exposure of service station attendants
averaged 1.79 mg/m3 (0.5 ppm). The long-term median value for
mechanics was 0.36 mg/m3 (0.1 ppm).
10.1.2 Human health effects
Consumers in some areas of the USA have complained about acute
health symptoms such as headache, eye and nose irritation, cough,
nausea, dizziness and disorientation associated with the use of
oxygenated fuels such as gasoline containing MTBE. Epidemiological
studies of human populations exposed under occupational and
non-occupational conditions, as well as experimental studies of human
volunteers exposed under controlled conditions, have not been able to
identify a basis for these complaints. Results of community studies
conducted in Alaska, New Jersey, Connecticut and Wisconsin, USA, have
been mixed and provided limited or no evidence of an association
between MTBE exposure and the prevalence of health complaints. In
addition, independently conducted experimental studies of volunteers
exposed in inhalation chambers to MTBE concentrations ranging from 5.0
mg/m3 (1.4 ppm) in one study to 180 mg/m3 (50 ppm) for 2 h in
another study have shown no evident effects in terms of either
subjective reports of symptoms or objective indicators of irritation
or other effects. Based on the collective evidence, it appears
unlikely that MTBE alone induces adverse acute health effects in the
general population under common inhalation exposure conditions.
However, the potential effects of mixtures of gasoline and MTBE, as
well as the manner in which most people are exposed to MTBE in
conjunction with the use of oxygenated fuels, have not been examined
experimentally or through prospective epidemiological methods.
Moreover, the role of factors such as awareness of MTBE, due in part
to its distinctive odour, for example, has not been investigated.
In studies on animals, MTBE is "moderately" acutely toxic, with
an oral LD50 in rats of approximately 3800 mg/kg bw and LC50 value
(15 min) of about 141 000 mg/m3 air in mice. Signs of intoxication
include CNS depression, ataxia and laboured respiration. The LD50 for
dermal toxicity in rabbits is >10 200 mg/kg bw.
MTBE is considered to be a mild skin and eye irritant but does
not induce skin sensitization.
Repeated exposure results primarily in increases in organ weights
and histopathological effects in the kidney of rats and the liver of
mice. Effect levels are compiled in Tables 22 and 23. Concentrations
or doses that induced significant increases in organ weights, for
which histopathological effects were observed at higher levels, were
considered LOELs. Doses at which histopathological effects were
observed were considered LOAELs (Tables 22 and 23).
Lowest reported effect levels for nephrotoxicity following
ingestion in subchronic studies were 440 mg/kg bw per day (increases
in relative kidney weight and hyaline droplet formation in
Sprague-Dawley rats). At 2860 mg/m3 (800 ppm), in a 90-day inhalation
study, there were increases in kidney weight associated at higher
concentrations with a mild increase in hyaline droplets in the
proximal tubules in Fischer-344 rats. At 1430 mg/m3 (400 ppm), in
inhalation oncogenicity studies, there was an increase in absolute
liver weight, which correlated with increased severity of
hepatocellular hypertrophy at higher concentrations and an increase in
relative kidney weight in male mice; in rats, incidence and severity
of chronic progressive nephropathy were increased at this level.
Exposure to MTBE also results in reversible central nervous
system effects including sedation, hypoactivity, ataxia and
anaesthesia at higher concentrations, and biphasic effects on motor
activity at lower concentrations. In a single 6-h inhalation exposure
study in rats, dose levels from 2860 mg/m3 (800 ppm) produced
reversible, non-monotonically dose-related changes in motor activity.
These effects were transient and not observed in longer-term studies.
Table 22. MTBE levels for non-neoplastic effects following oral exposure
Species Protocol Effect Level Basis of Effect Level Reference
(mg/kg bw/day)
Sprague-Dawley rats 28-day gavage LOAEL 440 males: increase in relative IITRI (1992)
(undiluted) kidney weight; hyaline droplet
formation in convoluted
NOAEL 90 tubules
Sprague-Dawley rats 90-day gavage in LOEL 900 increase in male absolute and Robinson et al. (1990)
corn oil relative kidney weight; chronic
nephropathy and increase in
NOAEL 300 hyaline droplets in proximal
tubular cells at the next
higher dose (1200 mg/kg bw/day)
Sprague-Dawley rats 104 weeks gavage NOEL 1000 no effects at any dose Belpoggi et al. (1995)
in olive oil;
maintained until
death
Table 23. MTBE levels for non-neoplastic effects following inhalation exposure
Species Protocol Effect level Basis for effect level Reference
Fischer-344 rats single exposure LOEL for reversible biphasic changes in motor Gill (1989);
neurological effects = activity in females Daughtrey et al.
2860 mg/m3 (800 ppm) (1997)
Fischer-344 rats 13 week study LOEL 2860 mg/m3 males: increase in relative Dodd & Kintigh (1989);
(800 ppm) weight of liver and kidney; Daughtrey et al. (1997)
no NOAEL males: at 28 600 mg/m3, increase
in lymphoid hyperplasia in
submandibular lymph nodes; mild
increase in hyaline droplets in
renal proximal tubules
Fischer-344 rats up to 104 weeks LOAEL 10 700 mg/m3 males: increased mortality and Chun et al. (1992);
(3000 ppm) decreased survival time at 10 700 Bird et al. (1997)
LOEL 1430 mg/m3 mg/m3; chronic progressive
(400 ppm) nephropathy was the major cause
of death at 10 700 mg/m3; increased
incidence and severity of chronic
progressive nephropathy at all
doses (significance not specified)
CD1 mice up to 18 months LOEL 1430 mg/m3 increase in absolute liver Chun et al. (1992)
(400 ppm) weight in male mice which Bird et al. (1997)
correlated with increased
severity of hepatocellular
hypertrophy at higher
concentrations; increase in
relative kidney weight in males
Specific adverse effects on reproduction have not been observed
in rats at concentrations up to 28 600 mg/m3 (8000 ppm). MTBE has not
induced developmental effects in rats, mice or rabbits at
concentrations less than those that were toxic to the mothers.
Decreases in uterine weight and increases in estrogen metabolism have
been observed at 28 600 mg/m3.
The weight of evidence indicates that MTBE is not genotoxic.
Identified oncogenicity studies include an inhalation study in rats
and mice and an oral study (gavage) in rats. In these investigations,
MTBE induced testicular (Leydig cell) tumours in male rats
(Fischer-344 and Sprague-Dawley), renal tumours in male rats
(Fischer-344) liver tumours in female mice (CD-1) and lymphomas and
leukaemias (combined) in female (Sprague-Dawley) rats.
All investigations on nephrotoxicity are consistent with the
renal tumours observed in Fischer-344 rats being related to
alpha2u-globulin nephropathy. alpha2u-Globulin nephropathy is
considered an effect specific to male rats and, therefore, these
tumours are of questionable relevance to humans.
Leydig cell tumours have been induced by MTBE in two strains of
rats. This type of tumour has been reported to be induced by
non-genotoxic carcinogens that disturb the hormonal balance of
testosterone, luteinizing hormone and luteinizing hormone releasing
factor in rats. Owing to differences between rats and humans in the
regulation of gonadotropins, it is questionable that a similar effect
will occur in humans. Although such a mechanism may be relevant, this
is not substantiated by experimental evidence, since these hormones
were not determined in any of the studies with MTBE.
Liver tumours have been induced by MTBE in female mice and
possibly in male mice (the data on male mice were not corrected for
increased mortality). The effect was modest and occurred only at
28 600 mg/m3 (8000 ppm) and in association with hepatocellular
hypertrophy (indicating enzyme induction) and altered estrogen
metabolism. The relevance of these mouse liver tumours for human risk
estimation is considered to be questionable.
In a single oral study in SD rats, the frequency of lymphomas and
leukaemias (combined) were increased in the high-dose group. This
observation was not supported by any indications of relevant
(preneoplastic) effects on the lymphoid system in other studies.
Moreover, the description of the study made it difficult to evaluate
adequately the results. However, since the effect observed appears to
be rather pronounced, it is not justified to neglect this finding,
based on presumed experimental deficiencies. For a proper evaluation,
additional information is required.
On the basis of these data, MTBE should be considered a rodent
carcinogen. MTBE is not genotoxic and the carcinogenic response is
only evident at high levels of exposure that also induce other adverse
effects. The available data are inconclusive and prohibit their use
for human carcinogenic risk assessment until outstanding complications
in their interpretation have been addressed.a
10.2 Evaluation of effects on the environment
MTBE emissions and leakages can be widespread in the environment
in areas where MTBE is used as an octane improver and oxygenate in
oxygenated gasoline.
MTBE is predominately emitted into air; however, it can be
released into the water and soil compartments. Ambient concentrations
in air are low. There are no terrestrial toxicity data for exposure to
MTBE in air; however, this appears not to be of concern to an
environmental evaluation since ambient air concentrations are low and
its half-life is relatively short.
Owing to its physical and chemical properties, MTBE can persist
longer in water and soil than in air. There are very limited data on
concentrations in ambient surface water. The biodegradation of MTBE in
water and soil is not well understood but is believed to be relatively
slow. MTBE in soil can leach into groundwater and persist there, due
to its lack of removal. MTBE has not been generally detected in deeper
groundwater or in shallow groundwater in agricultural areas. It is
more frequently found in shallower groundwater in urban areas where
MTBE is most extensively used.
Data available for ecotoxicological assessment refer almost
exclusively to MTBE in water. It can be classified as relatively
non-toxic for aquatic biota, with a lowest acute effect for several
aquatic organisms of more than 100 mg/litre. No long-term aquatic
toxicity tests at low concentrations have been identified. However,
the limited data on concentrations of MTBE in ambient surface water
have shown that concentrations range from non-detectable to 30
µg/litre. The maximum concentration is several orders of magnitude
below the effect level of the most sensitive organism tested to date.
It does not appear that the concentrations of MTBE in ambient water
are toxic to aquatic organisms, except during spills when very high
levels of MTBE may be found.
There are no data on concentrations of MTBE in soil or on
terrestrial toxicity. However, concentrations in this medium are
expected to be low except in the case of spills.
a MTBE was reviewed by an International Agency for Research on
Cancer (IARC) Working Group in October 1998. The conclusions were that
there was inadequate evidence for the carcinogenicity in humans of
MTBE, limited evidence for its carcinogenicity in experimental
animals, and the overall evaluation was that MTBE was not classifiable
as to its carcinogenicity for humans (Group 3).
11. RECOMMENDATIONS
To provide quantitative guidance on relevant limits of exposure
and to estimate risk, it is recommended that additional data be
acquired in the following areas:
a) additional information to evaluate the induction of
lymphomas/leukaemias in Sprague-Dawley rats;
b) mechanistic data on the induction of Leydig cell tumours and sex
specificity of liver tumours in mice;
c) controlled exposure studies to characterize the dose-response in
humans for MTBE and MTBE-containing mixtures;
d) monitoring data for better characterization of human exposure,
with particular attention to microenvironments;
e) potentiation studies of MTBE with BTX components of gasoline;
f) monitoring of environmental concentrations of MTBE in soil and
biota in areas adjacent to major sources and ambient areas in
order to verify theoretical values;
g) long-term toxicity tests in aquatic and possibly terrestrial
organisms;
h) field degradation tests to determine how persistent MTBE can be
in soil and groundwater under a range of redox conditions.
REFERENCES
ACGIH (1994) 1994-1995 threshold limit values for chemical substances
and physical agents and indices. Cincinnati, Ohio, American Conference
of Governmental Industrial Hygienists, p 27.
Adam G, Knuechel R, Vorwerk D, Held C, & Guenther RW (1990) Tissue
response of the biliary and digestive system of rabbits after MTBE
infusion into the gallbladder. Invest Radiol, 25(1): 58-61.
Adema DMM (1982) Tests and desk studies carried out by MT-TNO during
1980-1981 for Annex II of MARPOL 1973. Delft, The Netherlands,
Organization for Applied Scientific Research, 51 pp (Report CL
82/1499).
AFS (1994) Statute book of the Swedish National Board of Occupational
Safety and Health, Ordinance AFS 1993: 9: Occupational exposure limit
values. Solna, The Swedish National Board of Occupational Safety and
Health, pp 46, 52.
Akimoto R, Rieger E, Moossa AR, Hofmann AF, & Wahlstrom HE (1992)
Systemic and local toxicity in the rat of methyl tert-butyl ether: a
gallstone dissolution agent. J Surg Res, 53: 572-577.
Allard A-S, Remberger M, & Neilson AH (1996) The aerobic
biodegradation of tert-butyl methyl ether and tert-butanol: an
initiatory study. Stockholm, The Swedish Environmental Research
Institute, 9 pp (IVL Report No. B 1197).
Allen MK & Grande D (1995) Reformulated gasoline air monitoring study.
Madison, Wisconsin, Department of Natural Resources, Bureau of Air
Management, 50 pp (Publication No. AM-175-95).
Allen MJ, Borody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985a) Rapid dissolution of gallstones by methyl tert-butyl ether.
N Engl J Med, 312(4): 217-220.
Allen MJ, Barody TJ, Bugliosi TF, May GR, LaRusso NF, & Thistle JL
(1985b) Cholelitholysis using methyl tertiary butyl ether.
Gastroenterology, 88: 122-125.
Almaguer D (1993) Health hazard evaluation report HETA 93-0884-2344:
National Center for Environmental Health, Albany, New York.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 33 pp (NTIS/PB94-129020).
Anderson EV (1993) Health studies indicate MTBE is safe gasoline
additive. Chem Eng News, September 20: 9-18.
Anderson HA, Hanrahan L, Goldring J, & Delaney B (1995) An
investigation of health concerns attributed to reformulated gasoline
use in southwest Wisconsin - Final Report. Madison, Wisconsin,
Department of Health and Social Services, 50 pp.
