
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 49
CAPTAFOL
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1990
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Captafol : health and safety guide.
(Health and safety guide ; no. 49)
1. Captan - analogs & derivatives I. Series
ISBN 92 4 151049 8 (NLM Classification: WA 240)
ISSN 0259-7268
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Human exposure to captafol
2.2. Uptake, metabolism, and excretion
2.3. Effects on animals
2.4. Effects on human beings
2.5. Effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. Prevention and protection
4.1.2. First aid
4.2. Advice to physicians
4.3. Explosion and fire hazards
4.4. Storage and transport
4.5. Spillage and disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Exposure limit values
6.2. Specific restrictions
6.3. Transport and labelling
INTRODUCTION
This Health and Safety Guide is not based on an existing Environmental
Health Criteria document, but on critical national reviews. The
hazard evaluation in the Health and Safety Guide was made on the basis
of carefully selected studies, after scrutiny of the original
publications.
In order to assist the peer-review process of the present Health and
Safety Guide, a background companion document was prepared by the IPCS
and can be obtained from the Manager on request; the IPCS does not
intend that the background document should be published.
The first three sections of this Health and Safety Guide present
essential technical information and the hazard evaluation. Section 4
includes advice on preventive and protective measures and emergency
action; health workers should be thoroughly familiar with the medical
information to ensure that they can act efficiently in an emergency.
The section on regulatory information has been extracted from the
legal file of the International Register of Potentially Toxic
Chemicals (IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Manager
International Programme on Chemical Safety
Division of Environmental Health
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical formula: C10H9Cl4NO2S
Common name: captafol
Chemical structure:
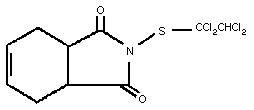 Relative molecular mass: 349.1
Common trade names Captafol; Captatol; Captofol; Captaspor;
(including formulations): Difolatan; Difosan; Merpafol; Folcid;
Ortho-5865; Ortho Difolatan 80W; Ortho
Difolatan 4 Flowable; Sanseal; Sanspor;
Sulfonimide; Sulpheimide
CAS chemical name: N-((1,1,2,2-tetrachloroethyl)thio)-4-
cyclo-hexene-1,2-dicarboximide
Synonyms: N-(1,1,2,2-tetrachloroethylthio)-
cyclohex-4-ene-1,2-dicarboximide;
N-(1,1,2,2-tetrachloraethylthio)-
tetra-hydrophthalamid;
N-1,1,2,2-tetrachloroethylmercapto-4-
cyclohexene-1,2-carboximide;
N-((1,1,2,2-tetrachloroethyl)-
sulfenyl)- cis-4-cyclohexene-1,2-
dicarboximide;
N-(1,1,2,2-tetra-chloroethylthio)-4-
cyclo-hexene-1,2-dicarboximide
CAS registry number: 2425-06-1
RTECS registry number: GW4900000
In the past, captafol was available in a wide range of mixtures and
formulations. However, information on currently available
formulations is not available.
Technical captafol, previously manufactured in the USA, contained
97-99% active ingredient with tetrahydrophthalimide (4-cyclohexene-
1,2-dicarboximide) as the main impurity (0.5-1.5%) together with
0.5-1.5% toluene and 0.1-0.2% of unknown chlorinated substances.
1.2 Physical and Chemical Properties
Technical captafol is a light tan powder with a characteristic odour.
It is stable at room temperature in the dry state, but is readily
hydrolysed, especially in an alkaline environment. The melting point
of the pure compound is 162 °C; a range of 156-161 °C has been
recorded for the technical product. The vapour pressure at room
temperature is negligible.
Captafol is practically insoluble in water (1.4 mg/litre) and only
slightly soluble in aliphatic hydrocarbon solvents: it has a
solubility of 25 g/litre at 77 °C in isopropanol, and of 428 g/litre
in toluene at 24 °C.
Sulfhydryl compounds, such as glutathione and cysteine, cause rapid
chemical decomposition of captafol.
1.3 Analytical Methods
Capillary gas-liquid chromatography with electron-capture detection is
a multi-residue method suitable for the routine determination of five
fungicides including captan, folpet, captafol, vinclozolin, and
iprodione.
