
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 78
DITHIOCARBAMATE PESTICIDES,
ETHYLENETHIOUREA AND PROPYLENETHIOUREA:
A GENERAL INTRODUCTION
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1988
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154278 0
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DITHIOCARBAMATE PESTICIDES,
ETHYLENETHIOUREA (ETU), AND PROPYLENETHIOUREA (PTU) - A GENERAL
INTRODUCTION
A. DITHIOCARBAMATE PESTICIDES: A GENERAL INTRODUCTION
B. ETHYLENETHIOUREA (ETU) AND PROPYLENETHIOUREA (PTU)
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
ANNEX I NAMES AND STRUCTURES OF SELECTED DITHIOCARBAMATES
ANNEX II NAMES AND STRUCTURES OF DEGRADATION PRODUCTS OF
ETHYLENE BISDITHIOCARBAMATES
ANNEX III DITHIOCARBAMATES AND ETU: JMPR REVIEWS, ADIs, EVALUATION
BY IARC, CLASSIFICATION BY HAZARD, FAO/WHO DATA SHEETS,
IRPTC DATA PROFILE AND LEGAL FILE
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DITHIOCARBAMATE
PESTICIDES, ETHYLENETHIOUREA (ETU), AND PROPYLENETHIOUREA (PTU)
Members
Dr U.G. Ahlborg, Unit of Toxicology, National Institute of
Environmental Medicine, Stockholm, Sweden (Vice-Chairman)
Dr H.H. Dieter, Federal Health Office, Institute for Water, Soil
and Air Hygiene, Berlin (West)
Dr R.C. Dougherty, Department of Chemistry, Florida State
University, Tallahassee, Florida, USA
Dr A.H. El Sabae, Pesticide Division, Faculty of Agriculture,
University of Alexandria, Alexandria, Egypta
Dr A. Furtado Rahde, Ministry of Public Health, Porto Alegre,
Brazil (Chairman)
Dr S. Gupta, Department of Zoology, Faculty of Basic Sciences,
Punjab Agricultural University, Ludhiana, Punjab, Indiaa
Dr L.V. Martson, All Union Scientific Research Institute of the
Hygiene and Toxicology of Pesticides, Polymers, and Plastics,
Kiev, USSRa
Dr U.G. Oleru, Department of Community Health, College of Medicine,
University of Lagos, Lagos, Nigeria
Dr Shou-Zheng Xue, Toxicology Programme, School of Public Health,
Shanghai Medical University, Shanghai, China
Observers
Dr R.F. Hertel, Fraunhöfer Institute for Toxicology and Aerosol
Research, Hanover, Federal Republic of Germany
Dr E. Kramer (European Chemical Industry Ecology and Toxicology
Centre), Dynamit Nobel A.G., Cologne, Federal Republic of
Germany
Mr G. Ozanne (European Chemical Industry Ecology and Toxicology
Centre), Rhone Poulenc DSE/TOX, Neuilly-sur-Seine, France
Mr V. Quarg, Federal Ministry for Environment, Nature Conservation
and Nuclear Safety, Bonn, Federal Republic of Germany
Dr U. Schlottmann, Chemical Safety, Federal Ministry for
Environment, Nature Conservation and Nuclear Safety, Bonn,
Federal Republic of Germany
Dr M. Sonneborn, Federal Health Office, Berlin (West)
--------------------------------------------------------------------------
a Invited but unable to attend.
Observers (contd.)
Dr W. Stöber, Fraunhöfer Institute for Toxicology and Aerosol
Research, Hanover, Federal Republic of Germany
Dr D. Streelman (International Group of National Associations of
Agrochemical Manufacturers), Agricultural Chemicals
Registration and Regulatory Affairs, Rohm & Haas, Philadelphia,
Pennsylvania, USA
Secretariat
Mrs B. Bender, International Register for Potentially Toxic
Chemicals, Geneva, Switzerland
Dr A. Gilman, Industrial Chemicals and Product Safety Section,
Health Protection Branch, Department of National Health and
Welfare, Tunney's Pasture, Ottawa, Ontario, Canada (Temporary
Adviser)
Dr L. Ivanova-Chemishanska, Institute of Hygiene and Occupational
Health, Medical Academy, Sofia, Bulgaria (Temporary Adviser)
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr E. Johnson, Unit of Analytical Epidemiology, International
Agency for Research on Cancer, Lyons, France
Dr G. Rosner, Fraunhöfer Institute for Toxicology and Aerosol
Research, Hanover, Federal Republic of Germany (Temporary
Adviser)
Dr G.J. Van Esch, Bilthoven, Netherlands (Temporary Adviser)
(Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
ENVIRONMENTAL HEALTH CRITERIA FOR DITHIOCARBAMATE PESTICIDES,
ETHYLENETHIOUREA (ETU), AND PROPYLENETHIOUREA (PTU)
A WHO Task Group on Environmental Health Criteria for
Dithiocarbamate Pesticides, Ethylenethiourea, and Propylene-
thiourea met at the Fraunhöfer Institute for Toxicology and Aerosol
Research, Hanover, Federal Republic of Germany, from 20 to 24
October 1986. Professor W. Stöber opened the meeting and welcomed
the members on behalf of the host Institute. Dr U. Schlottmann
spoke on behalf of the Federal Government, which sponsored the
meeting. Dr K.W. Jager addressed the meeting on behalf of the
three co-sponsoring organizations of the IPCS (UNEP/ILO/WHO). The
Task Group reviewed and revised the draft criteria document and
summarized the health risks of exposure to dithiocarbamate
pesticides.
The drafts of this document were prepared by DR L. IVANOVA-
CHEMISHANSKA, Institute of Hygiene and Occupational Health, Sofia,
Bulgaria, and DR G.J. VAN ESCH, Bilthoven, the Netherlands.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
ABBREVIATIONS
ADI acceptable daily intake
BSP sulfobromophthalein
DDC diethyldithiocarbamate
DIDTa 5,6-dihydro-3 H-imidazo(2,1- C)-1,2,4-dithiazole-3-thione
EBDC ethylene bisdithiocarbamate
EDA ethylenediamine
EDI ethylene diisothiocyanate
ETD ethylene bisthiuram disulfide
ETU ethylenethiourea
EU ethyleneurea
ip intraperitoneal
iv intravenous
JMPR Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and a WHO Expert
Group on Pesticide Residues
MIT methylisothiocyanate
NDDC sodium diethyldithiocarbamate
NDMA nitrosodimethylamine
NDMC sodium dimethyldithiocarbamate
PBI protein-bound iodine
PTU propylenethiourea
SGPT serum glutamic-pyruvic transaminase
T3 triiodothyronine
T4 thyroxine
TSH thyroid-stimulating hormone
----------------------------------------------------------------------
a In some older studies, DIDT is referred to as ethylene-
thiuram monosulfide (ETM). However, in 1974 the chemical
that had been referred to as ETM was shown to be DIDT, and
so the latter term has been used throughout this document.
PART A
DITHIOCARBAMATE PESTICIDES: A GENERAL INTRODUCTION
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DITHIOCARBAMATE PESTICIDES: A
GENERAL INTRODUCTION
INTRODUCTION
1. SUMMARY
1.1 General
1.2 Properties, uses, and analytical methods
1.3 Sources, environmental transport and distribution
1.4 Environmental levels and human exposure
1.5 Kinetics and metabolism
1.6 Effects on organisms in the environment
1.7 Effects on experimental animals and in vitro test systems
1.8 Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
3.2 Man-made sources
3.2.1 Production levels, processes, and uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Water
4.1.2 Soil
4.2 Biotransformation
4.2.1 Microbial degradation
4.2.2 Photodegradation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Food
5.2 Monitoring and market basket studies
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and excretion
6.2 Metabolic transformation
6.2.1 Mammals
6.3 Metabolism in plants
6.4 Decomposition in water and soil
6.5 Metabolism in microorganisms
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.2 Aquatic organisms
7.2.1 Acute toxicity
7.2.2 Short- and long-term toxicity and reproduction studies
7.2.2.1 Fish
7.2.2.2 Invertebrates
7.2.3 Bioconcentration (bioaccumulation)
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short- and long-term exposures
8.2.1 Oral exposure
8.2.1.1 Rat
8.2.1.2 Dog
8.2.1.3 Bird
8.2.2 Inhalation exposure
8.2.2.1 Rat
8.3 Skin and eye irritation; sensitization
8.4 Reproduction, embryotoxicity, and teratogenicity
8.4.1 Reproduction
8.4.1.1 Rat
8.4.1.2 Bird
8.4.2 Teratogenicity
8.4.2.1 Rat
8.4.2.2 Mouse
8.4.3 Embryotoxicity
8.5 Mutagenicity and related end-points
8.6 Carcinogenicity
8.6.1 Mouse
8.6.2 Rat
8.6.3 Dog
8.6.4 Dithiocarbamates in combination with nitrite
8.7 Mechanisms of toxicity; mode of action
8.7.1 Thyroid
8.7.2 Interaction of dithiocarbamates and alcohol
8.7.3 Neurotoxicity
8.7.4 Dithiocarbamates in combination with metals
8.7.5 Miscellaneous reactions
9. EFFECTS ON MAN
9.1 Occupational exposure
9.1.1 Acute toxicity - poisoning incidents
9.1.2 Case reports, short-term and epidemiological studies
9.1.2.1 Dermal
9.1.2.2 Exposure via different routes
INTRODUCTION
The dithiocarbamates included in this review are those that
are mainly used in agriculture and form part of the large group
of synthetic organic pesticides that have been developed and
produced on a large scale in the last 40 - 50 years. The
development of dithiocarbamate derivatives with pesticidal
properties occurred during and after the Second World War.
However, a few compounds, such as thiram and ziram, were
introduced in the 1930s.
The world-wide consumption of dithiocarbamates is between
25 000 and 35 000 metric tonnes per year. Dithiocarbamates are
used as fungicides, being effective against a broad spectrum of
fungi and plant diseases caused by fungi. In industry, they are
used as slimicides in water-cooling systems, in sugar, pulp, and
paper manufacturing, and as vulcanization accelerators and
antioxidants in rubber. Because of their chelating properties,
they are also used as scavengers in waste-water treatment. The
herbicidal compounds, which are an integral part of
industrialized agriculture, are used mostly in North and Central
America, and Europe, with little use reported in Asia, South
America, and Africa.
In this introductory document, an attempt has been made to
summarize the available data on the dithiocarbamates used as
pesticides, in order to indicate their impact on man, animals,
plants, and the environment. This overview is not complete, nor
is it intended to be. More details on certain aspects are given
in the JMPR and IARC (International Agency for Research on
Cancer) reports, which have already been published. It also
should be recognized that the design of a number of the studies
cited, especially the older ones, is inadequate.
1. SUMMARY
1.1. General
Dithiocarbamates are mainly used in agriculture as insecti-
cides, herbicides, and fungicides. Additional uses are as
biocides for industrial or other commercial applications, and in
household products. Some are used for vector control in public
health.
The general formula of dithiocarbamates is characterized by
the presence of:
R1 S
\ ||
N-C-S-R3
/
R2
Depending on the types of monoamines used in the synthesis of
these compounds, mono- or dialkyldithiocarbamates are formed.
Reactions with diamines result in the formation of two terminal
dithiocarbamate groups linked by an alkylene (ethylene) bridge.
Both alkyl and ethylene dithiocarbamates form salts with metals,
and both can be oxidized to the corresponding disulfides.
More than 15 dithiocarbamates are known. However, it is
beyond the scope of this publication to give complete informa-
tion on each compound. The intention is to cover the different
aspects of dithiocarbamates, making use of publications and
reports available on the compounds that are most used and best
known. Data on the carbamates or thiocarbamates are not
included, because these compounds have been covered in
Environmental Health Criteria 64: Carbamate Pesticides and 76:
Thiocarbamate Pesticides (WHO, 1986b; WHO, 1988)
1.2. Properties, Uses, and Analytical Methods
Dithiocarbamates with hydrophylic groups form water-soluble,
heavy-metal complexes, while some of the dithiocarbamate metal
complexes used as fungicides are insoluble in water but soluble
in non-polar solvents. Alkylene bisdithiocarbamates (containing
two donor CS2 groups), which form polymeric chelates, are
insoluble in both water and non-polar solvents.
The heavy-metal salts of ethylene bisdithiocarbamic acid
may polymerize. Dithiocarbamates may decompose under certain
circumstances into a number of compounds, such as sulfur,
5, 6-dihydro-3 H-imidazol [2,1-C]-1, 2, 4-dithiazole-3-thione,
ethylenethiourea (ETU), and ethylenediamine (EDA). ETU is
fairly stable, has a high water solubility, and is of particular
importance because of its specific toxicity. For this reason,
toxicological information on this compound is included in this
review.
Physical and chemical data for individual substances are
tabulated in the document, and analytical methods for dithio-
carbamates are described. Further details for individual
dithiocarbamates appear in the WHO Technical Report Series and
the IRPTC data profiles.
1.3. Sources, Environmental Transport and Distribution
Most dithiocarbamates were developed during and after World
War II. However, a few compounds (ziram and thiram) were
introduced around 1931. Dithiocarbamates, with their insecti-
cidal, herbicidal, and fungicidal properties, have a wide range
of applications and are produced in great quantities. Because
of their high biological activity, dithiocarbamates are also
used in medicine, the rubber industry, and in the treatment of
chronic alcoholism.
Alkyl dithiocarbamates are stable in an alkaline medium. By
splitting off carbon disulfide and hydrogen sulfide, as well as
by oxidative degradation, a number of break-down products, such
as ETU, are formed in soil and water. The rate of degradation
depends on a number of factors, including pH and type of cation.
Ethylene bisdithiocarbamates (EBDCs) are generally unstable in
the presence of moisture, oxygen, or biological systems, and
decompose rapidly in water.
The mobility of EBDCs in soil varies considerably, depending
on their individual water solubilities and the type of soil.
ETU is water-soluble and mobile. It is taken up by plant roots,
is translocated, and metabolized, forming ethyleneurea (EU),
other 2-imidazole derivatives, and various unidentified
metabolites. In addition, ETU is readily photooxidized to EU in
the presence of photosensitizers. Residues of EBDCs and ETU are
found in and/or on crops treated with EBDCs. The residue levels
change during storage, processing, and cooking due to environ-
mental factors. During these processes, the parent compound may
be converted to ETU.
1.4. Environmental Levels and Human Exposure
Information on the environmental impact of dithiocarbamates
with respect to persistence and bioaccumulation in the different
species and food chains is limited. On the basis of the
available information, it is likely that most of these compounds
are rapidly degraded in the presence of oxygen, moisture, etc.,
to form a number of compounds, some of which, e.g., ETU and
propylenethiourea (PTU), are toxicologically important.
When certain crops, such as spinach, carrots, and potatoes,
are treated with EBDCs, high levels of ETU can be found after
cooking. In general, however, the ETU levels are below 0.1 mg/kg
product.
Human exposure to EBDCs was calculated for the population of
the USA on the basis of estimated consumption of dietary
residues of ETU in treated crops. Upper limit (worst case) and
lower limit (lowest case) estimates of exposure to ETU were
3.65 µg/kg and 0.24 µg/kg body weight per day, respectively.
An estimate made for the Canadian population on the basis of
results of available market-basket surveys would be around
1 µg/kg body weight per day.
1.5. Kinetics and Metabolism
As a general rule, dithiocarbamates can be absorbed by the
organism via the skin, mucous membranes, and the respiratory and
gastrointestinal tracts. Whereas dithiocarbamates are absorbed
rapidly from the gastrointestinal tract, metal-complexed
alkylene bisdithiocarbamates are absorbed poorly both from the
gastrointestinal tract and through the skin.
Dialkyldithiocarbamates and EBDCs are metabolized via
different mechanisms. The metabolism of the former is
straightforward, dialkylthiocarbamic acid being formed as a free
acid or as S-glucuronide conjugate. Other metabolic products
include carbon disulfide, formaldehyde, sulfate, and dialkyl
amine.
The metabolic decomposition of EBDCs in mammals is complex
and results in the formation of carbon disulfide, EDA, a few
ethylene bisthiuram disulfides, hydrogen sulfide, ethylene
bisthiocyanate, and ETU. The latter is further broken down to
moieties that are incorporated into compounds such as oxalic
acid, glycine, urea, and lactose. Dithiocarbamates and their
metabolic products are found in certain organs, such as the
liver, kidneys, and, especially, the thyroid gland, but
accumulation of these compounds does not take place because of
their rapid metabolism.
After treating plants with dithiocarbamates, a large number
of metabolites are found, including ETU, EU, imidazole
derivatives, diisothiocyanates, diamines, disulfides, and other
metabolites, that are still unknown.
1.6. Effects on Organisms in the Environment
Soil microorganisms are capable of metabolizing dithio-
carbamates. From the limited information available, it seems
that the breakdown products can affect enzyme activities,
respiration, and nitrification at dose levels of the order of
10 mg/kg dry soil or more.
Dithiocarbamates have an LC50 of less than 1 mg/litre for
invertebrates (Daphnia) and between 1 and 4 mg/litre for algae
(Chlorella). The acute toxicity of dithiocarbamates for fish
is rather high. In general, the acute LC50 of dialkyldithio-
carbamates for fish is less than 1 mg/litre, and that of EBDCs
is in the range 1 - 8 mg/litre water. The sac fry and early fry
stages of the rainbow trout have a higher sensitivity than other
early life stages, and embryotoxic and teratogenic effects are
induced by certain dithiocarbamates. However, bioaccumulation
is low (bioconcentration factor < 100). The toxicity of ETU and
EU for fish, Daphnia, Chlorella, and two bacteria species is
very low, of the order of g/litre.
Several dithiocarbamates were shown to intervene with
testicular development and function and to cause nerve fibre
degeneration in domestic fowl.
Information on the influence of dithiocarbamates on honey
bees is lacking.
1.7. Effects on Experimental Animals and In Vitro Test Systems
The acute oral and dermal toxicities of the different
dithiocarbamates are generally low. Most compounds have a low
volatility, and only limited information concerning inhalation
toxicity is available. Local irritation of the respiratory
tract occurs when dithiocarbamates are inhaled as dust, which
can also induce eye and dermal irritation. Some dithiocarbamates
are sensitizing agents. ETU also has a low acute oral toxicity.
Many short- and long-term toxicity studies have been carried
out on different dithiocarbamates. In rats, some dithio-
carbamates tested at high dose levels induced dose-dependent
adverse effects on the reproduction and endocrine structures and
functions, thus reducing reproductive capacity. Some dithio-
carbamates also showed effects on reproduction in birds.
In teratogenicity studies on mice and rats, dithiocarbamates
induced an increase in resorption sites and somatic and skeletal
malformations (cleft palate, hydrocephaly, and other abnorm-
alities). The dose levels needed to produce these effects were
usually higher than 200 mg/kg body weight in rats, and above
100 mg/kg body weight in mice.
In general, the results of mutagenicity studies with
dithiocarbamates have been negative.
From the available long-term carcinogenicity studies on mice
and rats, there is no clear indication of a carcinogenic effect.
Some of the dithiocarbamates have shown a goitrogenic effect at
high dose levels.
There is evidence that certain dithiocarbamates may be
converted in vivo to N-nitroso derivatives, which are
considered to be both mutagenic and carcinogenic. However, the
levels of nitroso compounds that can be expected to result from
the dietary intake of dithiocarbamate pesticide residues are
negligible compared with those of the nitroso precursors, which
occur naturally in food and drinking-water.
In rats, high levels of dithiocarbamates produce an increase
in thyroid weight, a reduction in colloid in follicles, hyper-
plasia, and nodular goitre. These distinct morphological
changes are in agreement with an increase in thyroid-stimulating
hormone (TSH). Hypophyseal stimulation of the thyroid is the
consequence of a decreased blood level of thyroxin, the
synthesis of which is inhibited by dithiocarbamates. The
thyroid hyperplasia induced by dithiocarbamates is largely
reversible on cessation of exposure.
Another intriguing phenomenon is the induction of alcohol
intolerance by most of the alkyldithiocarbamates. This
phenomenon has been studied in rats and produced in man. It has
even led to the use of disulfiram in the treatment of chronic
alcoholism.
At dose levels above 50 mg/kg body weight, dithiocarbamates
produce neurotoxic effects in rats and rabbits, characterized by
ataxia and paralysis of the hind legs, and demyelination and
degeneration of peripheral nerves. In birds, paralysis and
muscular and peripheral nerve atrophy have also been observed.
Dithiocarbamates have been reported to cause a redistri-
bution of heavy metals, e.g., lead and cadmium, in organs such
as the brain. Furthermore, because of their chelating
properties, these dithiocarbamates may have an effect on the
function of enzymes containing metals, such as zinc and copper.
1.8. Effects on Man
Regular contact with dithiocarbamates can cause functional
changes in the nervous and hepatobiliary systems. Skin contact
with dithiocarbamates may induce contact dermatitis, and some of
these compounds will induce sensitization. Alcohol intolerance
can be induced by certain dithiocarbamates, as indicated in
section 1.7.
There are indications that the mean incidence of chromosomal
aberrations in lymphocytes is increased in workers exposed to
certain dithiocarbamates. Epidemiological studies on workers
exposed to dithiocarbamates or ETU did not show any increase in
the incidence of thyroid tumours. However, only a relatively
small number of workers was involved.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Dithiocarbamates are the disulfur analogues of carbamates,
and they are characterized by the presence of:
S
\ ||
N-C-S-
/
Secondary monoamines, e.g., dimethyl or diethyl amines,
react with carbon disulfide to give dialkyldithiocarbamates:
S
||
R2NH + CS2 + NaOH ---- R2N-C-S-
Reaction with monoalkylamines gives the corresponding
monoalkyldithiocarbamates. The reaction of carbon disulfide
with diamines (for instance, EDA) gives two terminal dithio-
carbamate groups linked by an alkylene bridge:
S
||
CH2-NH-C-S-
(CH2NH2)2 + CS2 + NaOH ----> |
CH2-NH-C-S-
||
S
Both alkyl and ethylene dithiocarbamates form salts with
metals and both can be oxidized to the corresponding disulfides.
EBDCs can form polymers, especially in the presence of certain
ubiquitous metallic ions (Engst & Schnaak, 1974).
The chemical structures and pesticidal activity of the
principal dithiocarbamates are listed in Table 1. CAS registry
numbers, chemical names, common names, molecular formulae,
relative molecular masses, and selected chemical and physical
properties are summarized in Annex I. Further information can
be obtained from the JMPR evaluations (Annex III).
2.2. Physical and Chemical Properties
Dithiocarbamates with hydrophylic groups, such as OH- and
COOH, form water-soluble heavy metal complexes. However,
dithiocarbamate metal complexes used as fungicides are all
insoluble in water, though they are soluble in non-polar
solvents. Alkylene bisdithiocarbamates containing two donor
CS2- groups, which form polymeric chelates, are insoluble in
both water and non-polar solvents.
R1 R1 R1
\ \ /
N-C-S-Metal N-C-S-S-C-N
/ || / || || \
R2 S R2 S S R2
Dithiocarbamate Thiuram disulfide
S
||
CH2-NH-C-S R1 R1
| \ \ /
| Metal N-C-S-C-N
| / / || || \
CH2-NH-C-S R2 S S R2
||
S
EBDC Thiuram monosulfide
[-CH2-CH2NH-C-S-C-NH-]x
|| ||
S S
Polymer
Table 1. Relationship of chemical structure and pesticidal activity
of dithiocarbamates
--------------------------------------------------------------------
Pesticidal Chemical structure Common or other name
activity
--------------------------------------------------------------------
Herbicides S sulfallatea
||
dialkyl-N-C-S-alkyl
Fungicides and/ S ferbam, mancozeb,
or insecticides || maneb, metam-sodiumb,
>N-C-S-Metal metiram, nabam,
propineb, zineb,
ziram
--------------------------------------------------------------------
a Pre-emergence herbicide.
b Soil fungicide, nematocide, and herbicide.
Dithiocarbamates are unstable in acidic conditions and
readily convert to the amine and carbon disulfide (Ludwig &
Thorn, 1962; Thorn & Ludwig, 1962). The heavy metal salts of
ethylene bisdithiocarbamic acid, i.e., maneb and zineb, may
polymerize, the extent of polymerization depending on the method
of preparation.
ETU may be formed during the manufacture of dithiocarba-
mates. Bontoyan & Looker (1973) studied the initial ETU content
of various EBDC products and the amount found after storage.
Lyman & Lacoste (1974) found that the average ETU content of 76
lots of mancozeb manufactured at six different locations was
0.07%. No significant ETU build-up was observed during normal
spray tank residence times.
2.3. Analytical Methods
Residue analysis consists of sampling the environmental
material or matrix, extracting the pesticide residue, removing
interfering substances from the extract, and identifying and
quantifying the pesticide residue. The manner in which the
matrix material is sampled, stored, and handled can affect the
results: samples should be truly representative, and their
handling and storage must not further contaminate or degrade the
residue being measured.
The dithiocarbamates, thiuram disulfides included, are
conveniently determined on the basis of their decomposition by
mineral acids to the amine and carbon disulfide. The amount of
either of these hydrolysis products can be determined, the
carbon disulfide being commonly measured iodometrically or
colorimetrically. This decomposition method is adaptable to
micro-determinations for the assay of pesticide residues on
crops or to the macro-methods, which are used to determine
concentrations of ingredients in pesticide formulations (Clarke
et al., 1951).
A polarographic method has been used to estimate residues of
maneb and zineb (detection limit, 0.5 mg/kg product) and
ethylene bisthiuram monosulfide (detection limit, 0.02 mg/kg
product) (Engst & Schnaak, 1969a,b,c, 1970b).
A number of procedures for the quantification of dithio-
carbamates are based on high-pressure liquid chromatography.
The limits of detection in water solutions for zineb, ziram, and
thiram are 0.05, 0.01, and 0.01 mg/kg, respectively (IARC, 1976;
Gustafsson & Thompson, 1981; Kirkbright & Mullins, 1984; Tetsumi
et al., 1985).
For further details of analytical methods for individual
dithiocarbamates, see Conkin & Gleason (1964), Fishbein (1975),
Ashworth et al. (1980), and Worthing & Walker (1983).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
No data are available.
3.2. Man-Made Sources
The development of mono- and dithiocarbamate derivatives
with pesticidal properties occurred during and after World War
II. However, a few compounds were introduced earlier, including
ziram in 1930 and thiram in 1931.
Dithiocarbamates were developed as practical field
fungicides in the United Kingdom in about 1936. The compounds
were already being explored as fungicides and insecticides in
the USA, where the classic Tisdale and Williams patent was
issued in 1934. This covered the use of compounds of the
formula X(Y)NCS2Z (where X is hydrogen or alkyl, Y is hydrogen,
alkyl, or aryl, and Z is metallic in nature) and thiuram
sulfides as bactericides and fungicides (Thorn & Ludwig, 1962).
3.2.1. Production levels, processes, and uses
Dithiocarbamates have also been used to control various
dermatophytes (Kligman & Rosensweig, 1948). For example,
tetramethylthiuram disulfide, incorporated in various soaps and
lotions, has been used since 1942 for the treatment of scabies
and other parasitic diseases of the skin in veterinary and human
medicine (Schultheiss, 1957). Dithiocarbamates also have
considerable biocidal activity against a number of protozoa.
An interesting development was the discovery of disulfiram
as a treatment for chronic alcoholism (Hald & Jacobsen, 1948).
Other important applications of dithiocarbamates are in the
field of rubber chemistry as antioxidants and accelerators
(Thorn & Ludwig, 1962).
Annual production and use figures for a number of dithio-
carbamates in various parts of the world are given in IARC
(1976); consumption figures are listed in Table 2.
Table 2. Consumption of dithiocarbamate pesticides
(in 100 kg)a
-----------------------------------------------------------
Area Dithiocarbamates
1974-76 1981 1982 1983
-----------------------------------------------------------
Africa
Egypt 30
Zimbabwe 795
North/Central America
Canada 10 977
Mexico 4531 38 350 34 000 33 050
USA 60 000 50 000
South America
Argentina 4890 8370
Uruguay 1454 822 1114 1668
Asia
Brunei 3 2 2
Cyprus 701 2242 1538
India 16 193 14 650 17 130
Israel 4177 3110 3370 3580
Jordan 27 500 28 748
Korea Republic 5027 18 380 18 233
Kuwait 6
Oman 115 62 120
Pakistan 24 370 881
Turkey 5906 8901 9346
Europe
Austria 2751 2334 2322 2207
Czechoslovakia 6927 8678 6501
Denmark 2187 11 485 13 747
Finland 504
Greece 12 763
Hungary 37 347 29 476 31 932 43 415
Italy 145 697 121 808 97 238
Malta 350
Norway 438 383 372 285
Poland 4007 11 386 14 102 12 517
Portugal 8114 8358 7592
Sweden 3283 3800 4380
-----------------------------------------------------------
a From: FAO (1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Dithiocarbamates, like all pesticides, can reach the soil
through many routes, ranging from direct application to drift
from foliage treatment. Generally, these compounds are not
persistent and undergo different types of degradation.
4.1. Transport and Distribution Between Media
In alkaline medium, alkyl dithiocarbamates are stable, but
EBDCs are not. EBDCs are also unstable in the presence of
moisture and oxygen as well as in biological systems. By
splitting off carbon disulfide and hydrogen sulfide, as well as
by oxidative degradation, a great number of secondary products
are formed, amongst them, ETU (Aldridge & Magos, 1978).
The rates of alkyl dithiocarbamates decomposition depends on
pH (Turner & Corden, 1963) and the cation present. The rate of
decomposition, and the production of carbon disulfide is
decreased by cations in the following order: Na+ > Zn2+ > Fe3+ >
Cu2+.
The release of carbon disulfide from EBDCs is influenced by
the chemical nature of the hydrolysing medium. It is low in
acetic acid and nearly 100% in sulfuric acid (Aldridge & Magos,
1978); it also depends on the temperature (Clarke et al.,
1951).
Decomposition to hydrogen sulfide seems to depend on the
presence of an N-H group. Monoalkyldithiocarbamates, such as
EBDCs, are not stable in alkaline medium and, in acidic medium,
decompose either to carbon disulfide or hydrogen sulfide (Joris
et al., 1970). The rate of decomposition to carbon disulfide is
two orders of magnitude lower in monoalkyldithiocarbamates
compared with that in the corresponding dialkyldithiocarbamate
(Zuman & Zahradnik, 1957). In the case of metiram sodium at pH
9.5, methylisothiocyanate (MIT) and sulfur are formed, whereas
in acid solution, the compound is decomposed into carbon
disulfide, hydrogen sulfide, N,N'-dimethylthiuram disulfide,
methylamine, and MIT (Turner & Corden, 1963).
4.1.1. Water
EBDCs decompose rapidly in water, mancozeb having a half-
life of less than 1 day in sterile water (pH range, 5 - 9). The
nature and abundance of the degradation products are pH-
dependent, and include ETU and EU (Lyman & Lacoste, 1974, 1975).
Photolytic degradation is a major pathway for ETU in water
(Cruickshank & Jarrow, 1973; Ross & Crosby, 1973), and is
enhanced by the presence of photosensitizers such as chlorophyll
(Ross & Crosby, 1973).
The half-life of thiram in water was 46.7 days at pH 7 and
9.4 h in an acid medium (pH 3.5). About 5.2% of a sample of
thiram was still present in water of pH 7 after 200 days.
4.1.2. Soil
The mobility of EBDCs in soil varies considerably, depending
on water solubility and soil type. They are generally more
mobile in wet and in sandy soils than in dry soil or soil rich
in organic matter (peat or muck). Thin-layer chromatography
studies have shown that nabam is more mobile than maneb, which
in turn is more mobile than zineb, zineb being almost immobile
(Helling et al., 1974).
The leaching of radioactive 14C-mancozeb and its degradation
products was studied in five different soils, the organic
content of which ranged from 0.4% to 15%, while the pH ranged
from 4.7 to 7.4. An aqueous slurry of 14C-mancozeb (15.6 mg)
was mixed with a soil sample and applied to the top of a column
of soil. Water (2.5 cm) was added to the top of the column once
a week for 9 weeks. The water was collected and its
radioactivity measured and, after 9 weeks, the columns were cut
into 2.5 cm sections. The results showed that no radioactivity
leached through four of the five columns (only 2 - 5% of the
activity leached through the Cecil clay column; the reason for
this is not known). Losses of radioactivity by volatilization
or by metabolism to carbon dioxide were significant in all soils
(Lyman & Lacoste, 1974).
4.2. Biotransformation
4.2.1. Microbial degradation
Sterilized and unsterilized samples of sewage, fresh water,
sea-water, and agricultural soil were incubated with 50 or
100 mg thiram per litre or kg. Thiram disappeared from sewage
and fresh water within 12 days, and from soil after 40 days.
After 8 months, 20% of the thiram was still present in sea-
water. Disappearance was faster in unsterilized than in
sterilized soil, indicating that microorganisms seem to be
involved (Odeyemi, 1980).
The results of a study on one soil (Hagerstown silt loam)
used in the leaching study mentioned in section 4.1.2, showed
that mancozeb is readily degraded by soil microorganisms,
releasing ethylene C atoms as carbon dioxide. No carbon dioxide
was released from sterile soil, but mancozeb was rapidly
degraded to carbon dioxide in non-sterile soil. The half-life
in soil at a concentration of 20 mg mancozeb/kg was 50 days; at
10 mg/kg, the half-life was 90 days (Lyman & Lacoste, 1974).
4.2.2. Photodegradation
Ziram is stable to ultraviolet radiation (UVR). It is slowly
photo-hydrolysed in water and is stable in media containing
quantities of organic acids. When precipitated to the bottom of
bodies of water, it remains toxic for a month (IRPTC, 1982).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The only exposure of the general population to dithio-
carbamates and their breakdown products results from occasional
residues in the diet. However, dithiocarbamates degrade rapidly
after application to crops, the rate being influenced by oxygen,
humidity, temperature, organic sensitizers, and pH. A number of
degradation products have been identified, including ETU,
ethylene thiuram disulfide (ETD), and DIDTa.
5.1. Food
Studies in Canada and the USA have shown that, when
vegetables, such as spinach, carrots, and potatoes, are treated
with EBDCs after harvest, a significant percentage of the EBDCs
is converted to ETU during subsequent cooking (Blazquez, 1973;
Newsome & Laver, 1973; Watts et al., 1974) (ETU section 5.1).
The results of a study by Phillips et al. (1977) to examine
the effects of food processing on EBDC residues confirmed and
extended the results described above. Washing the raw
agricultural products prior to processing removed 33 - 87% of
the EBDC residues and the majority of the ETU residues. The
results for raw and processed commodities are summarized in
Table 3.
Human exposure to EBDCs was calculated for the population of
the USA on the basis of estimated consumption of dietary
residues of ETU in treated crops. Upper limit (worst case) and
lower limit (lowest case) estimates of exposure to ETU were
3.65 µg/kg and 0.24 µg/kg body weight per day, respectively
(US EPA, 1982b).
EBDC residues would be expected to be lower in root crops,
such as carrots and potatoes, as they are not systemic and tend
to remain on the external portions of the plant. However, in
leafy crops, such as spinach and lettuce, EBDC residues are
generally higher. Culling, such as discarding the discoloured
leaves of lettuce and the rinds of melons, could presumably
reduce the residue level. Washing reduced the majority of EBDC
residues by at least 50%.
------------------------------------------------------------------
a In many publications, it has been stated that ethylene-
thiuram monosulfide (ETM) was identified in metabolic
studies; however, it is now clear that this metabolite is
5, 6-dihydro-3 H-imidazo [2, 1-C]-1,2,4-dithiazole-3-thione
(DIDT) (Pluygers et al., 1971; Benson et al., 1972; Alvarez
et al., 1973).
Table 3. Summary of EBDC/ETU residues (mg/kg
product) before and after processing
-----------------------------------------------
Eastern USA Western USA
EBDC ETU EBDC ETU
-----------------------------------------------
Tomatoes
Unwashed 0.3 - 2.1 0.01
Washed 0.2 - 0.6 0.01
Canned - 0.03 0.5 0.11
Carrots
Unwashed 0.6 - 0.1 0.01
Washed 0.3 - 0.1 0.01
Diced 0.1 - 0.1 -
Frozen - - - -
Canned - 0.03 0.1 -
Spinach
Unwashed 2.4 - 61.9 0.34
Washed 1.5 - 9.7 0.02
Frozen 0.1 0.04 0.6 0.50
Canned - 0.18 0.1 0.71
-----------------------------------------------
Note: Mancozeb was applied at the rate of
0.7 ai/0.5 ha in all cases. Spray
schedules were as follows: spinach, 1
treatment with 10-day pre-harvest
interval; carrot, 6 treatments at 7- to
10-day intervals (7-day pre-harvest
interval); tomato (eastern), 4 treatments
at 7- to 10-day intervals (16-day pre-
harvest interval); tomato (western), 3
treatments at 7-day intervals (5-day pre-
harvest interval). From: IUPAC (1977).
5.2. Monitoring and Market Basket Studies
In a market-basket study, over 500 samples of 34 foods were
analysed, together with 26 samples of drinking-water. The water
samples and 338 food samples did not contain any residues.
Doubtful positive values at approximately the limit of detection
were found in 110 food samples, and 53 samples were positive.
Only 21 of all the samples contained ETU residues.
Tomato products (203 samples) were analysed in a separate
market-basket study, and 19% contained dithiocarbamates in the
range of 0.2 - 0.5 mg/kg product (Gowers & Gordon, 1980).
A more realistic review of the actual exposure of the
general population was obtained by a "table-top" study in which
100 whole meals (60 from homes and 40 from restaurants) were
analysed for dithiocarbamates and ETU. In the 87 meals analysed
for dithiocarbamates, 11 contained residues of apparent
dithiocarbamates averaging 0.3 mg/kg, or 0.04 mg/kg if averaged
over the 87 meals. In a second study of 100 meals, 4 meals
contained apparent dithiocarbamates in the range of 0.2 - 0.4
mg/kg or 0.02 mg/kg as an average of the 100 meals. From these
studies, an overall average would be 0.03 mg dithiocarbamates/kg
meal. ETU residues were not found in either study (Gowers &
Gordon, 1980).
6. KINETICS AND METABOLISM
Dithiocarbamates penetrate the organism mainly via the
respiratory tract (aerosol, dust), skin and mucous membranes
(occupational exposure), and the digestive tract.
6.1. Absorption, Distribution, and Excretion
Thirty minutes after intragastric administration of 500 mg
ziram/kg body weight to rats, the compound was detected in the
blood, the liver, and the kidneys, the highest concentration
being in the liver (26.2 mg/kg tissue). After 16 h, the
concentration of ziram in the blood and liver (about 5.5 mg/kg
tissue) decreased considerably, while the concentration in the
intestines and the kidneys increased (in the kidneys, to 3 mg/kg
tissue). At the end of the first day, the ziram concentration
in the intestines reached a maximum and then dropped abruptly,
57% of unchanged ziram being detected in the faeces; the
compound was also detected in the spleen and the adrenal glands.
Maximum concentrations in the organs (6.8 mg/kg and 2.4 mg/kg,
respectively) were attained the following day. Ziram was no
longer present in the adrenal glands after 3 days, and in the
spleen after 6 days. The circulation of ziram in the blood
continued for 2 days (Vekshtein & Khitsenko, 1971).
After the oral administration of a dose of 2 mg 35S-
ziram per animal to white rats (100 - 120 g), the brain and
thyroid contained high levels of radioactivity during the first
2 days. During the 12 h following administration, higher
amounts of ziram (or its metabolites) were found in the ovaries
than in the uterus or the placenta. Ziram passed the placental
barrier and accumulated in the organs and tissues of the fetus
(skin, liver, heart) at levels several times higher than those
in the placenta and the uterus wall. The level of radioactivity
in the fetal liver exceeded the maximum level in the liver of
mature animals; at 12 h, it was more than 5 times higher
(Chernov & Khistenko, 1973). Twenty-four hours after
administering 35S-ziram to female rats, Izmirova & Marinov
(1972) found radioactivity in the thyroid, blood, kidneys,
spleen, ovaries, and liver.
When 14C-labelled maneb was orally administered to rats at a
dose of 360 mg/kg body weight by stomach tube, approximately 55%
of the radioactivity was eliminated in the faeces and urine
within 3 days. Almost no unmetabolized maneb was found. The
amounts of radioactivity in organs after day 1 and day 5 were
1.2% and 0.18%, respectively. The highest levels after 1 day
were found in blood (0.23%), liver (0.78%), and kidneys (0.18%).
Less was found in the thyroid (0.07%) (Seidler et al., 1970).
Similar results were obtained with rats administered 35S-
ferbam or 14C-ferbam. Approximately 50% was absorbed from the
gastrointestinal tract in the first 24 h. Rats receiving 35S-
ferbam showed 18%, 23%, and 1% in expired air, urine, and bile,
respectively, whereas with 14C-ferbam, the figures were < 0.1%,
43%, and 1.4%, respectively. Other tissues contained only small
amounts of labelled material. In addition, 14C was excreted in
the milk of lactating rats (Hodgson et al., 1974).
Blackwell-Smith et al. (1953) found that approximately 70 -
75% of ingested zineb passed through the gastrointestinal tract
of rats and appeared in the faeces within 24 - 72 h.
Rats dosed via a stomach tube with 20 mg 14C-mancozeb per
day for 7 days (equivalent to approximately 100 mg/kg body
weight) were killed one day after the last dose and the
radioactivity in excreta and organs was measured. In the
faeces, urine, organs and tissues, and carcass, 71%, 16%, 0.31%,
and 0.96% of the total radioactivity was detected, respectively.
Specifically, the liver contained 0.19%, the kidneys, 0.076%,
the thyroid gland, 0.003%, and all other organs, less than
0.01%. Most of the labelled material in the faeces was
mancozeb, indicating that mancozeb was poorly absorbed from the
gastrointestinal tract (Lyman, 1971).
6.2. Metabolic Transformation
Dialkyldithiocarbamates, such as thiram and disulfiram, and
EBDCs, such as nabam, maneb, and zineb, are metabolized via
different mechanisms.
6.2.1. Mammals
In general, the metabolism of dialkyldithiocarbamates (e.g.,
disulfiram) in mammals (including man) is straightforward,
diethylthiocarbamic acid being formed as the principal metabo-
lite. This is found either as the free acid or as the S-glucu-
ronide conjugate (Fig. 1) (Kaslander, 1963; Strömme, 1965;
Dekhuyzen et al., 1971; Aldridge & Magos, 1978) in the urine,
faeces, or tissues of animals. Other metabolic products include
carbon disulfide (Prickett & Johnston, 1953), methyldiethyldi-
thiocarbamate (Gessner & Jakubowski, 1972), and sulfate
(Strömme, 1965; Strömme & Eldjarn, 1966), but free disulfiram
was not detected.
One of the most important enzymatic processes in the meta-
bolism of dialkyldithiocarbamates is glucuronidation, which
takes place in the liver (Strömme, 1965). Glucuronic acid
conjugation might be overloaded after the administration of
diethyldithiocarbamate but not after the administration of
disulfiram, which is taken up by the liver at a much slower
rate. Methylation of diethyldithiocarbamates by S-adenosyl
methionine transmethylase in the kidneys and liver can occur
subsequently and leads to sulfate excretion (Gessner &
Jakubowski, 1972). In the case of 35S-disulfiram, more than 50%
of the 35S was recovered as sulfate in the urine, partly in the
free form and partly esterified (Eldjarn, 1950; Strömme, 1965).
A different enzymatic process is involved in the desulfuration
of the carbon disulfide formed from dithiocarbamates. After
administration of 14C-carbon disulfide, some label was exhaled
as 14C-carbon dioxide. This break-up of the carbon disulfide
molecule is catalysed by microsomal mixed-function oxidase (De
Matteis & Seawright, 1973; Dalvi et al., 1974).
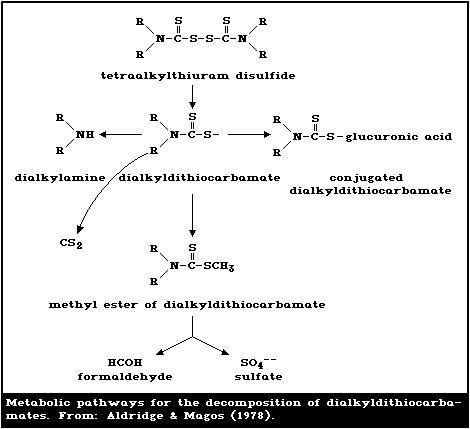 Thiram and the dimethylamine salt of dimethyldithiocarbamic
acid were the major metabolites in the urine, whereas carbon
disulfide and dimethylamine were detected in the expired air.
The body tissues contained tetramethylthiourea, the methylamine
salt of dimethyldithiocarbamic acid, carbon disulfide, and
methylamine. Overall, the results indicate that, in the rat,
ferbam and ziram are transformed into dimethyldithiocarbamic
acid, which is subsequently coupled to give thiram, or is broken
down to carbon disulfide and dimethylamine (Vekshtein &
Khitsenko, 1971; Hodgson et al., 1974).
The in vivo metabolic decomposition of EBDCs is complex and
results in the formation of carbon disulfide, hydrogen sulfide,
EDA, ethylene bisthiuram disulfide, DIDT, ethylene
diisothiocyanate (EDI) (unstable), ETU, EU, and 2-imidazoline
(Seidler et al., 1970; Lyman, 1971) (Fig. 2). The decomposition
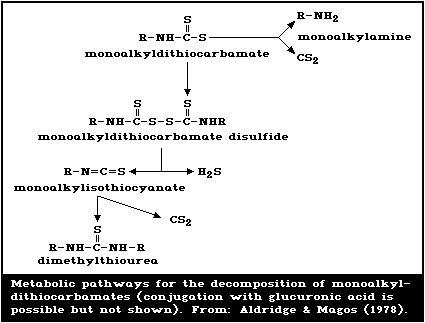
of monoalkyldithiocarbamates is detailed in Fig. 3.
When 14C-maneb was given to rats in a single oral dose of
390 mg/kg body weight, only 55% of the 14C was recovered in the
excreta. It was therefore suggested that a large part of the
dose might have been metabolized to carbon disulfide and 14C-
EDA, followed by oxidation of the latter to carbon disulfide.
The concentration of the radioactivity was highest after 24 h,
and EDA and ETU were identified in the excreta (Seidler et al.,
1970). ETU and DIDT were the major metabolites found in the
urine of rats treated with zineb, and carbon disulfide was
detected in the expired air.
6.3. Metabolism in Plants
ETU is one of several metabolites found when EBDCs are
applied to plants. In plants, nabam, maneb, and zineb are
transformed to ETU, DIDT, EU, 2-imidazoline, a diisothiocyanate
(EDI), and other metabolites (Fig. 2).
Nash & Beall (1980) have studied the fate of maneb and zineb
in microagroecosystem chambers (enclosed glass chambers), under
the following conditions: pH, 6.7; organic matter content, 5.2%;
soil type, Galestown sandy loam; soil water content, 15.6%. The
fungicides were applied twice to tomato plants at 2 kg/ha, and
the residual fungicides (measured as EDA and ETU) were monitored
on the fruit, leaves, and in the soil, water, and air for 100
days after treatment. ETU was detected at < 20 µg/kg on whole
fruit after 3 days, but had completely disappeared after 3
weeks. Maneb and zineb were present on whole fruit at < 1 mg/kg
and were still present in measurable amounts (as EDA) after 10
weeks. Both had half-concentration times (C“) of 14 days on
leaves. Half-concentration times for ETU, maneb, and zineb in
soil were < 3, 36, and 23 days, respectively, and that for ETU
in air was 9 days.
Thiram and the dimethylamine salt of dimethyldithiocarbamic
acid were the major metabolites in the urine, whereas carbon
disulfide and dimethylamine were detected in the expired air.
The body tissues contained tetramethylthiourea, the methylamine
salt of dimethyldithiocarbamic acid, carbon disulfide, and
methylamine. Overall, the results indicate that, in the rat,
ferbam and ziram are transformed into dimethyldithiocarbamic
acid, which is subsequently coupled to give thiram, or is broken
down to carbon disulfide and dimethylamine (Vekshtein &
Khitsenko, 1971; Hodgson et al., 1974).
The in vivo metabolic decomposition of EBDCs is complex and
results in the formation of carbon disulfide, hydrogen sulfide,
EDA, ethylene bisthiuram disulfide, DIDT, ethylene
diisothiocyanate (EDI) (unstable), ETU, EU, and 2-imidazoline
(Seidler et al., 1970; Lyman, 1971) (Fig. 2). The decomposition
of monoalkyldithiocarbamates is detailed in Fig. 3.
When 14C-maneb was given to rats in a single oral dose of
390 mg/kg body weight, only 55% of the 14C was recovered in the
excreta. It was therefore suggested that a large part of the
dose might have been metabolized to carbon disulfide and 14C-
EDA, followed by oxidation of the latter to carbon disulfide.
The concentration of the radioactivity was highest after 24 h,
and EDA and ETU were identified in the excreta (Seidler et al.,
1970). ETU and DIDT were the major metabolites found in the
urine of rats treated with zineb, and carbon disulfide was
detected in the expired air.
6.3. Metabolism in Plants
ETU is one of several metabolites found when EBDCs are
applied to plants. In plants, nabam, maneb, and zineb are
transformed to ETU, DIDT, EU, 2-imidazoline, a diisothiocyanate
(EDI), and other metabolites (Fig. 2).
Nash & Beall (1980) have studied the fate of maneb and zineb
in microagroecosystem chambers (enclosed glass chambers), under
the following conditions: pH, 6.7; organic matter content, 5.2%;
soil type, Galestown sandy loam; soil water content, 15.6%. The
fungicides were applied twice to tomato plants at 2 kg/ha, and
the residual fungicides (measured as EDA and ETU) were monitored
on the fruit, leaves, and in the soil, water, and air for 100
days after treatment. ETU was detected at < 20 µg/kg on whole
fruit after 3 days, but had completely disappeared after 3
weeks. Maneb and zineb were present on whole fruit at < 1 mg/kg
and were still present in measurable amounts (as EDA) after 10
weeks. Both had half-concentration times (C“) of 14 days on
leaves. Half-concentration times for ETU, maneb, and zineb in
soil were < 3, 36, and 23 days, respectively, and that for ETU
in air was 9 days.
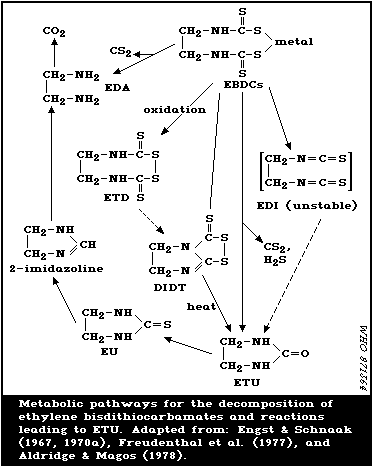 Note to Figure 2: Ion mechanisms leading to ETU formation are
not completely understood; however, a number of hypotheses have
been advanced. According to Marshall (1977), intermediary
products of the thermal bisdithiocarbamate degradation to ETU are
beta-amino ethylene dithiocarbamate and DIDT, but not ethylene
diisothiocyanate (EDI). EDI was, however, postulated and detected
several times as a secondary reaction product of the ethylene
bisdithiocarbamate degradation at normal temperatures (Engst &
Schnaak, 1970a). ----> indicates a postulated conversion.
Note to Figure 2: Ion mechanisms leading to ETU formation are
not completely understood; however, a number of hypotheses have
been advanced. According to Marshall (1977), intermediary
products of the thermal bisdithiocarbamate degradation to ETU are
beta-amino ethylene dithiocarbamate and DIDT, but not ethylene
diisothiocyanate (EDI). EDI was, however, postulated and detected
several times as a secondary reaction product of the ethylene
bisdithiocarbamate degradation at normal temperatures (Engst &
Schnaak, 1970a). ----> indicates a postulated conversion.
 Besides ETU, other degradation products of EBDCs include ETD
and DIDT. The main volatile components of zineb's decomposition
are carbon disulfide, carbonyl sulfide, and EDA. Almost all
zinc is converted into zinc sulfide and zinc oxide (Melnikov &
Trunov, 1966).
6.4. Decomposition in Water and Soil
Thiram and dimethyldithiocarbamic acid give rise in soil to
methyl isothiocyanate and sulfur, and, under acidic conditions,
to carbon disulfide, hydrogen sulfide, methylamine, methyl-
isocyanate, and the bisdisulfide of methyldithiocarbamic acid.
Two of the products, carbon disulfide and dimethylamine,
evaporated from the soil (Raghu et al., 1975). Dimethyldithio-
carbamic acid also binds with heavy metals in soil to form
complexes.
The various metal derivatives of ethylene bisdithiocarbamic
acid appear to be converted in the soil to DIDT, ETU, carbon
disulfide, hydrogen sulfide, and carbonyl sulfide (Moje et al.,
1964; Kaars Sijpesteijn & Vonk, 1970). The conversion by soil
bacteria and fungi of DIDT into ETU has been demonstrated (Vonk
& Kaars Sijpesteijn, 1976). Even though ETU is slowly converted
into EU in soil, pure cultures of soil bacteria and fungi were
unable to effect this transformation (Kaars Sijpesteijn & Vonk,
1970).
6.5. Metabolism in Microorganisms
Microorganisms readily form ETU from DIDT, a spontaneous
decomposition product of EBDCs. This conversion also takes
place after addition of reducing compounds such as cysteine,
glutathione, or ascorbic acid. It consists of the reduction of
the S-S bond of DIDT, with the subsequent release of carbon
disulfide to form ETU. It was shown by Vonk & Kaars Sijpesteijn
(1976) that DIDT was reduced by NADH in the presence of enzyme
extracts from Pseudomonas fluorescens, Escherichia coli,
Saccharomyces cerevisiae, or Aspergillus niger.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
There is some evidence that dithiocarbamates, at concentra-
tions 10 times that of normal field application, may reduce
microbial biomass and increase the bacterial:fungal ratio.
7.2. Aquatic Organisms
7.2.1. Acute toxicity
Toxicity studies using dithiocarbamates are hindered by the
fact that they are chemically and biologically degradable and
may also be contaminated with degradation products. Their
stability in water depends on the pH and on the presence of
metal ions with which they form complexes. The soluble dithio-
carbamates dissociate in water, whereas the polymers are only
slightly soluble in water. As the breakdown products will also
influence toxicity, toxicity testing of dithiocarbamates is
complex.
According to US EPA (1977, 1982a), the use of pesticide
products containing maneb against cranberry fruit rot at
application rates of up to 6.7 kg ai/ha would result in a
concentration of 4.4 mg/litre in a 15-cm layer of water. McCann
& Pitcher (1973) reported a 96-h LC50 of 1 mg/litre for
bluegills, while Worthing & Walker (1983) reported a 48-h LC50
for carp of 1.8 mg/litre. Zineb used against cranberry fruit
rot at an application rate of up to 5.4 kg ai/ha could result in
a concentration of 3.52 mg/litre in a 15-cm layer of water. A
26-h LC50 of 0.2 mg/litre has been reported for Daphnia magna.
Van Leeuwen (1986) carried out an extensive study with 18
dithiocarbamates and three metabolites of these compounds,
including ETU, in fish (Poecilia reticulata), crustacea (Daphnia
magna), algae ( Chlorella pyrenoidosa, Phytobacterium
phosphoreum), and two nitrifying bacteria (Nitrosomonas and
Nitrobacter). The results are summarized in Tables 4 and 5.
Worthing & Walker (1983) gave the following LC50 values:
propineb: rainbow trout, 1.9 mg/litre; golden orfe,
133 mg/litre; thiram: carp, 4 mg/litre; rainbow trout,
0.13 mg/litre; bluegill, 0.23 mg/litre; metiram: harlequin fish,
17 mg/litre.
The susceptibility to maneb of the early life stages of
rainbow trout has been studied using fertilized eggs (before and
after water hardening), early eye point eggs, late eye point
eggs, sac fry, and early fry. The sac fry and early fry stages
appeared to be the most sensitive. The 96-h LC50s for the
different stages were: for 0-h egg, 6 mg/litre; for 24-h egg,
5.6 mg/litre; for early eyed egg (14 days), 1.8 mg/litre; for
late eyed egg (28 days), 1.3 mg/litre; for sac fry (42 days),
0.32 mg/litre; and, for early fry (77 days), 0.34 mg/litre (Van
Leeuwen, 1986).
Table 4. Acute toxicity of dithiocarbamates and breakdown
products for fisha
-------------------------------------------------------------
Organism Compound 96-h LC50
(95% confidence
limit) (mg/litre)
-------------------------------------------------------------
Poecilia reticulatab nabam 5.8 (4 - 8.5)
maneb 3.7 (3.2 - 5.6)
zineb 7.2 (5 - 10.3)
mancozeb 2.6 (2.1 - 3.3)
metiram 6.4 (4 - 10.4)
Na-DMDC 2.6 (2.1 - 3.2)
ziram 0.75 (0.56 - 1)
ferbam 0.09 (0.06 - 0.18)
thiram 0.27 (0.22 - 0.33)
Na-DEDC 6.9 (5.5 - 8.5)
Zn-DEDC 0.49 (0.40 - 0.61)
disulfiram 0.32 (0.24 - 0.43)
ETU 7500 (5600 - 10 000)
EU 13 000 (10 000 - 18 000)
Rainbow troutc thiram 0.26 (0.24 - 0.32)
(Salmo gairdneri)
-------------------------------------------------------------
a From: Van Leeuwen (1986).
b Studies according to OECD guidelines 203.
c 24-h LC50; water temperature 15 ± 1 °C;
weight of fish, 34 ± 4.7 g.
Table 5. Short-term toxicity studies with dithiocarbamates and
breakdown productsa
--------------------------------------------------------------------
Compound Daphnia Chlorella Photobacterium Nitrosomonas
magna pyrenoidosa phosphoreum Nitrobacter
48-h LC50 96-h EC50 15-min EC50 3-h MIC
(mg/litre) (mg/litre) (mg/litre) (mg/litre)
--------------------------------------------------------------------
Nabam 0.44 2.4 102 32
Maneb 1 3.2 1.2 56
Zineb 0.97 1.8 6.2 18
Mancozeb 1.3 1.1 0.08 32
Metiram 2.2 1.8 0.37 32
Na-DMDC 0.67 0.8 0.51 26
Ziram 0.14 1.2 0.15 100
Ferbam 0.09 2.4 0.20 10
Thiram 0.21 1 0.10 18
Na-DECD 0.91 1.4 1.22 43
Zn-DEDC 0.24 1.1 1.70 > 320
Disulfiram 0.12 1.8 1.21 > 320
ETU 26.4 6600 2100 1
EU 5600 16 000 3300 1000
--------------------------------------------------------------------
a From: Van Leeuwen (1986).
7.2.2. Short- and long-term toxicity and reproduction studies
7.2.2.1. Fish
In sublethal toxicity studies carried out on Salmo
gairdneri, groups of 10 fish were exposed to thiram
(0.18 mg/litre) for 24 h. Blood parameters (decreased haemo-
globin and leukopenia, decreased glucose levels, and increased
glucose-6-phosphate dehydrogenase activity) and liver parameters
(increased lipid content, increased lactate dehydrogenase) were
changed, and it was concluded by the author that thiram is a
cytotoxic chemical (Van Leeuwen, 1986). In a further study, the
60-day toxicity for early life stages was tested on S. gairdneri
using a number of dialkyldithiocarbamates, EBDCs, and break-
down products. The LC50s ranged from approximately 1 to
9 µg/litre for dialkyldithiocarbamates and 211 - 2100 µg/litre
for EBDCs, but ETU and EU were not toxic, even at levels
exceeding 1000 mg/litre.
Embryotoxic and teratogenic effects were also observed for
all the compounds studied, and there was an overlap between the
responses for skeletal malformations and lethality over a wide
concentration range. The teratogenic effects in rainbow trout
proved to be in agreement with those observed in mammals.
Exposure of rainbow trout during embryo-larval development
revealed that malformations induced by dithiocarbamates were
almost exclusively confined to the notochord, which increased
considerably in both length and diameter. As a result, the
notochord became twisted and distorted. Ectopic osteogenesis
was observed in almost every affected notochord. Other effects,
such as the disruption of the integrity of myomeres and organ
dislocations, were closely related to the notochordal ano-
malies. Also, compression and fusion of vertebrae and "waviness"
of various skeletal elements were found. Concentration-related
changes in the liver were observed in short-term exposure of
juvenile rainbow trout, while at high levels proliferation of
bile duct epithelial cells and necrosis of hepatocytes were
seen. Ziram and thiram induced brain haemorrhages as well as
intraspinal extravasates of blood cells (Van Leeuwen, 1986).
7.2.2.2. Invertebrates
Short-term toxicity studies using the compounds listed in
Table 5 were carried out to investigate the effects of prolonged
exposure (21-day) on survival, fecundity, and growth of Daphnia
magna. Growth and reproduction were not specifically inhibited,
since effects on these characteristics were generally detected
at levels comparable with the 21-day LC50s. For dialkyldithio-
carbamates, the 21-day LC50s ranged from approximately 10 to
30 µg/litre, for EBDCs, from 80 to 110 µg/litre, and, for ETU
and EU, the levels were 18 and 3200 mg/litre, respectively (Van
Leeuwen, 1986).
7.2.3. Bioaccumulation
Van Leeuwen (1986) determined the log n-octanol/water par-
tition coefficient for a few dithiocarbamates and breakdown
products. The results are summarized in Table 6. In general,
the higher the log n-octanol/water partition coefficient, the
greater the tendency to bioaccumulate.
Table 6. log n-octanol/water partition
coefficients for some dithiocarbamates
------------------------------------------
Compound log n- octanol/water
partition coefficient
------------------------------------------
Disulfiram 4
Thiram 1.82
ETU 0.67
EU 0.96
------------------------------------------
In short-term studies on rainbow trout of uptake,
distribution, and retention of 14C-labelled zineb and ziram,
both compounds were found to be rapidly distributed throughout
the tissues. Whole-body accumulation was low, with bioconcen-
tration factors of less than 100. Relatively high radioactivity
levels were found in the liver, gall bladder, and intestinal
contents, suggesting the prominent role of hepatic biotransform-
ation and biliary excretion. With ziram, the eyes and skin also
appeared to be distribution sites. Whole-body elimination was
rapid, with about 75% of radioactivity being eliminated within
the first 4 days. With ziram, 45% of the initial total 14C
content in the body was still present at the end of the 16-day
depuration period. Differences in the extent of elimination
were most noteworthy for the eyes, skin, and kidneys. Whole-
body autoradiography showed the radioactivity in the digestive
tract, liver, bile, gills, thyroid follicles, melanophores of
the skin, choroid epithelium complex of the eyes, and in other
melanin-containing tissues, such as the kidneys (Van Leeuwen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
In general, the toxicity of dithiocarbamates for mammals is
relatively low. Some commonly used dithiocarbamates included in
the WHO recommended classification of pesticides by hazard (WHO,
1986a), which is based primarily on the acute oral and dermal
toxicity of the technical material for the rat, are given in
Annex III.
Acute oral and dermal toxicity data for a number of animal
species of various dithiocarbamates are given in Table 7. From
this Table, it is clear that nabam and metam-sodium are the most
toxic dithiocarbamates, other compounds having only low
toxicity. As for many compounds, the toxicity is often
influenced by the method of application, e.g., solvents used,
age and sex of animal, type of diet, etc. Thus, in rats fed an
isocaloric diet containing 3.5% protein, the LD50 of nabam was
210 mg/kg body weight, compared with 565 mg/kg in rats fed the
same diet plus 26% protein (Periquet & Derache, 1976).
Ivanova-Chemishanska (1969) found that rats treated with
zineb, maneb, or mancozeb showed dose-dependent signs of
depression, adynamia, decreased tonus, disturbances in
coordination, paresis, and paralysis of extremities combined
with general weakness, lack of appetite, and prostration.
Yin-Tak Woo (1983) has reviewed the structure-activity
relationships of different types of dithiocarbamates.
8.2. Short- and Long-Term Exposures
8.2.1. Oral exposure
8.2.1.1. Rat
In 1-month feeding tests, no growth retardation was noted in
rats fed diets containing 100 mg ferbam/kg diet, but decreased
growth occurred with 500 mg/kg diet and increased mortality with
5000 mg/kg diet (Hodge et al., 1952).
Groups of 40 weanling rats (20 females and 20 males) were
given diets containing 500, 1000, 2500, 5000, or 10 000 mg
zineb/kg diet for up to 30 days. Thyroid enlargement was seen
at all dose levels, but unequivocal histopathological changes
were observed only at 10 000 mg/kg diet (Blackwell-Smith et al.,
1953; Kampmeier & Haag, 1954).
Ferbam administered daily at 23, 66, or 109 mg/kg body
weight to male rats for 13 weeks caused death and weight loss at
the highest dose, but did not have any effect on reproduction.
Daily feeding (equivalent to 15 or 51 mg/kg body weight) to
females for 2 weeks caused severe weight loss at the highest
dose level (Minor et al., 1974).
In a 2-year feeding study, 25, 250, and 2500 mg ferbam or
ziram/kg diet shortened the life span of rats and caused growth
depression and neurological lesions (manifested at the highest
dose level by the crossing of hind legs when animals were lifted
by their tail) (Hodge et al., 1956).
Table 7. Acute toxicity (LD50) of dithiocarbamates for experimental
animals
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Ferbam mouse 1000 FAO/WHO (1965b)
rat > 4000 Hodge et al.
(1956)
guinea-pig 450 - 2000
rabbit 2000 - 3000
Metham-sodium mouse 285 Worthing & Walker
(vapam) (1983)
rat 1700 - 1800 Worthing & Walker
(1983)
rabbit 1300 Worthing & Walker
(1983)
Ziram rat 1400 Hodge et al.
(1952)
guinea-pig 100 - 150 Hodge et al.
(1952)
rabbit 100 - 1020 Hodge et al.
(1952)
Thiram mouse 1500 - 2000 Worthing & Walker
(1983)
rat 865 - 1300 Van Esch (1956)
rat 780 - 865 Worthing & Walker
(1983)
rat > 2000 Ben Dyke et al.
(1970)
rabbit 210 Lehman (1951)
cat 230
--------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Disulfiram rat > 4000 Van Esch (1956)
Zineb rat > 5200 Blackwell-Smith
(1953)
rat 9000 Ivanova-Chemi-
shanska (1969a)
Maneb mouse 4100 Engst et al.
(1971)
rat 4500 Engst et al.
(1971)
rat (male) 6750 Worthing & Walker
(1983)
Nabam rat 395 Blackwell-Smith et
al. (1953)
Mancozeb rat (female) 12 800 Ivanova-Chemi-
shanska (1969b)
rat (male) 14 000 Ivanova-Chemi-
shanska (1969)
rat > 8000 Worthing & Walker
(1983)
Propineb rat 8500 > 1000 Worthing & Walker
(1983)
rabbit 2500
cat 2500
hen 2500
Metiram mouse 5400 Worthing & Walker
(1983)
rat > 10 000
guinea-pig 2400 - 4800
(female)
--------------------------------------------------------------------------
Groups of 24 rats (12 females and 12 males) were given a
diet containing 48 mg thiram/kg diet for 2 years (a 3-generation
study). No effects on growth, reproduction, blood parameters,
or mortality rate were found, neither were there gross or
histological changes (Van Esch, 1956). In a further study, 12
female and 12 male rats given 200 mg/kg diet for 8 months did
not show any appreciable changes in growth or mortality rate,
and a dose of 300 mg/kg diet for 65 weeks did not give rise to
specific evidence of poisoning (Tollenaar, 1956). Groups of 24
rats (12 females and 12 males) fed diets containing 300, 1000,
or 2500 mg thiram/kg diet for 65 weeks showed weakness, ataxia,
various degrees of paralysis, and histological changes
(calcification in the brain stem and cerebellum and dystrophic
changes in the leg muscles). At 2500 mg/kg diet, there was an
increased mortality rate (Fitzhugh et al., 1952). Groups of 20
young rats administered diets containing 100, 300, or 500 mg
thiram/kg diet for 2 years all showed a small reduction in the
growth rate. At concentrations of 300 and 500 mg/kg diet, an
increased mortality rate was seen, while at 500 mg/kg diet,
convulsions, thyroid hyperplasia, and calcification in the
cerebellum, hypothalamus, and medulla oblongata were observed
(Griepentrog, 1962; IARC, 1976).
Groups of 25 male and 25 female rats were fed diets
containing 25, 250, 1250, or 2500 mg maneb/kg diet for 2 years.
At 1250 mg/kg diet, there was some depression, impaired food
consumption, and increased mortality rate. At the end of 2
years, the animals receiving 1250 mg/kg diet had an increased
liver/body weight ratio, and those receiving 2500 mg/kg diet
also showed thyroid hyperplasia and nodular goitre (Worthing &
Walker, 1983).
Groups of 10 young male and 10 young female rats were fed
diets containing 500, 1000, 2500, 5000, or 10 000 mg zineb/kg
diet for 2 years. At the two highest dose levels, there was an
apparent increase in the mortality rate among the female rats
and, at 10 000 mg/kg diet, there was a tendency towards
diminished growth in both sexes. The results of haematological
studies were normal, but a goitrogenic effect was seen at all
dose levels. Kidney damage was seen in 6 animals at the
10 000 mg/kg dose level and in one animal in each of the groups
receiving 1000, 2500, or 5000 mg/kg diet, but not at all at
500 mg/kg diet. The tumour incidence was not significantly
greater among any of the treated animals than it was in the
controls (Blackwell-Smith et al., 1953; Kampmeier & Haag,
1954).
Weanling rats in groups of 25 males and 25 females were fed
diets containing 25, 250, or 2500 mg ziram/kg diet for 2 years.
The growth rate and life span were normal in all groups, but
neurological changes were observed in the animals receiving
2500 mg/kg diet, though no cystic lesions were discovered in the
levels. In some of the male animals, the testes were atrophied,
and there was a slight indication of thyroid hyperplasia,
notably in the 2500 mg/kg diet group. However, there was no
increase in tumour incidence in the treated animals (Hodge et
al., 1956). A comparable study with the same dose levels was
carried out with ferbam, and again no increase in tumour
incidence was found (IARC, 1976).
8.2.1.2. Dog
A dog given ferbam and ziram together for one month, each at
a dose of 5 mg/kg body weight per day, remained healthy except
for slight anaemia. The same result was observed when ferbam
was given alone for one month at a dose of 25 mg/kg body weight
per day, or for one week at 50 mg/kg body weight per day.
Raising the dose to 100 mg/kg body weight per day, however,
immediately provoked severe vomiting and malaise (Hodge et al.,
1952). Pairs of adult dogs were given daily doses of 0.5, 5, or
25 mg ferbam/kg body weight for one year. Convulsions occurred
at the highest dose level, but urine analysis, blood parameters,
organ weights, and tissue histology (including that of the
thyroid gland) were normal (Hodge et al., 1956).
When pairs of dogs were fed maneb orally at the rate of 2,
20, 75, or 200 mg/kg body weight per day for one year, toxic
effects were observed at the two highest dose levels, but not at
20 mg/kg body weight (Worthing & Walker, 1983).
Three groups of three dogs each were fed diets containing
20, 2000, or 10 000 mg zineb/kg diet for one year. All the
animals survived, and no persistent changes in growth rate were
seen in any of the groups. There were no histopathological
changes in the tissues, except in the thyroid gland, and
haematological findings were normal. At 10 000 mg/kg diet,
thyroid hyperplasia was noted (Blackwell-Smith et al., 1953;
Kampmeier & Haag, 1954).
8.2.1.3. Bird
Sodium diethyldithiocarbamate (NDDC), the dimethyl compound
(NDMC), and ferbam, ziram, and thiram were given orally to young
and adult domestic fowl (Thorber's gog cockerels) at 330, 210,
205, 56, and 178 mg/kg body weight, respectively, and the birds
were killed after 6, 12, 18, or 20 weeks. All of the compounds
had an adverse effect on body weight gain, retarded testicular
development, and produced degeneration in the seminiferous epi-
thelium of mature birds. Nerve fibre degeneration was produced
in the medulla and spinal cord of chicks by NDDC and in those of
cocks by NDMC. Chicks exposed to thiram became lame and
exhibited swollen epiphyses of the long bones due to endo-
chondrial ossification giving rise to a thickened cartilaginous
epiphyseal plate (Rasul & Howell, 1974).
8.2.2. Inhalation exposure
8.2.2.1. Rat
Studies concerning toxicity following inhalation exposure
are scarce.
Ivanova-Chemishanska et al. (1972) studied the inhalation
toxicity in rats with zineb (70% purity), maneb (80% purity),
and mancozeb (80% purity), applied 6 days per week over a period
of 4“ months, at concentrations of 2, 10, 50, 100, or 135 mg/m3.
The pesticides were given in the form of dispersed aerosols,
with 95% of the dust particles ranging from 1 to 5 µm in size,
and the remainder from 5 to 10 µm. Local irritation of the
mucosa of the upper respiratory tract was noted and
concentration-related non-specific changes in the liver and
kidneys were evident. However, only slight changes were found
at a concentration of 2 mg/m3.
Davydova (1973) studied the influence of inhaled thiram on
the estrous cycle and genital function of rats. Groups of rats
were exposed to 0, 0.45, or 3.8 mg/m3 thiram for 6 h/day, 5
days/week, over a period of 4“ months. An extension of the
estrous cycle was seen at the highest dose level, and genital
function was disturbed, as shown by a reduction in the capacity
to conceive, a reduction in fertility, and of fetal weight
gain.
8.3. Skin and Eye Irritation; Sensitization
Nabam (19% solution) and zineb (65% wettable powder) were
each applied to the right eye of 10 rabbits, the left eye being
used as a control. Nabam did not produce signs of irritation,
while zineb produced mild irritation (erythema), which subsided
within 6 - 8 h. No oedema was seen. The mild irritation may
have been caused by the non-specific foreign body reaction to
the dry, insoluble powder. When this procedure was repeated
with both compounds diluted and suspended for agricultural use
(for nabam, 0.5% of the commercial 19% solution plus zinc
sulfate, 0.125% in water; for zineb, a 0.188% suspension of the
commercial 65% wettable powder in water), no irritation was seen
(Blackwell-Smith et al., 1953).
In studies performed on guinea-pigs, intracutaneous
injections, 10 times daily, followed by an epicutaneous
challenge test, provided evidence of the marked sensitizing and
cross-sensitizing properties of thiram and metiram (Griepentrog,
1960).
It has been reported that a number of dithiocarbamates
(mancozeb, metham-sodium, metiram, zineb, ziram, and thiram)
cause skin and/or eye irritation (Worthing & Walker, 1983).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
8.4.1. Reproduction
8.4.1.1. Rat
Groups of rats (16 male and 16 female Charles River-CD rats
per group) were fed maneb for 3 months at levels of 0, 125, or
250 mg/kg diet, and were mated in a standard 3-generation, 2-
litters-per-generation reproduction study. Groups of males and
females from the F1b and F2b litters were fed maneb for 3 months
after weaning and mated to become parents of the succeeding
generation. The major reproduction indices were unaffected by
maneb at dietary levels up to and including 250 mg/kg diet.
There was no histological evidence of congenital anomalies in a
variety of tissues and organs of the male and female rats of the
F3b litter subjected to histopathological examination (Sherman &
Zapp, 1966).
Maneb, zineb, and mancozeb exert dose-dependent damaging
effects on the gonads of rats of both sexes. The dose levels
were 96 - 960 mg zineb/kg body weight, 140 - 1400 mg mancozeb/kg
body weight, and 14 - 700 mg maneb/kg body weight, given twice a
week for 4.5 months. Both reproductive and endocrine structures
were affected at all dose levels, leading to decreased fertility
(Ivanova-Chemishanska et al., 1973, 1975a). In a 4-month
inhalation study on rats using maneb at 4.7 mg/m3, no effect on
sperm mobility was detected (Matokhnyuk, 1971).
Ivanova-Chemishanska & Antov (1980) studied the effects of
EndodanR (50% ethylenethiuram monosulfide) on the gonads and
reproduction in rats during long-term daily oral doses of 3.8 or
38 mg/kg body weight. The parental generation (F0) and 3
consecutive generations (F1 - F3) were examined. In F0, a
decrease in succinic dehydrogenase and ATPase activities in
testes homogenates was found, as well as an increase in glucose-
6-phosphate dehydrogenase (G6PDH) activity compared with control
levels. Changes in the liver and brain enzyme systems were also
noted.
The same results were obtained with zineb (78% purity). A
rapid loss of mobility and changed resistance (to osmotic and
acidic effects) of spermatozoa were found. A decreased index of
fertility was also found for both sexes in the F0 generation.
Decreased index of fertility and enzymatic changes in organ
homogenates were detectable in the F1 - F3 generations (Ivanova-
Chemishanska et al., 1973).
In extracts of testes of white rats, exposed by inhalation
to zineb and maneb at a concentration of 100 mg/m3 for 4 months,
Izmirova et al. (1969) found an increase in lactate dehydro-
genase (LDH), LDH2, and LDH4. Bogartykh et al. (1979) did not
find any changes in LDH or G6PDH activities in testes homo-
genates of Wistar rats orally treated with zineb (2.5 mg/kg body
weight) for 3 months.
Thiram at doses of 225, 300, 450, 600, 900, or 1200 mg/kg
diet given to male Wistar rats for 29 days produced changes in
many of the parameters studied. A significant effect on testes
and seminal vesicle weight was found at 450 mg/kg diet, and a
decrease in body weight was found at 300 mg/kg. The most
sensitive parameters studied were found to be the weights of the
epididymal and perirenal fat pads, which were decreased by
thiuram doses in the range 130 - 184 mg/kg diet. The no-effect
level, calculated using an extrapolation model, did not differ
significantly from the earlier reported value of 48 mg/kg diet
(Lowy et al., 1979, 1980).
Ferbam was fed to groups of 20 Charles River-CD male rats at
concentrations of 0, 500, 1200, or 2500 mg/kg diet for 13 weeks
before mating with untreated females. Six of the rats fed the
highest dose level died. The indices of fertility, gestation,
viability, and lactation for the females mated with treated
males were normal (Short et al., 1976).
When thiram was incorporated into the diet at concentrations
of 0, 500, 1000, and 2500 mg/kg diet and fed to male weanling
Charles River rats for 13 weeks prior to mating, food intake and
growth was mainly decreased at the two highest dose levels.
Loss of hair and rough coats were also seen in these groups. At
the highest dose level, high mortality occurred. Males in the
highest dose group failed to inseminate the females. In these
animals, there was evidence of testicular hypoplasia, tubular
degeneration, and atypical spermatoids in the epididymus. At
the two lower dose levels, no influence on reproduction was
found (Short et al., 1976).
Female rats fed 400 or 2000 mg thiram/kg diet for at least
14 days prior to mating showed a significant reduction in the
number of implants per dam and pups per dam. The delaying
effect on the estrous cycle was reversible. At the highest dose
level, a number of animals died. A comparable study with ferbam
using the same dose levels did not show any influence on
fertility, gestation, viability, or lactation (Short et al.,
1976).
Administration of 50 mg ziram/kg and 100 mg zineb/kg body
weight to rats for a period of 2, 4, or 6 months produced
delayed insemination, sterility, resorption of fetuses, and
anomalies in development (Rjazanova, 1967).
8.4.1.2. Bird
Thiram (99.9% purity) has been reported to decrease egg
production for the domestic chicken (Gallus domesticus), pigeon
(Columba livia), and pheasant (Phasianus colchicus torquatus).
A dose level of 8.8 mg/kg body weight per day caused a 50%
reduction in egg laying in bobwhite quail (Colinus virginianus).
During this period of reduced egg laying, it seems that an
alteration of hormone levels took place resulting in significant
weight losses of ovary and oviduct, decrease in serum calcium
level (which is controlled by estrogen), and alteration in
normal maturation of the ova (Wedig et al., 1968).
In a study by Van Steemis & Van Logten (1971), tecoram (an
oxidation product of disodium EBDC and sodium dimethyl dithio-
carbamate with ammonium persulfate) in propylene glycol or
saline was administered to chick embryos at doses of 0.01, 0.1,
1, or 10 mg/egg. Paralysis, shortening of the extremities,
muscular atrophy, dwarfing and death occurred. Microscopically,
signs of peripheral neuropathy confined to the distal parts of
the peripheral nerves, and muscular atrophy were found.
8.4.2. Teratogenicity
Some dithiocarbamates are potentially teratogenic in the
rat, but not in the mouse. In most cases, the teratogenic
effects have been observed at high dose levels.
8.4.2.1. Rat
Kaloyanova et al. (1967) have studied the effects on progeny
of albino rats of 0, 700, and 1400 mg maneb/kg body weight
administered twice per week for 4.5 months. Three groups of 20
rats (10 males and 10 females) were used and a first generation
was bred. Congenital deformities were found in the facial part
of the skull, caudal vertebrae, palates, limbs, and tail. The
same type of changes were also found after a single oral dose of
2000 - 8000 mg zineb or 1000 - 4000 mg maneb/kg body weight on
days 11 - 13 of pregnancy.
No teratogenic effects or adverse effects on the intra-
uterine development of progeny were observed when rats were
given 1000 mg zineb or 500 mg maneb/kg body weight, from days 2
to 21 of pregnancy, or were exposed in an inhalation chamber to
a concentration of 100 mg zineb/m3 for 4 h/day from day 4 of
pregnancy (Antonovich et al., 1972; Petrova-Vergieva & Ivanova-
Chemishanska, 1973; Ivanova-Chemishanska et al., 1975a).
In a study on Sprague Dawley rats using maneb at dose levels
of 0, 120, 240, or 480 mg/kg body weight on days 7 - 16 of
gestation, fetotoxic effects (reduced fetal weight, reduced
ossification, and hydrocephalus) were seen at the highest dose
level (Chernoff et al., 1979).
In studies by Larsson et al. (1976), maneb was administered
to Sprague Dawley rats at dose levels of 0, 400, 770, or
1420 mg/kg body weight, by gavage, as a single dose on day 11 of
gestation. Rats were sacrificed on day 18 of gestation and
fetuses were examined for reproductive and teratogenic abnorm-
alities. A substantially increased resorption rate was seen at
770 mg/kg body weight. Gross malformations occurred in all
surviving animals at 770 and 1420 mg/kg body weight, but no
malformations were observed in the single litter of the low-dose
group. These abnormalities included cleft palate, hydrocephaly,
and other serious defects. In another study, maternal admini-
stration of zinc acetate (made in an attempt to relieve the
incidence of teratogenic events) had some preventive effect at
750 mg/kg body weight, but, at 1380 mg/kg body weight, the
frequency and type of malformations were unchanged (Larsson et
al., 1976).
Mancozeb was administered to rats at dose levels of 0, 380,
730, or 1320 mg/kg body weight on day 11 of gestation in a study
similar to that reported above with maneb. Again, a substantial
increase in malformations, similar to those produced by maneb,
was observed at the highest dose level, but not at lower levels
(Larsson et al., 1976).
Propineb was administered to rats at dose levels of 0, 400,
760, or 2300 mg/kg body weight, by gavage, on day 11 of
gestation. The dams were sacrificed and fetuses examined for
gross external and internal malformations on day 18 of
pregnancy. Maternal toxicity was observed at all dose levels.
At the highest dose level, propineb was fetotoxic and induced a
variety of malformations in the surviving fetuses. At 760 mg/kg,
propineb was slightly fetotoxic but did not induce malformations
in surviving fetuses. The pattern of fetal abnormalities was
qualitatively similar to that noted in the maneb- and mancozeb-
treated rats (Larsson et al., 1976).
Cypromate (zinc propylene bisdithiocarbamate) was studied
for its teratogenic potential in white rats using either a
single oral dose of 250, 500, or 1000 mg/kg body weight on the
11th or 13th day of gestation or repeated treatment from the
first day of gestation through the whole pregnancy at 62, 250,
and 500 mg/kg body weight. A spectrum of malformations involving
the nervous and skeletal systems, facial cranium, extremities,
etc., were induced with a single dose of 500 mg/kg body weight
or more, and at all dose levels given repeatedly (Petrova-
Vergieva, 1976).
Groups of rats (26 - 27 pregnant CD1 rats per group) were
administered zineb (purity 85.5% containing 0.35% ETU) at dose
levels of 0, 200, 632, or 2000 mg/kg body weight per day on days
6 - 19 of gestation. Maternal body weight and food consumption
data were recorded. Pregnant rats were sacrificed at day 20 and
a laparotomy was performed. Fetal data included live, dead, and
resorbed fetuses as well as somatic and skeletal abnormalities.
There was no maternal mortality, but a substantial weight loss
was seen at the highest dose level. Fetuses from mothers
administered 2000 mg/kg also showed a reduced body weight.
Fetal mortality was not observed, and there were no significant
anomalies noted on gross external examination. However, a
higher incidence of teratogenic anomalies was noted at the
highest dose level (short and kinky tails, hydrocephalus, and
increased incidence of skeletal anomalies). At the 632 mg/kg
level, these teratogenic anomalies were absent. The abnorm-
alities found at the highest dose level may have been due, in
part, to the presence of ETU in the formulation (Short et al.,
1980).
Ferbam administered to rats on days 6 - 15 of gestation at
150 mg/kg body weight resulted in death, increased resorptions,
decreased fetal weights, and a slight increase in soft and
skeletal tissue anomalies (Minor et al., 1974). CD-1 rats were
treated on days 6 - 15 of gestation, by gavage, with 0, 11, or
114 mg ferbam/kg body weight. Twenty-five percent of the dams
administered 114 mg/kg died, but the surviving dams showed small
litters, increased resorptions, and decreased fetal weight.
Also, a number of malformations (unossified sternebrae,
malformed cranium, hydrocephalus, and cleft palate) were found.
When thiram was administered at doses of 0, 40, 90, 136,
164, or 200 mg/kg body weight on days 6 - 15 or 7 - 12 of
gestation, the 200 mg/kg dose reduced the number of mated rats
that delivered litters, and only 33% of the dams survived. At
doses of 136 mg/kg or more, a decrease in the number of implants
and fetuses per dam, an increase in resorptions, a decrease in
fetal body weight, and an increase in malformations, as
described for ferbam, were observed (Short et al., 1976).
8.4.2.2. Mouse
Pregnant female NMRI and Swiss-Webster mice were treated
orally during days 6 - 17 of pregnancy with thiram at 179, 357,
714, or 1071 mg/kg body weight and 250, 500, 1000, and
1500 mg/kg body weight, respectively. Increased resorption of
embryos, clearly retarded fetal development, and skeletal
malformation (cleft palate, wavy ribs, curved long bones of
extremities, and micrognathia) were seen in both strains. The
12th and 13th days seemed to be the most sensitive period of
embryonic development. The lowest dose had only a slight
effect, but the next dose level was clearly teratogenic (Roll,
1971; Matthiaschk, 1973).
Thiram did not reduce body weight gain during gestation at
doses of 100 or 300 mg/kg body weight, administered on days
6 - 14, and no changes in litter size, incidence of resorptions,
or fetal weight were observed. However, an increase in malform-
ations was seen (Short et al., 1976).
In studies by Larsson et al. (1976), doses of 0, 400, 770,
or 1420 mg maneb/kg body weight or 0, 380, 730, or 1330 mg
mancozeb/kg body weight were given on a single occasion to NMRI
mice on days 9 or 13, and mice were sacrificed on day 18 of
gestation. No adverse maternal or fetal effects could be
detected.
In a study on CD1 mice administered 0, 375, 750, or 1500 mg
maneb/kg body weight on days 7 - 16 of gestation, maternal
toxicity was found at the highest dose level, together with a
decrease in fetal caudal ossification centres at all dose levels
(Chernoff et al., 1979).
Ferbam administered to mice at 30 and 300 mg/kg body weight
on days 6 - 16 of gestation did not produce any teratogenic
effects (Minor et al., 1974), and, when given at 23 or 228 mg/kg
body weight on days 6 - 14, it did not affect the survival or
body weight of Swiss-Webster dams during gestation. No changes
in litter size, incidence of resorptions, or fetal weight were
observed, but, at the highest dose level, an increase in
malformations was seen (Short et al., 1976).
Groups of CD-1 mice were administered zineb (85.5% purity)
daily, at dose levels of 0, 200, 632, or 2000 mg/kg body weight
per day, for 11 days from day 6 of gestation and sacrificed on
day 18. Gross examination for maternal well-being and fetal
anomalies, both somatic and skeletal, failed to show any
teratogenic effects (Short et al., 1980).
8.4.3. Embryotoxicity
Korhonen et al. (1982a,b) used a system called chicken
embryo test to study the embryotoxic potential of dithio-
carbamates and found early and late death and malformed embryos.
It was thought that this test could have a predictive value as a
simple teratogenicity test, but many limitations were found in
doing so, and the interpretation of the results were difficult.
8.5. Mutagenicity and Related End-Points
Seiler (1973) studied the mutagenicity of maneb and ziram.
Maneb proved negative in tests with Salmonella strains his G46,
TA1530, TA1531, TA1532, and doubtful in TA1534. Ziram was
positive in TA1534, doubtful in TA1530, and negative in the
other strains.
In studies by Fahrig (1974), ziram was non-mutagenic in a
variety of other microorganisms (Escherichia coli, Serrata
marcescens, and Saccharomyces cerevisiae), but Pilinskaya (1971)
found that it induced chromosome breaks, most of them confined
to chromosome 2, in cultured peripheral human lymphocytes.
Shirasu et al. (1976) studied mutagenicity with the rec-
assay procedure, a sensitivity test using H17 rec+ and M45 rec-
strains of Bacillus subtilis, and with reversion assays on
plates using E. coli (WP2) and S. typhimurium TA1535, TA1536,
TA1537, and TA1538. In these tests, ferbam, thiram, and ziram
were non-mutagenic.
Certain dithiocarbamates given intraperitoneally to mice at
100 mg/kg body weight caused chromatid aberrations in bone
marrow cells (Kurinny & Kondratenko, 1972; Hedenstedt et al.,
1979), the order of effectiveness being thiram > ziram > maneb
and zineb. Thiram was also shown to induce gene mutations in
Salmonella and Aspergillus (Szymezyk, 1981; Zdienicka et al.,
1981).
Hedenstedt et al. (1979) found that the mutagenic effect of
tetramethylthiuram monosulfide (TMTM) was enhanced in the
presence of metabolizing systems (S-9 mix), but that tetraethyl-
thiuram disulfide (TETD or AntabuseR) was not mutagenic.
Propylene bisdithiocarbamate was tested for cytogenicity by
bone marrow analysis and for dominant lethal mutations by giving
a single oral dose to male rats. There was a considerable
increase in the number of chromosomal aberrations (chromatid
fragments), reaching a maximum after 24 h (Vachkova-Petrova,
1977). In studies on the cytotoxic effects of ziram on cultures
of human lymphocytes in vitro (Pilinskaya, 1971), the ratio of
chromatid-type aberrations to chromosome-type aberrations was
2.7:1, which suggests that most chromosomal damage took place at
the S-stage and the G2 stage of the mitotic cycle. Ziram-
induced chromosomal breaks were observed to be non-random, most
of them occurring in chromosome 2.
Propineb and its main metabolite propylenethiourea (PTU)
were investigated by the micronucleus test in mice. The
following doses were given by ip injection twice, with a 24-h
interval: propineb (unknown purity), 62.5, 125, or 250 mg/kg
body weight in a 5% aqueous solution of Tween 80; propineb (78%
purity), the same doses, but in 5% gum arabic; and PTU, 100,
200, 400, or 600 mg/kg body weight in distilled water. Controls
received methanesulfonate at doses of 10, 20, 40, or 80 mg/kg
(twice, in distilled water) and mitomycin at 1.75, 3.5, 7, or
14 mg/kg (twice, in distilled water). No statistically
significant increase in the percentage of micronuclei was
observed at any of the tested doses of propineb or PTU. The
positive control groups showed the expected dose-related
increase in the number of polychromatic erythrocytes with
micronuclei (Rolandi et al., 1984).
Vachkova-Petrova (1981) studied the mutagenic potential of
EndodanR (ethylenethiuram monosulfide) in short-term studies.
Several doses of EndodanR were administered to groups of 6 rats
either twice at an interval of 24 h or for 5 successive days,
and the animals were killed 6 h after the last dose. The cells
in metaphase were analysed for aneuploidy and aberrations, but
no mutagenic effects that could be attributed to the chemical
were detected.
Zineb and ziram were not mutagenic when tested in Drosophila
melanogaster (Benes & Sram, 1969).
8.6. Carcinogenicity
8.6.1. Mouse
In studies by Innes et al. (1969), groups of 18 mice of each
sex from two hybrid strains were given various dithiocarbamates
from 7 days of age up to 18 months. The compounds were given
daily, by gavage, from day 7 to weaning and thereafter added to
the diet. The compounds and the respective amounts were: ferbam
at 10 mg/kg body weight, then 32 mg/kg diet; maneb at 46.4 mg/kg
body weight, then 158 mg/kg diet; nabam at 21.5 mg/kg body
weight, then 73 mg/kg diet; thiram at 10 mg/kg body weight, then
26 mg/kg diet; and zineb at 464 mg/kg body weight, then
1298 mg/kg diet. No significant increase in tumours was found.
On the basis of all experimental data and experimental
designs, IARC (1976) suggested that there was no definite proof
for the carcinogenicity of maneb, though ETU, one of its meta-
bolites, was able to produce thyroid carcinomas. However, zineb
and maneb have been reported to induce pulmonary adenomas in
mice when treated orally (Chernov & Khistenko, 1969; Balin,
1970).
8.6.2. Rat
A 2-year feeding study of the effects of zineb on rats was
carried out using 60 young male and 60 female albino rats.
These were divided into groups of 10 each and administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg/kg diet.
Growth, mortality, haematology, and organ weights were examined
and histopathology was carried out. No clear influence on
growth and mortality was observed, and haematological findings
were within normal limits. A goitrogenic effect (hyperplasia)
was observed in 50% of the animals at 500 mg/kg, and, at
1000 mg/kg diet or more, this effect was more pronounced. The
interpretation of thyroid weight/body weight ratio was
complicated because of the small number of animals still alive.
