
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 99
DIFLUBENZURON
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1995
This is a companion volume to Environmental Health Criteria 184:
Diflubenzuron
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Health and safety guide for diflubenzuron
(Health and safety guide ; no. 99)
1.Diflubenzuron - toxicity 2.Insecticides
3.Environmental exposure 4. I.Series
ISBN 92 4 151099 4 (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Summary
2.1.1. Identity, physical and chemical properties, and
analytical methods
2.1.2. Sources of human and environmental exposure
2.1.3. Environmental transport, distribution, and
transformation
2.1.4. Environmental levels and human exposure
2.1.5. Kinetics and metabolism in laboratory animals
2.1.6. Effects on experimental mammals, and in vitro
test systems
2.1.7. Effects on humans
2.1.8. Effects on non-target organisms in the laboratory
and field
2.2. Evaluation
2.2.1. Toxicological assessment
2.2.2. Environmental assessment
2.2.3. Toxicological criteria for setting guideline values
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first
aid
4.1.1. Prevention and protection
4.1.2. First aid
4.2. Advice to physicians
4.3. Explosion and fire hazards
4.4. Storage and transport
4.5. Spillage
4.6. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Previous evaluations by international bodies
6.2. Exposure limit values
6.3. Specific restrictions
6.4. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who are
involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE SHOULD BE CONSIDERED AS A STARTING POINT
TO A COMPREHENSIVE HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1 Identity
Chemical structure:
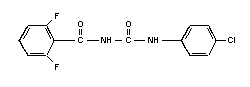 Molecular formula: C14H9ClF2N2O2
Common name: diflubenzuron (accepted by ISO, BSI, ANSI,
ESA)
Common trade names: Dimilin; Micromite; Vigilante
Common abbreviation: DFB
IUPAC name: 1-(4-chlorophenyl)-3-
(2,6-difluorobenzoyl)-urea
CAS chemical name: N-[[(4-chlorophenyl) amino]
carbonyl]-2,6-difluorobenzamide
CAS registry number: 35367-38-5
RTECS registry number: YS6200000
Technical diflubenzuron contains > 95% pure compound.
1.2 Physical and Chemical Properties
Diflubenzuron is an odourless, white, crystalline solid. It is almost
insoluble in water and poorly soluble in apolar organic solvents. In
polar to very polar solvents, the solubility is moderate to good,
e.g., in acetone 6.5 g/litre at 20°C. Diflubenzuron is highly soluble
in N-methylpyrolidone (200 g/litre), dimethylsulfoxide, and
dimethylformamide (both 120 g/litre).
Some physical and chemical properties of diflubenzuron are given in
Table 1.
Table 1. Physical and chemical properties of diflubenzuron
Relative molecular mass 310.7
Melting point technical > 95% 210-230°C
> 99% pure 230-232°C
Vapour pressure at 25°C 0.00012 mPa
Volatility: solid material <4%
from water pH 5.6 <2% virtually non-volatile
Specific gravity 1.56
Partition coefficient n-octanol/water (log Kow) 5000
Solubility in water (25°C) (pH 5.6) 8 × 10-5 g/litre
Stability in water after 3 weeks at pH 5 4% decomposition
(0.0001 g/litre) after 3 weeks at pH 7 8% decomposition
in the dark after 3 weeks at Ph 9 26% decomposition
Conversion factor: 1 ppm = 12.7 mg/m3 at 25°C
1.3 Analytical Methods
Two general types of assay procedures for residues of diflubenzuron in
crops, soil, water, and biological samples are available, i.e.,
high-pressure liquid chromatography and gas chromatography with
detection limits of approximately 0.01-0.05 mg/kg. The detection limit
in water is 0.1 µg/litre.
The Joint FAO/WHO Codex Alimentarius Commission has made
recommendations for the methods of analysis to be used for the
determination of diflubenzuron residues (FAO/WHO, 1989).
1.4 Production and Uses
The production figures are not available. Diflubenzuron is effective
as a stomach and contact insecticide, acting by inhibition of chitin
synthesis. It is usually applied directly on plants and in forest
areas, and in water for mosquito control.
2. SUMMARY AND EVALUATION
2.1 Summary
2.1.1 Identity, physical and chemical properties, and analytical
methods
Diflubenzuron is a member of the benzoylphenylurea group of
insecticides. Its insecticidal action is due to interaction with
chitin synthesis and/or deposition. It forms odourless white crystals
with a melting point of 230-232°C. It is sparingly soluble in water
(0.2 mg/litre at 20°C) and is virtually non-volatile. It is relatively
stable in acidic and neutral media but hydrolyses under alkaline
conditions.
Diflubenzuron is produced by the reaction of 2,6-difluorobenzamide
with 4-chlorophenylisocyanate.
Diflubenzuron residues may be measured in water, biological samples,
and soils using HPLC with UV detection, or GC with ECD for analysis of
the intact molecule or following derivatization of the liberated
4-chloroaniline with trifluoroacetic anhydride.
2.1.2 Sources of human and environmental exposure
Diflubenzuron is a synthetic compound used in agriculture, forestry,
and public health programmes to control insect pests and vectors.
Different formulations of diflubenzuron are available for these uses.
There is no relevant information on human exposure to diflubenzuron.
2.1.3 Environmental transport, distribution, and transformation
Diflubenzuron is usually applied directly to plants and water. Uptake
of diflubenzuron through plant leaves does not occur.
The adsorption of diflubenzuron on soil is rapid. It is immobilized in
the top 10-cm layer of soil to which it is applied and is unlikely to
leach. Diflubenzuron is degraded in soils of various types and origin
under aerobic or anaerobic conditions with a half-life of a few days.
The rate of degradation depends greatly on the diflubenzuron particle
size. The main metabolic pathway (over 90%) is hydrolysis leading to
2,6-difluorobenzoic acid and 4-chlorophenylurea; these are degraded
with half-lives of about 4 and 6 weeks, respectively. Free
4-chloroaniline has not been detected in soils.
Diflubenzuron degrades rapidly in neutral or alkaline waters. Studies
on the application of diflubenzuron to water showed rapid partition to
sediment; the parent compound and 4-chlorophenylurea may persist on
sediment for more than 30 days.
Diflubenzuron does not bioaccumulate in fish.
2.1.4 Environmental levels and human exposure
Exposure of the general population to diflubenzuron via water or food,
as a result of its use in agriculture, against forest insects, or in
mosquito control, is negligible.
2.1.5 Kinetics and metabolism in laboratory animals
In experimental animals, diflubenzuron is absorbed from the digestive
tract and to a lesser extent through the skin. There is a saturable
absorption mechanism in the rat gastrointestinal tract. Consequently,
a large proportion of orally administered diflubenzuron is found in
the faeces. Diflubenzuron is widely distributed in the tissues, but it
does not accumulate.