Angle CR (1991) If the tap water smells foul, think MTBE. J Am Med
Assoc, 266: 2985-2986.
API (1989a) Aboveground storage tank survey. Washington, DC, American
Petroleum Institute, Health and Environment Affairs Department, 44 pp
(Publication No. 301).
API (1989b) Monitoring near refineries for airborne chemicals - Volume
1: Validated ambient air concentrations around three refineries.
Washington, DC, American Petroleum Institute, Health and Environment
Affairs Department, 83 pp (Publication No. 4484).
API (1993) Gasoline vapor exposure assessment at service stations.
Washington, DC, American Petroleum Institute, 8 pp (Publication No.
4553).
API (1994) A survey of API members' aboveground storage tank
facilities. Washington, DC, American Petroleum Institute, 65 pp
(Publication No. 330).
API (1995a) Executive summary of a study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 5 pp (Publication No. 4619).
API (1995b) Petroleum Institute data characterizing occupational
exposures to methyl tertiary butyl ether (MTBE) 1983-1993. Washington,
DC, American Petroleum Institute, 21 pp (Publication No. 4622).
API (1995c) Service station personnel exposures to oxygenated fuel
components - 1994: Executive summary. Washington, DC, American
Petroleum Institute, 3 pp (Publication No. 4625).
Arashidani K, Katoh T, Yoshikawa M, Kikuchi M, Kawamoto J, & Kodama Y
(1993) LD50 and weight change in organs of mice following
intraperitoneal administration of methyl tertiary-butyl ether. Jpn J
Ind Health, 35: 404-405.
ARCO (1987) Methyl tert-butyl ether exposure monitoring study.
Channelview, Texas, ARCO Chemical Company, 39 pp.
Atkinson R (1990) Gas-phase tropospheric chemistry of organic
compounds : a review. Atmos Environ, 24A: 1-41.
Atkinson R & Pitts JN Jr (1978) Kinetics of the reactions of the OH
radical with HCHO and CH3CHO over the temperature range 299-246°K. J
Chem Phys, 68(8): 3581-3584.
Barreto RD, Gray KA, & Anders K (1995) Photocatalytic degradation of
methyl- tert-butyl ether in TiO2 slurries: a proposed reaction
scheme. Water Res, 29(5): 1243-1248.
Beller M & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska. Anchorage, Alaska, Department
of Health and Social Services, Section of Epidemiology, 5 pp.
Belpoggi F, Soffritti M, & Maltoni C (1995) Methyl-tertiary-butyl
ether (MTBE) - a gasoline additive - causes testicular and
lympho-haematopoietic cancers in rats. Toxicol Ind Health, 11(2):
119-149.
Bengtsson EB & Tarkpea M (1983) The acute aquatic toxicity of some
substances carried by ships. Mar Pollut Bull, 14(6): 213-214.
Bevan C, Neeper-Bradley TL, Tyl RW, Fisher LC, Panson RD, Kneiss JJ, &
Andrews LS (1997) Two-generation reproductive toxicity study of methyl
tertiary-butyl ether (MTBE) in rats. J Appl Toxicol, 17(suppl 1):
S13-S19.
Bianchi A & Varney MS (1989) Analysis of methyl tert-butyl ether and
1,2-dihaloethanes in estuarine water and sediments using
purge-and-trap/gas chromatography. J High Res Chromatogr, 12(3):
184-186.
Bianchi A, Varney MS, & Phillips J (1991) Analysis of volatile organic
compounds in estuarine sediments using dynamic headspace and gas
chromatography-mass spectrometry. J Chromatogr, 542: 413-450.
Biles RW, Schroeder RE, & Holdsworth CE (1987) Methyl tertiary butyl
ether inhalation in rats: a single generation reproduction study.
Toxicol Ind Health, 3(4): 519-534.
Biodynamics (1984) The metabolic fate of methyl-t-butyl ether (MTBE)
following an acute intraperitoneal injection (Project No. 80089).
Washington, DC, American Petroleum Institute, 97 pp.
Bird MG, Burleigh-Flayer HD, Chun JS, Douglas JF, Kneiss JJ, & Andrews
LS (1997) Oncogenicity studies of inhaled methyl tertiary-butyl ether
in CD1 mice and F-344 rats. J Appl Toxicol, 17(suppl 1): S45-S55.
Blackburn GR, Dooley JF III, Schreiner CA, & Mackerer CR (1991)
Specific identification of formaldehyde-mediated mutagenicity using
the mouse lymphoma L5178Y TK+/- assay supplemented with formaldehyde
dehydrogenase. In Vitro Toxicol, 4(2): 121-132.
Bonin MA, Ashley DL, Cardinali FL, McGraw JM, & Wooten JV (1994)
Measurement of methyl tert-butyl ether and tert-butyl alcohol in
human blood and urine by purge-and-trap gas chromatography-mass
spectrometry using an isotope dilution method. In: 208th National
Meeting of the American Chemical Society, 1994. Washington, DC,
American Chemical Society, 1 p (Abstract P102-ENVR).
Borghoff SJ & Lagarde WH (1993) Assessment of binding of
2,4,4-trimethyl-2-pentanol to low-molecular-weight proteins isolated
from kidneys of male rats and humans. Toxicol Appl Pharmacol, 119:
228-235.
Borghoff SJ, Short BG, & Swenberg JA (1990) Biochemical mechanisms and
pathobiology of alpha2u-globulin nephropathy. Annu Rev Pharmacol
Toxicol, 30: 264-275.
Borghoff SJ, Jayjock E, & Murphy JE (1995) Methyl tert-butyl ether
(MTBE) vs ethyl tert-butyl ether (ETBE): A comparison of blood and
tissue partition coefficients in male and female F344 rats.
Toxicologist, 30: 34.
Borghoff SJ, Murphy JA, & Medinsky MA (1996) Development of a
physiologically based pharmacokinetic model for methyl
tertiary-butyl ether and tertiary-butanol in male Fischer-344
rats. Fundam Appl Toxicol, 30: 264-275.
Borghoff SJ, Murphy JE, Turner M, & James AR (1998) Dosimetry of
methyl t-butyl ether (MTBE) and t-butyl alcohol in male and female
rats following inhalation exposure to MTBE. Toxicologist, 42(1-S):
A1049.
Brady JF, Xiao F, Ning SM, & Yang CS (1990) Metabolism of methyl
tertiary-butyl ether by rat hepatic microsomes. Arch Toxicol, 64:
157-160.
Brown SL (1997) Atmospheric and potable water exposures to methyl
tert-butyl ether (MTBE). Regul Toxicol Pharmacol, 25: 256-276.
Bruce BW & McMahon PB (1996) Shallow ground-water quality beneath a
major urban center: Denver, Colorado, USA. J Hydrol, 186: 129-151.
Buckley TJ, Prah JD, Ashley D, Zweidinger RA, & Wallace LA (1997) Body
burden measurements and models to assess inhalation exposure to methyl
tertiary butyl ether (MTBE). J Air Waste Manage Assoc, 47: 739-752.
Buchta TM (1993a) Health hazard evaluation report HETA 93-606-2336:
National Centers for Environmental Health, Fairbanks, Alaska.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 37 pp (NTIS/PB94-133667).
Buchta TM (1993b) Health hazard evaluation report HETA 93-802-2338:
National Centers for Environmental Health, Stamford Connecticut.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, 39 pp (NTIS/PB94-134343).
Budavari S, O'Heil MJ, Smith A, Heckelman PE, & Kinneary JF ed. (1996)
Methyl- tert-butyl ether. In: The Merck index, 12th ed. Whitehouse
Station, New Jersey, Merck & Co., Inc., p 1611.
Burleigh-Flayer HD, Chun JS, & Kintigh WJ (1992) Methyl tertiary butyl
ether: vapor inhalation oncogenicity study in CD-1 mice (Laboratory
project ID 91N0013A). Export, Pennsylvania, Bushy Run Research Center,
1068 pp (Report to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Cain WS, Leaderer BP, Ginsberg GL, Andrews LS, Cometto-Muniz JE, Gent
JF, Buck M, Berglund LG, Mohsenin V, Monahan E, & Kjaergaard S. (1996)
Acute exposure to low-level methyl tertiary-butyl ether (MTBE): human
reactions and pharmacokinetic response. Inhal Toxicol, 8: 21-48.
Calvert JG & Pitts JN Jr (1966) Photochemistry. New York, John Wiley &
Sons, Inc., pp 441-442.
CDC (1993) An investigation of exposure to methyl tertiary butyl ether
among motorists and exposed workers in Stamford, Connecticut.
Presented at the EPA Workshop on MTBE and other Oxygenates, Falls
Church, Virginia, 27 July 1993. Atlanta, Georgia, Centers for Disease
Control and Prevention, 35 pp.
Cederbaum AI & Cohen G (1980) Oxidative demethylation of t-butyl
alcohol by rat liver microsomes. Biochem Biophys Res Commun, 97:
730-736.
CEN (1996) Growth of top 50 chemicals slowed in 1995 from very high
1994 rate. Chem Eng News, April 8:16-20.
Chandler B & Middaugh J (1992) Potential illness due to exposure to
oxygenated fuels in Fairbanks, Alaska: Preliminary results. Anchorage,
Alaska, Department of Health and Social Services, 5 pp.
Charbonneau M, Strasser J Jr, Lock EA, Turner MJ Jr, & Swenberg JA
(1989) Involvement of reversible binding to alpha2u-globulin in
1,4-dichlorobenzene-induced nephrotoxicity. Toxicol Appl Pharmacol,
99: 122-132.
Chen CY, Chang KK, Chow NH, Leow TC, Chou TC, & Lin XZ (1995) Toxic
effects of cholelitholytic solvents on gallbladder and liver. Dig Dis
Sci, 40(2): 419-426.
Chiang CY, Loos KR, & Klopp RA (1992) Field determination of
geological/chemical properties of an aquifer by cone penetrometry and
headspace analysis. Ground Water, 30: 428-436.
Chun JS & Kintigh WJ (1993) Methyl tertiary-butyl ether: twenty-eight
day vapor inhalation study in rats and mice. Export, Pennsylvania,
Bushy Run Research Center, 387 pp (BRRC Report 93N1241).
Chun JS, Burleigh-Flayer HD, & Kintigh WJ (1992) Methyl tertiary butyl
ether (MTBE): vapor inhalation oncogenicity study in Fischer 344 rats
(Laboratory project ID 91N0013B). Export, Pennsylvania, Bushy Run
Research Center, 1463 pp (Report for the Methyl Tertiary Butyl Ether
Committee, Washington, DC).
Church CD, Isabelle LM, Pankow JF, Tratnyek PG, & Rose DL (1997)
Assessing the in situ degradation of methyl tert-butyl ether
(MTBE) byproduct identification at the sub-PPB level using direct
aqueous injection GC/MS: Extended abstract - Proceedings of ACS
National Meeting, San Francisco, California, 16-17 April 1997.
Washington, DC, American Chemical Society, 4 pp.
Cinelli S & Seeberg AH (1989) Reverse mutation in Salmonella
typhimurium. Test substance: MTBE. Rome, Life Science Research,
Toxicology Centre, 50 pp (LSR-RTC Report No. 216001-M-03489).
Cirvello JD, Radovsky A, Heath JE, Farnell DR, & Lindamood C (1995)
Toxicity and carcinogenicity of T-butyl alcohol in rats and mice
following chronic exposure in drinking water. Toxicol Ind Health, 11:
151-165.
Clark CR, Dutcher JS, Henderson TR, McClellan RO, Marshall WF, Naman
TM, & Seizinger DE (1984) Mutagenicity of automotive particulate
exhaust: influence of fuel extenders, additives, and aromatic content.
In: MacFarland HN, Holdsworth CE, MacGregor JA, Call RW, & Lane ML ed.
Applied toxicology of petroleum hydrocarbons. Princeton, New Jersey,
Princeton Scientific Publishers, pp 109-122.
Clayton Environmental Consultants (1991) Gasoline vapor exposure
assessment for the American Petroleum Institute (API) (Project No.
31774.00). Cypress, California, Clayton Environmental Consultants, 139
pp.
Cline PV, Delfino JJ, & Rao SC (1991) Partitioning of aromatic
constituents into water from gasoline and other complex solvent
mixtures. Environ Sci Technol, 25: 914-920.
Cochrane RA & Hillman DE (1984) Direct gas chromatographic
determination of alcohols and methyl tert-butyl ether in gasolines
using infrared detection. J Chromatogr, 287: 197-201.
Conaway CC, Schroeder RE, & Snyder NK (1985) Teratology evaluation of
methyl tertiary butyl ether in rats and mice. J Toxicol Environ
Health, 16: 797-809.
Cook CK & Kovein RJ (1995) NIOSH health hazard evaluation report No.
94-0220-2526: Exxon Company, Houston, Texas, USA. Cincinnati, Ohio,
National Institute for Occupational Safety and Health, 28 pp.
Cox RA & Goldstone A (1982) Atmospheric reactivity of oxygenated motor
fuel additives. In: Proceedings of the 2nd European Symposium on the
Physico-chemical Behaviour of Atmospheric Pollutants, Varese, Italy,
29 September-1 October 1981. Dordrecht, D Riedel Publishing Co, pp
112-119.
Cuthbert JA (1979) Safety tests on methyl- tert-butyl-ether.
Edinburgh, Inveresk Research International, 18 pp (IRI Report No.
1300).
Dale MS, Losee RF, Crofts EW, & Davis MK (1997) MTBE: Occurrence and
fate in source-water supplies. J Am Chem Soc, 37: 376-377.
Daughtrey WC, Gill MW, Pritts IM, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Neurotoxicological evaluation of methyl tertiary-butyl ether in
rats. J Appl Toxicol, 17(suppl 1): S57-S64.