Microscopically, no evidence of malignancies was present. At
the highest dose level, kidney damage (congestion, nephritis,
nephrosis) was seen. Although 10 000 mg zineb/kg diet produced
moderate goitrogenic effects, this effect was also seen at
500 mg/kg in some rats, and was not clearly dose dependent
(Blackwell-Smith et al., 1953).
Cases of goitre and thyroid adenoma were found in Sprague
Dawley rats fed for 2 years on a diet containing 120 mg or
360 mg metiram/kg diet (Griepentrog, 1962).
8.6.3. Dog
When nine mongrel dogs (three groups of three animals each)
were administered 20, 2000, or 10 000 mg zineb/kg diet for 1
year, no haematological changes were found. The thyroids of the
group given the highest dose level were enlarged and showed
hyper-plastic changes, but, at lower dose levels, they did not
show any histological changes (Blackwell-Smith et al., 1953).
Doses of 45 mg metiram/kg body weight for 90 days or
7.5 mg/kg body weight for 23 months did not cause any ill
effects (Worthing & Walker, 1983).
8.6.4. Dithiocarbamates in combination with nitrite
The above-mentioned studies were carried out with individual
dithiocarbamates. Other studies have shown that these
dithiocarbamates, in the presence of nitrite, can be converted
to N-nitroso derivatives, which may be carcinogenic.
Thiram, ferbam, ziram (Eisenbrand et al., 1974; Sen et al.,
1974), and disulfiram (Lijinsky et al., 1972; Elespuru &
Lijinsky, 1973) react with nitrite under mildly acidic con-
ditions to form N-nitroso compounds. Formation of N-nitroso-
dimethylamine (NDMA) by the action of microorganisms in sewage
and soil containing 0.1% thiram has been reported to occur under
experimental conditions (Ayanaba et al., 1973). Nitrite is
formed by the reduction of nitrate, which can be found in some
unrefrigerated vegetables (e.g., spinach and beets), especially
after cooking (Phillips, 1968), in human saliva (Tannenbaum et
al., 1974), and in cured meats. Thus, in vivo nitrosation of
dithiocarbamates in the stomach cannot be totally excluded.
As has been pointed out by IARC (1976), the extrapolation of
findings in experimental animals to man is complicated by many
factors. It is relatively easy to show that N-nitroso
derivatives can be formed and that these are mutagenic and/or
carcinogenic. The crucial information, however, is the quantity
produced in man under the prevailing conditions. The concen-
tration of both reactants, the pH, the influence of competing
reactions, and the presence of accelerators and inhibitors are
all important. In addition to these difficulties in defining
potential human exposure, the susceptibility of man, compared
with that of experimental animals, has to be considered.
Sen et al. (1974) concluded that it is unlikely that
significant amounts of NDMA would be produced from the ingestion
of trace amounts of the dithiocarbamates and the normal intake
of nitrite.
8.7. Mechanisms of Toxicity; Mode of Action
8.7.1. Thyroid
In weaning rats, a diet containing 500 mg nabam/kg given for
9 days caused thyroid hyperplasia and a decrease in the weight
of the thymus (Seifter & Ehrich, 1948).
Male and female albino rats were administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg zineb/kg diet
for up to 30 days. Animals were killed sequentially in order to
study the changes in the thyroid gland. Only in the 10 000 mg/kg
group were effects seen. In two out of five males and in one
out of five females, hyperplasia of the thyroid was observed
(Blackwell-Smith et al., 1953). Przezdziecki et al. (1969) fed
female Wistar rats a diet containing 1300 mg zineb/kg or 1875 mg
maneb/kg diet for 7 months. Significant increases in the weight
of the thyroid gland and decreases in the weight of the kidneys,
adrenal glands, and ovaries were observed.
Thyroid hyperplasia has been reported in rats given maneb,
zineb, or mancozeb in amounts ranging from 500 to 2500 mg/kg
diet for periods of up to 2 years (FAO/WHO, 1965b, 1971b). In a
2-year feeding study, 2500 mg maneb/kg diet produced thyroid
hyperplasia and nodular goitre and increased mortality, but 1250
and 250 mg/kg diet did not cause any ill effects (FAO/WHO,
1965b).
Zineb was given orally to white rats at dose levels of 96 or
960 mg/kg body weight for 4.5 months. Compared with that of
untreated animals, the thyroid was enlarged with microfollicles
and columnar cells. Succinic dehydrogenase and cytochrome
oxidase activities were raised in these cells, while the colloid
in the follicles showed reduced PASa-positive granules. These
changes were consistent with an increase in thyroid-stimulating
hormone (TSH). An increased number of basophilic cells con-
taining PASa-positive granules was observed in the adeno-
hypophysis (anterior pituitary). These effects were seen only
at the highest dose level. The uptake of 131iodine was also
increased at the highest dose level, and a high plasma TSH level
was recorded in treated animals. The changes observed in both
thyroid and pituitary were probably a compensatory response to
the antithyroid effect of the dithiocarbamate (Ivanova-
Chemishanska et al., 1975b).
After oral administration of zineb to rats at dose levels of
9.6 or 960 mg/kg body weight, twice a week, for 4“ months, the
gonadotropic and thyroid-stimulating functions of the adeno-
hypophysis were significantly increased compared with those of
control values, more markedly in those receiving the higher dose
(Ivanova-Chemishanska et al., 1974).
Albino rats were orally administered doses of 2400 mg
zineb/kg or 3500 mg maneb/kg body weight, and, after 24 h,
radioactive iodine was administered intraperitoneally. Reduced
assimilation of 131iodine by the thyroid was found, which
suggests that the dithiocarbamates, or certain of their
metabolites, possess a marked antithyroid effect and inhibit the
synthesis of thyroxine (Ivanova-Chemishanska et al., 1967,
1974). In similar studies with mancozeb, a single oral dose of
7000 mg/kg body weight resulted in a decreased uptake of
131iodine (Ivanova-Chemishanska et al., 1967).
The condition of the thyroid gland was studied in male
albino rats, which were administered 700 mg maneb/kg, 960 mg
zineb/kg, or 1400 mg mancozeb/kg body weight. After 30 days,
the distinct morphological changes observed indicated
stimulation of the thyroid by TSH. Hypophyseal stimulation is
the consequence of release from negative feedback by thyroxine,
the plasma level of which is depressed by the action of EBDCs
(Ivanova-Chemishanska et al., 1971, 1974).
Rats fed a diet containing 10 000 mg metiram/kg for 2 weeks
showed increased thyroid weight and a decrease in the uptake of
131iodine, but no ill effects were produced by 1000 mg/kg diet
(Worthing & Walker, 1983).
----------------------------------------------------------------
a PAS = periodic acid-Schift reagent.
8.7.2. Interaction of dithiocarbamates and alcohol
Hald et al. (1948) found that dithiocarbamates interact with
ethanol (ethyl alcohol), and since then, certain dithio-
carbamates, particularly disulfiram, have been used in the
treatment of chronic alcoholism. Disulfiram has been proposed
to act in two different ways. The first possibility is that the
drug or one of its metabolites (e.g., diethyldithiocarbamate,
carbon disulfide) interferes with the normal metabolism of
ethanol and, consequently, gives rise to an accumulation of
toxic amounts of intermediary products, such as acetaldehyde.
The second possible method of action is that ethanol interferes
with the normal metabolism of disulfiram and therefore makes
disulfiram more toxic in some way.
Ethanol is detoxified in many tissues, particularly the
liver, by oxidation, firstly to acetaldehyde, then to acetic
acid, and finally to carbon dioxide and water. Disulfiram
interferes with various enzyme systems including those involved
in the oxidation of ethanol. After administration of disulfiram,
the blood acetaldehyde level increases significantly.
Peripheral neuropathy and optic neuritis have been observed in
alcoholics treated with 125 - 150 mg disulfiram per day
(Gardner-Thorpe & Benjamin, 1971).
Van Logten (1972) studied this phenomenon of alcohol
intolerance extensively in rats. Zineb and maneb did not induce
alcohol intolerance, whereas most of the alkyldithiocarbamates
(such as ziram and nabam) and thiuram sulfides (such as thiram)
did. In general, the dithiocarbamates with a free H atom bound
to the N atom did not induce intolerance. Apart from the
accumulation of acetaldehyde in blood, disturbance of sulfo-
bromophthalein (BSP) elimination, increased serum glutamic-
pyruvic transaminase, hypothermia, increased glucose content,
changes in blood morphology, atrophy of spleen and thymus, and
increase in the weight of adrenals and brain were all observed.
Studies on adrenalectomized rats showed the involvement of the
adrenals in the alcohol intolerance. Hyperglycaemia, eosino-
penia, lymphopenia, and neutrophilia were not seen in the
animals without adrenals, and spleen and thymus atrophy was
reduced. However, the effect on the red blood cells was more
pronounced, and the accumulation of acetaldehyde in the blood
was unaffected by adrenalectomy.
Oral treatment of rats with ethanol after administration of
alkyldithiocarbamates or thiuram sulfides lowers the catechol-
amine content of the adrenals. Since the lowest level was not
reached until 24 h or more after alcohol treatment, it seems
likely that the influence on the adrenals of the dithio-
carbamate-ethanol interaction is of secondary importance. There
was no clear indication that the ethanol intolerance in the rat
is accompanied by changes in brain catecholamine level.
As in human beings, the dithiocarbamate-ethanol reaction in
the rat is characterized by a severe hypotension, which starts
almost immediately after the administration of the ethanol and
lasts at least 8 h. It is evident that, during alcohol
intolerance, body fluids shift from the plasma into the
interstitial tissue or cells, possibly thereby causing the hypo-
tension or shock. The observed hypothermia also starts almost
immediately after ethanol administration and may last for many
hours. Adrenalectomy did not prevent the hypothermia.
Therefore, this phenomenon must be considered as a primary
effect, along with the plasma accumulation of acetaldehyde.
Since heat loss is not increased during the dithiocarbamate-
ethanol reaction, the hypothermia is probably due to decreased
heat production. However, the serotonin concentration in the
brain was increased, and so a disturbance of the thermo-
regulation cannot be ruled out. It seems doubtful that the
dithiocarbamate-ethanol reaction is due to acetaldehyde per se.
Intraperitoneal injection of acetaldehyde, which resulted in a
blood level twice as high as during the dithiocarbamate-ethanol
reaction, did not influence BSP elimination, serum glucose
level, body temperature, organ weights, catecholamine content of
the adrenals, or blood pressure.
No indications were available that the accumulation of
acetaldehyde in the blood was due to an accelerated biotrans-
formation of alcohol. More work needs to be done to decide
whether the accumulation of acetaldehyde and pyruvate in the
blood is a consequence of a disturbance of carbohydrate
metabolism. The specificity of ethanol is remarkable. The
combination of thiram with either methanol or 1-propanol has no
effect on BSP elimination or on blood glucose level.
The sensitivity of the rat for the dithiocarbamate-alcohol
reaction is of the same magnitude as that of man. Administra-
tion of 1.9 mg thiram/kg body weight in the rat elicits an
accumulation of acetaldehyde in the blood. Thus, it may be
concluded that 60 ml of gin, 0.5 litre of beer, or even less
should be sufficient to induce alcohol intolerance.
Eight days after the administration of certain dithio-
carbamates, a dithiocarbamate-alcohol reaction may be observed
when ethanol is given. The maximum level of acetaldehyde in the
blood is reached within 15 min of ethanol treatment.
A 90-day study with several dietary levels of thiram
revealed a no-toxic-effect level of 100 mg/kg diet. After
feeding rats with 10 mg thiram/kg diet for 6 weeks, oral
administration of a single dose of 6 ml ethanol/kg body weight
caused a significant decrease in the body temperature. Higher
doses of thiram with alcohol induced hyperglycaemia, accumula-
tion of acetaldehyde in the blood, and other abnormalities. In
contrast, the combination of 100 mg thiram/kg diet and 5%
ethanol continuously in the drinking-water did not have any
effect (Van Logten, 1972).
8.7.3. Neurotoxicity
In an 80-week study on the neurotoxic and behavioural
effects of thiram, 12 male and 12 female rats per group were fed
thiram at dose levels of 0, 100, 400, or 1000 mg/kg diet (the
concentration of the compound in the diet was periodically
increased in order to give a relatively constant consumption on
the basis of body weight). A second study was carried out on
two groups of 24 female rats administered 0 or 1000 mg thiram/kg
diet, for 36 weeks. The neurotoxic effects were characterized
by ataxia and paralysis of the hind legs, although these effects
were only seen at the highest dose level (1000 mg/kg diet,
equivalent to 65 mg/kg body weight) in females. Demyelination,
degeneration of the axon cylinders, and the presence of
macrophages in the nerve bundle of the sciatic nerve were seen.
Degeneration in the ventral horn of the lower lumbar region of
the spinal cord was demonstrated by chromatolysis of motor
neurons, pyknosis, and satellitosis. Electromyograms indicated
a loss of motor unit function, and the histopathology suggested
that the peripheral nerve is the primary site of the lesion (Lee
& Peters, 1976).
In another study, groups of 12 males and 12 females were fed
ferbam in the dose levels that gave actual intake levels of
approximately 8.5, 34, and 87 mg/kg body weight (average of
males and females) per day. The neurotoxic effects of ferbam
are less than those of thiram. In this study, only 3 of the 24
rats fed the highest dose level developed ataxia or paralysis
(Lee & Peters, 1976). Neurotoxic effects have also been observed
for ziram by Hodge et al. (1956).
In a study on rats, zineb (490 and 2450 mg/kg body weight),
maneb (350 and 1750 mg/kg body weight), and mancozeb (700 and
3500 mg/kg body weight) were administered orally at twice weekly
doses for 4 months. Mortality was high, and paresis in the hind
limbs appeared in the third month of the study and progressed to
complete paralysis (Ivanova-Chemishanska, 1969a).
Dishovski & Ivanova-Chemishanska (1979) studied the ultra-
structural changes in the neocortex of rats repeatedly
administered propineb (70% purity) at 85 mg/kg and 425 mg/kg
body weight for 40 days. At the higher dose level, intense
ultrastructural changes in the sensorimotor neocortex were
detected using an electron microscope, primarily affecting the
pyramidal cells. The concentration of ribosomes and hypertrophy
of the Golgi apparatus suggested an increase in synthetic
processes in the neurons.
Edington & Howell (1966, 1969) found lesions in the central
nervous system of adult Dutch-New Zealand rabbits who were given
ip injections of sodium diethyldithiocarbamate (NDDC) at
330 mg/kg body weight, for 6 days/week, for 30 weeks. The first
changes were seen at 6 weeks in the accessory cuneate nucleus
and in Clarke's column; 12 weeks later, degeneration was seen in
the spinocerebellar tracts in the cerebellum medulla. After 24
weeks, severe nerve fibre degeneration in the peripheral white
matter of the spinal cord (both involved the axon and myelin
sheath) was observed. It was suggested that these changes might
be connected with changes in the level of copper in the serum.
Kim & Rizzuto (1975) studied the effect of NDDC (0.23, 2.3,
and 23 µg/ml nutrient medium) on myelinated cultures of new-
born mouse cerebellum. Exposure time was 24 - 120 h, and the
cultures were examined by light and electron microscopy.
Treatment of the cultures for 24 - 48 h produced swelling of
axons and presynaptic endings, morphologically characteristic of
dystrophic axons. Continued exposure induced an extensive
degeneration of axons and myelin sheath (Wallerian degeneration
in axons).
8.7.4. Dithiocarbamates in combination with metals
Truhaut et al. (1971) studied the chelating action of sodium
diethyldithiocarbamate to copper and the fact that this element
is indispensable for the activity of dopamine beta-hydroxylase.
The authors put forward the hypothesis that the inhibition of
this enzyme system, which catalyses the conversion of dopamine
to norepinephrine and participates in the biogenesis of cate-
cholamines in the central nervous system, may play a role in the
etiology of neurotoxic effects.
Maj et al. (1970) studied the effect of disulfiram, diethyl-
dithiocarbamate (DDC), and dimethyldithiocarbamate on serotonin
(5-HT) and 5-hydroxyindole-3-acetic acid (5-HIAA) in the brain
of rats. The total dose levels ranged from 150 to 500 mg/kg
body weight. It was concluded that these three compounds do not
affect the 5-HT level in the rat brain. The 5-HIAA levels
increased, but not significantly.
Possibly, reactions of carbon disulfide with pyridoxamine
could lead to the depletion of pyridoxal phosphate in the
tissues, which may, in turn, cause neurological changes. Long-
term poisoning of rabbits with carbon disulfide has been shown
to result in increased excretion of zinc in the urine and
disturbances of copper and zinc concentrations in the tissues.
Also, after NDDC treatment, increased levels of copper in the
liver and nervous tissue have been found (Cavanagh, 1973).
Aaseth et al. (1981) showed that oral treatment of Wistar
rats with tetramethylthiuram disulfide (TMTD) at 1000 mg/kg diet
for one week increased the brain levels of endogenous copper
and zinc. In further studies, rats were administered an iv
injection of 203HgCl2 (5 µmol/kg body weight in saline) at day
17 of pregnancy. DDC was given immediately after the mercury
injection (500 µmol/kg body weight). The maternal brain
concentration of mercury increased significantly, and the kidney
levels, measured after 24 and 48 h, also increased. In the
fetuses, the mercury in the brain, liver, kidneys, and blood
(but also in the placenta) were significantly increased after
24 h, but, after 72 h, only the levels in fetal blood were still
elevated. Mice of the NMRI strain were similarly injected with
203HgCl2 (2.5 µmol/kg body weight) and fed diets containing DDC
(10 000 mg/kg diet), disulfiram and TMTD (1000 mg/kg diet), or
carbon disulfide (3000 mg/kg diet) for 4 days. The brain level
of mercury was significantly increased after DDC or TMTD
treatment and marginally after disulfiram or carbon disulfide
treatment (Aaseth et al., 1981).
Lakomaa et al. (1982) studied the effect of DDC on copper
and zinc concentrations in different regions of the brain of
Long-Evans rats during acute or repeated treatment. Acute
treatment (250 mg/kg body weight) produced no effect after 24 h,
whereas repeated treatment (250 mg/kg, 5 times per week, for
4 weeks) increased copper levels in the brain stem, cortex,
hippocampus, and the rest of the brain, but did not alter zinc
concentrations.
Dithiocarbamates, with their metal-chelating properties, and
thiuram derivatives, have been demonstrated to cause a marked
increase in the concentration of lead in the brain as well as a
redistribution of lead in the rest of the body (Oskarsson, 1983,
1984; Danielsson et al., 1984). Thus, after injection of a dose
of labelled lead (203Pb), the brain concentrations were
increased by up to 100 times in thiuram-treated rats.
Male Sprague Dawley rats (10 groups of 5 rats each) were
administered different combinations of thiram, disulfiram, DDC,
or dimethyldithiocarbamate in combination with sodium or lead.
The study demonstrated that treatment with dithiocarbamates and
thiram derivatives in rats exposed for 6 weeks to lead causes a
substantial (up to 4-fold) increase in the lead concentration of
the brain. This effect can be explained by the formation of a
lipophilic lead-dithiocarbamate complex, which probably is
retained longer and has a higher capacity to penetrate the
blood-brain barrier and bind to lipid-rich brain tissue compo-
nents than inorganic lead itself. The chemical form of the lead
when it is in the brain remains uncertain. The lead complex may
decompose in the brain into inorganic lead, which exerts a
neurotoxic effect, or it may be very stable in the brain and of
low toxicity for the central nervous system (Oskarsson & Lind,
1985).
There are several reports on the effect of dithiocarbamates
on the distribution in the body of other metal ions such as
cadmium, thallium, nickel, copper, zinc, and mercury (Oskarsson
& Lind, 1985).
DDC has been shown to have a strong inducing effect on
levels of metallothionein, a low molecular weight, heavy-metal-
binding protein, in rat liver and kidney. The mechanism
probably reflects enhanced uptake of copper and depletion of
hepatic glutathione (Sunderman & Fraser, 1983).
8.7.5. Miscellaneous reactions
Dithiocarbamates, with their chelating capacity, also
interfere with a number of enzyme systems containing metals such
as zinc and copper (e.g., dopamine beta-hydroxylase). They also
inhibit sulfhydryl (SH)-containing enzymes and a number of other
enzyme systems involved in glucose metabolism (e.g., hexokinase,
glyceraldehyde-3-phosphate dehydrogenase, and glucose-6-
phosphate dehydrogenase). The effect of dithiocarbamates on
liver enzymes has consequences for the metabolism of other
chemicals. Thus, the toxicity of carbon tetrachloride is
decreased by diethyldithiocarbamate (Lange & Jung, 1971; Lutz et
al., 1973), and the toxicity of other chemicals, e.g., ethyl
alcohol, may be increased (section 8.7.2).
9. EFFECTS ON MAN
9.1. Occupational Exposure
9.1.1. Acute toxicity - poisoning incidents
The acute toxicity of dithiocarbamates is low and, there-
fore, acute intoxication in human beings is unlikely to occur.
A case was reported of a 62-year-old man with acute kidney
insufficiency after maneb application. However, the precise
cause of maneb exposure was not clear, since the patient had a
history of hypertension, cerebral infarction, gastrectomy
because of stomach cancer, and chemotherapy. The patient was
treated with haemodialysis and was discharged from hospital
(Koizumi et al., 1979).
Thiram (100 mg/m3) has been shown to cause headaches,
vertigo, impairment of mental capacity, muscle twitch, and
paraesthaesia (Sprecher & Grigorowa, 1967).
9.1.2. Case reports, short-term and epidemiological studies
9.1.2.1. Dermal
The irritant and allergic potential of most dithiocarbamates
is evident in occupational exposure. Skin irritation and
sensitization were studied in man using a conventional patch
test. A cotton square was dipped in 19% nabam solution and
placed on the inner surface of the forearm, and, 14 days later,
this procedure was repeated on the opposite forearm. Zineb was
tested in the same manner, except that the cotton square was
dipped in 65% wettable powder. The patches were left in place
for 48 h. Of the 25 subjects included in the nabam study, 2
showed irritation (mild erythema and itching). Thirteen of the
25 reacted to the retest (from mild erythema to severe erythema,
oedema, and vesiculation), indicating sensitization. Of the 50
subjects used in the zineb study, no reaction at all was seen in
49 of them. One reacted in such a way that it indicated primary
irritation rather than sensitization (Blackwell-Smith et al.,
1953). Schultheiss (1957) reported a case of contact dermatitis
with thiram. Zadorozhny et al. (1981) found dermatitis and
eczema in 241 industrial workers exposed to TMTD and other types
of pesticides. Twenty-one of them showed contact dermatitis, 25
allergic dermatitis, and 7 eczema.
Cases of diffuse erythema and eczematoid epidermatitis of
the eyelids and inguinal regions, probably with elements of sun
sensitization, were observed among agricultural workers (grape
and tobacco industries) in contact with zineb (Babini, 1966) or
maneb (Laborie & Laborie, 1966; Zorin, 1970). These were
largely allergic in character with only a few manifestations of
contact dermatitis. Decreased resistance of the workers,
vitamin deficiency, chronic liver disease, and other factors
apparently contributed to these effects.
9.1.2.2. Exposure via different routes
Kaskevich et al. (1981) carried out an epidemiological study
on 137 workers engaged in zineb manufacturing (51 men and 86
women). The duration of exposure to zineb for 52 workers was
between 1 and 3 years, and for 85 workers between 4 and 5 years.
Control groups in this study consisted of 193 persons, not
exposed to chemicals and matched for age, period of employment
rate, and sex. The concentrations in the air of the working
area never exceeded 1 mg/m3. Among workers occupationally
exposed to zineb, the following changes were found: hepato-
cholecystitis (28.4% of workers, versus 13.5% in controls);
vegetovascular dystonia connected with disorders in the central
nervous system (34.9%, versus 22.3% in controls); chronic
bronchitis (4.4%, versus 0.5% in controls); contact dermatitis
(11.9%, versus 0.1% in controls); and disorders in the menstrual
cycle (16.91%, versus 4.3% in controls). These studies indicate
a change in catecholamine metabolism.
In a study with cultured lymphocytes from 15 workers working
in different stages of zineb manufacture, the mean incidence of
aberrant metaphases was 6% greater than that in controls. The
incidence of chromosomal aberrations (chromatid breaks) in
cultured human lymphocytes treated with maneb (0.5, 15, or
30 µg/ml) was 10 - 20% greater than in controls (Antonovich et
al., 1972).
A number of studies on maneb and mancozeb production workers
have been carried out. In the earliest study (1965), 54 produc-
tion workers were given medical examinations that included blood
and urine analyses. Since this study predated the availability
of immunoassay techniques for thyroid hormone determination,
protein-bound iodine was used as a measure of thyroid function.
No thyroid or other medical abnormalities could be attributed to
EBDC exposure. In a second study (1975), 57 exposed and 98
unexposed production workers were examined for thyroid function
by measuring triiodothyronin (T3), thyroxine (T4), and TSH.
Again, no effects attributable to work-place exposure were
identified. Workers exposed to EBDC levels ranging from 0.13 to
5.46 mg/m3 were found to have elevated ETU and manganese levels
in the urine. In a 1976 mortality study, 992 past and present
production workers (over the period 1948-75) were studied.
Compared with the local general population, neither the overall
death rate nor the death rate due to cancer was elevated. The
number of cancer deaths observed (10) was too small to evaluate
cancer-specific mortality (Gowers & Gordon, 1980).
In the most extensive study, 42 currently exposed and 112
previously exposed workers were compared with equal size control
groups matched for age, period of employment, race, and type of
job. All participants were given thorough physical examinations
by specialists in diagnostic medicine, including detailed
questionnaires and interviews about health history and family
health. A separate thyroid examination was carried out by
thyroid specialists. Thyroid parameters that were measured
included total T3, T3 resin uptake, T4, TSH, free T4 index,
thyroglobulin antibodies, and microsomal antibodies. In
addition, urine was analysed for ETU, EBDC, zinc, manganese,
creatine, iodide, specific gravity, and pH. Blood levels of
glucose, urea nitrogen, sodium, potassium, calcium, chloride,
carbon dioxide, cholesterol, total protein, protein albumin,
bilirubin, uric acid, creatinine, inorganic phosphate, lactic
dehydrogenase, and serum glutamic oxaloacetic transaminase were
also determined. As in earlier studies, the occurrence of
unusually high levels of ETU in the urine of currently exposed
workers confirmed their exposure. However, a detailed
statistical analysis of the data revealed no differences in
thyroid function, blood and urine indicators of liver and kidney
function, or general health, between exposed and control groups
(Gowers & Gordon, 1980; Charkes et al., 1985).
PART B
ETHYLENETHIOUREA (ETU) AND PROPYLENETHIOUREA (PTU)
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLENETHIOUREA (ETU)
AND PROPYLENETHIOUREA (PTU)
INTRODUCTION
1. SUMMARY
1.1 Sources, environmental transport and distribution
1.2 Environmental levels and human exposure
1.3 Kinetics and metabolism
1.4 Effects on organisms in the environment
1.5 Effects on experimental animals and in vitro test systems
1.5.1 Ethylenethiourea
1.5.2 Propylenethiourea
1.6 Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
2.3.1 Extraction
2.3.2 Clean-up
2.3.3 Derivatization
2.3.4 Determination
2.3.4.1 Gas-liquid chromatography (GLC)
2.3.4.2 Thin-layer chromatography (TLC)
2.3.4.3 Polarography
2.3.4.4 Radioisotope dilution
2.3.4.5 High-pressure liquid chromatography (HPLC)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Soil
4.2 Water
4.3 Plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Food and drinking-water
5.2 Monitoring and market basket studies
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and excretion
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short- and long-term exposures
8.3 Teratogenicity
8.4 Mutagenicity
8.5 Carcinogenicity
8.5.1 Mouse
8.5.2 Rat
8.5.3 Hamster
8.6 ETU in combination with nitrite
8.7 Mechanisms of toxicity; mode of action
8.8 Probineb and Propylenethiourea (PTU)
8.8.1 General
8.8.2 Toxicological information
9. EFFECTS ON MAN
9.1 Epidemiological studies
INTRODUCTION
One of the metabolic products of ethylene bisdithiocarbamate
decomposition in mammals, plants, and lower organisms is
ethylenethiourea (ETU). It may also be present as an impurity
in these dithiocarbamates, and their residues on crops may be
partly transformed into ETU during food processing. A comparable
breakdown takes place with propineb, giving rise to propylene-
thiourea (PTU).
1. SUMMARY
1.1. Sources, Environmental Transport, and Distribution
Ethylenethiourea (ETU) is found together with residues of
the parent ethylene bisdithiocarbamates (EBDCs) in and on crops
that have been treated with these pesticides. During storage,
processing, and cooking, the amount of the parent compound
decreases while that of ETU increases. ETU is easily photo-
oxidized (in the presence of photosensitizers) to ethyleneurea
(EU).
1.2. Environmental Levels and Human Exposure
In certain crops, such as spinach, carrots, and potatoes,
treated with EBDCs, high levels of ETU can be found after
cooking. In general, however, the ETU levels are below
0.1 mg/kg product.
Estimates of the exposure of the general population of the
USA are of the order of 0.24 - 3.65 µg ETU/kg body weight per
day, and, in Canada, estimates based on market-basket studies
are around 1 µg ETU/kg body weight per day.
1.3. Kinetics and Metabolism
ETU is rapidly absorbed, metabolized, and excreted in
mammals. Up to 90% is eliminated via the urine and only a
small amount via the faeces. Distribution of ETU in the body
appears to be fairly uniform with the exception of a relative
accumulation in the thyroid. ETU is broken down to ethylene
diamine (EDA), urea, carbon dioxide, or oxalic acid, or is
transformed to imidazole derivatives in mammals, plants, and the
environment.
1.4. Effects on Organisms in the Environment
The available LC50 levels of ETU and EU for fish are in the
range of 7500 - 13 000 mg/litre.
1.5. Effects on Experimental Animals and In Vitro Test Systems
1.5.1. Ethylenethiourea
The acute oral toxicity in experimental animals is low, and
the long-term effects are mainly characterized by an antithyroid
action.
At dose levels > 25 mg/kg body weight, decreases in serum
T3, T4, and protein-bound iodine (PBI) and increases in thyroid-
stimulating hormone (TSH) have been found in studies on
experimental animals. At higher dose levels (> 100 mg/kg body
weight), increases in thyroid weight and hyperplasia occurred,
which finally resulted in the development of adenocarcinoma.
The effects of short-term exposure to low levels of ETU seem to
be reversible, but those of long-term exposure to higher levels
become, at a certain stage, irreversible. A level of
approximately 5 mg/kg body weight seems to be without effects.
Most mutagenicity studies on ETU, especially those with
mammalian test systems, have given negative results.
A number of carcinogenicity studies have been carried out on
mice, rats, and hamsters. In addition to an antithyroid action,
ETU has been found to induce, subsequently, thyroid tumours
(hyperplastic goitre, solid-cell adenomas, and follicular and
papillary carcinomas) in mice and rats. In an earlier study on
mice, liver tumours, lung tumours, and lymphomas were also
detected, but these findings have not been confirmed. No
tumours except thyroid tumours have been found in rats, and in
hamsters, no tumours of the thyroid gland or other organs were
observed, even at 200 mg/kg diet.
At dose levels above approximately 10 mg/kg body weight, ETU
has clear teratogenic effects in rats and hamsters, different
types of central nervous system and skeletal anomalies being
induced. However, in mice, no teratogenic effects were found at
much higher dose levels (up to 800 mg/kg body weight).
1.5.2. Propylenethiourea
In a long-term study on mice using propylenethiourea (PTU),
an increased incidence of hepatocellular adenomas was observed
at dose levels of 10 mg/kg diet or more. No thyroid tumours
were found, but increased thyroid hypercellularity occurred at a
dose level of 1000 mg/kg diet. In rats, goitrogenic effects
were seen with PTU at dose levels as low as 1 mg/kg diet.
1.6. Effects on Man
Epidemiological studies on workers exposed to ETU did not
reveal any increase in the incidence of thyroid tumours.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
The chemical structure of ethylenethiourea (ETU) is:
CH2-NH
| \
| C=S
| /
CH2-NH
2.2. Physical and Chemical Properties
ETU is a fairly stable compound. Some physical and chemical
properties are listed in Table 8.
Table 8. Some physical and chemical properties of ETU
-------------------------------------------------------------------
Empirical formula C3H6N2S
Common synonym 2-imidazolidinethione
Appearance white, crystalline
Relative molecular mass 102.17
Odour odourless
Melting point 203 - 204 °C
Solubility in water: 20 000 mg/litre at 30 °C;
in ethanol: moderately soluble;
in chloroform: nearly insolublea
CAS registry number 96-45-7
-------------------------------------------------------------------
a From: IARC (1974), IUPAC (1977), US EPA (1984).
2.3. Analytical Methods
Residue analysis consists of sampling the contaminated
material, extracting the pesticide residue, cleaning up the
extract of interfering substances, and identifying and
quantifying the pesticide residue. The main methods used are
summarized in Table 9.
2.3.1. Extraction
Methanol and ethanol have been used as extraction solvents
for biological samples, due to the high solubility of ETU in
polar solvents. Mixed solvents such as methanol/chloroform
(Onley & Yip, 1971) or methanol/acetone (Phillips et al., 1977)
have also been employed, and the addition of trichloroacetic
acid has been reported to improve recovery with the latter
solvent. Sodium ascorbate has also been found effective in
ensuring good recovery of ETU (IUPAC, 1977; Otto et al., 1977).
2.3.2. Clean-up
The simplest procedures involve extraction of a derivative
from aqueous acid and alkali (Newsome, 1972; Nash, 1974; King,
1977). Another approach has been to purify the initial extract
by column chromatography before proceeding with derivatization
(Onley & Yip, 1971; Haines & Adler, 1973; Onley, 1977) or
determination steps (Otto et al., 1977). Where ETU is determined
without derivatization, a solvent-partitioning step is included
to provide further clean-up (IUPAC, 1977; Otto et al., 1977).
2.3.3. Derivatization
In all cases, derivatization involves first an alkylation of
the thiocarbonyl group. The various derivatives that have been
used are given in Table 9. Careful attention to reagent purity
is essential to ensure quantitative results (Onley & Yip, 1971;
Pecka et al., 1975; King, 1977). The benzyl chlorides react
smoothly by refluxing in alcohol for 30 min, while alkylation
with butyl bromide is carried out at room temperature in aqueous
dimethylformamide containing sodium hydroxide and sodium
borohydride. Solutions of ETU in aqueous dimethylformamide have
been found to be extremely unstable and must be reacted
immediately (Phillips et al., 1977). The n-butyl (Onley & Yip,
1971) and m-trifluoromethyl benzyl (King, 1977) derivatives are
sufficiently volatile to be analysed directly by gas-liquid
chromatography, whereas the benzyl derivatives must be
concentrated and acetylated before quantifying. Care must be
exercised during the concentration step to prevent losses
through evaporation (Pecka et al., 1975). Pentafluorobenzoyl
chloride (Nash, 1974) and trifluoroacetic anhydride have been
used as acetylating reagents, the former requiring a column
chromatographic step to remove excess reagent and by-products
before moving to gas-liquid chromatography. Although the excess
trifluoroacetic anhydride is easily removed by evaporation, the
trifluoroacetate derivative is unstable in the presence of
moisture and must be determined soon after removal of the excess
reagent (IUPAC, 1977).
2.3.4. Determination
Gas-liquid chromatographic methods predominate because of
their greater sensitivity, specificity, and accuracy. Methods
of determining ETU in plant samples were reviewed in 1976 by the
IUPAC Commission on Pesticide Terminal Residues (IUPAC, 1977),
and an extensive review of methods for ETU determination has
been produced by Bottomley et al. (1985).
2.3.4.1. Gas-liquid chromatography (GLC)
A variety of column packings and conditions have been used
in the determination of ETU and its derivatives. Detectors used
include thermionic (Onley & Yip, 1971), flame photometric (FPD)
(Haines & Adler, 1973; Onley, 1977; Otto et al., 1977), and
electron capture (EC) (Nash, 1974; King, 1977). Although
quantification by GLC/EC enables the use of smaller samples
(5 - 10 g) for monitoring ETU residues at the 0.01 mg/kg level,
it requires confirmation of suspected residues by mass spectro-
metry (MS), a second derivative, or by element-selective
detectors. Methods employing GLC/FPD with large samples
(40 - 100 g) have the advantage of both quantifying and
confirming ETU residues.
2.3.4.2. Thin-layer chromatography (TLC)
A variety of adsorbents and developing solvents have been
used to detect ETU in plants (Vonk & Kaars Sijpesteijn, 1970;
Onley & Yip, 1971; Blazquez, 1973; Engst & Schnaak, 1974). The
limit of detection is 0.02 mg/kg using alumina plates and Grotes
reagent for visualization (Onley & Yip, 1971). Semi-
quantitative determinations are possible by comparison with ETU
standards run simultaneously (IUPAC, 1977).
2.3.4.3. Polarography
This technique involves clean-up on an alumina column,
followed by paper chromatography and determination of the
nitroso derivative by polarography (Engst & Schnaak, 1974).
2.3.4.4. Radioisotope dilution
A reverse isotope dilution method has been used to determine
ETU in the presence of its metabolites, and is useful in the low
milligram range (Graham & Bornak, 1973; IUPAC, 1977).
2.3.4.5. High-pressure liquid chromatography (HPLC)
High-pressure liquid chromatography has been used for the
determination of ETU without derivatization. Detection can be
by ultraviolet absorption or electro-conductivity measurement,
the minimum level being 0.025 mg ETU/litre or kg (Prince, 1985).
Massey et al. (1982) reported an HPLC method applied for ETU
determination in a beer extract with a detection limit of
10 µg/kg. The method has been found to give spuriously high
results in the determination of ETU in beer due to the presence
of co-eluting matrix components. The more powerful resolving
ability of column-switching high-performance liquid chromato-
graphy, using polar-bonded columns of different selectivities,
has proved highly effective in separating ETU from these co-
eluting materials.
Table 9. Methods for the determination of ETU in plant samplesa
----------------------------------------------------------------------------------------------------
Extraction Extraction/ Derivative formation Analysis Detect- Reference
solvent clean-up measurementb ability
(mg/kg)
----------------------------------------------------------------------------------------------------
Plant ethanol none silica gel/TLC 10.0 Vonk & Kaars Sij-
extract pesteijn (1970)
Plant ethanol none paper electro- - Vonk & Kaars Sij-
extract phoresis pesteijn (1971)
Ethanol and cellulose 2-(butylthio) - GLC/thermionic 0.02 Onley & Yip
chloroform column 2-imidazoline detector (1971)
Methanol cellulose GLC/FPD 0.002 Watts et al.
column (1974)
Methanol chloroform/ GLC/ECD 0.005 Newsome (1972)
HCl
Methanol Al2O3 column 2-(butylthio)- GLC/FPD 0.05 Haines & Adler
2-imidazoline (1973); Onley
(1977)
Dioxane and none none silica - Blazquez (1973)
water gel/TLC
Methanol/ Al2O3 column none GLC/FPD 0.01 Otto et al.
Na-ascorbate (1977)
Table 9 (contd.)
----------------------------------------------------------------------------------------------------
Extraction Extraction/ Derivative formation Analysis Detect- Reference
solvent clean-up measurementb ability
(mg/kg)
----------------------------------------------------------------------------------------------------
Methanol florisil 2-(benzylthio)-1- GLC/ECD 0.005 Nash (1974)
column (pentafluorobenzoyl)-
2-imidazoline
Ethanol ether/HCl 2-( m-trifluoromethyl- GLC/ECD 0.01 King (1977)
partition benzylthio)-ETU
Acetone methanol/ 2-(benzylthio)-1- GLC/ECD 0.01 Newsome (1978)
acetone; (pentafluorobenzyl)-
Al2O3 column 2-imidazoline
acetonitrile/
dichloromethane
silica column
----------------------------------------------------------------------------------------------------
a From: IUPAC (1977).
b TLC = thin-layer chromatography; GLC = gas-liquid chromatography; FPD = flame photometric
detection; ECD = electron capture detection.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
During recent years, much attention has been paid to the
finding that ETU may occur in plant samples following the use of
dithiocarbamate fungicides. It may be present in the fungicide
when applied, or may result from subsequent transformation
(Bontoyan et al., 1972). Similarly, propylenethiourea (PTU) may
occur in residues of the fungicide propineb (IUPAC, 1977). The
amounts of ETU present in commercial formulations vary from one
sample to another, and depend on the length of time between
manufacture and use and the storage conditions, especially
temperature and moisture. Bontoyan & Looker (1973) found that
ETU increased, during storage for 39 days at 49 °C and 80%
relative humidity, from an initial content of 0.02 - 2% to a
final level of 0.13 - 14.5%. The degradation dynamics of
formulations from different manufacturers varied, products
containing both manganese and zinc forming the least ETU (IUPAC,
1977).
ETU is one of the important residues in plants and in the
environment following the agricultural use of ethylene
bisdithiocarbamates (EBDCs). It is also a metabolite formed
when EBDCs are ingested by animals and man.
Sources of human and environmental exposure to ETU are also
discussed in sections 3, 4, and 5 of Part A and sections 4 and 5
of Part B.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
ETU is a fairly stable compound with respect to hydrolytic
reactions but is easily oxidized to ethyleneurea (EU).
Oxidation to EU takes place primarily in biological systems and
by photolytic reaction, especially in the presence of photo-
sensitizers (Cruickshank & Jarrow, 1973; Ross & Crosby, 1973).
In studies by Kaars Sijpesteijn & Vonk (1970), pure cultures of
soil bacteria and fungi were unable to effect this transform-
ation
After ultraviolet irradiation of ETU on silica gel,
Cruickshank & Jarrow (1973) found nine secondary reaction
products. 2-Imidazolidinone was identified as the main
degradation product and there were smaller amounts of 3-(2-
imidazolin-2-yl)-2-imidazolidinethione (Jaffe's base). Other
secondary reaction products of the photooxidation of ETU are 2-
imidazoline and glycine (Ross & Crosby, 1973) via the inter-
mediate hydantoin (IUPAC, 1977) (Fig. 4).
4.1. Soil
ETU degradation was found to be slower in autoclaved soils
than in non-sterile soils (Kaufman & Fletcher, 1973), and only
EU was identified. In biologically active soils, ETU was
oxidized to carbon dioxide and four other degradation products,
two of which were identified as hydantoin and Jaffe's base.
Degradation of ETU to carbon dioxide in non-sterile soils was
reported by Lyman & Lacoste (1974). These results indicate that
ETU is oxidized under both biological and non-biological
conditions to EU, which is considerably more stable than ETU and
can be considered a major breakdown product. EU, however, can
be oxidized photochemically, using a catalyst, to give glycine
and carbon dioxide (Ross & Crosby, 1973), or microbially in
soil. In this context, Jaffe's base might be considered as an
intermediate product in ETU degradation.
According to Lyman & Lacoste (1974) and Rhodes (1977), half
of the ETU (present at a concentration of 10 mg/kg) in
Hagerstown silt loam soil was degraded to carbon dioxide in 22
days. Normal microbial carbon dioxide production was unaffected
by ETU at this concentration. Because this value was determined
on the basis of 14C-carbon dioxide formation from 14C-labelled
precursor, it does not represent a half-life of ETU, since 14C-
carbon dioxide formation did not parallel the disappearance of
labelled starting material from the soil. The actual half-life
of ETU is less than one day.
According to Kaufman & Fletcher (1973), ETU is oxidized to
EU, whereas carbon dioxide is only formed slowly. In Hagerstown
silt loam, ETU at 2 or 20 mg/kg was entirely converted into EU
within 2 days, while 200 mg ETU/kg took 8 days. In contrast, 4
days after treatment of soil with 2, 20, and 200 mg ETU/kg, only
43.4%, 8.9%, and 0.9%, respectively, had been degraded to carbon
dioxide. A slow but constant conversion of ETU to EU was also
found in autoclaved soil, whereas the formation of carbon
dioxide was only observed in non-sterile soil (Kaufman &
Fletcher, 1973; Lyman & Lacoste, 1974).
Besides ETU, other degradation products of EBDCs include ETD
and DIDT. The main volatile components of zineb's decomposition
are carbon disulfide, carbonyl sulfide, and EDA. Almost all
zinc is converted into zinc sulfide and zinc oxide (Melnikov &
Trunov, 1966).
6.4. Decomposition in Water and Soil
Thiram and dimethyldithiocarbamic acid give rise in soil to
methyl isothiocyanate and sulfur, and, under acidic conditions,
to carbon disulfide, hydrogen sulfide, methylamine, methyl-
isocyanate, and the bisdisulfide of methyldithiocarbamic acid.
Two of the products, carbon disulfide and dimethylamine,
evaporated from the soil (Raghu et al., 1975). Dimethyldithio-
carbamic acid also binds with heavy metals in soil to form
complexes.
The various metal derivatives of ethylene bisdithiocarbamic
acid appear to be converted in the soil to DIDT, ETU, carbon
disulfide, hydrogen sulfide, and carbonyl sulfide (Moje et al.,
1964; Kaars Sijpesteijn & Vonk, 1970). The conversion by soil
bacteria and fungi of DIDT into ETU has been demonstrated (Vonk
& Kaars Sijpesteijn, 1976). Even though ETU is slowly converted
into EU in soil, pure cultures of soil bacteria and fungi were
unable to effect this transformation (Kaars Sijpesteijn & Vonk,
1970).
6.5. Metabolism in Microorganisms
Microorganisms readily form ETU from DIDT, a spontaneous
decomposition product of EBDCs. This conversion also takes
place after addition of reducing compounds such as cysteine,
glutathione, or ascorbic acid. It consists of the reduction of
the S-S bond of DIDT, with the subsequent release of carbon
disulfide to form ETU. It was shown by Vonk & Kaars Sijpesteijn
(1976) that DIDT was reduced by NADH in the presence of enzyme
extracts from Pseudomonas fluorescens, Escherichia coli,
Saccharomyces cerevisiae, or Aspergillus niger.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
There is some evidence that dithiocarbamates, at concentra-
tions 10 times that of normal field application, may reduce
microbial biomass and increase the bacterial:fungal ratio.
7.2. Aquatic Organisms
7.2.1. Acute toxicity
Toxicity studies using dithiocarbamates are hindered by the
fact that they are chemically and biologically degradable and
may also be contaminated with degradation products. Their
stability in water depends on the pH and on the presence of
metal ions with which they form complexes. The soluble dithio-
carbamates dissociate in water, whereas the polymers are only
slightly soluble in water. As the breakdown products will also
influence toxicity, toxicity testing of dithiocarbamates is
complex.
According to US EPA (1977, 1982a), the use of pesticide
products containing maneb against cranberry fruit rot at
application rates of up to 6.7 kg ai/ha would result in a
concentration of 4.4 mg/litre in a 15-cm layer of water. McCann
& Pitcher (1973) reported a 96-h LC50 of 1 mg/litre for
bluegills, while Worthing & Walker (1983) reported a 48-h LC50
for carp of 1.8 mg/litre. Zineb used against cranberry fruit
rot at an application rate of up to 5.4 kg ai/ha could result in
a concentration of 3.52 mg/litre in a 15-cm layer of water. A
26-h LC50 of 0.2 mg/litre has been reported for Daphnia magna.
Van Leeuwen (1986) carried out an extensive study with 18
dithiocarbamates and three metabolites of these compounds,
including ETU, in fish (Poecilia reticulata), crustacea (Daphnia
magna), algae ( Chlorella pyrenoidosa, Phytobacterium
phosphoreum), and two nitrifying bacteria (Nitrosomonas and
Nitrobacter). The results are summarized in Tables 4 and 5.
Worthing & Walker (1983) gave the following LC50 values:
propineb: rainbow trout, 1.9 mg/litre; golden orfe,
133 mg/litre; thiram: carp, 4 mg/litre; rainbow trout,
0.13 mg/litre; bluegill, 0.23 mg/litre; metiram: harlequin fish,
17 mg/litre.
The susceptibility to maneb of the early life stages of
rainbow trout has been studied using fertilized eggs (before and
after water hardening), early eye point eggs, late eye point
eggs, sac fry, and early fry. The sac fry and early fry stages
appeared to be the most sensitive. The 96-h LC50s for the
different stages were: for 0-h egg, 6 mg/litre; for 24-h egg,
5.6 mg/litre; for early eyed egg (14 days), 1.8 mg/litre; for
late eyed egg (28 days), 1.3 mg/litre; for sac fry (42 days),
0.32 mg/litre; and, for early fry (77 days), 0.34 mg/litre (Van
Leeuwen, 1986).
Table 4. Acute toxicity of dithiocarbamates and breakdown
products for fisha
-------------------------------------------------------------
Organism Compound 96-h LC50
(95% confidence
limit) (mg/litre)
-------------------------------------------------------------
Poecilia reticulatab nabam 5.8 (4 - 8.5)
maneb 3.7 (3.2 - 5.6)
zineb 7.2 (5 - 10.3)
mancozeb 2.6 (2.1 - 3.3)
metiram 6.4 (4 - 10.4)
Na-DMDC 2.6 (2.1 - 3.2)
ziram 0.75 (0.56 - 1)
ferbam 0.09 (0.06 - 0.18)
thiram 0.27 (0.22 - 0.33)
Na-DEDC 6.9 (5.5 - 8.5)
Zn-DEDC 0.49 (0.40 - 0.61)
disulfiram 0.32 (0.24 - 0.43)
ETU 7500 (5600 - 10 000)
EU 13 000 (10 000 - 18 000)
Rainbow troutc thiram 0.26 (0.24 - 0.32)
(Salmo gairdneri)
-------------------------------------------------------------
a From: Van Leeuwen (1986).
b Studies according to OECD guidelines 203.
c 24-h LC50; water temperature 15 ± 1 °C;
weight of fish, 34 ± 4.7 g.
Table 5. Short-term toxicity studies with dithiocarbamates and
breakdown productsa
--------------------------------------------------------------------
Compound Daphnia Chlorella Photobacterium Nitrosomonas
magna pyrenoidosa phosphoreum Nitrobacter
48-h LC50 96-h EC50 15-min EC50 3-h MIC
(mg/litre) (mg/litre) (mg/litre) (mg/litre)
--------------------------------------------------------------------
Nabam 0.44 2.4 102 32
Maneb 1 3.2 1.2 56
Zineb 0.97 1.8 6.2 18
Mancozeb 1.3 1.1 0.08 32
Metiram 2.2 1.8 0.37 32
Na-DMDC 0.67 0.8 0.51 26
Ziram 0.14 1.2 0.15 100
Ferbam 0.09 2.4 0.20 10
Thiram 0.21 1 0.10 18
Na-DECD 0.91 1.4 1.22 43
Zn-DEDC 0.24 1.1 1.70 > 320
Disulfiram 0.12 1.8 1.21 > 320
ETU 26.4 6600 2100 1
EU 5600 16 000 3300 1000
--------------------------------------------------------------------
a From: Van Leeuwen (1986).
7.2.2. Short- and long-term toxicity and reproduction studies
7.2.2.1. Fish
In sublethal toxicity studies carried out on Salmo
gairdneri, groups of 10 fish were exposed to thiram
(0.18 mg/litre) for 24 h. Blood parameters (decreased haemo-
globin and leukopenia, decreased glucose levels, and increased
glucose-6-phosphate dehydrogenase activity) and liver parameters
(increased lipid content, increased lactate dehydrogenase) were
changed, and it was concluded by the author that thiram is a
cytotoxic chemical (Van Leeuwen, 1986). In a further study, the
60-day toxicity for early life stages was tested on S. gairdneri
using a number of dialkyldithiocarbamates, EBDCs, and break-
down products. The LC50s ranged from approximately 1 to
9 µg/litre for dialkyldithiocarbamates and 211 - 2100 µg/litre
for EBDCs, but ETU and EU were not toxic, even at levels
exceeding 1000 mg/litre.
Embryotoxic and teratogenic effects were also observed for
all the compounds studied, and there was an overlap between the
responses for skeletal malformations and lethality over a wide
concentration range. The teratogenic effects in rainbow trout
proved to be in agreement with those observed in mammals.
Exposure of rainbow trout during embryo-larval development
revealed that malformations induced by dithiocarbamates were
almost exclusively confined to the notochord, which increased
considerably in both length and diameter. As a result, the
notochord became twisted and distorted. Ectopic osteogenesis
was observed in almost every affected notochord. Other effects,
such as the disruption of the integrity of myomeres and organ
dislocations, were closely related to the notochordal ano-
malies. Also, compression and fusion of vertebrae and "waviness"
of various skeletal elements were found. Concentration-related
changes in the liver were observed in short-term exposure of
juvenile rainbow trout, while at high levels proliferation of
bile duct epithelial cells and necrosis of hepatocytes were
seen. Ziram and thiram induced brain haemorrhages as well as
intraspinal extravasates of blood cells (Van Leeuwen, 1986).
7.2.2.2. Invertebrates
Short-term toxicity studies using the compounds listed in
Table 5 were carried out to investigate the effects of prolonged
exposure (21-day) on survival, fecundity, and growth of Daphnia
magna. Growth and reproduction were not specifically inhibited,
since effects on these characteristics were generally detected
at levels comparable with the 21-day LC50s. For dialkyldithio-
carbamates, the 21-day LC50s ranged from approximately 10 to
30 µg/litre, for EBDCs, from 80 to 110 µg/litre, and, for ETU
and EU, the levels were 18 and 3200 mg/litre, respectively (Van
Leeuwen, 1986).
7.2.3. Bioaccumulation
Van Leeuwen (1986) determined the log n-octanol/water par-
tition coefficient for a few dithiocarbamates and breakdown
products. The results are summarized in Table 6. In general,
the higher the log n-octanol/water partition coefficient, the
greater the tendency to bioaccumulate.
Table 6. log n-octanol/water partition
coefficients for some dithiocarbamates
------------------------------------------
Compound log n- octanol/water
partition coefficient
------------------------------------------
Disulfiram 4
Thiram 1.82
ETU 0.67
EU 0.96
------------------------------------------
In short-term studies on rainbow trout of uptake,
distribution, and retention of 14C-labelled zineb and ziram,
both compounds were found to be rapidly distributed throughout
the tissues. Whole-body accumulation was low, with bioconcen-
tration factors of less than 100. Relatively high radioactivity
levels were found in the liver, gall bladder, and intestinal
contents, suggesting the prominent role of hepatic biotransform-
ation and biliary excretion. With ziram, the eyes and skin also
appeared to be distribution sites. Whole-body elimination was
rapid, with about 75% of radioactivity being eliminated within
the first 4 days. With ziram, 45% of the initial total 14C
content in the body was still present at the end of the 16-day
depuration period. Differences in the extent of elimination
were most noteworthy for the eyes, skin, and kidneys. Whole-
body autoradiography showed the radioactivity in the digestive
tract, liver, bile, gills, thyroid follicles, melanophores of
the skin, choroid epithelium complex of the eyes, and in other
melanin-containing tissues, such as the kidneys (Van Leeuwen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
In general, the toxicity of dithiocarbamates for mammals is
relatively low. Some commonly used dithiocarbamates included in
the WHO recommended classification of pesticides by hazard (WHO,
1986a), which is based primarily on the acute oral and dermal
toxicity of the technical material for the rat, are given in
Annex III.
Acute oral and dermal toxicity data for a number of animal
species of various dithiocarbamates are given in Table 7. From
this Table, it is clear that nabam and metam-sodium are the most
toxic dithiocarbamates, other compounds having only low
toxicity. As for many compounds, the toxicity is often
influenced by the method of application, e.g., solvents used,
age and sex of animal, type of diet, etc. Thus, in rats fed an
isocaloric diet containing 3.5% protein, the LD50 of nabam was
210 mg/kg body weight, compared with 565 mg/kg in rats fed the
same diet plus 26% protein (Periquet & Derache, 1976).
Ivanova-Chemishanska (1969) found that rats treated with
zineb, maneb, or mancozeb showed dose-dependent signs of
depression, adynamia, decreased tonus, disturbances in
coordination, paresis, and paralysis of extremities combined
with general weakness, lack of appetite, and prostration.
Yin-Tak Woo (1983) has reviewed the structure-activity
relationships of different types of dithiocarbamates.
8.2. Short- and Long-Term Exposures
8.2.1. Oral exposure
8.2.1.1. Rat
In 1-month feeding tests, no growth retardation was noted in
rats fed diets containing 100 mg ferbam/kg diet, but decreased
growth occurred with 500 mg/kg diet and increased mortality with
5000 mg/kg diet (Hodge et al., 1952).
Groups of 40 weanling rats (20 females and 20 males) were
given diets containing 500, 1000, 2500, 5000, or 10 000 mg
zineb/kg diet for up to 30 days. Thyroid enlargement was seen
at all dose levels, but unequivocal histopathological changes
were observed only at 10 000 mg/kg diet (Blackwell-Smith et al.,
1953; Kampmeier & Haag, 1954).
Ferbam administered daily at 23, 66, or 109 mg/kg body
weight to male rats for 13 weeks caused death and weight loss at
the highest dose, but did not have any effect on reproduction.
Daily feeding (equivalent to 15 or 51 mg/kg body weight) to
females for 2 weeks caused severe weight loss at the highest
dose level (Minor et al., 1974).
In a 2-year feeding study, 25, 250, and 2500 mg ferbam or
ziram/kg diet shortened the life span of rats and caused growth
depression and neurological lesions (manifested at the highest
dose level by the crossing of hind legs when animals were lifted
by their tail) (Hodge et al., 1956).
Table 7. Acute toxicity (LD50) of dithiocarbamates for experimental
animals
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Ferbam mouse 1000 FAO/WHO (1965b)
rat > 4000 Hodge et al.
(1956)
guinea-pig 450 - 2000
rabbit 2000 - 3000
Metham-sodium mouse 285 Worthing & Walker
(vapam) (1983)
rat 1700 - 1800 Worthing & Walker
(1983)
rabbit 1300 Worthing & Walker
(1983)
Ziram rat 1400 Hodge et al.
(1952)
guinea-pig 100 - 150 Hodge et al.
(1952)
rabbit 100 - 1020 Hodge et al.
(1952)
Thiram mouse 1500 - 2000 Worthing & Walker
(1983)
rat 865 - 1300 Van Esch (1956)
rat 780 - 865 Worthing & Walker
(1983)
rat > 2000 Ben Dyke et al.
(1970)
rabbit 210 Lehman (1951)
cat 230
--------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Disulfiram rat > 4000 Van Esch (1956)
Zineb rat > 5200 Blackwell-Smith
(1953)
rat 9000 Ivanova-Chemi-
shanska (1969a)
Maneb mouse 4100 Engst et al.
(1971)
rat 4500 Engst et al.
(1971)
rat (male) 6750 Worthing & Walker
(1983)
Nabam rat 395 Blackwell-Smith et
al. (1953)
Mancozeb rat (female) 12 800 Ivanova-Chemi-
shanska (1969b)
rat (male) 14 000 Ivanova-Chemi-
shanska (1969)
rat > 8000 Worthing & Walker
(1983)
Propineb rat 8500 > 1000 Worthing & Walker
(1983)
rabbit 2500
cat 2500
hen 2500
Metiram mouse 5400 Worthing & Walker
(1983)
rat > 10 000
guinea-pig 2400 - 4800
(female)
--------------------------------------------------------------------------
Groups of 24 rats (12 females and 12 males) were given a
diet containing 48 mg thiram/kg diet for 2 years (a 3-generation
study). No effects on growth, reproduction, blood parameters,
or mortality rate were found, neither were there gross or
histological changes (Van Esch, 1956). In a further study, 12
female and 12 male rats given 200 mg/kg diet for 8 months did
not show any appreciable changes in growth or mortality rate,
and a dose of 300 mg/kg diet for 65 weeks did not give rise to
specific evidence of poisoning (Tollenaar, 1956). Groups of 24
rats (12 females and 12 males) fed diets containing 300, 1000,
or 2500 mg thiram/kg diet for 65 weeks showed weakness, ataxia,
various degrees of paralysis, and histological changes
(calcification in the brain stem and cerebellum and dystrophic
changes in the leg muscles). At 2500 mg/kg diet, there was an
increased mortality rate (Fitzhugh et al., 1952). Groups of 20
young rats administered diets containing 100, 300, or 500 mg
thiram/kg diet for 2 years all showed a small reduction in the
growth rate. At concentrations of 300 and 500 mg/kg diet, an
increased mortality rate was seen, while at 500 mg/kg diet,
convulsions, thyroid hyperplasia, and calcification in the
cerebellum, hypothalamus, and medulla oblongata were observed
(Griepentrog, 1962; IARC, 1976).
Groups of 25 male and 25 female rats were fed diets
containing 25, 250, 1250, or 2500 mg maneb/kg diet for 2 years.
At 1250 mg/kg diet, there was some depression, impaired food
consumption, and increased mortality rate. At the end of 2
years, the animals receiving 1250 mg/kg diet had an increased
liver/body weight ratio, and those receiving 2500 mg/kg diet
also showed thyroid hyperplasia and nodular goitre (Worthing &
Walker, 1983).
Groups of 10 young male and 10 young female rats were fed
diets containing 500, 1000, 2500, 5000, or 10 000 mg zineb/kg
diet for 2 years. At the two highest dose levels, there was an
apparent increase in the mortality rate among the female rats
and, at 10 000 mg/kg diet, there was a tendency towards
diminished growth in both sexes. The results of haematological
studies were normal, but a goitrogenic effect was seen at all
dose levels. Kidney damage was seen in 6 animals at the
10 000 mg/kg dose level and in one animal in each of the groups
receiving 1000, 2500, or 5000 mg/kg diet, but not at all at
500 mg/kg diet. The tumour incidence was not significantly
greater among any of the treated animals than it was in the
controls (Blackwell-Smith et al., 1953; Kampmeier & Haag,
1954).
Weanling rats in groups of 25 males and 25 females were fed
diets containing 25, 250, or 2500 mg ziram/kg diet for 2 years.
The growth rate and life span were normal in all groups, but
neurological changes were observed in the animals receiving
2500 mg/kg diet, though no cystic lesions were discovered in the
levels. In some of the male animals, the testes were atrophied,
and there was a slight indication of thyroid hyperplasia,
notably in the 2500 mg/kg diet group. However, there was no
increase in tumour incidence in the treated animals (Hodge et
al., 1956). A comparable study with the same dose levels was
carried out with ferbam, and again no increase in tumour
incidence was found (IARC, 1976).
8.2.1.2. Dog
A dog given ferbam and ziram together for one month, each at
a dose of 5 mg/kg body weight per day, remained healthy except
for slight anaemia. The same result was observed when ferbam
was given alone for one month at a dose of 25 mg/kg body weight
per day, or for one week at 50 mg/kg body weight per day.
Raising the dose to 100 mg/kg body weight per day, however,
immediately provoked severe vomiting and malaise (Hodge et al.,
1952). Pairs of adult dogs were given daily doses of 0.5, 5, or
25 mg ferbam/kg body weight for one year. Convulsions occurred
at the highest dose level, but urine analysis, blood parameters,
organ weights, and tissue histology (including that of the
thyroid gland) were normal (Hodge et al., 1956).
When pairs of dogs were fed maneb orally at the rate of 2,
20, 75, or 200 mg/kg body weight per day for one year, toxic
effects were observed at the two highest dose levels, but not at
20 mg/kg body weight (Worthing & Walker, 1983).
Three groups of three dogs each were fed diets containing
20, 2000, or 10 000 mg zineb/kg diet for one year. All the
animals survived, and no persistent changes in growth rate were
seen in any of the groups. There were no histopathological
changes in the tissues, except in the thyroid gland, and
haematological findings were normal. At 10 000 mg/kg diet,
thyroid hyperplasia was noted (Blackwell-Smith et al., 1953;
Kampmeier & Haag, 1954).
8.2.1.3. Bird
Sodium diethyldithiocarbamate (NDDC), the dimethyl compound
(NDMC), and ferbam, ziram, and thiram were given orally to young
and adult domestic fowl (Thorber's gog cockerels) at 330, 210,
205, 56, and 178 mg/kg body weight, respectively, and the birds
were killed after 6, 12, 18, or 20 weeks. All of the compounds
had an adverse effect on body weight gain, retarded testicular
development, and produced degeneration in the seminiferous epi-
thelium of mature birds. Nerve fibre degeneration was produced
in the medulla and spinal cord of chicks by NDDC and in those of
cocks by NDMC. Chicks exposed to thiram became lame and
exhibited swollen epiphyses of the long bones due to endo-
chondrial ossification giving rise to a thickened cartilaginous
epiphyseal plate (Rasul & Howell, 1974).
8.2.2. Inhalation exposure
8.2.2.1. Rat
Studies concerning toxicity following inhalation exposure
are scarce.
Ivanova-Chemishanska et al. (1972) studied the inhalation
toxicity in rats with zineb (70% purity), maneb (80% purity),
and mancozeb (80% purity), applied 6 days per week over a period
of 4“ months, at concentrations of 2, 10, 50, 100, or 135 mg/m3.
The pesticides were given in the form of dispersed aerosols,
with 95% of the dust particles ranging from 1 to 5 µm in size,
and the remainder from 5 to 10 µm. Local irritation of the
mucosa of the upper respiratory tract was noted and
concentration-related non-specific changes in the liver and
kidneys were evident. However, only slight changes were found
at a concentration of 2 mg/m3.
Davydova (1973) studied the influence of inhaled thiram on
the estrous cycle and genital function of rats. Groups of rats
were exposed to 0, 0.45, or 3.8 mg/m3 thiram for 6 h/day, 5
days/week, over a period of 4“ months. An extension of the
estrous cycle was seen at the highest dose level, and genital
function was disturbed, as shown by a reduction in the capacity
to conceive, a reduction in fertility, and of fetal weight
gain.
8.3. Skin and Eye Irritation; Sensitization
Nabam (19% solution) and zineb (65% wettable powder) were
each applied to the right eye of 10 rabbits, the left eye being
used as a control. Nabam did not produce signs of irritation,
while zineb produced mild irritation (erythema), which subsided
within 6 - 8 h. No oedema was seen. The mild irritation may
have been caused by the non-specific foreign body reaction to
the dry, insoluble powder. When this procedure was repeated
with both compounds diluted and suspended for agricultural use
(for nabam, 0.5% of the commercial 19% solution plus zinc
sulfate, 0.125% in water; for zineb, a 0.188% suspension of the
commercial 65% wettable powder in water), no irritation was seen
(Blackwell-Smith et al., 1953).
In studies performed on guinea-pigs, intracutaneous
injections, 10 times daily, followed by an epicutaneous
challenge test, provided evidence of the marked sensitizing and
cross-sensitizing properties of thiram and metiram (Griepentrog,
1960).
It has been reported that a number of dithiocarbamates
(mancozeb, metham-sodium, metiram, zineb, ziram, and thiram)
cause skin and/or eye irritation (Worthing & Walker, 1983).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
8.4.1. Reproduction
8.4.1.1. Rat
Groups of rats (16 male and 16 female Charles River-CD rats
per group) were fed maneb for 3 months at levels of 0, 125, or
250 mg/kg diet, and were mated in a standard 3-generation, 2-
litters-per-generation reproduction study. Groups of males and
females from the F1b and F2b litters were fed maneb for 3 months
after weaning and mated to become parents of the succeeding
generation. The major reproduction indices were unaffected by
maneb at dietary levels up to and including 250 mg/kg diet.
There was no histological evidence of congenital anomalies in a
variety of tissues and organs of the male and female rats of the
F3b litter subjected to histopathological examination (Sherman &
Zapp, 1966).
Maneb, zineb, and mancozeb exert dose-dependent damaging
effects on the gonads of rats of both sexes. The dose levels
were 96 - 960 mg zineb/kg body weight, 140 - 1400 mg mancozeb/kg
body weight, and 14 - 700 mg maneb/kg body weight, given twice a
week for 4.5 months. Both reproductive and endocrine structures
were affected at all dose levels, leading to decreased fertility
(Ivanova-Chemishanska et al., 1973, 1975a). In a 4-month
inhalation study on rats using maneb at 4.7 mg/m3, no effect on
sperm mobility was detected (Matokhnyuk, 1971).
Ivanova-Chemishanska & Antov (1980) studied the effects of
EndodanR (50% ethylenethiuram monosulfide) on the gonads and
reproduction in rats during long-term daily oral doses of 3.8 or
38 mg/kg body weight. The parental generation (F0) and 3
consecutive generations (F1 - F3) were examined. In F0, a
decrease in succinic dehydrogenase and ATPase activities in
testes homogenates was found, as well as an increase in glucose-
6-phosphate dehydrogenase (G6PDH) activity compared with control
levels. Changes in the liver and brain enzyme systems were also
noted.
The same results were obtained with zineb (78% purity). A
rapid loss of mobility and changed resistance (to osmotic and
acidic effects) of spermatozoa were found. A decreased index of
fertility was also found for both sexes in the F0 generation.
Decreased index of fertility and enzymatic changes in organ
homogenates were detectable in the F1 - F3 generations (Ivanova-
Chemishanska et al., 1973).
In extracts of testes of white rats, exposed by inhalation
to zineb and maneb at a concentration of 100 mg/m3 for 4 months,
Izmirova et al. (1969) found an increase in lactate dehydro-
genase (LDH), LDH2, and LDH4. Bogartykh et al. (1979) did not
find any changes in LDH or G6PDH activities in testes homo-
genates of Wistar rats orally treated with zineb (2.5 mg/kg body
weight) for 3 months.
Thiram at doses of 225, 300, 450, 600, 900, or 1200 mg/kg
diet given to male Wistar rats for 29 days produced changes in
many of the parameters studied. A significant effect on testes
and seminal vesicle weight was found at 450 mg/kg diet, and a
decrease in body weight was found at 300 mg/kg. The most
sensitive parameters studied were found to be the weights of the
epididymal and perirenal fat pads, which were decreased by
thiuram doses in the range 130 - 184 mg/kg diet. The no-effect
level, calculated using an extrapolation model, did not differ
significantly from the earlier reported value of 48 mg/kg diet
(Lowy et al., 1979, 1980).
Ferbam was fed to groups of 20 Charles River-CD male rats at
concentrations of 0, 500, 1200, or 2500 mg/kg diet for 13 weeks
before mating with untreated females. Six of the rats fed the
highest dose level died. The indices of fertility, gestation,
viability, and lactation for the females mated with treated
males were normal (Short et al., 1976).
When thiram was incorporated into the diet at concentrations
of 0, 500, 1000, and 2500 mg/kg diet and fed to male weanling
Charles River rats for 13 weeks prior to mating, food intake and
growth was mainly decreased at the two highest dose levels.
Loss of hair and rough coats were also seen in these groups. At
the highest dose level, high mortality occurred. Males in the
highest dose group failed to inseminate the females. In these
animals, there was evidence of testicular hypoplasia, tubular
degeneration, and atypical spermatoids in the epididymus. At
the two lower dose levels, no influence on reproduction was
found (Short et al., 1976).
Female rats fed 400 or 2000 mg thiram/kg diet for at least
14 days prior to mating showed a significant reduction in the
number of implants per dam and pups per dam. The delaying
effect on the estrous cycle was reversible. At the highest dose
level, a number of animals died. A comparable study with ferbam
using the same dose levels did not show any influence on
fertility, gestation, viability, or lactation (Short et al.,
1976).
Administration of 50 mg ziram/kg and 100 mg zineb/kg body
weight to rats for a period of 2, 4, or 6 months produced
delayed insemination, sterility, resorption of fetuses, and
anomalies in development (Rjazanova, 1967).
8.4.1.2. Bird
Thiram (99.9% purity) has been reported to decrease egg
production for the domestic chicken (Gallus domesticus), pigeon
(Columba livia), and pheasant (Phasianus colchicus torquatus).
A dose level of 8.8 mg/kg body weight per day caused a 50%
reduction in egg laying in bobwhite quail (Colinus virginianus).
During this period of reduced egg laying, it seems that an
alteration of hormone levels took place resulting in significant
weight losses of ovary and oviduct, decrease in serum calcium
level (which is controlled by estrogen), and alteration in
normal maturation of the ova (Wedig et al., 1968).
In a study by Van Steemis & Van Logten (1971), tecoram (an
oxidation product of disodium EBDC and sodium dimethyl dithio-
carbamate with ammonium persulfate) in propylene glycol or
saline was administered to chick embryos at doses of 0.01, 0.1,
1, or 10 mg/egg. Paralysis, shortening of the extremities,
muscular atrophy, dwarfing and death occurred. Microscopically,
signs of peripheral neuropathy confined to the distal parts of
the peripheral nerves, and muscular atrophy were found.
8.4.2. Teratogenicity
Some dithiocarbamates are potentially teratogenic in the
rat, but not in the mouse. In most cases, the teratogenic
effects have been observed at high dose levels.
8.4.2.1. Rat
Kaloyanova et al. (1967) have studied the effects on progeny
of albino rats of 0, 700, and 1400 mg maneb/kg body weight
administered twice per week for 4.5 months. Three groups of 20
rats (10 males and 10 females) were used and a first generation
was bred. Congenital deformities were found in the facial part
of the skull, caudal vertebrae, palates, limbs, and tail. The
same type of changes were also found after a single oral dose of
2000 - 8000 mg zineb or 1000 - 4000 mg maneb/kg body weight on
days 11 - 13 of pregnancy.
No teratogenic effects or adverse effects on the intra-
uterine development of progeny were observed when rats were
given 1000 mg zineb or 500 mg maneb/kg body weight, from days 2
to 21 of pregnancy, or were exposed in an inhalation chamber to
a concentration of 100 mg zineb/m3 for 4 h/day from day 4 of
pregnancy (Antonovich et al., 1972; Petrova-Vergieva & Ivanova-
Chemishanska, 1973; Ivanova-Chemishanska et al., 1975a).
In a study on Sprague Dawley rats using maneb at dose levels
of 0, 120, 240, or 480 mg/kg body weight on days 7 - 16 of
gestation, fetotoxic effects (reduced fetal weight, reduced
ossification, and hydrocephalus) were seen at the highest dose
level (Chernoff et al., 1979).
In studies by Larsson et al. (1976), maneb was administered
to Sprague Dawley rats at dose levels of 0, 400, 770, or
1420 mg/kg body weight, by gavage, as a single dose on day 11 of
gestation. Rats were sacrificed on day 18 of gestation and
fetuses were examined for reproductive and teratogenic abnorm-
alities. A substantially increased resorption rate was seen at
770 mg/kg body weight. Gross malformations occurred in all
surviving animals at 770 and 1420 mg/kg body weight, but no
malformations were observed in the single litter of the low-dose
group. These abnormalities included cleft palate, hydrocephaly,
and other serious defects. In another study, maternal admini-
stration of zinc acetate (made in an attempt to relieve the
incidence of teratogenic events) had some preventive effect at
750 mg/kg body weight, but, at 1380 mg/kg body weight, the
frequency and type of malformations were unchanged (Larsson et
al., 1976).
Mancozeb was administered to rats at dose levels of 0, 380,
730, or 1320 mg/kg body weight on day 11 of gestation in a study
similar to that reported above with maneb. Again, a substantial
increase in malformations, similar to those produced by maneb,
was observed at the highest dose level, but not at lower levels
(Larsson et al., 1976).
Propineb was administered to rats at dose levels of 0, 400,
760, or 2300 mg/kg body weight, by gavage, on day 11 of
gestation. The dams were sacrificed and fetuses examined for
gross external and internal malformations on day 18 of
pregnancy. Maternal toxicity was observed at all dose levels.
At the highest dose level, propineb was fetotoxic and induced a
variety of malformations in the surviving fetuses. At 760 mg/kg,
propineb was slightly fetotoxic but did not induce malformations
in surviving fetuses. The pattern of fetal abnormalities was
qualitatively similar to that noted in the maneb- and mancozeb-
treated rats (Larsson et al., 1976).
Cypromate (zinc propylene bisdithiocarbamate) was studied
for its teratogenic potential in white rats using either a
single oral dose of 250, 500, or 1000 mg/kg body weight on the
11th or 13th day of gestation or repeated treatment from the
first day of gestation through the whole pregnancy at 62, 250,
and 500 mg/kg body weight. A spectrum of malformations involving
the nervous and skeletal systems, facial cranium, extremities,
etc., were induced with a single dose of 500 mg/kg body weight
or more, and at all dose levels given repeatedly (Petrova-
Vergieva, 1976).
Groups of rats (26 - 27 pregnant CD1 rats per group) were
administered zineb (purity 85.5% containing 0.35% ETU) at dose
levels of 0, 200, 632, or 2000 mg/kg body weight per day on days
6 - 19 of gestation. Maternal body weight and food consumption
data were recorded. Pregnant rats were sacrificed at day 20 and
a laparotomy was performed. Fetal data included live, dead, and
resorbed fetuses as well as somatic and skeletal abnormalities.
There was no maternal mortality, but a substantial weight loss
was seen at the highest dose level. Fetuses from mothers
administered 2000 mg/kg also showed a reduced body weight.
Fetal mortality was not observed, and there were no significant
anomalies noted on gross external examination. However, a
higher incidence of teratogenic anomalies was noted at the
highest dose level (short and kinky tails, hydrocephalus, and
increased incidence of skeletal anomalies). At the 632 mg/kg
level, these teratogenic anomalies were absent. The abnorm-
alities found at the highest dose level may have been due, in
part, to the presence of ETU in the formulation (Short et al.,
1980).
Ferbam administered to rats on days 6 - 15 of gestation at
150 mg/kg body weight resulted in death, increased resorptions,
decreased fetal weights, and a slight increase in soft and
skeletal tissue anomalies (Minor et al., 1974). CD-1 rats were
treated on days 6 - 15 of gestation, by gavage, with 0, 11, or
114 mg ferbam/kg body weight. Twenty-five percent of the dams
administered 114 mg/kg died, but the surviving dams showed small
litters, increased resorptions, and decreased fetal weight.
Also, a number of malformations (unossified sternebrae,
malformed cranium, hydrocephalus, and cleft palate) were found.
When thiram was administered at doses of 0, 40, 90, 136,
164, or 200 mg/kg body weight on days 6 - 15 or 7 - 12 of
gestation, the 200 mg/kg dose reduced the number of mated rats
that delivered litters, and only 33% of the dams survived. At
doses of 136 mg/kg or more, a decrease in the number of implants
and fetuses per dam, an increase in resorptions, a decrease in
fetal body weight, and an increase in malformations, as
described for ferbam, were observed (Short et al., 1976).
8.4.2.2. Mouse
Pregnant female NMRI and Swiss-Webster mice were treated
orally during days 6 - 17 of pregnancy with thiram at 179, 357,
714, or 1071 mg/kg body weight and 250, 500, 1000, and
1500 mg/kg body weight, respectively. Increased resorption of
embryos, clearly retarded fetal development, and skeletal
malformation (cleft palate, wavy ribs, curved long bones of
extremities, and micrognathia) were seen in both strains. The
12th and 13th days seemed to be the most sensitive period of
embryonic development. The lowest dose had only a slight
effect, but the next dose level was clearly teratogenic (Roll,
1971; Matthiaschk, 1973).
Thiram did not reduce body weight gain during gestation at
doses of 100 or 300 mg/kg body weight, administered on days
6 - 14, and no changes in litter size, incidence of resorptions,
or fetal weight were observed. However, an increase in malform-
ations was seen (Short et al., 1976).
In studies by Larsson et al. (1976), doses of 0, 400, 770,
or 1420 mg maneb/kg body weight or 0, 380, 730, or 1330 mg
mancozeb/kg body weight were given on a single occasion to NMRI
mice on days 9 or 13, and mice were sacrificed on day 18 of
gestation. No adverse maternal or fetal effects could be
detected.
In a study on CD1 mice administered 0, 375, 750, or 1500 mg
maneb/kg body weight on days 7 - 16 of gestation, maternal
toxicity was found at the highest dose level, together with a
decrease in fetal caudal ossification centres at all dose levels
(Chernoff et al., 1979).
Ferbam administered to mice at 30 and 300 mg/kg body weight
on days 6 - 16 of gestation did not produce any teratogenic
effects (Minor et al., 1974), and, when given at 23 or 228 mg/kg
body weight on days 6 - 14, it did not affect the survival or
body weight of Swiss-Webster dams during gestation. No changes
in litter size, incidence of resorptions, or fetal weight were
observed, but, at the highest dose level, an increase in
malformations was seen (Short et al., 1976).
Groups of CD-1 mice were administered zineb (85.5% purity)
daily, at dose levels of 0, 200, 632, or 2000 mg/kg body weight
per day, for 11 days from day 6 of gestation and sacrificed on
day 18. Gross examination for maternal well-being and fetal
anomalies, both somatic and skeletal, failed to show any
teratogenic effects (Short et al., 1980).
8.4.3. Embryotoxicity
Korhonen et al. (1982a,b) used a system called chicken
embryo test to study the embryotoxic potential of dithio-
carbamates and found early and late death and malformed embryos.
It was thought that this test could have a predictive value as a
simple teratogenicity test, but many limitations were found in
doing so, and the interpretation of the results were difficult.
8.5. Mutagenicity and Related End-Points
Seiler (1973) studied the mutagenicity of maneb and ziram.
Maneb proved negative in tests with Salmonella strains his G46,
TA1530, TA1531, TA1532, and doubtful in TA1534. Ziram was
positive in TA1534, doubtful in TA1530, and negative in the
other strains.
In studies by Fahrig (1974), ziram was non-mutagenic in a
variety of other microorganisms (Escherichia coli, Serrata
marcescens, and Saccharomyces cerevisiae), but Pilinskaya (1971)
found that it induced chromosome breaks, most of them confined
to chromosome 2, in cultured peripheral human lymphocytes.
Shirasu et al. (1976) studied mutagenicity with the rec-
assay procedure, a sensitivity test using H17 rec+ and M45 rec-
strains of Bacillus subtilis, and with reversion assays on
plates using E. coli (WP2) and S. typhimurium TA1535, TA1536,
TA1537, and TA1538. In these tests, ferbam, thiram, and ziram
were non-mutagenic.
Certain dithiocarbamates given intraperitoneally to mice at
100 mg/kg body weight caused chromatid aberrations in bone
marrow cells (Kurinny & Kondratenko, 1972; Hedenstedt et al.,
1979), the order of effectiveness being thiram > ziram > maneb
and zineb. Thiram was also shown to induce gene mutations in
Salmonella and Aspergillus (Szymezyk, 1981; Zdienicka et al.,
1981).
Hedenstedt et al. (1979) found that the mutagenic effect of
tetramethylthiuram monosulfide (TMTM) was enhanced in the
presence of metabolizing systems (S-9 mix), but that tetraethyl-
thiuram disulfide (TETD or AntabuseR) was not mutagenic.
Propylene bisdithiocarbamate was tested for cytogenicity by
bone marrow analysis and for dominant lethal mutations by giving
a single oral dose to male rats. There was a considerable
increase in the number of chromosomal aberrations (chromatid
fragments), reaching a maximum after 24 h (Vachkova-Petrova,
1977). In studies on the cytotoxic effects of ziram on cultures
of human lymphocytes in vitro (Pilinskaya, 1971), the ratio of
chromatid-type aberrations to chromosome-type aberrations was
2.7:1, which suggests that most chromosomal damage took place at
the S-stage and the G2 stage of the mitotic cycle. Ziram-
induced chromosomal breaks were observed to be non-random, most
of them occurring in chromosome 2.
Propineb and its main metabolite propylenethiourea (PTU)
were investigated by the micronucleus test in mice. The
following doses were given by ip injection twice, with a 24-h
interval: propineb (unknown purity), 62.5, 125, or 250 mg/kg
body weight in a 5% aqueous solution of Tween 80; propineb (78%
purity), the same doses, but in 5% gum arabic; and PTU, 100,
200, 400, or 600 mg/kg body weight in distilled water. Controls
received methanesulfonate at doses of 10, 20, 40, or 80 mg/kg
(twice, in distilled water) and mitomycin at 1.75, 3.5, 7, or
14 mg/kg (twice, in distilled water). No statistically
significant increase in the percentage of micronuclei was
observed at any of the tested doses of propineb or PTU. The
positive control groups showed the expected dose-related
increase in the number of polychromatic erythrocytes with
micronuclei (Rolandi et al., 1984).
Vachkova-Petrova (1981) studied the mutagenic potential of
EndodanR (ethylenethiuram monosulfide) in short-term studies.
Several doses of EndodanR were administered to groups of 6 rats
either twice at an interval of 24 h or for 5 successive days,
and the animals were killed 6 h after the last dose. The cells
in metaphase were analysed for aneuploidy and aberrations, but
no mutagenic effects that could be attributed to the chemical
were detected.
Zineb and ziram were not mutagenic when tested in Drosophila
melanogaster (Benes & Sram, 1969).
8.6. Carcinogenicity
8.6.1. Mouse
In studies by Innes et al. (1969), groups of 18 mice of each
sex from two hybrid strains were given various dithiocarbamates
from 7 days of age up to 18 months. The compounds were given
daily, by gavage, from day 7 to weaning and thereafter added to
the diet. The compounds and the respective amounts were: ferbam
at 10 mg/kg body weight, then 32 mg/kg diet; maneb at 46.4 mg/kg
body weight, then 158 mg/kg diet; nabam at 21.5 mg/kg body
weight, then 73 mg/kg diet; thiram at 10 mg/kg body weight, then
26 mg/kg diet; and zineb at 464 mg/kg body weight, then
1298 mg/kg diet. No significant increase in tumours was found.
On the basis of all experimental data and experimental
designs, IARC (1976) suggested that there was no definite proof
for the carcinogenicity of maneb, though ETU, one of its meta-
bolites, was able to produce thyroid carcinomas. However, zineb
and maneb have been reported to induce pulmonary adenomas in
mice when treated orally (Chernov & Khistenko, 1969; Balin,
1970).
8.6.2. Rat
A 2-year feeding study of the effects of zineb on rats was
carried out using 60 young male and 60 female albino rats.
These were divided into groups of 10 each and administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg/kg diet.
Growth, mortality, haematology, and organ weights were examined
and histopathology was carried out. No clear influence on
growth and mortality was observed, and haematological findings
were within normal limits. A goitrogenic effect (hyperplasia)
was observed in 50% of the animals at 500 mg/kg, and, at
1000 mg/kg diet or more, this effect was more pronounced. The
interpretation of thyroid weight/body weight ratio was
complicated because of the small number of animals still alive.
Microscopically, no evidence of malignancies was present. At
the highest dose level, kidney damage (congestion, nephritis,
nephrosis) was seen. Although 10 000 mg zineb/kg diet produced
moderate goitrogenic effects, this effect was also seen at
500 mg/kg in some rats, and was not clearly dose dependent
(Blackwell-Smith et al., 1953).
Cases of goitre and thyroid adenoma were found in Sprague
Dawley rats fed for 2 years on a diet containing 120 mg or
360 mg metiram/kg diet (Griepentrog, 1962).
8.6.3. Dog
When nine mongrel dogs (three groups of three animals each)
were administered 20, 2000, or 10 000 mg zineb/kg diet for 1
year, no haematological changes were found. The thyroids of the
group given the highest dose level were enlarged and showed
hyper-plastic changes, but, at lower dose levels, they did not
show any histological changes (Blackwell-Smith et al., 1953).
Doses of 45 mg metiram/kg body weight for 90 days or
7.5 mg/kg body weight for 23 months did not cause any ill
effects (Worthing & Walker, 1983).
8.6.4. Dithiocarbamates in combination with nitrite
The above-mentioned studies were carried out with individual
dithiocarbamates. Other studies have shown that these
dithiocarbamates, in the presence of nitrite, can be converted
to N-nitroso derivatives, which may be carcinogenic.
Thiram, ferbam, ziram (Eisenbrand et al., 1974; Sen et al.,
1974), and disulfiram (Lijinsky et al., 1972; Elespuru &
Lijinsky, 1973) react with nitrite under mildly acidic con-
ditions to form N-nitroso compounds. Formation of N-nitroso-
dimethylamine (NDMA) by the action of microorganisms in sewage
and soil containing 0.1% thiram has been reported to occur under
experimental conditions (Ayanaba et al., 1973). Nitrite is
formed by the reduction of nitrate, which can be found in some
unrefrigerated vegetables (e.g., spinach and beets), especially
after cooking (Phillips, 1968), in human saliva (Tannenbaum et
al., 1974), and in cured meats. Thus, in vivo nitrosation of
dithiocarbamates in the stomach cannot be totally excluded.
As has been pointed out by IARC (1976), the extrapolation of
findings in experimental animals to man is complicated by many
factors. It is relatively easy to show that N-nitroso
derivatives can be formed and that these are mutagenic and/or
carcinogenic. The crucial information, however, is the quantity
produced in man under the prevailing conditions. The concen-
tration of both reactants, the pH, the influence of competing
reactions, and the presence of accelerators and inhibitors are
all important. In addition to these difficulties in defining
potential human exposure, the susceptibility of man, compared
with that of experimental animals, has to be considered.
Sen et al. (1974) concluded that it is unlikely that
significant amounts of NDMA would be produced from the ingestion
of trace amounts of the dithiocarbamates and the normal intake
of nitrite.
8.7. Mechanisms of Toxicity; Mode of Action
8.7.1. Thyroid
In weaning rats, a diet containing 500 mg nabam/kg given for
9 days caused thyroid hyperplasia and a decrease in the weight
of the thymus (Seifter & Ehrich, 1948).
Male and female albino rats were administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg zineb/kg diet
for up to 30 days. Animals were killed sequentially in order to
study the changes in the thyroid gland. Only in the 10 000 mg/kg
group were effects seen. In two out of five males and in one
out of five females, hyperplasia of the thyroid was observed
(Blackwell-Smith et al., 1953). Przezdziecki et al. (1969) fed
female Wistar rats a diet containing 1300 mg zineb/kg or 1875 mg
maneb/kg diet for 7 months. Significant increases in the weight
of the thyroid gland and decreases in the weight of the kidneys,
adrenal glands, and ovaries were observed.
Thyroid hyperplasia has been reported in rats given maneb,
zineb, or mancozeb in amounts ranging from 500 to 2500 mg/kg
diet for periods of up to 2 years (FAO/WHO, 1965b, 1971b). In a
2-year feeding study, 2500 mg maneb/kg diet produced thyroid
hyperplasia and nodular goitre and increased mortality, but 1250
and 250 mg/kg diet did not cause any ill effects (FAO/WHO,
1965b).
Zineb was given orally to white rats at dose levels of 96 or
960 mg/kg body weight for 4.5 months. Compared with that of
untreated animals, the thyroid was enlarged with microfollicles
and columnar cells. Succinic dehydrogenase and cytochrome
oxidase activities were raised in these cells, while the colloid
in the follicles showed reduced PASa-positive granules. These
changes were consistent with an increase in thyroid-stimulating
hormone (TSH). An increased number of basophilic cells con-
taining PASa-positive granules was observed in the adeno-
hypophysis (anterior pituitary). These effects were seen only
at the highest dose level. The uptake of 131iodine was also
increased at the highest dose level, and a high plasma TSH level
was recorded in treated animals. The changes observed in both
thyroid and pituitary were probably a compensatory response to
the antithyroid effect of the dithiocarbamate (Ivanova-
Chemishanska et al., 1975b).
After oral administration of zineb to rats at dose levels of
9.6 or 960 mg/kg body weight, twice a week, for 4“ months, the
gonadotropic and thyroid-stimulating functions of the adeno-
hypophysis were significantly increased compared with those of
control values, more markedly in those receiving the higher dose
(Ivanova-Chemishanska et al., 1974).
Albino rats were orally administered doses of 2400 mg
zineb/kg or 3500 mg maneb/kg body weight, and, after 24 h,
radioactive iodine was administered intraperitoneally. Reduced
assimilation of 131iodine by the thyroid was found, which
suggests that the dithiocarbamates, or certain of their
metabolites, possess a marked antithyroid effect and inhibit the
synthesis of thyroxine (Ivanova-Chemishanska et al., 1967,
1974). In similar studies with mancozeb, a single oral dose of
7000 mg/kg body weight resulted in a decreased uptake of
131iodine (Ivanova-Chemishanska et al., 1967).
The condition of the thyroid gland was studied in male
albino rats, which were administered 700 mg maneb/kg, 960 mg
zineb/kg, or 1400 mg mancozeb/kg body weight. After 30 days,
the distinct morphological changes observed indicated
stimulation of the thyroid by TSH. Hypophyseal stimulation is
the consequence of release from negative feedback by thyroxine,
the plasma level of which is depressed by the action of EBDCs
(Ivanova-Chemishanska et al., 1971, 1974).
Rats fed a diet containing 10 000 mg metiram/kg for 2 weeks
showed increased thyroid weight and a decrease in the uptake of
131iodine, but no ill effects were produced by 1000 mg/kg diet
(Worthing & Walker, 1983).
----------------------------------------------------------------
a PAS = periodic acid-Schift reagent.
8.7.2. Interaction of dithiocarbamates and alcohol
Hald et al. (1948) found that dithiocarbamates interact with
ethanol (ethyl alcohol), and since then, certain dithio-
carbamates, particularly disulfiram, have been used in the
treatment of chronic alcoholism. Disulfiram has been proposed
to act in two different ways. The first possibility is that the
drug or one of its metabolites (e.g., diethyldithiocarbamate,
carbon disulfide) interferes with the normal metabolism of
ethanol and, consequently, gives rise to an accumulation of
toxic amounts of intermediary products, such as acetaldehyde.
The second possible method of action is that ethanol interferes
with the normal metabolism of disulfiram and therefore makes
disulfiram more toxic in some way.
Ethanol is detoxified in many tissues, particularly the
liver, by oxidation, firstly to acetaldehyde, then to acetic
acid, and finally to carbon dioxide and water. Disulfiram
interferes with various enzyme systems including those involved
in the oxidation of ethanol. After administration of disulfiram,
the blood acetaldehyde level increases significantly.
