
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 14
HEPTACHLOR
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA
This is a companion volume to Environmental Health Criteria 38:
Heptachlor
This report contains the collective views of an international group
of experts and does not necessarily represent the decisions or the
stated policy of the United Nations Environment Programme, the
International Labour Organization, or the World Health Organization
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the
United Nations Environment Programme, the International Labour
Organisation, and the World Health Organization)
ISBN 92 4 154334 5
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Heptachlor toxicity
2.2. Human exposure to heptachlor
2.3. Evaluation of effects on the environment
3. CONCLUSIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Advice to physicians
4.1.1.1 Symptoms of poisoning
4.1.1.2 Medical advice
4.2. Health surveillance advice
4.3. Safety in use
4.4. Explosion and fire hazards
4.4.1. Explosion hazards
4.4.2. Fire hazards
4.5. Storage
4.5.1. Leaking containers in store
4.6. Transport
4.7. Spillage and disposal
4.7.1. Spillage
4.7.1.1 Solid products
4.7.1.2 Liquid products
4.7.1.3 All products
4.7.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. INTERNATIONAL CHEMICAL SAFETY CARD
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging, and transport
7.5. Waste disposal
7.6. Other measures
BIBLIOGRAPHY
INTRODUCTION
The International Programme on Chemical Safety prepares for
publication a series of Environmental Health Criteria (EHC)
documents, each of which includes an assessment of the effects on
human health and the environment of exposure to a specific chemical,
or group of chemicals, and gives guidelines for setting exposure
limits. The Health and Safety Guides complement the criteria
documents and are intended to facilitate the application of the
guidelines in national chemical safety programmes.
The first three sections of each Health and Safety Guide highlight
the relevant technical information from the corresponding EHC
document. Section 4 includes advice on preventive and protective
measures and action to be taken in an emergency. All health staff
should be thoroughly familiar with this information to ensure that
they can act rapidly and efficiently in an emergency. Hazards for
the environment and their prevention are discussed in section 5.
Each Guide indicates the information to be included in an
International Chemical Safety Card, which should be prominently
displayed in all areas where there is a possibility of exposure to
the chemical(s). The information included in the final section on
current national regulations and standards has been obtained from
the International Register of Potentially Toxic Chemicals (IRPTC)
and from other United Nations sources.
The target readership for the Health and Safety Guides includes the
staff of occupational health services and government ministries and
agencies, and personnel in industry and the trade unions who are
concerned with the safe use of chemicals and the avoidance of
environmental health hazards. The information on the prevention of,
and protection against, accidents will be of vital interest to all
workers who are involved in the production and handling of toxic
chemicals. A bibliography has been included for readers who require
further background information.
The information in this Guide will be revised in due course, and the
eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using this Guide would
be very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING
POINT TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY, PRODUCTION, AND USES
1.1 Identity
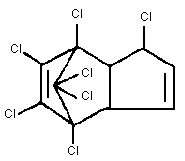
Common name: Heptachlor
Chemical formula: C10H5Cl7
Chemical structure:
 Common trade names: Aahepta, Agroceres, Basaklor, Drinox,
Eptacloro, E 3314, GPKh,
Heptachlorane, Heptagran, Heptagranox,
Heptamak, Heptamul, Heptasol, Heptox,
Rhodiachlor, Soleptax, Velsicol 104
CAS chemical name: 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-
tetra-hydro-4,7-methano-1H-indene
CAS registry number: 76-44-8
RTECS registry number: PC0700000
Relative molecular mass: 373.3
1.2 Physical and Chemical Properties
Heptachlor is a white crystalline solid with a mild odour of
camphor, a melting point of 93°C (46-74°C for the technical
product), and a density of 1.65-1.67 g/ml at 25°C. It has a boiling
point of 135-145°C and vapour pressure of 4 x 10-4 mmHg at 25°C.
It is virtually insoluble in water (0.056 mg/litre) but fairly
soluble in organic solvents, e.g., ethanol (45 g/litre), xylene
(1020 g/litre), acetone (750 g/litre), and benzene (1060 g/litre).
It is stable in daylight, air, moisture, and moderate heat (160°C)
but is oxidized biologically to heptachlor epoxide (Whetstone,
1964).
Technical heptachlor contains about 72-74% 1,4,5,6,7,8,8-
heptachloro-3a,4,7,7a-tetrahydro-4,7-methano-1H-indene, 20-22%
gamma-chlordane, and 4-8% gamma-nonachlor.
1.3 Analytical Methods
Extraction from crops, other plant products, dairy products and oils
can be achieved with hexane-acetone or acetonitrile.
The method of choice for the qualitative and quantitative
determination of heptachlor is gas-liquid chromatography with
electron capture detection.
1.4 Production and Uses
Heptachlor was isolated in 1946 from technical chlordane.
Production of heptachlor in the USA in 1971 was estimated to be 2.7
million kg. In 1970, the use of heptachlor throughout the world was
as follows: Africa 5%, Asia 15%, Canada and the USA 5%, Europe 60%,
and South America 15%. Recently, the use of heptachlor has been
increasingly restricted in many countries (section 7.3).
Formulations include emulsifiable concentrates, wettable powders,
dusts, and granules, containing various concentrations of active
material.
Heptachlor was first introduced as a contact insecticide in the USA
in 1952 for foliar, soil and structural application, and for the
control of malaria. It is a non-systemic stomach and contact
insecticide. The use of heptachlor is now confined almost
exclusively to the control of soil insects and termites.
2. SUMMARY AND EVALUATION
2.1 Heptachlor Toxicity
Heptachlor is readily absorbed following ingestion and skin contact
and is transported throughout the body. Heptachlor epoxide, the most
persistent metabolite, is rapidly formed and can be found in the
body, mainly in adipose tissue. The toxicity of heptachlor epoxide
is similar to that of heptachlor. Elimination takes place via both
the urine and faeces. Human milk can be a major excretion route for
heptachlor residues.
The acute toxicity of heptachlor in the rat ranges from 40 to
162 mg/kg body weight. Toxic symptoms are related to CNS-hyperactivity
and include tremors and convulsions. In experimental animals,
prolonged low-level exposure results in the induction of hepatic
microsomal enzymes and, at a later stage, in liver hypertrophy with
histological changes. At higher levels, heptachlor is hepatotoxic.
Heptachlor was not a teratogen in the experimental animal studies
conducted, but at higher exposure levels it may interfere with
reproduction and the viability of the offspring.
Heptachlor was not generally active in short-term tests for genetic
activity. There was evidence that it might have an effect on cell to
cell communication in vitro, a characteristic property of many
promoting agents.
There is limited evidence for the carcinogenicity of heptachlor and
heptachlor epoxide in experimental animals.
No cases of adverse effects in man or occupational poisoning have
been reported.
2.2 Human Exposure to Heptachlor
Food is the major source of exposure to heptachlor for the general
population, but residue intake in most countries is below the
advised acceptable daily intake. In areas where heptachlor is used,
the inhalation of dust or mist and the drinking of well-water may
account for some additional exposure.
Relatively high concentrations of heptachlor epoxide can be found in
human milk, especially in areas with high heptachlor exposure in the
general population.
Occupational exposure, especially via the skin and via inhalation,
can be considerable when the material is handled in installations or
situations with insufficient safety precautions.
2.3 Evaluation of Effects on the Environment
Heptachlor is persistent and relatively immobile in soil. However,
it may be lost from the soil by slow vaporization, by oxidation to
heptachlor epoxide (a more persistent degradation product of
comparable toxicity), by photoconversion to photo-heptachlor, or by
conversion to less toxic metabolites by soil bacteria. The rate at
which heptachlor is lost by these different mechanisms is influenced
by climate, soil type, and management practices (retention being
longest in undisturbed soil). The majority of heptachlor residues
are found in the top few centimetres of soil and are most likely to
be spread with dust particles by air currents.
