
Concise International Chemical Assessment Document 15
1,2-DIAMINOETHANE (ETHYLENEDIAMINE)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Concise International Chemical Assessment Document 15
1,2-DIAMINOETHANE (ETHYLENEDIAMINE)
First draft prepared by
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom,
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom, and
Dr J. Delic, Health and Safety Executive, Merseyside, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1999
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
1,2-Diaminoethane (Ethylenediamine).
(Concise international chemical assessment document ; 15)
1.Ethylenediamines 2.Environmental exposure 3.Risk assessment
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153015 4 (NLM classification: QV 275)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1999
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for EDA
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effect
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENTS
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of guidance
values for health-based exposure limits. Geneva, World Health
Organization (Environmental Health Criteria 170).
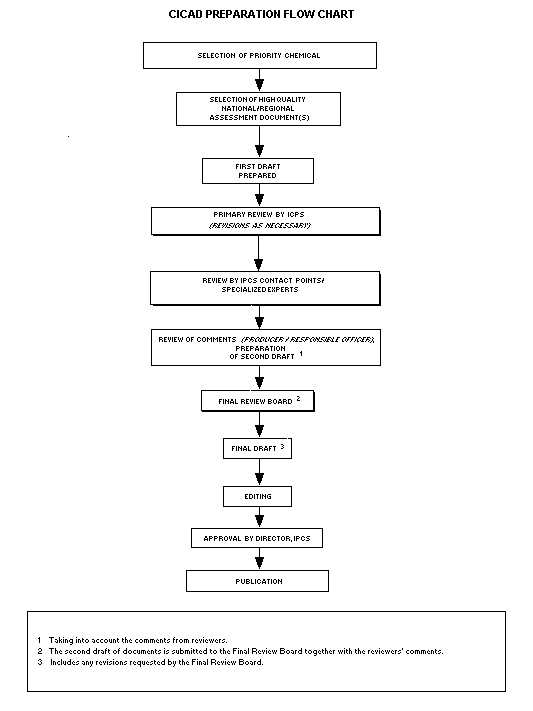
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on 1,2-diaminoethane (ethylenediamine) was based on a
review of human health concerns (primarily occupational, but also
including an environmental assessment) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997). Data
identified up to the end of 1994 were covered in the original review.
An additional literature search up to July 1997 was conducted to
identify any new information that had been published since the review
was completed. Information on environmental fate and effects was based
on the report of the German Chemical Society's Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1997). The
preparation and peer review of the source documents are described in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on
30 June - 2 July 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0269) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
1,2-Diaminoethane (CAS No. 107-15-3), commonly known as
ethylenediamine (EDA), is a synthetic colourless to yellowish liquid
at normal temperature and pressure. It is strongly alkaline and is
miscible with water and alcohol. The main use for EDA is as an
intermediate in the manufacture of tetraacetyl ethylenediamine,
ethylenediaminetetraacetic acid (EDTA), organic flocculants, urea
resins, and fatty bisamides. It is also used, to a much smaller
extent, in the production of formulations for use in the printed
circuit board and metal finishing industries, as an accelerator/curing
agent in epoxy coatings/resins, and in the manufacture of
pharmaceutical products. EDA is present as a contaminant (<0.5%) in
commercially supplied fatty amines, which are used as wetting agents
in bituminous emulsions. It is also used in the synthesis of carbamate
fungicides, in surfactant and dye manufacture, and in photography
development chemicals and cutting oils. EDA is a degradation product
of ethylenebis(dithiocarbamate) fungicides.
No atmospheric effects are expected, as reaction of EDA with
hydroxyl radicals is likely to be rapid (half-life 8.9 h), and washout
of volatilized EDA is expected. Volatilization to the atmosphere is
likely from soil but not from water. Adsorption to soil particulates
is strong through electrostatic binding; leaching through soil
profiles to groundwater is not expected. Complex formation with metals
and humic acids is expected. Biodegradation is the most likely source
of breakdown in the environment and should be quite rapid; adaptation
of microorganisms may improve degradation. Breakdown is less rapid in
seawater than in fresh water. Bioaccumulation is unlikely.
EDA has moderate acute toxicity in animals. It is a primary
irritant, being corrosive when undiluted, and is also a skin
sensitizer. EDA has not been tested for mutagenicity to current
regulatory standards, and there are no assays for clastogenic activity
or for the potential to express activity in somatic cells in vivo.
Thus, there is insufficient information to draw firm conclusions
regarding the mutagenic potential of EDA. EDA was not carcinogenic in
animals. Non-neoplastic effects on the liver (pleomorphic changes to
hepatocytes) have been observed in rats following oral dosing for 2
years at 45 mg EDA/kg body weight per day and above, with no effects
seen at 9 mg EDA/kg body weight per day. Although the significance of
these hepatic cell changes for human health is unclear, as well as
whether or not they are a consequence of oral exposure (i.e., they
might not occur via other routes, as they may be related to first-pass
effects), they cannot be discounted, and the risk of their development
should be characterized. In oral gavage dosing studies, effects on the
rat eye (retinal atrophy and, at higher doses, cataract formation)
were observed at doses of 100 mg EDA/kg body weight per day and above.
Doses of 200 and 100 mg EDA/kg body weight per day and above were
associated with renal damage in rats and mice, respectively. There was
also some indication of effects in the spleen in mice and rats at
doses of 400 mg EDA/kg body weight per day and above and in the thymus
in rats at 800 mg/kg body weight per day. In inhalation studies, no
effects were seen in rats at about 150 mg/m3 (60 ppm), and slight
depilation was the only treatment-related effect observed at about 330
mg/m3 (132 ppm).
Because diluted EDA is a skin irritant and a skin sensitizer,
there may be a risk of developing irritant and/or allergic dermatitis
if suitable personal protective equipment is not used in the
occupational environment where skin contact can occur. EDA is also
capable of inducing a state of respiratory tract hypersensitivity and
provoking asthma in the occupational environment, and this is
considered to be the major health effect of concern.
The mechanism for the induction of the hypersensitive state is
not proven, although the skin sensitizing potential of EDA and the
limited evidence of immunological involvement in workers with
EDA-provoked asthma are suggestive of an immunological mechanism.
However, irrespective of the mechanism involved, the available data do
not allow either elucidation of dose-response relationships or
identification of the thresholds for induction of the hypersensitive
state or provocation of an asthmatic response. The sample risk
characterization in this document has, in order to assess the risks of
other systemic effects, evaluated the risk of hepatic effects in
occupationally exposed individuals. It concludes that when EDA is used
in closed systems, the exposure, both measured and predicted from
models, is substantially (by 100-fold or greater) less than the
no-observed-effect level (NOEL) in rats; thus, adverse effects on the
liver are unlikely.
Exposure of the general public to EDA could not be evaluated
owing to the lack of available data.
Toxic thresholds for microorganisms may be as low as 0.1 mg
EDA/litre. However, toxicity tests in culture media should be treated
with caution, as the EDA may complex with metal ions. Effects may
therefore be indirect, resulting from the loss of bioavailability of
essential elements. LC50s for invertebrates and fish range from 14 to
>1000 mg/litre. A no-observed-effect concentration (NOEC) for
Daphnia reproduction has been reported at 0.16 mg/litre.
Given the wide range of acute and chronic test results, a
predicted no-effect concentration (PNEC) for aquatic organisms was
taken as 16 µg/litre, based on application of an uncertainty factor of
10 to the lowest reported NOEC for Daphnia reproduction.
Conservative assumptions for predicted environmental concentration
(PEC) produce PEC/PNEC ratios indicating some concern from initial
concentrations (i.e., at first release into the river or estuary).
However, more refined exposure estimates indicate low risk to aquatic
organisms.
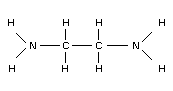
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,2-Diaminoethane (CAS No. 107-15-3) is more commonly known as
ethylenediamine, with EDA used as a common abbreviation. Other common
synonyms include dimethylenediamine, 1,2-ethanediamine,
1,2-ethylenediamine, beta-aminoethylamine, and ethane-1,2-diamine.
EDA's structural formula is shown below:
1. EXECUTIVE SUMMARY
This CICAD on 1,2-diaminoethane (ethylenediamine) was based on a
review of human health concerns (primarily occupational, but also
including an environmental assessment) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997). Data
identified up to the end of 1994 were covered in the original review.
An additional literature search up to July 1997 was conducted to
identify any new information that had been published since the review
was completed. Information on environmental fate and effects was based
on the report of the German Chemical Society's Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1997). The
preparation and peer review of the source documents are described in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on
30 June - 2 July 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0269) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
1,2-Diaminoethane (CAS No. 107-15-3), commonly known as
ethylenediamine (EDA), is a synthetic colourless to yellowish liquid
at normal temperature and pressure. It is strongly alkaline and is
miscible with water and alcohol. The main use for EDA is as an
intermediate in the manufacture of tetraacetyl ethylenediamine,
ethylenediaminetetraacetic acid (EDTA), organic flocculants, urea
resins, and fatty bisamides. It is also used, to a much smaller
extent, in the production of formulations for use in the printed
circuit board and metal finishing industries, as an accelerator/curing
agent in epoxy coatings/resins, and in the manufacture of
pharmaceutical products. EDA is present as a contaminant (<0.5%) in
commercially supplied fatty amines, which are used as wetting agents
in bituminous emulsions. It is also used in the synthesis of carbamate
fungicides, in surfactant and dye manufacture, and in photography
development chemicals and cutting oils. EDA is a degradation product
of ethylenebis(dithiocarbamate) fungicides.
No atmospheric effects are expected, as reaction of EDA with
hydroxyl radicals is likely to be rapid (half-life 8.9 h), and washout
of volatilized EDA is expected. Volatilization to the atmosphere is
likely from soil but not from water. Adsorption to soil particulates
is strong through electrostatic binding; leaching through soil
profiles to groundwater is not expected. Complex formation with metals
and humic acids is expected. Biodegradation is the most likely source
of breakdown in the environment and should be quite rapid; adaptation
of microorganisms may improve degradation. Breakdown is less rapid in
seawater than in fresh water. Bioaccumulation is unlikely.
EDA has moderate acute toxicity in animals. It is a primary
irritant, being corrosive when undiluted, and is also a skin
sensitizer. EDA has not been tested for mutagenicity to current
regulatory standards, and there are no assays for clastogenic activity
or for the potential to express activity in somatic cells in vivo.
Thus, there is insufficient information to draw firm conclusions
regarding the mutagenic potential of EDA. EDA was not carcinogenic in
animals. Non-neoplastic effects on the liver (pleomorphic changes to
hepatocytes) have been observed in rats following oral dosing for 2
years at 45 mg EDA/kg body weight per day and above, with no effects
seen at 9 mg EDA/kg body weight per day. Although the significance of
these hepatic cell changes for human health is unclear, as well as
whether or not they are a consequence of oral exposure (i.e., they
might not occur via other routes, as they may be related to first-pass
effects), they cannot be discounted, and the risk of their development
should be characterized. In oral gavage dosing studies, effects on the
rat eye (retinal atrophy and, at higher doses, cataract formation)
were observed at doses of 100 mg EDA/kg body weight per day and above.
Doses of 200 and 100 mg EDA/kg body weight per day and above were
associated with renal damage in rats and mice, respectively. There was
also some indication of effects in the spleen in mice and rats at
doses of 400 mg EDA/kg body weight per day and above and in the thymus
in rats at 800 mg/kg body weight per day. In inhalation studies, no
effects were seen in rats at about 150 mg/m3 (60 ppm), and slight
depilation was the only treatment-related effect observed at about 330
mg/m3 (132 ppm).
Because diluted EDA is a skin irritant and a skin sensitizer,
there may be a risk of developing irritant and/or allergic dermatitis
if suitable personal protective equipment is not used in the
occupational environment where skin contact can occur. EDA is also
capable of inducing a state of respiratory tract hypersensitivity and
provoking asthma in the occupational environment, and this is
considered to be the major health effect of concern.
The mechanism for the induction of the hypersensitive state is
not proven, although the skin sensitizing potential of EDA and the
limited evidence of immunological involvement in workers with
EDA-provoked asthma are suggestive of an immunological mechanism.
However, irrespective of the mechanism involved, the available data do
not allow either elucidation of dose-response relationships or
identification of the thresholds for induction of the hypersensitive
state or provocation of an asthmatic response. The sample risk
characterization in this document has, in order to assess the risks of
other systemic effects, evaluated the risk of hepatic effects in
occupationally exposed individuals. It concludes that when EDA is used
in closed systems, the exposure, both measured and predicted from
models, is substantially (by 100-fold or greater) less than the
no-observed-effect level (NOEL) in rats; thus, adverse effects on the
liver are unlikely.
Exposure of the general public to EDA could not be evaluated
owing to the lack of available data.
Toxic thresholds for microorganisms may be as low as 0.1 mg
EDA/litre. However, toxicity tests in culture media should be treated
with caution, as the EDA may complex with metal ions. Effects may
therefore be indirect, resulting from the loss of bioavailability of
essential elements. LC50s for invertebrates and fish range from 14 to
>1000 mg/litre. A no-observed-effect concentration (NOEC) for
Daphnia reproduction has been reported at 0.16 mg/litre.
Given the wide range of acute and chronic test results, a
predicted no-effect concentration (PNEC) for aquatic organisms was
taken as 16 µg/litre, based on application of an uncertainty factor of
10 to the lowest reported NOEC for Daphnia reproduction.
Conservative assumptions for predicted environmental concentration
(PEC) produce PEC/PNEC ratios indicating some concern from initial
concentrations (i.e., at first release into the river or estuary).
However, more refined exposure estimates indicate low risk to aquatic
organisms.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
1,2-Diaminoethane (CAS No. 107-15-3) is more commonly known as
ethylenediamine, with EDA used as a common abbreviation. Other common
synonyms include dimethylenediamine, 1,2-ethanediamine,
1,2-ethylenediamine, beta-aminoethylamine, and ethane-1,2-diamine.
EDA's structural formula is shown below:
 EDA is a colourless to yellowish hygroscopic liquid with an
ammonia-like odour. Its molecular weight is 60.12. It is a strongly
alkaline (pH of 25% EDA in water is 11.9), very volatile, pungent
material, which fumes profusely in air. It has a melting point of
about 8.5°C, a boiling point of 116°C (at 101.3 kPa), and a vapour
pressure of 1.7 kPa at 25°C. EDA is miscible with water and alcohol.
The log octanol/water partition coefficient (log Kow) ranges from
-1.2 to -1.52. p Ka1 and p Ka2 (calculated) are 10.71 and 7.56,
respectively, indicating protonation at environmentally relevant pH.
Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document.
Conversion factors for EDA at 20°C and 101.3 kPa are as follows:
1 ppm = 2.50 mg/m3
1 mg/m3 = 0.40 ppm
3. ANALYTICAL METHODS
For monitoring concentrations of EDA in workplace air, NIOSH
(1984-1989) uses a method that employs adsorption on silica gel and
analysis by gas chromatography with flame ionization detection. A
solvent-free sampling system is preferable because of more convenient
handling, and it is a great advantage if derivatization can be
achieved directly on the absorbent. The Health and Safety Laboratories
of the United Kingdom's Health and Safety Executive have evaluated a
published method (Andersson et al., 1985; Levin et al., 1989; Patel &
Rimmer, 1996). Air is sampled onto
1-naphthyl-isothiocyanate-impregnated filters, desorbed by
acetonitrile, and analysed by high-performance liquid chromatography
with ultraviolet detection. The method has a working range between 2.5
and 50 mg/m3 for a 5-litre air sample. The detection limit was found
to be 0.08 mg/m3. The method generally meets the Comité Européen de
Normalisation requirements on the overall uncertainty. Although the
Comité Européen de Normalisation requirements for desorption
efficiency were not satisfied at 25 and 50 mg/m3, a smaller sample
can be taken if necessary.
There are no reported methods for the biological monitoring of
occupational exposure to EDA. However, analytical techniques based on
solvent extraction of EDA and high-performance liquid chromatography
have been reported and used in pharmacological studies (Cotgreave &
Caldwell, 1983c), and these might form the basis for biological
monitoring methods.
EDA can be measured in water using reverse-phase high-performance
liquid chromatography with ultraviolet detection at 315 nm, following
derivatization with acetylacetone. The limit of detection was reported
to be 0.26 µg/litre (Nishikawa, 1987).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
EDA is not known to occur naturally. The main use for EDA is as
an intermediate in the manufacture of tetraacetyl ethylenediamine,
EDTA, organic flocculants, urea resins, and fatty bisamides. It is
also used, to a much smaller extent, in the production of formulations
for use in the printed circuit board and metal finishing industries,
as an accelerator/curing agent in epoxy coatings/resins, and in the
manufacture of pharmaceutical products. EDA is also present as a
contaminant (<0.5%) in commercially supplied fatty amines, which are
used as wetting agents in bituminous emulsions. It is also used in the
synthesis of carbamate fungicides, in surfactant and dye manufacture,
and in photography development chemicals and cutting oils. These are
believed to be minor uses in the United Kingdom and were not
investigated in this review. EDA is a degradation product of
ethylenebis(dithiocarbamate) fungicides.
Approximately 11 000 tonnes of EDA are imported into the United
Kingdom each year, with very little being re-exported (Brooke et al.,
1997). World production amounts to 100 000-500 000 tonnes annually.1
In 1992, annual production capacities were 18 000 tonnes for Germany,
54 000 tonnes for the Netherlands, 30 000 tonnes for Belgium, 25 000
tonnes for Sweden, about 159 000 tonnes for the United States, and
15 000 tonnes for Japan (BUA, 1997).
No measured concentrations of EDA in wastewater streams from
manufacture and use are available. However, estimates of EDA entering
waste treatment from four European manufacturing plants were 200, 287,
5000-10 000, and 1000 kg/year. Use in photochemicals was estimated to
lead to 1.1 tonnes being introduced into municipal sewage treatment
plants in Germany. All figures are for 1992 or 1993 (BUA, 1997).
1 IUCLID (European Union database), 1st ed., 1996.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few experimental data are available on the distribution,
transport, or fate of EDA in the environment. However, qualitative,
and some quantitative, estimates have been made on the basis of its
physicochemical properties.
EDA has a moderately high vapour pressure and is expected to
volatilize from soil (HSDB, 1997). In the atmosphere, it should react
rapidly with photochemically produced hydroxyl radicals; no
experimental rates are available for this proposed reaction, but a
half-life of 8.9 h has been calculated.1 EDA may react with carbon
dioxide to form an insoluble carbamate. The high water solubility of
EDA means that volatilized chemical is also likely to be washed out by
rain.2 The calculated dimensionless Henry's law constant (air/water
partition coefficient) is extremely low (7.08 × 10-8); therefore,
little evaporation would be expected from water. A half-life for
volatilization of 45 years was estimated for a model river 1 m
deep.2 An approximate Henry's law constant is given in BUA (1997) as
1.77 × 10-4 Pa.m3/mol.
Photodegradation is not expected, as the molecule contains no
chromophores, which absorb radiation (HSDB, 1997).
Despite their miscibility with water, ethyleneamines can bind
strongly to soil. There was a wide range of determined adsorption
coefficients in experimental studies on six soil types (Table 1). Some
reduction in variability occurred when results were normalized for
organic carbon content, although this was less marked with EDA than
with the other ethyleneamine studied. Sorption to soil was rapid, with
equilibrium occurring within a few hours. Electrostatic interaction
between the positively charged ethyleneamine and negatively charged
soils appeared to be the dominant factor in binding. Complex formation
with metals and humic acids is expected. Sorption is greater to soils
with high cation exchange capacity (Davis, 1993).
EDA at 200 mg/litre was incubated with adapted sewage sludge
until there was no further decrease in chemical oxygen demand (COD);
at that time (unspecified), 97.5% of the chemical had been degraded.
The rate of degradation was 9.8 mg COD/g per hour (Pitter, 1976).
EDA at 3, 7, and 10 mg/litre was incubated with sewage sludge
(adapted and non-adapted), and percent biodegradation was determined
5, 10, 15, and 20 days later. Degradation rates were comparable for
adapted and non-adapted sludge up to 15 days (at 56% and 55%,
respectively); at 20 days, however, the values were 70% and 47%,
respectively. Based on this single point, it is not possible to
conclude definitively that adaptation improves degradation. Nitrate
and nitrite were measured throughout the incubation to correct for
1 IUCLID (European Union database), 1st ed., 1996.
2 Syracuse Research Corporation modelling, summarized in HSDB
(1997).
oxygen demand due to conversion of ammonia or organic nitrogen to
these species. Such a correction was necessary for EDA alone out of
more than 50 compounds tested. Degradation was also tested in a
salt-water system using non-adapted sludge; EDA was degraded less
effectively, with 16% of theoretical degradation after 20 days (Price
et al., 1974). A comparable value at 16.6% was measured in seawater by
Takemoto et al. (1981). EDA incubated with microorganisms isolated
from river water and adapted to the compound over 28 days showed >80%
degradation relative to theoretical oxygen demand over 10 days (Mills
& Stack, 1955).
Brief descriptions of the following degradation tests were also
identified. EDA incubated with activated sludge at 100 mg/litre for 28
days showed 93-95% degradation relative to theoretical oxygen demand
in a modified Ministry of International Trade and Industry (MITI) test
(Japan Chemical Industry Ecology-Toxicology Information Center, 1992).
Incubation with activated sludge at a concentration of 50 mg/litre led
to degradation of 10%, 87.5%, and 94% after 5, 15, and 28 days,
respectively.1
The high water solubility and low octanol/water partition
coefficient indicate that bioaccumulation in organisms is unlikely.
1 Unpublished report from Akzo Research to Delamine, 1989 (cited in
IUCLID).
Table 1: Sorption of EDA to various soil types.a
Soil type pH Cation exchange Fraction of Freundlich adsorption Adsorption coefficient
capacity organic carbon coefficient (Kd)b normalized for organic
(meq/100 g) (Foc)b carbon (K = Kd/Foc)c
Sandy loam (Londo) 7.2 9.2 0.026 69 2700
Sandy clay loam 7.3 16.4 0.039 220 5600
Sandy loam (Cecil) 6.0 3.0 0.014 29 2100
Silty loam 6.0 15.6 0.034 238 7100
Clay 7.9 11.9 0.014 70 5000
Aquifer sand 9.6 6.9 0.0024 15 6200
a Data from Davis (1993).
b Values rounded.
c Mean 4800 + 2000 (SD).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no reports of monitoring of EDA levels in the aquatic
environment or of measurements in effluents.
Residues of EDA in soil, 15 days post-treatment with the
fungicide maneb, have been reported at 0.119 mg/kg for the top 1 cm
(approximately) and at 0.044 mg/kg down to about 5 cm. Residues on
tomatoes and beans were 0.053 and 0.239 mg/kg, respectively,
immediately after spraying, falling to 0.047 and 0.094 mg/kg,
respectively, after 14 days (Newsome et al., 1975).
6.2 Human exposure
The data available to the authors of this document are restricted
mainly to the occupational environment. The exposure assessments used
in this report are based on either limited data or data modelled using
the Estimation and Assessment of Substance Exposure (EASE) model. This
is a general-purpose predictive model developed by the United
Kingdom's Health and Safety Executive for exposure assessment in the
workplace. In its present form, the model is in widespread use across
the European Union for the occupational exposure assessment of new and
existing substances. Similarly, information on control measures has
been derived from United Kingdom industry sources. Where data gaps
exist, professional judgement has been used.
The number of employees exposed to EDA in the United Kingdom is
not accurately known. For use as an intermediate in the manufacture of
tetraacetyl ethylenediamine, EDTA, organic flocculants, urea resins,
and fatty bisamides, it is estimated that 140 employees will be
potentially exposed. During the production of formulations for use in
the printed circuit board and metal finishing industries and in the
manufacture of epoxy coatings/resins and pharmaceutical products, it
is estimated that 200 employees will be regularly exposed to EDA. The
number of employees potentially exposed from use of EDA-based
formulations in the printed circuit board and metal finishing
industries is estimated to be about 100. EDA can also be released when
industrial epoxy coatings/adhesives are applied, and this activity has
the potential to expose several thousand employees across a wide range
of industries.
There are very few measured occupational exposure data available.
EDA's use as an intermediate in chemical synthesis takes place in
closed systems. Measured exposures for these manufacturing processes
show that control is achieved to a level of less than 1.25 mg/m3
(0.5 ppm) 8-h time-weighted average (Hansen et al., 1984). Modelled
data (EASE) are in good agreement, predicting comparable values of
0.53-1.3 mg/m3 (0.21-0.52 ppm). Short-term peak exposures (sampling
and hose uncoupling operations) were predicted to range between 16.8
and 33.3 mg/m3 (6.7 and 13.3 ppm), 15-min time-weighted average.
EDA's use in the production of formulations usually takes place
in well-ventilated enclosed systems. Measured exposure data are not
available for these processes. However, modelled exposure data
indicate exposure levels of 5-20 mg/m3 (2-8 ppm) 8-h time-weighted
average in the presence of local exhaust ventilation and 38-75 mg/m3
(15-30 ppm) 8-h time-weighted average in the absence of local exhaust
ventilation. Corresponding short-term peak exposures during mixer
charging operations were estimated to be 5-25 mg/m3 (2-10 ppm) 15-min
time-weighted average in the presence of local exhaust ventilation and
50-103 mg/m3 (20-41 ppm) 15-min time-weighted average in the absence
of local exhaust ventilation.
The potential for exposure during the use of EDA formulations
will be moderated by the low concentration of EDA present in the
formulations. Very few exposure data are available, and there is scope
for widely different use scenarios. There will be no appreciable
occupational exposure if these products are used in enclosed
ventilated systems as indicated by measured exposure data
(<2.5 mg/m3 [<1 ppm] 8-h time-weighted average) and modelled exposure
data (0-0.25 mg/m3 [0-0.1 ppm] 8-h time-weighted average). Modelled
exposure data for immersion processes, in the presence of local
exhaust ventilation, predicted inhalation exposures of 0.5-2.5 mg/m3
(0.2-1 ppm) 8-h time-weighted average. The potential for greatest
inhalation exposure was predicted for situations where these
formulations are brushed in open systems with only general dilution
ventilation or sprayed in open systems in the presence of local
exhaust ventilation. Under these conditions, modelled exposure data
predict exposures in the range 2.5-5 mg/m3 (1-2 ppm) 8-h
time-weighted average. Short-term peak exposures during mixing and
loading operations were estimated to be 5-10 mg/m3 (2-4 ppm) 15-min
time-weighted average. Polyamines and alkanol polyamines, including
EDA, have been reported to be released from hot bitumen during road
paving (Levin et al., 1994). EDA concentrations generated during road
paving were below 0.025 mg/m3 (0.01 ppm).
There will also be a potential for dermal exposure across the
full range of industries handling EDA. Modelled data estimate dermal
exposures in the range 0-0.15 mg/cm2 per day. However, the use of
personal protective equipment is standard practice in all industries
using EDA. Therefore, in practice, dermal exposure will be
considerably reduced by the use of personal protective equipment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The toxicokinetics of EDA has received only limited study, and
there are no studies following inhalation exposure. Studies in humans
have been related to the clinical application of EDA and have
demonstrated rapid absorption via the gastrointestinal tract, with at
least 50% absorbed within the first 7 h; absorbed EDA is rapidly
removed from the plasma (Caldwell & Cotgreave, 1983; Cotgreave &
Caldwell, 1983a,b, 1985). At least half the amount absorbed is
excreted in the urine, largely as the acetylated metabolite
N-acetylethylenediamine and, in smaller amounts, as the unchanged
compound.
This toxicokinetic picture is supported and extended by data from
studies in experimental animals. Studies in rats and mice have
demonstrated rapid and extensive uptake via the oral route and also
via the respiratory tract following intratracheal instillation (about
70% or more of the applied dose was absorbed within 48 h) (McKelvey et
al., 1982; Yang & Tallant, 1982; Yang et al., 1984b). Some (about 12%
of the applied dose over 24 h) dermal absorption has also been
observed in rats at non-irritant concentrations, with greater
absorption at higher, skin-damaging concentrations (Yang et al.,
1987). These animal studies have also demonstrated that EDA and/or its
metabolites are widely distributed throughout the body and are rapidly
eliminated, largely via the urine but also as carbon dioxide in the
breath and a small amount via the faeces, providing evidence for some
biliary excretion. It would seem reasonable to conclude that a similar
situation with respect to distribution and excretion would pertain in
humans. Examination of urinary metabolites in these animal studies
demonstrated that EDA is also found in an acetylated conjugate form in
the rat and mouse. There is evidence that this pathway may become
saturated with increasing dose and that alternative metabolic pathways
may be involved at higher doses in the mouse.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A number of the available studies on both the toxicokinetics and
toxicity of EDA have employed the base substance (EDA) and/or the
hydrochloride salt (EDA.2HCl). The latter is used in pharmaceutical
preparations as a solubilizer to increase uptake of theophylline (this
complex being known as aminophylline) and has been used as a
preservative in skin creams (although it is unclear whether or not
this still occurs). In general, the presence of the hydrochloride has
little qualitative effect on the toxicokinetic or systemic toxicity
properties of EDA, particularly following oral dosing, as it is likely
that the hydrochloride salt would be formed anyway in the acidic
environment of the stomach. However, the hydrochloride does seem to
act in a neutralizing capacity to reduce the significant irritancy
potential of EDA. Studies using both forms of EDA are included in this
review.
8.1 Single exposure
Studies in various animal species have shown EDA to be of
moderate acute toxicity by the inhalation (rat 8-h LC50 estimated to
be in the range of 4916-9832 mg/m3 [1966-3933 ppm]), oral (rat LD50
values of 1160-3250 mg/kg body weight), and dermal (rabbit LD50
values of 550-2880 mg/kg body weight) routes of exposure (Smyth et
al., 1941, 1951; Boyd & Seymour, 1946; Carpenter et al., 1948; NTP,
1982a,b; Yang et al., 1983; Dubinina et al., 1997). Few details exist
of the toxic signs observed or of target organs.
8.2 Irritation and sensitization
There are a number of reports available on skin irritation in
animals, but in general they all repeat the information from one
original study (Smyth et al., 1951). In that study, 0.01 ml undiluted
EDA applied to the shaved backs of albino rabbits produced skin
necrosis within 24 h. A recent report has also indicated EDA to be a
skin irritant (Dubinina et al., 1997). Although no further information
is available, such a response is consistent with EDA being strongly
alkaline. Studies using EDA.2HCl have also resulted in skin
irritation, although the neutralizing action of the hydrochloride may
have influenced the severity of effects, particularly on dilution
(Yang et al., 1983, 1987).
As with skin irritation, the reports that are available for eye
irritation all largely reproduce data from one original study
(Carpenter & Smyth, 1946). In this study, 0.005-ml solutions of 5% EDA
or greater caused corneal injury, which again would be expected, given
the alkaline properties of the substance. More recently, Dubinina et
al. (1997) stated that inflammatory responses in the rabbit eye were
induced by "one drop" of EDA.
Overall, from the reports that are available, together with a
consideration of its alkaline properties, it is reasonable to conclude
that EDA is corrosive, with the capacity to produce severe chemical
burns to the skin and eye.
EDA has been demonstrated to possess skin sensitizing potential
in guinea-pig studies, generally using standard methodologies such as
the Magnusson and Kligman maximization and Buehler tests
(Thorgeirsson, 1978; Erikson, 1979; Maurer et al., 1979; Henck et al.,
1980; Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Dubinina et al., 1997; Leung & Auletta, 1997). In four of these
studies (Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Leung & Auletta, 1997), the investigators ensured that
non-irritant challenge concentrations of EDA were used, providing
clear evidence for a sensitization response. EDA also produced
positive results in the local lymph node assay (Basketter & Scholes,
1992). In contrast to these positive results, EDA consistently
produced negative results in the mouse ear swelling test (Gad et al.,
1986; Cornacoff et al., 1988; Dunn et al., 1990). One study
demonstrated the potential for EDA to cross-react with other
alkylamines either as the inducing or as the challenge agent (Leung &
Auletta, 1997).
No studies are available on respiratory sensitization in animals.
8.3 Short-term exposure
In a 12-day study (NTP, 1982b), mice received gavage doses of
between 50 and 600 mg EDA/kg body weight per day (administered as
EDA.2HCl). Deaths were observed at 400 and 600 mg EDA/kg body weight
per day. No effects were seen at 50 mg EDA/kg body weight per day.
Renal effects (nephrosis and tubule regeneration) were observed at 100
mg EDA/kg body weight per day and above. Lymphoid depletion in and
necrosis of splenic follicles were observed at 400 mg EDA/kg body
weight per day.
A 7 h/day, 30-day inhalation study in rats indicated that the
liver and kidney are potential target tissues, with local effects in
the lungs also likely (Pozzanni & Carpenter, 1954). No effects were
observed in this study at an airborne exposure concentration of about
150 mg/m3 (60 ppm). Slight depilatory effects were seen at 330 mg/m3
(132 ppm), becoming more marked at higher exposure concentrations.
Treatment-related deaths were observed at 563 mg/m3 (225 ppm) and
1210 mg/m3 (484 ppm) (all animals died at 1210 mg/m3 [484 ppm]).
Cloudy swelling of cells in the liver and convoluted tubules of the
kidneys were also observed at these exposure concentrations.
Degeneration of the convoluted tubules was seen in animals exposed to
1210 mg/m3 (484 ppm), as was congestion of the lungs and adrenals.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Dietary studies in rats have also indicated that the liver is a
target tissue, with changes in the size and shape of hepatocytes and
their nuclei being noted at 1000 mg/kg body weight per day in a 90-day
study (Yang et al., 1983).
Oral gavage studies using doses of between 100 and 1600 mg EDA/kg
body weight per day (administered as EDA.2HCl) have been carried out
in rats (NTP, 1982a). Deaths were observed after 12 doses at 800 and
1600 mg EDA/kg body weight per day and after 800 mg EDA/kg body weight
per day for 90 days. Renal tubular lesions (dilation of the lumen,
necrosis, degeneration and regeneration of the epithelium) were seen
at 200 mg EDA/kg body weight per day and above after 12 doses. Similar
renal lesions but of a less severe nature were seen only at 600 mg
EDA/kg body weight per day and above after 90 days. This indicates
recovery in the kidney, probably as a consequence of compensatory
regeneration. No effects were seen on the kidney at 100 mg EDA/kg body
weight per day in either study. Ocular effects including cataract
formation and retinal atrophy were observed in all dose groups.
Minimal to moderate focal retinal atrophy was observed in 3 out of 10
females at 100 mg EDA/kg body weight per day; 2 males had mild to
moderate retinal atrophy and 1 male had severe retinal atrophy at 200
mg EDA/kg body weight per day. Lymphoid depletion and/or necrosis in
spleen were observed at 800 mg EDA/kg body weight per day and in all
decedents following 12 doses, and thymus weight was reduced at 800 mg
EDA/kg body weight per day in the 90-day study. Uterine lesions
(reduced uterine horn size and atrophy of the myometrium and
endometrium) were seen after dosing for 90 days with 600 or 800 mg
EDA/kg body weight per day, and reduced ovarian size was seen after
800 mg EDA/kg body weight per day for 90 days. Overall, a
no-observed-adverse-effect level (NOAEL) was not identified from these
studies, as effects on the eyes were seen at all dose levels. Only
ocular effects were seen at the lowest-observed-adverse-effect level
(LOAEL) of 100 mg EDA/kg body weight per day, and these were of a
minimal to mild nature, suggesting that this dose represented the
lower end of the dose-response relationship for these effects.
In a 90-day study, mice received oral gavage doses of 25-400 mg
EDA/kg body weight per day (NTP, 1982b). No effects were seen at 100
mg EDA/kg body weight per day. Renal lesions (cortical tubular
degeneration and/or necrosis) were observed at 200 and 400 mg EDA/kg
body weight per day.
8.4.2 Chronic exposure and carcinogenicity
There are two carcinogenicity studies in animals. Both studies
were performed to reasonably adequate standards, including extensive
histopathology, and were negative for carcinogenic activity.
In the first study, groups of 99-225 F344 rats were orally dosed
with 0, 20, 100, or 350 mg EDA.2HCl (equivalent to 0, 9, 45, or 158
mg EDA/kg body weight per day) for 2 years (Yang et al., 1984a).
Non-neoplastic effects were similar to those described in studies of
shorter duration (Yang et al., 1993), as indicated above (section
8.4.1). Effects were seen at 45 mg EDA/kg body weight per day, with a
NOAEL of 9 mg EDA/kg body weight per day. Tracheitis was also
observed, probably as a consequence of exposure to EDA in airborne
dust derived from the diet.
In the second study, groups of 40-50 C3H/HeJ mice were dermally
administered 0 or 0.25 mg aqueous EDA 3 times per week for a lifetime
(DePass et al., 1984). The dermal study included a positive control
group that received 3-methylcholanthrene. Skin fibrosis and
hyper-keratosis were observed in EDA-treated mice.
8.5 Genotoxicity and related end-points
Only limited information is available on the genotoxic potential
of EDA. There is some evidence that EDA may be mutagenic in bacteria
with and without metabolic activation (Hedenstedt, 1978; Hulla et al.,
1981; Haworth et al., 1983; Leung, 1994). Although the most recent
study (Leung, 1994) appears to be negative, there was a small response
in Salmonella typhimurium TA100 and a positive, but not
reproducible, response in TA1535. Positive results have also been
reported in these strains from the other studies, although only one of
these (Haworth et al., 1983) was adequately reported. The only series
of studies performed on mammalian cell systems in vitro (gene
mutation and sister chromatid exchange in Chinese hamster ovary cells;
unscheduled DNA synthesis in rat primary hepatocytes) were
consistently negative (Slesinski et al., 1983), although there has
been no assay for clastogenic activity. A sex-linked recessive lethal
test in Drosophila melanogaster was negative following dosing by
feeding or injection (Zimmering et al., 1985). There are no in vivo
studies on somatic cells, but a dominant lethal study in rats up to
doses inducing signs of toxicity (up to 500 mg EDA.2HCl/kg body
weight per day in the diet) was negative (Slesinski et al., 1983).
Although there has been some evidence of mutagenicity in
bacterial systems in a few limited studies, the available evidence
indicates that EDA is not genotoxic, with all results in mammalian
cells in vitro and in vivo (dominant lethal assay) being negative.
It should be noted that the overall database is limited, with no
assays available for clastogenic activity or for genotoxic potential
in somatic cells in vivo.
8.6 Reproductive and developmental toxicity
The potential of EDA to affect fertility and development has been
studied in rats in investigations conducted to modern regulatory
standards. In a two-generation study in F344 rats, no effects on
fertility or development in any of the generations were observed up to
a dose level (225 mg EDA/kg body weight per day) that induced signs of
parental toxicity (Yang et al., 1984b). Dose levels used in this study
were 0, 50, 150, or 500 mg EDA.2HCl/kg body weight per day (equivalent
to 0, 23, 68, and 225 mg EDA/kg body weight per day). Effects on the
uterus and ovaries have been seen following gavage dosing of rats with
600 and 800 mg EDA/kg body weight per day for 90 days (see section
8.4.1; NTP, 1982a). In a series of developmental toxicity studies in
F344 rats, EDA was found to produce signs of fetotoxicity (increased
resorptions) and delays in development at high dose levels (450 mg
EDA/kg body weight per day) that induced clear signs of toxicity in
the dams (DePass et al., 1987). Dose levels used in this study were 0,
50, 250, or 1000 mg EDA.2HCl/kg body weight per day (equivalent to 0,
23, 113, and 450 mg EDA/kg body weight per day). Some of the
developmental effects appear to have been related, at least in part,
to the reduced nutritional status of the animals. However, a clear
NOAEL for developmental toxicity of 113 mg EDA/kg body weight per day
was observed in these studies.
The results of a preliminary screening study in mice indicated no
significant effects on development in the offspring of dams exposed to
toxic doses of EDA (400 mg/kg body weight per day) by oral gavage
(Hardin et al., 1987).
No effects on development were seen in the offspring of New
Zealand white rabbits dosed during pregnancy with up to 178 mg
EDA.2HCl/kg body weight per day (equivalent to 80 mg EDA/kg body
weight per day), a dose that did not induce maternal toxicity (NTP,
1991; Price et al., 1993). In a preliminary study, 2/20 pregnant
rabbits receiving 100 mg EDA/kg body weight per day by gavage died,
and decreased body weight was seen in survivors. At 400 mg/kg body
weight per day, all the dams died.
8.7 Immunological and neurological effects
No studies are available that have specifically investigated the
potential immunotoxicity of EDA. Effects on lymphoid tissue in the
spleen in mice and rats (see sections 8.3 and 8.4.1, respectively) and
on the thymus in rats (see section 8.4.1) were observed in oral gavage
dosing studies.