The metabolic fate of diflubenzuron has been studied in various
species. The major route of metabolism in mammals is via
hydroxylation. Hydrolysis of diflubenzuron may occur at any of the
three carbon-nitrogen bonds. In pigs and chickens, the major route of
hydrolysis is at the ureido bridge. In rats and cows, the major
metabolic pathway is hydroxylation. The major metabolites in sheep,
swine, and chickens are 2,6-difluorobenzoic acid and
4-chlorophenylurea; minor metabolites are 2,6-difluorobenzamide and
4-chloroaniline. In rats and cattle, 80% of the metabolites are
2,6-difluoro-3-hydroxydiflubenzuron, 4-chloro-2-hydroxy-diflubenzuron
and 4-chloro-3-hydroxydiflubenzuron. The metabolic studies indicate
that little or no 4-chloroaniline is formed in rats or cattle.
The major route of elimination is via the faeces, ranging from 70 to
85% in cats, pigs, and cattle. In sheep, elimination is roughly
equally distributed between the urine and faeces. Urinary excretion in
rats and mice decreases proportionally with increasing dosage level.
Less than 1% of an oral dose is recovered in exhaled air. Only trace
residues are found in milk.
No human studies on the kinetics and metabolism of diflubenzuron,
including the extent of biotransformation to 4-chloroaniline, are
available.
2.1.6 Effects on laboratory mammals, and in vitro test systems
The acute toxicity of diflubenzuron is low, by any route of exposure.
It has been classified by WHO as a "product unlikely to present an
acute hazard in normal use", based on an acute oral LD50 of more
than 4640 mg/kg body weight in rats. The acute dermal LD50 in rats
is greater than 10 000 mg/kg body weight, while the acute inhalation
LC50 for rats is greater than 2.49 mg/litre. No signs of
intoxication have been observed during the 14-day period following a
single administration of diflubenzuron, by various routes, to a
variety of animal species.
Diflubenzuron is neither a skin irritant (in rabbits) nor a skin
sensitizer (in guinea-pigs). It is marginally irritant to the eyes of
rabbits.
Diflubenzuron causes methaemoglobinaemia and sulfhaemoglobinaemia.
Dose-related methaemoglobinaemia has been demonstrated in various
species after oral, dermal, or inhalation exposure to diflubenzuron.
This effect is the most sensitive toxicological end-point in
experimental animals. The no-observed-effect level (NOEL), based on
methaemoglobin formation, is 2 mg/kg body weight per day in rats and
dogs and 2.4 mg/kg body weight per day in mice. In long-term toxicity
studies on mice and rats, treatment-related changes were principally
associated with oxidation of haemoglobin or with hepatocyte changes.
In carcinogenicity studies on mice and rats given dietary levels of up
to 10 000 mg/kg, there were no treatment-related effects on tumour
incidence. Specifically, there were no mesenchymal neoplasms of the
spleen or liver, as observed in carcinogenicity studies with
4-chloroaniline.
In several reproductive toxicity studies on rats, mice, rabbits, and
three avian species, no effects were seen on reproduction and there
was no embryotoxicity. Teratogenicity studies on rats and rabbits did
not reveal teratogenic effects.
Diflubenzuron and its main metabolites have been examined in a variety
of in vitro and in vivo mutagenicity tests. Neither diflubenzuron
nor its major metabolites have produced any mutagenic effects.
The minor metabolite, 4-chloroaniline, was shown to be positive in
several in vitro mutagenicity assays using various end-points. It is
carcinogenic in rats and mice. The neoplastic lesions related to
administration of 4-chloroaniline were benign and malignant
mesenchymal tumours in the spleens of male rats and haemangiomas and
haemangiosarcomas, primarily in the spleen and liver of male mice.
2.1.7 Effects on humans
The diflubenzuron metabolite, 4-chloroaniline, has been reported to
cause methaemoglobinaemia in exposed workers and in neonates
inadvertently exposed. Some individuals who are deficient in
NADH-methaemoglobin reductase may be particularly sensitive to
4-chloroaniline and, hence, to diflubenzuron exposure.
2.1.8 Effects on non-target organisms in the laboratory and field
All chitin-synthesizing organisms showed susceptibility to
diflubenzuron.
Bacteria were not affected by diflubenzuron at a concentration of
500 mg/kg soil; some stimulation of nitrogen fixation was seen.
Diflubenzuron acetone solutions were degraded; the acetone was used as
carbon source. Algal biomass increased at a diflubenzuron
concentration of 1 µg/litre. There were no adverse effects at
concentrations above the limit of diflubenzuron solubility. Fungi were
temporarily affected at 0.1 µg/litre in laboratory streams.
Aquatic invertebrates showed variable responses to diflubenzuron.
Molluscs were insensitive, the LC50 being greater than 200 mg/litre.
LC50s for other invertebrates ranged from 1 to > 1000 µg/litre,
reflecting the effects of the compound on juvenile, moulting stages. A
Maximum Acceptable Toxicant Concentration (MATC) for Daphnia has
been estimated at > 40 and < 93 ng/litre; as expected, larval
mayflies and other aquatic insect juveniles were highly susceptible.
Overspray of water bodies would be expected to kill some aquatic
invertebrates.
In ecosystems and field experiments, where diflubenzuron was applied
directly to the water, the effects on most taxa were less severe than
predicted from acute laboratory tests. No effects on aquatic organisms
have been found after aerial applications to forests.
The LC50s for fish are > 150 mg/litre. Fish kills have never been
recorded in field experiments.
The oral and contact LD50s for honey-bees were greater than
30 µg/bee. Honey-bee colonies were not affected after aerial
application of 350 g diflubenzuron/ha.
A 5-day dietary study on the mallard duck and bobwhite quail with
levels of up to 4640 mg/kg did not reveal any observable signs of
toxicity. Small songbirds in the forest ecosystem were not affected
after aerial application of diflubenzuron at 350 g/ha.
Small mammal species in a forest did not show any reductions in
numbers after application of diflubenzuron at 67 g/ha.
2.2 Evaluation
2.2.1 Toxicological assessment
The primary manifestation of diflubenzuron toxicity, i.e.,
methaemoglobin induction, occurred in a range of test animal species.
It is attributable to the metabolite, 4-chloroaniline, which is known
to induce methaemoglobin formation in several animal species and in
humans.
Diflubenzuron did not cause any other toxic effects following
long-term dietary administration. It was not mutagenic or carcinogenic
in mice or rats. However, its metabolite, 4-chloroaniline, is
mutagenic in vitro and is carcinogenic in mice and male rats.
Although 4-chloroaniline is a minor urinary metabolite of
diflubenzuron in rats, the extent to which it is formed in vivo in
various animal species remains unknown. Similarly, the comparative
extent of absorption of its parent compound in various species is not
known.
The sensitivity of human haemoglobin to methaemoglobin formation by
4-chloroaniline in vivo is not known. However, since induction of
methaemoglobinaemia has consistently been the most sensitive measure
of diflubenzuron toxicity in the various animal species tested, it may
be used as a basis to estimate levels causing no toxicological
effects.