Although there is no indication of widespread contamination of water
by heptachlor, its residues have been found in fish from various
bodies of water. Heptachlor is not very soluble in water and
persists in aquatic ecosystems by being adsorbed on sediments. It
has been shown to be toxic for aquatic life, but its toxicity varies
considerably with species. This is particularly so in marine
vertebrates, where acute LC50 values span three orders of
magnitude. Marine crustacea are particularly sensitive to
heptachlor; a concentration of 0.03 µg/litre may be lethal. Younger
life stages of both fish and invertebrates are the most sensitive to
heptachlor, "safe" concentrations being 0.1 and 0.01 µg/litre,
respectively.
Evaluation of the toxicity of heptachlor for wildlife depends solely
on extrapolation from studies on game birds and domestic species. In
these animals, toxicity is variable, with LD50 values ranging from
6 to 531 mg/kg body weight. Heptachlor is generally classified as a
neurotoxin.
Uptake of heptachlor in animals is fairly rapid. It is rapidly
metabolized to heptachlor epoxide, which can persist for extended
periods of time in body fat. The relative amounts of heptachlor
epoxide in tissues increase with length of exposure. Few data are
available on the toxicity of this metabolite, but indications are
that it is comparable to that of heptachlor.
Bioaccumulation and biomagnification occur and bioconcentration
factors of 200-37 000 have been reported from water into hydrobiota.
The marked persistence of heptachlor in the environment and its
tendency to accumulate in body fat make it a serious environmental
hazard.
3. CONCLUSIONS
Although there is no evidence that heptachlor is a human carcinogen,
the suspicion, principally arising from the mouse carcinogenicity
studies, cannot be ignored. Further research is required to look
into this problem. Nevertheless, with the present state of
knowledge, it is concluded that:
(a) As long as occupational hygiene procedures are maintained to
keep exposure levels to a minimum, whether or not by the
imposition of maximum allowable concentrations, there is little
reason to believe that workers will be at risk from handling,
or contact with, heptachlor.
(b) Members of the general population should not suffer any adverse
effects from heptachlor residues in food, provided that intake
is kept within the ADI set by the FAO/WHO Joint Meeting.
In certain regions of the world, the exposure of the general
population to heptachlor may be increased if heptachlor is used
in buildings for the control of termites.
The intake of heptachlor residues by breast-fed infants,
through human milk, in areas of high heptachlor use, remains a
concern.
(c) Environmentally, heptachlor causes concern because several
marine species are highly sensitive to it and because of the
persistence of the metabolite heptachlor epoxide in adipose
tissue and in the environment.
From: Environmental Health Criteria 38: Heptachlor
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Heptachlor is an organochlorine insecticide. It is toxic and can be
hazardous for human beings if incorrectly or carelessly handled. It
is therefore essential that the correct precautions are observed
during handling and use.
For details see the International Chemical Safety Card (pages 20-23).
4.1.1 Advice to Physicians
4.1.1.1 Symptoms of poisoning
Heptachlor is toxic by mouth, by skin contact (especially liquid
formulations) and by inhalation of dust from powder concentrates. It
acts as a stimulant of the central nervous system.
Following accidental ingestion or over-exposure, symptoms may
include headache, dizziness, nausea, vomiting, weakness in legs, and
convulsions.
4.1.1.2 Medical advice
Medical treatment is largely symptomatic and supportive and directed
against convulsions and anoxaemia. If swallowed, vomiting should not
be induced and emetics are contraindicated, because many liquid
formulations contain hydrocarbons and there is risk of aspiration
pneumonia. Instead, the stomach should be emptied as soon as
possible by careful gastric lavage, using a cuffed endotracheal tube
to avoid aspiration into the lungs. This should be followed by
intragastric administration of 3-4 tablespoons of activated charcoal
and 30 g of magnesium or sodium sulfate in a 30% aqueous solution.
Oily purgatives are contraindicated. No fats, oils, or milk should
be given.
If convulsions occur, anticonvulsants should be given, e.g.,
diazepam 10 mg, slowly, intravenously (children 1-5 mg), repeated as
necessary; or thiopental sodium, or hexobarbital sodium slowly,
intravenously, in a dose of 10 mg/kg body weight with a maximum
total dose of up to 750 mg for an adult, or paraldehyde at 5 ml by
injection. These short-acting anticonvulsants should always be
followed by phenobarbital given orally at 3 mg/kg body weight (up to
200 mg for anadult), or phenobarbital sodium given intramuscularly
at 3 mg/kg body weight (also up to 200 mg for an adult).
Morphine and its derivatives, epinephrine and noradrenaline should
never be given.
An unobstructed airway must be maintained. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
4.2 Health Surveillance Advice
A complete medical history should be taken, and physical examination
made, annually. Special attention should be paid to liver and kidney
function.
4.3 Safety in Use
Handling liquid formulations: Wear protective neoprene or PVC
gloves, cotton overalls, rubber
boots, and face shield.
Handling powder formulations: Avoid raising a dust cloud. Wear
protective neoprene or PVC
gloves, cotton overalls, and dust
mask.
4.4 Explosion and Fire Hazards
4.4.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation, or on the characteristics of the dust.
4.4.2 Fire hazards
Liquid formulations containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder.
With sufficient burning or external heat, heptachlor will decompose
emitting toxic fumes. Fire-fighters should wear self-contained
breathing apparatus, eye protection, and full protective clothing.
The use of water spray should be confined to the cooling of
unaffected stock, to avoid the accumulation of polluted run-off from
the site.
4.5 Storage
Products should be stored in locked buildings, preferably dedicated
to the storage of insecticides.
Products should be kept out of reach of children and unauthorized
personnel and should not be stored near foodstuffs or animal feed.
4.5.1 Leaking containers in store
The precautions described in section 4.3 should be followed. Any
product remaining in damaged/leaking containers should be emptied
into a clean empty drum, which should then be tightly closed and
suitably labelled.
Spillage should be swept up with sawdust, sand, or earth (moisten
for powders), and placed in a closed container for later disposal
(section 4.7.2).
Leaking containers that have been emptied should be rinsed with at
least 1 litre of water per 20-litre drum. Swirl round to rinse the
walls, empty and add the rinsings to the sawdust or earth. The
containers should be punctured to prevent re-use for any purpose.
4.6 Transport
Local requirements regarding movements of hazardous goods should be
complied with. The product should not be transported with feed or
food-stuffs. The containers should be checked before despatch to
make sure that they are in good condition and that the labels are
undamaged.
4.7 Spillage and Disposal
4.7.1 Spillage
Before dealing with any spillage, the precautions described in
section 4.3 should be followed.
4.7.1.1 Solid products
The spilled product should be absorbed in moist sawdust, sand, or
earth, swept up, and transferred in a suitable container to a safe
place for disposal.
4.7.1.2 Liquid products
The liquid should be prevented from spreading or contaminating other
cargo, vegetation, or waterways, by making a barrier of the most
suitable material available, e.g., earth or sand.
Spilled liquid should be absorbed in sawdust, sand, or earth, which
should then be swept up and placed in a closeable container for
later transfer to a safe place for disposal.
4.7.1.3 All products
As soon as possible after the spillage and before contaminated areas
are re-used, they should be covered with damp sawdust, sand, or
earth, which should then be swept up and placed in a closeable
container for later transfer to a safe place for disposal. Since
heptachlor is toxic for fish, care should be taken to avoid run-off
into water courses.
4.7.2 Disposal
Surplus product, and contaminated absorbants and containers should
be disposed of in an appropriate way. Heptachlor is not readily
decomposed chemically or biologically and is relatively persistent.
Waste material should be burned only in a proper incinerator
designed for organochlorine waste disposal (1000°C and 30-min
residence time with effluent gas scrubbing). If this is not
possible, it should be buried in an approved dump or landfill, where
there is no risk of contaminating surface or ground water. Any local
legislation regarding disposal of toxic wastes should be complied
with.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Heptachlor may pose a toxic hazard for many aquatic and terrestrial
species. It may give rise to bioaccumulation and biomagnification
and its main metabolite, heptachlor epoxide, is rather persistent in
the environment.
Industrial discharges during manufacture, formulation, and technical
use should not be allowed to pollute the environment and should be
treated properly.
