
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 79
DICHLORVOS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. J. Sekizawa
(National Institute of Hygienic Sciences, Japan)
and Dr. M. Eto (Kyushu University, Japan) with
the assistance of Dr. J. Miyamoto and
Dr. M. Matsuo (Sumitomo Chemical Company)
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1989
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154279 9
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DICHLORVOS
1. SUMMARY AND RECOMMENDATIONS
1.1. General
1.2. Environmental transport, distribution, and transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and in vitro test systems
1.7. Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Sampling methods
2.4.1.1 Food and feed
2.4.1.2 Blood
2.4.1.3 Air
2.4.2. Analytical methods
2.4.2.1 Analysis of technical and formulated
dichlorvos products
2.4.2.2 Determination of dichlorvos residues
2.4.2.3 Confirmatory tests
2.4.2.4 Food
2.4.2.5 Blood
2.4.2.6 Air
2.4.2.7 Soil and water
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 Worldwide production figures
3.2.1.2 Manufacturing process
3.2.2. Uses
3.2.3. Accidental release
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Biotransformation
4.2.1. Abiotic degradation
4.2.2. Biodegradation
4.2.3. Bioaccumulation and biomagnification
4.3. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Food
5.2. General population exposure
5.3. Occupational exposure during manufacture, formulation, or use
5.3.1. Air
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Human studies
6.2. Distribution
6.2.1. Studies on experimental animals
6.2.1.1 Oral
6.2.1.2 Inhalation
6.2.1.3 Intraperitoneal
6.2.1.4 Intravenous
6.3. Metabolic transformation
6.3.1. Metabolites
6.4. Elimination and excretion in expired air, faeces, and urine
6.4.1. Human studies
6.4.2. Studies on experimental animals
6.4.2.1 Oral
6.4.2.2 Parenteral
6.5. Retention and turnover
6.5.1. Biological half-life
6.5.2. Body burden
6.5.3. Indicator media
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.1.1. Algae and plankton
7.1.2. Fungi
7.1.3. Bacteria
7.2. Aquatic organisms
7.2.1. Fish
7.2.1.1 Acute toxicity
7.2.1.2 Short-term toxicity
7.2.2. Invertebrates
7.3. Terrestrial organisms
7.3.1. Birds
7.3.1.1 Acute oral toxicity
7.3.1.2 Short-term toxicity
7.3.1.3 Field experience
7.3.2. Invertebrates
7.3.3. Honey bees
7.3.4. Miscellaneous
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Domestic animals
8.1.2. Potentiation
8.2. Short-term exposures
8.2.1. Oral
8.2.1.1 Mouse
8.2.1.2 Rat
8.2.1.3 Rabbit
8.2.1.4 Cat
8.2.1.5 Dog
8.2.1.6 Pig
8.2.1.7 Cow
8.2.2. Dermal
8.2.2.1 Rat
8.2.2.2 Livestock
8.2.3. Inhalation
8.2.3.1 Experimental animals
8.2.3.2 Domestic animals
8.2.4. Studies on ChE activity
8.3. Skin and eye irritation; sensitization
8.4. Long-term exposure
8.4.1. Oral
8.4.1.1 Rat
8.4.1.2 Dog
8.4.2. Inhalation
8.4.2.1 Rat
8.5. Reproduction, embryotoxicity, and teratogenicity
8.5.1. Reproduction
8.5.1.1 Effects on testes
8.5.1.2 Effect on estrous cycle
8.5.1.3 Domestic animals
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Oral
8.5.2.2 Inhalation
8.5.2.3 Intraperitoneal
8.5.3. Résumé of reproduction, embryotoxicity, and
teratogenicity studies
8.6. Mutagenicity and related end-points
8.6.1. Methylating reactivity
8.6.1.1 In vitro studies
8.6.1.2 In vivo studies
8.6.1.3 Discussion of methylating reactivity
8.6.2. Mutagenicity
8.6.2.1 In vitro studies
8.6.2.2 In vivo studies
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1 Mouse
8.7.1.2 Rat
8.7.2. Inhalation
8.7.2.1 Rat
8.7.3. Appraisal of carcinogenicity
8.8. Mechanisms of toxicity; mode of action
8.9. Neurotoxicity
8.9.1. Delayed neurotoxicity
8.9.2. Mechanism of neurotoxicity
8.10. Other studies
8.10.1. Immunosuppressive action
8.11. Factors modifying toxicity; toxicity of metabolites
8.11.1. Factors modifying toxicity
8.11.2. Toxicity of metabolites
8.11.2.1 Acute toxicity
8.11.2.2 Short-term exposures
8.11.2.3 Long-term exposure
8.11.2.4 Mutagenicity
8.11.2.5 Metabolism
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Acute toxicity
9.1.1.1 Poisoning incidents
9.1.2. Effects of short- and long-term exposure
9.1.2.1 Studies on volunteers
9.1.2.2 Hospitalized patients
9.2. Occupational exposure
9.2.1. Acute toxicity
9.2.1.1 Poisoning incidents
9.2.2. Effects of short- and long-term exposure
9.2.2.1 Pesticide operators and factory workers
9.2.2.2 Mixed exposure
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
10.3. Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON DICHLORVOS
Members
Dr L. Albert, Environmental Pollution Programme, National Institute of
Biological Resource Research, Veracruz, Mexico
Dr E. Budd, Office of Pesticide Programs, US Environmental Protection
Agency, Washington DC, USA
Mr T.P. Bwititi, Ministry of Health, Causeway, Harare, Zimbabwe
Dr S. Deema, Ministry of Agriculture and Cooperatives, Bangkok,
Thailand
Dr I. Desi, Department of Hygiene and Epidemiology, Szeged University
Medical School, Szeged, Hungary
Dr A.K.H. El Sebae, Pesticides Division, Faculty of Agriculture,
Alexandria University, Alexandria, Egypt
Dr R. Goulding, Keats House, Guy's Hospital, London, United Kingdom
(Chairman)
Dr J. Jeyaratnam, National University of Singapore, Department of
Social Medicine and Public Health, Faculty of Medicine, National
University Hospital, Singapore (Vice-Chairman)
Dr Y. Osman, Occupational Health Department, Ministry of Health,
Khartoum, Sudan
Dr A. Takanaka, Division of Pharmacology, National Institute of
Hygienic Sciences, Tokyo, Japan
Observers
Dr N. Punja, European Chemical Industry, Ecology and Toxicology Centre
(ECETOC), Brussels, Belgium
Ms J. Shaw, International Group of National Associations of
Manufacturers of Agrochemical Products (GIFAP), Brussels, Belgium
Secretariat
Dr M. Gilbert, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme, Geneva, Switzerland
Dr K.W. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr T. Ng, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
Dr G. Quélennec, Pesticides Development and Safe Use Unit, World Health
Organization, Geneva, Switzerland
Dr R.C. Tincknell, Beaconsfield, Buckinghamshire, United Kingdom
(Temporary Adviser)
Dr G.J. Van Esch, Bilthoven, Netherlands (Temporary Adviser) (Co-
Rapporteur)
Dr E.A.H. Van Heemstra-Lequin, Laren, Netherlands (Temporary Adviser)
(Co-Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR DICHLORVOS
A WHO Task Group on Environmental Health Criteria for Dichlorvos
met in Geneva from 1 to 5 December 1986. Dr M. Mercier, Manager, IPCS,
opened the meeting and welcomed the participants on behalf of the heads
of the three IPCS co-sponsoring organizations (UNEP/ILO/WHO). The
Group reviewed and revised the draft criteria document and made an
evaluation of the risks for human health and the environment from
exposure to dichlorvos.
The drafts of the document were prepared by DR E.A.H. VAN
HEEMSTRA-LEQUIN and DR G.J. VAN ESCH of the Netherlands.
Draft summaries of Japanese studies on dichlorvos were prepared and
finalized by DR M. ETO (Kyushu University), and DR J. MIYAMOTO and
DR M. MATSUO (Sumitomo Chemical Co., Ltd), with the assistance of the
staff of the NATIONAL INSTITUTE OF HYGIENIC SCIENCES, Tokyo,
Japan and DR I. YAMAMOTO (Tokyo University of Agriculture).
The proprietary data mentioned in the document were made available
to the Central Unit of the IPCS by Temana International Ltd, Richmond,
United Kingdom for evaluation by the Task Group.
The efforts of all who helped in the preparation and finalization
of the document are gratefully acknowledged.
* * *
The proprietary information contained in this document cannot
replace documentation for registration purposes, because the latter has
to be closely linked to the source, the manufacturing route, and the
purity/impurities of the substance to be registered. The data should
be used in accordance with paragraphs 82-84 and recommendation
paragraph 90 of the 2nd FAO Government Consultation (FAO, 1982).
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of Health
and Human Services, through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA - a WHO Collaborating Centre for Environmental Health Effects. The
United Kingdom Department of Health and Social Security generously
supported the cost of printing.
1. SUMMARY AND RECOMMENDATIONS
1.1 General
Dichlorvos, an organophosphate, is a direct-acting cholinesterase
(ChE)a inhibitor. Since 1961, it has been commercially manufactured
and used throughout the world as a contact and stomach insecticide. It
is used to protect stored products and crops (mainly in greenhouses),
and to control internal and external parasites in livestock (granules
of impregnated resin) and insects in houses, buildings, aircraft, and
outdoor areas (as aerosols, liquid sprays, or impregnated cellulosic,
ceramic, or resin strips). The present worldwide production of
dichlorvos is about 4 million kg per year.
The purity of the technical grade product is at least 97%, and the
type of impurities depends on the manufacturing process. In the
presence of moisture, dichlorvos breaks down to form acidic products
that are eventually mineralized. Technical dichlorvos may be stabil-
ized, which improves the storage stability, but it is not normally
necessary to stabilize high purity products. In the past, 2 - 4%
epichlorohydrin has been used for this purpose. Dichlorvos is soluble
in water and miscible with most organic solvents and aerosol
propellants. The vapour pressure of dichlorvos is relatively high (1.6
Pa at 20 °C).
Methods for sampling and analysing dichlorvos in food, feed, and
the environment and for determining the inhibition of ChE activity in
blood, red blood cells, plasma, and brain are described.
1.2 Environmental Transport, Distribution, and Transformation
Dichlorvos is not directly applied to soil, but is added to water
to control invertebrate fish parasites encountered during intensive
fish farming. It breaks down rapidly in humid air, water, and soil,
both by abiotic and biotic processes, whereas on wooden surfaces it may
persist for a longer time (39% remaining after 33 days). It degrades
mainly to dichloro-ethanol, dichloroacetaldehyde (DCA), dichloroacetic
acid, dimethylphosphate, dimethylphosphoric acid, and other water-
soluble compounds, which are eventually mineralized.
Dichlorvos is rapidly lost from leaf surfaces by volatilization and
hydrolysis.
Accidental spillage of dichlorvos may have acute hazardous effects
on man and the environment. However, long-term effects are unlikely,
in view of the volatility and instability in humid environments.
Bioaccumulation or biomagnification do not occur.
------------------------------------------------------------------------
a Cholinesterase is the enzyme which breaks down acetylcholine (ACh),
the transmitter at cholinergic nerve synapses.
1.3 Environmental Levels and Human Exposure
The indoor air dichlorvos concentrations resulting from house-
hold and public health use depend on the method of application, tem-
perature, and humidity. For example, one impregnated resin strip per
30 m3 results in concentrations of the order of 0.1 - 0.3 mg/m3 the
first week (the latter only in special circumstances), subsequently
decreasing to 0.02 mg/m3 or less over the next few weeks.
Dichlorvos residues in food commodities are generally low and are
readily destroyed during processing. The metabolite DCA may also be
present in detectable amounts. Total-diet studies in the United
Kingdom and the USA have confirmed that no, or very little, dichlorvos
is found in prepared meals.
Exposure of the general population via food and drinking-water as a
result of agricultural or post-harvest use of dichlorvos is negligible.
However, household and public health use do give rise to exposure,
principally through inhalation and dermal absorption.
Similar routes of exposure occur in professional pest
control with dichlorvos. In warehouses, mushroom houses, and
greenhouses, the concentrations of dichlorvos in the air are in general
below 1 mg/m3 when the recommended application rates are used, but in
certain circumstances they may rise considerably above this level.
1.4 Kinetics and Metabolism
Dichlorvos is readily absorbed via all routes of exposure. After
oral administration, it is metabolized in the liver before it reaches
the systemic circulation.
One hour after the oral administration of 32P-dichlorvos, maximum
concentrations of radioactivity are found in the kidneys, liver,
stomach, and intestines. In bone, the increase is slower, due to
inorganic phosphate entering the phosphate pool of the organism.
Pigs administered a single oral dose of 14C-labelled dichlorvos as
a slow-release polyvinyl chloride (PVC) formulation, showed
radioactivity in all tissues, the highest level being in the liver
after 2 days, and the lowest being in the brain. Pregnant sows were
fed vinyl-1-14C-dichlorvos or 36Cl-dichlorvos in PVC pellets at 4 mg
dichlorvos/kg body weight per day during the last third of the
gestation period. Although the tissues of the sows and piglets
contained 14C or 36Cl ranging from 0.3 to 18 mg/kg tissue, no
radioactivity was associated with dichlorvos or its primary
metabolites.
Up to 70% of the dichlorvos inhaled by pigs is taken up into the
body. When rats and mice inhaled dichlorvos (90 mg/m3 for 4 h), none
or very little (up to 0.2 mg/kg) was found in blood, liver, testes,
lung, or brain. The highest concentrations (up to 2.4 mg/kg tissue)
were found in kidneys and adipose tissue. Dichlorvos rapidly
disappeared from the kidneys with a half-life of approximately 14 min.
Dichlorvos is metabolized mainly in the liver via 2 enzymatic
pathways: one, producing desmethyldichlorvos, is glutathione dependent,
while the other, resulting in dimethyl-phosphate and DCA, is
glutathione independent. The metabolism of dichlorvos in various
species, including man, is rapid and uses similar pathways.
Differences between species relate to the rate of metabolism rather
than to a difference of metabolites.
The major route of metabolism of the vinyl portion of dichlorvos
leads to (a) dichloroethanol glucuronide and (b) hippuric acid, urea,
carbon dioxide, and other endogenous chemicals, such as glycine and
serine, which give rise to high levels of radioactivity in the tissues.
No evidence of the accumulation of dichlorvos or potentially toxic
metabolites has been found.
The major route for the elimination of the phosphorus-containing
moiety is via the urine, with expired air being a less important route.
However, the vinyl moiety is mainly eliminated in the expired air, and
less so in the urine. In cows, elimination is roughly equally
distributed between urine and faeces.
1.5 Effects on Organisms in the Environment
The effect of dichlorvos on microorganisms is variable and species
dependent. Certain microorganisms have the ability to metabolize
dichlorvos but the pesticide may interfere with the endogenous
oxidative metabolism of the organism. In certain organisms it causes
growth inhibition, while in others it has no influence or may even
stimulate growth. Dichlorvos has little or no toxic effect on
microorganisms degrading organic matter in sewage. The above effects
have been seen over the wide dose range of 0.1 - 100 mg/litre.
The acute toxicity of dichlorvos for both freshwater and estuarine
species of fish is moderate to high (96-h LC50 values range from 0.2
to approximately 10 mg/litre). Brain and liver ChE inhibition in
certain fish was found at dose levels of 0.25 - 1.25 mg/litre, but
recovery of ChE activity took place when they were returned to clean
water.
Invertebrates are more sensitive to dichlorvos. Levels above
0.05 µg/litre may have deleterious effects. Dichlorvos also has a
high oral toxicity for birds. The LD50 values are in the range of
5 - 40 mg/kg body weight. In short-term dietary studies, the compound
was slightly to moderately toxic for birds. Brain ChE inhibition was
seen at 50 mg/kg diet or more and at 500 mg/kg diet, half of the birds
died. There have been instances when chickens and ducks have died
after accidental access to dichlorvos-contaminated feed and drinking-
water.
Dichlorvos is highly toxic for honey bees. The LD50 by oral
administration is 0.29 µg/g bee, and after topical application is
0.65 µg/g bee.
1.6 Effects on Experimental Animals and In Vitro Test Systems
Dichlorvos is moderately to highly toxic when administered in single
doses to a variety of animal species by several routes. It directly
inhibits acetylcholinesterase (AChE) activity in the nervous system and
in other tissues. Maximum inhibition generally occurs within 1 h, and
is followed by rapid recovery. The oral LD50 for the rat is 30 - 110
mg/kg body weight, depending on the solvent used. The hazard
classification of dichlorvos by WHO (1986a) is based on an oral
LD50 for the rat of 56 mg/kg body weight. The signs of intoxication
are typical of organophosphorus poisoning, i.e., salivation,
lachrymation, diarrhoea, tremors, and terminal convulsions, with death
occurring from respiratory failure. The signs of intoxication are
usually apparent shortly after dosing, and, at lethal doses, death
occurs within 1 h. Survivors recover completely within 24 h.
Potentiation is slight when dichlorvos is given orally in
combination with other organophosphates, but in combination with
malathion it is marked.
In short-term toxicity studies on the mouse, rat, dog, pig, and
monkey, inhibition of plasma, red blood cell, and brain ChE are the most
important signs of toxicity. After oral administration, approximately
0.5 mg/kg body weight (range, 0.3 - 0.7 mg/kg) did not produce ChE
inhibition. In a 2-year study on dogs, ChE inhibition was noted at
3.2 mg/kg body weight or more.
Flea collar dermatitis has been described in dogs and cats wearing
dichlorvos-impregnated PVC flea collars. This was a primary irritant
contact dermatitis which may have been caused by dichlorvos.
Many short-term inhalation studies on different animal species have
been carried out. Air concentrations in the range of 0.2 - 1 mg/m3 do
not affect ChE activity significantly. Other effects, such as growth
inhibition and increase in liver weight have been reported at dose
levels at least 10 - 20 times higher.
It is possible to produce clinical neuropathy in hens, but the doses
of dichlorvos required are far in excess of the LD50. The effects are
associated with high inhibition of neurotoxic esterase (NTE) in the
brain and spinal cord. In the rat, however, neuropathic changes in the
white matter of the brain have been reported following repeated daily
oral application of an LD50 dose.
Immune suppression has been reported in rabbits. At present, no
evaluation as to the relevance for human beings can be given; more
attention to this aspect is needed.
In a long-term study, rats fed dichlorvos in the diet for 2 years
showed no signs of intoxication. Hepatocellular fatty vacuolization of
the liver and ChE inhibition were significant at the two highest dose
levels (2.5 and 12.5 mg/kg body weight).
In a carefully conducted long-term inhalation study on rats
with whole body exposure (23 h/day, for 2 years), results were compar-
able with those seen in the oral study. No effects were seen at
0.05 mg/m3; inhibition of ChE activity took place at 0.48 mg/m3 or
more.
In several reproduction studies on rats and domestic animals, no
effects were seen on reproduction, and there was no embryotoxicity at
dose levels that did not cause maternal toxicity. At toxic doses,
dichlorvos may cause reversible disturbances of spermatogenesis in mice
and rats. It was not teratogenic in several studies carried out on
rats and rabbits.
Dichlorvos is an alkylating agent and binds in vitro to bacterial
and mammalian nucleic acids. It is mutagenic in a number of microbial
systems, but there is no evidence of mutagenicity in intact mammals,
where it is rapidly degraded by esterases in blood and other tissues.
Dichlorvos carcinogenicity has been investigated in mice (oral
studies) and rats (oral and inhalation studies). The dose levels used
in 2-year oral studies were up to 800 mg/litre drinking-water or
600 mg/kg diet for mice, and up to 280 mg/litre drinking-water or
234 mg/kg diet for rats. In a rat inhalation study, dichlorvos
concentrations in air of up to 4.7 mg/m3 were tested for 2 years. No
statistically significant increase in tumour incidence was found. In
two recent carcinogenicity studies on mice and rats, dichlorvos was
administered by intubation at dose levels between 10 and 40 mg/kg body
weight (mice) and 4 and 8 mg/kg body weight (rat) for up to 2 years.
Only preliminary information has been provided. The evidence for
carcinogenicity in these new studies is difficult to interpret at this
time. Only when complete and final reports become available will it be
possible to draw more definitive conclusions (in this context, see
footnote section 8.7.3).
From acute and short-term studies, it is clear that the metabolites
of dichlorvos are all less toxic than the parent compound. Only DCA
was positive in a few mutagenicity tests.
1.7 Effects on Man
A fatal case of dichlorvos poisoning has been described in the
general population: despite correct treatment, a suicide succeeded with
approximately 400 mg dichlorvos/kg body weight. In another poisoning
case, a woman ingested about 100 mg dichlorvos/kg and survived,
following intensive care for 14 days. Two workers who had skin
exposure to a concentrated dichlorvos formulation, and failed to wash
it off, died of poisoning.
There have been two clinical reports describing four patients
suffering from severe poisoning from dichlorvos, taken orally, who
survived after treatment and who showed delayed neurotoxic effects.
Thus although the possibility of neuropathy in man cannot be excluded,
it is likely to occur only after almost lethal oral doses.
Since the 1960s, field studies in malaria control have been carried
out and the interiors of aircraft have been sprayed with dichlorvos.
Exposure to concentrations in the air of up to 0.5 mg/m3 were without
clinical effects, and no, or only insignificant, inhibition of blood
ChE activity was noted.
When dichlorvos was administered orally to human volunteers (single
or repeated doses of a slow-release PVC formulation), significant
inhibition of red blood cell ChE activity was found at 4 mg/kg body
weight or more. At 1 mg/kg body weight or more, plasma ChE activity
was significantly inhibited. Daily oral doses of 2 mg
dichlorvos/person for 28 days reduced plasma ChE activity by 30%, but
red cell ChE activity was unaffected.
Human volunteers who were exposed to dichlorvos by inhalation for a
certain period per day for a number of consecutive days or weeks showed
ChE inhibition at a concentration of 1 mg/m3 or more, but not at
0.5 mg/m3. These results were confirmed in studies with pesticide
operators who came into contact with dichlorvos.
Hospitalized patients showed similar results after oral
administration or exposure by inhalation. Sick adults and children and
healthy pregnant women and babies in hospital wards treated with
dichlorvos strips (1 strip/30 or 40 m3) displayed normal ChE
activity. Only subjects exposed 24 h/day to concentrations above
0.1 mg/m3 or patients with liver insufficiency showed a moderate
decrease in plasma ChE activity.
No significant effects on plasma or red blood cell ChE activity
were observed in people exposed to the recommended rate of one
dichlorvos strip per 30 m3 in their homes over a period of 6 months,
even when the strips were replaced at shorter intervals than that
normally recommended. The maximum average concentration in the air was
approximately 0.1 mg/m3.
In factory workers exposed to an average of 0.7 mg/m3 for 8
months, significant inhibition of plasma and red blood cell ChE
activity was found.
Cases of dermatitis and skin sensitization due to dichlorvos have
been described in workers handling and spraying different types of
pesticides. In addition cross-sensitization with certain pesticides
has been seen.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Primary constituent
Chemical structure: O
||
Cl2C=CHOP(OCH3)2
Chemical formula: C4H7Cl2O4P
Chemical names: 2,2-dichloroethenyl dimethylphosphate (CAS);
2,2-dichlorovinyl dimethylphosphate (IUPAC)
Common synonyms: Bayer-19149, DDVF, DDVP, ENT-20738,
OMS-14, SD 1750, C-177
CAS registry number: 62-73-7
Technical product
Common trade names: Dedevap, Nogos, Nuvan, Phosvit, Vaponaa
Purity: should not be less than 97% (WHO, 1985)
Impurities: depend on the manufacturing process (section
3.2.1.2)
Additives: In the presence of traces of moisture,
dichlorvos slowly breaks down to form acidic
products that catalyse further decomposition
of the compound. In the past, 2 - 4%
epichlorohydrin was added to stabilize the
technical grade product (Melnikov, 1971).
Other stabilizers may now be used in some
products, but improved technology and purity
has largely eliminated the need for
stabilizers.
2.2 Physical and Chemical Properties
Dichlorvos is a colourless to amber liquid with an aromatic odour.
Some physical and chemical properties of dichlorvos are given in
Table 1.
----------------------------------------------------------------------------
a The Shell trademark Vapona was formerly used exclusively for dichlorvos
and dichlorvos-containing formulations. More recently, this trademark
has been used more widely to include formulations containing other
active ingredients.
Table 1. Some physical and chemical properties of dichlorvosa
-----------------------------------------------------------------------------
Relative molecular mass 221
Boiling point 35 °C at 6.7 Pa (0.05 mmHg);
74 °C at 133 Pa (1 mmHg)b
Vapour pressure (20 °C) 1.6 Pa (1.2 x 10-2 mmHg)
Density (25 °C) 1.415
Refractive index ND25 = 1.4523
Solubility about 10 g/litre water at 20 °C; 2 -
3 g/kg kerosene; miscible with most
organic solvents and aerosol propel-
lants
Stability dichlorvos is stable to heat but is
hydrolysed by water; a saturated
aqueous solution at room temperature
is converted to dimethylphosphate and
dichloroacetaldehyde at a rate of
about 3% per day, more rapidly in
alkali
Corrosivity corrosive to iron and mild steel
Log n-octanol/water partition 1.47c
coefficient
-----------------------------------------------------------------------------
a From: Worthing & Walker (1983).
b From: Melnikov (1971).
c From: Bowman & Sans (1983).
2.3 Conversion Factors
1 ppm = 10 mg/m3 at 25 °C and 101 kPa (760 mmHg);
1 mg/m3 = 0.1 ppm
2.4 Analytical Methods
The various analytical methods are summarized in Tables 2, 3, 4,
and 5.
Table 2. Analytical methods for dichlorvos residues in food and biological
media recommended by the Codex Working Group on Methods of Analysis
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
grain methanol gas-liquid 0.02 mg/kg Anon. (1973)
chromatography
with thermionic
phosphorus
detector or
flame
photometric
phosphorus
detector
cereal petroleum ether/ Florisil gas chromato- 70 - 80% 0.0025 mg/kg Mestres et al.
products ethyl ether column graphy with (1979b)
flame photo-
metric detector
or thermionic
ionization
detector
cereals hexane activated gas chromato- 72 - 83% 0.01 ng Aoki et al.
hexane/aceto- charcoal graphy with (sensitivity) (1975)
nitrile benzene column flame photo-
extraction metric
acetone/ detection
hexane
crops dichloromethane steam gas-liquid 80 - 100% 0.01 mg/kg Elgar et al.
or ethylacetate distil- chromato- (1970)
lation graphy with
flame photo-
metric
detector,
thermionic
ionization
detector, or
electron
capture
detector
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
ethylacetate/ Florisil gas chromato- 80% 0.002 - 0.05 Mestres et al.
dichloromethane column graphy with mg/kg (1979a)
flame photo-
metric detector
fruit and acetonitrile extraction gas-liquid approxi- Anon. (1977)
vegetables with chromato- mately 90%
chloroform; graphy with (at 0.5
residue flame photo- mg/kg)
in acetone metric detector
or thermionic
ionization
detector
onions acetonitrile amberlite gas chromato- 82% Iwata et al.
benzene XAD-8 graphy with (1981)
column flame photo-
benzene/ metric
dichloro- detection
methane
chloroform, HCl and gas-liquid approxi- 0.01 mg/kg Krause & Kirch-
methanol Celite chromatography mately 90% hoff (1970)
with thermionic (at 0.05 -
ionization 0.5 mg/kg)
detector
acetone and part- double gas-liquid 90% (at Luke et al.
ition with petro- concentration chromatography 0.1 mg/kg) (1981)
leum ether and with with flame
dichloromethane petroleum photometric
ether detector
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
eggplant water/methanol gas chromato- 95% 0.004 mg Nakamura &
fruit ether/petroleum graphy with Shiba (1980)
ether flame photo-
metric
detection
plants methanol ether/ gas-liquid 95 - 100% 0.1 mg/kg Dräger (1968)
petroleum chromatography
ether with phosphorus
detector
acetonitrile liquid-liquid thin-layer indo- bromo- Mendoza &
or partitioning chromatography phenyl- indoxyl- Shields (1971)
dichloromethane none enzymatic assay acetate acetate
or using: bee head 5 ng -
methanol/chloro- none extract, pig 5 ng 1 ng
form liver extract, 5 ng 0.1 ng
beef liver
extract
acetone or column thin-layer 1 - 2 ng Ambrus et al.
dichloromethane chromato- chromatography (1981)
graphy enzymatic
assay (horse
serum)
without thin-layer 100 ng
clean-up chromatography
silver nitrate
+ UV
gas-liquid 55 - 80% 0.1 - 1 ng
chromatography 0.01 - 0.05 ng
with thermionic typical limit
ionization of detection
detector or 0.005 - 0.02
electron mg/kg
capture
detector
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
vegetable acetone; dichloro- sweep co- gas chromato- 75 - 100% Eichner (1978)
and animal methane or aceto- distillation graphy with (at 0.03 -
food, nitrile; dichloro- thermionic 0.5 mg/kg)
tobacco methane phosphorus
detector
whole meal cereal: methanol; depending gas-liquid Abbott et al.
fats: hexane and on type chromatography (1970)
others: of sample with thermionic
acetonitrile phosphorus
detector,
caesium bromide
tips
homogenized silica gel gas chromato- 97 - 100% 0.005 mg/kg Dale et al.
sample, ethyl column; graphy with (sensitivity) (1973)
acetate-hexane elution flame
and HCl with acetone/ photometric
hexane detector
animal dichloromethane steam gas-liquid 80 - 100% 0.01 mg/kg Elgar et al.
tissues or ethylacetate distil- chromatography (1970)
lation with flame
photometric
detector,
thermionic
ionization
detector, or
electron
capture
detector
Table 2 (contd.)
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
milk methanol acetonitrile gas chromato- 80 - 90% 0.01 mg/kg Dräger (1968)
and ether/ graphy with (at 0.01 -
petroleum phosphorus 0.1 mg/kg)
ether detector
acetonitrile dichloro- gas-liquid Abbott et al.
methane; chromatography (1970)
methane; with thermionic
residue phosphorus
dissolved detector,
in acetone caesium bromide
tips
Table 3. Other analytical methods for dichlorvos residues in food and biological media
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
agricultural ethyl none except for gas-liquid chromato- food, crops: Anon.
crops, animal acetate oil extracts graphy with phosphorus 0.02 mg/kg (1972)
tissues, detector
beverages,
food
fruit, hexane/ aluminium thin-layer chromato- Wood &
vegetables acetone oxide column graphy; nitrobenzyl- Kanagasa-
pyridine/triaza un- bapathy
decamethylene diamine (1983)
organs/ ethanol none thin-layer chromato- 0.2 ng Ackerman
tissues; graphy enzymatic et al.
contents of none or none assay (beef liver) (1969)
stomach, chloroform
intestines;
urine
milk, dichloro- silica gel gas chromatography 95% 0.003 mg/kg Ivey &
methane column, mixed with flame photo- Claborn
solvents metric detector (1969)
fat, hexane 80% 0.002 mg/kg Ivey &
chicken, Claborn
skin (1969)
muscle, acetonitrile silica gel 80% 0.002 mg/kg Ivey &
eggs column Claborn
(1969)
animal depending on only for fat gas-liquid chromato- 0.05 - 0.1 Schultz
tissuesa sample tissues graphy with phosphorus mg/kg et al.
and fluids detector (1971)
milk silica gel col- polarography 85% 0.15 mg/kg Davidek
umn; alkaline et al.
condensation (1976)
with o -phenyl-
enediamine
---------------------------------------------------------------------------------------------------------
a Methods for analysing residues of four metabolites of dichlorvos are also given.
Table 4. Analytical methods for determining the dichlorvos concentration and ChE activity in blood
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
Dichlorvos concentrations
blood acetonitrile gas chromatography 86% Ivey & Claborn
hexane with flame photo- (1969)
metric detector
blood/serum chloroform none thin-layer chromato- Ackerman et al.
graphy enzymatic (1969)
assay (beef liver)
blooda water/ethanol none gas-liquid chromato- Schultz et al.
extracted with graphy with phosphorus (1971);
ethyl acetate detector Anon. (1972)
ChE activity
blood electrometric method Michel (1949)
(plasma and for ChE activity,
red cell) release of acetic
acid from ACh; pH change
whole blood ACh-perchlorate tintometric method Edson (1958)
ChE and bromothymol blue
whole blood dithiobis-nitro- colorimetry at Voss & Sachsse
and plasma benzoic acid (DTNB) 420 nm (1970)
ChE + acetylthiocholine
(animal blood) or
propionyl thiocholine
(human blood);
eserine salicylate
(esterase inhibitor)
whole blood DTNB + acetylthio- spectrophotometry Ellman et al.
and erythro- choline iodide at 412 nm (1961); Anderson
cyte ChE et al. (1978)
whole blood dithiodipyridine spectrophotometry Augustinsson et
and erythro- (DTPD) + propionyl at 324 nm al. (1978)
cyte ChE thiocholine; esterase
inhibitor
---------------------------------------------------------------------------------------------------------
a Methods for analysing concentrations of four metabolites of dichlorvos are also given.
Table 5. Analytical methods for the determination of dichlorvos in air, soil, and water
---------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
---------------------------------------------------------------------------------------------------------
Air
glass tubes containing:
water electrometric pH Elgar & Steer
method (1972)
ethyl none gas-liquid chromato- 0.01 mg/m3 Anon. (1972)
acetate graphy with phosphorus
detector
potassium elution with gas chromatography 80% Bryant &
nitrate hexane with flame photo- Minett
metric detector (1978)
XAD-2 (per- desorption with gas chromatography 0.2 µg NIOSH
sonal samp- toluene with flame photo- (1979);
ling) metric phosphorus Gunderson
detector (1981)
Soil
soil acetone column thin-layer chromato- 1 - 2 ng Ambrus
chromatography graphy enzymatic et al.
assay (horse serum) (1981)
soil without clean- thin-layer chromato- 100 ng Ambrus
up graphy; silver et al.
nitrate + UV (1981)
soil ether/acetone flame photometric 91% 5 µg Goto
(7:3) detector-gas (1977)
petroleum ether chromatography
Water
water dichloro- column thin-layer chromato- 1 - 2 ng Ambrus
methane chromato- graphy enzymatic et al.
graphy assay (horse serum) (1981)
Table 5 (contd.)
-------------------------------------------------------------------------------------------------------------------------
Sample Extraction Clean-up Detection and Recovery Limit of Reference
quantification detection
-------------------------------------------------------------------------------------------------------------------------
without clean- thin-layer chromato- 100 ng
up graphy; silver
nitrate + UV
gas-liquid chromato- 55 - 70% 0.01 - 0.05 ng;
graphy with electron
capture detector or
thermionic ionization 0.1 - 1 ng
detector typical limit of
detection 0.0001
mg/kg
-------------------------------------------------------------------------------------------------------------------------
2.4.1 Sampling methods
2.4.1.1 Food and feed
The "Codex Recommended Method of Sampling for the Determination of
Pesticide Residues" (Codex Alimentarius Commission, 1979; GIFAP, 1982)
describes sampling rates and acceptance criteria in relation to the
analytical sample and the Codex maximum residue limits (Codex
Alimentarius Commission, 1983).
2.4.1.2 Blood
Where samples cannot be determined immediately, e.g., samples taken
in the field, they must be frozen in order to prevent the reactivation
of inhibited plasma ChE or erythrocyte AChE. When freezing facilities
are limited, or where samples must be transported and/or stored for
several days, samples of whole blood are applied to filter paper.
These samples can be stored at room temperature for at least 2 weeks
and in a refrigerator for more than 6 weeks without reducing the
efficiency of elution from the filter paper (Eriksson & Fayersson,
1980).
2.4.1.3 Air
Methods of sampling air for pesticides have been reviewed by Miles
et al. (1970), Van Dijk & Visweswariah (1975), Lewis (1976), and Thomas
& Nishioka (1985).
Miles et al. (1970) compared the widely-used techniques and came to
the conclusion that, although each method has certain advantages, none
are ideal. Packed adsorption columns are very efficient for trapping
vapours, but recovery of the sample is frequently difficult. Glass
fibre filters or cellulose filter pads permit the collection of large
volumes of air in short periods of time, but their efficiency for
vapours is low, and unknown losses of aerosol samples occur. Membrane
filters are good for liquid aerosols and vapours, but the sampling rate
is slow. However, Tessari & Spencer (1971) considered collection on a
moist nylon net to be the best sampling method for aerosol and vapour-
phase pesticides. Freeze-out traps are of limited value in field work.
Impingers seem to offer a compromise; they can be operated at quite a
fast flow rate, they are efficient for collection of aerosols, and,
with correct solvent selection, they collect vapours efficiently.
Heuser & Scudamore (1966) used dry potassium nitrate in an adsorp-
tion tube and were able to measure less than 1 µg/m3 of dichlorvos
in air.
When Miles et al. (1970) used two Greenburg-Smith-type impingers
containing water, they trapped up to 97% of dichlorvos. However, when
ethylacetate was used instead of water, more than 95% of the available
dichlorvos was collected in the first impinger (Anon., 1972).
For personal sampling of dichlorvos in the work environment,
Gunderson (1981) collected air samples from the worker's breathing zone
in glass tubes packed with XAD-2 (a styrene-divinyl benzene cross-
linked porous polymer) as sorbent. A calibrated personal sampling pump
drew air through the filter.
2.4.2 Analytical methods
2.4.2.1 Analysis of technical and formulated dichlorvos products
Dichlorvos products can be analysed by gas-liquid chromatography,
infrared spectrometry (Oba & Kawabata, 1962), or by reaction with an
excess of iodine which is estimated by titration (CIPAC Handbook,
1980). A colorimetric method to estimate dichlorvos in formulations
was described by Mitsui et al. (1963) and improved by Ogata et al.
(1975). Formulated dichlorvos can be analysed by gas-liquid
chromatography after extraction or dilution with chloroform, or after
partitioning of the dichlorvos into acetonitrile (Anon., 1972). Heuser
& Scudamore (1975) described a method to assess the output of
dichlorvos slow-release strips for insect control. A method for the
analysis of dichlorvos in technical and formulated products was
reported in WHO (1985).
Qualitative methods to identify dichlorvos or to separate and
estimate it in the presence of other organophosphorus compounds were
described by Sera et al. (1959) and Yamashita (1961).
2.4.2.2 Determination of dichlorvos residues
The main methods for determining dichlorvos are:
(a) thin-layer chromatography (TLC);
(b) enzyme-inhibition detection, coupled with TLC;
(c) gas chromatography (GC) with electron capture detector (ECD)
(specificity is poor);
(d) GC with flame photometric detector (FPD) (the most widely-used
method for the determination of organophosphorus compounds);
(e) GC with thermionic alkaline flame ionization detector (TID),
which is more sensitive to phosphorous-containing compounds
than the FPD, but is less stable (Lewis, 1976).
Mendoza (1974) reviewed the applications of the TLC-enzyme-
inhibition technique for pesticide residues and metabolite analyses
involving determination and confirmation of pesticides.
IUPAC's Commission on Pesticide Chemistry examined simplified
analytical methods for screening pesticide residues and their
metabolites in food and environmental samples (Batora et al., 1981).
The Codex Committee on Pesticide Residues lists recommended methods
for the analysis of dichlorvos (FAO/WHO, 1986).
2.4.2.3 Confirmatory tests
Confirmation of the identity of the residue by an independent test
is an essential part of good laboratory practice. The ultimate choice
of a confirmatory test depends on the technique used in the initial
determination and on the available instrumentation and necessary
expertise. Details of various confirmatory tests have been published
(Mendoza & Shields, 1971; Shalik et al., 1971; Mestres et al., 1977;
Cochrane, 1979).
2.4.2.4 Food
The Working Group on Methods of Analysis of the Codex Committee on
Pesticide Residues has produced guidelines on good analytical practice
in residue analysis and Recommendations of Methods of Analysis for
Pesticide Residues (Codex Alimentarius Commission, 1983). The
recommended methods are mostly multiresidue ones and are suitable for
analysing as many pesticide product combinations as possible up to the
Codex maximum residue limits. The methods are summarized in Table 2.
Other methods for residue analysis are given in Table 3.
2.4.2.5 Blood
Methods for analysing dichlorvos concentrations in blood are given
in Table 4. The determination of the four metabolites of dichlorvos
was described by Schultz et al. (1971).
The most frequently used method for determining ChE activity in
blood is that of Ellman et al. (1961), subsequently modified by Voss &
Sachsse (1970) and Augustinsson et al. (1978). An improvement of this
spectrophotometric method for determining ChE activity in erythrocytes
and tissue homogenates was described by Anderson et al. (1978). The
method of Ellman et al. (1961) has been developed by WHO (1970) into a
field kit for the determination of blood ChE activity.
2.4.2.6 Air
A review of the analysis of airborne pesticides has been published
by Lewis (1976). Methods for determining dichlorvos concentrations in
air are given in Table 5.
2.4.2.7 Soil and water
Methods are summarized in Table 5.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural Occurrence
Dichlorvos does not occur as a natural product.
3.2 Man-Made Sources
3.2.1 Production levels and processes
3.2.1.1 Worldwide production figures
Dichlorvos has been manufactured commercially since 1961 in many
countries. Worldwide production figures for 1984 are given in Table 6.
Table 6. The worldwide production of dichlorvos in 1984
----------------------------------------------------------------------------
Country Production in tonnes
----------------------------------------------------------------------------
Eastern Europe 220
Japan 1100
Latin America 400
Middle East 1200
(including India and Pakistan)
South-East Asia 500
USA 500
Western Europe 300
Total 4220
-----------------------------------------------------------------------------
Of this total production, 60% is used in plant protection, 30% for
public hygiene and vector control, and 10% to protect stored products
(GIFAP, personal communication, 1986).
3.2.1.2 Manufacturing processes
Dichlorvos can be manufactured by the dehydrochlorination of
trichlorphon (chlorophos) through the action of caustic alkalis in
aqueous solution at 40 - 50 °C.
O O
|| ||
(CH3O)2PCH(OH)CCl3 + KOH -> (CH3O)2POCH=CCl2 + KCl + H2O
The yield of dichlorvos in this process does not exceed 60%.
Another process is the reaction of chloral with trimethyl-
phosphite:
O
||
(CH3O)3P + CCl3CHO -> (CH3O)2POCH=CCl2 + CH3Cl
Using this method, dichlorvos of 92 - 93% purity can be produced by
either a batch or a continuous process (Melnikov, 1971).
3.2.2 Uses
Dichlorvos is a contact and stomach insecticide with fumigant and
penetrant action. It is used for the protection of stored products and
crops (mainly greenhouse crops), and for the control of internal and
external parasites in livestock and insects in buildings, aircraft, and
outdoor areas.
As a household and public health insecticide with fumigant action,
dichlorvos has widespread use in the form of aerosol or liquid sprays,
or as impregnated cellulosic, ceramic, or resin strips, especially
against flies and mosquitos. For the control of fleas and ticks on
livestock and domestic animals (pets), impregnated resin collars are
used. A granular form of an impregnated resin strip is in use as an
anthelmintic in domestic animals.
The various formulations include emulsifiable and oil-soluble
concentrates, ready-for-use liquids, aerosols, granules, and
impregnated strips. Formulations containing mixtures of dichlorvos
with other insecticides, such as pyrethrins/piperonylbutoxide,
tetramethrin, allethrins, chlorpyriphos, diazinon, propoxur, or
fenitrothion, are also on the market.
3.2.3 Accidental release
Accidental spillages of dichlorvos could cause acute effects in
water (e.g., mortality of aquatic species), but long-term effects are
unlikely in view of its volatility and instability in humid
environments.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and Distribution Between Media
Dichlorvos is not generally used for direct application on soil or
to water. However, in intensive fish farming, dichlorvos is added
directly to water. Any residues in soil resulting from the treatment
of crops will be small and short-lived, due to volatilization and
degradation. Therefore, contamination of ground water or surface water
is unlikely to occur in normal practice. In air, dichlorvos is rapidly
degraded, the rate depending on the humidity of the air.
4.2 Biotransformation
4.2.1 Abiotic degradation
In water, dichlorvos hydrolyses into dimethylphosphoric acid and
DCA.
The photochemical degradation rate constant at environmentally
important wavelengths (around 300 nm) was 265 x 10-7/s at a
concentration of 0.67 µg dichlorvos/cm2 of glass plate, and the half-
life was 7 h (Chen et al., 1984).
The relative persistence of dichlorvos on concrete, glass, and wood
was investigated in the laboratory. The fastest loss occurred when it
was applied to concrete; after 1 h, only 0.7% of the applied amount was
present. This rapid loss was almost certainly due to alkaline
decomposition. The disappearance rate on glass was less rapid, with a
recovery of 1% dichlorvos 3 days after application. On wood,
dichlorvos showed the greatest persistence; 65% and 39% of the applied
dichlorvos still remained after one and 33 days, respectively (Hussey &
Hughes, 1964).
When houses were treated for pest control with a total of 230-330 g
dichlorvos as aerosol and 4 - 50 g as emulsion spray, the mean
dichlorvos residue on the surface was 24 µg/100 cm2 at the end of the
first day, and fell to 6 µg/100 cm2 by the end of 5 days (Das et al.,
1983).
4.2.2 Biodegradation
Two ponds containing 9200 and 25 000 µg plankton/litre water,
respectively, were treated with dichlorvos by spraying under the
surface of the water. The initial dichlorvos concentration in the
water was 325 µg/litre and the half-lives were 34 and 24 h,
respectively (Grahl, 1979).
The biodegradation of dichlorvos in soil was tested in the
laboratory using moist loam. The percentages of the applied amount
(200 mg/kg soil) remaining in the soil after 1, 2, and 3 days were 93%,
62%, and 37%, respectively. Concentrations of free DCA in the soil
were 9%, 7%, and 4%, respectively (Hussey & Hughes, 1964).
In studies on the fate of dichlorvos in soil, it was shown
that Bacillus cereus grown on a nutrient medium containing 1000 mg
dichlorvos/litre could use this compound as a sole carbon source but
not as a sole phosphorus source. When soil columns were perfused with
an aqueous solution containing 1000 mg dichlorvos/litre, the metabolic
activity of B. cereus accounted for 30% of the loss of dichlorvos from
the system over a 10-day period (Lamoreaux & Newland, 1978).
Dichlorvos in concentrations ranging from 0.1 to 100 mg/litre had
little or no toxicity, as measured by the oxygen depletion caused by
microorganisms degrading organic matter in sewage (Lieberman &
Alexander, 1981, 1983).
Dichlorvos was converted to dichloroethanol, dichloroacetic acid,
and ethyldichloroacetate by a microbial enrichment derived from sewage
containing, principally, two species of Pseudomonas and one
of Bacillus. The compounds were not formed in the absence of microbial
cells. Inorganic phosphate was also generated in the presence of
microorganisms, and dimethylphosphate was produced in the presence or
absence of microbial cells (Lieberman & Alexander, 1981, 1983).
Pseudomonas melophthora, the bacterial symbiont of the apple
maggot (Rhagoletis pomonella), degraded dichlorvos mainly into water-
soluble metabolites, using esterases (Boush & Matsumura, 1967). In
addition, a strain of Trichoderma viride, a fungus isolated from soil,
has the ability to degrade dichlorvos to water-soluble metabolites,
probably through an oxidative pathway (Matsumura & Boush, 1968).
Dichlorvos is rapidly lost from leaf surfaces by volatilization and
by hydrolysis, the half-life under laboratory conditions being of the
order of a few hours. A small percentage of the dichlorvos deposited
appears to penetrate into the waxy layers of plant tissues, where it
persists longer and undergoes hydrolysis to DCA (FAO/WHO, 1968a,
1971a).
4.2.3 Bioaccumulation and biomagnification
Due to the transient nature of dichlorvos, no bioaccumulation or
biomagnification occur in soil, water, plants, vertebrates, or
invertebrates.
4.3 Ultimate Fate Following Use
Direct application of dichlorvos on crops or animals will result in
residues disappearing rapidly by volatilization and hydrolysis.
Airborne dichlorvos arising from fogging, spraying, or volatilization
from impregnated strips is hydrolysed in the atmosphere to
dimethylphosphate and DCA. Losses occur through ventilation and by
absorption and hydrolysis on surfaces. Depending on the material,
dichlorvos may be absorbed and diffuse into the material, or it may be
hydrolysed on the surface.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental Levels
The occurrence of dichlorvos residues in the environment does not
necessarily originate from the use of dichlorvos. It can also occur as
a conversion product of trichlorphon (Miyamoto, 1959) and butonate
(Dedek et al., 1979).
5.1.1 Air
Examples of indoor air concentrations resulting from the household
and public health use of dichlorvos are given in Table 7. The air
concentration varies according to the method of application (strips,
spray cans, or fogging), the temperature, and humidity (Gillett et al.,
1972). Using strips (one strip per 30 m3), the concentration in the
first week is in the range 0.1 - 0.3 mg/m3, depending on the
ventilation. During succeeding weeks, the concentration decreases to
about 0.04 mg/m3 and after 3 months to 0.01 mg/m3 (Elgar & Steer,
1972).
5.1.2 Food
Data on residues in food commodities resulting from pre- or post-
harvest treatment and from use on animals have been summarized by
FAO/WHO (1967a, 1968a, 1971a, 1975a). Maximum residue limits, varying
from 0.02 to 5 mg/kg, have been recommended for a range of
commodities.
Frank et al. (1983) analysed 260 bovine and porcine fat samples
collected in the period 1973-81 in Ontario. Only one sample contained
a trace of dichlorvos.
Dichlorvos residues present in food commodities are readily
destroyed during processing, e.g., washing, cooking. Hence, the chance
that dichlorvos will occur in prepared meals is very low. This was
confirmed by Abbott et al. (1970) in a total-diet study in the United
Kingdom, in which no residues of dichlorvos were detected in the 462
sub-samples analysed.
In total-diet studies (including infant and toddler diets) carried
out from 1964 to 1979 by the US Food and Drug Administration, no
dichlorvos was found (Johnson et al., 1981a,b; Podrebarac, 1984).
Food samples, meals, and unwrapped ready-to-eat foodstuffs exposed
under practical conditions to dichlorvos generated by resin strips
showed mean residues of less than 0.05 mg/kg, with a range of < 0.01 -
0.1 mg/kg (Elgar et al., 1972a,b; Collins & de Vries, 1973). No
residues of DCA (< 0.03 mg/kg; limit of detection) were detected in the
ready-to-eat foodstuffs (Elgar et al., 1972b). Food and beverages
exposed to experimental air concentrations of 0.04 - 0.58 mg/m3 for
30 min contained dichlorvos residues of 0.005 - 0.5 mg/kg, with the
exception of margarine which contained up to 1.6 mg/kg (Dale et al.,
1973).
Table 7. Indoor air concentrations of dichlorvos following various applications
---------------------------------------------------------------------------------------------------------
Location Application Dosea Temper- RHb Ventilation Time after Concentration Reference
ature (%) application (mg/m3)
(°C)
---------------------------------------------------------------------------------------------------------
food shops resin strip 1 strip/ normal first week 0.03 Elgar et al.
30 m3 4 weeks 0.02 (1972b)
houses resin strip 1 strip/ 18-35 20-60 normal first week 0.06 - 0.17 Leary et al.
30 m3 2 - 3 weeks 0.01 (1974); Elgar
& Steer (1972);
Collins & de
Vries (1973)
hospital resin strip 1 strip/ 20-27 35-70 varied several days 0.10 - 0.28 Cavagna et al.
wards 30 m3 20 - 30 days 0.02 (1969)
hospital strips of paper 0.2 ml - - 2 h 3 days 0.06 Schulze (1979)
wards drenched in 50% ai/m3
dichlorvos sol- 0.2 ml 17 - 2 h 66 h 0.1 - 0.3
ution hanging ai/m3
in the room for 0.2 ml 17 2 h 90 h 0.3
24 - 36 h ai/m3
0.8 ml 30 high 2 h 3 h 3.7
ai/m3 46 h 0.6
houses 0.5% solution 225 or 26 47-60 none 0 0.4 Neuwirth & White
according to 1200 ml 8 h 0.2 (1961)
typical pest 24 h < 0.1
control practice
bathroom 0.5% solution 25 ml 26 60 none 0 1.1 Neuwirth & White
(sealed) wall spray 4 h 0.3 (1961)
24 h < 0.1
Table 7 (contd.)
----------------------------------------------------------------------------------------------------------------------------------
Location Application Dosea Temper- RHb Ventilation Time after Concentration Reference
ature (%) application (mg/m3)
(°C)
----------------------------------------------------------------------------------------------------------------------------------
living room spray cans 2.3 mg 20-22 30 min 0 0.24 Sagner &
(experimental) ai/m3 1 h 0 0.13 Schöndube (1982)
fogging 240 mg 20-22 none 1 h 37 Sagner &
ai/m3 none 24 h 5.5 Schöndube (1982)
1 h 1 h 2.5
120 h 1 h < 0.2
apartments 0.5% solution 190 mg 26 82 0 - 2 h 0.5 Gold et al.
ai/m2 2 - 24 h 0.2 (1984)
---------------------------------------------------------------------------------------------------------
a ai = active ingredient.
b RH = relative humidity.
5.2 General Population Exposure
Exposure of the general population to dichlorvos via air, water, or
food, as a result of its agricultural or post-harvest use, is
negligible. However, the household and public health use of
dichlorvos is a source of exposure. The dichlorvos slow-release resin
strip leads to exposure principally through inhalation from the air,
but dermal absorption by contact with surfaces and oral ingestion of
exposed food may also occur. Professional pest control with dichlorvos
in buildings results in the same routes of exposure but to lower levels
and for a shorter period (section 5.1).
Other sources of exposure are the use of household sprays and pet
collars.
The increased use of organophosphorus insecticide on lawns and turf
within parks and recreational areas presents a risk to human beings and
animals. They may be potentially exposed to toxic levels of residues,
although most product labels recommend that pets and children be kept
off treated turf until the spray has dried. To safeguard against
potential hazards, safe levels of dislodgeable residue have been
estimated so that safe reentry intervals or reentry precautions can be
established. In California, the estimated safe level of dislodgeable
foliar dichlorvos residue is 0.06 µg/cm2.
In studies carried out by Goh et al. (1986a,b), the dislodgeable
foliar dichlorvos residue level immediately after application dropped
rapidly during the first 2 - 6 h, and after 24 - 48 h, the residue was
undetectable.
5.3. Occupational Exposure During Manufacture, Formulation, or Use
5.3.1 Air
Employees in a vaporizer production plant and adjoining packing
rooms were exposed, on average, to 0.7 mg/m3 air. The highest single
value recorded was 3 mg/m3 (Menz et al., 1974).
When air was analysed by Wright & Leidy (1980) in office and
insecticide storage rooms in commercial pest control buildings
and in vehicles, the concentrations of dichlorvos did not exceed
0.001 mg/m3 air.
Gillenwater et al. (1971) measured maximum values of 2.4 -
7 mg/m3 of dichlorvos in a large warehouse during weekly 6-h
application periods. The amounts of dichlorvos dispersed per
application ranged from 25 to 59 mg/m3 and the average air
concentration after 8 applications was 4 mg/m3.
When the floors of a mushroom house were treated with a 10%
solution of a 50% (w/v) dichlorvos emulsion (2 g dichlorvos/m3 of
house volume), air concentrations of dichlorvos were well below
1 mg/m3. The air concentrations of DCA were approximately 1
mg/m3, decreasing over 14 days to 0.3 mg/m3 (Hussey & Hughes, 1964).
During thermal fogging by swingfog of 6 greenhouses (0.2 ml
dichlorvos/m3), the workplace concentration was 7 - 24 mg/m3 (mean:
16 mg/m3). Spraying of 12 glass and plastic green-
houses resulted in workplace concentrations between 0.7 and
2.7 mg/m3 (mean:1.3 mg/m3). Field application by spraying
resulted in air concentrations of 0.01 - 0.26 mg/m3 (mean :
0.08 mg/m3) (Wagner & Hoyer, 1975, 1976).
In a tobacco-drying unit used for mushroom production, dichlorvos
was sprayed at 8 ml aia/100 m3, and the unit was kept closed for 24
h. Air concentrations decreased from 3.3 mg/m3 to 0.006 mg/m3 in 24
h. Treatment of the unit with paper strips drenched in 50% dichlorvos
formulation (40 ml/100 m3) resulted in air concentrations of 0.38 and
0.024 mg/m3, 3 and 24 h, respectively, after treatment (Grübner,
1972).
Immediately after spraying plants in greenhouses with a 0.2 - 0.3%
dichlorvos solution, the air concentration was 1.2 mg/m3, decreasing
to 0.01 mg/m3 24 h later. When the plants were "shaken", air
concentrations increased by 10 - 26% (Zotov et al., 1977).
The air levels of dichlorvos in a room of a residence were moni-
tored during and after treatment with a pressurized home-fogger con-
tainer. The study was performed to determine if the prescribed 30 min
aeration period was sufficient to allow safe re-entry into a home or
room. The air levels were below the industrial workplace permissible
exposure level (PEL) of 1 mg/m3, recommended by US OSHA, at the end
of the aeration period. The dichlorvos dissipated quite slowly after
that. Without ventilation, it took 18 h to reach an acceptable level.
Because there is concern that infants and elderly or diseased persons
occupying rooms almost 24 h/day, 7 days per week, might be more
susceptible, the acceptable level for homes has been established at
1/40 of the PEL. Consequently, rooms treated with this type of
application device and ventilated after treatment should not be re-
entered for 10 h (Maddy et al., 1981a).
Dichlorvos is used to control Phorid flies in mushroom-growing
houses. After its use in one of these houses in Ventura County in the
USA in 1981, some workers complained of headaches and nausea upon re-
entry after 30 min of ventilation. Monitoring of the mushroom houses,
after the same treatment, revealed air concentrations of less than 0.1
mg/m3 (0.01 ppm). Swab samples of exposed horizontal surfaces
revealed a maximum of 0.026 µg/cm2 (Maddy et al., 1981b).
-----------------------------------------------------------------------
a ai = active ingredient.
6. KINETICS AND METABOLISM
6.1 Absorption
Dichlorvos is readily absorbed via all routes of exposure. In the
rat, dichlorvos taken orally is absorbed by the gastrointestinal tract,
transported via the hepatic portal venous system to the liver, and
detoxified before it reaches the systemic circulation (Gaines et al.,
1966; Laws, 1966).
Air exhaled by anaesthetized and tracheotomized pigs exposed by
inhalation to dichlorvos for up to 6 h revealed that, at dichlorvos
concentrations of 0.1 - 2 mg/m3, the pigs retained 15 - 70% of the
inhaled dichlorvos (Kirkland, 1971).
The percutaneous absorption of undiluted dichlorvos and solutions
of dichlorvos applied (under a glass cover slip) to rabbit skin was
calculated from the slope of the whole blood ChE activity inhibition
curve. Water and acetone solutions did not increase absorption,
whereas xylene and dimethylsulfoxide (DMSO) enhanced absorption
(Shellenberger et al., 1965; Shellenberger, 1980). The results are
summarized in Table 8.
Table 8. Effect of solvent on whole blood ChE activities and absorption
ratesa after percutaneous application of dichlorvos to rabbit skin
-----------------------------------------------------------------------------
Solvent ChE inhibition Time after Absorption
(%) application (mg/min per cm2)
-----------------------------------------------------------------------------
0.5 ml undiluted 30 2 h 3.8
dichlorvos
+0.5 ml acetone 45 2 h 4.08
+0.5 ml water 45 2 h 4.29
+0.5 ml xylene 100 40 min 11.96
+0.5 ml DMSO 100 35 min 16.08
-----------------------------------------------------------------------------
a Calculated from the slope of the enzyme inhibition curve.
6.1.1 Human studies
Dichlorvos was undetectable (less than 0.1 mg/litre) in the blood
of two men immediately after exposure, one to air concentrations of
0.25 mg dichlorvos/m3 for 10 h and one to 0.7 mg dichlorvos/m3 for
20 h (Blair et al., 1975).
6.2 Distribution
6.2.1 Studies on experimental animals
6.2.1.1 Oral
32P-Dichlorvos administered orally to rats at a single dose of
10 mg/kg body weight was found to be readily absorbed, distributed
among the tissues, hydrolysed, and rapidly metabolized. Radioactivity
was detected in the blood 15 min after administration, and the amount
slowly decreased over subsequent days. The concentrations of 32P in
kidneys, liver, stomach, and intestines reached their maximum 1 h after
dosing, and decreased within 1 day. The concentration in bone
increased slowly with time due to the 32P entering the inorganic
phosphate pool of the organism. No sex differences were found (Casida
et al., 1962).
When 1 mg of 14C-methyldichlorvos was administered orally to
rats, the gut, skin, and carcass contained 0.7%, 1.6%, and 5.2%,
respectively, of the administered radioactivity, 4 days after dosing
(Hutson & Hoadley, 1972b). In an earlier study on rats dosed orally
with 1 mg vinyl-1-14C-dichlorvos, the gut, skin, and carcass
contained 1.7%, 7.5%, and 14%, respectively, of the 14C, 4 days after
dosing (Hutson et al., 1971a,b).
Twenty-four hours after the administration of a single oral dose of
0.2 mg vinyl-1-14C-dichlorvos to mice, 26 - 34% of the radioactivity
was found in the carcass (Hutson & Hoadley, 1972a). Syrian hamsters
dosed with vinyl-1-14C-dichlorvos retained similar percentages in the
gut, skin, and carcass as did rats (Hutson & Hoadley, 1972a).
Fetuses from rabbits treated with daily oral doses of 5 mg
dichlorvos/kg body weight for 25 days of gestation were found to
contain no dichlorvos (Majewski et al., 1979).
In studies by Potter et al. (1973a), nine pigs received a single
oral dose of vinyl-1-14C-dichlorvos (approximately 40 mg
dichlorvos/kg feed) formulated as slow-release PVC pellets.
Sacrifices after 2, 7, and 14 days showed that all the tissues
contained 14C. The highest level of radioactivity, expressed as
dichlorvos equivalent, was found in liver tissue after 2 days
(33 mg/kg) and the lowest in brain tissue (2.5 mg/kg). In another
study, pregnant sows were fed vinyl-1-14C-dichlorvos or
36Cl-dichlorvos in PVC pellets at 4 mg dichlorvos/kg body weight per
day for the last third of the sow's gestation period. After farrowing,
the sows and piglets, nursing from their own mothers, were kept for 21
days before being sacrificed. The tissues of the sows and piglets
contained 14C and 36Cl residues ranging from 0.3 to 18 mg/kg tissue
equivalents. In neither study, were residues of dichlorvos, DCA,
desmethyldichlorvos, dichloroacetic acid, or dichloroethanol found in
the tissues (Potter et al., 1973a,b).
No dichlorvos was found in muscle (fat) tissue of rabbits treated
with daily oral doses of 5 mg dichlorvos/kg body weight for 2 weeks and
sacrificed at intervals up to 48 h after the last dose (Majewski et
al., 1979).
6.2.1.2 Inhalation
When groups of 3 rats and mice were exposed by inhalation to a
concentration of 90 mg dichlorvos/m3 air for 4 h, the rats exhibited
mild signs of intoxication (lethargy, pupillary constriction).
Concentrations of dichlorvos were very low or undetectable in blood
(< 0.2 mg/kg), liver, testes, lung, and brain (< 0.1 mg/kg), while the
kidneys and fat contained the highest concentrations (up to 2.4 and
0.4 mg/kg tissue, respectively). In rats, the values for the trachea
were higher than those for the lungs, indicating perhaps that some
dichlorvos is trapped in the trachea. When rats were exposed for 4 h
to 10 mg/m3 air, only the kidneys of the male animals contained
measurable or detectable dichlorvos concentrations (0.08 mg/kg). Mice
gave different results from rats, having higher concentrations of
dichlorvos in fat, lung, and testes, and much lower concentrations in
the kidneys. Exposure of male rats to 0.5 or 0.05 mg/m3 for 14 days
did not result in detectable residues (< 0.001 mg/kg) of dichlorvos in
blood, liver, kidneys, renal fat, or lung tissue. However, in male
rats exposed to approximately 50 mg dichlorvos/m3, dichlorvos (1.7
mg/kg) was found in the kidneys after 2 and 4 h exposure time. On
removal of the rats from the test atmosphere, the dichlorvos rapidly
disappeared from the kidneys, with a half-life of 13.5 min. The rate
of disappearance of dichlorvos in the blood was too rapid to measure;
it could not be detected 15 min after exposure (Blair et al., 1975).
Short-term inhalation trials in anaesthesized pigs did not
show the presence of intact dichlorvos or desmethyldichlorvos in
blood or lung tissues. Even in the 2- to 4-h trials, the
degradation proceeded to the stage where only methylphosphates and
phosphoric acid could be detected (Loeffler et al., 1971). When young
swine were exposed for 24 h to an atmosphere containing about 0.15 mg
vinyl-1-14C-dichlorvos/m3, the 14C content varied widely among the
different tissues, but none contained dichlorvos (Loeffler et al.,
1976).
6.2.1.3 Intraperitoneal
Nordgren et al. (1978) showed that within 1 min after a single
intraperitoneal injection of 10 mg dichlorvos/kg body weight to mice,
dichlorvos was detectable in the brain, but its concentration decreased
within a few minutes.
Mice and rats treated repeatedly by intraperitoneal injection with
10 or 4 mg 32P-dichlorvos/kg body weight showed hydrolysis products in
the tissues within 2 h (Casida et al., 1962). When male rats were
injected intraperitoneally with vinyl-1-14C-dichlorvos, the mean 24-h
retention percentages of administered radioactivity were: gut, 4%;
skin, 7%; and carcass, 23% (Hutson et al., 1971b). No differences in
the amount or distribution of radioactivity in the tissues of female
rats given either a single oral or intraperitoneal dose of 4 mg vinyl-
1-14C-dichlorvos/kg body weight were reported (Casida et al., 1962).
6.2.1.4 Intravenous
The dichlorvos concentrations in the kidneys of three male rats, 10
and 30 min after a single intravenous injection, showed a considerable
decrease, suggesting rapid metabolism of dichlorvos. As was the case
after oral administration, dichlorvos could not be detected in the
kidneys of female rats (Blair et al., 1975).
6.3 Metabolic Transformation
Early in vitro and in vivo studies indicated that detoxification of
dichlorvos occurs in the liver (Casida et al., 1962; Hodgson & Casida,
1962; Gaines et al., 1966; Laws, 1966). In vitro studies have shown
that rat liver degrades dichlorvos by two main enzymatic pathways, one
being glutathione dependent and producing desmethyldichlorvos, and the
other being glutathione independent and resulting in dimethylphosphate
and DCA. The degradation of desmethyldichlorvos to DCA and
monomethylphosphate was also found to be glutathione independent
(Dicowsky & Morello, 1971). Sakai & Matsumura (1971) demonstrated
the in vitro degradation of dichlorvos by human brain esterases.
Hodges & Casida (1962) have found that dichlorvos is hydrolysed by
the soluble and mitochondrial fractions of the rat liver but not by the
microsomes. DCA is reduced in the presence of NADH to dichloroethanol
and possibly to dichloroacetate.
The rapidity of dichlorvos metabolism has been demonstrated
in in vitro studies using fresh liver tissue. Ten minutes after
mixing 1 mg dichlorvos with 1 g of liver tissue, 50% dichlorvos was
recovered; after 123 min, only 0.4% remained (Majewski et al., 1979).
However, it is not only liver tissue that metabolizes dichlorvos.
32P-Dichlorvos was metabolized in the presence of blood and of
adrenal, kidney, lung, and spleen tissues, mainly to dimethylphosphate.
Desmethyldichlorvos, monomethylphosphate, and inorganic phosphate were
also found (Hodgson & Casida, 1962; Loeffler et al., 1971).
The identification of dichlorvos metabolites has been undertaken
in in vivo studies of mice (Casida et al., 1962; Hutson & Hoadley,
1972a,b), rats (Casida et al., 1962; Bull & Ridgeway, 1969; Hutson et
al., 1971b; Hutson & Hoadley, 1972b), Syrian hamsters (Hutson &
Hoadley, 1972a), pigs (Loeffler et al., 1971, 1976; Page et al., 1972;
Potter et al., 1973a,b), goats (Casida et al., 1962), cows (Casida et
al., 1962), and human beings (Hutson & Hoadley, 1972a), after different
routes of administration using radiolabelled dichlorvos. In general,
the metabolism of dichlorvos in the various species is similar and
rapid. Differences between species are related to the rate of
metabolite formation rather than to the nature of the metabolites.
In the mouse, O- desmethylation is a more important route of
dichlorvos detoxification than it is in the rat (Table 9), as indicated
by the larger amounts of radioactivity excreted in the mice as
desmethyldichlorvos.
Table 9. Isotope dilution analysis of urine from mammals treated orally
with vinyl-1-14C-dichlorvosa
-----------------------------------------------------------------------------
Metabolite Proportion of administered radioactivity as
measured urinary metabolite (%)
rat mouse hamster man
----------------------------------------------------------------------------
hippuric acid 1.7 0.6 1.0 0.4
desmethyldichlorvos 2.2 18.5 -b 0.15
urea (isolated as 0.6 0.6 -b 0.1
the nitrate salt)
-----------------------------------------------------------------------------
a From: Hutson & Hoadley (1972a).
b Not measured.
Desmethyldichlorvos arises from the hydrolysis of the methyl
oxygen-phosphate bond and is further degraded into DCA, mono-
methylphosphate, and dimethylphosphate (Casida et al., 1962; Hodgson &
Casida, 1962; Bradway et al., 1977). S- methyl-glutathione is formed
along with desmethyldichlorvos, and is degraded to methylmercapturic
acid and excreted in the urine (Hutson & Hoadley, 1972b).
The two major routes of metabolism of the vinyl portion of the
dichlorvos molecule lead to: (a) dichloroethanol glucuronide, and (b)
hippuric acid, urea, carbon dioxide, and other endogenous biochemicals
which give rise to high levels of radioactivity in the tissues for a
few days after dosing with vinyl-1-14C-dichlorvos. Both pathways
have been shown to occur in man, owing to the presence of these
compounds in the urine (Hutson & Hoadley, 1972a). In laboratory
animals most of the observed radioactivity in carcasses and tissues was
present as glycine, serine, and other normal body components,
indicating that the vinyl carbon atoms of dichlorvos enter the 2-carbon
metabolic pool (Hutson et al., 1971b; Page et al., 1971; Hutson &
Hoadley, 1972b; Loeffler et al., 1976). No evidence of accumulation of
dichlorvos or potentially toxic metabolites was found. A scheme of the
metabolites of dichlorvos in mammals is given in Fig. 1.
6.3.1 Metabolites
When 32P-dimethylphosphate (500 mg/kg body weight) was
administered orally to a male rat almost the entire dose was
eliminated. The urine contained about 50% unmetabolized
dimethylphosphate. On the other hand, a rat orally dosed with
32P-desmethyldichlorvos (500 mg/kg body weight) eliminated about
14% of the dose via urine in 90 h, 86% of the radioactivity being
phosphoric acid and 14% unchanged desmethyldichlorvos. The very
high proportion of radioactivity in the bone was indicative of rapid
degradation to phosphoric acid (Casida et al., 1962).
Following the intraperitoneal injection of 1-14C-DCA or
1-14C-dichloroethanol to female rats, 32% of the radioactivity was
expired as carbon dioxide within 24 h (Casida et al., 1962).
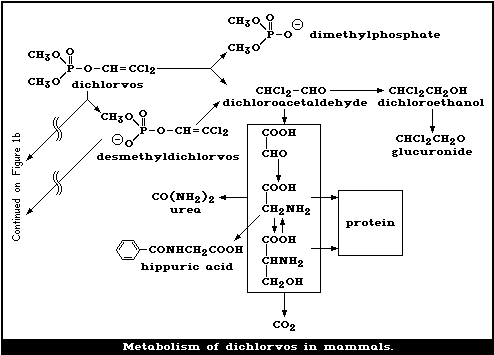
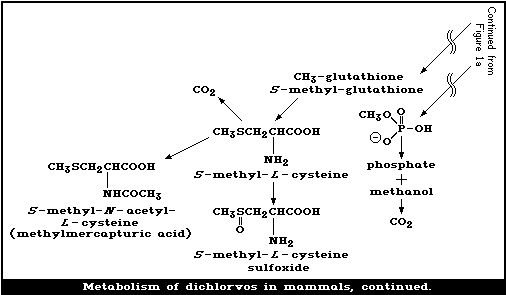 6.4 Elimination and Excretion in Expired Air, Faeces, and Urine
6.4.1 Human studies
Eight hours after a human male consumed 5 mg of vinyl-1-
14C-dichlorvos in orange juice, 27% of the radioactivity had been
eliminated as 14C-carbon dioxide. Approximately 8% had been excreted
by the urine within one day following dosing. Urinary excretion of
radioactivity decreased gradually and by day 9 none was detectable
(Hutson & Hoadley, 1972a).
The concentration of dimethylphosphate in the urine of three
pesticide control operators spraying houses with dichlorvos ranged from
0.32 to 1.4 µg at the end of the day's work (Das et al., 1983).
6.4.2 Studies on experimental animals
6.4.2.1 Oral
Dosing rats orally with 32P-dichlorvos (0.1 - 80 mg/kg body
weight) resulted in a recovery of 60 - 70% of the administered
radioactivity in the urine and approximately 10% in the faeces over a
6-day period following dosing (Casida et al., 1962).
After the oral administration of methyl-14C-dichlorvos to rats
(1 mg) and mice (0.5 mg), the excretion of radioactivity was rapid.
The major route of elimination after 4 days was the urine
(approximately 60%), followed by expired air (approximately 16%)
(Hutson & Hoadley, 1972b).
Rats given an oral dose of vinyl-1-14C-dichlorvos (1 mg per
animal) eliminated 10 - 20% of the 14C in the urine, 3 - 5% in the
faeces, and approximately 40% as expired carbon dioxide over 4 days
following dosing (Hutson et al., 1971a,b).
A comparison between the excretion by rat, mouse, hamster, and man
24 h after oral dosing with vinyl-1-14C-dichlorvos is given in
Table 10 (Hutson & Hoadley, 1972a).
A cow treated orally with 20 mg/kg body weight
32P-dichlorvos eliminated 40% of the radioactivity in the urine and
50% in the faeces. In the milk, the level of organosoluble radio-
activity was significantly above background only within the first 2 h
(Casida et al., 1962).
Table 10. Comparison of percentages of radioactivity excreted by males
24 h after oral ingestion of vinyl-1-14C-dichlorvosa
-----------------------------------------------------------------------------
Excretion route Rat (3) Mouse (1) Hamster (2) Man (1)
-----------------------------------------------------------------------------
urine 9.8 27.4 14.7 7.6
faeces 1.5 3.2 2.9 -
carbon dioxide 28.8 23.1 33.5 27 (8 h only)
-----------------------------------------------------------------------------
a Number of animals are given in parentheses.
6.4.2.2 Parenteral
The elimination of a single intraperitoneal injection of vinyl-1-
14C-dichlorvos (4 mg/kg body weight) from female rats was similar to
the elimination after oral dosing. A goat treated subcutaneously with
1.5 mg 32P-dichlorvos/kg body weight excreted 79% of the radioactivity
in the urine and 11% in the faeces. Two cows received an intravenous
or a subcutaneous injection with 1 mg 32P-dichlorvos/kg body weight.
Of the radioactivity which was recovered, 70 - 80% was in the urine and
approximately 14% in the faeces (Casida et al., 1962).
6.5 Retention and Turnover
6.5.1 Biological half-life
In studies by Blair et al. (1975), the metabolism of dichlorvos was
found to be so rapid that the biological half-life in blood could not
be determined. No intact dichlorvos could be demonstrated in the blood
or tissues of animals exposed by routes other than parenteral
injection. Only after exposure for 4 h to an atmospheric concentration
of 90 mg dichlorvos/m3 could dichlorvos be detected in most tissues of
the rat and mouse. Following exposure at 50 mg/m3, for 2 or 4 h, the
half-life in the rat kidney was 13.5 min.
The intraperitoneal injection of 10 mg dichlorvos/kg body weight
into mice increased the accumulation of ACh in the brain and caused an
inhibition of ChE activity. Symptoms of toxicity were clearly
recognizable after 15 min, and they disappeared almost completely after
60 min. The ChE activity and ACh levels reached their minimum and
maximum, respectively, at 15 min. The maximum concentration of
dichlorvos in the brain was reached after 1 min and decreased
thereafter, rapidly reaching the baseline level after 3 min (Nordgren
et al., 1978).
6.5.2 Body burden
There is no evidence for the storage of dichlorvos or its
metabolites in the tissues of animals. Small fractions of the carbon,
phosphorus, and chlorine derived from dichlorvos are retained in the
body for several days because their turnover rate is the same as that
for identical materials from other origins.
6.5.3 Indicator media
The determination of dichloroethanol in urine as a means of
monitoring the exposure of human beings to dichlorvos is not
sufficiently sensitive to detect levels arising from vapour exposure
through normal use. However, it could serve as the basis for a
specific detection method for the accidental ingestion of high levels
(Hutson & Hoadley, 1972a). Two other methods can be used: (a)
determination of the blood ChE activity; or (b) determination of
dimethylphosphate in urine by a rather complicated method (Blair &
Roderick, 1976). Neither method is specific when exposure to other
organophosphate or carbamate compounds, or to compounds that also
metabolize to dimethylphosphate, may have occurred.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
Lal (1982) reviewed the accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms.
Microorganisms undoubtedly have the ability to metabolize
organophosphorus insecticides; however, there are still large gaps in
our knowledge. It also seems clear that chemical, photochemical,
physical, and biological factors may influence the metabolism of
dichlorvos by microorganisms.
7.1.1 Algae and plankton
The dose of dichlorvos producing 50% growth inhibition of the
unicellular alga Euglena gracilis has been quoted as 3.5 mg/litre
(Butler, 1977).
Treating eutrophic carp ponds with 0.325 mg/litre killed
Cladocera (predominantly Bosmina and Daphnia species) and decreased
Copepoda (mainly Cyclops ). This was offset by increased development
of Rotatoria (mainly Polyarthra and Brachionus species) and
phytoplankton (mainly Scenedesmus and Pediastrum species), so that
the total plankton biomass changed only slightly (Grahl et al., 1981).
7.1.2 Fungi
Dichlorvos (in the range 10 - 80 mg/litre) has been found to affect
citric acid fermentation in Aspergillus niger grown in an artificial
medium. Inhibition of the fermentation was marked only at 40 and
80 mg/litre (Rahmatullah et al., 1978; Ali et al., 1979c). It appears
from the decreased uptake of inorganic phosphorus that dichlorvos may
have an interfering action on oxidative metabolism in A. niger. The
potential for inhibiting citrinin production by Penicillium
citrinum was investigated. Dichlorvos inhibited citrinin production by
76% at 100 µg/litre and by 48% at 10 µg/litre (Draughon & Ayres,
1978). The effect of dichlorvos on the survival time and the membrane
potential of the slime mould Physarum polycephalum was studied in a
laboratory test system. The threshold value for both these effects was
found to be 300 mg/litre for technical dichlorvos and 30 mg/litre for
the pure chemical (Terayama et al., 1978).
The influence of dichlorvos on 17 soil fungi, cultivated in
artificial medium, was tested. Dose levels of 0, 10, 30, 60, and
120 mg/kg were used during a test period of 21 days, and the effect on
the growth and morphology of the fungi was studied. In general, a
growth depression was found, but its extent depended on the fungal
strain. Occasionally growth was either unaffected or even stimulated
(Jakubowska & Nowak, 1973).
7.1.3 Bacteria
Dichlorvos has been found not to influence the overall metabolic
processes of Escherichia coli and Enterobacter aerogenes at doses up to
250 mg/litre (Grahl et al., 1980) and was not toxic for a sewage
isolate at up to 10 mg/litre (Rosenberg et al., 1979). In poultry
effluent slurry, concentrations of 100 and 1000 mg/litre did not
significantly reduce coliform populations, but 10 000 mg/litre caused
almost complete death. Therefore, residues of dichlorvos used for fly
control in layer houses can significantly reduce the enteric coliform
populations that are essential to the conversion of organic nitrogen to
inorganic nitrogen in poultry waste effluent (Ballington et al.,
1978).
In studies by Lieberman & Alexander (1981), dichlorvos (0.1 -
100 mg/litre) had little or no toxicity for microorganisms degrading
organic matter in sewage, as measured by respiratory activity,
degradation, and nitrification.
Incubation of dichlorvos with inocula of ruminal bacteria or
ciliated protozoa under anaerobic conditions suggests that dichlorvos
is not utilized by the organisms for growth, nor does it stimulate
endogenous gas production. However, it does, in certain instances,
affect volatile fatty acid production (Williams, 1977).
The growth of Bacillus thuringiens var th. was not inhibited by
dichlorvos (Dougherty et al., 1971).
7.2 Aquatic Organisms
Reviews of the acute and chronic effects of pesticides on aquatic
organisms have been made by Brungs et al. (1977), Livingston (1977),
and Kenaga (1979).
The toxicity of a chemical for aquatic organisms is influenced by
many factors such as the stage of development of the organism and the
composition, pH, oxygen content, and hardness of the water. In this
short review, these factors are not discussed in detail.
7.2.1 Fish
7.2.1.1 Acute toxicity
The acute toxicity of dichlorvos for both freshwater and estuarine
species of fish is moderate to high. The available data are summarized
in Table 11.
Variations in water hardness from 44 to 162 mg/litre and in pH from
6 to 9 did not alter the toxicity of dichlorvos for cutthroat or lake
trout (Johnson & Finley, 1980).
In studies by Yamane et al. (1974), young carp were exposed to a
concentration of 25 mg dichlorvos/litre water for 45 min. The ChE
activity (histochemically determined) of many tissues, including the
stratum griseum periventriculare, sarcolemma, and liver, was inhibited
or totally lost.
Table 11. Acute toxicity of dichlorvos for fish
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Freshwater
Clarias batrachus 26 - 31 g - 8.9 Verma et al. (1983)
Carp 8 mm 20 - 23 0.34 Verma et al. (1981d)
(Cyprinus carpio) 6 g 23 20a Yamane et al. (1974)
Mosquito fish 0.2 g 17 5.3 Johnson & Finley (1980)
(Gambusia affinis)
Blue gill 1.5 g 18 0.9 Johnson & Finley (1980)
(Lepomis macrochirus)
African catfish 6 - 10 g 18 0.5 Verma et al. (1980, 1981a)
(Mystus vittatus)
Ophiopcephalus punctatus 40 - 55 g 18 2.3 Verma et al. (1981a)
Fathead minnow 0.7 g 17 12 Johnson & Finley (1980)
(Pimephales promelas)
Harlequin fish - 20 7.8b Alabaster (1969)
(Rasbora heteromorpha)
Singii 5 - 10 g 18 6.6 Verma et al.
(Saccobranchus fossilis) (1982a)
Cutthroat trout 2.5 g 12 0.2 Johnson &
(Salmo clarki) Finley (1980)
Lake trout 0.3 g 12 0.2 Johnson &
(Salvelinus namaycush) Finley (1980)
Tilapia mossambica 3 - 10 cm 29 1.4 - 1.9 Rath & Misra
(1979a)
----------------------------------------------------------------------------------------------------
Table 11. (contd).
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Estuarine
American eel 0.14 g 20 1.8 Eisler (1970)
(Anguilla rostrata)
Mummichog 1.7 g 20 2.7 Eisler (1970)
(Fundulus heteroclitus)
Striped killifish 0.92 g 20 2.3 Eisler (1970)
(Fundulus majalis)
Atlantic silverside 0.8 g 20 1.3 Eisler (1970)
(Menidia menidia)
Striped mullet 1 - 6 g 20 0.23 Eisler (1970)
(Mugil cephalus)
Northern puffer 100 g 20 2.3 Eisler (1970)
(Sphaeroidus maculatus)
Bluehead 5.4 g 20 1.4 Eisler (1970)
(Thalassoma bifasciatum)
----------------------------------------------------------------------------------------------------
a 24-h LC50.
b 48-h LC50.
7.2.1.2 Short-term toxicity
Sublethal concentrations of dichlorvos (0.5 - 1 mg/litre) have
been found to decrease the respiratory rates of Tilapia mossambica (3
different age groups) exposed for up to 3 weeks. When the exposed
fish were transferred to fresh water, the rate did not completely
return to its pre-exposure value (Rath & Misra, 1979b). Liver and
brain ChE activity showed considerable inhibition when a group of T.
mossambica was exposed to dichlorvos (0.25 - 1.25 mg/litre water) for
periods of up to 4 weeks. At 7-day intervals, fish were studied or
transferred to clean water. The degree of enzyme inhibition was
related to the dichlorvos concentration and length of exposure. In all
age groups of fish, brain tissue exhibited a higher degree of ChE
inhibition than liver. Small fish were more susceptible to dichlorvos
with respect to AChE activity. When the fish were transferred to clean
water, most of the fish recovered their AChE activity, the recovery
being greater in liver than in brain. Small fish exhibited a
comparatively high level of recovery. The degree of recovery was
inversely related to the length of exposure (Rath & Misra, 1981).
Melanin dispersion in T. mossambica exposed to 1 mg/litre water for
15 days was stimulated indirectly by the inhibition of ChE activity.
The original colour was regained within 96 h after transfer of the fish
to clean water (Rath & Misra, 1980).
Exposure of Mystus vittatus (collected in the environment) to
sublethal concentrations of dichlorvos (0.045 or 0.09 mg/litre) for 30
days caused dose-related increases in serum glutamic-oxaloacetic and
glutamic-pyruvic transaminase levels (Verma et al., 1981b), increases
in alkaline phosphatase, acid phosphatase, and glucose-6-phosphatase
levels in serum (Verma et al., 1984), decreases in the levels of these
enzymes in liver, kidneys, and gills (Verma et al., 1981c), an increase
in glucose levels in blood, and a decrease in liver glycogen. Blood
lactate and muscle glycogen were unaffected (Verma et al., 1983). At
0.09 mg/litre, blood clotting time, mean corpuscular haemoglobin and
mean corpuscular haemoglobin concentration decreased, and the number of
leukocytes increased. Other haematological parameters did not show any
abnormalities (Verma et al., 1982b). From these results, a no-
observed-adverse-effect concentration of 0.03 mg/litre was derived.
In studies by Verma & Tonk (1984), Heteropneustes (Saccobranchus)
fossilis was exposed to dichlorvos for 30 days at a concentration of
0.44 mg/litre. Respiration, haematological parameters, and the
activities of two enzymes (one of them AChE) in liver, kidneys, and
gills were determined. The respiration rate decreased, and blood
concentrations of sodium and chloride ions and glucose increased
significantly, whereas the cholesterol level and clotting time were
decreased. A significant reduction in the AChE activity of the three
tissues was found.
Vadhva & Hasan (1986) studied the effect of dichlorvos (at 0, 3, 6,
and 9 mg/litre water) on various lipid fractions and lipid
peroxidation in the central nervous system of Heteropneustes
fossilis. After one week's exposure, the results indicated that
dichlorvos caused dose-related increases in total lipids, cholesterol-
esterified fatty acid, and lipid peroxidation in various regions of the
brain and spinal cord but a consistent decrease in the level of
phospholipids in these regions of the central nervous system.
Exposure of Clarias batrachus to 0.5 - 2.2 mg dichlorvos/litre
and Saccobranchus fossilis to 0.4 - 1.6 mg/litre for 30 days was found
to increase blood glucose and decrease liver glycogen, whereas blood
lactate and muscle glycogen were normal (Verma et al., 1983).
The estimated "maximum acceptable toxicant concentration"
(MATC)a for the larvae of Cyprinus carpio was 0.016 - 0.020 mg/litre
based on a 60-day study (Verma et al., 1981d).
7.2.2 Invertebrates
The acute toxicity of dichlorvos for aquatic insects and
crustaceans is extremely high (Table 12). As might be expected from an
organophosphorus insecticide, aquatic invertebrates are about three
orders of magnitude more susceptible to dichlorvos than are fish, and
freshwater crustaceans are particularly sensitive.
A study was carried out to determine the influence of a number of
pesticides on the "hatchability" of Artemia salina dry eggs. No effect
was found at 10 mg dichlorvos/litre in the aqueous system (Kuwabara et
al., 1980).
When prawns (Macrobrachium lamarrei) were exposed to dichlorvos at
concentrations of 0.31 or 0.62 mg/litre for 96 h, a decrease in hepatic
glycogen and an increase in the blood glucose level were found (Omkar &
Shukla, 1984). Possibly, the phosphorylase activity of the
hepatopancreas and muscle increased due to the inhibition of AChE
activity and the consequent accumulation of acetylcholine at
neurosynaptic junctions. The latter resulted in an induction of the
secretion of the sinus gland, which enhanced glycogenolysis.
7.3 Terrestrial Organisms
7.3.1 Birds
7.3.1.1 Acute oral toxicity
Dichlorvos has a high oral toxicity for birds (Table 13). The
signs of intoxication are typical of organophosphorus poisoning, namely
salivation, lachrymation, tremors, and terminal convulsions. They
usually appear shortly after dosing, and, at lethal doses, death occurs
within 1 h. Survivors appear to recover completely 24 h after dosing.
Various internal haemorrhages were found at autopsy in sacrificed
survivors of treated pheasants and Mallard ducks (Tucker & Crabtree,
1970).
-----------------------------------------------------------------------
a Maximum concentration at which no effect was seen.
Table 12. Acute toxicity of dichlorvos for non-target aquatic insects and Crustacea
-------------------------------------------------------------------------------------------------------
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (µg/litre) (µg/litre)
-------------------------------------------------------------------------------------------------------
Insects (stone flies)
Pteronarcys californica 15 - 0.1 Johnson & Finley (1980)
Crustacea (fresh water)
Water flea 15 0.07 - Johnson & Finley (1980)
(Daphnia pulex)
Water flea 21 0.28 - Johnson & Finley (1980)
(Simocephalus serrulatus)
Amphipod 21 - 0.5 Johnson & Finley (1980)
(Gammarus lacustris)
Crustacea (estuarine)
Sand shrimp - 12 4 Eisler (1969)
(Crangon septemspinosa)
Grass shrimp - 300 15 Eisler (1969)
(Palaemonetes vulgaris)
Hermit crab - 52 45 Eisler (1969)
(Pagurus longicarpus)
-------------------------------------------------------------------------------------------------------
Table 13. Acute oral LD50 values for birds
------------------------------------------------------------------------------------------------------------------------
Species Age Vehicle LD50 Reference
(mg/kg body weight)
------------------------------------------------------------------------------------------------------------------------
Red-winged blackbird propylene glycol 13.3 Schafer & Brunton (1979)
(Agelaius phoenicius) (male)
Mallard duck 5 - 7 months capsule 7.8 Tucker & Crabtree (1970)
(Anas platyrhynchos) (male)
Common pigeon propylene glycol 24 Schafer & Brunton (1979)
(Columba livia)
Quaila propylene glycol 24 Schafer & Brunton (1979)
(Coturnix coturnix) (female)
Domestic fowl 21 days aqueous suspension 6.5 Naidu et al. (1978)
(Gallus domesticus) (male) 6 - 8 months aqueous suspension 30 Dmitriev & Kozhemyakin
(1975)
House sparrow propylene glycol 17.8 Schafer & Brunton (1979)
(Passer domesticus)
Ring-necked pheasant 3 months capsule 11 Tucker & Crabtree (1970)
(Phasianus colchicus) (male)
Common grackle propylene glycol 13.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Starling adult propylene glycol 12 Schafer (1972)
(Sturnus vulgaris) - propylene glycol 42.1 Schafer & Brunton (1979)
------------------------------------------------------------------------------------------------------------------------
a Hattori et al. (1974) carried out studies on Japanese quail and found
LD50 values of 22 and 26 respectively, for male and female
(no details available).
7.3.1.2 Short-term toxicity
In short-term dietary studies, dichlorvos has been found to be
slightly to moderately toxic for birds (Table 14).
In the study with 7-day-old male chicks, there was weight loss and
50% mortality at 500 mg/kg diet. Marked, dose-related inhibition of
brain ChE activity occurred at 50, 100, and 500 mg/kg diet, but no
effects were noted at a level of 10 mg/kg diet (Naidu et al., 1978).
Canaries, Indian finches, and budgerigars, continuously exposed to
dichlorvos vapour (0.14 mg/m3) for 5 days, did not show any overt
signs of intoxication, but a reduction in ChE levels in plasma and
brain was observed in canaries and Indian finches (Brown et al.,
1968).
7.3.1.3 Field experience
Caution has been advised in the use and handling of dichlorvos
where birds might be exposed (Whitehead, 1971). The necessity for this
warning can be illustrated by the following cases. Adult mallards
feeding near horse mangers containing dichlorvos-treated feed were
found dead within a short time. At necropsy, excessive amounts of
mucus covering the mucosa of the proventriculus and scattered petechiae
along medial edges of liver lobes were observed. The small and large
intestines were markedly extended, and crystals were noted in the
gizzard. Brain ChE activity was inhibited by 75 - 80% (Ludke & Locke,
1976). Domestic fowl, which had accidental access to the faeces of a
horse dosed with dichlorvos pellets, picked out the pellets and more
than 30 birds died during the next 24 h (Lloyd, 1973). A mass
poisoning occurred in chickens following consumption of accidentally
contaminated drinking-water (Egyed & Bendheim, 1977). English game
bantams died after consuming wheat contaminated with 300 mg
dichlorvos/kg wheat (Reece, 1982).
7.3.2 Invertebrates
Dichlorvos was toxic for silkworm larvae when for 4 h they were fed
mulberry leaves previously sprayed with dilute dichlorvos emulsions.
Spray concentrations giving 50% mortality ranged from 1.56 to 6.25
mg/litre (Aratake & Kayamura, 1973).
No adverse effects were observed on the hatchability and general
condition of first instar silkworm larvae hatched in the following
generation when 5th instar larvae were fed mulberry leaves pre-treated
with 3 mg dichlorvos/kg of leaf (Yamanoi, 1980).
Table 14. Dietary LD50 values for birds
-------------------------------------------------------------------------------------
Species Age Duration LD50 Reference
(days) of feeding (mg/kg diet)
(days)
-------------------------------------------------------------------------------------
Mallard duck 16 8a 5000 Hill et al. (1975)
(Anas platyrhynchos) 5 8a 1310 Hill et al. (1975)
Japanese quail 14 8a 300 Hill et al. (1975)
(Coturnix japonica)
Domestic fowl 7 28 500 Naidu et al. (1978)
(Gallus domesticus) (male)
Ring-necked pheasant 10 8a 570 Hill et al. (1975)
(Phasianus colchicus)
-------------------------------------------------------------------------------------
a The median lethal dietary dose (LD50) during an 8-day test including
5 days of treated diet followed by 3 days of untreated diet.
7.3.3 Honey bees
Dichlorvos is highly toxic for honey bees. Atkins et al. (1973)
found in laboratory studies an LD50 of 0.495 µg/bee in 48 h (topical
application of dust; 26.7 °C; relative humidity 65%). Beran (1979)
obtained an oral LD50 of 0.29 µg/g body weight and an LD50 (topical
application) value of 0.65 µg/g body weight. This high toxicity has
led dichlorvos to be classified in the most toxic category for bees in
Austria and the USA.
When honeycombs were exposed to dichlorvos vapour from dichlorvos
resin strips for 4 months, the combs absorbed the insecticide and were
toxic to bees for approximately one month after exposure ceased.
Contamination of the bees appeared to be by fumigant rather than
contact action (Clinch, 1970).
7.3.4 Miscellaneous
Dichlorvos was highly toxic for the predatory mite Amblyseius
longispinosus in contact trials. Residual toxicity disappeared within
6 days, the susceptibility being the same as that of Phytoseiulus
persimilis (Shinkaji & Adachi, 1978).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides in general, especially their short- and long-term effects
on the nervous system, will be found in Environmental Health Criteria
63: Organophosphorus Insecticides - A General Introduction (WHO,
1986).
8.1 Single Exposures
Dichlorvos is moderately to highly toxic when administered in
single doses by various routes to a variety of animal species
(Table 15). It is less toxic via the dermal and oral routes than by
parenteral routes. The signs of intoxication by all exposure routes
are typical of organophosphorus poisoning, i.e., salivation,
lacrimation, diarrhoea, tremors, and terminal convulsions, with death
occurring from respiratory failure. In addition, lethargy, ataxia,
hypersensitivity to noise, splayed gait, and paresis may be observed.
The signs are usually apparent shortly after dosing and, at lethal
doses, death occurs within 1 h. Survivors appear to recover completely
24 h after dosing.
The acute inhalational LC50 values for mice and rats are
summarized in Table 16. The apparent differences in the LC50s may be
the result of the type of exposure of the animal (whole body or head
only), whether the studies were carried out with dichlorvos vapour or
atomized particles of spray (with or without vehicle), or differences
in the purity of the dichlorvos. Moreover, since dichlorvos adheres
strongly to surfaces including glass, the out-going air has a
significantly lower concentration of dichlorvos than the air coming
into the chamber, if the system is not yet equilibrated.
No macroscopic abnormalities were observed in mice or rats 2 weeks
after a single exposure (MacDonald, 1982).
8.1.1 Domestic animals
When cattle and sheep were treated orally with a single dose of
dichlorvos, 10 mg/kg body weight was toxic for calves and 25 mg/kg body
weight for sheep. For the latter a dose of 10 mg/kg body weight was
without effects (Radeleff & Woodard, 1957).
8.1.2 Potentiation
Potentiation studies on male rats indicated that oral
administration of dichlorvos with 22 other organophosphate pesticides
resulted in no (or very little) potentiation, while administration with
malathion showed a marked potentiation (Narcisse, 1967; Kimmerle &
Lorke, 1968). However, Cohen & Ehrich (1976) found that the anti-ChE
action of 800 mg malathion/kg body weight (injected intraperitoneally)
was not potentiated by pre-treatment (18 h previously) with 30 mg/kg
dichlorvos, nor did malathion pre-treatment potentiate the action of
dichlorvos.
Table 15. The acute toxicity (LD50) of dichlorvos for experimental animals
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Mouse (male) oral unknown Eryfor EL or other 68 - 90 Vrbovsky et al. (1959);
solvents Ueda et al. (1960)
Mouse oral 80% unknown 87 Sasinovich (1968, 1970)
Mouse oral 97% aqueous polysor- 133 - 139a Haley et al. (1975)
bate 80
Mouse (male) oral 98% corn oil 140 Isshiki et al. (1983)
Mouse oral unknown aqueous 124 - 275a Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) subcutaneous unknown propylene glycol 13 - 33 Ueda et al. (1960);
and other solvents Jaques (1964)
Mouse subcutaneous unknown aqueous 20 - 26 Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) dermal unknown different solvents 206 & 395b Ueda et al. (1960)
Mouse intraperitoneal technical corn oil 28 Vrbovsky et al. (1959);
Casida et al. (1962)
Mouse intraperitoneal unknown aqueous 28 - 41a Holmstedt et al. (1978)
Mouse intravenous unknown aqueous 8 - 10 Holmstedt et al. (1978)
Rat oral 90 - 99% peanut oil and 56 - 96a Durham et al. (1957);
other solvents Narcisse (1967); Gaines
(1969)
Rat (male) oral unknown Eryfor EL and 46 - 110 Vrbovsky et al. (1959);
other solvents Ueda et al. (1960)
Rat oral 80% unknown 65 Sasinovich (1968, 1970)
Rat oral unknown aqueous 30 Holmstedt et al. (1978)
---------------------------------------------------------------------------------------------------------
Table 15. (contd).
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Rat dermal unknown xylene 75 - 107a Durham et al. (1957);
Gaines (1969)
Rat dermal 80% unknown 113 Sasinovich (1968, 1970)
Rat subcutaneous 95% dimethylsulfoxide 72 Brown & Stevenson
(1962)
Rat (male) intraperitoneal unknown Eryfor EL 18 Vrbovsky et al. (1959)
Guinea-pig subcutaneous 95% undiluted 28 Brown & Stevenson
(1962)
Syrian hamster intraperitoneal - suspension in 30 Dzwonkowska & Hübner
water (1986)
Rabbit oral 93% unknown 12.5 Desi et al. (1978)
Rabbit oral 80% unknown 22.5 Sasinovich (1968, 1970)
Rabbit dermal 80% unknown 205 Sasinovich (1968, 1970)
Cat oral 80% unknown 28 Sasinovich (1968, 1970)
Dog oral in capsule 100 - 316 Kodama (1960)
Swine (40- to 60- oral technical in capsule 157 Stanton et al. (1979)
day-old)
Domestic fowl oral technical in capsule 15 Sherman & Ross (1961)
(chicken)
---------------------------------------------------------------------------------------------------------
a The LD50s are often slightly different in males and females. Furthermore, it is clear that the
purity of the dichlorvos tested and the vehicle used has an influence on the toxicity.
b The two values were obtained using two different solvents.
Table 16. Inhalational LC50 values for dichlorvos
---------------------------------------------------------------------------------------------------------
Species Purity Type of Duration LC50 (mg/m3) Reference
exposure
---------------------------------------------------------------------------------------------------------
Mouse 80% vapour 4 h 13 Sasinovich (1968, 1970)
whole body
Mouse 98% vapour 4 h > 218 MacDonald (1982)
head only
Mouse (male) unknown; in vapour 4 h 310 Ueda et al. (1960)
solvent Ca whole body
Rat (male) unknown unknown, but 1 h 455 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat (male) 84.1% whole body 1 h 140 Sakama & Nishimura
polyethylene (1977)
glycol
Rat (male) unknown unknown, but 4 h 340 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat 80%(?) vapour 4 h 15 Sasinovich (1968, 1970)
whole body
Rat 98% vapour 4 h > 198 MacDonald (1982)
head only
---------------------------------------------------------------------------------------------------------
a Solvent C = 0.5% Sorpol 2020 water solution.
In vitro studies using human erythrocytes and plasma as ChE
sources, with ACh as substrate, indicated no potentiationa when
dichlorvos was tested in combination with carbaryl, crotoxyphos,
phosphamidon, malathion, malaoxon, mevinphos, parathion, paraoxon,
physostigmine, and trichlorphon (Carter & Maddux, 1968, 1974).
8.2 Short-term Exposures
8.2.1 Oral
8.2.1.1 Mouse
A 10-week (range-finding) toxicity study was carried out on B6C3F1
mice given 0, 25, 50, 100, 200, or 400 mg dichlorvos/ litre drinking-
water. Each group consisted of 12 males and 12 females, except the
control group (10 males and 10 females). Growth and mortality were
comparable with controls. In a second study, groups of 10 males and 10
females received 400, 1600, 3200, 5000, or 10 000 mg dichlorvos/litre.
The animals given the two highest doses died within 2 weeks, while the
1600 and 3200 mg groups showed slight and clear growth depression,
respectively, after 10 weeks (Konishi et al., 1981).
8.2.1.2 Rat
For 15 weeks, groups of 15 male and 15 female Charles River rats
were fed diets containing 0, 0.1, 1, 10, 100, or 1000 mg dichlorvos
(93%)/kg diet, which were freshly prepared once each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1), it may be
assumed that the average concentration of dichlorvos in each diet was
approximately 47% of the amount that had been added. There were no
deaths or signs of intoxication. Only the rats fed the highest dose
exhibited decreased growth rates at the beginning of the study. At
termination, no differences were observed in haematology, serum
protein, urinalysis, or gross or histopathological examination. In the
highest dose group, marked inhibition of ChE activity in plasma,
erythrocytes, and brain was noted. In the 100 mg/kg group, only
erythrocyte ChE inhibition was observed, and the 10 mg/kg group did not
show any ChE inhibition (Witherup et al., 1964).
Daily doses of 3.5 or 7 mg dichlorvos (purity not stated)/kg body
weight administered intragastrically to rats for 4 months and 0.7 or
1.4 mg/kg for 12 months caused no deaths. There were signs of
intoxication in the 7 mg group, and decreases in food consumption and
body weight gain and increases in a number of organ weights were seen
in all the groups except the lowest dose group. Inhibition of plasma,
erythrocyte, and brain ChE activity was observed in all groups except
those given 0.7 mg/kg body weight. The inhibition was time and dose
dependent (Sasinovich, 1970).
-----------------------------------------------------------------------
a Potentiation is the phenomenon that results in the combined effect
of exposure to two or more chemical substances being greater than
the sum of the effects that would be produced by each substance
separately, as the result of synergistic action.
In a (range-finding) toxicity study, groups of F-344 rats (10 of
each sex) were administered 0, 5, 10, 20, 40, or 80 mg/kg body weight,
dissolved in 10 ml of drinking-water. During the study six animals
died, five of which were in the two highest doses groups (Enomoto,
1981).
Dichlorvos administered orally to rats at a concentration of 70
mg/kg body weight inhibited not only ChE, but also alkaline
phosphatase, lactate dehydrogenase, and glutamate dehydrogenase
competitively. It increased the activity of glutamic-pyruvic
transaminase, but leucine aminopeptidase was not affected (Ellinger,
1985).
Ellinger et al. (1985) also studied the influence of dichlorvos on
haematological parameters in acute and short-term toxicity studies. In
the acute study 70 mg dichlorvos/kg body weight and in the short-term
study 30 mg/kg body weight were administered for 12 weeks. Decreases
in haemoglobin, haematocrit, and mean corpuscular haemoglobin
concentration were found.
Seven rat mothers were administered different dose levels of
dichlorvos (1 g or 10 g/litre distilled water) by stomach tube, and
their litters (1 - 12 days old) were nursed for about 21 days, and
weaned after 35 days of age. Although doses of 10 and 20 mg/kg body
weight did not cause any symptoms of intoxication, the mothers showed
significant inhibition of erythrocyte ChE activity. Plasma ChE
activity was affected to a lesser extent. Dose levels of 30 and
40 mg/kg body weight caused severe inhibition of erythrocyte ChE, and
severe cholinergic symptoms were seen. The symptoms occurred 10 -
20 min after dichlorvos administration and continued for 30 - 90 min,
when recovery took place (Tracy et al., 1960).
When pregnant rats were given oral doses of 1 or 5 mg dichlorvos in
oil/kg body weight during days 14 - 21 of pregnancy, the plasma ChE
activity of the mothers was markedly inhibited, but that of the young
(up to 56 days old) resembled the activity in the control. Brain ChE
activity did not show any significant inhibition (Zalewska et al.,
1977).
8.2.1.3 Rabbit
The progeny of rabbits, treated orally with 6 mg dichlorvos/kg body
weight per day for the last 10 days of pregnancy showed a decrease in
brain ChE activity and an increase in plasma ChE activity throughout
days 1 - 16 of life (Maslinska & Zalewska, 1978).
8.2.1.4 Cat
"Flea collar dermatitis" has been described in cats and dogs
wearing dichlorvos-impregnated PVC flea collars. In most cases, flea
collar dermatitis is a primary irritant contact dermatitis to
dichlorvos. The symptoms may consist of mild local irritation
(localized erythema and itching), severe local irritation varying from
erythematous alopecia to erosions, ulceration, oedema, and purulent
discharges with crusting or, in the most severe cases, generalized
dermatitis with secondary pyoderma and systemic illness (Muller,
1970).
Two trials with six and five cats, respectively, were carried out
to study the occurrence of contact dermatitis and systemic
intoxication. The cats received either a placebo collar or a
dichlorvos collar. Two cats received two dichlorvos collars each and
each cat was observed for 21 days. In a third trial, four groups of 25
cats were used to study hepatotoxicity. The cats of the different
groups were treated as follows. Group 1: placebo collar; Group 2:
placebo collar and intraperitoneal treatment with carbon tetrachloride
(CCl4) 7 days later; Group 3: dichlorvos collar; Group 4: dichlorvos
collar and intraperitoneal injection of CCl4 7 days later. Serum
glutamic pyruvic transaminase and ChE activity was estimated on day 9
of exposure. In a fourth study, 24 cats were fitted with a placebo
collar to test the effect of PVC in relation to the contact
dermatitis. PVC did not have an effect. No clear effect of the
CCl4-induced hepatic dystrophy on the detoxification of dichlorvos
was found.
The predominant systemic abnormalities recorded in these studies
were bone-marrow depression, ataxia, demyelination, and depression.
Red cell and plasma ChE activity was severely depressed. Furthermore,
contact dermatitis was seen in animals with dichlorvos collars.
Ambient temperature and relative humidity may have had a marked
influence on these effects, especially in the case of high temperature
and low humidity (Bell et al., 1975).
A 90-day study on 90 mongrel cats was carried out to verify the
results of Bell et al. (1975) by using a larger number of animals over
a longer period. There were three groups of 30 cats; one group with a
PVC collar (without dichlorvos), one group with one dichlorvos collar,
and the third group with three dichlorvos collars. In this study, in
which all relevant parameters were studied, no confirmation of the
abnormalities described by Bell et al. (1975) were found, even under
conditions of high temperature and low humidity. The only effect was
contact dermatitis, but, in this study, this was also found in the PVC
collars (Allen et al., 1978).
8.2.1.5 Dog
Groups of three male and three female dogs received equivalents of
approximately 0, 0.3, 1, 1.5, or 3 mg dichlorvos (93% in olive oil by
gelatin capsule)/kg body weight, daily, for 90 days. No effects were
observed on mortality, growth, liver and kidney function, organ
weights, haematology, or at gross and histopathological examination.
In the two highest dose groups, the dogs showed excitement, increased
activity, and aggression. Plasma and erythrocyte ChE activities
(measured initially and at intervals of approximately 2 weeks) were
normal in the lowest dose group (0.3 mg/kg body weight) but reduced in
the other dose groups. Brain ChE activity at termination was reduced
only in the highest dose group (Hine, 1962).
8.2.1.6 Pig
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, and 16 mg dichlorvos/kg
body weight per day in the feed (divided over two daily doses) for 30
days. Body weight gain, blood cell counts, packed-cell volumes,
haemoglobin concentrations, plasma glucose, plasma fatty acids, hepatic
and muscle glycogen concentrations, plasma and skeletal muscle
concentrations of electrolytes, and plasma and pancreatic insulin were
all comparable with those of control swine fed a blank PVC formulation.
Plasma and erythrocyte ChE activities were significantly inhibited in
the 4 and 16 mg/kg groups only (Stanton et al., 1979).
8.2.1.7 Cow
Two cows with suckling calves fed 200 mg dichlorvos/kg grain in
their rations showed normal ChE activity in the erythrocytes. However,
severe inhibition of ChE activity was found at 500 mg/kg feed
(equivalent to 4.5 mg/kg body weight), and a single dose of 27 mg/kg
body weight caused cholinergic collapse, followed by rapid recovery.
The ChE values in the calves remained normal throughout the 78-day
test. Milk from these two cows contained less than 0.08 mg
dichlorvos/litre (Tracy et al., 1960).
8.2.2 Dermal
8.2.2.1 Rat
In studies by Dikshith et al. (1976), groups of 48 male rats
received daily topical applications of 21.4 mg/kg body weight
dichlorvos (96%) in ethanol (control animals received ethanol alone) 5
days per week for 90 days. At intervals of 7, 15, 30, 45, 60, and 90
days, eight test rats and two control rats were sacrificed for
histopathological examination of the skin and testes. None of the
animals showed signs of intoxication, but ChE activity was not
measured. The skin showed no irritation, and no histopathological
changes were seen in the testes or skin.
8.2.2.2 Livestock
Spray and dip studies have shown that a concentration of 1%
dichlorvos is not toxic for cattle (no further details are available)
(Radeleff & Woodard, 1957).
The effect of applying different suspensions (different solvents
with and without an emulsifier) of dichlorvos to the skin of cows has
been studied. The dose level was 5 mg/kg body weight, increasing after
7 days to 10 mg/kg. Dichlorvos at 5 mg/kg body weight caused a
significant decrease in ChE activity in blood serum. The higher dose
level additionally produced symptoms of intoxication. No dichlorvos
residues were found in milk (Majewski et al., 1978).
Two heifers were washed daily with either a 1% aqueous solution or
emulsion of dichlorvos or a 10% suspension of dichlorvos in water
(daily application of 1.8 g dichlorvos for 21 days). Two additional
heifers were used as controls. The ChE activity levels of the
erythrocytes remained at the lower limits of normal variation (Tracy et
al., 1960).
8.2.3 Inhalation
8.2.3.1 Experimental animals
In a report by Sasinovich (1968), groups of rats were exposed
(whole body) daily for 4 h to average dichlorvos concentrations
of 0.11 or 1.1 mg/m3 for 4 months, to 5.2 mg/m3 for 2
months, or to 8.2 mg/m3 for 45 days. The highest dose caused signs
of intoxication; two of the eight rats died. Exposure to
5.2 mg/m3 or more resulted in marked inhibition of ChE activity and
disturbance of the blood-sugar curve. In the 0.11 and
1.1 mg/m3 groups, no significant effects were observed.
Mice, rats, and guinea-pigs, exposed 23 h per day for 28 consecu-
tive days to actual dichlorvos concentrations of 0.03 mg/m3, did not
show gross pathological changes at the end of the study. Inhibition of
plasma and brain ChE activity occurred in male mice, male guinea-pigs
(plasma only), and female rats (brain only) after a 5-day exposure to
0.14 - 0.15 mg/m3 dichlorvos (Brown et al., 1968).
Guinea-pigs (P strain) exposed for 7 h per day for 5 consecutive
days to an actual concentration of 90 - 120 mg/m3 dichlorvos suffered
no visible effects to their health. Rats (CFE) and mice (CF1)
similarly exposed to 50 mg/m3 were not visibly affected. At
concentrations above 50 mg/m3, the mice became distressed and
prolonged exposure to 80 mg/m3 was frequently lethal. Rats were less
severely affected (Stevenson & Blair, 1969).
In studies by Vashkov et al. (1966), 100 mice, 50 rats, 22 rabbits,
and 13 cats were exposed to a mixture containing dichlorvos, kerosene,
xylene, and freons during a single 2-h exposure or for 2 h per
day for 40 days. Three concentrations, 16.5, 45, and 160 mg
dichlorvos/m3, were tested (the median gravimetric diameter of the
aerosol particles was about 5 µm). The overall condition of the
animals remained normal, and no effects on body weight, clinical
chemical blood analyses, blood ChE activity, or respiration rate, or
pathological changes were observed. Rabbits developed transient miosis
which disappeared at the end of the exposure period.
Mice (20), guinea-pigs (6), sheep (7), calves (39), and a heifer
were exposed to an increasing dichlorvos concentration in the air
generated by dichlorvos strips (20%). The number of strips was
increased each week for 6 weeks, reaching 80 strips per 140 m3, and
was then reduced over the next 6 weeks. During exposure to the
recommended number of strips, the dichlorvos concentration in the
air was 0.09 - 0.14 mg/m3. The highest concentration was
2.1 mg/m3 when the number of strips was 16 times the recommended
number. No mortality or signs of intoxication were observed, and mice
bore normal litters during the study. The serum ChE activities of the
calves and of those handling the animals were within normal values
(Henriksson et al., 1971).
When 10 rabbits, 8 cats, and 10 dogs (males and females) were
continuously exposed for 8 weeks to dichlorvos concentrations of 0.05 -
0.3 mg/m3 generated from impregnated PVC-resin strips, no effects were
found on general health, behaviour, plasma and erythrocyte ChE
activities, or electroencephalographic patterns in the brain of the
animals (Walker et al., 1972).
In studies by Coulston & Griffin (1977), four male and four female
Rhesus monkeys were continuously exposed to dichlorvos vapour at an
average actual concentration of 0.05 mg/m3 for 3 months. The control
group consisted of four males and one female. No adverse effects were
observed in appearance, behaviour, or haematological and clinical
chemical determinations. Plasma ChE activity was slightly inhibited
(up to 28% inhibition), whilst the greatest erythrocyte ChE activity
inhibition was 36%. No changes in nerve maximum conduction velocities
or muscle-evoked action potentials were induced by the exposure to
dichlorvos.
8.2.3.2 Domestic animals
Five horses, exposed continuously to dichlorvos for 22 days in a
closed barn that was treated daily with 17 mg dichlorvos/m3, dis-
played mild inhibition of erythrocyte ChE activity after 7 days,
followed by recovery at 11 - 22 days. However, plasma ChE activity was
not changed. The concentration in the barn varied between 0.24 and
1.48 mg/m3 (Tracy et al., 1960).
The influence on ChE activity in cattle exposed to an impregnated
plastic strip containing 20% of dichlorvos has been studied. Red blood
cell ChE activity was measured during the 85 days of exposure, and on
the 9th day an average inhibition of about 35% was found. After the
37th day the inhibition in ChE activity gradually declined to 20%. No
clinical signs were observed (Horvath et al., 1968),
8.2.4 Studies on ChE activity
Groups of 10 young female Sherman rats were fed for 90 days on
diets containing 0, 5, 20, 50, 200, 500, or 1000 mg dichlorvos (90%)/kg
diet (equivalent to 0, 0.4, 1.5, 3.5, 14.2, 35.7, and 69.9 mg/kg body
weight, respectively). Data on the stability of dichlorvos in the diet
were not reported. No clinical signs of intoxication were noted.
Blood samples were taken from two rats of each group for ChE activity
determination on day 3, 11, 60, and 90. In all test groups, a
decrease in plasma ChE activity was observed during the first 4 days,
gradually returning to normal, except in the rats receiving 14.2 mg/kg
body weight or more. At doses above 3.5 mg/kg body weight, erythrocyte
ChE activities were decreased throughout the test, but at 3.5 mg/kg
body weight, this was only so for the first 30 days of the study.
Lower dose levels produced comparable results to those of the control
group (Durham et al., 1957).
Thirty-two Rhesus monkeys were treated with 20% pelleted PVC-resin
formulations of dichlorvos at dosages ranging from 5 to 80 mg/kg of
formulation (equivalent to 1 - 16 mg dichlorvos/kg body weight) daily
or 8 and 20 mg/kg of formulation twice daily for 10 - 21 consecutive
days. None of the monkeys developed overt signs of intoxication,
though they ate less food and had soft faeces. Plasma and erythrocyte
ChE activities were reduced by approximately 80% in all animals, and
remained inhibited until completion of treatment. Plasma ChE
activities returned to normal within approximately 3 weeks, whereas the
erythrocyte ChE activities required 50 - 60 days to return to pre-
treatment values (Hass et al., 1972).
When groups of five male and five female guinea-pigs (P strain)
were given daily applications of 0, 25, 50, or 100 mg dichlorvos
(94%)/kg body weight on the shorn skin for 8 days, all the animals
survived. A significant dose-dependent inhibition of both plasma and
erythrocyte ChE activities occurred in all test groups. Recovery of
plasma ChE activities was complete within one week of the last
exposure, and that of erythrocyte ChE activities was complete within
one week in the females and 2 weeks in the males (Brown & Roberts,
1966).
Three Cynomolgus monkeys were treated daily with dermal doses of
50, 75, and 100 mg/kg body weight technical dichlorvos in xylene for 5
days per week until the animals died (after 8, 10, and 4 doses,
respectively). Symptoms of intoxication appeared in all monkeys,
beginning 10 - 20 min after administration of the first dose. The
erythrocyte ChE activity decreased rapidly and remained severely
inhibited for the period of the study, while plasma ChE activities
fluctuated throughout the study (Durham et al., 1957).
When male and female mice, rats, and guinea-pigs were exposed
continuously by inhalation for 5 consecutive days to actual
concentrations of 0, 0.14 - 0.15, or 1.1 - 1.3 mg/m3 dichlorvos, no
overt signs of intoxication were observed. In the high exposure
groups, plasma, erythrocyte, and brain ChE activities were inhibited in
all three species. Plasma ChE was the most sensitive in all three
species, with up to 70% inhibition in the female mice. The greatest
inhibition of brain ChE activity (30%) was found in mice (Brown et al.,
1968).
The effects of dichlorvos vapour inhalation on AChE activity was
investigated in the rat. Exposure to dichlorvos concentrations of 0.8
and 1.8 mg/m3 for 3 days reduced AChE activity in the bronchial tissue
(50 - 60% of control) but did not produce any changes in blood AChE
activity. However, at 4.3 mg/m3, blood AChE activity also declined
(38% of control). In histochemical preparations, staining of the
bronchial glands and smooth muscles revealed reduced enzyme activity
even at the lowest dose (0.2 mg/m3) tested. At this concentration,
no inhibition of bronchial homogenate ChE was observed (Schmidt et al.,
1975, 1979). The significance of bronchial ChE inhibition is not
clear.
Male mice were continuously exposed to actual concentrations of 0
or 0.03 mg dichlorvos/m3 for 28 consecutive days. Weekly assays of
ChE activities showed that brain ChE activity was slightly inhibited
(less than 20%) on the 28th day only, but plasma and erythrocyte ChE
activities were not significantly inhibited (Brown et al., 1968).
Rabbits exposed to an average concentration of 1 mg
dichlorvos/m3 (0.8 - 1.3 mg/m3) for 4 h per day for 4 months showed
up to 30% inhibition of serum and erythrocyte ChE activities during the
study. However, cats similarly exposed did not show significant
inhibition (Sasinovich, 1968).
Rats and monkeys were exposed for 2 weeks in an inhalation chamber
sprayed once with an emulsion of dichlorvos. The initial air
concentration was 6 mg dichlorvos/m3, decreasing over a few days to
about 0.1 - 0.2 mg/m3. No signs of intoxication were seen. The
plasma and erythrocyte ChE activities of the monkeys decreased to
approximately 50% of pre-exposure levels, but rapid recovery took place
after cessation of exposure. Comparable results were obtained when
rats and monkeys were continuously exposed for up to 7 days to a
maximum air concentration of 2.2 mg/m3. However, continuous exposure
for 4 days to 0.27 mg/m3 did not produce any effect on ChE activity
(Durham et al., 1957).
Groups of two monkeys were exposed to dichlorvos vapour 2 h per day
for 4 consecutive days. With concentrations up to 0.7 mg/m3, no
change in plasma or erythrocyte ChE activity was noted, while 1.2 -
3.3 mg/m3 caused a slight decrease in plasma ChE activity, and 7.5 -
18 mg/m3 (mean: 13 mg/m3) resulted in miosis and a pronounced
inhibition (40 - 70%) of plasma and erythrocyte ChE activities (Witter
et al., 1961).
Groups of 10 chickens were exposed to dichlorvos vapour from a
varying number of strips (20% dichlorvos) continually or intermittently
for 3 weeks. Exposure to a single strip in a room of 33 m3 (either
interrupted or for 16 h every day) produced no significant effect on
plasma or brain ChE activity. Exposure to more than one strip (2 - 5)
resulted in up to 50% inhibition of plasma ChE activity and
approximately 40% inhibition of brain ChE activity. Dichlorvos aerosol
sprays (0.7% dichlorvos) showed that daily excessive spraying for 6
seconds, 8 times per day, for 5 days, did not produce significant ChE
inhibition. However, a significant decrease in plasma ChE activity was
found when the study lasted for 21 days (Rauws & van Logten, 1973).
Three studies were carried out to determine the effect on the
laying performance of hens given dichlorvos in the feed for 4 weeks.
Plasma AChE levels were reduced by 70% at 20, 30, or 40 mg
dichlorvos/kg diet, although there was no clear influence on food
consumption, egg production, egg weight, or hatchability at these
doses. At 80 mg/kg diet, a decrease in food consumption and in egg
production was seen, the latter being a consequence, possibly, of the
former (Pym et al., 1984).
8.3 Skin and Eye Irritation; Sensitization
In the skin sensitization assay procedure of Stevens (flank/flank
technique), 1% dichlorvos in olive oil produced no visible effects in
male albino guinea-pigs. Negative results were also obtained when five
components of formulated dichlorvos/ PVC products were assayed for
their skin sensitization potential (Kodama, 1968).
A primary skin irritation test on dichlorvos was performed by using
male New Zealand white rabbits. Irritation observed on the skin after
the application of 5 - 20% water solutions of dichlorvos was relatively
severe compared with that caused by other organophosphorus insecticides
(Arimatsu et al., 1977).
In order to study the allergenicity of dichlorvos, the guinea-pig
maximization test was used. Threshold limit values of primary
irritancy tested on the skin of guinea-pigs (Hartley strain) were 2% or
more. In the maximization test, 0.05 and 0.5% were used. With 0.5%,
35% of the animals showed slight or discrete erythema. The
allergenicity rating as determined by the Kligman test was moderate. In
combination with methidathion, dichlorvos showed a stronger reaction,
indicating cross-sensitization (Fujita, 1985).
8.4 Long-term Exposure
8.4.1 Oral
8.4.1.1 Rat
In studies by Witherup et al. (1967, 1971), groups of 40 male and
40 female weanling CD rats were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg
diet for 2 years. Five males and five females from each group were
sacrificed after 6, 12, and 18 months, and analysis of diet samples
showed a considerable loss of dichlorvos. This was associated with a
gradual increase in DCA concentration which ranged in the different
groups from 0.014 to 28.6 mg/kg diet. The average actual
concentrations of dichlorvos in each diet were 0, 0.047, 0.467, 4.67,
46.7, and 234 mg/kg diet (equivalent to approximately 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). There were no signs of
intoxication, and no effects were seen on behaviour, mortality rate,
food consumption, weight gain, organ weights, haematology, or
urinalysis. Plasma and erythrocyte ChE activities were decreased
throughout the study in the two highest dose groups compared with
controls, but brain ChE activity was decreased in the highest dose
group only. Histological examination of major organs revealed
hepatocellular fatty vacuolization in the group with 234 mg/kg and in
some of the animals with 46.7 mg/kg diet. No effect was seen on serum
total proteins or albumin:globulin ratio, or on hexobarbital sleeping
time. It can be concluded that the actual average concentration of
4.7 mg/kg diet (equivalent to approximately 0.25 mg/kg body weight) was
without significant effect on any of the measured parameters. The
tumour incidence was comparable with that of the control group.
8.4.1.2 Dog
Groups of three male and three female beagle dogs were fed diets
containing 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg diet for 2
years, the average actual concentrations being 0, 0.09, 0.32, 3.2, 32,
and 256 mg dichlorvos/kg diet (equivalent to 0, 0.002, 0.008, 0.08,
0.8, and 6.4 mg/kg body weight). The average DCA concentration in the
diets with 10, 100, and 500 mg dichlorvos/kg diet was 0.6, 6.4, and 20
mg/kg diet. No effects were seen on general appearance, survival, food
consumption, weight gain, haematology, or urinalysis. However,
erythrocyte ChE was inhibited at dose levels of 3.2 mg/kg diet or more,
and plasma ChE activity was inhibited at the two highest dose levels.
Recovery to control values took place at the end of the feeding period.
Brain ChE activity, measured at the end of the study, was similar to
that of the controls, and liver weights were increased in both sexes in
the 256 mg/kg diet group. Histological examination of major organs
revealed slight dose-related alterations in the hepatic cells of one
female in the 3.2 mg/kg diet group, and greater alterations at higher
doses. No differences were seen in serum alkaline phosphatase, trans-
aminase activities, total serum proteins, or albumin:globulin ratios.
The actual average concentration of 0.32 mg/kg diet (equivalent to
0.008 mg/kg body weight) was without effect (Jolley et al., 1967;
Witherup et al., 1971).
