

This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 66
First draft prepared by Mr P.D. Howe, Dr S. Dobson, and Mr H.M. Malcolm, Centre for Ecology & Hydrology, Monks Wood, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2005
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
2,4,6-Tribromophenol and other simple brominated phenols.
(Concise international chemical assessment document ; 66)
1.Phenols - adverse effects 2.Phenols - toxicity 3.Environmental exposure 4.Risk
assessment I.International Programme on Chemical Safety II.Series
ISBN 92 4 153066 9 (NLM Classification: QV 223)
ISSN 1020-6167
©World Health Organization 2005
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and Swiss Agency for Environment, Forests and Landscape.
Technically and linguistically edited by Marla Sheffer, Ottawa, Canada, and printed by Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD2 on 2,4,6-tribromophenol and other simple brominated phenols was prepared by P.D. Howe, S. Dobson, and H.M. Malcolm from the Centre of Ecology & Hydrology, United Kingdom, and was based on data identified from a comprehensive literature search of relevant databases conducted up to January 2004. The CICAD and a United Kingdom national document were developed concurrently. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was discussed and approved as an international assessment at the 12th Final Review Board meeting, held in Hanoi, Viet Nam, on 28 September – 1 October 2004. Participants at the 12th Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Cards on 2,4,6-tribromophenol (ICSC 1563) and pentabromophenol (ICSC 1564), produced by the International Programme on Chemical Safety (IPCS, 2004a,b), have also been reproduced in this document.
The CICAD deals with 2,4,6-tribromophenol (2,4,6-TBP; CAS No.
Several species of marine algae are known to contain simple brominated phenols. It is known that brominated phenols occur naturally through production by marine benthic animals. Acorn worms (Enteropneusta) produce and excrete large amounts of bromophenols without any obvious dietary source of these compounds. Natural bromophenols, such as 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, are a consistent feature of pristine marine soft-bottom habitats, and their spatial and temporal abundance correlates with the abundance of infauna that produce these metabolites.
Brominated phenol production and use as a reactive flame retardant intermediate or as a wood preservative may result in release to the environment. No data are available on levels in and possible leaching of unreacted brominated phenols from plastics containing fire retardants derived from 2,4,6-TBP.
Estimated vapour pressures indicate that 2,4,6-TBP and PBP will exist in both the vapour and particulate phases in the ambient atmosphere. Vapour-phase brominated phenols are degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-lives for this reaction in air are estimated to be 13 h for 4-BP, 45 h for 2,4-DBP, and between 20 and 40 days for 2,4,6-TBP and PBP. Particulate-phase 2,4,6-TBP and PBP will be removed from the atmosphere by wet and dry deposition.
In water, PBP would be expected to adsorb to suspended solids and sediment. However, other less brominated phenols would tend to remain in the water phase. Volatilization of non-dissociated 2,4,6-TBP and PBP from water surfaces is not expected to be an important fate process. Henry’s law constants for mono- and dibrominated phenols would suggest little volatilization of these compounds.
All of the brominated phenols, if released to soil, essentially stay there and will not be mobile.
Brominated phenols are generally not readily biodegradable and will persist in the environment. However, adapted communities of microorganisms and specialist communities (such as anaerobic or sulfidogenic) may degrade the compounds.
Log Kow values for the bromophenols would give estimates for bioaccumulation potential that increase with increasing bromination. Predicted BCFs of 20, 24, 120, and 3100 for 4-BP, 2,4-DBP, 2,4,6-TBP, and PBP have been calculated. Measured BCFs for 2,4,6-TBP are similar to the predicted value.
Maximum reported concentrations in surface fresh water were 40, 3, and 0.3 µg/litre for 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, respectively; 4-BP has not been detected. Estuarine sediment concentrations range up to 3690 µg/kg dry weight for 2,4,6-TBP. 2,4-DBP and 2,6-DBP were not detected (detection limit 2 µg/kg).
Formation of brominated phenols from the chlorination of natural waters containing bromide ion can result in the production of brominated phenols. Measurements of brominated phenols in drinking-water are restricted to Canada, where the highest reported concentrations in treated water are 42, 60, and 20 ng/litre for 2-BP, 2,6-DBP, and 2,4,6-TBP, respectively, each in a single water sample. Generally, concentrations in drinking-water are less than 3 ng/litre, with levels higher in treated than in raw water.
Air concentrations of brominated phenols have been measured in the environment locally due to the combustion of halogenated waste, peat, and leaded fuel from vehicles; highest reported values were 380 and 4500 ng/m3 for 2,4,6-TBP from combustion of halogenated waste and vehicle fuel combustion, respectively, and 290 ng/m3 for 2,4-DBP from peat combustion.
Workplace air concentrations for 2,4,6-TBP in a production plant ranged from 0.6 to 6.3 mg/m3.
In biota likely to form part of human diets, edible portions contain mean 2,4,6-TBP concentrations up to 198 and 2360 µg/kg dry weight in molluscs and crustaceans, respectively, and up to 39 µg/kg dry weight in marine fish. Brominated phenols have been detected in human milk, blood, and adipose tissue.
2,4,6-TBP is rapidly absorbed from the gastrointestinal tract in mammals and also rapidly excreted via urine and faeces. No information was available on the absorption, distribution, and elimination of other brominated phenols.
Acute oral LD50 values for 2,4,6-TBP in rats ranged from 1486 to >5000 mg/kg body weight. Oral LD50s of 652 and 250–300 mg/kg body weight in rats were reported for 2-BP and PBP, respectively. The acute (4-h) inhalation LC50 in rats for 2,4,6-TBP was >50 000 mg/m3; exposure was to 2,4,6-TBP dust. The acute dermal LD50 values in rats and rabbits were >2000 mg/kg body weight.
2,4,6-TBP was not an irritant to rabbit skin, but was moderately irritating to the rabbit eye. 2,4,6-TBP was a skin sensitizer in guinea-pigs.
A combined repeated-dose oral toxicity study with a reproduction/developmental toxicity screening test on 2,4,6-TBP in rats showed, at 1000 mg/kg body weight per day, reduced body weight gain, increases in absolute and relative liver weights in both sexes, and increases in total protein, albumin, albumin/ globulin ratio and ALP in blood in male rats. At 300 mg/kg body weight per day, salivation was observed in both sexes and an increase in blood creatinine was observed in male rats. The NOAEL was considered to be 100 mg/kg body weight per day in rats of both sexes. No adverse effects were observed on estrous cyclicity, copulation index, fertility index, duration of gestation period, number of corpora lutea, number of implants, total number of pups and live pups, implantation index, or delivery index in any treated group. Neonatal viability on day 4 of lactation and neonatal body weights on days 0 and 4 of lactation in the 1000 mg/kg body weight per day group were lower than those in the control group. No reproductive or developmental effects were seen at 300 mg/kg body weight per day in rats.
No reliable studies were identified for repeated inhalation toxicity.
In vitro reverse mutation studies with 2,4,6-TBP in two types of bacteria were negative. One in vitro chromosomal aberration test was positive with and without metabolic activation. One in vivo micronucleus assay up to the maximum tolerated dose was negative.
2-BP and PBP are both nephrotoxic in rats at high doses, whereas 4-BP shows no nephrotoxicity.
No short-, medium-, or long-term toxicity data were identified for the lower brominated phenols or PBP. Most of the toxicity data relate to 2,4,6-TBP. Exposure of the general population to 2,4,6-TBP would be through drinking-water and the consumption of seafood (the latter from naturally occurring bromophenols). However, since the only reported short-term toxicity study by the oral route is considered a screening test, no reliable tolerable intakes for 2,4,6-TBP can be derived for drinking-water or food.
Seventy-two-hour EC50s in microalgae range from 0.4 to 1.6 mg/litre for 2,4,6-TBP; a 48-h EC50 for 2-BP was 110 mg/litre. Forty-eight-hour LC/EC50s in daphnids range from 0.9 to 6 mg/litre for 2- and 4-BP and from 0.3 to 5.5 mg/litre for 2,4,6-TBP. In chronic studies, 21-day NOECs for daphnid reproduction were 0.2 mg/litre for 2-BP and 0.1 mg/litre for 2,4,6-TBP. Ninety-six-hour LC50s in fish range from 0.2 to 6.8 mg/litre for 2,4,6-TBP. No studies were identified on the toxicity of lower brominated phenols to fish. A 96-h LC50 of 0.1 mg/litre was reported for PBP. For the terrestrial environment, only a single study on the effects of PBP on seed germination was identified.
PNECs of 2 µg/litre for 2-BP, 6 µg/litre for 4-BP, 2 µg/litre for 2,4,6-TBP, and 0.1 µg/litre for PBP have been calculated. It should be noted that there are insufficient or no data available for 3-BP, the dibromophenols, and 2,3,4,6-TeBP. Data on PBP have been included; however, without a full base set of information, the PNEC for PBP should not be used in a risk assessment.
The PEC/PNEC ratio for 2,4,6-TBP would be 0.15 based on a single monitored value in surface fresh waters. Risk factors cannot be calculated for the remaining brominated phenols because of lack of toxicity or exposure data. Only PBP would be predicted to bind preferentially to sediments; however, no measured values have been identified for PBP in sediment. No concentrations of monobrominated phenols in sediment were found; dibrominated phenols were not detected. Therefore, the only data available for sediments are for 2,4,6-TBP. On the basis of this very limited data set, the risk to aquatic organisms from 2,4,6-TBP in sediment would appear to be low. Insufficient data are available to make a meaningful risk assessment for the terrestrial environment.
Table 1 lists relevant physicochemical properties of the brominated phenols. 2-BP is a yellow to red, oily liquid with an unpleasant odour and a relative density of about 1.5 g/cm3. 4-BP is made up of tetragonal bipyramidal crystals with a density of 1.84 g/cm3 at 15 °C and 1.5875 g/cm3 at 80 °C. 2,4,6-TBP is a white to almost white crystalline powder with an acrid odour like that of phenol and a relative density of 2.55 g/cm3 (20 °C) (Merck Index, 2001).
Table 1: Physicochemical properties of bromophenols.a
|
Bromophenol |
Abbreviation |
CAS No. |
Molecular formula |
Relative molecular mass |
Melting point (°C) |
Boiling point |
|
2-Bromophenol |
2-BP |
|
C6H5BrO |
173 |
5.6b |
194c |
|
3-Bromophenol |
3-BP |
|
C6H5BrO |
173 |
33c |
235–236e |
|
4-Bromophenol |
4-BP |
|
C6H5BrO |
173 |
64c |
238e |
|
2,4-Dibromophenol |
2,4-DBP |
|
C6H4Br2O |
251.9 |
38b |
238b |
|
2,5-Dibromophenol |
2,5-DBP |
|
C6H4Br2O |
251.9 |
No data |
No data |
|
2,6-Dibromophenol |
2,6-DBP |
|
C6H4Br2O |
251.9 |
56.5b |
255b |
|
3,5-Dibromophenol |
3,5-DBP |
|
C6H4Br2O |
251.9 |
81b |
274b |
|
2,4,6-Tribromophenol |
2,4,6-TBP |
|
C6H3Br3O |
330.8 |
89k; 93.9l; |
244c; 290k |
|
2,3,4,6-Tetrabromophenol |
2,3,4,6-TeBP |
|
C6H2Br4O |
409.7 |
113.5b |
Sublimesb |
|
Pentabromophenol |
PBP |
|
C6HBr5O |
488.6 |
230b |
Sublimesb |
|
Bromophenol |
Vapour pressure at 25 °C (Pa) |
Aqueous solubility at 25 °C (mg/litre) |
Henry’s law constant (Pa·m3/mol) |
Log octanol/water partition coefficient (Kow) |
Log soil sorption coefficient (Koc) |
Dissociation constant (pKa) |
|
2-Bromophenol |
No data |
No data |
No data |
2.35d,e; 1.69f |
No data |
No data |
|
3-Bromophenol |
No data |
No data |
No data |
2.63d,e; 1.98f |
No data |
No data |
|
4-Bromophenol |
3.7g |
17 400g |
1.56 × 10−2 g |
2.62g; 2.63d; 2.59e; 1.94f |
2.41h; 2.64 |
9.17g |
|
2,4-Dibromophenol |
0.386g |
2080g |
3.65 × 10−2 g |
2.56e; 3.48i,g |
2.86 |
7.79g |
|
2,5-Dibromophenol |
No data |
No data |
No data |
2.56j |
No data |
No data |
|
2,6-Dibromophenol |
No data |
No data |
No data |
2.37f |
No data |
No data |
|
3,5-Dibromophenol |
No data |
No data |
No data |
No data |
No data |
No data |
|
2,4,6-Tribromophenol |
4.2 × 10−2 l; 0.76 × 10−2 m; 2.9 × 10−2 g |
59; 61g |
4.83 × 10−3; 3.59 × 10−3 n; 3.15 × 10−2 g |
4.13o; 3.89l; 4.02p; 4.23q; 3.74f; 4.24g |
3.07 |
6.08g |
|
2,3,4,6-Tetrabromophenol |
No data |
No data |
No data |
No data |
No data |
No data |
|
Pentabromophenol |
5 × 10−5 r |
0.1g |
8.41 × 10−4 n; 1.2 × 10−2 g |
5.30g; 5.96s |
3.53 |
4.4g |
a Where no source is given, values are modelled using EPIWIN.
b Lide (2002).
c Merck Index (2001).
d Jaworska & Schultz (1991).
e Leo et al. (1971).
f Boyle et al. (1992).
g Kuramochi et al. (2004) (at 25 °C).
h Meylan et al. (1992).
i Hansch & Leo (1979).
j Verschueren (1996).
k IUCLID (2003).
l CITI (1999).
m Neely & Blau (1985).
n Meylan & Howard (1991).
o Hansch et al. (1995)
p Broderius et al. (1995).
q Devillers et al. (1996).
r HSDB (2003)
s Meylan & Howard (1995).
Increasing bromination of the phenol decreases both solubility and vapour pressure. Dissociation of bromophenols varies with pH. Table 2 shows the degree of dissociation for each bromination level at a range of environmental pHs.
Table 2: Percentage dissociation of bromophenols at different environmental pH.
|
Bromophenol |
pKa |
Percentage dissociation |
||||
|
pH 5 |
pH 6 |
pH 7 |
pH 8 |
pH 9 |
||
|
4-BP |
9.17 |
0 |
0 |
1 |
6 |
40 |
|
2,4-DBP |
7.79 |
0 |
2 |
14 |
62 |
94 |
|
2,4,6-TBP |
6.08 |
8 |
45 |
89 |
99 |
100 |
|
PBP |
4.4 |
80 |
98 |
100 |
100 |
100 |
Tendency to bind to soil/sediment increases with bromination of the phenol. While the degree of dissociation would be expected to increase the solubility of the compounds, calculated binding to soil/sediment is insensitive to solubility and, therefore, pH.
Analysis of environmental media for brominated phenols is predominantly performed by GC-MS with ECD or SIM. Brominated phenols have been monitored in air samples using GC-MS with ECD (Müller & Buser, 1986; Thomsen et al., 2001b).
A range of brominated phenols was detected in raw and potable water using GC-MS with SIM mode; a detection limit of 1 ng/litre for 2-BP, 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP was reported (Sithole et al., 1986). Higher detection limits for 2-BP, 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP were reported using GC-ECD (Sithole et al., 1986). Watanabe et al. (1984) used GC-MS with ECD for analysing PBP in wastewater, with a detection limit of 1 ng/litre. Chatonnet et al. (2004) used GC-MS with SIM to analyse wines for 2,4,6-TBP, with a detection limit of 1.5 ng/litre.
GC-MS with ECD has been used to analyse sediment samples, with detection limits of 2 µg/kg for 2,4-DBP and 2,6-DBP and 0.5 µg/kg for 2,4,6-TBP (Watanabe et al., 1985). Fielman et al. (2001) used GC-MS with SIM mode to analyse for a variety of brominated phenols in sediment.
Brominated phenols have been detected in a variety of biological samples. GC-MS with electron ionization mode has been utilized to analyse biota samples, with a detection limit of 0.05 ng/g (Whitfield et al., 1992, 1995). Similarly, GC-MS with SIM mode has also been used (Adams et al., 1999; Flodin et al., 1999). A detection limit of 0.01 ng/g has been reported using GC-MS with multiple ion detection mode (Whitfield et al., 2002). The brominated phenols were extracted from plasma using solid-phase extraction; the plasma lipids were decomposed by treatment with concentrated sulfuric acid directly on the solid-phase extraction column, prior to the elution of the brominated phenols. Following diazo methane derivatization, the samples were determined by GC-MS with ECD, with a detection limit of 0.3 pg/g for 2,4,6-TBP and PBP (Thomsen et al., 2001a, 2002a).
The natural production of brominated organic chemicals is most abundant and diverse in the marine environment, where the precursors for their biosynthesis are readily available. Mono-, di-, and tribrominated phenols, in particular, are excreted by marine organisms such as algae, polychaetes, and hemichordates. Natural production of brominated phenols does not appear to occur in fresh waters. Many brominated organics are found in terrestrial ecosystems, in plants, bacteria, fungi, lichens, insects, and some higher animals; however, no information on the production of brominated phenols has been reported (Gribble, 2000).
Several species of marine algae are known to contain simple brominated phenols (Whitfield et al., 1999; Flodin & Whitfield, 2000). Biosynthesis of brominated phenols in marine algae has been observed (Flodin & Whitfield, 1999).
It is known that brominated phenols occur naturally through production by marine benthic animals. Acorn worms (Enteropneusta) produce and excrete large amounts of bromophenols without any obvious dietary source of these compounds (Higa et al., 1980). Bromophenols from natural sources, such as 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, are a consistent feature of pristine marine soft-bottom habitats, and their spatial and temporal abundance correlates with the abundance of infauna that produce these metabolites (Fielman et al., 2001). Marine sponges are natural sources of brominated organic compounds, including bromoindoles, bromophenols, and bromopyrroles, which may comprise up to 12% of the sponge dry weight (Ahn et al., 2003). Bromophenols are known as secondary metabolites in various Enteropneusta (King, 1986; Woodin et al., 1987), in Phoronida, Phoronopsis viridis (Sheikh & Djerassi, 1975), and in Polychaeta, Lanice conchilega and Arenicola cristata (Weber & Ernst, 1978; Woodin et al., 1987; Goerke & Weber, 1991). It has been reported that bromophenols can exhibit antimicrobial activity and are possibly of antiseptic importance for wound healing in bottom-living species (Sheikh & Djerassi, 1975). Jensen et al. (1992) found depletion of benthic metazoan fauna in the burrow wall lining of the deep sea enteropneust Stereobalanus canadensis and suggested that this was due to the presence of brominated metabolites excreted by the enteropneust. However, the effects of bromophenols on microbes vary depending on the group, process, or parameter examined (King, 1986, 1988). Giray & King (1997a) found that the differing potencies of bromophenol isomers as microbe inhibitors and differences in bromophenol excretion must be taken into account in comparisons of burrow wall biogeochemistry among the bromophenol-containing benthic fauna. However, Steward et al. (1996) found no significant impact of biogenic bromophenols on microbial biomass, activities, or community structure in sediments lining burrows of three species of marine worms. Surface and subsurface sediment communities were similarly unaffected (Steward et al., 1992). In further studies, 2,4-DBP was found not to be an effective antipredatory agent against hermit crabs and some predatory polychaetes (Giray & King, 1997b).
A unique flavin-containing chloroperoxidase, which can halogenate a wide variety of aromatic compounds, including phenol, to produce 4-BP, 2,4-DBP, and 2,4,6-TBP, has been isolated from the capitellid polychaete Notomastus lobatus (Chen et al., 1991).
Brominated phenols can be formed from the biodegradation of other pollutants, such as brominated benzenes and some brominated diphenyl ethers (Bergman, 1990). Further, brominated anisoles of both biological and anthropogenic origin can be demethylated to some extent under anaerobic conditions to the corresponding brominated phenols.
The production and use of 2,4,6-TBP as a reactive flame retardant intermediate or as a wood preservative may result in its release to the environment through various waste streams (HSDB, 2003). No data are available on levels in and possible leaching of unreacted brominated phenols from plastics containing fire retardants derived from 2,4,6-TBP. Imported treated wood is a possible source of exposure to 2,4,6-TBP for both organisms in the environment and humans handling the wood. Growing boxes for indoor crops also provide a potential source of exposure via contamination of the crop; no data are available.
2-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP have all been identified in vehicle emissions of leaded petrol (Müller & Buser, 1986). The raw flue gas from a hazardous waste incinerator fed brominated waste, municipal waste, or peat contained 2,4,6-TBP (Öberg et al., 1987).
Bromophenols can be formed as by-products during food processing and water treatment. Contact with weak halogen solutions can be made on cleansing raw materials and food processing lines and on dilution of juice concentrates. Chlorine is employed most often, but occasional use is made of solid "bromine donors," such as bromochlorodimethyl hydantoin, which hydrolyse to hypobromous acid, which can then come into contact with food (Adams et al., 1999). Bromophenols have very low sensory threshold values and have been found to cause "disinfectant" taints at nanogram per kilogram levels in fish products (Whitfield et al., 1988; Boyle et al., 1992). Bromophenols can be formed during the chlorination of natural water and wastewater containing phenol and bromide ions (Sweetman & Simmons, 1980; Watanabe et al., 1984). For example, the formation of 2,4,6-TBP has resulted from the chlorination of water containing phenol and bromine at pH 7.4. Direct bromination with hypobromous acid was compared with bromination by hypochlorous acid and bromide ion. Under conditions when hypochlorous acid was not limiting, a higher yield of bromine substitution products could be expected from the bromination by hypochlorous acid plus bromine than from direct bromination by hypobromous acid (Sweetman & Simmons, 1980). In power plants using seawater as a source of cooling water, Bean et al. (1983) identified monobrominated phenols, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP. The highest concentration reported was 0.15 µg/litre for 2,4,6-TBP. In a power plant drawing water from a reservoir on the Arkansas River, USA, and having a chlorine contact time of several hours, Grove et al. (1985) found dibromophenols and 2,4,6-TBP, each at concentrations of 0.4 µg/litre or less. The formation of both 2-BP and 4-BP has been demonstrated during the treatment of final effluent using peracetic acid (Booth & Lester, 1995).