Any spillage or unused product should be prevented from spreading to
vegetation or waterways and should be treated and disposed of
properly (see section 4.7).
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers
concerned with, and users of, heptachlor. It should be displayed
at, or near, entrances to areas where there is potential exposure
to heptachlor, and on processing equipment and containers. The card
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of
the National Poison Control Centre, and for local trade names.
HEPTACHLOR
(C10H5Cl7) (CAS chemical name: 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro-4,7-methano-1H-indene)
(CAS registry number: 76-44-8; RTECS registry number: PC0700000)
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) White crystalline solid with a mild odour of camphor; it
Pure 93 is stable in daylight, air, moisture, and moderate heat
Technical product 46-74 (160 °C); it is oxidized biologically to heptachlor epoxide;
Boiling point (°C) 135-145 technical heptachlor contains approximately 72-74%
Density (g/ml) 1.65-1.67 heptachlor, 20-22% gamma-chlordane, and 4-8%
Vapour pressure (mmHg at 25 °C) 4 œ 10-4 gamma-nonachlor; it is abroad-spectrum insecticide
Relative molecular mass 373.3 used almost exclusively for the control of soil insects and
Solubility in: termites; its uses have been increasingly restricted
water (virtually insoluble) 56 µg/litre
ethanol 45 g/litre
xylene 1020 g/litre
acetone 750 g/litre
benzene 1060 g/litre
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Overexposure may cause Avoid skin contact; wear protective Remove contaminated clothing immediately:
poisoning clothing, PVC or neoprene gloves, wash skin with soap and water
rubber boots
EYES: Irritation, redness Wear face shield or goggles Flush with clean water for 15 minutes; if
irritation persists, seek medical attention
INHALATION: Dust may irritate Wear dust mask
INGESTION: Unlikely occupational Do not eat, drink, or smoke during work
hazard
Accidental or intentional ingestion Obtain medical attention immediately; do not
may cause poisoning induce vomiting; keep at rest lying face
downwards
REPEATED EXPOSURE Precautions and personal protection as In case of poisoning, same as above
THROUGH SKIN, INHALATION above; take a shower and put on clean
OR INGESTION: Poisoning may clothing after work
occur after a considerable time
due to a slow build up of toxicant
in the body
ENVIRONMENT: Toxic for aquatic Do not spill on feed or in waterways
and terrestrial life
SPILLAGE STORAGE FIRE AND EXPLOSION
Take appropriate personal Products should be stores in Powder products will not burn; liquid products
precautions; prevent liquid from locked buildings, preferably will burn and emulsifiable concentrates are
spreading or contaminating other dedicated to insecticides miscible with water; extinguish fires with
cargo, vegetation, or waterways, alcohol-resistant foam, CO2, or powder; with
with a barrier of the most suitable Keep products out of reach of sufficient burning or external heat, heptachlor will
available material, e.g., earth or children and unauthorized personnel; decompose emitting toxic fumes; the smoke and fumes
sand do not store near foodstuffs or could be injurious through inhalation or
animal feed absorption through the skin, therefore,
Absorb spilled liquid with sawdust, protective clothing and self-contained breathing
sand, or earth; sweep up and place apparatus will be required; confine the use of
it in a suitable container for later water spray to cooling of unaffected stock, thus
safe disposal avoiding the accumulation of polluted run-off
from the site
WASTE DISPOSAL NATIONAL INFORMATION
Heptachlor is not readily National Occupational Exposure United Nations No. 2761, 2762, 2995, 2996
decomposed chemically or biologically Limit:
and is relatively persistent; waste
material should be burned in a
proper incinerator designed for
organochlorine waste disposal; if National Poison Control Centre:
this is not possible, bury in an
approved dump or landfill where
there is no risk of contamination of
surface or ground water; comply
with any local legislation regarding Local trade names
disposal of toxic wastes
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. The intention is to give the
reader a representative, but not an exhaustive, overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
IARC (1982) concluded that there is limited evidence for the
carcinogenicity of heptachlor in experimental animals and that human
data available "do not allow an evaluation of the carcinogenicity of
heptachlor or heptachlor epoxide to humans to be made". This
conclusion was confirmed in 1987.
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) has reviewed
residues and toxicity data on heptachlor on several occasions (in
1965, 1966, 1967, 1968, 1969, and 1970). In 1970, it set the
acceptable daily intake (ADI) for man at 0-0.0005 mg/kg body weight.
This was based on no-observed-adverse-effect levels of:
- 5 mg/kg diet, equivalent to 0.25 mg/kg body weight per day in the
rat; and
- 2.5 mg/kg diet, equivalent to 0.06 mg/kg body weight per day in
the dog.
WHO set a guideline value of 0-0.1 µg/litre for heptachlor and
heptachlor epoxide in drinking-water (WHO, 1982).
In the WHO Recommended Classification of Pesticides by Hazard, the
oral LD50 "for classification purposes" is 100 mg/kg body weight,
and the solid technical product is in Class II (moderately hazardous).
This means that solid formulations containing 15% active ingredient or
less, fall into
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
Class III (slightly hazardous). Liquid formulations containing over
50% active ingredient fall into Class Ib and those containing 3%
active ingredient or less into Class III.
A data sheet on heptachlor (WHO/FAO 1975-78) is available from WHO in
the series "Data Sheets on Pesticides".
IRPTC (1982) has issued a review on heptachlor in its series
"Scientific reviews of Soviet literature on toxicity and hazards of
chemicals".
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pages 26-30.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
7.3 Specific Restrictions
European Community legislation prohibits the marketing and use of
plant protection products containing heptachlor. The registration of
heptachlor in Canada was discontinued in 1985 (1987(r)). Cyprus,
Ecuador, Finland, the German Democratic Republic, Japan, the USSR, and
Yugoslavia prohibit the use of heptachlor in agriculture. The USA
prohibits its use in agriculture with some exceptions. The use of
heptachlor for various agricultural purposes is prohibited in
Argentina, Chile, and Venezuela. Use is permitted in agriculture but
prohibited in domestic sanitation in Brazil. It has never been
registered for use in Norway. The only accepted uses of heptachlor in
Finland are as a termiticide in particle-board and in the plywood
industry (for exported materials), and as a laboratory chemical.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place Argentina Maximum permissible concentration (MPC) 1979
- Time-weighted average (TWA) 0.5 mg/m3
- Short-term exposure limit (STEL) 1.5 mg/m3
Austria Threshold limit value (TLV) 1985 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Belgium Threshold limit value (TLV) 0.5 mg/m3 1985 (r)
Bulgaria Maximum permissible concentration (MPC) 0.1 mg/m3
Finland Exposure limit value 1981 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Germany, Maximum work-site concentration (MAK) 1986 (r)
Federal - Time-weighted average (TWA) 0.5 mg/m3
Republic of - Short-term exposure level (STEL) 5 mg/m3
Netherlands Maximum limit 1985 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Romania Maximum permissible concentration (MPC) 1985 (r)
- Time-weighted average (TWA) 0.3 mg/m3
- Ceiling value (CLV) 0.6 mg/m3
Switzerland Maximum work-site concentration (MAK) 1985 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place United Kingdom Recommended limit (RECL) 1985 (r)