8.4.2 Inhalation
8.4.2.1 Rat
When groups of 50 male and 50 female weanling CFE rats were
exposed for 23 h per day to air concentrations of 0, 0.05, 0.5, or
5 mg dichlorvos (97%)/m3 air (actual concentrations: 0, 0.05, 0.48,
and 4.7 mg/m3) for 2 years, body weight gain was reduced in the
two highest dose groups. After 2 years of exposure, plasma and
erythrocyte ChE activities were significantly reduced in the two
highest dose groups, but in the case of brain ChE activity, only in the
highest group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry values,
or organ weights, or upon gross or microscopic examination.
Ultrastructural examinations of bronchi and alveoli of rats exposed to
0 or 5 mg/m3 showed no differences between the two groups.
In connection with a reported correlation between brain ACh
concentration and the inhibition of brain ChE activity following acute
exposure to organophosphorus compounds, the brain tissue of three
female rats per group was examined for ACh and choline concentrations.
The dose level of 0.05 mg dichlorvos/m3 was without effect on any of
the measured parameters (Blair et al., 1976). It should be noted that
in this study the rats were not only exposed by inhalation but also via
their food, drinking-water, and by grooming. This resulted in
additional oral ingestion of dichlorvos (Stevenson & Blair, 1977).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
8.5.1 Reproduction
In a 3-generation reproduction study, weanling CD rats (six groups
of 30 animals) were fed dichlorvos (93%) at nominal concentrations of
0, 0.1, 1, 10, 100, or 500 mg/kg diet, prepared freshly each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1.1), it was
assumed that the average concentration of dichlorvos was approximately
47% of the nominal concentrations (equivalent to 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). No effects on fertility,
number and size of litters, body weight, or viability of the pups were
found. Gross and histopathological examination of 7-day-old pups from
F1a and F2a litters did not reveal any abnormalities (Witherup et
al., 1965, 1971).
Oral treatment of rats with 5.6 mg/kg body weight and rabbits with
6 mg/kg body weight during the last trimester of pregnancy had no
effect on offspring weight and development. However, the cerebral
cortices from the 1-day-old rabbits were less mature than those of
control rabbits, probably due to maternal toxicity (Dambska et al.,
1978, 1979).
Dambska & Maslinska (1982) observed impairment of the development
of the brain of rabbits dosed orally with 9 mg/kg body weight per day
from days 5 to 16 of life, the period of myelination.
8.5.1.1 Effects on testes
In studies by Krause & Homolo (1972, 1974), three groups of 14 male
NMRI/Han mice received either a single oral dose of 40 mg dichlorvos/kg
body weight, daily oral doses of 10 mg dichlorvos (in olive oil)/kg for
18 consecutive days, or daily oral doses of 0.5 ml olive oil for 18
days, respectively. On days 9, 18, 27, 36, 54, and 63, two animals
from each group were killed and their testes examined histologically.
Severe disturbances of spermatogenesis were observed in both test
groups; damaged seminiferous tubules were also seen and the supporting
Sertoli cells were damaged. In addition, there was an increase in the
number and hypertrophy of the Leydig cells. No explanation could be
offered for these effects.
A similar study was carried out on three groups of 16 male juvenile
Wistar rats. The rats received either 20 mg dichlorvos (in olive
oil)/kg body weight on days 4 and 5, 10 mg dichlorvos (in olive oil)/kg
daily from days 4 to 23, or 0.1 ml olive oil daily from days 4 to 23 of
life. On days 6, 12, 18, 26, 34, and 50 of life, two rats from each
group were sacrificed. Histological examination of the testes showed
slight reduction in the number of the spermatogenic cells and Leydig
cells. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells. All the changes were
reversed by the 50th day (Krause et al., 1976; Xing-Shu, 1983).
In order to examine the cause of the observed effect on the
spermatogenic and Leydig cells, the study was repeated with, in
addition, measurement of testosterone levels in serum and testes. The
testosterone concentrations in the testes, and leutinizing hormone (LH)
and follicle stimulating hormone (FSH) levels in serum were similar in
the presence or absence of dichlorvos (Krause, 1977). However, the use
of adult rats and a different dosing regimen prevented a strict
comparison with the earlier study by Krause et al. (1976).
In studies by Fujita et al. (1977), 55 male Wistar rats (aged 5
months) were orally administered dichlorvos at levels of 5 or 10 mg/kg
body weight every other day for 8 weeks. The rats were divided into
five groups, and one group of rats was killed every 4 weeks to study
the changes in several organs, including the testes. About 200
individual seminiferous tubules were examined in each rat. No change
was seen in body weight gain or testes weight. The score values of the
seminal cellular system decreased after 4 - 8 weeks of treatment, but
were restored 8 weeks after the end of treatment.
8.5.1.2 Effect on estrous cycle
Timmons et al. (1975) reported studies on female rats which were
exposed to an atmosphere containing 2.4 mg dichlorvos/m3 generated
from a dichlorvos strip placed on top of each cage. Exposure was
continuous from the birth of the first litter until the estrous cycle
began again. Controls were housed in a separate room. A significant
mean delay of 10 days in the onset of the estrous cycle, compared with
that of controls, was observed. However, the significance of the
results was complicated by different housing conditions.
8.5.1.3 Domestic animals
In studies by Bazer et al. (1969), sows were fed dichlorvos (9% in
resin pellets) at the level of 800 mg per animal per day beginning 21
days before breeding and continuing through gestation. No significant
differences in the numbers born alive or dead, the litter weight,
number weaned, or individual weaning weights were observed.
In a further study, dichlorvos was added to the rations of pregnant
sows at the level of 0 or 800 mg per animal per day. Resin pellets
containing 9% dichlorvos were fed either from 3 weeks before breeding
and throughout gestation, or from 18 to 56 days before parturition.
Data were collected from a total of 681 dams, representing eight
replicates over a period of 2 years. The dichlorvos-treated groups
scored consistently higher for individual farrowing weights, litter
farrowing weights, number weaned, individual weaning weights, and
litter weaning weights, and less consistently, for the percentage of
live births (Batte et al., 1969).
When dichlorvos (as a PVC-resin formulation) was administered to
sows in doses ranging from 4 to 13 mg/kg body weight per day for the
last 21 - 30 days of gestation, the average farrowing interval for the
live-born piglets was shorter in treated than in control animals.
There was also a dose-related increase in the mean birth weight of
live-born piglets from the dichlorvos-treated sows, while the incidence
of still births was lower than in controls (Bunding et al., 1972).
In studies by Collins et al. (1971), male and female swine were fed
for up to 3 years on diets containing a PVC-resin formulation at 0,
200, 250, 288, 400, or 500 mg dichlorvos/kg diet, and for at least 6
months prior to initial breeding. Two generations were raised, and no
effects were observed on number or size of litters, survival or growth
rate of offspring, urinalysis, haematology, hepatic and renal function,
physical structure, or calcium and phosphorus content of the femoral
bone, or in appearance during gross and microscopic examination. Organ
weights were normal except in the case of the liver, which was
generally increased. Whole blood ChE activity was inhibited and brain
ChE activity was slightly reduced, but no clinical evidence of
neurophysiological impairment was observed.
In studies reported by Stanton et al. (1979), pregnant sows were
given PVC-resin formulations of dichlorvos (10%) in the diet at a daily
dose of 0, 5, or 25 mg dichlorvos/kg body weight (divided between two
doses per day) during the last one-third of pregnancy (or 30 days).
Only about 50% of the total dichlorvos was released from this resin in
the gastrointestinal tract. All pigs were born alive, and their birth
weight was similar to that of control animals. In the group given
25 mg/kg body weight, plasma and erythrocyte ChE activities were
markedly inhibited (80 and 90%, respectively) in the sows, but not in
the newborn pigs. No changes in the packed cell volume or haemoglobin
concentration of the sows or their piglets were observed.
In a limited test reported by Darrow (1973), dichlorvos-impregnated
collars did not have any adverse effects on pregnant female goats or
later on their newborn young. Also, blood ChE activity was not
inhibited in either the nannies or the kids over a period of several
weeks. The acceptance of treated kids by the mothers was normal.
A pregnant non-lactating Holstein-Friesian cow was fed a nominal
concentration of 6.2 mg dichlorvos/kg body weight per day in the daily
ration from days 152 to 286 of pregnancy. A normal calf was delivered
(Macklin & Ribelin, 1971).
A herd of 54 dairy cows (two-thirds carrying calves) was accidently
sprayed with dichlorvos, resulting in a dosage of 50 mg dichlorvos/kg
body weight. All the animals showed symptoms of intoxication and some
had convulsions. However, they all recovered within a few hours, with
no abortions or other adverse effects except a temporary diminution in
milk production (Knapp & Graden, 1964).
When three pregnant sows were fed 8.5 mg dichlorvos/kg body weight
per day (as PVC-resin formulation) from days 41 to 70 of pregnancy, the
blood ChE activity of the sows was markedly inhibited, but no
demonstrable teratogenic effects or functional abnormalities in the
piglets were found (Wrathall et al., 1980).
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
In studies by Schwetz et al. (1979), CF1 mice and New Zealand
rabbits were given maximum tolerated doses of dichlorvos (96%) in corn
oil by gavage, at levels of 60 and 5 mg/kg body weight, respectively,
from days 6 to 15 and days 6 to 18 of gestation, respectively. Except
for an increased number of resorptions in rabbits, no significant
effect was observed on the mean number of live fetuses per litter, or
on fetal body measurements. There were no gross visceral or skeletal
alterations. In mice, no abnormalities were found.
Carson (1969) reported studies on a total of 168 New Zealand
rabbits, divided into 10 groups containing 15 - 26 animals. One group
received lactose capsules, three groups different PVC-placebo capsules,
and three groups PVC capsules containing 18, 54, or 93 mg
dichlorvos/animal. These capsules were provided twice daily, so that
the equivalent daily intake was 12, 36, or 62 mg/kg body weight,
respectively. The rabbits received 12 or 36 mg dichlorvos/kg body
weight on days 6 - 18 of gestation, or 62 mg/kg body weight on days 6 -
11 of gestation. Fetuses were obtained by Caesarian section. Maternal
mortality was increased in the highest dichlorvos group, and the
incidences of in utero and neonatal toxicity were also increased (but
not significantly) in the 62 mg group, compared with those in the
control groups. The fetal mortality was not clearly indicative of any
marked toxic effects other than those involving the dam, since whole
litters were not involved. Extensive skeletal and soft tissue
examinations were carried out on all viable neonates, but no adverse
effects on bone formation, articulation, or degree of ossification were
found in the dichlorvos groups. No teratogenic effects were observed.
When pregnant rabbits were given oral doses of PVC-resin
formulations during the critical days of organogenesis, doses of 34 mg
dichlorvos/kg body weight or more were found to cause maternal
toxicity. With doses of 12 mg/kg body weight or less, no significant
effect on nidation, in utero survival, or neonatal survival was found.
No evidence of teratogenic changes was observed on gross, visceral, or
skeletal examination of the fetuses (Vogin et al., 1971).
The effect of dichlorvos on embryonal and fetal development in
thyroparathyroidectomized, thyroxine-treated, and euthyroid control
rats has been investigated. On days 8 - 15 of gestation, 25 mg
dichlorvos/kg body weight per day was administered orally to pregnant
Charles River rats, and a slight decrease in fetal weight in all groups
was observed. No gross, visceral, or skeletal anomalies of the fetuses
were found as a result of dichlorvos administration to rats with
altered thryoid status (Baksi, 1978).
8.5.2.2 Inhalation
In studies by Thorpe et al. (1972), rats of E strain and Dutch
rabbits were exposed (23 h per day, 7 days per week) throughout
pregnancy to nominal concentrations of 0, 0.25, 1.25, or 6.25 mg
dichlorvos/m3. In an additional study, groups of 20 pregnant
rabbits were exposed to nominal concentrations of 2 or 4 mg
dichlorvos/m3. Maternal deaths occurred in the rabbits exposed to
2 mg/m3 or more, and ChE activities in plasma, erythrocytes, and brain
were markedly inhibited in both species exposed to 1.25 mg/m3 or
more. There was no indication of any dichlorvos-related teratogenic
effects.
When CF1 mice and New Zealand rabbits were exposed to dichlorvos at
an average actual concentration of 4 mg/m3 for 7 h per day from days
6 to 15 and from days 6 to 18 of gestation, respectively, no
significant effect on the mean number of live fetuses per litter, the
incidence or distribution of resorptions, or on fetal body measurements
was noted. No gross, visceral, or skeletal alterations were observed
(Schwetz et al., 1979).
8.5.2.3 Intraperitoneal
Kimbrough & Gaines (1968) reported a study on female Sherman rats
given a single intraperitoneal injection of 0 or 15 mg dichlorvos (in
peanut oil)/kg body weight on day 11 of pregnancy. The treated dams
showed toxic signs and weight loss. On day 20, the fetuses were
removed. There was no adverse effect on litter size, resorptions,
number of dead fetuses per litter, or average weight of fetuses, but 3
out of 41 fetuses had omphaloceles. This latter finding, observed at a
maternally toxic dose, is not in agreement with the other teratology
studies or the 3-generation reproduction study.
8.5.3 Résumé of reproduction, embryotoxicity, and teratogenicity studies
A 3-generation study on rats, fed dichlorvos at dose levels of up
to 500 mg/kg diet, did not show any effects on fertility, number and
size of litters, body weight, or viability of the pups.
In studies on mice and rats, high dose levels of dichlorvos (a
single dose of 40 mg/kg body weight or multiple doses of 5 or 10 mg/kg
body weight) induced disturbances in spermatogenesis, characterized by
damage to the seminiferous tubules and Sertoli cells and by hypertrophy
and increase in the number of Leydig cells. It was assumed, but not
confirmed, that testosterone synthesis was partially inhibited. After
dichlorvos treatment ceased, recovery was complete within about 2
months.
A number of reproduction studies on domestic animals, mainly sows,
have been carried out. Levels of 500 mg/kg diet for 3 years had no
effect on fertility. Inhibition of ChE activity in the sows, but not
in the newborn pigs, was induced by 25 mg/kg body weight. No
teratogenic effects were seen.
Teratogenicity studies on rats and rabbits, orally administered
62 mg/kg body weight during gestation, revealed symptoms of
intoxication and significant inhibition of ChE in the parent rabbits.
Except for an increased number of resorptions, no significant effect on
the mean number of live fetuses per litter or evidence of a teratogenic
effect was noted during gross, visceral, or skeletal examination.
There were no teratogenic effects after rats inhaled 6.25 mg
dichlorvos/m3 during gestation, though maternal deaths occurred. Mice
and rabbits exposed to 4 mg/m3 on days 6 - 18 of gestation did not
show any effects.
8.6 Mutagenicity and Related End-Points
8.6.1 Methylating reactivity
In a quantitative colour test for alkylating agents, dichlorvos
gave a positive response, whereas the metabolites desmethyldichlorvos,
dimethylphosphate, dichloroethanol, DCA, and dichloroacetic acid all
gave negative results (Bedford & Robinson, 1972).
8.6.1.1 In vitro studies
Lawley et al. (1974) have shown that methylation by dichlorvos of
DNA and RNA, using either isolated nucleic acids, Escherichia
coli cells, or human tumour HeLa cells, broadly resembled that by
methylmethanesulfonate (MMS) rather than methylation by
N-methyl- N-nitrosourea. In E. coli cells, 3-methyladenine, the
principal minor product in methylated DNA apart from 7-methylguanine,
was not detected, whereas it was present when pre-isolated DNA was
methylated. The overall extent of methylation achieved in cells was
small. Specific excision of 3-methyladenine was indicated in E.
coli cells (Lawley et al., 1974).
Labelled 7-methylguanine was present in both DNA and RNA isolated
from E. coli exposed to 3H-dichlorvos. The methylating capability of
dichlorvos was less, by a factor of 10 - 100, than that of strongly
genotoxic methylating compounds (Wennerberg & Löfroth, 1974).
Alkylation by dichlorvos of calf thymus DNA, resulting in the formation
of N-7-methylguanine, was reported by Löfroth (1970).
Shooter (1975) has applied the test for chain breaks in RNA to the
interaction of dichlorvos with bacteriophage R17. Breaks in the RNA
chain result from the hydrolysis of phosphotriesters and are thus a
measure of the extent of O-alkylation and of the SN1-type mechanism
of the reaction. The results so far suggest that the existence of O-
alkylation, as shown by degradation following phosphotriester
formation, does correlate with mutagenicity. Incubation of
bacteriophage R17 with 0 - 100 mmol dichlorvos/litre for 90 h did not
result in methylation of the phosphate group of the RNA to any
significant extent.
8.6.1.2 In vivo studies
Reviews of the literature on alkylating agents including dichlorvos
have been made by Bedford & Robinson (1972), Lohs et al. (1976), and
Hemminki (1983).
In studies in which mice were given intraperitoneal injections
of methyl-14C-dichlorvos (1.9 µmol/kg body weight), the degree
of alkylation of guanine- N-7 in DNA isolated from soft tissues
amounted to 8 x 10-13 mol methyl/g DNA. From this, a rate of
clearance of approximately 1.4 min was estimated (Segerbäck, 1981;
Segerbäck & Ehrenberg, 1981). DNA and RNA from the total soft tissues
of male CFE rats exposed to atmospheres containing 0.064 mg methyl-
14C-dichlorvos/m3 for 12 h did not show methylation of the N-7 atom
of guanine moieties. The exposure period constituted a significant
fraction of the half-life of the 7-methylguanine moieties in DNA. It
was concluded that dichlorvos does not pose a methylating hazard to
mammalian DNA in vivo (Wooder et al., 1977; Wooder & Wright, 1981).
The excretion of labelled 7-methyl guanine in the urine
by NMRI mice and R rats injected intraperitoneally with methyl-
14C-dichlorvos, or exposed by inhalation for 2 h (mice only), was
reported by Löfroth & Wennerberg (1974) and Wennerberg & Löfroth
(1974). In rat urine, labelled 3-methyladenine and 1-methyl-
nicotinamide were also detected (Löfroth & Wennerberg, 1974).
According to the authors, these results demonstrate the dichlorvos-
induced methylation of guanine and adenine moieties in nucleic acids.
However, the administration of radiolabelled adenine and guanine to
otherwise untreated rats gave rise to the excretion of radiolabelled
methylated purines in the urine. Therefore, the detection of
radiolabelled purines, per se, in the urine of animals exposed to
methyl-labelled methylating agents, does not constitute evidence for
the methylation of the purine moieties of nucleosides or nucleic acids
by methylating agents (Wooder et al., 1978). Moreover, the results of
metabolic studies have demonstrated the existence of a natural
biosynthetic pathway whereby the methyl carbon atoms of dichlorvos can,
with partial retention of hydrogen, become incorporated into the
heterocyclic rings and the methyl groups of urinary 7-methylguanine
after entering 1-C pools, in vivo. The results of preliminary studies
suggest the existence of a similar pathway for the production of
urinary 3-methyladenine (Wright et al., 1979).
Wooder & Creedy (1979) described a study on rats which investigated
the DNA-damaging potential of dichlorvos when administered as a single
intraperitoneal dose. Alkaline sucrose gradient profiles of rat liver
DNA showed that whereas MMS (a positive control) shifted the DNA
profile, dichlorvos at 10 mg/kg body weight (the maximum dose
consistent with survival) had no effect.
8.6.1.3 Discussion of methylating reactivity
Many alkylation tests and in vivo and in vitro mutagenicity studies
have been carried out with dichlorvos. It has been demonstrated that
dichlorvos has alkylating properties, and has been suggested from some
studies that the in vivo alkylating potential of dichlorvos is similar
to that of some known mutagens. However, this concern is misplaced
since alternative reactions were not considered (WHO, 1986b). The
phosphorus atom is markedly more electron-deficient and susceptible to
attack by nucleophiles than the alkyl carbon atom. Analysis by Bedford
& Robinson (1972) of the data of Löfroth et al. (1969) revealed that
the proposed rates of alkylation by potent nucleophiles were probably
combined rates of phosphorylation and alkylation, and that
phosphorylation was the totally dominant reaction in the case of the
hydroxide ion. The comparison with known mutagens is therefore
inappropriate. Two factors detract further from the toxicological
significance of the alkylation studies. The first is that mammalian
tissues (plasma, liver, etc.) contain active A-esterase enzymes, which
catalyse the phosphorylation of water by the organophosphorus esters.
Viewed inversely, these A-esterases catalyse the hydrolysis of the
organophosphorus esters, thereby rapidly reducing circulating levels of
hazardous material. Secondly, the comparative rate of reaction of most
of these esters with AChE is many orders of magnitude greater than the
rate of alkylation by the typical nucleophile 4-nitrobenzyl-pyridine:
for dichlorvos, the ratio of rates was 1 x 107 in favour of the
inhibitory phosphorylation of AChE (Aldridge & Johnson, 1977). It
follows that, at low exposure levels, in vivo phosphorylation of AChE
and other esterases will be the dominant reaction, with negligible
uncatalysed alkylation of nucleic acid. Indeed, no such alkylation has
been detected in sensitive in vivo studies designed to check this point
(Wooder et al., 1977).
8.6.2. Mutagenicity
Reviews of the existing literature have been published by Wild
(1975), Fishbein (1976, 1981, 1982), Leonard (1976), IARC (1979),
Sternberg (1979), Ramel et al. (1980), Lafontaine et al. (1981), Ramel
(1981), and Wildemauwe et al. (1983).
8.6.2.1 In vitro studies
(a) Microorganisms
Numerous mutagenicity studies using bacteria and fungi as test
organisms have been carried out (Table 17). In most of the studies,
only one dichlorvos concentration, often a high one, was tested,
sometimes resulting in low survival of the test organism. A
dose-response relationship was established in the few tests where a
range of concentrations was used. The results indicate that dichlorvos
induces base substitutions in bacteria and mitotic gene conversion in
yeast. The alkylating properties of dichlorvos (section 8.6.1) are
most probably the cause of the mutagenic action. This was the
conclusion of Bridges et al. (1973) from tests using both MMS and
dichlorvos with E. coli strains deficient at four different repair
loci.
In Aspergillus nidulans, dichlorvos has been found to induce point
mutations to 8-azaguanine resistance and a high frequency of mitotic
crossing-over and non-disjunction (Aulicino et al., 1976; Bignami et
al., 1976, 1977; Morpurgo et al., 1979). No mutagenic activity
using A. nidulans was found after incubating Nicotiana alata cell
cultures with dichlorvos for 21 days (simulating in vitro plant
metabolism). This confirms the rapid metabolism of dichlorvos (Benigni
et al., 1979).
The mutagenicity of dichlorvos has been extensively studied in
Japan, (Kawachi et al., 1980). Dichlorvos induced gene mutation
in Salmonella typhimurium TA100 as well as E. coli strains in the
absence of rat-liver S9 mix.
Dichlorvos (0.5 - 2 mg/ml) causes random strand breakage,
repairable by DNA polymerase I, in E. coli pol A as detected by the
alkaline sucrose sedimentation method. When pol+ bacteria and high
concentrations of dichlorvos (0.2 - 0.4%) were used, an all-or-none
breakdown of DNA molecules to fragments of very low relative molecular
mass occurred which correlated well with lethality. It has been
suggested that the major DNA damage resulting from dichlorvos treatment
arises indirectly through alkylation of other cellular constituents,
this leading to uncontrolled nuclease attack on DNA. However,
discontinuities in newly-synthesized DNA and mutagenesis following
dichlorvos treatment presumably result from direct alkylation of DNA
(Bridges et al., 1973; Green et al., 1973, 1974a,b). In the
standard E. coli DNA polymerase-deficient assay system, dichlorvos
(6.4 mmol/litre) gave a positive result (Rosenkranz, 1973; Rosenkranz &
Leifer, 1980). In measuring mutation to tryphophan independence in E.
coli strain WP2, it was found that 5 mg dichlorvos/litre was mutagenic
in this test system (Green et al., 1976). Griffin & Hall (1978) have
found that dichlorvos (1 mg/ml) causes breaks in colicinogenic plasmid
E1 DNA from E. coli. In a rec-type repair test with Proteus
mirabilis strains PG 713 (rec-hcr-) and PG 273 (wild type),
dichlorvos (10 or 40 µmol per plate) induced base-pair substitutions
and other DNA damage. In the same test, desmethyldichlorvos, at the
same concentrations, gave negative results (Adler et al., 1976; Braun
et al., 1982).
(b) Mammalian cells
In cultured V79 Chinese hamster cells, no induction of 8-
azaguanine-resistant mutations after treatment with up to 1 mmol/litre
dichlorvos (Wild, 1975), or of ouabain-resistant mutations after
treatment with 1.25 - 5 mmol/litre dichlorvos, was found (Aquilina et
al., 1984).
DNA strand breakage in cultured V79 Chinese hamster cells caused by
up to 0.2% v/v dichlorvos has been reported by Green et al. (1974a).
Dichlorvos (1 µl) decreased the sedimentation coefficient of calf
thymus DNA upon thermal denaturation, indicating a decrease in the DNA
molecular size (Rosenkranz & Rosenkranz, 1972). Incubation of
dichlorvos (45 mmol/litre) with calf thymus DNA did not result in
changes in thermal melting curves or DNA fractionation on a
hydroxyapatite column. However, changes were observed by means of
differential pulse polarography, indicating that single-stranded
segments and thermolabile regions were formed in the DNA. This
behaviour could perhaps be a consequence of guanine alkylation followed
by depurination and chain cutting at elevated temperatures (Olinski et
al., 1980).
The resistance of methylated DNA in Chinese hamster ovary cells,
labelled with 14C-thymidine and methyl-3H-1-methionine, to
micrococcal nuclease digestion was abolished when the cells were
treated with dichlorvos (10 mmol/litre) for 3 h. No effects were
observed on kinetics of total DNA digestion. These results indicate a
conformational change in chromatin induced by dichlorvos (Nishio &
Uyeki, 1982).
Table 17. Mutagenicity tests on microorganisms
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Bacillus subtilis
H17 rec+ 0.02 ml of plate none - Shirasu et al.
M45 rec- 10% solution plate none + (1976)
Citrobacter freundii
425 0.1% fluctuation none weak + Voogd et al. (1972)
0.05% none -
Enterobacter aerogenes
6 0.1% fluctuation none weak + Voogd et al. (1972)
Escherichia coli
B 5 - 25 mmol/litre liquid induc- none weak + Wild (1973)
1- to 10-h tion strepto- (dose-
exposure mycin-resistant and exposure-
mutants time related)
B/r WP2 22.6 mmol/litre plate reversion none + Moriya et al.
S9 mix + (1978)
L-cysteine +
CM 561, 571, 611 5 µl plate reversion none - Hanna & Dyer
(1975)
K12HfrH 0.1% fluctuation none weak + Voogd et al.
(1972)
K12(5-MT) 3.3 x 10-4 plate none + Mohn (1973)
mol/litre
WP2 micro drop plate none - Dean (1972a)
analytical and
technical grade,
aqueous solution
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Escherichia coli (contd).
WP2 try- approximately plate none + Ashwood-Smith et
20 mm2 dichlor- al. (1972)
vos strip
WP2 try- 20 - 25 µl of spot none + Nagy et al. (1975)
hcr- and hcr+ 50% emulsifiable back mutation
concentrate
0.1 ml of a 5% plate reversion none + Shirasu et al.
solution (1976)
WP2 hcr unknown (up to plate reversion none + Moriya et al.
5000 µg/plate) (1983)
WP2 uvrA, ca 5 µl plate reversion none + Hanna & Dyer
WP 67 (1975)
Klebsiella pneumoniae 0.1%; 0.05% fluctuation none weak + Voogd et al.
(1972)
Neurospora crassa
ad-3 exposed to air plate none - Michalek &
containing Brockman (1969)
dichlorvos
(strip)
Pseudomonas aeruginosa
PAO 38 0.08 mol/litre liquid reversion none + Dyer & Hanna
(1973)
Saccharomyces cerevisiae
D4 (ade2 and 4 mg/ml plate mitotic none + Dean et al.
trp5) 2 mg/ml gene conversion (1972)
D4 (ade2 and 19 mmol liquid mitotic none + Fahrig (1973,
trp5) gene conversion 1974)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium
64-320 0.05%; 0.1% suspension none + Voogd et al. (1972)
TA 98 20 or 40 mmol plate S9 male mice - Braun et al. (1982)
up to 5000 µg plate reversion none - Moriya et al. (1983)
TA 100 20 or 40 mmol plate S9 male mice (+) Braun et al. (1982)
low survival
up to 5000 µg plate reversion none + Moriya et al. (1983)
TA 1530 5 µl plate none + Hanna & Dyer (1975)
TA 1535 5 µl plate none + Hanna & Dyer (1975)
0.1 ml of a 5% plate none + Shirasu et al. (1976)
solution
0.1 ml of a 5% plate none + Moriya et al. (1978)
solution S9 mix -
L-cysteine -
2800 µg plate (spot) S9 male rat - Carere et al.
(1976, 1978a,b)
1.5 mg/ml liquid none + Carere et al.
(1978a,b)
20 or 40 mmol plate S9 male mice - Braun et al. (1982)
TA 1536, 1537, 0.1 ml of a 5% plate none - Shirasu et al.
1538 solution (1976)
TA 1536, 1537, 2800 µg plate (spot) S9 male rat - Carere et al.
1538 (1976, 1978a,b)
20 or 40 µg plate S9 male mice - Braun et al.
(1982)
up to 5000 µg plate none - Moriya et al.
(1983)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium (contd).
his C117 0.03 mol liquid none + Dyer & Hanna (1973)
LT2 his C117, 5 µl plate none - Hanna & Dyer (1975)
his G 46
Schizosaccharomyces pombe
ade6 1.5 - 14 mmol plate + + Gilot-Delhalle et
al. (1983)
Serratia marcescens
Hy alpha 13, 25 mg/ml plate (spot) none alpha 13 + Dean (1972a)
alpha 21 alpha 21 -
50, 100 mg/ml plate (spot) none + Dean (1972a)
saturated plate (spot) none + Dean (1972a)
aqueous solution
Streptomyces coelicolor
A 3(2) his Al 5600 µg spot none + Carere et al.
(1976, 1978a,b)
---------------------------------------------------------------------------------------------------------
Dichlorvos (0.03 and 0.1 mmol/litre) has been found to induce
sister chromatid exchanges (SCEs) in cultures of Chinese hamster ovary
cells (Nishio & Uyeki, 1981). On the other hand, in Chinese hamster
V79 cells (clone number 15), SCEs were not induced by 0.1 mmol
dichlorvos/litre, but only by 0.2 and 0.5 mmol/litre solutions. The
number of polyploid cells was increased at both 0.1 and 5 mmol/litre
(Tezuka et al., 1980).
Dichlorvos has been tested in two independent laboratories for its
ability to increase the transformation of Syrian hamster embryo cells
by simian adenovirus SA7. Pre-treatment of hamster cells with
dichlorvos at concentrations of 0.05 up to 0.45 mmol/litre produced a
significant enhancement of SA7 transformation at 0.11 mmol/litre and
higher (Hatch et al., 1986).
The mouse peripheral blood lymphocyte (PB) culture system has also
been used to test for SCE induction. Male B6C3F1 mice were injected
intraperitoneally with 5, 15, 25, or 35 mg dichlorvos/kg body weight,
but there was no change in the baseline SCE frequency (Kligerman et
al., 1985).
Dichlorvos (6.5 - 650 mmol/litre) has been found to induce dose-
dependent unscheduled DNA synthesis in the human epithelial-like cell
line EUE (Benigni & Dogliotti, 1980a,b; Aquilina et al., 1984). Both
scheduled and unscheduled DNA synthesis of human lymphocytes showed
dose-related inhibition by dichlorvos (5 -500 mg/litre), as measured by
3H-thymidine uptake (Perocco & Fini, 1980).
Dichlorvos (0.0001 - 0.1 mmol/litre) does not induce DNA repair in
human kidney T-cells. This was shown by a lack of dichlorvos-induced
3H-thymidine incorporation into T-cells in the G1- or G2-phase of the
cell cycle. No induction of single-strand breaks in the T-cell DNA, as
measured by alkaline sucrose gradients, was found following treatment
for 1, 2, or 4 h with 0.0001 - 1 mmol dichlorvos/litre (Bootsma et al.,
1971).
No clear effect on the frequency of SCEs in human lymphocytes or
human fetal lung fibroblasts was found after exposure to 2.5 - 10 mg
dichlorvos/litre for 72 h (Nicholas et al., 1978).
In studies by Dean (1972b), human blood samples were treated with
dichlorvos (0.0001 - 1 mmol/litre) for 1, 2, 4, and 20 h, after which
the lymphocytes were stimulated to divide using phyto-haemagglutinin.
The toxic effect of 1 mmol dichlorvos/litre was indicated by a
decreased mitotic index. Mitotic cells were analysed for chromosome
aberrations, but the number of dicentrics in dichlorvos-treated cells
was no different from that found in untreated cells (Bootsma et al.,
1971). When dichlorvos (1 - 40 mg/litre) was added at specific
intervals to cultures of human lymphocytes, cytotoxicity was found at
5 mg/litre or more, but no chromosome aberrations (chromatid gaps or
breaks) were detected (Dean, 1972b).
Negative results in cultured human lymphocytes were also reported
by Fahrig (1974) and Wild (1975).
8.6.2.2 In vivo studies
(a) Drosophila melanogaster
Negative results were obtained in the standard Muller-5 test for
sex-linked lethal mutations and in the induced crossing-over test with
the approximate LD50 concentration (0.035% dichlorvos) (Jayasuriya &
Ratnayake, 1973). Similarly, 0.0006 - 0.6 µmol dichlorvos gave
negative results in the standard Muller-5 test (Sobels & Todd, 1979).
In studies by Hanna & Dyer (1975), a Drosophila melanogaster
population was continuously exposed to gradually increasing
concentrations of dichlorvos (because of development of increased
resistance) in the food medium (up to 0.75 mg/kg food) for about 18
months. At the end of this period, an increased accumulation of
mutations was observed.
Gupta & Singh (1974) reported studies where female D.
melanogaster flies were kept on food with 1 - 50 mg dichlorvos (Nuvan
100 EC)/kg. No eggs were laid at 10 mg/kg or more, and at 1 mg/kg,
survival of the eggs was 45% of that of the controls. Salivary gland
chromosome abnormalities were observed in fully-grown larvae fed
1 mg/kg. However, Kramers & Knaap (1978), using the same route of
administration, found no induction of sex-linked recessive lethals by
dichlorvos (0.009, 0.048, and 0.09 mg/kg food).
(b) Host-mediated assays
Dichlorvos was not mutagenic in host-mediated assays in NMRI mice
using:
(i) S. typhimurium (G46 his-) and Serratia marcescens (a 21
leu-) after an intraperitoneal injection of 25 mg
dichlorvos/kg body weight (Buselmaier et al., 1972, 1973);
(ii) S. typhimurium (64-320) after an oral dose of 0.2 mg per
animal (equivalent to 8 - 10 mg/kg body weight) (Voogd et
al., 1972); or
(iii) Saccharomyces cerevisae (D4 ade2 and trp5 loci) after an
oral dose of 50 or 100 mg/kg body weight (or after exposure
of CF1 mice for 5 h to 60 or 90 mg dichlorvos/m3) (Dean et
al., 1972).
Although dichlorvos was mutagenic to S. cerevisae in in
vitro studies, these in vivo studies proved to be negative.
(c) Dominant lethal assays
Negative results were reported in a test for dominant lethal
mutations in ICR/Ha mice (expressed as an increase in early fetal
deaths or, indirectly, by pre-implantation losses), after a single
intraperitoneal injection of 13 or 16.5 mg/kg body weight or five
consecutive daily oral doses of 5 or 10 mg/kg body weight. The total
mating period was 8 weeks (Epstein et al., 1972). The same result was
obtained after exposure of male CF1 mice to 30 or 55 mg/m3 for 16 h or
to 2.1 or 5.8 mg/m3 for 23 h daily for 4 weeks (Dean & Thorpe,
1972b).
A statistically significant increase in the frequency of pre-
implantation losses in mice (Q strain) in the second week and fifth
week of mating has been observed after a single intraperitoneal
injection of 10 mg/kg body weight (Degraeve et al., 1980; Moutschen-
Dahmen et al., 1981).
In studies by Degraeve et al. (1982, 1984a), male Q strain mice
received drinking-water with 2 mg dichlorvos/litre (equivalent to
0.32 mg/kg body weight per day) 5 days per week, for 7 consecutive
weeks. At the end of this period, the males were mated for 1 week with
untreated virgin females, and the pregnant females were killed 14 days
after the start of pregnancy. No dominant lethal mutations were
induced. The same result was obtained when female CF1 mice were either
given single oral doses of 0, 25, or 50 mg dichlorvos/kg body weight or
continuously exposed to atmospheres containing 0, 2, or 8 mg
dichlorvos/m3 from weaning until 11 weeks of age. They were either
mated at the end of the dosing or inhalation period or at intervals of
5, 10, and 15 days thereafter (Dean & Blair, 1976).
(d) Chromosome abnormalities
Male Q strain mice receiving drinking-water containing 2 mg
dichlorvos/litre (equivalent to 0.32 mg/kg body weight), 5 days per
week for 7 weeks, did not show chromosome damage in bone marrow cells,
spermatogonia, or primary spermatocytes (Moutschen-Dahmen et al., 1981;
Degraeve et al., 1982, 1984a); neither did mice of the same strain
given a single intraperitoneal injection with 10 mg/kg body weight
(Moutschen-Dahmen et al., 1981; Degraeve et al., 1984b).
In a micronucleus test, Swiss mice were given daily intra-
peritoneal injections of dichlorvos (0.0075 - 0.015 mg/kg body weight
per day) for 2 days and killed 6 h after the last dose. No induction
of aberrations in the structure or number of chromosomes in bone marrow
cells was observed (Paik & Lee, 1977).
CF1 mice exposed to concentrations of 64 - 72 mg
dichlorvos/m3 for 16 h or to 5 mg/m3 per day, 23 h per day, for 21
days, did not show chromosome abnormalities in bone marrow or
spermatocytes. Similar results for Chinese hamsters exposed by the
inhalation route to 28 -36 mg/m3 for 16 h (males only), or by a single
oral dose of 15 mg/kg body weight (males) or 10 mg/kg body weight
(females), have been reported (Dean & Thorpe, 1972a).
Dichlorvos has been tested for its ability to induce in vivo
chromosomal aberrations in Syrian hamsters bone marrow cells. Four
dose levels (3, 6, 15, and 30 mg/kg body weight) were given
intraperitoneally. Statistically significant increases in the number
of cells with aberrant chromosomes (mainly breaks and gaps) were
observed (Dzwonkowska & Hübner, 1986).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1 Mouse
Carcinogenicity studies were carried out on two groups of 50 male
and 50 female B6C3F1 mice fed 1000 and 2000 mg dichlorvos (94%) in corn
oil/kg diet for 80 weeks. Due to severe signs of intoxication, doses
were lowered after 2 weeks to 300 and 600 mg/kg for the remaining 78
weeks. Samples of the diets analysed during the study showed
dichlorvos contents within 5% of the intended concentrations. Matched
controls consisted of 10 mice of each sex; the pooled controls
consisted of 100 male and 80 female mice. All surviving mice were
killed at 92 - 94 weeks. Hair loss and rough hair coats were noted in
many treated animals, particularly in the male groups, beginning at
week 20 and persisting throughout the study. The average body weights
of the high-dose mice of both sexes were slightly decreased compared
with controls. The low-dose female group showed the poorest survival;
74% of the animals lived to 90 weeks. There was no significant
increase in the incidence of tumours attributable to dichlorvos in
either sex, i.e., dichlorvos was not demonstrated to be carcinogenic
(NCI, 1977; Weisburger, 1982).
In other studies, groups of 50 male and 50 female B6C3F1 mice, 6
weeks of age, were given drinking-water with 0, 400, or 800 mg
dichlorvos/litre ad libitum. The drinking-water solutions were renewed
daily. All surviving animals were killed during week 102. A dose-
dependent inhibition of body weight increase was observed throughout
the study in both sexes at both dichlorvos concentrations. There was
no adverse effect on mortality. The survival rates at week 102 were
62%, 66%, and 84% in males (in controls, low-, and high-dose groups,
respectively), and 66%, 50%, and 80% in females. The corresponding
figures for tumour incidence were 22.4%, 39.1%, and 23.4% in males and
29.3%, 16.2%, and 9.1% in females. The main tumours that were found
were lung adenomas and tumours in the liver, spleen, thymus, and
salivary gland. These tumours occurred in all three groups. There was
no statistically significant difference in the incidence of tumours at
any site in any group (Konishi et al., 1981).
Preliminary results are available from a recently completed 2-year
mouse carcinogenicity study using dichlorvos. Groups of 50 male and 50
female B6C3Fl mice were given dichlorvos (dissolved in corn oil) by
oral gavage daily for 2 years. Dose levels for male mice were 0, 10,
or 20 mg/kg body weight per day and for female mice 0, 20, or 40 mg/kg.
There were no statistically significant differences in survival rates
between the treated and control male mice, and the same applied to
female mice. A statistically significant increase in the incidence of
forestomach squamous cell papillomas was observed in the female mice
receiving 40 mg/kg per day. Two forestomach squamous cell carcinomas
were also seen in this group, but none were observed in the controls or
the 20 mg/kg group. Considerably fewer forestomach squamous cell
papillomas were observed in male mice. Nevertheless, a non-significant
increase was observed in the 20 mg/kg per day group. No forestomach
squamous cell carcinomas were noted in any male group. Forestomach
hyperplasia occurred relatively frequently in both control and treated
male and female mice, the incidence being similar in all groups (NTP,
1987).
8.7.1.2 Rat
In studies reported by NCI (1977) and Weisburger (1982), two groups
each of 50 male and 50 female Osborne-Mendel rats were fed either 150
or 1000 mg dichlorvos (94%) in corn oil/kg diet for 80 weeks. Due to
the severe signs of intoxication, the 1000 mg/kg dose was reduced to
300 mg/kg diet after 3 weeks for the remaining 77 weeks. The diets
were stored under conditions designed to minimize loss of dichlorvos,
and the animals received fresh diets daily. Matched controls comprised
10 rats of each sex; the pooled controls consisted of 60 rats of each
sex. The surviving rats were killed after 110 weeks. The average body
weights of the rats receiving the high dose level were slightly
decreased compared with controls. There was no significant increase in
the incidence and type of tumours in either sex as a result of
dichlorvos treatment.
Enomoto et al. (1981) carried out studies on groups of 50 male and
50 female Fisher 344 rats, 6 weeks of age, given drinking-water
(renewed daily) containing 0, 140, or 280 mg dichlorvos/litre ad
libitum. All surviving animals were killed in week 108 (104 weeks of
exposure followed by a 4-week recovery). Slight inhibition of body
weight increase was observed in males in the high-dose group, but there
was no influence on mortality. The survival rates in week 108 were
82%, 75%, and 75% in males and 86%, 71%, and 82% in females,
respectively, in control, low-dose, and high-dose groups. A great
number of organs and tissues were selected for microscopy. The organs
and tissues of all animals which died or which were killed in moribund
conditions were microscopically examined, as were all tumours and
macroscopical lesions. A number of animals with no specific changes
were also examined. The overall tumour incidences were 100%, 96%, and
98% in males and 37%, 31%, and 33% in females, respectively, in
control, low-dose, and high-dose groups. High incidences of
interstitial cell tumours of the testes in males (49/51, 41/48, and
47/48, respectively) were observed in all three groups. Mononuclear
cell leukaemia was found in all groups at 4 - 12%. There was no
statistically significant difference in tumour incidence at any site in
any group.
Preliminary results are available from a recently completed 2-year
rat carcinogenicity study using dichlorvos. Dichlorvos, dissolved in
corn oil, was administered each day by oral gavage to groups of 50 male
and 50 female Fischer 344 rats at dose levels of 0, 4, or 8 mg/kg body
weight per day. Though survival rates were slightly decreased in the
treated male and female groups compared with those of their respective
controls groups, the differences were not statistically significant.
However, a statistically-significant and dose-related increase in the
incidence of pancreatic adenomas was observed in the male rats fed 4 or
8 mg/kg. In female rats fed 8 mg/kg, a non-significant increase in
pancreatic adenomas was observed. There was a relatively high
incidence of pancreatic hyperplasia and atrophy in all groups of male
rats, including the controls, and a lower incidence in all female
groups. A statistically significant (but not dose-related) increased
incidence of mononuclear leukaemia was observed in the male rats fed 4
or 8 mg/kg. A similar incidence was observed in all female groups,
including the control group. Since this is a common and variable
systemic lesion in Fischer-344 rats, the toxicological significance of
this finding is uncertain. It is possible that its incidence in the
male control group was unusually low. An increased incidence of
mammary gland fibroadenomas (not dose related) was observed in the
treated female rats, but these were not considered to be of
toxicological concern. In addition, mammary gland hyperplasia was
frequently observed in all control and treated female groups at similar
incidences (NTP, 1986).
As reported in section 8.4.1.1, Witherup (1967, 1971) found no
increase in tumour incidence following dichlorvos treatment of rats.
8.7.2. Inhalation
8.7.2.1 Rat
In the studies by Blair et al. (1976) described in section 8.4.2.1,
no dose-related increase in tumour incidence was found.
8.7.3. Appraisal of carcinogenicity
The majority of the mouse and rat studies using dichlorvos are
considered to be negative regarding carcinogenic potential (Witherup et
al., 1967, 1971; Blair et al., 1976; NCI, 1977; Enomoto et al., 1981;
Konishi et al., 1981; Weisburger, 1982). However, preliminary
information from two recent studies on the mouse and rat, respectively,
has provided equivocal evidence of carcinogenicity (NTP, 1986)a.
------------------------------------------------------------------------
a The NTP Peer Review Panel reviewed these studies and came to the
following conclusions:
"Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenic activity of dichlorvos for male F344/N rats, as
shown by increased incidences of adenomas of the exocrine pancreas and
mononuclear cell leukemia. There was equivocal evidence of carcinogenic
activity of dichlorvos for female F344/N rats, as shown by increased
incidence of adenomas of the exocrine pancreas and mammary gland
fibroadenomas. There was some evidence of carcinogenic activity of
dichlorvos for male B6C3F1 mice and clear evidence for female B6C3F1 mice,
as shown by increased incidences of forestomach squamous cell papillomas"
(NTP, 1988).
The increased incidence of forestomach tumours in the mouse study
is likely to be related to the route of administration (oral gavage),
which has been shown in other mouse studies to induce forestomach
tumours due to repeated and direct irritation of the gastric mucosa.
If so, ingestion of food by human beings could not result in such
direct effects on the stomach wall. Furthermore, human beings do not
possess a stomach wall comparable with the forestomach of the mouse,
except perhaps for the oesophagus. However, the transient passage of
food through the oesophagus would probably not allow sufficient time
for the carcinogenic event to occur.
Similarly, the pancreatic adenomas observed in the NTP rat study
may be related to the corn oil used in the study. Evidence from other
rat studies in which corn oil was used as a vehicle suggests that this
is a possibility. On the other hand, it does not explain why a higher
incidence of pancreatic tumours was observed in the treated animals
compared with the controls. Although increased incidences of
mononuclear leukaemia were observed in treated male rats, the
incidence in the control group of this common and variable tumour may
have been low. The evidence for carcinogenicity in these two recent
studies is difficult to interpret at present. When complete and final
reports of these studies become available, more definitive conclusions
may be drawn.
8.8. Mechanisms of Toxicity; Mode of Action
A full description of the mechanism of action can be found in
Environmental Health Criteria 63: Organophosphorus Insecticides - A
General Introduction (WHO, 1986b).
Dichlorvos directly inhibits AChE activity in the nervous system
and other tissues. This reaction takes place in three steps:
(a) reversible binding of dichlorvos to the enzyme;
(b) reaction with the enzyme to form a dimethylphospho-enzyme
derivative, with the loss of DCA; and
(c) "aging" of the phospho-enzyme compound to a more stable
methylphospho-enzyme derivative.
Reaction (a) is rapid, but reversible. Reaction (b) is also rapid,
but the phosphorylated enzyme can be returned to its native
state spontaneously only by hydrolysis at very slow rates or by agents
such as N-methyl 2-pyridinium aldoxime (2-PAM) at higher rates.
Reaction (c) is comparatively slow (t´ = about 2.5 h at 37 °C and pH
7.4), but the product has the same stability as that of erythrocytes
(t´ = about 120 days) (Gillett et al., 1972).
Death due to poisoning is caused by excessive cholinergic effects
such as bronchospasms, hypersecretion from cholinergic innervated
glands (especially critical in lungs and bronchi), and cardiac
disturbances caused by vagotonus and anoxia. Convulsions and paralysis
in skeletal musculature are caused by brain anoxia as well as
cholinergic effects within the central nervous system.
In vivo studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos, at various time
intervals, after dosing are summarized in Table 18. In general, the
maximum inhibition occurred within 1 h, and was followed by rapid
recovery. Dogs seemed to be more sensitive than rats, and showed a
slower recovery.
Dichlorvos, when infused intravenously (into the ear vein) of
adult male rabbits, produced dose- and time-related inhibition of whole
blood ChE activity during infusion. Spontaneous but incomplete
recovery to 60 - 80% of the normal activity occurred within 60 -
90 min of infusion (Shellenberger et al., 1965; Gough & Shellenberger,
1970, 1977-78; Shellenberger, 1980).
In a study on the influence of temperature on ChE activity, rats
were injected intraperitoneally with a single dose of 6.25 mg
dichlorvos (95%)/kg and kept at either 28 °C or 5 °C. The maximum
inhibition of whole blood ChE activity (40%) occurred after 0.5 h.
The animals at 5 °C showed less inhibition and a faster recovery of
whole blood ChE activity than those at 28 °C (Chattopadhyay et al.,
1982).
8.9. Neurotoxicity
8.9.1. Delayed neurotoxicity
A detailed description of the neurotoxic potential of
organophosphorus compounds can be found in Environmental Health
Criteria 63: Organophosphorus Insecticides - A General Introduction
(WHO, 1986b).
Several studies have shown that dichlorvos does not produce delayed
neurotoxicity in pre-medicated hens, whether it is administered orally
or subcutaneously (Durham et al., 1956; Aldridge & Barnes, 1966;
Johnson, 1969, 1975a,b, 1978, 1981; Aldridge & Johnson, 1971; Lotti &
Johnson, 1978). The inhibition of brain neurotoxic esterase (NTE)
without signs of ataxia has been observed (Aldridge & Johnson, 1971;
Johnson, 1978).
Caroldi & Lotti (1981) reported mild signs of ataxia in pre-
medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg body weight) and severe inhibition of NTE in peripheral
nerve, spinal cord, and brain. However, Johnson (1978) did not observe
ataxia in pre-medicated hens given the same dose in the same way.
These hens showed severe inhibition of brain NTE but far less
inhibition of spinal cord NTE. It appears that ataxia arises from the
inhibition of spinal cord NTE. When the dose was repeated 1 - 3 days
after the first dose, spinal cord NTE inhibition increased and the hens
became ataxic.
White leghorn hens have been used in a 90-day study on the
neurotoxic potential of dichlorvos (99.9%) after dermal or oral
administration. For oral administration, a 10 - 20% solution of
dichlorvos in corn oil in gelatin capsules was used whereas, dermally,
1 - 20% emulsifiable concentrates in technical grade xylene (containing
2% Triton X-100) were used. Dichlorvos at doses greater than 1 mg/kg
body weight per day (dermal) or 6 mg/kg (oral) led to cholinergic
symptoms including salivation, convulsions, and death after 2 - 3 days.
With oral doses of 3 - 6 mg/kg no ataxia or death was observed.
Dermally, dichlorvos was very toxic for hens. Dose levels of about 1.7
and 3.3 mg/kg for an average period of 37 days caused ataxia and death.
An average dermal dose level of 0.65 mg/kg body weight for 90 days did
not induce ataxia or death. Typical symptoms of organophosphorus-
ester-induced delayed neurotoxicity (OPIDN) were not observed (Francis
et al., 1985).
In summary, it is possible to produce clinical neuropathy in hens,
but the doses required are far in excess of the LD50. The effects
are associated with severe inhibition of NTE in brain and spinal cord,
measured shortly after dosing (Johnson, 1981).
8.9.2. Mechanism of neurotoxicity
Male rats given a lethal intraperitoneal injection of 40 mg
dichlorvos/kg body weight did not show electrocortical disturbances.
Deaths resulted from respiratory failure (Hyde et al., 1978).
In studies by Desi (1983), adult CFY rats were given daily oral
doses ranging from 1.25 to 4 mg dichlorvos/kg body weight mixed in the
diet for 3 months. Plasma, erythrocyte, and brain ChE activities were
comparable with those of control rats. Increased EEG activity and
enhanced central excitability were found in the male rats only.
No changes in reflex motor unit potential activity or in nerve
conduction velocity were noted in dogs 7 days after single oral doses
of 30, 59.5, or 148 mg dichlorvos/kg body weight. However, erythrocyte
ChE activity was inhibited at all doses, and the highest dose produced
signs of intoxication (Hazelwood et al., 1979).
A single oral dose of 40 mg dichlorvos/kg or repeated doses of
1.6 mg/kg body weight per day did not cause histological abnormalities
in the brain of adult Wistar rats. Repeated administration of 50% of
the LD50 (i.e., 40 mg/kg body weight per day for 10 - 21 days), caused
myelin pallor and micro-vacuolation of the white matter. It seems
likely that primary degeneration of axons and secondary myelin sheath
abnormalities caused the spongy tissue loosening observed under the
electron microscope (Zelman, 1977; Zelman & Majdecki, 1979).
In studies by Ali et al. (1979a) and Hasan et al. (1979), male rats
were given 3 mg dichlorvos/kg per day intraperitoneally for 10 days.
Following perfusion-fixation, sections of cerebellum and spinal cord
were studied with the electron microscope. An abnormal increase in the
number of mitochondria in the spinal cord was found. Myelin
degeneration was detected in the spinal cord and myelin figures were
occasionally noted within oedematous dendrite profiles.
Table 18. Time-related ChE activity in animals after administration of a single dose of dichlorvos
---------------------------------------------------------------------------------------------------------
Species/sex Route Dose (mg/kg Time Cholinesterase activity (%) Reference
body weight) plasma erythrocyte brain
---------------------------------------------------------------------------------------------------------
Mouse (male) intraperitoneal 30 15 min 37 Cohen & Ehrich
2 h 40 (1976)
5 h 69
18 h 92
Mouse (male) intraperitoneal 10 15 min 30 Nordgren et al.
60 min 50 (1978)
2 h 80
Rat (male) oral 50 15 min 30 20 10-15 Modak et al.
3 h 30 65 (1975)
24 h 60 75 60-70
Rat (male) oral 40 1 h 30 Teichert et al.
(1976)
Rat (male) oral 40 5 min 55 Pachecka et al.
15 min 20 (1977)
2 h 30
24 h 75
48 h 100
Rat (male) intravenous 2.5 30 min 40 15 Reiner & Plestina
90 min 60 35 (1979)
3 h 90 55
12 h 100 80
48 h 90
Dog (beagle) oral 50 2 h 32 63 Ward & Glicksberg
(sex not 24 h 63 74 (1971)
specified) 5 days 92 78
21 days 100 92
Dog oral 22 1 h 12 17 Snow & Watson
(greyhounds 3 h 24 34 (1973)
and crossbred) 24 h 70 65
72 h 95 65
---------------------------------------------------------------------------------------------------------
Another study of dichlorvos neurotoxicity involved the
investigation of lipid peroxidation. This entails the direct reaction
between oxygen and lipids to form free-radical intermediates and semi-
stable peroxides. Major cellular components, such as membranes and
subcellular organelles, are damaged by these free radicals. Hasan &
Ali (1980) found a dose-dependent increase in the rate of lipid
peroxidation in various regions of the brain of the rat after
intraperitoneal administration of dichlorvos (at concentrations ranging
from 0.6 to 3 mg/kg body weight, daily) for 10 days. Also, there was
an increased incidence of lipofuscin-like pigment in the Purkinje cells
of the cerebellar cortex.
Maslinska et al. (1984) found that dichlorvos (dose levels of 4 -
8 mg/kg body weight for 10 days) affected the phospholipid-protein
balance in the brain of rabbits. The animals were exposed during the
postnatal "critical" life period, which constitutes a turning point in
the development of the brain. At this time, the neurons have already
undergone considerable arborization, and myelination and
vascularization are expanding rapidly. In addition, the overall oxygen
consumption is reaching its steepest rate of increase. In the myelin
sheaths under formation, several phospholipids are deposited. The
authors found changes in the phospholipid-protein ratio which
correlated well with the observed delay in myelin sheath formation.
Ultrastructural changes in certain subcellular organelles may be
connected with the change in this ratio, since it is crucial to the
structural and functional properties of the membranes and enzymes bound
to them.
Dambska et al. (1984) have studied the influence of dichlorvos on
blood vessel walls, the perivascular area, and the permeability of the
blood-brain barrier in young rabbits. The young animals received 9 mg
dichlorvos/kg body weight for 16 days starting on the 6th day of life.
There was a decrease in ChE activity in brain capillary walls.
Electron microscopic studies showed lesions of the perivascular
astrocytes and changes in the endothelial cells. However, these
lesions did not disturb the blood-brain barrier mechanism for
horseradish peroxidase particles.
Studies on the central cholinergic system have revealed that the
inhibition of brain ChE activity and its subsequent recovery were
uniform in all brain regions studied in orally dosed (50 mg/kg) rats.
ACh concentrations were increased in brain areas within 15 min of
treatment. A biphasic effect was observed on choline metabolism in the
brain. The cortex was more cholinergic than the striatum in terms of
percentage increase in ACh and choline (Modak et al., 1975). In a
similar study on rats, either receiving a single oral dose (40 mg/kg)
or repeated oral doses (4 mg/kg), the activity of whole brain choline
acetyltransferase and the contents of ACh and choline in whole brain
were not altered, though brain ChE activity was markedly inhibited.
However, in the cerebral hemispheres, and especially the corpus
striatum, the ACh level was considerably increased, without a
concomitant change in choline (Teichert et al., 1976).
Kobayashi et al. (1980, 1986) investigated the concentration of
total, free, labile-bound and stable-bound ACh in the brain of rats
given single or multiple subcutaneous injections of dichlorvos (0.2 -
4 mg/kg body weight). The results suggest that alterations in the
mobilization and storage of ACh in the central cholinergic nerves may
be induced. The time course for ACh accumulation was measured in rat
brain regions after intravenous treatment with 15 mg dichlorvos/kg body
weight (Stavinoha et al., 1976). The striatum had the highest rate of
accumulation and the cerebellum the lowest. The calculated turnover
time for the different regions of the brain was between 0.9 and
5.6 min.
In studies by Ali & Hasan (1977) and Ali et al. (1979b, 1980), rats
were given intraperitoneally 3 mg dichlorvos/kg body weight per day for
10 or 15 days. The concentrations of dopamine, norepinephrine, and 5-
hydroxytryptamine (5-HT) were significantly decreased in different
parts of the brain, and 5-HT was significantly increased in the spinal
cord.
A single dose or short-term (12 weeks) treatment of rats with high
concentrations of dichlorvos, which produced brain ChE inhibition,
resulted in decreased norepinephrine levels in the brain (Brzezinski &
Wysocka-Paruszewska, 1980). From these studies, it was suggested that
the metabolism of catecholamines and 5-HT may be disturbed by
dichlorvos.
8.10. Other Studies
Many studies in different organ systems have been carried out. In
most of these studies, the route of administration was the oral or
intraperitoneal route. Single or repeated dosing was used, mainly with
high dose levels, in mice and rats. The influence on brain enzymes
(other than brain ChE), liver enzymes such as ChE (inhibition),
microsomal cytochrome P-450 activity (decrease), drug-metabolizing
enzyme activity, and UDP-glucuronyl transferase (no influence), and
many other enzyme systems were studied.
Furthermore, the influence of dichlorvos on adrenal steroidogenesis
has been investigated (Civen et al., 1980).
8.10.1. Immunosuppressive action
A dose-related suppression of the humoral immune response induced
by S. typhimurium was observed in rabbits orally administered 0.3 -
2.5 mg dichlorvos (93%)/kg body weight in capsules, 5 times a week for
6 weeks (Desi et al., 1978).
In a comparable study on rabbits, the cellular immune response was
estimated using the tuberculin skin test. The skin redness in the
tuberculin test and the serum antibody titres of treated animals showed
a dose-dependent decrease compared with those of controls (Desi et al.,
1980).
8.11. Factors Modifying Toxicity; Toxicity of Metabolites
8.11.1. Factors modifying toxicity
In studies on the effect of diet on the toxicity of dichlorvos,
young male rats were kept for 30 days on the following synthetic diets:
high protein (HPD), low protein (LPD), high fat (HFD), and standard
(SD). Growth rates were normal except for a slightly decreased body
weight gain in the HFD group. The composition of the diet, per se, did
not significantly affect plasma and erythrocyte ChE activity 24 h or 5
days after dosing. A single intraperitoneal injection of 50 mg
dichlorvos/kg body weight led to higher mortality in LPD rats (as
expected with a protein-deficient diet) and lower mortality in HPD
rats, compared with SD animals (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or HPD
for 30 days. At the end of this period, a single intraperitoneal dose
of dichlorvos (20 or 30 mg/kg body weight) was administered. Results
showed that diets, per se, did not affect initial plasma or erythrocyte
ChE activity, nor did the HPD or HFD diets protect against mortality
from dichlorvos. In the case of the HPD, the spontaneous recovery of
ChE activity was reduced in the plasma and erythrocytes of dichlorvos-
treated rats. However, with an HFD, this recovery was significantly
increased (Purshottam & Srivastava, 1984).
Costa & Murphy (1984) studied the interaction between acetaminophen
(which, like dichlorvos, is detoxified by glutathione transferase) and
dichlorvos (10 mg/kg body weight) in mice. Acetaminophen (600 mg/kg
body weight) pre-treatment did not have any affect on dichlorvos
toxicity. On the other hand, intraperitoneal pre-treatment with
diethylmaleate (1 ml/kg body weight) increased the acute toxicity of
dichlorvos.
8.11.2. Toxicity of metabolites
8.11.2.1 Acute toxicity
The toxicity of metabolites of dichlorvos in female mice, injected
intraperitoneally, is considerably less than that of dichlorvos
(Table 19).
Mice (male and female) survived a single exposure of 130 mg
DCA/m3 for 5 - 7 h (Stevenson & Blair, 1969).
8.11.2.2 Short-term exposures
Groups of 20 male and 20 female rats were exposed for 30 days to
actual concentrations of 0 or 0.5 - 1 mg DCA/m3. Half the animals
were then killed promptly and the remainder on day 35. After 30 days
of exposure, the male rats showed a slight decrease in body weight and
food intake, and a slight increase in absolute and relative liver
weight. There were no such changes in the rats left for 5 more days
unexposed to DCA. Histological examination of the lungs of exposed
males and females revealed a higher incidence of minor inflammatory
changes than in controls. No other changes attributable to DCA were
found in general health, behaviour, haematology, clinical chemistry,
organ weights, gross pathology, or histopathology. A similar study
using groups of 10 male and 10 female rats and actual concentrations of
0 or approximately 2 mg/m3 DCA revealed no abnormalities attributable
to DCA (Wilson & Dix, 1973).
Table 19. Intraperitoneal LD50 values of metabolites of dichlorvos in
female micea
-----------------------------------------------------------------------------
Compound Vehicle LD50 (mg/kg body weight)
-----------------------------------------------------------------------------
desmethyldichlorvos, water 1500
sodium salt
dichloroacetyaldehyde corn oil 440
(DCA)
dichloroethanol corn oil 890
dichloroacetic acid corn oil or water 250
sodium dichloroacetate water 3000
methyl and dimethyl water 1500
phosphoric acid mixture
sodium methyl- and water 3000
dimethylphosphate
mixture
-----------------------------------------------------------------------------
a From: Casida et al. (1962).
8.11.2.3 Long-term exposure
No long-term tests have been carried out with DCA as such.
However, in the 2-year oral studies on rats and dogs with dichlorvos
(section 8.4.1), the degradation product DCA was present in the test
diets in increasing amounts, so that possible effects of DCA were
included in the results of these studies.
8.11.2.4 Mutagenicity
DCA appears to be mutagenic in the Salmonella test using S.
typhimurium TA100. The mutagenicity decreased in the presence of a
microsomal activation system. Part of the decrease was dependent on
the presence of the co-factors NADP and glucose-6-phosphate. No
evidence for mutagenicity with 2,2-dichloroethanol was obtained in
this S. typhimurium strain (Löfroth, 1978).
In a dominant lethal assay, a single intraperitoneal injection of
176 mg DCA/kg in male mice (AB Jena-Halle strain) produced a decrease
in the number of total implants and live fetuses in the first 3 weeks
of the test, and an increase in post-implantation losses. The same
test was repeated with a strain of mice (DBA) less sensitive to
mutagenic effects. The same effects were found, but to a lesser
extent, and mainly in the fourth week (Fischer et al., 1977).
8.11.2.5 Metabolism
When 32P-dimethylphosphate (500 mg/kg in water) was administered
orally to a male rat, the autopsy (90 h after treatment)
indicated that almost the entire dose had been eliminated. The
urine, containing only unmetabolized dimethylphosphate, accounted for
about half of the radioactivity. The tissues were almost devoid
of 32P-containing material (Casida et al., 1962).
A rat, orally dosed with 500 mg/kg 32P-desmethyldichlorvos in
water, excreted about 14% of the dose in the urine in 90 h.
The tissue distribution of 32P was similar to that which followed
32P-dichlorvos administration. The very high proportion of
radioactivity in the bone was indicative of rapid degradation to
phosphoric acid (Casida et al., 1962).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity
9.1.1.1 Poisoning incidents
A 56-year-old woman ingested an estimated amount of 100 mg
dichlorvos/kg body weight and survived, following intensive care for 14
days (Watanabe et al., 1976). However, a suicide with a dichlorvos
dose of about 400 mg/kg succeeded in spite of treatment (Shinoda et
al., 1972).
A 35-year-old female patient accidently ingested 60 g fluid Divipan
(dichlorvos concentration not reported). She was comatose for one week
and recovered slowly. Clinical and electrophysiological examinations
(no details reported) showed a pure motor form of neuropathy, according
to the authors (Vasilescu & Florescu, 1980).
Two cases of poisoning with dichlorvos taken orally in unspecified,
but high, quantities have been reported. The patients first showed
signs of severe anti-ChE poisoning. After recovery, delayed
neurotoxicity developed. They showed a severe axonal degeneration
neuropathy. One of them recovered within 12 months (Wadia et al.,
1985).
Reeves et al. (1981) reported six cases, over an 8-year period, of
bone-marrow failure (pancytopenia) in children shortly after exposure
to dichlorvos and propoxur. Van Raalte & Jansen (1981) doubted the
causal relationship between dichlorvos and bone-marrow failure in the
children, because the disease has not been observed in workers with
high exposure, or in the general population. In addition, haematotoxic
effects have not been observed in experimental animals.
9.1.2. Effects of short- and long-term exposure
In the 1960s, field studies were carried out in several countries
to test dichlorvos as a residual insecticide for malaria control in
houses. Numerous residents of all ages and conditions were exposed
without any adverse effects attributable to dichlorvos being reported
(Escudié & Sales, 1963; Funckes et al., 1963; Gratz et al., 1963;
Quarterman et al., 1963; Foll et al., 1965). In two field studies, the
plasma and erythrocyte ChE activities were measured in adults and young
children. No abnormalities were found, though air concentrations of
dichlorvos in the treated houses rose to 0.8 mg/m3 (Funckes et al.,
1963; Gratz et al., 1963).
In a report by Gold et al. (1984), 20 single-family residences were
treated with a 0.5% solution of dichlorvos (at an average rate of
0.189 g/m2) to control the cockroach Blattella germanica L. The
average air concentration for the first 2 h after treatment was
548 µg/m3 and for the next 2 h was 183 µg/m3. Pesticide operators
and the residents of treated structures were monitored for evidence of
dichlorvos exposure, using exposure pads, air samples, serum and
erythrocyte ChE tests, and urinalyses. There was no evidence of
dichlorvos or dichloroacetic acid in urine. There were slight, but
statistically significant, changes in the mean serum ChE activity of
some of the residents of treated structures, but the mean erythrocyte
ChE was unchanged.
Passengers in an aircraft provided with an automatic insect-control
system, which released 0.15 - 0.30 mg dichlorvos/m3 for periods of
about 30 min, did not show any signs of discomfort (Jensen et al.,
1965). Tests have shown that man can withstand daily exposure to
concentrations of 0.5 mg/m3 without clinical effects, and with only a
slight depression of blood ChE activity (Hayes, 1961). Three men
exposed to approximately the same level during 24 tests did not show
any change in blood ChE activity (Schoof et al., 1961).
A case of chronic obstructive bronchitis ascribed to exposure to
dichlorvos was described by Barthel (1983). However, it could not be
excluded that other components of the unknown formulation could have
been the cause. ChE determinations were not performed.
9.1.2.1 Studies on volunteers
Single oral doses (1 - 32 mg/kg body weight) of dichlorvos in a
slow-release PVC formulation administered to 107 male volunteers
produced measurable reductions in erythrocyte ChE activity at dose
levels above 4 mg/kg, with a maximum reduction of 46% at the highest
dose. Plasma ChE activity was affected at lower doses, with 50%
reduction at 1 mg/kg and about 80% at 6 mg/kg or more. Repeated oral
doses of 1 - 16 mg/kg body weight per day were given to 38 volunteers
for up to 3 weeks. The plasma ChE activity was maximally depressed at
all dose levels, and the erythrocyte ChE activity depression was dose
related and significant at 2 mg/kg or more. Blood cell count, urine,
liver function, prothrombin time, and blood urea nitrogen were all
normal (Hine & Slomka, 1968, 1970; Slomka & Hine, 1981).
In studies by Rider et al. (1968), dichlorvos was given to groups
of five men at daily oral doses of 1, 1.5, 2, or 2.5 mg per man. The
plasma ChE activity of the 2.5 mg group was reduced by 30% after 20
days of treatment. Administration of 2 mg for 28 days resulted in a
reduction of 30% 2 days after the last dose. Erythrocyte ChE activity
was not significantly affected in either group (Rider et al., 1967).
Daily oral doses of 1.5 mg per man given to 10 volunteers for 60 days
caused a significant reduction (approximately 40%) in plasma ChE
activity, which returned to normal levels when dichlorvos
administration was discontinued.
Boyer et al. (1977) reported studies on two groups of six men (21 -
45 years of age) who received 0.9 mg of dichlorvos three times a day
for 21 days. One group received the dichlorvos in a gelatin salad, the
other in a pre-meal capsule filled with cottonseed oil. Two other
groups of six men received placebo treatment. No consistent
cholinomimetic signs or symptoms were observed, nor was erythrocyte ChE
inhibited. However, plasma ChE was significantly depressed within 20
days, although the extent depended on the method by which dichlorvos
was administered. Recovery was comparable in both groups, the half-
life for the regeneration of plasma ChE being 13.7 days.
Volunteers did not show inhibition of plasma or erythrocyte ChE
activity either when handling a dichlorvos strip for 30 min each day or
after having a piece of the strip applied to the arm for 30 min each
day for 5 consecutive days (Zavon & Kindel, 1966).
The wearing of dichlorvos-impregnated garments by babies for 48 -
84 h did not produce changes in either plasma or erythrocyte ChE
activity over a period of 5 days (Cavagna et al., 1969).
In studies on eight volunteers, carried out in an aircraft at
operational cabin altitude (2400 m), no changes in plasma or
erythrocyte ChE activity, dark adaptation, or bronchiolar resistance
were observed. Dichlorvos concentration in the air ranged from 0.73 to
1.18 mg/m3 during exposures of 45 min (Smith et al., 1972).
Hunter (1970a) reported studies on 26 men (21 - 57 years of
age) and 6 women (19 - 25 years of age) who were exposed in a chamber
for 2-7´ h to actual dichlorvos concentrations of approximately
1 mg/m3. Food and drink were served in the chamber. No clinical
signs were observed, and no effects on haematology, urinalysis, kidney
function, EEG, ECG, respiratory activities, or erythrocyte ChE activity
were found. Plasma ChE activities were markedly inhibited only when
the exposure lasted over 6 - 7 h (Hunter, 1970a).
In studies by Hunter (1969, 1970b), seven men (25 - 56 years of
age) were exposed (head and neck) to dichlorvos vapour (actual
concentrations of 1 - 52 mg/m3), and 6 men were exposed (head
exposure only) to 7 - 50 mg/m3, for periods from 10 min to 4 h. The
maximum dose level was 52 mg/m3 (for 65 min), and the maximum period
was 240 min (at 13 mg/m3). Symptoms were confined to irritation of
the throat, some rhinorrhoea, and substernal discomfort at the highest
concentrations. No effects on the pupil or on visual acuity were
recorded. Erythrocyte ChE activity was depressed in only one person.
However, there was a direct relationship between the reduction in
plasma ChE activity and the dichlorvos dose (concentration x time). No
changes were found in either kidney or pulmonary function or in the
overall metabolic rate.
When three men were exposed to dichlorvos (actual concentrations of
0.3 - 0.9 mg/m3 (mean, 0.5 mg/m3) or 0.9 - 3.5 mg/m3 (mean,
2.1 mg/m3) for 1 or 2 h per day for 4 consecutive days, plasma ChE
alone decreased slightly in the men exposed for 2 h per day to the
higher concentration (Witter et al., 1961).
A group of 15 men (23 - 61 years of age) was exposed for up to 6
half-hour intervals per night for 14 days (total of 39 doses) to actual
dichlorvos concentrations ranging from 0.14 to 0.33 mg/m3. No clear
changes in plasma ChE activity or other parameters such as reaction
time, airway resistance, or vision were found. The same results were
obtained when a similar group was exposed to actual concentrations of
0.1 - 0.6 mg/m3 for intervals of 8 - 10 h, 4 nights per week for 11
weeks (Rasmussen et al., 1963).
No significant effect on plasma or erythrocyte ChE activity was
observed in 14 persons exposed at the recommended rate of one
dichlorvos strip per 30 m3 in their homes over a period of 6 months.
The strips were replaced at much shorter intervals than normally
recommended. The air concentration 40 days after the installation of
the fourth strip was approximately 0.09 mg/m3 (Zavon & Kindel, 1966).
In three home studies involving 26 families, conducted in Arizona,
USA, no deleterious effects on health or plasma or erythrocyte ChE
activity were observed in the residents exposed to dichlorvos strips
all over the house (8 - 10 strips) for over a year. Even monthly
replacement of the strips resulted in only a slight inhibition of
plasma ChE activity. The maximum air concentrations of dichlorvos
averaged 0.13 mg/m3 (Leary et al., 1971, 1974).
9.1.2.2 Hospitalized patients
In a report by Pena Chavarra et al. (1969), a single oral dose of 6
or 12 mg dichlorvos/kg body weight was administered in the form of a
slow-release granular resin formulation as an anthelminthicum to 108
hospitalized adult patients, many of these debilitated and with severe
anaemia. Plasma ChE activity was markedly reduced, in some patients by
76 - 100%. However, erythrocyte ChE activity was much less inhibited.
No symptoms of intoxication were observed, except for brief mild
headaches in a few patients, and there were no abnormalities in
haematological studies or in hepatic and renal function tests.
In studies by Cervoni et al. (1969), single doses of dichlorvos
(PVC-resin formulations) were administered orally 2 h before breakfast
to 705 adults found to be harbouring infections of Trichuris, hookworm,
or Ascaris. Six or 12 mg dichlorvos/kg body weight resulted in
infection cure rates of approximately 70 - 100%. According to the
authors, minimum to modest plasma ChE depression and zero to minimum
erythrocyte ChE depression occurred at both dose levels. No clinical
symptoms or alterations in haematology or in liver and kidney function
were observed.
Sick adults and children and healthy pregnant women and babies in
hospital wards treated with dichlorvos strips (one strip per 30 or
40 m3) had normal erythrocyte ChE activities. Only subjects exposed
for 24 h per day to dichlorvos concentrations above 0.1 mg/m3 or
patients with liver insufficiency showed even a moderate decrease in
plasma ChE activity (Cavagna et al., 1969, 1970; Cavagna & Vigliani,
1970).
9.2. Occupational Exposure
9.2.1. Acute toxicity
9.2.1.1 Poisoning incidents
A number of fatal and non-fatal poisoning cases have been described
after concentrated formulations of dichlorvos splashed onto parts of
the body. Two workers who failed to wash it off promptly died
consequently. However, in those cases where the spilled solution was
washed off immediately, the victims showed symptoms of intoxication but
recovered after treatment. A serious non-fatal case occurred after a
spillage of 120 ml of a 3% formulation that was not washed off
immediately. After 1´ h, the victim developed slurred speech, became
drowsy, and collapsed. He recovered completely after treatment (Hayes,
1963, 1982).
A pest-control operator became contaminated with a 1% solution of
dichlorvos in mineral spirit after using a leaking knapsack sprayer.
The man changed his overalls and completed his day's work. He noticed
weakness, dizziness, and difficulty in breathing. Contact dermatitis
developed on the back skin. After the fourth day, his blood ChE was
36% of normal, but there were no signs of systemic illness. He
recovered without medication, and the ChE activity increased to 72% of
normal within one month. The acute dermatitis was probably caused by
the solvent (Bisby & Simpson, 1975).
A driver of a truck transporting a 5% commercial formulation of
dichlorvos (15% petroleum distillate and 80% trichloroethane)
developed persistent contact dermatitis for 2 months following
accidental skin contact. In addition, the patient experienced
headache, mild rhinorrhoea, burning of the tongue, and a bitter taste
in his mouth. Initial blood ChE levels were in the low normal range
returning to the high normal range within 2 weeks. Patch tests with 1%
and 0.1% dichlorvos in petroleum distillate were negative (Mathias,
1983). In view of the symptoms and the slight ChE inhibition, it seems
likely that trichloroethane caused the dermatitis.
Cronce & Alden (1968) described four people who handled dogs
wearing anti-flea dog collars containing 9 - 10% dichlorvos. Acute
primary contact dermatitis was described. Closed patch tests with
0.25, 0.5, and 1% dichlorvos in distilled water and in mineral oil were
positive in all four people. General experience with the collars
indicates that only a few people are susceptible to this kind of
irritation (Hayes, 1982).
9.2.2. Effects of short- and long-term exposure
9.2.2.1 Pesticide operators and factory workers
The effect on sprayers of dichlorvos fume, used inside a building
for cockroach control, was examined. When 0.3 - 0.6% dichlorvos oil
spray was used at the rate of 6 ml/m2 by the sprayers (7 - 9 people),
inhibition of ChE activity in the subjects was 15%, and conjunctival
injections or sore throats were observed in some sprayers after either
18 min or 4 h of spraying operation. To examine the effect of elevated
dichlorvos vapour pressure at higher temperatures, four men sprayed
0.6% oil spray for 2 h at the room temperature of 25 °C. The
inhibition of plasma and erythrocyte ChE activities was 22% and 7%,
respectively. In another study, 11 sprayers used 0.6% oil spray for
6 h of actual work time at 20 °C without rest. Two of them showed an
inhibition of plasma ChE activity of 38% while the others did not show
significant inhibition of erythrocyte ChE. It was concluded that
conjunctival injections and sore throats were attributable to the
kerosene solvent used and that the 38% depression in ChE activity was
probably due to long hours of continuous work. Therefore, the overall
effect on ChE activity was not considered to be severe (Ueda et al.,
1959, 1960).
Twelve fogging machine operators did not show any reduction in
plasma or erythrocyte ChE activity when applying 4% dichlorvos aerosols
in tobacco warehouses for 16 h per week, over a period of 2 - 4 months
(Witter, 1960).
Sixteen men replacing old dichlorvos dispensers and installing new
units in houses in Haiti, 5 days per week for 3 weeks, showed a
decrease of up to 60% in plasma ChE activity. No signs or symptoms of
intoxication were observed. Air concentrations ranged from 0.3 to
2.1 mg/m3 (Stein et al., 1966).
The blood ChE activity of sprayers, exposed during insect control
in grain stores to air concentrations of 1.9 - 3 mg/m3 dichlorvos,
was reduced to 19 - 23% (Sasinovich, 1970).
In a report by Das et al. (1983), each of 13 pesticide-control
operators carried out urban pest-control for one day in four houses
using 230 - 330 g dichlorvos as aerosol and 40 - 50 g dichlorvos as
emulsion spray. At the end of the day's work, an operator had an
average dichlorvos residue of 0.8 mg/m2 on the back, 0.4 mg/m2 on the
chest, and 11 mg/m2 on the respirator filter. Dimethylphosphate was
detected in the urine, but blood and urine analyses, including serum
ChE levels, did not reveal any other changes in clinical parameters.
In the course of either the production or processing of a
dichlorvos-releasing product, 11 male and 2 female factory workers were
exposed to an average dichlorvos concentration of 0.7 mg/m3 (highest
value 3 mg/m3) on each working day for a period of 8 months.
Inhibition of plasma ChE activity was noted within a few days of the
start of exposure, while inhibition of erythrocyte ChE activity
developed much more slowly. The maximum plasma ChE activities recorded
were 40% lower than the pre-exposure levels, but 60% lower than the
post-exposure levels. Erythrocyte ChE activity was reduced by
approximately 35% compared with pre- and post-exposure levels. One
month after exposure had ceased, plasma ChE and erythrocyte ChE
activities were found to have returned to normal physiological levels.
The other haematological investigations and the medical examinations
did not reveal any changes attributable to dichlorvos exposure (Menz et
al., 1974).
9.2.2.2 Mixed exposure
A number of articles have described the symptoms found in
individual workers or groups of workers exposed for a number of years
to different types of pesticides, including dichlorvos. In general, a
slight-to-moderate decrease in ChE activity occurred. Furthermore,
several complaints and symptoms were noticed, but no clear clinical
poisoning cases occurred. These case studies are, however, of little
relevance for the evaluation of dichlorvos because of the mixed
exposure to different pesticides (Bellin & Chow, 1974; Fournier et al.,
1976; Gupta et al., 1979; Ullmann et al., 1979; Hayes et al., 1980;
Hayes, 1982).
From 1975 to 1977, 88 patients with pesticide dermatitis, 15
suffering from photo-dermatitis, were studied. Patch tests using 29
different pesticides of different categories were carried out, but did
not lead to an identification of the responsible pesticides. Eight out
of 52 patients (15.4%) reacted positively to a photopatch (Horiuchi &
Ando, 1978; Horiuchi et al., 1978).
Stoermer (1985) described a case of contact dermatitis in a woman
who had worked as a pest controller for 3 months and had sprayed
dichlorvos and propoxur. She was treated and recovered within one
week.
A field survey of tea growers was made in the Chiran area of Japan
in 1982. Out of 84 tea growers examined (21 men and 63 women), 5 women
had contact dermatitis from agricultural work. Dichlorvos was among
the insecticides, fungicides, and herbicides used. Patch tests showed
relatively high rates of positive reactions with dichlorvos (27% of
women and 5% of men). Also, cross-sensitization was found between
methidathion and dichlorvos (Fujita, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Since 1961, dichlorvos, an organophosphate with anti-ChE activity,
has been used worldwide as a contact and stomach insecticide to control
insects on crops and domestic animals. It is also used as an
insecticide in houses and other buildings and for insect control in
aircraft.
Dichlorvos is readily absorbed by the body of mammals through all
routes of exposure, and is readily metabolized in the liver. Within
1 h of oral administration, dichlorvos is found in the liver, kidneys,
and other organs of experimental animals. It is rapidly eliminated via
the kidneys, with a half-life of 14 min.
The metabolism of dichlorvos in various species, including human
beings, follows similar pathways. Differences between species relate
only to the rate of metabolism, but this is always rapid.
Dichlorvos is moderately to highly toxic for mammals (the oral
LD50 for the rat is 30 - 110 mg/kg body weight). The classification
of dichlorvos by WHO (1986a) is based on an oral LD50 for the rat of
56 mg/kg body weight. Signs of intoxication usually occur shortly
after exposure and are typical of an organophosphorus pesticide.
Inhibition of ChE activity is a sensitive criterion of exposure. In
short-term toxicity studies on mammals, it was shown that ChE activity
was not decreased at oral dose levels below about 0.5 mg/kg body
weight. In long-term studies on rats at oral dose levels of 2.5 mg/kg
body weight or more, hepatocellular fatty vacuolization was seen. At
0.25 mg/kg body weight, no ChE inhibition was found, nor were there any
other effects.
Reproduction and teratogenicity studies using a wide range of dose
levels (6.25 - 500 mg/kg body weight) were negative. Dichlorvos showed
alkylating properties in in vitro but not in in vivo studies. Many in
vitro mutagenicity studies with bacteria and yeast were positive, while
the in vivo studies were mainly negative. From the available
mutagenicity studies, it is unlikely that dichlorvos constitutes a
mutagenic hazard for man. Carcinogenicity studies on mice and rats fed
dichlorvos (dose levels of up to 234 mg/kg diet) were negative. Two
recent carcinogenicity studies have been carried out on mice and rats
in which dichlorvos was administered by intubation for up to 2 years at
dose levels of 10 - 40 mg/kg body weight (mice) and 4 or 8 mg/kg body
weight (rats). Only preliminary information has been provided. The
evidence for carcinogenicity in these new studies is difficult to
interpret at this time. Only when complete and final reports become
available will it be possible to draw more definite conclusions (in
this context, see section 8.7.3).
From work on hens, the suspicion of delayed neurotoxicity from
dichlorvos has neither been established nor totally refuted. However,
there have been two clinical reports on four patients suffering intense
poisoning from dichlorvos taken orally who survived with treatment and
who then displayed neurotoxic effects. Thus, the possibility of
delayed neurotoxicity in man cannot be entirely discounted, but it is
likely to occur only with excessive oral doses.
Human volunteers who were given single or repeated oral doses of 2
mg/kg body weight or more showed significant inhibition of erythrocyte
ChE activity. At 1 mg/kg body weight, no such inhibition was found.
The application of dichlorvos to crops and animals results in
residues that rapidly disappear by volatilization and hydrolysis. In
general, residues of dichlorvos and the breakdown product DCA in food
commodities are low and will be further reduced during processing. The
exposure of the general population to dichlorvos by food and drinking-
water is negligible, as is confirmed in total-diet studies.
In short-term inhalation studies on mammals, 1 or 2 mg
dichlorvos/m3 did not inhibit ChE activity.
In a 2-year, 23 h/day, whole-body inhalation study on rats, 0.48
mg/m3 caused inhibition of plasma and erythrocyte activity, but brain
AChE activity was not inhibited and there were no clinical signs. An
unquantified, but considerable, extra exposure, resulting from the
grooming of contaminated fur and contamination of food and drinking-
water, had contributed to this effect. The no-observed-adverse-effect
level was 0.05 mg/m3. There was no evidence of carcinogenicity.
In a 6- to 7-h exposure of human volunteers to approximately 1 mg
dichlorvos/m3, only plasma ChE activity was inhibited. The
erythrocyte AChE activity, taken to be representative of the AChE
activity in the nervous tissue, was unaffected.
Residents exposed for over one year to an average air concentration
of 0.1 mg/m3 arising from slow-release strips showed no inhibition of
plasma or erythrocyte ChE activity and no deleterious effects on
health.
The main exposure of the general population is by inhaling
dichlorvos when used indoors to control insects. The recommended use
(one slow-release strip/ 30 m3) will give concentrations in the air
of up to 0.1 - 0.3 mg/m3 in the first few days, decreasing thereafter
to below 0.1 mg/m3. The air concentration depends on temperature,
humidity, and ventilation.
As long as approved slow-release strips are used according to the
label instructions, no health hazard can be expected for man. However,
special care may need to be taken with young children and sick or
elderly people who are especially vulnerable when continuously exposed
(24 h per day) in poorly ventilated rooms. Other methods of indoor
application should be equally safe if the label instructions are
followed.
There is some indication that dichlorvos may induce dermatitis and
cross-sensitization in people handling various types of pesticides
including dichlorvos.
In occupational conditions, the main route of exposure to
organophosphorus pesticides is usually the dermal route. In the case
of dichlorvos, with its high vapour pressure, exposure by inhalation is
also important. In these occupational situations, the dichlorvos
concentrations in the air are generally below 1 mg/m3 but, in certain
circumstances, they may rise considerably above this level. This
stresses the need for adequate protective measures to be taken during
occupational exposure and regular monitoring of ChE activity.
10.2. Evaluation of Effects on the Environment
The presence of dichlorvos in the environment as a result of
accidental losses or direct application on soil or in water will not
lead to long-term effects because of its fast breakdown and
evaporation. Furthermore, it is converted to a number of compounds,
such as dichloroacetic acid, by microorganisms. Certain bacteria
species can use dichlorvos as a sole carbon source, while others cannot
and are inhibited in their growth. Therefore, its influence on
microorganisms is complex.
Dichlorvos is moderately to highly toxic (range, 0.2 - 10 mg/litre)
for freshwater and estuarine species of fish and invertebrates. In
certain fish, concentrations of 0.25 - 1.25 mg/litre cause inhibition
of brain and liver ChE activity. Concentrations of as little as
0.05 mg/litre may have deleterious effects, particularly in
invertebrates. Dichlorvos has a high toxicity for birds and bees.
Caution is advised in the use and handling of dichlorvos where these
species might be exposed.
No bioaccumulation occurs in the different environmental
compartments and organisms.
10.3. Conclusions
1. Exposure of the general population to dichlorvos via food and
drinking-water is negligible and does not constitute a health hazard.
2. The in-house use of dichlorvos as an insecticide in the form of
sprays or slow-release strips (at recommended levels) does not
constitute a short- or long-term hazard for the general population.
However, continuous (24 h per day) exposure of young children and
diseased or elderly people in non-ventilated or poorly ventilated rooms
should be avoided.
3. In spite of their toxicity, dichlorvos and its formulations do not
contribute an undue hazard to those occupationally exposed, provided
that adequate ventilation and skin protection are used.
4. Except under conditions of gross spillage, the recommended use of
dichlorvos as an insecticide does not constitute an acute or long-term
hazard for aquatic or terrestrial organisms, although there may be an
acute hazard for birds and bees.
11. RECOMMENDATIONS
1. Continuous (24 h/day) exposure of young children and diseased or
elderly people to dichlorvos in non-ventilated or poorly ventilated
rooms should be avoided.
2. As dichlorvos from different sources may vary in purity and type of
impurities, attention should be paid to its composition. This should
conform to FAO and WHO specifications (FAO, 1977; WHO, 1985). In the
case of formulations, potential hazards of other components, such as
solvents and stabilizers, should also be considered.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dichlorvos was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1965, 1966, 1967, 1969, 1970, 1974, and 1977
(FAO/WHO, 1965a,b, 1967a,b, 1968a,b, 1970a,b, 1971a,b, 1975a,b,
1978a,b). In 1966, the JMPR established an Acceptable Daily Intake
(ADI) for human beings of 0 - 0.004 mg/kg body weight, which remains
unchanged.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified technical dichlorvos as
"highly hazardous" (Class IB) (Plestina, 1984; WHO, 1986a) and has
produced a safety sheet on dichlorvos (No. 75.2) (WHO/FAO, 1975-86).
Specifications for dichlorvos use in public health and in plant
protection have been published by WHO (1985) and FAO (1977),
respectively.
In 1979, the International Agency for Research on Cancer (IARC)
came to the following conclusions in considering the carcinogenicity of
dichlorvos:
(a) dichlorvos was tested in different animal species via
different routes; no conclusive evaluation on the basis of
these studies could be made;
(b dichlorvos is an alkylating agent and binds to bacterial and
mammalian nucleic acids;
(c) it is a mutagen in a number of microbial systems, but there is
no evidence of its mutagenicity in mammals, in which it is
rapidly degraded.
In the evaluation by IARC, it was stated that "the available data
do not allow an evaluation of the carcinogenicity of dichlorvos to be
made".
In its series "Scientific Reviews of Soviet Literature on Toxicity
and Hazards of Chemicals", the International Register of Potentially
Toxic Chemicals has published a volume on dichlorvos (IRPTC, 1984).
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O.G. (1970)
Pesticide residues in the total diet in England and Wales. III.
Organophosphorus pesticide residues in the total diet, 1966-67.
Pestic. Sci., 1: 10-13.
ACKERMAN, H., LEXOW, B., & PLEWKA, E. (1969) [Detection and
identification of the organic phosphate, phosphorothionate,
phosphonate, and carbamate insecticides in biological material.]
Arch. Toxicol., 24: 316-324 (in German).
ADLER, B., BRAUN, R., SCHONEICH, J., & BOHME, H. (1976) Repair-
defective mutants of Proteus mirabilis as a prescreening system for the
detection of potential carcinogens. Biol. Zentralbl., 95: 463-469.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides wetting agents and miscellaneous
substances. Int. pest Control, 11(2): 25-35.
ALDRIDGE, W.N. & BARNES, J.M. (1966) Further observations on the
neurotoxicity of organophosphorus compounds. Biochem. Pharmacol., 15:
541-548.
ALDRIDGE, W.N. & JOHNSON, M.K. (1971) Side effects of organo-
phosphorus compounds: delayed neurotoxicity. Bull. World Health
Organ., 44: 259-263.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and structure-
activity relationships providing a high safety factor for anti-
cholinesterase insecticides. In: Proceedings of the 1977 British Crop
Protection Conference - Pests and Diseases, Croyden, British Crop
Protection Council. pp. 721-729.
ALI, M.S., RAHMATULLAH, M., JAHAN, R., YUSUF, H.K.M., &
CHOWDHURY, A.A. (1979c) Effect of DDVP (insecticide) on citric acid
fermentation in Aspergillus niger. Enzyme Microb. Technol., 1: 127-
128.
ALI, S.F. & HASAN, M. (1977) Effect of organophosphate insecticide
dichlorvos on the amino acid content of different regions of the rat
brain and spinal cord. Indian J. exp. Biol., 15(9): 759-761.
ALI, S.F., HASAN, M., & MITRA, S.C. (1979a) Organophosphate DDVP-
induced changes in the rat spinal cord: an electron microscopic and
biochemical study. J. Anat. Soc. India, 28(1): 36.
ALI, S.F., HASAN, M., & TARIQ, M. (1979b) Levels of dopamine,
norepinephrine, and 5-hydroxytryptamine in different regions of rat
brain and spinal cord following chronic administration of organo-
phosphate pesticide dichlorvos. Indian J. exp. Biol., 17(4): 424-426.
ALI, S.F., CHANDRA, O., & HASAN, M. (1980) Effects of an
organophosphate (dichlorvos) on open-field behaviour and locomotor
activity: correlation with regional brain monoamine
levels. Psychopharmacology, 68(1): 37-42.
ALLEN, S.D., VANKAMPEN, K.R., & BROOKS, D.R. (1978) Evaluation of
the feline dichlorvos (DDVP) flea collar. Feline Pract., 8(3): 9-16.
AMBRUS, A., LANTOS, J., VISI, E., CSATLOS, I., & SARVARI, L. (1981)
General method for determination of pesticide residues in samples of
plant origin, soil, and water. J. Assoc. Off. Anal. Chem., 64(3):
733-768.
ANDERSON, R.A., BARSTAD, J.A.B., & LAAKE, K. (1978) An improvement
of the spectrophotometric method for determination of cholinesterase
activity in erythrocytes and tissue homogenates. Ann. Natl Inst. Publ.
Health (Norway), 1(1): 51-55.
ANON. (1972) Vapona insecticide. In: Analytical methods for
pesticides and plant growth regulators, Modesto, California, Shell
Development Company, Vol. 6, pp. 529-541.
ANON. (1973) The determination of malathion and dichlorvos residues
in grain. Report by the Panel on Malathion and Dichlorvos Residues in
Grain. Analyst, 98: 19-24.
ANON. (1977) Determination of residues of organophosphorus pesticides
in fruits and vegetables. Report by the Panel on Determination of
Residues of Certain Organophosphorus Pesticides in Fruits and
Vegetables. Analyst, 102: 858-868.
ANON. (1982) Pesticides: a safety guide, London, Shell International
Chemical Company Ltd, pp. 53-55.
AOKI, Y., TAKEDA, M., & UCHIYAMA, M. (1975) Comparative study of
methods for the extraction of eleven organophosphorus pesticide
residues in rice. J. Assoc. Off. Agric. Chem., 58(6): 1286-1293.
AQUILINA, G., BENIGNI, R., BIGNAMI, M., CALCAGNILE, A.,
DOGLIOTTI, E., FALCONE, E., & CARERE, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15:
439-442.
ARATAKE, Y. & KAYAMURA, T. (1973) [Toxicity of insecticides to
silkworm larvae.] Sanshi Kenkyu (Acta serol.), 87: 68-78 (in
Japanese).
ARIMATSU, S., HOSHIRO, Y., & NOMURA, T. (1977) [Studies on primary
irritation test of pesticides in rabbits.] Nippon Noson Igakkai
Zasshi, 26: 572-573 (in Japanese).
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees, Riverside,
University of California (Agricultural Extension Report No. M-16 Rev.
9/73).
AUGUSTINSSON, K.B., ERIKSSON, H., & FAIJERSSON, Y. (1978) A new
approach to determining cholinesterase activities in samples of whole
blood. Clin. Chim. Acta, 89: 239-252.
AULICINO, F., BIGNAMI, M., CARERE, A., CONTI, G., MORPURGO, G.,
& VELCICH, A. (1976) Mutational studies with some pesticides
in Aspergillus nidulans. Mutat. Res., 38(2): 138.
BAKSI, S.N. (1978) Effect of dichlorvos on embryonal and fetal
development in thyro-parathyroidectomized thyroxine-treated and
euthyroid rats. Toxicol. Lett., 2(4): 213-216.
BALLINGTON, P.E., SKIPPER, H.D., & HEGG, R.O. (1978) Response of
coliform populations in poultry waste digesters to three
insecticides. J. environ. Qual., 7(2): 262-264.
BARTHEL, E. (1983) [Irritative and allergic effects of pesticide
aerosols on the respiratory tract and problems of their evaluation.] Z.
gesamte Hyg., 29(11): 678-681 (in German).
BATORA, V., VITOROVIC, S.L.J., THIER, H.P., & KLISENKO, M.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BATTE, E.G., ROBINSON, O.W., & MONCOL, D.J. (1969) Influence of
dichlorvos on swine reproduction and performance of offspring to
weaning. J. Am. Vet. Med. Assoc., 154(11): 1397.
BAZER, F.W., ROBSON, O.W., & ULBERG, L.C. (1969) Effect of dichlorvos
and PMS on reproduction in swine. J. anim. Sci., 28: 145.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2(4): 307-337.
BELL, TH.G., FARRELL, K., PADGET, G.A., & LEENDERTSEN, L.W.
(1975) Ataxia, depression, and dermatitis associated with the use of
dichlorvos-impregnated collars in the laboratory cat. J. Am. Vet.
Med. Assoc., 167(7): 575-586.
BELLIN, J.S. & CHOW, I. (1974) Biochemical effects of chronic low-
level exposure to pesticides. Res. Commun. chem. Pathol.
Pharmacol., 9(2): 325-337.
BENIGNI, R. & DOGLIOTTI, E. (1980a) UDS studies on selected
environmental chemicals. Mutat. Res., 74: 248-249.
BENIGNI, R. & DOGLIOTTI, E. (1980b) UDS studies on selected
environmental chemicals. Mutat. Res., 74(3): 217.
BENIGNI, R., BIGNAMI, M., CAMONI, I., CARERE, A., CONTI, G.,
IACHETTA, R., MORPURGO, G., & ORTALI, V.A. (1979) A new in
vitro method for testing plant metabolism in mutagenicity studies. J.
Toxicol. environ. Health, 5: 809-819.
BERAN, F. (1970) [Present knowledge on the toxicity of our pesticides
to bees.] Gesunde Pflanz., 22(2): 21-31 (in German).
BIGNAMI, M., CONTI, L., MORPURGO, G., & VELCICH, A. (1976)
Comparative analysis of different test systems for somatic
recombination with Aspergillus nidulans. Mutat. Res., 38(2): 138-139.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of pesticides
in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BISBY, J.A. & SIMPSON, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Aust., 2: 394-395.
BLAIR, D. & RODERICK, H.R. (1976) An improved method for the
determination of urinary dimethyl phosphate. J. agric. food
Chem., 24(6): 1211-1223.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The distribution of
dichlorvos in the tissues of mammals after its inhalation or
intravenous administration. Toxicol. appl. Pharmacol., 31: 243-253.
BLAIR, D., DIX, K.M., HUNT, P.F., THORPE, E., STEVENSON, D.E., &
WALKER, A.I.T. (1976) Dichlorvos: a 2-year inhalation carcinogenesis
study in rats. Arch. Toxicol., 35: 281-294.
BOOTSMA, D., HEERING, H., KLEIJER, W.J., BUDKE, L., DE JONG,
L.P.A., & BERENDS, F. (1971) Effects of dichlorvos on human cells in
tissue culture. A progress report, Rijswijk, The Netherlands, Medical
Biological Laboratory TNO (Report No. MBL 1971-5).
BOUSH, G.M. & MATSUMURA, F. (1967) Insecticidal degradation by
Pseudomonas melophthora, the bacterial symbiote of the apple
maggot. J. econ. Entomol., 60: 918-920.
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol- water
partitioning coefficients (Kow) of 61 organophosphorus and carbamate
insecticides and their relationships to respective water solubility (S)
values. J. environ. Sci. Health, B18(6): 667-683.
BOYER, A.C., BROWN, L.J., SLOMKA, M.B., & HINE, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. appl. Pharmacol., 41: 389-
394.
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison of
cholinesterase activity, residue levels, and urinary metabolite
excretion of rats exposed to organophosphorus pesticides. J. agric.
food Chem., 25Z(6): 1353-1358.
BRAUN, R., SCHONEICH, J., WEISSFLOG, L., & DEDEK, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair: direct alkylation vs metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
desmethyldichlorvos, and demethylvinyl-butonate. Chem.-biol.
Interact., 39: 339-350.
BRIDGES, B.A., MOTTERSHEAD, R.P., GREEN, M.H.L., & GRAY, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulfonate
for Escherichia coli WP2 and some derivatives deficient in DNA
repair. Mutat. Res., 19: 295-303.
BROWN, V.K.H. & ROBERTS, M. (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to guinea-
pigs, Sittingbourne, Shell Research Ltd (Unpublished Report No. IRR
TL/40/66).
BROWN, V.K.H. & STEVENSON, D.E. (1962) Agricultural chemicals:
therapy of DDVP intoxication by atropine and cholinesterase
reactivators, Sittingbourne, Shell Research Ltd (Shell Technical
Memorandum No. TOX 31/62) (Unpublished Report).
BROWN, V.K.H., BLAIR, D., HOLMES, D.L., & PICKERING, R.G. (1968)
The toxicity of low concentrations of dichlorvos by inhalation in
rodent and avian species, Sittingbourne, Shell Research Ltd
(Unpublished Report No. TLGR.0015.68).
BRUNGS, W.A., MCCORMICK, J.H., NEIHEISEL, T.W., SPEHAR, R.L.,
STEPHAN, C.E., & STOKES, G.N. (1977) Effects of pollution on fresh-
water fish. J. Water Pollut. Control Fed., 49(6): 1425-1493.
BRYANT, R.J. & MINETT, W. (1978) A gas chromatographic method for the
determination of dichlorvos in air. Pestic. Sci., 9: 525-528.
BRZEZINSKI, J. & WYSOCKA-PARUSZEWSKA, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., 4(Suppl.): 475-478.
BULL, D.L. & RIDGWAY, R.L. (1969) Metabolism of trichlorfon in
animals and plants. J. agric. food Chem., 17(4): 837-841.
BUNDING, I.M., YOUNG, R., Jr, SCHOOLEY, M.A., & COLLINS, J.A.
(1972) Maternal dichlorvos effects on farrowing parameters. J. anim.
Sci., 35: 238.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1972) [Mutagenicity
investigations with pesticides in the host-mediated assay and the
dominant lethal test in mice.] Biol. Zentralbl., 91(3): 311-325 (in
German).
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973) Comparative
investigations on the mutagenicity of pesticides in mammalian test
systems. Mutat. Res., 21(1): 25-26.
BUTLER, G.L. (1977) Algae and pesticides. Residue Rev., 66: 40.
CARERE, A., CARDAMONE, G., ORTALI, V., BRUZZONE, M.L., & DI
GIUSEPPE, G. (1976) Mutational studies with some pesticides
in Streptomyces coelicolor and Salmonella typhimurium. Mutat.
Res., 38(2): 136.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978a) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARERE, A., ORTALI, V.A., CARDAMONE, G., & MORPURGO, G.
(1978b) Mutagenicity of dichlorvos and other structurally-related
pesticides in Salmonella and Streptomyces. Chem.-biol. Interact., 22:
297-308.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett., 9:
157-159.
CARSON, S. (1969) Teratology studies in rabbits (summary
appraisal), Maspeth, New York, Food and Drug Research Laboratories
(Unpublished Report, 30 June).
CARTER, M.K. & MADDUX, B. (1968) Effects of selected agents on the
in vitro inhibition of cholinesterase activity of dichlorvos.
Pharmacologist, 10: 222.
CARTER, M.K. & MADDUX, B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. appl. Pharmacol., 27: 456-463.
CASIDA, J.E., MCBRIDE, L., & NIEDERMEIER, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. agric. food Chem., 10(5): 370-377.
CAVAGNA, G. & VIGLIANI, E.C. (1970) Problčmes d'hygične et de
sécurité dans l'emploi du vapona comme insecticide dans les locaux
domestiques. Med. Lav., 61(8/9): 409-423.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch. environ.
Health, 19(1): 112-113.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1970) Exposure of new-
born babies to Vapona insecticide. Eur. J. Toxicol., 3(1): 49-57.
CERVONI, W.A., OLIVER-GONZALEZ, J., KAYE, S., & SLOMKA, M.B.
(1969) Dichlorvos as a single-dose intestinal anthelmintic therapy for
man. Am. J. trop. Med. Hyg., 18(6): 912-919.
CHATTOPADHYAY, D.P., DIGHE, S.K., DUBE, D.K., & PURNANAND
(1982) Changes in toxicity of DDVP, DFP, and parathion in rats under
cold environment. Bull. environ. Contam. Toxicol., 29: 605-610.
CHEN, Z.M., ZABIK, M.J., & LEAVITT, R.A. (1984) Comparative study of
thin film photodegradative rates for 36 pesticides. Ind. eng. chem.
Prod. Res. Dev., 23: 5-11.
CIPAC HANDBOOK (1980) Analysis of technical and formulated
pesticides, Cambridge, Wheffer and Sons, Collaborative International
Pesticides Analytical Council, Vol. 1A, pp. 1214-1224 (Addendum to
CIPAC 1).
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN, R.J.
(1980) Effects of low level administration of dichlorvos on adreno-
corticotrophic hormone secretion, adrenal cholesteryl ester, and
steroid metabolism. Biochem. Pharmacol., 29: 635-641.
CLINCH, P.G. (1970) Effect on honey bees of combs exposed to vapour
from dichlorvos slow-release strips. N.Z. J. agric. Res., 13(2): 448-
452.
COCHRANE, W.P. (1979) Application of chemical derivatisation
techniques for pesticide analysis. J. chromatogr. Sci., 17: 124-137.
CODEX ALIMENTARIUS COMMISSION (1979) Recommended method of
sampling for the determination of pesticide residues. Report of the
Tenth Session of the Codex Committee on Pesticide Residues, Rome, Food
and Agriculture Organization of the United Nations, Appendix IV, Annex
I, pp. 67-70 (Alinorm 79/24).
CODEX ALIMENTARIUS COMMISSION (1983) Guidelines on good
analytical practice in residue analysis and recommendations for methods
of analysis for pesticide residues, Rome, Food and Agriculture
Organization of the United Nations, p. 40.
COHEN, S.D. & EHRICH, M. (1976) Cholinesterase and carboxyl-esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. appl. Pharmacol., 37: 39-48.
COLLINS, J.A., SCHOOLEY, M.A., & SINGH, V.K. (1971) The effect of
dietary dichlorvos on swine reproduction and viability of their
offspring. Toxicol. appl. Pharmacol., 19: 377.
COLLINS, R.D. & DE VRIES, D.M. (1973) Air concentrations and food
residues from use of Shell No-PestR Insecticide Strip. Bull.
environ. Contam. Toxicol., 9(4): 227-233.
COSTA, L.G. & MURPHY, S.D. (1984) Interaction between acetaminophen
and organophosphate in mice. Chem. Pathol. Pharmacol., 44(3): 389-400.
COULSTON, F. & GRIFFIN, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP
vapours, Albany, New York, Institute of Comparative and Human
Toxicology and Holloman Air Force Base, International Center of
Environmental Safety (Unpublished report).
CRONCE, P.C. & ALDEN, H.S. (1968) Flea-collar dermatitis. J. Am.
Med. Assoc., 206(7): 1563-1564.
DALE, W.E., MILES, J.W., & WEATHERS, D.B. (1973) Measurement of
residues of dichlorvos absorbed by food exposed during disinsection of
aircraft. J. agric. food Chem., 21(5): 858-860.
DAMBSKA, M. & MASLINSKA, D. (1982) Effect of dichlorvos (DDVP)
intoxication of rabbit brain. Neuropathol. Pol. 20(1/2): 77-84.
DAMBSKA, M., MASLINSKA, D., RAKOWSKA, I., & RUTCZYNSKI, M.
(1978) [Evaluation of the effect of dichlorvos poisoning of pregnant
rabbits and rats on the condition and development of their
offspring.] Bromatol. Chem. Toksykol., 11(3): 355-357 (in Polish).
DAMBSKA, M., IWANOWSKI, L., & KOZLOWSKI, P. (1979) The effect of
transplacental intoxication with dichlorvos on the development of
cerebral cortex in newborn rabbits. Neuropathol. Pol. 17(4): 571-576.
DAMBSKA, M., IWANOWSKI, L., MASLINSKA, D., & OSTENDA, M. (1984)
Blood-brain barrier in young rabbit brain after dichlorvos
intoxication. Neuropathol. Pol. 22(1): 129-137.
DARROW, D.I. (1973) Biting lice of goats: control with dichlorvos-
impregnated resin neck collars. J. econ. Entomol., 66: 133-135.
DAS, Y.T., TASKAR, P.K., BROWN, H.D., & CHATTOPADHYAY, S.K.
(1983) Exposure of professional pest control operator to dichlorvos
(DDVP) and residue on house structures. Toxicol. Lett., 17: 95-99.
DAVIDEK, J., SEIFERT, J., & DOLEZALOVA, Z. (1976) The determination
of dichlorvos in milk by using classical and square-wave
polarography. Milchwissenschaft, 31(5): 267-270.
DEAN, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on microorganisms. Arch. Toxikol., 30: 67-74.
DEAN, B.J. (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol., 30: 75-85.
DEAN, B.J. & BLAIR, D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres containing
dichlorvos. Mutat. Res., 40(1): 67-72.
DEAN, B.J. & THORPE, E. (1972a) Cytogenic studies with dichlorvos in
mice and Chinese hamsters. Arch. Toxikol., 30: 39-49.
DEAN, B.J. & THORPE, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxikol., 30: 51-59.
DEAN, B.J., DOAK, S.M.A., & FUNNELL, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium
using Saccharomyces cerevisiae. Arch. Toxikol., 30: 61-66.
DEDEK, W., GEORGI, W., & GRAHL, R. (1979) Comparative degradation and
metabolism of 32P-labelled butonate, trichlorphone and dichlorvos in
crop plants. Biochem. Physiol. Pflanz., 174: 707-722.
DEGRAEVE, N., GILOT-DELHALLE, J., MOUTSCHEN, J.,
MOUTSCHENDAHMEN, M., COLIZZI, A., CHOLLET, M., &
HOUBRECHTS, N. (1980) Comparison of the mutagenic activity of
organophosphorus insecticides in mouse and in the
yeast Schizosaccharomyces pombe. Mutat. Res., 74(3): 201-202.
DEGRAEVE, N., CHOLLET, M.C., MOUTSCHEN, J., MOUTSCHEN-
DAHMEN, M., GILOT-DELHALLE, J., & COLIZZI, A. (1982) Genetic and
cytogenetic effects of chronic treatment with organophosphorus
insecticides. Mutat. Res., 97: 179-180.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984a) Cytogenetic
and genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56: 66-67.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Lett., 21: 315-319.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I., VARGA, L., & FARKAS, J. (1978) Studies on the
immunosuppressive effect of organochlorine and organophosphoric
pesticides in subacute experiments. J. Hyg. Epidemiol. Microbiol.
Immunol., 22: 115-122.
DESI, I., VARGA, L., & FARKAS, J. (1980) The effect of DDVP, an
organophosphorus pesticide, on the humoral and cell-mediated immunity
of rabbits. Arch. Toxicol., 4(Suppl.): 171-174.
DICOWSKY, L. & MORELLO, A. (1971) Glutathione-dependent degradation
of 2,2-dichlorovinyl dimethyl phosphate (DDVP) by the rat. Life
Sci., 10(2): 1031-1037.
DIKSHITH, T.S.S., DATTA, K.K., & CHANDRA, P. (1976) 90-day dermal
toxicity of DDVP in male rats. Bull. environ. Contam. Toxicol., 15(5):
574-580.
DOUGHERTY, E.M., REICHELDERFER, C.F., & FAUST, R.M. (1971)
Sensitivity of Bacillus thuringiensis var. thuringiensis to various
insecticides and herbicides. J. invertebr. Pathol., 17: 292-293.
DMITRIEV, A.I. & KOZEMJAKJN, N.G. (1975) [Veterinary expert report on
the meat of chicken poisoned by dichlorvos.] Veterinarija, 1975(1):
90-92 (in Russian).
DRAGER, G. (1968) [Gas chromatographic method for the determination
of dichlorvos residues in plants and milk.] Pflanzenschutz Nachr.
Bayer, 21(3): 377-384 (in German).
DRAUGHON, F.A. & AYRES, J.C. (1978) Effect of selected pesticides on
growth and citrinin production by Penicillium citrinum. J. food
Sci., 43: 576-578.
DURHAM, W.F., GAINES, TH.B., & HAYES, W.J. (1956) Paralytic and
related effects of certain organic phosphorus compounds. Arch. ind.
Health, 13: 326-330.
DURHAM, W.F., GAINES, TH.B., MCCAULEY, R.H., Jr, SEDLAK, V.A.,
MATTSON, A.M., & HAYES, W.J., Jr (1957) Studies on the toxicity
of O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP). Am. Med. Assoc.
Arch. Ind. Health, 15: 340-349.
DYER, K.F. & HANNA, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res., 21: 175-177.
DZWONKOWSKA, A. & HUBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo. Arch.
Toxicol., 58: 152-156.
EDSON, E.F. (1958) Blood tests for users of organophosphorus
insecticides. World Crops, 10 February: 49-51.
EGYED, M.N. & BENDHEIM, U. (1977) Mass poisoning in chickens by
consumption of organophosphorus (dichlorvos) contaminated drinking-
water. Refu. Vet., 34(3): 107-111.
EICHNER, M. (1978) [Quick residue control of plant and animal food,
tobacco, and tobacco products by Sweep-Co analysis 1.] Z. Lebensm.
Unters. Forsch., 167: 245-249 (in German).
EISLER, R. (1969) Acute toxicities of insecticides to marine decapod
crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish, Washington DC, US
Department of the Interior, Fish and Wildlife Service, Bureau of Sport
Fisheries and Wildlife (Technical Paper No. 46).
ELGAR, K.E. & STEER, B.D. (1972) Dichlorvos concentrations in the air
of houses arising from the use of dichlorvos PVC strips. Pestic.
Sci., 3: 591-600.
ELGAR, K.E., MARLOW, R.G., & MATHEWS, B.L. (1970) The determination
of residues of dichlorvos in crops and tissues. Analyst, 95: 875-878.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972a) Dichlorvos residues
in food arising from the domestic use of dichlorvos PVC strips. Pestic.
Sci., 3: 601-607.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972b) Vapona strips in
shops: residues in foodstuffs. Global aspects of chemistry, toxicology,
and technology as applied to the environment. Environ. Qual. Saf., 1:
217-221.
ELLINGER, CH. (1985) Enzyme activities after in vitro and in
vivo application of dichlorvos. Biomed. Biochim. Acta, 44(2): 311-
316.
ELLINGER, CH., KAISER, G., & HÖRING, H. (1985) [Haematological
changes to rats following exposure to dichlorvos.] Nahrung, 29(4): 351-
355 (in German).
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
ENOMOTO, M.F., NAKADATE, M., NINOMIYA, K., HAYAKAWA, Y.,
ITO, H., IGARASHI, S., UWANUMA, Y., NAKASATO, R., & HATANAKA,
J. (1981) Studies on carcinogenicity of DDVP (2,2-dichlorovinyl
dimethyl phosphate) mixed in drinking-water in rats, Tokyo, Ministry of
Health and Welfare (Cooperative Studies on Carcinogenicity Test on
Mutagens) (unpublished).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERIKSSON, H. & FAYERSSON, Y. (1980) A reliable way of estimating
cholinesterases from whole blood in the presence of
anticholinesterases. Clin. Chim. Acta, 100: 165-171.
ESCUDIE, E. & SALES, P. (1963) Premičres expériences en Haute Volta
sur le dichlorvos résiduel. Bull. World Health Organ., 29: 247-249.
FAHRIG, R. (1973) [Genetic effects of organophosphorus
insecticides.] Naturwissenschaften, 60(1): 50-51 (in German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomotis, L., ed. Chemical carcinogenesis essays,
Lyons, International Agency for Research on Cancer, pp. 161-181 (IARC
Scientific Publication No. 10).
FAO (1977) FAO Specifications for plant protection products,
dichlorvos and fenthion, Rome, Food and Agriculture Organization of the
United Nations.
FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10/1; WHO Food
Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Report of the Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL/1962/M/11; WHO Technical Report Series
No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1967/M11/1; WHO Food
Add./68.30).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1969 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (AGP 1974/M/11; WHO Pesticide Residue
Series No. 4).
FAO/WHO (1977a) Pesticide residues in food. Report of the 1976 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and the
Environment and the WHO Expert Group on Pesticide Residues, Geneva,
World Health Organization (FAO Food and Nutrition Series No. 9; FAO
Plant Production and Protection Series No. 8; WHO Technical Report
Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(ADP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977 Joint
Meeting on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 10
Rev.).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FISHBEIN, L. (1976) Potential hazards of fumigant residues.
Environ. Health Perspect., 14: 39-45.
FISHBEIN, L. (1981) Environmental sources of chemical mutagens. II.
Synthetic mutagens. In: Flamm, W.G. & Mehlman, M.A., ed. Mutagenesis:
advances in modern toxicology, Tokyo, Mishima and Kyoto, Vol. 5, pp.
257-348.
FISHBEIN, L. (1982) An overview of the structural features of
mutagenic S-chlorallylthio and dithiocarbamate pesticides and
trichlorfon, dichlorvos, and their metabolites. In: Proceedings of the
3rd International Conference on Environmental Mutagens and Carcinogens,
21-27 September 1981, Tokyo, Mishima and Kyoto, pp. 371-378.
FISCHER, G.W., SCHNEIDER, P., & SCHEUFLER, H. (1977) [Mutagenicity
of dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester: possible metabolites of the organophosphorus
pesticide trichlorphon.] Chem.-biol. Interact., 19: 205-213 (in
German).
FOLL, C.V., PANT, C.P., & LIETAERT, P.E. (1965) A large-scale field
trial with dichlorvos as a residual fumigant insecticide in northern
Nigeria. Bull. World Health Organ., 32: 531-550.
FOURNIER, E., DALLY, S., & CAMBIER, J. (1976) Neuropathie
périphérique probablement due aux insecticides anticholinesté-
rasiques. Nouv. Presse méd., 5(11): 718.
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Sci. Health, B20(1): 73-95.
FRANK, R., BRAUN, H.E., & FLEMING, G. (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969-81. J. food Prot., 46: 893-900.
FUJITA, K., MATSUSHIMA, S., ABE, E., SASAKI, K., & KUROSAWA, K.
(1977) [Examination of the effects of dichlorvos on the
testis.] Nippon Noson Igakkai Zasshi, 26(3): 328-329 (in Japanese).
FUJITA, Y. (1985) Studies on contact dermatitis from pesticides in
tea growers. Acta Med. Univ. Kagoshima, 27(1): 17-37.
FUNCKES, A.J., MILLER, S., & HAYES, W.J., Jr (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 243-246.
GAINES, TH.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14(3): 515-534.
GAINES, TH.B., HAYES, W.J., Jr, & LINDER, R.E. (1966) Liver
metabolism of anticholinesterase compounds in live rats: relation to
toxicity. Nature (Lond.), 209: 88-89.
GIFAP (1982) Guidelines on pesticide residues to provide data for the
registration of pesticides and the establishment of maximum residue
limits elaborated by Codex Committee on Pesticide Residues, Brussels,
International Group of National Associations of Manufacturers of
Agrochemical Products (Technical Monograph No. 4).
GILLENWATER, H.B., HAREIN, P.K., LOY, E.W., Jr, THOMPSON, J.F.,
LAUDANI, H., & EASON, G. (1971) Dichlorvos applied as a vapour in a
warehouse containing packaged foods. J. stored Prod. Res., 7: 45-56.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health hazards
on use of dichlorvos (DDVP), especially in resin strips. Residue
Rev., 44: 115-159.
GILOT-DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN-
DAHMEN, M. (1983) Mutagenicity of some organophosphorus compounds at
the ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117: 139-
148.
GOH, K.S., EDMISTON, S., MADDY, K.T., & MARGETICH, S. (1986a)
Dissipation of dislodgeable foliar residue for chlorpyrifos and
dichlorvos treated lawn: implication for safe reentry. Bull. environ.
Contam. Toxicol., 37: 33-40
GOH, K.S., EDMISTON, S., MADDY, K.T., MEINDERS, D.D., &
MARGETICH, S. (1986b) Dissipation of dislodgeable foliar residue of
chlorpyrifos and dichlorvos on turf. Bull. environ. Contam.
Toxicol., 37: 27-32.
GOLD, R.E., HOLCSLAW, T., TUPY, D., & BALLARD, J.B. (1984) Dermal
and respiratory exposure to applicators and occupants of residences
treated with dichlorvos (DDVP). J. econ. Entomol., 77: 430-436.
GOTO, S. (1977) [Variation of concentrations of pesticide residues in
field soil.] J. Pestic. Sci., 2: 319-321. (in Japanese, with English
summary).
GOUGH, B.J. & SHELLENBERGER, T.E. (1970) In vivo rabbit blood
cholinesterase inhibition and reactivation following organophosphate
infusion. Toxicol. appl. Pharmacol., 17(1): 302.
GOUGH, B.J. & SHELLENBERGER, T.E. (1977-78) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors and
reactivation with oximes. Drug Chem. Toxicol., 1(1): 25-43.
GRAHL, K. (1979) [The persistence of trichlorfon and dichlorvos in
ponds.] Z. Binnenfisch. DDR, 25(10): 312-316 (in German).
GRAHL, K., STELZER, W., & STOTTMEISTER, S. (1980) [Toxicity of
butonate, trichlorfon, and dichlorvos to Escherichia coli and
Enterobacter aerogenes.] Acta hydrochim. hydrobiol., 8: 19-28 (in
German).
GRAHL, K., HORN, H., & HALLEBACH, R. (1981) [Effect of butonate,
trichlorfon, and dichlorvos on plankton populations.] Acta hydrochim.
hydrobiol., 9(2): 147-161 (in German).
GRATZ, N.G., BRACHA, P., & CARMICHAEL, A. (1963) A village- scale
trial with dichlorvos as a residual fumigant insecticide in southern
Nigeria. Bull. World Health Organ., 29: 251-270.
GREEN, M.H.L., BRIDGES, B.A., GRAY, W.J.H., & MOTTERSHEAD, R.P.
(1973) Comparison of DNA damage caused by dichlorvos and methyl
methanesulfonate in pol A and pol A+ strains of Escherichia coli.
Genetics, 74: s100.
GREEN, M.H.L., MEDCALF, A.S.C., ARLETT, C.R., HARCOURT, S.A., &
LEHMANN, A.R. (1974a) DNA strand breakage caused by dichlorvos, methyl
methane-sulfonate and iodoacetamide in Escherichia coli and cultured
Chinese hamster cells. Mutat. Res., 24(3): 365-378.
GREEN, M.H.L., MEDCALF, A.S.C., & STEVENS, S.W. (1974b) Apparent
indirect DNA damage by dichlorvos and iodoacetamide in Escherichia
coli. Heredity, 33: 446.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens. Mutat.
Res., 38(1): 33-42.
GRIFFIN, D.E., III & HILL, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRUBNER, P. (1972) [Residue problems in the use of phosphoric acid
ester insecticides in mushroom cultures.] Nachrichtenbl.
Pflanzenschutzdienst (DDR), 26: 245-247 (in German).
GUNDERSON, E.C. (1981) Sampling methods for airborne pesticides,
Washington, DC, American Chemical Society, pp. 301-315 (ACS Symposium
Series No. 149).
GUPTA, A.K. & SINGH, J. (1974) Dichlorvos (DDVP) induced breaks in the
salivary gland chromosomes of Drosophila melanogaster. Curr.
Sci., 43(20): 661-662.
GUPTA, R.C., DAVE, S.K., SHAH, M.P., & KASHYAP, S.K. (1979) A
monitoring study of workers handling pesticides in warehouses and
godowns. J. environ. Sci. Health, B14(4): 405-416.
HALEY, T.J., FARMER, J.H., HARMON, J.R., & DOOLEY, K.L. (1975)
Estimation of the LD1 and extrapolation of the LD0.1 for five
organophosphate pesticides. Arch. Toxicol., 34: 103-109.
HANNA, P.J. & DYER, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res., 28: 405-420.
HASAN, M. & ALI, S.F. (1980) Organophosphate pesticide dichlorvos-
induced increase in the rate of lipid peroxidation in the different
regions of the rat brain: supporting ultra-structural
findings. Neurotoxicology, 2: 43-52.
HASAN, M., MAITRA, S.C., & ALI, S.F. (1979) Organophosphate pesticide
DDVP-induced alterations in the rat cerebellum and spinal cord: an
electron microscopic study. Exp. Pathol., 17(2): 88-94.
HASS, D.K., COLLINS, J.A., & KODAMA, J.K. (1972) Effects of orally
administered dichlorvos in rhesus monkeys. J. Am. Vet. Med.
Assoc., 161(6): 714-719.
HATCH, G.G., ANDERSON, T.M., HUBERT, R.A., KOURI, R.E.,
PUTMAN, D.L., CAMERON, J.W., NIMS, R.W., MOST, B., SPALDING, J.W.,
TENNANT, R.W., & SCHECHTMAN, L.M. (1986) Chemical enhancement of
sa7 virus transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagenesis.,
8: 515-531.
HATTORI, K., SATO, H., TSUCHIYA, K., YAMAMOTO, N., & OGAWA, E.
(1974) [Toxicological studies on the influences of chemicals to the
birds. I. Oral acute toxicity and cholinesterase inhibition of three
organophosphate insecticides in Japanese quail.] Hokkaidoritsu Eisei
Kenkyusho Ho, 24: 35-38 (in Japanese, with English summary).