DECOS (Dutch Expert Committee on Occupational Standards) (1994)
Methyl-t-butylether: Health based recommended occupational exposure
limit. The Hague, Health Council of the Netherlands, 75 pp
(Publication No. 1994/23).
Delzer GC, Zogorski JS, Lopes TJ, & Bosshart RL (1996) Occurrence of
the gasoline oxygenate MTBE and BTEX compounds in urban stormwater in
the United States, 1991-95: US Geological Survey. Rapid City, South
Dakota, US Geological Survey, 6 pp (Water-Resources Investigations
Report No. 96-4145).
Derwent RG, Jenkin ME, & Sandow SM (1996) Photochemical ozone creation
potentials for a large number of reactive hydrocarbons under European
conditions. Atmos Environ, 30(2): 181-199.
Dey JC, Brown RA, & McFarland WE (1991) Methyl tert-butyl ether.
Hazard Mater Control, 4: 32-39.
Diehl JW, Finkbeiner JW, & Di Sanzo FP (1995) Determination of ethers
and alcohols in reformulated gasolines by gas chromatography/atomic
emission detection. J High Res Chromatogr, 18: 108-110.
Di Padova C, Di Padova F, Montorsi W, & Tritapepe R (1986) Methyl
tert-butyl ether fails to dissolve retained radiolucent common bile
duct stones. Gastroenterology, 91: 1296-1300.
Dodd DE & Kintigh WJ (1989) Methyl tertiary butyl ether (MTBE):
repeated (13-week) vapor inhalation study in rats with neurotoxicity
evaluation. Export, Pennsylvania, Bushy Run Research Center, 502 pp
(Report No. 52-507 to the Methyl Tertiary Butyl Ether Committee,
Washington, DC).
Drivas PJ, Valberg PA, & Gauthier TD (1991) Health assessment of air
toxics emissions from alternative fuels. Paper presented at the 84th
Annual Meeting of the Air and Waste Management Association, Vancouver,
British Columbia, 16-21 June 1991. Washington, DC, Air and Waste
Management Association, 17 pp.
Du JT, Abernathy CO, Donohue J, Mahfouz A, & Khanna K (1998) A
drinking water advisory: consumer acceptability and advice and health
effects analysis on methyl tertiary-butyl ether (MTBE). Toxicologist,
42(1-S): A 1123.
Duffy LK (1994) Oxyfuel in Alaska: use of interleukins to monitor
effects on the immune system. Sci Total Environ, 151: 253-256.
ECETOC (1997) Methyl tert-butyl ether (MTBE): Health risk
characterization. Brussels, European Centre for Ecotoxicology and
Toxicology of Chemicals, 126 pp (Technical Report No. 72).
Environment Canada (1989) Analysis of shellfish for organic and
inorganic contaminants. Dartmouth, Nova Scotia, Environment Canada
Conservation and Protection, 10 pp (Report No. AN 893118).
Environment Canada (1992) Canadian Environmental Protection Act -
Priority substances list: Methyl tertiary-butyl ether. Ottawa,
Minister of Supply and Services, 19 pp (Assessment Report No. 5).
Environment Canada (1993) Canadian Environmental Protection Act -
Priority substances list: Supporting document methyl tertiary butyl
ether. Hull, Quebec, Environment Canada, 49 pp.
Environment Canada (1996a) Canadian Environmental Protection Act -
National pollutant release inventory: Summary report 1994. Hull,
Quebec, Environment Canada, 143 pp.
Environment Canada (1996b) MTBE concentrations at selected locations:
National air pollution surveillance programme 1995-1996. Hull, Quebec,
Environment Canada, 21 pp.
Erdal S, Gong H Jr, Linn WS, & Rykowski R (1997) Projection of health
benefits from ambient ozone reduction related to the use of methyl
tertiary butyl ether (MTBE) in the reformulated gasoline program.
Risk Anal, 17: 693-704.
Esch O, Spinosa JC, Hamilton RL, Crombie DL, Schteingart CD, Rondinone
JF, DAgostino HB, Lillienau J, & Hofmann AF (1992a) Acute effects of
topical methyl tert-butyl ether or ethyl propionate on gallbladder
histology in animals: a comparison of two solvents for contact
dissolution of cholesterol gallstones. Hepatology, 16(4): 984-991.
Esch O, Schteingart CD, Pappert D, Kirby D, Streich R, & Hofmann AF
(1992b) Increased blood levels of methyl tert-butyl ether but not of
ethyl propionate during instillation with contact gallstone
dissolution agents in the pig. Hepatology, 18(2): 373-379.
Ferdinandi ES, Houle J-M, Lalande M, Provencher A, & St-Onge Brault G
(1990a) Pharmacokinetics of methyl tert-butyl ether (MTBE) and
tert-butyl alcohol (TBA) in male and female Fischer-344 rats after
administration of MTBE by the intravenous, oral and dermal routes.
Montreal, Bio-Research Laboratories Ltd, 98 pp (Report No. 38842 to
the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lalande M, Houle J-M, Provencher A, & Ducharme S
(1990b) Mass balance of radioactivity and metabolism of methyl
ter-butyl ether (MTBE) in male and female Fischer 344 rats after
intravenous, oral and dermal administration of 14C-MTBE. Montreal,
Bio-Research Laboratories Ltd, 127 pp (Report No. 38843 to the MTBE
Task Force, Washington, DC).
Ferdinandi ES, Houle J-M, Lalande M, Lulham G, Pilon D, Provencher A,
Labbé R, & St-Onge Brault G (1990c) Pharmacokinetics of methyl
tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) in male and
female Fischer-344 rats after single and repeat inhalation nose-only
exposures to MTBE. Montreal, Bio-Research Laboratories Ltd, 172 pp
(Report No. 38844 to the MTBE Task Force, Washington, DC).
Ferdinandi ES, Lulham G, Pilon D, Labbé R, Lalande M, Provencher A,
Houle J-M, & Ducharme S (1990d) Disposition of radioactivity and
metabolism of methyl tert-butyl ether (MTBE) in male and female
Fischer 344 rats after nose-only inhalation exposure to 14C-MTBE.
Montreal, Bio-Research Laboratories Ltd, 89 pp (Report No. 38845 to
the MTBE Task Force, Washington, DC).
Fujiwara Y, Kinoshita T, Sato H, & Kojima I (1984) [Biodegradation and
bioconcentration of alkyl ethers.] Yukagatu, 33(2): 111-114 (in
Japanese with tables and abstract in English).
Garrett P, Moreau M, & Lowry JD (1986) MTBE as a groundwater
contaminant. In: Proceedings of the 1986 National Water Well
Association and American Petroleum Institute Conference on Petroleum
and Organic Chemicals in Groundwater, Houston, Texas, 12-14 November
1986. Dublin, Ohio, National Water Well Association, pp 227-238.
Geiger DL, Call DJ, & Brooks LT (1988) Acute toxicities of organic
chemicals to Fathead Minnows (Pimephales promelas). Superior,
Wisconsin, Center for Lake Superior Environmental Studies, University
of Wisconsin, vol 4, pp 75-76.
Giacomello P (1996) MTBE exposure in service stations: Italian study.
Paper presented at the 7th European Fuel Oxygenates Association
Conference, Sodehotel, 24-25 October 1996. Brussels, European Chemical
Industries Federation, 8 pp.
Gill MW (1989) Methyl tertiary butyl ether single exposure vapor
inhalation neurotoxicity study in rats. Export, Pennsylvania, Bushy
Run Research Center, 116 pp (Report No. 52-533 to the Methyl Tertiary
Butyl Ether Committee, Washington, DC).
Gillie AD (1993) Industrial hygiene monitoring methyl tertiary butyl
ether, benzene and gasoline. San Diego, California, Texaco Inc.,
Environmental Health and Safety Division, Industrial Hygiene, 31 pp.
Gordian ME & Guay G (1995) Benzene in Alaska. Alsk Med, 37: 25-28, 36.
Gordian ME, Huelsman MD, Brecht M-L, & Fisher DG (1995) Health effects
of methyl tertiary butyl ether (MTBE) in gasoline in Alaska. Alsk Med,
37: 101-103, 119.
Greenough RJ, McDonald P, Robinson P, Cowie JR, Maule W, Macnaughton
F, & Rushton A (1980) Methyl tertiary-butyl ether (Driveron): three
month inhalation toxicity in rats (Project No. 413038). Edinburgh,
Scotland, Inveresk Research International, 230 pp.
Grindstaff G, Henry M, Hernandez O, Hogan K, Lai D, & Siegel-Scott C
(1991) Formaldehyde risk assessment update. Washington DC, US
Environmental Protection Agency, Office of Toxic Substances, 140 pp.
Gupta G & & Lin YJ (1995) Toxicity of methyl tertiary butyl ether to
Daphnia magna and Photobacterium phosphoreum. Bull Environ Contam
Toxicol, 55: 618-620.
Hakkola M & Saarinen L (1996) Exposure of tanker drivers to gasoline
and some of its components. Ann Occup Hyg, 40: 1-10.
Hakkola M, Honkasalo ML, & Pulkkinen P (1996) Neuropsychological
symptoms among tanker drivers exposed to gasoline. J Occup Med, 46:
124-130.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, & Valcovic
LR (1993) Hazard evaluation of chemicals that cause accumulation of
alpha-globulin, hyaline droplet nephropathy, and tubule neoplasia in
the kidneys of male rats. Environ Health Perspect, 99: 313-349.
Harper M & Fiore AA (1995) Determination of methyl tert-butyl ether
in air using a diffusive sampler. Appl Occup Environ Hyg, 10: 616-619.
Hartle R (1993) Exposure to methyl tert-butyl ether and benzene
among service station attendants and operators. Environ Health
Perspect, 101(suppl 6): 23-26.
Haseman JK, Arnold J, & Eustis SL (1990) Tumor incidences in Fischer
344 rats: NTP historical data. In: Pathology of the Fischer rat:
Reference and atlas. McLean, Virginia, Academic Press, chapter 35, pp
555-564.
Hazleton Laboratories (1979) Acute eye irritation study in rabbits:
tert-butyl methyl ether (95% pure). Leesburg, Virginia, Hazleton
Laboratories America, Inc., 14 pp (Report No. 2024-132).
Hebert RS (1993) Industrial hygiene exposure assessment for methyl
tert-butyl ether (MTBE), benzene and gasoline. Houston, Texas, Star
Enterprise Environment, Health and Safety Department, 36 pp.
HEI (1996) The potential health effects of oxygenates added to
gasoline: a review of the current literature. A special report of the
Institute's oxygenates evaluation committee. Cambridge, Massachusetts,
Health Effects Institute, 158 pp.
Hinton J (1993) Occupational exposures. Presented at the MTBE
Conference in Washington, DC, 26-27 July 1993. Beacon, New York,
Texaco Inc., 37 pp.
Hoekman SK (1992) Speciated measurements and calculated reactivities
of vehicle exhaust emissions from conventional and reformulated
gasolines. Environ Sci Technol, 26: 1206-1216.
Hoekman SK (1993) Improved gas chromatography procedure for speciated
hydrocarbon measurements of vehicle emissions. J Chromatogr, 639:
239-253.
Hong J-Y, Yang CS, Lee M, Wang Y-Y, Huang WQ, Tan y, Patten CJ, &
Bondoc FY (1997) Role of cytochromes P450 in the metabolism of methyl
tert-butyl ether in human livers. Arch Toxicol, 71: 266-269.
Hong J-Y, Yang CS, Wang Y-Y, Bondoc FY, Pan J, Cokonis C, & Bao ZP
(1998) Use of CYP2E1 knock-out mice to assess the role of CYP2E1 in
metabolizing methyl tert-butyl ether (MTBE) and other gasoline ethers.
Toxicologist, 42(1-S): A87.
Howard PH, Boethling RS, Jarvis WF, Meylan WM, & Michalenko EM (1991)
Handbook of environmental degradation rates. Chelsea, Michigan, Lewis
Publishers, Inc., pp 653-654.
Hsieh CR & Ouimette JR (1994) Comparative study of multimedia modeling
for dynamic partitioning of fossil fuels-related pollutants. J Hazard
Mater, 37: 489-505.
Huber AH (1995) Human exposure estimates of methyl tertiary butyl
ether (MTBE). Presentation at Conference on MTBE and Other Oxygenates:
A Research Update, Falls Church, Virginia, 26-28 July 1993.
Washington, DC, National Technical Information Service, 32 pp (EPA
/600/A-94/255; NTIS/PB95-177150).
IITRI (1992) 28-day oral (gavage) toxicity of methyl tert-butyl
ether (MTBE) in rats (Project No. L08100). Chicago, Illinois, Illinois
Institute of Technology Research, 48 pp.
Imbriani M, Ghittori S, & Pezzagno G (1997) Partition coefficients of
methyl tert-butyl ether. G Ital Med Lav Ergon, 19: 63-65.
Industrial Bio-Test Laboratories (1969) Acute toxicity studies on
X-801-25. Northbrook, Illinois, Industrial Bio-Test Laboratories, 27
pp (Report No. A6809 to Sun Oil Company).
Japar SM, Wallington TJ, Richert JFO, & Ball JC (1990) The atmospheric
chemistry of oxygenated fuel additives: t-butyl alcohol, dimethyl
ether, and methyl t-butyl ether. Int J Chem Kinet, 22: 1257-1269.
Japar SM, Wallington TJ, Rudy SJ, & Chong TY (1991) Ozone-forming
potential of a series of oxygenated organic compounds. Environ Sci
Technol, 25: 415-420.
Johansen NG (1984) The analysis of C1-C4 alcohols, MTBE, and DIPE in
motor gasolines by multi-dimensional capillary column gas
chromatography. J High Res Chromatogr Chromatogr Commun, 7: 487-489.
Johanson G, Nihlén A, & Löf, A (1995) Toxicokinetics and acute effects
of MTBE and ETBE in male volunteers. Toxicol Lett, 82/83: 713-718.