- 8-h time-weighted average 0.5 mg/m3
- Short-term exposure level (STEL) 2 mg/m3
(10-min time-weighted average)
USA Permissible exposure limit (PEL) 1974
- Time-weighted average (TWA) 0.5 mg/m3
USSR Maximum allowable concentration (MAC) 1977
- Ceiling value (CLV) 0.01 mg/m3
Yugoslavia Maximum allowable concentration (MAC)
- Time-weighted average (TWA) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (MAC)
- 1 x per day 0.001 mg/m3
- Average per day 0.0002 mg/m3
FOOD Intake from FAO/WHO Acceptable daily intake (ADI)
- With heptachlor epoxide 0.0005mg/kg 1982 (r)
AIR Work-place Romania Maximum permissible concentration (MPC) 1985 (r)
- Time-weighted average (TWA) 0.3 mg/m3
FOOD Intake from USSR Acceptable daily intake (ADI) 0.0005 mg/kg 1983
FOOD General Argentina Maximum limit 0 - 0.1 mg/kg 1969
Czechoslovakia Maximum residue limit (MRL) 1978
- For imported food only 0.01 - 0.5 mg/kg
USSR Prohibited in all food products 1983
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD General USA Acceptable residue limit (ARL)
- Raw agricultural products 0 - 0.1 mg/kg
FOOD Plant Brazil Acceptable limit 0.01 - 0.2 mg/kg
European Maximum residue limit (cereals) 1988
Community - Cereals 0.01 mg/kg
(Heptachlor + heptachlor epoxide)
FAO/WHO Extraneous residue limit 0.01 - 0.5 mg/kg 1982 (r)
Germany, Maximum residue limit (MRL) 0.01 - 0.1 mg/kg
Federal
Republic of
India Maximum tolerable concentration 0.002 - 0.15 1976
mg/kg
Italy Maximum residue limit (MRL) 0.01 mg/kg 1987 (r)
(heptachlor + heptachlorepoxide)
Kenya Maximum limit 0.01 - 0.5
mg/kg
Netherlands Maximum residue limit (MRL) 0.01 - 0.1 1987 (r)
mg/kg
Sweden Maximum tolerable concentration 0.02 - 0.05
mg/kg
USA Acceptable residue limit (ARL) 0.01 - 0.1
mg/kg
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant Italy Maximum residue limit (MRL) 0.01 mg/kg 1987 (r)
- (Heptachlor + heptachlor epoxide)
Kenya Maximum limit 0.01 - 0.5 mg/kg
Netherlands Maximum residue limit (MRL) 0.01 - 0.1 mg/kg 1987 (r)
Sweden Maximum tolerable concentration 0.02 - 0.05
mg/kg
USA Acceptable residue limit (ARL) 0.01 - 0.1 mg/kg
FOOD Animal European Maximum residue limit (MRL) 1988
Community - In specified products 0.004 - 0.2 mg/kg
(section 7.6)
FAO/WHO Maximum residue limit (MRL) 1987 (r)
- In fat 0.15 - 0.2 mg/kg
Germany, Maximum residue limit (MRL) 1984
Federal - Heptachlor epoxide 0.01 - 0.2 mg/kg
Republic of
Netherlands Maximum residue limit (MRL) 1987 (r)
- In fat 0.15 - 0.5 mg/kg
Sweden Maximum tolerable concentration 0.005 - 0.1 1983
mg/kg
FEED Argentina Maximum limit 0 mg/kg 1969
USSR Prohibition 0 mg/kg 1981
Medium Specification Country/ Exposure limit description Value Effective
organization date
GOODS Argentina Maximum limit 1971
- Tobacco 0 mg/kg
Germany, Maximum residue limit (MRL) 1984
Federal - Tobacco 0.2 mg/kg
Republic of
WATER Ambient Mexico Maximum permissible concentration (MPC) 1973
- Coastal 0.2 µg/litre
- Estuarine 0.002 mg/litre
- Water treated for drinking 0.018 mg/litre
USSR Maximum allowable concentration (MAC) 0.05 mg/litre 1983
WATER Drinking- WHO Guideline value
- Heptachlor + heptachlor epoxide 0.1 µg/litre
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies heptachlor in:
Hazard class 6.1: poisonous substance;
Packing group II: a substance presenting a medium risk of
poisoning in transport (heptachlor
concentrations 80-100%);
Packing group III: a substance presenting a relatively low risk
of poisoning in transport (heptachlor
concentrations of 20-80% (solid) or 8-80%
(liquid)).
The label should read as follows:
Common trade names: Aahepta, Agroceres, Basaklor, Drinox,
Eptacloro, E 3314, GPKh,
Heptachlorane, Heptagran, Heptagranox,
Heptamak, Heptamul, Heptasol, Heptox,
Rhodiachlor, Soleptax, Velsicol 104
CAS chemical name: 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-
tetra-hydro-4,7-methano-1H-indene
CAS registry number: 76-44-8
RTECS registry number: PC0700000
Relative molecular mass: 373.3
1.2 Physical and Chemical Properties
Heptachlor is a white crystalline solid with a mild odour of
camphor, a melting point of 93°C (46-74°C for the technical
product), and a density of 1.65-1.67 g/ml at 25°C. It has a boiling
point of 135-145°C and vapour pressure of 4 x 10-4 mmHg at 25°C.
It is virtually insoluble in water (0.056 mg/litre) but fairly
soluble in organic solvents, e.g., ethanol (45 g/litre), xylene
(1020 g/litre), acetone (750 g/litre), and benzene (1060 g/litre).
It is stable in daylight, air, moisture, and moderate heat (160°C)
but is oxidized biologically to heptachlor epoxide (Whetstone,
1964).
Technical heptachlor contains about 72-74% 1,4,5,6,7,8,8-
heptachloro-3a,4,7,7a-tetrahydro-4,7-methano-1H-indene, 20-22%
gamma-chlordane, and 4-8% gamma-nonachlor.
1.3 Analytical Methods
Extraction from crops, other plant products, dairy products and oils
can be achieved with hexane-acetone or acetonitrile.
The method of choice for the qualitative and quantitative
determination of heptachlor is gas-liquid chromatography with
electron capture detection.
1.4 Production and Uses
Heptachlor was isolated in 1946 from technical chlordane.
Production of heptachlor in the USA in 1971 was estimated to be 2.7
million kg. In 1970, the use of heptachlor throughout the world was
as follows: Africa 5%, Asia 15%, Canada and the USA 5%, Europe 60%,
and South America 15%. Recently, the use of heptachlor has been
increasingly restricted in many countries (section 7.3).
Formulations include emulsifiable concentrates, wettable powders,
dusts, and granules, containing various concentrations of active
material.
Heptachlor was first introduced as a contact insecticide in the USA
in 1952 for foliar, soil and structural application, and for the
control of malaria. It is a non-systemic stomach and contact
insecticide. The use of heptachlor is now confined almost
exclusively to the control of soil insects and termites.
2. SUMMARY AND EVALUATION
2.1 Heptachlor Toxicity
Heptachlor is readily absorbed following ingestion and skin contact
and is transported throughout the body. Heptachlor epoxide, the most
persistent metabolite, is rapidly formed and can be found in the
body, mainly in adipose tissue. The toxicity of heptachlor epoxide
is similar to that of heptachlor. Elimination takes place via both
the urine and faeces. Human milk can be a major excretion route for
heptachlor residues.
The acute toxicity of heptachlor in the rat ranges from 40 to
162 mg/kg body weight. Toxic symptoms are related to CNS-hyperactivity
and include tremors and convulsions. In experimental animals,
prolonged low-level exposure results in the induction of hepatic
microsomal enzymes and, at a later stage, in liver hypertrophy with
histological changes. At higher levels, heptachlor is hepatotoxic.
Heptachlor was not a teratogen in the experimental animal studies
conducted, but at higher exposure levels it may interfere with
reproduction and the viability of the offspring.
Heptachlor was not generally active in short-term tests for genetic
activity. There was evidence that it might have an effect on cell to
cell communication in vitro, a characteristic property of many
promoting agents.
There is limited evidence for the carcinogenicity of heptachlor and
heptachlor epoxide in experimental animals.
No cases of adverse effects in man or occupational poisoning have
been reported.
2.2 Human Exposure to Heptachlor
Food is the major source of exposure to heptachlor for the general
population, but residue intake in most countries is below the
advised acceptable daily intake. In areas where heptachlor is used,
the inhalation of dust or mist and the drinking of well-water may
account for some additional exposure.
Relatively high concentrations of heptachlor epoxide can be found in
human milk, especially in areas with high heptachlor exposure in the
general population.
Occupational exposure, especially via the skin and via inhalation,
can be considerable when the material is handled in installations or
situations with insufficient safety precautions.
