
Concise International Chemical Assessment Document 21
2-FURALDEHYDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by
Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom,
Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United
Kingdom, and
Dr N. Gregg, Health and Safety Executive, Liverpool, United Kingdom
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
2-Furaldehyde.
(Concise international chemical assessment document ; 21)
1.Furaldehyde - toxicity 2.Risk assessment 3.Occupational
exposure 4.Environmental exposure I.International Programme
on Chemical Safety II.Series
ISBN 92 4 153021 9 (NLM Classification: QD 305.A6)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
6.2.1. Oral exposure
6.2.2. Inhalation exposure
6.2.3. Dermal exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic organisms
10.2. Terrestrial organisms
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting guidance values for 2-furaldehyde
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
11.2.1. Predicted environmental concentration
11.2.2. Predicted no-effect concentration
11.2.3. Environmental risk factors
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Health surveillance advice
13.2. Advice to physicians
13.3. Spillage
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 -- SOURCE DOCUMENT
APPENDIX 2 -- CICAD PEER REVIEW
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the relevant
scientific information concerning the potential effects of chemicals
upon human health and/or the environment. They are based on selected
national or regional evaluation documents or on existing EHCs. Before
acceptance for publication as CICADs by IPCS, these documents undergo
extensive peer review by internationally selected experts to ensure
their completeness, accuracy in the way in which the original data are
represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of
guidance values for health-based exposure limits. Geneva, World
Health Organization (Environmental Health Criteria 170).
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on 2-furaldehyde was based on a review of human health
concerns (primarily occupational) prepared by the United Kingdom's
Health and Safety Executive (Gregg et al., 1997), but it also contains
environmental information. Hence, this document focuses on exposures
via routes relevant to occupational settings but also includes an
environmental assessment. Data identified up to January 1997 were
covered in the review. A further literature search was performed up to
December 1997 to identify any new information published since the
review was completed, but no relevant studies were identified.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ISCS 0276) produced by the
International Programme on Chemical Safety (IPCS, 1993) has also been
reproduced in this document.
2-Furaldehyde (C5H4O2) (CAS No. 98-01-1) is a liquid with a
pungent "almond-like" odour. It is found in trace amounts in a number
of dietary sources and is produced commercially in batch or continuous
digesters where pentosans from agricultural residues are hydrolysed to
pentoses and the pentoses are subsequently cyclodehydrated to
2-furaldehyde. Industrial uses include the production of resins,
abrasive wheels, and refractories, refining of lubrication oils, and
solvent recovery. 2-Furaldehyde is also used, in very small
quantities, as a flavouring agent.
2-Furaldehyde is present in many food items as a natural product
or as a contaminant. Its presence in drinking-water and mothers' milk
has been reported, but levels were not sufficient for quantification.
Measured occupational inhalation exposures are available, and
estimations have also been made using a knowledge-based computer
system, Estimation and Assessment of Substance Exposure (EASE).
Generally, airborne exposure in all industries is below 8 mg/m3
(2 ppm), 8-h time-weighted average (TWA). There is insufficient
information to predict 15-min TWA exposures for most industries;
however, a 15-min TWA exposure between 1.2 and 6.8 mg/m3 (0.3 and 1.7
ppm) has been estimated in the flavouring industry. No measured data
are available for dermal exposure. EASE-predicted dermal exposures
between 0.1 and 1 mg/cm2 per day have been calculated for most
industries, with higher exposures of between 1 and 5 mg/cm2 per day
calculated for the industries manufacturing refractories and abrasive
wheels.
Toxicokinetic data are limited, but there are indications that
2-furaldehyde is readily absorbed via the inhalation and dermal
exposure routes. Animal studies demonstrate that, following oral
administration in rats, 2-furaldehyde is readily absorbed and rapidly
excreted mainly via the urine, although some elimination via exhaled
carbon dioxide also occurs. Metabolism is characterized by oxidation
or acetylation of the aldehyde group, followed by glycine conjugation.
2-Furoylglycine is the major urinary metabolite; other minor
metabolites include furoic acid, furanacrylic acid, and furanacryluric
acid.
In humans, absorption of the vapour via both the lungs and skin
has been demonstrated. Metabolism in humans appears similar to that in
rats, with the majority of the retained dose being excreted as urinary
2-furoylglycine. Furoic acid and furanacryluric acid are also detected
as minor metabolites. Dermal absorption from liquid 2-furaldehyde has
also been observed.
Acute toxicity data from animals are variable; overall, however,
2-furaldehyde is toxic by the inhalation and oral routes (4-h LC50,
940 mg/m3 [235 ppm]; oral LD50, about 120 mg/kg body weight), with
no clear information in relation to the dermal route. Respiratory
tract irritation and lung damage are consistently observed following
single and repeated inhalation exposure. Skin and eye irritation are
also reported. Apparently no throat or eye irritation was noted in
humans exposed to 40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for
4 h. In animals, no-observed-adverse-effect levels (NOAELs) of 80
mg/m3 (20 ppm) and 208 mg/m3 (52 ppm) in studies of up to 13 weeks'
duration have been identified in hamsters and rabbits, respectively,
for non-neoplastic effects. Malignant and benign tumours have been
observed in rats and mice following oral exposure to 60 and 50 mg/kg
body weight, respectively, for 103 weeks. 2-Furaldehyde is clearly
genotoxic in vitro in mammalian cells; although no firm conclusions
can be drawn on the genotoxic potential of 2-furaldehyde in vivo,
the possibility that genotoxicity could contribute to the carcinogenic
process cannot be discounted. These factors prevent the reliable
determination of a NOAEL for 2-furaldehyde.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to 2-furaldehyde from the general
environment precludes the characterization of potential cancer risks
for the general population.
In the occupational environment, there is a potential risk of
carcinogenic and genotoxic effects. The level of risk is uncertain; as
a result, there is a continuing requirement to reduce exposure levels
as much as is reasonably practicable with the technology that is
currently available.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The highest reported emissions of 2-furaldehyde to the
environment are from the wood pulp industry. 2-Furaldehyde will be
released to the atmosphere from natural and anthropogenic wood
burning.
No atmospheric effects are expected, as 2-furaldehyde is
destroyed by reaction with hydroxyl radicals at a calculated
atmospheric half-life of 0.44 days. In urban air, reaction with
nitrate radicals may be an additional degradation process. Direct
photo-oxidation may also occur. Low vapour pressure and low Henry's
law constant suggest only slow volatilization of 2-furaldehyde from
water and soil surfaces.
In water, hydrolysis is not expected to occur at environmental
pH. The low octanol/water partition coefficient (log Kow 0.41)
suggests low capacity for bioaccumulation. Sorption coefficients
( Koc) suggest little sorption to particulates and high mobility in
soils.
2-Furaldehyde is readily biodegraded in aerobic systems using
sewage sludge and in surface waters. Degradation also takes place
under anaerobic conditions, with a range of bacteria and other
microorganisms capable of degrading the compound as sole carbon
source. At high concentrations (>1000 mg/litre), 2-furaldehyde
inhibits growth and metabolic activity of unadapted anaerobic
cultures. However, acclimation increases the capacity of anaerobic
sludges to degrade the compound.
The highest reported concentrations of 2-furaldehyde in
industrial wastewaters are from sulfite evaporator condensate (about
15% of the waste stream from wood pulp mills), at an average of 274
mg/litre. A single study quantified 2-furaldehyde in indoor and
outdoor air at around 1 µg/m3; other studies have detected but not
quantified the compound.
Toxic thresholds for a variety of microorganisms have been
reported to range from 0.6 to 31 mg/litre. Acute LC50s for fish range
from 16 to 32 mg/litre.
Based on estimated predicted environmental concentrations from
wood pulp waste (expected to be the worst case) and the application of
an uncertainty factor of 1000 to the limited acute toxicity test data,
2-furaldehyde emissions are expected to pose a low risk to aquatic
organisms. There are no data on which to base a terrestrial risk
assessment, but emissions to land are expected to be low.
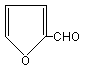
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
2-Furaldehyde (C5H4O2; molecular weight 96.09; CAS No.
98-01-1) is a liquid with a pungent "almond-like" odour. Its
structural formula is given below. A common synonym is furfural.
Others include furfurol, 2-furanaldehyde, fural, furfuraldehyde, and
2-furancarboxaldehyde. 2-Furaldehyde is colourless when freshly
distilled, but it darkens in contact with air. It is completely
miscible with most organic solvents, except saturated aliphatic
hydrocarbons, and is soluble in water (one source quotes 83 g/litre).
Its log Kow is 0.41, its vapour pressure is 0.144 kPa at 20°C, and
its dimensionless Henry's law constant (air/water partition
coefficient) is 1.5 × 10-4 (HSDB, 1998). Additional physical/chemical
properties are presented on the enclosed International Chemical Safety
Card (ISCS 0276).
1. EXECUTIVE SUMMARY
This CICAD on 2-furaldehyde was based on a review of human health
concerns (primarily occupational) prepared by the United Kingdom's
Health and Safety Executive (Gregg et al., 1997), but it also contains
environmental information. Hence, this document focuses on exposures
via routes relevant to occupational settings but also includes an
environmental assessment. Data identified up to January 1997 were
covered in the review. A further literature search was performed up to
December 1997 to identify any new information published since the
review was completed, but no relevant studies were identified.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ISCS 0276) produced by the
International Programme on Chemical Safety (IPCS, 1993) has also been
reproduced in this document.
2-Furaldehyde (C5H4O2) (CAS No. 98-01-1) is a liquid with a
pungent "almond-like" odour. It is found in trace amounts in a number
of dietary sources and is produced commercially in batch or continuous
digesters where pentosans from agricultural residues are hydrolysed to
pentoses and the pentoses are subsequently cyclodehydrated to
2-furaldehyde. Industrial uses include the production of resins,
abrasive wheels, and refractories, refining of lubrication oils, and
solvent recovery. 2-Furaldehyde is also used, in very small
quantities, as a flavouring agent.
2-Furaldehyde is present in many food items as a natural product
or as a contaminant. Its presence in drinking-water and mothers' milk
has been reported, but levels were not sufficient for quantification.
Measured occupational inhalation exposures are available, and
estimations have also been made using a knowledge-based computer
system, Estimation and Assessment of Substance Exposure (EASE).
Generally, airborne exposure in all industries is below 8 mg/m3
(2 ppm), 8-h time-weighted average (TWA). There is insufficient
information to predict 15-min TWA exposures for most industries;
however, a 15-min TWA exposure between 1.2 and 6.8 mg/m3 (0.3 and 1.7
ppm) has been estimated in the flavouring industry. No measured data
are available for dermal exposure. EASE-predicted dermal exposures
between 0.1 and 1 mg/cm2 per day have been calculated for most
industries, with higher exposures of between 1 and 5 mg/cm2 per day
calculated for the industries manufacturing refractories and abrasive
wheels.
Toxicokinetic data are limited, but there are indications that
2-furaldehyde is readily absorbed via the inhalation and dermal
exposure routes. Animal studies demonstrate that, following oral
administration in rats, 2-furaldehyde is readily absorbed and rapidly
excreted mainly via the urine, although some elimination via exhaled
carbon dioxide also occurs. Metabolism is characterized by oxidation
or acetylation of the aldehyde group, followed by glycine conjugation.
2-Furoylglycine is the major urinary metabolite; other minor
metabolites include furoic acid, furanacrylic acid, and furanacryluric
acid.
In humans, absorption of the vapour via both the lungs and skin
has been demonstrated. Metabolism in humans appears similar to that in
rats, with the majority of the retained dose being excreted as urinary
2-furoylglycine. Furoic acid and furanacryluric acid are also detected
as minor metabolites. Dermal absorption from liquid 2-furaldehyde has
also been observed.
Acute toxicity data from animals are variable; overall, however,
2-furaldehyde is toxic by the inhalation and oral routes (4-h LC50,
940 mg/m3 [235 ppm]; oral LD50, about 120 mg/kg body weight), with
no clear information in relation to the dermal route. Respiratory
tract irritation and lung damage are consistently observed following
single and repeated inhalation exposure. Skin and eye irritation are
also reported. Apparently no throat or eye irritation was noted in
humans exposed to 40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for
4 h. In animals, no-observed-adverse-effect levels (NOAELs) of 80
mg/m3 (20 ppm) and 208 mg/m3 (52 ppm) in studies of up to 13 weeks'
duration have been identified in hamsters and rabbits, respectively,
for non-neoplastic effects. Malignant and benign tumours have been
observed in rats and mice following oral exposure to 60 and 50 mg/kg
body weight, respectively, for 103 weeks. 2-Furaldehyde is clearly
genotoxic in vitro in mammalian cells; although no firm conclusions
can be drawn on the genotoxic potential of 2-furaldehyde in vivo,
the possibility that genotoxicity could contribute to the carcinogenic
process cannot be discounted. These factors prevent the reliable
determination of a NOAEL for 2-furaldehyde.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to 2-furaldehyde from the general
environment precludes the characterization of potential cancer risks
for the general population.
In the occupational environment, there is a potential risk of
carcinogenic and genotoxic effects. The level of risk is uncertain; as
a result, there is a continuing requirement to reduce exposure levels
as much as is reasonably practicable with the technology that is
currently available.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
The highest reported emissions of 2-furaldehyde to the
environment are from the wood pulp industry. 2-Furaldehyde will be
released to the atmosphere from natural and anthropogenic wood
burning.
No atmospheric effects are expected, as 2-furaldehyde is
destroyed by reaction with hydroxyl radicals at a calculated
atmospheric half-life of 0.44 days. In urban air, reaction with
nitrate radicals may be an additional degradation process. Direct
photo-oxidation may also occur. Low vapour pressure and low Henry's
law constant suggest only slow volatilization of 2-furaldehyde from
water and soil surfaces.
In water, hydrolysis is not expected to occur at environmental
pH. The low octanol/water partition coefficient (log Kow 0.41)
suggests low capacity for bioaccumulation. Sorption coefficients
( Koc) suggest little sorption to particulates and high mobility in
soils.
2-Furaldehyde is readily biodegraded in aerobic systems using
sewage sludge and in surface waters. Degradation also takes place
under anaerobic conditions, with a range of bacteria and other
microorganisms capable of degrading the compound as sole carbon
source. At high concentrations (>1000 mg/litre), 2-furaldehyde
inhibits growth and metabolic activity of unadapted anaerobic
cultures. However, acclimation increases the capacity of anaerobic
sludges to degrade the compound.
The highest reported concentrations of 2-furaldehyde in
industrial wastewaters are from sulfite evaporator condensate (about
15% of the waste stream from wood pulp mills), at an average of 274
mg/litre. A single study quantified 2-furaldehyde in indoor and
outdoor air at around 1 µg/m3; other studies have detected but not
quantified the compound.
Toxic thresholds for a variety of microorganisms have been
reported to range from 0.6 to 31 mg/litre. Acute LC50s for fish range
from 16 to 32 mg/litre.
Based on estimated predicted environmental concentrations from
wood pulp waste (expected to be the worst case) and the application of
an uncertainty factor of 1000 to the limited acute toxicity test data,
2-furaldehyde emissions are expected to pose a low risk to aquatic
organisms. There are no data on which to base a terrestrial risk
assessment, but emissions to land are expected to be low.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
2-Furaldehyde (C5H4O2; molecular weight 96.09; CAS No.
98-01-1) is a liquid with a pungent "almond-like" odour. Its
structural formula is given below. A common synonym is furfural.
