
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 59
First draft prepared by Ms Joann A. Wess, Dr Larry D. Olsen, and Dr Marie Haring Sweeney, National Institute for Occupational Safety and Health, Cincinnati, Ohio, USA
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2004
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Asphalt (bitumen).
(Concise international chemical assessment document ; 59)
1.Hydrocarbons - adverse effects 2.Risk assessment 3.Epidemiologic studies
4.Occupational exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153059 6 (LC/NLM Classification: QV 633)
ISSN 1020-6167
©World Health Organization 2004
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and the Swiss Agency for Environment, Forests and Landscape.
Technically and linguistically edited by Marla Sheffer, Ottawa, Canada, and printed by Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
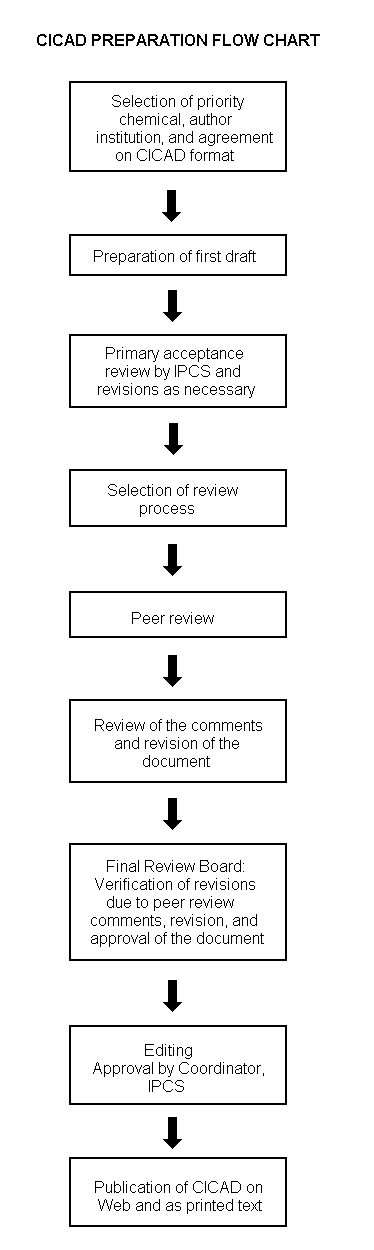
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on asphalt (bitumen) was based upon a review prepared by the US National Institute for Occupational Safety and Health (NIOSH, 2000). Additional data were identified through an updated literature search to February 2003. Information on the peer review of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Varna, Bulgaria, on 8–11 September 2003. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card on asphalt (ICSC 0162), produced by the International Programme on Chemical Safety (IPCS, 2002), has also been reproduced in this document.
Asphalt (CAS No.
When asphalts are heated, vapours are released; as these vapours cool, they condense. As such, these vapours are enriched in the more volatile components present in the asphalt and would be expected to be chemically and potentially toxicologically distinct from the parent material. Asphalt fumes are the cloud of small particles created by condensation from the gaseous state after volatilization of asphalt. However, because the components in the vapour do not condense all at once, workers are exposed not only to asphalt fumes but also to vapours. The physical nature of the fumes and vapours has not been well characterized. Nevertheless, a chemical analysis of oxidized roofing asphalt and non-oxidized paving asphalt fumes identified many of the same chemical classes. In addition, differences in the way in which asphalts are handled during paving and roofing operations probably influence the composition of asphalt fumes and vapours. Since the compositions of asphalts and asphalt fumes and vapours vary depending on temperature, manufacturing process, presence of additives and modifiers, and work practices, it should be no surprise to learn that laboratory-generated asphalt fumes that mimic asphalt fumes in the environment are difficult to produce. Researchers have concluded that temperature, rate of stirring, and pulling versus pushing the collection air all affect the chemical composition of the fumes.
The major types of asphalt products are paving asphalts and roofing asphalts. Asphalt is also used in asphalt-based paints as protective coatings to prevent corrosion of metals; in lining irrigation canals, water reservoirs, dams, and sea defence works; in adhesives in electrical laminates; and as a base for synthetic turf. In the USA, approximately 300 000 workers are employed at hot-mix asphalt facilities and paving sites; an estimated 50 000 workers are employed in asphalt roofing operations; and about 1500–2000 workers are employed in approximately 100 roofing manufacturing plants. In Western Europe, there are approximately 4000 asphalt mixing plants employing 5–10 individuals per plant. Approximately 100 000 members of paving crews apply these asphalt mixes to road surfaces across Western Europe.
Although a variety of sample collection and analytical methods are available for evaluating asphalt fume exposures, most of them are non-specific and cannot be used to characterize total asphalt fume exposure. Also, readily accessible body fluids and/or physiological functions have been sampled or monitored for biomarkers of exposure to asphalt fumes. Biomarkers specific to asphalt fume exposures have not yet been identified.
Limited data are available on the concentration of asphalt in environmental media. Characterization of concentrations of asphalt fractions in air samples and plant samples collected at various distances from a highway indicated that these concentrations were <4 × 10–3 mg/m3 and <4 mg/g dry plant material, respectively. An assessment of the effects of runoff from asphalt pavement on streams in California, USA, indicated that concentrations of all polycyclic aromatic hydrocarbon (PAH) analytes in all stream and road runoff samples were below the detection limit of 0.5 µg/litre. Although detectable levels of heavy metals were present in stream and runoff water, the authors concluded that no significant upstream versus downstream differences existed in the concentration of any heavy metal across all streams. Metal concentrations were elevated in runoff water from road surfaces relative to upstream samples. These elevated concentrations could be due to sources other than asphalt (e.g., vehicle emissions, crankcase oil drippings, etc.).
While asphalt fume concentrations associated with health effects have not been well characterized, symptoms of eye, nose, or throat irritation are reported by workers during open-air paving. In the occupational setting, results of recent studies indicate that, in general, most time-weighted average (TWA) air concentrations for total particulates (TP) and benzene-soluble particulates (BSP) ranged from 0.041 to 4.1 mg/m3 and from 0.05 to 1.26 mg/m3, respectively. Average personal exposures, calculated as full-shift TWAs, were generally below 1.0 mg/m3 for TP and 0.3 mg/m3 for BSP.
Asphalt fumes and vapours may be absorbed following inhalation and dermal exposure. Because asphalt is a complex mixture, its pharmacokinetic behaviour will vary depending upon the properties of the individual constituents. Therefore, it is inappropriate to make generalizations regarding the extent of absorption, distribution, and metabolism of asphalt.
Results of several in vitro studies indicate that while field-generated paving asphalt fume condensates were not mutagenic and did not induce DNA adduct formation, paving fume condensates generated in the laboratory were mutagenic and did induce DNA adduct formation. In contrast, one study reported that the particulate fractions of asphalt fumes collected in the personal breathing zone (PBZ) of workers during paving operations were mutagenic in the Ames Salmonella assay. Moreover, intratracheal exposure of rats to field-generated asphalt paving fumes caused a statistically significant increase in the level and activity of CYP1A1 (a major PAH-inducible isozyme of cytochrome P450) in the lung and increased micronuclei formation in bone marrow erythrocytes. Only laboratory-generated roofing asphalt fumes have been tested in genotoxicity studies. These fumes have been shown to be mutagenic, to cause increased micronuclei formation, and to inhibit intercellular communication in Chinese hamster lung fibroblasts (V79 cells) and in human epidermal keratinocytes. Equivocal results have been reported for asphalt-based paints. While in one study none of the asphalt-based paints examined demonstrated mutagenic activity, in another study other asphalt-based paints induced DNA adduct formation in adult and fetal human skin samples. Results of carcinogenicity studies indicated that laboratory-generated roofing asphalt fume condensates caused tumours when applied dermally to mice and that some asphalt-based paints contained chemicals capable of initiating tumours in mice. No animal studies have examined the carcinogenic potential of either field- or laboratory-generated paving asphalt fume condensates.
Acute effects of exposure to asphalt among workers in the various sectors of the asphalt industry (hot-mix plants, terminals, roofing application, paving, roofing manufacturing) include symptoms of irritation of the serous membranes of the conjunctivae (eye irritation) and the mucous membranes of the upper respiratory tract (nasal and throat irritation) and coughing. These health effects appear to be mild in severity and transient in nature. Additional symptoms include skin irritation, pruritus, rashes, nausea, stomach pain, decreased appetite, headaches, and fatigue, as reported by workers involved in paving operations, insulation of cables, and the manufacture of fluorescent light fixtures. Results from recent studies indicated that some workers involved in paving operations experienced lower respiratory tract symptoms (e.g., coughing, wheezing, and shortness of breath) and pulmonary function changes; bronchitis has also been reported. The lowest TP exposure that caused respiratory tract problems was 0.02 mg/m3. However, data from the available studies are insufficient to determine the relationship between asphalt fume exposures and the above reported health effects.
Burns may also occur when hot asphalt is handled. Burned areas usually include the head and neck, arms, hands, and legs.
The largest study to examine the health effects of occupational exposure to asphalt included a cohort of 29 820 workers from eight different countries engaged in road paving, asphalt mixing, roofing, waterproofing, or other specified jobs where exposure to asphalt fumes was possible. Overall mortality for the entire cohort (exposed and non-exposed workers) was below expected (standardized mortality ratio [SMR] = 0.92). For job classifications involving bitumen or asphalt exposure, overall mortality was not elevated (SMR = 0.96); mortality from lung cancer was increased among bitumen workers when compared with ground and building construction workers (SMR = 1.17, 95% confidence interval [CI] = 1.04–1.30). Overall mortality from head and neck cancer was elevated for bitumen workers only (SMR = 1.27, 95% CI = 1.02–1.56). Mortality from other malignant neoplasms was not increased. Further analysis suggested a slight increase in lung cancer mortality among road pavers after adjusting for coal tar pitch and allowing for a 15-year lag (SMR = 1.23, 95% CI = 1.02–1.48).
The investigators (Boffetta et al., 2003b) assessed two different metrics for exposure: average and cumulative exposure. For lung cancer, a positive association was observed for lagged average level of exposure, but not for lagged cumulative exposure. Corresponding indices of unlagged average and cumulative exposure showed a positive dose–response with lung cancer risk based on 63 deaths; relative risks [RRs] were 1.43 (95% CI = 0.87–2.33), 1.77 (0.99–3.19), and 3.53 (1.58–7.89) for 2.2–4.6, 4.7–9.6, and 9.7+ mg/m3 years of cumulative exposure and 2.77 (95% CI = 1.69–4.53), 2.43 (1.38–4.29), and 3.16 (1.83–5.47) for 1.03–1.23, 1.24–1.36, and 1.37+ mg/m3 average exposure (P-value of test for trend, 0.01 for both variables). The investigators concluded that the exposure–response analyses suggest an association between lung cancer mortality and indices of average level of exposure to bitumen fumes; however, they could not rule out that confounding played some role in this association.
A meta-analysis of 20 epidemiological studies failed to find overall evidence for a lung cancer risk among pavers and highway maintenance workers exposed to asphalt (RR = 0.87, 95% CI = 0.76–1.08). However, the analysis demonstrated an overall statistically significant excess of lung cancer among roofers (RR = 1.78, 95% CI = 1.5–2.1). Because, in the past, roofers have been exposed to coal tar and asbestos, which are known human carcinogens, it is uncertain to what extent these findings may be attributable to asphalt exposures.
The same meta-analysis reported increases in risk of bladder cancer (RR = 1.22, 95% CI = 0.95–1.53), stomach cancer (RR = 1.28, 95% CI = 1.03–1.59), and leukaemia (RR = 1.41, 95% CI = 1.05–1.85) in workers generally classified as asphalt workers, but not roofers. Interpretation of the findings of these 20 studies is limited by a lack of consistency among studies and the potential for confounding by other substances. Furthermore, many of these findings are from studies organized by broad job classifications that are prone to errors in defining asphalt exposures.
The extremely limited nature of the available data to serve as a basis for estimation of exposure of the general population should be borne in mind when attempting to determine exposure of the general population to asphalt, asphalt fumes and vapours, and asphalt-based paints. The concentrations of asphalt fractions — polar aromatics (polars), naphthene aromatics (aromatics), and saturates — measured in air samples collected 2.0–83.6 m from the highway were 0.54–3.96 × 10–3 mg/m3 air, 1.77–9.50 × 10–4 mg/m3 air, and 0.21–1.23 × 10–4 mg/m3 air, respectively. These values are extremely low in comparison with occupational exposures determined in the various sectors of the asphalt industry; personal exposures to TP and BSP ranged from 0.041 to 4.1 mg/m3 and from 0.05 to 1.26 mg/m3, respectively. However, the chemical composition of the air samples collected along the highway and at the worksites may differ. In addition to respiratory absorption, dermal absorption may also occur and play a pivotal role in asphalt exposure.
The frequency and concentration of potential asphalt exposures may be lower for the general population than for workers. However, in the general population, there are individuals who may be more sensitive to exposures and therefore exhibit more symptoms or other effects. The extent to which these symptoms occur in the general population has not been studied.
In weighing the available data that explore the relationship between exposure to asphalt and asphalt fumes and vapours and adverse health effects, it is important to consider them in the context of the overall limitations of the information. These uncertainties may be caused by the basic chemistry of asphalt, which is a mixture, the small number of in vivo studies, the inclusion of coal tar in roofing and paving asphalts in past decades (and the inclusion in some current formulations), and the mixed results of human studies. However, these limitations or uncertainties should not preclude a judgement regarding human and environmental health. Under various performance specifications, it is likely that asphalt fumes and paints contain carcinogenic substances.
Asphalt and some asphalt products are described below:
The European Committee for Standardization (CEN, 2000) has published a recommended terminology for bitumen and bituminous binders. The main classes of bitumens include paving bitumen, modified bitumen, special bitumen, industrial bitumen, petroleum cut-back bitumen, petroleum fluxed bitumen, and bitumen emulsion.
Performance specifications, not chemical composition, direct asphalt production. To meet these performance specifications, the asphalt may be air-blown or further processed by solvent precipitation or propane deasphalting. Additionally, the products of other refining processes may be blended with the asphalt to achieve the desired performance specifications. Therefore, the exact chemical composition of asphalt is dependent on the chemical complexity of the original crude petroleum and the manufacturing process. Crude petroleum consists mainly of aliphatic compounds, cyclic alkanes, aromatic hydrocarbons, PACs (a class of chemicals that includes PAHs and heterocyclic derivatives in which one or more of the carbon atoms in the PAH ring system have been replaced by a heteroatom of nitrogen [N-PAC], oxygen [O-PAC], or sulfur [S-PAC]; Vo-Dinh, 1989), and metals (e.g., iron, nickel, and vanadium). The proportions of these chemicals can vary greatly because of significant differences in crude petroleum from oil field to oil field or even at different locations in the same oil field (AI, 1990a).
While the manufacturing process may change the physical properties of asphalt dramatically, the chemical nature of the asphalt does not change unless thermal cracking occurs. Raising the temperature will increase the likelihood of cracking and cause more volatiles and even higher-boiling components to be released from the residuum. Solvent precipitation (usually using propane or butane) removes high-boiling components from a vacuum-processed asphalt, which are then used to make other products. Solvent precipitation results in a harder asphalt that is less resistant to temperature changes and often blended with straight-reduced or vacuum-processed asphalts. The air-blowing process can be a continuous or batch operation. Since the continuous operation is faster and results in a softer asphalt, a continuous operation is preferred for processing paving asphalts (Speight, 1992; Roberts et al., 1996). Air blowing combines oxygen with hydrogen in the asphalt, producing water vapour. This decreases saturation and increases cross-linking within and between different asphalt molecules. The process is exothermic (heat producing) and may cause a series of chemical reactions, such as oxidation, condensation, dehydration, dehydrogenation, and polymerization. These reactions cause the amount of asphaltenes (hexane-insoluble materials) to increase and the amounts of polar aromatics (hard resins), cycloalkanes, and non-polar aromatics (soft resins) to decrease, while the amounts of aliphatic compounds (oils and waxes) remain about the same (Table 1); at the same time, the oxygen content of the asphalt increases (Moschopedis & Speight, 1973; Corbett, 1975; Puzinauskas & Corbett, 1978; Boduszynski, 1981; Speight, 1992; Roberts et al., 1996).
Table 1: Changes in physical properties and chemical classes in an asphalta with increasingly longer air-blowing times.b
|
Air-blowing timesc |
||||
|
T0 |
T1 |
T2 |
T3 |
|
|
Physical properties |
||||
|
Softening point (°C) |
54.4 |
85 |
96.1 |
173.3 |
|
Penetration (mm/10) |
36 |
13 |
9 |
1 |
|
Chemical class, wt% |
||||
|
Asphaltenes |
14.8 |
26.9 |
31.4 |
51.3 |
|
Hard resins |
45.5 |
36.6 |
36.1 |
19.6 |
|
Soft resins |
25.0 |
22.3 |
20.9 |
16.9 |
|
Oils |
12.3 |
11.9 |
10.0 |
11.1 |
|
Waxes |
2.5 |
2.0 |
1.8 |
1.6 |
|
Total |
100.0 |
99.7 |
100.2 |
100.5 |
a Straight-reduced Arkansas asphalt.
b Adapted from Speight (1992).
c T0 = no air-blowing time; T0 < T1 < T2 < T3.
Although no two asphalts are chemically identical and chemical analysis cannot be used to define the exact chemical structure or chemical composition of asphalt, elemental analyses indicate that most asphalts contain 79–88 wt% carbon, 7–13 wt% hydrogen, traces to 3 wt% nitrogen, traces to 8 wt% sulfur, and traces to 8 wt% oxygen (examples shown in Table 2) (Speight, 1992). While heteroatoms (i.e., nitrogen, oxygen, and sulfur) make up only a minor component of most asphalts, the heteroatoms profoundly influence the differences in physical properties of asphalts from different crude sources (Speight, 1992; Roberts et al., 1996).
Table 2: Elemental analysis of asphalts from different crude sources.a
|
Crude sources |
Carbon (wt%) |
Hydrogen (wt%) |
Nitrogen (wt%) |
Sulfur (wt%) |
Oxygen (wt%) |
Vanadium (mg/kg) |
Nickel (mg/kg) |
|
Mexican blend |
83.77 |
9.91 |
0.28 |
5.25 |
0.77 |
180 |
22 |
|
Arkansas-Louisiana |
85.78 |
10.19 |
0.26 |
3.41 |
0.36 |
7 |
0.4 |
|
Boscan |
82.90 |
10.45 |
0.78 |
5.43 |
0.29 |
1380 |
109 |
|
California |
86.77 |
10.94 |
1.10 |
0.99 |
0.20 |
4 |
6 |
a Adapted from Speight (1992).
Asphalt is used for paving, roofing, industrial, and special purposes. Oxidized asphalt is used in roofing operations, pipe coating, undersealing for concrete pavements, hydraulic applications, membrane envelopes, some paving-grade mixes (AI, 1990b), and the manufacture of paints (Speight, 1992).
From a scientific point of view, asphalts probably should be classified as to whether or not they have been oxidized. However, most publications have classified asphalts according to the performance specifications for which they were manufactured (e.g., paving asphalts and roofing asphalts). This greatly complicates our understanding of the chemistry of asphalts and the presentation of materials in this document, because most asphalts used in paving are not made from oxidized asphalts, but most asphalts used in roofing are made from oxidized asphalts (Speight, 1992; Roberts et al., 1996). The situation is further complicated by the addition of additives and modifiers, differences in application temperatures, and work practices.
Three asphalt products are used in paving processes: asphalt cements, cutback asphalts, and emulsified asphalts. Cutback and emulsified asphalts are also called liquid asphalts because they are liquid at ambient temperatures. As mentioned previously, most asphalts used in paving operations are not oxidized. The asphalts are heated to about 149–177 °C and mixed with heated (143–163 °C) mineral aggregate. Once transported to the worksite, the hot-mix asphalt is applied to the roadway. The temperature of application is generally between 112 and 162 °C (AI, 1990a; FAA, 1991; Speight, 1992; Roberts et al., 1996).
Oxidized asphalts may or may not be used in roofing manufacturing plants to produce shingles, roll goods, built-up roofing felts, and underlayment felts; these asphalts are shipped hot and kept hot until used in the manufacturing process (AREC, 1999). In addition, some cutback and emulsified asphalts are also used in roofing operations (Speight, 1992). However, most oxidized asphalts are used to produce "mopping-grade" roofing asphalts. These asphalts are generally shipped as a solid and heated in a kettle at the worksite until they become a liquid. Table 3 lists the recommended application temperatures (Appendix C in AI, 1990a) and the recommended maximum heating temperatures (AREC, 1999) for these asphalts.
Table 3: Recommended application temperatures and recommended maximum heating temperatures used with "mopping-grade" roofing asphalts.
|
Type |
Recommended application temperaturea |
Recommended maximum heating temperatureb |
|
I |
166–179 |
246 |
|
II |
185–199 |
260 |
|
III |
202–216 |
274 |
|
IV |
221–229 |
274 |
a Adapted from AI (1990a, Appendix C) and AREC (1999).
b Adapted from AREC (1999).
Differences in the way in which asphalts are handled during paving and roofing operations probably influence the composition of asphalt fumes and vapours. When a hot-mix paving asphalt arrives at the worksite, the asphalt has been cooling since leaving the plant and may not be used immediately when it arrives at the worksite. Conversely, roofing asphalts are heated continuously and stirred occasionally at the worksite until the asphalt is needed.
Since the compositions of asphalts and asphalt fumes and vapours vary depending on temperature, manufacturing process, presence of additives and modifiers, and work practices, it should be no surprise to learn that laboratory-generated asphalt fumes that mimic asphalt fumes in the environment are difficult to produce. Researchers (Kriech & Kurek, 1993; Kriech et al., 1999) have shown how generation conditions can affect the composition of fumes. Using a variety of analytical techniques — gas chromatography with flame ionization detection (GC/FID), GC with flame photometric detection, GC with atomic emission detection, and GC with mass spectrometry (GC/MS) — they compared laboratory-generated asphalt fumes with fumes collected from the headspace in a storage tank at a hot-mix plant (paving asphalt), from the headspace in roofing kettles, and from PBZ samples. They concluded that temperature, rate of stirring, and pulling versus pushing the collection air all affected the chemical composition of the fumes.
When asphalts are heated, vapours are released; as these vapours cool, they condense. As such, these vapours are enriched in the more volatile components present in the asphalt and would be expected to be chemically and potentially toxicologically distinct from the parent material. Asphalt fumes are the cloud of small particles created by condensation from the gaseous state after volatilization of asphalt (NIOSH, 1977). However, because the components in the vapour do not condense all at once, workers are exposed not only to asphalt fumes but also to vapours. The physical nature of the fumes and vapours has not been well characterized, but the fume should be fairly viscous. The asphalt fume particles may collide and stick together, making it difficult to characterize the fume particle size. Some of the vapours may condense only to the liquid phase, thus forming a viscous liquid with some solids. Table 4 shows the results of a chemical analysis of a laboratory-generated oxidized asphalt fume (Lunsford & Cooper, 1989). A chemical analysis of non-oxidized paving asphalt fumes, PBZ samples from two sites, identified many of the same chemical classes as shown for the oxidized asphalt fume in Table 4 (Kriech et al., 2002a).