1.4 Production and Uses
Captafol was introduced in 1961; although it was withdrawn in many
countries in the late 1980s, production and use continue in some
countries.
Captafol is a non-systemic broad-spectrum fungicide, leaving
relatively stable deposits when applied to foliage. This pesticide
can be combined with commonly used insecticides and fungicides, except
for oil sprays and strongly alkaline materials. Captafol is mainly
used to control foliage and fruit diseases in various vegetable and
fruit crops. It has also been used in the lumber and timber
industries for the control of wood rot fungi on logs and wood
products.
Captafol is formulated as a wettable powder, dust, emulsifiable
concentrate, flowable suspension, and as water-dispersible granules.
It is applied by dusting, spraying, and misting, and by dipping under
pressure (wood treatment).
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Captafol
The highest exposures to captafol are occupational and are associated
with its use in agriculture. Dermal exposure can be important because
of local effects on the skin. Low-level exposure of the general
population may occur through residues in food. However, captafol is
extensively hydrolysed during thermal and other food processing.
Because of the nature of the captafol residues, they are readily
reduced by, for example, peeling, washing, and blanching.
2.2 Uptake, Metabolism, and Excretion
Captafol may be absorbed through ingestion as well as through
inhalation, and to a very limited extent through skin exposure.
Following oral administration, captafol appears to be extensively
hydrolysed to tetrahydrophthalimide (THPI; 4-cyclohexene-1,2-
dicarboximide) and tetrachloro-ethylmercaptan (TES). Captafol and its
metabolites do not accumulate in the tissues of animals and are
rapidly eliminated; after a single oral dose of labelled captafol,
most of the radioactivity appeared to be excreted within 3-4 days,
primarily in the urine. Tetrahydrophthalimide (THPI) has been
identified as the major metabolite of captafol in both animals and
plants, as is the case for the closely related fungicide, captan.
THPI is further metabolized into a large number of metabolites. More
important for the toxicity of captafol is the further metabolism of
TES. To explain the formation of the end metabolite, 2-chloro-
2-methyl-thioethylene sulfonic acid, the formation of a cyclic
sulfonium ion is assumed to be a transient intermediate. This
intermediate is a potential alkylating agent and is probably
responsible for the toxic and carcinogenic action of captafol.
2.3 Effects on Animals
The acute toxicity of captafol, when ingested, is low. However, the
inhalation toxicity is considerably higher, and it is irritating to
the skin and also to the mucous membranes of the respiratory tract.
The substance is highly irritating to the eye and may cause eye
damage. There are several reports indicating that captafol induces
sensitization in guinea-pigs.
After repeated administration in the diet to experimental animals,
captafol caused toxic effects involving several organs, notably kidney
damage, and pathological changes of the gastric mucosa. Long-term
administration to rats and mice induced tumours at multiple sites.
These results constitute sufficient evidence for the carcinogenicity
of captafol. Captafol, which is an alkylating agent, has produced
genotoxic effects in several in vitro systems, but, so far, it has
not been possible to demonstrate any mutagenic effects in vivo.
Thus, though captafol may induce genotoxic events in somatic cells,
the results obtained seem to indicate that the potential for causing
heritable effects in mammals is extremely low. There is no evidence
that captafol constitutes a teratogenic hazard, but it may induce
fetotoxicity at doses toxic for the mother.
2.4 Effects on Human Beings
Captafol has caused allergic and contact dermatitis in man. During
occupational exposure, it has also been reported to cause severe
irritation of the respiratory tract, eye damage, and other systemic
effects. In a limited study of employees involved in the manufacture
of captafol, no significant excess in mortality could be associated
with exposure to this pesticide.
2.5 Effects on the Environment
Captafol, administered as a single oral dose, or in short-term dietary
studies, was not toxic for birds (LC50 >5620 mg/kg diet). However,
high levels of exposure may cause reproductive impairment. The
toxicity of captafol for bees is low. Captafol is highly toxic for
fish and moderately to very highly toxic for freshwater invertebrates
(96-h LC50s ranged between 0.04 and 3 mg/litre). The 96-h LC50
reported in three investigations on rainbow trout ranged from 0.027 to
0.19 mg/litre.