2.3 Evaluation of Effects on the Environment
Heptachlor is persistent and relatively immobile in soil. However,
it may be lost from the soil by slow vaporization, by oxidation to
heptachlor epoxide (a more persistent degradation product of
comparable toxicity), by photoconversion to photo-heptachlor, or by
conversion to less toxic metabolites by soil bacteria. The rate at
which heptachlor is lost by these different mechanisms is influenced
by climate, soil type, and management practices (retention being
longest in undisturbed soil). The majority of heptachlor residues
are found in the top few centimetres of soil and are most likely to
be spread with dust particles by air currents.
Although there is no indication of widespread contamination of water
by heptachlor, its residues have been found in fish from various
bodies of water. Heptachlor is not very soluble in water and
persists in aquatic ecosystems by being adsorbed on sediments. It
has been shown to be toxic for aquatic life, but its toxicity varies
considerably with species. This is particularly so in marine
vertebrates, where acute LC50 values span three orders of
magnitude. Marine crustacea are particularly sensitive to
heptachlor; a concentration of 0.03 µg/litre may be lethal. Younger
life stages of both fish and invertebrates are the most sensitive to
heptachlor, "safe" concentrations being 0.1 and 0.01 µg/litre,
respectively.
Evaluation of the toxicity of heptachlor for wildlife depends solely
on extrapolation from studies on game birds and domestic species. In
these animals, toxicity is variable, with LD50 values ranging from
6 to 531 mg/kg body weight. Heptachlor is generally classified as a
neurotoxin.
Uptake of heptachlor in animals is fairly rapid. It is rapidly
metabolized to heptachlor epoxide, which can persist for extended
periods of time in body fat. The relative amounts of heptachlor
epoxide in tissues increase with length of exposure. Few data are
available on the toxicity of this metabolite, but indications are
that it is comparable to that of heptachlor.
Bioaccumulation and biomagnification occur and bioconcentration
factors of 200-37 000 have been reported from water into hydrobiota.
The marked persistence of heptachlor in the environment and its
tendency to accumulate in body fat make it a serious environmental
hazard.
3. CONCLUSIONS
Although there is no evidence that heptachlor is a human carcinogen,
the suspicion, principally arising from the mouse carcinogenicity
studies, cannot be ignored. Further research is required to look
into this problem. Nevertheless, with the present state of
knowledge, it is concluded that:
(a) As long as occupational hygiene procedures are maintained to
keep exposure levels to a minimum, whether or not by the
imposition of maximum allowable concentrations, there is little
reason to believe that workers will be at risk from handling,
or contact with, heptachlor.
(b) Members of the general population should not suffer any adverse
effects from heptachlor residues in food, provided that intake
is kept within the ADI set by the FAO/WHO Joint Meeting.
In certain regions of the world, the exposure of the general
population to heptachlor may be increased if heptachlor is used
in buildings for the control of termites.
The intake of heptachlor residues by breast-fed infants,
through human milk, in areas of high heptachlor use, remains a
concern.
(c) Environmentally, heptachlor causes concern because several
marine species are highly sensitive to it and because of the
persistence of the metabolite heptachlor epoxide in adipose
tissue and in the environment.
From: Environmental Health Criteria 38: Heptachlor
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Heptachlor is an organochlorine insecticide. It is toxic and can be
hazardous for human beings if incorrectly or carelessly handled. It
is therefore essential that the correct precautions are observed
during handling and use.
For details see the International Chemical Safety Card (pages 20-23).
4.1.1 Advice to Physicians
4.1.1.1 Symptoms of poisoning
Heptachlor is toxic by mouth, by skin contact (especially liquid
formulations) and by inhalation of dust from powder concentrates. It
acts as a stimulant of the central nervous system.
Following accidental ingestion or over-exposure, symptoms may
include headache, dizziness, nausea, vomiting, weakness in legs, and
convulsions.
4.1.1.2 Medical advice
Medical treatment is largely symptomatic and supportive and directed
against convulsions and anoxaemia. If swallowed, vomiting should not
be induced and emetics are contraindicated, because many liquid
formulations contain hydrocarbons and there is risk of aspiration
pneumonia. Instead, the stomach should be emptied as soon as
possible by careful gastric lavage, using a cuffed endotracheal tube
to avoid aspiration into the lungs. This should be followed by
intragastric administration of 3-4 tablespoons of activated charcoal
and 30 g of magnesium or sodium sulfate in a 30% aqueous solution.
Oily purgatives are contraindicated. No fats, oils, or milk should
be given.
If convulsions occur, anticonvulsants should be given, e.g.,
diazepam 10 mg, slowly, intravenously (children 1-5 mg), repeated as
necessary; or thiopental sodium, or hexobarbital sodium slowly,
intravenously, in a dose of 10 mg/kg body weight with a maximum
total dose of up to 750 mg for an adult, or paraldehyde at 5 ml by
injection. These short-acting anticonvulsants should always be
followed by phenobarbital given orally at 3 mg/kg body weight (up to
200 mg for anadult), or phenobarbital sodium given intramuscularly
at 3 mg/kg body weight (also up to 200 mg for an adult).
Morphine and its derivatives, epinephrine and noradrenaline should
never be given.
An unobstructed airway must be maintained. Respiratory inadequacy,
which may be accentuated by barbiturate anticonvulsants, should be
corrected, and oxygen and/or artificial ventilation may be needed.
4.2 Health Surveillance Advice
A complete medical history should be taken, and physical examination
made, annually. Special attention should be paid to liver and kidney
function.
4.3 Safety in Use
Handling liquid formulations: Wear protective neoprene or PVC
gloves, cotton overalls, rubber
boots, and face shield.
Handling powder formulations: Avoid raising a dust cloud. Wear
protective neoprene or PVC
gloves, cotton overalls, and dust
mask.
4.4 Explosion and Fire Hazards
4.4.1 Explosion hazards
The explosion hazard will depend on the solvent used in the
formulation, or on the characteristics of the dust.
4.4.2 Fire hazards
Liquid formulations containing organic solvents may be flammable.
Extinguish fires with alcohol-resistant foam, carbon dioxide, or
powder.
With sufficient burning or external heat, heptachlor will decompose
emitting toxic fumes. Fire-fighters should wear self-contained
breathing apparatus, eye protection, and full protective clothing.
The use of water spray should be confined to the cooling of
unaffected stock, to avoid the accumulation of polluted run-off from
the site.
4.5 Storage
Products should be stored in locked buildings, preferably dedicated
to the storage of insecticides.
Products should be kept out of reach of children and unauthorized
personnel and should not be stored near foodstuffs or animal feed.
4.5.1 Leaking containers in store
The precautions described in section 4.3 should be followed. Any
product remaining in damaged/leaking containers should be emptied
into a clean empty drum, which should then be tightly closed and
suitably labelled.
Spillage should be swept up with sawdust, sand, or earth (moisten
for powders), and placed in a closed container for later disposal
(section 4.7.2).
Leaking containers that have been emptied should be rinsed with at
least 1 litre of water per 20-litre drum. Swirl round to rinse the
walls, empty and add the rinsings to the sawdust or earth. The
containers should be punctured to prevent re-use for any purpose.
4.6 Transport
Local requirements regarding movements of hazardous goods should be
complied with. The product should not be transported with feed or
food-stuffs. The containers should be checked before despatch to
make sure that they are in good condition and that the labels are
undamaged.
4.7 Spillage and Disposal
4.7.1 Spillage
Before dealing with any spillage, the precautions described in
section 4.3 should be followed.
4.7.1.1 Solid products
The spilled product should be absorbed in moist sawdust, sand, or
earth, swept up, and transferred in a suitable container to a safe
place for disposal.
4.7.1.2 Liquid products
The liquid should be prevented from spreading or contaminating other
cargo, vegetation, or waterways, by making a barrier of the most
suitable material available, e.g., earth or sand.
Spilled liquid should be absorbed in sawdust, sand, or earth, which
should then be swept up and placed in a closeable container for
later transfer to a safe place for disposal.
4.7.1.3 All products
As soon as possible after the spillage and before contaminated areas
are re-used, they should be covered with damp sawdust, sand, or
earth, which should then be swept up and placed in a closeable
container for later transfer to a safe place for disposal. Since
heptachlor is toxic for fish, care should be taken to avoid run-off
into water courses.