Table 4: Analysis by GC/MS of chemical composition of asphalt fume fractions A–E from an oxidized "mopping-grade" (Type III) roofing asphalt collected during laboratory generation at 316 °C.a
|
Compound classb |
Fractionc,d |
||||
|
A |
B |
C |
D |
E |
|
|
Hydrocarbons |
|||||
|
Alkanes, C9–C27 |
++ |
+ |
+ |
– |
– |
|
Alkenes/cycloalkanes |
++ |
+ |
+ |
– |
– |
|
Benzenes, C2–C8 |
++ |
+ |
– |
– |
– |
|
Indanes, C0–C4 |
++ |
+ |
– |
– |
– |
|
Indenes, C0–C3 |
++ |
+ |
– |
– |
– |
|
Naphthalenes, C0–C5 |
++ |
+ |
– |
– |
– |
|
Biphenyls, C0–C2 |
++ |
+ |
– |
– |
– |
|
Fluorenes, C0–C3 |
++ |
+ |
– |
– |
– |
|
Anthracenes/phenanthrenes, C0–C4 |
++ |
+++ |
+ |
– |
– |
|
Pyrenes/fluoranthenes, C0–C2 |
– |
++ |
+ |
– |
– |
|
Chrysenes/benz[a]anthracenes, C0–C2 |
– |
– |
+ |
– |
– |
|
Sulfur-containing compounds |
|||||
|
Benzothiophenes, C0–C9 |
++ |
+ |
– |
– |
– |
|
Dibenzothiophenes/naphthothiophenes, C0–C4 |
++ |
+++ |
+ |
– |
– |
|
Tricarbocyclic fused-ring thiophenes, C0–C1 |
– |
– |
+ |
– |
– |
|
Oxygen-containing compounds |
|||||
|
Benzofurans, C0–C2 |
– |
+ |
– |
– |
– |
|
Dibenzofurans, C0–C2 |
– |
+ |
– |
– |
– |
|
Acetophenones, C0–C3 |
– |
+++ |
++ |
+ |
+ |
|
Fluorenones, C0–C3 |
– |
+ |
++ |
– |
– |
|
Dihydroindenones, C0–C4 |
– |
++ |
+++ |
+ |
+ |
|
Cycloalkenones, C6–C11 |
– |
+ |
+++ |
+ |
+ |
|
Dihydrofuranones |
– |
– |
+ |
++ |
– |
|
Isobenzofuranones, C0–C3 |
– |
– |
+ |
++ |
– |
|
Phenols, C0–C4 |
– |
– |
– |
+ |
– |
|
Naphthols, C0–C2 |
– |
– |
– |
+ |
– |
|
Furanones, C1–C3 |
– |
– |
– |
+ |
– |
|
Alkanones, C8–C22 |
– |
– |
– |
++ |
+ |
|
Alkanoic acids, C5–C14 |
– |
– |
+ |
++ |
+++ |
|
Benzoic acids, C0–C4 |
– |
– |
– |
– |
+ |
|
Nitrogen-containing compounds |
|||||
|
Carbazoles, C0–C4 |
– |
– |
– |
+ |
– |
|
Oxygen- and sulfur-containing compounds |
|||||
|
Hydroxybenzenethiols, C0 –C4 |
– |
– |
+ |
– |
– |
a Adapted from Lunsford & Cooper (1989).
b Degree of alkyl substitution given by Cn, where n = number of substituent carbon atoms.
c Relative abundance across fractions, but not classes, indicated by +++ > ++ > +.
d – = Not observed.
This section is not intended to be an all-inclusive list of the analytical sampling and analysis methods available for characterizing asphalt fumes and vapours. Emphasis is placed on validated methods that have been used in multiple studies.
Although a variety of sample collection and analytical methods are available for evaluating asphalt fume exposures, most of them are non-specific and cannot be used to characterize total asphalt fume exposure. Many studies have focused on TP and BSP determination for assessing asphalt fume exposures. NIOSH Methods 0500 (NIOSH, 1984, 1994) and 5023 (NIOSH, 1984) have commonly been used to determine these analytes, but on different samples. Using NIOSH Method 0500, the TP sample is collected by drawing a known volume of air through a tared polyvinyl chloride (PVC) filter; using NIOSH Method 5023, the BSP is collected by drawing a known volume of air through a polytetrafluoroethylene (PTFE) filter. The PVC filter is analysed gravimetrically to determine the TP, and the PTFE filter is analysed by extracting with benzene and gravimetrically determining the BSP. In some recent studies, because NIOSH Method 5023 had been withdrawn and because both TP and BSP can be determined on the same sampler, NIOSH Method 5042 (NIOSH, 1998) has been used. In this method, the TP and BSP sample is collected by drawing a known volume of air through a tared PTFE filter. After the tared PTFE filter is analysed gravimetrically to determine the TP, the filter is reanalysed by extracting with benzene and gravimetrically determining the BSP. The working range is 0.13–2 mg/m3 for a 1000-litre sample. The limit of detection (LOD) and limit of quantification (LOQ) for TP are 0.04 and 0.13 mg per sample, respectively; the LOD and LOQ for BSP are 0.04 and 0.14 mg per sample, respectively.
While other solvents have been used (such as cyclohexane, acetonitrile, and methylene chloride) to measure the soluble particulate, the results should not be compared, because the extraction capabilities of these solvents vary. In addition, these methods do not measure distinct chemical components or even a distinct class of chemicals in the asphalt fume sample. Although many researchers have reported results for PAHs in asphalt fumes, results obtained using high-performance liquid chromatography (HPLC)/fluorescence and GC/FID methods are suspect. Because asphalt fumes are composed of many alkylated isomers of PAHs, along with O-PACs and S-PACs, with the exception of naphthalene and some three-ring PAHs, they are so chemically complex that they cannot be separated into discrete compounds. The greater the lack of resolution between compounds, the less reliable the quantification results. Because of the poor resolution obtained with asphalt fume samples, quantification is unreliable when these methods are used. Moreover, an alternative method (such as GC/MS or HPLC/MS) is required to confirm the identity of any suspected PAHs, including naphthalene and other possible baseline-resolved PAHs. Any compounds reported using these methods are tentative identifications at best, and the more complex the matrix, the more unreliable these identifications become. Furthermore, since chromatographic software programs assign peak identification based on the largest peak in a given time window and not on retention time, the wrong peak may be assigned and analysed. Also, for HPLC, a gradient elution (e.g., mobile-phase composition varies during the chromatographic run) is used, which might result in varying retention times, thus further complicating the selection of the correct peak for identification and analysis (NIOSH, 2000).
NIOSH Method 5800 (NIOSH, 1998) can be used to estimate the total PAC content of asphalt fumes. This method uses an HPLC pump to provide a mobile phase into which a sample is injected. The sample then passes through two fluorescence detectors. Since no liquid chromatographic column is used, the entire sample reaches the flow cell at once, resulting in a rapid and sensitive analysis of the sample. The two fluorescence detectors are used to monitor different excitation and emission wavelengths. One set of wavelengths is more sensitive to two- and three-ring PACs, and the second set of wavelengths is more sensitive to four- and higher-ring PACs (NIOSH, 2000; Neumeister et al., 2003).
Readily accessible body fluids and/or physiological functions have been sampled or monitored for biomarkers of exposure to asphalt fumes. Biomarkers specific to asphalt fume exposures have not yet been identified. However, urinary 1-hydroxypyrene (1-OHP) (Hatjian et al., 1995a,b, 1997; Toraason et al., 2001, 2002), DNA strand breaks and oxidative damage in peripheral blood leukocytes (Toraason et al., 2001, 2002), and DNA or protein adducts (Herbert et al., 1990; Lee et al., 1991) have been used with limited success as general indicators of exposure to asphalt fumes and PAHs.
Natural asphalt deposits occur in various parts of the world, mainly as a result of mineral oil seepage from the ground. The best known natural deposit is Trinidad’s Pitch Lake; asphalt deposits can also be found in Venezuela, the Dead Sea, Switzerland, and the Athabasca oil sands in northeastern Alberta (IPCS, 1982; Budavari, 1989; Lewis, 1993). In addition, asphalt is produced from crude petroleum, and it is these petroleum-based asphalts that are the focus of this document.
A broad spectrum of asphalt modifiers and additives spanning categories such as antioxidants, antistripping agents, extenders, fibres, fillers, hydrocarbons, oxidants, plastics, rubbers, waste materials, and miscellaneous products are also employed with the various asphalts (Speight, 1992; Roberts et al., 1996). Their presence may affect the composition of asphalt fumes and vapours and worker exposure.
The major types of asphalt products are paving asphalts and roofing asphalts. Asphalt is also used in asphalt-based paints as protective coatings to prevent corrosion of metals; in lining irrigation canals, water reservoirs, dams, and sea defence works; in adhesives in electrical laminates; and as a base for synthetic turf (Lewis, 1993).
In the USA, approximately 30 million tonnes of asphalt materials were produced in 2000 for paving and non-paving applications (AI, 2001). In 2001, approximately 16 million tonnes of bitumen (asphalt) were produced in Western Europe, of which 14 million tonnes were used in road pavement applications (D. Lyall, Eurobitume, Brussels, personal communication, 2002).
No specific data are available relating to transport and distribution among media, environmental transformation and degradation, interaction with physical, chemical, or biological factors, and bioconcentration. However, a recent report by CONCAWE (2001) indicates that although constituents of bitumen (asphalt) have octanol/water partition coefficient (log Kow) values in excess of 6 and are potentially bioaccumulative, in practice, their very low water solubilities and high relative molecular masses (ranging from 500 to 15 000) are such that their bioavailability to aquatic organisms is expected to be limited. The bioaccumulation of bitumen components would therefore be highly unlikely.
Bitumens (asphalts) would not be readily degradable. However, basing toxicological conclusions on the activity of single components may not be relevant to the physical/chemical interactions of a complex mixture such as asphalt.
Cooper & Kratz (1997) determined the components of runoff from asphalt pavement in fish (rainbow trout Oncorhynchus mykiss, brown trout Salmo trutta, and Paiute sculpin Cottus beldingi) and invertebrates from streams in California, USA. Concentrations of the PAH analytes in fish and invertebrate tissues were below the detection limit of 0.2 mg/kg. While concentrations of lead and cadmium in fish tissues were below the detection limits of 0.5 and 0.05 mg/kg, respectively, concentrations of zinc were higher in invertebrate tissues than in fish tissues and also significantly elevated at downstream relative to upstream sites (P = 0.05), ranging from 26 to 98 mg/kg. Invertebrate tissue concentrations of cadmium were independent of collection sites within streams and ranged from below detectable limits to 0.28 mg/kg. However, there is a potential for contributions from fuel, combustion, and crankcase deposits, as well as metals contained in tire tread rubber dust from tire abrasions (see also section 6).
Limited data are available on the concentration of asphalt in environmental media.
Asphalt fractions, including polars, aromatics, and saturates, were characterized in airborne particles and air samples collected 2.0–83.6 m from a highway in Denmark and in plant samples (grass, leaves, and wheat straw) collected 2.0–10.0 m from the highway (Kebin et al., 1996). The percentage of asphalt in these airborne particles was 1.61–11.02%. Concentrations of asphalt fractions in air samples were 0.54–3.96 × 10–3 mg polars/m3 air, 1.77–9.50 × 10–4 mg aromatics/m3 air, and 0.21–1.23 × 10–4 mg saturates/m3 air. Concentrations of asphalt fractions for polars, aromatics, and saturates in mg/g dry plant material were: 0.96, 0.89, and 0.37 for grass; 0.93 and 3.07, 2.91 and 3.89, and 1.28 and 1.53 for leaves; and 1.19 and 0.29, 1.38 and 1.30, and 0.63 and 0.56 for wheat straw (at 5 m and 10 m, respectively), respectively. However, diesel and gasoline exhaust from nearby traffic may have contributed to the composition of these fractions.
An assessment was made of the effects of runoff from asphalt pavement on streams in California, USA (Cooper & Kratz, 1997). Concentrations of PAHs and selected heavy metals (lead, zinc, cadmium) were determined in water samples collected from water draining road surfaces and from waters upstream and downstream from the point where water discharged from road surfaces into stream sites. Results of analyses indicate that concentrations of all PAH analytes in all stream and road runoff samples were below the detection limit of 0.5 µg/litre. Although detectable levels of heavy metals were present in stream and runoff water, the authors concluded that no significant upstream versus downstream differences existed in the concentrations of any heavy metal across all streams. Furthermore, concentrations of metals were elevated in runoff waters from the road surfaces relative to upstream samples. Elevated metal concentrations could be due to sources other than asphalt (i.e., vehicle emissions, crankcase oil drippings, industrial operations, etc.).
Kriech et al. (2002b) conducted a laboratory study to determine 29 PACs in leachate water of six paving asphalt and four roofing asphalt samples. Samples were leached according to US Environmental Protection Agency (EPA) method SW846-1311. Results indicated that none of the roofing samples tested leached any of the 29 PACs. While four of the paving samples did not leach any of the 29 PACs, leachate of two paving samples contained detectable amounts of naphthalene and phenanthrene; however, the levels were well below drinking-water limits (0.015 mg/litre) in the USA. Similarly, Brantley & Townsend (1999) performed a series of leaching tests on samples of reclaimed asphalt from facilities in Florida, USA. None of 16 EPA priority pollutant PAHs were detected in the water leachates of any of these samples. The authors pointed out that during normal use of pavement, the asphalt may come in contact with vehicle exhaust, lube oils, gasoline, and metals from brake pads. In addition, Brandt & DeGroot (2001) demonstrated that PAH concentrations in leachate water from 10 asphalts were well below the European maximum tolerable concentration for potable water (0.1 μg/litre).
Quantitative information on levels of asphalt in drinking-water and foodstuffs has not been identified. However, experiments conducted to determine whether the use of asphalt seal coating in ductile-iron pipe would contribute significant concentrations of PACs in drinking-water indicated that the highest concentration found in three experiments was 5 ng/litre (Miller et al., 1982). The significance of these experiments is unclear, since they represented a worst-case scenario and the pipes were aged for only 1 month in a laboratory setting.
In the USA, approximately 300 000 workers are employed at hot-mix asphalt facilities and paving sites (APEC, 1999); an estimated 50 000 workers are employed in asphalt roofing operations; and about 1500–2000 workers are employed in approximately 100 roofing manufacturing plants (AREC, 1999). In Western Europe, there are approximately 4000 asphalt mixing plants employing 5–10 individuals per plant. Approximately 100 000 members of paving crews apply these asphalt mixes to road surfaces across Western Europe (Burstyn, 2001).
Data collected between 1994 and 1997 during seven paving surveys conducted in the USA by NIOSH (2000) indicated that, in general, most TWA PBZ air concentrations for both TP and BSP were below 0.5 mg/m3. Geometric mean (GM) full-shift PBZ samples for TP and BSP ranged from 0.041 to 0.48 mg/m3 and from 0.073 to 0.12 mg/m3, respectively. However, GM data collected during paving operations in a tunnel in Boston, Massachusetts, USA (Sylvain & Miller, 1996), indicated that PBZ exposures to TP and BSP were about 3 times higher than exposures measured during the seven NIOSH surveys at open-air roadway paving sites (NIOSH, 2000). Personal exposures to TP and BSP ranged from 1.09 to 2.17 mg/m3 and from 0.30 to 1.26 mg/m3, respectively (Sylvain & Miller, 1996).
Other studies examined exposures to asphalt not only at road paving sites, but also at hot-mix plants, refineries and terminals, roofing manufacturing plants, and roofing application sites in the USA (Hicks, 1995; Exxon, 1997; Gamble et al., 1999). GM exposures for TP and BSP at these sites are presented in Table 5. GM exposures for TP and BSP varied across all industry types: TP ranged from 0.18 to 1.40 mg/m3, and BSP ranged from 0.05 to 0.27 mg/m3. Heikkilä et al. (2002) reported GM exposures for TP from asphalt (described by the author as bitumen fume) of 0.4, 0.5, and 4.1 mg/m3 for paving operator, screed operator, and manual mastic paver, respectively. Similarly, Burstyn et al. (2000) reported higher GM asphalt fume exposures (described by the author as bitumen) during mastic laying operations (2.29 mg/m3) compared with exposures during paving operations (0.28 mg/m3). These values indicate that exposures may be higher in situations such as mastic laying.
Table 5: Geometric mean of personal exposures for total particulates (TP) and benzene-soluble particulates (BSP).
|
Type of industry |
Geometric mean of personal exposures (mg/m3) |
|||
|
TPa |
BSPa |
TPb |
BSPb |
|
|
Road paving |
0.37 |
0.24 |
0.33 |
0.09 |
|
Hot-mix plants |
0.78 |
0.15 |
0.45 |
0.06 |
|
Refineries and terminals |
0.18 |
0.16 |
0.19 |
0.05 |
|
Roofing manufacturing |
1.40 |
0.27 |
0.60 |
0.08 |
|
Roofing application |
0.55 |
0.25 |
0.34 |
0.12 |
a Adapted from Hicks (1995).
b Adapted from Exxon (1997) and Gamble et al. (1999).
Several investigators have attempted to assess asphalt exposure by the dermal route. Wolff et al. (1989) collected dermal wipe samples by wiping a 3 × 3 cm area of the forehead of workers exposed to asphalt during the application of hot asphalt to roofs in order to evaluate the extent to which dermal absorption of PAHs may contribute to the total body burden. These dermal wipe samples were analysed for specific PAHs. In the Wolff et al. (1989) study, PAH residues per square centimetre of skin were higher in postshift samples (6.1–31 ng/cm2) than in preshift samples (0.44–2.2 ng/cm2). However, workers monitored during the entire roofing application were potentially exposed to PAHs during both the removal of the old coal tar pitch roof and the application of hot asphalt for the new roof. Hicks (1995) collected dermal wipe samples by wiping a 4 × 8 cm area from the back of the hand or forehead of workers at the various asphalt sectors described in Table 5. The PAH concentrations determined from these postshift samples ranged from 2.2 to 520 ng/cm2. Workers in paving operations produced the largest number of PAHs detected (12 of 16), while refinery and roofing workers had the fewest (2 of 16). However, the HPLC/fluorescence technique used by these authors cannot reliably identify and quantify components of asphalt; their results are presented for completeness only.
Toraason et al. (2001, 2002) examined urinary 1-OHP concentrations at the beginning and end of the same work week (4 days later) in seven roofers who applied hot asphalt products but had no coal tar exposure during the preceding 3 months. All seven workers were smokers at the time of the study. Urinary 1-OHP concentrations were statistically significantly increased(P < 0.05) at the end of the work week (start of work week 0.26 ± 0.13 µmol/mol creatinine; end of work week 0.58 ± 0.29 µmol/mol creatinine). The average weekly TWA exposure for TP and BSP for a crew of six asphalt-only roofers was 0.24 ± 0.10 mg/m3 and 0.08 ± 0.02 mg/m3, respectively. The TWA exposures for TP and BSP for a seventh roofer in another crew were 0.31 mg/m3 and 0.18 mg/m3, respectively.
Heikkilä et al. (2002) measured preshift and postshift urinary 1-OHP concentrations in 32 road pavers participating in a study to evaluate asphalt fume exposures of workers employed at 13 paving sites where 11 different asphalt mixtures were applied. The mean TP exposure for the 11 asphalt mixtures ranged from 0.2 to 4.2 mg/m3 (AM [arithmetic mean] = 0.6 mg/m3; GM = 0.5 mg/m3). The mean TP exposure for all mixtures was below 0.5 mg/m3, with the exception of manual mastic paving (4.2 mg/m3) and stone mastic asphalt (2.0 mg/m3). The control group consisted of 78 smoking and non-smoking unexposed office workers obtained from a national reference group for 1-OHP in Finland. The authors reported that mean 1-OHP concentrations were statistically significantly higher (P < 0.05) among pavers (AM = 6.6 nmol/litre, standard deviation [SD] = 9.8) than in controls (AM = 1.6 nmol/litre, SD = 2.6) and twice as high among pavers who were smokers (preshift: AM = 8.5 nmol/litre, SD = 10.5) as among pavers who were non-smokers (preshift: AM = 4.0 nmol/litre, SD = 8.0) (P < 0.05) (P. Heikkilä, personal communication, Finnish Institute of Occupational Health, Helsinki, 2003). A similar trend was observed in postshift data (data not shown). There was no difference between non-smoking road pavers or non-smoking referents (data not shown), suggesting that smoking strongly influences urinary 1-OHP concentrations and may not be a sensitive measure of occupational asphalt fume exposure.
No studies that report exposures to cutback asphalts, emulsified asphalts, or asphalt-based paints (products applied at or near ambient temperatures) have been found. Because these products are liquids, workers may be exposed via inhalation and dermal contact.
Mixtures do not lend themselves to kinetic analyses. Because asphalt is a complex mixture, its pharmacokinetic pattern will vary depending upon the properties and interactions of the individual constituents. The pharmacokinetics of some asphalt components, particularly the PAHs, have been studied in considerable detail (Syracuse Research Corporation, 1985).
The long-chain aliphatic hydrocarbons constitute major components of asphalt; routes of uptake include inhalation, ingestion, and dermal uptake. Data indicate that following inhalation, hydrocarbons with 9–16 carbons were absorbed in the blood, brain, liver, kidneys, and fat of rats (ATSDR, 1998). Aerosols of hydrocarbons with more than 16 carbons were absorbed in liver and lungs of mice. These long-chain aliphatic compounds may be oxidatively metabolized via cytochrome P450 oxidases. Aliphatic hydrocarbons with between five and eight carbons may be oxidized to several alcohol, ketone, and carboxylic acid derivatives. Aliphatic hydrocarbons with 9–16 carbons are oxidatively metabolized via cytochrome P450 isozymes to fatty acids and alcohols. Evidence indicates that metabolism of these hydrocarbons may be quite slow. In general, these compounds are slowly eliminated in the urine and faeces.
The major routes of uptake of PAHs in humans are the lungs and respiratory tract after inhalation of PAH-containing aerosols or of particulates to which a PAH in the solid state has become absorbed; the gastrointestinal tract after ingestion of contaminated food or water; and the skin as a result of contact with PAH-bearing materials (IPCS, 1998). In general, the oxidative metabolism of PAHs involves epoxidation of double bonds, a reaction catalysed by cytochrome P450-dependent mono-oxygenases, rearrangement or hydration of the epoxides to yield phenols or diols, respectively, and conjugation of the hydroxylated derivatives with glutathione, sulfate, or glucuronic acid. However, in certain cases, radical cations and sulfate esters of hydroxymethyl derivatives may also be important (IPCS, 1998). Whole body distribution of PAHs has been studied in rodents. These studies have demonstrated that detectable levels of PAHs occur in almost all internal organs and that organs high in adipose tissue can serve as storage depots from which the PAHs are generally released (IPCS, 1998). In general, these compounds are eliminated by urinary or biliary excretion of metabolites.
In vivo and in vitro animal studies have evaluated the genotoxicity, carcinogenicity, and other toxic effects of asphalt-based paints and asphalt fumes. Because of the difficulty in obtaining a sufficient quantity of paving and roofing asphalt fumes in the field, many of the studies used laboratory-generated asphalt fume condensates.
Irritation studies (eye, skin, respiratory tract) have been reviewed previously in NIOSH (1977), IPCS (1982), and IARC (1985).
Exposure of rabbits to asphalt vapours was reviewed (NIOSH, 1977). The asphalts in the study were from the USA and England, with no further details provided. Additional experimental details (temperature of vapour generation, concentrations of the vapour, duration and frequencies of exposures) were not provided. Exposure to asphalt vapours caused only minor, transient conjunctivitis in the eyes of rabbits. After frequent exposures, a slight infiltration of the cornea was sometimes noted; however, this disappeared several days after exposures ceased. No other toxic effects were observed in the rabbits (NIOSH, 1977).
In a skin painting study summarized in IARC (1985), Swiss albino mice were exposed to samples of eight different bitumens (class 1). They received biweekly applications of 25 µl of bitumen solution (10% in benzene) to shaved areas of their backs for approximately 81 weeks. Skin effects included epidermal hyperplasia, along with inflammatory infiltration of the dermis and cutaneous ulceration with abscess formation.
In another study (Hueper & Payne, 1960), 30 guinea-pigs (Strain 13) and 65 Bethesda black rats were placed in chambers and exposed to roofing asphalt fumes and vapours for 5 h/day, 4 days/week, for 2 years. These fumes and vapours were derived from an air-blown petroleum asphalt by placing 700–10 000 g of the asphalt into an evaporating dish and heating it to 120–135 °C. Fresh asphalt was placed in the evaporating dish once a week, while on other days only the amount lost was replaced. (Asphalt typically would not be heated repeatedly during the course of a week; therefore, these fumes and vapours may not be representative of a typical exposure. Further experimental details were not provided.) Exposure to these asphalt fumes and vapours caused "extensive chronic fibrosing pneumonitis with peribronchial adenomatosis" (Hueper & Payne, 1960).