2.2.2 Environmental assessment
Diflubenzuron adsorbs readily on soil particles with little subsequent
desorption. Its mobility in soil is very low, practically all residues
remaining within 15 cm of the surface, even in sandy loam soils;
diflubenzuron does not leach. It is only partly removed from foliage
by heavy rainfall. Nevertheless, some diflubenzuron may be present in
surface water shortly after application, due to flooding of treatment
areas or agricultural run-off.
Dissipation of diflubenzuron from water is rapid. Adsorption on
sediment occurs within 4 days; both the parent compound and the
metabolite, 4-chlorophenylurea, may persist on sediment for at least
30 days.
Uptake of diflubenzuron through the leaves of plants, after aerial
application, does not occur. Some uptake of soil residues does occur
in plants and this may be translocated. At the highest application
rate (1 kg a.i./ha), following 1 month of aging of residues, up to
1 mg residue/kg was found in various crops.
Photolysis of diflubenzuron is slow with a calculated half-life of
40 days. Under environmental conditions, abiotic degradation in water
and soil represents a minimum route of break-down. Aerobic degradation
in water is a microbial process with a half-life of a few days, under
both laboratory and field conditions. In the field, degradation of
diflubenzuron, applied at practical rates, is influenced by pH,
temperature, formulation, organic matter content, and depth of the
water.
Degradation in soil through microbial hydrolysis is a rapid process,
with a half-life of a few days, depending on diflubenzuron particle
size. The major break-down products are 2,6-difluorobenzoic acid and
4-chlorophenylurea; a minor metabolite is parachloroaniline. All these
are irreversibly bound to soil and/or further metabolized.
The half-life of diflubenzuron residues on citrus fruits was
significantly decreased by high temperature and humidity.
Anaerobic degradation in water and sediment is slower than aerobic
degradation.
Fish bioconcentrate diflubenzuron and some bioaccumulation takes place
during extended exposure up to a plateau, depending on the water
concentration, due to fast degradation of diflubenzuron and the
excretion of metabolites; the depuration half-life is less than one
day. The 4-chloroaniline metabolite has not been detected in fish.
Fish are not sensitive to diflubenzuron. The toxicity of metabolites
of diflubenzuron for fish is also low. Long-term exposure to
diflubenzuron at recommended application rates did not produce any
effects on fish; the compound does not persist in water and no
long-term exposure is expected.
Diflubenzuron, at the solubility limit concentration, was not
phytotoxic for duckweed.
Honey-bees were not affected by topical applications of > 30 µg/bee
or dietary concentrations of up to 1000 mg/kg. Broods in hives were
reduced when bees were fed syrup at 59 mg diflubenzuron/kg. Broods
were also reduced following exposure of flying colonies.
Earthworms were not affected at a concentration of 780 mg
diflubenzuron/kg soil, which is at least three orders of magnitude
above reported soil residues.
The acute toxicity of diflubenzuron for birds is low with oral and
dietary LD(LC)50s greater than 3000 mg/kg. At recommended
application rates, diflubenzuron is not expected to pose a hazard for
birds.
Extensive field studies have shown minimal or reversible effects on
most aquatic invertebrates; daphnids were most seriously affected,
with short-term reductions in population of up to 75% following a
single application of diflubenzuron. Fish were not affected by water
overspraying. Neither bird nor mammal populations were adversely
affected following forest spraying with diflubenzuron.
Risk quotients for avian and fish risk categories are summarized in
Table 2.
Table 2. Toxicity-exposure ratios for birds and fish based on application rates
of 2.5 kg diflubenzuron a.i./ha to soybeans (worst case)
Risk category LC50 as Estimated exposurea,b Toxicity/exposure
mg/litre or as mg/litre or ratio (TER)c
mg/kg diet mg/kg diet
Acute bird 3762 73.7-535.7 51.0-7.0
Acute fish (stream) 150 0.0007 214 300
Acute fish (pond) 150 0.01 15 000
Acute aquatic
invertebrate (stream) 0.005 0.0007 7.1
Acute aquatic
invertebrate (pond) 0.005 0.01 0.5
a Estimated environmental concentration in the terrestrial environment
(for bird exposure) is based on the stated application rate and the assumption
of deposition on short grass, using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STEAM model, based on
a single application and estimated runoff into water; no direct
overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at, or below,
1.0 indicate the likely exposure to toxic concentrations of organisms in
the different risk categories.
2.2.3 Toxicological criteria for setting guideline values
The toxicological studies on diflubenzuron of relevance for setting
guideline values are shown in Table 3.
Table 3. Toxicological criteria for estimating guideline values for diflubenzuron
Exposure scenario Relevant route/effect/ Result/remarks
(technical diflubenzuron) species
Short-term dermal, irritation, rabbit non-irritant
(1-7 days) ocular, irritation, rabbit marginal, high dose
dermal, sensitization, non-sensitizing
guinea-pig
inhalational, toxicity, rat LC50 2.49 mg/litre
(single exposure)
Mid-term (1-26 weeks)
3 weeks; 5 days/week dermal, irritation, rabbit NOEL = 70 mg/kg body
weight per day
3 weeks; 5 days/week inhalational, methaemoglobin NOAEL = < 0.12 mg/litre
formation, rat
Long-term dietary, methaemoglobin NOEL = 2 mg/kg body
formation, rat weight per day
dietary, methaemoglobin NOEL = 2.4 mg/kg body
formation, mouse weight per day
dietary, methaemoglobin NOEL = 2 mg/kg body
formation, dog weight per day
3. CONCLUSIONS AND RECOMMENDATIONS
Considering the toxicological characteristics of diflubenzuron, both
qualitatively and quantitatively, the CAG concluded, on the basis of
the NOEL of 2 mg/kg body weight per day in long-term toxicity studies
on mice, rats, and dogs, and applying a 100-fold uncertainty factor,
that 0.02 mg/kg body weight per day will probably not cause adverse
effects in humans, whatever the route of exposure.
Biomonitoring of 4-chloroaniline during occupational exposures should
be carried out.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
For general information see ILO (1993).
4.1 Human Health Hazards, Prevention and Protection, First Aid
The acute oral and dermal toxicities of diflubenzuron for humans
appear to be low. Diflubenzuron is not a skin irritant or a
sensitizer; it was marginally irritant when tested on rabbit eyes.
4.1.1 Prevention and protection
The following precautions should be observed during handling and use,
in order to reduce the risk of accidental contamination.
* Avoid contact with skin and eyes.
* Do not smoke, drink, or eat in the workplace. Wash hands and any
exposed skin before eating, drinking, or smoking, and after work.
* Avoid raising a dust cloud when handling wettable powder
formulations.
* Avoid breathing the dust from powder products.
* When unloading and handling containers, wear protective PVC or
neoprene gloves.