A "plastic" or "chemical" taint has been reported in some drinking-waters, and this was attributed to the formation of 2,6-DBP (taste threshold 0.5 ng/litre). It was established that the relative ratios of phenol, bromide, and chlorine and pH were important determinants of whether the taste would or would not form and that primary sources of phenol are plastic appliances, especially kettles and refrigerators (Heitz et al., 2001). Further, it has been shown that organic and inorganic nitrogen-containing compounds, especially amines, have a significant retardant effect on the halogenation of phenols (Heitz et al., 2002).
Cork taint is a musty or mouldy off-odour in wine usually associated with 2,4,6-trichloroanisole, which is formed by the O methylation of 2,4,6-trichlorophenol by a variety of fungal species (Álvarez-Rodríguez et al., 2002). However, some wines have exactly the same type of tasting fault, but without sufficient quantities of chloroanisoles to account for the "musty" character. A "musty" off-odour was perceptible on smelling wine containing tribromoanisole produced by the O methylation of 2,4,6-TBP at concentrations as low as 4 ng/litre. Potential sources of 2,4,6-TBP include wooden or wood-based materials impregnated or surface-treated with 2,4,6-TBP that are in direct or indirect contact with wine (Chatonnet et al., 2004).
2,4,6-TBP is by far the most widely produced brominated phenol. The production volume of 2,4,6-TBP was estimated at approximately 2500 tonnes/year in Japan and 9500 tonnes/year worldwide in 2001 (IUCLID, 2003).
2,4-DBP has been produced, but at much lower volumes than 2,4,6-TBP. 4-BP, 2,4-DBP, and PBP have all been manufactured in the past by Bromine Compounds Ltd but are not manufactured by this company at present (DSBG/BCL, personal communication, 2004).
2,4,6-TBP is produced in closed reactors by a non-aqueous process and discharged as a melt, which is cooled and pelleted for easy handling (Weil, 1993). PBP is manufactured by the reaction of 2,4,6-TBP with anhydrous bromine in the presence of ferric bromide as a catalyst (HSDB, 2003).
2,4,6-TBP is not used directly as a flame retardant, but rather as an intermediate for such products as endstop for brominated epoxy resin made from tetrabromobisphenol A (probably the largest application), tribromophenyl allyl ether, and 1,2-bis(2,4,6-tribromophenoxyethane) (Weil, 1993). The latter is prepared by the reaction of 2,4,6-TBP and ethylene in the presence of a base. It is the second most prevalent flame retardant used in acrylonitrile-butadiene-styrene resins (Weil, 1993). 2,4,6-TBP is reacted with sodium hydroxide to form the salt sodium tribromophenol in water, which is used as a wood preservative. Standard application methods of pressure and vacuum impregnation, dipping, brushing, and spraying of the wood are used. The solution is very effective in controlling insects, fungi, and bacteria in construction lumber, plywood timbers, railroad ties, fence posts, utility poles, landscape materials, and foundation materials (DSBG/BCL, personal communication, 2004). 2,4,6-TBP is registered as a wood preservative in South America; for example, the current pesticide register for Chile reveals that three products based on the sodium tribromophenol salt are approved for use as a fungicide treatment (two manufacturers in Chile and one in Brazil). However, it is not registered in the EU or USA and is not known to be registered in other parts of the world (DSBG/BCL, personal communication, 2004).
PBP has been reportedly used as a chemical intermediate for pentabromophenoxy compounds (HSDB, 2003). It has also been reported to have been used as a molluscicide (Clayton & Clayton, 1993). Although there are indications of the efficacy of PBP as a biocide (similar to pentachlorophenol), there are no records of its registration as a biocide in Europe or the USA (DSBG/BCL, personal communication, 2004).
2,4-DBP has been used as a reactive intermediate in an epoxy-phenolic polymer (DSBG/BCL, personal communication, 2004).
According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, both 2,4,6-TBP and PBP, which have estimated vapour pressures of 4 × 10−2 Pa (at 25 °C) and 5 × 10−5 Pa (at 25 °C), respectively, determined from a fragment constant method, will exist in both the vapour and particulate phases in the ambient atmosphere (Lyman, 1985; Bidleman, 1988). Particulate-phase 2,4,6-TBP and PBP will be removed from the atmosphere by wet and dry deposition (HSDB, 2003).
Volatilization of non-dissociated 2,4,6-TBP and PBP from water surfaces is not expected to be an important fate process, given estimated Henry’s law constants of 3.6 × 10−3 Pa·m3/mol and 8.4 × 10−4 Pa·m3/mol, respectively, estimated using a fragment constant method (Lyman et al., 1990; Meylan & Howard, 1991; HSDB, 2003). Comparable Henry’s law constants for mono- and dibrominated phenols (see Table 1) would also suggest little volatilization of these compounds.
Modelling brominated phenols using the PCKOC model (v.1.66)3 shows increasing soil sorption coefficients with increasing bromination (Table 1). Estimated Koc is not sensitive to dissociation of phenols at different pHs. Anions generally do not adsorb to organic carbon and clay more strongly than their neutral counterparts.
Mackay Level III fugacity modelling (v.2.70; Canadian Environmental Modelling Centre, 2002) predicts the environmental distribution of the brominated phenols as shown in Table 3.
Table 3: Distribution of brominated phenols estimated by the Mackay Level III fugacity model.
|
Medium |
Percent distribution |
|||
|
4-BP |
2,4-DBP |
2,4,6-TBP |
PBP |
|
|
Release to water |
||||
|
Air |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
|
Water |
99.7 |
97.3 |
91.7 |
6.8 |
|
Soil |
0.02 |
0.03 |
0.03 |
0.06 |
|
Sediment |
0.3 |
2.7 |
8.3 |
93.2 |
|
Release to soil |
||||
|
Air |
<0.01 |
<0.01 |
<0.01 |
<0.01 |
|
Water |
5.9 |
0.9 |
0.4 |
0.06 |
|
Soil |
94.1 |
99 |
99.6 |
99.9 |
|
Sediment |
0.02 |
0.03 |
0.04 |
0.09 |
|
Release to air |
||||
|
Air |
2.5 |
2.9 |
1.1 |
2.6 |
|
Water |
7.8 |
2.9 |
2.0 |
0.3 |
|
Soil |
89.7 |
94.1 |
96.7 |
93.5 |
|
Sediment |
0.02 |
0.08 |
0.2 |
3.7 |
If released to soil, all of the brominated phenols essentially stay there and will not be mobile. Release into water leaves significant proportions of the lower brominated phenols in water, although PBP partitions almost entirely to sediment. Release to air leads to almost complete partitioning to soil.
Vapour-phase 2,4,6-TBP and PBP are degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-lives for this reaction in air were estimated to be 34 days and 36 days, respectively, calculated from rate constants of 4.8 × 10−13 cm3/molecule per second (at 25 °C) and 4.5 × 10−13 cm3/molecule per second (at 25 °C), respectively, and determined using a structure estimation method (Meylan & Howard, 1993; HSDB, 2003). Applying the AOP model (v.1.91), which uses comparable methodology, to a range of brominated phenols yields atmospheric half-lives of 13.2 h, 44.6 h, 22.5 days, and 23 days for 4-BP, 2,4-DBP, 2,4,6-TBP, and PBP, respectively.
Direct photolysis by UV light indicated a half-life for 2,4,6-TBP of 4.6 h (VCC, 1978a); however, this is not expected to be a significant route of degradation, since the brominated phenols partition predominantly to soil/sediment, where UV levels are likely to be low.
2,4,6-TBP is not expected to undergo hydrolysis in the environment due to the lack of hydrolysable functional groups (Lyman et al., 1990). Abiotically, 2,4,6-TBP is considered to be stable in water and not hydrolysed, regardless of pH (CITI, 1999).
Brominated phenols are generally not readily biodegradable and will persist in the environment. However, adapted communities of microorganisms and specialist communities (such as anaerobic or sulfidogenic) may degrade the compounds.
In 3-day biodegradation tests, 2-BP (1 mg/litre) was degraded by 2% and 3% in river water and seawater, respectively, whereas 2,4,6-TBP (10 mg/litre) was degraded by 82% in river water and by 9% in seawater (Kondo et al., 1988). 2,4,6-TBP, present at 100 mg/litre, reached 49% of its theoretical biochemical oxygen demand in 28 days using an activated sludge inoculum at 30 mg/litre in the Japanese MITI test, a result that fails the criterion for ready biodegradability (CITI, 1992). 2,4,6-TBP was not degraded over 14 days in a marine sediment slurry prepared from surface sediment (depth <1 cm) with equivalent amounts of filtered seawater (King, 1988). Percentage ring degradation by a soil inoculum of 100 mg of unspecified monobromophenol, 2,4-DBP, and PBP per litre was 25% (4 days), 81% (4 days), and 93% (1 day), respectively. No ring degradation was reported for 2,4,6-TBP over a 5-day period (Ingols et al., 1966). Water samples collected from two treatment ponds were unable to degrade 2,4,6-TBP over 32 days (VCC, 1990). However, 2,4,6-TBP has been reported to dehalogenate rapidly in anaerobic sediments, with a half-life of approximately 4 days (Peijnenburg et al., 1992).
Ronen et al. (2000) isolated a bacterium (Achromobacter piechaudii) from desert soil (from the northern Negev, Israel) contaminated with chemical industry waste that was capable of reductively dehalogenating 2,4,6-TBP to phenol, which was further metabolized under anaerobic conditions. The bacterium was unable to metabolize mono- or dibromophenols (Ronen & Abeliovich, 2000; Ronen et al., 2000).
Steward & Lovell (1997) found 4-BP to be a readily utilizable substrate for bacteria in estuarine sediments. Similar rates of 4-BP degradation at bromophenol-containing and non-bromophenol-containing locations show that adaptation of sediment bacteria by prior exposure to bromophenols is not required for degradation of these compounds.
Reinscheid et al. (1996) found that the thermophilic bacterium Bacillus sp. transformed 2-BP to 3-bromocatechol (365 µmol/litre after 8 h) and 3-BP to 3- and 4-bromocatechol (21 µmol/litre after 8 h). No transformation was observed for 4-BP. The growth of the bacterium Rhodococcus opacus on mixtures of 2-BP (0.3 mmol/litre) and 2-chlorophenol (0.3 mmol/litre) and on mixtures of 4-BP (0.28 mmol/litre), 4-chlorophenol (0.25 mmol/litre), and 4-iodophenol (0.25 mmol/litre) was accompanied by the consumption of the substrates and the excretion of halogen ions into the medium within 30 h and 35 h, respectively (Zaitsev & Surovtseva, 2000).
Sulfidogenic consortia enriched from an estuarine sediment were maintained on monochlorophenols as the only source of carbon and energy for over 5 years. The culture was capable of degrading 4-BP (100 µmol/litre) within 6 days. Utilization of 4-BP yielded stoichiometric release of bromide. To verify that 4-BP was mineralized under sulfate-reducing conditions, the evolution of 14CO2 from [14C]4-BP was examined. A 4-BP concentration of 275 µmol/litre was depleted within 30 days, with concomitant release of bromide at 228 µmol/litre; this demonstrated that [14C]4-BP was mineralized, with over 90% of the radiolabel recovered as carbon dioxide (Häggblom & Young, 1995).
The anaerobic biodegradation of 2-BP to phenol and the subsequent utilization of phenol by microorganisms enriched from marine and estuarine sediments from pristine (Lubec and Bay of Fundy, Canada) and polluted (Arthur Kill estuarine inlet between New York and New Jersey, USA) sites were determined under iron-reducing, sulfidogenic, and methanogenic conditions. 2-BP was debrominated with the subsequent utilization of phenol under all three reducing conditions. Debromination of 3-BP and 4-BP was also observed under sulfidogenic and methanogenic conditions, but not under iron-reducing conditions (Monserrate & Häggblom, 1997). The production of phenol as a transient intermediate demonstrated that reductive dehalogenation is the initial step in the biodegradation of bromophenols under iron- and sulfate-reducing conditions (Monserrate & Häggblom, 1997; Knight et al., 1999). In the presence of added sulfate, 2-BP and phenol were completely degraded by a sulfate-reducing consortium of bacteria. In the absence of sulfate, 2-BP was dehalogenated, and phenol accumulated (Fennell et al., 2004). Marine sponges are natural sources of brominated organic compounds, including bromophenols. Aplysina aerophoba sponges harbour large numbers of bacteria that can amount to 40% of the biomass of the animals and have been shown to be responsible for reductive dehalogenation of a number of brominated phenolic compounds. Reductive debromination of 2,6-DBP and 2,4,6-TBP has also been observed under methanogenic and sulfidogenic conditions. Debromination of 2,4,6-TBP and 2,6-DBP to 2-BP was more rapid than the debromination of the monobrominated phenols (Ahn et al., 2003). Boyle et al. (1999) isolated anaerobic bacteria (Strain TBP-1) from estuarine sediments of the Arthur Kill in the New York/New Jersey harbour, USA, that were capable of reductively dehalogenating 2,4,6-TBP to phenol. The organism was found to debrominate 2-BP, 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, but not 3-BP or 2,3-DBP. An anaerobic 2,4,6-TBP-debrominating bacterium was isolated from enrichment cultures inoculated with sediment from the burrows of the bromoaromatic-producing marine hemichordates Balanoglossus aurantiacus and Saccoglossus kowalewskyi. The bacterium preferentially removed ortho-position bromines, resulting in the transient appearance of 2,4-DBP and accumulation of 4-BP (Steward et al., 1995).
2,4,6-TBP was rapidly dehalogenated, with greater than 90% degradation in 2 days, in a marine sediment slurry prepared from surface sediment (depth 1–4 cm) with equivalent amounts of filtered seawater (King, 1988). 2,4-DBP was reported as a transient intermediate (King, 1988). A first-order rate constant of 0.19/day was reported for 2,4,6-TBP in anoxic sediment from Loosdrechtse Plassen (Peijnenburg et al., 1992). 2,4,6-TBP was degraded to 2,4-DBP and, to a lesser extent, 2,6-DBP in several sediment samples (Abrahamsson & Klick, 1991).
Penicillium simplicissimum SK9117, isolated from a sewage plant (Kaiserslauten, Germany), could not utilize monobromophenols as sole carbon and energy source. Under cometabolic conditions, 4-BP was metabolized by 90% within 28 days. Cometabolic transformation of 2- and 3-BP was not observed. In the presence of 2-BP, the phenol was used up within 15 days, whereas 3-BP inhibited the utilization of phenol and thus the growth of the fungus (Marr et al., 1996).
Prediction of the fate of brominated phenols in wastewater treatment plants based on the sewage treatment plant fugacity model are presented in Table 4. Most of the predicted removal is through adsorption to the sewage sludge.
Table 4: Fate of brominated phenols in wastewater treatment plants.
|
Bromophenol |
% in effluent |
% removed |
% biodegraded |
|
4-BP |
96 |
3.4 |
0 |
|
2,4-DBP |
92 |
8 |
0.1 |
|
2,4,6-TBP |
63.6 |
36.4 |
0.4 |
|
PBP |
8 |
92 |
0.8 |
Log Kow values for the bromophenols (see Table 1) would give estimates for bioaccumulation potential that increase with increasing bromination. Predicted BCFs of 20, 24, 120, and 3100 are generated for 4-BP, 2,4-DBP, 2,4,6-TBP, and PBP, respectively, using Bcfwin (v.2.15).
BCF values of 513 and 83 were measured in zebra fish (Brachydanio rerio) and fathead minnow (Pimephales promelas), respectively, for 2,4,6-TBP (Spehar et al., 1980; Devillers et al., 1996). These measured values suggest that the potential for bioconcentration of 2,4,6-TBP in aquatic organisms is moderate to high. Exposure of bluegills (Lepomis macrochirus) to 14C-labelled 2,4,6-TBP for 28 days resulted in 20-fold bioaccumulation in edible tissue and 140-fold bioconcentration in viscera. Plateau levels were reached by 3–7 days of exposure. The half-life for residues was less than 24 h following termination of exposure (Stoner Laboratories, 1978).
Levels of brominated phenols in air, water, and sediment are summarized in Table 5. 2-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP have been identified in vehicle emissions of leaded petrol (dibromoethane is added as a scavenger to prevent the deposition of lead compounds in the engine) at concentrations of 3.8, 4.2, 2.3, and 4.5 µg/m3, respectively (Müller & Buser, 1986).
Table 5: Brominated phenol concentrations in air, water, and sediment
|
Medium |
Location |
Year |
Bromophenol |
Concentration |
Notes |
Reference |
|
Air |
(µg/m3) |
|||||
|
flue gas from hazardous waste incinerator |
Norrtorp, Sweden |
2-BP |
0.036 |
The incinerator was fed chlorinated (mainly solvents) waste. |
Öberg et al. (1987) |
|
|
2-BP |
0.016–0.11 |
The incinerator was fed chlorinated (mainly solvents) and brominated waste (tetrabutylammonium bromide). |
Öberg et al. (1987) |
|||
|
3-/4-BP |
0.024 |
The incinerator was fed chlorinated (mainly solvents) waste. |
Öberg et al. (1987) |
|||
|
3-/4-BP |
0.031–0.23 |
The incinerator was fed chlorinated (mainly solvents) and brominated waste (tetrabutylammonium bromide). |
Öberg et al. (1987) |
|||
|
2,4-DBP |
0.018 |
The incinerator was fed chlorinated (mainly solvents) waste. |
Öberg et al. (1987) |
|||
|
2,4-DBP |
0.036–0.21 |
The incinerator was fed chlorinated (mainly solvents) and brominated waste (tetrabutylammonium bromide). |
Öberg et al. (1987) |
|||
|
2,6-DBP |
<0.004 |
The incinerator was fed chlorinated (mainly solvents) waste. |
Öberg et al. (1987) |
|||
|
2,6-DBP |
0.017–0.029 |
The incinerator was fed chlorinated (mainly solvents) and brominated waste (tetrabutylammonium bromide). |
Öberg et al. (1987) |
|||
|
2,4,6-TBP |
<0.014 |
The incinerator was fed chlorinated (mainly solvents) waste. |
Öberg et al. (1987) |
|||
|
2,4,6-TBP |
0.26–0.38 |
The incinerator was fed chlorinated (mainly solvents) and brominated waste (tetrabutylammonium bromide). |
Öberg et al. (1987) |
|||
|
2,4-DBP |
0.012–0.016 |
The incinerator was fed municipal waste. |
Öberg et al. (1987) |
|||
|
2,4,6-TBP |
0.04–0.05 |
The incinerator was fed municipal waste. |
Öberg et al. (1987) |
|||
|
2,4-DBP |
0.025–0.29 |
The incinerator was fed peat. |
Öberg et al. (1987) |
|||
|
2,4,6-TBP |
<0.005–0.06 |
The incinerator was fed peat. |
Öberg et al. (1987) |
|||
|
Water |
(µg/litre) |
|||||
|
River water |
India |
1988–1989 |
4-BP |
Not detected |
11 sampling points; 4 polluted rivers |
Nomani et al. (1996) |
|
India |
1988–1989 |
2,4-DBP |
40.3 (maximum) |
Detected in 4 of 11 samples |
Nomani et al. (1996) |
|
|
India |
1988–1989 |
2,6-DBP |
3 (maximum) |
Detected in 4 of 11 samples |
Nomani et al. (1996) |
|
|
Saitama Prefecture, Japan |
1996 |
2,4,6-TBP |
0.3 (maximum) |
Detected in 4 of 6 rivers |
Saitama Prefecture (1997) |
|
|
industrial liquid waste |
2,4,6-TBP |
0.08 (maximum) |
Detected in 3 of 6 wastewater samples |
|||
|
1997 |
2,4,6-TBP |
0.08 (maximum) |
Detected in 2 of 8 wastewater samples |
|||
|
raw water |
Canada |
1984–1985 |
2,4,6-TBP |
0.0002–0.0006 (maximum 0.01) |
Range of means. Water samples were collected once each season at 40 potable water treatments plants across Canada. |
Sithole & Williams (1986) |
|
treated water |
2,4,6-TBP |
0.0002–0.001 (maximum 0.02) |
Sithole & Williams (1986) |
|||
|
Sewage effluent |
(µg/litre) |
|||||
|
Untreated effluent |
Essex, United Kingdom |
2-BP |
0.003 |
Mean |
Booth & Lester (1995) |
|
|
3-BP |
0.01 |
Mean |
Booth & Lester (1995) |
|||
|
4-BP |
0.0003 |
Mean |
Booth & Lester (1995) |
|||
|
Treated effluent (with peracetic acid) |
2-BP |
0.03 |
Mean |
Booth & Lester (1995) |
||
|
3-BP |
0.001–0.007 |
Range of means |
Booth & Lester (1995) |
|||
|
4-BP |
0.04 |
Mean |
Booth & Lester (1995) |
|||
|
Sewage sludge |
(µg/kg) |
|||||
|
Municipal wastewater treatment plants |
Sweden |
1999–2000 |
2,4,6-TBP |
<0.3–0.9 (wet weight) |
57 samples; median <0.3 µg/kg |
Öberg et al. (2002) |
|
Sediment |
(µg/kg dry weight) |
|||||
|
non-industrial site |
Japan |
1986 |
2,4,6-TBP |
1.5–4 |
Detected in 1 of 11 sediments |
EAJ (1998) |
|
Upper river & estuarine |
Osaka Prefecture, Japan |
1981–1983 |
2,4-DBP |
Not detected |
12 samples; detection limit 2 µg/kg |
Watanabe et al. (1985) |
|
1981–1983 |
2,6-DBP |
Not detected |
12 samples; detection limit 2 µg/kg |
Watanabe et al. (1985) |
||
|
upper river |
Osaka Prefecture, Japan |
1981–1983 |
2,4,6-TBP |
0.9–36 |
Detected in 5 of 6 samples |
Watanabe et al. (1985) |
|
Estuarine |
Osaka Prefecture, Japan |
1981–1983 |
2,4,6-TBP |
0.8–1.3 |
Detected in 5 of 6 samples |
Watanabe et al. (1985) |
|
estuarine |
Rhone estuary, France |
1987–1988 |
2,4,6-TBP |
26–3690 |
Sediment samples were collected from 5 sites, and 2,4,6-TBP was detected in all samples. |
Tolosa et al. (1991) |
The raw flue gas from a Swedish hazardous waste incinerator (located at Norrtorp) fed chlorinated (mainly solvents) and brominated waste (tetrabutylammonium bromide) contained 2,4,6-TBP at <14, 380, and 260 ng/m3 over three tests; bromides were present initially at 32, 110, and 530 mg/m3. Flue gas from this incinerator fed municipal waste contained 2,4,6-TBP at 4–5 ng/m3. Peat combustion released 2,4,6-TBP at concentrations of <5–60 ng/m3 (Öberg et al., 1987); the highest reported value from peat combustion was 290 ng/m3 for the release of 2,4-DBP.