While exposure conditions in Simmers (1964) are not representative of real-world exposures, results are included for completeness. "The asphalt used in this study was a pooled sample from six different California refineries and contained both steam and air-blown samples." In the first experiment, 20 C57 Black mice were exposed to an asphalt aerosol made from an asphalt emulsion. Mice were exposed to this aerosol 30 min/day, 5 days/week, for up to 410 treatments. (Three mice survived 410 treatments, while 10 mice survived 280 or more treatments.) Effects included congestion, acute bronchitis, pneumonitis, bronchial dilatation, and some peribronchial round cell infiltration. In the second experiment, asphalt smoke was generated by placing 250–350 g of the asphalt sample into a tin container and heating to 120 °C, causing the asphalt to boil and give off a yellowish-brown smoke. Thirty C57 Black mice were exposed for 6–7.5 h/day, 5 days/week, for 21 months. Effects included peribronchial round cell infiltration, bronchitis, pneumonitis, loss of cilia, and epithelial atrophy.
A study to evaluate possible toxic effects of asphalt fumes after inhalation exposure of male and female Wistar WU rats was conducted by the Fraunhofer Institute (Fraunhofer, 2001) to determine concentrations and a maximally tolerated dose for a future carcinogenicity study. The composition of the asphalt fumes was designed to mimic exposure during road paving in Germany (Pohlmann et al., 2001). Rats (16 per group) were exposed nose only to clean air (control) or to target concentrations of 4, 20, or 100 mg/m3 of asphalt fumes for 6 h/day, 5 days/week, for 14 weeks. The mean actual concentrations (aerosol + vapour phase) analysed by infrared spectroscopy were 3.95, 20.12, and 106.55 mg/m3. The composition of the exposure atmosphere (% particulate/% vapour) was 24.6/75.4, 42.9/57.1, and 68.1/31.9 for 4, 20, and 100 mg/m3, respectively. The number median aerodynamic diameter as measured with the scanning mobility particle sizer system was 105 nm in the 4 mg/m3, 82 nm in the 20 mg/m3, and 86 nm in the 100 mg/m3 asphalt fumes. No mortality related to the asphalt fume exposure occurred. Results indicate that exposure to 100 mg/m3 asphalt fumes caused a significantly lower body weight in male rats and statistically significant (P-values not presented) exposure-related histopathological changes (e.g., hyalinosis, basal cell hyperplasia, mucous cell hyperplasia, inflammatory cell infiltration) in the nasal and paranasal cavities. Under the experimental conditions described above, the no-observed-adverse-effect level for asphalt fumes is 20 mg/m3.
A number of studies evaluated potential mutagenic effects of paving and roofing asphalt and asphalt-based paints using the Ames Salmonella assay. An evaluation of available data indicates that asphalt fumes collected at 146–157 °C from the headspace of an asphalt storage tank at a hot-mix asphalt production plant were not mutagenic in the modified Ames Salmonella assay, while fume condensates generated in the laboratory at 149 °C and 316 °C were mutagenic (Reinke & Swanson, 1993; Reinke et al., 2000). Asphalt fume condensates generated at 316 °C were more mutagenic than the fumes generated at 149 °C. In contrast, a study by Heikkilä et al. (2003) demonstrated that the particulate fractions of asphalt fumes collected in the PBZ of workers during paving operations were mutagenic in the Ames Salmonella assay, recycled asphalts being more mutagenic than the particulate fractions of new asphalt. Additionally, another study did not demonstrate any mutagenicity in mice exposed by nose only to paving asphalt fumes (Micillino et al., 2002). Asphalt fume samples collected above an open port of the heated cement storage tank at hot-mix plants were not mutagenic using a spiral Salmonella mutagenicity assay (Burr et al., 2002). In other studies, paving and roofing asphalt fumes generated in the laboratory under a variety of conditions were also mutagenic (AI, 1990a; NTP, 1990; Machado et al., 1993; De Méo et al., 1996). None of the asphalt-based paints examined by Robinson et al. (1984) demonstrated mutagenic activity in either the presence or absence of metabolic activation (S9).
Condensates of Type I and Type III roofing asphalt fumes generated in the laboratory at 316 °C using the same methodology as in Sivak et al. (1989) and roofing asphalt fumes generated by Sivak et al. (1989) (information on the methodology can be found in section 8.4) caused a dose-related increase in micronucleus formation in exponentially growing Chinese hamster lung fibroblasts (V79 cells) (Qian et al., 1996, 1999). The authors suggested that Type I and Type III roofing asphalt fume condensates are aneuploidogens and possess some clastogenic activities. These condensates caused mainly cytogenetic damage by spindle apparatus alterations in cultured mammalian cells. Ma et al. (2002) exposed male Sprague-Dawley rats intratracheally to asphalt fume condensates (saline control, 0.45 mg/kg body weight, or 8.8 mg/kg body weight) collected at the top of a paving storage tank (160 °C). Exposure to 0.45 mg asphalt fume condensate/kg body weight caused a non-significant increase in micronuclei formation, while 8.8 mg asphalt fume condensate/kg body weight (the highest concentration tested) caused a statistically significant (P < 0.05) increase in micronuclei formation in bone marrow polychromatic erythrocytes. However, all results were negative when three paving asphalt fume condensates generated in the field and in the laboratory were tested at 5, 10, 15, 20, 30, 40, 60, 80, and 120 µg/ml in a chromosomal aberration assay using Chinese hamster ovary cells (Reinke & Swanson, 1993; Reinke et al., 2000).
De Méo et al. (1996) and Genevois et al. (1996) tested paving asphalt fume condensates generated in the laboratory at 160 and 200 °C for their ability to induce DNA adduct formation in vitro and in vivo,respectively. All of the fume condensates induced DNA adduct formation in vitro when added to calf thymus DNA, although no specific DNA adducts were identified (De Méo et al., 1996). Additionally, the same paving asphalt fume condensates induced DNA adducts in the skin, lungs, and lymphocytes of BD4 rats treated with them dermally, but specific types of DNA adducts were not identified (Genevois et al., 1996). In a later study, Genevois-Charmeau et al. (2001) exposed three BD6 rats by nose only to paving asphalt fume condensates. A DNA adduct was detected only in the lungs of the exposed rats.
Male Parkes mice that received multiple topical applications of asphalt-based paints showed accumulations of DNA adducts in both skin and lung tissue (Schoket et al., 1988a). After topical application, asphalt-based paints also induced DNA adduct formation in adult and fetal human skin samples maintained in short-term tissue culture. A single 15-mg dose per skin patch of asphalt-based paint induced 0.22 fmol adducts (Schoket et al., 1988b). However, the specific types of DNA adducts were not identified in either study.
The five laboratory-generated asphalt roofing fume fractions used by Sivak et al. (1989) were tested for inhibition of intercellular communication. All fractions inhibited intercellular communication in Chinese hamster lung fibroblasts (V79 cells) (Toraason et al., 1991). Similarly, Wey et al. (1992) examined the effect of these fractions on intercellular communication in human epidermal keratinocytes. All fractions inhibited intercellular communication in a concentration-dependent fashion. Modulation of gap functional intercellular communication has been implicated as an important effect of tumour promoters. The inhibition of intercellular communication by a tumour promoter is believed to isolate an initiated or preneoplastic cell from the regulatory signals of surrounding cells, leading to the development of neoplasms (NIOSH, 2000).
Ma et al. (2002) exposed male Sprague-Dawley rats intratracheally to asphalt fume condensates (saline control or 0.45, 2.22, or 8.8 mg/kg body weight) collected at the top of a paving asphalt storage tank (160 °C). Exposure to 8.8 mg asphalt fume condensate/kg body weight, the highest concentration tested, caused a statistically significant (P < 0.05) dose-dependent increase in both the level and activity of CYP1A1 in the lung. However, CYP2B1 levels and activity were not significantly affected.
Several studies have reported carcinogenicity in mice following applications of laboratory-generated asphalt roofing fume condensates (Thayer et al., 1981; Niemeier et al., 1988; Sivak et al., 1989, 1997), raw roofing asphalt (Sivak et al., 1989, 1997), and asphalt-based paints (Robinson et al., 1984; Bull et al., 1985) to the skin of mice. However, in another study (Emmett et al., 1981), raw roofing asphalt applied dermally to mice was not carcinogenic.
Thayer et al. (1981) and Niemeier et al. (1988) investigated the tumorigenicity of fume condensates generated in the laboratory at 232 and 316 °C from Type I and Type III roofing asphalt
Table 6: Final histopathology of tumours induced in CD-1 mice treated dermally with roofing asphalt fume condensates.a
|
Material tested |
Sunlightb |
Tumour-bearing animals |
Tumours |
|||
|
Benign |
Malignant |
Papilloma |
Squamous cell carcinoma |
Totalc |
||
|
Type I asphalt at 232 °Cd |
– |
6 |
0 |
12 |
0 |
12 |
|
+ |
2 |
0 |
3 |
0 |
3 |
|
|
Type I asphalt at 316 °Cd |
– |
13 |
1 |
18 |
0 |
19 |
|
+ |
3 |
0 |
3 |
0 |
3 |
|
|
Type III asphalt at 232 °Cd |
– |
9 |
1 |
11 |
1 |
13 |
|
+ |
5 |
2 |
5 |
1 |
7 |
|
|
Type III asphalt at 316 °Cd |
– |
13 |
3 |
17 |
1 |
20 |
|
+ |
4 |
1 |
5 |
1 |
6 |
|
|
Benzo[a]pyrene (B(a)P)e |
– |
24 |
11 |
43 |
10 |
58 |
|
+ |
9 |
3 |
11 |
1 |
18 |
|
|
Cyclohexane/acetonef |
– |
0 |
||||
|
+ |
0 |
|||||
|
a |
Adapted from Thayer et al. (1981). |
|
b |
There were 50 animals per group, and half of each group was exposed to sunlight. |
|
c |
Other tumour types observed included fibrosarcomas, kerato-acanthomas, fibromas, and unclassified benign epitheliomas. |
|
d |
25 mg of total solid per application. |
|
e |
5 µg per application. |
|
f |
50 µl of a 1:1 solution. |
Table 7: Final histopathology of tumours induced in C3H/HeJ mice treated dermally with roofing asphalt fume condensates.a
|
Material tested |
Sunlightb |
Tumour-bearing animals |
Tumours |
|||
|
Benign |
Malignant |
Papilloma |
Squamous cell carcinoma |
Totalc |
||
|
Type I asphalt at 232 °Cd |
– |
24 |
22 |
34 |
26 |
76 |
|
+ |
14 |
27 |
22 |
25 |
62 |
|
|
Type I asphalt at 316 °Cd |
– |
13 |
31 |
27 |
31 |
78 |
|
+ |
18 |
26 |
36 |
26 |
73 |
|
|
Type III asphalt at 232 °Cd |
– |
15 |
25 |
32 |
19 |
66 |
|
+ |
11 |
20 |
14 |
19 |
54 |
|
|
Type III asphalt at 316 °Cd |
– |
12 |
28 |
24 |
36 |
82 |
|
+ |
20 |
18 |
34 |
20 |
65 |
|
|
Benzo[a]pyrene (B(a)P)e |
– |
11 |
27 |
12 |
29 |
53 |
|
+ |
7 |
27 |
11 |
22 |
43 |
|
|
Cyclohexane/acetonef |
– |
0 |
0 |
0 |
0 |
0 |
|
+ |
1 |
0 |
2 |
2 |
4 |
|
|
a |
Adapted from Thayer et al. (1981). |
|
b |
There were 50 animals per group, and half of each group was exposed to sunlight. |
|
c |
Other tumour types observed included fibrosarcomas, kerato-acanthomas, fibromas, and unclassified benign epitheliomas. |
|
d |
25 mg of total solid per application. |
|
e |
5 µg per application. |
|
f |
50 µl of a 1:1 solution. |
Sivak et al. (1989, 1997) heated Type III roofing asphalt from the same lot used by Niemeier et al. (1988) at 316 °C, generated fume condensates, and separated them by HPLC (see Belinky et al., 1988, for a description of this procedure). The chemical composition of fractions A through E, as analysed by GC/MS, can be found in Table 4. Raw roofing asphalt, neat asphalt fumes, asphalt heated to 316 °C with fumes allowed to escape, reconstituted asphalt fumes, and the asphalt fume fractions — individually and in various combinations — were then tested for their carcinogenic and tumour-promoting activity in male C3H/HeJ and Sencar mice. Fractions A through E were dissolved in a 1:1 solution of cyclohexane and acetone to yield concentrations proportional to their presence in the unfractionated (neat) asphalt fume condensate, i.e., 64.1%, 8.3%, 10.5%, 11.5%, and 5.6%, respectively. They were then applied biweekly to 40 groups of male C3H/HeJ mice and two groups of male Sencar mice (30 mice per group) for 104 weeks (2 years). Table 8 shows all the treatment groups, the number of papillomas and carcinomas per group, the number of tumour-bearing mice, and the average time (in weeks) to carcinoma development. The raw roofing asphalt and neat asphalt fumes induced carcinomas (local skin cancers) in 3 of 30 and 20 of 30 C3H/HeJ mice, respectively. However, the heated asphalt with fumes allowed to escape did not induce any tumours. Fractions B and C induced carcinomas in 10 of 30 and 17 of 30 C3H/HeJ mice, respectively, while fractions A, D, and E failed to induce any carcinomas when applied alone. All the combinations of the fractions induced carcinomas only if they included B or C. The A and D combination, the A and E combination, and the A, D, and E combination failed to induce any carcinomas. Furthermore, fractions A, D, and E failed to act as either tumour promoters or co-carcinogens. Eighteen of the 30 Sencar mice treated with the asphalt fume condensate developed carcinomas. Fractions contained PACs that included PAHs, S-PACs, and O-PACs, such as alkylated aryl thiophenes, alkylated phenanthrenes, alkylated acetophenones, and alkylated dihydrofuranones. Fraction B contained most of the S-PACs, and only a few were carried over to fraction C. Fraction C contained a small amount of four-ring PACs.
Table 8: Tumorigenic response in all treatment groups.a
|
Group numberb |
Treatment |
Asphalt dose (mg)c |
Total number of tumours per groupd |
Number of tumour-bearing mice |
Average time to carcinoma (weeks)e |
|
|
Papilloma |
Carcinoma |
|||||
|
1 |
Raw asphalt |
25 |
1 |
3 |
4 |
101 |
|
2 |
Heated asphalt (less fume) |
25 |
||||
|
3 |
Heated asphalt (plus fume) |
25 |
||||
|
4 |
Neat asphalt fume |
25 |
12f |
25f |
21 |
74 |
|
5 |
Solvent control |
0 |
||||
|
6 |
Fraction A |
16 |
||||
|
7 |
Fraction B |
2.3 |
2 |
10f |
11 |
98 |
|
8 |
Fraction C |
2.6 |
4 |
18f |
20 |
86 |
|
9 |
Fraction D |
2.3 |
||||
|
10 |
Fraction E |
1.6 |
||||
|
11 |
Fractions A + B + C + D + E |
24.8 |
30f |
23f |
25 |
75 |
|
12 |
Fractions A + B |
18.3 |
10f |
8f |
13 |
97 |
|
13 |
Fractions A + C |
18.6 |
12f |
16f |
15 |
90 |
|
14 |
Fractions A + D |
18.3 |
||||
|
15 |
Fractions A + E |
17.6 |
||||
|
16 |
Fractions B + C + D + E |
8.8 |
9f |
18f |
19 |
81 |
|
17 |
Fractions A + B + C + D |
23.2 |
17f |
22f |
24 |
80 |
|
18 |
Fractions A + B + C + E |
22.5 |
26f |
30f |
27 |
77 |
|
19 |
Fractions B + C + D |
7.2 |
15f |
22f |
21 |
86 |
|
20 |
Fractions B + C |
4.9 |
12f |
26f |
26 |
73 |
|
21 |
Fractions A + C + D + E |
22.5 |
5f |
14f |
17 |
89 |
|
22 |
Fractions A + B + D + E |
22.2 |
5 |
7f |
9 |
97 |
|
23 |
Fractions A + D + E |
19.9 |
2 |
|||
|
24 |
0.01% benzo[a]pyrene (B(a)P) |
0g |
1 |
28f |
27 |
56 |
|
25 |
0.001% B(a)P |
0g |
2 |
3 |
5 |
103 |
|
26 |
0.0001% B(a)P |
0g |
||||
|
27 |
Fraction A + 0.01% B(a)P |
16 |
7f |
28f |
24 |
70 |
|
28 |
Fraction A + 0.001% B(a)P |
16 |
1 |
1 |
2 |
106 |
|
29 |
Fraction A + 0.0001% B(a)P |
16 |
1 |
1 |
106 |
|
|
30 |
Fraction D + 0.01% B(a)P |
2.3 |
14f |
34f |
29 |
64 |
|
31 |
Fraction D + 0.001% B(a)P |
2.3 |
2 |
2 |
||
|
32 |
Fraction D + 0.0001% B(a)P |
2.3 |
1 |
1 |
106 |
|
|
33 |
Fraction E + 0.01% B(a)P |
1.6 |
111 |
23f |
24 |
61 |
|
34 |
Fraction E + 0.001% B(a)P |
1.6 |
2 |
2 |
106 |
|
|
35 |
Fraction E + 0.0001% B(a)P |
1.6 |
||||
|
36 |
B(a)P then fraction A |
16h |
||||
|
37 |
B(a)P then fraction D |
2.3h |
||||
|
38 |
B(a)P then fraction E |
1.6h |
||||
|
39 |
B(a)P alone |
0 |
||||
|
40 |
Sentinel micei |
0 |
||||
|
41 |
Sencar fume |
25 |
21f |
18f |
20 |
83 |
|
42 |
Sencar control |
0 |
||||
|
a |
Adapted from Sivak et al. (1989, 1997). |
|
b |
Groups 1–40 consisted of 30 male C3H/HeJ mice per group, and groups 41 and 42 consisted of 30 male Sencar mice per group. |
|
c |
Asphalt, asphalt plus fume, or asphalt fume alone were applied twice weekly for 104 weeks; 50 µl per application. |
|
d |
Only histologically confirmed skin tumours are given. |
|
e |
Based on gross observation. |
|
f |
There were significantly more tumours, earlier onset of tumours, or both in these groups compared with controls. |
|
g |
5, 0.5, 0.05 µg B(a)P per 50 µl application per group, respectively. |
|
h |
Mice were initiated with a single application of 200 mg B(a)P/50 µl followed by twice-weekly applications of indicated fractions. |
|
i |
Five mice were sacrificed prior to the initiation of the study and after 6, 12, 18, and 24 months. |
In an earlier study, Emmett et al. (1981) examined the carcinogenic potential of a standard roofing asphalt (asphalt type not provided) dissolved in redistilled toluene at a 1:1 ratio by weight. Fifty milligrams of this solution was applied twice a week to the shaved intrascapular region of the back of 50 male C3H/HeJ mice for 80 weeks. The vehicle control group received 50 mg of toluene biweekly, and the positive control group received 50 mg of 0.01% benzo[a]pyrene (B(a)P) in toluene biweekly. No tumours were observed in male C3H/HeJ mice that had been treated dermally with the roofing asphalt.
Asphalt-based paint formulations are used to prevent corrosion in drinking-water distribution systems (Miller et al., 1982). Four of these formulations (labelled A through D) were evaluated for their potential tumour-initiating ability using female Sencar mice in mouse skin bioassays (Robinson et al., 1984; Bull et al., 1985). The asphalt-based paints were formulations containing xylene or xylene and mineral spirits with between 89% and 98% cutback asphalt. These asphalt-based paints initiated tumour development in female Sencar mouse skin. Table 9 presents data demonstrating their tumour-initiating activity, provides gross tumour observations, and classifies tumours examined histologically. Asphalt paint formulation D was analysed for its ability to act as a complete carcinogen; a dose of 200 µl was applied to 40 female Sencar mice once a week for 30 weeks, and the mice were sacrificed after 52 weeks. Under the experimental conditions provided, of the female Sencar mice treated with asphalt D, only 1 in 40 (3%) developed a tumour (papilloma), while 3 of 40 mice in the group treated with mineral spirits developed papillomas. Robinson et al. (1984) concluded that the four asphalt-based paints contained chemicals capable of initiating tumours in mice and that a number of these tumours were carcinomas. However, asphalt D was not a complete carcinogen.
Table 9: Tumour-initiating activity of asphalt-based paints.a
|
Material tested |
Dose (µl) unless otherwise indicatedb |
Gross observations |
Squamous cell abnormalities in histopathology after 52 weeks |
||||
|
Animals with tumoursc |
Number of tumours |
Examined/ initiatedd |
Papillomas |
Carcinomase |
Tumourse |
||
|
Asphalt A |
200 |
18/40 (45) |
25 |
36 |
4 |
2 |
6 |
|
600 |
21/40 (53) |
31 |
38 |
8 |
2 |
10 |
|
|
Asphalt B |
200 |
17/40 (43) |
23 |
31 |
5 |
0 |
5 |
|
600 |
20/40 (50) |
34 |
35 |
4 |
2 |
6 |
|
|
Asphalt C |
200 |
19/40 (48) |
28 |
31 |
4 |
5 |
8 |
|
600 |
23/40 (58) |
51 |
36 |
11 |
4 |
13 |
|
|
Asphalt D |
200f |
21/40 (53) |
33 |
33 |
9 |
6 |
9 |
|
600 |
15/40 (38) |
22 |
35 |
2 |
3 |
4 |
|
|
Mineral spiritsg |
600 |
5/40 (13) |
6 |
37 |
1 |
0 |
1 |
|
Acetone |
200 |
6/30 (20) |
6 |
23 |
4 |
0 |
4 |
|
B(a)P |
10.0 µg |
22/30 (73) |
99 |
27 |
11 |
9 |
15 |
|
DMBAh |
2.65 µg |
Not given |
Not given |
8 |
3 |
6 |
8 |
|
a |
Adapted from Robinson et al. (1984) and Bull et al. (1985). |
|
b |
The 200-µl dose was administered in one dose, while the 600-µl dose was administered as three weekly 200-µl doses. All animals were treated with 1 µg tetradecanoyl phorbol acetate (TPA) in 200 µl of acetone 3 times weekly for 20 weeks beginning 2 weeks after the last initiating dose. |
|
c |
Data represent cumulative tumour counts through 40 weeks. Number in parentheses indicates percentage. |
|
d |
Each treatment group except the DMBA treatment group contained 40 female Sencar mice. Only 20 were in the DMBA treatment group. |
|
e |
The asphalt D group also had one animal with a fibrosarcoma and one with a basal cell carcinoma. Total number of animals having squamous cell papillomas and/or carcinomas does not agree with number of animals with squamous cell tumours because some animals had both types. |
|
f |
Also analysed for its ability to act as a complete carcinogen. Results indicated that 1 of 40 Sencar mice tested developed a tumour (papilloma). |
|
g |
Also tested (200 µl) as a complete carcinogen. Results indicated that 3 of 40 Sencar mice tested developed tumours (papillomas). |
|
h |
DMBA = dimethyl benzanthracene. |
Two studies conducted in the late 1990s assessed the relationship between airborne concentrations of TP and BSP from asphalt fumes and vapours and symptom prevalence and pulmonary function parameters among workers employed in one or another segment of the US asphalt industry (Exxon, 1997; Gamble et al., 1999; Burr et al., 2002). Gamble et al. (1999) examined 170 workers employed in five segments of the US asphalt industry — hot-mix asphalt manufacturing, hot-mix asphalt paving operations, asphalt distribution terminals, roofing manufacturing, and roofing application. On each of 2 consecutive days, pre- and postshift symptom prevalence surveys and forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and forced expiratory fraction (FEF25–75) were taken. Symptom prevalence was also obtained during the workshift on each of the days. PBZ monitors were used to evaluate airborne exposures. The results of the study and a comprehensive compilation of the methods are included in Exxon (1997).