Others include furfurol, 2-furanaldehyde, fural, furfuraldehyde, and
2-furancarboxaldehyde. 2-Furaldehyde is colourless when freshly
distilled, but it darkens in contact with air. It is completely
miscible with most organic solvents, except saturated aliphatic
hydrocarbons, and is soluble in water (one source quotes 83 g/litre).
Its log Kow is 0.41, its vapour pressure is 0.144 kPa at 20°C, and
its dimensionless Henry's law constant (air/water partition
coefficient) is 1.5 × 10-4 (HSDB, 1998). Additional physical/chemical
properties are presented on the enclosed International Chemical Safety
Card (ISCS 0276).
 The conversion factors for 2-furaldehyde in air at 20°C and 101.3
kPa are as follows:
1 ppm = 4.0 mg/m3
1 mg/m3 = 0.25 ppm
3. ANALYTICAL METHODS
Long- and short-term personal monitoring can be undertaken by
pumped sampling through XAD-2 resin impregnated with 2-(hydroxymethyl)
piperazine, desorbing with solvent (toluene), and analysing with gas
chromatography (NIOSH, 1987). The working range is 1.2-22 mg/m3
(0.3-5.5 ppm) for a 12-litre sample. Alternatively, a thermal
desorption tube may be used, in either the pumped or diffusive mode
(Patel et al., 1988). The working range for both is 4-40 mg/m3 (1-10
ppm) for a 10-litre sample. Screening measurements can be made with
colorimetric detector tubes, but these are unselective and not very
sensitive.
Biological monitoring of workers exposed to 2-furaldehyde is
possible by the analysis of 2-furoic acid (after alkaline hydrolysis
of the metabolite furoylglycine) in urine (Sedivec & Flek, 1978).
Although people not occupationally exposed to 2-furaldehyde have
background levels of 2-furoic acid in hydrolysed urine samples (from
dietary sources), these are low compared with those resulting from
occupational exposure (Nutley, 1989). The biological exposure index of
the American Conference of Government Industrial Hygienists is 200 mg
2-furoic acid/g creatinine (200 µmol/mmol creatinine), and a study in
the United Kingdom found that a 2-furoic acid concentration of over
160 mg/g creatinine (160 µmol/mmol creatinine) was likely to be due to
exposure to 2-furaldehyde at over 8 mg/m3 (2 ppm) (Nutley, 1989).
More sensitive analytical methods are required for monitoring in
food. Determination of the 2,4-dinitrophenylhydrazone derivative of
2-furaldehyde by high-performance liquid chromatography gives high
specificity, with a detection limit of 10 nmol/litre in spirits (Lo
Coco et al., 1992). A fast, semi-automatic, stopped-flow injection
analysis in which pretreatment of diversely coloured and turbid food
samples is not necessary has been developed (Espinosa-Mansilla et al.,
1993).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
2-Furaldehyde is found in a number of dietary sources. Because of
its formation during the thermal decomposition of carbohydrates,
2-furaldehyde is found in numerous processed foods and beverages,
including cocoa, coffee, tea, beer, wine, milk products, and bread
(Maga, 1979). It is also found in some fruits and vegetables, and it
is added as a flavouring agent to some foods. Industry in the United
Kingdom is voluntarily withdrawing 2-furaldehyde from addition to
food, although the chemical will still be present in food as a result
of its production during the thermal processing of anything containing
sugars. 2-Furaldehyde has also been found in the essential oils of
camphor, citronella, sassafras, lavender, and lime (Dunlop & Peters,
1953).
2-Furaldehyde is produced commercially in batch or continuous
digesters where pentosans from agricultural residues, including corn
cobs, oat hulls, rice hulls, and bagasse, are hydrolysed to pentoses
and the pentoses are subsequently cyclodehydrated to 2-furaldehyde.
2-Furaldehyde is shipped in steel tank cars, aluminium tank trucks,
and steel cans. When stored in bulk, the storage tanks normally have a
nitrogen blanket to prevent ingress of oxygen, which can cause
chemical degradation of the material.
Estimates of worldwide production are not available. US
production in 1983 was about 52 000 t (HSDB, 1998). The estimated
range of US production was 14 000-60 000 t in 1986, 2000-7000 t in
1990, and 11 000-45 000 t in 1994 (US EPA, 1998). Although
2-furaldehyde is not manufactured in the United Kingdom, it is
estimated that industry in the United Kingdom used approximately 3000
t in 1992. The official statistics indicate that imports into the
United Kingdom for the previous four years were 2800 t (1988), 3500 t
(1989), 3000 t (1990), and 3500 t (1991) (Gregg et al., 1997).
Industrial uses of 2-furaldehyde are summarized in Table 1. About
40% of 2-furaldehyde imported into the United Kingdom is used in the
production of resins, abrasive wheels, and refractories. The rest is
used in the refining of lubrication oils. Contacts with United Kingdom
industry in 1992 indicated that there was unlikely to be any
significant change in the pattern of use of 2-furaldehyde in the
foreseeable future.
Table 1: Industrial uses of 2-furaldehyde in the United Kingdom.
Industry Uses
1. Petrochemicals As a selective solvent in
the production of lubricating oils
2. Refractories As a reactive wetting agent in the
production of refractory components
3. Resin manufacture (a) Production of phenolic resins
(b) Production of cashew nutshell polymers
4. Abrasive materials As a reactive wetting agent for the resin
binder system in the production of abrasive wheels
5. Solvent recovery Steam distillation of spent 2-furaldehyde
for reuse by industry sectors 1, 2, and 4
6. Flavouring Used in very small quantities in
synthetic/natural oil blends
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The highest reported emissions of 2-furaldehyde to the
environment are from the wood pulp industry, with release to the
hydrosphere (section 6); release to water from other uses appears to
be substantially lower. The compound would be released to the
atmosphere from wood fires, a natural as well as anthropogenic source.
In the atmosphere, 2-furaldehyde will exist predominantly in the
vapour phase; the half-life for destruction by interaction with
hydroxyl radicals has been calculated at 0.44 days (HSDB, 1998).
Night-time destruction in the urban atmosphere may involve reaction
with nitrate radicals (Carter et al., 1981). Direct photo-oxidation
may occur in the atmosphere, based on experimental demonstration that
concentrations of 2-furaldehyde in wood smoke were reduced by
irradiation at sunlight and ultraviolet frequencies. Addition of
nitrogen oxides to wood smoke increased the rate of disappearance of
the compound (Kleindienst et al., 1986).
Virtually no degradation occurred in a solution of 2-furaldehyde
in distilled water over 30 days, suggesting that hydrolysis is not an
important process at environmental pH (Ettinger et al., 1954).
A Henry's law constant of 0.37 Pa.m3/mol at 25°C has been
calculated (HSDB, 1998), corresponding to a dimensionless Henry's law
constant (air/water partition coefficient) of 1.5 × 10-4. These
values indicate slow volatilization from water and damp soil surfaces;
an estimated half-life for volatilization from a model river of 9.9
days has been reported (HSDB, 1998).
2-Furaldehyde has a reported log Kow of 0.41, indicating low
capacity for bioaccumulation; calculated bioconcentration factors were
less than 1.2 (HSDB, 1998). The sorption coefficient ( Koc) can be
calculated as between 1.05 (Organisation for Economic Co-operation and
Development [OECD] Technical Guidance Manual) and 1.62 (Karickhoff et
al., 1979), indicating little sorption to particulates and a high
capacity for mobility in soil.
2-Furaldehyde was readily biodegraded in an aerobic batch culture
at 200 mg chemical oxygen demand (COD)/litre, with non-adapted sewage
sludge showing 96.3% degradation within 120 h and a degradation rate
of 37 mg COD/g per hour (Pitter, 1976). In a flow-through aerobic
laboratory bioreactor at an initial concentration of 300 mg/litre, 98%
degradation of 2-furaldehyde was reported using acclimated sewage
sludge. Degradation also occurred at a concentration of 1000 mg/litre
(Rowe & Tullos, 1980). 2-Furaldehyde was readily biodegradable in the
Japanese Ministry of International Trade and Industry (MITI) test
(Kawasaki, 1980). Wang et al. (1994) screened a range of
microorganisms for their ability to degrade 2-furaldehyde and reviewed
the earlier literature; some strains of the aerobic bacteria
Escherichia coli, Pseudomonas putida, and Rhodococcus
erythropolis and the yeast Hyphozyma rosoeniger were able to
degrade the compound to a greater or lesser degree. Inhibitory effects
of 2-furaldehyde on Pseudomonas putida were seen at concentrations
of 0.1% and above, with complete inhibition at 1% with exposure for 30
min (Kim et al., 1983).
2-Furaldehyde at 1 mg/litre was degraded completely in water from
the Great Miami, Little Miami, and Ohio rivers (USA) within 3 days
under aerobic conditions (Ettinger et al., 1954).
Under anaerobic conditions, 2-furaldehyde was completely degraded
to methane by unacclimated sewage sludge within 30 days at an initial
concentration of 580 mg/litre. The rate of methane production was
initially slower than in controls, indicating some interference of the
compound in the metabolism of the methanogenic bacteria. At an initial
concentration of 1160 mg/litre, gas production ceased after 5 days,
and none of the gas produced was methane; gas production did not
resume within the 28 days of the test, indicating that this
concentration was toxic to unacclimated microorganisms. Using
acclimated sludge (which had received 2-furaldehyde at 310 mg/litre
continuously for 8 months), full degradation occurred at 1160
mg/litre. At 2320 mg/litre, 2-furaldehyde was initially degraded
rapidly, but then gas production slowed for the remainder of the test
(Benjamin et al., 1984). A strictly anaerobic bacterium isolated from
a fermentor degrading sulfite evaporator condensate from wood pulp
production was able to use 2-furaldehyde as sole carbon source; the
organism was tentatively identified as Desulfovibrio sp. (Brune et
al., 1983). Methanococcus deltae was found to be able to use
2-furaldehyde as sole carbon source, degrading the compound to
furfuryl alcohol at initial concentrations of 5 and 10 mmol/litre (480
and 960 mg/litre); however, growth of the bacterium was inhibited at
concentrations of 20 and 25 mmol/litre (1920 and 2400 mg/litre). Other
methanogenic bacteria ( Methanobacterium thermoautotrophicum,
Methanosarcina barkeri, and Methanococcus thermolithotrophicus)
were unable to degrade the compound (Belay et al., 1997).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
2-Furaldehyde was detected in 1 out of 204 surface water samples
from heavily industrialized areas in the USA at 2 µg/litre (detection
limit 1 µg/litre) and in 1 out of 13 samples from the Lake Michigan
basin, USA, at 2 µg/litre in the late 1970s (HSDB, 1998).
2-Furaldehyde was not detected (detection limit 0.4 ng/litre) in
33 samples of surface waters in the 1996 monitoring of the general
environment in Japan (Japan Environment Agency, 1998). However, the
compound was detected in 6 out of 15 air samples (detection limit 40
ng/m3) in two cities (range 42-120 ng/m3). The analytical method was
not stated in the publication.
Levels of 2-furaldehyde in sulfite evaporator condensate, which
represents about 15% of the wastewater flow from pulp mills, have been
reported to range between 10 and 1280 mg/litre (Ruus, 1964) and
between 179 and 471 mg/litre (average 274 mg/litre) (Benjamin et al.,
1984). The 2-furaldehyde derives from pentoses in the wood pulp and is
formed during the waste treatment in the evaporator. 2-Furaldehyde was
detected in the wastewater of a synthetic rubber plant at 1.7 µg/litre
(Keith, 1974).
2-Furaldehyde was detected but not quantified in the air above
the Black Forest, Germany, in 1984-85 (Juttner, 1986). Although
2-furaldehyde has been identified in vehicle exhaust, it was not
detectable in air sampled from a road tunnel in the USA (Hampton et
al., 1982). The compound has been identified in smoke from burning
wood (Lipari et al., 1984; Kleindienst et al., 1986). Mean
concentrations of 2-furaldehyde measured in indoor and outdoor air in
suburban New Jersey, USA, in summer 1992 were 1.06 µg/m3 (0.27 ppb)
and 0.67 µg/m3 (0.17 ppb), respectively. The presence of the compound
in indoor air was presumed to be from emissions during cooking (Zhang
et al., 1994).
6.2 Human exposure
6.2.1 Oral exposure
2-Furaldehyde has been identified but not quantified as a major
flavour component in a range of food items, including beef, soy sauce,
roasted nuts, fried bacon, nectarines, baked potatoes, clove oil,
preserved mangoes, rum, roasted coffee, and blue cheese (HSDB, 1998).
Levels reported in food are as follows: non-alcoholic beverages, 4
mg/litre; alcoholic beverages, 10 mg/litre; ice creams, ices, etc., 13
mg/kg; candy, 12 mg/kg; baked goods (unspecified), 17 mg/kg; gelatins
and puddings, 0.8 mg/kg; chewing gum, 45 mg/kg; and syrups, 30
mg/litre (HSDB, 1998). 2-Furaldehyde was qualitatively identified in
two out of eight samples of mothers' milk from urban sites in the USA
(Pellizzari et al., 1982). Qualitative identification of 2-furaldehyde
in drinking-water has been reported from both the USA and Europe; no
quantification was reported (Kool et al., 1982).
6.2.2 Inhalation exposure
The remainder of the human exposure data available to the authors
of this CICAD are restricted to the occupational environment.
Measured inhalation exposure information was provided to the
United Kingdom's Health and Safety Executive by the petrochemical
industry. The Health and Safety Executive's National Exposure Data
Base contains information on exposure for the use of 2-furaldehyde in
the refractory industry and for a number of miscellaneous processes.
In all the exposure groupings below, when routine and breakdown
maintenance takes place and significant exposure to airborne
2-furaldehyde is anticipated, it is expected that appropriate
respiratory protective equipment would be used to minimize inhalation
exposure.
Exposures in the petrochemicals, resin and polymer manufacture,
and distillation industries are likely to be very similar. EASE
(Version 2) predictions seem to be in accord with the limited amount
of measured exposure information provided by the petrochemicals
industry (Gregg et al., 1997). The EASE predictions indicate that 8-h
TWA exposures will be somewhat below 8 mg/m3 (2 ppm). There is
insufficient information to predict 15-min TWA exposures for these
industries.
Exposures in the refractories and abrasive wheels manufacturing
industries are likely to be very similar. The high exposures measured
by the Health and Safety Executive in the refractories industry (20%
of 8-h TWA exposures are greater than 40 mg/m3 [10 ppm]) are likely
to be reduced following advice given about the application of
efficient local exhaust ventilation and the enclosure of the process
where possible (Gregg et al., 1997). The EASE-predicted 8-h TWA
exposures likely to result from these improvements will be between 2
and 12 mg/m3 (0.5 and 3 ppm) (Gregg et al., 1997). There is every
likelihood that exposures will actually be at the lower end of the
range -- i.e., less than 8 mg/m3 (2 ppm). There is insufficient
information to predict 15-min TWA exposures for these industries.
In the flavouring industry, very little 2-furaldehyde appears to
be used. The EASE model predicts exposures to be low: the 15-min TWA
exposure is between 1.2 and 6.8 mg/m3 (0.3 and 1.7 ppm), and the 8-h
TWA is between 0.04 and 0.2 mg/m3 (0.01 and 0.05 ppm) (Gregg et al.,
1997).
For oilseed residue application, no personal exposure was
detected. The extent of this industry is not known, but it seems that
8-h exposures to 2-furaldehyde are likely to be well below 8 mg/m3
(2 ppm) (Gregg et al., 1997). No comment can be made upon likely
short-term exposures.