Johnson T, McCoy M, & Wisbith T (1995) A study to characterize air
concentrations of methyl tertiary butyl ether (MTBE) at service
stations in the Northeast. Washington, DC, American Petroleum
Institute, 108 pp (API Publication No. 4619).
Katoh T, Arashidani K, Kikuchi M, Yoshikawa M, & Kodama Y (1993)
Effects of methyl tertiary butyl ether on hepatic lipid peroxidation
in mice. Jpn J Hyg, 48: 373-378.
Kelly TJ, Callahan PJ, Piell J, & Evans GF (1993) Method development
and field measurements for polar volatile organic compounds in ambient
air. Environ Sci Technol, 27: 1146-1153.
Kirchstetter TW, Singer BC, Harley RA, Kendall GR, & Chan W (1996)
Impact of oxygenated gasoline use on California light-duty vehicle
emissions. Environ Sci Technol, 30: 661-670.
Levy JM & Yancey JA (1986) Dual capillary gas chromatographic analysis
of alcohols and methyl tert-butyl ether in gasolines. J High Res
Chromatogr Chromatogr Commun, 9: 383-387.
Lewis RJ (1993) Methyl -tert-butyl ether (MTBE). In: Hawley's
condensed chemical dictionary, 12th ed. New York, Van Nostrand
Reinhold Co., p 760.
Lindamood C, Farnell DR, Giles HD, Prejean JD, Collins JJ, Takahashi
K, & Maronpot RR (1992) Subchronic toxicity studies to t-butyl
alcohol in rats and mice. Fundam Appl Toxicol, 19: 91-100.
Lindstrom AB & Pleil JD (1996) Alveolar breath sampling and analysis
to assess exposure to methyl tertiary butyl ether (MTBE) during motor
vehicle refuelling. J Air Waste Manage Assoc, 46: 676-682.
Lington AW, Dodd DE, Ridlon SA, Douglas JF, Kneiss JJ, & Andrews LS
(1997) Evaluation of 13-week inhalation toxicity study on methyl
t-butyl ether (MTBE) in Fischer 344 rats. J Appl Toxicol, 17 (suppl
1): S37-S44.
Lioy PJ, Weisel CP, Jo WK, Pellizzari E, & Raymer JH (1994)
Microenvironmental and personal measurements of methyl-tertiary butyl
ether (MTBE) associated with automobile use activities. J Expo Anal
Environ Epidemiol, 4(4): 427-444.
Litton Bionetics (1980) Guinea pig sensitization study (TBME-99).
Kensington, Maryland, Litton Bionetics, Inc., 30 pp (Report No.
22011).
Lyman WJ, Reehl WF, & Rosenblatt DH (1990) Handbook of organic
chemical property estimation methods: Environmental behavior of
organic compounds. Washington, DC, American Chemical Society, 960 pp.
McGahan JP, Tesluk H, Brock JM, Johnston R, & Shaul DB (1988)
Dissolution of gallstones using methyl tertiary-butyl ether in an
animal model. Invest Radiol, 23: 599-603.
Mackay D, Shiu WY, & Ma KC (1993) Illustrated handbook of
physical-chemical properties and environmental fate for organic
chemicals - volume 3: Volatile organic chemicals. Boca Raton, Florida,
Lewis Publishers, 916 pp.
Mackerer CR, Angelosanto FA, Blackburn GR, & Schreiner CA (1996)
Identification of formaldehyde as the metabolite responsible for the
mutagenicity of methyl tertiary-butyl ether in the activated mouse
lymphoma assay. Proc Soc Exp Biol Med, 212: 338-341.
Marsh DF & Leake CD (1950) The comparative anesthetic activity of the
aliphatic ethers. Anesthesiology, 11: 455-463.
Mihelcic JR (1990) Modeling the potential effect of additives on
enhancing the solubility of aromatic solutes contained in gasoline.
Ground Water Monit Rev, 10: 132-137.
Miller MJ, Ferdinandi ES, Andrews LS, Douglas JF, & Kneiss JJ (1997)
Pharmacokinetics and disposition of methyl t-butyl ether in
Fischer-344 rats. J Appl Toxicol, 17(suppl 1): S3-S12.
Mo K, Lora CO, Javanmardian M, Yang X, & Kulpa CF (1997)
Biodegradation of methyl t-butyl ether by pure bacterial cultures.
Appl Microbiol Biotechnol, 47: 69-72.
Mobil Oil Corporation (1993) Activated mouse lymphoma (L5178Y/TK/+/-)
mutagenicity assay supplemented with formaldehyde dehydrogenase for
methyl tertiary butyl ether. Princeton, New Jersey, Mobil Oil
Corporation, 17 pp (Status report No. 65579).
Mohr SN, Fiedler N, Weisel C, & Kelly-McNeil K (1994) Health effects
of MTBE among New Jersey garage workers. Inhal Toxicol, 6(6): 553-562.
Möller Jensen H & Arvin E (1990) Solubility and degradability of the
gasoline additive MTBE, methyl tert-butyl ether, and gasoline
compounds in water. In: Arendt F, Hinsewald M, & van der Brink WJ ed.
Contaminated soil 90. Dodrecht, Kluwer Academic Publishers, pp
445-448.
Moolenaar RL, Hefflin BJ, Ashley DL, Middaugh JP, & Etzel RA (1994)
Methyl tertiary butyl ether in human blood after exposure to
oxygenated fuel in Faibanks, Alaska. Arch Environ Health, 49(5):
402-409.
Mormile MR, Liu S, & Suflita JM (1994) Anaerobic biodegradation of
gasoline oxygenates: Extrapolation of information to multiple sites
and redox conditions. Environ Sci Technol, 28: 1727-1732.
Moser GJ, Wong BA, Wolf DC, Moss OR, & Goldsworthy TL (1996a)
Comparative short-term effects of methyl tertiary butyl ether and
unleaded gasoline vapor in female B6C3P1 mice. Fundam Appl Toxicol,
31: 173-183.
Moser GJ, Wong BA, Wolf DG, Fransson-Steen RL, & Goldsworthy TL
(1996b) Methyl tertiary butyl ether lacks tumor-promoting activity on
N-nitosodiethylamine-initiated B6C3F1 female mouse liver.
Carcinogenesis, 17: 2753-2761.
Munch JW & Eichelberger JW (1992) Evaluation of 48 compounds for
possible inclusion in U.S. EPA Method 524.2, Revision 3.0: expansion
of the method analyte list to a total of 83 compounds. J Chromatogr
Sci, 30: 471-477.
Murray WR, Laferla G, & Fullarton GM (1988) Choledolithiasis -
in vivo stone dissolution using methyl tertiary butyl ether (MTBE).
Gut, 29: 143-145.
Neeper-Bradley TL (1991) Two-generation reproduction study of inhaled
methyl tertiary butyl ether in CD (Sprague-Dawley) rats. Export,
Pennsylvania, Bushy Run Research Center, 499 pp (Report No. 53-594 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
Nihlén A, Löf A, & Johanson G (1995) Liquid/air partition coefficients
of methyl and ethyl t-butyl ethers, t-amyl methyl ether, and t-butyl
alcohol. J Expo Anal Environ Epidemiol, 5(4): 573-582.
Nihlén A, Löf A, & Johanson G (1998a) Experimental exposure to methyl
tertiary-butyl ether: I. Toxicokinetics in humans. Toxicol Appl
Pharmacol, 148: 281-287.
Nihlén A, Wålinder R, Löf A, & Johanson G (1998b) Experimental
exposure to methyl tertiary-butyl ether: II. Acute effects in humans.
Toxicol Appl Pharmacol, 148: 281-287.
Novak JT, Yeh C, Gullic D, Eichenberger J, & Benoit RE (1992). The
influence of microbial ecology on subsurface degradation of petroleum
contaminants. Blacksburg, Virginia, Virginia Polytechnic Institute and
State University, Water Resources Research Center, 86 pp (Bulletin No.
177).
Pankow JF, Rathbun RE, & Zogorski JS (1996) Calculated volatilization
rates of fuel oxygenate compounds and other gasoline-related compounds
from rivers and streams. Chemosphere, 33: 921-937.
Paulov S (1987) [Action of the anti-detonation preparation tert-butyl
methyl ether on the model species Rana temporaria L.] Biologia, 42:
185-189 (in Slovak with English abstract).
Pauls RE (1985) Determination of high octane components: methyl
t-butyl ether, benzene, toluene, and ethanol in gasoline by liquid
chromatography. J Chromatogr Sci, 23: 437-441.
Pekari K, Riihimaki V, Vainiotalo S, Teräväinen E, & Aitio A (1996)
Experimental exposure to methyl- tert-butyl ether (MTBE) and
methyl- tert-amyl ether (MTAE). In: Proceedings of the International
Symposium on Biological Monitoring in Occupational and Environmental
Health, Espoo, Finland, 11-13 September 1996. Helsinki, Institute of
Occupational Health, pp 27-28.
Pence VH (1987a) The evaluation of the biodegradation of 606610 using
a modified closed bottle method. Miamiville, Ohio, Hill Top Biolabs,
Inc., 92 pp (Report No. 87-0479-11, prepared for ARCO Chemical
Company).
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Poet TS & Borghoff SJ (1997) In vitro uptake of methyl tert-butyl
ether in male rat kidney: Use of a two-compartment model to describe
protein interactions. Toxicol Appl Pharmacol, 145: 340-348.
Pence VH (1987b) Anaerobic biodegradability test. Miamiville, Ohio,
Hill Top Biolabs, Inc., 99 pp (Report No. 87-0257-11, prepared for
ARCO Chemical Company).
Post G (1994) Methyl tertiary butyl ether: health-based maximum
contaminant level document. Trenton, New Jersey, New Jersey Department
of Environmental Protection, Division of Sciences and Research.
Poulsen M, Lemon L, & Barker JF (1992) Dissolution of monoaromatic
hydrocarbons into groundwater from gasoline-oxygenate mixtures.
Environ Sci Technol, 26: 2483-2489.
Prah JD, Goldstein GM, Devlin R, Otto D, Ashley D, House D, Cohen KL,
& Gerrity T (1994) Sensory, symptomatic, inflammatory, and ocular
responses to and the metabolism of methyl tertiary butyl ether in a
controlled human exposure experiment. Inhal Toxicol, 6(6): 521-538.
Prakash CB (1989) Motor vehicle emissions from gasoline containing
MTBE. Ottawa, Ontario, Environment Canada, Environmental Protection
Directorate, Conservation and Protection, Industrial Programs Branch,
25 pp (Report No. IP-97).
Prescott-Mathews JS, Wolf DC, Wong BA, & Borghoff SJ (1997) Methyl
tert-butyl ether causes alpha2u-globulin nephropathy in male
Fischer-344 rats. Toxicol Appl Pharmacol, 143: 301-314.
Raese JW, Rose DL, & Sandstrom MW (1995) US Geological Survey
laboratory method for methyl tert-butyl ether and other fuel
oxygenates. Rapid City, South Dakota, US Geological Survey, 4 pp (US
Geological Survey Fact Sheet FS-219-95).
Rao HV & Ginsberg GL (1997) Physiologically-based pharmacokinetic
(PBPK) model assessment of methyl t-butyl ether (MTBE) in groundwater
for a bathing and showering determination. Risk Anal, 17: 583-598.
Riihimaki V, Matikainen E, Akila R, Teräväinen E, Mutanen P, Pekari K,
Vainiotalo S, Vilhunen R, & Aitio A (1996) Central nervous system
effects of the gasoline additive methyl- tert-butyl ether (MTBE). In:
Proceedings of the Third International Symposium on Biological
Monitoring in Occupational and Environmental Health, Espoo, Finland,
11-13 September 1996. Helsinki, Institute of Occupational Health, p
75.
Robinson M, Bruner RH, & Olson GR (1990) Fourteen- and ninety-day oral
toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley
rats. J Am Coll Toxicol, 9(5): 525-540.
Roe VD, Lacy MJ, Stuart JD, & Robbins GA (1989) Manual headspace
method to analyze for the volatile aromatics of gasoline in
groundwater and soil samples. Anal Chem, 61: 2584-2585.
Rousch JM & Sommerfeld MR (1998) Liquid-gas partitioning of the
gasoline oxygenate methyl tert-butyl ether (MTBE) under laboratory
conditions and its effect on growth of selected algae. Arch Environ
Contam Toxicol, 34: 6-11.
Salanitro JP, Diaz LA, Williams MP, & Wisniewski HL (1994) Isolation
of a bacterial culture that degrades methyl t-butyl ether. Appl
Environ Microbiol, 60: 2593-2596.
Savolainen H, Pfäffli P, & Elovaara E (1985) Biochemical effects of
methyl tertiary-butyl ether in extended vapour exposure of rats.
Arch Toxicol, 57: 285-288.
Schroll R, Bierling B, Cao G, Dörfler U, Lahaniati M, Langenbach T,
Scheunert I, & Winkler R (1994) Uptake pathways of organic chemicals
from soil by agricultural plants. Chemosphere, 28: 297-303.
Schuberth J (1996) A full evaporation headspace technique with
capillary GC and ITD: a means for quantitating volatile organic
compounds in biological samples. J Chromatogr Soc, 34: 314-319.
Schuetzle D, Siegl WO, Trescott EJ, Jensen TE, Dearth MA, Kaiser EW,
Gorse R, Kreucher W, & Kulik E (1994) The relationship between
gasoline composition and vehicle hydrocarbon emissions: A review of
current studies and future research needs. Environ Health Perspect,
102(suppl 4): 3-12.
Seeberg AH (1989) Unscheduled DNA synthesis (UDS) in primary rat
hepatocytes (autoradiographic method). Test substance: MTBE. Rome,
Life Science Research, Toxicology Centre, 79 pp (LSR-RTC Report No.
216003-M-03689).