There are a few, mainly in vitro, studies on the effects of EDA
on the release of gamma-aminobutyric acid from the retina, gut, and
brain (Perkins & Stone, 1980; Forster et al., 1981; Lloyd et al.,
1982; Morgan & Stone, 1982; Sarthy, 1983; Kerr & Ong, 1984; Strain et
al., 1984; Hill, 1985; Erdo et al., 1986; Krantis et al., 1990; McKay
& Krantis, 1991). The general conclusion that can be drawn from these
studies is that EDA can cause a calcium-independent release of
gamma-minobutyric acid that is insensitive to the presence of
tetrodotoxin. EDA was also shown to have gamma-minobutyric acid
mimetic properties (i.e., reduction of neuronal firing rate). This
suggests that EDA could have a central nervous system depressant
effect, but studies were not performed to address this possibility. It
was reported that EDA elicited contraction of the guinea-pig ileum
that was mediated via neuronal release of gamma-aminobutyric acid.
However, in the rat ileum, EDA acted directly on the mucosa, resulting
in relaxation. Although these are interesting results, the
toxicological significance of these findings is unclear; they may,
however, partly explain the central nervous system depressant and
gastrointestinal effects seen in some of the animal studies at high
doses.
9. EFFECTS ON HUMANS
No studies are available in which the effects, other than the
respiratory effects summarized below, of repeated exposure of humans
to EDA are examined. No reports have been found in which genotoxicity,
carcinogenicity, or reproductive toxicity following exposure to EDA in
human populations has been studied.
A case report exists concerning a 36-year-old worker who died
from cardiac collapse 55 h after being splashed by an accidental
spillage of EDA (Niveau & Painchaux, 1973). Exposure to an
unquantified amount of EDA was in the order of a few minutes prior to
the patient being washed. Four hours after the exposure, he presented
with tachycardia (100 beats/min), anuria, and red/brown generalized
erythema. The tachycardia increased (up to 140 beats/min), anuria
persisted, and an expectorant cough, abdominal cramps, diarrhoea, and
blackish vomiting appeared. The patient became hyperkalaemic, and his
red blood cell count decreased. Overall, given the lack of information
with respect to levels of exposure, few useful conclusions can be
drawn from this case report.
The only information available on skin irritation in humans
either is anecdotal or does not involve direct surface skin contact
with EDA. In a brief report on the physicochemical properties of EDA,
it is noted anecdotally that "the liquid, if not washed from the skin,
causes blistering" (Boas-Traube et al., 1948). The other report
available documents the results of intradermal skin tests with
solutions (0.1-1%) of EDA on three individuals being tested for
hypersensitivity following treatment with aminophylline (Kradjan &
Lakshminarayan, 1981). The skin response in two patients consisted of
blistering rather than a weal and flare reaction, which normally
typifies a sensitization response. Punch biopsies were obtained from
one patient, and histopathological examination of these indicated
tissue necrosis and oedema of the epidermis and dermis. These
responses suggested a direct corrosive effect of EDA.
No specific reports were found of the effects of EDA on the eyes
of humans. In the original reports of the animal eye irritation study
(see section 8.3), it is claimed that EDA is known to have produced
loss of vision or slowly healing corneal burns in industrial use
(Carpenter & Smyth, 1946). However, no further information or
references were given.
EDA has been known for many years to be capable of inducing
allergic skin reactions in humans. This has been observed both in the
workplace and, most notably, in patients treated with aminophylline or
with skin creams in which EDA was used as a stabilizer (Epstein &
Maibach, 1968; Petrozzi & Shore, 1976; Booth et al., 1979; Wall, 1982;
Hardy et al., 1983; Balato et al., 1984; Edman & Moller, 1986; Nielsen
& Jorgensen, 1987; Terzian & Simon, 1992; Toal et al., 1992; Dias et
al., 1995; Simon et al., 1995; Sasseville & Al-Khenaizan, 1997). The
first reports of skin sensitizing effects in humans date back to the
late 1950s, when cases were described of eczematous reactions in
pharmacists who came into contact with EDA when using aminophylline
(Baer et al., 1958; Tas & Weissberg, 1958).
Subsequent to these early reports, numerous studies and case
reports have been published documenting the skin sensitizing
properties of EDA both following clinical use and within the
occupational setting, such that the substance has become incorporated
into standard series for patch testing (Fregert, 1981; Shehade et al.,
1991). An example from the clinical setting is that of the report on a
series of 13 patients who had used skin cream containing EDA for,
paradoxically, dermatitic conditions (Provost & Jillson, 1967). Use of
the cream in 11 of these patients had resulted in the sudden
appearance of a severe generalized patchy eczematous eruption
following, in all but one of the cases, an initial improvement upon
using the cream. Patch testing was conducted using a 1% aqueous
solution of EDA, producing skin reactions in all patients ranging from
erythema and oedema to erythema vesiculation and oedema vesiculation,
which extended beyond the patch test site. Other substances tested
also induced responses, but not in a consistent manner, with at most
only four individuals responding in any one test.
As well as the original reports in pharmacists working with EDA,
cases of skin sensitization to EDA have been reported in the
occupational environment in a number of different settings, including
use of floor polish remover (English & Rycroft, 1989), use of coolant
oils (Crow et al., 1978), and in wire-drawing (Matthieu et al., 1993;
Sasseville & Al-Khenaizan, 1997). Positive responses to patch testing
with EDA have also been observed in other occupational settings, such
as the offshore oil industry (Ormerod et al., 1989), but positive
responses to other substances, including other polyamines, were also
seen in such cases. Thus, it is unclear whether EDA had been
responsible for inducing the sensitized state and/or cross-reacting
following sensitization to another polyamine.
A large number of cases of occupational asthma reported to have
been caused by exposure to EDA are available in the literature
(Dernehl, 1951; Gelfand, 1963; Popa et al., 1969; Valeyeva et al.,
1975; Lam & Chan-Yeung, 1980; Chan-Yeung, 1982; Hagmar et al., 1982;
Matsui et al., 1986; Aldrich et al., 1987; Nakazawa & Matsui, 1990;
Lewinsohn & Ott, 1991; Ng et al., 1991, 1995). There are a few studies
in which the potential for EDA to cause respiratory hypersensitivity
has been examined using bronchial provocation testing and
investigation of antibody formation. As EDA is corrosive, the vapour
would be predicted to be a respiratory tract irritant, which is a
complicating factor in interpreting the data available and in
elucidating the underlying mechanism for any asthmatic responses seen.
Popa et al. (1969), in a well-conducted study, investigated 48
subjects with asthmatic symptoms caused by exposure to a number of low
molecular weight chemicals, including EDA. None of the subjects had a
history of respiratory disorder prior to occupational exposure, and
the asthmatic response was associated only with occupational exposure
in all cases. No information was given in the report on the workplace
airborne concentrations of EDA to which these workers were exposed. A
series of tests were performed in all subjects, including skin and
inhalation tests with the test agent at sub-irritant concentrations;
skin and inhalation tests to common allergens; skin tests
(intradermal, scratch, and patch tests) using sub-irritant
concentrations of the test substance; Prausnitz-Kustner transfer
reaction (to test for the presence of immunoglobulin E antibodies);
and determination of precipitating antibodies to EDA. For the
inhalation test, the sub-irritant concentration was determined in
control asthmatic subjects, and a 2- to 10-fold dilution of this was
used for the bronchial challenge. No information was given on the
airborne exposure concentrations generated under these test
conditions. Control inhalation tests with the diluent, physiological
saline, were also conducted. It is not stated in the report whether or
not the inhalation challenge tests were conducted in a blind manner.
Six subjects had an immediate, positive reaction to EDA in the
workplace. Of these, four showed an immediate, positive response
following inhalation testing with sub-irritant concentrations of EDA.
These subjects developed marked bronchoconstriction following
inhalation exposure to EDA, with a reduction in forced expiratory
volume in 1 s (FEV1) of 62% and an increase in respiratory resistance
of 44%, compared with controls. Although not stated in the report,
these values are presumed to be average changes. Intradermal skin
tests with EDA were positive in these four subjects, whereas patch
tests were negative. Inhalation challenges with common allergens were
negative. The Prausnitz-Kustner test was positive in all subjects, and
all had eosinophilia, determined in the sputum, although not, except
in one case, in the blood. No precipitating antibodies were found. In
the two other subjects, the inhalation challenge test was negative. No
precipitating antibodies were found, and the Prausnitz-Kustner test
was negative in both subjects. Eosinophilia was absent. Inhalation
challenges with other common allergens were also negative.
These data provide evidence that EDA may elicit an asthmatic
response at sub-irritant concentrations and that the response is
specific to EDA. Four out of six subjects responsive to EDA in the
workplace also had a positive response to inhaled EDA at sub-irritant
concentrations. This demonstrates that the reaction is not a
generalized response to an irritant. The positive Prausnitz-Kustner
reaction may be indicative of an immunological component, but the test
is not specific, and no firm conclusions can be drawn from it. It
provides supporting evidence in this case. The evidence suggests that
the subjects were hypersensitive to inhaled EDA and that a state of
respiratory hypersensitivity had been induced by the substance.
Although a number of other studies are available, the information
is of poor quality. Lam & Chan-Yeung (1980) and Chan-Yeung (1982)
describe the case of one worker in a photographic laboratory who
developed asthma after 2.5 years of exposure to a variety of
chemicals, including EDA, but also other irritant substances. The
worker developed symptoms of sneezing, nasal discharge, productive
cough and nocturnal cough, wheezing, and dyspnoea. The symptoms
coincided with the work shift and subsided at weekends. There was no
previous history of asthma. No information was given in the report of
the airborne concentrations of EDA (or other substances) to which the
man was exposed at work. A series of controlled inhalation challenge
exposures, designed to mimic work exposure conditions, were conducted
with each of the chemicals to which the subject was exposed at work.
The duration of exposure was determined by the patient's tolerance,
and exposure was terminated when eye irritation or cough was
experienced. No information on the airborne exposure concentrations of
EDA generated under these test conditions was given in the report. A
methacholine inhalation test for bronchial hyperreactivity was also
performed. Pulmonary function tests were conducted pre- and
post-challenge, and blood samples were taken before, during, and after
each challenge. The subject showed marked bronchial reactivity to
methacholine.
Exposure to an unknown concentration of vapour from a 1:25
solution of EDA was tolerated for 15 min. This exposure produced a
marked bronchoconstriction. A late asthmatic response developed 4 h
after the exposure, at which time FEV1 was reduced by 26% and
continued to decrease over the next 3 h towards a 40% reduction. A 26%
reduction was still apparent after 24 h, despite treatment with
bronchodilator drugs. This pattern of response to EDA was
reproducible. The patient did not respond similarly to any of the
other chemicals tested: formaldehyde, sulfur dioxide, and two colour
developing agents that were stated to be irritants. Exposure to
formaldehyde (vapour from a 1:4 solution) produced an immediate small
(<20%), transient reduction in FEV1, whereas exposure to sulfur
dioxide caused coughing and chest tightness and an immediate transient
reduction of 25% in FEV1. There was no increase in plasma histamine
concentration during the period of bronchoconstriction, although EDA
was shown to cause in vitro histamine release from whole blood taken
from the patient and from two control subjects. A skin test using
1:100 EDA and a precipitin test for antibodies to EDA were both
negative. The patient subsequently had to give up work because of
respiratory symptoms and became asymptomatic after 2 weeks. Subsequent
testing with methacholine, 2.4 months after ceasing work, showed that
the subject had a reduction in the previous bronchial hyperreactivity.
In conclusion, the subject showed an asthmatic response to EDA
but not to formaldehyde or the colour developing agents. The pattern
of response to sulfur dioxide was more immediate and suggestive of an
irritation response. Overall, a clear pattern of asthmatic response
that was specific to EDA was observed in this study. However, it is
not possible to distinguish with certainty between an irritant
response and a sensitization response, because it is possible that an
irritant concentration was used for the bronchial challenge exposure,
although little immediate response was observed. In addition, although
exposure to irritant concentrations of the other workplace chemicals
did not elicit the same pattern, magnitude, or severity of response as
that seen with EDA, since accurate exposure levels were not given, it
is not possible to determine whether or not the EDA concentration used
had the greatest irritant potential. No evidence for any immunological
involvement was found. In conclusion, this study provides only
circumstantial evidence that EDA caused a state of respiratory
hypersensitivity in this subject.
A number of other case reports are available of individuals who
exhibited asthmatic signs and symptoms associated with exposure to EDA
in the workplace (Gelfand, 1963; Valeyeva et al., 1975; Matsui et al.,
1986; Nakazawa & Matsui, 1990; Ng et al., 1991). Although bronchial
challenge testing with EDA produced asthmatic responses in these
subjects, they had personal and/or family histories of allergic
disease and/or they had worked with and responded on challenge to
other substances. Retrospective studies using the medical records of
populations of workers using EDA have indicated that about 10% of such
populations developed signs and symptoms of occupational asthma
(Aldrich et al., 1987; Lewinsohn & Ott, 1991). No challenge tests were
carried out with these surveys. Thus, these case reports and
population-based studies provide only supporting circumstantial
evidence for the involvement of EDA in producing occupational asthma.
Although it is clear from these reports that EDA can provoke an
asthma attack, in many cases there is insufficient information to
indicate whether or not the hypersensitive state was induced
specifically by EDA. However, from one well-conducted study, there is
evidence that a hypersensitive state specific to EDA has been induced
in workers and that an asthmatic response was provoked by sub-irritant
concentrations of the substance. Overall, the results of this study,
taken together with the supporting data from a substantial number of
other reports of occupational asthma, indicate that EDA is capable of
inducing a state of hypersensitivity in the airways, such that
subsequent exposure may trigger asthma. The mechanism by which the
hypersensitive state is induced is not proven. Given the skin
sensitizing potential of EDA and the limited evidence of immunological
involvement in workers with EDA-provoked occupational asthma, an
immunological mechanism would seem plausible. Irrespective of the
mechanism involved, the data available (specifically the lack of
information on airborne exposure concentrations under both work and
challenge test conditions) do not allow elucidation of a dose-response
relationship or the identification of levels of EDA that are not
capable of inducing a hypersensitive state or of provoking an
asthmatic response.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Results of acute ecotoxicity tests are given in Table 2.
A 21-day Daphnia magna reproduction test was conducted
according to the German Federal Environment Agency (Umweltbundesamt)
guidelines in a closed vessel. End-points measured included adult
mortality, onset of production of young, and reproduction rate. The
most sensitive end-point was for reproductive rate, and a NOEC of 0.16
mg/litre was established (Kuhn et al., 1989). In a second study
conducted according to Organisation for Economic Co-operation and
Development (OECD) guidelines, a NOEC for reproduction was reported at
2 mg/litre (Mark & Hantink-de Rooy, 1992). An early life stage test
conducted under OECD guidelines on three-spined stickleback
( Gasterosteus aculeatus) showed no effects of EDA at 10 mg/litre
(the highest concentration tested) over 28 days (Mark & Arends, 1992).
Growth of lettuce ( Lactuca sativa) plants over 7 days was
studied in tests conducted under OECD Guideline 208; EC50
concentrations for EDA in soil (nominal) were >1000 mg/kg for 7-day
and 692 mg/kg for 14-day growth periods (Hulzebos et al., 1993).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
EDA is of moderate acute toxicity by all routes. In studies in
animals, EDA is a primary skin and eye irritant, being corrosive when
undiluted. It is also a skin sensitizer. In repeated-dose toxicity
studies by the oral and inhalation routes, effects on the liver and
kidney have been observed, with pleomorphic changes to hepatocytes in
rats being reported at the lowest oral doses used (45 mg/kg body
weight per day and above for 2 years; NOAEL, 9 mg/kg body weight per
day). In inhalation studies, there were no effects at 150 mg/m3 (60
ppm), although slight depilation was observed at the next highest
concentration (330 mg/m3 [132 ppm]) and effects on the liver and
kidney at higher concentrations still (approximately 500 mg/m3 [200
ppm] and above).
There has been some evidence of mutagenicity in bacterial systems
in a few limited studies. However, much of the available evidence is
negative, although the overall database is limited, there being no
assays for clastogenic activity or for genotoxic potential in somatic
cells in vivo. EDA was not carcinogenic in adequate studies in
animals.
In humans, EDA has the potential to induce respiratory tract
hypersensitivity, and provocation of asthma is the major health effect
of concern in the occupational environment. The mechanism of induction
of the hypersensitive state is unknown, although the skin sensitizing
potential of EDA and limited evidence in workers with EDA-provoked
asthma are suggestive of an immunological mechanism. However,
irrespective of the mechanism involved, the available data
(particularly lack of information on exposure conditions either in the
workplace or on bronchial challenge testing) do not allow either
elucidation of dose-response relationships or identification of the
thresholds for induction of the hypersensitive state or provocation of
an asthmatic response.
11.1.2 Criteria for setting guidance values for EDA
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship for provocation of
an asthmatic response, the effect of greatest concern in the
occupational environment. As it is not possible to identify a level of
exposure that is without adverse effect, it is recommended that levels
be reduced to the extent possible.
Table 2: Acute toxicity of EDA to organisms other than laboratory mammals.
Species End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Microcystis aeruginosa Toxic threshold for cell multiplication 0.08 Bringmann & Kuhn, 1976
Pseudomonas putida 17-h EC50 (growth rate) 29 (23-35) van Ginkel, 1989
Nitrifying bacteria 3-h EC50 (respiration) 3.2 (0.6-5.7) Balk & Meuwsen, 1989a
NOEC 0.5
Activated sludge bacteria 1-h EC50 (respiration rate) 1600 van Ginkel & Stroo, 1989
Algae
Scenedesmus quadricauda Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Chlorella pyrenoidosa 96-h EC50 (growth) 100 van Leeuwen, 1986
Selenastrum capricornutum 72-h EC50 (biomass) 71 van Ginkel et al., 1990
72-h EC50 (growth rate) 645
NOEC approx. 3.2
96-h EC50 (growth rate) 151 van Wijk et al., 1994
Scenedesmus subspicatus 48-h EC50 (biomass and growth rate) >100 Kuhn & Pattard, 1990
Protozoa
Entosiphon sulcatum Toxic threshold for cell multiplication 1.8 Bringmann & Kuhn, 1980a
Uronemia parduczi Toxic threshold for cell multiplication 52 Bringmann & Kuhn, 1980b
Chilomonas paramaecium Toxic threshold for cell multiplication 103 Bringmann & Kuhn, 1980b
Invertebrates
Daphnia magna 48-h LC50 26.5 van Leeuwen, 1986
48-h LC50 46 van Wijk et al., 1994
48-h LC50 16.7 Balk & Meuwsen, 1989b
24-h LC50 14 Kuhn et al., 1989
Table 2 (continued)
Species End-point Concentration Reference
(mg/litre)
Brine shrimp (Artemia
salina) 24-h LC50 14 Price et al., 1974
Fish
Brown trout (Salmo trutta) 48-h LC50 230 Woodiwiss & Fretwell, 1974
Fathead minnow (Pimephales
promelas) 96-h LC50 115.7 Curtis & Ward, 1981
Guppy (Poecilia reticulata) 96-h LC50 275 van Leeuwen, 1986
96-h LC50 640 Balk & Meuwsen, 1989c
96-h LC50 1545 van Wijk et al., 1994
Medaka (Oryzias latipes) 48-h LC50 1000 Tonogai et al., 1982
With respect to systemic effects, the lowest NOAELs of 150 mg/m3
(60 ppm) (inhalation) and 9 mg/kg body weight per day (oral) can serve
as a basis for comparison with estimated exposure for characterization
of risk, either with application of appropriate uncertainty factors or
directly. An example of the latter (margin of exposure) approach is
presented in section 11.1.3.
11.1.3 Sample risk characterization
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship, and hence risk,
for provocation of an asthmatic response, the effect of greatest
concern in the occupational environment. For substances that are
asthmagens, it is also advisable to restrict peak exposures, as they
may have a role in the induction and triggering of asthmatic
phenomena. However, to help assess the risk to human health arising
from occupational exposures, a comparison is made with the NOAELs for
systemic effects from animal studies.
It should also be noted that because diluted EDA is a skin
irritant and sensitizer, there is a risk of developing irritant and/or
allergic dermatitis if suitable personal protective equipment is not
used.
A sample risk characterization for systemic effects in the
occupational environment in the United Kingdom is provided here.
Measured data on exposure to EDA (generally used in closed systems)
indicate that levels in industry in the United Kingdom are less than
1.25 mg/m3 (0.5 ppm), 8-h time-weighted average. Modelled data (EASE)
are in good agreement, predicting comparable values of 0.53-1.3 mg/m3
(0.21-0.52 ppm). Short-term peak exposures (sampling and hose
uncoupling operations) were predicted to be 16.8-33.3 mg/m3
(6.7-13.3 ppm), 15-min time-weighted average. The EASE model predicts
dermal exposures in the range of 0-0.15 mg/cm2 per day for an
operator transferring EDA into closed systems once a day (although
coveralls and well-maintained plastic gloves will significantly reduce
exposure from this source).
With respect to systemic effects, worst-case estimated and
measured exposures of 1.25 mg/m3 (0.5 ppm), 8-h time-weighted
average, are substantially less (by 100-fold or greater) than the
NOAEL in the inhalation study in rats. The combined body burden from
inhalation and dermal exposure for chemical synthesis can be estimated
to be about 0.3 mg/kg body weight per day (assuming a 70-kg worker
breathing 10 m3 of air containing 1.25 mg EDA/m3 [0.5 ppm] per day,
with 100% absorption; and 10% absorption from unprotected, undamaged
skin for a standard hand area of 840 cm2). This is 30-fold less than
the NOAEL for hepatic effects in the oral studies.
Available data on indirect exposure in the general environment or
from consumer products are inadequate to serve as a basis for a sample
characterization of risk for these scenarios.
11.2 Evaluation of environmental effects
No atmospheric effects are expected, as reaction with hydroxyl
radicals is likely to be rapid, and washout of volatilized EDA is
expected. Volatilization to the atmosphere is likely from soil but not
from water.
Adsorption to soil particulates is strong through electrostatic
binding; leaching through soil profiles to groundwater is not
expected. EDA is readily biodegradable, and this is the most likely
source of breakdown in the environment; adaptation of microorganisms
may improve degradation. Breakdown is less rapid in seawater than in
fresh water. Bioaccumulation is unlikely.
Toxic thresholds for microorganisms may be as low as 0.1
mg/litre. However, toxicity tests in culture media should be treated
with caution, as the EDA may complex with metal ions. Effects may
therefore be indirect, from loss of bioavailability of essential
elements. The "toxic thresholds" reported are lowest-observed-effect
concentrations (LOECs) for small changes in sublethal end-points; the
exact degree of effect at the reported concentrations is not always
clear, and these have not been used in the risk assessment.
The principal receiving compartment in the environment is the
hydrosphere, and this is the only compartment for which a quantitative
risk assessment can be attempted.
The distribution of acute (from Table 2) and chronic test results
is plotted in Figure 1. Chronic test results are available for fish (a
limit test only) and Daphnia (NOECs from 21-day reproduction tests).
Chronic EC50s for algal growth or biomass are also available, but no
NOEC was reported for these studies. Given the range of chronic test
results across three trophic levels, it is proposed that an
uncertainty factor of 10 be applied to the lowest reported NOEC (for
Daphnia reproduction at 0.16 mg/litre) to derive an estimated PNEC
for aquatic organisms of 0.016 mg/litre. This is in accord with OECD,
European Union, and US Environmental Protection Agency guidelines.
There are no reported test results for estuarine/marine organisms; it
is assumed that toxicity would be in the same range for these species.
No measured concentrations of EDA in surface waters have been
reported. A single quantitative risk assessment has been reported (van
Wijk, 1992) based on discharges into the Ems-Dollard estuary in the
Netherlands. The figure of 75 kg/day for release of EDA at this site
has been used here as a representative estimate of release; no
information on releases from industrial plants elsewhere is available.
EDA is a colourless to yellowish hygroscopic liquid with an
ammonia-like odour. Its molecular weight is 60.12. It is a strongly
alkaline (pH of 25% EDA in water is 11.9), very volatile, pungent
material, which fumes profusely in air. It has a melting point of
about 8.5°C, a boiling point of 116°C (at 101.3 kPa), and a vapour
pressure of 1.7 kPa at 25°C. EDA is miscible with water and alcohol.
The log octanol/water partition coefficient (log Kow) ranges from
-1.2 to -1.52. p Ka1 and p Ka2 (calculated) are 10.71 and 7.56,
respectively, indicating protonation at environmentally relevant pH.
Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document.
Conversion factors for EDA at 20°C and 101.3 kPa are as follows:
1 ppm = 2.50 mg/m3
1 mg/m3 = 0.40 ppm
3. ANALYTICAL METHODS
For monitoring concentrations of EDA in workplace air, NIOSH
(1984-1989) uses a method that employs adsorption on silica gel and
analysis by gas chromatography with flame ionization detection. A
solvent-free sampling system is preferable because of more convenient
handling, and it is a great advantage if derivatization can be
achieved directly on the absorbent. The Health and Safety Laboratories
of the United Kingdom's Health and Safety Executive have evaluated a
published method (Andersson et al., 1985; Levin et al., 1989; Patel &
Rimmer, 1996). Air is sampled onto
1-naphthyl-isothiocyanate-impregnated filters, desorbed by
acetonitrile, and analysed by high-performance liquid chromatography
with ultraviolet detection. The method has a working range between 2.5
and 50 mg/m3 for a 5-litre air sample. The detection limit was found
to be 0.08 mg/m3. The method generally meets the Comité Européen de
Normalisation requirements on the overall uncertainty. Although the
Comité Européen de Normalisation requirements for desorption
efficiency were not satisfied at 25 and 50 mg/m3, a smaller sample
can be taken if necessary.
There are no reported methods for the biological monitoring of
occupational exposure to EDA. However, analytical techniques based on
solvent extraction of EDA and high-performance liquid chromatography
have been reported and used in pharmacological studies (Cotgreave &
Caldwell, 1983c), and these might form the basis for biological
monitoring methods.
EDA can be measured in water using reverse-phase high-performance
liquid chromatography with ultraviolet detection at 315 nm, following
derivatization with acetylacetone. The limit of detection was reported
to be 0.26 µg/litre (Nishikawa, 1987).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
EDA is not known to occur naturally. The main use for EDA is as
an intermediate in the manufacture of tetraacetyl ethylenediamine,
EDTA, organic flocculants, urea resins, and fatty bisamides. It is
also used, to a much smaller extent, in the production of formulations
for use in the printed circuit board and metal finishing industries,
as an accelerator/curing agent in epoxy coatings/resins, and in the
manufacture of pharmaceutical products. EDA is also present as a
contaminant (<0.5%) in commercially supplied fatty amines, which are
used as wetting agents in bituminous emulsions. It is also used in the
synthesis of carbamate fungicides, in surfactant and dye manufacture,
and in photography development chemicals and cutting oils. These are
believed to be minor uses in the United Kingdom and were not
investigated in this review. EDA is a degradation product of
ethylenebis(dithiocarbamate) fungicides.
Approximately 11 000 tonnes of EDA are imported into the United
Kingdom each year, with very little being re-exported (Brooke et al.,
1997). World production amounts to 100 000-500 000 tonnes annually.1
In 1992, annual production capacities were 18 000 tonnes for Germany,
54 000 tonnes for the Netherlands, 30 000 tonnes for Belgium, 25 000
tonnes for Sweden, about 159 000 tonnes for the United States, and
15 000 tonnes for Japan (BUA, 1997).
No measured concentrations of EDA in wastewater streams from
manufacture and use are available. However, estimates of EDA entering
waste treatment from four European manufacturing plants were 200, 287,
5000-10 000, and 1000 kg/year. Use in photochemicals was estimated to
lead to 1.1 tonnes being introduced into municipal sewage treatment
plants in Germany. All figures are for 1992 or 1993 (BUA, 1997).
1 IUCLID (European Union database), 1st ed., 1996.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few experimental data are available on the distribution,
transport, or fate of EDA in the environment. However, qualitative,
and some quantitative, estimates have been made on the basis of its
physicochemical properties.
EDA has a moderately high vapour pressure and is expected to
volatilize from soil (HSDB, 1997). In the atmosphere, it should react
rapidly with photochemically produced hydroxyl radicals; no
experimental rates are available for this proposed reaction, but a
half-life of 8.9 h has been calculated.1 EDA may react with carbon
dioxide to form an insoluble carbamate. The high water solubility of
EDA means that volatilized chemical is also likely to be washed out by
rain.2 The calculated dimensionless Henry's law constant (air/water
partition coefficient) is extremely low (7.08 × 10-8); therefore,
little evaporation would be expected from water. A half-life for
volatilization of 45 years was estimated for a model river 1 m
deep.2 An approximate Henry's law constant is given in BUA (1997) as
1.77 × 10-4 Pa.m3/mol.
Photodegradation is not expected, as the molecule contains no
chromophores, which absorb radiation (HSDB, 1997).
Despite their miscibility with water, ethyleneamines can bind
strongly to soil. There was a wide range of determined adsorption
coefficients in experimental studies on six soil types (Table 1). Some
reduction in variability occurred when results were normalized for
organic carbon content, although this was less marked with EDA than
with the other ethyleneamine studied. Sorption to soil was rapid, with
equilibrium occurring within a few hours. Electrostatic interaction
between the positively charged ethyleneamine and negatively charged
soils appeared to be the dominant factor in binding. Complex formation
with metals and humic acids is expected. Sorption is greater to soils
with high cation exchange capacity (Davis, 1993).
EDA at 200 mg/litre was incubated with adapted sewage sludge
until there was no further decrease in chemical oxygen demand (COD);
at that time (unspecified), 97.5% of the chemical had been degraded.
The rate of degradation was 9.8 mg COD/g per hour (Pitter, 1976).
EDA at 3, 7, and 10 mg/litre was incubated with sewage sludge
(adapted and non-adapted), and percent biodegradation was determined
5, 10, 15, and 20 days later. Degradation rates were comparable for
adapted and non-adapted sludge up to 15 days (at 56% and 55%,
respectively); at 20 days, however, the values were 70% and 47%,
respectively. Based on this single point, it is not possible to
conclude definitively that adaptation improves degradation. Nitrate
and nitrite were measured throughout the incubation to correct for
1 IUCLID (European Union database), 1st ed., 1996.
2 Syracuse Research Corporation modelling, summarized in HSDB
(1997).
oxygen demand due to conversion of ammonia or organic nitrogen to
these species. Such a correction was necessary for EDA alone out of
more than 50 compounds tested. Degradation was also tested in a
salt-water system using non-adapted sludge; EDA was degraded less
effectively, with 16% of theoretical degradation after 20 days (Price
et al., 1974). A comparable value at 16.6% was measured in seawater by
Takemoto et al. (1981). EDA incubated with microorganisms isolated
from river water and adapted to the compound over 28 days showed >80%
degradation relative to theoretical oxygen demand over 10 days (Mills
& Stack, 1955).
Brief descriptions of the following degradation tests were also
identified. EDA incubated with activated sludge at 100 mg/litre for 28
days showed 93-95% degradation relative to theoretical oxygen demand
in a modified Ministry of International Trade and Industry (MITI) test
(Japan Chemical Industry Ecology-Toxicology Information Center, 1992).
Incubation with activated sludge at a concentration of 50 mg/litre led
to degradation of 10%, 87.5%, and 94% after 5, 15, and 28 days,
respectively.1
The high water solubility and low octanol/water partition
coefficient indicate that bioaccumulation in organisms is unlikely.
1 Unpublished report from Akzo Research to Delamine, 1989 (cited in
IUCLID).
Table 1: Sorption of EDA to various soil types.a
Soil type pH Cation exchange Fraction of Freundlich adsorption Adsorption coefficient
capacity organic carbon coefficient (Kd)b normalized for organic
(meq/100 g) (Foc)b carbon (K = Kd/Foc)c
Sandy loam (Londo) 7.2 9.2 0.026 69 2700
Sandy clay loam 7.3 16.4 0.039 220 5600
Sandy loam (Cecil) 6.0 3.0 0.014 29 2100
Silty loam 6.0 15.6 0.034 238 7100
Clay 7.9 11.9 0.014 70 5000
Aquifer sand 9.6 6.9 0.0024 15 6200
a Data from Davis (1993).
b Values rounded.
c Mean 4800 + 2000 (SD).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no reports of monitoring of EDA levels in the aquatic
environment or of measurements in effluents.
Residues of EDA in soil, 15 days post-treatment with the
fungicide maneb, have been reported at 0.119 mg/kg for the top 1 cm
(approximately) and at 0.044 mg/kg down to about 5 cm. Residues on
tomatoes and beans were 0.053 and 0.239 mg/kg, respectively,
immediately after spraying, falling to 0.047 and 0.094 mg/kg,
respectively, after 14 days (Newsome et al., 1975).
6.2 Human exposure
The data available to the authors of this document are restricted
mainly to the occupational environment. The exposure assessments used
in this report are based on either limited data or data modelled using
the Estimation and Assessment of Substance Exposure (EASE) model. This
is a general-purpose predictive model developed by the United
Kingdom's Health and Safety Executive for exposure assessment in the
workplace. In its present form, the model is in widespread use across
the European Union for the occupational exposure assessment of new and
existing substances. Similarly, information on control measures has
been derived from United Kingdom industry sources. Where data gaps
exist, professional judgement has been used.
The number of employees exposed to EDA in the United Kingdom is
not accurately known. For use as an intermediate in the manufacture of
tetraacetyl ethylenediamine, EDTA, organic flocculants, urea resins,
and fatty bisamides, it is estimated that 140 employees will be
potentially exposed. During the production of formulations for use in
the printed circuit board and metal finishing industries and in the
manufacture of epoxy coatings/resins and pharmaceutical products, it
is estimated that 200 employees will be regularly exposed to EDA. The
number of employees potentially exposed from use of EDA-based
formulations in the printed circuit board and metal finishing
industries is estimated to be about 100. EDA can also be released when
industrial epoxy coatings/adhesives are applied, and this activity has
the potential to expose several thousand employees across a wide range
of industries.
There are very few measured occupational exposure data available.
EDA's use as an intermediate in chemical synthesis takes place in
closed systems. Measured exposures for these manufacturing processes
show that control is achieved to a level of less than 1.25 mg/m3
(0.5 ppm) 8-h time-weighted average (Hansen et al., 1984). Modelled
data (EASE) are in good agreement, predicting comparable values of
0.53-1.3 mg/m3 (0.21-0.52 ppm). Short-term peak exposures (sampling
and hose uncoupling operations) were predicted to range between 16.8
and 33.3 mg/m3 (6.7 and 13.3 ppm), 15-min time-weighted average.
EDA's use in the production of formulations usually takes place
in well-ventilated enclosed systems. Measured exposure data are not
available for these processes. However, modelled exposure data
indicate exposure levels of 5-20 mg/m3 (2-8 ppm) 8-h time-weighted
average in the presence of local exhaust ventilation and 38-75 mg/m3
(15-30 ppm) 8-h time-weighted average in the absence of local exhaust
ventilation. Corresponding short-term peak exposures during mixer
charging operations were estimated to be 5-25 mg/m3 (2-10 ppm) 15-min
time-weighted average in the presence of local exhaust ventilation and
50-103 mg/m3 (20-41 ppm) 15-min time-weighted average in the absence
of local exhaust ventilation.
The potential for exposure during the use of EDA formulations
will be moderated by the low concentration of EDA present in the
formulations. Very few exposure data are available, and there is scope
for widely different use scenarios. There will be no appreciable
occupational exposure if these products are used in enclosed
ventilated systems as indicated by measured exposure data
(<2.5 mg/m3 [<1 ppm] 8-h time-weighted average) and modelled exposure
data (0-0.25 mg/m3 [0-0.1 ppm] 8-h time-weighted average). Modelled
exposure data for immersion processes, in the presence of local
exhaust ventilation, predicted inhalation exposures of 0.5-2.5 mg/m3
(0.2-1 ppm) 8-h time-weighted average. The potential for greatest
inhalation exposure was predicted for situations where these
formulations are brushed in open systems with only general dilution
ventilation or sprayed in open systems in the presence of local
exhaust ventilation. Under these conditions, modelled exposure data
predict exposures in the range 2.5-5 mg/m3 (1-2 ppm) 8-h
time-weighted average. Short-term peak exposures during mixing and
loading operations were estimated to be 5-10 mg/m3 (2-4 ppm) 15-min
time-weighted average. Polyamines and alkanol polyamines, including
EDA, have been reported to be released from hot bitumen during road
paving (Levin et al., 1994). EDA concentrations generated during road
paving were below 0.025 mg/m3 (0.01 ppm).
There will also be a potential for dermal exposure across the
full range of industries handling EDA. Modelled data estimate dermal
exposures in the range 0-0.15 mg/cm2 per day. However, the use of
personal protective equipment is standard practice in all industries
using EDA. Therefore, in practice, dermal exposure will be
considerably reduced by the use of personal protective equipment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The toxicokinetics of EDA has received only limited study, and
there are no studies following inhalation exposure. Studies in humans
have been related to the clinical application of EDA and have
demonstrated rapid absorption via the gastrointestinal tract, with at
least 50% absorbed within the first 7 h; absorbed EDA is rapidly
removed from the plasma (Caldwell & Cotgreave, 1983; Cotgreave &
Caldwell, 1983a,b, 1985). At least half the amount absorbed is
excreted in the urine, largely as the acetylated metabolite
N-acetylethylenediamine and, in smaller amounts, as the unchanged
compound.
This toxicokinetic picture is supported and extended by data from
studies in experimental animals. Studies in rats and mice have
demonstrated rapid and extensive uptake via the oral route and also
via the respiratory tract following intratracheal instillation (about
70% or more of the applied dose was absorbed within 48 h) (McKelvey et
al., 1982; Yang & Tallant, 1982; Yang et al., 1984b). Some (about 12%
of the applied dose over 24 h) dermal absorption has also been
observed in rats at non-irritant concentrations, with greater
absorption at higher, skin-damaging concentrations (Yang et al.,
1987). These animal studies have also demonstrated that EDA and/or its
metabolites are widely distributed throughout the body and are rapidly
eliminated, largely via the urine but also as carbon dioxide in the
breath and a small amount via the faeces, providing evidence for some
biliary excretion. It would seem reasonable to conclude that a similar
situation with respect to distribution and excretion would pertain in
humans. Examination of urinary metabolites in these animal studies
demonstrated that EDA is also found in an acetylated conjugate form in
the rat and mouse. There is evidence that this pathway may become
saturated with increasing dose and that alternative metabolic pathways
may be involved at higher doses in the mouse.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A number of the available studies on both the toxicokinetics and
toxicity of EDA have employed the base substance (EDA) and/or the
hydrochloride salt (EDA.2HCl). The latter is used in pharmaceutical
preparations as a solubilizer to increase uptake of theophylline (this
complex being known as aminophylline) and has been used as a
preservative in skin creams (although it is unclear whether or not
this still occurs). In general, the presence of the hydrochloride has
little qualitative effect on the toxicokinetic or systemic toxicity
properties of EDA, particularly following oral dosing, as it is likely
that the hydrochloride salt would be formed anyway in the acidic
environment of the stomach. However, the hydrochloride does seem to
act in a neutralizing capacity to reduce the significant irritancy
potential of EDA. Studies using both forms of EDA are included in this
review.
8.1 Single exposure
Studies in various animal species have shown EDA to be of
moderate acute toxicity by the inhalation (rat 8-h LC50 estimated to
be in the range of 4916-9832 mg/m3 [1966-3933 ppm]), oral (rat LD50
values of 1160-3250 mg/kg body weight), and dermal (rabbit LD50
values of 550-2880 mg/kg body weight) routes of exposure (Smyth et
al., 1941, 1951; Boyd & Seymour, 1946; Carpenter et al., 1948; NTP,
1982a,b; Yang et al., 1983; Dubinina et al., 1997). Few details exist
of the toxic signs observed or of target organs.
8.2 Irritation and sensitization
There are a number of reports available on skin irritation in
animals, but in general they all repeat the information from one
original study (Smyth et al., 1951). In that study, 0.01 ml undiluted
EDA applied to the shaved backs of albino rabbits produced skin
necrosis within 24 h. A recent report has also indicated EDA to be a
skin irritant (Dubinina et al., 1997). Although no further information
is available, such a response is consistent with EDA being strongly
alkaline. Studies using EDA.2HCl have also resulted in skin
irritation, although the neutralizing action of the hydrochloride may
have influenced the severity of effects, particularly on dilution
(Yang et al., 1983, 1987).
As with skin irritation, the reports that are available for eye
irritation all largely reproduce data from one original study
(Carpenter & Smyth, 1946). In this study, 0.005-ml solutions of 5% EDA
or greater caused corneal injury, which again would be expected, given
the alkaline properties of the substance. More recently, Dubinina et
al. (1997) stated that inflammatory responses in the rabbit eye were
induced by "one drop" of EDA.