In a partnership with the US Federal Highway Administration, NIOSH conducted a study to develop and field-test new methods to assess asphalt fume exposures, characterize and compare occupational exposure to crumb-rubber modified asphalt (CRM) and "conventional asphalt," and evaluate the potential health effects of exposure (Burr et al., 2002). For each site, the conventional asphalt had the same formulation as the CRM, but did not contain crumb-rubber. This CICAD contains information only from the analysis of seven conventional asphalt paving sites. Two groups of workers (exposed and unexposed) were recruited at each site; medical evaluations were conducted over 4 days — 2 days for CRM and 2 days for conventional asphalt. A general health questionnaire was completed by each participant at the beginning of a site survey, asking about recent history of eye, nose, or throat irritation, cough, shortness of breath, wheezing, and history of chronic respiratory conditions. Smoking and work histories were also obtained. A questionnaire addressing acute symptoms was distributed to workers pre- and postshift, 3 times during the workshift. Peak expiratory flow rate was measured just prior to completing the acute symptom questionnaire. Area and PBZ monitoring were conducted to evaluate airborne exposures. A total of 94 workers employed at any of the seven paving sites participated in the study. Results are presented in the following section.
Among worker populations, acute effects of exposure to asphalt fumes include symptoms of irritation of the serous membranes of the conjunctivae (eye irritation) and the mucous membranes of the upper respiratory tract (nasal and throat irritation). These effects are best described in asphalt road pavers (Norseth et al., 1991; Almaguer et al., 1996; Hanley & Miller, 1996a,b; Kinnes et al., 1996; Miller & Burr, 1996a,b, 1998; Sylvain & Miller, 1996; Exxon, 1997; Gamble et al., 1999; Burr et al., 2002) and typically appear to be of mild severity and transitory in nature (Almaguer et al., 1996; Hanley & Miller, 1996a,b; Kinnes et al., 1996; Miller & Burr, 1996a,b, 1998; Exxon, 1997). Similar symptoms have also been reported in workers exposed to asphalt fumes during the application of hot asphalt roofing material (Exxon, 1997), during the manufacture of asphalt roofing shingles (Apol & Okawa, 1977; Exxon, 1997), and at hot-mix asphalt plants and terminals (Exxon, 1997). Unexpected asphalt fume exposure was reported from fluorescent lights (Chase et al., 1994) and during cable insulating operations (Zeglio, 1950). Tavris et al. (1984) suggested that the 3-month outbreak of headache, eye irritation, sore throat, nasal congestion, and nausea in an office complex was due to malfunctioning fluorescent light ballast, which overheated and resulted in melting and volatilization of contained asphalt. Correction of the problem resulted in almost complete disappearance of symptoms within 2 weeks. In one study of five different asphalt exposure situations (hot-mix plants, terminals, roofing application, roofing manufacturing, and paving), although symptoms were reported, no significant dose–response associations were found between measured exposures and symptoms (Exxon, 1997). However, in the NIOSH study of conventional asphalt pavers, airborne concentrations of TP, BSP, and PACs were significantly higher on days when symptoms of the eye, nose, or throat were present compared with days when symptoms were not reported (P = 0.02, P < 0.01, and P < 0.01, respectively) (Burr et al., 2002). While asphalt fume concentrations associated with the health effects noted above have not been well characterized, symptoms of eye, nose, or throat irritation were reported by workers during open-air paving. Average personal exposures, calculated as full-shift TWAs, were generally below 1.0 mg/m3 for TP and 0.3 mg/m3 for BSP (Almaguer et al., 1996; Hanley & Miller, 1996a,b; Kinnes et al., 1996; Miller & Burr, 1996a,b, 1998; Exxon, 1997).
Lower respiratory tract symptoms (coughing, wheezing, shortness of breath) (Zeglio, 1950; Nyqvist, 1978; Almaguer et al., 1996; Hanley & Miller, 1996a,b; Kinnes et al., 1996; Miller & Burr, 1996a,b, 1998; Sylvain & Miller, 1996; Exxon, 1997) and changes in pulmonary function (e.g., bronchial lability) (Waage & Nielson, 1986; Hanley & Miller, 1996a; Kinnes et al., 1996; Miller & Burr, 1996b; Sylvain & Miller, 1996) have been described among workers exposed to asphalt fumes. Some workers experienced lower respiratory tract problems or changes in pulmonary function when exposed to TP at concentrations ranging between 0.02 and 1 mg/m3 during open-air highway paving (Almaguer et al., 1996; Hanley & Miller, 1996a,b; Kinnes et al., 1996; Miller & Burr, 1996a,b, 1998; Exxon, 1997; Gamble et al., 1999). Kinnes et al. (1996) reported significant changes in pulmonary function in one of seven workers engaged in open-air asphalt paving. In three of nine workers engaged in underground paving, increased bronchoreactivity was noted, although only one paver reported symptoms (Sylvain & Miller, 1996). TP concentrations ranged from 1.09 to 2.17 mg/m3 and BSP concentrations ranged from 0.3 to 1.26 mg/m3 during underground paving (Sylvain & Miller, 1996). The study of workers in five asphalt industry segments (Exxon, 1997; Gamble et al., 1999) found no significant association between pulmonary function measurements (FVC, FEV1, or FEF25–75 over a workshift) and asphalt exposures among workers. Some limited evidence suggests that personal health factors (e.g., pre-existing asthma) or exposures to greater amounts of asphalt fumes, such as those found during underground paving, may increase workers’ risk for lower respiratory tract symptoms or changes in pulmonary function (Norseth et al., 1991; Sylvain & Miller, 1996). However, the current data are insufficient to determine the relationship between asphalt fume exposures and these health effects. In addition, other potentially confounding exposures, such as gasoline and diesel exhaust and road and tire dust, may also contribute an as yet unquantified potential for respiratory irritation.
Acute and chronic bronchitis, possibly related to chronic lower respiratory tract irritation, have been reported among asphalt workers in several studies (Zeglio, 1950; Baylor & Weaver, 1968; Hasle et al., 1977; Nyqvist, 1978; Maintz et al., 1987; Hansen, 1991).
Skin irritation, pruritus, or rashes were also reported after exposure to asphalt-based materials (Tavris et al., 1984; Schaffer et al., 1985; Waage & Nielson, 1986; Chase et al., 1994; Miller & Burr, 1996a,b, 1998). Given the presence of confounding co-exposures (i.e., diesel fuel exhaust products, coal tar, fibreglass) and environmental conditions (wind, heat and humidity, ultraviolet radiation), the extent to which asphalt fumes may be associated with these skin problems is unclear.
Symptoms of nausea, stomach pain, decreased appetite, headaches, and fatigue have also been reported among workers exposed to asphalt (Tavris et al., 1984; Schaffer et al., 1985; Waage & Nielson, 1986; Norseth et al., 1991; Chase et al., 1994; Exxon, 1997; Gamble et al., 1999). With the exception of the studies by Norseth et al. (1991), Exxon (1997), and Gamble et al. (1999), none of the other reports examined comparison groups with which to analyse exposure–response effects. Norseth et al. (1991) found increased frequency of fatigue and reduced appetite among asphalt-exposed workers compared with controls. No significant dose–response associations were found between measured exposures and symptoms in any of five asphalt exposure situations (Exxon, 1997; Gamble et al., 1999). The extent to which asphalt fumes may be associated with the above symptoms is unclear, given potential confounders.
Although burns due to hot asphalt comprise a small percentage of all reported burns, they are often severe and difficult to treat (James & Moss, 1990; Baruchin et al., 1997). While hot asphalt cools quickly on contact, it can retain sufficient heat to continue to cause damage while it remains on the skin. Furthermore, as it cools, it hardens and adheres to the skin, making it difficult to remove. Burned areas usually include the extremities (head and neck, arms, hands, and legs); however, in a few cases, the burned areas also include the torso.
James & Moss (1990) conducted a retrospective review covering a 9-year period (between January 1, 1979, and December 31, 1987) of all inpatients treated for asphalt burns at the Burns Unit, Frenchay Hospital (Bristol, United Kingdom). Of the 24 inpatients (23 males, average age 33 years) treated, 22 were injured while working. Of the patients with reported occupation, 19 were employed as roofers or road workers. Injuries occurred as a result of explosions involving hot asphalt, tipping over of hot asphalt containers, falling off ladders into asphalt, or spilling hot asphalt while carrying it. The mean burn size was 3% (range 0.25–9.0%) of total body surface area. Sixteen patients (67%) required surgical treatment and skin grafts. The time between injury and surgery averaged 8 days (range 2–22 days), and the hospital stay averaged 9 days (range 1–21 days). Twenty-two patients were discharged from care and were able to return to work within 2 months; two were unemployed at the time of the injury.
Baruchin et al. (1997) conducted a retrospective review covering a 10-year period (between January 1, 1985, and January 1, 1995) of all inpatients treated for asphalt burns at either the Soroka Medical Center (Beer-Sheba, Israel) or the Barzilai Medical Center (Ashkelon, Israel). Of the 92 inpatients (all males, average age 29.6 years), 90 were injured while working. Eighty-four workers were injured when hot asphalt was spilled on them or they fell into it, four workers were injured when pipes carrying hot asphalt burst, and the remaining two workers were burned as a result of traffic accidents. The mean burn size was 3.87% of total body surface area. Fifty-three patients (58%) did not require surgical treatment, but did stay an average of 8.8 days in the hospital. Thirty-nine patients (42%) required surgical treatment and skin grafts. The average hospital stay for these patients was 10.7 days. On average, all patients lost 9.65 work days.
Cohort epidemiological studies of lung cancer among workers engaged in asphalt paving, asphalt mixing plants, or highway maintenance and exposed to asphalt fumes have yielded contradictory results (Table 10). That is, while several studies have reported an elevated risk of lung cancer (Hansen, 1989a, 1991; Engholm et al., 1991; Partanen et al., 1997), design limitations of some of these studies preclude drawing any strong conclusions. Of particular concern is the possible presence of confounding from co-exposures to coal tar and other potential lung carcinogens (e.g., diesel exhaust, silica, and asbestos) (Hansen, 1989a) as well as from smoking (Engholm et al., 1991). No excess of lung cancer was found among highway maintenance workers (Bender et al., 1989). A meta-analysis of 20 epidemiological studies failed to find overall evidence for a lung cancer risk among pavers and highway maintenance workers exposed to asphalt (RR = 0.87, 95% CI = 0.76–1.08) (Partanen & Boffetta, 1994).
Table 10: Epidemiological studies of asphalt exposure: cohort studies of diseases in pavers.a
|
Author, country, and occupation |
Number of study subjects |
Dates of case ascertainment |
Type or site of condition |
Number of deaths or cases |
Risk ratio |
95% CI or P value |
|
Hansen (1989a), Denmark, mastic asphalt workersb |
679 |
1959–1986 |
All cancers |
74 |
SIR 1.95c |
1.53–2.44 |
|
Lung cancer |
27 |
SIR 3.44d |
2.27–5.01 |
|||
|
Mouth |
2 |
SIR 11.11d |
1.35–40.14 |
|||
|
Oesophagus |
3 |
SIR 6.98d |
1.44–20.39 |
|||
|
Rectum |
7 |
SIR 3.18d |
1.28–6.56 |
|||
|
Hansen (1991), Denmark, mastic asphalt workerse |
679 |
1959–1986 |
All causes |
148 |
SMR 1.57d |
1.34–1.85 |
|
All cancers |
62 |
SMR 2.29d |
1.75–2.93 |
|||
|
Lung cancer |
25 |
SMR 2.90d |
1.88–4.29 |
|||
|
Non-lung cancer |
37 |
SMR 2.00d |
1.41–2.76 |
|||
|
Bronchitis, emphysema, asthma |
9 |
SMR 2.07d |
0.95–3.93 |
|||
|
Engholm et al. (1991), Sweden, paversf |
2572 |
1971–1985 |
All causes |
96 |
SMR 0.69 |
NR |
|
All cancers |
47 |
SIR 0.86 |
NR |
|||
|
Stomach cancer |
5 |
SMR 2.01 |
NR |
|||
|
Stomach cancer |
6 |
SIR 2.07 |
NR |
|||
|
Lung cancer |
7 |
SMR 1.10 |
NR |
|||
|
Lung cancer |
8 |
SIR 1.24 |
NR |
|||
|
Bender et al. (1989), USA, highway maintenance workersg,h |
4849 |
1945–1984 |
All causes |
1530 |
SMR 0.9 |
0.86–0.96 |
|
All cancers |
274 |
SMR 0.83 |
0.73–0.94 |
|||
|
Lung cancer |
57 |
SMR 0.69 |
0.52–0.90 |
|||
|
Mouth, pharyngeal cancer |
2i |
SMR 11.10 |
1.30–40.10 |
|||
|
Gastrointestinal cancer |
3j |
SMR 5.82 |
1.20–17.00 |
|||
|
Prostate cancer |
11k |
SMR 2.98 |
P < 0.01 |
|||
|
Kidney, bladder, other urinary organ cancers |
7l |
SMR 2.92 |
1.17–6.02 |
|||
|
Leukaemia |
8m |
SMR 4.49 |
1.94–8.84 |
|||
|
Partanen et al. (1997), Finland, road pavers (males only) |
Lung cancer |
NR |
SMR 1.5 |
1.2–1.9 |
||
|
Lung cancer |
NR |
SIR 1.4n |
0.9–1.9 |
|
a |
Abbreviations: CI = confidence interval; NR = not reported; SIR = standardized incidence ratio; SMR = standardized mortality ratio. |
|
b |
Possible exposure to coal tar pitch. Author concluded that smoking unlikely to account for 3-fold excess of cancer; exposure data limited to comparison population were based on Danish general population. |
|
c |
All mastic asphalt workers (n = 679). |
|
d |
Mastic asphalt workers aged 40–89 years (n = 547). |
|
e |
Follow-up of Hansen (1989a); limitations the same as in previous analysis. |
|
f |
Median follow-up for cohort 11.5 years; median age of cohort 42 years. No exposure assessment. Comparison populations for mortality and incidence studies were based on Swedish general population. Mortality and incidence studies did not control for smoking. |
|
g |
Follow-up case–control study found RR = 3 for lung cancer after adjusting for smoking, but limited by small number of cases. |
|
h |
Reference group was male population of Minnesota; quantitative exposure data limited. Highway maintenance workers employed as pavers, landscapers, mowers, garage workers, and office workers. No adjustment for smoking. |
|
i |
Employed >40 years. |
|
j |
Urban workers with 40–49 years of latency. |
|
k |
Started working 1955–1964. |
|
l |
Workers with 40–49 years of latency. |
|
m |
Employed 30–39 years. |
|
n |
Asphalt exposure. |
Cohort mortality and case–control studies of roofers have generally found an excess risk of lung cancer (Tables 11 and 12) (Hammond et al., 1976; Menck & Henderson, 1976; Schoenberg et al., 1987; Zahm et al., 1989; Engholm et al., 1991; Hrubec et al., 1992; Morabia et al., 1992; Pukkala, 1995). In contrast to pavers, the meta-analysis of 20 epidemiological studies by Partanen & Boffetta (1994) demonstrated an overall statistically significant excess of lung cancer among roofers (RR = 1.78, 95% CI = 1.5–2.1). However, it is uncertain to what extent these findings may be attributable to asphalt exposures. In the past, roofers have been exposed to coal tar and asbestos, which are known human lung carcinogens, as well as asphalt. In most studies, information on cigarette smoking was not available for analysis. Hence, while strong epidemiological evidence of an association between lung cancer and work as a roofer exists, it is uncertain whether asphalt or other substances are responsible for these findings.
Table 11: Epidemiological studies of asphalt exposure: cohort studies of diseases in roofers.a
|
Author, country, and occupation |
Number of study subjects |
Dates of case ascertainment |
Type or site of condition |
Number of deaths or cases |
Risk ratio |
95% CI or P value |
|
Hammond et al. (1976), USA, roofer, waterprooferb |
5939 |
1960–1971 |
Lung cancer |
99 |
SMR 1.59c |
NR |
|
Respiratory diseased |
71 |
SMR 1.67 |
NR |
|||
|
Menck & Henderson (1976), USA, rooferb |
2000 |
1968–1970 |
Lung cancer |
3 |
SMR 8.78 |
P > 0.01 |
|
Engholm et al. (1991), Sweden, roofere |
704 |
1971–1985 |
Lung cancer |
3 deaths |
SMR 2.79 |
NR |
|
4 cases |
SIR 3.62 |
NR |
||||
|
3 cases |
RR 6.0f |
NR |
||||
|
Stomach cancer |
5 deaths |
SMR 2.01 |
NR |
|||
|
1 case |
SIR 1.98 |
NR |
||||
|
Lymphatic, haematopoietic cancer |
2 deaths |
SMR 2.68 |
NR |
|||
|
Leukaemia |
1 case |
SIR 2.26 |
NR |
|||
|
Hrubec et al. (1992), USA, roofer, slaterg |
52 |
1954–1980 |
Lung cancer |
4 deaths |
RR 3.0 |
1.30–6.75h |
|
Pukkala (1995), Finland, asphalt roofer |
47 000 |
1971–1985 |
Lung cancer |
18 cases |
SIR 3.25i |
1.92–5.13 |
|
a |
Abbreviations: CI = confidence interval: NR = not reported; RR = relative risk; SIR = standardized incidence ratio; SMR = standardized mortality ratio. |
|
b |
Exposure to coal tar pitch; no adjustment for smoking or quantitative exposure assessment. Classified as roofer based on the usual occupation as recorded on death certificate. |
|
c |
More than 20 years since joining union. |
|
d |
Pneumonia, tuberculosis, influenza excluded. |
|
e |
Median follow-up for cohort 11.5 years; median age of cohort 42 years. No exposure assessment. Reference population was male Swedish population. Mortality and incidence studies did not control for smoking. Follow-up case–control study found RR = 6 after adjusting for smoking, but limited by small number of cases. |
|
f |
Adjusted for smoking. |
|
g |
Adjusted for smoking; occupation, industry of employment obtained through self-reports of 300 000 US Armed Forces veterans who served between 1917 and 1940. Fifty-two veterans reported occupational category as "roofers and slaters." |
|
h |
90% confidence interval. |
|
i |
Adjusted for age, calendar time, and social class. |
Table 12: Epidemiological studies of asphalt exposure: case–control studies of lung cancer in roofers.
|
Author, country, and occupation |
Dates of case ascertainment |
Number of study subjects |
Number of subjects with lung cancer |
Odds ratioa |
95% CIb |
||
|
Cases |
Controls |
Cases |
Controls |
||||
|
Zahm et al. (1989), USA, rooferc |
1980–1985 |
4431 |
11 326 |
6 |
7 |
2.1 |
0.6–8.2 |
|
Schoenberg et al. (1987), USA, roofer, slaterd |
1967–1976 |
763 |
900 |
13 |
8 |
1.7 |
0.7–4.4 |
|
Morabia et al. (1992), USA, roofer, slatere |
1980–1985 |
1793 |
3228 |
7 |
6 |
2.1 |
0.7–6.2 |
|
a |
Adjusted for smoking |
|
b |
CI = confidence interval. |
|
c |
Case identified from Missouri, USA, cancer registry. Matched controls were Missouri residents diagnosed with cancer, excluding cancers of lip, oral cavity, oesophagus, lung, bladder, ill defined and unspecified sites. Occupation was abstracted from cancer registry records. No exposure data available. |
|
d |
Cases were white male residents of six New Jersey, USA, municipalities with high lung cancer rates during 1967–1976. Matched controls were selected either by a random sample of New Jersey drivers’ licence files or through the state mortality files. Occupation was obtained from interviews of next-of-kin. |
|
e |
Cases diagnosed between 1980 and 1989 at 24 hospitals in metropolitan areas of the USA. Controls were matched by age, race, hospital, and admission date and were not admitted for a tobacco-related condition. "Usual occupation" was obtained from interviews of cases and controls. |
In an update of a study by Chiazze et al. (1993) of workers involved in roofing materials manufacturing and asphalt production, Watkins and colleagues (Watkins et al., 2002) conducted a case–control study of lung cancer and non-malignant respiratory disease (NMRD). Cases and controls were identified from among deaths occurring between 1977 and 1997 among active and retired workers. Controls were matched by age, race, and gender. History of smoking (ever/never) was available for approximately 65% of cases and controls. Quantitative data on worker asphalt exposure were not collected until 1977. For each worker, lifetime cumulative exposure to asphalt prior to 1977 was estimated using historical data and industrial hygiene measurements collected from 1977 to 1996. Each worker was assigned three exposure estimates — ever/never and number of years of exposure to asphalt — and two different estimates of cumulative exposure based on the extrapolation of measurements from 1977 to 1996 for the processes in which the workers were employed. The results of the analyses were presented as unadjusted odds ratios (unOR). For lung cancer, the unOR for smoking was 11.32 (95% CI = 1.87–∞), and all unORs for each asphalt exposure scenario exceeded unity. However, only the unOR for smoking was statistically significantly elevated. With the exception of smoking, the unORs for NMRD were generally less than 1. These data suggest that smoking contributes more strongly to lung cancer and NMRD than asphalt exposure. The authors caution that the study is limited by missing data on smoking for one-third of the cases and controls; that work history records were missing for 3 lung cancer cases and 19 lung cancer controls; and that exposure to asbestos, but not coal tar, occurred in both the asphalt and roofing plants. It should also be noted that cases and controls were selected only from among deaths of active and retired employees and not from among any workers ever employed at the facilities. What bias this might add to the study was not explained by the authors.
The largest study to examine health effects of occupational exposure to asphalt included a cohort of 29 820 workers engaged in road paving, asphalt mixing, roofing, waterproofing, or other specified jobs where exposure to asphalt fumes was possible. An underlying purpose of the study was to determine if asphalt (termed bitumen by the authors) was a human carcinogen. Historically, asphalt contained coal tar, a known lung carcinogen; over the past several decades, coal tar has been removed from paving and roofing asphalts. Additionally, this study, a meta-analysis, provided the opportunity to conduct a large study with a robust exposure assessment and sufficient power to detect excesses of lung cancer, if such an excess existed.
Workers were first employed between 1913 and 1999 in asphalt-exposed jobs in seven European countries (Denmark, Finland, France, Germany, Norway, Netherlands, Sweden) and Israel (IARC, 2001; Boffetta et al., 2003a,b). The cohort was divided into job exposure categories: 1) road paving (asphalt paving, surface dressing, mastic asphalt laying, emulsion paving, recycling, and other jobs in road paving; 2) asphalt mixing; 3) unspecified whether road paving or asphalt mixing; 4) waterproofing and roofing; 5) other and unspecified bitumen jobs. A comparison group was composed of 32 245 building and ground construction workers. All subjects were male and worked at least one full season in the target companies. A semiquantitative exposure matrix was constructed for each member of the cohort (Burstyn, 2001). The matrix took into account work history, exposure to bitumen fume, coal tar, and four- to six-ring PAHs, and other job-related exposures using data from industrial hygiene measurements and questionnaires. This study comprehensively analysed mortality using standard lifetable analyses and multivariate Poisson regression analyses to examine confounding effects of other workplace exposures as assessed by the exposure matrix. In total, 1 287 209 person-years of observation were accumulated by the entire cohort, of which 481 089 were from bitumen workers and 537 281 were from the comparison group. By the end of the follow-up period, 10 096 cohort members were deceased, of whom 3987 were bitumen workers and 3876 were building and ground construction workers.
In addition to the analysis of the entire cohort, separate country-specific analyses are available (Bergdahl & Järvholm, 2003; Hooiveld et al., 2003; Kauppinen et al., 2003; Randem et al., 2003a,b; Shaham et al., 2003; Stücker et al., 2003). Analysis by country found differences in site-specific mortality patterns, particularly increases in lung cancer in the German cohort (IARC, 2001); however, here we report only overall mortality and lung cancer mortality by job classification.