Captafol is not persistent. Its half-life in soil is less than 11
days. The environmental impact of the pesticide is likely to be
limited by its high chemical reactivity, high rate of biodegradation,
and lack of tendency to bioaccumulate. However, because of its
demonstrated high toxicity, exposure of aquatic organisms to captafol
through drift and/or run-off is a cause for concern. Fish kills have
been associated with the use of this pesticide.
3. CONCLUSIONS AND RECOMMENDATIONS
In view of the established carcinogenic potential of captafol and the
availability of alternatives, it is recommended that this pesticide
should not be used.
Captafol is highly toxic for fish, and moderately to highly toxic for
freshwater invertebrates. Because of this demonstrated high aquatic
toxicity, it is recommended that adequate precautions be taken to
prevent contamination of surface and ground water.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The acute oral toxicity of technical captafol for human beings is low.
The compound causes severe irritation of the respiratory tract, and
allergic dermatitis. Captafol also has a potential for causing eye
damage.
In view of the severe toxicity induced in experimental animals with
repeated exposure, including a proven carcinogenic action, exposure of
human beings should be kept to a minimum.
4.1.1 Prevention and protection
The following precautions should be observed during the handling and
use of captafol, in order to reduce the risk of accidental
contamination:
(a) Avoid contact with the skin and eyes.
(b) Do not smoke, drink, or eat in the work-place. Wash hands and
any exposed skin before eating, drinking, or smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable powder
formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear protective PVC or
neoprene gloves.
(f) When handling leaking containers, or when dealing with leaks and
spills, wear overalls, PVC or neoprene gloves, boots, and eye/face
protection. If overalls become contaminated, change and wash them
thoroughly before re-use.
(g) Store products in closed original containers, out of reach of
children, and away from food, drink, and animal feed.
4.1.2 First Aid
Acute poisoning by captafol is unlikely, unless large amounts are
ingested. In cases of over-exposure, apply routine first-aid
measures. If the compound has been spilled on the skin, immediately
remove the patient from the source of contamination, remove all
contaminated clothing, and wash affected areas with soap and running
water. If the material is in the eyes, flush with clean water for at
least 15 minutes. In case of ingestion of significant quantities, if
the patient is conscious, give several glasses of water. Do not
induce vomiting. In serious cases, medical attention should be
sought.
4.2 Advice to Physicians
The acute oral toxicity of captafol for human beings is low. There is
no specific antidote. Treat symptomatically, paying special attention
to respiratory and dermal symptoms when necessary. In cases of
ingestion of large amounts, gastric lavage may be indicated.
4.3 Explosion and Fire Hazards
Captafol is not flammable but, on heating, may produce toxic fumes,
such as sulfur dioxide, hydrochloric acid, and phosgene. Concentrated
products react violently with alkali.
Extinguish small fires with carbon dioxide, dry powder, or
alcohol-resistant foam. Water spray can be used for larger fires and
for the cooling of unaffected stock, but avoid the accumulation of
polluted run-off from the site. Fire service personnel should be
advised that self-contained breathing apparatus may be required,
because of the generation of noxious fumes.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
Avoid contact with the solid or dust. Keep spectators away from any
leakage. This pesticide is highly toxic for fish. Prevent
contamination of other goods or cargo, and of nearby vegetation and
waterways.
Absorb spilled liquid products using earth or sand. If available,
sawdust, peat, moss, or straw are also suitable absorbents; sweep up
and place in a separate container. Empty any product remaining in
damaged or leaking containers into a clean empty container, which
should be suitably labelled. Sweep up any spilled powder with damp
sawdust, taking care not to raise a dust cloud (use a vacuum cleaner).
Remove trapped material with suction hoses. Place in a separate
container for subsequent disposal. Use mechanical dredges or lifts to
remove immobilized masses of pollutants and precipitates.
Before disposal, captafol can be concentrated by gravity separation
followed by dual media filtration and activated carbon adsorption.
