
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 32
First draft prepared by
Dr Robert M. Bruce, National Center for Environmental Assessment, US Environmental Protection Agency, Cincinnati, OH, USA, and
Mr Mark Odin, Syracuse Research Corporation, Syracuse, NY, USA
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2001
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Beryllium and beryllium compounds
(Concise international chemical assessment document ; 32)
1. Beryllium – toxicity
2. Risk assessment
3. Environmental exposure
4. Occupational exposure
I. International Programme on Chemical Safety
II. Series
ISBN 92 4 153032 4
(NLM Classification: QV 275)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available.
©World Health Organization 2001
Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
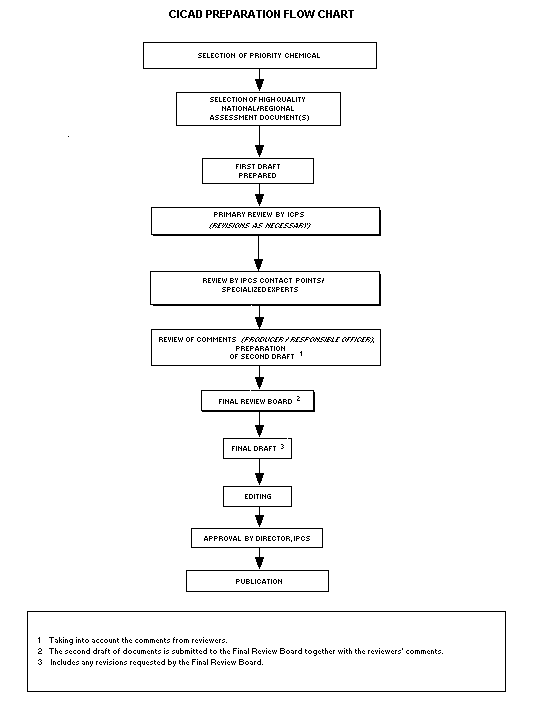
Procedures
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Co-ordinator, IPCS, on the selection of chemicals for an IPCS risk assessment, the appropriate form of the document (i.e., EHC or CICAD), and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.

The CICAD Final Review Board has several important functions:
|
– |
to ensure that each CICAD has been subjected to an appropriate and thorough peer review; |
|
– |
to verify that the peer reviewers’ comments have been addressed appropriately; |
|
– |
to provide guidance to those responsible for the preparation of CICADs on how to resolve any remaining issues if, in the opinion of the Board, the author has not adequately addressed all comments of the reviewers; and |
|
– |
to approve CICADs as international assessments. |
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on beryllium and beryllium compounds was prepared by the US Environmental Protection Agency (EPA), based principally on a review prepared to assess the cancer and non-cancer human health risks of beryllium and beryllium compounds (US EPA, 1998). Other sources included a 1993 review on beryllium prepared by the Agency for Toxic Substances and Disease Registry (ATSDR, 1993) to characterize information on adverse human health effects and public exposure, a review on the toxicity of beryllium and beryllium compounds prepared by the Health and Safety Executive of the United Kingdom (Delic, 1992; HSE, 1994), and a review prepared by the International Programme on Chemical Safety (IPCS, 1990) to evaluate the effects of beryllium on human health and the environment. Data available up to 1997 were considered in the US EPA (1998) review. The ATSDR (1993) and Health and Safety Executive (Delic, 1992; HSE, 1994) toxicity reviews were based on data available prior to 1992, and the IPCS (1990) review was based on data available prior to 1989. A literature search for information regarding ecological toxicity was conducted for the years 1988–1999 (February) because neither the US EPA (1998) review nor the ATSDR (1993) review included information on environmental effects. Information on the nature of the peer review and the availability of the source documents is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Helsinki, Finland, on 26–29 June 2000. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Cards for beryllium (ICSC 0226), beryllium oxide (ICSC 1325), beryllium sulfate (ICSC 1351), beryllium nitrate (ICSC 1352), beryllium carbonate (ICSC 1353), beryllium chloride (ICSC 1354), and beryllium fluoride (ICSC 1355), produced by the International Programme on Chemical Safety (IPCS, 1999a–g), have also been reproduced in this document.
Beryllium (Be; CAS No.
Beryllium is not significantly bioconcentrated from water by aquatic species. It is also apparently not bioaccumulated from sediment by bottom-feeding molluscs. Most plants take up beryllium from soil in small amounts, although a few species act as beryllium accumulators. The general population is exposed to beryllium primarily in food and drinking-water, with smaller contributions from air and incidental ingestion of dust. However, intake by the latter two pathways can be important in the vicinity of a source and can dominate exposure for workers in an industrial setting.
There are no human studies addressing the toxicokinetics of beryllium or beryllium compounds; however, beryllium has been found in the lungs and urine of non-occupationally exposed individuals. Beryllium and beryllium compounds are not metabolized. Animal studies have demonstrated that inhaled beryllium particles (insoluble) are cleared from the lungs slowly, so beryllium may remain in the lungs for many years after exposure. Pulmonary clearance of the soluble and sparingly soluble beryllium compounds via inhalation or intratracheal instillation appears to be biphasic, with a rapid first phase of a few days/weeks and a slower second phase, which may vary from a few weeks/months for the soluble compounds to months/years for the sparingly soluble compounds. Soluble beryllium compounds are absorbed to a greater degree (~20% of the initial lung burden) than sparingly soluble compounds (e.g., beryllium oxide) following inhalation or intratracheal instillation. The extent of absorption also varies with the calcining temperature of the oxide, which influences its particle size and solubility. Ingested beryllium is poorly absorbed (<1%) from the gastrointestinal tract. Absorbed beryllium is distributed primarily to the skeleton, where it accumulates. Elimination is very slow and occurs primarily in the urine. Unabsorbed beryllium is eliminated via the faeces shortly after exposure via inhalation and intratracheal instillation. However, urinary excretion becomes more important at later time points, especially for the more soluble beryllium compounds, as absorbed beryllium is removed from the body.
There are no reliable data on the oral toxicity of beryllium in humans. Acute oral exposures to single doses of soluble beryllium compounds are moderately toxic based on LD50 data; however, in the case of sparingly soluble beryllium compounds, no oral single-dose studies are available. Short-, medium-, and long-term studies in animals showed that the gastrointestinal and skeletal systems are target organs for beryllium following oral exposure. Dogs chronically exposed to soluble beryllium sulfate in the diet developed gastrointestinal lesions and bone marrow hypoplasia. Rickets were observed in rats exposed to sparingly soluble beryllium carbonate in the diet for 3–4 weeks, possibly due to decreased gastrointestinal absorption of phosphorus subsequent to formation of insoluble beryllium phosphate in the intestine. The calculated dose at the lower 95% confidence limit for a 10% incidence of lesions in the small intestine in dogs chronically exposed to beryllium sulfate tetrahydrate is 0.46 mg/kg body weight per day (BMD10). The oral tolerable intake of 0.002 mg/kg body weight per day was estimated from the BMD10 using an uncertainty factor of 300.
The lung is the primary target of inhalation exposure to beryllium in animals and humans. In animals, LC50 values could not be located for both soluble and sparingly soluble beryllium compounds. With respect to repeated or continuous exposures, the most marked effects (pneumonitis, fibrosis, proliferative lesions, metaplasia, and hyperplasia) were observed in the lungs of various animal species exposed to both soluble and sparingly soluble beryllium compounds. In humans, there is little information on the toxic effects of beryllium or its compounds following a single exposure via inhalation, although chemical pneumonitis (acute beryllium disease, or ABD) has been observed following single massive exposures. Short-term or repeated exposures of humans to beryllium or its compounds can result in an acute or chronic form of lung disease, depending upon the exposure concentration. ABD is generally associated with exposure levels above 100 µg beryllium/m3, which may be fatal in 10% of cases. In contrast to acute chemical pneumonitis, exposure to lower concentrations may produce, in about 1–5% of exposed individuals, a chronic form of the disease. Chronic beryllium disease (CBD) is characterized by the formation of granulomas, resulting from an immune reaction to beryllium particles in the lung. There is an extensive body of evidence documenting beryllium sensitization and CBD as the sensitive effects of inhalation exposure to beryllium. The tolerable concentration for the non-cancer health effects of beryllium is 0.02 µg/m3 and was estimated from the duration-adjusted lowest-observed-adverse-effect level (LOAEL) for CBD in exposed workers using a total uncertainty factor of 10 (3 for the use of a LOAEL rather than a no-observed-adverse-effect level, or NOAEL, based on the sensitive nature of the subclinical end-point [beryllium sensitization], and 3 for the poor quality of exposure monitoring of the co-principal studies).
Increases in lung cancer mortality were observed in cohort mortality studies of beryllium processing workers and in studies of entrants on the Beryllium Case Registry (BCR). These studies are considered to provide evidence of the carcinogenicity of beryllium in humans exposed by inhalation; the evidence is limited because of relatively small increases in lung cancer risks, poorly defined estimates of beryllium exposure, incomplete smoking data, and lack of control for potential exposure to other carcinogens. Regardless of the shortcomings of the epidemiological studies, the results of all the follow-up mortality studies on the same cohort and of the BCR cohort studies are suggestive of a causal relationship between beryllium exposure and an increased risk of lung cancer. This conclusion is strengthened by the increased incidences of lung cancers among workers with ABD (presumably these workers were exposed to very high concentrations of beryllium), the higher incidences of lung cancers among workers first employed when exposure levels were very high, a consistent finding of lung cancer excesses in six of seven beryllium processing facilities, and the occurrence of the highest risks for lung cancer in plants where the risk for non-malignant respiratory disease is the highest. An inhalation unit risk of 2.4 × 10–3 per µg/m3 was derived for beryllium based on the risk of lung cancer in exposed workers.
In animal studies, inhalation exposure to beryllium produced significant increases in lung cancer in rats and monkeys. Beryllium has also been shown to produce lung cancer in rats by intratracheal instillation and osteosarcomas in rabbits (and possibly mice) by intravenous injection and injection into the medullary cavity of bones.
There are no animal data available with respect to irritation of the skin and eyes from exposure to beryllium or beryllium compounds. However, both soluble and sparingly soluble compounds of beryllium have been shown to be skin sensitizers via various routes of exposure and in various animal species. Human data from exposure to soluble beryllium compounds are available with respect to skin and eye irritation. Dermal exposure of beryllium and its compounds in humans can result in a delayed-type (cell-mediated) hypersensitivity skin response.
Reproductive and developmental toxicity data are limited in animals; the few studies that are available have used parenteral routes of exposure and thus have limited relevance to humans exposed environmentally or in an occupational setting. Chronic oral studies were conducted in beagle dogs exposed to beryllium sulfate tetrahydrate. No gross or skeletal abnormalities were reported in the surviving first-litter pups upon examination of the cleared and stained preparations, which are no longer available. No animal experiments on the reproductive or developmental toxicity of inhaled beryllium are available.
Immunological effects of beryllium in humans involve a beryllium-specific cell-mediated immune response in the lung. The observation of beryllium-specific proliferation, using the beryllium lymphocyte transformation test, indicates sensitization that is highly correlated with CBD. However, sensitization is only one of the criteria used in diagnosis of CBD.
No studies were located regarding neurological effects in humans from inhalation, oral, or dermal routes of exposure to beryllium or beryllium compounds. There is no evidence of neurological effects in humans from occupational exposure; based on the minimal (<1%) absorption from the gastrointestinal tract and the lack of absorption from skin, neurological effects are not expected from breathing air, even in the workplace. In addition, oral exposure of some animal species does not result in the lesions normally associated with such exposures.
Beryllium is toxic to aquatic animals. The 96-h LC50 values ranged from 0.14 to 32.0 mg beryllium/litre, depending on the species studied and the test conditions, most notably hardness of the test water (higher toxicity in soft water). Chronic toxicity values of 0.05–1.10 mg beryllium/litre were reported in Daphnia magna at moderate water hardness (100–300 mg calcium carbonate/litre). Beryllium is phytotoxic to terrestrial plants, inhibiting growth and reducing yield at 0.5–5 mg/litre concentrations in nutrient culture solution under low- and neutral-pH conditions. In sandy soil, a concentration of 10 mg beryllium/kg reduced the yield of spring barley by 26%. At high pH, beryllium is less phytotoxic, due in part to precipitation as the phosphate salt, making it unavailable to plants. Most plants take up beryllium in small amounts, but very little is translocated within the plant. No data are available on the effects of beryllium on terrestrial animals. There is no evidence that beryllium biomagnifies within food chains.
Beryllium (Be; CAS No.
Selected physical and chemical properties of beryllium and some beryllium compounds are listed in Table 1. The metal is not soluble in water at neutral pH. Among the beryllium salts, the chloride (BeCl2), fluoride (BeF2), nitrate (Be(NO3)2), phosphate (Be3(PO4)2), and sulfate (tetrahydrate) (BeSO4 · 4H2O) are all water soluble, whereas the oxide (BeO), hydroxide (Be(OH)2), carbonate (Be2CO3(OH)2), and sulfate (anhydrous) (BeSO4) are either insoluble or slightly soluble. Aqueous solutions of the soluble beryllium salts are acidic as a result of the formation of Be(OH2)42+, the tetrahydrate, which will react to form insoluble hydroxides or hydrated complexes at pH values between 5 and 8 (US EPA, 1998).
Table 1a: Physical and chemical properties of beryllium and selected beryllium compounds.a,b
|
Property |
Beryllium |
Beryllium fluoride |
Beryllium chloride |
Beryllium oxide |
Beryllium hydroxide |
|
CAS No. |
|
|
|
|
|
|
Molecular formula |
Be |
BeF2 |
BeCl2 |
BeO |
Be(OH)2 |
|
Molecular mass |
9.012 |
47.01 |
79.93 |
25.01 |
43.03 |
|
Density (g/cm3) |
1.846 |
1.986 |
1.899 |
3.01 |
1.92 |
|
Melting point (°C) |
1287 |
555 |
405 |
2530 |
decomposes when heated |
|
Boiling point (°C) |
2970 |
1175 |
520 |
3787 |
ND |
|
Water solubility (mg/litre) |
insoluble |
extremely soluble |
very soluble |
very sparingly soluble (0.2) |
slightly soluble |
Table 1b:
|
Property |
Beryllium sulfate |
Beryllium sulfate |
Beryllium nitrate |
Beryllium carbonate |
Beryllium phosphate |
|
CAS No. |
|
77787-56-6 |
|
|
|
|
Molecular formula |
BeSO4 |
BeSO4 · 4H2O |
Be(NO3)2 |
BeCO3 + Be(OH)2 |
Be3(PO4)2 |
|
Molecular mass |
105.07 |
177.14 |
187.07 |
112.05 |
271.03 |
|
Density (g/cm3) |
2.443 |
1.713 |
1.557 |
ND |
ND |
|
Melting point (°C) |
550–600 |
100 |
60 |
ND |
ND |
|
Boiling point (°C) |
ND |
400 |
142 |
ND |
ND |
|
Water solubility (mg/litre) |
insoluble in cold water; converted to tetrahydrate in hot water |
extremely soluble |
very soluble |
insoluble in cold water; decomposes in hot water |
soluble |
a From IPCS (1990), ATSDR (1993), and US EPA (1998).
b ND = No data.
Figure 1 is a simplified chemical speciation diagram for beryllium hydroxide, Be2+, and HBeO2–, showing that minimal beryllium will be in soluble form at pH 7.5. Beryllium oxide is amphoteric (like aluminium, beryllium oxide behaves as an acid in the presence of a base, and vice versa) (Cartledge, 1928; Basolo, 1956). Because of its amphoteric character, beryllium oxide is soluble in dilute acids and alkalis, forming positive ions in dilute acids below pH 5 and negative ions called beryllates [(BeO2)2–] above pH 8 (Drury et al., 1978). Within the general physiological range (pH 5–8), beryllium tends to form insoluble hydroxides or hydrated complexes. The soluble cationic compounds, when dissolved in water, undergo hydrolysis, resulting in an acidic pH of 2.7 for iso-osmolar beryllium sulfate (Delic, 1992; HSE, 1994).
Beryllium shows a high affinity for oxygen in air and water, resulting in a thin surface film of beryllium oxide on the bare metal. The physical and chemical properties of beryllium oxide are worth noting, since it is used in many toxicological studies, and such properties are related to the firing temperatures and differences in crystal size. As one of the sparingly soluble beryllium compounds, beryllium oxide is prepared from beryllium hydroxide by calcining at temperatures between 500 and 1750 °C. Low-fired beryllium oxide is predominantly made up of poorly crystallized small particles, which are more soluble and reactive, whereas higher firing temperatures result in less reactivity due to increasing crystal size. Although the solubility of the low-fired crystals is 10 times that of the high-fired crystals, it should be pointed out that low-fired beryllium oxide is still only sparingly soluble (Delic, 1992; HSE, 1994).
Additional physical/chemical properties for beryllium (ICSC 0226), beryllium oxide (ICSC 1325), beryllium sulfate (ICSC 1351), beryllium nitrate (ICSC 1352), beryllium carbonate (ICSC 1353), beryllium chloride (ICSC 1354), and beryllium fluoride (ICSC 1355) are given in their International Chemical Safety Cards, which have been reproduced in this document.
Because most environmental samples contain only trace amounts of beryllium, proper collection and treatment of samples prior to analysis are essential (IPCS, 1990; ATSDR, 1993). Beryllium particulates in air are sampled by means of high-volume samplers using low-ash cellulose fibre, cellulose ester, or fibreglass filters. Water and urine samples are collected in borosilicate glass or plastic containers and adjusted to pH 5 or below to prevent losses from adsorption to the surface of the container. Particulate matter in the water is filtered out and analysed separately. Wet acid (nitric, sulfuric, or other acids) digestion of samples is performed to destroy organic materials, including air filters, and free the beryllium contents. Dry ashing is an alternative technique that is sometimes used to free beryllium from bone and tissue samples (Drury et al., 1978). Beryllium is separated from other elements by precipitation (this may involve considerable losses, so it is used only for separation of macro quantities of beryllium from small amounts of impurities) or chelation and extraction with an organic solvent (suitable for micro quantities of beryllium) (IPCS, 1990; ATSDR, 1993). Ion exchange techniques and electrolysis with a mercury cathode can also be used to remove interfering substances.
Detection and measurement of beryllium can be performed by many methods (reviewed by IPCS, 1990; Delic, 1992; ATSDR, 1993; HSE, 1994). As of 1992, there was no instrumentation suitable for directly detecting and measuring beryllium. However, sampling and analytical methods have been developed in the United Kingdom and the USA for measuring beryllium in air (Delic, 1992; HSE, 1994). Spectrophotometric techniques have detection limits of 100 ng beryllium and are limited by the non-specificity of the complexing agents employed (Fishbein, 1984). Fluorometric methods based on fluorescent dyes have very low detection limits (0.02 ng beryllium) but can be time-consuming and cumbersome. Emission spectroscopy is adequate in terms of both specificity and sensitivity, with limits of detection in the range of 0.5–5.0 ng beryllium (Drury et al., 1978; Fishbein, 1984). Flameless atomic absorption spectroscopy is a rapid and convenient method of beryllium analysis, with reported detection limits of 1 ng beryllium/g for faecal, hair, and fingernail samples, 0.01 ng beryllium/ml for urine samples, 0.01 ng beryllium/g for immunoelectrophoretic blood fractions, and 0.5–10.0 µg beryllium/m3 for air samples (Hurlbut, 1978; Stiefel et al., 1980; NIOSH, 1984). The highest sensitivity of any method is achieved with gas chromatography (using electron capture detectors or in combination with mass spectrometry). In preparation for this analysis, beryllium is chelated with trifluoroacetylacetone to make it volatile. Detection limits of 0.08 pg beryllium in human blood and 0.49–0.6 ng beryllium/m3 in air have been reported (Taylor & Arnold, 1971; Ross & Sievers, 1972; Wolf et al., 1972). Other techniques available include inductively coupled plasma atomic emission spectrometry (Schramel & Li-Qiang, 1982; Wolnik et al., 1984; Awadallah et al., 1986; Caroli et al., 1988), laser ion mass analysis for beryllium in tissue sections (Williams & Kelland, 1986), and laser spark spectroscopy for near real-time monitoring of trace quantities of beryllium in air (Cremers & Radziemski, 1985).
Beryllium is found in the Earth’s crust at an average concentration of approximately 2.8–5.0 mg/kg (ATSDR, 1993). It occurs in rocks and minerals at concentrations ranging from 0.038 to 11.4 mg/kg (Drury et al., 1978). The two beryllium minerals of economic significance are beryl, an aluminosilicate that contains up to 4% beryllium, and bertrandite, a beryllium silicate hydrate that contains less than 1% beryllium but is efficiently processed to beryllium hydroxide (IPCS, 1990). Total world reserves of beryllium recoverable by mining have been estimated at 200 000 tonnes (Petzow & Aldinger, 1974). Annual mining production of beryllium worldwide averaged around 400 tonnes from 1980 to 1984 but declined to less than 300 tonnes in 1991, with roughly 75% occurring in the USA (IPCS, 1990; IARC, 1993). The USA is also the leading producer and consumer of beryllium products, with Russia and Japan being the only other countries with beryllium ore processing facilities (IPCS, 1990). Beryllium metal, beryllium alloys, and beryllium oxide are the commercially important end products of beryllium processing, respectively representing 10%, 75%, and 15% of the total usage of the beryllium hydroxide obtained from ore processing (ATSDR, 1993). Beryllium metal is used primarily in the aerospace, weapons, and nuclear industries. Beryllium alloy, mostly beryllium–copper, is used in the aerospace, electronics, and mechanical industries due to its unique properties, such as high specific heat and excellent dimensional stability (low density yet very stiff). In various reactors in the nuclear industry (test, tokamak, and fusion), it is used because it has a combination of high neutron multiplication, low absorption, and high scattering characteristics (Rossman et al., 1991). Addition of only 2% of beryllium to copper forms alloys that are six times stronger than copper alone (LLNL, 1997). Beryllium oxide is used for ceramic applications, principally in electronics and microelectronics.
Annual atmospheric emissions of beryllium from production and processing average approximately 8.9 tonnes per year, representing only 4.4% of the total beryllium emissions to the air from all sources (IPCS, 1990). The primary source of beryllium in the atmosphere, responsible for emissions of 187.1 tonnes per year and 93% of all atmospheric beryllium, is the combustion of fossil fuels, especially coal (IPCS, 1990). Coal contains 1.8–2.2 mg beryllium/kg dry weight on average, and concentrations as high as 15 mg beryllium/kg have been reported (Lovblad, 1977; US EPA, 1987). Fuel oil can contain up to 100 µg beryllium/litre (Drury et al., 1978). Natural sources of beryllium release to the atmosphere, such as windblown dust and volcanic particles, are estimated to account for 5.2 tonnes per year, or 2.6% of total emissions (IPCS, 1990).
Beryllium particles produced from anthropogenic processes (ore crushing and coal combustion; i.e., over 99% of beryllium emitted into the atmosphere is the result of oil or coal combustion for electric power generation) are generally emitted as beryllium oxide (US EPA, 1987; ATSDR, 1993). Stack emissions from coal combustion consisted of beryllium particles, most of which were of a median aerodynamic diameter of <2.5 µm (Gladney & Owens, 1976). Natural and anthropogenic emissions of beryllium to the atmosphere are depicted in Table 2 (US EPA, 1987).
Beryllium is released to water in some industrial wastewater effluents, most notably treated wastewaters from iron and steel manufacturing and non-ferrous manufacturing industries (ATSDR, 1993). In 1988, 155 kg of beryllium were released to water in effluents from monitored industries, which do not include the beryllium ore processing industry (ATSDR, 1993). Other sources of beryllium in surface water include deposition of atmospheric beryllium and weathering of rocks and soils containing beryllium, although quantitative data are unavailable.
Table 2: Natural and anthropogenic emissions of beryllium to the atmosphere.a
|
Emission source |
Total US production |
Emission factor |
Emissions |
|
Natural |
|||
|
Windblown dust |
8.2 |
0.6 |
5.0 |
|
Volcanic particles |
0.41 |
0.6 |
0.2 |
|
Total |
|
|
5.2 |
|
Anthropogenic |
|||
|
Coal combustion |
640 |
0.28 |
180 |
|
Fuel oil |
148 |
0.048 |
7.1 |
|
Beryllium ore processing |
0.008 |
37.5b |
0.3 |
|
Total |
|
|
187.4 |
a
Source: US EPA (1987).b
The production of beryllium ore is expressed in equivalent tonnes of beryl; the emissions factor of 37.5 is hypothetical.Anthropogenic sources of beryllium in soil include landfill disposal of coal ash (about 100 mg beryllium/kg) (Griffitts et al., 1977) and municipal waste combustor ash, land burial of industrial wastes (22.2 tonnes from monitored industries in 1988; ATSDR, 1993), and land application of beryllium-enriched sewage sludge. Deposition of atmospheric beryllium is also a source of beryllium in soil. Quantitative data regarding the relative significance of each of these sources were not located.
Beryllium in the atmosphere is transported to water and soil by both dry and wet deposition (US EPA, 1987). It is not known if beryllium oxide in air reacts with sulfur or nitrogen oxides to produce beryllium sulfate or nitrate, but such a conversion to water-soluble compounds would accelerate removal of beryllium from the atmosphere by wet deposition. In most natural waters, the majority of beryllium will be sorbed to suspended matter or in the sediment, rather than dissolved. For example, in the US Great Lakes, beryllium is present in sediment at concentrations several orders of magnitude higher than its concentration in water (Bowen, 1979; Lum & Gammon, 1985; Rossman & Barres, 1988). Beryllium in sediment is primarily adsorbed to clay, but some beryllium may be in sediment as a result of the formation and precipitation of insoluble complexes (ATSDR, 1993). At neutral pH, most soluble beryllium salts dissolved in water will be hydrolysed to insoluble beryllium hydroxide (Callahan et al., 1979), and only trace quantities of dissolved beryllium will remain (Hem, 1970). However, at high pH, water-soluble complexes with hydroxide ions may form, increasing the solubility and mobility of beryllium. Solubility may also increase at low pH; detectable concentrations of dissolved beryllium have been found in acidified waters (US EPA, 1998).
Beryllium is not significantly bioconcentrated from water by aquatic species (Callahan et al., 1979; Kenaga, 1980; US EPA, 1980). It is also apparently not bioaccumulated from sediment by bottom feeders; beryllium levels in clams and oysters from Lake Pontchartrain, Louisiana, USA, were similar to levels in the surface sediments (Byrne & DeLeon, 1986). Most plants take up beryllium from soil in small amounts, although a few species (e.g., hickory, birch, larch) act as beryllium accumulators (Nikonova, 1967; Griffitts et al., 1977). The plant/soil transfer coefficient for beryllium has been estimated as 0.01–0.1, depending on plant species and soil properties (Kloke et al., 1984). Very little of the beryllium taken up by the roots is translocated to other plant parts (Romney & Childress, 1965), although above-ground plant parts can also be contaminated via atmospheric deposition. There is no evidence for significant biomagnification of beryllium within food chains (Callahan et al., 1979; Fishbein, 1981).
Atmospheric beryllium concentrations at rural sites in the USA ranged from 0.03 to 0.06 ng/m3 (Ross et al., 1977). These background levels probably reflect fossil fuel combustion, and lower levels may be found in less industrialized countries. Ross et al. (1977) reported beryllium concentrations of 0.04–0.07 ng/m3 at suburban sites and 0.1–0.2 ng/m3 at urban industrial sites in Dayton, Ohio, USA. Annual average beryllium concentrations at urban monitoring stations throughout the USA ranged from <0.1 to 6.7 ng/m3 during 1981–1986 (US EPA, 1987). A survey of beryllium concentrations in Japanese cities reported an average value of 0.042 ng/m3 and a maximum value of 0.222 ng/m3 (Ikebe et al., 1986). Urban areas in Germany had beryllium concentrations ranging from 0.06 to 0.33 ng/m3 (Mueller, 1979; Freise & Israel, 1987).
Higher concentrations have been reported in the vicinity of beryllium processing plants, including a mean of 15.5 ng/m3 and maximum of 82.7 ng/m3 near a Pennsylvania, USA, factory (Sussman et al., 1959), an average of 1 µg/m3 at a distance of 400 m from a beryllium extracting and processing plant in the former USSR that was not equipped with emission controls (decreasing to 10–100 ng/m3 at a distance of 1000 m) (Izmerov, 1985), and a mean of 8.4 ng/m3 (range 3.9–16.8 ng/m3) near a coal-fired power plant in Czechoslovakia (Bencko et al., 1980).
Using an atmospheric transport model, McGavran et al. (1999) estimated the air concentrations of beryllium resulting from emissions from the Rocky Flats (nuclear weapons) plant in Colorado, USA, during its operation from 1958 to 1989. Emissions passed through high-efficiency particulate air filters prior to release. The highest beryllium concentration, 6.8 ×10–2 ng/m3 (95th percentile), was predicted to occur on-site. The predicted 50th percentile air concentrations of beryllium at the predicted location of the highest off-site concentration ranged from 1.3 × 10–6 ng/m3 in 1986 to 7.3 × 10–4 ng/m3 in 1968, the year of the highest releases. These predicted off-site values do not exceed background.