* When handling leaking containers or when dealing with leaks and
spills, wear overalls and PVC or neoprene gloves and boots. If
overalls become contaminated, change and wash them thoroughly
before re-use.
* Store products in closed original containers, out of reach of
children and away from food, drink, and animal feed.
4.1.2 First aid
Acute poisoning by diflubenzuron is unlikely because of its low acute
toxicity.
When considering human health hazards and first aid, it is essential
to determine which product the victim has been exposed to. It is
essential to differentiate between dry products (diflubenzuron
technical, the 90% concentrate, the 25% wettable powder and other dry
products, such as wettable powders with lower active ingredient and
low percentage granular products, which may be available locally),
water-based products (DIMILIN SC-48, DIMILIN SC-15, DIMILIN 4L), and
oil-based products (DIMILIN ODC-45, DIMILIN OF 6 and DIMILIN 2F).
In cases of overexposure, apply routine first aid measures. If
material has been spilled on the skin, immediately remove the victim
from the source of contamination, remove all contaminated clothing,
and wash affected areas with soap and running water. If the material
is in the eyes, flush with clean water for at least 5-10 min. In case
of ingestion of significant quantities, medical attention should be
sought.
4.2 Advice to Physicians
The acute oral toxicity of diflubenzuron for humans is low. There is
no specific antidote. Treat symptomatically, when required. If
oil-based products have been ingested and the victim starts vomiting,
it may be advisable to perform gastric lavage, in order to avoid
aspiration into the lungs.
4.3 Explosion and Fire Hazards
Diflubenzuron is not flammable. If diflubenzuron is involved in a
small fire, extinguish with carbon dioxide, dry powder, or
alcohol-resistant foam.
4.4 Storage and Transport
All products should be stored under dry conditions under lock and key,
out of reach of children and animals, and local regulations should be
complied with. Containers should be sound and adequately labelled.
4.5 Spillage
Avoid contact with solid or dust. Keep spectators away from any
leakage. The pesticide is highly toxic for aquatic invertebrates.
Absorb any spillage with sand or any other inert absorbent, collect
the mixture into a clean, empty container, which should be adequately
labelled and sent for incineration. Empty any product remaining in
damaged or leaking containers in a clean, empty container, which
should be suitably labelled.
4.6 Disposal
Proper incineration is the method of choice for this compound.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Diflubenzuron is not persistent and is readily degraded in soil and
water. However, it is highly toxic for aquatic invertebrates. Water
surfaces should not be oversprayed when diflubenzuron is applied for
mosquito control.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1 Previous Evaluations by International Bodies
Diflubenzuron was classified by WHO (1992) as "a product unlikely to
present an acute hazard in normal use", on the basis of an acute oral
LD50 for rat greater than 4640 mg/kg body weight.
Diflubenzuron was evaluated by the FAO/WHO Joint Meeting on Pesticide
Residues (JMPR) in 1981, 1984, and 1985. An acceptable daily intake
(ADI) for man for diflubenzuron was estimated at 0-0.02 mg/kg body
weight per day in 1985.
A guideline value of 22.5 µg/litre for drinking-water was recommended,
on the basis of allocation of the tolerable daily intake (TDI) of
0.0075 mg/kg body weight (WHO, 1991).
In the WHO Recommended Classification of Pesticides by Hazard,
technical diflubenzuron has been classified as a product unlikely to
present an acute hazard in normal use.
6.2 Exposure Limit Values
Some tolerances for food and animal feed products are given in
Table 4.
For all agricultural uses, "pre-harvest intervals" have been defined
in most countries.
6.3 Specific Restrictions
Diflubenzuron is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents.
6.4 Waste Disposal
Incineration at high temperature in a unit with effluent gas scrubbing
is the method of choice.
Table 4. Tolerances and Maximum Residue Limits for food products
Country/ Food product Exposure limit description Value Effective
Organization (mg/kg) date
Brazil Specified plant products Acceptable limit 0.1-0.2 1984
FAO/WHO Apples, blackcurrants, brussels Maximum residue limit 1.0 1988
sprouts, cabbage, citrus fruits,
pears, plums, tomatoes
cottonseed 0.2
mushrooms, soybeans 0.1
carcass meat, eggs, milk,
meat by-products, poultry meat 0.05
Finland Mushrooms Maximum residue limit 0.1 1992
Germany Wild strawberries, 2.0 1989
stone fruits, cabbage 1.0
mushrooms 0.2
USA Raw agricultural products Acceptable residue limit 0.05-1.0 1984
(specified plant and animal
products)
Soybean hulls and soap stock 0.1-0.5
USSR Specified food products Maximum residue limit 0.1 1988
BIBLIOGRAPHY
CEC (1987) Legislation in dangerous substances - Classification and
labelling in the European Communities - Vol. 1 & 2. Commission of
the European Communities, London, Graham & Trotman, Ltd.
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964-present) Evaluations of pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1986) Codex Maximum Limits for pesticide residues. Codex
Alimentarius Commission, CAC/Vol. XIII., Supplement 1 & 2, 3rd ed.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues. 4th ed. Rome, Codex Commission on Pesticide
Residues, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packaging, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticides
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
HAYES, W.J., Jr & LAWS, E.R. Jr (1991) Handbook of pesticide
toxicology (3 vol.). New York, Academic Press.
IARC (1972-present) IARC Monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
ILO (1991) Safety and health in the use of agro-chemicals - a guide.
Geneva, International Labour Office.
ILO (1993) Safety and health in the use of chemicals at work - A
training manual, prepared by Abu Bakar Che Man & David Gold. Geneva,
International Labour Office.
IPCS (in preparation) Environmental Health Criteria No. 184
Diflubenzuron, Geneva, World Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (WHO
unpublished document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. Paris, United Nations
Environment Programme, Industry and Environment Office, 80 p.
UNITED NATIONS (1991) Consolidated list of products whose consumption
and/or sale have been banned, withdrawn, severely restricted or not
approved by Governments. 4th ed. New York, United Nations.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1992) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1992-93, Geneva, World Health
Organization (unpublished document WHO/PCS/92.14).
WORTHING, C.R. & HANCE, R.J. (1991) The pesticide manual. 9th ed.,
Farnham, United Kingdom, British Crop Protection Council.
Molecular formula: C14H9ClF2N2O2
Common name: diflubenzuron (accepted by ISO, BSI, ANSI,
ESA)
Common trade names: Dimilin; Micromite; Vigilante
Common abbreviation: DFB
IUPAC name: 1-(4-chlorophenyl)-3-
(2,6-difluorobenzoyl)-urea
CAS chemical name: N-[[(4-chlorophenyl) amino]
carbonyl]-2,6-difluorobenzamide
CAS registry number: 35367-38-5
RTECS registry number: YS6200000
Technical diflubenzuron contains > 95% pure compound.