Little is known about the extent of the use of 2-furaldehyde in
the glass reinforced plastics industry. It is very likely that the
application of appropriate controls would result in 8-h TWA exposures
to 2-furaldehyde being reduced to below 8 mg/m3 (2 ppm) (Gregg et
al., 1997). No comment can be made upon possible short-term exposures.
6.2.3 Dermal exposure
The industrial processes can be grouped in a similar manner to
that adopted for the general discussion of inhalation exposure above
(section 6.2.2). These predictions do not take account of personal
protective equipment that may be worn. Such equipment may
significantly reduce dermal exposure.
The EASE-predicted dermal exposures for the petrochemicals, resin
and polymer manufacture, and distillation industries are similar
-- namely, between 0.1 and 1 mg/cm2 per day (Gregg et al., 1997).
For the refractories and abrasive wheels manufacturing
industries, the EASE-predicted dermal exposures are between 1 and 5
mg/cm2 per day (Gregg et al., 1997). If improvements are made to the
process containment, opportunities for dermal contact will be reduced,
and predicted exposures will be reduced to between 0.1 and 1 mg/cm2
per day.
For the use of 2-furaldehyde in the flavourings industry, the
EASE prediction for dermal exposure is between 0 and 0.1 mg/cm2 per
day (Gregg et al., 1997). Insufficient information is provided for the
other minor uses to make predictions of dermal exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
No animal studies are available examining the toxicokinetics of
2-furaldehyde by the inhalation or dermal routes; however, signs of
systemic toxicity observed in animal studies (see section 8) indicate
that 2-furaldehyde is readily absorbed via both these routes of
exposure.
Animal studies demonstrate that, following oral administration in
rats, 2-furaldehyde is readily absorbed and rapidly excreted, with up
to 85% of the administered dose being detected in the urine within 24
h (Nomier et al., 1992). Some elimination via exhaled carbon dioxide
(7% of dose) also occurs. Metabolism is characterized by oxidation or
acetylation of the aldehyde group, followed by glycine conjugation.
2-Furoylglycine is the major urinary metabolite, accounting for
approximately 80% of the administered dose (Laham & Potvin, 1989;
Nomier et al., 1992; Parkash & Caldwell, 1994). Other minor
metabolites include furoic acid, furanacrylic acid, and furanacryluric
acid.
In humans, absorption of the vapour via both the lungs and skin
has been demonstrated (Flek & Sedivec, 1978a,b). Metabolism appears
similar to that in rats, with the majority of the retained dose being
excreted as urinary 2-furoylglycine. Furoic acid and furanacryluric
acid are also detected as minor metabolites. Dermal absorption from
liquid 2-furaldehyde has also been observed.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Unpublished or briefly reported papers indicate 1-, 4-, and 6-h
inhalation LC50 values in rats of 4148, 940, and 700 mg/m3 (1037,
235, and 175 ppm), respectively (Woods & Seevers, 1956; Terrill et
al., 1989). In contrast, an apparently well-conducted published study
indicated a 1-h LC50 value in rats of 756 mg/m3 (189 ppm) (Gupta et
al., 1991; Mishra et al., 1991). In the rat and other species,
respiratory tract irritation and lung damage were consistently
observed following inhalation exposure, with lung oedema and
congestion being reported in one study in rats immediately after
exposure to 380 mg/m3 (95 ppm) for 1 h. Oral LD50 values in rats
were in close agreement, ranging from 122 to 158 mg/kg body weight
(Woods & Seevers, 1955; SRI International, 1982). Toxic signs observed
indicated central nervous system depressant effects, with convulsions
also observed. The lungs also appeared to be a target organ via oral
dosing. No reliable information was available for the dermal route of
exposure.
8.2 Irritation and sensitization
No signs of skin irritation were noted in rabbits following a
single application of liquid 2-furaldehyde for 12 h, although mild
irritation was observed following a 48-h application (Woods & Seevers,
1955). Intense but reversible skin irritation was reported in
guinea-pigs after repeated application of undiluted liquid
2-furaldehyde (Agakishiyev, 1989, 1990).
A single instillation of liquid 2-furaldehyde produced gross
corneal opacities in rabbits (Woods & Seevers, 1955). 2-Furaldehyde
vapour produced eye irritation in several studies following repeated
exposure in different species (Gardner, 1925; Feron et al., 1979;
Gupta et al., 1991).
No information is available on the ability of 2-furaldehyde to
act as a skin or respiratory sensitizer.
8.3 Short-term exposure
The principal effects of repeated inhalation exposure were
similar to those seen in single-exposure experiments. Groups of rats
were exposed to 0 or 160 mg 2-furaldehyde/m3 (0 or 40 ppm) 1 h/day
for 5, 15, or 30 days (Gupta et al., 1991; Mishra et al., 1991), and
groups of rabbits were exposed to 208, 520, or 1040 mg/m3 (52, 130,
or 260 ppm) 4 h/day, 5 days/week, for at least 60 exposures
(Castellino et al., 1963). Respiratory irritation, hyperplasia and
degeneration of the olfactory epithelium, and lung congestion, oedema,
and inflammation were observed in rats following exposure to 160
mg/m3 (40 ppm) (Gupta et al., 1991; Mishra et al., 1991) and in
rabbits following exposure to 1040 mg/m3 (260 ppm) (Castellino et
al., 1963). There was also evidence of kidney damage seen
histopathologically and anaemia in rabbits following exposure to 520
mg 2-furaldehyde/m3 (130 ppm). A NOAEL of 208 mg/m3 (52 ppm) was
identified in rabbits; a NOAEL was not identified for rats.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
Groups of hamsters were exposed to 0, 80, 460, or 2208 mg/m3
(0, 20, 115, or 552 ppm) 6 h/day, 5 days/week, for 13 weeks (Feron et
al., 1979). Respiratory irritation, hyperplasia and degeneration of
the olfactory epithelium, and lung congestion, oedema, and
inflammation were observed in hamsters following exposure to 2208
mg/m3 (552 ppm) (Feron et al., 1979). Slight atrophy and hyperplasia
of the olfactory epithelium were also seen following exposure to 460
mg/m3 (115 ppm). A NOAEL of 80 mg/m3 (20 ppm) was identified.
Groups of 20 male and female rats received 0, 11, 22, 45, 90, or
180 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Mild to moderate centrilobular
vacuolation of hepatocytes was observed among all treated groups,
although there was no clear relationship between dose and incidence or
severity of the observed effect.
Groups of 20 male and 20 female mice received 0, 75, 150, 300,
600, or 1200 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Relative liver weights were
increased in a dose-related manner in all treated groups.
Centrilobular coagulative necrosis of hepatocytes was seen at
150 mg/kg body weight per day or more, and mild mononuclear
inflammatory cell infiltrate was seen in all treated groups.
8.4.2 Chronic exposure and carcinogenicity
No inhalation carcinogenicity studies in rats or mice were
available. An inhalation study in hamsters showed no evidence that
2-furaldehyde vapour had carcinogenic potential following 52 weeks'
exposure to 1000-1600 mg/m3 (250-400 ppm), 7 h/day, 5 days/week, by
this route (Feron & Kruysse, 1978). Nor was there any indication that
2-furaldehyde was carcinogenic to hamsters after 36 weekly
intratracheal instillations (Feron, 1972). However, both these studies
are not satisfactory negative studies, owing to their limited
duration.
In a gavage study, groups of 50 male and 50 female F344/N rats
were treated with 0, 30, or 60 mg 2-furaldehyde/kg body weight per
day, 5 days/week for 103 weeks (NTP, 1990). Animals were observed
twice daily, weighed monthly, and subjected to a thorough gross and
microscopic examination either at death or at the end of the study.
Mortality in males was not significantly affected by 2-furaldehyde
treatment throughout the study. Corresponding survival rates among
females were 18/50, 32/50, and 28/50. However, 19 of the 22 top-dose
deaths in females were attributed to accidental gavage errors. There
were no differences in body weight between test and control animals
throughout the study, and there were no treatment-related clinical
signs of toxicity. The main non-neoplastic changes observed in test
animals were minimal to mild centrilobular necrosis of the liver in
male rats (3/50, 9/50, 12/50) and biliary dysplasia with fibrosis in
two top-dose males.
Two cases of cholangiocarcinoma were seen in two other top-dose
males. The historical incidence of bile duct neoplasms in corn oil
vehicle control male F344/N rats in this laboratory was reported to be
3/2145 (0.1%). No other findings of biological significance were
observed. Hence, in this study, treatment by gavage with 2-furaldehyde
produced an increase in bile duct carcinoma in male rats only,
although a valid assessment of the carcinogenicity of 2-furaldehyde in
female rats cannot be made because of the low numbers of animals
surviving to the end of the study. Also, the high incidence of
accidental deaths casts doubt over the quality of the experimental
conditions.
Groups of 50 male and 50 female B6C3F1 mice were also dosed with
0, 50, 100, or 175 mg 2-furaldehyde/kg body weight per day by gavage,
5 days/week for 103 weeks, in the NTP study (NTP, 1990). Animals were
observed twice daily, weighed monthly, and subjected to a thorough
gross and microscopic examination either at death or at the end of the
study. No significant differences in survival were observed between
2-furaldehyde-treated and untreated animals throughout the study.
There were no differences in body weight gain between test and control
animals throughout the study, and there were no clinical signs of
treatment-related toxicity.
Non-neoplastic changes included chronic inflammation and
multifocal green-brown, granular pigmentation of the subserosa of the
liver in mid- and top-dose mice of each sex and forestomach
hyperplasia in 2-furaldehyde-treated female mice only. The principal
neoplastic changes observed were increases in hepatocellular adenomas
and hepatocellular carcinomas in males (adenoma: 9/50 [18%], 13/50
[26%], 11/49 [22%], 19/50 [38%]; carcinoma: 7/50 [14%], 12/50 [24%],
6/49 [12%], 21/50 [42%]; in control, low-, mid-, and top-dose animals,
respectively). Only adenomas were observed in females, the incidences
being dose-related: 1/50 (2%), 3/50 (6%), 5/50 (10%), 8/50 (16%).
Increases in adenomas and/or carcinomas were statistically significant
only in the top-dose animals of both sexes. An increase in squamous
cell papillomas of the forestomach was also seen in top-dose female
mice, although this increase was not statistically significant (1/50
[2%], 0/50 [0%], 1/50 [2%], 6/50 [12%] in control, low-, mid-, and
top-dose animals). No other findings of biological significance were
observed.
In an initiation/promotion study in mice using the dermal route,
an increased incidence of skin tumours was seen when 2-furaldehyde was
applied in combination with the promoting agent (5/20 compared with
1/20 in controls that received the promoting agent in combination with
dimethyl sulfoxide) (Miyakama et al., 1991). When 2-furaldehyde was
applied in combination with acetone, no skin tumours were seen.
Following the administration of an unstated amount of
2-furaldehyde to rats for 5 months, evidence of pre-neoplastic changes
(the formation of glutathione S-transferase placental form, GST-P,
foci) was seen in livers (Shimizu et al., 1989). Liver cirrhosis was
observed in treated rats; overall, however, it is impossible to reach
any conclusions about the possible mechanisms underlying cancer
induction from this study (e.g., to what extent cell proliferation or
direct mutagenicity may be involved in liver tumour formation).
8.5 Genotoxicity and related end-points
Experiments in cell-free systems have demonstrated that
2-furaldehyde causes DNA damage (Hadi & Rehman, 1989). Although
bacterial mutation studies with 2-furaldehyde have been largely
negative, they have in general been inadequately reported (Zdzienicka
& Tudek, 1978; McMahon et al., 1979; Loquet et al., 1981; Soska et
al., 1981; Marnett et al., 1985; Mortelmans et al., 1986; Shinohara &
Omura, 1986; Kim et al., 1987, 1988; Nakamura et al., 1987; Shane et
al., 1988; Kato et al., 1989). However, 2-furaldehyde is clearly
genotoxic in vitro in mammalian cell test systems, producing
chromosomal aberrations, gene mutations, and sister chromatid
exchanges (Stich et al., 1981; Gomez-Arroyo & Souza, 1985; McGregor et
al., 1988; Nishi et al., 1989; NTP, 1990). In addition, one positive
result and one negative result were obtained in Drosophila tests
(Woodruff et al., 1985; Rodriguez-Arnaiz et al., 1992).
The genotoxic potential of 2-furaldehyde in vivo is less
certain, with positive results being claimed in a briefly reported
cytogenetics study and negative results being reported in another
cytogenetics study and a sister chromatid exchange study (Subramanyam
et al., 1989; NTP, 1990). Further details of these studies are
provided in the source document to this CICAD (Gregg et al., 1997).
Overall, no firm conclusions can be drawn from these reports.
Point mutations in K-ras and H-ras genes were seen in the
2-furaldehyde-treated mouse livers taken from the NTP carcinogenicity
assay, demonstrating genotoxicity (possibly direct) at the tumour site
(Reynolds et al., 1987).
8.6 Reproductive and developmental toxicity
No studies specifically addressing these end-points are
available.
8.7 Immunological and neurological effects
No studies specifically addressing these end-points are
available.
9. EFFECTS ON HUMANS
The only effects reported following single exposure in humans
relate to irritation. Throat and eye irritation was apparently
experienced by a small group of workers exposed to 200 mg/m3 (50 ppm)
for at least 12 min.1 Apparently no irritation was noted with
40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h.
An unknown volume of 50% liquid 2-furaldehyde did not produce
skin irritation in workers (Nazyrov & Yampol'skaya, 1969).
Insufficient information is available on the ability of 2-furaldehyde
to act as a skin or respiratory sensitizer in humans.
A limited number of studies have investigated the effects of
repeated occupational exposure to 2-furaldehyde. One study suggested
that 2-furaldehyde led to eye, nose, and throat irritation in
situations where 10-min average 2-furaldehyde concentrations of 12-64
mg/m3 (3-16 ppm) were measured (Apol & Lucas, 1975). However, other
substances, including creosote and liquid resins, may have contributed
to the effects, and no firm conclusions with respect to the role of
2-furaldehyde can be drawn. In another study, no evidence of
respiratory effects in 2-furaldehyde-exposed workers was noted when
the highest average concentration was given as around 8 mg/m3 (2 ppm)
(Pawlowicz et al., 1984). However, no duration for this level of
exposure and no indication of peak concentrations were presented. No
conclusions can be drawn from other studies because of poor reporting
of exposure to 2-furaldehyde or because of the potential for
confounding factors to influence the findings.
No information was available on carcinogenicity in humans. The
only genotoxicity information available in humans comes from an
inadequate study that did not demonstrate any significant increase in
the incidence of sister chromatid exchanges in a group of only six
employees potentially exposed to unknown concentrations of
2-furaldehyde (Gomez-Arroyo & Souza, 1985).
No information is available on reproductive toxicity in humans.
1 QO Chemicals Inc. (1981) Summary of sensory response
criteria, furfural in air concentrations, Table 3.
Unpublished data.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic organisms
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
10.2 Terrestrial organisms
An estimated oral LD50 value of >98 mg/kg body weight has been
reported for the red-winged blackbird ( Agelaius phoeniceus)
(Schafer et al., 1983).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Acute toxicity data from animals are variable; overall, however,
2-furaldehyde is toxic by the inhalation and oral routes (4-h LC50,
940 mg/m3 [235 ppm]; oral LD50, about 120 mg/kg body weight), with
no clear information in relation to the dermal route.