Alkaline treatment of captafol leads to the formation of degradation
products of much lower toxicity. For treatment of large spills, or
for the decontamination of equipment, the use of an aqueous solution
of commercial low-foaming, hard-water detergent in 5% trisodium
phosphate or 10-25% sodium hydroxide is recommended. During
neutralization, hydrogen sulfide may be formed, if insufficient alkali
is used.
Do not deposit in landfill. Captafol is not amenable to biological
treatment at municipal sewage plants.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Captafol is not persistent and small quantities of the compound are
readily hydrolysed in soil and surface waters. However, it is highly
toxic for aquatic organisms. Contamination of ponds, waterways, and
ditches with captafol should be avoided. In case of spills, and for
the decontamination of equipment and containers, apply the methods
recommended in section 4.5.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with appropriate regulatory authorities
before application.
6.1 Exposure Limit Values
The threshold limit value (TWA) recommended by the US ACGIH for
captafol is 0.1 mg/m3 in air. This value is also enforced in
Argentina, Australia, Canada, the Netherlands, and the United Kingdom.
In 1985, the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
recommended that the temporary ADI should be withdrawn, saying that,
"In view of the established carcinogenic potential of this compound,
the meeting recommended that captafol should not be used where its
residues in food can arise."
Some tolerances for food and animal feed are given in the table
opposite.
6.2 Specific Restrictions
Captafol has never been granted registration in the German Democratic
Republic or Sweden. In 1987, the pesticide was voluntarily withdrawn
from the US market by the main producers. It was banned in the
Netherlands as from 21 May 1986 and also in Cyprus, the Federal
Republic of Germany, Hungary, and Italy.
TOLERANCES AND MAXIMUM RESIDUE LIMITS FOR FOOD PRODUCTS
Country/ Food product Exposure limit description Value Effective
organization (mg/kg) date
Brazil Specified plant products Acceptable limit 0.04-15
Czechoslovakia Plants products Maximum residue limit 2-15 October 1978
EEC Specified plant products Maximum residue limit 0.058a 1988
Sweden Fruits and vegetables Maximum acceptable 0.5-3.0 January 1985
concentration
USA Raw agricultural products Tolerance 0.25-100 May 1986
a Limit of detection.
6.3 Transport and Labelling
Conveyance labelling should be as follows:
Relative molecular mass: 349.1
Common trade names Captafol; Captatol; Captofol; Captaspor;
(including formulations): Difolatan; Difosan; Merpafol; Folcid;
Ortho-5865; Ortho Difolatan 80W; Ortho
Difolatan 4 Flowable; Sanseal; Sanspor;
Sulfonimide; Sulpheimide
CAS chemical name: N-((1,1,2,2-tetrachloroethyl)thio)-4-
cyclo-hexene-1,2-dicarboximide
Synonyms: N-(1,1,2,2-tetrachloroethylthio)-
cyclohex-4-ene-1,2-dicarboximide;
N-(1,1,2,2-tetrachloraethylthio)-
tetra-hydrophthalamid;
N-1,1,2,2-tetrachloroethylmercapto-4-
cyclohexene-1,2-carboximide;
N-((1,1,2,2-tetrachloroethyl)-
sulfenyl)- cis-4-cyclohexene-1,2-
dicarboximide;
N-(1,1,2,2-tetra-chloroethylthio)-4-
cyclo-hexene-1,2-dicarboximide
CAS registry number: 2425-06-1
RTECS registry number: GW4900000
In the past, captafol was available in a wide range of mixtures and
formulations. However, information on currently available
formulations is not available.
Technical captafol, previously manufactured in the USA, contained
97-99% active ingredient with tetrahydrophthalimide (4-cyclohexene-
1,2-dicarboximide) as the main impurity (0.5-1.5%) together with
0.5-1.5% toluene and 0.1-0.2% of unknown chlorinated substances.
1.2 Physical and Chemical Properties
Technical captafol is a light tan powder with a characteristic odour.
It is stable at room temperature in the dry state, but is readily
hydrolysed, especially in an alkaline environment. The melting point
of the pure compound is 162 °C; a range of 156-161 °C has been
recorded for the technical product. The vapour pressure at room
temperature is negligible.