Peripheral neuropathy and optic neuritis have been observed in
alcoholics treated with 125 - 150 mg disulfiram per day
(Gardner-Thorpe & Benjamin, 1971).
Van Logten (1972) studied this phenomenon of alcohol
intolerance extensively in rats. Zineb and maneb did not induce
alcohol intolerance, whereas most of the alkyldithiocarbamates
(such as ziram and nabam) and thiuram sulfides (such as thiram)
did. In general, the dithiocarbamates with a free H atom bound
to the N atom did not induce intolerance. Apart from the
accumulation of acetaldehyde in blood, disturbance of sulfo-
bromophthalein (BSP) elimination, increased serum glutamic-
pyruvic transaminase, hypothermia, increased glucose content,
changes in blood morphology, atrophy of spleen and thymus, and
increase in the weight of adrenals and brain were all observed.
Studies on adrenalectomized rats showed the involvement of the
adrenals in the alcohol intolerance. Hyperglycaemia, eosino-
penia, lymphopenia, and neutrophilia were not seen in the
animals without adrenals, and spleen and thymus atrophy was
reduced. However, the effect on the red blood cells was more
pronounced, and the accumulation of acetaldehyde in the blood
was unaffected by adrenalectomy.
Oral treatment of rats with ethanol after administration of
alkyldithiocarbamates or thiuram sulfides lowers the catechol-
amine content of the adrenals. Since the lowest level was not
reached until 24 h or more after alcohol treatment, it seems
likely that the influence on the adrenals of the dithio-
carbamate-ethanol interaction is of secondary importance. There
was no clear indication that the ethanol intolerance in the rat
is accompanied by changes in brain catecholamine level.
As in human beings, the dithiocarbamate-ethanol reaction in
the rat is characterized by a severe hypotension, which starts
almost immediately after the administration of the ethanol and
lasts at least 8 h. It is evident that, during alcohol
intolerance, body fluids shift from the plasma into the
interstitial tissue or cells, possibly thereby causing the hypo-
tension or shock. The observed hypothermia also starts almost
immediately after ethanol administration and may last for many
hours. Adrenalectomy did not prevent the hypothermia.
Therefore, this phenomenon must be considered as a primary
effect, along with the plasma accumulation of acetaldehyde.
Since heat loss is not increased during the dithiocarbamate-
ethanol reaction, the hypothermia is probably due to decreased
heat production. However, the serotonin concentration in the
brain was increased, and so a disturbance of the thermo-
regulation cannot be ruled out. It seems doubtful that the
dithiocarbamate-ethanol reaction is due to acetaldehyde per se.
Intraperitoneal injection of acetaldehyde, which resulted in a
blood level twice as high as during the dithiocarbamate-ethanol
reaction, did not influence BSP elimination, serum glucose
level, body temperature, organ weights, catecholamine content of
the adrenals, or blood pressure.
No indications were available that the accumulation of
acetaldehyde in the blood was due to an accelerated biotrans-
formation of alcohol. More work needs to be done to decide
whether the accumulation of acetaldehyde and pyruvate in the
blood is a consequence of a disturbance of carbohydrate
metabolism. The specificity of ethanol is remarkable. The
combination of thiram with either methanol or 1-propanol has no
effect on BSP elimination or on blood glucose level.
The sensitivity of the rat for the dithiocarbamate-alcohol
reaction is of the same magnitude as that of man. Administra-
tion of 1.9 mg thiram/kg body weight in the rat elicits an
accumulation of acetaldehyde in the blood. Thus, it may be
concluded that 60 ml of gin, 0.5 litre of beer, or even less
should be sufficient to induce alcohol intolerance.
Eight days after the administration of certain dithio-
carbamates, a dithiocarbamate-alcohol reaction may be observed
when ethanol is given. The maximum level of acetaldehyde in the
blood is reached within 15 min of ethanol treatment.
A 90-day study with several dietary levels of thiram
revealed a no-toxic-effect level of 100 mg/kg diet. After
feeding rats with 10 mg thiram/kg diet for 6 weeks, oral
administration of a single dose of 6 ml ethanol/kg body weight
caused a significant decrease in the body temperature. Higher
doses of thiram with alcohol induced hyperglycaemia, accumula-
tion of acetaldehyde in the blood, and other abnormalities. In
contrast, the combination of 100 mg thiram/kg diet and 5%
ethanol continuously in the drinking-water did not have any
effect (Van Logten, 1972).
8.7.3. Neurotoxicity
In an 80-week study on the neurotoxic and behavioural
effects of thiram, 12 male and 12 female rats per group were fed
thiram at dose levels of 0, 100, 400, or 1000 mg/kg diet (the
concentration of the compound in the diet was periodically
increased in order to give a relatively constant consumption on
the basis of body weight). A second study was carried out on
two groups of 24 female rats administered 0 or 1000 mg thiram/kg
diet, for 36 weeks. The neurotoxic effects were characterized
by ataxia and paralysis of the hind legs, although these effects
were only seen at the highest dose level (1000 mg/kg diet,
equivalent to 65 mg/kg body weight) in females. Demyelination,
degeneration of the axon cylinders, and the presence of
macrophages in the nerve bundle of the sciatic nerve were seen.
Degeneration in the ventral horn of the lower lumbar region of
the spinal cord was demonstrated by chromatolysis of motor
neurons, pyknosis, and satellitosis. Electromyograms indicated
a loss of motor unit function, and the histopathology suggested
that the peripheral nerve is the primary site of the lesion (Lee
& Peters, 1976).
In another study, groups of 12 males and 12 females were fed
ferbam in the dose levels that gave actual intake levels of
approximately 8.5, 34, and 87 mg/kg body weight (average of
males and females) per day. The neurotoxic effects of ferbam
are less than those of thiram. In this study, only 3 of the 24
rats fed the highest dose level developed ataxia or paralysis
(Lee & Peters, 1976). Neurotoxic effects have also been observed
for ziram by Hodge et al. (1956).
In a study on rats, zineb (490 and 2450 mg/kg body weight),
maneb (350 and 1750 mg/kg body weight), and mancozeb (700 and
3500 mg/kg body weight) were administered orally at twice weekly
doses for 4 months. Mortality was high, and paresis in the hind
limbs appeared in the third month of the study and progressed to
complete paralysis (Ivanova-Chemishanska, 1969a).
Dishovski & Ivanova-Chemishanska (1979) studied the ultra-
structural changes in the neocortex of rats repeatedly
administered propineb (70% purity) at 85 mg/kg and 425 mg/kg
body weight for 40 days. At the higher dose level, intense
ultrastructural changes in the sensorimotor neocortex were
detected using an electron microscope, primarily affecting the
pyramidal cells. The concentration of ribosomes and hypertrophy
of the Golgi apparatus suggested an increase in synthetic
processes in the neurons.
Edington & Howell (1966, 1969) found lesions in the central
nervous system of adult Dutch-New Zealand rabbits who were given
ip injections of sodium diethyldithiocarbamate (NDDC) at
330 mg/kg body weight, for 6 days/week, for 30 weeks. The first
changes were seen at 6 weeks in the accessory cuneate nucleus
and in Clarke's column; 12 weeks later, degeneration was seen in
the spinocerebellar tracts in the cerebellum medulla. After 24
weeks, severe nerve fibre degeneration in the peripheral white
matter of the spinal cord (both involved the axon and myelin
sheath) was observed. It was suggested that these changes might
be connected with changes in the level of copper in the serum.
Kim & Rizzuto (1975) studied the effect of NDDC (0.23, 2.3,
and 23 µg/ml nutrient medium) on myelinated cultures of new-
born mouse cerebellum. Exposure time was 24 - 120 h, and the
cultures were examined by light and electron microscopy.
Treatment of the cultures for 24 - 48 h produced swelling of
axons and presynaptic endings, morphologically characteristic of
dystrophic axons. Continued exposure induced an extensive
degeneration of axons and myelin sheath (Wallerian degeneration
in axons).
8.7.4. Dithiocarbamates in combination with metals
Truhaut et al. (1971) studied the chelating action of sodium
diethyldithiocarbamate to copper and the fact that this element
is indispensable for the activity of dopamine beta-hydroxylase.
The authors put forward the hypothesis that the inhibition of
this enzyme system, which catalyses the conversion of dopamine
to norepinephrine and participates in the biogenesis of cate-
cholamines in the central nervous system, may play a role in the
etiology of neurotoxic effects.
Maj et al. (1970) studied the effect of disulfiram, diethyl-
dithiocarbamate (DDC), and dimethyldithiocarbamate on serotonin
(5-HT) and 5-hydroxyindole-3-acetic acid (5-HIAA) in the brain
of rats. The total dose levels ranged from 150 to 500 mg/kg
body weight. It was concluded that these three compounds do not
affect the 5-HT level in the rat brain. The 5-HIAA levels
increased, but not significantly.
Possibly, reactions of carbon disulfide with pyridoxamine
could lead to the depletion of pyridoxal phosphate in the
tissues, which may, in turn, cause neurological changes. Long-
term poisoning of rabbits with carbon disulfide has been shown
to result in increased excretion of zinc in the urine and
disturbances of copper and zinc concentrations in the tissues.
Also, after NDDC treatment, increased levels of copper in the
liver and nervous tissue have been found (Cavanagh, 1973).
Aaseth et al. (1981) showed that oral treatment of Wistar
rats with tetramethylthiuram disulfide (TMTD) at 1000 mg/kg diet
for one week increased the brain levels of endogenous copper
and zinc. In further studies, rats were administered an iv
injection of 203HgCl2 (5 µmol/kg body weight in saline) at day
17 of pregnancy. DDC was given immediately after the mercury
injection (500 µmol/kg body weight). The maternal brain
concentration of mercury increased significantly, and the kidney
levels, measured after 24 and 48 h, also increased. In the
fetuses, the mercury in the brain, liver, kidneys, and blood
(but also in the placenta) were significantly increased after
24 h, but, after 72 h, only the levels in fetal blood were still
elevated. Mice of the NMRI strain were similarly injected with
203HgCl2 (2.5 µmol/kg body weight) and fed diets containing DDC
(10 000 mg/kg diet), disulfiram and TMTD (1000 mg/kg diet), or
carbon disulfide (3000 mg/kg diet) for 4 days. The brain level
of mercury was significantly increased after DDC or TMTD
treatment and marginally after disulfiram or carbon disulfide
treatment (Aaseth et al., 1981).
Lakomaa et al. (1982) studied the effect of DDC on copper
and zinc concentrations in different regions of the brain of
Long-Evans rats during acute or repeated treatment. Acute
treatment (250 mg/kg body weight) produced no effect after 24 h,
whereas repeated treatment (250 mg/kg, 5 times per week, for
4 weeks) increased copper levels in the brain stem, cortex,
hippocampus, and the rest of the brain, but did not alter zinc
concentrations.
Dithiocarbamates, with their metal-chelating properties, and
thiuram derivatives, have been demonstrated to cause a marked
increase in the concentration of lead in the brain as well as a
redistribution of lead in the rest of the body (Oskarsson, 1983,
1984; Danielsson et al., 1984). Thus, after injection of a dose
of labelled lead (203Pb), the brain concentrations were
increased by up to 100 times in thiuram-treated rats.
Male Sprague Dawley rats (10 groups of 5 rats each) were
administered different combinations of thiram, disulfiram, DDC,
or dimethyldithiocarbamate in combination with sodium or lead.
The study demonstrated that treatment with dithiocarbamates and
thiram derivatives in rats exposed for 6 weeks to lead causes a
substantial (up to 4-fold) increase in the lead concentration of
the brain. This effect can be explained by the formation of a
lipophilic lead-dithiocarbamate complex, which probably is
retained longer and has a higher capacity to penetrate the
blood-brain barrier and bind to lipid-rich brain tissue compo-
nents than inorganic lead itself. The chemical form of the lead
when it is in the brain remains uncertain. The lead complex may
decompose in the brain into inorganic lead, which exerts a
neurotoxic effect, or it may be very stable in the brain and of
low toxicity for the central nervous system (Oskarsson & Lind,
1985).
There are several reports on the effect of dithiocarbamates
on the distribution in the body of other metal ions such as
cadmium, thallium, nickel, copper, zinc, and mercury (Oskarsson
& Lind, 1985).
DDC has been shown to have a strong inducing effect on
levels of metallothionein, a low molecular weight, heavy-metal-
binding protein, in rat liver and kidney. The mechanism
probably reflects enhanced uptake of copper and depletion of
hepatic glutathione (Sunderman & Fraser, 1983).
8.7.5. Miscellaneous reactions
Dithiocarbamates, with their chelating capacity, also
interfere with a number of enzyme systems containing metals such
as zinc and copper (e.g., dopamine beta-hydroxylase). They also
inhibit sulfhydryl (SH)-containing enzymes and a number of other
enzyme systems involved in glucose metabolism (e.g., hexokinase,
glyceraldehyde-3-phosphate dehydrogenase, and glucose-6-
phosphate dehydrogenase). The effect of dithiocarbamates on
liver enzymes has consequences for the metabolism of other
chemicals. Thus, the toxicity of carbon tetrachloride is
decreased by diethyldithiocarbamate (Lange & Jung, 1971; Lutz et
al., 1973), and the toxicity of other chemicals, e.g., ethyl
alcohol, may be increased (section 8.7.2).
9. EFFECTS ON MAN
9.1. Occupational Exposure
9.1.1. Acute toxicity - poisoning incidents
The acute toxicity of dithiocarbamates is low and, there-
fore, acute intoxication in human beings is unlikely to occur.
A case was reported of a 62-year-old man with acute kidney
insufficiency after maneb application. However, the precise
cause of maneb exposure was not clear, since the patient had a
history of hypertension, cerebral infarction, gastrectomy
because of stomach cancer, and chemotherapy. The patient was
treated with haemodialysis and was discharged from hospital
(Koizumi et al., 1979).
Thiram (100 mg/m3) has been shown to cause headaches,
vertigo, impairment of mental capacity, muscle twitch, and
paraesthaesia (Sprecher & Grigorowa, 1967).
9.1.2. Case reports, short-term and epidemiological studies
9.1.2.1. Dermal
The irritant and allergic potential of most dithiocarbamates
is evident in occupational exposure. Skin irritation and
sensitization were studied in man using a conventional patch
test. A cotton square was dipped in 19% nabam solution and
placed on the inner surface of the forearm, and, 14 days later,
this procedure was repeated on the opposite forearm. Zineb was
tested in the same manner, except that the cotton square was
dipped in 65% wettable powder. The patches were left in place
for 48 h. Of the 25 subjects included in the nabam study, 2
showed irritation (mild erythema and itching). Thirteen of the
25 reacted to the retest (from mild erythema to severe erythema,
oedema, and vesiculation), indicating sensitization. Of the 50
subjects used in the zineb study, no reaction at all was seen in
49 of them. One reacted in such a way that it indicated primary
irritation rather than sensitization (Blackwell-Smith et al.,
1953). Schultheiss (1957) reported a case of contact dermatitis
with thiram. Zadorozhny et al. (1981) found dermatitis and
eczema in 241 industrial workers exposed to TMTD and other types
of pesticides. Twenty-one of them showed contact dermatitis, 25
allergic dermatitis, and 7 eczema.
Cases of diffuse erythema and eczematoid epidermatitis of
the eyelids and inguinal regions, probably with elements of sun
sensitization, were observed among agricultural workers (grape
and tobacco industries) in contact with zineb (Babini, 1966) or
maneb (Laborie & Laborie, 1966; Zorin, 1970). These were
largely allergic in character with only a few manifestations of
contact dermatitis. Decreased resistance of the workers,
vitamin deficiency, chronic liver disease, and other factors
apparently contributed to these effects.
9.1.2.2. Exposure via different routes
Kaskevich et al. (1981) carried out an epidemiological study
on 137 workers engaged in zineb manufacturing (51 men and 86
women). The duration of exposure to zineb for 52 workers was
between 1 and 3 years, and for 85 workers between 4 and 5 years.
Control groups in this study consisted of 193 persons, not
exposed to chemicals and matched for age, period of employment
rate, and sex. The concentrations in the air of the working
area never exceeded 1 mg/m3. Among workers occupationally
exposed to zineb, the following changes were found: hepato-
cholecystitis (28.4% of workers, versus 13.5% in controls);
vegetovascular dystonia connected with disorders in the central
nervous system (34.9%, versus 22.3% in controls); chronic
bronchitis (4.4%, versus 0.5% in controls); contact dermatitis
(11.9%, versus 0.1% in controls); and disorders in the menstrual
cycle (16.91%, versus 4.3% in controls). These studies indicate
a change in catecholamine metabolism.
In a study with cultured lymphocytes from 15 workers working
in different stages of zineb manufacture, the mean incidence of
aberrant metaphases was 6% greater than that in controls. The
incidence of chromosomal aberrations (chromatid breaks) in
cultured human lymphocytes treated with maneb (0.5, 15, or
30 µg/ml) was 10 - 20% greater than in controls (Antonovich et
al., 1972).
A number of studies on maneb and mancozeb production workers
have been carried out. In the earliest study (1965), 54 produc-
tion workers were given medical examinations that included blood
and urine analyses. Since this study predated the availability
of immunoassay techniques for thyroid hormone determination,
protein-bound iodine was used as a measure of thyroid function.
No thyroid or other medical abnormalities could be attributed to
EBDC exposure. In a second study (1975), 57 exposed and 98
unexposed production workers were examined for thyroid function
by measuring triiodothyronin (T3), thyroxine (T4), and TSH.
Again, no effects attributable to work-place exposure were
identified. Workers exposed to EBDC levels ranging from 0.13 to
5.46 mg/m3 were found to have elevated ETU and manganese levels
in the urine. In a 1976 mortality study, 992 past and present
production workers (over the period 1948-75) were studied.
Compared with the local general population, neither the overall
death rate nor the death rate due to cancer was elevated. The
number of cancer deaths observed (10) was too small to evaluate
cancer-specific mortality (Gowers & Gordon, 1980).
In the most extensive study, 42 currently exposed and 112
previously exposed workers were compared with equal size control
groups matched for age, period of employment, race, and type of
job. All participants were given thorough physical examinations
by specialists in diagnostic medicine, including detailed
questionnaires and interviews about health history and family
health. A separate thyroid examination was carried out by
thyroid specialists. Thyroid parameters that were measured
included total T3, T3 resin uptake, T4, TSH, free T4 index,
thyroglobulin antibodies, and microsomal antibodies. In
addition, urine was analysed for ETU, EBDC, zinc, manganese,
creatine, iodide, specific gravity, and pH. Blood levels of
glucose, urea nitrogen, sodium, potassium, calcium, chloride,
carbon dioxide, cholesterol, total protein, protein albumin,
bilirubin, uric acid, creatinine, inorganic phosphate, lactic
dehydrogenase, and serum glutamic oxaloacetic transaminase were
also determined. As in earlier studies, the occurrence of
unusually high levels of ETU in the urine of currently exposed
workers confirmed their exposure. However, a detailed
statistical analysis of the data revealed no differences in
thyroid function, blood and urine indicators of liver and kidney
function, or general health, between exposed and control groups
(Gowers & Gordon, 1980; Charkes et al., 1985).
PART B
ETHYLENETHIOUREA (ETU) AND PROPYLENETHIOUREA (PTU)
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLENETHIOUREA (ETU)
AND PROPYLENETHIOUREA (PTU)
INTRODUCTION
1. SUMMARY
1.1 Sources, environmental transport and distribution
1.2 Environmental levels and human exposure
1.3 Kinetics and metabolism
1.4 Effects on organisms in the environment
1.5 Effects on experimental animals and in vitro test systems
1.5.1 Ethylenethiourea
1.5.2 Propylenethiourea
1.6 Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
2.3.1 Extraction
2.3.2 Clean-up
2.3.3 Derivatization
2.3.4 Determination
2.3.4.1 Gas-liquid chromatography (GLC)
2.3.4.2 Thin-layer chromatography (TLC)
2.3.4.3 Polarography
2.3.4.4 Radioisotope dilution
2.3.4.5 High-pressure liquid chromatography (HPLC)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Soil
4.2 Water
4.3 Plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Food and drinking-water
5.2 Monitoring and market basket studies
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and excretion
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short- and long-term exposures
8.3 Teratogenicity
8.4 Mutagenicity
8.5 Carcinogenicity
8.5.1 Mouse
8.5.2 Rat
8.5.3 Hamster
8.6 ETU in combination with nitrite
8.7 Mechanisms of toxicity; mode of action
8.8 Probineb and Propylenethiourea (PTU)
8.8.1 General
8.8.2 Toxicological information
9. EFFECTS ON MAN
9.1 Epidemiological studies
INTRODUCTION
One of the metabolic products of ethylene bisdithiocarbamate
decomposition in mammals, plants, and lower organisms is
ethylenethiourea (ETU). It may also be present as an impurity
in these dithiocarbamates, and their residues on crops may be
partly transformed into ETU during food processing. A comparable
breakdown takes place with propineb, giving rise to propylene-
thiourea (PTU).
1. SUMMARY
1.1. Sources, Environmental Transport, and Distribution
Ethylenethiourea (ETU) is found together with residues of
the parent ethylene bisdithiocarbamates (EBDCs) in and on crops
that have been treated with these pesticides. During storage,
processing, and cooking, the amount of the parent compound
decreases while that of ETU increases. ETU is easily photo-
oxidized (in the presence of photosensitizers) to ethyleneurea
(EU).
1.2. Environmental Levels and Human Exposure
In certain crops, such as spinach, carrots, and potatoes,
treated with EBDCs, high levels of ETU can be found after
cooking. In general, however, the ETU levels are below
0.1 mg/kg product.
Estimates of the exposure of the general population of the
USA are of the order of 0.24 - 3.65 µg ETU/kg body weight per
day, and, in Canada, estimates based on market-basket studies
are around 1 µg ETU/kg body weight per day.
1.3. Kinetics and Metabolism
ETU is rapidly absorbed, metabolized, and excreted in
mammals. Up to 90% is eliminated via the urine and only a
small amount via the faeces. Distribution of ETU in the body
appears to be fairly uniform with the exception of a relative
accumulation in the thyroid. ETU is broken down to ethylene
diamine (EDA), urea, carbon dioxide, or oxalic acid, or is
transformed to imidazole derivatives in mammals, plants, and the
environment.
1.4. Effects on Organisms in the Environment
The available LC50 levels of ETU and EU for fish are in the
range of 7500 - 13 000 mg/litre.
1.5. Effects on Experimental Animals and In Vitro Test Systems
1.5.1. Ethylenethiourea
The acute oral toxicity in experimental animals is low, and
the long-term effects are mainly characterized by an antithyroid
action.
At dose levels > 25 mg/kg body weight, decreases in serum
T3, T4, and protein-bound iodine (PBI) and increases in thyroid-
stimulating hormone (TSH) have been found in studies on
experimental animals. At higher dose levels (> 100 mg/kg body
weight), increases in thyroid weight and hyperplasia occurred,
which finally resulted in the development of adenocarcinoma.
The effects of short-term exposure to low levels of ETU seem to
be reversible, but those of long-term exposure to higher levels
become, at a certain stage, irreversible. A level of
approximately 5 mg/kg body weight seems to be without effects.
Most mutagenicity studies on ETU, especially those with
mammalian test systems, have given negative results.
A number of carcinogenicity studies have been carried out on
mice, rats, and hamsters. In addition to an antithyroid action,
ETU has been found to induce, subsequently, thyroid tumours
(hyperplastic goitre, solid-cell adenomas, and follicular and
papillary carcinomas) in mice and rats. In an earlier study on
mice, liver tumours, lung tumours, and lymphomas were also
detected, but these findings have not been confirmed. No
tumours except thyroid tumours have been found in rats, and in
hamsters, no tumours of the thyroid gland or other organs were
observed, even at 200 mg/kg diet.
At dose levels above approximately 10 mg/kg body weight, ETU
has clear teratogenic effects in rats and hamsters, different
types of central nervous system and skeletal anomalies being
induced. However, in mice, no teratogenic effects were found at
much higher dose levels (up to 800 mg/kg body weight).
1.5.2. Propylenethiourea
In a long-term study on mice using propylenethiourea (PTU),
an increased incidence of hepatocellular adenomas was observed
at dose levels of 10 mg/kg diet or more. No thyroid tumours
were found, but increased thyroid hypercellularity occurred at a
dose level of 1000 mg/kg diet. In rats, goitrogenic effects
were seen with PTU at dose levels as low as 1 mg/kg diet.
1.6. Effects on Man
Epidemiological studies on workers exposed to ETU did not
reveal any increase in the incidence of thyroid tumours.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
The chemical structure of ethylenethiourea (ETU) is:
CH2-NH
| \
| C=S
| /
CH2-NH
2.2. Physical and Chemical Properties
ETU is a fairly stable compound. Some physical and chemical
properties are listed in Table 8.
Table 8. Some physical and chemical properties of ETU
-------------------------------------------------------------------
Empirical formula C3H6N2S
Common synonym 2-imidazolidinethione
Appearance white, crystalline
Relative molecular mass 102.17
Odour odourless
Melting point 203 - 204 °C
Solubility in water: 20 000 mg/litre at 30 °C;
in ethanol: moderately soluble;
in chloroform: nearly insolublea
CAS registry number 96-45-7
-------------------------------------------------------------------
a From: IARC (1974), IUPAC (1977), US EPA (1984).
2.3. Analytical Methods
Residue analysis consists of sampling the contaminated
material, extracting the pesticide residue, cleaning up the
extract of interfering substances, and identifying and
quantifying the pesticide residue. The main methods used are
summarized in Table 9.
2.3.1. Extraction
Methanol and ethanol have been used as extraction solvents
for biological samples, due to the high solubility of ETU in
polar solvents. Mixed solvents such as methanol/chloroform
(Onley & Yip, 1971) or methanol/acetone (Phillips et al., 1977)
have also been employed, and the addition of trichloroacetic
acid has been reported to improve recovery with the latter
solvent. Sodium ascorbate has also been found effective in
ensuring good recovery of ETU (IUPAC, 1977; Otto et al., 1977).
2.3.2. Clean-up
The simplest procedures involve extraction of a derivative
from aqueous acid and alkali (Newsome, 1972; Nash, 1974; King,
1977). Another approach has been to purify the initial extract
by column chromatography before proceeding with derivatization
(Onley & Yip, 1971; Haines & Adler, 1973; Onley, 1977) or
determination steps (Otto et al., 1977). Where ETU is determined
without derivatization, a solvent-partitioning step is included
to provide further clean-up (IUPAC, 1977; Otto et al., 1977).
2.3.3. Derivatization
In all cases, derivatization involves first an alkylation of
the thiocarbonyl group. The various derivatives that have been
used are given in Table 9. Careful attention to reagent purity
is essential to ensure quantitative results (Onley & Yip, 1971;
Pecka et al., 1975; King, 1977). The benzyl chlorides react
smoothly by refluxing in alcohol for 30 min, while alkylation
with butyl bromide is carried out at room temperature in aqueous
dimethylformamide containing sodium hydroxide and sodium
borohydride. Solutions of ETU in aqueous dimethylformamide have
been found to be extremely unstable and must be reacted
immediately (Phillips et al., 1977). The n-butyl (Onley & Yip,
1971) and m-trifluoromethyl benzyl (King, 1977) derivatives are
sufficiently volatile to be analysed directly by gas-liquid
chromatography, whereas the benzyl derivatives must be
concentrated and acetylated before quantifying. Care must be
exercised during the concentration step to prevent losses
through evaporation (Pecka et al., 1975). Pentafluorobenzoyl
chloride (Nash, 1974) and trifluoroacetic anhydride have been
used as acetylating reagents, the former requiring a column
chromatographic step to remove excess reagent and by-products
before moving to gas-liquid chromatography. Although the excess
trifluoroacetic anhydride is easily removed by evaporation, the
trifluoroacetate derivative is unstable in the presence of
moisture and must be determined soon after removal of the excess
reagent (IUPAC, 1977).
2.3.4. Determination
Gas-liquid chromatographic methods predominate because of
their greater sensitivity, specificity, and accuracy. Methods
of determining ETU in plant samples were reviewed in 1976 by the
IUPAC Commission on Pesticide Terminal Residues (IUPAC, 1977),
and an extensive review of methods for ETU determination has
been produced by Bottomley et al. (1985).
2.3.4.1. Gas-liquid chromatography (GLC)
A variety of column packings and conditions have been used
in the determination of ETU and its derivatives. Detectors used
include thermionic (Onley & Yip, 1971), flame photometric (FPD)
(Haines & Adler, 1973; Onley, 1977; Otto et al., 1977), and
electron capture (EC) (Nash, 1974; King, 1977). Although
quantification by GLC/EC enables the use of smaller samples
(5 - 10 g) for monitoring ETU residues at the 0.01 mg/kg level,
it requires confirmation of suspected residues by mass spectro-
metry (MS), a second derivative, or by element-selective
detectors. Methods employing GLC/FPD with large samples
(40 - 100 g) have the advantage of both quantifying and
confirming ETU residues.
2.3.4.2. Thin-layer chromatography (TLC)
A variety of adsorbents and developing solvents have been
used to detect ETU in plants (Vonk & Kaars Sijpesteijn, 1970;
Onley & Yip, 1971; Blazquez, 1973; Engst & Schnaak, 1974). The
limit of detection is 0.02 mg/kg using alumina plates and Grotes
reagent for visualization (Onley & Yip, 1971). Semi-
quantitative determinations are possible by comparison with ETU
standards run simultaneously (IUPAC, 1977).
2.3.4.3. Polarography
This technique involves clean-up on an alumina column,
followed by paper chromatography and determination of the
nitroso derivative by polarography (Engst & Schnaak, 1974).
2.3.4.4. Radioisotope dilution
A reverse isotope dilution method has been used to determine
ETU in the presence of its metabolites, and is useful in the low
milligram range (Graham & Bornak, 1973; IUPAC, 1977).
2.3.4.5. High-pressure liquid chromatography (HPLC)
High-pressure liquid chromatography has been used for the
determination of ETU without derivatization. Detection can be
by ultraviolet absorption or electro-conductivity measurement,
the minimum level being 0.025 mg ETU/litre or kg (Prince, 1985).
Massey et al. (1982) reported an HPLC method applied for ETU
determination in a beer extract with a detection limit of
10 µg/kg. The method has been found to give spuriously high
results in the determination of ETU in beer due to the presence
of co-eluting matrix components. The more powerful resolving
ability of column-switching high-performance liquid chromato-
graphy, using polar-bonded columns of different selectivities,
has proved highly effective in separating ETU from these co-
eluting materials.
Table 9. Methods for the determination of ETU in plant samplesa
----------------------------------------------------------------------------------------------------
Extraction Extraction/ Derivative formation Analysis Detect- Reference
solvent clean-up measurementb ability
(mg/kg)
----------------------------------------------------------------------------------------------------
Plant ethanol none silica gel/TLC 10.0 Vonk & Kaars Sij-
extract pesteijn (1970)
Plant ethanol none paper electro- - Vonk & Kaars Sij-
extract phoresis pesteijn (1971)
Ethanol and cellulose 2-(butylthio) - GLC/thermionic 0.02 Onley & Yip
chloroform column 2-imidazoline detector (1971)
Methanol cellulose GLC/FPD 0.002 Watts et al.
column (1974)
Methanol chloroform/ GLC/ECD 0.005 Newsome (1972)
HCl
Methanol Al2O3 column 2-(butylthio)- GLC/FPD 0.05 Haines & Adler
2-imidazoline (1973); Onley
(1977)
Dioxane and none none silica - Blazquez (1973)
water gel/TLC
Methanol/ Al2O3 column none GLC/FPD 0.01 Otto et al.
Na-ascorbate (1977)
Table 9 (contd.)
----------------------------------------------------------------------------------------------------
Extraction Extraction/ Derivative formation Analysis Detect- Reference
solvent clean-up measurementb ability
(mg/kg)
----------------------------------------------------------------------------------------------------
Methanol florisil 2-(benzylthio)-1- GLC/ECD 0.005 Nash (1974)
column (pentafluorobenzoyl)-
2-imidazoline
Ethanol ether/HCl 2-( m-trifluoromethyl- GLC/ECD 0.01 King (1977)
partition benzylthio)-ETU
Acetone methanol/ 2-(benzylthio)-1- GLC/ECD 0.01 Newsome (1978)
acetone; (pentafluorobenzyl)-
Al2O3 column 2-imidazoline
acetonitrile/
dichloromethane
silica column
----------------------------------------------------------------------------------------------------
a From: IUPAC (1977).
b TLC = thin-layer chromatography; GLC = gas-liquid chromatography; FPD = flame photometric
detection; ECD = electron capture detection.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
During recent years, much attention has been paid to the
finding that ETU may occur in plant samples following the use of
dithiocarbamate fungicides. It may be present in the fungicide
when applied, or may result from subsequent transformation
(Bontoyan et al., 1972). Similarly, propylenethiourea (PTU) may
occur in residues of the fungicide propineb (IUPAC, 1977). The
amounts of ETU present in commercial formulations vary from one
sample to another, and depend on the length of time between
manufacture and use and the storage conditions, especially
temperature and moisture. Bontoyan & Looker (1973) found that
ETU increased, during storage for 39 days at 49 °C and 80%
relative humidity, from an initial content of 0.02 - 2% to a
final level of 0.13 - 14.5%. The degradation dynamics of
formulations from different manufacturers varied, products
containing both manganese and zinc forming the least ETU (IUPAC,
1977).
ETU is one of the important residues in plants and in the
environment following the agricultural use of ethylene
bisdithiocarbamates (EBDCs). It is also a metabolite formed
when EBDCs are ingested by animals and man.
Sources of human and environmental exposure to ETU are also
discussed in sections 3, 4, and 5 of Part A and sections 4 and 5
of Part B.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
ETU is a fairly stable compound with respect to hydrolytic
reactions but is easily oxidized to ethyleneurea (EU).
Oxidation to EU takes place primarily in biological systems and
by photolytic reaction, especially in the presence of photo-
sensitizers (Cruickshank & Jarrow, 1973; Ross & Crosby, 1973).
In studies by Kaars Sijpesteijn & Vonk (1970), pure cultures of
soil bacteria and fungi were unable to effect this transform-
ation
After ultraviolet irradiation of ETU on silica gel,
Cruickshank & Jarrow (1973) found nine secondary reaction
products. 2-Imidazolidinone was identified as the main
degradation product and there were smaller amounts of 3-(2-
imidazolin-2-yl)-2-imidazolidinethione (Jaffe's base). Other
secondary reaction products of the photooxidation of ETU are 2-
imidazoline and glycine (Ross & Crosby, 1973) via the inter-
mediate hydantoin (IUPAC, 1977) (Fig. 4).
4.1. Soil
ETU degradation was found to be slower in autoclaved soils
than in non-sterile soils (Kaufman & Fletcher, 1973), and only
EU was identified. In biologically active soils, ETU was
oxidized to carbon dioxide and four other degradation products,
two of which were identified as hydantoin and Jaffe's base.
Degradation of ETU to carbon dioxide in non-sterile soils was
reported by Lyman & Lacoste (1974). These results indicate that
ETU is oxidized under both biological and non-biological
conditions to EU, which is considerably more stable than ETU and
can be considered a major breakdown product. EU, however, can
be oxidized photochemically, using a catalyst, to give glycine
and carbon dioxide (Ross & Crosby, 1973), or microbially in
soil. In this context, Jaffe's base might be considered as an
intermediate product in ETU degradation.
According to Lyman & Lacoste (1974) and Rhodes (1977), half
of the ETU (present at a concentration of 10 mg/kg) in
Hagerstown silt loam soil was degraded to carbon dioxide in 22
days. Normal microbial carbon dioxide production was unaffected
by ETU at this concentration. Because this value was determined
on the basis of 14C-carbon dioxide formation from 14C-labelled
precursor, it does not represent a half-life of ETU, since 14C-
carbon dioxide formation did not parallel the disappearance of
labelled starting material from the soil. The actual half-life
of ETU is less than one day.
According to Kaufman & Fletcher (1973), ETU is oxidized to
EU, whereas carbon dioxide is only formed slowly. In Hagerstown
silt loam, ETU at 2 or 20 mg/kg was entirely converted into EU
within 2 days, while 200 mg ETU/kg took 8 days. In contrast, 4
days after treatment of soil with 2, 20, and 200 mg ETU/kg, only
43.4%, 8.9%, and 0.9%, respectively, had been degraded to carbon
dioxide. A slow but constant conversion of ETU to EU was also
found in autoclaved soil, whereas the formation of carbon
dioxide was only observed in non-sterile soil (Kaufman &
Fletcher, 1973; Lyman & Lacoste, 1974).
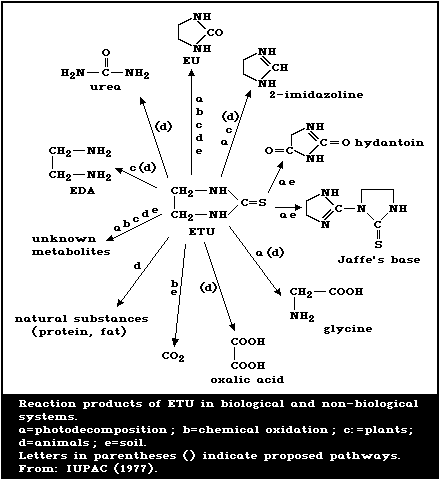 Rhodes (1977) found that when 14C-ETU was applied to soil
sections of Keyport silty loam at a rate of 2.2 kg/ha, total 14C
residues disappeared with a half-life of < 4 weeks. The half-
life of intact ETU was < 1 week. Most of the radioactivity was
confined to the top 2.5 - 12.5 cm of the soil column, and only
small amounts (0.2%) were found at depths of 20 - 30 cm after 12
weeks. It was concluded from this study that ETU did not leach
to any great extent.
Nash & Beall (1980) reported that ETU is weakly adsorbed to
soil, and is highly mobile in moist soil but immobile in dry
soil. The presence of organic matter in soil seems to be of
great importance in the leaching of ETU. Degradation appears to
be accomplished readily by both chemical and biological means
and, thus, ETU does not persist in soil.
Many studies have been carried out concerning the environ-
mental fate and transport of ETU (US EPA, 1984).
Parallel results were obtained in laboratory studies with
propineb, which, in a similar fashion, forms PTU, propyleneurea
(PU), and, eventually, carbon dioxide (Vogeler et al., 1977).
The results of these studies show that, under normal
practical conditions, it is unlikely that ETU or PTU will
accumulate in soil.
4.2. Water
ETU is stable in de-ionized water in the absence of photo-
sensitizers, but is rapidly oxidized in their presence. In
studies by Ross & Crosby (1973), several sensitizers were added
at 10 mg/litre to a 25 mg/litre solution of ETU and exposed to
sunlight. After 4 days with riboflavin as a sensitizer, the
concentration of ETU was less than 5% of that in the control
solution kept in darkness. To minimize microbial degradation,
the procedure was repeated after filtering and boiling the water
samples, with the same results. Furthermore, ETU degradation
was investigated in several boiled samples of agricultural
drainage water to which 0.5 mg ETU/litre had been added before
irradiation. The results are given in Table 10.
Numerous samples of natural water were collected from
rivers, lakes, and agricultural areas and, almost without
exception, they were found to degrade ETU to EU in sunlight.
The same samples degraded ETU in the dark but only after prior
exposure to sunlight, indicating that stable photo-oxidants had
been generated. The substances responsible for ETU oxidation
were isolated and identified as the amino acids tryptophane and
tyrosine. The pure amino acids also caused the conversion of
ETU to EU in the light, apparently by their ability to form
hydroperoxides or other strong oxidants (Ross & Crosby, 1973).
As both the amino acids and photosensitizers such as acetone,
riboflavin, and chlorophyll are known to occur world wide in
water and soil, and this photolysis also has been shown to take
place rapidly on a silica surface (Cruickshank & Jarrow, 1973),
the degradation of ETU to harmless products in the field seems
entirely plausible (IUPAC, 1977).
Table 10. Photodecomposition of ETU in agricultural
watersa
--------------------------------------------------------
Source Irradiation Remaining ETU (%)
--------------------------------------------------------
Irrigation ditch 3 days, lamp 10 - 20
(sugar beet) 3 days, dark 100
Paddy flooding 24 days, sun 25 - 50
ditch (rice) 24 days, dark 100
Paddy (rice) 24 days, sun 10 - 25
24 days, dark 100
--------------------------------------------------------
a From: Ross & Crosby (1973).
4.3. Plants
In studies in which the roots of corn, lettuce, tomato, and
pepper seedlings were treated with ETU, it was rapidly absorbed
by roots, translocated subsequently to the foliar tissues, and
then degraded very rapidly; virtually no ETU was detectable
after 20 days (Hoagland & Frear, 1976). When cucumber seedlings
were exposed to aqueous solutions of nabam or suspensions of
zineb or maneb, ETU was rapidly absorbed by the roots and
translocated within the plants. ETU appeared to be stable for
at least 2 weeks in seedlings and, inside the plant, a slow
conversion of ETU into 2-imidazoline was detected (Vonk & Kaars
Sijpesteijn, 1970, 1971).
In greenhouse studies, 14C-ETU was applied either to the
soil or to the leaves of 4-week-old potato plants and 8-week-old
dwarf tomato plants. Radioactivity was monitored in various
parts of the plant at different time intervals. The application
of 40 mg 14C-ETU/kg to the leaves of potato plants resulted in
negligible radioactivity in the roots and tubers 60 - 90 days
after application. The application of 17 - 22 kg/ha to soil
around the base of the plants resulted in a negligible amount of
radioactivity in the tubers, roots, and foliage of potato
plants, 60 - 90 days later. Comparable results were obtained
with tomato plants, using other dose levels and periods (Lyman &
Lacoste, 1974).
After systemic uptake of ETU by plants, EU and 2-imidazoline
were identified as metabolites. Surface deposits of ETU, which
may have occurred as a result of EBDC treatment, formed an
additional unidentified substance as the main metabolite and
ethylene diamine (EDA). Propineb and PTU also formed an
identical but unidentified major metabolite under similar
conditions (Vogeler et al., 1977).
Nash (1975) reported the presence of 7 - 10 different
degradation products in methanol extracts of soybeans after soil
or foliar treatment with EBDCs, as well as after treatment with
ETU. In these cases, EU was a degradation product.
More recently, Nash & Beall (1980) studied the fate of maneb
and zineb in microagroecosystem chambers (Part A, section 6.3).
ETU on the tomato fruit and leaves, and in the soil, water, and
air was monitored for 100 days after treatment. ETU was
detected at < 20 µg/kg on whole fruit after 3 days, but had
completely disappeared after 3 weeks. The half-life of ETU was
< 3 days.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The amount of ETU in commercial formulations of EBDCs has
been shown to increase with increasing temperature and humidity.
ETU formation during storage appears to be greatest in maneb
formulations (up to 14%), followed by zineb and mancozeb. The
relative proportions of degradation products appear to be
different for the various EBDCs.
Studies with propineb on apples and grapes, carried out by
Vogeler et al. (1977), showed that PTU could be detected
shortly after treatment, and that it was rapidly transformed to
an unknown metabolite, together with small amounts of PU, and
other unidentified reaction products.
5.1. Food and Drinking-Water
The residue levels of ETU are mainly below 0.1 mg/kg product
following treatment (with different formulations) at the maximum
recommended EBDC levels (Newsome, 1976; Phillips et al., 1977).
Studies in Canada and the USA have shown that vegetables,
such as spinach, carrots, and potatoes, that have been treated
with EBDCs contain high levels of ETU after cooking (Blazquez,
1973; Newsome & Laver, 1973; Watts et al., 1974). Snapbeans
treated with maneb were found to contain ETU after commercial
canning (US EPA, 1977). Farrow & Ralls (1970) demonstrated the
disappearance of zineb, ziram, and maneb residues from spinach
and apricots during normal canning operations. There have been
many studies on ETU residues in crops, such as those of Sato &
Tomizawa (1960) on zineb-treated cucumbers.
The levels of ETU formed from residues of mancozeb and
polyram present on apples that were processed to make apple
juice, apple sauce, and apple pomace were determined (IUPAC,
1977). The results showed that ETU residues were 0.17 mg/kg
pomace and 0.05 mg/kg juice. A surprisingly high level of
unchanged mancozeb remained in the pomace, despite heat
treatment for 15 h at 150 °C. However, it seems that ETU
residues diminish during storage.
Residues of intact 14C-labelled ETU were found to diminish
with time in canned tomato sauce, spinach, pickles, and apple
sauce (Rose et al., 1980). EU and more polar products accounted
for most of the 14C-labelled residues. These polar materials
were resistant to extraction and appeared to be bound.
A study sponsored by the US EPA (Phillips et al., 1977), on
the effects of food processing on EBDC residues, confirmed and
extended the results previously described. Washing the raw
agricultural products prior to processing removed 33 - 87% of
the EBDC residue and the majority of the ETU residue. An
interesting result was that although almost instantaneous
conversion of mancozeb to ETU took place in boiling water, field
weathered residues of mancozeb appeared to be more resistant to
degradation to ETU. A summary of the results for raw and
processed material is given in Table 3.
5.2. Monitoring and Market-Basket Studies
A monitoring programme initiated in 1972 by the Canadian
government showed that 33% of food samples contained detectable
ETU residues. In particular, samples of canned spinach and
orange peel had average values of 0.047 mg/kg product and
0.083 mg/kg, respectively (Pecka et al., 1975; US EPA, 1977).
Studies on the actual level of ETU in products prepared for
commercial sale show it to be generally present in small
amounts. The highest level, 0.61 mg/kg product, was found in
canned peaches, while levels in orange peel, tomato paste,
instant potatoes, strawberries, peaches, and cucumbers were less
than 0.2 mg/kg product (US EPA, 1982a,b).
The US EPA has estimated an upper limit for dietary exposure
to ETU in the general population of the USA to be 3.65 µg/kg
body weight per day. This estimate is a maximum value, since it
was assumed that residues are present at the tolerance level and
that all of the EBDC residue is quantitatively converted to ETU.
Using actual residue data and experimentally derived conversion
factors, the US EPA estimated the dietary intake of ETU to be
0.24 µg/kg body weight per day.
In a market-basket study, over 500 samples of 34 foods were
analysed, plus 26 samples of drinking-water. No water samples
and only 21 of the food samples contained ETU residues (Gowers &
Gordon, 1980). Exposure estimates based on market-basket
studies range from 0.01 to 1 µg ETU/kg body weight per day
(Gowers & Gordon, 1980; Rose et al., 1980).
Tomato products (203 samples) were analysed in another
market-basket study, but none contained ETU (Gowers & Gordon,
1980).
A more realistic review of the actual exposure of the
general population was obtained by a "table-top" study. Of 200
meals (some from homes and some from restaurants) which were
analysed for ETU, none contained any residues (Gowers & Gordon,
1980).
6. KINETICS AND METABOLISM
6.1. Absorption, Distribution, and Excretion
ETU is rapidly absorbed from the gastrointestinal tract and
cleared from the body in all the mammalian species that have
been tested. After only 5 min, ETU appeared in the blood of
rats administered an oral dose of 100 mg 14C-ETU/kg body weight.
Within 48 h, 82 - 99% of an oral dose was eliminated via the
urine and about 3% via the faeces (Kato et al., 1976; Rose et
al., 1980). Newsome (1974) and Ruddick et al. (1976a) found
that approximately 70% was eliminated in the urine and 1% in the
faeces. Comparable results were found for mice while, in
monkeys, 55% was eliminated via the urine within 48 h, and less
than 1.5% via the faeces (Allen et al., 1978).
To study the accumulation and elimination of radioactivity
by the thyroid gland of rats dosed with 14C-ETU, dose levels of
2 and 200 µg labelled ETU were administered daily for 14 days.
In another study, rats were dosed with 0, 0.1, 1, 10, 50, or 100
mg 14C-ETU/kg diet, daily, for 7 days. The first study showed
that the concentration of ETU and/or its metabolites in the
thyroid is dose dependent, and the second that the level of 14C
in the thyroid did not increase appreciably when the daily dose
was increased above 50 mg/kg diet. Withdrawal of ETU from the
diet led to an 80 - 94% reduction in the radioactivity in the
thyroid after 17 days (Lyman & Lacoste, 1974).
ETU and its metabolites have been found to have a half-life
of about 28 h in monkeys, 9 - 10 h in rats, and 5 h in mice
(Rose et al., 1980).
In cows administered 1 mg 14C-ETU/kg diet, Lyman (1971)
found a small quantity of unchanged ETU in both the urine and
the milk of the test animals. Higher levels of 14C were
detectable in metabolites, such as glycine and urea, and in the
lactose and protein in the milk (Table 11).
6.2. Metabolic Transformation
It has been demonstrated that ETU degradation leads to
traces of EU and other metabolites in the urine and that 14C-
carbon dioxide is exhaled following the administration of
labelled ETU. Kato et al. (1976) suggested that the metabolites
of ETU in the rat were produced primarily by fragmentation of
the imidazoline ring and decarboxylation of the fourth and fifth
carbon atoms. A small amount of radioactivity was also found in
a protein fraction of rat fetal tissue. Ruddick et al. (1976a),
however, concluded that ETU metabolism in the rat does not
appear to result in any release of 14C into the general
metabolic pool. Mice metabolize ETU to EU and other unknown
metabolites, while cats metabolize it to S-methyl-ETU and EU.
Lyman (1971) detected EU, EDA, oxalic acid, glycine, and
urea as major metabolites in cow urine. In addition, 14C
originating from 14C-ETU was found in the protein and lactose in
the milk (Table 11). From these results, it appears that the
metabolism of ETU in ruminants is different from that in non-
ruminants. The degradation products of ETU in plants are
similar to those found in animals.
A summary of the secondary metabolites of ETU in biological
and non-biological systems is given in Fig. 4 (Part B, section
4).
Table 11. 14C Activity in the milk and urine of
cows fed with 1 mg 14C-ETUa/kg diet for 6 weeks
------------------------------------------------------
Milk Urine
Substance Concentra- % of Concentra- % of
tion total tion total
(mg/litre) 14C (mg/litre) 14C
------------------------------------------------------
ETU 0.011 31 0.12 7
EU 0.0025 8 0.27 18
EDA - - 0.14 14
Glycine - - - 6
Oxalic acid - - - 12
Urea - - - 11
Fat - 3 - -
Protein - 18 - -
Lactose - 16 - -
Total (%) 76 68
------------------------------------------------------
a From: Lyman (1971) and IUPAC (1977).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only limited information is available on the effects of ETU
on organisms in the environment, and none is available
concerning the impact of ETU on terrestrial organisms. Data
concerning the toxicity for aquatic organisms of ETU and its
breakdown product EU are summarized in Tables 4 and 5. From
these results, ETU appears to have a low toxicity for bacteria,
algae, crustacea, and fish. Because of the low partition
coefficient (Table 6) and rapid biotransformation of ETU,
bioaccumulation will be insignificant or absent (Van Leewen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Lewerenz et al. (1975) reported an acute oral LD50 for the
rat of 900 mg/kg body weight. The values determined by Graham &
Hansen (1972) and Teramoto et al. (1978a) were 1832 mg/kg and
545 mg/kg body weight, respectively. Teramoto et al. (1978a)
reported values of 3000 mg/kg body weight for the mouse and
> 3000 mg/kg body weight for the hamster.
8.2. Short- and Long-term Exposures
Administration of ETU to laboratory rats causes enlargement
of the thyroid gland. This effect was noted by Seifter & Ehrich
(1948), and has since been confirmed in various short- and long-
term studies. The accumulation of ETU in the thyroid gland is
associated with biochemical and morphological effects comparable
with those induced by known antithyroid drugs such as
thiouracil.
Graham & Hansen (1972) fed rats with diets containing ETU at
dose levels of 0, 50, 100, 500, or 750 mg/kg diet for 30, 60,
90, or 120 days, and at 100 mg/kg or more, thyroid changes were
seen. Ross Hart & Valerio (1973) fed doses of up to 1000 mg
ETU/kg diet to rats. An increase in thyroid weight was seen at
159 mg/kg or more, and the larger doses produced hyperplasia.
When Freudenthal et al. (1977) carried out studies on rats
at dose levels of up to 625 mg ETU/kg diet for 30, 60, or 90
days, biochemical changes reflecting effects on thyroid function
were observed. A dose of 125 mg/kg diet produced, after 30
days, a decrease in T3, T4, and serum PBI levels and an increase
in TSH concentration. A dose of 25 mg/kg diet produced a
decrease in T4 content after 60 days. Thyroid weight was
increased in all groups receiving 25 mg/kg or more. After 90
days, tumours (adenomas) were found in the 125 mg/kg group. The
group receiving 625 mg/kg died within 7 weeks.
From these short-term studies, it can be concluded that the
no-observed-adverse-effect level lies below 25 mg ETU/kg diet
and is probably of the order of 5 mg/kg diet (equivalent to
0.25 mg/kg body weight).
8.3. Teratogenicity
ETU was administered orally to rats and rabbits in single
daily doses of 0, 5, 10, 20, 40, or 80 mg/kg body weight. Rats
were treated from 21 - 42 days before conception to day 15 of
pregnancy, or on days 6 - 15 or 7 - 20 of pregnancy, whereas
rabbits were treated on days 7 - 21 of pregnancy. ETU at dose
levels of 10 mg or more induced meningoencephalocele,
meningorrhagia, meningorrhea, hydrocephalus, obliterated neural
canal, abnormal pelvic limb posture with equinovarus, and short,
kinky tail in all rats. Fetal survival was not affected, and
fetal growth was retarded only at 40 and 80 mg/kg. Rabbits
showed an increased incidence of resorption sites and decreased
brain weight at 80 mg/kg body weight, but no malformations
(Khera, 1973).
ETU was studied in rats and mice for its ability to induce
perinatal toxicity and in guinea-pigs and golden hamsters for
its teratogenic potential. The ETU was administered by gavage
and during organogenesis. Table 12 summarizes the prenatal
treatments. Additional postnatal studies were performed on rats
using extended treatment periods (ETU at 0, 20, 25, or 30 mg/kg
body weight), including continuous exposure from day 7 of
gestation through parturition to day 15 of lactation. The pups
were weaned normally and postnatal studies on open-field
behaviour were performed at 6 weeks. ETU at 80 mg/kg body
weight induced maternal toxicity and reduced growth in the rat
and was teratogenic for the rat, inducing substantial fetal
effects at all dose levels above 10 mg/kg body weight. Gross
defects were seen in the skeletal and the central nervous
systems, and cleft palate was noted, mainly at the highest dose
level. At 20 and 30 mg/kg, an increased incidence of
hydrocephalus was the only defect noted. The maternal and fetal
toxicity of ETU for the mouse, guinea-pig, and hamster was
substantially less than for the rat. In mice, at the highest
dose, an increase in maternal liver weight and in fetal
supernumerary ribs was noted, but no effects were seen in other
species (Chernoff et al., 1979; FAO/WHO, 1980b).
Table 12. Summary of prenatal treatmentsa
---------------------------------------------------------
Compound Species Dose (mg/kg Treatment
body weight) (gestation days)
---------------------------------------------------------
ETU rat 0, 5, 10, 20, 7 - 21
30, 40, 80
mouse 0, 100, 200 7 - 16
hamster 0, 25, 50, 100 5 - 10
guinea-pig 0, 50, 100 7 - 25
---------------------------------------------------------
a Modified from: Chernoff et al. (1979).
ETU has been found to induce a variety of postnatal
effects, including reduction or absence of maternal milk
production, thereby causing pup mortality at doses of 30 mg/kg
body weight or more. However, no significant dose-related
behavioural abnormalities were observed in ETU-exposed pups
(Chernoff et al., 1979; FAO/WHO, 1980b).
In studies by Khera & Tryphonas (1977), groups of pregnant
rats were administered ETU at dose levels of 0, 15, 30, or
45 mg/kg body weight on day 15 of gestation and then subjected
to a variety of test conditions to evaluate pre- and postnatal
effects. Postnatal mortality occurred in pups from mothers
treated with dose levels exceeding 15 mg/kg or pups cross-
fostered to evaluate lactation exposure. All pups from mothers
treated with 45 mg/kg died within 4 weeks of birth. A high
incidence of hydrocephalus and microphthalmia was observed in
pups of mothers treated with 30 mg/kg and these pups died within
6 weeks of birth. Motor defects observed in some survivors
(16/65) of this group, were shown to result from the
hydrocephalic condition, which was accompanied by atrophy of the
cerebral cortex and subcortical white matter. These defects
were found to be a direct result of in utero exposure to ETU and
not of exposure during lactation (cross-fostered pups showed the
same effects as pups weaned from treated dams). When mated with
normal male rats, all female offspring of rats administered
30 mg/kg gave birth to normal offspring. The F2 generation was
not impaired, though some of the parents had neurological
defects. In these studies, no effects on the parameters
examined were observed at 15 mg/kg body weight (Khera &
Tryphonas, 1977).
Teramoto et al. (1978a) investigated the teratogenicity of
ETU in rats, mice, and hamsters. It was teratogenic when given
orally to rats at 20 - 50 mg/kg body weight per day on days 6 -
15 of pregnancy and to hamsters at 270 - 810 mg/kg body weight
per day on days 6 - 13 of pregnancy. However, no malformations
were induced in mice up to a daily oral dose of 800 mg/kg body
weight when given on days 7 - 15 of pregnancy. In hamsters,
cleft palate, kinky tail, oligodactyly, and anal atresia were
noted as gross external malformations. Skeletal examination
revealed a high incidence of defects in the ribs and vertebral
column, but no apparent defect was observed during visceral
examination. An oral dose of 100 or 200 mg ETU/kg body weight
given to pregnant rats consistently produced brain abnormalities
in the fetuses, when given on day 12 or 13 of pregnancy, and
forelimb abnormalities, when given on day 13 of pregnancy
(Teramoto et al., 1978a). Histological studies revealed
extensive cell necrosis in the brain and forelimbs of embryos
24 h after the treatment. These lesions were considered to be
the main cause of the abnormalities observed. However, neither
malformations nor cell necrosis were found in the fetuses that
had been injected with 200 µg ETU/conceptus into the amniotic
sac on day 12 of pregnancy (Teramoto et al., 1980). Studies
with 2-14C-ETU revealed that this dose was sufficient to test
the direct effects of ETU on the embryos, since the
incorporation of radioactive substance was five times higher in
the embryos injected with 200 µg into the amniotic sac than it
was in those embryos whose mothers were treated with an oral
dose of 100 mg/kg body weight (Teramoto et al., 1980).
The teratogenic potential of the ETU metabolite 1-
methylthiourea has been investigated by Teramoto et al. (1981).
It caused almost the same types of malformations in rat fetuses
when given orally to mothers at 250 - 500 mg/kg body weight on
day 12 or day 14 of pregnancy as those observed following
treatment with ETU. However, 1-methylthiourea did not induce
malformations in mouse fetuses whose mothers were given an oral
dose of 1000 mg/kg on day 10 of pregnancy. There is a
structural similarity between 1-methylthiourea and ETU: C=S,
and -NH- groups seem essential for producing teratogenic effects
(Teramoto et al., 1981). However, the structure of ETU seems
quite specific for the induction of teratogenicity since Ruddick
et al. (1976b) tested 16 compounds related to ETU, including
ethylenethiuram monosulfide, another metabolite, and only one,
4-methylenethiourea, was teratogenic.
8.4. Mutagenicity
Tests with a large number of S. typhimurium strains gave
mostly negative results, though a few (weak) positive results
were observed in the case of some strains of S. typhimurium
(Shirasu et al., 1977). The addition of rat liver microsomes
seemed to enhance the mutant reversion. Schüpbach & Hummler
(1976, 1977) concluded that ETU appeared to induce base-pair
mutations but not frameshift mutations in S. typhimurium
TA 1530, although frameshift mutations appeared in S.
typhimurium TA 98, TA 1537, and TA 1538 when exposed to ETU in
the presence of dimethylsulfoxide (DMSO) and/or rat liver
microsomes (Rose et al., 1980).
Teramoto et al. (1977) did not find mutagenicity with S.
typhimurium TA 1536, TA 1537, TA 1538, G46, E. coli WP2 hcr+ and
hcr-, or B. subtilis H17 rec+ and rec- at concentrations
of 10 000 µg ETU/plate. However, a weak reaction was seen with
S. typhimurium TA 1535, and Seiler (1974) reported weak
(dose-unrelated) mutagenicity in S. typhimurium strain G46.
ETU was also found to be mutagenic in a host-mediated assay
of S. typhimurium TA 1530 when mice were dosed with 6000 mg
ETU/kg body weight, but not at doses of 2000 mg/kg or less
(Schüpbach & Hummler, 1977). Cytogenetic effects of ETU have
been reported in bone marrow cells of mice and Chinese hamsters.
On the other hand, there was no significant evidence to suggest
that ETU was mutagenic in host-mediated assays of S.
typhimurium G 46 or in tests with other strains of bacteria, rat
bone marrow (including the micronucleus test) Chinese hamster
DON cells, rat lymphocytes, or human fibroblast cells.
Furthermore, ETU did not increase the frequency of dominant
lethal mutations in rodents or Drosophila melanogaster (Seiler,
1973, 1974; Schüpbach & Hummler, 1977; Shirasu et al., 1977;
Teramoto et al., 1978b; Rose et al., 1980). A large number of
mutagenicity tests are summarized in a report of the US EPA
(1984).
ETU has been tested in the hepatocyte DNA repair test,
which is used to determine pro-carcinogenic potential as well as
DNA damage. ETU did not induce DNA damage (Althaus et al.,
1982) nor cause chromosomal damage in cultured rat liver cells,
and it did not induce chromatid exchange in CHO cells in
vitro or in mice in vivo. A micronucleus test with mice bone
marrow cells in vivo also gave negative results (De Serres &
Ashby, 1981).
This evidence indicates that ETU is generally not
mutagenic, especially in mammalian test systems.
8.5. Carcinogenicity
The carcinogenicity of ETU has been evaluated by IARC
(1974, 1982). It was classified in group 2B, i.e., limited
evidence for activity in short-term tests; sufficient evidence
for carcinogenicity in animals; inadequate evidence for
carcinogenicity in human beings.
ETU has been studied for oncogenic potential in mice, rats,
and hamsters.
8.5.1. Mouse
In a comprehensive programme screening chemicals for
carcinogenicity, two strains of hybrid mice (X and Y) were given
215 mg ETU/kg body weight from day 7 until weaning, and
thereafter 646 mg/kg diet for more than 18 months. In the X
strain [(C57B1/6XC3H/Anf)F1], the incidence of lung tumours in
the ETU-treated females was higher than that of the control
group (3/18 versus 3/87), but it was lower than that of the
controls (0/16 versus 1/90) in the Y strain [(C57B1/6XAKR)F1].
In the males, the incidence was higher in the ETU-treated
animals (3/18 versus 1/90). The incidence of lymphomas was
slightly increased in treated Y-strain females. The hepatoma
incidence in the ETU-treated groups of both strains was
significantly higher than that of the control group (in the X
strain, 14/18 and 18/18, for males and females respectively; in
the Y strain, 18/18 and 9/18; in controls, 0/18 and 3/18). The
thyroid glands were not examined for histopathological changes
(Innes et al., 1969).
Graham et al. (1975), found that ETU induced thyroid
hyperplasia and other research groups have confirmed this
finding.
8.5.2. Rat
Ulland et al. (1972) and Weisburger et al. (1981) fed
groups of 26 male and female Charles River-CD rats diets
containing 0, 175, or 350 mg ETU (97%)/kg diet. Five females
and five males of the high-dose group were killed after 18
months and the remainder after 24 months. Hyperplastic goitre,
solid cell adenomas, and thyroid (follicular or papillary)
carcinomas were found. Two of the animals also had lung
tumours, which might have been metastases. The thyroid tumour
incidence was dose dependant; (in the 175 mg/kg group, it was
3/26 and 3/26, for males and females, respectively, and in the
350 mg/kg group it was 17/26 and 8/26). No thyroid carcinomas
were observed in the control animals. A few of the treated rats
had hyperplastic nodules in the liver.
Graham et al. (1973, 1975) studied the long-term effects on
the thyroid gland of ETU ingestion. Five groups of 68 male and
68 female Charles River rats were fed ETU at levels of 0, 5, 25,
125, 250, or 500 mg/kg diet for 2 years. Growth depression was
evident at the highest dose level. The thyroid/body weight
ratio was significantly increased at 250 and 500 mg/kg, and
slightly increased at 125 mg/kg after 24 months. Thyroidal
uptake of 131iodine per mg tissue was significantly decreased in
male rats fed 500 mg ETU/kg diet for 18 or 24 months. The
thyroids of females fed at the three highest dose levels were
hypofunctioning at 6 months, and hyperfunctioning at 12 months,
and at 24 months thyroid function was similar to that of the
controls. At the two highest dose levels (250 and 500 mg/kg),
thyroid adenomas and carcinomas were induced. At all lower dose
levels hyperplasia occurred more frequently than in the
controls, but there were no adenomas or carcinomas. No increase
in liver tumours was observed in this study.
Gak et al. (1976) studied the effects of feeding rats with
0, 5, 17, 60, or 200 mg ETU/kg diet for 24 months. Body weight,
food consumption, serum enzyme activities (e.g. glutamic pyruvic
transaminase, alkaline phosphatase), hepatic enzyme activities
(glutamic pyruvic transaminase, alkaline phosphatase, glucose-6-
phosphate dehydrogenase), cholesterol levels, weights of thyroid
and other organs, and histopathology were studied.
Hypercholesterolemia was found at dose levels of 5 mg/kg and
above. At 60 mg/kg or more, a significant increase in thyroid
tumours was found, but at lower levels the tumour incidence was
not significantly different from that of the controls.
8.5.3. Hamster
Gak et al. (1976) studied the effect of 0, 5, 17, 60, or
200 mg ETU/kg diet on hamsters for 18 months. Growth, food
intake, biochemical parameters in the serum and liver, organ
weights, and histology were studied. A significant increase in
thyroid tumours was found at 60 mg ETU/kg or more, but at lower
doses values were not significantly different from those of the
control group.
8.6. ETU in Combination with Nitrite
When the mutagenicity of ETU was assayed before and after
nitrosation with sodium nitrite under acid conditions,
nitrosation was found to cause a 160-fold increase in the number
of revertant colonies of S. typhimurium TA 1535 (Shirasu et
al., 1977). The interactive mutagenicity of ETU and nitrite was
also found in the mouse dominant lethal test by Teramoto et al.
(1978b). However, no dominant-lethal mutations were induced in
a group of mice treated with 30 mg ETU plus 10 mg nitrite/kg
body weight. A large increase in pre-implantation losses was
noted 5 and 6 weeks after completing a 5-day treatment of males
with a combined oral dose of 150 mg ETU/kg and 50 mg sodium
nitrite/kg body weight.
8.7. Mechanisms of Toxicity; Mode of Action
The biochemical changes induced by antithyroid drugs
include reduced production of thyroid hormones (T3 and T4),
followed by increased production of TSH in response to low
thyroid hormone levels in the blood. Pathological changes in
the thyroid gland begin with diffuse microfollicular hyper-
plasia, and are followed by diffuse and nodular hyperplasia and
later by nodular hyperplasia with papillary and cystic changes
induced by the TSH. If hyperstimulation of the thyroid by TSH
is severe and prolonged, it provides conditions conducive to the
formation of tumours.
Antithyroid drugs can inhibit T4 production in various
ways. The chemical similarity of ETU to thiourea and thiouracil
suggests that ETU acts by blocking the iodination of thyroxine
precursors, thus reducing the synthesis of the thyroid hormones.
Iodide peroxidase catalyses the iodination of tyrosine and the
coupling of the resultant iodotyrosyl residues to produce the
active hormones T3 and T4.
Graham & Hansen (1972) found that ETU inhibited
iodide peroxidase in vitro. The resulting decreased level of
thyroid hormones causes stimulatory feedback of the pituitary
gland and consequently an increased release of TSH (Rose et al.,
1980).
Lu & Staples (1978) studied the influence of ETU in
pregnant hypothyroid and euthyroid rats to determine whether ETU
teratogenicity occurs as a result of altered maternal thyroid
function. Doses of 40 mg ETU/kg body weight, administered on
days 7 - 15 of gestation, resulted in 84 - 100% of the fetuses
in all treated groups being malformed, regardless of the thyroid
status of the dams. The authors concluded that the thyroid
status of the mother is not of importance in causing teratogenic
effects.
Rose et al. (1980) reported that the effects of feeding
rats 125 - 625 mg ETU/kg diet for 2 - 12 weeks, which included
thyroid hyperplasia and dose related suppression of serum T3 and
T4 (with corresponding TSH elevation), were reversible within 22
weeks of placing on control diets.
Long-term studies using ETU showed significantly increased
thyroid/body weight ratios in rats fed 125, 250, or 500 mg/kg
diet for periods of up to 2 years (Graham et al., 1975). This
effect was not reversed in rats placed on a control diet after
66 weeks of continuous exposure to 5 - 500 mg ETU/kg diet. It
is likely that by that time the thyroid was severely damaged.
In studies by Arnold et al. (1982, 1983), decreased levels
of serum thyroid hormones and increased thyroid weights were
reversed in Sprague Dawley rats fed diets containing 0, 75, 100,
or 150 mg ETU/kg diet for 7 weeks. The reversibility of
microscopic changes in the thyroids of male rats exposed to ETU
was studied. The rats were fed diets containing 75 or 150 mg
ETU/kg diet for 7 - 82 weeks and then returned to a control diet
for periods ranging from 2 to 42 weeks. The severity and extent
of reversibility of thyroid hyperplasia were found to depend on
the duration of exposure to ETU. Above a certain threshold,
hyperplasia did not regress significantly.
Numerous studies with ETU suggest that the rat is more
sensitive than other species to the effects of the thyroid. A
recent study with propylthiouracil, a thyroid inhibitor with a
mode of action similar to that of ETU, has confirmed that
monkeys are much less sensitive than rats. The sensitivity
difference was not quantified in vivo, but, in an in
vitro study, the concentration of inhibitor required to produce
the same level of thyroid peroxidase inhibition was
approximately 100 times greater for monkey enzyme than it was
for rat enzyme (Takayama et al., 1986).
8.8. Propineb and Propylenethiourea (PTU)
8.8.1. General
The toxicology of propineb was reviewed at JMPR meetings in
1977, 1980, and 1983. Because of concern expressed at the 1977
meeting regarding the potential for thyrotoxicity and
tumourigenicity of propylenethiourea (PTU), a breakdown product
of propineb, the meeting estimated only a temporary ADI for man.
Further evaluation of propineb was postponed pending the
submission of additional data. Data submitted for evaluation in
1985 consisted of long-term mouse and rat studies, mutagenicity
studies, and a special study into the effects of PTU on DNA. In
addition, data previously submitted for evaluation in 1983 were
re-examined. These data included several studies on propineb
(acute toxicity studies, a short-term study on thyroid function
in rats, mutagenicity studies, and an oncogenicity study on
mice) and on PTU (pharmacokinetic studies on rats and a long-
term thyroid function study on rats) (FAO/WHO, 1986a,b).
8.8.2. Toxicological information
An oncogenicity study on mice with propineb indicated
increased hepatocellular adenomas in male mice and increased
pulmonary adenomas in female mice at 800 mg/kg diet, the highest
dose level tested. Thyroid tumours were not induced in treated
mice in this study. A no-observed-adverse-effect level for non-
neoplastic effects could not be determined in this study, owing
to insufficient data (FAO/WHO, 1986a, b).
In a long-term study into the effects of PTU on mice, an
increased incidence in male mice of hepatocellular adenomas was
observed at 1000 mg/kg diet (the highest dose level tested) and
of hepatocellular carcinomas at 10 mg/kg diet or more. In the
same study, increased incidences of hepatocellular adenomas and
carcinomas were observed in female mice at 100 mg/kg diet or
more. Thyroid tumours attributable to PTU were not observed,
but increased thyroid hypercellularity was noted in male mice at
1000 mg/kg diet (FAO/WHO, 1986a, b).
In long-term rat studies with propineb, previously reviewed
by the JMPR, an increased incidence of benign thyroid tumours
was observed at 1000 mg/kg diet or more. Non-neoplastic thyroid
effects were observed in the same study at 100 mg/kg diet or
more. In another study, increased liver and kidney weights were
observed at 100 mg/kg diet or more and a no-observed-adverse-
effect level of 10 mg/kg diet was determined. In a long-term
study on the effects of PTU on rats, thyroid tumours
attributable to PTU were only found at 1000 mg/kg diet.
Goitrogenic effects in the thyroid were observed, however, at
dose levels as low as 1 mg/kg diet, the lowest dose level
tested. A no-observed-adverse-effect level could not be
determined in this study (FAO/WHO, 1986a, b).
Short-term studies on the effects of propineb on thyroid
function in rats did not establish an unequivocal no-observed-
adverse-effect level for effects on the thyroid. In a long-term
study with PTU, effects on thyroid function were observed at
1000 mg/kg diet, but at lower dose levels effects were
ambiguous. Pharmacokinetic studies on rats demonstrated
preferential uptake of radioactivity from 14C-labelled PTU by
the thyroid (FAO/WHO, 1986a, b).
Mutagenicity studies on propineb and PTU produced negative
or inconclusive results. However, PTU has been shown to
increase DNA synthesis in mouse spleen cells, but it did not
bind to mouse liver cell DNA.
In view of the carcinogenic response to PTU in the liver of
mice and the lack of a no-observed-adverse-effect level for the
effects of propineb on the thyroid in a long-term study on mice
or short-term studies on rats, or for PTU in a long-term study
on rats, the JMPR recommended that the temporary ADI for
propineb should be withdrawn.
In view of the established carcinogenic potential of this
compound, the meeting recommended that propineb should not be
used where its residues can arise in food (FAO/WHO, 1986a,b).
9. EFFECTS ON MAN
9.1. Epidemiological Studies
Smith (1976) conducted a detailed study involving 1929
workers in rubber-compounding plants in Birmingham, England. No
thyroid cancers were found in the health records of these
workers.
Clinical examinations and thyroid function tests were
carried out over a period of 3 years on eight process workers
and five mixers in a factory producing ETU in the United
Kingdom. Matched controls were also examined. The results
showed that the exposed mixers, but not the process workers, had
significantly lower levels of T4 in their blood compared with
the controls. No effect was found on TSH or thyroid-binding
globulin (Smith, 1984).
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) and
the International Agency for Research on Cancer (IARC) have
evaluated the toxicity and carcinogenicity data for various
dithiocarbamates on several occasions. Annex III includes an
overview of the JMPR meetings in which these compounds, ETU, and
PTU have been evaluated, with their references, together with
the WHO recommended classification of pesticides by hazard for
individual dithiocarbamates. The existence of IARC evaluations
and the availability of WHO/FAO Data Sheets and IRPTC Data
Profiles and Legal Files are also indicated. These documents
include more detail concerning the product and legal aspects,
toxicological evaluation, and residues of individual
dithiocarbamates in different food items.
REFERENCES
AASETH, J., ALEXANDER, J., & WANNAG, A. (1981) Effect of
thiocarbamate derivatives on copper, zinc, and mercury
distribution in rats and mice. Arch. Toxicol., 48: 29-39.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarbamates,
dithiocarbamates, Luxembourg, Commission of the European
Communities (Report No. V/F/1/78/75 EN).
ALLEN, J.R., VAN MILLER, J.P., & SEYMOUR, J.L. (1978)
Absorption, tissue distribution, and excretion of 14C-ETU in the
rhesus monkey and the rat. Chem. Pathol. Pharmacol., 20: 109-
115.
ALTHAUS, F.R., LAWRENCE, S.D., SATTLER, G.L., LONGFELLOW, D.G.,
& PITOT, H.C. (1982) Chemical quantification of unscheduled
DNA synthesis in cultured hepatocytes on an assay for the rapid
screening of potential chemical carcinogens. Cancer Res., 42:
3010-3015.
ALVAREZ, M., CREEKMORE, R.W., & ROSEN, R.T. (1973) Structure
of ethylenethiuram monosulfide. Tetrahedron Lett., 12: 939-942.
ANTONOVICH, E.A., CHERNOV, O.V., SAMOSH, L.V., MARTSON, L.V.,
PILINSKAYA, M.A., KURINNY, L.I., VEKSHTEIN, M.S., MARTSON, V.S.,
BALIN, P.N., & KHITSENKO, I.I. (1972) [A comparative toxico-
logical assessment of dithiocarbamates.] Gig. i. Sanit., 37: 25-
30 (in Russian).
ARNOLD, D.L., BICKIS, M.G., NERA, E.A., MCGUIRE, P.F., & MUNRO,
I.C. (1982) Assessing the reversibility of thyroid lesions
that result from feeding ETU. Toxicologist, 2(1): 90 (Abstract
of Presentation at the SOT Meeting 1982).
ARNOLD, D.L., KREWSKI, D.R., JUNKINS, D.B., MCGUIRE, P.F.,
MOODIE, C.A., & MUNRO, I.C. (1983) Reversibility of ethylene-
thiourea-induced thyroid lesions. Toxicol. appl. Pharmacol., 67:
264-273.
ASHWORTH, R., DE B., HENRIET, J., LOVETT, J.F., & MARTIJN, A.,
ed. (1980) CIPAC handbook - Volume 1A: Analysis of technical
and formulated pesticides, Cambridge, United Kingdom, W. Heffer
and Sons Limited. England.
AYANABA, A., VERSTRAETE, W., & ALEXANDER, M. (1973) Formation
of dimethylnitrosamine, a carcinogen and mutagen, in soils
treated with nitrogen compounds. Soil Sci. Soc. Am. Proc., 37:
565-568.
BABINI, G. (1966) [A case of erythroderma caused by zinc
dithiocarbamate.] Arch. Ital. Dermatol. Venereol. Sessuol., 34:
230/238 (in Italian).
BALIN, P.N. (1970) [Experimental data on the blastomogenic
activity of the fungicide maneb.] Vrach. Delo, 4: 21-24 (in
Russian).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest. Control, 9: 119-
127.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg., 38: 50-
52.
BENSON, W.R., ROSS, R.D., CHEN JO-YUN, R., BARRON, R.P., &
MASTBROOK, D. (1972) Structure of ethylenethiuram monosulfide.
J. Assoc. Off. Anal. Chem., 55(1): 44-46.
BLACKWELL-SMITH, R., Jr, FINNEGAN, J.K., LARSON, P.S., SAHYOUN,
P.F., DREYFUSS, M.L., & HAAG, H.B. (1953) Toxicologic studies
on zinc and disodium ethylenebisdithiocarbamates. J. Pharmacol.
exp. Therap., 109: 159-166.
BLAZQUEZ, C.H. (1973) Residue determination of ethylene-
thiourea (2-imidazol-idinethione) from tomato foliage, soil, and
water. J. agric. food Chem., 21: 330-332.
BOGARTYKH, T.A., ORLOV, N.V., & KUZNETSOVA, T.V. (1979)
Activity of lactate dehydrogenase and glucose-6-phosphate
dehydrogenase in tissue of the testes under the action of some
pesticides. Vopr. Pitan., 5: 49-53.
BONTOYAN, W.R. & LOOKER, J.G. (1973) Degradation of commercial
ethylenebisdithiocarbamate formulations to ethylenethiourea
under elevated temperature and humidity. J. agric. food Chem.,
21: 338-342.
BONTOYAN, W.R., LOOKER, J.B., KAISER, T.E., GIANG, P., & OLIVE,
B.M. (1972) Survey of ethylenethiourea in commercial
ethylenebisdithiocarbamate formulations. J. Assoc. Off. Anal.
Chem., 55: 923-925.
BOTTOMLEY, P., HOODLESS, R.A., & SMART, N.A. (1985) Review of
methods for the determination of ethylenethiourea (imida-
zolidine-2-thione) residues. Residue Rev., 95: 45-89.
CAVANAGH, J.B. (1973) Peripheral neuropathy caused by chemical
agents. Crit. Rev. Toxicol., 2: 365-417.
CHARKES, N.D., BRAVERMAN, L.E., PENKO, K.F., GOWERS, D.S.,
GORDON, C.F., LIPWORTH, L., & MALMUD, L.S. (1985) Thyroid
function in male workers manufacturing dithane, an agricultural
fungicide, and in men not exposed to dithane. In: Proceedings of
the Ninth International Thyroid Congress, Sao Paulo, Brazil,
1-6 September 1985, p. 89.
CHERNOFF, N., KAVLOCK, R.J., ROGERS, E.H., CARVER, B.D., &
MURRAY, S. (1979) Perinatal toxicity of maneb, ethylene-
thiourea, and ethylene bisisothiocyanate sulfide in rodents. J.
Toxicol. environ. Health, 5: 821-834.
CHERNOV, O.V. & KHISTENKO, I.I. (1969) Blastomogenic
properties of some derivatives of dithiocarbamic acid. Vopr.
Onkol., 15(4): 71-74.
CHERNOV, O.V. & KHISTENKO, I.I. (1973) Some data on the
distribution and elimination of ziram-35S from the organism of
warm-blooded animals. In: Hygiene of using toxicology of
pesticides and the clinical picture of intoxication, Moscow,
Medizina, Vol. 10, pp. 250-254. (in Russian).
CLARKE, D.G., BAUM, H., STANLEY, E.L., & HESTER, W.F. (1951)
Determination of dithiocarbamates. Anal. Chem., 23(12): 1842-
1846.
CONKIN, R.A. & GLEASON, L.S. (1964) Vegadex. In: Zweig, G.,
ed. Analytical methods for pesticides, plant growth regulation
and food additives, New York, London, Academic Press, Vol. 4,
pp. 249-257.
CRUICKSHANK, P.A. & JARROW, H.C. (1973) Ethylenethiourea
degradation. J. agric. food Chem., 21 (3): 333-335.
DALVI, R.R., POORE, R.E., & NEAL, R.A. (1974) Studies of the
metabolism of carbon disulfide by rat liver microsomes. Life
Sci., 14: 1785-1796.
DANIELSSON, B.R.G., OSKARSSON, A., & DENCKER, L. (1984)
Placental transfer and fetal distribution of lead in mice after
treatment with dithiocarbamates. Arch. Toxicol., 55: 27-33.
DAVYDOVA, T.B. (1973) [The influence of tetramethylthiuram
disulfide on the estrous cycle and genital function of animals
when inhaled.] Gig. i Sanit., 38(4): 108-110 (in Russian).
DEKHUYZEN, H.M., VONK, J.W., & KAARS SIJPESTEIJN (1971)
Terminal residues of dithiocarbamate fungicides. In: Tahori,
A.S., ed. Proceedings of the International Symposium on Pesti-
cide Terminal Residues, Tel Aviv, Israel, 1971, London,
Butterworth, pp. 233-241.
DE MATTEIS, F. & SEAWRIGHT, A.A. (1973) Oxidative metabolism
of carbon disulfide by the rat. Effect of treatments which
modify the liver toxicity of carbon disulfide. Chem.-biol.
Interact., 7: 375-388.
DE SERRES, F.S. & ASHBY, J.C. (1981) Report of the
International Collaborative Program for Progress in Mutation
Research, Vol. 1, Amsterdam, Oxford, New York, Elsevier North-
Holland Inc.
DISHOVSKI, H. & IVANOVA-CHEMISHANSKA, L. (1979) Ultra-
structural changes in the neocortex of rats after chronic
dithiocarbamate Antracol. Activ. nerv. sup. (Praha), 21 (4):
279.
EDINGTON, N. & HOWELL, J.McC. (1966) Changes in the nervous
system of rabbits following the administration of sodium
diethyldithiocarbamate. Nature (Lond.), 210(5040): 1060-1062.
EDINGTON, N. & HOWELL, J.McC. (1969) The neurotoxicity of
sodium diethyldithiocarbamate in the rabbit. Acta neuropathol.
(Berlin), 12: 339-347.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N-nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under simulated gastric conditions and in vivo in the rat
stomach. Food Cosmet. Toxicol., 12: 229-232.
ELDJARN, L. (1950) The metabolism of tetraethylthiuram
disulfide (Antabuse, Aversan) in the rat, investigated by means
of radioactive sulfur. J. clin. lab. Invest., 2: 198-201.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ENGST, R. & SCHNAAK, W. (1967) [Studies on the metabolism of
the ethylenebisdithiocarbamate fungicides maneb and zineb.] Z.
Lebensm. Unters. Forsch., 134(4): 216-221 (in German).
ENGST, R. & SCHNAAK, W. (1969a) [Polarographic determination
of ethylene bisthiuram monosulfide.] Z. anal. Chem., 248: 321-
324 (in German).
ENGST, R. & SCHNAAK, W. (1969b) [Analysis of ethylene
bisthiuram monosulfide.] Z. anal. Chem., 244: 254-256 (in
German).
ENGST, R. & SCHNAAK, W. (1969c) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb
(polarographic determination of the undecomposed fraction of
active ingredient).] Z. Lebensm. Unters. Forsch., 139(3): 149-
152 (in German).
ENGST, R. & SCHNAAK, W. (1970a) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 143: 99-103 (in German).
ENGST, R. & SCHNAAK, W. (1970b) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 144(2): 81-85 (in German).
ENGST, R. & SCHNAAK, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev.,
52: 45-67.
ENGST, R., SCHNAAK, W., & LEWERENZ, H.J. (1971) [Studies on
the metabolism of the fungicidal
ethylenebisdiothiocarbamatesmaneb, zineb and nabam. V. The
toxicology of breakdown products.] Z. Lebensm Untersuch., 146,
91-97.
FAHRIG, R. (1974) Comparative mutagenicity studies with
pesticides. In: Montesano, R. & Tomatis, L., ed. Chemical
carcinogenesis assays, Lyons, International Agency for Research
on Cancer, pp. 161-181 (IARC Scientific Publication No. 10).
FAO (1985) Production yearbook 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO Meeting
Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL: 1967/M/1/1; WHO
Food Add./68.30).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO
Food Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 97; WHO Technical Report Studies No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10
Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations(FAO Plant Production and Protection Paper No. 10 Sup.).
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization (FAO
Plant Production and Protection Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20
Sup.).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 26 Sup.).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues
in food, Part II - Toxicology, Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 72).
FARROW, R.P. & RALLS, J.W. (1970) Investigation of changes in
pesticide residues during processing and storage of fruit and
vegetables, National Canners Association, 109 pp (Report No.
5).
FISHBEIN, L. (1975) Chromatography of environmental hazards.
III. Pesticides, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
FITZHUGH, O.G., WINTER, W.J., & NELSON, A.A. (1952) Some
observations on the chronic toxicity of antabuse (tetraethyl-
thiuramdisulfide). Fed. Proc., 11: 345-346.
FREUDENTHAL, R.I., KERCHNER, G.A., PERSING, R.L., BAUMEL, J., &
BARON, R.L. (1977) Subacute toxicity of ethylene bisisocyanate
sulfide in the laboratory rat. J. Toxicol. environ. Health, 2:
1067-1077.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Différence de
sensibilité du hamster et du rat vis-ą-vis des effets de
l'administration ą long terme de l'éthylčne thiourée. Eur. J.
Toxicol., 9: 303-312.
GARDNER-THORPE, C. & BENJAMIN, S. (1971) Peripheral neuropathy
after disulfiram administration. J. Neurol. Neurosurg.
Psychiatr., 34: 253-259.
GESSNER, T. & JAKUBOWSKI, M. (1972) Diethyldithiocarbamic acid
methyl ester, a metabolite of disulfiram. Biochem. Pharmacol.,
21: 219-230.
GOWERS, D.S. & GORDON, C.F. (1980) Some public health aspects
of the manufacture and use of zinc and manganese
ethylenebisdithiocarbamate fungicides Poznan, Poland, Institut
Ochrony Roslin, February 1979 pp. 491-522.
GRAHAM, S.L. & HANSEN, W.H. (1972) Effects of short-term
administration of ethylenethiourea on thyroid function of the
rat. Bull. environ. Contam. Toxicol., 7: 19-25.
GRAHAM, S.L., DAVIS, K.J., & PERRY, C.H. (1973) Effects of
one-year administration of ethylenethiourea upon thyroid
function of the rat. J. agric. food Chem., 21(3): 324-329.
GRAHAM, S.L., DAVIS, K.J., HANSEN, W.H., & GRAHAM, C.H. (1975)
Effects of prolonged ethylenethiourea ingestion on the thyroid
of the rat. Food Cosmet. Toxicol., 13: 493-499.
GRAHAM, W.H. & BORNAK, W.E. (1973) Improved experimental
technique for reverse isotope dilution method.
Anal. Chem., 45: 623-624.
GRIEPENTROG, F. (1960) [Allergy studies with simple chemical
substances.] Arzneimittelforschung, 10: 542-544 (in German).
GRIEPENTROG, F. (1962) [Tumor-like thyroid lesions in animal
studies of chronic toxicity of thiurams.] Beitr. Pathol.
Anat., 126: 243-255 (in German).
GUSTAFSSON, K.H. & THOMPSON, R.A. (1981) High-pressure liquid
chromatographic determination of fungicidal dithiocarbamates. J.
agric. food Chem., 29: 729-732.
HAINES, L.D. & ADLER, I.L. (1973) Gas chromatographic deter-
mination of ethylenethiourea residues. J. Assoc. Off. Anal.
Chem., 56 (2): 333-337.
HALD, J. & JACOBSEN, E. (1948) The formation of acetaldehyde
in the organism after ingestion of Antabuse (tetraethylthiuram
disulfide) and alcohol. Acta pharmacol. toxicol., 4: 305-310.
HALD, J., JACOBSEN, E., & LARSEN, V. (1948) The sensitizing
effect of tetraethylthiuram disulphide (Antabuse) to ethyl
alcohol. Acta pharmacol. toxicol., 4: 285-296.
HEDENSTEDT, A., RANNUG, U., LAMEL, C., & WACHTMEISTER, C.A.
(1979) Mutagenicity and metabolism studies on 12 thiram and
dithiocarbamate compounds used as accelerators in the Swedish
rubber industry. Mutat. Res., 68: 313.
HELLING, C.S., DENNISSON, D.C., & KAUFMAN, D.D. (1974)
Fungicide movement in soil. Phytopathology, 64(8): 1091-1100.
HOAGLAND, R.E. & FREAR, S.D. (1976) Behaviour and fate of
ethylenethiourea in plants. J. agric. food Chem., 24: 129-133.
HODGE, H.C., MAYNARD, Z.A., DOWNS, W., BLANCHET, H.J., Jr, &
JONES, C.K. (1952) Acute and short-term oral toxicity tests of
ferric dimethyldithiocarbamate (ferbam) and zinc dimethyldithio-
carbamate (ziram). J. Am. Pharmacol. Assoc., 41(12): 662-665.
HODGE, H.C., MAYNARD, E.A., DOWNS, W.L., COYE, R.D., Jr, &
STEADING, L.T. (1956) Chronic oral toxicity of ferric di-
methyldithiocarbamate (ferbam) and zinc dimethyldithiocarbamate
(ziram). J. Pharmacol. exp. Ther., 118: 174-181.
HODGSON, J.R., CASTLES, T.R., MURRILL, E., & LEE, C.C. (1974)
Distribution, excretion, and metabolism of the fungicide ferbam
in rats. Fed. Proc., 33: 537.
IARC (1974) Some antithyroid and related substances, nitro-
furans and industrial chemicals, Lyons, International Agency for
Research on Cancer, pp. 45-52 (Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 7).
IARC (1976) Some carbamates, thiocarbamates, and carbazides,
Lyons, International Agency for Research on Cancer (Monographs
on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol.
12).
IARC (1977) Some miscellaneous pharmaceutical substances,
Lyons, International Agency for Research on Cancer p. 243.
(Monographs on the evaluation of carcinogenic risk of chemicals
to humans, Vol. 13).
IARC (1983) Chemicals, industrial processes and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 128-130 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumour-
igenicity in mice: a preliminary note. J. Natl Cancer Inst., 42:
1101-1114.
IRPTC (1982) Data profile on ziram, Geneva, International
Register for Potentially Toxic Chemicals.
IRPTC (1983) Legal file - 2 vols, Geneva, International
Register for Potentially Toxic Chemicals.
IUPAC (International Union of Pure and Applied Chemistry) (1977)
Ethylenethiourea. Pure appl. Chem., 49: 675-689.
IVANOVA-CHEMISHANSKA, L. (1969a) Toxicological characteristics
of dithiocarbamates zineb, maneb, and mancozeb, Sofia,
(Dissertation).
IVANOVA-CHEMISHANSKA, L. (1969b) Toxicological characteristics
of mancozeb. Hig. i. Zdraveopazvane, 12(5): 417-426.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads reproduction of white rats under the effect of some
pesticides. Arch. Toxicol., Suppl. 4: 459-462.
IVANOVA-CHEMISHANSKA, L., SHEYTANOV, M., & MOSHEVA-IZMIROVA, N.
(1967) Changes in the functional state of the thyroid gland
upon acute intoxication with certain dithiocarbamates: zineb and
maneb. Med. Toxicol., 20(9): 1011-1013.
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., & DASHEV, G. (1971)
Light and electron microscopic observation on rat thyroid after
administration of some dithiocarbamates. Environ. Res., 4: 201-
212.
IVANOVA-CHEMISHANSKA, L., KALOYANOVA, F., IZMIROVA, N., ZLATEVA,
M., & VALCHEVA, V. (1972) [Experimental inhalatory toxicity and
substantiation of the maximum allowable concentration for some
dithiocarbamates in the air of the working area.] Truh. Dzihot.,
23: 55-64 (in Russian).
IVANOVA-CHEMISHANSKA, L., VALCHEVA, V., & TAKEVA, TZ. (1973)
Effect of chronic peroral poisoning with perozine (zineb) on the
reproduction of white rats. Acta med. soc., 1: 107-108.
IVANOVA-CHEMISHANSKA, L., MILANOV, ST., CHEMISHANSKI, G., &
DASHEV, G. (1974) Attempt of biological dose of some
hypophyseal hormones in white rats after experimental oral
administration of zineb. Environ. Res., 8: 160-165.