Beryllium is widely distributed in soils at low concentrations. An overall average concentration of 2.8–5.0 mg/kg has been estimated (ATSDR, 1993), but this figure is skewed by relatively rare areas with large deposits of beryllium minerals, where concentrations can reach up to 300 mg/kg and average 60 mg/kg (Shacklette et al., 1971). Agricultural soils in the USA contained <1–7 mg beryllium/kg and averaged 0.6 mg beryllium/kg (Shacklette et al., 1971). In Japan, the mean soil concentration was 1.31 mg beryllium/kg (Asami & Fukazawa, 1985).
Surface waters have been reported to contain beryllium at concentrations up to 1000 ng/litre (Bowen, 1979). Beryllium concentrations ranged from <4 to 120 ng/litre in the US Great Lakes (Rossman & Barres, 1988) and from <10 to 120 ng/litre (10–30 ng/litre average) in Australian river waters (Meehan & Smythe, 1967). Based on the US EPA’s STORET database for the years 1960–1988, the geometric mean concentration of total beryllium in US surface waters was estimated to be 70 ng/litre (Eckel & Jacob, 1988). Sediments from lakes in Illinois, USA, contained 1.4–7.4 mg beryllium/kg (Dreher et al., 1977). Groundwater in Germany contained an average beryllium concentration of 8 ng/litre (Reichert, 1974). Reported levels in seawater are lower than those in fresh water, ranging from 0.04 to 2 ng/litre (Merril et al., 1960; Meehan & Smythe, 1967; Measures & Edmond, 1982). Sediments in Tokyo and Sagami bays in Japan averaged 1.29 mg beryllium/kg (Asami & Fukazawa, 1985). Beryllium concentrations in water and sediment will be higher in the vicinity of point sources; concentrations of 30–170 µg/litre have been reported in industrial effluents (ATSDR, 1993).
Beryllium is generally found in plant samples at concentrations below 1 mg/kg dry weight (IPCS, 1990), although certain species that concentrate beryllium from soils (e.g., hickory, birch, larch) may have concentrations up to 10 mg/kg dry weight (Nikonova, 1967; Griffitts et al., 1977). Concentrations up to 100 µg beryllium/kg fresh weight have been reported in various fish and other marine organisms (Meehan & Smythe, 1967; Byrne & DeLeon, 1986).
The general population may be exposed to trace amounts of beryllium by inhalation of air, consumption of drinking-water and food, and inadvertent ingestion of dust. The US EPA (1987) estimated total daily beryllium intake as 423 ng, with the largest contributions from food (120 ng/day, based on daily consumption of 1200 g of food containing 0.1 ng beryllium/g fresh weight) and drinking-water (300 ng/day, based on daily intake of 1500 g of water containing 0.2 ng beryllium/g), and smaller contributions from air (1.6 ng/day, based on daily inhalation of 20 m3 of air containing 0.08 ng beryllium/m3) and dust (1.2 ng/day, based on daily intake of 0.02 g/day of dust containing 60 ng beryllium/g). The concentration used for beryllium in food was the midpoint of a range of values reported for a variety of foods in an Australian survey (Meehan & Smythe, 1967). The concentration used for beryllium in drinking-water was based on a survey of 1577 drinking-water samples throughout the USA, where beryllium was detected in 5.4% of samples with mean and maximum concentrations of 190 and 1220 ng/litre, respectively (US EPA, 1980). The concentration used for beryllium in air was taken as a likely average concentration in a residential area based on air sampling results reported above. The concentration used for beryllium in household dust was estimated by assuming an indoor air concentration of 0.1 ng/m3 and an air/dust ratio of 600. Although intake from air and dust are minor under background conditions, these can be important pathways of exposure in the vicinity of a point source. Beryllium intake through air and dust can be increased 2–3 orders of magnitude in the vicinity of a point source, such as a coal-fired power plant (IPCS, 1990).
Tobacco smoke is another potential source of exposure to beryllium in the general population. Beryllium levels of 0.47, 0.68, and 0.74 µg/cigarette were found in three brands of cigarettes (Zorn & Diem, 1974). Between 1.6 and 10% of the beryllium content, or 0.011–0.074 µg/cigarette, was reported to pass into the smoke during smoking. Assuming the smoke is entirely inhaled, an average smoker (20 cigarettes per day) might take in approximately 1.5 µg beryllium/day (3 times the combined total of the other routes). Other potential exposures to beryllium in the general population from consumer products are limited but may include leaching of beryllium from beryllium–nickel dental alloys (Covington et al., 1985) and emission of beryllium from the mantle of gas lanterns (Griggs, 1973).
Occupational exposure to beryllium occurs in a variety of industries (see section 4), ranging from mining to golf club manufacturing. In these industries, beryllium is released into the air by various processing techniques (melting, grinding, welding, drilling, etc.). In the USA, it was estimated that 13 869 workers were potentially exposed to beryllium metal and 4305 were potentially exposed to beryllium oxide during the years 1981–1983 (NIOSH, 1989). Occupational standards of 1–5 µg beryllium/m3 (time-weighted average, or TWA) have been promulgated in the USA and other countries but are not always achieved. For example, workers at a US precious metal refinery in 1983 had TWA personal air exposures that ranged from 0.22 up to 42.3 µg beryllium/m3 (Cullen et al., 1987). Workers at a metal processing plant in Germany in 1983 who worked on beryllium-containing alloys had breathing zone air samples containing 0.1–11.7 µg beryllium/m3 (Minkwitz et al., 1983). In general, however, exposures are much lower now than in previous years. TWA daily beryllium exposures for some workers at a metal extraction and production plant that had been >50 µg beryllium/m3 during the mid-1960s and >30 µg beryllium/m3 during the mid-1970s were reduced to <2 µg beryllium/m3 in the late 1970s in compliance with the occupational exposure standard (Kriebel et al., 1988a). Prior to 1950 and implementation of emission controls, workplace concentrations exceeding 1 mg/m3 were not unusual in the USA (Eisenbud & Lisson, 1983), and similar conditions existed in the former USSR (Izmerov, 1985).
Few data are available on the particle characteristics of beryllium under occupational exposure conditions. However, Hoover et al. (1990) found that 5.7% of the particles released during sawing of beryllium metal had aerodynamic diameters smaller than 25 µm but larger than 5 µm, and 0.3% were smaller than 5 µm. For milling of beryllium metal, 12–28% of the particles had aerodynamic diameters between 5 and 25 µm, and 4–9% were smaller than 5 µm, depending on the milling depth. More than 99% of the particles generated from operations conducted with beryllium alloys were larger than 25 µm.
The temperature at which beryllium oxide is calcined influences its particle size (surface area), its solubility, and, ultimately, its toxicity. Calcination of beryllium oxide at 500 °C produces a more toxic oxide than calcination at 1000 °C, which has been attributed to the oxide’s greater specific surface area compared with that of the material calcined at 1000 °C (Finch et al., 1988; Haley et al., 1989).
Although inhalation is the primary route of uptake of occupationally exposed persons, no human data are available on the deposition or absorption of inhaled beryllium. The deposition and clearance of beryllium, like those of other inhaled particles, are governed by important factors such as dose, size, and solubility. Particles formed from volatile emissions as a result of high temperature by either nucleation (where gas molecules come together) or condensation (where gas molecules condense onto an existing particle) tend to be fine and much smaller in size than those produced by mechanical processes, where small but coarser particles are produced from larger ones.
Atmospheric beryllium is primarily in the form of particulate matter. The respiratory tract, especially the lung, is the primary target of inhalation exposure in animals and humans. Inhaled beryllium particles are deposited in the respiratory tract and subsequently cleared. Absorption of beryllium occurs following its mobilization by clearance mechanisms. Significant absorption, approximately 20% of the initial lung burden, was noted via inhalation or intratracheal instillation of soluble beryllium salts; however, for sparingly soluble compounds (e.g., beryllium oxide), absorption is slower and less substantial (Delic, 1992; HSE, 1994). Animal studies have shown that clearance of soluble and sparingly soluble beryllium compounds is biphasic via both inhalation and intratracheal administration, with an initial rapid phase attributed to mucociliary transport of particles from the tracheobronchial tree to the gastrointestinal tract, followed by a prolonged slow phase of clearance via translocation to tracheobronchial lymph nodes, uptake by alveolar macrophages, and solubilization of beryllium (Camner et al., 1977; Sanders et al., 1978; Delic, 1992; HSE, 1994). In rats, the half-time for the rapid phase is on the order of 1–60 days, while that for the slow phase is generally in the range 0.6–2.3 years and is dependent upon the solubility of the beryllium compounds — namely, weeks/months and months/years for the soluble and sparingly soluble compounds, respectively (Reeves & Vorwald, 1967; Reeves et al., 1967; Zorn et al., 1977; Rhoads & Sanders, 1985). The slow clearance from the lungs means that beryllium may remain in the human lungs for many years after exposure, and this has been observed in human workers (e.g., Schepers, 1962). The amount of beryllium remaining in the lungs at any time after exposure is a function of the amount deposited and the rate of clearance, which depend in turn on the dose, size, and solubility of the specific beryllium particles inhaled. Studies in guinea-pigs and rats indicate that 40–50% of the inhaled soluble beryllium salts are retained in the respiratory tract; however, similar data by this exposure route do not appear to be available for the sparingly soluble inorganic beryllium compounds or the metal (Delic, 1992; HSE, 1994). Rats and mice were acutely exposed by the nose-only route to aerosolized beryllium metal. A comparison of the single-dose studies demonstrated that a single, acute inhalation exposure to beryllium metal can chronically retard particle clearance and induce lung damage in rats (Haley et al., 1990) and mice (Finch et al., 1998a). Other single nose-only inhalation studies by Finch et al. (1994) using male F344/N rats exposed to beryllium metal concentrations (and a 85Sr radioactive trace) sufficient to result in mean beryllium lung burdens of 1.8, 10, and 100 µg estimated a clearance half-life of between 250 and 380 days for these three groups of rats with these different lung burdens. In the case of mice (Finch et al., 1998a), lung clearance of beryllium was segregated into two discrete groups, with clearance half-times of 91–150 days (for 1.7- and 2.6-µg lung burden groups) or 360–400 days (for 12- and 34-µg lung burden groups). However, clearance half-times were similar for rats and mice in the two most affected groups. In general, more soluble beryllium compounds are cleared more rapidly than less soluble compounds (Van Cleave & Kaylor, 1955; Hart et al., 1980; Finch et al., 1990).
Gastrointestinal absorption can occur by both the inhalation and oral (diet, drinking) routes of exposure. In the case of inhalation, a portion of the inhaled material is transported to the gastrointestinal tract by the mucociliary escalator or by the swallowing of the insoluble material deposited in the upper respiratory tract (Kjellstrom & Kennedy, 1984). Unlike inhalation, where a significant part of the inhaled dose is incorporated into the skeleton (ultimate site of beryllium storage, half-life of 450 days), oral administration results in <1% absorption and storage (as reviewed by US EPA, 1991). Most of the beryllium taken up by the oral route passes through the gastrointestinal tract unabsorbed and is eliminated in the faeces.
Beryllium is poorly absorbed from the gastrointestinal tract, probably because as soluble beryllium sulfate passes into the intestine, which has a higher pH, the beryllium is precipitated as the insoluble phosphate and thus is no longer available for absorption (Reeves, 1965).
Beryllium is also poorly absorbed through the skin, which is likely due to the fact that beryllium is bound by epidermal (alkaline phosphatase and nucleic acids) constituents or converted to an insoluble beryllium compound at physiological pH (see Fig. 1). Only trace amounts of beryllium were absorbed through the tail skin of rats exposed to an aqueous solution of beryllium chloride (Petzow & Zorn, 1974).
Beryllium and its compounds are not biotransformed, but soluble beryllium salts are converted to less soluble forms in the lung (ATSDR, 1993). Insoluble beryllium, engulfed by activated phagocytes, can be ionized by myeloperoxidases (Leonard & Lauwerys, 1987; Lansdown, 1995).
Following inhalation exposure, beryllium cleared from the lungs is distributed to the tracheobronchial lymph nodes and the skeleton, which is the ultimate site of beryllium storage (Stokinger et al., 1953; Clary et al., 1975; Sanders et al., 1975; Finch et al., 1990). Trace amounts are distributed throughout the body (Zorn et al., 1977). Like inhaled beryllium, parenterally administered beryllium salts lead to accumulation in the skeletal system (Crowley et al., 1949; Scott et al., 1950). Following oral exposure, beryllium accumulates mainly in bone, but is also found in the stomach, intestines, liver, kidney, spleen, mesenteric lymph nodes, and other soft tissues (Furchner et al., 1973; Morgareidge et al., 1975; Watanabe et al., 1985; LeFevre & Joel, 1986). Systemic distribution of the more soluble compounds is greater than that of the insoluble compounds (Stokinger et al., 1953). Transport of beryllium across the placenta has been shown in rats and mice treated by intravenous injection (Bencko et al., 1979; Schulert et al., 1979).
Absorbed beryllium is eliminated primarily in the urine (Crowley et al., 1949; Scott et al., 1950; Furchner et al., 1973; Stiefel et al., 1980), whereas excretion of unabsorbed beryllium is primarily via the faecal route shortly after exposure by inhalation or intratracheal administration, through mucociliary clearance from the respiratory tract and ingestion of swallowed beryllium (Hart et al., 1980; Finch et al., 1990). In animal ingestion studies using radiolabelled beryllium chloride in rats, mice, dogs, and monkeys, the vast majority of the ingested dose was excreted in the faeces; in most studies, <1% of the administered radioactivity was excreted in the urine (Crowley et al., 1949; Furchner et al., 1973; LeFevre & Joel, 1986). Although biliary excretion of absorbed material can lead to high faecal elimination, this is not an important pathway for beryllium (Cikrt & Bencko, 1975). In parenteral studies using carrier-free 7Be, a far higher percentage of the dose was eliminated in the urine than in the faeces (Crowley et al., 1949; Scott et al., 1950; Furchner et al., 1973). This indicates that beryllium in the faeces following oral exposure is primarily unabsorbed material and that the best estimate for oral absorption is <1%, based on urinary excretion.
As with inhalation, the elimination of beryllium following percutaneous incorporation of soluble beryllium nitrate as 7Be demonstrated that more than 90% was eliminated via urine (Zorn et al., 1977). Mean daily excretion of beryllium metal was 4.6 × 10–6 % of the administered dose (intratracheal instillation) in baboons and 3.1 × 10–6 % in rats (Andre et al., 1987). Urinary excretion of beryllium following occupational exposure correlates qualitatively with degree of exposure (Klemperer et al., 1951). Elimination half-times of 890–1770 days (2.4–4.8 years) were calculated for mice, rats, monkeys, and dogs injected intravenously with beryllium chloride (Furchner et al., 1973). A half-life of 450 days has been estimated for beryllium in the human skeleton (ICRP, 1960). More than 99% of ingested radioberyllium as 7BeCl2 was excreted in the faeces, and 0.002% of the ingested amount was transferred to the milk in dairy cows (Mullen et al., 1972).
Inhaled beryllium is highly toxic by acute exposure. The acute 4-h LC50 value for beryllium sulfate was reported to be 0.15 mg beryllium/m3 in rats; that for beryllium phosphate was 0.86 mg/m3 (Venugopal & Luckey, 1977). In guinea-pigs, the 4-h LC50 for beryllium phosphate was 4.02 mg beryllium/m3. Single 1-h nose-only exposure to beryllium sulfate at 4.05 mg beryllium/m3 (mass median aerodynamic diameter 1.9 µm, geometric standard deviation [GSD] 1.89) resulted in the progressive development of pneumonitis and pleural plaques in rats (Sendelbach et al., 1989). Comparable exposure to sulfuric acid did not produce any significant pathological effects in the lungs, indicating that the effects were not due to the low pH of the beryllium sulfate dissolved in water (~2.7) or the anion.
Inhalation studies in rats and dogs exposed to beryllium oxide produced pneumonitis, granulomatous lesions, fibrosis, and hyperplasia. In beagle dogs, single, acute, nose-only inhalation exposure to beryllium oxide calcined at 500 or 1000 °C induced granulomatous pneumonia, lymphocytic infiltration into the lung, and positive beryllium-specific lymphocyte proliferative responses in vitro. The changes were more marked after exposure to beryllium oxide calcined at 1000 °C.
In testing the sensitivity of the transgenic heterozygous p53 knockout mouse model for predicting cancer via inhalation, mice of both sexes were exposed by nose-only inhalation to either air (controls) or beryllium metal (15 or 60 µg). Only the transgenic heterozygous mouse (p53+/–) was susceptible to carcinogenesis (Finch et al., 1998b).
Inflammatory and granulomatous lesions were observed after intratracheal instillation of beryllium oxide or beryllium hydroxide in rats and also in rats acutely exposed (nose-only) to beryllium metal via inhalation (Haley et al., 1990, 1992; Finch et al., 1994) or intratracheal instillation (LaBelle & Cucci, 1947).
A follow-up study demonstrated that short-term nose-only exposure to aerosolized beryllium metal can also retard particle clearance and induce lung damage in mice (Finch et al., 1998a). The mass of beryllium metal required to induce lung damage in mice is similar to that needed for rats. However, there is a significant accumulation of lymphocytes in mice, while no such change was observed in rats (Finch et al., 1994, 1996; Nikula et al., 1997).
A single exposure of A/J (H-2a haplotype) mice to beryllium sulfate via intratracheal instillation resulted in histological changes in the lungs but not in BALB/c or C57BL6/J mice. Similar histological changes were also induced in the lung by beryllium oxide, which correlated with bronchoalveolar lavage (BAL) cellularity, but these changes were greatly delayed and did not proceed to frank granulomas. Only BAL lymphocytes from mice preimmunized with beryllium sulfate/serum and challenged with beryllium sulfate/serum showed significant in vitro proliferation in response to beryllium sulfate (Huang et al., 1992).
Soluble beryllium compounds administered orally are moderately toxic. Oral LD50 values reported for beryllium compounds, including beryllium fluoride, beryllium chloride, beryllium sulfate, and a mixture of beryllium fluoride and beryllium oxide, ranged from 18.3 mg/kg body weight for the mixture (beryllium fluoride and beryllium oxide) to 200 mg/kg body weight for beryllium chloride in rats and 18–20 mg/kg body weight for beryllium fluoride to 140 mg/kg body weight for beryllium sulfate in mice (ATSDR, 1993). The sparingly soluble beryllium oxide had little or no effect on the LD50 of beryllium fluoride.With the exception of beryllium fluoride (fluoride ion also contributes to toxicity), the differences in the LD50 values of the other beryllium compounds are due to differences in solubility and the potential to form insoluble beryllium phosphate in the gastrointestinal tract (ATSDR, 1993).
No dermal single-dose studies were available for soluble and sparingly soluble beryllium compounds (Delic, 1992; HSE, 1994).
No data were located regarding the dermal or ocular irritancy of beryllium in laboratory animals. Both the soluble and sparingly soluble compounds of beryllium have been shown to be skin sensitizers in guinea-pigs, rabbits, mice, and pigs. Skin sensitization has been achieved following induction via the topical, intradermal, inhalation, or intratracheal routes of exposure. Cell-mediated passive transfer of the sensitized state has been demonstrated in guinea-pigs and mice. Several studies that have been identified in reviews (Delic, 1992; ATSDR, 1993; HSE, 1994) have demonstrated cutaneous hypersensitivity reactions to beryllium in guinea-pigs (Alekseeva, 1966; Belman, 1969; Marx & Burrell, 1973; Zissu et al., 1996). In these studies, guinea-pigs were sensitized by repeated intradermal injection or dermal application of small doses of soluble beryllium salts. In the study by Marx & Burrell (1973), skin reactions developed 6–8 h after the subsequent patch test challenge and lasted up to 3 weeks. The severity of the skin reaction was greater when a more soluble salt was used for the challenge (fluoride > sulfate > oxide). In a similar study, Krivanek & Reeves (1972) found that beryllium-sensitized guinea-pigs (beryllium sulfate) elicited different skin reactions depending on the beryllium compound used. The forms of beryllium used in the elicitation reaction were the sulfate, the hydrogencitrate, the albuminate, and the aurintricarboxylate. Each of these anions binds beryllium in solution with different tenacity. The beryllium–albuminate produced the greatest hypersensitivity, followed by beryllium sulfate, whereas beryllium–hydrogencitrate and beryllium–aurintricarboxylate produced essentially negative reactions due to the fact that the beryllium was strongly bound to the anion and therefore unavailable for interaction with the skin. Zissu et al. (1996) found hypersensitivity reactions in 30–60% of guinea-pigs sensitized with beryllium sulfate in response to challenge with beryllium–copper and beryllium–aluminium alloys. Vacher (1972) reported that skin contact was necessary for development of a hypersensitivity reaction to beryllium (parenteral administration did not elicit an immunological reaction) and that only forms of beryllium capable of complexing with skin constituents were immunogenic.
Respiratory sensitization studies in dogs exposed to aerosols of beryllium oxide demonstrated a sensitization that was specific to beryllium (based on immune responses of lymphocytes harvested from lung and blood) compared with a lack of a proliferative response when lymphocytes from treated dogs were tested against zinc sulfate and nickel sulfate and with a series of common canine antigens (Haley et al., 1997).
Other immunological studies that may not be relevant to human exposure via inhalation have been conducted. In the case of cynomolgus monkeys dosed intrabronchially with beryllium metal or beryllium oxide (calcined at 500 °C), beryllium-specific lymphocyte proliferation did not increase for lymphocytes from beryllium oxide-exposed (intrabronchiolar instillation) lung lobes, but did increase with exposure to the metal (Haley et al., 1994). In the case of mice (A/J and C3H/HeJ) exposed to a single, relatively high lung burden of inhaled beryllium metal, there were increases in lymphocyte proliferation in the lungs, but beryllium-specific proliferation of lymphocytes was not observed in the beryllium lymphocyte transformation test (BeLT) using lymphocytes from peripheral blood, the spleen, or bronchial lymph nodes. A lack of beryllium-specific proliferation of lymphocytes was also observed in mice (Balb/c or C57B1) administered a single intratracheal dose of beryllium sulfate using the BeLT with BAL lymphocytes (Huang et al., 1992). Although these two latter studies differed in the beryllium compound studied and neither demonstrated a beryllium-specific response, the observed granulomas did have an immune component.
With the exception of beryllium oxide and beryllium hydrogenphosphate (BeHPO4), few sparingly soluble salts of beryllium have been studied via this exposure route (Delic, 1992; ATSDR, 1993; HSE, 1994). Short-term inhalation exposure to beryllium produces acute chemical pneumonitis in laboratory animals. Schepers (1964) exposed groups of four monkeys to aerosols (particle size not reported) of beryllium sulfate, beryllium fluoride, or beryllium phosphate for 7–30 days. Similar effects were noted in all exposed monkeys; however, in the monkeys exposed for only 7 days, recovery was observed. In contrast to beryllium fluoride, no notable effects were observed in other extrapulmonary tissues following exposure to beryllium sulfate. Observed effects included severe weight loss, dyspnoea, pulmonary oedema, congestion, and marked changes in the liver (hepatocellular degeneration), kidneys (glomerular degeneration), and other internal organs (adrenals, pancreas, thyroid, and spleen). The soluble salts produced these effects at lower concentrations (184 µg beryllium/m3 for the fluoride, 198 µg beryllium/m3 for the sulfate) than the slightly soluble phosphate (1132 µg beryllium/m3). The excessive toxicity observed with beryllium fluoride compared with beryllium sulfate cannot be explained on the basis of solubility (roughly the same) but is probably due to the toxicity exhibited by the fluoride anion, as was previously noted in section 8.1.2 with respect to LD50 values of beryllium fluoride.
Insoluble beryllium oxide (low-fired at 400 °C) also produced pneumonitis in rats and dogs after a 40-day exposure to 3.6 mg beryllium/m3 (Hall et al., 1950). However, beryllium oxides high-fired at 1150 or 1350 °C did not produce pulmonary damage after a 360-h exposure to 32 mg beryllium/m3, possibly due to greater particle size and greater degree of aggregation in the high-fired beryllium oxides.
There appear to be no reports available on the short-term toxicity of soluble beryllium compounds and only a few addressing the toxicity of beryllium carbonate (BeCO3), a sparingly soluble compound. A number of experiments have demonstrated that rats fed between 0.5 and 6% of beryllium carbonate in their diet over periods of 14–168 days developed rickets of the bone and teeth (Delic, 1992; HSE, 1994). Guyatt et al. (1933) and Kay & Skill (1934) reported rickets in rats fed diets containing up to 3% beryllium carbonate for 20–28 days. The observed bone lesions were not attributed to any direct effects from beryllium itself, but to phosphorus deprivation due to precipitation as beryllium phosphate in the intestine. Matsumoto et al. (1991) fed groups of 10 male Wistar rats diets containing 0 or 3% beryllium carbonate for 4 weeks, at which time the rats fed the beryllium diet weighed approximately 18% less than the controls (statistical significance not reported) and had significantly reduced serum phosphate and alkaline phosphatase levels. Although this study did not include examination of bone for rickets, the observed changes are consistent with effects reported by Guyatt et al. (1933) and Kay & Skill (1934). Jacobson (1933) caused growing rats to develop osteoporosis upon feeding them food deficient in calcium and rickets upon the addition of beryllium carbonate to their food. He concluded, like the other authors, that rickets is caused by phosphorus deprivation.
With respect to the dermal route of exposure, no information was found on the effects of soluble and sparingly soluble beryllium compounds (Delic, 1992; HSE, 1994).
No medium-term (or long-term; see section 8.5) studies are available on non-neoplastic effects of beryllium oxide, the most environmentally relevant form of beryllium. Although a number of subchronic studies in laboratory animals have been conducted with other beryllium compounds, none has been done using modern criteria for high-quality toxicology studies. In addition, it is not clear which animal species, if any, is an appropriate model for humans. No laboratory animal model fully mimics all features of human chronic beryllium disease (CBD). In particular, animal models have not demonstrated a progressive granulomatous pulmonary response with a concomitant beryllium-specific immune response. However, several laboratory animal species (e.g., mice, guinea-pigs, dogs, and monkeys) respond to beryllium exposure with some of the features of human CBD, and one or more of these species may provide reasonable models for human CBD.
The pulmonary effects of beryllium following subchronic exposure in animals have been described by researchers. Stokinger et al. (1950) exposed rats, dogs, cats, rabbits, guinea-pigs, hamsters, monkeys, and goats via inhalation to 40, 430, or 2000 µg beryllium/m3 as beryllium sulfate tetrahydrate aerosols (particle size varied between 0.25 and 1.1 µm, with an average mass median diameter of the aerosol of 1 µm) for 6 h/day, 5 days/week, for 100, 95, or 51 days, respectively. Signs of toxicity in the exposed animals included weight loss, anaemia, and, in the two highest-dose groups, mortality. All histopathological lesions were confined to the lungs, with an interstitial and intra-alveolar infiltration of monocytes, polymorphonuclear leukocytes, lymphocytes, and plasma cells. Macrophages containing cellular debris were observed within the alveoli. The exposure levels at which histopathological lesions were observed in each species were not specified. Similar results were found in rats, rabbits, dogs, and cats exposed to 186 µg beryllium/m3 as beryllium fluoride for 6 h/day, 5 days/week, for 207 calendar days (Stokinger et al., 1953).
Vorwald & Reeves (1959) followed the time course of lung changes in rats exposed to an aerosol (particle size not reported) of beryllium sulfate at 6 or 54.7 µg beryllium/m3 for 6 h/day, 5 days/week, for over 9 months. Initially, inflammation consisted of histiocytes, lymphocytes, and plasma cells scattered throughout the lung parenchyma. Following more prolonged exposures, more focal lesions consisting primarily of histiocytes were observed. Subsequently, multinucleated giant cells, thickened alveolar walls, and fibrotic changes were also detected. Lung tumours, primarily adenomas and squamous cell cancers, developed in the animals sacrificed after 9 months of this exposure regime. Similarly, Schepers et al. (1959) found lung inflammation in monkeys after a 6-month (8 h/day, 5.5 days/week) exposure to beryllium sulfate at 28 µg beryllium/m3, followed by subsequent development of granulomas and adenomas months after the end of exposure (the study was continued until 18 months post-exposure).
Eight male albino rats (strain not specified) fed a standard diet and given a total dose of 20 mg (~0.14 mg/kg body weight per day) beryllium nitrate orally every third day for 2.5 months (40 doses administered) showed a number of histological alterations in the lungs relative to four male controls fed the standard diet (Goel et al., 1980). Although the method was not adequately described, it appears that the beryllium nitrate was placed on the food in a powder form; thus, the animals may have inhaled some of the beryllium. The histological alterations included congestion and ruptured ciliated epithelial cells of the respiratory bronchioles, thickened epithelial cells and necrosis in the alveoli, and damage to the arteriolar endothelium.
In a study to test the carcinogenicity of beryllium ores, Wagner et al. (1969) exposed groups of 12 male squirrel monkeys, 60 male CR-CD rats, 30 male Greenacres Controlled Flora (GA) rats, and 48 male golden Syrian hamsters to bertrandite or beryl ore at 0 or 15 mg/m3 for 6 h/day, 5 days/week, for 17 months (rats and hamsters) or 23 months (monkeys). The test atmospheres generated from the bertrandite ore (Be4Si2O7(OH)2; 1.4% beryllium) and beryl ore (Be3Al2Si6O18; 4.14% beryllium) contained 210 and 620 µg beryllium/m3, respectively, and the geometric mean diameters of the particles were 0.27 µm (GSD of 2.4) and 0.64 µm (GSD of 2.5). Both ores contained very high silicon dioxide (SiO2) levels (63.9% by weight). Exposed and control monkeys, rats, and hamsters were serially sacrificed upon completion of 6 and 12 months of exposure — rats and hamsters at the 17th month, and monkeys at the 23rd month. Five control rats and five rats from the 12- and 17-month exposure groups were sacrificed in order to determine the free silica content of the lung tissue. At exposure termination, beryllium concentrations in the lungs were 18.0 and 83 µg/g fresh tissue in the bertrandite- and beryl-exposed rats, 14.1 and 77.4 µg/g fresh tissue in the bertrandite- and beryl-exposed hamsters, and 33 and 280 µg/g fresh tissue in the bertrandite- and beryl-exposed monkeys. Free silica (silicon dioxide) levels in the rat lungs were 30–100 times higher in the beryllium ore-exposed rats than in the controls.