Captafol is practically insoluble in water (1.4 mg/litre) and only
slightly soluble in aliphatic hydrocarbon solvents: it has a
solubility of 25 g/litre at 77 °C in isopropanol, and of 428 g/litre
in toluene at 24 °C.
Sulfhydryl compounds, such as glutathione and cysteine, cause rapid
chemical decomposition of captafol.
1.3 Analytical Methods
Capillary gas-liquid chromatography with electron-capture detection is
a multi-residue method suitable for the routine determination of five
fungicides including captan, folpet, captafol, vinclozolin, and
iprodione.
1.4 Production and Uses
Captafol was introduced in 1961; although it was withdrawn in many
countries in the late 1980s, production and use continue in some
countries.
Captafol is a non-systemic broad-spectrum fungicide, leaving
relatively stable deposits when applied to foliage. This pesticide
can be combined with commonly used insecticides and fungicides, except
for oil sprays and strongly alkaline materials. Captafol is mainly
used to control foliage and fruit diseases in various vegetable and
fruit crops. It has also been used in the lumber and timber
industries for the control of wood rot fungi on logs and wood
products.
Captafol is formulated as a wettable powder, dust, emulsifiable
concentrate, flowable suspension, and as water-dispersible granules.
It is applied by dusting, spraying, and misting, and by dipping under
pressure (wood treatment).
2. SUMMARY AND EVALUATION
2.1 Human Exposure to Captafol
The highest exposures to captafol are occupational and are associated
with its use in agriculture. Dermal exposure can be important because
of local effects on the skin. Low-level exposure of the general
population may occur through residues in food. However, captafol is
extensively hydrolysed during thermal and other food processing.
Because of the nature of the captafol residues, they are readily
reduced by, for example, peeling, washing, and blanching.
2.2 Uptake, Metabolism, and Excretion
Captafol may be absorbed through ingestion as well as through
inhalation, and to a very limited extent through skin exposure.
Following oral administration, captafol appears to be extensively
hydrolysed to tetrahydrophthalimide (THPI; 4-cyclohexene-1,2-
dicarboximide) and tetrachloro-ethylmercaptan (TES). Captafol and its
metabolites do not accumulate in the tissues of animals and are
rapidly eliminated; after a single oral dose of labelled captafol,
most of the radioactivity appeared to be excreted within 3-4 days,
primarily in the urine. Tetrahydrophthalimide (THPI) has been
identified as the major metabolite of captafol in both animals and
plants, as is the case for the closely related fungicide, captan.
THPI is further metabolized into a large number of metabolites. More
important for the toxicity of captafol is the further metabolism of
TES. To explain the formation of the end metabolite, 2-chloro-
2-methyl-thioethylene sulfonic acid, the formation of a cyclic
sulfonium ion is assumed to be a transient intermediate. This
intermediate is a potential alkylating agent and is probably
responsible for the toxic and carcinogenic action of captafol.
2.3 Effects on Animals
The acute toxicity of captafol, when ingested, is low. However, the
inhalation toxicity is considerably higher, and it is irritating to
the skin and also to the mucous membranes of the respiratory tract.
The substance is highly irritating to the eye and may cause eye
damage. There are several reports indicating that captafol induces
sensitization in guinea-pigs.
After repeated administration in the diet to experimental animals,
captafol caused toxic effects involving several organs, notably kidney
damage, and pathological changes of the gastric mucosa. Long-term
administration to rats and mice induced tumours at multiple sites.
These results constitute sufficient evidence for the carcinogenicity
of captafol. Captafol, which is an alkylating agent, has produced
genotoxic effects in several in vitro systems, but, so far, it has
not been possible to demonstrate any mutagenic effects in vivo.
Thus, though captafol may induce genotoxic events in somatic cells,
the results obtained seem to indicate that the potential for causing
heritable effects in mammals is extremely low. There is no evidence
that captafol constitutes a teratogenic hazard, but it may induce
fetotoxicity at doses toxic for the mother.
2.4 Effects on Human Beings
Captafol has caused allergic and contact dermatitis in man. During
occupational exposure, it has also been reported to cause severe
irritation of the respiratory tract, eye damage, and other systemic
effects. In a limited study of employees involved in the manufacture
of captafol, no significant excess in mortality could be associated
with exposure to this pesticide.