Human data on effects of 2-furaldehyde are extremely limited and
of poor quality, although mucous membrane irritation has been
identified as the principal effect following exposure to
concentrations apparently as low as 12 mg/m3 (3 ppm) (10-min
average). However, peak exposure concentrations were not given, and
co-exposure with other substances was possible. In another study,
exposure to 40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h
apparently did not cause throat or eye irritancy.
From repeated-inhalation exposure studies in animals, the main
non-neoplastic effects are toxicity to the lung and respiratory tract.
NOAELs of 80 and 208 mg/m3 (20 and 52 ppm) have been identified in
hamsters and rabbits, respectively, from studies of up to 4-6 h/day, 5
days/week, for 13 weeks. Chronic oral exposure to 2-furaldehyde
produced increased incidences of bile duct carcinomas in male rats,
malignant and benign tumours in the liver in male mice, and benign
tumours in the liver and forestomach in female mice. The development
of the liver and forestomach tumours may be related to the chronic
inflammatory effects noted at the same organ sites.
The results of mutagenicity tests with bacteria were largely
negative, although 2-furaldehyde is clearly genotoxic in vitro in
mammalian cells, producing chromosomal aberrations, gene mutations,
and sister chromatid exchanges. In vivo genotoxicity has not been
adequately studied, and no firm conclusions can be drawn.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
11.1.2 Criteria for setting guidance values for 2-furaldehyde
2-Furaldehyde vapour and liquid are readily absorbed through the
skin, and experiments suggest that skin absorption of vapour may
account for a significant proportion of the total body burden. In view
of the potential for skin absorption, biological monitoring is an
important aspect of occupational exposure assessment. This is achieved
by measuring 2-furoic acid (after alkaline hydrolysis of the
metabolite furoylglycine) in urine.
Table 2: Acute toxicity of 2-furaldehyde to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold (16-h EC3, growth) 16 Bringmann & Kuhn (1976)
Microcystis aeruginosa Toxic threshold (8-day EC5, growth) 2.7 Bringmann & Kuhn (1976)
Yeast
Saccharomyces cerevisiae EC14, growth 1 Banerjee et al. (1981)
Algae
Scenedesmus quadricaudata Toxic threshold (7-day EC3, growth) 31 Bringmann & Kuhn (1980a)
Protozoa
Entosiphon sulcatum Toxic threshold (72-h EC5, growth) 0.6 Bringmann & Kuhn (1980a)
Uronema parduczi Toxic threshold (growth) 11 Bringmann & Kuhn (1980b)
Chilomonas paramaecium Toxic threshold (48-h EC5, growth) 3.9 Bringmann & Kuhn (1980c)
Fish
Mosquito fish 96-h LC50 2410 Wallen et al. (1957)
Gambusia affinis NOEC
Bluegill sunfish 96-h LC50 16 Turnbull et al. (1954)
Lepomis macrochirus
Fathead minnow 96-h LC50 32 Mattson et al. (1976)
Pimephales promelas
a ECn is the effective concentration inhibiting the stated end-point (growth) by n%.
The relative importance of genotoxicity and chronic inflammation
in tumorigenesis is uncertain; because of this uncertainty, it is not
possible to reliably identify a threshold below which exposure to
2-furaldehyde would not result in some risk to human health.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to 2-furaldehyde from the general
environment precludes the characterization of potential cancer risks
for the general population.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are a number of
models for characterizing potency, which may be of some benefit in
priority-setting schemes.
The scenario chosen here as an example is occupational exposure
in the United Kingdom. With respect to the irritant effects, in
general, in the industries where 2-furaldehyde is used, the measured
and predicted (using EASE modelling) exposure concentrations indicate
that there is little risk that irritation to the respiratory tract or
eyes will occur. However, in the manufacture of refractories and
abrasive wheels, current exposures in some parts of these industries
give rise to concern that there would be the risk of developing
irritation. Given the eye irritation potential of 2-furaldehyde, there
is a risk of developing eye irritation if appropriate personal
protective equipment is not employed to prevent eye contact with the
liquid.
With respect to the risk of genotoxicity and carcinogenicity, the
picture is unclear. Although the measured and predicted levels of
exposure to 2-furaldehyde generally are relatively low in these
industries, the risk of developing genetic damage at sites of initial
contact cannot be discounted, and thus there is cause for concern that
this may occur. The relative importance of genotoxicity and chronic
inflammation in tumorigenesis is uncertain, and a NOAEL cannot be
reliably identified because of this uncertainty. Consequently, it is
concluded that because of this toxicological picture, it is not
possible to identify a level of exposure at which one could be certain
that genetic damage leading to tumour formation would not occur; thus,
under contemporary occupational exposure conditions, there is cause
for concern for genotoxicity and carcinogenicity, although it is not
possible to reliably quantify this.
In view of the irritative effects reported in workers
occupationally exposed to low exposure concentrations of
2-furaldehyde, a short-term limit is likely to be appropriate in
addition to the 8-h TWA.
11.2 Evaluation of environmental effects
Although some emission to the atmosphere is expected from wood
burning, no atmospheric effects are expected given the short half-life
for reaction with hydroxyl and other radicals and possible
photodegradation of 2-furaldehyde. The low volatilization of the
compound from water and soil would not be expected to add
significantly to atmospheric levels.
The majority of 2-furaldehyde released to the environment will be
released to surface waters. Release from the wood pulp industry seems
to be the major source.
2-Furaldehyde has a low capacity for bioaccumulation. Binding to
particulates in soil and aquatic sediment is expected to be very low,
making 2-furaldehyde mobile in the environment.
2-Furaldehyde is readily biodegraded in aerobic sewage sludge and
has also been shown to degrade in anaerobic systems. Acclimation of
the sludge improves degradation. The compound is toxic to anaerobic
degrading bacteria at concentrations above 1000 mg/litre in
unacclimated sludges (these concentrations have been reported in wood
pulp waste), although acclimation allows full degradation at these
concentrations.
LC50s in fish range from 16 to 32 mg/litre, with a reported NOEC
in an acute test of 10 mg/litre. There are no test results for aquatic
invertebrates. Toxic thresholds (EC3 to EC5 for cell multiplication)
for bacteria, cyanobacteria, algae, and protozoa range from 0.6 to 31
mg/litre.
Only one estimated toxicity value is available for terrestrial
organisms, and little emission to land is expected; on this basis, no
quantitative risk assessment can be attempted for the terrestrial
environment.
11.2.1 Predicted environmental concentration
Very limited monitoring studies are available for surface waters,
with only two isolated measurements at 2 µg/litre reported. The sample
risk assessment will be based on emissions from the wood pulp
industry, the expected worst case. Reported concentrations in sulfite
evaporator condensate range up to 1280 mg/litre, with average
concentrations at 274 mg/litre in a more recent study. Since the
evaporator condensate represents 15% of the total wastewater flow, the
concentration in wastewater would be 41.1 mg/litre average.
Based on the average concentration and mainly default values from
the OECD Technical Guidance Manual, the initial concentration in
rivers receiving treated wastewater would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre),
calculated as Ceffluent = I × (100 - P)/100,
where:
I = input concentration to the wastewater treatment plant
(0.041 g/litre)
P = percent removal in the wastewater treatment plant (91%,
based on the "ready biodegradability" of the compound)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = foc(susp) × Koc, where:
foc(susp) = the fraction of organic carbon in suspended matter
(default 0.1)
Koc = the organic carbon/water partition coefficient
(1.05; see section 5)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (taken as 1000 minimum,
as the wood pulp process requires large water input and as plants
will normally be sited on moderate to large rivers)
Under these conservative conditions, PEClocal(water) = 3.7 µg/litre.
11.2.2 Predicted no-effect concentration
As no test results are available for aquatic invertebrates and no
long-term test results are available, an uncertainty factor of 1000
will be applied to the lowest reported acute LC50 of 16 mg/litre for
the bluegill sunfish ( Lepomis macrochirus) to give a predicted
no-effect concentration (PNEC) of 16 µg/litre. It is not considered
justifiable to base the PNEC on toxic threshold values.
11.2.3 Environmental risk factors
The risk factor (PEC/PNEC ratio) for aquatic organisms is,
therefore, 0.23, indicating low risk. The distribution of reported
toxicity test results against the worst-case PEC is plotted in Figure
1, illustrating the safety margin.
The conversion factors for 2-furaldehyde in air at 20°C and 101.3
kPa are as follows:
1 ppm = 4.0 mg/m3
1 mg/m3 = 0.25 ppm
3. ANALYTICAL METHODS
Long- and short-term personal monitoring can be undertaken by
pumped sampling through XAD-2 resin impregnated with 2-(hydroxymethyl)
piperazine, desorbing with solvent (toluene), and analysing with gas
chromatography (NIOSH, 1987). The working range is 1.2-22 mg/m3
(0.3-5.5 ppm) for a 12-litre sample. Alternatively, a thermal
desorption tube may be used, in either the pumped or diffusive mode
(Patel et al., 1988). The working range for both is 4-40 mg/m3 (1-10
ppm) for a 10-litre sample. Screening measurements can be made with
colorimetric detector tubes, but these are unselective and not very
sensitive.
Biological monitoring of workers exposed to 2-furaldehyde is
possible by the analysis of 2-furoic acid (after alkaline hydrolysis
of the metabolite furoylglycine) in urine (Sedivec & Flek, 1978).
Although people not occupationally exposed to 2-furaldehyde have
background levels of 2-furoic acid in hydrolysed urine samples (from
dietary sources), these are low compared with those resulting from
occupational exposure (Nutley, 1989). The biological exposure index of
the American Conference of Government Industrial Hygienists is 200 mg
2-furoic acid/g creatinine (200 µmol/mmol creatinine), and a study in
the United Kingdom found that a 2-furoic acid concentration of over
160 mg/g creatinine (160 µmol/mmol creatinine) was likely to be due to
exposure to 2-furaldehyde at over 8 mg/m3 (2 ppm) (Nutley, 1989).
More sensitive analytical methods are required for monitoring in
food. Determination of the 2,4-dinitrophenylhydrazone derivative of
2-furaldehyde by high-performance liquid chromatography gives high
specificity, with a detection limit of 10 nmol/litre in spirits (Lo
Coco et al., 1992). A fast, semi-automatic, stopped-flow injection
analysis in which pretreatment of diversely coloured and turbid food
samples is not necessary has been developed (Espinosa-Mansilla et al.,
1993).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
2-Furaldehyde is found in a number of dietary sources. Because of
its formation during the thermal decomposition of carbohydrates,
2-furaldehyde is found in numerous processed foods and beverages,
including cocoa, coffee, tea, beer, wine, milk products, and bread
(Maga, 1979). It is also found in some fruits and vegetables, and it
is added as a flavouring agent to some foods. Industry in the United
Kingdom is voluntarily withdrawing 2-furaldehyde from addition to
food, although the chemical will still be present in food as a result
of its production during the thermal processing of anything containing
sugars. 2-Furaldehyde has also been found in the essential oils of
camphor, citronella, sassafras, lavender, and lime (Dunlop & Peters,
1953).
2-Furaldehyde is produced commercially in batch or continuous
digesters where pentosans from agricultural residues, including corn
cobs, oat hulls, rice hulls, and bagasse, are hydrolysed to pentoses
and the pentoses are subsequently cyclodehydrated to 2-furaldehyde.
2-Furaldehyde is shipped in steel tank cars, aluminium tank trucks,
and steel cans. When stored in bulk, the storage tanks normally have a
nitrogen blanket to prevent ingress of oxygen, which can cause
chemical degradation of the material.
Estimates of worldwide production are not available. US
production in 1983 was about 52 000 t (HSDB, 1998). The estimated
range of US production was 14 000-60 000 t in 1986, 2000-7000 t in
1990, and 11 000-45 000 t in 1994 (US EPA, 1998). Although
2-furaldehyde is not manufactured in the United Kingdom, it is
estimated that industry in the United Kingdom used approximately 3000
t in 1992. The official statistics indicate that imports into the
United Kingdom for the previous four years were 2800 t (1988), 3500 t
(1989), 3000 t (1990), and 3500 t (1991) (Gregg et al., 1997).
Industrial uses of 2-furaldehyde are summarized in Table 1. About
40% of 2-furaldehyde imported into the United Kingdom is used in the
production of resins, abrasive wheels, and refractories. The rest is
used in the refining of lubrication oils. Contacts with United Kingdom
industry in 1992 indicated that there was unlikely to be any
significant change in the pattern of use of 2-furaldehyde in the
foreseeable future.
Table 1: Industrial uses of 2-furaldehyde in the United Kingdom.
Industry Uses
1. Petrochemicals As a selective solvent in
the production of lubricating oils
2. Refractories As a reactive wetting agent in the
production of refractory components
3. Resin manufacture (a) Production of phenolic resins
(b) Production of cashew nutshell polymers
4. Abrasive materials As a reactive wetting agent for the resin
binder system in the production of abrasive wheels
5. Solvent recovery Steam distillation of spent 2-furaldehyde
for reuse by industry sectors 1, 2, and 4
6. Flavouring Used in very small quantities in
synthetic/natural oil blends
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The highest reported emissions of 2-furaldehyde to the
environment are from the wood pulp industry, with release to the
hydrosphere (section 6); release to water from other uses appears to
be substantially lower. The compound would be released to the
atmosphere from wood fires, a natural as well as anthropogenic source.
In the atmosphere, 2-furaldehyde will exist predominantly in the
vapour phase; the half-life for destruction by interaction with
hydroxyl radicals has been calculated at 0.44 days (HSDB, 1998).
Night-time destruction in the urban atmosphere may involve reaction
with nitrate radicals (Carter et al., 1981). Direct photo-oxidation
may occur in the atmosphere, based on experimental demonstration that
concentrations of 2-furaldehyde in wood smoke were reduced by
irradiation at sunlight and ultraviolet frequencies. Addition of
nitrogen oxides to wood smoke increased the rate of disappearance of
the compound (Kleindienst et al., 1986).
Virtually no degradation occurred in a solution of 2-furaldehyde
in distilled water over 30 days, suggesting that hydrolysis is not an
important process at environmental pH (Ettinger et al., 1954).
A Henry's law constant of 0.37 Pa.m3/mol at 25°C has been
calculated (HSDB, 1998), corresponding to a dimensionless Henry's law
constant (air/water partition coefficient) of 1.5 × 10-4. These
values indicate slow volatilization from water and damp soil surfaces;
an estimated half-life for volatilization from a model river of 9.9
days has been reported (HSDB, 1998).
2-Furaldehyde has a reported log Kow of 0.41, indicating low
capacity for bioaccumulation; calculated bioconcentration factors were
less than 1.2 (HSDB, 1998). The sorption coefficient ( Koc) can be
calculated as between 1.05 (Organisation for Economic Co-operation and
Development [OECD] Technical Guidance Manual) and 1.62 (Karickhoff et
al., 1979), indicating little sorption to particulates and a high
capacity for mobility in soil.