1.2 Physical and Chemical Properties
Diflubenzuron is an odourless, white, crystalline solid. It is almost
insoluble in water and poorly soluble in apolar organic solvents. In
polar to very polar solvents, the solubility is moderate to good,
e.g., in acetone 6.5 g/litre at 20°C. Diflubenzuron is highly soluble
in N-methylpyrolidone (200 g/litre), dimethylsulfoxide, and
dimethylformamide (both 120 g/litre).
Some physical and chemical properties of diflubenzuron are given in
Table 1.
Table 1. Physical and chemical properties of diflubenzuron
Relative molecular mass 310.7
Melting point technical > 95% 210-230°C
> 99% pure 230-232°C
Vapour pressure at 25°C 0.00012 mPa
Volatility: solid material <4%
from water pH 5.6 <2% virtually non-volatile
Specific gravity 1.56
Partition coefficient n-octanol/water (log Kow) 5000
Solubility in water (25°C) (pH 5.6) 8 × 10-5 g/litre
Stability in water after 3 weeks at pH 5 4% decomposition
(0.0001 g/litre) after 3 weeks at pH 7 8% decomposition
in the dark after 3 weeks at Ph 9 26% decomposition
Conversion factor: 1 ppm = 12.7 mg/m3 at 25°C
1.3 Analytical Methods
Two general types of assay procedures for residues of diflubenzuron in
crops, soil, water, and biological samples are available, i.e.,
high-pressure liquid chromatography and gas chromatography with
detection limits of approximately 0.01-0.05 mg/kg. The detection limit
in water is 0.1 µg/litre.
The Joint FAO/WHO Codex Alimentarius Commission has made
recommendations for the methods of analysis to be used for the
determination of diflubenzuron residues (FAO/WHO, 1989).
1.4 Production and Uses
The production figures are not available. Diflubenzuron is effective
as a stomach and contact insecticide, acting by inhibition of chitin
synthesis. It is usually applied directly on plants and in forest
areas, and in water for mosquito control.
2. SUMMARY AND EVALUATION
2.1 Summary
2.1.1 Identity, physical and chemical properties, and analytical
methods
Diflubenzuron is a member of the benzoylphenylurea group of
insecticides. Its insecticidal action is due to interaction with
chitin synthesis and/or deposition. It forms odourless white crystals
with a melting point of 230-232°C. It is sparingly soluble in water
(0.2 mg/litre at 20°C) and is virtually non-volatile. It is relatively
stable in acidic and neutral media but hydrolyses under alkaline
conditions.
Diflubenzuron is produced by the reaction of 2,6-difluorobenzamide
with 4-chlorophenylisocyanate.
Diflubenzuron residues may be measured in water, biological samples,
and soils using HPLC with UV detection, or GC with ECD for analysis of
the intact molecule or following derivatization of the liberated
4-chloroaniline with trifluoroacetic anhydride.
2.1.2 Sources of human and environmental exposure
Diflubenzuron is a synthetic compound used in agriculture, forestry,
and public health programmes to control insect pests and vectors.
Different formulations of diflubenzuron are available for these uses.
There is no relevant information on human exposure to diflubenzuron.
2.1.3 Environmental transport, distribution, and transformation
Diflubenzuron is usually applied directly to plants and water. Uptake
of diflubenzuron through plant leaves does not occur.
The adsorption of diflubenzuron on soil is rapid. It is immobilized in
the top 10-cm layer of soil to which it is applied and is unlikely to
leach. Diflubenzuron is degraded in soils of various types and origin
under aerobic or anaerobic conditions with a half-life of a few days.
The rate of degradation depends greatly on the diflubenzuron particle
size. The main metabolic pathway (over 90%) is hydrolysis leading to
2,6-difluorobenzoic acid and 4-chlorophenylurea; these are degraded
with half-lives of about 4 and 6 weeks, respectively. Free
4-chloroaniline has not been detected in soils.
Diflubenzuron degrades rapidly in neutral or alkaline waters. Studies
on the application of diflubenzuron to water showed rapid partition to
sediment; the parent compound and 4-chlorophenylurea may persist on
sediment for more than 30 days.
Diflubenzuron does not bioaccumulate in fish.
2.1.4 Environmental levels and human exposure
Exposure of the general population to diflubenzuron via water or food,
as a result of its use in agriculture, against forest insects, or in
mosquito control, is negligible.
2.1.5 Kinetics and metabolism in laboratory animals
In experimental animals, diflubenzuron is absorbed from the digestive
tract and to a lesser extent through the skin. There is a saturable
absorption mechanism in the rat gastrointestinal tract. Consequently,
a large proportion of orally administered diflubenzuron is found in
the faeces. Diflubenzuron is widely distributed in the tissues, but it
does not accumulate.
The metabolic fate of diflubenzuron has been studied in various
species. The major route of metabolism in mammals is via
hydroxylation. Hydrolysis of diflubenzuron may occur at any of the
three carbon-nitrogen bonds. In pigs and chickens, the major route of
hydrolysis is at the ureido bridge. In rats and cows, the major
metabolic pathway is hydroxylation. The major metabolites in sheep,
swine, and chickens are 2,6-difluorobenzoic acid and
4-chlorophenylurea; minor metabolites are 2,6-difluorobenzamide and
4-chloroaniline. In rats and cattle, 80% of the metabolites are
2,6-difluoro-3-hydroxydiflubenzuron, 4-chloro-2-hydroxy-diflubenzuron
and 4-chloro-3-hydroxydiflubenzuron. The metabolic studies indicate
that little or no 4-chloroaniline is formed in rats or cattle.
The major route of elimination is via the faeces, ranging from 70 to
85% in cats, pigs, and cattle. In sheep, elimination is roughly
equally distributed between the urine and faeces. Urinary excretion in
rats and mice decreases proportionally with increasing dosage level.
Less than 1% of an oral dose is recovered in exhaled air. Only trace
residues are found in milk.
No human studies on the kinetics and metabolism of diflubenzuron,
including the extent of biotransformation to 4-chloroaniline, are
available.
2.1.6 Effects on laboratory mammals, and in vitro test systems
The acute toxicity of diflubenzuron is low, by any route of exposure.
It has been classified by WHO as a "product unlikely to present an
acute hazard in normal use", based on an acute oral LD50 of more
than 4640 mg/kg body weight in rats. The acute dermal LD50 in rats
is greater than 10 000 mg/kg body weight, while the acute inhalation
LC50 for rats is greater than 2.49 mg/litre. No signs of
intoxication have been observed during the 14-day period following a
single administration of diflubenzuron, by various routes, to a
variety of animal species.
Diflubenzuron is neither a skin irritant (in rabbits) nor a skin
sensitizer (in guinea-pigs). It is marginally irritant to the eyes of
rabbits.
Diflubenzuron causes methaemoglobinaemia and sulfhaemoglobinaemia.
Dose-related methaemoglobinaemia has been demonstrated in various
species after oral, dermal, or inhalation exposure to diflubenzuron.