HAYES, A.L., WISE, R.A., & WEIR, F.W. (1980) Assessment of
occupational exposure to organophosphates in pest control
operators. Am. Ind. Hyg. Assoc. J., 41(8): 568-575.
HAYES, W.J., Jr (1961) Safety of DDVP for the disinsection of
aircraft. Bull. World Health Organ., 24: 629-633.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons:
emergency information for treating poisoning, Atlanta, Georgia, US
Department of Health, Education and Welfare, Public Health Service,
Communicable Diseases Center (Public Health Service Publication No.
476).
HAYES, W.J., Jr (1982) Organic phosphorus pesticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and Wilkins,
pp. 343-351.
HAZELWOOD, J.C., STEFAN, G.E., & BOWEN, J.M. (1979) Motor unit
irritability in beagles before and after exposure to cholinesterase
inhibitors. Am. J. vet. Res., 40(6): 852-856.
HEMMINKI, K. (1983) Nucleic acid adducts of chemical carcinogens and
mutagens. Arch. Toxicol., 52: 249-285.
HENRIKSSON, K., KALLELA, K., VIRTAMO, M., & PFAFFLI, P. (1971)
[The toxicity of DDVP (dichlorvos) evaporated from Vapona strip.] J.
Sci. Agric. Soc. Finl., 43: 187-200 (in Finnish).
HEUSER, S.G. & SCUDAMORE, K.A. (1966) A rapid method for sampling
dichlorvos vapour in air. Chem. Ind., 50: 2093-2094.
HEUSER, S.G. & SCUDAMORE, K.A. (1975) A method for the assessment
under standard conditions of the output of dichlorvos slow-release
units used for insect control. Analyst, 100: 129-135.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp. 1-51
(Special Scientific Report: Wildlife No. 191).
HINE, C.H. (1962) 90-day chronic toxicity studies of
VaponaR insecticide for dogs, San Francisco, California, The Hine
Laboratories (Unpublished Report No. 1, 1 September).
HINE, C.H. & SLOMKA, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
HINE, C.H. & SLOMKA, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. appl. Pharmacol., 17(1):
304-305.
HODGSON, E. & CASIDA, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethylphosphate. J. agric. food
Chem., 10(3): 208-214.
HOLMSTEDT, B., NORDGREN, I., SANDOZ, M., & SUNDWALL, A. (1978)
Metrifonate. Summary of toxicological and pharmacological information
available. Arch. Toxicol., 41: 3-29.
HORIUCHI, N. & ANDO, Y. (1978) [Photosensitivity caused by
pesticides.] (original unpublished information used as a basis for
article by Horiuchi et al., 1978) (in Japanese).
HORIUCHI, N., ANDO, S., & SUZUKI, A. (1978) [Photosensitization due
to pesticides.] Nippon Noson Igakkai Zashi, 27(3): 450-451 (in
Japanese).
HORVATH, J., HEGATH, V., & PUCHA, K. (1968) A study of the influence
of cholinesterase with cattle housed in byres provided with DDVP
strips. Agrochemia, 8(9): 267-268.
HUNTER, C.G. (1969) Report on initial studies of deliberate exposures
to high concentrations of dichlorvos by human subjects, Sittingbourne,
Tunstall Laboratory Shell (Unpublished Report, July).
HUNTER, C.G. (1970a) Dichlorvos: inhalational exposures with human
subjects. Part 1, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0061.70).
HUNTER, C.G. (1970b) Dichlorvos: inhalational exposures with human
subjects. Part 2, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0067.70).
HUSSEY, N.W. & HUGHES, J.T. (1964) Investigations on the use of
dichlorvos in the control of the mushroom phorid Megaselia
halterata (Wood). Ann. appl. Biol., 54: 129-139.
HUTSON, D.H. & HOADLEY, E.C. (1972a) The comparative metabolism of
14C-vinyl-dichlorvos in animals and man. Arch. Toxikol., 30: 9-18.
HUTSON, D.H. & HOADLEY, E.C. (1972b) The metabolism of 14C-methyl-
dichlorvos in the rat and mouse. Xenobiotica, 2(2): 107-116.
HUTSON, D.H., BLAIR, D., HOADLEY, E.C., & PICKERING, B.A. (1971a)
The metabolism of 14C-VaponaR in rats after administration by oral
and inhalation routes. Toxicol. appl. Pharmacol., 19: 378-379.
HUTSON, D.H., HOADLEY, E.C., & PICKERING, B.A. (1971b) The
metabolic fate of vinyl-1-14C-dichlorvos in the rat after oral and
inhalation exposure. Xenobiotica, 1(6): 593-611.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K. (1978)
EEG, ECG, and respiratory response to acute insecticide exposure. Bull.
environ. Contam. Toxicol., 19: 47-55.
IARC (1979) Some halogenated hydrocarbons, Lyons, International Agency
for Research on Cancer, pp. 97-127 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IRPTC (1984) DDVP, Geneva, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme (Scientific
Reviews of Soviet Literature on Toxicity and Hazards of Chemicals, No.
79).
ISSHIKI, K., MIYATA, K., MATSUI, S., TSUTSUMI, M., & WATANABE, T.
(1983) [Effects of post-harvest fungicides and piperonyl butoxide on
the acute toxicity of pesticides in mice.] Shokuhin Eiseigaku
Zasshi, 24(3): 268-274 (in Japanese, with English summary).
IVEY, M.C. & CLABORN, H.V. (1969) GLC determination of dichlorvos in
milk, eggs and various body tissues of cattle and chickens. J. Assoc.
Off. Anal. Chem., 52(6): 1248-1251.
IWATA, Y., SUGITANI, A., & YAMADA, F. (1981) [An analytical method
for organophosphorus pesticides in the onion.] Shokuhin Eiseigaku
Zasshi, 22: 484-489 (in Japanese, with English summary).
JAKUBOWSKA, B. & NOWAK, A. (1973) [The effect of organophosphorus and
carbamate insecticides on the development of soil fungi.] Zesz. Nauk.
Akad. Roln. Szczecinie, 39(10): 141-150 (in Polish).
JAQUES, R. (1964) The protective effect of pyridine-2-aldoxime
methiodide (P2AM), bis-(4-hydroxyimino-methyl-pyridinium-1-methyl)-
ether dichloride (ToxogoninR) and atropine in experimental DDVP
poisoning. Helv. Physiol. Pharmacol. Acta, 22(3): 174-183.
JAYASURIYA, V.U., DE S. & RATNAYAKE, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inf. Serv., 50: 184-186.
JENSEN, J.A., FLURY, V.P., & SCHOOF, H.F. (1965) Dichlorvos vapour
disinsection of aircraft. Bull. World Health Organ., 32: 175-179.
JOHNSON, M.K. (1969) The delayed neurotoxic effect of some
organophosphorus compounds. Identification of the phosphorylation site
as an esterase. Biochem. J., 114(4): 711-717.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanisms and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing delayed
neurotoxic effects. Mechanism of action and structure/ activity
studies. Arch. Toxicol., 34: 259-288.
JOHNSON, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch.
Toxicol., 41: 107-110.
JOHNSON, M.K. (1981) Delayed neurotoxicity: do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol., 49(Suppl. 5): 87-98.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981a) Pesticide
metal and other chemical residues in adult total diet samples.
XII. Pestic. monit. J., 15(1): 54-69.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981b) Pesticide, heavy metal, and other chemical residues in infant
and toddler total diet samples. II. August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-50.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 137).
JOLLEY, W.P., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of VaponaR insecticide into their daily diet, Cincinnati,
Ohio, The Kettering Laboratory (Unpublished Report, 19 January).
KAWACHI, T., KOMATSU, T., KADA, T., ISHIDATE, M., SASAKI, M.,
SUGIYAMA, T., & TAZIMA, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In: G.M.
Williams et al., ed. The predictive value of short-term screening tests
in carcinogenicity evaluation, Amsterdam, Oxford, New York,
Elsevier/North Holland Biomedical Press, pp. 253-267.
KENAGA, E.E. (1979) Acute and chronic toxicity of 75 pesticide to
various animal species. Down Earth, 35(2): 25-31
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. environ.
Health, 16: 805-808.
KIMMERLE, G. & LORKE, D. (1968) [Toxicity of insecticidal phosphoric
acid esters.] Pflanzenschutz Nachr. Bayer, 21(1): 111-142 (in German).
KIRKLAND, V.L. (1971) Some aspects of acute inhalation pharmacology
of dichlorvos in swine. Paper presented at: American Chemical Society
for Pharmacology and Experimental Therapeutics and the Division of
Medical Chemistry, Burlington, Vermont, 25 August, 1971.
KLIGERMAN, A.D., EREXSON, G.L., & WILMER, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res., 157: 181-187.
KNAPP, F.W. & GRADEN, A.P. (1964) Accidental exposure of dairy cows
to excessive amount of dichlorvos. J. econ. Entomol., 57: 790-791.
KOBAYASHI, H., YUYAMA, A., IMAJO, S., & MATSUSAKA, N. (1980)
Effects of acute and chronic administration of DDVP (dichlorvos) on
distribution of brain acetylcholine in rats. J. toxicol. Sci., 5: 311-
319.
KOBAYASHI, H., YUYAMA, A., & CHIBA, K.I. (1986) Cholinergic system
of brain tissue in rats poisoned with the organophosphate
0,0-demethyl 0-(2,2-dichlorovinyl) phosphate. Toxicol. appl.
Pharmacol., 82: 32-39.
KODAMA, J.K. (1960) Technical DDVP. Acute oral administration:
dogs, Vienna, Virginia, Hazleton Laboratories (Unpublished Report, 20
January).
KODAMA, J.K. (1968) Experimental evaluation in guinea-pigs of skin-
sensitizing potential of components of formulated dichlorvos/
polyvinylchloride products, Modesto, California, Shell Development
Company (Unpublished Technical Progress Report No. M-67-68).
KONISHI, Y., DENDA, A., & KITAOKA, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3FI mice, Tokyo, Ministry of Health
and Welfare (Cooperative Studies on Carcinogenicity Tests on Mutagens)
(unpublished).
KRAMERS, P.G.N. & KNAAP, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of D rosophila melanogaster.
Mutat. Res., 57: 103-105.
KRAUSE, CHR., VON & KIRCHHOFF, J. (1970) [Gas chromatographic
determination of organophosphate residues in market samples of fruits
and vegetables.] Dtsch. Lebensm. Rundschau., 66(6): 194-199 (in
German).
KRAUSE, W. (1977) Influence of DDT, DDVP, and malathion on FSH, LH,
and testosterone serum levels and testosterone concentrations in
testis. Bull. environ. Contam. Toxicol., 18(2): 231-242.
KRAUSE, W. & HOMOLA, S. (1972) [Influence on spermatogenesis by DDVP
(dichlorvos).] Arch. Dermatol. Forsch., 244: 439-441 (in German).
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat* testis after the
application of dichlorvos (DDVP). Bull. environ. Contam.
Toxicol., 11(5): 429-433 (*study was performed on mice).
KRAUSE, W., HAMM, K., & WEISSMULLER, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. environ. Contam. Toxicol., 15(4): 458-462.
KUWABARA, K., NAKAMURA, A., & KASHIMOTO, T. (1980) Effect of
petroleum oil, pesticides, PCBs, and other environmental contaminants
on the hatchability of Artemia salina dry eggs. Bull. environ.
Contam. Toxicol., 25: 69-74.
LAFONTAINE, A., AERTS, J., & JACQUES, P. (1981) [Toxicity,
carcinogenic, mutagenic, and teratogenic action of dichlorvos.] Arch.
belg. Méd. soc. Hyg. Méd. Trav. Méd. lég., 39(3): 159-174 (in Dutch).
LAL, R. (1982) Accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms. Adv. appl.
Microbiol., 28: 149-200.
LAMOREAUX, R.J. & NEWLAND, L.W. (1978) The fate of dichlorvos in
soil. Chemosphere, 7(10): 807-814.
LAWLEY, P.D., SHAH, S.A., & ORR, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (dichlorvos,
DDVP). Chem.-biol. Interact., 8: 171-182.
LAWS, E.R., Jr (1966) Route of absorption of DDVP after oral
administration to rats. Toxicol. appl. Pharmacol., 8: 193-196.
LEARY, J.S., HIRSCH, L., LAVOR, E.M., FEICHTMEIR, E., SCHULTZ, D.,
KOOS, B., ROAN, C.R., FONTENOT, C., & HINE, C.H. (1971) An evaluation
of the safety of No-PestR strip insecticide with special reference to
respiratory and dietary exposure of occupants of homes in
Arizona. Toxicol. appl. Pharmacol., 19: 379.
LEARY, J.S., KEANE, W.T., FONTENOT, C., FEICHTMEIR, E.F.,
SCHULTZ, D., KOOS, B.A., HIRSCH, L., LAVOR, E.M., ROAN, C.C., &
HINE, C.H. (1974) Safety evaluation in the home of polyvinyl chloride
resin strips containing dichlorvos (DDVP). Arch. environ. Health, 29:
308-314.
LEONARD, A. (1976) Heritable chromosome aberrations in mammals after
exposure to chemicals. Radiat. environ. Biophys., 13: 1-8.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides. In:
Lee, R.L., ed. Air pollution from pesticides and agricultural
processes, Cleveland, Ohio, CRC Press, pp. 51-95.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides on
decomposition of organic matter nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LIEBERMAN, M.T. & ALEXANDER, M. (1983) Microbial and non-
enzymatic steps in the decomposition of dichlorvos (2,2-
dichlorovinyl O,O-dimethylphosphate). J. agric. food Chem., 31: 265-
267.
LIVINGSTON, R.J. (1977) Review of current literature concerning the
acute and chronic effects of pesticides on aquatic organisms. CRC crit.
Rev. environ. Control, 7: 325-351.
LLOYD, T.S. (1973) Accidental poisoning in birds. Vet. Rec., 5 May:
489.
LOEFFLER, J.E., DE VRIES, D.M., YOUNG, R., & PAGE, A.C. (1971)
Metabolic fate of inhaled dichlorvos in pigs. Toxicol. appl.
Pharmacol., 19: 378.
LOEFFLER, J.E., POTTER, J.C., SCORDELIS, S.L., HENDRICKSON, H.R.,
HUSTON, C.K., & PAGE, A.C. (1976) Long-term exposure of swine to a
14C-dichlorvos atmosphere. J. agric. food Chem., 24(2): 367-371.
LOFROTH, G. (1970) Alkylation of DNA by dichlorvos. Natur-
wissenschaften, 57(8): 393-394.
LOFROTH, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
Naturforsch., 33: 783-785.
LOFROTH, G. & WENNERBERG, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch., 29: 651.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating properties of
2,2-dichlorovinyl dimethyl phosphate: a disregarded hazard. Environ.
Mutat. Soc. Newslett., 2: 21-27.
LOHS, K., FISCHER, G.W., & DEDEK, W. (1976) [Chemical-toxicological
aspects of the alkylating properties of organophosphate pesticides.]
Sitzungsber. Akad. Wiss. (DDR), 11N: 1-58 (in German).
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of organophosphorus
pesticides: predictions can be based on in vitro studies with hen and
human enzymes. Arch. Toxicol., 41: 215-221.
LUDKE, J.L. & LOCKE, L.N. (1976) Duck deaths from accidental
ingestion of anthelmintic. Avian Dis., 20(3): 607-608.
LUKE, M.A., FROBERG, J.E., DOOSE, G.M., & MASUMOTO, H.T. (1981)
Improved multiresidue gas chromatographic determination of
organophosphorus organonitrogen, and organohalogen pesticides in
produce, using flame photometric and electrolytic conductivity
detectors. J. Assoc. Off. Anal. Chem., 64(5): 1187-1195.
MACDONALD, R. (1982) Toxicology of consumer products: the acute
(4-h) inhalation toxicity of dichlorvos vapour in rats and mice,
Sittingbourne, Shell Research Ltd (Unpublished Report SBGR.82.145).
MACKLIN, A.W. & RIBELIN, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc., 159(12): 1743-
1748.
MADDY, K.T., EDMISTON, S., & FREDRICKSON, A.S. (1981a) Monitoring
residue of DDVP in room air and on horizontal surfaces following use of
a room fogger, Sacramento, California, California Department of Food
and Agriculture (Unpublished Report No. HS-897).
MADDY, K.T., SCHNEIDER, F., LOWE, J., OCHI, E., FREDRICKSON, A.S.,
& MARGOTICH, S. (1981b) Vapona (DDVP) exposure potential to in
mushroom houses in Ventura County, California, in 1981, Sacramento,
California, California Department of Food and Agriculture (Unpublished
Report No. HS-861).
MAJEWSKI, T., PODGORSKI, W., BIALKOWSKI, Z., & TYCZKOWSKI, J.
(1978) [Effects of application of different forms of DDVP to
cows.] Rocz. Nauk. Zootech., 5(2): 65-74 (in Polish).
MAJEWSKI, T., PODGORSKI, W., & MICHALOWSKA, R. (1979) Retention
of dichlorvos (DDVP) in rabbits. Pol. Arch. Weter., 21(2): 249-255.
MASLINSKA, D. & ZALEWSKA, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia histochem. cytochem., 16(4): 335-341.
MASLINSKA, D., STROSZNAJDER, J., ZALEWSKA, T., & ORLEWSKI, P.
(1984) Phospholipid-protein ratio in brain of suckling rabbits treated
with an organophosphorus compound. Int. J. tissue React., 6(4): 317-
322.
MATHIAS, C.G.T. (1983) Persistent contact dermatitis from the
insecticide dichlorvos. Contact Dermatit., 9: 217-218.
MATSUMURA, F. & BOUSH, G.M. (1968) Degradation of insecticides by a
soil fungus Trichoderm viride. J. econ. Entomol., 61(3): 610-612.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
310-311.
MENDOZA, C.E. (1974) Analysis of pesticides by the thin-layer
chromatographic enzyme inhibition technique. Part II. Residue Rev., 50:
43-72.
MENDOZA, C.E. & SHIELDS, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin-layer chromatography. J. Assoc. Off.
Anal. Chem., 54: 507-512.
MENZ, M., LUETKEMEIER, H., & SACHSSE, K. (1974) Long-term exposure
of factory workers to dichlorvos (DDVP) insecticide. Arch. environ.
Health, 28: 72-76.
MESTRES, R., CHEVALLIER, CH., ESPINOZA, CL., & CORNET, R. (1977)
Application du couplage chromatographie gazeuse spectrométrie de masse
aux méthodes de recherche et de dosage des résidus de pesticides et de
micropolluants organiques dans les matériaux de l'environnement et les
matičres alimentaires. Ann. Fac. exp. Chim., 70(751): 177-188.
MESTRES, R., ILLES, S., CAMPO, M., & TOURTE, J. (1979a) Développement
de la méthode polyvalente d'analyse des résidus des pesticides dans les
plantes et les matičres alimentaires d'origine végétale. Trav. Soc.
Pharm. Montpellier, 39: 323-328.
MESTRES, R., ATMAWIJAYA, S., & CHEVALLIER, CH. (1979b) Méthode
de recherche et de dosage des résidus de pesticides dans les produits
céréaliers. I. Organochlorés, organophosphorés, pyréthrines, et
pyréthrinoďdes. Ann. Fac. exp. Chim., 72: 577-589.
MICHALEK, S.M. & BROCKMAN, H.E. (1969) A test for mutagenicity of
Shell "No-Pest Strip Insecticide" in Neurospora crassa. Neurospora
Newslett., 16: 8.
MICHEL, H.O. (1949) An electrometric method for the determination of
red blood cell and plasma cholinesterase activity. J. lab. clin.
Med., 34: 1564-1568.
MILES, J.W., FETZER, L.E., & PEARCE, G.W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ. Sci.
Technol., 4: 420-425.
MITSUI, T., OZAKI, H., KUMANO, H., & SANO, H. (1963) Insecticide
determination. I. Colormetric determination of dimethyl 2,2-
dichlorovinyl phosphate (DDVP). Chem. pharm. Bull., 11(5): 619-623.
MIYAMOTO, J. (1959) Non-enzymatic conversion of Dipterex into DDVP
and their inhibitory action on enzymes. Botyu-Kagaku (Sci. pestic.
Control), 24: 130-137.
MODAK, A.T., STAVINOHA, W.B., & WEINTRAUB, S.T. (1975) Dichlorvos
and the cholinergic system: effects on cholinesterase and acetylcholine
and choline contents of rat tissues. Arch. int. Pharmacodyn., 217:
293-301.
MOHN, G. (1973) S-methyltryptophan resistance mutations in
Escherichia coli K12. Mutagenic activity of mono-functional alkylating
agents including organophosphorus insecticides. Mutat. Res., 20: 7-
15.
MORIYA, M., KATO, K., & SHIRASU, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res., 57: 259-263.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MORPURGO, G., BELLINCAMPI, D., GUALANDI, G., BALDINELLI, L., &
SERLUPI CRESCENZI, O. (1979) Analysis of mitotic non-disjunction
with Aspergillus nidulans. Environ. Health Perspect., 31: 81-95.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., &
DEGRAEVE, N. (1981) Metrifonate and dichlorvos: cytogenetic
investigations. Acta pharmacol. toxicol., 49(Suppl. 5): 29-39.
MULLER, G.H. (1970) Flea collar dermatitis in animals. J. Am. Vet.
Med. Assoc., 157(11): 1616-1626.
NAGY, ZS., MILE, I., & ANTONI, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta microbiol. Acad. Sci.
Hung., 22: 309-314.
NAIDU, N.V., REDDY, K.S., JANARDHAN, A., & MURTHY, M.K. (1978)
Toxicological investigation of dichlorvos in chicks. Indian J.
Pharmacol., 10(14): 323-326.
NAKAMURA, K. & SHIBA, H. (1980) [Study on pesticide residues in fruit
and vegetables.] Bull. Saitama prefect. agric. exp. Stn, 36: 35-56 (in
Japanese).
NARCISSE, J.K. (1967) A potentiation study in rats of VaponaR with
26 other cholinesterase-inhibiting compounds, Menlo Park, California,
Stanford Research Institute (Unpublished Report, 2 June).
NCI (1977) Bioassay of dichlorvos for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute.
NEUWIRTH, E.A. & WHITE, T.T. (1961) Safety aspects of DDVP. Soap
chem. Spec., 37(3): 95-98.
NICHOLAS, A.H., VIENNE, M., & BERGHE, H., VAN DEN (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Lett., 2(5): 271-
275.
NIOSH (1979) NIOSH Manual of analytical methods. Analytical method
No. P and CAM 295, 2nd ed., Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. 5 (Publication No. 79-144).
NISHIO, A. & UYEKI, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphorus
insecticides and their oxygen analogs. J. Toxicol. environ. Health, 8:
939-946.
NISHIO, A. & UYEKI, E.M. (1982) Nuclease sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants. Biochem.
biophys. Res. Commun., 107(2): 485-491.
NORDGREN, I., BERGSTROM, M., HOLMSTEDT, B., & SANDOZ, M.
(1978) Transformation and action of metrifonate. Arch. Toxicol., 41:
31-41.
NOWELL, P.T., SCOTT, C.A., & WILSON, A. (1962) Hydrolysis of
neostigmine by plasma-cholinesterase. Br. J. Pharmacol., 19: 498-502.
NTP (1986) Carcinogenicity study in rats and mice with
dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (Unpublished data).
NTP (1987) NTP technical report on the toxicology and carcinogenesis
of dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (NTP-TR342).
OBA, T. & KAWABATA, G. (1962) [Application of infrared absorption
spectroscopy to examination of drugs and their preparations. XIV.
Determination of O,O-dimethyl 2,2-dichlorovinyl phosphate (DDVP) and
its preparation.] Eiseishikenjo-hokoku, 80: 1-3 (in Japanese, with
English summary).
OGATA, H., SAITO, E., MURATA, H., SHIBAZAKI, T., & INOUE, T. (1975)
[A new colorimetric method of determination of O,O-dimethyl 2,2-
dichlorovinyl phosphate in insecticidal preparations.] Yakugaku
Zasshi, 95(12): 1483-1491 (in Japanese, with English summary).
OLINSKI, R., WALTER, Z., WIADERKIEWICZ, R., LUKASOVA, E., &
PALECEK, E. (1980) Changes in DNA properties due to treatment with
the pesticides malathion and DDVP. Radiat. environ. Biophys., 18: 65-
72.
OMKAR, & SHUKLA, G.S. (1984) Alteration in carbohydrate metabolism of
fresh-water prawn Macrobianchium lamarrei after dichlorvos
exposure. Ind. Health, 22: 133-136.
PACHECKA, J., SULINSKI, A., & TRACZYKIEWICZ, K. (1977) The effect
of acute intoxication by dichlorvos and trichlorphon on the activities
of some rat brain esterases. Neuropathol. Polska, 15(1): 85-92.
PAGE, A.C., DE VRIES, D.M., YOUNG, R., & LOEFFLER, J.E. (1971)
Metabolic fate of ingested dichlorvos in swine. Toxicol. appl.
Pharmacol., 19: 378.
PAGE, A.C., LOEFFLER, J.E., HENDRICKSON, H.R., HUSTON, C.K., &
DEVRIES, D.M. (1972) Metabolic fate of dichlorvos in swine. Arch.
Toxikol., 30: 19-27.
PAIK, S.G. & LEE, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool., 20(1):
19-28.
PENA CHAVARRIA, A., SWARTZWELDER, J.C., VILLAREJOS, V.M.,
KOTCHER, E., & ARGUEDAS, J. (1969) Dichlorvos: an effective broad-
spectrum anthelmintic. Am. J. trop. Med. Hyg., 18(6): 907-911.
PEROCCO, P. & FINI, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (WHO Report
No. VBC/84.889).
PODREBARAC, D.S. (1984) Pesticide, heavy metals, and other chemical
residues in infant and toddler total diet samples. IV. October 1977 -
September 1978. J. Assoc. Off. Anal. Chem., 67(1): 166-175.
POTTER, J.C., LOEFFLER, J.E., COLLINS, R.D., YOUNG, R., & PAGE,
A.C. (1973a) Carbon-14 balance and residues of dichlorvos and its
metabolites in pigs dosed with dichlorvos-14C. J. agric. food
Chem., 21(2): 163-166.
POTTER, J.C., BOYER, A.C., MARXMILLER, R.E., YOUNG, R., &
LOEFFLER, J.E. (1973b). Radioisotope residues and residues of
dichlorvos and its metabolites in pregnant sows and their progeny dosed
with dichlorvos-14C or dichlorvos-36Cl formulated as PVC
pellets. J. agric. food Chem., 21(4): 734-738.
PURSHOTTAM, T. & KAVEESHWAR, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviat. Space
environ. Med., 50(6): 581-584.
PURSHOTTAM, T. & SRIVASTAVA, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
environ. Health, 39(6): 425-430.
PYM, R.A.E., SINGH, G., GILBERT, W.S., ARMSTRONG, J.P., &
MCCLEARY, B.V. (1984) Effects of dichlorvos, maldison, and
pirimiphos-methyl on food consumption, egg production, egg and tissue
residues, and plasma acetylcholinesterase inhibition in layer strain
hens. Aust. J. exp. Agric. anim. Husb., 24: 83-92.
QUARTERMAN, K.D., LOTTE, M., & SCHOOF, H.F. (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 231-235.
RADELEFF, R.D. & WOODARD, G.T. (1957) The toxicity of organic
phosphorus insecticides to livestock. J. Am. Vet. Med. Assoc., 130:
215-216.
RAHMATULLAH, M., JAHAN, R., CHOWDHURY, A.A., FARUK, A.B.M.,
& CHOWDHURY, M.S.K. (1978) Effect of organophosphorus insecticides on
fermentation processes in Aspergillus niger. J. ferment. Technol., 56:
169-172.
RAMEL, C. (1981) Does dichlorvos constitute a genotoxic hazard? In:
Kappas, A., ed. Progress in environmental mutagenesis and
carcinogenesis, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 69-78 (Progress in Mutation Research, Vol. 2.).
RAMEL, C., DRAKE, J., & SUGIMURA, T. (1980) An evaluation of the
genetic toxicity of dichlorvos. Mutat. Res., 76: 297-309.
RASMUSSEN, W.A., JENSEN, J.A., STEIN, W.J., & HAYES, W.J. (1963)
Toxicological studies of DDVP for disinsection of aircraft. Aerosp.
Med., 34(7): 593-600.
RATH, S. & MISRA, B.N. (1979a) Relative toxicity of dichlorvos (DDVP)
to Tilapia mossambica Peters of 3 different age groups. Exp.
Gerontol., 14: 307-309.
RATH, S. & MISRA, B.N. (1979b) Sub-lethal effects of dichlorvos (DDVP)
on respiratory metabolism of Tilapia mossambica of 3 age groups. Exp.
Gerontol., 14: 37-41.
RATH, S. & MISRA, B.N. (1980) Pigment disperson in Tilapia
mossambica Peters exposed to dichlorvos (DDVP). Curr. Sci., 49(23):
907-909.
RATH, S. & MISRA, B.N. (1981) Toxicological effects of dichlorvos
(DDVP) on brain and liver acetylcholinesterase (AChE) activity
of Tilapia mossambica Peters. Toxicology, 19: 239-245.
RAUWS, A.G. & LOGTEN, M.J., VAN (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in
chicken. Toxicology, 1: 29-41.
REECE, R.L. (1982) Observations on the accidental poisoning of birds
by organophosphate insecticides and other toxic substances. Vet.
Rec., 111(20): 453-455.
REEVES, J.D., DRIGGERS, D.A., & KILEY, V.A. (1981) Household
insecticide associated aplastic anaemia and acute leukaemia in
children. Lancet, 8 August: 300-301.
REINER, E. & PLESTINA, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by O,O-dimethyl-2,2-
dichlorovinyl phosphate. Toxicol. appl. Pharmacol., 49: 451-454.
RIDER, J.A., MOELLER, H.C., & PULETTI, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies with
Guthion, and determination of incipient toxicity level of dichlorvos in
humans. Fed. Proc., 26: 427.
RIDER, J.A., MOELLER, H.C., PULETTI, E.J., & SWADER, J. (1968)
Studies on the anticholinesterase effects of methyl parathion, guthion,
dichlorvos, and gardona in human subjects. Fed. Proc., 27: 597.
ROSENBERG, A., LIEBERMAN, M.T., & ALEXANDER, M. (1979)
Microbial degradation of pesticides, Springfield, Virginia, US
National Technical Information Service (PB 79-123156).
ROSENKRANZ, H.S. (1973) Preferential effect of dichlorvos
(VaponaR) on bacteria deficient in DNA polymerase. Cancer Res., 33:
458-459.
ROSENKRANZ, H.S. & LEIFER, Z. (1980) Determining the DNA-modifying
activity of chemicals using DNA polymerase-deficient Escherichia
coli. In: de Serres, F.J. & Hollander, A., ed. Chemical mutagens:
principles and methods for their detection, New York, Plenum Press,
Vol. 6, pp. 109-147.
ROSENKRANZ, H.S. & ROSENKRANZ, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia (Basel), 28(4): 386-387.
SAGNER, G. & SCHONDUBE, M. (1982) [Determination and toxicological
appraisal of indoor concentrations of dichlorvos after the application
of spraying products.] Schriftenr. Ver. Wasser Boden Lufthyg., 53:
359-368 (in German).
SAKAMA, K. & NISHIMURA, M. (1977) [Studies on inhalation toxicity of
organic phosphorus insecticides. II. Approach to establishment of
threshold limit value.] Jpn. J. ind. Health, 19: 357-358 (in
Japanese).
SASINOVICH, L.M. (1968) [Substantiation of the maximum permissible
concentration of DDVP in the air of the working zone.] Gig. i Sanit.,
33(12): 35-39 (in Russian).
SASINOVICH, L.M. (1970) [Toxicology of dimethyldichlorovinyl
phosphate and hygienic characteristics of its use.] Gig. Primen.
Toksikol. Pestits. Klin. Otravl., 8: 297-307 (in Russian).
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical, and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHAFER, E.W., Jr & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and management
materials, Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SCHMIDT, G., SCHMIDT, M., & NENNER, M. (1975) Inhibition of
acetylcholinesterase in the bronchial system of rats caused by
inhalation of dichlorvos (DDVP). Naunyn Schmiedeberg Arch.
Pharmacol., 287(Suppl.): R97.
SCHMIDT, G., SCHMIDT, M., NENNER, M., & VETTERLEIN, F. (1979)
Effects of dichlorvos (DDVP) inhalation on the activity of
acetylcholinesterase in the bronchial tissue of rats. Arch.
Toxicol., 42: 191-198.
SCHOOF, H.F., JENSEN, J.A., PORTER, J.E., & MADDOCK, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP
vapour. Bull. World Health Organ., 24: 623-628.
SCHULTZ, D.R., MARXMILLER, R.L., & KOOS, B.A. (1971) Residue
determination of dichlorvos and related metabolites in animal tissue
and fluids. J. agric. food Chem., 19(6): 1238-1243.
SCHULZE, H.D. (1979) [Current problems of pest control with
dichlorvos in hospital.] Z. gesamte Hyg., 25(5): 421-425 (in German).
SCHWETZ, B.A., IOSET, H.D., LEONG, B.K.J., & STAPLES, R.E. (1979)
Teratogenic potential of dichlorvos given by inhalation and gavage to
mice and rabbits. Teratology, 20: 383-388.
SEGERBACK, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP (2,2-dichlorovinyl
dimethylphosphate). Hereditas, 94: 73-76.
SEGERBACK, D. & EHRENBERG, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta pharmacol. toxicol., 49(Suppl. 5): 56-66.
SERA, K., MATSUNAGA, A., MURAKAMI, A., SATO, I., YAMASHITA,
K., & YOSHIMORI, H. (1959) Qualitative analysis of organic phosphorus
color reactions and simple detection. Kumamoto med. J., 12(3): 193-
213.
SHAFIK, M.T., BRADWAY, D., & ENOS, H.F. (1971) A method for
confirmation of organophosphorus compounds at the residue level.
Bull. environ. Contam. Toxicol., 6(1): 55-66.
SHELLENBERGER, T.E. (1980) Organophosphorus pesticide inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. environ. Sci. Health, B15(6): 795-822.
SHELLENBERGER, T.E., NEWELL, G.W., OKAMOTO, S.S., & SARROS, A.
(1965) Response of rabbit whole blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14: 943-
952.
SHERMAN, M. & ROSS, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. appl. Pharmacol., 3: 521-533.
SHINKAJI, N. & ADACHI, T. (1978) [The effect of certain pesticides on
the predaceous mite Amblyseius longispinosus (Evans) (Acarina:
Phytoseiidae).] Akitsu, 2: 99-108 (in Japanese, with English tables).
SHINODA, H., ITO, K., MATSUNAGA, T., TAKADA, T., & NOMURA, T.
(1972) Two suicides by acute pesticide intoxication. J. Jpn. Assoc.
Rural Med., 21: 242-249 (in Japanese).
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHOOTER, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating
agents. Chem.-biol. Interact., 11: 575-588.
SLOMKA, M.B. & HINE, C.H. (1981) Clinical pharmacology of
dichlorvos. Acta pharmacol. toxicol., 49(Suppl. 5): 105-108.
SMITH, P.W., MERTENS, H., LEWIS, M.F., FUNKHOUSER, G.E., HIGGINS,
E.A., CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & FLUX, M. (1972)
Toxicology of dichlorvos at operational aircraft cabin altitudes.
Aerosp. Med., 43(5): 473-478.
SNOW, D.H. & WATSON, A.D.J. (1973) The acute toxicity of dichlorvos
in the dog. I. Clinical observations and clinical pathology. Aust. vet.
J., 49: 113-119.
SOBELS, F.H. & TODD, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res., 67: 89-92.
STANTON, H.C., ALBERT, J.R., & MERSMANN, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows. Am. J.
vet. Res., 40(3): 315-320.
STAVINOHA, W.B., MODAK, A.T., & WEINTRAUB, S.T. (1976) Rate of
accumulation of acetylcholine in discrete regions of the rat brain
after dichlorvos treatment. J. Neurochem., 27: 1375-1378.
STEIN, W.J., MILLER, S., & FETZER, L.E., Jr (1966) Studies with
dichlorvos residual fumigant as a malaria eradication technique in
Haiti. III. Toxicological studies. Am. J. trop. Med. Hyg., 15(5): 672-
675.
STERNBERG, S.S. (1979) The carcinogenesis, mutagenesis, and
teratogenesis of insecticides. Review of studies in animals and
man. Pharmacol. Ther., 6: 147-166.
STEVENSON, D.E. & BLAIR, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0024.69).
STEVENSON, D.E. & BLAIR, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Toxicol., 18: 215-217.
STOERMER, D. (1985) [Occupational contact dermatitis from the
pesticide dichlorvos.] Dermatol. Monatsschr., 171(4): 245-249 (in
German).
TEICHERT, K., SZYMCZYK, T., CONSOLO, S., & LADINSKY, H. (1976)
Effect of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. appl. Pharmacol., 35: 77-81.
TERAYAMA, K., HONMA, H., & KAWARABAYASHI, T. (1978) Toxicity of
heavy metals and insecticides on slime mold Physarum polycephalum. J.
toxicol. Sci., 3: 293-304.
TESSARI, J.D. & SPENCER, D.L. (1971) Air sampling for pesticides in the
human environment. J. Assoc. Off. Anal. Chem., 54(6): 1376-1382.
TEZUKA, H., ANDO, N., SUZUKI, R., TERAHATA, M., MORIYA, M., &
SHIRASU, Y. (1980) Sister chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with pesticides
positive in microbial reversion assays. Mutat. Res., 78: 177-191.
THOMAS, T.C. & NISHIOKA, Y.A. (1985) Sampling of airborn pesticides
using chromosorb-102. Bull. environ. Contam. Toxicol., 35: 460-465.
THORPE, E., WILSON, A.B., DIX, K.M., & BLAIR, D. (1972) Teratological
studies with dichlorvos vapour in rabbits and rats. Arch. Toxicol.,
30(1): 29-38.
TIMMONS, E.M., CHAKLOS, R.J., BANNISTER, T.M., & KAPLAN, H.M.
(1975) Dichlorvos effects on estrous cycle onset in the rat. Lab.
anim. Sci., 25(1): 45-47.
TRACY, R.L., WOODCOCK, J.G., & CHODROFF, S. (1960) Toxicological
aspects of 2,2'-dichlorovinyl dimethylphosphate (DDVP) in cows,
horses, and white rats. J. econ. Entomol., 53(4): 593-601.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 43 pp (Resource Publication No. 84).
UEDA, K., SHIYO, K., MORI, T., KITAHARA, E., & URAGAMI, S. (1959)
[Effects of DDVP on man.] J. Jpn. Public Hyg. Assoc., 6: 315 (in
Japanese).
UEDA, K., SHIYO, K., IIZUKA, Y., KITAHARA, E., & OHASHI, A. (1960)
[The toxicity of organic phosphate "DDVP" for small animals and
man.] Igaku Seibutsugaku (Med. Biol.), 57(3): 98-101 (in Japanese).
ULLMANN, L., PHILLIPS, J., & SACHSSE, K. (1979) Cholinesterase
surveillance of aerial applicators and allied workers in the Democratic
Republic of the Sudan. Arch. environ. Contam. Toxicol., 8: 703-712.
VADHVA, P. & HASAN, M. (1986) Organophosphate dichlorvos induced
dose-related differential alterations in lipid levels and lipid
peroxidation in various regions of the fish brain and spinal cord. J.
environ. Sci. Health, B21(5): 413-424.
VAN DIJK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air: sampling
methods. Residue Rev., 55: 91-134.
VAN RAALTE, H.G.S. & JANSEN, J.D. (1981) Household insecticides and
the blood. Lancet, 10 October: 811.
VASHKOV, V.I., VOLKOVA, A.P., TSETLIN, V.M., & YANKOVSKII, E.Y.
(1966) [Assessment of the use of dimethyldichlorovinyl phosphate
(DDVP) in the insecticide mixture.] Gig. i Sanit., 9: 15-17 (in
Russian).
VASILESCU, C. & FLORESCU, A. (1980) Clinical and electro-
physiological study of neuropathy after organophosphorus compounds
poisoning. Arch. Toxicol., 43: 305-315.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination of
water by a sublethal concentration of pesticides. A system analysis
approach. Acta hydrochim. hydrobiol., 12(4): 399-499.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1980) Effects of
the pesticides thiotox, dichlorvos, and carbofuran on the test
fish Mystus vittatus. Water Air Soil Pollut., 13(2): 229-234.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1981a)
Evaluation of the comparative toxicity of thiotox, dichlorvos, and
carbofuran to two fresh water teleosts Ophiocephalus punctatus and
Mystus vittatus. Acta hydrochem. hydrobiol., 9(2): 119-129.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981b) Isolated and combined
effects of pesticides on serum transaminases in Mystus
vittatus (African catfish). Toxicol. Lett., 8: 67-71.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981c) Pesticide-induced
physiological alterations in certain tissues of a fish Mystus vittatus.
Toxicol. Lett., 9: 327-332.
VERMA, S.R., TONK, I.P., & DALELA, R.C. (1981d) Determination of the
maximum acceptable toxicant concentration (MATC) and the safe
concentration for certain aquatic pollutants. Acta hydrochim.
hydrobiol., 9(3): 247-254.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982a) Bioassay trials
with twenty-three pesticides to a fresh water teleost Saccobranchus
fossilis. Water Res., 16: 525-529.
VERMA, S.R., RANI, S., & DALELA, R.C. (1982b) Indicators of stress
induced by pesticides in Mystus vittatus: haematological
parameters. Indian J. environ. Health, 24(1): 58-64.
VERMA, S.R., RANI, S., TONK, I.P., & DALELA, R.C. (1983) Pesticide-
induced dysfunction in carbohydrate metabolism in three fresh water
fishes. Environ. Res., 32: 127-133.
VERMA, S.R., RANI, S., & DALELA, R.C. (1984) Effects of pesticides
and their combinations on three serum phosphatases of Mystus vittatus.
Water Air Soil Pollut., 21: 9-14.
VOGIN, E.E., CARSON, S., & SLOMKA, M.B. (1971) Teratology studies
with dichlorvos in rabbits. Toxicol. appl. Pharmacol., 19: 377-378.
VOOGD, C.E., JACOBS, J.J.J.A.A., & STEL, J.J., VAN DER (1972) On the
mutagenic action of dichlorvos. Mutat. Res., 16: 413-416.
VOSS, G. & SACHSSE, K. (1970) Red cell and plasma cholinesterase
activities in microsamples of human and animal blood determined
simultaneously by a modified acetylthiocholine/DNTB procedure.
Toxicol. appl. Pharmacol., 16: 764-772.
VRBOVSKY, L., SELECKY, FR.V., & ROSIVAL, L. (1959) [Toxicological and
pharmacological studies with phosphorester insecticides.] Naunyn
Schmiedeberg Arch. Pharmacol., 236: 202-204 (in German).
WADIA, R.S., SHINDE, S.N., & VAIDYA, (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurology (India), 33:
247-253.
WAGNER, R. & HOYER, J. (1975) [Method of determining workplace
concentrations and on occupational hygiene conditions during thermal
fogging atomization of pesticides in the greenhouse.] Z. gesamte
Hyg., 21(1): 18-20 (in German).
WALKER, A.I.T., BLAIR, D., STEVENSON, D.E., & CHAMBERS, P.L.
(1972) An inhalational toxicity study with dichlorvos. Arch.
Toxikol., 30: 1-7.
WARD, F.P. & GLICKSBERG, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Assoc., 158(4): 457-
461.
WATANABE, H., SHIRAI, O., & EBATA, N. (1976) A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care. Pestic. Abstr., 9(2): 94-95.
WEISBURGER, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 165-176.
WENNERBERG, R. & LOFROTH, G. (1974) Formation of 7-methyl- guanine
by dichlorvos in bacteria and mice. Chem.-biol. Interact., 8: 339-348.
WHITEHEAD, C.C. (1971) The effects of pesticides on production in
poultry. Vet. Rec., 30 January: 114-117.
WHO (1978) Spectrophotometric kit for measuring cholinesterase
activity, Geneva, World Health Organization (Report VBC/78.692).
WHO/FAO (1975-86) Data sheets on pesticides, Geneva, World Health
Organization (VBC).
WHO (1985) Technical dichlorvos. In: Specifications for pesticides
used in public health, 6th ed., Geneva, World Health Organization, pp.
163-174 (WHO/SIT/16.R2).
WHO (1986a) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87, Geneva, World Health
Organization, p. 11 (Report VBC/86.1 Rev.1)
WHO (1986b) Environmental Health Criteria 63: organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WILD, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of dichlorvos
and methyl methanesulfonate. Mutat. Res., 19: 33-41.
WILD, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res., 32: 133-150.
WILDEMAUWE, C., LONTIE, J.F., SCHOOFS, L., & LAREBEKE, N., VAN
(1983) The mutagenicity in procaryotes of insecticides, acaricides,
and nematicides. Residue Rev., 89: 129-178.
WILLIAMS, P.P. (1977) Metabolism of synthetic organic pesticides by
anaerobic microorganisms. Residue Rev., 66: 102.
WILSON, A.B. & DIX, K.M. (1973) Toxicity studies on dichlorvos: one
month inhalational exposure of rats to dichloroacetaldehyde,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0017.73).
WITHERUP, S., STEMMER, K.L., & CALDWELL, J.S. (1964) The effects
upon rats of being fed on diets containing VaponaR insecticide,
Cincinnati, Ohio, The Kettering Laboratory (Unpublished Report, 1
July).
WITHERUP, S., CALDWELL, J.S., & HULL, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their offspring by
the introduction of VaponaR insecticide into their diets, Cincinatti,
Ohio, The Kettering Laboratory (Unpublished Report, 12 April).
WITHERUP, S., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction of
VaponaR insecticide into their daily diets, Cincinnati, Ohio, The
Kettering Laboratory (Unpublished Report, 14 February).
WITHERUP, S., JOLLEY, W.J., STEMMER, K., & PFITZER, E.A. (1971)
Chronic toxicity studies with 2,2-dichlorovinyl dimethyl phosphate
(DDVP) in dogs and rats including observations on rat reproduction.
Toxicol. appl. Pharmacol., 19: 377.
WITTER, R.F. (1960) Effects of DDVP aerosols on blood cholinesterase
of fogging machine operators. Am. Med. Assoc. Arch. Ind. Health, 21:
7-9.
WITTER, R.F., GAINES, T.B., SHORT, J.G., SEDLAK, V.A., & MADDOCK,
D.R. (1961) Studies on the safety of DDVP for the disinsection of
commercial aircraft. Bull. World Health Organ., 24: 635-642.
WOOD, A.B. & KANAGASABAPATHY, L. (1983) Evaluation of inexpensive
thin layer chromatographic procedures for the estimation of some
organophosphorus and carbamate insecticide residues in fruit and
vegetables. Pestic. Sci., 14: 108-118.
WOODER, M.F. & CREEDY, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo, Sittingbourne,
Shell Research Ltd (Unpublished Report TLGR.79.089).
WOODER, M.F. & WRIGHT, A.S. (1981) Alkylation of DNA by organo-
phosphorus pesticides. Acta pharmacol. toxicol., 49(Suppl. 5): 51-55.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) I n vivo alkylation
studies with dichlorvos at practical use concentrations. Chem.-biol.
Interact., 19: 25-46.
WOODER, M.F., WRIGHT, A.S., & STEVENSON, D.E. (1978) Studies on
the in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol., 1: 505.
WORTHING, C.R. & WALKER, S.B. (1983) Pesticide manual, 7th ed.,
Croydon, British Crop Protection Council.
WRATHALL, A.E., WELLS, D.E., & ANDERSON, P.H. (1980) Effect of
feeding dichlorvos to sows in mid-pregnancy. Zbl. Vet. Med. A27: 662-
668.
WRIGHT, C.G. & LEIDY, R.B. (1980) Air samples in vehicles and
buildings turn up only very low levels of organic phosphate
insecticides. Pest Control, 48(7): 22-26, 68.
WRIGHT, A.S., HUTSON, D.H., & WOODER, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol., 42: 1-18.
XING-SHU, H. (1983) Preventing chemical damage to germ cells. Am.
Ind. Hyg. Assoc. J., 44: 699-703.
YAMANE, S., KINO, A., & TESHIMA, S. (1974) Histochemical
demonstration of cholinesterase activity in tissues of the carp
and effect of DDVP on its activity in situ. Acta histochem.
cytochem., 7(2): 167-174.
YAMANOI, F. (1980) [Effect of insecticides on the progeny in the
silkworm Bombyx mori. I. Effect of organophosphorus insecticides on egg
laying and their hatching.] Nippon Sanshigaku Zasshi, 49(5): 434-439
(in Japanese).
YAMASHITA, K. (1960) Studies on organic phosphorus. I. Toxicity of
dipterex and its vinyl derivative (DDVP). Kumamoto med. J., 13(4): 273-
279.
YAMASHITA, K. (1961) Studies on organic phosphorus dipterex. III.
Detection and discrimination of dipterex and its vinyl derivative
(DDVP). Kumamoto med. J., 14(1): 13-25.
YAMASHITA, K. (1962) Toxicity of dipterex and its vinyl derivative
(DDVP). Ind. Med. Surg., 31: 170-173.
ZALEWSKA, Z., RAKOWSKA, I., MATRASZEK, G., & SITKIEWICZ, D.
(1977) Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterases. Neuropathol.
Polska, 15(2): 255-262.
ZAVON, M.R. & KINDEL, E.A., Jr (1966) Potential hazard in using
dichlorvos insecticide resin. Adv. Chem. Ser., 60: 177-186.
ZELMAN, I.B. (1977) [Pathomorphology on the rat brain after
experimental intoxication with the phosphororganic pesticide dichlorvos
(DDVP).] Neuropathol. Polska, 15(4): 515-522 (in Polish).
ZELMAN, I.B. & MAJDECKI, T. (1979) [Ultrastructural changes in rat
brain following organophosphate insecticide dichlorvos (DDVP)
intoxication.] Neuropathol. Polska, 17(3): 443-453 (in Polish).
ZOTOV, V.M., SVIRIN, J.N., & PRUCAKOVA, R.M. (1977) [The develop-
ment of health regulations for occupational exposure in greenhouses
treated with chemical pesticides.] Gig. Tr. prof. Zabol., 3: 49-50 (in
Russian).
6.4 Elimination and Excretion in Expired Air, Faeces, and Urine
6.4.1 Human studies
Eight hours after a human male consumed 5 mg of vinyl-1-
14C-dichlorvos in orange juice, 27% of the radioactivity had been
eliminated as 14C-carbon dioxide. Approximately 8% had been excreted
by the urine within one day following dosing. Urinary excretion of
radioactivity decreased gradually and by day 9 none was detectable
(Hutson & Hoadley, 1972a).
The concentration of dimethylphosphate in the urine of three
pesticide control operators spraying houses with dichlorvos ranged from
0.32 to 1.4 µg at the end of the day's work (Das et al., 1983).
6.4.2 Studies on experimental animals
6.4.2.1 Oral
Dosing rats orally with 32P-dichlorvos (0.1 - 80 mg/kg body
weight) resulted in a recovery of 60 - 70% of the administered
radioactivity in the urine and approximately 10% in the faeces over a
6-day period following dosing (Casida et al., 1962).
After the oral administration of methyl-14C-dichlorvos to rats
(1 mg) and mice (0.5 mg), the excretion of radioactivity was rapid.
The major route of elimination after 4 days was the urine
(approximately 60%), followed by expired air (approximately 16%)
(Hutson & Hoadley, 1972b).
Rats given an oral dose of vinyl-1-14C-dichlorvos (1 mg per
animal) eliminated 10 - 20% of the 14C in the urine, 3 - 5% in the
faeces, and approximately 40% as expired carbon dioxide over 4 days
following dosing (Hutson et al., 1971a,b).
A comparison between the excretion by rat, mouse, hamster, and man
24 h after oral dosing with vinyl-1-14C-dichlorvos is given in
Table 10 (Hutson & Hoadley, 1972a).
A cow treated orally with 20 mg/kg body weight
32P-dichlorvos eliminated 40% of the radioactivity in the urine and
50% in the faeces. In the milk, the level of organosoluble radio-
activity was significantly above background only within the first 2 h
(Casida et al., 1962).
Table 10. Comparison of percentages of radioactivity excreted by males
24 h after oral ingestion of vinyl-1-14C-dichlorvosa
-----------------------------------------------------------------------------
Excretion route Rat (3) Mouse (1) Hamster (2) Man (1)
-----------------------------------------------------------------------------
urine 9.8 27.4 14.7 7.6
faeces 1.5 3.2 2.9 -
carbon dioxide 28.8 23.1 33.5 27 (8 h only)
-----------------------------------------------------------------------------
a Number of animals are given in parentheses.
6.4.2.2 Parenteral
The elimination of a single intraperitoneal injection of vinyl-1-
14C-dichlorvos (4 mg/kg body weight) from female rats was similar to
the elimination after oral dosing. A goat treated subcutaneously with
1.5 mg 32P-dichlorvos/kg body weight excreted 79% of the radioactivity
in the urine and 11% in the faeces. Two cows received an intravenous
or a subcutaneous injection with 1 mg 32P-dichlorvos/kg body weight.
Of the radioactivity which was recovered, 70 - 80% was in the urine and
approximately 14% in the faeces (Casida et al., 1962).
6.5 Retention and Turnover
6.5.1 Biological half-life
In studies by Blair et al. (1975), the metabolism of dichlorvos was
found to be so rapid that the biological half-life in blood could not
be determined. No intact dichlorvos could be demonstrated in the blood
or tissues of animals exposed by routes other than parenteral
injection. Only after exposure for 4 h to an atmospheric concentration
of 90 mg dichlorvos/m3 could dichlorvos be detected in most tissues of
the rat and mouse. Following exposure at 50 mg/m3, for 2 or 4 h, the
half-life in the rat kidney was 13.5 min.
The intraperitoneal injection of 10 mg dichlorvos/kg body weight
into mice increased the accumulation of ACh in the brain and caused an
inhibition of ChE activity. Symptoms of toxicity were clearly
recognizable after 15 min, and they disappeared almost completely after
60 min. The ChE activity and ACh levels reached their minimum and
maximum, respectively, at 15 min. The maximum concentration of
dichlorvos in the brain was reached after 1 min and decreased
thereafter, rapidly reaching the baseline level after 3 min (Nordgren
et al., 1978).
6.5.2 Body burden
There is no evidence for the storage of dichlorvos or its
metabolites in the tissues of animals. Small fractions of the carbon,
phosphorus, and chlorine derived from dichlorvos are retained in the
body for several days because their turnover rate is the same as that
for identical materials from other origins.
6.5.3 Indicator media
The determination of dichloroethanol in urine as a means of
monitoring the exposure of human beings to dichlorvos is not
sufficiently sensitive to detect levels arising from vapour exposure
through normal use. However, it could serve as the basis for a
specific detection method for the accidental ingestion of high levels
(Hutson & Hoadley, 1972a). Two other methods can be used: (a)
determination of the blood ChE activity; or (b) determination of
dimethylphosphate in urine by a rather complicated method (Blair &
Roderick, 1976). Neither method is specific when exposure to other
organophosphate or carbamate compounds, or to compounds that also
metabolize to dimethylphosphate, may have occurred.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
Lal (1982) reviewed the accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms.
Microorganisms undoubtedly have the ability to metabolize
organophosphorus insecticides; however, there are still large gaps in
our knowledge. It also seems clear that chemical, photochemical,
physical, and biological factors may influence the metabolism of
dichlorvos by microorganisms.
7.1.1 Algae and plankton
The dose of dichlorvos producing 50% growth inhibition of the
unicellular alga Euglena gracilis has been quoted as 3.5 mg/litre
(Butler, 1977).
Treating eutrophic carp ponds with 0.325 mg/litre killed
Cladocera (predominantly Bosmina and Daphnia species) and decreased
Copepoda (mainly Cyclops ). This was offset by increased development
of Rotatoria (mainly Polyarthra and Brachionus species) and
phytoplankton (mainly Scenedesmus and Pediastrum species), so that
the total plankton biomass changed only slightly (Grahl et al., 1981).
7.1.2 Fungi
Dichlorvos (in the range 10 - 80 mg/litre) has been found to affect
citric acid fermentation in Aspergillus niger grown in an artificial
medium. Inhibition of the fermentation was marked only at 40 and
80 mg/litre (Rahmatullah et al., 1978; Ali et al., 1979c). It appears
from the decreased uptake of inorganic phosphorus that dichlorvos may
have an interfering action on oxidative metabolism in A. niger. The
potential for inhibiting citrinin production by Penicillium
citrinum was investigated. Dichlorvos inhibited citrinin production by
76% at 100 µg/litre and by 48% at 10 µg/litre (Draughon & Ayres,
1978). The effect of dichlorvos on the survival time and the membrane
potential of the slime mould Physarum polycephalum was studied in a
laboratory test system. The threshold value for both these effects was
found to be 300 mg/litre for technical dichlorvos and 30 mg/litre for
the pure chemical (Terayama et al., 1978).
The influence of dichlorvos on 17 soil fungi, cultivated in
artificial medium, was tested. Dose levels of 0, 10, 30, 60, and
120 mg/kg were used during a test period of 21 days, and the effect on
the growth and morphology of the fungi was studied. In general, a
growth depression was found, but its extent depended on the fungal
strain. Occasionally growth was either unaffected or even stimulated
(Jakubowska & Nowak, 1973).
7.1.3 Bacteria
Dichlorvos has been found not to influence the overall metabolic
processes of Escherichia coli and Enterobacter aerogenes at doses up to
250 mg/litre (Grahl et al., 1980) and was not toxic for a sewage
isolate at up to 10 mg/litre (Rosenberg et al., 1979). In poultry
effluent slurry, concentrations of 100 and 1000 mg/litre did not
significantly reduce coliform populations, but 10 000 mg/litre caused
almost complete death. Therefore, residues of dichlorvos used for fly
control in layer houses can significantly reduce the enteric coliform
populations that are essential to the conversion of organic nitrogen to
inorganic nitrogen in poultry waste effluent (Ballington et al.,
1978).
In studies by Lieberman & Alexander (1981), dichlorvos (0.1 -
100 mg/litre) had little or no toxicity for microorganisms degrading
organic matter in sewage, as measured by respiratory activity,
degradation, and nitrification.
Incubation of dichlorvos with inocula of ruminal bacteria or
ciliated protozoa under anaerobic conditions suggests that dichlorvos
is not utilized by the organisms for growth, nor does it stimulate
endogenous gas production. However, it does, in certain instances,
affect volatile fatty acid production (Williams, 1977).
The growth of Bacillus thuringiens var th. was not inhibited by
dichlorvos (Dougherty et al., 1971).
7.2 Aquatic Organisms
Reviews of the acute and chronic effects of pesticides on aquatic
organisms have been made by Brungs et al. (1977), Livingston (1977),
and Kenaga (1979).
The toxicity of a chemical for aquatic organisms is influenced by
many factors such as the stage of development of the organism and the
composition, pH, oxygen content, and hardness of the water. In this
short review, these factors are not discussed in detail.
7.2.1 Fish
7.2.1.1 Acute toxicity
The acute toxicity of dichlorvos for both freshwater and estuarine
species of fish is moderate to high. The available data are summarized
in Table 11.
Variations in water hardness from 44 to 162 mg/litre and in pH from
6 to 9 did not alter the toxicity of dichlorvos for cutthroat or lake
trout (Johnson & Finley, 1980).
In studies by Yamane et al. (1974), young carp were exposed to a
concentration of 25 mg dichlorvos/litre water for 45 min. The ChE
activity (histochemically determined) of many tissues, including the
stratum griseum periventriculare, sarcolemma, and liver, was inhibited
or totally lost.
Table 11. Acute toxicity of dichlorvos for fish
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Freshwater
Clarias batrachus 26 - 31 g - 8.9 Verma et al. (1983)
Carp 8 mm 20 - 23 0.34 Verma et al. (1981d)
(Cyprinus carpio) 6 g 23 20a Yamane et al. (1974)
Mosquito fish 0.2 g 17 5.3 Johnson & Finley (1980)
(Gambusia affinis)
Blue gill 1.5 g 18 0.9 Johnson & Finley (1980)
(Lepomis macrochirus)
African catfish 6 - 10 g 18 0.5 Verma et al. (1980, 1981a)
(Mystus vittatus)
Ophiopcephalus punctatus 40 - 55 g 18 2.3 Verma et al. (1981a)
Fathead minnow 0.7 g 17 12 Johnson & Finley (1980)
(Pimephales promelas)
Harlequin fish - 20 7.8b Alabaster (1969)
(Rasbora heteromorpha)
Singii 5 - 10 g 18 6.6 Verma et al.
(Saccobranchus fossilis) (1982a)
Cutthroat trout 2.5 g 12 0.2 Johnson &
(Salmo clarki) Finley (1980)
Lake trout 0.3 g 12 0.2 Johnson &
(Salvelinus namaycush) Finley (1980)
Tilapia mossambica 3 - 10 cm 29 1.4 - 1.9 Rath & Misra
(1979a)
----------------------------------------------------------------------------------------------------
Table 11. (contd).
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Estuarine
American eel 0.14 g 20 1.8 Eisler (1970)
(Anguilla rostrata)
Mummichog 1.7 g 20 2.7 Eisler (1970)
(Fundulus heteroclitus)
Striped killifish 0.92 g 20 2.3 Eisler (1970)
(Fundulus majalis)
Atlantic silverside 0.8 g 20 1.3 Eisler (1970)
(Menidia menidia)
Striped mullet 1 - 6 g 20 0.23 Eisler (1970)
(Mugil cephalus)
Northern puffer 100 g 20 2.3 Eisler (1970)
(Sphaeroidus maculatus)
Bluehead 5.4 g 20 1.4 Eisler (1970)
(Thalassoma bifasciatum)
----------------------------------------------------------------------------------------------------
a 24-h LC50.
b 48-h LC50.
7.2.1.2 Short-term toxicity
Sublethal concentrations of dichlorvos (0.5 - 1 mg/litre) have
been found to decrease the respiratory rates of Tilapia mossambica (3
different age groups) exposed for up to 3 weeks. When the exposed
fish were transferred to fresh water, the rate did not completely
return to its pre-exposure value (Rath & Misra, 1979b). Liver and
brain ChE activity showed considerable inhibition when a group of T.
mossambica was exposed to dichlorvos (0.25 - 1.25 mg/litre water) for
periods of up to 4 weeks. At 7-day intervals, fish were studied or
transferred to clean water. The degree of enzyme inhibition was
related to the dichlorvos concentration and length of exposure. In all
age groups of fish, brain tissue exhibited a higher degree of ChE
inhibition than liver. Small fish were more susceptible to dichlorvos
with respect to AChE activity. When the fish were transferred to clean
water, most of the fish recovered their AChE activity, the recovery
being greater in liver than in brain. Small fish exhibited a
comparatively high level of recovery. The degree of recovery was
inversely related to the length of exposure (Rath & Misra, 1981).
Melanin dispersion in T. mossambica exposed to 1 mg/litre water for
15 days was stimulated indirectly by the inhibition of ChE activity.
The original colour was regained within 96 h after transfer of the fish
to clean water (Rath & Misra, 1980).
Exposure of Mystus vittatus (collected in the environment) to
sublethal concentrations of dichlorvos (0.045 or 0.09 mg/litre) for 30
days caused dose-related increases in serum glutamic-oxaloacetic and
glutamic-pyruvic transaminase levels (Verma et al., 1981b), increases
in alkaline phosphatase, acid phosphatase, and glucose-6-phosphatase
levels in serum (Verma et al., 1984), decreases in the levels of these
enzymes in liver, kidneys, and gills (Verma et al., 1981c), an increase
in glucose levels in blood, and a decrease in liver glycogen. Blood
lactate and muscle glycogen were unaffected (Verma et al., 1983). At
0.09 mg/litre, blood clotting time, mean corpuscular haemoglobin and
mean corpuscular haemoglobin concentration decreased, and the number of
leukocytes increased. Other haematological parameters did not show any
abnormalities (Verma et al., 1982b). From these results, a no-
observed-adverse-effect concentration of 0.03 mg/litre was derived.
In studies by Verma & Tonk (1984), Heteropneustes (Saccobranchus)
fossilis was exposed to dichlorvos for 30 days at a concentration of
0.44 mg/litre. Respiration, haematological parameters, and the
activities of two enzymes (one of them AChE) in liver, kidneys, and
gills were determined. The respiration rate decreased, and blood
concentrations of sodium and chloride ions and glucose increased
significantly, whereas the cholesterol level and clotting time were
decreased. A significant reduction in the AChE activity of the three
tissues was found.
Vadhva & Hasan (1986) studied the effect of dichlorvos (at 0, 3, 6,
and 9 mg/litre water) on various lipid fractions and lipid
peroxidation in the central nervous system of Heteropneustes
fossilis. After one week's exposure, the results indicated that
dichlorvos caused dose-related increases in total lipids, cholesterol-
esterified fatty acid, and lipid peroxidation in various regions of the
brain and spinal cord but a consistent decrease in the level of
phospholipids in these regions of the central nervous system.
Exposure of Clarias batrachus to 0.5 - 2.2 mg dichlorvos/litre
and Saccobranchus fossilis to 0.4 - 1.6 mg/litre for 30 days was found
to increase blood glucose and decrease liver glycogen, whereas blood
lactate and muscle glycogen were normal (Verma et al., 1983).
The estimated "maximum acceptable toxicant concentration"
(MATC)a for the larvae of Cyprinus carpio was 0.016 - 0.020 mg/litre
based on a 60-day study (Verma et al., 1981d).
7.2.2 Invertebrates
The acute toxicity of dichlorvos for aquatic insects and
crustaceans is extremely high (Table 12). As might be expected from an
organophosphorus insecticide, aquatic invertebrates are about three
orders of magnitude more susceptible to dichlorvos than are fish, and
freshwater crustaceans are particularly sensitive.
A study was carried out to determine the influence of a number of
pesticides on the "hatchability" of Artemia salina dry eggs. No effect
was found at 10 mg dichlorvos/litre in the aqueous system (Kuwabara et
al., 1980).
When prawns (Macrobrachium lamarrei) were exposed to dichlorvos at
concentrations of 0.31 or 0.62 mg/litre for 96 h, a decrease in hepatic
glycogen and an increase in the blood glucose level were found (Omkar &
Shukla, 1984). Possibly, the phosphorylase activity of the
hepatopancreas and muscle increased due to the inhibition of AChE
activity and the consequent accumulation of acetylcholine at
neurosynaptic junctions. The latter resulted in an induction of the
secretion of the sinus gland, which enhanced glycogenolysis.
7.3 Terrestrial Organisms
7.3.1 Birds
7.3.1.1 Acute oral toxicity
Dichlorvos has a high oral toxicity for birds (Table 13). The
signs of intoxication are typical of organophosphorus poisoning, namely
salivation, lachrymation, tremors, and terminal convulsions. They
usually appear shortly after dosing, and, at lethal doses, death occurs
within 1 h. Survivors appear to recover completely 24 h after dosing.
Various internal haemorrhages were found at autopsy in sacrificed
survivors of treated pheasants and Mallard ducks (Tucker & Crabtree,
1970).
-----------------------------------------------------------------------
a Maximum concentration at which no effect was seen.
Table 12. Acute toxicity of dichlorvos for non-target aquatic insects and Crustacea
-------------------------------------------------------------------------------------------------------
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (µg/litre) (µg/litre)
-------------------------------------------------------------------------------------------------------
Insects (stone flies)
Pteronarcys californica 15 - 0.1 Johnson & Finley (1980)
Crustacea (fresh water)
Water flea 15 0.07 - Johnson & Finley (1980)
(Daphnia pulex)
Water flea 21 0.28 - Johnson & Finley (1980)
(Simocephalus serrulatus)
Amphipod 21 - 0.5 Johnson & Finley (1980)
(Gammarus lacustris)
Crustacea (estuarine)
Sand shrimp - 12 4 Eisler (1969)
(Crangon septemspinosa)
Grass shrimp - 300 15 Eisler (1969)
(Palaemonetes vulgaris)
Hermit crab - 52 45 Eisler (1969)
(Pagurus longicarpus)
-------------------------------------------------------------------------------------------------------
Table 13. Acute oral LD50 values for birds
------------------------------------------------------------------------------------------------------------------------
Species Age Vehicle LD50 Reference
(mg/kg body weight)
------------------------------------------------------------------------------------------------------------------------
Red-winged blackbird propylene glycol 13.3 Schafer & Brunton (1979)
(Agelaius phoenicius) (male)
Mallard duck 5 - 7 months capsule 7.8 Tucker & Crabtree (1970)
(Anas platyrhynchos) (male)
Common pigeon propylene glycol 24 Schafer & Brunton (1979)
(Columba livia)
Quaila propylene glycol 24 Schafer & Brunton (1979)
(Coturnix coturnix) (female)
Domestic fowl 21 days aqueous suspension 6.5 Naidu et al. (1978)
(Gallus domesticus) (male) 6 - 8 months aqueous suspension 30 Dmitriev & Kozhemyakin
(1975)
House sparrow propylene glycol 17.8 Schafer & Brunton (1979)
(Passer domesticus)
Ring-necked pheasant 3 months capsule 11 Tucker & Crabtree (1970)
(Phasianus colchicus) (male)
Common grackle propylene glycol 13.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Starling adult propylene glycol 12 Schafer (1972)
(Sturnus vulgaris) - propylene glycol 42.1 Schafer & Brunton (1979)
------------------------------------------------------------------------------------------------------------------------
a Hattori et al. (1974) carried out studies on Japanese quail and found
LD50 values of 22 and 26 respectively, for male and female
(no details available).
7.3.1.2 Short-term toxicity
In short-term dietary studies, dichlorvos has been found to be
slightly to moderately toxic for birds (Table 14).
In the study with 7-day-old male chicks, there was weight loss and
50% mortality at 500 mg/kg diet. Marked, dose-related inhibition of
brain ChE activity occurred at 50, 100, and 500 mg/kg diet, but no
effects were noted at a level of 10 mg/kg diet (Naidu et al., 1978).
Canaries, Indian finches, and budgerigars, continuously exposed to
dichlorvos vapour (0.14 mg/m3) for 5 days, did not show any overt
signs of intoxication, but a reduction in ChE levels in plasma and
brain was observed in canaries and Indian finches (Brown et al.,
1968).
7.3.1.3 Field experience
Caution has been advised in the use and handling of dichlorvos
where birds might be exposed (Whitehead, 1971). The necessity for this
warning can be illustrated by the following cases. Adult mallards
feeding near horse mangers containing dichlorvos-treated feed were
found dead within a short time. At necropsy, excessive amounts of
mucus covering the mucosa of the proventriculus and scattered petechiae
along medial edges of liver lobes were observed. The small and large
intestines were markedly extended, and crystals were noted in the
gizzard. Brain ChE activity was inhibited by 75 - 80% (Ludke & Locke,
1976). Domestic fowl, which had accidental access to the faeces of a
horse dosed with dichlorvos pellets, picked out the pellets and more
than 30 birds died during the next 24 h (Lloyd, 1973). A mass
poisoning occurred in chickens following consumption of accidentally
contaminated drinking-water (Egyed & Bendheim, 1977). English game
bantams died after consuming wheat contaminated with 300 mg
dichlorvos/kg wheat (Reece, 1982).
7.3.2 Invertebrates
Dichlorvos was toxic for silkworm larvae when for 4 h they were fed
mulberry leaves previously sprayed with dilute dichlorvos emulsions.
Spray concentrations giving 50% mortality ranged from 1.56 to 6.25
mg/litre (Aratake & Kayamura, 1973).
No adverse effects were observed on the hatchability and general
condition of first instar silkworm larvae hatched in the following
generation when 5th instar larvae were fed mulberry leaves pre-treated
with 3 mg dichlorvos/kg of leaf (Yamanoi, 1980).
Table 14. Dietary LD50 values for birds
-------------------------------------------------------------------------------------
Species Age Duration LD50 Reference
(days) of feeding (mg/kg diet)
(days)
-------------------------------------------------------------------------------------
Mallard duck 16 8a 5000 Hill et al. (1975)
(Anas platyrhynchos) 5 8a 1310 Hill et al. (1975)
Japanese quail 14 8a 300 Hill et al. (1975)
(Coturnix japonica)
Domestic fowl 7 28 500 Naidu et al. (1978)
(Gallus domesticus) (male)
Ring-necked pheasant 10 8a 570 Hill et al. (1975)
(Phasianus colchicus)
-------------------------------------------------------------------------------------
a The median lethal dietary dose (LD50) during an 8-day test including
5 days of treated diet followed by 3 days of untreated diet.
7.3.3 Honey bees
Dichlorvos is highly toxic for honey bees. Atkins et al. (1973)
found in laboratory studies an LD50 of 0.495 µg/bee in 48 h (topical
application of dust; 26.7 °C; relative humidity 65%). Beran (1979)
obtained an oral LD50 of 0.29 µg/g body weight and an LD50 (topical
application) value of 0.65 µg/g body weight. This high toxicity has
led dichlorvos to be classified in the most toxic category for bees in
Austria and the USA.
When honeycombs were exposed to dichlorvos vapour from dichlorvos
resin strips for 4 months, the combs absorbed the insecticide and were
toxic to bees for approximately one month after exposure ceased.
Contamination of the bees appeared to be by fumigant rather than
contact action (Clinch, 1970).
7.3.4 Miscellaneous
Dichlorvos was highly toxic for the predatory mite Amblyseius
longispinosus in contact trials. Residual toxicity disappeared within
6 days, the susceptibility being the same as that of Phytoseiulus
persimilis (Shinkaji & Adachi, 1978).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides in general, especially their short- and long-term effects
on the nervous system, will be found in Environmental Health Criteria
63: Organophosphorus Insecticides - A General Introduction (WHO,
1986).
8.1 Single Exposures
Dichlorvos is moderately to highly toxic when administered in
single doses by various routes to a variety of animal species
(Table 15). It is less toxic via the dermal and oral routes than by
parenteral routes. The signs of intoxication by all exposure routes
are typical of organophosphorus poisoning, i.e., salivation,
lacrimation, diarrhoea, tremors, and terminal convulsions, with death
occurring from respiratory failure. In addition, lethargy, ataxia,
hypersensitivity to noise, splayed gait, and paresis may be observed.
The signs are usually apparent shortly after dosing and, at lethal
doses, death occurs within 1 h. Survivors appear to recover completely
24 h after dosing.
The acute inhalational LC50 values for mice and rats are
summarized in Table 16. The apparent differences in the LC50s may be
the result of the type of exposure of the animal (whole body or head
only), whether the studies were carried out with dichlorvos vapour or
atomized particles of spray (with or without vehicle), or differences
in the purity of the dichlorvos. Moreover, since dichlorvos adheres
strongly to surfaces including glass, the out-going air has a
significantly lower concentration of dichlorvos than the air coming
into the chamber, if the system is not yet equilibrated.
No macroscopic abnormalities were observed in mice or rats 2 weeks
after a single exposure (MacDonald, 1982).
8.1.1 Domestic animals
When cattle and sheep were treated orally with a single dose of
dichlorvos, 10 mg/kg body weight was toxic for calves and 25 mg/kg body
weight for sheep. For the latter a dose of 10 mg/kg body weight was
without effects (Radeleff & Woodard, 1957).
8.1.2 Potentiation
Potentiation studies on male rats indicated that oral
administration of dichlorvos with 22 other organophosphate pesticides
resulted in no (or very little) potentiation, while administration with
malathion showed a marked potentiation (Narcisse, 1967; Kimmerle &
Lorke, 1968). However, Cohen & Ehrich (1976) found that the anti-ChE
action of 800 mg malathion/kg body weight (injected intraperitoneally)
was not potentiated by pre-treatment (18 h previously) with 30 mg/kg
dichlorvos, nor did malathion pre-treatment potentiate the action of
dichlorvos.
Table 15. The acute toxicity (LD50) of dichlorvos for experimental animals
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Mouse (male) oral unknown Eryfor EL or other 68 - 90 Vrbovsky et al. (1959);
solvents Ueda et al. (1960)
Mouse oral 80% unknown 87 Sasinovich (1968, 1970)
Mouse oral 97% aqueous polysor- 133 - 139a Haley et al. (1975)
bate 80
Mouse (male) oral 98% corn oil 140 Isshiki et al. (1983)
Mouse oral unknown aqueous 124 - 275a Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) subcutaneous unknown propylene glycol 13 - 33 Ueda et al. (1960);
and other solvents Jaques (1964)
Mouse subcutaneous unknown aqueous 20 - 26 Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) dermal unknown different solvents 206 & 395b Ueda et al. (1960)
Mouse intraperitoneal technical corn oil 28 Vrbovsky et al. (1959);
Casida et al. (1962)
Mouse intraperitoneal unknown aqueous 28 - 41a Holmstedt et al. (1978)
Mouse intravenous unknown aqueous 8 - 10 Holmstedt et al. (1978)
Rat oral 90 - 99% peanut oil and 56 - 96a Durham et al. (1957);
other solvents Narcisse (1967); Gaines
(1969)
Rat (male) oral unknown Eryfor EL and 46 - 110 Vrbovsky et al. (1959);
other solvents Ueda et al. (1960)
Rat oral 80% unknown 65 Sasinovich (1968, 1970)
Rat oral unknown aqueous 30 Holmstedt et al. (1978)
---------------------------------------------------------------------------------------------------------
Table 15. (contd).
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Rat dermal unknown xylene 75 - 107a Durham et al. (1957);
Gaines (1969)
Rat dermal 80% unknown 113 Sasinovich (1968, 1970)
Rat subcutaneous 95% dimethylsulfoxide 72 Brown & Stevenson
(1962)
Rat (male) intraperitoneal unknown Eryfor EL 18 Vrbovsky et al. (1959)
Guinea-pig subcutaneous 95% undiluted 28 Brown & Stevenson
(1962)
Syrian hamster intraperitoneal - suspension in 30 Dzwonkowska & Hübner
water (1986)
Rabbit oral 93% unknown 12.5 Desi et al. (1978)
Rabbit oral 80% unknown 22.5 Sasinovich (1968, 1970)
Rabbit dermal 80% unknown 205 Sasinovich (1968, 1970)
Cat oral 80% unknown 28 Sasinovich (1968, 1970)
Dog oral in capsule 100 - 316 Kodama (1960)
Swine (40- to 60- oral technical in capsule 157 Stanton et al. (1979)
day-old)
Domestic fowl oral technical in capsule 15 Sherman & Ross (1961)
(chicken)
---------------------------------------------------------------------------------------------------------
a The LD50s are often slightly different in males and females. Furthermore, it is clear that the
purity of the dichlorvos tested and the vehicle used has an influence on the toxicity.
b The two values were obtained using two different solvents.
Table 16. Inhalational LC50 values for dichlorvos
---------------------------------------------------------------------------------------------------------
Species Purity Type of Duration LC50 (mg/m3) Reference
exposure
---------------------------------------------------------------------------------------------------------
Mouse 80% vapour 4 h 13 Sasinovich (1968, 1970)
whole body
Mouse 98% vapour 4 h > 218 MacDonald (1982)
head only
Mouse (male) unknown; in vapour 4 h 310 Ueda et al. (1960)
solvent Ca whole body
Rat (male) unknown unknown, but 1 h 455 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat (male) 84.1% whole body 1 h 140 Sakama & Nishimura
polyethylene (1977)
glycol
Rat (male) unknown unknown, but 4 h 340 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat 80%(?) vapour 4 h 15 Sasinovich (1968, 1970)
whole body
Rat 98% vapour 4 h > 198 MacDonald (1982)
head only
---------------------------------------------------------------------------------------------------------
a Solvent C = 0.5% Sorpol 2020 water solution.
In vitro studies using human erythrocytes and plasma as ChE
sources, with ACh as substrate, indicated no potentiationa when
dichlorvos was tested in combination with carbaryl, crotoxyphos,
phosphamidon, malathion, malaoxon, mevinphos, parathion, paraoxon,
physostigmine, and trichlorphon (Carter & Maddux, 1968, 1974).
8.2 Short-term Exposures
8.2.1 Oral
8.2.1.1 Mouse
A 10-week (range-finding) toxicity study was carried out on B6C3F1
mice given 0, 25, 50, 100, 200, or 400 mg dichlorvos/ litre drinking-
water. Each group consisted of 12 males and 12 females, except the
control group (10 males and 10 females). Growth and mortality were
comparable with controls. In a second study, groups of 10 males and 10
females received 400, 1600, 3200, 5000, or 10 000 mg dichlorvos/litre.
The animals given the two highest doses died within 2 weeks, while the
1600 and 3200 mg groups showed slight and clear growth depression,
respectively, after 10 weeks (Konishi et al., 1981).
8.2.1.2 Rat
For 15 weeks, groups of 15 male and 15 female Charles River rats
were fed diets containing 0, 0.1, 1, 10, 100, or 1000 mg dichlorvos
(93%)/kg diet, which were freshly prepared once each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1), it may be
assumed that the average concentration of dichlorvos in each diet was
approximately 47% of the amount that had been added. There were no
deaths or signs of intoxication. Only the rats fed the highest dose
exhibited decreased growth rates at the beginning of the study. At
termination, no differences were observed in haematology, serum
protein, urinalysis, or gross or histopathological examination. In the
highest dose group, marked inhibition of ChE activity in plasma,
erythrocytes, and brain was noted. In the 100 mg/kg group, only
erythrocyte ChE inhibition was observed, and the 10 mg/kg group did not
show any ChE inhibition (Witherup et al., 1964).
Daily doses of 3.5 or 7 mg dichlorvos (purity not stated)/kg body
weight administered intragastrically to rats for 4 months and 0.7 or
1.4 mg/kg for 12 months caused no deaths. There were signs of
intoxication in the 7 mg group, and decreases in food consumption and
body weight gain and increases in a number of organ weights were seen
in all the groups except the lowest dose group. Inhibition of plasma,
erythrocyte, and brain ChE activity was observed in all groups except
those given 0.7 mg/kg body weight. The inhibition was time and dose
dependent (Sasinovich, 1970).
-----------------------------------------------------------------------
a Potentiation is the phenomenon that results in the combined effect
of exposure to two or more chemical substances being greater than
the sum of the effects that would be produced by each substance
separately, as the result of synergistic action.
In a (range-finding) toxicity study, groups of F-344 rats (10 of
each sex) were administered 0, 5, 10, 20, 40, or 80 mg/kg body weight,
dissolved in 10 ml of drinking-water. During the study six animals
died, five of which were in the two highest doses groups (Enomoto,
1981).
Dichlorvos administered orally to rats at a concentration of 70
mg/kg body weight inhibited not only ChE, but also alkaline
phosphatase, lactate dehydrogenase, and glutamate dehydrogenase
competitively. It increased the activity of glutamic-pyruvic
transaminase, but leucine aminopeptidase was not affected (Ellinger,
1985).
Ellinger et al. (1985) also studied the influence of dichlorvos on
haematological parameters in acute and short-term toxicity studies. In
the acute study 70 mg dichlorvos/kg body weight and in the short-term
study 30 mg/kg body weight were administered for 12 weeks. Decreases
in haemoglobin, haematocrit, and mean corpuscular haemoglobin
concentration were found.
Seven rat mothers were administered different dose levels of
dichlorvos (1 g or 10 g/litre distilled water) by stomach tube, and
their litters (1 - 12 days old) were nursed for about 21 days, and
weaned after 35 days of age. Although doses of 10 and 20 mg/kg body
weight did not cause any symptoms of intoxication, the mothers showed
significant inhibition of erythrocyte ChE activity. Plasma ChE
activity was affected to a lesser extent. Dose levels of 30 and
40 mg/kg body weight caused severe inhibition of erythrocyte ChE, and
severe cholinergic symptoms were seen. The symptoms occurred 10 -
20 min after dichlorvos administration and continued for 30 - 90 min,
when recovery took place (Tracy et al., 1960).
When pregnant rats were given oral doses of 1 or 5 mg dichlorvos in
oil/kg body weight during days 14 - 21 of pregnancy, the plasma ChE
activity of the mothers was markedly inhibited, but that of the young
(up to 56 days old) resembled the activity in the control. Brain ChE
activity did not show any significant inhibition (Zalewska et al.,
1977).
8.2.1.3 Rabbit
The progeny of rabbits, treated orally with 6 mg dichlorvos/kg body
weight per day for the last 10 days of pregnancy showed a decrease in
brain ChE activity and an increase in plasma ChE activity throughout
days 1 - 16 of life (Maslinska & Zalewska, 1978).
8.2.1.4 Cat
"Flea collar dermatitis" has been described in cats and dogs
wearing dichlorvos-impregnated PVC flea collars. In most cases, flea
collar dermatitis is a primary irritant contact dermatitis to
dichlorvos. The symptoms may consist of mild local irritation
(localized erythema and itching), severe local irritation varying from
erythematous alopecia to erosions, ulceration, oedema, and purulent
discharges with crusting or, in the most severe cases, generalized
dermatitis with secondary pyoderma and systemic illness (Muller,
1970).
Two trials with six and five cats, respectively, were carried out
to study the occurrence of contact dermatitis and systemic
intoxication. The cats received either a placebo collar or a
dichlorvos collar. Two cats received two dichlorvos collars each and
each cat was observed for 21 days. In a third trial, four groups of 25
cats were used to study hepatotoxicity. The cats of the different
groups were treated as follows. Group 1: placebo collar; Group 2:
placebo collar and intraperitoneal treatment with carbon tetrachloride
(CCl4) 7 days later; Group 3: dichlorvos collar; Group 4: dichlorvos
collar and intraperitoneal injection of CCl4 7 days later. Serum
glutamic pyruvic transaminase and ChE activity was estimated on day 9
of exposure. In a fourth study, 24 cats were fitted with a placebo
collar to test the effect of PVC in relation to the contact
dermatitis. PVC did not have an effect. No clear effect of the
CCl4-induced hepatic dystrophy on the detoxification of dichlorvos
was found.
The predominant systemic abnormalities recorded in these studies
were bone-marrow depression, ataxia, demyelination, and depression.
Red cell and plasma ChE activity was severely depressed. Furthermore,
contact dermatitis was seen in animals with dichlorvos collars.
Ambient temperature and relative humidity may have had a marked
influence on these effects, especially in the case of high temperature
and low humidity (Bell et al., 1975).
A 90-day study on 90 mongrel cats was carried out to verify the
results of Bell et al. (1975) by using a larger number of animals over
a longer period. There were three groups of 30 cats; one group with a
PVC collar (without dichlorvos), one group with one dichlorvos collar,
and the third group with three dichlorvos collars. In this study, in
which all relevant parameters were studied, no confirmation of the
abnormalities described by Bell et al. (1975) were found, even under
conditions of high temperature and low humidity. The only effect was
contact dermatitis, but, in this study, this was also found in the PVC
collars (Allen et al., 1978).
8.2.1.5 Dog
Groups of three male and three female dogs received equivalents of
approximately 0, 0.3, 1, 1.5, or 3 mg dichlorvos (93% in olive oil by
gelatin capsule)/kg body weight, daily, for 90 days. No effects were
observed on mortality, growth, liver and kidney function, organ
weights, haematology, or at gross and histopathological examination.
In the two highest dose groups, the dogs showed excitement, increased
activity, and aggression. Plasma and erythrocyte ChE activities
(measured initially and at intervals of approximately 2 weeks) were
normal in the lowest dose group (0.3 mg/kg body weight) but reduced in
the other dose groups. Brain ChE activity at termination was reduced
only in the highest dose group (Hine, 1962).
8.2.1.6 Pig
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, and 16 mg dichlorvos/kg
body weight per day in the feed (divided over two daily doses) for 30
days. Body weight gain, blood cell counts, packed-cell volumes,
haemoglobin concentrations, plasma glucose, plasma fatty acids, hepatic
and muscle glycogen concentrations, plasma and skeletal muscle
concentrations of electrolytes, and plasma and pancreatic insulin were
all comparable with those of control swine fed a blank PVC formulation.
Plasma and erythrocyte ChE activities were significantly inhibited in
the 4 and 16 mg/kg groups only (Stanton et al., 1979).
8.2.1.7 Cow
Two cows with suckling calves fed 200 mg dichlorvos/kg grain in
their rations showed normal ChE activity in the erythrocytes. However,
severe inhibition of ChE activity was found at 500 mg/kg feed
(equivalent to 4.5 mg/kg body weight), and a single dose of 27 mg/kg
body weight caused cholinergic collapse, followed by rapid recovery.
The ChE values in the calves remained normal throughout the 78-day
test. Milk from these two cows contained less than 0.08 mg
dichlorvos/litre (Tracy et al., 1960).
8.2.2 Dermal
8.2.2.1 Rat
In studies by Dikshith et al. (1976), groups of 48 male rats
received daily topical applications of 21.4 mg/kg body weight
dichlorvos (96%) in ethanol (control animals received ethanol alone) 5
days per week for 90 days. At intervals of 7, 15, 30, 45, 60, and 90
days, eight test rats and two control rats were sacrificed for
histopathological examination of the skin and testes. None of the
animals showed signs of intoxication, but ChE activity was not
measured. The skin showed no irritation, and no histopathological
changes were seen in the testes or skin.
8.2.2.2 Livestock
Spray and dip studies have shown that a concentration of 1%
dichlorvos is not toxic for cattle (no further details are available)
(Radeleff & Woodard, 1957).
The effect of applying different suspensions (different solvents
with and without an emulsifier) of dichlorvos to the skin of cows has
been studied. The dose level was 5 mg/kg body weight, increasing after
7 days to 10 mg/kg. Dichlorvos at 5 mg/kg body weight caused a
significant decrease in ChE activity in blood serum. The higher dose
level additionally produced symptoms of intoxication. No dichlorvos
residues were found in milk (Majewski et al., 1978).
Two heifers were washed daily with either a 1% aqueous solution or
emulsion of dichlorvos or a 10% suspension of dichlorvos in water
(daily application of 1.8 g dichlorvos for 21 days). Two additional
heifers were used as controls. The ChE activity levels of the
erythrocytes remained at the lower limits of normal variation (Tracy et
al., 1960).
8.2.3 Inhalation
8.2.3.1 Experimental animals
In a report by Sasinovich (1968), groups of rats were exposed
(whole body) daily for 4 h to average dichlorvos concentrations
of 0.11 or 1.1 mg/m3 for 4 months, to 5.2 mg/m3 for 2
months, or to 8.2 mg/m3 for 45 days. The highest dose caused signs
of intoxication; two of the eight rats died. Exposure to
5.2 mg/m3 or more resulted in marked inhibition of ChE activity and
disturbance of the blood-sugar curve. In the 0.11 and
1.1 mg/m3 groups, no significant effects were observed.
Mice, rats, and guinea-pigs, exposed 23 h per day for 28 consecu-
tive days to actual dichlorvos concentrations of 0.03 mg/m3, did not
show gross pathological changes at the end of the study. Inhibition of
plasma and brain ChE activity occurred in male mice, male guinea-pigs
(plasma only), and female rats (brain only) after a 5-day exposure to
0.14 - 0.15 mg/m3 dichlorvos (Brown et al., 1968).
Guinea-pigs (P strain) exposed for 7 h per day for 5 consecutive
days to an actual concentration of 90 - 120 mg/m3 dichlorvos suffered
no visible effects to their health. Rats (CFE) and mice (CF1)
similarly exposed to 50 mg/m3 were not visibly affected. At
concentrations above 50 mg/m3, the mice became distressed and
prolonged exposure to 80 mg/m3 was frequently lethal. Rats were less
severely affected (Stevenson & Blair, 1969).
In studies by Vashkov et al. (1966), 100 mice, 50 rats, 22 rabbits,
and 13 cats were exposed to a mixture containing dichlorvos, kerosene,
xylene, and freons during a single 2-h exposure or for 2 h per
day for 40 days. Three concentrations, 16.5, 45, and 160 mg
dichlorvos/m3, were tested (the median gravimetric diameter of the
aerosol particles was about 5 µm). The overall condition of the
animals remained normal, and no effects on body weight, clinical
chemical blood analyses, blood ChE activity, or respiration rate, or
pathological changes were observed. Rabbits developed transient miosis
which disappeared at the end of the exposure period.
Mice (20), guinea-pigs (6), sheep (7), calves (39), and a heifer
were exposed to an increasing dichlorvos concentration in the air
generated by dichlorvos strips (20%). The number of strips was
increased each week for 6 weeks, reaching 80 strips per 140 m3, and
was then reduced over the next 6 weeks. During exposure to the
recommended number of strips, the dichlorvos concentration in the
air was 0.09 - 0.14 mg/m3. The highest concentration was
2.1 mg/m3 when the number of strips was 16 times the recommended
number. No mortality or signs of intoxication were observed, and mice
bore normal litters during the study. The serum ChE activities of the
calves and of those handling the animals were within normal values
(Henriksson et al., 1971).
When 10 rabbits, 8 cats, and 10 dogs (males and females) were
continuously exposed for 8 weeks to dichlorvos concentrations of 0.05 -
0.3 mg/m3 generated from impregnated PVC-resin strips, no effects were
found on general health, behaviour, plasma and erythrocyte ChE
activities, or electroencephalographic patterns in the brain of the
animals (Walker et al., 1972).
In studies by Coulston & Griffin (1977), four male and four female
Rhesus monkeys were continuously exposed to dichlorvos vapour at an
average actual concentration of 0.05 mg/m3 for 3 months. The control
group consisted of four males and one female. No adverse effects were
observed in appearance, behaviour, or haematological and clinical
chemical determinations. Plasma ChE activity was slightly inhibited
(up to 28% inhibition), whilst the greatest erythrocyte ChE activity
inhibition was 36%. No changes in nerve maximum conduction velocities
or muscle-evoked action potentials were induced by the exposure to
dichlorvos.
8.2.3.2 Domestic animals
Five horses, exposed continuously to dichlorvos for 22 days in a
closed barn that was treated daily with 17 mg dichlorvos/m3, dis-
played mild inhibition of erythrocyte ChE activity after 7 days,
followed by recovery at 11 - 22 days. However, plasma ChE activity was
not changed. The concentration in the barn varied between 0.24 and
1.48 mg/m3 (Tracy et al., 1960).
The influence on ChE activity in cattle exposed to an impregnated
plastic strip containing 20% of dichlorvos has been studied. Red blood
cell ChE activity was measured during the 85 days of exposure, and on
the 9th day an average inhibition of about 35% was found. After the
37th day the inhibition in ChE activity gradually declined to 20%. No
clinical signs were observed (Horvath et al., 1968),
8.2.4 Studies on ChE activity
Groups of 10 young female Sherman rats were fed for 90 days on
diets containing 0, 5, 20, 50, 200, 500, or 1000 mg dichlorvos (90%)/kg
diet (equivalent to 0, 0.4, 1.5, 3.5, 14.2, 35.7, and 69.9 mg/kg body
weight, respectively). Data on the stability of dichlorvos in the diet
were not reported. No clinical signs of intoxication were noted.
Blood samples were taken from two rats of each group for ChE activity
determination on day 3, 11, 60, and 90. In all test groups, a
decrease in plasma ChE activity was observed during the first 4 days,
gradually returning to normal, except in the rats receiving 14.2 mg/kg
body weight or more. At doses above 3.5 mg/kg body weight, erythrocyte
ChE activities were decreased throughout the test, but at 3.5 mg/kg
body weight, this was only so for the first 30 days of the study.
Lower dose levels produced comparable results to those of the control
group (Durham et al., 1957).
Thirty-two Rhesus monkeys were treated with 20% pelleted PVC-resin
formulations of dichlorvos at dosages ranging from 5 to 80 mg/kg of
formulation (equivalent to 1 - 16 mg dichlorvos/kg body weight) daily
or 8 and 20 mg/kg of formulation twice daily for 10 - 21 consecutive
days. None of the monkeys developed overt signs of intoxication,
though they ate less food and had soft faeces. Plasma and erythrocyte
ChE activities were reduced by approximately 80% in all animals, and
remained inhibited until completion of treatment. Plasma ChE
activities returned to normal within approximately 3 weeks, whereas the
erythrocyte ChE activities required 50 - 60 days to return to pre-
treatment values (Hass et al., 1972).
When groups of five male and five female guinea-pigs (P strain)
were given daily applications of 0, 25, 50, or 100 mg dichlorvos
(94%)/kg body weight on the shorn skin for 8 days, all the animals
survived. A significant dose-dependent inhibition of both plasma and
erythrocyte ChE activities occurred in all test groups. Recovery of
plasma ChE activities was complete within one week of the last
exposure, and that of erythrocyte ChE activities was complete within
one week in the females and 2 weeks in the males (Brown & Roberts,
1966).
Three Cynomolgus monkeys were treated daily with dermal doses of
50, 75, and 100 mg/kg body weight technical dichlorvos in xylene for 5
days per week until the animals died (after 8, 10, and 4 doses,
respectively). Symptoms of intoxication appeared in all monkeys,
beginning 10 - 20 min after administration of the first dose. The
erythrocyte ChE activity decreased rapidly and remained severely
inhibited for the period of the study, while plasma ChE activities
fluctuated throughout the study (Durham et al., 1957).
When male and female mice, rats, and guinea-pigs were exposed
continuously by inhalation for 5 consecutive days to actual
concentrations of 0, 0.14 - 0.15, or 1.1 - 1.3 mg/m3 dichlorvos, no
overt signs of intoxication were observed. In the high exposure
groups, plasma, erythrocyte, and brain ChE activities were inhibited in
all three species. Plasma ChE was the most sensitive in all three
species, with up to 70% inhibition in the female mice. The greatest
inhibition of brain ChE activity (30%) was found in mice (Brown et al.,
1968).
The effects of dichlorvos vapour inhalation on AChE activity was
investigated in the rat. Exposure to dichlorvos concentrations of 0.8
and 1.8 mg/m3 for 3 days reduced AChE activity in the bronchial tissue
(50 - 60% of control) but did not produce any changes in blood AChE
activity. However, at 4.3 mg/m3, blood AChE activity also declined
(38% of control). In histochemical preparations, staining of the
bronchial glands and smooth muscles revealed reduced enzyme activity
even at the lowest dose (0.2 mg/m3) tested. At this concentration,
no inhibition of bronchial homogenate ChE was observed (Schmidt et al.,
1975, 1979). The significance of bronchial ChE inhibition is not
clear.
Male mice were continuously exposed to actual concentrations of 0
or 0.03 mg dichlorvos/m3 for 28 consecutive days. Weekly assays of
ChE activities showed that brain ChE activity was slightly inhibited
(less than 20%) on the 28th day only, but plasma and erythrocyte ChE
activities were not significantly inhibited (Brown et al., 1968).
Rabbits exposed to an average concentration of 1 mg
dichlorvos/m3 (0.8 - 1.3 mg/m3) for 4 h per day for 4 months showed
up to 30% inhibition of serum and erythrocyte ChE activities during the
study. However, cats similarly exposed did not show significant
inhibition (Sasinovich, 1968).
Rats and monkeys were exposed for 2 weeks in an inhalation chamber
sprayed once with an emulsion of dichlorvos. The initial air
concentration was 6 mg dichlorvos/m3, decreasing over a few days to
about 0.1 - 0.2 mg/m3. No signs of intoxication were seen. The
plasma and erythrocyte ChE activities of the monkeys decreased to
approximately 50% of pre-exposure levels, but rapid recovery took place
after cessation of exposure. Comparable results were obtained when
rats and monkeys were continuously exposed for up to 7 days to a
maximum air concentration of 2.2 mg/m3. However, continuous exposure
for 4 days to 0.27 mg/m3 did not produce any effect on ChE activity
(Durham et al., 1957).
Groups of two monkeys were exposed to dichlorvos vapour 2 h per day
for 4 consecutive days. With concentrations up to 0.7 mg/m3, no
change in plasma or erythrocyte ChE activity was noted, while 1.2 -
3.3 mg/m3 caused a slight decrease in plasma ChE activity, and 7.5 -
18 mg/m3 (mean: 13 mg/m3) resulted in miosis and a pronounced
inhibition (40 - 70%) of plasma and erythrocyte ChE activities (Witter
et al., 1961).
Groups of 10 chickens were exposed to dichlorvos vapour from a
varying number of strips (20% dichlorvos) continually or intermittently
for 3 weeks. Exposure to a single strip in a room of 33 m3 (either
interrupted or for 16 h every day) produced no significant effect on
plasma or brain ChE activity. Exposure to more than one strip (2 - 5)
resulted in up to 50% inhibition of plasma ChE activity and
approximately 40% inhibition of brain ChE activity. Dichlorvos aerosol
sprays (0.7% dichlorvos) showed that daily excessive spraying for 6
seconds, 8 times per day, for 5 days, did not produce significant ChE
inhibition. However, a significant decrease in plasma ChE activity was
found when the study lasted for 21 days (Rauws & van Logten, 1973).
Three studies were carried out to determine the effect on the
laying performance of hens given dichlorvos in the feed for 4 weeks.
Plasma AChE levels were reduced by 70% at 20, 30, or 40 mg
dichlorvos/kg diet, although there was no clear influence on food
consumption, egg production, egg weight, or hatchability at these
doses. At 80 mg/kg diet, a decrease in food consumption and in egg
production was seen, the latter being a consequence, possibly, of the
former (Pym et al., 1984).
8.3 Skin and Eye Irritation; Sensitization
In the skin sensitization assay procedure of Stevens (flank/flank
technique), 1% dichlorvos in olive oil produced no visible effects in
male albino guinea-pigs. Negative results were also obtained when five
components of formulated dichlorvos/ PVC products were assayed for
their skin sensitization potential (Kodama, 1968).
A primary skin irritation test on dichlorvos was performed by using
male New Zealand white rabbits. Irritation observed on the skin after
the application of 5 - 20% water solutions of dichlorvos was relatively
severe compared with that caused by other organophosphorus insecticides
(Arimatsu et al., 1977).
In order to study the allergenicity of dichlorvos, the guinea-pig
maximization test was used. Threshold limit values of primary
irritancy tested on the skin of guinea-pigs (Hartley strain) were 2% or
more. In the maximization test, 0.05 and 0.5% were used. With 0.5%,
35% of the animals showed slight or discrete erythema. The
allergenicity rating as determined by the Kligman test was moderate. In
combination with methidathion, dichlorvos showed a stronger reaction,
indicating cross-sensitization (Fujita, 1985).
8.4 Long-term Exposure
8.4.1 Oral
8.4.1.1 Rat
In studies by Witherup et al. (1967, 1971), groups of 40 male and
40 female weanling CD rats were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg
diet for 2 years. Five males and five females from each group were
sacrificed after 6, 12, and 18 months, and analysis of diet samples
showed a considerable loss of dichlorvos. This was associated with a
gradual increase in DCA concentration which ranged in the different
groups from 0.014 to 28.6 mg/kg diet. The average actual
concentrations of dichlorvos in each diet were 0, 0.047, 0.467, 4.67,
46.7, and 234 mg/kg diet (equivalent to approximately 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). There were no signs of
intoxication, and no effects were seen on behaviour, mortality rate,
food consumption, weight gain, organ weights, haematology, or
urinalysis. Plasma and erythrocyte ChE activities were decreased
throughout the study in the two highest dose groups compared with
controls, but brain ChE activity was decreased in the highest dose
group only. Histological examination of major organs revealed
hepatocellular fatty vacuolization in the group with 234 mg/kg and in
some of the animals with 46.7 mg/kg diet. No effect was seen on serum
total proteins or albumin:globulin ratio, or on hexobarbital sleeping
time. It can be concluded that the actual average concentration of
4.7 mg/kg diet (equivalent to approximately 0.25 mg/kg body weight) was
without significant effect on any of the measured parameters. The
tumour incidence was comparable with that of the control group.
8.4.1.2 Dog
Groups of three male and three female beagle dogs were fed diets
containing 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg diet for 2
years, the average actual concentrations being 0, 0.09, 0.32, 3.2, 32,
and 256 mg dichlorvos/kg diet (equivalent to 0, 0.002, 0.008, 0.08,
0.8, and 6.4 mg/kg body weight). The average DCA concentration in the
diets with 10, 100, and 500 mg dichlorvos/kg diet was 0.6, 6.4, and 20
mg/kg diet. No effects were seen on general appearance, survival, food
consumption, weight gain, haematology, or urinalysis. However,
erythrocyte ChE was inhibited at dose levels of 3.2 mg/kg diet or more,
and plasma ChE activity was inhibited at the two highest dose levels.
Recovery to control values took place at the end of the feeding period.
Brain ChE activity, measured at the end of the study, was similar to
that of the controls, and liver weights were increased in both sexes in
the 256 mg/kg diet group. Histological examination of major organs
revealed slight dose-related alterations in the hepatic cells of one
female in the 3.2 mg/kg diet group, and greater alterations at higher
doses. No differences were seen in serum alkaline phosphatase, trans-
aminase activities, total serum proteins, or albumin:globulin ratios.
The actual average concentration of 0.32 mg/kg diet (equivalent to
0.008 mg/kg body weight) was without effect (Jolley et al., 1967;
Witherup et al., 1971).
8.4.2 Inhalation
8.4.2.1 Rat
When groups of 50 male and 50 female weanling CFE rats were
exposed for 23 h per day to air concentrations of 0, 0.05, 0.5, or
5 mg dichlorvos (97%)/m3 air (actual concentrations: 0, 0.05, 0.48,
and 4.7 mg/m3) for 2 years, body weight gain was reduced in the
two highest dose groups. After 2 years of exposure, plasma and
erythrocyte ChE activities were significantly reduced in the two
highest dose groups, but in the case of brain ChE activity, only in the
highest group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry values,
or organ weights, or upon gross or microscopic examination.
Ultrastructural examinations of bronchi and alveoli of rats exposed to
0 or 5 mg/m3 showed no differences between the two groups.
In connection with a reported correlation between brain ACh
concentration and the inhibition of brain ChE activity following acute
exposure to organophosphorus compounds, the brain tissue of three
female rats per group was examined for ACh and choline concentrations.
The dose level of 0.05 mg dichlorvos/m3 was without effect on any of
the measured parameters (Blair et al., 1976). It should be noted that
in this study the rats were not only exposed by inhalation but also via
their food, drinking-water, and by grooming. This resulted in
additional oral ingestion of dichlorvos (Stevenson & Blair, 1977).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
8.5.1 Reproduction
In a 3-generation reproduction study, weanling CD rats (six groups
of 30 animals) were fed dichlorvos (93%) at nominal concentrations of
0, 0.1, 1, 10, 100, or 500 mg/kg diet, prepared freshly each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1.1), it was
assumed that the average concentration of dichlorvos was approximately
47% of the nominal concentrations (equivalent to 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). No effects on fertility,
number and size of litters, body weight, or viability of the pups were
found. Gross and histopathological examination of 7-day-old pups from
F1a and F2a litters did not reveal any abnormalities (Witherup et
al., 1965, 1971).
Oral treatment of rats with 5.6 mg/kg body weight and rabbits with
6 mg/kg body weight during the last trimester of pregnancy had no
effect on offspring weight and development. However, the cerebral
cortices from the 1-day-old rabbits were less mature than those of
control rabbits, probably due to maternal toxicity (Dambska et al.,
1978, 1979).
Dambska & Maslinska (1982) observed impairment of the development
of the brain of rabbits dosed orally with 9 mg/kg body weight per day
from days 5 to 16 of life, the period of myelination.
8.5.1.1 Effects on testes
In studies by Krause & Homolo (1972, 1974), three groups of 14 male
NMRI/Han mice received either a single oral dose of 40 mg dichlorvos/kg
body weight, daily oral doses of 10 mg dichlorvos (in olive oil)/kg for
18 consecutive days, or daily oral doses of 0.5 ml olive oil for 18
days, respectively. On days 9, 18, 27, 36, 54, and 63, two animals
from each group were killed and their testes examined histologically.
Severe disturbances of spermatogenesis were observed in both test
groups; damaged seminiferous tubules were also seen and the supporting
Sertoli cells were damaged. In addition, there was an increase in the
number and hypertrophy of the Leydig cells. No explanation could be
offered for these effects.
A similar study was carried out on three groups of 16 male juvenile
Wistar rats. The rats received either 20 mg dichlorvos (in olive
oil)/kg body weight on days 4 and 5, 10 mg dichlorvos (in olive oil)/kg
daily from days 4 to 23, or 0.1 ml olive oil daily from days 4 to 23 of
life. On days 6, 12, 18, 26, 34, and 50 of life, two rats from each
group were sacrificed. Histological examination of the testes showed
slight reduction in the number of the spermatogenic cells and Leydig
cells. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells. All the changes were
reversed by the 50th day (Krause et al., 1976; Xing-Shu, 1983).
In order to examine the cause of the observed effect on the
spermatogenic and Leydig cells, the study was repeated with, in
addition, measurement of testosterone levels in serum and testes. The
testosterone concentrations in the testes, and leutinizing hormone (LH)
and follicle stimulating hormone (FSH) levels in serum were similar in
the presence or absence of dichlorvos (Krause, 1977). However, the use
of adult rats and a different dosing regimen prevented a strict
comparison with the earlier study by Krause et al. (1976).
In studies by Fujita et al. (1977), 55 male Wistar rats (aged 5
months) were orally administered dichlorvos at levels of 5 or 10 mg/kg
body weight every other day for 8 weeks. The rats were divided into
five groups, and one group of rats was killed every 4 weeks to study
the changes in several organs, including the testes. About 200
individual seminiferous tubules were examined in each rat. No change
was seen in body weight gain or testes weight. The score values of the
seminal cellular system decreased after 4 - 8 weeks of treatment, but
were restored 8 weeks after the end of treatment.
8.5.1.2 Effect on estrous cycle
Timmons et al. (1975) reported studies on female rats which were
exposed to an atmosphere containing 2.4 mg dichlorvos/m3 generated
from a dichlorvos strip placed on top of each cage. Exposure was
continuous from the birth of the first litter until the estrous cycle
began again. Controls were housed in a separate room. A significant
mean delay of 10 days in the onset of the estrous cycle, compared with
that of controls, was observed. However, the significance of the
results was complicated by different housing conditions.
8.5.1.3 Domestic animals
In studies by Bazer et al. (1969), sows were fed dichlorvos (9% in
resin pellets) at the level of 800 mg per animal per day beginning 21
days before breeding and continuing through gestation. No significant
differences in the numbers born alive or dead, the litter weight,
number weaned, or individual weaning weights were observed.
In a further study, dichlorvos was added to the rations of pregnant
sows at the level of 0 or 800 mg per animal per day. Resin pellets
containing 9% dichlorvos were fed either from 3 weeks before breeding
and throughout gestation, or from 18 to 56 days before parturition.
Data were collected from a total of 681 dams, representing eight
replicates over a period of 2 years. The dichlorvos-treated groups
scored consistently higher for individual farrowing weights, litter
farrowing weights, number weaned, individual weaning weights, and
litter weaning weights, and less consistently, for the percentage of
live births (Batte et al., 1969).
When dichlorvos (as a PVC-resin formulation) was administered to
sows in doses ranging from 4 to 13 mg/kg body weight per day for the
last 21 - 30 days of gestation, the average farrowing interval for the
live-born piglets was shorter in treated than in control animals.
There was also a dose-related increase in the mean birth weight of
live-born piglets from the dichlorvos-treated sows, while the incidence
of still births was lower than in controls (Bunding et al., 1972).
In studies by Collins et al. (1971), male and female swine were fed
for up to 3 years on diets containing a PVC-resin formulation at 0,
200, 250, 288, 400, or 500 mg dichlorvos/kg diet, and for at least 6
months prior to initial breeding. Two generations were raised, and no
effects were observed on number or size of litters, survival or growth
rate of offspring, urinalysis, haematology, hepatic and renal function,
physical structure, or calcium and phosphorus content of the femoral
bone, or in appearance during gross and microscopic examination. Organ
weights were normal except in the case of the liver, which was
generally increased. Whole blood ChE activity was inhibited and brain
ChE activity was slightly reduced, but no clinical evidence of
neurophysiological impairment was observed.
In studies reported by Stanton et al. (1979), pregnant sows were
given PVC-resin formulations of dichlorvos (10%) in the diet at a daily
dose of 0, 5, or 25 mg dichlorvos/kg body weight (divided between two
doses per day) during the last one-third of pregnancy (or 30 days).
Only about 50% of the total dichlorvos was released from this resin in
the gastrointestinal tract. All pigs were born alive, and their birth
weight was similar to that of control animals. In the group given
25 mg/kg body weight, plasma and erythrocyte ChE activities were
markedly inhibited (80 and 90%, respectively) in the sows, but not in
the newborn pigs. No changes in the packed cell volume or haemoglobin
concentration of the sows or their piglets were observed.
In a limited test reported by Darrow (1973), dichlorvos-impregnated
collars did not have any adverse effects on pregnant female goats or
later on their newborn young. Also, blood ChE activity was not
inhibited in either the nannies or the kids over a period of several
weeks. The acceptance of treated kids by the mothers was normal.
A pregnant non-lactating Holstein-Friesian cow was fed a nominal
concentration of 6.2 mg dichlorvos/kg body weight per day in the daily
ration from days 152 to 286 of pregnancy. A normal calf was delivered
(Macklin & Ribelin, 1971).
A herd of 54 dairy cows (two-thirds carrying calves) was accidently
sprayed with dichlorvos, resulting in a dosage of 50 mg dichlorvos/kg
body weight. All the animals showed symptoms of intoxication and some
had convulsions. However, they all recovered within a few hours, with
no abortions or other adverse effects except a temporary diminution in
milk production (Knapp & Graden, 1964).
When three pregnant sows were fed 8.5 mg dichlorvos/kg body weight
per day (as PVC-resin formulation) from days 41 to 70 of pregnancy, the
blood ChE activity of the sows was markedly inhibited, but no
demonstrable teratogenic effects or functional abnormalities in the
piglets were found (Wrathall et al., 1980).
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
In studies by Schwetz et al. (1979), CF1 mice and New Zealand
rabbits were given maximum tolerated doses of dichlorvos (96%) in corn
oil by gavage, at levels of 60 and 5 mg/kg body weight, respectively,
from days 6 to 15 and days 6 to 18 of gestation, respectively. Except
for an increased number of resorptions in rabbits, no significant
effect was observed on the mean number of live fetuses per litter, or
on fetal body measurements. There were no gross visceral or skeletal
alterations. In mice, no abnormalities were found.
Carson (1969) reported studies on a total of 168 New Zealand
rabbits, divided into 10 groups containing 15 - 26 animals. One group
received lactose capsules, three groups different PVC-placebo capsules,
and three groups PVC capsules containing 18, 54, or 93 mg
dichlorvos/animal. These capsules were provided twice daily, so that
the equivalent daily intake was 12, 36, or 62 mg/kg body weight,
respectively. The rabbits received 12 or 36 mg dichlorvos/kg body
weight on days 6 - 18 of gestation, or 62 mg/kg body weight on days 6 -
11 of gestation. Fetuses were obtained by Caesarian section. Maternal
mortality was increased in the highest dichlorvos group, and the
incidences of in utero and neonatal toxicity were also increased (but
not significantly) in the 62 mg group, compared with those in the
control groups. The fetal mortality was not clearly indicative of any
marked toxic effects other than those involving the dam, since whole
litters were not involved. Extensive skeletal and soft tissue
examinations were carried out on all viable neonates, but no adverse
effects on bone formation, articulation, or degree of ossification were
found in the dichlorvos groups. No teratogenic effects were observed.
When pregnant rabbits were given oral doses of PVC-resin
formulations during the critical days of organogenesis, doses of 34 mg
dichlorvos/kg body weight or more were found to cause maternal
toxicity. With doses of 12 mg/kg body weight or less, no significant
effect on nidation, in utero survival, or neonatal survival was found.
No evidence of teratogenic changes was observed on gross, visceral, or
skeletal examination of the fetuses (Vogin et al., 1971).
The effect of dichlorvos on embryonal and fetal development in
thyroparathyroidectomized, thyroxine-treated, and euthyroid control
rats has been investigated. On days 8 - 15 of gestation, 25 mg
dichlorvos/kg body weight per day was administered orally to pregnant
Charles River rats, and a slight decrease in fetal weight in all groups
was observed. No gross, visceral, or skeletal anomalies of the fetuses
were found as a result of dichlorvos administration to rats with
altered thryoid status (Baksi, 1978).
8.5.2.2 Inhalation
In studies by Thorpe et al. (1972), rats of E strain and Dutch
rabbits were exposed (23 h per day, 7 days per week) throughout
pregnancy to nominal concentrations of 0, 0.25, 1.25, or 6.25 mg
dichlorvos/m3. In an additional study, groups of 20 pregnant
rabbits were exposed to nominal concentrations of 2 or 4 mg
dichlorvos/m3. Maternal deaths occurred in the rabbits exposed to
2 mg/m3 or more, and ChE activities in plasma, erythrocytes, and brain
were markedly inhibited in both species exposed to 1.25 mg/m3 or
more. There was no indication of any dichlorvos-related teratogenic
effects.
When CF1 mice and New Zealand rabbits were exposed to dichlorvos at
an average actual concentration of 4 mg/m3 for 7 h per day from days
6 to 15 and from days 6 to 18 of gestation, respectively, no
significant effect on the mean number of live fetuses per litter, the
incidence or distribution of resorptions, or on fetal body measurements
was noted. No gross, visceral, or skeletal alterations were observed
(Schwetz et al., 1979).
8.5.2.3 Intraperitoneal
Kimbrough & Gaines (1968) reported a study on female Sherman rats
given a single intraperitoneal injection of 0 or 15 mg dichlorvos (in
peanut oil)/kg body weight on day 11 of pregnancy. The treated dams
showed toxic signs and weight loss. On day 20, the fetuses were
removed. There was no adverse effect on litter size, resorptions,
number of dead fetuses per litter, or average weight of fetuses, but 3
out of 41 fetuses had omphaloceles. This latter finding, observed at a
maternally toxic dose, is not in agreement with the other teratology
studies or the 3-generation reproduction study.
8.5.3 Résumé of reproduction, embryotoxicity, and teratogenicity studies
A 3-generation study on rats, fed dichlorvos at dose levels of up
to 500 mg/kg diet, did not show any effects on fertility, number and
size of litters, body weight, or viability of the pups.
In studies on mice and rats, high dose levels of dichlorvos (a
single dose of 40 mg/kg body weight or multiple doses of 5 or 10 mg/kg
body weight) induced disturbances in spermatogenesis, characterized by
damage to the seminiferous tubules and Sertoli cells and by hypertrophy
and increase in the number of Leydig cells. It was assumed, but not
confirmed, that testosterone synthesis was partially inhibited. After
dichlorvos treatment ceased, recovery was complete within about 2
months.
A number of reproduction studies on domestic animals, mainly sows,
have been carried out. Levels of 500 mg/kg diet for 3 years had no
effect on fertility. Inhibition of ChE activity in the sows, but not
in the newborn pigs, was induced by 25 mg/kg body weight. No
teratogenic effects were seen.
Teratogenicity studies on rats and rabbits, orally administered
62 mg/kg body weight during gestation, revealed symptoms of
intoxication and significant inhibition of ChE in the parent rabbits.
Except for an increased number of resorptions, no significant effect on
the mean number of live fetuses per litter or evidence of a teratogenic
effect was noted during gross, visceral, or skeletal examination.
There were no teratogenic effects after rats inhaled 6.25 mg
dichlorvos/m3 during gestation, though maternal deaths occurred. Mice
and rabbits exposed to 4 mg/m3 on days 6 - 18 of gestation did not
show any effects.
8.6 Mutagenicity and Related End-Points
8.6.1 Methylating reactivity
In a quantitative colour test for alkylating agents, dichlorvos
gave a positive response, whereas the metabolites desmethyldichlorvos,
dimethylphosphate, dichloroethanol, DCA, and dichloroacetic acid all
gave negative results (Bedford & Robinson, 1972).
8.6.1.1 In vitro studies
Lawley et al. (1974) have shown that methylation by dichlorvos of
DNA and RNA, using either isolated nucleic acids, Escherichia
coli cells, or human tumour HeLa cells, broadly resembled that by
methylmethanesulfonate (MMS) rather than methylation by
N-methyl- N-nitrosourea. In E. coli cells, 3-methyladenine, the
principal minor product in methylated DNA apart from 7-methylguanine,
was not detected, whereas it was present when pre-isolated DNA was
methylated. The overall extent of methylation achieved in cells was
small. Specific excision of 3-methyladenine was indicated in E.
coli cells (Lawley et al., 1974).
Labelled 7-methylguanine was present in both DNA and RNA isolated
from E. coli exposed to 3H-dichlorvos. The methylating capability of
dichlorvos was less, by a factor of 10 - 100, than that of strongly
genotoxic methylating compounds (Wennerberg & Löfroth, 1974).
Alkylation by dichlorvos of calf thymus DNA, resulting in the formation
of N-7-methylguanine, was reported by Löfroth (1970).
Shooter (1975) has applied the test for chain breaks in RNA to the
interaction of dichlorvos with bacteriophage R17. Breaks in the RNA
chain result from the hydrolysis of phosphotriesters and are thus a
measure of the extent of O-alkylation and of the SN1-type mechanism
of the reaction. The results so far suggest that the existence of O-
alkylation, as shown by degradation following phosphotriester
formation, does correlate with mutagenicity. Incubation of
bacteriophage R17 with 0 - 100 mmol dichlorvos/litre for 90 h did not
result in methylation of the phosphate group of the RNA to any
significant extent.
8.6.1.2 In vivo studies
Reviews of the literature on alkylating agents including dichlorvos
have been made by Bedford & Robinson (1972), Lohs et al. (1976), and
Hemminki (1983).
In studies in which mice were given intraperitoneal injections
of methyl-14C-dichlorvos (1.9 µmol/kg body weight), the degree
of alkylation of guanine- N-7 in DNA isolated from soft tissues
amounted to 8 x 10-13 mol methyl/g DNA. From this, a rate of
clearance of approximately 1.4 min was estimated (Segerbäck, 1981;
Segerbäck & Ehrenberg, 1981). DNA and RNA from the total soft tissues
of male CFE rats exposed to atmospheres containing 0.064 mg methyl-
14C-dichlorvos/m3 for 12 h did not show methylation of the N-7 atom
of guanine moieties. The exposure period constituted a significant
fraction of the half-life of the 7-methylguanine moieties in DNA. It
was concluded that dichlorvos does not pose a methylating hazard to
mammalian DNA in vivo (Wooder et al., 1977; Wooder & Wright, 1981).
The excretion of labelled 7-methyl guanine in the urine
by NMRI mice and R rats injected intraperitoneally with methyl-
14C-dichlorvos, or exposed by inhalation for 2 h (mice only), was
reported by Löfroth & Wennerberg (1974) and Wennerberg & Löfroth
(1974). In rat urine, labelled 3-methyladenine and 1-methyl-
nicotinamide were also detected (Löfroth & Wennerberg, 1974).
According to the authors, these results demonstrate the dichlorvos-
induced methylation of guanine and adenine moieties in nucleic acids.
However, the administration of radiolabelled adenine and guanine to
otherwise untreated rats gave rise to the excretion of radiolabelled
methylated purines in the urine. Therefore, the detection of
radiolabelled purines, per se, in the urine of animals exposed to
methyl-labelled methylating agents, does not constitute evidence for
the methylation of the purine moieties of nucleosides or nucleic acids
by methylating agents (Wooder et al., 1978). Moreover, the results of
metabolic studies have demonstrated the existence of a natural
biosynthetic pathway whereby the methyl carbon atoms of dichlorvos can,
with partial retention of hydrogen, become incorporated into the
heterocyclic rings and the methyl groups of urinary 7-methylguanine
after entering 1-C pools, in vivo. The results of preliminary studies
suggest the existence of a similar pathway for the production of
urinary 3-methyladenine (Wright et al., 1979).
Wooder & Creedy (1979) described a study on rats which investigated
the DNA-damaging potential of dichlorvos when administered as a single
intraperitoneal dose. Alkaline sucrose gradient profiles of rat liver
DNA showed that whereas MMS (a positive control) shifted the DNA
profile, dichlorvos at 10 mg/kg body weight (the maximum dose
consistent with survival) had no effect.
8.6.1.3 Discussion of methylating reactivity
Many alkylation tests and in vivo and in vitro mutagenicity studies
have been carried out with dichlorvos. It has been demonstrated that
dichlorvos has alkylating properties, and has been suggested from some
studies that the in vivo alkylating potential of dichlorvos is similar
to that of some known mutagens. However, this concern is misplaced
since alternative reactions were not considered (WHO, 1986b). The
phosphorus atom is markedly more electron-deficient and susceptible to
attack by nucleophiles than the alkyl carbon atom. Analysis by Bedford
& Robinson (1972) of the data of Löfroth et al. (1969) revealed that
the proposed rates of alkylation by potent nucleophiles were probably
combined rates of phosphorylation and alkylation, and that
phosphorylation was the totally dominant reaction in the case of the
hydroxide ion. The comparison with known mutagens is therefore
inappropriate. Two factors detract further from the toxicological
significance of the alkylation studies. The first is that mammalian
tissues (plasma, liver, etc.) contain active A-esterase enzymes, which
catalyse the phosphorylation of water by the organophosphorus esters.
Viewed inversely, these A-esterases catalyse the hydrolysis of the
organophosphorus esters, thereby rapidly reducing circulating levels of
hazardous material. Secondly, the comparative rate of reaction of most
of these esters with AChE is many orders of magnitude greater than the
rate of alkylation by the typical nucleophile 4-nitrobenzyl-pyridine:
for dichlorvos, the ratio of rates was 1 x 107 in favour of the
inhibitory phosphorylation of AChE (Aldridge & Johnson, 1977). It
follows that, at low exposure levels, in vivo phosphorylation of AChE
and other esterases will be the dominant reaction, with negligible
uncatalysed alkylation of nucleic acid. Indeed, no such alkylation has
been detected in sensitive in vivo studies designed to check this point
(Wooder et al., 1977).
8.6.2. Mutagenicity
Reviews of the existing literature have been published by Wild
(1975), Fishbein (1976, 1981, 1982), Leonard (1976), IARC (1979),
Sternberg (1979), Ramel et al. (1980), Lafontaine et al. (1981), Ramel
(1981), and Wildemauwe et al. (1983).
8.6.2.1 In vitro studies
(a) Microorganisms
Numerous mutagenicity studies using bacteria and fungi as test
organisms have been carried out (Table 17). In most of the studies,
only one dichlorvos concentration, often a high one, was tested,
sometimes resulting in low survival of the test organism. A
dose-response relationship was established in the few tests where a
range of concentrations was used. The results indicate that dichlorvos
induces base substitutions in bacteria and mitotic gene conversion in
yeast. The alkylating properties of dichlorvos (section 8.6.1) are
most probably the cause of the mutagenic action. This was the
conclusion of Bridges et al. (1973) from tests using both MMS and
dichlorvos with E. coli strains deficient at four different repair
loci.
In Aspergillus nidulans, dichlorvos has been found to induce point
mutations to 8-azaguanine resistance and a high frequency of mitotic
crossing-over and non-disjunction (Aulicino et al., 1976; Bignami et
al., 1976, 1977; Morpurgo et al., 1979). No mutagenic activity
using A. nidulans was found after incubating Nicotiana alata cell
cultures with dichlorvos for 21 days (simulating in vitro plant
metabolism). This confirms the rapid metabolism of dichlorvos (Benigni
et al., 1979).