IVANOVA-CHEMISHANSKA, L., PETROVA-VERGIEVA, T., & MIRKOVA, E.
(1975a) [Embryotoxic and teratogenic action of some
pesticides.] Eksp. Med. Morfol., 14(1): 29-33 (in Russian).
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., MILANOV, S.,
STRASHIMIROV, D.D., DASHEV, G.I., & CHEMISHANKSKA, G.A. (1975b)
Effects of subacute oral administration of zinc ethylene bisdi-
thiocarbamate on the thyroid gland and adenohypophysis of the
rat. Food Cosmetol. Toxicol., 13: 445-447.
IZMIROVA, N. & MARINOV, V. (1972) [Distribution and excretion
of 35S-ziram and metabolic products after 24 hours following
oral administration of the preparation in female rats.] Eksp.
Med. Morfol., 11(3): 152-156 (in Russian).
IZMIROVA, N., IZMIROVA, I., & IVANOVA, L. (1969) The effect of
zineb and maneb on the isoenzymes of lactate dehydrogenase in
the testes of rats. C. R. Acad. Bulg. Sci., 22(2): 225-227.
JORIS, S.J., ASPILA, K.I., & CHAKRABARTI, C.L. (1970) Decompo-
sition of monoalkyl dithiocarbamates. Anal. Chem., 42(6): 647-
651.
KAARS SIJPESTEIJN, A.K. & VONK, J.W. (1970) Microbial conver-
sion of dithiocarbamate fungicides. Med. Fac. Landbouwwet.
Gent, 35(2): 799-804.
KALOYANOVA, F., IVANOVA, L., & ALEXIEV, B. (1967) The
influence of large doses of maneb on the progeny of albino rats.
Med. Toxicol., 20(10): 1109-1112.
KAMPMEIER, C. & HAAG, H.B. (1954) Toxicological considerations
in use of dithiocarbamates. Agric. Chem., April: 49-50, 133.
KASKEVICH, L.M., KOMAROVA, L.I., ILINA, V.I., IVANICKI, V.A., &
SHILINA, V.F. (1981) [Clinical-functional changes due to
exposure to zineb,] pp. 109-111 (in Russian).
KASLANDER, J. (1963) Formation of an S-glucuronide from
tetraethylthiuram disulfide (Antabuse) in man. Biochem. Biophys.
Acta, 71: 730-732.
KATO, Y., ODANAKA, Y., TERAMOTO, S., & MATANO, O. (1976) Meta-
bolic fate of ethylenethiourea in pregnant rats. Bull. environ.
Contam. Toxicol., 16(5): 546-555.
KAUFMAN, D.D. & FLETCHER, C.L. (1973) Title? In: Proceedings
of the Second International Congress on Plant Pathology,
Minneapolis, Minnesota (Abstract No. 1018).
KHERA, K.S. (1973) Ethylenethiourea: teratogenicity study in
rats and rabbits. Teratology, 7: 243-252.
KHERA, K.S. & TRYPHONAS, L. (1977) Ethylenethiourea induced
hydrocephalus: pre- and postnatal pathogenesis in offspring from
rats given a single oral dose during pregnancy. Toxicol. appl.
Pharmacol., 42: 85-97.
KIM, S.U. & RIZZUTO, N. (1975) Neuroaxonal degeneration
induced by sodium diethyldithiocarbamate in cultures of central
nervous tissue. J. Neuropath. exp. Neurol., 34: 531-541.
KING, R.R. (1977) Derivatization of ethylenethiourea with m-
trifluoromethylbenzylchloride for analysis by electron capture
gas chromatography. J. agric. food Chem., 25(1): 73-75.
KIRKBRIGHT, G.F. & MULLINS, F.G.P. (1984) Separation of
dithiocarbamates by high-performance liquid chromatography using
a micellar mobile phase. Analyst, 109: 493-496.
KLIGMAN, A.M. & ROSENSWEIG, W. (1948) Studies with new
fungistatic agents. I. For the treatment of superficial
mycoses. J. invest. Derm., 10: 59-68.
KOIZUMI, A., SHIOJIMA, S., OMIYA, M., NAKANO, S., SATO, N., &
IDEDA, M. (1979) Acute renal failure and maneb (manganous
ethylene bisdithiocarbamate) exposure. J. Am. Med. Assoc.,
242(23): 2583-2585.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982a) Embryo-
toxicity of industrial chemicals on the chicken embryo:
thiourea derivatives. Acta pharmacol. toxicol., 51: 38-44.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982b) Application
of the chicken embryo in testing for embryotoxicity. Scand. J.
Work environ. Health, 8: 63-69.
KURINNY, A.I. & KONDRATENKO, T.I. (1972) [Effect of some
fungicides (dithiocarbamic acid derivatives) on chromosomes of
bone marrow cells in mice.] Tsitol. Genet., 6: 225-228 (in
Russian).
LABORIE, F. & LABORIE, R. (1966) Allergies aux fongicides et
prophylaxie biochimique (relations entre allergie et
intoxications). Rev. Pathol. comp., 66: 105-109.
LAKOMAA, E.L., SATO, S., GOLDBERG, A.M., & FRAZIER, J.M. (1982)
The effect of sodium diethyldithiocarbamate treatment on copper
and zinc concentrations in rat brain. Toxicol. appl. Pharmacol.,
65: 286-290.
LANGE, P. & JUNG, F. (1971) [Reduction of the toxicity to the
liver of carbon tetrachloride by diethyldithiocarbamate and 3-
(O-tolyl)-4-(nitro)-sydnone.] Acta biol. med. Ger., 27: 425-434
(in German).
LARSSON, K.S., ARNANDER, C., CEKANOVA, E., & KJILLBERG, M.
(1976) Studies of teratogenic effects of the dithiocarbamates
maneb, mancozeb, and propineb. Teratology, 14: 171-183.
LEE, C.C. & PETERS, P.J. (1976) Neurotoxicity and behavioural
effects of thiram in rats. Environ. Health Perspect., 17: 35-
42.
LEHMAN, A.J. (1951) Chemicals in food: a report of the
association of food and drug officials on current developments,
II, Pesticides, Q. Bull. Fed. Drug Off.U.S., 15: 122-133.
LEWERENZ, H.J., BLEYL, D., PLASS, R., & ENGST, R. (1975)
Toxicology of ETU , Potsdam, Rehbrüke, German Democratic
Republic, Central Institute for Nutrition, Academy of Science.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972) Carcino-
genic nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LOWY, R., GRIFFATON, G., BRIGANT, L., ARDONIN, B., & DUPUY, F.
(1979) The dietary no-effect level of a dithiocarbamate
fungicide, thiram, as evaluated from measurement data on rats.
II. The various sensitivities of the various parameters.
Toxicology, 14: 39-53.
LOWY, R., GRIFFATON, G., & PELISIER, M.A. (1980) The dose-
response relationship of rat organ weights under dietary thiram
influence. Toxicol. Lett., 5(Suppl. 1): 148.
LU, M.H. & STAPLES, R.E. (1978) Teratogenicity of ethylene-
thiourea and thyroid function in the rat. Teratology, 17: 171-
178.
LUDWIG, R.A. & THORN, G.D. (1962) Chemistry and mode of action
of dithiocarbamate fungicides. Adv. Pestic. Control Res., 3:
219-252.
LUTZ, L.M., GLENDA, E.A., Jr, & RECKNAGEL, R.O. (1973) Protec-
tion by diethyldithiocarbamate against carbon tetrachloride
lethality in rats and against carbon tetrachloride-induced lipid
peroxidation in vitro. Biochem. Pharmacol., 22: 1729-1734.
LYMAN, W.R. (1971) The metabolic fate of Dithan M-45
(coordination product of zinc ion and manganous ethylene
bisdithiocarbamate). In: Tahori, A.S., ed. International
Symposium on Pesticide Terminal Residues, Tel Aviv, 1971,
Israel, London, Butterworth, pp. 243-256.
LYMAN, W.R. & LACOSTE, R.J. (1974) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
In: Proceedings of the 3rd International IUPAC Congress on
Pesticide Chemistry, Helsinki, 3-9 July, 1974, Stuttgart, George
Thieme Publishers, pp. 67-74.
LYMAN, W.R. & LACOSTE, R.J. (1975) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
Environ. Qual. Saf., Suppl. 3: 67-74.
McCANN, J. & PITCHER, F. (1973) Fish toxicity laboratory
reports Nos. 535 and 541, Beltsville, Maryland, US Environmental
Protection Agency, Animal Biology Laboratory.
MAJ, J., GRABOWSKA, M., & KWIEK, J. (1970) The effect of
disulfiram, diethyldithiocarbamate, and dimethyldithiocarbamate
on serotonin and 5-hydroxyindole-3-acetic acid brain levels in
rats. Biochem. Pharmacol., 19: 2517-2519.
MARSHALL, W.D. (1977) Thermal decomposition of ethylene
bisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. agric. food Chem., 25: 357-361.
MASSEY, R.C., KEY, P.E., & MCWEENY, D.J. (1982) Analysis of
ethylenethiourea in beer by high-performance liquid
chromatography. J. Chromatogr., 240: 254-256.
MATOKHNYUK, L.A. (1971) Toxicity of the fungicide maneb on
inhalation. Hyg. Sanit., 36(5): 195-199.
MATTHIASCHK, G. (1973) [Influence of L-cysteine on
teratogenisis caused by thiram (TMTD) in NMRI mice.] Arch.
Toxikol., 30: 251-262 (in German).
MELNIKOV, N.N. & TRUNOV, P.P. (1966) Persistence of residual
quantities of preparations on the basis of dithiocarbamate acid
derivatives. Chem. Agric. IV, 10(36): 21-24.
MINOR, J.L., RUSSEL, J.Q., & LEE, C.C. (1974) Reproduction and
teratology studies with the fungicide ferbam. Toxicol. appl.
Pharmacol., 29(16): 120.
MOJE, W., MUNNECKE, D.E., & RICHARDSON, L.T. (1964) Carbonyl
sulfide, a volatile fungitoxicant from nabam in soil. Nature
(Lond.), 202: 831-832.
NASH, R.G. (1974) Improved gas-liquid chromatographic method
for determining ethylenethiourea in plants. J. Assoc. Off. Anal.
Chem., 57(5): 1015-1021.
NASH, R.G. (1975) In: Proceedings of the 170th Meeting of the
American Chemical Society for Pesticides (Abstract No. 1).
NASH, R.G. & BEALL, M.L., Jr (1980) Fate of maneb and zineb
fungicides in microagroecosystem chambers. J. agric. food Chem.,
28: 322-330.
NEWSOME, W.H. (1972) Determination of ethylenethiourea
residues in apples. J. agric. food Chem., 20: 967-969.
NEWSOME, W.H. (1974) The excretion of ethylenethiourea by rat
and guinea-pig. Bull. environ. Contam. Toxicol., 11: 174-176.
NEWSOME, W.H. (1976) Residues of four ethylene bisdithio-
carbamates and their decomposition products on field-sprayed
tomatoes. J. agric. food Chem., 24(5): 999-1001.
NEWSOME, W.H. (1978) A method for the determination of
ethyleneurea in foods as the pentafluorobenzamide. J. agric.
food Chem., 26(6): 1325-1327.
NEWSOME, W.H. & LAVER, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb-treated foods. Bull.
environ. Contam. Toxicol., 10(3): 151-154.
ODEYEMI, O. (1980) Persistence of arasan pesticide in aquatic
ecosystems in Nigeria. Pestic. Abstr., 80: 1276.
ONLEY, J.H. (1977) Gas-liquid chromatographic method for
determining ethylenethiourea in potatoes, spinach, apple sauce,
and milk. Collaborative study. J. Assoc. Off. Anal. Chem.,
60(5): 1111-1115.
ONLEY, J.H. & YIP, G. (1971) Determination of ethylenethiourea
residues in foods using thin-layer and gas chromatography. J.
Assoc. Off. Anal. Chem., 54(1): 165-169.
OSKARSSON, A. (1983) Redistribution and increased brain uptake
of lead in rats after treatment with diethyldithiocarbamate.
Arch. Toxicol., 6: 279-284.
OSKARSSON, A. (1984) Dithiocarbamate induced redistribution
and increased brain uptake of lead in rats. Neurotoxicology,
5(3): 283-294.
OSKARSSON, A. & LIND, B. (1985) Increased lead levels in brain
after long-term treatment with lead and dithiocarbamate or
thiuram derivatives in rats. Acta pharmacol. toxicol., 56: 1-6.
OTTO, S., KELLER, W., & DRESCHER, N. (1977) A new gas
chromatographic determination of ethylenethiourea residues
without derivatization. J. environ. Sci. Health, B12(3): 179-
191.
PECKA, Z., BAULU, P., & NEWSOME, H. (1975) Preliminary survey
of ethlenethiourea residues in the Canadian food supply, 1972.
Pestic. monit. J., 8(4): 232-234.
PERIQUET, A. & DERACHE, R. (1976) Influence d'un régime
alimentaire hypoprotéique sur la toxicité aigue d'un pesticide,
le nabame. Ann. Nutr. Aliment., 30: 29-44.
PETROVA-VERGIEVA, T. (1976) On the teratogenic activity of
zinc-propylene bisdithiocarbamate. Hig. i. Zdraveopazvane,
19(5): 435-442.
PETROVA-VERGIEVA, T. & IVANOVA-CHEMISHANSKA, L. (1973)
Assessment of the teratogenic activity of dithiocarbamate
fungicides. Food Cosmet. Toxicol., 11: 239-244.
PHILLIPS, W.E.J. (1968) Changes in the nitrate and nitrite
contents of fresh and processed spinach during storage. J.
agric. food Chem., 16: 88-91.
PHILLIPS, W.F., GRADY, M.D., & FREUDENTHAL, R. (1977) Effects
of food processing on residues of ethylene bisdithiocarbamate
fungicides and ethylenethiourea, Washington DC, US Environmental
Protection Agency (EPA No. 600/1-77-021).
PILINSKAYA, M.A. (1971) [Cytogenetic effect of the fungicide
ziram in the culture of human lymphocytes in vitro. ] Genetika,
7: 138-143 (in Russian).
PLUYGERS, C.W., VONK, J.W., & THORN, G.D. (1971) Re-
examination of the structure of ethylenethiuram monosulfide.
Tetrahedron Lett., 18: 1317-1318.
PRZEZDZIECKI, Z., BANKOWSKA, J., KOMOROWSKA-MALEWSKA, W., &
JANICKA, T. (1969) Studies of the chronic short-term toxicity
of zineb, maneb, and kaptan. Rocz. Panstw. Zakl. Hlg., 20: 133-
140.
PRICKETT, C.S. & JOHNSTON, C.D. (1953) The in vivo production
of carbon disulfide from tetraethylthiuram disulfide
(AntabuseR). Biochem. Biophys. Acta, 12: 542-546.
PRINCE, J.L. (1985) Analysis of ethylenethiourea in urine by
high-performance liquid chromatography. J. agric. food Chem.,
33: 93-94.
RAGHU, K., MURTHY, N.B.K., KUMARASWAMY, R., RAO, R.S., & SANE,
P.V. (1975) In: Proceedings of the Joint FAO/IAEA, Division of
Atomic Energy in Food and Agriculture, Vienna, p. 137.
RASUL, A.R. & HOWELL, J.M. (1974) The toxicity of some
dithiocarbamate compounds in young and adult domestic fowl.
Toxicol. appl. Pharmacol., 30: 63-78.
RHODES, R.C. (1977) Studies with manganese 14C-ethylene
bisdithiocarbamate (14C-maneb) fungicide and 14C-ethylenethio-
urea (14C-ETU) in plants, soil, and water. J. agric. food Chem.,
25(3): 528-533.
RJAZANOVA, R.A. (1967) [The effects of the fungicides ziram and
zineb on the generative function of test animals.] Gig. i
Sanit., 2: 26-30 (in Russian).
ROLANDI, A., DE MARINIS, E., & DE CATERINA, M. (1984) Dithio-
carbamate pesticides: activity of propineb in the micronucleus
test in mice. Mutat. Res., 135: 193-197.
ROLL, R. (1971) [Teratological experiments with thiram (TMTD)
on two mouse strains.] Arch. Toxikol., 27: 173-186 (in German).
ROSE, D., PEARSON, C.M., ZUKER, M., & ROBERTS, J.R. (1980)
Ethylenethiourea: criteria for the assessment of its effects on
man, Ottawa, National Research Council, Associate Committee on
Scientific Criteria for Environmental Quality (NRCC Publication
No. 18469).
ROSS, R.D. & CROSBY, D.G. (1973) Photolysis of ethylene-
thiourea. J. agric. food Chem., 21(3): 335-337.
ROSS HART, E. & VALERIO, M.G. (1973) Evaluation of goitrogenic
activity in rats: Ethylene thiourea, practical and Dithane M-45,
Communication from Litton Bionetics to Rohm and Haas Co. Cited
in Van Leeuwen et al., (1982)
RUDDICK, J.A., WILLIAMS, D.T., HIERLIHY, L., & KHERA, K.S.
(1976a) 14C-ethylenethiourea: distribution, excretion, and
metabolism in pregnant rats. Teratology, 13: 35-39.
RUDDICK, J.A., NEWSOME, W.H., & NASH, L. (1976b) Correlation
of teratogenicity and molecular structure: ethylenethiourea and
related compounds. Teratology, 13: 263-266.
SATO, F. & TOMIZAWA, C. (1960) Decomposition of zinc ethylene
bisdithiocarbamate (zineb) applied to plants. Bull. Natl Inst.
Agric. Sci. (Tokyo) Ser. C, 12: 181-187.
SCHULTHEISS, E. (1957) [The therapeutic use of
tetramethylthiuram disulfide.] Dermatol. Wochenschr., 7: 160-162
(in German).
SCHUPBACH, M. & HUMMLER, H. (1976) Evaluation of the
mutagenicity of ethylenethiourea using bacterial and mammalian
test systems. Mutat. Res., 28: 122.
SCHUPBACH, M. & HUMMLER, H. (1977) A comparative study on the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutat. Res., 56: 111-120.
SEIDLER, H., HARTIG, M., SCHNAAK, W., & ENGST, R. (1970)
[Studies on the metabolism of certain insecticides and
fungicides in the rat. II. Mean distribution and breakdown of
14C-labelled maneb.] Nahrung, 14(5): 363-373 (in German).
SEIFTER, J. & EHRICH, W.E. (1948) Goitrogenic compounds:
pharmacological and pathological effects. J. Pharmacol. exp.
Ther., 92: 303-314.
SEILER, J.P. (1973) A survey on the mutagenicity of various
pesticides. Experientia, (Basel) 29: 622-623.
SEILER, J.P. (1974) Ethylenethiourea (ETU): a carcinogenic and
mutagenic metabolite of ethylene bisdithiocarbamate. Mutat.
Res., 26: 189-191.
SEN, N.P., DONALDSON, B.A., & CHARBONNEAU, C. (1974) Formation
of nitrosodimethylamine form the interaction of certain
pesticides and nitrite. In: Bogorski, P., Walker, E.A., & Davis,
W., ed. N-nitroso compounds in the environment, Lyons,
International Agency for Research on Cancer, pp. 75-79 (IARC
Scientific Publication No. 9).
SHERMAN, H. & ZAPP, J.A. (1966) Three generation reproduction
study, Manzate DR (88% maneb). Unpublished report from Haskell
Laboratory submitted to the Word Health Organization by E.I. du
Pont de Nemours and Co.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H.,
TERAMOTO, S., & KADA, T. (1977) Mutagenicity screening on
pesticides and modification products: a basis of carcinogenicity
evaluation. In: Hiatt, H.H., Watson, J.D., & Winsen, J.A.,
ed. Origins of human cancer, Book A, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, Vol.4, pp. 267-285.
SHORT, R.D., Jr, RUSSEL, J.Q., MINOR, J.L., & LEE, C.C. (1976)
Developmental toxicity of ferric dimethyldithiocarbamate and
bis(dimethylthiocarbamoyl) disulfide in rats and mice. Toxicol.
appl. Pharmacol., 35: 83-94.
SHORT, R.D., MINOR, J.L., UNGER, T.M., BREEDEN, B., VAN GOETHEM,
D., & LEE C.C. (1980) Teratology of a zineb formulation.
(Results of a study performed at Midwest Research Institute for
the US Environmental Protection Agency submitted to the World
Health Organization) (EPA-600/1-80-017).
SMITH, D. (1976) Ethylenethiourea: a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.,
26: 92-94.
SMITH, D.M. (1984) Ethylenethiourea: thyroid function in two
groups of exposed workers. Br. J. ind. Med., 41: 362-366.
SPRECHER, D. & GRIGOROWA, R. (1967) [Maximum workplace
concentration of thiuram.] Z. gesamte Hyg., 13: 241-245 (in
German).
STROMME, J.H. (1965) Metabolism of disulfiram and diethyl-
dithiocarbamate in rats with demonstration of an in vivo
ethanol-induced inhibition of the glucuronic acid conjugation of
the thiol. Biochem. Pharmacol., 14: 393-410.
STROMME, J.H. & ELDJARN, L. (1966) Distribution and chemical
forms of diethyldithiocarbamate and tetraethylthiuram disulfide
(disulfiram) in mice in relation to radioprotein. Biochem.
Pharmacol., 15: 287-297.
SUNDERMAN, F.W., Jr & FRASER, C.B. (1983) Effects of nickel
chloride and diethyl dithiocarbamate on metallothionein in rat
liver and kidney. Ann. clin. lab. Sci., 13 (6): 489-495.
SZYMEZYK, T. (1981) The mutagenicity of the fungicide thiram.
Mutat. Res., 85: 223-224.
TAKAYAMA, S., AIHARA, K., ONODERA, T., & AKIMOTO, T. (1986)
Antithyroid effects of propylthiouracil and sulfamonomethoxine
in rats and monkeys. Toxicol. appl. Pharmacol., 82: 191-199.
TANNENBAUM, S.R., SINSKEY, A.J., WEISSMAN, M., & BISHOP, W.
(1974) Nitrite in human saliva: its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TERAMOTO, S., MORIYA, M., KATO, K., TEZUKA, H., NAKAMURA, S.,
SHINGU, A., & SHIRASU, Y. (1977) Mutagenicity testing on
ethylenethiourea. Mutat. Res., 56: 121-129.
TERAMOTO, S., SHINGU, A., KANEDA, M., & SAITO, R. (1978a)
Teratogenicity studies with ethylenethiourea in rats, mice, and
hamsters. Congenital Anom., 18: 11-17.
TERAMOTO, S., SHINGU, A., & SHIRASU, Y. (1978b) Induction of
dominant lethal mutations after administration of ethylene-
thiourea in combination with nitrite or of N-nitrosoethylene-
thiourea in mice. Mutat. Res., 56: 335-340.
TERAMOTO, S., KANEDA, M., KATO Y. & SHIRASU Y. (1980) Failure
of inducing malformations after intro-amniotic injections of
ethylenethiourea in the rat. Congenital Anom., 20: 17-23.
TERAMOTO, S., KANEDA, H., AOYAMA, & SHIRASU, Y. (1981)
Correlation between the molecular structure of N-alkylureas and
N-alkylthioureas and their teratogenic properties.
Teratology, 23: 335-342.
TETSUMI, T., SUMI, M., TANAKA, M., & SHONO, T. (1985) Reversed
phase ion interaction chromatography of sodium dialkyldithio-
carbamates in the presence of tetraalkylammonium salts. J.
Chromatogr., 348: 389-395.
THORN, G.D & LUDWIG, R.A. (1962) The dithiocarbamates and
related compounds, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 298.
TOLLENAAR, F.D. (1956) [Recent developments in the use of
antioxidants in foodstuffs.] Dtsch. Lebensm. Rundschau, 12:
307-313 (in German).
TRUHAUT, R., GUERINOT, F., & BOHUON, C. (1971) Mécanisme bio-
chimique de toxicité des fongicides de la série des dithiocarba-
mates: L'action inhibitrice de la dopamine beta-hydroxylase. Ann.
pharm. fr., 29(2): 117-124.
TURNER, N.J. & CORDEN, M.E. (1963) Decomposition of sodium-
N-methyldithiocarbamate in soil. Phytopathology, 53: 1388-
1394.
ULLAND, B.M., WEISBURGER, J.H., WEISBURGER, E.K., RICE, J.M., &
CYPHER, R. (1972) Brief communication: thyroid cancer in rats
from ethylenethiourea intake. J. Natl Cancer Inst., 49: 583-
584.
US EPA (1977) Rebuttable presumption against registration and
continued registration of pesticide products containing ethylene
bisdithiocarbamates. Fed. Reg., 42(154): 40618-40627.
US EPA (1982a) Ethylene bisdithiocarbamates: final resolution
of rebuttable presumption against registration, Washington
DC, US Environmental Protection Agency, Office of Pesticides and
Toxic Substances (Unpublished report).
US EPA (1982b) Ethylene bisdithiocarbamates: notice of
determination concluding the presumption against registration;
notice of availability of decision document, Washington DC, US
Environmental Protection Agency (Unpublished report 82P-2851).
US EPA (1984) Health and environmental effects: profile for
ethylenethiourea (2-imidazolidinethione), Cincinnati, Ohio, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office (Program Office Draft ECAO-CIN-019).
VACHKOVA-PETROVA, R. (1977) [A study on mutagenic activity of
basfungine on white rats.] Hig. i. Zdraveopazvane, 20(5): 422-
426 (in Russian).
VACHKOVA-PETROVA, R. (1981) Mutagenicity study of endodan in
rats. Mutat. Res., 85(4): 288.
VAN ESCH, G.J. (1956) [Toxicity of tetramethylthiuram
disulfide and tetraethylthiuram disulfide.] Versl.
Volksgezondheid, Vol. No. 166-169 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN LEEUWEN, F.X.R., VOOGD, C.E., KNAAP, A.G.A.C., DEN
TONKELAAR, E.M., & VAN DER HEIJDEN, C.A. (1982) Toxicological
evaluation of ethylenethiourea (ETU), Bilthoven, National
Institute of Public Health (Unpublished Report No. 627915/008).
VAN LOGTEN, M.J. (1972) [ Thesis: the dithiocarbamate-alcohol
reaction by the rat. ] Terborg, The Netherland, Fa. Lammers (in
Dutch).
VAN STEENIS, G. & VAN LOGTEN, M.J. (1971) Neurotoxic effect of
the dithiocarbamate tecoram on the chick embryo. Toxicol. appl.
Pharmacol., 19: 675-686.
VEKSHTEIN, M.SH. & KHITSENKO, I.L. (1971) Ziram metabolism in
warm-blooded animals. Gig. i Sanit., 36: 28-33.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index: JMPR 1961-84, Geneva, World Health Organization
(Unpublished report).
VOGELER, K., DREZE, Ph., RAPP, A., STEFFAN, H., & ULLEMEYER, H.
(1977) Distribution and metabolism of propineb in apples and
grapes, including their breakdown products propylenethiourea and
ethylenethiourea in apples. Pflanzenschutz-Nachrichten Bayer
30: 72-97.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1970) Studies on the fate
in plants of ethylene bisthiocarbamate fungicides and their
decomposition products. Ann. appl. Biol., 65: 489-496.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1971) Tentative
identification of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicides. Pestic. Biochem.
Physiol., 1: 163-165.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1976) Formation of
ethylenethiourea from 5,6-dihydro-3 H-imidazo[2,1-C]-1,2,4-
dithiazole-3-thione by microorganisms and reducing agents. J.
environ. Sci. Health, B11: 33-47.
WATTS, R.R., STORHERR, R.W., & ONLEY, J.H. (1974) Effects of
cooking on ethylene bisdithiocarbamate degradation to
ethylenethiourea. Bull. environ. Contam. Toxicol., 12: 224-226.
WEDIG, J., COWAN, A., & HARTUNG, R. (1968) Some of the effects
of tetramethylthiuram disulfide (TMTD) on reproduction of the
bobwhite quail (Colinus virginianus). Toxicol. appl. Pharmacol.,
12: 293.
WEISBURGER, E.K., ULLAND, B.M., NAM, J.M., GART, J.J., &
WEISBURGER, J.H. (1981) Carcinogenicity tests of certain
environmental and industrial chemicals. J. Natl Cancer Inst.,
67: 75-88.
WHO (1986a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1986-1987, Geneva, World
Health Organization (Unpublished report VBC/86.1, rev. 1).
WHO (1986b) Environmental Health Criteria 64: Carbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 137.
WHO (1988) Environmental Health Criteria 76: Thiocarbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 49.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., Croydon, British Crop Protection
Council.
YIN-TAK WOO (1983) Carcinogenicity, mutagenicity, and
teratogenicity of carbamates, thiocarbamates, and related
compounds. An overview of structure-activity relationships and
environmental concerns. J. environ. Sci. Health, C1(1): 97-133.
ZADOROZHNY, B.A., PETROV, B.R., OLTIRENKO, V.A., & KIRILKO, V.A.
(1981) [Occupational dermatoses and their control under
conditions of pesticide manufacture.] Vestn Dermatol. Venerol.,
6: 48-51 (in Russian).
ZDIENICKA, M., ZIELENSKA, M., HRYMIEWICA, M., TROJANOWSKA, M.,
ZALEJSKA, M., & SZYMCZYK, T. (1981) The mutagenicity of the
fungicide thiram. In: Kappas, A., ed. Progress in environmental
mutagenesis and carcinogenesis, Amsterdam, Oxford, New York,
Elsevier Science Publishers, Vol. 2, pp. 79-86.
ZORIN, P.M. (1970) [Allergic dermatitis arising as a result of
contact with zineb.] Vestn Dermatol. Venerol., 44: 65-68 (in
Russian).
ZUMAN, P. & ZAHRADNIK, R. (1957) [Kinetics and decomposition
mechanism of dithiocarbamic acids solutions. Polarographic
study.] Z. Phys. Chem., 208: 135-140 (in German).
Annex I. Names and structures of selected dithiocarbamates
--------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
dibam Methylnamate S sodium dimethyldi- C3H6NS2Na 143.21
|| thiocarbamate
(CH3)2N-C-SNa (128-04-1)
disul- Antabuse S S tetraethylthiuram C10H20N2S4 2 mg/
firam || || disulfide litrea
(C2H5)2NC-SS-CN(C2H5)2 (29925-58-4)
ferbam Fermate S iron, tris(dimethyl- C9H18FeN3S6 416.51 130
Fuklasin || carbamodithioato- mg/litre
Hokmate [(CH3)2N-C-S-]3Fe S,S' )-,
Karbam Black (14484-64-1)
Niacide
mancozeb Aazimag manganese, [[1,2- indefinite, insol-
Fore [-SCSNHCH2CH2NHCSSMn-]x(Zn)y ethanediylbis-[carba- variable uble
Dithane M-45 modithioato]](2-)]-,
Manzate 200 in combination with
[[1,2-ethanediylbis-
[carbamodithioato]]-
(2-)]zinc
(8018-01-7)
maneb Amazin manganese, [[1,2- C4H6MnN2S4 265.29 insol-
Blitex [-SCSNHCH2CH2NHCSS-Mn-]x ethanediylbis- uble
Dithane M-22 [carbamodi-
Manzate thioato]](2-)]
Martemick (12427-38-2)
Mancid
Tubothane
---------------------------------------------------------------------------------------------------------
Annex I. (contd.)
---------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
metam- Carbam S carbamodithioic C2H4NaNS2 129.18 722
sodium Masposol || acid, methyl-, mg/
Sistan CH3NH-C-S- Na+ sodium salt litreb
Trapex (137-42-8)
Vapam
metiram Zinc-metiram ammonia complex of indefinite, insol-
Polyram zineb and poly variable uble
(ethylene thiuram
disulfide),
zineb ethylene
thiuram disulfide
(9006-42-2)
nabam Nabasan S S carbamodithioic acid, C4H6Na2N2S4 256.34 200 g/
Parzate || || 1,2-ethanediylbis-, litre
Spring-Bak Na+ -S-C-NHC2H4-NH-C-S- Na+ disodium salt
(142-59-6)
polyram (see metiram)
propineb Antracol S S zinc, [[(1-methyl- C5H8N2S4Zn 289.9 insol-
Cypromate || || 1,2-ethanediyl)-bis uble
Mezineb [-S-C-NHCH2CH-NH-C-S-Zn-]x [carbamodithioato]]
| (2-)]-,
CH3 (12071-83-9)
sulfal- Vegadex S carbamodithioic acid, C8H14NClS2 223.8 92 mg/
late CDEC || diethyl-, 2-chloro- litre
(C2H5)2N-C-S-CH2-C=CH2 2-propenyl ester
| (95-06-7)
Cl
---------------------------------------------------------------------------------------------------------
Annex I. (contd.)
---------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
thiram Arasan S S thioperoxydicarbonic C6H12N2S4 240.44 30 mg/
Cyuram || || diamide, tetramethyl litre
Fernasan (CH3)2N-C-S-S-C-N(CH3)2 (137-26-8)
Mercuram
Normersan
TMTD
zineb Aspor-Z S S zinc, [[1,2-ethane- C4H6N2S4Zn 275.73 10 mg/
Carbane || || diylbis[carbamodi- litre
Dithane-Z78 [-S-C-NHC2H4-NH-C-S-Zn-]x thioato]](2-)]-
Lonacol (12122-67-7)
Murphane
Novozir
Parzate
Perozine 75B
Sudothane
Zebenide
Zelmone
ziram Cuman S zinc, bis(dimethyl- C6H12N2S4Zn 305.81 65 mg/
Fuklasin || carbamodithioato- litre
Milbam [(CH3)2N-C-S-]2Zn S,S')-
Zerlate (137-30-4)
---------------------------------------------------------------------------------------------------------
a At 38 °C.
b At 20 °C.
Annex II. Names and structures of degradation products of ethylene
bisdithiocarbamates
-------------------------------------------------------------------------------------
Common name Chemical CAS chemical name/ Molecular Relative
structure CAS registry number formula molecular
mass
-------------------------------------------------------------------------------------
Ethylenethiourea CH2-NH 2-imidazolidinethione C3H6N2S 102.2
(ETU) | \ (96-45-7)
| C=S
| /
CH2-NH
S
||
C-S
DIDT / | 5,6-dihydro-3- H-imidazo[2, C4H4N2S3 176.3
CH2-N | 1-C]1,2,4-dithiazole-3-thione,
| \ | (33813-20-6)
| C-S
| //
CH2-N
S
||
CH2-NH-C-S
Ethylenethiuram | | 1,2,4,7-dithiadiazocine- C4H6N2S4 210.3
disulfide (ETD) | | 3,8-dithione, tetrahydro
CH2-NH-C-S (3082-38-0)
||
S
-------------------------------------------------------------------------------------
Annex III. Dithiocarbamates and ETU: JMPR reviews, ADIs, Evaluation by IARC, Classification by Hazard,
WHO/FAO Data Sheets, IRPTC Data Profile and Legal Filea
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Ferbam 1983 0-0.02 1984a Vol. 12 + + 0
page 121
1980 0-0.02 1981b Vol. 13
page 243
1981a
1977 0-0.02 1978b
1978a
1974 0-0.05 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compounds
only, and to the sum
of all the dithio-
carbamate fungicides
if more than one is
present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with other
dimethyl-dithiocar-
bamates (thiram and
ziram))
1965 no ADI 1965b
1965a
1963 no ADI 1964
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Mancozeb 1983 0-0.05i 1984a + + 0
1980 0-0.05i (indivi- 1981b
dually of the sum 1981a
of mancozeb, maneb,
and zineb)
1977 0-0.005 1978b
(temporary)
(sum of mancozeb, 1978a
maneb, and zineb)
1974 0-0.005 1975b
(temporary)
(sum of dithio- 1975b
carbamates) 1975b
1975a
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and the sum
of all the ethylene
bisdithiocarbamate
fungicides if more
than one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other ethylene
bisdithiocarbamates
(maneb and zineb),
including zineb
derived from nabam
plus zinc sulfate)
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Maneb 1983 0-0.05i 1984a + + 0
1980 0-0.05 1981b
(individual or 1981a Vol. 12
the sum of manco- page 137
zeb, maneb, and
zineb)
1977 0-0.005 1978b
(temporary)
(sum of manco- 1978a
zeb, maneb, and
zineb)
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other ethylene
bisdithiocarbamates
(mancozeb and zineb)
including zineb
derived from nabam
plus zinc sulfate)
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Maneb 1965 no ADI 1965b
(contd.) 1965a
1963 no ADI 1964
Nabam 1983 no ADI 1984a + + II
1977 no ADI 1978b
1978a
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(as nabam alone 1968a
or in combination
with other ethylene
bisdithiocarbamates
(mancozeb, maneb,
and zineb) including
zineb derived from
nabam plus zinc
sulfate)
1965 no ADI 1965b
1965a
1963 no ADI 1964
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Propineb 1985 ADI withdrawn 1986b 0
1984 0-0.005 1985b
(temporary)
1983 0-0.005 1984b
(temporary)
1980 0-0.005 1981b
(temporary)
1977 0-0.005 1978b
Thiram 1983 0-0.005 1984a Vol. 12 + + III
(temporary) page 225
1980 0-0.005 1981b
(temporary) 1981a
1977 0-0.005 1978a
(temporary)
1974 0-0.005 1975b
(temporary)
(sum of all 1975a
dithiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other dimethyl di-
thiocarbamates
(ferbam and ziram))
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Thiram 1965 0-0.025 1965b
(contd.) 1965a
1963 0-0.025 1964
Zineb 1983 0-0.05i 1984a Vol. 12 + + 0
1980 0-0.05 1981b page 245
(individually 1981a
or the sum of
mancozeb, maneb,
and zineb)
1977 0-0.005 1978b
(temporary)
(sum of mancozeb, 1978a
maneb, and zineb)
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
1967 (alone or in com- 1968a
bination with
other ethylene
bisdithiocarbamates
(mancozeb and maneb)
including zineb
derived from nabam
plus zinc sulfate)
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Zineb 1965 no ADI 1965b
(contd.) 1965a
1963 no ADI 1964
Ziram 1983 0-0.02 1984a Vol. 12 + + III No. 73
page 259 (in
prepar-
ation)
1980 0-0.02 1981b
1981a
1977 0-0.02 1978b
1978a
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other dimethyl
dithiocarbamates
(ferbam and thiram))
1965 no ADI 1965b
1965a
1963 no ADI 1964
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
ETU (see 1980 0.002 1981b Vol. 7,
dithio- p. 45
carbamates) 1974 - 1975b Suppl. 4,
p. 128
PTU (see 1985 no ADI 1986a
propineb) (withdrawn)
--------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register for Potentially Toxic Chemicals (UNEP, Geneva).
f WHO/FAO Data Sheets on Pesticides with number and year of appearance.
g From: IRPTC (1983).
h From: WHO (1986a).
i Not more than 0.002 mg/kg body weight may be present as ETU.
The hazard referred to in this Classification is the acute risk for health
(that is, the risk of single or multiple exposures over a relatively short
period of time) that might be encountered accidentally by an person
handling the for storage and transportation by competent international
bodies.
Classification relates to the technical material, and not to the formulated product:
-----------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
-----------------------------------------------------------------------------------
1A Extremely hazardous 5 or less 20 or less 10 or less 40 or less
1B Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 over 4000
O Unlikely to present
acute hazard in
normal use
-----------------------------------------------------------------------------------
Rhodes (1977) found that when 14C-ETU was applied to soil
sections of Keyport silty loam at a rate of 2.2 kg/ha, total 14C
residues disappeared with a half-life of < 4 weeks. The half-
life of intact ETU was < 1 week. Most of the radioactivity was
confined to the top 2.5 - 12.5 cm of the soil column, and only
small amounts (0.2%) were found at depths of 20 - 30 cm after 12
weeks. It was concluded from this study that ETU did not leach
to any great extent.
Nash & Beall (1980) reported that ETU is weakly adsorbed to
soil, and is highly mobile in moist soil but immobile in dry
soil. The presence of organic matter in soil seems to be of
great importance in the leaching of ETU. Degradation appears to
be accomplished readily by both chemical and biological means
and, thus, ETU does not persist in soil.
Many studies have been carried out concerning the environ-
mental fate and transport of ETU (US EPA, 1984).
Parallel results were obtained in laboratory studies with
propineb, which, in a similar fashion, forms PTU, propyleneurea
(PU), and, eventually, carbon dioxide (Vogeler et al., 1977).
The results of these studies show that, under normal
practical conditions, it is unlikely that ETU or PTU will
accumulate in soil.
4.2. Water
ETU is stable in de-ionized water in the absence of photo-
sensitizers, but is rapidly oxidized in their presence. In
studies by Ross & Crosby (1973), several sensitizers were added
at 10 mg/litre to a 25 mg/litre solution of ETU and exposed to
sunlight. After 4 days with riboflavin as a sensitizer, the
concentration of ETU was less than 5% of that in the control
solution kept in darkness. To minimize microbial degradation,
the procedure was repeated after filtering and boiling the water
samples, with the same results. Furthermore, ETU degradation
was investigated in several boiled samples of agricultural
drainage water to which 0.5 mg ETU/litre had been added before
irradiation. The results are given in Table 10.
Numerous samples of natural water were collected from
rivers, lakes, and agricultural areas and, almost without
exception, they were found to degrade ETU to EU in sunlight.
The same samples degraded ETU in the dark but only after prior
exposure to sunlight, indicating that stable photo-oxidants had
been generated. The substances responsible for ETU oxidation
were isolated and identified as the amino acids tryptophane and
tyrosine. The pure amino acids also caused the conversion of
ETU to EU in the light, apparently by their ability to form
hydroperoxides or other strong oxidants (Ross & Crosby, 1973).
As both the amino acids and photosensitizers such as acetone,
riboflavin, and chlorophyll are known to occur world wide in
water and soil, and this photolysis also has been shown to take
place rapidly on a silica surface (Cruickshank & Jarrow, 1973),
the degradation of ETU to harmless products in the field seems
entirely plausible (IUPAC, 1977).
Table 10. Photodecomposition of ETU in agricultural
watersa
--------------------------------------------------------
Source Irradiation Remaining ETU (%)
--------------------------------------------------------
Irrigation ditch 3 days, lamp 10 - 20
(sugar beet) 3 days, dark 100
Paddy flooding 24 days, sun 25 - 50
ditch (rice) 24 days, dark 100
Paddy (rice) 24 days, sun 10 - 25
24 days, dark 100
--------------------------------------------------------
a From: Ross & Crosby (1973).
4.3. Plants
In studies in which the roots of corn, lettuce, tomato, and
pepper seedlings were treated with ETU, it was rapidly absorbed
by roots, translocated subsequently to the foliar tissues, and
then degraded very rapidly; virtually no ETU was detectable
after 20 days (Hoagland & Frear, 1976). When cucumber seedlings
were exposed to aqueous solutions of nabam or suspensions of
zineb or maneb, ETU was rapidly absorbed by the roots and
translocated within the plants. ETU appeared to be stable for
at least 2 weeks in seedlings and, inside the plant, a slow
conversion of ETU into 2-imidazoline was detected (Vonk & Kaars
Sijpesteijn, 1970, 1971).
In greenhouse studies, 14C-ETU was applied either to the
soil or to the leaves of 4-week-old potato plants and 8-week-old
dwarf tomato plants. Radioactivity was monitored in various
parts of the plant at different time intervals. The application
of 40 mg 14C-ETU/kg to the leaves of potato plants resulted in
negligible radioactivity in the roots and tubers 60 - 90 days
after application. The application of 17 - 22 kg/ha to soil
around the base of the plants resulted in a negligible amount of
radioactivity in the tubers, roots, and foliage of potato
plants, 60 - 90 days later. Comparable results were obtained
with tomato plants, using other dose levels and periods (Lyman &
Lacoste, 1974).
After systemic uptake of ETU by plants, EU and 2-imidazoline
were identified as metabolites. Surface deposits of ETU, which
may have occurred as a result of EBDC treatment, formed an
additional unidentified substance as the main metabolite and
ethylene diamine (EDA). Propineb and PTU also formed an
identical but unidentified major metabolite under similar
conditions (Vogeler et al., 1977).
Nash (1975) reported the presence of 7 - 10 different
degradation products in methanol extracts of soybeans after soil
or foliar treatment with EBDCs, as well as after treatment with
ETU. In these cases, EU was a degradation product.
More recently, Nash & Beall (1980) studied the fate of maneb
and zineb in microagroecosystem chambers (Part A, section 6.3).
ETU on the tomato fruit and leaves, and in the soil, water, and
air was monitored for 100 days after treatment. ETU was
detected at < 20 µg/kg on whole fruit after 3 days, but had
completely disappeared after 3 weeks. The half-life of ETU was
< 3 days.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The amount of ETU in commercial formulations of EBDCs has
been shown to increase with increasing temperature and humidity.
ETU formation during storage appears to be greatest in maneb
formulations (up to 14%), followed by zineb and mancozeb. The
relative proportions of degradation products appear to be
different for the various EBDCs.
Studies with propineb on apples and grapes, carried out by
Vogeler et al. (1977), showed that PTU could be detected
shortly after treatment, and that it was rapidly transformed to
an unknown metabolite, together with small amounts of PU, and
other unidentified reaction products.
5.1. Food and Drinking-Water
The residue levels of ETU are mainly below 0.1 mg/kg product
following treatment (with different formulations) at the maximum
recommended EBDC levels (Newsome, 1976; Phillips et al., 1977).
Studies in Canada and the USA have shown that vegetables,
such as spinach, carrots, and potatoes, that have been treated
with EBDCs contain high levels of ETU after cooking (Blazquez,
1973; Newsome & Laver, 1973; Watts et al., 1974). Snapbeans
treated with maneb were found to contain ETU after commercial
canning (US EPA, 1977). Farrow & Ralls (1970) demonstrated the
disappearance of zineb, ziram, and maneb residues from spinach
and apricots during normal canning operations. There have been
many studies on ETU residues in crops, such as those of Sato &
Tomizawa (1960) on zineb-treated cucumbers.
The levels of ETU formed from residues of mancozeb and
polyram present on apples that were processed to make apple
juice, apple sauce, and apple pomace were determined (IUPAC,
1977). The results showed that ETU residues were 0.17 mg/kg
pomace and 0.05 mg/kg juice. A surprisingly high level of
unchanged mancozeb remained in the pomace, despite heat
treatment for 15 h at 150 °C. However, it seems that ETU
residues diminish during storage.
Residues of intact 14C-labelled ETU were found to diminish
with time in canned tomato sauce, spinach, pickles, and apple
sauce (Rose et al., 1980). EU and more polar products accounted
for most of the 14C-labelled residues. These polar materials
were resistant to extraction and appeared to be bound.
A study sponsored by the US EPA (Phillips et al., 1977), on
the effects of food processing on EBDC residues, confirmed and
extended the results previously described. Washing the raw
agricultural products prior to processing removed 33 - 87% of
the EBDC residue and the majority of the ETU residue. An
interesting result was that although almost instantaneous
conversion of mancozeb to ETU took place in boiling water, field
weathered residues of mancozeb appeared to be more resistant to
degradation to ETU. A summary of the results for raw and
processed material is given in Table 3.
5.2. Monitoring and Market-Basket Studies
A monitoring programme initiated in 1972 by the Canadian
government showed that 33% of food samples contained detectable
ETU residues. In particular, samples of canned spinach and
orange peel had average values of 0.047 mg/kg product and
0.083 mg/kg, respectively (Pecka et al., 1975; US EPA, 1977).
Studies on the actual level of ETU in products prepared for
commercial sale show it to be generally present in small
amounts. The highest level, 0.61 mg/kg product, was found in
canned peaches, while levels in orange peel, tomato paste,
instant potatoes, strawberries, peaches, and cucumbers were less
than 0.2 mg/kg product (US EPA, 1982a,b).
The US EPA has estimated an upper limit for dietary exposure
to ETU in the general population of the USA to be 3.65 µg/kg
body weight per day. This estimate is a maximum value, since it
was assumed that residues are present at the tolerance level and
that all of the EBDC residue is quantitatively converted to ETU.
Using actual residue data and experimentally derived conversion
factors, the US EPA estimated the dietary intake of ETU to be
0.24 µg/kg body weight per day.
In a market-basket study, over 500 samples of 34 foods were
analysed, plus 26 samples of drinking-water. No water samples
and only 21 of the food samples contained ETU residues (Gowers &
Gordon, 1980). Exposure estimates based on market-basket
studies range from 0.01 to 1 µg ETU/kg body weight per day
(Gowers & Gordon, 1980; Rose et al., 1980).
Tomato products (203 samples) were analysed in another
market-basket study, but none contained ETU (Gowers & Gordon,
1980).
A more realistic review of the actual exposure of the
general population was obtained by a "table-top" study. Of 200
meals (some from homes and some from restaurants) which were
analysed for ETU, none contained any residues (Gowers & Gordon,
1980).
6. KINETICS AND METABOLISM
6.1. Absorption, Distribution, and Excretion
ETU is rapidly absorbed from the gastrointestinal tract and
cleared from the body in all the mammalian species that have
been tested. After only 5 min, ETU appeared in the blood of
rats administered an oral dose of 100 mg 14C-ETU/kg body weight.
Within 48 h, 82 - 99% of an oral dose was eliminated via the
urine and about 3% via the faeces (Kato et al., 1976; Rose et
al., 1980). Newsome (1974) and Ruddick et al. (1976a) found
that approximately 70% was eliminated in the urine and 1% in the
faeces. Comparable results were found for mice while, in
monkeys, 55% was eliminated via the urine within 48 h, and less
than 1.5% via the faeces (Allen et al., 1978).
To study the accumulation and elimination of radioactivity
by the thyroid gland of rats dosed with 14C-ETU, dose levels of
2 and 200 µg labelled ETU were administered daily for 14 days.
In another study, rats were dosed with 0, 0.1, 1, 10, 50, or 100
mg 14C-ETU/kg diet, daily, for 7 days. The first study showed
that the concentration of ETU and/or its metabolites in the
thyroid is dose dependent, and the second that the level of 14C
in the thyroid did not increase appreciably when the daily dose
was increased above 50 mg/kg diet. Withdrawal of ETU from the
diet led to an 80 - 94% reduction in the radioactivity in the
thyroid after 17 days (Lyman & Lacoste, 1974).
ETU and its metabolites have been found to have a half-life
of about 28 h in monkeys, 9 - 10 h in rats, and 5 h in mice
(Rose et al., 1980).
In cows administered 1 mg 14C-ETU/kg diet, Lyman (1971)
found a small quantity of unchanged ETU in both the urine and
the milk of the test animals. Higher levels of 14C were
detectable in metabolites, such as glycine and urea, and in the
lactose and protein in the milk (Table 11).
6.2. Metabolic Transformation
It has been demonstrated that ETU degradation leads to
traces of EU and other metabolites in the urine and that 14C-
carbon dioxide is exhaled following the administration of
labelled ETU. Kato et al. (1976) suggested that the metabolites
of ETU in the rat were produced primarily by fragmentation of
the imidazoline ring and decarboxylation of the fourth and fifth
carbon atoms. A small amount of radioactivity was also found in
a protein fraction of rat fetal tissue. Ruddick et al. (1976a),
however, concluded that ETU metabolism in the rat does not
appear to result in any release of 14C into the general
metabolic pool. Mice metabolize ETU to EU and other unknown
metabolites, while cats metabolize it to S-methyl-ETU and EU.
Lyman (1971) detected EU, EDA, oxalic acid, glycine, and
urea as major metabolites in cow urine. In addition, 14C
originating from 14C-ETU was found in the protein and lactose in
the milk (Table 11). From these results, it appears that the
metabolism of ETU in ruminants is different from that in non-
ruminants. The degradation products of ETU in plants are
similar to those found in animals.
A summary of the secondary metabolites of ETU in biological
and non-biological systems is given in Fig. 4 (Part B, section
4).
Table 11. 14C Activity in the milk and urine of
cows fed with 1 mg 14C-ETUa/kg diet for 6 weeks
------------------------------------------------------
Milk Urine
Substance Concentra- % of Concentra- % of
tion total tion total
(mg/litre) 14C (mg/litre) 14C
------------------------------------------------------
ETU 0.011 31 0.12 7
EU 0.0025 8 0.27 18
EDA - - 0.14 14
Glycine - - - 6
Oxalic acid - - - 12
Urea - - - 11
Fat - 3 - -
Protein - 18 - -
Lactose - 16 - -
Total (%) 76 68
------------------------------------------------------
a From: Lyman (1971) and IUPAC (1977).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only limited information is available on the effects of ETU
on organisms in the environment, and none is available
concerning the impact of ETU on terrestrial organisms. Data
concerning the toxicity for aquatic organisms of ETU and its
breakdown product EU are summarized in Tables 4 and 5. From
these results, ETU appears to have a low toxicity for bacteria,
algae, crustacea, and fish. Because of the low partition
coefficient (Table 6) and rapid biotransformation of ETU,
bioaccumulation will be insignificant or absent (Van Leewen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Lewerenz et al. (1975) reported an acute oral LD50 for the
rat of 900 mg/kg body weight. The values determined by Graham &
Hansen (1972) and Teramoto et al. (1978a) were 1832 mg/kg and
545 mg/kg body weight, respectively. Teramoto et al. (1978a)
reported values of 3000 mg/kg body weight for the mouse and
> 3000 mg/kg body weight for the hamster.
8.2. Short- and Long-term Exposures
Administration of ETU to laboratory rats causes enlargement
of the thyroid gland. This effect was noted by Seifter & Ehrich
(1948), and has since been confirmed in various short- and long-
term studies. The accumulation of ETU in the thyroid gland is
associated with biochemical and morphological effects comparable
with those induced by known antithyroid drugs such as
thiouracil.
Graham & Hansen (1972) fed rats with diets containing ETU at
dose levels of 0, 50, 100, 500, or 750 mg/kg diet for 30, 60,
90, or 120 days, and at 100 mg/kg or more, thyroid changes were
seen. Ross Hart & Valerio (1973) fed doses of up to 1000 mg
ETU/kg diet to rats. An increase in thyroid weight was seen at
159 mg/kg or more, and the larger doses produced hyperplasia.
When Freudenthal et al. (1977) carried out studies on rats
at dose levels of up to 625 mg ETU/kg diet for 30, 60, or 90
days, biochemical changes reflecting effects on thyroid function
were observed. A dose of 125 mg/kg diet produced, after 30
days, a decrease in T3, T4, and serum PBI levels and an increase
in TSH concentration. A dose of 25 mg/kg diet produced a
decrease in T4 content after 60 days. Thyroid weight was
increased in all groups receiving 25 mg/kg or more. After 90
days, tumours (adenomas) were found in the 125 mg/kg group. The
group receiving 625 mg/kg died within 7 weeks.
From these short-term studies, it can be concluded that the
no-observed-adverse-effect level lies below 25 mg ETU/kg diet
and is probably of the order of 5 mg/kg diet (equivalent to
0.25 mg/kg body weight).
8.3. Teratogenicity
ETU was administered orally to rats and rabbits in single
daily doses of 0, 5, 10, 20, 40, or 80 mg/kg body weight. Rats
were treated from 21 - 42 days before conception to day 15 of
pregnancy, or on days 6 - 15 or 7 - 20 of pregnancy, whereas
rabbits were treated on days 7 - 21 of pregnancy. ETU at dose
levels of 10 mg or more induced meningoencephalocele,
meningorrhagia, meningorrhea, hydrocephalus, obliterated neural
canal, abnormal pelvic limb posture with equinovarus, and short,
kinky tail in all rats. Fetal survival was not affected, and
fetal growth was retarded only at 40 and 80 mg/kg. Rabbits
showed an increased incidence of resorption sites and decreased
brain weight at 80 mg/kg body weight, but no malformations
(Khera, 1973).
ETU was studied in rats and mice for its ability to induce
perinatal toxicity and in guinea-pigs and golden hamsters for
its teratogenic potential. The ETU was administered by gavage
and during organogenesis. Table 12 summarizes the prenatal
treatments. Additional postnatal studies were performed on rats
using extended treatment periods (ETU at 0, 20, 25, or 30 mg/kg
body weight), including continuous exposure from day 7 of
gestation through parturition to day 15 of lactation. The pups
were weaned normally and postnatal studies on open-field
behaviour were performed at 6 weeks. ETU at 80 mg/kg body
weight induced maternal toxicity and reduced growth in the rat
and was teratogenic for the rat, inducing substantial fetal
effects at all dose levels above 10 mg/kg body weight. Gross
defects were seen in the skeletal and the central nervous
systems, and cleft palate was noted, mainly at the highest dose
level. At 20 and 30 mg/kg, an increased incidence of
hydrocephalus was the only defect noted. The maternal and fetal
toxicity of ETU for the mouse, guinea-pig, and hamster was
substantially less than for the rat. In mice, at the highest
dose, an increase in maternal liver weight and in fetal
supernumerary ribs was noted, but no effects were seen in other
species (Chernoff et al., 1979; FAO/WHO, 1980b).
Table 12. Summary of prenatal treatmentsa
---------------------------------------------------------
Compound Species Dose (mg/kg Treatment
body weight) (gestation days)
---------------------------------------------------------
ETU rat 0, 5, 10, 20, 7 - 21
30, 40, 80
mouse 0, 100, 200 7 - 16
hamster 0, 25, 50, 100 5 - 10
guinea-pig 0, 50, 100 7 - 25
---------------------------------------------------------
a Modified from: Chernoff et al. (1979).
ETU has been found to induce a variety of postnatal
effects, including reduction or absence of maternal milk
production, thereby causing pup mortality at doses of 30 mg/kg
body weight or more. However, no significant dose-related
behavioural abnormalities were observed in ETU-exposed pups
(Chernoff et al., 1979; FAO/WHO, 1980b).
In studies by Khera & Tryphonas (1977), groups of pregnant
rats were administered ETU at dose levels of 0, 15, 30, or
45 mg/kg body weight on day 15 of gestation and then subjected
to a variety of test conditions to evaluate pre- and postnatal
effects. Postnatal mortality occurred in pups from mothers
treated with dose levels exceeding 15 mg/kg or pups cross-
fostered to evaluate lactation exposure. All pups from mothers
treated with 45 mg/kg died within 4 weeks of birth. A high
incidence of hydrocephalus and microphthalmia was observed in
pups of mothers treated with 30 mg/kg and these pups died within
6 weeks of birth. Motor defects observed in some survivors
(16/65) of this group, were shown to result from the
hydrocephalic condition, which was accompanied by atrophy of the
cerebral cortex and subcortical white matter. These defects
were found to be a direct result of in utero exposure to ETU and
not of exposure during lactation (cross-fostered pups showed the
same effects as pups weaned from treated dams). When mated with
normal male rats, all female offspring of rats administered
30 mg/kg gave birth to normal offspring. The F2 generation was
not impaired, though some of the parents had neurological
defects. In these studies, no effects on the parameters
examined were observed at 15 mg/kg body weight (Khera &
Tryphonas, 1977).
Teramoto et al. (1978a) investigated the teratogenicity of
ETU in rats, mice, and hamsters. It was teratogenic when given
orally to rats at 20 - 50 mg/kg body weight per day on days 6 -
15 of pregnancy and to hamsters at 270 - 810 mg/kg body weight
per day on days 6 - 13 of pregnancy. However, no malformations
were induced in mice up to a daily oral dose of 800 mg/kg body
weight when given on days 7 - 15 of pregnancy. In hamsters,
cleft palate, kinky tail, oligodactyly, and anal atresia were
noted as gross external malformations. Skeletal examination
revealed a high incidence of defects in the ribs and vertebral
column, but no apparent defect was observed during visceral
examination. An oral dose of 100 or 200 mg ETU/kg body weight
given to pregnant rats consistently produced brain abnormalities
in the fetuses, when given on day 12 or 13 of pregnancy, and
forelimb abnormalities, when given on day 13 of pregnancy
(Teramoto et al., 1978a). Histological studies revealed
extensive cell necrosis in the brain and forelimbs of embryos
24 h after the treatment. These lesions were considered to be
the main cause of the abnormalities observed. However, neither
malformations nor cell necrosis were found in the fetuses that
had been injected with 200 µg ETU/conceptus into the amniotic
sac on day 12 of pregnancy (Teramoto et al., 1980). Studies
with 2-14C-ETU revealed that this dose was sufficient to test
the direct effects of ETU on the embryos, since the
incorporation of radioactive substance was five times higher in
the embryos injected with 200 µg into the amniotic sac than it
was in those embryos whose mothers were treated with an oral
dose of 100 mg/kg body weight (Teramoto et al., 1980).
The teratogenic potential of the ETU metabolite 1-
methylthiourea has been investigated by Teramoto et al. (1981).
It caused almost the same types of malformations in rat fetuses
when given orally to mothers at 250 - 500 mg/kg body weight on
day 12 or day 14 of pregnancy as those observed following
treatment with ETU. However, 1-methylthiourea did not induce
malformations in mouse fetuses whose mothers were given an oral
dose of 1000 mg/kg on day 10 of pregnancy. There is a
structural similarity between 1-methylthiourea and ETU: C=S,
and -NH- groups seem essential for producing teratogenic effects
(Teramoto et al., 1981). However, the structure of ETU seems
quite specific for the induction of teratogenicity since Ruddick
et al. (1976b) tested 16 compounds related to ETU, including
ethylenethiuram monosulfide, another metabolite, and only one,
4-methylenethiourea, was teratogenic.
8.4. Mutagenicity
Tests with a large number of S. typhimurium strains gave
mostly negative results, though a few (weak) positive results
were observed in the case of some strains of S. typhimurium
(Shirasu et al., 1977). The addition of rat liver microsomes
seemed to enhance the mutant reversion. Schüpbach & Hummler
(1976, 1977) concluded that ETU appeared to induce base-pair
mutations but not frameshift mutations in S. typhimurium
TA 1530, although frameshift mutations appeared in S.
typhimurium TA 98, TA 1537, and TA 1538 when exposed to ETU in
the presence of dimethylsulfoxide (DMSO) and/or rat liver
microsomes (Rose et al., 1980).
Teramoto et al. (1977) did not find mutagenicity with S.
typhimurium TA 1536, TA 1537, TA 1538, G46, E. coli WP2 hcr+ and
hcr-, or B. subtilis H17 rec+ and rec- at concentrations
of 10 000 µg ETU/plate. However, a weak reaction was seen with
S. typhimurium TA 1535, and Seiler (1974) reported weak
(dose-unrelated) mutagenicity in S. typhimurium strain G46.
ETU was also found to be mutagenic in a host-mediated assay
of S. typhimurium TA 1530 when mice were dosed with 6000 mg
ETU/kg body weight, but not at doses of 2000 mg/kg or less
(Schüpbach & Hummler, 1977). Cytogenetic effects of ETU have
been reported in bone marrow cells of mice and Chinese hamsters.
On the other hand, there was no significant evidence to suggest
that ETU was mutagenic in host-mediated assays of S.
typhimurium G 46 or in tests with other strains of bacteria, rat
bone marrow (including the micronucleus test) Chinese hamster
DON cells, rat lymphocytes, or human fibroblast cells.
Furthermore, ETU did not increase the frequency of dominant
lethal mutations in rodents or Drosophila melanogaster (Seiler,
1973, 1974; Schüpbach & Hummler, 1977; Shirasu et al., 1977;
Teramoto et al., 1978b; Rose et al., 1980). A large number of
mutagenicity tests are summarized in a report of the US EPA
(1984).
ETU has been tested in the hepatocyte DNA repair test,
which is used to determine pro-carcinogenic potential as well as
DNA damage. ETU did not induce DNA damage (Althaus et al.,
1982) nor cause chromosomal damage in cultured rat liver cells,
and it did not induce chromatid exchange in CHO cells in
vitro or in mice in vivo. A micronucleus test with mice bone
marrow cells in vivo also gave negative results (De Serres &
Ashby, 1981).
This evidence indicates that ETU is generally not
mutagenic, especially in mammalian test systems.
8.5. Carcinogenicity
The carcinogenicity of ETU has been evaluated by IARC
(1974, 1982). It was classified in group 2B, i.e., limited
evidence for activity in short-term tests; sufficient evidence
for carcinogenicity in animals; inadequate evidence for
carcinogenicity in human beings.
ETU has been studied for oncogenic potential in mice, rats,
and hamsters.
8.5.1. Mouse
In a comprehensive programme screening chemicals for
carcinogenicity, two strains of hybrid mice (X and Y) were given
215 mg ETU/kg body weight from day 7 until weaning, and
thereafter 646 mg/kg diet for more than 18 months. In the X
strain [(C57B1/6XC3H/Anf)F1], the incidence of lung tumours in
the ETU-treated females was higher than that of the control
group (3/18 versus 3/87), but it was lower than that of the
controls (0/16 versus 1/90) in the Y strain [(C57B1/6XAKR)F1].
In the males, the incidence was higher in the ETU-treated
animals (3/18 versus 1/90). The incidence of lymphomas was
slightly increased in treated Y-strain females. The hepatoma
incidence in the ETU-treated groups of both strains was
significantly higher than that of the control group (in the X
strain, 14/18 and 18/18, for males and females respectively; in
the Y strain, 18/18 and 9/18; in controls, 0/18 and 3/18). The
thyroid glands were not examined for histopathological changes
(Innes et al., 1969).
Graham et al. (1975), found that ETU induced thyroid
hyperplasia and other research groups have confirmed this
finding.
8.5.2. Rat
Ulland et al. (1972) and Weisburger et al. (1981) fed
groups of 26 male and female Charles River-CD rats diets
containing 0, 175, or 350 mg ETU (97%)/kg diet. Five females
and five males of the high-dose group were killed after 18
months and the remainder after 24 months. Hyperplastic goitre,
solid cell adenomas, and thyroid (follicular or papillary)
carcinomas were found. Two of the animals also had lung
tumours, which might have been metastases. The thyroid tumour
incidence was dose dependant; (in the 175 mg/kg group, it was
3/26 and 3/26, for males and females, respectively, and in the
350 mg/kg group it was 17/26 and 8/26). No thyroid carcinomas
were observed in the control animals. A few of the treated rats
had hyperplastic nodules in the liver.
Graham et al. (1973, 1975) studied the long-term effects on
the thyroid gland of ETU ingestion. Five groups of 68 male and
68 female Charles River rats were fed ETU at levels of 0, 5, 25,
125, 250, or 500 mg/kg diet for 2 years. Growth depression was
evident at the highest dose level. The thyroid/body weight
ratio was significantly increased at 250 and 500 mg/kg, and
slightly increased at 125 mg/kg after 24 months. Thyroidal
uptake of 131iodine per mg tissue was significantly decreased in
male rats fed 500 mg ETU/kg diet for 18 or 24 months. The
thyroids of females fed at the three highest dose levels were
hypofunctioning at 6 months, and hyperfunctioning at 12 months,
and at 24 months thyroid function was similar to that of the
controls. At the two highest dose levels (250 and 500 mg/kg),
thyroid adenomas and carcinomas were induced. At all lower dose
levels hyperplasia occurred more frequently than in the
controls, but there were no adenomas or carcinomas. No increase
in liver tumours was observed in this study.
Gak et al. (1976) studied the effects of feeding rats with
0, 5, 17, 60, or 200 mg ETU/kg diet for 24 months. Body weight,
food consumption, serum enzyme activities (e.g. glutamic pyruvic
transaminase, alkaline phosphatase), hepatic enzyme activities
(glutamic pyruvic transaminase, alkaline phosphatase, glucose-6-
phosphate dehydrogenase), cholesterol levels, weights of thyroid
and other organs, and histopathology were studied.
Hypercholesterolemia was found at dose levels of 5 mg/kg and
above. At 60 mg/kg or more, a significant increase in thyroid
tumours was found, but at lower levels the tumour incidence was
not significantly different from that of the controls.
8.5.3. Hamster
Gak et al. (1976) studied the effect of 0, 5, 17, 60, or
200 mg ETU/kg diet on hamsters for 18 months. Growth, food
intake, biochemical parameters in the serum and liver, organ
weights, and histology were studied. A significant increase in
thyroid tumours was found at 60 mg ETU/kg or more, but at lower
doses values were not significantly different from those of the
control group.
8.6. ETU in Combination with Nitrite
When the mutagenicity of ETU was assayed before and after
nitrosation with sodium nitrite under acid conditions,
nitrosation was found to cause a 160-fold increase in the number
of revertant colonies of S. typhimurium TA 1535 (Shirasu et
al., 1977). The interactive mutagenicity of ETU and nitrite was
also found in the mouse dominant lethal test by Teramoto et al.
(1978b). However, no dominant-lethal mutations were induced in
a group of mice treated with 30 mg ETU plus 10 mg nitrite/kg
body weight. A large increase in pre-implantation losses was
noted 5 and 6 weeks after completing a 5-day treatment of males
with a combined oral dose of 150 mg ETU/kg and 50 mg sodium
nitrite/kg body weight.
8.7. Mechanisms of Toxicity; Mode of Action
The biochemical changes induced by antithyroid drugs
include reduced production of thyroid hormones (T3 and T4),
followed by increased production of TSH in response to low
thyroid hormone levels in the blood. Pathological changes in
the thyroid gland begin with diffuse microfollicular hyper-
plasia, and are followed by diffuse and nodular hyperplasia and
later by nodular hyperplasia with papillary and cystic changes
induced by the TSH. If hyperstimulation of the thyroid by TSH
is severe and prolonged, it provides conditions conducive to the
formation of tumours.
Antithyroid drugs can inhibit T4 production in various
ways. The chemical similarity of ETU to thiourea and thiouracil
suggests that ETU acts by blocking the iodination of thyroxine
precursors, thus reducing the synthesis of the thyroid hormones.
Iodide peroxidase catalyses the iodination of tyrosine and the
coupling of the resultant iodotyrosyl residues to produce the
active hormones T3 and T4.
Graham & Hansen (1972) found that ETU inhibited
iodide peroxidase in vitro. The resulting decreased level of
thyroid hormones causes stimulatory feedback of the pituitary
gland and consequently an increased release of TSH (Rose et al.,
1980).
Lu & Staples (1978) studied the influence of ETU in
pregnant hypothyroid and euthyroid rats to determine whether ETU
teratogenicity occurs as a result of altered maternal thyroid
function. Doses of 40 mg ETU/kg body weight, administered on
days 7 - 15 of gestation, resulted in 84 - 100% of the fetuses
in all treated groups being malformed, regardless of the thyroid
status of the dams. The authors concluded that the thyroid
status of the mother is not of importance in causing teratogenic
effects.
Rose et al. (1980) reported that the effects of feeding
rats 125 - 625 mg ETU/kg diet for 2 - 12 weeks, which included
thyroid hyperplasia and dose related suppression of serum T3 and
T4 (with corresponding TSH elevation), were reversible within 22
weeks of placing on control diets.
Long-term studies using ETU showed significantly increased
thyroid/body weight ratios in rats fed 125, 250, or 500 mg/kg
diet for periods of up to 2 years (Graham et al., 1975). This
effect was not reversed in rats placed on a control diet after
66 weeks of continuous exposure to 5 - 500 mg ETU/kg diet. It
is likely that by that time the thyroid was severely damaged.
In studies by Arnold et al. (1982, 1983), decreased levels
of serum thyroid hormones and increased thyroid weights were
reversed in Sprague Dawley rats fed diets containing 0, 75, 100,
or 150 mg ETU/kg diet for 7 weeks. The reversibility of
microscopic changes in the thyroids of male rats exposed to ETU
was studied. The rats were fed diets containing 75 or 150 mg
ETU/kg diet for 7 - 82 weeks and then returned to a control diet
for periods ranging from 2 to 42 weeks. The severity and extent
of reversibility of thyroid hyperplasia were found to depend on
the duration of exposure to ETU. Above a certain threshold,
hyperplasia did not regress significantly.
Numerous studies with ETU suggest that the rat is more
sensitive than other species to the effects of the thyroid. A
recent study with propylthiouracil, a thyroid inhibitor with a
mode of action similar to that of ETU, has confirmed that
monkeys are much less sensitive than rats. The sensitivity
difference was not quantified in vivo, but, in an in
vitro study, the concentration of inhibitor required to produce
the same level of thyroid peroxidase inhibition was
approximately 100 times greater for monkey enzyme than it was
for rat enzyme (Takayama et al., 1986).
8.8. Propineb and Propylenethiourea (PTU)
8.8.1. General
The toxicology of propineb was reviewed at JMPR meetings in
1977, 1980, and 1983. Because of concern expressed at the 1977
meeting regarding the potential for thyrotoxicity and
tumourigenicity of propylenethiourea (PTU), a breakdown product
of propineb, the meeting estimated only a temporary ADI for man.
Further evaluation of propineb was postponed pending the
submission of additional data. Data submitted for evaluation in
1985 consisted of long-term mouse and rat studies, mutagenicity
studies, and a special study into the effects of PTU on DNA. In
addition, data previously submitted for evaluation in 1983 were
re-examined. These data included several studies on propineb
(acute toxicity studies, a short-term study on thyroid function
in rats, mutagenicity studies, and an oncogenicity study on
mice) and on PTU (pharmacokinetic studies on rats and a long-
term thyroid function study on rats) (FAO/WHO, 1986a,b).
8.8.2. Toxicological information
An oncogenicity study on mice with propineb indicated
increased hepatocellular adenomas in male mice and increased
pulmonary adenomas in female mice at 800 mg/kg diet, the highest
dose level tested. Thyroid tumours were not induced in treated
mice in this study. A no-observed-adverse-effect level for non-
neoplastic effects could not be determined in this study, owing
to insufficient data (FAO/WHO, 1986a, b).
In a long-term study into the effects of PTU on mice, an
increased incidence in male mice of hepatocellular adenomas was
observed at 1000 mg/kg diet (the highest dose level tested) and
of hepatocellular carcinomas at 10 mg/kg diet or more. In the
same study, increased incidences of hepatocellular adenomas and
carcinomas were observed in female mice at 100 mg/kg diet or
more. Thyroid tumours attributable to PTU were not observed,
but increased thyroid hypercellularity was noted in male mice at
1000 mg/kg diet (FAO/WHO, 1986a, b).
In long-term rat studies with propineb, previously reviewed
by the JMPR, an increased incidence of benign thyroid tumours
was observed at 1000 mg/kg diet or more. Non-neoplastic thyroid
effects were observed in the same study at 100 mg/kg diet or
more. In another study, increased liver and kidney weights were
observed at 100 mg/kg diet or more and a no-observed-adverse-
effect level of 10 mg/kg diet was determined. In a long-term
study on the effects of PTU on rats, thyroid tumours
attributable to PTU were only found at 1000 mg/kg diet.
Goitrogenic effects in the thyroid were observed, however, at
dose levels as low as 1 mg/kg diet, the lowest dose level
tested. A no-observed-adverse-effect level could not be
determined in this study (FAO/WHO, 1986a, b).
Short-term studies on the effects of propineb on thyroid
function in rats did not establish an unequivocal no-observed-
adverse-effect level for effects on the thyroid. In a long-term
study with PTU, effects on thyroid function were observed at
1000 mg/kg diet, but at lower dose levels effects were
ambiguous. Pharmacokinetic studies on rats demonstrated
preferential uptake of radioactivity from 14C-labelled PTU by
the thyroid (FAO/WHO, 1986a, b).
Mutagenicity studies on propineb and PTU produced negative
or inconclusive results. However, PTU has been shown to
increase DNA synthesis in mouse spleen cells, but it did not
bind to mouse liver cell DNA.
In view of the carcinogenic response to PTU in the liver of
mice and the lack of a no-observed-adverse-effect level for the
effects of propineb on the thyroid in a long-term study on mice
or short-term studies on rats, or for PTU in a long-term study
on rats, the JMPR recommended that the temporary ADI for
propineb should be withdrawn.
In view of the established carcinogenic potential of this
compound, the meeting recommended that propineb should not be
used where its residues can arise in food (FAO/WHO, 1986a,b).
9. EFFECTS ON MAN
9.1. Epidemiological Studies
Smith (1976) conducted a detailed study involving 1929
workers in rubber-compounding plants in Birmingham, England. No
thyroid cancers were found in the health records of these
workers.
Clinical examinations and thyroid function tests were
carried out over a period of 3 years on eight process workers
and five mixers in a factory producing ETU in the United
Kingdom. Matched controls were also examined. The results
showed that the exposed mixers, but not the process workers, had
significantly lower levels of T4 in their blood compared with
the controls. No effect was found on TSH or thyroid-binding
globulin (Smith, 1984).
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) and
the International Agency for Research on Cancer (IARC) have
evaluated the toxicity and carcinogenicity data for various
dithiocarbamates on several occasions. Annex III includes an
overview of the JMPR meetings in which these compounds, ETU, and
PTU have been evaluated, with their references, together with
the WHO recommended classification of pesticides by hazard for
individual dithiocarbamates. The existence of IARC evaluations
and the availability of WHO/FAO Data Sheets and IRPTC Data
Profiles and Legal Files are also indicated. These documents
include more detail concerning the product and legal aspects,
toxicological evaluation, and residues of individual
dithiocarbamates in different food items.
REFERENCES
AASETH, J., ALEXANDER, J., & WANNAG, A. (1981) Effect of
thiocarbamate derivatives on copper, zinc, and mercury
distribution in rats and mice. Arch. Toxicol., 48: 29-39.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarbamates,
dithiocarbamates, Luxembourg, Commission of the European
Communities (Report No. V/F/1/78/75 EN).
ALLEN, J.R., VAN MILLER, J.P., & SEYMOUR, J.L. (1978)
Absorption, tissue distribution, and excretion of 14C-ETU in the
rhesus monkey and the rat. Chem. Pathol. Pharmacol., 20: 109-
115.
ALTHAUS, F.R., LAWRENCE, S.D., SATTLER, G.L., LONGFELLOW, D.G.,
& PITOT, H.C. (1982) Chemical quantification of unscheduled
DNA synthesis in cultured hepatocytes on an assay for the rapid
screening of potential chemical carcinogens. Cancer Res., 42:
3010-3015.
ALVAREZ, M., CREEKMORE, R.W., & ROSEN, R.T. (1973) Structure
of ethylenethiuram monosulfide. Tetrahedron Lett., 12: 939-942.
ANTONOVICH, E.A., CHERNOV, O.V., SAMOSH, L.V., MARTSON, L.V.,
PILINSKAYA, M.A., KURINNY, L.I., VEKSHTEIN, M.S., MARTSON, V.S.,
BALIN, P.N., & KHITSENKO, I.I. (1972) [A comparative toxico-
logical assessment of dithiocarbamates.] Gig. i. Sanit., 37: 25-
30 (in Russian).
ARNOLD, D.L., BICKIS, M.G., NERA, E.A., MCGUIRE, P.F., & MUNRO,
I.C. (1982) Assessing the reversibility of thyroid lesions
that result from feeding ETU. Toxicologist, 2(1): 90 (Abstract
of Presentation at the SOT Meeting 1982).
ARNOLD, D.L., KREWSKI, D.R., JUNKINS, D.B., MCGUIRE, P.F.,
MOODIE, C.A., & MUNRO, I.C. (1983) Reversibility of ethylene-
thiourea-induced thyroid lesions. Toxicol. appl. Pharmacol., 67:
264-273.
ASHWORTH, R., DE B., HENRIET, J., LOVETT, J.F., & MARTIJN, A.,
ed. (1980) CIPAC handbook - Volume 1A: Analysis of technical
and formulated pesticides, Cambridge, United Kingdom, W. Heffer
and Sons Limited. England.
AYANABA, A., VERSTRAETE, W., & ALEXANDER, M. (1973) Formation
of dimethylnitrosamine, a carcinogen and mutagen, in soils
treated with nitrogen compounds. Soil Sci. Soc. Am. Proc., 37:
565-568.
BABINI, G. (1966) [A case of erythroderma caused by zinc
dithiocarbamate.] Arch. Ital. Dermatol. Venereol. Sessuol., 34:
230/238 (in Italian).
BALIN, P.N. (1970) [Experimental data on the blastomogenic
activity of the fungicide maneb.] Vrach. Delo, 4: 21-24 (in
Russian).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest. Control, 9: 119-
127.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg., 38: 50-
52.
BENSON, W.R., ROSS, R.D., CHEN JO-YUN, R., BARRON, R.P., &
MASTBROOK, D. (1972) Structure of ethylenethiuram monosulfide.
J. Assoc. Off. Anal. Chem., 55(1): 44-46.
BLACKWELL-SMITH, R., Jr, FINNEGAN, J.K., LARSON, P.S., SAHYOUN,
P.F., DREYFUSS, M.L., & HAAG, H.B. (1953) Toxicologic studies
on zinc and disodium ethylenebisdithiocarbamates. J. Pharmacol.
exp. Therap., 109: 159-166.
BLAZQUEZ, C.H. (1973) Residue determination of ethylene-
thiourea (2-imidazol-idinethione) from tomato foliage, soil, and
water. J. agric. food Chem., 21: 330-332.
BOGARTYKH, T.A., ORLOV, N.V., & KUZNETSOVA, T.V. (1979)
Activity of lactate dehydrogenase and glucose-6-phosphate
dehydrogenase in tissue of the testes under the action of some
pesticides. Vopr. Pitan., 5: 49-53.
BONTOYAN, W.R. & LOOKER, J.G. (1973) Degradation of commercial
ethylenebisdithiocarbamate formulations to ethylenethiourea
under elevated temperature and humidity. J. agric. food Chem.,
21: 338-342.
BONTOYAN, W.R., LOOKER, J.B., KAISER, T.E., GIANG, P., & OLIVE,
B.M. (1972) Survey of ethylenethiourea in commercial
ethylenebisdithiocarbamate formulations. J. Assoc. Off. Anal.
Chem., 55: 923-925.
BOTTOMLEY, P., HOODLESS, R.A., & SMART, N.A. (1985) Review of
methods for the determination of ethylenethiourea (imida-
zolidine-2-thione) residues. Residue Rev., 95: 45-89.
CAVANAGH, J.B. (1973) Peripheral neuropathy caused by chemical
agents. Crit. Rev. Toxicol., 2: 365-417.
CHARKES, N.D., BRAVERMAN, L.E., PENKO, K.F., GOWERS, D.S.,
GORDON, C.F., LIPWORTH, L., & MALMUD, L.S. (1985) Thyroid
function in male workers manufacturing dithane, an agricultural
fungicide, and in men not exposed to dithane. In: Proceedings of
the Ninth International Thyroid Congress, Sao Paulo, Brazil,
1-6 September 1985, p. 89.
CHERNOFF, N., KAVLOCK, R.J., ROGERS, E.H., CARVER, B.D., &
MURRAY, S. (1979) Perinatal toxicity of maneb, ethylene-
thiourea, and ethylene bisisothiocyanate sulfide in rodents. J.
Toxicol. environ. Health, 5: 821-834.
CHERNOV, O.V. & KHISTENKO, I.I. (1969) Blastomogenic
properties of some derivatives of dithiocarbamic acid. Vopr.
Onkol., 15(4): 71-74.
CHERNOV, O.V. & KHISTENKO, I.I. (1973) Some data on the
distribution and elimination of ziram-35S from the organism of
warm-blooded animals. In: Hygiene of using toxicology of
pesticides and the clinical picture of intoxication, Moscow,
Medizina, Vol. 10, pp. 250-254. (in Russian).
CLARKE, D.G., BAUM, H., STANLEY, E.L., & HESTER, W.F. (1951)
Determination of dithiocarbamates. Anal. Chem., 23(12): 1842-
1846.
CONKIN, R.A. & GLEASON, L.S. (1964) Vegadex. In: Zweig, G.,
ed. Analytical methods for pesticides, plant growth regulation
and food additives, New York, London, Academic Press, Vol. 4,
pp. 249-257.
CRUICKSHANK, P.A. & JARROW, H.C. (1973) Ethylenethiourea
degradation. J. agric. food Chem., 21 (3): 333-335.
DALVI, R.R., POORE, R.E., & NEAL, R.A. (1974) Studies of the
metabolism of carbon disulfide by rat liver microsomes. Life
Sci., 14: 1785-1796.
DANIELSSON, B.R.G., OSKARSSON, A., & DENCKER, L. (1984)
Placental transfer and fetal distribution of lead in mice after
treatment with dithiocarbamates. Arch. Toxicol., 55: 27-33.
DAVYDOVA, T.B. (1973) [The influence of tetramethylthiuram
disulfide on the estrous cycle and genital function of animals
when inhaled.] Gig. i Sanit., 38(4): 108-110 (in Russian).
DEKHUYZEN, H.M., VONK, J.W., & KAARS SIJPESTEIJN (1971)
Terminal residues of dithiocarbamate fungicides. In: Tahori,
A.S., ed. Proceedings of the International Symposium on Pesti-
cide Terminal Residues, Tel Aviv, Israel, 1971, London,
Butterworth, pp. 233-241.
DE MATTEIS, F. & SEAWRIGHT, A.A. (1973) Oxidative metabolism
of carbon disulfide by the rat. Effect of treatments which
modify the liver toxicity of carbon disulfide. Chem.-biol.
Interact., 7: 375-388.
DE SERRES, F.S. & ASHBY, J.C. (1981) Report of the
International Collaborative Program for Progress in Mutation
Research, Vol. 1, Amsterdam, Oxford, New York, Elsevier North-
Holland Inc.
DISHOVSKI, H. & IVANOVA-CHEMISHANSKA, L. (1979) Ultra-
structural changes in the neocortex of rats after chronic
dithiocarbamate Antracol. Activ. nerv. sup. (Praha), 21 (4):
279.
EDINGTON, N. & HOWELL, J.McC. (1966) Changes in the nervous
system of rabbits following the administration of sodium
diethyldithiocarbamate. Nature (Lond.), 210(5040): 1060-1062.
EDINGTON, N. & HOWELL, J.McC. (1969) The neurotoxicity of
sodium diethyldithiocarbamate in the rabbit. Acta neuropathol.
(Berlin), 12: 339-347.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N-nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under simulated gastric conditions and in vivo in the rat
stomach. Food Cosmet. Toxicol., 12: 229-232.
ELDJARN, L. (1950) The metabolism of tetraethylthiuram
disulfide (Antabuse, Aversan) in the rat, investigated by means
of radioactive sulfur. J. clin. lab. Invest., 2: 198-201.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ENGST, R. & SCHNAAK, W. (1967) [Studies on the metabolism of
the ethylenebisdithiocarbamate fungicides maneb and zineb.] Z.
Lebensm. Unters. Forsch., 134(4): 216-221 (in German).
ENGST, R. & SCHNAAK, W. (1969a) [Polarographic determination
of ethylene bisthiuram monosulfide.] Z. anal. Chem., 248: 321-
324 (in German).
ENGST, R. & SCHNAAK, W. (1969b) [Analysis of ethylene
bisthiuram monosulfide.] Z. anal. Chem., 244: 254-256 (in
German).
ENGST, R. & SCHNAAK, W. (1969c) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb
(polarographic determination of the undecomposed fraction of
active ingredient).] Z. Lebensm. Unters. Forsch., 139(3): 149-
152 (in German).
ENGST, R. & SCHNAAK, W. (1970a) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 143: 99-103 (in German).
ENGST, R. & SCHNAAK, W. (1970b) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 144(2): 81-85 (in German).
ENGST, R. & SCHNAAK, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev.,
52: 45-67.
ENGST, R., SCHNAAK, W., & LEWERENZ, H.J. (1971) [Studies on
the metabolism of the fungicidal
ethylenebisdiothiocarbamatesmaneb, zineb and nabam. V. The
toxicology of breakdown products.] Z. Lebensm Untersuch., 146,
91-97.
FAHRIG, R. (1974) Comparative mutagenicity studies with
pesticides. In: Montesano, R. & Tomatis, L., ed. Chemical
carcinogenesis assays, Lyons, International Agency for Research
on Cancer, pp. 161-181 (IARC Scientific Publication No. 10).
FAO (1985) Production yearbook 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO Meeting
Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL: 1967/M/1/1; WHO
Food Add./68.30).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO
Food Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 97; WHO Technical Report Studies No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10
Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations(FAO Plant Production and Protection Paper No. 10 Sup.).
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization (FAO
Plant Production and Protection Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20
Sup.).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 26 Sup.).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues
in food, Part II - Toxicology, Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 72).
FARROW, R.P. & RALLS, J.W. (1970) Investigation of changes in
pesticide residues during processing and storage of fruit and
vegetables, National Canners Association, 109 pp (Report No.
5).
FISHBEIN, L. (1975) Chromatography of environmental hazards.
III. Pesticides, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
FITZHUGH, O.G., WINTER, W.J., & NELSON, A.A. (1952) Some
observations on the chronic toxicity of antabuse (tetraethyl-
thiuramdisulfide). Fed. Proc., 11: 345-346.
FREUDENTHAL, R.I., KERCHNER, G.A., PERSING, R.L., BAUMEL, J., &
BARON, R.L. (1977) Subacute toxicity of ethylene bisisocyanate
sulfide in the laboratory rat. J. Toxicol. environ. Health, 2:
1067-1077.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Différence de
sensibilité du hamster et du rat vis-ą-vis des effets de
l'administration ą long terme de l'éthylčne thiourée. Eur. J.
Toxicol., 9: 303-312.
GARDNER-THORPE, C. & BENJAMIN, S. (1971) Peripheral neuropathy
after disulfiram administration. J. Neurol. Neurosurg.
Psychiatr., 34: 253-259.
GESSNER, T. & JAKUBOWSKI, M. (1972) Diethyldithiocarbamic acid
methyl ester, a metabolite of disulfiram. Biochem. Pharmacol.,
21: 219-230.
GOWERS, D.S. & GORDON, C.F. (1980) Some public health aspects
of the manufacture and use of zinc and manganese
ethylenebisdithiocarbamate fungicides Poznan, Poland, Institut
Ochrony Roslin, February 1979 pp. 491-522.
GRAHAM, S.L. & HANSEN, W.H. (1972) Effects of short-term
administration of ethylenethiourea on thyroid function of the
rat. Bull. environ. Contam. Toxicol., 7: 19-25.
GRAHAM, S.L., DAVIS, K.J., & PERRY, C.H. (1973) Effects of
one-year administration of ethylenethiourea upon thyroid
function of the rat. J. agric. food Chem., 21(3): 324-329.
GRAHAM, S.L., DAVIS, K.J., HANSEN, W.H., & GRAHAM, C.H. (1975)
Effects of prolonged ethylenethiourea ingestion on the thyroid
of the rat. Food Cosmet. Toxicol., 13: 493-499.
GRAHAM, W.H. & BORNAK, W.E. (1973) Improved experimental
technique for reverse isotope dilution method.
Anal. Chem., 45: 623-624.
GRIEPENTROG, F. (1960) [Allergy studies with simple chemical
substances.] Arzneimittelforschung, 10: 542-544 (in German).
GRIEPENTROG, F. (1962) [Tumor-like thyroid lesions in animal
studies of chronic toxicity of thiurams.] Beitr. Pathol.
Anat., 126: 243-255 (in German).
GUSTAFSSON, K.H. & THOMPSON, R.A. (1981) High-pressure liquid
chromatographic determination of fungicidal dithiocarbamates. J.
agric. food Chem., 29: 729-732.
HAINES, L.D. & ADLER, I.L. (1973) Gas chromatographic deter-
mination of ethylenethiourea residues. J. Assoc. Off. Anal.
Chem., 56 (2): 333-337.
HALD, J. & JACOBSEN, E. (1948) The formation of acetaldehyde
in the organism after ingestion of Antabuse (tetraethylthiuram
disulfide) and alcohol. Acta pharmacol. toxicol., 4: 305-310.
HALD, J., JACOBSEN, E., & LARSEN, V. (1948) The sensitizing
effect of tetraethylthiuram disulphide (Antabuse) to ethyl
alcohol. Acta pharmacol. toxicol., 4: 285-296.
HEDENSTEDT, A., RANNUG, U., LAMEL, C., & WACHTMEISTER, C.A.
(1979) Mutagenicity and metabolism studies on 12 thiram and
dithiocarbamate compounds used as accelerators in the Swedish
rubber industry. Mutat. Res., 68: 313.
HELLING, C.S., DENNISSON, D.C., & KAUFMAN, D.D. (1974)
Fungicide movement in soil. Phytopathology, 64(8): 1091-1100.
HOAGLAND, R.E. & FREAR, S.D. (1976) Behaviour and fate of
ethylenethiourea in plants. J. agric. food Chem., 24: 129-133.
HODGE, H.C., MAYNARD, Z.A., DOWNS, W., BLANCHET, H.J., Jr, &
JONES, C.K. (1952) Acute and short-term oral toxicity tests of
ferric dimethyldithiocarbamate (ferbam) and zinc dimethyldithio-
carbamate (ziram). J. Am. Pharmacol. Assoc., 41(12): 662-665.
HODGE, H.C., MAYNARD, E.A., DOWNS, W.L., COYE, R.D., Jr, &
STEADING, L.T. (1956) Chronic oral toxicity of ferric di-
methyldithiocarbamate (ferbam) and zinc dimethyldithiocarbamate
(ziram). J. Pharmacol. exp. Ther., 118: 174-181.
HODGSON, J.R., CASTLES, T.R., MURRILL, E., & LEE, C.C. (1974)
Distribution, excretion, and metabolism of the fungicide ferbam
in rats. Fed. Proc., 33: 537.
IARC (1974) Some antithyroid and related substances, nitro-
furans and industrial chemicals, Lyons, International Agency for
Research on Cancer, pp. 45-52 (Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 7).
IARC (1976) Some carbamates, thiocarbamates, and carbazides,
Lyons, International Agency for Research on Cancer (Monographs
on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol.
12).
IARC (1977) Some miscellaneous pharmaceutical substances,
Lyons, International Agency for Research on Cancer p. 243.
(Monographs on the evaluation of carcinogenic risk of chemicals
to humans, Vol. 13).
IARC (1983) Chemicals, industrial processes and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 128-130 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumour-
igenicity in mice: a preliminary note. J. Natl Cancer Inst., 42:
1101-1114.
IRPTC (1982) Data profile on ziram, Geneva, International
Register for Potentially Toxic Chemicals.
IRPTC (1983) Legal file - 2 vols, Geneva, International
Register for Potentially Toxic Chemicals.