Overall mortality for the entire cohort (exposed and non-exposed workers) was below expected (SMR = 0.92, 95% CI = 0.90–0.94) (Boffetta et al., 2003a). For job classifications involving bitumen or asphalt exposure, overall mortality was not statistically significantly elevated (SMR = 0.96, 95% CI = 0.93–0.99); mortality from lung cancer4 among bitumen workers was increased compared with ground and building construction workers (SMR = 1.17, 95% CI = 1.04–1.30) (Table 13). Overall mortality from head and neck cancer was elevated for bitumen workers only (SMR = 1.27, 95% CI = 1.02–1.56). Mortality from other malignant neoplasms was not increased. Further analysis suggested a slight increase in lung cancer mortality among road pavers after adjusting for coal tar pitch and allowing for a 15-year lag (SMR = 1.23, 95% CI = 1.02–1.48) (Boffetta et al., 2003b).
Table 13: IARC epidemiological cohort study of cancer mortality among European asphalt workers by job class.a,b,c,d
|
Cause of death (ICD) |
Bitumen worker (job class 1) |
Road paver (job class 11) |
Asphalt paver (job class 111) |
Asphalt mixer (job class 12) |
Unspecified paver/mixer (job class 13) |
Roofer (job class 14) |
Unspecified bitumen worker (job class 15) |
|
All causes |
0.96e |
0.94 |
0.89 |
0.77 |
0.80 |
0.88 |
1.08 |
|
All malignant neoplasms |
0.95 |
0.96 |
0.95 |
0.66 |
0.73 |
1.21 |
1.01 |
|
Trachea, bronchus, and lung |
1.17 |
1.17 |
1.15 |
1.12 |
1.18 |
1.33 |
1.13 |
|
a |
Adapted from IARC (2001). |
|
b |
Abbreviations: ICD = International Classification of Diseases; IARC = International Agency for Research on Cancer. |
|
c |
All countries. |
|
d |
More than one season of employment. |
|
e |
Standardized mortality ratio. |
|
f |
95% confidence interval. |
|
g |
Observed/expected. |
|
h |
Person-years. |
The investigators (Boffetta et al., 2003b) assessed two different metrics for exposure: average and cumulative exposure. For lung cancer, a positive association was observed for lagged average level of exposure, but not for lagged cumulative exposure. Corresponding indices of unlagged average and cumulative exposure showed a positive dose–response with lung cancer risk based on 63 deaths: RRs were 1.43 (95% CI = 0.87–2.33), 1.77 (0.99–3.19), and 3.53 (1.58–7.89) for 2.2–4.6, 4.7–9.6, and 9.7+ mg/m3 years of cumulative exposure, 2.77 (95% CI = 1.69–4.53), 2.43 (1.38–4.29), and 3.16 (1.83–5.47) for 1.03–1.23, 1.24–1.36, and 1.37+ mg/m3 average exposure (P-value of test for trend, 0.01 for both variables). The investigators concluded that the exposure–response analyses suggest an association between lung cancer mortality and indices of average level of exposure to bitumen fume; however, they could not rule out that confounding played some role in this association.
Studies have reported increased risk of cancer among workers in occupations with the potential for exposures to asphalt (Tables 10, 14–16). Case–control studies of renal pelvis, ureter, and bladder cancers found elevated risk among occupations with reported exposures to asphalt or tar (Jensen et al., 1988; Risch et al., 1988) or petroleum or asphalt (Mommsen et al., 1983) and in road or highway maintenance workers (Bonassi et al., 1989). Isolated studies have reported elevated risk estimates for cancers of the brain (Hansen, 1989b), bladder and other urinary organs (Bender et al., 1989; Hansen 1989b), mouth and pharynx (Bender et al., 1989; Hansen, 1989a), stomach (Engholm et al., 1991), liver (Austin et al., 1987), and other digestive organs (Bender et al., 1989; Hansen, 1989a,b; Siemiatycki, 1991), leukaemia (Bender et al., 1989; Engholm et al., 1991), respiratory cancer (Hansen, 1989b), and lung cancer (Vineis et al., 1988). However, two other case–control studies found no excess of lung cancer (Zahm et al., 1989; Chiazze et al., 1993).
Table 14: Epidemiological studies of asphalt exposure: case–control studies of bladder, renal pelvis, and ureter cancer.a
|
Author, country, and exposure or occupations |
Dates of case ascertainment |
Site |
Number of study subjects |
Number of study subjects with cancer |
Risk ratio |
95% CI |
||
|
Cases |
Controls |
Cases |
Controls |
|||||
|
Mommsen et al. (1983), Denmark, petroleum or asphaltb |
Not given |
Bladder |
212 |
259 |
2 |
3 |
RR 2.36 |
NS |
|
Risch et al. (1988), Canada, asphalt or tarc |
1979–1982 |
Bladder |
739 |
781 |
739 |
781 |
OR 1.44d |
0.78–2.74 |
|
OR 3.11e |
1.19–9.68 |
|||||||
|
OR 2.02f |
1.08–4.97 |
|||||||
|
Bonassi et al. (1989), USA, road mendersg |
Not given |
Bladder |
121 |
342 |
2 |
6 |
OR 1.40 |
0.27–7.28 |
|
Jensen et al. (1988), Denmark, asphalt or tarh |
1979–1982 |
Renal pelvis, ureter |
96 |
294 |
9 |
6 |
RR 5.5 |
1.6–19.6 |
|
a |
Abbreviations: CI = confidence interval; NS = not statistically significant; OR = odds ratio; RR = relative risk. |
|
b |
Cases identified as patients of Department of Oncology and Radiotherapy in Aarhus, Denmark. Controls matched by age, gender, geographic region, and urbanization were identified by the National Registry in Denmark. Occupational exposures and smoking history were obtained for cases and controls by questionnaire. |
|
c |
Cases diagnosed in metropolitan areas of Canada. Population controls matched by age, sex, area of residence. Lifetime occupational history and smoking history were obtained for all cases and controls by questionnaire. Study limited by low participation rate. |
|
d |
Ever exposed to "tar and asphalts" (n = 46). |
|
e |
Exposed during full-time job of at least 6 months 8–28 years before diagnosis (n = 23). |
|
f |
Trend with duration. Odds ratio for trend at 10 years’ duration. |
|
g |
Cases diagnosed in the Bormida Valley, Italy. Population controls selected from demographic registries of the cases matched by age at year of bladder cancer diagnosis and by sex. Interview of subject or next-of-kin obtained data on smoking history and occupation. Jobs were classified into categories with potential for PAH exposure. |
|
h |
Cases diagnosed in eastern Denmark. Hospital-based controls were matched by sex and age and did not have renal diseases or diseases related to smoking. Occupational history and smoking history were obtained on cases and controls through questionnaire. |
Table 15: Epidemiological cohort study of asphalt exposure during manufacture of asphalt products, Denmark.a,b,c,d
|
Number of study subjects |
Type of condition |
Number of deaths or cases |
SMR |
95% CI |
|
|
Exposed |
Unexposed |
||||
|
1320 |
43 024 |
All cancers |
29 |
1.59e |
1.06–2.28 |
|
Digestive cancer |
6 |
1.57 |
0.58–3.43 |
||
|
Respiratory cancer |
11 |
1.52 |
0.76–2.71 |
||
|
Bladder cancer |
3 |
2.91 |
0.60–8.51 |
||
|
Brain cancer |
3 |
5.00 |
1.03–14.61 |
||
|
a |
From Hansen (1989b). |
|
b |
Abbreviations: CI = confidence interval; SMR = standardized mortality ratio. |
|
c |
Case ascertainment was for 1970–1980. |
|
d |
Workers employed at asphalt plants, roofing felt plants, and one tar plant compared with the Danish general population. Study limited by lack of data on length of employment in the asphalt industry and extent of asphalt exposure. Smoking data were not available. |
|
e |
Workers >45 years of age between 1975 and 1980. |
Table 16: Epidemiological studies on asphalt exposure: case–control studies of respiratory cancer and other diseases.a
|
Author, country, and occupation |
Dates of case ascertainment |
Site |
Number of study subjects |
Number of study subjects with disease |
Odds ratio |
95% CI |
||
|
Cases |
Controls |
Cases |
Controls |
|||||
|
Vineis et al. (1988), USA, roofers and asphalt workersb |
1974–1981 |
Lung cancer |
2973 |
3210 |
45 |
37 |
1.4 |
0.9–2.3 |
|
Zahm et al. (1989), USA, pavers, surfacers, materials-moving equipment operatorsc |
1980–1985 |
Lung cancer |
4431 |
11 326 |
32 |
64 |
0.9 |
0.6–1.5 |
|
Chiazze et al. (1993), USAd,e |
Not given |
Lung cancer |
144 |
260 |
111 |
251 |
0.96 |
0.65–1.42 |
|
NMRD |
101 |
183 |
79 |
171 |
1.34 |
0.82–2.2 |
||
|
Austin et al. (1987), USAf,g |
Not given |
Hepatocellular carcinoma |
80 |
146 |
7 |
5 |
3.2 |
0.9–11 |
|
Siemiatycki (1991), Canadah |
Not given |
Colon cancer |
3730 |
533i |
22 |
i |
1.6 |
1.1–2.5 |
|
a |
Abbreviations: CI = confidence interval; NMRD = non-malignant respiratory diseases. |
|
b |
Meta-analysis of lung cancer cases studied in five case–control studies and identified through cancer registries, hospital registries, or admissions or from death certificates. Controls were identified through similar sources as the cases and matched at least by age and gender. Smoking history was obtained for 98% of cases and controls. |
|
c |
Cases identified from Missouri, USA, cancer registry. Matched controls were Missouri residents diagnosed with cancer, excluding cancers of lip, oral cavity, oesophagus, lung, bladder, ill defined and unspecified sites. Occupation was abstracted from cancer registry records. No exposure data were available. |
|
d |
Cases and controls matched by age and survival to end of follow-up or death identified from historical cohort of production and maintenance workers who died while employed or who retired at a fibreglass manufacturing facility that also produced asphalt-coated roofing products. Complete occupational demographics and personal histories including smoking history were obtained by questionnaire for cases and controls. Historical exposure reconstruction was conducted and an estimate of cumulative exposure to asphalt and other products was developed for each cohort member. Potential confounding exposures include respirable silica, talc (fibre contamination), and formaldehyde. |
|
e |
Exposed to asphalt fumes of >0.01 mg/m3 cumulative exposure concentration. |
|
f |
Exposed to asphalt. |
|
g |
Cases diagnosed at five US hospitals. Hospital-based controls were matched on age, sex, race, and hospital location. Occupational history was obtained from cases and controls by interview. Subjects were also asked about exposure to substances on the job, including asphalt. No exposure data were collected. |
|
h |
Cases identified among males aged 35–70 residing in Montreal metropolitan area. Population-based controls were stratified to the age distribution of the cases. Comprehensive interviews obtained detailed occupational history including exposure to chemicals and smoking history from cases and controls. No quantitative exposure data were collected. |
|
i |
Number of controls not available. |
In a meta-analysis of 20 epidemiological studies of workers generally classified as asphalt workers, but not roofers, Partanen & Boffetta (1994) reported increases in risk of bladder cancer (RR = 1.22, 95% CI = 0.95–1.53), stomach cancer (RR = 1.28, 95% CI = 1.03–1.59), and leukaemia (RR = 1.41, 95% CI = 1.05–1.85).
Interpretation of the findings of these studies is limited by a lack of consistency among studies and the potential for confounding by other substances. Furthermore, many of these findings are from studies organized by broad job classifications that are prone to errors in defining asphalt exposures (Mommsen et al., 1983; Jensen et al., 1988; Risch et al., 1988; Bender et al., 1989; Bonassi et al., 1989; Siemiatycki, 1991; Partanen & Boffetta, 1994). Thus, the evidence for an association between exposure to asphalt and cancers is weak and requires further confirmation by studies with better control of confounding variables and better identification of asphalt exposures.
Toraason et al. (2001, 2002) examined DNA strand breaks in leukocytes at the beginning and end of the same work week (4 days later) in seven roofers (all were smokers at time of study) who applied hot asphalt products, but had no coal tar exposure during the preceding 3 months. Using the comet assay, estimates of DNA strand breaks were significantly (P < 0.05) increased at the end of the work week (start of work week 13.6 ± 1.9; end of work week 16.7 ± 1.4). Exposure data related to this study were presented previously in section 6.2.
Fuchs et al. (1996) measured primary DNA damage (strand breaks) and DNA adducts in mononuclear cells of workers exposed to asphalt. These workers included roofers (n = 7), pavers (n = 18), and asphalt painters (n = 9). The control group (n = 34) consisted of students and office workers. All roofers and 10 members of the control group smoked. The roofers studied had significantly greater (P < 0.002) numbers of DNA strand breaks, and these were found to increase during the work week. Because the type of roofing work and materials used were not defined, exposure to coal tar could not be excluded. Pavers and asphalt painters did not differ statistically from controls in the incidence of DNA strand breaks; however, the number of strand breaks was found to increase during the work week in the group of pavers. DNA adducts were found in 10 of 14 samples obtained from pavers and asphalt painters, and DNA adduct concentrations were positively correlated with age and years of exposure. Technical problems prohibited analysis of DNA adducts in other subjects.
Järvholm et al. (1999) examined sister chromatid exchanges and micronuclei in peripheral lymphocytes of non-smoking (non-smokers or ex-smokers who had stopped smoking at least 3 years before the examination) Swedish road pavement workers. The study included 28 non-smoking road pavers and 30 non-smoking referents. Asphalt paving operations were performed in teams that consisted of 4–7 members with different tasks. Although the study showed that the Swedish road pavers have an increased exposure to PAHs from bitumen fumes, no significant increases occurred in the sister chromatid exchanges or micronuclei of exposed workers compared with referents.
Data on the effects of asphalt on other organisms in the laboratory and field are limited. Assessment of quantitative structure–activity relationships (QSAR) relating log Kow values of single hydrocarbons to toxicity indicate that bitumens (asphalts) would not be expected to cause acute toxicity in aquatic organisms (CONCAWE, 2001).
A 56-day laboratory study (Miller et al., 1980) using natural soil media was conducted with Phasiolus vulgaris (bean) seeds or Zea mays (corn) seeds. The bean and corn seeds were exposed to either 4.09 or 20.5 g of asphalt/1.8 kg of soil for 56 days. Results indicated that the asphalt had no effect on the growth of either bean or corn seeds.
Asphalt fumes and vapours cause irritation of the eyes, nose, and respiratory tract in animals and humans. Available data indicate that while laboratory-generated paving and roofing asphalt fume condensates were mutagenic in the Ames Salmonella assay, field-generated paving asphalt fume condensates were not mutagenic. Paving asphalt fumes generated in the laboratory induced DNA adduct formation in vitro and in vivo. A field-generated fume collected from the headspace of an asphalt storage tank was not mutagenic in the Ames assay; however, asphalt fume condensates collected at the top of a paving storage tank caused chromosomal damage when administered intratracheally to rats. Laboratory-generated roofing asphalt fume condensates also induced micronuclei formation and inhibited intracellular communication in mammalian cells. No studies using asphalt fumes generated during roofing operations have been reported.
Data from studies in animals indicated that roofing asphalt fume condensates generated in the laboratory and applied dermally caused benign and malignant skin tumours in several strains of mice. No animal studies have examined the carcinogenic potential of asphalt fumes collected during roofing and paving operations or of laboratory-generated paving asphalt fume condensates. Additionally, several formulations of asphalt-based paints caused benign and malignant skin tumours in mice, but were not mutagenic in the Ames Salmonella assay. Results of several animal studies are conflicting with regard to the carcinogenicity of raw asphalt. Raw asphalt induced a weak carcinogenic response when applied to the skin of mice in one study, while in the other studies it was not carcinogenic.
Although burns due to hot asphalt comprise a small percentage of all reported burns, they are often severe and difficult to treat. Burned areas usually include the extremities (head and neck, arms, hands, and legs); however, in a few cases, the burned areas also include the torso.
Some workers exposed to asphalt fumes during paving operations experienced lower respiratory tract symptoms such as coughing, wheezing, shortness of breath, and changes in pulmonary function. The lowest TP exposure that caused respiratory tract problems was 0.02 mg/m3.
A meta-analysis of epidemiological studies of roofers indicated an excess of lung cancer among them, but it was uncertain whether this excess was related to asphalt fumes and vapours and/or to carcinogens such as coal tar or asbestos or cigarette smoking. Epidemiological studies of pavers exposed to asphalt yielded contradictory results regarding lung cancer. Design limitations and confounders such as smoking and diesel exhaust precluded any strong conclusions regarding an association between lung cancer and working as an asphalt paver. Furthermore, a meta-analysis of these studies failed to find overall evidence for a lung cancer risk among pavers exposed to asphalt fumes and vapours. A few studies reported an association between bladder, renal pelvis, ureter, brain, liver, and other digestive cancers and occupations having potential exposures to asphalt fumes and vapours. However, because of limitations in study design and lack of exposure data, no association can be made at this time between exposure to asphalt fumes and vapours and the induction of these types of cancers.
No human data are available to serve as a basis for characterization of a dose–response relationship between asphalt fume and vapour exposure and the occurrence of either acute or chronic effects. Studies of asphalt pavers and roofers are limited by study design and the inability to account for appropriate confounders, making it difficult to establish a clear dose–response relationship. Available worker exposure data are only suggestive of a possible dose–response relationship between asphalt fume and vapour exposure and the occurrence of acute effects and cannot be extrapolated to the general population at this time.
The extremely limited nature of the available data to serve as a basis for estimation of exposure of the general population should be borne in mind when attempting to determine exposure of the general population to asphalt, asphalt fumes and vapours, and asphalt-based paints. The concentrations of asphalt fractions — polars, aromatics, and saturates — measured in air samples collected 2.0–83.6 m from the highway were 0.54–3.96 × 10–3 mg/m3 air, 1.77–9.50 × 10–4 mg/m3 air, and 0.21–1.23 × 10–4 mg/m3 air, respectively. As noted previously, these values are extremely low in comparison with occupational exposures determined in the various sectors of the asphalt industry; personal exposures to TP and BSP ranged from 0.041 to 4.1 mg/m3 and from 0.05 to 1.26 mg/m3, respectively. However, the chemical composition of the air samples collected along the highway and at the worksites may differ. In addition to respiratory absorption, dermal absorption may also occur and play a pivotal role in asphalt exposure.
The uncertainties identified in this section apply to all types of asphalt, as the commonalities generally outweigh the differences. In weighing the available data that explore the relationship between exposure to asphalt or asphalt fumes and vapours and adverse health effects, it is important to consider them in the context of the overall limitations of the information. These uncertainties may be caused by the basic chemistry of asphalt, which is a mixture, the small number of in vivo studies, the inclusion of coal tar in roofing and paving asphalts in past decades (and the inclusion in some current formulations), and the mixed results of human studies, to name a few. However, these limitations or uncertainties should not preclude a judgement regarding human and environmental health.
The chemical properties of asphalts present a recognized source of uncertainty. Many aspects affect the chemical properties and constituents of the asphalt used for paving, roofing, and other applications. These issues are discussed at length in the source document. Recognized items that affect chemical properties of asphalt include the source of the crude petroleum, manufacturing and refining processes (oxidized versus non-oxidized), modifiers and additives, and application temperature. However, it must be kept in mind that the chemical composition of all types of asphalts is similar in many respects. Elemental analysis indicates that most asphalts contain 79–88 wt% carbon, 7–13 wt% hydrogen, traces to 8 wt% sulfur, 2–8 wt% oxygen, and traces to 3 wt% nitrogen.
The use of results from studies in experimental animals and in vitro assays demonstrates the difficulty in ascribing the occurrence of adverse health effects in humans to exposure to asphalt and asphalt fumes and vapours. While all laboratory-generated roofing and paving asphalt fume condensates were positive for mutagenicity in the Ames Salmonella assay, fumes collected above the headspace of an asphalt storage tank and during paving operations were not. In contrast, the particulate fractions of asphalt fumes collected in the PBZ of workers during paving operations were mutagenic, and intratracheal instillation of field-generated paving asphalt fume condensates in rats caused not only increased micronuclei formation in bone marrow erythrocytes, but also a statistically significant increase in the level and activity of CYP1A1 in the lung. Data on asphalt-based paints are equivocal; while some of the paint formulations exhibited mutagenicity, others did not.
Data on chronic health effects in laboratory studies are limited. Although several studies have examined the carcinogenic potential of laboratory-generated roofing asphalt fume condensates administered dermally and found them to be carcinogenic, there are no studies that have examined the carcinogenic potential of field-generated roofing and paving asphalt fume condensates administered via inhalation, recognized as the primary route of human exposure. None of the above data lends itself to development of a dose–response curve for quantifying an identified end-point in laboratory animals that can be extrapolated to humans.
It is likely that the concern over human exposure to chemical constituents of asphalt or asphalt fumes and vapours originates from the discovery of scrotal cancer among chimney sweeps by Sir Percival Pott in 1775. The exposure of the sweeps was not to asphalt, per se, but to a class of chemicals generated as by-products of combustion of coal and other fossil fuels, namely PAHs. Some of the earlier studies of asphalt exposure examined skin cancers as a possible consequence of exposure. A series of toxicological studies of roofing asphalt, without coal tar, found evidence of carcinogenic potential, whereas the human studies found little evidence of increased risk of skin cancers.
Risk of increased mortality from lung cancer in roofers was assessed as early as the 1970s. Early studies of pavers or roofers are generally less informative because of small study population sizes, insufficient and unspecific data on exposure to bitumen or asphalt, and residual confounding due to co-exposure to coal tar pitch and other lung carcinogens (e.g., asbestos, PAHs, tobacco smoke). Roofers and pavers may also have differing risks. In a meta-analysis of 20 epidemiological studies, the overall mortality risk from lung cancer was statistically significantly increased for roofers, but not for pavers. It is not clear whether these differences are due to differences in the type of asphalt used in roofing or paving operations or to study design issues.
Furthermore, there are no epidemiological studies of workers exposed only to asphalt or asphalt fumes or vapours. The largest and most recent mortality study of a cohort of more than 30 000 workers with exposure to asphalt or asphalt fumes and vapours found slight increases in lung cancer mortality; however, even after controlling for potential coal tar exposure, they could not with certainty attribute a causal relationship to bitumen exposure. Further assessment of the cohort using a case–control design of lung cancer cases may clarify the results.
With respect to acute effects, in a series of studies, workers applying paving asphalt exposed to higher TP and BSP fractions report increased nose, throat, and eye irritation. In addition, case reports of other types of asphalt fume exposure suggest that irritation may be due to the asphalt exposure. In one instance, symptoms resolved within 2 weeks after exposure ceased. One other study of five segments of the asphalt industry reported symptoms among workers, but they were not statistically associated with airborne concentrations of TP or BSP. One might conclude that if there were few symptoms at the concentrations at which the workers in this study were exposed, the reported airborne concentrations could be considered an upper limit for occupational exposures.
The potential for exposure to bitumen/asphalt fumes or vapours varies with the type of asphalt application (e.g., paving or roofing) and the task of the worker. For example, the kettle operator on roofing jobs has been shown to have a higher likelihood of exposure than someone who is applying the roofing material. The kettle operator must, at times, open the kettle to add new asphalt, check viscosity, etc. In some studies, individuals who operate the paving machines generally have higher exposures than other paving workers; in other studies, however, labourers or truck drivers had higher exposures. Furthermore, work practices and types of machinery available to the crew may also increase or decrease potential for exposure. Nevertheless, in the mix of things, these differences may even out over the years as individuals move from one job to another. Although this may be considered an uncertainty, it most likely has a lesser impact on an individual’s overall risk.
It is possible to show, with certainty, that workers exposed to asphalt or asphalt fumes and vapours do indeed take in and metabolize the chemical constituents of asphalt. While the current biomarker methodology is in its infancy and the specificity and sensitivity of the tests need improvement, it is clear that asphalt-exposed workers have exposure through both dermal and respiratory routes. In situations where individuals from the general population live or work near asphalt production facilities or roofing or paving operations, the potential for dermal and/or respiratory exposure to asphalt fumes and vapours exists. The frequency and concentration of these potential exposures may be lower for the general population than for workers. However, in the general population, there are individuals who may be more sensitive to exposures and therefore exhibit more symptoms or other effects. The extent to which these symptoms occur in the general population has not been studied.