2-Furaldehyde was readily biodegraded in an aerobic batch culture
at 200 mg chemical oxygen demand (COD)/litre, with non-adapted sewage
sludge showing 96.3% degradation within 120 h and a degradation rate
of 37 mg COD/g per hour (Pitter, 1976). In a flow-through aerobic
laboratory bioreactor at an initial concentration of 300 mg/litre, 98%
degradation of 2-furaldehyde was reported using acclimated sewage
sludge. Degradation also occurred at a concentration of 1000 mg/litre
(Rowe & Tullos, 1980). 2-Furaldehyde was readily biodegradable in the
Japanese Ministry of International Trade and Industry (MITI) test
(Kawasaki, 1980). Wang et al. (1994) screened a range of
microorganisms for their ability to degrade 2-furaldehyde and reviewed
the earlier literature; some strains of the aerobic bacteria
Escherichia coli, Pseudomonas putida, and Rhodococcus
erythropolis and the yeast Hyphozyma rosoeniger were able to
degrade the compound to a greater or lesser degree. Inhibitory effects
of 2-furaldehyde on Pseudomonas putida were seen at concentrations
of 0.1% and above, with complete inhibition at 1% with exposure for 30
min (Kim et al., 1983).
2-Furaldehyde at 1 mg/litre was degraded completely in water from
the Great Miami, Little Miami, and Ohio rivers (USA) within 3 days
under aerobic conditions (Ettinger et al., 1954).
Under anaerobic conditions, 2-furaldehyde was completely degraded
to methane by unacclimated sewage sludge within 30 days at an initial
concentration of 580 mg/litre. The rate of methane production was
initially slower than in controls, indicating some interference of the
compound in the metabolism of the methanogenic bacteria. At an initial
concentration of 1160 mg/litre, gas production ceased after 5 days,
and none of the gas produced was methane; gas production did not
resume within the 28 days of the test, indicating that this
concentration was toxic to unacclimated microorganisms. Using
acclimated sludge (which had received 2-furaldehyde at 310 mg/litre
continuously for 8 months), full degradation occurred at 1160
mg/litre. At 2320 mg/litre, 2-furaldehyde was initially degraded
rapidly, but then gas production slowed for the remainder of the test
(Benjamin et al., 1984). A strictly anaerobic bacterium isolated from
a fermentor degrading sulfite evaporator condensate from wood pulp
production was able to use 2-furaldehyde as sole carbon source; the
organism was tentatively identified as Desulfovibrio sp. (Brune et
al., 1983). Methanococcus deltae was found to be able to use
2-furaldehyde as sole carbon source, degrading the compound to
furfuryl alcohol at initial concentrations of 5 and 10 mmol/litre (480
and 960 mg/litre); however, growth of the bacterium was inhibited at
concentrations of 20 and 25 mmol/litre (1920 and 2400 mg/litre). Other
methanogenic bacteria ( Methanobacterium thermoautotrophicum,
Methanosarcina barkeri, and Methanococcus thermolithotrophicus)
were unable to degrade the compound (Belay et al., 1997).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
2-Furaldehyde was detected in 1 out of 204 surface water samples
from heavily industrialized areas in the USA at 2 µg/litre (detection
limit 1 µg/litre) and in 1 out of 13 samples from the Lake Michigan
basin, USA, at 2 µg/litre in the late 1970s (HSDB, 1998).
2-Furaldehyde was not detected (detection limit 0.4 ng/litre) in
33 samples of surface waters in the 1996 monitoring of the general
environment in Japan (Japan Environment Agency, 1998). However, the
compound was detected in 6 out of 15 air samples (detection limit 40
ng/m3) in two cities (range 42-120 ng/m3). The analytical method was
not stated in the publication.
Levels of 2-furaldehyde in sulfite evaporator condensate, which
represents about 15% of the wastewater flow from pulp mills, have been
reported to range between 10 and 1280 mg/litre (Ruus, 1964) and
between 179 and 471 mg/litre (average 274 mg/litre) (Benjamin et al.,
1984). The 2-furaldehyde derives from pentoses in the wood pulp and is
formed during the waste treatment in the evaporator. 2-Furaldehyde was
detected in the wastewater of a synthetic rubber plant at 1.7 µg/litre
(Keith, 1974).
2-Furaldehyde was detected but not quantified in the air above
the Black Forest, Germany, in 1984-85 (Juttner, 1986). Although
2-furaldehyde has been identified in vehicle exhaust, it was not
detectable in air sampled from a road tunnel in the USA (Hampton et
al., 1982). The compound has been identified in smoke from burning
wood (Lipari et al., 1984; Kleindienst et al., 1986). Mean
concentrations of 2-furaldehyde measured in indoor and outdoor air in
suburban New Jersey, USA, in summer 1992 were 1.06 µg/m3 (0.27 ppb)
and 0.67 µg/m3 (0.17 ppb), respectively. The presence of the compound
in indoor air was presumed to be from emissions during cooking (Zhang
et al., 1994).
6.2 Human exposure
6.2.1 Oral exposure
2-Furaldehyde has been identified but not quantified as a major
flavour component in a range of food items, including beef, soy sauce,
roasted nuts, fried bacon, nectarines, baked potatoes, clove oil,
preserved mangoes, rum, roasted coffee, and blue cheese (HSDB, 1998).
Levels reported in food are as follows: non-alcoholic beverages, 4
mg/litre; alcoholic beverages, 10 mg/litre; ice creams, ices, etc., 13
mg/kg; candy, 12 mg/kg; baked goods (unspecified), 17 mg/kg; gelatins
and puddings, 0.8 mg/kg; chewing gum, 45 mg/kg; and syrups, 30
mg/litre (HSDB, 1998). 2-Furaldehyde was qualitatively identified in
two out of eight samples of mothers' milk from urban sites in the USA
(Pellizzari et al., 1982). Qualitative identification of 2-furaldehyde
in drinking-water has been reported from both the USA and Europe; no
quantification was reported (Kool et al., 1982).
6.2.2 Inhalation exposure
The remainder of the human exposure data available to the authors
of this CICAD are restricted to the occupational environment.
Measured inhalation exposure information was provided to the
United Kingdom's Health and Safety Executive by the petrochemical
industry. The Health and Safety Executive's National Exposure Data
Base contains information on exposure for the use of 2-furaldehyde in
the refractory industry and for a number of miscellaneous processes.
In all the exposure groupings below, when routine and breakdown
maintenance takes place and significant exposure to airborne
2-furaldehyde is anticipated, it is expected that appropriate
respiratory protective equipment would be used to minimize inhalation
exposure.
Exposures in the petrochemicals, resin and polymer manufacture,
and distillation industries are likely to be very similar. EASE
(Version 2) predictions seem to be in accord with the limited amount
of measured exposure information provided by the petrochemicals
industry (Gregg et al., 1997). The EASE predictions indicate that 8-h
TWA exposures will be somewhat below 8 mg/m3 (2 ppm). There is
insufficient information to predict 15-min TWA exposures for these
industries.
Exposures in the refractories and abrasive wheels manufacturing
industries are likely to be very similar. The high exposures measured
by the Health and Safety Executive in the refractories industry (20%
of 8-h TWA exposures are greater than 40 mg/m3 [10 ppm]) are likely
to be reduced following advice given about the application of
efficient local exhaust ventilation and the enclosure of the process
where possible (Gregg et al., 1997). The EASE-predicted 8-h TWA
exposures likely to result from these improvements will be between 2
and 12 mg/m3 (0.5 and 3 ppm) (Gregg et al., 1997). There is every
likelihood that exposures will actually be at the lower end of the
range -- i.e., less than 8 mg/m3 (2 ppm). There is insufficient
information to predict 15-min TWA exposures for these industries.
In the flavouring industry, very little 2-furaldehyde appears to
be used. The EASE model predicts exposures to be low: the 15-min TWA
exposure is between 1.2 and 6.8 mg/m3 (0.3 and 1.7 ppm), and the 8-h
TWA is between 0.04 and 0.2 mg/m3 (0.01 and 0.05 ppm) (Gregg et al.,
1997).
For oilseed residue application, no personal exposure was
detected. The extent of this industry is not known, but it seems that
8-h exposures to 2-furaldehyde are likely to be well below 8 mg/m3
(2 ppm) (Gregg et al., 1997). No comment can be made upon likely
short-term exposures.
Little is known about the extent of the use of 2-furaldehyde in
the glass reinforced plastics industry. It is very likely that the
application of appropriate controls would result in 8-h TWA exposures
to 2-furaldehyde being reduced to below 8 mg/m3 (2 ppm) (Gregg et
al., 1997). No comment can be made upon possible short-term exposures.
6.2.3 Dermal exposure
The industrial processes can be grouped in a similar manner to
that adopted for the general discussion of inhalation exposure above
(section 6.2.2). These predictions do not take account of personal
protective equipment that may be worn. Such equipment may
significantly reduce dermal exposure.
The EASE-predicted dermal exposures for the petrochemicals, resin
and polymer manufacture, and distillation industries are similar
-- namely, between 0.1 and 1 mg/cm2 per day (Gregg et al., 1997).
For the refractories and abrasive wheels manufacturing
industries, the EASE-predicted dermal exposures are between 1 and 5
mg/cm2 per day (Gregg et al., 1997). If improvements are made to the
process containment, opportunities for dermal contact will be reduced,
and predicted exposures will be reduced to between 0.1 and 1 mg/cm2
per day.
For the use of 2-furaldehyde in the flavourings industry, the
EASE prediction for dermal exposure is between 0 and 0.1 mg/cm2 per
day (Gregg et al., 1997). Insufficient information is provided for the
other minor uses to make predictions of dermal exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
No animal studies are available examining the toxicokinetics of
2-furaldehyde by the inhalation or dermal routes; however, signs of
systemic toxicity observed in animal studies (see section 8) indicate
that 2-furaldehyde is readily absorbed via both these routes of
exposure.
Animal studies demonstrate that, following oral administration in
rats, 2-furaldehyde is readily absorbed and rapidly excreted, with up
to 85% of the administered dose being detected in the urine within 24
h (Nomier et al., 1992). Some elimination via exhaled carbon dioxide
(7% of dose) also occurs. Metabolism is characterized by oxidation or
acetylation of the aldehyde group, followed by glycine conjugation.
2-Furoylglycine is the major urinary metabolite, accounting for
approximately 80% of the administered dose (Laham & Potvin, 1989;
Nomier et al., 1992; Parkash & Caldwell, 1994). Other minor
metabolites include furoic acid, furanacrylic acid, and furanacryluric
acid.
In humans, absorption of the vapour via both the lungs and skin
has been demonstrated (Flek & Sedivec, 1978a,b). Metabolism appears
similar to that in rats, with the majority of the retained dose being
excreted as urinary 2-furoylglycine. Furoic acid and furanacryluric
acid are also detected as minor metabolites. Dermal absorption from
liquid 2-furaldehyde has also been observed.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Unpublished or briefly reported papers indicate 1-, 4-, and 6-h
inhalation LC50 values in rats of 4148, 940, and 700 mg/m3 (1037,
235, and 175 ppm), respectively (Woods & Seevers, 1956; Terrill et
al., 1989). In contrast, an apparently well-conducted published study
indicated a 1-h LC50 value in rats of 756 mg/m3 (189 ppm) (Gupta et
al., 1991; Mishra et al., 1991). In the rat and other species,
respiratory tract irritation and lung damage were consistently
observed following inhalation exposure, with lung oedema and
congestion being reported in one study in rats immediately after
exposure to 380 mg/m3 (95 ppm) for 1 h. Oral LD50 values in rats
were in close agreement, ranging from 122 to 158 mg/kg body weight
(Woods & Seevers, 1955; SRI International, 1982). Toxic signs observed
indicated central nervous system depressant effects, with convulsions
also observed. The lungs also appeared to be a target organ via oral
dosing. No reliable information was available for the dermal route of
exposure.
8.2 Irritation and sensitization
No signs of skin irritation were noted in rabbits following a
single application of liquid 2-furaldehyde for 12 h, although mild
irritation was observed following a 48-h application (Woods & Seevers,
1955). Intense but reversible skin irritation was reported in
guinea-pigs after repeated application of undiluted liquid
2-furaldehyde (Agakishiyev, 1989, 1990).
A single instillation of liquid 2-furaldehyde produced gross
corneal opacities in rabbits (Woods & Seevers, 1955). 2-Furaldehyde
vapour produced eye irritation in several studies following repeated
exposure in different species (Gardner, 1925; Feron et al., 1979;
Gupta et al., 1991).
No information is available on the ability of 2-furaldehyde to
act as a skin or respiratory sensitizer.
8.3 Short-term exposure
The principal effects of repeated inhalation exposure were
similar to those seen in single-exposure experiments. Groups of rats
were exposed to 0 or 160 mg 2-furaldehyde/m3 (0 or 40 ppm) 1 h/day
for 5, 15, or 30 days (Gupta et al., 1991; Mishra et al., 1991), and
groups of rabbits were exposed to 208, 520, or 1040 mg/m3 (52, 130,
or 260 ppm) 4 h/day, 5 days/week, for at least 60 exposures
(Castellino et al., 1963). Respiratory irritation, hyperplasia and
degeneration of the olfactory epithelium, and lung congestion, oedema,
and inflammation were observed in rats following exposure to 160
mg/m3 (40 ppm) (Gupta et al., 1991; Mishra et al., 1991) and in
rabbits following exposure to 1040 mg/m3 (260 ppm) (Castellino et
al., 1963). There was also evidence of kidney damage seen
histopathologically and anaemia in rabbits following exposure to 520
mg 2-furaldehyde/m3 (130 ppm). A NOAEL of 208 mg/m3 (52 ppm) was
identified in rabbits; a NOAEL was not identified for rats.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
Groups of hamsters were exposed to 0, 80, 460, or 2208 mg/m3
(0, 20, 115, or 552 ppm) 6 h/day, 5 days/week, for 13 weeks (Feron et
al., 1979). Respiratory irritation, hyperplasia and degeneration of
the olfactory epithelium, and lung congestion, oedema, and
inflammation were observed in hamsters following exposure to 2208
mg/m3 (552 ppm) (Feron et al., 1979). Slight atrophy and hyperplasia
of the olfactory epithelium were also seen following exposure to 460
mg/m3 (115 ppm). A NOAEL of 80 mg/m3 (20 ppm) was identified.
Groups of 20 male and female rats received 0, 11, 22, 45, 90, or
180 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Mild to moderate centrilobular
vacuolation of hepatocytes was observed among all treated groups,
although there was no clear relationship between dose and incidence or
severity of the observed effect.
Groups of 20 male and 20 female mice received 0, 75, 150, 300,
600, or 1200 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Relative liver weights were
increased in a dose-related manner in all treated groups.
Centrilobular coagulative necrosis of hepatocytes was seen at
150 mg/kg body weight per day or more, and mild mononuclear
inflammatory cell infiltrate was seen in all treated groups.
8.4.2 Chronic exposure and carcinogenicity
No inhalation carcinogenicity studies in rats or mice were
available. An inhalation study in hamsters showed no evidence that
2-furaldehyde vapour had carcinogenic potential following 52 weeks'
exposure to 1000-1600 mg/m3 (250-400 ppm), 7 h/day, 5 days/week, by
this route (Feron & Kruysse, 1978). Nor was there any indication that
2-furaldehyde was carcinogenic to hamsters after 36 weekly
intratracheal instillations (Feron, 1972). However, both these studies
are not satisfactory negative studies, owing to their limited
duration.
In a gavage study, groups of 50 male and 50 female F344/N rats
were treated with 0, 30, or 60 mg 2-furaldehyde/kg body weight per
day, 5 days/week for 103 weeks (NTP, 1990). Animals were observed
twice daily, weighed monthly, and subjected to a thorough gross and
microscopic examination either at death or at the end of the study.