2.5 Effects on the Environment
Captafol, administered as a single oral dose, or in short-term dietary
studies, was not toxic for birds (LC50 >5620 mg/kg diet). However,
high levels of exposure may cause reproductive impairment. The
toxicity of captafol for bees is low. Captafol is highly toxic for
fish and moderately to very highly toxic for freshwater invertebrates
(96-h LC50s ranged between 0.04 and 3 mg/litre). The 96-h LC50
reported in three investigations on rainbow trout ranged from 0.027 to
0.19 mg/litre.
Captafol is not persistent. Its half-life in soil is less than 11
days. The environmental impact of the pesticide is likely to be
limited by its high chemical reactivity, high rate of biodegradation,
and lack of tendency to bioaccumulate. However, because of its
demonstrated high toxicity, exposure of aquatic organisms to captafol
through drift and/or run-off is a cause for concern. Fish kills have
been associated with the use of this pesticide.
3. CONCLUSIONS AND RECOMMENDATIONS
In view of the established carcinogenic potential of captafol and the
availability of alternatives, it is recommended that this pesticide
should not be used.
Captafol is highly toxic for fish, and moderately to highly toxic for
freshwater invertebrates. Because of this demonstrated high aquatic
toxicity, it is recommended that adequate precautions be taken to
prevent contamination of surface and ground water.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
The acute oral toxicity of technical captafol for human beings is low.
The compound causes severe irritation of the respiratory tract, and
allergic dermatitis. Captafol also has a potential for causing eye
damage.
In view of the severe toxicity induced in experimental animals with
repeated exposure, including a proven carcinogenic action, exposure of
human beings should be kept to a minimum.
4.1.1 Prevention and protection
The following precautions should be observed during the handling and
use of captafol, in order to reduce the risk of accidental
contamination:
(a) Avoid contact with the skin and eyes.
(b) Do not smoke, drink, or eat in the work-place. Wash hands and
any exposed skin before eating, drinking, or smoking, and after work.
(c) Avoid raising a dust cloud when handling wettable powder
formulations.
(d) Avoid breathing dust from powder products.
(e) When unloading and handling containers, wear protective PVC or
neoprene gloves.
(f) When handling leaking containers, or when dealing with leaks and
spills, wear overalls, PVC or neoprene gloves, boots, and eye/face
protection. If overalls become contaminated, change and wash them
thoroughly before re-use.
(g) Store products in closed original containers, out of reach of
children, and away from food, drink, and animal feed.
4.1.2 First Aid
Acute poisoning by captafol is unlikely, unless large amounts are
ingested. In cases of over-exposure, apply routine first-aid
measures. If the compound has been spilled on the skin, immediately
remove the patient from the source of contamination, remove all
contaminated clothing, and wash affected areas with soap and running
water. If the material is in the eyes, flush with clean water for at
least 15 minutes. In case of ingestion of significant quantities, if
the patient is conscious, give several glasses of water. Do not
induce vomiting. In serious cases, medical attention should be
sought.
4.2 Advice to Physicians
The acute oral toxicity of captafol for human beings is low. There is
no specific antidote. Treat symptomatically, paying special attention
to respiratory and dermal symptoms when necessary. In cases of
ingestion of large amounts, gastric lavage may be indicated.
4.3 Explosion and Fire Hazards
Captafol is not flammable but, on heating, may produce toxic fumes,
such as sulfur dioxide, hydrochloric acid, and phosgene. Concentrated
products react violently with alkali.
Extinguish small fires with carbon dioxide, dry powder, or
alcohol-resistant foam. Water spray can be used for larger fires and
for the cooling of unaffected stock, but avoid the accumulation of
polluted run-off from the site. Fire service personnel should be
advised that self-contained breathing apparatus may be required,
because of the generation of noxious fumes.
4.4 Storage and Transport
All products should be stored in secure buildings, out of reach of
children and animals, and local regulations should be complied with.
Containers should be sound and adequately labelled.