4.7.2 Disposal
Surplus product, and contaminated absorbants and containers should
be disposed of in an appropriate way. Heptachlor is not readily
decomposed chemically or biologically and is relatively persistent.
Waste material should be burned only in a proper incinerator
designed for organochlorine waste disposal (1000°C and 30-min
residence time with effluent gas scrubbing). If this is not
possible, it should be buried in an approved dump or landfill, where
there is no risk of contaminating surface or ground water. Any local
legislation regarding disposal of toxic wastes should be complied
with.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Heptachlor may pose a toxic hazard for many aquatic and terrestrial
species. It may give rise to bioaccumulation and biomagnification
and its main metabolite, heptachlor epoxide, is rather persistent in
the environment.
Industrial discharges during manufacture, formulation, and technical
use should not be allowed to pollute the environment and should be
treated properly.
Any spillage or unused product should be prevented from spreading to
vegetation or waterways and should be treated and disposed of
properly (see section 4.7).
6. INTERNATIONAL CHEMICAL SAFETY CARD
This card should be easily available to all health workers
concerned with, and users of, heptachlor. It should be displayed
at, or near, entrances to areas where there is potential exposure
to heptachlor, and on processing equipment and containers. The card
should be translated into the appropriate language(s). All persons
potentially exposed to the chemical should also have the
instructions on the chemical safety card clearly explained.
Space is available on the card for insertion of the National
Occupational Exposure Limit, the address and telephone number of
the National Poison Control Centre, and for local trade names.
HEPTACHLOR
(C10H5Cl7) (CAS chemical name: 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro-4,7-methano-1H-indene)
(CAS registry number: 76-44-8; RTECS registry number: PC0700000)
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Melting point (°C) White crystalline solid with a mild odour of camphor; it
Pure 93 is stable in daylight, air, moisture, and moderate heat
Technical product 46-74 (160 °C); it is oxidized biologically to heptachlor epoxide;
Boiling point (°C) 135-145 technical heptachlor contains approximately 72-74%
Density (g/ml) 1.65-1.67 heptachlor, 20-22% gamma-chlordane, and 4-8%
Vapour pressure (mmHg at 25 °C) 4 œ 10-4 gamma-nonachlor; it is abroad-spectrum insecticide
Relative molecular mass 373.3 used almost exclusively for the control of soil insects and
Solubility in: termites; its uses have been increasingly restricted
water (virtually insoluble) 56 µg/litre
ethanol 45 g/litre
xylene 1020 g/litre
acetone 750 g/litre
benzene 1060 g/litre
HAZARDS/SYMPTOMS PREVENTION AND PROTECTION FIRST AID
SKIN: Overexposure may cause Avoid skin contact; wear protective Remove contaminated clothing immediately:
poisoning clothing, PVC or neoprene gloves, wash skin with soap and water
rubber boots
EYES: Irritation, redness Wear face shield or goggles Flush with clean water for 15 minutes; if
irritation persists, seek medical attention
INHALATION: Dust may irritate Wear dust mask
INGESTION: Unlikely occupational Do not eat, drink, or smoke during work
hazard
Accidental or intentional ingestion Obtain medical attention immediately; do not
may cause poisoning induce vomiting; keep at rest lying face
downwards
REPEATED EXPOSURE Precautions and personal protection as In case of poisoning, same as above
THROUGH SKIN, INHALATION above; take a shower and put on clean
OR INGESTION: Poisoning may clothing after work
occur after a considerable time
due to a slow build up of toxicant
in the body
ENVIRONMENT: Toxic for aquatic Do not spill on feed or in waterways
and terrestrial life
SPILLAGE STORAGE FIRE AND EXPLOSION
Take appropriate personal Products should be stores in Powder products will not burn; liquid products
precautions; prevent liquid from locked buildings, preferably will burn and emulsifiable concentrates are
spreading or contaminating other dedicated to insecticides miscible with water; extinguish fires with
cargo, vegetation, or waterways, alcohol-resistant foam, CO2, or powder; with
with a barrier of the most suitable Keep products out of reach of sufficient burning or external heat, heptachlor will
available material, e.g., earth or children and unauthorized personnel; decompose emitting toxic fumes; the smoke and fumes
sand do not store near foodstuffs or could be injurious through inhalation or
animal feed absorption through the skin, therefore,
Absorb spilled liquid with sawdust, protective clothing and self-contained breathing
sand, or earth; sweep up and place apparatus will be required; confine the use of
it in a suitable container for later water spray to cooling of unaffected stock, thus
safe disposal avoiding the accumulation of polluted run-off
from the site
WASTE DISPOSAL NATIONAL INFORMATION
Heptachlor is not readily National Occupational Exposure United Nations No. 2761, 2762, 2995, 2996
decomposed chemically or biologically Limit:
and is relatively persistent; waste
material should be burned in a
proper incinerator designed for
organochlorine waste disposal; if National Poison Control Centre:
this is not possible, bury in an
approved dump or landfill where
there is no risk of contamination of
surface or ground water; comply
with any local legislation regarding Local trade names
disposal of toxic wastes
7. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. The intention is to give the
reader a representative, but not an exhaustive, overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country.a
7.1 Previous Evaluations by International Bodies
IARC (1982) concluded that there is limited evidence for the
carcinogenicity of heptachlor in experimental animals and that human
data available "do not allow an evaluation of the carcinogenicity of
heptachlor or heptachlor epoxide to humans to be made". This
conclusion was confirmed in 1987.
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR) has reviewed
residues and toxicity data on heptachlor on several occasions (in
1965, 1966, 1967, 1968, 1969, and 1970). In 1970, it set the
acceptable daily intake (ADI) for man at 0-0.0005 mg/kg body weight.
This was based on no-observed-adverse-effect levels of:
- 5 mg/kg diet, equivalent to 0.25 mg/kg body weight per day in the
rat; and
- 2.5 mg/kg diet, equivalent to 0.06 mg/kg body weight per day in
the dog.
WHO set a guideline value of 0-0.1 µg/litre for heptachlor and
heptachlor epoxide in drinking-water (WHO, 1982).
In the WHO Recommended Classification of Pesticides by Hazard, the
oral LD50 "for classification purposes" is 100 mg/kg body weight,
and the solid technical product is in Class II (moderately hazardous).
This means that solid formulations containing 15% active ingredient or
less, fall into
a The regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate
regulatory authorities before application.
Class III (slightly hazardous). Liquid formulations containing over
50% active ingredient fall into Class Ib and those containing 3%
active ingredient or less into Class III.
A data sheet on heptachlor (WHO/FAO 1975-78) is available from WHO in
the series "Data Sheets on Pesticides".
IRPTC (1982) has issued a review on heptachlor in its series
"Scientific reviews of Soviet literature on toxicity and hazards of
chemicals".
7.2 Exposure Limit Values
Some exposure limit values are given in the table on pages 26-30.
When no effective date appears in the IRPTC legal file, the year of
the reference from which the data are taken is indicated by (r).
7.3 Specific Restrictions
European Community legislation prohibits the marketing and use of
plant protection products containing heptachlor. The registration of
heptachlor in Canada was discontinued in 1985 (1987(r)). Cyprus,
Ecuador, Finland, the German Democratic Republic, Japan, the USSR, and
Yugoslavia prohibit the use of heptachlor in agriculture. The USA
prohibits its use in agriculture with some exceptions. The use of
heptachlor for various agricultural purposes is prohibited in
Argentina, Chile, and Venezuela. Use is permitted in agriculture but
prohibited in domestic sanitation in Brazil. It has never been
registered for use in Norway. The only accepted uses of heptachlor in
Finland are as a termiticide in particle-board and in the plywood
industry (for exported materials), and as a laboratory chemical.