Increased mortality was observed in the monkeys (11%), rats (13%), and hamsters (25%) exposed to either bertrandite or beryl ore, with the highest mortality rates in the bertrandite ore-exposed animals (no further details provided) (Wagner et al., 1969). No significant alterations in body weight gain were observed in the monkeys or hamsters. In the rats, decreased body weight gains were observed beginning after 6 months of exposure; terminal body weights were 15% lower than in controls. In the beryl-exposed rats, small foci of squamous metaplasia or tiny epidermoid tumours were observed in the lungs of 5/11 rats killed after 12 months of exposure. At exposure termination, lung tumours were observed in 18/19 rats (18 had bronchiolar alveolar cell tumours, 7 had adenomas, 9 had adenocarcinomas, and 4 had epidermoid tumours). Additional alterations in the lungs included loose collections of foamy macrophages and cell breakdown products, lymphocyte infiltrates around the bronchi, and polymorphonuclear leukocytes and lymphocytes present in most of the bronchiolar alveolar cell tumours. In the bertrandite-exposed rats, granulomatous lesions composed of several large, tightly packed, dust-laden macrophages were observed in all rats exposed for 6, 12, or 17 months. No tumours were observed. Neoplastic or granulomatous pulmonary lesions were not observed in the control rats. In the beryl- and bertrandite-exposed monkeys, the histological alterations consisted of aggregates of dust-laden macrophages, lymphocytes, and plasma cells near respiratory bronchioles and small blood vessels. No tumours were found. In the bertrandite-exposed hamsters, granulomatous lesions consisting of tightly packed, dust-laden macrophages were observed after 6 months, and the number did not increase after 17 months. These alterations were not observed in the beryl-exposed or control hamsters. Atypical proliferation and lesions, which were considered bronchiolar alveolar cell tumours except for their size, were observed in the hamsters after 12 months of exposure to beryl or bertrandite. After 17 months of exposure, these lesions became larger and more adenomatous in the beryl-exposed hamsters. It should be noted that silicosis was not observed in any of the animals exposed to the beryllium ores that contained a large amount of free silica. No significant gross or histological alterations were observed in the thymus, spleen, liver, or kidneys of the beryllium-exposed rats, hamsters, or monkeys.
Exposure to 35 µg beryllium/m3 as beryllium sulfate mist for as many as 4070 h over a 7-year period produced lung tumours in 8/12 rhesus monkeys (age 18 months at the start of the study) that survived the first 2 months of the study (four animals died of acute chemical pneumonitis during the first 2 months) (Vorwald, 1968). Several other studies also reported lung tumours (adenoma, adenocarcinoma, squamous cell cancers) in rats exposed to beryllium sulfate aerosols (Schepers et al., 1959; Vorwald & Reeves, 1959; Reeves et al., 1967; Reeves & Deitch, 1969). In addition to the proliferative lung response, an inflammatory lung response (accumulation of histiocytic elements, thickened alveolar septa, increased lung weight) was also typically present. Exposure concentrations in these studies ranged from 6 to 54.7 µg beryllium/m3. Exposure durations that produced tumours were as short as 3 months, although the tumours generally did not develop before 9 months. The earliest proliferative response was hyperplasia, which was observed as soon as 1 month after the start of exposure. Age at the initiation of exposure may be a more important variable than duration of exposure for tumour development. Reeves & Deitch (1969) found that lung tumour incidence in young rats exposed for 3 months (19/22, 86%) was the same as in young rats exposed for 18 months (13/15, 86%), but was higher than in older rats exposed for 3 months (3–10/20–25, 15–40%). Nickell-Brady et al. (1994) showed that a single brief exposure (8–48 min) to a high concentration of beryllium metal aerosol (410–980 mg beryllium/m3), producing a lung burden of 40–430 µg of beryllium, was sufficient to induce subsequent development of lung tumours in rats. In limited studies, beryllium oxide and beryllium chloride have also induced lung tumours in rodents after inhalation exposure (IARC, 1993).
Morgareidge et al. (1976) conducted a long-term feeding study in which groups of five male and five female beagle dogs (aged 8–12 months) were fed diets containing 0, 5, 50, or 500 ppm (mg/kg) of beryllium as beryllium sulfate tetrahydrate for 172 weeks. Because of overt signs of toxicity, the 500 ppm group was terminated at 33 weeks. At this time, a group of five male and five female dogs was added to the study and fed a diet containing 1 ppm beryllium for 143 weeks. Using estimated TWA body weights and the reported average food intake, the 1, 5, 50, and 500 ppm concentrations correspond to doses of 0.023, 0.12, 1.1, and 12.2 mg beryllium/kg body weight per day for male dogs and 0.029, 0.15, 1.3, and 17.4 mg beryllium/kg body weight per day for females. The following parameters were used to assess toxicity: daily observations of appearance and behaviour, food consumption, body weight, haematology, serial serum clinical chemistry, serial urinalysis, organ weights, and extensive histopathology.
Two moribund animals in the 500 ppm group were sacrificed during week 26; the remainder of the animals in the 500 ppm group were killed during week 33 (Morgareidge et al., 1976). Overt signs of toxicity observed in the 500 ppm group included lassitude, weight loss, anorexia, and visibly bloody faeces, indicating that the maximum tolerated dose (MTD) is less than 500 ppm. Four other animals died during the course of the study or were killed moribund; two dogs died during parturition (dose groups not reported), and one male and one female dog in the 50 ppm group died. The appearance, behaviour, food intake, and body weight gain of the animals in the other beryllium groups did not differ from controls. No beryllium-related haematological, serum chemistry, or urinalysis alterations were observed in the 1, 5, or 50 ppm groups. In the 500 ppm group, a slight anaemia (slight decreases in erythrocyte, haemoglobin, and haematocrit; statistical analysis not reported), more apparent in the females than in the males, was observed after 3 and 6 months of exposure; however, no bone marrow changes were noted, and none of the animals was seriously affected. The authors suggested that the anaemia may have been related to the gastrointestinal tract haemorrhages (see below) rather than a direct effect of beryllium on the haematological system. No alterations in organ weights were observed. All animals in the 500 ppm group showed fairly extensive erosive (ulcerative) and inflammatory lesions in the gastrointestinal tract. These occurred predominantly in the small intestine and to a lesser extent in the stomach and large intestine and were regarded by the authors as treatment-related. The female dog that died in the 50 ppm group (after 70 weeks of treatment) showed gastrointestinal lesions occurring in the same locations and appearing to be the same types, but less severe, as those in dogs administered 500 ppm. The authors stated that the death of this animal appeared to be related to beryllium administration. Gastrointestinal lesions were not reported in the male that died at this dose level. Other animals in this treatment group survived until study termination and had no remarkable gross or microscopic findings. No treatment-related effects were reported in the 1 or 5 ppm group dogs. Combined incidence rates for gastrointestinal lesions in male and female dogs were 0/10, 0/10, 0/10, 1/10, and 9/10 in the 0, 1, 5, 50, and 500 ppm groups, respectively. No neoplasms were observed in the beryllium-exposed dogs. Reproductive end-points are discussed in section 8.7. An independent review of the study report supported the researchers’ conclusion that the gastrointestinal lesions were treatment-related.8.5.3 Other routes
Beryllium metal, passivated beryllium metal (99% beryllium, 0.26% chromium as chromate), beryllium–aluminium alloy, beryllium hydroxide, and low- and high-fired beryllium oxide have been shown to produce lung cancer in rats by intratracheal instillation. Beryllium metal, zinc beryllium silicate, beryllium silicate, beryllium oxide, and beryllium phosphate produced osteosarcomas in rabbits by intravenous injection, and beryllium oxide, zinc beryllium silicate, and beryllium carbonate produced osteosarcomas in rabbits after injection into the medullary cavity of bones (US EPA, 1987; IARC, 1993).
Genotoxicity studies of beryllium have produced mixed results. Most studies have found that beryllium chloride, beryllium nitrate, beryllium sulfate, and beryllium oxide did not induce gene mutations in bacterial assays, with or without metabolic activation. In the case of beryllium sulfate, all mutagenicity studies (Ames Salmonella typhimurium [Simmon, 1979; Dunkel et al., 1984; Arlauskas et al., 1985; Ashby et al., 1990]; Escherichia coli pol A [Rosenkranz & Poirer, 1979]; E. coli WP2 uvr A [Dunkel et al., 1984]; and Saccharomyces cerevisiae [Simmon, 1979]) were negative, with the exception of results reported for Bacillus subtilis rec assay (Kada et al., 1980; Kanematsu et al., 1980) and E. coli rec assay (Dylevoi, 1990).
Beryllium nitrate was negative in the Ames assay (Tso & Fung, 1981; Kuroda et al., 1991) but positive in a B. subtilis rec assay (Kuroda et al., 1991). Beryllium chloride was negative in a variety of studies (Ames [Ogawa et al., 1987; Kuroda et al., 1991]; E. coli WP2 uvr A [Rossman & Molina, 1986]; and B. subtilis rec assay [Nishioka, 1975]). In addition, beryllium chloride failed to induce SOS3repair in E. coli (Rossman et al., 1984). However, positive results were reported for B. subtilis rec assay using spores (Kuroda et al., 1991), E. coli KMBL 3835, lacI gene (Zakour & Glickman, 1984), and hprt locus in Chinese hamster lung V79 cells. Beryllium oxide was negative in the Ames assay and B. subtilis rec assays (Kuroda et al., 1991).
Gene mutations have been observed in mammalian cells cultured with beryllium chloride (Hsie et al., 1979a,b; Miyaki et al., 1979), and culturing mammalian cells with beryllium chloride (Vegni-Talluri & Guiggiani, 1967), beryllium sulfate (Larramendy et al., 1981; Brooks et al., 1989), or beryllium nitrate has resulted in clastogenic alterations. Beryllium sulfate did not induce unscheduled DNA synthesis in primary rat hepatocytes (Williams et al., 1982), but did induce morphological transformations in cultured mammalian cells. Data on the in vivo genotoxicity of beryllium are limited to two studies. Beryllium sulfate (1.4 and 2.3 g/kg body weight, 50% and 80% of median lethal dose) administered by gavage did not induce micronuclei in the bone marrow of CBA mice, although a marked depression of erythropoiesis suggestive of bone marrow toxicity was evident 24 h after dosing. No increase in mutations was found in p53 or c-raf-1, and only a small increase in mutations was detected in K-ras, in lung carcinomas (tumours became apparent by 14 months after exposure, and the incidence, apparently for all groups combined, was 64% over the lifetime of the rats) from F344/N rats given a single nose-only exposure to beryllium metal (Nickell-Brady et al., 1994).
Ionized beryllium can bind to nucleic acids (Leonard & Lauwerys, 1987; Lansdown, 1995), and beryllium sulfate and beryllium chloride have been shown to affect enzymes required for DNA synthesis (Leonard & Lauwerys, 1987).
There are limited data available regarding the reproductive and developmental toxicity of beryllium compounds in animals by routes of exposure that are relevant to humans. In the chronic dog oral exposure study conducted by Morgareidge et al. (1976) (described in section 8.5.2), the male and female dogs exposed to 1, 5, or 50 ppm (mg/kg) beryllium sulfate in the diet (0.023, 0.12, or 1.1 mg beryllium/kg body weight per day for males and 0.029, 0.15, or 1.3 mg beryllium/kg body weight per day for females) were housed together at the time of the second heat after treatment initiation and allowed to mate and wean (at 6 weeks of age) their pups, which were then returned to community floor pens (with the exception of the first litter, which was killed 5 days after whelping). The number of pregnant females exposed to 0, 1, 5, and 50 ppm was 3, 2, 5, and 3, respectively. Treated females had between 1 and 3 litters; controls had 1–4, each litter by the same sire. First-litter pups surviving to postnatal day 5 were sacrificed for soft tissue gross examination and were stained for evaluation of skeletal malformations. Pups from subsequent litters were grossly examined at weaning. Beryllium did not appear to adversely affect reproductive or developmental end-points (number of pregnancies, number of pups, number of live pups, pup weight) in the beryllium-exposed dogs. No beryllium-related decreases in postnatal survival (day 7 or weaning) were observed. The authors reported no gross or skeletal abnormalities in the surviving first litter pups, but data were not shown; stillborn or cannibalized pups dying within the first few postnatal days were not examined.
No animal experiments of the reproductive or developmental toxicity of inhaled beryllium are available.
Studies by parenteral routes of administration have produced mixed results regarding the reproductive and developmental toxicity of beryllium. Selivanova & Savinova (1986) reported increased fetal mortality, decreased fetal weight, and increased percentage of pups with internal abnormalities in rats treated with 50 mg beryllium/kg body weight as beryllium chloride or beryllium oxide by intratracheal injection on days 3, 5, 8, and 20 of gestation. Mathur et al. (1987) administered intravenous injections of 0.021 mg beryllium/kg body weight as beryllium nitrate to mated Sprague-Dawley rats (n = 5–8 per group) on postcoital day 1, 11, 12, 13, 15, or 17. Rats were laparotomized on gestation days 10 and 20 and then allowed to deliver. All experimental groups were contrasted with control groups, which received an equal volume of distilled water through the intravenous route at day 1 postcoitum and were laparotomized on day 20 postcoitum. All pups died within 2–3 days of birth, and all pups in the group injected on postcoital day 11 died in utero, but these effects may have been due to the repeated surgeries. Clary et al. (1975) found no effect on the average number of pregnancies, number of live or dead pups per litter, lactation index, or fetal body weight in continuous breeding experiments using male and female Sprague-Dawley rats given a single intratracheal administration of beryllium oxide (200 µg beryllium) calcined at 960 °C or 500 °C prior to the first mating. A review of these three parenteral studies reveals no information regarding maternal toxicity.
An immune component has also been demonstrated in several animal models for CBD: mice (Huang et al., 1992), guinea-pigs (Barna et al., 1981, 1984), dogs (Haley et al., 1989), and monkeys (Haley et al., 1994) (rats are the notable exception). In guinea-pigs, for example, Barna et al. (1981, 1984) demonstrated that intratracheal instillation of beryllium oxide can induce both immune granulomas containing a T-lymphocyte component and a beryllium-specific immune response. The studies in mice and guinea-pigs demonstrated genetic control of the immune response in these species because the response was observed in some strains, but not others. In the case of the guinea-pigs, the immune response was seen in strain 2, but not strain 13, which differs from strain 2 at a single locus (MHC Ia) (Barna et al., 1984). Dermal hypersensitivity studies in laboratory animals are discussed in section 8.2. In addition to the above studies, other immunological studies via parenteral routes of exposure to various beryllium compounds (beryllium sulfate, beryllium hydroxide, or beryllium oxide) are cited in Delic (1992) and HSE (1994).
Lesions that would be associated with neurological effects in the brain and spinal cord were absent in rats exposed orally (diet) for 2 years to ł 31 mg beryllium/kg body weight per day as beryllium sulfate tetrahydrate.
The lung is the primary target of inhalation exposure to beryllium in humans. Information on the subchronic toxicity of beryllium following inhalation exposure to humans was unavailable; however, information regarding acute and chronic exposure via inhalation is extensive, especially chronic data. Short-term or repeated exposures of humans to beryllium or its compounds can result in an acute (chemical pneumonitis) or chronic (berylliosis) form of lung disease, depending upon the exposure concentration. Both acute beryllium disease (ABD) and CBD result from exposure to both soluble and insoluble forms of beryllium (ATSDR, 1993).
ABD is defined as beryllium-induced pulmonary disease of less than 1 year’s duration (Hamilton & Hardy, 1974; Sprince & Kazemi, 1980) and is likely to be due to direct toxicity, unlike the immune mechanism of CBD. ABD, which is in fact a chemical pneumonitis, may be fatal in 10% of cases. The severity of ABD appears to depend directly on the magnitude of the exposures received. Early investigations by Eisenbud et al. (1948) found that chemical pneumonitis occurred in almost all workers exposed to 1000 µg beryllium/m3 and above and in none exposed to less than 100 µg/m3 and appears to be reversible at concentrations of less than 1000 µg beryllium/m3 (Delic, 1992; HSE, 1994).
CBD, formerly known as "berylliosis" or "chronic berylliosis," is an inflammatory lung disease that results from inhalation exposure to both soluble and insoluble forms of beryllium. CBD in humans exposed to beryllium involves a cell-mediated immune response to beryllium in the lung. It is characterized by the formation of granulomas (pathological clusters of immune cells) with varying degrees of interstitial fibrosis and involves a beryllium-specific immune response. With respect to the present knowledge of the mechanism of this chronic disease, the initial response is of an inflammatory nature caused by beryllium-induced phagocytosis and the subsequent release of lysosomal enzymes. The release of such enzymes is thought to be responsible for the damaging effects observed with respect to lung architecture. Unlike other particles (e.g., alpha quartz), beryllium is unusual in that it also initiates cell-mediated immune responses. Because the beryllium ion is too small to be antigenic per se, it may function as a hapten, binding with a large carrier molecule (e.g., protein) to form an antigen. There is ample evidence for the existence of cell-mediated immune responses to beryllium (Kriebel et al., 1988b). Once the antigen is created by the macrophage, it is presented to helper T-cells, which form sensitized T-cells. Once a population of sensitized T-cells is created, these cells transform and actively secrete lymphokines, which actively regulate subsequent events, leading to the formation of clusters of macrophages commonly known as "granulomas."
A particularly important part of the diagnosis of CBD is to distinguish it from sarcoidosis, a granulomatous lung disease of unknown cause. The Beryllium Case Registry (BCR) lists the following criteria for diagnosing CBD:
|
(1) |
establishment of significant beryllium exposure based on sound epidemiological history; |
|
(2) |
objective evidence of lower respiratory tract disease and clinical course consistent with beryllium disease; |
|
(3) |
chest X-ray films with radiological evidence of interstitial fibronodular disease; |
|
(4) |
evidence of restrictive or obstructive defect with diminished carbon monoxide diffusing capacity by physiological studies of lung function; |
|
(5a) |
pathological changes consistent with beryllium disease on examination of lung tissue; and |
|
(5b) |
presence of beryllium in lung tissue or thoracic lymph nodes. |
Cases were entered into the registry if they met at least three of the criteria (Hasan & Kazemi, 1974).
The criteria for diagnosis of CBD have evolved with time, as more advanced diagnostic technology has become available. These varying definitions of CBD should be considered in comparing results from different studies. More recent criteria have both higher specificity than earlier methods and higher sensitivity, identifying subclinical effects. Recent studies typically use the following criteria:
|
(1) |
history of beryllium exposure; |
|
(2) |
histopathological evidence of non-caseating granulomas or mononuclear cell infiltrates in the absence of infection; and |
|
(3) |
a positive blood or BAL lymphocyte transformation test (Newman et al., 1989). |
The availability of transbronchial lung biopsy facilitates the evaluation of the second criterion, by making histopathological confirmation possible in almost all cases.
A key aspect of the identification of CBD is the demonstration of beryllium sensitization in the BeLT (also known as the LTT, BeLPT) (Newman, 1996). In this test, lymphocytes obtained from either BAL fluid or peripheral blood are cultured in vitro and then exposed to soluble beryllium sulfate to stimulate lymphocyte proliferation. The observation of beryllium-specific proliferation indicates beryllium sensitization. Early versions of the test had high variability, but the use of tritiated thymidine to identify proliferating cells has led to a more reliable test (Rossman et al., 1988; Mroz et al., 1991). In recent years, the peripheral blood test has been found to be as sensitive as the BAL assay, although larger abnormal responses are generally observed in the BAL assay (Kreiss et al., 1993; Pappas & Newman, 1993). False-negative results can occur with the BAL BeLT in cigarette smokers, who have a marked excess of alveolar macrophages in lavage fluid (Kreiss et al., 1993). The BeLT has also been used in animal studies to identify those species with a beryllium-specific immune response (see section 8.8). As described below, the BeLT test can detect beryllium sensitization and has a higher predictive value in CBD screening than clinical exam, spirometry, or chest radiography.
Occupational studies show compound-specific differences in beryllium toxicity, but are less clear about whether beryllium metal or beryllium oxide is more toxic, probably due to variability in particle size or solubility differences. Eisenbud & Lisson (1983) found a higher prevalence of CBD in people who worked with beryllium metal than in those who worked with beryllium oxide, and Sterner & Eisenbud (1951) found a much higher prevalence of CBD in people who worked with beryllium oxide than in those who worked with other beryllium compounds. By contrast, Cullen et al. (1987) found a greater frequency of CBD in workers presumably exposed to beryllium oxide fume compared with the beryllium metal, but the small particle size of the fume compared with the beryllium metal dust may have contributed to the higher toxicity of the beryllium oxide in this study. Owing to the markedly decreased levels of occupational exposure to beryllium, acute chemical pneumonitis is now quite rare, except in instances where there are accidental exposures that exceed the US occupational standard (Eisenbud et al., 1949).
Evaluation of the exposure–response to beryllium has been made more difficult because CBD is an immune disease, and only a small percentage of the population (1–5%) appears to be susceptible. Nonetheless, exposure–response relationships are evident. Several studies have observed CBD in people chronically exposed in modern plants, which are generally in compliance with the beryllium permissible exposure limit of 2 µg/m3.
In contrast to inhalation and oral routes of exposure, exposure of humans to beryllium compounds has been shown to cause skin and eye irritation (Van Orstrand et al., 1945; De Nardi et al., 1953; Nishimura, 1966; Epstein, 1990). Direct skin contact with soluble beryllium compounds, but not beryllium hydroxide or beryllium metal, can cause dermal lesions (reddened, elevated, or fluid-filled lesions on exposed body surfaces) in susceptible persons (McCord, 1951). The lesions appear after a latent period of 1–2 weeks, suggesting a delayed allergic reaction. Curtis (1951) conducted patch tests showing that soluble beryllium compounds do produce an allergic contact dermatitis. The dermal reaction occurs more rapidly and in response to smaller amounts of beryllium in sensitized individuals (Van Orstrand et al., 1945). Introduction of soluble or insoluble beryllium compounds into or under the skin as a result of abrasions or cuts at work can result in chronic ulcerations with granuloma formation (Van Orstrand et al., 1945; Lederer & Savage, 1954).
Kreiss et al. (1996) conducted a cross-sectional study of 136 of the 139 beryllium workers in a plant that made beryllia ceramics from beryllium oxide powder. An additional 15 workers who had been exposed to beryllium at other jobs were excluded from exposure calculations because their earlier exposure was not known. Because the plant opened in 1980, high-quality industrial hygiene measurements were available for almost the entire exposure period. Measurements from 1981 and later (for each job title) were reviewed and included area samples, process breathing zone samples, and personal lapel samples (the last year only). Cumulative beryllium exposure for individuals (quarterly daily-weighted average [DWA]) was estimated by summing the quarterly DWAs for their job titles, weighted by days of employment in the job title during the quarter. However, general area and breathing zone samples were not recorded for machining processes until the last quarter of 1985, soon after machining production was transferred to that plant, even though a limited amount of machining had been conducted since 1982. Although total beryllium exposure was generally well characterized for most of the affected workers, two of the seven beryllium-sensitized machinists started machining prior to the systematic environmental monitoring. Since exposure levels generally declined with time, exposure of these two subjects may have been underestimated. The median breathing zone measurement of beryllium was 0.6 µg beryllium/m3 for machining and 0.3 µg beryllium/m3 for other processes. The frequency of excursions to higher exposure levels decreased with time, with the percentage of machining breathing zone measurements above 5 µg beryllium/m3 falling from 7.7% during early sampling years to 2.1% during later sampling years.
BeLTs were performed by two different laboratories on blood samples collected from 136 employees (Kreiss et al., 1996). Positive results from one or both laboratories were confirmed by analysing a subsequent blood sample. Of the 136 tested employees, 5 had consistently abnormal blood BeLT results and were diagnosed with CBD based on observation of granulomas in lung biopsy samples. An additional two employees had abnormal blood results from one of the two laboratories and had no granulomas in lung biopsy samples. Both employees developed abnormal blood results in other laboratory tests within 2 years. One of these two employees also developed symptoms of CBD. The other employee declined clinical follow-up. An additional case of CBD was found during the study in an employee hired in 1991, who had a non-healing granulomatous response to a beryllium-contaminated skin wound. This subject had a confirmed abnormal blood test and after several additional months developed lung granulomas. Only one CBD case had an abnormal chest X-ray (defined as small opacity profusion of 1/0 or greater). An additional 11 former employees had CBD, for a total prevalence of 19/709 (2.7%). Beryllium-sensitized cases were similar to non-sensitized ones in terms of age, ethnic background, and smoking status, but did have significantly fewer pack-years of smoking. There was also no significant difference in the percentage exposed to beryllium dust or mist in an accident or unusual incident, or those in areas with a posted high air count. Of the eight sensitized workers, seven had worked in machining at some point, while one had never worked in a production job. The beryllium sensitization rate was 14.3% among the machinists, compared with 1.2% among all other employees. The individual average beryllium exposures for the six CBD cases and two sensitized cases among current employees ranged from 0.2 to 1.1 µg beryllium/m3, and the cumulative exposure ranged from 92.6 to 1945 µg beryllium/m3-days. The median of estimated average beryllium exposure for the sensitized cases was about 0.55 µg beryllium/m3. The sensitized cases without disease did not have lower exposures than the CBD cases. Machinists may have been more susceptible than other groups because of their higher overall exposure or because the particles produced during machining were primarily respirable in size, while other exposures were to particles larger than the respirable range. Other characteristics of the machining exposure, such as the particle morphology and surface properties or adjuvants in machining fluids, may also have affected sensitization. The study authors noted that median breathing zone levels tended to be lower than the DWAs derived from these levels, because much of the day was typically spent in high-exposure tasks. This study identified a lowest-observed-adverse-effect level (LOAEL) of 0.55 µg beryllium/m3, adjusted for occupational exposure (i.e., 5 days/7 days), and a duration-adjusted LOAEL (LOAEL [adj]) of 0.20 µg beryllium/m3 (adjusted for occupational exposure, i.e., 5 days/7 days, and 10 m3 per 8-h workday/20 m3 per day; US EPA, 1994).
Similar results were found in other groups of beryllium workers. Cullen et al. (1987) identified five cases of likely CBD among workers at a precious metals refinery. Four of the five cases worked predominantly in the furnace area, where exposure (based on personal air samples over a 2-week period during the study) was to beryllium oxide fumes at 0.52 ± 0.44 µg beryllium/m3 (maximum measurement 1.7 µg beryllium/m3). The fifth case worked as a crusher with exposure to beryllium metal dust at 2.7 ± 7.2 µg beryllium/m3. This study identified a LOAEL of 0.52 µg beryllium/m3 for CBD in the exposed workers (LOAEL [adj] = 0.19 µg beryllium/m3). Stange et al. (1996) reported that the total incidence of beryllium sensitization (positive BeLT results) and CBD among current and former employees at the Rocky Flats Environmental Technology site was 107/4397 (2.43%). Worker exposure concentrations measured at the site between 1984 and 1987 using personal air monitoring devices averaged 1.04 µg beryllium/m3 (95% confidence interval [CI] = 0.79–1.29 µg beryllium/m3). Thus, this study identified a LOAEL of 1.04 µg beryllium/m3 for beryllium sensitization and CBD (LOAEL [adj] = 0.37 µg beryllium/m3).
Kreiss et al. (1997) reported a prevalence of 4.6% (29/632) for CBD at a beryllium metal, alloy, and oxide production plant, where median average beryllium exposure of the entire workforce was 1.0 µg beryllium/m3 and that of the five CBD cases employed since 1984 was 1.3 µg beryllium/m3 (LOAEL [adj] = 0.46 µg beryllium/m3). Cotes et al. (1983) identified two definite cases of CBD and several more probable cases among 130 workers at a beryllium manufacturing plant. The two definite cases were exposed to an estimated average beryllium concentration of 0.1 µg beryllium/m3 over a 6-year period. The LOAEL of 0.1 µg beryllium/m3 corresponds to a LOAEL [adj] of 0.036 µg beryllium/m3. A 4-year survey was conducted at a Japanese beryllium–copper alloy factory to clarify the relationship between beryllium levels in the working environment and the BeLT value of workers in the work environment of beryllium–copper alloy manufacturing processes. Such an investigation found a positive correlation between beryllium exposure in the workplace and BeLT values in the exposed workers. The present results suggest that T-cells of workers continuously exposed to beryllium concentrations of more than 0.01 µg beryllium/m3 may be activated and the cell-mediated response of workers may be promoted. On the other hand, the BeLT values of workers exposed to beryllium concentrations of less than 0.01 µg beryllium/m3 were found to be unaffected. Such data imply that a threshold beryllium concentration exists for sensitization and subsequent promotion of cell-mediated response (Yoshida et al., 1997).