Overall, from the reports that are available, together with a
consideration of its alkaline properties, it is reasonable to conclude
that EDA is corrosive, with the capacity to produce severe chemical
burns to the skin and eye.
EDA has been demonstrated to possess skin sensitizing potential
in guinea-pig studies, generally using standard methodologies such as
the Magnusson and Kligman maximization and Buehler tests
(Thorgeirsson, 1978; Erikson, 1979; Maurer et al., 1979; Henck et al.,
1980; Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Dubinina et al., 1997; Leung & Auletta, 1997). In four of these
studies (Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Leung & Auletta, 1997), the investigators ensured that
non-irritant challenge concentrations of EDA were used, providing
clear evidence for a sensitization response. EDA also produced
positive results in the local lymph node assay (Basketter & Scholes,
1992). In contrast to these positive results, EDA consistently
produced negative results in the mouse ear swelling test (Gad et al.,
1986; Cornacoff et al., 1988; Dunn et al., 1990). One study
demonstrated the potential for EDA to cross-react with other
alkylamines either as the inducing or as the challenge agent (Leung &
Auletta, 1997).
No studies are available on respiratory sensitization in animals.
8.3 Short-term exposure
In a 12-day study (NTP, 1982b), mice received gavage doses of
between 50 and 600 mg EDA/kg body weight per day (administered as
EDA.2HCl). Deaths were observed at 400 and 600 mg EDA/kg body weight
per day. No effects were seen at 50 mg EDA/kg body weight per day.
Renal effects (nephrosis and tubule regeneration) were observed at 100
mg EDA/kg body weight per day and above. Lymphoid depletion in and
necrosis of splenic follicles were observed at 400 mg EDA/kg body
weight per day.
A 7 h/day, 30-day inhalation study in rats indicated that the
liver and kidney are potential target tissues, with local effects in
the lungs also likely (Pozzanni & Carpenter, 1954). No effects were
observed in this study at an airborne exposure concentration of about
150 mg/m3 (60 ppm). Slight depilatory effects were seen at 330 mg/m3
(132 ppm), becoming more marked at higher exposure concentrations.
Treatment-related deaths were observed at 563 mg/m3 (225 ppm) and
1210 mg/m3 (484 ppm) (all animals died at 1210 mg/m3 [484 ppm]).
Cloudy swelling of cells in the liver and convoluted tubules of the
kidneys were also observed at these exposure concentrations.
Degeneration of the convoluted tubules was seen in animals exposed to
1210 mg/m3 (484 ppm), as was congestion of the lungs and adrenals.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Dietary studies in rats have also indicated that the liver is a
target tissue, with changes in the size and shape of hepatocytes and
their nuclei being noted at 1000 mg/kg body weight per day in a 90-day
study (Yang et al., 1983).
Oral gavage studies using doses of between 100 and 1600 mg EDA/kg
body weight per day (administered as EDA.2HCl) have been carried out
in rats (NTP, 1982a). Deaths were observed after 12 doses at 800 and
1600 mg EDA/kg body weight per day and after 800 mg EDA/kg body weight
per day for 90 days. Renal tubular lesions (dilation of the lumen,
necrosis, degeneration and regeneration of the epithelium) were seen
at 200 mg EDA/kg body weight per day and above after 12 doses. Similar
renal lesions but of a less severe nature were seen only at 600 mg
EDA/kg body weight per day and above after 90 days. This indicates
recovery in the kidney, probably as a consequence of compensatory
regeneration. No effects were seen on the kidney at 100 mg EDA/kg body
weight per day in either study. Ocular effects including cataract
formation and retinal atrophy were observed in all dose groups.
Minimal to moderate focal retinal atrophy was observed in 3 out of 10
females at 100 mg EDA/kg body weight per day; 2 males had mild to
moderate retinal atrophy and 1 male had severe retinal atrophy at 200
mg EDA/kg body weight per day. Lymphoid depletion and/or necrosis in
spleen were observed at 800 mg EDA/kg body weight per day and in all
decedents following 12 doses, and thymus weight was reduced at 800 mg
EDA/kg body weight per day in the 90-day study. Uterine lesions
(reduced uterine horn size and atrophy of the myometrium and
endometrium) were seen after dosing for 90 days with 600 or 800 mg
EDA/kg body weight per day, and reduced ovarian size was seen after
800 mg EDA/kg body weight per day for 90 days. Overall, a
no-observed-adverse-effect level (NOAEL) was not identified from these
studies, as effects on the eyes were seen at all dose levels. Only
ocular effects were seen at the lowest-observed-adverse-effect level
(LOAEL) of 100 mg EDA/kg body weight per day, and these were of a
minimal to mild nature, suggesting that this dose represented the
lower end of the dose-response relationship for these effects.
In a 90-day study, mice received oral gavage doses of 25-400 mg
EDA/kg body weight per day (NTP, 1982b). No effects were seen at 100
mg EDA/kg body weight per day. Renal lesions (cortical tubular
degeneration and/or necrosis) were observed at 200 and 400 mg EDA/kg
body weight per day.
8.4.2 Chronic exposure and carcinogenicity
There are two carcinogenicity studies in animals. Both studies
were performed to reasonably adequate standards, including extensive
histopathology, and were negative for carcinogenic activity.
In the first study, groups of 99-225 F344 rats were orally dosed
with 0, 20, 100, or 350 mg EDA.2HCl (equivalent to 0, 9, 45, or 158
mg EDA/kg body weight per day) for 2 years (Yang et al., 1984a).
Non-neoplastic effects were similar to those described in studies of
shorter duration (Yang et al., 1993), as indicated above (section
8.4.1). Effects were seen at 45 mg EDA/kg body weight per day, with a
NOAEL of 9 mg EDA/kg body weight per day. Tracheitis was also
observed, probably as a consequence of exposure to EDA in airborne
dust derived from the diet.
In the second study, groups of 40-50 C3H/HeJ mice were dermally
administered 0 or 0.25 mg aqueous EDA 3 times per week for a lifetime
(DePass et al., 1984). The dermal study included a positive control
group that received 3-methylcholanthrene. Skin fibrosis and
hyper-keratosis were observed in EDA-treated mice.
8.5 Genotoxicity and related end-points
Only limited information is available on the genotoxic potential
of EDA. There is some evidence that EDA may be mutagenic in bacteria
with and without metabolic activation (Hedenstedt, 1978; Hulla et al.,
1981; Haworth et al., 1983; Leung, 1994). Although the most recent
study (Leung, 1994) appears to be negative, there was a small response
in Salmonella typhimurium TA100 and a positive, but not
reproducible, response in TA1535. Positive results have also been
reported in these strains from the other studies, although only one of
these (Haworth et al., 1983) was adequately reported. The only series
of studies performed on mammalian cell systems in vitro (gene
mutation and sister chromatid exchange in Chinese hamster ovary cells;
unscheduled DNA synthesis in rat primary hepatocytes) were
consistently negative (Slesinski et al., 1983), although there has
been no assay for clastogenic activity. A sex-linked recessive lethal
test in Drosophila melanogaster was negative following dosing by
feeding or injection (Zimmering et al., 1985). There are no in vivo
studies on somatic cells, but a dominant lethal study in rats up to
doses inducing signs of toxicity (up to 500 mg EDA.2HCl/kg body
weight per day in the diet) was negative (Slesinski et al., 1983).
Although there has been some evidence of mutagenicity in
bacterial systems in a few limited studies, the available evidence
indicates that EDA is not genotoxic, with all results in mammalian
cells in vitro and in vivo (dominant lethal assay) being negative.
It should be noted that the overall database is limited, with no
assays available for clastogenic activity or for genotoxic potential
in somatic cells in vivo.
8.6 Reproductive and developmental toxicity
The potential of EDA to affect fertility and development has been
studied in rats in investigations conducted to modern regulatory
standards. In a two-generation study in F344 rats, no effects on
fertility or development in any of the generations were observed up to
a dose level (225 mg EDA/kg body weight per day) that induced signs of
parental toxicity (Yang et al., 1984b). Dose levels used in this study
were 0, 50, 150, or 500 mg EDA.2HCl/kg body weight per day (equivalent
to 0, 23, 68, and 225 mg EDA/kg body weight per day). Effects on the
uterus and ovaries have been seen following gavage dosing of rats with
600 and 800 mg EDA/kg body weight per day for 90 days (see section
8.4.1; NTP, 1982a). In a series of developmental toxicity studies in
F344 rats, EDA was found to produce signs of fetotoxicity (increased
resorptions) and delays in development at high dose levels (450 mg
EDA/kg body weight per day) that induced clear signs of toxicity in
the dams (DePass et al., 1987). Dose levels used in this study were 0,
50, 250, or 1000 mg EDA.2HCl/kg body weight per day (equivalent to 0,
23, 113, and 450 mg EDA/kg body weight per day). Some of the
developmental effects appear to have been related, at least in part,
to the reduced nutritional status of the animals. However, a clear
NOAEL for developmental toxicity of 113 mg EDA/kg body weight per day
was observed in these studies.
The results of a preliminary screening study in mice indicated no
significant effects on development in the offspring of dams exposed to
toxic doses of EDA (400 mg/kg body weight per day) by oral gavage
(Hardin et al., 1987).
No effects on development were seen in the offspring of New
Zealand white rabbits dosed during pregnancy with up to 178 mg
EDA.2HCl/kg body weight per day (equivalent to 80 mg EDA/kg body
weight per day), a dose that did not induce maternal toxicity (NTP,
1991; Price et al., 1993). In a preliminary study, 2/20 pregnant
rabbits receiving 100 mg EDA/kg body weight per day by gavage died,
and decreased body weight was seen in survivors. At 400 mg/kg body
weight per day, all the dams died.
8.7 Immunological and neurological effects
No studies are available that have specifically investigated the
potential immunotoxicity of EDA. Effects on lymphoid tissue in the
spleen in mice and rats (see sections 8.3 and 8.4.1, respectively) and
on the thymus in rats (see section 8.4.1) were observed in oral gavage
dosing studies.
There are a few, mainly in vitro, studies on the effects of EDA
on the release of gamma-aminobutyric acid from the retina, gut, and
brain (Perkins & Stone, 1980; Forster et al., 1981; Lloyd et al.,
1982; Morgan & Stone, 1982; Sarthy, 1983; Kerr & Ong, 1984; Strain et
al., 1984; Hill, 1985; Erdo et al., 1986; Krantis et al., 1990; McKay
& Krantis, 1991). The general conclusion that can be drawn from these
studies is that EDA can cause a calcium-independent release of
gamma-minobutyric acid that is insensitive to the presence of
tetrodotoxin. EDA was also shown to have gamma-minobutyric acid
mimetic properties (i.e., reduction of neuronal firing rate). This
suggests that EDA could have a central nervous system depressant
effect, but studies were not performed to address this possibility. It
was reported that EDA elicited contraction of the guinea-pig ileum
that was mediated via neuronal release of gamma-aminobutyric acid.
However, in the rat ileum, EDA acted directly on the mucosa, resulting
in relaxation. Although these are interesting results, the
toxicological significance of these findings is unclear; they may,
however, partly explain the central nervous system depressant and
gastrointestinal effects seen in some of the animal studies at high
doses.
9. EFFECTS ON HUMANS
No studies are available in which the effects, other than the
respiratory effects summarized below, of repeated exposure of humans
to EDA are examined. No reports have been found in which genotoxicity,
carcinogenicity, or reproductive toxicity following exposure to EDA in
human populations has been studied.
A case report exists concerning a 36-year-old worker who died
from cardiac collapse 55 h after being splashed by an accidental
spillage of EDA (Niveau & Painchaux, 1973). Exposure to an
unquantified amount of EDA was in the order of a few minutes prior to
the patient being washed. Four hours after the exposure, he presented
with tachycardia (100 beats/min), anuria, and red/brown generalized
erythema. The tachycardia increased (up to 140 beats/min), anuria
persisted, and an expectorant cough, abdominal cramps, diarrhoea, and
blackish vomiting appeared. The patient became hyperkalaemic, and his
red blood cell count decreased. Overall, given the lack of information
with respect to levels of exposure, few useful conclusions can be
drawn from this case report.
The only information available on skin irritation in humans
either is anecdotal or does not involve direct surface skin contact
with EDA. In a brief report on the physicochemical properties of EDA,
it is noted anecdotally that "the liquid, if not washed from the skin,
causes blistering" (Boas-Traube et al., 1948). The other report
available documents the results of intradermal skin tests with
solutions (0.1-1%) of EDA on three individuals being tested for
hypersensitivity following treatment with aminophylline (Kradjan &
Lakshminarayan, 1981). The skin response in two patients consisted of
blistering rather than a weal and flare reaction, which normally
typifies a sensitization response. Punch biopsies were obtained from
one patient, and histopathological examination of these indicated
tissue necrosis and oedema of the epidermis and dermis. These
responses suggested a direct corrosive effect of EDA.
No specific reports were found of the effects of EDA on the eyes
of humans. In the original reports of the animal eye irritation study
(see section 8.3), it is claimed that EDA is known to have produced
loss of vision or slowly healing corneal burns in industrial use
(Carpenter & Smyth, 1946). However, no further information or
references were given.
EDA has been known for many years to be capable of inducing
allergic skin reactions in humans. This has been observed both in the
workplace and, most notably, in patients treated with aminophylline or
with skin creams in which EDA was used as a stabilizer (Epstein &
Maibach, 1968; Petrozzi & Shore, 1976; Booth et al., 1979; Wall, 1982;
Hardy et al., 1983; Balato et al., 1984; Edman & Moller, 1986; Nielsen
& Jorgensen, 1987; Terzian & Simon, 1992; Toal et al., 1992; Dias et
al., 1995; Simon et al., 1995; Sasseville & Al-Khenaizan, 1997). The
first reports of skin sensitizing effects in humans date back to the
late 1950s, when cases were described of eczematous reactions in
pharmacists who came into contact with EDA when using aminophylline
(Baer et al., 1958; Tas & Weissberg, 1958).
Subsequent to these early reports, numerous studies and case
reports have been published documenting the skin sensitizing
properties of EDA both following clinical use and within the
occupational setting, such that the substance has become incorporated
into standard series for patch testing (Fregert, 1981; Shehade et al.,
1991). An example from the clinical setting is that of the report on a
series of 13 patients who had used skin cream containing EDA for,
paradoxically, dermatitic conditions (Provost & Jillson, 1967). Use of
the cream in 11 of these patients had resulted in the sudden
appearance of a severe generalized patchy eczematous eruption
following, in all but one of the cases, an initial improvement upon
using the cream. Patch testing was conducted using a 1% aqueous
solution of EDA, producing skin reactions in all patients ranging from
erythema and oedema to erythema vesiculation and oedema vesiculation,
which extended beyond the patch test site. Other substances tested
also induced responses, but not in a consistent manner, with at most
only four individuals responding in any one test.
As well as the original reports in pharmacists working with EDA,
cases of skin sensitization to EDA have been reported in the
occupational environment in a number of different settings, including
use of floor polish remover (English & Rycroft, 1989), use of coolant
oils (Crow et al., 1978), and in wire-drawing (Matthieu et al., 1993;
Sasseville & Al-Khenaizan, 1997). Positive responses to patch testing
with EDA have also been observed in other occupational settings, such
as the offshore oil industry (Ormerod et al., 1989), but positive
responses to other substances, including other polyamines, were also
seen in such cases. Thus, it is unclear whether EDA had been
responsible for inducing the sensitized state and/or cross-reacting
following sensitization to another polyamine.
A large number of cases of occupational asthma reported to have
been caused by exposure to EDA are available in the literature
(Dernehl, 1951; Gelfand, 1963; Popa et al., 1969; Valeyeva et al.,
1975; Lam & Chan-Yeung, 1980; Chan-Yeung, 1982; Hagmar et al., 1982;
Matsui et al., 1986; Aldrich et al., 1987; Nakazawa & Matsui, 1990;
Lewinsohn & Ott, 1991; Ng et al., 1991, 1995). There are a few studies
in which the potential for EDA to cause respiratory hypersensitivity
has been examined using bronchial provocation testing and
investigation of antibody formation. As EDA is corrosive, the vapour
would be predicted to be a respiratory tract irritant, which is a
complicating factor in interpreting the data available and in
elucidating the underlying mechanism for any asthmatic responses seen.
Popa et al. (1969), in a well-conducted study, investigated 48
subjects with asthmatic symptoms caused by exposure to a number of low
molecular weight chemicals, including EDA. None of the subjects had a
history of respiratory disorder prior to occupational exposure, and
the asthmatic response was associated only with occupational exposure
in all cases. No information was given in the report on the workplace
airborne concentrations of EDA to which these workers were exposed. A
series of tests were performed in all subjects, including skin and
inhalation tests with the test agent at sub-irritant concentrations;
skin and inhalation tests to common allergens; skin tests
(intradermal, scratch, and patch tests) using sub-irritant
concentrations of the test substance; Prausnitz-Kustner transfer
reaction (to test for the presence of immunoglobulin E antibodies);
and determination of precipitating antibodies to EDA. For the
inhalation test, the sub-irritant concentration was determined in
control asthmatic subjects, and a 2- to 10-fold dilution of this was
used for the bronchial challenge. No information was given on the
airborne exposure concentrations generated under these test
conditions. Control inhalation tests with the diluent, physiological
saline, were also conducted. It is not stated in the report whether or
not the inhalation challenge tests were conducted in a blind manner.
Six subjects had an immediate, positive reaction to EDA in the
workplace. Of these, four showed an immediate, positive response
following inhalation testing with sub-irritant concentrations of EDA.
These subjects developed marked bronchoconstriction following
inhalation exposure to EDA, with a reduction in forced expiratory
volume in 1 s (FEV1) of 62% and an increase in respiratory resistance
of 44%, compared with controls. Although not stated in the report,
these values are presumed to be average changes. Intradermal skin
tests with EDA were positive in these four subjects, whereas patch
tests were negative. Inhalation challenges with common allergens were
negative. The Prausnitz-Kustner test was positive in all subjects, and
all had eosinophilia, determined in the sputum, although not, except
in one case, in the blood. No precipitating antibodies were found. In
the two other subjects, the inhalation challenge test was negative. No
precipitating antibodies were found, and the Prausnitz-Kustner test
was negative in both subjects. Eosinophilia was absent. Inhalation
challenges with other common allergens were also negative.
These data provide evidence that EDA may elicit an asthmatic
response at sub-irritant concentrations and that the response is
specific to EDA. Four out of six subjects responsive to EDA in the
workplace also had a positive response to inhaled EDA at sub-irritant
concentrations. This demonstrates that the reaction is not a
generalized response to an irritant. The positive Prausnitz-Kustner
reaction may be indicative of an immunological component, but the test
is not specific, and no firm conclusions can be drawn from it. It
provides supporting evidence in this case. The evidence suggests that
the subjects were hypersensitive to inhaled EDA and that a state of
respiratory hypersensitivity had been induced by the substance.
Although a number of other studies are available, the information
is of poor quality. Lam & Chan-Yeung (1980) and Chan-Yeung (1982)
describe the case of one worker in a photographic laboratory who
developed asthma after 2.5 years of exposure to a variety of
chemicals, including EDA, but also other irritant substances. The
worker developed symptoms of sneezing, nasal discharge, productive
cough and nocturnal cough, wheezing, and dyspnoea. The symptoms
coincided with the work shift and subsided at weekends. There was no
previous history of asthma. No information was given in the report of
the airborne concentrations of EDA (or other substances) to which the
man was exposed at work. A series of controlled inhalation challenge
exposures, designed to mimic work exposure conditions, were conducted
with each of the chemicals to which the subject was exposed at work.
The duration of exposure was determined by the patient's tolerance,
and exposure was terminated when eye irritation or cough was
experienced. No information on the airborne exposure concentrations of
EDA generated under these test conditions was given in the report. A
methacholine inhalation test for bronchial hyperreactivity was also
performed. Pulmonary function tests were conducted pre- and
post-challenge, and blood samples were taken before, during, and after
each challenge. The subject showed marked bronchial reactivity to
methacholine.
Exposure to an unknown concentration of vapour from a 1:25
solution of EDA was tolerated for 15 min. This exposure produced a
marked bronchoconstriction. A late asthmatic response developed 4 h
after the exposure, at which time FEV1 was reduced by 26% and
continued to decrease over the next 3 h towards a 40% reduction. A 26%
reduction was still apparent after 24 h, despite treatment with
bronchodilator drugs. This pattern of response to EDA was
reproducible. The patient did not respond similarly to any of the
other chemicals tested: formaldehyde, sulfur dioxide, and two colour
developing agents that were stated to be irritants. Exposure to
formaldehyde (vapour from a 1:4 solution) produced an immediate small
(<20%), transient reduction in FEV1, whereas exposure to sulfur
dioxide caused coughing and chest tightness and an immediate transient
reduction of 25% in FEV1. There was no increase in plasma histamine
concentration during the period of bronchoconstriction, although EDA
was shown to cause in vitro histamine release from whole blood taken
from the patient and from two control subjects. A skin test using
1:100 EDA and a precipitin test for antibodies to EDA were both
negative. The patient subsequently had to give up work because of
respiratory symptoms and became asymptomatic after 2 weeks. Subsequent
testing with methacholine, 2.4 months after ceasing work, showed that
the subject had a reduction in the previous bronchial hyperreactivity.
In conclusion, the subject showed an asthmatic response to EDA
but not to formaldehyde or the colour developing agents. The pattern
of response to sulfur dioxide was more immediate and suggestive of an
irritation response. Overall, a clear pattern of asthmatic response
that was specific to EDA was observed in this study. However, it is
not possible to distinguish with certainty between an irritant
response and a sensitization response, because it is possible that an
irritant concentration was used for the bronchial challenge exposure,
although little immediate response was observed. In addition, although
exposure to irritant concentrations of the other workplace chemicals
did not elicit the same pattern, magnitude, or severity of response as
that seen with EDA, since accurate exposure levels were not given, it
is not possible to determine whether or not the EDA concentration used
had the greatest irritant potential. No evidence for any immunological
involvement was found. In conclusion, this study provides only
circumstantial evidence that EDA caused a state of respiratory
hypersensitivity in this subject.
A number of other case reports are available of individuals who
exhibited asthmatic signs and symptoms associated with exposure to EDA
in the workplace (Gelfand, 1963; Valeyeva et al., 1975; Matsui et al.,
1986; Nakazawa & Matsui, 1990; Ng et al., 1991). Although bronchial
challenge testing with EDA produced asthmatic responses in these
subjects, they had personal and/or family histories of allergic
disease and/or they had worked with and responded on challenge to
other substances. Retrospective studies using the medical records of
populations of workers using EDA have indicated that about 10% of such
populations developed signs and symptoms of occupational asthma
(Aldrich et al., 1987; Lewinsohn & Ott, 1991). No challenge tests were
carried out with these surveys. Thus, these case reports and
population-based studies provide only supporting circumstantial
evidence for the involvement of EDA in producing occupational asthma.
Although it is clear from these reports that EDA can provoke an
asthma attack, in many cases there is insufficient information to
indicate whether or not the hypersensitive state was induced
specifically by EDA. However, from one well-conducted study, there is
evidence that a hypersensitive state specific to EDA has been induced
in workers and that an asthmatic response was provoked by sub-irritant
concentrations of the substance. Overall, the results of this study,
taken together with the supporting data from a substantial number of
other reports of occupational asthma, indicate that EDA is capable of
inducing a state of hypersensitivity in the airways, such that
subsequent exposure may trigger asthma. The mechanism by which the
hypersensitive state is induced is not proven. Given the skin
sensitizing potential of EDA and the limited evidence of immunological
involvement in workers with EDA-provoked occupational asthma, an
immunological mechanism would seem plausible. Irrespective of the
mechanism involved, the data available (specifically the lack of
information on airborne exposure concentrations under both work and
challenge test conditions) do not allow elucidation of a dose-response
relationship or the identification of levels of EDA that are not
capable of inducing a hypersensitive state or of provoking an
asthmatic response.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Results of acute ecotoxicity tests are given in Table 2.
A 21-day Daphnia magna reproduction test was conducted
according to the German Federal Environment Agency (Umweltbundesamt)
guidelines in a closed vessel. End-points measured included adult
mortality, onset of production of young, and reproduction rate. The
most sensitive end-point was for reproductive rate, and a NOEC of 0.16
mg/litre was established (Kuhn et al., 1989). In a second study
conducted according to Organisation for Economic Co-operation and
Development (OECD) guidelines, a NOEC for reproduction was reported at
2 mg/litre (Mark & Hantink-de Rooy, 1992). An early life stage test
conducted under OECD guidelines on three-spined stickleback
( Gasterosteus aculeatus) showed no effects of EDA at 10 mg/litre
(the highest concentration tested) over 28 days (Mark & Arends, 1992).
Growth of lettuce ( Lactuca sativa) plants over 7 days was
studied in tests conducted under OECD Guideline 208; EC50
concentrations for EDA in soil (nominal) were >1000 mg/kg for 7-day
and 692 mg/kg for 14-day growth periods (Hulzebos et al., 1993).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
EDA is of moderate acute toxicity by all routes. In studies in
animals, EDA is a primary skin and eye irritant, being corrosive when
undiluted. It is also a skin sensitizer. In repeated-dose toxicity
studies by the oral and inhalation routes, effects on the liver and
kidney have been observed, with pleomorphic changes to hepatocytes in
rats being reported at the lowest oral doses used (45 mg/kg body
weight per day and above for 2 years; NOAEL, 9 mg/kg body weight per
day). In inhalation studies, there were no effects at 150 mg/m3 (60
ppm), although slight depilation was observed at the next highest
concentration (330 mg/m3 [132 ppm]) and effects on the liver and
kidney at higher concentrations still (approximately 500 mg/m3 [200
ppm] and above).
There has been some evidence of mutagenicity in bacterial systems
in a few limited studies. However, much of the available evidence is
negative, although the overall database is limited, there being no
assays for clastogenic activity or for genotoxic potential in somatic
cells in vivo. EDA was not carcinogenic in adequate studies in
animals.
In humans, EDA has the potential to induce respiratory tract
hypersensitivity, and provocation of asthma is the major health effect
of concern in the occupational environment. The mechanism of induction
of the hypersensitive state is unknown, although the skin sensitizing
potential of EDA and limited evidence in workers with EDA-provoked
asthma are suggestive of an immunological mechanism. However,
irrespective of the mechanism involved, the available data
(particularly lack of information on exposure conditions either in the
workplace or on bronchial challenge testing) do not allow either
elucidation of dose-response relationships or identification of the
thresholds for induction of the hypersensitive state or provocation of
an asthmatic response.
11.1.2 Criteria for setting guidance values for EDA
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship for provocation of
an asthmatic response, the effect of greatest concern in the
occupational environment. As it is not possible to identify a level of
exposure that is without adverse effect, it is recommended that levels
be reduced to the extent possible.
Table 2: Acute toxicity of EDA to organisms other than laboratory mammals.
Species End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Microcystis aeruginosa Toxic threshold for cell multiplication 0.08 Bringmann & Kuhn, 1976
Pseudomonas putida 17-h EC50 (growth rate) 29 (23-35) van Ginkel, 1989
Nitrifying bacteria 3-h EC50 (respiration) 3.2 (0.6-5.7) Balk & Meuwsen, 1989a
NOEC 0.5
Activated sludge bacteria 1-h EC50 (respiration rate) 1600 van Ginkel & Stroo, 1989
Algae
Scenedesmus quadricauda Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Chlorella pyrenoidosa 96-h EC50 (growth) 100 van Leeuwen, 1986
Selenastrum capricornutum 72-h EC50 (biomass) 71 van Ginkel et al., 1990
72-h EC50 (growth rate) 645
NOEC approx. 3.2
96-h EC50 (growth rate) 151 van Wijk et al., 1994
Scenedesmus subspicatus 48-h EC50 (biomass and growth rate) >100 Kuhn & Pattard, 1990
Protozoa
Entosiphon sulcatum Toxic threshold for cell multiplication 1.8 Bringmann & Kuhn, 1980a
Uronemia parduczi Toxic threshold for cell multiplication 52 Bringmann & Kuhn, 1980b
Chilomonas paramaecium Toxic threshold for cell multiplication 103 Bringmann & Kuhn, 1980b
Invertebrates
Daphnia magna 48-h LC50 26.5 van Leeuwen, 1986
48-h LC50 46 van Wijk et al., 1994
48-h LC50 16.7 Balk & Meuwsen, 1989b
24-h LC50 14 Kuhn et al., 1989
Table 2 (continued)
Species End-point Concentration Reference
(mg/litre)
Brine shrimp (Artemia
salina) 24-h LC50 14 Price et al., 1974
Fish
Brown trout (Salmo trutta) 48-h LC50 230 Woodiwiss & Fretwell, 1974
Fathead minnow (Pimephales
promelas) 96-h LC50 115.7 Curtis & Ward, 1981
Guppy (Poecilia reticulata) 96-h LC50 275 van Leeuwen, 1986
96-h LC50 640 Balk & Meuwsen, 1989c
96-h LC50 1545 van Wijk et al., 1994
Medaka (Oryzias latipes) 48-h LC50 1000 Tonogai et al., 1982
With respect to systemic effects, the lowest NOAELs of 150 mg/m3
(60 ppm) (inhalation) and 9 mg/kg body weight per day (oral) can serve
as a basis for comparison with estimated exposure for characterization
of risk, either with application of appropriate uncertainty factors or
directly. An example of the latter (margin of exposure) approach is
presented in section 11.1.3.
11.1.3 Sample risk characterization
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship, and hence risk,
for provocation of an asthmatic response, the effect of greatest
concern in the occupational environment. For substances that are
asthmagens, it is also advisable to restrict peak exposures, as they
may have a role in the induction and triggering of asthmatic
phenomena. However, to help assess the risk to human health arising
from occupational exposures, a comparison is made with the NOAELs for
systemic effects from animal studies.
It should also be noted that because diluted EDA is a skin
irritant and sensitizer, there is a risk of developing irritant and/or
allergic dermatitis if suitable personal protective equipment is not
used.
A sample risk characterization for systemic effects in the
occupational environment in the United Kingdom is provided here.
Measured data on exposure to EDA (generally used in closed systems)
indicate that levels in industry in the United Kingdom are less than
1.25 mg/m3 (0.5 ppm), 8-h time-weighted average. Modelled data (EASE)
are in good agreement, predicting comparable values of 0.53-1.3 mg/m3
(0.21-0.52 ppm). Short-term peak exposures (sampling and hose
uncoupling operations) were predicted to be 16.8-33.3 mg/m3
(6.7-13.3 ppm), 15-min time-weighted average. The EASE model predicts
dermal exposures in the range of 0-0.15 mg/cm2 per day for an
operator transferring EDA into closed systems once a day (although
coveralls and well-maintained plastic gloves will significantly reduce
exposure from this source).
With respect to systemic effects, worst-case estimated and
measured exposures of 1.25 mg/m3 (0.5 ppm), 8-h time-weighted
average, are substantially less (by 100-fold or greater) than the
NOAEL in the inhalation study in rats. The combined body burden from
inhalation and dermal exposure for chemical synthesis can be estimated
to be about 0.3 mg/kg body weight per day (assuming a 70-kg worker
breathing 10 m3 of air containing 1.25 mg EDA/m3 [0.5 ppm] per day,
with 100% absorption; and 10% absorption from unprotected, undamaged
skin for a standard hand area of 840 cm2). This is 30-fold less than
the NOAEL for hepatic effects in the oral studies.
Available data on indirect exposure in the general environment or
from consumer products are inadequate to serve as a basis for a sample
characterization of risk for these scenarios.
11.2 Evaluation of environmental effects
No atmospheric effects are expected, as reaction with hydroxyl
radicals is likely to be rapid, and washout of volatilized EDA is
expected. Volatilization to the atmosphere is likely from soil but not
from water.
Adsorption to soil particulates is strong through electrostatic
binding; leaching through soil profiles to groundwater is not
expected. EDA is readily biodegradable, and this is the most likely
source of breakdown in the environment; adaptation of microorganisms
may improve degradation. Breakdown is less rapid in seawater than in
fresh water. Bioaccumulation is unlikely.
Toxic thresholds for microorganisms may be as low as 0.1
mg/litre. However, toxicity tests in culture media should be treated
with caution, as the EDA may complex with metal ions. Effects may
therefore be indirect, from loss of bioavailability of essential
elements. The "toxic thresholds" reported are lowest-observed-effect
concentrations (LOECs) for small changes in sublethal end-points; the
exact degree of effect at the reported concentrations is not always
clear, and these have not been used in the risk assessment.
The principal receiving compartment in the environment is the
hydrosphere, and this is the only compartment for which a quantitative
risk assessment can be attempted.
The distribution of acute (from Table 2) and chronic test results
is plotted in Figure 1. Chronic test results are available for fish (a
limit test only) and Daphnia (NOECs from 21-day reproduction tests).
Chronic EC50s for algal growth or biomass are also available, but no
NOEC was reported for these studies. Given the range of chronic test
results across three trophic levels, it is proposed that an
uncertainty factor of 10 be applied to the lowest reported NOEC (for
Daphnia reproduction at 0.16 mg/litre) to derive an estimated PNEC
for aquatic organisms of 0.016 mg/litre. This is in accord with OECD,
European Union, and US Environmental Protection Agency guidelines.
There are no reported test results for estuarine/marine organisms; it
is assumed that toxicity would be in the same range for these species.
No measured concentrations of EDA in surface waters have been
reported. A single quantitative risk assessment has been reported (van
Wijk, 1992) based on discharges into the Ems-Dollard estuary in the
Netherlands. The figure of 75 kg/day for release of EDA at this site
has been used here as a representative estimate of release; no
information on releases from industrial plants elsewhere is available.
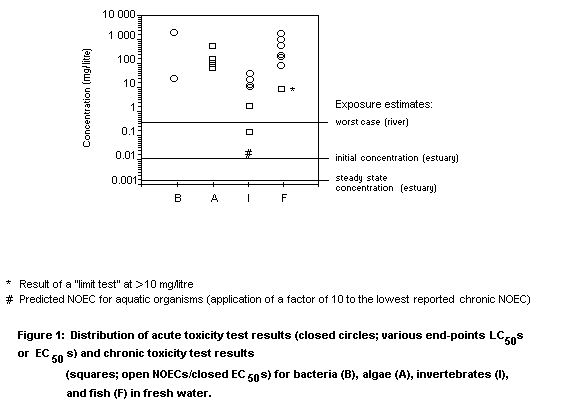 Based on this emission rate, and using default values from the
OECD Technical Guidance Manual, the initial concentration in river
water would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
= 337.5 µg/litre
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q),
where:
W = emission rate (75 kg/day)
P = percent removal in the wastewater treatment plant
(91%, based on a classification of the chemical as
"readily biodegradable")
Q = volume of wastewater in m3/day (default 200
litres [0.2 m3] per person per day for a
population of 10 000 inhabitants)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Foc(susp) × Koc, where:
Foc(susp)= the fraction of organic carbon in suspended
matter (0.01)
Koc = 0.411 × Kow
where:
Kow = the octanol/water partition coefficient (0.063)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (default value of 10)
Calculation of initial PEC in the compartment of the estuary
receiving emissions from the actual plant in the Netherlands gives a
PEC(initial near field) of 10.9 µg/litre. This is based on a local
volume at mean tide of 6.9 × 109 litres, a residence time of 1 day,
and a wastewater flow from the plant of 500 m3/day, realistic for the
local conditions.
More refined modelling, taking into account tidal dilution and
expected biodegradation with a half-life of 5 days, predicts a
steady-state concentration of 1.3 µg EDA/litre (van Wijk, 1992). This
is likely to be a closer reflection of the real situation in the
estuary.
The risk factors shown in Table 3 can be calculated for
conservative worst-case and refined estimates of environmental
concentrations for a river and estuary.
Table 3: PEC/PNEC ratios.
PEC (µg/litre) PNEC (µg/litre) PEC/PNEC
ratio
River, worst case 337.5 16 21.1
Estuary, initial
worst case 10.9 16 0.68
Estuary, refined PEC 1.3 16 0.08
The PEC/PNEC ratio for the river indicates some cause for concern
(ratio greater than 1). However, the PEC is based on very conservative
assumptions, and both estimates assume low adsorption to sediment
based on water solubility. Refined estimates for the estuary indicate
low risk to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0269) reproduced in this
document.
13.1 Human health hazards
Repeated or prolonged contact with EDA may cause skin
sensitization and asthma.
13.2 Advice to physicians
EDA is corrosive. Inhalation of the vapour may cause irritation
of the respiratory tract and even lung oedema and could mask asthmatic
reaction.
13.3 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of EDA as a human asthmagen.
13.4 Spillage
In the case of spillage, emergency crews need to wear proper
equipment and prevent EDA from reaching drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENEDIAMINE ICSC: 0269
June 1999
CAS # 107-15-3 1,2-Diaminoethane
RTECS # KH8575000 1,2-Ethanediamine
UN # 1604 H2NCH2CH2NH2
EC # 612-006-00-6 Molecular mass: 60.1
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Flammable. Gives off NO open flames, NO Powder, alcohol-resistant
irritating or toxic sparks, and NO foam, water spray, carbon
fumes (or gases) in smoking. dioxide.
a fire.
EXPLOSION Above 34°C explosive Above 34°C closed In case of fire: keep drums,
vapour/air mixtures system, ventilation, etc., cool by spraying with
may be formed. and explosion-proof water.
electrical equipment.
EXPOSURE STRICT HYGIENE!
Inhalation Burning sensation. Ventilation, local exhaust, Fresh air, rest. Artificial
Cough. Laboured breathing. or breathing protection. respiration if indicated.
Shortness of breath. Sore Refer for medical attention.
Throat.
Skin MAY BE ABSORBED! Redness. Protective gloves. Remove contaminated
Skin burns. Pain. Protective clothing. clothes. Rinse skin with
plenty of water or shower.
Refer for medical
attention.
Eyes Redness. Pain. Blurred Face shield. First rinse with plenty
vision. of water for several minutes
(remove contact lenses if
easily possible), then take to
a doctor.
Ingestion Abdominal pain. Diarrhoea. Do not eat, drink, Rinse mouth. Give plenty of
Sore throat. Vomiting or smoke during work. water to drink. Refer for medical
attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid in sealable EU Classification
containers as far as possible. Absorb remaining Symbol: C
liquid in sand or inert absorbent and remove R: 10-21/22-34-42/43
to safe place (extra personal protection: S: (1/2-)23-26-36/37/39-45
complete protective clothing including UN Classification
self-contained breathing apparatus). UN Hazard Class: 8
UN Subsidiary Risks: 3
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-77 Fireproof. Separated from incompatible materials
NFPA Code: H 3; F 2; R O; (see Chemical Dangers). Dry.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS TO YELLOW HYGROSCOPIC LIQUID, WITH The substance can be absorbed into the body by
CHARACTERISTIC ODOUR. inhalation, through the skin and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating producing A harmful contamination of the air can be reached
toxic fumes (nitrogen oxides). The substance rather quickly on evaporation of this substance
is a medium strong base. Reacts violently at 20°C.
with chlorinated organic compounds strong
oxidants.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV (as TWA): 10 ppm; 25 mg/m3 A4 The substance is corrosive to the eyes, the skin
(ACGIH 1999). and the respiratory tract. Inhalation of vapour or
fumes may cause lung oedema (see Notes).
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact with skin may cause
dermatitis.
Repeated or prolonged contact may cause skin
sensitization.
Repeated or prolonged inhalation exposure may cause
asthma.
PHYSICAL PROPERTIES
Boiling point: 116°C Auto-ignition temperature: 385°C
Melting point: 8.5°C Explosive limits, vol% in air: 2.7-16.6
Relative density (water = 1) 0.90 Octanol/water partition
Solubility in water: miscible coefficient as log Pow: -1.2
Vapour pressure, kPa at 20°C: 1.2
Relative vapour density
(air = 1): 2.1
Relative density of the
vapour/air-mixture at 20°C
(air = 1): 1.02
Flash point: 34°C (c.c.)
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should
be given to water organisms.
NOTES
The symptoms of lung oedema often do not become manifest until a few hours have passed and they
are aggravated by physical effort. Rest and medical observation are therefore essential.
Immediate administration of an appropriate spray, by a doctor or a person authorized by
him/her, should be considered. The symptoms of asthma often do not become manifest until a
few hours have passed and they are aggravated by physical effort. Rest and medical observation
are therefore essential. Anyone who has shown symptoms of asthma due to this substance should
never again come into contact with this substance. Do NOT take working clothes home.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the
CEC or the IPCS is responsible for the use which might be made of this
information.
REFERENCES
Aldrich F, Stange A, Geesaman R (1987) Smoking and ethylene diamine
sensitization in an industrial population. Journal of occupational
medicine, 29:311-314.