The mutagenicity of dichlorvos has been extensively studied in
Japan, (Kawachi et al., 1980). Dichlorvos induced gene mutation
in Salmonella typhimurium TA100 as well as E. coli strains in the
absence of rat-liver S9 mix.
Dichlorvos (0.5 - 2 mg/ml) causes random strand breakage,
repairable by DNA polymerase I, in E. coli pol A as detected by the
alkaline sucrose sedimentation method. When pol+ bacteria and high
concentrations of dichlorvos (0.2 - 0.4%) were used, an all-or-none
breakdown of DNA molecules to fragments of very low relative molecular
mass occurred which correlated well with lethality. It has been
suggested that the major DNA damage resulting from dichlorvos treatment
arises indirectly through alkylation of other cellular constituents,
this leading to uncontrolled nuclease attack on DNA. However,
discontinuities in newly-synthesized DNA and mutagenesis following
dichlorvos treatment presumably result from direct alkylation of DNA
(Bridges et al., 1973; Green et al., 1973, 1974a,b). In the
standard E. coli DNA polymerase-deficient assay system, dichlorvos
(6.4 mmol/litre) gave a positive result (Rosenkranz, 1973; Rosenkranz &
Leifer, 1980). In measuring mutation to tryphophan independence in E.
coli strain WP2, it was found that 5 mg dichlorvos/litre was mutagenic
in this test system (Green et al., 1976). Griffin & Hall (1978) have
found that dichlorvos (1 mg/ml) causes breaks in colicinogenic plasmid
E1 DNA from E. coli. In a rec-type repair test with Proteus
mirabilis strains PG 713 (rec-hcr-) and PG 273 (wild type),
dichlorvos (10 or 40 µmol per plate) induced base-pair substitutions
and other DNA damage. In the same test, desmethyldichlorvos, at the
same concentrations, gave negative results (Adler et al., 1976; Braun
et al., 1982).
(b) Mammalian cells
In cultured V79 Chinese hamster cells, no induction of 8-
azaguanine-resistant mutations after treatment with up to 1 mmol/litre
dichlorvos (Wild, 1975), or of ouabain-resistant mutations after
treatment with 1.25 - 5 mmol/litre dichlorvos, was found (Aquilina et
al., 1984).
DNA strand breakage in cultured V79 Chinese hamster cells caused by
up to 0.2% v/v dichlorvos has been reported by Green et al. (1974a).
Dichlorvos (1 µl) decreased the sedimentation coefficient of calf
thymus DNA upon thermal denaturation, indicating a decrease in the DNA
molecular size (Rosenkranz & Rosenkranz, 1972). Incubation of
dichlorvos (45 mmol/litre) with calf thymus DNA did not result in
changes in thermal melting curves or DNA fractionation on a
hydroxyapatite column. However, changes were observed by means of
differential pulse polarography, indicating that single-stranded
segments and thermolabile regions were formed in the DNA. This
behaviour could perhaps be a consequence of guanine alkylation followed
by depurination and chain cutting at elevated temperatures (Olinski et
al., 1980).
The resistance of methylated DNA in Chinese hamster ovary cells,
labelled with 14C-thymidine and methyl-3H-1-methionine, to
micrococcal nuclease digestion was abolished when the cells were
treated with dichlorvos (10 mmol/litre) for 3 h. No effects were
observed on kinetics of total DNA digestion. These results indicate a
conformational change in chromatin induced by dichlorvos (Nishio &
Uyeki, 1982).
Table 17. Mutagenicity tests on microorganisms
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Bacillus subtilis
H17 rec+ 0.02 ml of plate none - Shirasu et al.
M45 rec- 10% solution plate none + (1976)
Citrobacter freundii
425 0.1% fluctuation none weak + Voogd et al. (1972)
0.05% none -
Enterobacter aerogenes
6 0.1% fluctuation none weak + Voogd et al. (1972)
Escherichia coli
B 5 - 25 mmol/litre liquid induc- none weak + Wild (1973)
1- to 10-h tion strepto- (dose-
exposure mycin-resistant and exposure-
mutants time related)
B/r WP2 22.6 mmol/litre plate reversion none + Moriya et al.
S9 mix + (1978)
L-cysteine +
CM 561, 571, 611 5 µl plate reversion none - Hanna & Dyer
(1975)
K12HfrH 0.1% fluctuation none weak + Voogd et al.
(1972)
K12(5-MT) 3.3 x 10-4 plate none + Mohn (1973)
mol/litre
WP2 micro drop plate none - Dean (1972a)
analytical and
technical grade,
aqueous solution
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Escherichia coli (contd).
WP2 try- approximately plate none + Ashwood-Smith et
20 mm2 dichlor- al. (1972)
vos strip
WP2 try- 20 - 25 µl of spot none + Nagy et al. (1975)
hcr- and hcr+ 50% emulsifiable back mutation
concentrate
0.1 ml of a 5% plate reversion none + Shirasu et al.
solution (1976)
WP2 hcr unknown (up to plate reversion none + Moriya et al.
5000 µg/plate) (1983)
WP2 uvrA, ca 5 µl plate reversion none + Hanna & Dyer
WP 67 (1975)
Klebsiella pneumoniae 0.1%; 0.05% fluctuation none weak + Voogd et al.
(1972)
Neurospora crassa
ad-3 exposed to air plate none - Michalek &
containing Brockman (1969)
dichlorvos
(strip)
Pseudomonas aeruginosa
PAO 38 0.08 mol/litre liquid reversion none + Dyer & Hanna
(1973)
Saccharomyces cerevisiae
D4 (ade2 and 4 mg/ml plate mitotic none + Dean et al.
trp5) 2 mg/ml gene conversion (1972)
D4 (ade2 and 19 mmol liquid mitotic none + Fahrig (1973,
trp5) gene conversion 1974)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium
64-320 0.05%; 0.1% suspension none + Voogd et al. (1972)
TA 98 20 or 40 mmol plate S9 male mice - Braun et al. (1982)
up to 5000 µg plate reversion none - Moriya et al. (1983)
TA 100 20 or 40 mmol plate S9 male mice (+) Braun et al. (1982)
low survival
up to 5000 µg plate reversion none + Moriya et al. (1983)
TA 1530 5 µl plate none + Hanna & Dyer (1975)
TA 1535 5 µl plate none + Hanna & Dyer (1975)
0.1 ml of a 5% plate none + Shirasu et al. (1976)
solution
0.1 ml of a 5% plate none + Moriya et al. (1978)
solution S9 mix -
L-cysteine -
2800 µg plate (spot) S9 male rat - Carere et al.
(1976, 1978a,b)
1.5 mg/ml liquid none + Carere et al.
(1978a,b)
20 or 40 mmol plate S9 male mice - Braun et al. (1982)
TA 1536, 1537, 0.1 ml of a 5% plate none - Shirasu et al.
1538 solution (1976)
TA 1536, 1537, 2800 µg plate (spot) S9 male rat - Carere et al.
1538 (1976, 1978a,b)
20 or 40 µg plate S9 male mice - Braun et al.
(1982)
up to 5000 µg plate none - Moriya et al.
(1983)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium (contd).
his C117 0.03 mol liquid none + Dyer & Hanna (1973)
LT2 his C117, 5 µl plate none - Hanna & Dyer (1975)
his G 46
Schizosaccharomyces pombe
ade6 1.5 - 14 mmol plate + + Gilot-Delhalle et
al. (1983)
Serratia marcescens
Hy alpha 13, 25 mg/ml plate (spot) none alpha 13 + Dean (1972a)
alpha 21 alpha 21 -
50, 100 mg/ml plate (spot) none + Dean (1972a)
saturated plate (spot) none + Dean (1972a)
aqueous solution
Streptomyces coelicolor
A 3(2) his Al 5600 µg spot none + Carere et al.
(1976, 1978a,b)
---------------------------------------------------------------------------------------------------------
Dichlorvos (0.03 and 0.1 mmol/litre) has been found to induce
sister chromatid exchanges (SCEs) in cultures of Chinese hamster ovary
cells (Nishio & Uyeki, 1981). On the other hand, in Chinese hamster
V79 cells (clone number 15), SCEs were not induced by 0.1 mmol
dichlorvos/litre, but only by 0.2 and 0.5 mmol/litre solutions. The
number of polyploid cells was increased at both 0.1 and 5 mmol/litre
(Tezuka et al., 1980).
Dichlorvos has been tested in two independent laboratories for its
ability to increase the transformation of Syrian hamster embryo cells
by simian adenovirus SA7. Pre-treatment of hamster cells with
dichlorvos at concentrations of 0.05 up to 0.45 mmol/litre produced a
significant enhancement of SA7 transformation at 0.11 mmol/litre and
higher (Hatch et al., 1986).
The mouse peripheral blood lymphocyte (PB) culture system has also
been used to test for SCE induction. Male B6C3F1 mice were injected
intraperitoneally with 5, 15, 25, or 35 mg dichlorvos/kg body weight,
but there was no change in the baseline SCE frequency (Kligerman et
al., 1985).
Dichlorvos (6.5 - 650 mmol/litre) has been found to induce dose-
dependent unscheduled DNA synthesis in the human epithelial-like cell
line EUE (Benigni & Dogliotti, 1980a,b; Aquilina et al., 1984). Both
scheduled and unscheduled DNA synthesis of human lymphocytes showed
dose-related inhibition by dichlorvos (5 -500 mg/litre), as measured by
3H-thymidine uptake (Perocco & Fini, 1980).
Dichlorvos (0.0001 - 0.1 mmol/litre) does not induce DNA repair in
human kidney T-cells. This was shown by a lack of dichlorvos-induced
3H-thymidine incorporation into T-cells in the G1- or G2-phase of the
cell cycle. No induction of single-strand breaks in the T-cell DNA, as
measured by alkaline sucrose gradients, was found following treatment
for 1, 2, or 4 h with 0.0001 - 1 mmol dichlorvos/litre (Bootsma et al.,
1971).
No clear effect on the frequency of SCEs in human lymphocytes or
human fetal lung fibroblasts was found after exposure to 2.5 - 10 mg
dichlorvos/litre for 72 h (Nicholas et al., 1978).
In studies by Dean (1972b), human blood samples were treated with
dichlorvos (0.0001 - 1 mmol/litre) for 1, 2, 4, and 20 h, after which
the lymphocytes were stimulated to divide using phyto-haemagglutinin.
The toxic effect of 1 mmol dichlorvos/litre was indicated by a
decreased mitotic index. Mitotic cells were analysed for chromosome
aberrations, but the number of dicentrics in dichlorvos-treated cells
was no different from that found in untreated cells (Bootsma et al.,
1971). When dichlorvos (1 - 40 mg/litre) was added at specific
intervals to cultures of human lymphocytes, cytotoxicity was found at
5 mg/litre or more, but no chromosome aberrations (chromatid gaps or
breaks) were detected (Dean, 1972b).
Negative results in cultured human lymphocytes were also reported
by Fahrig (1974) and Wild (1975).
8.6.2.2 In vivo studies
(a) Drosophila melanogaster
Negative results were obtained in the standard Muller-5 test for
sex-linked lethal mutations and in the induced crossing-over test with
the approximate LD50 concentration (0.035% dichlorvos) (Jayasuriya &
Ratnayake, 1973). Similarly, 0.0006 - 0.6 µmol dichlorvos gave
negative results in the standard Muller-5 test (Sobels & Todd, 1979).
In studies by Hanna & Dyer (1975), a Drosophila melanogaster
population was continuously exposed to gradually increasing
concentrations of dichlorvos (because of development of increased
resistance) in the food medium (up to 0.75 mg/kg food) for about 18
months. At the end of this period, an increased accumulation of
mutations was observed.
Gupta & Singh (1974) reported studies where female D.
melanogaster flies were kept on food with 1 - 50 mg dichlorvos (Nuvan
100 EC)/kg. No eggs were laid at 10 mg/kg or more, and at 1 mg/kg,
survival of the eggs was 45% of that of the controls. Salivary gland
chromosome abnormalities were observed in fully-grown larvae fed
1 mg/kg. However, Kramers & Knaap (1978), using the same route of
administration, found no induction of sex-linked recessive lethals by
dichlorvos (0.009, 0.048, and 0.09 mg/kg food).
(b) Host-mediated assays
Dichlorvos was not mutagenic in host-mediated assays in NMRI mice
using:
(i) S. typhimurium (G46 his-) and Serratia marcescens (a 21
leu-) after an intraperitoneal injection of 25 mg
dichlorvos/kg body weight (Buselmaier et al., 1972, 1973);
(ii) S. typhimurium (64-320) after an oral dose of 0.2 mg per
animal (equivalent to 8 - 10 mg/kg body weight) (Voogd et
al., 1972); or
(iii) Saccharomyces cerevisae (D4 ade2 and trp5 loci) after an
oral dose of 50 or 100 mg/kg body weight (or after exposure
of CF1 mice for 5 h to 60 or 90 mg dichlorvos/m3) (Dean et
al., 1972).
Although dichlorvos was mutagenic to S. cerevisae in in
vitro studies, these in vivo studies proved to be negative.
(c) Dominant lethal assays
Negative results were reported in a test for dominant lethal
mutations in ICR/Ha mice (expressed as an increase in early fetal
deaths or, indirectly, by pre-implantation losses), after a single
intraperitoneal injection of 13 or 16.5 mg/kg body weight or five
consecutive daily oral doses of 5 or 10 mg/kg body weight. The total
mating period was 8 weeks (Epstein et al., 1972). The same result was
obtained after exposure of male CF1 mice to 30 or 55 mg/m3 for 16 h or
to 2.1 or 5.8 mg/m3 for 23 h daily for 4 weeks (Dean & Thorpe,
1972b).
A statistically significant increase in the frequency of pre-
implantation losses in mice (Q strain) in the second week and fifth
week of mating has been observed after a single intraperitoneal
injection of 10 mg/kg body weight (Degraeve et al., 1980; Moutschen-
Dahmen et al., 1981).
In studies by Degraeve et al. (1982, 1984a), male Q strain mice
received drinking-water with 2 mg dichlorvos/litre (equivalent to
0.32 mg/kg body weight per day) 5 days per week, for 7 consecutive
weeks. At the end of this period, the males were mated for 1 week with
untreated virgin females, and the pregnant females were killed 14 days
after the start of pregnancy. No dominant lethal mutations were
induced. The same result was obtained when female CF1 mice were either
given single oral doses of 0, 25, or 50 mg dichlorvos/kg body weight or
continuously exposed to atmospheres containing 0, 2, or 8 mg
dichlorvos/m3 from weaning until 11 weeks of age. They were either
mated at the end of the dosing or inhalation period or at intervals of
5, 10, and 15 days thereafter (Dean & Blair, 1976).
(d) Chromosome abnormalities
Male Q strain mice receiving drinking-water containing 2 mg
dichlorvos/litre (equivalent to 0.32 mg/kg body weight), 5 days per
week for 7 weeks, did not show chromosome damage in bone marrow cells,
spermatogonia, or primary spermatocytes (Moutschen-Dahmen et al., 1981;
Degraeve et al., 1982, 1984a); neither did mice of the same strain
given a single intraperitoneal injection with 10 mg/kg body weight
(Moutschen-Dahmen et al., 1981; Degraeve et al., 1984b).
In a micronucleus test, Swiss mice were given daily intra-
peritoneal injections of dichlorvos (0.0075 - 0.015 mg/kg body weight
per day) for 2 days and killed 6 h after the last dose. No induction
of aberrations in the structure or number of chromosomes in bone marrow
cells was observed (Paik & Lee, 1977).
CF1 mice exposed to concentrations of 64 - 72 mg
dichlorvos/m3 for 16 h or to 5 mg/m3 per day, 23 h per day, for 21
days, did not show chromosome abnormalities in bone marrow or
spermatocytes. Similar results for Chinese hamsters exposed by the
inhalation route to 28 -36 mg/m3 for 16 h (males only), or by a single
oral dose of 15 mg/kg body weight (males) or 10 mg/kg body weight
(females), have been reported (Dean & Thorpe, 1972a).
Dichlorvos has been tested for its ability to induce in vivo
chromosomal aberrations in Syrian hamsters bone marrow cells. Four
dose levels (3, 6, 15, and 30 mg/kg body weight) were given
intraperitoneally. Statistically significant increases in the number
of cells with aberrant chromosomes (mainly breaks and gaps) were
observed (Dzwonkowska & Hübner, 1986).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1 Mouse
Carcinogenicity studies were carried out on two groups of 50 male
and 50 female B6C3F1 mice fed 1000 and 2000 mg dichlorvos (94%) in corn
oil/kg diet for 80 weeks. Due to severe signs of intoxication, doses
were lowered after 2 weeks to 300 and 600 mg/kg for the remaining 78
weeks. Samples of the diets analysed during the study showed
dichlorvos contents within 5% of the intended concentrations. Matched
controls consisted of 10 mice of each sex; the pooled controls
consisted of 100 male and 80 female mice. All surviving mice were
killed at 92 - 94 weeks. Hair loss and rough hair coats were noted in
many treated animals, particularly in the male groups, beginning at
week 20 and persisting throughout the study. The average body weights
of the high-dose mice of both sexes were slightly decreased compared
with controls. The low-dose female group showed the poorest survival;
74% of the animals lived to 90 weeks. There was no significant
increase in the incidence of tumours attributable to dichlorvos in
either sex, i.e., dichlorvos was not demonstrated to be carcinogenic
(NCI, 1977; Weisburger, 1982).
In other studies, groups of 50 male and 50 female B6C3F1 mice, 6
weeks of age, were given drinking-water with 0, 400, or 800 mg
dichlorvos/litre ad libitum. The drinking-water solutions were renewed
daily. All surviving animals were killed during week 102. A dose-
dependent inhibition of body weight increase was observed throughout
the study in both sexes at both dichlorvos concentrations. There was
no adverse effect on mortality. The survival rates at week 102 were
62%, 66%, and 84% in males (in controls, low-, and high-dose groups,
respectively), and 66%, 50%, and 80% in females. The corresponding
figures for tumour incidence were 22.4%, 39.1%, and 23.4% in males and
29.3%, 16.2%, and 9.1% in females. The main tumours that were found
were lung adenomas and tumours in the liver, spleen, thymus, and
salivary gland. These tumours occurred in all three groups. There was
no statistically significant difference in the incidence of tumours at
any site in any group (Konishi et al., 1981).
Preliminary results are available from a recently completed 2-year
mouse carcinogenicity study using dichlorvos. Groups of 50 male and 50
female B6C3Fl mice were given dichlorvos (dissolved in corn oil) by
oral gavage daily for 2 years. Dose levels for male mice were 0, 10,
or 20 mg/kg body weight per day and for female mice 0, 20, or 40 mg/kg.
There were no statistically significant differences in survival rates
between the treated and control male mice, and the same applied to
female mice. A statistically significant increase in the incidence of
forestomach squamous cell papillomas was observed in the female mice
receiving 40 mg/kg per day. Two forestomach squamous cell carcinomas
were also seen in this group, but none were observed in the controls or
the 20 mg/kg group. Considerably fewer forestomach squamous cell
papillomas were observed in male mice. Nevertheless, a non-significant
increase was observed in the 20 mg/kg per day group. No forestomach
squamous cell carcinomas were noted in any male group. Forestomach
hyperplasia occurred relatively frequently in both control and treated
male and female mice, the incidence being similar in all groups (NTP,
1987).
8.7.1.2 Rat
In studies reported by NCI (1977) and Weisburger (1982), two groups
each of 50 male and 50 female Osborne-Mendel rats were fed either 150
or 1000 mg dichlorvos (94%) in corn oil/kg diet for 80 weeks. Due to
the severe signs of intoxication, the 1000 mg/kg dose was reduced to
300 mg/kg diet after 3 weeks for the remaining 77 weeks. The diets
were stored under conditions designed to minimize loss of dichlorvos,
and the animals received fresh diets daily. Matched controls comprised
10 rats of each sex; the pooled controls consisted of 60 rats of each
sex. The surviving rats were killed after 110 weeks. The average body
weights of the rats receiving the high dose level were slightly
decreased compared with controls. There was no significant increase in
the incidence and type of tumours in either sex as a result of
dichlorvos treatment.
Enomoto et al. (1981) carried out studies on groups of 50 male and
50 female Fisher 344 rats, 6 weeks of age, given drinking-water
(renewed daily) containing 0, 140, or 280 mg dichlorvos/litre ad
libitum. All surviving animals were killed in week 108 (104 weeks of
exposure followed by a 4-week recovery). Slight inhibition of body
weight increase was observed in males in the high-dose group, but there
was no influence on mortality. The survival rates in week 108 were
82%, 75%, and 75% in males and 86%, 71%, and 82% in females,
respectively, in control, low-dose, and high-dose groups. A great
number of organs and tissues were selected for microscopy. The organs
and tissues of all animals which died or which were killed in moribund
conditions were microscopically examined, as were all tumours and
macroscopical lesions. A number of animals with no specific changes
were also examined. The overall tumour incidences were 100%, 96%, and
98% in males and 37%, 31%, and 33% in females, respectively, in
control, low-dose, and high-dose groups. High incidences of
interstitial cell tumours of the testes in males (49/51, 41/48, and
47/48, respectively) were observed in all three groups. Mononuclear
cell leukaemia was found in all groups at 4 - 12%. There was no
statistically significant difference in tumour incidence at any site in
any group.
Preliminary results are available from a recently completed 2-year
rat carcinogenicity study using dichlorvos. Dichlorvos, dissolved in
corn oil, was administered each day by oral gavage to groups of 50 male
and 50 female Fischer 344 rats at dose levels of 0, 4, or 8 mg/kg body
weight per day. Though survival rates were slightly decreased in the
treated male and female groups compared with those of their respective
controls groups, the differences were not statistically significant.
However, a statistically-significant and dose-related increase in the
incidence of pancreatic adenomas was observed in the male rats fed 4 or
8 mg/kg. In female rats fed 8 mg/kg, a non-significant increase in
pancreatic adenomas was observed. There was a relatively high
incidence of pancreatic hyperplasia and atrophy in all groups of male
rats, including the controls, and a lower incidence in all female
groups. A statistically significant (but not dose-related) increased
incidence of mononuclear leukaemia was observed in the male rats fed 4
or 8 mg/kg. A similar incidence was observed in all female groups,
including the control group. Since this is a common and variable
systemic lesion in Fischer-344 rats, the toxicological significance of
this finding is uncertain. It is possible that its incidence in the
male control group was unusually low. An increased incidence of
mammary gland fibroadenomas (not dose related) was observed in the
treated female rats, but these were not considered to be of
toxicological concern. In addition, mammary gland hyperplasia was
frequently observed in all control and treated female groups at similar
incidences (NTP, 1986).
As reported in section 8.4.1.1, Witherup (1967, 1971) found no
increase in tumour incidence following dichlorvos treatment of rats.
8.7.2. Inhalation
8.7.2.1 Rat
In the studies by Blair et al. (1976) described in section 8.4.2.1,
no dose-related increase in tumour incidence was found.
8.7.3. Appraisal of carcinogenicity
The majority of the mouse and rat studies using dichlorvos are
considered to be negative regarding carcinogenic potential (Witherup et
al., 1967, 1971; Blair et al., 1976; NCI, 1977; Enomoto et al., 1981;
Konishi et al., 1981; Weisburger, 1982). However, preliminary
information from two recent studies on the mouse and rat, respectively,
has provided equivocal evidence of carcinogenicity (NTP, 1986)a.
------------------------------------------------------------------------
a The NTP Peer Review Panel reviewed these studies and came to the
following conclusions:
"Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenic activity of dichlorvos for male F344/N rats, as
shown by increased incidences of adenomas of the exocrine pancreas and
mononuclear cell leukemia. There was equivocal evidence of carcinogenic
activity of dichlorvos for female F344/N rats, as shown by increased
incidence of adenomas of the exocrine pancreas and mammary gland
fibroadenomas. There was some evidence of carcinogenic activity of
dichlorvos for male B6C3F1 mice and clear evidence for female B6C3F1 mice,
as shown by increased incidences of forestomach squamous cell papillomas"
(NTP, 1988).
The increased incidence of forestomach tumours in the mouse study
is likely to be related to the route of administration (oral gavage),
which has been shown in other mouse studies to induce forestomach
tumours due to repeated and direct irritation of the gastric mucosa.
If so, ingestion of food by human beings could not result in such
direct effects on the stomach wall. Furthermore, human beings do not
possess a stomach wall comparable with the forestomach of the mouse,
except perhaps for the oesophagus. However, the transient passage of
food through the oesophagus would probably not allow sufficient time
for the carcinogenic event to occur.
Similarly, the pancreatic adenomas observed in the NTP rat study
may be related to the corn oil used in the study. Evidence from other
rat studies in which corn oil was used as a vehicle suggests that this
is a possibility. On the other hand, it does not explain why a higher
incidence of pancreatic tumours was observed in the treated animals
compared with the controls. Although increased incidences of
mononuclear leukaemia were observed in treated male rats, the
incidence in the control group of this common and variable tumour may
have been low. The evidence for carcinogenicity in these two recent
studies is difficult to interpret at present. When complete and final
reports of these studies become available, more definitive conclusions
may be drawn.
8.8. Mechanisms of Toxicity; Mode of Action
A full description of the mechanism of action can be found in
Environmental Health Criteria 63: Organophosphorus Insecticides - A
General Introduction (WHO, 1986b).
Dichlorvos directly inhibits AChE activity in the nervous system
and other tissues. This reaction takes place in three steps:
(a) reversible binding of dichlorvos to the enzyme;
(b) reaction with the enzyme to form a dimethylphospho-enzyme
derivative, with the loss of DCA; and
(c) "aging" of the phospho-enzyme compound to a more stable
methylphospho-enzyme derivative.
Reaction (a) is rapid, but reversible. Reaction (b) is also rapid,
but the phosphorylated enzyme can be returned to its native
state spontaneously only by hydrolysis at very slow rates or by agents
such as N-methyl 2-pyridinium aldoxime (2-PAM) at higher rates.
Reaction (c) is comparatively slow (t´ = about 2.5 h at 37 °C and pH
7.4), but the product has the same stability as that of erythrocytes
(t´ = about 120 days) (Gillett et al., 1972).
Death due to poisoning is caused by excessive cholinergic effects
such as bronchospasms, hypersecretion from cholinergic innervated
glands (especially critical in lungs and bronchi), and cardiac
disturbances caused by vagotonus and anoxia. Convulsions and paralysis
in skeletal musculature are caused by brain anoxia as well as
cholinergic effects within the central nervous system.
In vivo studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos, at various time
intervals, after dosing are summarized in Table 18. In general, the
maximum inhibition occurred within 1 h, and was followed by rapid
recovery. Dogs seemed to be more sensitive than rats, and showed a
slower recovery.
Dichlorvos, when infused intravenously (into the ear vein) of
adult male rabbits, produced dose- and time-related inhibition of whole
blood ChE activity during infusion. Spontaneous but incomplete
recovery to 60 - 80% of the normal activity occurred within 60 -
90 min of infusion (Shellenberger et al., 1965; Gough & Shellenberger,
1970, 1977-78; Shellenberger, 1980).
In a study on the influence of temperature on ChE activity, rats
were injected intraperitoneally with a single dose of 6.25 mg
dichlorvos (95%)/kg and kept at either 28 °C or 5 °C. The maximum
inhibition of whole blood ChE activity (40%) occurred after 0.5 h.
The animals at 5 °C showed less inhibition and a faster recovery of
whole blood ChE activity than those at 28 °C (Chattopadhyay et al.,
1982).
8.9. Neurotoxicity
8.9.1. Delayed neurotoxicity
A detailed description of the neurotoxic potential of
organophosphorus compounds can be found in Environmental Health
Criteria 63: Organophosphorus Insecticides - A General Introduction
(WHO, 1986b).
Several studies have shown that dichlorvos does not produce delayed
neurotoxicity in pre-medicated hens, whether it is administered orally
or subcutaneously (Durham et al., 1956; Aldridge & Barnes, 1966;
Johnson, 1969, 1975a,b, 1978, 1981; Aldridge & Johnson, 1971; Lotti &
Johnson, 1978). The inhibition of brain neurotoxic esterase (NTE)
without signs of ataxia has been observed (Aldridge & Johnson, 1971;
Johnson, 1978).
Caroldi & Lotti (1981) reported mild signs of ataxia in pre-
medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg body weight) and severe inhibition of NTE in peripheral
nerve, spinal cord, and brain. However, Johnson (1978) did not observe
ataxia in pre-medicated hens given the same dose in the same way.
These hens showed severe inhibition of brain NTE but far less
inhibition of spinal cord NTE. It appears that ataxia arises from the
inhibition of spinal cord NTE. When the dose was repeated 1 - 3 days
after the first dose, spinal cord NTE inhibition increased and the hens
became ataxic.
White leghorn hens have been used in a 90-day study on the
neurotoxic potential of dichlorvos (99.9%) after dermal or oral
administration. For oral administration, a 10 - 20% solution of
dichlorvos in corn oil in gelatin capsules was used whereas, dermally,
1 - 20% emulsifiable concentrates in technical grade xylene (containing
2% Triton X-100) were used. Dichlorvos at doses greater than 1 mg/kg
body weight per day (dermal) or 6 mg/kg (oral) led to cholinergic
symptoms including salivation, convulsions, and death after 2 - 3 days.
With oral doses of 3 - 6 mg/kg no ataxia or death was observed.
Dermally, dichlorvos was very toxic for hens. Dose levels of about 1.7
and 3.3 mg/kg for an average period of 37 days caused ataxia and death.
An average dermal dose level of 0.65 mg/kg body weight for 90 days did
not induce ataxia or death. Typical symptoms of organophosphorus-
ester-induced delayed neurotoxicity (OPIDN) were not observed (Francis
et al., 1985).
In summary, it is possible to produce clinical neuropathy in hens,
but the doses required are far in excess of the LD50. The effects
are associated with severe inhibition of NTE in brain and spinal cord,
measured shortly after dosing (Johnson, 1981).
8.9.2. Mechanism of neurotoxicity
Male rats given a lethal intraperitoneal injection of 40 mg
dichlorvos/kg body weight did not show electrocortical disturbances.
Deaths resulted from respiratory failure (Hyde et al., 1978).
In studies by Desi (1983), adult CFY rats were given daily oral
doses ranging from 1.25 to 4 mg dichlorvos/kg body weight mixed in the
diet for 3 months. Plasma, erythrocyte, and brain ChE activities were
comparable with those of control rats. Increased EEG activity and
enhanced central excitability were found in the male rats only.
No changes in reflex motor unit potential activity or in nerve
conduction velocity were noted in dogs 7 days after single oral doses
of 30, 59.5, or 148 mg dichlorvos/kg body weight. However, erythrocyte
ChE activity was inhibited at all doses, and the highest dose produced
signs of intoxication (Hazelwood et al., 1979).
A single oral dose of 40 mg dichlorvos/kg or repeated doses of
1.6 mg/kg body weight per day did not cause histological abnormalities
in the brain of adult Wistar rats. Repeated administration of 50% of
the LD50 (i.e., 40 mg/kg body weight per day for 10 - 21 days), caused
myelin pallor and micro-vacuolation of the white matter. It seems
likely that primary degeneration of axons and secondary myelin sheath
abnormalities caused the spongy tissue loosening observed under the
electron microscope (Zelman, 1977; Zelman & Majdecki, 1979).
In studies by Ali et al. (1979a) and Hasan et al. (1979), male rats
were given 3 mg dichlorvos/kg per day intraperitoneally for 10 days.
Following perfusion-fixation, sections of cerebellum and spinal cord
were studied with the electron microscope. An abnormal increase in the
number of mitochondria in the spinal cord was found. Myelin
degeneration was detected in the spinal cord and myelin figures were
occasionally noted within oedematous dendrite profiles.
Table 18. Time-related ChE activity in animals after administration of a single dose of dichlorvos
---------------------------------------------------------------------------------------------------------
Species/sex Route Dose (mg/kg Time Cholinesterase activity (%) Reference
body weight) plasma erythrocyte brain
---------------------------------------------------------------------------------------------------------
Mouse (male) intraperitoneal 30 15 min 37 Cohen & Ehrich
2 h 40 (1976)
5 h 69
18 h 92
Mouse (male) intraperitoneal 10 15 min 30 Nordgren et al.
60 min 50 (1978)
2 h 80
Rat (male) oral 50 15 min 30 20 10-15 Modak et al.
3 h 30 65 (1975)
24 h 60 75 60-70
Rat (male) oral 40 1 h 30 Teichert et al.
(1976)
Rat (male) oral 40 5 min 55 Pachecka et al.
15 min 20 (1977)
2 h 30
24 h 75
48 h 100
Rat (male) intravenous 2.5 30 min 40 15 Reiner & Plestina
90 min 60 35 (1979)
3 h 90 55
12 h 100 80
48 h 90
Dog (beagle) oral 50 2 h 32 63 Ward & Glicksberg
(sex not 24 h 63 74 (1971)
specified) 5 days 92 78
21 days 100 92
Dog oral 22 1 h 12 17 Snow & Watson
(greyhounds 3 h 24 34 (1973)
and crossbred) 24 h 70 65
72 h 95 65
---------------------------------------------------------------------------------------------------------
Another study of dichlorvos neurotoxicity involved the
investigation of lipid peroxidation. This entails the direct reaction
between oxygen and lipids to form free-radical intermediates and semi-
stable peroxides. Major cellular components, such as membranes and
subcellular organelles, are damaged by these free radicals. Hasan &
Ali (1980) found a dose-dependent increase in the rate of lipid
peroxidation in various regions of the brain of the rat after
intraperitoneal administration of dichlorvos (at concentrations ranging
from 0.6 to 3 mg/kg body weight, daily) for 10 days. Also, there was
an increased incidence of lipofuscin-like pigment in the Purkinje cells
of the cerebellar cortex.
Maslinska et al. (1984) found that dichlorvos (dose levels of 4 -
8 mg/kg body weight for 10 days) affected the phospholipid-protein
balance in the brain of rabbits. The animals were exposed during the
postnatal "critical" life period, which constitutes a turning point in
the development of the brain. At this time, the neurons have already
undergone considerable arborization, and myelination and
vascularization are expanding rapidly. In addition, the overall oxygen
consumption is reaching its steepest rate of increase. In the myelin
sheaths under formation, several phospholipids are deposited. The
authors found changes in the phospholipid-protein ratio which
correlated well with the observed delay in myelin sheath formation.
Ultrastructural changes in certain subcellular organelles may be
connected with the change in this ratio, since it is crucial to the
structural and functional properties of the membranes and enzymes bound
to them.
Dambska et al. (1984) have studied the influence of dichlorvos on
blood vessel walls, the perivascular area, and the permeability of the
blood-brain barrier in young rabbits. The young animals received 9 mg
dichlorvos/kg body weight for 16 days starting on the 6th day of life.
There was a decrease in ChE activity in brain capillary walls.
Electron microscopic studies showed lesions of the perivascular
astrocytes and changes in the endothelial cells. However, these
lesions did not disturb the blood-brain barrier mechanism for
horseradish peroxidase particles.
Studies on the central cholinergic system have revealed that the
inhibition of brain ChE activity and its subsequent recovery were
uniform in all brain regions studied in orally dosed (50 mg/kg) rats.
ACh concentrations were increased in brain areas within 15 min of
treatment. A biphasic effect was observed on choline metabolism in the
brain. The cortex was more cholinergic than the striatum in terms of
percentage increase in ACh and choline (Modak et al., 1975). In a
similar study on rats, either receiving a single oral dose (40 mg/kg)
or repeated oral doses (4 mg/kg), the activity of whole brain choline
acetyltransferase and the contents of ACh and choline in whole brain
were not altered, though brain ChE activity was markedly inhibited.
However, in the cerebral hemispheres, and especially the corpus
striatum, the ACh level was considerably increased, without a
concomitant change in choline (Teichert et al., 1976).
Kobayashi et al. (1980, 1986) investigated the concentration of
total, free, labile-bound and stable-bound ACh in the brain of rats
given single or multiple subcutaneous injections of dichlorvos (0.2 -
4 mg/kg body weight). The results suggest that alterations in the
mobilization and storage of ACh in the central cholinergic nerves may
be induced. The time course for ACh accumulation was measured in rat
brain regions after intravenous treatment with 15 mg dichlorvos/kg body
weight (Stavinoha et al., 1976). The striatum had the highest rate of
accumulation and the cerebellum the lowest. The calculated turnover
time for the different regions of the brain was between 0.9 and
5.6 min.
In studies by Ali & Hasan (1977) and Ali et al. (1979b, 1980), rats
were given intraperitoneally 3 mg dichlorvos/kg body weight per day for
10 or 15 days. The concentrations of dopamine, norepinephrine, and 5-
hydroxytryptamine (5-HT) were significantly decreased in different
parts of the brain, and 5-HT was significantly increased in the spinal
cord.
A single dose or short-term (12 weeks) treatment of rats with high
concentrations of dichlorvos, which produced brain ChE inhibition,
resulted in decreased norepinephrine levels in the brain (Brzezinski &
Wysocka-Paruszewska, 1980). From these studies, it was suggested that
the metabolism of catecholamines and 5-HT may be disturbed by
dichlorvos.
8.10. Other Studies
Many studies in different organ systems have been carried out. In
most of these studies, the route of administration was the oral or
intraperitoneal route. Single or repeated dosing was used, mainly with
high dose levels, in mice and rats. The influence on brain enzymes
(other than brain ChE), liver enzymes such as ChE (inhibition),
microsomal cytochrome P-450 activity (decrease), drug-metabolizing
enzyme activity, and UDP-glucuronyl transferase (no influence), and
many other enzyme systems were studied.
Furthermore, the influence of dichlorvos on adrenal steroidogenesis
has been investigated (Civen et al., 1980).
8.10.1. Immunosuppressive action
A dose-related suppression of the humoral immune response induced
by S. typhimurium was observed in rabbits orally administered 0.3 -
2.5 mg dichlorvos (93%)/kg body weight in capsules, 5 times a week for
6 weeks (Desi et al., 1978).
In a comparable study on rabbits, the cellular immune response was
estimated using the tuberculin skin test. The skin redness in the
tuberculin test and the serum antibody titres of treated animals showed
a dose-dependent decrease compared with those of controls (Desi et al.,
1980).
8.11. Factors Modifying Toxicity; Toxicity of Metabolites
8.11.1. Factors modifying toxicity
In studies on the effect of diet on the toxicity of dichlorvos,
young male rats were kept for 30 days on the following synthetic diets:
high protein (HPD), low protein (LPD), high fat (HFD), and standard
(SD). Growth rates were normal except for a slightly decreased body
weight gain in the HFD group. The composition of the diet, per se, did
not significantly affect plasma and erythrocyte ChE activity 24 h or 5
days after dosing. A single intraperitoneal injection of 50 mg
dichlorvos/kg body weight led to higher mortality in LPD rats (as
expected with a protein-deficient diet) and lower mortality in HPD
rats, compared with SD animals (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or HPD
for 30 days. At the end of this period, a single intraperitoneal dose
of dichlorvos (20 or 30 mg/kg body weight) was administered. Results
showed that diets, per se, did not affect initial plasma or erythrocyte
ChE activity, nor did the HPD or HFD diets protect against mortality
from dichlorvos. In the case of the HPD, the spontaneous recovery of
ChE activity was reduced in the plasma and erythrocytes of dichlorvos-
treated rats. However, with an HFD, this recovery was significantly
increased (Purshottam & Srivastava, 1984).
Costa & Murphy (1984) studied the interaction between acetaminophen
(which, like dichlorvos, is detoxified by glutathione transferase) and
dichlorvos (10 mg/kg body weight) in mice. Acetaminophen (600 mg/kg
body weight) pre-treatment did not have any affect on dichlorvos
toxicity. On the other hand, intraperitoneal pre-treatment with
diethylmaleate (1 ml/kg body weight) increased the acute toxicity of
dichlorvos.
8.11.2. Toxicity of metabolites
8.11.2.1 Acute toxicity
The toxicity of metabolites of dichlorvos in female mice, injected
intraperitoneally, is considerably less than that of dichlorvos
(Table 19).
Mice (male and female) survived a single exposure of 130 mg
DCA/m3 for 5 - 7 h (Stevenson & Blair, 1969).
8.11.2.2 Short-term exposures
Groups of 20 male and 20 female rats were exposed for 30 days to
actual concentrations of 0 or 0.5 - 1 mg DCA/m3. Half the animals
were then killed promptly and the remainder on day 35. After 30 days
of exposure, the male rats showed a slight decrease in body weight and
food intake, and a slight increase in absolute and relative liver
weight. There were no such changes in the rats left for 5 more days
unexposed to DCA. Histological examination of the lungs of exposed
males and females revealed a higher incidence of minor inflammatory
changes than in controls. No other changes attributable to DCA were
found in general health, behaviour, haematology, clinical chemistry,
organ weights, gross pathology, or histopathology. A similar study
using groups of 10 male and 10 female rats and actual concentrations of
0 or approximately 2 mg/m3 DCA revealed no abnormalities attributable
to DCA (Wilson & Dix, 1973).
Table 19. Intraperitoneal LD50 values of metabolites of dichlorvos in
female micea
-----------------------------------------------------------------------------
Compound Vehicle LD50 (mg/kg body weight)
-----------------------------------------------------------------------------
desmethyldichlorvos, water 1500
sodium salt
dichloroacetyaldehyde corn oil 440
(DCA)
dichloroethanol corn oil 890
dichloroacetic acid corn oil or water 250
sodium dichloroacetate water 3000
methyl and dimethyl water 1500
phosphoric acid mixture
sodium methyl- and water 3000
dimethylphosphate
mixture
-----------------------------------------------------------------------------
a From: Casida et al. (1962).
8.11.2.3 Long-term exposure
No long-term tests have been carried out with DCA as such.
However, in the 2-year oral studies on rats and dogs with dichlorvos
(section 8.4.1), the degradation product DCA was present in the test
diets in increasing amounts, so that possible effects of DCA were
included in the results of these studies.
8.11.2.4 Mutagenicity
DCA appears to be mutagenic in the Salmonella test using S.
typhimurium TA100. The mutagenicity decreased in the presence of a
microsomal activation system. Part of the decrease was dependent on
the presence of the co-factors NADP and glucose-6-phosphate. No
evidence for mutagenicity with 2,2-dichloroethanol was obtained in
this S. typhimurium strain (Löfroth, 1978).
In a dominant lethal assay, a single intraperitoneal injection of
176 mg DCA/kg in male mice (AB Jena-Halle strain) produced a decrease
in the number of total implants and live fetuses in the first 3 weeks
of the test, and an increase in post-implantation losses. The same
test was repeated with a strain of mice (DBA) less sensitive to
mutagenic effects. The same effects were found, but to a lesser
extent, and mainly in the fourth week (Fischer et al., 1977).
8.11.2.5 Metabolism
When 32P-dimethylphosphate (500 mg/kg in water) was administered
orally to a male rat, the autopsy (90 h after treatment)
indicated that almost the entire dose had been eliminated. The
urine, containing only unmetabolized dimethylphosphate, accounted for
about half of the radioactivity. The tissues were almost devoid
of 32P-containing material (Casida et al., 1962).
A rat, orally dosed with 500 mg/kg 32P-desmethyldichlorvos in
water, excreted about 14% of the dose in the urine in 90 h.
The tissue distribution of 32P was similar to that which followed
32P-dichlorvos administration. The very high proportion of
radioactivity in the bone was indicative of rapid degradation to
phosphoric acid (Casida et al., 1962).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity
9.1.1.1 Poisoning incidents
A 56-year-old woman ingested an estimated amount of 100 mg
dichlorvos/kg body weight and survived, following intensive care for 14
days (Watanabe et al., 1976). However, a suicide with a dichlorvos
dose of about 400 mg/kg succeeded in spite of treatment (Shinoda et
al., 1972).
A 35-year-old female patient accidently ingested 60 g fluid Divipan
(dichlorvos concentration not reported). She was comatose for one week
and recovered slowly. Clinical and electrophysiological examinations
(no details reported) showed a pure motor form of neuropathy, according
to the authors (Vasilescu & Florescu, 1980).
Two cases of poisoning with dichlorvos taken orally in unspecified,
but high, quantities have been reported. The patients first showed
signs of severe anti-ChE poisoning. After recovery, delayed
neurotoxicity developed. They showed a severe axonal degeneration
neuropathy. One of them recovered within 12 months (Wadia et al.,
1985).
Reeves et al. (1981) reported six cases, over an 8-year period, of
bone-marrow failure (pancytopenia) in children shortly after exposure
to dichlorvos and propoxur. Van Raalte & Jansen (1981) doubted the
causal relationship between dichlorvos and bone-marrow failure in the
children, because the disease has not been observed in workers with
high exposure, or in the general population. In addition, haematotoxic
effects have not been observed in experimental animals.
9.1.2. Effects of short- and long-term exposure
In the 1960s, field studies were carried out in several countries
to test dichlorvos as a residual insecticide for malaria control in
houses. Numerous residents of all ages and conditions were exposed
without any adverse effects attributable to dichlorvos being reported
(Escudié & Sales, 1963; Funckes et al., 1963; Gratz et al., 1963;
Quarterman et al., 1963; Foll et al., 1965). In two field studies, the
plasma and erythrocyte ChE activities were measured in adults and young
children. No abnormalities were found, though air concentrations of
dichlorvos in the treated houses rose to 0.8 mg/m3 (Funckes et al.,
1963; Gratz et al., 1963).
In a report by Gold et al. (1984), 20 single-family residences were
treated with a 0.5% solution of dichlorvos (at an average rate of
0.189 g/m2) to control the cockroach Blattella germanica L. The
average air concentration for the first 2 h after treatment was
548 µg/m3 and for the next 2 h was 183 µg/m3. Pesticide operators
and the residents of treated structures were monitored for evidence of
dichlorvos exposure, using exposure pads, air samples, serum and
erythrocyte ChE tests, and urinalyses. There was no evidence of
dichlorvos or dichloroacetic acid in urine. There were slight, but
statistically significant, changes in the mean serum ChE activity of
some of the residents of treated structures, but the mean erythrocyte
ChE was unchanged.
Passengers in an aircraft provided with an automatic insect-control
system, which released 0.15 - 0.30 mg dichlorvos/m3 for periods of
about 30 min, did not show any signs of discomfort (Jensen et al.,
1965). Tests have shown that man can withstand daily exposure to
concentrations of 0.5 mg/m3 without clinical effects, and with only a
slight depression of blood ChE activity (Hayes, 1961). Three men
exposed to approximately the same level during 24 tests did not show
any change in blood ChE activity (Schoof et al., 1961).
A case of chronic obstructive bronchitis ascribed to exposure to
dichlorvos was described by Barthel (1983). However, it could not be
excluded that other components of the unknown formulation could have
been the cause. ChE determinations were not performed.
9.1.2.1 Studies on volunteers
Single oral doses (1 - 32 mg/kg body weight) of dichlorvos in a
slow-release PVC formulation administered to 107 male volunteers
produced measurable reductions in erythrocyte ChE activity at dose
levels above 4 mg/kg, with a maximum reduction of 46% at the highest
dose. Plasma ChE activity was affected at lower doses, with 50%
reduction at 1 mg/kg and about 80% at 6 mg/kg or more. Repeated oral
doses of 1 - 16 mg/kg body weight per day were given to 38 volunteers
for up to 3 weeks. The plasma ChE activity was maximally depressed at
all dose levels, and the erythrocyte ChE activity depression was dose
related and significant at 2 mg/kg or more. Blood cell count, urine,
liver function, prothrombin time, and blood urea nitrogen were all
normal (Hine & Slomka, 1968, 1970; Slomka & Hine, 1981).
In studies by Rider et al. (1968), dichlorvos was given to groups
of five men at daily oral doses of 1, 1.5, 2, or 2.5 mg per man. The
plasma ChE activity of the 2.5 mg group was reduced by 30% after 20
days of treatment. Administration of 2 mg for 28 days resulted in a
reduction of 30% 2 days after the last dose. Erythrocyte ChE activity
was not significantly affected in either group (Rider et al., 1967).
Daily oral doses of 1.5 mg per man given to 10 volunteers for 60 days
caused a significant reduction (approximately 40%) in plasma ChE
activity, which returned to normal levels when dichlorvos
administration was discontinued.
Boyer et al. (1977) reported studies on two groups of six men (21 -
45 years of age) who received 0.9 mg of dichlorvos three times a day
for 21 days. One group received the dichlorvos in a gelatin salad, the
other in a pre-meal capsule filled with cottonseed oil. Two other
groups of six men received placebo treatment. No consistent
cholinomimetic signs or symptoms were observed, nor was erythrocyte ChE
inhibited. However, plasma ChE was significantly depressed within 20
days, although the extent depended on the method by which dichlorvos
was administered. Recovery was comparable in both groups, the half-
life for the regeneration of plasma ChE being 13.7 days.
Volunteers did not show inhibition of plasma or erythrocyte ChE
activity either when handling a dichlorvos strip for 30 min each day or
after having a piece of the strip applied to the arm for 30 min each
day for 5 consecutive days (Zavon & Kindel, 1966).
The wearing of dichlorvos-impregnated garments by babies for 48 -
84 h did not produce changes in either plasma or erythrocyte ChE
activity over a period of 5 days (Cavagna et al., 1969).
In studies on eight volunteers, carried out in an aircraft at
operational cabin altitude (2400 m), no changes in plasma or
erythrocyte ChE activity, dark adaptation, or bronchiolar resistance
were observed. Dichlorvos concentration in the air ranged from 0.73 to
1.18 mg/m3 during exposures of 45 min (Smith et al., 1972).
Hunter (1970a) reported studies on 26 men (21 - 57 years of
age) and 6 women (19 - 25 years of age) who were exposed in a chamber
for 2-7´ h to actual dichlorvos concentrations of approximately
1 mg/m3. Food and drink were served in the chamber. No clinical
signs were observed, and no effects on haematology, urinalysis, kidney
function, EEG, ECG, respiratory activities, or erythrocyte ChE activity
were found. Plasma ChE activities were markedly inhibited only when
the exposure lasted over 6 - 7 h (Hunter, 1970a).
In studies by Hunter (1969, 1970b), seven men (25 - 56 years of
age) were exposed (head and neck) to dichlorvos vapour (actual
concentrations of 1 - 52 mg/m3), and 6 men were exposed (head
exposure only) to 7 - 50 mg/m3, for periods from 10 min to 4 h. The
maximum dose level was 52 mg/m3 (for 65 min), and the maximum period
was 240 min (at 13 mg/m3). Symptoms were confined to irritation of
the throat, some rhinorrhoea, and substernal discomfort at the highest
concentrations. No effects on the pupil or on visual acuity were
recorded. Erythrocyte ChE activity was depressed in only one person.
However, there was a direct relationship between the reduction in
plasma ChE activity and the dichlorvos dose (concentration x time). No
changes were found in either kidney or pulmonary function or in the
overall metabolic rate.
When three men were exposed to dichlorvos (actual concentrations of
0.3 - 0.9 mg/m3 (mean, 0.5 mg/m3) or 0.9 - 3.5 mg/m3 (mean,
2.1 mg/m3) for 1 or 2 h per day for 4 consecutive days, plasma ChE
alone decreased slightly in the men exposed for 2 h per day to the
higher concentration (Witter et al., 1961).
A group of 15 men (23 - 61 years of age) was exposed for up to 6
half-hour intervals per night for 14 days (total of 39 doses) to actual
dichlorvos concentrations ranging from 0.14 to 0.33 mg/m3. No clear
changes in plasma ChE activity or other parameters such as reaction
time, airway resistance, or vision were found. The same results were
obtained when a similar group was exposed to actual concentrations of
0.1 - 0.6 mg/m3 for intervals of 8 - 10 h, 4 nights per week for 11
weeks (Rasmussen et al., 1963).
No significant effect on plasma or erythrocyte ChE activity was
observed in 14 persons exposed at the recommended rate of one
dichlorvos strip per 30 m3 in their homes over a period of 6 months.
The strips were replaced at much shorter intervals than normally
recommended. The air concentration 40 days after the installation of
the fourth strip was approximately 0.09 mg/m3 (Zavon & Kindel, 1966).
In three home studies involving 26 families, conducted in Arizona,
USA, no deleterious effects on health or plasma or erythrocyte ChE
activity were observed in the residents exposed to dichlorvos strips
all over the house (8 - 10 strips) for over a year. Even monthly
replacement of the strips resulted in only a slight inhibition of
plasma ChE activity. The maximum air concentrations of dichlorvos
averaged 0.13 mg/m3 (Leary et al., 1971, 1974).
9.1.2.2 Hospitalized patients
In a report by Pena Chavarra et al. (1969), a single oral dose of 6
or 12 mg dichlorvos/kg body weight was administered in the form of a
slow-release granular resin formulation as an anthelminthicum to 108
hospitalized adult patients, many of these debilitated and with severe
anaemia. Plasma ChE activity was markedly reduced, in some patients by
76 - 100%. However, erythrocyte ChE activity was much less inhibited.
No symptoms of intoxication were observed, except for brief mild
headaches in a few patients, and there were no abnormalities in
haematological studies or in hepatic and renal function tests.
In studies by Cervoni et al. (1969), single doses of dichlorvos
(PVC-resin formulations) were administered orally 2 h before breakfast
to 705 adults found to be harbouring infections of Trichuris, hookworm,
or Ascaris. Six or 12 mg dichlorvos/kg body weight resulted in
infection cure rates of approximately 70 - 100%. According to the
authors, minimum to modest plasma ChE depression and zero to minimum
erythrocyte ChE depression occurred at both dose levels. No clinical
symptoms or alterations in haematology or in liver and kidney function
were observed.
Sick adults and children and healthy pregnant women and babies in
hospital wards treated with dichlorvos strips (one strip per 30 or
40 m3) had normal erythrocyte ChE activities. Only subjects exposed
for 24 h per day to dichlorvos concentrations above 0.1 mg/m3 or
patients with liver insufficiency showed even a moderate decrease in
plasma ChE activity (Cavagna et al., 1969, 1970; Cavagna & Vigliani,
1970).
9.2. Occupational Exposure
9.2.1. Acute toxicity
9.2.1.1 Poisoning incidents
A number of fatal and non-fatal poisoning cases have been described
after concentrated formulations of dichlorvos splashed onto parts of
the body. Two workers who failed to wash it off promptly died
consequently. However, in those cases where the spilled solution was
washed off immediately, the victims showed symptoms of intoxication but
recovered after treatment. A serious non-fatal case occurred after a
spillage of 120 ml of a 3% formulation that was not washed off
immediately. After 1´ h, the victim developed slurred speech, became
drowsy, and collapsed. He recovered completely after treatment (Hayes,
1963, 1982).
A pest-control operator became contaminated with a 1% solution of
dichlorvos in mineral spirit after using a leaking knapsack sprayer.
The man changed his overalls and completed his day's work. He noticed
weakness, dizziness, and difficulty in breathing. Contact dermatitis
developed on the back skin. After the fourth day, his blood ChE was
36% of normal, but there were no signs of systemic illness. He
recovered without medication, and the ChE activity increased to 72% of
normal within one month. The acute dermatitis was probably caused by
the solvent (Bisby & Simpson, 1975).
A driver of a truck transporting a 5% commercial formulation of
dichlorvos (15% petroleum distillate and 80% trichloroethane)
developed persistent contact dermatitis for 2 months following
accidental skin contact. In addition, the patient experienced
headache, mild rhinorrhoea, burning of the tongue, and a bitter taste
in his mouth. Initial blood ChE levels were in the low normal range
returning to the high normal range within 2 weeks. Patch tests with 1%
and 0.1% dichlorvos in petroleum distillate were negative (Mathias,
1983). In view of the symptoms and the slight ChE inhibition, it seems
likely that trichloroethane caused the dermatitis.
Cronce & Alden (1968) described four people who handled dogs
wearing anti-flea dog collars containing 9 - 10% dichlorvos. Acute
primary contact dermatitis was described. Closed patch tests with
0.25, 0.5, and 1% dichlorvos in distilled water and in mineral oil were
positive in all four people. General experience with the collars
indicates that only a few people are susceptible to this kind of
irritation (Hayes, 1982).
9.2.2. Effects of short- and long-term exposure
9.2.2.1 Pesticide operators and factory workers
The effect on sprayers of dichlorvos fume, used inside a building
for cockroach control, was examined. When 0.3 - 0.6% dichlorvos oil
spray was used at the rate of 6 ml/m2 by the sprayers (7 - 9 people),
inhibition of ChE activity in the subjects was 15%, and conjunctival
injections or sore throats were observed in some sprayers after either
18 min or 4 h of spraying operation. To examine the effect of elevated
dichlorvos vapour pressure at higher temperatures, four men sprayed
0.6% oil spray for 2 h at the room temperature of 25 °C. The
inhibition of plasma and erythrocyte ChE activities was 22% and 7%,
respectively. In another study, 11 sprayers used 0.6% oil spray for
6 h of actual work time at 20 °C without rest. Two of them showed an
inhibition of plasma ChE activity of 38% while the others did not show
significant inhibition of erythrocyte ChE. It was concluded that
conjunctival injections and sore throats were attributable to the
kerosene solvent used and that the 38% depression in ChE activity was
probably due to long hours of continuous work. Therefore, the overall
effect on ChE activity was not considered to be severe (Ueda et al.,
1959, 1960).
Twelve fogging machine operators did not show any reduction in
plasma or erythrocyte ChE activity when applying 4% dichlorvos aerosols
in tobacco warehouses for 16 h per week, over a period of 2 - 4 months
(Witter, 1960).
Sixteen men replacing old dichlorvos dispensers and installing new
units in houses in Haiti, 5 days per week for 3 weeks, showed a
decrease of up to 60% in plasma ChE activity. No signs or symptoms of
intoxication were observed. Air concentrations ranged from 0.3 to
2.1 mg/m3 (Stein et al., 1966).
The blood ChE activity of sprayers, exposed during insect control
in grain stores to air concentrations of 1.9 - 3 mg/m3 dichlorvos,
was reduced to 19 - 23% (Sasinovich, 1970).
In a report by Das et al. (1983), each of 13 pesticide-control
operators carried out urban pest-control for one day in four houses
using 230 - 330 g dichlorvos as aerosol and 40 - 50 g dichlorvos as
emulsion spray. At the end of the day's work, an operator had an
average dichlorvos residue of 0.8 mg/m2 on the back, 0.4 mg/m2 on the
chest, and 11 mg/m2 on the respirator filter. Dimethylphosphate was
detected in the urine, but blood and urine analyses, including serum
ChE levels, did not reveal any other changes in clinical parameters.
In the course of either the production or processing of a
dichlorvos-releasing product, 11 male and 2 female factory workers were
exposed to an average dichlorvos concentration of 0.7 mg/m3 (highest
value 3 mg/m3) on each working day for a period of 8 months.
Inhibition of plasma ChE activity was noted within a few days of the
start of exposure, while inhibition of erythrocyte ChE activity
developed much more slowly. The maximum plasma ChE activities recorded
were 40% lower than the pre-exposure levels, but 60% lower than the
post-exposure levels. Erythrocyte ChE activity was reduced by
approximately 35% compared with pre- and post-exposure levels. One
month after exposure had ceased, plasma ChE and erythrocyte ChE
activities were found to have returned to normal physiological levels.
The other haematological investigations and the medical examinations
did not reveal any changes attributable to dichlorvos exposure (Menz et
al., 1974).
9.2.2.2 Mixed exposure
A number of articles have described the symptoms found in
individual workers or groups of workers exposed for a number of years
to different types of pesticides, including dichlorvos. In general, a
slight-to-moderate decrease in ChE activity occurred. Furthermore,
several complaints and symptoms were noticed, but no clear clinical
poisoning cases occurred. These case studies are, however, of little
relevance for the evaluation of dichlorvos because of the mixed
exposure to different pesticides (Bellin & Chow, 1974; Fournier et al.,
1976; Gupta et al., 1979; Ullmann et al., 1979; Hayes et al., 1980;
Hayes, 1982).
From 1975 to 1977, 88 patients with pesticide dermatitis, 15
suffering from photo-dermatitis, were studied. Patch tests using 29
different pesticides of different categories were carried out, but did
not lead to an identification of the responsible pesticides. Eight out
of 52 patients (15.4%) reacted positively to a photopatch (Horiuchi &
Ando, 1978; Horiuchi et al., 1978).
Stoermer (1985) described a case of contact dermatitis in a woman
who had worked as a pest controller for 3 months and had sprayed
dichlorvos and propoxur. She was treated and recovered within one
week.
A field survey of tea growers was made in the Chiran area of Japan
in 1982. Out of 84 tea growers examined (21 men and 63 women), 5 women
had contact dermatitis from agricultural work. Dichlorvos was among
the insecticides, fungicides, and herbicides used. Patch tests showed
relatively high rates of positive reactions with dichlorvos (27% of
women and 5% of men). Also, cross-sensitization was found between
methidathion and dichlorvos (Fujita, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Since 1961, dichlorvos, an organophosphate with anti-ChE activity,
has been used worldwide as a contact and stomach insecticide to control
insects on crops and domestic animals. It is also used as an
insecticide in houses and other buildings and for insect control in
aircraft.
Dichlorvos is readily absorbed by the body of mammals through all
routes of exposure, and is readily metabolized in the liver. Within
1 h of oral administration, dichlorvos is found in the liver, kidneys,
and other organs of experimental animals. It is rapidly eliminated via
the kidneys, with a half-life of 14 min.
The metabolism of dichlorvos in various species, including human
beings, follows similar pathways. Differences between species relate
only to the rate of metabolism, but this is always rapid.
Dichlorvos is moderately to highly toxic for mammals (the oral
LD50 for the rat is 30 - 110 mg/kg body weight). The classification
of dichlorvos by WHO (1986a) is based on an oral LD50 for the rat of
56 mg/kg body weight. Signs of intoxication usually occur shortly
after exposure and are typical of an organophosphorus pesticide.
Inhibition of ChE activity is a sensitive criterion of exposure. In
short-term toxicity studies on mammals, it was shown that ChE activity
was not decreased at oral dose levels below about 0.5 mg/kg body
weight. In long-term studies on rats at oral dose levels of 2.5 mg/kg
body weight or more, hepatocellular fatty vacuolization was seen. At
0.25 mg/kg body weight, no ChE inhibition was found, nor were there any
other effects.
Reproduction and teratogenicity studies using a wide range of dose
levels (6.25 - 500 mg/kg body weight) were negative. Dichlorvos showed
alkylating properties in in vitro but not in in vivo studies. Many in
vitro mutagenicity studies with bacteria and yeast were positive, while
the in vivo studies were mainly negative. From the available
mutagenicity studies, it is unlikely that dichlorvos constitutes a
mutagenic hazard for man. Carcinogenicity studies on mice and rats fed
dichlorvos (dose levels of up to 234 mg/kg diet) were negative. Two
recent carcinogenicity studies have been carried out on mice and rats
in which dichlorvos was administered by intubation for up to 2 years at
dose levels of 10 - 40 mg/kg body weight (mice) and 4 or 8 mg/kg body
weight (rats). Only preliminary information has been provided. The
evidence for carcinogenicity in these new studies is difficult to
interpret at this time. Only when complete and final reports become
available will it be possible to draw more definite conclusions (in
this context, see section 8.7.3).
From work on hens, the suspicion of delayed neurotoxicity from
dichlorvos has neither been established nor totally refuted. However,
there have been two clinical reports on four patients suffering intense
poisoning from dichlorvos taken orally who survived with treatment and
who then displayed neurotoxic effects. Thus, the possibility of
delayed neurotoxicity in man cannot be entirely discounted, but it is
likely to occur only with excessive oral doses.
Human volunteers who were given single or repeated oral doses of 2
mg/kg body weight or more showed significant inhibition of erythrocyte
ChE activity. At 1 mg/kg body weight, no such inhibition was found.
The application of dichlorvos to crops and animals results in
residues that rapidly disappear by volatilization and hydrolysis. In
general, residues of dichlorvos and the breakdown product DCA in food
commodities are low and will be further reduced during processing. The
exposure of the general population to dichlorvos by food and drinking-
water is negligible, as is confirmed in total-diet studies.
In short-term inhalation studies on mammals, 1 or 2 mg
dichlorvos/m3 did not inhibit ChE activity.
In a 2-year, 23 h/day, whole-body inhalation study on rats, 0.48
mg/m3 caused inhibition of plasma and erythrocyte activity, but brain
AChE activity was not inhibited and there were no clinical signs. An
unquantified, but considerable, extra exposure, resulting from the
grooming of contaminated fur and contamination of food and drinking-
water, had contributed to this effect. The no-observed-adverse-effect
level was 0.05 mg/m3. There was no evidence of carcinogenicity.
In a 6- to 7-h exposure of human volunteers to approximately 1 mg
dichlorvos/m3, only plasma ChE activity was inhibited. The
erythrocyte AChE activity, taken to be representative of the AChE
activity in the nervous tissue, was unaffected.
Residents exposed for over one year to an average air concentration
of 0.1 mg/m3 arising from slow-release strips showed no inhibition of
plasma or erythrocyte ChE activity and no deleterious effects on
health.
The main exposure of the general population is by inhaling
dichlorvos when used indoors to control insects. The recommended use
(one slow-release strip/ 30 m3) will give concentrations in the air
of up to 0.1 - 0.3 mg/m3 in the first few days, decreasing thereafter
to below 0.1 mg/m3. The air concentration depends on temperature,
humidity, and ventilation.
As long as approved slow-release strips are used according to the
label instructions, no health hazard can be expected for man. However,
special care may need to be taken with young children and sick or
elderly people who are especially vulnerable when continuously exposed
(24 h per day) in poorly ventilated rooms. Other methods of indoor
application should be equally safe if the label instructions are
followed.
There is some indication that dichlorvos may induce dermatitis and
cross-sensitization in people handling various types of pesticides
including dichlorvos.
In occupational conditions, the main route of exposure to
organophosphorus pesticides is usually the dermal route. In the case
of dichlorvos, with its high vapour pressure, exposure by inhalation is
also important. In these occupational situations, the dichlorvos
concentrations in the air are generally below 1 mg/m3 but, in certain
circumstances, they may rise considerably above this level. This
stresses the need for adequate protective measures to be taken during
occupational exposure and regular monitoring of ChE activity.
10.2. Evaluation of Effects on the Environment
The presence of dichlorvos in the environment as a result of
accidental losses or direct application on soil or in water will not
lead to long-term effects because of its fast breakdown and
evaporation. Furthermore, it is converted to a number of compounds,
such as dichloroacetic acid, by microorganisms. Certain bacteria
species can use dichlorvos as a sole carbon source, while others cannot
and are inhibited in their growth. Therefore, its influence on
microorganisms is complex.
Dichlorvos is moderately to highly toxic (range, 0.2 - 10 mg/litre)
for freshwater and estuarine species of fish and invertebrates. In
certain fish, concentrations of 0.25 - 1.25 mg/litre cause inhibition
of brain and liver ChE activity. Concentrations of as little as
0.05 mg/litre may have deleterious effects, particularly in
invertebrates. Dichlorvos has a high toxicity for birds and bees.
Caution is advised in the use and handling of dichlorvos where these
species might be exposed.
No bioaccumulation occurs in the different environmental
compartments and organisms.
10.3. Conclusions
1. Exposure of the general population to dichlorvos via food and
drinking-water is negligible and does not constitute a health hazard.
2. The in-house use of dichlorvos as an insecticide in the form of
sprays or slow-release strips (at recommended levels) does not
constitute a short- or long-term hazard for the general population.
However, continuous (24 h per day) exposure of young children and
diseased or elderly people in non-ventilated or poorly ventilated rooms
should be avoided.
3. In spite of their toxicity, dichlorvos and its formulations do not
contribute an undue hazard to those occupationally exposed, provided
that adequate ventilation and skin protection are used.
4. Except under conditions of gross spillage, the recommended use of
dichlorvos as an insecticide does not constitute an acute or long-term
hazard for aquatic or terrestrial organisms, although there may be an
acute hazard for birds and bees.
11. RECOMMENDATIONS
1. Continuous (24 h/day) exposure of young children and diseased or
elderly people to dichlorvos in non-ventilated or poorly ventilated
rooms should be avoided.
2. As dichlorvos from different sources may vary in purity and type of
impurities, attention should be paid to its composition. This should
conform to FAO and WHO specifications (FAO, 1977; WHO, 1985). In the
case of formulations, potential hazards of other components, such as
solvents and stabilizers, should also be considered.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dichlorvos was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1965, 1966, 1967, 1969, 1970, 1974, and 1977
(FAO/WHO, 1965a,b, 1967a,b, 1968a,b, 1970a,b, 1971a,b, 1975a,b,
1978a,b). In 1966, the JMPR established an Acceptable Daily Intake
(ADI) for human beings of 0 - 0.004 mg/kg body weight, which remains
unchanged.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified technical dichlorvos as
"highly hazardous" (Class IB) (Plestina, 1984; WHO, 1986a) and has
produced a safety sheet on dichlorvos (No. 75.2) (WHO/FAO, 1975-86).
Specifications for dichlorvos use in public health and in plant
protection have been published by WHO (1985) and FAO (1977),
respectively.
In 1979, the International Agency for Research on Cancer (IARC)
came to the following conclusions in considering the carcinogenicity of
dichlorvos:
(a) dichlorvos was tested in different animal species via
different routes; no conclusive evaluation on the basis of
these studies could be made;
(b dichlorvos is an alkylating agent and binds to bacterial and
mammalian nucleic acids;
(c) it is a mutagen in a number of microbial systems, but there is
no evidence of its mutagenicity in mammals, in which it is
rapidly degraded.
In the evaluation by IARC, it was stated that "the available data
do not allow an evaluation of the carcinogenicity of dichlorvos to be
made".
In its series "Scientific Reviews of Soviet Literature on Toxicity
and Hazards of Chemicals", the International Register of Potentially
Toxic Chemicals has published a volume on dichlorvos (IRPTC, 1984).
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O.G. (1970)
Pesticide residues in the total diet in England and Wales. III.
Organophosphorus pesticide residues in the total diet, 1966-67.
Pestic. Sci., 1: 10-13.
ACKERMAN, H., LEXOW, B., & PLEWKA, E. (1969) [Detection and
identification of the organic phosphate, phosphorothionate,
phosphonate, and carbamate insecticides in biological material.]
Arch. Toxicol., 24: 316-324 (in German).
ADLER, B., BRAUN, R., SCHONEICH, J., & BOHME, H. (1976) Repair-
defective mutants of Proteus mirabilis as a prescreening system for the
detection of potential carcinogens. Biol. Zentralbl., 95: 463-469.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides wetting agents and miscellaneous
substances. Int. pest Control, 11(2): 25-35.
ALDRIDGE, W.N. & BARNES, J.M. (1966) Further observations on the
neurotoxicity of organophosphorus compounds. Biochem. Pharmacol., 15:
541-548.
ALDRIDGE, W.N. & JOHNSON, M.K. (1971) Side effects of organo-
phosphorus compounds: delayed neurotoxicity. Bull. World Health
Organ., 44: 259-263.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and structure-
activity relationships providing a high safety factor for anti-
cholinesterase insecticides. In: Proceedings of the 1977 British Crop
Protection Conference - Pests and Diseases, Croyden, British Crop
Protection Council. pp. 721-729.
ALI, M.S., RAHMATULLAH, M., JAHAN, R., YUSUF, H.K.M., &
CHOWDHURY, A.A. (1979c) Effect of DDVP (insecticide) on citric acid
fermentation in Aspergillus niger. Enzyme Microb. Technol., 1: 127-
128.
ALI, S.F. & HASAN, M. (1977) Effect of organophosphate insecticide
dichlorvos on the amino acid content of different regions of the rat
brain and spinal cord. Indian J. exp. Biol., 15(9): 759-761.
ALI, S.F., HASAN, M., & MITRA, S.C. (1979a) Organophosphate DDVP-
induced changes in the rat spinal cord: an electron microscopic and
biochemical study. J. Anat. Soc. India, 28(1): 36.
ALI, S.F., HASAN, M., & TARIQ, M. (1979b) Levels of dopamine,
norepinephrine, and 5-hydroxytryptamine in different regions of rat
brain and spinal cord following chronic administration of organo-
phosphate pesticide dichlorvos. Indian J. exp. Biol., 17(4): 424-426.
ALI, S.F., CHANDRA, O., & HASAN, M. (1980) Effects of an
organophosphate (dichlorvos) on open-field behaviour and locomotor
activity: correlation with regional brain monoamine
levels. Psychopharmacology, 68(1): 37-42.
ALLEN, S.D., VANKAMPEN, K.R., & BROOKS, D.R. (1978) Evaluation of
the feline dichlorvos (DDVP) flea collar. Feline Pract., 8(3): 9-16.
AMBRUS, A., LANTOS, J., VISI, E., CSATLOS, I., & SARVARI, L. (1981)
General method for determination of pesticide residues in samples of
plant origin, soil, and water. J. Assoc. Off. Anal. Chem., 64(3):
733-768.
ANDERSON, R.A., BARSTAD, J.A.B., & LAAKE, K. (1978) An improvement
of the spectrophotometric method for determination of cholinesterase
activity in erythrocytes and tissue homogenates. Ann. Natl Inst. Publ.
Health (Norway), 1(1): 51-55.
ANON. (1972) Vapona insecticide. In: Analytical methods for
pesticides and plant growth regulators, Modesto, California, Shell
Development Company, Vol. 6, pp. 529-541.
ANON. (1973) The determination of malathion and dichlorvos residues
in grain. Report by the Panel on Malathion and Dichlorvos Residues in
Grain. Analyst, 98: 19-24.
ANON. (1977) Determination of residues of organophosphorus pesticides
in fruits and vegetables. Report by the Panel on Determination of
Residues of Certain Organophosphorus Pesticides in Fruits and
Vegetables. Analyst, 102: 858-868.
ANON. (1982) Pesticides: a safety guide, London, Shell International
Chemical Company Ltd, pp. 53-55.
AOKI, Y., TAKEDA, M., & UCHIYAMA, M. (1975) Comparative study of
methods for the extraction of eleven organophosphorus pesticide
residues in rice. J. Assoc. Off. Agric. Chem., 58(6): 1286-1293.
AQUILINA, G., BENIGNI, R., BIGNAMI, M., CALCAGNILE, A.,
DOGLIOTTI, E., FALCONE, E., & CARERE, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15:
439-442.
ARATAKE, Y. & KAYAMURA, T. (1973) [Toxicity of insecticides to
silkworm larvae.] Sanshi Kenkyu (Acta serol.), 87: 68-78 (in
Japanese).
ARIMATSU, S., HOSHIRO, Y., & NOMURA, T. (1977) [Studies on primary
irritation test of pesticides in rabbits.] Nippon Noson Igakkai
Zasshi, 26: 572-573 (in Japanese).
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees, Riverside,
University of California (Agricultural Extension Report No. M-16 Rev.
9/73).
AUGUSTINSSON, K.B., ERIKSSON, H., & FAIJERSSON, Y. (1978) A new
approach to determining cholinesterase activities in samples of whole
blood. Clin. Chim. Acta, 89: 239-252.
AULICINO, F., BIGNAMI, M., CARERE, A., CONTI, G., MORPURGO, G.,
& VELCICH, A. (1976) Mutational studies with some pesticides
in Aspergillus nidulans. Mutat. Res., 38(2): 138.
BAKSI, S.N. (1978) Effect of dichlorvos on embryonal and fetal
development in thyro-parathyroidectomized thyroxine-treated and
euthyroid rats. Toxicol. Lett., 2(4): 213-216.
BALLINGTON, P.E., SKIPPER, H.D., & HEGG, R.O. (1978) Response of
coliform populations in poultry waste digesters to three
insecticides. J. environ. Qual., 7(2): 262-264.
BARTHEL, E. (1983) [Irritative and allergic effects of pesticide
aerosols on the respiratory tract and problems of their evaluation.] Z.
gesamte Hyg., 29(11): 678-681 (in German).
BATORA, V., VITOROVIC, S.L.J., THIER, H.P., & KLISENKO, M.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BATTE, E.G., ROBINSON, O.W., & MONCOL, D.J. (1969) Influence of
dichlorvos on swine reproduction and performance of offspring to
weaning. J. Am. Vet. Med. Assoc., 154(11): 1397.
BAZER, F.W., ROBSON, O.W., & ULBERG, L.C. (1969) Effect of dichlorvos
and PMS on reproduction in swine. J. anim. Sci., 28: 145.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2(4): 307-337.
BELL, TH.G., FARRELL, K., PADGET, G.A., & LEENDERTSEN, L.W.
(1975) Ataxia, depression, and dermatitis associated with the use of
dichlorvos-impregnated collars in the laboratory cat. J. Am. Vet.
Med. Assoc., 167(7): 575-586.
BELLIN, J.S. & CHOW, I. (1974) Biochemical effects of chronic low-
level exposure to pesticides. Res. Commun. chem. Pathol.
Pharmacol., 9(2): 325-337.
BENIGNI, R. & DOGLIOTTI, E. (1980a) UDS studies on selected
environmental chemicals. Mutat. Res., 74: 248-249.
BENIGNI, R. & DOGLIOTTI, E. (1980b) UDS studies on selected
environmental chemicals. Mutat. Res., 74(3): 217.
BENIGNI, R., BIGNAMI, M., CAMONI, I., CARERE, A., CONTI, G.,
IACHETTA, R., MORPURGO, G., & ORTALI, V.A. (1979) A new in
vitro method for testing plant metabolism in mutagenicity studies. J.
Toxicol. environ. Health, 5: 809-819.
BERAN, F. (1970) [Present knowledge on the toxicity of our pesticides
to bees.] Gesunde Pflanz., 22(2): 21-31 (in German).
BIGNAMI, M., CONTI, L., MORPURGO, G., & VELCICH, A. (1976)
Comparative analysis of different test systems for somatic
recombination with Aspergillus nidulans. Mutat. Res., 38(2): 138-139.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of pesticides
in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BISBY, J.A. & SIMPSON, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Aust., 2: 394-395.
BLAIR, D. & RODERICK, H.R. (1976) An improved method for the
determination of urinary dimethyl phosphate. J. agric. food
Chem., 24(6): 1211-1223.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The distribution of
dichlorvos in the tissues of mammals after its inhalation or
intravenous administration. Toxicol. appl. Pharmacol., 31: 243-253.
BLAIR, D., DIX, K.M., HUNT, P.F., THORPE, E., STEVENSON, D.E., &
WALKER, A.I.T. (1976) Dichlorvos: a 2-year inhalation carcinogenesis
study in rats. Arch. Toxicol., 35: 281-294.
BOOTSMA, D., HEERING, H., KLEIJER, W.J., BUDKE, L., DE JONG,
L.P.A., & BERENDS, F. (1971) Effects of dichlorvos on human cells in
tissue culture. A progress report, Rijswijk, The Netherlands, Medical
Biological Laboratory TNO (Report No. MBL 1971-5).
BOUSH, G.M. & MATSUMURA, F. (1967) Insecticidal degradation by
Pseudomonas melophthora, the bacterial symbiote of the apple
maggot. J. econ. Entomol., 60: 918-920.
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol- water
partitioning coefficients (Kow) of 61 organophosphorus and carbamate
insecticides and their relationships to respective water solubility (S)
values. J. environ. Sci. Health, B18(6): 667-683.
BOYER, A.C., BROWN, L.J., SLOMKA, M.B., & HINE, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. appl. Pharmacol., 41: 389-
394.
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison of
cholinesterase activity, residue levels, and urinary metabolite
excretion of rats exposed to organophosphorus pesticides. J. agric.
food Chem., 25Z(6): 1353-1358.
BRAUN, R., SCHONEICH, J., WEISSFLOG, L., & DEDEK, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair: direct alkylation vs metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
desmethyldichlorvos, and demethylvinyl-butonate. Chem.-biol.
Interact., 39: 339-350.
BRIDGES, B.A., MOTTERSHEAD, R.P., GREEN, M.H.L., & GRAY, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulfonate
for Escherichia coli WP2 and some derivatives deficient in DNA
repair. Mutat. Res., 19: 295-303.
BROWN, V.K.H. & ROBERTS, M. (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to guinea-
pigs, Sittingbourne, Shell Research Ltd (Unpublished Report No. IRR
TL/40/66).
BROWN, V.K.H. & STEVENSON, D.E. (1962) Agricultural chemicals:
therapy of DDVP intoxication by atropine and cholinesterase
reactivators, Sittingbourne, Shell Research Ltd (Shell Technical
Memorandum No. TOX 31/62) (Unpublished Report).
BROWN, V.K.H., BLAIR, D., HOLMES, D.L., & PICKERING, R.G. (1968)
The toxicity of low concentrations of dichlorvos by inhalation in
rodent and avian species, Sittingbourne, Shell Research Ltd
(Unpublished Report No. TLGR.0015.68).
BRUNGS, W.A., MCCORMICK, J.H., NEIHEISEL, T.W., SPEHAR, R.L.,
STEPHAN, C.E., & STOKES, G.N. (1977) Effects of pollution on fresh-
water fish. J. Water Pollut. Control Fed., 49(6): 1425-1493.
BRYANT, R.J. & MINETT, W. (1978) A gas chromatographic method for the
determination of dichlorvos in air. Pestic. Sci., 9: 525-528.
BRZEZINSKI, J. & WYSOCKA-PARUSZEWSKA, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., 4(Suppl.): 475-478.
BULL, D.L. & RIDGWAY, R.L. (1969) Metabolism of trichlorfon in
animals and plants. J. agric. food Chem., 17(4): 837-841.
BUNDING, I.M., YOUNG, R., Jr, SCHOOLEY, M.A., & COLLINS, J.A.
(1972) Maternal dichlorvos effects on farrowing parameters. J. anim.
Sci., 35: 238.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1972) [Mutagenicity
investigations with pesticides in the host-mediated assay and the
dominant lethal test in mice.] Biol. Zentralbl., 91(3): 311-325 (in
German).
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973) Comparative
investigations on the mutagenicity of pesticides in mammalian test
systems. Mutat. Res., 21(1): 25-26.
BUTLER, G.L. (1977) Algae and pesticides. Residue Rev., 66: 40.
CARERE, A., CARDAMONE, G., ORTALI, V., BRUZZONE, M.L., & DI
GIUSEPPE, G. (1976) Mutational studies with some pesticides
in Streptomyces coelicolor and Salmonella typhimurium. Mutat.
Res., 38(2): 136.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978a) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARERE, A., ORTALI, V.A., CARDAMONE, G., & MORPURGO, G.
(1978b) Mutagenicity of dichlorvos and other structurally-related
pesticides in Salmonella and Streptomyces. Chem.-biol. Interact., 22:
297-308.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett., 9:
157-159.
CARSON, S. (1969) Teratology studies in rabbits (summary
appraisal), Maspeth, New York, Food and Drug Research Laboratories
(Unpublished Report, 30 June).
CARTER, M.K. & MADDUX, B. (1968) Effects of selected agents on the
in vitro inhibition of cholinesterase activity of dichlorvos.
Pharmacologist, 10: 222.
CARTER, M.K. & MADDUX, B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. appl. Pharmacol., 27: 456-463.
CASIDA, J.E., MCBRIDE, L., & NIEDERMEIER, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. agric. food Chem., 10(5): 370-377.
CAVAGNA, G. & VIGLIANI, E.C. (1970) Problčmes d'hygične et de
sécurité dans l'emploi du vapona comme insecticide dans les locaux
domestiques. Med. Lav., 61(8/9): 409-423.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch. environ.
Health, 19(1): 112-113.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1970) Exposure of new-
born babies to Vapona insecticide. Eur. J. Toxicol., 3(1): 49-57.
CERVONI, W.A., OLIVER-GONZALEZ, J., KAYE, S., & SLOMKA, M.B.
(1969) Dichlorvos as a single-dose intestinal anthelmintic therapy for
man. Am. J. trop. Med. Hyg., 18(6): 912-919.
CHATTOPADHYAY, D.P., DIGHE, S.K., DUBE, D.K., & PURNANAND
(1982) Changes in toxicity of DDVP, DFP, and parathion in rats under
cold environment. Bull. environ. Contam. Toxicol., 29: 605-610.
CHEN, Z.M., ZABIK, M.J., & LEAVITT, R.A. (1984) Comparative study of
thin film photodegradative rates for 36 pesticides. Ind. eng. chem.
Prod. Res. Dev., 23: 5-11.
CIPAC HANDBOOK (1980) Analysis of technical and formulated
pesticides, Cambridge, Wheffer and Sons, Collaborative International
Pesticides Analytical Council, Vol. 1A, pp. 1214-1224 (Addendum to
CIPAC 1).
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN, R.J.
(1980) Effects of low level administration of dichlorvos on adreno-
corticotrophic hormone secretion, adrenal cholesteryl ester, and
steroid metabolism. Biochem. Pharmacol., 29: 635-641.
CLINCH, P.G. (1970) Effect on honey bees of combs exposed to vapour
from dichlorvos slow-release strips. N.Z. J. agric. Res., 13(2): 448-
452.
COCHRANE, W.P. (1979) Application of chemical derivatisation
techniques for pesticide analysis. J. chromatogr. Sci., 17: 124-137.
CODEX ALIMENTARIUS COMMISSION (1979) Recommended method of
sampling for the determination of pesticide residues. Report of the
Tenth Session of the Codex Committee on Pesticide Residues, Rome, Food
and Agriculture Organization of the United Nations, Appendix IV, Annex
I, pp. 67-70 (Alinorm 79/24).
CODEX ALIMENTARIUS COMMISSION (1983) Guidelines on good
analytical practice in residue analysis and recommendations for methods
of analysis for pesticide residues, Rome, Food and Agriculture
Organization of the United Nations, p. 40.
COHEN, S.D. & EHRICH, M. (1976) Cholinesterase and carboxyl-esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. appl. Pharmacol., 37: 39-48.
COLLINS, J.A., SCHOOLEY, M.A., & SINGH, V.K. (1971) The effect of
dietary dichlorvos on swine reproduction and viability of their
offspring. Toxicol. appl. Pharmacol., 19: 377.
COLLINS, R.D. & DE VRIES, D.M. (1973) Air concentrations and food
residues from use of Shell No-PestR Insecticide Strip. Bull.
environ. Contam. Toxicol., 9(4): 227-233.
COSTA, L.G. & MURPHY, S.D. (1984) Interaction between acetaminophen
and organophosphate in mice. Chem. Pathol. Pharmacol., 44(3): 389-400.
COULSTON, F. & GRIFFIN, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP
vapours, Albany, New York, Institute of Comparative and Human
Toxicology and Holloman Air Force Base, International Center of
Environmental Safety (Unpublished report).
CRONCE, P.C. & ALDEN, H.S. (1968) Flea-collar dermatitis. J. Am.
Med. Assoc., 206(7): 1563-1564.
DALE, W.E., MILES, J.W., & WEATHERS, D.B. (1973) Measurement of
residues of dichlorvos absorbed by food exposed during disinsection of
aircraft. J. agric. food Chem., 21(5): 858-860.
DAMBSKA, M. & MASLINSKA, D. (1982) Effect of dichlorvos (DDVP)
intoxication of rabbit brain. Neuropathol. Pol. 20(1/2): 77-84.
DAMBSKA, M., MASLINSKA, D., RAKOWSKA, I., & RUTCZYNSKI, M.
(1978) [Evaluation of the effect of dichlorvos poisoning of pregnant
rabbits and rats on the condition and development of their
offspring.] Bromatol. Chem. Toksykol., 11(3): 355-357 (in Polish).
DAMBSKA, M., IWANOWSKI, L., & KOZLOWSKI, P. (1979) The effect of
transplacental intoxication with dichlorvos on the development of
cerebral cortex in newborn rabbits. Neuropathol. Pol. 17(4): 571-576.
DAMBSKA, M., IWANOWSKI, L., MASLINSKA, D., & OSTENDA, M. (1984)
Blood-brain barrier in young rabbit brain after dichlorvos
intoxication. Neuropathol. Pol. 22(1): 129-137.
DARROW, D.I. (1973) Biting lice of goats: control with dichlorvos-
impregnated resin neck collars. J. econ. Entomol., 66: 133-135.
DAS, Y.T., TASKAR, P.K., BROWN, H.D., & CHATTOPADHYAY, S.K.
(1983) Exposure of professional pest control operator to dichlorvos
(DDVP) and residue on house structures. Toxicol. Lett., 17: 95-99.
DAVIDEK, J., SEIFERT, J., & DOLEZALOVA, Z. (1976) The determination
of dichlorvos in milk by using classical and square-wave
polarography. Milchwissenschaft, 31(5): 267-270.
DEAN, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on microorganisms. Arch. Toxikol., 30: 67-74.
DEAN, B.J. (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol., 30: 75-85.
DEAN, B.J. & BLAIR, D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres containing
dichlorvos. Mutat. Res., 40(1): 67-72.
DEAN, B.J. & THORPE, E. (1972a) Cytogenic studies with dichlorvos in
mice and Chinese hamsters. Arch. Toxikol., 30: 39-49.
DEAN, B.J. & THORPE, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxikol., 30: 51-59.
DEAN, B.J., DOAK, S.M.A., & FUNNELL, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium
using Saccharomyces cerevisiae. Arch. Toxikol., 30: 61-66.
DEDEK, W., GEORGI, W., & GRAHL, R. (1979) Comparative degradation and
metabolism of 32P-labelled butonate, trichlorphone and dichlorvos in
crop plants. Biochem. Physiol. Pflanz., 174: 707-722.
DEGRAEVE, N., GILOT-DELHALLE, J., MOUTSCHEN, J.,
MOUTSCHENDAHMEN, M., COLIZZI, A., CHOLLET, M., &
HOUBRECHTS, N. (1980) Comparison of the mutagenic activity of
organophosphorus insecticides in mouse and in the
yeast Schizosaccharomyces pombe. Mutat. Res., 74(3): 201-202.
DEGRAEVE, N., CHOLLET, M.C., MOUTSCHEN, J., MOUTSCHEN-
DAHMEN, M., GILOT-DELHALLE, J., & COLIZZI, A. (1982) Genetic and
cytogenetic effects of chronic treatment with organophosphorus
insecticides. Mutat. Res., 97: 179-180.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984a) Cytogenetic
and genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56: 66-67.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Lett., 21: 315-319.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I., VARGA, L., & FARKAS, J. (1978) Studies on the
immunosuppressive effect of organochlorine and organophosphoric
pesticides in subacute experiments. J. Hyg. Epidemiol. Microbiol.
Immunol., 22: 115-122.
DESI, I., VARGA, L., & FARKAS, J. (1980) The effect of DDVP, an
organophosphorus pesticide, on the humoral and cell-mediated immunity
of rabbits. Arch. Toxicol., 4(Suppl.): 171-174.
DICOWSKY, L. & MORELLO, A. (1971) Glutathione-dependent degradation
of 2,2-dichlorovinyl dimethyl phosphate (DDVP) by the rat. Life
Sci., 10(2): 1031-1037.
DIKSHITH, T.S.S., DATTA, K.K., & CHANDRA, P. (1976) 90-day dermal
toxicity of DDVP in male rats. Bull. environ. Contam. Toxicol., 15(5):
574-580.
DOUGHERTY, E.M., REICHELDERFER, C.F., & FAUST, R.M. (1971)
Sensitivity of Bacillus thuringiensis var. thuringiensis to various
insecticides and herbicides. J. invertebr. Pathol., 17: 292-293.
DMITRIEV, A.I. & KOZEMJAKJN, N.G. (1975) [Veterinary expert report on
the meat of chicken poisoned by dichlorvos.] Veterinarija, 1975(1):
90-92 (in Russian).
DRAGER, G. (1968) [Gas chromatographic method for the determination
of dichlorvos residues in plants and milk.] Pflanzenschutz Nachr.
Bayer, 21(3): 377-384 (in German).
DRAUGHON, F.A. & AYRES, J.C. (1978) Effect of selected pesticides on
growth and citrinin production by Penicillium citrinum. J. food
Sci., 43: 576-578.
DURHAM, W.F., GAINES, TH.B., & HAYES, W.J. (1956) Paralytic and
related effects of certain organic phosphorus compounds. Arch. ind.
Health, 13: 326-330.
DURHAM, W.F., GAINES, TH.B., MCCAULEY, R.H., Jr, SEDLAK, V.A.,
MATTSON, A.M., & HAYES, W.J., Jr (1957) Studies on the toxicity
of O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP). Am. Med. Assoc.
Arch. Ind. Health, 15: 340-349.
DYER, K.F. & HANNA, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res., 21: 175-177.
DZWONKOWSKA, A. & HUBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo. Arch.
Toxicol., 58: 152-156.
EDSON, E.F. (1958) Blood tests for users of organophosphorus
insecticides. World Crops, 10 February: 49-51.
EGYED, M.N. & BENDHEIM, U. (1977) Mass poisoning in chickens by
consumption of organophosphorus (dichlorvos) contaminated drinking-
water. Refu. Vet., 34(3): 107-111.
EICHNER, M. (1978) [Quick residue control of plant and animal food,
tobacco, and tobacco products by Sweep-Co analysis 1.] Z. Lebensm.
Unters. Forsch., 167: 245-249 (in German).
EISLER, R. (1969) Acute toxicities of insecticides to marine decapod
crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish, Washington DC, US
Department of the Interior, Fish and Wildlife Service, Bureau of Sport
Fisheries and Wildlife (Technical Paper No. 46).
ELGAR, K.E. & STEER, B.D. (1972) Dichlorvos concentrations in the air
of houses arising from the use of dichlorvos PVC strips. Pestic.
Sci., 3: 591-600.
ELGAR, K.E., MARLOW, R.G., & MATHEWS, B.L. (1970) The determination
of residues of dichlorvos in crops and tissues. Analyst, 95: 875-878.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972a) Dichlorvos residues
in food arising from the domestic use of dichlorvos PVC strips. Pestic.
Sci., 3: 601-607.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972b) Vapona strips in
shops: residues in foodstuffs. Global aspects of chemistry, toxicology,
and technology as applied to the environment. Environ. Qual. Saf., 1:
217-221.
ELLINGER, CH. (1985) Enzyme activities after in vitro and in
vivo application of dichlorvos. Biomed. Biochim. Acta, 44(2): 311-
316.
ELLINGER, CH., KAISER, G., & HÖRING, H. (1985) [Haematological
changes to rats following exposure to dichlorvos.] Nahrung, 29(4): 351-
355 (in German).
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
ENOMOTO, M.F., NAKADATE, M., NINOMIYA, K., HAYAKAWA, Y.,
ITO, H., IGARASHI, S., UWANUMA, Y., NAKASATO, R., & HATANAKA,
J. (1981) Studies on carcinogenicity of DDVP (2,2-dichlorovinyl
dimethyl phosphate) mixed in drinking-water in rats, Tokyo, Ministry of
Health and Welfare (Cooperative Studies on Carcinogenicity Test on
Mutagens) (unpublished).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERIKSSON, H. & FAYERSSON, Y. (1980) A reliable way of estimating
cholinesterases from whole blood in the presence of
anticholinesterases. Clin. Chim. Acta, 100: 165-171.
ESCUDIE, E. & SALES, P. (1963) Premičres expériences en Haute Volta
sur le dichlorvos résiduel. Bull. World Health Organ., 29: 247-249.
FAHRIG, R. (1973) [Genetic effects of organophosphorus
insecticides.] Naturwissenschaften, 60(1): 50-51 (in German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomotis, L., ed. Chemical carcinogenesis essays,
Lyons, International Agency for Research on Cancer, pp. 161-181 (IARC
Scientific Publication No. 10).
FAO (1977) FAO Specifications for plant protection products,
dichlorvos and fenthion, Rome, Food and Agriculture Organization of the
United Nations.
FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10/1; WHO Food
Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Report of the Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL/1962/M/11; WHO Technical Report Series
No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1967/M11/1; WHO Food
Add./68.30).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1969 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (AGP 1974/M/11; WHO Pesticide Residue
Series No. 4).
FAO/WHO (1977a) Pesticide residues in food. Report of the 1976 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and the
Environment and the WHO Expert Group on Pesticide Residues, Geneva,
World Health Organization (FAO Food and Nutrition Series No. 9; FAO
Plant Production and Protection Series No. 8; WHO Technical Report
Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(ADP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977 Joint
Meeting on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 10
Rev.).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FISHBEIN, L. (1976) Potential hazards of fumigant residues.
Environ. Health Perspect., 14: 39-45.
FISHBEIN, L. (1981) Environmental sources of chemical mutagens. II.
Synthetic mutagens. In: Flamm, W.G. & Mehlman, M.A., ed. Mutagenesis:
advances in modern toxicology, Tokyo, Mishima and Kyoto, Vol. 5, pp.
257-348.
FISHBEIN, L. (1982) An overview of the structural features of
mutagenic S-chlorallylthio and dithiocarbamate pesticides and
trichlorfon, dichlorvos, and their metabolites. In: Proceedings of the
3rd International Conference on Environmental Mutagens and Carcinogens,
21-27 September 1981, Tokyo, Mishima and Kyoto, pp. 371-378.
FISCHER, G.W., SCHNEIDER, P., & SCHEUFLER, H. (1977) [Mutagenicity
of dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester: possible metabolites of the organophosphorus
pesticide trichlorphon.] Chem.-biol. Interact., 19: 205-213 (in
German).
FOLL, C.V., PANT, C.P., & LIETAERT, P.E. (1965) A large-scale field
trial with dichlorvos as a residual fumigant insecticide in northern
Nigeria. Bull. World Health Organ., 32: 531-550.
FOURNIER, E., DALLY, S., & CAMBIER, J. (1976) Neuropathie
périphérique probablement due aux insecticides anticholinesté-
rasiques. Nouv. Presse méd., 5(11): 718.
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Sci. Health, B20(1): 73-95.
FRANK, R., BRAUN, H.E., & FLEMING, G. (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969-81. J. food Prot., 46: 893-900.
FUJITA, K., MATSUSHIMA, S., ABE, E., SASAKI, K., & KUROSAWA, K.
(1977) [Examination of the effects of dichlorvos on the
testis.] Nippon Noson Igakkai Zasshi, 26(3): 328-329 (in Japanese).
FUJITA, Y. (1985) Studies on contact dermatitis from pesticides in
tea growers. Acta Med. Univ. Kagoshima, 27(1): 17-37.
FUNCKES, A.J., MILLER, S., & HAYES, W.J., Jr (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 243-246.
GAINES, TH.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14(3): 515-534.
GAINES, TH.B., HAYES, W.J., Jr, & LINDER, R.E. (1966) Liver
metabolism of anticholinesterase compounds in live rats: relation to
toxicity. Nature (Lond.), 209: 88-89.
GIFAP (1982) Guidelines on pesticide residues to provide data for the
registration of pesticides and the establishment of maximum residue
limits elaborated by Codex Committee on Pesticide Residues, Brussels,
International Group of National Associations of Manufacturers of
Agrochemical Products (Technical Monograph No. 4).
GILLENWATER, H.B., HAREIN, P.K., LOY, E.W., Jr, THOMPSON, J.F.,
LAUDANI, H., & EASON, G. (1971) Dichlorvos applied as a vapour in a
warehouse containing packaged foods. J. stored Prod. Res., 7: 45-56.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health hazards
on use of dichlorvos (DDVP), especially in resin strips. Residue
Rev., 44: 115-159.
GILOT-DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN-
DAHMEN, M. (1983) Mutagenicity of some organophosphorus compounds at
the ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117: 139-
148.
GOH, K.S., EDMISTON, S., MADDY, K.T., & MARGETICH, S. (1986a)
Dissipation of dislodgeable foliar residue for chlorpyrifos and
dichlorvos treated lawn: implication for safe reentry. Bull. environ.
Contam. Toxicol., 37: 33-40
GOH, K.S., EDMISTON, S., MADDY, K.T., MEINDERS, D.D., &
MARGETICH, S. (1986b) Dissipation of dislodgeable foliar residue of
chlorpyrifos and dichlorvos on turf. Bull. environ. Contam.
Toxicol., 37: 27-32.
GOLD, R.E., HOLCSLAW, T., TUPY, D., & BALLARD, J.B. (1984) Dermal
and respiratory exposure to applicators and occupants of residences
treated with dichlorvos (DDVP). J. econ. Entomol., 77: 430-436.
GOTO, S. (1977) [Variation of concentrations of pesticide residues in
field soil.] J. Pestic. Sci., 2: 319-321. (in Japanese, with English
summary).
GOUGH, B.J. & SHELLENBERGER, T.E. (1970) In vivo rabbit blood
cholinesterase inhibition and reactivation following organophosphate
infusion. Toxicol. appl. Pharmacol., 17(1): 302.
GOUGH, B.J. & SHELLENBERGER, T.E. (1977-78) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors and
reactivation with oximes. Drug Chem. Toxicol., 1(1): 25-43.
GRAHL, K. (1979) [The persistence of trichlorfon and dichlorvos in
ponds.] Z. Binnenfisch. DDR, 25(10): 312-316 (in German).
GRAHL, K., STELZER, W., & STOTTMEISTER, S. (1980) [Toxicity of
butonate, trichlorfon, and dichlorvos to Escherichia coli and
Enterobacter aerogenes.] Acta hydrochim. hydrobiol., 8: 19-28 (in
German).
GRAHL, K., HORN, H., & HALLEBACH, R. (1981) [Effect of butonate,
trichlorfon, and dichlorvos on plankton populations.] Acta hydrochim.
hydrobiol., 9(2): 147-161 (in German).
GRATZ, N.G., BRACHA, P., & CARMICHAEL, A. (1963) A village- scale
trial with dichlorvos as a residual fumigant insecticide in southern
Nigeria. Bull. World Health Organ., 29: 251-270.
GREEN, M.H.L., BRIDGES, B.A., GRAY, W.J.H., & MOTTERSHEAD, R.P.
(1973) Comparison of DNA damage caused by dichlorvos and methyl
methanesulfonate in pol A and pol A+ strains of Escherichia coli.
Genetics, 74: s100.
GREEN, M.H.L., MEDCALF, A.S.C., ARLETT, C.R., HARCOURT, S.A., &
LEHMANN, A.R. (1974a) DNA strand breakage caused by dichlorvos, methyl
methane-sulfonate and iodoacetamide in Escherichia coli and cultured
Chinese hamster cells. Mutat. Res., 24(3): 365-378.
GREEN, M.H.L., MEDCALF, A.S.C., & STEVENS, S.W. (1974b) Apparent
indirect DNA damage by dichlorvos and iodoacetamide in Escherichia
coli. Heredity, 33: 446.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens. Mutat.
Res., 38(1): 33-42.
GRIFFIN, D.E., III & HILL, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRUBNER, P. (1972) [Residue problems in the use of phosphoric acid
ester insecticides in mushroom cultures.] Nachrichtenbl.
Pflanzenschutzdienst (DDR), 26: 245-247 (in German).
GUNDERSON, E.C. (1981) Sampling methods for airborne pesticides,
Washington, DC, American Chemical Society, pp. 301-315 (ACS Symposium
Series No. 149).
GUPTA, A.K. & SINGH, J. (1974) Dichlorvos (DDVP) induced breaks in the
salivary gland chromosomes of Drosophila melanogaster. Curr.
Sci., 43(20): 661-662.
GUPTA, R.C., DAVE, S.K., SHAH, M.P., & KASHYAP, S.K. (1979) A
monitoring study of workers handling pesticides in warehouses and
godowns. J. environ. Sci. Health, B14(4): 405-416.
HALEY, T.J., FARMER, J.H., HARMON, J.R., & DOOLEY, K.L. (1975)
Estimation of the LD1 and extrapolation of the LD0.1 for five
organophosphate pesticides. Arch. Toxicol., 34: 103-109.
HANNA, P.J. & DYER, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res., 28: 405-420.
HASAN, M. & ALI, S.F. (1980) Organophosphate pesticide dichlorvos-
induced increase in the rate of lipid peroxidation in the different
regions of the rat brain: supporting ultra-structural
findings. Neurotoxicology, 2: 43-52.
HASAN, M., MAITRA, S.C., & ALI, S.F. (1979) Organophosphate pesticide
DDVP-induced alterations in the rat cerebellum and spinal cord: an
electron microscopic study. Exp. Pathol., 17(2): 88-94.
HASS, D.K., COLLINS, J.A., & KODAMA, J.K. (1972) Effects of orally
administered dichlorvos in rhesus monkeys. J. Am. Vet. Med.
Assoc., 161(6): 714-719.
HATCH, G.G., ANDERSON, T.M., HUBERT, R.A., KOURI, R.E.,
PUTMAN, D.L., CAMERON, J.W., NIMS, R.W., MOST, B., SPALDING, J.W.,
TENNANT, R.W., & SCHECHTMAN, L.M. (1986) Chemical enhancement of
sa7 virus transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagenesis.,
8: 515-531.
HATTORI, K., SATO, H., TSUCHIYA, K., YAMAMOTO, N., & OGAWA, E.
(1974) [Toxicological studies on the influences of chemicals to the
birds. I. Oral acute toxicity and cholinesterase inhibition of three
organophosphate insecticides in Japanese quail.] Hokkaidoritsu Eisei
Kenkyusho Ho, 24: 35-38 (in Japanese, with English summary).
HAYES, A.L., WISE, R.A., & WEIR, F.W. (1980) Assessment of
occupational exposure to organophosphates in pest control
operators. Am. Ind. Hyg. Assoc. J., 41(8): 568-575.
HAYES, W.J., Jr (1961) Safety of DDVP for the disinsection of
aircraft. Bull. World Health Organ., 24: 629-633.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons:
emergency information for treating poisoning, Atlanta, Georgia, US
Department of Health, Education and Welfare, Public Health Service,
Communicable Diseases Center (Public Health Service Publication No.
476).
HAYES, W.J., Jr (1982) Organic phosphorus pesticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and Wilkins,
pp. 343-351.
HAZELWOOD, J.C., STEFAN, G.E., & BOWEN, J.M. (1979) Motor unit
irritability in beagles before and after exposure to cholinesterase
inhibitors. Am. J. vet. Res., 40(6): 852-856.
HEMMINKI, K. (1983) Nucleic acid adducts of chemical carcinogens and
mutagens. Arch. Toxicol., 52: 249-285.
HENRIKSSON, K., KALLELA, K., VIRTAMO, M., & PFAFFLI, P. (1971)
[The toxicity of DDVP (dichlorvos) evaporated from Vapona strip.] J.
Sci. Agric. Soc. Finl., 43: 187-200 (in Finnish).
HEUSER, S.G. & SCUDAMORE, K.A. (1966) A rapid method for sampling
dichlorvos vapour in air. Chem. Ind., 50: 2093-2094.
HEUSER, S.G. & SCUDAMORE, K.A. (1975) A method for the assessment
under standard conditions of the output of dichlorvos slow-release
units used for insect control. Analyst, 100: 129-135.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp. 1-51
(Special Scientific Report: Wildlife No. 191).
HINE, C.H. (1962) 90-day chronic toxicity studies of
VaponaR insecticide for dogs, San Francisco, California, The Hine
Laboratories (Unpublished Report No. 1, 1 September).
HINE, C.H. & SLOMKA, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
HINE, C.H. & SLOMKA, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. appl. Pharmacol., 17(1):
304-305.
HODGSON, E. & CASIDA, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethylphosphate. J. agric. food
Chem., 10(3): 208-214.
HOLMSTEDT, B., NORDGREN, I., SANDOZ, M., & SUNDWALL, A. (1978)
Metrifonate. Summary of toxicological and pharmacological information
available. Arch. Toxicol., 41: 3-29.
HORIUCHI, N. & ANDO, Y. (1978) [Photosensitivity caused by
pesticides.] (original unpublished information used as a basis for
article by Horiuchi et al., 1978) (in Japanese).
HORIUCHI, N., ANDO, S., & SUZUKI, A. (1978) [Photosensitization due
to pesticides.] Nippon Noson Igakkai Zashi, 27(3): 450-451 (in
Japanese).
HORVATH, J., HEGATH, V., & PUCHA, K. (1968) A study of the influence
of cholinesterase with cattle housed in byres provided with DDVP
strips. Agrochemia, 8(9): 267-268.
HUNTER, C.G. (1969) Report on initial studies of deliberate exposures
to high concentrations of dichlorvos by human subjects, Sittingbourne,
Tunstall Laboratory Shell (Unpublished Report, July).
HUNTER, C.G. (1970a) Dichlorvos: inhalational exposures with human
subjects. Part 1, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0061.70).
HUNTER, C.G. (1970b) Dichlorvos: inhalational exposures with human
subjects. Part 2, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0067.70).
HUSSEY, N.W. & HUGHES, J.T. (1964) Investigations on the use of
dichlorvos in the control of the mushroom phorid Megaselia
halterata (Wood). Ann. appl. Biol., 54: 129-139.
HUTSON, D.H. & HOADLEY, E.C. (1972a) The comparative metabolism of
14C-vinyl-dichlorvos in animals and man. Arch. Toxikol., 30: 9-18.
HUTSON, D.H. & HOADLEY, E.C. (1972b) The metabolism of 14C-methyl-
dichlorvos in the rat and mouse. Xenobiotica, 2(2): 107-116.
HUTSON, D.H., BLAIR, D., HOADLEY, E.C., & PICKERING, B.A. (1971a)
The metabolism of 14C-VaponaR in rats after administration by oral
and inhalation routes. Toxicol. appl. Pharmacol., 19: 378-379.
HUTSON, D.H., HOADLEY, E.C., & PICKERING, B.A. (1971b) The
metabolic fate of vinyl-1-14C-dichlorvos in the rat after oral and
inhalation exposure. Xenobiotica, 1(6): 593-611.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K. (1978)
EEG, ECG, and respiratory response to acute insecticide exposure. Bull.
environ. Contam. Toxicol., 19: 47-55.
IARC (1979) Some halogenated hydrocarbons, Lyons, International Agency
for Research on Cancer, pp. 97-127 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IRPTC (1984) DDVP, Geneva, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme (Scientific
Reviews of Soviet Literature on Toxicity and Hazards of Chemicals, No.
79).
ISSHIKI, K., MIYATA, K., MATSUI, S., TSUTSUMI, M., & WATANABE, T.
(1983) [Effects of post-harvest fungicides and piperonyl butoxide on
the acute toxicity of pesticides in mice.] Shokuhin Eiseigaku
Zasshi, 24(3): 268-274 (in Japanese, with English summary).
IVEY, M.C. & CLABORN, H.V. (1969) GLC determination of dichlorvos in
milk, eggs and various body tissues of cattle and chickens. J. Assoc.
Off. Anal. Chem., 52(6): 1248-1251.
IWATA, Y., SUGITANI, A., & YAMADA, F. (1981) [An analytical method
for organophosphorus pesticides in the onion.] Shokuhin Eiseigaku
Zasshi, 22: 484-489 (in Japanese, with English summary).
JAKUBOWSKA, B. & NOWAK, A. (1973) [The effect of organophosphorus and
carbamate insecticides on the development of soil fungi.] Zesz. Nauk.
Akad. Roln. Szczecinie, 39(10): 141-150 (in Polish).
JAQUES, R. (1964) The protective effect of pyridine-2-aldoxime
methiodide (P2AM), bis-(4-hydroxyimino-methyl-pyridinium-1-methyl)-
ether dichloride (ToxogoninR) and atropine in experimental DDVP
poisoning. Helv. Physiol. Pharmacol. Acta, 22(3): 174-183.
JAYASURIYA, V.U., DE S. & RATNAYAKE, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inf. Serv., 50: 184-186.
JENSEN, J.A., FLURY, V.P., & SCHOOF, H.F. (1965) Dichlorvos vapour
disinsection of aircraft. Bull. World Health Organ., 32: 175-179.
JOHNSON, M.K. (1969) The delayed neurotoxic effect of some
organophosphorus compounds. Identification of the phosphorylation site
as an esterase. Biochem. J., 114(4): 711-717.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanisms and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing delayed
neurotoxic effects. Mechanism of action and structure/ activity
studies. Arch. Toxicol., 34: 259-288.
JOHNSON, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch.
Toxicol., 41: 107-110.
JOHNSON, M.K. (1981) Delayed neurotoxicity: do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol., 49(Suppl. 5): 87-98.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981a) Pesticide
metal and other chemical residues in adult total diet samples.
XII. Pestic. monit. J., 15(1): 54-69.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981b) Pesticide, heavy metal, and other chemical residues in infant
and toddler total diet samples. II. August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-50.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 137).
JOLLEY, W.P., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of VaponaR insecticide into their daily diet, Cincinnati,
Ohio, The Kettering Laboratory (Unpublished Report, 19 January).
KAWACHI, T., KOMATSU, T., KADA, T., ISHIDATE, M., SASAKI, M.,
SUGIYAMA, T., & TAZIMA, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In: G.M.
Williams et al., ed. The predictive value of short-term screening tests
in carcinogenicity evaluation, Amsterdam, Oxford, New York,
Elsevier/North Holland Biomedical Press, pp. 253-267.
KENAGA, E.E. (1979) Acute and chronic toxicity of 75 pesticide to
various animal species. Down Earth, 35(2): 25-31
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. environ.
Health, 16: 805-808.
KIMMERLE, G. & LORKE, D. (1968) [Toxicity of insecticidal phosphoric
acid esters.] Pflanzenschutz Nachr. Bayer, 21(1): 111-142 (in German).
KIRKLAND, V.L. (1971) Some aspects of acute inhalation pharmacology
of dichlorvos in swine. Paper presented at: American Chemical Society
for Pharmacology and Experimental Therapeutics and the Division of
Medical Chemistry, Burlington, Vermont, 25 August, 1971.
KLIGERMAN, A.D., EREXSON, G.L., & WILMER, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res., 157: 181-187.
KNAPP, F.W. & GRADEN, A.P. (1964) Accidental exposure of dairy cows
to excessive amount of dichlorvos. J. econ. Entomol., 57: 790-791.
KOBAYASHI, H., YUYAMA, A., IMAJO, S., & MATSUSAKA, N. (1980)
Effects of acute and chronic administration of DDVP (dichlorvos) on
distribution of brain acetylcholine in rats. J. toxicol. Sci., 5: 311-
319.
KOBAYASHI, H., YUYAMA, A., & CHIBA, K.I. (1986) Cholinergic system
of brain tissue in rats poisoned with the organophosphate
0,0-demethyl 0-(2,2-dichlorovinyl) phosphate. Toxicol. appl.
Pharmacol., 82: 32-39.
KODAMA, J.K. (1960) Technical DDVP. Acute oral administration:
dogs, Vienna, Virginia, Hazleton Laboratories (Unpublished Report, 20
January).
KODAMA, J.K. (1968) Experimental evaluation in guinea-pigs of skin-
sensitizing potential of components of formulated dichlorvos/
polyvinylchloride products, Modesto, California, Shell Development
Company (Unpublished Technical Progress Report No. M-67-68).
KONISHI, Y., DENDA, A., & KITAOKA, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3FI mice, Tokyo, Ministry of Health
and Welfare (Cooperative Studies on Carcinogenicity Tests on Mutagens)
(unpublished).
KRAMERS, P.G.N. & KNAAP, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of D rosophila melanogaster.
Mutat. Res., 57: 103-105.
KRAUSE, CHR., VON & KIRCHHOFF, J. (1970) [Gas chromatographic
determination of organophosphate residues in market samples of fruits
and vegetables.] Dtsch. Lebensm. Rundschau., 66(6): 194-199 (in
German).
KRAUSE, W. (1977) Influence of DDT, DDVP, and malathion on FSH, LH,
and testosterone serum levels and testosterone concentrations in
testis. Bull. environ. Contam. Toxicol., 18(2): 231-242.
KRAUSE, W. & HOMOLA, S. (1972) [Influence on spermatogenesis by DDVP
(dichlorvos).] Arch. Dermatol. Forsch., 244: 439-441 (in German).
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat* testis after the
application of dichlorvos (DDVP). Bull. environ. Contam.
Toxicol., 11(5): 429-433 (*study was performed on mice).
KRAUSE, W., HAMM, K., & WEISSMULLER, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. environ. Contam. Toxicol., 15(4): 458-462.
KUWABARA, K., NAKAMURA, A., & KASHIMOTO, T. (1980) Effect of
petroleum oil, pesticides, PCBs, and other environmental contaminants
on the hatchability of Artemia salina dry eggs. Bull. environ.
Contam. Toxicol., 25: 69-74.
LAFONTAINE, A., AERTS, J., & JACQUES, P. (1981) [Toxicity,
carcinogenic, mutagenic, and teratogenic action of dichlorvos.] Arch.
belg. Méd. soc. Hyg. Méd. Trav. Méd. lég., 39(3): 159-174 (in Dutch).
LAL, R. (1982) Accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms. Adv. appl.
Microbiol., 28: 149-200.
LAMOREAUX, R.J. & NEWLAND, L.W. (1978) The fate of dichlorvos in
soil. Chemosphere, 7(10): 807-814.
LAWLEY, P.D., SHAH, S.A., & ORR, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (dichlorvos,
DDVP). Chem.-biol. Interact., 8: 171-182.
LAWS, E.R., Jr (1966) Route of absorption of DDVP after oral
administration to rats. Toxicol. appl. Pharmacol., 8: 193-196.
LEARY, J.S., HIRSCH, L., LAVOR, E.M., FEICHTMEIR, E., SCHULTZ, D.,
KOOS, B., ROAN, C.R., FONTENOT, C., & HINE, C.H. (1971) An evaluation
of the safety of No-PestR strip insecticide with special reference to
respiratory and dietary exposure of occupants of homes in
Arizona. Toxicol. appl. Pharmacol., 19: 379.
LEARY, J.S., KEANE, W.T., FONTENOT, C., FEICHTMEIR, E.F.,
SCHULTZ, D., KOOS, B.A., HIRSCH, L., LAVOR, E.M., ROAN, C.C., &
HINE, C.H. (1974) Safety evaluation in the home of polyvinyl chloride
resin strips containing dichlorvos (DDVP). Arch. environ. Health, 29:
308-314.
LEONARD, A. (1976) Heritable chromosome aberrations in mammals after
exposure to chemicals. Radiat. environ. Biophys., 13: 1-8.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides. In:
Lee, R.L., ed. Air pollution from pesticides and agricultural
processes, Cleveland, Ohio, CRC Press, pp. 51-95.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides on
decomposition of organic matter nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LIEBERMAN, M.T. & ALEXANDER, M. (1983) Microbial and non-
enzymatic steps in the decomposition of dichlorvos (2,2-
dichlorovinyl O,O-dimethylphosphate). J. agric. food Chem., 31: 265-
267.
LIVINGSTON, R.J. (1977) Review of current literature concerning the
acute and chronic effects of pesticides on aquatic organisms. CRC crit.
Rev. environ. Control, 7: 325-351.
LLOYD, T.S. (1973) Accidental poisoning in birds. Vet. Rec., 5 May:
489.
LOEFFLER, J.E., DE VRIES, D.M., YOUNG, R., & PAGE, A.C. (1971)
Metabolic fate of inhaled dichlorvos in pigs. Toxicol. appl.
Pharmacol., 19: 378.
LOEFFLER, J.E., POTTER, J.C., SCORDELIS, S.L., HENDRICKSON, H.R.,
HUSTON, C.K., & PAGE, A.C. (1976) Long-term exposure of swine to a
14C-dichlorvos atmosphere. J. agric. food Chem., 24(2): 367-371.
LOFROTH, G. (1970) Alkylation of DNA by dichlorvos. Natur-
wissenschaften, 57(8): 393-394.
LOFROTH, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
Naturforsch., 33: 783-785.
LOFROTH, G. & WENNERBERG, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch., 29: 651.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating properties of
2,2-dichlorovinyl dimethyl phosphate: a disregarded hazard. Environ.
Mutat. Soc. Newslett., 2: 21-27.
LOHS, K., FISCHER, G.W., & DEDEK, W. (1976) [Chemical-toxicological
aspects of the alkylating properties of organophosphate pesticides.]
Sitzungsber. Akad. Wiss. (DDR), 11N: 1-58 (in German).
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of organophosphorus
pesticides: predictions can be based on in vitro studies with hen and
human enzymes. Arch. Toxicol., 41: 215-221.
LUDKE, J.L. & LOCKE, L.N. (1976) Duck deaths from accidental
ingestion of anthelmintic. Avian Dis., 20(3): 607-608.
LUKE, M.A., FROBERG, J.E., DOOSE, G.M., & MASUMOTO, H.T. (1981)
Improved multiresidue gas chromatographic determination of
organophosphorus organonitrogen, and organohalogen pesticides in
produce, using flame photometric and electrolytic conductivity
detectors. J. Assoc. Off. Anal. Chem., 64(5): 1187-1195.
MACDONALD, R. (1982) Toxicology of consumer products: the acute
(4-h) inhalation toxicity of dichlorvos vapour in rats and mice,
Sittingbourne, Shell Research Ltd (Unpublished Report SBGR.82.145).
MACKLIN, A.W. & RIBELIN, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc., 159(12): 1743-
1748.
MADDY, K.T., EDMISTON, S., & FREDRICKSON, A.S. (1981a) Monitoring
residue of DDVP in room air and on horizontal surfaces following use of
a room fogger, Sacramento, California, California Department of Food
and Agriculture (Unpublished Report No. HS-897).
MADDY, K.T., SCHNEIDER, F., LOWE, J., OCHI, E., FREDRICKSON, A.S.,
& MARGOTICH, S. (1981b) Vapona (DDVP) exposure potential to in
mushroom houses in Ventura County, California, in 1981, Sacramento,
California, California Department of Food and Agriculture (Unpublished
Report No. HS-861).
MAJEWSKI, T., PODGORSKI, W., BIALKOWSKI, Z., & TYCZKOWSKI, J.
(1978) [Effects of application of different forms of DDVP to
cows.] Rocz. Nauk. Zootech., 5(2): 65-74 (in Polish).
MAJEWSKI, T., PODGORSKI, W., & MICHALOWSKA, R. (1979) Retention
of dichlorvos (DDVP) in rabbits. Pol. Arch. Weter., 21(2): 249-255.
MASLINSKA, D. & ZALEWSKA, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia histochem. cytochem., 16(4): 335-341.
MASLINSKA, D., STROSZNAJDER, J., ZALEWSKA, T., & ORLEWSKI, P.
(1984) Phospholipid-protein ratio in brain of suckling rabbits treated
with an organophosphorus compound. Int. J. tissue React., 6(4): 317-
322.
MATHIAS, C.G.T. (1983) Persistent contact dermatitis from the
insecticide dichlorvos. Contact Dermatit., 9: 217-218.
MATSUMURA, F. & BOUSH, G.M. (1968) Degradation of insecticides by a
soil fungus Trichoderm viride. J. econ. Entomol., 61(3): 610-612.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
310-311.
MENDOZA, C.E. (1974) Analysis of pesticides by the thin-layer
chromatographic enzyme inhibition technique. Part II. Residue Rev., 50:
43-72.
MENDOZA, C.E. & SHIELDS, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin-layer chromatography. J. Assoc. Off.
Anal. Chem., 54: 507-512.
MENZ, M., LUETKEMEIER, H., & SACHSSE, K. (1974) Long-term exposure
of factory workers to dichlorvos (DDVP) insecticide. Arch. environ.
Health, 28: 72-76.
MESTRES, R., CHEVALLIER, CH., ESPINOZA, CL., & CORNET, R. (1977)
Application du couplage chromatographie gazeuse spectrométrie de masse
aux méthodes de recherche et de dosage des résidus de pesticides et de
micropolluants organiques dans les matériaux de l'environnement et les
matičres alimentaires. Ann. Fac. exp. Chim., 70(751): 177-188.
MESTRES, R., ILLES, S., CAMPO, M., & TOURTE, J. (1979a) Développement
de la méthode polyvalente d'analyse des résidus des pesticides dans les
plantes et les matičres alimentaires d'origine végétale. Trav. Soc.
Pharm. Montpellier, 39: 323-328.
MESTRES, R., ATMAWIJAYA, S., & CHEVALLIER, CH. (1979b) Méthode
de recherche et de dosage des résidus de pesticides dans les produits
céréaliers. I. Organochlorés, organophosphorés, pyréthrines, et
pyréthrinoďdes. Ann. Fac. exp. Chim., 72: 577-589.
MICHALEK, S.M. & BROCKMAN, H.E. (1969) A test for mutagenicity of
Shell "No-Pest Strip Insecticide" in Neurospora crassa. Neurospora
Newslett., 16: 8.
MICHEL, H.O. (1949) An electrometric method for the determination of
red blood cell and plasma cholinesterase activity. J. lab. clin.
Med., 34: 1564-1568.
MILES, J.W., FETZER, L.E., & PEARCE, G.W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ. Sci.
Technol., 4: 420-425.
MITSUI, T., OZAKI, H., KUMANO, H., & SANO, H. (1963) Insecticide
determination. I. Colormetric determination of dimethyl 2,2-
dichlorovinyl phosphate (DDVP). Chem. pharm. Bull., 11(5): 619-623.
MIYAMOTO, J. (1959) Non-enzymatic conversion of Dipterex into DDVP
and their inhibitory action on enzymes. Botyu-Kagaku (Sci. pestic.
Control), 24: 130-137.
MODAK, A.T., STAVINOHA, W.B., & WEINTRAUB, S.T. (1975) Dichlorvos
and the cholinergic system: effects on cholinesterase and acetylcholine
and choline contents of rat tissues. Arch. int. Pharmacodyn., 217:
293-301.
MOHN, G. (1973) S-methyltryptophan resistance mutations in
Escherichia coli K12. Mutagenic activity of mono-functional alkylating
agents including organophosphorus insecticides. Mutat. Res., 20: 7-
15.
MORIYA, M., KATO, K., & SHIRASU, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res., 57: 259-263.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MORPURGO, G., BELLINCAMPI, D., GUALANDI, G., BALDINELLI, L., &
SERLUPI CRESCENZI, O. (1979) Analysis of mitotic non-disjunction
with Aspergillus nidulans. Environ. Health Perspect., 31: 81-95.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., &
DEGRAEVE, N. (1981) Metrifonate and dichlorvos: cytogenetic
investigations. Acta pharmacol. toxicol., 49(Suppl. 5): 29-39.
MULLER, G.H. (1970) Flea collar dermatitis in animals. J. Am. Vet.
Med. Assoc., 157(11): 1616-1626.
NAGY, ZS., MILE, I., & ANTONI, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta microbiol. Acad. Sci.
Hung., 22: 309-314.
NAIDU, N.V., REDDY, K.S., JANARDHAN, A., & MURTHY, M.K. (1978)
Toxicological investigation of dichlorvos in chicks. Indian J.
Pharmacol., 10(14): 323-326.
NAKAMURA, K. & SHIBA, H. (1980) [Study on pesticide residues in fruit
and vegetables.] Bull. Saitama prefect. agric. exp. Stn, 36: 35-56 (in
Japanese).
NARCISSE, J.K. (1967) A potentiation study in rats of VaponaR with
26 other cholinesterase-inhibiting compounds, Menlo Park, California,
Stanford Research Institute (Unpublished Report, 2 June).
NCI (1977) Bioassay of dichlorvos for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute.
NEUWIRTH, E.A. & WHITE, T.T. (1961) Safety aspects of DDVP. Soap
chem. Spec., 37(3): 95-98.