Studying the possible health effects attributed to chemical mixtures, including resulting fumes and vapours, is complex. Despite the uncertainties, limitations, and mixed study results, what is clear is that asphalt fume condensates produce malignant skin tumours in mice; and that, when exposed to airborne concentrations of asphalt or asphalt fumes and vapours, workers report symptoms of irritation of the eyes, nose, and throat and, in some, lower airway changes and demonstrate metabolism of the chemical constituents of asphalt fumes and vapours. Taken as a whole, these results suggest that effects do occur in mammalian systems and that the limitations or uncertainties should not preclude taking steps to manage human exposures. Under various performance specifications, it is likely that asphalt fumes and paints contain carcinogenic substances.
The lack of available information precludes adequate assessment of potential risks to environmental organisms.
In 1987, the IARC evaluation on bitumens was as follows: There is inadequate evidence for the carcinogenicity of bitumens to humans. There is limited evidence for the carcinogenicity of undiluted steam-refined and cracking-residue bitumens to experimental animals, inadequate evidence for the carcinogenicity of undiluted air-refined bitumens to experimental animals, and sufficient evidence for the carcinogenicity of extracts of steam-refined and air-refined bitumens to experimental animals. The overall evaluation of carcinogenicity was Group 3: bitumens are not classifiable as to their carcinogenicity to humans (IARC, 1987).
AI (1990a) Report to OSHA and NIOSH: Status of Asphalt Industry Steering Committee research program on the health effects of asphalt fumes and recommendation for a worker health standard. Lexington, KY, The Asphalt Institute.
AI (1990b) Introduction to asphalt, 8th ed. Lexington, KY, The Asphalt Institute (Manual Series No. 5).
AI (2001) 2000 U.S. asphalt usage (short tons). Lexington, KY, The Asphalt Institute.
Almaguer D, Miller AK, Hanley KW (1996) Health hazard evaluation report: Martin Paving Company, Yeehaw Junction, Florida. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 95-0118-2565).
APEC (1999) Comments of the Asphalt Paving Environmental Council on NIOSH’s September 1998 hazard review document: Health effects of occupational exposure to asphalt. The Asphalt Institute and National Asphalt Pavement Association (unpublished).
Apol AG, Okawa MT (1977) Health hazard evaluation report: Certain-Teed Products, Inc., Tacoma, Washington. Cincinnati, OH, US Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health (NIOSH HHE No. 76-55-443; NTIS No. PB-81-143-984/A02).
AREC (1999) Comments of the Asphalt Roofing Environmental Council on NIOSH’s September 1998 hazard review document: Health effects of occupational exposure to asphalt. The Asphalt Institute, Asphalt Roofing Manufacturers’ Association, National Roofing Contractors Association, and Roof Coating Manufacturers’ Association (unpublished).
ATSDR (1998) Toxicological profile for total petroleum hydrocarbons (TPH). Draft for public comment. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, September.
Austin H, Delzell E, Grufferman S, Levine R, Morrison AS, Stolley PD, Cole P (1987) Case–control study of hepatocellular carcinoma, occupation, and chemical exposures. Journal of Occupational Medicine, 29(8):665–669.
Baruchin AM, Schraf S, Rosenberg L, Sagi AA (1997) Hot bitumen burns: 92 hospitalized patients. Burns, 23(5):438–441.
Baylor CH, Weaver NK (1968) A health survey of petroleum asphalt workers. Archives of Environmental Health, 17:210–214.
Belinky BR, Cooper CV, Niemeier RW (1988) Fractionation and analysis of asphalt fumes for carcinogenicity testing. In: Cameron TP, Adamson RH, Blackwood IC, eds. Proceedings of the 4th NCI/EPA/NIOSH Collaborative Workshop: Progress on Joint Environmental and Occupational Cancer Studies. Washington, DC, National Institutes of Health (NIH Publication No. 88-2960).
Bender AP, Parker DL, Johnson RA, Scharber WK, Williams AN, Marbury MC, Mandel JS (1989) Minnesota highway maintenance worker study: cancer mortality. American Journal of Industrial Medicine, 15:545–556.
Bergdahl IA, Järvholm B (2003) Cancer morbidity in Swedish asphalt workers. American Journal of Industrial Medicine, 43:104–108.
Boduszynski MW (1981) Asphaltenes in petroleum asphalts, composition and formation. In: Bunger JW, Li NC, eds. Chemistry of asphaltenes. Washington, DC, American Chemical Society, pp. 119–135.
Boffetta P, Burstyn I, Partanen T, Kromhout H, Svane O, Langård S, Järvholm B, Frentzel-Beyme R, Kauppinen T, Stücker I, Shaham J, Heederik D, Ahrens W, Bergdahl IA, Cenée S, Ferro G, Heikkilä P, Hooiveld M, Johansen C, Randem BG, Schill W (2003a) Cancer mortality among European asphalt workers: an international epidemiological study. I. Results of the analysis based on job titles. American Journal of Industrial Medicine, 43:18–27.
Boffetta P, Burstyn I, Partanen T, Kromhout H, Svane O, Langård S, Järvholm B, Frentzel-Beyme R, Kauppinen T, Stücker I, Shaham J, Heederik D, Ahrens W, Bergdahl IA, Cenée S, Ferro G, Heikkilä P, Hooiveld M, Johansen C, Randem BG, Schill W (2003b) Cancer mortality among European asphalt workers: an international epidemiological study. II. Exposure to bitumen fume and other agents. American Journal of Industrial Medicine, 43:28–39.
Bonassi S, Merlo F, Pearce N, Puntoni R (1989) Bladder cancer and occupational exposure to polycyclic aromatic hydrocarbons. International Journal of Cancer, 44:648–651.
Brandt HCA, DeGroot PC (2001) Aqueous leaching of polycyclic aromatic hydrocarbons from bitumen and asphalt. Water Research, 35(17):4200–4207.
Brantley AS, Townsend TG (1999) Leaching of pollutants from reclaimed asphalt pavement. Environmental Engineering Science, 16(2):105–116.
Budavari S, ed. (1989) The Merck index — Encyclopedia of chemicals, drugs, and biologicals, 11th ed. Rahway, NJ, Merck and Co.
Bull RJ, Robinson M, Munch J, Meier J (1985) Coal tar and asphalt paints [letter to the editor]. Journal of Applied Toxicology, 5(6):423–424.
Burr G, Tepper A, Feng A, Olsen L, Miller A (2002) Health hazard evaluation report: Crumb-rubber modified asphalt paving: occupational exposures and acute health effects. Cincinnati, OH, US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 2001-0536-2864).
Burstyn I (2001) Exposure assessment for a multicentric cohort study of cancer risk among European asphalt workers [PhD thesis]. Utrecht, Utrecht University (ISBN 90-393-2700-9).
Burstyn I, Kromhout H, Kauppinen T, Heikkilä P, Boffetta P (2000) Statistical modelling of the determinants of historical exposure to bitumen and polycyclic aromatic hydrocarbons among paving workers. Annals of Occupational Hygiene, 44(1):43–56.
CEN (2000) Bitumen and bituminous binders — Terminology. Brussels, European Committee for Standardization, 14 pp. (European Standard EN 12597).
Chase RM, Liss GM, Cole DC, Heath B (1994) Toxic health effects including reversible macrothrombocytosis in workers exposed to asphalt fumes. American Journal of Industrial Medicine, 25:279–289.
Chiazze L Jr, Watkins DK, Fryar C, Kozono J (1993) A case–control study of malignant and non-malignant respiratory disease among employees of a fibreglass manufacturing facility. II. Exposure assessment. British Journal of Industrial Medicine, 50:717–725.
CONCAWE (1992) Bitumens and bitumen derivatives. Prepared by CONCAWE’s Petroleum Products and Health Management Groups, Brussels, December. Available at http://www.concawe.be (Product Dossier No. 92/104).
CONCAWE (2001) Environmental classification of petroleum substances — Summary data and rationale. Prepared by CONCAWE Petroleum Products Ecology Group, Brussels, October. Available at http://www.concawe.be (Report No. 01/54).
Cooper SD, Kratz KW (1997) Impact of runoff from asphaltic products on stream communities in California. Marine Science Institute, University of California, Santa Barbara, CA (Government Reports Announcements & Index (GRA&I) Issue 15).
Corbett LW (1975) Reaction variables in the air blowing of asphalt. Industrial and Engineering Chemistry, Process Design and Development, 14(2):181–187.
De Méo M, Genevois C, Brandt H, Laget M, Bartsch H, Castegnaro M (1996) In vitro studies of the genotoxic effects of bitumen and coal-tar fume condensates: comparison of data obtained by mutagenicity testing and DNA analysis by 32P-postlabeling. Chemico-Biological Interactions, 101:73–88.
Emmett EA, Bingham EM, Barkley W (1981) A carcinogenic bioassay of certain roofing materials. American Journal of Industrial Medicine, 2(1):59–64.
Engholm G, Englund A, Linder B (1991) Mortality and cancer incidence in Swedish road paving asphalt workers and roofers. Health and Environment, 1:62–68.
Exxon (1997) Shift study of pulmonary function and symptoms in workers exposed to asphalt fumes. Final report submitted to Asphalt Industry Oversight Committee. East Millstone, NJ, Exxon Biomedical Sciences, Inc. (Report No. 97TP31).
FAA (1991) Hot mix asphalt paving handbook. Washington, DC, US Department of Transportation, Federal Aviation Administration (FAA Advisory Circular No. 150/5370-14).
Fraunhofer (2001) Final report. 13 weeks inhalation toxicity study of bitumen fumes in Wistar (WU) rats. Hanover, Fraunhofer Institute of Toxicology and Aerosol Research (Fraunhofer ITA Study No. 02G01005).
Fries AS, Knudsen L (1990) Bitumen fumes — impacts: review of the scientific results worldwide. Presented at the European Asphalt Producers Association Symposium on Health Aspects of Asphalt Production and Paving, 7–8 June, Stockholm.
Fuchs J, Hengstler JG, Boettler G, Oesch F (1996) Primary DNA damage in peripheral mononuclear blood cells of workers exposed to bitumen-based products. International Archives of Occupational and Environmental Health, 68(3):141–146.
Gamble JF, Nicolich MJ, Barone NJ, Vincent WJ (1999) Exposure–response of asphalt fumes with changes in pulmonary function and symptoms. Scandinavian Journal of Work, Environment and Health, 25(3):186–206.
Genevois C, Brandt HCA, Bartsch H, Obrecht-Pflumio S, Wild CP, Castegnaro M (1996) Formation of DNA adducts in skin, lung, and lymphocytes after skin painting of rats with undiluted bitumen or coal-tar fume condensates. Polycyclic Aromatic Compounds, 8:75–92.
Genevois-Charmeau C, Binet S, Bonnet P, Lafontaine M, Brandt H, Kriech A, DeGroot PC, Wissel H, Garren L, Morele Y, Nunge H, Castegnaro M (2001) Inhalation study on exposure to bitumen fumes: formation of DNA adducts in various rat tissues following nose-only inhalation. Polycyclic Aromatic Compounds, 18:427–449.
Hammond EC, Selikoff IF, Lawther PL, Seidman H (1976) Inhalation of benzpyrene and cancer in man. Annals of the New York Academy of Sciences, 271:116–124.
Hanley KW, Miller AK (1996a) Health hazard evaluation report: Spartan Paving Company, Lansing, Michigan. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 94-0365-2563).
Hanley KW, Miller AK (1996b) Health hazard evaluation report: Granite Construction Company, Sacramento, California. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 94-0408-2564).
Hansen ES (1989a) Cancer incidence in an occupational cohort exposed to bitumen fumes. Scandinavian Journal of Work, Environment and Health, 15(2):101–105.
Hansen ES (1989b) Cancer mortality in the asphalt industry: A ten-year follow up of an occupational cohort. British Journal of Industrial Medicine, 46(8):582–585.
Hansen ES (1991) Mortality of mastic asphalt workers. Scandinavian Journal of Work, Environment and Health, 17:20–24.
Hasle P et al. (1977) Asfaltarbejdeen arbejdsmiljeundersr gelse. Projektrapport fra Roskilde Universitetscenter. Roskilde Universitetsforlag [cited in Fries & Knudsen, 1990].
Hatjian BA, Edwards JW, Williams FM, Harrison J, Blain PG (1995a) Risk assessment of occupational exposure to bitumen fumes in the road paving and roofing industries. Paper presented at the 1995 Pacific Rim Conference on Occupational and Environmental Health, Sydney, Australia, 4–6 October.
Hatjian BA, Edwards JW, Harrison J, Williams FM, Blain PG (1995b) Ambient, biological, and biological effect monitoring of exposure to polycyclic aromatic hydrocarbons (PAHs). Toxicology Letters, 77:271–279.
Hatjian BA, Edwards JW, Williams FM, Harrison J, Blain PG (1997) Risk assessment of occupational exposure to bitumen fumes in the road paving and roofing industries. Journal of Occupational Health and Safety Australia–New Zealand, 13(1):65–78.
Heikkilä P, Riitta R, Hameila M, Hykyri E, Pfaffi P (2002) Occupational exposure to bitumen during road paving. American Industrial Hygiene Association Journal, 63:156–165.
Heikkilä PR, Väänänen V, Hämeilä M, Linnainmas K (2003) Mutagenicity of bitumen and asphalt fumes. Toxicology In Vitro, 17(4):403–412.
Herbert R, Marcus M, Wolff MS, Perara FP, Andrews L, Godbold JH, Rivera M, Stefanidis M, Lu XQ, Landrigan PJ, Santella RM (1990) Detection of adducts of deoxyribonucleic acid in white blood cells of roofers by 32P-postlabeling. Scandinavian Journal of Work, Environment and Health, 16:135–143.
Hicks JB (1995) Asphalt industry cross-sectional exposure assessment study. Applied Occupational and Environmental Hygiene, 10(10):840–848.
Hooiveld M, Spee T, Burstyn I, Kromhout H, Heederik D (2003) Lung cancer mortality in a Dutch cohort of asphalt workers: evaluation of possible confounding by smoking. American Journal of Industrial Medicine, 43:79–87.
Hrubec Z, Blair AE, Rogot E, Vaught J (1992) Mortality risks by occupation among U.S. veterans of known smoking status (1954–1980). Bethesda, MD, US Department of Health and Human Services, Public Health Service, National Institutes of Health (NIH Publication No. 92-3407).
Hueper WC, Payne WW (1960) Carcinogenic studies on petroleum asphalt, cooking oil, and coal tar. Archives of Pathology, 70:372–384.
IARC (1985) Polynuclear aromatic compounds: Bitumens, coal tars, derived products, shale-oils, and soots. Lyon, International Agency for Research on Cancer, pp. 39–81 (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 35).
IARC (1987) Overall evaluations of carcinogenicity: an updating of IARC monographs, Volumes 1 to 42. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl. 7).
IARC (2001) IARC epidemiological study of cancer mortality among European asphalt workers. Lyon, International Agency for Research on Cancer, October (IARC International Report No. 00/003).
IPCS (1982) Selected petroleum products. Geneva, World Health Organization, International Programme on Chemical Safety, pp. 101–111 (Environmental Health Criteria 20).
IPCS (1998) Selected non-heterocyclic polycyclic aromatic hydrocarbons. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 202).
IPCS (2002) Asphalt. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0612).
James NK, Moss ALH (1990) Review of burns caused by bitumen and the problems of its removal. Burns, 16(3):214–216.
Järvholm B, Nordström G, Levin J-O, Wahlström J, Östman C, Bergendahl C (1999) Exposure to polycyclic aromatic hydrocarbons and genotoxic effects on nonsmoking Swedish road pavement workers. Scandinavian Journal of Work, Environment and Health, 25(2):131–136.
Jensen OM, Knudsen JB, McLaughlin JK, Sorensen BL (1988) The Copenhagen case–control study of renal pelvis and ureter cancer: Role of smoking and occupational exposures. International Journal of Cancer, 41(4):557–561.
Kauppinen T, Heikkilä P, Partanen T, Virtanen SV, Pukkala E, Ylöstalo P, Burstyn I, Ferro G, Boffetta P (2003) Mortality and cancer incidence of workers in Finnish road paving companies. American Journal of Industrial Medicine, 43:49–57.
Kebin H, Mosbaek H, Tjell JC (1996) Asphalt fractions in airborne particles from highway traffic and the accumulation in plants. Journal of Environmental Science (China), 8(2):196–202.
Kinnes GM, Miller AK, Burr GA (1996) Health hazard evaluation report: The Sim J. Harris Company, San Diego, California. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 96-0130-2619).
Kriech AJ, Kurek JT (1993) A comparison of field versus laboratory generated asphalt fumes. A report submitted to the NIOSH Docket by the Heritage Research Group, Indianapolis, IN (unpublished).
Kriech AJ, Kurek JT, Wissel HT (1999) Effects of mode of generation on the composition of asphalt fumes. Polycyclic Aromatic Compounds, 14–15:179–188.
Kriech AJ, Kurek JT, Wissel HL, Osborn LV, Blackburn GR (2002a) Evaluation of worker exposure to asphalt paving fumes using traditional and nontraditional techniques. American Industrial Hygiene Association Journal, 63:625–638.
Kriech AJ, Kurek JT, Osborn LV, Wissel HL, Sweeney BJ (2002b) Determination of polycyclic aromatic compounds in asphalt and in corresponding leachate water. Polycyclic Aromatic Compounds, 22(3–4):517–535.
Lee BM, Baoyun Y, Herbert R, Hemminki K, Perera FP, Santella RM (1991) Immunologic measurement of polycyclic aromatic hydrocarbon–albumin adducts in foundry workers and roofers. Scandinavian Journal of Work, Environment and Health, 17:190–194.
Lewis RJ Sr, ed. (1993) Hawley’s condensed chemical dictionary, 12th ed. New York, NY, Van Nostrand Reinhold.
Lunsford RA, Cooper CV (1989) Characterization of petroleum asphalt fume fractions by gas chromatography/mass spectrometry. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health (unpublished).
Ma JYC, Yang H-M, Barger MW, Siegel PD, Zhong B-Z, Kriech AJ, Castranova V (2002) Alteration of pulmonary cytochrome P-450 system: Effects of asphalt fume condensate exposure. Journal of Toxicology and Environmental Health Part A, 65:1247–1260.
Machado ML, Beatty PW, Fetzer JC, Glickman AH, McGinnis EL (1993) Evaluation of the relationship between PAH content and mutagenic activity of fumes from roofing and paving asphalts and coal tar pitch. Fundamental and Applied Toxicology, 21:492–499.
Maintz G, Schneider WD, Maczek P (1987) Chronic obstructive lung disease caused by long lasting occupational exposure to asphalt fumes. Zeitschrift für Erkrankungen der Atmungorgane, 168:71–76.
Menck HR, Henderson BE (1976) Occupational differences in rates of lung cancer. Journal of Occupational Medicine, 18(12):797–801.
Micillino JC, Coulais C, Binet S, Bottini MC, Keith G, Moulin D, Rihn BH (2002) Lack of genotoxicity of bitumen fumes in transgenic mouse lung. Toxicology, 170:11–20.
Miller AK, Burr GA (1996a) Health hazard evaluation report: Koester Equipment Company, Evansville, Indiana. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 95-0307-2602).
Miller AK, Burr GA (1996b) Health hazard evaluation report: Staker Construction Company, Casa Grande, Arizona. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 96-0072-2603).
Miller AK, Burr GA (1998) Health hazard evaluation report: Bardon-Trimount, Stoughton, Massachusetts. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 97-0232-2674).
Miller HC, Barrett WJ, James RH (1982) Investigating potential water contamination by petroleum-asphalt coatings in ductile-iron pipes. Journal of the American Water Works Association, 74:151–156.
Miller RW, Honarvar S, Hunsaker B (1980) Effects of drilling fluids on soils and plants: I. Individual fluid components. Journal of Environmental Quality, 9(4):547–552.
Mommsen S, Aagaard J, Sell A (1983) An epidemiological study of bladder cancer in a predominantly rural district. Scandinavian Journal of Urology and Nephrology, 17(3):307–312.
Morabia A, Markowitz S, Garibaldi K, Wynder EL (1992) Lung cancer and occupation: Results of a multicentre case–control study. British Journal of Industrial Medicine, 49:721–727.
Moschopedis SE, Speight JG (1973) Oxidation of petroleum fractions. Fuel, 52:83.
Neumeister CE, Olsen LD, Dollberg DD ( 2003) Development of a flow-injection fluorescence method for estimation of total polycyclic aromatic compounds in asphalt fumes. American Industrial Hygiene Association Journal, 64:618–624.
Niemeier RW, Thayer PS, Menzies KT, VonThuna P, Moss CE, Burg J (1988) A comparison of the skin carcinogenicity of condensed roofing asphalt and coal tar pitch fumes. In: Cooke M, Dennis AJ, eds. Polynuclear aromatic hydrocarbons: a decade of progress. 10th International Symposium on Polynuclear Aromatic Hydrocarbons. Columbus, OH, Batelle Press, pp. 609–647.
NIOSH (1977) Criteria for a recommended standard: occupational exposure to asphalt fumes. Cincinnati, OH, US Department of Health, Education and Welfare, Public Health Service, Center for Disease Control, National Institute for Occupational Safety and Health (DHEW (NIOSH) Publication No. 78-106; NTIS Publication No. PB-277-333).
NIOSH (1984) NIOSH manual of analytical methods, 3rd ed. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health (DHHS (NIOSH) 84-100).
NIOSH (1994) NIOSH manual of analytical methods, 4th ed. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (DHHS (NIOSH) 94-113).
NIOSH (1998) NIOSH manual of analytical methods. Supplement 2. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication No. 98-119).
NIOSH (2000) Hazard review: health effects of occupational exposure to asphalt. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication No. 2001-110).
Norseth T, Waage J, Dale I (1991) Acute effects and exposure to organic compounds in road maintenance workers exposed to asphalt. American Journal of Industrial Medicine, 20:737–744.
NTP (1990) NTP results report. Results and status information on all NTP chemicals produced from NTP Chemtrack System. Washington, DC, US Department of Health and Human Services, National Institutes of Health, National Toxicology Program.
Nyqvist B (1978) Respiratory tract symptoms of asphalt workers. Another occupational bronchitis? Laekartidningen, 75(12):1173–1175.
Partanen T, Boffetta P (1994) Cancer risk in asphalt workers and roofers: Review and meta-analysis of epidemiologic studies. American Journal of Industrial Medicine, 26:721–740.
Partanen T, Pukkala E, Kauppinen T, Heikkilä P, Ojajärvi A, Boffetta P (1997) Cancer risk in employees in road paving in Finland. 12th International Symposium on Epidemiology in Occupational Health, ISEOH 1997, 16–19 September 1997, Harare (abstract).
Pohlmann G, Koch W, Levsen K, Preib A, Fuhst R, Muhle H, Heinrich U (2001) Sammlung von Kondensat aus Bitumendampf und Erzeugung von Atmosphären zur tierexperimentellen Inhalation. Gefahrstoffe Reinhaltung der Luft, 61(11/12):507–508.
Pukkala E (1995) Cancer risk by social class and occupation. New York, NY, Karger, pp. 12–53.
Puzinauskas VP, Corbett LW (1978) Differences between petroleum asphalt, coal-tar pitch, and road tar. College Park, MD, The Asphalt Institute (Research Report No. 78-1).
Qian HW, Ong T, Whong WZ (1996) Induction of micronuclei in cultured mammalian cells by fume condensates of roofing asphalt. American Journal of Industrial Medicine, 29:554–559.
Qian HW, Whong WZ, Olsen L, Nath J, Ong T (1999) Induction of micronuclei in V79 cells by fractions of roofing asphalt fume condensate. Mutation Research, 441:163–170.
Randem BG, Langärd S, Dale I, Kongerud J, Martinsen JI, Andersen A (2003a) Cancer incidence among male Norwegian asphalt workers. American Journal of Industrial Medicine, 43:88–95.
Randem BG, Langärd S, Kongerud J, Dale I, Burstyn I, Martinsen JI, Andersen A (2003b) Mortality from non-malignant diseases among male Norwegian asphalt workers. American Journal of Industrial Medicine, 43:96–103.