The most complete investigation of community cases of CBD was conducted by Eisenbud et al. (1949), who evaluated exposure related to 11 cases of CBD. Radiological screening of 10 000 residents was conducted, with questionable cases undergoing clinical evaluation. CBD was diagnosed based on radiological and clinical findings and on a consensus of specialists. One case was exposed to beryllium dust on worker clothes and will not be discussed further. Of the other cases, five lived within 0.4 km of a beryllium production plant, and all lived within 1.2 km of the plant. The prevalence of CBD for the population of approximately 500 people living within 0.4 km of the plant was 5/500 or 1%. The incidence for the area within 1.2 km of the plant could not be estimated because of a lack of population data for this area. A follow-up to this study reported three additional cases at less than 1.2 km from the plant but no additional cases of CBD at greater than 1.2 km (Sterner & Eisenbud, 1951). Based on a limited number of downwind measurements from a mobile sampler stationed at 0.4–1.2 km from the plant, on continuous 10-week monitoring at fixed stations located up to 0.23 km from the plant, and on exposure levels modelled from emissions data, stack heights, and wind data, Eisenbud et al. (1949) estimated that the average exposure levels at 1.2 km from the plant during the period of exposure monitoring were 0.004–0.02 µg beryllium/m3. Averaging these values to 0.01 µg beryllium/m3 and noting that both plant production and emissions were about 10-fold higher in earlier years, the authors estimated that the concentration at 1.2 km was 0.01–0.1 µg beryllium/m3. Eisenbud & Lisson (1983) were quite certain that a population of approximately 500 people was exposed to levels of 0.1 µg beryllium/m3. Beyond 0.4 km, estimates of exposure are very uncertain for the period from 1940, when significant beryllium production began, through the time of the study. The similar prevalence of CBD in the community compared with workers exposed to much higher levels (up to 100 µg beryllium/m3) was attributed to the smaller particle size of beryllium emitted to the outside air compared with beryllium particles inside the plant (also discussed in Eisenbud & Lisson, 1983). Thus, this study establishes a no-observed-adverse-effect level (NOAEL) [adj] of 0.01–0.1 µg beryllium/m3 for the development of CBD in a population exposed to beryllium in ambient air.
A number of cohort mortality studies have investigated the carcinogenic potential of beryllium in beryllium processing workers employed in seven facilities in the USA. Several studies published in 1971, 1979, and 1980 were critically reviewed in US EPA (1987). A brief description of these studies as well as a discussion of the major criticisms are included in this section. Two more recent studies (Steenland & Ward, 1991; Ward et al., 1992) not previously reviewed by the US EPA are also discussed in this section. MacMahon (1994), in a review funded by the Beryllium Industry Scientific Advisory Committee, cited serious defects in the methodology of the early (pre-1987) epidemiological studies that linked occupational beryllium exposure to lung cancer and questioned the interpretation of the more recent, and generally better grounded, epidemiological studies (Steenland & Ward, 1991; Ward et al., 1992), which the International Agency for Research on Cancer reviewed in 1993 (IARC, 1993).
The Ward et al. (1992) study of the seven beryllium-producing plants in Ohio and Pennsylvania, USA, is basically an update of several earlier retrospective cohort studies (Bayliss et al., 1971; Mancuso, 1979, 1980; Wagoner et al., 1980). Since these studies are follow-up studies and not independent of each other, they will be discussed in chronological sequence, beginning with some of the earlier studies.
Bayliss et al. (1971) studied ~7000 past and current workers (out of 10 356) in the beryllium processing industry in Ohio and Pennsylvania. There was an elevated risk of lung cancer (36 observed versus 34.06 expected), but this risk was not statistically significant. The report does not specify the plants but implies, as does MacMahon (1994), that all the beryllium processing facilities were included. Limitations of this study include the elimination of >2000 workers because of incomplete data, lack of analysis of the data according to length of time since initial employment, and the combining of populations from several different plants into one cohort (US EPA, 1987). Follow-up studies conducted by Mancuso (1979, 1980) and Wagoner et al. (1980) focused on cohorts from only one or two of the plants.
A cohort mortality study of 3055 white males employed between 1942 and 1967 at a beryllium extraction, processing, and fabrication facility in Reading, Pennsylvania, was conducted by Wagoner et al. (1980).4 The study cohort was followed through 1975. The total number of deaths (875) was not significantly different from the number expected on the basis of age and calendar period for the general white male US population (vital statistics data for the period 1965–1967 were assumed to apply to 1968–1975). Significant (P < 0.05) increases in the number of deaths due to malignant neoplasm of trachea, bronchus, and lung (47 deaths observed versus 34.29 expected, standardized mortality ratio [SMR] = 1.37), heart disease (SMR = 1.13), and non-neoplastic respiratory disease (excluding influenza and pneumonia) (SMR = 1.65) were observed in the study cohort. When deaths from lung cancer were segregated by latency (interval since onset of employment) and duration of employment, significant increases were observed for workers with a >25-year latency employed for <5 years (17 observed versus 9.07 expected, SMR = 1.87) and across all employment durations (20 observed versus 10.79 expected, SMR = 1.87). (It should be noted that 83% of the cohort was employed for <5 years.) When lung cancer mortalities were partitioned based on initial date of employment, lung cancer deaths were significantly higher in workers hired before 1950 (SMR = 1.35; P < 0.05); an increase in deaths of workers hired after 1950 was also found, but it was not statistically significant (SMR = 1.52). (Prior to 1950, beryllium exposures were not controlled, and it is likely that the workers were exposed to high concentrations of beryllium.) Similar findings were reported when non-neoplastic respiratory disease mortalities were segregated; a significant increase in mortality was observed in the workers in the >25-year latency and <5-year employment category (SMR = 2.13). In the workers who were hired prior to 1950, a significant increase in mortality from non-neoplastic respiratory disease was observed (SMR = 1.85). This was not observed for workers whose initial date of employment was after 1950 (0 deaths observed versus 2.03 expected). Wagoner et al. (1980) noted that using US population data rather than county mortality data resulted in an overestimation of expected lung cancer deaths by a factor of 19% (and thus a corresponding underestimation of lung cancer risk for the cohort) because residents of Berks County (where most of the workers lived) have a lower lung cancer rate (31.8 per 100 000) than the US population (38.0 per 100 000). The percentage of beryllium workers living in the city of Reading, however, was higher than for county residents in general, and higher lung cancer rates are observed in urban residents compared with residents living in rural areas. US EPA (1987) estimated that comparing lung cancer mortality in the beryllium cohort with county rates weighted towards the city of Reading rates, which were 12% higher than the national rates, would result in an increased number of expected deaths. It is generally preferable to use county data, although the issue of urban residence may dictate using general US data if the cohort is more urban-oriented.
Wagoner et al. (1980) compared cigarette smoking histories of the cohort and the US population using smoking habit information collected during a medical survey in 1968 and cigarette smoking data for white males from a Public Health Service survey conducted in 1964–1965. A smaller percentage of the cohort was current smokers (49.6% never smoked or were former smokers versus 45.2% in the US population), but the percentage of current smokers smoking more than one pack per day was higher (21.4% versus 15.3%). The investigators estimated that not adjusting lung cancer incidence data for differences in cigarette smoking habits resulted in an overestimation of lung cancer risks by a factor of 14%.
This study has severe limitations, including the following:
|
(1) |
the use of US white male mortality data for the period 1941–1967, which resulted in an underestimation of the number of expected lung cancer deaths, because lung cancer death rates in the USA were increasing during the period 1968–1975; the expected number of lung cancer deaths should have been 10–11% higher (Saracci, 1985; US EPA, 1987); |
|
(2) |
the inclusion of one lung cancer death of an individual who was paid for the pre-employment physical but was not hired (US EPA, 1987); |
|
(3) |
the exclusion of approximately 300 white males employed at the Reading facility in jobs similar to those of the workers included in the cohort (US EPA, 1987); and |
|
(4) |
the inadequate discussion of confounding effects from other potential lung carcinogens (US EPA, 1987). |
The above limitations tended to exaggerate the risk of lung cancer in this population of workers potentially exposed to beryllium (US EPA, 1987; MacMahon, 1994).
US EPA (1987) adjusted the standardized mortality ratios for lung cancer from the Wagoner et al. (1980) study to take these issues into account. The expected lung cancer deaths were increased by 11% to account for the underestimation that occurred from using older vital statistics and by 4.1–9.8% to account for differences in smoking habits between the beryllium cohort and the US population. One ineligible lung cancer death was removed from the observed deaths. Although the SMRs for latency ł 25 years remained elevated after this adjustment (SMR = 1.42 for <5 years’ employment and 1.36 across all durations of employment), they were no longer statistically significant (Table 3).
Ward et al. (1992) conducted a retrospective cohort mortality study of 9225 men (5681 alive and 3240 dead) employed for at least 2 days between 1 January 1940 and 31 December 1969 and followed through 31 December 1988 at any one of seven beryllium processing facilities located in Reading (this is the same facility studied by Wagoner et al. [1980]) and Hazelton, Pennsylvania, and Lorain, Cleveland (data for Perkins and St. Clair plants combined), Lucky, and Elmore, Ohio. This study is basically an update of several earlier retrospective cohort studies (Bayliss et al., 1971; Mancuso et al., 1979, 1980; Wagoner et al., 1980). Cohort members were identified from quarterly earning reports from the Social Security Administration and compared with personnel files. Workers identified from quarterly earning reports without personnel files were included in the cohort only if they appeared on at least two quarterly earning reports. Workers who worked at more than one facility were placed into a seventh category termed "multiple plant." Vital statistics for the workers were obtained from the Social Security Administration, Internal Revenue Service, post office cards mailed to the last known address, Veterans Administration, Health Care Financing Administration, and the National Death Index. Vital statistics were not located for 304 (3.3%) individuals, and death certificates were not obtained for 46 (0.4%) individuals known to be deceased.
Table 3: Observed and expected deaths due to lung cancer in beryllium processing facility workers.a
|
Interval since onset of employment |
Duration of employmentb,c,d |
||||||||
|
<5 years |
ł 5 years |
Total |
|||||||
|
O |
E |
SMR |
O |
E |
SMR |
O |
E |
SMR |
|
|
<15 years |
7 |
8.88 |
0.79 |
1 |
1.76 |
0.57 |
8 |
10.64 |
0.75 |
|
15–24 years |
15 |
13.44 |
1.12 |
3 |
3.15 |
0.95 |
18 |
16.59 |
1.08 |
|
ł 25 years |
17 |
12 |
1.42 |
3 |
2.67 |
1.12 |
20 |
14.67 |
1.36 |
|
Total |
39 |
34.32 |
1.14 |
7 |
7.58 |
0.92 |
46 |
41.9 |
1.1 |
a
Modified from Wagoner et al. (1980) by US EPA (1987).b
Workers employed during 1942 through 1967 and followed through 1975.c
No comparisons were statistically significant at P < 0.05.d
O = observed deaths; E = expected deaths; SMR = standardized mortality ratio.The workers at the beryllium processing facilities were involved in the extraction of beryllium hydroxide from beryl ore; the production of beryllium oxide, pure beryllium metal, and beryllium–copper alloy; and the machining of beryllium-containing products. The beryllium compounds to which the workers were potentially exposed include beryllium sulfate mists and fumes, beryllium oxide dusts, beryllium ammonium fluoride and beryllium fluoride dusts, beryllium metal, and beryllium–copper alloy dusts and fumes. In addition to exposure to beryllium, the workers were also potentially exposed to ore dust, silicon dioxide fumes, lead sulfide, copper sulfide, sulfur trioxide, acid fluoride mists, hydrogen fluoride, and ammonium fluoride. In addition, according to BISAC (1997), exposure to sulfuric acid mists and fumes occurred in the Lorain facility. Because no occupational history data other than starting and ending dates of employment were coded and no individual monitoring data were available, the study could not address the relationship of degree of beryllium exposure or type of beryllium compound to lung cancer risk. Ward et al. (1992) noted that prior to 1949, when controls were not mandated, air concentrations of beryllium were very high, frequently exceeding 1000 µg/m3.
When mortality from all causes in the entire cohort was compared with mortality rates from the US population, a significant (P < 0.05) increase in risk was observed (SMR = 1.05, 95% CI = 1.01–1.08). Excess mortality was also observed for malignant neoplasm of the trachea, bronchus, and lung (SMR = 1.26, 95% CI = 1.12–1.42). Examination of the cause of death on a per plant basis revealed that only the Lorain and Reading facilities (the two oldest plants) had significant excesses in lung cancer: total SMRs of 1.69 and 1.24, respectively. In addition, the Cleveland and Hazelton facilities had non-significant excesses in lung cancer (total SMRs >1). Data on lung cancer deaths for the whole cohort and for each plant are presented in Table 4. Increased employment duration was not associated with an increase in lung cancer SMR; when lung cancer mortalities were stratified by employment duration category, the only significant increase in lung cancer SMRs was for workers employed <1 year. However, there was a tendency for lung cancer SMRs to increase with increasing latency; SMRs were statistically significantly elevated in the >30-year latency category for all employment durations combined (SMR = 1.46) and for workers employed for <1 year (SMR = 1.52) and in the 25- to 30-year latency category for workers employed for <1 year. Additionally, decade of hire influenced lung cancer mortality. The SMR (1.42) was statistically elevated in workers hired before 1950; this was mainly influenced by mortality in the Lorain plant, which closed in 1948. Of the two other facilities in operation before 1950 (Reading and Cleveland), an increased lung cancer rate was found at the Reading facility (SMR = 1.26 for workers hired before 1950). With the exception of those hired before 1950 (total SMR = 1.42), no other significant increases in lung cancer deaths were observed when workers were grouped by decade of hire. Non-significant increases were seen for the 1950s decade at the Reading (SMR = 1.42), Cleveland (SMR = 1.32), Elmore (SMR = 1.42), and Hazelton (SMR = 1.86) facilities. Regression analysis (controlling for age, race, and calendar period of risk) showed that decade of hire was independent of potential latency (time since first employment). The influence of geographic variation in lung cancer mortality was evaluated, comparing lung cancer mortality found in the beryllium cohort with lung cancer rates for the counties where most of the workers resided. Lung cancer mortality was significantly elevated in workers at the Lorain (SMR = 1.60, 95% CI = 1.21–2.08) and Reading (SMR = 1.42, 95% CI = 1.18–1.69) facilities compared with residents of Lorain County and Berks County, respectively. The investigators noted that county residents may not serve as a better referent group than the US population because the percentage of workers at the Lorain and Reading facilities residing in an urban area was approximately 3 times higher than the percentage of county residents living in urban areas. A significant excess of pneumoconiosis and other respiratory disease was also observed at the Lorain facility (SMR = 1.94).
Table 4: Lung cancer mortality in male beryllium workers employed between 1940 and 1969 and followed through 1988 by facility worked and latency (time since first employment)a,b
|
Facility |
Latency <15 years |
Latency 15–30 years |
Latency >30 years |
Total |
||||||||
|
O |
E |
SMR |
O |
E |
SMR |
O |
E |
SMR |
O |
E |
SMR |
|
|
Loraine |
1 |
2.6 |
0.38 |
21 |
10 |
2.09c |
35 |
21.1 |
1.66d |
57 |
33.8 |
1.69d |
|
Reading |
9 |
12 |
0.78 |
44 |
37.5 |
1.17 |
67 |
47.9 |
1.40d |
120 |
96.9 |
1.24d |
|
Lucky |
1 |
1 |
0.96 |
4 |
4.7 |
0.85 |
4 |
5.3 |
0.76 |
9 |
11 |
0.82 |
|
Cleveland |
9 |
6.9 |
1.3 |
20 |
22 |
0.91 |
15 |
11.8 |
1.27 |
44 |
40.7 |
1.08 |
|
Elmore |
2 |
3.9 |
0.51 |
12 |
10.5 |
1.14 |
1 |
0.8 |
1.31 |
15 |
15.2 |
0.99 |
|
Hazelton |
4 |
2.1 |
1.91 |
9 |
7.1 |
1.26 |
0 |
0.2 |
– |
13 |
9.4 |
1.39 |
|
Multiple plants |
0 |
0.7 |
– |
4 |
3.2 |
1.23 |
9 |
3.8 |
2.38 |
13 |
7.6 |
1.67 |
|
Unknown |
1 |
1.6 |
0.64 |
5 |
3.9 |
1.28 |
3 |
1.3 |
2.3 |
9 |
6.8 |
1.33 |
|
Total |
27 |
30 |
0.89 |
119 |
99.1 |
1.20d |
134 |
92.1 |
1.46c |
280 |
222 |
1.26c |
|
a |
From Ward et al. (1992). |
|
b |
O = observed deaths; E = expected deaths; SMR = standardized mortality ratio. SMRs not adjusted for differences in smoking habits between exposed cohort and US population. |
|
c |
Two-sided P < 0.01. |
|
d |
Two-sided P < 0.05. |
Data on the smoking habits of the entire beryllium cohort were not available (Ward et al., 1992). Some information was available from a 1968 Public Health Service survey conducted at the Reading, Hazelton, Elmore, and St. Clair facilities (it included 15.9% of the cohort members). These data were compared with smoking habits of the US population obtained from averaging smoking surveys conducted in 1965 (National Center for Health Statistics) and 1970 (Office of Health Research, Statistics, and Technology). A comparison of the SMRs for malignant neoplasms of the trachea, bronchus, and lung using county and US rates is presented in Table 5. The estimated relative risk ratio for lung cancer for the beryllium cohort compared with the US population was calculated using estimated risks of 1 for non-smokers, 6.5 for current smokers of 1 pack per day, 13.8 for smokers of >1 pack per day, and 6.2 for former smokers. The relative risk ratio or smoking adjustment was 1.1323, which indicates that smoking alone could account for an SMR of 1.13. Using the smoking adjustment factor, smoking-adjusted SMRs were calculated for the entire cohort and the Lorain and Reading facilities. The resultant SMRs were 1.12, 1.49, and 1.09, respectively (Table 6). One process exposure, to which BISAC (1997) attributed the excess cancer (SMR 1.49) after adjustment for smoking, and which was briefly mentioned by Ward et al. (1992), is exposure to mists and vapours from sulfuric acid and the related sulfur oxide gases. Such exposure, according to BISAC (1997), was very high in the Lorain plant. This was the only plant that used a sulfuric acid-dependent process with limited ventilation (Kjellgren, 1946). Because the 1968 survey data are the only information available on the smoking habits of the beryllium cohort, an assumption was made that the smoking habit difference between the cohort and the US population found in the late 1960s was the same in the 1940s and 1950s. Other investigators have shown that increased smoking is unlikely to account for SMRs greater than 1.3 for lung cancer and other smoking-related diseases (Siemiatycki et al., 1988). Ward et al. (1992) concluded that the most plausible explanation for the increased lung cancer rates is occupational exposure to beryllium. Although the results of this study are suggestive that occupational exposure to beryllium can result in an increase in lung cancer mortality, interpretation of this study is limited by the lack of exposure data and scarcity of smoking data.
Table 5: SMRs for malignant neoplasm of the trachea, bronchus, and lung among US male beryllium workers employed in 1940–1969 and followed through 31 December 1988 using county death rates (1950–1983) for comparison.a
|
Plant location |
Observed |
SMR based on county rates |
95% CI |
SMR based on US rates |
|
|
City |
County |
||||
|
1. Lorain |
Lorain, OH |
57 |
1.60b |
1.21–2.08 |
1.69b |
|
2. Reading |
Berks, PA |
120 |
1.42b |
1.18–1.69 |
1.24c |
|
3. Lucky |
Ottawa, OH |
9 |
0.84 |
0.38–1.59 |
0.82 |
|
|
Sandusky, OH |
|
|
|
|
|
|
Wood, OH |
|
|
|
|
|
4. Clevelandd |
Cuyahoga, OH |
44 |
1.05 |
0.76–1.41 |
1.08 |
|
5. Elmore |
Ottawa, OH |
15 |
1.06 |
0.59–1.75 |
0.99 |
|
|
Sandusky, OH |
|
|
|
|
|
|
Wood, OH |
|
|
|
|
|
6. Hazelton |
Carbon, PA |
13 |
1.5 |
0.80–2.57 |
1.39 |
|
Sum of six locations |
258 |
1.32c,e |
1.19–1.46 |
1.26b,e |
|
|
a |
From Ward et al. (1992). |
|
b |
Two-sided P < 0.01. |
|
c |
Two-sided P < 0.05. |
|
d |
St. Clair and Perkins combined. |
|
e |
Total study population (n = 9225, 280 lung cancers); six locations population (n = 8672, 258 lung cancers). |
Table 6: Observed and expected lung cancer cases, before and after external adjustment for differences in smoking habits between exposed cohorts and US population, and corresponding standardized mortality ratios with 95% confidence intervals.a
|
Beryllium plant |
Lung cancer observed cases |
No adjustment for smoking |
Adjustment for smoking |
||||
|
Expected cases |
SMR (CI) |
P -value |
Expected cases |
SMR (CI) |
P -value |
||
|
Lorain |
57 |
33.8 |
1.69 (1.28–2.19) |
0 |
38.2 |
1.49 (1.13–1.93) |
0.005 |
|
Reading |
120 |
96.9 |
1.24 (1.03–1.48) |
0.026 |
109.8 |
1.09 (0.91–1.31) |
0.353 |
|
All other plants |
103 |
90.8 |
1.13 (0.93–1.38) |
0.222 |
102.8 |
1.00 (0.82–1.22) |
0.99 |
|
Total |
280 |
221.5 |
1.26 (1.12–1.42) |
0 |
250.8 |
1.12 (0.99–1.26) |
0.074 |
a
From BISAC (1997), using data from Ward et al. (1992).Formal epidemiological studies of the BCR enrollees have been undertaken to assess long-term mortality patterns, particularly carcinogenic risk, among a beryllium-exposed population. The first of these studies included all white males alive at the time of entry into the BCR, with a follow-up through 1975 (Infante et al., 1980). This study examined the possible relationship between beryllium exposure and lung cancer in a cohort mortality study of 421 white males entered into the BCR between July 1952 and December 1975 with the diagnosis of beryllium disease. However, this study deviated from the official criteria previously cited to define "chronic beryllium disease" (Hasan & Kazemi, 1974) by eliminating three of the five criteria (US EPA, 1987). These self-imposed constraints caused them to eliminate from the cohort subjects who were deceased at the time of BCR entry and all non-white and female subjects because of their lack of "statistical sensitivity." No information on occupations was provided in the report, but IARC (1993), in its review of this study, mentioned that the majority of individuals in the BCR worked in beryllium extraction and smelting, metal production, and fluorescent tube production, and a small number were not exposed occupationally but lived near the plants. Cause-specific mortality data from the US population vital statistics for the period of 1965–1967 (matched for race, sex, age, and calendar period) were used for comparison. A significant (P < 0.05) increase in deaths from cancer was observed (SMR = 1.53), but the number of lung cancer (includes cancer of the trachea, bronchus, and lung) deaths (7 observed versus 3.3 expected, SMR = 2.11) was not significantly increased in the cohort in comparison with US national statistics. A significant increase in non-malignant respiratory disease (excludes influenza and pneumonia) mortality was observed (SMR = 32.1). Cancer mortalities were segregated into workers with a diagnosis of acute beryllium-related respiratory illness (n = 223) and those with chronic beryllium-related diseases (n = 198). Acute beryllium illness was defined as a diagnosis of chemical bronchitis, pneumonitis, or other acute respiratory illness at the time of entry into the registry. Chronic beryllium illness was defined as a diagnosis of pulmonary fibrosis or some recognized chronic lung condition at the time of entry into the registry. For subjects without a clear diagnosis, categorization was based on the interval between initial exposure and first respiratory symptoms (within 1 year of initial exposure was considered acute). Significant increases in deaths from lung cancer were observed in the acute beryllium illness group (SMR = 3.14). Most of these deaths were observed in workers with >15-year latencies (SMR = 3.21). Deaths from lung cancer were not elevated in the group with chronic respiratory illness (SMR = 0.72). The authors note that this may be due to the high case fatality rate for non-neoplastic respiratory disease in the workers with chronic beryllium illnesses. Significant increases in deaths from non-neoplastic respiratory disease were observed in the group with acute beryllium illness (SMR = 10.3) and those in the chronic beryllium illness group (SMR = 64.6). The lung cancer mortality rates were not adjusted for cigarette smoking because smoking habit information was not obtained from the cohort. The investigators noted that it was highly unlikely that workers with acute beryllium illnesses had smoking habits of sufficient magnitude to account for the excessive lung cancer risk observed in that group. As with the Wagoner et al. (1980) and Mancuso (1980) studies, using US mortality rates for the period ending in 1967 probably resulted in an underestimation of expected lung cancer deaths. It is likely that this distortion was similar to the one found in the Wagoner et al. (1980) study (11%). On the other hand, Steenland & Ward’s (1991) analysis of smoking habit information as of 1965 for their cohort from the BCR (see below) indicated that not correcting for differences in smoking habits between the cohort and the US population may overestimate the expected deaths.
Steenland & Ward (1991) extended this earlier study of BCR enrollees by including females and by adding 13 years of follow-up. Cancer mortality was examined in a cohort of 689 males and females (66% of the cohort was male; 261 alive and 428 dead) who were entered in the BCR. This high rate of mortality was due to pneumoconiosis, primarily CBD (SMR = 34.23, 95% CI = 29.1–40.0, 158 deaths). Similar results (SMR = 16.40, P < 0.05, 52 deaths versus 3.17 expected) were observed in an earlier analysis of mortality in the BCR due to non-neoplastic respiratory disease (Infante et al., 1980). All members of the cohort were alive at the time of entry and were followed until the time of death or until 1988. Among the cohort members, CBD (64%; 50% in males and 91% in females) was more common than acute disease. The members had worked in the fluorescent tube industry (34%) or basic manufacturing (36%) or were members of a community exposed to high beryllium levels (6%), or their records lacked industry/exposure scenario information (10%). Mortality rates for the cohort were compared with the US population (stratified by age, race, sex, and calendar period). The SMR for lung cancer mortality was increased for the total cohort (2.00, 95% CI = 1.33–2.89) and for males (1.76, 95% CI = 1.02–2.67) and females (4.04, 95% CI = 1.47–8.81). When the cohort was divided into groups based on duration of beryllium exposure (Ł 4 years and >4 years) and time since first exposure (Ł 20 years and >20 years), significant trends in lung cancer rates were not observed. However, the authors noted that the duration of exposure information in the registry was likely to contain a number of inaccuracies. An increased SMR was also observed for pneumoconiosis and other respiratory diseases (26.30, 95% CI = 20.6–33.1); the SMR for the group exposed to beryllium for >4 years (45.78, 95% CI = 36.6–56.5) was significantly (P < 0.001) higher than for the group exposed for Ł 4 years (26.30, 95% CI = 20.6–33.1). Dividing the cohort based on whether they were diagnosed with ABD or CBD revealed a higher lung cancer SMR for workers in the acute disease group (presumably they were exposed to higher levels of beryllium; SMR = 2.32, 95% CI = 1.35–3.72) than in the chronic disease group (SMR = 1.57, 95% CI = 0.75–2.89). The SMR for pneumoconiosis and other respiratory diseases was higher in the chronic disease group (68.64, 95% CI = 57.8–81.0) than in the acute disease group (6.55, 95% CI = 3.74–10.6). The smoking habits of 32% of the cohort were available from direct interviews, interviews with next-of-kin, or registry records. The cohort smoking habits as of 1965 were compared with US population smoking habits as of 1965. The authors note that 1965 was chosen as a time point "because smoking habits in the 1960s are considered to have been most relevant for lung cancer mortality in the 1980s." The cohort contained fewer current smokers and more former smokers than the comparison US population, perhaps because the presence of respiratory disease in this cohort deterred smoking. Taking into account the known relative risks for various smoking habit categories for the cohort compared with the US population, the SMR for the cohort based on smoking alone was 0.98 for men and 0.86 for women. The investigators concluded that if the smoking habits of the entire cohort were represented by the 32% with smoking habit data, then it "would be unlikely that smoking was a cause of the observed lung cancer excess." Other studies (Wagoner et al., 1980; Ward et al., 1992) found that not correcting for differences in cigarette smoking-related lung cancer deaths between the exposed cohort and comparison population would result in an underestimation of expected deaths, which differs from the conclusions of Steenland & Ward (1991).
Ward et al. (1992) examined the BCR mortality study file to determine how many of the members of the cohort were registered (i.e., had a history of beryllium disease). The Lorain plant had the highest percentage of registrants — 8.2% (98 of 1192 workers); 93% of these were listed as having had ABD, which is associated with very high exposure (Eisenbud & Lisson, 1983). The lung cancer SMR for the Lorain workers in the BCR was 3.33 (95% CI = 1.66–5.95), compared with 1.51 (95% CI = 1.11–2.02) for the remaining Lorain workers. Ward et al. (1992) concluded that a plausible explanation for the observed increased lung cancer rates is occupational exposure to beryllium. Although the results of this study are suggestive that occupational exposure to beryllium can result in an increase in lung cancer mortality, interpretation of this study is limited by a number of factors:
|
(1) |
No data (including job history data) were available to associate beryllium exposure levels, exposure to specific beryllium compounds, or concomitant exposure to other chemicals with members of the cohort. |
|
(2) |
Because of the lack of job history data, it is possible that the cohort contained salaried workers and other non-production personnel who may not have been exposed to beryllium. |
|
(3) |
The limitations in the available smoking habit data, as discussed above, may have led to an over- or underestimation of the contribution of smoking to the lung cancer rates. |
|
(4) |
A large percentage (73.1%) of the workers was employed in the beryllium industry for <5 years. This is particularly true at the Lorain facility, where 84.6% of the workers were employed for <1 year. US EPA (1987) points out that there is a possibility that the workers were exposed to other potential carcinogens at jobs held before or after the beryllium job; the two facilities with the highest cancer rates (Lorain and Reading) are located in or near heavily industrialized areas. |
Savitz et al. (1989) found no association between paternal occupational exposure to beryllium and the risk of stillbirth, preterm delivery, or small-for-gestational-age infants in a case–control study using National Natality and National Fetal Mortality Survey data. Analyses were conducted for 2096 mothers and 3170 fathers of stillbirths, 363 mothers and 552 fathers of preterm babies, and 218 mothers and 371 fathers of small-for-gestational-age babies. In light of the small population exposed to beryllium, case–control studies have limited sensitivity for reproductive effects.