Maximum reported concentrations in surface fresh water were 40, 3, and 0.3 µg/litre for 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, respectively; 4-BP has not been detected.
Chlorination of natural water containing bromide ion can result in the production of dibromo-, bromodichloro-, dibromochloro-, and tribromophenols (Bean et al., 1980; Sweetman & Simmons, 1980; Rivera & Ventura, 1984; Sithole & Williams, 1986). In a survey of 40 potable water treatment plants located in 39 cities distributed geographically in proportion to population and covering about 40% of Canadian consumers, between October 1984 and June 1985, mean concentrations of 2,4-DBP ranged from 0.6 to 1.2 ng/litre for raw water and from 0.4 to 2.5 ng/litre for treated water; mean 2,4,6-TBP concentrations ranged from 0.2 to 0.6 ng/litre (maximum 10 ng/litre) and from 0.2 to 1.3 ng/litre (maximum 20 ng/litre) for raw and treated water, respectively (Sithole & Williams, 1986). Raw water from water treatment plants in six Canadian cities and treated water from water treatment plants in five of six Canadian cities, collected in February 1985, contained 2-BP, 2,6-DBP, and 2,4,6-TBP at concentrations below the quantification limit of 2–4 ng/litre; samples of treated water from one city contained 2,4,6-TBP at a mean concentration of 5 ng/litre, while samples of treated water from another city contained 2-BP and 2,6-DBP at mean concentrations of 42 and 60 ng/litre, respectively (Sithole et al., 1986).
One hundred and sixteen sewage samples from 22 municipal wastewater treatment plants in Sweden were analysed for brominated flame retardants. Fifty-seven samples were analysed for 2,4,6-TBP, and concentrations ranged from <0.3–0.9 ng/g wet weight, with a median concentration of <0.3 ng/g (Öberg et al., 2002).
An effluent sample collected from an advanced waste treatment plant in Blue Plains, Washington, DC, USA, contained an unreported concentration of 2,4,6-TBP (Lucas, 1984).
Samples of upper river and marine sediment layers in Osaka Prefecture, Japan, collected in 1981 through 1983 at 12 different locations contained 2,4,6-TBP (found in 10 of 12 locations) at concentrations ranging from <0.2 to 36 µg/kg dry weight; 2,4-DBP and 2,6-DBP were not detected above the detection limit of 2 µg/kg (Watanabe et al., 1985). Surficial sediments from the Rhone estuary, collected in 1987–1988, contained 2,4,6-TBP at concentrations of 26–3690 µg/kg dry weight, from five sampling sites (Tolosa et al., 1991).
Gutiérrez et al. (2002) analysed sawdust and soil from the vicinity of Chilean sawmills and reported 2,4,6-TBP concentrations in sawdust of 0.6–1.7 mg/kg and in soil of up to 0.006 mg/kg.
Brominated phenols appear to be widely distributed in marine organisms (Boyle et al., 1992). Levels of brominated phenols in biota are summarized in Table 6. Levels of 2,4,6-TBP in a green marine macroalga (Ulva lactuca) showed extreme seasonal variations, with levels that were 10–100 times higher in summer than in winter (Flodin et al., 1999). Mono- and dibromophenol levels were consistently low, and no seasonal trend could be established. Concentrations of 2,4,6-TBP were measured in brown and red algae (4.5–68 µg/kg), bryozoa (24 and 27 µg/kg wet weight), a hydroid (29 µg/kg wet weight), and sponges (0.2–240 µg/kg wet weight) collected from Exmouth Gulf, Australia, in October 1990 (Whitfield et al., 1992).
Table 6: Brominated phenol concentrations in biota
|
Organism |
Location |
Year |
Bromophenol |
Concentrationa (µg/kg) |
Notes |
Reference |
|
Brown and red macroalgal species |
Exmouth Gulf, Western Australia |
1990 |
2-BP |
0.36–17 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|
4-BP |
0.1–13 (ww) |
Detected in 6 of 8 samples |
Whitfield et al. (1992) |
|||
|
2,4-DBP |
1.9– 25 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
2,6-DBP |
0.29–5.6 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
2,4,6-TBP |
4.5–68 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
Red macroalga (Polysiphonia sphaerocarpa) |
Turimetta Head, Sydney, Australia |
1997–1998 |
2-BP |
0.4–1.0 (ww) |
Flodin & Whitfield (2000) |
|
|
4-BP |
1–8 (ww) |
Flodin & Whitfield (2000) |
||||
|
2,4-DBP |
1–6 (ww) |
Flodin & Whitfield (2000) |
||||
|
2,6-DBP |
3–12 (ww) |
Flodin & Whitfield (2000) |
||||
|
2,4,6-TBP |
7–16 (ww) |
Flodin & Whitfield (2000) |
||||
|
Green macroalga (Ulva lactuca) |
Turimetta Head, Sydney, Australia |
1997–1998 |
2-BP |
0.1–3 (ww) |
Detected in all 18 samples |
Flodin et al. (1999) |
|
4-BP |
0.2–70 (ww) |
Detected in 15 of 18 samples |
Flodin et al. (1999) |
|||
|
2,4-DBP |
0.9–23 (ww) |
Detected in all 18 samples |
Flodin et al. (1999) |
|||
|
2,6-DBP |
0.7–9 (ww) |
Detected in all 18 samples |
Flodin et al. (1999) |
|||
|
2,4,6-TBP |
10–1600 (ww) |
Detected in all 18 samples |
Flodin et al. (1999) |
|||
|
bryozoa |
Exmouth Gulf, Western Australia |
1981 |
2-BP |
1.3–2.4 (ww) |
Detected in 2 of 2 bryozoa |
Whitfield et al. (1992) |
|
4-BP |
2.3–18 (ww) |
Detected in 2 of 2 bryozoa |
Whitfield et al. (1992) |
|||
|
2,4-DBP |
6.7–8.3 (ww) |
Detected in 2 of 2 bryozoa |
Whitfield et al. (1992) |
|||
|
2,6-DBP |
54–69 (ww) |
Detected in 2 of 2 bryozoa |
Whitfield et al. (1992) |
|||
|
2,4,6-TBP |
24–27 (ww) |
Detected in 2 of 2 bryozoa |
Whitfield et al. (1992) |
|||
|
hydroid |
Exmouth Gulf, Western Australia |
1990 |
2-BP |
2.2 (ww) |
One hydroid was investigated |
Whitfield et al. (1992) |
|
4-BP |
4.9 (ww) |
One hydroid was investigated |
Whitfield et al. (1992) |
|||
|
2,4-DBP |
21 (ww) |
One hydroid was investigated |
Whitfield et al. (1992) |
|||
|
2,6-DBP |
41 (ww) |
One hydroid was investigated |
Whitfield et al. (1992) |
|||
|
2,4,6-TBP |
29 (ww) |
One hydroid was investigated |
Whitfield et al. (1992) |
|||
|
sponge |
Exmouth Gulf, Western Australia |
1990 |
2-BP |
0.2–5.8 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|
4-BP |
0.4–62 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
2,4-DBP |
2.1–110 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
2,6-DBP |
0.7–9.6 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
2,4,6-TBP |
0.2–240 (ww) |
Detected in all 8 samples |
Whitfield et al. (1992) |
|||
|
Polychaete annelids |
German Bight/English Channel |
2,4-DBP |
10–610 (ww) |
Range of means |
Goerke & Weber (1991) |
|
|
|
2,4,6-TBP |
40–3220 (ww) |
Range of means |
Goerke & Weber (1991) |
||
|
Norwegian Sea |
1988 |
2,4,6-TBP |
500–7000 (ww) |
Range |
Jensen et al. (1992) |
|
|
Molluscs |
Hong Kong |
1999–2000 |
2-BP |
0.2–17.2 (dw) |
Range of means; detected in all 39 samples |
Chung et al. (2003b) |
|
4-BP |
4.6–55.6 (dw) |
Range of means; detected in 9 of 39 samples |
Chung et al. (2003b) |
|||
|
2,4-DBP |
2.5–195 (dw) |
Range of means; detected in all 39 samples |
Chung et al. (2003b) |
|||
|
2,6-DBP |
0.2–11.9 (dw) |
Range of means; detected in all 39 samples |
Chung et al. (2003b) |
|||
|
2,4,6-TBP |
1.4–198 (dw) |
Range of means; detected in all 39 samples |
Chung et al. (2003b) |
|||
|
Crustacea |
2-BP |
1.1–1.6 (dw) |
Detected in 3 of 6 samples |
Boyle et al. (1992) |
||
|
3-/4-BP |
1.3–1.8 (dw) |
Detected in 2 of 6 samples |
Boyle et al. (1992) |
|||
|
2,4-DBP |
1.2–>100 (dw) |
Detected in 5 of 6 samples |
Boyle et al. (1992) |
|||
|
2,6-DBP |
1.2–1.9 (dw) |
Detected in 3 of 6 samples |
Boyle et al. (1992) |
|||
|
2,4,6-TBP |
1.7–18.9 (dw) |
Detected in 5 of 6 samples |
Boyle et al. (1992) |
|||
|
2-BP |
1.7–2.2 (dw) |
Detected in 3 of 5 samples |
Boyle et al. (1992) |
|||
|
3-/4-BP |
1.6 (dw) |
Detected in 1 of 6 samples |
Boyle et al. (1992) |
|||
|
2,4-DBP |
0.9–10.1 (dw) |
Detected in 5 of 5 samples |
Boyle et al. (1992) |
|||
|
2,6-DBP |
1–5.6 (dw) |
Detected in 5 of 5 samples |
Boyle et al. (1992) |
|||
|
2,4,6-TBP |
0.9–2.1 (dw) |
Detected in 5 of 5 samples |
Boyle et al. (1992) |
|||
|
Hong Kong |
1999–2000 |
2-BP |
0.4–34.4 (dw) |
Range of means; detected in all 63 samples |
Chung et al. (2003b) |
|
|
4-BP |
1.7–47.9 (dw) |
Range of means; detected in 24 of 63 samples |
Chung et al. (2003b) |
|||
|
2,4-DBP |
0.6–214 (dw) |
Range of means; detected in 60 of 63 samples |
Chung et al. (2003b) |
|||
|
2,6-DBP |
0.3–77.3 (dw) |
Range of means; detected in 60 of 63 samples |
Chung et al. (2003b) |
|||
|
2,4,6-TBP |
6.4–2360 (dw) |
Range of means; detected in all 63 samples |
Chung et al. (2003b) |
|||
|
eastern coast of Australia |
1993–1996 |
2,4,6-TBP |
0.07–170b |
Detected in 28 of 30 samples; 9 species of prawns |
Whitfield et al. (1997) |
|
|
2,4,6-TBP |
0.1–0.5c |
Detected in 28 of 30 samples; 9 species of prawns |
Whitfield et al. (1997) |
|||
|
Freshwater fish |
Genessee River, NY, USA |
1984 |
DBP |
76 (lw) |
Isomers not specified |
Jaffe & Hites (1986) |
|
1984 |
2,4,6-TBP |
130 (lw) |
– |
Jaffe & Hites (1986) |
||
|
Marine fish |
Anchor Point, AK, USA |
2-BP |
1.4–1.6 (dw) |
Detected in 2 of 4 samples; 4 species of salmon |
Boyle et al. (1992) |
|
|
3-/4-BP |
1.0 (dw) |
Detected in 1 of 4 samples; 4 species of salmon |
Boyle et al. (1992) |
|||
|
2,4-DP |
0.8 (dw) |
Detected in 1 of 4 samples; 4 species of salmon |
Boyle et al. (1992) |
|||
|
2,6-DP |
Not detected |
Not detected in any of 4 samples; 4 species of salmon |
Boyle et al. (1992) |
|||
|
2,4,6-TBP |
5.1–33.2 (dw) |
Detected in all 4 samples; 4 species of salmon |
Boyle et al. (1992) |
|||
|
eastern coast of Australia |
1992 |
2-BP |
0.1–5.2d (dw) |
Detected in 6 of 10 samples |
Whitfield et al. (1995) |
|
|
4-BP |
0.5–100d (ww) |
Detected in 4 of 10 samples |
Whitfield et al. (1995) |
|||
|
2,4-DBP |
1.5–150d (ww) |
Detected in 7 of 10 samples |
Whitfield et al. (1995) |
|||
|
2,6-DBP |
0.4–18d (ww) |
Detected in 7 of 10 samples |
Whitfield et al. (1995) |
|||
|
2,4,6-TBP |
5.7–170d (ww) |
Detected in 8 of 10 samples |
Whitfield et al. (1995) |
|||
|
2-BP |
0.1e (ww) |
Detected in 2 of 10 samples |
Whitfield et al. (1995) |
|||
|
4-BP |
0.2e (ww) |
Detected in 1 of 10 samples |
Whitfield et al. (1995) |
|||
|
2,4-DBP |
0.1–2.0e (ww) |
Detected in 5 of 10 samples |
Whitfield et al. (1995) |
|||
|
2,6-DBP |
0.1–0.6e (ww) |
Detected in 4 of 10 samples |
Whitfield et al. (1995) |
|||
|
2,4,6-TBP |
0.1–3e (ww) |
Detected in 6 of 10 samples |
Whitfield et al. (1995) |
|||
|
New South Wales, Australia |
1994–1995 |
2,4,6-TBP |
0.4–230d (ww) |
Detected in 22 of 32 samples |
Whitfield et al. (1998) |
|
|
New South Wales, Australia |
2,4,6-TBP |
0.1–1.2f (ww) |
Detected in 19 of 32 samples |
Whitfield et al. (1998) |
||
|
Hong Kong |
1999–2000 |
2-BP |
0.5–30.8d (dw) |
Range of means; detected in 36 of 42 samples |
Chung et al. (2003b) |
|
|
4-BP |
206d |
Mean; detected in 3 of 42 samples |
Chung et al. (2003b) |
|||
|
2,4-DBP |
3.4–97.1d (dw) |
Range of means; detected in all 42 samples |
Chung et al. (2003b) |
|||
|
2,6-DBP |
0.3–15.3d (dw) |
Range of means; detected in 33 of 42 samples |
Chung et al. (2003b) |
|||
|
2,4,6-TBP |
2.2–155d (dw) |
Range of means; detected in all 42 samples |
Chung et al. (2003b) |
|||
|
|
Hong Kong |
1999–2000 |
2-BP |
0.2–10.7f (dw) |
Range of means; detected in 30 of 42 samples |
Chung et al. (2003b) |
|
4-BP |
Not detectedf |
Not detected in any of 42 samples |
Chung et al. (2003b) |
|||
|
|
Hong Kong |
1999–2000 |
2,4-DBP |
0.3–9.5f (dw) |
Range of means; detected in 39 of 42 samples |
Chung et al. (2003b) |
|
2,6-DBP |
0.1–3.5f (dw) |
Range of means; detected in 63 of 42 samples |
Chung et al. (2003b) |
|||
|
2,4,6-TBP |
2.4–39.2f (dw) |
Range of means; detected in all 42 samples |
Chung et al. (2003b) |
a ww = wet weight; dw = dry weight; lw = lipid weight.
b Natural.
c Cultivated.
d Gut.
e Carcass.
f Flesh.
Forty-nine species (87 samples) of red, brown, and green marine microalgae from eastern Australia (principally Bateau Bay, Batemans Bay) were analysed by GC-MS for the key seafood flavour components (2-BP, 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP). All five bromophenols were found in 62% of samples, four in 32% of the samples, and three in the remaining 6% of samples. 2,4,6-TBP was found in all samples and, with few exceptions, was present in the highest concentrations. The total bromophenol content determined on a wet weight basis varied widely across species, from 0.9 ng/g in the green alga Codium fragile to 2590 ng/g in the red alga Pterocladiella capillacea (Whitfield et al., 1999).
Chung et al. (2003a) investigated distributions and seasonal variations of the key seafood flavour compounds, including 2-BP, 4-BP, 2,4-DBP, and 2,4,6-TBP, in three species of brown algae (Padina arborescens, Sargassum siliquastrum, and Lobophora variegata) in Hong Kong waters from 1999 to 2000. On a dry weight basis, the total bromophenol content determined varied widely from 40.9 to 7030 ng/g, being higher in winter and lower in summer. With the exception of 2-BP, the bromophenols were detected in all of the algal samples.
In biota likely to form part of human diets, edible portions contain mean 2,4,6-TBP concentrations up to 198 and 2360 µg/kg dry weight in molluscs and crustaceans, respectively, and up to 39 µg/kg dry weight in marine fish (Table 6).
Endeavour prawns (Metapenaeus endeavouri) from Exmouth Gulf, Shark Bay, and Groote Elylandt, Australia, contained 2,4,6-TBP at concentrations of 41–97, 7.8, and 8.5 µg/kg, respectively (Whitfield et al., 1992). Concentrations of bromophenols in polychaete annelids are not related to sex and generally do not vary with the weight of the animals or with the season (Goerke & Weber, 1991). However, Chung et al. (2003b) found that the total bromophenol content in oyster, crab, shrimp, and fish varied with season. Bromophenol levels were highest in the winter months and lowest during the hot summer months. These seasonal fluctuations generally coincided with the normal growth cycle of the bromophenol-synthesizing seaweeds in the region (Chung et al., 2003a). There are indications that bromophenol concentrations are significantly different in different geographical regions (Goerke & Weber, 1990). Ten different species of fish, collected in August 1992 from the eastern coast of Australia, contained 2,4,6-TBP at concentrations of <0.05–3.4 ng/g for the carcass and <0.05–170 ng/g for the whole gut (analysis of a single fish from each species) (Whitfield et al., 1995). Ocean fish were separated by species into pelagic carnivores, benthic carnivores, diverse omnivores, and restricted omnivores; concentrations in the flesh ranged from <0.01 to 0.9 ng/g, from <0.01 to 12 ng/g, from <0.01 to 4.3 ng/g, and from 0.1 to 1.4 ng/g, respectively, while concentrations in the gut ranged from <0.01 to 11 ng/g, from <0.01 to 230 ng/g, from 0.04 to 55 ng/g, and from 7 to 45 ng/g, respectively (Whitfield et al., 1998). Thirty samples of nine species of prawns, collected from the eastern coast of Australia from 1993 to 1996, contained 2,4,6-TBP at concentrations of <0.01–170 ng/g. 2,4,6-TBP concentrations in cultivated prawns ranged from <0.01 to 0.53 ng/g (Whitfield et al., 1997).
The distribution of bromophenols appears widespread in marine fish and seafoods, and it has been suggested that their presence likely involves biomagnification in the food-chain (Boyle et al., 1992). Whitfield et al. (1988) and Whitfield (1990) provided some evidence that bromophenols in adult prawns were initially derived from ingested small animals, which either biosynthesize or accumulate them from other animals or plants. Thus, the entry of bromophenols into the food-chain at the single-cell level provides an opportunity for widespread distribution of these bromophenolic flavour compounds in all marine fish and seafoods (Whitfield, 1990; Boyle et al., 1992).
Chatonnet et al. (2004) analysed red wines suspected of musty, corky off-flavours and found 2,4,6-TBP concentrations ranging up to 392.6 ng/litre. Neither 2,3,4,6-TeBP nor PBP was detected in any of the wine samples. Based on concerns regarding the contamination of agricultural crops in Chile by 2,4,6-TBP-treated wood via irrigation water, air, and soil, Mardonnes et al. (2003) analysed asparagus (Asparagus officinalis) plants. Six of 10 samples contained detectable levels of 2,4,6-TBP, with concentrations ranging from 0.4 to 1.5 µg/kg. All samples were found to be below the maximal allowed concentration in vegetables of 10 µg/kg.
Sources of exposure of the general population are discussed in section 4.
Smeds & Saukko (2003) analysed human adipose tissue and found PBP in 2 of 29 samples at 5.1 and 11.7 µg/kg lipid weight (detection limit 2 µg/kg); 2,4-DBP and 2,4,6-TBP were not detected (detection limit approximately 0.5 µg/kg).
A pooled sample of human milk from Norway contained a 2,4,6-TBP concentration of 0.6 µg/kg lipid weight; the PBP concentration was below the detection limit of 0.003 µg/kg (Thomsen et al., 2002a).