Reinke G, Swanson M (1993) Investigation of the chemical and mutagenic properties of an asphalt fume condensate generated under laboratory and field conditions. Paper presented at Peer Review Meeting on Asphalt, National Institute for Occupational Safety and Health, Cincinnati, OH, December (unpublished).
Reinke G, Swanson M, Paustenbach D, Beach J (2000) Chemical and mutagenic properties of asphalt fume condensates generated under laboratory and field conditions. Mutation Research, 469:41–50.
Risch HA, Burch JD, Miller AB, Hill GB, Steele R, Howe GR (1988) Occupational factors and the incidence of cancer of the bladder in Canada. British Journal of Industrial Medicine, 45(6):361–367.
Roberts FL, Kandhal PS, Brown ER, Lee D-Y, Kennedy TW (1996) Hot mix asphalt materials, mixture design and construction, 2nd ed. Lanham, MD, NAPA Research and Education Foundation.
Robinson M, Bull RJ, Munch J, Meier J (1984) Comparative carcinogenic and mutagenic activity of coal tar and petroleum asphalt paints used in potable water supply systems. Journal of Applied Toxicology, 4(1):49–56.
Sax NI, Lewis RJ, eds. (1987) Hawley’s condensed chemical dictionary, 11th ed. New York, NY, Van Nostrand Reinhold, pp. 102–103, 290, 320.
Schaffer von A, Shafrir A, Suprun H, Calabrezzi R (1985) Ergebnisse der gesundheitsüberwachung von bitumen-asphalt-arbeitern. Arbeitsmedizin, Sozialmedizin, Präventivmedizin, 20(9):205–207 [cited in Fries & Knudsen, 1990].
Schoenberg JB, Stemhagen A, Mason TJ, Patterson J, Bill J, Altman R (1987) Occupation and lung cancer risk among New Jersey white males. Journal of the National Cancer Institute, 79(1):13–21.
Schoket B, Hewer A, Grover PL, Phillips DH (1988a) Covalent binding of components of coal-tar, creosote and bitumen to the DNA of the skin and lungs of mice following topical application. Carcinogenesis, 9(7):1253–1258.
Schoket B, Hewer A, Grover PL, Phillips DH (1988b) Formation of DNA adducts in human skin maintained in short-term organ culture and treated with coal-tar, creosote, or bitumen. International Journal of Cancer, 42(4):622–626.
Shaham J, Knecht Y, Burstyn I, Kromhout H, Ferro G, Partanen T, Boffetta P (2003) Epidemiologic study of cancer mortality among Israeli asphalt workers. American Journal of Industrial Medicine, 43:69–78.
Siemiatycki J, ed. (1991) Risk factors for cancer in the workplace. Boca Raton, FL, CRC Press.
Simmers MH (1964) Petroleum asphalt inhalation by mice: effects of aerosols and smoke on the tracheobronchial tree and lungs. Archives of Environmental Health, 9:727–734.
Sivak A, Menzies K, Beltis K, Worthington J, Ross A, Latta R (1989) Assessment of the cocarcinogenic/promoting activity of asphalt fumes. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health (NIOSH No. 200-83-2612; NTIS Publication No. PB-91-110-213).
Sivak A, Niemeier R, Lynch D, Beltis K, Simon S, Salomon R, Latta R, Belinky B, Menzies K, Lunsford A, Cooper C, Ross A, Bruner R (1997) Skin carcinogenicity of condensed asphalt roofing fumes and their fractions following dermal applications to mice. Cancer Letters, 117:113–123.
Speight JG (1992) Asphalt. In: Kirk-Othmer encyclopedia of chemical technology, 4th ed. Vol. 3. New York, NY, John Wiley & Sons, pp. 689–724.
Stein JS (1980) Construction glossary: an encyclopedic reference and manual. New York, NY, John Wiley & Sons.
Stücker I, Meguellati D, Boffeta P, Cénée S, Margelin D, Hémon D (2003) Cohort mortality study among French asphalt workers. American Journal of Industrial Medicine, 43:58–68.
Sylvain DC, Miller AK (1996) Health hazard evaluation report: Walsh Construction Company, Boston, Massachusetts. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (NIOSH HETA No. 94-0219-2620).
Syracuse Research Corporation (1985) Monograph on human exposure to chemicals in the workplace: Asphalt. Final report prepared for Division of Cancer Etiology, National Cancer Institute, Bethesda, MD, July. Syracuse, NY, Syracuse Research Corporation, Center for Chemical Hazard Assessment (Contract No. 1-CP-26002-03).
Tavris DR, Field L, Brumback CL (1984) Outbreak of illness due to volatilized asphalt coming from a malfunctioning fluorescent lighting fixture. American Journal of Public Health, 74(6):614–615.
Thayer PS, Menzies KT, VonThuna PC (1981) Roofing asphalts, pitch and UVL carcinogenesis. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health, Division of Biomedical and Behavioral Science (NIOSH No. 210-78-0035).
Toraason M, Bohrman JS, Elmore E, Wyatt G, McGregor D, Willington SE, Zajac W (1991) Inhibition of intercellular communication in Chinese hamster V79 cells by fractionated asphalt fume condensates. Journal of Toxicology and Environmental Health, 34:95–102.
Toraason M, Hayden C, Marlow D, Rinehart R, Mathias P, Werren D, DeBord DG, Reid TM (2001) DNA strand breaks, oxidative damage, and 1-OH pyrene in roofers with coal-tar pitch dust and/or asphalt fume exposure. International Archives of Occupational and Environmental Health, 74:396–404.
Toraason M, Hayden C, Marlow D, Rinehart R, Mathias P, Werren D, Olsen LD, Neumeister CE, Mathews ES, Cheever KL, Marlow KL, DeBord DG, Reid TM (2002) DNA strand breaks, oxidative damage, and 1-OH pyrene in roofers with coal-tar pitch dust and/or asphalt fume exposure. International Archives of Occupational and Environmental Health, 75:279.
Vineis P, Thomas T, Hayes RB, Blot WJ, Mason TJ, Rickle LW, Correa P, Fontham ETH, Schoenberg J (1988) Proportion of lung cancers in males, due to occupation, in different areas of the USA. International Journal of Cancer, 42:851–856.
Vo-Dinh T (1989) Significance of chemical analysis of polycyclic aromatic compounds and related biological systems. In: Vo-Dinh T, ed. Chemical analysis of polycyclic aromatic compounds. A series of monographs on analytical chemistry and its applications. Vol. 101. New York, NY, John Wiley & Sons, pp. 1–5.
Waage J, Nielson E (1986) En undersøkelse over løsemiddeleksponering undere asfaltutleggning. Specialopgave. Hordaland vegkontor. Bergen [cited in Fries & Knudsen, 1990].
Watkins DK, Chiazze L Jr, Fryar CD, Fayerweather W (2002) A case control study of lung cancer and non-malignant respiratory disease among employees in asphalt roofing manufacturing and asphalt production. Journal of Occupational and Environmental Medicine, 44(6):551–558.
Wey HE, Breitenstein MJ, Toraason MA (1992) Inhibition of intercellular communication in human keratinocytes by fractionated asphalt fume condensates. Carcinogenesis, 13(6):1047–1050.
Wolff MS, Herbert R, Marcus M, Rivera M, Landrigan PJ (1989) Polycyclic aromatic hydrocarbon (PAH) residues on skin in relation to air levels among roofers. Archives of Environmental Health, 44(3):157–163.
Zahm SH, Brownson RC, Chang JC, Davis JR (1989) Study of lung cancer histologic types, occupation, and smoking in Missouri. American Journal of Industrial Medicine, 15:565–578.
Zeglio P (1950) Changes in the respiratory tract from bitumen vapors. Rassegna di Medicina Industriale, 19:268–273.
NIOSH (2000) Hazard review: health effects of occupational exposure to asphalt. Cincinnati, OH, US Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health (DHHS (NIOSH) Publication No. 2001-110)
The source document may be obtained from:
NIOSH — Publications Dissemination
4676 Columbia Parkway
Cincinnati, OH 45226–1998
USA
Telephone: 1-800-35-NIOSH (1-800-356-4674)
Fax: 513-533-8573
E-mail: pubstaft@cdc.gov
The document is also available online at http://www.cdc.gov/niosh/pdfs/01-110.pdf.
All draft publications are first reviewed by NIOSH scientists who have expertise in the area of interest. Comments are incorporated into the next iteration of the document. Final drafts are reviewed externally by scientists with expertise in the field and representatives of stakeholder industries, labour organizations, government entities, and interested members of the public. Comments and reviews are incorporated into the final text. Subsequent to publication and dissemination, the document is reviewed and approved by the Director of NIOSH.
The document on asphalt was reviewed by:
Asphalt Institute
Asphalt Paving Environmental Council
Asphalt Roofing Environmental Council
Asphalt Roofing Manufacturers’ Association
National Asphalt Pavement Association
National Roofing Contractors’ Association
Roof Coating Manufacturers’ Association
Ernest J. Bastian Jr, Federal Highway, Washington, DC
M.J.J. Castegnaro, International Agency for Research on Cancer, Lyon, France
Gary Foureman, US Environmental Protection Agency, Research Triangle Park, NC
Frank Hanley, International Union of Operating Engineers, Washington, DC
Berj A. Hatjian, University of Balamand, Beirut, Lebanon
Jill Järnberg, National Institute for Working Life, Umea, Sweden
Bengt Järvholm, National Institute for Working Life, Umea, Sweden
William Kojola, Department of Occupational Safety and Health, American Federation of Labor – Congress of Industrial Organizations, Washington, DC
Earl J. Kruse, United Union of Roofers, Waterproofers, and Allied Workers, Washington, DC
Jan-Olof Levin, National Institute for Working Life, Umea, Sweden
Joellen Lewtas, US Environmental Protection Agency, Seattle, WA
David M. Lyall, Eurobitume, Brussels, Belgium
James Melius, Laborers’ Health and Safety Fund of North America, Washington, DC
Timo Partanen, Finnish Institute of Occupational Health, Helsinki, Finland
Richard Rinehart, Harvard School of Public Health, Boston, MA
Max von Devivere, European Asphalt Association, Breukelen, The Netherlands
William Wagner, American Conference of Governmental Industrial Hygienists, Cincinnati, OH
Dave Warshawsky, University of Cincinnati, Cincinnati, OH
Sheila Zahm, National Cancer Institute, Rockville, MD
The draft CICAD on asphalt (bitumen) was sent for review to IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
Asphalt Institute
Asphalt Paving Environmental Council
Asphalt Roofing Environmental Council
European Bitumen Association
International Union of Operating Engineers
Laborers’ Health & Safety Fund of North America
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
B. Boffetta, International Agency for Research on Cancer, Lyons, France
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
I. Desi, Department of Public Health, University of Szeged, Hungary
L. Fishbein, Fairfax, VA, USA
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
P. Heikkilä, Finnish Institute of Occupational Health, Helsinki, Finland
R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
J. Hopkins, Toxicology Advice & Consulting Ltd, Sutton, United Kingdom
P. Howden, Health and Safety Executive, Bootle, Merseyside, United Kingdom
B. Jernström, Karolinska Institute, Stockholm, Sweden
J. Kielhorn, Fraunhofer Institute, Hanover, Germany
J. Ma, National Institute for Occupational Safety and Health, Morgantown, WV, USA
P. Siegel, National Institute for Occupational Safety and Health, Morgantown, WV, USA
J.L. Stauber, Commonwealth Scientific & Industrial Research Organisation, Bangor, NSW, Australia
H.W. Thielmann, Deutsches Krebsforschungszentrum, Heidelberg, Germany
D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, Australia
K. Ziegler-Skylakakis, Commission of the European Communities, Luxembourg
Varna, Bulgaria
8–11 September 2003
Members
Dr I. Benchev, Sofia, Bulgaria
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environment, József Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Hygienic Sciences, Tokyo, Japan
Dr D. Kanungo, Central Insecticides Board, Directorate of Plant Protection, Quarantine & Storage, Ministry of Agriculture, Haryana, India
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
Ms B. Meek, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr T. Morita, Division of Safety Information on Drug, Food and Chemicals, National Institute of Hygienic Sciences, Tokyo, Japan
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr L. Olsen, Biological Monitoring & Health Assessment Branch, Division of Applied Research & Technology, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr N. Rizov, National Center of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Dr P. Schulte, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Sekizawa, Faculty of Integrated Arts and Sciences, Tokushima University, Tokushima, Japan
Dr F. Petrova Simeonova, Sofia, Bulgaria
Dr S. Soliman, Faculty of Agriculture, Alexandria University, El Shatby, Alexandria, Egypt
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, NSW, Australia
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, NSW, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Observers
Dr S. Jacobi, Degussa AG, Fine Chemicals, Hanau-Wolfgang, Germany
Mr M. Southern, Shell International Petroleum Company Ltd, London, United Kingdom
Dr W. ten Berge, DSM, Heerlen, The Netherlands
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
|
1-OHP |
1-hydroxypyrene |
|
AM |
arithmetic mean |
|
B(a)P |
benzo[a]pyrene |
|
BSP |
benzene-soluble particulates |
|
CAS |
Chemical Abstracts Service |
|
CI |
confidence interval |
|
CICAD |
Concise International Chemical Assessment Document |
|
CRM |
crumb-rubber modified asphalt |
|
CYP1A1 |
cytochrome P450, subfamily I (aromatic compound-inducible), polypeptide 1 |
|
DMBA |
dimethyl benzanthracene |
|
DNA |
deoxyribonucleic acid |
|
EHC |
Environmental Health Criteria |
|
EPA |
Environmental Protection Agency (USA) |
|
FEF25–75 |
forced expiratory fraction |
|
FEV1 |
forced expiratory volume in 1 s |
|
FID |
flame ionization detection |
|
FVC |
forced vital capacity |
|
GC |
gas chromatography |
|
GM |
geometric mean |
|
HPLC |
high-performance liquid chromatography |
|
IARC |
International Agency for Research on Cancer |
|
ICD |
International Classification of Diseases |
|
ILO |
International Labour Organization |
|
IPCS |
International Programme on Chemical Safety |
|
Kow |
octanol/water partition coefficient |
|
LOD |
limit of detection |
|
LOQ |
limit of quantification |
|
MS |
mass spectrometry |
|
NIOSH |
National Institute for Occupational Safety and Health (USA) |
|
NMRD |
non-malignant respiratory disease |
|
N-PAC |
polycyclic aromatic compound in which one or more of the carbon atoms in the PAH ring system have been replaced by a heteroatom of nitrogen |
|
NR |
not reported |
|
NS |
not statistically significant |
|
O-PAC |
polycyclic aromatic compound in which one or more of the carbon atoms in the PAH ring system have been replaced by a heteroatom of oxygen |
|
OR |
odds ratio |
|
PAC |
polycyclic aromatic compound |
|
PAH |
polycyclic aromatic hydrocarbon |
|
PBZ |
personal breathing zone |
|
PIM |
Poison Information Monograph |
|
PTFE |
polytetrafluoroethylene |
|
PVC |
polyvinyl chloride |
|
QSAR |
quantitative structure–activity relationship |
|
RR |
relative risk |
|
SD |
standard deviation |
|
SIR |
standardized incidence ratio |
|
SMR |
standardized mortality ratio |
|
S-PAC |
polycyclic aromatic compound in which one or more of the carbon atoms in the PAH ring system have been replaced by a heteroatom of sulfur |
|
TP |
total particulates |
|
TPA |
tetradecanoyl phorbol acetate |
|
TWA |
time-weighted average |
|
UNEP |
United Nations Environment Programme |
|
unOR |
unadjusted odds ratio |
|
USA |
United States of America |
|
US EPA |
United States Environmental Protection Agency |
|
WHO |
World Health Organization |
|
wt% |
weight per cent |
Le présent CICAD consacré à l’asphalte (bitume) s’inspire d’une mise au point préparée par le National Institute for Occupational Safety and Health (NIOSH) des Etats-Unis (NIOSH, 2000). Le dépouillement de références bibliographiques plus récentes arrêté à février 2003 a permis de compléter les données présentées. Des renseignements sur l’examen par des pairs du document original sont donnés à l’appendice 1. L’appendice 2 fournit des informations sur l’examen de ce CICAD par des pairs. Ce CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Varna (Bulgarie) du 8 au 11 septembre 2003. La liste des participants à cette réunion figure à l’appendice 3. La fiche internationale sur la sécurité chimique de l’asphalte (ICSC 0162), établie par le Programme international sur la sécurité chimique (IPCS, 2002), est également reproduite dans le présent document.
L’asphalte (No CAS
Lorsqu’il est chauffé, l’asphalte émet des vapeurs qui se condensent par refroidissement. Ces vapeurs contiennent évidemment une forte proportion des constituants les plus volatils de l’asphalte et on peut s’attendre à ce qu’elles soient chimiquement et toxicologiquement distinctes du matériau initial. Les fumées d’asphalte sont constituées de nuages de petites particules qui se forment par condensation de la phase gazeuse après volatilisation de l’asphalte. Toutefois, comme les constituants de la vapeur ne se condensent pas tous immédiatement, les travailleurs sont exposés non seulement aux fumées mais aussi aux vapeurs. La nature physique de ces fumées et vapeurs n’est pas parfaitement déterminée. Toutefois, l’analyse chimique de l’asphalte oxydé utilisé pour les toitures et celle de l’asphalte non oxydé utilisé pour le revêtement des sols a permis de mettre en évidence un grand nombre de composés appartenant à la même classe. Par ailleurs, l’asphalte pour le revêtement du sol et l’asphalte pour toitures ne subissent pas les mêmes manipulations et cela influe sans doute sur la composition des fumées et des vapeurs. Comme la composition de ces fumées et vapeurs dépend de la température, du procédé de fabrication, de la présence d’additifs et d’agents modificateurs ou encore de la manière de les travailler, il n’est pas surprenant qu’il soit difficile de produire en laboratoire des fumées d’asphalte identiques à celles que l’on retrouve dans l’environnement. Selon les chercheurs, la température, la vitesse d’agitation, et le fait d’aspirer l’air pour capter les fumées au lieu de le chasser sont autant de facteurs qui influent sur la composition des fumées.
On distingue principalement l’asphalte destiné au revêtement des sols et celui qui est utilisée pour les toitures. On utilise également de l’asphalte pour la confection de certaines peintures destinées à servir d’enduits protecteurs contre la corrosion des métaux. On l’emploie aussi pour le revêtement des canaux, des réservoirs d’eau, des barrages et des ouvrages maritimes de protection contre la houle, comme adhésif dans les stratifiés pour isolation électrique et comme base dans le gazon artificiel. Aux Etats-Unis, environ 300 000 personnes travaillent dans des ateliers de fabrication à chaud ou au revêtement des sols; on estime que 50 000 couvreurs utilisent de l’asphalte pour des travaux de toiture et qu’environ 1500 à 2000 personnes travaillent dans quelque 100 ateliers de fabrication de couvertures bituminées. En Europe occidentale, il y a environ 4000 ateliers de production d’asphalte employant chacun de 5 à 10 personnes et environ 100 000 cantonniers travaillent à l’asphaltage des chaussées.
Pour déterminer l’exposition aux fumées d’asphalte, on dispose de diverses méthodes de prélèvement et d’analyse, mais la plupart d’entre elles manquent de spécificité et ne peuvent pas être utilisées pour caractériser l’exposition totale à ces fumées. On a procédé à des prélèvements de liquides biologiques ou à l’exploration de fonctions physiologiques facilement accessibles en vue de mettre en évidence des biomarqueurs de l’exposition aux fumées d’asphalte. En fait, on n’a pas encore trouvé de biomarqueurs qui soient spécifiques de l’exposition aux fumées d’asphalte.
Les données dont on dispose sur la concentration d’asphalte dans les divers compartiments du milieu restent limitées. La mesure de la concentration d’asphalte dans des échantillons d’air et de végétaux prélevés à différentes distances d’une autoroute donne les valeurs respectives suivantes : < 4 × 10–3 mg/m3 dans l’air et < 4 mg/g par de substance végétale sèche. Selon une étude portant sur l’effet des eaux de ruissellement qui se déversent dans les cours d’eau après passage sur des revêtements d’asphalte, la concentration en HAP (hydrocarbures aromatiques polycycliques) dans ces eaux et dans celles de tous les cours d’eau est inférieure à la limite de détection qui est de 0,5 μg/litre. Malgrι la présence de métaux lourds dans l’eau des cours d’eau et dans l’eau de ruissellement, les auteurs de cette étude ont conclu que dans tous les cours d’eau, la différence de teneur en métaux lourds entre les échantillons d’aval et les échantillons d’amont n’était pas significative. On constatait cependant une concentration élevée de métaux lourds dans les échantillons d’eaux de ruissellement par rapport aux valeurs obtenues en amont de ces ruissellements. Ces teneurs élevées en métaux lourds pourraient avoir une autre origine que l’asphalte du revêtement routier (par exemple, les échappements de véhicules à moteur, des fuites d’huile de carter, etc.).
On n’a pas parfaitement caractérisé les concentrations de fumées d’asphalte qui sont susceptibles d’avoir des effets sur la santé, mais les cantonniers qui procèdent au revêtement des chaussées en plein air se plaignent d’irritation au niveau des yeux, du nez et de la gorge. Des études récentes effectuées en milieu professionnel montrent qu’en général, la concentration moyenne pondérée par rapport au temps (TWA) des particules aéroportées totales et des particules solubles dans le benzène vont la plupart du temps de 0,041 à 4,1 mg/m3 et de 0,05 à 1,26 mg/m3, respectivement. La durée moyenne d’exposition individuelle, exprimée par la TWA sur toute la durée d’un poste, est généralement inférieure à 1,0 mg/m3 dans le cas des particules aéroportées totale et à 0,3 mg/m3 dans le cas des particules solubles dans le benzène.
Il peut y avoir absorption de vapeurs et de fumées d’asphalte après inhalation ou exposition cutanée. Comme l’asphalte est un mélange complexe, son comportement pharmacocinétique varie en fonction des propriétés de chacun de ses constituants. Il est donc tout à fait vain de tenter de tirer des conclusions générales quant au taux d’absorption, à la distribution et au métabolisme de ce produit.
Les résultats d’un certain nombre d’études in vitro montrent que les condensats de fumées d’asphalte provenant du revêtement des sols ne sont pas mutagènes et ne conduisent pas à la formation d’adduits avec l’ADN, mais que ces mêmes condensats se révèlent mutagènes et générateurs d’adduits avec l’ADN lorsqu’ils sont produits en laboratoire. Selon une autre étude, en revanche, les fractions particulaires de fumées d’asphalte recueillies dans la zone de respiration individuelle de cantonniers en train de travailler au revêtement d’une chaussée, se sont révélées mutagènes dans le test d’Ames sur Salmonella. De plus, chez des rats exposés par voie intratrachéale à des fumées d’asphalte prélévées sur le terrain lors du revêtement de sols, on a observé une augmentation statistiquement significative du taux et de l’activité de la CYP1A1 (une importante isozyme du cytochrome P450 inductible par les HAP) dans le poumon et un accroissement de la formation de micronoyaux dans les érythrocytes médullaires. Seules les fumées d’asphalte pour toiture produites en laboratoire ont subi des tests de génotoxicité. Ces fumées se sont révélées mutagènes, elles ont provoqué la formation de micronoyaux en quantités accrues et inhibé la communication intercellulaire chez des fibroblastes pulmonaires de hamster chinois (cellules V79) et des kératinocytes épidermiques humains. Les peintures à base d’asphalte ont donné des résultats ambigus. Selon une étude, aucune des peintures examinées n’a présenté d’activité mutagène, tandis que selon une autre, d’autres peintures de ce type ont provoqué la formation d’adduits avec l’ADN dans des échantillons de peau humaine adulte et foetale. Les résultats des études de cancérogénicité indiquent que les condensats de fumées d’asphalte pour toiture obtenus en laboratoire entraînent l’apparition de tumeurs lorsqu’on les applique sur la peau de souris et que certaines peintures à base d’asphalte contiennent des substances chimiques capables de déclencher la formation de tumeurs chez ces animaux. Aucune étude sur l’animal n’a été effectuée en vue de déterminer le pouvoir cancérogène des condensats de fumées d’asphalte pour revêtements de sols produites sur le terrain ou en laboratoire.