Minimal new information has become available since the publication of the Environmental Health Criteria document on beryllium (IPCS, 1990).
Data regarding the acute toxicity of beryllium (soluble salts) to freshwater fish are compiled in Table 7. The 96-h LC50 values ranged from 0.15 to 32.0 mg beryllium/litre, depending on the species studied and the test conditions, most notably hardness of the test water. In guppies (Poecilia reticulata), the LC50 decreased from 19.0–32.0 mg beryllium/litre in hard water (hardness = 450 mg/litre as calcium carbonate [CaCO3]) by roughly 2 orders of magnitude to 0.16 mg beryllium/litre in soft water (hardness = 22 mg/litre as calcium carbonate) (Slonim & Slonim, 1973). Similar results were reported in fathead minnows (Pimephales promelas) and bluegills (Lepomis macrochirus) tested in both hard and soft water (Tarzwell & Henderson, 1960).
Toxicity to fish was even greater in very soft (2 mg calcium/litre), acidified water (Jagoe et al., 1993). At pH 5.5, larval European perch (Perca fluviatilis) experienced significantly increased mortality (80% dead) at ł 100 µg beryllium/litre (as beryllium sulfate), while juvenile roach (Rutilis rutilis) had significantly increased mortality (90% dead) at 150 µg beryllium/litre. At pH 4.5, perch mortality was significantly increased (75% dead) at ł 10 µg beryllium/litre, while roach mortality was significantly increased (60% mortality) only at 150 µg beryllium/litre. Gill abnormalities were observed in perch exposed to concentrations as low as 10 µg beryllium/litre (pH 5.5).
Table 7: Acute toxicity of beryllium to freshwater fish.
|
Test species |
Test type |
Test chemical |
Hardness |
96-h LC50 |
Reference |
|
Bluegill |
static |
beryllium sulfate |
20 |
1.3 |
Tarzwell & Henderson, 1960 |
|
Fathead minnow |
static |
beryllium sulfate |
20 |
0.15–0.2 |
Tarzwell & Henderson, 1960 |
|
Fathead minnow |
flow-through |
beryllium sulfate |
140 |
3.25 |
Cardwell et al., 1976 |
|
Flagfish |
flow-through |
beryllium sulfate |
140 |
3.5–4.4 |
Cardwell et al., 1976 |
|
Goldfish |
flow-through |
beryllium sulfate |
147 |
4.8 |
Cardwell et al., 1976 |
|
Channel catfish |
static |
beryllium sulfate |
140 |
5 |
Cardwell et al., 1976 |
|
Brook trout |
static |
beryllium sulfate |
140 |
5 |
Cardwell et al., 1976 |
|
Guppy |
static |
beryllium sulfate |
22 |
0.16 |
Slonim, 1973; Slonim & Slonim, 1973 |
Salamander (Ambystoma spp.) larvae showed a sensitivity to beryllium that was similar to that exhibited by fish, with 96-h LC50 values of 18–31 mg beryllium/litre (as beryllium sulfate) in hard water and 3.2–8.3 mg beryllium/litre in soft water (Slonim & Ray, 1975). Acute toxicity in Daphnia magna was comparable to acute toxicity in vertebrate species, with 48-h EC50 values for beryllium sulfate and beryllium chloride ranging from 1.19 to 7.9 mg beryllium/litre, depending on the study and the water hardness (US EPA, 1978, 1980; Buikema, 1986; Khangarot & Ray, 1989). Findings in other invertebrate species included a 96-h LC50 of 0.14 mg beryllium/litre (as beryllium sulfate) in the free-living nematode Caenorhabditis elegans (Williams & Dusenberry, 1990) and a 96-h LC50 of 10.25 mg beryllium/litre (as beryllium sulfate) in the freshwater tubificid worm Tubifex tubifex (Khangarot, 1991).
US EPA (1980) reported a chronic toxicity value of 5.3 µg beryllium/litre (28-day maximum allowable toxicant concentration, or MATC5) for beryllium sulfate in Daphnia magna at a water hardness of 220 mg calcium carbonate/litre (21-day life cycle test, critical end-point not reported). The acute toxicity value (48-h EC50) in this same test water was 2.5 mg beryllium/litre, so an acute/chronic ratio of 472 can be calculated for beryllium based on this study. However, Buikema (1986) found much less difference between chronic and acute toxicity values for beryllium sulfate in D. magna. In this study (21-day life cycle test), chronic toxicity values were 0.051 mg beryllium/litre at a hardness of 100 mg/litre, 0.288 mg beryllium/litre at a hardness of 200 mg/litre, and 1.10 mg beryllium/litre at a hardness of 300 mg/litre, leading to acute/chronic ratios decreasing from 23.33 at a hardness of 100 mg/litre to 5.75 at a hardness of 300 mg/litre. Survival, body length, and reproduction were all affected to the same degree at a hardness of 100 or 200 mg/litre, but body length was the critical effect at a hardness of 300 mg/litre. Chronic toxicity data were not located for aquatic vertebrates. Early life stage studies found hatching success of carp (Cyprinus carpio) eggs reduced to 0% at 0.2 mg beryllium/litre, with no effect at 0.08 mg beryllium/litre (hardness, 50 mg calcium carbonate/litre) (Hildebrand & Cushman, 1978), and there was no effect of beryllium nitrate on frog (species not defined) egg development at 0.9–4.5 mg beryllium/litre or tadpole development at 0.09–0.2 mg beryllium/litre (Dilling & Healey, 1926).
Aquatic microalgae are less sensitive to beryllium than aquatic animals. Growth of the green alga Chlorella vannieli was inhibited by beryllium chloride at a concentration of 100 mg beryllium/litre (Karlander & Krauss, 1972). A beryllium sulfate concentration of 1.8–2.7 mg beryllium/litre produced only slight (5.6%) growth inhibition in Chlorella pyrenoidosa (Hoagland, 1952).
No data were located regarding the toxicity of beryllium to terrestrial wildlife species. Studies on laboratory mammals (described in section 8) showed that inhaled beryllium could be acutely toxic at concentrations as low as 0.15 mg beryllium/m3 and could produce pulmonary effects (proliferative and inflammatory changes) following prolonged exposure to concentrations as low as 6 µg beryllium/m3. Ingested beryllium was acutely toxic at doses as low as 18 mg/kg body weight. A NOAEL of 0.1 mg beryllium/kg body weight per day was identified for chronic ingestion of beryllium in dogs based on lesions of the small intestine.
Beryllium is phytotoxic to terrestrial plants, inhibiting growth and reducing yield at mg/litre concentrations under low- and neutral-pH conditions. The yield of bush beans grown in nutrient culture solution (pH 5.3) was reduced 33% by the addition of 0.5 mg beryllium/litre and 88% by the addition of 5 mg beryllium/litre (Romney et al., 1962). Similar effects on yield were observed in nutrient culture solution studies using beryllium sulfate in kale (Williams & Le Riche, 1968) and beryllium nitrate in cabbage (Hara et al., 1977). In cabbage, the critical concentration producing a 50% reduction in yield corresponded to 3000 mg beryllium/kg in the roots and 6 mg beryllium/kg dry weight in the leaves (very little of the beryllium taken up by the roots was translocated to the upper parts of the plant). A critical level of 0.6 mg beryllium/kg dry weight in the leaves was reported for spring barley (Davis et al., 1978). Yield reductions (stunting of both roots and foliage, but without chlorosis or mottling of foliage) were also seen in beans, wheat, and clover exposed to beryllium in soil cultures at levels corresponding to 4% of the cation exchange capacity in the soil (Romney & Childress, 1965). The study found that beryllium was strongly adsorbed by bentonite and various soils (displacing barium, calcium, magnesium, and strontium), but not by kaolinite. A concentration of 10 mg beryllium/kg (as beryllium chloride) reduced the yield of spring barley grown in sandy soil by 26% (Kick et al., 1980). At high pH, beryllium is less phytotoxic, due in part to precipitation as the phosphate salt, making it unavailable to plants (Williams & Le Riche, 1968). At high pH, beryllium can also reduce the magnesium requirements of plants to a limited extent, enhancing the growth of plants (and algae) cultured in magnesium-deficient media (Hoagland, 1952).
The effect of beryllium sulfate on soil microorganisms was studied by Wilke (1987), who found that soil biomass was reduced by 40% and nitrogen mineralization by 43% at a soil concentration of 30 mg beryllium/kg. At a soil concentration of 80 mg beryllium/kg, dehydrogenase, saccharase, and protease were also inhibited. No data regarding the toxicity of beryllium to earthworms or other soil organisms were located.
The toxicity thresholds of beryllium nitrate tetrahydrate (Be(NO3)2 · 4H2O) were 0.004 mg beryllium/litre for the flagellate Entosiphon sulcatum Stein, 0.017 mg/litre for the cilicate Uronema parduczi Chatton-Lwoff, and 0.51 mg/litre for the flagellate Chilomonas paramaecium Ehrenberg (Bringmann et al., 1980). When beryllium phosphate was added to fertilizers, the biomass was reduced to 60% and nitrogen mineralization to 57% of the control at the level of 30 mg beryllium/kg soil (Wilke, 1987).
There are no reliable data on the oral toxicity of beryllium in humans.
Dogs fed 500 ppm (mg/kg) beryllium as beryllium sulfate tetrahydrate (12.2 and 17.4 mg beryllium/kg body weight per day for males and females, respectively) developed gastrointestinal lesions. Similar, but less severe, gastrointestinal tract lesions were observed in one female given 50 ppm (1.3 mg/kg body weight per day) beryllium, which died during week 70. The remaining animals at this dose showed no histopathological alterations in the gastrointestinal tract related to treatment. A NOAEL of approximately 0.1 mg beryllium/kg body weight per day and a frank effect level 6 of 12 mg beryllium/kg body weight per day for gastrointestinal tract lesions, anorexia, and weight loss in moribund dogs can be derived from this study. The LOAEL is not clear, as the findings are limited to one animal. To decrease reliance on findings on individual animals, the benchmark dose (BMD) approach was used to derive a BMD10. The average of the male and female doses and the combined male and female incidence for small intestinal lesions were modelled by the exponential polynomial, THRESH, and Weibull models; a 10% change (extra risk), BMD10, was calculated to be 0.46 mg beryllium/kg body weight per day (US EPA, 1998; Appendix 4).
Gastrointestinal effects were not observed in rats or mice exposed to dietary beryllium sulfate (Morgareidge et al., 1975, 1977; Schroeder & Mitchener, 1975a,b), and the gastrointestinal tract was not examined in the beryllium carbonate studies.
"Beryllium rickets" was observed in young rats fed a "normal" stock diet for 3–4 weeks containing 0.125–3.0% beryllium carbonate (13–300 mg beryllium/kg body weight per day using a food factor of 0.05 [US EPA, 1986] and the authors’ estimate that the beryllium carbonate used in the study contained 20% beryllium) (Guyatt et al., 1933; Kay & Skill, 1934). It is not known if exposure to beryllium compounds other than beryllium carbonate will result in rickets, because the available studies on beryllium sulfate (the only other beryllium compound with available oral toxicity data) did not examine the skeletal system or measure serum phosphate levels. Schroeder & Mitchener (1975a) noted that rickets was not observed in their beryllium-exposed rats, but the criteria used to assess potential rachitic effects were not reported. Morgareidge et al. (1976) did not mention the occurrence of rickets in dogs that were observed daily and that underwent histological examination of the bone.
The potential of beryllium to induce developmental and/or reproductive effects has not been adequately assessed. In the only oral exposure study examining reproductive or developmental end-points, beryllium did not affect fertility or pup survival, weight, or skeletal formation in dogs (Morgareidge et al., 1976). However, only small numbers of animals were evaluated, visceral examinations of pups and examination of dying pups were not conducted, and postnatal development was not evaluated.
Measures of immune response or dysfunction have not been evaluated in oral exposure studies in animals.
In humans, the lung is the primary target of inhalation exposure to beryllium. Exposure to beryllium may result in the development of CBD, characterized by the formation of granulomas (Cotes et al., 1983; Cullen et al., 1987; Kreiss et al., 1996). These granulomas result from an immune reaction, primarily based on cell-mediated immunity. A genetic component to CBD susceptibility has been identified (Sterner & Eisenbud, 1951; Richeldi et al., 1993; Stubbs et al., 1996; US EPA, 1998). The toxicity of beryllium compounds increases with increasing water solubility (Finch et al., 1988; Haley et al., 1989). Beryllium oxide calcined at 500 °C is more soluble, is more toxic, and has a greater surface area than beryllium calcined at 1000 °C. The toxicity of inhaled aerosolized beryllium metal appears to resemble that of beryllium oxide calcined at 500 °C because of a thin layer of oxide on the beryllium metal particles (Hoover et al., 1989).
An animal model of human CBD is defined by the development of immune granulomas, a beryllium-specific immune response, and a disease progression that mimics the human disease. Based on these criteria in single-exposure studies, the beagle dog appears to model several aspects of CBD (Haley et al., 1989). Monkeys (Haley et al., 1994), mice (Huang et al., 1992), and guinea-pigs (Barna et al., 1984), although they have not been studied in as great detail, also appear to develop immune granulomas. Rats form granulomas after inhaling beryllium compounds, but the granulomas do not have an immune component, and rats do not mount a beryllium-specific immune response (Hart et al., 1984; Haley et al., 1990; Finch et al., 1994). Using mice and guinea-pigs gives the advantage of being able to use larger numbers of animals in experiments; of these two species, however, a beryllium-specific immune response has been shown only in guinea-pigs (i.e., the immune reaction in mice has not been shown to involve beryllium-specific sensitization). No exposure–response studies have been published using species that are appropriate models for CBD, and all studies using appropriate models have been conducted only with acute exposures.
There is an extensive body of evidence documenting beryllium sensitization and CBD as the most sensitive effect of inhalation exposure to beryllium. The Kreiss et al. (1996) occupational exposure study, which identified a LOAEL [adj] of 0.20 µg beryllium/m3, and the Eisenbud et al. (1949) community monitoring study, which identified a NOAEL [adj] of 0.01–0.1 µg beryllium/m3, were selected as the co-principal studies. Although the method of identifying CBD cases in the Eisenbud et al. (1949) study was relatively insensitive compared with modern methods, this study has the advantage of being conducted with the general population, rather than a worker population. In addition, because the incidence of CBD was evaluated at different distances from the plant (and hence at different estimated exposure levels), this was the only study that was able to identify a NOAEL for CBD. The NOAEL [adj] range reflects the uncertainty associated with the estimations of exposure level.
Occupational exposure studies by Cullen et al. (1987) and Cotes et al. (1983) identified low LOAEL [adj] values for CBD. Using the BCR definition of CBD, Cullen et al. (1987) identified a LOAEL [adj] of 0.19 µg beryllium/m3. Although the LOAEL [adj] identified in this study was similar to that found in Kreiss et al. (1996), the Cullen et al. (1987) study did not have historical exposure monitoring data, and worker exposure levels were estimated using only a small number of recent monitoring data. Cotes et al. (1983) reported a LOAEL [adj] of 0.036 µg beryllium/m3, but the CBD used in this study was not well defined, only two cases of CBD were identified, and the exposure concentrations were estimated using area samplers rather than personal and/or breathing zone samplers.
Studies regarding the potential carcinogenicity of ingested beryllium to humans are not available. Increases in lung cancer mortality were observed in cohort mortality studies of beryllium processing workers (Mancuso, 1979, 1980; Wagoner et al., 1980; Ward et al., 1992) and in studies of entrants on the BCR (Infante et al., 1980; Steenland & Ward, 1991). No increases in other types of cancer were found, but increases in deaths from non-malignant respiratory disease were also observed. These studies are considered to provide evidence of carcinogenicity in humans exposed by inhalation; the evidence is limited because of relatively small increases in lung cancer risks, poorly defined estimates of beryllium exposure, incomplete smoking data, and lack of control for potential exposure to other carcinogens, including co-exposure to sulfuric or hydrofluoric acid mists during employment in the beryllium industry. Regardless of the shortcomings of the epidemiological studies of beryllium exposure, the results of all the follow-up mortality studies on the same cohort and of the BCR cohort studies are suggestive of a causal relationship between beryllium exposure and an increased risk of lung cancer. This conclusion is strengthened by the increased incidences of lung cancers among workers with ABD (presumably these workers were exposed to very high concentrations of beryllium), the higher incidences of lung cancers among workers first employed when exposure levels were very high, a consistent finding of lung cancer excesses in six of seven beryllium processing facilities, and the occurrence of the highest risks for lung cancer in plants where the risk for non-malignant respiratory disease is the highest.
Studies of beryllium carcinogenicity in animals are available by inhalation, intratracheal, oral, and parenteral exposure. Inhalation exposure to beryllium (metal, ores, and sulfate compounds) produced significant increases in lung cancer in rats and monkeys (Reeves et al., 1967; Vorwald, 1968; Reeves & Deitch, 1969; Wagner et al., 1969; Nickell-Brady et al., 1994). These observations support the possible causal association noted in the occupational studies. Beryllium (metal, alloys, and compounds) has also been shown to produce lung cancer in rats by intratracheal instillation and osteosarcomas in rabbits by intravenous and intramedullary injection (US EPA, 1987). Oral exposure studies using the sulfate tetrahydrate in rats (Morgareidge et al., 1975, 1977; Schroeder & Mitchener, 1975a) and mice (Schroeder & Mitchener, 1975b) did not find significant increases in tumour incidences, but were inadequate for assessment of carcinogenicity due to the use of doses below the MTD. Overall, the animal data are considered to provide sufficient evidence of beryllium carcinogenicity in animals.
Genotoxicity data for beryllium are mixed (US EPA, 1998). Beryllium did not produce gene mutations in the majority of bacterial assays, with or without metabolic activation. However, gene mutations were observed in mammalian cells cultured with beryllium chloride, and clastogenic alterations were found in mammalian cells cultured with beryllium chloride, beryllium sulfate, and beryllium nitrate.
An oral tolerable intake of 0.002 mg/kg body weight per day was estimated from the BMD10 (0.46 mg/kg body weight per day, dose calculated at the lower 95% confidence interval for a 10% incidence [response] of small intestinal lesions; assumed to be equal to a NOAEL) in dogs chronically exposed to beryllium sulfate tetrahydrate using the benchmark dose approach and an uncertainty factor of 300. The uncertainty factor of 300 was composed of 10-fold factors each for intra- and interspecies variation and a 3-fold factor for database deficiencies (no studies available on developmental effects and no mechanistic/mode of action data to suggest this may be an issue). Although there are several chronic oral animal studies, there is a lack of human toxicity data by the oral route, reproductive/developmental end-points have not been adequately assessed, and oral studies examining immunological end-points, the most sensitive end-point by the inhalation route, are lacking. Since the principal study is of chronic duration and a benchmark dose was used, there are no uncertainty factors for duration or NOAEL/LOAEL extrapolation.
There is an extensive body of evidence documenting beryllium sensitization and CBD as the most sensitive effects of inhalation exposure to beryllium in humans. A NOAEL [adj] of 0.01–0.1 µg beryllium/m3 was observed based on general population inhalation exposure to beryllium near the Lorain beryllium plant (1.2 km), using insensitive screening methods (Eisenbud et al., 1949). An occupational study by Kreiss et al. (1996) found a LOAEL of 0.55 µg beryllium/m3 (LOAEL [adj] of 0.20 µg beryllium/m3) using more sensitive screening methods.
An inhalation tolerable concentration for the non-cancer health effects of beryllium was estimated at 0.02 µg/m3 from the duration-adjusted LOAEL (0.20 µg/m3) for CBD in exposed workers using a total uncertainty factor of 10. This value was derived from the Kreiss et al. (1996) LOAEL rather than the Eisenbud et al. (1949) NOAEL because the Kreiss et al. (1996) study used a more sensitive screening method. The uncertainty factor of 10 (rounded value; US EPA, 1994) includes a factor of 3 for use of a LOAEL (a full factor of 10 was not used due to the sensitive nature of the subclinical end-point [beryllium sensitization]), a database uncertainty factor of 3 to account for the poor quality of exposure monitoring in the co-principal studies and other epidemiological studies that assessed the incidence of beryllium sensitization and CBD among exposed workers and community residents, a factor of 1 to adjust for the less-than-chronic exposure duration of the Kreiss et al. (1996) study based on evidence that the occurrence of CBD does not appear to be related to exposure duration, and a factor of 1 to account for human variability, because individuals developing beryllium sensitization and CBD (1–5% of the exposed population) are the most sensitive subpopulation.
Although there are no developmental studies or two-generation reproduction studies, a limited continuous breeding study found that beryllium does not cause reproductive or developmental effects following intratracheal administration (Clary et al., 1975). In addition, systemic distribution of beryllium is less than 1% (US EPA, 1987), and any systemic effects would be expected to occur at exposure levels much above the very low levels at which CBD is observed.
The oral carcinogenicity database is considered inadequate for assessing the carcinogenic potential of ingested beryllium. No human data are available, and the animal studies (Morgareidge et al., 1975, 1976, 1977; Schroeder & Mitchener, 1975a,b) produced only negative results and were limited by failure to achieve the MTD. Derivation of a quantitative cancer risk estimate for oral exposure is therefore precluded.
The inhalation carcinogenicity database includes both animal studies and epidemiology studies in exposed humans. With the possible exception of the Wagner et al. (1969) study, the results of the animal carcinogenicity studies are incompletely reported and are not of sufficient quality to be used as the basis for quantitative cancer risk estimates. Because Wagner et al. (1969) exposed the rats to ores with relatively low beryllium levels and high levels of silicon dioxide, this study would not be an appropriate basis for a risk estimate for general population exposure to beryllium.
The epidemiology study by Wagoner et al. (1980) was used to estimate the lifetime cancer risk from exposure to beryllium based on the lower and upper bounds of median exposure estimated by NIOSH (1972) — namely, 100 and 1000 µg/m3. The "effective" dose was determined by adjusting each NIOSH (1972) exposure estimate for duration of daily (8/24 h) and annual (240/365 days) exposure (US EPA, 1987) and the ratio of exposure duration to duration at risk, i.e., f years out of a period of L years at risk (from onset of employment to termination of follow-up). Two values of f/L were used in the calculations, namely, f/L = 1 and f/L = 0.25. For a given "effective" dose d and a relative risk R, the unit risk B is calculated by the formula B = (R – 1) × (0.036/d), where 0.036 is the background lifetime probability of death from lung cancer in the USA. The relative risk values used in this calculation were the 95% upper confidence limits on the relative risk estimates of 1.36 (P > 0.05) and 1.44 (P > 0.05) derived from the Wagoner et al. (1980) study, which were 1.98 and 2.09, respectively. Although these relative risk estimates were not statistically significant (after the adjustments for smoking and mortality described in section 9.5), the data were considered to be adequate to calculate an upper limit on lung cancer risk.
In recognition of the great uncertainty associated with the exposure estimation, four different "effective" levels of exposure that reflect various uncertainties, along with two relative risk estimates, were used in the present calculations (see Table 8). As a result, eight potency estimates were calculated, ranging from 1.6 × 10–4 per µg beryllium/m3 to 7.2 × 10–3 per µg beryllium/m3, with the geometric mean of the eight estimates being 2.4 × 10–3 per µg beryllium/m3. This "unit risk" estimate could be considered an upper-bound estimate of the cancer risk because low-dose linearity is assumed in the extrapolation and the 95% upper confidence limits (1.98 and 2.09) are used in the calculations.
Table 8: Derivation of unit risk estimates for beryllium based on exposure estimates from NIOSH (1972) and relative risk estimates derived from Wagoner et al. (1980).a
|
Beryllium concentration in workplace (µg/m3) |
Ratio of years of exposure to years at risk (f/L) |
Effective dose (µg/m3) |
95% upper-bound estimate of relative risk |
Unit risk per µg/m3 |
|
100 |
1 |
21.92 |
1.98 |
0.00161 |
|
|
|
|
2.09 |
0.00179 |
|
|
0.25 |
5.48 |
1.98 |
0.00644 |
|
|
|
|
2.09 |
0.00716 |
|
1000 |
1 |
219.18 |
1.98 |
0.000161 |
|
|
|
|
2.09 |
0.000179 |
|
|
0.25 |
54.79 |
1.98 |
0.000644 |
|
|
|
|
2.09 |
0.000716 |
a
See text for explanation of calculations.The inhalation tolerable concentration of 0.02 µg beryllium/m3 is an estimate of a daily inhalation exposure of the human population (including sensitive subgroups) that is likely to be without appreciable risk of deleterious non-cancer effects during a lifetime. For beryllium, therefore, an average lifetime exposure to 0.02 µg beryllium/m3 (20 ng beryllium/m3) is likely to be without appreciable risk of deleterious effects. The general population is exposed to beryllium concentrations well below the tolerable concentration. Ross et al. (1977) reported beryllium concentrations of 0.03–0.06 ng/m3 at rural sites (background), 0.04–0.07 ng/m3 at suburban sites, and 0.1–0.2 ng/m3 at urban industrial sites in the USA. The highest annual average beryllium concentration recorded at urban monitoring stations throughout the USA during 1981–1986 was 6.7 ng/m3 (US EPA, 1987). People who live near a point source, such as a coal-fired power generating plant, or work in an industry with beryllium exposure may be exposed to much higher levels.
The cancer unit risk of 2.4 × 10–3 per µg beryllium/m3 can be used with air concentration data (Ł 4 µg/m3; US EPA, 1987) to produce estimates of cancer risk in exposed populations (assuming lifetime average exposure at that concentration). For example, taking 0.045 ng/m3 as the ambient concentration of beryllium at rural sites, a cancer risk of 1.1 × 10–7 is calculated. The risk at an urban site with 0.15 ng/m3 beryllium in air is 3.6 × 10–7. The risks can be much higher for people who live near a beryllium point source or are exposed occupationally. While an occupational exposure limit of 1 µg beryllium/m3 as a TWA for a 40-h work week would produce a lifetime average exposure well below 1 µg beryllium/m3, available data suggest that some workers may be exposed at levels that would produce lifetime average exposures of 1 µg beryllium/m3 or more.
The overall uncertainty of the risk estimates is probably 1 order of magnitude.
Although a number of subchronic studies in laboratory animals have been conducted with beryllium compounds, none has been done using modern criteria for high-quality toxicology studies.
Whereas several laboratory animal species respond to beryllium exposure with some of the features of human CBD, no laboratory animal model fully mimics all features of human CBD. In particular, the animal models fail to demonstrate a progressive granulomatous pulmonary response with a concomitant beryllium-specific immune response.
Gastrointestinal effects have been observed in dogs, but not — in limited studies — in rodents. It is not clear if these effects are relevant to humans. There is an important uncertainty concerning the LOAELs of the gastrointestinal effects; this uncertainty was diminished by using the benchmark dose approach. Rickets was induced by beryllium carbonate in rats; again, it is not clear whether this effect, the mechanism of which apparently was an indirect one, is relevant to humans.
The major source of uncertainty in the risk characterizations from human studies — both for CBD and for respiratory cancer — is the exposure assessment. In addition to the uncertainty of the quantitation of exposure, the exact chemical species to which humans have been exposed is not clear; it is possible that different beryllium compounds have varying potencies in the induction of the immunological reactions that are the pathogenic mechanism of CBD.
The mode of action of beryllium in the production of CBD is not fully elucidated, but it apparently includes an individual susceptibility component. The prevalence of the disease, approximately 1%, is rather similar in populations with widely different exposure levels.
Beryllium is toxic to aquatic animals. Acute toxicity values (96-h LC50s) ranged from 0.14 mg beryllium/litre (free-living nematode) to 32.0 mg beryllium/litre (guppy), depending on the species studied and the test conditions, most notably hardness of the test water (Tarzwell & Henderson, 1960; Slonim, 1973; Slonim & Slonim, 1973; Cardwell et al., 1976; US EPA, 1978, 1980; Buikema, 1986; Khangarot & Ray, 1989; Williams & Dusenberry, 1990; Khangarot, 1991). In very soft, acidic waters, beryllium produced significant acute mortality (75% dead) in perch at ł 10 µg beryllium/litre (Jagoe et al., 1993). The lowest chronic toxicity value reported was 5 µg beryllium/litre in Daphnia magna at moderate water hardness (220 mg calcium carbonate/litre) (US EPA, 1980). Available data suggest that beryllium levels in surface waters are typically much lower. Beryllium concentrations ranged from <4 to 120 ng/litre in the US Great Lakes (Rossman & Barres, 1988) and from <10 to 120 ng/litre (10–30 ng/litre average) in Australian river waters (Meehan & Smythe, 1967). Based on US EPA’s STORET database for the years 1960–1988, the geometric mean concentration of total beryllium in US surface waters was estimated to be 70 ng/litre (Eckel & Jacob, 1988), although surface water concentrations as high as 1 µg/litre have been reported (Bowen, 1979). Waters near point sources, however, may contain higher beryllium concentrations. Concentrations of 30–170 µg/litre have been reported in industrial effluents (ATSDR, 1993). It is possible that beryllium concentrations near point sources would be toxic to aquatic animals. The greatest potential for beryllium toxicity to aquatic animals occurs in acidic lakes and streams with very soft water (Jagoe et al., 1993). Aquatic microalgae are considerably less sensitive than aquatic animals to beryllium (Hoagland, 1952; Karlander & Krauss, 1972), so adverse effects from beryllium on algae would not be expected at environmental levels, even in the vicinity of point sources.