NICHOLAS, A.H., VIENNE, M., & BERGHE, H., VAN DEN (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Lett., 2(5): 271-
275.
NIOSH (1979) NIOSH Manual of analytical methods. Analytical method
No. P and CAM 295, 2nd ed., Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. 5 (Publication No. 79-144).
NISHIO, A. & UYEKI, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphorus
insecticides and their oxygen analogs. J. Toxicol. environ. Health, 8:
939-946.
NISHIO, A. & UYEKI, E.M. (1982) Nuclease sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants. Biochem.
biophys. Res. Commun., 107(2): 485-491.
NORDGREN, I., BERGSTROM, M., HOLMSTEDT, B., & SANDOZ, M.
(1978) Transformation and action of metrifonate. Arch. Toxicol., 41:
31-41.
NOWELL, P.T., SCOTT, C.A., & WILSON, A. (1962) Hydrolysis of
neostigmine by plasma-cholinesterase. Br. J. Pharmacol., 19: 498-502.
NTP (1986) Carcinogenicity study in rats and mice with
dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (Unpublished data).
NTP (1987) NTP technical report on the toxicology and carcinogenesis
of dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (NTP-TR342).
OBA, T. & KAWABATA, G. (1962) [Application of infrared absorption
spectroscopy to examination of drugs and their preparations. XIV.
Determination of O,O-dimethyl 2,2-dichlorovinyl phosphate (DDVP) and
its preparation.] Eiseishikenjo-hokoku, 80: 1-3 (in Japanese, with
English summary).
OGATA, H., SAITO, E., MURATA, H., SHIBAZAKI, T., & INOUE, T. (1975)
[A new colorimetric method of determination of O,O-dimethyl 2,2-
dichlorovinyl phosphate in insecticidal preparations.] Yakugaku
Zasshi, 95(12): 1483-1491 (in Japanese, with English summary).
OLINSKI, R., WALTER, Z., WIADERKIEWICZ, R., LUKASOVA, E., &
PALECEK, E. (1980) Changes in DNA properties due to treatment with
the pesticides malathion and DDVP. Radiat. environ. Biophys., 18: 65-
72.
OMKAR, & SHUKLA, G.S. (1984) Alteration in carbohydrate metabolism of
fresh-water prawn Macrobianchium lamarrei after dichlorvos
exposure. Ind. Health, 22: 133-136.
PACHECKA, J., SULINSKI, A., & TRACZYKIEWICZ, K. (1977) The effect
of acute intoxication by dichlorvos and trichlorphon on the activities
of some rat brain esterases. Neuropathol. Polska, 15(1): 85-92.
PAGE, A.C., DE VRIES, D.M., YOUNG, R., & LOEFFLER, J.E. (1971)
Metabolic fate of ingested dichlorvos in swine. Toxicol. appl.
Pharmacol., 19: 378.
PAGE, A.C., LOEFFLER, J.E., HENDRICKSON, H.R., HUSTON, C.K., &
DEVRIES, D.M. (1972) Metabolic fate of dichlorvos in swine. Arch.
Toxikol., 30: 19-27.
PAIK, S.G. & LEE, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool., 20(1):
19-28.
PENA CHAVARRIA, A., SWARTZWELDER, J.C., VILLAREJOS, V.M.,
KOTCHER, E., & ARGUEDAS, J. (1969) Dichlorvos: an effective broad-
spectrum anthelmintic. Am. J. trop. Med. Hyg., 18(6): 907-911.
PEROCCO, P. & FINI, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (WHO Report
No. VBC/84.889).
PODREBARAC, D.S. (1984) Pesticide, heavy metals, and other chemical
residues in infant and toddler total diet samples. IV. October 1977 -
September 1978. J. Assoc. Off. Anal. Chem., 67(1): 166-175.
POTTER, J.C., LOEFFLER, J.E., COLLINS, R.D., YOUNG, R., & PAGE,
A.C. (1973a) Carbon-14 balance and residues of dichlorvos and its
metabolites in pigs dosed with dichlorvos-14C. J. agric. food
Chem., 21(2): 163-166.
POTTER, J.C., BOYER, A.C., MARXMILLER, R.E., YOUNG, R., &
LOEFFLER, J.E. (1973b). Radioisotope residues and residues of
dichlorvos and its metabolites in pregnant sows and their progeny dosed
with dichlorvos-14C or dichlorvos-36Cl formulated as PVC
pellets. J. agric. food Chem., 21(4): 734-738.
PURSHOTTAM, T. & KAVEESHWAR, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviat. Space
environ. Med., 50(6): 581-584.
PURSHOTTAM, T. & SRIVASTAVA, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
environ. Health, 39(6): 425-430.
PYM, R.A.E., SINGH, G., GILBERT, W.S., ARMSTRONG, J.P., &
MCCLEARY, B.V. (1984) Effects of dichlorvos, maldison, and
pirimiphos-methyl on food consumption, egg production, egg and tissue
residues, and plasma acetylcholinesterase inhibition in layer strain
hens. Aust. J. exp. Agric. anim. Husb., 24: 83-92.
QUARTERMAN, K.D., LOTTE, M., & SCHOOF, H.F. (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 231-235.
RADELEFF, R.D. & WOODARD, G.T. (1957) The toxicity of organic
phosphorus insecticides to livestock. J. Am. Vet. Med. Assoc., 130:
215-216.
RAHMATULLAH, M., JAHAN, R., CHOWDHURY, A.A., FARUK, A.B.M.,
& CHOWDHURY, M.S.K. (1978) Effect of organophosphorus insecticides on
fermentation processes in Aspergillus niger. J. ferment. Technol., 56:
169-172.
RAMEL, C. (1981) Does dichlorvos constitute a genotoxic hazard? In:
Kappas, A., ed. Progress in environmental mutagenesis and
carcinogenesis, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 69-78 (Progress in Mutation Research, Vol. 2.).
RAMEL, C., DRAKE, J., & SUGIMURA, T. (1980) An evaluation of the
genetic toxicity of dichlorvos. Mutat. Res., 76: 297-309.
RASMUSSEN, W.A., JENSEN, J.A., STEIN, W.J., & HAYES, W.J. (1963)
Toxicological studies of DDVP for disinsection of aircraft. Aerosp.
Med., 34(7): 593-600.
RATH, S. & MISRA, B.N. (1979a) Relative toxicity of dichlorvos (DDVP)
to Tilapia mossambica Peters of 3 different age groups. Exp.
Gerontol., 14: 307-309.
RATH, S. & MISRA, B.N. (1979b) Sub-lethal effects of dichlorvos (DDVP)
on respiratory metabolism of Tilapia mossambica of 3 age groups. Exp.
Gerontol., 14: 37-41.
RATH, S. & MISRA, B.N. (1980) Pigment disperson in Tilapia
mossambica Peters exposed to dichlorvos (DDVP). Curr. Sci., 49(23):
907-909.
RATH, S. & MISRA, B.N. (1981) Toxicological effects of dichlorvos
(DDVP) on brain and liver acetylcholinesterase (AChE) activity
of Tilapia mossambica Peters. Toxicology, 19: 239-245.
RAUWS, A.G. & LOGTEN, M.J., VAN (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in
chicken. Toxicology, 1: 29-41.
REECE, R.L. (1982) Observations on the accidental poisoning of birds
by organophosphate insecticides and other toxic substances. Vet.
Rec., 111(20): 453-455.
REEVES, J.D., DRIGGERS, D.A., & KILEY, V.A. (1981) Household
insecticide associated aplastic anaemia and acute leukaemia in
children. Lancet, 8 August: 300-301.
REINER, E. & PLESTINA, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by O,O-dimethyl-2,2-
dichlorovinyl phosphate. Toxicol. appl. Pharmacol., 49: 451-454.
RIDER, J.A., MOELLER, H.C., & PULETTI, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies with
Guthion, and determination of incipient toxicity level of dichlorvos in
humans. Fed. Proc., 26: 427.
RIDER, J.A., MOELLER, H.C., PULETTI, E.J., & SWADER, J. (1968)
Studies on the anticholinesterase effects of methyl parathion, guthion,
dichlorvos, and gardona in human subjects. Fed. Proc., 27: 597.
ROSENBERG, A., LIEBERMAN, M.T., & ALEXANDER, M. (1979)
Microbial degradation of pesticides, Springfield, Virginia, US
National Technical Information Service (PB 79-123156).
ROSENKRANZ, H.S. (1973) Preferential effect of dichlorvos
(VaponaR) on bacteria deficient in DNA polymerase. Cancer Res., 33:
458-459.
ROSENKRANZ, H.S. & LEIFER, Z. (1980) Determining the DNA-modifying
activity of chemicals using DNA polymerase-deficient Escherichia
coli. In: de Serres, F.J. & Hollander, A., ed. Chemical mutagens:
principles and methods for their detection, New York, Plenum Press,
Vol. 6, pp. 109-147.
ROSENKRANZ, H.S. & ROSENKRANZ, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia (Basel), 28(4): 386-387.
SAGNER, G. & SCHONDUBE, M. (1982) [Determination and toxicological
appraisal of indoor concentrations of dichlorvos after the application
of spraying products.] Schriftenr. Ver. Wasser Boden Lufthyg., 53:
359-368 (in German).
SAKAMA, K. & NISHIMURA, M. (1977) [Studies on inhalation toxicity of
organic phosphorus insecticides. II. Approach to establishment of
threshold limit value.] Jpn. J. ind. Health, 19: 357-358 (in
Japanese).
SASINOVICH, L.M. (1968) [Substantiation of the maximum permissible
concentration of DDVP in the air of the working zone.] Gig. i Sanit.,
33(12): 35-39 (in Russian).
SASINOVICH, L.M. (1970) [Toxicology of dimethyldichlorovinyl
phosphate and hygienic characteristics of its use.] Gig. Primen.
Toksikol. Pestits. Klin. Otravl., 8: 297-307 (in Russian).
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical, and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHAFER, E.W., Jr & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and management
materials, Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SCHMIDT, G., SCHMIDT, M., & NENNER, M. (1975) Inhibition of
acetylcholinesterase in the bronchial system of rats caused by
inhalation of dichlorvos (DDVP). Naunyn Schmiedeberg Arch.
Pharmacol., 287(Suppl.): R97.
SCHMIDT, G., SCHMIDT, M., NENNER, M., & VETTERLEIN, F. (1979)
Effects of dichlorvos (DDVP) inhalation on the activity of
acetylcholinesterase in the bronchial tissue of rats. Arch.
Toxicol., 42: 191-198.
SCHOOF, H.F., JENSEN, J.A., PORTER, J.E., & MADDOCK, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP
vapour. Bull. World Health Organ., 24: 623-628.
SCHULTZ, D.R., MARXMILLER, R.L., & KOOS, B.A. (1971) Residue
determination of dichlorvos and related metabolites in animal tissue
and fluids. J. agric. food Chem., 19(6): 1238-1243.
SCHULZE, H.D. (1979) [Current problems of pest control with
dichlorvos in hospital.] Z. gesamte Hyg., 25(5): 421-425 (in German).
SCHWETZ, B.A., IOSET, H.D., LEONG, B.K.J., & STAPLES, R.E. (1979)
Teratogenic potential of dichlorvos given by inhalation and gavage to
mice and rabbits. Teratology, 20: 383-388.
SEGERBACK, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP (2,2-dichlorovinyl
dimethylphosphate). Hereditas, 94: 73-76.
SEGERBACK, D. & EHRENBERG, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta pharmacol. toxicol., 49(Suppl. 5): 56-66.
SERA, K., MATSUNAGA, A., MURAKAMI, A., SATO, I., YAMASHITA,
K., & YOSHIMORI, H. (1959) Qualitative analysis of organic phosphorus
color reactions and simple detection. Kumamoto med. J., 12(3): 193-
213.
SHAFIK, M.T., BRADWAY, D., & ENOS, H.F. (1971) A method for
confirmation of organophosphorus compounds at the residue level.
Bull. environ. Contam. Toxicol., 6(1): 55-66.
SHELLENBERGER, T.E. (1980) Organophosphorus pesticide inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. environ. Sci. Health, B15(6): 795-822.
SHELLENBERGER, T.E., NEWELL, G.W., OKAMOTO, S.S., & SARROS, A.
(1965) Response of rabbit whole blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14: 943-
952.
SHERMAN, M. & ROSS, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. appl. Pharmacol., 3: 521-533.
SHINKAJI, N. & ADACHI, T. (1978) [The effect of certain pesticides on
the predaceous mite Amblyseius longispinosus (Evans) (Acarina:
Phytoseiidae).] Akitsu, 2: 99-108 (in Japanese, with English tables).
SHINODA, H., ITO, K., MATSUNAGA, T., TAKADA, T., & NOMURA, T.
(1972) Two suicides by acute pesticide intoxication. J. Jpn. Assoc.
Rural Med., 21: 242-249 (in Japanese).
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHOOTER, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating
agents. Chem.-biol. Interact., 11: 575-588.
SLOMKA, M.B. & HINE, C.H. (1981) Clinical pharmacology of
dichlorvos. Acta pharmacol. toxicol., 49(Suppl. 5): 105-108.
SMITH, P.W., MERTENS, H., LEWIS, M.F., FUNKHOUSER, G.E., HIGGINS,
E.A., CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & FLUX, M. (1972)
Toxicology of dichlorvos at operational aircraft cabin altitudes.
Aerosp. Med., 43(5): 473-478.
SNOW, D.H. & WATSON, A.D.J. (1973) The acute toxicity of dichlorvos
in the dog. I. Clinical observations and clinical pathology. Aust. vet.
J., 49: 113-119.
SOBELS, F.H. & TODD, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res., 67: 89-92.
STANTON, H.C., ALBERT, J.R., & MERSMANN, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows. Am. J.
vet. Res., 40(3): 315-320.
STAVINOHA, W.B., MODAK, A.T., & WEINTRAUB, S.T. (1976) Rate of
accumulation of acetylcholine in discrete regions of the rat brain
after dichlorvos treatment. J. Neurochem., 27: 1375-1378.
STEIN, W.J., MILLER, S., & FETZER, L.E., Jr (1966) Studies with
dichlorvos residual fumigant as a malaria eradication technique in
Haiti. III. Toxicological studies. Am. J. trop. Med. Hyg., 15(5): 672-
675.
STERNBERG, S.S. (1979) The carcinogenesis, mutagenesis, and
teratogenesis of insecticides. Review of studies in animals and
man. Pharmacol. Ther., 6: 147-166.
STEVENSON, D.E. & BLAIR, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0024.69).
STEVENSON, D.E. & BLAIR, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Toxicol., 18: 215-217.
STOERMER, D. (1985) [Occupational contact dermatitis from the
pesticide dichlorvos.] Dermatol. Monatsschr., 171(4): 245-249 (in
German).
TEICHERT, K., SZYMCZYK, T., CONSOLO, S., & LADINSKY, H. (1976)
Effect of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. appl. Pharmacol., 35: 77-81.
TERAYAMA, K., HONMA, H., & KAWARABAYASHI, T. (1978) Toxicity of
heavy metals and insecticides on slime mold Physarum polycephalum. J.
toxicol. Sci., 3: 293-304.
TESSARI, J.D. & SPENCER, D.L. (1971) Air sampling for pesticides in the
human environment. J. Assoc. Off. Anal. Chem., 54(6): 1376-1382.
TEZUKA, H., ANDO, N., SUZUKI, R., TERAHATA, M., MORIYA, M., &
SHIRASU, Y. (1980) Sister chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with pesticides
positive in microbial reversion assays. Mutat. Res., 78: 177-191.
THOMAS, T.C. & NISHIOKA, Y.A. (1985) Sampling of airborn pesticides
using chromosorb-102. Bull. environ. Contam. Toxicol., 35: 460-465.
THORPE, E., WILSON, A.B., DIX, K.M., & BLAIR, D. (1972) Teratological
studies with dichlorvos vapour in rabbits and rats. Arch. Toxicol.,
30(1): 29-38.
TIMMONS, E.M., CHAKLOS, R.J., BANNISTER, T.M., & KAPLAN, H.M.
(1975) Dichlorvos effects on estrous cycle onset in the rat. Lab.
anim. Sci., 25(1): 45-47.
TRACY, R.L., WOODCOCK, J.G., & CHODROFF, S. (1960) Toxicological
aspects of 2,2'-dichlorovinyl dimethylphosphate (DDVP) in cows,
horses, and white rats. J. econ. Entomol., 53(4): 593-601.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 43 pp (Resource Publication No. 84).
UEDA, K., SHIYO, K., MORI, T., KITAHARA, E., & URAGAMI, S. (1959)
[Effects of DDVP on man.] J. Jpn. Public Hyg. Assoc., 6: 315 (in
Japanese).
UEDA, K., SHIYO, K., IIZUKA, Y., KITAHARA, E., & OHASHI, A. (1960)
[The toxicity of organic phosphate "DDVP" for small animals and
man.] Igaku Seibutsugaku (Med. Biol.), 57(3): 98-101 (in Japanese).
ULLMANN, L., PHILLIPS, J., & SACHSSE, K. (1979) Cholinesterase
surveillance of aerial applicators and allied workers in the Democratic
Republic of the Sudan. Arch. environ. Contam. Toxicol., 8: 703-712.
VADHVA, P. & HASAN, M. (1986) Organophosphate dichlorvos induced
dose-related differential alterations in lipid levels and lipid
peroxidation in various regions of the fish brain and spinal cord. J.
environ. Sci. Health, B21(5): 413-424.
VAN DIJK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air: sampling
methods. Residue Rev., 55: 91-134.
VAN RAALTE, H.G.S. & JANSEN, J.D. (1981) Household insecticides and
the blood. Lancet, 10 October: 811.
VASHKOV, V.I., VOLKOVA, A.P., TSETLIN, V.M., & YANKOVSKII, E.Y.
(1966) [Assessment of the use of dimethyldichlorovinyl phosphate
(DDVP) in the insecticide mixture.] Gig. i Sanit., 9: 15-17 (in
Russian).
VASILESCU, C. & FLORESCU, A. (1980) Clinical and electro-
physiological study of neuropathy after organophosphorus compounds
poisoning. Arch. Toxicol., 43: 305-315.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination of
water by a sublethal concentration of pesticides. A system analysis
approach. Acta hydrochim. hydrobiol., 12(4): 399-499.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1980) Effects of
the pesticides thiotox, dichlorvos, and carbofuran on the test
fish Mystus vittatus. Water Air Soil Pollut., 13(2): 229-234.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1981a)
Evaluation of the comparative toxicity of thiotox, dichlorvos, and
carbofuran to two fresh water teleosts Ophiocephalus punctatus and
Mystus vittatus. Acta hydrochem. hydrobiol., 9(2): 119-129.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981b) Isolated and combined
effects of pesticides on serum transaminases in Mystus
vittatus (African catfish). Toxicol. Lett., 8: 67-71.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981c) Pesticide-induced
physiological alterations in certain tissues of a fish Mystus vittatus.
Toxicol. Lett., 9: 327-332.
VERMA, S.R., TONK, I.P., & DALELA, R.C. (1981d) Determination of the
maximum acceptable toxicant concentration (MATC) and the safe
concentration for certain aquatic pollutants. Acta hydrochim.
hydrobiol., 9(3): 247-254.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982a) Bioassay trials
with twenty-three pesticides to a fresh water teleost Saccobranchus
fossilis. Water Res., 16: 525-529.
VERMA, S.R., RANI, S., & DALELA, R.C. (1982b) Indicators of stress
induced by pesticides in Mystus vittatus: haematological
parameters. Indian J. environ. Health, 24(1): 58-64.
VERMA, S.R., RANI, S., TONK, I.P., & DALELA, R.C. (1983) Pesticide-
induced dysfunction in carbohydrate metabolism in three fresh water
fishes. Environ. Res., 32: 127-133.
VERMA, S.R., RANI, S., & DALELA, R.C. (1984) Effects of pesticides
and their combinations on three serum phosphatases of Mystus vittatus.
Water Air Soil Pollut., 21: 9-14.
VOGIN, E.E., CARSON, S., & SLOMKA, M.B. (1971) Teratology studies
with dichlorvos in rabbits. Toxicol. appl. Pharmacol., 19: 377-378.
VOOGD, C.E., JACOBS, J.J.J.A.A., & STEL, J.J., VAN DER (1972) On the
mutagenic action of dichlorvos. Mutat. Res., 16: 413-416.
VOSS, G. & SACHSSE, K. (1970) Red cell and plasma cholinesterase
activities in microsamples of human and animal blood determined
simultaneously by a modified acetylthiocholine/DNTB procedure.
Toxicol. appl. Pharmacol., 16: 764-772.
VRBOVSKY, L., SELECKY, FR.V., & ROSIVAL, L. (1959) [Toxicological and
pharmacological studies with phosphorester insecticides.] Naunyn
Schmiedeberg Arch. Pharmacol., 236: 202-204 (in German).
WADIA, R.S., SHINDE, S.N., & VAIDYA, (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurology (India), 33:
247-253.
WAGNER, R. & HOYER, J. (1975) [Method of determining workplace
concentrations and on occupational hygiene conditions during thermal
fogging atomization of pesticides in the greenhouse.] Z. gesamte
Hyg., 21(1): 18-20 (in German).
WALKER, A.I.T., BLAIR, D., STEVENSON, D.E., & CHAMBERS, P.L.
(1972) An inhalational toxicity study with dichlorvos. Arch.
Toxikol., 30: 1-7.
WARD, F.P. & GLICKSBERG, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Assoc., 158(4): 457-
461.
WATANABE, H., SHIRAI, O., & EBATA, N. (1976) A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care. Pestic. Abstr., 9(2): 94-95.
WEISBURGER, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 165-176.
WENNERBERG, R. & LOFROTH, G. (1974) Formation of 7-methyl- guanine
by dichlorvos in bacteria and mice. Chem.-biol. Interact., 8: 339-348.
WHITEHEAD, C.C. (1971) The effects of pesticides on production in
poultry. Vet. Rec., 30 January: 114-117.
WHO (1978) Spectrophotometric kit for measuring cholinesterase
activity, Geneva, World Health Organization (Report VBC/78.692).
WHO/FAO (1975-86) Data sheets on pesticides, Geneva, World Health
Organization (VBC).
WHO (1985) Technical dichlorvos. In: Specifications for pesticides
used in public health, 6th ed., Geneva, World Health Organization, pp.
163-174 (WHO/SIT/16.R2).
WHO (1986a) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87, Geneva, World Health
Organization, p. 11 (Report VBC/86.1 Rev.1)
WHO (1986b) Environmental Health Criteria 63: organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WILD, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of dichlorvos
and methyl methanesulfonate. Mutat. Res., 19: 33-41.
WILD, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res., 32: 133-150.
WILDEMAUWE, C., LONTIE, J.F., SCHOOFS, L., & LAREBEKE, N., VAN
(1983) The mutagenicity in procaryotes of insecticides, acaricides,
and nematicides. Residue Rev., 89: 129-178.
WILLIAMS, P.P. (1977) Metabolism of synthetic organic pesticides by
anaerobic microorganisms. Residue Rev., 66: 102.
WILSON, A.B. & DIX, K.M. (1973) Toxicity studies on dichlorvos: one
month inhalational exposure of rats to dichloroacetaldehyde,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0017.73).
WITHERUP, S., STEMMER, K.L., & CALDWELL, J.S. (1964) The effects
upon rats of being fed on diets containing VaponaR insecticide,
Cincinnati, Ohio, The Kettering Laboratory (Unpublished Report, 1
July).
WITHERUP, S., CALDWELL, J.S., & HULL, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their offspring by
the introduction of VaponaR insecticide into their diets, Cincinatti,
Ohio, The Kettering Laboratory (Unpublished Report, 12 April).
WITHERUP, S., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction of
VaponaR insecticide into their daily diets, Cincinnati, Ohio, The
Kettering Laboratory (Unpublished Report, 14 February).
WITHERUP, S., JOLLEY, W.J., STEMMER, K., & PFITZER, E.A. (1971)
Chronic toxicity studies with 2,2-dichlorovinyl dimethyl phosphate
(DDVP) in dogs and rats including observations on rat reproduction.
Toxicol. appl. Pharmacol., 19: 377.
WITTER, R.F. (1960) Effects of DDVP aerosols on blood cholinesterase
of fogging machine operators. Am. Med. Assoc. Arch. Ind. Health, 21:
7-9.
WITTER, R.F., GAINES, T.B., SHORT, J.G., SEDLAK, V.A., & MADDOCK,
D.R. (1961) Studies on the safety of DDVP for the disinsection of
commercial aircraft. Bull. World Health Organ., 24: 635-642.
WOOD, A.B. & KANAGASABAPATHY, L. (1983) Evaluation of inexpensive
thin layer chromatographic procedures for the estimation of some
organophosphorus and carbamate insecticide residues in fruit and
vegetables. Pestic. Sci., 14: 108-118.
WOODER, M.F. & CREEDY, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo, Sittingbourne,
Shell Research Ltd (Unpublished Report TLGR.79.089).
WOODER, M.F. & WRIGHT, A.S. (1981) Alkylation of DNA by organo-
phosphorus pesticides. Acta pharmacol. toxicol., 49(Suppl. 5): 51-55.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) I n vivo alkylation
studies with dichlorvos at practical use concentrations. Chem.-biol.
Interact., 19: 25-46.
WOODER, M.F., WRIGHT, A.S., & STEVENSON, D.E. (1978) Studies on
the in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol., 1: 505.
WORTHING, C.R. & WALKER, S.B. (1983) Pesticide manual, 7th ed.,
Croydon, British Crop Protection Council.
WRATHALL, A.E., WELLS, D.E., & ANDERSON, P.H. (1980) Effect of
feeding dichlorvos to sows in mid-pregnancy. Zbl. Vet. Med. A27: 662-
668.
WRIGHT, C.G. & LEIDY, R.B. (1980) Air samples in vehicles and
buildings turn up only very low levels of organic phosphate
insecticides. Pest Control, 48(7): 22-26, 68.
WRIGHT, A.S., HUTSON, D.H., & WOODER, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol., 42: 1-18.
XING-SHU, H. (1983) Preventing chemical damage to germ cells. Am.
Ind. Hyg. Assoc. J., 44: 699-703.
YAMANE, S., KINO, A., & TESHIMA, S. (1974) Histochemical
demonstration of cholinesterase activity in tissues of the carp
and effect of DDVP on its activity in situ. Acta histochem.
cytochem., 7(2): 167-174.
YAMANOI, F. (1980) [Effect of insecticides on the progeny in the
silkworm Bombyx mori. I. Effect of organophosphorus insecticides on egg
laying and their hatching.] Nippon Sanshigaku Zasshi, 49(5): 434-439
(in Japanese).
YAMASHITA, K. (1960) Studies on organic phosphorus. I. Toxicity of
dipterex and its vinyl derivative (DDVP). Kumamoto med. J., 13(4): 273-
279.
YAMASHITA, K. (1961) Studies on organic phosphorus dipterex. III.
Detection and discrimination of dipterex and its vinyl derivative
(DDVP). Kumamoto med. J., 14(1): 13-25.
YAMASHITA, K. (1962) Toxicity of dipterex and its vinyl derivative
(DDVP). Ind. Med. Surg., 31: 170-173.
ZALEWSKA, Z., RAKOWSKA, I., MATRASZEK, G., & SITKIEWICZ, D.
(1977) Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterases. Neuropathol.
Polska, 15(2): 255-262.
ZAVON, M.R. & KINDEL, E.A., Jr (1966) Potential hazard in using
dichlorvos insecticide resin. Adv. Chem. Ser., 60: 177-186.
ZELMAN, I.B. (1977) [Pathomorphology on the rat brain after
experimental intoxication with the phosphororganic pesticide dichlorvos
(DDVP).] Neuropathol. Polska, 15(4): 515-522 (in Polish).
ZELMAN, I.B. & MAJDECKI, T. (1979) [Ultrastructural changes in rat
brain following organophosphate insecticide dichlorvos (DDVP)
intoxication.] Neuropathol. Polska, 17(3): 443-453 (in Polish).
ZOTOV, V.M., SVIRIN, J.N., & PRUCAKOVA, R.M. (1977) [The develop-
ment of health regulations for occupational exposure in greenhouses
treated with chemical pesticides.] Gig. Tr. prof. Zabol., 3: 49-50 (in
Russian).

 6.4 Elimination and Excretion in Expired Air, Faeces, and Urine
6.4.1 Human studies
Eight hours after a human male consumed 5 mg of vinyl-1-
14C-dichlorvos in orange juice, 27% of the radioactivity had been
eliminated as 14C-carbon dioxide. Approximately 8% had been excreted
by the urine within one day following dosing. Urinary excretion of
radioactivity decreased gradually and by day 9 none was detectable
(Hutson & Hoadley, 1972a).
The concentration of dimethylphosphate in the urine of three
pesticide control operators spraying houses with dichlorvos ranged from
0.32 to 1.4 µg at the end of the day's work (Das et al., 1983).
6.4.2 Studies on experimental animals
6.4.2.1 Oral
Dosing rats orally with 32P-dichlorvos (0.1 - 80 mg/kg body
weight) resulted in a recovery of 60 - 70% of the administered
radioactivity in the urine and approximately 10% in the faeces over a
6-day period following dosing (Casida et al., 1962).
After the oral administration of methyl-14C-dichlorvos to rats
(1 mg) and mice (0.5 mg), the excretion of radioactivity was rapid.
The major route of elimination after 4 days was the urine
(approximately 60%), followed by expired air (approximately 16%)
(Hutson & Hoadley, 1972b).
Rats given an oral dose of vinyl-1-14C-dichlorvos (1 mg per
animal) eliminated 10 - 20% of the 14C in the urine, 3 - 5% in the
faeces, and approximately 40% as expired carbon dioxide over 4 days
following dosing (Hutson et al., 1971a,b).
A comparison between the excretion by rat, mouse, hamster, and man
24 h after oral dosing with vinyl-1-14C-dichlorvos is given in
Table 10 (Hutson & Hoadley, 1972a).
A cow treated orally with 20 mg/kg body weight
32P-dichlorvos eliminated 40% of the radioactivity in the urine and
50% in the faeces. In the milk, the level of organosoluble radio-
activity was significantly above background only within the first 2 h
(Casida et al., 1962).
Table 10. Comparison of percentages of radioactivity excreted by males
24 h after oral ingestion of vinyl-1-14C-dichlorvosa
-----------------------------------------------------------------------------
Excretion route Rat (3) Mouse (1) Hamster (2) Man (1)
-----------------------------------------------------------------------------
urine 9.8 27.4 14.7 7.6
faeces 1.5 3.2 2.9 -
carbon dioxide 28.8 23.1 33.5 27 (8 h only)
-----------------------------------------------------------------------------
a Number of animals are given in parentheses.
6.4.2.2 Parenteral
The elimination of a single intraperitoneal injection of vinyl-1-
14C-dichlorvos (4 mg/kg body weight) from female rats was similar to
the elimination after oral dosing. A goat treated subcutaneously with
1.5 mg 32P-dichlorvos/kg body weight excreted 79% of the radioactivity
in the urine and 11% in the faeces. Two cows received an intravenous
or a subcutaneous injection with 1 mg 32P-dichlorvos/kg body weight.
Of the radioactivity which was recovered, 70 - 80% was in the urine and
approximately 14% in the faeces (Casida et al., 1962).
6.5 Retention and Turnover
6.5.1 Biological half-life
In studies by Blair et al. (1975), the metabolism of dichlorvos was
found to be so rapid that the biological half-life in blood could not
be determined. No intact dichlorvos could be demonstrated in the blood
or tissues of animals exposed by routes other than parenteral
injection. Only after exposure for 4 h to an atmospheric concentration
of 90 mg dichlorvos/m3 could dichlorvos be detected in most tissues of
the rat and mouse. Following exposure at 50 mg/m3, for 2 or 4 h, the
half-life in the rat kidney was 13.5 min.
The intraperitoneal injection of 10 mg dichlorvos/kg body weight
into mice increased the accumulation of ACh in the brain and caused an
inhibition of ChE activity. Symptoms of toxicity were clearly
recognizable after 15 min, and they disappeared almost completely after
60 min. The ChE activity and ACh levels reached their minimum and
maximum, respectively, at 15 min. The maximum concentration of
dichlorvos in the brain was reached after 1 min and decreased
thereafter, rapidly reaching the baseline level after 3 min (Nordgren
et al., 1978).
6.5.2 Body burden
There is no evidence for the storage of dichlorvos or its
metabolites in the tissues of animals. Small fractions of the carbon,
phosphorus, and chlorine derived from dichlorvos are retained in the
body for several days because their turnover rate is the same as that
for identical materials from other origins.
6.5.3 Indicator media
The determination of dichloroethanol in urine as a means of
monitoring the exposure of human beings to dichlorvos is not
sufficiently sensitive to detect levels arising from vapour exposure
through normal use. However, it could serve as the basis for a
specific detection method for the accidental ingestion of high levels
(Hutson & Hoadley, 1972a). Two other methods can be used: (a)
determination of the blood ChE activity; or (b) determination of
dimethylphosphate in urine by a rather complicated method (Blair &
Roderick, 1976). Neither method is specific when exposure to other
organophosphate or carbamate compounds, or to compounds that also
metabolize to dimethylphosphate, may have occurred.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
Lal (1982) reviewed the accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms.
Microorganisms undoubtedly have the ability to metabolize
organophosphorus insecticides; however, there are still large gaps in
our knowledge. It also seems clear that chemical, photochemical,
physical, and biological factors may influence the metabolism of
dichlorvos by microorganisms.
7.1.1 Algae and plankton
The dose of dichlorvos producing 50% growth inhibition of the
unicellular alga Euglena gracilis has been quoted as 3.5 mg/litre
(Butler, 1977).
Treating eutrophic carp ponds with 0.325 mg/litre killed
Cladocera (predominantly Bosmina and Daphnia species) and decreased
Copepoda (mainly Cyclops ). This was offset by increased development
of Rotatoria (mainly Polyarthra and Brachionus species) and
phytoplankton (mainly Scenedesmus and Pediastrum species), so that
the total plankton biomass changed only slightly (Grahl et al., 1981).
7.1.2 Fungi
Dichlorvos (in the range 10 - 80 mg/litre) has been found to affect
citric acid fermentation in Aspergillus niger grown in an artificial
medium. Inhibition of the fermentation was marked only at 40 and
80 mg/litre (Rahmatullah et al., 1978; Ali et al., 1979c). It appears
from the decreased uptake of inorganic phosphorus that dichlorvos may
have an interfering action on oxidative metabolism in A. niger. The
potential for inhibiting citrinin production by Penicillium
citrinum was investigated. Dichlorvos inhibited citrinin production by
76% at 100 µg/litre and by 48% at 10 µg/litre (Draughon & Ayres,
1978). The effect of dichlorvos on the survival time and the membrane
potential of the slime mould Physarum polycephalum was studied in a
laboratory test system. The threshold value for both these effects was
found to be 300 mg/litre for technical dichlorvos and 30 mg/litre for
the pure chemical (Terayama et al., 1978).
The influence of dichlorvos on 17 soil fungi, cultivated in
artificial medium, was tested. Dose levels of 0, 10, 30, 60, and
120 mg/kg were used during a test period of 21 days, and the effect on
the growth and morphology of the fungi was studied. In general, a
growth depression was found, but its extent depended on the fungal
strain. Occasionally growth was either unaffected or even stimulated
(Jakubowska & Nowak, 1973).
7.1.3 Bacteria
Dichlorvos has been found not to influence the overall metabolic
processes of Escherichia coli and Enterobacter aerogenes at doses up to
250 mg/litre (Grahl et al., 1980) and was not toxic for a sewage
isolate at up to 10 mg/litre (Rosenberg et al., 1979). In poultry
effluent slurry, concentrations of 100 and 1000 mg/litre did not
significantly reduce coliform populations, but 10 000 mg/litre caused
almost complete death. Therefore, residues of dichlorvos used for fly
control in layer houses can significantly reduce the enteric coliform
populations that are essential to the conversion of organic nitrogen to
inorganic nitrogen in poultry waste effluent (Ballington et al.,
1978).
In studies by Lieberman & Alexander (1981), dichlorvos (0.1 -
100 mg/litre) had little or no toxicity for microorganisms degrading
organic matter in sewage, as measured by respiratory activity,
degradation, and nitrification.
Incubation of dichlorvos with inocula of ruminal bacteria or
ciliated protozoa under anaerobic conditions suggests that dichlorvos
is not utilized by the organisms for growth, nor does it stimulate
endogenous gas production. However, it does, in certain instances,
affect volatile fatty acid production (Williams, 1977).
The growth of Bacillus thuringiens var th. was not inhibited by
dichlorvos (Dougherty et al., 1971).
7.2 Aquatic Organisms
Reviews of the acute and chronic effects of pesticides on aquatic
organisms have been made by Brungs et al. (1977), Livingston (1977),
and Kenaga (1979).
The toxicity of a chemical for aquatic organisms is influenced by
many factors such as the stage of development of the organism and the
composition, pH, oxygen content, and hardness of the water. In this
short review, these factors are not discussed in detail.
7.2.1 Fish
7.2.1.1 Acute toxicity
The acute toxicity of dichlorvos for both freshwater and estuarine
species of fish is moderate to high. The available data are summarized
in Table 11.
Variations in water hardness from 44 to 162 mg/litre and in pH from
6 to 9 did not alter the toxicity of dichlorvos for cutthroat or lake
trout (Johnson & Finley, 1980).
In studies by Yamane et al. (1974), young carp were exposed to a
concentration of 25 mg dichlorvos/litre water for 45 min. The ChE
activity (histochemically determined) of many tissues, including the
stratum griseum periventriculare, sarcolemma, and liver, was inhibited
or totally lost.
Table 11. Acute toxicity of dichlorvos for fish
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Freshwater
Clarias batrachus 26 - 31 g - 8.9 Verma et al. (1983)
Carp 8 mm 20 - 23 0.34 Verma et al. (1981d)
(Cyprinus carpio) 6 g 23 20a Yamane et al. (1974)
Mosquito fish 0.2 g 17 5.3 Johnson & Finley (1980)
(Gambusia affinis)
Blue gill 1.5 g 18 0.9 Johnson & Finley (1980)
(Lepomis macrochirus)
African catfish 6 - 10 g 18 0.5 Verma et al. (1980, 1981a)
(Mystus vittatus)
Ophiopcephalus punctatus 40 - 55 g 18 2.3 Verma et al. (1981a)
Fathead minnow 0.7 g 17 12 Johnson & Finley (1980)
(Pimephales promelas)
Harlequin fish - 20 7.8b Alabaster (1969)
(Rasbora heteromorpha)
Singii 5 - 10 g 18 6.6 Verma et al.
(Saccobranchus fossilis) (1982a)
Cutthroat trout 2.5 g 12 0.2 Johnson &
(Salmo clarki) Finley (1980)
Lake trout 0.3 g 12 0.2 Johnson &
(Salvelinus namaycush) Finley (1980)
Tilapia mossambica 3 - 10 cm 29 1.4 - 1.9 Rath & Misra
(1979a)
----------------------------------------------------------------------------------------------------
Table 11. (contd).
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Estuarine
American eel 0.14 g 20 1.8 Eisler (1970)
(Anguilla rostrata)
Mummichog 1.7 g 20 2.7 Eisler (1970)
(Fundulus heteroclitus)
Striped killifish 0.92 g 20 2.3 Eisler (1970)
(Fundulus majalis)
Atlantic silverside 0.8 g 20 1.3 Eisler (1970)
(Menidia menidia)
Striped mullet 1 - 6 g 20 0.23 Eisler (1970)
(Mugil cephalus)
Northern puffer 100 g 20 2.3 Eisler (1970)
(Sphaeroidus maculatus)
Bluehead 5.4 g 20 1.4 Eisler (1970)
(Thalassoma bifasciatum)
----------------------------------------------------------------------------------------------------
a 24-h LC50.
b 48-h LC50.
7.2.1.2 Short-term toxicity
Sublethal concentrations of dichlorvos (0.5 - 1 mg/litre) have
been found to decrease the respiratory rates of Tilapia mossambica (3
different age groups) exposed for up to 3 weeks. When the exposed
fish were transferred to fresh water, the rate did not completely
return to its pre-exposure value (Rath & Misra, 1979b). Liver and
brain ChE activity showed considerable inhibition when a group of T.
mossambica was exposed to dichlorvos (0.25 - 1.25 mg/litre water) for
periods of up to 4 weeks. At 7-day intervals, fish were studied or
transferred to clean water. The degree of enzyme inhibition was
related to the dichlorvos concentration and length of exposure. In all
age groups of fish, brain tissue exhibited a higher degree of ChE
inhibition than liver. Small fish were more susceptible to dichlorvos
with respect to AChE activity. When the fish were transferred to clean
water, most of the fish recovered their AChE activity, the recovery
being greater in liver than in brain. Small fish exhibited a
comparatively high level of recovery. The degree of recovery was
inversely related to the length of exposure (Rath & Misra, 1981).
Melanin dispersion in T. mossambica exposed to 1 mg/litre water for
15 days was stimulated indirectly by the inhibition of ChE activity.
The original colour was regained within 96 h after transfer of the fish
to clean water (Rath & Misra, 1980).
Exposure of Mystus vittatus (collected in the environment) to
sublethal concentrations of dichlorvos (0.045 or 0.09 mg/litre) for 30
days caused dose-related increases in serum glutamic-oxaloacetic and
glutamic-pyruvic transaminase levels (Verma et al., 1981b), increases
in alkaline phosphatase, acid phosphatase, and glucose-6-phosphatase
levels in serum (Verma et al., 1984), decreases in the levels of these
enzymes in liver, kidneys, and gills (Verma et al., 1981c), an increase
in glucose levels in blood, and a decrease in liver glycogen. Blood
lactate and muscle glycogen were unaffected (Verma et al., 1983). At
0.09 mg/litre, blood clotting time, mean corpuscular haemoglobin and
mean corpuscular haemoglobin concentration decreased, and the number of
leukocytes increased. Other haematological parameters did not show any
abnormalities (Verma et al., 1982b). From these results, a no-
observed-adverse-effect concentration of 0.03 mg/litre was derived.
In studies by Verma & Tonk (1984), Heteropneustes (Saccobranchus)
fossilis was exposed to dichlorvos for 30 days at a concentration of
0.44 mg/litre. Respiration, haematological parameters, and the
activities of two enzymes (one of them AChE) in liver, kidneys, and
gills were determined. The respiration rate decreased, and blood
concentrations of sodium and chloride ions and glucose increased
significantly, whereas the cholesterol level and clotting time were
decreased. A significant reduction in the AChE activity of the three
tissues was found.
Vadhva & Hasan (1986) studied the effect of dichlorvos (at 0, 3, 6,
and 9 mg/litre water) on various lipid fractions and lipid
peroxidation in the central nervous system of Heteropneustes
fossilis. After one week's exposure, the results indicated that
dichlorvos caused dose-related increases in total lipids, cholesterol-
esterified fatty acid, and lipid peroxidation in various regions of the
brain and spinal cord but a consistent decrease in the level of
phospholipids in these regions of the central nervous system.
Exposure of Clarias batrachus to 0.5 - 2.2 mg dichlorvos/litre
and Saccobranchus fossilis to 0.4 - 1.6 mg/litre for 30 days was found
to increase blood glucose and decrease liver glycogen, whereas blood
lactate and muscle glycogen were normal (Verma et al., 1983).
The estimated "maximum acceptable toxicant concentration"
(MATC)a for the larvae of Cyprinus carpio was 0.016 - 0.020 mg/litre
based on a 60-day study (Verma et al., 1981d).
7.2.2 Invertebrates
The acute toxicity of dichlorvos for aquatic insects and
crustaceans is extremely high (Table 12). As might be expected from an
organophosphorus insecticide, aquatic invertebrates are about three
orders of magnitude more susceptible to dichlorvos than are fish, and
freshwater crustaceans are particularly sensitive.
A study was carried out to determine the influence of a number of
pesticides on the "hatchability" of Artemia salina dry eggs. No effect
was found at 10 mg dichlorvos/litre in the aqueous system (Kuwabara et
al., 1980).
When prawns (Macrobrachium lamarrei) were exposed to dichlorvos at
concentrations of 0.31 or 0.62 mg/litre for 96 h, a decrease in hepatic
glycogen and an increase in the blood glucose level were found (Omkar &
Shukla, 1984). Possibly, the phosphorylase activity of the
hepatopancreas and muscle increased due to the inhibition of AChE
activity and the consequent accumulation of acetylcholine at
neurosynaptic junctions. The latter resulted in an induction of the
secretion of the sinus gland, which enhanced glycogenolysis.
7.3 Terrestrial Organisms
7.3.1 Birds
7.3.1.1 Acute oral toxicity
Dichlorvos has a high oral toxicity for birds (Table 13). The
signs of intoxication are typical of organophosphorus poisoning, namely
salivation, lachrymation, tremors, and terminal convulsions. They
usually appear shortly after dosing, and, at lethal doses, death occurs
within 1 h. Survivors appear to recover completely 24 h after dosing.
Various internal haemorrhages were found at autopsy in sacrificed
survivors of treated pheasants and Mallard ducks (Tucker & Crabtree,
1970).
-----------------------------------------------------------------------
a Maximum concentration at which no effect was seen.
Table 12. Acute toxicity of dichlorvos for non-target aquatic insects and Crustacea
-------------------------------------------------------------------------------------------------------
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (µg/litre) (µg/litre)
-------------------------------------------------------------------------------------------------------
Insects (stone flies)
Pteronarcys californica 15 - 0.1 Johnson & Finley (1980)
Crustacea (fresh water)
Water flea 15 0.07 - Johnson & Finley (1980)
(Daphnia pulex)
Water flea 21 0.28 - Johnson & Finley (1980)
(Simocephalus serrulatus)
Amphipod 21 - 0.5 Johnson & Finley (1980)
(Gammarus lacustris)
Crustacea (estuarine)
Sand shrimp - 12 4 Eisler (1969)
(Crangon septemspinosa)
Grass shrimp - 300 15 Eisler (1969)
(Palaemonetes vulgaris)
Hermit crab - 52 45 Eisler (1969)
(Pagurus longicarpus)
-------------------------------------------------------------------------------------------------------
Table 13. Acute oral LD50 values for birds
------------------------------------------------------------------------------------------------------------------------
Species Age Vehicle LD50 Reference
(mg/kg body weight)
------------------------------------------------------------------------------------------------------------------------
Red-winged blackbird propylene glycol 13.3 Schafer & Brunton (1979)
(Agelaius phoenicius) (male)
Mallard duck 5 - 7 months capsule 7.8 Tucker & Crabtree (1970)
(Anas platyrhynchos) (male)
Common pigeon propylene glycol 24 Schafer & Brunton (1979)
(Columba livia)
Quaila propylene glycol 24 Schafer & Brunton (1979)
(Coturnix coturnix) (female)
Domestic fowl 21 days aqueous suspension 6.5 Naidu et al. (1978)
(Gallus domesticus) (male) 6 - 8 months aqueous suspension 30 Dmitriev & Kozhemyakin
(1975)
House sparrow propylene glycol 17.8 Schafer & Brunton (1979)
(Passer domesticus)
Ring-necked pheasant 3 months capsule 11 Tucker & Crabtree (1970)
(Phasianus colchicus) (male)
Common grackle propylene glycol 13.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Starling adult propylene glycol 12 Schafer (1972)
(Sturnus vulgaris) - propylene glycol 42.1 Schafer & Brunton (1979)
------------------------------------------------------------------------------------------------------------------------
a Hattori et al. (1974) carried out studies on Japanese quail and found
LD50 values of 22 and 26 respectively, for male and female
(no details available).
7.3.1.2 Short-term toxicity
In short-term dietary studies, dichlorvos has been found to be
slightly to moderately toxic for birds (Table 14).
In the study with 7-day-old male chicks, there was weight loss and
50% mortality at 500 mg/kg diet. Marked, dose-related inhibition of
brain ChE activity occurred at 50, 100, and 500 mg/kg diet, but no
effects were noted at a level of 10 mg/kg diet (Naidu et al., 1978).
Canaries, Indian finches, and budgerigars, continuously exposed to
dichlorvos vapour (0.14 mg/m3) for 5 days, did not show any overt
signs of intoxication, but a reduction in ChE levels in plasma and
brain was observed in canaries and Indian finches (Brown et al.,
1968).
7.3.1.3 Field experience
Caution has been advised in the use and handling of dichlorvos
where birds might be exposed (Whitehead, 1971). The necessity for this
warning can be illustrated by the following cases. Adult mallards
feeding near horse mangers containing dichlorvos-treated feed were
found dead within a short time. At necropsy, excessive amounts of
mucus covering the mucosa of the proventriculus and scattered petechiae
along medial edges of liver lobes were observed. The small and large
intestines were markedly extended, and crystals were noted in the
gizzard. Brain ChE activity was inhibited by 75 - 80% (Ludke & Locke,
1976). Domestic fowl, which had accidental access to the faeces of a
horse dosed with dichlorvos pellets, picked out the pellets and more
than 30 birds died during the next 24 h (Lloyd, 1973). A mass
poisoning occurred in chickens following consumption of accidentally
contaminated drinking-water (Egyed & Bendheim, 1977). English game
bantams died after consuming wheat contaminated with 300 mg
dichlorvos/kg wheat (Reece, 1982).
7.3.2 Invertebrates
Dichlorvos was toxic for silkworm larvae when for 4 h they were fed
mulberry leaves previously sprayed with dilute dichlorvos emulsions.
Spray concentrations giving 50% mortality ranged from 1.56 to 6.25
mg/litre (Aratake & Kayamura, 1973).
No adverse effects were observed on the hatchability and general
condition of first instar silkworm larvae hatched in the following
generation when 5th instar larvae were fed mulberry leaves pre-treated
with 3 mg dichlorvos/kg of leaf (Yamanoi, 1980).
Table 14. Dietary LD50 values for birds
-------------------------------------------------------------------------------------
Species Age Duration LD50 Reference
(days) of feeding (mg/kg diet)
(days)
-------------------------------------------------------------------------------------
Mallard duck 16 8a 5000 Hill et al. (1975)
(Anas platyrhynchos) 5 8a 1310 Hill et al. (1975)
Japanese quail 14 8a 300 Hill et al. (1975)
(Coturnix japonica)
Domestic fowl 7 28 500 Naidu et al. (1978)
(Gallus domesticus) (male)
Ring-necked pheasant 10 8a 570 Hill et al. (1975)
(Phasianus colchicus)
-------------------------------------------------------------------------------------
a The median lethal dietary dose (LD50) during an 8-day test including
5 days of treated diet followed by 3 days of untreated diet.
7.3.3 Honey bees
Dichlorvos is highly toxic for honey bees. Atkins et al. (1973)
found in laboratory studies an LD50 of 0.495 µg/bee in 48 h (topical
application of dust; 26.7 °C; relative humidity 65%). Beran (1979)
obtained an oral LD50 of 0.29 µg/g body weight and an LD50 (topical
application) value of 0.65 µg/g body weight. This high toxicity has
led dichlorvos to be classified in the most toxic category for bees in
Austria and the USA.
When honeycombs were exposed to dichlorvos vapour from dichlorvos
resin strips for 4 months, the combs absorbed the insecticide and were
toxic to bees for approximately one month after exposure ceased.
Contamination of the bees appeared to be by fumigant rather than
contact action (Clinch, 1970).
7.3.4 Miscellaneous
Dichlorvos was highly toxic for the predatory mite Amblyseius
longispinosus in contact trials. Residual toxicity disappeared within
6 days, the susceptibility being the same as that of Phytoseiulus
persimilis (Shinkaji & Adachi, 1978).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides in general, especially their short- and long-term effects
on the nervous system, will be found in Environmental Health Criteria
63: Organophosphorus Insecticides - A General Introduction (WHO,
1986).
8.1 Single Exposures
Dichlorvos is moderately to highly toxic when administered in
single doses by various routes to a variety of animal species
(Table 15). It is less toxic via the dermal and oral routes than by
parenteral routes. The signs of intoxication by all exposure routes
are typical of organophosphorus poisoning, i.e., salivation,
lacrimation, diarrhoea, tremors, and terminal convulsions, with death
occurring from respiratory failure. In addition, lethargy, ataxia,
hypersensitivity to noise, splayed gait, and paresis may be observed.
The signs are usually apparent shortly after dosing and, at lethal
doses, death occurs within 1 h. Survivors appear to recover completely
24 h after dosing.
The acute inhalational LC50 values for mice and rats are
summarized in Table 16. The apparent differences in the LC50s may be
the result of the type of exposure of the animal (whole body or head
only), whether the studies were carried out with dichlorvos vapour or
atomized particles of spray (with or without vehicle), or differences
in the purity of the dichlorvos. Moreover, since dichlorvos adheres
strongly to surfaces including glass, the out-going air has a
significantly lower concentration of dichlorvos than the air coming
into the chamber, if the system is not yet equilibrated.
No macroscopic abnormalities were observed in mice or rats 2 weeks
after a single exposure (MacDonald, 1982).
8.1.1 Domestic animals
When cattle and sheep were treated orally with a single dose of
dichlorvos, 10 mg/kg body weight was toxic for calves and 25 mg/kg body
weight for sheep. For the latter a dose of 10 mg/kg body weight was
without effects (Radeleff & Woodard, 1957).
8.1.2 Potentiation
Potentiation studies on male rats indicated that oral
administration of dichlorvos with 22 other organophosphate pesticides
resulted in no (or very little) potentiation, while administration with
malathion showed a marked potentiation (Narcisse, 1967; Kimmerle &
Lorke, 1968). However, Cohen & Ehrich (1976) found that the anti-ChE
action of 800 mg malathion/kg body weight (injected intraperitoneally)
was not potentiated by pre-treatment (18 h previously) with 30 mg/kg
dichlorvos, nor did malathion pre-treatment potentiate the action of
dichlorvos.
Table 15. The acute toxicity (LD50) of dichlorvos for experimental animals
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Mouse (male) oral unknown Eryfor EL or other 68 - 90 Vrbovsky et al. (1959);
solvents Ueda et al. (1960)
Mouse oral 80% unknown 87 Sasinovich (1968, 1970)
Mouse oral 97% aqueous polysor- 133 - 139a Haley et al. (1975)
bate 80
Mouse (male) oral 98% corn oil 140 Isshiki et al. (1983)
Mouse oral unknown aqueous 124 - 275a Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) subcutaneous unknown propylene glycol 13 - 33 Ueda et al. (1960);
and other solvents Jaques (1964)
Mouse subcutaneous unknown aqueous 20 - 26 Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) dermal unknown different solvents 206 & 395b Ueda et al. (1960)
Mouse intraperitoneal technical corn oil 28 Vrbovsky et al. (1959);
Casida et al. (1962)
Mouse intraperitoneal unknown aqueous 28 - 41a Holmstedt et al. (1978)
Mouse intravenous unknown aqueous 8 - 10 Holmstedt et al. (1978)
Rat oral 90 - 99% peanut oil and 56 - 96a Durham et al. (1957);
other solvents Narcisse (1967); Gaines
(1969)
Rat (male) oral unknown Eryfor EL and 46 - 110 Vrbovsky et al. (1959);
other solvents Ueda et al. (1960)
Rat oral 80% unknown 65 Sasinovich (1968, 1970)
Rat oral unknown aqueous 30 Holmstedt et al. (1978)
---------------------------------------------------------------------------------------------------------
Table 15. (contd).
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Rat dermal unknown xylene 75 - 107a Durham et al. (1957);
Gaines (1969)
Rat dermal 80% unknown 113 Sasinovich (1968, 1970)
Rat subcutaneous 95% dimethylsulfoxide 72 Brown & Stevenson
(1962)
Rat (male) intraperitoneal unknown Eryfor EL 18 Vrbovsky et al. (1959)
Guinea-pig subcutaneous 95% undiluted 28 Brown & Stevenson
(1962)
Syrian hamster intraperitoneal - suspension in 30 Dzwonkowska & Hübner
water (1986)
Rabbit oral 93% unknown 12.5 Desi et al. (1978)
Rabbit oral 80% unknown 22.5 Sasinovich (1968, 1970)
Rabbit dermal 80% unknown 205 Sasinovich (1968, 1970)
Cat oral 80% unknown 28 Sasinovich (1968, 1970)
Dog oral in capsule 100 - 316 Kodama (1960)
Swine (40- to 60- oral technical in capsule 157 Stanton et al. (1979)
day-old)
Domestic fowl oral technical in capsule 15 Sherman & Ross (1961)
(chicken)
---------------------------------------------------------------------------------------------------------
a The LD50s are often slightly different in males and females. Furthermore, it is clear that the
purity of the dichlorvos tested and the vehicle used has an influence on the toxicity.
b The two values were obtained using two different solvents.
Table 16. Inhalational LC50 values for dichlorvos
---------------------------------------------------------------------------------------------------------
Species Purity Type of Duration LC50 (mg/m3) Reference
exposure
---------------------------------------------------------------------------------------------------------
Mouse 80% vapour 4 h 13 Sasinovich (1968, 1970)
whole body
Mouse 98% vapour 4 h > 218 MacDonald (1982)
head only
Mouse (male) unknown; in vapour 4 h 310 Ueda et al. (1960)
solvent Ca whole body
Rat (male) unknown unknown, but 1 h 455 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat (male) 84.1% whole body 1 h 140 Sakama & Nishimura
polyethylene (1977)
glycol
Rat (male) unknown unknown, but 4 h 340 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat 80%(?) vapour 4 h 15 Sasinovich (1968, 1970)
whole body
Rat 98% vapour 4 h > 198 MacDonald (1982)
head only
---------------------------------------------------------------------------------------------------------
a Solvent C = 0.5% Sorpol 2020 water solution.
In vitro studies using human erythrocytes and plasma as ChE
sources, with ACh as substrate, indicated no potentiationa when
dichlorvos was tested in combination with carbaryl, crotoxyphos,
phosphamidon, malathion, malaoxon, mevinphos, parathion, paraoxon,
physostigmine, and trichlorphon (Carter & Maddux, 1968, 1974).
8.2 Short-term Exposures
8.2.1 Oral
8.2.1.1 Mouse
A 10-week (range-finding) toxicity study was carried out on B6C3F1
mice given 0, 25, 50, 100, 200, or 400 mg dichlorvos/ litre drinking-
water. Each group consisted of 12 males and 12 females, except the
control group (10 males and 10 females). Growth and mortality were
comparable with controls. In a second study, groups of 10 males and 10
females received 400, 1600, 3200, 5000, or 10 000 mg dichlorvos/litre.
The animals given the two highest doses died within 2 weeks, while the
1600 and 3200 mg groups showed slight and clear growth depression,
respectively, after 10 weeks (Konishi et al., 1981).
8.2.1.2 Rat
For 15 weeks, groups of 15 male and 15 female Charles River rats
were fed diets containing 0, 0.1, 1, 10, 100, or 1000 mg dichlorvos
(93%)/kg diet, which were freshly prepared once each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1), it may be
assumed that the average concentration of dichlorvos in each diet was
approximately 47% of the amount that had been added. There were no
deaths or signs of intoxication. Only the rats fed the highest dose
exhibited decreased growth rates at the beginning of the study. At
termination, no differences were observed in haematology, serum
protein, urinalysis, or gross or histopathological examination. In the
highest dose group, marked inhibition of ChE activity in plasma,
erythrocytes, and brain was noted. In the 100 mg/kg group, only
erythrocyte ChE inhibition was observed, and the 10 mg/kg group did not
show any ChE inhibition (Witherup et al., 1964).
Daily doses of 3.5 or 7 mg dichlorvos (purity not stated)/kg body
weight administered intragastrically to rats for 4 months and 0.7 or
1.4 mg/kg for 12 months caused no deaths. There were signs of
intoxication in the 7 mg group, and decreases in food consumption and
body weight gain and increases in a number of organ weights were seen
in all the groups except the lowest dose group. Inhibition of plasma,
erythrocyte, and brain ChE activity was observed in all groups except
those given 0.7 mg/kg body weight. The inhibition was time and dose
dependent (Sasinovich, 1970).
-----------------------------------------------------------------------
a Potentiation is the phenomenon that results in the combined effect
of exposure to two or more chemical substances being greater than
the sum of the effects that would be produced by each substance
separately, as the result of synergistic action.
In a (range-finding) toxicity study, groups of F-344 rats (10 of
each sex) were administered 0, 5, 10, 20, 40, or 80 mg/kg body weight,
dissolved in 10 ml of drinking-water. During the study six animals
died, five of which were in the two highest doses groups (Enomoto,
1981).
Dichlorvos administered orally to rats at a concentration of 70
mg/kg body weight inhibited not only ChE, but also alkaline
phosphatase, lactate dehydrogenase, and glutamate dehydrogenase
competitively. It increased the activity of glutamic-pyruvic
transaminase, but leucine aminopeptidase was not affected (Ellinger,
1985).
Ellinger et al. (1985) also studied the influence of dichlorvos on
haematological parameters in acute and short-term toxicity studies. In
the acute study 70 mg dichlorvos/kg body weight and in the short-term
study 30 mg/kg body weight were administered for 12 weeks. Decreases
in haemoglobin, haematocrit, and mean corpuscular haemoglobin
concentration were found.
Seven rat mothers were administered different dose levels of
dichlorvos (1 g or 10 g/litre distilled water) by stomach tube, and
their litters (1 - 12 days old) were nursed for about 21 days, and
weaned after 35 days of age. Although doses of 10 and 20 mg/kg body
weight did not cause any symptoms of intoxication, the mothers showed
significant inhibition of erythrocyte ChE activity. Plasma ChE
activity was affected to a lesser extent. Dose levels of 30 and
40 mg/kg body weight caused severe inhibition of erythrocyte ChE, and
severe cholinergic symptoms were seen. The symptoms occurred 10 -
20 min after dichlorvos administration and continued for 30 - 90 min,
when recovery took place (Tracy et al., 1960).
When pregnant rats were given oral doses of 1 or 5 mg dichlorvos in
oil/kg body weight during days 14 - 21 of pregnancy, the plasma ChE
activity of the mothers was markedly inhibited, but that of the young
(up to 56 days old) resembled the activity in the control. Brain ChE
activity did not show any significant inhibition (Zalewska et al.,
1977).
8.2.1.3 Rabbit
The progeny of rabbits, treated orally with 6 mg dichlorvos/kg body
weight per day for the last 10 days of pregnancy showed a decrease in
brain ChE activity and an increase in plasma ChE activity throughout
days 1 - 16 of life (Maslinska & Zalewska, 1978).
8.2.1.4 Cat
"Flea collar dermatitis" has been described in cats and dogs
wearing dichlorvos-impregnated PVC flea collars. In most cases, flea
collar dermatitis is a primary irritant contact dermatitis to
dichlorvos. The symptoms may consist of mild local irritation
(localized erythema and itching), severe local irritation varying from
erythematous alopecia to erosions, ulceration, oedema, and purulent
discharges with crusting or, in the most severe cases, generalized
dermatitis with secondary pyoderma and systemic illness (Muller,
1970).
Two trials with six and five cats, respectively, were carried out
to study the occurrence of contact dermatitis and systemic
intoxication. The cats received either a placebo collar or a
dichlorvos collar. Two cats received two dichlorvos collars each and
each cat was observed for 21 days. In a third trial, four groups of 25
cats were used to study hepatotoxicity. The cats of the different
groups were treated as follows. Group 1: placebo collar; Group 2:
placebo collar and intraperitoneal treatment with carbon tetrachloride
(CCl4) 7 days later; Group 3: dichlorvos collar; Group 4: dichlorvos
collar and intraperitoneal injection of CCl4 7 days later. Serum
glutamic pyruvic transaminase and ChE activity was estimated on day 9
of exposure. In a fourth study, 24 cats were fitted with a placebo
collar to test the effect of PVC in relation to the contact
dermatitis. PVC did not have an effect. No clear effect of the
CCl4-induced hepatic dystrophy on the detoxification of dichlorvos
was found.
The predominant systemic abnormalities recorded in these studies
were bone-marrow depression, ataxia, demyelination, and depression.
Red cell and plasma ChE activity was severely depressed. Furthermore,
contact dermatitis was seen in animals with dichlorvos collars.
Ambient temperature and relative humidity may have had a marked
influence on these effects, especially in the case of high temperature
and low humidity (Bell et al., 1975).
A 90-day study on 90 mongrel cats was carried out to verify the
results of Bell et al. (1975) by using a larger number of animals over
a longer period. There were three groups of 30 cats; one group with a
PVC collar (without dichlorvos), one group with one dichlorvos collar,
and the third group with three dichlorvos collars. In this study, in
which all relevant parameters were studied, no confirmation of the
abnormalities described by Bell et al. (1975) were found, even under
conditions of high temperature and low humidity. The only effect was
contact dermatitis, but, in this study, this was also found in the PVC
collars (Allen et al., 1978).
8.2.1.5 Dog
Groups of three male and three female dogs received equivalents of
approximately 0, 0.3, 1, 1.5, or 3 mg dichlorvos (93% in olive oil by
gelatin capsule)/kg body weight, daily, for 90 days. No effects were
observed on mortality, growth, liver and kidney function, organ
weights, haematology, or at gross and histopathological examination.
In the two highest dose groups, the dogs showed excitement, increased
activity, and aggression. Plasma and erythrocyte ChE activities
(measured initially and at intervals of approximately 2 weeks) were
normal in the lowest dose group (0.3 mg/kg body weight) but reduced in
the other dose groups. Brain ChE activity at termination was reduced
only in the highest dose group (Hine, 1962).
8.2.1.6 Pig
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, and 16 mg dichlorvos/kg
body weight per day in the feed (divided over two daily doses) for 30
days. Body weight gain, blood cell counts, packed-cell volumes,
haemoglobin concentrations, plasma glucose, plasma fatty acids, hepatic
and muscle glycogen concentrations, plasma and skeletal muscle
concentrations of electrolytes, and plasma and pancreatic insulin were
all comparable with those of control swine fed a blank PVC formulation.
Plasma and erythrocyte ChE activities were significantly inhibited in
the 4 and 16 mg/kg groups only (Stanton et al., 1979).
8.2.1.7 Cow
Two cows with suckling calves fed 200 mg dichlorvos/kg grain in
their rations showed normal ChE activity in the erythrocytes. However,
severe inhibition of ChE activity was found at 500 mg/kg feed
(equivalent to 4.5 mg/kg body weight), and a single dose of 27 mg/kg
body weight caused cholinergic collapse, followed by rapid recovery.
The ChE values in the calves remained normal throughout the 78-day
test. Milk from these two cows contained less than 0.08 mg
dichlorvos/litre (Tracy et al., 1960).
8.2.2 Dermal
8.2.2.1 Rat
In studies by Dikshith et al. (1976), groups of 48 male rats
received daily topical applications of 21.4 mg/kg body weight
dichlorvos (96%) in ethanol (control animals received ethanol alone) 5
days per week for 90 days. At intervals of 7, 15, 30, 45, 60, and 90
days, eight test rats and two control rats were sacrificed for
histopathological examination of the skin and testes. None of the
animals showed signs of intoxication, but ChE activity was not
measured. The skin showed no irritation, and no histopathological
changes were seen in the testes or skin.
8.2.2.2 Livestock
Spray and dip studies have shown that a concentration of 1%
dichlorvos is not toxic for cattle (no further details are available)
(Radeleff & Woodard, 1957).
The effect of applying different suspensions (different solvents
with and without an emulsifier) of dichlorvos to the skin of cows has
been studied. The dose level was 5 mg/kg body weight, increasing after
7 days to 10 mg/kg. Dichlorvos at 5 mg/kg body weight caused a
significant decrease in ChE activity in blood serum. The higher dose
level additionally produced symptoms of intoxication. No dichlorvos
residues were found in milk (Majewski et al., 1978).
Two heifers were washed daily with either a 1% aqueous solution or
emulsion of dichlorvos or a 10% suspension of dichlorvos in water
(daily application of 1.8 g dichlorvos for 21 days). Two additional
heifers were used as controls. The ChE activity levels of the
erythrocytes remained at the lower limits of normal variation (Tracy et
al., 1960).
8.2.3 Inhalation
8.2.3.1 Experimental animals
In a report by Sasinovich (1968), groups of rats were exposed
(whole body) daily for 4 h to average dichlorvos concentrations
of 0.11 or 1.1 mg/m3 for 4 months, to 5.2 mg/m3 for 2
months, or to 8.2 mg/m3 for 45 days. The highest dose caused signs
of intoxication; two of the eight rats died. Exposure to
5.2 mg/m3 or more resulted in marked inhibition of ChE activity and
disturbance of the blood-sugar curve. In the 0.11 and
1.1 mg/m3 groups, no significant effects were observed.
Mice, rats, and guinea-pigs, exposed 23 h per day for 28 consecu-
tive days to actual dichlorvos concentrations of 0.03 mg/m3, did not
show gross pathological changes at the end of the study. Inhibition of
plasma and brain ChE activity occurred in male mice, male guinea-pigs
(plasma only), and female rats (brain only) after a 5-day exposure to
0.14 - 0.15 mg/m3 dichlorvos (Brown et al., 1968).
Guinea-pigs (P strain) exposed for 7 h per day for 5 consecutive
days to an actual concentration of 90 - 120 mg/m3 dichlorvos suffered
no visible effects to their health. Rats (CFE) and mice (CF1)
similarly exposed to 50 mg/m3 were not visibly affected. At
concentrations above 50 mg/m3, the mice became distressed and
prolonged exposure to 80 mg/m3 was frequently lethal. Rats were less
severely affected (Stevenson & Blair, 1969).
In studies by Vashkov et al. (1966), 100 mice, 50 rats, 22 rabbits,
and 13 cats were exposed to a mixture containing dichlorvos, kerosene,
xylene, and freons during a single 2-h exposure or for 2 h per
day for 40 days. Three concentrations, 16.5, 45, and 160 mg
dichlorvos/m3, were tested (the median gravimetric diameter of the
aerosol particles was about 5 µm). The overall condition of the
animals remained normal, and no effects on body weight, clinical
chemical blood analyses, blood ChE activity, or respiration rate, or
pathological changes were observed. Rabbits developed transient miosis
which disappeared at the end of the exposure period.
Mice (20), guinea-pigs (6), sheep (7), calves (39), and a heifer
were exposed to an increasing dichlorvos concentration in the air
generated by dichlorvos strips (20%). The number of strips was
increased each week for 6 weeks, reaching 80 strips per 140 m3, and
was then reduced over the next 6 weeks. During exposure to the
recommended number of strips, the dichlorvos concentration in the
air was 0.09 - 0.14 mg/m3. The highest concentration was
2.1 mg/m3 when the number of strips was 16 times the recommended
number. No mortality or signs of intoxication were observed, and mice
bore normal litters during the study. The serum ChE activities of the
calves and of those handling the animals were within normal values
(Henriksson et al., 1971).
When 10 rabbits, 8 cats, and 10 dogs (males and females) were
continuously exposed for 8 weeks to dichlorvos concentrations of 0.05 -
0.3 mg/m3 generated from impregnated PVC-resin strips, no effects were
found on general health, behaviour, plasma and erythrocyte ChE
activities, or electroencephalographic patterns in the brain of the
animals (Walker et al., 1972).
In studies by Coulston & Griffin (1977), four male and four female
Rhesus monkeys were continuously exposed to dichlorvos vapour at an
average actual concentration of 0.05 mg/m3 for 3 months. The control
group consisted of four males and one female. No adverse effects were
observed in appearance, behaviour, or haematological and clinical
chemical determinations. Plasma ChE activity was slightly inhibited
(up to 28% inhibition), whilst the greatest erythrocyte ChE activity
inhibition was 36%. No changes in nerve maximum conduction velocities
or muscle-evoked action potentials were induced by the exposure to
dichlorvos.
8.2.3.2 Domestic animals
Five horses, exposed continuously to dichlorvos for 22 days in a
closed barn that was treated daily with 17 mg dichlorvos/m3, dis-
played mild inhibition of erythrocyte ChE activity after 7 days,
followed by recovery at 11 - 22 days. However, plasma ChE activity was
not changed. The concentration in the barn varied between 0.24 and
1.48 mg/m3 (Tracy et al., 1960).
The influence on ChE activity in cattle exposed to an impregnated
plastic strip containing 20% of dichlorvos has been studied. Red blood
cell ChE activity was measured during the 85 days of exposure, and on
the 9th day an average inhibition of about 35% was found. After the
37th day the inhibition in ChE activity gradually declined to 20%. No
clinical signs were observed (Horvath et al., 1968),
8.2.4 Studies on ChE activity
Groups of 10 young female Sherman rats were fed for 90 days on
diets containing 0, 5, 20, 50, 200, 500, or 1000 mg dichlorvos (90%)/kg
diet (equivalent to 0, 0.4, 1.5, 3.5, 14.2, 35.7, and 69.9 mg/kg body
weight, respectively). Data on the stability of dichlorvos in the diet
were not reported. No clinical signs of intoxication were noted.
Blood samples were taken from two rats of each group for ChE activity
determination on day 3, 11, 60, and 90. In all test groups, a
decrease in plasma ChE activity was observed during the first 4 days,
gradually returning to normal, except in the rats receiving 14.2 mg/kg
body weight or more. At doses above 3.5 mg/kg body weight, erythrocyte
ChE activities were decreased throughout the test, but at 3.5 mg/kg
body weight, this was only so for the first 30 days of the study.
Lower dose levels produced comparable results to those of the control
group (Durham et al., 1957).
Thirty-two Rhesus monkeys were treated with 20% pelleted PVC-resin
formulations of dichlorvos at dosages ranging from 5 to 80 mg/kg of
formulation (equivalent to 1 - 16 mg dichlorvos/kg body weight) daily
or 8 and 20 mg/kg of formulation twice daily for 10 - 21 consecutive
days. None of the monkeys developed overt signs of intoxication,
though they ate less food and had soft faeces. Plasma and erythrocyte
ChE activities were reduced by approximately 80% in all animals, and
remained inhibited until completion of treatment. Plasma ChE
activities returned to normal within approximately 3 weeks, whereas the
erythrocyte ChE activities required 50 - 60 days to return to pre-
treatment values (Hass et al., 1972).
When groups of five male and five female guinea-pigs (P strain)
were given daily applications of 0, 25, 50, or 100 mg dichlorvos
(94%)/kg body weight on the shorn skin for 8 days, all the animals
survived. A significant dose-dependent inhibition of both plasma and
erythrocyte ChE activities occurred in all test groups. Recovery of
plasma ChE activities was complete within one week of the last
exposure, and that of erythrocyte ChE activities was complete within
one week in the females and 2 weeks in the males (Brown & Roberts,
1966).
Three Cynomolgus monkeys were treated daily with dermal doses of
50, 75, and 100 mg/kg body weight technical dichlorvos in xylene for 5
days per week until the animals died (after 8, 10, and 4 doses,
respectively). Symptoms of intoxication appeared in all monkeys,
beginning 10 - 20 min after administration of the first dose. The
erythrocyte ChE activity decreased rapidly and remained severely
inhibited for the period of the study, while plasma ChE activities
fluctuated throughout the study (Durham et al., 1957).
When male and female mice, rats, and guinea-pigs were exposed
continuously by inhalation for 5 consecutive days to actual
concentrations of 0, 0.14 - 0.15, or 1.1 - 1.3 mg/m3 dichlorvos, no
overt signs of intoxication were observed. In the high exposure
groups, plasma, erythrocyte, and brain ChE activities were inhibited in
all three species. Plasma ChE was the most sensitive in all three
species, with up to 70% inhibition in the female mice. The greatest
inhibition of brain ChE activity (30%) was found in mice (Brown et al.,
1968).
The effects of dichlorvos vapour inhalation on AChE activity was
investigated in the rat. Exposure to dichlorvos concentrations of 0.8
and 1.8 mg/m3 for 3 days reduced AChE activity in the bronchial tissue
(50 - 60% of control) but did not produce any changes in blood AChE
activity. However, at 4.3 mg/m3, blood AChE activity also declined
(38% of control). In histochemical preparations, staining of the
bronchial glands and smooth muscles revealed reduced enzyme activity
even at the lowest dose (0.2 mg/m3) tested. At this concentration,
no inhibition of bronchial homogenate ChE was observed (Schmidt et al.,
1975, 1979). The significance of bronchial ChE inhibition is not
clear.
Male mice were continuously exposed to actual concentrations of 0
or 0.03 mg dichlorvos/m3 for 28 consecutive days. Weekly assays of
ChE activities showed that brain ChE activity was slightly inhibited
(less than 20%) on the 28th day only, but plasma and erythrocyte ChE
activities were not significantly inhibited (Brown et al., 1968).
Rabbits exposed to an average concentration of 1 mg
dichlorvos/m3 (0.8 - 1.3 mg/m3) for 4 h per day for 4 months showed
up to 30% inhibition of serum and erythrocyte ChE activities during the
study. However, cats similarly exposed did not show significant
inhibition (Sasinovich, 1968).
Rats and monkeys were exposed for 2 weeks in an inhalation chamber
sprayed once with an emulsion of dichlorvos. The initial air
concentration was 6 mg dichlorvos/m3, decreasing over a few days to
about 0.1 - 0.2 mg/m3. No signs of intoxication were seen. The
plasma and erythrocyte ChE activities of the monkeys decreased to
approximately 50% of pre-exposure levels, but rapid recovery took place
after cessation of exposure. Comparable results were obtained when
rats and monkeys were continuously exposed for up to 7 days to a
maximum air concentration of 2.2 mg/m3. However, continuous exposure
for 4 days to 0.27 mg/m3 did not produce any effect on ChE activity
(Durham et al., 1957).
Groups of two monkeys were exposed to dichlorvos vapour 2 h per day
for 4 consecutive days. With concentrations up to 0.7 mg/m3, no
change in plasma or erythrocyte ChE activity was noted, while 1.2 -
3.3 mg/m3 caused a slight decrease in plasma ChE activity, and 7.5 -
18 mg/m3 (mean: 13 mg/m3) resulted in miosis and a pronounced
inhibition (40 - 70%) of plasma and erythrocyte ChE activities (Witter
et al., 1961).
Groups of 10 chickens were exposed to dichlorvos vapour from a
varying number of strips (20% dichlorvos) continually or intermittently
for 3 weeks. Exposure to a single strip in a room of 33 m3 (either
interrupted or for 16 h every day) produced no significant effect on
plasma or brain ChE activity. Exposure to more than one strip (2 - 5)
resulted in up to 50% inhibition of plasma ChE activity and
approximately 40% inhibition of brain ChE activity. Dichlorvos aerosol
sprays (0.7% dichlorvos) showed that daily excessive spraying for 6
seconds, 8 times per day, for 5 days, did not produce significant ChE
inhibition. However, a significant decrease in plasma ChE activity was
found when the study lasted for 21 days (Rauws & van Logten, 1973).
Three studies were carried out to determine the effect on the
laying performance of hens given dichlorvos in the feed for 4 weeks.
Plasma AChE levels were reduced by 70% at 20, 30, or 40 mg
dichlorvos/kg diet, although there was no clear influence on food
consumption, egg production, egg weight, or hatchability at these
doses. At 80 mg/kg diet, a decrease in food consumption and in egg
production was seen, the latter being a consequence, possibly, of the
former (Pym et al., 1984).
8.3 Skin and Eye Irritation; Sensitization
In the skin sensitization assay procedure of Stevens (flank/flank
technique), 1% dichlorvos in olive oil produced no visible effects in
male albino guinea-pigs. Negative results were also obtained when five
components of formulated dichlorvos/ PVC products were assayed for
their skin sensitization potential (Kodama, 1968).
A primary skin irritation test on dichlorvos was performed by using
male New Zealand white rabbits. Irritation observed on the skin after
the application of 5 - 20% water solutions of dichlorvos was relatively
severe compared with that caused by other organophosphorus insecticides
(Arimatsu et al., 1977).
In order to study the allergenicity of dichlorvos, the guinea-pig
maximization test was used. Threshold limit values of primary
irritancy tested on the skin of guinea-pigs (Hartley strain) were 2% or
more. In the maximization test, 0.05 and 0.5% were used. With 0.5%,
35% of the animals showed slight or discrete erythema. The
allergenicity rating as determined by the Kligman test was moderate. In
combination with methidathion, dichlorvos showed a stronger reaction,
indicating cross-sensitization (Fujita, 1985).
8.4 Long-term Exposure
8.4.1 Oral
8.4.1.1 Rat
In studies by Witherup et al. (1967, 1971), groups of 40 male and
40 female weanling CD rats were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg
diet for 2 years. Five males and five females from each group were
sacrificed after 6, 12, and 18 months, and analysis of diet samples
showed a considerable loss of dichlorvos. This was associated with a
gradual increase in DCA concentration which ranged in the different
groups from 0.014 to 28.6 mg/kg diet. The average actual
concentrations of dichlorvos in each diet were 0, 0.047, 0.467, 4.67,
46.7, and 234 mg/kg diet (equivalent to approximately 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). There were no signs of
intoxication, and no effects were seen on behaviour, mortality rate,
food consumption, weight gain, organ weights, haematology, or
urinalysis. Plasma and erythrocyte ChE activities were decreased
throughout the study in the two highest dose groups compared with
controls, but brain ChE activity was decreased in the highest dose
group only. Histological examination of major organs revealed
hepatocellular fatty vacuolization in the group with 234 mg/kg and in
some of the animals with 46.7 mg/kg diet. No effect was seen on serum
total proteins or albumin:globulin ratio, or on hexobarbital sleeping
time. It can be concluded that the actual average concentration of
4.7 mg/kg diet (equivalent to approximately 0.25 mg/kg body weight) was
without significant effect on any of the measured parameters. The
tumour incidence was comparable with that of the control group.
8.4.1.2 Dog
Groups of three male and three female beagle dogs were fed diets
containing 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg diet for 2
years, the average actual concentrations being 0, 0.09, 0.32, 3.2, 32,
and 256 mg dichlorvos/kg diet (equivalent to 0, 0.002, 0.008, 0.08,
0.8, and 6.4 mg/kg body weight). The average DCA concentration in the
diets with 10, 100, and 500 mg dichlorvos/kg diet was 0.6, 6.4, and 20
mg/kg diet. No effects were seen on general appearance, survival, food
consumption, weight gain, haematology, or urinalysis. However,
erythrocyte ChE was inhibited at dose levels of 3.2 mg/kg diet or more,
and plasma ChE activity was inhibited at the two highest dose levels.
Recovery to control values took place at the end of the feeding period.
Brain ChE activity, measured at the end of the study, was similar to
that of the controls, and liver weights were increased in both sexes in
the 256 mg/kg diet group. Histological examination of major organs
revealed slight dose-related alterations in the hepatic cells of one
female in the 3.2 mg/kg diet group, and greater alterations at higher
doses. No differences were seen in serum alkaline phosphatase, trans-
aminase activities, total serum proteins, or albumin:globulin ratios.
The actual average concentration of 0.32 mg/kg diet (equivalent to
0.008 mg/kg body weight) was without effect (Jolley et al., 1967;
Witherup et al., 1971).
8.4.2 Inhalation
8.4.2.1 Rat
When groups of 50 male and 50 female weanling CFE rats were
exposed for 23 h per day to air concentrations of 0, 0.05, 0.5, or
5 mg dichlorvos (97%)/m3 air (actual concentrations: 0, 0.05, 0.48,
and 4.7 mg/m3) for 2 years, body weight gain was reduced in the
two highest dose groups. After 2 years of exposure, plasma and
erythrocyte ChE activities were significantly reduced in the two
highest dose groups, but in the case of brain ChE activity, only in the
highest group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry values,
or organ weights, or upon gross or microscopic examination.
Ultrastructural examinations of bronchi and alveoli of rats exposed to
0 or 5 mg/m3 showed no differences between the two groups.
In connection with a reported correlation between brain ACh
concentration and the inhibition of brain ChE activity following acute
exposure to organophosphorus compounds, the brain tissue of three
female rats per group was examined for ACh and choline concentrations.
The dose level of 0.05 mg dichlorvos/m3 was without effect on any of
the measured parameters (Blair et al., 1976). It should be noted that
in this study the rats were not only exposed by inhalation but also via
their food, drinking-water, and by grooming. This resulted in
additional oral ingestion of dichlorvos (Stevenson & Blair, 1977).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
8.5.1 Reproduction
In a 3-generation reproduction study, weanling CD rats (six groups
of 30 animals) were fed dichlorvos (93%) at nominal concentrations of
0, 0.1, 1, 10, 100, or 500 mg/kg diet, prepared freshly each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1.1), it was
assumed that the average concentration of dichlorvos was approximately
47% of the nominal concentrations (equivalent to 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). No effects on fertility,
number and size of litters, body weight, or viability of the pups were
found. Gross and histopathological examination of 7-day-old pups from
F1a and F2a litters did not reveal any abnormalities (Witherup et
al., 1965, 1971).
Oral treatment of rats with 5.6 mg/kg body weight and rabbits with
6 mg/kg body weight during the last trimester of pregnancy had no
effect on offspring weight and development. However, the cerebral
cortices from the 1-day-old rabbits were less mature than those of
control rabbits, probably due to maternal toxicity (Dambska et al.,
1978, 1979).
Dambska & Maslinska (1982) observed impairment of the development
of the brain of rabbits dosed orally with 9 mg/kg body weight per day
from days 5 to 16 of life, the period of myelination.
8.5.1.1 Effects on testes
In studies by Krause & Homolo (1972, 1974), three groups of 14 male
NMRI/Han mice received either a single oral dose of 40 mg dichlorvos/kg
body weight, daily oral doses of 10 mg dichlorvos (in olive oil)/kg for
18 consecutive days, or daily oral doses of 0.5 ml olive oil for 18
days, respectively. On days 9, 18, 27, 36, 54, and 63, two animals
from each group were killed and their testes examined histologically.
Severe disturbances of spermatogenesis were observed in both test
groups; damaged seminiferous tubules were also seen and the supporting
Sertoli cells were damaged. In addition, there was an increase in the
number and hypertrophy of the Leydig cells. No explanation could be
offered for these effects.
A similar study was carried out on three groups of 16 male juvenile
Wistar rats. The rats received either 20 mg dichlorvos (in olive
oil)/kg body weight on days 4 and 5, 10 mg dichlorvos (in olive oil)/kg
daily from days 4 to 23, or 0.1 ml olive oil daily from days 4 to 23 of
life. On days 6, 12, 18, 26, 34, and 50 of life, two rats from each
group were sacrificed. Histological examination of the testes showed
slight reduction in the number of the spermatogenic cells and Leydig
cells. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells. All the changes were
reversed by the 50th day (Krause et al., 1976; Xing-Shu, 1983).
In order to examine the cause of the observed effect on the
spermatogenic and Leydig cells, the study was repeated with, in
addition, measurement of testosterone levels in serum and testes. The
testosterone concentrations in the testes, and leutinizing hormone (LH)
and follicle stimulating hormone (FSH) levels in serum were similar in
the presence or absence of dichlorvos (Krause, 1977). However, the use
of adult rats and a different dosing regimen prevented a strict
comparison with the earlier study by Krause et al. (1976).
In studies by Fujita et al. (1977), 55 male Wistar rats (aged 5
months) were orally administered dichlorvos at levels of 5 or 10 mg/kg
body weight every other day for 8 weeks. The rats were divided into
five groups, and one group of rats was killed every 4 weeks to study
the changes in several organs, including the testes. About 200
individual seminiferous tubules were examined in each rat. No change
was seen in body weight gain or testes weight. The score values of the
seminal cellular system decreased after 4 - 8 weeks of treatment, but
were restored 8 weeks after the end of treatment.
8.5.1.2 Effect on estrous cycle
Timmons et al. (1975) reported studies on female rats which were
exposed to an atmosphere containing 2.4 mg dichlorvos/m3 generated
from a dichlorvos strip placed on top of each cage. Exposure was
continuous from the birth of the first litter until the estrous cycle
began again. Controls were housed in a separate room. A significant
mean delay of 10 days in the onset of the estrous cycle, compared with
that of controls, was observed. However, the significance of the
results was complicated by different housing conditions.
8.5.1.3 Domestic animals
In studies by Bazer et al. (1969), sows were fed dichlorvos (9% in
resin pellets) at the level of 800 mg per animal per day beginning 21
days before breeding and continuing through gestation. No significant
differences in the numbers born alive or dead, the litter weight,
number weaned, or individual weaning weights were observed.
In a further study, dichlorvos was added to the rations of pregnant
sows at the level of 0 or 800 mg per animal per day. Resin pellets
containing 9% dichlorvos were fed either from 3 weeks before breeding
and throughout gestation, or from 18 to 56 days before parturition.
Data were collected from a total of 681 dams, representing eight
replicates over a period of 2 years. The dichlorvos-treated groups
scored consistently higher for individual farrowing weights, litter
farrowing weights, number weaned, individual weaning weights, and
litter weaning weights, and less consistently, for the percentage of
live births (Batte et al., 1969).
When dichlorvos (as a PVC-resin formulation) was administered to
sows in doses ranging from 4 to 13 mg/kg body weight per day for the
last 21 - 30 days of gestation, the average farrowing interval for the
live-born piglets was shorter in treated than in control animals.
There was also a dose-related increase in the mean birth weight of
live-born piglets from the dichlorvos-treated sows, while the incidence
of still births was lower than in controls (Bunding et al., 1972).
In studies by Collins et al. (1971), male and female swine were fed
for up to 3 years on diets containing a PVC-resin formulation at 0,
200, 250, 288, 400, or 500 mg dichlorvos/kg diet, and for at least 6
months prior to initial breeding. Two generations were raised, and no
effects were observed on number or size of litters, survival or growth
rate of offspring, urinalysis, haematology, hepatic and renal function,
physical structure, or calcium and phosphorus content of the femoral
bone, or in appearance during gross and microscopic examination. Organ
weights were normal except in the case of the liver, which was
generally increased. Whole blood ChE activity was inhibited and brain
ChE activity was slightly reduced, but no clinical evidence of
neurophysiological impairment was observed.
In studies reported by Stanton et al. (1979), pregnant sows were
given PVC-resin formulations of dichlorvos (10%) in the diet at a daily
dose of 0, 5, or 25 mg dichlorvos/kg body weight (divided between two
doses per day) during the last one-third of pregnancy (or 30 days).
Only about 50% of the total dichlorvos was released from this resin in
the gastrointestinal tract. All pigs were born alive, and their birth
weight was similar to that of control animals. In the group given
25 mg/kg body weight, plasma and erythrocyte ChE activities were
markedly inhibited (80 and 90%, respectively) in the sows, but not in
the newborn pigs. No changes in the packed cell volume or haemoglobin
concentration of the sows or their piglets were observed.
In a limited test reported by Darrow (1973), dichlorvos-impregnated
collars did not have any adverse effects on pregnant female goats or
later on their newborn young. Also, blood ChE activity was not
inhibited in either the nannies or the kids over a period of several
weeks. The acceptance of treated kids by the mothers was normal.
A pregnant non-lactating Holstein-Friesian cow was fed a nominal
concentration of 6.2 mg dichlorvos/kg body weight per day in the daily
ration from days 152 to 286 of pregnancy. A normal calf was delivered
(Macklin & Ribelin, 1971).
A herd of 54 dairy cows (two-thirds carrying calves) was accidently
sprayed with dichlorvos, resulting in a dosage of 50 mg dichlorvos/kg
body weight. All the animals showed symptoms of intoxication and some
had convulsions. However, they all recovered within a few hours, with
no abortions or other adverse effects except a temporary diminution in
milk production (Knapp & Graden, 1964).
When three pregnant sows were fed 8.5 mg dichlorvos/kg body weight
per day (as PVC-resin formulation) from days 41 to 70 of pregnancy, the
blood ChE activity of the sows was markedly inhibited, but no
demonstrable teratogenic effects or functional abnormalities in the
piglets were found (Wrathall et al., 1980).
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
In studies by Schwetz et al. (1979), CF1 mice and New Zealand
rabbits were given maximum tolerated doses of dichlorvos (96%) in corn
oil by gavage, at levels of 60 and 5 mg/kg body weight, respectively,
from days 6 to 15 and days 6 to 18 of gestation, respectively. Except
for an increased number of resorptions in rabbits, no significant
effect was observed on the mean number of live fetuses per litter, or
on fetal body measurements. There were no gross visceral or skeletal
alterations. In mice, no abnormalities were found.
Carson (1969) reported studies on a total of 168 New Zealand
rabbits, divided into 10 groups containing 15 - 26 animals. One group
received lactose capsules, three groups different PVC-placebo capsules,
and three groups PVC capsules containing 18, 54, or 93 mg
dichlorvos/animal. These capsules were provided twice daily, so that
the equivalent daily intake was 12, 36, or 62 mg/kg body weight,
respectively. The rabbits received 12 or 36 mg dichlorvos/kg body
weight on days 6 - 18 of gestation, or 62 mg/kg body weight on days 6 -
11 of gestation. Fetuses were obtained by Caesarian section. Maternal
mortality was increased in the highest dichlorvos group, and the
incidences of in utero and neonatal toxicity were also increased (but
not significantly) in the 62 mg group, compared with those in the
control groups. The fetal mortality was not clearly indicative of any
marked toxic effects other than those involving the dam, since whole
litters were not involved. Extensive skeletal and soft tissue
examinations were carried out on all viable neonates, but no adverse
effects on bone formation, articulation, or degree of ossification were
found in the dichlorvos groups. No teratogenic effects were observed.
When pregnant rabbits were given oral doses of PVC-resin
formulations during the critical days of organogenesis, doses of 34 mg
dichlorvos/kg body weight or more were found to cause maternal
toxicity. With doses of 12 mg/kg body weight or less, no significant
effect on nidation, in utero survival, or neonatal survival was found.
No evidence of teratogenic changes was observed on gross, visceral, or
skeletal examination of the fetuses (Vogin et al., 1971).
The effect of dichlorvos on embryonal and fetal development in
thyroparathyroidectomized, thyroxine-treated, and euthyroid control
rats has been investigated. On days 8 - 15 of gestation, 25 mg
dichlorvos/kg body weight per day was administered orally to pregnant
Charles River rats, and a slight decrease in fetal weight in all groups
was observed. No gross, visceral, or skeletal anomalies of the fetuses
were found as a result of dichlorvos administration to rats with
altered thryoid status (Baksi, 1978).
8.5.2.2 Inhalation
In studies by Thorpe et al. (1972), rats of E strain and Dutch
rabbits were exposed (23 h per day, 7 days per week) throughout
pregnancy to nominal concentrations of 0, 0.25, 1.25, or 6.25 mg
dichlorvos/m3. In an additional study, groups of 20 pregnant
rabbits were exposed to nominal concentrations of 2 or 4 mg
dichlorvos/m3. Maternal deaths occurred in the rabbits exposed to
2 mg/m3 or more, and ChE activities in plasma, erythrocytes, and brain
were markedly inhibited in both species exposed to 1.25 mg/m3 or
more. There was no indication of any dichlorvos-related teratogenic
effects.
When CF1 mice and New Zealand rabbits were exposed to dichlorvos at
an average actual concentration of 4 mg/m3 for 7 h per day from days
6 to 15 and from days 6 to 18 of gestation, respectively, no
significant effect on the mean number of live fetuses per litter, the
incidence or distribution of resorptions, or on fetal body measurements
was noted. No gross, visceral, or skeletal alterations were observed
(Schwetz et al., 1979).
8.5.2.3 Intraperitoneal
Kimbrough & Gaines (1968) reported a study on female Sherman rats
given a single intraperitoneal injection of 0 or 15 mg dichlorvos (in
peanut oil)/kg body weight on day 11 of pregnancy. The treated dams
showed toxic signs and weight loss. On day 20, the fetuses were
removed. There was no adverse effect on litter size, resorptions,
number of dead fetuses per litter, or average weight of fetuses, but 3
out of 41 fetuses had omphaloceles. This latter finding, observed at a
maternally toxic dose, is not in agreement with the other teratology
studies or the 3-generation reproduction study.
8.5.3 Résumé of reproduction, embryotoxicity, and teratogenicity studies
A 3-generation study on rats, fed dichlorvos at dose levels of up
to 500 mg/kg diet, did not show any effects on fertility, number and
size of litters, body weight, or viability of the pups.
In studies on mice and rats, high dose levels of dichlorvos (a
single dose of 40 mg/kg body weight or multiple doses of 5 or 10 mg/kg
body weight) induced disturbances in spermatogenesis, characterized by
damage to the seminiferous tubules and Sertoli cells and by hypertrophy
and increase in the number of Leydig cells. It was assumed, but not
confirmed, that testosterone synthesis was partially inhibited. After
dichlorvos treatment ceased, recovery was complete within about 2
months.
A number of reproduction studies on domestic animals, mainly sows,
have been carried out. Levels of 500 mg/kg diet for 3 years had no
effect on fertility. Inhibition of ChE activity in the sows, but not
in the newborn pigs, was induced by 25 mg/kg body weight. No
teratogenic effects were seen.
Teratogenicity studies on rats and rabbits, orally administered
62 mg/kg body weight during gestation, revealed symptoms of
intoxication and significant inhibition of ChE in the parent rabbits.
Except for an increased number of resorptions, no significant effect on
the mean number of live fetuses per litter or evidence of a teratogenic
effect was noted during gross, visceral, or skeletal examination.
There were no teratogenic effects after rats inhaled 6.25 mg
dichlorvos/m3 during gestation, though maternal deaths occurred. Mice
and rabbits exposed to 4 mg/m3 on days 6 - 18 of gestation did not
show any effects.
8.6 Mutagenicity and Related End-Points
8.6.1 Methylating reactivity
In a quantitative colour test for alkylating agents, dichlorvos
gave a positive response, whereas the metabolites desmethyldichlorvos,
dimethylphosphate, dichloroethanol, DCA, and dichloroacetic acid all
gave negative results (Bedford & Robinson, 1972).
8.6.1.1 In vitro studies
Lawley et al. (1974) have shown that methylation by dichlorvos of
DNA and RNA, using either isolated nucleic acids, Escherichia
coli cells, or human tumour HeLa cells, broadly resembled that by
methylmethanesulfonate (MMS) rather than methylation by
N-methyl- N-nitrosourea. In E. coli cells, 3-methyladenine, the
principal minor product in methylated DNA apart from 7-methylguanine,
was not detected, whereas it was present when pre-isolated DNA was
methylated. The overall extent of methylation achieved in cells was
small. Specific excision of 3-methyladenine was indicated in E.
coli cells (Lawley et al., 1974).
Labelled 7-methylguanine was present in both DNA and RNA isolated
from E. coli exposed to 3H-dichlorvos. The methylating capability of
dichlorvos was less, by a factor of 10 - 100, than that of strongly
genotoxic methylating compounds (Wennerberg & Löfroth, 1974).
Alkylation by dichlorvos of calf thymus DNA, resulting in the formation
of N-7-methylguanine, was reported by Löfroth (1970).
Shooter (1975) has applied the test for chain breaks in RNA to the
interaction of dichlorvos with bacteriophage R17. Breaks in the RNA
chain result from the hydrolysis of phosphotriesters and are thus a
measure of the extent of O-alkylation and of the SN1-type mechanism
of the reaction. The results so far suggest that the existence of O-
alkylation, as shown by degradation following phosphotriester
formation, does correlate with mutagenicity. Incubation of
bacteriophage R17 with 0 - 100 mmol dichlorvos/litre for 90 h did not
result in methylation of the phosphate group of the RNA to any
significant extent.
8.6.1.2 In vivo studies
Reviews of the literature on alkylating agents including dichlorvos
have been made by Bedford & Robinson (1972), Lohs et al. (1976), and
Hemminki (1983).
In studies in which mice were given intraperitoneal injections
of methyl-14C-dichlorvos (1.9 µmol/kg body weight), the degree
of alkylation of guanine- N-7 in DNA isolated from soft tissues
amounted to 8 x 10-13 mol methyl/g DNA. From this, a rate of
clearance of approximately 1.4 min was estimated (Segerbäck, 1981;
Segerbäck & Ehrenberg, 1981). DNA and RNA from the total soft tissues
of male CFE rats exposed to atmospheres containing 0.064 mg methyl-
14C-dichlorvos/m3 for 12 h did not show methylation of the N-7 atom
of guanine moieties. The exposure period constituted a significant
fraction of the half-life of the 7-methylguanine moieties in DNA. It
was concluded that dichlorvos does not pose a methylating hazard to
mammalian DNA in vivo (Wooder et al., 1977; Wooder & Wright, 1981).
The excretion of labelled 7-methyl guanine in the urine
by NMRI mice and R rats injected intraperitoneally with methyl-
14C-dichlorvos, or exposed by inhalation for 2 h (mice only), was
reported by Löfroth & Wennerberg (1974) and Wennerberg & Löfroth
(1974). In rat urine, labelled 3-methyladenine and 1-methyl-
nicotinamide were also detected (Löfroth & Wennerberg, 1974).
According to the authors, these results demonstrate the dichlorvos-
induced methylation of guanine and adenine moieties in nucleic acids.
However, the administration of radiolabelled adenine and guanine to
otherwise untreated rats gave rise to the excretion of radiolabelled
methylated purines in the urine. Therefore, the detection of
radiolabelled purines, per se, in the urine of animals exposed to
methyl-labelled methylating agents, does not constitute evidence for
the methylation of the purine moieties of nucleosides or nucleic acids
by methylating agents (Wooder et al., 1978). Moreover, the results of
metabolic studies have demonstrated the existence of a natural
biosynthetic pathway whereby the methyl carbon atoms of dichlorvos can,
with partial retention of hydrogen, become incorporated into the
heterocyclic rings and the methyl groups of urinary 7-methylguanine
after entering 1-C pools, in vivo. The results of preliminary studies
suggest the existence of a similar pathway for the production of
urinary 3-methyladenine (Wright et al., 1979).
Wooder & Creedy (1979) described a study on rats which investigated
the DNA-damaging potential of dichlorvos when administered as a single
intraperitoneal dose. Alkaline sucrose gradient profiles of rat liver
DNA showed that whereas MMS (a positive control) shifted the DNA
profile, dichlorvos at 10 mg/kg body weight (the maximum dose
consistent with survival) had no effect.
8.6.1.3 Discussion of methylating reactivity
Many alkylation tests and in vivo and in vitro mutagenicity studies
have been carried out with dichlorvos. It has been demonstrated that
dichlorvos has alkylating properties, and has been suggested from some
studies that the in vivo alkylating potential of dichlorvos is similar
to that of some known mutagens. However, this concern is misplaced
since alternative reactions were not considered (WHO, 1986b). The
phosphorus atom is markedly more electron-deficient and susceptible to
attack by nucleophiles than the alkyl carbon atom. Analysis by Bedford
& Robinson (1972) of the data of Löfroth et al. (1969) revealed that
the proposed rates of alkylation by potent nucleophiles were probably
combined rates of phosphorylation and alkylation, and that
phosphorylation was the totally dominant reaction in the case of the
hydroxide ion. The comparison with known mutagens is therefore
inappropriate. Two factors detract further from the toxicological
significance of the alkylation studies. The first is that mammalian
tissues (plasma, liver, etc.) contain active A-esterase enzymes, which
catalyse the phosphorylation of water by the organophosphorus esters.
Viewed inversely, these A-esterases catalyse the hydrolysis of the
organophosphorus esters, thereby rapidly reducing circulating levels of
hazardous material. Secondly, the comparative rate of reaction of most
of these esters with AChE is many orders of magnitude greater than the
rate of alkylation by the typical nucleophile 4-nitrobenzyl-pyridine:
for dichlorvos, the ratio of rates was 1 x 107 in favour of the
inhibitory phosphorylation of AChE (Aldridge & Johnson, 1977). It
follows that, at low exposure levels, in vivo phosphorylation of AChE
and other esterases will be the dominant reaction, with negligible
uncatalysed alkylation of nucleic acid. Indeed, no such alkylation has
been detected in sensitive in vivo studies designed to check this point
(Wooder et al., 1977).
8.6.2. Mutagenicity
Reviews of the existing literature have been published by Wild
(1975), Fishbein (1976, 1981, 1982), Leonard (1976), IARC (1979),
Sternberg (1979), Ramel et al. (1980), Lafontaine et al. (1981), Ramel
(1981), and Wildemauwe et al. (1983).
8.6.2.1 In vitro studies
(a) Microorganisms
Numerous mutagenicity studies using bacteria and fungi as test
organisms have been carried out (Table 17). In most of the studies,
only one dichlorvos concentration, often a high one, was tested,
sometimes resulting in low survival of the test organism. A
dose-response relationship was established in the few tests where a
range of concentrations was used. The results indicate that dichlorvos
induces base substitutions in bacteria and mitotic gene conversion in
yeast. The alkylating properties of dichlorvos (section 8.6.1) are
most probably the cause of the mutagenic action. This was the
conclusion of Bridges et al. (1973) from tests using both MMS and
dichlorvos with E. coli strains deficient at four different repair
loci.
In Aspergillus nidulans, dichlorvos has been found to induce point
mutations to 8-azaguanine resistance and a high frequency of mitotic
crossing-over and non-disjunction (Aulicino et al., 1976; Bignami et
al., 1976, 1977; Morpurgo et al., 1979). No mutagenic activity
using A. nidulans was found after incubating Nicotiana alata cell
cultures with dichlorvos for 21 days (simulating in vitro plant
metabolism). This confirms the rapid metabolism of dichlorvos (Benigni
et al., 1979).
The mutagenicity of dichlorvos has been extensively studied in
Japan, (Kawachi et al., 1980). Dichlorvos induced gene mutation
in Salmonella typhimurium TA100 as well as E. coli strains in the
absence of rat-liver S9 mix.
Dichlorvos (0.5 - 2 mg/ml) causes random strand breakage,
repairable by DNA polymerase I, in E. coli pol A as detected by the
alkaline sucrose sedimentation method. When pol+ bacteria and high
concentrations of dichlorvos (0.2 - 0.4%) were used, an all-or-none
breakdown of DNA molecules to fragments of very low relative molecular
mass occurred which correlated well with lethality. It has been
suggested that the major DNA damage resulting from dichlorvos treatment
arises indirectly through alkylation of other cellular constituents,
this leading to uncontrolled nuclease attack on DNA. However,
discontinuities in newly-synthesized DNA and mutagenesis following
dichlorvos treatment presumably result from direct alkylation of DNA
(Bridges et al., 1973; Green et al., 1973, 1974a,b). In the
standard E. coli DNA polymerase-deficient assay system, dichlorvos
(6.4 mmol/litre) gave a positive result (Rosenkranz, 1973; Rosenkranz &
Leifer, 1980). In measuring mutation to tryphophan independence in E.
coli strain WP2, it was found that 5 mg dichlorvos/litre was mutagenic
in this test system (Green et al., 1976). Griffin & Hall (1978) have
found that dichlorvos (1 mg/ml) causes breaks in colicinogenic plasmid
E1 DNA from E. coli. In a rec-type repair test with Proteus
mirabilis strains PG 713 (rec-hcr-) and PG 273 (wild type),
dichlorvos (10 or 40 µmol per plate) induced base-pair substitutions
and other DNA damage. In the same test, desmethyldichlorvos, at the
same concentrations, gave negative results (Adler et al., 1976; Braun
et al., 1982).
(b) Mammalian cells
In cultured V79 Chinese hamster cells, no induction of 8-
azaguanine-resistant mutations after treatment with up to 1 mmol/litre
dichlorvos (Wild, 1975), or of ouabain-resistant mutations after
treatment with 1.25 - 5 mmol/litre dichlorvos, was found (Aquilina et
al., 1984).
DNA strand breakage in cultured V79 Chinese hamster cells caused by
up to 0.2% v/v dichlorvos has been reported by Green et al. (1974a).
Dichlorvos (1 µl) decreased the sedimentation coefficient of calf
thymus DNA upon thermal denaturation, indicating a decrease in the DNA
molecular size (Rosenkranz & Rosenkranz, 1972). Incubation of
dichlorvos (45 mmol/litre) with calf thymus DNA did not result in
changes in thermal melting curves or DNA fractionation on a
hydroxyapatite column. However, changes were observed by means of
differential pulse polarography, indicating that single-stranded
segments and thermolabile regions were formed in the DNA. This
behaviour could perhaps be a consequence of guanine alkylation followed
by depurination and chain cutting at elevated temperatures (Olinski et
al., 1980).
The resistance of methylated DNA in Chinese hamster ovary cells,
labelled with 14C-thymidine and methyl-3H-1-methionine, to
micrococcal nuclease digestion was abolished when the cells were
treated with dichlorvos (10 mmol/litre) for 3 h. No effects were
observed on kinetics of total DNA digestion. These results indicate a
conformational change in chromatin induced by dichlorvos (Nishio &
Uyeki, 1982).
Table 17. Mutagenicity tests on microorganisms
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Bacillus subtilis
H17 rec+ 0.02 ml of plate none - Shirasu et al.
M45 rec- 10% solution plate none + (1976)
Citrobacter freundii
425 0.1% fluctuation none weak + Voogd et al. (1972)
0.05% none -
Enterobacter aerogenes
6 0.1% fluctuation none weak + Voogd et al. (1972)
Escherichia coli
B 5 - 25 mmol/litre liquid induc- none weak + Wild (1973)
1- to 10-h tion strepto- (dose-
exposure mycin-resistant and exposure-
mutants time related)
B/r WP2 22.6 mmol/litre plate reversion none + Moriya et al.
S9 mix + (1978)
L-cysteine +
CM 561, 571, 611 5 µl plate reversion none - Hanna & Dyer
(1975)
K12HfrH 0.1% fluctuation none weak + Voogd et al.
(1972)
K12(5-MT) 3.3 x 10-4 plate none + Mohn (1973)
mol/litre
WP2 micro drop plate none - Dean (1972a)
analytical and
technical grade,
aqueous solution
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Escherichia coli (contd).
WP2 try- approximately plate none + Ashwood-Smith et
20 mm2 dichlor- al. (1972)
vos strip
WP2 try- 20 - 25 µl of spot none + Nagy et al. (1975)
hcr- and hcr+ 50% emulsifiable back mutation
concentrate
0.1 ml of a 5% plate reversion none + Shirasu et al.
solution (1976)
WP2 hcr unknown (up to plate reversion none + Moriya et al.
5000 µg/plate) (1983)
WP2 uvrA, ca 5 µl plate reversion none + Hanna & Dyer
WP 67 (1975)
Klebsiella pneumoniae 0.1%; 0.05% fluctuation none weak + Voogd et al.
(1972)
Neurospora crassa
ad-3 exposed to air plate none - Michalek &
containing Brockman (1969)
dichlorvos
(strip)
Pseudomonas aeruginosa
PAO 38 0.08 mol/litre liquid reversion none + Dyer & Hanna
(1973)
Saccharomyces cerevisiae
D4 (ade2 and 4 mg/ml plate mitotic none + Dean et al.
trp5) 2 mg/ml gene conversion (1972)
D4 (ade2 and 19 mmol liquid mitotic none + Fahrig (1973,
trp5) gene conversion 1974)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium
64-320 0.05%; 0.1% suspension none + Voogd et al. (1972)
TA 98 20 or 40 mmol plate S9 male mice - Braun et al. (1982)
up to 5000 µg plate reversion none - Moriya et al. (1983)
TA 100 20 or 40 mmol plate S9 male mice (+) Braun et al. (1982)
low survival
up to 5000 µg plate reversion none + Moriya et al. (1983)
TA 1530 5 µl plate none + Hanna & Dyer (1975)
TA 1535 5 µl plate none + Hanna & Dyer (1975)
0.1 ml of a 5% plate none + Shirasu et al. (1976)
solution
0.1 ml of a 5% plate none + Moriya et al. (1978)
solution S9 mix -
L-cysteine -
2800 µg plate (spot) S9 male rat - Carere et al.
(1976, 1978a,b)
1.5 mg/ml liquid none + Carere et al.
(1978a,b)
20 or 40 mmol plate S9 male mice - Braun et al. (1982)
TA 1536, 1537, 0.1 ml of a 5% plate none - Shirasu et al.
1538 solution (1976)
TA 1536, 1537, 2800 µg plate (spot) S9 male rat - Carere et al.
1538 (1976, 1978a,b)
20 or 40 µg plate S9 male mice - Braun et al.
(1982)
up to 5000 µg plate none - Moriya et al.
(1983)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium (contd).
his C117 0.03 mol liquid none + Dyer & Hanna (1973)
LT2 his C117, 5 µl plate none - Hanna & Dyer (1975)
his G 46
Schizosaccharomyces pombe
ade6 1.5 - 14 mmol plate + + Gilot-Delhalle et
al. (1983)
Serratia marcescens
Hy alpha 13, 25 mg/ml plate (spot) none alpha 13 + Dean (1972a)
alpha 21 alpha 21 -
50, 100 mg/ml plate (spot) none + Dean (1972a)
saturated plate (spot) none + Dean (1972a)
aqueous solution
Streptomyces coelicolor
A 3(2) his Al 5600 µg spot none + Carere et al.
(1976, 1978a,b)
---------------------------------------------------------------------------------------------------------
Dichlorvos (0.03 and 0.1 mmol/litre) has been found to induce
sister chromatid exchanges (SCEs) in cultures of Chinese hamster ovary
cells (Nishio & Uyeki, 1981). On the other hand, in Chinese hamster
V79 cells (clone number 15), SCEs were not induced by 0.1 mmol
dichlorvos/litre, but only by 0.2 and 0.5 mmol/litre solutions. The
number of polyploid cells was increased at both 0.1 and 5 mmol/litre
(Tezuka et al., 1980).
Dichlorvos has been tested in two independent laboratories for its
ability to increase the transformation of Syrian hamster embryo cells
by simian adenovirus SA7. Pre-treatment of hamster cells with
dichlorvos at concentrations of 0.05 up to 0.45 mmol/litre produced a
significant enhancement of SA7 transformation at 0.11 mmol/litre and
higher (Hatch et al., 1986).
The mouse peripheral blood lymphocyte (PB) culture system has also
been used to test for SCE induction. Male B6C3F1 mice were injected
intraperitoneally with 5, 15, 25, or 35 mg dichlorvos/kg body weight,
but there was no change in the baseline SCE frequency (Kligerman et
al., 1985).
Dichlorvos (6.5 - 650 mmol/litre) has been found to induce dose-
dependent unscheduled DNA synthesis in the human epithelial-like cell
line EUE (Benigni & Dogliotti, 1980a,b; Aquilina et al., 1984). Both
scheduled and unscheduled DNA synthesis of human lymphocytes showed
dose-related inhibition by dichlorvos (5 -500 mg/litre), as measured by
3H-thymidine uptake (Perocco & Fini, 1980).
Dichlorvos (0.0001 - 0.1 mmol/litre) does not induce DNA repair in
human kidney T-cells. This was shown by a lack of dichlorvos-induced
3H-thymidine incorporation into T-cells in the G1- or G2-phase of the
cell cycle. No induction of single-strand breaks in the T-cell DNA, as
measured by alkaline sucrose gradients, was found following treatment
for 1, 2, or 4 h with 0.0001 - 1 mmol dichlorvos/litre (Bootsma et al.,
1971).
No clear effect on the frequency of SCEs in human lymphocytes or
human fetal lung fibroblasts was found after exposure to 2.5 - 10 mg
dichlorvos/litre for 72 h (Nicholas et al., 1978).
In studies by Dean (1972b), human blood samples were treated with
dichlorvos (0.0001 - 1 mmol/litre) for 1, 2, 4, and 20 h, after which
the lymphocytes were stimulated to divide using phyto-haemagglutinin.
The toxic effect of 1 mmol dichlorvos/litre was indicated by a
decreased mitotic index. Mitotic cells were analysed for chromosome
aberrations, but the number of dicentrics in dichlorvos-treated cells
was no different from that found in untreated cells (Bootsma et al.,
1971). When dichlorvos (1 - 40 mg/litre) was added at specific
intervals to cultures of human lymphocytes, cytotoxicity was found at
5 mg/litre or more, but no chromosome aberrations (chromatid gaps or
breaks) were detected (Dean, 1972b).
Negative results in cultured human lymphocytes were also reported
by Fahrig (1974) and Wild (1975).
8.6.2.2 In vivo studies
(a) Drosophila melanogaster
Negative results were obtained in the standard Muller-5 test for
sex-linked lethal mutations and in the induced crossing-over test with
the approximate LD50 concentration (0.035% dichlorvos) (Jayasuriya &
Ratnayake, 1973). Similarly, 0.0006 - 0.6 µmol dichlorvos gave
negative results in the standard Muller-5 test (Sobels & Todd, 1979).
In studies by Hanna & Dyer (1975), a Drosophila melanogaster
population was continuously exposed to gradually increasing
concentrations of dichlorvos (because of development of increased
resistance) in the food medium (up to 0.75 mg/kg food) for about 18
months. At the end of this period, an increased accumulation of
mutations was observed.
Gupta & Singh (1974) reported studies where female D.
melanogaster flies were kept on food with 1 - 50 mg dichlorvos (Nuvan
100 EC)/kg. No eggs were laid at 10 mg/kg or more, and at 1 mg/kg,
survival of the eggs was 45% of that of the controls. Salivary gland
chromosome abnormalities were observed in fully-grown larvae fed
1 mg/kg. However, Kramers & Knaap (1978), using the same route of
administration, found no induction of sex-linked recessive lethals by
dichlorvos (0.009, 0.048, and 0.09 mg/kg food).
(b) Host-mediated assays
Dichlorvos was not mutagenic in host-mediated assays in NMRI mice
using:
(i) S. typhimurium (G46 his-) and Serratia marcescens (a 21
leu-) after an intraperitoneal injection of 25 mg
dichlorvos/kg body weight (Buselmaier et al., 1972, 1973);
(ii) S. typhimurium (64-320) after an oral dose of 0.2 mg per
animal (equivalent to 8 - 10 mg/kg body weight) (Voogd et
al., 1972); or
(iii) Saccharomyces cerevisae (D4 ade2 and trp5 loci) after an
oral dose of 50 or 100 mg/kg body weight (or after exposure
of CF1 mice for 5 h to 60 or 90 mg dichlorvos/m3) (Dean et
al., 1972).
Although dichlorvos was mutagenic to S. cerevisae in in
vitro studies, these in vivo studies proved to be negative.
(c) Dominant lethal assays
Negative results were reported in a test for dominant lethal
mutations in ICR/Ha mice (expressed as an increase in early fetal
deaths or, indirectly, by pre-implantation losses), after a single
intraperitoneal injection of 13 or 16.5 mg/kg body weight or five
consecutive daily oral doses of 5 or 10 mg/kg body weight. The total
mating period was 8 weeks (Epstein et al., 1972). The same result was
obtained after exposure of male CF1 mice to 30 or 55 mg/m3 for 16 h or
to 2.1 or 5.8 mg/m3 for 23 h daily for 4 weeks (Dean & Thorpe,
1972b).
A statistically significant increase in the frequency of pre-
implantation losses in mice (Q strain) in the second week and fifth
week of mating has been observed after a single intraperitoneal
injection of 10 mg/kg body weight (Degraeve et al., 1980; Moutschen-
Dahmen et al., 1981).
In studies by Degraeve et al. (1982, 1984a), male Q strain mice
received drinking-water with 2 mg dichlorvos/litre (equivalent to
0.32 mg/kg body weight per day) 5 days per week, for 7 consecutive
weeks. At the end of this period, the males were mated for 1 week with
untreated virgin females, and the pregnant females were killed 14 days
after the start of pregnancy. No dominant lethal mutations were
induced. The same result was obtained when female CF1 mice were either
given single oral doses of 0, 25, or 50 mg dichlorvos/kg body weight or
continuously exposed to atmospheres containing 0, 2, or 8 mg
dichlorvos/m3 from weaning until 11 weeks of age. They were either
mated at the end of the dosing or inhalation period or at intervals of
5, 10, and 15 days thereafter (Dean & Blair, 1976).
(d) Chromosome abnormalities
Male Q strain mice receiving drinking-water containing 2 mg
dichlorvos/litre (equivalent to 0.32 mg/kg body weight), 5 days per
week for 7 weeks, did not show chromosome damage in bone marrow cells,
spermatogonia, or primary spermatocytes (Moutschen-Dahmen et al., 1981;
Degraeve et al., 1982, 1984a); neither did mice of the same strain
given a single intraperitoneal injection with 10 mg/kg body weight
(Moutschen-Dahmen et al., 1981; Degraeve et al., 1984b).
In a micronucleus test, Swiss mice were given daily intra-
peritoneal injections of dichlorvos (0.0075 - 0.015 mg/kg body weight
per day) for 2 days and killed 6 h after the last dose. No induction
of aberrations in the structure or number of chromosomes in bone marrow
cells was observed (Paik & Lee, 1977).
CF1 mice exposed to concentrations of 64 - 72 mg
dichlorvos/m3 for 16 h or to 5 mg/m3 per day, 23 h per day, for 21
days, did not show chromosome abnormalities in bone marrow or
spermatocytes. Similar results for Chinese hamsters exposed by the
inhalation route to 28 -36 mg/m3 for 16 h (males only), or by a single
oral dose of 15 mg/kg body weight (males) or 10 mg/kg body weight
(females), have been reported (Dean & Thorpe, 1972a).
Dichlorvos has been tested for its ability to induce in vivo
chromosomal aberrations in Syrian hamsters bone marrow cells. Four
dose levels (3, 6, 15, and 30 mg/kg body weight) were given
intraperitoneally. Statistically significant increases in the number
of cells with aberrant chromosomes (mainly breaks and gaps) were
observed (Dzwonkowska & Hübner, 1986).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1 Mouse
Carcinogenicity studies were carried out on two groups of 50 male
and 50 female B6C3F1 mice fed 1000 and 2000 mg dichlorvos (94%) in corn
oil/kg diet for 80 weeks. Due to severe signs of intoxication, doses
were lowered after 2 weeks to 300 and 600 mg/kg for the remaining 78
weeks. Samples of the diets analysed during the study showed
dichlorvos contents within 5% of the intended concentrations. Matched
controls consisted of 10 mice of each sex; the pooled controls
consisted of 100 male and 80 female mice. All surviving mice were
killed at 92 - 94 weeks. Hair loss and rough hair coats were noted in
many treated animals, particularly in the male groups, beginning at
week 20 and persisting throughout the study. The average body weights
of the high-dose mice of both sexes were slightly decreased compared
with controls. The low-dose female group showed the poorest survival;
74% of the animals lived to 90 weeks. There was no significant
increase in the incidence of tumours attributable to dichlorvos in
either sex, i.e., dichlorvos was not demonstrated to be carcinogenic
(NCI, 1977; Weisburger, 1982).
In other studies, groups of 50 male and 50 female B6C3F1 mice, 6
weeks of age, were given drinking-water with 0, 400, or 800 mg
dichlorvos/litre ad libitum. The drinking-water solutions were renewed
daily. All surviving animals were killed during week 102. A dose-
dependent inhibition of body weight increase was observed throughout
the study in both sexes at both dichlorvos concentrations. There was
no adverse effect on mortality. The survival rates at week 102 were
62%, 66%, and 84% in males (in controls, low-, and high-dose groups,
respectively), and 66%, 50%, and 80% in females. The corresponding
figures for tumour incidence were 22.4%, 39.1%, and 23.4% in males and
29.3%, 16.2%, and 9.1% in females. The main tumours that were found
were lung adenomas and tumours in the liver, spleen, thymus, and
salivary gland. These tumours occurred in all three groups. There was
no statistically significant difference in the incidence of tumours at
any site in any group (Konishi et al., 1981).
Preliminary results are available from a recently completed 2-year
mouse carcinogenicity study using dichlorvos. Groups of 50 male and 50
female B6C3Fl mice were given dichlorvos (dissolved in corn oil) by
oral gavage daily for 2 years. Dose levels for male mice were 0, 10,
or 20 mg/kg body weight per day and for female mice 0, 20, or 40 mg/kg.
There were no statistically significant differences in survival rates
between the treated and control male mice, and the same applied to
female mice. A statistically significant increase in the incidence of
forestomach squamous cell papillomas was observed in the female mice
receiving 40 mg/kg per day. Two forestomach squamous cell carcinomas
were also seen in this group, but none were observed in the controls or
the 20 mg/kg group. Considerably fewer forestomach squamous cell
papillomas were observed in male mice. Nevertheless, a non-significant
increase was observed in the 20 mg/kg per day group. No forestomach
squamous cell carcinomas were noted in any male group. Forestomach
hyperplasia occurred relatively frequently in both control and treated
male and female mice, the incidence being similar in all groups (NTP,
1987).
8.7.1.2 Rat
In studies reported by NCI (1977) and Weisburger (1982), two groups
each of 50 male and 50 female Osborne-Mendel rats were fed either 150
or 1000 mg dichlorvos (94%) in corn oil/kg diet for 80 weeks. Due to
the severe signs of intoxication, the 1000 mg/kg dose was reduced to
300 mg/kg diet after 3 weeks for the remaining 77 weeks. The diets
were stored under conditions designed to minimize loss of dichlorvos,
and the animals received fresh diets daily. Matched controls comprised
10 rats of each sex; the pooled controls consisted of 60 rats of each
sex. The surviving rats were killed after 110 weeks. The average body
weights of the rats receiving the high dose level were slightly
decreased compared with controls. There was no significant increase in
the incidence and type of tumours in either sex as a result of
dichlorvos treatment.
Enomoto et al. (1981) carried out studies on groups of 50 male and
50 female Fisher 344 rats, 6 weeks of age, given drinking-water
(renewed daily) containing 0, 140, or 280 mg dichlorvos/litre ad
libitum. All surviving animals were killed in week 108 (104 weeks of
exposure followed by a 4-week recovery). Slight inhibition of body
weight increase was observed in males in the high-dose group, but there
was no influence on mortality. The survival rates in week 108 were
82%, 75%, and 75% in males and 86%, 71%, and 82% in females,
respectively, in control, low-dose, and high-dose groups. A great
number of organs and tissues were selected for microscopy. The organs
and tissues of all animals which died or which were killed in moribund
conditions were microscopically examined, as were all tumours and
macroscopical lesions. A number of animals with no specific changes
were also examined. The overall tumour incidences were 100%, 96%, and
98% in males and 37%, 31%, and 33% in females, respectively, in
control, low-dose, and high-dose groups. High incidences of
interstitial cell tumours of the testes in males (49/51, 41/48, and
47/48, respectively) were observed in all three groups. Mononuclear
cell leukaemia was found in all groups at 4 - 12%. There was no
statistically significant difference in tumour incidence at any site in
any group.
Preliminary results are available from a recently completed 2-year
rat carcinogenicity study using dichlorvos. Dichlorvos, dissolved in
corn oil, was administered each day by oral gavage to groups of 50 male
and 50 female Fischer 344 rats at dose levels of 0, 4, or 8 mg/kg body
weight per day. Though survival rates were slightly decreased in the
treated male and female groups compared with those of their respective
controls groups, the differences were not statistically significant.