Mortality in males was not significantly affected by 2-furaldehyde
treatment throughout the study. Corresponding survival rates among
females were 18/50, 32/50, and 28/50. However, 19 of the 22 top-dose
deaths in females were attributed to accidental gavage errors. There
were no differences in body weight between test and control animals
throughout the study, and there were no treatment-related clinical
signs of toxicity. The main non-neoplastic changes observed in test
animals were minimal to mild centrilobular necrosis of the liver in
male rats (3/50, 9/50, 12/50) and biliary dysplasia with fibrosis in
two top-dose males.
Two cases of cholangiocarcinoma were seen in two other top-dose
males. The historical incidence of bile duct neoplasms in corn oil
vehicle control male F344/N rats in this laboratory was reported to be
3/2145 (0.1%). No other findings of biological significance were
observed. Hence, in this study, treatment by gavage with 2-furaldehyde
produced an increase in bile duct carcinoma in male rats only,
although a valid assessment of the carcinogenicity of 2-furaldehyde in
female rats cannot be made because of the low numbers of animals
surviving to the end of the study. Also, the high incidence of
accidental deaths casts doubt over the quality of the experimental
conditions.
Groups of 50 male and 50 female B6C3F1 mice were also dosed with
0, 50, 100, or 175 mg 2-furaldehyde/kg body weight per day by gavage,
5 days/week for 103 weeks, in the NTP study (NTP, 1990). Animals were
observed twice daily, weighed monthly, and subjected to a thorough
gross and microscopic examination either at death or at the end of the
study. No significant differences in survival were observed between
2-furaldehyde-treated and untreated animals throughout the study.
There were no differences in body weight gain between test and control
animals throughout the study, and there were no clinical signs of
treatment-related toxicity.
Non-neoplastic changes included chronic inflammation and
multifocal green-brown, granular pigmentation of the subserosa of the
liver in mid- and top-dose mice of each sex and forestomach
hyperplasia in 2-furaldehyde-treated female mice only. The principal
neoplastic changes observed were increases in hepatocellular adenomas
and hepatocellular carcinomas in males (adenoma: 9/50 [18%], 13/50
[26%], 11/49 [22%], 19/50 [38%]; carcinoma: 7/50 [14%], 12/50 [24%],
6/49 [12%], 21/50 [42%]; in control, low-, mid-, and top-dose animals,
respectively). Only adenomas were observed in females, the incidences
being dose-related: 1/50 (2%), 3/50 (6%), 5/50 (10%), 8/50 (16%).
Increases in adenomas and/or carcinomas were statistically significant
only in the top-dose animals of both sexes. An increase in squamous
cell papillomas of the forestomach was also seen in top-dose female
mice, although this increase was not statistically significant (1/50
[2%], 0/50 [0%], 1/50 [2%], 6/50 [12%] in control, low-, mid-, and
top-dose animals). No other findings of biological significance were
observed.
In an initiation/promotion study in mice using the dermal route,
an increased incidence of skin tumours was seen when 2-furaldehyde was
applied in combination with the promoting agent (5/20 compared with
1/20 in controls that received the promoting agent in combination with
dimethyl sulfoxide) (Miyakama et al., 1991). When 2-furaldehyde was
applied in combination with acetone, no skin tumours were seen.
Following the administration of an unstated amount of
2-furaldehyde to rats for 5 months, evidence of pre-neoplastic changes
(the formation of glutathione S-transferase placental form, GST-P,
foci) was seen in livers (Shimizu et al., 1989). Liver cirrhosis was
observed in treated rats; overall, however, it is impossible to reach
any conclusions about the possible mechanisms underlying cancer
induction from this study (e.g., to what extent cell proliferation or
direct mutagenicity may be involved in liver tumour formation).
8.5 Genotoxicity and related end-points
Experiments in cell-free systems have demonstrated that
2-furaldehyde causes DNA damage (Hadi & Rehman, 1989). Although
bacterial mutation studies with 2-furaldehyde have been largely
negative, they have in general been inadequately reported (Zdzienicka
& Tudek, 1978; McMahon et al., 1979; Loquet et al., 1981; Soska et
al., 1981; Marnett et al., 1985; Mortelmans et al., 1986; Shinohara &
Omura, 1986; Kim et al., 1987, 1988; Nakamura et al., 1987; Shane et
al., 1988; Kato et al., 1989). However, 2-furaldehyde is clearly
genotoxic in vitro in mammalian cell test systems, producing
chromosomal aberrations, gene mutations, and sister chromatid
exchanges (Stich et al., 1981; Gomez-Arroyo & Souza, 1985; McGregor et
al., 1988; Nishi et al., 1989; NTP, 1990). In addition, one positive
result and one negative result were obtained in Drosophila tests
(Woodruff et al., 1985; Rodriguez-Arnaiz et al., 1992).
The genotoxic potential of 2-furaldehyde in vivo is less
certain, with positive results being claimed in a briefly reported
cytogenetics study and negative results being reported in another
cytogenetics study and a sister chromatid exchange study (Subramanyam
et al., 1989; NTP, 1990). Further details of these studies are
provided in the source document to this CICAD (Gregg et al., 1997).
Overall, no firm conclusions can be drawn from these reports.
Point mutations in K-ras and H-ras genes were seen in the
2-furaldehyde-treated mouse livers taken from the NTP carcinogenicity
assay, demonstrating genotoxicity (possibly direct) at the tumour site
(Reynolds et al., 1987).
8.6 Reproductive and developmental toxicity
No studies specifically addressing these end-points are
available.
8.7 Immunological and neurological effects
No studies specifically addressing these end-points are
available.
9. EFFECTS ON HUMANS
The only effects reported following single exposure in humans
relate to irritation. Throat and eye irritation was apparently
experienced by a small group of workers exposed to 200 mg/m3 (50 ppm)
for at least 12 min.1 Apparently no irritation was noted with
40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h.
An unknown volume of 50% liquid 2-furaldehyde did not produce
skin irritation in workers (Nazyrov & Yampol'skaya, 1969).
Insufficient information is available on the ability of 2-furaldehyde
to act as a skin or respiratory sensitizer in humans.
A limited number of studies have investigated the effects of
repeated occupational exposure to 2-furaldehyde. One study suggested
that 2-furaldehyde led to eye, nose, and throat irritation in
situations where 10-min average 2-furaldehyde concentrations of 12-64
mg/m3 (3-16 ppm) were measured (Apol & Lucas, 1975). However, other
substances, including creosote and liquid resins, may have contributed
to the effects, and no firm conclusions with respect to the role of
2-furaldehyde can be drawn. In another study, no evidence of
respiratory effects in 2-furaldehyde-exposed workers was noted when
the highest average concentration was given as around 8 mg/m3 (2 ppm)
(Pawlowicz et al., 1984). However, no duration for this level of
exposure and no indication of peak concentrations were presented. No
conclusions can be drawn from other studies because of poor reporting
of exposure to 2-furaldehyde or because of the potential for
confounding factors to influence the findings.
No information was available on carcinogenicity in humans. The
only genotoxicity information available in humans comes from an
inadequate study that did not demonstrate any significant increase in
the incidence of sister chromatid exchanges in a group of only six
employees potentially exposed to unknown concentrations of
2-furaldehyde (Gomez-Arroyo & Souza, 1985).
No information is available on reproductive toxicity in humans.
1 QO Chemicals Inc. (1981) Summary of sensory response
criteria, furfural in air concentrations, Table 3.
Unpublished data.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic organisms
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
10.2 Terrestrial organisms
An estimated oral LD50 value of >98 mg/kg body weight has been
reported for the red-winged blackbird ( Agelaius phoeniceus)
(Schafer et al., 1983).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Acute toxicity data from animals are variable; overall, however,
2-furaldehyde is toxic by the inhalation and oral routes (4-h LC50,
940 mg/m3 [235 ppm]; oral LD50, about 120 mg/kg body weight), with
no clear information in relation to the dermal route.
Human data on effects of 2-furaldehyde are extremely limited and
of poor quality, although mucous membrane irritation has been
identified as the principal effect following exposure to
concentrations apparently as low as 12 mg/m3 (3 ppm) (10-min
average). However, peak exposure concentrations were not given, and
co-exposure with other substances was possible. In another study,
exposure to 40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h
apparently did not cause throat or eye irritancy.
From repeated-inhalation exposure studies in animals, the main
non-neoplastic effects are toxicity to the lung and respiratory tract.
NOAELs of 80 and 208 mg/m3 (20 and 52 ppm) have been identified in
hamsters and rabbits, respectively, from studies of up to 4-6 h/day, 5
days/week, for 13 weeks. Chronic oral exposure to 2-furaldehyde
produced increased incidences of bile duct carcinomas in male rats,
malignant and benign tumours in the liver in male mice, and benign
tumours in the liver and forestomach in female mice. The development
of the liver and forestomach tumours may be related to the chronic
inflammatory effects noted at the same organ sites.
The results of mutagenicity tests with bacteria were largely
negative, although 2-furaldehyde is clearly genotoxic in vitro in
mammalian cells, producing chromosomal aberrations, gene mutations,
and sister chromatid exchanges. In vivo genotoxicity has not been
adequately studied, and no firm conclusions can be drawn.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
11.1.2 Criteria for setting guidance values for 2-furaldehyde
2-Furaldehyde vapour and liquid are readily absorbed through the
skin, and experiments suggest that skin absorption of vapour may
account for a significant proportion of the total body burden. In view
of the potential for skin absorption, biological monitoring is an
important aspect of occupational exposure assessment. This is achieved
by measuring 2-furoic acid (after alkaline hydrolysis of the
metabolite furoylglycine) in urine.
Table 2: Acute toxicity of 2-furaldehyde to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold (16-h EC3, growth) 16 Bringmann & Kuhn (1976)
Microcystis aeruginosa Toxic threshold (8-day EC5, growth) 2.7 Bringmann & Kuhn (1976)
Yeast
Saccharomyces cerevisiae EC14, growth 1 Banerjee et al. (1981)
Algae
Scenedesmus quadricaudata Toxic threshold (7-day EC3, growth) 31 Bringmann & Kuhn (1980a)
Protozoa
Entosiphon sulcatum Toxic threshold (72-h EC5, growth) 0.6 Bringmann & Kuhn (1980a)
Uronema parduczi Toxic threshold (growth) 11 Bringmann & Kuhn (1980b)
Chilomonas paramaecium Toxic threshold (48-h EC5, growth) 3.9 Bringmann & Kuhn (1980c)
Fish
Mosquito fish 96-h LC50 2410 Wallen et al. (1957)
Gambusia affinis NOEC
Bluegill sunfish 96-h LC50 16 Turnbull et al. (1954)
Lepomis macrochirus
Fathead minnow 96-h LC50 32 Mattson et al. (1976)
Pimephales promelas
a ECn is the effective concentration inhibiting the stated end-point (growth) by n%.
The relative importance of genotoxicity and chronic inflammation
in tumorigenesis is uncertain; because of this uncertainty, it is not
possible to reliably identify a threshold below which exposure to
2-furaldehyde would not result in some risk to human health.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to 2-furaldehyde from the general
environment precludes the characterization of potential cancer risks
for the general population.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are a number of
models for characterizing potency, which may be of some benefit in
priority-setting schemes.
The scenario chosen here as an example is occupational exposure
in the United Kingdom. With respect to the irritant effects, in
general, in the industries where 2-furaldehyde is used, the measured
and predicted (using EASE modelling) exposure concentrations indicate
that there is little risk that irritation to the respiratory tract or
eyes will occur. However, in the manufacture of refractories and
abrasive wheels, current exposures in some parts of these industries
give rise to concern that there would be the risk of developing
irritation. Given the eye irritation potential of 2-furaldehyde, there
is a risk of developing eye irritation if appropriate personal
protective equipment is not employed to prevent eye contact with the
liquid.
With respect to the risk of genotoxicity and carcinogenicity, the
picture is unclear. Although the measured and predicted levels of
exposure to 2-furaldehyde generally are relatively low in these
industries, the risk of developing genetic damage at sites of initial
contact cannot be discounted, and thus there is cause for concern that
this may occur. The relative importance of genotoxicity and chronic
inflammation in tumorigenesis is uncertain, and a NOAEL cannot be
reliably identified because of this uncertainty. Consequently, it is
concluded that because of this toxicological picture, it is not
possible to identify a level of exposure at which one could be certain
that genetic damage leading to tumour formation would not occur; thus,
under contemporary occupational exposure conditions, there is cause
for concern for genotoxicity and carcinogenicity, although it is not
possible to reliably quantify this.
In view of the irritative effects reported in workers
occupationally exposed to low exposure concentrations of
2-furaldehyde, a short-term limit is likely to be appropriate in
addition to the 8-h TWA.
11.2 Evaluation of environmental effects
Although some emission to the atmosphere is expected from wood
burning, no atmospheric effects are expected given the short half-life
for reaction with hydroxyl and other radicals and possible
photodegradation of 2-furaldehyde. The low volatilization of the
compound from water and soil would not be expected to add
significantly to atmospheric levels.
The majority of 2-furaldehyde released to the environment will be
released to surface waters. Release from the wood pulp industry seems
to be the major source.
2-Furaldehyde has a low capacity for bioaccumulation. Binding to
particulates in soil and aquatic sediment is expected to be very low,
making 2-furaldehyde mobile in the environment.
2-Furaldehyde is readily biodegraded in aerobic sewage sludge and
has also been shown to degrade in anaerobic systems. Acclimation of
the sludge improves degradation. The compound is toxic to anaerobic
degrading bacteria at concentrations above 1000 mg/litre in
unacclimated sludges (these concentrations have been reported in wood
pulp waste), although acclimation allows full degradation at these
concentrations.
LC50s in fish range from 16 to 32 mg/litre, with a reported NOEC
in an acute test of 10 mg/litre. There are no test results for aquatic
invertebrates. Toxic thresholds (EC3 to EC5 for cell multiplication)
for bacteria, cyanobacteria, algae, and protozoa range from 0.6 to 31
mg/litre.
Only one estimated toxicity value is available for terrestrial
organisms, and little emission to land is expected; on this basis, no
quantitative risk assessment can be attempted for the terrestrial
environment.
11.2.1 Predicted environmental concentration
Very limited monitoring studies are available for surface waters,
with only two isolated measurements at 2 µg/litre reported. The sample
risk assessment will be based on emissions from the wood pulp
industry, the expected worst case. Reported concentrations in sulfite
evaporator condensate range up to 1280 mg/litre, with average
concentrations at 274 mg/litre in a more recent study. Since the
evaporator condensate represents 15% of the total wastewater flow, the
concentration in wastewater would be 41.1 mg/litre average.
Based on the average concentration and mainly default values from
the OECD Technical Guidance Manual, the initial concentration in
rivers receiving treated wastewater would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre),
calculated as Ceffluent = I × (100 - P)/100,
where:
I = input concentration to the wastewater treatment plant
(0.041 g/litre)
P = percent removal in the wastewater treatment plant (91%,
based on the "ready biodegradability" of the compound)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = foc(susp) × Koc, where:
foc(susp) = the fraction of organic carbon in suspended matter
(default 0.1)
Koc = the organic carbon/water partition coefficient
(1.05; see section 5)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (taken as 1000 minimum,
as the wood pulp process requires large water input and as plants
will normally be sited on moderate to large rivers)
Under these conservative conditions, PEClocal(water) = 3.7 µg/litre.
11.2.2 Predicted no-effect concentration
As no test results are available for aquatic invertebrates and no
long-term test results are available, an uncertainty factor of 1000
will be applied to the lowest reported acute LC50 of 16 mg/litre for
the bluegill sunfish ( Lepomis macrochirus) to give a predicted
no-effect concentration (PNEC) of 16 µg/litre. It is not considered
justifiable to base the PNEC on toxic threshold values.