4.5 Spillage and Disposal
Avoid contact with the solid or dust. Keep spectators away from any
leakage. This pesticide is highly toxic for fish. Prevent
contamination of other goods or cargo, and of nearby vegetation and
waterways.
Absorb spilled liquid products using earth or sand. If available,
sawdust, peat, moss, or straw are also suitable absorbents; sweep up
and place in a separate container. Empty any product remaining in
damaged or leaking containers into a clean empty container, which
should be suitably labelled. Sweep up any spilled powder with damp
sawdust, taking care not to raise a dust cloud (use a vacuum cleaner).
Remove trapped material with suction hoses. Place in a separate
container for subsequent disposal. Use mechanical dredges or lifts to
remove immobilized masses of pollutants and precipitates.
Before disposal, captafol can be concentrated by gravity separation
followed by dual media filtration and activated carbon adsorption.
Alkaline treatment of captafol leads to the formation of degradation
products of much lower toxicity. For treatment of large spills, or
for the decontamination of equipment, the use of an aqueous solution
of commercial low-foaming, hard-water detergent in 5% trisodium
phosphate or 10-25% sodium hydroxide is recommended. During
neutralization, hydrogen sulfide may be formed, if insufficient alkali
is used.
Do not deposit in landfill. Captafol is not amenable to biological
treatment at municipal sewage plants.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Captafol is not persistent and small quantities of the compound are
readily hydrolysed in soil and surface waters. However, it is highly
toxic for aquatic organisms. Contamination of ponds, waterways, and
ditches with captafol should be avoided. In case of spills, and for
the decontamination of equipment and containers, apply the methods
recommended in section 4.5.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file. A full reference to the original national document from which
the information was extracted can be obtained from IRPTC. When no
effective date appears in the IRPTC legal file, the year of the
reference from which the data are taken is indicated by (r).
The reader should be aware that regulatory decisions about chemicals
taken in a certain country can only be fully understood in the
framework of the legislation of that country. Furthermore, the
regulations and guidelines of all countries are subject to change and
should always be verified with appropriate regulatory authorities
before application.
6.1 Exposure Limit Values
The threshold limit value (TWA) recommended by the US ACGIH for
captafol is 0.1 mg/m3 in air. This value is also enforced in
Argentina, Australia, Canada, the Netherlands, and the United Kingdom.
In 1985, the Joint FAO/WHO Meeting on Pesticide Residues (JMPR)
recommended that the temporary ADI should be withdrawn, saying that,
"In view of the established carcinogenic potential of this compound,
the meeting recommended that captafol should not be used where its
residues in food can arise."
Some tolerances for food and animal feed are given in the table
opposite.
6.2 Specific Restrictions
Captafol has never been granted registration in the German Democratic
Republic or Sweden. In 1987, the pesticide was voluntarily withdrawn
from the US market by the main producers. It was banned in the
Netherlands as from 21 May 1986 and also in Cyprus, the Federal
Republic of Germany, Hungary, and Italy.
TOLERANCES AND MAXIMUM RESIDUE LIMITS FOR FOOD PRODUCTS
Country/ Food product Exposure limit description Value Effective
organization (mg/kg) date
Brazil Specified plant products Acceptable limit 0.04-15
Czechoslovakia Plants products Maximum residue limit 2-15 October 1978
EEC Specified plant products Maximum residue limit 0.058a 1988
Sweden Fruits and vegetables Maximum acceptable 0.5-3.0 January 1985
concentration
USA Raw agricultural products Tolerance 0.25-100 May 1986
a Limit of detection.