Chez les travailleurs des diverses branches de l’industrie de l’asphalte (ateliers de production par malaxage à chaud, terminaux, étanchéification des toitures, asphaltage des sols, fabrication de bardeaux d’asphalte etc.) qui sont exposés à ce produit, on observe des symptômes d’irritation des membranes séreuses de la conjonctive (irritation oculaire) et des muqueuses des voies respiratoires supérieures (irritation du nez et de la gorge) ainsi que de la toux. Les effets sur la santé des travailleurs sont passagers et sans gravité. Des ouvriers employés à des travaux de revêtement des sols, à l’isolation de cables et à la fabrication d’appareillage électrique comme les tubes fluorescents par exemple ont fait état d’autres symptômes tels qu’une irritation cutanée, un prurit, un érythème, des nausées, des douleurs gastriques, une perte d’appétit, des céphalées et de la fatigue. Les résultats d’études récentes indiquent que certains travailleurs employés à l’asphaltage des sols ont présenté des symptômes témoignant d’effets au niveau des voies respiratoires inférieures (par ex. de la toux, une respiration sifflante et de la dyspnée) et d’anomalies de la fonction pulmonaire. Des cas de bronchite ont également été signalés. L’exposition la plus faible aux particules totales qui ait causé des problèmes respiratoire était de 0,02 mg/m3. Toutefois, les données fournies par les études existantes sont insuffisantes pour déterminer s’il y a une relation entre l’exposition aux fumées d’asphalte et les effets indiqués ci-dessus.
Des brûlures peuvent également se produire lors de la manipulation à chaud de l’asphalte. Les territoires cutanés où se produisent ces brûlures se situent généralement au niveau du cou et de la tête, des bras, des mains et des jambes.
La plus vaste étude relative aux effets sanitaires de l’exposition professionnelle à l’asphalte a porté sur une cohorte de 29 820 ouvriers appartenant à huit pays qui étaient employés au revêtement des chaussées, au malaxage de l’asphalte, à la confection de bardeaux, à des travaux d’étanchéification ou à d’autres travaux susceptibles de les exposer à des fumées d’asphalte. La mortalité globale pour l’ensemble de la cohorte (c’est-à-dire les ouvriers exposés et non exposés) s’est révélée inférieure aux prévisions avec un taux comparatif de mortalité (SMR) de 0,92. En ce qui concerne les activités professionnelles comportant une exposition au bitume ou à l’asphalte, la mortalité globale n’était pas très élevée (SMR = 0,96); la mortalité par cancer du poumon était plus élevée chez les ouvriers utilisant du bitume que chez les travailleurs du BTP (bâtiments et travaux publics) (SMR de 1,17 avec un intervalle de confiance à 95 % de 1,04-1,30). La mortalité globale imputable à des cancers de la tête et du cou n’était élevée que chez les ouvriers utilisant du bitume (SMR = 1,27, intervalle de confiance à 95 % = 1,02-1,56). Il n’y avait pas d’accroissement de la mortalité imputable à d’autres types de cancers. Une analyse plus poussée indique une légère augmentation de la mortalité chez les cantonniers après correction pour tenir compte de l’exposition au goudron et en prenant un recul 15 ans (SMR = 1,23, IC à 95 % = 1,02-1,48).
Les chercheurs (Boffetta et al., 2003b) ont évalué deux manières différentes de quantifier l’exposition : l’exposition moyenne ou l’exposition cumulée. Dans le cas du cancer du poumon, ils ont observé une association positive avec le niveau moyen d’exposition avec un certain recul, mais aucune association avec le niveau cumulé avec le même recul. Les indices correspondants d’exposition moyenne et cumulée avec recul ont révélé pour 63 décès l’existence d’une relation dose-réponse avec le risque de cancer du poumon. Le risque relatif (RR) était de 1,43 (IC à 95 % = 0,87-2,33), de 1,77 (0,99-3,19) et de 3,53 (1,58-7,89) respectivement pour 2,2-4,6, 4,7-9,6 et 9,7+ mg/m3 années d’exposition cumulée et 2,77 (IC à 95 % = 1,69-4,53), 2,43 (1,38-4,29) et 3,16 (1,83-5,47) respectivement pour une exposition moyenne de 1,03-1,23, 1,24-1,36 et 1,37+ mg/m3 (valeur de P dans le test de tendance égale à 0,01 pour les deux variables). Les chercheurs ont conclu de leurs analyse exposition-réponse qu’il y avait une association entre la mortalité par cancer du poumon et les indices d’exposition moyenne aux fumées de bitume; toutefois, ils n’excluent pas qu’un facteur de confusion soit pour quelque chose dans l’association constatée.
Une méta-analyse de 20 études épidémiologiques n’a pas pu mettre en évidence un risque de cancer du poumon chez des cantonniers et des personnels d’entretien de chaussées exposés à de l’asphalte (RR = 0,87; IC à 95 % = 0,76-1,08). Toutefois, l’analyse a montré l’existence d’un excès général statistiquement significatif de cancers du poumon chez des couvreurs utilisant de l’asphalte (RR = 1,78, IC à 95 % = 1,5-2,1). Comme ces couvreurs avaient été précédemment exposés à du goudron et à de l’amiante, qui sont notoirement cancérogènes pour l’Homme, on ignore dans quelle mesure ces observations relatives au cancer du poumon sont attribuables à l’asphalte.
Cette même méta-analyse a conclu à une augmentation du risque de cancer de la vessie (RR = 1,22, IC à 95 % = 0,95-1,53), de cancer de l’estomac (RR = 1,28, IC 95% = 1,03-1,59) et de leucémie (RR = 1,41, IC à 95 % = 1,05-1,85) chez des ouvriers généralement classés comme travailleurs utilisant de l’asphalte, mais sans être des couvreurs. L’interprétation des résultats de ces 20 études épidémiologiques est limitée par leur manque de cohérence et par le fait que la présence d’autres substances pourrait constituer un facteur de confusion. De plus, nombre de ces résultats proviennent d’études organisées par grandes classes professionnelles, ce qui peut conduire à des erreurs concernant l’exposition à l’asphalte.
Il ne faut pas perdre de vue le caractère extrêmement limité des données qui servent à estimer l’exposition de la population dans son ensemble lorsqu’on cherche à déterminer l’exposition de cette population à l’asphalte, à ses fumées et à ses vapeurs, ainsi qu’aux peintures à base d’asphalte. La concentration des différentes fractions de l’asphalte - composés aromatiques polaires (polaires), les dérivés aromatiques naphténiques (composés aromatiques) et composés saturés - mesurée dans les échantillons d’air prélevés à 2,0-83,6 m d’une autoroute était respectivement égale à 0,54-3,96 × 10–3 mg/m3, 1,77-9,50 × 10–4 mg/m3 et 0,21-1,23 × 10–4 mg/m3. Ces valeurs sont extrêmement faibles comparativement à celles de l’exposition déterminées dans les diverses branches de l’industrie de l’asphalte; l’exposition du personnel aux particules totales et aux particules solubles dans le benzène vont respectivement de 0,041 à 4,1 mg/m3 et de 0,05 à 1,26 mg/m3. Cela étant, la composition chimique des échantillons d’air prélevés le long de l’autoroute ou sur des sites industriels n’est pas forcément identique. Outre l’absorption par la voie respiratoire, l’absorption cutanée peut également intervenir de façon importante dans l’exposition à l’asphalte.
Il est possible que l’exposition à l’asphalte soit moins fréquente et moins forte pour la population générale que pour les travailleurs. Toutefois, dans cette population, certains individus peuvent être plus sensibles à l’exposition et par conséquent, présenter davantages de symptômes et autres effets. On n’a pas étudié quelle est la proportion de ces symptômes dans la population générale.
Lorsqu’on évalue les données existantes qui permettent d’étudier la relation entre l’exposition à l’asphalte, ses fumées et ses vapeurs et certains effets sanitaires indésirables, il faut garder à l’esprit leurs limites. Ces incertitudes peuvent découler de la chimie de base de l’asphalte - qui est un mélange -, du nombre peu élevé d’études in vivo, du fait que les couvreurs et les cantonniers ont aussi utilisé du goudron au cours des dernières décennies (et utilisent encore des produits qui en contiennent) et enfin, des résultats mitigés fournis par les études sur des sujets humains. Néanmoins, ces limites et ces incertitudes ne devraient pas empêcher d’émettre un jugement au sujet du risque que ce produit représente pour la santé humaine et la salubrité de l’environnement. Il est vraisemblable que les fumées d’asphalte et les peintures à base d’asphalte contiennent des substances cancérogènes selon les diverses spécifications d’utilisation.
Este CICAD sobre el asfalto (betún) se basó en un examen preparado por el Instituto Nacional de Salud y Seguridad en el Trabajo de los Estados Unidos (NIOSH, 2000). Se obtuvieron datos adicionales mediante una búsqueda bibliográfica actualizada hasta febrero de 2003. La información acerca del carácter del examen colegiado del documento original figura en el apéndice 1. La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Varna (Bulgaria) del 8 al 11 de septiembre de 2003. La lista de participantes en esta reunión figura en el apéndice 3. La Ficha internacional de seguridad química sobre el asfalto (ICSC Nş 0162), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2002), también se reproduce en el presente documento.
El asfalto (CAS Nş
Cuando se calienta el asfalto se desprenden vapores, que al enfriarse se condensan. En estos vapores como tales abundan sobre todo los componentes más volátiles presentes en el asfalto y cabría esperar que fueran distintos del material original desde el punto de vista químico y posiblemente desde el toxicológico. Los humos de asfalto son la nube de pequeñas partículas formadas por condensación a partir del estado gaseoso tras la volatilización del asfalto. Sin embargo, dado que no todos los componentes del vapor se condensan al mismo tiempo, los trabajadores no sólo están expuestos a los humos de asfalto, sino también a los vapores. No se ha determinado bien el carácter físico de los humos y los vapores. No obstante, en un análisis químico de los humos de asfalto oxidado para techado y de asfalto no oxidado para pavimentación se identificaron numerosas sustancias químicas del mismo tipo. Además, la manera en la cual se utiliza el asfalto durante las operaciones de pavimentación y de techado probablemente influye en la composición de los humos y los vapores. Habida cuenta de que la composición del asfalto y de sus humos y vapores varía en función de la temperatura, el proceso de fabricación, la presencia de aditivos y modificadores y las prácticas de trabajo, no resulta sorprendente que en el laboratorio sea difícil generar humos de asfalto semejantes a los que se producen en el medio ambiente. Los investigadores han llegado a la conclusión de que la temperatura, la velocidad de agitación y la aspiración frente a la expulsión del aire que se recoge son factores que influyen en la composición química de los humos.
Los principales tipos de productos son el asfalto para pavimentación y para techado. El asfalto también se utiliza en pinturas para revestimientos de protección contra la corrosión de los metales; en el recubrimiento de canales de riego, depósitos de agua, presas y obras de defensa contra el mar; en adhesivos de laminados eléctricos; y como base para la turba sintética. En los Estados Unidos hay unos 300 000 trabajadores en instalaciones que utilizan asfalto en mezcla caliente y en operaciones de pavimentación; se calcula que hay 50 000 trabajadores en actividades de techado con asfalto; y son alrededor de 1500-2000 las personas que trabajan en unas 100 instalaciones de fabricación de techados. En Europa occidental existen unas 4000 instalaciones de mezcla de asfalto, en cada una de las cuales trabajan entre 5 y 10 personas. Alrededor de 100 000 miembros de equipos de pavimentación aplican estas mezclas de asfalto a la superficie de las carreteras en toda Europa occidental.
Aunque se dispone de una serie de métodos de recogida y análisis de muestras para la evaluación de la exposición a los humos de asfalto, la mayor parte de ellos no son específicos y no se pueden utilizar para caracterizar la exposición total a este tipo de humo. También se han obtenido muestras de fluidos corporales y/o funciones fisiológicas fácilmente accesibles o se han analizado en busca de biomarcadores de la exposición a los humos de asfalto. No se han encontrado todavía biomarcadores específicos de dicha exposición.
Se dispone de datos limitados sobre la concentración de asfalto en los distintos compartimentos del medio ambiente. La caracterización de la concentración de fracciones de asfalto en muestras de aire y de plantas recogidas a distintas distancias de una autopista puso de manifiesto que dichas concentraciones eran <4 × 10–3 mg/m3 y <4 mg/g de material vegetal seco, respectivamente. En una evaluación de los efectos de la escorrentía del pavimento de asfalto hacia las corrientes de agua en California (Estados Unidos) se observó que las concentraciones de todos los analitos de hidrocarburos aromáticos policíclicos en todas las muestras de corrientes de agua y escorrentías de carreteras eran inferiores al límite de detección de 0,5 µg/l. Aunque había niveles detectables de metales pesados en el agua de las corrientes y la escorrentía, los autores llegaron a la conclusión de que en ningún caso había diferencias significativas entre corriente arriba y corriente abajo en cuanto a la concentración de ningún metal pesado. La concentración de metales era más elevada en el agua de escorrentía de la superficie de las carreteras que en las muestras tomadas corriente arriba. Estas concentraciones elevadas se podrían derivar de fuentes distintas del asfalto (por ejemplo, emisiones de los vehículos, pérdidas de lubrificante en el cárter, etc.).
Aunque no se han caracterizado bien las concentraciones de humos de asfalto asociadas con efectos en la salud, se han notificado síntomas de irritación ocular, nasal o de la garganta en asfaltadores que trabajaban al aire libre. Los resultados de estudios recientes realizados en el entorno ocupacional indican que, en general, la mayor parte de las concentraciones en el aire como promedio ponderado por el tiempo del total de partículas y de partículas solubles en benceno eran de 0,041 a 4,1 mg/m3 y de 0,05 a 1,26 mg/m3, respectivamente. El promedio de la exposición de las personas, calculado como promedio ponderado por el tiempo en turnos completos, fue en general inferior a 1,0 mg/m3 para el total de partículas y de 0,3 mg/m3 para las partículas solubles en benceno.
Puede haber absorción de humos y vapores de asfalto tras la exposición por inhalación y cutánea. Debido a que el asfalto es una mezcla compleja, su comportamiento farmacocinético variará en función de las propiedades de sus distintos componentes. Por consiguiente, no se pueden hacer generalizaciones con respecto a su grado de absorción, distribución y metabolismo.
Los resultados de varios estudios in vitro indican que, si bien los humos condensados de asfalto para pavimentación generados sobre el terreno no eran mutagénicos ni inducían la formación de aductos de ADN, los obtenidos en el laboratorio producían ambos efectos. En cambio, en un estudio se notificó que las fracciones partículadas de los humos de asfalto recogidos en la zona de respiración de los trabajadores durante las operaciones de pavimentación eran mutagénicas en la valoración de Ames con Salmonella. Además, la exposición intratraqueal de ratas a los humos de la pavimentación con asfalto generados sobre el terreno provocó un aumento estadísticamente significativo del nivel y la actividad de la CYP1A1 (isoenzima importante del citocromo P450 inducible por hidrocarburos aromáticos policíclicos) en el pulmón y una mayor formación de micronúcleos en los eritrocitos de la médula ósea. Solamente se han realizado pruebas de genotoxicidad con humos de asfalto para techado generados en el laboratorio. Se ha demostrado que estos humos son mutagénicos, determinan una mayor formación de micronúcleos e inhiben la comunicación intercelular en los fibroblastos del pulmón del hámster chino (células V79) y en queratinocitos epidérmicos humanos. Se han notificado resultados contradictorios para las pinturas con base de asfalto. Si bien en un estudio ninguna de las pinturas examinadas demostró una actividad mutagénica, en otro estudio otras pinturas indujeron la formación de aductos de ADN en muestras de piel humana de adultos y de efectos. Los resultados de los estudios de carcinogenicidad indicaron que los humos condensados de asfalto para techado generados en el laboratorio provocaban tumores cuando se aplicaban por vía cutánea a ratones y que algunas pinturas con base de asfalto contenían sustancias químicas capaces de inducir tumores en ratones. En ningún estudio con animales se ha examinado el potencial carcinogénico de los humos condensados de asfalto para pavimentación generados sobre el terreno o en el laboratorio.
Los efectos agudos de la exposición al asfalto de los trabajadores de los distintos sectores de esta industria (instalaciones de mezcla en caliente, terminales, aplicación del asfalto para techado, pavimentación, fabricación de asfalto para techado) incluyen síntomas de irritación de las membranas serosas de la conjuntiva (irritación ocular) y de las membranas mucosas de las vías respiratorias superiores (irritación nasal y de la garganta) y tos. Estos efectos en la salud parecen ser de carácter leve y transitorio. Otros síntomas son irritación cutánea, prurito, eritemas, náuseas, dolor de estómago, disminución del apetito, dolor de cabeza y fatiga, según han informado trabajadores que intervenían en operaciones de pavimentación, aislamiento de cables y fabricación de aparatos de luz fluorescente. Los resultados de estudios recientes han indicado que algunos trabajadores que intervenían en operaciones de pavimentación experimentaron síntomas en las vías respiratorias inferiores (por ejemplo tos, sibilancia y disnea) y cambios en la función pulmonar; también se ha notificado bronquitis. La exposición más baja al total de partículas que provocó problemas en las vías respiratorias fue de 0,02 mg/m3. Sin embargo, los datos obtenidos de los estudios disponibles no son suficientes para determinar la relación entre la exposición a los humos de asfalto y los efectos en la salud señalados más arriba.
Se pueden producir quemaduras cuando se maneja asfalto caliente. Las zonas afectadas suelen ser la cabeza y el cuello, los brazos, las manos y las piernas.
El estudio más amplio para examinar los efectos en la salud de la exposición ocupacional al asfalto se realizó con una cohorte de 29 820 trabajadores procedentes de ocho países distintos que participaban en la pavimentación de carreteras, la mezcla de asfaltos, el techado, la impermeabilización u otras tareas específicas en las que era posible la exposición a los humos de asfalto. La mortalidad global en la cohorte completa (trabajadores expuestos y no expuestos) fue inferior a la prevista (razón normalizada de mortalidad [SMR] = 0,92). En la clasificación de los trabajos relacionados con la exposición al betún o el asfalto, la mortalidad global no fue elevada (SMR= 0,96); la mortalidad por cáncer de pulmón fue mayor entre los trabajadores del betún que en los de la construcción de superficie y de edificios (SMR = 1,17, intervalo de confianza [IC] del 95% = 1,04-1,30). La mortalidad global por cáncer de cabeza y cuello fue elevada sólo en los trabajadores del betún (SMR= 1,27, IC del 95% = 1,02-1,56). No se observó aumento de la mortalidad por otros neoplasmas malignos. Análisis ulteriores parecían indicar un ligero aumento de la mortalidad por cáncer de pulmón en los trabajadores de la pavimentación de carreteras tras el ajuste de la brea de alquitrán mineral y dejando transcurrir 15 años (SMR= 1,23; IC del 95%= 1,02-1,48).
Los investigadores (Boffeta et al., 2003b) evaluaron dos sistemas de medición diferentes para la exposición: exposición media y acumulativa. En el caso del cáncer de pulmón, se observó una asociación positiva para un nivel de exposición media diferida, pero no para una exposición acumulativa diferida. Los índices correspondientes de la exposición media y acumulativa no diferidas pusieron de manifiesto una relación dosis-respuesta positiva con riesgo de cáncer de pulmón basada en 63 fallecimientos; los riesgos relativos [RR] fueron 1,43 (IC del 95% = 0,87-2,33), 1,77 (0,99-3,19) y 3,53 (1,58-7,89) para 2,2-4,6, 4,7-9,6 y 9,7+ mg/m3 años de exposición acumulativa y 2,77 (IC del 95% = 1,69-4,53), 2,43 (1,38-4,29) y 3,16 (1,83-5,47) para 1,03-1,23, 1,24-1,36 y 1,37+ mg/m3 de exposición media (valor P de la prueba para la tendencia, 0,01 para ambas variables). Los investigadores llegaron a la conclusión de que el análisis de la relación exposición-respuesta parecía indicar una asociación entre la mortalidad por cáncer de pulmón y los índices del nivel medio de exposición a los humos de betún; sin embargo, no pudieron desechar la posibilidad de que hubiera factores de confusión que desempeñaran alguna función en esta asociación.
En un metaanálisis de 20 estudios epidemiológicos no se logró encontrar una prueba global de riesgo de cáncer de pulmón en los pavimentadores y trabajadores encargados del mantenimiento de las autopistas expuestos al asfalto (RR = 0,87, IC del 95% = 0,76-1,08). Sin embargo, el análisis demostró un exceso global estadísticamente significativo de cáncer de pulmón en los techadores (RR = 1,78, IC del 95% = 1,5-2,1). Dado que en el pasado los techadores han estado expuestos al alquitrán de hulla y al amianto, que son carcinógenos humanos conocidos, es difícil saber en qué medida estos resultados pueden ser atribuibles a la exposición al asfalto.
En el mismo metaanálisis se notificó un aumento del riesgo de cáncer de vejiga (RR = 1,22, IC del 95% = 0,95-1,53), cáncer de estómago (RR = 1,28, IC del 95% = 1,03-1,59) y leucemia (RR = 1,41, IC del 95% = 1,05-1,85) en trabajadores generalmente clasificados como del asfalto, pero no en los techadores. La interpretación de los resultados de estos 20 estudios se ve limitada por la falta de coherencia entre los estudios y el potencial de confusión atribuible a otras sustancias. Además, muchos de estos resultados proceden de estudios organizados para clasificaciones amplias de trabajo, con posibilidad de errores en la definición de la exposición al asfalto.
Hay que tener en cuenta el carácter extremadamente limitado de los datos disponibles que sirven de base para la estimación de la exposición de la población general a la hora de intentar determinar su exposición al asfalto, los humos y vapores de asfalto y las pinturas con base de asfalto. La concentración de las fracciones de asfalto –aromáticos polares (polares), aromáticos naftalénicos (aromáticos) y saturados- medidas en muestras de aire recogidas a 2,0-83,6 metros de distancia de la autopista fueron de 0,54-3,96 × 10–3 mg/m3 de aire, 1,77-9,50 × 10–4 mg/m3 de aire y 0,21-1,23 × 10–4 mg/m3 de aire, respectivamente. Estos valores son enormemente bajos en comparación con las exposiciones ocupacionales determinadas en diversos sectores de la industria del asfalto; la exposición personal a las partículas totales y las partículas solubles en benceno oscilaban entre 0,041 y 4,1 mg/m3 y entre 0,05 y 1,26 mg/m3, respectivamente. Sin embargo, la composición química de las muestras de aire recogidas junto a la autopista y en los lugares de trabajo puede variar. También se puede producir una absorción cutánea, además de la respiratoria, que desempeñe una función esencial en la exposición al asfalto.
La frecuencia y la concentración de la posible exposición al asfalto tal vez sean más bajas para la población general que para los trabajadores. Sin embargo, en la población general hay personas que podrían ser más sensibles a la exposición y por este motivo presentar más síntomas u otros efectos. No se ha estudiado en qué medida se presentan estos síntomas en la población general.
A la hora de ponderar los datos disponibles relativos a la relación entre la exposición al asfalto y sus humos y vapores y los efectos adversos en la salud, es importante tenerlos en cuenta en el marco de las limitaciones globales de la información. Estas incertidumbres pueden deberse a la química básica del asfalto, que es una mezcla, el pequeño número de estudios in vivo, la inclusión de alquitrán de hulla en los asfaltos para techado y pavimentación en decenios pasados (y en algunas formulaciones actuales) y los desiguales resultados de los estudios en personas. Sin embargo, estas limitaciones o incertidumbres no deben impedir un juicio con respecto a la salud de las personas y el medio ambiente. Es probable que los humos y las pinturas de asfalto de diversas especificaciones de rendimiento contengan sustancias carcinogénicas.
ENDNOTES:
See Also:
Toxicological Abbreviations