11.2.3 Environmental risk factors
The risk factor (PEC/PNEC ratio) for aquatic organisms is,
therefore, 0.23, indicating low risk. The distribution of reported
toxicity test results against the worst-case PEC is plotted in Figure
1, illustrating the safety margin.
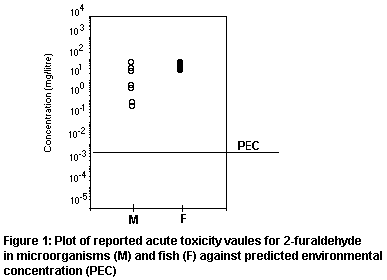 12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer concluded that
"There is inadequate evidence in humans for the carcinogenicity of
2-furaldehyde" and that "There is limited evidence in experimental
animals for the carcinogenicity of 2-furaldehyde." Its overall
evaluation is that "2-Furaldehyde is not classifiable as to its
carcinogenicity to humans" (IARC, 1995).
2-Furaldehyde has previously been evaluated by the Joint Expert
Committee on Food Additives (JECFA, 1999), whose conclusions are
summarized as follows:
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL [no-observed-effect level] was
identified for hepatotoxicity in rats, the Committee was unable
to allocate an ADI [acceptable daily intake]. Before reviewing
the substance again, the Committee would wish to review the
results of studies of DNA binding in mice and of a 90-day study
of toxicity in rats to identify a NOEL for hepatotoxicity.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0276) reproduced in this
document.
13.1 Health surveillance advice
An exposure surveillance program could include monitoring of
2-furoic acid in urine after alkaline hydrolysis of the main
metabolite of 2-furaldehyde, furoylglycine, immediately following
exposure.
13.2 Advice to physicians
In case of poisoning, treatment is supportive.
13.3 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from mixing with acids, bases, or oxidants
because of the risk of fire or explosion.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals, taken in a certain country, can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
2-Furaldehyde
INTERNATIONAL CHEMICAL SAFETY CARD
FURFURAL ICSC: 0276
November 1998
CAS # 98-01-1 2-Furancarboxyaldehyde
RTECS # LT7000000 2-Furaldehyde
UN # 1199 2-Furylmethanal
EC # 605-010-00-4 C5H4O2/C4H3OCHO
Molecular mass: 96.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE
/EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. NO open flames. Powder, alcohol-resistant foam,
water spray, carbon dioxide.
EXPLOSION Above 60°C explosive vapour/air Above 60°C use a dosed system,
mixtures may be formed. ventilation, and explosion-proof
electrical equipment.
EXPOSURE
Inhalation Cough. Headache. Laboured Ventilation, local exhaust, or Fresh air, rest. Refer for
breathing. Shortness of breath. breathing protection. medical attention.
Sore throat.
Skin MAY BE ABSORBED! Dry skin. Protective clothing. Remove contaminated clothes.
Redness. Pain. Rinse skin with plenty of water
or shower. Refer for
medical attention.
Eyes Redness. Pain. Face shield. First rinse with plenty of water
for several minutes (remove contact
lenses if easily
possible), then take to a doctor.
Ingestion Abdominal pain. Diarrhoea. Do not eat, drink, or smoke Rinse mouth. Give plenty of water
Headache. Sore throat. during work. to drink. Refer for medical attention.
Vomiting.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking liquid in sealable Do not transport with food and feedstuffs.
containers. Absorb remaining liquid in EU Classification
sand or inert absorbent and remove to safe place. Symbol: T
Do NOT let this chemical enter the environment. R: 21-23/25-36137-40
(Extra personal protection: self-contained S: (1/2-)26-36137/39-45
breathing apparatus). UN Classification
UN Hazard Class: 6.1
UN Subsidiary Risks: 3
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-84 Separated from strong bases, strong acids,
NFPA Code: H2; F2; R0; strong oxidants, food and feedstuffs. Keep
in the dark. Well closed. Ventilation
along the floor.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS TO YELLOW LIQUID, WITH CHARACTERISTIC The substance can be absorbed Into the body
ODOUR. TURNS RED-BROWN ON EXPOSURE TO AIR AND by inhalation, through the skin and by
LIGHT. ingestion.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air. The substance irritates the eyes, the skin
and the respiratory tract.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance polymerizes under the The liquid defats the skin. The substance may
influence of acids) or base(s) have effects on the liver.
with fire or explosion hazard. Reacts
violently with oxidants. Attacks
many plastics.
OCCUPATIONAL EXPOSURE LIMITS:
TLV (as TWA): 2 ppm; 7.9 mg/m3 (skin) (ACGIH 1998).
PHYSICAL PROPERTIES
Boiling point: 162°C Explosive limits, vol% in air: 2.1-19.3
Melting point: -36.5°C Octanol/water partition coefficient as log Pow: 0.41
Relative density (water = 1): 1.16
Solubility In water, g/100 ml at 20°C: 8.3
Vapour pressure, kPa at 20°C: 0.144
Relative vapour density (air = 1): 3.31
Flash point: 60°C (c.c.)
Auto-ignition temperature: 315°C
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should be given to water organisms.
NOTES
The odour warning when the exposure limit value is exceeded is insufficient.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible for the use
which might be made of this information
(c) IPCS, CEC 1999
REFERENCES
Agakishiyev D (1989) Skin irritation of laboratory animals caused by
single and combined applications of petroleum refinery (furfural and
D-11 mineral oil distillate). Vestnik Dermatologii i Venerologii,
65:51-56 (HSE Translation No. 14358A).
Agakishiyev D (1990) Changes in guinea-pig skin and visceral
morphology after multiple epicutaneous exposure to furfural and D-11
mineral oil distillate and to a combination of the two. Vestnik
Dermatologii i Venerologii, 66(12):16-20 (HSE Translation No.
14397A).
Apol A, Lucas J (1975) Health hazard evaluation/toxicity
determination report, Pacific Grinding Wheel Co., Marysville,
Washington. Cincinnati, OH, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and Health (Report
No. 73-18-171).
Banerjee N, Bhatnagar R, Viswanathan L (1981) Inhibition of glycolysis
by furfural in Saccharomyces cerevisiae. European journal of applied
microbiology and biotechnology, 11:226-228.
Belay N, Boopathy R, Voskuilen G (1997) Anaerobic transformation of
furfural by Methanococcus deltae. Applied environmental
microbiology, 63:2092-2094.
Benjamin M, Woods S, Ferguson J (1984) Anaerobic toxicity and
biodegradability of pulp mill waste constituents. Water research,
18:601-607.
Bringmann G, Kuhn R (1976) Vergleichende Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Bakterien ( Pseudomonas putida) und
Blaualgen ( Microcystis aeruginosa). GasWasserfach-Wasser Abwasser,
117:410-413.
Bringmann G, Kuhn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication test. Water research, 14:231-241.
Bringmann G, Kuhn R (1980b) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen II Backterienfressende
Ciliaten. Zeitschrift fuer Wasser und Abwasser Forschung, 13:26-31.
Bringmann G, Kuhn R (1980c) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen III Saprozoische
Flagellaten. Zeitschrift fuer Wasser und Abwasser Forschung,
13:170-173.
Brune G, Schoberth S, Sahm H (1983) Growth of a strictly anaerobic
bacterium on furfural (2-furaldehyde). Applied environmental
microbiology, 46:1187-1192.
Carter W, Winer A, Pitts J (1981) Effect of peroxyacetyl nitrate on
the initiation of photochemical smog. Environmental science and
technology, 15:829-831.
Castellino N, Elmino O, Rozera G (1963) Experimental research on
toxicity of furfural. Archives of environmental health, 7:574-582.
Dunlop AP, Peters FN (1953) The furans. New York, NY, Reinhold
Publishing Corporation (American Chemical Society Monograph Series
Vol. 119).
Espinosa-Mansilla A, Muno de la Pena A, Salinas F (1993) Semiautomatic
determination of furanic aldehydes in food and pharmaceutical samples
by a stopped-flow injection analysis method. Journal of the
Association of Official Analytical Chemists International,
76:1255-1261.
Ettinger M et al. (1954) In: Proceedings of the 8th Industrial Waste
Conference, Purdue University, West Lafayette, IN [cited in HSDB,
1998].
Feron V (1972) Respiratory tract tumours in hamsters after
intratracheal instillations of benzo(a)pyrene alone and with furfural.
Cancer research, 32:28-36.
Feron V, Kruysse A (1978) Effects of exposure to furfural vapour in
hamsters simultaneously treated with benzo(a)pyrene or
diethylnitrosamine. Toxicology, 11:127-144.
Feron V, Kruysse A, Dreef-van-der-Meulen H (1979) Repeated exposure to
furfural vapour: 13 week study in Syrian golden hamsters.
Zentralblatt fuer Bakteriologie, Parasitenkunde,
Infektionskrankheiten und Hygiene, Abteilung 1, Originale, Reihe B,
168:442-451.
Flek J, Sedivec V (1978a) Absorption, metabolism and excretion of
furfural in man. Prac-Lek, 30 (5):172-177 (HSE Translation No. 143
60B).
Flek J, Sedivec V (l978b) Absorption, metabolism and excretion of
furfural in man. International archives of occupational and
environmental health, 41:159-168.
Gardner H (1925) Physiological effects of vapours from a few
solvents used in paints, varnishes and lacquers. Scientific
Section, Educational Bureau, Paint Manufacturers' Association of the
U.S. (Circulation No. 250).
Gomez-Arroyo S, Souza V (1985) In vitro and occupational induction
of SCE in human lymphocytes with furfuryl alcohol and furfural.
Mutation research, 156:233-238.
Gregg C, Rajan R, Cocker J, Groves J (1997) 2-Furaldehyde. Sudbury,
Suffolk, UK, HSE Books (Risk Assessment Document EH72/6; ISBN
0-7176-1358-5).
Gupta G, Mishra A, Agarwal D (1991) Inhalation toxicology of furfural
vapours: an assessment of biochemical response in rat lungs.
Journal of applied toxicology, 11:343-347.
Hadi S, Rehman S (1989) Specificity of the interaction of furfural
with DNA. Mutation research, 225:101-106.
Hampton C, Pierson W, Harvey T, Updegrove W, Marano R (1982)
Hydrocarbon gases emitted from vehicles on the road. I. A qualitative
gas-chromatography mass-spectrometry survey. Environmental science
and technology, 16:287-298.
HSDB (1998) Hazardous substances data bank. Published by Micromedex
Inc. (Copyright 1998). Accessed via the CD-ROM version.
IARC (1995) Dry cleaning, some chlorinated solvents and other
industrial chemicals. IARC monographs on the evaluation of
carcinogenic risks to man, 63:409-429.
IPCS (1993) International Chemical Safety Card -- Furfural. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0276).
Japan Environment Agency (1998) Chemicals in the environment, 1997
ed. Tokyo, Japan Environment Agency, Environmental Health and Safety
Division.
JECFA (1999) Toxicological evaluation of certain food additives.
Geneva, World Health Organization, International Programme on Chemical
Safety, Joint FAO/WHO Expert Committee on Food Additives, pp. 33-56
(WHO Food Additives Series 42).
Juttner F (1986) Analysis of organic-compounds (VOC) in the forest air
of the southern Black Forest. Chemosphere, 15:985-992.
Karickhoff S, Brown D, Scott T (1979) Sorption of hydrophobic
pollutants on natural sediments. Water research, 13:241-248.
Kato F, Araki A, Nozaki K, Matsushima T (1989) Mutagenicity of
aldehydes and diketones. Mutation research, 216:366-367.
Kawasaki M (1980) Experiences with the test scheme under the Chemical
Control Law of Japan: An approach to structure-activity correlations.
Ecotoxicology and environmental safety, 4:444-454.
Keith L (1974) Chemical characterization of industrial wastewaters by
gas chromatography-mass spectrometry. Science of the total
environment, 3:87-102.
Kim S, Hayase F, Kato H (1987) Desmutagenic effect of alpha-dicarbonyl
and alpha-hydroxycarbonyl compounds against mutagenic heterocyclic
amines. Mutation research, 177:9-15.
Kim S, Kim I, Yeum D, Park Y (1988) Desmutagenic action of sugar
degradation products. Korean journal of food science and technology,
20 (1):119-124.
Kim T, Hah Y, Hong S (1983) Toxic effects of furfural on
Pseudomonas fluorescens. Korean journal of microbiology, 21:149-155.
Kleindienst T, Shepson P, Edney E, Claxton L, Cupitt L (1986) Wood
smoke -- measurement of the mutagenic activity of its gas-phase and
particulate-phase photooxidation products. Environmental science and
technology, 20:493-501.
Kool HJ, van Kreijl CF, Zoetman BCJ (1982) Toxicological assessment of
organic compounds in drinking water. Critical reviews in toxicology,
12:307-357.
Laham S, Potvin M (1989) Metabolism of furfural in the Sprague-Dawley
rat. Toxicology and environmental chemistry, 24:35-47.
Lipari F, Dasch JM, Scruggs WF (1984) Aldehyde emissions from
wood-burning fireplaces. Environmental science and technology,
18:326-330.
Lo Coco F, Ceccon L, Valentini C, Novelli V (1992) High performance
liquid chromatographic determination of 2-furaldehyde in spirits.
Journal of chromatography, 590:235-240.
Loquet C, Toussaint G, LeTalaer J (1981) Studies on mutagenic
constituents of apple brandy and various alcoholic beverages collected
in Western France, a high incidence area for oesophageal cancer.
Mutation research, 88:155-164.
Maga J (1979) Furans in foods. Critical review. Food science and
nutrition, 4:355-399.
Marnett L, Hurd H, Hollstein M, Levin D, Esterbauer H, Ames B (1985)
Naturally occurring carbonyl compounds are mutagens in Salmonella
tester strain TA 104. Mutation research, 148:25-34.
Mattson V, Arthur J, Walbridge C (1976) Acute toxicity of selected
organic compounds to fathead minnows. Duluth, MN, US Environment
Protection Agency, Environmental Research Laboratory (Report No.
EPA/600/3-76-097).
McGregor D, Brown A, Cattanach P, Edwards I, McBride D, Caspary W
(1988) Responses of the L5l78Y tk+/tk- mouse lymphoma cell forward
mutation assay II: 18 coded chemicals. Environmental molecular
mutagenesis, 11:91-118.
McMahon R, Cline J, Thompson C (1979) Assay of 855 test chemicals in
ten tester strains using a new modification of the Ames test for
bacterial mutagens. Cancer research, 39:682-693.
Mishra A, Dwivedi P, Verma A, Sinha M, Mishra J, Lal K, Pandya K,
Dutta K (1991) Pathological and biochemical alterations induced by
inhalation of furfural vapour in rat lung. Bulletin of environmental
contamination and toxicology, 47:668-674.
Miyakama Y, Nishi Y, Kato K, Sato H, Takahashi M, Hayashi Y (1991)
Initiating activity of eight pyrolysates of carbohydrates in a two
stage mouse skin tumorigenesis model. Carcinogenesis, 12:1169-1173.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E (1986)
Salmonella mutagenicity tests 11: Results from the testing of 270
chemicals. Environmental mutagenesis, 8 (Suppl. 7):1-119.
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA 1535/pSK1002. Examination of 151 chemicals.
Mutation research, 192:239-246.
Nazyrov G, Yampol'skaya Y (1969) The effect of furan resins on the
skin and skin allergies of persons employed in their manufacture.