Mean 2,4,6-TBP concentrations ranging from 0.08 to 26 µg/kg lipid weight were found in serum samples collected in Norway from 40- to 50-year-old males between 1977 and 1999 and from eight groups of people of differing age and gender during 1998. No trend related to age or increase in concentration of 2,4,6-TBP was seen during the study period (Thomsen et al., 2002b). In a survey of three occupational groups in Norway, a range of brominated flame retardants was monitored, with 2,4,6-TBP generally the most abundant brominated compound present in plasma samples. Levels of 2,4,6-TBP exceeded concentrations of the other brominated compounds by 10–100 times. Mean plasma 2,4,6-TBP concentrations were 24, 31, and 11 µg/kg lipid weight for electronics dismantlers, circuit board producers, and laboratory personnel, respectively (Thomsen et al., 2001c). There was no significant difference in concentrations between the three occupational groups; therefore, the authors concluded that the occurrence of 2,4,6-TBP in the plasma could be due to a general exposure via food rather than an occupational exposure (Thomsen et al., 2001c).
Occupational exposures at 2,4,6-TBP production sites may occur by both inhalation and dermal routes. Workplace air concentrations were measured at one production site using 5-min sampling times (JISHA, 2002); data are presented in Table 7.
Table 7: Workplace monitoring data for 2,4,6-TBP.a
|
Operation |
Monitoring data (mg/m3) |
Frequency of activity (times/day) |
Working time (h/day) |
|
Recovery work I (recovering residue on transfer pipes) |
1.357 |
2 |
0.33 |
|
Recovery work II (recovering residue on solidification equipment) |
6.280 |
1 |
0.25 |
|
Drum filling |
1.243 |
10 |
1.67 |
|
Filling machine operation |
0.600 |
10 |
1.67 |
|
Analysis work |
<0.019 |
1 |
0.17 |
a From JISHA (2002). The monitoring method used was as follows: Air sample was suctioned at the breathing zone of the worker at the suction rate of 0.4 litres/min for 5 min, adsorbed through a collection can, and analysed by GC.
Information on the kinetics and metabolism of the brominated phenols is very limited.
2-BP, 3-BP, and 4-BP can all be formed during the metabolism of bromobenzene in both rats and guinea-pigs. 2-BP is formed predominantly by spontaneous isomerization of the 2,3-oxide. 3-BP is formed via the sulfur-series pathway to phenols, which involves the enterohepatic circulation, with the key intermediate being S-(2-hydroxy-4-bromocyclohexa-3,5-dienyl)-L-cysteine, derived from the 4-S-glutathione conjugate of the 3,4-oxide. 4-BP is formed by the sulfur-series route from the S-(2-hydroxy-5-bromocyclohexa-3,5-dienyl)-L-cysteine. Additional suggested in vivo routes to 3- and 4-BP involve dehydration/aromatization of the 3,4-dihydro-3,4-diol, possibly by way of conjugates (Lertratanangkoon et al., 1993).
Absorption, distribution, and elimination of 2,4,6-TBP were examined in 2 male and 10 female Holzman’s albino rats (2 or 3 rats per group) after a single oral administration at doses from 4 to 5.3 mg/kg body weight. 2,4,6-TBP was rapidly absorbed, with the concentration in blood peaking after 1 h at 4.6 mg/kg body weight. The bulk of radioactivity (50–91%) was rapidly excreted via urine, with 4–14% eliminated in the faeces within 48 h. The 2,4,6-TBP concentration in blood fell to 0.002 mg/kg within 24 h. About 0.01% of the administered dose was retained in all tissues after 48 h, with detectable residues (>2 µg/kg) in the kidneys (27 µg/kg), liver (6 µg/kg), and lungs (14 µg/kg). The pharmacokinetics in the rats appeared to follow a one-compartment model system. 2,4,6-TBP was rapidly distributed in the body, and the rate of elimination in urine was proportional to the concentration in the blood. The rate constant for elimination was 0.3, and the half-life in blood was 2.03 h (VCC, 1978b). No information is available on the metabolites of 2,4,6-TBP or on its absorption, distribution, and elimination following its administration via other exposure routes.
4-BP is metabolized in rat liver microsomes, in part to 4-bromocatechol. The catechol undergoes autoxidation to the corresponding quinine or semiquinone, which can either covalently bind to microsomal protein or, in the presence of glutathione, form a glutathione conjugate (Monks et al., 1984).
No studies on the kinetics or metabolism of brominated phenols in humans were identified.
Data on the acute toxicity of brominated phenols to laboratory mammals are limited to 2-BP, 2,4,6-TBP, and PBP. Available information is summarized in Table 8.
Table 8: Acute toxicity of brominated phenols to laboratory animals
|
Bromophenol |
Route |
Species |
Test type |
Concentration |
Reference |
|
2-BP |
Oral |
Mouse |
LD50 |
652 mg/kg body weight |
Anon (1979) |
|
2,4,6-TBP |
Oral |
Rat |
LD50 |
1486 mg/kg body weight (both sexes) |
Fujishima & Fujiwara (1999) |
|
2,4,6-TBP |
Oral |
Rat |
LD50 |
>5000 mg/kg body weight (both sexes) |
DSBG/BCL (1985a) |
|
2,4,6-TBP |
Oral |
Rat |
LD50 |
1995 mg/kg body weight (male rats) |
IRDC (1978a) |
|
2,4,6-TBP |
Oral |
Rat |
LD50 |
1819 mg/kg body weight (female rats) |
IRDC (1978a) |
|
2,4,6-TBP |
Oral |
Rat |
LD50 |
5012 mg/kg body weight (both sexes) |
IRDC (1974c) |
|
PBP |
Oral |
Rat |
LD50 |
251 mg/kg body weight (male rats) |
Anon (1992) |
|
PBP |
Oral |
Rat |
LD50 |
302 mg/kg body weight (female rats) |
Anon (1992) |
|
PBP |
Oral |
Rat |
LD50 |
275 mg/kg body weight (both sexes) |
Anon (1992) |
|
2,4,6-TBP |
Inhalation |
Rat |
4-h LC50 |
>50 000 mg/m3 (>50 mg/litre) |
IRDC (1974b) |
|
2,4,6-TBP |
Inhalation |
Rat |
1-h LC50 |
>200 000 mg/m3 (>200 mg/litre) |
IRDC (1973) |
|
2,4-DBP |
Dermal |
Rabbit |
LD50 |
>2000 mg/kg body weight |
DCC (1975) |
|
2,4,6-TBP |
Dermal |
Rat |
LD50 |
>2000 mg/kg body weight (both sexes) |
DSBG/BCL (1997a) |
|
2,4,6-TBP |
Dermal |
Rabbit |
LD50 |
>2000 mg/kg body weight (both sexes) |
IRDC (1973) |
|
2,4,6-TBP |
Dermal |
Rabbit |
LD50 |
>8000 mg/kg body weight (both sexes) |
IRDC (1974a) |
Acute oral LD50 values in rats for 2,4,6-TBP ranged from 1486 to >5000 mg/kg body weight. The oral rat LD50 value of 1486 mg/kg body weight (Fujishima & Fujiwara, 1999) was derived from a study conducted according to internationally accepted regulatory guidelines (OECD Test Guideline 401) and GLP. Deaths occurred in both sexes within 1 day following administration of 1300 mg/kg body weight or more.
Signs of toxicity during high acute oral exposure of rats to 2,4,6-TBP included hypoactivity, salivation, decreased motor activity, nasal discharge, lacrimation, decreased motor activity, tremors, prostration, clonic convulsions, and death (IRDC, 1974c; Fujishima & Fujiwara, 1999).
Rat oral LD50s of 652 and 250–300 mg/kg body weight were reported for 2-BP and PBP, respectively. In a range-finding study with 2,4-DBP, no deaths of guinea-pigs were reported at 3000 mg/kg body weight (DCC, 1946). In a further range-finding study with 2,4-DBP (10% solution in corn oil), one of two rats died at 2000 mg/kg body weight (both rats survived at 1000 mg/kg body weight) (DCC, 1958). Symptoms from PBP poisoning in rats after oral administration included increased respiratory rate and amplitude, with general body tremors, occasional convulsions, and death. The pathological changes were most marked in the lungs, with mild to severe congestion and petechial haemorrhages noted (Clayton & Clayton, 1993). Gross necropsy of rats exposed to oral doses of PBP revealed grey foci, congestion and focal haemorrhage of the lungs, congestion of the liver, and petechiation of the thymus (Anon, 1992).
The acute (4-h) inhalation LC50 value in rats for 2,4,6-TBP (as dust) was reported as >50 000 mg/m3. In this study, decreased motor activity was observed; in addition, eye squint, slight dyspnoea, erythema, ocular porphyrin discharge, and diarrhoea were also noted at high exposure concentrations. No changes in mortality rate or body weight gain were found. Necropsy of all rats following the 14-day observation period did not reveal any compound-related findings (IRDC, 1974b).
The acute dermal LD50 in rats for 2,4,6-TBP was considered to be >2000 mg/kg body weight. There were no deaths or signs of systemic toxicity observed during and after the 14-day test duration. No abnormalities were noted at necropsy (DSBG/BCL, 1997a). Two studies in rabbits gave LD50 values of >2000 and > 8000 mg/kg body weight (IRDC, 1973, 1974a).
Studies on skin irritation with 2,4,6-TBP were identified (see Table 9). The report by DSBG/BCL (1985b), conducted to OECD Test Guideline 404 and GLP, tested one dose of 2,4,6-TBP at 0.5 g, which was applied for 4 h to the intact and abraded skin of rabbits under occluded conditions. Reactions of the test sites were scored according to the criteria of Draize (1959). No signs of skin irritation were observed at any of the test sites. The result was classified as "not irritating" to skin (DSBG/BCL, 1985b). In the rat dermal toxicity test with 2,4,6-TBP reported in the previous section (DSBG/BCL, 1997a), no signs of irritation were reported. In a series of range-finding tests with rabbits, slight hyperaemia with moderate oedema was reported following a single application of undiluted 2,4-DBP to intact skin for 24 h. Repeated (6–10) applications of a 10% solution of 2,4-DBP to intact skin caused slight hyperaemia. There was no indication that 2,4-DBP was absorbed through the skin in toxic amounts (DCC, 1958).
Table 9: The skin irritation of 2,4,6-TBP.
|
Species |
Method |
Result |
Reference |
|
Rabbit |
OECD TG 404 |
Not irritating |
DSBG/BCL (1985b) |
|
Rabbit |
No data |
Not irritating |
IRDC (1974e) |
a Mean of erythema score after 24 and 72 h for abraded and unabraded skin; each scored from 0 to 1. No oedema observed; score zero.
Two available reports of eye irritation with 2,4,6-TBP are summarized in Table 10. The study by DSBG/BCL (1997b) was conducted to OECD Test Guideline 405 and GLP; a single application to the non-irrigated eye of the three rabbits produced diffuse corneal opacity, iridial inflammation, and moderate conjunctival irritation. The test material produced a maximum group mean score of 27.0 (out of a possible maximum of 39). 2,4,6-TBP was classified as a "moderate irritant" to the eye. In a series of range-finding tests with rabbits, extensive conjunctival and corneal damage was reported following an application of undiluted 2,4-DBP to the eye. Extensive conjunctival irritation with moderate corneal damage was reported for a 10% solution of 2,4-DBP (DCC, 1958).
Table 10: The eye irritation of 2,4,6-TBP.
|
Species |
Method |
Result |
Reference |
|
Rabbit |
OECD TG 405 |
Moderately irritating |
DSBG/BCL (1997b) |
|
Rabbit |
No data |
Irritating |
IRDC (1974d) |
There are two available reports on sensitization with 2,4,6-TBP. One was conducted to OECD Test Guideline 406 and GLP (DSBG/BCL, 1997c). Twenty test and 10 control guinea-pigs were used for the main study. Based on the results of sighting tests, the concentrations of the test material for the induction and challenge phases were selected as follows: intradermal induction, 10% w/v in arachis oil; topical induction, 50% w/w in arachis oil; topical challenge, 75% and 50% w/w in arachis oil. The test material produced 75% (15/20) sensitization. 2,4,6-TBP was classified as a strong sensitizer to guinea-pig skin. Another study (IRDC, 1975) reported slight sensitization (50% responded to the challenge with a flare response slightly greater than the initial sensitizing regime) in guinea-pigs.
Only one oral administration study (Tanaka et al., 1999) was available for 2,4,6-TBP.4 Following OECD Test Guideline 422 for a combined repeated-dose toxicity study with a reproduction/developmental toxicity screening test, groups of 12 male and 11–12 female SD (Crj: CD) rats were administered doses of 0 (vehicle; corn oil), 100, 300, or 1000 mg/kg body weight per day by gavage. The dosing period for males was 48 days starting from 14 days before mating, and that for females was 41–45 days starting from 14 days before mating to day 3 of lactation. For females unsuccessfully mated, the dosing period was 48 days. At 1000 mg/kg body weight per day, salivation, significant suppression of body weight gain (14% for males and 7.5% for females), decreased food consumption, increased absolute and relative liver weights, and increased relative kidney weight were observed in both sexes. Significant increases in total protein, albumin, albumin/globulin ratio, and ALP, decreases in total bilirubin and potassium in blood, significant decreases in absolute thymus weight, enlargement of liver, increase in the incidence of hepatocyte hypertrophy, and decrease of fatty change in liver, renal papillary necrosis, dilatation of tubules, lymphocyte infiltration, basophilic tubular epithelium, and hyaline casts in kidney were observed in males at 1000 mg/kg body weight per day. No biochemical or histopathological studies were performed with females. At 300 mg/kg body weight per day, salivation was observed in both sexes, and a significant increase in creatinine in blood was observed in males. At 100 mg/kg body weight per day, no adverse effects were observed in either sex. The NOAEL for the repeated-dose oral toxicity is therefore considered to be 100 mg/kg body weight per day for both sexes (Tanaka et al., 1999). See section 8.6 for a discussion of the reproduction/developmental toxicity part of this study.
PBP was given in drinking-water to three young bulls at a dosage of 7.6 mg/kg body weight per day for 5 weeks. No significant signs of intoxication and no micropathological changes were noted. No further details were reported (Herdt et al., 1951).
No long-term exposure or carcinogenicity studies on brominated phenols were identified.
Results are summarized in Table 11. These comprise two in vitro reverse mutation tests on two types of bacteria, one mammalian cell in vitro test, and one genotoxicity in vivo test for 2,4,6-TBP, as well as a single in vitro reverse mutation test for PBP. All tests were conducted to appropriate guidelines, included positive and solvent controls, and are considered reliable. All of the tests, except the mammalian cell in vitro (chromosomal aberration) test, were negative.
Table 11: Summary of genotoxicity studies.
|
Type of test |
Test system |
Bromophenol |
Dose |
Result |
Reference |
|
Bacterial in vitro test |
|||||
|
Reverse mutation |
S. typhimurium (strains TA100, TA1535, TA98, TA1537) |
2,4,6-TBP |
Up to 1000 µg/plate; concurrent solvent and positive controls |
Negative for all strains at all doses with and without metabolic activation |
Shibuya et al. (1999) |
|
E. coli WP2 uvrA |
Up to 5000 µg/plate |
||||
|
Reverse mutation |
S. typhimurium (strains TA1535, TA1537, TA1538, TA98, and TA100) |
2,4,6-TBP |
Up to 1500 µg/plate; concurrent solvent and positive controls |
Negative for all strains at all doses with and without metabolic activation |
DSBG/BCL (1996) |
|
Reverse mutation |
S. typhimurium (strains TA1535, TA1537, TA1538, TA98, and TA100) |
2,4,6-TBP |
Up to 1000 µg/plate; concurrent solvent and positive controls |
Negative for all strains at all doses with and without metabolic activation |
Litton Bionetics (1978) |
|
Reverse mutation |
S. typhimurium (strains TA1535, TA1537, TA98, and TA100) |
PBP |
Up to 333 µg/plate; concurrent solvent and positive controls |
Negative for all strains at all doses with and without metabolic activation |
Zeiger et al. (1987) |
|
Mammalian cells in vitro test |
|||||
|
Chromosomal aberration test |
CHL/IU cells |
2,4,6-TBP |
Up to 1.6 mg/ml |
Positive (with and without metabolic activation) |
Sasaki et al. (1999) |
|
Mammalian in vivo test |
|||||
|
Micronucleus test |
Mouse, bone marrow |
2,4,6-TBP |
75, 150, 300 mg/kg body weight intraperitoneal |
Negative |
DSBG/BCL (2002) |
No adverse effects were observed on estrous cyclicity, copulation index, fertility index, duration of gestation period, number of corpora lutea, delivery findings, number of implants, number of total pups and live pups born, implantation index, or delivery index in any treated groups of the repeated-dose toxicity study with the reproduction/developmental screening test described in section 8.3. Neonatal viability on day 4 of lactation and neonatal body weights on days 0 and 4 of lactation in the 1000 mg/kg body weight per day group were lower than those in the control group (~50% for viability; body weights were reduced by 17–19% in males and 19–25% in females). No reproductive or developmental effects were seen at 300 mg/kg body weight per day in rats. The NOELs for reproductive/ developmental toxicity were considered to be 1000 mg/kg body weight per day for parents and 300 mg/kg body weight per day for pups (Tanaka et al., 1999).
Developmental toxicity was evaluated in six groups of five pregnant Charles River CD rats receiving 2,4,6-TBP via oral gavage at dose levels of 10, 30, 100, 300, 1000, or 3000 mg/kg body weight per day on gestation days 6 through 15. A control group received the vehicle, corn oil, at 10 mg/kg body weight per day. During gestation, the females were observed for clinical signs of effect, mortality, and body weight changes. These rats were sacrificed on gestation day 20 and their uterine contents examined for viable and non-viable fetuses, early and late resorptions, and total implantations. No effects were noted on maternal behaviour or appearance of the group that received 1000 mg/kg body weight per day or less. Total mortality was observed at the 3000 mg/kg body weight per day dose. There were no effects on maternal body weights, food consumption, number of corpora lutea, viable or non-viable fetuses, resorptions, or implantantions at doses of 300 mg/kg body weight per day or less. There were slight decreases in body weight gains between gestation days 6 and 12, an increase in post-implantation losses, and a slight decrease in the number of viable fetuses in the 1000 mg/kg body weight per day group. Therefore, the NOAELs for maternal and developmental toxicity were 1000 and 300 mg/kg body weight per day, respectively (IRDC, 1978b).
Pregnant Wistar rats were exposed to 2,4,6-TBP by whole-body inhalation (at 0, 0.03, 0.1, 0.3, or 1.0 mg/m3) for 24 h/day, 7 days/week, from day 1 to day 21 of gestation (Lyubimov et al., 1998). The authors reported that pre-implantation and post-implantation embryo losses were significantly increased in a dose-dependent manner and were seen in all treated groups except the lowest concentration (0.03 mg/m3, equivalent to 0.015 mg/kg body weight per day) group. Fetal body weight decreased with increasing 2,4,6-TBP concentrations from 0.1 to 1.0 mg/m3. Behavioural effects were seen in progeny, giving a NOAEL of <0.03 mg/m3. No effects were found on non-specific immunological parameters. However, the method for generating the airborne substance, analysis of air concentrations in the test chambers, and physical state of the test substance were not reported in the paper. The maternal NOAEL is reported as both 0.1 and 0.3 mg/m3; it is unclear on which end-point the estimates were based.
Olsen et al. (2002) characterized the estrogen-like activity of 4-BP, 2,4-DBP, and 2,4,6-TBP using estrogen-dependent human breast cancer cell line MCF-7. 4-BP and 2,4-DBP bind to the estrogen receptor with approximately 10 000-fold less affinity than 17beta-estradiol. 2,4,6-TBP was able to displace only 43% of radiolabelled estrogen when tested at concentrations up to 1 µmol/litre. The brominated phenols, although binding to the estrogen receptor, did not stimulate cell growth, increase the levels of progesterone receptor or estrogen-regulated secretorial proteins such as pS2, or reduce the level of 17beta-estrogen-induced pS2. The lack of estrogen-mediated cellular responses in vitro suggests that these brominated phenols will most likely not directly interact with estrogen-mediated processes in vivo, despite binding to estrogen receptors.
The nephrotoxicity of 2-BP has been investigated because it is a metabolite of bromobenzene, a known nephrotoxin (Lau et al., 1984a,b; Rush et al. 1984). Bruchajzar et al. (2002) studied its nephrotoxicity in female rats administered intragastrically both in a single dose of 750 or 1125 mg/kg body weight and following repeat dosing (at 7, 14, 21, and 28 days) at 30 or 150 mg/kg body weight. Following single acute dosing, the protein concentration in urine was significantly elevated at 24 h, and numbers of epithelial cells were elevated at 72 and 120 h. The renal reduced glutathione level was depressed for 72 h after dosing. Similar, but less pronounced, effects were seen with the repeat dosing. However, the transition from single to repeated exposure did not result in enhanced nephrotoxicity. The authors concluded that 2-BP is mildly nephrotoxic at high doses. In vitro studies on hepatocytes showed that 4-BP did not contribute significantly to bromobenzene toxicity (Dankovic & Billings, 1985).