Andersson K, Hallgren C, Levin J, Nilsson C (1985) Determination of
ethylenediamine in air using reagent-coated adsorbent tubes and
high-performance liquid chromatography on the naphthylisothiourea
derivative. American Industrial Hygiene Association journal,
46:225-229.
Babiuk C, Hastings K, Dean J (1987) Induction of ethylenediamine
hypersensitivity in the guinea-pig and the development of ELISA and
lymphocyte blastogenesis techniques for its characterisation.
Fundamental and applied toxicology, 9:623-634.
Baer R, Cohen H, Neidorff A (1958) Allergic eczematous sensitivity to
aminophylline. American Medical Association archives of dermatology,
79:647-648.
Balato N, Cusano F, Lembo G, Ayala F (1984) Ethylenediamine contact
dermatitis. Contact dermatitis, 11:112-114.
Balk F, Meuwsen I (1989a) Respiration inhibition test with
nitrifying bacteria, ethylenediamine (EDA). Arnhem, Akzo Chemicals
(Unpublished Report No. CRL F89082).
Balk F, Meuwsen I (1989b) Acute toxicity of ethylenediamine (EDA) for
Daphnia. Arnhem, Akzo Chemicals (Unpublished Report No. CRL 189040).
Balk F, Meuwsen I (1989c) Acute toxicity of ethylenediamine (EDA)
for fish. Arnhem, Akzo Chemicals (Unpublished Report No. CRL
189032).
Basketter D, Scholes E (1992) Comparison of the local lymph node assay
with the guinea pig maximisation test for the detection of a range of
contact allergens. Food and chemical toxicology, 30:65-69.
Boas-Traube S, Dresel E, Dryden I (1948) Properties of ethylene
diamine. Nature, 162:960.
Booth B, Coleman W, Mitchell D (1979) Urticaria following intravenous
aminophylline. Annals of allergy, 43:289-290.
Boyd E, Seymour K (1946) Ethylenediamine dihydrochloride or
"Chlor-ethamine." II: Untoward and toxic reactions. Experimental
medical surgery, 4:223-227.
Bringmann G, Kuhn R (1976) Vergleichende Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Backterien ( Pseudomonas putida) und
Blaualgen ( Microcystis aeruginosa) . Gwf-wasser/Abwasser,
117:410-413.
Bringmann G, Kuhn R (1980a) Comparison of the toxic thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Bringmann G, Kuhn R (1980b) Bestimmung der Biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen. II Bakterienfressende
Ciliaten. Zeitschrift fuer Wasser und Abwasser Forschung, 1:26-31.
Brooke I, Saleem A, Stewart T, Delic J, Cocker J, Patel S, Ogunbiyi A
(1997) 1,2-Diaminoethane. Sudbury, Suffolk, HSE Books (Risk
Assessment Document EH72/7; ISBN 0 7176 1338 0).
BUA (1997) Ethylenediamine. Stuttgart, S. Hirzel (GDCh-Advisory
Committee on Existing Chemicals of Environmental Relevance Report No.
184; ISBN 3-7776-0811-4).
Caldwell J, Cotgreave I (1983) Disposition in human volunteers of
theophylline and ethylenediamine given combined as aminophylline.
Clinical practice, 23:22-25.
Carpenter C, Smyth H (1946) Chemical burns of the rabbit cornea.
American journal of ophthalmology, 29:1363-1372.
Carpenter C, Smyth R, Shaffer C (1948) The acute toxicity of ethylene
imine to small animals. Journal of industrial hygiene and
toxicology, 30:2-6.
Chan-Yeung M (1982) Occupational assessment of asthma. Chest,
82:24S.
Cornacoff J, House R, Dean J (1988) Comparison of a radioisotopic
incorporation method and the mouse ear swelling test (MEST) for
contact sensitivity to weak sensitisers. Fundamental and applied
toxicology, 10:40-44.
Cotgreave I, Caldwell J (1983a) Physicochemical and in-vitro
biological studies on the possible association between theophylline
and ethylenediamine in solution. Journal of pharmacy and
pharmacology, 35:774-779.
Cotgreave I, Caldwell J (1983b) Comparative plasma pharmacokinetics of
theophylline and ethylenediamine after the administration of
aminophylline to man. Journal of pharmacy and pharmacology,
35:378-382.
Cotgreave I, Caldwell J (1983c) Studies on aminophylline disposition.
1. A rapid and sensitive HPLC assay for ethylenediamine in plasma and
urine. Biopharmacy and drug disposition, 4:53-62.
Cotgreave I, Caldwell J (1985) Comparative pharmacokinetics of
theophylline and ethylenediamine following single and repeated doses
of a sustained-release aminophylline preparation to volunteers.
Journal of pharmacy and pharmacology, 37:618-621.
Crow K, Peachey R, Adams J (1978) Coolant oil dermatitis due to
ethylenediamine. Contact dermatitis, 4:359-361.
Curtis M, Ward C (1981) Aquatic toxicity of forty industrial
chemicals: testing in support of hazardous substance spill prevention
regulations. Journal of hydrology, 51:359-367.
Davis J (1993) Physico-chemical factors influencing ethyleneamine
sorption to soil. Environmental toxicology and chemistry, 12:27-35.
DePass L, Fowler E, Yang R (1984) Dermal oncogenicity studies on
ethylenediamine in male C3H mice. Fundamental and applied
toxicology, 4:641-645.
DePass L, Yang R, Woodside M (1987) Evaluation of the teratogenicity
of ethylenediamine HCl in F344 rats by conventional and pair-feeding
studies. Fundamental and applied toxicology, 9:687-697.
Dernehl C (1951) Clinical experiences with exposures to ethylene
amines. Industrial and medical surgery, 20:541-546.
Dias M, Fernandes C, Pereira F, Pacheco A (1995) Occupational
dermatitis from ethylenediamine. Contact dermatitis, 33:129-130.
Dubinina O, Galeeva L, Trubnikova L, Varlamova T, Tlacheva S (1997)
Experimental studies towards a possible adjustment to the MAC for
ethylenediamine in workplace air. Meditsina Truda i Promyshlennaia
Ekologiia, 1:38-41.
Dunn B, Rusch G, Siglin J, Blasczcak D (1990) Variability of a mouse
ear-swelling test (MEST) in predicting weak and moderate contact
sensitization. Fundamental and applied toxicology, 15:242-248.
Edman B, Moller H (1986) Medicament contact allergy. Dermatosen,
34:139-142.
English J, Rycroft R (1989) Occupational sensitization to
ethylene-diamine in a floor polish remover. Contact dermatitis,
20:220-221.
Epstein E, Maibach H (1968) Ethylenediamine. Allergic contact
dermatitis. Archives of dermatology, 98:476-477.
Erdo S, Kiss B, Riesz M, Szporny L (1986) Stimulus-evoked efflux of
GABA from preloaded slices of the rabbit oviduct. European journal
of pharmacology, 130:295-303.
Erikson K (1979) Sensitization capacity of ethylenediamine in the
guinea pig and induction response. Contact dermatitis, 5:293-296.
Forster P, Lloyd H, Morgan P, Parker M, Perkins M, Stone T (1981)
Ethylenediamine acts upon GABA receptors and uptake sites.
Proceedings of the British Pharmacology Society, 1-3 April 1981, p.
274.
Fregert S (1981) Manual of contact dermatitis, 2nd ed. Copenhagen,
Munksgaard.
Gad S, Dunn B, Dobbs D, Reilly C, Walsh R (1986) Development and
validation of an alternative dermal sensitization test: the mouse
ear-swelling test (MEST). Toxicology and applied pharmacology,
84:93-114.
Gelfand H (1963) Respiratory allergy due to chemical compounds
encountered in the rubber, lacquer, shellac, and beauty culture
industries. Journal of allergy, 34:374-381.
Goodwin B, Crevel R, Johnson A (1981) A comparison of three guinea-pig
sensitisation procedures for the detection of 19 reported human
contact sensitisers. Contact dermatitis, 7:248-258.
Hagmar L, Bellander T, Berg B, Simonsson B (1982) Piperazine-induced
occupational asthma. Journal of occupational medicine, 24:193-197.
Hansen L, Kristiansson B, Sollenberg J (1984) A method for the
determination of ethylenediamine in workroom air. Scandinavian
journal of environmental health, 10:95-98.
Hardin B, Schuler R, Burg J, Booth G, Hazeldean K, MacKenzie K,
Piccirrillo V, Smith K (1987) Evaluation of 60 chemicals in a
preliminary developmental toxicity test. Teratogenesis,
Carcinogenesis, Mutagenesis, 7:29-48.
Hardy C, Schofield O, George C (1983) Allergy to aminophylline.
British medical journal, 286:2051-2052.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, Suppl. 1:3-142.
Hedenstedt A (1978) Mutagenicity screening of industrial chemicals.
Seven aliphatic amines and one amide tested in the Salmonella
microsome assay. Mutation research, 53:198-199.
Henck J, Lockwood D, Olsen K (1980) Skin sensitisation potential of
trisodium ethylenediaminetetraacetate. Drug chemistry and
toxicology, 3:99-103.
Hill D (1985) The influence of bicarbonate ions on the GABA-mimetic
activity of ethylenediamine. Neuropharmacology, 24:147-155.
HSDB (1997) Hazardous substances data bank. Bethesda, MD, National
Library of Medicine (CD-ROM: Micromedex Inc., Englewood, CO, Edition
V35).
Hulla J, Rogers S, Warren G (1981) Mutagenicity of a series of
polyamines. Environmental mutagenesis, 3:332-333.
Hulzebos E, Adema D, Dirven-van Breeman E, Henzen L, Van Dis W,
Herbold H, Hoekstra J, Baerselman R, Van Gestel C (1993) Phytotoxicity
studies with Lactuca sativa in soil and nutrient solution.
Environmental toxicology and chemistry, 12:1079-1094.
IPCS (1993) International Chemical Safety Card -- Ethylenediamine.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 0269).
Japan Chemical Industry Ecology-Toxicology Information Center (1992)
Biodegradation and bioaccumulation data of existing chemicals based
on the CSCL Japan. Ministry of International Trade and Industry,
Chemicals Inspection and Testing Institute (ISBN 4-89074-101-2).
Kerr D, Ong J (1984) Evidence that ethylenediamine acts in the
isolated ileum of the guinea pig by releasing endogenous GABA.
British journal of pharmacology, 83:169-177.
Kradjan W, Lakshminarayan S (1981) Allergy to aminophylline: lack of
predictability by skin testing. American journal of hospital
pharmacology, 38:1031-1033.
Krantis A, Khalil A, McKay A (1990) Differences in the effects ( in
vitro) of ethylenediamine on the guinea pig and rat intestine.
European journal of pharmacology, 177:9-17.
Kuhn R, Pattard M (1990) Results of the harmful effects of water
pollutants to green algae ( Scenedesmus subspicatus) in the cell
multiplication inhibition test. Water research, 24:31-38.
Kuhn R, Pattard M, Pernak K, Winter A (1989) Results of the harmful
effects of water pollutants to Daphnia magna in the 21 day
reproduction test. Water research, 23:501-510.
Lam S, Chan-Yeung M (1980) Ethylenediamine-induced asthma. American
review of respiratory disease, 121:151-155.
Leung H-W (1994) Evaluation of the genotoxic potential of
alkylene-amines. Mutation research, 320:31-43.
Leung H, Auletta C (1997) Evaluation of skin sensitisation and
cross-reaction of nine alkyleneamines in the guinea pig maximization
test. Journal of toxicology -- Cutaneous and ocular toxicology,
16:189-195.
Levin J, Andersson K, Fangmark I, Hallgren C (1989) Determination of
gaseous and particulate polyamines in air using sorbent or filter
coated with naphthylisothiocyanate. Applied Industrial Hygiene
Association journal, 4:98-100.
Levin J, Andersson K, Hallgren C (1994) Exposure to low molecular
weight polyamines during road paving. Annals of occupational
hygiene, 38:257-264.
Lewinsohn H, Ott M (1991) A review of medical surveillance records of
employees exposed to ethyleneamines. Journal of occupational
medicine, 33:148-154.
Lloyd H, Perkins M, Stone T (1982) Ethylenediamine as a specific
releasing agent of gamma-aminobutyric acid in rat striatal slices.
Journal of neurochemistry, 38:1168-1169.
Mark U, Arends I (1992) Fish early life stage test with
ethylene-diamine. Arnhem, Akzo Chemicals (Unpublished Report No. CRL
F91180).
Mark U, Hantink-de Rooy E (1992) Prolonged toxicity of
ethylenediamine to Daphnia magna. Arnhem, Akzo Chemicals
(Unpublished Report No. CRL F91182).
Matsui S, Nakazawa T, Umagae Y, Kanatani K, Fujiwara T, Fueki R,
Kobayashi S (1986) Two cases of late response bronchial asthma caused
by ethylenediamine. Arerugi, 35:40-46.
Matthieu L, Weyler J, Deckers I, van Sprundel M, van Andel A, Dockx P
(1993) Occupational contact sensitization to ethylenediamine in a
wire-drawing factory. Contact dermatitis, 29:39.
Maurer T, Thomann P, Weirich E, Hess R (1979) Predictive evaluation in
animals of the contact allergenic potential of medically important
substances. Contact dermatitis, 5:1-10.
McKay A, Krantis A (1991) The effects of ethylenediamine in the rat
small intestine: a powerful relaxant of the muscularis. Canadian
journal of physiology and pharmacology, 69:199-204.
McKelvey J, Tallant M, Yang R (1982) The fate of
14C-ethylene-diamine in the mouse following different routes of
administration. Philadelphia, PA, Union Carbide Corporation, Bushy
Run Research Center (Project Report 45-191).
Mills E, Stack V (1955) Suggested procedure for evaluation of
biological oxidation of organic chemicals. Sewage and industrial
wastes, 27:1061-1064.
Morgan P, Stone T (1982) Anticonvulsant actions of the putative
gamma-aminobutyric acid (GABA)-mimetic, ethylenediamine. British
journal of pharmacology, 77:525-529.
Nakazawa T, Matsui S (1990) Ethylenediamine-induced late asthmatic
responses. Journal of asthma, 27:207-212.
Newsome W, Shields J, Villeneuve D (1975) Residues of maneb,
ethylenethiuram monosulfide, ethylenethiourea and ethylenediamine on
beans and tomatoes field-treated with maneb. Journal of agricultural
and food chemistry, 23:756-758.
Ng T, Lee H, Lee F, Wang Y, Tay V, Tan K (1991) Occupational asthma
due to ethylenediamine. Annals of the Academy of Medicine,
20:397-402.
Ng T, Lee H, Malik M, Chee Q, Cheong T, Wang Y (1995) Asthma in
chemical workers exposed to aliphatic polyamines. Occupational
medicine, 45:45-48.
Nielsen M, Jorgensen J (1987) Persistence of contact sensitivity to
ethylenediamine. Contact dermatitis, 16:275-276.
NIOSH (1984-1989) Manual of analytical methods. Method 3509.
Cincinnati, OH, US Department of Health and Human Services, Public
Health Service, National Institute for Occupational Safety and Health.
Nishikawa Y (1987) Liquid chromatographic determination of aliphatic
diamines in water via derivatization with acetylacetone. Journal of
chromatography, 392:349-359.
Niveau J, Painchaux J (1973) Intoxication mortelle par éthylène
diamine. Archives des Maladies Professionnelles du Travail et de
Securité Sociale, 34:523-528.
NTP (1982a) Report on prechronic studies of ethylenediamine acute,
repeated dose and subchronic in rats. Contract NO1 CP 95653-02 to
National Toxicology Program, National Institutes of Health, Public
Health Service, US Department of Health and Human Services, Research
Triangle Park, NC. Columbus, OH, Batelle Columbus Laboratories.
NTP (1982b) Report on prechronic studies of ethylenediamine acute,
repeated dose and subchronic in mice. Contract NO1 CP 95653-02 to
National Toxicology Program, National Institutes of Health, Public
Health Service, US Department of Health and Human Services, Research
Triangle Park, NC. Columbus, OH, Batelle Columbus Laboratories.
NTP (1991) Developmental toxicity of ethylene diamine in New Zealand
white rabbits. Research Triangle Park, NC, US Department of Health
and Human Services, Public Health Service, National Institutes of
Health, National Toxicology Program (Contract NIEHS-N01-ES-95255;
Report No. RTI-411).
Ormerod A, Wakeel R, Mann T, Main R, Aldridge R (1989) Polyamine
sensitization in offshore workers handling drilling muds.
Contact dermatitis, 21:326-329.
Patel S, Rimmer D (1996) Development of an air monitoring method for
the measurement of ethylenediamine. Sheffield, Health and Safety
Executive (Internal Report IR/L/IA/95/6).
Perkins M, Stone T (1980) Aminophylline and theophylline derivatives
as antagonists of neuronal depression by adenosine: a
micro-iontophoretic study. Archives of international
pharmacodynamics, 246:205-214.
Petrozzi J, Shore R (1976) Generalized exfoliative dermatitis from
ethylenediamine. Sensitization and induction. Archives of
dermatology, 112:525-526.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Popa V, Teculescu D, Stanescu D, Gavrilescu N (1969) Bronchial asthma
and asthmatic bronchitis determined by simple chemicals. Diseases of
the chest, 56:395-404.
Pozzanni U, Carpenter C (1954) Response of rats to repeated
inhalations of ethylenediamine vapours. Archives of industrial
hygiene and occupational medicine, 9:223-226.
Price K, Waggy G, Conway R (1974) Brine shrimp bioassay and seawater
BOD of petrochemicals. Journal of the Water Pollution Control
Federation, 46:63-77.
Price Q, George J, Marr M, Myers C, Heindel J, Schwetz B (1993)
Developmental toxicity evaluation of ethylenediamine (EDA) in New
Zealand white rabbits. Teratology, 47:432-433.
Provost T, Jillson O (1967) Ethylenediamine contact dermatitis.
Archives of dermatology, 96:231-234.
Robinson M, Fletcher E, Johnson G, Wyder W, Maurer J (1990) Value of
cutaneous basophil hypersensitivity (CBH) response for distinguishing
weak contact sensitization from irritation reactions in the
guinea-pig. Journal of investigative dermatology, 94:636-643.
Sarthy P (1983) Release of [3H] gamma-aminobutyric acid from glial
(Muller) cells of the rat retina: effects of K+, veratridine, and
ethylenediamine. Journal of neuroscience, 3:2494-2503.
Sasseville D, Al-Khenaizan S (1997) Occupational contact dermatitis
from ethylenediamine in a wire-drawing lubricant. Contact
dermatitis, 36:228-229.
Shehade S, Beck M, Hillier V (1991) Epidemiological survey of standard
series patch test results and observations on day 2 and day 4
readings. Contact dermatitis, 24:119-122.
Simon P, Terzian C, Frome B (1995) Skin reactions to topical
aminophylline. Journal of the American Medical Association,
273:1737-1738.
Slesinski R, Guzzie P, Hengler W, Watanabe P, Woodside M, Yang R
(1983) Assessment of genotoxic potential of ethylenediamine: in
vitro and in vivo studies. Mutation research, 124:299-314.
Smyth H Jr, Seaton J, Fischer L (1941) The single dose toxicity of
some glycols and derivatives. Journal of industrial hygiene and
toxicology, 23:259-268.
Smyth H Jr, Carpenter C, Weil C (1951) Range-finding toxicity data:
List IV. American Medical Association archives of industrial
hygiene, 4:119-122.
Strain G, Flory W, Tucker T (1984) Inhibition of synaptosomal uptake
of amino acid transmitters by diamines. Neuropharmacology,
23:971-975.
Takemoto S, Kuge Y, Nakamoto M (1981) The measurement of BOD in sea
water. Suishitsu Okagu Kenkyu [Japanese journal of water
pollution research], 4:80-90.
Tas J, Weissberg D (1958) Allergy to aminophylline. Acta
Allergologica, 12:39-42.
Terzian C, Simon P (1992) Aminophylline hypersensitivity apparently
due to ethylenediamine. Annals of emergency medicine, 21:312-314.
Thorgeirsson A (1978) Sensitization capacity of epoxy resin hardeners
in the guinea pig. Acta Dermatologica, 58:332-336.
Toal M, Kinney A, Fulton R (1992) Allergy to the ethylenediamine
component of aminophylline. Ulster medical journal, 61:205-206.
Tonogai Y, Ogawa S, Ito Y, Iwaida M (1982) Actual survey on Tlm
(median tolerance limit) values of environmental pollutants,
especially on amines, nitriles, aromatic nitrogen compounds and
artificial dyes. Journal of toxicological sciences, 7:193-203.
Valeyeva K, Belomyteseva, L, Khafizullin E (1975) Occupational
bronchial asthma in employees engaged in the production of
ethylenediamine. Aktual'nye Voprosy Gigieny Truda Professional' -n
Patologii i Toksikologii v Neftyanoi Nefte-khimicheskoi
Promyshlennosti, 121-124.
van Ginkel C (1989) Toxicity of ethylenediamine for Pseudomonas
putida. Arnhem, Akzo Chemicals (Unpublished Report No. CRL F89091).
van Ginkel CG, Stroo CA (1989) Toxicity of ethylenediamine for
activated sludge. Arnhem, Akzo Chemicals (Unpublished Report No.
F89054).
van Ginkel CG, Kroon AGM, Mark U (1990) Algal inhibition test with
ethylenediamine. Arnhem, Akzo Chemicals (Unpublished Report No.
F90086).
van Leeuwen C (1986) Ecotoxicological aspects of dithiocarbamates.
Thesis, University of Utrecht. The Hague, Rijkswaterstaat
(Rijkswaterstaat Communications No. 44).
van Wijk R (1992) Environmental risk assessment of ethylenediamine
and diethylenetriamine wastewater discharges in the Ems-Dollard
estuary. Arnhem, Akzo Chemicals (Unpublished Report No. CRL F92009).
van Wijk R, Postman J, Van Houwelingen H (1994) Joint toxicity of
ethyleneamines to algae, daphnids and fish. Environmental toxicology
and chemistry, 13:167-171.
Wall L (1982) Lymphatoid contact dermatitis due to ethylenediamine
dihydrochloride. Contact dermatitis, 8:51-54.
Woodiwiss F, Fretwell G (1974) The toxicities of sewage effluents,
industrial discharges and some chemical substances to brown trout in
the Trent River Authority area. Water pollution control (Great
Britain), 73:396.
Yang R, Tallant M (1982) Metabolism and pharmacokinetics of
ethylenediamine in the rat following oral, endotracheal or intravenous
administration. Fundamental and applied toxicology, 2:252-260.
Yang R, Garman R, Maronpot R, McKelvey J, Weil C, Woodside M (1983)
Acute and subchronic toxicity of ethylenediamine in laboratory
animals. Fundamental and applied toxicology, 3:512-520.
Yang R, Garman R, Maronpot R, Mirro E, Woodside M (1984a) Chronic
toxicity/carcinogenicity study of ethylenediamine in Fischer 344 rats.
Toxicologist, 4:53.
Yang R, Tallant M, McKelvey J (1984b) Age-dependent pharmacokinetic
changes of ethylenediamine in Fischer 344 rats parallel to a two-year
chronic toxicity study. Fundamental and applied toxicology,
4:663-670.
Yang R, Anuszkiewicz C, Chu S, Garman R, McKelvey J, Tallant M (1987)
Biochemical and morphological studies on the percutaneous uptake of
[14C]ethylenediamine in the rat. Journal of toxicology and
environmental health, 20:261-272.
Zimmering S, Mason M, Valencia R, Woodruff R (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:87-100.
APPENDIX 1 -- SOURCE DOCUMENTS
Brooke et al. (1997): 1,2-Diaminoethane (Risk Assessment Document EH72/7)
The authors' draft version of this Health and Safety Executive
report was initially reviewed internally by a group of approximately
10 Health and Safety Executive experts (mainly toxicologists, but also
scientists from other relevant disciplines, such as epidemiology and
occupational hygiene). The toxicology section of the amended draft was
then reviewed by toxicologists from the United Kingdom Department of
Health. Subsequently, the entire risk assessment document was reviewed
by a tripartite advisory committee to the United Kingdom Health and
Safety Commission, the Working Group for the Assessment of Toxic
Chemicals (WATCH). This committee is composed of experts in toxicology
and occupational health and hygiene from industry, trade unions, and
academia.
The members of the WATCH committee at the time of the peer review
were Mr Steve Bailey, Independent Consultant; Dr Hilary Cross, Trade
Unions Congress; Mr David Farrar, Independent Consultant; Dr Tony
Fletcher, Trade Unions Congress; Dr Alastair Hay, Trade Unions
Congress; Dr Jenny Leeser, Chemical Industries Association; Dr Len
Levy, Institute of Occupational Hygiene, Birmingham; Dr Mike Molyneux,
Chemical Industries Association; Mr Alan Moses, Chemical Industries
Association; and Mr Jim Sanderson, Independent Consultant.
BUA (1997): Ethylenediamine (GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance Report No. 184)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on 1,2-diaminoethane (ethylenediamine) was sent
for review to institutions and organizations identified by IPCS after
contact with IPCS national Contact Points and Participating
Institutions, as well as to identified experts. Comments were received
from:
Akzo Novel nv, Arnhem, Netherlands
Department of Health, London, United Kingdom
Environment Agency, Wallingford, United Kingdom
Ethyleneamines Product Stewardship Discussion Group, Michigan,
USA
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
Nofer Institute of Occupational Medicine, Lodz, Poland
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
United States Environmental Protection Agency (Office of Research
and Development, Washington, DC, USA)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikasei Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au 1,2-diaminoéthane (éthylènediamine) a été
préparé à partir d'une étude du Health and Safety Executive du
Royaume-Uni sur les risques pour la santé humaine (risques
professionnels pour l'essentiel, mais comportant également un volet
écologique) (Brooke et al., 1997). La bibliographie sur laquelle
s'appuie l'étude originale a été arrêtée à fin 1994. Une analyse de la
littérature a été ensuite effectuée jusqu'à juillet 1997 à la
recherche de données qui auraient pu être publiées depuis la fin de
l'étude. Les données relatives à la destinée du composé dans
l'environnement et à son impact écologique sont tirées d'un rapport du
Comité consultatif de la Société allemande de Chimie pour les
substances chimiques d'intérêt écologique (BUA, 1997). On trouvera à
l'appendice 1 des indications sur les sources documentaires utilisées
et sur leur mode de dépouillement. Les renseignements concernant
l'examen du CICAD par des pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors d'une
réunion du Comité d'évaluation finale qui s'est tenue à Tokyo (Japon)
du 30 juin au 2 juillet 1998. La liste des participants à cette
réunion figure à l'appendice 3. La fiche d'information internationale
sur la sécurité chimique (ICSC No 0269) établie par le Programme
international sur la Sécurité chimique (IPCS, 1993) est également
reproduite dans ce document.
Le 1,2-diaminoéthane (No CAS 107-15-3), couramment désigné sous
le nom d'éthylènediamine (EDA), est un produit de synthèse qui se
présente sous la forme d'un liquide incolore à jaunâtre dans les
conditions normales de température et de pression. Il présente une
réaction fortement alcaline et il est miscible à l'eau et à l'alcool.
On l'utilise principalement comme intermédiaire dans la fabrication de
la tétra-acétyl-éthylènediamine, de l'acide
éthylènediamine-tétra-acétique (EDTA), des floculants organiques, des
résines à base d'urée et des diamides gras. Dans des proportions
beaucoup plus faibles, il entre également dans la composition de
formulations destinées à la fabrication des supports de circuits
imprimés ou utilisées dans l'industrie de finissage des métaux. Il
peut aussi être utilisé comme accélérateur ou agent de réticulation
dans les résines époxy employées notamment comme revêtements ainsi que
pour la préparation de certains produits pharmaceutiques. L'EDA est
présent sous la forme d'impureté (<0,5 %) dans les amines grasses du
commerce utilisées comme agents mouillants dans les émulsions
bitumineuses. On l'emploie également dans la synthèse des fongicides à
base de carbamates, dans la fabrication des agents de surface et des
colorants ainsi que dans la préparation de produits de développement
photographique et d'huiles de coupe. L'EDA est un produit de
décomposition des éthylènebis(dithiocarbamates) utilisés comme
fongicides.
Il ne devrait pas y avoir d'effets atmosphériques puisque la
réaction de l'EDA avec les radicaux hydroxyles est vraisemblablement
rapide (demi-vie 8,9 h) et que la fraction volatilisée devrait être
éliminée par les précipitations. Ce passage dans l'atmosphère à l'état
de vapeur est probable à partir du sol, mais pas à partir de l'eau.
L'EDA adhère fortement aux particules du sol par attraction
électrostatique; on pense qu'il ne devrait donc pas y avoir de passage
dans les eaux souterraines par lessivage des sols. Il peut sans doute
former des complexes avec les métaux et les acides humiques. La voie
de décomposition la plus probable dans l'environnement est la voie
biologique et alle devrait être assez rapide; l'adaptation des
microorganismes pourrait accélérer le processus. La décomposition est
plus lente dans l'eau de mer que dans l'eau douce. Il n'y a
probablement pas de bioaccumulation.
L'EDA présente une toxicité aiguë modérée pour les animaux. C'est
surtout une substance irritante, qui est corrosive quand elle n'est
pas diluée et qui provoque également une sensibilisation cutanée. On
n'a pas procédé à la recherche de son pouvoir mutagène dans les
conditions prescrites par la réglementation actuelle et on ne dispose
pas non plus d'études sur son activité clastogène ou sur une action
qui s'exercerait sur les cellules somatiques in vivo. Il n'existe
donc pas de données suffisantes pour que l'on puisse se prononcer avec
certitude sur le pouvoir mutagène éventuel de l'EDA. Quoi qu'il en
soit, le composé ne s'est pas révélé cancérogène chez l'animal. On a
observé des effets non néoplasiques au niveau du foie (modifications
pléomorphes des hépatocytes) chez des rats auxquels on avait fait
ingérer du 1,2-diaminoéthane pendant 2 ans. Ces effets ont été
observés à des doses quotidiennes supérieures ou égales à 45 mg d'EDA
par kg de poids corporel, aucune anomalie ne se manifestant à la dose
quotidienne de 9 mg par kg de poids corporel. On voit pas très
clairement ce que cette observation peut signifier pour la santé
humaine et l'on ne peut d'ailleurs pas se prononcer non plus sur le
point de savoir si les effets rapportés sont effectivement dus à
l'ingestion de l'EDA (par exemple, ils pourraient ne pas se produire
si on changeait la voie d'administration ou être liés à des effets de
premier passage), mais on ne peut les négliger pour autant et il faut
déterminer les conditions de leur apparition. Lors d'études où le
composé a été administré par gavage, on a observé des effets oculaires
chez le rat (atrophie rétinienne et à dose élevée, formation de
cataractes) à des doses quotidiennes supérieures ou égales à 100 mg
par kg de poids corporel. Chez des rats et des souris, on a constaté
la présence de lésions rénales aux doses quotidiennes respectives de
200 et 100 mg de composé par kg de poids corporel et au-delà. On a
également trouvé quelques indices d'effets sur la rate chez des souris
et des rats à des doses quotidiennes supérieures ou égales à 400 mg
d'EDA par kg de poids corporel, de même que chez des rats, au niveau
du thymus, à la dose quotidienne de 800 mg/kg de poids corporel. Les
études d'inhalation effectuées sur des rats n'ont pas permis
d'observer d'effets à la dose d'environ 150 mg/m3 (60 ppm) et le seul
effet imputable au traitement a été une légère dépilation à la dose
d'environ 330 mg/m3 (132 ppm).
Comme l'EDA a un effet irritant et sensibilisateur sur
l'épiderme, il pourrait y avoir un risque d'apparition de dermatites
d'irritation ou de dermatites allergiques si l'on porte pas
d'équipement protecteur individuel sur les lieux de travail où il y a
possibilité de contact cutané. L'EDA peut également provoquer une
hypersensibilité des voies respiratoires et de l'asthme chez les
personnes professionnellement exposées et c'est d'ailleurs cet effet
que l'on considère comme le plus préoccupant sur le plan sanitaire.
On ne sait pas avec exactement par quel mécanisme se développe
cet état d'hypersensibilité, mais le pouvoir sensibilisateur cutané de
l'EDA et les quelques indications dont on dispose sur l'existence
d'une composante immunologique chez les ouvriers souffrant d'un asthme
provoqué par ce composé, incitent à penser que ce mécanisme serait
justement de nature immunologique. Quoi qu'il en soit et quel que
puisse être la nature du mécanisme en question, les données
disponibles ne permettent pas de mettre en évidence une relation
dose-réponse ou de déterminer le seuil d'apparition d'un état
d'hypersensibilité ou d'une réaction asthmatiforme. Dans le présent
document, le risque imputable au composé est caractérisé par des
effets hépatiques dont on a évalué la probabilité chez des sujets
exposés à l'EDA de par leur profession, le but étant d'apprécier le
risque d'autres effets généraux. La conclusion en est que lorsque
l'EDA est utilisé en vase clos, l'exposition - mesurée ou calculée par
modélisation - est très sensiblement inférieure (d'un facteur 100 au
moins) à la valeur sans effet observable (NOEL) chez le rat et que,
par conséquent, des effets hépatiques indésirables sont improbables.
Faute de données, on ne peut évaluer le niveau d'exposition de la
population générale à l'EDA.
Le seuil de toxicité pour les microorganismes pourrait ne pas
dépasser 0,1 mg/litre. Cependant, il faut interpréter avec prudence
les résultats des tests toxicologiques en milieu de culture car l'EDA
est susceptible de former des complexes avec les ions métalliques. Ses
effets pourraient donc être indirects et résulter de ce que certains
éléments essentiels cessent alors d'être biodisponibles. Pour les
invertébrés et les poissons, la valeur de la CL50 va de 14 à >1000
mg/litre. On a trouvé une valeur de 0,16 mg/litre pour la dose sans
effet observable (NOEC) sur la reproduction de Daphnia.
Etant donné que les résultats des épreuves de toxicité aiguë et
chronique varient très largement, on a fixé à 16 µg/litre la valeur de
la concentration sans effet observable prévisible (PNEC), en
appliquant un coefficient d'incertitude de 10 à la valeur publiée la
plus faible de la concentration sans effet observable (NOEC) sur la
reproduction de Daphnia. Des hypothèses prudentes relatives à la
concentration prévisible dans l'environnement (PEC) permettent
d'aboutir à une valeur du rapport PEC/PNEC justifiant quelques
craintes eu égard aux concentrations initiales (par ex. lors de la
décharge initiale dans un cours d'eau ou un estuaire). Néanmoins, une
estimation plus élaborée de l'exposition probable indique un faible
risque pour les organismes aquatiques.
RESUMEN DE ORIENTACION
Este CICAD sobre el 1,2 diaminoetano (etilendiamina) se basa en
un examen de los problemas relativos a la salud humana
(fundamentalmente ocupacional, pero con la inclusión también de una
evaluación en el medio ambiente) preparado por la Dirección de Salud y
Seguridad del Reino Unido (Brooke et al., 1997). En el documento
original se incorporaron los datos obtenidos hasta el final de 1994.
Se realizó asimismo una búsqueda bibliográfica amplia hasta julio de
1997 para identificar cualquier información que se hubiera publicado
después de la terminación del informe. La información sobre el destino
y los efectos en el medio ambiente se basa en el informe del Comité
Consultivo sobre Sustancias Químicas Existentes Importantes para el
Medio Ambiente de la Sociedad Alemana de Química (BUA, 1997). La
información sobre la preparación del documento original y su examen
colegiado figura en el apéndice 1. La información acerca del examen
colegiado de este CICAD se presenta en el apéndice 2. Este CICAD se
aprobó como evaluación internacional en una reunión de la Junta de
Evaluación Final celebrada en Tokio, Japón, del 30 de junio al 2 de
julio de 1998. La lista de participantes en esta reunión figura en el
apéndice 3. La Ficha internacional de seguridad química (ICSC 0269),
preparada por el Programa Internacional de Seguridad de las Sustancia
Químicas (IPCS, 1993), también se reproduce en este documento.
El 1,2-diaminoetano (CAS No 107-15-3), conocido normalmente
como etilendiamina (EDA), es un líquido sintético entre incoloro y
amarillento a temperatura y presión normales. Es fuertemente alcalino
y miscible con agua y con alcohol. Se utiliza fundamentalmente como
intermediario en la fabricación de tetracetil etilendiamina, ácido
etilendiaminotetracético (EDTA), floculantes orgánicos, resinas de
urea y bisamidas grasas. También se usa, en proporción mucho menor, en
la producción de formulaciones con destino a las industrias de
tarjetas de circuitos impresos y acabado de metales, como agente
acelerador o de curado en revestimientos/resinas de epóxido y en la
fabricación de productos farmacéuticos. Se encuentra como contaminante
(<0,5%) en las aminas grasas de suministro comercial, que se utilizan
como agentes humectantes en emulsiones bituminosas. También se emplea
en la síntesis de fungicidas a base de carbamato, en la fabricación de
surfactantes y tintes y en productos químicos para el revelado
fotográfico, así como en lubricantes para cuchillas. La EDA es un
producto de la degradación de los fungicidas de
etilenbis(ditiocarbamato).
No cabe prever efectos atmosféricos, puesto que la reacción de la
EDA con los radicales hidroxilo es probablemente rápida (semivida de
8,9 horas) y se supone que la EDA volatilizada se arrastra. Es
probable la volatilización a la atmósfera a partir del suelo, pero no
del agua. Se adsorbe fuertemente a las partículas del suelo mediante
enlaces electrostáticos; no parece haber lixiviación a través de los
perfiles del suelo hacia el agua freática. Es posible la formación de
complejos con metales y ácidos húmicos. La biodegradación es el
mecanismo más probable de descomposición en el medio ambiente y
debería ser bastante rápida; la adaptación de los microorganismos
puede aumentar la degradación. La descomposición es menos rápida en el
agua de mar que en el agua dulce. No es probable la bioacumulación.
La toxicidad aguda de la EDA en los animales es moderada. Es
irritante primario, de propiedades corrosivas cuando no está diluido,
y es también sensibilizador cutáneo. La EDA no se ha sometido a
pruebas de mutagenicidad con arreglo a las normas reglamentarias
actuales y no se han realizado valoraciones para determinar la
actividad clastogénica o el potencial para expresar su actividad en
células somáticas in vivo. Así pues, no se dispone de información
suficiente para llegar a conclusiones firmes sobre el potencial
mutagénico de la EDA. No fue carcinogénico en animales. Se han
observado efectos no neoplásicos en el hígado de ratas (cambios
pleomórficos a hepatocitos), tras la administración oral durante dos
años con concentraciones de 45 mg de EDA/kg de peso corporal al día y
superiores, sin que se vieran efectos con 9 mg EDA/kg de peso corporal
al día. Aunque no está clara la importancia de estos cambios de las
células hepáticas para la salud humana, así como si son consecuencia
de la exposición oral o no (es decir, podrían no producirse por otras
vías, porque pueden estar relacionados con los efectos del primer
paso), no se pueden ignorar y se debería caracterizar el riesgo de su
aparición. En estudios de administración oral por sonda se observaron
efectos oculares en las ratas (atrofia de la retina y con dosis más
altas formación de cataratas) con dosis de 100 mg de EDA/kg de peso
corporal al día y superiores. Las dosis de 200 mg y 100 mg de EDA/kg
de peso corporal al día y superiores se asociaron con daños renales en
ratas y ratones, respectivamente. También se encontraron signos de
efectos en el bazo de ratones y ratas con dosis de 400 mg de EDA/kg de
peso corporal al día y superiores y en el timo de ratas con 800 mg/kg
de peso corporal al día. En estudios de inhalación realizados con
ratas no se detectaron efectos con concentraciones de unos 150 mg/m3
(60 ppm), y con 330 mg/m3 (132 ppm) el único efecto relacionado con
la dosis fue una ligera depilación.
Puesto que la EDA diluida es irritante y sensibilizador cutáneo,
si en el lugar de trabajo no se utiliza un equipo de protección
personal adecuado y se produce un contacto cutáneo se corre el riesgo
de contraer una dermatitis irritante y/o alérgica. La EDA puede
inducir además un estado de hipersensibilidad de las vías
respiratorias y provocar asma en el entorno ocupacional, y se
considera que éste es el efecto en la salud que despierta mayor
preocupación.