This effect is the most sensitive toxicological end-point in
experimental animals. The no-observed-effect level (NOEL), based on
methaemoglobin formation, is 2 mg/kg body weight per day in rats and
dogs and 2.4 mg/kg body weight per day in mice. In long-term toxicity
studies on mice and rats, treatment-related changes were principally
associated with oxidation of haemoglobin or with hepatocyte changes.
In carcinogenicity studies on mice and rats given dietary levels of up
to 10 000 mg/kg, there were no treatment-related effects on tumour
incidence. Specifically, there were no mesenchymal neoplasms of the
spleen or liver, as observed in carcinogenicity studies with
4-chloroaniline.
In several reproductive toxicity studies on rats, mice, rabbits, and
three avian species, no effects were seen on reproduction and there
was no embryotoxicity. Teratogenicity studies on rats and rabbits did
not reveal teratogenic effects.
Diflubenzuron and its main metabolites have been examined in a variety
of in vitro and in vivo mutagenicity tests. Neither diflubenzuron
nor its major metabolites have produced any mutagenic effects.
The minor metabolite, 4-chloroaniline, was shown to be positive in
several in vitro mutagenicity assays using various end-points. It is
carcinogenic in rats and mice. The neoplastic lesions related to
administration of 4-chloroaniline were benign and malignant
mesenchymal tumours in the spleens of male rats and haemangiomas and
haemangiosarcomas, primarily in the spleen and liver of male mice.
2.1.7 Effects on humans
The diflubenzuron metabolite, 4-chloroaniline, has been reported to
cause methaemoglobinaemia in exposed workers and in neonates
inadvertently exposed. Some individuals who are deficient in
NADH-methaemoglobin reductase may be particularly sensitive to
4-chloroaniline and, hence, to diflubenzuron exposure.
2.1.8 Effects on non-target organisms in the laboratory and field
All chitin-synthesizing organisms showed susceptibility to
diflubenzuron.
Bacteria were not affected by diflubenzuron at a concentration of
500 mg/kg soil; some stimulation of nitrogen fixation was seen.
Diflubenzuron acetone solutions were degraded; the acetone was used as
carbon source. Algal biomass increased at a diflubenzuron
concentration of 1 µg/litre. There were no adverse effects at
concentrations above the limit of diflubenzuron solubility. Fungi were
temporarily affected at 0.1 µg/litre in laboratory streams.
Aquatic invertebrates showed variable responses to diflubenzuron.
Molluscs were insensitive, the LC50 being greater than 200 mg/litre.
LC50s for other invertebrates ranged from 1 to > 1000 µg/litre,
reflecting the effects of the compound on juvenile, moulting stages. A
Maximum Acceptable Toxicant Concentration (MATC) for Daphnia has
been estimated at > 40 and < 93 ng/litre; as expected, larval
mayflies and other aquatic insect juveniles were highly susceptible.
Overspray of water bodies would be expected to kill some aquatic
invertebrates.
In ecosystems and field experiments, where diflubenzuron was applied
directly to the water, the effects on most taxa were less severe than
predicted from acute laboratory tests. No effects on aquatic organisms
have been found after aerial applications to forests.
The LC50s for fish are > 150 mg/litre. Fish kills have never been
recorded in field experiments.
The oral and contact LD50s for honey-bees were greater than
30 µg/bee. Honey-bee colonies were not affected after aerial
application of 350 g diflubenzuron/ha.
A 5-day dietary study on the mallard duck and bobwhite quail with
levels of up to 4640 mg/kg did not reveal any observable signs of
toxicity. Small songbirds in the forest ecosystem were not affected
after aerial application of diflubenzuron at 350 g/ha.
Small mammal species in a forest did not show any reductions in
numbers after application of diflubenzuron at 67 g/ha.
2.2 Evaluation
2.2.1 Toxicological assessment
The primary manifestation of diflubenzuron toxicity, i.e.,
methaemoglobin induction, occurred in a range of test animal species.
It is attributable to the metabolite, 4-chloroaniline, which is known
to induce methaemoglobin formation in several animal species and in
humans.
Diflubenzuron did not cause any other toxic effects following
long-term dietary administration. It was not mutagenic or carcinogenic
in mice or rats. However, its metabolite, 4-chloroaniline, is
mutagenic in vitro and is carcinogenic in mice and male rats.
Although 4-chloroaniline is a minor urinary metabolite of
diflubenzuron in rats, the extent to which it is formed in vivo in
various animal species remains unknown. Similarly, the comparative
extent of absorption of its parent compound in various species is not
known.
The sensitivity of human haemoglobin to methaemoglobin formation by
4-chloroaniline in vivo is not known. However, since induction of
methaemoglobinaemia has consistently been the most sensitive measure
of diflubenzuron toxicity in the various animal species tested, it may
be used as a basis to estimate levels causing no toxicological
effects.
2.2.2 Environmental assessment
Diflubenzuron adsorbs readily on soil particles with little subsequent
desorption. Its mobility in soil is very low, practically all residues
remaining within 15 cm of the surface, even in sandy loam soils;
diflubenzuron does not leach. It is only partly removed from foliage
by heavy rainfall. Nevertheless, some diflubenzuron may be present in
surface water shortly after application, due to flooding of treatment
areas or agricultural run-off.
Dissipation of diflubenzuron from water is rapid. Adsorption on
sediment occurs within 4 days; both the parent compound and the
metabolite, 4-chlorophenylurea, may persist on sediment for at least
30 days.
Uptake of diflubenzuron through the leaves of plants, after aerial
application, does not occur. Some uptake of soil residues does occur
in plants and this may be translocated. At the highest application
rate (1 kg a.i./ha), following 1 month of aging of residues, up to
1 mg residue/kg was found in various crops.
Photolysis of diflubenzuron is slow with a calculated half-life of
40 days. Under environmental conditions, abiotic degradation in water
and soil represents a minimum route of break-down. Aerobic degradation
in water is a microbial process with a half-life of a few days, under
both laboratory and field conditions. In the field, degradation of
diflubenzuron, applied at practical rates, is influenced by pH,
temperature, formulation, organic matter content, and depth of the
water.
Degradation in soil through microbial hydrolysis is a rapid process,
with a half-life of a few days, depending on diflubenzuron particle
size. The major break-down products are 2,6-difluorobenzoic acid and
4-chlorophenylurea; a minor metabolite is parachloroaniline. All these
are irreversibly bound to soil and/or further metabolized.
The half-life of diflubenzuron residues on citrus fruits was
significantly decreased by high temperature and humidity.
Anaerobic degradation in water and sediment is slower than aerobic
degradation.
Fish bioconcentrate diflubenzuron and some bioaccumulation takes place
during extended exposure up to a plateau, depending on the water
concentration, due to fast degradation of diflubenzuron and the
excretion of metabolites; the depuration half-life is less than one
day. The 4-chloroaniline metabolite has not been detected in fish.