However, a statistically-significant and dose-related increase in the
incidence of pancreatic adenomas was observed in the male rats fed 4 or
8 mg/kg. In female rats fed 8 mg/kg, a non-significant increase in
pancreatic adenomas was observed. There was a relatively high
incidence of pancreatic hyperplasia and atrophy in all groups of male
rats, including the controls, and a lower incidence in all female
groups. A statistically significant (but not dose-related) increased
incidence of mononuclear leukaemia was observed in the male rats fed 4
or 8 mg/kg. A similar incidence was observed in all female groups,
including the control group. Since this is a common and variable
systemic lesion in Fischer-344 rats, the toxicological significance of
this finding is uncertain. It is possible that its incidence in the
male control group was unusually low. An increased incidence of
mammary gland fibroadenomas (not dose related) was observed in the
treated female rats, but these were not considered to be of
toxicological concern. In addition, mammary gland hyperplasia was
frequently observed in all control and treated female groups at similar
incidences (NTP, 1986).
As reported in section 8.4.1.1, Witherup (1967, 1971) found no
increase in tumour incidence following dichlorvos treatment of rats.
8.7.2. Inhalation
8.7.2.1 Rat
In the studies by Blair et al. (1976) described in section 8.4.2.1,
no dose-related increase in tumour incidence was found.
8.7.3. Appraisal of carcinogenicity
The majority of the mouse and rat studies using dichlorvos are
considered to be negative regarding carcinogenic potential (Witherup et
al., 1967, 1971; Blair et al., 1976; NCI, 1977; Enomoto et al., 1981;
Konishi et al., 1981; Weisburger, 1982). However, preliminary
information from two recent studies on the mouse and rat, respectively,
has provided equivocal evidence of carcinogenicity (NTP, 1986)a.
------------------------------------------------------------------------
a The NTP Peer Review Panel reviewed these studies and came to the
following conclusions:
"Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenic activity of dichlorvos for male F344/N rats, as
shown by increased incidences of adenomas of the exocrine pancreas and
mononuclear cell leukemia. There was equivocal evidence of carcinogenic
activity of dichlorvos for female F344/N rats, as shown by increased
incidence of adenomas of the exocrine pancreas and mammary gland
fibroadenomas. There was some evidence of carcinogenic activity of
dichlorvos for male B6C3F1 mice and clear evidence for female B6C3F1 mice,
as shown by increased incidences of forestomach squamous cell papillomas"
(NTP, 1988).
The increased incidence of forestomach tumours in the mouse study
is likely to be related to the route of administration (oral gavage),
which has been shown in other mouse studies to induce forestomach
tumours due to repeated and direct irritation of the gastric mucosa.
If so, ingestion of food by human beings could not result in such
direct effects on the stomach wall. Furthermore, human beings do not
possess a stomach wall comparable with the forestomach of the mouse,
except perhaps for the oesophagus. However, the transient passage of
food through the oesophagus would probably not allow sufficient time
for the carcinogenic event to occur.
Similarly, the pancreatic adenomas observed in the NTP rat study
may be related to the corn oil used in the study. Evidence from other
rat studies in which corn oil was used as a vehicle suggests that this
is a possibility. On the other hand, it does not explain why a higher
incidence of pancreatic tumours was observed in the treated animals
compared with the controls. Although increased incidences of
mononuclear leukaemia were observed in treated male rats, the
incidence in the control group of this common and variable tumour may
have been low. The evidence for carcinogenicity in these two recent
studies is difficult to interpret at present. When complete and final
reports of these studies become available, more definitive conclusions
may be drawn.
8.8. Mechanisms of Toxicity; Mode of Action
A full description of the mechanism of action can be found in
Environmental Health Criteria 63: Organophosphorus Insecticides - A
General Introduction (WHO, 1986b).
Dichlorvos directly inhibits AChE activity in the nervous system
and other tissues. This reaction takes place in three steps:
(a) reversible binding of dichlorvos to the enzyme;
(b) reaction with the enzyme to form a dimethylphospho-enzyme
derivative, with the loss of DCA; and
(c) "aging" of the phospho-enzyme compound to a more stable
methylphospho-enzyme derivative.
Reaction (a) is rapid, but reversible. Reaction (b) is also rapid,
but the phosphorylated enzyme can be returned to its native
state spontaneously only by hydrolysis at very slow rates or by agents
such as N-methyl 2-pyridinium aldoxime (2-PAM) at higher rates.
Reaction (c) is comparatively slow (t´ = about 2.5 h at 37 °C and pH
7.4), but the product has the same stability as that of erythrocytes
(t´ = about 120 days) (Gillett et al., 1972).
Death due to poisoning is caused by excessive cholinergic effects
such as bronchospasms, hypersecretion from cholinergic innervated
glands (especially critical in lungs and bronchi), and cardiac
disturbances caused by vagotonus and anoxia. Convulsions and paralysis
in skeletal musculature are caused by brain anoxia as well as
cholinergic effects within the central nervous system.
In vivo studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos, at various time
intervals, after dosing are summarized in Table 18. In general, the
maximum inhibition occurred within 1 h, and was followed by rapid
recovery. Dogs seemed to be more sensitive than rats, and showed a
slower recovery.
Dichlorvos, when infused intravenously (into the ear vein) of
adult male rabbits, produced dose- and time-related inhibition of whole
blood ChE activity during infusion. Spontaneous but incomplete
recovery to 60 - 80% of the normal activity occurred within 60 -
90 min of infusion (Shellenberger et al., 1965; Gough & Shellenberger,
1970, 1977-78; Shellenberger, 1980).
In a study on the influence of temperature on ChE activity, rats
were injected intraperitoneally with a single dose of 6.25 mg
dichlorvos (95%)/kg and kept at either 28 °C or 5 °C. The maximum
inhibition of whole blood ChE activity (40%) occurred after 0.5 h.
The animals at 5 °C showed less inhibition and a faster recovery of
whole blood ChE activity than those at 28 °C (Chattopadhyay et al.,
1982).
8.9. Neurotoxicity
8.9.1. Delayed neurotoxicity
A detailed description of the neurotoxic potential of
organophosphorus compounds can be found in Environmental Health
Criteria 63: Organophosphorus Insecticides - A General Introduction
(WHO, 1986b).
Several studies have shown that dichlorvos does not produce delayed
neurotoxicity in pre-medicated hens, whether it is administered orally
or subcutaneously (Durham et al., 1956; Aldridge & Barnes, 1966;
Johnson, 1969, 1975a,b, 1978, 1981; Aldridge & Johnson, 1971; Lotti &
Johnson, 1978). The inhibition of brain neurotoxic esterase (NTE)
without signs of ataxia has been observed (Aldridge & Johnson, 1971;
Johnson, 1978).
Caroldi & Lotti (1981) reported mild signs of ataxia in pre-
medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg body weight) and severe inhibition of NTE in peripheral
nerve, spinal cord, and brain. However, Johnson (1978) did not observe
ataxia in pre-medicated hens given the same dose in the same way.
These hens showed severe inhibition of brain NTE but far less
inhibition of spinal cord NTE. It appears that ataxia arises from the
inhibition of spinal cord NTE. When the dose was repeated 1 - 3 days
after the first dose, spinal cord NTE inhibition increased and the hens
became ataxic.
White leghorn hens have been used in a 90-day study on the
neurotoxic potential of dichlorvos (99.9%) after dermal or oral
administration. For oral administration, a 10 - 20% solution of
dichlorvos in corn oil in gelatin capsules was used whereas, dermally,
1 - 20% emulsifiable concentrates in technical grade xylene (containing
2% Triton X-100) were used. Dichlorvos at doses greater than 1 mg/kg
body weight per day (dermal) or 6 mg/kg (oral) led to cholinergic
symptoms including salivation, convulsions, and death after 2 - 3 days.
With oral doses of 3 - 6 mg/kg no ataxia or death was observed.
Dermally, dichlorvos was very toxic for hens. Dose levels of about 1.7
and 3.3 mg/kg for an average period of 37 days caused ataxia and death.
An average dermal dose level of 0.65 mg/kg body weight for 90 days did
not induce ataxia or death. Typical symptoms of organophosphorus-
ester-induced delayed neurotoxicity (OPIDN) were not observed (Francis
et al., 1985).
In summary, it is possible to produce clinical neuropathy in hens,
but the doses required are far in excess of the LD50. The effects
are associated with severe inhibition of NTE in brain and spinal cord,
measured shortly after dosing (Johnson, 1981).
8.9.2. Mechanism of neurotoxicity
Male rats given a lethal intraperitoneal injection of 40 mg
dichlorvos/kg body weight did not show electrocortical disturbances.
Deaths resulted from respiratory failure (Hyde et al., 1978).
In studies by Desi (1983), adult CFY rats were given daily oral
doses ranging from 1.25 to 4 mg dichlorvos/kg body weight mixed in the
diet for 3 months. Plasma, erythrocyte, and brain ChE activities were
comparable with those of control rats. Increased EEG activity and
enhanced central excitability were found in the male rats only.
No changes in reflex motor unit potential activity or in nerve
conduction velocity were noted in dogs 7 days after single oral doses
of 30, 59.5, or 148 mg dichlorvos/kg body weight. However, erythrocyte
ChE activity was inhibited at all doses, and the highest dose produced
signs of intoxication (Hazelwood et al., 1979).
A single oral dose of 40 mg dichlorvos/kg or repeated doses of
1.6 mg/kg body weight per day did not cause histological abnormalities
in the brain of adult Wistar rats. Repeated administration of 50% of
the LD50 (i.e., 40 mg/kg body weight per day for 10 - 21 days), caused
myelin pallor and micro-vacuolation of the white matter. It seems
likely that primary degeneration of axons and secondary myelin sheath
abnormalities caused the spongy tissue loosening observed under the
electron microscope (Zelman, 1977; Zelman & Majdecki, 1979).
In studies by Ali et al. (1979a) and Hasan et al. (1979), male rats
were given 3 mg dichlorvos/kg per day intraperitoneally for 10 days.
Following perfusion-fixation, sections of cerebellum and spinal cord
were studied with the electron microscope. An abnormal increase in the
number of mitochondria in the spinal cord was found. Myelin
degeneration was detected in the spinal cord and myelin figures were
occasionally noted within oedematous dendrite profiles.
Table 18. Time-related ChE activity in animals after administration of a single dose of dichlorvos
---------------------------------------------------------------------------------------------------------
Species/sex Route Dose (mg/kg Time Cholinesterase activity (%) Reference
body weight) plasma erythrocyte brain
---------------------------------------------------------------------------------------------------------
Mouse (male) intraperitoneal 30 15 min 37 Cohen & Ehrich
2 h 40 (1976)
5 h 69
18 h 92
Mouse (male) intraperitoneal 10 15 min 30 Nordgren et al.
60 min 50 (1978)
2 h 80
Rat (male) oral 50 15 min 30 20 10-15 Modak et al.
3 h 30 65 (1975)
24 h 60 75 60-70
Rat (male) oral 40 1 h 30 Teichert et al.
(1976)
Rat (male) oral 40 5 min 55 Pachecka et al.
15 min 20 (1977)
2 h 30
24 h 75
48 h 100
Rat (male) intravenous 2.5 30 min 40 15 Reiner & Plestina
90 min 60 35 (1979)
3 h 90 55
12 h 100 80
48 h 90
Dog (beagle) oral 50 2 h 32 63 Ward & Glicksberg
(sex not 24 h 63 74 (1971)
specified) 5 days 92 78
21 days 100 92
Dog oral 22 1 h 12 17 Snow & Watson
(greyhounds 3 h 24 34 (1973)
and crossbred) 24 h 70 65
72 h 95 65
---------------------------------------------------------------------------------------------------------
Another study of dichlorvos neurotoxicity involved the
investigation of lipid peroxidation. This entails the direct reaction
between oxygen and lipids to form free-radical intermediates and semi-
stable peroxides. Major cellular components, such as membranes and
subcellular organelles, are damaged by these free radicals. Hasan &
Ali (1980) found a dose-dependent increase in the rate of lipid
peroxidation in various regions of the brain of the rat after
intraperitoneal administration of dichlorvos (at concentrations ranging
from 0.6 to 3 mg/kg body weight, daily) for 10 days. Also, there was
an increased incidence of lipofuscin-like pigment in the Purkinje cells
of the cerebellar cortex.
Maslinska et al. (1984) found that dichlorvos (dose levels of 4 -
8 mg/kg body weight for 10 days) affected the phospholipid-protein
balance in the brain of rabbits. The animals were exposed during the
postnatal "critical" life period, which constitutes a turning point in
the development of the brain. At this time, the neurons have already
undergone considerable arborization, and myelination and
vascularization are expanding rapidly. In addition, the overall oxygen
consumption is reaching its steepest rate of increase. In the myelin
sheaths under formation, several phospholipids are deposited. The
authors found changes in the phospholipid-protein ratio which
correlated well with the observed delay in myelin sheath formation.
Ultrastructural changes in certain subcellular organelles may be
connected with the change in this ratio, since it is crucial to the
structural and functional properties of the membranes and enzymes bound
to them.
Dambska et al. (1984) have studied the influence of dichlorvos on
blood vessel walls, the perivascular area, and the permeability of the
blood-brain barrier in young rabbits. The young animals received 9 mg
dichlorvos/kg body weight for 16 days starting on the 6th day of life.
There was a decrease in ChE activity in brain capillary walls.
Electron microscopic studies showed lesions of the perivascular
astrocytes and changes in the endothelial cells. However, these
lesions did not disturb the blood-brain barrier mechanism for
horseradish peroxidase particles.
Studies on the central cholinergic system have revealed that the
inhibition of brain ChE activity and its subsequent recovery were
uniform in all brain regions studied in orally dosed (50 mg/kg) rats.
ACh concentrations were increased in brain areas within 15 min of
treatment. A biphasic effect was observed on choline metabolism in the
brain. The cortex was more cholinergic than the striatum in terms of
percentage increase in ACh and choline (Modak et al., 1975). In a
similar study on rats, either receiving a single oral dose (40 mg/kg)
or repeated oral doses (4 mg/kg), the activity of whole brain choline
acetyltransferase and the contents of ACh and choline in whole brain
were not altered, though brain ChE activity was markedly inhibited.
However, in the cerebral hemispheres, and especially the corpus
striatum, the ACh level was considerably increased, without a
concomitant change in choline (Teichert et al., 1976).
Kobayashi et al. (1980, 1986) investigated the concentration of
total, free, labile-bound and stable-bound ACh in the brain of rats
given single or multiple subcutaneous injections of dichlorvos (0.2 -
4 mg/kg body weight). The results suggest that alterations in the
mobilization and storage of ACh in the central cholinergic nerves may
be induced. The time course for ACh accumulation was measured in rat
brain regions after intravenous treatment with 15 mg dichlorvos/kg body
weight (Stavinoha et al., 1976). The striatum had the highest rate of
accumulation and the cerebellum the lowest. The calculated turnover
time for the different regions of the brain was between 0.9 and
5.6 min.
In studies by Ali & Hasan (1977) and Ali et al. (1979b, 1980), rats
were given intraperitoneally 3 mg dichlorvos/kg body weight per day for
10 or 15 days. The concentrations of dopamine, norepinephrine, and 5-
hydroxytryptamine (5-HT) were significantly decreased in different
parts of the brain, and 5-HT was significantly increased in the spinal
cord.
A single dose or short-term (12 weeks) treatment of rats with high
concentrations of dichlorvos, which produced brain ChE inhibition,
resulted in decreased norepinephrine levels in the brain (Brzezinski &
Wysocka-Paruszewska, 1980). From these studies, it was suggested that
the metabolism of catecholamines and 5-HT may be disturbed by
dichlorvos.
8.10. Other Studies
Many studies in different organ systems have been carried out. In
most of these studies, the route of administration was the oral or
intraperitoneal route. Single or repeated dosing was used, mainly with
high dose levels, in mice and rats. The influence on brain enzymes
(other than brain ChE), liver enzymes such as ChE (inhibition),
microsomal cytochrome P-450 activity (decrease), drug-metabolizing
enzyme activity, and UDP-glucuronyl transferase (no influence), and
many other enzyme systems were studied.
Furthermore, the influence of dichlorvos on adrenal steroidogenesis
has been investigated (Civen et al., 1980).
8.10.1. Immunosuppressive action
A dose-related suppression of the humoral immune response induced
by S. typhimurium was observed in rabbits orally administered 0.3 -
2.5 mg dichlorvos (93%)/kg body weight in capsules, 5 times a week for
6 weeks (Desi et al., 1978).
In a comparable study on rabbits, the cellular immune response was
estimated using the tuberculin skin test. The skin redness in the
tuberculin test and the serum antibody titres of treated animals showed
a dose-dependent decrease compared with those of controls (Desi et al.,
1980).
8.11. Factors Modifying Toxicity; Toxicity of Metabolites
8.11.1. Factors modifying toxicity
In studies on the effect of diet on the toxicity of dichlorvos,
young male rats were kept for 30 days on the following synthetic diets:
high protein (HPD), low protein (LPD), high fat (HFD), and standard
(SD). Growth rates were normal except for a slightly decreased body
weight gain in the HFD group. The composition of the diet, per se, did
not significantly affect plasma and erythrocyte ChE activity 24 h or 5
days after dosing. A single intraperitoneal injection of 50 mg
dichlorvos/kg body weight led to higher mortality in LPD rats (as
expected with a protein-deficient diet) and lower mortality in HPD
rats, compared with SD animals (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or HPD
for 30 days. At the end of this period, a single intraperitoneal dose
of dichlorvos (20 or 30 mg/kg body weight) was administered. Results
showed that diets, per se, did not affect initial plasma or erythrocyte
ChE activity, nor did the HPD or HFD diets protect against mortality
from dichlorvos. In the case of the HPD, the spontaneous recovery of
ChE activity was reduced in the plasma and erythrocytes of dichlorvos-
treated rats. However, with an HFD, this recovery was significantly
increased (Purshottam & Srivastava, 1984).
Costa & Murphy (1984) studied the interaction between acetaminophen
(which, like dichlorvos, is detoxified by glutathione transferase) and
dichlorvos (10 mg/kg body weight) in mice. Acetaminophen (600 mg/kg
body weight) pre-treatment did not have any affect on dichlorvos
toxicity. On the other hand, intraperitoneal pre-treatment with
diethylmaleate (1 ml/kg body weight) increased the acute toxicity of
dichlorvos.
8.11.2. Toxicity of metabolites
8.11.2.1 Acute toxicity
The toxicity of metabolites of dichlorvos in female mice, injected
intraperitoneally, is considerably less than that of dichlorvos
(Table 19).
Mice (male and female) survived a single exposure of 130 mg
DCA/m3 for 5 - 7 h (Stevenson & Blair, 1969).
8.11.2.2 Short-term exposures
Groups of 20 male and 20 female rats were exposed for 30 days to
actual concentrations of 0 or 0.5 - 1 mg DCA/m3. Half the animals
were then killed promptly and the remainder on day 35. After 30 days
of exposure, the male rats showed a slight decrease in body weight and
food intake, and a slight increase in absolute and relative liver
weight. There were no such changes in the rats left for 5 more days
unexposed to DCA. Histological examination of the lungs of exposed
males and females revealed a higher incidence of minor inflammatory
changes than in controls. No other changes attributable to DCA were
found in general health, behaviour, haematology, clinical chemistry,
organ weights, gross pathology, or histopathology. A similar study
using groups of 10 male and 10 female rats and actual concentrations of
0 or approximately 2 mg/m3 DCA revealed no abnormalities attributable
to DCA (Wilson & Dix, 1973).
Table 19. Intraperitoneal LD50 values of metabolites of dichlorvos in
female micea
-----------------------------------------------------------------------------
Compound Vehicle LD50 (mg/kg body weight)
-----------------------------------------------------------------------------
desmethyldichlorvos, water 1500
sodium salt
dichloroacetyaldehyde corn oil 440
(DCA)
dichloroethanol corn oil 890
dichloroacetic acid corn oil or water 250
sodium dichloroacetate water 3000
methyl and dimethyl water 1500
phosphoric acid mixture
sodium methyl- and water 3000
dimethylphosphate
mixture
-----------------------------------------------------------------------------
a From: Casida et al. (1962).
8.11.2.3 Long-term exposure
No long-term tests have been carried out with DCA as such.
However, in the 2-year oral studies on rats and dogs with dichlorvos
(section 8.4.1), the degradation product DCA was present in the test
diets in increasing amounts, so that possible effects of DCA were
included in the results of these studies.
8.11.2.4 Mutagenicity
DCA appears to be mutagenic in the Salmonella test using S.
typhimurium TA100. The mutagenicity decreased in the presence of a
microsomal activation system. Part of the decrease was dependent on
the presence of the co-factors NADP and glucose-6-phosphate. No
evidence for mutagenicity with 2,2-dichloroethanol was obtained in
this S. typhimurium strain (Löfroth, 1978).
In a dominant lethal assay, a single intraperitoneal injection of
176 mg DCA/kg in male mice (AB Jena-Halle strain) produced a decrease
in the number of total implants and live fetuses in the first 3 weeks
of the test, and an increase in post-implantation losses. The same
test was repeated with a strain of mice (DBA) less sensitive to
mutagenic effects. The same effects were found, but to a lesser
extent, and mainly in the fourth week (Fischer et al., 1977).
8.11.2.5 Metabolism
When 32P-dimethylphosphate (500 mg/kg in water) was administered
orally to a male rat, the autopsy (90 h after treatment)
indicated that almost the entire dose had been eliminated. The
urine, containing only unmetabolized dimethylphosphate, accounted for
about half of the radioactivity. The tissues were almost devoid
of 32P-containing material (Casida et al., 1962).
A rat, orally dosed with 500 mg/kg 32P-desmethyldichlorvos in
water, excreted about 14% of the dose in the urine in 90 h.
The tissue distribution of 32P was similar to that which followed
32P-dichlorvos administration. The very high proportion of
radioactivity in the bone was indicative of rapid degradation to
phosphoric acid (Casida et al., 1962).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity
9.1.1.1 Poisoning incidents
A 56-year-old woman ingested an estimated amount of 100 mg
dichlorvos/kg body weight and survived, following intensive care for 14
days (Watanabe et al., 1976). However, a suicide with a dichlorvos
dose of about 400 mg/kg succeeded in spite of treatment (Shinoda et
al., 1972).
A 35-year-old female patient accidently ingested 60 g fluid Divipan
(dichlorvos concentration not reported). She was comatose for one week
and recovered slowly. Clinical and electrophysiological examinations
(no details reported) showed a pure motor form of neuropathy, according
to the authors (Vasilescu & Florescu, 1980).
Two cases of poisoning with dichlorvos taken orally in unspecified,
but high, quantities have been reported. The patients first showed
signs of severe anti-ChE poisoning. After recovery, delayed
neurotoxicity developed. They showed a severe axonal degeneration
neuropathy. One of them recovered within 12 months (Wadia et al.,
1985).
Reeves et al. (1981) reported six cases, over an 8-year period, of
bone-marrow failure (pancytopenia) in children shortly after exposure
to dichlorvos and propoxur. Van Raalte & Jansen (1981) doubted the
causal relationship between dichlorvos and bone-marrow failure in the
children, because the disease has not been observed in workers with
high exposure, or in the general population. In addition, haematotoxic
effects have not been observed in experimental animals.
9.1.2. Effects of short- and long-term exposure
In the 1960s, field studies were carried out in several countries
to test dichlorvos as a residual insecticide for malaria control in
houses. Numerous residents of all ages and conditions were exposed
without any adverse effects attributable to dichlorvos being reported
(Escudié & Sales, 1963; Funckes et al., 1963; Gratz et al., 1963;
Quarterman et al., 1963; Foll et al., 1965). In two field studies, the
plasma and erythrocyte ChE activities were measured in adults and young
children. No abnormalities were found, though air concentrations of
dichlorvos in the treated houses rose to 0.8 mg/m3 (Funckes et al.,
1963; Gratz et al., 1963).
In a report by Gold et al. (1984), 20 single-family residences were
treated with a 0.5% solution of dichlorvos (at an average rate of
0.189 g/m2) to control the cockroach Blattella germanica L. The
average air concentration for the first 2 h after treatment was
548 µg/m3 and for the next 2 h was 183 µg/m3. Pesticide operators
and the residents of treated structures were monitored for evidence of
dichlorvos exposure, using exposure pads, air samples, serum and
erythrocyte ChE tests, and urinalyses. There was no evidence of
dichlorvos or dichloroacetic acid in urine. There were slight, but
statistically significant, changes in the mean serum ChE activity of
some of the residents of treated structures, but the mean erythrocyte
ChE was unchanged.
Passengers in an aircraft provided with an automatic insect-control
system, which released 0.15 - 0.30 mg dichlorvos/m3 for periods of
about 30 min, did not show any signs of discomfort (Jensen et al.,
1965). Tests have shown that man can withstand daily exposure to
concentrations of 0.5 mg/m3 without clinical effects, and with only a
slight depression of blood ChE activity (Hayes, 1961). Three men
exposed to approximately the same level during 24 tests did not show
any change in blood ChE activity (Schoof et al., 1961).
A case of chronic obstructive bronchitis ascribed to exposure to
dichlorvos was described by Barthel (1983). However, it could not be
excluded that other components of the unknown formulation could have
been the cause. ChE determinations were not performed.
9.1.2.1 Studies on volunteers
Single oral doses (1 - 32 mg/kg body weight) of dichlorvos in a
slow-release PVC formulation administered to 107 male volunteers
produced measurable reductions in erythrocyte ChE activity at dose
levels above 4 mg/kg, with a maximum reduction of 46% at the highest
dose. Plasma ChE activity was affected at lower doses, with 50%
reduction at 1 mg/kg and about 80% at 6 mg/kg or more. Repeated oral
doses of 1 - 16 mg/kg body weight per day were given to 38 volunteers
for up to 3 weeks. The plasma ChE activity was maximally depressed at
all dose levels, and the erythrocyte ChE activity depression was dose
related and significant at 2 mg/kg or more. Blood cell count, urine,
liver function, prothrombin time, and blood urea nitrogen were all
normal (Hine & Slomka, 1968, 1970; Slomka & Hine, 1981).
In studies by Rider et al. (1968), dichlorvos was given to groups
of five men at daily oral doses of 1, 1.5, 2, or 2.5 mg per man. The
plasma ChE activity of the 2.5 mg group was reduced by 30% after 20
days of treatment. Administration of 2 mg for 28 days resulted in a
reduction of 30% 2 days after the last dose. Erythrocyte ChE activity
was not significantly affected in either group (Rider et al., 1967).
Daily oral doses of 1.5 mg per man given to 10 volunteers for 60 days
caused a significant reduction (approximately 40%) in plasma ChE
activity, which returned to normal levels when dichlorvos
administration was discontinued.
Boyer et al. (1977) reported studies on two groups of six men (21 -
45 years of age) who received 0.9 mg of dichlorvos three times a day
for 21 days. One group received the dichlorvos in a gelatin salad, the
other in a pre-meal capsule filled with cottonseed oil. Two other
groups of six men received placebo treatment. No consistent
cholinomimetic signs or symptoms were observed, nor was erythrocyte ChE
inhibited. However, plasma ChE was significantly depressed within 20
days, although the extent depended on the method by which dichlorvos
was administered. Recovery was comparable in both groups, the half-
life for the regeneration of plasma ChE being 13.7 days.
Volunteers did not show inhibition of plasma or erythrocyte ChE
activity either when handling a dichlorvos strip for 30 min each day or
after having a piece of the strip applied to the arm for 30 min each
day for 5 consecutive days (Zavon & Kindel, 1966).
The wearing of dichlorvos-impregnated garments by babies for 48 -
84 h did not produce changes in either plasma or erythrocyte ChE
activity over a period of 5 days (Cavagna et al., 1969).
In studies on eight volunteers, carried out in an aircraft at
operational cabin altitude (2400 m), no changes in plasma or
erythrocyte ChE activity, dark adaptation, or bronchiolar resistance
were observed. Dichlorvos concentration in the air ranged from 0.73 to
1.18 mg/m3 during exposures of 45 min (Smith et al., 1972).
Hunter (1970a) reported studies on 26 men (21 - 57 years of
age) and 6 women (19 - 25 years of age) who were exposed in a chamber
for 2-7´ h to actual dichlorvos concentrations of approximately
1 mg/m3. Food and drink were served in the chamber. No clinical
signs were observed, and no effects on haematology, urinalysis, kidney
function, EEG, ECG, respiratory activities, or erythrocyte ChE activity
were found. Plasma ChE activities were markedly inhibited only when
the exposure lasted over 6 - 7 h (Hunter, 1970a).
In studies by Hunter (1969, 1970b), seven men (25 - 56 years of
age) were exposed (head and neck) to dichlorvos vapour (actual
concentrations of 1 - 52 mg/m3), and 6 men were exposed (head
exposure only) to 7 - 50 mg/m3, for periods from 10 min to 4 h. The
maximum dose level was 52 mg/m3 (for 65 min), and the maximum period
was 240 min (at 13 mg/m3). Symptoms were confined to irritation of
the throat, some rhinorrhoea, and substernal discomfort at the highest
concentrations. No effects on the pupil or on visual acuity were
recorded. Erythrocyte ChE activity was depressed in only one person.
However, there was a direct relationship between the reduction in
plasma ChE activity and the dichlorvos dose (concentration x time). No
changes were found in either kidney or pulmonary function or in the
overall metabolic rate.
When three men were exposed to dichlorvos (actual concentrations of
0.3 - 0.9 mg/m3 (mean, 0.5 mg/m3) or 0.9 - 3.5 mg/m3 (mean,
2.1 mg/m3) for 1 or 2 h per day for 4 consecutive days, plasma ChE
alone decreased slightly in the men exposed for 2 h per day to the
higher concentration (Witter et al., 1961).
A group of 15 men (23 - 61 years of age) was exposed for up to 6
half-hour intervals per night for 14 days (total of 39 doses) to actual
dichlorvos concentrations ranging from 0.14 to 0.33 mg/m3. No clear
changes in plasma ChE activity or other parameters such as reaction
time, airway resistance, or vision were found. The same results were
obtained when a similar group was exposed to actual concentrations of
0.1 - 0.6 mg/m3 for intervals of 8 - 10 h, 4 nights per week for 11
weeks (Rasmussen et al., 1963).
No significant effect on plasma or erythrocyte ChE activity was
observed in 14 persons exposed at the recommended rate of one
dichlorvos strip per 30 m3 in their homes over a period of 6 months.
The strips were replaced at much shorter intervals than normally
recommended. The air concentration 40 days after the installation of
the fourth strip was approximately 0.09 mg/m3 (Zavon & Kindel, 1966).
In three home studies involving 26 families, conducted in Arizona,
USA, no deleterious effects on health or plasma or erythrocyte ChE
activity were observed in the residents exposed to dichlorvos strips
all over the house (8 - 10 strips) for over a year. Even monthly
replacement of the strips resulted in only a slight inhibition of
plasma ChE activity. The maximum air concentrations of dichlorvos
averaged 0.13 mg/m3 (Leary et al., 1971, 1974).
9.1.2.2 Hospitalized patients
In a report by Pena Chavarra et al. (1969), a single oral dose of 6
or 12 mg dichlorvos/kg body weight was administered in the form of a
slow-release granular resin formulation as an anthelminthicum to 108
hospitalized adult patients, many of these debilitated and with severe
anaemia. Plasma ChE activity was markedly reduced, in some patients by
76 - 100%. However, erythrocyte ChE activity was much less inhibited.
No symptoms of intoxication were observed, except for brief mild
headaches in a few patients, and there were no abnormalities in
haematological studies or in hepatic and renal function tests.
In studies by Cervoni et al. (1969), single doses of dichlorvos
(PVC-resin formulations) were administered orally 2 h before breakfast
to 705 adults found to be harbouring infections of Trichuris, hookworm,
or Ascaris. Six or 12 mg dichlorvos/kg body weight resulted in
infection cure rates of approximately 70 - 100%. According to the
authors, minimum to modest plasma ChE depression and zero to minimum
erythrocyte ChE depression occurred at both dose levels. No clinical
symptoms or alterations in haematology or in liver and kidney function
were observed.
Sick adults and children and healthy pregnant women and babies in
hospital wards treated with dichlorvos strips (one strip per 30 or
40 m3) had normal erythrocyte ChE activities. Only subjects exposed
for 24 h per day to dichlorvos concentrations above 0.1 mg/m3 or
patients with liver insufficiency showed even a moderate decrease in
plasma ChE activity (Cavagna et al., 1969, 1970; Cavagna & Vigliani,
1970).
9.2. Occupational Exposure
9.2.1. Acute toxicity
9.2.1.1 Poisoning incidents
A number of fatal and non-fatal poisoning cases have been described
after concentrated formulations of dichlorvos splashed onto parts of
the body. Two workers who failed to wash it off promptly died
consequently. However, in those cases where the spilled solution was
washed off immediately, the victims showed symptoms of intoxication but
recovered after treatment. A serious non-fatal case occurred after a
spillage of 120 ml of a 3% formulation that was not washed off
immediately. After 1´ h, the victim developed slurred speech, became
drowsy, and collapsed. He recovered completely after treatment (Hayes,
1963, 1982).
A pest-control operator became contaminated with a 1% solution of
dichlorvos in mineral spirit after using a leaking knapsack sprayer.
The man changed his overalls and completed his day's work. He noticed
weakness, dizziness, and difficulty in breathing. Contact dermatitis
developed on the back skin. After the fourth day, his blood ChE was
36% of normal, but there were no signs of systemic illness. He
recovered without medication, and the ChE activity increased to 72% of
normal within one month. The acute dermatitis was probably caused by
the solvent (Bisby & Simpson, 1975).
A driver of a truck transporting a 5% commercial formulation of
dichlorvos (15% petroleum distillate and 80% trichloroethane)
developed persistent contact dermatitis for 2 months following
accidental skin contact. In addition, the patient experienced
headache, mild rhinorrhoea, burning of the tongue, and a bitter taste
in his mouth. Initial blood ChE levels were in the low normal range
returning to the high normal range within 2 weeks. Patch tests with 1%
and 0.1% dichlorvos in petroleum distillate were negative (Mathias,
1983). In view of the symptoms and the slight ChE inhibition, it seems
likely that trichloroethane caused the dermatitis.
Cronce & Alden (1968) described four people who handled dogs
wearing anti-flea dog collars containing 9 - 10% dichlorvos. Acute
primary contact dermatitis was described. Closed patch tests with
0.25, 0.5, and 1% dichlorvos in distilled water and in mineral oil were
positive in all four people. General experience with the collars
indicates that only a few people are susceptible to this kind of
irritation (Hayes, 1982).
9.2.2. Effects of short- and long-term exposure
9.2.2.1 Pesticide operators and factory workers
The effect on sprayers of dichlorvos fume, used inside a building
for cockroach control, was examined. When 0.3 - 0.6% dichlorvos oil
spray was used at the rate of 6 ml/m2 by the sprayers (7 - 9 people),
inhibition of ChE activity in the subjects was 15%, and conjunctival
injections or sore throats were observed in some sprayers after either
18 min or 4 h of spraying operation. To examine the effect of elevated
dichlorvos vapour pressure at higher temperatures, four men sprayed
0.6% oil spray for 2 h at the room temperature of 25 °C. The
inhibition of plasma and erythrocyte ChE activities was 22% and 7%,
respectively. In another study, 11 sprayers used 0.6% oil spray for
6 h of actual work time at 20 °C without rest. Two of them showed an
inhibition of plasma ChE activity of 38% while the others did not show
significant inhibition of erythrocyte ChE. It was concluded that
conjunctival injections and sore throats were attributable to the
kerosene solvent used and that the 38% depression in ChE activity was
probably due to long hours of continuous work. Therefore, the overall
effect on ChE activity was not considered to be severe (Ueda et al.,
1959, 1960).
Twelve fogging machine operators did not show any reduction in
plasma or erythrocyte ChE activity when applying 4% dichlorvos aerosols
in tobacco warehouses for 16 h per week, over a period of 2 - 4 months
(Witter, 1960).
Sixteen men replacing old dichlorvos dispensers and installing new
units in houses in Haiti, 5 days per week for 3 weeks, showed a
decrease of up to 60% in plasma ChE activity. No signs or symptoms of
intoxication were observed. Air concentrations ranged from 0.3 to
2.1 mg/m3 (Stein et al., 1966).
The blood ChE activity of sprayers, exposed during insect control
in grain stores to air concentrations of 1.9 - 3 mg/m3 dichlorvos,
was reduced to 19 - 23% (Sasinovich, 1970).
In a report by Das et al. (1983), each of 13 pesticide-control
operators carried out urban pest-control for one day in four houses
using 230 - 330 g dichlorvos as aerosol and 40 - 50 g dichlorvos as
emulsion spray. At the end of the day's work, an operator had an
average dichlorvos residue of 0.8 mg/m2 on the back, 0.4 mg/m2 on the
chest, and 11 mg/m2 on the respirator filter. Dimethylphosphate was
detected in the urine, but blood and urine analyses, including serum
ChE levels, did not reveal any other changes in clinical parameters.
In the course of either the production or processing of a
dichlorvos-releasing product, 11 male and 2 female factory workers were
exposed to an average dichlorvos concentration of 0.7 mg/m3 (highest
value 3 mg/m3) on each working day for a period of 8 months.
Inhibition of plasma ChE activity was noted within a few days of the
start of exposure, while inhibition of erythrocyte ChE activity
developed much more slowly. The maximum plasma ChE activities recorded
were 40% lower than the pre-exposure levels, but 60% lower than the
post-exposure levels. Erythrocyte ChE activity was reduced by
approximately 35% compared with pre- and post-exposure levels. One
month after exposure had ceased, plasma ChE and erythrocyte ChE
activities were found to have returned to normal physiological levels.
The other haematological investigations and the medical examinations
did not reveal any changes attributable to dichlorvos exposure (Menz et
al., 1974).
9.2.2.2 Mixed exposure
A number of articles have described the symptoms found in
individual workers or groups of workers exposed for a number of years
to different types of pesticides, including dichlorvos. In general, a
slight-to-moderate decrease in ChE activity occurred. Furthermore,
several complaints and symptoms were noticed, but no clear clinical
poisoning cases occurred. These case studies are, however, of little
relevance for the evaluation of dichlorvos because of the mixed
exposure to different pesticides (Bellin & Chow, 1974; Fournier et al.,
1976; Gupta et al., 1979; Ullmann et al., 1979; Hayes et al., 1980;
Hayes, 1982).
From 1975 to 1977, 88 patients with pesticide dermatitis, 15
suffering from photo-dermatitis, were studied. Patch tests using 29
different pesticides of different categories were carried out, but did
not lead to an identification of the responsible pesticides. Eight out
of 52 patients (15.4%) reacted positively to a photopatch (Horiuchi &
Ando, 1978; Horiuchi et al., 1978).
Stoermer (1985) described a case of contact dermatitis in a woman
who had worked as a pest controller for 3 months and had sprayed
dichlorvos and propoxur. She was treated and recovered within one
week.
A field survey of tea growers was made in the Chiran area of Japan
in 1982. Out of 84 tea growers examined (21 men and 63 women), 5 women
had contact dermatitis from agricultural work. Dichlorvos was among
the insecticides, fungicides, and herbicides used. Patch tests showed
relatively high rates of positive reactions with dichlorvos (27% of
women and 5% of men). Also, cross-sensitization was found between
methidathion and dichlorvos (Fujita, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Since 1961, dichlorvos, an organophosphate with anti-ChE activity,
has been used worldwide as a contact and stomach insecticide to control
insects on crops and domestic animals. It is also used as an
insecticide in houses and other buildings and for insect control in
aircraft.
Dichlorvos is readily absorbed by the body of mammals through all
routes of exposure, and is readily metabolized in the liver. Within
1 h of oral administration, dichlorvos is found in the liver, kidneys,
and other organs of experimental animals. It is rapidly eliminated via
the kidneys, with a half-life of 14 min.
The metabolism of dichlorvos in various species, including human
beings, follows similar pathways. Differences between species relate
only to the rate of metabolism, but this is always rapid.
Dichlorvos is moderately to highly toxic for mammals (the oral
LD50 for the rat is 30 - 110 mg/kg body weight). The classification
of dichlorvos by WHO (1986a) is based on an oral LD50 for the rat of
56 mg/kg body weight. Signs of intoxication usually occur shortly
after exposure and are typical of an organophosphorus pesticide.
Inhibition of ChE activity is a sensitive criterion of exposure. In
short-term toxicity studies on mammals, it was shown that ChE activity
was not decreased at oral dose levels below about 0.5 mg/kg body
weight. In long-term studies on rats at oral dose levels of 2.5 mg/kg
body weight or more, hepatocellular fatty vacuolization was seen. At
0.25 mg/kg body weight, no ChE inhibition was found, nor were there any
other effects.
Reproduction and teratogenicity studies using a wide range of dose
levels (6.25 - 500 mg/kg body weight) were negative. Dichlorvos showed
alkylating properties in in vitro but not in in vivo studies. Many in
vitro mutagenicity studies with bacteria and yeast were positive, while
the in vivo studies were mainly negative. From the available
mutagenicity studies, it is unlikely that dichlorvos constitutes a
mutagenic hazard for man. Carcinogenicity studies on mice and rats fed
dichlorvos (dose levels of up to 234 mg/kg diet) were negative. Two
recent carcinogenicity studies have been carried out on mice and rats
in which dichlorvos was administered by intubation for up to 2 years at
dose levels of 10 - 40 mg/kg body weight (mice) and 4 or 8 mg/kg body
weight (rats). Only preliminary information has been provided. The
evidence for carcinogenicity in these new studies is difficult to
interpret at this time. Only when complete and final reports become
available will it be possible to draw more definite conclusions (in
this context, see section 8.7.3).
From work on hens, the suspicion of delayed neurotoxicity from
dichlorvos has neither been established nor totally refuted. However,
there have been two clinical reports on four patients suffering intense
poisoning from dichlorvos taken orally who survived with treatment and
who then displayed neurotoxic effects. Thus, the possibility of
delayed neurotoxicity in man cannot be entirely discounted, but it is
likely to occur only with excessive oral doses.
Human volunteers who were given single or repeated oral doses of 2
mg/kg body weight or more showed significant inhibition of erythrocyte
ChE activity. At 1 mg/kg body weight, no such inhibition was found.
The application of dichlorvos to crops and animals results in
residues that rapidly disappear by volatilization and hydrolysis. In
general, residues of dichlorvos and the breakdown product DCA in food
commodities are low and will be further reduced during processing. The
exposure of the general population to dichlorvos by food and drinking-
water is negligible, as is confirmed in total-diet studies.
In short-term inhalation studies on mammals, 1 or 2 mg
dichlorvos/m3 did not inhibit ChE activity.
In a 2-year, 23 h/day, whole-body inhalation study on rats, 0.48
mg/m3 caused inhibition of plasma and erythrocyte activity, but brain
AChE activity was not inhibited and there were no clinical signs. An
unquantified, but considerable, extra exposure, resulting from the
grooming of contaminated fur and contamination of food and drinking-
water, had contributed to this effect. The no-observed-adverse-effect
level was 0.05 mg/m3. There was no evidence of carcinogenicity.
In a 6- to 7-h exposure of human volunteers to approximately 1 mg
dichlorvos/m3, only plasma ChE activity was inhibited. The
erythrocyte AChE activity, taken to be representative of the AChE
activity in the nervous tissue, was unaffected.
Residents exposed for over one year to an average air concentration
of 0.1 mg/m3 arising from slow-release strips showed no inhibition of
plasma or erythrocyte ChE activity and no deleterious effects on
health.
The main exposure of the general population is by inhaling
dichlorvos when used indoors to control insects. The recommended use
(one slow-release strip/ 30 m3) will give concentrations in the air
of up to 0.1 - 0.3 mg/m3 in the first few days, decreasing thereafter
to below 0.1 mg/m3. The air concentration depends on temperature,
humidity, and ventilation.
As long as approved slow-release strips are used according to the
label instructions, no health hazard can be expected for man. However,
special care may need to be taken with young children and sick or
elderly people who are especially vulnerable when continuously exposed
(24 h per day) in poorly ventilated rooms. Other methods of indoor
application should be equally safe if the label instructions are
followed.
There is some indication that dichlorvos may induce dermatitis and
cross-sensitization in people handling various types of pesticides
including dichlorvos.
In occupational conditions, the main route of exposure to
organophosphorus pesticides is usually the dermal route. In the case
of dichlorvos, with its high vapour pressure, exposure by inhalation is
also important. In these occupational situations, the dichlorvos
concentrations in the air are generally below 1 mg/m3 but, in certain
circumstances, they may rise considerably above this level. This
stresses the need for adequate protective measures to be taken during
occupational exposure and regular monitoring of ChE activity.
10.2. Evaluation of Effects on the Environment
The presence of dichlorvos in the environment as a result of
accidental losses or direct application on soil or in water will not
lead to long-term effects because of its fast breakdown and
evaporation. Furthermore, it is converted to a number of compounds,
such as dichloroacetic acid, by microorganisms. Certain bacteria
species can use dichlorvos as a sole carbon source, while others cannot
and are inhibited in their growth. Therefore, its influence on
microorganisms is complex.
Dichlorvos is moderately to highly toxic (range, 0.2 - 10 mg/litre)
for freshwater and estuarine species of fish and invertebrates. In
certain fish, concentrations of 0.25 - 1.25 mg/litre cause inhibition
of brain and liver ChE activity. Concentrations of as little as
0.05 mg/litre may have deleterious effects, particularly in
invertebrates. Dichlorvos has a high toxicity for birds and bees.
Caution is advised in the use and handling of dichlorvos where these
species might be exposed.
No bioaccumulation occurs in the different environmental
compartments and organisms.
10.3. Conclusions
1. Exposure of the general population to dichlorvos via food and
drinking-water is negligible and does not constitute a health hazard.
2. The in-house use of dichlorvos as an insecticide in the form of
sprays or slow-release strips (at recommended levels) does not
constitute a short- or long-term hazard for the general population.
However, continuous (24 h per day) exposure of young children and
diseased or elderly people in non-ventilated or poorly ventilated rooms
should be avoided.
3. In spite of their toxicity, dichlorvos and its formulations do not
contribute an undue hazard to those occupationally exposed, provided
that adequate ventilation and skin protection are used.
4. Except under conditions of gross spillage, the recommended use of
dichlorvos as an insecticide does not constitute an acute or long-term
hazard for aquatic or terrestrial organisms, although there may be an
acute hazard for birds and bees.
11. RECOMMENDATIONS
1. Continuous (24 h/day) exposure of young children and diseased or
elderly people to dichlorvos in non-ventilated or poorly ventilated
rooms should be avoided.
2. As dichlorvos from different sources may vary in purity and type of
impurities, attention should be paid to its composition. This should
conform to FAO and WHO specifications (FAO, 1977; WHO, 1985). In the
case of formulations, potential hazards of other components, such as
solvents and stabilizers, should also be considered.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dichlorvos was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1965, 1966, 1967, 1969, 1970, 1974, and 1977
(FAO/WHO, 1965a,b, 1967a,b, 1968a,b, 1970a,b, 1971a,b, 1975a,b,
1978a,b). In 1966, the JMPR established an Acceptable Daily Intake
(ADI) for human beings of 0 - 0.004 mg/kg body weight, which remains
unchanged.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified technical dichlorvos as
"highly hazardous" (Class IB) (Plestina, 1984; WHO, 1986a) and has
produced a safety sheet on dichlorvos (No. 75.2) (WHO/FAO, 1975-86).
Specifications for dichlorvos use in public health and in plant
protection have been published by WHO (1985) and FAO (1977),
respectively.
In 1979, the International Agency for Research on Cancer (IARC)
came to the following conclusions in considering the carcinogenicity of
dichlorvos:
(a) dichlorvos was tested in different animal species via
different routes; no conclusive evaluation on the basis of
these studies could be made;
(b dichlorvos is an alkylating agent and binds to bacterial and
mammalian nucleic acids;
(c) it is a mutagen in a number of microbial systems, but there is
no evidence of its mutagenicity in mammals, in which it is
rapidly degraded.
In the evaluation by IARC, it was stated that "the available data
do not allow an evaluation of the carcinogenicity of dichlorvos to be
made".
In its series "Scientific Reviews of Soviet Literature on Toxicity
and Hazards of Chemicals", the International Register of Potentially
Toxic Chemicals has published a volume on dichlorvos (IRPTC, 1984).
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O.G. (1970)
Pesticide residues in the total diet in England and Wales. III.
Organophosphorus pesticide residues in the total diet, 1966-67.
Pestic. Sci., 1: 10-13.
ACKERMAN, H., LEXOW, B., & PLEWKA, E. (1969) [Detection and
identification of the organic phosphate, phosphorothionate,
phosphonate, and carbamate insecticides in biological material.]
Arch. Toxicol., 24: 316-324 (in German).
ADLER, B., BRAUN, R., SCHONEICH, J., & BOHME, H. (1976) Repair-
defective mutants of Proteus mirabilis as a prescreening system for the
detection of potential carcinogens. Biol. Zentralbl., 95: 463-469.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides wetting agents and miscellaneous
substances. Int. pest Control, 11(2): 25-35.
ALDRIDGE, W.N. & BARNES, J.M. (1966) Further observations on the
neurotoxicity of organophosphorus compounds. Biochem. Pharmacol., 15:
541-548.
ALDRIDGE, W.N. & JOHNSON, M.K. (1971) Side effects of organo-
phosphorus compounds: delayed neurotoxicity. Bull. World Health
Organ., 44: 259-263.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and structure-
activity relationships providing a high safety factor for anti-
cholinesterase insecticides. In: Proceedings of the 1977 British Crop
Protection Conference - Pests and Diseases, Croyden, British Crop
Protection Council. pp. 721-729.
ALI, M.S., RAHMATULLAH, M., JAHAN, R., YUSUF, H.K.M., &
CHOWDHURY, A.A. (1979c) Effect of DDVP (insecticide) on citric acid
fermentation in Aspergillus niger. Enzyme Microb. Technol., 1: 127-
128.
ALI, S.F. & HASAN, M. (1977) Effect of organophosphate insecticide
dichlorvos on the amino acid content of different regions of the rat
brain and spinal cord. Indian J. exp. Biol., 15(9): 759-761.
ALI, S.F., HASAN, M., & MITRA, S.C. (1979a) Organophosphate DDVP-
induced changes in the rat spinal cord: an electron microscopic and
biochemical study. J. Anat. Soc. India, 28(1): 36.
ALI, S.F., HASAN, M., & TARIQ, M. (1979b) Levels of dopamine,
norepinephrine, and 5-hydroxytryptamine in different regions of rat
brain and spinal cord following chronic administration of organo-
phosphate pesticide dichlorvos. Indian J. exp. Biol., 17(4): 424-426.
ALI, S.F., CHANDRA, O., & HASAN, M. (1980) Effects of an
organophosphate (dichlorvos) on open-field behaviour and locomotor
activity: correlation with regional brain monoamine
levels. Psychopharmacology, 68(1): 37-42.
ALLEN, S.D., VANKAMPEN, K.R., & BROOKS, D.R. (1978) Evaluation of
the feline dichlorvos (DDVP) flea collar. Feline Pract., 8(3): 9-16.
AMBRUS, A., LANTOS, J., VISI, E., CSATLOS, I., & SARVARI, L. (1981)
General method for determination of pesticide residues in samples of
plant origin, soil, and water. J. Assoc. Off. Anal. Chem., 64(3):
733-768.
ANDERSON, R.A., BARSTAD, J.A.B., & LAAKE, K. (1978) An improvement
of the spectrophotometric method for determination of cholinesterase
activity in erythrocytes and tissue homogenates. Ann. Natl Inst. Publ.
Health (Norway), 1(1): 51-55.
ANON. (1972) Vapona insecticide. In: Analytical methods for
pesticides and plant growth regulators, Modesto, California, Shell
Development Company, Vol. 6, pp. 529-541.
ANON. (1973) The determination of malathion and dichlorvos residues
in grain. Report by the Panel on Malathion and Dichlorvos Residues in
Grain. Analyst, 98: 19-24.
ANON. (1977) Determination of residues of organophosphorus pesticides
in fruits and vegetables. Report by the Panel on Determination of
Residues of Certain Organophosphorus Pesticides in Fruits and
Vegetables. Analyst, 102: 858-868.
ANON. (1982) Pesticides: a safety guide, London, Shell International
Chemical Company Ltd, pp. 53-55.
AOKI, Y., TAKEDA, M., & UCHIYAMA, M. (1975) Comparative study of
methods for the extraction of eleven organophosphorus pesticide
residues in rice. J. Assoc. Off. Agric. Chem., 58(6): 1286-1293.
AQUILINA, G., BENIGNI, R., BIGNAMI, M., CALCAGNILE, A.,
DOGLIOTTI, E., FALCONE, E., & CARERE, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15:
439-442.
ARATAKE, Y. & KAYAMURA, T. (1973) [Toxicity of insecticides to
silkworm larvae.] Sanshi Kenkyu (Acta serol.), 87: 68-78 (in
Japanese).
ARIMATSU, S., HOSHIRO, Y., & NOMURA, T. (1977) [Studies on primary
irritation test of pesticides in rabbits.] Nippon Noson Igakkai
Zasshi, 26: 572-573 (in Japanese).
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees, Riverside,
University of California (Agricultural Extension Report No. M-16 Rev.
9/73).
AUGUSTINSSON, K.B., ERIKSSON, H., & FAIJERSSON, Y. (1978) A new
approach to determining cholinesterase activities in samples of whole
blood. Clin. Chim. Acta, 89: 239-252.
AULICINO, F., BIGNAMI, M., CARERE, A., CONTI, G., MORPURGO, G.,
& VELCICH, A. (1976) Mutational studies with some pesticides
in Aspergillus nidulans. Mutat. Res., 38(2): 138.
BAKSI, S.N. (1978) Effect of dichlorvos on embryonal and fetal
development in thyro-parathyroidectomized thyroxine-treated and
euthyroid rats. Toxicol. Lett., 2(4): 213-216.
BALLINGTON, P.E., SKIPPER, H.D., & HEGG, R.O. (1978) Response of
coliform populations in poultry waste digesters to three
insecticides. J. environ. Qual., 7(2): 262-264.
BARTHEL, E. (1983) [Irritative and allergic effects of pesticide
aerosols on the respiratory tract and problems of their evaluation.] Z.
gesamte Hyg., 29(11): 678-681 (in German).
BATORA, V., VITOROVIC, S.L.J., THIER, H.P., & KLISENKO, M.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BATTE, E.G., ROBINSON, O.W., & MONCOL, D.J. (1969) Influence of
dichlorvos on swine reproduction and performance of offspring to
weaning. J. Am. Vet. Med. Assoc., 154(11): 1397.
BAZER, F.W., ROBSON, O.W., & ULBERG, L.C. (1969) Effect of dichlorvos
and PMS on reproduction in swine. J. anim. Sci., 28: 145.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2(4): 307-337.
BELL, TH.G., FARRELL, K., PADGET, G.A., & LEENDERTSEN, L.W.
(1975) Ataxia, depression, and dermatitis associated with the use of
dichlorvos-impregnated collars in the laboratory cat. J. Am. Vet.
Med. Assoc., 167(7): 575-586.
BELLIN, J.S. & CHOW, I. (1974) Biochemical effects of chronic low-
level exposure to pesticides. Res. Commun. chem. Pathol.
Pharmacol., 9(2): 325-337.
BENIGNI, R. & DOGLIOTTI, E. (1980a) UDS studies on selected
environmental chemicals. Mutat. Res., 74: 248-249.
BENIGNI, R. & DOGLIOTTI, E. (1980b) UDS studies on selected
environmental chemicals. Mutat. Res., 74(3): 217.
BENIGNI, R., BIGNAMI, M., CAMONI, I., CARERE, A., CONTI, G.,
IACHETTA, R., MORPURGO, G., & ORTALI, V.A. (1979) A new in
vitro method for testing plant metabolism in mutagenicity studies. J.
Toxicol. environ. Health, 5: 809-819.
BERAN, F. (1970) [Present knowledge on the toxicity of our pesticides
to bees.] Gesunde Pflanz., 22(2): 21-31 (in German).
BIGNAMI, M., CONTI, L., MORPURGO, G., & VELCICH, A. (1976)
Comparative analysis of different test systems for somatic
recombination with Aspergillus nidulans. Mutat. Res., 38(2): 138-139.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of pesticides
in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BISBY, J.A. & SIMPSON, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Aust., 2: 394-395.
BLAIR, D. & RODERICK, H.R. (1976) An improved method for the
determination of urinary dimethyl phosphate. J. agric. food
Chem., 24(6): 1211-1223.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The distribution of
dichlorvos in the tissues of mammals after its inhalation or
intravenous administration. Toxicol. appl. Pharmacol., 31: 243-253.
BLAIR, D., DIX, K.M., HUNT, P.F., THORPE, E., STEVENSON, D.E., &
WALKER, A.I.T. (1976) Dichlorvos: a 2-year inhalation carcinogenesis
study in rats. Arch. Toxicol., 35: 281-294.
BOOTSMA, D., HEERING, H., KLEIJER, W.J., BUDKE, L., DE JONG,
L.P.A., & BERENDS, F. (1971) Effects of dichlorvos on human cells in
tissue culture. A progress report, Rijswijk, The Netherlands, Medical
Biological Laboratory TNO (Report No. MBL 1971-5).
BOUSH, G.M. & MATSUMURA, F. (1967) Insecticidal degradation by
Pseudomonas melophthora, the bacterial symbiote of the apple
maggot. J. econ. Entomol., 60: 918-920.
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol- water
partitioning coefficients (Kow) of 61 organophosphorus and carbamate
insecticides and their relationships to respective water solubility (S)
values. J. environ. Sci. Health, B18(6): 667-683.
BOYER, A.C., BROWN, L.J., SLOMKA, M.B., & HINE, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. appl. Pharmacol., 41: 389-
394.
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison of
cholinesterase activity, residue levels, and urinary metabolite
excretion of rats exposed to organophosphorus pesticides. J. agric.
food Chem., 25Z(6): 1353-1358.
BRAUN, R., SCHONEICH, J., WEISSFLOG, L., & DEDEK, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair: direct alkylation vs metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
desmethyldichlorvos, and demethylvinyl-butonate. Chem.-biol.
Interact., 39: 339-350.
BRIDGES, B.A., MOTTERSHEAD, R.P., GREEN, M.H.L., & GRAY, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulfonate
for Escherichia coli WP2 and some derivatives deficient in DNA
repair. Mutat. Res., 19: 295-303.
BROWN, V.K.H. & ROBERTS, M. (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to guinea-
pigs, Sittingbourne, Shell Research Ltd (Unpublished Report No. IRR
TL/40/66).
BROWN, V.K.H. & STEVENSON, D.E. (1962) Agricultural chemicals:
therapy of DDVP intoxication by atropine and cholinesterase
reactivators, Sittingbourne, Shell Research Ltd (Shell Technical
Memorandum No. TOX 31/62) (Unpublished Report).
BROWN, V.K.H., BLAIR, D., HOLMES, D.L., & PICKERING, R.G. (1968)
The toxicity of low concentrations of dichlorvos by inhalation in
rodent and avian species, Sittingbourne, Shell Research Ltd
(Unpublished Report No. TLGR.0015.68).
BRUNGS, W.A., MCCORMICK, J.H., NEIHEISEL, T.W., SPEHAR, R.L.,
STEPHAN, C.E., & STOKES, G.N. (1977) Effects of pollution on fresh-
water fish. J. Water Pollut. Control Fed., 49(6): 1425-1493.
BRYANT, R.J. & MINETT, W. (1978) A gas chromatographic method for the
determination of dichlorvos in air. Pestic. Sci., 9: 525-528.
BRZEZINSKI, J. & WYSOCKA-PARUSZEWSKA, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., 4(Suppl.): 475-478.
BULL, D.L. & RIDGWAY, R.L. (1969) Metabolism of trichlorfon in
animals and plants. J. agric. food Chem., 17(4): 837-841.
BUNDING, I.M., YOUNG, R., Jr, SCHOOLEY, M.A., & COLLINS, J.A.
(1972) Maternal dichlorvos effects on farrowing parameters. J. anim.
Sci., 35: 238.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1972) [Mutagenicity
investigations with pesticides in the host-mediated assay and the
dominant lethal test in mice.] Biol. Zentralbl., 91(3): 311-325 (in
German).
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973) Comparative
investigations on the mutagenicity of pesticides in mammalian test
systems. Mutat. Res., 21(1): 25-26.
BUTLER, G.L. (1977) Algae and pesticides. Residue Rev., 66: 40.
CARERE, A., CARDAMONE, G., ORTALI, V., BRUZZONE, M.L., & DI
GIUSEPPE, G. (1976) Mutational studies with some pesticides
in Streptomyces coelicolor and Salmonella typhimurium. Mutat.
Res., 38(2): 136.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978a) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARERE, A., ORTALI, V.A., CARDAMONE, G., & MORPURGO, G.
(1978b) Mutagenicity of dichlorvos and other structurally-related
pesticides in Salmonella and Streptomyces. Chem.-biol. Interact., 22:
297-308.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett., 9:
157-159.
CARSON, S. (1969) Teratology studies in rabbits (summary
appraisal), Maspeth, New York, Food and Drug Research Laboratories
(Unpublished Report, 30 June).
CARTER, M.K. & MADDUX, B. (1968) Effects of selected agents on the
in vitro inhibition of cholinesterase activity of dichlorvos.
Pharmacologist, 10: 222.
CARTER, M.K. & MADDUX, B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. appl. Pharmacol., 27: 456-463.
CASIDA, J.E., MCBRIDE, L., & NIEDERMEIER, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. agric. food Chem., 10(5): 370-377.
CAVAGNA, G. & VIGLIANI, E.C. (1970) Problčmes d'hygične et de
sécurité dans l'emploi du vapona comme insecticide dans les locaux
domestiques. Med. Lav., 61(8/9): 409-423.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch. environ.
Health, 19(1): 112-113.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1970) Exposure of new-
born babies to Vapona insecticide. Eur. J. Toxicol., 3(1): 49-57.
CERVONI, W.A., OLIVER-GONZALEZ, J., KAYE, S., & SLOMKA, M.B.
(1969) Dichlorvos as a single-dose intestinal anthelmintic therapy for
man. Am. J. trop. Med. Hyg., 18(6): 912-919.
CHATTOPADHYAY, D.P., DIGHE, S.K., DUBE, D.K., & PURNANAND
(1982) Changes in toxicity of DDVP, DFP, and parathion in rats under
cold environment. Bull. environ. Contam. Toxicol., 29: 605-610.
CHEN, Z.M., ZABIK, M.J., & LEAVITT, R.A. (1984) Comparative study of
thin film photodegradative rates for 36 pesticides. Ind. eng. chem.
Prod. Res. Dev., 23: 5-11.
CIPAC HANDBOOK (1980) Analysis of technical and formulated
pesticides, Cambridge, Wheffer and Sons, Collaborative International
Pesticides Analytical Council, Vol. 1A, pp. 1214-1224 (Addendum to
CIPAC 1).
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN, R.J.
(1980) Effects of low level administration of dichlorvos on adreno-
corticotrophic hormone secretion, adrenal cholesteryl ester, and
steroid metabolism. Biochem. Pharmacol., 29: 635-641.
CLINCH, P.G. (1970) Effect on honey bees of combs exposed to vapour
from dichlorvos slow-release strips. N.Z. J. agric. Res., 13(2): 448-
452.
COCHRANE, W.P. (1979) Application of chemical derivatisation
techniques for pesticide analysis. J. chromatogr. Sci., 17: 124-137.
CODEX ALIMENTARIUS COMMISSION (1979) Recommended method of
sampling for the determination of pesticide residues. Report of the
Tenth Session of the Codex Committee on Pesticide Residues, Rome, Food
and Agriculture Organization of the United Nations, Appendix IV, Annex
I, pp. 67-70 (Alinorm 79/24).
CODEX ALIMENTARIUS COMMISSION (1983) Guidelines on good
analytical practice in residue analysis and recommendations for methods
of analysis for pesticide residues, Rome, Food and Agriculture
Organization of the United Nations, p. 40.
COHEN, S.D. & EHRICH, M. (1976) Cholinesterase and carboxyl-esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. appl. Pharmacol., 37: 39-48.
COLLINS, J.A., SCHOOLEY, M.A., & SINGH, V.K. (1971) The effect of
dietary dichlorvos on swine reproduction and viability of their
offspring. Toxicol. appl. Pharmacol., 19: 377.
COLLINS, R.D. & DE VRIES, D.M. (1973) Air concentrations and food
residues from use of Shell No-PestR Insecticide Strip. Bull.
environ. Contam. Toxicol., 9(4): 227-233.
COSTA, L.G. & MURPHY, S.D. (1984) Interaction between acetaminophen
and organophosphate in mice. Chem. Pathol. Pharmacol., 44(3): 389-400.
COULSTON, F. & GRIFFIN, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP
vapours, Albany, New York, Institute of Comparative and Human
Toxicology and Holloman Air Force Base, International Center of
Environmental Safety (Unpublished report).
CRONCE, P.C. & ALDEN, H.S. (1968) Flea-collar dermatitis. J. Am.
Med. Assoc., 206(7): 1563-1564.
DALE, W.E., MILES, J.W., & WEATHERS, D.B. (1973) Measurement of
residues of dichlorvos absorbed by food exposed during disinsection of
aircraft. J. agric. food Chem., 21(5): 858-860.
DAMBSKA, M. & MASLINSKA, D. (1982) Effect of dichlorvos (DDVP)
intoxication of rabbit brain. Neuropathol. Pol. 20(1/2): 77-84.
DAMBSKA, M., MASLINSKA, D., RAKOWSKA, I., & RUTCZYNSKI, M.
(1978) [Evaluation of the effect of dichlorvos poisoning of pregnant
rabbits and rats on the condition and development of their
offspring.] Bromatol. Chem. Toksykol., 11(3): 355-357 (in Polish).
DAMBSKA, M., IWANOWSKI, L., & KOZLOWSKI, P. (1979) The effect of
transplacental intoxication with dichlorvos on the development of
cerebral cortex in newborn rabbits. Neuropathol. Pol. 17(4): 571-576.
DAMBSKA, M., IWANOWSKI, L., MASLINSKA, D., & OSTENDA, M. (1984)
Blood-brain barrier in young rabbit brain after dichlorvos
intoxication. Neuropathol. Pol. 22(1): 129-137.
DARROW, D.I. (1973) Biting lice of goats: control with dichlorvos-
impregnated resin neck collars. J. econ. Entomol., 66: 133-135.
DAS, Y.T., TASKAR, P.K., BROWN, H.D., & CHATTOPADHYAY, S.K.
(1983) Exposure of professional pest control operator to dichlorvos
(DDVP) and residue on house structures. Toxicol. Lett., 17: 95-99.
DAVIDEK, J., SEIFERT, J., & DOLEZALOVA, Z. (1976) The determination
of dichlorvos in milk by using classical and square-wave
polarography. Milchwissenschaft, 31(5): 267-270.
DEAN, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on microorganisms. Arch. Toxikol., 30: 67-74.
DEAN, B.J. (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol., 30: 75-85.
DEAN, B.J. & BLAIR, D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres containing
dichlorvos. Mutat. Res., 40(1): 67-72.
DEAN, B.J. & THORPE, E. (1972a) Cytogenic studies with dichlorvos in
mice and Chinese hamsters. Arch. Toxikol., 30: 39-49.
DEAN, B.J. & THORPE, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxikol., 30: 51-59.
DEAN, B.J., DOAK, S.M.A., & FUNNELL, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium
using Saccharomyces cerevisiae. Arch. Toxikol., 30: 61-66.
DEDEK, W., GEORGI, W., & GRAHL, R. (1979) Comparative degradation and
metabolism of 32P-labelled butonate, trichlorphone and dichlorvos in
crop plants. Biochem. Physiol. Pflanz., 174: 707-722.
DEGRAEVE, N., GILOT-DELHALLE, J., MOUTSCHEN, J.,
MOUTSCHENDAHMEN, M., COLIZZI, A., CHOLLET, M., &
HOUBRECHTS, N. (1980) Comparison of the mutagenic activity of
organophosphorus insecticides in mouse and in the
yeast Schizosaccharomyces pombe. Mutat. Res., 74(3): 201-202.
DEGRAEVE, N., CHOLLET, M.C., MOUTSCHEN, J., MOUTSCHEN-
DAHMEN, M., GILOT-DELHALLE, J., & COLIZZI, A. (1982) Genetic and
cytogenetic effects of chronic treatment with organophosphorus
insecticides. Mutat. Res., 97: 179-180.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984a) Cytogenetic
and genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56: 66-67.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Lett., 21: 315-319.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I., VARGA, L., & FARKAS, J. (1978) Studies on the
immunosuppressive effect of organochlorine and organophosphoric
pesticides in subacute experiments. J. Hyg. Epidemiol. Microbiol.
Immunol., 22: 115-122.
DESI, I., VARGA, L., & FARKAS, J. (1980) The effect of DDVP, an
organophosphorus pesticide, on the humoral and cell-mediated immunity
of rabbits. Arch. Toxicol., 4(Suppl.): 171-174.
DICOWSKY, L. & MORELLO, A. (1971) Glutathione-dependent degradation
of 2,2-dichlorovinyl dimethyl phosphate (DDVP) by the rat. Life
Sci., 10(2): 1031-1037.
DIKSHITH, T.S.S., DATTA, K.K., & CHANDRA, P. (1976) 90-day dermal
toxicity of DDVP in male rats. Bull. environ. Contam. Toxicol., 15(5):
574-580.
DOUGHERTY, E.M., REICHELDERFER, C.F., & FAUST, R.M. (1971)
Sensitivity of Bacillus thuringiensis var. thuringiensis to various
insecticides and herbicides. J. invertebr. Pathol., 17: 292-293.
DMITRIEV, A.I. & KOZEMJAKJN, N.G. (1975) [Veterinary expert report on
the meat of chicken poisoned by dichlorvos.] Veterinarija, 1975(1):
90-92 (in Russian).
DRAGER, G. (1968) [Gas chromatographic method for the determination
of dichlorvos residues in plants and milk.] Pflanzenschutz Nachr.
Bayer, 21(3): 377-384 (in German).
DRAUGHON, F.A. & AYRES, J.C. (1978) Effect of selected pesticides on
growth and citrinin production by Penicillium citrinum. J. food
Sci., 43: 576-578.
DURHAM, W.F., GAINES, TH.B., & HAYES, W.J. (1956) Paralytic and
related effects of certain organic phosphorus compounds. Arch. ind.
Health, 13: 326-330.
DURHAM, W.F., GAINES, TH.B., MCCAULEY, R.H., Jr, SEDLAK, V.A.,
MATTSON, A.M., & HAYES, W.J., Jr (1957) Studies on the toxicity
of O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP). Am. Med. Assoc.
Arch. Ind. Health, 15: 340-349.
DYER, K.F. & HANNA, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res., 21: 175-177.
DZWONKOWSKA, A. & HUBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo. Arch.
Toxicol., 58: 152-156.
EDSON, E.F. (1958) Blood tests for users of organophosphorus
insecticides. World Crops, 10 February: 49-51.
EGYED, M.N. & BENDHEIM, U. (1977) Mass poisoning in chickens by
consumption of organophosphorus (dichlorvos) contaminated drinking-
water. Refu. Vet., 34(3): 107-111.
EICHNER, M. (1978) [Quick residue control of plant and animal food,
tobacco, and tobacco products by Sweep-Co analysis 1.] Z. Lebensm.
Unters. Forsch., 167: 245-249 (in German).
EISLER, R. (1969) Acute toxicities of insecticides to marine decapod
crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish, Washington DC, US
Department of the Interior, Fish and Wildlife Service, Bureau of Sport
Fisheries and Wildlife (Technical Paper No. 46).
ELGAR, K.E. & STEER, B.D. (1972) Dichlorvos concentrations in the air
of houses arising from the use of dichlorvos PVC strips. Pestic.
Sci., 3: 591-600.
ELGAR, K.E., MARLOW, R.G., & MATHEWS, B.L. (1970) The determination
of residues of dichlorvos in crops and tissues. Analyst, 95: 875-878.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972a) Dichlorvos residues
in food arising from the domestic use of dichlorvos PVC strips. Pestic.
Sci., 3: 601-607.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972b) Vapona strips in
shops: residues in foodstuffs. Global aspects of chemistry, toxicology,
and technology as applied to the environment. Environ. Qual. Saf., 1:
217-221.
ELLINGER, CH. (1985) Enzyme activities after in vitro and in
vivo application of dichlorvos. Biomed. Biochim. Acta, 44(2): 311-
316.
ELLINGER, CH., KAISER, G., & HÖRING, H. (1985) [Haematological
changes to rats following exposure to dichlorvos.] Nahrung, 29(4): 351-
355 (in German).
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
ENOMOTO, M.F., NAKADATE, M., NINOMIYA, K., HAYAKAWA, Y.,
ITO, H., IGARASHI, S., UWANUMA, Y., NAKASATO, R., & HATANAKA,
J. (1981) Studies on carcinogenicity of DDVP (2,2-dichlorovinyl
dimethyl phosphate) mixed in drinking-water in rats, Tokyo, Ministry of
Health and Welfare (Cooperative Studies on Carcinogenicity Test on
Mutagens) (unpublished).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERIKSSON, H. & FAYERSSON, Y. (1980) A reliable way of estimating
cholinesterases from whole blood in the presence of
anticholinesterases. Clin. Chim. Acta, 100: 165-171.
ESCUDIE, E. & SALES, P. (1963) Premičres expériences en Haute Volta
sur le dichlorvos résiduel. Bull. World Health Organ., 29: 247-249.
FAHRIG, R. (1973) [Genetic effects of organophosphorus
insecticides.] Naturwissenschaften, 60(1): 50-51 (in German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomotis, L., ed. Chemical carcinogenesis essays,
Lyons, International Agency for Research on Cancer, pp. 161-181 (IARC
Scientific Publication No. 10).
FAO (1977) FAO Specifications for plant protection products,
dichlorvos and fenthion, Rome, Food and Agriculture Organization of the
United Nations.
FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10/1; WHO Food
Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Report of the Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL/1962/M/11; WHO Technical Report Series
No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1967/M11/1; WHO Food
Add./68.30).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1969 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (AGP 1974/M/11; WHO Pesticide Residue
Series No. 4).
FAO/WHO (1977a) Pesticide residues in food. Report of the 1976 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and the
Environment and the WHO Expert Group on Pesticide Residues, Geneva,
World Health Organization (FAO Food and Nutrition Series No. 9; FAO
Plant Production and Protection Series No. 8; WHO Technical Report
Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(ADP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977 Joint
Meeting on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 10
Rev.).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FISHBEIN, L. (1976) Potential hazards of fumigant residues.
Environ. Health Perspect., 14: 39-45.
FISHBEIN, L. (1981) Environmental sources of chemical mutagens. II.
Synthetic mutagens. In: Flamm, W.G. & Mehlman, M.A., ed. Mutagenesis:
advances in modern toxicology, Tokyo, Mishima and Kyoto, Vol. 5, pp.
257-348.
FISHBEIN, L. (1982) An overview of the structural features of
mutagenic S-chlorallylthio and dithiocarbamate pesticides and
trichlorfon, dichlorvos, and their metabolites. In: Proceedings of the
3rd International Conference on Environmental Mutagens and Carcinogens,
21-27 September 1981, Tokyo, Mishima and Kyoto, pp. 371-378.
FISCHER, G.W., SCHNEIDER, P., & SCHEUFLER, H. (1977) [Mutagenicity
of dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester: possible metabolites of the organophosphorus
pesticide trichlorphon.] Chem.-biol. Interact., 19: 205-213 (in
German).
FOLL, C.V., PANT, C.P., & LIETAERT, P.E. (1965) A large-scale field
trial with dichlorvos as a residual fumigant insecticide in northern
Nigeria. Bull. World Health Organ., 32: 531-550.
FOURNIER, E., DALLY, S., & CAMBIER, J. (1976) Neuropathie
périphérique probablement due aux insecticides anticholinesté-
rasiques. Nouv. Presse méd., 5(11): 718.
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Sci. Health, B20(1): 73-95.
FRANK, R., BRAUN, H.E., & FLEMING, G. (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969-81. J. food Prot., 46: 893-900.
FUJITA, K., MATSUSHIMA, S., ABE, E., SASAKI, K., & KUROSAWA, K.
(1977) [Examination of the effects of dichlorvos on the
testis.] Nippon Noson Igakkai Zasshi, 26(3): 328-329 (in Japanese).
FUJITA, Y. (1985) Studies on contact dermatitis from pesticides in
tea growers. Acta Med. Univ. Kagoshima, 27(1): 17-37.
FUNCKES, A.J., MILLER, S., & HAYES, W.J., Jr (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 243-246.
GAINES, TH.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14(3): 515-534.
GAINES, TH.B., HAYES, W.J., Jr, & LINDER, R.E. (1966) Liver
metabolism of anticholinesterase compounds in live rats: relation to
toxicity. Nature (Lond.), 209: 88-89.
GIFAP (1982) Guidelines on pesticide residues to provide data for the
registration of pesticides and the establishment of maximum residue
limits elaborated by Codex Committee on Pesticide Residues, Brussels,
International Group of National Associations of Manufacturers of
Agrochemical Products (Technical Monograph No. 4).
GILLENWATER, H.B., HAREIN, P.K., LOY, E.W., Jr, THOMPSON, J.F.,
LAUDANI, H., & EASON, G. (1971) Dichlorvos applied as a vapour in a
warehouse containing packaged foods. J. stored Prod. Res., 7: 45-56.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health hazards
on use of dichlorvos (DDVP), especially in resin strips. Residue
Rev., 44: 115-159.
GILOT-DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN-
DAHMEN, M. (1983) Mutagenicity of some organophosphorus compounds at
the ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117: 139-
148.
GOH, K.S., EDMISTON, S., MADDY, K.T., & MARGETICH, S. (1986a)
Dissipation of dislodgeable foliar residue for chlorpyrifos and
dichlorvos treated lawn: implication for safe reentry. Bull. environ.
Contam. Toxicol., 37: 33-40
GOH, K.S., EDMISTON, S., MADDY, K.T., MEINDERS, D.D., &
MARGETICH, S. (1986b) Dissipation of dislodgeable foliar residue of
chlorpyrifos and dichlorvos on turf. Bull. environ. Contam.
Toxicol., 37: 27-32.
GOLD, R.E., HOLCSLAW, T., TUPY, D., & BALLARD, J.B. (1984) Dermal
and respiratory exposure to applicators and occupants of residences
treated with dichlorvos (DDVP). J. econ. Entomol., 77: 430-436.
GOTO, S. (1977) [Variation of concentrations of pesticide residues in
field soil.] J. Pestic. Sci., 2: 319-321. (in Japanese, with English
summary).
GOUGH, B.J. & SHELLENBERGER, T.E. (1970) In vivo rabbit blood
cholinesterase inhibition and reactivation following organophosphate
infusion. Toxicol. appl. Pharmacol., 17(1): 302.
GOUGH, B.J. & SHELLENBERGER, T.E. (1977-78) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors and
reactivation with oximes. Drug Chem. Toxicol., 1(1): 25-43.
GRAHL, K. (1979) [The persistence of trichlorfon and dichlorvos in
ponds.] Z. Binnenfisch. DDR, 25(10): 312-316 (in German).
GRAHL, K., STELZER, W., & STOTTMEISTER, S. (1980) [Toxicity of
butonate, trichlorfon, and dichlorvos to Escherichia coli and
Enterobacter aerogenes.] Acta hydrochim. hydrobiol., 8: 19-28 (in
German).
GRAHL, K., HORN, H., & HALLEBACH, R. (1981) [Effect of butonate,
trichlorfon, and dichlorvos on plankton populations.] Acta hydrochim.
hydrobiol., 9(2): 147-161 (in German).
GRATZ, N.G., BRACHA, P., & CARMICHAEL, A. (1963) A village- scale
trial with dichlorvos as a residual fumigant insecticide in southern
Nigeria. Bull. World Health Organ., 29: 251-270.
GREEN, M.H.L., BRIDGES, B.A., GRAY, W.J.H., & MOTTERSHEAD, R.P.
(1973) Comparison of DNA damage caused by dichlorvos and methyl
methanesulfonate in pol A and pol A+ strains of Escherichia coli.
Genetics, 74: s100.
GREEN, M.H.L., MEDCALF, A.S.C., ARLETT, C.R., HARCOURT, S.A., &
LEHMANN, A.R. (1974a) DNA strand breakage caused by dichlorvos, methyl
methane-sulfonate and iodoacetamide in Escherichia coli and cultured
Chinese hamster cells. Mutat. Res., 24(3): 365-378.
GREEN, M.H.L., MEDCALF, A.S.C., & STEVENS, S.W. (1974b) Apparent
indirect DNA damage by dichlorvos and iodoacetamide in Escherichia
coli. Heredity, 33: 446.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens. Mutat.
Res., 38(1): 33-42.
GRIFFIN, D.E., III & HILL, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRUBNER, P. (1972) [Residue problems in the use of phosphoric acid
ester insecticides in mushroom cultures.] Nachrichtenbl.
Pflanzenschutzdienst (DDR), 26: 245-247 (in German).
GUNDERSON, E.C. (1981) Sampling methods for airborne pesticides,
Washington, DC, American Chemical Society, pp. 301-315 (ACS Symposium
Series No. 149).
GUPTA, A.K. & SINGH, J. (1974) Dichlorvos (DDVP) induced breaks in the
salivary gland chromosomes of Drosophila melanogaster. Curr.
Sci., 43(20): 661-662.
GUPTA, R.C., DAVE, S.K., SHAH, M.P., & KASHYAP, S.K. (1979) A
monitoring study of workers handling pesticides in warehouses and
godowns. J. environ. Sci. Health, B14(4): 405-416.
HALEY, T.J., FARMER, J.H., HARMON, J.R., & DOOLEY, K.L. (1975)
Estimation of the LD1 and extrapolation of the LD0.1 for five
organophosphate pesticides. Arch. Toxicol., 34: 103-109.
HANNA, P.J. & DYER, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res., 28: 405-420.
HASAN, M. & ALI, S.F. (1980) Organophosphate pesticide dichlorvos-
induced increase in the rate of lipid peroxidation in the different
regions of the rat brain: supporting ultra-structural
findings. Neurotoxicology, 2: 43-52.
HASAN, M., MAITRA, S.C., & ALI, S.F. (1979) Organophosphate pesticide
DDVP-induced alterations in the rat cerebellum and spinal cord: an
electron microscopic study. Exp. Pathol., 17(2): 88-94.
HASS, D.K., COLLINS, J.A., & KODAMA, J.K. (1972) Effects of orally
administered dichlorvos in rhesus monkeys. J. Am. Vet. Med.
Assoc., 161(6): 714-719.
HATCH, G.G., ANDERSON, T.M., HUBERT, R.A., KOURI, R.E.,
PUTMAN, D.L., CAMERON, J.W., NIMS, R.W., MOST, B., SPALDING, J.W.,
TENNANT, R.W., & SCHECHTMAN, L.M. (1986) Chemical enhancement of
sa7 virus transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagenesis.,
8: 515-531.
HATTORI, K., SATO, H., TSUCHIYA, K., YAMAMOTO, N., & OGAWA, E.
(1974) [Toxicological studies on the influences of chemicals to the
birds. I. Oral acute toxicity and cholinesterase inhibition of three
organophosphate insecticides in Japanese quail.] Hokkaidoritsu Eisei
Kenkyusho Ho, 24: 35-38 (in Japanese, with English summary).
HAYES, A.L., WISE, R.A., & WEIR, F.W. (1980) Assessment of
occupational exposure to organophosphates in pest control
operators. Am. Ind. Hyg. Assoc. J., 41(8): 568-575.
HAYES, W.J., Jr (1961) Safety of DDVP for the disinsection of
aircraft. Bull. World Health Organ., 24: 629-633.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons:
emergency information for treating poisoning, Atlanta, Georgia, US
Department of Health, Education and Welfare, Public Health Service,
Communicable Diseases Center (Public Health Service Publication No.
476).
HAYES, W.J., Jr (1982) Organic phosphorus pesticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and Wilkins,
pp. 343-351.
HAZELWOOD, J.C., STEFAN, G.E., & BOWEN, J.M. (1979) Motor unit
irritability in beagles before and after exposure to cholinesterase
inhibitors. Am. J. vet. Res., 40(6): 852-856.
HEMMINKI, K. (1983) Nucleic acid adducts of chemical carcinogens and
mutagens. Arch. Toxicol., 52: 249-285.
HENRIKSSON, K., KALLELA, K., VIRTAMO, M., & PFAFFLI, P. (1971)
[The toxicity of DDVP (dichlorvos) evaporated from Vapona strip.] J.
Sci. Agric. Soc. Finl., 43: 187-200 (in Finnish).
HEUSER, S.G. & SCUDAMORE, K.A. (1966) A rapid method for sampling
dichlorvos vapour in air. Chem. Ind., 50: 2093-2094.
HEUSER, S.G. & SCUDAMORE, K.A. (1975) A method for the assessment
under standard conditions of the output of dichlorvos slow-release
units used for insect control. Analyst, 100: 129-135.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp. 1-51
(Special Scientific Report: Wildlife No. 191).
HINE, C.H. (1962) 90-day chronic toxicity studies of
VaponaR insecticide for dogs, San Francisco, California, The Hine
Laboratories (Unpublished Report No. 1, 1 September).
HINE, C.H. & SLOMKA, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
HINE, C.H. & SLOMKA, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. appl. Pharmacol., 17(1):
304-305.
HODGSON, E. & CASIDA, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethylphosphate. J. agric. food
Chem., 10(3): 208-214.
HOLMSTEDT, B., NORDGREN, I., SANDOZ, M., & SUNDWALL, A. (1978)
Metrifonate. Summary of toxicological and pharmacological information
available. Arch. Toxicol., 41: 3-29.
HORIUCHI, N. & ANDO, Y. (1978) [Photosensitivity caused by
pesticides.] (original unpublished information used as a basis for
article by Horiuchi et al., 1978) (in Japanese).
HORIUCHI, N., ANDO, S., & SUZUKI, A. (1978) [Photosensitization due
to pesticides.] Nippon Noson Igakkai Zashi, 27(3): 450-451 (in
Japanese).
HORVATH, J., HEGATH, V., & PUCHA, K. (1968) A study of the influence
of cholinesterase with cattle housed in byres provided with DDVP
strips. Agrochemia, 8(9): 267-268.
HUNTER, C.G. (1969) Report on initial studies of deliberate exposures
to high concentrations of dichlorvos by human subjects, Sittingbourne,
Tunstall Laboratory Shell (Unpublished Report, July).
HUNTER, C.G. (1970a) Dichlorvos: inhalational exposures with human
subjects. Part 1, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0061.70).
HUNTER, C.G. (1970b) Dichlorvos: inhalational exposures with human
subjects. Part 2, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0067.70).
HUSSEY, N.W. & HUGHES, J.T. (1964) Investigations on the use of
dichlorvos in the control of the mushroom phorid Megaselia
halterata (Wood). Ann. appl. Biol., 54: 129-139.
HUTSON, D.H. & HOADLEY, E.C. (1972a) The comparative metabolism of
14C-vinyl-dichlorvos in animals and man. Arch. Toxikol., 30: 9-18.
HUTSON, D.H. & HOADLEY, E.C. (1972b) The metabolism of 14C-methyl-
dichlorvos in the rat and mouse. Xenobiotica, 2(2): 107-116.
HUTSON, D.H., BLAIR, D., HOADLEY, E.C., & PICKERING, B.A. (1971a)
The metabolism of 14C-VaponaR in rats after administration by oral
and inhalation routes. Toxicol. appl. Pharmacol., 19: 378-379.
HUTSON, D.H., HOADLEY, E.C., & PICKERING, B.A. (1971b) The
metabolic fate of vinyl-1-14C-dichlorvos in the rat after oral and
inhalation exposure. Xenobiotica, 1(6): 593-611.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K. (1978)
EEG, ECG, and respiratory response to acute insecticide exposure. Bull.
environ. Contam. Toxicol., 19: 47-55.
IARC (1979) Some halogenated hydrocarbons, Lyons, International Agency
for Research on Cancer, pp. 97-127 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IRPTC (1984) DDVP, Geneva, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme (Scientific
Reviews of Soviet Literature on Toxicity and Hazards of Chemicals, No.
79).
ISSHIKI, K., MIYATA, K., MATSUI, S., TSUTSUMI, M., & WATANABE, T.
(1983) [Effects of post-harvest fungicides and piperonyl butoxide on
the acute toxicity of pesticides in mice.] Shokuhin Eiseigaku
Zasshi, 24(3): 268-274 (in Japanese, with English summary).
IVEY, M.C. & CLABORN, H.V. (1969) GLC determination of dichlorvos in
milk, eggs and various body tissues of cattle and chickens. J. Assoc.
Off. Anal. Chem., 52(6): 1248-1251.
IWATA, Y., SUGITANI, A., & YAMADA, F. (1981) [An analytical method
for organophosphorus pesticides in the onion.] Shokuhin Eiseigaku
Zasshi, 22: 484-489 (in Japanese, with English summary).
JAKUBOWSKA, B. & NOWAK, A. (1973) [The effect of organophosphorus and
carbamate insecticides on the development of soil fungi.] Zesz. Nauk.
Akad. Roln. Szczecinie, 39(10): 141-150 (in Polish).
JAQUES, R. (1964) The protective effect of pyridine-2-aldoxime
methiodide (P2AM), bis-(4-hydroxyimino-methyl-pyridinium-1-methyl)-
ether dichloride (ToxogoninR) and atropine in experimental DDVP
poisoning. Helv. Physiol. Pharmacol. Acta, 22(3): 174-183.
JAYASURIYA, V.U., DE S. & RATNAYAKE, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inf. Serv., 50: 184-186.
JENSEN, J.A., FLURY, V.P., & SCHOOF, H.F. (1965) Dichlorvos vapour
disinsection of aircraft. Bull. World Health Organ., 32: 175-179.
JOHNSON, M.K. (1969) The delayed neurotoxic effect of some
organophosphorus compounds. Identification of the phosphorylation site
as an esterase. Biochem. J., 114(4): 711-717.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanisms and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing delayed
neurotoxic effects. Mechanism of action and structure/ activity
studies. Arch. Toxicol., 34: 259-288.
JOHNSON, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch.
Toxicol., 41: 107-110.
JOHNSON, M.K. (1981) Delayed neurotoxicity: do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol., 49(Suppl. 5): 87-98.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981a) Pesticide
metal and other chemical residues in adult total diet samples.
XII. Pestic. monit. J., 15(1): 54-69.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981b) Pesticide, heavy metal, and other chemical residues in infant
and toddler total diet samples. II. August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-50.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 137).
JOLLEY, W.P., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of VaponaR insecticide into their daily diet, Cincinnati,
Ohio, The Kettering Laboratory (Unpublished Report, 19 January).
KAWACHI, T., KOMATSU, T., KADA, T., ISHIDATE, M., SASAKI, M.,
SUGIYAMA, T., & TAZIMA, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In: G.M.
Williams et al., ed. The predictive value of short-term screening tests
in carcinogenicity evaluation, Amsterdam, Oxford, New York,
Elsevier/North Holland Biomedical Press, pp. 253-267.
KENAGA, E.E. (1979) Acute and chronic toxicity of 75 pesticide to
various animal species. Down Earth, 35(2): 25-31
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. environ.
Health, 16: 805-808.
KIMMERLE, G. & LORKE, D. (1968) [Toxicity of insecticidal phosphoric
acid esters.] Pflanzenschutz Nachr. Bayer, 21(1): 111-142 (in German).
KIRKLAND, V.L. (1971) Some aspects of acute inhalation pharmacology
of dichlorvos in swine. Paper presented at: American Chemical Society
for Pharmacology and Experimental Therapeutics and the Division of
Medical Chemistry, Burlington, Vermont, 25 August, 1971.
KLIGERMAN, A.D., EREXSON, G.L., & WILMER, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res., 157: 181-187.
KNAPP, F.W. & GRADEN, A.P. (1964) Accidental exposure of dairy cows
to excessive amount of dichlorvos. J. econ. Entomol., 57: 790-791.
KOBAYASHI, H., YUYAMA, A., IMAJO, S., & MATSUSAKA, N. (1980)
Effects of acute and chronic administration of DDVP (dichlorvos) on
distribution of brain acetylcholine in rats. J. toxicol. Sci., 5: 311-
319.
KOBAYASHI, H., YUYAMA, A., & CHIBA, K.I. (1986) Cholinergic system
of brain tissue in rats poisoned with the organophosphate
0,0-demethyl 0-(2,2-dichlorovinyl) phosphate. Toxicol. appl.
Pharmacol., 82: 32-39.
KODAMA, J.K. (1960) Technical DDVP. Acute oral administration:
dogs, Vienna, Virginia, Hazleton Laboratories (Unpublished Report, 20
January).
KODAMA, J.K. (1968) Experimental evaluation in guinea-pigs of skin-
sensitizing potential of components of formulated dichlorvos/
polyvinylchloride products, Modesto, California, Shell Development
Company (Unpublished Technical Progress Report No. M-67-68).
KONISHI, Y., DENDA, A., & KITAOKA, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3FI mice, Tokyo, Ministry of Health
and Welfare (Cooperative Studies on Carcinogenicity Tests on Mutagens)
(unpublished).
KRAMERS, P.G.N. & KNAAP, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of D rosophila melanogaster.
Mutat. Res., 57: 103-105.
KRAUSE, CHR., VON & KIRCHHOFF, J. (1970) [Gas chromatographic
determination of organophosphate residues in market samples of fruits
and vegetables.] Dtsch. Lebensm. Rundschau., 66(6): 194-199 (in
German).
KRAUSE, W. (1977) Influence of DDT, DDVP, and malathion on FSH, LH,
and testosterone serum levels and testosterone concentrations in
testis. Bull. environ. Contam. Toxicol., 18(2): 231-242.
KRAUSE, W. & HOMOLA, S. (1972) [Influence on spermatogenesis by DDVP
(dichlorvos).] Arch. Dermatol. Forsch., 244: 439-441 (in German).
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat* testis after the
application of dichlorvos (DDVP). Bull. environ. Contam.
Toxicol., 11(5): 429-433 (*study was performed on mice).
KRAUSE, W., HAMM, K., & WEISSMULLER, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. environ. Contam. Toxicol., 15(4): 458-462.
KUWABARA, K., NAKAMURA, A., & KASHIMOTO, T. (1980) Effect of
petroleum oil, pesticides, PCBs, and other environmental contaminants
on the hatchability of Artemia salina dry eggs. Bull. environ.
Contam. Toxicol., 25: 69-74.
LAFONTAINE, A., AERTS, J., & JACQUES, P. (1981) [Toxicity,
carcinogenic, mutagenic, and teratogenic action of dichlorvos.] Arch.
belg. Méd. soc. Hyg. Méd. Trav. Méd. lég., 39(3): 159-174 (in Dutch).
LAL, R. (1982) Accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms. Adv. appl.
Microbiol., 28: 149-200.
LAMOREAUX, R.J. & NEWLAND, L.W. (1978) The fate of dichlorvos in
soil. Chemosphere, 7(10): 807-814.
LAWLEY, P.D., SHAH, S.A., & ORR, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (dichlorvos,
DDVP). Chem.-biol. Interact., 8: 171-182.
LAWS, E.R., Jr (1966) Route of absorption of DDVP after oral
administration to rats. Toxicol. appl. Pharmacol., 8: 193-196.
LEARY, J.S., HIRSCH, L., LAVOR, E.M., FEICHTMEIR, E., SCHULTZ, D.,
KOOS, B., ROAN, C.R., FONTENOT, C., & HINE, C.H. (1971) An evaluation
of the safety of No-PestR strip insecticide with special reference to
respiratory and dietary exposure of occupants of homes in
Arizona. Toxicol. appl. Pharmacol., 19: 379.
LEARY, J.S., KEANE, W.T., FONTENOT, C., FEICHTMEIR, E.F.,
SCHULTZ, D., KOOS, B.A., HIRSCH, L., LAVOR, E.M., ROAN, C.C., &
HINE, C.H. (1974) Safety evaluation in the home of polyvinyl chloride
resin strips containing dichlorvos (DDVP). Arch. environ. Health, 29:
308-314.
LEONARD, A. (1976) Heritable chromosome aberrations in mammals after
exposure to chemicals. Radiat. environ. Biophys., 13: 1-8.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides. In:
Lee, R.L., ed. Air pollution from pesticides and agricultural
processes, Cleveland, Ohio, CRC Press, pp. 51-95.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides on
decomposition of organic matter nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LIEBERMAN, M.T. & ALEXANDER, M. (1983) Microbial and non-
enzymatic steps in the decomposition of dichlorvos (2,2-
dichlorovinyl O,O-dimethylphosphate). J. agric. food Chem., 31: 265-
267.
LIVINGSTON, R.J. (1977) Review of current literature concerning the
acute and chronic effects of pesticides on aquatic organisms. CRC crit.
Rev. environ. Control, 7: 325-351.
LLOYD, T.S. (1973) Accidental poisoning in birds. Vet. Rec., 5 May:
489.
LOEFFLER, J.E., DE VRIES, D.M., YOUNG, R., & PAGE, A.C. (1971)
Metabolic fate of inhaled dichlorvos in pigs. Toxicol. appl.
Pharmacol., 19: 378.
LOEFFLER, J.E., POTTER, J.C., SCORDELIS, S.L., HENDRICKSON, H.R.,
HUSTON, C.K., & PAGE, A.C. (1976) Long-term exposure of swine to a
14C-dichlorvos atmosphere. J. agric. food Chem., 24(2): 367-371.
LOFROTH, G. (1970) Alkylation of DNA by dichlorvos. Natur-
wissenschaften, 57(8): 393-394.
LOFROTH, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
Naturforsch., 33: 783-785.
LOFROTH, G. & WENNERBERG, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch., 29: 651.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating properties of
2,2-dichlorovinyl dimethyl phosphate: a disregarded hazard. Environ.
Mutat. Soc. Newslett., 2: 21-27.
LOHS, K., FISCHER, G.W., & DEDEK, W. (1976) [Chemical-toxicological
aspects of the alkylating properties of organophosphate pesticides.]
Sitzungsber. Akad. Wiss. (DDR), 11N: 1-58 (in German).
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of organophosphorus
pesticides: predictions can be based on in vitro studies with hen and
human enzymes. Arch. Toxicol., 41: 215-221.
LUDKE, J.L. & LOCKE, L.N. (1976) Duck deaths from accidental
ingestion of anthelmintic. Avian Dis., 20(3): 607-608.
LUKE, M.A., FROBERG, J.E., DOOSE, G.M., & MASUMOTO, H.T. (1981)
Improved multiresidue gas chromatographic determination of
organophosphorus organonitrogen, and organohalogen pesticides in
produce, using flame photometric and electrolytic conductivity
detectors. J. Assoc. Off. Anal. Chem., 64(5): 1187-1195.
MACDONALD, R. (1982) Toxicology of consumer products: the acute
(4-h) inhalation toxicity of dichlorvos vapour in rats and mice,
Sittingbourne, Shell Research Ltd (Unpublished Report SBGR.82.145).
MACKLIN, A.W. & RIBELIN, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc., 159(12): 1743-
1748.
MADDY, K.T., EDMISTON, S., & FREDRICKSON, A.S. (1981a) Monitoring
residue of DDVP in room air and on horizontal surfaces following use of
a room fogger, Sacramento, California, California Department of Food
and Agriculture (Unpublished Report No. HS-897).
MADDY, K.T., SCHNEIDER, F., LOWE, J., OCHI, E., FREDRICKSON, A.S.,
& MARGOTICH, S. (1981b) Vapona (DDVP) exposure potential to in
mushroom houses in Ventura County, California, in 1981, Sacramento,
California, California Department of Food and Agriculture (Unpublished
Report No. HS-861).
MAJEWSKI, T., PODGORSKI, W., BIALKOWSKI, Z., & TYCZKOWSKI, J.
(1978) [Effects of application of different forms of DDVP to
cows.] Rocz. Nauk. Zootech., 5(2): 65-74 (in Polish).
MAJEWSKI, T., PODGORSKI, W., & MICHALOWSKA, R. (1979) Retention
of dichlorvos (DDVP) in rabbits. Pol. Arch. Weter., 21(2): 249-255.
MASLINSKA, D. & ZALEWSKA, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia histochem. cytochem., 16(4): 335-341.
MASLINSKA, D., STROSZNAJDER, J., ZALEWSKA, T., & ORLEWSKI, P.
(1984) Phospholipid-protein ratio in brain of suckling rabbits treated
with an organophosphorus compound. Int. J. tissue React., 6(4): 317-
322.
MATHIAS, C.G.T. (1983) Persistent contact dermatitis from the
insecticide dichlorvos. Contact Dermatit., 9: 217-218.
MATSUMURA, F. & BOUSH, G.M. (1968) Degradation of insecticides by a
soil fungus Trichoderm viride. J. econ. Entomol., 61(3): 610-612.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
310-311.
MENDOZA, C.E. (1974) Analysis of pesticides by the thin-layer
chromatographic enzyme inhibition technique. Part II. Residue Rev., 50:
43-72.
MENDOZA, C.E. & SHIELDS, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin-layer chromatography. J. Assoc. Off.
Anal. Chem., 54: 507-512.
MENZ, M., LUETKEMEIER, H., & SACHSSE, K. (1974) Long-term exposure
of factory workers to dichlorvos (DDVP) insecticide. Arch. environ.
Health, 28: 72-76.
MESTRES, R., CHEVALLIER, CH., ESPINOZA, CL., & CORNET, R. (1977)
Application du couplage chromatographie gazeuse spectrométrie de masse
aux méthodes de recherche et de dosage des résidus de pesticides et de
micropolluants organiques dans les matériaux de l'environnement et les
matičres alimentaires. Ann. Fac. exp. Chim., 70(751): 177-188.
MESTRES, R., ILLES, S., CAMPO, M., & TOURTE, J. (1979a) Développement
de la méthode polyvalente d'analyse des résidus des pesticides dans les
plantes et les matičres alimentaires d'origine végétale. Trav. Soc.
Pharm. Montpellier, 39: 323-328.
MESTRES, R., ATMAWIJAYA, S., & CHEVALLIER, CH. (1979b) Méthode
de recherche et de dosage des résidus de pesticides dans les produits
céréaliers. I. Organochlorés, organophosphorés, pyréthrines, et
pyréthrinoďdes. Ann. Fac. exp. Chim., 72: 577-589.
MICHALEK, S.M. & BROCKMAN, H.E. (1969) A test for mutagenicity of
Shell "No-Pest Strip Insecticide" in Neurospora crassa. Neurospora
Newslett., 16: 8.
MICHEL, H.O. (1949) An electrometric method for the determination of
red blood cell and plasma cholinesterase activity. J. lab. clin.
Med., 34: 1564-1568.
MILES, J.W., FETZER, L.E., & PEARCE, G.W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ. Sci.
Technol., 4: 420-425.
MITSUI, T., OZAKI, H., KUMANO, H., & SANO, H. (1963) Insecticide
determination. I. Colormetric determination of dimethyl 2,2-
dichlorovinyl phosphate (DDVP). Chem. pharm. Bull., 11(5): 619-623.
MIYAMOTO, J. (1959) Non-enzymatic conversion of Dipterex into DDVP
and their inhibitory action on enzymes. Botyu-Kagaku (Sci. pestic.
Control), 24: 130-137.
MODAK, A.T., STAVINOHA, W.B., & WEINTRAUB, S.T. (1975) Dichlorvos
and the cholinergic system: effects on cholinesterase and acetylcholine
and choline contents of rat tissues. Arch. int. Pharmacodyn., 217:
293-301.
MOHN, G. (1973) S-methyltryptophan resistance mutations in
Escherichia coli K12. Mutagenic activity of mono-functional alkylating
agents including organophosphorus insecticides. Mutat. Res., 20: 7-
15.
MORIYA, M., KATO, K., & SHIRASU, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res., 57: 259-263.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MORPURGO, G., BELLINCAMPI, D., GUALANDI, G., BALDINELLI, L., &
SERLUPI CRESCENZI, O. (1979) Analysis of mitotic non-disjunction
with Aspergillus nidulans. Environ. Health Perspect., 31: 81-95.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., &
DEGRAEVE, N. (1981) Metrifonate and dichlorvos: cytogenetic
investigations. Acta pharmacol. toxicol., 49(Suppl. 5): 29-39.
MULLER, G.H. (1970) Flea collar dermatitis in animals. J. Am. Vet.
Med. Assoc., 157(11): 1616-1626.
NAGY, ZS., MILE, I., & ANTONI, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta microbiol. Acad. Sci.
Hung., 22: 309-314.
NAIDU, N.V., REDDY, K.S., JANARDHAN, A., & MURTHY, M.K. (1978)
Toxicological investigation of dichlorvos in chicks. Indian J.
Pharmacol., 10(14): 323-326.
NAKAMURA, K. & SHIBA, H. (1980) [Study on pesticide residues in fruit
and vegetables.] Bull. Saitama prefect. agric. exp. Stn, 36: 35-56 (in
Japanese).
NARCISSE, J.K. (1967) A potentiation study in rats of VaponaR with
26 other cholinesterase-inhibiting compounds, Menlo Park, California,
Stanford Research Institute (Unpublished Report, 2 June).
NCI (1977) Bioassay of dichlorvos for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute.
NEUWIRTH, E.A. & WHITE, T.T. (1961) Safety aspects of DDVP. Soap
chem. Spec., 37(3): 95-98.
NICHOLAS, A.H., VIENNE, M., & BERGHE, H., VAN DEN (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Lett., 2(5): 271-
275.
NIOSH (1979) NIOSH Manual of analytical methods. Analytical method
No. P and CAM 295, 2nd ed., Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. 5 (Publication No. 79-144).
NISHIO, A. & UYEKI, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphorus
insecticides and their oxygen analogs. J. Toxicol. environ. Health, 8:
939-946.
NISHIO, A. & UYEKI, E.M. (1982) Nuclease sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants. Biochem.
biophys. Res. Commun., 107(2): 485-491.
NORDGREN, I., BERGSTROM, M., HOLMSTEDT, B., & SANDOZ, M.
(1978) Transformation and action of metrifonate. Arch. Toxicol., 41:
31-41.
NOWELL, P.T., SCOTT, C.A., & WILSON, A. (1962) Hydrolysis of
neostigmine by plasma-cholinesterase. Br. J. Pharmacol., 19: 498-502.
NTP (1986) Carcinogenicity study in rats and mice with
dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (Unpublished data).
NTP (1987) NTP technical report on the toxicology and carcinogenesis
of dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (NTP-TR342).
OBA, T. & KAWABATA, G. (1962) [Application of infrared absorption
spectroscopy to examination of drugs and their preparations. XIV.
Determination of O,O-dimethyl 2,2-dichlorovinyl phosphate (DDVP) and
its preparation.] Eiseishikenjo-hokoku, 80: 1-3 (in Japanese, with
English summary).
OGATA, H., SAITO, E., MURATA, H., SHIBAZAKI, T., & INOUE, T. (1975)
[A new colorimetric method of determination of O,O-dimethyl 2,2-
dichlorovinyl phosphate in insecticidal preparations.] Yakugaku
Zasshi, 95(12): 1483-1491 (in Japanese, with English summary).
OLINSKI, R., WALTER, Z., WIADERKIEWICZ, R., LUKASOVA, E., &
PALECEK, E. (1980) Changes in DNA properties due to treatment with
the pesticides malathion and DDVP. Radiat. environ. Biophys., 18: 65-
72.
OMKAR, & SHUKLA, G.S. (1984) Alteration in carbohydrate metabolism of
fresh-water prawn Macrobianchium lamarrei after dichlorvos
exposure. Ind. Health, 22: 133-136.
PACHECKA, J., SULINSKI, A., & TRACZYKIEWICZ, K. (1977) The effect
of acute intoxication by dichlorvos and trichlorphon on the activities
of some rat brain esterases. Neuropathol. Polska, 15(1): 85-92.
PAGE, A.C., DE VRIES, D.M., YOUNG, R., & LOEFFLER, J.E. (1971)
Metabolic fate of ingested dichlorvos in swine. Toxicol. appl.
Pharmacol., 19: 378.
PAGE, A.C., LOEFFLER, J.E., HENDRICKSON, H.R., HUSTON, C.K., &
DEVRIES, D.M. (1972) Metabolic fate of dichlorvos in swine. Arch.
Toxikol., 30: 19-27.
PAIK, S.G. & LEE, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool., 20(1):
19-28.
PENA CHAVARRIA, A., SWARTZWELDER, J.C., VILLAREJOS, V.M.,
KOTCHER, E., & ARGUEDAS, J. (1969) Dichlorvos: an effective broad-
spectrum anthelmintic. Am. J. trop. Med. Hyg., 18(6): 907-911.
PEROCCO, P. & FINI, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (WHO Report
No. VBC/84.889).
PODREBARAC, D.S. (1984) Pesticide, heavy metals, and other chemical
residues in infant and toddler total diet samples. IV. October 1977 -
September 1978. J. Assoc. Off. Anal. Chem., 67(1): 166-175.
POTTER, J.C., LOEFFLER, J.E., COLLINS, R.D., YOUNG, R., & PAGE,
A.C. (1973a) Carbon-14 balance and residues of dichlorvos and its
metabolites in pigs dosed with dichlorvos-14C. J. agric. food
Chem., 21(2): 163-166.
POTTER, J.C., BOYER, A.C., MARXMILLER, R.E., YOUNG, R., &
LOEFFLER, J.E. (1973b). Radioisotope residues and residues of
dichlorvos and its metabolites in pregnant sows and their progeny dosed
with dichlorvos-14C or dichlorvos-36Cl formulated as PVC
pellets. J. agric. food Chem., 21(4): 734-738.
PURSHOTTAM, T. & KAVEESHWAR, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviat. Space
environ. Med., 50(6): 581-584.
PURSHOTTAM, T. & SRIVASTAVA, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
environ. Health, 39(6): 425-430.
PYM, R.A.E., SINGH, G., GILBERT, W.S., ARMSTRONG, J.P., &
MCCLEARY, B.V. (1984) Effects of dichlorvos, maldison, and
pirimiphos-methyl on food consumption, egg production, egg and tissue
residues, and plasma acetylcholinesterase inhibition in layer strain
hens. Aust. J. exp. Agric. anim. Husb., 24: 83-92.
QUARTERMAN, K.D., LOTTE, M., & SCHOOF, H.F. (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 231-235.
RADELEFF, R.D. & WOODARD, G.T. (1957) The toxicity of organic
phosphorus insecticides to livestock. J. Am. Vet. Med. Assoc., 130:
215-216.
RAHMATULLAH, M., JAHAN, R., CHOWDHURY, A.A., FARUK, A.B.M.,
& CHOWDHURY, M.S.K. (1978) Effect of organophosphorus insecticides on
fermentation processes in Aspergillus niger. J. ferment. Technol., 56:
169-172.
RAMEL, C. (1981) Does dichlorvos constitute a genotoxic hazard? In:
Kappas, A., ed. Progress in environmental mutagenesis and
carcinogenesis, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 69-78 (Progress in Mutation Research, Vol. 2.).
RAMEL, C., DRAKE, J., & SUGIMURA, T. (1980) An evaluation of the
genetic toxicity of dichlorvos. Mutat. Res., 76: 297-309.
RASMUSSEN, W.A., JENSEN, J.A., STEIN, W.J., & HAYES, W.J. (1963)
Toxicological studies of DDVP for disinsection of aircraft. Aerosp.
Med., 34(7): 593-600.
RATH, S. & MISRA, B.N. (1979a) Relative toxicity of dichlorvos (DDVP)
to Tilapia mossambica Peters of 3 different age groups. Exp.
Gerontol., 14: 307-309.
RATH, S. & MISRA, B.N. (1979b) Sub-lethal effects of dichlorvos (DDVP)
on respiratory metabolism of Tilapia mossambica of 3 age groups. Exp.
Gerontol., 14: 37-41.
RATH, S. & MISRA, B.N. (1980) Pigment disperson in Tilapia
mossambica Peters exposed to dichlorvos (DDVP). Curr. Sci., 49(23):
907-909.
RATH, S. & MISRA, B.N. (1981) Toxicological effects of dichlorvos
(DDVP) on brain and liver acetylcholinesterase (AChE) activity
of Tilapia mossambica Peters. Toxicology, 19: 239-245.
RAUWS, A.G. & LOGTEN, M.J., VAN (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in
chicken. Toxicology, 1: 29-41.
REECE, R.L. (1982) Observations on the accidental poisoning of birds
by organophosphate insecticides and other toxic substances. Vet.
Rec., 111(20): 453-455.
REEVES, J.D., DRIGGERS, D.A., & KILEY, V.A. (1981) Household
insecticide associated aplastic anaemia and acute leukaemia in
children. Lancet, 8 August: 300-301.
REINER, E. & PLESTINA, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by O,O-dimethyl-2,2-
dichlorovinyl phosphate. Toxicol. appl. Pharmacol., 49: 451-454.
RIDER, J.A., MOELLER, H.C., & PULETTI, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies with
Guthion, and determination of incipient toxicity level of dichlorvos in
humans. Fed. Proc., 26: 427.
RIDER, J.A., MOELLER, H.C., PULETTI, E.J., & SWADER, J. (1968)
Studies on the anticholinesterase effects of methyl parathion, guthion,
dichlorvos, and gardona in human subjects. Fed. Proc., 27: 597.
ROSENBERG, A., LIEBERMAN, M.T., & ALEXANDER, M. (1979)
Microbial degradation of pesticides, Springfield, Virginia, US
National Technical Information Service (PB 79-123156).
ROSENKRANZ, H.S. (1973) Preferential effect of dichlorvos
(VaponaR) on bacteria deficient in DNA polymerase. Cancer Res., 33:
458-459.
ROSENKRANZ, H.S. & LEIFER, Z. (1980) Determining the DNA-modifying
activity of chemicals using DNA polymerase-deficient Escherichia
coli. In: de Serres, F.J. & Hollander, A., ed. Chemical mutagens:
principles and methods for their detection, New York, Plenum Press,
Vol. 6, pp. 109-147.
ROSENKRANZ, H.S. & ROSENKRANZ, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia (Basel), 28(4): 386-387.
SAGNER, G. & SCHONDUBE, M. (1982) [Determination and toxicological
appraisal of indoor concentrations of dichlorvos after the application
of spraying products.] Schriftenr. Ver. Wasser Boden Lufthyg., 53:
359-368 (in German).
SAKAMA, K. & NISHIMURA, M. (1977) [Studies on inhalation toxicity of
organic phosphorus insecticides. II. Approach to establishment of
threshold limit value.] Jpn. J. ind. Health, 19: 357-358 (in
Japanese).
SASINOVICH, L.M. (1968) [Substantiation of the maximum permissible
concentration of DDVP in the air of the working zone.] Gig. i Sanit.,
33(12): 35-39 (in Russian).
SASINOVICH, L.M. (1970) [Toxicology of dimethyldichlorovinyl
phosphate and hygienic characteristics of its use.] Gig. Primen.
Toksikol. Pestits. Klin. Otravl., 8: 297-307 (in Russian).
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical, and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHAFER, E.W., Jr & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and management
materials, Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SCHMIDT, G., SCHMIDT, M., & NENNER, M. (1975) Inhibition of
acetylcholinesterase in the bronchial system of rats caused by
inhalation of dichlorvos (DDVP). Naunyn Schmiedeberg Arch.
Pharmacol., 287(Suppl.): R97.
SCHMIDT, G., SCHMIDT, M., NENNER, M., & VETTERLEIN, F. (1979)
Effects of dichlorvos (DDVP) inhalation on the activity of
acetylcholinesterase in the bronchial tissue of rats. Arch.
Toxicol., 42: 191-198.
SCHOOF, H.F., JENSEN, J.A., PORTER, J.E., & MADDOCK, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP
vapour. Bull. World Health Organ., 24: 623-628.
SCHULTZ, D.R., MARXMILLER, R.L., & KOOS, B.A. (1971) Residue
determination of dichlorvos and related metabolites in animal tissue
and fluids. J. agric. food Chem., 19(6): 1238-1243.
SCHULZE, H.D. (1979) [Current problems of pest control with
dichlorvos in hospital.] Z. gesamte Hyg., 25(5): 421-425 (in German).
SCHWETZ, B.A., IOSET, H.D., LEONG, B.K.J., & STAPLES, R.E. (1979)
Teratogenic potential of dichlorvos given by inhalation and gavage to
mice and rabbits. Teratology, 20: 383-388.
SEGERBACK, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP (2,2-dichlorovinyl
dimethylphosphate). Hereditas, 94: 73-76.
SEGERBACK, D. & EHRENBERG, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta pharmacol. toxicol., 49(Suppl. 5): 56-66.
SERA, K., MATSUNAGA, A., MURAKAMI, A., SATO, I., YAMASHITA,
K., & YOSHIMORI, H. (1959) Qualitative analysis of organic phosphorus
color reactions and simple detection. Kumamoto med. J., 12(3): 193-
213.
SHAFIK, M.T., BRADWAY, D., & ENOS, H.F. (1971) A method for
confirmation of organophosphorus compounds at the residue level.
Bull. environ. Contam. Toxicol., 6(1): 55-66.
SHELLENBERGER, T.E. (1980) Organophosphorus pesticide inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. environ. Sci. Health, B15(6): 795-822.
SHELLENBERGER, T.E., NEWELL, G.W., OKAMOTO, S.S., & SARROS, A.
(1965) Response of rabbit whole blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14: 943-
952.
SHERMAN, M. & ROSS, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. appl. Pharmacol., 3: 521-533.
SHINKAJI, N. & ADACHI, T. (1978) [The effect of certain pesticides on
the predaceous mite Amblyseius longispinosus (Evans) (Acarina:
Phytoseiidae).] Akitsu, 2: 99-108 (in Japanese, with English tables).
SHINODA, H., ITO, K., MATSUNAGA, T., TAKADA, T., & NOMURA, T.
(1972) Two suicides by acute pesticide intoxication. J. Jpn. Assoc.
Rural Med., 21: 242-249 (in Japanese).
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHOOTER, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating
agents. Chem.-biol. Interact., 11: 575-588.
SLOMKA, M.B. & HINE, C.H. (1981) Clinical pharmacology of
dichlorvos. Acta pharmacol. toxicol., 49(Suppl. 5): 105-108.
SMITH, P.W., MERTENS, H., LEWIS, M.F., FUNKHOUSER, G.E., HIGGINS,
E.A., CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & FLUX, M. (1972)
Toxicology of dichlorvos at operational aircraft cabin altitudes.
Aerosp. Med., 43(5): 473-478.
SNOW, D.H. & WATSON, A.D.J. (1973) The acute toxicity of dichlorvos
in the dog. I. Clinical observations and clinical pathology. Aust. vet.
J., 49: 113-119.
SOBELS, F.H. & TODD, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res., 67: 89-92.
STANTON, H.C., ALBERT, J.R., & MERSMANN, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows. Am. J.
vet. Res., 40(3): 315-320.
STAVINOHA, W.B., MODAK, A.T., & WEINTRAUB, S.T. (1976) Rate of
accumulation of acetylcholine in discrete regions of the rat brain
after dichlorvos treatment. J. Neurochem., 27: 1375-1378.
STEIN, W.J., MILLER, S., & FETZER, L.E., Jr (1966) Studies with
dichlorvos residual fumigant as a malaria eradication technique in
Haiti. III. Toxicological studies. Am. J. trop. Med. Hyg., 15(5): 672-
675.
STERNBERG, S.S. (1979) The carcinogenesis, mutagenesis, and
teratogenesis of insecticides. Review of studies in animals and
man. Pharmacol. Ther., 6: 147-166.
STEVENSON, D.E. & BLAIR, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0024.69).
STEVENSON, D.E. & BLAIR, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Toxicol., 18: 215-217.
STOERMER, D. (1985) [Occupational contact dermatitis from the
pesticide dichlorvos.] Dermatol. Monatsschr., 171(4): 245-249 (in
German).
TEICHERT, K., SZYMCZYK, T., CONSOLO, S., & LADINSKY, H. (1976)
Effect of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. appl. Pharmacol., 35: 77-81.
TERAYAMA, K., HONMA, H., & KAWARABAYASHI, T. (1978) Toxicity of
heavy metals and insecticides on slime mold Physarum polycephalum. J.
toxicol. Sci., 3: 293-304.
TESSARI, J.D. & SPENCER, D.L. (1971) Air sampling for pesticides in the
human environment. J. Assoc. Off. Anal. Chem., 54(6): 1376-1382.
TEZUKA, H., ANDO, N., SUZUKI, R., TERAHATA, M., MORIYA, M., &
SHIRASU, Y. (1980) Sister chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with pesticides
positive in microbial reversion assays. Mutat. Res., 78: 177-191.
THOMAS, T.C. & NISHIOKA, Y.A. (1985) Sampling of airborn pesticides
using chromosorb-102. Bull. environ. Contam. Toxicol., 35: 460-465.
THORPE, E., WILSON, A.B., DIX, K.M., & BLAIR, D. (1972) Teratological
studies with dichlorvos vapour in rabbits and rats. Arch. Toxicol.,
30(1): 29-38.
TIMMONS, E.M., CHAKLOS, R.J., BANNISTER, T.M., & KAPLAN, H.M.
(1975) Dichlorvos effects on estrous cycle onset in the rat. Lab.
anim. Sci., 25(1): 45-47.
TRACY, R.L., WOODCOCK, J.G., & CHODROFF, S. (1960) Toxicological
aspects of 2,2'-dichlorovinyl dimethylphosphate (DDVP) in cows,
horses, and white rats. J. econ. Entomol., 53(4): 593-601.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 43 pp (Resource Publication No. 84).
UEDA, K., SHIYO, K., MORI, T., KITAHARA, E., & URAGAMI, S. (1959)
[Effects of DDVP on man.] J. Jpn. Public Hyg. Assoc., 6: 315 (in
Japanese).
UEDA, K., SHIYO, K., IIZUKA, Y., KITAHARA, E., & OHASHI, A. (1960)
[The toxicity of organic phosphate "DDVP" for small animals and
man.] Igaku Seibutsugaku (Med. Biol.), 57(3): 98-101 (in Japanese).
ULLMANN, L., PHILLIPS, J., & SACHSSE, K. (1979) Cholinesterase
surveillance of aerial applicators and allied workers in the Democratic
Republic of the Sudan. Arch. environ. Contam. Toxicol., 8: 703-712.
VADHVA, P. & HASAN, M. (1986) Organophosphate dichlorvos induced
dose-related differential alterations in lipid levels and lipid
peroxidation in various regions of the fish brain and spinal cord. J.
environ. Sci. Health, B21(5): 413-424.
VAN DIJK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air: sampling
methods. Residue Rev., 55: 91-134.
VAN RAALTE, H.G.S. & JANSEN, J.D. (1981) Household insecticides and
the blood. Lancet, 10 October: 811.
VASHKOV, V.I., VOLKOVA, A.P., TSETLIN, V.M., & YANKOVSKII, E.Y.
(1966) [Assessment of the use of dimethyldichlorovinyl phosphate
(DDVP) in the insecticide mixture.] Gig. i Sanit., 9: 15-17 (in
Russian).
VASILESCU, C. & FLORESCU, A. (1980) Clinical and electro-
physiological study of neuropathy after organophosphorus compounds
poisoning. Arch. Toxicol., 43: 305-315.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination of
water by a sublethal concentration of pesticides. A system analysis
approach. Acta hydrochim. hydrobiol., 12(4): 399-499.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1980) Effects of
the pesticides thiotox, dichlorvos, and carbofuran on the test
fish Mystus vittatus. Water Air Soil Pollut., 13(2): 229-234.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1981a)
Evaluation of the comparative toxicity of thiotox, dichlorvos, and
carbofuran to two fresh water teleosts Ophiocephalus punctatus and
Mystus vittatus. Acta hydrochem. hydrobiol., 9(2): 119-129.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981b) Isolated and combined
effects of pesticides on serum transaminases in Mystus
vittatus (African catfish). Toxicol. Lett., 8: 67-71.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981c) Pesticide-induced
physiological alterations in certain tissues of a fish Mystus vittatus.
Toxicol. Lett., 9: 327-332.
VERMA, S.R., TONK, I.P., & DALELA, R.C. (1981d) Determination of the
maximum acceptable toxicant concentration (MATC) and the safe
concentration for certain aquatic pollutants. Acta hydrochim.
hydrobiol., 9(3): 247-254.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982a) Bioassay trials
with twenty-three pesticides to a fresh water teleost Saccobranchus
fossilis. Water Res., 16: 525-529.
VERMA, S.R., RANI, S., & DALELA, R.C. (1982b) Indicators of stress
induced by pesticides in Mystus vittatus: haematological
parameters. Indian J. environ. Health, 24(1): 58-64.
VERMA, S.R., RANI, S., TONK, I.P., & DALELA, R.C. (1983) Pesticide-
induced dysfunction in carbohydrate metabolism in three fresh water
fishes. Environ. Res., 32: 127-133.
VERMA, S.R., RANI, S., & DALELA, R.C. (1984) Effects of pesticides
and their combinations on three serum phosphatases of Mystus vittatus.
Water Air Soil Pollut., 21: 9-14.
VOGIN, E.E., CARSON, S., & SLOMKA, M.B. (1971) Teratology studies
with dichlorvos in rabbits. Toxicol. appl. Pharmacol., 19: 377-378.
VOOGD, C.E., JACOBS, J.J.J.A.A., & STEL, J.J., VAN DER (1972) On the
mutagenic action of dichlorvos. Mutat. Res., 16: 413-416.
VOSS, G. & SACHSSE, K. (1970) Red cell and plasma cholinesterase
activities in microsamples of human and animal blood determined
simultaneously by a modified acetylthiocholine/DNTB procedure.
Toxicol. appl. Pharmacol., 16: 764-772.
VRBOVSKY, L., SELECKY, FR.V., & ROSIVAL, L. (1959) [Toxicological and
pharmacological studies with phosphorester insecticides.] Naunyn
Schmiedeberg Arch. Pharmacol., 236: 202-204 (in German).
WADIA, R.S., SHINDE, S.N., & VAIDYA, (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurology (India), 33:
247-253.
WAGNER, R. & HOYER, J. (1975) [Method of determining workplace
concentrations and on occupational hygiene conditions during thermal
fogging atomization of pesticides in the greenhouse.] Z. gesamte
Hyg., 21(1): 18-20 (in German).
WALKER, A.I.T., BLAIR, D., STEVENSON, D.E., & CHAMBERS, P.L.
(1972) An inhalational toxicity study with dichlorvos. Arch.
Toxikol., 30: 1-7.
WARD, F.P. & GLICKSBERG, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Assoc., 158(4): 457-
461.
WATANABE, H., SHIRAI, O., & EBATA, N. (1976) A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care. Pestic. Abstr., 9(2): 94-95.
WEISBURGER, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 165-176.
WENNERBERG, R. & LOFROTH, G. (1974) Formation of 7-methyl- guanine
by dichlorvos in bacteria and mice. Chem.-biol. Interact., 8: 339-348.
WHITEHEAD, C.C. (1971) The effects of pesticides on production in
poultry. Vet. Rec., 30 January: 114-117.
WHO (1978) Spectrophotometric kit for measuring cholinesterase
activity, Geneva, World Health Organization (Report VBC/78.692).
WHO/FAO (1975-86) Data sheets on pesticides, Geneva, World Health
Organization (VBC).