IUPAC (International Union of Pure and Applied Chemistry) (1977)
Ethylenethiourea. Pure appl. Chem., 49: 675-689.
IVANOVA-CHEMISHANSKA, L. (1969a) Toxicological characteristics
of dithiocarbamates zineb, maneb, and mancozeb, Sofia,
(Dissertation).
IVANOVA-CHEMISHANSKA, L. (1969b) Toxicological characteristics
of mancozeb. Hig. i. Zdraveopazvane, 12(5): 417-426.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads reproduction of white rats under the effect of some
pesticides. Arch. Toxicol., Suppl. 4: 459-462.
IVANOVA-CHEMISHANSKA, L., SHEYTANOV, M., & MOSHEVA-IZMIROVA, N.
(1967) Changes in the functional state of the thyroid gland
upon acute intoxication with certain dithiocarbamates: zineb and
maneb. Med. Toxicol., 20(9): 1011-1013.
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., & DASHEV, G. (1971)
Light and electron microscopic observation on rat thyroid after
administration of some dithiocarbamates. Environ. Res., 4: 201-
212.
IVANOVA-CHEMISHANSKA, L., KALOYANOVA, F., IZMIROVA, N., ZLATEVA,
M., & VALCHEVA, V. (1972) [Experimental inhalatory toxicity and
substantiation of the maximum allowable concentration for some
dithiocarbamates in the air of the working area.] Truh. Dzihot.,
23: 55-64 (in Russian).
IVANOVA-CHEMISHANSKA, L., VALCHEVA, V., & TAKEVA, TZ. (1973)
Effect of chronic peroral poisoning with perozine (zineb) on the
reproduction of white rats. Acta med. soc., 1: 107-108.
IVANOVA-CHEMISHANSKA, L., MILANOV, ST., CHEMISHANSKI, G., &
DASHEV, G. (1974) Attempt of biological dose of some
hypophyseal hormones in white rats after experimental oral
administration of zineb. Environ. Res., 8: 160-165.
IVANOVA-CHEMISHANSKA, L., PETROVA-VERGIEVA, T., & MIRKOVA, E.
(1975a) [Embryotoxic and teratogenic action of some
pesticides.] Eksp. Med. Morfol., 14(1): 29-33 (in Russian).
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., MILANOV, S.,
STRASHIMIROV, D.D., DASHEV, G.I., & CHEMISHANKSKA, G.A. (1975b)
Effects of subacute oral administration of zinc ethylene bisdi-
thiocarbamate on the thyroid gland and adenohypophysis of the
rat. Food Cosmetol. Toxicol., 13: 445-447.
IZMIROVA, N. & MARINOV, V. (1972) [Distribution and excretion
of 35S-ziram and metabolic products after 24 hours following
oral administration of the preparation in female rats.] Eksp.
Med. Morfol., 11(3): 152-156 (in Russian).
IZMIROVA, N., IZMIROVA, I., & IVANOVA, L. (1969) The effect of
zineb and maneb on the isoenzymes of lactate dehydrogenase in
the testes of rats. C. R. Acad. Bulg. Sci., 22(2): 225-227.
JORIS, S.J., ASPILA, K.I., & CHAKRABARTI, C.L. (1970) Decompo-
sition of monoalkyl dithiocarbamates. Anal. Chem., 42(6): 647-
651.
KAARS SIJPESTEIJN, A.K. & VONK, J.W. (1970) Microbial conver-
sion of dithiocarbamate fungicides. Med. Fac. Landbouwwet.
Gent, 35(2): 799-804.
KALOYANOVA, F., IVANOVA, L., & ALEXIEV, B. (1967) The
influence of large doses of maneb on the progeny of albino rats.
Med. Toxicol., 20(10): 1109-1112.
KAMPMEIER, C. & HAAG, H.B. (1954) Toxicological considerations
in use of dithiocarbamates. Agric. Chem., April: 49-50, 133.
KASKEVICH, L.M., KOMAROVA, L.I., ILINA, V.I., IVANICKI, V.A., &
SHILINA, V.F. (1981) [Clinical-functional changes due to
exposure to zineb,] pp. 109-111 (in Russian).
KASLANDER, J. (1963) Formation of an S-glucuronide from
tetraethylthiuram disulfide (Antabuse) in man. Biochem. Biophys.
Acta, 71: 730-732.
KATO, Y., ODANAKA, Y., TERAMOTO, S., & MATANO, O. (1976) Meta-
bolic fate of ethylenethiourea in pregnant rats. Bull. environ.
Contam. Toxicol., 16(5): 546-555.
KAUFMAN, D.D. & FLETCHER, C.L. (1973) Title? In: Proceedings
of the Second International Congress on Plant Pathology,
Minneapolis, Minnesota (Abstract No. 1018).
KHERA, K.S. (1973) Ethylenethiourea: teratogenicity study in
rats and rabbits. Teratology, 7: 243-252.
KHERA, K.S. & TRYPHONAS, L. (1977) Ethylenethiourea induced
hydrocephalus: pre- and postnatal pathogenesis in offspring from
rats given a single oral dose during pregnancy. Toxicol. appl.
Pharmacol., 42: 85-97.
KIM, S.U. & RIZZUTO, N. (1975) Neuroaxonal degeneration
induced by sodium diethyldithiocarbamate in cultures of central
nervous tissue. J. Neuropath. exp. Neurol., 34: 531-541.
KING, R.R. (1977) Derivatization of ethylenethiourea with m-
trifluoromethylbenzylchloride for analysis by electron capture
gas chromatography. J. agric. food Chem., 25(1): 73-75.
KIRKBRIGHT, G.F. & MULLINS, F.G.P. (1984) Separation of
dithiocarbamates by high-performance liquid chromatography using
a micellar mobile phase. Analyst, 109: 493-496.
KLIGMAN, A.M. & ROSENSWEIG, W. (1948) Studies with new
fungistatic agents. I. For the treatment of superficial
mycoses. J. invest. Derm., 10: 59-68.
KOIZUMI, A., SHIOJIMA, S., OMIYA, M., NAKANO, S., SATO, N., &
IDEDA, M. (1979) Acute renal failure and maneb (manganous
ethylene bisdithiocarbamate) exposure. J. Am. Med. Assoc.,
242(23): 2583-2585.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982a) Embryo-
toxicity of industrial chemicals on the chicken embryo:
thiourea derivatives. Acta pharmacol. toxicol., 51: 38-44.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982b) Application
of the chicken embryo in testing for embryotoxicity. Scand. J.
Work environ. Health, 8: 63-69.
KURINNY, A.I. & KONDRATENKO, T.I. (1972) [Effect of some
fungicides (dithiocarbamic acid derivatives) on chromosomes of
bone marrow cells in mice.] Tsitol. Genet., 6: 225-228 (in
Russian).
LABORIE, F. & LABORIE, R. (1966) Allergies aux fongicides et
prophylaxie biochimique (relations entre allergie et
intoxications). Rev. Pathol. comp., 66: 105-109.
LAKOMAA, E.L., SATO, S., GOLDBERG, A.M., & FRAZIER, J.M. (1982)
The effect of sodium diethyldithiocarbamate treatment on copper
and zinc concentrations in rat brain. Toxicol. appl. Pharmacol.,
65: 286-290.
LANGE, P. & JUNG, F. (1971) [Reduction of the toxicity to the
liver of carbon tetrachloride by diethyldithiocarbamate and 3-
(O-tolyl)-4-(nitro)-sydnone.] Acta biol. med. Ger., 27: 425-434
(in German).
LARSSON, K.S., ARNANDER, C., CEKANOVA, E., & KJILLBERG, M.
(1976) Studies of teratogenic effects of the dithiocarbamates
maneb, mancozeb, and propineb. Teratology, 14: 171-183.
LEE, C.C. & PETERS, P.J. (1976) Neurotoxicity and behavioural
effects of thiram in rats. Environ. Health Perspect., 17: 35-
42.
LEHMAN, A.J. (1951) Chemicals in food: a report of the
association of food and drug officials on current developments,
II, Pesticides, Q. Bull. Fed. Drug Off.U.S., 15: 122-133.
LEWERENZ, H.J., BLEYL, D., PLASS, R., & ENGST, R. (1975)
Toxicology of ETU , Potsdam, Rehbrüke, German Democratic
Republic, Central Institute for Nutrition, Academy of Science.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972) Carcino-
genic nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LOWY, R., GRIFFATON, G., BRIGANT, L., ARDONIN, B., & DUPUY, F.
(1979) The dietary no-effect level of a dithiocarbamate
fungicide, thiram, as evaluated from measurement data on rats.
II. The various sensitivities of the various parameters.
Toxicology, 14: 39-53.
LOWY, R., GRIFFATON, G., & PELISIER, M.A. (1980) The dose-
response relationship of rat organ weights under dietary thiram
influence. Toxicol. Lett., 5(Suppl. 1): 148.
LU, M.H. & STAPLES, R.E. (1978) Teratogenicity of ethylene-
thiourea and thyroid function in the rat. Teratology, 17: 171-
178.
LUDWIG, R.A. & THORN, G.D. (1962) Chemistry and mode of action
of dithiocarbamate fungicides. Adv. Pestic. Control Res., 3:
219-252.
LUTZ, L.M., GLENDA, E.A., Jr, & RECKNAGEL, R.O. (1973) Protec-
tion by diethyldithiocarbamate against carbon tetrachloride
lethality in rats and against carbon tetrachloride-induced lipid
peroxidation in vitro. Biochem. Pharmacol., 22: 1729-1734.
LYMAN, W.R. (1971) The metabolic fate of Dithan M-45
(coordination product of zinc ion and manganous ethylene
bisdithiocarbamate). In: Tahori, A.S., ed. International
Symposium on Pesticide Terminal Residues, Tel Aviv, 1971,
Israel, London, Butterworth, pp. 243-256.
LYMAN, W.R. & LACOSTE, R.J. (1974) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
In: Proceedings of the 3rd International IUPAC Congress on
Pesticide Chemistry, Helsinki, 3-9 July, 1974, Stuttgart, George
Thieme Publishers, pp. 67-74.
LYMAN, W.R. & LACOSTE, R.J. (1975) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
Environ. Qual. Saf., Suppl. 3: 67-74.
McCANN, J. & PITCHER, F. (1973) Fish toxicity laboratory
reports Nos. 535 and 541, Beltsville, Maryland, US Environmental
Protection Agency, Animal Biology Laboratory.
MAJ, J., GRABOWSKA, M., & KWIEK, J. (1970) The effect of
disulfiram, diethyldithiocarbamate, and dimethyldithiocarbamate
on serotonin and 5-hydroxyindole-3-acetic acid brain levels in
rats. Biochem. Pharmacol., 19: 2517-2519.
MARSHALL, W.D. (1977) Thermal decomposition of ethylene
bisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. agric. food Chem., 25: 357-361.
MASSEY, R.C., KEY, P.E., & MCWEENY, D.J. (1982) Analysis of
ethylenethiourea in beer by high-performance liquid
chromatography. J. Chromatogr., 240: 254-256.
MATOKHNYUK, L.A. (1971) Toxicity of the fungicide maneb on
inhalation. Hyg. Sanit., 36(5): 195-199.
MATTHIASCHK, G. (1973) [Influence of L-cysteine on
teratogenisis caused by thiram (TMTD) in NMRI mice.] Arch.
Toxikol., 30: 251-262 (in German).
MELNIKOV, N.N. & TRUNOV, P.P. (1966) Persistence of residual
quantities of preparations on the basis of dithiocarbamate acid
derivatives. Chem. Agric. IV, 10(36): 21-24.
MINOR, J.L., RUSSEL, J.Q., & LEE, C.C. (1974) Reproduction and
teratology studies with the fungicide ferbam. Toxicol. appl.
Pharmacol., 29(16): 120.
MOJE, W., MUNNECKE, D.E., & RICHARDSON, L.T. (1964) Carbonyl
sulfide, a volatile fungitoxicant from nabam in soil. Nature
(Lond.), 202: 831-832.
NASH, R.G. (1974) Improved gas-liquid chromatographic method
for determining ethylenethiourea in plants. J. Assoc. Off. Anal.
Chem., 57(5): 1015-1021.
NASH, R.G. (1975) In: Proceedings of the 170th Meeting of the
American Chemical Society for Pesticides (Abstract No. 1).
NASH, R.G. & BEALL, M.L., Jr (1980) Fate of maneb and zineb
fungicides in microagroecosystem chambers. J. agric. food Chem.,
28: 322-330.
NEWSOME, W.H. (1972) Determination of ethylenethiourea
residues in apples. J. agric. food Chem., 20: 967-969.
NEWSOME, W.H. (1974) The excretion of ethylenethiourea by rat
and guinea-pig. Bull. environ. Contam. Toxicol., 11: 174-176.
NEWSOME, W.H. (1976) Residues of four ethylene bisdithio-
carbamates and their decomposition products on field-sprayed
tomatoes. J. agric. food Chem., 24(5): 999-1001.
NEWSOME, W.H. (1978) A method for the determination of
ethyleneurea in foods as the pentafluorobenzamide. J. agric.
food Chem., 26(6): 1325-1327.
NEWSOME, W.H. & LAVER, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb-treated foods. Bull.
environ. Contam. Toxicol., 10(3): 151-154.
ODEYEMI, O. (1980) Persistence of arasan pesticide in aquatic
ecosystems in Nigeria. Pestic. Abstr., 80: 1276.
ONLEY, J.H. (1977) Gas-liquid chromatographic method for
determining ethylenethiourea in potatoes, spinach, apple sauce,
and milk. Collaborative study. J. Assoc. Off. Anal. Chem.,
60(5): 1111-1115.
ONLEY, J.H. & YIP, G. (1971) Determination of ethylenethiourea
residues in foods using thin-layer and gas chromatography. J.
Assoc. Off. Anal. Chem., 54(1): 165-169.
OSKARSSON, A. (1983) Redistribution and increased brain uptake
of lead in rats after treatment with diethyldithiocarbamate.
Arch. Toxicol., 6: 279-284.
OSKARSSON, A. (1984) Dithiocarbamate induced redistribution
and increased brain uptake of lead in rats. Neurotoxicology,
5(3): 283-294.
OSKARSSON, A. & LIND, B. (1985) Increased lead levels in brain
after long-term treatment with lead and dithiocarbamate or
thiuram derivatives in rats. Acta pharmacol. toxicol., 56: 1-6.
OTTO, S., KELLER, W., & DRESCHER, N. (1977) A new gas
chromatographic determination of ethylenethiourea residues
without derivatization. J. environ. Sci. Health, B12(3): 179-
191.
PECKA, Z., BAULU, P., & NEWSOME, H. (1975) Preliminary survey
of ethlenethiourea residues in the Canadian food supply, 1972.
Pestic. monit. J., 8(4): 232-234.
PERIQUET, A. & DERACHE, R. (1976) Influence d'un régime
alimentaire hypoprotéique sur la toxicité aigue d'un pesticide,
le nabame. Ann. Nutr. Aliment., 30: 29-44.
PETROVA-VERGIEVA, T. (1976) On the teratogenic activity of
zinc-propylene bisdithiocarbamate. Hig. i. Zdraveopazvane,
19(5): 435-442.
PETROVA-VERGIEVA, T. & IVANOVA-CHEMISHANSKA, L. (1973)
Assessment of the teratogenic activity of dithiocarbamate
fungicides. Food Cosmet. Toxicol., 11: 239-244.
PHILLIPS, W.E.J. (1968) Changes in the nitrate and nitrite
contents of fresh and processed spinach during storage. J.
agric. food Chem., 16: 88-91.
PHILLIPS, W.F., GRADY, M.D., & FREUDENTHAL, R. (1977) Effects
of food processing on residues of ethylene bisdithiocarbamate
fungicides and ethylenethiourea, Washington DC, US Environmental
Protection Agency (EPA No. 600/1-77-021).
PILINSKAYA, M.A. (1971) [Cytogenetic effect of the fungicide
ziram in the culture of human lymphocytes in vitro. ] Genetika,
7: 138-143 (in Russian).
PLUYGERS, C.W., VONK, J.W., & THORN, G.D. (1971) Re-
examination of the structure of ethylenethiuram monosulfide.
Tetrahedron Lett., 18: 1317-1318.
PRZEZDZIECKI, Z., BANKOWSKA, J., KOMOROWSKA-MALEWSKA, W., &
JANICKA, T. (1969) Studies of the chronic short-term toxicity
of zineb, maneb, and kaptan. Rocz. Panstw. Zakl. Hlg., 20: 133-
140.
PRICKETT, C.S. & JOHNSTON, C.D. (1953) The in vivo production
of carbon disulfide from tetraethylthiuram disulfide
(AntabuseR). Biochem. Biophys. Acta, 12: 542-546.
PRINCE, J.L. (1985) Analysis of ethylenethiourea in urine by
high-performance liquid chromatography. J. agric. food Chem.,
33: 93-94.
RAGHU, K., MURTHY, N.B.K., KUMARASWAMY, R., RAO, R.S., & SANE,
P.V. (1975) In: Proceedings of the Joint FAO/IAEA, Division of
Atomic Energy in Food and Agriculture, Vienna, p. 137.
RASUL, A.R. & HOWELL, J.M. (1974) The toxicity of some
dithiocarbamate compounds in young and adult domestic fowl.
Toxicol. appl. Pharmacol., 30: 63-78.
RHODES, R.C. (1977) Studies with manganese 14C-ethylene
bisdithiocarbamate (14C-maneb) fungicide and 14C-ethylenethio-
urea (14C-ETU) in plants, soil, and water. J. agric. food Chem.,
25(3): 528-533.
RJAZANOVA, R.A. (1967) [The effects of the fungicides ziram and
zineb on the generative function of test animals.] Gig. i
Sanit., 2: 26-30 (in Russian).
ROLANDI, A., DE MARINIS, E., & DE CATERINA, M. (1984) Dithio-
carbamate pesticides: activity of propineb in the micronucleus
test in mice. Mutat. Res., 135: 193-197.
ROLL, R. (1971) [Teratological experiments with thiram (TMTD)
on two mouse strains.] Arch. Toxikol., 27: 173-186 (in German).
ROSE, D., PEARSON, C.M., ZUKER, M., & ROBERTS, J.R. (1980)
Ethylenethiourea: criteria for the assessment of its effects on
man, Ottawa, National Research Council, Associate Committee on
Scientific Criteria for Environmental Quality (NRCC Publication
No. 18469).
ROSS, R.D. & CROSBY, D.G. (1973) Photolysis of ethylene-
thiourea. J. agric. food Chem., 21(3): 335-337.
ROSS HART, E. & VALERIO, M.G. (1973) Evaluation of goitrogenic
activity in rats: Ethylene thiourea, practical and Dithane M-45,
Communication from Litton Bionetics to Rohm and Haas Co. Cited
in Van Leeuwen et al., (1982)
RUDDICK, J.A., WILLIAMS, D.T., HIERLIHY, L., & KHERA, K.S.
(1976a) 14C-ethylenethiourea: distribution, excretion, and
metabolism in pregnant rats. Teratology, 13: 35-39.
RUDDICK, J.A., NEWSOME, W.H., & NASH, L. (1976b) Correlation
of teratogenicity and molecular structure: ethylenethiourea and
related compounds. Teratology, 13: 263-266.
SATO, F. & TOMIZAWA, C. (1960) Decomposition of zinc ethylene
bisdithiocarbamate (zineb) applied to plants. Bull. Natl Inst.
Agric. Sci. (Tokyo) Ser. C, 12: 181-187.
SCHULTHEISS, E. (1957) [The therapeutic use of
tetramethylthiuram disulfide.] Dermatol. Wochenschr., 7: 160-162
(in German).
SCHUPBACH, M. & HUMMLER, H. (1976) Evaluation of the
mutagenicity of ethylenethiourea using bacterial and mammalian
test systems. Mutat. Res., 28: 122.
SCHUPBACH, M. & HUMMLER, H. (1977) A comparative study on the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutat. Res., 56: 111-120.
SEIDLER, H., HARTIG, M., SCHNAAK, W., & ENGST, R. (1970)
[Studies on the metabolism of certain insecticides and
fungicides in the rat. II. Mean distribution and breakdown of
14C-labelled maneb.] Nahrung, 14(5): 363-373 (in German).
SEIFTER, J. & EHRICH, W.E. (1948) Goitrogenic compounds:
pharmacological and pathological effects. J. Pharmacol. exp.
Ther., 92: 303-314.
SEILER, J.P. (1973) A survey on the mutagenicity of various
pesticides. Experientia, (Basel) 29: 622-623.
SEILER, J.P. (1974) Ethylenethiourea (ETU): a carcinogenic and
mutagenic metabolite of ethylene bisdithiocarbamate. Mutat.
Res., 26: 189-191.
SEN, N.P., DONALDSON, B.A., & CHARBONNEAU, C. (1974) Formation
of nitrosodimethylamine form the interaction of certain
pesticides and nitrite. In: Bogorski, P., Walker, E.A., & Davis,
W., ed. N-nitroso compounds in the environment, Lyons,
International Agency for Research on Cancer, pp. 75-79 (IARC
Scientific Publication No. 9).
SHERMAN, H. & ZAPP, J.A. (1966) Three generation reproduction
study, Manzate DR (88% maneb). Unpublished report from Haskell
Laboratory submitted to the Word Health Organization by E.I. du
Pont de Nemours and Co.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H.,
TERAMOTO, S., & KADA, T. (1977) Mutagenicity screening on
pesticides and modification products: a basis of carcinogenicity
evaluation. In: Hiatt, H.H., Watson, J.D., & Winsen, J.A.,
ed. Origins of human cancer, Book A, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, Vol.4, pp. 267-285.
SHORT, R.D., Jr, RUSSEL, J.Q., MINOR, J.L., & LEE, C.C. (1976)
Developmental toxicity of ferric dimethyldithiocarbamate and
bis(dimethylthiocarbamoyl) disulfide in rats and mice. Toxicol.
appl. Pharmacol., 35: 83-94.
SHORT, R.D., MINOR, J.L., UNGER, T.M., BREEDEN, B., VAN GOETHEM,
D., & LEE C.C. (1980) Teratology of a zineb formulation.
(Results of a study performed at Midwest Research Institute for
the US Environmental Protection Agency submitted to the World
Health Organization) (EPA-600/1-80-017).
SMITH, D. (1976) Ethylenethiourea: a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.,
26: 92-94.
SMITH, D.M. (1984) Ethylenethiourea: thyroid function in two
groups of exposed workers. Br. J. ind. Med., 41: 362-366.
SPRECHER, D. & GRIGOROWA, R. (1967) [Maximum workplace
concentration of thiuram.] Z. gesamte Hyg., 13: 241-245 (in
German).
STROMME, J.H. (1965) Metabolism of disulfiram and diethyl-
dithiocarbamate in rats with demonstration of an in vivo
ethanol-induced inhibition of the glucuronic acid conjugation of
the thiol. Biochem. Pharmacol., 14: 393-410.
STROMME, J.H. & ELDJARN, L. (1966) Distribution and chemical
forms of diethyldithiocarbamate and tetraethylthiuram disulfide
(disulfiram) in mice in relation to radioprotein. Biochem.
Pharmacol., 15: 287-297.
SUNDERMAN, F.W., Jr & FRASER, C.B. (1983) Effects of nickel
chloride and diethyl dithiocarbamate on metallothionein in rat
liver and kidney. Ann. clin. lab. Sci., 13 (6): 489-495.
SZYMEZYK, T. (1981) The mutagenicity of the fungicide thiram.
Mutat. Res., 85: 223-224.
TAKAYAMA, S., AIHARA, K., ONODERA, T., & AKIMOTO, T. (1986)
Antithyroid effects of propylthiouracil and sulfamonomethoxine
in rats and monkeys. Toxicol. appl. Pharmacol., 82: 191-199.
TANNENBAUM, S.R., SINSKEY, A.J., WEISSMAN, M., & BISHOP, W.
(1974) Nitrite in human saliva: its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TERAMOTO, S., MORIYA, M., KATO, K., TEZUKA, H., NAKAMURA, S.,
SHINGU, A., & SHIRASU, Y. (1977) Mutagenicity testing on
ethylenethiourea. Mutat. Res., 56: 121-129.
TERAMOTO, S., SHINGU, A., KANEDA, M., & SAITO, R. (1978a)
Teratogenicity studies with ethylenethiourea in rats, mice, and
hamsters. Congenital Anom., 18: 11-17.
TERAMOTO, S., SHINGU, A., & SHIRASU, Y. (1978b) Induction of
dominant lethal mutations after administration of ethylene-
thiourea in combination with nitrite or of N-nitrosoethylene-
thiourea in mice. Mutat. Res., 56: 335-340.
TERAMOTO, S., KANEDA, M., KATO Y. & SHIRASU Y. (1980) Failure
of inducing malformations after intro-amniotic injections of
ethylenethiourea in the rat. Congenital Anom., 20: 17-23.
TERAMOTO, S., KANEDA, H., AOYAMA, & SHIRASU, Y. (1981)
Correlation between the molecular structure of N-alkylureas and
N-alkylthioureas and their teratogenic properties.
Teratology, 23: 335-342.
TETSUMI, T., SUMI, M., TANAKA, M., & SHONO, T. (1985) Reversed
phase ion interaction chromatography of sodium dialkyldithio-
carbamates in the presence of tetraalkylammonium salts. J.
Chromatogr., 348: 389-395.
THORN, G.D & LUDWIG, R.A. (1962) The dithiocarbamates and
related compounds, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 298.
TOLLENAAR, F.D. (1956) [Recent developments in the use of
antioxidants in foodstuffs.] Dtsch. Lebensm. Rundschau, 12:
307-313 (in German).
TRUHAUT, R., GUERINOT, F., & BOHUON, C. (1971) Mécanisme bio-
chimique de toxicité des fongicides de la série des dithiocarba-
mates: L'action inhibitrice de la dopamine beta-hydroxylase. Ann.
pharm. fr., 29(2): 117-124.
TURNER, N.J. & CORDEN, M.E. (1963) Decomposition of sodium-
N-methyldithiocarbamate in soil. Phytopathology, 53: 1388-
1394.
ULLAND, B.M., WEISBURGER, J.H., WEISBURGER, E.K., RICE, J.M., &
CYPHER, R. (1972) Brief communication: thyroid cancer in rats
from ethylenethiourea intake. J. Natl Cancer Inst., 49: 583-
584.
US EPA (1977) Rebuttable presumption against registration and
continued registration of pesticide products containing ethylene
bisdithiocarbamates. Fed. Reg., 42(154): 40618-40627.
US EPA (1982a) Ethylene bisdithiocarbamates: final resolution
of rebuttable presumption against registration, Washington
DC, US Environmental Protection Agency, Office of Pesticides and
Toxic Substances (Unpublished report).
US EPA (1982b) Ethylene bisdithiocarbamates: notice of
determination concluding the presumption against registration;
notice of availability of decision document, Washington DC, US
Environmental Protection Agency (Unpublished report 82P-2851).
US EPA (1984) Health and environmental effects: profile for
ethylenethiourea (2-imidazolidinethione), Cincinnati, Ohio, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office (Program Office Draft ECAO-CIN-019).
VACHKOVA-PETROVA, R. (1977) [A study on mutagenic activity of
basfungine on white rats.] Hig. i. Zdraveopazvane, 20(5): 422-
426 (in Russian).
VACHKOVA-PETROVA, R. (1981) Mutagenicity study of endodan in
rats. Mutat. Res., 85(4): 288.
VAN ESCH, G.J. (1956) [Toxicity of tetramethylthiuram
disulfide and tetraethylthiuram disulfide.] Versl.
Volksgezondheid, Vol. No. 166-169 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN LEEUWEN, F.X.R., VOOGD, C.E., KNAAP, A.G.A.C., DEN
TONKELAAR, E.M., & VAN DER HEIJDEN, C.A. (1982) Toxicological
evaluation of ethylenethiourea (ETU), Bilthoven, National
Institute of Public Health (Unpublished Report No. 627915/008).
VAN LOGTEN, M.J. (1972) [ Thesis: the dithiocarbamate-alcohol
reaction by the rat. ] Terborg, The Netherland, Fa. Lammers (in
Dutch).
VAN STEENIS, G. & VAN LOGTEN, M.J. (1971) Neurotoxic effect of
the dithiocarbamate tecoram on the chick embryo. Toxicol. appl.
Pharmacol., 19: 675-686.
VEKSHTEIN, M.SH. & KHITSENKO, I.L. (1971) Ziram metabolism in
warm-blooded animals. Gig. i Sanit., 36: 28-33.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index: JMPR 1961-84, Geneva, World Health Organization
(Unpublished report).
VOGELER, K., DREZE, Ph., RAPP, A., STEFFAN, H., & ULLEMEYER, H.
(1977) Distribution and metabolism of propineb in apples and
grapes, including their breakdown products propylenethiourea and
ethylenethiourea in apples. Pflanzenschutz-Nachrichten Bayer
30: 72-97.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1970) Studies on the fate
in plants of ethylene bisthiocarbamate fungicides and their
decomposition products. Ann. appl. Biol., 65: 489-496.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1971) Tentative
identification of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicides. Pestic. Biochem.
Physiol., 1: 163-165.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1976) Formation of
ethylenethiourea from 5,6-dihydro-3 H-imidazo[2,1-C]-1,2,4-
dithiazole-3-thione by microorganisms and reducing agents. J.
environ. Sci. Health, B11: 33-47.
WATTS, R.R., STORHERR, R.W., & ONLEY, J.H. (1974) Effects of
cooking on ethylene bisdithiocarbamate degradation to
ethylenethiourea. Bull. environ. Contam. Toxicol., 12: 224-226.
WEDIG, J., COWAN, A., & HARTUNG, R. (1968) Some of the effects
of tetramethylthiuram disulfide (TMTD) on reproduction of the
bobwhite quail (Colinus virginianus). Toxicol. appl. Pharmacol.,
12: 293.
WEISBURGER, E.K., ULLAND, B.M., NAM, J.M., GART, J.J., &
WEISBURGER, J.H. (1981) Carcinogenicity tests of certain
environmental and industrial chemicals. J. Natl Cancer Inst.,
67: 75-88.
WHO (1986a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1986-1987, Geneva, World
Health Organization (Unpublished report VBC/86.1, rev. 1).
WHO (1986b) Environmental Health Criteria 64: Carbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 137.
WHO (1988) Environmental Health Criteria 76: Thiocarbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 49.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., Croydon, British Crop Protection
Council.
YIN-TAK WOO (1983) Carcinogenicity, mutagenicity, and
teratogenicity of carbamates, thiocarbamates, and related
compounds. An overview of structure-activity relationships and
environmental concerns. J. environ. Sci. Health, C1(1): 97-133.
ZADOROZHNY, B.A., PETROV, B.R., OLTIRENKO, V.A., & KIRILKO, V.A.
(1981) [Occupational dermatoses and their control under
conditions of pesticide manufacture.] Vestn Dermatol. Venerol.,
6: 48-51 (in Russian).
ZDIENICKA, M., ZIELENSKA, M., HRYMIEWICA, M., TROJANOWSKA, M.,
ZALEJSKA, M., & SZYMCZYK, T. (1981) The mutagenicity of the
fungicide thiram. In: Kappas, A., ed. Progress in environmental
mutagenesis and carcinogenesis, Amsterdam, Oxford, New York,
Elsevier Science Publishers, Vol. 2, pp. 79-86.
ZORIN, P.M. (1970) [Allergic dermatitis arising as a result of
contact with zineb.] Vestn Dermatol. Venerol., 44: 65-68 (in
Russian).
ZUMAN, P. & ZAHRADNIK, R. (1957) [Kinetics and decomposition
mechanism of dithiocarbamic acids solutions. Polarographic
study.] Z. Phys. Chem., 208: 135-140 (in German).
Annex I. Names and structures of selected dithiocarbamates
--------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
dibam Methylnamate S sodium dimethyldi- C3H6NS2Na 143.21
|| thiocarbamate
(CH3)2N-C-SNa (128-04-1)
disul- Antabuse S S tetraethylthiuram C10H20N2S4 2 mg/
firam || || disulfide litrea
(C2H5)2NC-SS-CN(C2H5)2 (29925-58-4)
ferbam Fermate S iron, tris(dimethyl- C9H18FeN3S6 416.51 130
Fuklasin || carbamodithioato- mg/litre
Hokmate [(CH3)2N-C-S-]3Fe S,S' )-,
Karbam Black (14484-64-1)
Niacide
mancozeb Aazimag manganese, [[1,2- indefinite, insol-
Fore [-SCSNHCH2CH2NHCSSMn-]x(Zn)y ethanediylbis-[carba- variable uble
Dithane M-45 modithioato]](2-)]-,
Manzate 200 in combination with
[[1,2-ethanediylbis-
[carbamodithioato]]-
(2-)]zinc
(8018-01-7)
maneb Amazin manganese, [[1,2- C4H6MnN2S4 265.29 insol-
Blitex [-SCSNHCH2CH2NHCSS-Mn-]x ethanediylbis- uble
Dithane M-22 [carbamodi-
Manzate thioato]](2-)]
Martemick (12427-38-2)
Mancid
Tubothane
---------------------------------------------------------------------------------------------------------
Annex I. (contd.)
---------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
metam- Carbam S carbamodithioic C2H4NaNS2 129.18 722
sodium Masposol || acid, methyl-, mg/
Sistan CH3NH-C-S- Na+ sodium salt litreb
Trapex (137-42-8)
Vapam
metiram Zinc-metiram ammonia complex of indefinite, insol-
Polyram zineb and poly variable uble
(ethylene thiuram
disulfide),
zineb ethylene
thiuram disulfide
(9006-42-2)
nabam Nabasan S S carbamodithioic acid, C4H6Na2N2S4 256.34 200 g/
Parzate || || 1,2-ethanediylbis-, litre
Spring-Bak Na+ -S-C-NHC2H4-NH-C-S- Na+ disodium salt
(142-59-6)
polyram (see metiram)
propineb Antracol S S zinc, [[(1-methyl- C5H8N2S4Zn 289.9 insol-
Cypromate || || 1,2-ethanediyl)-bis uble
Mezineb [-S-C-NHCH2CH-NH-C-S-Zn-]x [carbamodithioato]]
| (2-)]-,
CH3 (12071-83-9)
sulfal- Vegadex S carbamodithioic acid, C8H14NClS2 223.8 92 mg/
late CDEC || diethyl-, 2-chloro- litre
(C2H5)2N-C-S-CH2-C=CH2 2-propenyl ester
| (95-06-7)
Cl
---------------------------------------------------------------------------------------------------------
Annex I. (contd.)
---------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
thiram Arasan S S thioperoxydicarbonic C6H12N2S4 240.44 30 mg/
Cyuram || || diamide, tetramethyl litre
Fernasan (CH3)2N-C-S-S-C-N(CH3)2 (137-26-8)
Mercuram
Normersan
TMTD
zineb Aspor-Z S S zinc, [[1,2-ethane- C4H6N2S4Zn 275.73 10 mg/
Carbane || || diylbis[carbamodi- litre
Dithane-Z78 [-S-C-NHC2H4-NH-C-S-Zn-]x thioato]](2-)]-
Lonacol (12122-67-7)
Murphane
Novozir
Parzate
Perozine 75B
Sudothane
Zebenide
Zelmone
ziram Cuman S zinc, bis(dimethyl- C6H12N2S4Zn 305.81 65 mg/
Fuklasin || carbamodithioato- litre
Milbam [(CH3)2N-C-S-]2Zn S,S')-
Zerlate (137-30-4)
---------------------------------------------------------------------------------------------------------
a At 38 °C.
b At 20 °C.
Annex II. Names and structures of degradation products of ethylene
bisdithiocarbamates
-------------------------------------------------------------------------------------
Common name Chemical CAS chemical name/ Molecular Relative
structure CAS registry number formula molecular
mass
-------------------------------------------------------------------------------------
Ethylenethiourea CH2-NH 2-imidazolidinethione C3H6N2S 102.2
(ETU) | \ (96-45-7)
| C=S
| /
CH2-NH
S
||
C-S
DIDT / | 5,6-dihydro-3- H-imidazo[2, C4H4N2S3 176.3
CH2-N | 1-C]1,2,4-dithiazole-3-thione,
| \ | (33813-20-6)
| C-S
| //
CH2-N
S
||
CH2-NH-C-S
Ethylenethiuram | | 1,2,4,7-dithiadiazocine- C4H6N2S4 210.3
disulfide (ETD) | | 3,8-dithione, tetrahydro
CH2-NH-C-S (3082-38-0)
||
S
-------------------------------------------------------------------------------------
Annex III. Dithiocarbamates and ETU: JMPR reviews, ADIs, Evaluation by IARC, Classification by Hazard,
WHO/FAO Data Sheets, IRPTC Data Profile and Legal Filea
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Ferbam 1983 0-0.02 1984a Vol. 12 + + 0
page 121
1980 0-0.02 1981b Vol. 13
page 243
1981a
1977 0-0.02 1978b
1978a
1974 0-0.05 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compounds
only, and to the sum
of all the dithio-
carbamate fungicides
if more than one is
present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with other
dimethyl-dithiocar-
bamates (thiram and
ziram))
1965 no ADI 1965b
1965a
1963 no ADI 1964
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Mancozeb 1983 0-0.05i 1984a + + 0
1980 0-0.05i (indivi- 1981b
dually of the sum 1981a
of mancozeb, maneb,
and zineb)
1977 0-0.005 1978b
(temporary)
(sum of mancozeb, 1978a
maneb, and zineb)
1974 0-0.005 1975b
(temporary)
(sum of dithio- 1975b
carbamates) 1975b
1975a
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and the sum
of all the ethylene
bisdithiocarbamate
fungicides if more
than one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other ethylene
bisdithiocarbamates
(maneb and zineb),
including zineb
derived from nabam
plus zinc sulfate)
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Maneb 1983 0-0.05i 1984a + + 0
1980 0-0.05 1981b
(individual or 1981a Vol. 12
the sum of manco- page 137
zeb, maneb, and
zineb)
1977 0-0.005 1978b
(temporary)
(sum of manco- 1978a
zeb, maneb, and
zineb)
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other ethylene
bisdithiocarbamates
(mancozeb and zineb)
including zineb
derived from nabam
plus zinc sulfate)
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Maneb 1965 no ADI 1965b
(contd.) 1965a
1963 no ADI 1964
Nabam 1983 no ADI 1984a + + II
1977 no ADI 1978b
1978a
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(as nabam alone 1968a
or in combination
with other ethylene
bisdithiocarbamates
(mancozeb, maneb,
and zineb) including
zineb derived from
nabam plus zinc
sulfate)
1965 no ADI 1965b
1965a
1963 no ADI 1964
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Propineb 1985 ADI withdrawn 1986b 0
1984 0-0.005 1985b
(temporary)
1983 0-0.005 1984b
(temporary)
1980 0-0.005 1981b
(temporary)
1977 0-0.005 1978b
Thiram 1983 0-0.005 1984a Vol. 12 + + III
(temporary) page 225
1980 0-0.005 1981b
(temporary) 1981a
1977 0-0.005 1978a
(temporary)
1974 0-0.005 1975b
(temporary)
(sum of all 1975a
dithiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other dimethyl di-
thiocarbamates
(ferbam and ziram))
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Thiram 1965 0-0.025 1965b
(contd.) 1965a
1963 0-0.025 1964
Zineb 1983 0-0.05i 1984a Vol. 12 + + 0
1980 0-0.05 1981b page 245
(individually 1981a
or the sum of
mancozeb, maneb,
and zineb)
1977 0-0.005 1978b
(temporary)
(sum of mancozeb, 1978a
maneb, and zineb)
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
1967 (alone or in com- 1968a
bination with
other ethylene
bisdithiocarbamates
(mancozeb and maneb)
including zineb
derived from nabam
plus zinc sulfate)
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
Zineb 1965 no ADI 1965b
(contd.) 1965a
1963 no ADI 1964
Ziram 1983 0-0.02 1984a Vol. 12 + + III No. 73
page 259 (in
prepar-
ation)
1980 0-0.02 1981b
1981a
1977 0-0.02 1978b
1978a
1974 0-0.005 1975b
(temporary)
(sum of all di- 1975a
thiocarbamates)
1970 0-0.025 1971b
(temporary)
(applicable to the 1971a
parent compound
only, and to the
sum of all the di-
thiocarbamate fung-
icides if more than
one is present)
1967 0-0.025 1968b
(temporary)
(alone or in com- 1968a
bination with
other dimethyl
dithiocarbamates
(ferbam and thiram))
1965 no ADI 1965b
1965a
1963 no ADI 1964
--------------------------------------------------------------------------------------------------------
Annex III. (contd.)
--------------------------------------------------------------------------------------------------------
Compound Year ADIb Evaluation IARCd Availability WHO recom- WHO/FAO
of (mg/kg by JMPRc: Evaluation of IRPTCe: mended clas- Data
JMPR body Published of Carcino- Data Legal sification Sheets
meet- weight) in: genicity Profile fileg of pesticides on Pest-
ing FAO/WHO by hazardh icidesf
--------------------------------------------------------------------------------------------------------
ETU (see 1980 0.002 1981b Vol. 7,
dithio- p. 45
carbamates) 1974 - 1975b Suppl. 4,
p. 128
PTU (see 1985 no ADI 1986a
propineb) (withdrawn)
--------------------------------------------------------------------------------------------------------
a Adapted from: Vettorazzi & van den Hurk (1984).
b ADI = acceptable daily intake.
c JMPR = Joint Meeting on Pesticide Residues (FAO/WHO).
d IARC = International Agency for Research on Cancer (WHO, Lyons, France).
e IRPTC = International Register for Potentially Toxic Chemicals (UNEP, Geneva).
f WHO/FAO Data Sheets on Pesticides with number and year of appearance.
g From: IRPTC (1983).
h From: WHO (1986a).
i Not more than 0.002 mg/kg body weight may be present as ETU.
The hazard referred to in this Classification is the acute risk for health
(that is, the risk of single or multiple exposures over a relatively short
period of time) that might be encountered accidentally by an person
handling the for storage and transportation by competent international
bodies.
Classification relates to the technical material, and not to the formulated product:
-----------------------------------------------------------------------------------
Class LD50 for the rat (mg/kg body weight)
Oral Dermal
Solids Liquids Solids Liquids
-----------------------------------------------------------------------------------
1A Extremely hazardous 5 or less 20 or less 10 or less 40 or less
1B Highly hazardous 5 - 50 20 - 200 10 - 100 40 - 400
II Moderately hazardous 50 - 500 200 - 2000 100 - 1000 400 - 4000
III Slightly hazardous over 500 over 2000 over 1000 over 4000
O Unlikely to present
acute hazard in
normal use
-----------------------------------------------------------------------------------
 Thiram and the dimethylamine salt of dimethyldithiocarbamic
acid were the major metabolites in the urine, whereas carbon
disulfide and dimethylamine were detected in the expired air.
The body tissues contained tetramethylthiourea, the methylamine
salt of dimethyldithiocarbamic acid, carbon disulfide, and
methylamine. Overall, the results indicate that, in the rat,
ferbam and ziram are transformed into dimethyldithiocarbamic
acid, which is subsequently coupled to give thiram, or is broken
down to carbon disulfide and dimethylamine (Vekshtein &
Khitsenko, 1971; Hodgson et al., 1974).
The in vivo metabolic decomposition of EBDCs is complex and
results in the formation of carbon disulfide, hydrogen sulfide,
EDA, ethylene bisthiuram disulfide, DIDT, ethylene
diisothiocyanate (EDI) (unstable), ETU, EU, and 2-imidazoline
(Seidler et al., 1970; Lyman, 1971) (Fig. 2). The decomposition
of monoalkyldithiocarbamates is detailed in Fig. 3.
When 14C-maneb was given to rats in a single oral dose of
390 mg/kg body weight, only 55% of the 14C was recovered in the
excreta. It was therefore suggested that a large part of the
dose might have been metabolized to carbon disulfide and 14C-
EDA, followed by oxidation of the latter to carbon disulfide.
The concentration of the radioactivity was highest after 24 h,
and EDA and ETU were identified in the excreta (Seidler et al.,
1970). ETU and DIDT were the major metabolites found in the
urine of rats treated with zineb, and carbon disulfide was
detected in the expired air.
6.3. Metabolism in Plants
ETU is one of several metabolites found when EBDCs are
applied to plants. In plants, nabam, maneb, and zineb are
transformed to ETU, DIDT, EU, 2-imidazoline, a diisothiocyanate
(EDI), and other metabolites (Fig. 2).
Nash & Beall (1980) have studied the fate of maneb and zineb
in microagroecosystem chambers (enclosed glass chambers), under
the following conditions: pH, 6.7; organic matter content, 5.2%;
soil type, Galestown sandy loam; soil water content, 15.6%. The
fungicides were applied twice to tomato plants at 2 kg/ha, and
the residual fungicides (measured as EDA and ETU) were monitored
on the fruit, leaves, and in the soil, water, and air for 100
days after treatment. ETU was detected at < 20 µg/kg on whole
fruit after 3 days, but had completely disappeared after 3
weeks. Maneb and zineb were present on whole fruit at < 1 mg/kg
and were still present in measurable amounts (as EDA) after 10
weeks. Both had half-concentration times (C“) of 14 days on
leaves. Half-concentration times for ETU, maneb, and zineb in
soil were < 3, 36, and 23 days, respectively, and that for ETU
in air was 9 days.
Thiram and the dimethylamine salt of dimethyldithiocarbamic
acid were the major metabolites in the urine, whereas carbon
disulfide and dimethylamine were detected in the expired air.
The body tissues contained tetramethylthiourea, the methylamine
salt of dimethyldithiocarbamic acid, carbon disulfide, and
methylamine. Overall, the results indicate that, in the rat,
ferbam and ziram are transformed into dimethyldithiocarbamic
acid, which is subsequently coupled to give thiram, or is broken
down to carbon disulfide and dimethylamine (Vekshtein &
Khitsenko, 1971; Hodgson et al., 1974).
The in vivo metabolic decomposition of EBDCs is complex and
results in the formation of carbon disulfide, hydrogen sulfide,
EDA, ethylene bisthiuram disulfide, DIDT, ethylene
diisothiocyanate (EDI) (unstable), ETU, EU, and 2-imidazoline
(Seidler et al., 1970; Lyman, 1971) (Fig. 2). The decomposition
of monoalkyldithiocarbamates is detailed in Fig. 3.
When 14C-maneb was given to rats in a single oral dose of
390 mg/kg body weight, only 55% of the 14C was recovered in the
excreta. It was therefore suggested that a large part of the
dose might have been metabolized to carbon disulfide and 14C-
EDA, followed by oxidation of the latter to carbon disulfide.
The concentration of the radioactivity was highest after 24 h,
and EDA and ETU were identified in the excreta (Seidler et al.,
1970). ETU and DIDT were the major metabolites found in the
urine of rats treated with zineb, and carbon disulfide was
detected in the expired air.
6.3. Metabolism in Plants
ETU is one of several metabolites found when EBDCs are
applied to plants. In plants, nabam, maneb, and zineb are
transformed to ETU, DIDT, EU, 2-imidazoline, a diisothiocyanate
(EDI), and other metabolites (Fig. 2).
Nash & Beall (1980) have studied the fate of maneb and zineb
in microagroecosystem chambers (enclosed glass chambers), under
the following conditions: pH, 6.7; organic matter content, 5.2%;
soil type, Galestown sandy loam; soil water content, 15.6%. The
fungicides were applied twice to tomato plants at 2 kg/ha, and
the residual fungicides (measured as EDA and ETU) were monitored
on the fruit, leaves, and in the soil, water, and air for 100
days after treatment. ETU was detected at < 20 µg/kg on whole
fruit after 3 days, but had completely disappeared after 3
weeks. Maneb and zineb were present on whole fruit at < 1 mg/kg
and were still present in measurable amounts (as EDA) after 10
weeks. Both had half-concentration times (C“) of 14 days on
leaves. Half-concentration times for ETU, maneb, and zineb in
soil were < 3, 36, and 23 days, respectively, and that for ETU
in air was 9 days.
 Note to Figure 2: Ion mechanisms leading to ETU formation are
not completely understood; however, a number of hypotheses have
been advanced. According to Marshall (1977), intermediary
products of the thermal bisdithiocarbamate degradation to ETU are
beta-amino ethylene dithiocarbamate and DIDT, but not ethylene
diisothiocyanate (EDI). EDI was, however, postulated and detected
several times as a secondary reaction product of the ethylene
bisdithiocarbamate degradation at normal temperatures (Engst &
Schnaak, 1970a). ----> indicates a postulated conversion.
Note to Figure 2: Ion mechanisms leading to ETU formation are
not completely understood; however, a number of hypotheses have
been advanced. According to Marshall (1977), intermediary
products of the thermal bisdithiocarbamate degradation to ETU are
beta-amino ethylene dithiocarbamate and DIDT, but not ethylene
diisothiocyanate (EDI). EDI was, however, postulated and detected
several times as a secondary reaction product of the ethylene
bisdithiocarbamate degradation at normal temperatures (Engst &
Schnaak, 1970a). ----> indicates a postulated conversion.
 Besides ETU, other degradation products of EBDCs include ETD
and DIDT. The main volatile components of zineb's decomposition
are carbon disulfide, carbonyl sulfide, and EDA. Almost all
zinc is converted into zinc sulfide and zinc oxide (Melnikov &
Trunov, 1966).
6.4. Decomposition in Water and Soil
Thiram and dimethyldithiocarbamic acid give rise in soil to
methyl isothiocyanate and sulfur, and, under acidic conditions,
to carbon disulfide, hydrogen sulfide, methylamine, methyl-
isocyanate, and the bisdisulfide of methyldithiocarbamic acid.
Two of the products, carbon disulfide and dimethylamine,
evaporated from the soil (Raghu et al., 1975). Dimethyldithio-
carbamic acid also binds with heavy metals in soil to form
complexes.
The various metal derivatives of ethylene bisdithiocarbamic
acid appear to be converted in the soil to DIDT, ETU, carbon
disulfide, hydrogen sulfide, and carbonyl sulfide (Moje et al.,
1964; Kaars Sijpesteijn & Vonk, 1970). The conversion by soil
bacteria and fungi of DIDT into ETU has been demonstrated (Vonk
& Kaars Sijpesteijn, 1976). Even though ETU is slowly converted
into EU in soil, pure cultures of soil bacteria and fungi were
unable to effect this transformation (Kaars Sijpesteijn & Vonk,
1970).
6.5. Metabolism in Microorganisms
Microorganisms readily form ETU from DIDT, a spontaneous
decomposition product of EBDCs. This conversion also takes
place after addition of reducing compounds such as cysteine,
glutathione, or ascorbic acid. It consists of the reduction of
the S-S bond of DIDT, with the subsequent release of carbon
disulfide to form ETU. It was shown by Vonk & Kaars Sijpesteijn
(1976) that DIDT was reduced by NADH in the presence of enzyme
extracts from Pseudomonas fluorescens, Escherichia coli,
Saccharomyces cerevisiae, or Aspergillus niger.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
There is some evidence that dithiocarbamates, at concentra-
tions 10 times that of normal field application, may reduce
microbial biomass and increase the bacterial:fungal ratio.
7.2. Aquatic Organisms
7.2.1. Acute toxicity
Toxicity studies using dithiocarbamates are hindered by the
fact that they are chemically and biologically degradable and
may also be contaminated with degradation products. Their
stability in water depends on the pH and on the presence of
metal ions with which they form complexes. The soluble dithio-
carbamates dissociate in water, whereas the polymers are only
slightly soluble in water. As the breakdown products will also
influence toxicity, toxicity testing of dithiocarbamates is
complex.
According to US EPA (1977, 1982a), the use of pesticide
products containing maneb against cranberry fruit rot at
application rates of up to 6.7 kg ai/ha would result in a
concentration of 4.4 mg/litre in a 15-cm layer of water. McCann
& Pitcher (1973) reported a 96-h LC50 of 1 mg/litre for
bluegills, while Worthing & Walker (1983) reported a 48-h LC50
for carp of 1.8 mg/litre. Zineb used against cranberry fruit
rot at an application rate of up to 5.4 kg ai/ha could result in
a concentration of 3.52 mg/litre in a 15-cm layer of water. A
26-h LC50 of 0.2 mg/litre has been reported for Daphnia magna.
Van Leeuwen (1986) carried out an extensive study with 18
dithiocarbamates and three metabolites of these compounds,
including ETU, in fish (Poecilia reticulata), crustacea (Daphnia
magna), algae ( Chlorella pyrenoidosa, Phytobacterium
phosphoreum), and two nitrifying bacteria (Nitrosomonas and
Nitrobacter). The results are summarized in Tables 4 and 5.
Worthing & Walker (1983) gave the following LC50 values:
propineb: rainbow trout, 1.9 mg/litre; golden orfe,
133 mg/litre; thiram: carp, 4 mg/litre; rainbow trout,
0.13 mg/litre; bluegill, 0.23 mg/litre; metiram: harlequin fish,
17 mg/litre.
The susceptibility to maneb of the early life stages of
rainbow trout has been studied using fertilized eggs (before and
after water hardening), early eye point eggs, late eye point
eggs, sac fry, and early fry. The sac fry and early fry stages
appeared to be the most sensitive. The 96-h LC50s for the
different stages were: for 0-h egg, 6 mg/litre; for 24-h egg,
5.6 mg/litre; for early eyed egg (14 days), 1.8 mg/litre; for
late eyed egg (28 days), 1.3 mg/litre; for sac fry (42 days),
0.32 mg/litre; and, for early fry (77 days), 0.34 mg/litre (Van
Leeuwen, 1986).
Table 4. Acute toxicity of dithiocarbamates and breakdown
products for fisha
-------------------------------------------------------------
Organism Compound 96-h LC50
(95% confidence
limit) (mg/litre)
-------------------------------------------------------------
Poecilia reticulatab nabam 5.8 (4 - 8.5)
maneb 3.7 (3.2 - 5.6)
zineb 7.2 (5 - 10.3)
mancozeb 2.6 (2.1 - 3.3)
metiram 6.4 (4 - 10.4)
Na-DMDC 2.6 (2.1 - 3.2)
ziram 0.75 (0.56 - 1)
ferbam 0.09 (0.06 - 0.18)
thiram 0.27 (0.22 - 0.33)
Na-DEDC 6.9 (5.5 - 8.5)
Zn-DEDC 0.49 (0.40 - 0.61)
disulfiram 0.32 (0.24 - 0.43)
ETU 7500 (5600 - 10 000)
EU 13 000 (10 000 - 18 000)
Rainbow troutc thiram 0.26 (0.24 - 0.32)
(Salmo gairdneri)
-------------------------------------------------------------
a From: Van Leeuwen (1986).
b Studies according to OECD guidelines 203.
c 24-h LC50; water temperature 15 ± 1 °C;
weight of fish, 34 ± 4.7 g.
Table 5. Short-term toxicity studies with dithiocarbamates and
breakdown productsa
--------------------------------------------------------------------
Compound Daphnia Chlorella Photobacterium Nitrosomonas
magna pyrenoidosa phosphoreum Nitrobacter
48-h LC50 96-h EC50 15-min EC50 3-h MIC
(mg/litre) (mg/litre) (mg/litre) (mg/litre)
--------------------------------------------------------------------
Nabam 0.44 2.4 102 32
Maneb 1 3.2 1.2 56
Zineb 0.97 1.8 6.2 18
Mancozeb 1.3 1.1 0.08 32
Metiram 2.2 1.8 0.37 32
Na-DMDC 0.67 0.8 0.51 26
Ziram 0.14 1.2 0.15 100
Ferbam 0.09 2.4 0.20 10
Thiram 0.21 1 0.10 18
Na-DECD 0.91 1.4 1.22 43
Zn-DEDC 0.24 1.1 1.70 > 320
Disulfiram 0.12 1.8 1.21 > 320
ETU 26.4 6600 2100 1
EU 5600 16 000 3300 1000
--------------------------------------------------------------------
a From: Van Leeuwen (1986).
7.2.2. Short- and long-term toxicity and reproduction studies
7.2.2.1. Fish
In sublethal toxicity studies carried out on Salmo
gairdneri, groups of 10 fish were exposed to thiram
(0.18 mg/litre) for 24 h. Blood parameters (decreased haemo-
globin and leukopenia, decreased glucose levels, and increased
glucose-6-phosphate dehydrogenase activity) and liver parameters
(increased lipid content, increased lactate dehydrogenase) were
changed, and it was concluded by the author that thiram is a
cytotoxic chemical (Van Leeuwen, 1986). In a further study, the
60-day toxicity for early life stages was tested on S. gairdneri
using a number of dialkyldithiocarbamates, EBDCs, and break-
down products. The LC50s ranged from approximately 1 to
9 µg/litre for dialkyldithiocarbamates and 211 - 2100 µg/litre
for EBDCs, but ETU and EU were not toxic, even at levels
exceeding 1000 mg/litre.
Embryotoxic and teratogenic effects were also observed for
all the compounds studied, and there was an overlap between the
responses for skeletal malformations and lethality over a wide
concentration range. The teratogenic effects in rainbow trout
proved to be in agreement with those observed in mammals.
Exposure of rainbow trout during embryo-larval development
revealed that malformations induced by dithiocarbamates were
almost exclusively confined to the notochord, which increased
considerably in both length and diameter. As a result, the
notochord became twisted and distorted. Ectopic osteogenesis
was observed in almost every affected notochord. Other effects,
such as the disruption of the integrity of myomeres and organ
dislocations, were closely related to the notochordal ano-
malies. Also, compression and fusion of vertebrae and "waviness"
of various skeletal elements were found. Concentration-related
changes in the liver were observed in short-term exposure of
juvenile rainbow trout, while at high levels proliferation of
bile duct epithelial cells and necrosis of hepatocytes were
seen. Ziram and thiram induced brain haemorrhages as well as
intraspinal extravasates of blood cells (Van Leeuwen, 1986).
7.2.2.2. Invertebrates
Short-term toxicity studies using the compounds listed in
Table 5 were carried out to investigate the effects of prolonged
exposure (21-day) on survival, fecundity, and growth of Daphnia
magna. Growth and reproduction were not specifically inhibited,
since effects on these characteristics were generally detected
at levels comparable with the 21-day LC50s. For dialkyldithio-
carbamates, the 21-day LC50s ranged from approximately 10 to
30 µg/litre, for EBDCs, from 80 to 110 µg/litre, and, for ETU
and EU, the levels were 18 and 3200 mg/litre, respectively (Van
Leeuwen, 1986).
7.2.3. Bioaccumulation
Van Leeuwen (1986) determined the log n-octanol/water par-
tition coefficient for a few dithiocarbamates and breakdown
products. The results are summarized in Table 6. In general,
the higher the log n-octanol/water partition coefficient, the
greater the tendency to bioaccumulate.
Table 6. log n-octanol/water partition
coefficients for some dithiocarbamates
------------------------------------------
Compound log n- octanol/water
partition coefficient
------------------------------------------
Disulfiram 4
Thiram 1.82
ETU 0.67
EU 0.96
------------------------------------------
In short-term studies on rainbow trout of uptake,
distribution, and retention of 14C-labelled zineb and ziram,
both compounds were found to be rapidly distributed throughout
the tissues. Whole-body accumulation was low, with bioconcen-
tration factors of less than 100. Relatively high radioactivity
levels were found in the liver, gall bladder, and intestinal
contents, suggesting the prominent role of hepatic biotransform-
ation and biliary excretion. With ziram, the eyes and skin also
appeared to be distribution sites. Whole-body elimination was
rapid, with about 75% of radioactivity being eliminated within
the first 4 days. With ziram, 45% of the initial total 14C
content in the body was still present at the end of the 16-day
depuration period. Differences in the extent of elimination
were most noteworthy for the eyes, skin, and kidneys. Whole-
body autoradiography showed the radioactivity in the digestive
tract, liver, bile, gills, thyroid follicles, melanophores of
the skin, choroid epithelium complex of the eyes, and in other
melanin-containing tissues, such as the kidneys (Van Leeuwen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
In general, the toxicity of dithiocarbamates for mammals is
relatively low. Some commonly used dithiocarbamates included in
the WHO recommended classification of pesticides by hazard (WHO,
1986a), which is based primarily on the acute oral and dermal
toxicity of the technical material for the rat, are given in
Annex III.
Acute oral and dermal toxicity data for a number of animal
species of various dithiocarbamates are given in Table 7. From
this Table, it is clear that nabam and metam-sodium are the most
toxic dithiocarbamates, other compounds having only low
toxicity. As for many compounds, the toxicity is often
influenced by the method of application, e.g., solvents used,
age and sex of animal, type of diet, etc. Thus, in rats fed an
isocaloric diet containing 3.5% protein, the LD50 of nabam was
210 mg/kg body weight, compared with 565 mg/kg in rats fed the
same diet plus 26% protein (Periquet & Derache, 1976).
Ivanova-Chemishanska (1969) found that rats treated with
zineb, maneb, or mancozeb showed dose-dependent signs of
depression, adynamia, decreased tonus, disturbances in
coordination, paresis, and paralysis of extremities combined
with general weakness, lack of appetite, and prostration.
Yin-Tak Woo (1983) has reviewed the structure-activity
relationships of different types of dithiocarbamates.
8.2. Short- and Long-Term Exposures
8.2.1. Oral exposure
8.2.1.1. Rat
In 1-month feeding tests, no growth retardation was noted in
rats fed diets containing 100 mg ferbam/kg diet, but decreased
growth occurred with 500 mg/kg diet and increased mortality with
5000 mg/kg diet (Hodge et al., 1952).
Groups of 40 weanling rats (20 females and 20 males) were
given diets containing 500, 1000, 2500, 5000, or 10 000 mg
zineb/kg diet for up to 30 days. Thyroid enlargement was seen
at all dose levels, but unequivocal histopathological changes
were observed only at 10 000 mg/kg diet (Blackwell-Smith et al.,
1953; Kampmeier & Haag, 1954).
Ferbam administered daily at 23, 66, or 109 mg/kg body
weight to male rats for 13 weeks caused death and weight loss at
the highest dose, but did not have any effect on reproduction.
Daily feeding (equivalent to 15 or 51 mg/kg body weight) to
females for 2 weeks caused severe weight loss at the highest
dose level (Minor et al., 1974).
In a 2-year feeding study, 25, 250, and 2500 mg ferbam or
ziram/kg diet shortened the life span of rats and caused growth
depression and neurological lesions (manifested at the highest
dose level by the crossing of hind legs when animals were lifted
by their tail) (Hodge et al., 1956).
Table 7. Acute toxicity (LD50) of dithiocarbamates for experimental
animals
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Ferbam mouse 1000 FAO/WHO (1965b)
rat > 4000 Hodge et al.
(1956)
guinea-pig 450 - 2000
rabbit 2000 - 3000
Metham-sodium mouse 285 Worthing & Walker
(vapam) (1983)
rat 1700 - 1800 Worthing & Walker
(1983)
rabbit 1300 Worthing & Walker
(1983)
Ziram rat 1400 Hodge et al.
(1952)
guinea-pig 100 - 150 Hodge et al.
(1952)
rabbit 100 - 1020 Hodge et al.
(1952)
Thiram mouse 1500 - 2000 Worthing & Walker
(1983)
rat 865 - 1300 Van Esch (1956)
rat 780 - 865 Worthing & Walker
(1983)
rat > 2000 Ben Dyke et al.
(1970)
rabbit 210 Lehman (1951)
cat 230
--------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Disulfiram rat > 4000 Van Esch (1956)
Zineb rat > 5200 Blackwell-Smith
(1953)
rat 9000 Ivanova-Chemi-
shanska (1969a)
Maneb mouse 4100 Engst et al.
(1971)
rat 4500 Engst et al.
(1971)
rat (male) 6750 Worthing & Walker
(1983)
Nabam rat 395 Blackwell-Smith et
al. (1953)
Mancozeb rat (female) 12 800 Ivanova-Chemi-
shanska (1969b)
rat (male) 14 000 Ivanova-Chemi-
shanska (1969)
rat > 8000 Worthing & Walker
(1983)
Propineb rat 8500 > 1000 Worthing & Walker
(1983)
rabbit 2500
cat 2500
hen 2500
Metiram mouse 5400 Worthing & Walker
(1983)
rat > 10 000
guinea-pig 2400 - 4800
(female)
--------------------------------------------------------------------------
Groups of 24 rats (12 females and 12 males) were given a
diet containing 48 mg thiram/kg diet for 2 years (a 3-generation
study). No effects on growth, reproduction, blood parameters,
or mortality rate were found, neither were there gross or
histological changes (Van Esch, 1956). In a further study, 12
female and 12 male rats given 200 mg/kg diet for 8 months did
not show any appreciable changes in growth or mortality rate,
and a dose of 300 mg/kg diet for 65 weeks did not give rise to
specific evidence of poisoning (Tollenaar, 1956). Groups of 24
rats (12 females and 12 males) fed diets containing 300, 1000,
or 2500 mg thiram/kg diet for 65 weeks showed weakness, ataxia,
various degrees of paralysis, and histological changes
(calcification in the brain stem and cerebellum and dystrophic
changes in the leg muscles). At 2500 mg/kg diet, there was an
increased mortality rate (Fitzhugh et al., 1952). Groups of 20
young rats administered diets containing 100, 300, or 500 mg
thiram/kg diet for 2 years all showed a small reduction in the
growth rate. At concentrations of 300 and 500 mg/kg diet, an
increased mortality rate was seen, while at 500 mg/kg diet,
convulsions, thyroid hyperplasia, and calcification in the
cerebellum, hypothalamus, and medulla oblongata were observed
(Griepentrog, 1962; IARC, 1976).
Groups of 25 male and 25 female rats were fed diets
containing 25, 250, 1250, or 2500 mg maneb/kg diet for 2 years.
At 1250 mg/kg diet, there was some depression, impaired food
consumption, and increased mortality rate. At the end of 2
years, the animals receiving 1250 mg/kg diet had an increased
liver/body weight ratio, and those receiving 2500 mg/kg diet
also showed thyroid hyperplasia and nodular goitre (Worthing &
Walker, 1983).
Groups of 10 young male and 10 young female rats were fed
diets containing 500, 1000, 2500, 5000, or 10 000 mg zineb/kg
diet for 2 years. At the two highest dose levels, there was an
apparent increase in the mortality rate among the female rats
and, at 10 000 mg/kg diet, there was a tendency towards
diminished growth in both sexes. The results of haematological
studies were normal, but a goitrogenic effect was seen at all
dose levels. Kidney damage was seen in 6 animals at the
10 000 mg/kg dose level and in one animal in each of the groups
receiving 1000, 2500, or 5000 mg/kg diet, but not at all at
500 mg/kg diet. The tumour incidence was not significantly
greater among any of the treated animals than it was in the
controls (Blackwell-Smith et al., 1953; Kampmeier & Haag,
1954).
Weanling rats in groups of 25 males and 25 females were fed
diets containing 25, 250, or 2500 mg ziram/kg diet for 2 years.
The growth rate and life span were normal in all groups, but
neurological changes were observed in the animals receiving
2500 mg/kg diet, though no cystic lesions were discovered in the
levels. In some of the male animals, the testes were atrophied,
and there was a slight indication of thyroid hyperplasia,
notably in the 2500 mg/kg diet group. However, there was no
increase in tumour incidence in the treated animals (Hodge et
al., 1956). A comparable study with the same dose levels was
carried out with ferbam, and again no increase in tumour
incidence was found (IARC, 1976).
8.2.1.2. Dog
A dog given ferbam and ziram together for one month, each at
a dose of 5 mg/kg body weight per day, remained healthy except
for slight anaemia. The same result was observed when ferbam
was given alone for one month at a dose of 25 mg/kg body weight
per day, or for one week at 50 mg/kg body weight per day.
Raising the dose to 100 mg/kg body weight per day, however,
immediately provoked severe vomiting and malaise (Hodge et al.,
1952). Pairs of adult dogs were given daily doses of 0.5, 5, or
25 mg ferbam/kg body weight for one year. Convulsions occurred
at the highest dose level, but urine analysis, blood parameters,
organ weights, and tissue histology (including that of the
thyroid gland) were normal (Hodge et al., 1956).
When pairs of dogs were fed maneb orally at the rate of 2,
20, 75, or 200 mg/kg body weight per day for one year, toxic
effects were observed at the two highest dose levels, but not at
20 mg/kg body weight (Worthing & Walker, 1983).
Three groups of three dogs each were fed diets containing
20, 2000, or 10 000 mg zineb/kg diet for one year. All the
animals survived, and no persistent changes in growth rate were
seen in any of the groups. There were no histopathological
changes in the tissues, except in the thyroid gland, and
haematological findings were normal. At 10 000 mg/kg diet,
thyroid hyperplasia was noted (Blackwell-Smith et al., 1953;
Kampmeier & Haag, 1954).
8.2.1.3. Bird
Sodium diethyldithiocarbamate (NDDC), the dimethyl compound
(NDMC), and ferbam, ziram, and thiram were given orally to young
and adult domestic fowl (Thorber's gog cockerels) at 330, 210,
205, 56, and 178 mg/kg body weight, respectively, and the birds
were killed after 6, 12, 18, or 20 weeks. All of the compounds
had an adverse effect on body weight gain, retarded testicular
development, and produced degeneration in the seminiferous epi-
thelium of mature birds. Nerve fibre degeneration was produced
in the medulla and spinal cord of chicks by NDDC and in those of
cocks by NDMC. Chicks exposed to thiram became lame and
exhibited swollen epiphyses of the long bones due to endo-
chondrial ossification giving rise to a thickened cartilaginous
epiphyseal plate (Rasul & Howell, 1974).
8.2.2. Inhalation exposure
8.2.2.1. Rat
Studies concerning toxicity following inhalation exposure
are scarce.
Ivanova-Chemishanska et al. (1972) studied the inhalation
toxicity in rats with zineb (70% purity), maneb (80% purity),
and mancozeb (80% purity), applied 6 days per week over a period
of 4“ months, at concentrations of 2, 10, 50, 100, or 135 mg/m3.
The pesticides were given in the form of dispersed aerosols,
with 95% of the dust particles ranging from 1 to 5 µm in size,
and the remainder from 5 to 10 µm. Local irritation of the
mucosa of the upper respiratory tract was noted and
concentration-related non-specific changes in the liver and
kidneys were evident. However, only slight changes were found
at a concentration of 2 mg/m3.
Davydova (1973) studied the influence of inhaled thiram on
the estrous cycle and genital function of rats. Groups of rats
were exposed to 0, 0.45, or 3.8 mg/m3 thiram for 6 h/day, 5
days/week, over a period of 4“ months. An extension of the
estrous cycle was seen at the highest dose level, and genital
function was disturbed, as shown by a reduction in the capacity
to conceive, a reduction in fertility, and of fetal weight
gain.
8.3. Skin and Eye Irritation; Sensitization
Nabam (19% solution) and zineb (65% wettable powder) were
each applied to the right eye of 10 rabbits, the left eye being
used as a control. Nabam did not produce signs of irritation,
while zineb produced mild irritation (erythema), which subsided
within 6 - 8 h. No oedema was seen. The mild irritation may
have been caused by the non-specific foreign body reaction to
the dry, insoluble powder. When this procedure was repeated
with both compounds diluted and suspended for agricultural use
(for nabam, 0.5% of the commercial 19% solution plus zinc
sulfate, 0.125% in water; for zineb, a 0.188% suspension of the
commercial 65% wettable powder in water), no irritation was seen
(Blackwell-Smith et al., 1953).
In studies performed on guinea-pigs, intracutaneous
injections, 10 times daily, followed by an epicutaneous
challenge test, provided evidence of the marked sensitizing and
cross-sensitizing properties of thiram and metiram (Griepentrog,
1960).
It has been reported that a number of dithiocarbamates
(mancozeb, metham-sodium, metiram, zineb, ziram, and thiram)
cause skin and/or eye irritation (Worthing & Walker, 1983).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
8.4.1. Reproduction
8.4.1.1. Rat
Groups of rats (16 male and 16 female Charles River-CD rats
per group) were fed maneb for 3 months at levels of 0, 125, or
250 mg/kg diet, and were mated in a standard 3-generation, 2-
litters-per-generation reproduction study. Groups of males and
females from the F1b and F2b litters were fed maneb for 3 months
after weaning and mated to become parents of the succeeding
generation. The major reproduction indices were unaffected by
maneb at dietary levels up to and including 250 mg/kg diet.
There was no histological evidence of congenital anomalies in a
variety of tissues and organs of the male and female rats of the
F3b litter subjected to histopathological examination (Sherman &
Zapp, 1966).
Maneb, zineb, and mancozeb exert dose-dependent damaging
effects on the gonads of rats of both sexes. The dose levels
were 96 - 960 mg zineb/kg body weight, 140 - 1400 mg mancozeb/kg
body weight, and 14 - 700 mg maneb/kg body weight, given twice a
week for 4.5 months. Both reproductive and endocrine structures
were affected at all dose levels, leading to decreased fertility
(Ivanova-Chemishanska et al., 1973, 1975a). In a 4-month
inhalation study on rats using maneb at 4.7 mg/m3, no effect on
sperm mobility was detected (Matokhnyuk, 1971).
Ivanova-Chemishanska & Antov (1980) studied the effects of
EndodanR (50% ethylenethiuram monosulfide) on the gonads and
reproduction in rats during long-term daily oral doses of 3.8 or
38 mg/kg body weight. The parental generation (F0) and 3
consecutive generations (F1 - F3) were examined. In F0, a
decrease in succinic dehydrogenase and ATPase activities in
testes homogenates was found, as well as an increase in glucose-
6-phosphate dehydrogenase (G6PDH) activity compared with control
levels. Changes in the liver and brain enzyme systems were also
noted.
The same results were obtained with zineb (78% purity). A
rapid loss of mobility and changed resistance (to osmotic and
acidic effects) of spermatozoa were found. A decreased index of
fertility was also found for both sexes in the F0 generation.
Decreased index of fertility and enzymatic changes in organ
homogenates were detectable in the F1 - F3 generations (Ivanova-
Chemishanska et al., 1973).
In extracts of testes of white rats, exposed by inhalation
to zineb and maneb at a concentration of 100 mg/m3 for 4 months,
Izmirova et al. (1969) found an increase in lactate dehydro-
genase (LDH), LDH2, and LDH4. Bogartykh et al. (1979) did not
find any changes in LDH or G6PDH activities in testes homo-
genates of Wistar rats orally treated with zineb (2.5 mg/kg body
weight) for 3 months.
Thiram at doses of 225, 300, 450, 600, 900, or 1200 mg/kg
diet given to male Wistar rats for 29 days produced changes in
many of the parameters studied. A significant effect on testes
and seminal vesicle weight was found at 450 mg/kg diet, and a
decrease in body weight was found at 300 mg/kg. The most
sensitive parameters studied were found to be the weights of the
epididymal and perirenal fat pads, which were decreased by
thiuram doses in the range 130 - 184 mg/kg diet. The no-effect
level, calculated using an extrapolation model, did not differ
significantly from the earlier reported value of 48 mg/kg diet
(Lowy et al., 1979, 1980).
Ferbam was fed to groups of 20 Charles River-CD male rats at
concentrations of 0, 500, 1200, or 2500 mg/kg diet for 13 weeks
before mating with untreated females. Six of the rats fed the
highest dose level died. The indices of fertility, gestation,
viability, and lactation for the females mated with treated
males were normal (Short et al., 1976).
When thiram was incorporated into the diet at concentrations
of 0, 500, 1000, and 2500 mg/kg diet and fed to male weanling
Charles River rats for 13 weeks prior to mating, food intake and
growth was mainly decreased at the two highest dose levels.
Loss of hair and rough coats were also seen in these groups. At
the highest dose level, high mortality occurred. Males in the
highest dose group failed to inseminate the females. In these
animals, there was evidence of testicular hypoplasia, tubular
degeneration, and atypical spermatoids in the epididymus. At
the two lower dose levels, no influence on reproduction was
found (Short et al., 1976).
Female rats fed 400 or 2000 mg thiram/kg diet for at least
14 days prior to mating showed a significant reduction in the
number of implants per dam and pups per dam. The delaying
effect on the estrous cycle was reversible. At the highest dose
level, a number of animals died. A comparable study with ferbam
using the same dose levels did not show any influence on
fertility, gestation, viability, or lactation (Short et al.,
1976).
Administration of 50 mg ziram/kg and 100 mg zineb/kg body
weight to rats for a period of 2, 4, or 6 months produced
delayed insemination, sterility, resorption of fetuses, and
anomalies in development (Rjazanova, 1967).
8.4.1.2. Bird
Thiram (99.9% purity) has been reported to decrease egg
production for the domestic chicken (Gallus domesticus), pigeon
(Columba livia), and pheasant (Phasianus colchicus torquatus).
A dose level of 8.8 mg/kg body weight per day caused a 50%
reduction in egg laying in bobwhite quail (Colinus virginianus).
During this period of reduced egg laying, it seems that an
alteration of hormone levels took place resulting in significant
weight losses of ovary and oviduct, decrease in serum calcium
level (which is controlled by estrogen), and alteration in
normal maturation of the ova (Wedig et al., 1968).
In a study by Van Steemis & Van Logten (1971), tecoram (an
oxidation product of disodium EBDC and sodium dimethyl dithio-
carbamate with ammonium persulfate) in propylene glycol or
saline was administered to chick embryos at doses of 0.01, 0.1,
1, or 10 mg/egg. Paralysis, shortening of the extremities,
muscular atrophy, dwarfing and death occurred. Microscopically,
signs of peripheral neuropathy confined to the distal parts of
the peripheral nerves, and muscular atrophy were found.
8.4.2. Teratogenicity
Some dithiocarbamates are potentially teratogenic in the
rat, but not in the mouse. In most cases, the teratogenic
effects have been observed at high dose levels.
8.4.2.1. Rat
Kaloyanova et al. (1967) have studied the effects on progeny
of albino rats of 0, 700, and 1400 mg maneb/kg body weight
administered twice per week for 4.5 months. Three groups of 20
rats (10 males and 10 females) were used and a first generation
was bred. Congenital deformities were found in the facial part
of the skull, caudal vertebrae, palates, limbs, and tail. The
same type of changes were also found after a single oral dose of
2000 - 8000 mg zineb or 1000 - 4000 mg maneb/kg body weight on
days 11 - 13 of pregnancy.
No teratogenic effects or adverse effects on the intra-
uterine development of progeny were observed when rats were
given 1000 mg zineb or 500 mg maneb/kg body weight, from days 2
to 21 of pregnancy, or were exposed in an inhalation chamber to
a concentration of 100 mg zineb/m3 for 4 h/day from day 4 of
pregnancy (Antonovich et al., 1972; Petrova-Vergieva & Ivanova-
Chemishanska, 1973; Ivanova-Chemishanska et al., 1975a).
In a study on Sprague Dawley rats using maneb at dose levels
of 0, 120, 240, or 480 mg/kg body weight on days 7 - 16 of
gestation, fetotoxic effects (reduced fetal weight, reduced
ossification, and hydrocephalus) were seen at the highest dose
level (Chernoff et al., 1979).
In studies by Larsson et al. (1976), maneb was administered
to Sprague Dawley rats at dose levels of 0, 400, 770, or
1420 mg/kg body weight, by gavage, as a single dose on day 11 of
gestation. Rats were sacrificed on day 18 of gestation and
fetuses were examined for reproductive and teratogenic abnorm-
alities. A substantially increased resorption rate was seen at
770 mg/kg body weight. Gross malformations occurred in all
surviving animals at 770 and 1420 mg/kg body weight, but no
malformations were observed in the single litter of the low-dose
group. These abnormalities included cleft palate, hydrocephaly,
and other serious defects. In another study, maternal admini-
stration of zinc acetate (made in an attempt to relieve the
incidence of teratogenic events) had some preventive effect at
750 mg/kg body weight, but, at 1380 mg/kg body weight, the
frequency and type of malformations were unchanged (Larsson et
al., 1976).
Mancozeb was administered to rats at dose levels of 0, 380,
730, or 1320 mg/kg body weight on day 11 of gestation in a study
similar to that reported above with maneb. Again, a substantial
increase in malformations, similar to those produced by maneb,
was observed at the highest dose level, but not at lower levels
(Larsson et al., 1976).
Propineb was administered to rats at dose levels of 0, 400,
760, or 2300 mg/kg body weight, by gavage, on day 11 of
gestation. The dams were sacrificed and fetuses examined for
gross external and internal malformations on day 18 of
pregnancy. Maternal toxicity was observed at all dose levels.
At the highest dose level, propineb was fetotoxic and induced a
variety of malformations in the surviving fetuses. At 760 mg/kg,
propineb was slightly fetotoxic but did not induce malformations
in surviving fetuses. The pattern of fetal abnormalities was
qualitatively similar to that noted in the maneb- and mancozeb-
treated rats (Larsson et al., 1976).
Cypromate (zinc propylene bisdithiocarbamate) was studied
for its teratogenic potential in white rats using either a
single oral dose of 250, 500, or 1000 mg/kg body weight on the
11th or 13th day of gestation or repeated treatment from the
first day of gestation through the whole pregnancy at 62, 250,
and 500 mg/kg body weight. A spectrum of malformations involving
the nervous and skeletal systems, facial cranium, extremities,
etc., were induced with a single dose of 500 mg/kg body weight
or more, and at all dose levels given repeatedly (Petrova-
Vergieva, 1976).
Groups of rats (26 - 27 pregnant CD1 rats per group) were
administered zineb (purity 85.5% containing 0.35% ETU) at dose
levels of 0, 200, 632, or 2000 mg/kg body weight per day on days
6 - 19 of gestation. Maternal body weight and food consumption
data were recorded. Pregnant rats were sacrificed at day 20 and
a laparotomy was performed. Fetal data included live, dead, and
resorbed fetuses as well as somatic and skeletal abnormalities.
There was no maternal mortality, but a substantial weight loss
was seen at the highest dose level. Fetuses from mothers
administered 2000 mg/kg also showed a reduced body weight.
Fetal mortality was not observed, and there were no significant
anomalies noted on gross external examination. However, a
higher incidence of teratogenic anomalies was noted at the
highest dose level (short and kinky tails, hydrocephalus, and
increased incidence of skeletal anomalies). At the 632 mg/kg
level, these teratogenic anomalies were absent. The abnorm-
alities found at the highest dose level may have been due, in
part, to the presence of ETU in the formulation (Short et al.,
1980).
Ferbam administered to rats on days 6 - 15 of gestation at
150 mg/kg body weight resulted in death, increased resorptions,
decreased fetal weights, and a slight increase in soft and
skeletal tissue anomalies (Minor et al., 1974). CD-1 rats were
treated on days 6 - 15 of gestation, by gavage, with 0, 11, or
114 mg ferbam/kg body weight. Twenty-five percent of the dams
administered 114 mg/kg died, but the surviving dams showed small
litters, increased resorptions, and decreased fetal weight.
Also, a number of malformations (unossified sternebrae,
malformed cranium, hydrocephalus, and cleft palate) were found.
When thiram was administered at doses of 0, 40, 90, 136,
164, or 200 mg/kg body weight on days 6 - 15 or 7 - 12 of
gestation, the 200 mg/kg dose reduced the number of mated rats
that delivered litters, and only 33% of the dams survived. At
doses of 136 mg/kg or more, a decrease in the number of implants
and fetuses per dam, an increase in resorptions, a decrease in
fetal body weight, and an increase in malformations, as
described for ferbam, were observed (Short et al., 1976).
8.4.2.2. Mouse
Pregnant female NMRI and Swiss-Webster mice were treated
orally during days 6 - 17 of pregnancy with thiram at 179, 357,
714, or 1071 mg/kg body weight and 250, 500, 1000, and
1500 mg/kg body weight, respectively. Increased resorption of
embryos, clearly retarded fetal development, and skeletal
malformation (cleft palate, wavy ribs, curved long bones of
extremities, and micrognathia) were seen in both strains. The
12th and 13th days seemed to be the most sensitive period of
embryonic development. The lowest dose had only a slight
effect, but the next dose level was clearly teratogenic (Roll,
1971; Matthiaschk, 1973).
Thiram did not reduce body weight gain during gestation at
doses of 100 or 300 mg/kg body weight, administered on days
6 - 14, and no changes in litter size, incidence of resorptions,
or fetal weight were observed. However, an increase in malform-
ations was seen (Short et al., 1976).
In studies by Larsson et al. (1976), doses of 0, 400, 770,
or 1420 mg maneb/kg body weight or 0, 380, 730, or 1330 mg
mancozeb/kg body weight were given on a single occasion to NMRI
mice on days 9 or 13, and mice were sacrificed on day 18 of
gestation. No adverse maternal or fetal effects could be
detected.
In a study on CD1 mice administered 0, 375, 750, or 1500 mg
maneb/kg body weight on days 7 - 16 of gestation, maternal
toxicity was found at the highest dose level, together with a
decrease in fetal caudal ossification centres at all dose levels
(Chernoff et al., 1979).
Ferbam administered to mice at 30 and 300 mg/kg body weight
on days 6 - 16 of gestation did not produce any teratogenic
effects (Minor et al., 1974), and, when given at 23 or 228 mg/kg
body weight on days 6 - 14, it did not affect the survival or
body weight of Swiss-Webster dams during gestation. No changes
in litter size, incidence of resorptions, or fetal weight were
observed, but, at the highest dose level, an increase in
malformations was seen (Short et al., 1976).
Groups of CD-1 mice were administered zineb (85.5% purity)
daily, at dose levels of 0, 200, 632, or 2000 mg/kg body weight
per day, for 11 days from day 6 of gestation and sacrificed on
day 18. Gross examination for maternal well-being and fetal
anomalies, both somatic and skeletal, failed to show any
teratogenic effects (Short et al., 1980).
8.4.3. Embryotoxicity
Korhonen et al. (1982a,b) used a system called chicken
embryo test to study the embryotoxic potential of dithio-
carbamates and found early and late death and malformed embryos.
It was thought that this test could have a predictive value as a
simple teratogenicity test, but many limitations were found in
doing so, and the interpretation of the results were difficult.
8.5. Mutagenicity and Related End-Points
Seiler (1973) studied the mutagenicity of maneb and ziram.
Maneb proved negative in tests with Salmonella strains his G46,
TA1530, TA1531, TA1532, and doubtful in TA1534. Ziram was
positive in TA1534, doubtful in TA1530, and negative in the
other strains.
In studies by Fahrig (1974), ziram was non-mutagenic in a
variety of other microorganisms (Escherichia coli, Serrata
marcescens, and Saccharomyces cerevisiae), but Pilinskaya (1971)
found that it induced chromosome breaks, most of them confined
to chromosome 2, in cultured peripheral human lymphocytes.
Shirasu et al. (1976) studied mutagenicity with the rec-
assay procedure, a sensitivity test using H17 rec+ and M45 rec-
strains of Bacillus subtilis, and with reversion assays on
plates using E. coli (WP2) and S. typhimurium TA1535, TA1536,
TA1537, and TA1538. In these tests, ferbam, thiram, and ziram
were non-mutagenic.
Certain dithiocarbamates given intraperitoneally to mice at
100 mg/kg body weight caused chromatid aberrations in bone
marrow cells (Kurinny & Kondratenko, 1972; Hedenstedt et al.,
1979), the order of effectiveness being thiram > ziram > maneb
and zineb. Thiram was also shown to induce gene mutations in
Salmonella and Aspergillus (Szymezyk, 1981; Zdienicka et al.,
1981).
Hedenstedt et al. (1979) found that the mutagenic effect of
tetramethylthiuram monosulfide (TMTM) was enhanced in the
presence of metabolizing systems (S-9 mix), but that tetraethyl-
thiuram disulfide (TETD or AntabuseR) was not mutagenic.
Propylene bisdithiocarbamate was tested for cytogenicity by
bone marrow analysis and for dominant lethal mutations by giving
a single oral dose to male rats. There was a considerable
increase in the number of chromosomal aberrations (chromatid
fragments), reaching a maximum after 24 h (Vachkova-Petrova,
1977). In studies on the cytotoxic effects of ziram on cultures
of human lymphocytes in vitro (Pilinskaya, 1971), the ratio of
chromatid-type aberrations to chromosome-type aberrations was
2.7:1, which suggests that most chromosomal damage took place at
the S-stage and the G2 stage of the mitotic cycle. Ziram-
induced chromosomal breaks were observed to be non-random, most
of them occurring in chromosome 2.
Propineb and its main metabolite propylenethiourea (PTU)
were investigated by the micronucleus test in mice. The
following doses were given by ip injection twice, with a 24-h
interval: propineb (unknown purity), 62.5, 125, or 250 mg/kg
body weight in a 5% aqueous solution of Tween 80; propineb (78%
purity), the same doses, but in 5% gum arabic; and PTU, 100,
200, 400, or 600 mg/kg body weight in distilled water. Controls
received methanesulfonate at doses of 10, 20, 40, or 80 mg/kg
(twice, in distilled water) and mitomycin at 1.75, 3.5, 7, or
14 mg/kg (twice, in distilled water). No statistically
significant increase in the percentage of micronuclei was
observed at any of the tested doses of propineb or PTU. The
positive control groups showed the expected dose-related
increase in the number of polychromatic erythrocytes with
micronuclei (Rolandi et al., 1984).
Vachkova-Petrova (1981) studied the mutagenic potential of
EndodanR (ethylenethiuram monosulfide) in short-term studies.
Several doses of EndodanR were administered to groups of 6 rats
either twice at an interval of 24 h or for 5 successive days,
and the animals were killed 6 h after the last dose. The cells
in metaphase were analysed for aneuploidy and aberrations, but
no mutagenic effects that could be attributed to the chemical
were detected.
Zineb and ziram were not mutagenic when tested in Drosophila
melanogaster (Benes & Sram, 1969).
8.6. Carcinogenicity
8.6.1. Mouse
In studies by Innes et al. (1969), groups of 18 mice of each
sex from two hybrid strains were given various dithiocarbamates
from 7 days of age up to 18 months. The compounds were given
daily, by gavage, from day 7 to weaning and thereafter added to
the diet. The compounds and the respective amounts were: ferbam
at 10 mg/kg body weight, then 32 mg/kg diet; maneb at 46.4 mg/kg
body weight, then 158 mg/kg diet; nabam at 21.5 mg/kg body
weight, then 73 mg/kg diet; thiram at 10 mg/kg body weight, then
26 mg/kg diet; and zineb at 464 mg/kg body weight, then
1298 mg/kg diet. No significant increase in tumours was found.
On the basis of all experimental data and experimental
designs, IARC (1976) suggested that there was no definite proof
for the carcinogenicity of maneb, though ETU, one of its meta-
bolites, was able to produce thyroid carcinomas. However, zineb
and maneb have been reported to induce pulmonary adenomas in
mice when treated orally (Chernov & Khistenko, 1969; Balin,
1970).
8.6.2. Rat
A 2-year feeding study of the effects of zineb on rats was
carried out using 60 young male and 60 female albino rats.
These were divided into groups of 10 each and administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg/kg diet.
Growth, mortality, haematology, and organ weights were examined
and histopathology was carried out. No clear influence on
growth and mortality was observed, and haematological findings
were within normal limits. A goitrogenic effect (hyperplasia)
was observed in 50% of the animals at 500 mg/kg, and, at
1000 mg/kg diet or more, this effect was more pronounced. The
interpretation of thyroid weight/body weight ratio was
complicated because of the small number of animals still alive.
Microscopically, no evidence of malignancies was present. At
the highest dose level, kidney damage (congestion, nephritis,
nephrosis) was seen. Although 10 000 mg zineb/kg diet produced
moderate goitrogenic effects, this effect was also seen at
500 mg/kg in some rats, and was not clearly dose dependent
(Blackwell-Smith et al., 1953).
Cases of goitre and thyroid adenoma were found in Sprague
Dawley rats fed for 2 years on a diet containing 120 mg or
360 mg metiram/kg diet (Griepentrog, 1962).
8.6.3. Dog
When nine mongrel dogs (three groups of three animals each)
were administered 20, 2000, or 10 000 mg zineb/kg diet for 1
year, no haematological changes were found. The thyroids of the
group given the highest dose level were enlarged and showed
hyper-plastic changes, but, at lower dose levels, they did not
show any histological changes (Blackwell-Smith et al., 1953).
Doses of 45 mg metiram/kg body weight for 90 days or
7.5 mg/kg body weight for 23 months did not cause any ill
effects (Worthing & Walker, 1983).
8.6.4. Dithiocarbamates in combination with nitrite
The above-mentioned studies were carried out with individual
dithiocarbamates. Other studies have shown that these
dithiocarbamates, in the presence of nitrite, can be converted
to N-nitroso derivatives, which may be carcinogenic.
Thiram, ferbam, ziram (Eisenbrand et al., 1974; Sen et al.,
1974), and disulfiram (Lijinsky et al., 1972; Elespuru &
Lijinsky, 1973) react with nitrite under mildly acidic con-
ditions to form N-nitroso compounds. Formation of N-nitroso-
dimethylamine (NDMA) by the action of microorganisms in sewage
and soil containing 0.1% thiram has been reported to occur under
experimental conditions (Ayanaba et al., 1973). Nitrite is
formed by the reduction of nitrate, which can be found in some
unrefrigerated vegetables (e.g., spinach and beets), especially
after cooking (Phillips, 1968), in human saliva (Tannenbaum et
al., 1974), and in cured meats. Thus, in vivo nitrosation of
dithiocarbamates in the stomach cannot be totally excluded.
As has been pointed out by IARC (1976), the extrapolation of
findings in experimental animals to man is complicated by many
factors. It is relatively easy to show that N-nitroso
derivatives can be formed and that these are mutagenic and/or
carcinogenic. The crucial information, however, is the quantity
produced in man under the prevailing conditions. The concen-
tration of both reactants, the pH, the influence of competing
reactions, and the presence of accelerators and inhibitors are
all important. In addition to these difficulties in defining
potential human exposure, the susceptibility of man, compared
with that of experimental animals, has to be considered.
Sen et al. (1974) concluded that it is unlikely that
significant amounts of NDMA would be produced from the ingestion
of trace amounts of the dithiocarbamates and the normal intake
of nitrite.