EXPOSURE LIMIT VALUES
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place Argentina Maximum permissible concentration (MPC) 1979
- Time-weighted average (TWA) 0.5 mg/m3
- Short-term exposure limit (STEL) 1.5 mg/m3
Austria Threshold limit value (TLV) 1985 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Belgium Threshold limit value (TLV) 0.5 mg/m3 1985 (r)
Bulgaria Maximum permissible concentration (MPC) 0.1 mg/m3
Finland Exposure limit value 1981 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Germany, Maximum work-site concentration (MAK) 1986 (r)
Federal - Time-weighted average (TWA) 0.5 mg/m3
Republic of - Short-term exposure level (STEL) 5 mg/m3
Netherlands Maximum limit 1985 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Romania Maximum permissible concentration (MPC) 1985 (r)
- Time-weighted average (TWA) 0.3 mg/m3
- Ceiling value (CLV) 0.6 mg/m3
Switzerland Maximum work-site concentration (MAK) 1985 (r)
- Time-weighted average (TWA) 0.5 mg/m3
Medium Specification Country/ Exposure limit description Value Effective
organization date
AIR Work-place United Kingdom Recommended limit (RECL) 1985 (r)
- 8-h time-weighted average 0.5 mg/m3
- Short-term exposure level (STEL) 2 mg/m3
(10-min time-weighted average)
USA Permissible exposure limit (PEL) 1974
- Time-weighted average (TWA) 0.5 mg/m3
USSR Maximum allowable concentration (MAC) 1977
- Ceiling value (CLV) 0.01 mg/m3
Yugoslavia Maximum allowable concentration (MAC)
- Time-weighted average (TWA) 0.5 mg/m3
AIR Ambient USSR Maximum allowable concentration (MAC)
- 1 x per day 0.001 mg/m3
- Average per day 0.0002 mg/m3
FOOD Intake from FAO/WHO Acceptable daily intake (ADI)
- With heptachlor epoxide 0.0005mg/kg 1982 (r)
AIR Work-place Romania Maximum permissible concentration (MPC) 1985 (r)
- Time-weighted average (TWA) 0.3 mg/m3
FOOD Intake from USSR Acceptable daily intake (ADI) 0.0005 mg/kg 1983
FOOD General Argentina Maximum limit 0 - 0.1 mg/kg 1969
Czechoslovakia Maximum residue limit (MRL) 1978
- For imported food only 0.01 - 0.5 mg/kg
USSR Prohibited in all food products 1983
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD General USA Acceptable residue limit (ARL)
- Raw agricultural products 0 - 0.1 mg/kg
FOOD Plant Brazil Acceptable limit 0.01 - 0.2 mg/kg
European Maximum residue limit (cereals) 1988
Community - Cereals 0.01 mg/kg
(Heptachlor + heptachlor epoxide)
FAO/WHO Extraneous residue limit 0.01 - 0.5 mg/kg 1982 (r)
Germany, Maximum residue limit (MRL) 0.01 - 0.1 mg/kg
Federal
Republic of
India Maximum tolerable concentration 0.002 - 0.15 1976
mg/kg
Italy Maximum residue limit (MRL) 0.01 mg/kg 1987 (r)
(heptachlor + heptachlorepoxide)
Kenya Maximum limit 0.01 - 0.5
mg/kg
Netherlands Maximum residue limit (MRL) 0.01 - 0.1 1987 (r)
mg/kg
Sweden Maximum tolerable concentration 0.02 - 0.05
mg/kg
USA Acceptable residue limit (ARL) 0.01 - 0.1
mg/kg
Medium Specification Country/ Exposure limit description Value Effective
organization date
FOOD Plant Italy Maximum residue limit (MRL) 0.01 mg/kg 1987 (r)
- (Heptachlor + heptachlor epoxide)
Kenya Maximum limit 0.01 - 0.5 mg/kg
Netherlands Maximum residue limit (MRL) 0.01 - 0.1 mg/kg 1987 (r)
Sweden Maximum tolerable concentration 0.02 - 0.05
mg/kg
USA Acceptable residue limit (ARL) 0.01 - 0.1 mg/kg
FOOD Animal European Maximum residue limit (MRL) 1988
Community - In specified products 0.004 - 0.2 mg/kg
(section 7.6)
FAO/WHO Maximum residue limit (MRL) 1987 (r)
- In fat 0.15 - 0.2 mg/kg
Germany, Maximum residue limit (MRL) 1984
Federal - Heptachlor epoxide 0.01 - 0.2 mg/kg
Republic of
Netherlands Maximum residue limit (MRL) 1987 (r)
- In fat 0.15 - 0.5 mg/kg
Sweden Maximum tolerable concentration 0.005 - 0.1 1983
mg/kg
FEED Argentina Maximum limit 0 mg/kg 1969
USSR Prohibition 0 mg/kg 1981
Medium Specification Country/ Exposure limit description Value Effective
organization date
GOODS Argentina Maximum limit 1971
- Tobacco 0 mg/kg
Germany, Maximum residue limit (MRL) 1984
Federal - Tobacco 0.2 mg/kg
Republic of
WATER Ambient Mexico Maximum permissible concentration (MPC) 1973
- Coastal 0.2 µg/litre
- Estuarine 0.002 mg/litre
- Water treated for drinking 0.018 mg/litre
USSR Maximum allowable concentration (MAC) 0.05 mg/litre 1983
WATER Drinking- WHO Guideline value
- Heptachlor + heptachlor epoxide 0.1 µg/litre
7.4 Labelling, Packaging, and Transport
The United Nations Committee of Experts on the Transportation of
Dangerous Goods classifies heptachlor in:
Hazard class 6.1: poisonous substance;
Packing group II: a substance presenting a medium risk of
poisoning in transport (heptachlor
concentrations 80-100%);
Packing group III: a substance presenting a relatively low risk
of poisoning in transport (heptachlor
concentrations of 20-80% (solid) or 8-80%
(liquid)).
The label should read as follows:
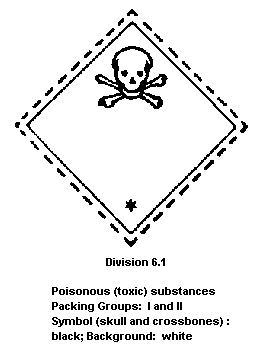
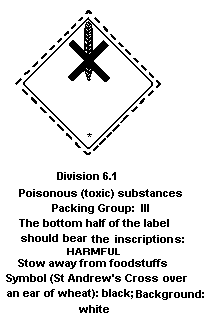 The FAO specifications for plant protection products for heptachlor
(technical product and formulations) indicate the appropriate
composition and purity of heptachlor and recommend methods for
checking these values. The technical product should contain
approximately 75% heptachlor - this should be stated on the label and
the composition should not deviate by more than 2% from this. For
formulations, the heptachlor content should be stated and not deviate
by more than 10% from this.
The European Community legislation requires that both heptachlor and
heptachlor epoxide should be labelled as dangerous substances using
the symbol:
The FAO specifications for plant protection products for heptachlor
(technical product and formulations) indicate the appropriate
composition and purity of heptachlor and recommend methods for
checking these values. The technical product should contain
approximately 75% heptachlor - this should be stated on the label and
the composition should not deviate by more than 2% from this. For
formulations, the heptachlor content should be stated and not deviate
by more than 10% from this.
The European Community legislation requires that both heptachlor and
heptachlor epoxide should be labelled as dangerous substances using
the symbol:
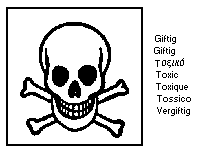 The label must read:
Toxic in contact with skin and if swallowed; danger of
cumulative effects; possible risks of irreversible effects; wear
suitable protective clothing and gloves; if you feel unwell,
seek medical advice (show the label where possible).
The European Community legislation on labelling of pesticide
preparations classifies heptachlor in Class Ib for the purposes of
determining the label for preparations containing heptachlor and other
active ingredients.
7.5 Waste Disposal
In the USA, heptachlor (technical and formulations) is classified as a
toxic pollutant for which the EPA has set effluent limitations and
pretreatment standards. Permits are required for discharge from any
point source into US waters. Hazardous-waste incinerators must achieve
99.99% destruction of this substance.