No se ha demostrado el mecanismo de inducción de la
hipersensibilidad, aunque el potencial de sensibilización cutánea de
la EDA y las pruebas limitadas de actuación inmunitaria en los
trabajadores con asma a causa de esta sustancia hacen pensar en un
mecanismo inmunitario. Sin embargo, con independencia del mecanismo de
que se trate, los datos disponibles no permiten dilucidar las
relaciones dosis-respuesta o identificar los umbrales para la
inducción del estado de hipersensibilidad o la provocación de una
respuesta asmática. A fin de determinar los riesgos de otros efectos
sistémicos, en la caracterización del riesgo de muestras en este
documento se evaluó el riesgo de efectos hepáticos en personas con
exposición ocupacional. Se ha llegado a la conclusión de que, cuando
la EDA se utiliza en sistemas cerrados, la exposición, tanto medida
como pronosticada a partir de modelos, es fundamentalmente inferior
(100 veces o más) a la concentración sin efectos observados (NOEL) en
ratas; así pues, son poco probables los efectos hepáticos adversos.
No se pudo evaluar la exposición del público general a la EDA
debido a la falta de datos disponibles.
El umbral tóxico para los microorganismos puede ser de sólo 0,1
mg de EDA/litro. Sin embargo, las pruebas de toxicidad en medios de
cultivo se han de interpretar con precaución, porque la EDA puede
formar complejos con iones metálicos. Por consiguiente, los efectos
pueden ser indirectos debido a una pérdida de biodisponibilidad de
elementos esenciales. Las CL50 para invertebrados y peces oscila
entre 14 y >1000 mg/litro. Se ha notificado una concentración sin
efectos observados (NOEC) para la reproducción en Daphnia de 0,16
mg/litro.
Habida cuenta de la gran variedad de resultados de las pruebas de
toxicidad aguda y crónica, se determinó una concentración prevista sin
efectos observados (PNEC) para los organismos acuáticos de 16
µg/litro, basada en la aplicación de un factor de incertidumbre de 10
a la NOEC más baja notificada para la reproducción de Daphnia.
Hipótesis prudentes para la concentración prevista en el medio
ambiente (PEC) establecen razones PEC/PNEC que ponen de manifiesto
alguna preocupación a partir de concentraciones iniciales (es decir,
en el primer vertido en el río o el estuario). Sin embargo,
estimaciones más precisas de la exposición indican un riesgo escaso
para los organismos acuáticos.
Based on this emission rate, and using default values from the
OECD Technical Guidance Manual, the initial concentration in river
water would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
= 337.5 µg/litre
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q),
where:
W = emission rate (75 kg/day)
P = percent removal in the wastewater treatment plant
(91%, based on a classification of the chemical as
"readily biodegradable")
Q = volume of wastewater in m3/day (default 200
litres [0.2 m3] per person per day for a
population of 10 000 inhabitants)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Foc(susp) × Koc, where:
Foc(susp)= the fraction of organic carbon in suspended
matter (0.01)
Koc = 0.411 × Kow
where:
Kow = the octanol/water partition coefficient (0.063)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (default value of 10)
Calculation of initial PEC in the compartment of the estuary
receiving emissions from the actual plant in the Netherlands gives a
PEC(initial near field) of 10.9 µg/litre. This is based on a local
volume at mean tide of 6.9 × 109 litres, a residence time of 1 day,
and a wastewater flow from the plant of 500 m3/day, realistic for the
local conditions.
More refined modelling, taking into account tidal dilution and
expected biodegradation with a half-life of 5 days, predicts a
steady-state concentration of 1.3 µg EDA/litre (van Wijk, 1992). This
is likely to be a closer reflection of the real situation in the
estuary.
The risk factors shown in Table 3 can be calculated for
conservative worst-case and refined estimates of environmental
concentrations for a river and estuary.
Table 3: PEC/PNEC ratios.
PEC (µg/litre) PNEC (µg/litre) PEC/PNEC
ratio
River, worst case 337.5 16 21.1
Estuary, initial
worst case 10.9 16 0.68
Estuary, refined PEC 1.3 16 0.08
The PEC/PNEC ratio for the river indicates some cause for concern
(ratio greater than 1). However, the PEC is based on very conservative
assumptions, and both estimates assume low adsorption to sediment
based on water solubility. Refined estimates for the estuary indicate
low risk to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0269) reproduced in this
document.
13.1 Human health hazards
Repeated or prolonged contact with EDA may cause skin
sensitization and asthma.
13.2 Advice to physicians
EDA is corrosive. Inhalation of the vapour may cause irritation
of the respiratory tract and even lung oedema and could mask asthmatic
reaction.
13.3 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of EDA as a human asthmagen.
13.4 Spillage
In the case of spillage, emergency crews need to wear proper
equipment and prevent EDA from reaching drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENEDIAMINE ICSC: 0269
June 1999
CAS # 107-15-3 1,2-Diaminoethane
RTECS # KH8575000 1,2-Ethanediamine
UN # 1604 H2NCH2CH2NH2
EC # 612-006-00-6 Molecular mass: 60.1
TYPES OF HAZARD/ ACUTE HAZARDS/ PREVENTION FIRST AID/
EXPOSURE SYMPTOMS FIRE FIGHTING
FIRE Flammable. Gives off NO open flames, NO Powder, alcohol-resistant
irritating or toxic sparks, and NO foam, water spray, carbon
fumes (or gases) in smoking. dioxide.
a fire.
EXPLOSION Above 34°C explosive Above 34°C closed In case of fire: keep drums,
vapour/air mixtures system, ventilation, etc., cool by spraying with
may be formed. and explosion-proof water.
electrical equipment.
EXPOSURE STRICT HYGIENE!
Inhalation Burning sensation. Ventilation, local exhaust, Fresh air, rest. Artificial
Cough. Laboured breathing. or breathing protection. respiration if indicated.
Shortness of breath. Sore Refer for medical attention.
Throat.
Skin MAY BE ABSORBED! Redness. Protective gloves. Remove contaminated
Skin burns. Pain. Protective clothing. clothes. Rinse skin with
plenty of water or shower.
Refer for medical
attention.
Eyes Redness. Pain. Blurred Face shield. First rinse with plenty
vision. of water for several minutes
(remove contact lenses if
easily possible), then take to
a doctor.
Ingestion Abdominal pain. Diarrhoea. Do not eat, drink, Rinse mouth. Give plenty of
Sore throat. Vomiting or smoke during work. water to drink. Refer for medical
attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking and spilled liquid in sealable EU Classification
containers as far as possible. Absorb remaining Symbol: C
liquid in sand or inert absorbent and remove R: 10-21/22-34-42/43
to safe place (extra personal protection: S: (1/2-)23-26-36/37/39-45
complete protective clothing including UN Classification
self-contained breathing apparatus). UN Hazard Class: 8
UN Subsidiary Risks: 3
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-77 Fireproof. Separated from incompatible materials
NFPA Code: H 3; F 2; R O; (see Chemical Dangers). Dry.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS TO YELLOW HYGROSCOPIC LIQUID, WITH The substance can be absorbed into the body by
CHARACTERISTIC ODOUR. inhalation, through the skin and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating producing A harmful contamination of the air can be reached
toxic fumes (nitrogen oxides). The substance rather quickly on evaporation of this substance
is a medium strong base. Reacts violently at 20°C.
with chlorinated organic compounds strong
oxidants.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF SHORT-TERM EXPOSURE:
TLV (as TWA): 10 ppm; 25 mg/m3 A4 The substance is corrosive to the eyes, the skin
(ACGIH 1999). and the respiratory tract. Inhalation of vapour or
fumes may cause lung oedema (see Notes).
EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
Repeated or prolonged contact with skin may cause
dermatitis.
Repeated or prolonged contact may cause skin
sensitization.
Repeated or prolonged inhalation exposure may cause
asthma.
PHYSICAL PROPERTIES
Boiling point: 116°C Auto-ignition temperature: 385°C
Melting point: 8.5°C Explosive limits, vol% in air: 2.7-16.6
Relative density (water = 1) 0.90 Octanol/water partition
Solubility in water: miscible coefficient as log Pow: -1.2
Vapour pressure, kPa at 20°C: 1.2
Relative vapour density
(air = 1): 2.1
Relative density of the
vapour/air-mixture at 20°C
(air = 1): 1.02
Flash point: 34°C (c.c.)
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should
be given to water organisms.
NOTES
The symptoms of lung oedema often do not become manifest until a few hours have passed and they
are aggravated by physical effort. Rest and medical observation are therefore essential.
Immediate administration of an appropriate spray, by a doctor or a person authorized by
him/her, should be considered. The symptoms of asthma often do not become manifest until a
few hours have passed and they are aggravated by physical effort. Rest and medical observation
are therefore essential. Anyone who has shown symptoms of asthma due to this substance should
never again come into contact with this substance. Do NOT take working clothes home.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the
CEC or the IPCS is responsible for the use which might be made of this
information.
REFERENCES
Aldrich F, Stange A, Geesaman R (1987) Smoking and ethylene diamine
sensitization in an industrial population. Journal of occupational
medicine, 29:311-314.
Andersson K, Hallgren C, Levin J, Nilsson C (1985) Determination of
ethylenediamine in air using reagent-coated adsorbent tubes and
high-performance liquid chromatography on the naphthylisothiourea
derivative. American Industrial Hygiene Association journal,
46:225-229.
Babiuk C, Hastings K, Dean J (1987) Induction of ethylenediamine
hypersensitivity in the guinea-pig and the development of ELISA and
lymphocyte blastogenesis techniques for its characterisation.
Fundamental and applied toxicology, 9:623-634.
Baer R, Cohen H, Neidorff A (1958) Allergic eczematous sensitivity to
aminophylline. American Medical Association archives of dermatology,
79:647-648.
Balato N, Cusano F, Lembo G, Ayala F (1984) Ethylenediamine contact
dermatitis. Contact dermatitis, 11:112-114.
Balk F, Meuwsen I (1989a) Respiration inhibition test with
nitrifying bacteria, ethylenediamine (EDA). Arnhem, Akzo Chemicals
(Unpublished Report No. CRL F89082).
Balk F, Meuwsen I (1989b) Acute toxicity of ethylenediamine (EDA) for
Daphnia. Arnhem, Akzo Chemicals (Unpublished Report No. CRL 189040).
Balk F, Meuwsen I (1989c) Acute toxicity of ethylenediamine (EDA)
for fish. Arnhem, Akzo Chemicals (Unpublished Report No. CRL
189032).
Basketter D, Scholes E (1992) Comparison of the local lymph node assay
with the guinea pig maximisation test for the detection of a range of
contact allergens. Food and chemical toxicology, 30:65-69.
Boas-Traube S, Dresel E, Dryden I (1948) Properties of ethylene
diamine. Nature, 162:960.
Booth B, Coleman W, Mitchell D (1979) Urticaria following intravenous
aminophylline. Annals of allergy, 43:289-290.
Boyd E, Seymour K (1946) Ethylenediamine dihydrochloride or
"Chlor-ethamine." II: Untoward and toxic reactions. Experimental
medical surgery, 4:223-227.
Bringmann G, Kuhn R (1976) Vergleichende Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Backterien ( Pseudomonas putida) und
Blaualgen ( Microcystis aeruginosa) . Gwf-wasser/Abwasser,
117:410-413.
Bringmann G, Kuhn R (1980a) Comparison of the toxic thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication inhibition test. Water research, 14:231-241.
Bringmann G, Kuhn R (1980b) Bestimmung der Biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen. II Bakterienfressende
Ciliaten. Zeitschrift fuer Wasser und Abwasser Forschung, 1:26-31.
Brooke I, Saleem A, Stewart T, Delic J, Cocker J, Patel S, Ogunbiyi A
(1997) 1,2-Diaminoethane. Sudbury, Suffolk, HSE Books (Risk
Assessment Document EH72/7; ISBN 0 7176 1338 0).
BUA (1997) Ethylenediamine. Stuttgart, S. Hirzel (GDCh-Advisory
Committee on Existing Chemicals of Environmental Relevance Report No.
184; ISBN 3-7776-0811-4).
Caldwell J, Cotgreave I (1983) Disposition in human volunteers of
theophylline and ethylenediamine given combined as aminophylline.
Clinical practice, 23:22-25.
Carpenter C, Smyth H (1946) Chemical burns of the rabbit cornea.
American journal of ophthalmology, 29:1363-1372.
Carpenter C, Smyth R, Shaffer C (1948) The acute toxicity of ethylene
imine to small animals. Journal of industrial hygiene and
toxicology, 30:2-6.
Chan-Yeung M (1982) Occupational assessment of asthma. Chest,
82:24S.
Cornacoff J, House R, Dean J (1988) Comparison of a radioisotopic
incorporation method and the mouse ear swelling test (MEST) for
contact sensitivity to weak sensitisers. Fundamental and applied
toxicology, 10:40-44.
Cotgreave I, Caldwell J (1983a) Physicochemical and in-vitro
biological studies on the possible association between theophylline
and ethylenediamine in solution. Journal of pharmacy and
pharmacology, 35:774-779.
Cotgreave I, Caldwell J (1983b) Comparative plasma pharmacokinetics of
theophylline and ethylenediamine after the administration of
aminophylline to man. Journal of pharmacy and pharmacology,
35:378-382.
Cotgreave I, Caldwell J (1983c) Studies on aminophylline disposition.
1. A rapid and sensitive HPLC assay for ethylenediamine in plasma and
urine. Biopharmacy and drug disposition, 4:53-62.
Cotgreave I, Caldwell J (1985) Comparative pharmacokinetics of
theophylline and ethylenediamine following single and repeated doses
of a sustained-release aminophylline preparation to volunteers.
Journal of pharmacy and pharmacology, 37:618-621.
Crow K, Peachey R, Adams J (1978) Coolant oil dermatitis due to
ethylenediamine. Contact dermatitis, 4:359-361.
Curtis M, Ward C (1981) Aquatic toxicity of forty industrial
chemicals: testing in support of hazardous substance spill prevention
regulations. Journal of hydrology, 51:359-367.
Davis J (1993) Physico-chemical factors influencing ethyleneamine
sorption to soil. Environmental toxicology and chemistry, 12:27-35.
DePass L, Fowler E, Yang R (1984) Dermal oncogenicity studies on
ethylenediamine in male C3H mice. Fundamental and applied
toxicology, 4:641-645.
DePass L, Yang R, Woodside M (1987) Evaluation of the teratogenicity
of ethylenediamine HCl in F344 rats by conventional and pair-feeding
studies. Fundamental and applied toxicology, 9:687-697.
Dernehl C (1951) Clinical experiences with exposures to ethylene
amines. Industrial and medical surgery, 20:541-546.
Dias M, Fernandes C, Pereira F, Pacheco A (1995) Occupational
dermatitis from ethylenediamine. Contact dermatitis, 33:129-130.
Dubinina O, Galeeva L, Trubnikova L, Varlamova T, Tlacheva S (1997)
Experimental studies towards a possible adjustment to the MAC for
ethylenediamine in workplace air. Meditsina Truda i Promyshlennaia
Ekologiia, 1:38-41.
Dunn B, Rusch G, Siglin J, Blasczcak D (1990) Variability of a mouse
ear-swelling test (MEST) in predicting weak and moderate contact
sensitization. Fundamental and applied toxicology, 15:242-248.
Edman B, Moller H (1986) Medicament contact allergy. Dermatosen,
34:139-142.
English J, Rycroft R (1989) Occupational sensitization to
ethylene-diamine in a floor polish remover. Contact dermatitis,
20:220-221.
Epstein E, Maibach H (1968) Ethylenediamine. Allergic contact
dermatitis. Archives of dermatology, 98:476-477.
Erdo S, Kiss B, Riesz M, Szporny L (1986) Stimulus-evoked efflux of
GABA from preloaded slices of the rabbit oviduct. European journal
of pharmacology, 130:295-303.
Erikson K (1979) Sensitization capacity of ethylenediamine in the
guinea pig and induction response. Contact dermatitis, 5:293-296.
Forster P, Lloyd H, Morgan P, Parker M, Perkins M, Stone T (1981)
Ethylenediamine acts upon GABA receptors and uptake sites.
Proceedings of the British Pharmacology Society, 1-3 April 1981, p.
274.
Fregert S (1981) Manual of contact dermatitis, 2nd ed. Copenhagen,
Munksgaard.
Gad S, Dunn B, Dobbs D, Reilly C, Walsh R (1986) Development and
validation of an alternative dermal sensitization test: the mouse
ear-swelling test (MEST). Toxicology and applied pharmacology,
84:93-114.
Gelfand H (1963) Respiratory allergy due to chemical compounds
encountered in the rubber, lacquer, shellac, and beauty culture
industries. Journal of allergy, 34:374-381.
Goodwin B, Crevel R, Johnson A (1981) A comparison of three guinea-pig
sensitisation procedures for the detection of 19 reported human
contact sensitisers. Contact dermatitis, 7:248-258.
Hagmar L, Bellander T, Berg B, Simonsson B (1982) Piperazine-induced
occupational asthma. Journal of occupational medicine, 24:193-197.
Hansen L, Kristiansson B, Sollenberg J (1984) A method for the
determination of ethylenediamine in workroom air. Scandinavian
journal of environmental health, 10:95-98.
Hardin B, Schuler R, Burg J, Booth G, Hazeldean K, MacKenzie K,
Piccirrillo V, Smith K (1987) Evaluation of 60 chemicals in a
preliminary developmental toxicity test. Teratogenesis,
Carcinogenesis, Mutagenesis, 7:29-48.
Hardy C, Schofield O, George C (1983) Allergy to aminophylline.
British medical journal, 286:2051-2052.
Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983)
Salmonella mutagenicity test results for 250 chemicals.
Environmental mutagenesis, Suppl. 1:3-142.
Hedenstedt A (1978) Mutagenicity screening of industrial chemicals.
Seven aliphatic amines and one amide tested in the Salmonella
microsome assay. Mutation research, 53:198-199.
Henck J, Lockwood D, Olsen K (1980) Skin sensitisation potential of
trisodium ethylenediaminetetraacetate. Drug chemistry and
toxicology, 3:99-103.
Hill D (1985) The influence of bicarbonate ions on the GABA-mimetic
activity of ethylenediamine. Neuropharmacology, 24:147-155.
HSDB (1997) Hazardous substances data bank. Bethesda, MD, National
Library of Medicine (CD-ROM: Micromedex Inc., Englewood, CO, Edition
V35).
Hulla J, Rogers S, Warren G (1981) Mutagenicity of a series of
polyamines. Environmental mutagenesis, 3:332-333.
Hulzebos E, Adema D, Dirven-van Breeman E, Henzen L, Van Dis W,
Herbold H, Hoekstra J, Baerselman R, Van Gestel C (1993) Phytotoxicity
studies with Lactuca sativa in soil and nutrient solution.
Environmental toxicology and chemistry, 12:1079-1094.
IPCS (1993) International Chemical Safety Card -- Ethylenediamine.
Geneva, World Health Organization, International Programme on Chemical
Safety (ICSC 0269).
Japan Chemical Industry Ecology-Toxicology Information Center (1992)
Biodegradation and bioaccumulation data of existing chemicals based
on the CSCL Japan. Ministry of International Trade and Industry,
Chemicals Inspection and Testing Institute (ISBN 4-89074-101-2).
Kerr D, Ong J (1984) Evidence that ethylenediamine acts in the
isolated ileum of the guinea pig by releasing endogenous GABA.
British journal of pharmacology, 83:169-177.
Kradjan W, Lakshminarayan S (1981) Allergy to aminophylline: lack of
predictability by skin testing. American journal of hospital
pharmacology, 38:1031-1033.
Krantis A, Khalil A, McKay A (1990) Differences in the effects ( in
vitro) of ethylenediamine on the guinea pig and rat intestine.
European journal of pharmacology, 177:9-17.
Kuhn R, Pattard M (1990) Results of the harmful effects of water
pollutants to green algae ( Scenedesmus subspicatus) in the cell
multiplication inhibition test. Water research, 24:31-38.
Kuhn R, Pattard M, Pernak K, Winter A (1989) Results of the harmful
effects of water pollutants to Daphnia magna in the 21 day
reproduction test. Water research, 23:501-510.
Lam S, Chan-Yeung M (1980) Ethylenediamine-induced asthma. American
review of respiratory disease, 121:151-155.
Leung H-W (1994) Evaluation of the genotoxic potential of
alkylene-amines. Mutation research, 320:31-43.
Leung H, Auletta C (1997) Evaluation of skin sensitisation and
cross-reaction of nine alkyleneamines in the guinea pig maximization
test. Journal of toxicology -- Cutaneous and ocular toxicology,
16:189-195.
Levin J, Andersson K, Fangmark I, Hallgren C (1989) Determination of
gaseous and particulate polyamines in air using sorbent or filter
coated with naphthylisothiocyanate. Applied Industrial Hygiene
Association journal, 4:98-100.
Levin J, Andersson K, Hallgren C (1994) Exposure to low molecular
weight polyamines during road paving. Annals of occupational
hygiene, 38:257-264.
Lewinsohn H, Ott M (1991) A review of medical surveillance records of
employees exposed to ethyleneamines. Journal of occupational
medicine, 33:148-154.
Lloyd H, Perkins M, Stone T (1982) Ethylenediamine as a specific
releasing agent of gamma-aminobutyric acid in rat striatal slices.
Journal of neurochemistry, 38:1168-1169.
Mark U, Arends I (1992) Fish early life stage test with
ethylene-diamine. Arnhem, Akzo Chemicals (Unpublished Report No. CRL
F91180).
Mark U, Hantink-de Rooy E (1992) Prolonged toxicity of
ethylenediamine to Daphnia magna. Arnhem, Akzo Chemicals
(Unpublished Report No. CRL F91182).
Matsui S, Nakazawa T, Umagae Y, Kanatani K, Fujiwara T, Fueki R,
Kobayashi S (1986) Two cases of late response bronchial asthma caused
by ethylenediamine. Arerugi, 35:40-46.
Matthieu L, Weyler J, Deckers I, van Sprundel M, van Andel A, Dockx P
(1993) Occupational contact sensitization to ethylenediamine in a
wire-drawing factory. Contact dermatitis, 29:39.
Maurer T, Thomann P, Weirich E, Hess R (1979) Predictive evaluation in
animals of the contact allergenic potential of medically important
substances. Contact dermatitis, 5:1-10.
McKay A, Krantis A (1991) The effects of ethylenediamine in the rat
small intestine: a powerful relaxant of the muscularis. Canadian
journal of physiology and pharmacology, 69:199-204.
McKelvey J, Tallant M, Yang R (1982) The fate of
14C-ethylene-diamine in the mouse following different routes of
administration. Philadelphia, PA, Union Carbide Corporation, Bushy
Run Research Center (Project Report 45-191).
Mills E, Stack V (1955) Suggested procedure for evaluation of
biological oxidation of organic chemicals. Sewage and industrial
wastes, 27:1061-1064.
Morgan P, Stone T (1982) Anticonvulsant actions of the putative
gamma-aminobutyric acid (GABA)-mimetic, ethylenediamine. British
journal of pharmacology, 77:525-529.
Nakazawa T, Matsui S (1990) Ethylenediamine-induced late asthmatic
responses. Journal of asthma, 27:207-212.
Newsome W, Shields J, Villeneuve D (1975) Residues of maneb,
ethylenethiuram monosulfide, ethylenethiourea and ethylenediamine on
beans and tomatoes field-treated with maneb. Journal of agricultural
and food chemistry, 23:756-758.
Ng T, Lee H, Lee F, Wang Y, Tay V, Tan K (1991) Occupational asthma
due to ethylenediamine. Annals of the Academy of Medicine,
20:397-402.
Ng T, Lee H, Malik M, Chee Q, Cheong T, Wang Y (1995) Asthma in
chemical workers exposed to aliphatic polyamines. Occupational
medicine, 45:45-48.
Nielsen M, Jorgensen J (1987) Persistence of contact sensitivity to
ethylenediamine. Contact dermatitis, 16:275-276.
NIOSH (1984-1989) Manual of analytical methods. Method 3509.
Cincinnati, OH, US Department of Health and Human Services, Public
Health Service, National Institute for Occupational Safety and Health.
Nishikawa Y (1987) Liquid chromatographic determination of aliphatic
diamines in water via derivatization with acetylacetone. Journal of
chromatography, 392:349-359.
Niveau J, Painchaux J (1973) Intoxication mortelle par éthylène
diamine. Archives des Maladies Professionnelles du Travail et de
Securité Sociale, 34:523-528.
NTP (1982a) Report on prechronic studies of ethylenediamine acute,
repeated dose and subchronic in rats. Contract NO1 CP 95653-02 to
National Toxicology Program, National Institutes of Health, Public
Health Service, US Department of Health and Human Services, Research
Triangle Park, NC. Columbus, OH, Batelle Columbus Laboratories.
NTP (1982b) Report on prechronic studies of ethylenediamine acute,
repeated dose and subchronic in mice. Contract NO1 CP 95653-02 to
National Toxicology Program, National Institutes of Health, Public
Health Service, US Department of Health and Human Services, Research
Triangle Park, NC. Columbus, OH, Batelle Columbus Laboratories.
NTP (1991) Developmental toxicity of ethylene diamine in New Zealand
white rabbits. Research Triangle Park, NC, US Department of Health
and Human Services, Public Health Service, National Institutes of
Health, National Toxicology Program (Contract NIEHS-N01-ES-95255;
Report No. RTI-411).
Ormerod A, Wakeel R, Mann T, Main R, Aldridge R (1989) Polyamine
sensitization in offshore workers handling drilling muds.
Contact dermatitis, 21:326-329.
Patel S, Rimmer D (1996) Development of an air monitoring method for
the measurement of ethylenediamine. Sheffield, Health and Safety
Executive (Internal Report IR/L/IA/95/6).
Perkins M, Stone T (1980) Aminophylline and theophylline derivatives
as antagonists of neuronal depression by adenosine: a
micro-iontophoretic study. Archives of international
pharmacodynamics, 246:205-214.
Petrozzi J, Shore R (1976) Generalized exfoliative dermatitis from
ethylenediamine. Sensitization and induction. Archives of
dermatology, 112:525-526.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Popa V, Teculescu D, Stanescu D, Gavrilescu N (1969) Bronchial asthma
and asthmatic bronchitis determined by simple chemicals. Diseases of
the chest, 56:395-404.
Pozzanni U, Carpenter C (1954) Response of rats to repeated
inhalations of ethylenediamine vapours. Archives of industrial
hygiene and occupational medicine, 9:223-226.
Price K, Waggy G, Conway R (1974) Brine shrimp bioassay and seawater
BOD of petrochemicals. Journal of the Water Pollution Control
Federation, 46:63-77.
Price Q, George J, Marr M, Myers C, Heindel J, Schwetz B (1993)
Developmental toxicity evaluation of ethylenediamine (EDA) in New
Zealand white rabbits. Teratology, 47:432-433.
Provost T, Jillson O (1967) Ethylenediamine contact dermatitis.
Archives of dermatology, 96:231-234.
Robinson M, Fletcher E, Johnson G, Wyder W, Maurer J (1990) Value of
cutaneous basophil hypersensitivity (CBH) response for distinguishing
weak contact sensitization from irritation reactions in the
guinea-pig. Journal of investigative dermatology, 94:636-643.
Sarthy P (1983) Release of [3H] gamma-aminobutyric acid from glial
(Muller) cells of the rat retina: effects of K+, veratridine, and
ethylenediamine. Journal of neuroscience, 3:2494-2503.
Sasseville D, Al-Khenaizan S (1997) Occupational contact dermatitis
from ethylenediamine in a wire-drawing lubricant. Contact
dermatitis, 36:228-229.
Shehade S, Beck M, Hillier V (1991) Epidemiological survey of standard
series patch test results and observations on day 2 and day 4
readings. Contact dermatitis, 24:119-122.
Simon P, Terzian C, Frome B (1995) Skin reactions to topical
aminophylline. Journal of the American Medical Association,
273:1737-1738.
Slesinski R, Guzzie P, Hengler W, Watanabe P, Woodside M, Yang R
(1983) Assessment of genotoxic potential of ethylenediamine: in
vitro and in vivo studies. Mutation research, 124:299-314.
Smyth H Jr, Seaton J, Fischer L (1941) The single dose toxicity of
some glycols and derivatives. Journal of industrial hygiene and
toxicology, 23:259-268.
Smyth H Jr, Carpenter C, Weil C (1951) Range-finding toxicity data:
List IV. American Medical Association archives of industrial
hygiene, 4:119-122.
Strain G, Flory W, Tucker T (1984) Inhibition of synaptosomal uptake
of amino acid transmitters by diamines. Neuropharmacology,
23:971-975.
Takemoto S, Kuge Y, Nakamoto M (1981) The measurement of BOD in sea
water. Suishitsu Okagu Kenkyu [Japanese journal of water
pollution research], 4:80-90.
Tas J, Weissberg D (1958) Allergy to aminophylline. Acta
Allergologica, 12:39-42.
Terzian C, Simon P (1992) Aminophylline hypersensitivity apparently
due to ethylenediamine. Annals of emergency medicine, 21:312-314.
Thorgeirsson A (1978) Sensitization capacity of epoxy resin hardeners
in the guinea pig. Acta Dermatologica, 58:332-336.
Toal M, Kinney A, Fulton R (1992) Allergy to the ethylenediamine
component of aminophylline. Ulster medical journal, 61:205-206.
Tonogai Y, Ogawa S, Ito Y, Iwaida M (1982) Actual survey on Tlm
(median tolerance limit) values of environmental pollutants,
especially on amines, nitriles, aromatic nitrogen compounds and
artificial dyes. Journal of toxicological sciences, 7:193-203.
Valeyeva K, Belomyteseva, L, Khafizullin E (1975) Occupational
bronchial asthma in employees engaged in the production of
ethylenediamine. Aktual'nye Voprosy Gigieny Truda Professional' -n
Patologii i Toksikologii v Neftyanoi Nefte-khimicheskoi
Promyshlennosti, 121-124.
van Ginkel C (1989) Toxicity of ethylenediamine for Pseudomonas
putida. Arnhem, Akzo Chemicals (Unpublished Report No. CRL F89091).
van Ginkel CG, Stroo CA (1989) Toxicity of ethylenediamine for
activated sludge. Arnhem, Akzo Chemicals (Unpublished Report No.
F89054).
van Ginkel CG, Kroon AGM, Mark U (1990) Algal inhibition test with
ethylenediamine. Arnhem, Akzo Chemicals (Unpublished Report No.
F90086).
van Leeuwen C (1986) Ecotoxicological aspects of dithiocarbamates.
Thesis, University of Utrecht. The Hague, Rijkswaterstaat
(Rijkswaterstaat Communications No. 44).
van Wijk R (1992) Environmental risk assessment of ethylenediamine
and diethylenetriamine wastewater discharges in the Ems-Dollard
estuary. Arnhem, Akzo Chemicals (Unpublished Report No. CRL F92009).
van Wijk R, Postman J, Van Houwelingen H (1994) Joint toxicity of
ethyleneamines to algae, daphnids and fish. Environmental toxicology
and chemistry, 13:167-171.
Wall L (1982) Lymphatoid contact dermatitis due to ethylenediamine
dihydrochloride. Contact dermatitis, 8:51-54.
Woodiwiss F, Fretwell G (1974) The toxicities of sewage effluents,
industrial discharges and some chemical substances to brown trout in
the Trent River Authority area. Water pollution control (Great
Britain), 73:396.
Yang R, Tallant M (1982) Metabolism and pharmacokinetics of
ethylenediamine in the rat following oral, endotracheal or intravenous
administration. Fundamental and applied toxicology, 2:252-260.
Yang R, Garman R, Maronpot R, McKelvey J, Weil C, Woodside M (1983)
Acute and subchronic toxicity of ethylenediamine in laboratory
animals. Fundamental and applied toxicology, 3:512-520.
Yang R, Garman R, Maronpot R, Mirro E, Woodside M (1984a) Chronic
toxicity/carcinogenicity study of ethylenediamine in Fischer 344 rats.
Toxicologist, 4:53.
Yang R, Tallant M, McKelvey J (1984b) Age-dependent pharmacokinetic
changes of ethylenediamine in Fischer 344 rats parallel to a two-year
chronic toxicity study. Fundamental and applied toxicology,
4:663-670.
Yang R, Anuszkiewicz C, Chu S, Garman R, McKelvey J, Tallant M (1987)
Biochemical and morphological studies on the percutaneous uptake of
[14C]ethylenediamine in the rat. Journal of toxicology and
environmental health, 20:261-272.
Zimmering S, Mason M, Valencia R, Woodruff R (1985) Chemical
mutagenesis testing in Drosophila. II. Results of 20 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:87-100.
APPENDIX 1 -- SOURCE DOCUMENTS
Brooke et al. (1997): 1,2-Diaminoethane (Risk Assessment Document EH72/7)
The authors' draft version of this Health and Safety Executive
report was initially reviewed internally by a group of approximately
10 Health and Safety Executive experts (mainly toxicologists, but also
scientists from other relevant disciplines, such as epidemiology and
occupational hygiene). The toxicology section of the amended draft was
then reviewed by toxicologists from the United Kingdom Department of
Health. Subsequently, the entire risk assessment document was reviewed
by a tripartite advisory committee to the United Kingdom Health and
Safety Commission, the Working Group for the Assessment of Toxic
Chemicals (WATCH). This committee is composed of experts in toxicology
and occupational health and hygiene from industry, trade unions, and
academia.
The members of the WATCH committee at the time of the peer review
were Mr Steve Bailey, Independent Consultant; Dr Hilary Cross, Trade
Unions Congress; Mr David Farrar, Independent Consultant; Dr Tony
Fletcher, Trade Unions Congress; Dr Alastair Hay, Trade Unions
Congress; Dr Jenny Leeser, Chemical Industries Association; Dr Len
Levy, Institute of Occupational Hygiene, Birmingham; Dr Mike Molyneux,
Chemical Industries Association; Mr Alan Moses, Chemical Industries
Association; and Mr Jim Sanderson, Independent Consultant.
BUA (1997): Ethylenediamine (GDCh-Advisory Committee on Existing
Chemicals of Environmental Relevance Report No. 184)
For the BUA review process, the company that is in charge of
writing the report (usually the largest producer in Germany) prepares
a draft report using literature from an extensive literature search as
well as internal company studies. This draft is subject to a peer
review during several readings of a working group consisting of
representatives from government agencies, the scientific community,
and industry.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on 1,2-diaminoethane (ethylenediamine) was sent
for review to institutions and organizations identified by IPCS after
contact with IPCS national Contact Points and Participating
Institutions, as well as to identified experts. Comments were received
from:
Akzo Novel nv, Arnhem, Netherlands
Department of Health, London, United Kingdom
Environment Agency, Wallingford, United Kingdom
Ethyleneamines Product Stewardship Discussion Group, Michigan,
USA
Health Canada, Ottawa, Canada
International Agency for Research on Cancer, Lyon, France
National Chemicals Inspectorate (KEMI), Solna, Sweden
National Institute for Working Life, Solna, Sweden
National Institute of Public Health and Environmental Protection,
Bilthoven, The Netherlands
Nofer Institute of Occupational Medicine, Lodz, Poland
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati, USA;
National Institute of Environmental Health Sciences, Research
Triangle Park, USA)
United States Environmental Protection Agency (Office of Research
and Development, Washington, DC, USA)
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Tokyo, Japan, 30 June - 2 July 1998
Members
Dr R. Benson, Drinking Water Program, United States Environmental
Protection Agency, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Mr R. Cary, Health Directorate, Health and Safety Executive,
Merseyside, United Kingdom
Dr C. DeRosa, Agency for Toxic Substances and Disease Registry, Center
for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Cambridgeshire, United
Kingdom
Dr H. Gibb, National Center for Environmental Assessment, United
States Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Ms M.E. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada ( Chairperson)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan ( Vice-Chairperson)
Professor S.A. Soliman, Department of Pesticide Chemistry, Alexandria
University, Alexandria, Egypt
Ms D. Willcocks, Chemical Assessment Division, Worksafe Australia,
Camperdown, Australia ( Rapporteur)
Professor P. Yao, Chinese Academy of Preventive Medicine, Institute of
Occupational Medicine, Beijing, People's Republic of China
Observers
Professor F.M.C. Carpanini,1 Secretary-General, ECETOC (European
Centre for Ecotoxicology and Toxicology of Chemicals), Brussels,
Belgium
Dr M. Ema, Division of Biological Evaluation, National Institute of
Health Sciences, Osakai, Japan
Mr R. Green,1 International Federation of Chemical, Energy, Mine and
General Workers' Unions, Brussels, Belgium
Dr B. Hansen,1 European Chemicals Bureau, European Commission, Ispra,
Italy
Mr T. Jacob,1 Dupont, Washington, DC, USA
Dr H. Koeter, Organisation for Economic Co-operation and Development,
Paris, France
Mr H. Kondo, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Ms J. Matsui, Chemical Safety Policy Office, Ministry of International
Trade and Industry, Tokyo, Japan
Mr R. Montaigne,1 European Chemical Industry Council (CEFIC),
Brussels, Belgium
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr H. Nishimura, Environmental Health Science Laboratory, National
Institute of Health Sciences, Osaka, Japan
Ms C. Ohtake, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr T. Suzuki, Division of Food, National Institute of Health Sciences,
Tokyo, Japan
Dr K. Takeda, Mitsubishikasei Institute of Toxicological and
Environmental Sciences, Yokohama, Japan
Dr K. Tasaka, Department of Chemistry, International Christian
University, Tokyo, Japan
Dr H. Yamada, Environment Conservation Division, National Research
Institute of Fisheries Science, Kanagawa, Japan
Dr M. Yamamoto, Chem-Bio Informatics, National Institute of Health
Sciences, Tokyo, Japan
Dr M. Yasuno, School of Environmental Science, The University of Shiga
Prefecture, Hikone, Japan
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Ms L. Regis, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au 1,2-diaminoéthane (éthylènediamine) a été
préparé à partir d'une étude du Health and Safety Executive du
Royaume-Uni sur les risques pour la santé humaine (risques
professionnels pour l'essentiel, mais comportant également un volet
écologique) (Brooke et al., 1997). La bibliographie sur laquelle
s'appuie l'étude originale a été arrêtée à fin 1994. Une analyse de la
littérature a été ensuite effectuée jusqu'à juillet 1997 à la
recherche de données qui auraient pu être publiées depuis la fin de
l'étude. Les données relatives à la destinée du composé dans
l'environnement et à son impact écologique sont tirées d'un rapport du
Comité consultatif de la Société allemande de Chimie pour les
substances chimiques d'intérêt écologique (BUA, 1997). On trouvera à
l'appendice 1 des indications sur les sources documentaires utilisées
et sur leur mode de dépouillement. Les renseignements concernant
l'examen du CICAD par des pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors d'une
réunion du Comité d'évaluation finale qui s'est tenue à Tokyo (Japon)
du 30 juin au 2 juillet 1998. La liste des participants à cette
réunion figure à l'appendice 3. La fiche d'information internationale
sur la sécurité chimique (ICSC No 0269) établie par le Programme
international sur la Sécurité chimique (IPCS, 1993) est également
reproduite dans ce document.
Le 1,2-diaminoéthane (No CAS 107-15-3), couramment désigné sous
le nom d'éthylènediamine (EDA), est un produit de synthèse qui se
présente sous la forme d'un liquide incolore à jaunâtre dans les
conditions normales de température et de pression. Il présente une
réaction fortement alcaline et il est miscible à l'eau et à l'alcool.
On l'utilise principalement comme intermédiaire dans la fabrication de
la tétra-acétyl-éthylènediamine, de l'acide
éthylènediamine-tétra-acétique (EDTA), des floculants organiques, des
résines à base d'urée et des diamides gras. Dans des proportions
beaucoup plus faibles, il entre également dans la composition de
formulations destinées à la fabrication des supports de circuits
imprimés ou utilisées dans l'industrie de finissage des métaux. Il
peut aussi être utilisé comme accélérateur ou agent de réticulation
dans les résines époxy employées notamment comme revêtements ainsi que
pour la préparation de certains produits pharmaceutiques. L'EDA est
présent sous la forme d'impureté (<0,5 %) dans les amines grasses du
commerce utilisées comme agents mouillants dans les émulsions
bitumineuses. On l'emploie également dans la synthèse des fongicides à
base de carbamates, dans la fabrication des agents de surface et des
colorants ainsi que dans la préparation de produits de développement
photographique et d'huiles de coupe. L'EDA est un produit de
décomposition des éthylènebis(dithiocarbamates) utilisés comme
fongicides.
Il ne devrait pas y avoir d'effets atmosphériques puisque la
réaction de l'EDA avec les radicaux hydroxyles est vraisemblablement
rapide (demi-vie 8,9 h) et que la fraction volatilisée devrait être
éliminée par les précipitations. Ce passage dans l'atmosphère à l'état
de vapeur est probable à partir du sol, mais pas à partir de l'eau.