Studies on laboratory mammals (described above) showed that ingested beryllium could be acutely toxic to laboratory mammals at doses as low as 18 mg/kg body weight and identified a NOAEL of 0.1 mg beryllium/kg body weight per day for chronic ingestion of beryllium in dogs based on lesions of the small intestine. Assuming a drinking-water concentration of 1 µg beryllium/litre, the highest concentration reported for surface water (Bowen, 1979), and a food concentration of 10 mg/kg dry weight, the highest concentration of beryllium reported in a terrestrial plant species (i.e., a tree) (Nikonova, 1967; Griffitts et al., 1977), a reference deer mouse (US EPA, 1993) with a body weight normalized water consumption of 0.19 kg/kg body weight per day and food consumption of 0.22 kg/kg body weight per day would receive a dose of 2.2 mg beryllium/kg body weight per day, which is greater than the chronic dog NOAEL of 0.1 mg/kg body weight per day, but well below the chronic rat NOAEL of 37 mg beryllium/kg body weight per day. This scenario suggests that there could be some risk to wildlife species that, like the dog, are especially susceptible to beryllium toxicity and consume food having very high levels of beryllium over a long period of time. However, since beryllium levels are concentrated in the upper portions of the tree and not at ground level, there appears to be no risk to small animals eating food at ground level, but there may be a risk for large mammals browsing at higher levels.
Beryllium is phytotoxic to terrestrial plants, inhibiting growth and reducing yield at 0.5–5 mg beryllium/litre concentrations in nutrient culture solution under low- and neutral-pH conditions. At high pH, beryllium is less phytotoxic, due in part to precipitation as the phosphate salt, making it unavailable to plants (Williams & Le Riche, 1968). At high pH, beryllium can also reduce the magnesium requirements of plants to a limited extent, enhancing growth of plants (and algae) cultured in magnesium-deficient media (Hoagland, 1952). Kick et al. (1980) reported an effect level in soil of 10 mg beryllium/kg for reduced yield of spring barley in sandy soil. It is expected that higher concentrations would be required to produce effects in other soils where adsorption would be greater and bioavailability of beryllium lower. Soil concentrations are typically lower than the phytotoxic effect level of 10 mg beryllium/kg identified by Kick et al. (1980). Agricultural soils in the USA contained <1–7 mg beryllium/kg and averaged 0.6 mg beryllium/kg (Shacklette et al., 1971). In Japan, the mean soil concentration was 1.31 mg beryllium/kg (Asami & Fukazawa, 1985). These data suggest that beryllium in the environment would have to be roughly 10-fold higher than normal to be a hazard to terrestrial plants. Most plants take up beryllium in small amounts, but very little is translocated within the plant (Romney & Childress, 1965; Kloke et al., 1984). From the few data available, there is no evidence that beryllium biomagnifies within food chains (Callahan et al., 1979; Kenaga, 1980; US EPA, 1980; Fishbein, 1981; Byrne & DeLeon, 1986). Therefore, the risk to wildlife from food chain transfer of beryllium is low.
The International Agency for Research on Cancer (IARC, 1993) evaluated the carcinogenicity of beryllium and assigned beryllium and beryllium compounds to Group 1, concluding that they are carcinogenic to humans. The assessment was based on sufficient evidence for carcinogenicity in humans and sufficient evidence for carcinogenicity in animals.
In the absence of suitable oral toxicity data, no drinking-water quality guideline could be established for beryllium (WHO, 1996).
Alekseeva OG (1966) Ability of beryllium compounds to cause allergy of the delayed type. Federation proceedings, 25:843–846.
Andre SM, Metivier H, Lantenois G, Boyer M, Nolibe D, Masse R (1987) Beryllium metal solubility in the lung: comparison of metal hot-pressed forms by in-vivo and in-vitro dissolution bioassays. Human toxicology, 6(3):233–240.
Arlauskas A, Baker RS, Bonin AM, Tandon RK, Crisp PT, Elllis J (1985) Mutagenicity of metal ions in bacteria. Environmental research, 36:379–388.
Asami T, Fukazawa F (1985) Beryllium contents of uncontaminated soil and sediments in Japan. Soil science and plant nutrition, 31:43–54.
Ashby J, Ishidate M Jr, Stoner GD, Morgan MA, Ratpan F, Callander RD (1990) Studies on the genotoxicity of beryllium sulphate in vitro and in vivo. Mutation research, 240:217–225.
ATSDR (1993) Toxicological profile for beryllium. Update. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry (ATSDR/TP-92/08; NTIS Accession No. PB93-182434).
Awadallah RM, Sherif MK, Amrallah AH, Grass F (1986) Determination of trace elements of some Egyptian crops by instrumental neutron activation, inductively coupled plasma–atomic emission spectrometric and flameless atomic absorption spectrophotometric analysis. Journal of radioanalysts and nuclear chemists, 98(2):235–246.
Barna BP, Chiang T, Pillarisetti SG, Deodhar SD (1981) Immunologic studies of experimental beryllium lung disease in the guinea pig. Clinical immunology and immunopathology, 20:402–411.
Barna BP, Deodhar SD, Chiang T, Gautam S, Edinger M (1984) Experimental beryllium-induced lung disease. I. Differences in immunologic responses to beryllium compounds in strains 2 and 13 guinea pigs. International archives of allergy and applied immunology, 73(1):42–48.
Basolo F (1956) Theories of acids, bases, amphoteric hydroxides and basic salts as applied to the chemistry of complex compounds. In: Bailar JC Jr, ed. The chemistry of the coordination compounds. New York, NY, Reinhold Publishing Corp., p. 834.
Bayliss DL, Lainhart WS, Crally LJ, Ligo R, Ayer H, Hunter F (1971) Mortality pattern in a group of former beryllium workers. In: Transactions of the 33rd Annual Meeting of the American Conference of Governmental Industrial Hygienists, Toronto, Ontario, 24–28 May 1971. Cincinnati, OH, American Conference of Governmental Industrial Hygienists, pp. 94–107.
Belman S (1969) Beryllium binding of epidermal constituents. Journal of occupational medicine, 11:175–183.
Bencko V, Brezina M, Benes B, Cikrt M (1979) Penetration of beryllium through the placenta and its distribution in the mouse. Journal of hygiene, epidemiology, microbiology and immunology, 23:361–367.
Bencko V, Vasileva EV, Symon K (1980) Immunological aspects of exposure to emissions from burning coal of high beryllium content. Environmental research, 22:439–449.
BISAC (Beryllium Industry Scientific Advisory Committee) (1997) Is beryllium carcinogenic in humans? Journal of occupational and environmental medicine, 39:25–208.
Bowen HJM (1979) Environmental chemistry of the elements. New York, NY, Academic Press.
Bringmann G, Kühn R, Winter A (1980) [Determination of biological damage from water pollutants to protozoa. III. Saprozoic flagellates.] Zeitschrift für Wasser und Abwasser Forschung, 13(5):170–173 (in German, with English abstract).
Brooks AL, Griffith WC, Johnson NF, Finch GL, Cuddihy RG (1989) The induction of chromosome damage in CHO cells by beryllium and radiation given alone and in combination. Radiation research, 120:494–507.
Buikema A (1986) Toxicity of beryllium to the cladoceran, Daphnia magna, as a function of water hardness. Blacksburg, VA, Virginia Polytechnic Institute and State University, Department of Biology, 24 pp.
Byrne CJ, DeLeon LR (1986) Trace metal residues in biota and sediments from Lake Pontchartrain, Louisiana. Bulletin of environmental contamination and toxicology, 37(1):151–158.
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR, Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, Gould C (1979) Water-related environmental fate of 129 priority pollutants. Washington, DC, US Environmental Protection Agency (EPA-440/4-79-029a).
Camner P, Hellstrom PA, Lundborg M, Philipson K (1977) Lung clearance of 4µm particles coated with silver, carbon or beryllium. Archives of environmental health, 32:58–62.
Cardwell RD, Foreman DG, Payne TR, Wilbur DJ (1976) Acute toxicity of selected toxicants to six species of fish. Duluth, MN, US Environmental Protection Agency (Report No. 600/3-76-008).
Caroli S, Coni E, Alimonti A, Beccaloni E, Sabbioni E, Pietra R (1988) Determination of trace elements in human lungs by ICP-AES and NAA. Analysis, 16:656–661.
Cartledge GH (1928) Studies on the periodic system: II. The ionic potential and related properties. Journal of the American Chemical Society, 50:2863–2872.
Cikrt M, Bencko V (1975) Biliary excretion of 7Be and its distribution after intravenous administration of 7BeCl2 in rats. Archives of toxicology, 34:53–60.
Clary JJ, Bland LS, Stokinger HF (1975) The effect of reproduction and lactation on the onset of latent chronic beryllium disease. Toxicology and applied pharmacology, 33(2):214–211.
Cotes JE, Gilson JC, McKerrow CB, Oldham PD (1983) A long-term follow up of workers exposed to beryllium. British journal of industrial medicine, 40(1):13–21.
Cotton FA, Wilkinson G (1972) Advanced inorganic chemistry; a comprehensive text. New York, NY, Interscience Publishers [cited in Callahan et al., 1979].
Covington JS, McBride MA, Slagle WF, Disney AL (1985) Beryllium localization in base metal dental casting alloys. Journal of biomedical materials research, 19(7):747–750.
Cremers DA, Radziemski LJ (1985) Direct detection of beryllium on filters using the laser spark. Applied spectroscopy, 39(1):57–63.
Crowley JF, Hamilton JG, Scott KG (1949) The metabolism of carrier-free radioberyllium in the rat. Journal of biological chemistry, 177:975–984.
Cullen MR, Kominsky JR, Rossman MD, Cherniack MG, Rankin JA, Balmes JR, Kern JA, Daniele RP, Palmer L, Naegel GP, McMagnus K, Cruz R (1987) Chronic beryllium disease in a precious metal refinery. Clinical epidemiologic and immunologic evidence for continuing risk from exposure to low level beryllium fume. American reviews of respiratory diseases, 135(1):201–208.
Curtis GH (1951) Cutaneous hypersensitivity due to beryllium. Archives of dermatology and syphilology, 64:470–482.
Davis RD, Beckett PHT, Wollan E (1978) Critical levels of twenty potentially toxic elements in young spring barley. Plant and soil, 49:395–408.
Delic J (1992) Toxicity Review 27 (Part 2): Beryllium and beryllium compounds. London, Her Majesty’s Stationery Office (ISBN 0 11 886343 6).
De Nardi JM, Van Orstrand HS, Curtis GH, Zielinski J (1953) Berylliosis: Summary and survey of all clinical types observed in a twelve-year period. American Medical Association archives of industrial hygiene and occupational medicine, 8:1–24.
Dilling WJ, Healey CW (1926) Influence of lead and the metallic ions of copper, zinc, thorium, beryllium, and thallium on the germination of frogs’ spawn and on the growth of tadpoles. Annals of applied biology, 13:177–188.
Dreher GB, Muchmore CB, Stover DW (1977) Major, minor, and trace elements of bottom sediments in lake Du Quoin, Johnston City Lake, and Little Grassy Lake in Southern Illinois. Urbana, IL, Illinois State Geological Survey, 38 pp. (Environmental Geology Notes No. 82).
Drury JS, Shriner CR, Lewis EB, Towill LE, Hammons AS (1978) Reviews of the environmental effects of pollutants: VI. Beryllium. Prepared under IAG-D5-0403 by Oak Ridge National Laboratory, Union Carbide Corp., Oak Ridge, TN (EPA 600/1-78-028; NTIS PB-290966).
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K, Rosenkranz HS, Simmon VF (1984) Reproducibility of microbial mutagenicity assays: I. Tests with Salmonella typhimurium and Escherichia coli using a standardized protocol. Environmental mutagenesis, 6(Suppl. 2):1–254.
Dylevoi MV (1990) [Evaluation of the DNA-damaging action of the carcinogenic metal beryllium by means of bacterial repair test.] Mikrobiologicheskii Zhurnal (Kiev), 52:34–38 (in Russian).
Eckel WP, Jacob TA (1988) Ambient levels of 24 dissolved metals in USA surface and ground waters. American Chemical Society Division of Environmental Chemistry 196th Meeting Preprints, 28:371–372.
Eisenbud M, Lisson J (1983) Epidemiological aspects of beryllium-induced non-malignant lung disease: A 30-year update. Journal of occupational medicine, 25:196–202.
Eisenbud M, Berghout CF, Steadman LT (1948) Environmental studies in plants and laboratories using beryllium: The acute disease. Journal of industrial hygiene and toxicology, 30:281–285.
Eisenbud M, Wanta RC, Dastan C, Steadman LT, Harris WB, Wolfe BS (1949) Non-occupational berylliosis. Journal of industrial hygiene and toxicology, 31:282–294.
Epstein WL (1990) Cutaneous effects of beryllium. In: Rossman MD, Preuss OP, Powers MB, eds. Beryllium: Biomedical and environmental aspects. Baltimore, MD, Williams and Wilkins, pp. 113–117.
Finch GL, Brooks AL, Hoover MD, Cuddihy RG (1988) Influence of physicochemical properties of beryllium particles on toxicity to cultured cells. In vitro toxicology, 2:287–297.
Finch GL, Mewhinney JA, Hoover MD, Eidson AF, Haley PJ, Bice DE (1990) Clearance, translocation, and excretion of beryllium following acute inhalation of beryllium oxide by beagle dogs. Fundamental and applied toxicology, 15:231–241.
Finch GL, Haley PJ, Hoover MD, Snipes MD, Cuddihy RG (1994) Responses of rat lungs to low lung burdens of inhaled beryllium metal. Inhalation toxicology, 6(3):205–224.
Finch GL, Hoover MD, Hahn FF, Nikula KJ, Belinsky SA, Haley PJ, Griffith WC (1996) Animal models of beryllium-induced lung disease. Environmental health perspectives, 104(Suppl. 5):973–979.
Finch GL, Nikula KJ, Hoover MD (1998a) Dose–response relationships between inhaled beryllium metal and lung toxicity in C3H mice. Toxicological sciences, 42:36–48.
Finch GL, March TH, Hahn FF, Barr EB, Belinsky SA, Hoover MD, Lechner JF, Nikula KJ, Hobbs CH (1998b) Carcinogenic responses of transgenic heterozygous p53 knockout mice to inhaled 239PuO2 or metallic beryllium. Toxicologic pathology, 26:484–491.
Fishbein L (1981) Sources, transport and alterations of metal compounds: an overview. 1. Arsenic, beryllium, cadmium, chromium, and nickel. Environmental health perspectives, 40:43–64.
Fishbein L (1984) Overview of analysis of carcinogenic and/or mutagenic metals in biological and environmental samples. I. Arsenic, beryllium, cadmium, chromium and selenium. International journal of environmental and analytical chemistry, 17:113–170.
Freise R, Israel GW (1987) [Investigations into the suspended dust load in Berlin (West).] Berlin (West), Technische Universität Berlin, Institut für Technischen Umweltschutz (in German).
Furchner JE, Richmond CR, London JE (1973) Comparative metabolism of radionuclides in mammals. Part 8: Retention of beryllium in the mouse, rat, monkey and dog. Health physics, 24(3):293–300.
Gladney ES, Owens JW (1976) Beryllium emissions from a coal-fired power plant. Journal of environmental science and health, 11:297–311 [cited in ATSDR, 1993].
Goel KA, Agrawal VP, Garg V (1980) Pulmonary toxicity of beryllium in albino rats. Bulletin of environmental contamination and toxicology, 24:59–64.
Greene TM, Lanzisera DV, Andrews L, Downs AJ (1998) Matrix-isolation and density functional theory study of the reactions of laser-abated beryllium, magnesium, and calcium atoms with methane. Journal of the American Chemical Society, 120(24):6097–6104.
Griffitts WR, Allaway WH, Groth DH (1977) Beryllium. In: Geochemistry and the environment. Vol. II. The relation of other selected trace elements to health and disease. Washington, DC, National Academy of Sciences, US National Committee for Geochemistry, pp. 7–10.
Griggs K (1973) Toxic metal fumes from mantle-type camp lanterns. Science, 181(4102):842–843.
Guyatt BL, Kay HD, Branion HD (1933) Beryllium "rickets." Journal of nutrition, 6:313–324.
Haley PJ, Finch GL, Mewhinney JA, Harmsen AG, Hahn FF, Hoover MD, Muggenburg BA, Bice DE (1989) A canine model of beryllium-induced granulomatous lung disease. Laboratory investigation, 61(2):219–227.
Haley PJ, Finch GL, Hoover MD, Cuddihy RG (1990) The acute toxicity of inhaled beryllium metal in rats. Fundamental and applied toxicology, 15:767–778.
Haley PJ, Finch GL, Hoover MD, Mewhinney JA, Bice DE, Muggenburg BA (1992) Beryllium-induced lung disease in the dog following two exposures to BeO. Environmental research, 59(2):400–415.
Haley PJ, Pavia KF, Swafford DS, Davila DR, Hoover MD, Finch GL (1994) Comparative pulmonary toxicity of beryllium metal and beryllium-oxide in cynomolgus monkeys. Immunopharmacology and immunotoxicology, 16(4):627–644.
Haley PJ, Swafford DS, Finch GL, Hoover MD, Muggenburg BA, Johnson NF (1997) Immunologic specificity of lymphocyte cell lines from dogs exposed to beryllium oxide. Immunopharmacology and immunotoxicology, 19(4):459–471.
Hall RH, Scott JK, Laskin S, Stroud CA, Stokinger HE (1950) Acute toxicity of inhaled beryllium. III. Observations correlating toxicity with the physicochemical properties of beryllium oxide dust. Archives of industrial hygiene and occupational medicine, 2:25–33.
Hamilton A, Hardy H (1974) Industrial toxicology, 3rd ed. Acton, MA, Publishing Sciences Group.
Hara T, Sonoda Y, Iwai I (1977) Growth response of cabbage plants to beryllium and strontium under water culture conditions. Soil science and plant nutrition, 23:373–380.
Hart BA, Bickford PC, Whatlen MC, Hemanway D (1980) Distribution and retention of beryllium in guinea pigs after administration of a beryllium chloride aerosol. US Department of Energy symposium series (pulmonary toxicology of respirable particulates), 53:87–102.
Hart BA, Harmsen AG, Low RB, Emerson R (1984) Biochemical, cytological, and histological alterations in rat lung following acute beryllium aerosol exposure. Toxicology and applied pharmacology, 75(3):454–465.
Hasan FM, Kazemi H (1974) Chronic beryllium disease: a continuing epidemiologic hazard. Chest, 65:289–293.
Hem JD (1970) Study and interpretation of the chemical characteristics of natural water. Washington, DC, US Geological Survey (Geological Survey Water Paper 1473).
Hildebrand SG, Cushman RM (1978) Toxicity of gallium and beryllium to developing carp eggs (Cyprinus carpio) utilizing copper as a reference. Toxicology letters, 2(2):91–95.
Hoagland MB (1952) Beryllium and growth II. The effect of beryllium on plant growth. Archives of biochemistry and biophysics, 35:249–258.
Hoover MD, Castorina BT, Finch GL, Rothenberg SJ (1989) Determination of the oxide layer thickness on beryllium metal particles. American industrial hygiene association journal, 50(10):550–553.
Hoover MD, Finch GL, Mewhinney JA, Eidson AF (1990) Release of aerosols during sawing and milling of beryllium metal and beryllium alloys. Applied occupational and environmental hygiene, 5(11):787–791.
Howe RB (1995) THRESH: A computer program to compute a reference dose from quantal animal toxicity data using the benchmark dose method. Ruston, LA, ICF Kaiser Engineers, Inc.
HSE (1994) EH64 summary criteria for occupational exposure standards: Beryllium and beryllium compounds. Sudbury, Suffolk, Health and Safety Executive Books (ISBN 0 7176 1800 5).
Hsie AW, Johnson NP, Couch DB, San Sabastian JR, O’Neill JP, Hoeschele JD, Rahn RO, Forbes NL (1979a) Quantitative mammalian cell mutagenesis and a preliminary study of the mutagenic potential of metallic compounds. In: Kharasch N, ed. Trace metals in health and disease. New York, NY, Raven Press, pp. 55–69.
Hsie AW, O’Neill JP, San Sebastian JR, Couch DB, Brimer PA, Sun WNC, Fuscoe JC, Forbes NL, Machanoff R, Riddle JC, Hsie MH (1979b) Quantitative mammalian cell genetic toxicology: study of the cytotoxicity and mutagenicity of seventy individual environmental agents related to energy technologies and three subfractions of crude synthetic oil in the CHO/HGPRT system. Environmental science and research, 15:291–315.
Huang H, Meyer KC, Kubai L, Auerbach R (1992) An immune model of beryllium-induced pulmonary granulomata in mice. Histopathology, immune reactivity, and flow-cytometric analysis of bronchoalveolar lavage-derived cells. Laboratory investigation, 67(1):138–146.
Hurlbut JA (1978) Determination of beryllium in biological tissues and fluids by flameless atomic absorption spectroscopy. Atomic absorption newsletter, 17:121–124.
IARC (1993) Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Lyon, International Agency for Research on Cancer, pp. 41–118 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 58).
ICRP (1960) Report of ICRP Committee II on Permissible Dose for Internal Radiation. Health physics, 3:154–155.
Ikebe K, Tanaka R, Kuzuhara Y, Suenaga S, Takabatake E (1986) Studies on the behavior of beryllium in environment. Behavior of beryllium and strontium in atmospheric air. Eisei Kagaku, 32:159–166.
Infante PF, Wagoner JK, Sprince NL (1980) Mortality patterns from lung cancer and nonneoplastic respiratory disease among white males in the beryllium case registry. Environmental research, 21:35–43.
IPCS (1990) Beryllium. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 106).
IPCS (1999a) International Chemical Safety Card — Beryllium. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0226).
IPCS (1999b) International Chemical Safety Card — Beryllium oxide. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1325).
IPCS (1999c) International Chemical Safety Card — Beryllium sulfate. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1351).
IPCS (1999d) International Chemical Safety Card — Beryllium nitrate. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1352).
IPCS (1999e) International Chemical Safety Card — Beryllium carbonate. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1353).
IPCS (1999f) International Chemical Safety Card — Beryllium chloride. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1354).
IPCS (1999g) International Chemical Safety Card — Beryllium fluoride. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 1355).
Izmerov NF, ed. (1985) Beryllium. Geneva, International Register of Potentially Toxic Chemicals; Moscow, Centre of International Projects (Scientific Reviews of Soviet Literature on Toxicity and Hazards of Chemicals Series).
Jacobson SA (1933) Bone lesions in rats produced by the substitution of beryllium for calcium in the diet. Archives of pathology, 15:18–26.
Jagoe CH, Matey VE, Haines TA, Komov VT (1993) Effect of beryllium on fish in acid water is analogous to aluminum toxicity. Aquatic toxicology, 24:241–256.
Kada T, Hirano K, Shirasu Y (1980) Screening of environmental chemical mutagens by the rec-assay system with Bacillus subtilis. In: deSerres FJ, Hollander A, eds. Chemical mutagens. Principles and methods for their detection. Vol. 6. New York, NY, Plenum Press, pp. 149–173.
Kanematsu N, Hara M, Kada T (1980) REC assay and mutagenicity study on metal compounds. Mutation research, 77:109–116.
Karlander EP, Krauss RW (1972) Absorption and toxicity of beryllium and lithium in Chlorella vannielii Shihira and Krauss. Chesapeake science, 13:245–253.
Kay HD, Skill DL (1934) Prevention and cure of beryllium rickets. Biochemistry journal, 28:1222–1229.
Kenaga E (1980) Predicted bioconcentration factors and soil sorption coefficients of pesticides and other chemicals. Ecotoxicology and environmental safety, 4:26–38.
Khangarot BS (1991) Toxicity of metals to a freshwater tubificid worm, Tubifex tubifex (Muller). Bulletin of environmental contamination and toxicology, 46:906–912.
Khangarot BS, Ray PK (1989) Investigation of correlation between physiochemical properties of metals and their toxicity to the water flea Daphnia magna Straus. Ecotoxicology and environmental safety, 18:109–120.
Kick H, Burger H, Sommer K (1980) [Plant experiments on the uptake of beryllium and thallium by barley and rape.] Landwirtschaftliche Forschung, 37:186–190 (in German).
Kjellgren BRF (1946) The production of beryllium oxide and beryllium copper. Transactions of the Electrochemists Society, 89:247–261.
Kjellstrom T, Kennedy P (1984) Criteria document for Swedish occupational standards: Beryllium. Solna, Arbetarsdyddsstyrelsen, Publikationsservice, 60 pp.
Klemperer FW, Martin AP, Van Riper J (1951) Beryllium excretion in humans. Archives of industrial hygiene and occupational medicine, 4:251–256.
Kloke A, Sauerbeck DR, Vetter H (1984) The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In: Nriagu JO, ed. Changing metal cycles and human health. Report of the Dahlem Workshop, Berlin (FRG), 20–25 March 1983. Berlin, Springer-Verlag, pp. 113–141.
Kreiss K, Wasserman S, Mroz MM, Newman LS (1993) Beryllium disease screening in the ceramics industry: blood lymphocyte test performance and exposure–disease relations. Journal of occupational medicine, 35:267–274.
Kreiss K, Mroz MM, Newman LS, Martyny J, Zhen B (1996) Machining risk of beryllium disease and sensitization with median exposures below 2 µg/m3. American journal of industrial medicine, 30(1):16–25.
Kreiss K, Mroz MM, Zhen BG, Wiedemann H, Barna B (1997) Risks of beryllium disease related to work processes at a metal, alloy, and oxide production plant. Occupational and environmental medicine, 54:605–612.
Kriebel D, Sprince NL, Eisen EA, Greaves IA, Feldman HA, Greene RE (1988a) Beryllium exposure and pulmonary function: a cross sectional study of beryllium workers. British journal of industrial medicine, 45:167–173.
Kriebel D, Sprince NL, Eisen EA, Greaves IA (1988b) Pulmonary function in beryllium workers: Assessment of exposure. British journal of industrial medicine, 45(2):83–92.
Krivanek ND, Reeves AL (1972) The effect of chemical forms of beryllium on the production of the immunologic response. American industrial hygiene association journal, 33:45–52.
Kuroda K, Endo G, Okamoto A, Yoo YS, Horiguchi S (1991) Genotoxicity of beryllium, gallium, and antimony in short-term assays. Mutation research, 264:163–170.
LaBelle CW, Cucci MR (1947) Preliminary studies in the toxicology of beryllium: The effect of intratracheal injection of beryllium in experimental animals. Washington, DC, US Atomic Energy Commission (Report MDDC-1232) [cited in Delic, 1992].
Lansdown ABG (1995) Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Critical reviews in toxicology, 25(5):397–462.
Larramendy ML, Popescu NC, DiPaolo JA (1981) Induction by inorganic metal salts of sister chromatid exchanges and chromosome aberrations in human and Syrian hamster cell strains. Environmental mutagenesis, 3:597–606.
Lederer H, Savage J (1954) Beryllium granuloma of the skin. British journal of industrial medicine, 11:45–51.
LeFevre ME, Joel DD (1986) Distribution of label after intragastric administration of 7Be-labeled carbon to weanling and aged mice. Proceedings of the Society of Experimental Biological Medicine, 182:112–119.
Leonard A, Lauwerys R (1987) Mutagenicity, carcinogenicity and teratogenicity of beryllium. Mutation research, 186:35–42.
LLNL (1997) Livermore, CA, Lawrence Livermore National Laboratory. www.llnl.gov/.
Lovblad G (1977) Trace elements concentrations in some coal samples and possible emissions from coal combustion in Sweden. Gothenburg, Swedish Water and Air Pollution Research Laboratory, 20 pp.
Lum KR, Gammon KL (1985) Geochemical availability of some trace and major elements in surficial sediments of the Detroit River and western Lake Erie. Journal of Great Lakes research, 11:328–338.
MacMahon B (1994) The epidemiological evidence on the carcinogenicity of beryllium in humans. Journal of occupational medicine, 36:15–24.
Mancuso TF (1979) Occupational lung cancer among beryllium workers. In: Lemen R, Dement J, eds. Conference on occupational exposures to fibrous and particle dust and their extension into the environment. Washington, DC, Society for Occupational and Environmental Health, pp. 463–482.
Mancuso TF (1980) Mortality study of beryllium industry workers’ occupational lung cancer. Environmental research, 21:48–55.
Marx JJ, Burrell R (1973) Delayed hypersensitivity to beryllium compounds. Journal of immunology, 111:590–598.