WHO (1985) Technical dichlorvos. In: Specifications for pesticides
used in public health, 6th ed., Geneva, World Health Organization, pp.
163-174 (WHO/SIT/16.R2).
WHO (1986a) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87, Geneva, World Health
Organization, p. 11 (Report VBC/86.1 Rev.1)
WHO (1986b) Environmental Health Criteria 63: organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WILD, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of dichlorvos
and methyl methanesulfonate. Mutat. Res., 19: 33-41.
WILD, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res., 32: 133-150.
WILDEMAUWE, C., LONTIE, J.F., SCHOOFS, L., & LAREBEKE, N., VAN
(1983) The mutagenicity in procaryotes of insecticides, acaricides,
and nematicides. Residue Rev., 89: 129-178.
WILLIAMS, P.P. (1977) Metabolism of synthetic organic pesticides by
anaerobic microorganisms. Residue Rev., 66: 102.
WILSON, A.B. & DIX, K.M. (1973) Toxicity studies on dichlorvos: one
month inhalational exposure of rats to dichloroacetaldehyde,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0017.73).
WITHERUP, S., STEMMER, K.L., & CALDWELL, J.S. (1964) The effects
upon rats of being fed on diets containing VaponaR insecticide,
Cincinnati, Ohio, The Kettering Laboratory (Unpublished Report, 1
July).
WITHERUP, S., CALDWELL, J.S., & HULL, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their offspring by
the introduction of VaponaR insecticide into their diets, Cincinatti,
Ohio, The Kettering Laboratory (Unpublished Report, 12 April).
WITHERUP, S., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction of
VaponaR insecticide into their daily diets, Cincinnati, Ohio, The
Kettering Laboratory (Unpublished Report, 14 February).
WITHERUP, S., JOLLEY, W.J., STEMMER, K., & PFITZER, E.A. (1971)
Chronic toxicity studies with 2,2-dichlorovinyl dimethyl phosphate
(DDVP) in dogs and rats including observations on rat reproduction.
Toxicol. appl. Pharmacol., 19: 377.
WITTER, R.F. (1960) Effects of DDVP aerosols on blood cholinesterase
of fogging machine operators. Am. Med. Assoc. Arch. Ind. Health, 21:
7-9.
WITTER, R.F., GAINES, T.B., SHORT, J.G., SEDLAK, V.A., & MADDOCK,
D.R. (1961) Studies on the safety of DDVP for the disinsection of
commercial aircraft. Bull. World Health Organ., 24: 635-642.
WOOD, A.B. & KANAGASABAPATHY, L. (1983) Evaluation of inexpensive
thin layer chromatographic procedures for the estimation of some
organophosphorus and carbamate insecticide residues in fruit and
vegetables. Pestic. Sci., 14: 108-118.
WOODER, M.F. & CREEDY, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo, Sittingbourne,
Shell Research Ltd (Unpublished Report TLGR.79.089).
WOODER, M.F. & WRIGHT, A.S. (1981) Alkylation of DNA by organo-
phosphorus pesticides. Acta pharmacol. toxicol., 49(Suppl. 5): 51-55.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) I n vivo alkylation
studies with dichlorvos at practical use concentrations. Chem.-biol.
Interact., 19: 25-46.
WOODER, M.F., WRIGHT, A.S., & STEVENSON, D.E. (1978) Studies on
the in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol., 1: 505.
WORTHING, C.R. & WALKER, S.B. (1983) Pesticide manual, 7th ed.,
Croydon, British Crop Protection Council.
WRATHALL, A.E., WELLS, D.E., & ANDERSON, P.H. (1980) Effect of
feeding dichlorvos to sows in mid-pregnancy. Zbl. Vet. Med. A27: 662-
668.
WRIGHT, C.G. & LEIDY, R.B. (1980) Air samples in vehicles and
buildings turn up only very low levels of organic phosphate
insecticides. Pest Control, 48(7): 22-26, 68.
WRIGHT, A.S., HUTSON, D.H., & WOODER, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol., 42: 1-18.
XING-SHU, H. (1983) Preventing chemical damage to germ cells. Am.
Ind. Hyg. Assoc. J., 44: 699-703.
YAMANE, S., KINO, A., & TESHIMA, S. (1974) Histochemical
demonstration of cholinesterase activity in tissues of the carp
and effect of DDVP on its activity in situ. Acta histochem.
cytochem., 7(2): 167-174.
YAMANOI, F. (1980) [Effect of insecticides on the progeny in the
silkworm Bombyx mori. I. Effect of organophosphorus insecticides on egg
laying and their hatching.] Nippon Sanshigaku Zasshi, 49(5): 434-439
(in Japanese).
YAMASHITA, K. (1960) Studies on organic phosphorus. I. Toxicity of
dipterex and its vinyl derivative (DDVP). Kumamoto med. J., 13(4): 273-
279.
YAMASHITA, K. (1961) Studies on organic phosphorus dipterex. III.
Detection and discrimination of dipterex and its vinyl derivative
(DDVP). Kumamoto med. J., 14(1): 13-25.
YAMASHITA, K. (1962) Toxicity of dipterex and its vinyl derivative
(DDVP). Ind. Med. Surg., 31: 170-173.
ZALEWSKA, Z., RAKOWSKA, I., MATRASZEK, G., & SITKIEWICZ, D.
(1977) Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterases. Neuropathol.
Polska, 15(2): 255-262.
ZAVON, M.R. & KINDEL, E.A., Jr (1966) Potential hazard in using
dichlorvos insecticide resin. Adv. Chem. Ser., 60: 177-186.
ZELMAN, I.B. (1977) [Pathomorphology on the rat brain after
experimental intoxication with the phosphororganic pesticide dichlorvos
(DDVP).] Neuropathol. Polska, 15(4): 515-522 (in Polish).
ZELMAN, I.B. & MAJDECKI, T. (1979) [Ultrastructural changes in rat
brain following organophosphate insecticide dichlorvos (DDVP)
intoxication.] Neuropathol. Polska, 17(3): 443-453 (in Polish).
ZOTOV, V.M., SVIRIN, J.N., & PRUCAKOVA, R.M. (1977) [The develop-
ment of health regulations for occupational exposure in greenhouses
treated with chemical pesticides.] Gig. Tr. prof. Zabol., 3: 49-50 (in
Russian).
6.4 Elimination and Excretion in Expired Air, Faeces, and Urine
6.4.1 Human studies
Eight hours after a human male consumed 5 mg of vinyl-1-
14C-dichlorvos in orange juice, 27% of the radioactivity had been
eliminated as 14C-carbon dioxide. Approximately 8% had been excreted
by the urine within one day following dosing. Urinary excretion of
radioactivity decreased gradually and by day 9 none was detectable
(Hutson & Hoadley, 1972a).
The concentration of dimethylphosphate in the urine of three
pesticide control operators spraying houses with dichlorvos ranged from
0.32 to 1.4 µg at the end of the day's work (Das et al., 1983).
6.4.2 Studies on experimental animals
6.4.2.1 Oral
Dosing rats orally with 32P-dichlorvos (0.1 - 80 mg/kg body
weight) resulted in a recovery of 60 - 70% of the administered
radioactivity in the urine and approximately 10% in the faeces over a
6-day period following dosing (Casida et al., 1962).
After the oral administration of methyl-14C-dichlorvos to rats
(1 mg) and mice (0.5 mg), the excretion of radioactivity was rapid.
The major route of elimination after 4 days was the urine
(approximately 60%), followed by expired air (approximately 16%)
(Hutson & Hoadley, 1972b).
Rats given an oral dose of vinyl-1-14C-dichlorvos (1 mg per
animal) eliminated 10 - 20% of the 14C in the urine, 3 - 5% in the
faeces, and approximately 40% as expired carbon dioxide over 4 days
following dosing (Hutson et al., 1971a,b).
A comparison between the excretion by rat, mouse, hamster, and man
24 h after oral dosing with vinyl-1-14C-dichlorvos is given in
Table 10 (Hutson & Hoadley, 1972a).
A cow treated orally with 20 mg/kg body weight
32P-dichlorvos eliminated 40% of the radioactivity in the urine and
50% in the faeces. In the milk, the level of organosoluble radio-
activity was significantly above background only within the first 2 h
(Casida et al., 1962).
Table 10. Comparison of percentages of radioactivity excreted by males
24 h after oral ingestion of vinyl-1-14C-dichlorvosa
-----------------------------------------------------------------------------
Excretion route Rat (3) Mouse (1) Hamster (2) Man (1)
-----------------------------------------------------------------------------
urine 9.8 27.4 14.7 7.6
faeces 1.5 3.2 2.9 -
carbon dioxide 28.8 23.1 33.5 27 (8 h only)
-----------------------------------------------------------------------------
a Number of animals are given in parentheses.
6.4.2.2 Parenteral
The elimination of a single intraperitoneal injection of vinyl-1-
14C-dichlorvos (4 mg/kg body weight) from female rats was similar to
the elimination after oral dosing. A goat treated subcutaneously with
1.5 mg 32P-dichlorvos/kg body weight excreted 79% of the radioactivity
in the urine and 11% in the faeces. Two cows received an intravenous
or a subcutaneous injection with 1 mg 32P-dichlorvos/kg body weight.
Of the radioactivity which was recovered, 70 - 80% was in the urine and
approximately 14% in the faeces (Casida et al., 1962).
6.5 Retention and Turnover
6.5.1 Biological half-life
In studies by Blair et al. (1975), the metabolism of dichlorvos was
found to be so rapid that the biological half-life in blood could not
be determined. No intact dichlorvos could be demonstrated in the blood
or tissues of animals exposed by routes other than parenteral
injection. Only after exposure for 4 h to an atmospheric concentration
of 90 mg dichlorvos/m3 could dichlorvos be detected in most tissues of
the rat and mouse. Following exposure at 50 mg/m3, for 2 or 4 h, the
half-life in the rat kidney was 13.5 min.
The intraperitoneal injection of 10 mg dichlorvos/kg body weight
into mice increased the accumulation of ACh in the brain and caused an
inhibition of ChE activity. Symptoms of toxicity were clearly
recognizable after 15 min, and they disappeared almost completely after
60 min. The ChE activity and ACh levels reached their minimum and
maximum, respectively, at 15 min. The maximum concentration of
dichlorvos in the brain was reached after 1 min and decreased
thereafter, rapidly reaching the baseline level after 3 min (Nordgren
et al., 1978).
6.5.2 Body burden
There is no evidence for the storage of dichlorvos or its
metabolites in the tissues of animals. Small fractions of the carbon,
phosphorus, and chlorine derived from dichlorvos are retained in the
body for several days because their turnover rate is the same as that
for identical materials from other origins.
6.5.3 Indicator media
The determination of dichloroethanol in urine as a means of
monitoring the exposure of human beings to dichlorvos is not
sufficiently sensitive to detect levels arising from vapour exposure
through normal use. However, it could serve as the basis for a
specific detection method for the accidental ingestion of high levels
(Hutson & Hoadley, 1972a). Two other methods can be used: (a)
determination of the blood ChE activity; or (b) determination of
dimethylphosphate in urine by a rather complicated method (Blair &
Roderick, 1976). Neither method is specific when exposure to other
organophosphate or carbamate compounds, or to compounds that also
metabolize to dimethylphosphate, may have occurred.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
Lal (1982) reviewed the accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms.
Microorganisms undoubtedly have the ability to metabolize
organophosphorus insecticides; however, there are still large gaps in
our knowledge. It also seems clear that chemical, photochemical,
physical, and biological factors may influence the metabolism of
dichlorvos by microorganisms.
7.1.1 Algae and plankton
The dose of dichlorvos producing 50% growth inhibition of the
unicellular alga Euglena gracilis has been quoted as 3.5 mg/litre
(Butler, 1977).
Treating eutrophic carp ponds with 0.325 mg/litre killed
Cladocera (predominantly Bosmina and Daphnia species) and decreased
Copepoda (mainly Cyclops ). This was offset by increased development
of Rotatoria (mainly Polyarthra and Brachionus species) and
phytoplankton (mainly Scenedesmus and Pediastrum species), so that
the total plankton biomass changed only slightly (Grahl et al., 1981).
7.1.2 Fungi
Dichlorvos (in the range 10 - 80 mg/litre) has been found to affect
citric acid fermentation in Aspergillus niger grown in an artificial
medium. Inhibition of the fermentation was marked only at 40 and
80 mg/litre (Rahmatullah et al., 1978; Ali et al., 1979c). It appears
from the decreased uptake of inorganic phosphorus that dichlorvos may
have an interfering action on oxidative metabolism in A. niger. The
potential for inhibiting citrinin production by Penicillium
citrinum was investigated. Dichlorvos inhibited citrinin production by
76% at 100 µg/litre and by 48% at 10 µg/litre (Draughon & Ayres,
1978). The effect of dichlorvos on the survival time and the membrane
potential of the slime mould Physarum polycephalum was studied in a
laboratory test system. The threshold value for both these effects was
found to be 300 mg/litre for technical dichlorvos and 30 mg/litre for
the pure chemical (Terayama et al., 1978).
The influence of dichlorvos on 17 soil fungi, cultivated in
artificial medium, was tested. Dose levels of 0, 10, 30, 60, and
120 mg/kg were used during a test period of 21 days, and the effect on
the growth and morphology of the fungi was studied. In general, a
growth depression was found, but its extent depended on the fungal
strain. Occasionally growth was either unaffected or even stimulated
(Jakubowska & Nowak, 1973).
7.1.3 Bacteria
Dichlorvos has been found not to influence the overall metabolic
processes of Escherichia coli and Enterobacter aerogenes at doses up to
250 mg/litre (Grahl et al., 1980) and was not toxic for a sewage
isolate at up to 10 mg/litre (Rosenberg et al., 1979). In poultry
effluent slurry, concentrations of 100 and 1000 mg/litre did not
significantly reduce coliform populations, but 10 000 mg/litre caused
almost complete death. Therefore, residues of dichlorvos used for fly
control in layer houses can significantly reduce the enteric coliform
populations that are essential to the conversion of organic nitrogen to
inorganic nitrogen in poultry waste effluent (Ballington et al.,
1978).
In studies by Lieberman & Alexander (1981), dichlorvos (0.1 -
100 mg/litre) had little or no toxicity for microorganisms degrading
organic matter in sewage, as measured by respiratory activity,
degradation, and nitrification.
Incubation of dichlorvos with inocula of ruminal bacteria or
ciliated protozoa under anaerobic conditions suggests that dichlorvos
is not utilized by the organisms for growth, nor does it stimulate
endogenous gas production. However, it does, in certain instances,
affect volatile fatty acid production (Williams, 1977).
The growth of Bacillus thuringiens var th. was not inhibited by
dichlorvos (Dougherty et al., 1971).
7.2 Aquatic Organisms
Reviews of the acute and chronic effects of pesticides on aquatic
organisms have been made by Brungs et al. (1977), Livingston (1977),
and Kenaga (1979).
The toxicity of a chemical for aquatic organisms is influenced by
many factors such as the stage of development of the organism and the
composition, pH, oxygen content, and hardness of the water. In this
short review, these factors are not discussed in detail.
7.2.1 Fish
7.2.1.1 Acute toxicity
The acute toxicity of dichlorvos for both freshwater and estuarine
species of fish is moderate to high. The available data are summarized
in Table 11.
Variations in water hardness from 44 to 162 mg/litre and in pH from
6 to 9 did not alter the toxicity of dichlorvos for cutthroat or lake
trout (Johnson & Finley, 1980).
In studies by Yamane et al. (1974), young carp were exposed to a
concentration of 25 mg dichlorvos/litre water for 45 min. The ChE
activity (histochemically determined) of many tissues, including the
stratum griseum periventriculare, sarcolemma, and liver, was inhibited
or totally lost.
Table 11. Acute toxicity of dichlorvos for fish
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Freshwater
Clarias batrachus 26 - 31 g - 8.9 Verma et al. (1983)
Carp 8 mm 20 - 23 0.34 Verma et al. (1981d)
(Cyprinus carpio) 6 g 23 20a Yamane et al. (1974)
Mosquito fish 0.2 g 17 5.3 Johnson & Finley (1980)
(Gambusia affinis)
Blue gill 1.5 g 18 0.9 Johnson & Finley (1980)
(Lepomis macrochirus)
African catfish 6 - 10 g 18 0.5 Verma et al. (1980, 1981a)
(Mystus vittatus)
Ophiopcephalus punctatus 40 - 55 g 18 2.3 Verma et al. (1981a)
Fathead minnow 0.7 g 17 12 Johnson & Finley (1980)
(Pimephales promelas)
Harlequin fish - 20 7.8b Alabaster (1969)
(Rasbora heteromorpha)
Singii 5 - 10 g 18 6.6 Verma et al.
(Saccobranchus fossilis) (1982a)
Cutthroat trout 2.5 g 12 0.2 Johnson &
(Salmo clarki) Finley (1980)
Lake trout 0.3 g 12 0.2 Johnson &
(Salvelinus namaycush) Finley (1980)
Tilapia mossambica 3 - 10 cm 29 1.4 - 1.9 Rath & Misra
(1979a)
----------------------------------------------------------------------------------------------------
Table 11. (contd).
----------------------------------------------------------------------------------------------------
Species Mass or Temperature 96-h LC50 Reference
length (°C) (mg/litre)
----------------------------------------------------------------------------------------------------
Estuarine
American eel 0.14 g 20 1.8 Eisler (1970)
(Anguilla rostrata)
Mummichog 1.7 g 20 2.7 Eisler (1970)
(Fundulus heteroclitus)
Striped killifish 0.92 g 20 2.3 Eisler (1970)
(Fundulus majalis)
Atlantic silverside 0.8 g 20 1.3 Eisler (1970)
(Menidia menidia)
Striped mullet 1 - 6 g 20 0.23 Eisler (1970)
(Mugil cephalus)
Northern puffer 100 g 20 2.3 Eisler (1970)
(Sphaeroidus maculatus)
Bluehead 5.4 g 20 1.4 Eisler (1970)
(Thalassoma bifasciatum)
----------------------------------------------------------------------------------------------------
a 24-h LC50.
b 48-h LC50.
7.2.1.2 Short-term toxicity
Sublethal concentrations of dichlorvos (0.5 - 1 mg/litre) have
been found to decrease the respiratory rates of Tilapia mossambica (3
different age groups) exposed for up to 3 weeks. When the exposed
fish were transferred to fresh water, the rate did not completely
return to its pre-exposure value (Rath & Misra, 1979b). Liver and
brain ChE activity showed considerable inhibition when a group of T.
mossambica was exposed to dichlorvos (0.25 - 1.25 mg/litre water) for
periods of up to 4 weeks. At 7-day intervals, fish were studied or
transferred to clean water. The degree of enzyme inhibition was
related to the dichlorvos concentration and length of exposure. In all
age groups of fish, brain tissue exhibited a higher degree of ChE
inhibition than liver. Small fish were more susceptible to dichlorvos
with respect to AChE activity. When the fish were transferred to clean
water, most of the fish recovered their AChE activity, the recovery
being greater in liver than in brain. Small fish exhibited a
comparatively high level of recovery. The degree of recovery was
inversely related to the length of exposure (Rath & Misra, 1981).
Melanin dispersion in T. mossambica exposed to 1 mg/litre water for
15 days was stimulated indirectly by the inhibition of ChE activity.
The original colour was regained within 96 h after transfer of the fish
to clean water (Rath & Misra, 1980).
Exposure of Mystus vittatus (collected in the environment) to
sublethal concentrations of dichlorvos (0.045 or 0.09 mg/litre) for 30
days caused dose-related increases in serum glutamic-oxaloacetic and
glutamic-pyruvic transaminase levels (Verma et al., 1981b), increases
in alkaline phosphatase, acid phosphatase, and glucose-6-phosphatase
levels in serum (Verma et al., 1984), decreases in the levels of these
enzymes in liver, kidneys, and gills (Verma et al., 1981c), an increase
in glucose levels in blood, and a decrease in liver glycogen. Blood
lactate and muscle glycogen were unaffected (Verma et al., 1983). At
0.09 mg/litre, blood clotting time, mean corpuscular haemoglobin and
mean corpuscular haemoglobin concentration decreased, and the number of
leukocytes increased. Other haematological parameters did not show any
abnormalities (Verma et al., 1982b). From these results, a no-
observed-adverse-effect concentration of 0.03 mg/litre was derived.
In studies by Verma & Tonk (1984), Heteropneustes (Saccobranchus)
fossilis was exposed to dichlorvos for 30 days at a concentration of
0.44 mg/litre. Respiration, haematological parameters, and the
activities of two enzymes (one of them AChE) in liver, kidneys, and
gills were determined. The respiration rate decreased, and blood
concentrations of sodium and chloride ions and glucose increased
significantly, whereas the cholesterol level and clotting time were
decreased. A significant reduction in the AChE activity of the three
tissues was found.
Vadhva & Hasan (1986) studied the effect of dichlorvos (at 0, 3, 6,
and 9 mg/litre water) on various lipid fractions and lipid
peroxidation in the central nervous system of Heteropneustes
fossilis. After one week's exposure, the results indicated that
dichlorvos caused dose-related increases in total lipids, cholesterol-
esterified fatty acid, and lipid peroxidation in various regions of the
brain and spinal cord but a consistent decrease in the level of
phospholipids in these regions of the central nervous system.
Exposure of Clarias batrachus to 0.5 - 2.2 mg dichlorvos/litre
and Saccobranchus fossilis to 0.4 - 1.6 mg/litre for 30 days was found
to increase blood glucose and decrease liver glycogen, whereas blood
lactate and muscle glycogen were normal (Verma et al., 1983).
The estimated "maximum acceptable toxicant concentration"
(MATC)a for the larvae of Cyprinus carpio was 0.016 - 0.020 mg/litre
based on a 60-day study (Verma et al., 1981d).
7.2.2 Invertebrates
The acute toxicity of dichlorvos for aquatic insects and
crustaceans is extremely high (Table 12). As might be expected from an
organophosphorus insecticide, aquatic invertebrates are about three
orders of magnitude more susceptible to dichlorvos than are fish, and
freshwater crustaceans are particularly sensitive.
A study was carried out to determine the influence of a number of
pesticides on the "hatchability" of Artemia salina dry eggs. No effect
was found at 10 mg dichlorvos/litre in the aqueous system (Kuwabara et
al., 1980).
When prawns (Macrobrachium lamarrei) were exposed to dichlorvos at
concentrations of 0.31 or 0.62 mg/litre for 96 h, a decrease in hepatic
glycogen and an increase in the blood glucose level were found (Omkar &
Shukla, 1984). Possibly, the phosphorylase activity of the
hepatopancreas and muscle increased due to the inhibition of AChE
activity and the consequent accumulation of acetylcholine at
neurosynaptic junctions. The latter resulted in an induction of the
secretion of the sinus gland, which enhanced glycogenolysis.
7.3 Terrestrial Organisms
7.3.1 Birds
7.3.1.1 Acute oral toxicity
Dichlorvos has a high oral toxicity for birds (Table 13). The
signs of intoxication are typical of organophosphorus poisoning, namely
salivation, lachrymation, tremors, and terminal convulsions. They
usually appear shortly after dosing, and, at lethal doses, death occurs
within 1 h. Survivors appear to recover completely 24 h after dosing.
Various internal haemorrhages were found at autopsy in sacrificed
survivors of treated pheasants and Mallard ducks (Tucker & Crabtree,
1970).
-----------------------------------------------------------------------
a Maximum concentration at which no effect was seen.
Table 12. Acute toxicity of dichlorvos for non-target aquatic insects and Crustacea
-------------------------------------------------------------------------------------------------------
Species Temperature 48-h LC50 96-h LC50 Reference
(°C) (µg/litre) (µg/litre)
-------------------------------------------------------------------------------------------------------
Insects (stone flies)
Pteronarcys californica 15 - 0.1 Johnson & Finley (1980)
Crustacea (fresh water)
Water flea 15 0.07 - Johnson & Finley (1980)
(Daphnia pulex)
Water flea 21 0.28 - Johnson & Finley (1980)
(Simocephalus serrulatus)
Amphipod 21 - 0.5 Johnson & Finley (1980)
(Gammarus lacustris)
Crustacea (estuarine)
Sand shrimp - 12 4 Eisler (1969)
(Crangon septemspinosa)
Grass shrimp - 300 15 Eisler (1969)
(Palaemonetes vulgaris)
Hermit crab - 52 45 Eisler (1969)
(Pagurus longicarpus)
-------------------------------------------------------------------------------------------------------
Table 13. Acute oral LD50 values for birds
------------------------------------------------------------------------------------------------------------------------
Species Age Vehicle LD50 Reference
(mg/kg body weight)
------------------------------------------------------------------------------------------------------------------------
Red-winged blackbird propylene glycol 13.3 Schafer & Brunton (1979)
(Agelaius phoenicius) (male)
Mallard duck 5 - 7 months capsule 7.8 Tucker & Crabtree (1970)
(Anas platyrhynchos) (male)
Common pigeon propylene glycol 24 Schafer & Brunton (1979)
(Columba livia)
Quaila propylene glycol 24 Schafer & Brunton (1979)
(Coturnix coturnix) (female)
Domestic fowl 21 days aqueous suspension 6.5 Naidu et al. (1978)
(Gallus domesticus) (male) 6 - 8 months aqueous suspension 30 Dmitriev & Kozhemyakin
(1975)
House sparrow propylene glycol 17.8 Schafer & Brunton (1979)
(Passer domesticus)
Ring-necked pheasant 3 months capsule 11 Tucker & Crabtree (1970)
(Phasianus colchicus) (male)
Common grackle propylene glycol 13.3 Schafer & Brunton (1979)
(Quiscalus quiscula)
Starling adult propylene glycol 12 Schafer (1972)
(Sturnus vulgaris) - propylene glycol 42.1 Schafer & Brunton (1979)
------------------------------------------------------------------------------------------------------------------------
a Hattori et al. (1974) carried out studies on Japanese quail and found
LD50 values of 22 and 26 respectively, for male and female
(no details available).
7.3.1.2 Short-term toxicity
In short-term dietary studies, dichlorvos has been found to be
slightly to moderately toxic for birds (Table 14).
In the study with 7-day-old male chicks, there was weight loss and
50% mortality at 500 mg/kg diet. Marked, dose-related inhibition of
brain ChE activity occurred at 50, 100, and 500 mg/kg diet, but no
effects were noted at a level of 10 mg/kg diet (Naidu et al., 1978).
Canaries, Indian finches, and budgerigars, continuously exposed to
dichlorvos vapour (0.14 mg/m3) for 5 days, did not show any overt
signs of intoxication, but a reduction in ChE levels in plasma and
brain was observed in canaries and Indian finches (Brown et al.,
1968).
7.3.1.3 Field experience
Caution has been advised in the use and handling of dichlorvos
where birds might be exposed (Whitehead, 1971). The necessity for this
warning can be illustrated by the following cases. Adult mallards
feeding near horse mangers containing dichlorvos-treated feed were
found dead within a short time. At necropsy, excessive amounts of
mucus covering the mucosa of the proventriculus and scattered petechiae
along medial edges of liver lobes were observed. The small and large
intestines were markedly extended, and crystals were noted in the
gizzard. Brain ChE activity was inhibited by 75 - 80% (Ludke & Locke,
1976). Domestic fowl, which had accidental access to the faeces of a
horse dosed with dichlorvos pellets, picked out the pellets and more
than 30 birds died during the next 24 h (Lloyd, 1973). A mass
poisoning occurred in chickens following consumption of accidentally
contaminated drinking-water (Egyed & Bendheim, 1977). English game
bantams died after consuming wheat contaminated with 300 mg
dichlorvos/kg wheat (Reece, 1982).
7.3.2 Invertebrates
Dichlorvos was toxic for silkworm larvae when for 4 h they were fed
mulberry leaves previously sprayed with dilute dichlorvos emulsions.
Spray concentrations giving 50% mortality ranged from 1.56 to 6.25
mg/litre (Aratake & Kayamura, 1973).
No adverse effects were observed on the hatchability and general
condition of first instar silkworm larvae hatched in the following
generation when 5th instar larvae were fed mulberry leaves pre-treated
with 3 mg dichlorvos/kg of leaf (Yamanoi, 1980).
Table 14. Dietary LD50 values for birds
-------------------------------------------------------------------------------------
Species Age Duration LD50 Reference
(days) of feeding (mg/kg diet)
(days)
-------------------------------------------------------------------------------------
Mallard duck 16 8a 5000 Hill et al. (1975)
(Anas platyrhynchos) 5 8a 1310 Hill et al. (1975)
Japanese quail 14 8a 300 Hill et al. (1975)
(Coturnix japonica)
Domestic fowl 7 28 500 Naidu et al. (1978)
(Gallus domesticus) (male)
Ring-necked pheasant 10 8a 570 Hill et al. (1975)
(Phasianus colchicus)
-------------------------------------------------------------------------------------
a The median lethal dietary dose (LD50) during an 8-day test including
5 days of treated diet followed by 3 days of untreated diet.
7.3.3 Honey bees
Dichlorvos is highly toxic for honey bees. Atkins et al. (1973)
found in laboratory studies an LD50 of 0.495 µg/bee in 48 h (topical
application of dust; 26.7 °C; relative humidity 65%). Beran (1979)
obtained an oral LD50 of 0.29 µg/g body weight and an LD50 (topical
application) value of 0.65 µg/g body weight. This high toxicity has
led dichlorvos to be classified in the most toxic category for bees in
Austria and the USA.
When honeycombs were exposed to dichlorvos vapour from dichlorvos
resin strips for 4 months, the combs absorbed the insecticide and were
toxic to bees for approximately one month after exposure ceased.
Contamination of the bees appeared to be by fumigant rather than
contact action (Clinch, 1970).
7.3.4 Miscellaneous
Dichlorvos was highly toxic for the predatory mite Amblyseius
longispinosus in contact trials. Residual toxicity disappeared within
6 days, the susceptibility being the same as that of Phytoseiulus
persimilis (Shinkaji & Adachi, 1978).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
A more complete treatise on the effects of organophosphorus
insecticides in general, especially their short- and long-term effects
on the nervous system, will be found in Environmental Health Criteria
63: Organophosphorus Insecticides - A General Introduction (WHO,
1986).
8.1 Single Exposures
Dichlorvos is moderately to highly toxic when administered in
single doses by various routes to a variety of animal species
(Table 15). It is less toxic via the dermal and oral routes than by
parenteral routes. The signs of intoxication by all exposure routes
are typical of organophosphorus poisoning, i.e., salivation,
lacrimation, diarrhoea, tremors, and terminal convulsions, with death
occurring from respiratory failure. In addition, lethargy, ataxia,
hypersensitivity to noise, splayed gait, and paresis may be observed.
The signs are usually apparent shortly after dosing and, at lethal
doses, death occurs within 1 h. Survivors appear to recover completely
24 h after dosing.
The acute inhalational LC50 values for mice and rats are
summarized in Table 16. The apparent differences in the LC50s may be
the result of the type of exposure of the animal (whole body or head
only), whether the studies were carried out with dichlorvos vapour or
atomized particles of spray (with or without vehicle), or differences
in the purity of the dichlorvos. Moreover, since dichlorvos adheres
strongly to surfaces including glass, the out-going air has a
significantly lower concentration of dichlorvos than the air coming
into the chamber, if the system is not yet equilibrated.
No macroscopic abnormalities were observed in mice or rats 2 weeks
after a single exposure (MacDonald, 1982).
8.1.1 Domestic animals
When cattle and sheep were treated orally with a single dose of
dichlorvos, 10 mg/kg body weight was toxic for calves and 25 mg/kg body
weight for sheep. For the latter a dose of 10 mg/kg body weight was
without effects (Radeleff & Woodard, 1957).
8.1.2 Potentiation
Potentiation studies on male rats indicated that oral
administration of dichlorvos with 22 other organophosphate pesticides
resulted in no (or very little) potentiation, while administration with
malathion showed a marked potentiation (Narcisse, 1967; Kimmerle &
Lorke, 1968). However, Cohen & Ehrich (1976) found that the anti-ChE
action of 800 mg malathion/kg body weight (injected intraperitoneally)
was not potentiated by pre-treatment (18 h previously) with 30 mg/kg
dichlorvos, nor did malathion pre-treatment potentiate the action of
dichlorvos.
Table 15. The acute toxicity (LD50) of dichlorvos for experimental animals
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Mouse (male) oral unknown Eryfor EL or other 68 - 90 Vrbovsky et al. (1959);
solvents Ueda et al. (1960)
Mouse oral 80% unknown 87 Sasinovich (1968, 1970)
Mouse oral 97% aqueous polysor- 133 - 139a Haley et al. (1975)
bate 80
Mouse (male) oral 98% corn oil 140 Isshiki et al. (1983)
Mouse oral unknown aqueous 124 - 275a Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) subcutaneous unknown propylene glycol 13 - 33 Ueda et al. (1960);
and other solvents Jaques (1964)
Mouse subcutaneous unknown aqueous 20 - 26 Yamashita (1960, 1962);
Holmstedt et al. (1978)
Mouse (male) dermal unknown different solvents 206 & 395b Ueda et al. (1960)
Mouse intraperitoneal technical corn oil 28 Vrbovsky et al. (1959);
Casida et al. (1962)
Mouse intraperitoneal unknown aqueous 28 - 41a Holmstedt et al. (1978)
Mouse intravenous unknown aqueous 8 - 10 Holmstedt et al. (1978)
Rat oral 90 - 99% peanut oil and 56 - 96a Durham et al. (1957);
other solvents Narcisse (1967); Gaines
(1969)
Rat (male) oral unknown Eryfor EL and 46 - 110 Vrbovsky et al. (1959);
other solvents Ueda et al. (1960)
Rat oral 80% unknown 65 Sasinovich (1968, 1970)
Rat oral unknown aqueous 30 Holmstedt et al. (1978)
---------------------------------------------------------------------------------------------------------
Table 15. (contd).
---------------------------------------------------------------------------------------------------------
Species Route Purity Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------------------------------------
Rat dermal unknown xylene 75 - 107a Durham et al. (1957);
Gaines (1969)
Rat dermal 80% unknown 113 Sasinovich (1968, 1970)
Rat subcutaneous 95% dimethylsulfoxide 72 Brown & Stevenson
(1962)
Rat (male) intraperitoneal unknown Eryfor EL 18 Vrbovsky et al. (1959)
Guinea-pig subcutaneous 95% undiluted 28 Brown & Stevenson
(1962)
Syrian hamster intraperitoneal - suspension in 30 Dzwonkowska & Hübner
water (1986)
Rabbit oral 93% unknown 12.5 Desi et al. (1978)
Rabbit oral 80% unknown 22.5 Sasinovich (1968, 1970)
Rabbit dermal 80% unknown 205 Sasinovich (1968, 1970)
Cat oral 80% unknown 28 Sasinovich (1968, 1970)
Dog oral in capsule 100 - 316 Kodama (1960)
Swine (40- to 60- oral technical in capsule 157 Stanton et al. (1979)
day-old)
Domestic fowl oral technical in capsule 15 Sherman & Ross (1961)
(chicken)
---------------------------------------------------------------------------------------------------------
a The LD50s are often slightly different in males and females. Furthermore, it is clear that the
purity of the dichlorvos tested and the vehicle used has an influence on the toxicity.
b The two values were obtained using two different solvents.
Table 16. Inhalational LC50 values for dichlorvos
---------------------------------------------------------------------------------------------------------
Species Purity Type of Duration LC50 (mg/m3) Reference
exposure
---------------------------------------------------------------------------------------------------------
Mouse 80% vapour 4 h 13 Sasinovich (1968, 1970)
whole body
Mouse 98% vapour 4 h > 218 MacDonald (1982)
head only
Mouse (male) unknown; in vapour 4 h 310 Ueda et al. (1960)
solvent Ca whole body
Rat (male) unknown unknown, but 1 h 455 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat (male) 84.1% whole body 1 h 140 Sakama & Nishimura
polyethylene (1977)
glycol
Rat (male) unknown unknown, but 4 h 340 Kimmerle & Lorke (1968)
uptake by in-
halation only
Rat 80%(?) vapour 4 h 15 Sasinovich (1968, 1970)
whole body
Rat 98% vapour 4 h > 198 MacDonald (1982)
head only
---------------------------------------------------------------------------------------------------------
a Solvent C = 0.5% Sorpol 2020 water solution.
In vitro studies using human erythrocytes and plasma as ChE
sources, with ACh as substrate, indicated no potentiationa when
dichlorvos was tested in combination with carbaryl, crotoxyphos,
phosphamidon, malathion, malaoxon, mevinphos, parathion, paraoxon,
physostigmine, and trichlorphon (Carter & Maddux, 1968, 1974).
8.2 Short-term Exposures
8.2.1 Oral
8.2.1.1 Mouse
A 10-week (range-finding) toxicity study was carried out on B6C3F1
mice given 0, 25, 50, 100, 200, or 400 mg dichlorvos/ litre drinking-
water. Each group consisted of 12 males and 12 females, except the
control group (10 males and 10 females). Growth and mortality were
comparable with controls. In a second study, groups of 10 males and 10
females received 400, 1600, 3200, 5000, or 10 000 mg dichlorvos/litre.
The animals given the two highest doses died within 2 weeks, while the
1600 and 3200 mg groups showed slight and clear growth depression,
respectively, after 10 weeks (Konishi et al., 1981).
8.2.1.2 Rat
For 15 weeks, groups of 15 male and 15 female Charles River rats
were fed diets containing 0, 0.1, 1, 10, 100, or 1000 mg dichlorvos
(93%)/kg diet, which were freshly prepared once each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1), it may be
assumed that the average concentration of dichlorvos in each diet was
approximately 47% of the amount that had been added. There were no
deaths or signs of intoxication. Only the rats fed the highest dose
exhibited decreased growth rates at the beginning of the study. At
termination, no differences were observed in haematology, serum
protein, urinalysis, or gross or histopathological examination. In the
highest dose group, marked inhibition of ChE activity in plasma,
erythrocytes, and brain was noted. In the 100 mg/kg group, only
erythrocyte ChE inhibition was observed, and the 10 mg/kg group did not
show any ChE inhibition (Witherup et al., 1964).
Daily doses of 3.5 or 7 mg dichlorvos (purity not stated)/kg body
weight administered intragastrically to rats for 4 months and 0.7 or
1.4 mg/kg for 12 months caused no deaths. There were signs of
intoxication in the 7 mg group, and decreases in food consumption and
body weight gain and increases in a number of organ weights were seen
in all the groups except the lowest dose group. Inhibition of plasma,
erythrocyte, and brain ChE activity was observed in all groups except
those given 0.7 mg/kg body weight. The inhibition was time and dose
dependent (Sasinovich, 1970).
-----------------------------------------------------------------------
a Potentiation is the phenomenon that results in the combined effect
of exposure to two or more chemical substances being greater than
the sum of the effects that would be produced by each substance
separately, as the result of synergistic action.
In a (range-finding) toxicity study, groups of F-344 rats (10 of
each sex) were administered 0, 5, 10, 20, 40, or 80 mg/kg body weight,
dissolved in 10 ml of drinking-water. During the study six animals
died, five of which were in the two highest doses groups (Enomoto,
1981).
Dichlorvos administered orally to rats at a concentration of 70
mg/kg body weight inhibited not only ChE, but also alkaline
phosphatase, lactate dehydrogenase, and glutamate dehydrogenase
competitively. It increased the activity of glutamic-pyruvic
transaminase, but leucine aminopeptidase was not affected (Ellinger,
1985).
Ellinger et al. (1985) also studied the influence of dichlorvos on
haematological parameters in acute and short-term toxicity studies. In
the acute study 70 mg dichlorvos/kg body weight and in the short-term
study 30 mg/kg body weight were administered for 12 weeks. Decreases
in haemoglobin, haematocrit, and mean corpuscular haemoglobin
concentration were found.
Seven rat mothers were administered different dose levels of
dichlorvos (1 g or 10 g/litre distilled water) by stomach tube, and
their litters (1 - 12 days old) were nursed for about 21 days, and
weaned after 35 days of age. Although doses of 10 and 20 mg/kg body
weight did not cause any symptoms of intoxication, the mothers showed
significant inhibition of erythrocyte ChE activity. Plasma ChE
activity was affected to a lesser extent. Dose levels of 30 and
40 mg/kg body weight caused severe inhibition of erythrocyte ChE, and
severe cholinergic symptoms were seen. The symptoms occurred 10 -
20 min after dichlorvos administration and continued for 30 - 90 min,
when recovery took place (Tracy et al., 1960).
When pregnant rats were given oral doses of 1 or 5 mg dichlorvos in
oil/kg body weight during days 14 - 21 of pregnancy, the plasma ChE
activity of the mothers was markedly inhibited, but that of the young
(up to 56 days old) resembled the activity in the control. Brain ChE
activity did not show any significant inhibition (Zalewska et al.,
1977).
8.2.1.3 Rabbit
The progeny of rabbits, treated orally with 6 mg dichlorvos/kg body
weight per day for the last 10 days of pregnancy showed a decrease in
brain ChE activity and an increase in plasma ChE activity throughout
days 1 - 16 of life (Maslinska & Zalewska, 1978).
8.2.1.4 Cat
"Flea collar dermatitis" has been described in cats and dogs
wearing dichlorvos-impregnated PVC flea collars. In most cases, flea
collar dermatitis is a primary irritant contact dermatitis to
dichlorvos. The symptoms may consist of mild local irritation
(localized erythema and itching), severe local irritation varying from
erythematous alopecia to erosions, ulceration, oedema, and purulent
discharges with crusting or, in the most severe cases, generalized
dermatitis with secondary pyoderma and systemic illness (Muller,
1970).
Two trials with six and five cats, respectively, were carried out
to study the occurrence of contact dermatitis and systemic
intoxication. The cats received either a placebo collar or a
dichlorvos collar. Two cats received two dichlorvos collars each and
each cat was observed for 21 days. In a third trial, four groups of 25
cats were used to study hepatotoxicity. The cats of the different
groups were treated as follows. Group 1: placebo collar; Group 2:
placebo collar and intraperitoneal treatment with carbon tetrachloride
(CCl4) 7 days later; Group 3: dichlorvos collar; Group 4: dichlorvos
collar and intraperitoneal injection of CCl4 7 days later. Serum
glutamic pyruvic transaminase and ChE activity was estimated on day 9
of exposure. In a fourth study, 24 cats were fitted with a placebo
collar to test the effect of PVC in relation to the contact
dermatitis. PVC did not have an effect. No clear effect of the
CCl4-induced hepatic dystrophy on the detoxification of dichlorvos
was found.
The predominant systemic abnormalities recorded in these studies
were bone-marrow depression, ataxia, demyelination, and depression.
Red cell and plasma ChE activity was severely depressed. Furthermore,
contact dermatitis was seen in animals with dichlorvos collars.
Ambient temperature and relative humidity may have had a marked
influence on these effects, especially in the case of high temperature
and low humidity (Bell et al., 1975).
A 90-day study on 90 mongrel cats was carried out to verify the
results of Bell et al. (1975) by using a larger number of animals over
a longer period. There were three groups of 30 cats; one group with a
PVC collar (without dichlorvos), one group with one dichlorvos collar,
and the third group with three dichlorvos collars. In this study, in
which all relevant parameters were studied, no confirmation of the
abnormalities described by Bell et al. (1975) were found, even under
conditions of high temperature and low humidity. The only effect was
contact dermatitis, but, in this study, this was also found in the PVC
collars (Allen et al., 1978).
8.2.1.5 Dog
Groups of three male and three female dogs received equivalents of
approximately 0, 0.3, 1, 1.5, or 3 mg dichlorvos (93% in olive oil by
gelatin capsule)/kg body weight, daily, for 90 days. No effects were
observed on mortality, growth, liver and kidney function, organ
weights, haematology, or at gross and histopathological examination.
In the two highest dose groups, the dogs showed excitement, increased
activity, and aggression. Plasma and erythrocyte ChE activities
(measured initially and at intervals of approximately 2 weeks) were
normal in the lowest dose group (0.3 mg/kg body weight) but reduced in
the other dose groups. Brain ChE activity at termination was reduced
only in the highest dose group (Hine, 1962).
8.2.1.6 Pig
Young swine (35 days old) were fed a PVC-resin formulation of
dichlorvos (10%) in dosages equivalent to 1, 4, and 16 mg dichlorvos/kg
body weight per day in the feed (divided over two daily doses) for 30
days. Body weight gain, blood cell counts, packed-cell volumes,
haemoglobin concentrations, plasma glucose, plasma fatty acids, hepatic
and muscle glycogen concentrations, plasma and skeletal muscle
concentrations of electrolytes, and plasma and pancreatic insulin were
all comparable with those of control swine fed a blank PVC formulation.
Plasma and erythrocyte ChE activities were significantly inhibited in
the 4 and 16 mg/kg groups only (Stanton et al., 1979).
8.2.1.7 Cow
Two cows with suckling calves fed 200 mg dichlorvos/kg grain in
their rations showed normal ChE activity in the erythrocytes. However,
severe inhibition of ChE activity was found at 500 mg/kg feed
(equivalent to 4.5 mg/kg body weight), and a single dose of 27 mg/kg
body weight caused cholinergic collapse, followed by rapid recovery.
The ChE values in the calves remained normal throughout the 78-day
test. Milk from these two cows contained less than 0.08 mg
dichlorvos/litre (Tracy et al., 1960).
8.2.2 Dermal
8.2.2.1 Rat
In studies by Dikshith et al. (1976), groups of 48 male rats
received daily topical applications of 21.4 mg/kg body weight
dichlorvos (96%) in ethanol (control animals received ethanol alone) 5
days per week for 90 days. At intervals of 7, 15, 30, 45, 60, and 90
days, eight test rats and two control rats were sacrificed for
histopathological examination of the skin and testes. None of the
animals showed signs of intoxication, but ChE activity was not
measured. The skin showed no irritation, and no histopathological
changes were seen in the testes or skin.
8.2.2.2 Livestock
Spray and dip studies have shown that a concentration of 1%
dichlorvos is not toxic for cattle (no further details are available)
(Radeleff & Woodard, 1957).
The effect of applying different suspensions (different solvents
with and without an emulsifier) of dichlorvos to the skin of cows has
been studied. The dose level was 5 mg/kg body weight, increasing after
7 days to 10 mg/kg. Dichlorvos at 5 mg/kg body weight caused a
significant decrease in ChE activity in blood serum. The higher dose
level additionally produced symptoms of intoxication. No dichlorvos
residues were found in milk (Majewski et al., 1978).
Two heifers were washed daily with either a 1% aqueous solution or
emulsion of dichlorvos or a 10% suspension of dichlorvos in water
(daily application of 1.8 g dichlorvos for 21 days). Two additional
heifers were used as controls. The ChE activity levels of the
erythrocytes remained at the lower limits of normal variation (Tracy et
al., 1960).
8.2.3 Inhalation
8.2.3.1 Experimental animals
In a report by Sasinovich (1968), groups of rats were exposed
(whole body) daily for 4 h to average dichlorvos concentrations
of 0.11 or 1.1 mg/m3 for 4 months, to 5.2 mg/m3 for 2
months, or to 8.2 mg/m3 for 45 days. The highest dose caused signs
of intoxication; two of the eight rats died. Exposure to
5.2 mg/m3 or more resulted in marked inhibition of ChE activity and
disturbance of the blood-sugar curve. In the 0.11 and
1.1 mg/m3 groups, no significant effects were observed.
Mice, rats, and guinea-pigs, exposed 23 h per day for 28 consecu-
tive days to actual dichlorvos concentrations of 0.03 mg/m3, did not
show gross pathological changes at the end of the study. Inhibition of
plasma and brain ChE activity occurred in male mice, male guinea-pigs
(plasma only), and female rats (brain only) after a 5-day exposure to
0.14 - 0.15 mg/m3 dichlorvos (Brown et al., 1968).
Guinea-pigs (P strain) exposed for 7 h per day for 5 consecutive
days to an actual concentration of 90 - 120 mg/m3 dichlorvos suffered
no visible effects to their health. Rats (CFE) and mice (CF1)
similarly exposed to 50 mg/m3 were not visibly affected. At
concentrations above 50 mg/m3, the mice became distressed and
prolonged exposure to 80 mg/m3 was frequently lethal. Rats were less
severely affected (Stevenson & Blair, 1969).
In studies by Vashkov et al. (1966), 100 mice, 50 rats, 22 rabbits,
and 13 cats were exposed to a mixture containing dichlorvos, kerosene,
xylene, and freons during a single 2-h exposure or for 2 h per
day for 40 days. Three concentrations, 16.5, 45, and 160 mg
dichlorvos/m3, were tested (the median gravimetric diameter of the
aerosol particles was about 5 µm). The overall condition of the
animals remained normal, and no effects on body weight, clinical
chemical blood analyses, blood ChE activity, or respiration rate, or
pathological changes were observed. Rabbits developed transient miosis
which disappeared at the end of the exposure period.
Mice (20), guinea-pigs (6), sheep (7), calves (39), and a heifer
were exposed to an increasing dichlorvos concentration in the air
generated by dichlorvos strips (20%). The number of strips was
increased each week for 6 weeks, reaching 80 strips per 140 m3, and
was then reduced over the next 6 weeks. During exposure to the
recommended number of strips, the dichlorvos concentration in the
air was 0.09 - 0.14 mg/m3. The highest concentration was
2.1 mg/m3 when the number of strips was 16 times the recommended
number. No mortality or signs of intoxication were observed, and mice
bore normal litters during the study. The serum ChE activities of the
calves and of those handling the animals were within normal values
(Henriksson et al., 1971).
When 10 rabbits, 8 cats, and 10 dogs (males and females) were
continuously exposed for 8 weeks to dichlorvos concentrations of 0.05 -
0.3 mg/m3 generated from impregnated PVC-resin strips, no effects were
found on general health, behaviour, plasma and erythrocyte ChE
activities, or electroencephalographic patterns in the brain of the
animals (Walker et al., 1972).
In studies by Coulston & Griffin (1977), four male and four female
Rhesus monkeys were continuously exposed to dichlorvos vapour at an
average actual concentration of 0.05 mg/m3 for 3 months. The control
group consisted of four males and one female. No adverse effects were
observed in appearance, behaviour, or haematological and clinical
chemical determinations. Plasma ChE activity was slightly inhibited
(up to 28% inhibition), whilst the greatest erythrocyte ChE activity
inhibition was 36%. No changes in nerve maximum conduction velocities
or muscle-evoked action potentials were induced by the exposure to
dichlorvos.
8.2.3.2 Domestic animals
Five horses, exposed continuously to dichlorvos for 22 days in a
closed barn that was treated daily with 17 mg dichlorvos/m3, dis-
played mild inhibition of erythrocyte ChE activity after 7 days,
followed by recovery at 11 - 22 days. However, plasma ChE activity was
not changed. The concentration in the barn varied between 0.24 and
1.48 mg/m3 (Tracy et al., 1960).
The influence on ChE activity in cattle exposed to an impregnated
plastic strip containing 20% of dichlorvos has been studied. Red blood
cell ChE activity was measured during the 85 days of exposure, and on
the 9th day an average inhibition of about 35% was found. After the
37th day the inhibition in ChE activity gradually declined to 20%. No
clinical signs were observed (Horvath et al., 1968),
8.2.4 Studies on ChE activity
Groups of 10 young female Sherman rats were fed for 90 days on
diets containing 0, 5, 20, 50, 200, 500, or 1000 mg dichlorvos (90%)/kg
diet (equivalent to 0, 0.4, 1.5, 3.5, 14.2, 35.7, and 69.9 mg/kg body
weight, respectively). Data on the stability of dichlorvos in the diet
were not reported. No clinical signs of intoxication were noted.
Blood samples were taken from two rats of each group for ChE activity
determination on day 3, 11, 60, and 90. In all test groups, a
decrease in plasma ChE activity was observed during the first 4 days,
gradually returning to normal, except in the rats receiving 14.2 mg/kg
body weight or more. At doses above 3.5 mg/kg body weight, erythrocyte
ChE activities were decreased throughout the test, but at 3.5 mg/kg
body weight, this was only so for the first 30 days of the study.
Lower dose levels produced comparable results to those of the control
group (Durham et al., 1957).
Thirty-two Rhesus monkeys were treated with 20% pelleted PVC-resin
formulations of dichlorvos at dosages ranging from 5 to 80 mg/kg of
formulation (equivalent to 1 - 16 mg dichlorvos/kg body weight) daily
or 8 and 20 mg/kg of formulation twice daily for 10 - 21 consecutive
days. None of the monkeys developed overt signs of intoxication,
though they ate less food and had soft faeces. Plasma and erythrocyte
ChE activities were reduced by approximately 80% in all animals, and
remained inhibited until completion of treatment. Plasma ChE
activities returned to normal within approximately 3 weeks, whereas the
erythrocyte ChE activities required 50 - 60 days to return to pre-
treatment values (Hass et al., 1972).
When groups of five male and five female guinea-pigs (P strain)
were given daily applications of 0, 25, 50, or 100 mg dichlorvos
(94%)/kg body weight on the shorn skin for 8 days, all the animals
survived. A significant dose-dependent inhibition of both plasma and
erythrocyte ChE activities occurred in all test groups. Recovery of
plasma ChE activities was complete within one week of the last
exposure, and that of erythrocyte ChE activities was complete within
one week in the females and 2 weeks in the males (Brown & Roberts,
1966).
Three Cynomolgus monkeys were treated daily with dermal doses of
50, 75, and 100 mg/kg body weight technical dichlorvos in xylene for 5
days per week until the animals died (after 8, 10, and 4 doses,
respectively). Symptoms of intoxication appeared in all monkeys,
beginning 10 - 20 min after administration of the first dose. The
erythrocyte ChE activity decreased rapidly and remained severely
inhibited for the period of the study, while plasma ChE activities
fluctuated throughout the study (Durham et al., 1957).
When male and female mice, rats, and guinea-pigs were exposed
continuously by inhalation for 5 consecutive days to actual
concentrations of 0, 0.14 - 0.15, or 1.1 - 1.3 mg/m3 dichlorvos, no
overt signs of intoxication were observed. In the high exposure
groups, plasma, erythrocyte, and brain ChE activities were inhibited in
all three species. Plasma ChE was the most sensitive in all three
species, with up to 70% inhibition in the female mice. The greatest
inhibition of brain ChE activity (30%) was found in mice (Brown et al.,
1968).
The effects of dichlorvos vapour inhalation on AChE activity was
investigated in the rat. Exposure to dichlorvos concentrations of 0.8
and 1.8 mg/m3 for 3 days reduced AChE activity in the bronchial tissue
(50 - 60% of control) but did not produce any changes in blood AChE
activity. However, at 4.3 mg/m3, blood AChE activity also declined
(38% of control). In histochemical preparations, staining of the
bronchial glands and smooth muscles revealed reduced enzyme activity
even at the lowest dose (0.2 mg/m3) tested. At this concentration,
no inhibition of bronchial homogenate ChE was observed (Schmidt et al.,
1975, 1979). The significance of bronchial ChE inhibition is not
clear.
Male mice were continuously exposed to actual concentrations of 0
or 0.03 mg dichlorvos/m3 for 28 consecutive days. Weekly assays of
ChE activities showed that brain ChE activity was slightly inhibited
(less than 20%) on the 28th day only, but plasma and erythrocyte ChE
activities were not significantly inhibited (Brown et al., 1968).
Rabbits exposed to an average concentration of 1 mg
dichlorvos/m3 (0.8 - 1.3 mg/m3) for 4 h per day for 4 months showed
up to 30% inhibition of serum and erythrocyte ChE activities during the
study. However, cats similarly exposed did not show significant
inhibition (Sasinovich, 1968).
Rats and monkeys were exposed for 2 weeks in an inhalation chamber
sprayed once with an emulsion of dichlorvos. The initial air
concentration was 6 mg dichlorvos/m3, decreasing over a few days to
about 0.1 - 0.2 mg/m3. No signs of intoxication were seen. The
plasma and erythrocyte ChE activities of the monkeys decreased to
approximately 50% of pre-exposure levels, but rapid recovery took place
after cessation of exposure. Comparable results were obtained when
rats and monkeys were continuously exposed for up to 7 days to a
maximum air concentration of 2.2 mg/m3. However, continuous exposure
for 4 days to 0.27 mg/m3 did not produce any effect on ChE activity
(Durham et al., 1957).
Groups of two monkeys were exposed to dichlorvos vapour 2 h per day
for 4 consecutive days. With concentrations up to 0.7 mg/m3, no
change in plasma or erythrocyte ChE activity was noted, while 1.2 -
3.3 mg/m3 caused a slight decrease in plasma ChE activity, and 7.5 -
18 mg/m3 (mean: 13 mg/m3) resulted in miosis and a pronounced
inhibition (40 - 70%) of plasma and erythrocyte ChE activities (Witter
et al., 1961).
Groups of 10 chickens were exposed to dichlorvos vapour from a
varying number of strips (20% dichlorvos) continually or intermittently
for 3 weeks. Exposure to a single strip in a room of 33 m3 (either
interrupted or for 16 h every day) produced no significant effect on
plasma or brain ChE activity. Exposure to more than one strip (2 - 5)
resulted in up to 50% inhibition of plasma ChE activity and
approximately 40% inhibition of brain ChE activity. Dichlorvos aerosol
sprays (0.7% dichlorvos) showed that daily excessive spraying for 6
seconds, 8 times per day, for 5 days, did not produce significant ChE
inhibition. However, a significant decrease in plasma ChE activity was
found when the study lasted for 21 days (Rauws & van Logten, 1973).
Three studies were carried out to determine the effect on the
laying performance of hens given dichlorvos in the feed for 4 weeks.
Plasma AChE levels were reduced by 70% at 20, 30, or 40 mg
dichlorvos/kg diet, although there was no clear influence on food
consumption, egg production, egg weight, or hatchability at these
doses. At 80 mg/kg diet, a decrease in food consumption and in egg
production was seen, the latter being a consequence, possibly, of the
former (Pym et al., 1984).
8.3 Skin and Eye Irritation; Sensitization
In the skin sensitization assay procedure of Stevens (flank/flank
technique), 1% dichlorvos in olive oil produced no visible effects in
male albino guinea-pigs. Negative results were also obtained when five
components of formulated dichlorvos/ PVC products were assayed for
their skin sensitization potential (Kodama, 1968).
A primary skin irritation test on dichlorvos was performed by using
male New Zealand white rabbits. Irritation observed on the skin after
the application of 5 - 20% water solutions of dichlorvos was relatively
severe compared with that caused by other organophosphorus insecticides
(Arimatsu et al., 1977).
In order to study the allergenicity of dichlorvos, the guinea-pig
maximization test was used. Threshold limit values of primary
irritancy tested on the skin of guinea-pigs (Hartley strain) were 2% or
more. In the maximization test, 0.05 and 0.5% were used. With 0.5%,
35% of the animals showed slight or discrete erythema. The
allergenicity rating as determined by the Kligman test was moderate. In
combination with methidathion, dichlorvos showed a stronger reaction,
indicating cross-sensitization (Fujita, 1985).
8.4 Long-term Exposure
8.4.1 Oral
8.4.1.1 Rat
In studies by Witherup et al. (1967, 1971), groups of 40 male and
40 female weanling CD rats were fed diets containing nominal
concentrations of 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg
diet for 2 years. Five males and five females from each group were
sacrificed after 6, 12, and 18 months, and analysis of diet samples
showed a considerable loss of dichlorvos. This was associated with a
gradual increase in DCA concentration which ranged in the different
groups from 0.014 to 28.6 mg/kg diet. The average actual
concentrations of dichlorvos in each diet were 0, 0.047, 0.467, 4.67,
46.7, and 234 mg/kg diet (equivalent to approximately 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). There were no signs of
intoxication, and no effects were seen on behaviour, mortality rate,
food consumption, weight gain, organ weights, haematology, or
urinalysis. Plasma and erythrocyte ChE activities were decreased
throughout the study in the two highest dose groups compared with
controls, but brain ChE activity was decreased in the highest dose
group only. Histological examination of major organs revealed
hepatocellular fatty vacuolization in the group with 234 mg/kg and in
some of the animals with 46.7 mg/kg diet. No effect was seen on serum
total proteins or albumin:globulin ratio, or on hexobarbital sleeping
time. It can be concluded that the actual average concentration of
4.7 mg/kg diet (equivalent to approximately 0.25 mg/kg body weight) was
without significant effect on any of the measured parameters. The
tumour incidence was comparable with that of the control group.
8.4.1.2 Dog
Groups of three male and three female beagle dogs were fed diets
containing 0, 0.1, 1, 10, 100, or 500 mg dichlorvos (93%)/kg diet for 2
years, the average actual concentrations being 0, 0.09, 0.32, 3.2, 32,
and 256 mg dichlorvos/kg diet (equivalent to 0, 0.002, 0.008, 0.08,
0.8, and 6.4 mg/kg body weight). The average DCA concentration in the
diets with 10, 100, and 500 mg dichlorvos/kg diet was 0.6, 6.4, and 20
mg/kg diet. No effects were seen on general appearance, survival, food
consumption, weight gain, haematology, or urinalysis. However,
erythrocyte ChE was inhibited at dose levels of 3.2 mg/kg diet or more,
and plasma ChE activity was inhibited at the two highest dose levels.
Recovery to control values took place at the end of the feeding period.
Brain ChE activity, measured at the end of the study, was similar to
that of the controls, and liver weights were increased in both sexes in
the 256 mg/kg diet group. Histological examination of major organs
revealed slight dose-related alterations in the hepatic cells of one
female in the 3.2 mg/kg diet group, and greater alterations at higher
doses. No differences were seen in serum alkaline phosphatase, trans-
aminase activities, total serum proteins, or albumin:globulin ratios.
The actual average concentration of 0.32 mg/kg diet (equivalent to
0.008 mg/kg body weight) was without effect (Jolley et al., 1967;
Witherup et al., 1971).
8.4.2 Inhalation
8.4.2.1 Rat
When groups of 50 male and 50 female weanling CFE rats were
exposed for 23 h per day to air concentrations of 0, 0.05, 0.5, or
5 mg dichlorvos (97%)/m3 air (actual concentrations: 0, 0.05, 0.48,
and 4.7 mg/m3) for 2 years, body weight gain was reduced in the
two highest dose groups. After 2 years of exposure, plasma and
erythrocyte ChE activities were significantly reduced in the two
highest dose groups, but in the case of brain ChE activity, only in the
highest group. No effects attributable to dichlorvos were seen on
appearance, food consumption, haematological or blood chemistry values,
or organ weights, or upon gross or microscopic examination.
Ultrastructural examinations of bronchi and alveoli of rats exposed to
0 or 5 mg/m3 showed no differences between the two groups.
In connection with a reported correlation between brain ACh
concentration and the inhibition of brain ChE activity following acute
exposure to organophosphorus compounds, the brain tissue of three
female rats per group was examined for ACh and choline concentrations.
The dose level of 0.05 mg dichlorvos/m3 was without effect on any of
the measured parameters (Blair et al., 1976). It should be noted that
in this study the rats were not only exposed by inhalation but also via
their food, drinking-water, and by grooming. This resulted in
additional oral ingestion of dichlorvos (Stevenson & Blair, 1977).
8.5 Reproduction, Embryotoxicity, and Teratogenicity
8.5.1 Reproduction
In a 3-generation reproduction study, weanling CD rats (six groups
of 30 animals) were fed dichlorvos (93%) at nominal concentrations of
0, 0.1, 1, 10, 100, or 500 mg/kg diet, prepared freshly each week. The
stability of dichlorvos in the diets was not reported but, in
accordance with the 2-year oral rat study (section 8.4.1.1), it was
assumed that the average concentration of dichlorvos was approximately
47% of the nominal concentrations (equivalent to 0, 0.0025, 0.025,
0.25, 2.5, and 12.5 mg/kg body weight). No effects on fertility,
number and size of litters, body weight, or viability of the pups were
found. Gross and histopathological examination of 7-day-old pups from
F1a and F2a litters did not reveal any abnormalities (Witherup et
al., 1965, 1971).
Oral treatment of rats with 5.6 mg/kg body weight and rabbits with
6 mg/kg body weight during the last trimester of pregnancy had no
effect on offspring weight and development. However, the cerebral
cortices from the 1-day-old rabbits were less mature than those of
control rabbits, probably due to maternal toxicity (Dambska et al.,
1978, 1979).
Dambska & Maslinska (1982) observed impairment of the development
of the brain of rabbits dosed orally with 9 mg/kg body weight per day
from days 5 to 16 of life, the period of myelination.
8.5.1.1 Effects on testes
In studies by Krause & Homolo (1972, 1974), three groups of 14 male
NMRI/Han mice received either a single oral dose of 40 mg dichlorvos/kg
body weight, daily oral doses of 10 mg dichlorvos (in olive oil)/kg for
18 consecutive days, or daily oral doses of 0.5 ml olive oil for 18
days, respectively. On days 9, 18, 27, 36, 54, and 63, two animals
from each group were killed and their testes examined histologically.
Severe disturbances of spermatogenesis were observed in both test
groups; damaged seminiferous tubules were also seen and the supporting
Sertoli cells were damaged. In addition, there was an increase in the
number and hypertrophy of the Leydig cells. No explanation could be
offered for these effects.
A similar study was carried out on three groups of 16 male juvenile
Wistar rats. The rats received either 20 mg dichlorvos (in olive
oil)/kg body weight on days 4 and 5, 10 mg dichlorvos (in olive oil)/kg
daily from days 4 to 23, or 0.1 ml olive oil daily from days 4 to 23 of
life. On days 6, 12, 18, 26, 34, and 50 of life, two rats from each
group were sacrificed. Histological examination of the testes showed
slight reduction in the number of the spermatogenic cells and Leydig
cells. It was assumed that a reduction in testosterone synthesis
resulted in damage to the spermatogenic cells. All the changes were
reversed by the 50th day (Krause et al., 1976; Xing-Shu, 1983).
In order to examine the cause of the observed effect on the
spermatogenic and Leydig cells, the study was repeated with, in
addition, measurement of testosterone levels in serum and testes. The
testosterone concentrations in the testes, and leutinizing hormone (LH)
and follicle stimulating hormone (FSH) levels in serum were similar in
the presence or absence of dichlorvos (Krause, 1977). However, the use
of adult rats and a different dosing regimen prevented a strict
comparison with the earlier study by Krause et al. (1976).
In studies by Fujita et al. (1977), 55 male Wistar rats (aged 5
months) were orally administered dichlorvos at levels of 5 or 10 mg/kg
body weight every other day for 8 weeks. The rats were divided into
five groups, and one group of rats was killed every 4 weeks to study
the changes in several organs, including the testes. About 200
individual seminiferous tubules were examined in each rat. No change
was seen in body weight gain or testes weight. The score values of the
seminal cellular system decreased after 4 - 8 weeks of treatment, but
were restored 8 weeks after the end of treatment.
8.5.1.2 Effect on estrous cycle
Timmons et al. (1975) reported studies on female rats which were
exposed to an atmosphere containing 2.4 mg dichlorvos/m3 generated
from a dichlorvos strip placed on top of each cage. Exposure was
continuous from the birth of the first litter until the estrous cycle
began again. Controls were housed in a separate room. A significant
mean delay of 10 days in the onset of the estrous cycle, compared with
that of controls, was observed. However, the significance of the
results was complicated by different housing conditions.
8.5.1.3 Domestic animals
In studies by Bazer et al. (1969), sows were fed dichlorvos (9% in
resin pellets) at the level of 800 mg per animal per day beginning 21
days before breeding and continuing through gestation. No significant
differences in the numbers born alive or dead, the litter weight,
number weaned, or individual weaning weights were observed.
In a further study, dichlorvos was added to the rations of pregnant
sows at the level of 0 or 800 mg per animal per day. Resin pellets
containing 9% dichlorvos were fed either from 3 weeks before breeding
and throughout gestation, or from 18 to 56 days before parturition.
Data were collected from a total of 681 dams, representing eight
replicates over a period of 2 years. The dichlorvos-treated groups
scored consistently higher for individual farrowing weights, litter
farrowing weights, number weaned, individual weaning weights, and
litter weaning weights, and less consistently, for the percentage of
live births (Batte et al., 1969).
When dichlorvos (as a PVC-resin formulation) was administered to
sows in doses ranging from 4 to 13 mg/kg body weight per day for the
last 21 - 30 days of gestation, the average farrowing interval for the
live-born piglets was shorter in treated than in control animals.
There was also a dose-related increase in the mean birth weight of
live-born piglets from the dichlorvos-treated sows, while the incidence
of still births was lower than in controls (Bunding et al., 1972).
In studies by Collins et al. (1971), male and female swine were fed
for up to 3 years on diets containing a PVC-resin formulation at 0,
200, 250, 288, 400, or 500 mg dichlorvos/kg diet, and for at least 6
months prior to initial breeding. Two generations were raised, and no
effects were observed on number or size of litters, survival or growth
rate of offspring, urinalysis, haematology, hepatic and renal function,
physical structure, or calcium and phosphorus content of the femoral
bone, or in appearance during gross and microscopic examination. Organ
weights were normal except in the case of the liver, which was
generally increased. Whole blood ChE activity was inhibited and brain
ChE activity was slightly reduced, but no clinical evidence of
neurophysiological impairment was observed.
In studies reported by Stanton et al. (1979), pregnant sows were
given PVC-resin formulations of dichlorvos (10%) in the diet at a daily
dose of 0, 5, or 25 mg dichlorvos/kg body weight (divided between two
doses per day) during the last one-third of pregnancy (or 30 days).
Only about 50% of the total dichlorvos was released from this resin in
the gastrointestinal tract. All pigs were born alive, and their birth
weight was similar to that of control animals. In the group given
25 mg/kg body weight, plasma and erythrocyte ChE activities were
markedly inhibited (80 and 90%, respectively) in the sows, but not in
the newborn pigs. No changes in the packed cell volume or haemoglobin
concentration of the sows or their piglets were observed.
In a limited test reported by Darrow (1973), dichlorvos-impregnated
collars did not have any adverse effects on pregnant female goats or
later on their newborn young. Also, blood ChE activity was not
inhibited in either the nannies or the kids over a period of several
weeks. The acceptance of treated kids by the mothers was normal.
A pregnant non-lactating Holstein-Friesian cow was fed a nominal
concentration of 6.2 mg dichlorvos/kg body weight per day in the daily
ration from days 152 to 286 of pregnancy. A normal calf was delivered
(Macklin & Ribelin, 1971).
A herd of 54 dairy cows (two-thirds carrying calves) was accidently
sprayed with dichlorvos, resulting in a dosage of 50 mg dichlorvos/kg
body weight. All the animals showed symptoms of intoxication and some
had convulsions. However, they all recovered within a few hours, with
no abortions or other adverse effects except a temporary diminution in
milk production (Knapp & Graden, 1964).
When three pregnant sows were fed 8.5 mg dichlorvos/kg body weight
per day (as PVC-resin formulation) from days 41 to 70 of pregnancy, the
blood ChE activity of the sows was markedly inhibited, but no
demonstrable teratogenic effects or functional abnormalities in the
piglets were found (Wrathall et al., 1980).
8.5.2 Embryotoxicity and teratogenicity
8.5.2.1 Oral
In studies by Schwetz et al. (1979), CF1 mice and New Zealand
rabbits were given maximum tolerated doses of dichlorvos (96%) in corn
oil by gavage, at levels of 60 and 5 mg/kg body weight, respectively,
from days 6 to 15 and days 6 to 18 of gestation, respectively. Except
for an increased number of resorptions in rabbits, no significant
effect was observed on the mean number of live fetuses per litter, or
on fetal body measurements. There were no gross visceral or skeletal
alterations. In mice, no abnormalities were found.
Carson (1969) reported studies on a total of 168 New Zealand
rabbits, divided into 10 groups containing 15 - 26 animals. One group
received lactose capsules, three groups different PVC-placebo capsules,
and three groups PVC capsules containing 18, 54, or 93 mg
dichlorvos/animal. These capsules were provided twice daily, so that
the equivalent daily intake was 12, 36, or 62 mg/kg body weight,
respectively. The rabbits received 12 or 36 mg dichlorvos/kg body
weight on days 6 - 18 of gestation, or 62 mg/kg body weight on days 6 -
11 of gestation. Fetuses were obtained by Caesarian section. Maternal
mortality was increased in the highest dichlorvos group, and the
incidences of in utero and neonatal toxicity were also increased (but
not significantly) in the 62 mg group, compared with those in the
control groups. The fetal mortality was not clearly indicative of any
marked toxic effects other than those involving the dam, since whole
litters were not involved. Extensive skeletal and soft tissue
examinations were carried out on all viable neonates, but no adverse
effects on bone formation, articulation, or degree of ossification were
found in the dichlorvos groups. No teratogenic effects were observed.
When pregnant rabbits were given oral doses of PVC-resin
formulations during the critical days of organogenesis, doses of 34 mg
dichlorvos/kg body weight or more were found to cause maternal
toxicity. With doses of 12 mg/kg body weight or less, no significant
effect on nidation, in utero survival, or neonatal survival was found.
No evidence of teratogenic changes was observed on gross, visceral, or
skeletal examination of the fetuses (Vogin et al., 1971).
The effect of dichlorvos on embryonal and fetal development in
thyroparathyroidectomized, thyroxine-treated, and euthyroid control
rats has been investigated. On days 8 - 15 of gestation, 25 mg
dichlorvos/kg body weight per day was administered orally to pregnant
Charles River rats, and a slight decrease in fetal weight in all groups
was observed. No gross, visceral, or skeletal anomalies of the fetuses
were found as a result of dichlorvos administration to rats with
altered thryoid status (Baksi, 1978).
8.5.2.2 Inhalation
In studies by Thorpe et al. (1972), rats of E strain and Dutch
rabbits were exposed (23 h per day, 7 days per week) throughout
pregnancy to nominal concentrations of 0, 0.25, 1.25, or 6.25 mg
dichlorvos/m3. In an additional study, groups of 20 pregnant
rabbits were exposed to nominal concentrations of 2 or 4 mg
dichlorvos/m3. Maternal deaths occurred in the rabbits exposed to
2 mg/m3 or more, and ChE activities in plasma, erythrocytes, and brain
were markedly inhibited in both species exposed to 1.25 mg/m3 or
more. There was no indication of any dichlorvos-related teratogenic
effects.
When CF1 mice and New Zealand rabbits were exposed to dichlorvos at
an average actual concentration of 4 mg/m3 for 7 h per day from days
6 to 15 and from days 6 to 18 of gestation, respectively, no
significant effect on the mean number of live fetuses per litter, the
incidence or distribution of resorptions, or on fetal body measurements
was noted. No gross, visceral, or skeletal alterations were observed
(Schwetz et al., 1979).
8.5.2.3 Intraperitoneal
Kimbrough & Gaines (1968) reported a study on female Sherman rats
given a single intraperitoneal injection of 0 or 15 mg dichlorvos (in
peanut oil)/kg body weight on day 11 of pregnancy. The treated dams
showed toxic signs and weight loss. On day 20, the fetuses were
removed. There was no adverse effect on litter size, resorptions,
number of dead fetuses per litter, or average weight of fetuses, but 3
out of 41 fetuses had omphaloceles. This latter finding, observed at a
maternally toxic dose, is not in agreement with the other teratology
studies or the 3-generation reproduction study.
8.5.3 Résumé of reproduction, embryotoxicity, and teratogenicity studies
A 3-generation study on rats, fed dichlorvos at dose levels of up
to 500 mg/kg diet, did not show any effects on fertility, number and
size of litters, body weight, or viability of the pups.
In studies on mice and rats, high dose levels of dichlorvos (a
single dose of 40 mg/kg body weight or multiple doses of 5 or 10 mg/kg
body weight) induced disturbances in spermatogenesis, characterized by
damage to the seminiferous tubules and Sertoli cells and by hypertrophy
and increase in the number of Leydig cells. It was assumed, but not
confirmed, that testosterone synthesis was partially inhibited. After
dichlorvos treatment ceased, recovery was complete within about 2
months.
A number of reproduction studies on domestic animals, mainly sows,
have been carried out. Levels of 500 mg/kg diet for 3 years had no
effect on fertility. Inhibition of ChE activity in the sows, but not
in the newborn pigs, was induced by 25 mg/kg body weight. No
teratogenic effects were seen.
Teratogenicity studies on rats and rabbits, orally administered
62 mg/kg body weight during gestation, revealed symptoms of
intoxication and significant inhibition of ChE in the parent rabbits.
Except for an increased number of resorptions, no significant effect on
the mean number of live fetuses per litter or evidence of a teratogenic
effect was noted during gross, visceral, or skeletal examination.
There were no teratogenic effects after rats inhaled 6.25 mg
dichlorvos/m3 during gestation, though maternal deaths occurred. Mice
and rabbits exposed to 4 mg/m3 on days 6 - 18 of gestation did not
show any effects.
8.6 Mutagenicity and Related End-Points
8.6.1 Methylating reactivity
In a quantitative colour test for alkylating agents, dichlorvos
gave a positive response, whereas the metabolites desmethyldichlorvos,
dimethylphosphate, dichloroethanol, DCA, and dichloroacetic acid all
gave negative results (Bedford & Robinson, 1972).
8.6.1.1 In vitro studies
Lawley et al. (1974) have shown that methylation by dichlorvos of
DNA and RNA, using either isolated nucleic acids, Escherichia
coli cells, or human tumour HeLa cells, broadly resembled that by
methylmethanesulfonate (MMS) rather than methylation by
N-methyl- N-nitrosourea. In E. coli cells, 3-methyladenine, the
principal minor product in methylated DNA apart from 7-methylguanine,
was not detected, whereas it was present when pre-isolated DNA was
methylated. The overall extent of methylation achieved in cells was
small. Specific excision of 3-methyladenine was indicated in E.
coli cells (Lawley et al., 1974).
Labelled 7-methylguanine was present in both DNA and RNA isolated
from E. coli exposed to 3H-dichlorvos. The methylating capability of
dichlorvos was less, by a factor of 10 - 100, than that of strongly
genotoxic methylating compounds (Wennerberg & Löfroth, 1974).
Alkylation by dichlorvos of calf thymus DNA, resulting in the formation
of N-7-methylguanine, was reported by Löfroth (1970).
Shooter (1975) has applied the test for chain breaks in RNA to the
interaction of dichlorvos with bacteriophage R17. Breaks in the RNA
chain result from the hydrolysis of phosphotriesters and are thus a
measure of the extent of O-alkylation and of the SN1-type mechanism
of the reaction. The results so far suggest that the existence of O-
alkylation, as shown by degradation following phosphotriester
formation, does correlate with mutagenicity. Incubation of
bacteriophage R17 with 0 - 100 mmol dichlorvos/litre for 90 h did not
result in methylation of the phosphate group of the RNA to any
significant extent.
8.6.1.2 In vivo studies
Reviews of the literature on alkylating agents including dichlorvos
have been made by Bedford & Robinson (1972), Lohs et al. (1976), and
Hemminki (1983).
In studies in which mice were given intraperitoneal injections
of methyl-14C-dichlorvos (1.9 µmol/kg body weight), the degree
of alkylation of guanine- N-7 in DNA isolated from soft tissues
amounted to 8 x 10-13 mol methyl/g DNA. From this, a rate of
clearance of approximately 1.4 min was estimated (Segerbäck, 1981;
Segerbäck & Ehrenberg, 1981). DNA and RNA from the total soft tissues
of male CFE rats exposed to atmospheres containing 0.064 mg methyl-
14C-dichlorvos/m3 for 12 h did not show methylation of the N-7 atom
of guanine moieties. The exposure period constituted a significant
fraction of the half-life of the 7-methylguanine moieties in DNA. It
was concluded that dichlorvos does not pose a methylating hazard to
mammalian DNA in vivo (Wooder et al., 1977; Wooder & Wright, 1981).
The excretion of labelled 7-methyl guanine in the urine
by NMRI mice and R rats injected intraperitoneally with methyl-
14C-dichlorvos, or exposed by inhalation for 2 h (mice only), was
reported by Löfroth & Wennerberg (1974) and Wennerberg & Löfroth
(1974). In rat urine, labelled 3-methyladenine and 1-methyl-
nicotinamide were also detected (Löfroth & Wennerberg, 1974).
According to the authors, these results demonstrate the dichlorvos-
induced methylation of guanine and adenine moieties in nucleic acids.
However, the administration of radiolabelled adenine and guanine to
otherwise untreated rats gave rise to the excretion of radiolabelled
methylated purines in the urine. Therefore, the detection of
radiolabelled purines, per se, in the urine of animals exposed to
methyl-labelled methylating agents, does not constitute evidence for
the methylation of the purine moieties of nucleosides or nucleic acids
by methylating agents (Wooder et al., 1978). Moreover, the results of
metabolic studies have demonstrated the existence of a natural
biosynthetic pathway whereby the methyl carbon atoms of dichlorvos can,
with partial retention of hydrogen, become incorporated into the
heterocyclic rings and the methyl groups of urinary 7-methylguanine
after entering 1-C pools, in vivo. The results of preliminary studies
suggest the existence of a similar pathway for the production of
urinary 3-methyladenine (Wright et al., 1979).
Wooder & Creedy (1979) described a study on rats which investigated
the DNA-damaging potential of dichlorvos when administered as a single
intraperitoneal dose. Alkaline sucrose gradient profiles of rat liver
DNA showed that whereas MMS (a positive control) shifted the DNA
profile, dichlorvos at 10 mg/kg body weight (the maximum dose
consistent with survival) had no effect.
8.6.1.3 Discussion of methylating reactivity
Many alkylation tests and in vivo and in vitro mutagenicity studies
have been carried out with dichlorvos. It has been demonstrated that
dichlorvos has alkylating properties, and has been suggested from some
studies that the in vivo alkylating potential of dichlorvos is similar
to that of some known mutagens. However, this concern is misplaced
since alternative reactions were not considered (WHO, 1986b). The
phosphorus atom is markedly more electron-deficient and susceptible to
attack by nucleophiles than the alkyl carbon atom. Analysis by Bedford
& Robinson (1972) of the data of Löfroth et al. (1969) revealed that
the proposed rates of alkylation by potent nucleophiles were probably
combined rates of phosphorylation and alkylation, and that
phosphorylation was the totally dominant reaction in the case of the
hydroxide ion. The comparison with known mutagens is therefore
inappropriate. Two factors detract further from the toxicological
significance of the alkylation studies. The first is that mammalian
tissues (plasma, liver, etc.) contain active A-esterase enzymes, which
catalyse the phosphorylation of water by the organophosphorus esters.
Viewed inversely, these A-esterases catalyse the hydrolysis of the
organophosphorus esters, thereby rapidly reducing circulating levels of
hazardous material. Secondly, the comparative rate of reaction of most
of these esters with AChE is many orders of magnitude greater than the
rate of alkylation by the typical nucleophile 4-nitrobenzyl-pyridine:
for dichlorvos, the ratio of rates was 1 x 107 in favour of the
inhibitory phosphorylation of AChE (Aldridge & Johnson, 1977). It
follows that, at low exposure levels, in vivo phosphorylation of AChE
and other esterases will be the dominant reaction, with negligible
uncatalysed alkylation of nucleic acid. Indeed, no such alkylation has
been detected in sensitive in vivo studies designed to check this point
(Wooder et al., 1977).
8.6.2. Mutagenicity
Reviews of the existing literature have been published by Wild
(1975), Fishbein (1976, 1981, 1982), Leonard (1976), IARC (1979),
Sternberg (1979), Ramel et al. (1980), Lafontaine et al. (1981), Ramel
(1981), and Wildemauwe et al. (1983).
8.6.2.1 In vitro studies
(a) Microorganisms
Numerous mutagenicity studies using bacteria and fungi as test
organisms have been carried out (Table 17). In most of the studies,
only one dichlorvos concentration, often a high one, was tested,
sometimes resulting in low survival of the test organism. A
dose-response relationship was established in the few tests where a
range of concentrations was used. The results indicate that dichlorvos
induces base substitutions in bacteria and mitotic gene conversion in
yeast. The alkylating properties of dichlorvos (section 8.6.1) are
most probably the cause of the mutagenic action. This was the
conclusion of Bridges et al. (1973) from tests using both MMS and
dichlorvos with E. coli strains deficient at four different repair
loci.
In Aspergillus nidulans, dichlorvos has been found to induce point
mutations to 8-azaguanine resistance and a high frequency of mitotic
crossing-over and non-disjunction (Aulicino et al., 1976; Bignami et
al., 1976, 1977; Morpurgo et al., 1979). No mutagenic activity
using A. nidulans was found after incubating Nicotiana alata cell
cultures with dichlorvos for 21 days (simulating in vitro plant
metabolism). This confirms the rapid metabolism of dichlorvos (Benigni
et al., 1979).
The mutagenicity of dichlorvos has been extensively studied in
Japan, (Kawachi et al., 1980). Dichlorvos induced gene mutation
in Salmonella typhimurium TA100 as well as E. coli strains in the
absence of rat-liver S9 mix.
Dichlorvos (0.5 - 2 mg/ml) causes random strand breakage,
repairable by DNA polymerase I, in E. coli pol A as detected by the
alkaline sucrose sedimentation method. When pol+ bacteria and high
concentrations of dichlorvos (0.2 - 0.4%) were used, an all-or-none
breakdown of DNA molecules to fragments of very low relative molecular
mass occurred which correlated well with lethality. It has been
suggested that the major DNA damage resulting from dichlorvos treatment
arises indirectly through alkylation of other cellular constituents,
this leading to uncontrolled nuclease attack on DNA. However,
discontinuities in newly-synthesized DNA and mutagenesis following
dichlorvos treatment presumably result from direct alkylation of DNA
(Bridges et al., 1973; Green et al., 1973, 1974a,b). In the
standard E. coli DNA polymerase-deficient assay system, dichlorvos
(6.4 mmol/litre) gave a positive result (Rosenkranz, 1973; Rosenkranz &
Leifer, 1980). In measuring mutation to tryphophan independence in E.
coli strain WP2, it was found that 5 mg dichlorvos/litre was mutagenic
in this test system (Green et al., 1976). Griffin & Hall (1978) have
found that dichlorvos (1 mg/ml) causes breaks in colicinogenic plasmid
E1 DNA from E. coli. In a rec-type repair test with Proteus
mirabilis strains PG 713 (rec-hcr-) and PG 273 (wild type),
dichlorvos (10 or 40 µmol per plate) induced base-pair substitutions
and other DNA damage. In the same test, desmethyldichlorvos, at the
same concentrations, gave negative results (Adler et al., 1976; Braun
et al., 1982).
(b) Mammalian cells
In cultured V79 Chinese hamster cells, no induction of 8-
azaguanine-resistant mutations after treatment with up to 1 mmol/litre
dichlorvos (Wild, 1975), or of ouabain-resistant mutations after
treatment with 1.25 - 5 mmol/litre dichlorvos, was found (Aquilina et
al., 1984).
DNA strand breakage in cultured V79 Chinese hamster cells caused by
up to 0.2% v/v dichlorvos has been reported by Green et al. (1974a).
Dichlorvos (1 µl) decreased the sedimentation coefficient of calf
thymus DNA upon thermal denaturation, indicating a decrease in the DNA
molecular size (Rosenkranz & Rosenkranz, 1972). Incubation of
dichlorvos (45 mmol/litre) with calf thymus DNA did not result in
changes in thermal melting curves or DNA fractionation on a
hydroxyapatite column. However, changes were observed by means of
differential pulse polarography, indicating that single-stranded
segments and thermolabile regions were formed in the DNA. This
behaviour could perhaps be a consequence of guanine alkylation followed
by depurination and chain cutting at elevated temperatures (Olinski et
al., 1980).
The resistance of methylated DNA in Chinese hamster ovary cells,
labelled with 14C-thymidine and methyl-3H-1-methionine, to
micrococcal nuclease digestion was abolished when the cells were
treated with dichlorvos (10 mmol/litre) for 3 h. No effects were
observed on kinetics of total DNA digestion. These results indicate a
conformational change in chromatin induced by dichlorvos (Nishio &
Uyeki, 1982).
Table 17. Mutagenicity tests on microorganisms
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Bacillus subtilis
H17 rec+ 0.02 ml of plate none - Shirasu et al.
M45 rec- 10% solution plate none + (1976)
Citrobacter freundii
425 0.1% fluctuation none weak + Voogd et al. (1972)
0.05% none -
Enterobacter aerogenes
6 0.1% fluctuation none weak + Voogd et al. (1972)
Escherichia coli
B 5 - 25 mmol/litre liquid induc- none weak + Wild (1973)
1- to 10-h tion strepto- (dose-
exposure mycin-resistant and exposure-
mutants time related)
B/r WP2 22.6 mmol/litre plate reversion none + Moriya et al.
S9 mix + (1978)
L-cysteine +
CM 561, 571, 611 5 µl plate reversion none - Hanna & Dyer
(1975)
K12HfrH 0.1% fluctuation none weak + Voogd et al.
(1972)
K12(5-MT) 3.3 x 10-4 plate none + Mohn (1973)
mol/litre
WP2 micro drop plate none - Dean (1972a)
analytical and
technical grade,
aqueous solution
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Escherichia coli (contd).
WP2 try- approximately plate none + Ashwood-Smith et
20 mm2 dichlor- al. (1972)
vos strip
WP2 try- 20 - 25 µl of spot none + Nagy et al. (1975)
hcr- and hcr+ 50% emulsifiable back mutation
concentrate
0.1 ml of a 5% plate reversion none + Shirasu et al.
solution (1976)
WP2 hcr unknown (up to plate reversion none + Moriya et al.
5000 µg/plate) (1983)
WP2 uvrA, ca 5 µl plate reversion none + Hanna & Dyer
WP 67 (1975)
Klebsiella pneumoniae 0.1%; 0.05% fluctuation none weak + Voogd et al.
(1972)
Neurospora crassa
ad-3 exposed to air plate none - Michalek &
containing Brockman (1969)
dichlorvos
(strip)
Pseudomonas aeruginosa
PAO 38 0.08 mol/litre liquid reversion none + Dyer & Hanna
(1973)
Saccharomyces cerevisiae
D4 (ade2 and 4 mg/ml plate mitotic none + Dean et al.
trp5) 2 mg/ml gene conversion (1972)
D4 (ade2 and 19 mmol liquid mitotic none + Fahrig (1973,
trp5) gene conversion 1974)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium
64-320 0.05%; 0.1% suspension none + Voogd et al. (1972)
TA 98 20 or 40 mmol plate S9 male mice - Braun et al. (1982)
up to 5000 µg plate reversion none - Moriya et al. (1983)
TA 100 20 or 40 mmol plate S9 male mice (+) Braun et al. (1982)
low survival
up to 5000 µg plate reversion none + Moriya et al. (1983)
TA 1530 5 µl plate none + Hanna & Dyer (1975)
TA 1535 5 µl plate none + Hanna & Dyer (1975)
0.1 ml of a 5% plate none + Shirasu et al. (1976)
solution
0.1 ml of a 5% plate none + Moriya et al. (1978)
solution S9 mix -
L-cysteine -
2800 µg plate (spot) S9 male rat - Carere et al.
(1976, 1978a,b)
1.5 mg/ml liquid none + Carere et al.
(1978a,b)
20 or 40 mmol plate S9 male mice - Braun et al. (1982)
TA 1536, 1537, 0.1 ml of a 5% plate none - Shirasu et al.
1538 solution (1976)
TA 1536, 1537, 2800 µg plate (spot) S9 male rat - Carere et al.
1538 (1976, 1978a,b)
20 or 40 µg plate S9 male mice - Braun et al.
(1982)
up to 5000 µg plate none - Moriya et al.
(1983)
Table 17. (contd).
---------------------------------------------------------------------------------------------------------
Organism/strain Dose Type of test Metabolic Result Reference
activation
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium (contd).
his C117 0.03 mol liquid none + Dyer & Hanna (1973)
LT2 his C117, 5 µl plate none - Hanna & Dyer (1975)
his G 46
Schizosaccharomyces pombe
ade6 1.5 - 14 mmol plate + + Gilot-Delhalle et
al. (1983)
Serratia marcescens
Hy alpha 13, 25 mg/ml plate (spot) none alpha 13 + Dean (1972a)
alpha 21 alpha 21 -
50, 100 mg/ml plate (spot) none + Dean (1972a)
saturated plate (spot) none + Dean (1972a)
aqueous solution
Streptomyces coelicolor
A 3(2) his Al 5600 µg spot none + Carere et al.
(1976, 1978a,b)
---------------------------------------------------------------------------------------------------------
Dichlorvos (0.03 and 0.1 mmol/litre) has been found to induce
sister chromatid exchanges (SCEs) in cultures of Chinese hamster ovary
cells (Nishio & Uyeki, 1981). On the other hand, in Chinese hamster
V79 cells (clone number 15), SCEs were not induced by 0.1 mmol
dichlorvos/litre, but only by 0.2 and 0.5 mmol/litre solutions. The
number of polyploid cells was increased at both 0.1 and 5 mmol/litre
(Tezuka et al., 1980).
Dichlorvos has been tested in two independent laboratories for its
ability to increase the transformation of Syrian hamster embryo cells
by simian adenovirus SA7. Pre-treatment of hamster cells with
dichlorvos at concentrations of 0.05 up to 0.45 mmol/litre produced a
significant enhancement of SA7 transformation at 0.11 mmol/litre and
higher (Hatch et al., 1986).
The mouse peripheral blood lymphocyte (PB) culture system has also
been used to test for SCE induction. Male B6C3F1 mice were injected
intraperitoneally with 5, 15, 25, or 35 mg dichlorvos/kg body weight,
but there was no change in the baseline SCE frequency (Kligerman et
al., 1985).
Dichlorvos (6.5 - 650 mmol/litre) has been found to induce dose-
dependent unscheduled DNA synthesis in the human epithelial-like cell
line EUE (Benigni & Dogliotti, 1980a,b; Aquilina et al., 1984). Both
scheduled and unscheduled DNA synthesis of human lymphocytes showed
dose-related inhibition by dichlorvos (5 -500 mg/litre), as measured by
3H-thymidine uptake (Perocco & Fini, 1980).
Dichlorvos (0.0001 - 0.1 mmol/litre) does not induce DNA repair in
human kidney T-cells. This was shown by a lack of dichlorvos-induced
3H-thymidine incorporation into T-cells in the G1- or G2-phase of the
cell cycle. No induction of single-strand breaks in the T-cell DNA, as
measured by alkaline sucrose gradients, was found following treatment
for 1, 2, or 4 h with 0.0001 - 1 mmol dichlorvos/litre (Bootsma et al.,
1971).
No clear effect on the frequency of SCEs in human lymphocytes or
human fetal lung fibroblasts was found after exposure to 2.5 - 10 mg
dichlorvos/litre for 72 h (Nicholas et al., 1978).
In studies by Dean (1972b), human blood samples were treated with
dichlorvos (0.0001 - 1 mmol/litre) for 1, 2, 4, and 20 h, after which
the lymphocytes were stimulated to divide using phyto-haemagglutinin.
The toxic effect of 1 mmol dichlorvos/litre was indicated by a
decreased mitotic index. Mitotic cells were analysed for chromosome
aberrations, but the number of dicentrics in dichlorvos-treated cells
was no different from that found in untreated cells (Bootsma et al.,
1971). When dichlorvos (1 - 40 mg/litre) was added at specific
intervals to cultures of human lymphocytes, cytotoxicity was found at
5 mg/litre or more, but no chromosome aberrations (chromatid gaps or
breaks) were detected (Dean, 1972b).
Negative results in cultured human lymphocytes were also reported
by Fahrig (1974) and Wild (1975).
8.6.2.2 In vivo studies
(a) Drosophila melanogaster
Negative results were obtained in the standard Muller-5 test for
sex-linked lethal mutations and in the induced crossing-over test with
the approximate LD50 concentration (0.035% dichlorvos) (Jayasuriya &
Ratnayake, 1973). Similarly, 0.0006 - 0.6 µmol dichlorvos gave
negative results in the standard Muller-5 test (Sobels & Todd, 1979).
In studies by Hanna & Dyer (1975), a Drosophila melanogaster
population was continuously exposed to gradually increasing
concentrations of dichlorvos (because of development of increased
resistance) in the food medium (up to 0.75 mg/kg food) for about 18
months. At the end of this period, an increased accumulation of
mutations was observed.
Gupta & Singh (1974) reported studies where female D.
melanogaster flies were kept on food with 1 - 50 mg dichlorvos (Nuvan
100 EC)/kg. No eggs were laid at 10 mg/kg or more, and at 1 mg/kg,
survival of the eggs was 45% of that of the controls. Salivary gland
chromosome abnormalities were observed in fully-grown larvae fed
1 mg/kg. However, Kramers & Knaap (1978), using the same route of
administration, found no induction of sex-linked recessive lethals by
dichlorvos (0.009, 0.048, and 0.09 mg/kg food).
(b) Host-mediated assays
Dichlorvos was not mutagenic in host-mediated assays in NMRI mice
using:
(i) S. typhimurium (G46 his-) and Serratia marcescens (a 21
leu-) after an intraperitoneal injection of 25 mg
dichlorvos/kg body weight (Buselmaier et al., 1972, 1973);
(ii) S. typhimurium (64-320) after an oral dose of 0.2 mg per
animal (equivalent to 8 - 10 mg/kg body weight) (Voogd et
al., 1972); or
(iii) Saccharomyces cerevisae (D4 ade2 and trp5 loci) after an
oral dose of 50 or 100 mg/kg body weight (or after exposure
of CF1 mice for 5 h to 60 or 90 mg dichlorvos/m3) (Dean et
al., 1972).
Although dichlorvos was mutagenic to S. cerevisae in in
vitro studies, these in vivo studies proved to be negative.
(c) Dominant lethal assays
Negative results were reported in a test for dominant lethal
mutations in ICR/Ha mice (expressed as an increase in early fetal
deaths or, indirectly, by pre-implantation losses), after a single
intraperitoneal injection of 13 or 16.5 mg/kg body weight or five
consecutive daily oral doses of 5 or 10 mg/kg body weight. The total
mating period was 8 weeks (Epstein et al., 1972). The same result was
obtained after exposure of male CF1 mice to 30 or 55 mg/m3 for 16 h or
to 2.1 or 5.8 mg/m3 for 23 h daily for 4 weeks (Dean & Thorpe,
1972b).
A statistically significant increase in the frequency of pre-
implantation losses in mice (Q strain) in the second week and fifth
week of mating has been observed after a single intraperitoneal
injection of 10 mg/kg body weight (Degraeve et al., 1980; Moutschen-
Dahmen et al., 1981).
In studies by Degraeve et al. (1982, 1984a), male Q strain mice
received drinking-water with 2 mg dichlorvos/litre (equivalent to
0.32 mg/kg body weight per day) 5 days per week, for 7 consecutive
weeks. At the end of this period, the males were mated for 1 week with
untreated virgin females, and the pregnant females were killed 14 days
after the start of pregnancy. No dominant lethal mutations were
induced. The same result was obtained when female CF1 mice were either
given single oral doses of 0, 25, or 50 mg dichlorvos/kg body weight or
continuously exposed to atmospheres containing 0, 2, or 8 mg
dichlorvos/m3 from weaning until 11 weeks of age. They were either
mated at the end of the dosing or inhalation period or at intervals of
5, 10, and 15 days thereafter (Dean & Blair, 1976).
(d) Chromosome abnormalities
Male Q strain mice receiving drinking-water containing 2 mg
dichlorvos/litre (equivalent to 0.32 mg/kg body weight), 5 days per
week for 7 weeks, did not show chromosome damage in bone marrow cells,
spermatogonia, or primary spermatocytes (Moutschen-Dahmen et al., 1981;
Degraeve et al., 1982, 1984a); neither did mice of the same strain
given a single intraperitoneal injection with 10 mg/kg body weight
(Moutschen-Dahmen et al., 1981; Degraeve et al., 1984b).
In a micronucleus test, Swiss mice were given daily intra-
peritoneal injections of dichlorvos (0.0075 - 0.015 mg/kg body weight
per day) for 2 days and killed 6 h after the last dose. No induction
of aberrations in the structure or number of chromosomes in bone marrow
cells was observed (Paik & Lee, 1977).
CF1 mice exposed to concentrations of 64 - 72 mg
dichlorvos/m3 for 16 h or to 5 mg/m3 per day, 23 h per day, for 21
days, did not show chromosome abnormalities in bone marrow or
spermatocytes. Similar results for Chinese hamsters exposed by the
inhalation route to 28 -36 mg/m3 for 16 h (males only), or by a single
oral dose of 15 mg/kg body weight (males) or 10 mg/kg body weight
(females), have been reported (Dean & Thorpe, 1972a).
Dichlorvos has been tested for its ability to induce in vivo
chromosomal aberrations in Syrian hamsters bone marrow cells. Four
dose levels (3, 6, 15, and 30 mg/kg body weight) were given
intraperitoneally. Statistically significant increases in the number
of cells with aberrant chromosomes (mainly breaks and gaps) were
observed (Dzwonkowska & Hübner, 1986).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1 Mouse
Carcinogenicity studies were carried out on two groups of 50 male
and 50 female B6C3F1 mice fed 1000 and 2000 mg dichlorvos (94%) in corn
oil/kg diet for 80 weeks. Due to severe signs of intoxication, doses
were lowered after 2 weeks to 300 and 600 mg/kg for the remaining 78
weeks. Samples of the diets analysed during the study showed
dichlorvos contents within 5% of the intended concentrations. Matched
controls consisted of 10 mice of each sex; the pooled controls
consisted of 100 male and 80 female mice. All surviving mice were
killed at 92 - 94 weeks. Hair loss and rough hair coats were noted in
many treated animals, particularly in the male groups, beginning at
week 20 and persisting throughout the study. The average body weights
of the high-dose mice of both sexes were slightly decreased compared
with controls. The low-dose female group showed the poorest survival;
74% of the animals lived to 90 weeks. There was no significant
increase in the incidence of tumours attributable to dichlorvos in
either sex, i.e., dichlorvos was not demonstrated to be carcinogenic
(NCI, 1977; Weisburger, 1982).
In other studies, groups of 50 male and 50 female B6C3F1 mice, 6
weeks of age, were given drinking-water with 0, 400, or 800 mg
dichlorvos/litre ad libitum. The drinking-water solutions were renewed
daily. All surviving animals were killed during week 102. A dose-
dependent inhibition of body weight increase was observed throughout
the study in both sexes at both dichlorvos concentrations. There was
no adverse effect on mortality. The survival rates at week 102 were
62%, 66%, and 84% in males (in controls, low-, and high-dose groups,
respectively), and 66%, 50%, and 80% in females. The corresponding
figures for tumour incidence were 22.4%, 39.1%, and 23.4% in males and
29.3%, 16.2%, and 9.1% in females. The main tumours that were found
were lung adenomas and tumours in the liver, spleen, thymus, and
salivary gland. These tumours occurred in all three groups. There was
no statistically significant difference in the incidence of tumours at
any site in any group (Konishi et al., 1981).
Preliminary results are available from a recently completed 2-year
mouse carcinogenicity study using dichlorvos. Groups of 50 male and 50
female B6C3Fl mice were given dichlorvos (dissolved in corn oil) by
oral gavage daily for 2 years. Dose levels for male mice were 0, 10,
or 20 mg/kg body weight per day and for female mice 0, 20, or 40 mg/kg.
There were no statistically significant differences in survival rates
between the treated and control male mice, and the same applied to
female mice. A statistically significant increase in the incidence of
forestomach squamous cell papillomas was observed in the female mice
receiving 40 mg/kg per day. Two forestomach squamous cell carcinomas
were also seen in this group, but none were observed in the controls or
the 20 mg/kg group. Considerably fewer forestomach squamous cell
papillomas were observed in male mice. Nevertheless, a non-significant
increase was observed in the 20 mg/kg per day group. No forestomach
squamous cell carcinomas were noted in any male group. Forestomach
hyperplasia occurred relatively frequently in both control and treated
male and female mice, the incidence being similar in all groups (NTP,
1987).
8.7.1.2 Rat
In studies reported by NCI (1977) and Weisburger (1982), two groups
each of 50 male and 50 female Osborne-Mendel rats were fed either 150
or 1000 mg dichlorvos (94%) in corn oil/kg diet for 80 weeks. Due to
the severe signs of intoxication, the 1000 mg/kg dose was reduced to
300 mg/kg diet after 3 weeks for the remaining 77 weeks. The diets
were stored under conditions designed to minimize loss of dichlorvos,
and the animals received fresh diets daily. Matched controls comprised
10 rats of each sex; the pooled controls consisted of 60 rats of each
sex. The surviving rats were killed after 110 weeks. The average body
weights of the rats receiving the high dose level were slightly
decreased compared with controls. There was no significant increase in
the incidence and type of tumours in either sex as a result of
dichlorvos treatment.
Enomoto et al. (1981) carried out studies on groups of 50 male and
50 female Fisher 344 rats, 6 weeks of age, given drinking-water
(renewed daily) containing 0, 140, or 280 mg dichlorvos/litre ad
libitum. All surviving animals were killed in week 108 (104 weeks of
exposure followed by a 4-week recovery). Slight inhibition of body
weight increase was observed in males in the high-dose group, but there
was no influence on mortality. The survival rates in week 108 were
82%, 75%, and 75% in males and 86%, 71%, and 82% in females,
respectively, in control, low-dose, and high-dose groups. A great
number of organs and tissues were selected for microscopy. The organs
and tissues of all animals which died or which were killed in moribund
conditions were microscopically examined, as were all tumours and
macroscopical lesions. A number of animals with no specific changes
were also examined. The overall tumour incidences were 100%, 96%, and
98% in males and 37%, 31%, and 33% in females, respectively, in
control, low-dose, and high-dose groups. High incidences of
interstitial cell tumours of the testes in males (49/51, 41/48, and
47/48, respectively) were observed in all three groups. Mononuclear
cell leukaemia was found in all groups at 4 - 12%. There was no
statistically significant difference in tumour incidence at any site in
any group.
Preliminary results are available from a recently completed 2-year
rat carcinogenicity study using dichlorvos. Dichlorvos, dissolved in
corn oil, was administered each day by oral gavage to groups of 50 male
and 50 female Fischer 344 rats at dose levels of 0, 4, or 8 mg/kg body
weight per day. Though survival rates were slightly decreased in the
treated male and female groups compared with those of their respective
controls groups, the differences were not statistically significant.
However, a statistically-significant and dose-related increase in the
incidence of pancreatic adenomas was observed in the male rats fed 4 or
8 mg/kg. In female rats fed 8 mg/kg, a non-significant increase in
pancreatic adenomas was observed. There was a relatively high
incidence of pancreatic hyperplasia and atrophy in all groups of male
rats, including the controls, and a lower incidence in all female
groups. A statistically significant (but not dose-related) increased
incidence of mononuclear leukaemia was observed in the male rats fed 4
or 8 mg/kg. A similar incidence was observed in all female groups,
including the control group. Since this is a common and variable
systemic lesion in Fischer-344 rats, the toxicological significance of
this finding is uncertain. It is possible that its incidence in the
male control group was unusually low. An increased incidence of
mammary gland fibroadenomas (not dose related) was observed in the
treated female rats, but these were not considered to be of
toxicological concern. In addition, mammary gland hyperplasia was
frequently observed in all control and treated female groups at similar
incidences (NTP, 1986).
As reported in section 8.4.1.1, Witherup (1967, 1971) found no
increase in tumour incidence following dichlorvos treatment of rats.
8.7.2. Inhalation
8.7.2.1 Rat
In the studies by Blair et al. (1976) described in section 8.4.2.1,
no dose-related increase in tumour incidence was found.
8.7.3. Appraisal of carcinogenicity
The majority of the mouse and rat studies using dichlorvos are
considered to be negative regarding carcinogenic potential (Witherup et
al., 1967, 1971; Blair et al., 1976; NCI, 1977; Enomoto et al., 1981;
Konishi et al., 1981; Weisburger, 1982). However, preliminary
information from two recent studies on the mouse and rat, respectively,
has provided equivocal evidence of carcinogenicity (NTP, 1986)a.
------------------------------------------------------------------------
a The NTP Peer Review Panel reviewed these studies and came to the
following conclusions:
"Under the conditions of these 2-year gavage studies, there was some
evidence of carcinogenic activity of dichlorvos for male F344/N rats, as
shown by increased incidences of adenomas of the exocrine pancreas and
mononuclear cell leukemia. There was equivocal evidence of carcinogenic
activity of dichlorvos for female F344/N rats, as shown by increased
incidence of adenomas of the exocrine pancreas and mammary gland
fibroadenomas. There was some evidence of carcinogenic activity of
dichlorvos for male B6C3F1 mice and clear evidence for female B6C3F1 mice,
as shown by increased incidences of forestomach squamous cell papillomas"
(NTP, 1988).
The increased incidence of forestomach tumours in the mouse study
is likely to be related to the route of administration (oral gavage),
which has been shown in other mouse studies to induce forestomach
tumours due to repeated and direct irritation of the gastric mucosa.
If so, ingestion of food by human beings could not result in such
direct effects on the stomach wall. Furthermore, human beings do not
possess a stomach wall comparable with the forestomach of the mouse,
except perhaps for the oesophagus. However, the transient passage of
food through the oesophagus would probably not allow sufficient time
for the carcinogenic event to occur.
Similarly, the pancreatic adenomas observed in the NTP rat study
may be related to the corn oil used in the study. Evidence from other
rat studies in which corn oil was used as a vehicle suggests that this
is a possibility. On the other hand, it does not explain why a higher
incidence of pancreatic tumours was observed in the treated animals
compared with the controls. Although increased incidences of
mononuclear leukaemia were observed in treated male rats, the
incidence in the control group of this common and variable tumour may
have been low. The evidence for carcinogenicity in these two recent
studies is difficult to interpret at present. When complete and final
reports of these studies become available, more definitive conclusions
may be drawn.
8.8. Mechanisms of Toxicity; Mode of Action
A full description of the mechanism of action can be found in
Environmental Health Criteria 63: Organophosphorus Insecticides - A
General Introduction (WHO, 1986b).
Dichlorvos directly inhibits AChE activity in the nervous system
and other tissues. This reaction takes place in three steps:
(a) reversible binding of dichlorvos to the enzyme;
(b) reaction with the enzyme to form a dimethylphospho-enzyme
derivative, with the loss of DCA; and
(c) "aging" of the phospho-enzyme compound to a more stable
methylphospho-enzyme derivative.
Reaction (a) is rapid, but reversible. Reaction (b) is also rapid,
but the phosphorylated enzyme can be returned to its native
state spontaneously only by hydrolysis at very slow rates or by agents
such as N-methyl 2-pyridinium aldoxime (2-PAM) at higher rates.
Reaction (c) is comparatively slow (t´ = about 2.5 h at 37 °C and pH
7.4), but the product has the same stability as that of erythrocytes
(t´ = about 120 days) (Gillett et al., 1972).
Death due to poisoning is caused by excessive cholinergic effects
such as bronchospasms, hypersecretion from cholinergic innervated
glands (especially critical in lungs and bronchi), and cardiac
disturbances caused by vagotonus and anoxia. Convulsions and paralysis
in skeletal musculature are caused by brain anoxia as well as
cholinergic effects within the central nervous system.
In vivo studies on the inhibition of ChE activity resulting from a
single oral or parenteral dose of dichlorvos, at various time
intervals, after dosing are summarized in Table 18. In general, the
maximum inhibition occurred within 1 h, and was followed by rapid
recovery. Dogs seemed to be more sensitive than rats, and showed a
slower recovery.
Dichlorvos, when infused intravenously (into the ear vein) of
adult male rabbits, produced dose- and time-related inhibition of whole
blood ChE activity during infusion. Spontaneous but incomplete
recovery to 60 - 80% of the normal activity occurred within 60 -
90 min of infusion (Shellenberger et al., 1965; Gough & Shellenberger,
1970, 1977-78; Shellenberger, 1980).
In a study on the influence of temperature on ChE activity, rats
were injected intraperitoneally with a single dose of 6.25 mg
dichlorvos (95%)/kg and kept at either 28 °C or 5 °C. The maximum
inhibition of whole blood ChE activity (40%) occurred after 0.5 h.
The animals at 5 °C showed less inhibition and a faster recovery of
whole blood ChE activity than those at 28 °C (Chattopadhyay et al.,
1982).
8.9. Neurotoxicity
8.9.1. Delayed neurotoxicity
A detailed description of the neurotoxic potential of
organophosphorus compounds can be found in Environmental Health
Criteria 63: Organophosphorus Insecticides - A General Introduction
(WHO, 1986b).
Several studies have shown that dichlorvos does not produce delayed
neurotoxicity in pre-medicated hens, whether it is administered orally
or subcutaneously (Durham et al., 1956; Aldridge & Barnes, 1966;
Johnson, 1969, 1975a,b, 1978, 1981; Aldridge & Johnson, 1971; Lotti &
Johnson, 1978). The inhibition of brain neurotoxic esterase (NTE)
without signs of ataxia has been observed (Aldridge & Johnson, 1971;
Johnson, 1978).
Caroldi & Lotti (1981) reported mild signs of ataxia in pre-
medicated hens 2 weeks after a single massive subcutaneous dose
(100 mg/kg body weight) and severe inhibition of NTE in peripheral
nerve, spinal cord, and brain. However, Johnson (1978) did not observe
ataxia in pre-medicated hens given the same dose in the same way.
These hens showed severe inhibition of brain NTE but far less
inhibition of spinal cord NTE. It appears that ataxia arises from the
inhibition of spinal cord NTE. When the dose was repeated 1 - 3 days
after the first dose, spinal cord NTE inhibition increased and the hens
became ataxic.
White leghorn hens have been used in a 90-day study on the
neurotoxic potential of dichlorvos (99.9%) after dermal or oral
administration. For oral administration, a 10 - 20% solution of
dichlorvos in corn oil in gelatin capsules was used whereas, dermally,
1 - 20% emulsifiable concentrates in technical grade xylene (containing
2% Triton X-100) were used. Dichlorvos at doses greater than 1 mg/kg
body weight per day (dermal) or 6 mg/kg (oral) led to cholinergic
symptoms including salivation, convulsions, and death after 2 - 3 days.
With oral doses of 3 - 6 mg/kg no ataxia or death was observed.
Dermally, dichlorvos was very toxic for hens. Dose levels of about 1.7
and 3.3 mg/kg for an average period of 37 days caused ataxia and death.
An average dermal dose level of 0.65 mg/kg body weight for 90 days did
not induce ataxia or death. Typical symptoms of organophosphorus-
ester-induced delayed neurotoxicity (OPIDN) were not observed (Francis
et al., 1985).
In summary, it is possible to produce clinical neuropathy in hens,
but the doses required are far in excess of the LD50. The effects
are associated with severe inhibition of NTE in brain and spinal cord,
measured shortly after dosing (Johnson, 1981).
8.9.2. Mechanism of neurotoxicity
Male rats given a lethal intraperitoneal injection of 40 mg
dichlorvos/kg body weight did not show electrocortical disturbances.
Deaths resulted from respiratory failure (Hyde et al., 1978).
In studies by Desi (1983), adult CFY rats were given daily oral
doses ranging from 1.25 to 4 mg dichlorvos/kg body weight mixed in the
diet for 3 months. Plasma, erythrocyte, and brain ChE activities were
comparable with those of control rats. Increased EEG activity and
enhanced central excitability were found in the male rats only.
No changes in reflex motor unit potential activity or in nerve
conduction velocity were noted in dogs 7 days after single oral doses
of 30, 59.5, or 148 mg dichlorvos/kg body weight. However, erythrocyte
ChE activity was inhibited at all doses, and the highest dose produced
signs of intoxication (Hazelwood et al., 1979).
A single oral dose of 40 mg dichlorvos/kg or repeated doses of
1.6 mg/kg body weight per day did not cause histological abnormalities
in the brain of adult Wistar rats. Repeated administration of 50% of
the LD50 (i.e., 40 mg/kg body weight per day for 10 - 21 days), caused
myelin pallor and micro-vacuolation of the white matter. It seems
likely that primary degeneration of axons and secondary myelin sheath
abnormalities caused the spongy tissue loosening observed under the
electron microscope (Zelman, 1977; Zelman & Majdecki, 1979).
In studies by Ali et al. (1979a) and Hasan et al. (1979), male rats
were given 3 mg dichlorvos/kg per day intraperitoneally for 10 days.
Following perfusion-fixation, sections of cerebellum and spinal cord
were studied with the electron microscope. An abnormal increase in the
number of mitochondria in the spinal cord was found. Myelin
degeneration was detected in the spinal cord and myelin figures were
occasionally noted within oedematous dendrite profiles.
Table 18. Time-related ChE activity in animals after administration of a single dose of dichlorvos
---------------------------------------------------------------------------------------------------------
Species/sex Route Dose (mg/kg Time Cholinesterase activity (%) Reference
body weight) plasma erythrocyte brain
---------------------------------------------------------------------------------------------------------
Mouse (male) intraperitoneal 30 15 min 37 Cohen & Ehrich
2 h 40 (1976)
5 h 69
18 h 92
Mouse (male) intraperitoneal 10 15 min 30 Nordgren et al.
60 min 50 (1978)
2 h 80
Rat (male) oral 50 15 min 30 20 10-15 Modak et al.
3 h 30 65 (1975)
24 h 60 75 60-70
Rat (male) oral 40 1 h 30 Teichert et al.
(1976)
Rat (male) oral 40 5 min 55 Pachecka et al.
15 min 20 (1977)
2 h 30
24 h 75
48 h 100
Rat (male) intravenous 2.5 30 min 40 15 Reiner & Plestina
90 min 60 35 (1979)
3 h 90 55
12 h 100 80
48 h 90
Dog (beagle) oral 50 2 h 32 63 Ward & Glicksberg
(sex not 24 h 63 74 (1971)
specified) 5 days 92 78
21 days 100 92
Dog oral 22 1 h 12 17 Snow & Watson
(greyhounds 3 h 24 34 (1973)
and crossbred) 24 h 70 65
72 h 95 65
---------------------------------------------------------------------------------------------------------
Another study of dichlorvos neurotoxicity involved the
investigation of lipid peroxidation. This entails the direct reaction
between oxygen and lipids to form free-radical intermediates and semi-
stable peroxides. Major cellular components, such as membranes and
subcellular organelles, are damaged by these free radicals. Hasan &
Ali (1980) found a dose-dependent increase in the rate of lipid
peroxidation in various regions of the brain of the rat after
intraperitoneal administration of dichlorvos (at concentrations ranging
from 0.6 to 3 mg/kg body weight, daily) for 10 days. Also, there was
an increased incidence of lipofuscin-like pigment in the Purkinje cells
of the cerebellar cortex.
Maslinska et al. (1984) found that dichlorvos (dose levels of 4 -
8 mg/kg body weight for 10 days) affected the phospholipid-protein
balance in the brain of rabbits. The animals were exposed during the
postnatal "critical" life period, which constitutes a turning point in
the development of the brain. At this time, the neurons have already
undergone considerable arborization, and myelination and
vascularization are expanding rapidly. In addition, the overall oxygen
consumption is reaching its steepest rate of increase. In the myelin
sheaths under formation, several phospholipids are deposited. The
authors found changes in the phospholipid-protein ratio which
correlated well with the observed delay in myelin sheath formation.
Ultrastructural changes in certain subcellular organelles may be
connected with the change in this ratio, since it is crucial to the
structural and functional properties of the membranes and enzymes bound
to them.
Dambska et al. (1984) have studied the influence of dichlorvos on
blood vessel walls, the perivascular area, and the permeability of the
blood-brain barrier in young rabbits. The young animals received 9 mg
dichlorvos/kg body weight for 16 days starting on the 6th day of life.
There was a decrease in ChE activity in brain capillary walls.
Electron microscopic studies showed lesions of the perivascular
astrocytes and changes in the endothelial cells. However, these
lesions did not disturb the blood-brain barrier mechanism for
horseradish peroxidase particles.
Studies on the central cholinergic system have revealed that the
inhibition of brain ChE activity and its subsequent recovery were
uniform in all brain regions studied in orally dosed (50 mg/kg) rats.
ACh concentrations were increased in brain areas within 15 min of
treatment. A biphasic effect was observed on choline metabolism in the
brain. The cortex was more cholinergic than the striatum in terms of
percentage increase in ACh and choline (Modak et al., 1975). In a
similar study on rats, either receiving a single oral dose (40 mg/kg)
or repeated oral doses (4 mg/kg), the activity of whole brain choline
acetyltransferase and the contents of ACh and choline in whole brain
were not altered, though brain ChE activity was markedly inhibited.
However, in the cerebral hemispheres, and especially the corpus
striatum, the ACh level was considerably increased, without a
concomitant change in choline (Teichert et al., 1976).
Kobayashi et al. (1980, 1986) investigated the concentration of
total, free, labile-bound and stable-bound ACh in the brain of rats
given single or multiple subcutaneous injections of dichlorvos (0.2 -
4 mg/kg body weight). The results suggest that alterations in the
mobilization and storage of ACh in the central cholinergic nerves may
be induced. The time course for ACh accumulation was measured in rat
brain regions after intravenous treatment with 15 mg dichlorvos/kg body
weight (Stavinoha et al., 1976). The striatum had the highest rate of
accumulation and the cerebellum the lowest. The calculated turnover
time for the different regions of the brain was between 0.9 and
5.6 min.
In studies by Ali & Hasan (1977) and Ali et al. (1979b, 1980), rats
were given intraperitoneally 3 mg dichlorvos/kg body weight per day for
10 or 15 days. The concentrations of dopamine, norepinephrine, and 5-
hydroxytryptamine (5-HT) were significantly decreased in different
parts of the brain, and 5-HT was significantly increased in the spinal
cord.
A single dose or short-term (12 weeks) treatment of rats with high
concentrations of dichlorvos, which produced brain ChE inhibition,
resulted in decreased norepinephrine levels in the brain (Brzezinski &
Wysocka-Paruszewska, 1980). From these studies, it was suggested that
the metabolism of catecholamines and 5-HT may be disturbed by
dichlorvos.
8.10. Other Studies
Many studies in different organ systems have been carried out. In
most of these studies, the route of administration was the oral or
intraperitoneal route. Single or repeated dosing was used, mainly with
high dose levels, in mice and rats. The influence on brain enzymes
(other than brain ChE), liver enzymes such as ChE (inhibition),
microsomal cytochrome P-450 activity (decrease), drug-metabolizing
enzyme activity, and UDP-glucuronyl transferase (no influence), and
many other enzyme systems were studied.
Furthermore, the influence of dichlorvos on adrenal steroidogenesis
has been investigated (Civen et al., 1980).
8.10.1. Immunosuppressive action
A dose-related suppression of the humoral immune response induced
by S. typhimurium was observed in rabbits orally administered 0.3 -
2.5 mg dichlorvos (93%)/kg body weight in capsules, 5 times a week for
6 weeks (Desi et al., 1978).
In a comparable study on rabbits, the cellular immune response was
estimated using the tuberculin skin test. The skin redness in the
tuberculin test and the serum antibody titres of treated animals showed
a dose-dependent decrease compared with those of controls (Desi et al.,
1980).
8.11. Factors Modifying Toxicity; Toxicity of Metabolites
8.11.1. Factors modifying toxicity
In studies on the effect of diet on the toxicity of dichlorvos,
young male rats were kept for 30 days on the following synthetic diets:
high protein (HPD), low protein (LPD), high fat (HFD), and standard
(SD). Growth rates were normal except for a slightly decreased body
weight gain in the HFD group. The composition of the diet, per se, did
not significantly affect plasma and erythrocyte ChE activity 24 h or 5
days after dosing. A single intraperitoneal injection of 50 mg
dichlorvos/kg body weight led to higher mortality in LPD rats (as
expected with a protein-deficient diet) and lower mortality in HPD
rats, compared with SD animals (Purshottam & Kaveeshwar, 1979).
In a further study, growing male rats were kept on an HFD or HPD
for 30 days. At the end of this period, a single intraperitoneal dose
of dichlorvos (20 or 30 mg/kg body weight) was administered. Results
showed that diets, per se, did not affect initial plasma or erythrocyte
ChE activity, nor did the HPD or HFD diets protect against mortality
from dichlorvos. In the case of the HPD, the spontaneous recovery of
ChE activity was reduced in the plasma and erythrocytes of dichlorvos-
treated rats. However, with an HFD, this recovery was significantly
increased (Purshottam & Srivastava, 1984).
Costa & Murphy (1984) studied the interaction between acetaminophen
(which, like dichlorvos, is detoxified by glutathione transferase) and
dichlorvos (10 mg/kg body weight) in mice. Acetaminophen (600 mg/kg
body weight) pre-treatment did not have any affect on dichlorvos
toxicity. On the other hand, intraperitoneal pre-treatment with
diethylmaleate (1 ml/kg body weight) increased the acute toxicity of
dichlorvos.
8.11.2. Toxicity of metabolites
8.11.2.1 Acute toxicity
The toxicity of metabolites of dichlorvos in female mice, injected
intraperitoneally, is considerably less than that of dichlorvos
(Table 19).
Mice (male and female) survived a single exposure of 130 mg
DCA/m3 for 5 - 7 h (Stevenson & Blair, 1969).
8.11.2.2 Short-term exposures
Groups of 20 male and 20 female rats were exposed for 30 days to
actual concentrations of 0 or 0.5 - 1 mg DCA/m3. Half the animals
were then killed promptly and the remainder on day 35. After 30 days
of exposure, the male rats showed a slight decrease in body weight and
food intake, and a slight increase in absolute and relative liver
weight. There were no such changes in the rats left for 5 more days
unexposed to DCA. Histological examination of the lungs of exposed
males and females revealed a higher incidence of minor inflammatory
changes than in controls. No other changes attributable to DCA were
found in general health, behaviour, haematology, clinical chemistry,
organ weights, gross pathology, or histopathology. A similar study
using groups of 10 male and 10 female rats and actual concentrations of
0 or approximately 2 mg/m3 DCA revealed no abnormalities attributable
to DCA (Wilson & Dix, 1973).
Table 19. Intraperitoneal LD50 values of metabolites of dichlorvos in
female micea
-----------------------------------------------------------------------------
Compound Vehicle LD50 (mg/kg body weight)
-----------------------------------------------------------------------------
desmethyldichlorvos, water 1500
sodium salt
dichloroacetyaldehyde corn oil 440
(DCA)
dichloroethanol corn oil 890
dichloroacetic acid corn oil or water 250
sodium dichloroacetate water 3000
methyl and dimethyl water 1500
phosphoric acid mixture
sodium methyl- and water 3000
dimethylphosphate
mixture
-----------------------------------------------------------------------------
a From: Casida et al. (1962).
8.11.2.3 Long-term exposure
No long-term tests have been carried out with DCA as such.
However, in the 2-year oral studies on rats and dogs with dichlorvos
(section 8.4.1), the degradation product DCA was present in the test
diets in increasing amounts, so that possible effects of DCA were
included in the results of these studies.
8.11.2.4 Mutagenicity
DCA appears to be mutagenic in the Salmonella test using S.
typhimurium TA100. The mutagenicity decreased in the presence of a
microsomal activation system. Part of the decrease was dependent on
the presence of the co-factors NADP and glucose-6-phosphate. No
evidence for mutagenicity with 2,2-dichloroethanol was obtained in
this S. typhimurium strain (Löfroth, 1978).