Monks et al. (1984) found that 4-BP was not nephrotoxic in rats. An initial in vitro study showed that 4-BP is metabolized in rat liver microsomes, in part to 4-bromocatechol. The catechol undergoes autoxidation to the corresponding quinine or semiquinone, which can either covalently bind to microsomal protein or, in the presence of glutathione, form a glutathione conjugate. However, conditions that increased the in vitro covalent binding of 4-BP (i.e., prior phenobarbital treatment and the absence of glutathione) did not cause toxicity in vivo following administration at 0.6, 1.27, 1.9, or 2.55 mmol/kg body weight intraperitoneally. Thus, chemically reactive metabolites of 4-BP do not play a role in bromobenzene-mediated hepatoxicity. In contrast, 2-BP (1.6 mmol/kg; intraperitoneal) caused severe renal damage in non-induced rats (Lau et al., 1984b). When [14C]2-BP was administered to rats, the amount of radioactive material covalently bound to kidney proteins was 4 times greater than that bound to liver proteins. Liver microsomes converted 2-BP to covalently bound material and 2-bromohydroquinone, whereas kidney microsomes did not. The observations were consistent with the view that a hepatic metabolite of bromobenzene and of 2-BP (2-bromohydroquinone or a conjugate) might be formed in the liver and transported by the blood to the kidney, where it elicited toxicity (Lau et al., 1984a,b). Monks et al. (1985), in later studies with glutathione conjugates of 2-bromohydroquinone, suggested that the kidney necrosis observed after the administration of bromobenzene, 2-bromophenol, or 2-bromohydroquinone might be due in part to 2-bromohydroquinone glutathione conjugates formed in the liver and subsequently transported to the kidney and converted to ultimate nephrotoxic metabolite(s).
PBP was administered in single (20, 40, or 80 mg/kg body weight) or repeated (3, 6, or 12 mg/kg body weight; once per day for 7 days) intraperitoneal doses to male BALB/c strain mice or in a single (per os) dose (90 or 135 mg/kg body weight) to female Wistar rats. Slight changes were noted in the level of SGPT in mouse serum and glutathione in the liver, with a more pronounced increase of malondialdehyde after a single dose. Following repeated administration, levels of gamma-glutamyl-transferase and malondialdehyde were elevated. The nephrotoxic action of PBP in rats was manifested by the decrease of renal glutathione levels, as well as by an increase in protein contents and the number of renal epithelial cells in urine. As a result, limited hepatotoxicity was found only in mice. The nephrotoxicity in rats was comparable with that for 2-BP (Szymanska et al., 1995).
PBP showed very potent competition binding to human transthyretin (one of the thyroid hormone binding transport proteins in plasma of vertebrates) in vitro compared with thyroxine (7.1-fold stronger than the natural thyroxine ligand). The investigators suggested that this might have effects on thyroid hormone homeostasis in vivo (Meerts et al., 2000). The relative potency of 2,4,6-TBP was 1.2, whereas 2,4-DBP had a relative potency 17 times lower than that of thyroxine. The maximum competition reached by 2,4,6-TBP and PBP at 500 nmol/litre exceeded that of thyroxine at the same concentration. The maximum thyroxine-transthyretin binding competition for 2,4-DBP was only 50% at a concentration of 25 µmol/litre.
2,4,6-TBP reduced cell growth and increased acetylcholinesterase activity in cultured SH-SY5Y human neuroblastoma cells at concentrations of 0.1 µmol/litre and above. Apoptosis was observed at higher concentrations. Differentiated cells were more sensitive to 2,4,6-TBP than naive cells (Rios et al., 2003).
No studies have been identified on the effects of brominated phenols on human health.
The toxicity of brominated phenols to aquatic organisms is summarized in Table 12. Seventy-two-hour EC50s in microalgae range from 0.4 to 1.6 mg/litre for 2,4,6-TBP; a 48-h EC50 for 2-BP was 110 mg/litre. Forty-eight-hour LC/EC50s in daphnids range from 0.9 to 6 mg/litre for 2- and 4-BP and from 0.3 to 5.5 mg/litre for 2,4,6-TBP. In chronic studies, 21-day NOECs for daphnid reproduction were 0.2 mg/litre for 2-BP and 0.1 mg/litre for 2,4,6-TBP. Ninety-six-hour LC50s in fish range from 0.2 to 6.8 mg/litre for 2,4,6-TBP. No studies were identified on the toxicity of lower brominated phenols to fish. A 96-h LC50 of 0.1 mg/litre was reported for PBP. Liu et al. (1982) reported IC50s, based on inhibition of bacterial dehydrogenase, of 550, 380, 400, 60, and 500 mg/litre for 2-BP, 3-BP, 4-BP, 2,4-DBP, and 2,6-DBP, respectively. Twenty-four-hour IC50s for methanogenic bacteria, based on gas production, were 104.2, 137.4, 353.2, 7.4, and 0.03 mg/litre for 2-BP, 3-BP, 4-BP, 2,4,6-TBP, and PBP, respectively. For Nitrosomonas sp., 24-h IC50s, based on ammonia consumption, were 0.4, 0.8, 7.8, and 0.3 mg/litre for 2-BP, 4-BP, 2,4,6-TBP, and PBP, respectively. A 24-h IC50, based on oxygen consumption, for 4-BP of 125 mg/litre was reported for an aerobic heterotroph (Blum & Speece, 1991). Microtox test (5 min) results were 20, 3.9, 0.4, 5.5, 1.2, and 0.003 mg/litre for 2-BP, 3-BP, 4-BP, 2,6-DBP, 2,4,6-TBP, and PBP, respectively (Blum & Speece, 1991).
Table 12: Toxicity of brominated phenols to aquatic species.
|
Organism |
Bromophenol |
End-point |
Concentration (mg/litre) |
Reference |
|
Green algae (Selenastrum capricornutum) |
2,4,6-TBP |
72-h EC50 (biomass) |
0.8a |
EAJ (2000c) |
|
2,4,6-TBP |
72-h NOEC (biomass) |
0.2a |
EAJ (2000c) |
|
|
2,4,6-TBP |
48-h EC50 (growth inhibition) |
1.1a |
EAJ (2000c) |
|
|
2,4,6-TBP |
72-h EC50 (growth inhibition) |
1.6a |
EAJ (2000c) |
|
|
2,4,6-TBP |
72-h NOEC (growth inhibition) |
1.0a |
EAJ (2000c) |
|
|
2,4,6-TBP |
72-h EC50 (growth inhibition) |
0.4a |
DSBG/BCL (1998c) |
|
|
Green algae (Scenedesmus subspicatus) |
2-BP |
48-h EC50 (growth rate) |
110 |
Kühn & Pattard (1990) |
|
Protozoa (Tetrahymena pyriformis) |
2-BP |
60-h EC50 (growth inhibition) |
54.2b |
Schultz & Riggin (1985) |
|
4-BP |
60-h EC50 (growth inhibition) |
36.1b |
Schultz & Riggin (1985) |
|
|
2,4-DBP |
60-h EC50 (growth inhibition) |
10b |
Schultz & Riggin (1985) |
|
|
2,4,6-TBP |
60-h EC50 (growth inhibition) |
3b |
Schultz & Riggin (1985) |
|
|
PBP |
48-h EC50 (growth inhibition) |
1.1 |
Schultz (1987) |
|
|
Water flea (Daphnia magna) |
2-BP |
24-h EC50 |
13b |
Kühn et al. (1989b) |
|
2-BP |
24-h EC50 |
1.6 |
Kühn et al. (1989a) |
|
|
2-BP |
48-h EC50 |
0.9 |
Kühn et al. (1989a) |
|
|
2-BP |
21-day NOEC (reproduction) |
0.2c |
Kühn et al. (1989b) |
|
|
4-BP |
48-h EC50 |
6 |
Kopperman et al. (1974) |
|
|
2,4,6-TBP |
48-h LC50 |
5.5 |
Industrial Bio-Test (1976a) |
|
|
2,4,6-TBP |
48-h EC50 |
2.2a |
EAJ (2000a) |
|
|
2,4,6-TBP |
48-h EC50 |
0.3a |
DSBG/BCL (1998b) |
|
|
2,4,6-TBP |
48-h EC50 |
1.3c |
Kopperman et al. (1974) |
|
|
2,4,6-TBP |
21-day NOEC (reproduction) |
0.1c,d |
EAJ (2000a) |
|
|
Rainbow trout (Oncorhynchus mykiss) |
2,4,6-TBP |
96-h LC50 |
0.2 |
Industrial Bio-Test (1976b) |
|
Bluegill (Lepomis macrochirus) |
2,4,6-TBP |
96-h LC50 |
0.3 |
Industrial Bio-Test (1976b) |
|
Medaka (Oryzias latipes) |
2,4,6-TBP |
96-h LC50 |
1.5a |
EAJ (2000b) |
|
Fathead minnow (Pimephales promelas) |
2,4,6-TBP |
96-h LC50 |
6.5–6.8b |
Phipps et al. (1981) |
|
2,4,6-TBP |
96-h LC50 |
6.3b |
Broderius et al. (1995) |
|
|
PBP |
96-h LC50 |
0.1 |
Geiger et al. (1988) |
|
|
2,4,6-TBP |
192-h LC50 |
4.5–4.9b |
Phipps et al. (1981) |
|
|
Goldfish (Cyprinus carpio) |
2,4,6-TBP |
96-h LC50 |
1.1c |
DSBG/BCL (1998a) |
a Based on nominal concentrations (within 20% of measured values).
b Based on nominal concentrations.
c Based on measured concentrations.
d Highest concentration tested.
Photosynthesis in estuarine phytoplankton was significantly reduced at a 2,4,6-TBP concentration of 0.5 mg/litre, whereas significant adverse effects of PBP were observed at 0.125 mg/litre in some species. No effects on photosynthesis were found at 4-BP concentrations of 2 mg/litre (Erickson & Hawkins, 1980).
Many marine infaunal hemichordates and polychaetes produce bromophenol metabolites. Lovell et al. (1999) found no effect of a common bromometabolite, 4-BP (10 mg/kg dry weight), on substrate respiration and assimilation by undisturbed sediment bacterial communities during 6- to 8-h exposures.
Applegate et al. (1957) exposed rainbow trout (Oncorhynchus mykiss), bluegill (Lepomis macrochirus), and sea lamprey (Petromyzon marinus) to 2,4,6-TBP and PBP at 5 mg/litre for 24 h. For 2,4,6-TBP, deaths occurred at 3 h for trout and bluegill and at 12 h for sea lamprey; no toxic effects were observed for PBP.
Sund & Nomura (1963) reported 5-day EC50s for PBP, based on inhibition of seed germination, of 1.17 × 10−4 mol/litre and 8.59 × 10−5 mol/litre for cucumber (Raphanus sativus) and Sudan grass (Sorghum sudanese), respectively.
No short-, medium, or long-term toxicity data were identified for the lower brominated phenols. Most of the data relate 2,4,6-TBP; therefore, it is possible to evaluate the health effects of 2,4,6-TBP only.
2,4,6-TBP is rapidly absorbed from the gastrointestinal tract in mammals and also rapidly excreted via urine and faeces. Little information was available on the kinetics and metabolism of other brominated phenols.
2,4,6-TBP is considered to be non-irritating to the skin, but moderately irritating to the eye; it is considered a skin sensitizer in guinea-pigs.
A combined repeated-dose oral toxicity study with a reproduction/developmental toxicity screening test on 2,4,6-TBP in rats showed, at 1000 mg/kg body weight per day, body weight gain suppression and increased absolute and relative liver weights in both sexes and increases in total protein, albumin, albumin/ globulin ratio, and ALP in blood in male rats. At 300 mg/kg body weight per day, salivation was observed in both sexes, and an increase in blood creatinine was observed in male rats. The NOAEL was considered to be 100 mg/kg body weight per day in rats of both sexes. No adverse effects were observed in estrous cyclicity, copulation index, fertility index, duration of gestation period, number of corpora lutea, number of implants, total number of pups and live pups, implantation index, or delivery index in any treated group. Neonatal viability on day 4 of lactation and neonatal body weights on days 0 and 4 of lactation in the 1000 mg/kg body weight per day group were lower than those in the control group. No reproductive or developmental effects were seen at 300 mg/kg body weight per day in rats (Tanaka et al., 1999).
No reliable studies were identified for repeated inhalation toxicity.
In vitro reverse mutation studies with 2,4,6-TBP in two types of bacteria were negative. One in vitro chromosomal aberration test was positive with and without metabolic activation. One in vivo micronucleus assay up to the maximum tolerated dose was negative.
2-BP and PBP are both nephrotoxic in rats at high doses, whereas 4-BP shows no nephrotoxicity.
There are no long-term repeated-dose studies or carcinogenicity studies, and no human data were identified.
Lack of a reliable study to determine a NOAEL by the inhalation route precludes the derivation of a tolerable concentration.
Since the only reported short-term toxicity study by the oral route is considered a screening test, no reliable tolerable intakes for 2,4,6-TBP can be derived for drinking-water or food.
Air concentrations of brominated phenols have been measured in the environment locally due to the combustion of halogenated waste, peat, and leaded fuel from vehicles; highest reported values were 380 and 4500 ng/m3 for 2,4,6-TBP from combustion of halogenated waste and vehicle fuel combustion, respectively, and 290 ng/m3 for 2,4-DBP from peat combustion. These are inadequate to estimate exposure of the general population through inhalation.
Exposure of the general population would be through drinking-water and the consumption of seafood (the latter from naturally occurring bromophenols).
Measurements of brominated phenols in drinking-water are restricted to Canada, where the highest reported concentrations in treated water are 42, 60, and 20 ng/litre for 2-BP, 2,6-DBP, and 2,4,6-TBP, respectively, each in a single water sample. Generally, concentrations in drinking-water are less than 3 ng/litre, with levels higher in treated than in raw water.
In biota likely to form part of human diets, edible portions contain mean 2,4,6-TBP concentrations up to 198 and 2360 µg/kg dry weight in molluscs and crustaceans, respectively, and up to 39 µg/kg dry weight in marine fish.
Several species of marine algae are known to contain simple brominated phenols. It is known that brominated phenols occur naturally through production by marine benthic animals. Acorn worms (Enteropneusta) produce and excrete large amounts of bromophenols without any obvious dietary source of these compounds. Bromophenols from natural sources, such as 4-BP, 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, are a consistent feature of pristine marine soft-bottom habitats, and their spatial and temporal abundance correlates with the abundance of infauna that produce these metabolites.
Estimated vapour pressures indicate that 2,4,6-TBP and PBP will exist in both the vapour and particulate phases in the ambient atmosphere. Vapour-phase brominated phenols are degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; the half-lives for this reaction in air are estimated to be 13 h for 4-BP, 45 h for 2,4-DBP, and between 20 and 40 days for 2,4,6-TBP and PBP. Particulate-phase 2,4,6-TBP and PBP will be removed from the atmosphere by wet and dry deposition.
In water, PBP would be expected to adsorb to suspended solids and sediment. However, other less brominated phenols would tend to remain in the water phase. Volatilization of non-dissociated 2,4,6-TBP and PBP from water surfaces is not expected to be an important fate process. Henry’s law constants for mono- and dibrominated phenols would suggest little volatilization of these compounds.
All of the brominated phenols, if released to soil, essentially stay there and will not be mobile.
Brominated phenols are generally not readily biodegradable and will persist in the environment. However, adapted communities of microorganisms and specialist communities (such as anaerobic or sulfidogenic) may degrade the compounds.
Log Kow values for the bromophenols would give estimates for bioaccumulation potential that increase with increasing bromination. Predicted BCFs of 20, 24, 120, and 3100 for 4-BP, 2,4-DBP, 2,4,6-TBP, and PBP have been calculated. Measured BCFs for 2,4,6-TBP are similar to the predicted value.
Maximum reported concentrations in surface fresh water were 40, 3, and 0.3 µg/litre for 2,4-DBP, 2,6-DBP, and 2,4,6-TBP, respectively; 4-BP has not been detected. Estuarine sediment concentrations range up to 3690 µg/kg dry weight for 2,4,6-TBP. 2,4-DBP and 2,6-DBP were not detected (detection limit 2 µg/kg). No PBP concentrations have been reported.
Air concentrations of brominated phenols have been measured in the environment locally due to the combustion of halogenated waste, peat, and leaded fuel from vehicles; highest reported values were 380 and 4500 ng/m3 for 2,4,6-TBP from the combustion of halogenated waste and vehicle fuel combustion and 290 ng/m3 for 2,4-DBP from peat combustion.
Seventy-two-hour EC50s in microalgae range from 0.4 to 1.6 mg/litre for 2,4,6-TBP; a 48-h EC50 for 2-BP is 110 mg/litre. Forty-eight-hour LC/EC50s in daphnids range from 0.9 to 6 mg/litre for 2- and 4-BP and from 0.3 to 5.5 mg/litre for 2,4,6-TBP. In chronic studies, 21-day NOECs for daphnid reproduction were 0.2 mg/litre for 2-BP and 0.1 mg/litre for 2,4,6-TBP. Ninety-six-hour LC50s in fish range from 0.2 to 6.8 mg/litre for 2,4,6-TBP. No studies were identified on the toxicity of lower brominated phenols to fish. A 96-h LC50 of 0.1 mg/litre was reported for PBP. For the terrestrial environment, only a single study on the effects of PBP on seed germination was identified.
All acute and chronic toxicity test results for the various brominated phenols are plotted in Figure 1. It might be expected that toxicity increases with increasing bromination of the phenol. However, the data set is limited, and only one study, on the protozoan Tetrahymena pyriformis, demonstrates this trend clearly. For daphnids, there is no apparent trend with bromination.
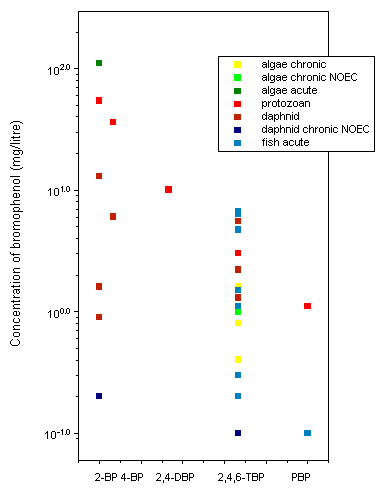
Figure 1: Concentration of brominated phenols causing acute or chronic toxic effects in a range of organisms.
PNECs for brominated phenols are summarized in Table 13. Assessment factors are based on criteria set out in CEC (2003). It should be noted that there are insufficient or no data available for 3-BP, the dibromophenols, and 2,3,4,6-TeBP. Data on PBP have been included; however, without a full base set of information, the PNEC for PBP should not be used in a risk assessment.
Table 13: PNECs for brominated phenols in aquatic organisms.
|
Bromophenol |
Lowest reported toxicity value and organism |
Assessment factor |
PNEC (µg/litre) |
|
2-BP |
0.2 mg/litre; 21-day NOEC for reproduction in Daphnia magna |
100 |
2 |
|
3-BP |
No data |
– |
– |
|
4-BP |
6 mg/litre; 48-h EC50 in Daphnia magna |
1000 |
6 |
|
2,4-DBP |
No adequate data |
– |
– |
|
2,5-DBP |
No adequate data |
– |
– |
|
2,6-DBP |
No adequate data |
– |
– |
|
3,5-DBP |
No adequate data |
– |
– |
|
2,4,6-TBP |
0.1 mg/litre; 21-day NOEC for reproduction in Daphnia magna |
50 |
2 |
|
2,3,4,6-TeBP |
No data |
– |
– |
|
PBP |
0.1 mg/litre; 96-h LC50 in fathead minnow (only usable data point for this compound) |
1000 |
0.1a |
|
a |
A complete base set of data was not available for PBP, and so the PNEC is provided for information only and should not be used in a risk assessment. |
Although it is possible to produce rather conservative PNECs for the monobrominated phenols (based on a limited data set), it is not possible to give a PEC/PNEC ratio, because there are no measured concentrations of these compounds (4-BP has been looked for but not detected). There are measured concentrations of one of the dibrominated phenols (2,4-DBP); however, there are no adequate toxicity data, and again a PEC/PNEC ratio cannot be calculated. There are very limited toxicity data on PBP; however, again there are no measured concentrations of this compound. The only PEC/PNEC ratio that could be calculated based on measured results was for 2,4,6-TBP. The PEC/PNEC ratio for 2,4,6-TBP would be 0.15 based on a maximum measured value in surface fresh waters of 0.3 µg/litre. It should be noted that the exposure information is extremely limited, and, thus, this risk factor should only be used with caution as a crude indicator. There were very limited production figures available and no release figures, and, therefore, modelling exposure concentrations was felt to be inappropriate at this stage.
Only PBP would be predicted to bind preferentially to sediments; however, no measured values have been identified for PBP in sediment. No concentrations of the monobrominated phenols in sediment were reported; dibrominated phenols were not detected. Therefore, the only data available for sediments are for 2,4,6-TBP. Sediment concentrations in fresh water (upper river water in Japan, with no information on the nature of the sediment) range up to 36 µg/kg for 2,4,6-TBP. On the basis of this very limited data set, the risk to aquatic organisms from 2,4,6-TBP in sediment would appear to be low.
A marine risk assessment is not possible because there are no toxicity data available and there is natural production of bromophenols by biota, making measured concentrations difficult to interpret.
Insufficient data are available to make a meaningful risk assessment for the terrestrial environment.
There are very limited production figures available and no release figures.
There are very limited exposure data for the brominated phenols.
Few toxicity data exist other than for 2,4,6-TBP.
It was not possible to set human health tolerable intakes/concentrations for 2,4,6-TBP because the critical studies were not suitable for the following reasons: the OECD Test Guideline 422 is based on a screening protocol, the studies by Lyubimov and fellow workers were mainly reported as abstracts with insufficient detail (and so were not included in this document), and the test results from the Industrial Bio-Test Laboratories (Industrial Bio-Test, 1976c, 1977) were considered to be too unreliable.
All calculations of environmental risk are based on a very limited data set. Although it is possible to produce rather conservative PNECs for the monobrominated phenols, it is not possible to give a PEC/PNEC ratio because there are no measured concentrations of these compounds. There are measured concentrations of one of the dibrominated phenols (2,4-DBP); however, there are no adequate toxicity data, and again a PEC/PNEC ratio cannot be calculated. There are very limited toxicity data on PBP; however, again there are no measured concentrations of this compound. The only PEC/PNEC ratio that could be calculated based on very limited exposure data was for 2,4,6-TBP.