8.7. Mechanisms of Toxicity; Mode of Action
8.7.1. Thyroid
In weaning rats, a diet containing 500 mg nabam/kg given for
9 days caused thyroid hyperplasia and a decrease in the weight
of the thymus (Seifter & Ehrich, 1948).
Male and female albino rats were administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg zineb/kg diet
for up to 30 days. Animals were killed sequentially in order to
study the changes in the thyroid gland. Only in the 10 000 mg/kg
group were effects seen. In two out of five males and in one
out of five females, hyperplasia of the thyroid was observed
(Blackwell-Smith et al., 1953). Przezdziecki et al. (1969) fed
female Wistar rats a diet containing 1300 mg zineb/kg or 1875 mg
maneb/kg diet for 7 months. Significant increases in the weight
of the thyroid gland and decreases in the weight of the kidneys,
adrenal glands, and ovaries were observed.
Thyroid hyperplasia has been reported in rats given maneb,
zineb, or mancozeb in amounts ranging from 500 to 2500 mg/kg
diet for periods of up to 2 years (FAO/WHO, 1965b, 1971b). In a
2-year feeding study, 2500 mg maneb/kg diet produced thyroid
hyperplasia and nodular goitre and increased mortality, but 1250
and 250 mg/kg diet did not cause any ill effects (FAO/WHO,
1965b).
Zineb was given orally to white rats at dose levels of 96 or
960 mg/kg body weight for 4.5 months. Compared with that of
untreated animals, the thyroid was enlarged with microfollicles
and columnar cells. Succinic dehydrogenase and cytochrome
oxidase activities were raised in these cells, while the colloid
in the follicles showed reduced PASa-positive granules. These
changes were consistent with an increase in thyroid-stimulating
hormone (TSH). An increased number of basophilic cells con-
taining PASa-positive granules was observed in the adeno-
hypophysis (anterior pituitary). These effects were seen only
at the highest dose level. The uptake of 131iodine was also
increased at the highest dose level, and a high plasma TSH level
was recorded in treated animals. The changes observed in both
thyroid and pituitary were probably a compensatory response to
the antithyroid effect of the dithiocarbamate (Ivanova-
Chemishanska et al., 1975b).
After oral administration of zineb to rats at dose levels of
9.6 or 960 mg/kg body weight, twice a week, for 4“ months, the
gonadotropic and thyroid-stimulating functions of the adeno-
hypophysis were significantly increased compared with those of
control values, more markedly in those receiving the higher dose
(Ivanova-Chemishanska et al., 1974).
Albino rats were orally administered doses of 2400 mg
zineb/kg or 3500 mg maneb/kg body weight, and, after 24 h,
radioactive iodine was administered intraperitoneally. Reduced
assimilation of 131iodine by the thyroid was found, which
suggests that the dithiocarbamates, or certain of their
metabolites, possess a marked antithyroid effect and inhibit the
synthesis of thyroxine (Ivanova-Chemishanska et al., 1967,
1974). In similar studies with mancozeb, a single oral dose of
7000 mg/kg body weight resulted in a decreased uptake of
131iodine (Ivanova-Chemishanska et al., 1967).
The condition of the thyroid gland was studied in male
albino rats, which were administered 700 mg maneb/kg, 960 mg
zineb/kg, or 1400 mg mancozeb/kg body weight. After 30 days,
the distinct morphological changes observed indicated
stimulation of the thyroid by TSH. Hypophyseal stimulation is
the consequence of release from negative feedback by thyroxine,
the plasma level of which is depressed by the action of EBDCs
(Ivanova-Chemishanska et al., 1971, 1974).
Rats fed a diet containing 10 000 mg metiram/kg for 2 weeks
showed increased thyroid weight and a decrease in the uptake of
131iodine, but no ill effects were produced by 1000 mg/kg diet
(Worthing & Walker, 1983).
----------------------------------------------------------------
a PAS = periodic acid-Schift reagent.
8.7.2. Interaction of dithiocarbamates and alcohol
Hald et al. (1948) found that dithiocarbamates interact with
ethanol (ethyl alcohol), and since then, certain dithio-
carbamates, particularly disulfiram, have been used in the
treatment of chronic alcoholism. Disulfiram has been proposed
to act in two different ways. The first possibility is that the
drug or one of its metabolites (e.g., diethyldithiocarbamate,
carbon disulfide) interferes with the normal metabolism of
ethanol and, consequently, gives rise to an accumulation of
toxic amounts of intermediary products, such as acetaldehyde.
The second possible method of action is that ethanol interferes
with the normal metabolism of disulfiram and therefore makes
disulfiram more toxic in some way.
Ethanol is detoxified in many tissues, particularly the
liver, by oxidation, firstly to acetaldehyde, then to acetic
acid, and finally to carbon dioxide and water. Disulfiram
interferes with various enzyme systems including those involved
in the oxidation of ethanol. After administration of disulfiram,
the blood acetaldehyde level increases significantly.
Peripheral neuropathy and optic neuritis have been observed in
alcoholics treated with 125 - 150 mg disulfiram per day
(Gardner-Thorpe & Benjamin, 1971).
Van Logten (1972) studied this phenomenon of alcohol
intolerance extensively in rats. Zineb and maneb did not induce
alcohol intolerance, whereas most of the alkyldithiocarbamates
(such as ziram and nabam) and thiuram sulfides (such as thiram)
did. In general, the dithiocarbamates with a free H atom bound
to the N atom did not induce intolerance. Apart from the
accumulation of acetaldehyde in blood, disturbance of sulfo-
bromophthalein (BSP) elimination, increased serum glutamic-
pyruvic transaminase, hypothermia, increased glucose content,
changes in blood morphology, atrophy of spleen and thymus, and
increase in the weight of adrenals and brain were all observed.
Studies on adrenalectomized rats showed the involvement of the
adrenals in the alcohol intolerance. Hyperglycaemia, eosino-
penia, lymphopenia, and neutrophilia were not seen in the
animals without adrenals, and spleen and thymus atrophy was
reduced. However, the effect on the red blood cells was more
pronounced, and the accumulation of acetaldehyde in the blood
was unaffected by adrenalectomy.
Oral treatment of rats with ethanol after administration of
alkyldithiocarbamates or thiuram sulfides lowers the catechol-
amine content of the adrenals. Since the lowest level was not
reached until 24 h or more after alcohol treatment, it seems
likely that the influence on the adrenals of the dithio-
carbamate-ethanol interaction is of secondary importance. There
was no clear indication that the ethanol intolerance in the rat
is accompanied by changes in brain catecholamine level.
As in human beings, the dithiocarbamate-ethanol reaction in
the rat is characterized by a severe hypotension, which starts
almost immediately after the administration of the ethanol and
lasts at least 8 h. It is evident that, during alcohol
intolerance, body fluids shift from the plasma into the
interstitial tissue or cells, possibly thereby causing the hypo-
tension or shock. The observed hypothermia also starts almost
immediately after ethanol administration and may last for many
hours. Adrenalectomy did not prevent the hypothermia.
Therefore, this phenomenon must be considered as a primary
effect, along with the plasma accumulation of acetaldehyde.
Since heat loss is not increased during the dithiocarbamate-
ethanol reaction, the hypothermia is probably due to decreased
heat production. However, the serotonin concentration in the
brain was increased, and so a disturbance of the thermo-
regulation cannot be ruled out. It seems doubtful that the
dithiocarbamate-ethanol reaction is due to acetaldehyde per se.
Intraperitoneal injection of acetaldehyde, which resulted in a
blood level twice as high as during the dithiocarbamate-ethanol
reaction, did not influence BSP elimination, serum glucose
level, body temperature, organ weights, catecholamine content of
the adrenals, or blood pressure.
No indications were available that the accumulation of
acetaldehyde in the blood was due to an accelerated biotrans-
formation of alcohol. More work needs to be done to decide
whether the accumulation of acetaldehyde and pyruvate in the
blood is a consequence of a disturbance of carbohydrate
metabolism. The specificity of ethanol is remarkable. The
combination of thiram with either methanol or 1-propanol has no
effect on BSP elimination or on blood glucose level.
The sensitivity of the rat for the dithiocarbamate-alcohol
reaction is of the same magnitude as that of man. Administra-
tion of 1.9 mg thiram/kg body weight in the rat elicits an
accumulation of acetaldehyde in the blood. Thus, it may be
concluded that 60 ml of gin, 0.5 litre of beer, or even less
should be sufficient to induce alcohol intolerance.
Eight days after the administration of certain dithio-
carbamates, a dithiocarbamate-alcohol reaction may be observed
when ethanol is given. The maximum level of acetaldehyde in the
blood is reached within 15 min of ethanol treatment.
A 90-day study with several dietary levels of thiram
revealed a no-toxic-effect level of 100 mg/kg diet. After
feeding rats with 10 mg thiram/kg diet for 6 weeks, oral
administration of a single dose of 6 ml ethanol/kg body weight
caused a significant decrease in the body temperature. Higher
doses of thiram with alcohol induced hyperglycaemia, accumula-
tion of acetaldehyde in the blood, and other abnormalities. In
contrast, the combination of 100 mg thiram/kg diet and 5%
ethanol continuously in the drinking-water did not have any
effect (Van Logten, 1972).
8.7.3. Neurotoxicity
In an 80-week study on the neurotoxic and behavioural
effects of thiram, 12 male and 12 female rats per group were fed
thiram at dose levels of 0, 100, 400, or 1000 mg/kg diet (the
concentration of the compound in the diet was periodically
increased in order to give a relatively constant consumption on
the basis of body weight). A second study was carried out on
two groups of 24 female rats administered 0 or 1000 mg thiram/kg
diet, for 36 weeks. The neurotoxic effects were characterized
by ataxia and paralysis of the hind legs, although these effects
were only seen at the highest dose level (1000 mg/kg diet,
equivalent to 65 mg/kg body weight) in females. Demyelination,
degeneration of the axon cylinders, and the presence of
macrophages in the nerve bundle of the sciatic nerve were seen.
Degeneration in the ventral horn of the lower lumbar region of
the spinal cord was demonstrated by chromatolysis of motor
neurons, pyknosis, and satellitosis. Electromyograms indicated
a loss of motor unit function, and the histopathology suggested
that the peripheral nerve is the primary site of the lesion (Lee
& Peters, 1976).
In another study, groups of 12 males and 12 females were fed
ferbam in the dose levels that gave actual intake levels of
approximately 8.5, 34, and 87 mg/kg body weight (average of
males and females) per day. The neurotoxic effects of ferbam
are less than those of thiram. In this study, only 3 of the 24
rats fed the highest dose level developed ataxia or paralysis
(Lee & Peters, 1976). Neurotoxic effects have also been observed
for ziram by Hodge et al. (1956).
In a study on rats, zineb (490 and 2450 mg/kg body weight),
maneb (350 and 1750 mg/kg body weight), and mancozeb (700 and
3500 mg/kg body weight) were administered orally at twice weekly
doses for 4 months. Mortality was high, and paresis in the hind
limbs appeared in the third month of the study and progressed to
complete paralysis (Ivanova-Chemishanska, 1969a).
Dishovski & Ivanova-Chemishanska (1979) studied the ultra-
structural changes in the neocortex of rats repeatedly
administered propineb (70% purity) at 85 mg/kg and 425 mg/kg
body weight for 40 days. At the higher dose level, intense
ultrastructural changes in the sensorimotor neocortex were
detected using an electron microscope, primarily affecting the
pyramidal cells. The concentration of ribosomes and hypertrophy
of the Golgi apparatus suggested an increase in synthetic
processes in the neurons.
Edington & Howell (1966, 1969) found lesions in the central
nervous system of adult Dutch-New Zealand rabbits who were given
ip injections of sodium diethyldithiocarbamate (NDDC) at
330 mg/kg body weight, for 6 days/week, for 30 weeks. The first
changes were seen at 6 weeks in the accessory cuneate nucleus
and in Clarke's column; 12 weeks later, degeneration was seen in
the spinocerebellar tracts in the cerebellum medulla. After 24
weeks, severe nerve fibre degeneration in the peripheral white
matter of the spinal cord (both involved the axon and myelin
sheath) was observed. It was suggested that these changes might
be connected with changes in the level of copper in the serum.
Kim & Rizzuto (1975) studied the effect of NDDC (0.23, 2.3,
and 23 µg/ml nutrient medium) on myelinated cultures of new-
born mouse cerebellum. Exposure time was 24 - 120 h, and the
cultures were examined by light and electron microscopy.
Treatment of the cultures for 24 - 48 h produced swelling of
axons and presynaptic endings, morphologically characteristic of
dystrophic axons. Continued exposure induced an extensive
degeneration of axons and myelin sheath (Wallerian degeneration
in axons).
8.7.4. Dithiocarbamates in combination with metals
Truhaut et al. (1971) studied the chelating action of sodium
diethyldithiocarbamate to copper and the fact that this element
is indispensable for the activity of dopamine beta-hydroxylase.
The authors put forward the hypothesis that the inhibition of
this enzyme system, which catalyses the conversion of dopamine
to norepinephrine and participates in the biogenesis of cate-
cholamines in the central nervous system, may play a role in the
etiology of neurotoxic effects.
Maj et al. (1970) studied the effect of disulfiram, diethyl-
dithiocarbamate (DDC), and dimethyldithiocarbamate on serotonin
(5-HT) and 5-hydroxyindole-3-acetic acid (5-HIAA) in the brain
of rats. The total dose levels ranged from 150 to 500 mg/kg
body weight. It was concluded that these three compounds do not
affect the 5-HT level in the rat brain. The 5-HIAA levels
increased, but not significantly.
Possibly, reactions of carbon disulfide with pyridoxamine
could lead to the depletion of pyridoxal phosphate in the
tissues, which may, in turn, cause neurological changes. Long-
term poisoning of rabbits with carbon disulfide has been shown
to result in increased excretion of zinc in the urine and
disturbances of copper and zinc concentrations in the tissues.
Also, after NDDC treatment, increased levels of copper in the
liver and nervous tissue have been found (Cavanagh, 1973).
Aaseth et al. (1981) showed that oral treatment of Wistar
rats with tetramethylthiuram disulfide (TMTD) at 1000 mg/kg diet
for one week increased the brain levels of endogenous copper
and zinc. In further studies, rats were administered an iv
injection of 203HgCl2 (5 µmol/kg body weight in saline) at day
17 of pregnancy. DDC was given immediately after the mercury
injection (500 µmol/kg body weight). The maternal brain
concentration of mercury increased significantly, and the kidney
levels, measured after 24 and 48 h, also increased. In the
fetuses, the mercury in the brain, liver, kidneys, and blood
(but also in the placenta) were significantly increased after
24 h, but, after 72 h, only the levels in fetal blood were still
elevated. Mice of the NMRI strain were similarly injected with
203HgCl2 (2.5 µmol/kg body weight) and fed diets containing DDC
(10 000 mg/kg diet), disulfiram and TMTD (1000 mg/kg diet), or
carbon disulfide (3000 mg/kg diet) for 4 days. The brain level
of mercury was significantly increased after DDC or TMTD
treatment and marginally after disulfiram or carbon disulfide
treatment (Aaseth et al., 1981).
Lakomaa et al. (1982) studied the effect of DDC on copper
and zinc concentrations in different regions of the brain of
Long-Evans rats during acute or repeated treatment. Acute
treatment (250 mg/kg body weight) produced no effect after 24 h,
whereas repeated treatment (250 mg/kg, 5 times per week, for
4 weeks) increased copper levels in the brain stem, cortex,
hippocampus, and the rest of the brain, but did not alter zinc
concentrations.
Dithiocarbamates, with their metal-chelating properties, and
thiuram derivatives, have been demonstrated to cause a marked
increase in the concentration of lead in the brain as well as a
redistribution of lead in the rest of the body (Oskarsson, 1983,
1984; Danielsson et al., 1984). Thus, after injection of a dose
of labelled lead (203Pb), the brain concentrations were
increased by up to 100 times in thiuram-treated rats.
Male Sprague Dawley rats (10 groups of 5 rats each) were
administered different combinations of thiram, disulfiram, DDC,
or dimethyldithiocarbamate in combination with sodium or lead.
The study demonstrated that treatment with dithiocarbamates and
thiram derivatives in rats exposed for 6 weeks to lead causes a
substantial (up to 4-fold) increase in the lead concentration of
the brain. This effect can be explained by the formation of a
lipophilic lead-dithiocarbamate complex, which probably is
retained longer and has a higher capacity to penetrate the
blood-brain barrier and bind to lipid-rich brain tissue compo-
nents than inorganic lead itself. The chemical form of the lead
when it is in the brain remains uncertain. The lead complex may
decompose in the brain into inorganic lead, which exerts a
neurotoxic effect, or it may be very stable in the brain and of
low toxicity for the central nervous system (Oskarsson & Lind,
1985).
There are several reports on the effect of dithiocarbamates
on the distribution in the body of other metal ions such as
cadmium, thallium, nickel, copper, zinc, and mercury (Oskarsson
& Lind, 1985).
DDC has been shown to have a strong inducing effect on
levels of metallothionein, a low molecular weight, heavy-metal-
binding protein, in rat liver and kidney. The mechanism
probably reflects enhanced uptake of copper and depletion of
hepatic glutathione (Sunderman & Fraser, 1983).
8.7.5. Miscellaneous reactions
Dithiocarbamates, with their chelating capacity, also
interfere with a number of enzyme systems containing metals such
as zinc and copper (e.g., dopamine beta-hydroxylase). They also
inhibit sulfhydryl (SH)-containing enzymes and a number of other
enzyme systems involved in glucose metabolism (e.g., hexokinase,
glyceraldehyde-3-phosphate dehydrogenase, and glucose-6-
phosphate dehydrogenase). The effect of dithiocarbamates on
liver enzymes has consequences for the metabolism of other
chemicals. Thus, the toxicity of carbon tetrachloride is
decreased by diethyldithiocarbamate (Lange & Jung, 1971; Lutz et
al., 1973), and the toxicity of other chemicals, e.g., ethyl
alcohol, may be increased (section 8.7.2).
9. EFFECTS ON MAN
9.1. Occupational Exposure
9.1.1. Acute toxicity - poisoning incidents
The acute toxicity of dithiocarbamates is low and, there-
fore, acute intoxication in human beings is unlikely to occur.
A case was reported of a 62-year-old man with acute kidney
insufficiency after maneb application. However, the precise
cause of maneb exposure was not clear, since the patient had a
history of hypertension, cerebral infarction, gastrectomy
because of stomach cancer, and chemotherapy. The patient was
treated with haemodialysis and was discharged from hospital
(Koizumi et al., 1979).
Thiram (100 mg/m3) has been shown to cause headaches,
vertigo, impairment of mental capacity, muscle twitch, and
paraesthaesia (Sprecher & Grigorowa, 1967).
9.1.2. Case reports, short-term and epidemiological studies
9.1.2.1. Dermal
The irritant and allergic potential of most dithiocarbamates
is evident in occupational exposure. Skin irritation and
sensitization were studied in man using a conventional patch
test. A cotton square was dipped in 19% nabam solution and
placed on the inner surface of the forearm, and, 14 days later,
this procedure was repeated on the opposite forearm. Zineb was
tested in the same manner, except that the cotton square was
dipped in 65% wettable powder. The patches were left in place
for 48 h. Of the 25 subjects included in the nabam study, 2
showed irritation (mild erythema and itching). Thirteen of the
25 reacted to the retest (from mild erythema to severe erythema,
oedema, and vesiculation), indicating sensitization. Of the 50
subjects used in the zineb study, no reaction at all was seen in
49 of them. One reacted in such a way that it indicated primary
irritation rather than sensitization (Blackwell-Smith et al.,
1953). Schultheiss (1957) reported a case of contact dermatitis
with thiram. Zadorozhny et al. (1981) found dermatitis and
eczema in 241 industrial workers exposed to TMTD and other types
of pesticides. Twenty-one of them showed contact dermatitis, 25
allergic dermatitis, and 7 eczema.
Cases of diffuse erythema and eczematoid epidermatitis of
the eyelids and inguinal regions, probably with elements of sun
sensitization, were observed among agricultural workers (grape
and tobacco industries) in contact with zineb (Babini, 1966) or
maneb (Laborie & Laborie, 1966; Zorin, 1970). These were
largely allergic in character with only a few manifestations of
contact dermatitis. Decreased resistance of the workers,
vitamin deficiency, chronic liver disease, and other factors
apparently contributed to these effects.
9.1.2.2. Exposure via different routes
Kaskevich et al. (1981) carried out an epidemiological study
on 137 workers engaged in zineb manufacturing (51 men and 86
women). The duration of exposure to zineb for 52 workers was
between 1 and 3 years, and for 85 workers between 4 and 5 years.
Control groups in this study consisted of 193 persons, not
exposed to chemicals and matched for age, period of employment
rate, and sex. The concentrations in the air of the working
area never exceeded 1 mg/m3. Among workers occupationally
exposed to zineb, the following changes were found: hepato-
cholecystitis (28.4% of workers, versus 13.5% in controls);
vegetovascular dystonia connected with disorders in the central
nervous system (34.9%, versus 22.3% in controls); chronic
bronchitis (4.4%, versus 0.5% in controls); contact dermatitis
(11.9%, versus 0.1% in controls); and disorders in the menstrual
cycle (16.91%, versus 4.3% in controls). These studies indicate
a change in catecholamine metabolism.
In a study with cultured lymphocytes from 15 workers working
in different stages of zineb manufacture, the mean incidence of
aberrant metaphases was 6% greater than that in controls. The
incidence of chromosomal aberrations (chromatid breaks) in
cultured human lymphocytes treated with maneb (0.5, 15, or
30 µg/ml) was 10 - 20% greater than in controls (Antonovich et
al., 1972).
A number of studies on maneb and mancozeb production workers
have been carried out. In the earliest study (1965), 54 produc-
tion workers were given medical examinations that included blood
and urine analyses. Since this study predated the availability
of immunoassay techniques for thyroid hormone determination,
protein-bound iodine was used as a measure of thyroid function.
No thyroid or other medical abnormalities could be attributed to
EBDC exposure. In a second study (1975), 57 exposed and 98
unexposed production workers were examined for thyroid function
by measuring triiodothyronin (T3), thyroxine (T4), and TSH.
Again, no effects attributable to work-place exposure were
identified. Workers exposed to EBDC levels ranging from 0.13 to
5.46 mg/m3 were found to have elevated ETU and manganese levels
in the urine. In a 1976 mortality study, 992 past and present
production workers (over the period 1948-75) were studied.
Compared with the local general population, neither the overall
death rate nor the death rate due to cancer was elevated. The
number of cancer deaths observed (10) was too small to evaluate
cancer-specific mortality (Gowers & Gordon, 1980).
In the most extensive study, 42 currently exposed and 112
previously exposed workers were compared with equal size control
groups matched for age, period of employment, race, and type of
job. All participants were given thorough physical examinations
by specialists in diagnostic medicine, including detailed
questionnaires and interviews about health history and family
health. A separate thyroid examination was carried out by
thyroid specialists. Thyroid parameters that were measured
included total T3, T3 resin uptake, T4, TSH, free T4 index,
thyroglobulin antibodies, and microsomal antibodies. In
addition, urine was analysed for ETU, EBDC, zinc, manganese,
creatine, iodide, specific gravity, and pH. Blood levels of
glucose, urea nitrogen, sodium, potassium, calcium, chloride,
carbon dioxide, cholesterol, total protein, protein albumin,
bilirubin, uric acid, creatinine, inorganic phosphate, lactic
dehydrogenase, and serum glutamic oxaloacetic transaminase were
also determined. As in earlier studies, the occurrence of
unusually high levels of ETU in the urine of currently exposed
workers confirmed their exposure. However, a detailed
statistical analysis of the data revealed no differences in
thyroid function, blood and urine indicators of liver and kidney
function, or general health, between exposed and control groups
(Gowers & Gordon, 1980; Charkes et al., 1985).
PART B
ETHYLENETHIOUREA (ETU) AND PROPYLENETHIOUREA (PTU)
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLENETHIOUREA (ETU)
AND PROPYLENETHIOUREA (PTU)
INTRODUCTION
1. SUMMARY
1.1 Sources, environmental transport and distribution
1.2 Environmental levels and human exposure
1.3 Kinetics and metabolism
1.4 Effects on organisms in the environment
1.5 Effects on experimental animals and in vitro test systems
1.5.1 Ethylenethiourea
1.5.2 Propylenethiourea
1.6 Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
2.3.1 Extraction
2.3.2 Clean-up
2.3.3 Derivatization
2.3.4 Determination
2.3.4.1 Gas-liquid chromatography (GLC)
2.3.4.2 Thin-layer chromatography (TLC)
2.3.4.3 Polarography
2.3.4.4 Radioisotope dilution
2.3.4.5 High-pressure liquid chromatography (HPLC)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Soil
4.2 Water
4.3 Plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Food and drinking-water
5.2 Monitoring and market basket studies
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and excretion
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short- and long-term exposures
8.3 Teratogenicity
8.4 Mutagenicity
8.5 Carcinogenicity
8.5.1 Mouse
8.5.2 Rat
8.5.3 Hamster
8.6 ETU in combination with nitrite
8.7 Mechanisms of toxicity; mode of action
8.8 Probineb and Propylenethiourea (PTU)
8.8.1 General
8.8.2 Toxicological information
9. EFFECTS ON MAN
9.1 Epidemiological studies
INTRODUCTION
One of the metabolic products of ethylene bisdithiocarbamate
decomposition in mammals, plants, and lower organisms is
ethylenethiourea (ETU). It may also be present as an impurity
in these dithiocarbamates, and their residues on crops may be
partly transformed into ETU during food processing. A comparable
breakdown takes place with propineb, giving rise to propylene-
thiourea (PTU).
1. SUMMARY
1.1. Sources, Environmental Transport, and Distribution
Ethylenethiourea (ETU) is found together with residues of
the parent ethylene bisdithiocarbamates (EBDCs) in and on crops
that have been treated with these pesticides. During storage,
processing, and cooking, the amount of the parent compound
decreases while that of ETU increases. ETU is easily photo-
oxidized (in the presence of photosensitizers) to ethyleneurea
(EU).
1.2. Environmental Levels and Human Exposure
In certain crops, such as spinach, carrots, and potatoes,
treated with EBDCs, high levels of ETU can be found after
cooking. In general, however, the ETU levels are below
0.1 mg/kg product.
Estimates of the exposure of the general population of the
USA are of the order of 0.24 - 3.65 µg ETU/kg body weight per
day, and, in Canada, estimates based on market-basket studies
are around 1 µg ETU/kg body weight per day.
1.3. Kinetics and Metabolism
ETU is rapidly absorbed, metabolized, and excreted in
mammals. Up to 90% is eliminated via the urine and only a
small amount via the faeces. Distribution of ETU in the body
appears to be fairly uniform with the exception of a relative
accumulation in the thyroid. ETU is broken down to ethylene
diamine (EDA), urea, carbon dioxide, or oxalic acid, or is
transformed to imidazole derivatives in mammals, plants, and the
environment.
1.4. Effects on Organisms in the Environment
The available LC50 levels of ETU and EU for fish are in the
range of 7500 - 13 000 mg/litre.
1.5. Effects on Experimental Animals and In Vitro Test Systems
1.5.1. Ethylenethiourea
The acute oral toxicity in experimental animals is low, and
the long-term effects are mainly characterized by an antithyroid
action.
At dose levels > 25 mg/kg body weight, decreases in serum
T3, T4, and protein-bound iodine (PBI) and increases in thyroid-
stimulating hormone (TSH) have been found in studies on
experimental animals. At higher dose levels (> 100 mg/kg body
weight), increases in thyroid weight and hyperplasia occurred,
which finally resulted in the development of adenocarcinoma.
The effects of short-term exposure to low levels of ETU seem to
be reversible, but those of long-term exposure to higher levels
become, at a certain stage, irreversible. A level of
approximately 5 mg/kg body weight seems to be without effects.
Most mutagenicity studies on ETU, especially those with
mammalian test systems, have given negative results.
A number of carcinogenicity studies have been carried out on
mice, rats, and hamsters. In addition to an antithyroid action,
ETU has been found to induce, subsequently, thyroid tumours
(hyperplastic goitre, solid-cell adenomas, and follicular and
papillary carcinomas) in mice and rats. In an earlier study on
mice, liver tumours, lung tumours, and lymphomas were also
detected, but these findings have not been confirmed. No
tumours except thyroid tumours have been found in rats, and in
hamsters, no tumours of the thyroid gland or other organs were
observed, even at 200 mg/kg diet.
At dose levels above approximately 10 mg/kg body weight, ETU
has clear teratogenic effects in rats and hamsters, different
types of central nervous system and skeletal anomalies being
induced. However, in mice, no teratogenic effects were found at
much higher dose levels (up to 800 mg/kg body weight).
1.5.2. Propylenethiourea
In a long-term study on mice using propylenethiourea (PTU),
an increased incidence of hepatocellular adenomas was observed
at dose levels of 10 mg/kg diet or more. No thyroid tumours
were found, but increased thyroid hypercellularity occurred at a
dose level of 1000 mg/kg diet. In rats, goitrogenic effects
were seen with PTU at dose levels as low as 1 mg/kg diet.
1.6. Effects on Man
Epidemiological studies on workers exposed to ETU did not
reveal any increase in the incidence of thyroid tumours.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
The chemical structure of ethylenethiourea (ETU) is:
CH2-NH
| \
| C=S
| /
CH2-NH
2.2. Physical and Chemical Properties
ETU is a fairly stable compound. Some physical and chemical
properties are listed in Table 8.
Table 8. Some physical and chemical properties of ETU
-------------------------------------------------------------------
Empirical formula C3H6N2S
Common synonym 2-imidazolidinethione
Appearance white, crystalline
Relative molecular mass 102.17
Odour odourless
Melting point 203 - 204 °C
Solubility in water: 20 000 mg/litre at 30 °C;
in ethanol: moderately soluble;
in chloroform: nearly insolublea
CAS registry number
Besides ETU, other degradation products of EBDCs include ETD
and DIDT. The main volatile components of zineb's decomposition
are carbon disulfide, carbonyl sulfide, and EDA. Almost all
zinc is converted into zinc sulfide and zinc oxide (Melnikov &
Trunov, 1966).
6.4. Decomposition in Water and Soil
Thiram and dimethyldithiocarbamic acid give rise in soil to
methyl isothiocyanate and sulfur, and, under acidic conditions,
to carbon disulfide, hydrogen sulfide, methylamine, methyl-
isocyanate, and the bisdisulfide of methyldithiocarbamic acid.
Two of the products, carbon disulfide and dimethylamine,
evaporated from the soil (Raghu et al., 1975). Dimethyldithio-
carbamic acid also binds with heavy metals in soil to form
complexes.
The various metal derivatives of ethylene bisdithiocarbamic
acid appear to be converted in the soil to DIDT, ETU, carbon
disulfide, hydrogen sulfide, and carbonyl sulfide (Moje et al.,
1964; Kaars Sijpesteijn & Vonk, 1970). The conversion by soil
bacteria and fungi of DIDT into ETU has been demonstrated (Vonk
& Kaars Sijpesteijn, 1976). Even though ETU is slowly converted
into EU in soil, pure cultures of soil bacteria and fungi were
unable to effect this transformation (Kaars Sijpesteijn & Vonk,
1970).
6.5. Metabolism in Microorganisms
Microorganisms readily form ETU from DIDT, a spontaneous
decomposition product of EBDCs. This conversion also takes
place after addition of reducing compounds such as cysteine,
glutathione, or ascorbic acid. It consists of the reduction of
the S-S bond of DIDT, with the subsequent release of carbon
disulfide to form ETU. It was shown by Vonk & Kaars Sijpesteijn
(1976) that DIDT was reduced by NADH in the presence of enzyme
extracts from Pseudomonas fluorescens, Escherichia coli,
Saccharomyces cerevisiae, or Aspergillus niger.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
There is some evidence that dithiocarbamates, at concentra-
tions 10 times that of normal field application, may reduce
microbial biomass and increase the bacterial:fungal ratio.
7.2. Aquatic Organisms
7.2.1. Acute toxicity
Toxicity studies using dithiocarbamates are hindered by the
fact that they are chemically and biologically degradable and
may also be contaminated with degradation products. Their
stability in water depends on the pH and on the presence of
metal ions with which they form complexes. The soluble dithio-
carbamates dissociate in water, whereas the polymers are only
slightly soluble in water. As the breakdown products will also
influence toxicity, toxicity testing of dithiocarbamates is
complex.
According to US EPA (1977, 1982a), the use of pesticide
products containing maneb against cranberry fruit rot at
application rates of up to 6.7 kg ai/ha would result in a
concentration of 4.4 mg/litre in a 15-cm layer of water. McCann
& Pitcher (1973) reported a 96-h LC50 of 1 mg/litre for
bluegills, while Worthing & Walker (1983) reported a 48-h LC50
for carp of 1.8 mg/litre. Zineb used against cranberry fruit
rot at an application rate of up to 5.4 kg ai/ha could result in
a concentration of 3.52 mg/litre in a 15-cm layer of water. A
26-h LC50 of 0.2 mg/litre has been reported for Daphnia magna.
Van Leeuwen (1986) carried out an extensive study with 18
dithiocarbamates and three metabolites of these compounds,
including ETU, in fish (Poecilia reticulata), crustacea (Daphnia
magna), algae ( Chlorella pyrenoidosa, Phytobacterium
phosphoreum), and two nitrifying bacteria (Nitrosomonas and
Nitrobacter). The results are summarized in Tables 4 and 5.
Worthing & Walker (1983) gave the following LC50 values:
propineb: rainbow trout, 1.9 mg/litre; golden orfe,
133 mg/litre; thiram: carp, 4 mg/litre; rainbow trout,
0.13 mg/litre; bluegill, 0.23 mg/litre; metiram: harlequin fish,
17 mg/litre.
The susceptibility to maneb of the early life stages of
rainbow trout has been studied using fertilized eggs (before and
after water hardening), early eye point eggs, late eye point
eggs, sac fry, and early fry. The sac fry and early fry stages
appeared to be the most sensitive. The 96-h LC50s for the
different stages were: for 0-h egg, 6 mg/litre; for 24-h egg,
5.6 mg/litre; for early eyed egg (14 days), 1.8 mg/litre; for
late eyed egg (28 days), 1.3 mg/litre; for sac fry (42 days),
0.32 mg/litre; and, for early fry (77 days), 0.34 mg/litre (Van
Leeuwen, 1986).
Table 4. Acute toxicity of dithiocarbamates and breakdown
products for fisha
-------------------------------------------------------------
Organism Compound 96-h LC50
(95% confidence
limit) (mg/litre)
-------------------------------------------------------------
Poecilia reticulatab nabam 5.8 (4 - 8.5)
maneb 3.7 (3.2 - 5.6)
zineb 7.2 (5 - 10.3)
mancozeb 2.6 (2.1 - 3.3)
metiram 6.4 (4 - 10.4)
Na-DMDC 2.6 (2.1 - 3.2)
ziram 0.75 (0.56 - 1)
ferbam 0.09 (0.06 - 0.18)
thiram 0.27 (0.22 - 0.33)
Na-DEDC 6.9 (5.5 - 8.5)
Zn-DEDC 0.49 (0.40 - 0.61)
disulfiram 0.32 (0.24 - 0.43)
ETU 7500 (5600 - 10 000)
EU 13 000 (10 000 - 18 000)
Rainbow troutc thiram 0.26 (0.24 - 0.32)
(Salmo gairdneri)
-------------------------------------------------------------
a From: Van Leeuwen (1986).
b Studies according to OECD guidelines 203.
c 24-h LC50; water temperature 15 ± 1 °C;
weight of fish, 34 ± 4.7 g.
Table 5. Short-term toxicity studies with dithiocarbamates and
breakdown productsa
--------------------------------------------------------------------
Compound Daphnia Chlorella Photobacterium Nitrosomonas
magna pyrenoidosa phosphoreum Nitrobacter
48-h LC50 96-h EC50 15-min EC50 3-h MIC
(mg/litre) (mg/litre) (mg/litre) (mg/litre)
--------------------------------------------------------------------
Nabam 0.44 2.4 102 32
Maneb 1 3.2 1.2 56
Zineb 0.97 1.8 6.2 18
Mancozeb 1.3 1.1 0.08 32
Metiram 2.2 1.8 0.37 32
Na-DMDC 0.67 0.8 0.51 26
Ziram 0.14 1.2 0.15 100
Ferbam 0.09 2.4 0.20 10
Thiram 0.21 1 0.10 18
Na-DECD 0.91 1.4 1.22 43
Zn-DEDC 0.24 1.1 1.70 > 320
Disulfiram 0.12 1.8 1.21 > 320
ETU 26.4 6600 2100 1
EU 5600 16 000 3300 1000
--------------------------------------------------------------------
a From: Van Leeuwen (1986).
7.2.2. Short- and long-term toxicity and reproduction studies
7.2.2.1. Fish
In sublethal toxicity studies carried out on Salmo
gairdneri, groups of 10 fish were exposed to thiram
(0.18 mg/litre) for 24 h. Blood parameters (decreased haemo-
globin and leukopenia, decreased glucose levels, and increased
glucose-6-phosphate dehydrogenase activity) and liver parameters
(increased lipid content, increased lactate dehydrogenase) were
changed, and it was concluded by the author that thiram is a
cytotoxic chemical (Van Leeuwen, 1986). In a further study, the
60-day toxicity for early life stages was tested on S. gairdneri
using a number of dialkyldithiocarbamates, EBDCs, and break-
down products. The LC50s ranged from approximately 1 to
9 µg/litre for dialkyldithiocarbamates and 211 - 2100 µg/litre
for EBDCs, but ETU and EU were not toxic, even at levels
exceeding 1000 mg/litre.
Embryotoxic and teratogenic effects were also observed for
all the compounds studied, and there was an overlap between the
responses for skeletal malformations and lethality over a wide
concentration range. The teratogenic effects in rainbow trout
proved to be in agreement with those observed in mammals.
Exposure of rainbow trout during embryo-larval development
revealed that malformations induced by dithiocarbamates were
almost exclusively confined to the notochord, which increased
considerably in both length and diameter. As a result, the
notochord became twisted and distorted. Ectopic osteogenesis
was observed in almost every affected notochord. Other effects,
such as the disruption of the integrity of myomeres and organ
dislocations, were closely related to the notochordal ano-
malies. Also, compression and fusion of vertebrae and "waviness"
of various skeletal elements were found. Concentration-related
changes in the liver were observed in short-term exposure of
juvenile rainbow trout, while at high levels proliferation of
bile duct epithelial cells and necrosis of hepatocytes were
seen. Ziram and thiram induced brain haemorrhages as well as
intraspinal extravasates of blood cells (Van Leeuwen, 1986).
7.2.2.2. Invertebrates
Short-term toxicity studies using the compounds listed in
Table 5 were carried out to investigate the effects of prolonged
exposure (21-day) on survival, fecundity, and growth of Daphnia
magna. Growth and reproduction were not specifically inhibited,
since effects on these characteristics were generally detected
at levels comparable with the 21-day LC50s. For dialkyldithio-
carbamates, the 21-day LC50s ranged from approximately 10 to
30 µg/litre, for EBDCs, from 80 to 110 µg/litre, and, for ETU
and EU, the levels were 18 and 3200 mg/litre, respectively (Van
Leeuwen, 1986).
7.2.3. Bioaccumulation
Van Leeuwen (1986) determined the log n-octanol/water par-
tition coefficient for a few dithiocarbamates and breakdown
products. The results are summarized in Table 6. In general,
the higher the log n-octanol/water partition coefficient, the
greater the tendency to bioaccumulate.
Table 6. log n-octanol/water partition
coefficients for some dithiocarbamates
------------------------------------------
Compound log n- octanol/water
partition coefficient
------------------------------------------
Disulfiram 4
Thiram 1.82
ETU 0.67
EU 0.96
------------------------------------------
In short-term studies on rainbow trout of uptake,
distribution, and retention of 14C-labelled zineb and ziram,
both compounds were found to be rapidly distributed throughout
the tissues. Whole-body accumulation was low, with bioconcen-
tration factors of less than 100. Relatively high radioactivity
levels were found in the liver, gall bladder, and intestinal
contents, suggesting the prominent role of hepatic biotransform-
ation and biliary excretion. With ziram, the eyes and skin also
appeared to be distribution sites. Whole-body elimination was
rapid, with about 75% of radioactivity being eliminated within
the first 4 days. With ziram, 45% of the initial total 14C
content in the body was still present at the end of the 16-day
depuration period. Differences in the extent of elimination
were most noteworthy for the eyes, skin, and kidneys. Whole-
body autoradiography showed the radioactivity in the digestive
tract, liver, bile, gills, thyroid follicles, melanophores of
the skin, choroid epithelium complex of the eyes, and in other
melanin-containing tissues, such as the kidneys (Van Leeuwen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
In general, the toxicity of dithiocarbamates for mammals is
relatively low. Some commonly used dithiocarbamates included in
the WHO recommended classification of pesticides by hazard (WHO,
1986a), which is based primarily on the acute oral and dermal
toxicity of the technical material for the rat, are given in
Annex III.
Acute oral and dermal toxicity data for a number of animal
species of various dithiocarbamates are given in Table 7. From
this Table, it is clear that nabam and metam-sodium are the most
toxic dithiocarbamates, other compounds having only low
toxicity. As for many compounds, the toxicity is often
influenced by the method of application, e.g., solvents used,
age and sex of animal, type of diet, etc. Thus, in rats fed an
isocaloric diet containing 3.5% protein, the LD50 of nabam was
210 mg/kg body weight, compared with 565 mg/kg in rats fed the
same diet plus 26% protein (Periquet & Derache, 1976).
Ivanova-Chemishanska (1969) found that rats treated with
zineb, maneb, or mancozeb showed dose-dependent signs of
depression, adynamia, decreased tonus, disturbances in
coordination, paresis, and paralysis of extremities combined
with general weakness, lack of appetite, and prostration.
Yin-Tak Woo (1983) has reviewed the structure-activity
relationships of different types of dithiocarbamates.
8.2. Short- and Long-Term Exposures
8.2.1. Oral exposure
8.2.1.1. Rat
In 1-month feeding tests, no growth retardation was noted in
rats fed diets containing 100 mg ferbam/kg diet, but decreased
growth occurred with 500 mg/kg diet and increased mortality with
5000 mg/kg diet (Hodge et al., 1952).
Groups of 40 weanling rats (20 females and 20 males) were
given diets containing 500, 1000, 2500, 5000, or 10 000 mg
zineb/kg diet for up to 30 days. Thyroid enlargement was seen
at all dose levels, but unequivocal histopathological changes
were observed only at 10 000 mg/kg diet (Blackwell-Smith et al.,
1953; Kampmeier & Haag, 1954).
Ferbam administered daily at 23, 66, or 109 mg/kg body
weight to male rats for 13 weeks caused death and weight loss at
the highest dose, but did not have any effect on reproduction.
Daily feeding (equivalent to 15 or 51 mg/kg body weight) to
females for 2 weeks caused severe weight loss at the highest
dose level (Minor et al., 1974).
In a 2-year feeding study, 25, 250, and 2500 mg ferbam or
ziram/kg diet shortened the life span of rats and caused growth
depression and neurological lesions (manifested at the highest
dose level by the crossing of hind legs when animals were lifted
by their tail) (Hodge et al., 1956).
Table 7. Acute toxicity (LD50) of dithiocarbamates for experimental
animals
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Ferbam mouse 1000 FAO/WHO (1965b)
rat > 4000 Hodge et al.
(1956)
guinea-pig 450 - 2000
rabbit 2000 - 3000
Metham-sodium mouse 285 Worthing & Walker
(vapam) (1983)
rat 1700 - 1800 Worthing & Walker
(1983)
rabbit 1300 Worthing & Walker
(1983)
Ziram rat 1400 Hodge et al.
(1952)
guinea-pig 100 - 150 Hodge et al.
(1952)
rabbit 100 - 1020 Hodge et al.
(1952)
Thiram mouse 1500 - 2000 Worthing & Walker
(1983)
rat 865 - 1300 Van Esch (1956)
rat 780 - 865 Worthing & Walker
(1983)
rat > 2000 Ben Dyke et al.
(1970)
rabbit 210 Lehman (1951)
cat 230
--------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------
Compound Animal Dose Reference
(mg/kg body weight)
oral dermal
--------------------------------------------------------------------------
Disulfiram rat > 4000 Van Esch (1956)
Zineb rat > 5200 Blackwell-Smith
(1953)
rat 9000 Ivanova-Chemi-
shanska (1969a)
Maneb mouse 4100 Engst et al.
(1971)
rat 4500 Engst et al.
(1971)
rat (male) 6750 Worthing & Walker
(1983)
Nabam rat 395 Blackwell-Smith et
al. (1953)
Mancozeb rat (female) 12 800 Ivanova-Chemi-
shanska (1969b)
rat (male) 14 000 Ivanova-Chemi-
shanska (1969)
rat > 8000 Worthing & Walker
(1983)
Propineb rat 8500 > 1000 Worthing & Walker
(1983)
rabbit 2500
cat 2500
hen 2500
Metiram mouse 5400 Worthing & Walker
(1983)
rat > 10 000
guinea-pig 2400 - 4800
(female)
--------------------------------------------------------------------------
Groups of 24 rats (12 females and 12 males) were given a
diet containing 48 mg thiram/kg diet for 2 years (a 3-generation
study). No effects on growth, reproduction, blood parameters,
or mortality rate were found, neither were there gross or
histological changes (Van Esch, 1956). In a further study, 12
female and 12 male rats given 200 mg/kg diet for 8 months did
not show any appreciable changes in growth or mortality rate,
and a dose of 300 mg/kg diet for 65 weeks did not give rise to
specific evidence of poisoning (Tollenaar, 1956). Groups of 24
rats (12 females and 12 males) fed diets containing 300, 1000,
or 2500 mg thiram/kg diet for 65 weeks showed weakness, ataxia,
various degrees of paralysis, and histological changes
(calcification in the brain stem and cerebellum and dystrophic
changes in the leg muscles). At 2500 mg/kg diet, there was an
increased mortality rate (Fitzhugh et al., 1952). Groups of 20
young rats administered diets containing 100, 300, or 500 mg
thiram/kg diet for 2 years all showed a small reduction in the
growth rate. At concentrations of 300 and 500 mg/kg diet, an
increased mortality rate was seen, while at 500 mg/kg diet,
convulsions, thyroid hyperplasia, and calcification in the
cerebellum, hypothalamus, and medulla oblongata were observed
(Griepentrog, 1962; IARC, 1976).
Groups of 25 male and 25 female rats were fed diets
containing 25, 250, 1250, or 2500 mg maneb/kg diet for 2 years.
At 1250 mg/kg diet, there was some depression, impaired food
consumption, and increased mortality rate. At the end of 2
years, the animals receiving 1250 mg/kg diet had an increased
liver/body weight ratio, and those receiving 2500 mg/kg diet
also showed thyroid hyperplasia and nodular goitre (Worthing &
Walker, 1983).
Groups of 10 young male and 10 young female rats were fed
diets containing 500, 1000, 2500, 5000, or 10 000 mg zineb/kg
diet for 2 years. At the two highest dose levels, there was an
apparent increase in the mortality rate among the female rats
and, at 10 000 mg/kg diet, there was a tendency towards
diminished growth in both sexes. The results of haematological
studies were normal, but a goitrogenic effect was seen at all
dose levels. Kidney damage was seen in 6 animals at the
10 000 mg/kg dose level and in one animal in each of the groups
receiving 1000, 2500, or 5000 mg/kg diet, but not at all at
500 mg/kg diet. The tumour incidence was not significantly
greater among any of the treated animals than it was in the
controls (Blackwell-Smith et al., 1953; Kampmeier & Haag,
1954).
Weanling rats in groups of 25 males and 25 females were fed
diets containing 25, 250, or 2500 mg ziram/kg diet for 2 years.
The growth rate and life span were normal in all groups, but
neurological changes were observed in the animals receiving
2500 mg/kg diet, though no cystic lesions were discovered in the
levels. In some of the male animals, the testes were atrophied,
and there was a slight indication of thyroid hyperplasia,
notably in the 2500 mg/kg diet group. However, there was no
increase in tumour incidence in the treated animals (Hodge et
al., 1956). A comparable study with the same dose levels was
carried out with ferbam, and again no increase in tumour
incidence was found (IARC, 1976).
8.2.1.2. Dog
A dog given ferbam and ziram together for one month, each at
a dose of 5 mg/kg body weight per day, remained healthy except
for slight anaemia. The same result was observed when ferbam
was given alone for one month at a dose of 25 mg/kg body weight
per day, or for one week at 50 mg/kg body weight per day.
Raising the dose to 100 mg/kg body weight per day, however,
immediately provoked severe vomiting and malaise (Hodge et al.,
1952). Pairs of adult dogs were given daily doses of 0.5, 5, or
25 mg ferbam/kg body weight for one year. Convulsions occurred
at the highest dose level, but urine analysis, blood parameters,
organ weights, and tissue histology (including that of the
thyroid gland) were normal (Hodge et al., 1956).
When pairs of dogs were fed maneb orally at the rate of 2,
20, 75, or 200 mg/kg body weight per day for one year, toxic
effects were observed at the two highest dose levels, but not at
20 mg/kg body weight (Worthing & Walker, 1983).
Three groups of three dogs each were fed diets containing
20, 2000, or 10 000 mg zineb/kg diet for one year. All the
animals survived, and no persistent changes in growth rate were
seen in any of the groups. There were no histopathological
changes in the tissues, except in the thyroid gland, and
haematological findings were normal. At 10 000 mg/kg diet,
thyroid hyperplasia was noted (Blackwell-Smith et al., 1953;
Kampmeier & Haag, 1954).
8.2.1.3. Bird
Sodium diethyldithiocarbamate (NDDC), the dimethyl compound
(NDMC), and ferbam, ziram, and thiram were given orally to young
and adult domestic fowl (Thorber's gog cockerels) at 330, 210,
205, 56, and 178 mg/kg body weight, respectively, and the birds
were killed after 6, 12, 18, or 20 weeks. All of the compounds
had an adverse effect on body weight gain, retarded testicular
development, and produced degeneration in the seminiferous epi-
thelium of mature birds. Nerve fibre degeneration was produced
in the medulla and spinal cord of chicks by NDDC and in those of
cocks by NDMC. Chicks exposed to thiram became lame and
exhibited swollen epiphyses of the long bones due to endo-
chondrial ossification giving rise to a thickened cartilaginous
epiphyseal plate (Rasul & Howell, 1974).
8.2.2. Inhalation exposure
8.2.2.1. Rat
Studies concerning toxicity following inhalation exposure
are scarce.
Ivanova-Chemishanska et al. (1972) studied the inhalation
toxicity in rats with zineb (70% purity), maneb (80% purity),
and mancozeb (80% purity), applied 6 days per week over a period
of 4“ months, at concentrations of 2, 10, 50, 100, or 135 mg/m3.
The pesticides were given in the form of dispersed aerosols,
with 95% of the dust particles ranging from 1 to 5 µm in size,
and the remainder from 5 to 10 µm. Local irritation of the
mucosa of the upper respiratory tract was noted and
concentration-related non-specific changes in the liver and
kidneys were evident. However, only slight changes were found
at a concentration of 2 mg/m3.
Davydova (1973) studied the influence of inhaled thiram on
the estrous cycle and genital function of rats. Groups of rats
were exposed to 0, 0.45, or 3.8 mg/m3 thiram for 6 h/day, 5
days/week, over a period of 4“ months. An extension of the
estrous cycle was seen at the highest dose level, and genital
function was disturbed, as shown by a reduction in the capacity
to conceive, a reduction in fertility, and of fetal weight
gain.
8.3. Skin and Eye Irritation; Sensitization
Nabam (19% solution) and zineb (65% wettable powder) were
each applied to the right eye of 10 rabbits, the left eye being
used as a control. Nabam did not produce signs of irritation,
while zineb produced mild irritation (erythema), which subsided
within 6 - 8 h. No oedema was seen. The mild irritation may
have been caused by the non-specific foreign body reaction to
the dry, insoluble powder. When this procedure was repeated
with both compounds diluted and suspended for agricultural use
(for nabam, 0.5% of the commercial 19% solution plus zinc
sulfate, 0.125% in water; for zineb, a 0.188% suspension of the
commercial 65% wettable powder in water), no irritation was seen
(Blackwell-Smith et al., 1953).
In studies performed on guinea-pigs, intracutaneous
injections, 10 times daily, followed by an epicutaneous
challenge test, provided evidence of the marked sensitizing and
cross-sensitizing properties of thiram and metiram (Griepentrog,
1960).
It has been reported that a number of dithiocarbamates
(mancozeb, metham-sodium, metiram, zineb, ziram, and thiram)
cause skin and/or eye irritation (Worthing & Walker, 1983).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
8.4.1. Reproduction
8.4.1.1. Rat
Groups of rats (16 male and 16 female Charles River-CD rats
per group) were fed maneb for 3 months at levels of 0, 125, or
250 mg/kg diet, and were mated in a standard 3-generation, 2-
litters-per-generation reproduction study. Groups of males and
females from the F1b and F2b litters were fed maneb for 3 months
after weaning and mated to become parents of the succeeding
generation. The major reproduction indices were unaffected by
maneb at dietary levels up to and including 250 mg/kg diet.
There was no histological evidence of congenital anomalies in a
variety of tissues and organs of the male and female rats of the
F3b litter subjected to histopathological examination (Sherman &
Zapp, 1966).
Maneb, zineb, and mancozeb exert dose-dependent damaging
effects on the gonads of rats of both sexes. The dose levels
were 96 - 960 mg zineb/kg body weight, 140 - 1400 mg mancozeb/kg
body weight, and 14 - 700 mg maneb/kg body weight, given twice a
week for 4.5 months. Both reproductive and endocrine structures
were affected at all dose levels, leading to decreased fertility
(Ivanova-Chemishanska et al., 1973, 1975a). In a 4-month
inhalation study on rats using maneb at 4.7 mg/m3, no effect on
sperm mobility was detected (Matokhnyuk, 1971).
Ivanova-Chemishanska & Antov (1980) studied the effects of
EndodanR (50% ethylenethiuram monosulfide) on the gonads and
reproduction in rats during long-term daily oral doses of 3.8 or
38 mg/kg body weight. The parental generation (F0) and 3
consecutive generations (F1 - F3) were examined. In F0, a
decrease in succinic dehydrogenase and ATPase activities in
testes homogenates was found, as well as an increase in glucose-
6-phosphate dehydrogenase (G6PDH) activity compared with control
levels. Changes in the liver and brain enzyme systems were also
noted.
The same results were obtained with zineb (78% purity). A
rapid loss of mobility and changed resistance (to osmotic and
acidic effects) of spermatozoa were found. A decreased index of
fertility was also found for both sexes in the F0 generation.
Decreased index of fertility and enzymatic changes in organ
homogenates were detectable in the F1 - F3 generations (Ivanova-
Chemishanska et al., 1973).
In extracts of testes of white rats, exposed by inhalation
to zineb and maneb at a concentration of 100 mg/m3 for 4 months,
Izmirova et al. (1969) found an increase in lactate dehydro-
genase (LDH), LDH2, and LDH4. Bogartykh et al. (1979) did not
find any changes in LDH or G6PDH activities in testes homo-
genates of Wistar rats orally treated with zineb (2.5 mg/kg body
weight) for 3 months.
Thiram at doses of 225, 300, 450, 600, 900, or 1200 mg/kg
diet given to male Wistar rats for 29 days produced changes in
many of the parameters studied. A significant effect on testes
and seminal vesicle weight was found at 450 mg/kg diet, and a
decrease in body weight was found at 300 mg/kg. The most
sensitive parameters studied were found to be the weights of the
epididymal and perirenal fat pads, which were decreased by
thiuram doses in the range 130 - 184 mg/kg diet. The no-effect
level, calculated using an extrapolation model, did not differ
significantly from the earlier reported value of 48 mg/kg diet
(Lowy et al., 1979, 1980).
Ferbam was fed to groups of 20 Charles River-CD male rats at
concentrations of 0, 500, 1200, or 2500 mg/kg diet for 13 weeks
before mating with untreated females. Six of the rats fed the
highest dose level died. The indices of fertility, gestation,
viability, and lactation for the females mated with treated
males were normal (Short et al., 1976).
When thiram was incorporated into the diet at concentrations
of 0, 500, 1000, and 2500 mg/kg diet and fed to male weanling
Charles River rats for 13 weeks prior to mating, food intake and
growth was mainly decreased at the two highest dose levels.
Loss of hair and rough coats were also seen in these groups. At
the highest dose level, high mortality occurred. Males in the
highest dose group failed to inseminate the females. In these
animals, there was evidence of testicular hypoplasia, tubular
degeneration, and atypical spermatoids in the epididymus. At
the two lower dose levels, no influence on reproduction was
found (Short et al., 1976).
Female rats fed 400 or 2000 mg thiram/kg diet for at least
14 days prior to mating showed a significant reduction in the
number of implants per dam and pups per dam. The delaying
effect on the estrous cycle was reversible. At the highest dose
level, a number of animals died. A comparable study with ferbam
using the same dose levels did not show any influence on
fertility, gestation, viability, or lactation (Short et al.,
1976).
Administration of 50 mg ziram/kg and 100 mg zineb/kg body
weight to rats for a period of 2, 4, or 6 months produced
delayed insemination, sterility, resorption of fetuses, and
anomalies in development (Rjazanova, 1967).
8.4.1.2. Bird
Thiram (99.9% purity) has been reported to decrease egg
production for the domestic chicken (Gallus domesticus), pigeon
(Columba livia), and pheasant (Phasianus colchicus torquatus).
A dose level of 8.8 mg/kg body weight per day caused a 50%
reduction in egg laying in bobwhite quail (Colinus virginianus).
During this period of reduced egg laying, it seems that an
alteration of hormone levels took place resulting in significant
weight losses of ovary and oviduct, decrease in serum calcium
level (which is controlled by estrogen), and alteration in
normal maturation of the ova (Wedig et al., 1968).
In a study by Van Steemis & Van Logten (1971), tecoram (an
oxidation product of disodium EBDC and sodium dimethyl dithio-
carbamate with ammonium persulfate) in propylene glycol or
saline was administered to chick embryos at doses of 0.01, 0.1,
1, or 10 mg/egg. Paralysis, shortening of the extremities,
muscular atrophy, dwarfing and death occurred. Microscopically,
signs of peripheral neuropathy confined to the distal parts of
the peripheral nerves, and muscular atrophy were found.
8.4.2. Teratogenicity
Some dithiocarbamates are potentially teratogenic in the
rat, but not in the mouse. In most cases, the teratogenic
effects have been observed at high dose levels.
8.4.2.1. Rat
Kaloyanova et al. (1967) have studied the effects on progeny
of albino rats of 0, 700, and 1400 mg maneb/kg body weight
administered twice per week for 4.5 months. Three groups of 20
rats (10 males and 10 females) were used and a first generation
was bred. Congenital deformities were found in the facial part
of the skull, caudal vertebrae, palates, limbs, and tail. The
same type of changes were also found after a single oral dose of
2000 - 8000 mg zineb or 1000 - 4000 mg maneb/kg body weight on
days 11 - 13 of pregnancy.
No teratogenic effects or adverse effects on the intra-
uterine development of progeny were observed when rats were
given 1000 mg zineb or 500 mg maneb/kg body weight, from days 2
to 21 of pregnancy, or were exposed in an inhalation chamber to
a concentration of 100 mg zineb/m3 for 4 h/day from day 4 of
pregnancy (Antonovich et al., 1972; Petrova-Vergieva & Ivanova-
Chemishanska, 1973; Ivanova-Chemishanska et al., 1975a).
In a study on Sprague Dawley rats using maneb at dose levels
of 0, 120, 240, or 480 mg/kg body weight on days 7 - 16 of
gestation, fetotoxic effects (reduced fetal weight, reduced
ossification, and hydrocephalus) were seen at the highest dose
level (Chernoff et al., 1979).
In studies by Larsson et al. (1976), maneb was administered
to Sprague Dawley rats at dose levels of 0, 400, 770, or
1420 mg/kg body weight, by gavage, as a single dose on day 11 of
gestation. Rats were sacrificed on day 18 of gestation and
fetuses were examined for reproductive and teratogenic abnorm-
alities. A substantially increased resorption rate was seen at
770 mg/kg body weight. Gross malformations occurred in all
surviving animals at 770 and 1420 mg/kg body weight, but no
malformations were observed in the single litter of the low-dose
group. These abnormalities included cleft palate, hydrocephaly,
and other serious defects. In another study, maternal admini-
stration of zinc acetate (made in an attempt to relieve the
incidence of teratogenic events) had some preventive effect at
750 mg/kg body weight, but, at 1380 mg/kg body weight, the
frequency and type of malformations were unchanged (Larsson et
al., 1976).
Mancozeb was administered to rats at dose levels of 0, 380,
730, or 1320 mg/kg body weight on day 11 of gestation in a study
similar to that reported above with maneb. Again, a substantial
increase in malformations, similar to those produced by maneb,
was observed at the highest dose level, but not at lower levels
(Larsson et al., 1976).
Propineb was administered to rats at dose levels of 0, 400,
760, or 2300 mg/kg body weight, by gavage, on day 11 of
gestation. The dams were sacrificed and fetuses examined for
gross external and internal malformations on day 18 of
pregnancy. Maternal toxicity was observed at all dose levels.
At the highest dose level, propineb was fetotoxic and induced a
variety of malformations in the surviving fetuses. At 760 mg/kg,
propineb was slightly fetotoxic but did not induce malformations
in surviving fetuses. The pattern of fetal abnormalities was
qualitatively similar to that noted in the maneb- and mancozeb-
treated rats (Larsson et al., 1976).
Cypromate (zinc propylene bisdithiocarbamate) was studied
for its teratogenic potential in white rats using either a
single oral dose of 250, 500, or 1000 mg/kg body weight on the
11th or 13th day of gestation or repeated treatment from the
first day of gestation through the whole pregnancy at 62, 250,
and 500 mg/kg body weight. A spectrum of malformations involving
the nervous and skeletal systems, facial cranium, extremities,
etc., were induced with a single dose of 500 mg/kg body weight
or more, and at all dose levels given repeatedly (Petrova-
Vergieva, 1976).
Groups of rats (26 - 27 pregnant CD1 rats per group) were
administered zineb (purity 85.5% containing 0.35% ETU) at dose
levels of 0, 200, 632, or 2000 mg/kg body weight per day on days
6 - 19 of gestation. Maternal body weight and food consumption
data were recorded. Pregnant rats were sacrificed at day 20 and
a laparotomy was performed. Fetal data included live, dead, and
resorbed fetuses as well as somatic and skeletal abnormalities.
There was no maternal mortality, but a substantial weight loss
was seen at the highest dose level. Fetuses from mothers
administered 2000 mg/kg also showed a reduced body weight.
Fetal mortality was not observed, and there were no significant
anomalies noted on gross external examination. However, a
higher incidence of teratogenic anomalies was noted at the
highest dose level (short and kinky tails, hydrocephalus, and
increased incidence of skeletal anomalies). At the 632 mg/kg
level, these teratogenic anomalies were absent. The abnorm-
alities found at the highest dose level may have been due, in
part, to the presence of ETU in the formulation (Short et al.,
1980).
Ferbam administered to rats on days 6 - 15 of gestation at
150 mg/kg body weight resulted in death, increased resorptions,
decreased fetal weights, and a slight increase in soft and
skeletal tissue anomalies (Minor et al., 1974). CD-1 rats were
treated on days 6 - 15 of gestation, by gavage, with 0, 11, or
114 mg ferbam/kg body weight. Twenty-five percent of the dams
administered 114 mg/kg died, but the surviving dams showed small
litters, increased resorptions, and decreased fetal weight.
Also, a number of malformations (unossified sternebrae,
malformed cranium, hydrocephalus, and cleft palate) were found.
When thiram was administered at doses of 0, 40, 90, 136,
164, or 200 mg/kg body weight on days 6 - 15 or 7 - 12 of
gestation, the 200 mg/kg dose reduced the number of mated rats
that delivered litters, and only 33% of the dams survived. At
doses of 136 mg/kg or more, a decrease in the number of implants
and fetuses per dam, an increase in resorptions, a decrease in
fetal body weight, and an increase in malformations, as
described for ferbam, were observed (Short et al., 1976).
8.4.2.2. Mouse
Pregnant female NMRI and Swiss-Webster mice were treated
orally during days 6 - 17 of pregnancy with thiram at 179, 357,
714, or 1071 mg/kg body weight and 250, 500, 1000, and
1500 mg/kg body weight, respectively. Increased resorption of
embryos, clearly retarded fetal development, and skeletal
malformation (cleft palate, wavy ribs, curved long bones of
extremities, and micrognathia) were seen in both strains. The
12th and 13th days seemed to be the most sensitive period of
embryonic development. The lowest dose had only a slight
effect, but the next dose level was clearly teratogenic (Roll,
1971; Matthiaschk, 1973).
Thiram did not reduce body weight gain during gestation at
doses of 100 or 300 mg/kg body weight, administered on days
6 - 14, and no changes in litter size, incidence of resorptions,
or fetal weight were observed. However, an increase in malform-
ations was seen (Short et al., 1976).
In studies by Larsson et al. (1976), doses of 0, 400, 770,
or 1420 mg maneb/kg body weight or 0, 380, 730, or 1330 mg
mancozeb/kg body weight were given on a single occasion to NMRI
mice on days 9 or 13, and mice were sacrificed on day 18 of
gestation. No adverse maternal or fetal effects could be
detected.
In a study on CD1 mice administered 0, 375, 750, or 1500 mg
maneb/kg body weight on days 7 - 16 of gestation, maternal
toxicity was found at the highest dose level, together with a
decrease in fetal caudal ossification centres at all dose levels
(Chernoff et al., 1979).
Ferbam administered to mice at 30 and 300 mg/kg body weight
on days 6 - 16 of gestation did not produce any teratogenic
effects (Minor et al., 1974), and, when given at 23 or 228 mg/kg
body weight on days 6 - 14, it did not affect the survival or
body weight of Swiss-Webster dams during gestation. No changes
in litter size, incidence of resorptions, or fetal weight were
observed, but, at the highest dose level, an increase in
malformations was seen (Short et al., 1976).
Groups of CD-1 mice were administered zineb (85.5% purity)
daily, at dose levels of 0, 200, 632, or 2000 mg/kg body weight
per day, for 11 days from day 6 of gestation and sacrificed on
day 18. Gross examination for maternal well-being and fetal
anomalies, both somatic and skeletal, failed to show any
teratogenic effects (Short et al., 1980).
8.4.3. Embryotoxicity
Korhonen et al. (1982a,b) used a system called chicken
embryo test to study the embryotoxic potential of dithio-
carbamates and found early and late death and malformed embryos.
It was thought that this test could have a predictive value as a
simple teratogenicity test, but many limitations were found in
doing so, and the interpretation of the results were difficult.
8.5. Mutagenicity and Related End-Points
Seiler (1973) studied the mutagenicity of maneb and ziram.
Maneb proved negative in tests with Salmonella strains his G46,
TA1530, TA1531, TA1532, and doubtful in TA1534. Ziram was
positive in TA1534, doubtful in TA1530, and negative in the
other strains.
In studies by Fahrig (1974), ziram was non-mutagenic in a
variety of other microorganisms (Escherichia coli, Serrata
marcescens, and Saccharomyces cerevisiae), but Pilinskaya (1971)
found that it induced chromosome breaks, most of them confined
to chromosome 2, in cultured peripheral human lymphocytes.
Shirasu et al. (1976) studied mutagenicity with the rec-
assay procedure, a sensitivity test using H17 rec+ and M45 rec-
strains of Bacillus subtilis, and with reversion assays on
plates using E. coli (WP2) and S. typhimurium TA1535, TA1536,
TA1537, and TA1538. In these tests, ferbam, thiram, and ziram
were non-mutagenic.
Certain dithiocarbamates given intraperitoneally to mice at
100 mg/kg body weight caused chromatid aberrations in bone
marrow cells (Kurinny & Kondratenko, 1972; Hedenstedt et al.,
1979), the order of effectiveness being thiram > ziram > maneb
and zineb. Thiram was also shown to induce gene mutations in
Salmonella and Aspergillus (Szymezyk, 1981; Zdienicka et al.,
1981).
Hedenstedt et al. (1979) found that the mutagenic effect of
tetramethylthiuram monosulfide (TMTM) was enhanced in the
presence of metabolizing systems (S-9 mix), but that tetraethyl-
thiuram disulfide (TETD or AntabuseR) was not mutagenic.
Propylene bisdithiocarbamate was tested for cytogenicity by
bone marrow analysis and for dominant lethal mutations by giving
a single oral dose to male rats. There was a considerable
increase in the number of chromosomal aberrations (chromatid
fragments), reaching a maximum after 24 h (Vachkova-Petrova,
1977). In studies on the cytotoxic effects of ziram on cultures
of human lymphocytes in vitro (Pilinskaya, 1971), the ratio of
chromatid-type aberrations to chromosome-type aberrations was
2.7:1, which suggests that most chromosomal damage took place at
the S-stage and the G2 stage of the mitotic cycle. Ziram-
induced chromosomal breaks were observed to be non-random, most
of them occurring in chromosome 2.
Propineb and its main metabolite propylenethiourea (PTU)
were investigated by the micronucleus test in mice. The
following doses were given by ip injection twice, with a 24-h
interval: propineb (unknown purity), 62.5, 125, or 250 mg/kg
body weight in a 5% aqueous solution of Tween 80; propineb (78%
purity), the same doses, but in 5% gum arabic; and PTU, 100,
200, 400, or 600 mg/kg body weight in distilled water. Controls
received methanesulfonate at doses of 10, 20, 40, or 80 mg/kg
(twice, in distilled water) and mitomycin at 1.75, 3.5, 7, or
14 mg/kg (twice, in distilled water). No statistically
significant increase in the percentage of micronuclei was
observed at any of the tested doses of propineb or PTU. The
positive control groups showed the expected dose-related
increase in the number of polychromatic erythrocytes with
micronuclei (Rolandi et al., 1984).
Vachkova-Petrova (1981) studied the mutagenic potential of
EndodanR (ethylenethiuram monosulfide) in short-term studies.
Several doses of EndodanR were administered to groups of 6 rats
either twice at an interval of 24 h or for 5 successive days,
and the animals were killed 6 h after the last dose. The cells
in metaphase were analysed for aneuploidy and aberrations, but
no mutagenic effects that could be attributed to the chemical
were detected.
Zineb and ziram were not mutagenic when tested in Drosophila
melanogaster (Benes & Sram, 1969).
8.6. Carcinogenicity
8.6.1. Mouse
In studies by Innes et al. (1969), groups of 18 mice of each
sex from two hybrid strains were given various dithiocarbamates
from 7 days of age up to 18 months. The compounds were given
daily, by gavage, from day 7 to weaning and thereafter added to
the diet. The compounds and the respective amounts were: ferbam
at 10 mg/kg body weight, then 32 mg/kg diet; maneb at 46.4 mg/kg
body weight, then 158 mg/kg diet; nabam at 21.5 mg/kg body
weight, then 73 mg/kg diet; thiram at 10 mg/kg body weight, then
26 mg/kg diet; and zineb at 464 mg/kg body weight, then
1298 mg/kg diet. No significant increase in tumours was found.
On the basis of all experimental data and experimental
designs, IARC (1976) suggested that there was no definite proof
for the carcinogenicity of maneb, though ETU, one of its meta-
bolites, was able to produce thyroid carcinomas. However, zineb
and maneb have been reported to induce pulmonary adenomas in
mice when treated orally (Chernov & Khistenko, 1969; Balin,
1970).
8.6.2. Rat
A 2-year feeding study of the effects of zineb on rats was
carried out using 60 young male and 60 female albino rats.
These were divided into groups of 10 each and administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg/kg diet.
Growth, mortality, haematology, and organ weights were examined
and histopathology was carried out. No clear influence on
growth and mortality was observed, and haematological findings
were within normal limits. A goitrogenic effect (hyperplasia)
was observed in 50% of the animals at 500 mg/kg, and, at
1000 mg/kg diet or more, this effect was more pronounced. The
interpretation of thyroid weight/body weight ratio was
complicated because of the small number of animals still alive.
Microscopically, no evidence of malignancies was present. At
the highest dose level, kidney damage (congestion, nephritis,
nephrosis) was seen. Although 10 000 mg zineb/kg diet produced
moderate goitrogenic effects, this effect was also seen at
500 mg/kg in some rats, and was not clearly dose dependent
(Blackwell-Smith et al., 1953).
Cases of goitre and thyroid adenoma were found in Sprague
Dawley rats fed for 2 years on a diet containing 120 mg or
360 mg metiram/kg diet (Griepentrog, 1962).
8.6.3. Dog
When nine mongrel dogs (three groups of three animals each)
were administered 20, 2000, or 10 000 mg zineb/kg diet for 1
year, no haematological changes were found. The thyroids of the
group given the highest dose level were enlarged and showed
hyper-plastic changes, but, at lower dose levels, they did not
show any histological changes (Blackwell-Smith et al., 1953).
Doses of 45 mg metiram/kg body weight for 90 days or
7.5 mg/kg body weight for 23 months did not cause any ill
effects (Worthing & Walker, 1983).
8.6.4. Dithiocarbamates in combination with nitrite
The above-mentioned studies were carried out with individual
dithiocarbamates. Other studies have shown that these
dithiocarbamates, in the presence of nitrite, can be converted
to N-nitroso derivatives, which may be carcinogenic.
Thiram, ferbam, ziram (Eisenbrand et al., 1974; Sen et al.,
1974), and disulfiram (Lijinsky et al., 1972; Elespuru &
Lijinsky, 1973) react with nitrite under mildly acidic con-
ditions to form N-nitroso compounds. Formation of N-nitroso-
dimethylamine (NDMA) by the action of microorganisms in sewage
and soil containing 0.1% thiram has been reported to occur under
experimental conditions (Ayanaba et al., 1973). Nitrite is
formed by the reduction of nitrate, which can be found in some
unrefrigerated vegetables (e.g., spinach and beets), especially
after cooking (Phillips, 1968), in human saliva (Tannenbaum et
al., 1974), and in cured meats. Thus, in vivo nitrosation of
dithiocarbamates in the stomach cannot be totally excluded.
As has been pointed out by IARC (1976), the extrapolation of
findings in experimental animals to man is complicated by many
factors. It is relatively easy to show that N-nitroso
derivatives can be formed and that these are mutagenic and/or
carcinogenic. The crucial information, however, is the quantity
produced in man under the prevailing conditions. The concen-
tration of both reactants, the pH, the influence of competing
reactions, and the presence of accelerators and inhibitors are
all important. In addition to these difficulties in defining
potential human exposure, the susceptibility of man, compared
with that of experimental animals, has to be considered.
Sen et al. (1974) concluded that it is unlikely that
significant amounts of NDMA would be produced from the ingestion
of trace amounts of the dithiocarbamates and the normal intake
of nitrite.
8.7. Mechanisms of Toxicity; Mode of Action
8.7.1. Thyroid
In weaning rats, a diet containing 500 mg nabam/kg given for
9 days caused thyroid hyperplasia and a decrease in the weight
of the thymus (Seifter & Ehrich, 1948).
Male and female albino rats were administered diets
containing 0, 500, 1000, 2500, 5000, or 10 000 mg zineb/kg diet
for up to 30 days. Animals were killed sequentially in order to
study the changes in the thyroid gland. Only in the 10 000 mg/kg
group were effects seen. In two out of five males and in one
out of five females, hyperplasia of the thyroid was observed
(Blackwell-Smith et al., 1953). Przezdziecki et al. (1969) fed
female Wistar rats a diet containing 1300 mg zineb/kg or 1875 mg
maneb/kg diet for 7 months. Significant increases in the weight
of the thyroid gland and decreases in the weight of the kidneys,
adrenal glands, and ovaries were observed.
Thyroid hyperplasia has been reported in rats given maneb,
zineb, or mancozeb in amounts ranging from 500 to 2500 mg/kg
diet for periods of up to 2 years (FAO/WHO, 1965b, 1971b). In a
2-year feeding study, 2500 mg maneb/kg diet produced thyroid
hyperplasia and nodular goitre and increased mortality, but 1250
and 250 mg/kg diet did not cause any ill effects (FAO/WHO,
1965b).
Zineb was given orally to white rats at dose levels of 96 or
960 mg/kg body weight for 4.5 months. Compared with that of
untreated animals, the thyroid was enlarged with microfollicles
and columnar cells. Succinic dehydrogenase and cytochrome
oxidase activities were raised in these cells, while the colloid
in the follicles showed reduced PASa-positive granules. These
changes were consistent with an increase in thyroid-stimulating
hormone (TSH). An increased number of basophilic cells con-
taining PASa-positive granules was observed in the adeno-
hypophysis (anterior pituitary). These effects were seen only
at the highest dose level. The uptake of 131iodine was also
increased at the highest dose level, and a high plasma TSH level
was recorded in treated animals. The changes observed in both
thyroid and pituitary were probably a compensatory response to
the antithyroid effect of the dithiocarbamate (Ivanova-
Chemishanska et al., 1975b).
After oral administration of zineb to rats at dose levels of
9.6 or 960 mg/kg body weight, twice a week, for 4“ months, the
gonadotropic and thyroid-stimulating functions of the adeno-
hypophysis were significantly increased compared with those of
control values, more markedly in those receiving the higher dose
(Ivanova-Chemishanska et al., 1974).
Albino rats were orally administered doses of 2400 mg
zineb/kg or 3500 mg maneb/kg body weight, and, after 24 h,
radioactive iodine was administered intraperitoneally. Reduced
assimilation of 131iodine by the thyroid was found, which
suggests that the dithiocarbamates, or certain of their
metabolites, possess a marked antithyroid effect and inhibit the
synthesis of thyroxine (Ivanova-Chemishanska et al., 1967,
1974). In similar studies with mancozeb, a single oral dose of
7000 mg/kg body weight resulted in a decreased uptake of
131iodine (Ivanova-Chemishanska et al., 1967).
The condition of the thyroid gland was studied in male
albino rats, which were administered 700 mg maneb/kg, 960 mg
zineb/kg, or 1400 mg mancozeb/kg body weight. After 30 days,
the distinct morphological changes observed indicated
stimulation of the thyroid by TSH. Hypophyseal stimulation is
the consequence of release from negative feedback by thyroxine,
the plasma level of which is depressed by the action of EBDCs
(Ivanova-Chemishanska et al., 1971, 1974).
Rats fed a diet containing 10 000 mg metiram/kg for 2 weeks
showed increased thyroid weight and a decrease in the uptake of
131iodine, but no ill effects were produced by 1000 mg/kg diet
(Worthing & Walker, 1983).
----------------------------------------------------------------
a PAS = periodic acid-Schift reagent.
8.7.2. Interaction of dithiocarbamates and alcohol
Hald et al. (1948) found that dithiocarbamates interact with
ethanol (ethyl alcohol), and since then, certain dithio-
carbamates, particularly disulfiram, have been used in the
treatment of chronic alcoholism. Disulfiram has been proposed
to act in two different ways. The first possibility is that the
drug or one of its metabolites (e.g., diethyldithiocarbamate,
carbon disulfide) interferes with the normal metabolism of
ethanol and, consequently, gives rise to an accumulation of
toxic amounts of intermediary products, such as acetaldehyde.
The second possible method of action is that ethanol interferes
with the normal metabolism of disulfiram and therefore makes
disulfiram more toxic in some way.
Ethanol is detoxified in many tissues, particularly the
liver, by oxidation, firstly to acetaldehyde, then to acetic
acid, and finally to carbon dioxide and water. Disulfiram
interferes with various enzyme systems including those involved
in the oxidation of ethanol. After administration of disulfiram,
the blood acetaldehyde level increases significantly.
Peripheral neuropathy and optic neuritis have been observed in
alcoholics treated with 125 - 150 mg disulfiram per day
(Gardner-Thorpe & Benjamin, 1971).
Van Logten (1972) studied this phenomenon of alcohol
intolerance extensively in rats. Zineb and maneb did not induce
alcohol intolerance, whereas most of the alkyldithiocarbamates
(such as ziram and nabam) and thiuram sulfides (such as thiram)
did. In general, the dithiocarbamates with a free H atom bound
to the N atom did not induce intolerance. Apart from the
accumulation of acetaldehyde in blood, disturbance of sulfo-
bromophthalein (BSP) elimination, increased serum glutamic-
pyruvic transaminase, hypothermia, increased glucose content,
changes in blood morphology, atrophy of spleen and thymus, and
increase in the weight of adrenals and brain were all observed.
Studies on adrenalectomized rats showed the involvement of the
adrenals in the alcohol intolerance. Hyperglycaemia, eosino-
penia, lymphopenia, and neutrophilia were not seen in the
animals without adrenals, and spleen and thymus atrophy was
reduced. However, the effect on the red blood cells was more
pronounced, and the accumulation of acetaldehyde in the blood
was unaffected by adrenalectomy.
Oral treatment of rats with ethanol after administration of
alkyldithiocarbamates or thiuram sulfides lowers the catechol-
amine content of the adrenals. Since the lowest level was not
reached until 24 h or more after alcohol treatment, it seems
likely that the influence on the adrenals of the dithio-
carbamate-ethanol interaction is of secondary importance. There
was no clear indication that the ethanol intolerance in the rat
is accompanied by changes in brain catecholamine level.
As in human beings, the dithiocarbamate-ethanol reaction in
the rat is characterized by a severe hypotension, which starts
almost immediately after the administration of the ethanol and
lasts at least 8 h. It is evident that, during alcohol
intolerance, body fluids shift from the plasma into the
interstitial tissue or cells, possibly thereby causing the hypo-
tension or shock. The observed hypothermia also starts almost
immediately after ethanol administration and may last for many
hours. Adrenalectomy did not prevent the hypothermia.
Therefore, this phenomenon must be considered as a primary
effect, along with the plasma accumulation of acetaldehyde.
Since heat loss is not increased during the dithiocarbamate-
ethanol reaction, the hypothermia is probably due to decreased
heat production. However, the serotonin concentration in the
brain was increased, and so a disturbance of the thermo-
regulation cannot be ruled out. It seems doubtful that the
dithiocarbamate-ethanol reaction is due to acetaldehyde per se.
Intraperitoneal injection of acetaldehyde, which resulted in a
blood level twice as high as during the dithiocarbamate-ethanol
reaction, did not influence BSP elimination, serum glucose
level, body temperature, organ weights, catecholamine content of
the adrenals, or blood pressure.
No indications were available that the accumulation of
acetaldehyde in the blood was due to an accelerated biotrans-
formation of alcohol. More work needs to be done to decide
whether the accumulation of acetaldehyde and pyruvate in the
blood is a consequence of a disturbance of carbohydrate
metabolism. The specificity of ethanol is remarkable. The
combination of thiram with either methanol or 1-propanol has no
effect on BSP elimination or on blood glucose level.
The sensitivity of the rat for the dithiocarbamate-alcohol
reaction is of the same magnitude as that of man. Administra-
tion of 1.9 mg thiram/kg body weight in the rat elicits an
accumulation of acetaldehyde in the blood. Thus, it may be
concluded that 60 ml of gin, 0.5 litre of beer, or even less
should be sufficient to induce alcohol intolerance.
Eight days after the administration of certain dithio-
carbamates, a dithiocarbamate-alcohol reaction may be observed
when ethanol is given. The maximum level of acetaldehyde in the
blood is reached within 15 min of ethanol treatment.
A 90-day study with several dietary levels of thiram
revealed a no-toxic-effect level of 100 mg/kg diet. After
feeding rats with 10 mg thiram/kg diet for 6 weeks, oral
administration of a single dose of 6 ml ethanol/kg body weight
caused a significant decrease in the body temperature. Higher
doses of thiram with alcohol induced hyperglycaemia, accumula-
tion of acetaldehyde in the blood, and other abnormalities. In
contrast, the combination of 100 mg thiram/kg diet and 5%
ethanol continuously in the drinking-water did not have any
effect (Van Logten, 1972).
8.7.3. Neurotoxicity
In an 80-week study on the neurotoxic and behavioural
effects of thiram, 12 male and 12 female rats per group were fed
thiram at dose levels of 0, 100, 400, or 1000 mg/kg diet (the
concentration of the compound in the diet was periodically
increased in order to give a relatively constant consumption on
the basis of body weight). A second study was carried out on
two groups of 24 female rats administered 0 or 1000 mg thiram/kg
diet, for 36 weeks. The neurotoxic effects were characterized
by ataxia and paralysis of the hind legs, although these effects
were only seen at the highest dose level (1000 mg/kg diet,
equivalent to 65 mg/kg body weight) in females. Demyelination,
degeneration of the axon cylinders, and the presence of
macrophages in the nerve bundle of the sciatic nerve were seen.
Degeneration in the ventral horn of the lower lumbar region of
the spinal cord was demonstrated by chromatolysis of motor
neurons, pyknosis, and satellitosis. Electromyograms indicated
a loss of motor unit function, and the histopathology suggested
that the peripheral nerve is the primary site of the lesion (Lee
& Peters, 1976).
In another study, groups of 12 males and 12 females were fed
ferbam in the dose levels that gave actual intake levels of
approximately 8.5, 34, and 87 mg/kg body weight (average of
males and females) per day. The neurotoxic effects of ferbam
are less than those of thiram. In this study, only 3 of the 24
rats fed the highest dose level developed ataxia or paralysis
(Lee & Peters, 1976). Neurotoxic effects have also been observed
for ziram by Hodge et al. (1956).
In a study on rats, zineb (490 and 2450 mg/kg body weight),
maneb (350 and 1750 mg/kg body weight), and mancozeb (700 and
3500 mg/kg body weight) were administered orally at twice weekly
doses for 4 months. Mortality was high, and paresis in the hind
limbs appeared in the third month of the study and progressed to
complete paralysis (Ivanova-Chemishanska, 1969a).
Dishovski & Ivanova-Chemishanska (1979) studied the ultra-
structural changes in the neocortex of rats repeatedly
administered propineb (70% purity) at 85 mg/kg and 425 mg/kg
body weight for 40 days. At the higher dose level, intense
ultrastructural changes in the sensorimotor neocortex were
detected using an electron microscope, primarily affecting the
pyramidal cells. The concentration of ribosomes and hypertrophy
of the Golgi apparatus suggested an increase in synthetic
processes in the neurons.
Edington & Howell (1966, 1969) found lesions in the central
nervous system of adult Dutch-New Zealand rabbits who were given
ip injections of sodium diethyldithiocarbamate (NDDC) at
330 mg/kg body weight, for 6 days/week, for 30 weeks. The first
changes were seen at 6 weeks in the accessory cuneate nucleus
and in Clarke's column; 12 weeks later, degeneration was seen in
the spinocerebellar tracts in the cerebellum medulla. After 24
weeks, severe nerve fibre degeneration in the peripheral white
matter of the spinal cord (both involved the axon and myelin
sheath) was observed. It was suggested that these changes might
be connected with changes in the level of copper in the serum.
Kim & Rizzuto (1975) studied the effect of NDDC (0.23, 2.3,
and 23 µg/ml nutrient medium) on myelinated cultures of new-
born mouse cerebellum. Exposure time was 24 - 120 h, and the
cultures were examined by light and electron microscopy.
Treatment of the cultures for 24 - 48 h produced swelling of
axons and presynaptic endings, morphologically characteristic of
dystrophic axons. Continued exposure induced an extensive
degeneration of axons and myelin sheath (Wallerian degeneration
in axons).
8.7.4. Dithiocarbamates in combination with metals
Truhaut et al. (1971) studied the chelating action of sodium
diethyldithiocarbamate to copper and the fact that this element
is indispensable for the activity of dopamine beta-hydroxylase.
The authors put forward the hypothesis that the inhibition of
this enzyme system, which catalyses the conversion of dopamine
to norepinephrine and participates in the biogenesis of cate-
cholamines in the central nervous system, may play a role in the
etiology of neurotoxic effects.
Maj et al. (1970) studied the effect of disulfiram, diethyl-
dithiocarbamate (DDC), and dimethyldithiocarbamate on serotonin
(5-HT) and 5-hydroxyindole-3-acetic acid (5-HIAA) in the brain
of rats. The total dose levels ranged from 150 to 500 mg/kg
body weight. It was concluded that these three compounds do not
affect the 5-HT level in the rat brain. The 5-HIAA levels
increased, but not significantly.
Possibly, reactions of carbon disulfide with pyridoxamine
could lead to the depletion of pyridoxal phosphate in the
tissues, which may, in turn, cause neurological changes. Long-
term poisoning of rabbits with carbon disulfide has been shown
to result in increased excretion of zinc in the urine and
disturbances of copper and zinc concentrations in the tissues.
Also, after NDDC treatment, increased levels of copper in the
liver and nervous tissue have been found (Cavanagh, 1973).
Aaseth et al. (1981) showed that oral treatment of Wistar
rats with tetramethylthiuram disulfide (TMTD) at 1000 mg/kg diet
for one week increased the brain levels of endogenous copper
and zinc. In further studies, rats were administered an iv
injection of 203HgCl2 (5 µmol/kg body weight in saline) at day
17 of pregnancy. DDC was given immediately after the mercury
injection (500 µmol/kg body weight). The maternal brain
concentration of mercury increased significantly, and the kidney
levels, measured after 24 and 48 h, also increased. In the
fetuses, the mercury in the brain, liver, kidneys, and blood
(but also in the placenta) were significantly increased after
24 h, but, after 72 h, only the levels in fetal blood were still
elevated. Mice of the NMRI strain were similarly injected with
203HgCl2 (2.5 µmol/kg body weight) and fed diets containing DDC
(10 000 mg/kg diet), disulfiram and TMTD (1000 mg/kg diet), or
carbon disulfide (3000 mg/kg diet) for 4 days. The brain level
of mercury was significantly increased after DDC or TMTD
treatment and marginally after disulfiram or carbon disulfide
treatment (Aaseth et al., 1981).
Lakomaa et al. (1982) studied the effect of DDC on copper
and zinc concentrations in different regions of the brain of
Long-Evans rats during acute or repeated treatment. Acute
treatment (250 mg/kg body weight) produced no effect after 24 h,
whereas repeated treatment (250 mg/kg, 5 times per week, for
4 weeks) increased copper levels in the brain stem, cortex,
hippocampus, and the rest of the brain, but did not alter zinc
concentrations.
Dithiocarbamates, with their metal-chelating properties, and
thiuram derivatives, have been demonstrated to cause a marked
increase in the concentration of lead in the brain as well as a
redistribution of lead in the rest of the body (Oskarsson, 1983,
1984; Danielsson et al., 1984). Thus, after injection of a dose
of labelled lead (203Pb), the brain concentrations were
increased by up to 100 times in thiuram-treated rats.
Male Sprague Dawley rats (10 groups of 5 rats each) were
administered different combinations of thiram, disulfiram, DDC,
or dimethyldithiocarbamate in combination with sodium or lead.
The study demonstrated that treatment with dithiocarbamates and
thiram derivatives in rats exposed for 6 weeks to lead causes a
substantial (up to 4-fold) increase in the lead concentration of
the brain. This effect can be explained by the formation of a
lipophilic lead-dithiocarbamate complex, which probably is
retained longer and has a higher capacity to penetrate the
blood-brain barrier and bind to lipid-rich brain tissue compo-
nents than inorganic lead itself. The chemical form of the lead
when it is in the brain remains uncertain. The lead complex may
decompose in the brain into inorganic lead, which exerts a
neurotoxic effect, or it may be very stable in the brain and of
low toxicity for the central nervous system (Oskarsson & Lind,
1985).
There are several reports on the effect of dithiocarbamates
on the distribution in the body of other metal ions such as
cadmium, thallium, nickel, copper, zinc, and mercury (Oskarsson
& Lind, 1985).
DDC has been shown to have a strong inducing effect on
levels of metallothionein, a low molecular weight, heavy-metal-
binding protein, in rat liver and kidney. The mechanism
probably reflects enhanced uptake of copper and depletion of
hepatic glutathione (Sunderman & Fraser, 1983).
8.7.5. Miscellaneous reactions
Dithiocarbamates, with their chelating capacity, also
interfere with a number of enzyme systems containing metals such
as zinc and copper (e.g., dopamine beta-hydroxylase). They also
inhibit sulfhydryl (SH)-containing enzymes and a number of other
enzyme systems involved in glucose metabolism (e.g., hexokinase,
glyceraldehyde-3-phosphate dehydrogenase, and glucose-6-
phosphate dehydrogenase). The effect of dithiocarbamates on
liver enzymes has consequences for the metabolism of other
chemicals. Thus, the toxicity of carbon tetrachloride is
decreased by diethyldithiocarbamate (Lange & Jung, 1971; Lutz et
al., 1973), and the toxicity of other chemicals, e.g., ethyl
alcohol, may be increased (section 8.7.2).
9. EFFECTS ON MAN
9.1. Occupational Exposure
9.1.1. Acute toxicity - poisoning incidents
The acute toxicity of dithiocarbamates is low and, there-
fore, acute intoxication in human beings is unlikely to occur.
A case was reported of a 62-year-old man with acute kidney
insufficiency after maneb application. However, the precise
cause of maneb exposure was not clear, since the patient had a
history of hypertension, cerebral infarction, gastrectomy
because of stomach cancer, and chemotherapy. The patient was
treated with haemodialysis and was discharged from hospital
(Koizumi et al., 1979).
Thiram (100 mg/m3) has been shown to cause headaches,
vertigo, impairment of mental capacity, muscle twitch, and
paraesthaesia (Sprecher & Grigorowa, 1967).