Mathur R, Sharma S, Mathur S, Prakash AO (1987) Effect of beryllium nitrate on early and late pregnancy in rats. Bulletin of environmental contamination and toxicology, 38(1):73–77.
Matsumoto A, Hisada Y, Yoshimura Y (1991) Calcium and phosphate concentrations, and alkaline and acid phosphatase activities in serum of the rat fed with low calcium and beryllium diets. Oral therapeutics and pharmacology, 10:253–259.
McCord CP (1951) Beryllium as a sensitizing agent. Industrial medicine and surgery, 20:336.
McGavran PD, Rood AS, Till JE (1999) Chronic beryllium disease and cancer risk estimates with uncertainty for beryllium released to the air from the Rocky Flats plant. Environmental health perspectives, 107(9):73–144.
Measures CI, Edmond JM (1982) Beryllium in the water column of the central North Pacific. Nature, 297:51–53.
Meehan WR, Smythe LE (1967) Occurrence of beryllium as a trace element in environmental materials. Environmental science and technology, 1:839–844.
Merril JR, Lyden EFX, Honda M, Arnold JR (1960) Sedimentary geochemistry of the beryllium isotopes. Geochimica Cosmochimica Acta, 18:108–129.
Minkwitz R, Fohlich N, Lehmann E (1983) [Examination of charges of harmful substances at work places during the production and processing of metals: beryllium, cobalt and their alloys.] Dortmund, Bundesanstalt für Arbeitsschutz, 107 pp. (Forschungsbericht No. 367) (in German) [cited by IPCS, 1990].
Miyaki M, Akamatsu N, Ono T, Koyama H (1979) Mutagenicity of metal cations in cultured cells from Chinese hamster. Mutation research, 68:259–263.
Morgareidge K, Cox GE, Bailey DE (1975) Chronic feeding studies with beryllium sulfate in rats: evaluation of carcinogenic potential. Submitted to Alcan Research and Development, Ltd. by Food and Drug Research Laboratories, Inc.
Morgareidge K, Cox GE, Gallo MA (1976) Chronic feeding studies with beryllium in dogs. Submitted to the Aluminum Company of America, Alcan Research & Development, Ltd., Kawecki-Berylco Industries, Inc., and Brush-Wellman, Inc. by Food and Drug Research Laboratories, Inc.
Morgareidge K, Cox GE, Bailey DE, Gallo M (1977) Chronic oral toxicity of beryllium in the rat. Toxicology and applied pharmacology, 41(1):204–205.
Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS (1991) Re-examination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. Journal of allergy and clinical immunology, 88:54–60.
Mueller J (1979) Beryllium, cobalt, chromium and nickel in particulate matter of ambient air. In: Proceedings of the international conference on heavy metals in the environment, London, September 1979. Edinburgh, CEP Consultants Ltd., pp. 300–303.
Mullen AL, Stanley RE, Lloyd SR, Moghissi AA (1972) Radioberyllium metabolism by the dairy cow. Health physics, 22:17–22.
Newman LS (1996) Immunology, genetics, and epidemiology of beryllium disease. Chest, 109(Suppl. 3):40S–43S.
Newman LS, Kreiss K, King TE Jr, Seay S, Campbell PA (1989) Pathologic and immunologic alterations in early stages of beryllium disease. American review of respiratory disease, 139:1479–1486.
Nickell-Brady C, Hahn FF, Finch GL, Belinsky SA (1994) Analysis of K-ras, p53 and c-raf-1 mutations in beryllium-induced rat lung tumors. Carcinogenesis, 15:257–262.
Nikonova NN (1967) [On the accumulation of beryllium, molybdenum, zirconium, yttrium, and other rare elements in plants of South Ural.] Izvestiya Sibirskogo Akademii Nauk SSSR, Seriya Biologicheskikh Nauk, 3:25–29 (in Russian).
Nikula KJ, Swafford DS, Hoover MD, Tohulka MD, Finch GL (1997) Chronic granulomatous pneumonia and lymphocytic responses induced by inhaled beryllium metal in A/J and C3H/HeJ mice. Toxicologic pathology, 25(1):2–12.
NIOSH (1972) Criteria document: Recommendations for an occupational exposure standard for beryllium. Rockville, MD, National Institute for Occupational Safety and Health, 128 pp. (NIOSH Report No. TR00372: HSM 7210268).
NIOSH (1984) Manual of analytical methods, Vol. 1, 3rd ed. Cincinnati, OH, National Institute for Occupational Safety and Health, pp. 71021–71023.
NIOSH (1989) National Occupational Exposure Survey (NOES). Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, 29 March 1989.
Nishimura M (1966) Clinical and experimental studies on acute beryllium disease. Nagoya journal of medical sciences, 28:17–44.
Nishioka H (1975) Mutagenic activities of metal compounds in bacteria. Mutation research, 31:185–189.
Ogawa HI, Tsuruta S, Niyitani Y, Mino H, Sakata K, Kato Y (1987) Mutagenicity of metal salts in combination with 9-aminoacridine in Salmonella typhimurium. Japanese journal of genetics, 62:159–162.
Pappas GP, Newman LS (1993) Early pulmonary physiologic abnormalities in beryllium disease. American review of respiratory disease, 148:661–666.
Petzow G, Aldinger F (1974) [Beryllium and beryllium compounds.] In: Bartholomé E, Bickert E, Hellmann H, Ley H, eds. [Ullmann’s encyclopedia of technical chemistry.] Weinheim, Verlag Chemie, pp. 442–458 (in German).
Petzow G, Zorn H (1974) [Toxicology of beryllium containing materials.] Chemiker-Zeitung, 98:236–241 (in German).
Reeves AL (1965) The absorption of beryllium from the gastrointestinal tract. Archives of environmental health, 11:209–214.
Reeves AL, Deitch D (1969) Influence of age on the carcinogenic response to beryllium inhalation. In: Harishima S, ed. Proceedings of the 16th international congress on occupational health. Tokyo, Japan Industrial Safety Association, pp. 651–652 [cited by US EPA, 1987].
Reeves AL, Vorwald AJ (1967) Beryllium carcinogenesis. II. Pulmonary deposition and clearance of inhaled beryllium sulfate in the rat. Cancer research, 27:446–451.
Reeves AL, Deitch D, Vorwald AJ (1967) Beryllium carcinogenesis. I. Inhalation exposure of rats to beryllium sulfate aerosol. Cancer research, 27:439–445.
Reichert JK (1974) [Beryllium: A toxic element in the human environment with special regard to its occurrence in water.] Vom Wasser, 41:209–216 (in German).
Rhoads K, Sanders CL (1985) Lung clearance, translocation, and acute toxicity of arsenic, beryllium, cadmium, cobalt, lead, selenium, vanadium, and ytterbium oxides following deposition in rat lung. Environmental research, 36(2):359–378.
Richeldi L, Sorrentino R, Saltini C (1993) HLA-DPB1 glutamate 69: a genetic marker of beryllium disease. Science, 262:242–244.
Romney EM, Childress JD (1965) Effects of beryllium in plants and soils. Soil science, 100:210–217.
Romney EM, Childress JD, Alexander GV (1962) Beryllium and the growth of bush beans. Science, 135:786–787.
Rosenkranz HS, Poirer LA (1979) Evaluation of the mutagenicity and DNA-modifying activity of carcinogens and noncarcinogens in microbial systems. Journal of the National Cancer Institute, 62:873–892.
Ross WD, Sievers RE (1972) Environmental air analysis for ultratrace concentrations of beryllium by gas chromatography. Environmental science and technology, 6:155–160.
Ross WD, Pyle JL, Sievers RE (1977) Analysis for beryllium in ambient air particulates by gas chromatography. Environmental science and technology, 11:467–471.
Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, Markham TN, Daniele RP (1988) Proliferative response of bronchoalveolar lymphocytes to beryllium: a test for chronic beryllium disease. Annals of internal medicine, 108(5):687–693.
Rossman MD, Preuss OP, Powers MB (1991) Beryllium: Biomedical and environmental aspects. Baltimore, MD, Williams and Wilkins.
Rossman R, Barres J (1988) Trace element concentration in near-surface waters of the Great Lakes and methods of collection storage and analysis. Journal of Great Lakes research, 14:188–204.
Rossman TG, Molina M (1986) The genetic toxicology of metal compounds: II. Enhancement by ultraviolet light-induced mutagenesis in Escherichia coli WP2. Environmental mutagenesis, 8:263–271.
Rossman TG, Molina M, Meyer LW (1984) The genetic toxicology of metal compounds: I. Induction of lambda prophage in Escherichia coli WP2s(lambda). Environmental mutagenesis, 6:59–69.
Sanders CL, Cannon WC, Powers GJ, Adu RR, Meier DM (1975) Toxicology of high-fired beryllium oxide inhaled by rodents. I. Metabolism and early effects. Archives of environmental health, 30:546–551.
Sanders CL, Cannon WC, Powers GJ (1978) Lung carcinogenesis induced by inhaled high-fired oxides of beryllium and plutonium. Health physics, 35:193–199.
Sanderson WT (1997) Lung cancer case–control study of beryllium workers. Unpublished Ph.D. dissertation submitted to the faculty of the University of North Carolina, Chapel Hill, NC.
Saracci R (1985) Beryllium: epidemiological evidence. In: Wald NJ, Doll R, eds. Interpretation of negative epidemiological evidence for carcinogenicity. Lyon, International Agency for Research on Cancer, pp. 203–219 (IARC Science Publication No. 65).
Savitz DA, Whelan EA, Kleckner RC (1989) Effects of parents’ occupational exposures on risk of stillbirth, preterm delivery, and small-for-gestational age infants. Archives of industrial health, 129:1201–1218.
Schepers GWH (1962) The mineral content of the lung in chronic berylliosis. Diseases of the chest, 42:600–607.
Schepers GWH (1964) Biological action of beryllium: reaction of the monkey to inhaled aerosols. Industrial medicine and surgery, 33:1–16.
Schepers GWH, Durkan TM, Delahunt AB, Creedon FT (1959) The biological action of inhaled beryllium sulfate: a preliminary chronic toxicity study on rats. Archives of industrial health, 15:32–58.
Schramel P, Li-Qiang X (1982) Determination of beryllium in the parts-per-billion range in three standard reference materials by inductively coupled plasma atomic emission spectrometry. Analytical chemistry, 54:1333–1336.
Schroeder HA, Mitchener M (1975a) Life-term studies in rats: effects of aluminum, barium, beryllium, and tungsten. Journal of nutrition, 105:421–427.
Schroeder HA, Mitchener M (1975b) Life-term effects of mercury, methyl mercury, and nine other trace metals on mice. Journal of nutrition, 105:452–458.
Schulert AR, Glasser SR, Stant EG Jr, Brill AB, Koshakji RP (1969) Development of placental discrimination among homologous elements. Washington, DC, US Atomic Energy Commission, pp. 145–152 (Symposium Series 17; US Government Research and Development Report 70(7), 1970 52 JPRS-49653, 1970).
Scott JK, Neumann WF, Allen R (1950) The effect of added carrier on the distribution and excretion of soluble beryllium. Journal of biological chemistry, 182:291–298.
Selivanova LN, Savinova TB (1986) [Effects of beryllium chloride and oxide on the sexual function of female rats and development of their progeny.] Gigiena i sanitariya, 8:44–46 (in Russian) [cited by ATSDR, 1993].
Sendelbach LE, Witschi HP (1987) Bronchoalveolar lavage in rats and mice following beryllium sulfate inhalation. Toxicology and applied pharmacology, 90:322–332.
Sendelbach LE, Tyrka AF, Witschi H (1989) Progressive lung injury over a one-year period after a single inhalation exposure to beryllium sulfate. American review of respiratory disease, 139:1003–1009.
Shacklette HT, Hamilton JG, Boerngen JG, Bowles JM (1971) Elemental composition of surficial materials in the conterminous United States. Washington, DC, US Geological Survey, US Government Printing Office, 71 pp. (Professional Paper 574-D).
Siemiatycki J, Wacholder S, Dewar R, Cardis E, Greenwood C, Richardson L (1988) Degree of confounding bias related to smoking, ethnic group, and socioeconomic status in estimates of the associations between occupation and cancer. Journal of occupational medicine, 30:617–625.
Simmon VF (1979) In vitro mutagenicity assays of chemical carcinogens and related compounds with Salmonella typhimurium. Journal of the National Cancer Institute, 63:893–899.
Slonim AR (1973) Acute toxicity of beryllium sulfate to the common guppy. Journal of the Water Pollution Control Federation, 45(10):2110–2122.
Slonim AR, Ray EE (1975) Acute toxicity of beryllium sulfate to salamander larvae (Ambystoma spp.). Bulletin of environmental contamination and toxicology, 13:307–312.
Slonim CB, Slonim AR (1973) Effect of water hardness on the tolerance of the guppy to beryllium sulfate. Bulletin of environmental contamination and toxicology, 10:295–301.
Sprince NL, Kazemi H (1980) U.S. beryllium case registry through 1977. Environmental research, 21:44–47.
Stange AW, Hilmas DE, Furman FJ (1996) Possible health risks from low level exposure to beryllium. Toxicology, 111:213–224.
Steenland K, Ward E (1991) Lung cancer incidence among patients with beryllium disease: a cohort mortality study. Journal of the National Cancer Institute, 83:1380–1385.
Sterner JH, Eisenbud M (1951) Epidemiology of beryllium intoxication. Archives of industrial hygiene and occupational medicine, 4:123–151.
Stiefel T, Schulze K, Zorn H, Tolg G (1980) Toxicokinetics and toxicodynamic studies of beryllium. Archives of toxicology, 45:81–92.
Stokinger HE, Sprague GF, Hall RH, Ashenburg NJ, Scott JK, Steadman LT (1950) Acute inhalation toxicity of beryllium. I. Four definitive studies of beryllium sulfate at exposure concentrations of 100, 50, 10, 1 mg. per cubic meter. Archives of industrial hygiene and occupational medicine, 1:379–397.
Stokinger HE, Spiegl CJ, Root RE, Hall RH, Steadman LT, Stroud CA, Scott JK, Smith EA, Gardner DE (1953) Acute inhalation toxicity of beryllium. IV. Beryllium fluoride at exposure concentrations of one and ten milligrams per cubic meter. Archives of industrial hygiene and occupational medicine, 8:493–506.
Stubbs J, Argyris E, Lee CW, Monos D, Rossman MD (1996) Genetic markers in beryllium hypersensitivity. Chest, 109(Suppl. 3):45S.
Sussman VH, Lieben J, Cleland JG (1959) An air pollution study of a community surrounding a beryllium plant. American Industrial Hygiene Association journal, 20:504–508.
Tarzwell CM, Henderson C (1960) Toxicity of less common metals to fishes. Industrial wastes, 5:12.
Taylor ML, Arnold EL (1971) Ultratrace determination of metals in biological specimens: quantitative determination of beryllium by gas chromatography. Analytical chemistry, 43(10):1328–1331.
Tso WW, Fung WP (1981) Mutagenicity of metallic cations. Toxicology letters, 8:195–200.
US EPA (1978) In-depth studies on health and environmental impacts of selected water pollutants. Washington, DC, US Environmental Protection Agency (Contract No. 68-01-4646).
US EPA (1980) Ambient water quality criteria for beryllium. Washington, DC, US Environmental Protection Agency, Division of Water Planning and Standards (EPA Report 440/580024).
US EPA (Environmental Protection Agency) (1986) Guidelines for carcinogen risk assessment. Federal register, 51:33992–34003.
US EPA (1987) Health assessment document for beryllium. Research Triangle Park, NC, US Environmental Protection Agency, Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office (EPA/600/8-84/026F).
US EPA (1988) Recommendations for and documentation of biological values for use in risk assessment. Prepared by the US Environmental Protection Agency, Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Cincinnati, OH, for the Office of Solid Waste and Emergency Response, Washington, DC (EPA/600/6-87/008; NTIS PB88-79874/AS).
US EPA (1991) Drinking water criteria document for beryllium. Prepared by the Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Cincinnati, OH, for the Office of Drinking Water, Washington, DC (NTIS PB92-173301).
US EPA (1993) Wildlife exposure factors handbook. Washington, DC, US Environmental Protection Agency, Office of Research and Development (EPA/600/R-93/187a).
US EPA (1994) Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. Washington, DC, US Environmental Protection Agency, Office of Research and Development (EPA/600/8-90/066F).
US EPA (1998) Toxicological review of beryllium and compounds (CAS No.
Vacher J (1972) Immunological responses of guinea pigs to beryllium salts. Journal of medical microbiology, 5(1):91–108.
Van Cleave CD, Kaylor CT (1955) Distribution, retention, and elimination of Be in the rat after intratracheal injection. Archives of industrial health, 11:375–392.
Van Orstrand HS, Hughes R, De Nardi JM, Carmody MG (1945) Beryllium poisoning. Journal of the American Medical Association, 129:1084–1090.
Vegni-Talluri M, Guiggiani V (1967) Action of beryllium ions on primary cultures of swine cells. Caryologia, 20:355–367.
Venugopal B, Luckey TD (1977) Metal toxicity in mammals. 2. Chemical toxicity of metals and metalloids. New York, NY, Plenum Press.
Vorwald AJ (1968) Biologic manifestations of toxic inhalants in monkeys. In: Vagtborg H, ed. Use of nonhuman primates in drug evaluation. Austin, TX, University of Texas Press, pp. 222–228.
Vorwald AJ, Reeves AL (1959) Pathologic changes induced by beryllium compounds: experimental studies. Archives of industrial health, 19:190–199.
Wagner WD, Groth DH, Holtz JL, Madden GE, Stokinger HE (1969) Comparative chronic inhalation toxicity of beryllium ores, bertrandite and beryl, with production of pulmonary tumors by beryl. Toxicology and applied pharmacology, 15:10–129.
Wagoner JK, Infante PF, Bayliss DL (1980) Beryllium: an etiologic agent in the induction of lung cancer, nonneoplastic respiratory disease, and heart disease among industrially exposed workers. Environmental research, 21:15–34.
Ward E, Okun A, Ruder A, Fingerhut M, Steenland K (1992) A mortality study of workers at seven beryllium processing plants. American journal of industrial medicine, 22:885–904.
Watanabe K, Shima S, Tachikawa S, Kato Y, Hidaka K, Taniwaki H, Ito T (1985) [Biotoxicity and beryllium distribution in organs by oral administration of beryllium compounds for long periods. II. Experimental study on oral administration of beryllium compounds.] Rodo Kagaku, 61:235–246 (in Japanese).
WHO (1996) Guidelines for drinking-water quality, 2nd ed. Vol. 2. Health criteria and other supporting information. Geneva, World Health Organization, 973 pp.
Wilke B-M (1987) [Effects of inorganic pollutants on microbial processes.] Berlin (West), Umweltbundesmt (Research Report No. 10701006) (in German) [cited by IPCS, 1990].
Williams GM, Laspia MF, Dunkel VC (1982) Reliability of the hepatocyte primary culture/DNA repair test in testing of coded carcinogens and noncarcinogens. Mutagenesis, 97:359–370.
Williams PL, Dusenberry DB (1990) Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environmental toxicology and chemistry, 9:1285–1290.
Williams RJB, Le Riche HH (1968) The effect of traces of beryllium on the growth of kale, grass and mustard. Plant and soil, 29:317–326.
Williams WJ, Kelland D (1986) New aid for diagnosing chronic beryllium disease (CBD): laser ion mass analysis (LIMA). Journal of clinical pathology, 39:900–901.
Witkin EM (1976) Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriological reviews, 40:869–907.
Wolf WR, Taylor MI, Hughes BM, Tiernan TO, Sievers RE (1972) Determination of chromium and beryllium at the picogram level by gas chromatography/mass spectrometry. Analytical chemistry, 44:616–618.
Wolnik KA, Fricke FL, Gaston CM (1984) Quality assurance in the elemental analysis of foods by inductively coupled plasma spectroscopy. Spectrochimica Acta, Part B, 398:649–655.
Yoshida T, Shima S, Nagaoka K, Taniwaki H, Wada A, Kurita H, Morita K (1997) A study on the beryllium lymphocyte transformation test and the beryllium levels in the working environment. Industrial health, 35:374–379.
Zakour RA, Glickman BW (1984) Metal-induced mutagenesis in the lacI gene of Escherichia coli. Mutation research, 126:9–18.
Zissu D, Binet S, Cavelier C (1996) Patch testing with beryllium alloy samples in guinea pigs. Contact dermatitis, 34:196–200.
Zorn H, Diem H (1974) [Importance of beryllium and its compounds in occupational medicine.] Zentralblatt für Arbeitsmedizin und Arbeitsschutz, 24:38 (in German).
Zorn H, Stiefel T, Diem H (1977) [Importance of beryllium and its compounds in occupational medicine, 2nd report.] Zentralblatt für Arbeitsmedizin, 27:83–88 (in German).
US Environmental Protection Agency (US EPA, 1998)
Copies of the EPA Toxicological review of beryllium and compounds (US EPA, 1998) may be obtained from:
US Environmental Protection Agency
National Center for Environmental Assessment
26 West Martin Luther King Drive
Cincinnati, Ohio 45268
USA
The toxicological review document has received peer review both by EPA scientists and by independent scientists external to EPA. External reviewers included Dr M. Dourson (Toxicology Excellence for Risk Assessment, USA), Dr G. Finch (Lovelace Inhalation Toxicology Research Institute, USA), Dr V. Hasselblad (Duke University, USA), Ms M. Mroz (National Jewish Medical Research Center, USA), Dr P. Mushak (PB Associates, USA), Dr J. Pounds (Wayne State University, USA), Dr R. Ratney (Mabbett & Associates, USA), Ms F. Rice (National Institute for Occupational Safety and Health, USA), and Mr W. Sanderson (National Institute for Occupational Safety and Health, USA). Subsequent to external peer review and incorporation of comments, this assessment underwent an Agency-wide review process whereby the Integrated Risk Information System (IRIS) Program Manager achieved a consensus approval among the Office of Research and Development; Office of Air and Radiation; Office of Prevention, Pesticides, and Toxic Substances; Office of Solid Waste and Emergency Response; Office of Water; Office of Policy, Planning, and Evaluation; and the Regional Offices.
Agency for Toxic Substances and Disease Registry (ATSDR, 1993)
Copies of the updated ATSDR Toxicological profile for beryllium (ATSDR, 1993) may be obtained from:
Agency for Toxic Substances and Disease Registry
Division of Toxicology/Toxicology Information Branch
1600 Clifton Road, NE, E-29
Atlanta, Georgia 30333
USA
The profile has undergone the following ATSDR internal reviews: Green Border Review, Health Effects Review, Minimal Risk Level Review, and Quality Assurance Review. In addition, an external peer review panel was assembled to review the document, including Dr F. Cavender (Abilene Christian University, USA), Dr G. Finch (Lovelace Inhalation Toxicology Research Institute, USA), Dr J. Gallo (University of Georgia, USA), Dr A. Hall (private consultant, USA), Dr P. Lacouture (Purdue Frederick Co., USA), Dr A. Reeves (Wayne State University, USA), and Dr M. Rossman (University of Pennsylvania, USA).
International Programme on Chemical Safety (IPCS, 1990)
Copies of the Environmental Health Criteria document on beryllium (IPCS, 1990) can be obtained from:
International Programme on Chemical Safety
World Health Organization
Geneva, Switzerland
This document was reviewed by the WHO Task Group on Environmental Health Criteria for Beryllium, consisting of Dr V. Bencko (Institute of Tropical Health, Czechoslovakia), Dr A. Choudhry (Kenya Medical Research Centre, Kenya), Dr R. Hertel (Fraunhofer Institute for Toxicology and Aerosol Research, Germany), Dr P. Infante (Occupational Safety and Health Administration, USA), Prof. A. Massoud (Ain Shams University, Egypt), Dr L. Naumova (Institute of Industrial Hygiene and Occupational Diseases, Russia), Prof. A. Reeves (Wayne State University, USA), and Dr G. Rosner (Fraunhofer Institute for Toxicology and Aerosol Research, Germany).
Health and Safety Executive of the United Kingdom (Delic, 1992; HSE, 1994)
The Health and Safety Executive documentation on beryllium and beryllium compounds is composed of two parts. The first is a summary (HSE, 1994), which presents data on exposure and human health effects as well as provides the logic underpinning the occupational exposure limit that is established. The main document (Delic, 1992) is the review of the toxicological data on beryllium and beryllium compounds.
The authors’ draft version of the Health and Safety Executive summary report was initially reviewed internally by a group of approximately 10 Health and Safety Executive experts (mainly toxicologists, but also scientists from other relevant disciplines, such as epidemiology and occupational hygiene). The toxicology section of the amended draft was then reviewed by toxicologists from the United Kingdom Department of Health. Subsequently, the entire risk assessment document was reviewed by a tripartite advisory committee to the United Kingdom Health and Safety Commission, the Working Group for the Assessment of Toxic Chemicals (WATCH). This committee is composed of experts in toxicology and occupational health and hygiene from industry, trade unions, and academia.
The members of the WATCH committee at the time of the peer review were:
Mr S. Bailey, National Hygiene Services Ltd.
Mr R. Bibbings, Social Insurance and Industrial Welfare Department
Dr A. Hay, University of Leeds
Dr L. Levy, University of Birmingham
Dr M. Molyneux, Shell UK
Mr A. Moses, ICI plc
Dr R. Owen, Consultant to the Trades Union Congress
Mr J. Sanderson, Exxon Company International
Dr M. Sharratt, BP Group
Dr A. Smith, Ciba-Geigy plc
The draft CICAD on beryllium and beryllium compounds was sent for review to institutions and organizations identified by IPCS after contact with IPCS national contact points and Participating Institutions, as well as to identified experts. Comments were received from:
A. Aitio, International Programme on Chemical Safety, World Health Organization, Switzerland
M. Baril, Institut de Recherche en Santé et en Sécurité du Travail du Québec, Canada
D. Bayliss, National Center for Environmental Assessment, US Environmental Protection Agency, USA
R. Benson, Drinking Water Program, US Environmental Protection Agency, USA
T. Berzins, National Chemicals Inspectorate (KEMI), Sweden
R. Cary, Health and Safety Executive, United Kingdom
R.S. Chhabra, National Institute of Environmental Health, National Institutes of Health, USA
S. Dobson, Institute of Terrestrial Ecology, United Kingdom
P. Edwards, Department of Health, United Kingdom
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine (BgVV), Germany
J. Hurych, National Institute of Public Health, Czech Republic
J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol Research, Germany
H. Nagy, Centers for Disease Control and Prevention, USA
E. Ohanian, US Environmental Protection Agency, USA
W. Taylor, Brush Wellman Ltd., United Kingdom
P. Yao, Chinese Academy of Preventive Medicine, Institute of Occupational Medicine, People’s Republic of China
K. Ziegler-Skylakakis, Beratergremium für Umweltrelevante Altstoffe (BUA), Germany
Helsinki, Finland, 26–29 June 2000
Members
Mr H. Ahlers, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, Office of Research and Development, National Center for Environmental Assessment, US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom (Rapporteur)
Dr R.S. Chhabra, General Toxicology Group, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr H. Choudhury, National Center for Environmental Assessment, US Environmental Protection Agency, Cincinnati, OH, USA
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, United Kingdom (Chairman)
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Ms K. Hughes, Priority Substances Section, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr G. Koennecker, Chemical Risk Assessment, Fraunhofer Institute for Toxicology and Aerosol Research, Hannover, Germany
Ms M. Meek, Existing Substances Division, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr A. Nishikawa, Division of Pathology, Biological Safety Research Centre, National Institute of Health Sciences, Tokyo, Japan
Dr V. Riihimäki, Finnish Institute of Occupational Health, Helsinki, Finland
Dr J. Risher, Agency for Toxic Substances and Disease Registry, Division of Toxicology, US Department of Health and Human Services, Atlanta, GA, USA
Professor K. Savolainen, Finnish Institute of Occupational Health, Helsinki, Finland (Vice-Chairman)
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S. Soliman, Department of Pesticide Chemistry, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, NSW, Australia
Observers
Dr R.J. Lewis (representative of European Centre for Ecotoxicology and Toxicology of Chemicals), Epidemiology and Health Surveillance, ExxonMobil Biomedical Sciences, Inc., Annandale, NJ, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland (Secretary)
Dr P.G. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr M. Younes, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Computational models — quantal data for small-intestine lesions in male and female dogs (Morgareidge et al., 1976)
The polynomial mean response regression model (THRESH; Howe, 1995), the exponential polynomial model, and the Weibull model were used to fit data by the maximum likelihood method. The following are the forms of the three equations used:
THRESH
P(d) = 1- exp[q1(d – d0)1 – . . . – qk(d – d0)k]
Exponential polynomial
P(d) = 1 – exp[– q1d1 – . . . – qkdk]
Weibull
P(d) = 1 – exp[– á – âd j]
where:
d = dose
d0 = threshold
P(d) = probability of a response (health effect) at dose d
P(0) = probability of response in the absence of exposure (d = 0)
q1...qk, d0, á, â, k, j = estimated parameters.
For data input to THRESH and polynomial exponential models, the degree of the polynomial k = 2 gave the best representation of the data, and the response type was extra risk [P(d) – P(0)] / [1 – P(0)]. For the THRESH model, a threshold was estimated.
Data
|
Group |
Dose (mg/kg body weight per day) |
No. of responses/ |
|
1 |
0 |
36900 |
|
2 |
0.026 |
36900 |
|
3 |
0.135 |
36900 |
|
4 |
1.2 |
36900 |
|
5 |
14.8 |
37143 |
Doses are average of male and female doses. Incidence is combined for males and females.