The natural production of brominated phenols by marine/estuarine biota precludes risk assessment of the marine environment. It has been assumed that natural production does not occur in freshwater environments. Brominated phenols are breakdown products of other brominated compounds — for example, bromobenzene and some brominated diphenyl ethers; measured concentrations may reflect this degradation rather than release of brominated phenols directly.
A SIDS Initial Assessment Report has been produced on 2,4,6-TBP for the OECD SIDS programme. It was discussed at the 17th SIDS Initial Assessment Meeting and subsequently revised.
Abrahamsson K, Klick S (1991) Degradation of halogenated phenols in anoxic natural marine sediments. Marine Pollution Bulletin, 22(5):227–233.
Adams JB, Lock SJ, Toward MR, Williams BM (1999) Bromophenol formation as a potential cause of "disinfectant" taint in foods. Food Chemistry, 64(3):377–381.
Ahn YB, Rhee SK, Fennell DE, Kerkhof LJ, Hentschel U, Häggblom MM (2003) Reductive dehalogenation of brominated phenolic compounds by microorganisms associated with the marine sponge Aplysina aerophoba. Applied and Environmental Microbiology, 69(7):4159–4166.
Álvarez-Rodríguez ML, López-Ocana L, López-Coronado JM, Rodríguez E, Martínez MJ, Coque JJR (2002) Cork taint of wines: role of the filamentous fungi isolated from cork in the formation of 2,4,6-trichloroanisole by O methylation of 2,4,6-tribromophenol. Applied and Environmental Microbiology, 68(12):5860–5869.
Anon (1979) Gigiena i Sanitariia, 44:19 [cited in NIOSH, 1991].
Anon (1992) Initial submission: letter to US EPA regarding information on pentabromophenol enclosed acute oral toxicity study (LD50) in albino rats with attachments (Report No. EPA/OTS 88-920003856S; NTIS/OTS 0536705).
Applegate VC, Howell JH, Hall AE, Smith MA (1957) Toxicity of 4,346 chemicals to larval lampreys and fishes. Washington, DC, US Department of the Interior, Fish and Wildlife Service (Report No. 207).
Bean RM, Mann DC, Wilson BW, Riley RG, Lusty EW, Thatcher TO (1980) Organohalogen production from chlorination of natural waters under simulated biofouling control conditions. In: Jolley RL, Brungs WA, Cumming RB, eds. Water chlorination: Environmental impact and health effects. Vol. 3. Ann Arbor, MI, Ann Arbor Science Publishers, pp. 369–377.
Bean RM, Mann DC, Neitzel DA (1983) Organohalogens in chlorinated cooling waters discharged from nuclear power station. In: Jolley RL, Brungs WA, Cotruvo JA, Cumming RB, Mattice JS, Jacobs VA, eds. Water chlorination: Environmental impact and health effects. Vol. 4. Ann Arbor, MI, Ann Arbor Science Publishers, pp. 382–390.
Bergman A (1990) Brominated flame retardants in a global environmental perspective. In: Freij L, ed. Proceedings of the workshop on brominated aromatic flame retardants, Skokloster, 24–26 October 1989. Solna, National Chemicals Inspectorate (KEMI).
BIBRA (1992) Toxicity profile. 2-Bromophenol. Carshalton, Surrey, BIBRA International Ltd, 3 pp.
Bidleman TF (1988) Atmospheric processes — wet and dry deposition of organic compounds are controlled by their vapor particle partitioning. Environmental Science & Technology, 22(4):361–367.
Blum DJW, Speece RE (1991) Quantitative structure–activity relationships for chemical toxicity to environmental bacteria. Ecotoxicology and Environmental Safety, 22:198–224.
Booth RA, Lester JN (1995) The potential formation of halogenated by-products during peracetic acid treatment of final sewage effluent. Water Research, 29(7):1793–1801.
Boyle AW, Phelps CD, Young LY (1999) Isolation from estuarine sediments of a Desulfovibrio strain which can grow on lactate coupled to the reductive dehalogenation of 2,4,6-tribromophenol. Applied and Environmental Microbiology, 65(3):1133–1140.
Boyle JL, Lindsay RC, Stuiber DA (1992) Bromophenol distribution in salmon and selected seafoods of fresh water and saltwater origin. Journal of Food Science, 57(4):918–922.
Broderius SJ, Kahl MD, Hoglund MD (1995) Use of joint toxic response to define the primary mode of toxic action for diverse industrial organic chemicals. Environmental Toxicology and Chemistry, 14(9):1591–1605.
Bruchajzar E, Szymanska JA, Piotrowski JK (2002) Acute and subacute nephrotoxicity of 2-bromophenol in rats. Toxicology Letters, 134:245–252.
Canadian Environmental Modelling Centre (2002) Level III model. Version 2.70. Peterborough, Ontario, Trent University, March.
CEC (2003) Technical guidance document on risk asssessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No 1488/94 on risk assessment for existing substances and Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Luxembourg, European Commission, Office for Official Publications of the European Communities.
Chatonnet P, Bonnet S, Boutou S, Labadie MD (2004) Identification and responsibility of 2,4,6-tribromoanisole in musty, corked odors in wine. Journal of Agricultural and Food Chemistry, 52:1255–1262.
Chen YP, Lincoln DE, Woodin SA, Lovell CR (1991) Purification and properties of a unique flavin-containing chloroperoxidase from the capitellid polychaete, Notomastus lobatus. Journal of Biological Chemistry, 266:23909–23915.
Chung HY, Ma WCJ, Ang PO, Kim JS, Chen F (2003a) Seasonal variations of bromophenols in brown algae (Padina arborescens, Sargassum siliquastrum, and Lobophora variegata) collected in Hong Kong. Journal of Agricultural and Food Chemistry, 51(9):2619–2624.
Chung HY, Ma WCJ, Kim JS (2003b) Seasonal distribution of bromophenols in selected Hong Kong seafood. Journal of Agricultural and Food Chemistry, 51:6752–6760.
CITI (1992) Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo, Chemical Inspection and Testing Institute.
CITI (1999) Report number 80492K. Tokyo, Chemical Inspection and Testing Institute [cited in IUCLID, 2003].
Clayton GD, Clayton FE, eds. (1993) Patty’s industrial hygiene and toxicology. Vol. 2. Toxicology. New York, NY, John Wiley and Sons.
Dankovic DA, Billings RE (1985) The role of 4-bromophenol and 4-bromocatechol in bromobenzene covalent binding and toxicity in isolated rat hepatocytes. Toxicology and Applied Pharmacology, 79:323–331.
DCC (1946) Results of range finding toxicological tests on 2,4-dibromophenol with cover letter and attachments. Midland, MI, Dow Chemical Corporation (Report No. EPA/OTS 86-910000333S; NTIS/OTS 0530105; submitted to US EPA by Dow Chemical Corporation in December 1990).
DCC (1958) Results of range finding toxicological tests on 2,4-dibromophenol with cover letter and attachments. Midland, MI, Dow Chemical Corporation (Report No. EPA/OTS 86-910000334S; NTIS/OTS 0530106; submitted to US EPA by Dow Chemical Corporation in December 1990).
DCC (1975) Acute dermal toxicity and industrial handling hazards of 2,4-dibromophenol with cover letter and attachments. Midland, MI, Dow Chemical Corporation (Report No. EPA/OTS 86-910000335S; NTIS/OTS 0530107; submitted to US EPA by Dow Chemical Corporation in December 1990).
Devillers J, Bintein S, Domine D (1996) Comparison of BCF models based on log P. Chemosphere, 33:1047–1065.
Draize JH (1959) Dermal toxicity. Appraisal of the safety of chemicals in foods, drugs and cosmetics. Austin, Texas, Texas State Department of Health, Association of Food and Drug Officials of the United States.
DSBG/BCL (1985a) Acute oral toxicity in the rat. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1985b) Primary skin irritation study in rabbits. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1996) Reverse mutation assay "Ames test" using Salmonella typhimurium. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1997a) Acute dermal toxicity (limit test) in rat. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1997b) Acute eye irritation test in the rabbit. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1997c) Magnusson and Kligman maximisation study in the guinea pig. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1998a) 96-hour acute toxicity study in carp with 2,4,6-tribromophenol (FR-613). Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1998b) Acute toxicity study in Daphnia magna with 2,4,6-tribromophenol (FR-613). Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (1998c) Fresh water algal growth inhibition test with 2,4,6-tribromophenol (FR-613). Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
DSBG/BCL (2002) Micronucleus test in bone marrow cells of the mouse with tribromophenol. Unpublished study of Dead Sea Bromine Group / Bromine Compounds Ltd [cited in IUCLID, 2003].
EAJ (1998) Chemicals in the environment. Tokyo, Environment Agency of Japan, Environmental Health Department, Environmental Health and Safety Division [cited in IUCLID 2003].
EAJ (2000a) Acute toxicity to daphnid (Daphnia magna). Ecotoxicity testing report (unpublished) (Test number 10032). Tokyo, Environment Agency of Japan, Japan Food Research Laboratories.
EAJ (2000b) Acute toxicity to himedaka (Oryzias latipes). Ecotoxicity testing report (unpublished) (Test number 10034). Tokyo, Environment Agency of Japan, Japan Food Research Laboratories.
EAJ (2000c) Growth inhibition test to algae (Selenastrum capricornutum) (Test number 10031). Tokyo, Environment Agency of Japan, Japan Food Research Laboratories.
Erickson SJ, Hawkins CE (1980) Effects of halogenated organic compounds on photosynthesis in estuarine phytoplankton. Bulletin of Environmental Contamination and Toxicology, 24:910–915.
Fennell DE, Rhee SK, Ahn YB, Haggblom MM, Kerkhof LJ (2004) Detection and characterization of a dehalogenating microorganism by terminal restriction fragment length polymorphism fingerprinting of 16S rRNA in a sulfidogenic, 2-bromophenol-utilizing enrichment. Applied and Environmental Microbiology, 70(2):1169–1175.
Fielman KT, Woodin SA, Lincoln DE (2001) Polychaete indicator species as a source of natural halogenated organic compounds in marine sediments. Environmental Toxicology and Chemistry, 20(4):738–747.
Flodin C, Whitfield FB (1999) Biosynthesis of bromophenols in marine algae. Water Science and Technology, 40(6):53–58.
Flodin C, Whitfield FB (2000) Brominated anisoles and cresols in the red alga Polysiphonia sphaerocarpa. Phytochemistry, 53(1):77–80.
Flodin C, Helidoniotis F, Whitfield FB (1999) Seasonal variation in bromophenol content and bromoperoxidase activity in Ulva lactuca. Phytochemistry, 51(1):135–138.
Fujishima A, Fujiwara M (1999) Single dose oral toxicity test of 2,4,6-tribromophenol in rats. Shizuoka, Japan Ministry of Health & Welfare, Biosafety Research Centre (Toxicity Testing Reports of Environmental Chemicals No. 7; (http://wwwdb.mhlw.go.jp/ginc/dbfile1/paper/paper118-79-6a.html) (in Japanese).
Geiger DL, Call DJ, Brooke LT, eds (1988) Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas). Vol. IV. Superior, WI, University of Wisconsin–Superior.
Giray C, King GM (1997a) Effect of naturally occurring bromophenols on sulfate reduction and ammonia oxidation in intertidal sediments. Aquatic Microbial Ecology, 13(3):295–301.
Giray C, King GM (1997b) Predator deterrence and 2,4-dibromophenol conservation by the enteropneusts Saccoglossus bromophenolosus and Protoglossus graveolens. Marine Ecology - Progress Series, 159:229–238.
Goerke H, Weber K (1990) Locality-dependent concentrations of bromophenols in Lanice conchilega (Polychaeta, Terebellidae). Comparative Biochemistry and Physiology B - Biochemistry & Molecular Biology, 97(4):741–744.
Goerke H, Weber K (1991) Bromophenols in Lanice conchilega (Polychaeta, Terebellidae) — the influence of sex, weight and season. Bulletin of Marine Science, 48(2):517–523.
Gribble GJ (2000) The natural production of organobromine compounds. Environmental Science and Pollution Research, 7(1):37–49.
Grove RS, Faeder EJ, Ospital J, Bean M (1985) Halogenated compounds discharged from a coastal power plant. In: Jolley RL, Bull RJ, Davies WP, Katz S, Roberts MH, Jacobs VA, eds. Water chlorination: Chemistry, environmental impact and health effects. Vol. 5. Chelsea, MI, Lewis Publishers, pp. 1371–1379.
Gutiérrez M, Becerra J, Barra R (2002) [Tribromophenol in sawmills: methods of analysis, physico-chemical properties and levels in environment components.] Boletin de la Sociedad Chilena de Química, 47(4):485–493 (in Spanish).
Häggblom MM, Young LY (1995) Anaerobic degradation of halogenated phenols by sulfate-reducing consortia. Applied and Environmental Microbiology, 61(4):1546–1550.
Hansch C, Leo A (1979) Substituent constants for correlation analysis in chemistry and biology. New York, NY, Wiley-Interscience.
Hansch C, Leo A, Hoekman D (1995) Exploring QSAR. In: Heller SR, ed. Hydrophobic, electronic, and steric constants. Washington, DC, American Chemical Society, p. 16 (American Chemical Society Professional Reference Book).
Heitz A, Allpike B, Joll CA, Blythe J, Kagi RI (2001) Plastic taste in drinking water caused by bromophenols. In: Proceedings of the Australian Water Association 19th federal convention, Canberra. Sydney, Australian Water Association.
Heitz A, Blyth J, Allpike B, Joll CA, Kagi R (2002) Plastic tastes in drinking water: factors affecting the chemistry of bromophenol formation. Water Supply, 2(5–6):179–184.
Herdt JR, Loomis LN, Nolan MO (1951) Public Health Reports, 66:1313.
Higa T, Fujiyama T, Scheuer PJ (1980) Halogenated phenol and indole constituents of acorn worms. Comparative Biochemistry and Physiology B - Biochemistry & Molecular Biology, 65:525–530.
HSDB (2003) Hazardous substances data bank. Bethesda, MD, National Institutes of Health, National Library of Medicine.
Industrial Bio-Test (1976a) Report to Michigan Chemical Corporation 48 hour dynamic aquatic toxicity study with 2,4,6-tribromophenol in Daphnia. Northbrook, IL, Industrial Bio-Test Laboratories, Inc. (Report No. EPA/OTS 86-900000310; NTIS/OTS0523302; submitted to US EPA by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Industrial Bio-Test (1976b) Report to Michigan Chemical Corporation four-day static aquatic toxicity studies with 2,4,6-tribromophenol in rainbow trout and bluegills. Northbrook, IL, Industrial Bio-Test Laboratories, Inc. (Report No. EPA/OTS 86-900000311; NTIS/OTS0523303; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Industrial Bio-Test (1976c) 21-day subacute dust inhalation toxicity study with 2,4,6-tribromophenol in albino rats. Northbrook, IL, Industrial Bio-Test Laboratories, Inc. (Report No. EPA/OTS 86-900000313; NTIS/OTS 0523305; submitted to US EPA by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Industrial Bio-Test (1977) 28 day subacute dermal toxicity study with 2,4,6-tribromophenol in albino rabbits. Northbrook, IL, Industrial Bio-Test Laboratories, Inc. (Report No. 8530-09439A; Report No. EPA/OTS 86-900000314; NTIS/OTS 0523306; submitted to US EPA by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Ingols RS, Gaffney PE, Stevenson PC (1966) Biological activity of chlorophenols. Journal of the Water Pollution Control Federation, 38:629–635.
IPCS (2004a) 2,4,6-Tribromophenol. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1563; http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc15/icsc1563.pdf).
IPCS (2004b) Pentabromophenol. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1564; http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc15/icsc1564.pdf).
IRDC (1973) Acute toxicity studies in rats and rabbits. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000302; NTIS/OTS 0523294; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1974a) Acute dermal toxicity in male and female albino rabbits. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000306; NTIS/OTS 0523298; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1974b) Acute inhalation toxicity in the albino rat. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000305; NTIS/OTS 0523297; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1974c) Acute oral toxicity LD50 study in albino rats. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000307; NTIS/OTS 0523299; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1974d) Eye irritation study in albino rabbits. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000304; NTIS/OTS 0523296; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1974e) Primary skin irritation study in albino rabbits. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000303; NTIS/OTS 0523295; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1975) Dermal sensitization study in the albino guinea pig. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000308; NTIS/OTS 0523300; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1978a) Acute oral toxicity LD50 study in rats. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000318; NTIS/OTS 0523310; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IRDC (1978b) Pilot teratology study in rats. Mattawan, MI, International Research Development Corporation (Report No. EPA/OTS 86-900000316; NTIS/OTS 0523308; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
IUCLID (2003) Data set for 2,4,6-tribromophenol. Ispra, European Chemicals Bureau, International Uniform Chemical Information Database.
Jaffe R, Hites RA (1986) Anthropogenic, polyhalogenated, organic compounds in non-migratory fish from the Niagara River area and tributaries to Lake Ontario (USA, Canada). Journal of Great Lakes Research, 12(1):63–71.
Jaworska JS, Schultz TW (1991) Comparative toxicity and structure–activity in Chlorella and Tetrahymena monosubstituted phenols. Bulletin of Environmental Contamination and Toxicology, 47(1):57–62.
Jensen P, Emrich R, Weber K (1992) Brominated metabolites and reduced numbers of meiofauna organisms in the burrow wall lining of the deep-sea enteropneust Stereobalanus canadensis. Deep-Sea Research Part A - Oceanographic Research Papers, 39(7–8):1247–1253.
JISHA (2002) Unpublished report. Tokyo, Japan Industrial Safety and Health Association [cited in IUCLID, 2003].
King GM (1986) Inhibition of microbial activity in marine sediments by a bromophenol from a hemichordate. Nature, 323:257–259.
King GM (1988) Dehalogenation in marine sediments containing natural sources of halophenols. Applied and Environmental Microbiology, 54(12):3079–3085.
Knight VK, Kerkhof LJ, Hägblom MM (1999) Community analyses of sulfidogenic 2-bromophenol-dehalogenating and phenol-degrading microbial consortia. FEMS Microbiology Ecology, 29:137–147.
Kondo M, Nishihara T, Shimamoto T, Koshikawa T, Itio T, Sawamura R, Tanaka K (1988) Biodegradation test of chemicals by cultivation methods. Eisei Kagaku, 34(2):188–195.
Kopperman HL, Carlson RM, Caple R (1974) Aqueous chlorination and ozonation studies. I. Structure–toxicity correlations of phenolic compounds to Daphnia magna. Chemico-Biological Interactions, 9(4):245–251.
Kühn R, Pattard M, Pernak KD, Winter A (1989a) Results of the harmful effects of selected water pollutants (anilines, phenols, aliphatic compounds) to Daphnia magna. Water Research, 23(4):495–499.
Kühn R, Pattard M, Pernak KD, Winter A (1989b) Results of the harmful effects of water pollutants to Daphnia magna in the 21 day reproduction test. Water Research, 23(4):501–510.
Kühn R, Pattard M (1990) Results of the harmful effects of water pollutants to green algae (Scenedesmus subspicatus) in the cell multiplication inhibition test. Water Research, 24(1):31–38.
Kuramochi H, Maeda K, Kawamoto K (2004) Water solubility and partitioning behavior of brominated phenols. Environmental Toxicology and Chemistry, 23(6):1386–1393.
Lau SS, Monks TJ, Gillette JR (1984a) Identification of 2-bromohydroquinone as a metabolite of bromobenzene and o-bromophenol: implications for bromobenzene-induced nephrotoxicity. Journal of Pharmacology and Experimental Therapeutics, 230:360–366.
Lau SS, Monks TJ, Greene KE, Gillette JR (1984b) The role of ortho-bromophenol in the nephrotoxicity of bromobenzene in the rat. Toxicology and Applied Pharmacology, 72:539–549.
Leo A, Hansch C, Elkins D (1971) Partition coefficients and their uses. Chemical Reviews, 71:525–616.
Lertratanangkoon K, Horning EC, Horning MG (1993) Pathways of formation of 2-bromophenol, 3-bromophenol and 4-bromophenol from bromobenzene — proposed mechanism for C–S lyase reactions of cysteine conjugates. Research Communications in Chemical Pathology and Pharmacology, 80(3):259–282.
Lide DR, ed. (2002) CRC handbook of chemistry and physics, 83rd ed. Boca Raton, FL, CRC Press.
Litton Bionetics (1978) Mutagenicity evaluation of 2,4,6-tribromophenol lot #3287 in the Ames Salmonella/microsome plate test (final). Kensington, MD, Litton Bionetics Inc. (Report No. EPA/OTS 86-900000317; NTIS/OTS 0523309; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Liu D, Thomson K, Kaiser K (1982) Quantitative structure toxicity relationship of halogenated phenols on bacteria. Bulletin of Environmental Contamination and Toxicology, 29(2):130–136.
Lovell CR, Steward CC, Phillips T (1999) Activity of marine sediment bacterial communities exposed to 4-bromophenol, a polychaete secondary metabolite. Marine Ecology - Progress Series, 179:241–246.
Lucas SV (1984) GC/MS analysis of organics in drinking water concentrates and advanced waste treatment concentrates. Vol. 1. Analysis results for 17 drinking water, 16 advanced waste treatment and 3 process blank concentrates. Columbus, OH, Health Effects Research Laboratory (Report No. EPA-600/1-84-020A).
Lyman WJ (1985) Chapter 2. In: Neely WB, Blau GE, eds. Environmental exposure from chemicals. Vol. 1. Boca Raton, FL, CRC Press, p. 31.
Lyman WJ, Reehl WF, Rosenblatt DH (1990) Handbook of chemical property estimation methods: Environmental behavior of organic compounds. Washington, DC, American Chemical Society.
Lyubimov AV, Babin VV, Kartashov AI (1998) Developmental neurotoxicity of 2,4,6-tribromophenol in Wistar rats. Neurotoxicology, 19(2):303–312.