6.3 Transport and Labelling
Conveyance labelling should be as follows:
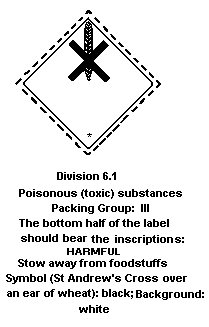 Supply and use labelling
European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
Supply and use labelling
European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
 The label must read:
R20 Harmful by inhalation
R36/37/38 Irritating to eyes, respiratory system and skin
R40 Possible risk of irreversible effects
R41 Risk of serious damage to eyes
R43 May cause sensitization by skin contact
R45 May cause cancer
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feeding
stuffs
S20/21 When using do not eat, drink, or smoke
S22 Do not breathe dust
S24/25 Avoid contact with skin and eyes
S36/37/39 Wear suitable protective clothing gloves and
eye/face protection
The label must read:
R20 Harmful by inhalation
R36/37/38 Irritating to eyes, respiratory system and skin
R40 Possible risk of irreversible effects
R41 Risk of serious damage to eyes
R43 May cause sensitization by skin contact
R45 May cause cancer
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feeding
stuffs
S20/21 When using do not eat, drink, or smoke
S22 Do not breathe dust
S24/25 Avoid contact with skin and eyes
S36/37/39 Wear suitable protective clothing gloves and
eye/face protection
 Relative molecular mass: 349.1
Common trade names Captafol; Captatol; Captofol; Captaspor;
(including formulations): Difolatan; Difosan; Merpafol; Folcid;
Ortho-5865; Ortho Difolatan 80W; Ortho
Difolatan 4 Flowable; Sanseal; Sanspor;
Sulfonimide; Sulpheimide
CAS chemical name: N-((1,1,2,2-tetrachloroethyl)thio)-4-
cyclo-hexene-1,2-dicarboximide
Synonyms: N-(1,1,2,2-tetrachloroethylthio)-
cyclohex-4-ene-1,2-dicarboximide;
N-(1,1,2,2-tetrachloraethylthio)-
tetra-hydrophthalamid;
N-1,1,2,2-tetrachloroethylmercapto-4-
cyclohexene-1,2-carboximide;
N-((1,1,2,2-tetrachloroethyl)-
sulfenyl)- cis-4-cyclohexene-1,2-
dicarboximide;
N-(1,1,2,2-tetra-chloroethylthio)-4-
cyclo-hexene-1,2-dicarboximide
CAS registry number:
Relative molecular mass: 349.1
Common trade names Captafol; Captatol; Captofol; Captaspor;
(including formulations): Difolatan; Difosan; Merpafol; Folcid;
Ortho-5865; Ortho Difolatan 80W; Ortho
Difolatan 4 Flowable; Sanseal; Sanspor;
Sulfonimide; Sulpheimide
CAS chemical name: N-((1,1,2,2-tetrachloroethyl)thio)-4-
cyclo-hexene-1,2-dicarboximide
Synonyms: N-(1,1,2,2-tetrachloroethylthio)-
cyclohex-4-ene-1,2-dicarboximide;
N-(1,1,2,2-tetrachloraethylthio)-
tetra-hydrophthalamid;
N-1,1,2,2-tetrachloroethylmercapto-4-
cyclohexene-1,2-carboximide;
N-((1,1,2,2-tetrachloroethyl)-
sulfenyl)- cis-4-cyclohexene-1,2-
dicarboximide;
N-(1,1,2,2-tetra-chloroethylthio)-4-
cyclo-hexene-1,2-dicarboximide
CAS registry number:  Supply and use labelling
European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
Supply and use labelling
European Economic Community legislation requires labelling as a
dangerous substance using the symbol:
 The label must read:
R20 Harmful by inhalation
R36/37/38 Irritating to eyes, respiratory system and skin
R40 Possible risk of irreversible effects
R41 Risk of serious damage to eyes
R43 May cause sensitization by skin contact
R45 May cause cancer
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feeding
stuffs
S20/21 When using do not eat, drink, or smoke
S22 Do not breathe dust
S24/25 Avoid contact with skin and eyes
S36/37/39 Wear suitable protective clothing gloves and
eye/face protection
The label must read:
R20 Harmful by inhalation
R36/37/38 Irritating to eyes, respiratory system and skin
R40 Possible risk of irreversible effects
R41 Risk of serious damage to eyes
R43 May cause sensitization by skin contact
R45 May cause cancer
S2 Keep out of reach of children
S13 Keep away from food, drink, and animal feeding
stuffs
S20/21 When using do not eat, drink, or smoke
S22 Do not breathe dust
S24/25 Avoid contact with skin and eyes
S36/37/39 Wear suitable protective clothing gloves and
eye/face protection