L'EDA adhère fortement aux particules du sol par attraction
électrostatique; on pense qu'il ne devrait donc pas y avoir de passage
dans les eaux souterraines par lessivage des sols. Il peut sans doute
former des complexes avec les métaux et les acides humiques. La voie
de décomposition la plus probable dans l'environnement est la voie
biologique et alle devrait être assez rapide; l'adaptation des
microorganismes pourrait accélérer le processus. La décomposition est
plus lente dans l'eau de mer que dans l'eau douce. Il n'y a
probablement pas de bioaccumulation.
L'EDA présente une toxicité aiguë modérée pour les animaux. C'est
surtout une substance irritante, qui est corrosive quand elle n'est
pas diluée et qui provoque également une sensibilisation cutanée. On
n'a pas procédé à la recherche de son pouvoir mutagène dans les
conditions prescrites par la réglementation actuelle et on ne dispose
pas non plus d'études sur son activité clastogène ou sur une action
qui s'exercerait sur les cellules somatiques in vivo. Il n'existe
donc pas de données suffisantes pour que l'on puisse se prononcer avec
certitude sur le pouvoir mutagène éventuel de l'EDA. Quoi qu'il en
soit, le composé ne s'est pas révélé cancérogène chez l'animal. On a
observé des effets non néoplasiques au niveau du foie (modifications
pléomorphes des hépatocytes) chez des rats auxquels on avait fait
ingérer du 1,2-diaminoéthane pendant 2 ans. Ces effets ont été
observés à des doses quotidiennes supérieures ou égales à 45 mg d'EDA
par kg de poids corporel, aucune anomalie ne se manifestant à la dose
quotidienne de 9 mg par kg de poids corporel. On voit pas très
clairement ce que cette observation peut signifier pour la santé
humaine et l'on ne peut d'ailleurs pas se prononcer non plus sur le
point de savoir si les effets rapportés sont effectivement dus à
l'ingestion de l'EDA (par exemple, ils pourraient ne pas se produire
si on changeait la voie d'administration ou être liés à des effets de
premier passage), mais on ne peut les négliger pour autant et il faut
déterminer les conditions de leur apparition. Lors d'études où le
composé a été administré par gavage, on a observé des effets oculaires
chez le rat (atrophie rétinienne et à dose élevée, formation de
cataractes) à des doses quotidiennes supérieures ou égales à 100 mg
par kg de poids corporel. Chez des rats et des souris, on a constaté
la présence de lésions rénales aux doses quotidiennes respectives de
200 et 100 mg de composé par kg de poids corporel et au-delà. On a
également trouvé quelques indices d'effets sur la rate chez des souris
et des rats à des doses quotidiennes supérieures ou égales à 400 mg
d'EDA par kg de poids corporel, de même que chez des rats, au niveau
du thymus, à la dose quotidienne de 800 mg/kg de poids corporel. Les
études d'inhalation effectuées sur des rats n'ont pas permis
d'observer d'effets à la dose d'environ 150 mg/m3 (60 ppm) et le seul
effet imputable au traitement a été une légère dépilation à la dose
d'environ 330 mg/m3 (132 ppm).
Comme l'EDA a un effet irritant et sensibilisateur sur
l'épiderme, il pourrait y avoir un risque d'apparition de dermatites
d'irritation ou de dermatites allergiques si l'on porte pas
d'équipement protecteur individuel sur les lieux de travail où il y a
possibilité de contact cutané. L'EDA peut également provoquer une
hypersensibilité des voies respiratoires et de l'asthme chez les
personnes professionnellement exposées et c'est d'ailleurs cet effet
que l'on considère comme le plus préoccupant sur le plan sanitaire.
On ne sait pas avec exactement par quel mécanisme se développe
cet état d'hypersensibilité, mais le pouvoir sensibilisateur cutané de
l'EDA et les quelques indications dont on dispose sur l'existence
d'une composante immunologique chez les ouvriers souffrant d'un asthme
provoqué par ce composé, incitent à penser que ce mécanisme serait
justement de nature immunologique. Quoi qu'il en soit et quel que
puisse être la nature du mécanisme en question, les données
disponibles ne permettent pas de mettre en évidence une relation
dose-réponse ou de déterminer le seuil d'apparition d'un état
d'hypersensibilité ou d'une réaction asthmatiforme. Dans le présent
document, le risque imputable au composé est caractérisé par des
effets hépatiques dont on a évalué la probabilité chez des sujets
exposés à l'EDA de par leur profession, le but étant d'apprécier le
risque d'autres effets généraux. La conclusion en est que lorsque
l'EDA est utilisé en vase clos, l'exposition - mesurée ou calculée par
modélisation - est très sensiblement inférieure (d'un facteur 100 au
moins) à la valeur sans effet observable (NOEL) chez le rat et que,
par conséquent, des effets hépatiques indésirables sont improbables.
Faute de données, on ne peut évaluer le niveau d'exposition de la
population générale à l'EDA.
Le seuil de toxicité pour les microorganismes pourrait ne pas
dépasser 0,1 mg/litre. Cependant, il faut interpréter avec prudence
les résultats des tests toxicologiques en milieu de culture car l'EDA
est susceptible de former des complexes avec les ions métalliques. Ses
effets pourraient donc être indirects et résulter de ce que certains
éléments essentiels cessent alors d'être biodisponibles. Pour les
invertébrés et les poissons, la valeur de la CL50 va de 14 à >1000
mg/litre. On a trouvé une valeur de 0,16 mg/litre pour la dose sans
effet observable (NOEC) sur la reproduction de Daphnia.
Etant donné que les résultats des épreuves de toxicité aiguë et
chronique varient très largement, on a fixé à 16 µg/litre la valeur de
la concentration sans effet observable prévisible (PNEC), en
appliquant un coefficient d'incertitude de 10 à la valeur publiée la
plus faible de la concentration sans effet observable (NOEC) sur la
reproduction de Daphnia. Des hypothèses prudentes relatives à la
concentration prévisible dans l'environnement (PEC) permettent
d'aboutir à une valeur du rapport PEC/PNEC justifiant quelques
craintes eu égard aux concentrations initiales (par ex. lors de la
décharge initiale dans un cours d'eau ou un estuaire). Néanmoins, une
estimation plus élaborée de l'exposition probable indique un faible
risque pour les organismes aquatiques.
RESUMEN DE ORIENTACION
Este CICAD sobre el 1,2 diaminoetano (etilendiamina) se basa en
un examen de los problemas relativos a la salud humana
(fundamentalmente ocupacional, pero con la inclusión también de una
evaluación en el medio ambiente) preparado por la Dirección de Salud y
Seguridad del Reino Unido (Brooke et al., 1997). En el documento
original se incorporaron los datos obtenidos hasta el final de 1994.
Se realizó asimismo una búsqueda bibliográfica amplia hasta julio de
1997 para identificar cualquier información que se hubiera publicado
después de la terminación del informe. La información sobre el destino
y los efectos en el medio ambiente se basa en el informe del Comité
Consultivo sobre Sustancias Químicas Existentes Importantes para el
Medio Ambiente de la Sociedad Alemana de Química (BUA, 1997). La
información sobre la preparación del documento original y su examen
colegiado figura en el apéndice 1. La información acerca del examen
colegiado de este CICAD se presenta en el apéndice 2. Este CICAD se
aprobó como evaluación internacional en una reunión de la Junta de
Evaluación Final celebrada en Tokio, Japón, del 30 de junio al 2 de
julio de 1998. La lista de participantes en esta reunión figura en el
apéndice 3. La Ficha internacional de seguridad química (ICSC 0269),
preparada por el Programa Internacional de Seguridad de las Sustancia
Químicas (IPCS, 1993), también se reproduce en este documento.
El 1,2-diaminoetano (CAS No 107-15-3), conocido normalmente
como etilendiamina (EDA), es un líquido sintético entre incoloro y
amarillento a temperatura y presión normales. Es fuertemente alcalino
y miscible con agua y con alcohol. Se utiliza fundamentalmente como
intermediario en la fabricación de tetracetil etilendiamina, ácido
etilendiaminotetracético (EDTA), floculantes orgánicos, resinas de
urea y bisamidas grasas. También se usa, en proporción mucho menor, en
la producción de formulaciones con destino a las industrias de
tarjetas de circuitos impresos y acabado de metales, como agente
acelerador o de curado en revestimientos/resinas de epóxido y en la
fabricación de productos farmacéuticos. Se encuentra como contaminante
(<0,5%) en las aminas grasas de suministro comercial, que se utilizan
como agentes humectantes en emulsiones bituminosas. También se emplea
en la síntesis de fungicidas a base de carbamato, en la fabricación de
surfactantes y tintes y en productos químicos para el revelado
fotográfico, así como en lubricantes para cuchillas. La EDA es un
producto de la degradación de los fungicidas de
etilenbis(ditiocarbamato).
No cabe prever efectos atmosféricos, puesto que la reacción de la
EDA con los radicales hidroxilo es probablemente rápida (semivida de
8,9 horas) y se supone que la EDA volatilizada se arrastra. Es
probable la volatilización a la atmósfera a partir del suelo, pero no
del agua. Se adsorbe fuertemente a las partículas del suelo mediante
enlaces electrostáticos; no parece haber lixiviación a través de los
perfiles del suelo hacia el agua freática. Es posible la formación de
complejos con metales y ácidos húmicos. La biodegradación es el
mecanismo más probable de descomposición en el medio ambiente y
debería ser bastante rápida; la adaptación de los microorganismos
puede aumentar la degradación. La descomposición es menos rápida en el
agua de mar que en el agua dulce. No es probable la bioacumulación.
La toxicidad aguda de la EDA en los animales es moderada. Es
irritante primario, de propiedades corrosivas cuando no está diluido,
y es también sensibilizador cutáneo. La EDA no se ha sometido a
pruebas de mutagenicidad con arreglo a las normas reglamentarias
actuales y no se han realizado valoraciones para determinar la
actividad clastogénica o el potencial para expresar su actividad en
células somáticas in vivo. Así pues, no se dispone de información
suficiente para llegar a conclusiones firmes sobre el potencial
mutagénico de la EDA. No fue carcinogénico en animales. Se han
observado efectos no neoplásicos en el hígado de ratas (cambios
pleomórficos a hepatocitos), tras la administración oral durante dos
años con concentraciones de 45 mg de EDA/kg de peso corporal al día y
superiores, sin que se vieran efectos con 9 mg EDA/kg de peso corporal
al día. Aunque no está clara la importancia de estos cambios de las
células hepáticas para la salud humana, así como si son consecuencia
de la exposición oral o no (es decir, podrían no producirse por otras
vías, porque pueden estar relacionados con los efectos del primer
paso), no se pueden ignorar y se debería caracterizar el riesgo de su
aparición. En estudios de administración oral por sonda se observaron
efectos oculares en las ratas (atrofia de la retina y con dosis más
altas formación de cataratas) con dosis de 100 mg de EDA/kg de peso
corporal al día y superiores. Las dosis de 200 mg y 100 mg de EDA/kg
de peso corporal al día y superiores se asociaron con daños renales en
ratas y ratones, respectivamente. También se encontraron signos de
efectos en el bazo de ratones y ratas con dosis de 400 mg de EDA/kg de
peso corporal al día y superiores y en el timo de ratas con 800 mg/kg
de peso corporal al día. En estudios de inhalación realizados con
ratas no se detectaron efectos con concentraciones de unos 150 mg/m3
(60 ppm), y con 330 mg/m3 (132 ppm) el único efecto relacionado con
la dosis fue una ligera depilación.
Puesto que la EDA diluida es irritante y sensibilizador cutáneo,
si en el lugar de trabajo no se utiliza un equipo de protección
personal adecuado y se produce un contacto cutáneo se corre el riesgo
de contraer una dermatitis irritante y/o alérgica. La EDA puede
inducir además un estado de hipersensibilidad de las vías
respiratorias y provocar asma en el entorno ocupacional, y se
considera que éste es el efecto en la salud que despierta mayor
preocupación.
No se ha demostrado el mecanismo de inducción de la
hipersensibilidad, aunque el potencial de sensibilización cutánea de
la EDA y las pruebas limitadas de actuación inmunitaria en los
trabajadores con asma a causa de esta sustancia hacen pensar en un
mecanismo inmunitario. Sin embargo, con independencia del mecanismo de
que se trate, los datos disponibles no permiten dilucidar las
relaciones dosis-respuesta o identificar los umbrales para la
inducción del estado de hipersensibilidad o la provocación de una
respuesta asmática. A fin de determinar los riesgos de otros efectos
sistémicos, en la caracterización del riesgo de muestras en este
documento se evaluó el riesgo de efectos hepáticos en personas con
exposición ocupacional. Se ha llegado a la conclusión de que, cuando
la EDA se utiliza en sistemas cerrados, la exposición, tanto medida
como pronosticada a partir de modelos, es fundamentalmente inferior
(100 veces o más) a la concentración sin efectos observados (NOEL) en
ratas; así pues, son poco probables los efectos hepáticos adversos.
No se pudo evaluar la exposición del público general a la EDA
debido a la falta de datos disponibles.
El umbral tóxico para los microorganismos puede ser de sólo 0,1
mg de EDA/litro. Sin embargo, las pruebas de toxicidad en medios de
cultivo se han de interpretar con precaución, porque la EDA puede
formar complejos con iones metálicos. Por consiguiente, los efectos
pueden ser indirectos debido a una pérdida de biodisponibilidad de
elementos esenciales. Las CL50 para invertebrados y peces oscila
entre 14 y >1000 mg/litro. Se ha notificado una concentración sin
efectos observados (NOEC) para la reproducción en Daphnia de 0,16
mg/litro.
Habida cuenta de la gran variedad de resultados de las pruebas de
toxicidad aguda y crónica, se determinó una concentración prevista sin
efectos observados (PNEC) para los organismos acuáticos de 16
µg/litro, basada en la aplicación de un factor de incertidumbre de 10
a la NOEC más baja notificada para la reproducción de Daphnia.
Hipótesis prudentes para la concentración prevista en el medio
ambiente (PEC) establecen razones PEC/PNEC que ponen de manifiesto
alguna preocupación a partir de concentraciones iniciales (es decir,
en el primer vertido en el río o el estuario). Sin embargo,
estimaciones más precisas de la exposición indican un riesgo escaso
para los organismos acuáticos.
 1. EXECUTIVE SUMMARY
This CICAD on 1,2-diaminoethane (ethylenediamine) was based on a
review of human health concerns (primarily occupational, but also
including an environmental assessment) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997). Data
identified up to the end of 1994 were covered in the original review.
An additional literature search up to July 1997 was conducted to
identify any new information that had been published since the review
was completed. Information on environmental fate and effects was based
on the report of the German Chemical Society's Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1997). The
preparation and peer review of the source documents are described in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on
30 June - 2 July 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0269) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
1,2-Diaminoethane (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on 1,2-diaminoethane (ethylenediamine) was based on a
review of human health concerns (primarily occupational, but also
including an environmental assessment) prepared by the United
Kingdom's Health and Safety Executive (Brooke et al., 1997). Data
identified up to the end of 1994 were covered in the original review.
An additional literature search up to July 1997 was conducted to
identify any new information that had been published since the review
was completed. Information on environmental fate and effects was based
on the report of the German Chemical Society's Advisory Committee on
Existing Chemicals of Environmental Relevance (BUA, 1997). The
preparation and peer review of the source documents are described in
Appendix 1. Information on the peer review of this CICAD is presented
in Appendix 2. This CICAD was approved as an international assessment
at a meeting of the Final Review Board, held in Tokyo, Japan, on
30 June - 2 July 1998. Participants at the Final Review Board meeting
are listed in Appendix 3. The International Chemical Safety Card (ICSC
0269) produced by the International Programme on Chemical Safety
(IPCS, 1993) has also been reproduced in this document.
1,2-Diaminoethane (CAS No.  EDA is a colourless to yellowish hygroscopic liquid with an
ammonia-like odour. Its molecular weight is 60.12. It is a strongly
alkaline (pH of 25% EDA in water is 11.9), very volatile, pungent
material, which fumes profusely in air. It has a melting point of
about 8.5°C, a boiling point of 116°C (at 101.3 kPa), and a vapour
pressure of 1.7 kPa at 25°C. EDA is miscible with water and alcohol.
The log octanol/water partition coefficient (log Kow) ranges from
-1.2 to -1.52. p Ka1 and p Ka2 (calculated) are 10.71 and 7.56,
respectively, indicating protonation at environmentally relevant pH.
Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document.
Conversion factors for EDA at 20°C and 101.3 kPa are as follows:
1 ppm = 2.50 mg/m3
1 mg/m3 = 0.40 ppm
3. ANALYTICAL METHODS
For monitoring concentrations of EDA in workplace air, NIOSH
(1984-1989) uses a method that employs adsorption on silica gel and
analysis by gas chromatography with flame ionization detection. A
solvent-free sampling system is preferable because of more convenient
handling, and it is a great advantage if derivatization can be
achieved directly on the absorbent. The Health and Safety Laboratories
of the United Kingdom's Health and Safety Executive have evaluated a
published method (Andersson et al., 1985; Levin et al., 1989; Patel &
Rimmer, 1996). Air is sampled onto
1-naphthyl-isothiocyanate-impregnated filters, desorbed by
acetonitrile, and analysed by high-performance liquid chromatography
with ultraviolet detection. The method has a working range between 2.5
and 50 mg/m3 for a 5-litre air sample. The detection limit was found
to be 0.08 mg/m3. The method generally meets the Comité Européen de
Normalisation requirements on the overall uncertainty. Although the
Comité Européen de Normalisation requirements for desorption
efficiency were not satisfied at 25 and 50 mg/m3, a smaller sample
can be taken if necessary.
There are no reported methods for the biological monitoring of
occupational exposure to EDA. However, analytical techniques based on
solvent extraction of EDA and high-performance liquid chromatography
have been reported and used in pharmacological studies (Cotgreave &
Caldwell, 1983c), and these might form the basis for biological
monitoring methods.
EDA can be measured in water using reverse-phase high-performance
liquid chromatography with ultraviolet detection at 315 nm, following
derivatization with acetylacetone. The limit of detection was reported
to be 0.26 µg/litre (Nishikawa, 1987).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
EDA is not known to occur naturally. The main use for EDA is as
an intermediate in the manufacture of tetraacetyl ethylenediamine,
EDTA, organic flocculants, urea resins, and fatty bisamides. It is
also used, to a much smaller extent, in the production of formulations
for use in the printed circuit board and metal finishing industries,
as an accelerator/curing agent in epoxy coatings/resins, and in the
manufacture of pharmaceutical products. EDA is also present as a
contaminant (<0.5%) in commercially supplied fatty amines, which are
used as wetting agents in bituminous emulsions. It is also used in the
synthesis of carbamate fungicides, in surfactant and dye manufacture,
and in photography development chemicals and cutting oils. These are
believed to be minor uses in the United Kingdom and were not
investigated in this review. EDA is a degradation product of
ethylenebis(dithiocarbamate) fungicides.
Approximately 11 000 tonnes of EDA are imported into the United
Kingdom each year, with very little being re-exported (Brooke et al.,
1997). World production amounts to 100 000-500 000 tonnes annually.1
In 1992, annual production capacities were 18 000 tonnes for Germany,
54 000 tonnes for the Netherlands, 30 000 tonnes for Belgium, 25 000
tonnes for Sweden, about 159 000 tonnes for the United States, and
15 000 tonnes for Japan (BUA, 1997).
No measured concentrations of EDA in wastewater streams from
manufacture and use are available. However, estimates of EDA entering
waste treatment from four European manufacturing plants were 200, 287,
5000-10 000, and 1000 kg/year. Use in photochemicals was estimated to
lead to 1.1 tonnes being introduced into municipal sewage treatment
plants in Germany. All figures are for 1992 or 1993 (BUA, 1997).
1 IUCLID (European Union database), 1st ed., 1996.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few experimental data are available on the distribution,
transport, or fate of EDA in the environment. However, qualitative,
and some quantitative, estimates have been made on the basis of its
physicochemical properties.
EDA has a moderately high vapour pressure and is expected to
volatilize from soil (HSDB, 1997). In the atmosphere, it should react
rapidly with photochemically produced hydroxyl radicals; no
experimental rates are available for this proposed reaction, but a
half-life of 8.9 h has been calculated.1 EDA may react with carbon
dioxide to form an insoluble carbamate. The high water solubility of
EDA means that volatilized chemical is also likely to be washed out by
rain.2 The calculated dimensionless Henry's law constant (air/water
partition coefficient) is extremely low (7.08 × 10-8); therefore,
little evaporation would be expected from water. A half-life for
volatilization of 45 years was estimated for a model river 1 m
deep.2 An approximate Henry's law constant is given in BUA (1997) as
1.77 × 10-4 Pa.m3/mol.
Photodegradation is not expected, as the molecule contains no
chromophores, which absorb radiation (HSDB, 1997).
Despite their miscibility with water, ethyleneamines can bind
strongly to soil. There was a wide range of determined adsorption
coefficients in experimental studies on six soil types (Table 1). Some
reduction in variability occurred when results were normalized for
organic carbon content, although this was less marked with EDA than
with the other ethyleneamine studied. Sorption to soil was rapid, with
equilibrium occurring within a few hours. Electrostatic interaction
between the positively charged ethyleneamine and negatively charged
soils appeared to be the dominant factor in binding. Complex formation
with metals and humic acids is expected. Sorption is greater to soils
with high cation exchange capacity (Davis, 1993).
EDA at 200 mg/litre was incubated with adapted sewage sludge
until there was no further decrease in chemical oxygen demand (COD);
at that time (unspecified), 97.5% of the chemical had been degraded.
The rate of degradation was 9.8 mg COD/g per hour (Pitter, 1976).
EDA at 3, 7, and 10 mg/litre was incubated with sewage sludge
(adapted and non-adapted), and percent biodegradation was determined
5, 10, 15, and 20 days later. Degradation rates were comparable for
adapted and non-adapted sludge up to 15 days (at 56% and 55%,
respectively); at 20 days, however, the values were 70% and 47%,
respectively. Based on this single point, it is not possible to
conclude definitively that adaptation improves degradation. Nitrate
and nitrite were measured throughout the incubation to correct for
1 IUCLID (European Union database), 1st ed., 1996.
2 Syracuse Research Corporation modelling, summarized in HSDB
(1997).
oxygen demand due to conversion of ammonia or organic nitrogen to
these species. Such a correction was necessary for EDA alone out of
more than 50 compounds tested. Degradation was also tested in a
salt-water system using non-adapted sludge; EDA was degraded less
effectively, with 16% of theoretical degradation after 20 days (Price
et al., 1974). A comparable value at 16.6% was measured in seawater by
Takemoto et al. (1981). EDA incubated with microorganisms isolated
from river water and adapted to the compound over 28 days showed >80%
degradation relative to theoretical oxygen demand over 10 days (Mills
& Stack, 1955).
Brief descriptions of the following degradation tests were also
identified. EDA incubated with activated sludge at 100 mg/litre for 28
days showed 93-95% degradation relative to theoretical oxygen demand
in a modified Ministry of International Trade and Industry (MITI) test
(Japan Chemical Industry Ecology-Toxicology Information Center, 1992).
Incubation with activated sludge at a concentration of 50 mg/litre led
to degradation of 10%, 87.5%, and 94% after 5, 15, and 28 days,
respectively.1
The high water solubility and low octanol/water partition
coefficient indicate that bioaccumulation in organisms is unlikely.
1 Unpublished report from Akzo Research to Delamine, 1989 (cited in
IUCLID).
Table 1: Sorption of EDA to various soil types.a
Soil type pH Cation exchange Fraction of Freundlich adsorption Adsorption coefficient
capacity organic carbon coefficient (Kd)b normalized for organic
(meq/100 g) (Foc)b carbon (K = Kd/Foc)c
Sandy loam (Londo) 7.2 9.2 0.026 69 2700
Sandy clay loam 7.3 16.4 0.039 220 5600
Sandy loam (Cecil) 6.0 3.0 0.014 29 2100
Silty loam 6.0 15.6 0.034 238 7100
Clay 7.9 11.9 0.014 70 5000
Aquifer sand 9.6 6.9 0.0024 15 6200
a Data from Davis (1993).
b Values rounded.
c Mean 4800 + 2000 (SD).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no reports of monitoring of EDA levels in the aquatic
environment or of measurements in effluents.
Residues of EDA in soil, 15 days post-treatment with the
fungicide maneb, have been reported at 0.119 mg/kg for the top 1 cm
(approximately) and at 0.044 mg/kg down to about 5 cm. Residues on
tomatoes and beans were 0.053 and 0.239 mg/kg, respectively,
immediately after spraying, falling to 0.047 and 0.094 mg/kg,
respectively, after 14 days (Newsome et al., 1975).
6.2 Human exposure
The data available to the authors of this document are restricted
mainly to the occupational environment. The exposure assessments used
in this report are based on either limited data or data modelled using
the Estimation and Assessment of Substance Exposure (EASE) model. This
is a general-purpose predictive model developed by the United
Kingdom's Health and Safety Executive for exposure assessment in the
workplace. In its present form, the model is in widespread use across
the European Union for the occupational exposure assessment of new and
existing substances. Similarly, information on control measures has
been derived from United Kingdom industry sources. Where data gaps
exist, professional judgement has been used.
The number of employees exposed to EDA in the United Kingdom is
not accurately known. For use as an intermediate in the manufacture of
tetraacetyl ethylenediamine, EDTA, organic flocculants, urea resins,
and fatty bisamides, it is estimated that 140 employees will be
potentially exposed. During the production of formulations for use in
the printed circuit board and metal finishing industries and in the
manufacture of epoxy coatings/resins and pharmaceutical products, it
is estimated that 200 employees will be regularly exposed to EDA. The
number of employees potentially exposed from use of EDA-based
formulations in the printed circuit board and metal finishing
industries is estimated to be about 100. EDA can also be released when
industrial epoxy coatings/adhesives are applied, and this activity has
the potential to expose several thousand employees across a wide range
of industries.
There are very few measured occupational exposure data available.
EDA's use as an intermediate in chemical synthesis takes place in
closed systems. Measured exposures for these manufacturing processes
show that control is achieved to a level of less than 1.25 mg/m3
(0.5 ppm) 8-h time-weighted average (Hansen et al., 1984). Modelled
data (EASE) are in good agreement, predicting comparable values of
0.53-1.3 mg/m3 (0.21-0.52 ppm). Short-term peak exposures (sampling
and hose uncoupling operations) were predicted to range between 16.8
and 33.3 mg/m3 (6.7 and 13.3 ppm), 15-min time-weighted average.
EDA's use in the production of formulations usually takes place
in well-ventilated enclosed systems. Measured exposure data are not
available for these processes. However, modelled exposure data
indicate exposure levels of 5-20 mg/m3 (2-8 ppm) 8-h time-weighted
average in the presence of local exhaust ventilation and 38-75 mg/m3
(15-30 ppm) 8-h time-weighted average in the absence of local exhaust
ventilation. Corresponding short-term peak exposures during mixer
charging operations were estimated to be 5-25 mg/m3 (2-10 ppm) 15-min
time-weighted average in the presence of local exhaust ventilation and
50-103 mg/m3 (20-41 ppm) 15-min time-weighted average in the absence
of local exhaust ventilation.
The potential for exposure during the use of EDA formulations
will be moderated by the low concentration of EDA present in the
formulations. Very few exposure data are available, and there is scope
for widely different use scenarios. There will be no appreciable
occupational exposure if these products are used in enclosed
ventilated systems as indicated by measured exposure data
(<2.5 mg/m3 [<1 ppm] 8-h time-weighted average) and modelled exposure
data (0-0.25 mg/m3 [0-0.1 ppm] 8-h time-weighted average). Modelled
exposure data for immersion processes, in the presence of local
exhaust ventilation, predicted inhalation exposures of 0.5-2.5 mg/m3
(0.2-1 ppm) 8-h time-weighted average. The potential for greatest
inhalation exposure was predicted for situations where these
formulations are brushed in open systems with only general dilution
ventilation or sprayed in open systems in the presence of local
exhaust ventilation. Under these conditions, modelled exposure data
predict exposures in the range 2.5-5 mg/m3 (1-2 ppm) 8-h
time-weighted average. Short-term peak exposures during mixing and
loading operations were estimated to be 5-10 mg/m3 (2-4 ppm) 15-min
time-weighted average. Polyamines and alkanol polyamines, including
EDA, have been reported to be released from hot bitumen during road
paving (Levin et al., 1994). EDA concentrations generated during road
paving were below 0.025 mg/m3 (0.01 ppm).
There will also be a potential for dermal exposure across the
full range of industries handling EDA. Modelled data estimate dermal
exposures in the range 0-0.15 mg/cm2 per day. However, the use of
personal protective equipment is standard practice in all industries
using EDA. Therefore, in practice, dermal exposure will be
considerably reduced by the use of personal protective equipment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The toxicokinetics of EDA has received only limited study, and
there are no studies following inhalation exposure. Studies in humans
have been related to the clinical application of EDA and have
demonstrated rapid absorption via the gastrointestinal tract, with at
least 50% absorbed within the first 7 h; absorbed EDA is rapidly
removed from the plasma (Caldwell & Cotgreave, 1983; Cotgreave &
Caldwell, 1983a,b, 1985). At least half the amount absorbed is
excreted in the urine, largely as the acetylated metabolite
N-acetylethylenediamine and, in smaller amounts, as the unchanged
compound.
This toxicokinetic picture is supported and extended by data from
studies in experimental animals. Studies in rats and mice have
demonstrated rapid and extensive uptake via the oral route and also
via the respiratory tract following intratracheal instillation (about
70% or more of the applied dose was absorbed within 48 h) (McKelvey et
al., 1982; Yang & Tallant, 1982; Yang et al., 1984b). Some (about 12%
of the applied dose over 24 h) dermal absorption has also been
observed in rats at non-irritant concentrations, with greater
absorption at higher, skin-damaging concentrations (Yang et al.,
1987). These animal studies have also demonstrated that EDA and/or its
metabolites are widely distributed throughout the body and are rapidly
eliminated, largely via the urine but also as carbon dioxide in the
breath and a small amount via the faeces, providing evidence for some
biliary excretion. It would seem reasonable to conclude that a similar
situation with respect to distribution and excretion would pertain in
humans. Examination of urinary metabolites in these animal studies
demonstrated that EDA is also found in an acetylated conjugate form in
the rat and mouse. There is evidence that this pathway may become
saturated with increasing dose and that alternative metabolic pathways
may be involved at higher doses in the mouse.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A number of the available studies on both the toxicokinetics and
toxicity of EDA have employed the base substance (EDA) and/or the
hydrochloride salt (EDA.2HCl). The latter is used in pharmaceutical
preparations as a solubilizer to increase uptake of theophylline (this
complex being known as aminophylline) and has been used as a
preservative in skin creams (although it is unclear whether or not
this still occurs). In general, the presence of the hydrochloride has
little qualitative effect on the toxicokinetic or systemic toxicity
properties of EDA, particularly following oral dosing, as it is likely
that the hydrochloride salt would be formed anyway in the acidic
environment of the stomach. However, the hydrochloride does seem to
act in a neutralizing capacity to reduce the significant irritancy
potential of EDA. Studies using both forms of EDA are included in this
review.
8.1 Single exposure
Studies in various animal species have shown EDA to be of
moderate acute toxicity by the inhalation (rat 8-h LC50 estimated to
be in the range of 4916-9832 mg/m3 [1966-3933 ppm]), oral (rat LD50
values of 1160-3250 mg/kg body weight), and dermal (rabbit LD50
values of 550-2880 mg/kg body weight) routes of exposure (Smyth et
al., 1941, 1951; Boyd & Seymour, 1946; Carpenter et al., 1948; NTP,
1982a,b; Yang et al., 1983; Dubinina et al., 1997). Few details exist
of the toxic signs observed or of target organs.
8.2 Irritation and sensitization
There are a number of reports available on skin irritation in
animals, but in general they all repeat the information from one
original study (Smyth et al., 1951). In that study, 0.01 ml undiluted
EDA applied to the shaved backs of albino rabbits produced skin
necrosis within 24 h. A recent report has also indicated EDA to be a
skin irritant (Dubinina et al., 1997). Although no further information
is available, such a response is consistent with EDA being strongly
alkaline. Studies using EDA.2HCl have also resulted in skin
irritation, although the neutralizing action of the hydrochloride may
have influenced the severity of effects, particularly on dilution
(Yang et al., 1983, 1987).
As with skin irritation, the reports that are available for eye
irritation all largely reproduce data from one original study
(Carpenter & Smyth, 1946). In this study, 0.005-ml solutions of 5% EDA
or greater caused corneal injury, which again would be expected, given
the alkaline properties of the substance. More recently, Dubinina et
al. (1997) stated that inflammatory responses in the rabbit eye were
induced by "one drop" of EDA.
Overall, from the reports that are available, together with a
consideration of its alkaline properties, it is reasonable to conclude
that EDA is corrosive, with the capacity to produce severe chemical
burns to the skin and eye.
EDA has been demonstrated to possess skin sensitizing potential
in guinea-pig studies, generally using standard methodologies such as
the Magnusson and Kligman maximization and Buehler tests
(Thorgeirsson, 1978; Erikson, 1979; Maurer et al., 1979; Henck et al.,
1980; Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Dubinina et al., 1997; Leung & Auletta, 1997). In four of these
studies (Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Leung & Auletta, 1997), the investigators ensured that
non-irritant challenge concentrations of EDA were used, providing
clear evidence for a sensitization response. EDA also produced
positive results in the local lymph node assay (Basketter & Scholes,
1992). In contrast to these positive results, EDA consistently
produced negative results in the mouse ear swelling test (Gad et al.,
1986; Cornacoff et al., 1988; Dunn et al., 1990). One study
demonstrated the potential for EDA to cross-react with other
alkylamines either as the inducing or as the challenge agent (Leung &
Auletta, 1997).
No studies are available on respiratory sensitization in animals.
8.3 Short-term exposure
In a 12-day study (NTP, 1982b), mice received gavage doses of
between 50 and 600 mg EDA/kg body weight per day (administered as
EDA.2HCl). Deaths were observed at 400 and 600 mg EDA/kg body weight
per day. No effects were seen at 50 mg EDA/kg body weight per day.
Renal effects (nephrosis and tubule regeneration) were observed at 100
mg EDA/kg body weight per day and above. Lymphoid depletion in and
necrosis of splenic follicles were observed at 400 mg EDA/kg body
weight per day.
A 7 h/day, 30-day inhalation study in rats indicated that the
liver and kidney are potential target tissues, with local effects in
the lungs also likely (Pozzanni & Carpenter, 1954). No effects were
observed in this study at an airborne exposure concentration of about
150 mg/m3 (60 ppm). Slight depilatory effects were seen at 330 mg/m3
(132 ppm), becoming more marked at higher exposure concentrations.
Treatment-related deaths were observed at 563 mg/m3 (225 ppm) and
1210 mg/m3 (484 ppm) (all animals died at 1210 mg/m3 [484 ppm]).
Cloudy swelling of cells in the liver and convoluted tubules of the
kidneys were also observed at these exposure concentrations.
Degeneration of the convoluted tubules was seen in animals exposed to
1210 mg/m3 (484 ppm), as was congestion of the lungs and adrenals.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Dietary studies in rats have also indicated that the liver is a
target tissue, with changes in the size and shape of hepatocytes and
their nuclei being noted at 1000 mg/kg body weight per day in a 90-day
study (Yang et al., 1983).
Oral gavage studies using doses of between 100 and 1600 mg EDA/kg
body weight per day (administered as EDA.2HCl) have been carried out
in rats (NTP, 1982a). Deaths were observed after 12 doses at 800 and
1600 mg EDA/kg body weight per day and after 800 mg EDA/kg body weight
per day for 90 days. Renal tubular lesions (dilation of the lumen,
necrosis, degeneration and regeneration of the epithelium) were seen
at 200 mg EDA/kg body weight per day and above after 12 doses. Similar
renal lesions but of a less severe nature were seen only at 600 mg
EDA/kg body weight per day and above after 90 days. This indicates
recovery in the kidney, probably as a consequence of compensatory
regeneration. No effects were seen on the kidney at 100 mg EDA/kg body
weight per day in either study. Ocular effects including cataract
formation and retinal atrophy were observed in all dose groups.
Minimal to moderate focal retinal atrophy was observed in 3 out of 10
females at 100 mg EDA/kg body weight per day; 2 males had mild to
moderate retinal atrophy and 1 male had severe retinal atrophy at 200
mg EDA/kg body weight per day. Lymphoid depletion and/or necrosis in
spleen were observed at 800 mg EDA/kg body weight per day and in all
decedents following 12 doses, and thymus weight was reduced at 800 mg
EDA/kg body weight per day in the 90-day study. Uterine lesions
(reduced uterine horn size and atrophy of the myometrium and
endometrium) were seen after dosing for 90 days with 600 or 800 mg
EDA/kg body weight per day, and reduced ovarian size was seen after
800 mg EDA/kg body weight per day for 90 days. Overall, a
no-observed-adverse-effect level (NOAEL) was not identified from these
studies, as effects on the eyes were seen at all dose levels. Only
ocular effects were seen at the lowest-observed-adverse-effect level
(LOAEL) of 100 mg EDA/kg body weight per day, and these were of a
minimal to mild nature, suggesting that this dose represented the
lower end of the dose-response relationship for these effects.
In a 90-day study, mice received oral gavage doses of 25-400 mg
EDA/kg body weight per day (NTP, 1982b). No effects were seen at 100
mg EDA/kg body weight per day. Renal lesions (cortical tubular
degeneration and/or necrosis) were observed at 200 and 400 mg EDA/kg
body weight per day.
8.4.2 Chronic exposure and carcinogenicity
There are two carcinogenicity studies in animals. Both studies
were performed to reasonably adequate standards, including extensive
histopathology, and were negative for carcinogenic activity.
In the first study, groups of 99-225 F344 rats were orally dosed
with 0, 20, 100, or 350 mg EDA.2HCl (equivalent to 0, 9, 45, or 158
mg EDA/kg body weight per day) for 2 years (Yang et al., 1984a).
Non-neoplastic effects were similar to those described in studies of
shorter duration (Yang et al., 1993), as indicated above (section
8.4.1). Effects were seen at 45 mg EDA/kg body weight per day, with a
NOAEL of 9 mg EDA/kg body weight per day. Tracheitis was also
observed, probably as a consequence of exposure to EDA in airborne
dust derived from the diet.
In the second study, groups of 40-50 C3H/HeJ mice were dermally
administered 0 or 0.25 mg aqueous EDA 3 times per week for a lifetime
(DePass et al., 1984). The dermal study included a positive control
group that received 3-methylcholanthrene. Skin fibrosis and
hyper-keratosis were observed in EDA-treated mice.
8.5 Genotoxicity and related end-points
Only limited information is available on the genotoxic potential
of EDA. There is some evidence that EDA may be mutagenic in bacteria
with and without metabolic activation (Hedenstedt, 1978; Hulla et al.,
1981; Haworth et al., 1983; Leung, 1994). Although the most recent
study (Leung, 1994) appears to be negative, there was a small response
in Salmonella typhimurium TA100 and a positive, but not
reproducible, response in TA1535. Positive results have also been
reported in these strains from the other studies, although only one of
these (Haworth et al., 1983) was adequately reported. The only series
of studies performed on mammalian cell systems in vitro (gene
mutation and sister chromatid exchange in Chinese hamster ovary cells;
unscheduled DNA synthesis in rat primary hepatocytes) were
consistently negative (Slesinski et al., 1983), although there has
been no assay for clastogenic activity. A sex-linked recessive lethal
test in Drosophila melanogaster was negative following dosing by
feeding or injection (Zimmering et al., 1985). There are no in vivo
studies on somatic cells, but a dominant lethal study in rats up to
doses inducing signs of toxicity (up to 500 mg EDA.2HCl/kg body
weight per day in the diet) was negative (Slesinski et al., 1983).
Although there has been some evidence of mutagenicity in
bacterial systems in a few limited studies, the available evidence
indicates that EDA is not genotoxic, with all results in mammalian
cells in vitro and in vivo (dominant lethal assay) being negative.
It should be noted that the overall database is limited, with no
assays available for clastogenic activity or for genotoxic potential
in somatic cells in vivo.
8.6 Reproductive and developmental toxicity
The potential of EDA to affect fertility and development has been
studied in rats in investigations conducted to modern regulatory
standards. In a two-generation study in F344 rats, no effects on
fertility or development in any of the generations were observed up to
a dose level (225 mg EDA/kg body weight per day) that induced signs of
parental toxicity (Yang et al., 1984b). Dose levels used in this study
were 0, 50, 150, or 500 mg EDA.2HCl/kg body weight per day (equivalent
to 0, 23, 68, and 225 mg EDA/kg body weight per day). Effects on the
uterus and ovaries have been seen following gavage dosing of rats with
600 and 800 mg EDA/kg body weight per day for 90 days (see section
8.4.1; NTP, 1982a). In a series of developmental toxicity studies in
F344 rats, EDA was found to produce signs of fetotoxicity (increased
resorptions) and delays in development at high dose levels (450 mg
EDA/kg body weight per day) that induced clear signs of toxicity in
the dams (DePass et al., 1987). Dose levels used in this study were 0,
50, 250, or 1000 mg EDA.2HCl/kg body weight per day (equivalent to 0,
23, 113, and 450 mg EDA/kg body weight per day). Some of the
developmental effects appear to have been related, at least in part,
to the reduced nutritional status of the animals. However, a clear
NOAEL for developmental toxicity of 113 mg EDA/kg body weight per day
was observed in these studies.