Sernau RC (1989) Mutagenicity test on methyl tertiary butyl ether
Drosophila melanogaster sex-linked recessive test (Study No.
10484-0-461). Kensington, Maryland, Hazleton Laboratories America,
Inc., 23 pp (Report to the Methyl Tertiary Butyl Ether Toxicology
Committee, Washington, DC).
Simer MM (1986) Texaco Chemical Corporation - Safety (SAF) studies,
surveys and reports: methanol, tertiary butyl alcohol, 1,3-butadiene,
methyl tertiary butyl ether, total other hydrocarbons. Port Neches,
Texas, Texaco Chemical Corporation, 15 pp.
Smith Sl & Duffy LK (1995) Odor and health complaints with Alaskan
gasolines. Chem Health Saf, 2: 32-38.
Smith DF, Kleindienst TE, Hudgens EE, McIver CD & Bufalini JJ (1991)
The photooxidation of methyl tertiary butyl ether. Int J Chem Kinet,
23: 907-924.
Snamprogetti (1980) MTBE toxicological data book. Rome, Snamprogetti
Engineering Company S.p.A., 202 pp (Report No. LICE/0086/AA/1g).
SNV (1993) [Better environmental qualities in petrol - a proposal for
environmental classification.] Stockholm, National Environmental
Protection Agency, 69 pp (in Swedish).
Spitzer HL (1997) An analysis of health benefits associated with the
use of MTBE reformulated gasoline and oxygenated fuels in reducing
atmospheric concentrations of selected volatile organic compounds.
Risk Anal, 17: 683-691.
Squillace PJ, Pope DA, & Price CV (1995a) Occurrence of the gasoline
additive MTBE in shallow ground water in urban and agricultural areas.
Rapid City, South Dakota, US Geological Survey, 4 pp (US Geological
Survey Fact Sheet FS-114-95).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1995b) A preliminary
assessment of the occurrence and possible sources of MTBE in ground
water of the United States, 1993-94. Rapid City, South Dakota, US
Geological Survey, 29 pp (File Report No. 95-456).
Squillace PJ, Zogorski JS, Wilber WG, & Price CV (1996) Preliminary
assessment of occurrence and possible sources of MTBE in groundwater
in the United States, 1993-1994. Environ Sci Technol, 30: 1721-1730.
Stackelberg PE, O'Brien AK, & Terracciano SA (1997) Occurrence of MTBE
in surface and ground water, Long Island, New York and New Jersey.
Proceedings of American Chemical Society meeting, San Francisco,
California, 16-17 April 1996. Washington, DC, American Chemical
Society, 4 pp.
Standeven AM & Goldsworthy TL (1993) Promotion of preneoplastic
lesions and induction of CYP2B by unleaded gasoline vapor in female
B6C3F1 mouse liver. Carcinogenesis, 14: 2137-2141.
State of Connecticut (1987) The incidence of MTBE in Connecticut.
Hertford, Connecticut, Department of Health Services, 3 pp (Document
No. FYI-OTS 0987-0574).
Steffan RJ, McClay K, Vainberg S, Condee CW, & Zhang D (1997)
Biodegradation of the gasoline oxygenates methyl tert-butyl ether,
ethyl tert-butyl ether, and tert-amyl methyl ether by propane
oxidising bacteria. Appl Environ Microbiol, 63: 4216-4222.
Stern BR & Tardiff RG (1997) Risk characterization of methyl
tertiary butyl ether (MTBE) in tap water. Risk Anal, 17: 727-743.
Sternal RS & Davis MA (1992) In vitro dissolution of cholesterol
gallstones. Invest Radiol, 27(12): 1040-1043.
Storck WJ, Layman PL, Reisch MS, Thayer AM, Kirschner EM, Peaff G, &
Tremblay J-F (1996) Facts and figures for the chemical industry. Chem
Eng News, June 24: 38-46.
Streete PJ, Ruprah M, Ramsey JD, & Flanagan RJ (1992) Detection and
identification of volatile substances by headspace capillary gas
chromatography to aid diagnosis of acute poisoning. Analyst, 117:
1111-1127.
Suflita JM & Mormile MR (1993) Anaerobic biodegradation of known and
potential gasoline oxygenates in the terrestrial subsurface. Environ
Sci Technol, 27: 976-978.
Swenberg JA & Dietrich DR (1991) Immunohistochemical localization of
alpha 2µ-globulin in kidneys of treated and control rats of a 13-week
vapor inhalation study undertaken with methyl tertiary butyl ether.
Washington, DC, Synthetic Organic Chemical Manufacturers Association,
5 pp (Report to the MTBE Health Effects Testing Task Force,
Washington, DC).
Tarkpea M & Svanberg O (1982) The acute toxicity of motor fuels to
brackish water organisms. Mar Pollut Bull, 13(4): 125-127.
Tepper JS, Jackson MC, McGee JK, Costa DL, & Graham JA (1994)
Estimation of respiratory irritancy from inhaled methyl tertiary butyl
ether in mice. Inhal Toxicol, 6(6): 563-569.
Texaco (1993) Industrial hygiene air sampling results: Guatemala
Refinery - methyl tert-butyl ether (MTBE). Beacon, New York, Texaco
Inc., Environment Health and Safety Division, 3 pp.
THE (1989) Biomonitoring results using MTBE. Wheat Ridge, Colorado,
THE Consultants, 8 pp.
TRC (1993) Final report to ARCO Chemical Company on the odor and taste
threshold studies performed with methyl tertiary-butyl ether (MTBE)
and ethyl tertiary-butyl ether (ETBE). Windsor, Connecticut, TRC
Environmental Corporation, 37 pp.
Tuazon EC, Carter WPL, Aschmann SM, & Atkinson R (1991) Products of
the gas-phase reaction of methyl tert-butyl ether with the OH
radical in the presence of NOx. Int J Chem Kinet, 23: 1003-1015.
Tyl RW (1989) Developmental toxicity study of inhaled methyl tertiary
butyl ether in New Zealand White rabbits. Export, Pennsylvania, Bushy
Run Research Center, 260 pp (Report No. 51-628 to the Methyl Tertiary
butyl Ether Toxicology Committee, Washington, DC).
Tyl RW & Neeper-Bradley TL (1989) Developmental toxicity study of
inhaled methyl tertiary butyl ether in CD-1 mice. Export,
Pennsylvania, Bushy Run Research Center, 342 pp (Report No. 52-526 to
the Methyl Tertiary Butyl Ether Toxicology Committee, Washington, DC).
US EPA (1989) Chemical rate constants for superfund health evaluation
manual chemicals. Washington, DC, US Environmental Protection Agency,
pp 645-646 (Report No. 68-02-4254).
US Interagency Assessment (1997) Interagency assessment of oxygenated
fuels. Washington, DC, National Science and Technology Council, 256
pp.
Vainiotalo S, Peltonen Y, & Pfäffli P (1996) MTBE exposure in service
stations. Paper presented at the 7th European Fuel Oxygenates
Association Conference, Sodehotel, 24-25 October 1996. Brussels,
European Chemical Industries Federation, 7 pp.
Veith GD & Kosian P (1983) Estimating bioconcentration potential from
octanol/water partition coefficients. In: Mackay D, Paterson S,
Eisenreich SJ, & Simmons MS ed. Physical behavior of PCBs in the Great
Lakes. Ann Arbor, Michigan, Ann Arbor Science Publishers, Chapter 15,
pp 269-282.
Veith GD, Call DJ, & Brooke LT (1983) Structure-toxicity relationships
for the fathead minnow, Pimephales promelas: narcotic industrial
chemicals. Can J Fish Aquat Sci, 40(6): 743-748.
Verga GR, Sironi A, Schneider W, & Frohne JCh (1988) Selective
determination of oxygenates in complex samples with the O-FID analyze.
J High Res Chromatogr Chromatogr Commun, 11: 248-252.
Vergnes JS & Chun JS (1994) Methyl tertiary butyl ether: in vivo-
in vitro hepatocyte unscheduled DNA synthesis assay in mice
(Laboratory project ID 93N1316). Export, Pennsylvania, Bushy Run
Research Center, 67 pp (Report to the MTBE Effects Testing Task Force,
Washington, DC).
Vergnes JS & Kintigh WJ (1993) Methyl tertiary butyl ether: bone
marrow micronucleus test in mice (Laboratory project ID 93N1244).
Export, Pennsylvania, Bushy Run Research Center, 99 pp (Report to the
MTBE Effects Testing Task Force, Washington, DC).
Vergnes JS & Morabit ER (1989) Methyl tertiary butyl ether repeated
exposure vapor inhalation study in rats: in vivo cytogenetic
evaluation. Export, Pennsylvania, Bushy Run Research Center, 40 pp
(Report No. 51-635 to the MTBE Effects Testing Task Force, Washington,
DC).
Wallington TJ, Dagaut P, Liu R, & Kurylo MJ (1988) Gas-phase reactions
of hydroxyl radicals with the fuel additives methyl tert-butyl ether
and tert-butyl alcohol over the temperature range 240-440 K. Environ
Sci Technol, 22(7): 842-844.
Wallington TJ, Andino JM, Skewes LM, Siegl WO, & Japar SM (1989)
Kinetics of the reaction of OH radicals with a series of ethers under
simulated atmospheric conditions at 295 K. Int J Chem Kinet, 21:
993-1001.
Ward JB, Au WW, Whorton EB, & Legator MS (1994) Genetic toxicology of
methyl tertiary butyl ether. Galveston, Texas, University of Texas, pp
57-134 (Final Report to the Agency for Toxic Substances and Disease
Registry).
Watson JG, Chow JC, Pritchett LC, Houck JA, Ragazzi RA, & Burns S
(1990) Chemical source profiles for particulate motor vehicle exhaust
under cold and high altitude operationg conditions. Sci Total Environ,
93: 183-190.
White MC, Johnson CA, Ashley DL, Buchta TM, & Pelletier DJ (1995)
Exposure to methyl tertiary-butyl ether from oxygenated gasoline in
Stamford, Connecticut. Arch Environ Health, 50: 183-189.
Wibowo AAE (1994) DEFOS (Dutch Expert Committee for Occupational
Standards) and NEG (Nordic Expert Group for Criteria Documentation of
Health Risks from Chemicals) basis for an occupational standard:
methyl-tert-butyl-ether. Solna, Sweden, National Institute of
Occupational Health, 21 pp.
Windholz M ed. (1983) The Merck index: an encyclopedia of chemicals,
drugs, and biologicals, 10th ed. Rahway, New Jersey, Merck and Co.,
Inc., p 865.
Yeh CK & Novak JT (1994) Anaerobic biodegradation of gasoline
oxygenates in soils. Water Environ Res, 66: 744-752.
Yoshikawa M, Arashidani K, Katoh T, Kawamoto T, & Kodama Y (1994)
Pulmonary elimination of methyl tertiary-butyl ether after
intraperitoneal administration in mice. Arch Toxicol, 68(8): 517-519.
Zogorski JS, Morduchowitz A, Baehr AL, Bauman BJ, Drew RT, Korte NE,
Lepham WW, Pankow JF, & Washington ER (1996) Fuel oxygenates and water
quality: Current understanding of sources, occurrence in natural
waters, environmental behavior, fate, and significance. Washington,
DC, Office of Science and Technology Policy, 91 pp (Final report
prepared for the Interagency Oxygenated Fuel Assessment).
Zweidinger RB (1993) Air quality measurements in Fairbanks, Stamford,
and Albany. Research Triangle Park, North Carolina, United States
Environmental Protection Agency, Atmospheric Research and
Environmental Assessment Laboratory, 19 pp (Report ACC-1539).
RÉSUMÉ
Le méthyltertiobutyléther (MTBE) est actuellement le plus utilisé
des éthers que l'on peut employer comme additifs de l'essence.
L'éthyltertiobutyléther (ETBE), le tertioamylméthyléther (TAME), le
tertioamyléthyléther (TAEE) et le diisopropyléther (DIPE), entre
autres, peuvent être ajoutés ou substitués au MTBE afin d'améliorer
l'oxygénation et l'indice d'octane, aussi peut-on en trouver à côté du
MTBE.
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le MTBE est un composé volatil et incolore, à l'odeur terpénique,
qui est liquide à la température ambiante. Sa viscosité est faible et
son point d'ébullition est de 55,2°C. Son point de congélation est de
-109°C. Sa densité est de 0,7404 à 20°C. Sa tension de vapeur est
relativement élevée: 33 500 Pa à 25°C. C'est une substance inflammable
qui peut en outre former des mélanges explosifs avec l'air. Il est
très soluble dans les autres éthers et dans l'alcool. Miscible à
l'essence, il est également soluble dans l'eau (42 000 g/m3 à
19,8°C). Son coefficient de partage entre l'octanol et l'eau (log
Kow) est de 0,94-1,3. Il est instable en solution acide.
La recherche et le dosage du MTBE se font dans tous types de
matrices par chromatographie en phase gazeuse au moyen de détecteurs
et de colonnes capillaires adaptés à la matrice en cause. On a
également recours à la chromatographie liquide à phases inversées pour
l'analyse des échantillons d'essence. On utilise aussi, pour la
préparation des échantillons d'air, d'eau et de sédiments ou encore
des échantillons biologiques, divers systèmes de purge et de piégeage,
la sorption/désorption et des méthodes basées sur l'espace de tête.
2. Sources d'exposition humaine et environnementale
Autant qu'on sache, le MTBE n'existe pas à l'état naturel. Dans
l'industrie, on l'obtient par l'action du méthanol sur l'isobutylène
en présence d'un catalyseur. Un certain nombre de pays le produisent
en quantités croissantes depuis la fin des années 70. Il compte
actuellement parmi les 50 produits chimiques dont le volume de
production est le plus élevé. En 1996, la capacité de production des
Etats-Unis était de 10,6 millions de tonnes et on estime que la
demande de MTBE va encore augmenter. Environ 25% de l'essence vendue
aux Etats-Unis est additionnée de MTBE. On l'utilise presque
exclusivement pour améliorer l'indice d'octane et accroître la teneur
de l'essence en oxygène. On en ajoute à l'essence jusqu'à 17% en
volume.