Fish are not sensitive to diflubenzuron. The toxicity of metabolites
of diflubenzuron for fish is also low. Long-term exposure to
diflubenzuron at recommended application rates did not produce any
effects on fish; the compound does not persist in water and no
long-term exposure is expected.
Diflubenzuron, at the solubility limit concentration, was not
phytotoxic for duckweed.
Honey-bees were not affected by topical applications of > 30 µg/bee
or dietary concentrations of up to 1000 mg/kg. Broods in hives were
reduced when bees were fed syrup at 59 mg diflubenzuron/kg. Broods
were also reduced following exposure of flying colonies.
Earthworms were not affected at a concentration of 780 mg
diflubenzuron/kg soil, which is at least three orders of magnitude
above reported soil residues.
The acute toxicity of diflubenzuron for birds is low with oral and
dietary LD(LC)50s greater than 3000 mg/kg. At recommended
application rates, diflubenzuron is not expected to pose a hazard for
birds.
Extensive field studies have shown minimal or reversible effects on
most aquatic invertebrates; daphnids were most seriously affected,
with short-term reductions in population of up to 75% following a
single application of diflubenzuron. Fish were not affected by water
overspraying. Neither bird nor mammal populations were adversely
affected following forest spraying with diflubenzuron.
Risk quotients for avian and fish risk categories are summarized in
Table 2.
Table 2. Toxicity-exposure ratios for birds and fish based on application rates
of 2.5 kg diflubenzuron a.i./ha to soybeans (worst case)
Risk category LC50 as Estimated exposurea,b Toxicity/exposure
mg/litre or as mg/litre or ratio (TER)c
mg/kg diet mg/kg diet
Acute bird 3762 73.7-535.7 51.0-7.0
Acute fish (stream) 150 0.0007 214 300
Acute fish (pond) 150 0.01 15 000
Acute aquatic
invertebrate (stream) 0.005 0.0007 7.1
Acute aquatic
invertebrate (pond) 0.005 0.01 0.5
a Estimated environmental concentration in the terrestrial environment
(for bird exposure) is based on the stated application rate and the assumption
of deposition on short grass, using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STEAM model, based on
a single application and estimated runoff into water; no direct
overspray is included.
c TER is the toxicity (as LC50) divided by the exposure; values at, or below,
1.0 indicate the likely exposure to toxic concentrations of organisms in
the different risk categories.
2.2.3 Toxicological criteria for setting guideline values
The toxicological studies on diflubenzuron of relevance for setting
guideline values are shown in Table 3.
Table 3. Toxicological criteria for estimating guideline values for diflubenzuron
Exposure scenario Relevant route/effect/ Result/remarks
(technical diflubenzuron) species
Short-term dermal, irritation, rabbit non-irritant
(1-7 days) ocular, irritation, rabbit marginal, high dose
dermal, sensitization, non-sensitizing
guinea-pig
inhalational, toxicity, rat LC50 2.49 mg/litre
(single exposure)
Mid-term (1-26 weeks)
3 weeks; 5 days/week dermal, irritation, rabbit NOEL = 70 mg/kg body
weight per day
3 weeks; 5 days/week inhalational, methaemoglobin NOAEL = < 0.12 mg/litre
formation, rat
Long-term dietary, methaemoglobin NOEL = 2 mg/kg body
formation, rat weight per day
dietary, methaemoglobin NOEL = 2.4 mg/kg body
formation, mouse weight per day
dietary, methaemoglobin NOEL = 2 mg/kg body
formation, dog weight per day
3. CONCLUSIONS AND RECOMMENDATIONS
Considering the toxicological characteristics of diflubenzuron, both
qualitatively and quantitatively, the CAG concluded, on the basis of
the NOEL of 2 mg/kg body weight per day in long-term toxicity studies
on mice, rats, and dogs, and applying a 100-fold uncertainty factor,
that 0.02 mg/kg body weight per day will probably not cause adverse
effects in humans, whatever the route of exposure.
Biomonitoring of 4-chloroaniline during occupational exposures should
be carried out.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
For general information see ILO (1993).
4.1 Human Health Hazards, Prevention and Protection, First Aid
The acute oral and dermal toxicities of diflubenzuron for humans
appear to be low. Diflubenzuron is not a skin irritant or a
sensitizer; it was marginally irritant when tested on rabbit eyes.
4.1.1 Prevention and protection
The following precautions should be observed during handling and use,
in order to reduce the risk of accidental contamination.
* Avoid contact with skin and eyes.
* Do not smoke, drink, or eat in the workplace. Wash hands and any
exposed skin before eating, drinking, or smoking, and after work.
* Avoid raising a dust cloud when handling wettable powder
formulations.
* Avoid breathing the dust from powder products.
* When unloading and handling containers, wear protective PVC or
neoprene gloves.
* When handling leaking containers or when dealing with leaks and
spills, wear overalls and PVC or neoprene gloves and boots. If
overalls become contaminated, change and wash them thoroughly
before re-use.
* Store products in closed original containers, out of reach of
children and away from food, drink, and animal feed.
4.1.2 First aid
Acute poisoning by diflubenzuron is unlikely because of its low acute
toxicity.
When considering human health hazards and first aid, it is essential
to determine which product the victim has been exposed to. It is
essential to differentiate between dry products (diflubenzuron
technical, the 90% concentrate, the 25% wettable powder and other dry
products, such as wettable powders with lower active ingredient and
low percentage granular products, which may be available locally),
water-based products (DIMILIN SC-48, DIMILIN SC-15, DIMILIN 4L), and
oil-based products (DIMILIN ODC-45, DIMILIN OF 6 and DIMILIN 2F).
In cases of overexposure, apply routine first aid measures. If
material has been spilled on the skin, immediately remove the victim
from the source of contamination, remove all contaminated clothing,
and wash affected areas with soap and running water. If the material
is in the eyes, flush with clean water for at least 5-10 min. In case
of ingestion of significant quantities, medical attention should be
sought.
4.2 Advice to Physicians
The acute oral toxicity of diflubenzuron for humans is low. There is
no specific antidote. Treat symptomatically, when required. If
oil-based products have been ingested and the victim starts vomiting,
it may be advisable to perform gastric lavage, in order to avoid
aspiration into the lungs.
4.3 Explosion and Fire Hazards
Diflubenzuron is not flammable. If diflubenzuron is involved in a
small fire, extinguish with carbon dioxide, dry powder, or
alcohol-resistant foam.
4.4 Storage and Transport
All products should be stored under dry conditions under lock and key,
out of reach of children and animals, and local regulations should be
complied with. Containers should be sound and adequately labelled.
4.5 Spillage
Avoid contact with solid or dust. Keep spectators away from any
leakage. The pesticide is highly toxic for aquatic invertebrates.