The results of a preliminary screening study in mice indicated no
significant effects on development in the offspring of dams exposed to
toxic doses of EDA (400 mg/kg body weight per day) by oral gavage
(Hardin et al., 1987).
No effects on development were seen in the offspring of New
Zealand white rabbits dosed during pregnancy with up to 178 mg
EDA.2HCl/kg body weight per day (equivalent to 80 mg EDA/kg body
weight per day), a dose that did not induce maternal toxicity (NTP,
1991; Price et al., 1993). In a preliminary study, 2/20 pregnant
rabbits receiving 100 mg EDA/kg body weight per day by gavage died,
and decreased body weight was seen in survivors. At 400 mg/kg body
weight per day, all the dams died.
8.7 Immunological and neurological effects
No studies are available that have specifically investigated the
potential immunotoxicity of EDA. Effects on lymphoid tissue in the
spleen in mice and rats (see sections 8.3 and 8.4.1, respectively) and
on the thymus in rats (see section 8.4.1) were observed in oral gavage
dosing studies.
There are a few, mainly in vitro, studies on the effects of EDA
on the release of gamma-aminobutyric acid from the retina, gut, and
brain (Perkins & Stone, 1980; Forster et al., 1981; Lloyd et al.,
1982; Morgan & Stone, 1982; Sarthy, 1983; Kerr & Ong, 1984; Strain et
al., 1984; Hill, 1985; Erdo et al., 1986; Krantis et al., 1990; McKay
& Krantis, 1991). The general conclusion that can be drawn from these
studies is that EDA can cause a calcium-independent release of
gamma-minobutyric acid that is insensitive to the presence of
tetrodotoxin. EDA was also shown to have gamma-minobutyric acid
mimetic properties (i.e., reduction of neuronal firing rate). This
suggests that EDA could have a central nervous system depressant
effect, but studies were not performed to address this possibility. It
was reported that EDA elicited contraction of the guinea-pig ileum
that was mediated via neuronal release of gamma-aminobutyric acid.
However, in the rat ileum, EDA acted directly on the mucosa, resulting
in relaxation. Although these are interesting results, the
toxicological significance of these findings is unclear; they may,
however, partly explain the central nervous system depressant and
gastrointestinal effects seen in some of the animal studies at high
doses.
9. EFFECTS ON HUMANS
No studies are available in which the effects, other than the
respiratory effects summarized below, of repeated exposure of humans
to EDA are examined. No reports have been found in which genotoxicity,
carcinogenicity, or reproductive toxicity following exposure to EDA in
human populations has been studied.
A case report exists concerning a 36-year-old worker who died
from cardiac collapse 55 h after being splashed by an accidental
spillage of EDA (Niveau & Painchaux, 1973). Exposure to an
unquantified amount of EDA was in the order of a few minutes prior to
the patient being washed. Four hours after the exposure, he presented
with tachycardia (100 beats/min), anuria, and red/brown generalized
erythema. The tachycardia increased (up to 140 beats/min), anuria
persisted, and an expectorant cough, abdominal cramps, diarrhoea, and
blackish vomiting appeared. The patient became hyperkalaemic, and his
red blood cell count decreased. Overall, given the lack of information
with respect to levels of exposure, few useful conclusions can be
drawn from this case report.
The only information available on skin irritation in humans
either is anecdotal or does not involve direct surface skin contact
with EDA. In a brief report on the physicochemical properties of EDA,
it is noted anecdotally that "the liquid, if not washed from the skin,
causes blistering" (Boas-Traube et al., 1948). The other report
available documents the results of intradermal skin tests with
solutions (0.1-1%) of EDA on three individuals being tested for
hypersensitivity following treatment with aminophylline (Kradjan &
Lakshminarayan, 1981). The skin response in two patients consisted of
blistering rather than a weal and flare reaction, which normally
typifies a sensitization response. Punch biopsies were obtained from
one patient, and histopathological examination of these indicated
tissue necrosis and oedema of the epidermis and dermis. These
responses suggested a direct corrosive effect of EDA.
No specific reports were found of the effects of EDA on the eyes
of humans. In the original reports of the animal eye irritation study
(see section 8.3), it is claimed that EDA is known to have produced
loss of vision or slowly healing corneal burns in industrial use
(Carpenter & Smyth, 1946). However, no further information or
references were given.
EDA has been known for many years to be capable of inducing
allergic skin reactions in humans. This has been observed both in the
workplace and, most notably, in patients treated with aminophylline or
with skin creams in which EDA was used as a stabilizer (Epstein &
Maibach, 1968; Petrozzi & Shore, 1976; Booth et al., 1979; Wall, 1982;
Hardy et al., 1983; Balato et al., 1984; Edman & Moller, 1986; Nielsen
& Jorgensen, 1987; Terzian & Simon, 1992; Toal et al., 1992; Dias et
al., 1995; Simon et al., 1995; Sasseville & Al-Khenaizan, 1997). The
first reports of skin sensitizing effects in humans date back to the
late 1950s, when cases were described of eczematous reactions in
pharmacists who came into contact with EDA when using aminophylline
(Baer et al., 1958; Tas & Weissberg, 1958).
Subsequent to these early reports, numerous studies and case
reports have been published documenting the skin sensitizing
properties of EDA both following clinical use and within the
occupational setting, such that the substance has become incorporated
into standard series for patch testing (Fregert, 1981; Shehade et al.,
1991). An example from the clinical setting is that of the report on a
series of 13 patients who had used skin cream containing EDA for,
paradoxically, dermatitic conditions (Provost & Jillson, 1967). Use of
the cream in 11 of these patients had resulted in the sudden
appearance of a severe generalized patchy eczematous eruption
following, in all but one of the cases, an initial improvement upon
using the cream. Patch testing was conducted using a 1% aqueous
solution of EDA, producing skin reactions in all patients ranging from
erythema and oedema to erythema vesiculation and oedema vesiculation,
which extended beyond the patch test site. Other substances tested
also induced responses, but not in a consistent manner, with at most
only four individuals responding in any one test.
As well as the original reports in pharmacists working with EDA,
cases of skin sensitization to EDA have been reported in the
occupational environment in a number of different settings, including
use of floor polish remover (English & Rycroft, 1989), use of coolant
oils (Crow et al., 1978), and in wire-drawing (Matthieu et al., 1993;
Sasseville & Al-Khenaizan, 1997). Positive responses to patch testing
with EDA have also been observed in other occupational settings, such
as the offshore oil industry (Ormerod et al., 1989), but positive
responses to other substances, including other polyamines, were also
seen in such cases. Thus, it is unclear whether EDA had been
responsible for inducing the sensitized state and/or cross-reacting
following sensitization to another polyamine.
A large number of cases of occupational asthma reported to have
been caused by exposure to EDA are available in the literature
(Dernehl, 1951; Gelfand, 1963; Popa et al., 1969; Valeyeva et al.,
1975; Lam & Chan-Yeung, 1980; Chan-Yeung, 1982; Hagmar et al., 1982;
Matsui et al., 1986; Aldrich et al., 1987; Nakazawa & Matsui, 1990;
Lewinsohn & Ott, 1991; Ng et al., 1991, 1995). There are a few studies
in which the potential for EDA to cause respiratory hypersensitivity
has been examined using bronchial provocation testing and
investigation of antibody formation. As EDA is corrosive, the vapour
would be predicted to be a respiratory tract irritant, which is a
complicating factor in interpreting the data available and in
elucidating the underlying mechanism for any asthmatic responses seen.
Popa et al. (1969), in a well-conducted study, investigated 48
subjects with asthmatic symptoms caused by exposure to a number of low
molecular weight chemicals, including EDA. None of the subjects had a
history of respiratory disorder prior to occupational exposure, and
the asthmatic response was associated only with occupational exposure
in all cases. No information was given in the report on the workplace
airborne concentrations of EDA to which these workers were exposed. A
series of tests were performed in all subjects, including skin and
inhalation tests with the test agent at sub-irritant concentrations;
skin and inhalation tests to common allergens; skin tests
(intradermal, scratch, and patch tests) using sub-irritant
concentrations of the test substance; Prausnitz-Kustner transfer
reaction (to test for the presence of immunoglobulin E antibodies);
and determination of precipitating antibodies to EDA. For the
inhalation test, the sub-irritant concentration was determined in
control asthmatic subjects, and a 2- to 10-fold dilution of this was
used for the bronchial challenge. No information was given on the
airborne exposure concentrations generated under these test
conditions. Control inhalation tests with the diluent, physiological
saline, were also conducted. It is not stated in the report whether or
not the inhalation challenge tests were conducted in a blind manner.
Six subjects had an immediate, positive reaction to EDA in the
workplace. Of these, four showed an immediate, positive response
following inhalation testing with sub-irritant concentrations of EDA.
These subjects developed marked bronchoconstriction following
inhalation exposure to EDA, with a reduction in forced expiratory
volume in 1 s (FEV1) of 62% and an increase in respiratory resistance
of 44%, compared with controls. Although not stated in the report,
these values are presumed to be average changes. Intradermal skin
tests with EDA were positive in these four subjects, whereas patch
tests were negative. Inhalation challenges with common allergens were
negative. The Prausnitz-Kustner test was positive in all subjects, and
all had eosinophilia, determined in the sputum, although not, except
in one case, in the blood. No precipitating antibodies were found. In
the two other subjects, the inhalation challenge test was negative. No
precipitating antibodies were found, and the Prausnitz-Kustner test
was negative in both subjects. Eosinophilia was absent. Inhalation
challenges with other common allergens were also negative.
These data provide evidence that EDA may elicit an asthmatic
response at sub-irritant concentrations and that the response is
specific to EDA. Four out of six subjects responsive to EDA in the
workplace also had a positive response to inhaled EDA at sub-irritant
concentrations. This demonstrates that the reaction is not a
generalized response to an irritant. The positive Prausnitz-Kustner
reaction may be indicative of an immunological component, but the test
is not specific, and no firm conclusions can be drawn from it. It
provides supporting evidence in this case. The evidence suggests that
the subjects were hypersensitive to inhaled EDA and that a state of
respiratory hypersensitivity had been induced by the substance.
Although a number of other studies are available, the information
is of poor quality. Lam & Chan-Yeung (1980) and Chan-Yeung (1982)
describe the case of one worker in a photographic laboratory who
developed asthma after 2.5 years of exposure to a variety of
chemicals, including EDA, but also other irritant substances. The
worker developed symptoms of sneezing, nasal discharge, productive
cough and nocturnal cough, wheezing, and dyspnoea. The symptoms
coincided with the work shift and subsided at weekends. There was no
previous history of asthma. No information was given in the report of
the airborne concentrations of EDA (or other substances) to which the
man was exposed at work. A series of controlled inhalation challenge
exposures, designed to mimic work exposure conditions, were conducted
with each of the chemicals to which the subject was exposed at work.
The duration of exposure was determined by the patient's tolerance,
and exposure was terminated when eye irritation or cough was
experienced. No information on the airborne exposure concentrations of
EDA generated under these test conditions was given in the report. A
methacholine inhalation test for bronchial hyperreactivity was also
performed. Pulmonary function tests were conducted pre- and
post-challenge, and blood samples were taken before, during, and after
each challenge. The subject showed marked bronchial reactivity to
methacholine.
Exposure to an unknown concentration of vapour from a 1:25
solution of EDA was tolerated for 15 min. This exposure produced a
marked bronchoconstriction. A late asthmatic response developed 4 h
after the exposure, at which time FEV1 was reduced by 26% and
continued to decrease over the next 3 h towards a 40% reduction. A 26%
reduction was still apparent after 24 h, despite treatment with
bronchodilator drugs. This pattern of response to EDA was
reproducible. The patient did not respond similarly to any of the
other chemicals tested: formaldehyde, sulfur dioxide, and two colour
developing agents that were stated to be irritants. Exposure to
formaldehyde (vapour from a 1:4 solution) produced an immediate small
(<20%), transient reduction in FEV1, whereas exposure to sulfur
dioxide caused coughing and chest tightness and an immediate transient
reduction of 25% in FEV1. There was no increase in plasma histamine
concentration during the period of bronchoconstriction, although EDA
was shown to cause in vitro histamine release from whole blood taken
from the patient and from two control subjects. A skin test using
1:100 EDA and a precipitin test for antibodies to EDA were both
negative. The patient subsequently had to give up work because of
respiratory symptoms and became asymptomatic after 2 weeks. Subsequent
testing with methacholine, 2.4 months after ceasing work, showed that
the subject had a reduction in the previous bronchial hyperreactivity.
In conclusion, the subject showed an asthmatic response to EDA
but not to formaldehyde or the colour developing agents. The pattern
of response to sulfur dioxide was more immediate and suggestive of an
irritation response. Overall, a clear pattern of asthmatic response
that was specific to EDA was observed in this study. However, it is
not possible to distinguish with certainty between an irritant
response and a sensitization response, because it is possible that an
irritant concentration was used for the bronchial challenge exposure,
although little immediate response was observed. In addition, although
exposure to irritant concentrations of the other workplace chemicals
did not elicit the same pattern, magnitude, or severity of response as
that seen with EDA, since accurate exposure levels were not given, it
is not possible to determine whether or not the EDA concentration used
had the greatest irritant potential. No evidence for any immunological
involvement was found. In conclusion, this study provides only
circumstantial evidence that EDA caused a state of respiratory
hypersensitivity in this subject.
A number of other case reports are available of individuals who
exhibited asthmatic signs and symptoms associated with exposure to EDA
in the workplace (Gelfand, 1963; Valeyeva et al., 1975; Matsui et al.,
1986; Nakazawa & Matsui, 1990; Ng et al., 1991). Although bronchial
challenge testing with EDA produced asthmatic responses in these
subjects, they had personal and/or family histories of allergic
disease and/or they had worked with and responded on challenge to
other substances. Retrospective studies using the medical records of
populations of workers using EDA have indicated that about 10% of such
populations developed signs and symptoms of occupational asthma
(Aldrich et al., 1987; Lewinsohn & Ott, 1991). No challenge tests were
carried out with these surveys. Thus, these case reports and
population-based studies provide only supporting circumstantial
evidence for the involvement of EDA in producing occupational asthma.
Although it is clear from these reports that EDA can provoke an
asthma attack, in many cases there is insufficient information to
indicate whether or not the hypersensitive state was induced
specifically by EDA. However, from one well-conducted study, there is
evidence that a hypersensitive state specific to EDA has been induced
in workers and that an asthmatic response was provoked by sub-irritant
concentrations of the substance. Overall, the results of this study,
taken together with the supporting data from a substantial number of
other reports of occupational asthma, indicate that EDA is capable of
inducing a state of hypersensitivity in the airways, such that
subsequent exposure may trigger asthma. The mechanism by which the
hypersensitive state is induced is not proven. Given the skin
sensitizing potential of EDA and the limited evidence of immunological
involvement in workers with EDA-provoked occupational asthma, an
immunological mechanism would seem plausible. Irrespective of the
mechanism involved, the data available (specifically the lack of
information on airborne exposure concentrations under both work and
challenge test conditions) do not allow elucidation of a dose-response
relationship or the identification of levels of EDA that are not
capable of inducing a hypersensitive state or of provoking an
asthmatic response.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Results of acute ecotoxicity tests are given in Table 2.
A 21-day Daphnia magna reproduction test was conducted
according to the German Federal Environment Agency (Umweltbundesamt)
guidelines in a closed vessel. End-points measured included adult
mortality, onset of production of young, and reproduction rate. The
most sensitive end-point was for reproductive rate, and a NOEC of 0.16
mg/litre was established (Kuhn et al., 1989). In a second study
conducted according to Organisation for Economic Co-operation and
Development (OECD) guidelines, a NOEC for reproduction was reported at
2 mg/litre (Mark & Hantink-de Rooy, 1992). An early life stage test
conducted under OECD guidelines on three-spined stickleback
( Gasterosteus aculeatus) showed no effects of EDA at 10 mg/litre
(the highest concentration tested) over 28 days (Mark & Arends, 1992).
Growth of lettuce ( Lactuca sativa) plants over 7 days was
studied in tests conducted under OECD Guideline 208; EC50
concentrations for EDA in soil (nominal) were >1000 mg/kg for 7-day
and 692 mg/kg for 14-day growth periods (Hulzebos et al., 1993).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
EDA is of moderate acute toxicity by all routes. In studies in
animals, EDA is a primary skin and eye irritant, being corrosive when
undiluted. It is also a skin sensitizer. In repeated-dose toxicity
studies by the oral and inhalation routes, effects on the liver and
kidney have been observed, with pleomorphic changes to hepatocytes in
rats being reported at the lowest oral doses used (45 mg/kg body
weight per day and above for 2 years; NOAEL, 9 mg/kg body weight per
day). In inhalation studies, there were no effects at 150 mg/m3 (60
ppm), although slight depilation was observed at the next highest
concentration (330 mg/m3 [132 ppm]) and effects on the liver and
kidney at higher concentrations still (approximately 500 mg/m3 [200
ppm] and above).
There has been some evidence of mutagenicity in bacterial systems
in a few limited studies. However, much of the available evidence is
negative, although the overall database is limited, there being no
assays for clastogenic activity or for genotoxic potential in somatic
cells in vivo. EDA was not carcinogenic in adequate studies in
animals.
In humans, EDA has the potential to induce respiratory tract
hypersensitivity, and provocation of asthma is the major health effect
of concern in the occupational environment. The mechanism of induction
of the hypersensitive state is unknown, although the skin sensitizing
potential of EDA and limited evidence in workers with EDA-provoked
asthma are suggestive of an immunological mechanism. However,
irrespective of the mechanism involved, the available data
(particularly lack of information on exposure conditions either in the
workplace or on bronchial challenge testing) do not allow either
elucidation of dose-response relationships or identification of the
thresholds for induction of the hypersensitive state or provocation of
an asthmatic response.
11.1.2 Criteria for setting guidance values for EDA
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship for provocation of
an asthmatic response, the effect of greatest concern in the
occupational environment. As it is not possible to identify a level of
exposure that is without adverse effect, it is recommended that levels
be reduced to the extent possible.
Table 2: Acute toxicity of EDA to organisms other than laboratory mammals.
Species End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Microcystis aeruginosa Toxic threshold for cell multiplication 0.08 Bringmann & Kuhn, 1976
Pseudomonas putida 17-h EC50 (growth rate) 29 (23-35) van Ginkel, 1989
Nitrifying bacteria 3-h EC50 (respiration) 3.2 (0.6-5.7) Balk & Meuwsen, 1989a
NOEC 0.5
Activated sludge bacteria 1-h EC50 (respiration rate) 1600 van Ginkel & Stroo, 1989
Algae
Scenedesmus quadricauda Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Chlorella pyrenoidosa 96-h EC50 (growth) 100 van Leeuwen, 1986
Selenastrum capricornutum 72-h EC50 (biomass) 71 van Ginkel et al., 1990
72-h EC50 (growth rate) 645
NOEC approx. 3.2
96-h EC50 (growth rate) 151 van Wijk et al., 1994
Scenedesmus subspicatus 48-h EC50 (biomass and growth rate) >100 Kuhn & Pattard, 1990
Protozoa
Entosiphon sulcatum Toxic threshold for cell multiplication 1.8 Bringmann & Kuhn, 1980a
Uronemia parduczi Toxic threshold for cell multiplication 52 Bringmann & Kuhn, 1980b
Chilomonas paramaecium Toxic threshold for cell multiplication 103 Bringmann & Kuhn, 1980b
Invertebrates
Daphnia magna 48-h LC50 26.5 van Leeuwen, 1986
48-h LC50 46 van Wijk et al., 1994
48-h LC50 16.7 Balk & Meuwsen, 1989b
24-h LC50 14 Kuhn et al., 1989
Table 2 (continued)
Species End-point Concentration Reference
(mg/litre)
Brine shrimp (Artemia
salina) 24-h LC50 14 Price et al., 1974
Fish
Brown trout (Salmo trutta) 48-h LC50 230 Woodiwiss & Fretwell, 1974
Fathead minnow (Pimephales
promelas) 96-h LC50 115.7 Curtis & Ward, 1981
Guppy (Poecilia reticulata) 96-h LC50 275 van Leeuwen, 1986
96-h LC50 640 Balk & Meuwsen, 1989c
96-h LC50 1545 van Wijk et al., 1994
Medaka (Oryzias latipes) 48-h LC50 1000 Tonogai et al., 1982
With respect to systemic effects, the lowest NOAELs of 150 mg/m3
(60 ppm) (inhalation) and 9 mg/kg body weight per day (oral) can serve
as a basis for comparison with estimated exposure for characterization
of risk, either with application of appropriate uncertainty factors or
directly. An example of the latter (margin of exposure) approach is
presented in section 11.1.3.
11.1.3 Sample risk characterization
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship, and hence risk,
for provocation of an asthmatic response, the effect of greatest
concern in the occupational environment. For substances that are
asthmagens, it is also advisable to restrict peak exposures, as they
may have a role in the induction and triggering of asthmatic
phenomena. However, to help assess the risk to human health arising
from occupational exposures, a comparison is made with the NOAELs for
systemic effects from animal studies.
It should also be noted that because diluted EDA is a skin
irritant and sensitizer, there is a risk of developing irritant and/or
allergic dermatitis if suitable personal protective equipment is not
used.
A sample risk characterization for systemic effects in the
occupational environment in the United Kingdom is provided here.
Measured data on exposure to EDA (generally used in closed systems)
indicate that levels in industry in the United Kingdom are less than
1.25 mg/m3 (0.5 ppm), 8-h time-weighted average. Modelled data (EASE)
are in good agreement, predicting comparable values of 0.53-1.3 mg/m3
(0.21-0.52 ppm). Short-term peak exposures (sampling and hose
uncoupling operations) were predicted to be 16.8-33.3 mg/m3
(6.7-13.3 ppm), 15-min time-weighted average. The EASE model predicts
dermal exposures in the range of 0-0.15 mg/cm2 per day for an
operator transferring EDA into closed systems once a day (although
coveralls and well-maintained plastic gloves will significantly reduce
exposure from this source).
With respect to systemic effects, worst-case estimated and
measured exposures of 1.25 mg/m3 (0.5 ppm), 8-h time-weighted
average, are substantially less (by 100-fold or greater) than the
NOAEL in the inhalation study in rats. The combined body burden from
inhalation and dermal exposure for chemical synthesis can be estimated
to be about 0.3 mg/kg body weight per day (assuming a 70-kg worker
breathing 10 m3 of air containing 1.25 mg EDA/m3 [0.5 ppm] per day,
with 100% absorption; and 10% absorption from unprotected, undamaged
skin for a standard hand area of 840 cm2). This is 30-fold less than
the NOAEL for hepatic effects in the oral studies.
Available data on indirect exposure in the general environment or
from consumer products are inadequate to serve as a basis for a sample
characterization of risk for these scenarios.
11.2 Evaluation of environmental effects
No atmospheric effects are expected, as reaction with hydroxyl
radicals is likely to be rapid, and washout of volatilized EDA is
expected. Volatilization to the atmosphere is likely from soil but not
from water.
Adsorption to soil particulates is strong through electrostatic
binding; leaching through soil profiles to groundwater is not
expected. EDA is readily biodegradable, and this is the most likely
source of breakdown in the environment; adaptation of microorganisms
may improve degradation. Breakdown is less rapid in seawater than in
fresh water. Bioaccumulation is unlikely.
Toxic thresholds for microorganisms may be as low as 0.1
mg/litre. However, toxicity tests in culture media should be treated
with caution, as the EDA may complex with metal ions. Effects may
therefore be indirect, from loss of bioavailability of essential
elements. The "toxic thresholds" reported are lowest-observed-effect
concentrations (LOECs) for small changes in sublethal end-points; the
exact degree of effect at the reported concentrations is not always
clear, and these have not been used in the risk assessment.
The principal receiving compartment in the environment is the
hydrosphere, and this is the only compartment for which a quantitative
risk assessment can be attempted.
The distribution of acute (from Table 2) and chronic test results
is plotted in Figure 1. Chronic test results are available for fish (a
limit test only) and Daphnia (NOECs from 21-day reproduction tests).
Chronic EC50s for algal growth or biomass are also available, but no
NOEC was reported for these studies. Given the range of chronic test
results across three trophic levels, it is proposed that an
uncertainty factor of 10 be applied to the lowest reported NOEC (for
Daphnia reproduction at 0.16 mg/litre) to derive an estimated PNEC
for aquatic organisms of 0.016 mg/litre. This is in accord with OECD,
European Union, and US Environmental Protection Agency guidelines.
There are no reported test results for estuarine/marine organisms; it
is assumed that toxicity would be in the same range for these species.
No measured concentrations of EDA in surface waters have been
reported. A single quantitative risk assessment has been reported (van
Wijk, 1992) based on discharges into the Ems-Dollard estuary in the
Netherlands. The figure of 75 kg/day for release of EDA at this site
has been used here as a representative estimate of release; no
information on releases from industrial plants elsewhere is available.
EDA is a colourless to yellowish hygroscopic liquid with an
ammonia-like odour. Its molecular weight is 60.12. It is a strongly
alkaline (pH of 25% EDA in water is 11.9), very volatile, pungent
material, which fumes profusely in air. It has a melting point of
about 8.5°C, a boiling point of 116°C (at 101.3 kPa), and a vapour
pressure of 1.7 kPa at 25°C. EDA is miscible with water and alcohol.
The log octanol/water partition coefficient (log Kow) ranges from
-1.2 to -1.52. p Ka1 and p Ka2 (calculated) are 10.71 and 7.56,
respectively, indicating protonation at environmentally relevant pH.
Additional physical/chemical properties are presented in the
International Chemical Safety Card reproduced in this document.
Conversion factors for EDA at 20°C and 101.3 kPa are as follows:
1 ppm = 2.50 mg/m3
1 mg/m3 = 0.40 ppm
3. ANALYTICAL METHODS
For monitoring concentrations of EDA in workplace air, NIOSH
(1984-1989) uses a method that employs adsorption on silica gel and
analysis by gas chromatography with flame ionization detection. A
solvent-free sampling system is preferable because of more convenient
handling, and it is a great advantage if derivatization can be
achieved directly on the absorbent. The Health and Safety Laboratories
of the United Kingdom's Health and Safety Executive have evaluated a
published method (Andersson et al., 1985; Levin et al., 1989; Patel &
Rimmer, 1996). Air is sampled onto
1-naphthyl-isothiocyanate-impregnated filters, desorbed by
acetonitrile, and analysed by high-performance liquid chromatography
with ultraviolet detection. The method has a working range between 2.5
and 50 mg/m3 for a 5-litre air sample. The detection limit was found
to be 0.08 mg/m3. The method generally meets the Comité Européen de
Normalisation requirements on the overall uncertainty. Although the
Comité Européen de Normalisation requirements for desorption
efficiency were not satisfied at 25 and 50 mg/m3, a smaller sample
can be taken if necessary.
There are no reported methods for the biological monitoring of
occupational exposure to EDA. However, analytical techniques based on
solvent extraction of EDA and high-performance liquid chromatography
have been reported and used in pharmacological studies (Cotgreave &
Caldwell, 1983c), and these might form the basis for biological
monitoring methods.
EDA can be measured in water using reverse-phase high-performance
liquid chromatography with ultraviolet detection at 315 nm, following
derivatization with acetylacetone. The limit of detection was reported
to be 0.26 µg/litre (Nishikawa, 1987).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
EDA is not known to occur naturally. The main use for EDA is as
an intermediate in the manufacture of tetraacetyl ethylenediamine,
EDTA, organic flocculants, urea resins, and fatty bisamides. It is
also used, to a much smaller extent, in the production of formulations
for use in the printed circuit board and metal finishing industries,
as an accelerator/curing agent in epoxy coatings/resins, and in the
manufacture of pharmaceutical products. EDA is also present as a
contaminant (<0.5%) in commercially supplied fatty amines, which are
used as wetting agents in bituminous emulsions. It is also used in the
synthesis of carbamate fungicides, in surfactant and dye manufacture,
and in photography development chemicals and cutting oils. These are
believed to be minor uses in the United Kingdom and were not
investigated in this review. EDA is a degradation product of
ethylenebis(dithiocarbamate) fungicides.
Approximately 11 000 tonnes of EDA are imported into the United
Kingdom each year, with very little being re-exported (Brooke et al.,
1997). World production amounts to 100 000-500 000 tonnes annually.1
In 1992, annual production capacities were 18 000 tonnes for Germany,
54 000 tonnes for the Netherlands, 30 000 tonnes for Belgium, 25 000
tonnes for Sweden, about 159 000 tonnes for the United States, and
15 000 tonnes for Japan (BUA, 1997).
No measured concentrations of EDA in wastewater streams from
manufacture and use are available. However, estimates of EDA entering
waste treatment from four European manufacturing plants were 200, 287,
5000-10 000, and 1000 kg/year. Use in photochemicals was estimated to
lead to 1.1 tonnes being introduced into municipal sewage treatment
plants in Germany. All figures are for 1992 or 1993 (BUA, 1997).
1 IUCLID (European Union database), 1st ed., 1996.
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Few experimental data are available on the distribution,
transport, or fate of EDA in the environment. However, qualitative,
and some quantitative, estimates have been made on the basis of its
physicochemical properties.
EDA has a moderately high vapour pressure and is expected to
volatilize from soil (HSDB, 1997). In the atmosphere, it should react
rapidly with photochemically produced hydroxyl radicals; no
experimental rates are available for this proposed reaction, but a
half-life of 8.9 h has been calculated.1 EDA may react with carbon
dioxide to form an insoluble carbamate. The high water solubility of
EDA means that volatilized chemical is also likely to be washed out by
rain.2 The calculated dimensionless Henry's law constant (air/water
partition coefficient) is extremely low (7.08 × 10-8); therefore,
little evaporation would be expected from water. A half-life for
volatilization of 45 years was estimated for a model river 1 m
deep.2 An approximate Henry's law constant is given in BUA (1997) as
1.77 × 10-4 Pa.m3/mol.
Photodegradation is not expected, as the molecule contains no
chromophores, which absorb radiation (HSDB, 1997).
Despite their miscibility with water, ethyleneamines can bind
strongly to soil. There was a wide range of determined adsorption
coefficients in experimental studies on six soil types (Table 1). Some
reduction in variability occurred when results were normalized for
organic carbon content, although this was less marked with EDA than
with the other ethyleneamine studied. Sorption to soil was rapid, with
equilibrium occurring within a few hours. Electrostatic interaction
between the positively charged ethyleneamine and negatively charged
soils appeared to be the dominant factor in binding. Complex formation
with metals and humic acids is expected. Sorption is greater to soils
with high cation exchange capacity (Davis, 1993).
EDA at 200 mg/litre was incubated with adapted sewage sludge
until there was no further decrease in chemical oxygen demand (COD);
at that time (unspecified), 97.5% of the chemical had been degraded.
The rate of degradation was 9.8 mg COD/g per hour (Pitter, 1976).
EDA at 3, 7, and 10 mg/litre was incubated with sewage sludge
(adapted and non-adapted), and percent biodegradation was determined
5, 10, 15, and 20 days later. Degradation rates were comparable for
adapted and non-adapted sludge up to 15 days (at 56% and 55%,
respectively); at 20 days, however, the values were 70% and 47%,
respectively. Based on this single point, it is not possible to
conclude definitively that adaptation improves degradation. Nitrate
and nitrite were measured throughout the incubation to correct for
1 IUCLID (European Union database), 1st ed., 1996.
2 Syracuse Research Corporation modelling, summarized in HSDB
(1997).
oxygen demand due to conversion of ammonia or organic nitrogen to
these species. Such a correction was necessary for EDA alone out of
more than 50 compounds tested. Degradation was also tested in a
salt-water system using non-adapted sludge; EDA was degraded less
effectively, with 16% of theoretical degradation after 20 days (Price
et al., 1974). A comparable value at 16.6% was measured in seawater by
Takemoto et al. (1981). EDA incubated with microorganisms isolated
from river water and adapted to the compound over 28 days showed >80%
degradation relative to theoretical oxygen demand over 10 days (Mills
& Stack, 1955).
Brief descriptions of the following degradation tests were also
identified. EDA incubated with activated sludge at 100 mg/litre for 28
days showed 93-95% degradation relative to theoretical oxygen demand
in a modified Ministry of International Trade and Industry (MITI) test
(Japan Chemical Industry Ecology-Toxicology Information Center, 1992).
Incubation with activated sludge at a concentration of 50 mg/litre led
to degradation of 10%, 87.5%, and 94% after 5, 15, and 28 days,
respectively.1
The high water solubility and low octanol/water partition
coefficient indicate that bioaccumulation in organisms is unlikely.
1 Unpublished report from Akzo Research to Delamine, 1989 (cited in
IUCLID).
Table 1: Sorption of EDA to various soil types.a
Soil type pH Cation exchange Fraction of Freundlich adsorption Adsorption coefficient
capacity organic carbon coefficient (Kd)b normalized for organic
(meq/100 g) (Foc)b carbon (K = Kd/Foc)c
Sandy loam (Londo) 7.2 9.2 0.026 69 2700
Sandy clay loam 7.3 16.4 0.039 220 5600
Sandy loam (Cecil) 6.0 3.0 0.014 29 2100
Silty loam 6.0 15.6 0.034 238 7100
Clay 7.9 11.9 0.014 70 5000
Aquifer sand 9.6 6.9 0.0024 15 6200
a Data from Davis (1993).
b Values rounded.
c Mean 4800 + 2000 (SD).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
There are no reports of monitoring of EDA levels in the aquatic
environment or of measurements in effluents.
Residues of EDA in soil, 15 days post-treatment with the
fungicide maneb, have been reported at 0.119 mg/kg for the top 1 cm
(approximately) and at 0.044 mg/kg down to about 5 cm. Residues on
tomatoes and beans were 0.053 and 0.239 mg/kg, respectively,
immediately after spraying, falling to 0.047 and 0.094 mg/kg,
respectively, after 14 days (Newsome et al., 1975).
6.2 Human exposure
The data available to the authors of this document are restricted
mainly to the occupational environment. The exposure assessments used
in this report are based on either limited data or data modelled using
the Estimation and Assessment of Substance Exposure (EASE) model. This
is a general-purpose predictive model developed by the United
Kingdom's Health and Safety Executive for exposure assessment in the
workplace. In its present form, the model is in widespread use across
the European Union for the occupational exposure assessment of new and
existing substances. Similarly, information on control measures has
been derived from United Kingdom industry sources. Where data gaps
exist, professional judgement has been used.
The number of employees exposed to EDA in the United Kingdom is
not accurately known. For use as an intermediate in the manufacture of
tetraacetyl ethylenediamine, EDTA, organic flocculants, urea resins,
and fatty bisamides, it is estimated that 140 employees will be
potentially exposed. During the production of formulations for use in
the printed circuit board and metal finishing industries and in the
manufacture of epoxy coatings/resins and pharmaceutical products, it
is estimated that 200 employees will be regularly exposed to EDA. The
number of employees potentially exposed from use of EDA-based
formulations in the printed circuit board and metal finishing
industries is estimated to be about 100. EDA can also be released when
industrial epoxy coatings/adhesives are applied, and this activity has
the potential to expose several thousand employees across a wide range
of industries.
There are very few measured occupational exposure data available.
EDA's use as an intermediate in chemical synthesis takes place in
closed systems. Measured exposures for these manufacturing processes
show that control is achieved to a level of less than 1.25 mg/m3
(0.5 ppm) 8-h time-weighted average (Hansen et al., 1984). Modelled
data (EASE) are in good agreement, predicting comparable values of
0.53-1.3 mg/m3 (0.21-0.52 ppm). Short-term peak exposures (sampling
and hose uncoupling operations) were predicted to range between 16.8
and 33.3 mg/m3 (6.7 and 13.3 ppm), 15-min time-weighted average.
EDA's use in the production of formulations usually takes place
in well-ventilated enclosed systems. Measured exposure data are not
available for these processes. However, modelled exposure data
indicate exposure levels of 5-20 mg/m3 (2-8 ppm) 8-h time-weighted
average in the presence of local exhaust ventilation and 38-75 mg/m3
(15-30 ppm) 8-h time-weighted average in the absence of local exhaust
ventilation. Corresponding short-term peak exposures during mixer
charging operations were estimated to be 5-25 mg/m3 (2-10 ppm) 15-min
time-weighted average in the presence of local exhaust ventilation and
50-103 mg/m3 (20-41 ppm) 15-min time-weighted average in the absence
of local exhaust ventilation.
The potential for exposure during the use of EDA formulations
will be moderated by the low concentration of EDA present in the
formulations. Very few exposure data are available, and there is scope
for widely different use scenarios. There will be no appreciable
occupational exposure if these products are used in enclosed
ventilated systems as indicated by measured exposure data
(<2.5 mg/m3 [<1 ppm] 8-h time-weighted average) and modelled exposure
data (0-0.25 mg/m3 [0-0.1 ppm] 8-h time-weighted average). Modelled
exposure data for immersion processes, in the presence of local
exhaust ventilation, predicted inhalation exposures of 0.5-2.5 mg/m3
(0.2-1 ppm) 8-h time-weighted average. The potential for greatest
inhalation exposure was predicted for situations where these
formulations are brushed in open systems with only general dilution
ventilation or sprayed in open systems in the presence of local
exhaust ventilation. Under these conditions, modelled exposure data
predict exposures in the range 2.5-5 mg/m3 (1-2 ppm) 8-h
time-weighted average. Short-term peak exposures during mixing and
loading operations were estimated to be 5-10 mg/m3 (2-4 ppm) 15-min
time-weighted average. Polyamines and alkanol polyamines, including
EDA, have been reported to be released from hot bitumen during road
paving (Levin et al., 1994). EDA concentrations generated during road
paving were below 0.025 mg/m3 (0.01 ppm).
There will also be a potential for dermal exposure across the
full range of industries handling EDA. Modelled data estimate dermal
exposures in the range 0-0.15 mg/cm2 per day. However, the use of
personal protective equipment is standard practice in all industries
using EDA. Therefore, in practice, dermal exposure will be
considerably reduced by the use of personal protective equipment.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
The toxicokinetics of EDA has received only limited study, and
there are no studies following inhalation exposure. Studies in humans
have been related to the clinical application of EDA and have
demonstrated rapid absorption via the gastrointestinal tract, with at
least 50% absorbed within the first 7 h; absorbed EDA is rapidly
removed from the plasma (Caldwell & Cotgreave, 1983; Cotgreave &
Caldwell, 1983a,b, 1985). At least half the amount absorbed is
excreted in the urine, largely as the acetylated metabolite
N-acetylethylenediamine and, in smaller amounts, as the unchanged
compound.
This toxicokinetic picture is supported and extended by data from
studies in experimental animals. Studies in rats and mice have
demonstrated rapid and extensive uptake via the oral route and also
via the respiratory tract following intratracheal instillation (about
70% or more of the applied dose was absorbed within 48 h) (McKelvey et
al., 1982; Yang & Tallant, 1982; Yang et al., 1984b). Some (about 12%
of the applied dose over 24 h) dermal absorption has also been
observed in rats at non-irritant concentrations, with greater
absorption at higher, skin-damaging concentrations (Yang et al.,
1987). These animal studies have also demonstrated that EDA and/or its
metabolites are widely distributed throughout the body and are rapidly
eliminated, largely via the urine but also as carbon dioxide in the
breath and a small amount via the faeces, providing evidence for some
biliary excretion. It would seem reasonable to conclude that a similar
situation with respect to distribution and excretion would pertain in
humans. Examination of urinary metabolites in these animal studies
demonstrated that EDA is also found in an acetylated conjugate form in
the rat and mouse. There is evidence that this pathway may become
saturated with increasing dose and that alternative metabolic pathways
may be involved at higher doses in the mouse.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A number of the available studies on both the toxicokinetics and
toxicity of EDA have employed the base substance (EDA) and/or the
hydrochloride salt (EDA.2HCl). The latter is used in pharmaceutical
preparations as a solubilizer to increase uptake of theophylline (this
complex being known as aminophylline) and has been used as a
preservative in skin creams (although it is unclear whether or not
this still occurs). In general, the presence of the hydrochloride has
little qualitative effect on the toxicokinetic or systemic toxicity
properties of EDA, particularly following oral dosing, as it is likely
that the hydrochloride salt would be formed anyway in the acidic
environment of the stomach. However, the hydrochloride does seem to
act in a neutralizing capacity to reduce the significant irritancy
potential of EDA. Studies using both forms of EDA are included in this
review.