7.6 Other Measures
The European Community legislation requires that Member States should
prescribe that, from the time cereals (wheat, rye, barley, oats,
maize, paddy rice, buckwheat, millet, grain sorghum, triticale) are
put into circulation,they may not contain (in and on) heptachlor
residue levels (sum of heptachlor and heptachlor epoxide expressed as
heptachlor) exceeding 0.01 mg/kg (applicable latest by June 1988).
The European Community legislation also requires that Member States
should prescribe that, from the time that foodstuffs of animal origin
(meat, poultry, milk, butter, cheese, etc.) are put into circulation,
they may not contain heptachlor residue levels (sum of heptachlor and
heptachlor epoxide expressed as heptachlor) exceeding 0.2 mg/kg in fat
of meat and poultry or exceeding 0.004 mg/kg in raw cow's milk, whole
cream cow's milk, butter, cheese (applicable latest by June 1988).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (Report No.
VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) EHC No. 38: Heptachlor, Geneva, World Health
Organization, 81 pp.
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The label must read:
Toxic in contact with skin and if swallowed; danger of
cumulative effects; possible risks of irreversible effects; wear
suitable protective clothing and gloves; if you feel unwell,
seek medical advice (show the label where possible).
The European Community legislation on labelling of pesticide
preparations classifies heptachlor in Class Ib for the purposes of
determining the label for preparations containing heptachlor and other
active ingredients.
7.5 Waste Disposal
In the USA, heptachlor (technical and formulations) is classified as a
toxic pollutant for which the EPA has set effluent limitations and
pretreatment standards. Permits are required for discharge from any
point source into US waters. Hazardous-waste incinerators must achieve
99.99% destruction of this substance.
7.6 Other Measures
The European Community legislation requires that Member States should
prescribe that, from the time cereals (wheat, rye, barley, oats,
maize, paddy rice, buckwheat, millet, grain sorghum, triticale) are
put into circulation,they may not contain (in and on) heptachlor
residue levels (sum of heptachlor and heptachlor epoxide expressed as
heptachlor) exceeding 0.01 mg/kg (applicable latest by June 1988).
The European Community legislation also requires that Member States
should prescribe that, from the time that foodstuffs of animal origin
(meat, poultry, milk, butter, cheese, etc.) are put into circulation,
they may not contain heptachlor residue levels (sum of heptachlor and
heptachlor epoxide expressed as heptachlor) exceeding 0.2 mg/kg in fat
of meat and poultry or exceeding 0.004 mg/kg in raw cow's milk, whole
cream cow's milk, butter, cheese (applicable latest by June 1988).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (Report No.
VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) EHC No. 38: Heptachlor, Geneva, World Health
Organization, 81 pp.
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
 Common trade names: Aahepta, Agroceres, Basaklor, Drinox,
Eptacloro, E 3314, GPKh,
Heptachlorane, Heptagran, Heptagranox,
Heptamak, Heptamul, Heptasol, Heptox,
Rhodiachlor, Soleptax, Velsicol 104
CAS chemical name: 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-
tetra-hydro-4,7-methano-1H-indene
CAS registry number:
Common trade names: Aahepta, Agroceres, Basaklor, Drinox,
Eptacloro, E 3314, GPKh,
Heptachlorane, Heptagran, Heptagranox,
Heptamak, Heptamul, Heptasol, Heptox,
Rhodiachlor, Soleptax, Velsicol 104
CAS chemical name: 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-
tetra-hydro-4,7-methano-1H-indene
CAS registry number: 
 The FAO specifications for plant protection products for heptachlor
(technical product and formulations) indicate the appropriate
composition and purity of heptachlor and recommend methods for
checking these values. The technical product should contain
approximately 75% heptachlor - this should be stated on the label and
the composition should not deviate by more than 2% from this. For
formulations, the heptachlor content should be stated and not deviate
by more than 10% from this.
The European Community legislation requires that both heptachlor and
heptachlor epoxide should be labelled as dangerous substances using
the symbol:
The FAO specifications for plant protection products for heptachlor
(technical product and formulations) indicate the appropriate
composition and purity of heptachlor and recommend methods for
checking these values. The technical product should contain
approximately 75% heptachlor - this should be stated on the label and
the composition should not deviate by more than 2% from this. For
formulations, the heptachlor content should be stated and not deviate
by more than 10% from this.
The European Community legislation requires that both heptachlor and
heptachlor epoxide should be labelled as dangerous substances using
the symbol:
 The label must read:
Toxic in contact with skin and if swallowed; danger of
cumulative effects; possible risks of irreversible effects; wear
suitable protective clothing and gloves; if you feel unwell,
seek medical advice (show the label where possible).
The European Community legislation on labelling of pesticide
preparations classifies heptachlor in Class Ib for the purposes of
determining the label for preparations containing heptachlor and other
active ingredients.
7.5 Waste Disposal
In the USA, heptachlor (technical and formulations) is classified as a
toxic pollutant for which the EPA has set effluent limitations and
pretreatment standards. Permits are required for discharge from any
point source into US waters. Hazardous-waste incinerators must achieve
99.99% destruction of this substance.
7.6 Other Measures
The European Community legislation requires that Member States should
prescribe that, from the time cereals (wheat, rye, barley, oats,
maize, paddy rice, buckwheat, millet, grain sorghum, triticale) are
put into circulation,they may not contain (in and on) heptachlor
residue levels (sum of heptachlor and heptachlor epoxide expressed as
heptachlor) exceeding 0.01 mg/kg (applicable latest by June 1988).
The European Community legislation also requires that Member States
should prescribe that, from the time that foodstuffs of animal origin
(meat, poultry, milk, butter, cheese, etc.) are put into circulation,
they may not contain heptachlor residue levels (sum of heptachlor and
heptachlor epoxide expressed as heptachlor) exceeding 0.2 mg/kg in fat
of meat and poultry or exceeding 0.004 mg/kg in raw cow's milk, whole
cream cow's milk, butter, cheese (applicable latest by June 1988).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (Report No.
VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) EHC No. 38: Heptachlor, Geneva, World Health
Organization, 81 pp.
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
The label must read:
Toxic in contact with skin and if swallowed; danger of
cumulative effects; possible risks of irreversible effects; wear
suitable protective clothing and gloves; if you feel unwell,
seek medical advice (show the label where possible).
The European Community legislation on labelling of pesticide
preparations classifies heptachlor in Class Ib for the purposes of
determining the label for preparations containing heptachlor and other
active ingredients.
7.5 Waste Disposal
In the USA, heptachlor (technical and formulations) is classified as a
toxic pollutant for which the EPA has set effluent limitations and
pretreatment standards. Permits are required for discharge from any
point source into US waters. Hazardous-waste incinerators must achieve
99.99% destruction of this substance.
7.6 Other Measures
The European Community legislation requires that Member States should
prescribe that, from the time cereals (wheat, rye, barley, oats,
maize, paddy rice, buckwheat, millet, grain sorghum, triticale) are
put into circulation,they may not contain (in and on) heptachlor
residue levels (sum of heptachlor and heptachlor epoxide expressed as
heptachlor) exceeding 0.01 mg/kg (applicable latest by June 1988).
The European Community legislation also requires that Member States
should prescribe that, from the time that foodstuffs of animal origin
(meat, poultry, milk, butter, cheese, etc.) are put into circulation,
they may not contain heptachlor residue levels (sum of heptachlor and
heptachlor epoxide expressed as heptachlor) exceeding 0.2 mg/kg in fat
of meat and poultry or exceeding 0.004 mg/kg in raw cow's milk, whole
cream cow's milk, butter, cheese (applicable latest by June 1988).
BIBLIOGRAPHY
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packing, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticide
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
IRPTC (1983) IRPTC legal file 1983. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
PESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (Report No.
VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, Van Nostrand Reinhold Company, Inc.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed. New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vols. Washington, DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1984) EHC No. 38: Heptachlor, Geneva, World Health
Organization, 81 pp.
WHO (1986) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1986-87. Geneva, World Health
Organization (Unpublished report VBC/86.1).
WHO/FAO (1975-87) Data sheets on pesticides. Geneva, World Health
Organization.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.
Lavenham, Lavenham Press Limited, British Crop Protection Council.