3. Transport, distribution et transformation dans l'environnement
Une fois libéré dans l'air, le MTBE y reste en majeure partie
avec seulement de petites quantités qui passent dans le sol et dans
l'eau. Le MTBE présent dans l'atmosphère peut passer en partie dans
l'eau de pluie, mais la proportion qui s'élimine ainsi reste faible.
Dans l'atmosphère, l'action des radicaux hydroxyle entraîne la
formation d'un certain nombre de composés et en particulier de
formiate de tertiobutyle, photochimiquement stable, et de
2-méthoxy-2-méthylpropanol, qui doit réagir énergiquement avec les
radicaux hydroxyles pour donner du CO2, du formaldéhyde, de l'acétone
et de l'eau. Lorsque du MTBE est libéré dans l'eau, il se dissout
partiellement, une partie passant dans l'air. Les quantités qui
passent dans les biotes et les sédiments sont faibles. Les épreuves
classiques indiquent une faible biodégradabilité. On pense que d'une
façon générale, la biodégradation est lente dans l'environnement.
Lorsque du MTBE est libéré dans le sol, il passe dans l'air par
volatilisation, dans les eaux de surface par entraînement et dans les
eaux souterraines par lessivage. Le MTBE peut persister dans les eaux
souterraines.
4. Concentrations dans l'environnement et exposition humaine
Les données relatives aux concentrations dans l'environnement et
à l'exposition humaine sont peu nombreuses.
Dans des études portant sur l'air de certaines villes où les
véhicules utilisaient de l'essence oxygénée contenant 15% de MTBE, on
a relevé des concentrations ambiantes allant de "non décelable" à
100,9 µg/m3 (0,028 ppm), avec plusieurs concentrations médianes
allant de 0,47 à 14,4 µg/m3 (0,00013 à 0,004 ppm). Dans l'air de
quelques villes où le MBTE était utilisé à plus faible teneur pour
augmenter l'indice d'octane, la concentration de ce composé allait de
non décelable à 26,4 µg/m3 (0,0073).
Au niveau du sol ou à proximité de raffineries de pétrole, la
concentration allait de 15 à 281 µg/m3. Dans l'air urbain, à
proximité d'ateliers où l'on procédait au mélange de cet additif à
l'essence, la concentration était de 1508 µg/m3 (0,419 ppm), avec des
valeurs extrêmes de 216-35 615 µg/m3 (0,06-9,8 ppm).
Dans les stations service situées dans des zones où l'on
utilisait de l'essence oxygénée à 10-15% de MTBE, c'est dans la zone
de respiration des consommateurs, au moment des pleins, que la
concentration de l'additif était la plus forte (300 à 136 000 µg/m3,
soit 0,09 à 38 ppm), les valeurs dépassant toutefois rarement 3600
µg/m3 (10 ppm) et tombant un peu plus bas au niveau des pompes (de
non décelable à 5700 µg/m3, soit 1,6 ppm). Les valeurs les plus
faibles ont été relevées sur le périmètre de la station (de non
décelable à 500 µg/m3, soit 0,14 ppm). Les concentrations relevées
dans les stations services dépourvues de système de récupération des
vapeurs étaient généralement plus élevées.
A l'intérieur d'une automobile, on a relevé des valeurs de 7 à 60
µg/m3 (0,002 à 0,017 ppm) au cours de navettes et de 20 610 µg/m3
(0,006 à 0,172 ppm) lors des pleins.
D'après des données de surveillance qui se limitent
presqu'exclusivement aux Etats-Unis, la présence de MTBE a été décelée
dans de la neige, des eaux d'orage, des eaux de surface (ruisseaux,
rivières et retenues), des eaux souterraines et dans de l'eau de
boisson. Les valeurs relevées dans les eaux d'orage allaient de 0,2 à
8,7 µg/litre avec une valeur médiane inférieure à 1,0 µg/litre. Dans
le cas des ruisseaux, rivières et retenues, les concentrations
s'étageaient entre 0,2 et 30 µg/litre, les valeurs médianes obtenues
dans diverses études allant de 0,24 à 7,75 µg/litre.
La présence de MTBE n'a généralement pas été décelée dans les
eaux souterraines profondes ou non des zones agricoles. Lorsqu'on en a
trouvé, la concentration était inférieure à 2,0 µg/litre. La présence
de MTBE est plus fréquente dans eaux souterraines de faible profondeur
des régions urbanisées (dans les premiers 1,5 à 3 m des nappes
phréatiques). On trouve alors des concentrations de moins de 0,2
µg/litre à 23 µg/litre, avec une valeur médiane de moins de 0,2
µg/litre.
On trouve rarement du MTBE dans l'eau des réseaux d'adduction qui
est pompée dans les nappes souterraines. Sur 51 réseaux contrôlés sauf
3, la concentration était inférieure ou égale à 20 µg/litre. Il est
difficile de donner des valeurs caractéristiques pour l'eau
d'adduction captée en surface car les données sont insuffisantes. On a
trouvé de fortes concentrations de MTBE dans quelques puits privés
utilisés comme source d'eau potable (soit >1000 µg/m3). On peut
toutefois douter que de l'eau contenant plus de 50 à 100 µg/litre de
MTBE soit encore buvable, car le seuil organoleptique du MTBE est bas.
Parmi les travailleurs exposés au MTBE, on peut citer ceux qui
produisent, distribuent ou utilisent ce composé ou de l'essence qui en
contient, y compris les pompistes et les mécaniciens des stations
service.
En ce qui concerne l'exposition de courte durée (<30 min) lors
d'opérations habituelles de production ou de stockage de MTBE pur, les
chiffres vont de 715 à 43 000 µg/m3 (0,2 à 12 ppm), avec une valeur
médiane moyenne de 3400 µg/m3 (0,95 ppm). Pour l'exposition de plus
longue durée (30 min à 8 h), les valeurs vont de 360 à 890 000 µg/m3
(0,01 à 250 ppm), avec une valeur médiane d'environ 540 µg/m3 (0,15
ppm). Chez les ouvriers qui mélangent l'additif à l'essence, les
valeurs de l'exposition de courte durée vont de non décelable à
360 000 µg/m3 (100 ppm), la médiane se situant à environ 5700 µg/m3
(1,6 ppm). Dans le cas d'une exposition de plus longue durée, les
valeurs obtenues vont de non décelable à 257 000 µg/m3 (72 ppm), avec
une valeur médiane moyenne d'environ 2000 µg/m3 (0,6 ppm).
C'est lors du transport de MTBE pur ou en mélange avec des
carburants, dans des canalisations, sur des péniches, des wagons de
chemin de fer ou des camions (MTBE pur seulement) que l'on a
enregistré les expositions les plus fortes, avec des valeurs à court
terme allant de 3750 mg/m3 (0,001 à 1050 ppm) et une valeur médiane
moyenne de 140 mg/m3 (39 ppm). Dans le cas d'expositions à long
terme, les valeurs allaient de 0,036 à 2540 mg/m3 (0,01 à 712 ppm),
la valeur médiane moyenne se situant à 2,85 mg/m3 (0,8 ppm). Lors de
la distribution (c'est-à-dire du chargement de mélanges carburant-MTBE
sur des camions et de leur livraison et déchargement dans des stations
service), on a relevé des valeurs à court terme allant de non
décelable à 225 mg/m3 (63 ppm), les valeurs médianes moyennes se
situant autour de 21 mg/m3 (6 ppm). Les valeurs à long terme allaient
de 0,036 à 22 mg/m3 (0,01 à 6,2 ppm), avec une valeur médiane moyenne
de 1,79 mg/m3 (0,5 ppm).
L'exposition médiane moyenne à court terme des pompistes de
stations service allait, selon certaines mesures, généralement de
1,071 à 21,42 mg/m3 (0,3 à 6 ppm) et dépassait rarement 35,7 mg/m3
(10 ppm). Dans le cas de l'exposition médiane à long terme, on a
obtenu la valeur de 1,79 mg/m3 (0,5 ppm). L'exposition médiane des
mécaniciens est restée inférieure au seuil de détection dans une étude
à court terme; dans le cas de l'exposition à long terme, la valeur
était d'environ 360 µg/m3 (0,1 ppm).
5. Cinétique et métabolisme
Les données toxicocinétiques relatives aux effets du MTBE sur
l'Homme proviennent essentiellement d'études contrôlées pratiquées sur
des volontaires adultes ou sur une population exposée à de l'essence
oxygénée. Après inhalation, le MTBE passe rapidement dans le courant
sanguin. Chez des volontaires humains en bonne santé exposés par voie
respiratoire, on constate que la cinétique est linéaire jusqu'à la
concentration de 268 mg/m3 (75 ppm). On a procédé au dosage de
l'alcool tertiobutylique (en abrégé TBA, un métabolite du MTBE) dans
le sang et les urines. Chez des sujets humains exposés à des
concentrations de MTBE allant de 5,0 à 178,5 mg/m3 (1,4 à 50 ppm), la
concentration maximale de MTBE et de TBA allait respectivement de 17,2
à 1144 µg/litre et de 7,8 à 925 µg/litre. En utilisant un modèle
monocompartimental, on a pu constater qu'intervenaient dans la
demi-vie globale du MTBE des constituants à demi-vie brève (36-90 min)
et des constituants à demi-vie longue (19 h).
Chez les rongeurs, le MTBE est bien résorbé et réparti après
administration per os ou exposition par la voie respiratoire.
L'absorption est moindre par voie percutanée. A la dose de 400 mg/kg
per os et de 28 800 mg/m3 (8000 ppm) par inhalation, la proportion
de la dose totale absorbée qui était éliminée dans l'air expiré
augmentait à mesure que diminuait la proportion éliminée dans les
urines, ce qui est le signe d'une saturation du métabolisme. On n'a
pas mis en évidence de TBA dans l'urine des rats exposés. La présence
de 2-méthyl-1,2-propanediol et d'acide alpha-hydroxyisobutyrique dans
l'urine indique que le TBA est également métabolisé. Les études
in vitro montrent que le MTBE est métabolisé en TBA, formaldéhyde et
acétone.
6. Effets sur les animaux de laboratoire et les systèmes d'épreuve
in vitro
Chez le rat, la dose létale médiane aiguë par voie buccale
(DL50) est égale à environ 3 800 mg/kg de poids corporel. La
concentration létale médiane aiguë (CL50) pour une exposition de 15
minutes par inhalation se situe aux environs de 141 000 mg/m3 d'air
chez la souris. Parmi les signes d'intoxication on peut citer une
dépression du SNC, une ataxie et des difficultés respiratoires. Aux
doses non létales, la récupération a été complète. Par voie
percutanée, la DL50 est >10 200 mg/kg de poids corporel chez le
lapin.
On n'a trouvé qu'une seule étude où il soit question d'un effet
"modérément" irritant pour la peau, l'irritation consistant en un
érythème et un oedème modérés après application sur la peau de lapins.
Chez ce même animal, le MTBE s'est également révélé irritant pour la
muqueuse oculaire, les effets produits étant bénins et réversibles.
Dans la seule étude retrouvée, le MTBE a provoqué une irritation
légère à forte des voies respiratoires lors de l'exposition de souris
à des doses de 300 à 30 000 mg/m3. Il n'a pas produit de
sensibilisation cutanée chez le cobaye.
L'expérimentation sur des rats et des souris montre que des
expositions réitérées conduisent principalement à une augmentation du
poids des organes et ont des effets histopathologiques sur le rein
(rat) et sur le foie (souris). Une étude d'ingestion de 90 jours a
montré que la limite inférieure d'apparition d'effets néphrotoxiques
se situe à 440 mg/kg p.c. par jour (augmentation du poids des reins et
dégénérescence hyaline chez des rats Sprague-Dawley). En exposant des
rats Fischer-344 par inhalation à une concentration de 2880 mg/m3
(800 ppm) de MTBE, on a obtenu une augmentation du poids rénal
accompagnée, lorsqu'on accroissait la concentration, d'une
augmentation modérée de la dégénérescence hyaline au niveau des
tubules proximaux. Lors d'études d'oncogénicité comportant
l'exposition des animaux par inhalation, on a observé à la dose de
1440 mg/m3 (400 ppm), un accroissement de la fréquence et de la
gravité des néphropathies progressives chroniques chez les rats mâles,
alors que chez les souris mâles, il y avait à cette même dose
augmentation du poids absolu du foie (corrélée avec une augmentation
des hypertrophies hépatocellulaires à plus forte concentration) et du
poids relatif des reins.
L'exposition au MTBE provoque également des effets irréversibles
sur le système nerveux central (SNC) consistant notamment en sédation,
diminution de l'activité, ataxie et anesthésie à forte concentration.
Des effets biphasiques s'observent également sur l'activité motrice à
plus faible concentration. Lors d'une étude sur des rats comportant
une exposition de 6 h par inhalation, on a constaté qu'à la dose de
2880 mg/m3 (800 ppm), il se produisait, chez les animaux d'un des
deux sexes, des modifications réversibles et liées à la dose de
l'activité motrice. Ces effets étaient passagers et n'apparaissaient
plus guère lors des études à long terme.
On a pu retrouver des études de reproduction portant sur une ou
deux générations de rats ainsi que quatre études relatives au
développement de rats, de souris et de lapins exposés à du MTBE. Ces
études n'ont pas permis de mettre en évidence d'effets spécifiques sur
la reproduction des rats à des concentrations allant jusqu'à 28 800
mg/m3. En outre, aux concentrations inférieures à celles qui se
révélaient toxiques pour les mères, le MTBE n'a pas eu non plus
d'effets sur le développement de la progéniture. A la dose de 28 800
mg/m3, on a constaté, chez la souris, une augmentation du poids de
l'utérus et un accroissement du métabolisme des estrogènes.