8.1 Single exposure
Studies in various animal species have shown EDA to be of
moderate acute toxicity by the inhalation (rat 8-h LC50 estimated to
be in the range of 4916-9832 mg/m3 [1966-3933 ppm]), oral (rat LD50
values of 1160-3250 mg/kg body weight), and dermal (rabbit LD50
values of 550-2880 mg/kg body weight) routes of exposure (Smyth et
al., 1941, 1951; Boyd & Seymour, 1946; Carpenter et al., 1948; NTP,
1982a,b; Yang et al., 1983; Dubinina et al., 1997). Few details exist
of the toxic signs observed or of target organs.
8.2 Irritation and sensitization
There are a number of reports available on skin irritation in
animals, but in general they all repeat the information from one
original study (Smyth et al., 1951). In that study, 0.01 ml undiluted
EDA applied to the shaved backs of albino rabbits produced skin
necrosis within 24 h. A recent report has also indicated EDA to be a
skin irritant (Dubinina et al., 1997). Although no further information
is available, such a response is consistent with EDA being strongly
alkaline. Studies using EDA.2HCl have also resulted in skin
irritation, although the neutralizing action of the hydrochloride may
have influenced the severity of effects, particularly on dilution
(Yang et al., 1983, 1987).
As with skin irritation, the reports that are available for eye
irritation all largely reproduce data from one original study
(Carpenter & Smyth, 1946). In this study, 0.005-ml solutions of 5% EDA
or greater caused corneal injury, which again would be expected, given
the alkaline properties of the substance. More recently, Dubinina et
al. (1997) stated that inflammatory responses in the rabbit eye were
induced by "one drop" of EDA.
Overall, from the reports that are available, together with a
consideration of its alkaline properties, it is reasonable to conclude
that EDA is corrosive, with the capacity to produce severe chemical
burns to the skin and eye.
EDA has been demonstrated to possess skin sensitizing potential
in guinea-pig studies, generally using standard methodologies such as
the Magnusson and Kligman maximization and Buehler tests
(Thorgeirsson, 1978; Erikson, 1979; Maurer et al., 1979; Henck et al.,
1980; Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Dubinina et al., 1997; Leung & Auletta, 1997). In four of these
studies (Goodwin et al., 1981; Babiuk et al., 1987; Robinson et al.,
1990; Leung & Auletta, 1997), the investigators ensured that
non-irritant challenge concentrations of EDA were used, providing
clear evidence for a sensitization response. EDA also produced
positive results in the local lymph node assay (Basketter & Scholes,
1992). In contrast to these positive results, EDA consistently
produced negative results in the mouse ear swelling test (Gad et al.,
1986; Cornacoff et al., 1988; Dunn et al., 1990). One study
demonstrated the potential for EDA to cross-react with other
alkylamines either as the inducing or as the challenge agent (Leung &
Auletta, 1997).
No studies are available on respiratory sensitization in animals.
8.3 Short-term exposure
In a 12-day study (NTP, 1982b), mice received gavage doses of
between 50 and 600 mg EDA/kg body weight per day (administered as
EDA.2HCl). Deaths were observed at 400 and 600 mg EDA/kg body weight
per day. No effects were seen at 50 mg EDA/kg body weight per day.
Renal effects (nephrosis and tubule regeneration) were observed at 100
mg EDA/kg body weight per day and above. Lymphoid depletion in and
necrosis of splenic follicles were observed at 400 mg EDA/kg body
weight per day.
A 7 h/day, 30-day inhalation study in rats indicated that the
liver and kidney are potential target tissues, with local effects in
the lungs also likely (Pozzanni & Carpenter, 1954). No effects were
observed in this study at an airborne exposure concentration of about
150 mg/m3 (60 ppm). Slight depilatory effects were seen at 330 mg/m3
(132 ppm), becoming more marked at higher exposure concentrations.
Treatment-related deaths were observed at 563 mg/m3 (225 ppm) and
1210 mg/m3 (484 ppm) (all animals died at 1210 mg/m3 [484 ppm]).
Cloudy swelling of cells in the liver and convoluted tubules of the
kidneys were also observed at these exposure concentrations.
Degeneration of the convoluted tubules was seen in animals exposed to
1210 mg/m3 (484 ppm), as was congestion of the lungs and adrenals.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Dietary studies in rats have also indicated that the liver is a
target tissue, with changes in the size and shape of hepatocytes and
their nuclei being noted at 1000 mg/kg body weight per day in a 90-day
study (Yang et al., 1983).
Oral gavage studies using doses of between 100 and 1600 mg EDA/kg
body weight per day (administered as EDA.2HCl) have been carried out
in rats (NTP, 1982a). Deaths were observed after 12 doses at 800 and
1600 mg EDA/kg body weight per day and after 800 mg EDA/kg body weight
per day for 90 days. Renal tubular lesions (dilation of the lumen,
necrosis, degeneration and regeneration of the epithelium) were seen
at 200 mg EDA/kg body weight per day and above after 12 doses. Similar
renal lesions but of a less severe nature were seen only at 600 mg
EDA/kg body weight per day and above after 90 days. This indicates
recovery in the kidney, probably as a consequence of compensatory
regeneration. No effects were seen on the kidney at 100 mg EDA/kg body
weight per day in either study. Ocular effects including cataract
formation and retinal atrophy were observed in all dose groups.
Minimal to moderate focal retinal atrophy was observed in 3 out of 10
females at 100 mg EDA/kg body weight per day; 2 males had mild to
moderate retinal atrophy and 1 male had severe retinal atrophy at 200
mg EDA/kg body weight per day. Lymphoid depletion and/or necrosis in
spleen were observed at 800 mg EDA/kg body weight per day and in all
decedents following 12 doses, and thymus weight was reduced at 800 mg
EDA/kg body weight per day in the 90-day study. Uterine lesions
(reduced uterine horn size and atrophy of the myometrium and
endometrium) were seen after dosing for 90 days with 600 or 800 mg
EDA/kg body weight per day, and reduced ovarian size was seen after
800 mg EDA/kg body weight per day for 90 days. Overall, a
no-observed-adverse-effect level (NOAEL) was not identified from these
studies, as effects on the eyes were seen at all dose levels. Only
ocular effects were seen at the lowest-observed-adverse-effect level
(LOAEL) of 100 mg EDA/kg body weight per day, and these were of a
minimal to mild nature, suggesting that this dose represented the
lower end of the dose-response relationship for these effects.
In a 90-day study, mice received oral gavage doses of 25-400 mg
EDA/kg body weight per day (NTP, 1982b). No effects were seen at 100
mg EDA/kg body weight per day. Renal lesions (cortical tubular
degeneration and/or necrosis) were observed at 200 and 400 mg EDA/kg
body weight per day.
8.4.2 Chronic exposure and carcinogenicity
There are two carcinogenicity studies in animals. Both studies
were performed to reasonably adequate standards, including extensive
histopathology, and were negative for carcinogenic activity.
In the first study, groups of 99-225 F344 rats were orally dosed
with 0, 20, 100, or 350 mg EDA.2HCl (equivalent to 0, 9, 45, or 158
mg EDA/kg body weight per day) for 2 years (Yang et al., 1984a).
Non-neoplastic effects were similar to those described in studies of
shorter duration (Yang et al., 1993), as indicated above (section
8.4.1). Effects were seen at 45 mg EDA/kg body weight per day, with a
NOAEL of 9 mg EDA/kg body weight per day. Tracheitis was also
observed, probably as a consequence of exposure to EDA in airborne
dust derived from the diet.
In the second study, groups of 40-50 C3H/HeJ mice were dermally
administered 0 or 0.25 mg aqueous EDA 3 times per week for a lifetime
(DePass et al., 1984). The dermal study included a positive control
group that received 3-methylcholanthrene. Skin fibrosis and
hyper-keratosis were observed in EDA-treated mice.
8.5 Genotoxicity and related end-points
Only limited information is available on the genotoxic potential
of EDA. There is some evidence that EDA may be mutagenic in bacteria
with and without metabolic activation (Hedenstedt, 1978; Hulla et al.,
1981; Haworth et al., 1983; Leung, 1994). Although the most recent
study (Leung, 1994) appears to be negative, there was a small response
in Salmonella typhimurium TA100 and a positive, but not
reproducible, response in TA1535. Positive results have also been
reported in these strains from the other studies, although only one of
these (Haworth et al., 1983) was adequately reported. The only series
of studies performed on mammalian cell systems in vitro (gene
mutation and sister chromatid exchange in Chinese hamster ovary cells;
unscheduled DNA synthesis in rat primary hepatocytes) were
consistently negative (Slesinski et al., 1983), although there has
been no assay for clastogenic activity. A sex-linked recessive lethal
test in Drosophila melanogaster was negative following dosing by
feeding or injection (Zimmering et al., 1985). There are no in vivo
studies on somatic cells, but a dominant lethal study in rats up to
doses inducing signs of toxicity (up to 500 mg EDA.2HCl/kg body
weight per day in the diet) was negative (Slesinski et al., 1983).
Although there has been some evidence of mutagenicity in
bacterial systems in a few limited studies, the available evidence
indicates that EDA is not genotoxic, with all results in mammalian
cells in vitro and in vivo (dominant lethal assay) being negative.
It should be noted that the overall database is limited, with no
assays available for clastogenic activity or for genotoxic potential
in somatic cells in vivo.
8.6 Reproductive and developmental toxicity
The potential of EDA to affect fertility and development has been
studied in rats in investigations conducted to modern regulatory
standards. In a two-generation study in F344 rats, no effects on
fertility or development in any of the generations were observed up to
a dose level (225 mg EDA/kg body weight per day) that induced signs of
parental toxicity (Yang et al., 1984b). Dose levels used in this study
were 0, 50, 150, or 500 mg EDA.2HCl/kg body weight per day (equivalent
to 0, 23, 68, and 225 mg EDA/kg body weight per day). Effects on the
uterus and ovaries have been seen following gavage dosing of rats with
600 and 800 mg EDA/kg body weight per day for 90 days (see section
8.4.1; NTP, 1982a). In a series of developmental toxicity studies in
F344 rats, EDA was found to produce signs of fetotoxicity (increased
resorptions) and delays in development at high dose levels (450 mg
EDA/kg body weight per day) that induced clear signs of toxicity in
the dams (DePass et al., 1987). Dose levels used in this study were 0,
50, 250, or 1000 mg EDA.2HCl/kg body weight per day (equivalent to 0,
23, 113, and 450 mg EDA/kg body weight per day). Some of the
developmental effects appear to have been related, at least in part,
to the reduced nutritional status of the animals. However, a clear
NOAEL for developmental toxicity of 113 mg EDA/kg body weight per day
was observed in these studies.
The results of a preliminary screening study in mice indicated no
significant effects on development in the offspring of dams exposed to
toxic doses of EDA (400 mg/kg body weight per day) by oral gavage
(Hardin et al., 1987).
No effects on development were seen in the offspring of New
Zealand white rabbits dosed during pregnancy with up to 178 mg
EDA.2HCl/kg body weight per day (equivalent to 80 mg EDA/kg body
weight per day), a dose that did not induce maternal toxicity (NTP,
1991; Price et al., 1993). In a preliminary study, 2/20 pregnant
rabbits receiving 100 mg EDA/kg body weight per day by gavage died,
and decreased body weight was seen in survivors. At 400 mg/kg body
weight per day, all the dams died.
8.7 Immunological and neurological effects
No studies are available that have specifically investigated the
potential immunotoxicity of EDA. Effects on lymphoid tissue in the
spleen in mice and rats (see sections 8.3 and 8.4.1, respectively) and
on the thymus in rats (see section 8.4.1) were observed in oral gavage
dosing studies.
There are a few, mainly in vitro, studies on the effects of EDA
on the release of gamma-aminobutyric acid from the retina, gut, and
brain (Perkins & Stone, 1980; Forster et al., 1981; Lloyd et al.,
1982; Morgan & Stone, 1982; Sarthy, 1983; Kerr & Ong, 1984; Strain et
al., 1984; Hill, 1985; Erdo et al., 1986; Krantis et al., 1990; McKay
& Krantis, 1991). The general conclusion that can be drawn from these
studies is that EDA can cause a calcium-independent release of
gamma-minobutyric acid that is insensitive to the presence of
tetrodotoxin. EDA was also shown to have gamma-minobutyric acid
mimetic properties (i.e., reduction of neuronal firing rate). This
suggests that EDA could have a central nervous system depressant
effect, but studies were not performed to address this possibility. It
was reported that EDA elicited contraction of the guinea-pig ileum
that was mediated via neuronal release of gamma-aminobutyric acid.
However, in the rat ileum, EDA acted directly on the mucosa, resulting
in relaxation. Although these are interesting results, the
toxicological significance of these findings is unclear; they may,
however, partly explain the central nervous system depressant and
gastrointestinal effects seen in some of the animal studies at high
doses.
9. EFFECTS ON HUMANS
No studies are available in which the effects, other than the
respiratory effects summarized below, of repeated exposure of humans
to EDA are examined. No reports have been found in which genotoxicity,
carcinogenicity, or reproductive toxicity following exposure to EDA in
human populations has been studied.
A case report exists concerning a 36-year-old worker who died
from cardiac collapse 55 h after being splashed by an accidental
spillage of EDA (Niveau & Painchaux, 1973). Exposure to an
unquantified amount of EDA was in the order of a few minutes prior to
the patient being washed. Four hours after the exposure, he presented
with tachycardia (100 beats/min), anuria, and red/brown generalized
erythema. The tachycardia increased (up to 140 beats/min), anuria
persisted, and an expectorant cough, abdominal cramps, diarrhoea, and
blackish vomiting appeared. The patient became hyperkalaemic, and his
red blood cell count decreased. Overall, given the lack of information
with respect to levels of exposure, few useful conclusions can be
drawn from this case report.
The only information available on skin irritation in humans
either is anecdotal or does not involve direct surface skin contact
with EDA. In a brief report on the physicochemical properties of EDA,
it is noted anecdotally that "the liquid, if not washed from the skin,
causes blistering" (Boas-Traube et al., 1948). The other report
available documents the results of intradermal skin tests with
solutions (0.1-1%) of EDA on three individuals being tested for
hypersensitivity following treatment with aminophylline (Kradjan &
Lakshminarayan, 1981). The skin response in two patients consisted of
blistering rather than a weal and flare reaction, which normally
typifies a sensitization response. Punch biopsies were obtained from
one patient, and histopathological examination of these indicated
tissue necrosis and oedema of the epidermis and dermis. These
responses suggested a direct corrosive effect of EDA.
No specific reports were found of the effects of EDA on the eyes
of humans. In the original reports of the animal eye irritation study
(see section 8.3), it is claimed that EDA is known to have produced
loss of vision or slowly healing corneal burns in industrial use
(Carpenter & Smyth, 1946). However, no further information or
references were given.
EDA has been known for many years to be capable of inducing
allergic skin reactions in humans. This has been observed both in the
workplace and, most notably, in patients treated with aminophylline or
with skin creams in which EDA was used as a stabilizer (Epstein &
Maibach, 1968; Petrozzi & Shore, 1976; Booth et al., 1979; Wall, 1982;
Hardy et al., 1983; Balato et al., 1984; Edman & Moller, 1986; Nielsen
& Jorgensen, 1987; Terzian & Simon, 1992; Toal et al., 1992; Dias et
al., 1995; Simon et al., 1995; Sasseville & Al-Khenaizan, 1997). The
first reports of skin sensitizing effects in humans date back to the
late 1950s, when cases were described of eczematous reactions in
pharmacists who came into contact with EDA when using aminophylline
(Baer et al., 1958; Tas & Weissberg, 1958).
Subsequent to these early reports, numerous studies and case
reports have been published documenting the skin sensitizing
properties of EDA both following clinical use and within the
occupational setting, such that the substance has become incorporated
into standard series for patch testing (Fregert, 1981; Shehade et al.,
1991). An example from the clinical setting is that of the report on a
series of 13 patients who had used skin cream containing EDA for,
paradoxically, dermatitic conditions (Provost & Jillson, 1967). Use of
the cream in 11 of these patients had resulted in the sudden
appearance of a severe generalized patchy eczematous eruption
following, in all but one of the cases, an initial improvement upon
using the cream. Patch testing was conducted using a 1% aqueous
solution of EDA, producing skin reactions in all patients ranging from
erythema and oedema to erythema vesiculation and oedema vesiculation,
which extended beyond the patch test site. Other substances tested
also induced responses, but not in a consistent manner, with at most
only four individuals responding in any one test.
As well as the original reports in pharmacists working with EDA,
cases of skin sensitization to EDA have been reported in the
occupational environment in a number of different settings, including
use of floor polish remover (English & Rycroft, 1989), use of coolant
oils (Crow et al., 1978), and in wire-drawing (Matthieu et al., 1993;
Sasseville & Al-Khenaizan, 1997). Positive responses to patch testing
with EDA have also been observed in other occupational settings, such
as the offshore oil industry (Ormerod et al., 1989), but positive
responses to other substances, including other polyamines, were also
seen in such cases. Thus, it is unclear whether EDA had been
responsible for inducing the sensitized state and/or cross-reacting
following sensitization to another polyamine.
A large number of cases of occupational asthma reported to have
been caused by exposure to EDA are available in the literature
(Dernehl, 1951; Gelfand, 1963; Popa et al., 1969; Valeyeva et al.,
1975; Lam & Chan-Yeung, 1980; Chan-Yeung, 1982; Hagmar et al., 1982;
Matsui et al., 1986; Aldrich et al., 1987; Nakazawa & Matsui, 1990;
Lewinsohn & Ott, 1991; Ng et al., 1991, 1995). There are a few studies
in which the potential for EDA to cause respiratory hypersensitivity
has been examined using bronchial provocation testing and
investigation of antibody formation. As EDA is corrosive, the vapour
would be predicted to be a respiratory tract irritant, which is a
complicating factor in interpreting the data available and in
elucidating the underlying mechanism for any asthmatic responses seen.
Popa et al. (1969), in a well-conducted study, investigated 48
subjects with asthmatic symptoms caused by exposure to a number of low
molecular weight chemicals, including EDA. None of the subjects had a
history of respiratory disorder prior to occupational exposure, and
the asthmatic response was associated only with occupational exposure
in all cases. No information was given in the report on the workplace
airborne concentrations of EDA to which these workers were exposed. A
series of tests were performed in all subjects, including skin and
inhalation tests with the test agent at sub-irritant concentrations;
skin and inhalation tests to common allergens; skin tests
(intradermal, scratch, and patch tests) using sub-irritant
concentrations of the test substance; Prausnitz-Kustner transfer
reaction (to test for the presence of immunoglobulin E antibodies);
and determination of precipitating antibodies to EDA. For the
inhalation test, the sub-irritant concentration was determined in
control asthmatic subjects, and a 2- to 10-fold dilution of this was
used for the bronchial challenge. No information was given on the
airborne exposure concentrations generated under these test
conditions. Control inhalation tests with the diluent, physiological
saline, were also conducted. It is not stated in the report whether or
not the inhalation challenge tests were conducted in a blind manner.
Six subjects had an immediate, positive reaction to EDA in the
workplace. Of these, four showed an immediate, positive response
following inhalation testing with sub-irritant concentrations of EDA.
These subjects developed marked bronchoconstriction following
inhalation exposure to EDA, with a reduction in forced expiratory
volume in 1 s (FEV1) of 62% and an increase in respiratory resistance
of 44%, compared with controls. Although not stated in the report,
these values are presumed to be average changes. Intradermal skin
tests with EDA were positive in these four subjects, whereas patch
tests were negative. Inhalation challenges with common allergens were
negative. The Prausnitz-Kustner test was positive in all subjects, and
all had eosinophilia, determined in the sputum, although not, except
in one case, in the blood. No precipitating antibodies were found. In
the two other subjects, the inhalation challenge test was negative. No
precipitating antibodies were found, and the Prausnitz-Kustner test
was negative in both subjects. Eosinophilia was absent. Inhalation
challenges with other common allergens were also negative.
These data provide evidence that EDA may elicit an asthmatic
response at sub-irritant concentrations and that the response is
specific to EDA. Four out of six subjects responsive to EDA in the
workplace also had a positive response to inhaled EDA at sub-irritant
concentrations. This demonstrates that the reaction is not a
generalized response to an irritant. The positive Prausnitz-Kustner
reaction may be indicative of an immunological component, but the test
is not specific, and no firm conclusions can be drawn from it. It
provides supporting evidence in this case. The evidence suggests that
the subjects were hypersensitive to inhaled EDA and that a state of
respiratory hypersensitivity had been induced by the substance.
Although a number of other studies are available, the information
is of poor quality. Lam & Chan-Yeung (1980) and Chan-Yeung (1982)
describe the case of one worker in a photographic laboratory who
developed asthma after 2.5 years of exposure to a variety of
chemicals, including EDA, but also other irritant substances. The
worker developed symptoms of sneezing, nasal discharge, productive
cough and nocturnal cough, wheezing, and dyspnoea. The symptoms
coincided with the work shift and subsided at weekends. There was no
previous history of asthma. No information was given in the report of
the airborne concentrations of EDA (or other substances) to which the
man was exposed at work. A series of controlled inhalation challenge
exposures, designed to mimic work exposure conditions, were conducted
with each of the chemicals to which the subject was exposed at work.
The duration of exposure was determined by the patient's tolerance,
and exposure was terminated when eye irritation or cough was
experienced. No information on the airborne exposure concentrations of
EDA generated under these test conditions was given in the report. A
methacholine inhalation test for bronchial hyperreactivity was also
performed. Pulmonary function tests were conducted pre- and
post-challenge, and blood samples were taken before, during, and after
each challenge. The subject showed marked bronchial reactivity to
methacholine.
Exposure to an unknown concentration of vapour from a 1:25
solution of EDA was tolerated for 15 min. This exposure produced a
marked bronchoconstriction. A late asthmatic response developed 4 h
after the exposure, at which time FEV1 was reduced by 26% and
continued to decrease over the next 3 h towards a 40% reduction. A 26%
reduction was still apparent after 24 h, despite treatment with
bronchodilator drugs. This pattern of response to EDA was
reproducible. The patient did not respond similarly to any of the
other chemicals tested: formaldehyde, sulfur dioxide, and two colour
developing agents that were stated to be irritants. Exposure to
formaldehyde (vapour from a 1:4 solution) produced an immediate small
(<20%), transient reduction in FEV1, whereas exposure to sulfur
dioxide caused coughing and chest tightness and an immediate transient
reduction of 25% in FEV1. There was no increase in plasma histamine
concentration during the period of bronchoconstriction, although EDA
was shown to cause in vitro histamine release from whole blood taken
from the patient and from two control subjects. A skin test using
1:100 EDA and a precipitin test for antibodies to EDA were both
negative. The patient subsequently had to give up work because of
respiratory symptoms and became asymptomatic after 2 weeks. Subsequent
testing with methacholine, 2.4 months after ceasing work, showed that
the subject had a reduction in the previous bronchial hyperreactivity.
In conclusion, the subject showed an asthmatic response to EDA
but not to formaldehyde or the colour developing agents. The pattern
of response to sulfur dioxide was more immediate and suggestive of an
irritation response. Overall, a clear pattern of asthmatic response
that was specific to EDA was observed in this study. However, it is
not possible to distinguish with certainty between an irritant
response and a sensitization response, because it is possible that an
irritant concentration was used for the bronchial challenge exposure,
although little immediate response was observed. In addition, although
exposure to irritant concentrations of the other workplace chemicals
did not elicit the same pattern, magnitude, or severity of response as
that seen with EDA, since accurate exposure levels were not given, it
is not possible to determine whether or not the EDA concentration used
had the greatest irritant potential. No evidence for any immunological
involvement was found. In conclusion, this study provides only
circumstantial evidence that EDA caused a state of respiratory
hypersensitivity in this subject.
A number of other case reports are available of individuals who
exhibited asthmatic signs and symptoms associated with exposure to EDA
in the workplace (Gelfand, 1963; Valeyeva et al., 1975; Matsui et al.,
1986; Nakazawa & Matsui, 1990; Ng et al., 1991). Although bronchial
challenge testing with EDA produced asthmatic responses in these
subjects, they had personal and/or family histories of allergic
disease and/or they had worked with and responded on challenge to
other substances. Retrospective studies using the medical records of
populations of workers using EDA have indicated that about 10% of such
populations developed signs and symptoms of occupational asthma
(Aldrich et al., 1987; Lewinsohn & Ott, 1991). No challenge tests were
carried out with these surveys. Thus, these case reports and
population-based studies provide only supporting circumstantial
evidence for the involvement of EDA in producing occupational asthma.
Although it is clear from these reports that EDA can provoke an
asthma attack, in many cases there is insufficient information to
indicate whether or not the hypersensitive state was induced
specifically by EDA. However, from one well-conducted study, there is
evidence that a hypersensitive state specific to EDA has been induced
in workers and that an asthmatic response was provoked by sub-irritant
concentrations of the substance. Overall, the results of this study,
taken together with the supporting data from a substantial number of
other reports of occupational asthma, indicate that EDA is capable of
inducing a state of hypersensitivity in the airways, such that
subsequent exposure may trigger asthma. The mechanism by which the
hypersensitive state is induced is not proven. Given the skin
sensitizing potential of EDA and the limited evidence of immunological
involvement in workers with EDA-provoked occupational asthma, an
immunological mechanism would seem plausible. Irrespective of the
mechanism involved, the data available (specifically the lack of
information on airborne exposure concentrations under both work and
challenge test conditions) do not allow elucidation of a dose-response
relationship or the identification of levels of EDA that are not
capable of inducing a hypersensitive state or of provoking an
asthmatic response.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Results of acute ecotoxicity tests are given in Table 2.
A 21-day Daphnia magna reproduction test was conducted
according to the German Federal Environment Agency (Umweltbundesamt)
guidelines in a closed vessel. End-points measured included adult
mortality, onset of production of young, and reproduction rate. The
most sensitive end-point was for reproductive rate, and a NOEC of 0.16
mg/litre was established (Kuhn et al., 1989). In a second study
conducted according to Organisation for Economic Co-operation and
Development (OECD) guidelines, a NOEC for reproduction was reported at
2 mg/litre (Mark & Hantink-de Rooy, 1992). An early life stage test
conducted under OECD guidelines on three-spined stickleback
( Gasterosteus aculeatus) showed no effects of EDA at 10 mg/litre
(the highest concentration tested) over 28 days (Mark & Arends, 1992).
Growth of lettuce ( Lactuca sativa) plants over 7 days was
studied in tests conducted under OECD Guideline 208; EC50
concentrations for EDA in soil (nominal) were >1000 mg/kg for 7-day
and 692 mg/kg for 14-day growth periods (Hulzebos et al., 1993).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
EDA is of moderate acute toxicity by all routes. In studies in
animals, EDA is a primary skin and eye irritant, being corrosive when
undiluted. It is also a skin sensitizer. In repeated-dose toxicity
studies by the oral and inhalation routes, effects on the liver and
kidney have been observed, with pleomorphic changes to hepatocytes in
rats being reported at the lowest oral doses used (45 mg/kg body
weight per day and above for 2 years; NOAEL, 9 mg/kg body weight per
day). In inhalation studies, there were no effects at 150 mg/m3 (60
ppm), although slight depilation was observed at the next highest
concentration (330 mg/m3 [132 ppm]) and effects on the liver and
kidney at higher concentrations still (approximately 500 mg/m3 [200
ppm] and above).
There has been some evidence of mutagenicity in bacterial systems
in a few limited studies. However, much of the available evidence is
negative, although the overall database is limited, there being no
assays for clastogenic activity or for genotoxic potential in somatic
cells in vivo. EDA was not carcinogenic in adequate studies in
animals.
In humans, EDA has the potential to induce respiratory tract
hypersensitivity, and provocation of asthma is the major health effect
of concern in the occupational environment. The mechanism of induction
of the hypersensitive state is unknown, although the skin sensitizing
potential of EDA and limited evidence in workers with EDA-provoked
asthma are suggestive of an immunological mechanism. However,
irrespective of the mechanism involved, the available data
(particularly lack of information on exposure conditions either in the
workplace or on bronchial challenge testing) do not allow either
elucidation of dose-response relationships or identification of the
thresholds for induction of the hypersensitive state or provocation of
an asthmatic response.
11.1.2 Criteria for setting guidance values for EDA
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship for provocation of
an asthmatic response, the effect of greatest concern in the
occupational environment. As it is not possible to identify a level of
exposure that is without adverse effect, it is recommended that levels
be reduced to the extent possible.
Table 2: Acute toxicity of EDA to organisms other than laboratory mammals.
Species End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Microcystis aeruginosa Toxic threshold for cell multiplication 0.08 Bringmann & Kuhn, 1976
Pseudomonas putida 17-h EC50 (growth rate) 29 (23-35) van Ginkel, 1989
Nitrifying bacteria 3-h EC50 (respiration) 3.2 (0.6-5.7) Balk & Meuwsen, 1989a
NOEC 0.5
Activated sludge bacteria 1-h EC50 (respiration rate) 1600 van Ginkel & Stroo, 1989
Algae
Scenedesmus quadricauda Toxic threshold for cell multiplication 0.85 Bringmann & Kuhn, 1980a
Chlorella pyrenoidosa 96-h EC50 (growth) 100 van Leeuwen, 1986
Selenastrum capricornutum 72-h EC50 (biomass) 71 van Ginkel et al., 1990
72-h EC50 (growth rate) 645
NOEC approx. 3.2
96-h EC50 (growth rate) 151 van Wijk et al., 1994
Scenedesmus subspicatus 48-h EC50 (biomass and growth rate) >100 Kuhn & Pattard, 1990
Protozoa
Entosiphon sulcatum Toxic threshold for cell multiplication 1.8 Bringmann & Kuhn, 1980a
Uronemia parduczi Toxic threshold for cell multiplication 52 Bringmann & Kuhn, 1980b
Chilomonas paramaecium Toxic threshold for cell multiplication 103 Bringmann & Kuhn, 1980b
Invertebrates
Daphnia magna 48-h LC50 26.5 van Leeuwen, 1986
48-h LC50 46 van Wijk et al., 1994
48-h LC50 16.7 Balk & Meuwsen, 1989b
24-h LC50 14 Kuhn et al., 1989
Table 2 (continued)
Species End-point Concentration Reference
(mg/litre)
Brine shrimp (Artemia
salina) 24-h LC50 14 Price et al., 1974
Fish
Brown trout (Salmo trutta) 48-h LC50 230 Woodiwiss & Fretwell, 1974
Fathead minnow (Pimephales
promelas) 96-h LC50 115.7 Curtis & Ward, 1981
Guppy (Poecilia reticulata) 96-h LC50 275 van Leeuwen, 1986
96-h LC50 640 Balk & Meuwsen, 1989c
96-h LC50 1545 van Wijk et al., 1994
Medaka (Oryzias latipes) 48-h LC50 1000 Tonogai et al., 1982
With respect to systemic effects, the lowest NOAELs of 150 mg/m3
(60 ppm) (inhalation) and 9 mg/kg body weight per day (oral) can serve
as a basis for comparison with estimated exposure for characterization
of risk, either with application of appropriate uncertainty factors or
directly. An example of the latter (margin of exposure) approach is
presented in section 11.1.3.
11.1.3 Sample risk characterization
Available data are inadequate to serve as a basis for
characterization of the dose-response relationship, and hence risk,
for provocation of an asthmatic response, the effect of greatest
concern in the occupational environment. For substances that are
asthmagens, it is also advisable to restrict peak exposures, as they
may have a role in the induction and triggering of asthmatic
phenomena. However, to help assess the risk to human health arising
from occupational exposures, a comparison is made with the NOAELs for
systemic effects from animal studies.
It should also be noted that because diluted EDA is a skin
irritant and sensitizer, there is a risk of developing irritant and/or
allergic dermatitis if suitable personal protective equipment is not
used.
A sample risk characterization for systemic effects in the
occupational environment in the United Kingdom is provided here.
Measured data on exposure to EDA (generally used in closed systems)
indicate that levels in industry in the United Kingdom are less than
1.25 mg/m3 (0.5 ppm), 8-h time-weighted average. Modelled data (EASE)
are in good agreement, predicting comparable values of 0.53-1.3 mg/m3
(0.21-0.52 ppm). Short-term peak exposures (sampling and hose
uncoupling operations) were predicted to be 16.8-33.3 mg/m3
(6.7-13.3 ppm), 15-min time-weighted average. The EASE model predicts
dermal exposures in the range of 0-0.15 mg/cm2 per day for an
operator transferring EDA into closed systems once a day (although
coveralls and well-maintained plastic gloves will significantly reduce
exposure from this source).
With respect to systemic effects, worst-case estimated and
measured exposures of 1.25 mg/m3 (0.5 ppm), 8-h time-weighted
average, are substantially less (by 100-fold or greater) than the
NOAEL in the inhalation study in rats. The combined body burden from
inhalation and dermal exposure for chemical synthesis can be estimated
to be about 0.3 mg/kg body weight per day (assuming a 70-kg worker
breathing 10 m3 of air containing 1.25 mg EDA/m3 [0.5 ppm] per day,
with 100% absorption; and 10% absorption from unprotected, undamaged
skin for a standard hand area of 840 cm2). This is 30-fold less than
the NOAEL for hepatic effects in the oral studies.
Available data on indirect exposure in the general environment or
from consumer products are inadequate to serve as a basis for a sample
characterization of risk for these scenarios.
11.2 Evaluation of environmental effects
No atmospheric effects are expected, as reaction with hydroxyl
radicals is likely to be rapid, and washout of volatilized EDA is
expected. Volatilization to the atmosphere is likely from soil but not
from water.
Adsorption to soil particulates is strong through electrostatic
binding; leaching through soil profiles to groundwater is not
expected. EDA is readily biodegradable, and this is the most likely
source of breakdown in the environment; adaptation of microorganisms
may improve degradation. Breakdown is less rapid in seawater than in
fresh water. Bioaccumulation is unlikely.
Toxic thresholds for microorganisms may be as low as 0.1
mg/litre. However, toxicity tests in culture media should be treated
with caution, as the EDA may complex with metal ions. Effects may
therefore be indirect, from loss of bioavailability of essential
elements. The "toxic thresholds" reported are lowest-observed-effect
concentrations (LOECs) for small changes in sublethal end-points; the
exact degree of effect at the reported concentrations is not always
clear, and these have not been used in the risk assessment.
The principal receiving compartment in the environment is the
hydrosphere, and this is the only compartment for which a quantitative
risk assessment can be attempted.
The distribution of acute (from Table 2) and chronic test results
is plotted in Figure 1. Chronic test results are available for fish (a
limit test only) and Daphnia (NOECs from 21-day reproduction tests).
Chronic EC50s for algal growth or biomass are also available, but no
NOEC was reported for these studies. Given the range of chronic test
results across three trophic levels, it is proposed that an
uncertainty factor of 10 be applied to the lowest reported NOEC (for
Daphnia reproduction at 0.16 mg/litre) to derive an estimated PNEC
for aquatic organisms of 0.016 mg/litre. This is in accord with OECD,
European Union, and US Environmental Protection Agency guidelines.
There are no reported test results for estuarine/marine organisms; it
is assumed that toxicity would be in the same range for these species.
No measured concentrations of EDA in surface waters have been
reported. A single quantitative risk assessment has been reported (van
Wijk, 1992) based on discharges into the Ems-Dollard estuary in the
Netherlands. The figure of 75 kg/day for release of EDA at this site
has been used here as a representative estimate of release; no
information on releases from industrial plants elsewhere is available.
 Based on this emission rate, and using default values from the
OECD Technical Guidance Manual, the initial concentration in river
water would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
= 337.5 µg/litre
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q),
where:
W = emission rate (75 kg/day)
P = percent removal in the wastewater treatment plant
(91%, based on a classification of the chemical as
"readily biodegradable")
Q = volume of wastewater in m3/day (default 200
litres [0.2 m3] per person per day for a
population of 10 000 inhabitants)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Foc(susp) × Koc, where:
Foc(susp)= the fraction of organic carbon in suspended
matter (0.01)
Koc = 0.411 × Kow
where:
Kow = the octanol/water partition coefficient (0.063)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (default value of 10)
Calculation of initial PEC in the compartment of the estuary
receiving emissions from the actual plant in the Netherlands gives a
PEC(initial near field) of 10.9 µg/litre. This is based on a local
volume at mean tide of 6.9 × 109 litres, a residence time of 1 day,
and a wastewater flow from the plant of 500 m3/day, realistic for the
local conditions.
More refined modelling, taking into account tidal dilution and
expected biodegradation with a half-life of 5 days, predicts a
steady-state concentration of 1.3 µg EDA/litre (van Wijk, 1992). This
is likely to be a closer reflection of the real situation in the
estuary.
The risk factors shown in Table 3 can be calculated for
conservative worst-case and refined estimates of environmental
concentrations for a river and estuary.
Table 3: PEC/PNEC ratios.
PEC (µg/litre) PNEC (µg/litre) PEC/PNEC
ratio
River, worst case 337.5 16 21.1
Estuary, initial
worst case 10.9 16 0.68
Estuary, refined PEC 1.3 16 0.08
The PEC/PNEC ratio for the river indicates some cause for concern
(ratio greater than 1). However, the PEC is based on very conservative
assumptions, and both estimates assume low adsorption to sediment
based on water solubility. Refined estimates for the estuary indicate
low risk to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0269) reproduced in this
document.
13.1 Human health hazards
Repeated or prolonged contact with EDA may cause skin
sensitization and asthma.
13.2 Advice to physicians
EDA is corrosive. Inhalation of the vapour may cause irritation
of the respiratory tract and even lung oedema and could mask asthmatic
reaction.
13.3 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of EDA as a human asthmagen.
13.4 Spillage
In the case of spillage, emergency crews need to wear proper
equipment and prevent EDA from reaching drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENEDIAMINE ICSC: 0269
June 1999
CAS #
Based on this emission rate, and using default values from the
OECD Technical Guidance Manual, the initial concentration in river
water would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
= 337.5 µg/litre
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre), calculated as
Ceffluent = W × (100 - P)/(100 × Q),
where:
W = emission rate (75 kg/day)
P = percent removal in the wastewater treatment plant
(91%, based on a classification of the chemical as
"readily biodegradable")
Q = volume of wastewater in m3/day (default 200
litres [0.2 m3] per person per day for a
population of 10 000 inhabitants)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = Foc(susp) × Koc, where:
Foc(susp)= the fraction of organic carbon in suspended
matter (0.01)
Koc = 0.411 × Kow
where:
Kow = the octanol/water partition coefficient (0.063)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (default value of 10)
Calculation of initial PEC in the compartment of the estuary
receiving emissions from the actual plant in the Netherlands gives a
PEC(initial near field) of 10.9 µg/litre. This is based on a local
volume at mean tide of 6.9 × 109 litres, a residence time of 1 day,
and a wastewater flow from the plant of 500 m3/day, realistic for the
local conditions.
More refined modelling, taking into account tidal dilution and
expected biodegradation with a half-life of 5 days, predicts a
steady-state concentration of 1.3 µg EDA/litre (van Wijk, 1992). This
is likely to be a closer reflection of the real situation in the
estuary.
The risk factors shown in Table 3 can be calculated for
conservative worst-case and refined estimates of environmental
concentrations for a river and estuary.
Table 3: PEC/PNEC ratios.
PEC (µg/litre) PNEC (µg/litre) PEC/PNEC
ratio
River, worst case 337.5 16 21.1
Estuary, initial
worst case 10.9 16 0.68
Estuary, refined PEC 1.3 16 0.08
The PEC/PNEC ratio for the river indicates some cause for concern
(ratio greater than 1). However, the PEC is based on very conservative
assumptions, and both estimates assume low adsorption to sediment
based on water solubility. Refined estimates for the estuary indicate
low risk to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Previous evaluations by international bodies were not identified.
Information on international hazard classification and labelling is
included in the International Chemical Safety Card reproduced in this
document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0269) reproduced in this
document.
13.1 Human health hazards
Repeated or prolonged contact with EDA may cause skin
sensitization and asthma.
13.2 Advice to physicians
EDA is corrosive. Inhalation of the vapour may cause irritation
of the respiratory tract and even lung oedema and could mask asthmatic
reaction.
13.3 Health surveillance advice
Physicians involved in worker health surveillance programmes
should be aware of the potential of EDA as a human asthmagen.
13.4 Spillage
In the case of spillage, emergency crews need to wear proper
equipment and prevent EDA from reaching drains or watercourses.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards
may be obtained from UNEP Chemicals (IRPTC), Geneva.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
ETHYLENEDIAMINE ICSC: 0269
June 1999
CAS # 