Model fit
Model fit was judged by the P-values associated with the Chi2 goodness-of-fit generated by the models.
Results
|
Model |
BMD10 |
Maximum likelihood estimate |
Estimated parameters |
P -value |
Chi2 goodness-of-fit |
Degrees of freedom |
|
Exponential polynomial |
0.46 |
1.4 |
q 1 = 6.9E-2q2 = 5.9E-3 |
0.94 |
0.13 |
2 |
|
THRESH |
0.47 |
1.2 |
q 1 = 9.4E-2q2 = 4.3E-3 d0 = 1.4E-1 |
1 |
8.7000000e-18 |
1 |
|
Weibull |
0.46 |
1.3 |
á = 0â = 7.3E-2 |
0.96 |
0.08 |
2 |
Discussion
There was good correlation among the three models for the BMD10. The BMD10 of 0.46 mg/kg body weight per day is used for further quantitation of the inhalation tolerable concentration.
Ce CICAD relatif au béryllium et à ses composés a été préparé par l’Environmental Protection Agency des Etats-Unis (EPA), principalement sur la base d’une mise au point qui avait pour but l’estimation du risque de pathologies néoplasiques ou autres en cas d’exposition humaine à ces substances (US EPA, 1998). Autres sources documentaires utilisées : un avant-projet d’étude sur le béryllium préparé par l’Agency for Toxic Substances and Disease Registry (ATSDR, 1993) en vue de rencenser les informations concernant l’exposition de la population et les effets nocifs de ce métal; une mise au point relative à la toxicité du béryllium et de ses dérivés préparée par le Health and Safety Executive du Royaume-Uni (Delic, 1992; HSE, 1994) et enfin, une évaluation des effets du béryllium sur la santé humaine publiée par le Programme international sur la sécurité chimique (IPCS, 1990). La mise au point de l’EPA (US EPA, 1998) prend en compte les données publiées jusqu’en 1997. Les études de l’ATSDR (1993) et du Health and Safety Executive du Royaume-Uni (Delic, 1992; HSE, 1994) reposent sur des données antérieures à 1992, celle de l’IPCS (1990), sur des données antérieures à 1989. On a procédé à un dépouillement de la littérature afin de rechercher les données écotoxicologiques correspondant à la période 1989 – février 1999 car ni la mise au point de l’EPA (US EPA, 1998), ni celle de l’ATSDR (1993) ne fournissent de renseignements sur les effets environnementaux du béryllium et de ses dérivés. Des renseignements sur la nature de l’examen par des pairs et sur les sources documentaires existantes sont donnés à l’appendice 1. Les informations concernant l’examen par des pairs du présent CICAD figurent à l’appendice 2. Ce CICAD a été approuvé en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Helsinki (Finlande) du 26 au 29 juin 2000. La liste des participants à cette réunion est donnée à l’appendice 3. Les fiches internationales sur la sécurité chimique relatives au béryllium (ICSC 0226), à l’oxyde de béryllium (ICSC 1325), au sulfate de béryllium (ICSC 1351), au nitrate de béryllium (ICSC 1352), au carbonate de béryllium (ICSC 1353), au chlorure de béryllium (ICSC 1354) et au fluorure de béryllium (ICSC 1355) établies par le Programme international sur la sécurité chimique (IPCS, 1999 a-g) sont également reproduite dans le présent document.
Le béryllium (symbole Be; No CAS
Le béryllium présent dans l’eau ne subit pas une importante bioconcentration par les organismes aquatiques. Les mollusques qui se nourrissent de vase ne l’accumulent pas non plus à partir des sédiments. La plupart des végétaux ne fixent qu’un peu du béryllium présent dans le sol mais quelques espèces ont cependant tendance à l’accumuler. L’exposition de la population générale au béryllium s’effectue principalement par l’intermédiaire des aliments et de l’eau de boisson, et dans une moindre mesure par l’intermédiaire de l’air ou par ingestion accidentelle de poussière. Cependant, ces deux dernières voies d’exposition peuvent se révéler importantes au voisinage d’une source de béryllium ou même être prédominantes chez les travailleurs de certaines industries.
Il n’existe pas d’études consacrées à la toxicocinétique du béryllium ou de ses dérivés chez l’Homme; pourtant du béryllium a été retrouvé dans les poumons et les urines de sujets non exposés de par leur profession. Le béryllium et ses composés ne sont pas métabolisés. L’expérimentation animale montre qu’une fois inhalées, les particules de béryllium (insolubles) ne sont que lentement éliminées des poumons, de sorte que le métal peut y subsister de nombreuses années après l’exposition. L’élimination pulmonaire des composés solubles ou modérément solubles du béryllium après inhalation ou instillation intratrachéenne s’effectue en deux phases. Il y a tout d’abord une phase rapide de quelques jours ou semaines, puis une deuxième phase plus lente qui peut durer de quelques semaines ou quelques mois pour les composés solubles, à plusieurs mois voire plusieurs années dans le cas des composés modérément solubles. Les composés solubles sont davantage absorbés (~ 20 % de la charge pulmonaire initiale) que les composés modérément solubles (comme, par exemple, l’oxyde de béryllium) après inhalation ou instillation intratrachéenne. Le taux d’absorption varie également en fonction de la température de calcination de l’oxyde, qui conditionne la granulométrie des particules et leur solubilité. Une fois ingéré, le béryllium est difficilement résorbé (< 1 %) au niveau des voies digestives. La fraction absorbée se répartit principalement dans le squelette, où elle s’accumule. L’élimination, très lente, s’effectue essentiellement par la voie urinaire. Peu après l’inhalation ou l’instillation intratrachéenne, la fraction non résorbée s’élimine dans les matières fécales. Toutefois, à mesure que le temps passe, l’excrétion par la voie urinaire devient prédominante, notamment dans le cas des dérivés les plus solubles.
On ne possède pas de données sur la toxicité du béryllium chez l’Homme après exposition par voie orale. La valeur de la DL50 montre qu’une exposition unique par voie orale à des composés solubles est modérément toxique, mais pour ce qui est des composés peu solubles, on ne dispose d’aucune étude toxicologique concernant l’ingestion d’une seule dose. Des études à court, moyen et long terme sur l’animal ont montré qu’après exposition par la voie orale, ce sont les voies digestives et le squelette qui sont les organes cibles. Des chiens à qui l’on avait fait ingérer pendant une assez longue période du sulfate de béryllium soluble mêlé à leur nourriture, ont présenté des lésions gastrointestinales et une hypoplasie de la moelle osseuse. Chez des rats exposés par la voie alimentaire à du carbonate de béryllium soluble pendant 3 à 4 semaines, on a observé un rachitisme qui pourrait s’expliquer par une diminution de l’absorption gastrointestinale du phosphore consécutive à la formation, dans l’intestin, de phosphate de béryllium insoluble. Chez des chiens exposés pendant une période prolongée à du sulfate de béryllium à quatre molécules d’eau, on a calculé que la dose correspondant à la limite inférieure de l’intervalle de confiance à 95 % pour une incidence de 10 % des lésions de l’intestin grêle, était de 0,46 mg de béryllium par kg de poids corporel et par jour (dose de référence 10 %) A partir de la valeur de la dose de référence 10 % et avec un coefficient d’incertitude de 300, on obtient une dose journalière tolérable de 0,002 mg/kg.
En cas d’exposition par la voie respiratoire, c’est le poumon qui est le principal organe cible chez l’Homme et l’animal. On n’a pas pu trouver de valeurs de la CL50 chez l’animal pour les composés solubles ou modérément solubles. En cas d’exposition répétée ou continue, les effets les plus marqués (pneumopathie, fibrose, lésions prolifératives, métaplasie et hyperplasie) ont été observés au niveau du poumon chez diverses espèces animales exposées à des composés solubles ou modérément solubles. En ce qui concerne l’Homme, on connaît mal les effets toxiques du béryllium et de ses dérivés après une seule exposition par la voie respiratoire, mais on a observé des cas de pneumopathie chimique (bérylliose aiguë) consécutive à une inhalation massive. Une exposition de courte durée ou des expositions répétées au béryllium ou à ses dérivés peut entraîner chez l’Homme une pneumopathie aiguë ou chronique, selon la concentration. La bérylliose aiguë est généralement consécutive à une exposition à des concentrations supérieures à 100 µg de béryllium par m3; elle est mortelle dans 10 % des cas. A côté de cette forme aiguë, il existe une forme de bérylliose chronique qui s’observe lors d’exposition à des concentrations plus faibles, chez environ 1 à 5 % des individus. La bérylliose chronique se caractérise par la formation de granulomes qui résultent d’une réaction immunitaire aux particules de béryllium présentes dans le poumon. On possède toute une somme de données attestant que les effets sensibles d’une exposition au béryllium par la voie respiratoire peuvent aller d’une sensibilisation à une bérylliose chronique. La limite de tolérance pour les effets non néoplasiques se situe à 0,02 µg/m3. Pour obtenir cette estimation, on s’est basé sur la valeur de la LOAEL (dose la plus faible produisant un effet nocif observable corrigée du facteur temps) en prenant comme critère la bérylliose chronique chez des travailleurs exposés et en appliquant un coefficient d’incertitude de 10 (à savoir, 3 pour tenir compte du fait qu’on a utilisé la LOAEL plutôt que la NOAEL – dose sans effet nocif observable-basée sur le caractère sensible du point d’aboutissement infraclinique – la sensibilisation – et encore 3 pour tenir compte de la qualité médiocre du suivi de l’exposition dans les études coprincipales).
Des statistiques de mortalité effectuées sur une cohorte d’ouvriers travaillant dans l’industrie du béryllium et l’étude des cas enregistrés dans le Beryllium Case Registry (BCR) on permis de mettre en évidence un accroissement de la mortalité par cancer du poumon. On estime que ces travaux fournissent la preuve de la cancérogénicité du béryllium chez les sujets humains exposés par la voie respiratoire; ces éléments de preuve sont néanmoins limités du fait que : l’accroissement du risque de cancer pulmonaire est relativement faible, l’exposition au béryllium n’est pas très bien définie, les données relative au tabagisme des sujets sont incomplètes et enfin il n’est pas tenu compte d’une exposition éventuelle à d’autres substances cancérogènes. Quels que soient les insuffisances des études épidémiologiques, les résultats de toutes les études de mortalité longitudinales sur la même cohorte et celles qui portent sur les cohortes du BCR donnent à penser qu’il existe une relation de cause à effet entre l’exposition au béryllium et l’augmentation du risque de cancer du poumon. Un certain nombre de faits appuient cette conclusion : l’augmentation de l’incidence des cancers pulmonaires chez les ouvriers souffrant de bérylliose aiguë (il est vraisemblable que ces ouvriers ont été exposés à de très fortes concentration de béryllium), une incidence plus élevée de ces cancers chez des ouvriers qui avaient été embauchés lorsque les niveaux d’exposition étaient très élevés, la présence systématique d’un excès de cancers du poumon dans six ateliers de traitement du béryllium sur sept et enfin, la constatation que le risque de cancer du poumon est maximal dans les usines où le risque d’affections respiratoires non malignes est justement maximal. En se basant sur le risque de cancer du poumon chez les ouvriers exposés, on a calculé que le risque respiratoire unitaire pour le béryllium était de 2,4 ´ 10–3 par µg/m3.
L’expérimentation animale montre qu’une exposition au béryllium par la voie respiratoire détermine une augmentation sensible des cancers du poumon chez le rat et le singe. On a également montré que le béryllium produit des cancers du poumon lorsqu’il est instillé à des rats par voie intratrachéenne et des ostéosarcomes chez le lapin (et peut-être aussi chez la souris) après injection intraveineuse ou dans la cavité médullaire des os.
On ne possède aucune donnée tirée de l’expérimentation animale quant à l’irritation de la peau et des yeux résultant d’une exposition au béryllium et à ses dérivés. On a cependant montré que les dérivés solubles ou modérément solubles se révélaient avoir une action sensibilisatrice sur l’épiderme chez diverses espèces animales et par diverses voies d’exposition. En ce qui concerne l’Homme, on dispose en revanche de données concernant l’irritation de la peau et des yeux consécutive à une exposition à des composés solubles du béryllium. Chez les sujets humains, une exposition cutanée au béryllium ou à ses dérivés peut provoquer une réponse d’hypersensibilité cutanée retardée à médiation cellulaire.
Les données relatives à l’action toxique du béryllium sur la reproduction et le développement sont limitées chez l’animal; les quelques études dont on dispose reposent sur une exposition par la voie parentérale et sont donc de peu d’intérêt en ce qui concerne l’exposition humaine dans l’environnement ou sur le lieu de travail. Des études de longue durée ont été effectuées sur des chiens beagle exposés par la voie orale à du sulfate de béryllium à quatre molécules d’eau. Chez les chiots survivants de la première portée, on n’a observé ni anomalies macroscopiques ni anomalies au niveau du squelette en examinant des préparations colorées ou non et qui n’existent d’ailleurs plus. On n’a connaissance d’aucune expérimentation animale consacrée aux effets toxiques sur la reproduction et le développement d’une exposition au béryllium par la voie respiratoire.
Chez l’Homme, les effets immunologiques du béryllium consistent en une réaction immunitaire spécifique à médiation cellulaire qui se produit au niveau pulmonaire. Le test de transformation des leucocytes a permis d’observer une prolifération provoquée spécifiquement par le béryllium, ce qui est la marque d’une sensibilisation étroitement corrélée avec la bérylliose chronique. Toutefois, la sensibilisation n’est qu’un des critères utilisés pour le diagnostic de la bérylliose chronique.
On n’a pas pu trouver d’études consacrées aux effets neurologiques qui seraient consécutifs, chez l’Homme, à l’inhalation de béryllium ou de ses dérivés. Rien n’indique qu’une exposition sur le lieu de travail puisse avoir des effets de ce genre; si l’on considère le taux d’absorption minime (< 1 %) de ces substances au niveau des voies digestives et l’absence d’absorption percutanée, on ne peut guère s’attendre à des effets neurologiques consécutifs à l’inhalation d’air contenant du béryllium, même sur le lieu de travail. Par ailleurs, l’exposition de certaines espèces animales par la voie orale n’entraîne pas les lésions habituellement constatées en pareil cas.
Le béryllium est toxique pour la faune aquatique. On a obtenu, pour la CL50 à 96 h, des valeurs allant de 0,14 à 32,0 mg de béryllium par litre, selon les espèces étudiées et les conditions expérimentales, en particulier la dureté de l’eau utilisée pour les épreuves (la toxicité est plus forte dans une eau de faible dureté). En ce qui concerne la toxicité chronique, on a fait état de valeurs allant de 0,05 à 1,10 mg de béryllium par litre pour des eaux de dureté modérée (100 à 300 mg de carbonate de calcium par litre). Le béryllium est également toxique pour les végétaux terrestres, dont il bloque la croissance ou réduit le rendement lorsqu’il est présent à la concen tration de 0,5-5 mg/litre dans des solutions de nutriments de pH faible à neutre. Dans un sol sablonneux une concentration de béryllium de 10 mg par kg a réduit de 26 % le rendement de l’orge de printemps. A pH élevé, le béryllium est moins toxique, pour une part en raison de sa précipitation sous forme de phosphate, ce qui empêche les plantes de le fixer. La plupart des végétaux fixent de petites quantités de béryllium, mais la proportion transportée à l’intérieur de la plante est très faible. On ne possède aucune donnée au sujet des effets du béryllium sur les animaux terrestres. Rien n’indique en tout cas que le béryllium subisse une bioamplification par passage dans la chaîne alimentaire.
Este CICAD sobre el berilio y sus compuestos, preparado por la Agencia para la Protección del Medio Ambiente de los Estados Unidos (EPA), se basó principalmente en un examen realizado a fin de evaluar el riesgo de cáncer y otros tipos de riesgos para la salud humana del berilio y sus compuestos (US EPA, 1998). Otras fuentes fueron un proyecto de examen sobre el berilio preparado por la Agencia para el Registro de Sustancias Tóxicas y Enfermedades (ATSDR, 1993), que tenía por objeto caracterizar la información sobre los efectos adversos para la salud humana y la exposición del público, un examen sobre la toxicidad del berilio y sus compuestos preparado por la Dirección de Salud y Seguridad del Reino Unido (Delic, 1992; HSE, 1994) y un examen preparado por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 1990) para evaluar los efectos del berilio en la salud humana y en el medio ambiente. En el examen de la US EPA (1998) se analizaron los datos disponibles hasta 1997. Los exámenes de la toxicidad realizados por la ATSDR (1993) y la Dirección de Salud y Seguridad del Reino Unido (Delic, 1992; HSE, 1994) se basaron en los datos disponibles antes de 1992 y el examen del IPCS (1990) en los de antes de 1989. Se realizó una búsqueda bibliográfica de información relativa a la toxicidad ecológica para los años 1988-1999 (febrero), porque ni el examen de la US EPA (1998) ni el de la ATSDR (1993) incluía información sobre los efectos en el medio ambiente. La información relativa al carácter del examen colegiado y a la disponibilidad de los documentos originales figura en el apéndice 1. La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Helsinki (Finlandia) del 26 al 29 de junio de 2000. La lista de participantes en esta reunión figura en el apéndice 3. Las Fichas internacionales de seguridad química para el berilio (ICSC 0226), el óxido de berilio (ICSC 1325), el sulfato de berilio (ICSC 1351), el nitrato de berilio (ICSC 1352), el carbonato de berilio (ICSC 1353), el cloruro de berilio (ICSC 1354) y el fluoruro de berilio (ICSC 1355), preparadas por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 1999 a-g), también se reproducen en el presente documento.
El berilio (Be; CAS Nş
No se ha observado una bioconcentración importante del berilio del agua en las especies acuáticas. El berilio del sedimento tampoco parece bioacumularse en los moluscos que se alimentan en el lecho marino. La mayor parte de las plantas absorben berilio del suelo en pequeñas cantidades, aunque un reducido número de especies actúan como acumuladoras de berilio. La población general está expuesta al berilio fundamentalmente a través de los alimentos y el agua de bebida, con una contribución menor del aire y la ingestión accidental de polvo. Sin embargo, estos dos últimos tipos de ingesta pueden ser importantes en las cercanías de una fuente y pueden predominar en la exposición de los trabajadores en un entorno industrial.
No hay estudios en el ser humano en los que se aborde la toxicocinética del berilio o sus compuestos; sin embargo, se ha detectado berilio en los pulmones y en la orina de personas no expuestas en el trabajo. El berilio y sus compuestos no se metabolizan. Los estudios en animales han demostrado que las partículas de berilio inhaladas (insolubles) se eliminan de los pulmones lentamente, de manera que después de la exposición pueden permanecer en ellos durante muchos años. La eliminación de los pulmones de los compuestos de berilio solubles y poco solubles por inhalación o instilación intratraqueal parece ser bifásica, con una primera fase rápida de unos días/semanas y una segunda fase más lenta, que puede variar entre varias semanas/meses para los compuestos solubles y meses/años para los poco solubles. Los compuestos de berilio solubles se absorben en mayor grado (~20% de la concentración inicial en el pulmón) que los poco solubles (por ejemplo, el óxido de berilio) tras la inhalación o la instilación intratraqueal. El grado de absorción varía también con la temperatura de calcinación del óxido, que influye en el tamaño y la solubilidad de sus partículas. La absorción del berilio ingerido a partir del tracto gastrointestinal es escasa (<1%). El berilio absorbido se distribuye fundamentalmente en el esqueleto, donde se acumula. La eliminación es muy lenta y se produce sobre todo en la orina. El berilio no absorbido se elimina en las heces poco después de la exposición por inhalación o la instilación intratraqueal. Sin embargo, la excreción urinaria adquiere mayor importancia más adelante, especialmente para los compuestos de berilio más solubles, a medida que el berilio absorbido se elimina del organismo.
No hay datos fidedignos sobre la toxicidad oral del berilio en las personas. La exposición aguda por vía oral a dosis aisladas de compuestos de berilio solubles es moderadamente tóxica, teniendo cuenta los datos de la DL50; sin embargo, no se dispone de estudios de este tipo en el caso de los compuestos de berilio poco solubles. Los estudios de duración breve, media y prolongada en animales pusieron de manifiesto que, tras la exposición oral, los sistemas gastrointestinal y esquelético son los órganos destinatarios del berilio. En perros con exposición crónica a sulfato de berilio soluble en los alimentos se observaron lesiones gastrointestinales e hiperplasia de la médula ósea. En ratas expuestas al carbonato de berilio poco soluble en los alimentos durante 3-4 semanas se detectó raquitismo, posiblemente debido a la disminución de la absorción gastrointestinal de fósforo tras la formación de fosfato de berilio insoluble en el intestino. La dosis calculada con el límite de confianza más bajo del 95% para una incidencia de lesiones del 10% en el intestino delgado de perros con exposición crónica al sulfato de berilio tetrahidrato es de 0,46 mg/kg de peso corporal al día (BMD10). A partir de la BMD10 se estimó una ingestión tolerable por vía oral de 0,002 mg/kg de peso corporal al día, utilizando un factor de incertidumbre de 300.
El pulmón es el destino primordial de la exposición al berilio por inhalación en los animales y en las personas. En los animales no se pudieron determinar los valores de la CL50 para los compuestos de berilio solubles, y tampoco para los poco solubles. Con respecto a exposiciones repetidas o continuas, los efectos más acentuados (neumonitis, fibrosis, lesiones proliferativas, metaplasia e hiperplasia) se observaron en los pulmones de varias especies de animales expuestas a compuestos de berilio tanto solubles como poco solubles. En el ser humano, hay poca información sobre los efectos tóxicos del berilio o sus compuestos tras una exposición única por inhalación, aunque se ha observado neumonitis química (beriliosis aguda) tras exposiciones aisladas masivas. Las exposiciones breves o repetidas de las personas al berilio o sus compuestos pueden producir una forma aguda o crónica de enfermedad pulmonar, en función de la concentración de la exposición. La beriliosis aguda suele estar asociada con niveles de exposición superiores a 100 µg de berilio/m3, que puede ser mortal en el 10% de los casos. En contraposición a la neumonitis química aguda, la exposición a concentraciones más bajas puede producir, en alrededor del 1%-5% de las personas expuestas, una forma crónica de la enfer medad. La beriliosis crónica se caracteriza por la formación de granulomas, debido a la reacción inmunitaria frente a las partículas de berilio en el pulmón. Hay abundantes pruebas que documentan la sensibilización al berilio y la beriliosis crónica como los efectos sensibles de la exposición a él por inhalación. La concentración tolerable para los efectos en la salud distintos del cáncer es de 0,02 µg/m3, habiéndose estimado a partir de la concentración más baja con efectos adversos observados (LOAEL) ajustada a la duración para la beriliosis crónica en trabajadores expuestos utilizando un factor de incertidumbre total de 10 (3 por la utilización de una LOAEL en lugar de la concentración sin efectos adversos observados o NOAEL, basada en el carácter sensible del efecto subclínico final [sensibilización al berilio], y 3 por la escasa calidad de la vigilancia de la exposición de los estudios coprincipales).
Se observó un aumento de la mortalidad por cáncer de pulmón en estudios de mortalidad de cohortes de trabajadores de la elaboración del berilio y en estudios de las personas inscritas en el Registro de Casos de Berilio. Se considera que estos estudios proporcionan pruebas de la carcinogenicidad del berilio en las personas expuestas por inhalación; las pruebas son limitadas, debido al aumento relativamente pequeño del riesgo de cáncer de pulmón, las estimaciones escasamente definidas de la exposición al berilio, los datos incompletos sobre el hábito de fumar y la falta de control sobre la exposición potencial a otros carcinógenos. Con independencia de las deficiencias de los estudios epidemiológicos, los resultados de todos los estudios de mortalidad complementarios sobre la misma cohorte y de los estudios de cohortes del Registro parecen indicar una relación causal entre la exposición al berilio y un mayor riesgo de cáncer pulmón. Esta conclusión se ve reforzada por la mayor incidencia de casos de cáncer de pulmón entre los trabajadores con beriliosis aguda (posiblemente estos trabajadores estuvieron expuestos a concentraciones muy altas de berilio), la mayor incidencia de cáncer de pulmón entre los trabajadores empleados por primera vez cuando los niveles de exposición eran muy altos, el resultado sistemático de un exceso de cáncer de pulmón en seis de las siete instalaciones de elaboración de berilio y la aparición del mayor riesgo de cáncer de pulmón en las instalaciones donde el riesgo de enfermedades respiratorias no malignas es el más alto. Se obtuvo un riesgo por unidad de inhalación de 2,4 ´ 10–3 por µg/m3 para el berilio tomando como base el riesgo de cáncer de pulmón de los trabajadores expuestos.
En estudios con animales, la exposición al berilio por inhalación produjo un aumento significativo de casos de cáncer de pulmón en las ratas y los monos. También se ha puesto de manifiesto que el berilio produce cáncer de pulmón en las ratas por instilación intratraqueal y osteosarcoma en los conejos (y posiblemente en los ratones) por inyección intravenosa e inyección en la cavidad medular de los huesos.
No se dispone de datos sobre irritación cutánea y ocular en animales por exposición al berilio o a sus compuestos. Sin embargo, se ha comprobado que tanto los compuestos de berilio solubles como los poco solubles son sensibilizadores cutáneos mediante diversas vías de exposición y en distintas especies animales. Hay datos de irritación cutánea y ocular en personas tras su exposición a compuestos de berilio solubles. La exposición de la piel humana al berilio y sus compuestos puede producir una respuesta cutánea de hipersensibilidad de tipo retardado (mediada por células).
Los datos sobre la toxicidad reproductiva y del desarrollo son limitados en animales; en los pocos estudios disponibles se ha utilizado la vía parenteral de exposición, por lo que tienen un interés limitado para las personas expuestas en el medio ambiente o en el entorno del trabajo. Se realizaron estudios crónicos por vía oral en perros pachones expuestos al sulfato de berilio tetrahidrato. No se notificó ninguna anomalía grave o esquelética en las crías de la primera camada tras el examen de las preparaciones aclaradas y teñidas, que ya no están disponibles. No hay datos experimentales con animales sobre la toxicidad reproductiva o del desarrollo del berilio inhalado.
En los efectos inmunológicos del berilio en las personas interviene una respuesta inmunitaria mediada por células específica del berilio. La observación de proliferación específica del berilio, utilizando la prueba de transformación de los linfocitos con berilio, indica que hay una sensibilización estrechamente relacionada con la beriliosis aguda. Sin embargo, la sensibilización es solamente uno de los criterios utilizados en el diagnóstico de esta enfermedad.
No se localizaron estudios relativos a los efectos neurológicos en las personas debidos a la exposición al berilio o sus compuestos por inhalación o por vía oral o cutánea. No hay pruebas de efectos neurológicos en las personas a causa de la exposición ocupacional; sobre la base de la absorción mínima (<1%) a partir del tracto gastrointestinal y de la ausencia de absorción cutánea, no se prevén efectos neurológicos a causa del aire respirado, incluso en el lugar de trabajo. Además, en la exposición oral de algunas especies de animales no se han observado las lesiones normalmente asociadas con tales exposiciones.
El berilio es tóxico para los animales acuáticos. Los valores de la CL50 a las 96 horas oscilaron entre 0,14 y 32,0 mg de berilio/litro, en función de la especie estudiada y de las condiciones de la prueba, en particular de la dureza del agua utilizada (mayor toxicidad en aguas blandas). En Daphnia magna se notificaron valores de la toxicidad crónica de 0,05-1,10 mg de berilio/litro en aguas de dureza moderada (100-300 mg de carbonato de calcio/litro). El berilio es fitotóxico para las plantas terrestres, inhibiendo el crecimiento y reduciendo el rendimiento a concentraciones de 0,5-5 mg/litro en una solución de cultivo con nutrientes en condiciones de pH bajo y neutro. En suelos arenosos, una concentración de 10 mg de berilio/kg redujo en un 26% el rendimiento de la cebada de primavera. Con un pH alto, el berilio es menos fitotóxico debido en parte a que precipita como sal fosfato, por lo que la planta no puede absorberlo. La mayoría de las plantas absorben berilio en pequeñas cantidades, pero se desplaza muy poco dentro de ellas. No hay datos sobre los efectos del berilio en los animales terrestres. No hay pruebas de bioamplificación del berilio en las cadenas alimentarias.
ENDNOTES
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170). |
|
|
Letter to P.M. McGinnis, Syracuse Research Corporation, from D.G. Goodman, Integrated Risk Information System (IRIS) peer review of beryllium, September 25, 1997. |
|
|
The designation SOS (the international distress signal) implies that damage to DNA initiates a regulatory signal that causes the simultaneous depression of various functions, all of which presumably promote the survival of the cell or of its phages (Witkin, 1976). |
|
|
A case–control study within the Reading cohort, with a refined exposure assessment, has been finalized (Sanderson, 1997) and is expected to be published in the near future. |
|
|
The MATC is equal to the geometric mean of the highest test concentration that did not significantly affect growth or reproduction and the lowest test concentration that did significantly affect growth or reproduction. |
|
|
The exposure level that produces frankly apparent and unmistakable adverse effects, such as irreversible functional impairment or mortality, at a statistically and biologically significant increase in frequency or severity between an exposed population and its appropriate control (US EPA, 1994). |
See Also:
Toxicological Abbreviations
Beryllium and Beryllium Compounds (IARC Summary & Evaluation, Volume 58, 1993)