Mardonnes C, Palma J, Sepúlveda C, von Baer D (2003) Determination of tribromophenol and pentachlorophenol and its metabolite pentachloroanisole in Asparagus officinalis by gas chromatography/mass spectrometry. Journal of Separation Science, 26:923–926.
Marr J, Kremer S, Sterner O, Anke H (1996) Transformation and mineralization of halophenols by Penicillium simplicissimum SK9117. Biodegradation, 7(2):165–171.
Meerts I, van Zanden JJ, Luijks EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A (2000) Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicological Sciences, 56(1):95–104.
Merck Index (2001) The Merck index — An encyclopedia of chemicals, drugs, and biologicals, 13th ed. Whitehouse Station, NJ, Merck & Co. Inc.
Meylan WM, Howard PH (1991) Bond contribution method for estimating Henry’s law constants. Environmental Toxicology and Chemistry, 10(10):1283–1293.
Meylan WM, Howard PH (1993) Computer estimation of the atmospheric gas-phase reaction rate of organic compounds with hydroxyl radicals and ozone. Chemosphere, 26(12):2293–2299.
Meylan WM, Howard PH (1995) Atom fragment contribution method for estimating octanol–water partition coefficients. Journal of Pharmaceutical Sciences, 84(1):83–92.
Meylan WM, Howard PH, Boethling RS (1992) Molecular topology fragment contribution method for predicting soil sorption coefficients. Environmental Science & Technology, 26(8):1560–1567.
Monks TJ, Lau SS, Highet RJ (1984) Formation of nontoxic reactive metabolites of p-bromophenol. Identification of a new glutathione conjugate. Drug Metabolism and Disposition, 12:432–437.
Monks TJ, Lau SS, Highet RJ, Gillette JR (1985) Glutathione conjugates of 2-bromohydroquinone are nephrotoxic. Drug Metabolism and Disposition, 13(5):553–559.
Monserrate E, Häggblom MM (1997) Dehalogenation and biodegradation of brominated phenols and benzoic acids under iron-reducing, sulfidogenic, and methanogenic conditions. Applied and Environmental Microbiology, 63(10):3911–3915.
Müller MD, Buser HR (1986) Halogenated aromatic compounds in automotive emissions from leaded gasoline additives. Environmental Science & Technology, 20(11):1151–1157.
Neely WB, Blau GE, eds (1985) Environmental exposure from chemicals. Boca Raton, FL, CRC Press.
NIOSH (1991) Registry of toxic effects of chemical substances, November 1991 ed. Cincinnati, OH, US Department of Health and Human Services, National Institute for Occupational Safety and Health [cited in BIBRA, 1992].
Nomani AA, Ajmal M, Ahmad S (1996) Gas chromatography mass spectrometric analysis of four polluted river waters for phenolic and organic compounds. Environmental Monitoring and Assessment, 40(1):1–9.
Öberg K, Warman K, Öberg T (2002) Distribution and levels of brominated flame retardants in sewage sludge. Chemosphere, 48(8):805–809.
Öberg T, Warman K, Bergström J (1987) Brominated aromatics from combustion. Chemosphere, 16:2451–2465.
Olsen CM, Meussen-Elholm ETM, Holme JA, Hongslo JK (2002) Brominated phenols: characterization of estrogen-like activity in the human breast cancer cell-line MCF-7. Toxicology Letters, 129(1–2):55–63.
Peijnenburg WJGM, Thart MJ, Denhollander HA, Vandemeent D, Verboom HH, Wolfe NL (1992) Reductive transformations of halogenated aromatic hydrocarbons in anaerobic water sediment systems — kinetics, mechanisms and products. Environmental Toxicology and Chemistry, 11(3):289–300.
Phipps GL, Holcombe GW, Fiandt JT (1981) Acute toxicity of phenol and substituted phenols to the fathead minnow. Bulletin of Environmental Contamination and Toxicology, 26(5):585–593.
Reinscheid UM, Bauer MP, Muller R (1996) Biotransformation of halophenols by a thermophilic Bacillus sp. Biodegradation, 7(6):455–461.
Richardson ML, Gangolli S, eds (1994) The dictionary of substances and their effects. Vol. 7. Cambridge, Royal Society of Chemistry, p. 522.
Ríos JC, Repetto G, Jos A, del Peso A, Salguero M, Cameán A, Repetto M (2003) Tribromophenol induces the differentiation of SH-SY5Y human neuroblastoma cells in vitro. Toxicology in Vitro, 17:635–641.
Rivera J, Ventura F (1984) In: Analysis of organic micropollutants. Angeletti G, Pjorseth A, eds. Dordrecht, D. Reidel Publishing Co., pp. 294–300.
Ronen Z, Abeliovich A (2000) Anaerobic–aerobic process for microbial degradation of tetrabromobisphenol-A. Applied and Environmental Microbiology, 66(6): 2372–2377.
Ronen Z, Vasiluk L, Abeliovich A, Nejidat A (2000) Activity and survival of tribromophenol-degrading bacteria in a contaminated desert soil. Soil Biology & Biochemistry, 32(11–12):1643–1650.
Rush GF, Newton JR, Maita K, Kuo C-H, Hook JB (1984) Nephrotoxicity of phenolic bromobenzene metabolites in the mouse. Toxicology, 30: 259–272.
Saitama Prefecture (1997) Saitama Prefecture white paper on the environment. Japan [cited in IUCLID, 2003].
Sasaki K, Kusakabe H, Takahashi T, Hashimoto K (1999) In vitro chromosomal aberration test of 2,4,6-tribromophenol on cultured Chinese hamster cells. Kanagawa Ministry of Health & Welfare, Hatano Research Institute (Toxicity Testing Reports of Environmental Chemicals No. 7; http://wwwdb.mhlw.go.jp/ginc/dbfile1/paper/paper118-79-6f.html) (in Japanese).
Schultz TW (1987) The use of the ionization constant (pKa) in selecting models of toxicity in phenols. Ecotoxicology and Environmental Safety, 14(2):178–183.
Schultz TW, Riggin GW (1985) Predictive correlations for the toxicity of alkyl- and halogen-substituted phenols. Toxicology Letters, 25:47–54.
Sheikh YM, Djerassi C (1975) 2,6-Dibromophenol and 2,4,6-tribromophenols — antiseptic secondary metabolites of Phoronopsis viridis. Experientia, 31:265–266.
Shibuya T, Hara T, Kawakami K (1999) Reverse mutation test of 2,4,6-tribromophenol on bacteria. Kanagawa, Japan Ministry of Health & Welfare, Hatano Research Institute (Toxicity Testing Reports of Environmental Chemicals No. 7; http://wwwdb.mhlw.go.jp/ginc/dbfile1/paper/paper118-79-6e.html) (in Japanese).
Sithole BB, Williams DT (1986) Halogenated phenols in water at forty Canadian potable water treatment facilities. Journal of the Association of Official Analytical Chemists, 69:807–810.
Sithole BB, Williams DT, Lastoria C, Robertson JL (1986) Determination of halogenated phenols in raw and potable water by selected ion gas chromatography–mass spectrometry. Journal of the Association of Official Analytical Chemists, 69:466–473.
Smeds A, Saukko P (2003) Brominated flame retardants and phenolic endocrine disrupters in Finnish human adipose tissue. Chemosphere, 53(9):1123–1130.
Spehar RL, Carlson RW, Lemke AE, Mount DI, Pickering QH, Snarski VM (1980) Effects of pollution on freshwater fish. Journal of the Water Pollution Control Federation, 52(6):1703–1768.
Steward CC, Lovell CR (1997) Respiration and assimilation of 4-bromophenol by estuarine sediment bacteria. Microbial Ecology, 33(3):198–205.
Steward CC, Pinckney J, Piceno Y, Lovell CR (1992) Bacterial numbers and activity, microalgal biomass and productivity and meiofaunal distribution in sediments naturally contaminated with biogenic bromophenols. Marine Ecology - Progress Series, 90:61–72.
Steward CC, Dixon TC, Chen YP, Lovell CR (1995) Enrichment and isolation of a reductively debrominating bacterium from the burrow of a bromometabolite-producing marine Hemichordate. Canadian Journal of Microbiology, 41(7):637–642.
Steward CC, Nold SC, Ringelberg DB, White DC, Lovell CR (1996) Microbial biomass and community structures in the burrows of bromophenol producing and non-producing marine worms and surrounding sediments. Marine Ecology - Progress Series, 133(1–3):149–165.
Stoner Laboratories (1978) The bioaccumulation of 2,4,6-tribromophenol in the bluegill sunfish. Santa Clara, CA, Stoner Laboratories, Inc. (Report No. EPA/OTS 86-900000319; NTIS/OTS 0523311; submitted to US EPA by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Sund KA, Nomura N (1963) Laboratory evaluation of several herbicides. Weed Research, 3:35–43.
Sweetman JA, Simmons MS (1980) The production of bromophenols resulting from chlorination of waters containing bromide ion and phenol. Water Research, 14(3):287–290.
Szymanska JA, Bruchajzer E, Piotrowski JK (1995) Investigations on acute hepato- and nephrotoxicity of pentabromophenol. International Journal of Occupational and Environmental Health, 8(3):245–254.
Tanaka R, Yamada R, Oba K, Iga T, Mikami S (1999) Combined repeat dose and reproductive/developmental toxicity screening test of 2,4,6-tribromophenol by oral administration in rats. Shizuoka, Japan Ministry of Health & Welfare, Biosafety Research Centre (Toxicity Testing Reports of Environmental Chemicals No. 7; http://wwwdb.mhlw.go.jp/ginc/dbfile1/paper/paper118-79-6d.html) (in Japanese).
Thomsen C, Janak K, Lundanes E, Becher G (2001a) Determination of phenolic flame retardants in human plasma using solid-phase extraction and gas chromatography–electron- capture mass spectrometry. Journal of Chromatography B -Analytical Technologies in the Biomedical and Life Sciences, 750(1):1–11.
Thomsen C, Leknes H, Lundanes E, Becher G (2001b) Brominated flame retardants in laboratory air. Journal of Chromatography A, 923(1–2):299–304.
Thomsen C, Lundanes E, Becher G (2001c) Brominated flame retardants in plasma samples from three different occupational groups in Norway. Journal of Environmental Monitoring, 3(4):366–370.
Thomsen C, Leknes H, Lundanes E, Becher G (2002a) A new method for determination of halogenated flame retardants in human milk using solid-phase extraction. Journal of Analytical Toxicology, 26(3):129–137.
Thomsen C, Lundanes E, Becher G (2002b) Brominated flame retardants in archived serum samples from Norway: A study on temporal trends and the role of age. Environmental Science & Technology, 36(7):1414–1418.
Tolosa I, Bayona JM, Albaiges J (1991) Identification and occurrence of brominated and nitrated phenols in estuarine sediments. Marine Pollution Bulletin, 22:603–607.
US EPA (2000) EPI Suite v. 3.11. Downloaded from http://www.epa.gov/oppt/exposure/docs/episuitedl.htm in July 2004. Washington, DC, US Environmental Protection Agency, Office of Pollution Prevention & Toxics.
VCC (1978a) Photolysis of 2,4,6-tribromophenol. Chicago, IL, Velsicol Chemical Corporation (Report No. EPA/OTS 86-900000323; NTIS/OTS 0523315; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
VCC (1978b) Pharmacokinetic study of 2,4,6-tribromophenol in rats. Chicago, IL, Velsicol Chemical Corporation (Report No. EPA/OTS 86-900000322; NTIS/OTS 0523314; submitted to US EPA by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
VCC (1990) 2,4,6-Tribromophenol (TBP) biodegradation. Chicago, IL, Velsicol Chemical Corporation (Report No. EPA/OTS 86-900000321; NTS/OTS 0523313; submitted to US EPA with test data and cover letter by Great Lakes Chemical Corporation, West Lafayette, IN, March 1990).
Verschueren K (1996) Handbook of environmental data on organic chemicals, 3rd ed. New York, NY, Von Nostrand Reinhold.
Watanabe I, Kashimoto T, Tatsukawa R (1984) Brominated phenol production from the chlorination of wastewater containing bromide ions. Bulletin of Environmental Contamination and Toxicology, 33(4):395–399.
Watanabe I, Takahashi K, Tatsukawa R (1985) Brominated phenols and anisoles in river and marine sediments in Japan. Bulletin of Environmental Contamination and Toxicology, 35:272–278.
Weber K, Ernst W (1978) Occurrence of brominated phenols in the marine polychaete Lanice conchilega. Naturwissenschaften, 65:262.
Weil ED (1993) Halogenated flame retardants. In: Kirk-Othmer encyclopedia of chemical technology, 4th ed. Vol. 10. Howe-Grant M, ed. New York, NY, John Wiley & Sons, pp. 954–958.
Whitfield FB (1990) Flavour of prawns and lobsters. Food Reviews International, 6(4):505–519.
Whitfield FB, Last JH, Shaw KJ, Tindale CR (1988) 2,6-Dibromophenols: the cause of an iodoform-like flavour in some Australian crustacea. Journal of the Science of Food and Agriculture, 46:29–42.
Whitfield FB, Shaw KJ, Walker DI (1992) The source of 2,6-dibromophenol: cause of an iodoform taint in Australian prawns. Water Science and Technology, 25(2):131–138.
Whitfield FB, Helidoniotis F, Svoronos D, Shaw KJ, Ford GL (1995) The source of bromophenols in some species of Australian ocean fish. Water Science and Technology, 31(11):113–120.
Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D (1997) Distribution of bromophenols in Australian wild-harvested and cultivated prawns (shrimp). Journal of Agricultural and Food Chemistry, 45(11):4398–4405.
Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D (1998) Distribution of bromophenols in species of ocean fish from eastern Australia. Journal of Agricultural and Food Chemistry, 46(9):3750–3757.
Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D (1999) Distribution of bromophenols in species of marine algae from eastern Australia. Journal of Agricultural and Food Chemistry, 47(6):2367–2373.
Whitfield FB, Helidoniotis F, Smith D (2002) Role of feed ingredients in the bromophenol content of cultured prawns. Food Chemistry, 79(3):355–365.
Woodin SA, Walla MD, Lincoln DE (1987) Occurrence of brominated compounds in soft-bottom benthic organisms. Journal of Experimental Marine Biology and Ecology, 107:209–217.
Zaitsev GM, Surovtseva EG (2000) Growth of Rhodococcus opacus on mixtures of monohalogenated benzenes and phenols. Microbiology, 69(4):401–405.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W (1987) Salmonella mutagenicity tests. 3. Results from the testing of 255 chemicals. Environmental Mutagenesis, 9:1–109.
|
ALP |
alkaline phosphatase |
|
BCF |
bioconcentration factor |
|
BP |
bromophenol |
|
CAS |
Chemical Abstracts Service |
|
CICAD |
Concise International Chemical Assessment Document |
|
DBP |
dibromophenol |
|
EC50 |
median effective concentration |
|
ECD |
electron capture detection |
|
EPA |
Environmental Protection Agency (USA) |
|
EU |
European Union |
|
GC |
gas chromatography |
|
GLP |
Good Laboratory Practice |
|
IC50 |
median inhibitory concentration |
|
ICSC |
International Chemical Safety Card |
|
IOMC |
Inter-Organization Programme for the Sound Management of Chemicals |
|
Koc |
soil–sediment partition coefficient |
|
Kow |
octanol–water partition coefficient |
|
LC50 |
median lethal concentration |
|
LD50 |
median lethal dose |
|
MITI |
Ministry of International Trade and Industry (Japan) |
|
MS |
mass spectrometry |
|
NOAEL |
no-observed-adverse-effect level |
|
NOEC |
no-observed-effect concentration |
|
NOEL |
no-observed-effect level |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PBP |
pentabromophenol |
|
PEC |
predicted exposure concentration |
|
PNEC |
predicted no-effect concentration |
|
SGPT |
serum glutamic–pyruvic transaminase |
|
SIDS |
screening information data set |
|
SIM |
selected ion monitoring |
|
TBP |
tribromophenol |
|
TeBP |
tetrabromophenol |
|
TG |
Test Guideline |
|
USA |
United States of America |
|
UV |
ultraviolet |
The draft CICAD on 2,4,6-TBP and other simple brominated phenols was sent for review to IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, Institut de recherche Robert Sauvé en santé et en sécurité du travail, Montreal, Canada
R. Benson, US Environmental Protection Agency, Denver, CO, USA
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
T.W. Cohen, Bromine Compounds Ltd, for European Chemical Industry Council (CEFIC), Beer-Sheva, Israel
P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
I. Desi, University of Szeged, Szeged, Hungary
S. Dungey, Environment Agency, Wallingford, United Kingdom
L. Fishbein, Fairfax, VA, USA
E. Frantik, Institute of Public Health, Prague, Czech Republic
H. Galal-Gorchev, US Environmental Protection Agency, Washington, DC, USA
H. Gibb, Sciences International, Alexandria, VA, USA
R. Henrich, Great Lakes Chemical Corporation, West Lafayette, IN, USA
R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
S. Humphreys, US Food and Drug Administration, College Park, MD, USA
O. Sabzevari, Ministry of Health and Medical Education, Tehran, Iran
H.V.T. Santonen, Institute of Occupational Health, Helsinki, Finland
H. Savolainen, Ministry of Social Affairs & Health, Tampere, Finland
S. Schmidt, University of Hamburg, Hamburg, Germany
P. Schulte, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
A. Smith, Health and Safety Executive, Bootle, Merseyside, United Kingdom
J.L. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
U. Stenius, Karolinska Institute, Stockholm, Sweden
H. Sweeney, US Health Attaché, Hanoi, Viet Nam
Deborah Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
K. Ziegler-Skylakakis, European Commission, Luxembourg
Hanoi, Viet Nam
28 September – 1 October 2004
Members
Mr D.T. Bai, Centre of Environmental Protection & Chemical Safety, Institute of Industrial Chemistry, Hanoi, Viet Nam
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Mr P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centres for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health of József Fodor Public Health Centre, Budapest, Hungary
Ms C.W. Fang, National Institute of Occupational Safety and Health Malaysia, Selangor, Malaysia
Dr L. Fishbein, Fairfax, VA, USA
Dr L. Fruchtengarten, Poison Control Center of Sao Paulo, Sao Paulo, Brazil
Dr C.L. Geraci, Document Development Branch, Centers for Disease Control and Prevention / National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr H. Gibb, Sciences International, Alexandria, VA, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
Mr P. Howe, Centre for Ecology & Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Health Sciences, Tokyo, Japan
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
Dr S. Kunarattanapruke, Food & Drug Administration, Ministry of Public Health, Nonthaburi, Thailand
Dr Y. Liang, Department of Occupational Health, Fudan University School of Public Health, Shanghai, China
Ms B. Meek, Existing Substances Division, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Food and Drug Control Labs, Ministry of Health & Medical Education, Tehran, Islamic Republic of Iran
Dr J. Stauber, CSIRO Energy Technology, Menai, New South Wales, Australia
Dr M.H. Sweeney, US Embassy, Hanoi, Viet Nam
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, New South Wales, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
2,4,6-TRIBROMOPHENOL ICSC:1563
Le présent CICAD,5 consacré au 2,4,6-tribromophénol et autres phénols bromés simples, a été élaboré par P. D. Howe, S. Dobson et H. M. Malcolm du Centre of Ecology & Hydrology, Royaume-Uni, et repose sur les données fournies par une recherche bibliographique très complète, menée sur des bases de données intéressantes jusqu’en janvier 2004. Ce CICAD et un document s’appliquant au Royaume-Uni ont été mis au point en parallèle. Des informations relatives à l’examen par des pairs du présent CICAD sont fournies à l’appendice 2. Ce CICAD a fait l’objet d’une discussion et d’une approbation en tant qu’évaluation internationale lors de la 12e réunion du Comité d’évaluation finale, qui s’est tenue à Hanoi (Vietnam), du 28 septembre au 1er octobre 2004. La liste des participants à la 12e réunion du Comité d’évaluation finale est donnée à l’appendice 3. Les fiches internationales de sécurité chimique relatives au 2,4,6-tribromophénol (ICSC 1563) et au pentabromophénol (ICSC 1564), établies par le Programme international sur la sécurité chimique (IPCS, 2004a, b) sont également reproduites dans le présent document.
Le présent CICAD porte sur le 2,4,6-tribromophénol (2,4,6-TBP, N° CAS
On sait que plusieurs espèces d’algues marines contiennent des phénols bromés simples. La présence de phénols bromés d’origine naturelle, produits par des animaux benthiques marins, est connue. Les vers appartenant à la classe Enteropneusta produisent et excrètent de grandes quantités de bromophénols, sans qu’aucune source alimentaire de ces composés puisse être mise en évidence. Les bromophénols naturels, comme le 4-BP, le 2,4-DBP, le 2,6-DBP et le 2,4,6-TBP, sont régulièrement présents dans les habitats constitués d’anciens fonds marins meubles et leur abondance dans l’espace et dans le temps est corrélée à celle de l’endofaune produisant ces métabolites.
La production de phénols bromés ou leur utilisation comme intermédiaire réactif dans la préparation d’agents ignifugeants ou comme produits conservateurs pour le bois peuvent entraîner des rejets de ces composés dans l’environnement. On ne dispose d’aucune donnée sur les concentrations de phénols bromés dans les matières plastiques contenant des agents ignifugeants dérivés du 2,4,6-TBP et sur l’éventuelle lixiviation des phénols bromés n’ayant pas réagi à partir de ces matières plastiques.