Le MTBE a fait l'objet d'un grand nombre d'épreuves valables de
mutagénicité et autres études de génotoxicité. Les résultats obtenus
montrent que le composé n'est pas génotoxique, même si un résultat
positif a été obtenu dans l'épreuve de mutation portant sur le locus
tk des cellules lymphomateuses. Ce résultat s'explique en effet par la
métabolisation du MTBE en formaldéhyde.
Pour les études de cancérogénicité, on a exposé par inhalation
des rats Fischer-344 et des souris CD-1 ou gavé des rats
Sprague-Dawley avec une nourriture contenant du MTBE. Dans aucune des
études d'exposition par inhalation on a procédé à une correction
statistique pour tenir compte des différences de survie. Dans les
trois études, on a constaté une augmentation sensible de l'incidence
des tumeurs, localisées, chez les rats mâles Fischer-344, au niveau
des tubules rénaux et des cellules de Leydig, chez les rats mâles
Sprague-Dawley, au niveau des cellules de Leydig (lymphomes et
leucémies chez les femelles) et chez les souris femelles CD-1, au
niveau du foie. Les tumeurs des tubules rénaux et les
leucémies/lymphomes n'ont donc pas été observées systématiquement chez
le rat lors des différentes études. En outre, les tumeurs rénales
sexospécifiques étaient associées à une néphropathie également
sexospécifique mettant en jeu l'alpha2u-globuline, qui a été observée
dans plusieurs études de courte durée. L'augmentation des tumeurs des
cellules de Leydig a été observée à la dose la plus élevée chez les
rats Sprague-Dawley (1000 mg/kg p.c.), mais chez les rats Fischer-344,
l'interprétation de cet accroissement est rendu délicate par la très
forte incidence tumorale également observée chez les témoins
concomitants et les témoins historiques. Les tumeurs hépatiques ont
été observées dans les groupes témoins et à la dose de 28 800 mg/m3
(8000 ppm) dans les groupes exposés avec des incidences respectives de
2/50 et 10/50 chez les femelles et de 12/49 et 16/49 chez les mâles.
L'accroissement d'incidence était modeste et s'accompagnait d'une
hypertrophie hépatocellulaire.
7. Effets sur l'Homme
Après la mise sur le marché, aux Etats-Unis, de deux types
d'essence nécessitant l'utilisation d'additifs d'oxygénation (pas
obligatoirement du MTBE), on a constaté que les usagers se plaignaient
de symptômes aigus tels que maux de tête, irritation des yeux et du
nez, toux, nausées, vertiges et désorientation. Les études
épidémiologiques effectuées sur des populations humaines en milieu
professionnel ou non, de même que les études expérimentales sur
volontaires humains exposés dans des conditions contrôlées, n'ont pas
permis de découvrir si ces plaintes étaient fondées. Des études
intracommunautaires menées en Alaska, au New Jersey, dans le
Connecticut et dans le Wisconsin ont, avec des résultats divers il est
vrai, montré qu'il n'y avait guère de relation entre l'exposition au
MTBE et les symptômes dont la population se plaignait.
Des volontaires adultes ont été placés, dans le cadre d'études
expérimentales contrôlées, dans des chambres d'inhalation où on leur a
fait respirer du MTBE à des concentrations allant de 5,0 mg/m3
(1,4 ppm) à 270 mg/m3 (75 ppm). Aucun effet patent n'a été relevé,
qu'il s'agisse de la relation subjective de symptômes ou d'indicateurs
objectifs tels qu'une irritation ou d'autres signes, à des
concentrations allant jusqu'à 180 mg/m3 (50 ppm) et pendant une durée
pouvant atteindre 2 h. A en juger d'après ces résultats, il est peu
probable que le MTBE puisse à lui seul exercer des effets toxiques
aigus sur la population générale dans les conditions habituelles
d'exposition par la voie respiratoire. Il est cependant à noter que
les effets potentiels d'essences additionnées de MTBE, dans les
conditions où la plupart des gens sont exposés à cet additif
lorsqu'ils utilisent des carburants oxygénés, n'ont été étudiés ni
expérimentalement, ni par le biais de méthodes épidémiologiques
prospectives. Par ailleurs, le rôle de facteurs tels que la perception
de la présence de MTBE, explicable en partie par l'odeur particulière
de ce composé, n'a pas été étudié non plus.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Expérimentalement, la toxicité aiguë (exprimée par la CL50) du
MTBE pour les poissons, les amphibiens et les crustacés se révèle
supérieure à 100 mg/litre. On ne possède pas de données sur la
toxicité chronique ou subléthale de ce composé pour les organismes
aquatiques, ni sur sa toxicité pour les organismes terrestres.
9. Evaluation des risques pour la santé humaine et des effets sur
l'environnement
A en juger par les données collectives, il semble peu probable
que le MTBE puisse à lui seul et dans les conditions usuelles
d'exposition, provoquer des effets toxiques aigus dans la population
générale.
D'après les études effectuées sur l'animal, le MTBE possède une
toxicité aiguë "modérée" et il provoque une légère irritation cutanée
et oculaire, mais pas de sensibilisation. Des expositions répétées
entraînent des effets essentiellement localisés au rein chez le rat et
au foie chez la souris, la dose nocive la plus faible étant de 440
mg/kg p.c. par jour chez le rat après ingestion et de 1440 mg/m3
(400 ppm) après inhalation. Aux concentrations inférieures au seuil de
toxicité parentale, le MTBE n'a pas eu d'effets nocifs sur la
reproduction ou le développement.
Le MTBE n'est pas génotoxique mais il peut provoquer la formation
de tumeurs chez les rongeurs, surtout aux concentrations suffisamment
élevées pour avoir d'autres effets toxiques. On considère actuellement
que ces données ne sont pas suffisantes pour que l'on puisse en tirer
une évaluation du risque cancérogène chez l'Homme. Le Groupe spécial a
conclu que, pour être en mesure de donner des indications
quantitatives concernant les limites d'exposition et d'évaluer le
risque, il fallait obtenir des données supplémentaires sur un certain
nombre de points.
Il ne semble pas que le MTBE, aux concentrations auxquelles il se
trouve dans l'eau, puisse être toxique pour les organismes aquatiques,
sauf en cas de déversement. On ne possède pas de données sur la
toxicité du MTBE pour les organismes terrestres mais il n'y a
vraisemblablement pas lieu de s'alarmer, étant donné que sa
concentration est faible dans l'air ambiant et que sa demi-vie est
relativement brève.
RESUMEN
El éter metil- terciario-butílico (MTBE) es uno de los distintos
éteres que pueden utilizarse como aditivos de combustibles y en la
actualidad es con gran diferencia el más usado. El éter
etil- terciario-butílico (ETBE), el éter terciario-amil-metílico
(TAME), el éter terciario-amil-etílico (TAEE) y el éter
diisopropílico (DIPE), entre otros, pueden ser suplementos del MTBE o
sustituirlo para fines de oxigenación o mejora de los octanos y, en
consecuencia, pueden hallarse en asociación con el MTBE.
1. Identidad, propiedades físicas y químicas y métodos analíticos
El MTBE es un líquido volátil e incoloro a la temperatura
ambiente, de olor parecido al terpeno. Su viscosidad es baja y tiene
un punto de ebullición de 55,2°C. El punto de congelación es de -
109°C. La densidad es de 0,7404 a 20°C. La presión de vapor es
relativamente alta: 33 500 Pa a 25°C. El MTBE es inflamable y puede
formar mezclas explosivas con el aire. Es muy soluble en otros éteres
y alcohol. Se mezcla con la gasolina y es soluble en agua (42 000
g/m3 a 19,8°C). El coeficiente de partición log n-octanol/agua es
de 0,94-1,3. Es inestable en solución ácida.
El MTBE se analiza en todas las matrices en general por
cromatografía de gases, utilizando una gama de columnas capilares y
sistemas detectores que son apropiados para la matriz específica.
También se ha utilizado la cromatografía inversa en fase líquida para
el análisis de las muestras de gasolina. Se han empleado sistemas de
sorción-desorción, incluidos sistemas de purga y captación, así como
procedimientos de recámara, a fin de preparar muestras de aire, agua,
sedimento y biológicas para el análisis.
2. Fuentes de exposición humana y ambiental
No se conoce la presencia natural de MTBE en el medio ambiente.
En la industria deriva de la reacción catalítica del metanol y el
isobutileno, y en varios países se ha producido en volúmenes
crecientes desde los últimos años setenta. El MTBE figura actualmente
entre los 50 productos químicos de mayor producción en volumen. En
1996, la capacidad estadounidense de producción fue aproximadamente de
10,6 millones de toneladas, previéndose un constante aumento del uso
de MTBE. El 25% aproximadamente de la gasolina en los EE.UU está
mezclada con MTBE. El MTBE se utiliza casi exclusivamente para el
refuerzo de los octanos y para aumentar el contenido de la gasolina en
oxígeno. El MTBE se ha añadido a la gasolina en concentraciones de
hasta el 17% en volumen.
3. Transporte, distribución y transformación en el medio ambiente
Tras su eliminación en el aire, el MTBE permanecerá en gran parte
en este medio, penetrando cantidades menores en el suelo y el agua. En
la atmósfera, el MTBE puede ser arrastrado por la lluvia. Sin embargo,
sólo una pequeña cantidad es eliminada de la atmósfera de este modo.
La transformación atmosférica por radicales hidroxilos produce varios
productos, entre los que figuran el formato terciario-butílico (TBF)
estable y el 2-metoxi-2-metilpropanol, que se supone que son muy
reactivos con los radicales hidroxilos, dando CO2, formaldehido,
acetona y agua. Cuando el MTBE pasa al agua se disuelve una cantidad
significativa, con cierta proporción en el aire. La proporción que
pasa a los biota y el sedimento es escasa. La biodegradabilidad en
ensayos convencionales es limitada. Se cree que por lo general es
lenta en el medio ambiente. Cuando el MTBE pasa al suelo, es
transportado al aire por volatilización, al agua superficial por
escurrimiento y al agua subterránea como resultado de la lixiviación.
El MTBE puede persistir en el agua subterránea.
4. Niveles medioambientales y exposición humana
Se dispone de escasos datos sobre los niveles medioambientales y
la exposición humana.
En los estudios sobre el MTBE en el aire de algunas ciudades que
utilizan gasolina oxigenada con MTBE al 15%, las concentraciones
ambientales iban del nivel indetectable a 100,9 µg/m3 (0,028 ppm),
con varias concentraciones medianas de 0,47 a 14,4 µg/m3 (0,00013 a
0,004 ppm). Las concentraciones de MTBE en el aire de algunas ciudades
en donde se utiliza MTBE como reforzador de octanos en concentraciones
inferiores van del nivel no detectable a 26,4 µg/m3 (0,0073 ppm).
Las concentraciones a nivel del suelo o cerca de las refinerías
eran de 15 a 281 µg/m3. Los niveles medianos en el aire urbano cerca
de instalaciones de mezclado eran de 1508 µg/m3 (0,419 ppm), con
gamas de 216-35 615 µg/m3 (0,06 a 9,8 ppm).
En las estaciones de servicio situadas en zonas en donde la
gasolina oxigenada contiene el 10-15% de MTBE, las concentraciones
alcanzaban el nivel máximo en la zona de respiración durante el
llenado de los depósitos por los consumidores (gama de 300 a
136 000 µg/m3 (0,09 a 38 ppm)), con niveles que rara vez pasaban de
3600 µg/m3 (10 ppm), siendo ligeramente inferiores en la zona de
bombas (indetectables a 5700 µg/m3 (1,6 ppm)) y mínimos en el
perímetro de la estación (indetectables a 550 µg/m3 (0,14 ppm)). En
general las concentraciones eran superiores en las estaciones de
servicio sin sistemas de recuperación de vapores.
En la cabina del automóvil, las concentraciones eran de 7 a
60 µg/m3 (0,002 a 0,017 ppm) durante la conducción y de 20 a
610 µg/m3 (0,006 a 0,172 ppm) al llenar el depósito.
Basándose en operaciones limitadas de vigilancia realizadas casi
exclusivamente en los EE.UU., se ha detectado el MTBE en la nieve, el
agua de tormenta, las aguas superficiales (riachuelos, ríos y
embalses), las aguas subterráneas y el agua de beber. Las
concentraciones de MTBE detectadas en el agua de tormenta iban de 0,02
a 8,7 µg/litro, con un valor mediano de menos de 1,0 µg/litro. En los
riachuelos, ríos y embalses, la gama de detección era de 0,2 a
30 µg/litro y la gama de valores medianos en varios estudios era de
0,24 a 7,75 µg/litro.
En general no se ha detectado el MTBE en las aguas subterráneas
profundas o cercanas a la superficie en zonas agrícolas. Cuando se ha
detectado, la concentración era inferior a 2,0 µg/litro. El MTBE se
halla con más frecuencia en las aguas subterráneas cercanas a la
superficie (1,6 a 3,2 metros de estos acuíferos) de las zonas urbanas.
En este entorno, las concentraciones van de menos de 0,2 µg/litro a 2
mg/litro, con un valor mediano inferior a 0,2 µg/litro.
El MTBE se halla poco frecuentemente en sistemas de
abastecimiento público de agua procedente de capas freáticas. Entre 51
sistemas estudiados, en 48 la concentración era de <20 µg/litro.
Son insuficientes los datos disponibles para caracterizar la
concentración de MTBE en los sistemas de abastecimiento público de
agua procedentes de aguas superficiales. El MTBE se ha hallado en
concentraciones altas (esto es, >1000 µg/litro) en algunos pozos
privados utilizados para obtener agua de beber. Sin embargo, es dudoso
que las personas puedan consumir agua con concentraciones de MTBE
superiores a unos 50-100 µg/litro debido al bajo umbral de su gusto y
olor.