9.1.2. Case reports, short-term and epidemiological studies
9.1.2.1. Dermal
The irritant and allergic potential of most dithiocarbamates
is evident in occupational exposure. Skin irritation and
sensitization were studied in man using a conventional patch
test. A cotton square was dipped in 19% nabam solution and
placed on the inner surface of the forearm, and, 14 days later,
this procedure was repeated on the opposite forearm. Zineb was
tested in the same manner, except that the cotton square was
dipped in 65% wettable powder. The patches were left in place
for 48 h. Of the 25 subjects included in the nabam study, 2
showed irritation (mild erythema and itching). Thirteen of the
25 reacted to the retest (from mild erythema to severe erythema,
oedema, and vesiculation), indicating sensitization. Of the 50
subjects used in the zineb study, no reaction at all was seen in
49 of them. One reacted in such a way that it indicated primary
irritation rather than sensitization (Blackwell-Smith et al.,
1953). Schultheiss (1957) reported a case of contact dermatitis
with thiram. Zadorozhny et al. (1981) found dermatitis and
eczema in 241 industrial workers exposed to TMTD and other types
of pesticides. Twenty-one of them showed contact dermatitis, 25
allergic dermatitis, and 7 eczema.
Cases of diffuse erythema and eczematoid epidermatitis of
the eyelids and inguinal regions, probably with elements of sun
sensitization, were observed among agricultural workers (grape
and tobacco industries) in contact with zineb (Babini, 1966) or
maneb (Laborie & Laborie, 1966; Zorin, 1970). These were
largely allergic in character with only a few manifestations of
contact dermatitis. Decreased resistance of the workers,
vitamin deficiency, chronic liver disease, and other factors
apparently contributed to these effects.
9.1.2.2. Exposure via different routes
Kaskevich et al. (1981) carried out an epidemiological study
on 137 workers engaged in zineb manufacturing (51 men and 86
women). The duration of exposure to zineb for 52 workers was
between 1 and 3 years, and for 85 workers between 4 and 5 years.
Control groups in this study consisted of 193 persons, not
exposed to chemicals and matched for age, period of employment
rate, and sex. The concentrations in the air of the working
area never exceeded 1 mg/m3. Among workers occupationally
exposed to zineb, the following changes were found: hepato-
cholecystitis (28.4% of workers, versus 13.5% in controls);
vegetovascular dystonia connected with disorders in the central
nervous system (34.9%, versus 22.3% in controls); chronic
bronchitis (4.4%, versus 0.5% in controls); contact dermatitis
(11.9%, versus 0.1% in controls); and disorders in the menstrual
cycle (16.91%, versus 4.3% in controls). These studies indicate
a change in catecholamine metabolism.
In a study with cultured lymphocytes from 15 workers working
in different stages of zineb manufacture, the mean incidence of
aberrant metaphases was 6% greater than that in controls. The
incidence of chromosomal aberrations (chromatid breaks) in
cultured human lymphocytes treated with maneb (0.5, 15, or
30 µg/ml) was 10 - 20% greater than in controls (Antonovich et
al., 1972).
A number of studies on maneb and mancozeb production workers
have been carried out. In the earliest study (1965), 54 produc-
tion workers were given medical examinations that included blood
and urine analyses. Since this study predated the availability
of immunoassay techniques for thyroid hormone determination,
protein-bound iodine was used as a measure of thyroid function.
No thyroid or other medical abnormalities could be attributed to
EBDC exposure. In a second study (1975), 57 exposed and 98
unexposed production workers were examined for thyroid function
by measuring triiodothyronin (T3), thyroxine (T4), and TSH.
Again, no effects attributable to work-place exposure were
identified. Workers exposed to EBDC levels ranging from 0.13 to
5.46 mg/m3 were found to have elevated ETU and manganese levels
in the urine. In a 1976 mortality study, 992 past and present
production workers (over the period 1948-75) were studied.
Compared with the local general population, neither the overall
death rate nor the death rate due to cancer was elevated. The
number of cancer deaths observed (10) was too small to evaluate
cancer-specific mortality (Gowers & Gordon, 1980).
In the most extensive study, 42 currently exposed and 112
previously exposed workers were compared with equal size control
groups matched for age, period of employment, race, and type of
job. All participants were given thorough physical examinations
by specialists in diagnostic medicine, including detailed
questionnaires and interviews about health history and family
health. A separate thyroid examination was carried out by
thyroid specialists. Thyroid parameters that were measured
included total T3, T3 resin uptake, T4, TSH, free T4 index,
thyroglobulin antibodies, and microsomal antibodies. In
addition, urine was analysed for ETU, EBDC, zinc, manganese,
creatine, iodide, specific gravity, and pH. Blood levels of
glucose, urea nitrogen, sodium, potassium, calcium, chloride,
carbon dioxide, cholesterol, total protein, protein albumin,
bilirubin, uric acid, creatinine, inorganic phosphate, lactic
dehydrogenase, and serum glutamic oxaloacetic transaminase were
also determined. As in earlier studies, the occurrence of
unusually high levels of ETU in the urine of currently exposed
workers confirmed their exposure. However, a detailed
statistical analysis of the data revealed no differences in
thyroid function, blood and urine indicators of liver and kidney
function, or general health, between exposed and control groups
(Gowers & Gordon, 1980; Charkes et al., 1985).
PART B
ETHYLENETHIOUREA (ETU) AND PROPYLENETHIOUREA (PTU)
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ETHYLENETHIOUREA (ETU)
AND PROPYLENETHIOUREA (PTU)
INTRODUCTION
1. SUMMARY
1.1 Sources, environmental transport and distribution
1.2 Environmental levels and human exposure
1.3 Kinetics and metabolism
1.4 Effects on organisms in the environment
1.5 Effects on experimental animals and in vitro test systems
1.5.1 Ethylenethiourea
1.5.2 Propylenethiourea
1.6 Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
2.2 Physical and chemical properties
2.3 Analytical methods
2.3.1 Extraction
2.3.2 Clean-up
2.3.3 Derivatization
2.3.4 Determination
2.3.4.1 Gas-liquid chromatography (GLC)
2.3.4.2 Thin-layer chromatography (TLC)
2.3.4.3 Polarography
2.3.4.4 Radioisotope dilution
2.3.4.5 High-pressure liquid chromatography (HPLC)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Soil
4.2 Water
4.3 Plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Food and drinking-water
5.2 Monitoring and market basket studies
6. KINETICS AND METABOLISM
6.1 Absorption, distribution, and excretion
6.2 Metabolic transformation
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposures
8.2 Short- and long-term exposures
8.3 Teratogenicity
8.4 Mutagenicity
8.5 Carcinogenicity
8.5.1 Mouse
8.5.2 Rat
8.5.3 Hamster
8.6 ETU in combination with nitrite
8.7 Mechanisms of toxicity; mode of action
8.8 Probineb and Propylenethiourea (PTU)
8.8.1 General
8.8.2 Toxicological information
9. EFFECTS ON MAN
9.1 Epidemiological studies
INTRODUCTION
One of the metabolic products of ethylene bisdithiocarbamate
decomposition in mammals, plants, and lower organisms is
ethylenethiourea (ETU). It may also be present as an impurity
in these dithiocarbamates, and their residues on crops may be
partly transformed into ETU during food processing. A comparable
breakdown takes place with propineb, giving rise to propylene-
thiourea (PTU).
1. SUMMARY
1.1. Sources, Environmental Transport, and Distribution
Ethylenethiourea (ETU) is found together with residues of
the parent ethylene bisdithiocarbamates (EBDCs) in and on crops
that have been treated with these pesticides. During storage,
processing, and cooking, the amount of the parent compound
decreases while that of ETU increases. ETU is easily photo-
oxidized (in the presence of photosensitizers) to ethyleneurea
(EU).
1.2. Environmental Levels and Human Exposure
In certain crops, such as spinach, carrots, and potatoes,
treated with EBDCs, high levels of ETU can be found after
cooking. In general, however, the ETU levels are below
0.1 mg/kg product.
Estimates of the exposure of the general population of the
USA are of the order of 0.24 - 3.65 µg ETU/kg body weight per
day, and, in Canada, estimates based on market-basket studies
are around 1 µg ETU/kg body weight per day.
1.3. Kinetics and Metabolism
ETU is rapidly absorbed, metabolized, and excreted in
mammals. Up to 90% is eliminated via the urine and only a
small amount via the faeces. Distribution of ETU in the body
appears to be fairly uniform with the exception of a relative
accumulation in the thyroid. ETU is broken down to ethylene
diamine (EDA), urea, carbon dioxide, or oxalic acid, or is
transformed to imidazole derivatives in mammals, plants, and the
environment.
1.4. Effects on Organisms in the Environment
The available LC50 levels of ETU and EU for fish are in the
range of 7500 - 13 000 mg/litre.
1.5. Effects on Experimental Animals and In Vitro Test Systems
1.5.1. Ethylenethiourea
The acute oral toxicity in experimental animals is low, and
the long-term effects are mainly characterized by an antithyroid
action.
At dose levels > 25 mg/kg body weight, decreases in serum
T3, T4, and protein-bound iodine (PBI) and increases in thyroid-
stimulating hormone (TSH) have been found in studies on
experimental animals. At higher dose levels (> 100 mg/kg body
weight), increases in thyroid weight and hyperplasia occurred,
which finally resulted in the development of adenocarcinoma.
The effects of short-term exposure to low levels of ETU seem to
be reversible, but those of long-term exposure to higher levels
become, at a certain stage, irreversible. A level of
approximately 5 mg/kg body weight seems to be without effects.
Most mutagenicity studies on ETU, especially those with
mammalian test systems, have given negative results.
A number of carcinogenicity studies have been carried out on
mice, rats, and hamsters. In addition to an antithyroid action,
ETU has been found to induce, subsequently, thyroid tumours
(hyperplastic goitre, solid-cell adenomas, and follicular and
papillary carcinomas) in mice and rats. In an earlier study on
mice, liver tumours, lung tumours, and lymphomas were also
detected, but these findings have not been confirmed. No
tumours except thyroid tumours have been found in rats, and in
hamsters, no tumours of the thyroid gland or other organs were
observed, even at 200 mg/kg diet.
At dose levels above approximately 10 mg/kg body weight, ETU
has clear teratogenic effects in rats and hamsters, different
types of central nervous system and skeletal anomalies being
induced. However, in mice, no teratogenic effects were found at
much higher dose levels (up to 800 mg/kg body weight).
1.5.2. Propylenethiourea
In a long-term study on mice using propylenethiourea (PTU),
an increased incidence of hepatocellular adenomas was observed
at dose levels of 10 mg/kg diet or more. No thyroid tumours
were found, but increased thyroid hypercellularity occurred at a
dose level of 1000 mg/kg diet. In rats, goitrogenic effects
were seen with PTU at dose levels as low as 1 mg/kg diet.
1.6. Effects on Man
Epidemiological studies on workers exposed to ETU did not
reveal any increase in the incidence of thyroid tumours.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
The chemical structure of ethylenethiourea (ETU) is:
CH2-NH
| \
| C=S
| /
CH2-NH
2.2. Physical and Chemical Properties
ETU is a fairly stable compound. Some physical and chemical
properties are listed in Table 8.
Table 8. Some physical and chemical properties of ETU
-------------------------------------------------------------------
Empirical formula C3H6N2S
Common synonym 2-imidazolidinethione
Appearance white, crystalline
Relative molecular mass 102.17
Odour odourless
Melting point 203 - 204 °C
Solubility in water: 20 000 mg/litre at 30 °C;
in ethanol: moderately soluble;
in chloroform: nearly insolublea
CAS registry number  Rhodes (1977) found that when 14C-ETU was applied to soil
sections of Keyport silty loam at a rate of 2.2 kg/ha, total 14C
residues disappeared with a half-life of < 4 weeks. The half-
life of intact ETU was < 1 week. Most of the radioactivity was
confined to the top 2.5 - 12.5 cm of the soil column, and only
small amounts (0.2%) were found at depths of 20 - 30 cm after 12
weeks. It was concluded from this study that ETU did not leach
to any great extent.
Nash & Beall (1980) reported that ETU is weakly adsorbed to
soil, and is highly mobile in moist soil but immobile in dry
soil. The presence of organic matter in soil seems to be of
great importance in the leaching of ETU. Degradation appears to
be accomplished readily by both chemical and biological means
and, thus, ETU does not persist in soil.
Many studies have been carried out concerning the environ-
mental fate and transport of ETU (US EPA, 1984).
Parallel results were obtained in laboratory studies with
propineb, which, in a similar fashion, forms PTU, propyleneurea
(PU), and, eventually, carbon dioxide (Vogeler et al., 1977).
The results of these studies show that, under normal
practical conditions, it is unlikely that ETU or PTU will
accumulate in soil.
4.2. Water
ETU is stable in de-ionized water in the absence of photo-
sensitizers, but is rapidly oxidized in their presence. In
studies by Ross & Crosby (1973), several sensitizers were added
at 10 mg/litre to a 25 mg/litre solution of ETU and exposed to
sunlight. After 4 days with riboflavin as a sensitizer, the
concentration of ETU was less than 5% of that in the control
solution kept in darkness. To minimize microbial degradation,
the procedure was repeated after filtering and boiling the water
samples, with the same results. Furthermore, ETU degradation
was investigated in several boiled samples of agricultural
drainage water to which 0.5 mg ETU/litre had been added before
irradiation. The results are given in Table 10.
Numerous samples of natural water were collected from
rivers, lakes, and agricultural areas and, almost without
exception, they were found to degrade ETU to EU in sunlight.
The same samples degraded ETU in the dark but only after prior
exposure to sunlight, indicating that stable photo-oxidants had
been generated. The substances responsible for ETU oxidation
were isolated and identified as the amino acids tryptophane and
tyrosine. The pure amino acids also caused the conversion of
ETU to EU in the light, apparently by their ability to form
hydroperoxides or other strong oxidants (Ross & Crosby, 1973).
As both the amino acids and photosensitizers such as acetone,
riboflavin, and chlorophyll are known to occur world wide in
water and soil, and this photolysis also has been shown to take
place rapidly on a silica surface (Cruickshank & Jarrow, 1973),
the degradation of ETU to harmless products in the field seems
entirely plausible (IUPAC, 1977).
Table 10. Photodecomposition of ETU in agricultural
watersa
--------------------------------------------------------
Source Irradiation Remaining ETU (%)
--------------------------------------------------------
Irrigation ditch 3 days, lamp 10 - 20
(sugar beet) 3 days, dark 100
Paddy flooding 24 days, sun 25 - 50
ditch (rice) 24 days, dark 100
Paddy (rice) 24 days, sun 10 - 25
24 days, dark 100
--------------------------------------------------------
a From: Ross & Crosby (1973).
4.3. Plants
In studies in which the roots of corn, lettuce, tomato, and
pepper seedlings were treated with ETU, it was rapidly absorbed
by roots, translocated subsequently to the foliar tissues, and
then degraded very rapidly; virtually no ETU was detectable
after 20 days (Hoagland & Frear, 1976). When cucumber seedlings
were exposed to aqueous solutions of nabam or suspensions of
zineb or maneb, ETU was rapidly absorbed by the roots and
translocated within the plants. ETU appeared to be stable for
at least 2 weeks in seedlings and, inside the plant, a slow
conversion of ETU into 2-imidazoline was detected (Vonk & Kaars
Sijpesteijn, 1970, 1971).
In greenhouse studies, 14C-ETU was applied either to the
soil or to the leaves of 4-week-old potato plants and 8-week-old
dwarf tomato plants. Radioactivity was monitored in various
parts of the plant at different time intervals. The application
of 40 mg 14C-ETU/kg to the leaves of potato plants resulted in
negligible radioactivity in the roots and tubers 60 - 90 days
after application. The application of 17 - 22 kg/ha to soil
around the base of the plants resulted in a negligible amount of
radioactivity in the tubers, roots, and foliage of potato
plants, 60 - 90 days later. Comparable results were obtained
with tomato plants, using other dose levels and periods (Lyman &
Lacoste, 1974).
After systemic uptake of ETU by plants, EU and 2-imidazoline
were identified as metabolites. Surface deposits of ETU, which
may have occurred as a result of EBDC treatment, formed an
additional unidentified substance as the main metabolite and
ethylene diamine (EDA). Propineb and PTU also formed an
identical but unidentified major metabolite under similar
conditions (Vogeler et al., 1977).
Nash (1975) reported the presence of 7 - 10 different
degradation products in methanol extracts of soybeans after soil
or foliar treatment with EBDCs, as well as after treatment with
ETU. In these cases, EU was a degradation product.
More recently, Nash & Beall (1980) studied the fate of maneb
and zineb in microagroecosystem chambers (Part A, section 6.3).
ETU on the tomato fruit and leaves, and in the soil, water, and
air was monitored for 100 days after treatment. ETU was
detected at < 20 µg/kg on whole fruit after 3 days, but had
completely disappeared after 3 weeks. The half-life of ETU was
< 3 days.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The amount of ETU in commercial formulations of EBDCs has
been shown to increase with increasing temperature and humidity.
ETU formation during storage appears to be greatest in maneb
formulations (up to 14%), followed by zineb and mancozeb. The
relative proportions of degradation products appear to be
different for the various EBDCs.
Studies with propineb on apples and grapes, carried out by
Vogeler et al. (1977), showed that PTU could be detected
shortly after treatment, and that it was rapidly transformed to
an unknown metabolite, together with small amounts of PU, and
other unidentified reaction products.
5.1. Food and Drinking-Water
The residue levels of ETU are mainly below 0.1 mg/kg product
following treatment (with different formulations) at the maximum
recommended EBDC levels (Newsome, 1976; Phillips et al., 1977).
Studies in Canada and the USA have shown that vegetables,
such as spinach, carrots, and potatoes, that have been treated
with EBDCs contain high levels of ETU after cooking (Blazquez,
1973; Newsome & Laver, 1973; Watts et al., 1974). Snapbeans
treated with maneb were found to contain ETU after commercial
canning (US EPA, 1977). Farrow & Ralls (1970) demonstrated the
disappearance of zineb, ziram, and maneb residues from spinach
and apricots during normal canning operations. There have been
many studies on ETU residues in crops, such as those of Sato &
Tomizawa (1960) on zineb-treated cucumbers.
The levels of ETU formed from residues of mancozeb and
polyram present on apples that were processed to make apple
juice, apple sauce, and apple pomace were determined (IUPAC,
1977). The results showed that ETU residues were 0.17 mg/kg
pomace and 0.05 mg/kg juice. A surprisingly high level of
unchanged mancozeb remained in the pomace, despite heat
treatment for 15 h at 150 °C. However, it seems that ETU
residues diminish during storage.
Residues of intact 14C-labelled ETU were found to diminish
with time in canned tomato sauce, spinach, pickles, and apple
sauce (Rose et al., 1980). EU and more polar products accounted
for most of the 14C-labelled residues. These polar materials
were resistant to extraction and appeared to be bound.
A study sponsored by the US EPA (Phillips et al., 1977), on
the effects of food processing on EBDC residues, confirmed and
extended the results previously described. Washing the raw
agricultural products prior to processing removed 33 - 87% of
the EBDC residue and the majority of the ETU residue. An
interesting result was that although almost instantaneous
conversion of mancozeb to ETU took place in boiling water, field
weathered residues of mancozeb appeared to be more resistant to
degradation to ETU. A summary of the results for raw and
processed material is given in Table 3.
5.2. Monitoring and Market-Basket Studies
A monitoring programme initiated in 1972 by the Canadian
government showed that 33% of food samples contained detectable
ETU residues. In particular, samples of canned spinach and
orange peel had average values of 0.047 mg/kg product and
0.083 mg/kg, respectively (Pecka et al., 1975; US EPA, 1977).
Studies on the actual level of ETU in products prepared for
commercial sale show it to be generally present in small
amounts. The highest level, 0.61 mg/kg product, was found in
canned peaches, while levels in orange peel, tomato paste,
instant potatoes, strawberries, peaches, and cucumbers were less
than 0.2 mg/kg product (US EPA, 1982a,b).
The US EPA has estimated an upper limit for dietary exposure
to ETU in the general population of the USA to be 3.65 µg/kg
body weight per day. This estimate is a maximum value, since it
was assumed that residues are present at the tolerance level and
that all of the EBDC residue is quantitatively converted to ETU.
Using actual residue data and experimentally derived conversion
factors, the US EPA estimated the dietary intake of ETU to be
0.24 µg/kg body weight per day.
In a market-basket study, over 500 samples of 34 foods were
analysed, plus 26 samples of drinking-water. No water samples
and only 21 of the food samples contained ETU residues (Gowers &
Gordon, 1980). Exposure estimates based on market-basket
studies range from 0.01 to 1 µg ETU/kg body weight per day
(Gowers & Gordon, 1980; Rose et al., 1980).
Tomato products (203 samples) were analysed in another
market-basket study, but none contained ETU (Gowers & Gordon,
1980).
A more realistic review of the actual exposure of the
general population was obtained by a "table-top" study. Of 200
meals (some from homes and some from restaurants) which were
analysed for ETU, none contained any residues (Gowers & Gordon,
1980).
6. KINETICS AND METABOLISM
6.1. Absorption, Distribution, and Excretion
ETU is rapidly absorbed from the gastrointestinal tract and
cleared from the body in all the mammalian species that have
been tested. After only 5 min, ETU appeared in the blood of
rats administered an oral dose of 100 mg 14C-ETU/kg body weight.
Within 48 h, 82 - 99% of an oral dose was eliminated via the
urine and about 3% via the faeces (Kato et al., 1976; Rose et
al., 1980). Newsome (1974) and Ruddick et al. (1976a) found
that approximately 70% was eliminated in the urine and 1% in the
faeces. Comparable results were found for mice while, in
monkeys, 55% was eliminated via the urine within 48 h, and less
than 1.5% via the faeces (Allen et al., 1978).
To study the accumulation and elimination of radioactivity
by the thyroid gland of rats dosed with 14C-ETU, dose levels of
2 and 200 µg labelled ETU were administered daily for 14 days.
In another study, rats were dosed with 0, 0.1, 1, 10, 50, or 100
mg 14C-ETU/kg diet, daily, for 7 days. The first study showed
that the concentration of ETU and/or its metabolites in the
thyroid is dose dependent, and the second that the level of 14C
in the thyroid did not increase appreciably when the daily dose
was increased above 50 mg/kg diet. Withdrawal of ETU from the
diet led to an 80 - 94% reduction in the radioactivity in the
thyroid after 17 days (Lyman & Lacoste, 1974).
ETU and its metabolites have been found to have a half-life
of about 28 h in monkeys, 9 - 10 h in rats, and 5 h in mice
(Rose et al., 1980).
In cows administered 1 mg 14C-ETU/kg diet, Lyman (1971)
found a small quantity of unchanged ETU in both the urine and
the milk of the test animals. Higher levels of 14C were
detectable in metabolites, such as glycine and urea, and in the
lactose and protein in the milk (Table 11).
6.2. Metabolic Transformation
It has been demonstrated that ETU degradation leads to
traces of EU and other metabolites in the urine and that 14C-
carbon dioxide is exhaled following the administration of
labelled ETU. Kato et al. (1976) suggested that the metabolites
of ETU in the rat were produced primarily by fragmentation of
the imidazoline ring and decarboxylation of the fourth and fifth
carbon atoms. A small amount of radioactivity was also found in
a protein fraction of rat fetal tissue. Ruddick et al. (1976a),
however, concluded that ETU metabolism in the rat does not
appear to result in any release of 14C into the general
metabolic pool. Mice metabolize ETU to EU and other unknown
metabolites, while cats metabolize it to S-methyl-ETU and EU.
Lyman (1971) detected EU, EDA, oxalic acid, glycine, and
urea as major metabolites in cow urine. In addition, 14C
originating from 14C-ETU was found in the protein and lactose in
the milk (Table 11). From these results, it appears that the
metabolism of ETU in ruminants is different from that in non-
ruminants. The degradation products of ETU in plants are
similar to those found in animals.
A summary of the secondary metabolites of ETU in biological
and non-biological systems is given in Fig. 4 (Part B, section
4).
Table 11. 14C Activity in the milk and urine of
cows fed with 1 mg 14C-ETUa/kg diet for 6 weeks
------------------------------------------------------
Milk Urine
Substance Concentra- % of Concentra- % of
tion total tion total
(mg/litre) 14C (mg/litre) 14C
------------------------------------------------------
ETU 0.011 31 0.12 7
EU 0.0025 8 0.27 18
EDA - - 0.14 14
Glycine - - - 6
Oxalic acid - - - 12
Urea - - - 11
Fat - 3 - -
Protein - 18 - -
Lactose - 16 - -
Total (%) 76 68
------------------------------------------------------
a From: Lyman (1971) and IUPAC (1977).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only limited information is available on the effects of ETU
on organisms in the environment, and none is available
concerning the impact of ETU on terrestrial organisms. Data
concerning the toxicity for aquatic organisms of ETU and its
breakdown product EU are summarized in Tables 4 and 5. From
these results, ETU appears to have a low toxicity for bacteria,
algae, crustacea, and fish. Because of the low partition
coefficient (Table 6) and rapid biotransformation of ETU,
bioaccumulation will be insignificant or absent (Van Leewen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Lewerenz et al. (1975) reported an acute oral LD50 for the
rat of 900 mg/kg body weight. The values determined by Graham &
Hansen (1972) and Teramoto et al. (1978a) were 1832 mg/kg and
545 mg/kg body weight, respectively. Teramoto et al. (1978a)
reported values of 3000 mg/kg body weight for the mouse and
> 3000 mg/kg body weight for the hamster.
8.2. Short- and Long-term Exposures
Administration of ETU to laboratory rats causes enlargement
of the thyroid gland. This effect was noted by Seifter & Ehrich
(1948), and has since been confirmed in various short- and long-
term studies. The accumulation of ETU in the thyroid gland is
associated with biochemical and morphological effects comparable
with those induced by known antithyroid drugs such as
thiouracil.
Graham & Hansen (1972) fed rats with diets containing ETU at
dose levels of 0, 50, 100, 500, or 750 mg/kg diet for 30, 60,
90, or 120 days, and at 100 mg/kg or more, thyroid changes were
seen. Ross Hart & Valerio (1973) fed doses of up to 1000 mg
ETU/kg diet to rats. An increase in thyroid weight was seen at
159 mg/kg or more, and the larger doses produced hyperplasia.
When Freudenthal et al. (1977) carried out studies on rats
at dose levels of up to 625 mg ETU/kg diet for 30, 60, or 90
days, biochemical changes reflecting effects on thyroid function
were observed. A dose of 125 mg/kg diet produced, after 30
days, a decrease in T3, T4, and serum PBI levels and an increase
in TSH concentration. A dose of 25 mg/kg diet produced a
decrease in T4 content after 60 days. Thyroid weight was
increased in all groups receiving 25 mg/kg or more. After 90
days, tumours (adenomas) were found in the 125 mg/kg group. The
group receiving 625 mg/kg died within 7 weeks.
From these short-term studies, it can be concluded that the
no-observed-adverse-effect level lies below 25 mg ETU/kg diet
and is probably of the order of 5 mg/kg diet (equivalent to
0.25 mg/kg body weight).
8.3. Teratogenicity
ETU was administered orally to rats and rabbits in single
daily doses of 0, 5, 10, 20, 40, or 80 mg/kg body weight. Rats
were treated from 21 - 42 days before conception to day 15 of
pregnancy, or on days 6 - 15 or 7 - 20 of pregnancy, whereas
rabbits were treated on days 7 - 21 of pregnancy. ETU at dose
levels of 10 mg or more induced meningoencephalocele,
meningorrhagia, meningorrhea, hydrocephalus, obliterated neural
canal, abnormal pelvic limb posture with equinovarus, and short,
kinky tail in all rats. Fetal survival was not affected, and
fetal growth was retarded only at 40 and 80 mg/kg. Rabbits
showed an increased incidence of resorption sites and decreased
brain weight at 80 mg/kg body weight, but no malformations
(Khera, 1973).
ETU was studied in rats and mice for its ability to induce
perinatal toxicity and in guinea-pigs and golden hamsters for
its teratogenic potential. The ETU was administered by gavage
and during organogenesis. Table 12 summarizes the prenatal
treatments. Additional postnatal studies were performed on rats
using extended treatment periods (ETU at 0, 20, 25, or 30 mg/kg
body weight), including continuous exposure from day 7 of
gestation through parturition to day 15 of lactation. The pups
were weaned normally and postnatal studies on open-field
behaviour were performed at 6 weeks. ETU at 80 mg/kg body
weight induced maternal toxicity and reduced growth in the rat
and was teratogenic for the rat, inducing substantial fetal
effects at all dose levels above 10 mg/kg body weight. Gross
defects were seen in the skeletal and the central nervous
systems, and cleft palate was noted, mainly at the highest dose
level. At 20 and 30 mg/kg, an increased incidence of
hydrocephalus was the only defect noted. The maternal and fetal
toxicity of ETU for the mouse, guinea-pig, and hamster was
substantially less than for the rat. In mice, at the highest
dose, an increase in maternal liver weight and in fetal
supernumerary ribs was noted, but no effects were seen in other
species (Chernoff et al., 1979; FAO/WHO, 1980b).
Table 12. Summary of prenatal treatmentsa
---------------------------------------------------------
Compound Species Dose (mg/kg Treatment
body weight) (gestation days)
---------------------------------------------------------
ETU rat 0, 5, 10, 20, 7 - 21
30, 40, 80
mouse 0, 100, 200 7 - 16
hamster 0, 25, 50, 100 5 - 10
guinea-pig 0, 50, 100 7 - 25
---------------------------------------------------------
a Modified from: Chernoff et al. (1979).
ETU has been found to induce a variety of postnatal
effects, including reduction or absence of maternal milk
production, thereby causing pup mortality at doses of 30 mg/kg
body weight or more. However, no significant dose-related
behavioural abnormalities were observed in ETU-exposed pups
(Chernoff et al., 1979; FAO/WHO, 1980b).
In studies by Khera & Tryphonas (1977), groups of pregnant
rats were administered ETU at dose levels of 0, 15, 30, or
45 mg/kg body weight on day 15 of gestation and then subjected
to a variety of test conditions to evaluate pre- and postnatal
effects. Postnatal mortality occurred in pups from mothers
treated with dose levels exceeding 15 mg/kg or pups cross-
fostered to evaluate lactation exposure. All pups from mothers
treated with 45 mg/kg died within 4 weeks of birth. A high
incidence of hydrocephalus and microphthalmia was observed in
pups of mothers treated with 30 mg/kg and these pups died within
6 weeks of birth. Motor defects observed in some survivors
(16/65) of this group, were shown to result from the
hydrocephalic condition, which was accompanied by atrophy of the
cerebral cortex and subcortical white matter. These defects
were found to be a direct result of in utero exposure to ETU and
not of exposure during lactation (cross-fostered pups showed the
same effects as pups weaned from treated dams). When mated with
normal male rats, all female offspring of rats administered
30 mg/kg gave birth to normal offspring. The F2 generation was
not impaired, though some of the parents had neurological
defects. In these studies, no effects on the parameters
examined were observed at 15 mg/kg body weight (Khera &
Tryphonas, 1977).
Teramoto et al. (1978a) investigated the teratogenicity of
ETU in rats, mice, and hamsters. It was teratogenic when given
orally to rats at 20 - 50 mg/kg body weight per day on days 6 -
15 of pregnancy and to hamsters at 270 - 810 mg/kg body weight
per day on days 6 - 13 of pregnancy. However, no malformations
were induced in mice up to a daily oral dose of 800 mg/kg body
weight when given on days 7 - 15 of pregnancy. In hamsters,
cleft palate, kinky tail, oligodactyly, and anal atresia were
noted as gross external malformations. Skeletal examination
revealed a high incidence of defects in the ribs and vertebral
column, but no apparent defect was observed during visceral
examination. An oral dose of 100 or 200 mg ETU/kg body weight
given to pregnant rats consistently produced brain abnormalities
in the fetuses, when given on day 12 or 13 of pregnancy, and
forelimb abnormalities, when given on day 13 of pregnancy
(Teramoto et al., 1978a). Histological studies revealed
extensive cell necrosis in the brain and forelimbs of embryos
24 h after the treatment. These lesions were considered to be
the main cause of the abnormalities observed. However, neither
malformations nor cell necrosis were found in the fetuses that
had been injected with 200 µg ETU/conceptus into the amniotic
sac on day 12 of pregnancy (Teramoto et al., 1980). Studies
with 2-14C-ETU revealed that this dose was sufficient to test
the direct effects of ETU on the embryos, since the
incorporation of radioactive substance was five times higher in
the embryos injected with 200 µg into the amniotic sac than it
was in those embryos whose mothers were treated with an oral
dose of 100 mg/kg body weight (Teramoto et al., 1980).
The teratogenic potential of the ETU metabolite 1-
methylthiourea has been investigated by Teramoto et al. (1981).
It caused almost the same types of malformations in rat fetuses
when given orally to mothers at 250 - 500 mg/kg body weight on
day 12 or day 14 of pregnancy as those observed following
treatment with ETU. However, 1-methylthiourea did not induce
malformations in mouse fetuses whose mothers were given an oral
dose of 1000 mg/kg on day 10 of pregnancy. There is a
structural similarity between 1-methylthiourea and ETU: C=S,
and -NH- groups seem essential for producing teratogenic effects
(Teramoto et al., 1981). However, the structure of ETU seems
quite specific for the induction of teratogenicity since Ruddick
et al. (1976b) tested 16 compounds related to ETU, including
ethylenethiuram monosulfide, another metabolite, and only one,
4-methylenethiourea, was teratogenic.
8.4. Mutagenicity
Tests with a large number of S. typhimurium strains gave
mostly negative results, though a few (weak) positive results
were observed in the case of some strains of S. typhimurium
(Shirasu et al., 1977). The addition of rat liver microsomes
seemed to enhance the mutant reversion. Schüpbach & Hummler
(1976, 1977) concluded that ETU appeared to induce base-pair
mutations but not frameshift mutations in S. typhimurium
TA 1530, although frameshift mutations appeared in S.
typhimurium TA 98, TA 1537, and TA 1538 when exposed to ETU in
the presence of dimethylsulfoxide (DMSO) and/or rat liver
microsomes (Rose et al., 1980).
Teramoto et al. (1977) did not find mutagenicity with S.
typhimurium TA 1536, TA 1537, TA 1538, G46, E. coli WP2 hcr+ and
hcr-, or B. subtilis H17 rec+ and rec- at concentrations
of 10 000 µg ETU/plate. However, a weak reaction was seen with
S. typhimurium TA 1535, and Seiler (1974) reported weak
(dose-unrelated) mutagenicity in S. typhimurium strain G46.
ETU was also found to be mutagenic in a host-mediated assay
of S. typhimurium TA 1530 when mice were dosed with 6000 mg
ETU/kg body weight, but not at doses of 2000 mg/kg or less
(Schüpbach & Hummler, 1977). Cytogenetic effects of ETU have
been reported in bone marrow cells of mice and Chinese hamsters.
On the other hand, there was no significant evidence to suggest
that ETU was mutagenic in host-mediated assays of S.
typhimurium G 46 or in tests with other strains of bacteria, rat
bone marrow (including the micronucleus test) Chinese hamster
DON cells, rat lymphocytes, or human fibroblast cells.
Furthermore, ETU did not increase the frequency of dominant
lethal mutations in rodents or Drosophila melanogaster (Seiler,
1973, 1974; Schüpbach & Hummler, 1977; Shirasu et al., 1977;
Teramoto et al., 1978b; Rose et al., 1980). A large number of
mutagenicity tests are summarized in a report of the US EPA
(1984).
ETU has been tested in the hepatocyte DNA repair test,
which is used to determine pro-carcinogenic potential as well as
DNA damage. ETU did not induce DNA damage (Althaus et al.,
1982) nor cause chromosomal damage in cultured rat liver cells,
and it did not induce chromatid exchange in CHO cells in
vitro or in mice in vivo. A micronucleus test with mice bone
marrow cells in vivo also gave negative results (De Serres &
Ashby, 1981).
This evidence indicates that ETU is generally not
mutagenic, especially in mammalian test systems.
8.5. Carcinogenicity
The carcinogenicity of ETU has been evaluated by IARC
(1974, 1982). It was classified in group 2B, i.e., limited
evidence for activity in short-term tests; sufficient evidence
for carcinogenicity in animals; inadequate evidence for
carcinogenicity in human beings.
ETU has been studied for oncogenic potential in mice, rats,
and hamsters.
8.5.1. Mouse
In a comprehensive programme screening chemicals for
carcinogenicity, two strains of hybrid mice (X and Y) were given
215 mg ETU/kg body weight from day 7 until weaning, and
thereafter 646 mg/kg diet for more than 18 months. In the X
strain [(C57B1/6XC3H/Anf)F1], the incidence of lung tumours in
the ETU-treated females was higher than that of the control
group (3/18 versus 3/87), but it was lower than that of the
controls (0/16 versus 1/90) in the Y strain [(C57B1/6XAKR)F1].
In the males, the incidence was higher in the ETU-treated
animals (3/18 versus 1/90). The incidence of lymphomas was
slightly increased in treated Y-strain females. The hepatoma
incidence in the ETU-treated groups of both strains was
significantly higher than that of the control group (in the X
strain, 14/18 and 18/18, for males and females respectively; in
the Y strain, 18/18 and 9/18; in controls, 0/18 and 3/18). The
thyroid glands were not examined for histopathological changes
(Innes et al., 1969).
Graham et al. (1975), found that ETU induced thyroid
hyperplasia and other research groups have confirmed this
finding.
8.5.2. Rat
Ulland et al. (1972) and Weisburger et al. (1981) fed
groups of 26 male and female Charles River-CD rats diets
containing 0, 175, or 350 mg ETU (97%)/kg diet. Five females
and five males of the high-dose group were killed after 18
months and the remainder after 24 months. Hyperplastic goitre,
solid cell adenomas, and thyroid (follicular or papillary)
carcinomas were found. Two of the animals also had lung
tumours, which might have been metastases. The thyroid tumour
incidence was dose dependant; (in the 175 mg/kg group, it was
3/26 and 3/26, for males and females, respectively, and in the
350 mg/kg group it was 17/26 and 8/26). No thyroid carcinomas
were observed in the control animals. A few of the treated rats
had hyperplastic nodules in the liver.
Graham et al. (1973, 1975) studied the long-term effects on
the thyroid gland of ETU ingestion. Five groups of 68 male and
68 female Charles River rats were fed ETU at levels of 0, 5, 25,
125, 250, or 500 mg/kg diet for 2 years. Growth depression was
evident at the highest dose level. The thyroid/body weight
ratio was significantly increased at 250 and 500 mg/kg, and
slightly increased at 125 mg/kg after 24 months. Thyroidal
uptake of 131iodine per mg tissue was significantly decreased in
male rats fed 500 mg ETU/kg diet for 18 or 24 months. The
thyroids of females fed at the three highest dose levels were
hypofunctioning at 6 months, and hyperfunctioning at 12 months,
and at 24 months thyroid function was similar to that of the
controls. At the two highest dose levels (250 and 500 mg/kg),
thyroid adenomas and carcinomas were induced. At all lower dose
levels hyperplasia occurred more frequently than in the
controls, but there were no adenomas or carcinomas. No increase
in liver tumours was observed in this study.
Gak et al. (1976) studied the effects of feeding rats with
0, 5, 17, 60, or 200 mg ETU/kg diet for 24 months. Body weight,
food consumption, serum enzyme activities (e.g. glutamic pyruvic
transaminase, alkaline phosphatase), hepatic enzyme activities
(glutamic pyruvic transaminase, alkaline phosphatase, glucose-6-
phosphate dehydrogenase), cholesterol levels, weights of thyroid
and other organs, and histopathology were studied.
Hypercholesterolemia was found at dose levels of 5 mg/kg and
above. At 60 mg/kg or more, a significant increase in thyroid
tumours was found, but at lower levels the tumour incidence was
not significantly different from that of the controls.
8.5.3. Hamster
Gak et al. (1976) studied the effect of 0, 5, 17, 60, or
200 mg ETU/kg diet on hamsters for 18 months. Growth, food
intake, biochemical parameters in the serum and liver, organ
weights, and histology were studied. A significant increase in
thyroid tumours was found at 60 mg ETU/kg or more, but at lower
doses values were not significantly different from those of the
control group.
8.6. ETU in Combination with Nitrite
When the mutagenicity of ETU was assayed before and after
nitrosation with sodium nitrite under acid conditions,
nitrosation was found to cause a 160-fold increase in the number
of revertant colonies of S. typhimurium TA 1535 (Shirasu et
al., 1977). The interactive mutagenicity of ETU and nitrite was
also found in the mouse dominant lethal test by Teramoto et al.
(1978b). However, no dominant-lethal mutations were induced in
a group of mice treated with 30 mg ETU plus 10 mg nitrite/kg
body weight. A large increase in pre-implantation losses was
noted 5 and 6 weeks after completing a 5-day treatment of males
with a combined oral dose of 150 mg ETU/kg and 50 mg sodium
nitrite/kg body weight.
8.7. Mechanisms of Toxicity; Mode of Action
The biochemical changes induced by antithyroid drugs
include reduced production of thyroid hormones (T3 and T4),
followed by increased production of TSH in response to low
thyroid hormone levels in the blood. Pathological changes in
the thyroid gland begin with diffuse microfollicular hyper-
plasia, and are followed by diffuse and nodular hyperplasia and
later by nodular hyperplasia with papillary and cystic changes
induced by the TSH. If hyperstimulation of the thyroid by TSH
is severe and prolonged, it provides conditions conducive to the
formation of tumours.
Antithyroid drugs can inhibit T4 production in various
ways. The chemical similarity of ETU to thiourea and thiouracil
suggests that ETU acts by blocking the iodination of thyroxine
precursors, thus reducing the synthesis of the thyroid hormones.
Iodide peroxidase catalyses the iodination of tyrosine and the
coupling of the resultant iodotyrosyl residues to produce the
active hormones T3 and T4.
Graham & Hansen (1972) found that ETU inhibited
iodide peroxidase in vitro. The resulting decreased level of
thyroid hormones causes stimulatory feedback of the pituitary
gland and consequently an increased release of TSH (Rose et al.,
1980).
Lu & Staples (1978) studied the influence of ETU in
pregnant hypothyroid and euthyroid rats to determine whether ETU
teratogenicity occurs as a result of altered maternal thyroid
function. Doses of 40 mg ETU/kg body weight, administered on
days 7 - 15 of gestation, resulted in 84 - 100% of the fetuses
in all treated groups being malformed, regardless of the thyroid
status of the dams. The authors concluded that the thyroid
status of the mother is not of importance in causing teratogenic
effects.
Rose et al. (1980) reported that the effects of feeding
rats 125 - 625 mg ETU/kg diet for 2 - 12 weeks, which included
thyroid hyperplasia and dose related suppression of serum T3 and
T4 (with corresponding TSH elevation), were reversible within 22
weeks of placing on control diets.
Long-term studies using ETU showed significantly increased
thyroid/body weight ratios in rats fed 125, 250, or 500 mg/kg
diet for periods of up to 2 years (Graham et al., 1975). This
effect was not reversed in rats placed on a control diet after
66 weeks of continuous exposure to 5 - 500 mg ETU/kg diet. It
is likely that by that time the thyroid was severely damaged.
In studies by Arnold et al. (1982, 1983), decreased levels
of serum thyroid hormones and increased thyroid weights were
reversed in Sprague Dawley rats fed diets containing 0, 75, 100,
or 150 mg ETU/kg diet for 7 weeks. The reversibility of
microscopic changes in the thyroids of male rats exposed to ETU
was studied. The rats were fed diets containing 75 or 150 mg
ETU/kg diet for 7 - 82 weeks and then returned to a control diet
for periods ranging from 2 to 42 weeks. The severity and extent
of reversibility of thyroid hyperplasia were found to depend on
the duration of exposure to ETU. Above a certain threshold,
hyperplasia did not regress significantly.
Numerous studies with ETU suggest that the rat is more
sensitive than other species to the effects of the thyroid. A
recent study with propylthiouracil, a thyroid inhibitor with a
mode of action similar to that of ETU, has confirmed that
monkeys are much less sensitive than rats. The sensitivity
difference was not quantified in vivo, but, in an in
vitro study, the concentration of inhibitor required to produce
the same level of thyroid peroxidase inhibition was
approximately 100 times greater for monkey enzyme than it was
for rat enzyme (Takayama et al., 1986).
8.8. Propineb and Propylenethiourea (PTU)
8.8.1. General
The toxicology of propineb was reviewed at JMPR meetings in
1977, 1980, and 1983. Because of concern expressed at the 1977
meeting regarding the potential for thyrotoxicity and
tumourigenicity of propylenethiourea (PTU), a breakdown product
of propineb, the meeting estimated only a temporary ADI for man.
Further evaluation of propineb was postponed pending the
submission of additional data. Data submitted for evaluation in
1985 consisted of long-term mouse and rat studies, mutagenicity
studies, and a special study into the effects of PTU on DNA. In
addition, data previously submitted for evaluation in 1983 were
re-examined. These data included several studies on propineb
(acute toxicity studies, a short-term study on thyroid function
in rats, mutagenicity studies, and an oncogenicity study on
mice) and on PTU (pharmacokinetic studies on rats and a long-
term thyroid function study on rats) (FAO/WHO, 1986a,b).
8.8.2. Toxicological information
An oncogenicity study on mice with propineb indicated
increased hepatocellular adenomas in male mice and increased
pulmonary adenomas in female mice at 800 mg/kg diet, the highest
dose level tested. Thyroid tumours were not induced in treated
mice in this study. A no-observed-adverse-effect level for non-
neoplastic effects could not be determined in this study, owing
to insufficient data (FAO/WHO, 1986a, b).
In a long-term study into the effects of PTU on mice, an
increased incidence in male mice of hepatocellular adenomas was
observed at 1000 mg/kg diet (the highest dose level tested) and
of hepatocellular carcinomas at 10 mg/kg diet or more. In the
same study, increased incidences of hepatocellular adenomas and
carcinomas were observed in female mice at 100 mg/kg diet or
more. Thyroid tumours attributable to PTU were not observed,
but increased thyroid hypercellularity was noted in male mice at
1000 mg/kg diet (FAO/WHO, 1986a, b).
In long-term rat studies with propineb, previously reviewed
by the JMPR, an increased incidence of benign thyroid tumours
was observed at 1000 mg/kg diet or more. Non-neoplastic thyroid
effects were observed in the same study at 100 mg/kg diet or
more. In another study, increased liver and kidney weights were
observed at 100 mg/kg diet or more and a no-observed-adverse-
effect level of 10 mg/kg diet was determined. In a long-term
study on the effects of PTU on rats, thyroid tumours
attributable to PTU were only found at 1000 mg/kg diet.
Goitrogenic effects in the thyroid were observed, however, at
dose levels as low as 1 mg/kg diet, the lowest dose level
tested. A no-observed-adverse-effect level could not be
determined in this study (FAO/WHO, 1986a, b).
Short-term studies on the effects of propineb on thyroid
function in rats did not establish an unequivocal no-observed-
adverse-effect level for effects on the thyroid. In a long-term
study with PTU, effects on thyroid function were observed at
1000 mg/kg diet, but at lower dose levels effects were
ambiguous. Pharmacokinetic studies on rats demonstrated
preferential uptake of radioactivity from 14C-labelled PTU by
the thyroid (FAO/WHO, 1986a, b).
Mutagenicity studies on propineb and PTU produced negative
or inconclusive results. However, PTU has been shown to
increase DNA synthesis in mouse spleen cells, but it did not
bind to mouse liver cell DNA.
In view of the carcinogenic response to PTU in the liver of
mice and the lack of a no-observed-adverse-effect level for the
effects of propineb on the thyroid in a long-term study on mice
or short-term studies on rats, or for PTU in a long-term study
on rats, the JMPR recommended that the temporary ADI for
propineb should be withdrawn.
In view of the established carcinogenic potential of this
compound, the meeting recommended that propineb should not be
used where its residues can arise in food (FAO/WHO, 1986a,b).
9. EFFECTS ON MAN
9.1. Epidemiological Studies
Smith (1976) conducted a detailed study involving 1929
workers in rubber-compounding plants in Birmingham, England. No
thyroid cancers were found in the health records of these
workers.
Clinical examinations and thyroid function tests were
carried out over a period of 3 years on eight process workers
and five mixers in a factory producing ETU in the United
Kingdom. Matched controls were also examined. The results
showed that the exposed mixers, but not the process workers, had
significantly lower levels of T4 in their blood compared with
the controls. No effect was found on TSH or thyroid-binding
globulin (Smith, 1984).
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) and
the International Agency for Research on Cancer (IARC) have
evaluated the toxicity and carcinogenicity data for various
dithiocarbamates on several occasions. Annex III includes an
overview of the JMPR meetings in which these compounds, ETU, and
PTU have been evaluated, with their references, together with
the WHO recommended classification of pesticides by hazard for
individual dithiocarbamates. The existence of IARC evaluations
and the availability of WHO/FAO Data Sheets and IRPTC Data
Profiles and Legal Files are also indicated. These documents
include more detail concerning the product and legal aspects,
toxicological evaluation, and residues of individual
dithiocarbamates in different food items.
REFERENCES
AASETH, J., ALEXANDER, J., & WANNAG, A. (1981) Effect of
thiocarbamate derivatives on copper, zinc, and mercury
distribution in rats and mice. Arch. Toxicol., 48: 29-39.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarbamates,
dithiocarbamates, Luxembourg, Commission of the European
Communities (Report No. V/F/1/78/75 EN).
ALLEN, J.R., VAN MILLER, J.P., & SEYMOUR, J.L. (1978)
Absorption, tissue distribution, and excretion of 14C-ETU in the
rhesus monkey and the rat. Chem. Pathol. Pharmacol., 20: 109-
115.
ALTHAUS, F.R., LAWRENCE, S.D., SATTLER, G.L., LONGFELLOW, D.G.,
& PITOT, H.C. (1982) Chemical quantification of unscheduled
DNA synthesis in cultured hepatocytes on an assay for the rapid
screening of potential chemical carcinogens. Cancer Res., 42:
3010-3015.
ALVAREZ, M., CREEKMORE, R.W., & ROSEN, R.T. (1973) Structure
of ethylenethiuram monosulfide. Tetrahedron Lett., 12: 939-942.
ANTONOVICH, E.A., CHERNOV, O.V., SAMOSH, L.V., MARTSON, L.V.,
PILINSKAYA, M.A., KURINNY, L.I., VEKSHTEIN, M.S., MARTSON, V.S.,
BALIN, P.N., & KHITSENKO, I.I. (1972) [A comparative toxico-
logical assessment of dithiocarbamates.] Gig. i. Sanit., 37: 25-
30 (in Russian).
ARNOLD, D.L., BICKIS, M.G., NERA, E.A., MCGUIRE, P.F., & MUNRO,
I.C. (1982) Assessing the reversibility of thyroid lesions
that result from feeding ETU. Toxicologist, 2(1): 90 (Abstract
of Presentation at the SOT Meeting 1982).
ARNOLD, D.L., KREWSKI, D.R., JUNKINS, D.B., MCGUIRE, P.F.,
MOODIE, C.A., & MUNRO, I.C. (1983) Reversibility of ethylene-
thiourea-induced thyroid lesions. Toxicol. appl. Pharmacol., 67:
264-273.
ASHWORTH, R., DE B., HENRIET, J., LOVETT, J.F., & MARTIJN, A.,
ed. (1980) CIPAC handbook - Volume 1A: Analysis of technical
and formulated pesticides, Cambridge, United Kingdom, W. Heffer
and Sons Limited. England.
AYANABA, A., VERSTRAETE, W., & ALEXANDER, M. (1973) Formation
of dimethylnitrosamine, a carcinogen and mutagen, in soils
treated with nitrogen compounds. Soil Sci. Soc. Am. Proc., 37:
565-568.
BABINI, G. (1966) [A case of erythroderma caused by zinc
dithiocarbamate.] Arch. Ital. Dermatol. Venereol. Sessuol., 34:
230/238 (in Italian).
BALIN, P.N. (1970) [Experimental data on the blastomogenic
activity of the fungicide maneb.] Vrach. Delo, 4: 21-24 (in
Russian).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest. Control, 9: 119-
127.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg., 38: 50-
52.
BENSON, W.R., ROSS, R.D., CHEN JO-YUN, R., BARRON, R.P., &
MASTBROOK, D. (1972) Structure of ethylenethiuram monosulfide.
J. Assoc. Off. Anal. Chem., 55(1): 44-46.
BLACKWELL-SMITH, R., Jr, FINNEGAN, J.K., LARSON, P.S., SAHYOUN,
P.F., DREYFUSS, M.L., & HAAG, H.B. (1953) Toxicologic studies
on zinc and disodium ethylenebisdithiocarbamates. J. Pharmacol.
exp. Therap., 109: 159-166.
BLAZQUEZ, C.H. (1973) Residue determination of ethylene-
thiourea (2-imidazol-idinethione) from tomato foliage, soil, and
water. J. agric. food Chem., 21: 330-332.
BOGARTYKH, T.A., ORLOV, N.V., & KUZNETSOVA, T.V. (1979)
Activity of lactate dehydrogenase and glucose-6-phosphate
dehydrogenase in tissue of the testes under the action of some
pesticides. Vopr. Pitan., 5: 49-53.
BONTOYAN, W.R. & LOOKER, J.G. (1973) Degradation of commercial
ethylenebisdithiocarbamate formulations to ethylenethiourea
under elevated temperature and humidity. J. agric. food Chem.,
21: 338-342.
BONTOYAN, W.R., LOOKER, J.B., KAISER, T.E., GIANG, P., & OLIVE,
B.M. (1972) Survey of ethylenethiourea in commercial
ethylenebisdithiocarbamate formulations. J. Assoc. Off. Anal.
Chem., 55: 923-925.
BOTTOMLEY, P., HOODLESS, R.A., & SMART, N.A. (1985) Review of
methods for the determination of ethylenethiourea (imida-
zolidine-2-thione) residues. Residue Rev., 95: 45-89.
CAVANAGH, J.B. (1973) Peripheral neuropathy caused by chemical
agents. Crit. Rev. Toxicol., 2: 365-417.
CHARKES, N.D., BRAVERMAN, L.E., PENKO, K.F., GOWERS, D.S.,
GORDON, C.F., LIPWORTH, L., & MALMUD, L.S. (1985) Thyroid
function in male workers manufacturing dithane, an agricultural
fungicide, and in men not exposed to dithane. In: Proceedings of
the Ninth International Thyroid Congress, Sao Paulo, Brazil,
1-6 September 1985, p. 89.
CHERNOFF, N., KAVLOCK, R.J., ROGERS, E.H., CARVER, B.D., &
MURRAY, S. (1979) Perinatal toxicity of maneb, ethylene-
thiourea, and ethylene bisisothiocyanate sulfide in rodents. J.
Toxicol. environ. Health, 5: 821-834.
CHERNOV, O.V. & KHISTENKO, I.I. (1969) Blastomogenic
properties of some derivatives of dithiocarbamic acid. Vopr.
Onkol., 15(4): 71-74.
CHERNOV, O.V. & KHISTENKO, I.I. (1973) Some data on the
distribution and elimination of ziram-35S from the organism of
warm-blooded animals. In: Hygiene of using toxicology of
pesticides and the clinical picture of intoxication, Moscow,
Medizina, Vol. 10, pp. 250-254. (in Russian).
CLARKE, D.G., BAUM, H., STANLEY, E.L., & HESTER, W.F. (1951)
Determination of dithiocarbamates. Anal. Chem., 23(12): 1842-
1846.
CONKIN, R.A. & GLEASON, L.S. (1964) Vegadex. In: Zweig, G.,
ed. Analytical methods for pesticides, plant growth regulation
and food additives, New York, London, Academic Press, Vol. 4,
pp. 249-257.
CRUICKSHANK, P.A. & JARROW, H.C. (1973) Ethylenethiourea
degradation. J. agric. food Chem., 21 (3): 333-335.
DALVI, R.R., POORE, R.E., & NEAL, R.A. (1974) Studies of the
metabolism of carbon disulfide by rat liver microsomes. Life
Sci., 14: 1785-1796.
DANIELSSON, B.R.G., OSKARSSON, A., & DENCKER, L. (1984)
Placental transfer and fetal distribution of lead in mice after
treatment with dithiocarbamates. Arch. Toxicol., 55: 27-33.
DAVYDOVA, T.B. (1973) [The influence of tetramethylthiuram
disulfide on the estrous cycle and genital function of animals
when inhaled.] Gig. i Sanit., 38(4): 108-110 (in Russian).
DEKHUYZEN, H.M., VONK, J.W., & KAARS SIJPESTEIJN (1971)
Terminal residues of dithiocarbamate fungicides. In: Tahori,
A.S., ed. Proceedings of the International Symposium on Pesti-
cide Terminal Residues, Tel Aviv, Israel, 1971, London,
Butterworth, pp. 233-241.
DE MATTEIS, F. & SEAWRIGHT, A.A. (1973) Oxidative metabolism
of carbon disulfide by the rat. Effect of treatments which
modify the liver toxicity of carbon disulfide. Chem.-biol.
Interact., 7: 375-388.
DE SERRES, F.S. & ASHBY, J.C. (1981) Report of the
International Collaborative Program for Progress in Mutation
Research, Vol. 1, Amsterdam, Oxford, New York, Elsevier North-
Holland Inc.
DISHOVSKI, H. & IVANOVA-CHEMISHANSKA, L. (1979) Ultra-
structural changes in the neocortex of rats after chronic
dithiocarbamate Antracol. Activ. nerv. sup. (Praha), 21 (4):
279.
EDINGTON, N. & HOWELL, J.McC. (1966) Changes in the nervous
system of rabbits following the administration of sodium
diethyldithiocarbamate. Nature (Lond.), 210(5040): 1060-1062.
EDINGTON, N. & HOWELL, J.McC. (1969) The neurotoxicity of
sodium diethyldithiocarbamate in the rabbit. Acta neuropathol.
(Berlin), 12: 339-347.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N-nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under simulated gastric conditions and in vivo in the rat
stomach. Food Cosmet. Toxicol., 12: 229-232.
ELDJARN, L. (1950) The metabolism of tetraethylthiuram
disulfide (Antabuse, Aversan) in the rat, investigated by means
of radioactive sulfur. J. clin. lab. Invest., 2: 198-201.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ENGST, R. & SCHNAAK, W. (1967) [Studies on the metabolism of
the ethylenebisdithiocarbamate fungicides maneb and zineb.] Z.
Lebensm. Unters. Forsch., 134(4): 216-221 (in German).
ENGST, R. & SCHNAAK, W. (1969a) [Polarographic determination
of ethylene bisthiuram monosulfide.] Z. anal. Chem., 248: 321-
324 (in German).
ENGST, R. & SCHNAAK, W. (1969b) [Analysis of ethylene
bisthiuram monosulfide.] Z. anal. Chem., 244: 254-256 (in
German).
ENGST, R. & SCHNAAK, W. (1969c) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb
(polarographic determination of the undecomposed fraction of
active ingredient).] Z. Lebensm. Unters. Forsch., 139(3): 149-
152 (in German).
ENGST, R. & SCHNAAK, W. (1970a) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 143: 99-103 (in German).
ENGST, R. & SCHNAAK, W. (1970b) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 144(2): 81-85 (in German).
ENGST, R. & SCHNAAK, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev.,
52: 45-67.
ENGST, R., SCHNAAK, W., & LEWERENZ, H.J. (1971) [Studies on
the metabolism of the fungicidal
ethylenebisdiothiocarbamatesmaneb, zineb and nabam. V. The
toxicology of breakdown products.] Z. Lebensm Untersuch., 146,
91-97.
FAHRIG, R. (1974) Comparative mutagenicity studies with
pesticides. In: Montesano, R. & Tomatis, L., ed. Chemical
carcinogenesis assays, Lyons, International Agency for Research
on Cancer, pp. 161-181 (IARC Scientific Publication No. 10).
FAO (1985) Production yearbook 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO Meeting
Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL: 1967/M/1/1; WHO
Food Add./68.30).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO
Food Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 97; WHO Technical Report Studies No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10
Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations(FAO Plant Production and Protection Paper No. 10 Sup.).
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization (FAO
Plant Production and Protection Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20
Sup.).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 26 Sup.).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues
in food, Part II - Toxicology, Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 72).
FARROW, R.P. & RALLS, J.W. (1970) Investigation of changes in
pesticide residues during processing and storage of fruit and
vegetables, National Canners Association, 109 pp (Report No.
5).
FISHBEIN, L. (1975) Chromatography of environmental hazards.
III. Pesticides, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
FITZHUGH, O.G., WINTER, W.J., & NELSON, A.A. (1952) Some
observations on the chronic toxicity of antabuse (tetraethyl-
thiuramdisulfide). Fed. Proc., 11: 345-346.
FREUDENTHAL, R.I., KERCHNER, G.A., PERSING, R.L., BAUMEL, J., &
BARON, R.L. (1977) Subacute toxicity of ethylene bisisocyanate
sulfide in the laboratory rat. J. Toxicol. environ. Health, 2:
1067-1077.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Différence de
sensibilité du hamster et du rat vis-ą-vis des effets de
l'administration ą long terme de l'éthylčne thiourée. Eur. J.
Toxicol., 9: 303-312.
GARDNER-THORPE, C. & BENJAMIN, S. (1971) Peripheral neuropathy
after disulfiram administration. J. Neurol. Neurosurg.
Psychiatr., 34: 253-259.
GESSNER, T. & JAKUBOWSKI, M. (1972) Diethyldithiocarbamic acid
methyl ester, a metabolite of disulfiram. Biochem. Pharmacol.,
21: 219-230.
GOWERS, D.S. & GORDON, C.F. (1980) Some public health aspects
of the manufacture and use of zinc and manganese
ethylenebisdithiocarbamate fungicides Poznan, Poland, Institut
Ochrony Roslin, February 1979 pp. 491-522.
GRAHAM, S.L. & HANSEN, W.H. (1972) Effects of short-term
administration of ethylenethiourea on thyroid function of the
rat. Bull. environ. Contam. Toxicol., 7: 19-25.
GRAHAM, S.L., DAVIS, K.J., & PERRY, C.H. (1973) Effects of
one-year administration of ethylenethiourea upon thyroid
function of the rat. J. agric. food Chem., 21(3): 324-329.
GRAHAM, S.L., DAVIS, K.J., HANSEN, W.H., & GRAHAM, C.H. (1975)
Effects of prolonged ethylenethiourea ingestion on the thyroid
of the rat. Food Cosmet. Toxicol., 13: 493-499.
GRAHAM, W.H. & BORNAK, W.E. (1973) Improved experimental
technique for reverse isotope dilution method.
Anal. Chem., 45: 623-624.
GRIEPENTROG, F. (1960) [Allergy studies with simple chemical
substances.] Arzneimittelforschung, 10: 542-544 (in German).
GRIEPENTROG, F. (1962) [Tumor-like thyroid lesions in animal
studies of chronic toxicity of thiurams.] Beitr. Pathol.
Anat., 126: 243-255 (in German).
GUSTAFSSON, K.H. & THOMPSON, R.A. (1981) High-pressure liquid
chromatographic determination of fungicidal dithiocarbamates. J.
agric. food Chem., 29: 729-732.
HAINES, L.D. & ADLER, I.L. (1973) Gas chromatographic deter-
mination of ethylenethiourea residues. J. Assoc. Off. Anal.
Chem., 56 (2): 333-337.
HALD, J. & JACOBSEN, E. (1948) The formation of acetaldehyde
in the organism after ingestion of Antabuse (tetraethylthiuram
disulfide) and alcohol. Acta pharmacol. toxicol., 4: 305-310.
HALD, J., JACOBSEN, E., & LARSEN, V. (1948) The sensitizing
effect of tetraethylthiuram disulphide (Antabuse) to ethyl
alcohol. Acta pharmacol. toxicol., 4: 285-296.
HEDENSTEDT, A., RANNUG, U., LAMEL, C., & WACHTMEISTER, C.A.
(1979) Mutagenicity and metabolism studies on 12 thiram and
dithiocarbamate compounds used as accelerators in the Swedish
rubber industry. Mutat. Res., 68: 313.
HELLING, C.S., DENNISSON, D.C., & KAUFMAN, D.D. (1974)
Fungicide movement in soil. Phytopathology, 64(8): 1091-1100.
HOAGLAND, R.E. & FREAR, S.D. (1976) Behaviour and fate of
ethylenethiourea in plants. J. agric. food Chem., 24: 129-133.
HODGE, H.C., MAYNARD, Z.A., DOWNS, W., BLANCHET, H.J., Jr, &
JONES, C.K. (1952) Acute and short-term oral toxicity tests of
ferric dimethyldithiocarbamate (ferbam) and zinc dimethyldithio-
carbamate (ziram). J. Am. Pharmacol. Assoc., 41(12): 662-665.
HODGE, H.C., MAYNARD, E.A., DOWNS, W.L., COYE, R.D., Jr, &
STEADING, L.T. (1956) Chronic oral toxicity of ferric di-
methyldithiocarbamate (ferbam) and zinc dimethyldithiocarbamate
(ziram). J. Pharmacol. exp. Ther., 118: 174-181.
HODGSON, J.R., CASTLES, T.R., MURRILL, E., & LEE, C.C. (1974)
Distribution, excretion, and metabolism of the fungicide ferbam
in rats. Fed. Proc., 33: 537.
IARC (1974) Some antithyroid and related substances, nitro-
furans and industrial chemicals, Lyons, International Agency for
Research on Cancer, pp. 45-52 (Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 7).
IARC (1976) Some carbamates, thiocarbamates, and carbazides,
Lyons, International Agency for Research on Cancer (Monographs
on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol.
12).
IARC (1977) Some miscellaneous pharmaceutical substances,
Lyons, International Agency for Research on Cancer p. 243.
(Monographs on the evaluation of carcinogenic risk of chemicals
to humans, Vol. 13).
IARC (1983) Chemicals, industrial processes and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 128-130 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumour-
igenicity in mice: a preliminary note. J. Natl Cancer Inst., 42:
1101-1114.
IRPTC (1982) Data profile on ziram, Geneva, International
Register for Potentially Toxic Chemicals.
IRPTC (1983) Legal file - 2 vols, Geneva, International
Register for Potentially Toxic Chemicals.
IUPAC (International Union of Pure and Applied Chemistry) (1977)
Ethylenethiourea. Pure appl. Chem., 49: 675-689.
IVANOVA-CHEMISHANSKA, L. (1969a) Toxicological characteristics
of dithiocarbamates zineb, maneb, and mancozeb, Sofia,
(Dissertation).
IVANOVA-CHEMISHANSKA, L. (1969b) Toxicological characteristics
of mancozeb. Hig. i. Zdraveopazvane, 12(5): 417-426.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads reproduction of white rats under the effect of some
pesticides. Arch. Toxicol., Suppl. 4: 459-462.
IVANOVA-CHEMISHANSKA, L., SHEYTANOV, M., & MOSHEVA-IZMIROVA, N.
(1967) Changes in the functional state of the thyroid gland
upon acute intoxication with certain dithiocarbamates: zineb and
maneb. Med. Toxicol., 20(9): 1011-1013.
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., & DASHEV, G. (1971)
Light and electron microscopic observation on rat thyroid after
administration of some dithiocarbamates. Environ. Res., 4: 201-
212.
IVANOVA-CHEMISHANSKA, L., KALOYANOVA, F., IZMIROVA, N., ZLATEVA,
M., & VALCHEVA, V. (1972) [Experimental inhalatory toxicity and
substantiation of the maximum allowable concentration for some
dithiocarbamates in the air of the working area.] Truh. Dzihot.,
23: 55-64 (in Russian).
IVANOVA-CHEMISHANSKA, L., VALCHEVA, V., & TAKEVA, TZ. (1973)
Effect of chronic peroral poisoning with perozine (zineb) on the
reproduction of white rats. Acta med. soc., 1: 107-108.
IVANOVA-CHEMISHANSKA, L., MILANOV, ST., CHEMISHANSKI, G., &
DASHEV, G. (1974) Attempt of biological dose of some
hypophyseal hormones in white rats after experimental oral
administration of zineb. Environ. Res., 8: 160-165.
IVANOVA-CHEMISHANSKA, L., PETROVA-VERGIEVA, T., & MIRKOVA, E.
(1975a) [Embryotoxic and teratogenic action of some
pesticides.] Eksp. Med. Morfol., 14(1): 29-33 (in Russian).
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., MILANOV, S.,
STRASHIMIROV, D.D., DASHEV, G.I., & CHEMISHANKSKA, G.A. (1975b)
Effects of subacute oral administration of zinc ethylene bisdi-
thiocarbamate on the thyroid gland and adenohypophysis of the
rat. Food Cosmetol. Toxicol., 13: 445-447.
IZMIROVA, N. & MARINOV, V. (1972) [Distribution and excretion
of 35S-ziram and metabolic products after 24 hours following
oral administration of the preparation in female rats.] Eksp.
Med. Morfol., 11(3): 152-156 (in Russian).
IZMIROVA, N., IZMIROVA, I., & IVANOVA, L. (1969) The effect of
zineb and maneb on the isoenzymes of lactate dehydrogenase in
the testes of rats. C. R. Acad. Bulg. Sci., 22(2): 225-227.
JORIS, S.J., ASPILA, K.I., & CHAKRABARTI, C.L. (1970) Decompo-
sition of monoalkyl dithiocarbamates. Anal. Chem., 42(6): 647-
651.
KAARS SIJPESTEIJN, A.K. & VONK, J.W. (1970) Microbial conver-
sion of dithiocarbamate fungicides. Med. Fac. Landbouwwet.
Gent, 35(2): 799-804.
KALOYANOVA, F., IVANOVA, L., & ALEXIEV, B. (1967) The
influence of large doses of maneb on the progeny of albino rats.
Med. Toxicol., 20(10): 1109-1112.
KAMPMEIER, C. & HAAG, H.B. (1954) Toxicological considerations
in use of dithiocarbamates. Agric. Chem., April: 49-50, 133.
KASKEVICH, L.M., KOMAROVA, L.I., ILINA, V.I., IVANICKI, V.A., &
SHILINA, V.F. (1981) [Clinical-functional changes due to
exposure to zineb,] pp. 109-111 (in Russian).
KASLANDER, J. (1963) Formation of an S-glucuronide from
tetraethylthiuram disulfide (Antabuse) in man. Biochem. Biophys.
Acta, 71: 730-732.
KATO, Y., ODANAKA, Y., TERAMOTO, S., & MATANO, O. (1976) Meta-
bolic fate of ethylenethiourea in pregnant rats. Bull. environ.
Contam. Toxicol., 16(5): 546-555.
KAUFMAN, D.D. & FLETCHER, C.L. (1973) Title? In: Proceedings
of the Second International Congress on Plant Pathology,
Minneapolis, Minnesota (Abstract No. 1018).
KHERA, K.S. (1973) Ethylenethiourea: teratogenicity study in
rats and rabbits. Teratology, 7: 243-252.
KHERA, K.S. & TRYPHONAS, L. (1977) Ethylenethiourea induced
hydrocephalus: pre- and postnatal pathogenesis in offspring from
rats given a single oral dose during pregnancy. Toxicol. appl.
Pharmacol., 42: 85-97.
KIM, S.U. & RIZZUTO, N. (1975) Neuroaxonal degeneration
induced by sodium diethyldithiocarbamate in cultures of central
nervous tissue. J. Neuropath. exp. Neurol., 34: 531-541.
KING, R.R. (1977) Derivatization of ethylenethiourea with m-
trifluoromethylbenzylchloride for analysis by electron capture
gas chromatography. J. agric. food Chem., 25(1): 73-75.
KIRKBRIGHT, G.F. & MULLINS, F.G.P. (1984) Separation of
dithiocarbamates by high-performance liquid chromatography using
a micellar mobile phase. Analyst, 109: 493-496.
KLIGMAN, A.M. & ROSENSWEIG, W. (1948) Studies with new
fungistatic agents. I. For the treatment of superficial
mycoses. J. invest. Derm., 10: 59-68.
KOIZUMI, A., SHIOJIMA, S., OMIYA, M., NAKANO, S., SATO, N., &
IDEDA, M. (1979) Acute renal failure and maneb (manganous
ethylene bisdithiocarbamate) exposure. J. Am. Med. Assoc.,
242(23): 2583-2585.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982a) Embryo-
toxicity of industrial chemicals on the chicken embryo:
thiourea derivatives. Acta pharmacol. toxicol., 51: 38-44.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982b) Application
of the chicken embryo in testing for embryotoxicity. Scand. J.
Work environ. Health, 8: 63-69.
KURINNY, A.I. & KONDRATENKO, T.I. (1972) [Effect of some
fungicides (dithiocarbamic acid derivatives) on chromosomes of
bone marrow cells in mice.] Tsitol. Genet., 6: 225-228 (in
Russian).
LABORIE, F. & LABORIE, R. (1966) Allergies aux fongicides et
prophylaxie biochimique (relations entre allergie et
intoxications). Rev. Pathol. comp., 66: 105-109.
LAKOMAA, E.L., SATO, S., GOLDBERG, A.M., & FRAZIER, J.M. (1982)
The effect of sodium diethyldithiocarbamate treatment on copper
and zinc concentrations in rat brain. Toxicol. appl. Pharmacol.,
65: 286-290.
LANGE, P. & JUNG, F. (1971) [Reduction of the toxicity to the
liver of carbon tetrachloride by diethyldithiocarbamate and 3-
(O-tolyl)-4-(nitro)-sydnone.] Acta biol. med. Ger., 27: 425-434
(in German).
LARSSON, K.S., ARNANDER, C., CEKANOVA, E., & KJILLBERG, M.
(1976) Studies of teratogenic effects of the dithiocarbamates
maneb, mancozeb, and propineb. Teratology, 14: 171-183.
LEE, C.C. & PETERS, P.J. (1976) Neurotoxicity and behavioural
effects of thiram in rats. Environ. Health Perspect., 17: 35-
42.
LEHMAN, A.J. (1951) Chemicals in food: a report of the
association of food and drug officials on current developments,
II, Pesticides, Q. Bull. Fed. Drug Off.U.S., 15: 122-133.
LEWERENZ, H.J., BLEYL, D., PLASS, R., & ENGST, R. (1975)
Toxicology of ETU , Potsdam, Rehbrüke, German Democratic
Republic, Central Institute for Nutrition, Academy of Science.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972) Carcino-
genic nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LOWY, R., GRIFFATON, G., BRIGANT, L., ARDONIN, B., & DUPUY, F.
(1979) The dietary no-effect level of a dithiocarbamate
fungicide, thiram, as evaluated from measurement data on rats.
II. The various sensitivities of the various parameters.
Toxicology, 14: 39-53.
LOWY, R., GRIFFATON, G., & PELISIER, M.A. (1980) The dose-
response relationship of rat organ weights under dietary thiram
influence. Toxicol. Lett., 5(Suppl. 1): 148.
LU, M.H. & STAPLES, R.E. (1978) Teratogenicity of ethylene-
thiourea and thyroid function in the rat. Teratology, 17: 171-
178.
LUDWIG, R.A. & THORN, G.D. (1962) Chemistry and mode of action
of dithiocarbamate fungicides. Adv. Pestic. Control Res., 3:
219-252.
LUTZ, L.M., GLENDA, E.A., Jr, & RECKNAGEL, R.O. (1973) Protec-
tion by diethyldithiocarbamate against carbon tetrachloride
lethality in rats and against carbon tetrachloride-induced lipid
peroxidation in vitro. Biochem. Pharmacol., 22: 1729-1734.
LYMAN, W.R. (1971) The metabolic fate of Dithan M-45
(coordination product of zinc ion and manganous ethylene
bisdithiocarbamate). In: Tahori, A.S., ed. International
Symposium on Pesticide Terminal Residues, Tel Aviv, 1971,
Israel, London, Butterworth, pp. 243-256.
LYMAN, W.R. & LACOSTE, R.J. (1974) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
In: Proceedings of the 3rd International IUPAC Congress on
Pesticide Chemistry, Helsinki, 3-9 July, 1974, Stuttgart, George
Thieme Publishers, pp. 67-74.
LYMAN, W.R. & LACOSTE, R.J. (1975) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
Environ. Qual. Saf., Suppl. 3: 67-74.
McCANN, J. & PITCHER, F. (1973) Fish toxicity laboratory
reports Nos. 535 and 541, Beltsville, Maryland, US Environmental
Protection Agency, Animal Biology Laboratory.
MAJ, J., GRABOWSKA, M., & KWIEK, J. (1970) The effect of
disulfiram, diethyldithiocarbamate, and dimethyldithiocarbamate
on serotonin and 5-hydroxyindole-3-acetic acid brain levels in
rats. Biochem. Pharmacol., 19: 2517-2519.
MARSHALL, W.D. (1977) Thermal decomposition of ethylene
bisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. agric. food Chem., 25: 357-361.
MASSEY, R.C., KEY, P.E., & MCWEENY, D.J. (1982) Analysis of
ethylenethiourea in beer by high-performance liquid
chromatography. J. Chromatogr., 240: 254-256.
MATOKHNYUK, L.A. (1971) Toxicity of the fungicide maneb on
inhalation. Hyg. Sanit., 36(5): 195-199.
MATTHIASCHK, G. (1973) [Influence of L-cysteine on
teratogenisis caused by thiram (TMTD) in NMRI mice.] Arch.
Toxikol., 30: 251-262 (in German).
MELNIKOV, N.N. & TRUNOV, P.P. (1966) Persistence of residual
quantities of preparations on the basis of dithiocarbamate acid
derivatives. Chem. Agric. IV, 10(36): 21-24.
MINOR, J.L., RUSSEL, J.Q., & LEE, C.C. (1974) Reproduction and
teratology studies with the fungicide ferbam. Toxicol. appl.
Pharmacol., 29(16): 120.
MOJE, W., MUNNECKE, D.E., & RICHARDSON, L.T. (1964) Carbonyl
sulfide, a volatile fungitoxicant from nabam in soil. Nature
(Lond.), 202: 831-832.
NASH, R.G. (1974) Improved gas-liquid chromatographic method
for determining ethylenethiourea in plants. J. Assoc. Off. Anal.
Chem., 57(5): 1015-1021.
NASH, R.G. (1975) In: Proceedings of the 170th Meeting of the
American Chemical Society for Pesticides (Abstract No. 1).
NASH, R.G. & BEALL, M.L., Jr (1980) Fate of maneb and zineb
fungicides in microagroecosystem chambers. J. agric. food Chem.,
28: 322-330.
NEWSOME, W.H. (1972) Determination of ethylenethiourea
residues in apples. J. agric. food Chem., 20: 967-969.
NEWSOME, W.H. (1974) The excretion of ethylenethiourea by rat
and guinea-pig. Bull. environ. Contam. Toxicol., 11: 174-176.
NEWSOME, W.H. (1976) Residues of four ethylene bisdithio-
carbamates and their decomposition products on field-sprayed
tomatoes. J. agric. food Chem., 24(5): 999-1001.
NEWSOME, W.H. (1978) A method for the determination of
ethyleneurea in foods as the pentafluorobenzamide. J. agric.
food Chem., 26(6): 1325-1327.
NEWSOME, W.H. & LAVER, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb-treated foods. Bull.
environ. Contam. Toxicol., 10(3): 151-154.
ODEYEMI, O. (1980) Persistence of arasan pesticide in aquatic
ecosystems in Nigeria. Pestic. Abstr., 80: 1276.
ONLEY, J.H. (1977) Gas-liquid chromatographic method for
determining ethylenethiourea in potatoes, spinach, apple sauce,
and milk. Collaborative study. J. Assoc. Off. Anal. Chem.,
60(5): 1111-1115.
ONLEY, J.H. & YIP, G. (1971) Determination of ethylenethiourea
residues in foods using thin-layer and gas chromatography. J.
Assoc. Off. Anal. Chem., 54(1): 165-169.
OSKARSSON, A. (1983) Redistribution and increased brain uptake
of lead in rats after treatment with diethyldithiocarbamate.
Arch. Toxicol., 6: 279-284.
OSKARSSON, A. (1984) Dithiocarbamate induced redistribution
and increased brain uptake of lead in rats. Neurotoxicology,
5(3): 283-294.
OSKARSSON, A. & LIND, B. (1985) Increased lead levels in brain
after long-term treatment with lead and dithiocarbamate or
thiuram derivatives in rats. Acta pharmacol. toxicol., 56: 1-6.
OTTO, S., KELLER, W., & DRESCHER, N. (1977) A new gas
chromatographic determination of ethylenethiourea residues
without derivatization. J. environ. Sci. Health, B12(3): 179-
191.
PECKA, Z., BAULU, P., & NEWSOME, H. (1975) Preliminary survey
of ethlenethiourea residues in the Canadian food supply, 1972.
Pestic. monit. J., 8(4): 232-234.
PERIQUET, A. & DERACHE, R. (1976) Influence d'un régime
alimentaire hypoprotéique sur la toxicité aigue d'un pesticide,
le nabame. Ann. Nutr. Aliment., 30: 29-44.
PETROVA-VERGIEVA, T. (1976) On the teratogenic activity of
zinc-propylene bisdithiocarbamate. Hig. i. Zdraveopazvane,
19(5): 435-442.
PETROVA-VERGIEVA, T. & IVANOVA-CHEMISHANSKA, L. (1973)
Assessment of the teratogenic activity of dithiocarbamate
fungicides. Food Cosmet. Toxicol., 11: 239-244.
PHILLIPS, W.E.J. (1968) Changes in the nitrate and nitrite
contents of fresh and processed spinach during storage. J.
agric. food Chem., 16: 88-91.
PHILLIPS, W.F., GRADY, M.D., & FREUDENTHAL, R. (1977) Effects
of food processing on residues of ethylene bisdithiocarbamate
fungicides and ethylenethiourea, Washington DC, US Environmental
Protection Agency (EPA No. 600/1-77-021).
PILINSKAYA, M.A. (1971) [Cytogenetic effect of the fungicide
ziram in the culture of human lymphocytes in vitro. ] Genetika,
7: 138-143 (in Russian).
PLUYGERS, C.W., VONK, J.W., & THORN, G.D. (1971) Re-
examination of the structure of ethylenethiuram monosulfide.
Tetrahedron Lett., 18: 1317-1318.
PRZEZDZIECKI, Z., BANKOWSKA, J., KOMOROWSKA-MALEWSKA, W., &
JANICKA, T. (1969) Studies of the chronic short-term toxicity
of zineb, maneb, and kaptan. Rocz. Panstw. Zakl. Hlg., 20: 133-
140.
PRICKETT, C.S. & JOHNSTON, C.D. (1953) The in vivo production
of carbon disulfide from tetraethylthiuram disulfide
(AntabuseR). Biochem. Biophys. Acta, 12: 542-546.
PRINCE, J.L. (1985) Analysis of ethylenethiourea in urine by
high-performance liquid chromatography. J. agric. food Chem.,
33: 93-94.
RAGHU, K., MURTHY, N.B.K., KUMARASWAMY, R., RAO, R.S., & SANE,
P.V. (1975) In: Proceedings of the Joint FAO/IAEA, Division of
Atomic Energy in Food and Agriculture, Vienna, p. 137.
RASUL, A.R. & HOWELL, J.M. (1974) The toxicity of some
dithiocarbamate compounds in young and adult domestic fowl.
Toxicol. appl. Pharmacol., 30: 63-78.
RHODES, R.C. (1977) Studies with manganese 14C-ethylene
bisdithiocarbamate (14C-maneb) fungicide and 14C-ethylenethio-
urea (14C-ETU) in plants, soil, and water. J. agric. food Chem.,
25(3): 528-533.
RJAZANOVA, R.A. (1967) [The effects of the fungicides ziram and
zineb on the generative function of test animals.] Gig. i
Sanit., 2: 26-30 (in Russian).
ROLANDI, A., DE MARINIS, E., & DE CATERINA, M. (1984) Dithio-
carbamate pesticides: activity of propineb in the micronucleus
test in mice. Mutat. Res., 135: 193-197.
ROLL, R. (1971) [Teratological experiments with thiram (TMTD)
on two mouse strains.] Arch. Toxikol., 27: 173-186 (in German).
ROSE, D., PEARSON, C.M., ZUKER, M., & ROBERTS, J.R. (1980)
Ethylenethiourea: criteria for the assessment of its effects on
man, Ottawa, National Research Council, Associate Committee on
Scientific Criteria for Environmental Quality (NRCC Publication
No. 18469).
ROSS, R.D. & CROSBY, D.G. (1973) Photolysis of ethylene-
thiourea. J. agric. food Chem., 21(3): 335-337.
ROSS HART, E. & VALERIO, M.G. (1973) Evaluation of goitrogenic
activity in rats: Ethylene thiourea, practical and Dithane M-45,
Communication from Litton Bionetics to Rohm and Haas Co. Cited
in Van Leeuwen et al., (1982)
RUDDICK, J.A., WILLIAMS, D.T., HIERLIHY, L., & KHERA, K.S.
(1976a) 14C-ethylenethiourea: distribution, excretion, and
metabolism in pregnant rats. Teratology, 13: 35-39.
RUDDICK, J.A., NEWSOME, W.H., & NASH, L. (1976b) Correlation
of teratogenicity and molecular structure: ethylenethiourea and
related compounds. Teratology, 13: 263-266.
SATO, F. & TOMIZAWA, C. (1960) Decomposition of zinc ethylene
bisdithiocarbamate (zineb) applied to plants. Bull. Natl Inst.
Agric. Sci. (Tokyo) Ser. C, 12: 181-187.
SCHULTHEISS, E. (1957) [The therapeutic use of
tetramethylthiuram disulfide.] Dermatol. Wochenschr., 7: 160-162
(in German).
SCHUPBACH, M. & HUMMLER, H. (1976) Evaluation of the
mutagenicity of ethylenethiourea using bacterial and mammalian
test systems. Mutat. Res., 28: 122.
SCHUPBACH, M. & HUMMLER, H. (1977) A comparative study on the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutat. Res., 56: 111-120.
SEIDLER, H., HARTIG, M., SCHNAAK, W., & ENGST, R. (1970)
[Studies on the metabolism of certain insecticides and
fungicides in the rat. II. Mean distribution and breakdown of
14C-labelled maneb.] Nahrung, 14(5): 363-373 (in German).
SEIFTER, J. & EHRICH, W.E. (1948) Goitrogenic compounds:
pharmacological and pathological effects. J. Pharmacol. exp.
Ther., 92: 303-314.
SEILER, J.P. (1973) A survey on the mutagenicity of various
pesticides. Experientia, (Basel) 29: 622-623.
SEILER, J.P. (1974) Ethylenethiourea (ETU): a carcinogenic and
mutagenic metabolite of ethylene bisdithiocarbamate. Mutat.
Res., 26: 189-191.
SEN, N.P., DONALDSON, B.A., & CHARBONNEAU, C. (1974) Formation
of nitrosodimethylamine form the interaction of certain
pesticides and nitrite. In: Bogorski, P., Walker, E.A., & Davis,
W., ed. N-nitroso compounds in the environment, Lyons,
International Agency for Research on Cancer, pp. 75-79 (IARC
Scientific Publication No. 9).
SHERMAN, H. & ZAPP, J.A. (1966) Three generation reproduction
study, Manzate DR (88% maneb). Unpublished report from Haskell
Laboratory submitted to the Word Health Organization by E.I. du
Pont de Nemours and Co.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H.,
TERAMOTO, S., & KADA, T. (1977) Mutagenicity screening on
pesticides and modification products: a basis of carcinogenicity
evaluation. In: Hiatt, H.H., Watson, J.D., & Winsen, J.A.,
ed. Origins of human cancer, Book A, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, Vol.4, pp. 267-285.
SHORT, R.D., Jr, RUSSEL, J.Q., MINOR, J.L., & LEE, C.C. (1976)
Developmental toxicity of ferric dimethyldithiocarbamate and
bis(dimethylthiocarbamoyl) disulfide in rats and mice. Toxicol.
appl. Pharmacol., 35: 83-94.
SHORT, R.D., MINOR, J.L., UNGER, T.M., BREEDEN, B., VAN GOETHEM,
D., & LEE C.C. (1980) Teratology of a zineb formulation.
(Results of a study performed at Midwest Research Institute for
the US Environmental Protection Agency submitted to the World
Health Organization) (EPA-600/1-80-017).
SMITH, D. (1976) Ethylenethiourea: a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.,
26: 92-94.
SMITH, D.M. (1984) Ethylenethiourea: thyroid function in two
groups of exposed workers. Br. J. ind. Med., 41: 362-366.
SPRECHER, D. & GRIGOROWA, R. (1967) [Maximum workplace
concentration of thiuram.] Z. gesamte Hyg., 13: 241-245 (in
German).
STROMME, J.H. (1965) Metabolism of disulfiram and diethyl-
dithiocarbamate in rats with demonstration of an in vivo
ethanol-induced inhibition of the glucuronic acid conjugation of
the thiol. Biochem. Pharmacol., 14: 393-410.
STROMME, J.H. & ELDJARN, L. (1966) Distribution and chemical
forms of diethyldithiocarbamate and tetraethylthiuram disulfide
(disulfiram) in mice in relation to radioprotein. Biochem.
Pharmacol., 15: 287-297.
SUNDERMAN, F.W., Jr & FRASER, C.B. (1983) Effects of nickel
chloride and diethyl dithiocarbamate on metallothionein in rat
liver and kidney. Ann. clin. lab. Sci., 13 (6): 489-495.
SZYMEZYK, T. (1981) The mutagenicity of the fungicide thiram.
Mutat. Res., 85: 223-224.
TAKAYAMA, S., AIHARA, K., ONODERA, T., & AKIMOTO, T. (1986)
Antithyroid effects of propylthiouracil and sulfamonomethoxine
in rats and monkeys. Toxicol. appl. Pharmacol., 82: 191-199.
TANNENBAUM, S.R., SINSKEY, A.J., WEISSMAN, M., & BISHOP, W.
(1974) Nitrite in human saliva: its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TERAMOTO, S., MORIYA, M., KATO, K., TEZUKA, H., NAKAMURA, S.,
SHINGU, A., & SHIRASU, Y. (1977) Mutagenicity testing on
ethylenethiourea. Mutat. Res., 56: 121-129.
TERAMOTO, S., SHINGU, A., KANEDA, M., & SAITO, R. (1978a)
Teratogenicity studies with ethylenethiourea in rats, mice, and
hamsters. Congenital Anom., 18: 11-17.
TERAMOTO, S., SHINGU, A., & SHIRASU, Y. (1978b) Induction of
dominant lethal mutations after administration of ethylene-
thiourea in combination with nitrite or of N-nitrosoethylene-
thiourea in mice. Mutat. Res., 56: 335-340.
TERAMOTO, S., KANEDA, M., KATO Y. & SHIRASU Y. (1980) Failure
of inducing malformations after intro-amniotic injections of
ethylenethiourea in the rat. Congenital Anom., 20: 17-23.
TERAMOTO, S., KANEDA, H., AOYAMA, & SHIRASU, Y. (1981)
Correlation between the molecular structure of N-alkylureas and
N-alkylthioureas and their teratogenic properties.
Teratology, 23: 335-342.
TETSUMI, T., SUMI, M., TANAKA, M., & SHONO, T. (1985) Reversed
phase ion interaction chromatography of sodium dialkyldithio-
carbamates in the presence of tetraalkylammonium salts. J.
Chromatogr., 348: 389-395.
THORN, G.D & LUDWIG, R.A. (1962) The dithiocarbamates and
related compounds, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 298.
TOLLENAAR, F.D. (1956) [Recent developments in the use of
antioxidants in foodstuffs.] Dtsch. Lebensm. Rundschau, 12:
307-313 (in German).
TRUHAUT, R., GUERINOT, F., & BOHUON, C. (1971) Mécanisme bio-
chimique de toxicité des fongicides de la série des dithiocarba-
mates: L'action inhibitrice de la dopamine beta-hydroxylase. Ann.
pharm. fr., 29(2): 117-124.
TURNER, N.J. & CORDEN, M.E. (1963) Decomposition of sodium-
N-methyldithiocarbamate in soil. Phytopathology, 53: 1388-
1394.
ULLAND, B.M., WEISBURGER, J.H., WEISBURGER, E.K., RICE, J.M., &
CYPHER, R. (1972) Brief communication: thyroid cancer in rats
from ethylenethiourea intake. J. Natl Cancer Inst., 49: 583-
584.
US EPA (1977) Rebuttable presumption against registration and
continued registration of pesticide products containing ethylene
bisdithiocarbamates. Fed. Reg., 42(154): 40618-40627.
US EPA (1982a) Ethylene bisdithiocarbamates: final resolution
of rebuttable presumption against registration, Washington
DC, US Environmental Protection Agency, Office of Pesticides and
Toxic Substances (Unpublished report).
US EPA (1982b) Ethylene bisdithiocarbamates: notice of
determination concluding the presumption against registration;
notice of availability of decision document, Washington DC, US
Environmental Protection Agency (Unpublished report 82P-2851).
US EPA (1984) Health and environmental effects: profile for
ethylenethiourea (2-imidazolidinethione), Cincinnati, Ohio, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office (Program Office Draft ECAO-CIN-019).
VACHKOVA-PETROVA, R. (1977) [A study on mutagenic activity of
basfungine on white rats.] Hig. i. Zdraveopazvane, 20(5): 422-
426 (in Russian).
VACHKOVA-PETROVA, R. (1981) Mutagenicity study of endodan in
rats. Mutat. Res., 85(4): 288.
VAN ESCH, G.J. (1956) [Toxicity of tetramethylthiuram
disulfide and tetraethylthiuram disulfide.] Versl.
Volksgezondheid, Vol. No. 166-169 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN LEEUWEN, F.X.R., VOOGD, C.E., KNAAP, A.G.A.C., DEN
TONKELAAR, E.M., & VAN DER HEIJDEN, C.A. (1982) Toxicological
evaluation of ethylenethiourea (ETU), Bilthoven, National
Institute of Public Health (Unpublished Report No. 627915/008).
VAN LOGTEN, M.J. (1972) [ Thesis: the dithiocarbamate-alcohol
reaction by the rat. ] Terborg, The Netherland, Fa. Lammers (in
Dutch).