In a dominant lethal assay, a single intraperitoneal injection of
176 mg DCA/kg in male mice (AB Jena-Halle strain) produced a decrease
in the number of total implants and live fetuses in the first 3 weeks
of the test, and an increase in post-implantation losses. The same
test was repeated with a strain of mice (DBA) less sensitive to
mutagenic effects. The same effects were found, but to a lesser
extent, and mainly in the fourth week (Fischer et al., 1977).
8.11.2.5 Metabolism
When 32P-dimethylphosphate (500 mg/kg in water) was administered
orally to a male rat, the autopsy (90 h after treatment)
indicated that almost the entire dose had been eliminated. The
urine, containing only unmetabolized dimethylphosphate, accounted for
about half of the radioactivity. The tissues were almost devoid
of 32P-containing material (Casida et al., 1962).
A rat, orally dosed with 500 mg/kg 32P-desmethyldichlorvos in
water, excreted about 14% of the dose in the urine in 90 h.
The tissue distribution of 32P was similar to that which followed
32P-dichlorvos administration. The very high proportion of
radioactivity in the bone was indicative of rapid degradation to
phosphoric acid (Casida et al., 1962).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity
9.1.1.1 Poisoning incidents
A 56-year-old woman ingested an estimated amount of 100 mg
dichlorvos/kg body weight and survived, following intensive care for 14
days (Watanabe et al., 1976). However, a suicide with a dichlorvos
dose of about 400 mg/kg succeeded in spite of treatment (Shinoda et
al., 1972).
A 35-year-old female patient accidently ingested 60 g fluid Divipan
(dichlorvos concentration not reported). She was comatose for one week
and recovered slowly. Clinical and electrophysiological examinations
(no details reported) showed a pure motor form of neuropathy, according
to the authors (Vasilescu & Florescu, 1980).
Two cases of poisoning with dichlorvos taken orally in unspecified,
but high, quantities have been reported. The patients first showed
signs of severe anti-ChE poisoning. After recovery, delayed
neurotoxicity developed. They showed a severe axonal degeneration
neuropathy. One of them recovered within 12 months (Wadia et al.,
1985).
Reeves et al. (1981) reported six cases, over an 8-year period, of
bone-marrow failure (pancytopenia) in children shortly after exposure
to dichlorvos and propoxur. Van Raalte & Jansen (1981) doubted the
causal relationship between dichlorvos and bone-marrow failure in the
children, because the disease has not been observed in workers with
high exposure, or in the general population. In addition, haematotoxic
effects have not been observed in experimental animals.
9.1.2. Effects of short- and long-term exposure
In the 1960s, field studies were carried out in several countries
to test dichlorvos as a residual insecticide for malaria control in
houses. Numerous residents of all ages and conditions were exposed
without any adverse effects attributable to dichlorvos being reported
(Escudié & Sales, 1963; Funckes et al., 1963; Gratz et al., 1963;
Quarterman et al., 1963; Foll et al., 1965). In two field studies, the
plasma and erythrocyte ChE activities were measured in adults and young
children. No abnormalities were found, though air concentrations of
dichlorvos in the treated houses rose to 0.8 mg/m3 (Funckes et al.,
1963; Gratz et al., 1963).
In a report by Gold et al. (1984), 20 single-family residences were
treated with a 0.5% solution of dichlorvos (at an average rate of
0.189 g/m2) to control the cockroach Blattella germanica L. The
average air concentration for the first 2 h after treatment was
548 µg/m3 and for the next 2 h was 183 µg/m3. Pesticide operators
and the residents of treated structures were monitored for evidence of
dichlorvos exposure, using exposure pads, air samples, serum and
erythrocyte ChE tests, and urinalyses. There was no evidence of
dichlorvos or dichloroacetic acid in urine. There were slight, but
statistically significant, changes in the mean serum ChE activity of
some of the residents of treated structures, but the mean erythrocyte
ChE was unchanged.
Passengers in an aircraft provided with an automatic insect-control
system, which released 0.15 - 0.30 mg dichlorvos/m3 for periods of
about 30 min, did not show any signs of discomfort (Jensen et al.,
1965). Tests have shown that man can withstand daily exposure to
concentrations of 0.5 mg/m3 without clinical effects, and with only a
slight depression of blood ChE activity (Hayes, 1961). Three men
exposed to approximately the same level during 24 tests did not show
any change in blood ChE activity (Schoof et al., 1961).
A case of chronic obstructive bronchitis ascribed to exposure to
dichlorvos was described by Barthel (1983). However, it could not be
excluded that other components of the unknown formulation could have
been the cause. ChE determinations were not performed.
9.1.2.1 Studies on volunteers
Single oral doses (1 - 32 mg/kg body weight) of dichlorvos in a
slow-release PVC formulation administered to 107 male volunteers
produced measurable reductions in erythrocyte ChE activity at dose
levels above 4 mg/kg, with a maximum reduction of 46% at the highest
dose. Plasma ChE activity was affected at lower doses, with 50%
reduction at 1 mg/kg and about 80% at 6 mg/kg or more. Repeated oral
doses of 1 - 16 mg/kg body weight per day were given to 38 volunteers
for up to 3 weeks. The plasma ChE activity was maximally depressed at
all dose levels, and the erythrocyte ChE activity depression was dose
related and significant at 2 mg/kg or more. Blood cell count, urine,
liver function, prothrombin time, and blood urea nitrogen were all
normal (Hine & Slomka, 1968, 1970; Slomka & Hine, 1981).
In studies by Rider et al. (1968), dichlorvos was given to groups
of five men at daily oral doses of 1, 1.5, 2, or 2.5 mg per man. The
plasma ChE activity of the 2.5 mg group was reduced by 30% after 20
days of treatment. Administration of 2 mg for 28 days resulted in a
reduction of 30% 2 days after the last dose. Erythrocyte ChE activity
was not significantly affected in either group (Rider et al., 1967).
Daily oral doses of 1.5 mg per man given to 10 volunteers for 60 days
caused a significant reduction (approximately 40%) in plasma ChE
activity, which returned to normal levels when dichlorvos
administration was discontinued.
Boyer et al. (1977) reported studies on two groups of six men (21 -
45 years of age) who received 0.9 mg of dichlorvos three times a day
for 21 days. One group received the dichlorvos in a gelatin salad, the
other in a pre-meal capsule filled with cottonseed oil. Two other
groups of six men received placebo treatment. No consistent
cholinomimetic signs or symptoms were observed, nor was erythrocyte ChE
inhibited. However, plasma ChE was significantly depressed within 20
days, although the extent depended on the method by which dichlorvos
was administered. Recovery was comparable in both groups, the half-
life for the regeneration of plasma ChE being 13.7 days.
Volunteers did not show inhibition of plasma or erythrocyte ChE
activity either when handling a dichlorvos strip for 30 min each day or
after having a piece of the strip applied to the arm for 30 min each
day for 5 consecutive days (Zavon & Kindel, 1966).
The wearing of dichlorvos-impregnated garments by babies for 48 -
84 h did not produce changes in either plasma or erythrocyte ChE
activity over a period of 5 days (Cavagna et al., 1969).
In studies on eight volunteers, carried out in an aircraft at
operational cabin altitude (2400 m), no changes in plasma or
erythrocyte ChE activity, dark adaptation, or bronchiolar resistance
were observed. Dichlorvos concentration in the air ranged from 0.73 to
1.18 mg/m3 during exposures of 45 min (Smith et al., 1972).
Hunter (1970a) reported studies on 26 men (21 - 57 years of
age) and 6 women (19 - 25 years of age) who were exposed in a chamber
for 2-7´ h to actual dichlorvos concentrations of approximately
1 mg/m3. Food and drink were served in the chamber. No clinical
signs were observed, and no effects on haematology, urinalysis, kidney
function, EEG, ECG, respiratory activities, or erythrocyte ChE activity
were found. Plasma ChE activities were markedly inhibited only when
the exposure lasted over 6 - 7 h (Hunter, 1970a).
In studies by Hunter (1969, 1970b), seven men (25 - 56 years of
age) were exposed (head and neck) to dichlorvos vapour (actual
concentrations of 1 - 52 mg/m3), and 6 men were exposed (head
exposure only) to 7 - 50 mg/m3, for periods from 10 min to 4 h. The
maximum dose level was 52 mg/m3 (for 65 min), and the maximum period
was 240 min (at 13 mg/m3). Symptoms were confined to irritation of
the throat, some rhinorrhoea, and substernal discomfort at the highest
concentrations. No effects on the pupil or on visual acuity were
recorded. Erythrocyte ChE activity was depressed in only one person.
However, there was a direct relationship between the reduction in
plasma ChE activity and the dichlorvos dose (concentration x time). No
changes were found in either kidney or pulmonary function or in the
overall metabolic rate.
When three men were exposed to dichlorvos (actual concentrations of
0.3 - 0.9 mg/m3 (mean, 0.5 mg/m3) or 0.9 - 3.5 mg/m3 (mean,
2.1 mg/m3) for 1 or 2 h per day for 4 consecutive days, plasma ChE
alone decreased slightly in the men exposed for 2 h per day to the
higher concentration (Witter et al., 1961).
A group of 15 men (23 - 61 years of age) was exposed for up to 6
half-hour intervals per night for 14 days (total of 39 doses) to actual
dichlorvos concentrations ranging from 0.14 to 0.33 mg/m3. No clear
changes in plasma ChE activity or other parameters such as reaction
time, airway resistance, or vision were found. The same results were
obtained when a similar group was exposed to actual concentrations of
0.1 - 0.6 mg/m3 for intervals of 8 - 10 h, 4 nights per week for 11
weeks (Rasmussen et al., 1963).
No significant effect on plasma or erythrocyte ChE activity was
observed in 14 persons exposed at the recommended rate of one
dichlorvos strip per 30 m3 in their homes over a period of 6 months.
The strips were replaced at much shorter intervals than normally
recommended. The air concentration 40 days after the installation of
the fourth strip was approximately 0.09 mg/m3 (Zavon & Kindel, 1966).
In three home studies involving 26 families, conducted in Arizona,
USA, no deleterious effects on health or plasma or erythrocyte ChE
activity were observed in the residents exposed to dichlorvos strips
all over the house (8 - 10 strips) for over a year. Even monthly
replacement of the strips resulted in only a slight inhibition of
plasma ChE activity. The maximum air concentrations of dichlorvos
averaged 0.13 mg/m3 (Leary et al., 1971, 1974).
9.1.2.2 Hospitalized patients
In a report by Pena Chavarra et al. (1969), a single oral dose of 6
or 12 mg dichlorvos/kg body weight was administered in the form of a
slow-release granular resin formulation as an anthelminthicum to 108
hospitalized adult patients, many of these debilitated and with severe
anaemia. Plasma ChE activity was markedly reduced, in some patients by
76 - 100%. However, erythrocyte ChE activity was much less inhibited.
No symptoms of intoxication were observed, except for brief mild
headaches in a few patients, and there were no abnormalities in
haematological studies or in hepatic and renal function tests.
In studies by Cervoni et al. (1969), single doses of dichlorvos
(PVC-resin formulations) were administered orally 2 h before breakfast
to 705 adults found to be harbouring infections of Trichuris, hookworm,
or Ascaris. Six or 12 mg dichlorvos/kg body weight resulted in
infection cure rates of approximately 70 - 100%. According to the
authors, minimum to modest plasma ChE depression and zero to minimum
erythrocyte ChE depression occurred at both dose levels. No clinical
symptoms or alterations in haematology or in liver and kidney function
were observed.
Sick adults and children and healthy pregnant women and babies in
hospital wards treated with dichlorvos strips (one strip per 30 or
40 m3) had normal erythrocyte ChE activities. Only subjects exposed
for 24 h per day to dichlorvos concentrations above 0.1 mg/m3 or
patients with liver insufficiency showed even a moderate decrease in
plasma ChE activity (Cavagna et al., 1969, 1970; Cavagna & Vigliani,
1970).
9.2. Occupational Exposure
9.2.1. Acute toxicity
9.2.1.1 Poisoning incidents
A number of fatal and non-fatal poisoning cases have been described
after concentrated formulations of dichlorvos splashed onto parts of
the body. Two workers who failed to wash it off promptly died
consequently. However, in those cases where the spilled solution was
washed off immediately, the victims showed symptoms of intoxication but
recovered after treatment. A serious non-fatal case occurred after a
spillage of 120 ml of a 3% formulation that was not washed off
immediately. After 1´ h, the victim developed slurred speech, became
drowsy, and collapsed. He recovered completely after treatment (Hayes,
1963, 1982).
A pest-control operator became contaminated with a 1% solution of
dichlorvos in mineral spirit after using a leaking knapsack sprayer.
The man changed his overalls and completed his day's work. He noticed
weakness, dizziness, and difficulty in breathing. Contact dermatitis
developed on the back skin. After the fourth day, his blood ChE was
36% of normal, but there were no signs of systemic illness. He
recovered without medication, and the ChE activity increased to 72% of
normal within one month. The acute dermatitis was probably caused by
the solvent (Bisby & Simpson, 1975).
A driver of a truck transporting a 5% commercial formulation of
dichlorvos (15% petroleum distillate and 80% trichloroethane)
developed persistent contact dermatitis for 2 months following
accidental skin contact. In addition, the patient experienced
headache, mild rhinorrhoea, burning of the tongue, and a bitter taste
in his mouth. Initial blood ChE levels were in the low normal range
returning to the high normal range within 2 weeks. Patch tests with 1%
and 0.1% dichlorvos in petroleum distillate were negative (Mathias,
1983). In view of the symptoms and the slight ChE inhibition, it seems
likely that trichloroethane caused the dermatitis.
Cronce & Alden (1968) described four people who handled dogs
wearing anti-flea dog collars containing 9 - 10% dichlorvos. Acute
primary contact dermatitis was described. Closed patch tests with
0.25, 0.5, and 1% dichlorvos in distilled water and in mineral oil were
positive in all four people. General experience with the collars
indicates that only a few people are susceptible to this kind of
irritation (Hayes, 1982).
9.2.2. Effects of short- and long-term exposure
9.2.2.1 Pesticide operators and factory workers
The effect on sprayers of dichlorvos fume, used inside a building
for cockroach control, was examined. When 0.3 - 0.6% dichlorvos oil
spray was used at the rate of 6 ml/m2 by the sprayers (7 - 9 people),
inhibition of ChE activity in the subjects was 15%, and conjunctival
injections or sore throats were observed in some sprayers after either
18 min or 4 h of spraying operation. To examine the effect of elevated
dichlorvos vapour pressure at higher temperatures, four men sprayed
0.6% oil spray for 2 h at the room temperature of 25 °C. The
inhibition of plasma and erythrocyte ChE activities was 22% and 7%,
respectively. In another study, 11 sprayers used 0.6% oil spray for
6 h of actual work time at 20 °C without rest. Two of them showed an
inhibition of plasma ChE activity of 38% while the others did not show
significant inhibition of erythrocyte ChE. It was concluded that
conjunctival injections and sore throats were attributable to the
kerosene solvent used and that the 38% depression in ChE activity was
probably due to long hours of continuous work. Therefore, the overall
effect on ChE activity was not considered to be severe (Ueda et al.,
1959, 1960).
Twelve fogging machine operators did not show any reduction in
plasma or erythrocyte ChE activity when applying 4% dichlorvos aerosols
in tobacco warehouses for 16 h per week, over a period of 2 - 4 months
(Witter, 1960).
Sixteen men replacing old dichlorvos dispensers and installing new
units in houses in Haiti, 5 days per week for 3 weeks, showed a
decrease of up to 60% in plasma ChE activity. No signs or symptoms of
intoxication were observed. Air concentrations ranged from 0.3 to
2.1 mg/m3 (Stein et al., 1966).
The blood ChE activity of sprayers, exposed during insect control
in grain stores to air concentrations of 1.9 - 3 mg/m3 dichlorvos,
was reduced to 19 - 23% (Sasinovich, 1970).
In a report by Das et al. (1983), each of 13 pesticide-control
operators carried out urban pest-control for one day in four houses
using 230 - 330 g dichlorvos as aerosol and 40 - 50 g dichlorvos as
emulsion spray. At the end of the day's work, an operator had an
average dichlorvos residue of 0.8 mg/m2 on the back, 0.4 mg/m2 on the
chest, and 11 mg/m2 on the respirator filter. Dimethylphosphate was
detected in the urine, but blood and urine analyses, including serum
ChE levels, did not reveal any other changes in clinical parameters.
In the course of either the production or processing of a
dichlorvos-releasing product, 11 male and 2 female factory workers were
exposed to an average dichlorvos concentration of 0.7 mg/m3 (highest
value 3 mg/m3) on each working day for a period of 8 months.
Inhibition of plasma ChE activity was noted within a few days of the
start of exposure, while inhibition of erythrocyte ChE activity
developed much more slowly. The maximum plasma ChE activities recorded
were 40% lower than the pre-exposure levels, but 60% lower than the
post-exposure levels. Erythrocyte ChE activity was reduced by
approximately 35% compared with pre- and post-exposure levels. One
month after exposure had ceased, plasma ChE and erythrocyte ChE
activities were found to have returned to normal physiological levels.
The other haematological investigations and the medical examinations
did not reveal any changes attributable to dichlorvos exposure (Menz et
al., 1974).
9.2.2.2 Mixed exposure
A number of articles have described the symptoms found in
individual workers or groups of workers exposed for a number of years
to different types of pesticides, including dichlorvos. In general, a
slight-to-moderate decrease in ChE activity occurred. Furthermore,
several complaints and symptoms were noticed, but no clear clinical
poisoning cases occurred. These case studies are, however, of little
relevance for the evaluation of dichlorvos because of the mixed
exposure to different pesticides (Bellin & Chow, 1974; Fournier et al.,
1976; Gupta et al., 1979; Ullmann et al., 1979; Hayes et al., 1980;
Hayes, 1982).
From 1975 to 1977, 88 patients with pesticide dermatitis, 15
suffering from photo-dermatitis, were studied. Patch tests using 29
different pesticides of different categories were carried out, but did
not lead to an identification of the responsible pesticides. Eight out
of 52 patients (15.4%) reacted positively to a photopatch (Horiuchi &
Ando, 1978; Horiuchi et al., 1978).
Stoermer (1985) described a case of contact dermatitis in a woman
who had worked as a pest controller for 3 months and had sprayed
dichlorvos and propoxur. She was treated and recovered within one
week.
A field survey of tea growers was made in the Chiran area of Japan
in 1982. Out of 84 tea growers examined (21 men and 63 women), 5 women
had contact dermatitis from agricultural work. Dichlorvos was among
the insecticides, fungicides, and herbicides used. Patch tests showed
relatively high rates of positive reactions with dichlorvos (27% of
women and 5% of men). Also, cross-sensitization was found between
methidathion and dichlorvos (Fujita, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Since 1961, dichlorvos, an organophosphate with anti-ChE activity,
has been used worldwide as a contact and stomach insecticide to control
insects on crops and domestic animals. It is also used as an
insecticide in houses and other buildings and for insect control in
aircraft.
Dichlorvos is readily absorbed by the body of mammals through all
routes of exposure, and is readily metabolized in the liver. Within
1 h of oral administration, dichlorvos is found in the liver, kidneys,
and other organs of experimental animals. It is rapidly eliminated via
the kidneys, with a half-life of 14 min.
The metabolism of dichlorvos in various species, including human
beings, follows similar pathways. Differences between species relate
only to the rate of metabolism, but this is always rapid.
Dichlorvos is moderately to highly toxic for mammals (the oral
LD50 for the rat is 30 - 110 mg/kg body weight). The classification
of dichlorvos by WHO (1986a) is based on an oral LD50 for the rat of
56 mg/kg body weight. Signs of intoxication usually occur shortly
after exposure and are typical of an organophosphorus pesticide.
Inhibition of ChE activity is a sensitive criterion of exposure. In
short-term toxicity studies on mammals, it was shown that ChE activity
was not decreased at oral dose levels below about 0.5 mg/kg body
weight. In long-term studies on rats at oral dose levels of 2.5 mg/kg
body weight or more, hepatocellular fatty vacuolization was seen. At
0.25 mg/kg body weight, no ChE inhibition was found, nor were there any
other effects.
Reproduction and teratogenicity studies using a wide range of dose
levels (6.25 - 500 mg/kg body weight) were negative. Dichlorvos showed
alkylating properties in in vitro but not in in vivo studies. Many in
vitro mutagenicity studies with bacteria and yeast were positive, while
the in vivo studies were mainly negative. From the available
mutagenicity studies, it is unlikely that dichlorvos constitutes a
mutagenic hazard for man. Carcinogenicity studies on mice and rats fed
dichlorvos (dose levels of up to 234 mg/kg diet) were negative. Two
recent carcinogenicity studies have been carried out on mice and rats
in which dichlorvos was administered by intubation for up to 2 years at
dose levels of 10 - 40 mg/kg body weight (mice) and 4 or 8 mg/kg body
weight (rats). Only preliminary information has been provided. The
evidence for carcinogenicity in these new studies is difficult to
interpret at this time. Only when complete and final reports become
available will it be possible to draw more definite conclusions (in
this context, see section 8.7.3).
From work on hens, the suspicion of delayed neurotoxicity from
dichlorvos has neither been established nor totally refuted. However,
there have been two clinical reports on four patients suffering intense
poisoning from dichlorvos taken orally who survived with treatment and
who then displayed neurotoxic effects. Thus, the possibility of
delayed neurotoxicity in man cannot be entirely discounted, but it is
likely to occur only with excessive oral doses.
Human volunteers who were given single or repeated oral doses of 2
mg/kg body weight or more showed significant inhibition of erythrocyte
ChE activity. At 1 mg/kg body weight, no such inhibition was found.
The application of dichlorvos to crops and animals results in
residues that rapidly disappear by volatilization and hydrolysis. In
general, residues of dichlorvos and the breakdown product DCA in food
commodities are low and will be further reduced during processing. The
exposure of the general population to dichlorvos by food and drinking-
water is negligible, as is confirmed in total-diet studies.
In short-term inhalation studies on mammals, 1 or 2 mg
dichlorvos/m3 did not inhibit ChE activity.
In a 2-year, 23 h/day, whole-body inhalation study on rats, 0.48
mg/m3 caused inhibition of plasma and erythrocyte activity, but brain
AChE activity was not inhibited and there were no clinical signs. An
unquantified, but considerable, extra exposure, resulting from the
grooming of contaminated fur and contamination of food and drinking-
water, had contributed to this effect. The no-observed-adverse-effect
level was 0.05 mg/m3. There was no evidence of carcinogenicity.
In a 6- to 7-h exposure of human volunteers to approximately 1 mg
dichlorvos/m3, only plasma ChE activity was inhibited. The
erythrocyte AChE activity, taken to be representative of the AChE
activity in the nervous tissue, was unaffected.
Residents exposed for over one year to an average air concentration
of 0.1 mg/m3 arising from slow-release strips showed no inhibition of
plasma or erythrocyte ChE activity and no deleterious effects on
health.
The main exposure of the general population is by inhaling
dichlorvos when used indoors to control insects. The recommended use
(one slow-release strip/ 30 m3) will give concentrations in the air
of up to 0.1 - 0.3 mg/m3 in the first few days, decreasing thereafter
to below 0.1 mg/m3. The air concentration depends on temperature,
humidity, and ventilation.
As long as approved slow-release strips are used according to the
label instructions, no health hazard can be expected for man. However,
special care may need to be taken with young children and sick or
elderly people who are especially vulnerable when continuously exposed
(24 h per day) in poorly ventilated rooms. Other methods of indoor
application should be equally safe if the label instructions are
followed.
There is some indication that dichlorvos may induce dermatitis and
cross-sensitization in people handling various types of pesticides
including dichlorvos.
In occupational conditions, the main route of exposure to
organophosphorus pesticides is usually the dermal route. In the case
of dichlorvos, with its high vapour pressure, exposure by inhalation is
also important. In these occupational situations, the dichlorvos
concentrations in the air are generally below 1 mg/m3 but, in certain
circumstances, they may rise considerably above this level. This
stresses the need for adequate protective measures to be taken during
occupational exposure and regular monitoring of ChE activity.
10.2. Evaluation of Effects on the Environment
The presence of dichlorvos in the environment as a result of
accidental losses or direct application on soil or in water will not
lead to long-term effects because of its fast breakdown and
evaporation. Furthermore, it is converted to a number of compounds,
such as dichloroacetic acid, by microorganisms. Certain bacteria
species can use dichlorvos as a sole carbon source, while others cannot
and are inhibited in their growth. Therefore, its influence on
microorganisms is complex.
Dichlorvos is moderately to highly toxic (range, 0.2 - 10 mg/litre)
for freshwater and estuarine species of fish and invertebrates. In
certain fish, concentrations of 0.25 - 1.25 mg/litre cause inhibition
of brain and liver ChE activity. Concentrations of as little as
0.05 mg/litre may have deleterious effects, particularly in
invertebrates. Dichlorvos has a high toxicity for birds and bees.
Caution is advised in the use and handling of dichlorvos where these
species might be exposed.
No bioaccumulation occurs in the different environmental
compartments and organisms.
10.3. Conclusions
1. Exposure of the general population to dichlorvos via food and
drinking-water is negligible and does not constitute a health hazard.
2. The in-house use of dichlorvos as an insecticide in the form of
sprays or slow-release strips (at recommended levels) does not
constitute a short- or long-term hazard for the general population.
However, continuous (24 h per day) exposure of young children and
diseased or elderly people in non-ventilated or poorly ventilated rooms
should be avoided.
3. In spite of their toxicity, dichlorvos and its formulations do not
contribute an undue hazard to those occupationally exposed, provided
that adequate ventilation and skin protection are used.
4. Except under conditions of gross spillage, the recommended use of
dichlorvos as an insecticide does not constitute an acute or long-term
hazard for aquatic or terrestrial organisms, although there may be an
acute hazard for birds and bees.
11. RECOMMENDATIONS
1. Continuous (24 h/day) exposure of young children and diseased or
elderly people to dichlorvos in non-ventilated or poorly ventilated
rooms should be avoided.
2. As dichlorvos from different sources may vary in purity and type of
impurities, attention should be paid to its composition. This should
conform to FAO and WHO specifications (FAO, 1977; WHO, 1985). In the
case of formulations, potential hazards of other components, such as
solvents and stabilizers, should also be considered.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Dichlorvos was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1965, 1966, 1967, 1969, 1970, 1974, and 1977
(FAO/WHO, 1965a,b, 1967a,b, 1968a,b, 1970a,b, 1971a,b, 1975a,b,
1978a,b). In 1966, the JMPR established an Acceptable Daily Intake
(ADI) for human beings of 0 - 0.004 mg/kg body weight, which remains
unchanged.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, has classified technical dichlorvos as
"highly hazardous" (Class IB) (Plestina, 1984; WHO, 1986a) and has
produced a safety sheet on dichlorvos (No. 75.2) (WHO/FAO, 1975-86).
Specifications for dichlorvos use in public health and in plant
protection have been published by WHO (1985) and FAO (1977),
respectively.
In 1979, the International Agency for Research on Cancer (IARC)
came to the following conclusions in considering the carcinogenicity of
dichlorvos:
(a) dichlorvos was tested in different animal species via
different routes; no conclusive evaluation on the basis of
these studies could be made;
(b dichlorvos is an alkylating agent and binds to bacterial and
mammalian nucleic acids;
(c) it is a mutagen in a number of microbial systems, but there is
no evidence of its mutagenicity in mammals, in which it is
rapidly degraded.
In the evaluation by IARC, it was stated that "the available data
do not allow an evaluation of the carcinogenicity of dichlorvos to be
made".
In its series "Scientific Reviews of Soviet Literature on Toxicity
and Hazards of Chemicals", the International Register of Potentially
Toxic Chemicals has published a volume on dichlorvos (IRPTC, 1984).
REFERENCES
ABBOTT, D.C., CRISP, S., TARRANT, K.R., & TATTON, J.O.G. (1970)
Pesticide residues in the total diet in England and Wales. III.
Organophosphorus pesticide residues in the total diet, 1966-67.
Pestic. Sci., 1: 10-13.
ACKERMAN, H., LEXOW, B., & PLEWKA, E. (1969) [Detection and
identification of the organic phosphate, phosphorothionate,
phosphonate, and carbamate insecticides in biological material.]
Arch. Toxicol., 24: 316-324 (in German).
ADLER, B., BRAUN, R., SCHONEICH, J., & BOHME, H. (1976) Repair-
defective mutants of Proteus mirabilis as a prescreening system for the
detection of potential carcinogens. Biol. Zentralbl., 95: 463-469.
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides wetting agents and miscellaneous
substances. Int. pest Control, 11(2): 25-35.
ALDRIDGE, W.N. & BARNES, J.M. (1966) Further observations on the
neurotoxicity of organophosphorus compounds. Biochem. Pharmacol., 15:
541-548.
ALDRIDGE, W.N. & JOHNSON, M.K. (1971) Side effects of organo-
phosphorus compounds: delayed neurotoxicity. Bull. World Health
Organ., 44: 259-263.
ALDRIDGE, W.N. & JOHNSON, M.K. (1977) Mechanisms and structure-
activity relationships providing a high safety factor for anti-
cholinesterase insecticides. In: Proceedings of the 1977 British Crop
Protection Conference - Pests and Diseases, Croyden, British Crop
Protection Council. pp. 721-729.
ALI, M.S., RAHMATULLAH, M., JAHAN, R., YUSUF, H.K.M., &
CHOWDHURY, A.A. (1979c) Effect of DDVP (insecticide) on citric acid
fermentation in Aspergillus niger. Enzyme Microb. Technol., 1: 127-
128.
ALI, S.F. & HASAN, M. (1977) Effect of organophosphate insecticide
dichlorvos on the amino acid content of different regions of the rat
brain and spinal cord. Indian J. exp. Biol., 15(9): 759-761.
ALI, S.F., HASAN, M., & MITRA, S.C. (1979a) Organophosphate DDVP-
induced changes in the rat spinal cord: an electron microscopic and
biochemical study. J. Anat. Soc. India, 28(1): 36.
ALI, S.F., HASAN, M., & TARIQ, M. (1979b) Levels of dopamine,
norepinephrine, and 5-hydroxytryptamine in different regions of rat
brain and spinal cord following chronic administration of organo-
phosphate pesticide dichlorvos. Indian J. exp. Biol., 17(4): 424-426.
ALI, S.F., CHANDRA, O., & HASAN, M. (1980) Effects of an
organophosphate (dichlorvos) on open-field behaviour and locomotor
activity: correlation with regional brain monoamine
levels. Psychopharmacology, 68(1): 37-42.
ALLEN, S.D., VANKAMPEN, K.R., & BROOKS, D.R. (1978) Evaluation of
the feline dichlorvos (DDVP) flea collar. Feline Pract., 8(3): 9-16.
AMBRUS, A., LANTOS, J., VISI, E., CSATLOS, I., & SARVARI, L. (1981)
General method for determination of pesticide residues in samples of
plant origin, soil, and water. J. Assoc. Off. Anal. Chem., 64(3):
733-768.
ANDERSON, R.A., BARSTAD, J.A.B., & LAAKE, K. (1978) An improvement
of the spectrophotometric method for determination of cholinesterase
activity in erythrocytes and tissue homogenates. Ann. Natl Inst. Publ.
Health (Norway), 1(1): 51-55.
ANON. (1972) Vapona insecticide. In: Analytical methods for
pesticides and plant growth regulators, Modesto, California, Shell
Development Company, Vol. 6, pp. 529-541.
ANON. (1973) The determination of malathion and dichlorvos residues
in grain. Report by the Panel on Malathion and Dichlorvos Residues in
Grain. Analyst, 98: 19-24.
ANON. (1977) Determination of residues of organophosphorus pesticides
in fruits and vegetables. Report by the Panel on Determination of
Residues of Certain Organophosphorus Pesticides in Fruits and
Vegetables. Analyst, 102: 858-868.
ANON. (1982) Pesticides: a safety guide, London, Shell International
Chemical Company Ltd, pp. 53-55.
AOKI, Y., TAKEDA, M., & UCHIYAMA, M. (1975) Comparative study of
methods for the extraction of eleven organophosphorus pesticide
residues in rice. J. Assoc. Off. Agric. Chem., 58(6): 1286-1293.
AQUILINA, G., BENIGNI, R., BIGNAMI, M., CALCAGNILE, A.,
DOGLIOTTI, E., FALCONE, E., & CARERE, A. (1984) Genotoxic activity of
dichlorvos, trichlorfon, and dichloroacetaldehyde. Pestic. Sci., 15:
439-442.
ARATAKE, Y. & KAYAMURA, T. (1973) [Toxicity of insecticides to
silkworm larvae.] Sanshi Kenkyu (Acta serol.), 87: 68-78 (in
Japanese).
ARIMATSU, S., HOSHIRO, Y., & NOMURA, T. (1977) [Studies on primary
irritation test of pesticides in rabbits.] Nippon Noson Igakkai
Zasshi, 26: 572-573 (in Japanese).
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ATKINS, E.L., GREYWOOD, E.A., & MACDONALD, R.L. (1973) Toxicity of
pesticides and other agricultural chemicals to honey bees, Riverside,
University of California (Agricultural Extension Report No. M-16 Rev.
9/73).
AUGUSTINSSON, K.B., ERIKSSON, H., & FAIJERSSON, Y. (1978) A new
approach to determining cholinesterase activities in samples of whole
blood. Clin. Chim. Acta, 89: 239-252.
AULICINO, F., BIGNAMI, M., CARERE, A., CONTI, G., MORPURGO, G.,
& VELCICH, A. (1976) Mutational studies with some pesticides
in Aspergillus nidulans. Mutat. Res., 38(2): 138.
BAKSI, S.N. (1978) Effect of dichlorvos on embryonal and fetal
development in thyro-parathyroidectomized thyroxine-treated and
euthyroid rats. Toxicol. Lett., 2(4): 213-216.
BALLINGTON, P.E., SKIPPER, H.D., & HEGG, R.O. (1978) Response of
coliform populations in poultry waste digesters to three
insecticides. J. environ. Qual., 7(2): 262-264.
BARTHEL, E. (1983) [Irritative and allergic effects of pesticide
aerosols on the respiratory tract and problems of their evaluation.] Z.
gesamte Hyg., 29(11): 678-681 (in German).
BATORA, V., VITOROVIC, S.L.J., THIER, H.P., & KLISENKO, M.A. (1981)
Development and evaluation of simplified approaches to residue
analysis. Pure appl. Chem., 53: 1039-1049.
BATTE, E.G., ROBINSON, O.W., & MONCOL, D.J. (1969) Influence of
dichlorvos on swine reproduction and performance of offspring to
weaning. J. Am. Vet. Med. Assoc., 154(11): 1397.
BAZER, F.W., ROBSON, O.W., & ULBERG, L.C. (1969) Effect of dichlorvos
and PMS on reproduction in swine. J. anim. Sci., 28: 145.
BEDFORD, C.T. & ROBINSON, J. (1972) The alkylating properties of
organophosphates. Xenobiotica, 2(4): 307-337.
BELL, TH.G., FARRELL, K., PADGET, G.A., & LEENDERTSEN, L.W.
(1975) Ataxia, depression, and dermatitis associated with the use of
dichlorvos-impregnated collars in the laboratory cat. J. Am. Vet.
Med. Assoc., 167(7): 575-586.
BELLIN, J.S. & CHOW, I. (1974) Biochemical effects of chronic low-
level exposure to pesticides. Res. Commun. chem. Pathol.
Pharmacol., 9(2): 325-337.
BENIGNI, R. & DOGLIOTTI, E. (1980a) UDS studies on selected
environmental chemicals. Mutat. Res., 74: 248-249.
BENIGNI, R. & DOGLIOTTI, E. (1980b) UDS studies on selected
environmental chemicals. Mutat. Res., 74(3): 217.
BENIGNI, R., BIGNAMI, M., CAMONI, I., CARERE, A., CONTI, G.,
IACHETTA, R., MORPURGO, G., & ORTALI, V.A. (1979) A new in
vitro method for testing plant metabolism in mutagenicity studies. J.
Toxicol. environ. Health, 5: 809-819.
BERAN, F. (1970) [Present knowledge on the toxicity of our pesticides
to bees.] Gesunde Pflanz., 22(2): 21-31 (in German).
BIGNAMI, M., CONTI, L., MORPURGO, G., & VELCICH, A. (1976)
Comparative analysis of different test systems for somatic
recombination with Aspergillus nidulans. Mutat. Res., 38(2): 138-139.
BIGNAMI, M., AULICINO, F., VELCICH, A., CARERE, A., &
MORPURGO, G. (1977) Mutagenic and recombinogenic action of pesticides
in Aspergillus nidulans. Mutat. Res., 46: 395-402.
BISBY, J.A. & SIMPSON, G.R. (1975) An unusual presentation of
systemic organophosphate poisoning. Med. J. Aust., 2: 394-395.
BLAIR, D. & RODERICK, H.R. (1976) An improved method for the
determination of urinary dimethyl phosphate. J. agric. food
Chem., 24(6): 1211-1223.
BLAIR, D., HOADLEY, E.C., & HUTSON, D.H. (1975) The distribution of
dichlorvos in the tissues of mammals after its inhalation or
intravenous administration. Toxicol. appl. Pharmacol., 31: 243-253.
BLAIR, D., DIX, K.M., HUNT, P.F., THORPE, E., STEVENSON, D.E., &
WALKER, A.I.T. (1976) Dichlorvos: a 2-year inhalation carcinogenesis
study in rats. Arch. Toxicol., 35: 281-294.
BOOTSMA, D., HEERING, H., KLEIJER, W.J., BUDKE, L., DE JONG,
L.P.A., & BERENDS, F. (1971) Effects of dichlorvos on human cells in
tissue culture. A progress report, Rijswijk, The Netherlands, Medical
Biological Laboratory TNO (Report No. MBL 1971-5).
BOUSH, G.M. & MATSUMURA, F. (1967) Insecticidal degradation by
Pseudomonas melophthora, the bacterial symbiote of the apple
maggot. J. econ. Entomol., 60: 918-920.
BOWMAN, B.T. & SANS, W.W. (1983) Determination of octanol- water
partitioning coefficients (Kow) of 61 organophosphorus and carbamate
insecticides and their relationships to respective water solubility (S)
values. J. environ. Sci. Health, B18(6): 667-683.
BOYER, A.C., BROWN, L.J., SLOMKA, M.B., & HINE, C.H. (1977)
Inhibition of human plasma cholinesterase by ingested dichlorvos:
effect of formulation vehicle. Toxicol. appl. Pharmacol., 41: 389-
394.
BRADWAY, D.E., SHAFIK, T.M., & LORES, E.M. (1977) Comparison of
cholinesterase activity, residue levels, and urinary metabolite
excretion of rats exposed to organophosphorus pesticides. J. agric.
food Chem., 25Z(6): 1353-1358.
BRAUN, R., SCHONEICH, J., WEISSFLOG, L., & DEDEK, W. (1982) Activity
of organophosphorus insecticides in bacterial tests for mutagenicity
and DNA repair: direct alkylation vs metabolic activation and
breakdown. I. Butonate, vinylbutonate, trichlorfon, dichlorvos,
desmethyldichlorvos, and demethylvinyl-butonate. Chem.-biol.
Interact., 39: 339-350.
BRIDGES, B.A., MOTTERSHEAD, R.P., GREEN, M.H.L., & GRAY, W.J.H.
(1973) Mutagenicity of dichlorvos and methyl methanesulfonate
for Escherichia coli WP2 and some derivatives deficient in DNA
repair. Mutat. Res., 19: 295-303.
BROWN, V.K.H. & ROBERTS, M. (1966) The anti-acetylcholinesterase
activity of technical DDVP when administered percutaneously to guinea-
pigs, Sittingbourne, Shell Research Ltd (Unpublished Report No. IRR
TL/40/66).
BROWN, V.K.H. & STEVENSON, D.E. (1962) Agricultural chemicals:
therapy of DDVP intoxication by atropine and cholinesterase
reactivators, Sittingbourne, Shell Research Ltd (Shell Technical
Memorandum No. TOX 31/62) (Unpublished Report).
BROWN, V.K.H., BLAIR, D., HOLMES, D.L., & PICKERING, R.G. (1968)
The toxicity of low concentrations of dichlorvos by inhalation in
rodent and avian species, Sittingbourne, Shell Research Ltd
(Unpublished Report No. TLGR.0015.68).
BRUNGS, W.A., MCCORMICK, J.H., NEIHEISEL, T.W., SPEHAR, R.L.,
STEPHAN, C.E., & STOKES, G.N. (1977) Effects of pollution on fresh-
water fish. J. Water Pollut. Control Fed., 49(6): 1425-1493.
BRYANT, R.J. & MINETT, W. (1978) A gas chromatographic method for the
determination of dichlorvos in air. Pestic. Sci., 9: 525-528.
BRZEZINSKI, J. & WYSOCKA-PARUSZEWSKA, B. (1980) Neurochemical
alterations in rat brain as a test for studying the neurotoxicity of
organophosphorus insecticides. Arch. Toxicol., 4(Suppl.): 475-478.
BULL, D.L. & RIDGWAY, R.L. (1969) Metabolism of trichlorfon in
animals and plants. J. agric. food Chem., 17(4): 837-841.
BUNDING, I.M., YOUNG, R., Jr, SCHOOLEY, M.A., & COLLINS, J.A.
(1972) Maternal dichlorvos effects on farrowing parameters. J. anim.
Sci., 35: 238.
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1972) [Mutagenicity
investigations with pesticides in the host-mediated assay and the
dominant lethal test in mice.] Biol. Zentralbl., 91(3): 311-325 (in
German).
BUSELMAIER, W., ROHRBORN, G., & PROPPING, P. (1973) Comparative
investigations on the mutagenicity of pesticides in mammalian test
systems. Mutat. Res., 21(1): 25-26.
BUTLER, G.L. (1977) Algae and pesticides. Residue Rev., 66: 40.
CARERE, A., CARDAMONE, G., ORTALI, V., BRUZZONE, M.L., & DI
GIUSEPPE, G. (1976) Mutational studies with some pesticides
in Streptomyces coelicolor and Salmonella typhimurium. Mutat.
Res., 38(2): 136.
CARERE, A., ORTALI, V.A., CARDAMONE, G., TORRACCA, A.M., &
RASCHETTI, R. (1978a) Microbiological mutagenicity studies of
pesticides in vitro. Mutat. Res., 57: 277-286.
CARERE, A., ORTALI, V.A., CARDAMONE, G., & MORPURGO, G.
(1978b) Mutagenicity of dichlorvos and other structurally-related
pesticides in Salmonella and Streptomyces. Chem.-biol. Interact., 22:
297-308.
CAROLDI, S. & LOTTI, M. (1981) Delayed neurotoxicity caused by a
single massive dose of dichlorvos to adult hens. Toxicol. Lett., 9:
157-159.
CARSON, S. (1969) Teratology studies in rabbits (summary
appraisal), Maspeth, New York, Food and Drug Research Laboratories
(Unpublished Report, 30 June).
CARTER, M.K. & MADDUX, B. (1968) Effects of selected agents on the
in vitro inhibition of cholinesterase activity of dichlorvos.
Pharmacologist, 10: 222.
CARTER, M.K. & MADDUX, B. (1974) Interaction of dichlorvos and
anticholinesterases on the in vitro inhibition of human blood
cholinesterases. Toxicol. appl. Pharmacol., 27: 456-463.
CASIDA, J.E., MCBRIDE, L., & NIEDERMEIER, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. agric. food Chem., 10(5): 370-377.
CAVAGNA, G. & VIGLIANI, E.C. (1970) Problčmes d'hygične et de
sécurité dans l'emploi du vapona comme insecticide dans les locaux
domestiques. Med. Lav., 61(8/9): 409-423.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch. environ.
Health, 19(1): 112-113.
CAVAGNA, G., LOCATI, G., & VIGLIANI, E.C. (1970) Exposure of new-
born babies to Vapona insecticide. Eur. J. Toxicol., 3(1): 49-57.
CERVONI, W.A., OLIVER-GONZALEZ, J., KAYE, S., & SLOMKA, M.B.
(1969) Dichlorvos as a single-dose intestinal anthelmintic therapy for
man. Am. J. trop. Med. Hyg., 18(6): 912-919.
CHATTOPADHYAY, D.P., DIGHE, S.K., DUBE, D.K., & PURNANAND
(1982) Changes in toxicity of DDVP, DFP, and parathion in rats under
cold environment. Bull. environ. Contam. Toxicol., 29: 605-610.
CHEN, Z.M., ZABIK, M.J., & LEAVITT, R.A. (1984) Comparative study of
thin film photodegradative rates for 36 pesticides. Ind. eng. chem.
Prod. Res. Dev., 23: 5-11.
CIPAC HANDBOOK (1980) Analysis of technical and formulated
pesticides, Cambridge, Wheffer and Sons, Collaborative International
Pesticides Analytical Council, Vol. 1A, pp. 1214-1224 (Addendum to
CIPAC 1).
CIVEN, M., LEEB, J.E., WISHNOW, R.M., WOLFSEN, A., & MORIN, R.J.
(1980) Effects of low level administration of dichlorvos on adreno-
corticotrophic hormone secretion, adrenal cholesteryl ester, and
steroid metabolism. Biochem. Pharmacol., 29: 635-641.
CLINCH, P.G. (1970) Effect on honey bees of combs exposed to vapour
from dichlorvos slow-release strips. N.Z. J. agric. Res., 13(2): 448-
452.
COCHRANE, W.P. (1979) Application of chemical derivatisation
techniques for pesticide analysis. J. chromatogr. Sci., 17: 124-137.
CODEX ALIMENTARIUS COMMISSION (1979) Recommended method of
sampling for the determination of pesticide residues. Report of the
Tenth Session of the Codex Committee on Pesticide Residues, Rome, Food
and Agriculture Organization of the United Nations, Appendix IV, Annex
I, pp. 67-70 (Alinorm 79/24).
CODEX ALIMENTARIUS COMMISSION (1983) Guidelines on good
analytical practice in residue analysis and recommendations for methods
of analysis for pesticide residues, Rome, Food and Agriculture
Organization of the United Nations, p. 40.
COHEN, S.D. & EHRICH, M. (1976) Cholinesterase and carboxyl-esterase
inhibition by dichlorvos and interactions with malathion and
triorthotolyl phosphate. Toxicol. appl. Pharmacol., 37: 39-48.
COLLINS, J.A., SCHOOLEY, M.A., & SINGH, V.K. (1971) The effect of
dietary dichlorvos on swine reproduction and viability of their
offspring. Toxicol. appl. Pharmacol., 19: 377.
COLLINS, R.D. & DE VRIES, D.M. (1973) Air concentrations and food
residues from use of Shell No-PestR Insecticide Strip. Bull.
environ. Contam. Toxicol., 9(4): 227-233.
COSTA, L.G. & MURPHY, S.D. (1984) Interaction between acetaminophen
and organophosphate in mice. Chem. Pathol. Pharmacol., 44(3): 389-400.
COULSTON, F. & GRIFFIN, T. (1977) Cholinesterase activity and
neuromuscular functions of Rhesus monkeys exposed to DDVP
vapours, Albany, New York, Institute of Comparative and Human
Toxicology and Holloman Air Force Base, International Center of
Environmental Safety (Unpublished report).
CRONCE, P.C. & ALDEN, H.S. (1968) Flea-collar dermatitis. J. Am.
Med. Assoc., 206(7): 1563-1564.
DALE, W.E., MILES, J.W., & WEATHERS, D.B. (1973) Measurement of
residues of dichlorvos absorbed by food exposed during disinsection of
aircraft. J. agric. food Chem., 21(5): 858-860.
DAMBSKA, M. & MASLINSKA, D. (1982) Effect of dichlorvos (DDVP)
intoxication of rabbit brain. Neuropathol. Pol. 20(1/2): 77-84.
DAMBSKA, M., MASLINSKA, D., RAKOWSKA, I., & RUTCZYNSKI, M.
(1978) [Evaluation of the effect of dichlorvos poisoning of pregnant
rabbits and rats on the condition and development of their
offspring.] Bromatol. Chem. Toksykol., 11(3): 355-357 (in Polish).
DAMBSKA, M., IWANOWSKI, L., & KOZLOWSKI, P. (1979) The effect of
transplacental intoxication with dichlorvos on the development of
cerebral cortex in newborn rabbits. Neuropathol. Pol. 17(4): 571-576.
DAMBSKA, M., IWANOWSKI, L., MASLINSKA, D., & OSTENDA, M. (1984)
Blood-brain barrier in young rabbit brain after dichlorvos
intoxication. Neuropathol. Pol. 22(1): 129-137.
DARROW, D.I. (1973) Biting lice of goats: control with dichlorvos-
impregnated resin neck collars. J. econ. Entomol., 66: 133-135.
DAS, Y.T., TASKAR, P.K., BROWN, H.D., & CHATTOPADHYAY, S.K.
(1983) Exposure of professional pest control operator to dichlorvos
(DDVP) and residue on house structures. Toxicol. Lett., 17: 95-99.
DAVIDEK, J., SEIFERT, J., & DOLEZALOVA, Z. (1976) The determination
of dichlorvos in milk by using classical and square-wave
polarography. Milchwissenschaft, 31(5): 267-270.
DEAN, B.J. (1972a) The mutagenic effects of organophosphorus
pesticides on microorganisms. Arch. Toxikol., 30: 67-74.
DEAN, B.J. (1972b) The effect of dichlorvos on cultured human
lymphocytes. Arch. Toxikol., 30: 75-85.
DEAN, B.J. & BLAIR, D. (1976) Dominant lethal assay in female mice
after oral dosing with dichlorvos or exposure to atmospheres containing
dichlorvos. Mutat. Res., 40(1): 67-72.
DEAN, B.J. & THORPE, E. (1972a) Cytogenic studies with dichlorvos in
mice and Chinese hamsters. Arch. Toxikol., 30: 39-49.
DEAN, B.J. & THORPE, E. (1972b) Studies with dichlorvos vapour in
dominant lethal mutation tests on mice. Arch. Toxikol., 30: 51-59.
DEAN, B.J., DOAK, S.M.A., & FUNNELL, J. (1972) Genetic studies with
dichlorvos in the host-mediated assay and in liquid medium
using Saccharomyces cerevisiae. Arch. Toxikol., 30: 61-66.
DEDEK, W., GEORGI, W., & GRAHL, R. (1979) Comparative degradation and
metabolism of 32P-labelled butonate, trichlorphone and dichlorvos in
crop plants. Biochem. Physiol. Pflanz., 174: 707-722.
DEGRAEVE, N., GILOT-DELHALLE, J., MOUTSCHEN, J.,
MOUTSCHENDAHMEN, M., COLIZZI, A., CHOLLET, M., &
HOUBRECHTS, N. (1980) Comparison of the mutagenic activity of
organophosphorus insecticides in mouse and in the
yeast Schizosaccharomyces pombe. Mutat. Res., 74(3): 201-202.
DEGRAEVE, N., CHOLLET, M.C., MOUTSCHEN, J., MOUTSCHEN-
DAHMEN, M., GILOT-DELHALLE, J., & COLIZZI, A. (1982) Genetic and
cytogenetic effects of chronic treatment with organophosphorus
insecticides. Mutat. Res., 97: 179-180.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984a) Cytogenetic
and genetic effects of subchronic treatments with organophosphorus
insecticides. Arch. Toxicol., 56: 66-67.
DEGRAEVE, N., CHOLLET, M.C., & MOUTSCHEN, J. (1984b) Cytogenetic
effects induced by organophosphorus pesticides in mouse
spermatocytes. Toxicol. Lett., 21: 315-319.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I., VARGA, L., & FARKAS, J. (1978) Studies on the
immunosuppressive effect of organochlorine and organophosphoric
pesticides in subacute experiments. J. Hyg. Epidemiol. Microbiol.
Immunol., 22: 115-122.
DESI, I., VARGA, L., & FARKAS, J. (1980) The effect of DDVP, an
organophosphorus pesticide, on the humoral and cell-mediated immunity
of rabbits. Arch. Toxicol., 4(Suppl.): 171-174.
DICOWSKY, L. & MORELLO, A. (1971) Glutathione-dependent degradation
of 2,2-dichlorovinyl dimethyl phosphate (DDVP) by the rat. Life
Sci., 10(2): 1031-1037.
DIKSHITH, T.S.S., DATTA, K.K., & CHANDRA, P. (1976) 90-day dermal
toxicity of DDVP in male rats. Bull. environ. Contam. Toxicol., 15(5):
574-580.
DOUGHERTY, E.M., REICHELDERFER, C.F., & FAUST, R.M. (1971)
Sensitivity of Bacillus thuringiensis var. thuringiensis to various
insecticides and herbicides. J. invertebr. Pathol., 17: 292-293.
DMITRIEV, A.I. & KOZEMJAKJN, N.G. (1975) [Veterinary expert report on
the meat of chicken poisoned by dichlorvos.] Veterinarija, 1975(1):
90-92 (in Russian).
DRAGER, G. (1968) [Gas chromatographic method for the determination
of dichlorvos residues in plants and milk.] Pflanzenschutz Nachr.
Bayer, 21(3): 377-384 (in German).
DRAUGHON, F.A. & AYRES, J.C. (1978) Effect of selected pesticides on
growth and citrinin production by Penicillium citrinum. J. food
Sci., 43: 576-578.
DURHAM, W.F., GAINES, TH.B., & HAYES, W.J. (1956) Paralytic and
related effects of certain organic phosphorus compounds. Arch. ind.
Health, 13: 326-330.
DURHAM, W.F., GAINES, TH.B., MCCAULEY, R.H., Jr, SEDLAK, V.A.,
MATTSON, A.M., & HAYES, W.J., Jr (1957) Studies on the toxicity
of O,O-dimethyl-2,2-dichlorovinyl phosphate (DDVP). Am. Med. Assoc.
Arch. Ind. Health, 15: 340-349.
DYER, K.F. & HANNA, P.J. (1973) Comparative mutagenic activity and
toxicity of triethylphosphate and dichlorvos in bacteria and
Drosophila. Mutat. Res., 21: 175-177.
DZWONKOWSKA, A. & HUBNER, H. (1986) Induction of chromosomal
aberrations in the Syrian hamster by insecticides tested in vivo. Arch.
Toxicol., 58: 152-156.
EDSON, E.F. (1958) Blood tests for users of organophosphorus
insecticides. World Crops, 10 February: 49-51.
EGYED, M.N. & BENDHEIM, U. (1977) Mass poisoning in chickens by
consumption of organophosphorus (dichlorvos) contaminated drinking-
water. Refu. Vet., 34(3): 107-111.
EICHNER, M. (1978) [Quick residue control of plant and animal food,
tobacco, and tobacco products by Sweep-Co analysis 1.] Z. Lebensm.
Unters. Forsch., 167: 245-249 (in German).
EISLER, R. (1969) Acute toxicities of insecticides to marine decapod
crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Acute toxicities of organochlorine and
organophosphorus insecticides to estuarine fish, Washington DC, US
Department of the Interior, Fish and Wildlife Service, Bureau of Sport
Fisheries and Wildlife (Technical Paper No. 46).
ELGAR, K.E. & STEER, B.D. (1972) Dichlorvos concentrations in the air
of houses arising from the use of dichlorvos PVC strips. Pestic.
Sci., 3: 591-600.
ELGAR, K.E., MARLOW, R.G., & MATHEWS, B.L. (1970) The determination
of residues of dichlorvos in crops and tissues. Analyst, 95: 875-878.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972a) Dichlorvos residues
in food arising from the domestic use of dichlorvos PVC strips. Pestic.
Sci., 3: 601-607.
ELGAR, K.E., MATHEWS, B.L., & BOSIO, P. (1972b) Vapona strips in
shops: residues in foodstuffs. Global aspects of chemistry, toxicology,
and technology as applied to the environment. Environ. Qual. Saf., 1:
217-221.
ELLINGER, CH. (1985) Enzyme activities after in vitro and in
vivo application of dichlorvos. Biomed. Biochim. Acta, 44(2): 311-
316.
ELLINGER, CH., KAISER, G., & HÖRING, H. (1985) [Haematological
changes to rats following exposure to dichlorvos.] Nahrung, 29(4): 351-
355 (in German).
ELLMAN, G.L., COURTNEY, K.D., ANDRES, V., Jr, & FEATHERSTONE,
R.M. (1961) A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmacol., 7: 88-95.
ENOMOTO, M.F., NAKADATE, M., NINOMIYA, K., HAYAKAWA, Y.,
ITO, H., IGARASHI, S., UWANUMA, Y., NAKASATO, R., & HATANAKA,
J. (1981) Studies on carcinogenicity of DDVP (2,2-dichlorovinyl
dimethyl phosphate) mixed in drinking-water in rats, Tokyo, Ministry of
Health and Welfare (Cooperative Studies on Carcinogenicity Test on
Mutagens) (unpublished).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y. (1972)
Detection of chemical mutagens by the dominant lethal assay in the
mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERIKSSON, H. & FAYERSSON, Y. (1980) A reliable way of estimating
cholinesterases from whole blood in the presence of
anticholinesterases. Clin. Chim. Acta, 100: 165-171.
ESCUDIE, E. & SALES, P. (1963) Premičres expériences en Haute Volta
sur le dichlorvos résiduel. Bull. World Health Organ., 29: 247-249.
FAHRIG, R. (1973) [Genetic effects of organophosphorus
insecticides.] Naturwissenschaften, 60(1): 50-51 (in German).
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides.
In: Montesano, R. & Tomotis, L., ed. Chemical carcinogenesis essays,
Lyons, International Agency for Research on Cancer, pp. 161-181 (IARC
Scientific Publication No. 10).
FAO (1977) FAO Specifications for plant protection products,
dichlorvos and fenthion, Rome, Food and Agriculture Organization of the
United Nations.
FAO (1982) Report of the Second Government Consultation on
International Harmonization of Pesticide Registration Requirements,
Rome, 11-15 October, Rome, Food and Agriculture Organization of the
United Nations.
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10; WHO Food
Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO PL/1965/10/1; WHO Food
Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Report of the Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Meeting Report No. PL/1962/M/11; WHO Technical Report Series
No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1967/M11/1; WHO Food
Add./68.30).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1969 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 84; WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (FAO PL/1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 87; WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluation of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71.42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974 Joint
Meeting on Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 97; WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (AGP 1974/M/11; WHO Pesticide Residue
Series No. 4).
FAO/WHO (1977a) Pesticide residues in food. Report of the 1976 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues and the
Environment and the WHO Expert Group on Pesticide Residues, Geneva,
World Health Organization (FAO Food and Nutrition Series No. 9; FAO
Plant Production and Protection Series No. 8; WHO Technical Report
Series No. 612).
FAO/WHO (1977b) 1976 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(ADP 1977/M/14).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977 Joint
Meeting on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 10
Rev.).
FAO/WHO (1978b) 1977 Evaluation of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Suppl.).
FAO/WHO (1986) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FISHBEIN, L. (1976) Potential hazards of fumigant residues.
Environ. Health Perspect., 14: 39-45.
FISHBEIN, L. (1981) Environmental sources of chemical mutagens. II.
Synthetic mutagens. In: Flamm, W.G. & Mehlman, M.A., ed. Mutagenesis:
advances in modern toxicology, Tokyo, Mishima and Kyoto, Vol. 5, pp.
257-348.
FISHBEIN, L. (1982) An overview of the structural features of
mutagenic S-chlorallylthio and dithiocarbamate pesticides and
trichlorfon, dichlorvos, and their metabolites. In: Proceedings of the
3rd International Conference on Environmental Mutagens and Carcinogens,
21-27 September 1981, Tokyo, Mishima and Kyoto, pp. 371-378.
FISCHER, G.W., SCHNEIDER, P., & SCHEUFLER, H. (1977) [Mutagenicity
of dichloroacetaldehyde and 2,2-dichloro-1,1-dihydroxyethane phosphoric
acid methyl ester: possible metabolites of the organophosphorus
pesticide trichlorphon.] Chem.-biol. Interact., 19: 205-213 (in
German).
FOLL, C.V., PANT, C.P., & LIETAERT, P.E. (1965) A large-scale field
trial with dichlorvos as a residual fumigant insecticide in northern
Nigeria. Bull. World Health Organ., 32: 531-550.
FOURNIER, E., DALLY, S., & CAMBIER, J. (1976) Neuropathie
périphérique probablement due aux insecticides anticholinesté-
rasiques. Nouv. Presse méd., 5(11): 718.
FRANCIS, B.M., METCALF, R.L., & HANSEN, L.G. (1985) Toxicity of
organophosphorus esters to laying hens after oral and dermal
administration. J. environ. Sci. Health, B20(1): 73-95.
FRANK, R., BRAUN, H.E., & FLEMING, G. (1983) Organochlorine and
organophosphorus residues in fat of bovine and porcine carcasses
marketed in Ontario, Canada from 1969-81. J. food Prot., 46: 893-900.
FUJITA, K., MATSUSHIMA, S., ABE, E., SASAKI, K., & KUROSAWA, K.
(1977) [Examination of the effects of dichlorvos on the
testis.] Nippon Noson Igakkai Zasshi, 26(3): 328-329 (in Japanese).
FUJITA, Y. (1985) Studies on contact dermatitis from pesticides in
tea growers. Acta Med. Univ. Kagoshima, 27(1): 17-37.
FUNCKES, A.J., MILLER, S., & HAYES, W.J., Jr (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 243-246.
GAINES, TH.B. (1969) Acute toxicity of pesticides. Toxicol. appl.
Pharmacol., 14(3): 515-534.
GAINES, TH.B., HAYES, W.J., Jr, & LINDER, R.E. (1966) Liver
metabolism of anticholinesterase compounds in live rats: relation to
toxicity. Nature (Lond.), 209: 88-89.
GIFAP (1982) Guidelines on pesticide residues to provide data for the
registration of pesticides and the establishment of maximum residue
limits elaborated by Codex Committee on Pesticide Residues, Brussels,
International Group of National Associations of Manufacturers of
Agrochemical Products (Technical Monograph No. 4).
GILLENWATER, H.B., HAREIN, P.K., LOY, E.W., Jr, THOMPSON, J.F.,
LAUDANI, H., & EASON, G. (1971) Dichlorvos applied as a vapour in a
warehouse containing packaged foods. J. stored Prod. Res., 7: 45-56.
GILLETT, J.W., HARR, J.R., LINDSTROM, F.T., MOUNT, D.A., ST.
CLAIR, A.D., & WEBER, L.J. (1972) Evaluation of human health hazards
on use of dichlorvos (DDVP), especially in resin strips. Residue
Rev., 44: 115-159.
GILOT-DELHALLE, J., COLIZZI, A., MOUTSCHEN, J., & MOUTSCHEN-
DAHMEN, M. (1983) Mutagenicity of some organophosphorus compounds at
the ade6 locus of Schizosaccharomyces pombe. Mutat. Res., 117: 139-
148.
GOH, K.S., EDMISTON, S., MADDY, K.T., & MARGETICH, S. (1986a)
Dissipation of dislodgeable foliar residue for chlorpyrifos and
dichlorvos treated lawn: implication for safe reentry. Bull. environ.
Contam. Toxicol., 37: 33-40
GOH, K.S., EDMISTON, S., MADDY, K.T., MEINDERS, D.D., &
MARGETICH, S. (1986b) Dissipation of dislodgeable foliar residue of
chlorpyrifos and dichlorvos on turf. Bull. environ. Contam.
Toxicol., 37: 27-32.
GOLD, R.E., HOLCSLAW, T., TUPY, D., & BALLARD, J.B. (1984) Dermal
and respiratory exposure to applicators and occupants of residences
treated with dichlorvos (DDVP). J. econ. Entomol., 77: 430-436.
GOTO, S. (1977) [Variation of concentrations of pesticide residues in
field soil.] J. Pestic. Sci., 2: 319-321. (in Japanese, with English
summary).
GOUGH, B.J. & SHELLENBERGER, T.E. (1970) In vivo rabbit blood
cholinesterase inhibition and reactivation following organophosphate
infusion. Toxicol. appl. Pharmacol., 17(1): 302.
GOUGH, B.J. & SHELLENBERGER, T.E. (1977-78) In vivo inhibition of
rabbit whole blood cholinesterase with organophosphate inhibitors and
reactivation with oximes. Drug Chem. Toxicol., 1(1): 25-43.
GRAHL, K. (1979) [The persistence of trichlorfon and dichlorvos in
ponds.] Z. Binnenfisch. DDR, 25(10): 312-316 (in German).
GRAHL, K., STELZER, W., & STOTTMEISTER, S. (1980) [Toxicity of
butonate, trichlorfon, and dichlorvos to Escherichia coli and
Enterobacter aerogenes.] Acta hydrochim. hydrobiol., 8: 19-28 (in
German).
GRAHL, K., HORN, H., & HALLEBACH, R. (1981) [Effect of butonate,
trichlorfon, and dichlorvos on plankton populations.] Acta hydrochim.
hydrobiol., 9(2): 147-161 (in German).
GRATZ, N.G., BRACHA, P., & CARMICHAEL, A. (1963) A village- scale
trial with dichlorvos as a residual fumigant insecticide in southern
Nigeria. Bull. World Health Organ., 29: 251-270.
GREEN, M.H.L., BRIDGES, B.A., GRAY, W.J.H., & MOTTERSHEAD, R.P.
(1973) Comparison of DNA damage caused by dichlorvos and methyl
methanesulfonate in pol A and pol A+ strains of Escherichia coli.
Genetics, 74: s100.
GREEN, M.H.L., MEDCALF, A.S.C., ARLETT, C.R., HARCOURT, S.A., &
LEHMANN, A.R. (1974a) DNA strand breakage caused by dichlorvos, methyl
methane-sulfonate and iodoacetamide in Escherichia coli and cultured
Chinese hamster cells. Mutat. Res., 24(3): 365-378.
GREEN, M.H.L., MEDCALF, A.S.C., & STEVENS, S.W. (1974b) Apparent
indirect DNA damage by dichlorvos and iodoacetamide in Escherichia
coli. Heredity, 33: 446.
GREEN, M.H.L., MURIEL, W.J., & BRIDGES, B.A. (1976) Use of a
simplified fluctuation test to detect low levels of mutagens. Mutat.
Res., 38(1): 33-42.
GRIFFIN, D.E., III & HILL, W.E. (1978) In vitro breakage of plasmid
DNA by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRUBNER, P. (1972) [Residue problems in the use of phosphoric acid
ester insecticides in mushroom cultures.] Nachrichtenbl.
Pflanzenschutzdienst (DDR), 26: 245-247 (in German).
GUNDERSON, E.C. (1981) Sampling methods for airborne pesticides,
Washington, DC, American Chemical Society, pp. 301-315 (ACS Symposium
Series No. 149).
GUPTA, A.K. & SINGH, J. (1974) Dichlorvos (DDVP) induced breaks in the
salivary gland chromosomes of Drosophila melanogaster. Curr.
Sci., 43(20): 661-662.
GUPTA, R.C., DAVE, S.K., SHAH, M.P., & KASHYAP, S.K. (1979) A
monitoring study of workers handling pesticides in warehouses and
godowns. J. environ. Sci. Health, B14(4): 405-416.
HALEY, T.J., FARMER, J.H., HARMON, J.R., & DOOLEY, K.L. (1975)
Estimation of the LD1 and extrapolation of the LD0.1 for five
organophosphate pesticides. Arch. Toxicol., 34: 103-109.
HANNA, P.J. & DYER, K.F. (1975) Mutagenicity of organophosphorus
compounds in bacteria and Drosophila. Mutat. Res., 28: 405-420.
HASAN, M. & ALI, S.F. (1980) Organophosphate pesticide dichlorvos-
induced increase in the rate of lipid peroxidation in the different
regions of the rat brain: supporting ultra-structural
findings. Neurotoxicology, 2: 43-52.
HASAN, M., MAITRA, S.C., & ALI, S.F. (1979) Organophosphate pesticide
DDVP-induced alterations in the rat cerebellum and spinal cord: an
electron microscopic study. Exp. Pathol., 17(2): 88-94.
HASS, D.K., COLLINS, J.A., & KODAMA, J.K. (1972) Effects of orally
administered dichlorvos in rhesus monkeys. J. Am. Vet. Med.
Assoc., 161(6): 714-719.
HATCH, G.G., ANDERSON, T.M., HUBERT, R.A., KOURI, R.E.,
PUTMAN, D.L., CAMERON, J.W., NIMS, R.W., MOST, B., SPALDING, J.W.,
TENNANT, R.W., & SCHECHTMAN, L.M. (1986) Chemical enhancement of
sa7 virus transformation of hamster embryo cells: evaluation by
interlaboratory testing of diverse chemicals. Environ. Mutagenesis.,
8: 515-531.
HATTORI, K., SATO, H., TSUCHIYA, K., YAMAMOTO, N., & OGAWA, E.
(1974) [Toxicological studies on the influences of chemicals to the
birds. I. Oral acute toxicity and cholinesterase inhibition of three
organophosphate insecticides in Japanese quail.] Hokkaidoritsu Eisei
Kenkyusho Ho, 24: 35-38 (in Japanese, with English summary).
HAYES, A.L., WISE, R.A., & WEIR, F.W. (1980) Assessment of
occupational exposure to organophosphates in pest control
operators. Am. Ind. Hyg. Assoc. J., 41(8): 568-575.
HAYES, W.J., Jr (1961) Safety of DDVP for the disinsection of
aircraft. Bull. World Health Organ., 24: 629-633.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons:
emergency information for treating poisoning, Atlanta, Georgia, US
Department of Health, Education and Welfare, Public Health Service,
Communicable Diseases Center (Public Health Service Publication No.
476).
HAYES, W.J., Jr (1982) Organic phosphorus pesticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and Wilkins,
pp. 343-351.
HAZELWOOD, J.C., STEFAN, G.E., & BOWEN, J.M. (1979) Motor unit
irritability in beagles before and after exposure to cholinesterase
inhibitors. Am. J. vet. Res., 40(6): 852-856.
HEMMINKI, K. (1983) Nucleic acid adducts of chemical carcinogens and
mutagens. Arch. Toxicol., 52: 249-285.
HENRIKSSON, K., KALLELA, K., VIRTAMO, M., & PFAFFLI, P. (1971)
[The toxicity of DDVP (dichlorvos) evaporated from Vapona strip.] J.
Sci. Agric. Soc. Finl., 43: 187-200 (in Finnish).
HEUSER, S.G. & SCUDAMORE, K.A. (1966) A rapid method for sampling
dichlorvos vapour in air. Chem. Ind., 50: 2093-2094.
HEUSER, S.G. & SCUDAMORE, K.A. (1975) A method for the assessment
under standard conditions of the output of dichlorvos slow-release
units used for insect control. Analyst, 100: 129-135.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975) Lethal
dietary toxicities of environmental pollutants to birds, Washington DC,
US Department of the Interior, Fish and Wildlife Service, pp. 1-51
(Special Scientific Report: Wildlife No. 191).
HINE, C.H. (1962) 90-day chronic toxicity studies of
VaponaR insecticide for dogs, San Francisco, California, The Hine
Laboratories (Unpublished Report No. 1, 1 September).
HINE, C.H. & SLOMKA, M.B. (1968) Human tolerance of the acute and
subacute oral administration of a polyvinylchloride formulation of
dichlorvos (V-3 and V-12). Pharmacologist, 10: 222.
HINE, C.H. & SLOMKA, M.B. (1970) Human toxicity studies on polyvinyl
chloride formulation of dichlorvos. Toxicol. appl. Pharmacol., 17(1):
304-305.
HODGSON, E. & CASIDA, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethylphosphate. J. agric. food
Chem., 10(3): 208-214.
HOLMSTEDT, B., NORDGREN, I., SANDOZ, M., & SUNDWALL, A. (1978)
Metrifonate. Summary of toxicological and pharmacological information
available. Arch. Toxicol., 41: 3-29.
HORIUCHI, N. & ANDO, Y. (1978) [Photosensitivity caused by
pesticides.] (original unpublished information used as a basis for
article by Horiuchi et al., 1978) (in Japanese).
HORIUCHI, N., ANDO, S., & SUZUKI, A. (1978) [Photosensitization due
to pesticides.] Nippon Noson Igakkai Zashi, 27(3): 450-451 (in
Japanese).
HORVATH, J., HEGATH, V., & PUCHA, K. (1968) A study of the influence
of cholinesterase with cattle housed in byres provided with DDVP
strips. Agrochemia, 8(9): 267-268.
HUNTER, C.G. (1969) Report on initial studies of deliberate exposures
to high concentrations of dichlorvos by human subjects, Sittingbourne,
Tunstall Laboratory Shell (Unpublished Report, July).
HUNTER, C.G. (1970a) Dichlorvos: inhalational exposures with human
subjects. Part 1, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0061.70).
HUNTER, C.G. (1970b) Dichlorvos: inhalational exposures with human
subjects. Part 2, Sittingbourne, Shell Research Ltd (Unpublished Report
No. TLGR.0067.70).
HUSSEY, N.W. & HUGHES, J.T. (1964) Investigations on the use of
dichlorvos in the control of the mushroom phorid Megaselia
halterata (Wood). Ann. appl. Biol., 54: 129-139.
HUTSON, D.H. & HOADLEY, E.C. (1972a) The comparative metabolism of
14C-vinyl-dichlorvos in animals and man. Arch. Toxikol., 30: 9-18.
HUTSON, D.H. & HOADLEY, E.C. (1972b) The metabolism of 14C-methyl-
dichlorvos in the rat and mouse. Xenobiotica, 2(2): 107-116.
HUTSON, D.H., BLAIR, D., HOADLEY, E.C., & PICKERING, B.A. (1971a)
The metabolism of 14C-VaponaR in rats after administration by oral
and inhalation routes. Toxicol. appl. Pharmacol., 19: 378-379.
HUTSON, D.H., HOADLEY, E.C., & PICKERING, B.A. (1971b) The
metabolic fate of vinyl-1-14C-dichlorvos in the rat after oral and
inhalation exposure. Xenobiotica, 1(6): 593-611.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K. (1978)
EEG, ECG, and respiratory response to acute insecticide exposure. Bull.
environ. Contam. Toxicol., 19: 47-55.
IARC (1979) Some halogenated hydrocarbons, Lyons, International Agency
for Research on Cancer, pp. 97-127 (Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Vol. 20).
IRPTC (1984) DDVP, Geneva, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme (Scientific
Reviews of Soviet Literature on Toxicity and Hazards of Chemicals, No.
79).
ISSHIKI, K., MIYATA, K., MATSUI, S., TSUTSUMI, M., & WATANABE, T.
(1983) [Effects of post-harvest fungicides and piperonyl butoxide on
the acute toxicity of pesticides in mice.] Shokuhin Eiseigaku
Zasshi, 24(3): 268-274 (in Japanese, with English summary).
IVEY, M.C. & CLABORN, H.V. (1969) GLC determination of dichlorvos in
milk, eggs and various body tissues of cattle and chickens. J. Assoc.
Off. Anal. Chem., 52(6): 1248-1251.
IWATA, Y., SUGITANI, A., & YAMADA, F. (1981) [An analytical method
for organophosphorus pesticides in the onion.] Shokuhin Eiseigaku
Zasshi, 22: 484-489 (in Japanese, with English summary).
JAKUBOWSKA, B. & NOWAK, A. (1973) [The effect of organophosphorus and
carbamate insecticides on the development of soil fungi.] Zesz. Nauk.
Akad. Roln. Szczecinie, 39(10): 141-150 (in Polish).
JAQUES, R. (1964) The protective effect of pyridine-2-aldoxime
methiodide (P2AM), bis-(4-hydroxyimino-methyl-pyridinium-1-methyl)-
ether dichloride (ToxogoninR) and atropine in experimental DDVP
poisoning. Helv. Physiol. Pharmacol. Acta, 22(3): 174-183.
JAYASURIYA, V.U., DE S. & RATNAYAKE, W.E. (1973) Screening of some
pesticides on Drosophila melanogaster for toxic and genetic
effects. Drosophila Inf. Serv., 50: 184-186.
JENSEN, J.A., FLURY, V.P., & SCHOOF, H.F. (1965) Dichlorvos vapour
disinsection of aircraft. Bull. World Health Organ., 32: 175-179.
JOHNSON, M.K. (1969) The delayed neurotoxic effect of some
organophosphorus compounds. Identification of the phosphorylation site
as an esterase. Biochem. J., 114(4): 711-717.
JOHNSON, M.K. (1975a) The delayed neuropathy caused by some
organophosphorus esters: mechanisms and challenge. CRC Crit. Rev.
Toxicol., 3: 289-316.
JOHNSON, M.K. (1975b) Organophosphorus esters causing delayed
neurotoxic effects. Mechanism of action and structure/ activity
studies. Arch. Toxicol., 34: 259-288.
JOHNSON, M.K. (1978) The anomalous behaviour of dimethyl phosphates
in the biochemical test for delayed neurotoxicity. Arch.
Toxicol., 41: 107-110.
JOHNSON, M.K. (1981) Delayed neurotoxicity: do trichlorphon and/or
dichlorvos cause delayed neuropathy in man or in test animals? Acta
pharmacol. toxicol., 49(Suppl. 5): 87-98.
JOHNSON, R.D., MANSKE, D.D., & PODREBARAC, D.S. (1981a) Pesticide
metal and other chemical residues in adult total diet samples.
XII. Pestic. monit. J., 15(1): 54-69.
JOHNSON, R.D., MANSKE, D.D., NEW, D.H., & PODREBARAC, D.S.
(1981b) Pesticide, heavy metal, and other chemical residues in infant
and toddler total diet samples. II. August 1975 - July 1976. Pestic.
monit. J., 15(1): 39-50.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication No. 137).
JOLLEY, W.P., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon beagle dogs during a period of two years by the
introduction of VaponaR insecticide into their daily diet, Cincinnati,
Ohio, The Kettering Laboratory (Unpublished Report, 19 January).
KAWACHI, T., KOMATSU, T., KADA, T., ISHIDATE, M., SASAKI, M.,
SUGIYAMA, T., & TAZIMA, Y. (1980) Results of recent studies on the
relevance of various short-term screening tests in Japan. In: G.M.
Williams et al., ed. The predictive value of short-term screening tests
in carcinogenicity evaluation, Amsterdam, Oxford, New York,
Elsevier/North Holland Biomedical Press, pp. 253-267.
KENAGA, E.E. (1979) Acute and chronic toxicity of 75 pesticide to
various animal species. Down Earth, 35(2): 25-31
KIMBROUGH, R.D. & GAINES, T.B. (1968) Effect of organic phosphorus
compounds and alkylating agents on the rat fetus. Arch. environ.
Health, 16: 805-808.
KIMMERLE, G. & LORKE, D. (1968) [Toxicity of insecticidal phosphoric
acid esters.] Pflanzenschutz Nachr. Bayer, 21(1): 111-142 (in German).
KIRKLAND, V.L. (1971) Some aspects of acute inhalation pharmacology
of dichlorvos in swine. Paper presented at: American Chemical Society
for Pharmacology and Experimental Therapeutics and the Division of
Medical Chemistry, Burlington, Vermont, 25 August, 1971.
KLIGERMAN, A.D., EREXSON, G.L., & WILMER, J.L. (1985) Induction of
sister-chromatid exchange (SCE) and cell-cycle inhibition in mouse
peripheral blood B lymphocytes exposed to mutagenic carcinogens in
vivo. Mutat. Res., 157: 181-187.
KNAPP, F.W. & GRADEN, A.P. (1964) Accidental exposure of dairy cows
to excessive amount of dichlorvos. J. econ. Entomol., 57: 790-791.
KOBAYASHI, H., YUYAMA, A., IMAJO, S., & MATSUSAKA, N. (1980)
Effects of acute and chronic administration of DDVP (dichlorvos) on
distribution of brain acetylcholine in rats. J. toxicol. Sci., 5: 311-
319.
KOBAYASHI, H., YUYAMA, A., & CHIBA, K.I. (1986) Cholinergic system
of brain tissue in rats poisoned with the organophosphate
0,0-demethyl 0-(2,2-dichlorovinyl) phosphate. Toxicol. appl.
Pharmacol., 82: 32-39.
KODAMA, J.K. (1960) Technical DDVP. Acute oral administration:
dogs, Vienna, Virginia, Hazleton Laboratories (Unpublished Report, 20
January).
KODAMA, J.K. (1968) Experimental evaluation in guinea-pigs of skin-
sensitizing potential of components of formulated dichlorvos/
polyvinylchloride products, Modesto, California, Shell Development
Company (Unpublished Technical Progress Report No. M-67-68).
KONISHI, Y., DENDA, A., & KITAOKA, R. (1981) Studies on
carcinogenicity of dichlorvos in B6C3FI mice, Tokyo, Ministry of Health
and Welfare (Cooperative Studies on Carcinogenicity Tests on Mutagens)
(unpublished).
KRAMERS, P.G.N. & KNAAP, A.G.A.C. (1978) Absence of a mutagenic
effect after feeding dichlorvos to larvae of D rosophila melanogaster.
Mutat. Res., 57: 103-105.
KRAUSE, CHR., VON & KIRCHHOFF, J. (1970) [Gas chromatographic
determination of organophosphate residues in market samples of fruits
and vegetables.] Dtsch. Lebensm. Rundschau., 66(6): 194-199 (in
German).
KRAUSE, W. (1977) Influence of DDT, DDVP, and malathion on FSH, LH,
and testosterone serum levels and testosterone concentrations in
testis. Bull. environ. Contam. Toxicol., 18(2): 231-242.
KRAUSE, W. & HOMOLA, S. (1972) [Influence on spermatogenesis by DDVP
(dichlorvos).] Arch. Dermatol. Forsch., 244: 439-441 (in German).
KRAUSE, W. & HOMOLA, S. (1974) Alterations of the seminiferous
epithelium and the Leydig cells of the rat* testis after the
application of dichlorvos (DDVP). Bull. environ. Contam.
Toxicol., 11(5): 429-433 (*study was performed on mice).
KRAUSE, W., HAMM, K., & WEISSMULLER, J. (1976) Damage to
spermatogenesis in juvenile rat treated with DDVP and malathion.
Bull. environ. Contam. Toxicol., 15(4): 458-462.
KUWABARA, K., NAKAMURA, A., & KASHIMOTO, T. (1980) Effect of
petroleum oil, pesticides, PCBs, and other environmental contaminants
on the hatchability of Artemia salina dry eggs. Bull. environ.
Contam. Toxicol., 25: 69-74.
LAFONTAINE, A., AERTS, J., & JACQUES, P. (1981) [Toxicity,
carcinogenic, mutagenic, and teratogenic action of dichlorvos.] Arch.
belg. Méd. soc. Hyg. Méd. Trav. Méd. lég., 39(3): 159-174 (in Dutch).
LAL, R. (1982) Accumulation, metabolism, and effects of
organophosphorus insecticides on microorganisms. Adv. appl.
Microbiol., 28: 149-200.
LAMOREAUX, R.J. & NEWLAND, L.W. (1978) The fate of dichlorvos in
soil. Chemosphere, 7(10): 807-814.
LAWLEY, P.D., SHAH, S.A., & ORR, D.J. (1974) Methylation of nucleic
acids by 2,2-dichlorovinyl dimethyl phosphate (dichlorvos,
DDVP). Chem.-biol. Interact., 8: 171-182.
LAWS, E.R., Jr (1966) Route of absorption of DDVP after oral
administration to rats. Toxicol. appl. Pharmacol., 8: 193-196.
LEARY, J.S., HIRSCH, L., LAVOR, E.M., FEICHTMEIR, E., SCHULTZ, D.,
KOOS, B., ROAN, C.R., FONTENOT, C., & HINE, C.H. (1971) An evaluation
of the safety of No-PestR strip insecticide with special reference to
respiratory and dietary exposure of occupants of homes in
Arizona. Toxicol. appl. Pharmacol., 19: 379.
LEARY, J.S., KEANE, W.T., FONTENOT, C., FEICHTMEIR, E.F.,
SCHULTZ, D., KOOS, B.A., HIRSCH, L., LAVOR, E.M., ROAN, C.C., &
HINE, C.H. (1974) Safety evaluation in the home of polyvinyl chloride
resin strips containing dichlorvos (DDVP). Arch. environ. Health, 29:
308-314.
LEONARD, A. (1976) Heritable chromosome aberrations in mammals after
exposure to chemicals. Radiat. environ. Biophys., 13: 1-8.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides. In:
Lee, R.L., ed. Air pollution from pesticides and agricultural
processes, Cleveland, Ohio, CRC Press, pp. 51-95.
LIEBERMAN, M.T. & ALEXANDER, M. (1981) Effects of pesticides on
decomposition of organic matter nitrification in sewage. Bull.
environ. Contam. Toxicol., 26: 554-560.
LIEBERMAN, M.T. & ALEXANDER, M. (1983) Microbial and non-
enzymatic steps in the decomposition of dichlorvos (2,2-
dichlorovinyl O,O-dimethylphosphate). J. agric. food Chem., 31: 265-
267.
LIVINGSTON, R.J. (1977) Review of current literature concerning the
acute and chronic effects of pesticides on aquatic organisms. CRC crit.
Rev. environ. Control, 7: 325-351.
LLOYD, T.S. (1973) Accidental poisoning in birds. Vet. Rec., 5 May:
489.
LOEFFLER, J.E., DE VRIES, D.M., YOUNG, R., & PAGE, A.C. (1971)
Metabolic fate of inhaled dichlorvos in pigs. Toxicol. appl.
Pharmacol., 19: 378.
LOEFFLER, J.E., POTTER, J.C., SCORDELIS, S.L., HENDRICKSON, H.R.,
HUSTON, C.K., & PAGE, A.C. (1976) Long-term exposure of swine to a
14C-dichlorvos atmosphere. J. agric. food Chem., 24(2): 367-371.
LOFROTH, G. (1970) Alkylation of DNA by dichlorvos. Natur-
wissenschaften, 57(8): 393-394.
LOFROTH, G. (1978) The mutagenicity of dichloroacetaldehyde. Z.
Naturforsch., 33: 783-785.
LOFROTH, G. & WENNERBERG, R. (1974) Methylation of purines and
nicotinamide in the rat by dichlorvos. Z. Naturforsch., 29: 651.
LOFROTH, G., KIM, C.H., & HUSSAIN, S. (1969) Alkylating properties of
2,2-dichlorovinyl dimethyl phosphate: a disregarded hazard. Environ.
Mutat. Soc. Newslett., 2: 21-27.
LOHS, K., FISCHER, G.W., & DEDEK, W. (1976) [Chemical-toxicological
aspects of the alkylating properties of organophosphate pesticides.]
Sitzungsber. Akad. Wiss. (DDR), 11N: 1-58 (in German).
LOTTI, M. & JOHNSON, M.K. (1978) Neurotoxicity of organophosphorus
pesticides: predictions can be based on in vitro studies with hen and
human enzymes. Arch. Toxicol., 41: 215-221.
LUDKE, J.L. & LOCKE, L.N. (1976) Duck deaths from accidental
ingestion of anthelmintic. Avian Dis., 20(3): 607-608.
LUKE, M.A., FROBERG, J.E., DOOSE, G.M., & MASUMOTO, H.T. (1981)
Improved multiresidue gas chromatographic determination of
organophosphorus organonitrogen, and organohalogen pesticides in
produce, using flame photometric and electrolytic conductivity
detectors. J. Assoc. Off. Anal. Chem., 64(5): 1187-1195.
MACDONALD, R. (1982) Toxicology of consumer products: the acute
(4-h) inhalation toxicity of dichlorvos vapour in rats and mice,
Sittingbourne, Shell Research Ltd (Unpublished Report SBGR.82.145).
MACKLIN, A.W. & RIBELIN, W.E. (1971) The relation of pesticides to
abortion in dairy cattle. J. Am. Vet. Med. Assoc., 159(12): 1743-
1748.
MADDY, K.T., EDMISTON, S., & FREDRICKSON, A.S. (1981a) Monitoring
residue of DDVP in room air and on horizontal surfaces following use of
a room fogger, Sacramento, California, California Department of Food
and Agriculture (Unpublished Report No. HS-897).
MADDY, K.T., SCHNEIDER, F., LOWE, J., OCHI, E., FREDRICKSON, A.S.,
& MARGOTICH, S. (1981b) Vapona (DDVP) exposure potential to in
mushroom houses in Ventura County, California, in 1981, Sacramento,
California, California Department of Food and Agriculture (Unpublished
Report No. HS-861).
MAJEWSKI, T., PODGORSKI, W., BIALKOWSKI, Z., & TYCZKOWSKI, J.
(1978) [Effects of application of different forms of DDVP to
cows.] Rocz. Nauk. Zootech., 5(2): 65-74 (in Polish).
MAJEWSKI, T., PODGORSKI, W., & MICHALOWSKA, R. (1979) Retention
of dichlorvos (DDVP) in rabbits. Pol. Arch. Weter., 21(2): 249-255.
MASLINSKA, D. & ZALEWSKA, Z. (1978) Effect of dichlorvos administered
to the pregnant rabbits on the cholinesterase activity in the
progeny. Folia histochem. cytochem., 16(4): 335-341.
MASLINSKA, D., STROSZNAJDER, J., ZALEWSKA, T., & ORLEWSKI, P.
(1984) Phospholipid-protein ratio in brain of suckling rabbits treated
with an organophosphorus compound. Int. J. tissue React., 6(4): 317-
322.
MATHIAS, C.G.T. (1983) Persistent contact dermatitis from the
insecticide dichlorvos. Contact Dermatit., 9: 217-218.
MATSUMURA, F. & BOUSH, G.M. (1968) Degradation of insecticides by a
soil fungus Trichoderm viride. J. econ. Entomol., 61(3): 610-612.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
310-311.
MENDOZA, C.E. (1974) Analysis of pesticides by the thin-layer
chromatographic enzyme inhibition technique. Part II. Residue Rev., 50:
43-72.
MENDOZA, C.E. & SHIELDS, J.B. (1971) Esterase specificity and
sensitivity to organophosphorus and carbamate pesticides: factors
affecting determination by thin-layer chromatography. J. Assoc. Off.
Anal. Chem., 54: 507-512.
MENZ, M., LUETKEMEIER, H., & SACHSSE, K. (1974) Long-term exposure
of factory workers to dichlorvos (DDVP) insecticide. Arch. environ.
Health, 28: 72-76.
MESTRES, R., CHEVALLIER, CH., ESPINOZA, CL., & CORNET, R. (1977)
Application du couplage chromatographie gazeuse spectrométrie de masse
aux méthodes de recherche et de dosage des résidus de pesticides et de
micropolluants organiques dans les matériaux de l'environnement et les
matičres alimentaires. Ann. Fac. exp. Chim., 70(751): 177-188.
MESTRES, R., ILLES, S., CAMPO, M., & TOURTE, J. (1979a) Développement
de la méthode polyvalente d'analyse des résidus des pesticides dans les
plantes et les matičres alimentaires d'origine végétale. Trav. Soc.
Pharm. Montpellier, 39: 323-328.
MESTRES, R., ATMAWIJAYA, S., & CHEVALLIER, CH. (1979b) Méthode
de recherche et de dosage des résidus de pesticides dans les produits
céréaliers. I. Organochlorés, organophosphorés, pyréthrines, et
pyréthrinoďdes. Ann. Fac. exp. Chim., 72: 577-589.
MICHALEK, S.M. & BROCKMAN, H.E. (1969) A test for mutagenicity of
Shell "No-Pest Strip Insecticide" in Neurospora crassa. Neurospora
Newslett., 16: 8.
MICHEL, H.O. (1949) An electrometric method for the determination of
red blood cell and plasma cholinesterase activity. J. lab. clin.
Med., 34: 1564-1568.
MILES, J.W., FETZER, L.E., & PEARCE, G.W. (1970) Collection and
determination of trace quantities of pesticides in air. Environ. Sci.
Technol., 4: 420-425.
MITSUI, T., OZAKI, H., KUMANO, H., & SANO, H. (1963) Insecticide
determination. I. Colormetric determination of dimethyl 2,2-
dichlorovinyl phosphate (DDVP). Chem. pharm. Bull., 11(5): 619-623.
MIYAMOTO, J. (1959) Non-enzymatic conversion of Dipterex into DDVP
and their inhibitory action on enzymes. Botyu-Kagaku (Sci. pestic.
Control), 24: 130-137.
MODAK, A.T., STAVINOHA, W.B., & WEINTRAUB, S.T. (1975) Dichlorvos
and the cholinergic system: effects on cholinesterase and acetylcholine
and choline contents of rat tissues. Arch. int. Pharmacodyn., 217:
293-301.
MOHN, G. (1973) S-methyltryptophan resistance mutations in
Escherichia coli K12. Mutagenic activity of mono-functional alkylating
agents including organophosphorus insecticides. Mutat. Res., 20: 7-
15.
MORIYA, M., KATO, K., & SHIRASU, Y. (1978) Effects of cysteine and a
liver metabolic activation system on the activities of mutagenic
pesticides. Mutat. Res., 57: 259-263.
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MORPURGO, G., BELLINCAMPI, D., GUALANDI, G., BALDINELLI, L., &
SERLUPI CRESCENZI, O. (1979) Analysis of mitotic non-disjunction
with Aspergillus nidulans. Environ. Health Perspect., 31: 81-95.
MOUTSCHEN-DAHMEN, J., MOUTSCHEN-DAHMEN, M., &
DEGRAEVE, N. (1981) Metrifonate and dichlorvos: cytogenetic
investigations. Acta pharmacol. toxicol., 49(Suppl. 5): 29-39.
MULLER, G.H. (1970) Flea collar dermatitis in animals. J. Am. Vet.
Med. Assoc., 157(11): 1616-1626.
NAGY, ZS., MILE, I., & ANTONI, F. (1975) The mutagenic effect of
pesticides on Escherichia coli WP2 try-. Acta microbiol. Acad. Sci.
Hung., 22: 309-314.
NAIDU, N.V., REDDY, K.S., JANARDHAN, A., & MURTHY, M.K. (1978)
Toxicological investigation of dichlorvos in chicks. Indian J.
Pharmacol., 10(14): 323-326.
NAKAMURA, K. & SHIBA, H. (1980) [Study on pesticide residues in fruit
and vegetables.] Bull. Saitama prefect. agric. exp. Stn, 36: 35-56 (in
Japanese).
NARCISSE, J.K. (1967) A potentiation study in rats of VaponaR with
26 other cholinesterase-inhibiting compounds, Menlo Park, California,
Stanford Research Institute (Unpublished Report, 2 June).
NCI (1977) Bioassay of dichlorvos for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute.
NEUWIRTH, E.A. & WHITE, T.T. (1961) Safety aspects of DDVP. Soap
chem. Spec., 37(3): 95-98.
NICHOLAS, A.H., VIENNE, M., & BERGHE, H., VAN DEN (1978) Sister
chromatid exchange frequencies in cultured human cells exposed to an
organophosphorus insecticide: dichlorvos. Toxicol. Lett., 2(5): 271-
275.
NIOSH (1979) NIOSH Manual of analytical methods. Analytical method
No. P and CAM 295, 2nd ed., Cincinnati, Ohio, National Institute for
Occupational Safety and Health, Vol. 5 (Publication No. 79-144).
NISHIO, A. & UYEKI, E.M. (1981) Induction of sister chromatid
exchanges in Chinese hamster ovary cells by organophosphorus
insecticides and their oxygen analogs. J. Toxicol. environ. Health, 8:
939-946.
NISHIO, A. & UYEKI, E.M. (1982) Nuclease sensitivity of methylated
DNA as a probe for chromatin reconstitution by genotoxicants. Biochem.
biophys. Res. Commun., 107(2): 485-491.
NORDGREN, I., BERGSTROM, M., HOLMSTEDT, B., & SANDOZ, M.
(1978) Transformation and action of metrifonate. Arch. Toxicol., 41:
31-41.
NOWELL, P.T., SCOTT, C.A., & WILSON, A. (1962) Hydrolysis of
neostigmine by plasma-cholinesterase. Br. J. Pharmacol., 19: 498-502.
NTP (1986) Carcinogenicity study in rats and mice with
dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (Unpublished data).
NTP (1987) NTP technical report on the toxicology and carcinogenesis
of dichlorvos, Research Triangle Park, North Carolina, US National
Toxicology Program (NTP-TR342).
OBA, T. & KAWABATA, G. (1962) [Application of infrared absorption
spectroscopy to examination of drugs and their preparations. XIV.
Determination of O,O-dimethyl 2,2-dichlorovinyl phosphate (DDVP) and
its preparation.] Eiseishikenjo-hokoku, 80: 1-3 (in Japanese, with
English summary).
OGATA, H., SAITO, E., MURATA, H., SHIBAZAKI, T., & INOUE, T. (1975)
[A new colorimetric method of determination of O,O-dimethyl 2,2-
dichlorovinyl phosphate in insecticidal preparations.] Yakugaku
Zasshi, 95(12): 1483-1491 (in Japanese, with English summary).
OLINSKI, R., WALTER, Z., WIADERKIEWICZ, R., LUKASOVA, E., &
PALECEK, E. (1980) Changes in DNA properties due to treatment with
the pesticides malathion and DDVP. Radiat. environ. Biophys., 18: 65-
72.
OMKAR, & SHUKLA, G.S. (1984) Alteration in carbohydrate metabolism of
fresh-water prawn Macrobianchium lamarrei after dichlorvos
exposure. Ind. Health, 22: 133-136.
PACHECKA, J., SULINSKI, A., & TRACZYKIEWICZ, K. (1977) The effect
of acute intoxication by dichlorvos and trichlorphon on the activities
of some rat brain esterases. Neuropathol. Polska, 15(1): 85-92.
PAGE, A.C., DE VRIES, D.M., YOUNG, R., & LOEFFLER, J.E. (1971)
Metabolic fate of ingested dichlorvos in swine. Toxicol. appl.
Pharmacol., 19: 378.
PAGE, A.C., LOEFFLER, J.E., HENDRICKSON, H.R., HUSTON, C.K., &
DEVRIES, D.M. (1972) Metabolic fate of dichlorvos in swine. Arch.
Toxikol., 30: 19-27.
PAIK, S.G. & LEE, S.Y. (1977) Genetic effects of pesticides in the
mammalian cells. I. Induction of micronucleus. Korean J. Zool., 20(1):
19-28.
PENA CHAVARRIA, A., SWARTZWELDER, J.C., VILLAREJOS, V.M.,
KOTCHER, E., & ARGUEDAS, J. (1969) Dichlorvos: an effective broad-
spectrum anthelmintic. Am. J. trop. Med. Hyg., 18(6): 907-911.
PEROCCO, P. & FINI, A. (1980) Damage by dichlorvos of human
lymphocyte DNA. Tumori, 66: 425-430.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization (WHO Report
No. VBC/84.889).
PODREBARAC, D.S. (1984) Pesticide, heavy metals, and other chemical
residues in infant and toddler total diet samples. IV. October 1977 -
September 1978. J. Assoc. Off. Anal. Chem., 67(1): 166-175.
POTTER, J.C., LOEFFLER, J.E., COLLINS, R.D., YOUNG, R., & PAGE,
A.C. (1973a) Carbon-14 balance and residues of dichlorvos and its
metabolites in pigs dosed with dichlorvos-14C. J. agric. food
Chem., 21(2): 163-166.
POTTER, J.C., BOYER, A.C., MARXMILLER, R.E., YOUNG, R., &
LOEFFLER, J.E. (1973b). Radioisotope residues and residues of
dichlorvos and its metabolites in pregnant sows and their progeny dosed
with dichlorvos-14C or dichlorvos-36Cl formulated as PVC
pellets. J. agric. food Chem., 21(4): 734-738.
PURSHOTTAM, T. & KAVEESHWAR, U. (1979) Effect of diet on
dichlorovinyl dimethyl phosphate toxicity in rats. Aviat. Space
environ. Med., 50(6): 581-584.
PURSHOTTAM, T. & SRIVASTAVA, R.K. (1984) Effect of high-fat and
high-protein diets on toxicity of parathion and dichlorvos. Arch.
environ. Health, 39(6): 425-430.
PYM, R.A.E., SINGH, G., GILBERT, W.S., ARMSTRONG, J.P., &
MCCLEARY, B.V. (1984) Effects of dichlorvos, maldison, and
pirimiphos-methyl on food consumption, egg production, egg and tissue
residues, and plasma acetylcholinesterase inhibition in layer strain
hens. Aust. J. exp. Agric. anim. Husb., 24: 83-92.
QUARTERMAN, K.D., LOTTE, M., & SCHOOF, H.F. (1963) Initial field
studies in Upper Volta with dichlorvos residual fumigant as a malaria
eradication technique. Bull. World Health Organ., 29: 231-235.
RADELEFF, R.D. & WOODARD, G.T. (1957) The toxicity of organic
phosphorus insecticides to livestock. J. Am. Vet. Med. Assoc., 130:
215-216.
RAHMATULLAH, M., JAHAN, R., CHOWDHURY, A.A., FARUK, A.B.M.,
& CHOWDHURY, M.S.K. (1978) Effect of organophosphorus insecticides on
fermentation processes in Aspergillus niger. J. ferment. Technol., 56:
169-172.
RAMEL, C. (1981) Does dichlorvos constitute a genotoxic hazard? In:
Kappas, A., ed. Progress in environmental mutagenesis and
carcinogenesis, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 69-78 (Progress in Mutation Research, Vol. 2.).
RAMEL, C., DRAKE, J., & SUGIMURA, T. (1980) An evaluation of the
genetic toxicity of dichlorvos. Mutat. Res., 76: 297-309.
RASMUSSEN, W.A., JENSEN, J.A., STEIN, W.J., & HAYES, W.J. (1963)
Toxicological studies of DDVP for disinsection of aircraft. Aerosp.
Med., 34(7): 593-600.
RATH, S. & MISRA, B.N. (1979a) Relative toxicity of dichlorvos (DDVP)
to Tilapia mossambica Peters of 3 different age groups. Exp.
Gerontol., 14: 307-309.
RATH, S. & MISRA, B.N. (1979b) Sub-lethal effects of dichlorvos (DDVP)
on respiratory metabolism of Tilapia mossambica of 3 age groups. Exp.
Gerontol., 14: 37-41.
RATH, S. & MISRA, B.N. (1980) Pigment disperson in Tilapia
mossambica Peters exposed to dichlorvos (DDVP). Curr. Sci., 49(23):
907-909.
RATH, S. & MISRA, B.N. (1981) Toxicological effects of dichlorvos
(DDVP) on brain and liver acetylcholinesterase (AChE) activity
of Tilapia mossambica Peters. Toxicology, 19: 239-245.
RAUWS, A.G. & LOGTEN, M.J., VAN (1973) The influence of dichlorvos
from strips or sprays on cholinesterase activity in
chicken. Toxicology, 1: 29-41.
REECE, R.L. (1982) Observations on the accidental poisoning of birds
by organophosphate insecticides and other toxic substances. Vet.
Rec., 111(20): 453-455.
REEVES, J.D., DRIGGERS, D.A., & KILEY, V.A. (1981) Household
insecticide associated aplastic anaemia and acute leukaemia in
children. Lancet, 8 August: 300-301.
REINER, E. & PLESTINA, R. (1979) Regeneration of cholinesterase
activities in humans and rats after inhibition by O,O-dimethyl-2,2-
dichlorovinyl phosphate. Toxicol. appl. Pharmacol., 49: 451-454.
RIDER, J.A., MOELLER, H.C., & PULETTI, E.J. (1967) Continuing studies
on anticholinesterase effects of methyl parathion, initial studies with
Guthion, and determination of incipient toxicity level of dichlorvos in
humans. Fed. Proc., 26: 427.
RIDER, J.A., MOELLER, H.C., PULETTI, E.J., & SWADER, J. (1968)
Studies on the anticholinesterase effects of methyl parathion, guthion,
dichlorvos, and gardona in human subjects. Fed. Proc., 27: 597.
ROSENBERG, A., LIEBERMAN, M.T., & ALEXANDER, M. (1979)
Microbial degradation of pesticides, Springfield, Virginia, US
National Technical Information Service (PB 79-123156).
ROSENKRANZ, H.S. (1973) Preferential effect of dichlorvos
(VaponaR) on bacteria deficient in DNA polymerase. Cancer Res., 33:
458-459.
ROSENKRANZ, H.S. & LEIFER, Z. (1980) Determining the DNA-modifying
activity of chemicals using DNA polymerase-deficient Escherichia
coli. In: de Serres, F.J. & Hollander, A., ed. Chemical mutagens:
principles and methods for their detection, New York, Plenum Press,
Vol. 6, pp. 109-147.
ROSENKRANZ, H.S. & ROSENKRANZ, S. (1972) Reaction of DNA with
phosphoric acid esters: gasoline additive and insecticides.
Experientia (Basel), 28(4): 386-387.
SAGNER, G. & SCHONDUBE, M. (1982) [Determination and toxicological
appraisal of indoor concentrations of dichlorvos after the application
of spraying products.] Schriftenr. Ver. Wasser Boden Lufthyg., 53:
359-368 (in German).
SAKAMA, K. & NISHIMURA, M. (1977) [Studies on inhalation toxicity of
organic phosphorus insecticides. II. Approach to establishment of
threshold limit value.] Jpn. J. ind. Health, 19: 357-358 (in
Japanese).
SASINOVICH, L.M. (1968) [Substantiation of the maximum permissible
concentration of DDVP in the air of the working zone.] Gig. i Sanit.,
33(12): 35-39 (in Russian).
SASINOVICH, L.M. (1970) [Toxicology of dimethyldichlorovinyl
phosphate and hygienic characteristics of its use.] Gig. Primen.
Toksikol. Pestits. Klin. Otravl., 8: 297-307 (in Russian).
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical, and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHAFER, E.W., Jr & BRUNTON, R.B. (1979) Indicator bird species for
toxicity determinations: is the technique usable in test method
development? In: Beck, J.R., ed. Vertebrate pest control and management
materials, Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 157-168 (ASTM STP 680).
SCHMIDT, G., SCHMIDT, M., & NENNER, M. (1975) Inhibition of
acetylcholinesterase in the bronchial system of rats caused by
inhalation of dichlorvos (DDVP). Naunyn Schmiedeberg Arch.
Pharmacol., 287(Suppl.): R97.
SCHMIDT, G., SCHMIDT, M., NENNER, M., & VETTERLEIN, F. (1979)
Effects of dichlorvos (DDVP) inhalation on the activity of
acetylcholinesterase in the bronchial tissue of rats. Arch.
Toxicol., 42: 191-198.
SCHOOF, H.F., JENSEN, J.A., PORTER, J.E., & MADDOCK, D.R. (1961)
Disinsection of aircraft with a mechanical dispenser of DDVP
vapour. Bull. World Health Organ., 24: 623-628.
SCHULTZ, D.R., MARXMILLER, R.L., & KOOS, B.A. (1971) Residue
determination of dichlorvos and related metabolites in animal tissue
and fluids. J. agric. food Chem., 19(6): 1238-1243.
SCHULZE, H.D. (1979) [Current problems of pest control with
dichlorvos in hospital.] Z. gesamte Hyg., 25(5): 421-425 (in German).
SCHWETZ, B.A., IOSET, H.D., LEONG, B.K.J., & STAPLES, R.E. (1979)
Teratogenic potential of dichlorvos given by inhalation and gavage to
mice and rabbits. Teratology, 20: 383-388.
SEGERBACK, D. (1981) Estimation of genetic risks of alkylating
agents. V. Methylation of DNA in the mouse by DDVP (2,2-dichlorovinyl
dimethylphosphate). Hereditas, 94: 73-76.
SEGERBACK, D. & EHRENBERG, L. (1981) Alkylating properties of
dichlorvos (DDVP). Acta pharmacol. toxicol., 49(Suppl. 5): 56-66.
SERA, K., MATSUNAGA, A., MURAKAMI, A., SATO, I., YAMASHITA,
K., & YOSHIMORI, H. (1959) Qualitative analysis of organic phosphorus
color reactions and simple detection. Kumamoto med. J., 12(3): 193-
213.
SHAFIK, M.T., BRADWAY, D., & ENOS, H.F. (1971) A method for
confirmation of organophosphorus compounds at the residue level.
Bull. environ. Contam. Toxicol., 6(1): 55-66.
SHELLENBERGER, T.E. (1980) Organophosphorus pesticide inhibition of
cholinesterase in laboratory animals and man and effects of oxime
reactivators. J. environ. Sci. Health, B15(6): 795-822.
SHELLENBERGER, T.E., NEWELL, G.W., OKAMOTO, S.S., & SARROS, A.
(1965) Response of rabbit whole blood cholinesterase in vivo after
continuous intravenous infusion and percutaneous application of
dimethyl organophosphate inhibitors. Biochem. Pharmacol., 14: 943-
952.
SHERMAN, M. & ROSS, E. (1961) Acute and subacute toxicity of
insecticides to chicks. Toxicol. appl. Pharmacol., 3: 521-533.
SHINKAJI, N. & ADACHI, T. (1978) [The effect of certain pesticides on
the predaceous mite Amblyseius longispinosus (Evans) (Acarina:
Phytoseiidae).] Akitsu, 2: 99-108 (in Japanese, with English tables).
SHINODA, H., ITO, K., MATSUNAGA, T., TAKADA, T., & NOMURA, T.
(1972) Two suicides by acute pesticide intoxication. J. Jpn. Assoc.
Rural Med., 21: 242-249 (in Japanese).
SHIRASU, Y., MORIYA, M., KATO, K., FURUHASHI, A., & KADA, T.
(1976) Mutagenicity screening of pesticides in the microbial
system. Mutat. Res., 40: 19-30.
SHOOTER, K.V. (1975) Assays for phosphotriester formation in the
reaction of bacteriophage R17 with a group of alkylating
agents. Chem.-biol. Interact., 11: 575-588.
SLOMKA, M.B. & HINE, C.H. (1981) Clinical pharmacology of
dichlorvos. Acta pharmacol. toxicol., 49(Suppl. 5): 105-108.
SMITH, P.W., MERTENS, H., LEWIS, M.F., FUNKHOUSER, G.E., HIGGINS,
E.A., CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & FLUX, M. (1972)
Toxicology of dichlorvos at operational aircraft cabin altitudes.
Aerosp. Med., 43(5): 473-478.
SNOW, D.H. & WATSON, A.D.J. (1973) The acute toxicity of dichlorvos
in the dog. I. Clinical observations and clinical pathology. Aust. vet.
J., 49: 113-119.
SOBELS, F.H. & TODD, N.K. (1979) Absence of a mutagenic effect of
dichlorvos on Drosophila melanogaster. Mutat. Res., 67: 89-92.
STANTON, H.C., ALBERT, J.R., & MERSMANN, H.J. (1979) Studies on the
pharmacology and safety of dichlorvos in pigs and pregnant sows. Am. J.
vet. Res., 40(3): 315-320.
STAVINOHA, W.B., MODAK, A.T., & WEINTRAUB, S.T. (1976) Rate of
accumulation of acetylcholine in discrete regions of the rat brain
after dichlorvos treatment. J. Neurochem., 27: 1375-1378.
STEIN, W.J., MILLER, S., & FETZER, L.E., Jr (1966) Studies with
dichlorvos residual fumigant as a malaria eradication technique in
Haiti. III. Toxicological studies. Am. J. trop. Med. Hyg., 15(5): 672-
675.
STERNBERG, S.S. (1979) The carcinogenesis, mutagenesis, and
teratogenesis of insecticides. Review of studies in animals and
man. Pharmacol. Ther., 6: 147-166.
STEVENSON, D.E. & BLAIR, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0024.69).
STEVENSON, D.E. & BLAIR, D. (1977) The uptake of dichlorvos during
long-term inhalation studies. Proc. Eur. Soc. Toxicol., 18: 215-217.
STOERMER, D. (1985) [Occupational contact dermatitis from the
pesticide dichlorvos.] Dermatol. Monatsschr., 171(4): 245-249 (in
German).
TEICHERT, K., SZYMCZYK, T., CONSOLO, S., & LADINSKY, H. (1976)
Effect of acute and chronic treatment with dichlorvos on rat brain
cholinergic parameters. Toxicol. appl. Pharmacol., 35: 77-81.
TERAYAMA, K., HONMA, H., & KAWARABAYASHI, T. (1978) Toxicity of
heavy metals and insecticides on slime mold Physarum polycephalum. J.
toxicol. Sci., 3: 293-304.
TESSARI, J.D. & SPENCER, D.L. (1971) Air sampling for pesticides in the
human environment. J. Assoc. Off. Anal. Chem., 54(6): 1376-1382.
TEZUKA, H., ANDO, N., SUZUKI, R., TERAHATA, M., MORIYA, M., &
SHIRASU, Y. (1980) Sister chromatid exchanges and chromosomal
aberrations in cultured Chinese hamster cells treated with pesticides
positive in microbial reversion assays. Mutat. Res., 78: 177-191.
THOMAS, T.C. & NISHIOKA, Y.A. (1985) Sampling of airborn pesticides
using chromosorb-102. Bull. environ. Contam. Toxicol., 35: 460-465.
THORPE, E., WILSON, A.B., DIX, K.M., & BLAIR, D. (1972) Teratological
studies with dichlorvos vapour in rabbits and rats. Arch. Toxicol.,
30(1): 29-38.
TIMMONS, E.M., CHAKLOS, R.J., BANNISTER, T.M., & KAPLAN, H.M.
(1975) Dichlorvos effects on estrous cycle onset in the rat. Lab.
anim. Sci., 25(1): 45-47.
TRACY, R.L., WOODCOCK, J.G., & CHODROFF, S. (1960) Toxicological
aspects of 2,2'-dichlorovinyl dimethylphosphate (DDVP) in cows,
horses, and white rats. J. econ. Entomol., 53(4): 593-601.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 43 pp (Resource Publication No. 84).
UEDA, K., SHIYO, K., MORI, T., KITAHARA, E., & URAGAMI, S. (1959)
[Effects of DDVP on man.] J. Jpn. Public Hyg. Assoc., 6: 315 (in
Japanese).
UEDA, K., SHIYO, K., IIZUKA, Y., KITAHARA, E., & OHASHI, A. (1960)
[The toxicity of organic phosphate "DDVP" for small animals and
man.] Igaku Seibutsugaku (Med. Biol.), 57(3): 98-101 (in Japanese).
ULLMANN, L., PHILLIPS, J., & SACHSSE, K. (1979) Cholinesterase
surveillance of aerial applicators and allied workers in the Democratic
Republic of the Sudan. Arch. environ. Contam. Toxicol., 8: 703-712.
VADHVA, P. & HASAN, M. (1986) Organophosphate dichlorvos induced
dose-related differential alterations in lipid levels and lipid
peroxidation in various regions of the fish brain and spinal cord. J.
environ. Sci. Health, B21(5): 413-424.
VAN DIJK, L.P. & VISWESWARIAH, K. (1975) Pesticides in air: sampling
methods. Residue Rev., 55: 91-134.
VAN RAALTE, H.G.S. & JANSEN, J.D. (1981) Household insecticides and
the blood. Lancet, 10 October: 811.
VASHKOV, V.I., VOLKOVA, A.P., TSETLIN, V.M., & YANKOVSKII, E.Y.
(1966) [Assessment of the use of dimethyldichlorovinyl phosphate
(DDVP) in the insecticide mixture.] Gig. i Sanit., 9: 15-17 (in
Russian).
VASILESCU, C. & FLORESCU, A. (1980) Clinical and electro-
physiological study of neuropathy after organophosphorus compounds
poisoning. Arch. Toxicol., 43: 305-315.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination of
water by a sublethal concentration of pesticides. A system analysis
approach. Acta hydrochim. hydrobiol., 12(4): 399-499.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1980) Effects of
the pesticides thiotox, dichlorvos, and carbofuran on the test
fish Mystus vittatus. Water Air Soil Pollut., 13(2): 229-234.
VERMA, S.R., RANI, S., BANSAL, S.K., & DALELA, R.C. (1981a)
Evaluation of the comparative toxicity of thiotox, dichlorvos, and
carbofuran to two fresh water teleosts Ophiocephalus punctatus and
Mystus vittatus. Acta hydrochem. hydrobiol., 9(2): 119-129.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981b) Isolated and combined
effects of pesticides on serum transaminases in Mystus
vittatus (African catfish). Toxicol. Lett., 8: 67-71.
VERMA, S.R., RANI, S., & DALELA, R.C. (1981c) Pesticide-induced
physiological alterations in certain tissues of a fish Mystus vittatus.
Toxicol. Lett., 9: 327-332.
VERMA, S.R., TONK, I.P., & DALELA, R.C. (1981d) Determination of the
maximum acceptable toxicant concentration (MATC) and the safe
concentration for certain aquatic pollutants. Acta hydrochim.
hydrobiol., 9(3): 247-254.
VERMA, S.R., BANSAL, S.K., GUPTA, A.K., PAL, N., TYAGI, A.K.,
BHATNAGAR, M.C., KUMAR, V., & DALELA, R.C. (1982a) Bioassay trials
with twenty-three pesticides to a fresh water teleost Saccobranchus
fossilis. Water Res., 16: 525-529.
VERMA, S.R., RANI, S., & DALELA, R.C. (1982b) Indicators of stress
induced by pesticides in Mystus vittatus: haematological
parameters. Indian J. environ. Health, 24(1): 58-64.
VERMA, S.R., RANI, S., TONK, I.P., & DALELA, R.C. (1983) Pesticide-
induced dysfunction in carbohydrate metabolism in three fresh water
fishes. Environ. Res., 32: 127-133.
VERMA, S.R., RANI, S., & DALELA, R.C. (1984) Effects of pesticides
and their combinations on three serum phosphatases of Mystus vittatus.
Water Air Soil Pollut., 21: 9-14.
VOGIN, E.E., CARSON, S., & SLOMKA, M.B. (1971) Teratology studies
with dichlorvos in rabbits. Toxicol. appl. Pharmacol., 19: 377-378.
VOOGD, C.E., JACOBS, J.J.J.A.A., & STEL, J.J., VAN DER (1972) On the
mutagenic action of dichlorvos. Mutat. Res., 16: 413-416.
VOSS, G. & SACHSSE, K. (1970) Red cell and plasma cholinesterase
activities in microsamples of human and animal blood determined
simultaneously by a modified acetylthiocholine/DNTB procedure.
Toxicol. appl. Pharmacol., 16: 764-772.
VRBOVSKY, L., SELECKY, FR.V., & ROSIVAL, L. (1959) [Toxicological and
pharmacological studies with phosphorester insecticides.] Naunyn
Schmiedeberg Arch. Pharmacol., 236: 202-204 (in German).
WADIA, R.S., SHINDE, S.N., & VAIDYA, (1985) Delayed neurotoxicity
after an episode of poisoning with dichlorvos. Neurology (India), 33:
247-253.
WAGNER, R. & HOYER, J. (1975) [Method of determining workplace
concentrations and on occupational hygiene conditions during thermal
fogging atomization of pesticides in the greenhouse.] Z. gesamte
Hyg., 21(1): 18-20 (in German).
WALKER, A.I.T., BLAIR, D., STEVENSON, D.E., & CHAMBERS, P.L.
(1972) An inhalational toxicity study with dichlorvos. Arch.
Toxikol., 30: 1-7.
WARD, F.P. & GLICKSBERG, C.L. (1971) Effects of dichlorvos on blood
cholinesterase activity in dogs. J. Am. Vet. Med. Assoc., 158(4): 457-
461.
WATANABE, H., SHIRAI, O., & EBATA, N. (1976) A case report of
organophosphorus insecticide (DDVP) intoxication with respiratory
intensive care. Pestic. Abstr., 9(2): 94-95.
WEISBURGER, E.K. (1982) Carcinogenicity tests on pesticides. In:
Chambers, J.E. & Yarbrough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 165-176.
WENNERBERG, R. & LOFROTH, G. (1974) Formation of 7-methyl- guanine
by dichlorvos in bacteria and mice. Chem.-biol. Interact., 8: 339-348.
WHITEHEAD, C.C. (1971) The effects of pesticides on production in
poultry. Vet. Rec., 30 January: 114-117.
WHO (1978) Spectrophotometric kit for measuring cholinesterase
activity, Geneva, World Health Organization (Report VBC/78.692).
WHO/FAO (1975-86) Data sheets on pesticides, Geneva, World Health
Organization (VBC).
WHO (1985) Technical dichlorvos. In: Specifications for pesticides
used in public health, 6th ed., Geneva, World Health Organization, pp.
163-174 (WHO/SIT/16.R2).
WHO (1986a) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1986-87, Geneva, World Health
Organization, p. 11 (Report VBC/86.1 Rev.1)
WHO (1986b) Environmental Health Criteria 63: organophosphorus
insecticides: a general introduction, Geneva, World Health
Organization, 181 pp.
WILD, D. (1973) Chemical induction of streptomycin-resistant
mutations in Escherichia coli. Dose and mutagenic effects of dichlorvos
and methyl methanesulfonate. Mutat. Res., 19: 33-41.
WILD, D. (1975) Mutagenicity studies on organophosphorus
insecticides. Mutat. Res., 32: 133-150.
WILDEMAUWE, C., LONTIE, J.F., SCHOOFS, L., & LAREBEKE, N., VAN
(1983) The mutagenicity in procaryotes of insecticides, acaricides,
and nematicides. Residue Rev., 89: 129-178.
WILLIAMS, P.P. (1977) Metabolism of synthetic organic pesticides by
anaerobic microorganisms. Residue Rev., 66: 102.
WILSON, A.B. & DIX, K.M. (1973) Toxicity studies on dichlorvos: one
month inhalational exposure of rats to dichloroacetaldehyde,
Sittingbourne, Shell Research Ltd (Unpublished Report TLGR.0017.73).
WITHERUP, S., STEMMER, K.L., & CALDWELL, J.S. (1964) The effects
upon rats of being fed on diets containing VaponaR insecticide,
Cincinnati, Ohio, The Kettering Laboratory (Unpublished Report, 1
July).
WITHERUP, S., CALDWELL, J.S., & HULL, L. (1965) The effects exerted
upon the fertility of rats and upon the viability of their offspring by
the introduction of VaponaR insecticide into their diets, Cincinatti,
Ohio, The Kettering Laboratory (Unpublished Report, 12 April).
WITHERUP, S., STEMMER, K.L., & PFITZER, E.A. (1967) The effects
exerted upon rats during a period of two years by the introduction of
VaponaR insecticide into their daily diets, Cincinnati, Ohio, The
Kettering Laboratory (Unpublished Report, 14 February).
WITHERUP, S., JOLLEY, W.J., STEMMER, K., & PFITZER, E.A. (1971)
Chronic toxicity studies with 2,2-dichlorovinyl dimethyl phosphate
(DDVP) in dogs and rats including observations on rat reproduction.
Toxicol. appl. Pharmacol., 19: 377.
WITTER, R.F. (1960) Effects of DDVP aerosols on blood cholinesterase
of fogging machine operators. Am. Med. Assoc. Arch. Ind. Health, 21:
7-9.
WITTER, R.F., GAINES, T.B., SHORT, J.G., SEDLAK, V.A., & MADDOCK,
D.R. (1961) Studies on the safety of DDVP for the disinsection of
commercial aircraft. Bull. World Health Organ., 24: 635-642.
WOOD, A.B. & KANAGASABAPATHY, L. (1983) Evaluation of inexpensive
thin layer chromatographic procedures for the estimation of some
organophosphorus and carbamate insecticide residues in fruit and
vegetables. Pestic. Sci., 14: 108-118.
WOODER, M.F. & CREEDY, C.L. (1979) Studies on the effects of
dichlorvos on the integrity of rat liver DNA in vivo, Sittingbourne,
Shell Research Ltd (Unpublished Report TLGR.79.089).
WOODER, M.F. & WRIGHT, A.S. (1981) Alkylation of DNA by organo-
phosphorus pesticides. Acta pharmacol. toxicol., 49(Suppl. 5): 51-55.
WOODER, M.F., WRIGHT, A.S., & KING, L.J. (1977) I n vivo alkylation
studies with dichlorvos at practical use concentrations. Chem.-biol.
Interact., 19: 25-46.
WOODER, M.F., WRIGHT, A.S., & STEVENSON, D.E. (1978) Studies on
the in vivo reactivity of methylating agents for mammalian DNA in
relation to the assessment of genetic risk. Proc. Int. Congr.
Toxicol., 1: 505.
WORTHING, C.R. & WALKER, S.B. (1983) Pesticide manual, 7th ed.,
Croydon, British Crop Protection Council.
WRATHALL, A.E., WELLS, D.E., & ANDERSON, P.H. (1980) Effect of
feeding dichlorvos to sows in mid-pregnancy. Zbl. Vet. Med. A27: 662-
668.
WRIGHT, C.G. & LEIDY, R.B. (1980) Air samples in vehicles and
buildings turn up only very low levels of organic phosphate
insecticides. Pest Control, 48(7): 22-26, 68.
WRIGHT, A.S., HUTSON, D.H., & WOODER, M.F. (1979) The chemical and
biochemical reactivity of dichlorvos. Arch. Toxicol., 42: 1-18.
XING-SHU, H. (1983) Preventing chemical damage to germ cells. Am.
Ind. Hyg. Assoc. J., 44: 699-703.
YAMANE, S., KINO, A., & TESHIMA, S. (1974) Histochemical
demonstration of cholinesterase activity in tissues of the carp
and effect of DDVP on its activity in situ. Acta histochem.
cytochem., 7(2): 167-174.
YAMANOI, F. (1980) [Effect of insecticides on the progeny in the
silkworm Bombyx mori. I. Effect of organophosphorus insecticides on egg
laying and their hatching.] Nippon Sanshigaku Zasshi, 49(5): 434-439
(in Japanese).
YAMASHITA, K. (1960) Studies on organic phosphorus. I. Toxicity of
dipterex and its vinyl derivative (DDVP). Kumamoto med. J., 13(4): 273-
279.
YAMASHITA, K. (1961) Studies on organic phosphorus dipterex. III.
Detection and discrimination of dipterex and its vinyl derivative
(DDVP). Kumamoto med. J., 14(1): 13-25.
YAMASHITA, K. (1962) Toxicity of dipterex and its vinyl derivative
(DDVP). Ind. Med. Surg., 31: 170-173.
ZALEWSKA, Z., RAKOWSKA, I., MATRASZEK, G., & SITKIEWICZ, D.
(1977) Effect of dichlorvos on some enzyme activities of the rat brain
during postnatal development. I. Cholinesterases. Neuropathol.
Polska, 15(2): 255-262.
ZAVON, M.R. & KINDEL, E.A., Jr (1966) Potential hazard in using
dichlorvos insecticide resin. Adv. Chem. Ser., 60: 177-186.
ZELMAN, I.B. (1977) [Pathomorphology on the rat brain after
experimental intoxication with the phosphororganic pesticide dichlorvos
(DDVP).] Neuropathol. Polska, 15(4): 515-522 (in Polish).
ZELMAN, I.B. & MAJDECKI, T. (1979) [Ultrastructural changes in rat
brain following organophosphate insecticide dichlorvos (DDVP)
intoxication.] Neuropathol. Polska, 17(3): 443-453 (in Polish).
ZOTOV, V.M., SVIRIN, J.N., & PRUCAKOVA, R.M. (1977) [The develop-
ment of health regulations for occupational exposure in greenhouses
treated with chemical pesticides.] Gig. Tr. prof. Zabol., 3: 49-50 (in
Russian).