Entre los trabajadores con posible exposición al MTBE figuran los
ocupados en la producción, distribución y uso de MTBE y de gasolina
con MTBE, que incluye el personal de estaciones de servicio y los
mecánicos.
La exposición a corto plazo (<30 min) en operaciones corrientes
de fabricación y mantenimiento de MTBE puro iba de 715 a 43 000 µg/m3
(0,2 a 12 ppm), siendo el promedio de los valores medianos de
3400 µg/m3 aproximadamente (0,95 ppm). La exposición a largo plazo
(30 min a 8 h) era de 360 a 890 000 µg/m3 (0,01 ppm a 250 ppm), con
valores medianos de aproximadamente 540 µg/m3 (0,15 ppm). En el caso
de los trabajadores de operaciones de mezclado, los valores a corto
plazo oscilaban entre niveles indetectables y 360 000 µg/m3
(100 ppm), siendo el promedio de los valores medianos de 5700 µg/m3
aproximadamente (1,6 ppm). Los valores a largo plazo comprendían desde
niveles indetectables hasta 257 000 µg/m3 (72 ppm), siendo el
promedio de los valores medianos de 2000 µg/m3 aproximadamente
(0,6 ppm).
La exposición alcanzó el nivel máximo durante el transporte de
MTBE puro y de mezclas de combustible en oleoductos, barcazas, vagones
de ferrocarril y camiones (sólo MTBE puro), variando los valores a
corto plazo entre 4 y 3750 mg/m3 (0,001 a 1050 ppm), con un promedio
de los valores medianos de 140 mg/m3 (39 ppm). Los valores a largo
plazo fueron de 0,036 a 2540 mg/m3 (0,01 a 712 ppm), con un promedio
de los valores medianos de 2,85 mg/m3 (0,8 ppm). En las operaciones
de distribución (esto es, carga de mezclas de combustible y MTBE en
camiones y entrega y descarga en las estaciones de servicio), los
valores a corto plazo oscilaron entre niveles indetectables y
225 mg/m3 (63 ppm), siendo el promedio de los valores medianos de
21 mg/m3 aproximadamente (6 ppm). Los valores a largo plazo fueron de
0,036 a 22 mg/m3 (0,01 a 6,2 ppm), siendo el promedio de los valores
medianos de 1,79 mg/m3 (0,5 ppm).
Los valores medianos de la exposición a corto plazo de operarios
de estaciones de servicio fueron en general de 1,071 a 21,42 mg/m3
(0,3 a 6 ppm), excediendo rara vez de 35,7 mg/m3 (10 ppm). Los
valores medianos de la exposición a largo plazo en operarios de
estaciones de servicio presentaron un promedio de 1,79 mg/m3
(0,5 ppm). Los valores medianos de la exposición de mecánicos estaban
por debajo de los niveles de detección en un estudio a corto plazo; el
promedio de los valores medianos para la exposición a largo plazo fue
aproximadamente de 360 µg/m3 (0,1 ppm).
5. Cinética y metabolismo
Los datos toxicocinéticos sobre el MTBE en personas proceden
principalmente de estudios controlados en voluntarios adultos sanos y
en una población expuesta a la gasolina oxigenada. El MTBE pasa
rápidamente a la circulación después de la exposición por inhalación.
En voluntarios sanos expuestos a la inhalación, la cinética del MTBE
era lineal hasta concentraciones de 268 mg/m3 (75 ppm). Se midió en
la sangre y orina de personas expuestas el alcohol
terciario-butílico, metabolito del MTBE. Las concentraciones
sanguíneas máximas del MTBE y el alcohol terciario-butílico fueron
de 17,2 a 1144 µg/m3 y de 7,8 a 925 µg/m3, respectivamente, en
personas expuestas a 5,0 a 178,5 mg/m3 (1,4 a 50 ppm) de MTBE.
Basándose en un modelo de monocompartimiento se identificaron
componentes rápidos (36- 90 min) y lentos (19 h) de la semivida del
MTBE.
En los roedores, el MTBE se absorbe y distribuye bien después de
la administración oral y la exposición por inhalación, con menor
absorción cutánea. En la administración oral de 400 mg/kg y en la
inhalación de 28 800 mg/m3 (8000 ppm) aumentó el porcentaje de la
dosis absorbida total eliminado en el aire espirado, con un descenso
correspondiente del porcentaje eliminado por la orina, indicando la
saturación metabólica. No se identificó la presencia de alcohol
terciario-butílico (TBA) en la orina de ratas expuestas. Hubo
indicios de un metabolismo adicional del TBA, basados en la
identificación de 2-metil-1,2-propanodiol y de ácido
alpha-hidroxiisobutírico eliminado por la orina. Los estudios
in vitro prueban que el MTBE se metaboliza hasta TBA, formaldehido y
acetona.
6. Efectos en los animales de laboratorio y en los sistemas
in vitro
En las ratas, la dosis letal oral mediana aguda (DL50) es
aproximadamente de 3800 mg/kg de peso corporal. La concentración letal
mediana aguda (CL50) para una exposición por inhalación de 15 minutos
es de aproximadamente 141 000 mg/m3 de aire en ratones. Entre los
signos de intoxicación figuran la depresión del SNC, la ataxia y la
respiración laboriosa. Si la dosis no es letal, la recuperación es
completa. La DL50 para la toxicidad cutánea en conejos es de
>10 200 mg/kg de peso corporal.
En un solo estudio identificado, el MTBE resultó "moderadamente"
irritante para la piel, produciendo eritema moderado y edema después
de la aplicación cutánea en conejos. También resultó irritante para
los ojos de los conejos, produciendo lesiones leves y reversibles. En
el único estudio identificado, el MTBE produjo irritación respiratoria
ligera a intensa después de la exposición de ratones a 300 y
30 000 mg/m3, respectivamente. No causó sensibilización cutánea en
estudios en cobayos.
La exposición repetida produce fundamentalmente aumentos del peso
de los órganos y lesiones histopatológicas en el riñón de ratas y en
el hígado de ratones. Los niveles de mínimo efecto señalado de
nefrotoxicidad tras la ingestión en estudios de 90 días fueron de
400 mg/kg de peso corporal por día (aumentos del peso renal relativo y
formación de gotas hialinas en ratas Sprague-Dawley). En la exposición
por inhalación a 2880 mg/m3 (800 ppm) se produjeron aumentos del peso
del riñón asociados a las concentraciones más altas, con moderado
aumento de las gotas hialinas en los túbulos proximales en ratas
Fischer-344. En estudios de oncogenicidad por inhalación en dosis de
1440 mg/m3 (400 ppm), la incidencia y la gravedad de la nefropatía
progresiva crónica aumentó en ratas machos; en esta concentración, en
ratones machos se observó un aumento del peso absoluto del hígado (que
guardaba correlación con el aumento de la hipertrofia hepatocelular en
concentraciones superiores) y un aumento del peso renal relativo.
La exposición al MTBE también produjo lesiones reversibles del
sistema nervioso central (SNC), incluidas sedación, hipoactividad,
ataxia y anestesia en concentraciones superiores y efectos bifásicos
sobre la actividad motriz en concentraciones inferiores. En un estudio
de una sola exposición de 6 horas en ratas, las concentraciones de
2880 mg/m3 (800 ppm) produjeron cambios reversibles de la actividad
motriz relacionados con la dosis en sexos separados. Esos efectos
fueron transitorios y no se pusieron de manifiesto en estudios a largo
plazo.
Se han efectuado estudios reproductivos por inhalación de una y
dos generaciones y estudios de cuatro generaciones en ratas, ratones y
conejos. En esos estudios no se hallaron efectos reproductivos
específicos en ratas en concentraciones de hasta 28 800 mg/m3. El
MTBE no ha producido efectos en el desarrollo en concentraciones
inferiores a las que resultaron tóxicas en las madres. Se han
observado disminuciones del peso del útero y aumentos del metabolismo
estrogénico en ratonas con dosis de 28 000 mg/m3.
El MTBE ha sido sometido a pruebas apropiadas en una amplia gama
de ensayos de mutagenicidad y de genotoxicidad. Los resultados
muestran que el MTBE no es genotóxico, aunque resultó positiva una
prueba de mutación del locus tk en células linfomatosas de ratón
debido al paso metabólico de MTBE a formaldehido.
Se han realizado estudios de carcinogenicidad que comprendieron
la exposición por inhalación de ratas Fischer-344 y de ratones CD-1 y
el cebado de ratas Sprague-Dawley. En ninguno de los dos estudios de
inhalación se utilizaron métodos de análisis estadístico que
efectuaran el reajuste de las diferencias de supervivencia. En los
tres estudios se produjeron aumentos significativos de la incidencia
de tumores, esto es, tumores de células tubulares renales y tumores de
células de Leydig en ratas Fischer-344 machos, tumores de células de
Leydig en ratas Sprague-Dawley machos y linfomas-leucemias
(combinadas) en ratas hembras de la misma especie, y tumores de
células hepáticas en ratones CD-1 hembras. Así pues, no se observaron
constantemente tumores de células tubulares renales ni
leucemias-linfomas en los distintos estudios en ratas. Además, los
tumores renales específicos del sexo se asociaron a la nefropatía de
la alpha2u-globulina específica del sexo, observada en varios estudios
de breve duración. Se observaron aumentos de los tumores de células de
Leydig con la dosis más alta (1000 mg/kg de peso corporal) en la ratas
Sprague-Dawley, pero la interpretación de los aumentos registrados en
las ratas Fischer-344 resultó compleja por las incidencias muy altas
concurrentes y de los testigos históricos. En los ratones, las
incidencias de los tumores hepáticos fueron en los testigos y en los
grupos expuestos a 28 800 mg/m3 (8000 ppm), respectivamente, de 2/50
y 10/50 en las hembras y de 12/49 y 16/49 en los machos. Los aumentos
fueron moderados y acompañados de hipertrofia hepatocelular.
7. Efectos en el ser humano
Tras la introducción de dos programas separados relativos a los
combustibles en los EE.UU., que requieren el empleo de productos de
oxigenación de la gasolina (no necesariamente MTBE), los consumidores
de algunas zonas se han quejado de trastornos agudos de la salud, como
dolor de cabeza, irritación de los ojos y la nariz, tos, náuseas,
mareos y desorientación. Los estudios epidemiológicos de poblaciones
humanas expuestas en condiciones profesionales o no profesionales, así
como los estudios experimentales de voluntarios expuestos en
condiciones controladas, no han podido identificar la base de esos
trastornos. Aunque los resultados son variados, los estudios
comunitarios efectuados en Alaska, New Jersey, Connecticut y Wisconsin
(EE.UU.) no han proporcionado indicios, o éstos han sido limitados, de
la asociación entre la exposición al MTBE y la prevalencia de
trastornos de la salud.
En estudios experimentales controlados en voluntarios humanos
expuestos en cámaras de inhalación al MTBE en concentraciones de 5,0
mg/m3 (1,4 ppm) a 270 mg/m3 (75 ppm) no hubo efectos manifiestos en
términos de presencia subjetiva de síntomas o de indicadores objetivos
de irritación u otros efectos en concentraciones de hasta 180 mg/m3
(50 ppm) durante dos horas. Partiendo de esos datos parece improbable
que el MTBE por sí solo produzca efectos agudos adversos en la salud
en la población general en las condiciones corrientes de exposición
por inhalación. Sin embargo, los posibles efectos de las mezclas de
gasolina y MTBE y el modo de exposición de la mayor parte de las
personas al MTBE en asociación con el empleo de combustibles
oxigenados, no se han examinado experimentalmente ni por métodos
epidemiológicos prospectivos. Por otra parte, no se ha investigado,
por ejemplo, la función de factores tales como la percepción del MTBE,
debida en parte a su olor distintivo.
8. Efectos en otros organismos en el laboratorio y sobre el
terreno
La toxicidad aguda experimental (CL50) del MTBE en los peces,
los anfibios y los crustáceos es >100 mg/litro. No existen datos
sobre la toxicidad crónica o subletal para las especies acuáticas ni
la toxicidad para los organismos terrestres.
9. Evaluación de los riesgos para la salud humana y efectos en el
medio ambiente
Basándose en datos de observación colectiva, parece improbable
que el MTBE por sí solo induzca efectos agudos adversos en la salud de
la población general en las condiciones corrientes de exposición.
En estudios en animales, el MTBE es "moderadamente" tóxico en
forma aguda y produce irritación cutánea y ocular moderada, pero no
sensibilización. La exposición repetida afecta fundamentalmente al
riñón de ratas y al hígado de ratones, observándose los efectos
adversos mínimos con concentraciones de 440 mg/kg de peso corporal por
día en ratas después de la ingestión y de 1400 mg/m3 (400 ppm)
después de la inhalación. El MTBE no ha inducido efectos adversos en
la reproducción o el desarrollo en concentraciones inferiores a las
que eran tóxicas para los padres.
El MTBE no es genotóxico, pero ha producido tumores en roedores,
principalmente con concentraciones altas, que también inducen otros
efectos adversos. Esos datos se consideran en la actualidad
insuficientes para la evaluación del riesgo carcinogénico en seres
humanos. El Grupo Especial llegó a la conclusión de que para
proporcionar orientación cuantitativa sobre los límites pertinentes de
exposición y para estimar el riesgo se necesita adquirir datos
adicionales en distintos sectores.
No parece que las concentraciones de MTBE en el agua ambiental
sean tóxicas para los organismos acuáticos, excepto en caso de
escapes. Aunque no hay datos sobre la toxicidad terrestre del MTBE,
parece que no es preocupante ya que las concentraciones en el aire
ambiental son bajas y la semivida del MTBE es relativamente breve.