VAN STEENIS, G. & VAN LOGTEN, M.J. (1971) Neurotoxic effect of
the dithiocarbamate tecoram on the chick embryo. Toxicol. appl.
Pharmacol., 19: 675-686.
VEKSHTEIN, M.SH. & KHITSENKO, I.L. (1971) Ziram metabolism in
warm-blooded animals. Gig. i Sanit., 36: 28-33.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index: JMPR 1961-84, Geneva, World Health Organization
(Unpublished report).
VOGELER, K., DREZE, Ph., RAPP, A., STEFFAN, H., & ULLEMEYER, H.
(1977) Distribution and metabolism of propineb in apples and
grapes, including their breakdown products propylenethiourea and
ethylenethiourea in apples. Pflanzenschutz-Nachrichten Bayer
30: 72-97.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1970) Studies on the fate
in plants of ethylene bisthiocarbamate fungicides and their
decomposition products. Ann. appl. Biol., 65: 489-496.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1971) Tentative
identification of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicides. Pestic. Biochem.
Physiol., 1: 163-165.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1976) Formation of
ethylenethiourea from 5,6-dihydro-3 H-imidazo[2,1-C]-1,2,4-
dithiazole-3-thione by microorganisms and reducing agents. J.
environ. Sci. Health, B11: 33-47.
WATTS, R.R., STORHERR, R.W., & ONLEY, J.H. (1974) Effects of
cooking on ethylene bisdithiocarbamate degradation to
ethylenethiourea. Bull. environ. Contam. Toxicol., 12: 224-226.
WEDIG, J., COWAN, A., & HARTUNG, R. (1968) Some of the effects
of tetramethylthiuram disulfide (TMTD) on reproduction of the
bobwhite quail (Colinus virginianus). Toxicol. appl. Pharmacol.,
12: 293.
WEISBURGER, E.K., ULLAND, B.M., NAM, J.M., GART, J.J., &
WEISBURGER, J.H. (1981) Carcinogenicity tests of certain
environmental and industrial chemicals. J. Natl Cancer Inst.,
67: 75-88.
WHO (1986a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1986-1987, Geneva, World
Health Organization (Unpublished report VBC/86.1, rev. 1).
WHO (1986b) Environmental Health Criteria 64: Carbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 137.
WHO (1988) Environmental Health Criteria 76: Thiocarbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 49.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., Croydon, British Crop Protection
Council.
YIN-TAK WOO (1983) Carcinogenicity, mutagenicity, and
teratogenicity of carbamates, thiocarbamates, and related
compounds. An overview of structure-activity relationships and
environmental concerns. J. environ. Sci. Health, C1(1): 97-133.
ZADOROZHNY, B.A., PETROV, B.R., OLTIRENKO, V.A., & KIRILKO, V.A.
(1981) [Occupational dermatoses and their control under
conditions of pesticide manufacture.] Vestn Dermatol. Venerol.,
6: 48-51 (in Russian).
ZDIENICKA, M., ZIELENSKA, M., HRYMIEWICA, M., TROJANOWSKA, M.,
ZALEJSKA, M., & SZYMCZYK, T. (1981) The mutagenicity of the
fungicide thiram. In: Kappas, A., ed. Progress in environmental
mutagenesis and carcinogenesis, Amsterdam, Oxford, New York,
Elsevier Science Publishers, Vol. 2, pp. 79-86.
ZORIN, P.M. (1970) [Allergic dermatitis arising as a result of
contact with zineb.] Vestn Dermatol. Venerol., 44: 65-68 (in
Russian).
ZUMAN, P. & ZAHRADNIK, R. (1957) [Kinetics and decomposition
mechanism of dithiocarbamic acids solutions. Polarographic
study.] Z. Phys. Chem., 208: 135-140 (in German).
Annex I. Names and structures of selected dithiocarbamates
--------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
dibam Methylnamate S sodium dimethyldi- C3H6NS2Na 143.21
|| thiocarbamate
(CH3)2N-C-SNa (
Rhodes (1977) found that when 14C-ETU was applied to soil
sections of Keyport silty loam at a rate of 2.2 kg/ha, total 14C
residues disappeared with a half-life of < 4 weeks. The half-
life of intact ETU was < 1 week. Most of the radioactivity was
confined to the top 2.5 - 12.5 cm of the soil column, and only
small amounts (0.2%) were found at depths of 20 - 30 cm after 12
weeks. It was concluded from this study that ETU did not leach
to any great extent.
Nash & Beall (1980) reported that ETU is weakly adsorbed to
soil, and is highly mobile in moist soil but immobile in dry
soil. The presence of organic matter in soil seems to be of
great importance in the leaching of ETU. Degradation appears to
be accomplished readily by both chemical and biological means
and, thus, ETU does not persist in soil.
Many studies have been carried out concerning the environ-
mental fate and transport of ETU (US EPA, 1984).
Parallel results were obtained in laboratory studies with
propineb, which, in a similar fashion, forms PTU, propyleneurea
(PU), and, eventually, carbon dioxide (Vogeler et al., 1977).
The results of these studies show that, under normal
practical conditions, it is unlikely that ETU or PTU will
accumulate in soil.
4.2. Water
ETU is stable in de-ionized water in the absence of photo-
sensitizers, but is rapidly oxidized in their presence. In
studies by Ross & Crosby (1973), several sensitizers were added
at 10 mg/litre to a 25 mg/litre solution of ETU and exposed to
sunlight. After 4 days with riboflavin as a sensitizer, the
concentration of ETU was less than 5% of that in the control
solution kept in darkness. To minimize microbial degradation,
the procedure was repeated after filtering and boiling the water
samples, with the same results. Furthermore, ETU degradation
was investigated in several boiled samples of agricultural
drainage water to which 0.5 mg ETU/litre had been added before
irradiation. The results are given in Table 10.
Numerous samples of natural water were collected from
rivers, lakes, and agricultural areas and, almost without
exception, they were found to degrade ETU to EU in sunlight.
The same samples degraded ETU in the dark but only after prior
exposure to sunlight, indicating that stable photo-oxidants had
been generated. The substances responsible for ETU oxidation
were isolated and identified as the amino acids tryptophane and
tyrosine. The pure amino acids also caused the conversion of
ETU to EU in the light, apparently by their ability to form
hydroperoxides or other strong oxidants (Ross & Crosby, 1973).
As both the amino acids and photosensitizers such as acetone,
riboflavin, and chlorophyll are known to occur world wide in
water and soil, and this photolysis also has been shown to take
place rapidly on a silica surface (Cruickshank & Jarrow, 1973),
the degradation of ETU to harmless products in the field seems
entirely plausible (IUPAC, 1977).
Table 10. Photodecomposition of ETU in agricultural
watersa
--------------------------------------------------------
Source Irradiation Remaining ETU (%)
--------------------------------------------------------
Irrigation ditch 3 days, lamp 10 - 20
(sugar beet) 3 days, dark 100
Paddy flooding 24 days, sun 25 - 50
ditch (rice) 24 days, dark 100
Paddy (rice) 24 days, sun 10 - 25
24 days, dark 100
--------------------------------------------------------
a From: Ross & Crosby (1973).
4.3. Plants
In studies in which the roots of corn, lettuce, tomato, and
pepper seedlings were treated with ETU, it was rapidly absorbed
by roots, translocated subsequently to the foliar tissues, and
then degraded very rapidly; virtually no ETU was detectable
after 20 days (Hoagland & Frear, 1976). When cucumber seedlings
were exposed to aqueous solutions of nabam or suspensions of
zineb or maneb, ETU was rapidly absorbed by the roots and
translocated within the plants. ETU appeared to be stable for
at least 2 weeks in seedlings and, inside the plant, a slow
conversion of ETU into 2-imidazoline was detected (Vonk & Kaars
Sijpesteijn, 1970, 1971).
In greenhouse studies, 14C-ETU was applied either to the
soil or to the leaves of 4-week-old potato plants and 8-week-old
dwarf tomato plants. Radioactivity was monitored in various
parts of the plant at different time intervals. The application
of 40 mg 14C-ETU/kg to the leaves of potato plants resulted in
negligible radioactivity in the roots and tubers 60 - 90 days
after application. The application of 17 - 22 kg/ha to soil
around the base of the plants resulted in a negligible amount of
radioactivity in the tubers, roots, and foliage of potato
plants, 60 - 90 days later. Comparable results were obtained
with tomato plants, using other dose levels and periods (Lyman &
Lacoste, 1974).
After systemic uptake of ETU by plants, EU and 2-imidazoline
were identified as metabolites. Surface deposits of ETU, which
may have occurred as a result of EBDC treatment, formed an
additional unidentified substance as the main metabolite and
ethylene diamine (EDA). Propineb and PTU also formed an
identical but unidentified major metabolite under similar
conditions (Vogeler et al., 1977).
Nash (1975) reported the presence of 7 - 10 different
degradation products in methanol extracts of soybeans after soil
or foliar treatment with EBDCs, as well as after treatment with
ETU. In these cases, EU was a degradation product.
More recently, Nash & Beall (1980) studied the fate of maneb
and zineb in microagroecosystem chambers (Part A, section 6.3).
ETU on the tomato fruit and leaves, and in the soil, water, and
air was monitored for 100 days after treatment. ETU was
detected at < 20 µg/kg on whole fruit after 3 days, but had
completely disappeared after 3 weeks. The half-life of ETU was
< 3 days.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
The amount of ETU in commercial formulations of EBDCs has
been shown to increase with increasing temperature and humidity.
ETU formation during storage appears to be greatest in maneb
formulations (up to 14%), followed by zineb and mancozeb. The
relative proportions of degradation products appear to be
different for the various EBDCs.
Studies with propineb on apples and grapes, carried out by
Vogeler et al. (1977), showed that PTU could be detected
shortly after treatment, and that it was rapidly transformed to
an unknown metabolite, together with small amounts of PU, and
other unidentified reaction products.
5.1. Food and Drinking-Water
The residue levels of ETU are mainly below 0.1 mg/kg product
following treatment (with different formulations) at the maximum
recommended EBDC levels (Newsome, 1976; Phillips et al., 1977).
Studies in Canada and the USA have shown that vegetables,
such as spinach, carrots, and potatoes, that have been treated
with EBDCs contain high levels of ETU after cooking (Blazquez,
1973; Newsome & Laver, 1973; Watts et al., 1974). Snapbeans
treated with maneb were found to contain ETU after commercial
canning (US EPA, 1977). Farrow & Ralls (1970) demonstrated the
disappearance of zineb, ziram, and maneb residues from spinach
and apricots during normal canning operations. There have been
many studies on ETU residues in crops, such as those of Sato &
Tomizawa (1960) on zineb-treated cucumbers.
The levels of ETU formed from residues of mancozeb and
polyram present on apples that were processed to make apple
juice, apple sauce, and apple pomace were determined (IUPAC,
1977). The results showed that ETU residues were 0.17 mg/kg
pomace and 0.05 mg/kg juice. A surprisingly high level of
unchanged mancozeb remained in the pomace, despite heat
treatment for 15 h at 150 °C. However, it seems that ETU
residues diminish during storage.
Residues of intact 14C-labelled ETU were found to diminish
with time in canned tomato sauce, spinach, pickles, and apple
sauce (Rose et al., 1980). EU and more polar products accounted
for most of the 14C-labelled residues. These polar materials
were resistant to extraction and appeared to be bound.
A study sponsored by the US EPA (Phillips et al., 1977), on
the effects of food processing on EBDC residues, confirmed and
extended the results previously described. Washing the raw
agricultural products prior to processing removed 33 - 87% of
the EBDC residue and the majority of the ETU residue. An
interesting result was that although almost instantaneous
conversion of mancozeb to ETU took place in boiling water, field
weathered residues of mancozeb appeared to be more resistant to
degradation to ETU. A summary of the results for raw and
processed material is given in Table 3.
5.2. Monitoring and Market-Basket Studies
A monitoring programme initiated in 1972 by the Canadian
government showed that 33% of food samples contained detectable
ETU residues. In particular, samples of canned spinach and
orange peel had average values of 0.047 mg/kg product and
0.083 mg/kg, respectively (Pecka et al., 1975; US EPA, 1977).
Studies on the actual level of ETU in products prepared for
commercial sale show it to be generally present in small
amounts. The highest level, 0.61 mg/kg product, was found in
canned peaches, while levels in orange peel, tomato paste,
instant potatoes, strawberries, peaches, and cucumbers were less
than 0.2 mg/kg product (US EPA, 1982a,b).
The US EPA has estimated an upper limit for dietary exposure
to ETU in the general population of the USA to be 3.65 µg/kg
body weight per day. This estimate is a maximum value, since it
was assumed that residues are present at the tolerance level and
that all of the EBDC residue is quantitatively converted to ETU.
Using actual residue data and experimentally derived conversion
factors, the US EPA estimated the dietary intake of ETU to be
0.24 µg/kg body weight per day.
In a market-basket study, over 500 samples of 34 foods were
analysed, plus 26 samples of drinking-water. No water samples
and only 21 of the food samples contained ETU residues (Gowers &
Gordon, 1980). Exposure estimates based on market-basket
studies range from 0.01 to 1 µg ETU/kg body weight per day
(Gowers & Gordon, 1980; Rose et al., 1980).
Tomato products (203 samples) were analysed in another
market-basket study, but none contained ETU (Gowers & Gordon,
1980).
A more realistic review of the actual exposure of the
general population was obtained by a "table-top" study. Of 200
meals (some from homes and some from restaurants) which were
analysed for ETU, none contained any residues (Gowers & Gordon,
1980).
6. KINETICS AND METABOLISM
6.1. Absorption, Distribution, and Excretion
ETU is rapidly absorbed from the gastrointestinal tract and
cleared from the body in all the mammalian species that have
been tested. After only 5 min, ETU appeared in the blood of
rats administered an oral dose of 100 mg 14C-ETU/kg body weight.
Within 48 h, 82 - 99% of an oral dose was eliminated via the
urine and about 3% via the faeces (Kato et al., 1976; Rose et
al., 1980). Newsome (1974) and Ruddick et al. (1976a) found
that approximately 70% was eliminated in the urine and 1% in the
faeces. Comparable results were found for mice while, in
monkeys, 55% was eliminated via the urine within 48 h, and less
than 1.5% via the faeces (Allen et al., 1978).
To study the accumulation and elimination of radioactivity
by the thyroid gland of rats dosed with 14C-ETU, dose levels of
2 and 200 µg labelled ETU were administered daily for 14 days.
In another study, rats were dosed with 0, 0.1, 1, 10, 50, or 100
mg 14C-ETU/kg diet, daily, for 7 days. The first study showed
that the concentration of ETU and/or its metabolites in the
thyroid is dose dependent, and the second that the level of 14C
in the thyroid did not increase appreciably when the daily dose
was increased above 50 mg/kg diet. Withdrawal of ETU from the
diet led to an 80 - 94% reduction in the radioactivity in the
thyroid after 17 days (Lyman & Lacoste, 1974).
ETU and its metabolites have been found to have a half-life
of about 28 h in monkeys, 9 - 10 h in rats, and 5 h in mice
(Rose et al., 1980).
In cows administered 1 mg 14C-ETU/kg diet, Lyman (1971)
found a small quantity of unchanged ETU in both the urine and
the milk of the test animals. Higher levels of 14C were
detectable in metabolites, such as glycine and urea, and in the
lactose and protein in the milk (Table 11).
6.2. Metabolic Transformation
It has been demonstrated that ETU degradation leads to
traces of EU and other metabolites in the urine and that 14C-
carbon dioxide is exhaled following the administration of
labelled ETU. Kato et al. (1976) suggested that the metabolites
of ETU in the rat were produced primarily by fragmentation of
the imidazoline ring and decarboxylation of the fourth and fifth
carbon atoms. A small amount of radioactivity was also found in
a protein fraction of rat fetal tissue. Ruddick et al. (1976a),
however, concluded that ETU metabolism in the rat does not
appear to result in any release of 14C into the general
metabolic pool. Mice metabolize ETU to EU and other unknown
metabolites, while cats metabolize it to S-methyl-ETU and EU.
Lyman (1971) detected EU, EDA, oxalic acid, glycine, and
urea as major metabolites in cow urine. In addition, 14C
originating from 14C-ETU was found in the protein and lactose in
the milk (Table 11). From these results, it appears that the
metabolism of ETU in ruminants is different from that in non-
ruminants. The degradation products of ETU in plants are
similar to those found in animals.
A summary of the secondary metabolites of ETU in biological
and non-biological systems is given in Fig. 4 (Part B, section
4).
Table 11. 14C Activity in the milk and urine of
cows fed with 1 mg 14C-ETUa/kg diet for 6 weeks
------------------------------------------------------
Milk Urine
Substance Concentra- % of Concentra- % of
tion total tion total
(mg/litre) 14C (mg/litre) 14C
------------------------------------------------------
ETU 0.011 31 0.12 7
EU 0.0025 8 0.27 18
EDA - - 0.14 14
Glycine - - - 6
Oxalic acid - - - 12
Urea - - - 11
Fat - 3 - -
Protein - 18 - -
Lactose - 16 - -
Total (%) 76 68
------------------------------------------------------
a From: Lyman (1971) and IUPAC (1977).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Only limited information is available on the effects of ETU
on organisms in the environment, and none is available
concerning the impact of ETU on terrestrial organisms. Data
concerning the toxicity for aquatic organisms of ETU and its
breakdown product EU are summarized in Tables 4 and 5. From
these results, ETU appears to have a low toxicity for bacteria,
algae, crustacea, and fish. Because of the low partition
coefficient (Table 6) and rapid biotransformation of ETU,
bioaccumulation will be insignificant or absent (Van Leewen,
1986).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Lewerenz et al. (1975) reported an acute oral LD50 for the
rat of 900 mg/kg body weight. The values determined by Graham &
Hansen (1972) and Teramoto et al. (1978a) were 1832 mg/kg and
545 mg/kg body weight, respectively. Teramoto et al. (1978a)
reported values of 3000 mg/kg body weight for the mouse and
> 3000 mg/kg body weight for the hamster.
8.2. Short- and Long-term Exposures
Administration of ETU to laboratory rats causes enlargement
of the thyroid gland. This effect was noted by Seifter & Ehrich
(1948), and has since been confirmed in various short- and long-
term studies. The accumulation of ETU in the thyroid gland is
associated with biochemical and morphological effects comparable
with those induced by known antithyroid drugs such as
thiouracil.
Graham & Hansen (1972) fed rats with diets containing ETU at
dose levels of 0, 50, 100, 500, or 750 mg/kg diet for 30, 60,
90, or 120 days, and at 100 mg/kg or more, thyroid changes were
seen. Ross Hart & Valerio (1973) fed doses of up to 1000 mg
ETU/kg diet to rats. An increase in thyroid weight was seen at
159 mg/kg or more, and the larger doses produced hyperplasia.
When Freudenthal et al. (1977) carried out studies on rats
at dose levels of up to 625 mg ETU/kg diet for 30, 60, or 90
days, biochemical changes reflecting effects on thyroid function
were observed. A dose of 125 mg/kg diet produced, after 30
days, a decrease in T3, T4, and serum PBI levels and an increase
in TSH concentration. A dose of 25 mg/kg diet produced a
decrease in T4 content after 60 days. Thyroid weight was
increased in all groups receiving 25 mg/kg or more. After 90
days, tumours (adenomas) were found in the 125 mg/kg group. The
group receiving 625 mg/kg died within 7 weeks.
From these short-term studies, it can be concluded that the
no-observed-adverse-effect level lies below 25 mg ETU/kg diet
and is probably of the order of 5 mg/kg diet (equivalent to
0.25 mg/kg body weight).
8.3. Teratogenicity
ETU was administered orally to rats and rabbits in single
daily doses of 0, 5, 10, 20, 40, or 80 mg/kg body weight. Rats
were treated from 21 - 42 days before conception to day 15 of
pregnancy, or on days 6 - 15 or 7 - 20 of pregnancy, whereas
rabbits were treated on days 7 - 21 of pregnancy. ETU at dose
levels of 10 mg or more induced meningoencephalocele,
meningorrhagia, meningorrhea, hydrocephalus, obliterated neural
canal, abnormal pelvic limb posture with equinovarus, and short,
kinky tail in all rats. Fetal survival was not affected, and
fetal growth was retarded only at 40 and 80 mg/kg. Rabbits
showed an increased incidence of resorption sites and decreased
brain weight at 80 mg/kg body weight, but no malformations
(Khera, 1973).
ETU was studied in rats and mice for its ability to induce
perinatal toxicity and in guinea-pigs and golden hamsters for
its teratogenic potential. The ETU was administered by gavage
and during organogenesis. Table 12 summarizes the prenatal
treatments. Additional postnatal studies were performed on rats
using extended treatment periods (ETU at 0, 20, 25, or 30 mg/kg
body weight), including continuous exposure from day 7 of
gestation through parturition to day 15 of lactation. The pups
were weaned normally and postnatal studies on open-field
behaviour were performed at 6 weeks. ETU at 80 mg/kg body
weight induced maternal toxicity and reduced growth in the rat
and was teratogenic for the rat, inducing substantial fetal
effects at all dose levels above 10 mg/kg body weight. Gross
defects were seen in the skeletal and the central nervous
systems, and cleft palate was noted, mainly at the highest dose
level. At 20 and 30 mg/kg, an increased incidence of
hydrocephalus was the only defect noted. The maternal and fetal
toxicity of ETU for the mouse, guinea-pig, and hamster was
substantially less than for the rat. In mice, at the highest
dose, an increase in maternal liver weight and in fetal
supernumerary ribs was noted, but no effects were seen in other
species (Chernoff et al., 1979; FAO/WHO, 1980b).
Table 12. Summary of prenatal treatmentsa
---------------------------------------------------------
Compound Species Dose (mg/kg Treatment
body weight) (gestation days)
---------------------------------------------------------
ETU rat 0, 5, 10, 20, 7 - 21
30, 40, 80
mouse 0, 100, 200 7 - 16
hamster 0, 25, 50, 100 5 - 10
guinea-pig 0, 50, 100 7 - 25
---------------------------------------------------------
a Modified from: Chernoff et al. (1979).
ETU has been found to induce a variety of postnatal
effects, including reduction or absence of maternal milk
production, thereby causing pup mortality at doses of 30 mg/kg
body weight or more. However, no significant dose-related
behavioural abnormalities were observed in ETU-exposed pups
(Chernoff et al., 1979; FAO/WHO, 1980b).
In studies by Khera & Tryphonas (1977), groups of pregnant
rats were administered ETU at dose levels of 0, 15, 30, or
45 mg/kg body weight on day 15 of gestation and then subjected
to a variety of test conditions to evaluate pre- and postnatal
effects. Postnatal mortality occurred in pups from mothers
treated with dose levels exceeding 15 mg/kg or pups cross-
fostered to evaluate lactation exposure. All pups from mothers
treated with 45 mg/kg died within 4 weeks of birth. A high
incidence of hydrocephalus and microphthalmia was observed in
pups of mothers treated with 30 mg/kg and these pups died within
6 weeks of birth. Motor defects observed in some survivors
(16/65) of this group, were shown to result from the
hydrocephalic condition, which was accompanied by atrophy of the
cerebral cortex and subcortical white matter. These defects
were found to be a direct result of in utero exposure to ETU and
not of exposure during lactation (cross-fostered pups showed the
same effects as pups weaned from treated dams). When mated with
normal male rats, all female offspring of rats administered
30 mg/kg gave birth to normal offspring. The F2 generation was
not impaired, though some of the parents had neurological
defects. In these studies, no effects on the parameters
examined were observed at 15 mg/kg body weight (Khera &
Tryphonas, 1977).
Teramoto et al. (1978a) investigated the teratogenicity of
ETU in rats, mice, and hamsters. It was teratogenic when given
orally to rats at 20 - 50 mg/kg body weight per day on days 6 -
15 of pregnancy and to hamsters at 270 - 810 mg/kg body weight
per day on days 6 - 13 of pregnancy. However, no malformations
were induced in mice up to a daily oral dose of 800 mg/kg body
weight when given on days 7 - 15 of pregnancy. In hamsters,
cleft palate, kinky tail, oligodactyly, and anal atresia were
noted as gross external malformations. Skeletal examination
revealed a high incidence of defects in the ribs and vertebral
column, but no apparent defect was observed during visceral
examination. An oral dose of 100 or 200 mg ETU/kg body weight
given to pregnant rats consistently produced brain abnormalities
in the fetuses, when given on day 12 or 13 of pregnancy, and
forelimb abnormalities, when given on day 13 of pregnancy
(Teramoto et al., 1978a). Histological studies revealed
extensive cell necrosis in the brain and forelimbs of embryos
24 h after the treatment. These lesions were considered to be
the main cause of the abnormalities observed. However, neither
malformations nor cell necrosis were found in the fetuses that
had been injected with 200 µg ETU/conceptus into the amniotic
sac on day 12 of pregnancy (Teramoto et al., 1980). Studies
with 2-14C-ETU revealed that this dose was sufficient to test
the direct effects of ETU on the embryos, since the
incorporation of radioactive substance was five times higher in
the embryos injected with 200 µg into the amniotic sac than it
was in those embryos whose mothers were treated with an oral
dose of 100 mg/kg body weight (Teramoto et al., 1980).
The teratogenic potential of the ETU metabolite 1-
methylthiourea has been investigated by Teramoto et al. (1981).
It caused almost the same types of malformations in rat fetuses
when given orally to mothers at 250 - 500 mg/kg body weight on
day 12 or day 14 of pregnancy as those observed following
treatment with ETU. However, 1-methylthiourea did not induce
malformations in mouse fetuses whose mothers were given an oral
dose of 1000 mg/kg on day 10 of pregnancy. There is a
structural similarity between 1-methylthiourea and ETU: C=S,
and -NH- groups seem essential for producing teratogenic effects
(Teramoto et al., 1981). However, the structure of ETU seems
quite specific for the induction of teratogenicity since Ruddick
et al. (1976b) tested 16 compounds related to ETU, including
ethylenethiuram monosulfide, another metabolite, and only one,
4-methylenethiourea, was teratogenic.
8.4. Mutagenicity
Tests with a large number of S. typhimurium strains gave
mostly negative results, though a few (weak) positive results
were observed in the case of some strains of S. typhimurium
(Shirasu et al., 1977). The addition of rat liver microsomes
seemed to enhance the mutant reversion. Schüpbach & Hummler
(1976, 1977) concluded that ETU appeared to induce base-pair
mutations but not frameshift mutations in S. typhimurium
TA 1530, although frameshift mutations appeared in S.
typhimurium TA 98, TA 1537, and TA 1538 when exposed to ETU in
the presence of dimethylsulfoxide (DMSO) and/or rat liver
microsomes (Rose et al., 1980).
Teramoto et al. (1977) did not find mutagenicity with S.
typhimurium TA 1536, TA 1537, TA 1538, G46, E. coli WP2 hcr+ and
hcr-, or B. subtilis H17 rec+ and rec- at concentrations
of 10 000 µg ETU/plate. However, a weak reaction was seen with
S. typhimurium TA 1535, and Seiler (1974) reported weak
(dose-unrelated) mutagenicity in S. typhimurium strain G46.
ETU was also found to be mutagenic in a host-mediated assay
of S. typhimurium TA 1530 when mice were dosed with 6000 mg
ETU/kg body weight, but not at doses of 2000 mg/kg or less
(Schüpbach & Hummler, 1977). Cytogenetic effects of ETU have
been reported in bone marrow cells of mice and Chinese hamsters.
On the other hand, there was no significant evidence to suggest
that ETU was mutagenic in host-mediated assays of S.
typhimurium G 46 or in tests with other strains of bacteria, rat
bone marrow (including the micronucleus test) Chinese hamster
DON cells, rat lymphocytes, or human fibroblast cells.
Furthermore, ETU did not increase the frequency of dominant
lethal mutations in rodents or Drosophila melanogaster (Seiler,
1973, 1974; Schüpbach & Hummler, 1977; Shirasu et al., 1977;
Teramoto et al., 1978b; Rose et al., 1980). A large number of
mutagenicity tests are summarized in a report of the US EPA
(1984).
ETU has been tested in the hepatocyte DNA repair test,
which is used to determine pro-carcinogenic potential as well as
DNA damage. ETU did not induce DNA damage (Althaus et al.,
1982) nor cause chromosomal damage in cultured rat liver cells,
and it did not induce chromatid exchange in CHO cells in
vitro or in mice in vivo. A micronucleus test with mice bone
marrow cells in vivo also gave negative results (De Serres &
Ashby, 1981).
This evidence indicates that ETU is generally not
mutagenic, especially in mammalian test systems.
8.5. Carcinogenicity
The carcinogenicity of ETU has been evaluated by IARC
(1974, 1982). It was classified in group 2B, i.e., limited
evidence for activity in short-term tests; sufficient evidence
for carcinogenicity in animals; inadequate evidence for
carcinogenicity in human beings.
ETU has been studied for oncogenic potential in mice, rats,
and hamsters.
8.5.1. Mouse
In a comprehensive programme screening chemicals for
carcinogenicity, two strains of hybrid mice (X and Y) were given
215 mg ETU/kg body weight from day 7 until weaning, and
thereafter 646 mg/kg diet for more than 18 months. In the X
strain [(C57B1/6XC3H/Anf)F1], the incidence of lung tumours in
the ETU-treated females was higher than that of the control
group (3/18 versus 3/87), but it was lower than that of the
controls (0/16 versus 1/90) in the Y strain [(C57B1/6XAKR)F1].
In the males, the incidence was higher in the ETU-treated
animals (3/18 versus 1/90). The incidence of lymphomas was
slightly increased in treated Y-strain females. The hepatoma
incidence in the ETU-treated groups of both strains was
significantly higher than that of the control group (in the X
strain, 14/18 and 18/18, for males and females respectively; in
the Y strain, 18/18 and 9/18; in controls, 0/18 and 3/18). The
thyroid glands were not examined for histopathological changes
(Innes et al., 1969).
Graham et al. (1975), found that ETU induced thyroid
hyperplasia and other research groups have confirmed this
finding.
8.5.2. Rat
Ulland et al. (1972) and Weisburger et al. (1981) fed
groups of 26 male and female Charles River-CD rats diets
containing 0, 175, or 350 mg ETU (97%)/kg diet. Five females
and five males of the high-dose group were killed after 18
months and the remainder after 24 months. Hyperplastic goitre,
solid cell adenomas, and thyroid (follicular or papillary)
carcinomas were found. Two of the animals also had lung
tumours, which might have been metastases. The thyroid tumour
incidence was dose dependant; (in the 175 mg/kg group, it was
3/26 and 3/26, for males and females, respectively, and in the
350 mg/kg group it was 17/26 and 8/26). No thyroid carcinomas
were observed in the control animals. A few of the treated rats
had hyperplastic nodules in the liver.
Graham et al. (1973, 1975) studied the long-term effects on
the thyroid gland of ETU ingestion. Five groups of 68 male and
68 female Charles River rats were fed ETU at levels of 0, 5, 25,
125, 250, or 500 mg/kg diet for 2 years. Growth depression was
evident at the highest dose level. The thyroid/body weight
ratio was significantly increased at 250 and 500 mg/kg, and
slightly increased at 125 mg/kg after 24 months. Thyroidal
uptake of 131iodine per mg tissue was significantly decreased in
male rats fed 500 mg ETU/kg diet for 18 or 24 months. The
thyroids of females fed at the three highest dose levels were
hypofunctioning at 6 months, and hyperfunctioning at 12 months,
and at 24 months thyroid function was similar to that of the
controls. At the two highest dose levels (250 and 500 mg/kg),
thyroid adenomas and carcinomas were induced. At all lower dose
levels hyperplasia occurred more frequently than in the
controls, but there were no adenomas or carcinomas. No increase
in liver tumours was observed in this study.
Gak et al. (1976) studied the effects of feeding rats with
0, 5, 17, 60, or 200 mg ETU/kg diet for 24 months. Body weight,
food consumption, serum enzyme activities (e.g. glutamic pyruvic
transaminase, alkaline phosphatase), hepatic enzyme activities
(glutamic pyruvic transaminase, alkaline phosphatase, glucose-6-
phosphate dehydrogenase), cholesterol levels, weights of thyroid
and other organs, and histopathology were studied.
Hypercholesterolemia was found at dose levels of 5 mg/kg and
above. At 60 mg/kg or more, a significant increase in thyroid
tumours was found, but at lower levels the tumour incidence was
not significantly different from that of the controls.
8.5.3. Hamster
Gak et al. (1976) studied the effect of 0, 5, 17, 60, or
200 mg ETU/kg diet on hamsters for 18 months. Growth, food
intake, biochemical parameters in the serum and liver, organ
weights, and histology were studied. A significant increase in
thyroid tumours was found at 60 mg ETU/kg or more, but at lower
doses values were not significantly different from those of the
control group.
8.6. ETU in Combination with Nitrite
When the mutagenicity of ETU was assayed before and after
nitrosation with sodium nitrite under acid conditions,
nitrosation was found to cause a 160-fold increase in the number
of revertant colonies of S. typhimurium TA 1535 (Shirasu et
al., 1977). The interactive mutagenicity of ETU and nitrite was
also found in the mouse dominant lethal test by Teramoto et al.
(1978b). However, no dominant-lethal mutations were induced in
a group of mice treated with 30 mg ETU plus 10 mg nitrite/kg
body weight. A large increase in pre-implantation losses was
noted 5 and 6 weeks after completing a 5-day treatment of males
with a combined oral dose of 150 mg ETU/kg and 50 mg sodium
nitrite/kg body weight.
8.7. Mechanisms of Toxicity; Mode of Action
The biochemical changes induced by antithyroid drugs
include reduced production of thyroid hormones (T3 and T4),
followed by increased production of TSH in response to low
thyroid hormone levels in the blood. Pathological changes in
the thyroid gland begin with diffuse microfollicular hyper-
plasia, and are followed by diffuse and nodular hyperplasia and
later by nodular hyperplasia with papillary and cystic changes
induced by the TSH. If hyperstimulation of the thyroid by TSH
is severe and prolonged, it provides conditions conducive to the
formation of tumours.
Antithyroid drugs can inhibit T4 production in various
ways. The chemical similarity of ETU to thiourea and thiouracil
suggests that ETU acts by blocking the iodination of thyroxine
precursors, thus reducing the synthesis of the thyroid hormones.
Iodide peroxidase catalyses the iodination of tyrosine and the
coupling of the resultant iodotyrosyl residues to produce the
active hormones T3 and T4.
Graham & Hansen (1972) found that ETU inhibited
iodide peroxidase in vitro. The resulting decreased level of
thyroid hormones causes stimulatory feedback of the pituitary
gland and consequently an increased release of TSH (Rose et al.,
1980).
Lu & Staples (1978) studied the influence of ETU in
pregnant hypothyroid and euthyroid rats to determine whether ETU
teratogenicity occurs as a result of altered maternal thyroid
function. Doses of 40 mg ETU/kg body weight, administered on
days 7 - 15 of gestation, resulted in 84 - 100% of the fetuses
in all treated groups being malformed, regardless of the thyroid
status of the dams. The authors concluded that the thyroid
status of the mother is not of importance in causing teratogenic
effects.
Rose et al. (1980) reported that the effects of feeding
rats 125 - 625 mg ETU/kg diet for 2 - 12 weeks, which included
thyroid hyperplasia and dose related suppression of serum T3 and
T4 (with corresponding TSH elevation), were reversible within 22
weeks of placing on control diets.
Long-term studies using ETU showed significantly increased
thyroid/body weight ratios in rats fed 125, 250, or 500 mg/kg
diet for periods of up to 2 years (Graham et al., 1975). This
effect was not reversed in rats placed on a control diet after
66 weeks of continuous exposure to 5 - 500 mg ETU/kg diet. It
is likely that by that time the thyroid was severely damaged.
In studies by Arnold et al. (1982, 1983), decreased levels
of serum thyroid hormones and increased thyroid weights were
reversed in Sprague Dawley rats fed diets containing 0, 75, 100,
or 150 mg ETU/kg diet for 7 weeks. The reversibility of
microscopic changes in the thyroids of male rats exposed to ETU
was studied. The rats were fed diets containing 75 or 150 mg
ETU/kg diet for 7 - 82 weeks and then returned to a control diet
for periods ranging from 2 to 42 weeks. The severity and extent
of reversibility of thyroid hyperplasia were found to depend on
the duration of exposure to ETU. Above a certain threshold,
hyperplasia did not regress significantly.
Numerous studies with ETU suggest that the rat is more
sensitive than other species to the effects of the thyroid. A
recent study with propylthiouracil, a thyroid inhibitor with a
mode of action similar to that of ETU, has confirmed that
monkeys are much less sensitive than rats. The sensitivity
difference was not quantified in vivo, but, in an in
vitro study, the concentration of inhibitor required to produce
the same level of thyroid peroxidase inhibition was
approximately 100 times greater for monkey enzyme than it was
for rat enzyme (Takayama et al., 1986).
8.8. Propineb and Propylenethiourea (PTU)
8.8.1. General
The toxicology of propineb was reviewed at JMPR meetings in
1977, 1980, and 1983. Because of concern expressed at the 1977
meeting regarding the potential for thyrotoxicity and
tumourigenicity of propylenethiourea (PTU), a breakdown product
of propineb, the meeting estimated only a temporary ADI for man.
Further evaluation of propineb was postponed pending the
submission of additional data. Data submitted for evaluation in
1985 consisted of long-term mouse and rat studies, mutagenicity
studies, and a special study into the effects of PTU on DNA. In
addition, data previously submitted for evaluation in 1983 were
re-examined. These data included several studies on propineb
(acute toxicity studies, a short-term study on thyroid function
in rats, mutagenicity studies, and an oncogenicity study on
mice) and on PTU (pharmacokinetic studies on rats and a long-
term thyroid function study on rats) (FAO/WHO, 1986a,b).
8.8.2. Toxicological information
An oncogenicity study on mice with propineb indicated
increased hepatocellular adenomas in male mice and increased
pulmonary adenomas in female mice at 800 mg/kg diet, the highest
dose level tested. Thyroid tumours were not induced in treated
mice in this study. A no-observed-adverse-effect level for non-
neoplastic effects could not be determined in this study, owing
to insufficient data (FAO/WHO, 1986a, b).
In a long-term study into the effects of PTU on mice, an
increased incidence in male mice of hepatocellular adenomas was
observed at 1000 mg/kg diet (the highest dose level tested) and
of hepatocellular carcinomas at 10 mg/kg diet or more. In the
same study, increased incidences of hepatocellular adenomas and
carcinomas were observed in female mice at 100 mg/kg diet or
more. Thyroid tumours attributable to PTU were not observed,
but increased thyroid hypercellularity was noted in male mice at
1000 mg/kg diet (FAO/WHO, 1986a, b).
In long-term rat studies with propineb, previously reviewed
by the JMPR, an increased incidence of benign thyroid tumours
was observed at 1000 mg/kg diet or more. Non-neoplastic thyroid
effects were observed in the same study at 100 mg/kg diet or
more. In another study, increased liver and kidney weights were
observed at 100 mg/kg diet or more and a no-observed-adverse-
effect level of 10 mg/kg diet was determined. In a long-term
study on the effects of PTU on rats, thyroid tumours
attributable to PTU were only found at 1000 mg/kg diet.
Goitrogenic effects in the thyroid were observed, however, at
dose levels as low as 1 mg/kg diet, the lowest dose level
tested. A no-observed-adverse-effect level could not be
determined in this study (FAO/WHO, 1986a, b).
Short-term studies on the effects of propineb on thyroid
function in rats did not establish an unequivocal no-observed-
adverse-effect level for effects on the thyroid. In a long-term
study with PTU, effects on thyroid function were observed at
1000 mg/kg diet, but at lower dose levels effects were
ambiguous. Pharmacokinetic studies on rats demonstrated
preferential uptake of radioactivity from 14C-labelled PTU by
the thyroid (FAO/WHO, 1986a, b).
Mutagenicity studies on propineb and PTU produced negative
or inconclusive results. However, PTU has been shown to
increase DNA synthesis in mouse spleen cells, but it did not
bind to mouse liver cell DNA.
In view of the carcinogenic response to PTU in the liver of
mice and the lack of a no-observed-adverse-effect level for the
effects of propineb on the thyroid in a long-term study on mice
or short-term studies on rats, or for PTU in a long-term study
on rats, the JMPR recommended that the temporary ADI for
propineb should be withdrawn.
In view of the established carcinogenic potential of this
compound, the meeting recommended that propineb should not be
used where its residues can arise in food (FAO/WHO, 1986a,b).
9. EFFECTS ON MAN
9.1. Epidemiological Studies
Smith (1976) conducted a detailed study involving 1929
workers in rubber-compounding plants in Birmingham, England. No
thyroid cancers were found in the health records of these
workers.
Clinical examinations and thyroid function tests were
carried out over a period of 3 years on eight process workers
and five mixers in a factory producing ETU in the United
Kingdom. Matched controls were also examined. The results
showed that the exposed mixers, but not the process workers, had
significantly lower levels of T4 in their blood compared with
the controls. No effect was found on TSH or thyroid-binding
globulin (Smith, 1984).
PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) and
the International Agency for Research on Cancer (IARC) have
evaluated the toxicity and carcinogenicity data for various
dithiocarbamates on several occasions. Annex III includes an
overview of the JMPR meetings in which these compounds, ETU, and
PTU have been evaluated, with their references, together with
the WHO recommended classification of pesticides by hazard for
individual dithiocarbamates. The existence of IARC evaluations
and the availability of WHO/FAO Data Sheets and IRPTC Data
Profiles and Legal Files are also indicated. These documents
include more detail concerning the product and legal aspects,
toxicological evaluation, and residues of individual
dithiocarbamates in different food items.
REFERENCES
AASETH, J., ALEXANDER, J., & WANNAG, A. (1981) Effect of
thiocarbamate derivatives on copper, zinc, and mercury
distribution in rats and mice. Arch. Toxicol., 48: 29-39.
ALDRIDGE, W.N. & MAGOS, L. (1978) Carbamates, thiocarbamates,
dithiocarbamates, Luxembourg, Commission of the European
Communities (Report No. V/F/1/78/75 EN).
ALLEN, J.R., VAN MILLER, J.P., & SEYMOUR, J.L. (1978)
Absorption, tissue distribution, and excretion of 14C-ETU in the
rhesus monkey and the rat. Chem. Pathol. Pharmacol., 20: 109-
115.
ALTHAUS, F.R., LAWRENCE, S.D., SATTLER, G.L., LONGFELLOW, D.G.,
& PITOT, H.C. (1982) Chemical quantification of unscheduled
DNA synthesis in cultured hepatocytes on an assay for the rapid
screening of potential chemical carcinogens. Cancer Res., 42:
3010-3015.
ALVAREZ, M., CREEKMORE, R.W., & ROSEN, R.T. (1973) Structure
of ethylenethiuram monosulfide. Tetrahedron Lett., 12: 939-942.
ANTONOVICH, E.A., CHERNOV, O.V., SAMOSH, L.V., MARTSON, L.V.,
PILINSKAYA, M.A., KURINNY, L.I., VEKSHTEIN, M.S., MARTSON, V.S.,
BALIN, P.N., & KHITSENKO, I.I. (1972) [A comparative toxico-
logical assessment of dithiocarbamates.] Gig. i. Sanit., 37: 25-
30 (in Russian).
ARNOLD, D.L., BICKIS, M.G., NERA, E.A., MCGUIRE, P.F., & MUNRO,
I.C. (1982) Assessing the reversibility of thyroid lesions
that result from feeding ETU. Toxicologist, 2(1): 90 (Abstract
of Presentation at the SOT Meeting 1982).
ARNOLD, D.L., KREWSKI, D.R., JUNKINS, D.B., MCGUIRE, P.F.,
MOODIE, C.A., & MUNRO, I.C. (1983) Reversibility of ethylene-
thiourea-induced thyroid lesions. Toxicol. appl. Pharmacol., 67:
264-273.
ASHWORTH, R., DE B., HENRIET, J., LOVETT, J.F., & MARTIJN, A.,
ed. (1980) CIPAC handbook - Volume 1A: Analysis of technical
and formulated pesticides, Cambridge, United Kingdom, W. Heffer
and Sons Limited. England.
AYANABA, A., VERSTRAETE, W., & ALEXANDER, M. (1973) Formation
of dimethylnitrosamine, a carcinogen and mutagen, in soils
treated with nitrogen compounds. Soil Sci. Soc. Am. Proc., 37:
565-568.
BABINI, G. (1966) [A case of erythroderma caused by zinc
dithiocarbamate.] Arch. Ital. Dermatol. Venereol. Sessuol., 34:
230/238 (in Italian).
BALIN, P.N. (1970) [Experimental data on the blastomogenic
activity of the fungicide maneb.] Vrach. Delo, 4: 21-24 (in
Russian).
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute
toxicity data for pesticides. World Rev. Pest. Control, 9: 119-
127.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some
pesticides in Drosophila melanogaster. Ind. Med. Surg., 38: 50-
52.
BENSON, W.R., ROSS, R.D., CHEN JO-YUN, R., BARRON, R.P., &
MASTBROOK, D. (1972) Structure of ethylenethiuram monosulfide.
J. Assoc. Off. Anal. Chem., 55(1): 44-46.
BLACKWELL-SMITH, R., Jr, FINNEGAN, J.K., LARSON, P.S., SAHYOUN,
P.F., DREYFUSS, M.L., & HAAG, H.B. (1953) Toxicologic studies
on zinc and disodium ethylenebisdithiocarbamates. J. Pharmacol.
exp. Therap., 109: 159-166.
BLAZQUEZ, C.H. (1973) Residue determination of ethylene-
thiourea (2-imidazol-idinethione) from tomato foliage, soil, and
water. J. agric. food Chem., 21: 330-332.
BOGARTYKH, T.A., ORLOV, N.V., & KUZNETSOVA, T.V. (1979)
Activity of lactate dehydrogenase and glucose-6-phosphate
dehydrogenase in tissue of the testes under the action of some
pesticides. Vopr. Pitan., 5: 49-53.
BONTOYAN, W.R. & LOOKER, J.G. (1973) Degradation of commercial
ethylenebisdithiocarbamate formulations to ethylenethiourea
under elevated temperature and humidity. J. agric. food Chem.,
21: 338-342.
BONTOYAN, W.R., LOOKER, J.B., KAISER, T.E., GIANG, P., & OLIVE,
B.M. (1972) Survey of ethylenethiourea in commercial
ethylenebisdithiocarbamate formulations. J. Assoc. Off. Anal.
Chem., 55: 923-925.
BOTTOMLEY, P., HOODLESS, R.A., & SMART, N.A. (1985) Review of
methods for the determination of ethylenethiourea (imida-
zolidine-2-thione) residues. Residue Rev., 95: 45-89.
CAVANAGH, J.B. (1973) Peripheral neuropathy caused by chemical
agents. Crit. Rev. Toxicol., 2: 365-417.
CHARKES, N.D., BRAVERMAN, L.E., PENKO, K.F., GOWERS, D.S.,
GORDON, C.F., LIPWORTH, L., & MALMUD, L.S. (1985) Thyroid
function in male workers manufacturing dithane, an agricultural
fungicide, and in men not exposed to dithane. In: Proceedings of
the Ninth International Thyroid Congress, Sao Paulo, Brazil,
1-6 September 1985, p. 89.
CHERNOFF, N., KAVLOCK, R.J., ROGERS, E.H., CARVER, B.D., &
MURRAY, S. (1979) Perinatal toxicity of maneb, ethylene-
thiourea, and ethylene bisisothiocyanate sulfide in rodents. J.
Toxicol. environ. Health, 5: 821-834.
CHERNOV, O.V. & KHISTENKO, I.I. (1969) Blastomogenic
properties of some derivatives of dithiocarbamic acid. Vopr.
Onkol., 15(4): 71-74.
CHERNOV, O.V. & KHISTENKO, I.I. (1973) Some data on the
distribution and elimination of ziram-35S from the organism of
warm-blooded animals. In: Hygiene of using toxicology of
pesticides and the clinical picture of intoxication, Moscow,
Medizina, Vol. 10, pp. 250-254. (in Russian).
CLARKE, D.G., BAUM, H., STANLEY, E.L., & HESTER, W.F. (1951)
Determination of dithiocarbamates. Anal. Chem., 23(12): 1842-
1846.
CONKIN, R.A. & GLEASON, L.S. (1964) Vegadex. In: Zweig, G.,
ed. Analytical methods for pesticides, plant growth regulation
and food additives, New York, London, Academic Press, Vol. 4,
pp. 249-257.
CRUICKSHANK, P.A. & JARROW, H.C. (1973) Ethylenethiourea
degradation. J. agric. food Chem., 21 (3): 333-335.
DALVI, R.R., POORE, R.E., & NEAL, R.A. (1974) Studies of the
metabolism of carbon disulfide by rat liver microsomes. Life
Sci., 14: 1785-1796.
DANIELSSON, B.R.G., OSKARSSON, A., & DENCKER, L. (1984)
Placental transfer and fetal distribution of lead in mice after
treatment with dithiocarbamates. Arch. Toxicol., 55: 27-33.
DAVYDOVA, T.B. (1973) [The influence of tetramethylthiuram
disulfide on the estrous cycle and genital function of animals
when inhaled.] Gig. i Sanit., 38(4): 108-110 (in Russian).
DEKHUYZEN, H.M., VONK, J.W., & KAARS SIJPESTEIJN (1971)
Terminal residues of dithiocarbamate fungicides. In: Tahori,
A.S., ed. Proceedings of the International Symposium on Pesti-
cide Terminal Residues, Tel Aviv, Israel, 1971, London,
Butterworth, pp. 233-241.
DE MATTEIS, F. & SEAWRIGHT, A.A. (1973) Oxidative metabolism
of carbon disulfide by the rat. Effect of treatments which
modify the liver toxicity of carbon disulfide. Chem.-biol.
Interact., 7: 375-388.
DE SERRES, F.S. & ASHBY, J.C. (1981) Report of the
International Collaborative Program for Progress in Mutation
Research, Vol. 1, Amsterdam, Oxford, New York, Elsevier North-
Holland Inc.
DISHOVSKI, H. & IVANOVA-CHEMISHANSKA, L. (1979) Ultra-
structural changes in the neocortex of rats after chronic
dithiocarbamate Antracol. Activ. nerv. sup. (Praha), 21 (4):
279.
EDINGTON, N. & HOWELL, J.McC. (1966) Changes in the nervous
system of rabbits following the administration of sodium
diethyldithiocarbamate. Nature (Lond.), 210(5040): 1060-1062.
EDINGTON, N. & HOWELL, J.McC. (1969) The neurotoxicity of
sodium diethyldithiocarbamate in the rabbit. Acta neuropathol.
(Berlin), 12: 339-347.
EISENBRAND, G., UNGERER, O., & PREUSSMANN, R. (1974) Rapid
formation of carcinogenic N-nitrosamines by interaction of
nitrite with fungicides derived from dithiocarbamic acid in
vitro under simulated gastric conditions and in vivo in the rat
stomach. Food Cosmet. Toxicol., 12: 229-232.
ELDJARN, L. (1950) The metabolism of tetraethylthiuram
disulfide (Antabuse, Aversan) in the rat, investigated by means
of radioactive sulfur. J. clin. lab. Invest., 2: 198-201.
ELESPURU, R.K. & LIJINSKY, W. (1973) The formation of
carcinogenic nitroso compounds from nitrite and some types of
agricultural chemicals. Food Cosmet. Toxicol., 11: 807-817.
ENGST, R. & SCHNAAK, W. (1967) [Studies on the metabolism of
the ethylenebisdithiocarbamate fungicides maneb and zineb.] Z.
Lebensm. Unters. Forsch., 134(4): 216-221 (in German).
ENGST, R. & SCHNAAK, W. (1969a) [Polarographic determination
of ethylene bisthiuram monosulfide.] Z. anal. Chem., 248: 321-
324 (in German).
ENGST, R. & SCHNAAK, W. (1969b) [Analysis of ethylene
bisthiuram monosulfide.] Z. anal. Chem., 244: 254-256 (in
German).
ENGST, R. & SCHNAAK, W. (1969c) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb
(polarographic determination of the undecomposed fraction of
active ingredient).] Z. Lebensm. Unters. Forsch., 139(3): 149-
152 (in German).
ENGST, R. & SCHNAAK, W. (1970a) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 143: 99-103 (in German).
ENGST, R. & SCHNAAK, W. (1970b) [Studies on the metabolism of
the ethylenebisdiothiocarbamate fungicides maneb and zineb.]
Z. Lebensm. Unters. Forsch., 144(2): 81-85 (in German).
ENGST, R. & SCHNAAK, W. (1974) Residues of dithiocarbamate
fungicides and their metabolites on plant foods. Residue Rev.,
52: 45-67.
ENGST, R., SCHNAAK, W., & LEWERENZ, H.J. (1971) [Studies on
the metabolism of the fungicidal
ethylenebisdiothiocarbamatesmaneb, zineb and nabam. V. The
toxicology of breakdown products.] Z. Lebensm Untersuch., 146,
91-97.
FAHRIG, R. (1974) Comparative mutagenicity studies with
pesticides. In: Montesano, R. & Tomatis, L., ed. Chemical
carcinogenesis assays, Lyons, International Agency for Research
on Cancer, pp. 161-181 (IARC Scientific Publication No. 10).
FAO (1985) Production yearbook 1984, Rome, Food and
Agriculture Organization of the United Nations.
FAO/WHO (1964) Evaluation of the toxicity of pesticide
residues in food. Report of a Joint Meeting of the FAO Committee
on Pesticides in Agriculture and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide
residues in food. Report of the Second Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert
Committee on Pesticide Residues, Geneva, World Health
Organization (FAO Meeting Report No. PL:1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide
residues in food, Geneva, World Health Organization (FAO Meeting
Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL: 1967/M/1/1; WHO
Food Add./68.30).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO
Food Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies
No. 97; WHO Technical Report Studies No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 10
Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations(FAO Plant Production and Protection Paper No. 10 Sup.).
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization (FAO
Plant Production and Protection Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20
Sup.).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 26 Sup.).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 67).
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985
Joint Meeting of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the WHO Expert Group on
Pesticide Residues, Rome, Food and Agriculture Organization of
the United Nations (FAO Plant Production and Protection Paper
No. 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues
in food, Part II - Toxicology, Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper No. 72).
FARROW, R.P. & RALLS, J.W. (1970) Investigation of changes in
pesticide residues during processing and storage of fruit and
vegetables, National Canners Association, 109 pp (Report No.
5).
FISHBEIN, L. (1975) Chromatography of environmental hazards.
III. Pesticides, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
FITZHUGH, O.G., WINTER, W.J., & NELSON, A.A. (1952) Some
observations on the chronic toxicity of antabuse (tetraethyl-
thiuramdisulfide). Fed. Proc., 11: 345-346.
FREUDENTHAL, R.I., KERCHNER, G.A., PERSING, R.L., BAUMEL, J., &
BARON, R.L. (1977) Subacute toxicity of ethylene bisisocyanate
sulfide in the laboratory rat. J. Toxicol. environ. Health, 2:
1067-1077.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Différence de
sensibilité du hamster et du rat vis-ą-vis des effets de
l'administration ą long terme de l'éthylčne thiourée. Eur. J.
Toxicol., 9: 303-312.
GARDNER-THORPE, C. & BENJAMIN, S. (1971) Peripheral neuropathy
after disulfiram administration. J. Neurol. Neurosurg.
Psychiatr., 34: 253-259.
GESSNER, T. & JAKUBOWSKI, M. (1972) Diethyldithiocarbamic acid
methyl ester, a metabolite of disulfiram. Biochem. Pharmacol.,
21: 219-230.
GOWERS, D.S. & GORDON, C.F. (1980) Some public health aspects
of the manufacture and use of zinc and manganese
ethylenebisdithiocarbamate fungicides Poznan, Poland, Institut
Ochrony Roslin, February 1979 pp. 491-522.
GRAHAM, S.L. & HANSEN, W.H. (1972) Effects of short-term
administration of ethylenethiourea on thyroid function of the
rat. Bull. environ. Contam. Toxicol., 7: 19-25.
GRAHAM, S.L., DAVIS, K.J., & PERRY, C.H. (1973) Effects of
one-year administration of ethylenethiourea upon thyroid
function of the rat. J. agric. food Chem., 21(3): 324-329.
GRAHAM, S.L., DAVIS, K.J., HANSEN, W.H., & GRAHAM, C.H. (1975)
Effects of prolonged ethylenethiourea ingestion on the thyroid
of the rat. Food Cosmet. Toxicol., 13: 493-499.
GRAHAM, W.H. & BORNAK, W.E. (1973) Improved experimental
technique for reverse isotope dilution method.
Anal. Chem., 45: 623-624.
GRIEPENTROG, F. (1960) [Allergy studies with simple chemical
substances.] Arzneimittelforschung, 10: 542-544 (in German).
GRIEPENTROG, F. (1962) [Tumor-like thyroid lesions in animal
studies of chronic toxicity of thiurams.] Beitr. Pathol.
Anat., 126: 243-255 (in German).
GUSTAFSSON, K.H. & THOMPSON, R.A. (1981) High-pressure liquid
chromatographic determination of fungicidal dithiocarbamates. J.
agric. food Chem., 29: 729-732.
HAINES, L.D. & ADLER, I.L. (1973) Gas chromatographic deter-
mination of ethylenethiourea residues. J. Assoc. Off. Anal.
Chem., 56 (2): 333-337.
HALD, J. & JACOBSEN, E. (1948) The formation of acetaldehyde
in the organism after ingestion of Antabuse (tetraethylthiuram
disulfide) and alcohol. Acta pharmacol. toxicol., 4: 305-310.
HALD, J., JACOBSEN, E., & LARSEN, V. (1948) The sensitizing
effect of tetraethylthiuram disulphide (Antabuse) to ethyl
alcohol. Acta pharmacol. toxicol., 4: 285-296.
HEDENSTEDT, A., RANNUG, U., LAMEL, C., & WACHTMEISTER, C.A.
(1979) Mutagenicity and metabolism studies on 12 thiram and
dithiocarbamate compounds used as accelerators in the Swedish
rubber industry. Mutat. Res., 68: 313.
HELLING, C.S., DENNISSON, D.C., & KAUFMAN, D.D. (1974)
Fungicide movement in soil. Phytopathology, 64(8): 1091-1100.
HOAGLAND, R.E. & FREAR, S.D. (1976) Behaviour and fate of
ethylenethiourea in plants. J. agric. food Chem., 24: 129-133.
HODGE, H.C., MAYNARD, Z.A., DOWNS, W., BLANCHET, H.J., Jr, &
JONES, C.K. (1952) Acute and short-term oral toxicity tests of
ferric dimethyldithiocarbamate (ferbam) and zinc dimethyldithio-
carbamate (ziram). J. Am. Pharmacol. Assoc., 41(12): 662-665.
HODGE, H.C., MAYNARD, E.A., DOWNS, W.L., COYE, R.D., Jr, &
STEADING, L.T. (1956) Chronic oral toxicity of ferric di-
methyldithiocarbamate (ferbam) and zinc dimethyldithiocarbamate
(ziram). J. Pharmacol. exp. Ther., 118: 174-181.
HODGSON, J.R., CASTLES, T.R., MURRILL, E., & LEE, C.C. (1974)
Distribution, excretion, and metabolism of the fungicide ferbam
in rats. Fed. Proc., 33: 537.
IARC (1974) Some antithyroid and related substances, nitro-
furans and industrial chemicals, Lyons, International Agency for
Research on Cancer, pp. 45-52 (Monographs on the Evaluation of
Carcinogenic Risk of Chemicals to Man, Vol. 7).
IARC (1976) Some carbamates, thiocarbamates, and carbazides,
Lyons, International Agency for Research on Cancer (Monographs
on the Evaluation of Carcinogenic Risk of Chemicals to Man, Vol.
12).
IARC (1977) Some miscellaneous pharmaceutical substances,
Lyons, International Agency for Research on Cancer p. 243.
(Monographs on the evaluation of carcinogenic risk of chemicals
to humans, Vol. 13).
IARC (1983) Chemicals, industrial processes and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 128-130 (Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
INNES, J.R.M., ULLAND, B.M., VALERIO, M.G., PETRUCELLI, L.,
FISHBEIN, L., HART, E.R., PALLOTTA, A.J., BATES, R.R., FALK,
H.L., GART, J.J., KLEIN, M., MITCHELL, I., & PETERS, J. (1969)
Bioassay of pesticides and industrial chemicals for tumour-
igenicity in mice: a preliminary note. J. Natl Cancer Inst., 42:
1101-1114.
IRPTC (1982) Data profile on ziram, Geneva, International
Register for Potentially Toxic Chemicals.
IRPTC (1983) Legal file - 2 vols, Geneva, International
Register for Potentially Toxic Chemicals.
IUPAC (International Union of Pure and Applied Chemistry) (1977)
Ethylenethiourea. Pure appl. Chem., 49: 675-689.
IVANOVA-CHEMISHANSKA, L. (1969a) Toxicological characteristics
of dithiocarbamates zineb, maneb, and mancozeb, Sofia,
(Dissertation).
IVANOVA-CHEMISHANSKA, L. (1969b) Toxicological characteristics
of mancozeb. Hig. i. Zdraveopazvane, 12(5): 417-426.
IVANOVA-CHEMISHANSKA, L. & ANTOV, G. (1980) Changes in the
gonads reproduction of white rats under the effect of some
pesticides. Arch. Toxicol., Suppl. 4: 459-462.
IVANOVA-CHEMISHANSKA, L., SHEYTANOV, M., & MOSHEVA-IZMIROVA, N.
(1967) Changes in the functional state of the thyroid gland
upon acute intoxication with certain dithiocarbamates: zineb and
maneb. Med. Toxicol., 20(9): 1011-1013.
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., & DASHEV, G. (1971)
Light and electron microscopic observation on rat thyroid after
administration of some dithiocarbamates. Environ. Res., 4: 201-
212.
IVANOVA-CHEMISHANSKA, L., KALOYANOVA, F., IZMIROVA, N., ZLATEVA,
M., & VALCHEVA, V. (1972) [Experimental inhalatory toxicity and
substantiation of the maximum allowable concentration for some
dithiocarbamates in the air of the working area.] Truh. Dzihot.,
23: 55-64 (in Russian).
IVANOVA-CHEMISHANSKA, L., VALCHEVA, V., & TAKEVA, TZ. (1973)
Effect of chronic peroral poisoning with perozine (zineb) on the
reproduction of white rats. Acta med. soc., 1: 107-108.
IVANOVA-CHEMISHANSKA, L., MILANOV, ST., CHEMISHANSKI, G., &
DASHEV, G. (1974) Attempt of biological dose of some
hypophyseal hormones in white rats after experimental oral
administration of zineb. Environ. Res., 8: 160-165.
IVANOVA-CHEMISHANSKA, L., PETROVA-VERGIEVA, T., & MIRKOVA, E.
(1975a) [Embryotoxic and teratogenic action of some
pesticides.] Eksp. Med. Morfol., 14(1): 29-33 (in Russian).
IVANOVA-CHEMISHANSKA, L., MARKOV, D.V., MILANOV, S.,
STRASHIMIROV, D.D., DASHEV, G.I., & CHEMISHANKSKA, G.A. (1975b)
Effects of subacute oral administration of zinc ethylene bisdi-
thiocarbamate on the thyroid gland and adenohypophysis of the
rat. Food Cosmetol. Toxicol., 13: 445-447.
IZMIROVA, N. & MARINOV, V. (1972) [Distribution and excretion
of 35S-ziram and metabolic products after 24 hours following
oral administration of the preparation in female rats.] Eksp.
Med. Morfol., 11(3): 152-156 (in Russian).
IZMIROVA, N., IZMIROVA, I., & IVANOVA, L. (1969) The effect of
zineb and maneb on the isoenzymes of lactate dehydrogenase in
the testes of rats. C. R. Acad. Bulg. Sci., 22(2): 225-227.
JORIS, S.J., ASPILA, K.I., & CHAKRABARTI, C.L. (1970) Decompo-
sition of monoalkyl dithiocarbamates. Anal. Chem., 42(6): 647-
651.
KAARS SIJPESTEIJN, A.K. & VONK, J.W. (1970) Microbial conver-
sion of dithiocarbamate fungicides. Med. Fac. Landbouwwet.
Gent, 35(2): 799-804.
KALOYANOVA, F., IVANOVA, L., & ALEXIEV, B. (1967) The
influence of large doses of maneb on the progeny of albino rats.
Med. Toxicol., 20(10): 1109-1112.
KAMPMEIER, C. & HAAG, H.B. (1954) Toxicological considerations
in use of dithiocarbamates. Agric. Chem., April: 49-50, 133.
KASKEVICH, L.M., KOMAROVA, L.I., ILINA, V.I., IVANICKI, V.A., &
SHILINA, V.F. (1981) [Clinical-functional changes due to
exposure to zineb,] pp. 109-111 (in Russian).
KASLANDER, J. (1963) Formation of an S-glucuronide from
tetraethylthiuram disulfide (Antabuse) in man. Biochem. Biophys.
Acta, 71: 730-732.
KATO, Y., ODANAKA, Y., TERAMOTO, S., & MATANO, O. (1976) Meta-
bolic fate of ethylenethiourea in pregnant rats. Bull. environ.
Contam. Toxicol., 16(5): 546-555.
KAUFMAN, D.D. & FLETCHER, C.L. (1973) Title? In: Proceedings
of the Second International Congress on Plant Pathology,
Minneapolis, Minnesota (Abstract No. 1018).
KHERA, K.S. (1973) Ethylenethiourea: teratogenicity study in
rats and rabbits. Teratology, 7: 243-252.
KHERA, K.S. & TRYPHONAS, L. (1977) Ethylenethiourea induced
hydrocephalus: pre- and postnatal pathogenesis in offspring from
rats given a single oral dose during pregnancy. Toxicol. appl.
Pharmacol., 42: 85-97.
KIM, S.U. & RIZZUTO, N. (1975) Neuroaxonal degeneration
induced by sodium diethyldithiocarbamate in cultures of central
nervous tissue. J. Neuropath. exp. Neurol., 34: 531-541.
KING, R.R. (1977) Derivatization of ethylenethiourea with m-
trifluoromethylbenzylchloride for analysis by electron capture
gas chromatography. J. agric. food Chem., 25(1): 73-75.
KIRKBRIGHT, G.F. & MULLINS, F.G.P. (1984) Separation of
dithiocarbamates by high-performance liquid chromatography using
a micellar mobile phase. Analyst, 109: 493-496.
KLIGMAN, A.M. & ROSENSWEIG, W. (1948) Studies with new
fungistatic agents. I. For the treatment of superficial
mycoses. J. invest. Derm., 10: 59-68.
KOIZUMI, A., SHIOJIMA, S., OMIYA, M., NAKANO, S., SATO, N., &
IDEDA, M. (1979) Acute renal failure and maneb (manganous
ethylene bisdithiocarbamate) exposure. J. Am. Med. Assoc.,
242(23): 2583-2585.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982a) Embryo-
toxicity of industrial chemicals on the chicken embryo:
thiourea derivatives. Acta pharmacol. toxicol., 51: 38-44.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1982b) Application
of the chicken embryo in testing for embryotoxicity. Scand. J.
Work environ. Health, 8: 63-69.
KURINNY, A.I. & KONDRATENKO, T.I. (1972) [Effect of some
fungicides (dithiocarbamic acid derivatives) on chromosomes of
bone marrow cells in mice.] Tsitol. Genet., 6: 225-228 (in
Russian).
LABORIE, F. & LABORIE, R. (1966) Allergies aux fongicides et
prophylaxie biochimique (relations entre allergie et
intoxications). Rev. Pathol. comp., 66: 105-109.
LAKOMAA, E.L., SATO, S., GOLDBERG, A.M., & FRAZIER, J.M. (1982)
The effect of sodium diethyldithiocarbamate treatment on copper
and zinc concentrations in rat brain. Toxicol. appl. Pharmacol.,
65: 286-290.
LANGE, P. & JUNG, F. (1971) [Reduction of the toxicity to the
liver of carbon tetrachloride by diethyldithiocarbamate and 3-
(O-tolyl)-4-(nitro)-sydnone.] Acta biol. med. Ger., 27: 425-434
(in German).
LARSSON, K.S., ARNANDER, C., CEKANOVA, E., & KJILLBERG, M.
(1976) Studies of teratogenic effects of the dithiocarbamates
maneb, mancozeb, and propineb. Teratology, 14: 171-183.
LEE, C.C. & PETERS, P.J. (1976) Neurotoxicity and behavioural
effects of thiram in rats. Environ. Health Perspect., 17: 35-
42.
LEHMAN, A.J. (1951) Chemicals in food: a report of the
association of food and drug officials on current developments,
II, Pesticides, Q. Bull. Fed. Drug Off.U.S., 15: 122-133.
LEWERENZ, H.J., BLEYL, D., PLASS, R., & ENGST, R. (1975)
Toxicology of ETU , Potsdam, Rehbrüke, German Democratic
Republic, Central Institute for Nutrition, Academy of Science.
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972) Carcino-
genic nitrosamines formed by drug/nitrite interactions. Nature
(Lond.), 239: 165-167.
LOWY, R., GRIFFATON, G., BRIGANT, L., ARDONIN, B., & DUPUY, F.
(1979) The dietary no-effect level of a dithiocarbamate
fungicide, thiram, as evaluated from measurement data on rats.
II. The various sensitivities of the various parameters.
Toxicology, 14: 39-53.
LOWY, R., GRIFFATON, G., & PELISIER, M.A. (1980) The dose-
response relationship of rat organ weights under dietary thiram
influence. Toxicol. Lett., 5(Suppl. 1): 148.
LU, M.H. & STAPLES, R.E. (1978) Teratogenicity of ethylene-
thiourea and thyroid function in the rat. Teratology, 17: 171-
178.
LUDWIG, R.A. & THORN, G.D. (1962) Chemistry and mode of action
of dithiocarbamate fungicides. Adv. Pestic. Control Res., 3:
219-252.
LUTZ, L.M., GLENDA, E.A., Jr, & RECKNAGEL, R.O. (1973) Protec-
tion by diethyldithiocarbamate against carbon tetrachloride
lethality in rats and against carbon tetrachloride-induced lipid
peroxidation in vitro. Biochem. Pharmacol., 22: 1729-1734.
LYMAN, W.R. (1971) The metabolic fate of Dithan M-45
(coordination product of zinc ion and manganous ethylene
bisdithiocarbamate). In: Tahori, A.S., ed. International
Symposium on Pesticide Terminal Residues, Tel Aviv, 1971,
Israel, London, Butterworth, pp. 243-256.
LYMAN, W.R. & LACOSTE, R.J. (1974) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
In: Proceedings of the 3rd International IUPAC Congress on
Pesticide Chemistry, Helsinki, 3-9 July, 1974, Stuttgart, George
Thieme Publishers, pp. 67-74.
LYMAN, W.R. & LACOSTE, R.J. (1975) New developments in the
chemistry and fate of ethylene bisdithiocarbamate fungicides.
Environ. Qual. Saf., Suppl. 3: 67-74.
McCANN, J. & PITCHER, F. (1973) Fish toxicity laboratory
reports Nos. 535 and 541, Beltsville, Maryland, US Environmental
Protection Agency, Animal Biology Laboratory.
MAJ, J., GRABOWSKA, M., & KWIEK, J. (1970) The effect of
disulfiram, diethyldithiocarbamate, and dimethyldithiocarbamate
on serotonin and 5-hydroxyindole-3-acetic acid brain levels in
rats. Biochem. Pharmacol., 19: 2517-2519.
MARSHALL, W.D. (1977) Thermal decomposition of ethylene
bisdithiocarbamate fungicides to ethylenethiourea in aqueous
media. J. agric. food Chem., 25: 357-361.
MASSEY, R.C., KEY, P.E., & MCWEENY, D.J. (1982) Analysis of
ethylenethiourea in beer by high-performance liquid
chromatography. J. Chromatogr., 240: 254-256.
MATOKHNYUK, L.A. (1971) Toxicity of the fungicide maneb on
inhalation. Hyg. Sanit., 36(5): 195-199.
MATTHIASCHK, G. (1973) [Influence of L-cysteine on
teratogenisis caused by thiram (TMTD) in NMRI mice.] Arch.
Toxikol., 30: 251-262 (in German).
MELNIKOV, N.N. & TRUNOV, P.P. (1966) Persistence of residual
quantities of preparations on the basis of dithiocarbamate acid
derivatives. Chem. Agric. IV, 10(36): 21-24.
MINOR, J.L., RUSSEL, J.Q., & LEE, C.C. (1974) Reproduction and
teratology studies with the fungicide ferbam. Toxicol. appl.
Pharmacol., 29(16): 120.
MOJE, W., MUNNECKE, D.E., & RICHARDSON, L.T. (1964) Carbonyl
sulfide, a volatile fungitoxicant from nabam in soil. Nature
(Lond.), 202: 831-832.
NASH, R.G. (1974) Improved gas-liquid chromatographic method
for determining ethylenethiourea in plants. J. Assoc. Off. Anal.
Chem., 57(5): 1015-1021.
NASH, R.G. (1975) In: Proceedings of the 170th Meeting of the
American Chemical Society for Pesticides (Abstract No. 1).
NASH, R.G. & BEALL, M.L., Jr (1980) Fate of maneb and zineb
fungicides in microagroecosystem chambers. J. agric. food Chem.,
28: 322-330.
NEWSOME, W.H. (1972) Determination of ethylenethiourea
residues in apples. J. agric. food Chem., 20: 967-969.
NEWSOME, W.H. (1974) The excretion of ethylenethiourea by rat
and guinea-pig. Bull. environ. Contam. Toxicol., 11: 174-176.
NEWSOME, W.H. (1976) Residues of four ethylene bisdithio-
carbamates and their decomposition products on field-sprayed
tomatoes. J. agric. food Chem., 24(5): 999-1001.
NEWSOME, W.H. (1978) A method for the determination of
ethyleneurea in foods as the pentafluorobenzamide. J. agric.
food Chem., 26(6): 1325-1327.
NEWSOME, W.H. & LAVER, G.W. (1973) Effect of boiling on the
formation of ethylenethiourea in zineb-treated foods. Bull.
environ. Contam. Toxicol., 10(3): 151-154.
ODEYEMI, O. (1980) Persistence of arasan pesticide in aquatic
ecosystems in Nigeria. Pestic. Abstr., 80: 1276.
ONLEY, J.H. (1977) Gas-liquid chromatographic method for
determining ethylenethiourea in potatoes, spinach, apple sauce,
and milk. Collaborative study. J. Assoc. Off. Anal. Chem.,
60(5): 1111-1115.
ONLEY, J.H. & YIP, G. (1971) Determination of ethylenethiourea
residues in foods using thin-layer and gas chromatography. J.
Assoc. Off. Anal. Chem., 54(1): 165-169.
OSKARSSON, A. (1983) Redistribution and increased brain uptake
of lead in rats after treatment with diethyldithiocarbamate.
Arch. Toxicol., 6: 279-284.
OSKARSSON, A. (1984) Dithiocarbamate induced redistribution
and increased brain uptake of lead in rats. Neurotoxicology,
5(3): 283-294.
OSKARSSON, A. & LIND, B. (1985) Increased lead levels in brain
after long-term treatment with lead and dithiocarbamate or
thiuram derivatives in rats. Acta pharmacol. toxicol., 56: 1-6.
OTTO, S., KELLER, W., & DRESCHER, N. (1977) A new gas
chromatographic determination of ethylenethiourea residues
without derivatization. J. environ. Sci. Health, B12(3): 179-
191.
PECKA, Z., BAULU, P., & NEWSOME, H. (1975) Preliminary survey
of ethlenethiourea residues in the Canadian food supply, 1972.
Pestic. monit. J., 8(4): 232-234.
PERIQUET, A. & DERACHE, R. (1976) Influence d'un régime
alimentaire hypoprotéique sur la toxicité aigue d'un pesticide,
le nabame. Ann. Nutr. Aliment., 30: 29-44.
PETROVA-VERGIEVA, T. (1976) On the teratogenic activity of
zinc-propylene bisdithiocarbamate. Hig. i. Zdraveopazvane,
19(5): 435-442.
PETROVA-VERGIEVA, T. & IVANOVA-CHEMISHANSKA, L. (1973)
Assessment of the teratogenic activity of dithiocarbamate
fungicides. Food Cosmet. Toxicol., 11: 239-244.
PHILLIPS, W.E.J. (1968) Changes in the nitrate and nitrite
contents of fresh and processed spinach during storage. J.
agric. food Chem., 16: 88-91.
PHILLIPS, W.F., GRADY, M.D., & FREUDENTHAL, R. (1977) Effects
of food processing on residues of ethylene bisdithiocarbamate
fungicides and ethylenethiourea, Washington DC, US Environmental
Protection Agency (EPA No. 600/1-77-021).
PILINSKAYA, M.A. (1971) [Cytogenetic effect of the fungicide
ziram in the culture of human lymphocytes in vitro. ] Genetika,
7: 138-143 (in Russian).
PLUYGERS, C.W., VONK, J.W., & THORN, G.D. (1971) Re-
examination of the structure of ethylenethiuram monosulfide.
Tetrahedron Lett., 18: 1317-1318.
PRZEZDZIECKI, Z., BANKOWSKA, J., KOMOROWSKA-MALEWSKA, W., &
JANICKA, T. (1969) Studies of the chronic short-term toxicity
of zineb, maneb, and kaptan. Rocz. Panstw. Zakl. Hlg., 20: 133-
140.
PRICKETT, C.S. & JOHNSTON, C.D. (1953) The in vivo production
of carbon disulfide from tetraethylthiuram disulfide
(AntabuseR). Biochem. Biophys. Acta, 12: 542-546.
PRINCE, J.L. (1985) Analysis of ethylenethiourea in urine by
high-performance liquid chromatography. J. agric. food Chem.,
33: 93-94.
RAGHU, K., MURTHY, N.B.K., KUMARASWAMY, R., RAO, R.S., & SANE,
P.V. (1975) In: Proceedings of the Joint FAO/IAEA, Division of
Atomic Energy in Food and Agriculture, Vienna, p. 137.
RASUL, A.R. & HOWELL, J.M. (1974) The toxicity of some
dithiocarbamate compounds in young and adult domestic fowl.
Toxicol. appl. Pharmacol., 30: 63-78.
RHODES, R.C. (1977) Studies with manganese 14C-ethylene
bisdithiocarbamate (14C-maneb) fungicide and 14C-ethylenethio-
urea (14C-ETU) in plants, soil, and water. J. agric. food Chem.,
25(3): 528-533.
RJAZANOVA, R.A. (1967) [The effects of the fungicides ziram and
zineb on the generative function of test animals.] Gig. i
Sanit., 2: 26-30 (in Russian).
ROLANDI, A., DE MARINIS, E., & DE CATERINA, M. (1984) Dithio-
carbamate pesticides: activity of propineb in the micronucleus
test in mice. Mutat. Res., 135: 193-197.
ROLL, R. (1971) [Teratological experiments with thiram (TMTD)
on two mouse strains.] Arch. Toxikol., 27: 173-186 (in German).
ROSE, D., PEARSON, C.M., ZUKER, M., & ROBERTS, J.R. (1980)
Ethylenethiourea: criteria for the assessment of its effects on
man, Ottawa, National Research Council, Associate Committee on
Scientific Criteria for Environmental Quality (NRCC Publication
No. 18469).
ROSS, R.D. & CROSBY, D.G. (1973) Photolysis of ethylene-
thiourea. J. agric. food Chem., 21(3): 335-337.
ROSS HART, E. & VALERIO, M.G. (1973) Evaluation of goitrogenic
activity in rats: Ethylene thiourea, practical and Dithane M-45,
Communication from Litton Bionetics to Rohm and Haas Co. Cited
in Van Leeuwen et al., (1982)
RUDDICK, J.A., WILLIAMS, D.T., HIERLIHY, L., & KHERA, K.S.
(1976a) 14C-ethylenethiourea: distribution, excretion, and
metabolism in pregnant rats. Teratology, 13: 35-39.
RUDDICK, J.A., NEWSOME, W.H., & NASH, L. (1976b) Correlation
of teratogenicity and molecular structure: ethylenethiourea and
related compounds. Teratology, 13: 263-266.
SATO, F. & TOMIZAWA, C. (1960) Decomposition of zinc ethylene
bisdithiocarbamate (zineb) applied to plants. Bull. Natl Inst.
Agric. Sci. (Tokyo) Ser. C, 12: 181-187.
SCHULTHEISS, E. (1957) [The therapeutic use of
tetramethylthiuram disulfide.] Dermatol. Wochenschr., 7: 160-162
(in German).
SCHUPBACH, M. & HUMMLER, H. (1976) Evaluation of the
mutagenicity of ethylenethiourea using bacterial and mammalian
test systems. Mutat. Res., 28: 122.
SCHUPBACH, M. & HUMMLER, H. (1977) A comparative study on the
mutagenicity of ethylenethiourea in bacterial and mammalian test
systems. Mutat. Res., 56: 111-120.
SEIDLER, H., HARTIG, M., SCHNAAK, W., & ENGST, R. (1970)
[Studies on the metabolism of certain insecticides and
fungicides in the rat. II. Mean distribution and breakdown of
14C-labelled maneb.] Nahrung, 14(5): 363-373 (in German).
SEIFTER, J. & EHRICH, W.E. (1948) Goitrogenic compounds:
pharmacological and pathological effects. J. Pharmacol. exp.
Ther., 92: 303-314.
SEILER, J.P. (1973) A survey on the mutagenicity of various
pesticides. Experientia, (Basel) 29: 622-623.
SEILER, J.P. (1974) Ethylenethiourea (ETU): a carcinogenic and
mutagenic metabolite of ethylene bisdithiocarbamate. Mutat.
Res., 26: 189-191.
SEN, N.P., DONALDSON, B.A., & CHARBONNEAU, C. (1974) Formation
of nitrosodimethylamine form the interaction of certain
pesticides and nitrite. In: Bogorski, P., Walker, E.A., & Davis,
W., ed. N-nitroso compounds in the environment, Lyons,
International Agency for Research on Cancer, pp. 75-79 (IARC
Scientific Publication No. 9).
SHERMAN, H. & ZAPP, J.A. (1966) Three generation reproduction
study, Manzate DR (88% maneb). Unpublished report from Haskell
Laboratory submitted to the Word Health Organization by E.I. du
Pont de Nemours and Co.
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHIRASU, Y., MORIYA, M., KATO, K., LIENARD, F., TEZUKA, H.,
TERAMOTO, S., & KADA, T. (1977) Mutagenicity screening on
pesticides and modification products: a basis of carcinogenicity
evaluation. In: Hiatt, H.H., Watson, J.D., & Winsen, J.A.,
ed. Origins of human cancer, Book A, Cold Spring Harbor, New
York, Cold Spring Harbor Laboratory, Vol.4, pp. 267-285.
SHORT, R.D., Jr, RUSSEL, J.Q., MINOR, J.L., & LEE, C.C. (1976)
Developmental toxicity of ferric dimethyldithiocarbamate and
bis(dimethylthiocarbamoyl) disulfide in rats and mice. Toxicol.
appl. Pharmacol., 35: 83-94.
SHORT, R.D., MINOR, J.L., UNGER, T.M., BREEDEN, B., VAN GOETHEM,
D., & LEE C.C. (1980) Teratology of a zineb formulation.
(Results of a study performed at Midwest Research Institute for
the US Environmental Protection Agency submitted to the World
Health Organization) (EPA-600/1-80-017).
SMITH, D. (1976) Ethylenethiourea: a study of possible
teratogenicity and thyroid carcinogenicity. J. Soc. Occup. Med.,
26: 92-94.
SMITH, D.M. (1984) Ethylenethiourea: thyroid function in two
groups of exposed workers. Br. J. ind. Med., 41: 362-366.
SPRECHER, D. & GRIGOROWA, R. (1967) [Maximum workplace
concentration of thiuram.] Z. gesamte Hyg., 13: 241-245 (in
German).
STROMME, J.H. (1965) Metabolism of disulfiram and diethyl-
dithiocarbamate in rats with demonstration of an in vivo
ethanol-induced inhibition of the glucuronic acid conjugation of
the thiol. Biochem. Pharmacol., 14: 393-410.
STROMME, J.H. & ELDJARN, L. (1966) Distribution and chemical
forms of diethyldithiocarbamate and tetraethylthiuram disulfide
(disulfiram) in mice in relation to radioprotein. Biochem.
Pharmacol., 15: 287-297.
SUNDERMAN, F.W., Jr & FRASER, C.B. (1983) Effects of nickel
chloride and diethyl dithiocarbamate on metallothionein in rat
liver and kidney. Ann. clin. lab. Sci., 13 (6): 489-495.
SZYMEZYK, T. (1981) The mutagenicity of the fungicide thiram.
Mutat. Res., 85: 223-224.
TAKAYAMA, S., AIHARA, K., ONODERA, T., & AKIMOTO, T. (1986)
Antithyroid effects of propylthiouracil and sulfamonomethoxine
in rats and monkeys. Toxicol. appl. Pharmacol., 82: 191-199.
TANNENBAUM, S.R., SINSKEY, A.J., WEISSMAN, M., & BISHOP, W.
(1974) Nitrite in human saliva: its possible relationship to
nitrosamine formation. J. Natl Cancer Inst., 53: 79-84.
TERAMOTO, S., MORIYA, M., KATO, K., TEZUKA, H., NAKAMURA, S.,
SHINGU, A., & SHIRASU, Y. (1977) Mutagenicity testing on
ethylenethiourea. Mutat. Res., 56: 121-129.
TERAMOTO, S., SHINGU, A., KANEDA, M., & SAITO, R. (1978a)
Teratogenicity studies with ethylenethiourea in rats, mice, and
hamsters. Congenital Anom., 18: 11-17.
TERAMOTO, S., SHINGU, A., & SHIRASU, Y. (1978b) Induction of
dominant lethal mutations after administration of ethylene-
thiourea in combination with nitrite or of N-nitrosoethylene-
thiourea in mice. Mutat. Res., 56: 335-340.
TERAMOTO, S., KANEDA, M., KATO Y. & SHIRASU Y. (1980) Failure
of inducing malformations after intro-amniotic injections of
ethylenethiourea in the rat. Congenital Anom., 20: 17-23.
TERAMOTO, S., KANEDA, H., AOYAMA, & SHIRASU, Y. (1981)
Correlation between the molecular structure of N-alkylureas and
N-alkylthioureas and their teratogenic properties.
Teratology, 23: 335-342.
TETSUMI, T., SUMI, M., TANAKA, M., & SHONO, T. (1985) Reversed
phase ion interaction chromatography of sodium dialkyldithio-
carbamates in the presence of tetraalkylammonium salts. J.
Chromatogr., 348: 389-395.
THORN, G.D & LUDWIG, R.A. (1962) The dithiocarbamates and
related compounds, Amsterdam, Oxford, New York, Elsevier
Science Publishers, pp. 298.
TOLLENAAR, F.D. (1956) [Recent developments in the use of
antioxidants in foodstuffs.] Dtsch. Lebensm. Rundschau, 12:
307-313 (in German).
TRUHAUT, R., GUERINOT, F., & BOHUON, C. (1971) Mécanisme bio-
chimique de toxicité des fongicides de la série des dithiocarba-
mates: L'action inhibitrice de la dopamine beta-hydroxylase. Ann.
pharm. fr., 29(2): 117-124.
TURNER, N.J. & CORDEN, M.E. (1963) Decomposition of sodium-
N-methyldithiocarbamate in soil. Phytopathology, 53: 1388-
1394.
ULLAND, B.M., WEISBURGER, J.H., WEISBURGER, E.K., RICE, J.M., &
CYPHER, R. (1972) Brief communication: thyroid cancer in rats
from ethylenethiourea intake. J. Natl Cancer Inst., 49: 583-
584.
US EPA (1977) Rebuttable presumption against registration and
continued registration of pesticide products containing ethylene
bisdithiocarbamates. Fed. Reg., 42(154): 40618-40627.
US EPA (1982a) Ethylene bisdithiocarbamates: final resolution
of rebuttable presumption against registration, Washington
DC, US Environmental Protection Agency, Office of Pesticides and
Toxic Substances (Unpublished report).
US EPA (1982b) Ethylene bisdithiocarbamates: notice of
determination concluding the presumption against registration;
notice of availability of decision document, Washington DC, US
Environmental Protection Agency (Unpublished report 82P-2851).
US EPA (1984) Health and environmental effects: profile for
ethylenethiourea (2-imidazolidinethione), Cincinnati, Ohio, US
Environmental Protection Agency, Environmental Criteria and
Assessment Office (Program Office Draft ECAO-CIN-019).
VACHKOVA-PETROVA, R. (1977) [A study on mutagenic activity of
basfungine on white rats.] Hig. i. Zdraveopazvane, 20(5): 422-
426 (in Russian).
VACHKOVA-PETROVA, R. (1981) Mutagenicity study of endodan in
rats. Mutat. Res., 85(4): 288.
VAN ESCH, G.J. (1956) [Toxicity of tetramethylthiuram
disulfide and tetraethylthiuram disulfide.] Versl.
Volksgezondheid, Vol. No. 166-169 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN LEEUWEN, F.X.R., VOOGD, C.E., KNAAP, A.G.A.C., DEN
TONKELAAR, E.M., & VAN DER HEIJDEN, C.A. (1982) Toxicological
evaluation of ethylenethiourea (ETU), Bilthoven, National
Institute of Public Health (Unpublished Report No. 627915/008).
VAN LOGTEN, M.J. (1972) [ Thesis: the dithiocarbamate-alcohol
reaction by the rat. ] Terborg, The Netherland, Fa. Lammers (in
Dutch).
VAN STEENIS, G. & VAN LOGTEN, M.J. (1971) Neurotoxic effect of
the dithiocarbamate tecoram on the chick embryo. Toxicol. appl.
Pharmacol., 19: 675-686.
VEKSHTEIN, M.SH. & KHITSENKO, I.L. (1971) Ziram metabolism in
warm-blooded animals. Gig. i Sanit., 36: 28-33.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index: JMPR 1961-84, Geneva, World Health Organization
(Unpublished report).
VOGELER, K., DREZE, Ph., RAPP, A., STEFFAN, H., & ULLEMEYER, H.
(1977) Distribution and metabolism of propineb in apples and
grapes, including their breakdown products propylenethiourea and
ethylenethiourea in apples. Pflanzenschutz-Nachrichten Bayer
30: 72-97.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1970) Studies on the fate
in plants of ethylene bisthiocarbamate fungicides and their
decomposition products. Ann. appl. Biol., 65: 489-496.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1971) Tentative
identification of 2-imidazoline as a transformation product of
ethylenebisdithiocarbamate fungicides. Pestic. Biochem.
Physiol., 1: 163-165.
VONK, J.W. & KAARS SIJPESTEIJN, A. (1976) Formation of
ethylenethiourea from 5,6-dihydro-3 H-imidazo[2,1-C]-1,2,4-
dithiazole-3-thione by microorganisms and reducing agents. J.
environ. Sci. Health, B11: 33-47.
WATTS, R.R., STORHERR, R.W., & ONLEY, J.H. (1974) Effects of
cooking on ethylene bisdithiocarbamate degradation to
ethylenethiourea. Bull. environ. Contam. Toxicol., 12: 224-226.
WEDIG, J., COWAN, A., & HARTUNG, R. (1968) Some of the effects
of tetramethylthiuram disulfide (TMTD) on reproduction of the
bobwhite quail (Colinus virginianus). Toxicol. appl. Pharmacol.,
12: 293.
WEISBURGER, E.K., ULLAND, B.M., NAM, J.M., GART, J.J., &
WEISBURGER, J.H. (1981) Carcinogenicity tests of certain
environmental and industrial chemicals. J. Natl Cancer Inst.,
67: 75-88.
WHO (1986a) The WHO recommended classification of pesticides
by hazard. Guidelines to classification 1986-1987, Geneva, World
Health Organization (Unpublished report VBC/86.1, rev. 1).
WHO (1986b) Environmental Health Criteria 64: Carbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 137.
WHO (1988) Environmental Health Criteria 76: Thiocarbamate
pesticides: a general introduction, Geneva, World Health
Organization, pp. 49.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a
world compendium, 7th ed., Croydon, British Crop Protection
Council.
YIN-TAK WOO (1983) Carcinogenicity, mutagenicity, and
teratogenicity of carbamates, thiocarbamates, and related
compounds. An overview of structure-activity relationships and
environmental concerns. J. environ. Sci. Health, C1(1): 97-133.
ZADOROZHNY, B.A., PETROV, B.R., OLTIRENKO, V.A., & KIRILKO, V.A.
(1981) [Occupational dermatoses and their control under
conditions of pesticide manufacture.] Vestn Dermatol. Venerol.,
6: 48-51 (in Russian).
ZDIENICKA, M., ZIELENSKA, M., HRYMIEWICA, M., TROJANOWSKA, M.,
ZALEJSKA, M., & SZYMCZYK, T. (1981) The mutagenicity of the
fungicide thiram. In: Kappas, A., ed. Progress in environmental
mutagenesis and carcinogenesis, Amsterdam, Oxford, New York,
Elsevier Science Publishers, Vol. 2, pp. 79-86.
ZORIN, P.M. (1970) [Allergic dermatitis arising as a result of
contact with zineb.] Vestn Dermatol. Venerol., 44: 65-68 (in
Russian).
ZUMAN, P. & ZAHRADNIK, R. (1957) [Kinetics and decomposition
mechanism of dithiocarbamic acids solutions. Polarographic
study.] Z. Phys. Chem., 208: 135-140 (in German).
Annex I. Names and structures of selected dithiocarbamates
--------------------------------------------------------------------------------------------------------
Common Trade/other Chemical structure CAS chemical name/ Molecular Relative Water
name name CAS registry number formula mole- solu-
cular bility
mass (25°C)
---------------------------------------------------------------------------------------------------------
dibam Methylnamate S sodium dimethyldi- C3H6NS2Na 143.21
|| thiocarbamate
(CH3)2N-C-SNa (