D’après les valeurs estimées de leur tension de vapeur, le 2,4,6-TBP et le PBP seraient présents à la fois en phase vapeur et sous forme particulaire dans l’atmosphère ambiante. Les phénols bromés en phase vapeur sont dégradés dans l’atmosphère par réaction des radicaux hydroxyle produits par voie photochimique, les demi-vies pour cette réaction dans l’air étant estimées à 13 h pour le 4-BP, à 45 h pour le 2,4-DBP et à une valeur comprise entre 20 et 40 jours pour le 2,4,6-TBP et le PBP. Le 2,4,6-TBP et le PBP en phase particulaire sont éliminés de l’atmosphère par dépôt humide ou sec.
Dans l’eau, on s’attend à ce que le PBP s’adsorbe sur les matières solides en suspension et les sédiments. Cependant, d’autres phénols ayant subi une bromation moins poussée auraient tendance à rester dans la phase vapeur. On ne s’attend pas à ce que la volatilisation du 2,4,6-TBP et du PBP non dissociés à partir des surfaces aqueuses constitue un processus important dans le devenir de ces espèces. Les valeurs des constantes de Henry pour les phénols monobromés et dibromés laissent prévoir une légère volatilisation de ces composés.
En cas de rejet dans le sol, l’ensemble des phénols bromés demeure essentiellement en place et ne sont pas mobilisés.
En général, les phénols bromés ne sont pas facilement biodégradables et persistent dans l’environnement. Néanmoins, des communautés adaptées et des communautés spécialisées de micro-organismes (tels que des micro-organismes anaérobies ou sulfurogènes) sont capables de dégrader ces composés.
D’après les valeurs de log Kow pour les bromophénols, le potentiel de bioaccumulation devrait augmenter avec le degré de bromation. On a calculé des valeurs prédictives du facteur de bioconcentration (BCF) de 20, 24, 120 et 3100 respectivement pour le 4-BP, le 2,4-DBP, le 2,4,6-TBP et le PBP. Les valeurs du BCF mesurées pour le 2,4,6-TBP sont proches de la valeur prévue.
Les concentrations maximales relevées dans l’eau douce de surface sont respectivement de 40, de 3 et de 0,3 µg/litre pour le 2,4-DBP, le 2,6-DBP et le 2,4,6-TBP. La présence de 4-BP n’a pas été détectée. Les concentrations dans les sédiments estuairiens atteignent jusqu’à 3690 µg/kg de poids sec pour le 2,4,6-TBP. On ne détecte pas de 2,4-DBP ou de 2,6-DBP dans ces sédiments (limite de détection 2 µg/kg).
Des phénols bromés peuvent se former par chloration des eaux naturelles contenant des ions bromure. On ne dispose de mesures des concentrations de phénols bromés dans l’eau de boisson que pour le Canada, où l’on signale des concentrations dans l’eau traitée atteignant au maximum 42, 60 et 20 ng/litre pour le 2-BP, le 2,6-DBP et le 2,4,6-TBP respectivement, chacune de ces valeurs concernant un échantillon d’eau unique. En général, les concentrations de phénols bromés dans l’eau de boisson sont inférieures à 3 ng/l, avec des valeurs plus fortes dans l’eau traitée que dans l’eau non traitée.
On a mesuré des concentrations de phénols bromés dans l’atmosphère ambiante résultant, au niveau local, de la combustion de déchets halogénés, de tourbe ou de carburant au plomb provenant de véhicules. Les valeurs relevées vont jusqu’à 380 ng/m3 et 4500 ng/m3 pour le 2,4,6-TBP provenant respectivement de la combustion de déchets halogénés et de la combustion de carburant pour véhicules, et jusqu’à 290 ng/m3 pour le 2,4-DBP provenant de la combustion de tourbe.
On a mesuré des concentrations de 2,4,6-TBP dans l’atmosphère des postes de travail d’une installation de production allant de 0,6 à 6,3 mg/m3.
Parmi les biotes susceptibles de faire partie de l’alimentation humaine, les fractions consommables contiennent en moyenne jusqu’à 198 µg/kg de poids sec de 2,4,6-TBP, jusqu’à 2360 µg/kg de poids sec de la même substance pour les mollusques et les crustacés et jusqu’à 39 µg/kg de poids sec pour les poissons de mer. On a détecté des phénols bromés dans le lait, le sang et les tissus adipeux humains.
L’absorption du 2,4,6-TBP à partir du tractus gastro-intestinal des mammifères et son excrétion via l’urine et les fèces s’effectuent rapidement. On ne dispose d’aucune donnée sur l’absorption, la distribution et l’élimination des autres phénols bromés.
Pour le 2,4,6-TBP, les valeurs de la DL50 orale aiguë chez le rat vont de 1486 à plus de 5000 mg/kg de poids corporel. Pour ce même animal, des valeurs de la LD50 orale de 652 et de 250 à 300 mg/kg de poids corporel ont été rapportées respectivement pour le 2-BP et le PBP. Chez le rat exposé à de la poussière de 2,4,6-TBP, on a déterminé une CL50 aiguë (4 h) par inhalation supérieure à 50 000 mg/m3. Les valeurs de la DL50 cutanée aiguë chez le rat et le lapin dépassent 2000 mg/kg de poids corporel.
Le 2,4,6-TBP n’est pas un irritant cutané, mais provoque une irritation oculaire modérée chez le lapin. Le 2,4,6-TBP provoque une sensibilisation cutanée chez le cochon d’Inde.
Une étude de toxicité orale à doses répétées, associée à un test préliminaire de toxicité pour la reproduction et pour l’environnement et menée sur des rats exposés à du 2,4,6-TBP, a mis en évidence, pour une dose de 1000 mg/kg de poids corporel/j, une diminution de la prise de poids, une augmentation en valeur absolue et relative du poids du foie chez les deux sexes et un accroissement des protéines totales, du taux d’albumine, du rapport albumine/globuline et du taux sanguin de phosphatase alcaline chez les rats mâles. A la dose de 300 mg/kg de poids corporel/j, on observe une salivation chez les deux sexes et une augmentation du taux de créatinine sanguin chez les rats mâles. On a considéré que la DSEIO était de 100 mg/kg/j chez les rats des deux sexes. Dans aucun des groupes traités, on n’a observé d’effet préjudiciable sur la cyclicité oestrale, le taux de copulation, le taux de fertilité, la durée de la période de gestation, le nombre de corps jaunes (corpora lutea), le nombre d’implants, le nombre total de jeunes et le nombre de jeunes vivants, le taux d’implantation ou le taux de mise bas. Parmi le groupe recevant une dose de 1000 mg/kg de poids corporel/j, la viabilité néonatale au 4e jour de lactation et le poids corporel au premier et au 4e jour de lactation étaient plus faibles que chez les sujets du groupe témoin. Aucun effet sur la reproduction ou sur le développement n’a été observé pour la dose de 300 mg/kg de poids corporel/j chez le rat.
S’agissant de la toxicité par inhalation répétée, aucune étude fiable n’a pu être retenue.
Les épreuves de mutation inverse réalisées in vitro avec le 2,4,6-TBP sur deux types de bactérie se sont révélées négatives. Un test d’aberration chromosomique in vitro a donné un résultat positif, avec ou sans activation métabolique. Un test du micronoyau mené in vivo jusqu’à la dose maximale tolérée s’est révélé négatif.
Le 2-BP, comme le PBP, présentent à forte dose chez le rat des effets néphrotoxiques, tandis que le 4-BP ne manifeste aucune toxicité pour les reins.
Les recherches bibliographiques n’ont relevé aucune donnée quant à la toxicité à court, moyen ou long terme des phénols bromés ou du PBP. La plupart des données sur la toxicité se réfèrent à 2,4,6-TBP. La population générale est exposée au 2,4,6-TBP via l’eau de boisson et la consommation de fruits de mer (dans le cas de ce composé, il s’agit d’un bromophénol d’origine naturelle). Néanmoins, la seule étude de toxicité par voie orale rapportée étant considérée comme un travail préliminaire, il n’est pas possible d’établir des doses admissibles fiables pour le 2,4,6-TBP dans l’eau de boisson et les aliments.
La CE50 à 72 h pour les microalgues va de 0,4 à 1,6 mg/l pour le 2,4,6-TBP. On a déterminé une CE50 à 48 h pour le 2-BP de 110 mg/l. La CL50 ou la CE50 à 48 h chez les daphnés va de 0,9 à 6 mg/l pour le 2-BP et le 4-BP et de 0,3 à 5,5 mg/l pour le 2,4,6-TBP. Les études chroniques fournissent une valeur de la NOEC à 21 jours pour la reproduction des daphnés de 0,2 mg/l pour le 2-BP et de 0,1 mg/l pour le 2,4,6-TBP. La CL50 à 96 h chez le poisson va de 0,2 à 6,8 mg/l pour le 2,4,6-TBP. Aucune étude n’a pu être retenue à propos de la toxicité pour les poissons des phénols bromés inférieurs. Pour le PBP, une CL50 à 96 h de 0,1 mg/l a été rapportée. Concernant l’environnement terrestre, seule une étude sur les effets du PBP sur la germination des semences a été retenue.
On a calculé une PNEC de 2 µg/l pour le 2-BP, de 6 µg/l pour le 4-BP, de 2 µg/l pour le 2,4,6-TBP et de 0,1 µg/l pour le PBP. Il convient de noter l’insuffisance des données, voire leur absence, à propos du 3-BP, des dibromophénols et du 2,3,4,6-TeBP. Les données relatives au PBP ont été prises en compte. Néanmoins, faute de disposer d’un jeu complet de données de base, il est impossible d’utiliser la PNEC concernant le PBP dans le cadre d’une évaluation des risques.
Le rapport PEC/PNEC devrait être de 0,15 pour le 2,4,6-TBP d’après une valeur unique relevée dans des eaux douces de surface. Il est impossible de calculer des facteurs de risque pour les autres phénols bromés en raison du manque de données de toxicité ou d’exposition. On prévoit que seul le PBP se lie préférentiellement aux sédiments. Cependant, les recherches bibliographiques n’ont pas retenu de valeur mesurée de la concentration de PBP dans les sédiments. On n’a trouvé ni phénols monobromés, ni phénols dibromés dans les sédiments. Par conséquent, les seules données disponibles pour les sédiments concernent le 2,4,6-TBP. D’après ce jeu de données très limité, le risque pour les organismes aquatiques semblerait faible. Les données disponibles sont insuffisantes pour permettre une évaluation correcte des risques pour l’environnement terrestre.
El presente CICAD6 sobre el 2,4,6-tribromofenol y otros bromofenoles simples, preparado por P. D. Howe, S. Dobson y H. M. Malcolm, del Centro de Ecología e Hidrología del Reino Unido, se basó en la información identificada en una búsqueda bibliográfica amplia de las bases de datos pertinentes realizada hasta enero de 2004. Se elaboraron al mismo tiempo el CICAD y un documento nacional del Reino Unido. La información sobre el examen colegiado de este CICAD figura en el apéndice 2. Este CICAD se examinó y aprobó como evaluación internacional en la 12Ş reunión de la Junta de Evaluación Final, celebrada en Hanoi, Viet Nam, del 28 de septiembre al 1ş de octubre de 2004. La lista de participantes en esta reunión figura en el apéndice 3. También se reproducen en este documento las fichas internacionales de seguridad química para el 2,4,6- tribromofenol (ICSC 1563) y el pentabromofenol (ICSC 1564), preparadas por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2004a,b).
En este CICAD se examinan el 2,4,6-tribromofenol (2,4,6-TBF; CAS Nş
Se sabe que varias especies de algas marinas contienen bromofenoles simples. Es también conocido que en la naturaleza hay bromofenoles porque los producen animales bentónicos marinos. Los gusanos espirales (Enteropneusta) producen y excretan grandes cantidades de bromofenoles sin ninguna fuente de alimentos evidente de estos compuestos. Los bromofenoles naturales, como el 4-BF, el 2,4-DBF, el 2,6-DBF y el 2,4,6-TBF son una característica constante de los hábitat marinos naturales de fondo blando y su riqueza espacial y temporal está en relación con la abundancia de la endofauna que produce estos metabolitos.
La producción de bromofenoles y su utilización como intermediarios reactivos pirorretardantes o como conservadores de la madera puede dar lugar a emisiones al medio ambiente. No se dispone de datos sobre sus concentraciones en la naturaleza ni sobre la posible lixiviación de bromofenoles que no han reaccionado procedentes de plásticos con pirorretardantes derivados del 2,4,6-TBF.
Las presiones de vapor estimadas indican que el 2,4,6-TBF y el PBF pueden existir en el medio ambiente atmosférico tanto en fase de vapor como sólida. Los bromofenoles en fase de vapor se degradan en la atmósfera por reacción con los radicales hidroxilo que se generan por la acción fotoquímica; se estima que la semivida de esta reacción en el aire es de 13 h para el 4-BF, de 45 h para el 2,4-DBF y de entre 20 y 40 días para el 2,4,6-TBF y el PBF. La fase sólida del 2,4,6-TBF y el PBF se elimina de la atmósfera mediante la deposición en medios húmedos o secos.
En el agua, cabría prever que el PBF se absorbería en partículas sólidas suspendidas y los sedimentos. Sin embargo, otros fenoles menos bromados tenderían a permanecer en la fase acuosa. No se prevé que la volatilización del 2,4,6-TBF y el PBF no disociados procedentes de superficies acuosas sea un proceso de destino final importante. Las constantes de la ley de Henry para los fenoles monobromados y dibromados parecen indicar una escasa volatilización de estos compuestos.
En caso de emisiones al suelo, todos los bromofenoles se mantienen básicamente allí y no se desplazan.
Los bromofenoles no suelen ser fácilmente biodegradables y persisten en el medio ambiente. Sin embargo, pueden degradar los compuestos comunidades adaptadas de microorganismos y otras especializadas (como las de anaerobios y sulfidogénicos).
Los valores del Log Kow para los bromofenoles dan estimaciones del potencial de bioacumulación que aumentan al elevarse el grado de bromación. Se han calculado factores de bioconcentración previstos de 20, 24, 120 y 3100 para el 4-BF, el 2,4-DBF, el 2,4,6-TBF y el PBF. Los factores de bioconcentración medidos para el 2,4,6-TBF son semejantes a los valores previstos.
Las concentraciones máximas notificadas en las aguas dulces superficiales fueron de 40, 3 y 0.3 µg/l para el 2,4-DBF, el 2,6-DBF y el 2,4,6-TBF, respectivamente; no se ha detectado 4-BF. Las concentraciones en el sedimento de los estuarios alcanzaron un valor de hasta 3690 µg/kg de peso seco para el 2,4,6-TBF. No se detectaron el 2,4-DBF y el 2,6-DBF (límite de detección de 2 µg/kg).
La cloración de aguas naturales que contienen iones bromuro puede generar bromofenoles. La medición de las concentraciones de éstos en el agua de bebida se limita al Canadá, donde las concentraciones más altas notificadas en el agua tratada son de 42, 60 y 20 ng/l para el 2-BF, el 2,6-DBF y el 2,4,6-TBF, respectivamente, cada una de ellas en una sola muestra de agua. En general, las concentraciones en el agua de bebida son inferiores a 3 ng/l, con niveles más altos en el agua tratada que en el agua sin tratar.
Se han medido las concentraciones de bromofenoles en el aire del medio ambiente local derivadas de la combustión de desechos halogenados, turba y combustible con plomo de los vehículos; los valores más altos notificados fueron de 380 y 4500 ng/m3 para el 2,4,6-TBF procedente de la combustión de desechos halogenados y del combustible de los vehículos, respectivamente, y de 290 ng/m3 para el 2,4-DBF derivado de la combustión de la turba.
Las concentraciones de 2,4,6-TBF en el aire del lugar de trabajo de una instalación de producción oscilaron entre 0,6 y 6,3 mg/m3.
Es probable que en la biota formen parte de la alimentación humana, con porciones comestibles que contienen concentraciones medias de 2,4,6-TBF de hasta 198 y 2360 µg/kg de peso seco en los moluscos y los crustáceos, respectivamente, y de hasta 39 µg/kg de peso seco en los peces marinos. Se han detectado bromofenoles en la leche, la sangre y el tejido adiposo humanos.
El 2,4,6-TBF se absorbe con rapidez del tracto gastrointestinal de los mamíferos y también se excreta con rapidez por la orina y las heces. No se disponía de información sobre la absorción, distribución y eliminación de otros bromofenoles.
Los valores de la DL50 aguda por vía oral para el 2,4,6-TBF en ratas fueron de 1486 a >5000 mg/kg de peso corporal. Para el 2-BF y el PBF se notificaron valores de la DL50 por vía oral en ratas de 652 y de 250–300 mg/kg de peso corporal, respectivamente. La CL50 aguda en ratas por inhalación (4 h) para el 2,4,6-TBF fue >50 000 mg/m3; se trataba de exposición a polvo de 2,4,6-TBF. Los valores de la DL50 aguda por vía cutánea en ratas y conejos fueron de >2000 mg/kg de peso corporal.
El 2,4,6-TBF no provocó irritación cutánea en conejos, pero sí ligeros efectos oculares; también fue sensibilizador cutáneo en cobayas.
En un estudio de la toxicidad oral con dosis repetidas combinado con una prueba de detección de la toxicidad en la reproducción/desarrollo del 2,4,6-TBF en ratas, con 1000 mg/kg de peso corporal al día se produjo una disminución del aumento de peso corporal, aumento del peso del hígado en valores absolutos y relativos en ambos sexos y aumento de las proteínas totales, la albúmina, la razón albúmina/globulina y la fosfatasa alcalina en la sangre de las ratas macho. Con 300 mg/kg de peso corporal al día se observó salivación en ambos sexos y un aumento de la concentración de creatinina en la sangre de las ratas macho. Se calculó una NOAEL de 100 mg/kg de peso corporal al día en ratas de ambos sexos. No se detectaron efectos adversos en la variación cíclica del estro, el índice de copulación, el índice de fecundidad, la duración del periodo de gestación, el número de cuerpos lúteos, el número de implantes, el número total de crías y de crías vivas, el índice de implantación o el índice de partos en ninguno de los grupos tratados. La viabilidad neonatal al cuarto día de la lactación y el peso al nacer y al cuarto día de la lactación en el grupo que recibía 1000 mg/kg de peso corporal al día fueron inferiores a los observados en el grupo testigo. No se detectaron efectos en la reproducción o el desarrollo de las ratas con 300 mg/kg de peso corporal al día.
No se encontró ningún estudio fidedigno sobre la toxicidad por inhalación repetida.
Los estudios de mutación inversa in vitro con 2,4,6-TBF en dos tipos de bacterias dieron resultados negativos. Una prueba de aberración cromosómica in vitro dio un resultado positivo con y sin activación metabólica. En una valoración de micronúcleos in vivo hasta la dosis máxima tolerada dio un resultado negativo.
Las dosis elevadas tanto de 2-BF como de PBF tienen efectos nefrotóxicos en las ratas, mientras que el 4-BF no muestra síntomas de nefrotoxicidad.
No se identificaron datos sobre la toxicidad a corto, medio o largo plazo de los fenoles menos bromados o del PBF. La mayoría de los datos sobre la toxicidad se refieren al 2,4,6-TBF. La exposición de la población general al 2,4,6-TBF tendría lugar a través del agua de bebida y del consumo de pescado y marisco (estos últimos por los bromofenoles que se producen de manera natural). Sin embargo, dado que el único estudio notificado de toxicidad a corto plazo por vía oral se considera una prueba de detección, no se pueden derivar valores fidedignos de la ingesta tolerable para el 2,4,6-TBF a partir del agua de bebida o los alimentos.
Las CE50 a las 72 h en las microalgas oscilan entre 0,4 y 1,6 mg/l para el 2,4,6-TBF; la CE50 a las 48 h para el 2-BF fue de 110 mg/l. Los valores de la CL/CE50 a las 48 horas en dáfnidos varían entre 0,9 y 6 mg/l para el 2-BF y el 4-BF y entre 0,3 y 5,5 mg/l para el 2,4,6-TBF. En estudios crónicos, los valores de la NOEC para la reproducción de los dáfnidos a los 21 días fueron de 0,2 mg/l para el 2-BF y de 0,1 mg/l para el 2,4,6-TBF. Los valores de la CL50 a las 96 h en los peces oscilan entre 0,2 y 6,8 mg/l para el 2,4,6-TBF. No se identificaron estudios sobre la toxicidad de los fenoles menos bromados en los peces. Se notificó una CL50 a las 96 h de 0,1 mg/l para el PBF. Para el medio terrestre, se encontró un solo estudio sobre los efectos del PBF en la germinación de las semillas.
Se han calculado valores de la PNEC de 2 µg/l para el 2-BF, de 6 µg/l para el 4-BF, de 2 µg/l para el 2,4,6-TBF y de 0,1 µg/l para el PBF. Hay que señalar que los datos para el 3-BF, los dibromofenoles y el 2,3,4,6-TeBF son insuficientes o no existen. Se han incluido datos sobre el PBF; sin embargo, no se debería utilizar la PNEC para el PBF en una evaluación del riesgo sin una base completa de información.
La razón PEC/PNEC para el 2,4,6-TBF sería de 0,15 basándose en un valor de vigilancia único de las aguas dulces superficiales. No se pueden calcular los factores de riesgo para el resto de los bromofenoles debido a la falta de datos sobre la toxicidad o la exposición. Sólo se podría pronosticar la unión preferente a los sedimentos para el PBF; sin embargo, no se han identificado valores medidos al respecto. No se encontró ninguna concentración de fenoles monobromados y dibromados en el sedimento. Por consiguiente, los únicos datos disponibles en relación con los sedimentos son los del 2,4,6-TBF. Sobre la base de esta serie de datos, muy limitada, el riesgo para los organismos acuáticos derivado de la presencia del 2,4,6-TBF parece ser bajo. No se dispone de datos suficientes para hacer una evaluación del riesgo bien fundamentada en el medio ambiente terrestre.
FOOTNOTES:
See Also:
Toxicological Abbreviations