Absorb any spillage with sand or any other inert absorbent, collect
the mixture into a clean, empty container, which should be adequately
labelled and sent for incineration. Empty any product remaining in
damaged or leaking containers in a clean, empty container, which
should be suitably labelled.
4.6 Disposal
Proper incineration is the method of choice for this compound.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
Diflubenzuron is not persistent and is readily degraded in soil and
water. However, it is highly toxic for aquatic invertebrates. Water
surfaces should not be oversprayed when diflubenzuron is applied for
mosquito control.
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1 Previous Evaluations by International Bodies
Diflubenzuron was classified by WHO (1992) as "a product unlikely to
present an acute hazard in normal use", on the basis of an acute oral
LD50 for rat greater than 4640 mg/kg body weight.
Diflubenzuron was evaluated by the FAO/WHO Joint Meeting on Pesticide
Residues (JMPR) in 1981, 1984, and 1985. An acceptable daily intake
(ADI) for man for diflubenzuron was estimated at 0-0.02 mg/kg body
weight per day in 1985.
A guideline value of 22.5 µg/litre for drinking-water was recommended,
on the basis of allocation of the tolerable daily intake (TDI) of
0.0075 mg/kg body weight (WHO, 1991).
In the WHO Recommended Classification of Pesticides by Hazard,
technical diflubenzuron has been classified as a product unlikely to
present an acute hazard in normal use.
6.2 Exposure Limit Values
Some tolerances for food and animal feed products are given in
Table 4.
For all agricultural uses, "pre-harvest intervals" have been defined
in most countries.
6.3 Specific Restrictions
Diflubenzuron is approved as a pesticide in many countries. Specific
uses, limitations, and precautions are listed in national regulatory
documents.
6.4 Waste Disposal
Incineration at high temperature in a unit with effluent gas scrubbing
is the method of choice.
Table 4. Tolerances and Maximum Residue Limits for food products
Country/ Food product Exposure limit description Value Effective
Organization (mg/kg) date
Brazil Specified plant products Acceptable limit 0.1-0.2 1984
FAO/WHO Apples, blackcurrants, brussels Maximum residue limit 1.0 1988
sprouts, cabbage, citrus fruits,
pears, plums, tomatoes
cottonseed 0.2
mushrooms, soybeans 0.1
carcass meat, eggs, milk,
meat by-products, poultry meat 0.05
Finland Mushrooms Maximum residue limit 0.1 1992
Germany Wild strawberries, 2.0 1989
stone fruits, cabbage 1.0
mushrooms 0.2
USA Raw agricultural products Acceptable residue limit 0.05-1.0 1984
(specified plant and animal
products)
Soybean hulls and soap stock 0.1-0.5
USSR Specified food products Maximum residue limit 0.1 1988
BIBLIOGRAPHY
CEC (1987) Legislation in dangerous substances - Classification and
labelling in the European Communities - Vol. 1 & 2. Commission of
the European Communities, London, Graham & Trotman, Ltd.
FAO (1985a) Guidelines for the packaging and storage of pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1985b) Guidelines for the disposal of waste pesticides and
pesticide containers on the farm. Rome, Food and Agriculture
Organization of the United Nations.
FAO (1985c) Guidelines on good labelling practice for pesticides.
Rome, Food and Agriculture Organization of the United Nations.
FAO (1986) International code of conduct on the distribution and use
of pesticides. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964-present) Evaluations of pesticide residues in food.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1986) Codex Maximum Limits for pesticide residues. Codex
Alimentarius Commission, CAC/Vol. XIII., Supplement 1 & 2, 3rd ed.
Rome, Food and Agriculture Organization of the United Nations.
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues. 4th ed. Rome, Codex Commission on Pesticide
Residues, Food and Agriculture Organization of the United Nations.
GIFAP (1982) Guidelines for the safe handling of pesticides during
their formulation, packaging, storage and transport. Brussels,
Groupement International des Associations Nationales des Fabricants de
Produits Agrochimiques.
GIFAP (1983) Guidelines for the safe and effective use of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
GIFAP (1984) Guidelines for emergency measures in cases of pesticides
poisoning. Brussels, Groupement International des Associations
Nationales des Fabricants de Produits Agrochimiques.
GIFAP (1987) Guidelines for the safe transport of pesticides.
Brussels, Groupement International des Associations Nationales des
Fabricants de Produits Agrochimiques.
HAYES, W.J., Jr & LAWS, E.R. Jr (1991) Handbook of pesticide
toxicology (3 vol.). New York, Academic Press.
IARC (1972-present) IARC Monographs on the evaluation of carcinogenic
risk of chemicals to man. Lyon, International Agency for Research on
Cancer.
ILO (1991) Safety and health in the use of agro-chemicals - a guide.
Geneva, International Labour Office.
ILO (1993) Safety and health in the use of chemicals at work - A
training manual, prepared by Abu Bakar Che Man & David Gold. Geneva,
International Labour Office.
IPCS (in preparation) Environmental Health Criteria No. 184
Diflubenzuron, Geneva, World Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals. Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1987) IRPTC legal file 1986. Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment Programme.
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization (WHO
unpublished document VBC/84.889).
SAX, N.I. (1984) Dangerous properties of industrial materials. New
York, van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide for
safe warehousing of hazardous materials. Paris, United Nations
Environment Programme, Industry and Environment Office, 80 p.
UNITED NATIONS (1991) Consolidated list of products whose consumption
and/or sale have been banned, withdrawn, severely restricted or not
approved by Governments. 4th ed. New York, United Nations.
UNITED NATIONS (1989) Recommendations on the transport of dangerous
goods. 6th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards. 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS(NIOSH) 01-123).
WHO (1992) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1992-93, Geneva, World Health
Organization (unpublished document WHO/PCS/92.14).
WORTHING, C.R. & HANCE, R.J. (1991) The pesticide manual. 9th ed.,
Farnham, United Kingdom, British Crop Protection Council.
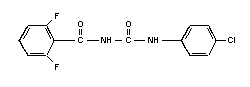 Molecular formula: C14H9ClF2N2O2
Common name: diflubenzuron (accepted by ISO, BSI, ANSI,
ESA)
Common trade names: Dimilin; Micromite; Vigilante
Common abbreviation: DFB
IUPAC name: 1-(4-chlorophenyl)-3-
(2,6-difluorobenzoyl)-urea
CAS chemical name: N-[[(4-chlorophenyl) amino]
carbonyl]-2,6-difluorobenzamide
CAS registry number:
Molecular formula: C14H9ClF2N2O2
Common name: diflubenzuron (accepted by ISO, BSI, ANSI,
ESA)
Common trade names: Dimilin; Micromite; Vigilante
Common abbreviation: DFB
IUPAC name: 1-(4-chlorophenyl)-3-
(2,6-difluorobenzoyl)-urea
CAS chemical name: N-[[(4-chlorophenyl) amino]
carbonyl]-2,6-difluorobenzamide
CAS registry number: 