Trudy Institut Gigiena Truda i Professionalyne Zabolevaniya, 10:
19-21 (HSE Translation No. 14480A).
NIOSH (1987) NIOSH Proficiency Analytical Testing (PAT) Program.
Cincinnati, OH, US Department of Health, Education and Welfare,
National Institute for Occupational Safety and Health (DHEW
Publication No. 77-173).
Nishi Y, Miyakama Y, Kato K (1989) Chromosome aberrations induced by
pyrolysates of carbohydrates in Chinese hamster V79 cells.
Mutation research, 227:117-123.
Nomier A, Silveira D, McComish M, Chadwick M (1992) Comparative
metabolism and disposition of furfural and furfural alcohol in rats.
Drug metabolism and disposition, 20:198-204.
NTP (1990) Toxicology and carcinogenesis studies of furfural in
F344/N rats and B6C3F1 mice (gavage studies). Research Triangle
Park, NC, US Department of Health and Human Services, Public Health
Service, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 382; NIH Publication No. 90-2837).
Nutley B (1989) An analytical method for biological monitoring of
furfural exposure. Sheffield, UK, Health and Safety Executive,
Health and Safety Laboratories (Section Report IR/L/OT/89/1).
Parkash M, Caldwell J (1994) Metabolism and excretion of
[14C]furfural in the rat and mouse. Food and chemical toxicology,
32(10):887-895.
Patel S, Robertson S, Brown R (1988) Method validation for atmospheric
monitoring -- an illustration with furfural. In: Proceedings of the
Sixth Thermal Desorption Symposium, Anughaha, Windsor, UK.
Pawlowicz A, Droszcz W, Garlicki A, Kutyla J, Kowalczyk M (1984)
Effect of furfural on the respiratory system. Medycyna Pracy,
35:39-45 (HSE Translation No. 14361B).
Pellizzari E, Hartwell T, Harris B, Waddell R, Whitaker D, Erickson M
(1982) Purgeable organic compounds in mothers' milk. Bulletin of
environmental contamination and toxicology, 28:322-328.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Reynolds S, Stower S, Patterson R, Maronpot R, Aaronson S, Anderson M
(1987) Activated oncogenes in B6C3F1 mouse liver tumours:
implications for risk assessment. Science, 237:1309-1316.
Rodriguez-Arnaiz R, Morales P, Zimmering S (1992) Evaluation in
Drosophila melanogaster of the mutagenic potential of furfural in
the mei-9a test for chromosome loss in germ-line cells and the wing
spot test for mutational activity in somatic cells.
Mutation research, 280:75-80.
Rowe E, Tullos L (1980) Lube solvents no threat to waste treatment.
Hydrocarbon processing, 59:63-65.
Ruus L (1964) A study of waste waters from the forest products
industry. 4. Composition of biochemical oxygen demand of condensate
from spent sulfite liquor evaporation. Svensk Papperstidning,
67:221-225.
Schafer E, Bowles W, Hurlbut J (1983) The acute oral toxicity,
repellency and hazard potential of 998 chemicals to one or more
species of wild and domestic birds. Archives of environmental
contamination and toxicology, 12:355-382.
Sedivec V, Flek J (1978) Biologic monitoring of persons exposed to
furfural vapours. International archives of occupational and
environmental health, 42:41-49.
Shane B, Troxclair A, McMillin D, Henry C (1988) Comparative
mutagenicity of 9 brands of coffee to Salmonella typhimurium TA100,
TA102, TA104. Environmental molecular mutagenesis, 11:195-206.
Shimizu A, Nakamura Y, Harada M, Ono T, Sato K, Inoue T, Kanisawa M
(1989) Positive foci of glutathione S-transferase placental form in
the liver of rats given furfural by oral administration.
Japanese journal of cancer research, 80:608-611.
Shinohara K, Omura H (1986) Furans as mutagens formed by
amino-carbonyl reactions. Developmental food science, 3:353-362.
Soska J, Koukalova B, Ebringer L (1981) Mutagenic activities of simple
nitrofuran derivatives. Comparison of related compounds in the phage
inductest, chloroplast-bleaching and bacterial-repair and mutagenicity
tests. Mutation research, 81:21-26.
SRI International (1982) The acute oral toxicity of furfural in
various vehicles in the rat. Menlo Park, California (SRI Project No.
LSC-4357; Compound Report No. 1, August 1982).
Stich H, Rosin M, Wu C, Powrie W (1981) Clastogenicity of furans found
in food. Cancer letters, 13:89-95.
Subramanyam S, Sailaja D, Rathnaprabha D (1989) Genotoxic assay of two
dietary furans by some in vivo cytogenetic parameters.
Environmental molecular mutagenesis, 14 (Suppl. 15):239.
Terrill J, Van Horn W, Robinson D, Thomas D (1989) Acute inhalation
toxicity of furan, 2-methyl furan, furfuryl alcohol and furfural in
the rat. American Industrial Hygiene Association journal,
50:A359-A361.
Turnbull H, DeMann J, Weston R (1954) Toxicity of various refinery
materials to fresh water fish. Industrial and engineering chemistry,
46:324-333.
US EPA (1998) Toxic Substances Control Act: Inventory update rule 51.
In: Federal register. Washington, DC, US Environmental Protection
Agency.
Wallen I, Greer W, Lasater R (1957) Toxicity to Gambusia affinis of
certain pure chemicals in turbid waters. Sewage and industrial
wastes, 29:695-711.
Wang P, Brenchley J, Humphrey A (1994) Screening microorganisms for
utilization of furfural and possible intermediates in its degradation
pathway. Biotechnology letters, 16:977-982.
Woodruff R, Mason J, Valencia R, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:677-702.
Woods L, Seevers M (1955) Toxicity of furfural. Unpublished report,
University of Michigan Medical School, Ann Arbor, MI. 28 March 1955.
Woods L, Seevers M (1956) Toxicity of furfural. Unpublished report,
University of Michigan Medical School, Ann Arbor, MI. February 1956.
Zdzienicka M, Tudek B (1978) Mutagenic activity of furfural in
Salmonella typhimurium TA 100. Mutation research, 58:205-209.
Zhang J, He Q, Lioy P (1994) Characteristics of aldehydes --
concentrations, sources and exposures for indoor and outdoor
residential microenvironments. Environmental science and technology,
28:146-152.
APPENDIX 1 -- SOURCE DOCUMENT
Gregg et al. (1997): 2-Furaldehyde (Risk Assessment Document EH72/6)
The authors' draft version of this Health and Safety Executive
report was initially reviewed internally by a group of approximately
10 Health and Safety Executive experts (mainly toxicologists, but also
scientists from other relevant disciplines, such as epidemiology and
occupational hygiene). The toxicology section of the amended draft was
then reviewed by toxicologists from the United Kingdom Department of
Health. Subsequently, the entire risk assessment document was reviewed
by a tripartite advisory committee to the United Kingdom Health and
Safety Commission, the Working Group for the Assessment of Toxic
Chemicals (WATCH). This committee is composed of experts in toxicology
and occupational health and hygiene from industry, trade unions, and
academia.
The members of the WATCH committee at the time of the peer review
were Mr Steve Bailey, Confederation of British Industries; Professor
Jim Bridges, University of Surrey; Dr Ian Guest, Confederation of
British Industries; Dr Alastair Hay, Trade Unions Congress; Dr Jenny
Leeser, Confederation of British Industries; Dr Len Levy, Institute of
Occupational Hygiene, Birmingham; Dr Mike Molyneux, Confederation of
British Industries; Mr Alan Moses, Confederation of British
Industries; Dr Ron Owen, Trade Unions Congress; Mr Jim Sanderson,
Independent Consultant; and Dr Mike Sharratt, University of Surrey.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on 2-furaldehyde was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Chinese Academy of Preventive Medicine, Ministry of Health,
Beijing, People's Republic of China
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health and Environmental Protection
(RIVM), Bilthoven, The Netherlands
Senatskommission der Deutschen Forschungsgemeinschaft, Bonn,
Germany
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati;
National Institute of Environmental Health Sciences, Research
Triangle Park), USA
United States Environmental Protection Agency (Region VIII;
National Center for Environmental Assessment, Washington, DC),
USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au 2-furaldéhyde est basé sur une mise au point
rédigée par le Health and Safety Executive du Royaume-Uni au sujet des
effets que ce composé pourrait avoir sur la santé humaine,
principalement en milieu professionnel, mais aussi sur l'environnement
en général (Gregg et al., 1997). Ce document est donc principalement
consacré aux voies d'exposition qui existent sur lieux de travail,
mais il comporte également une évaluation d'ordre écologique. Les
données prises en compte vont jusqu'en janvier 1997. Une bibliographie
complémentaire a été effectuée jusqu'à décembre 1997 afin de relever
les données qui auraient pu être publiées après la première recherche
bibliographique, mais sans résultat. On trouvera à l'appendice 1 des
indications sur le mode d'examen par des pairs ainsi que sur les
sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC No 0276) relative au 2-furaldéhyde, établie
par le Programme internationale sur la sécurité chimique (IPCS, 1993),
est également reproduite dans ce document.
Le 2-furaldéhyde (C5H4O2) (No CAS 98-01-1) se présente sous la
forme d'un liquide à l'odeur piquante, rappelant celle des amandes. On
le trouve à l'état de traces dans diverses denrées alimentaires et il
est produit industriellement par digestion en continu ou en discontinu
des pentosanes contenus dans les déchets agricoles. Ces pentosanes
sont hydrolysés en pentoses qui subissent ensuite une
cyclodéshydratation en 2-furaldéhyde. On utilise ce composé dans
l'industrie pour la production de résines, de meules et de substances
réfractaires, pour le raffinage des huiles lubrifiantes et la
récupération des solvants. On l'emploie également en très petites
quantités comme aromatisant.
Le 2-furaldéhyde est présent dans de nombreuses denrées
alimentaires, soit naturellement soit comme contaminant. On en a
signalé la présence dans l'eau destinée à la boisson et le lait
maternel, mais en quantités non mesurables.
On dispose de mesures portant sur l'exposition professionnelle
par inhalation et des estimations ont été obtenues au moyen d'un
système expert appelé Estimation and Assessment of Substance Exposure
(EASE). En règle générale, dans l'ensemble des industries,
l'exposition au 2-furaldéhyde présent dans l'air est inférieure à 8
mg/m3 (2 ppm) en moyenne pondérée par rapport au temps calculée sur 8
h (TWA). Pour la plupart des industries, cette donnée ne permet pas de
prévoir l'exposition moyenne pondérée sur 15 minutes. Toutefois, on a
calculé que dans l'industrie des aromatisants, l'exposition moyenne
pondérée sur 15 min devait se situer entre 1,2 et 6,8 mg/m3
(c'est-à-dire entre 0,3 et 1,7 ppm). On ne dispose d'aucune mesure de
l'exposition par voie cutanée. Le système EASE donne une exposition
cutanée comprise entre 0,1 et 1 mg/cm2 j-1 pour la plupart des
industries, avec des valeurs plus élevées (entre 1 et 5 mg/cm2 j-1)
dans la fabrication de matériaux abrasifs et de meules.
Les données toxicocinétiques sont limitées, mais on est fondé à
penser que le 2-furaldéhyde est facilement résorbé par la voie
respiratoire et la voie percutanée. L'expérimentation animale montre
qu'après administration par voie orale à des rats, le composé est
facilement absorbé et rapidement excrété, principalement dans les
urines, mais une petite partie passe également dans l'air expiré sous
la forme de dioxyde de carbone. Le métabolisme du 2-furaldéhyde se
caractérise par l'oxydation ou l'acétylation du groupement aldéhyde,
suivie par une conjugaison avec la glycine. La 2-furoylglycine est le
principal métabolite urinaire à côté de l'acide furoïque, de l'acide
furanacrylique et de l'acide furanacrylurique, dont l'importance est
moindre.
Chez l'Homme, on a mis en évidence une résorption du
2-furaldéhyde gazeux au niveau des poumons et de la peau. L'organisme
humain métabolise ce composé de manière analogue à celui du rat, la
majeure partie de la dose absorbée se retrouvant dans les urines sous
la forme de 2-furoylglycine. L'acide furoïque et l'acide
furanacrylurique sont également présents en moindre quantité. On
observé une résorption du 2-furanaldéhyde par la voie percutanée.
Chez l'animal, la toxicité aiguë du composé est variable :
globalement, le 2-furaldéhyde est toxique par la voie respiratoire et
la voie orale (CL50 à 4 h, 940 mg/m3 [235 ppm]; DL50 par voie orale,
environ 120 mg/kg de poids corporel) et l'on ne possède pas de données
claire au sujet de la voie percutanée. Après exposition unique ou
répétée au produit par inhalation, on observe systématiquement une
irritation des voies respiratoires et des lésions pulmonaires. On a
également fait état d'irritation de la peau et des yeux. Il semble en
revanche qu'on n'ait pas noté d'irritation de la gorge ou des yeux
chez des sujets humains exposés à une concentration de 40 mg/m3 (10
ppm) pendant 8 h ou de 80 mg/m3 pendant 4 h. Chez l'animal, on a
obtenu, pour la dose sans effet nocif observable (NOAEL) relative aux
effets cancérogènes, une valeur de 80 mg/m3 (20 ppm) dans le cas du
hamster et de 208 mg/m3 (52 ppm) dans le cas du hamster, lors
d'études qui se sont prolongées jusqu'à 13 semaines. Après avoir fait
ingérer le composé à rats et des souris pendant 103 semaines, à des
doses respectivement égales à 60 et 50 mg/kg de poids corporel, on a
observé
des tumeurs malignes et bénignes. Le 2-furaldéhyde est indéniablement
génotoxique in vitro sur des cultures de mammifères; on ne peut
tirer de conclusions définitives quant à la génotoxicité de ce composé
in vivo, mais on ne peut pas exclure non plus que cette génotoxicité
soit réelle et qu'elle puisse contribuer au processus de
cancérisation. Dans ces conditions, il n'est pas possible de
déterminer avec certitude la valeur de la NOAEL.
Faute de données utilisables pour l'estimation de l'exposition
individuelle indirecte au 2-furaldéhyde présent dans l'environnement
général, il est impossible de préciser le risque cancérogène que ce
composé représente pour la population dans son ensemble.
En milieu professionnel, il existe un risque d'effets
cancérogènes et génotoxiques. Il y cependant incertitude quant au
degré de risque; il est donc nécessaire de continuer à réduire
l'exposition autant qu'il est techniquement possible de le faire.
On ne dispose pas de données suffisantes concernant les effets du
composé sur la reproduction et le développement; il n'est donc pas
possible de déterminer s'il existe des risques de cette nature pour
l'Homme.
C'est l'industrie de la pâte à papier qui rejette le plus de
2-furaldéhyde dans l'environnement. La combustion du bois, qu'elle
soit naturelle ou d'origine humaine, entraîne également la libération
de se composé dans l'atmosphère.
On ne prévoit pas d'effets atmosphériques étant donné que le
2-furaldéhyde est détruit par réaction sur les radicaux hydroxyle, sa
demi-vie calculée dans l'atmosphère étant égale à 0,44 jours. Dans
l'air des villes, il peut également être décomposé par réaction avec
des radicaux nitrate. Une photo-oxydation directe n'est pas non plus à
exclure. Etant donné la faible valeur de la tension de vapeur et de la
constante de Henry, sa volatilisation à partir de l'eau et du sol
devrait être lente.
Il ne devrait pas y avoir d'hydrolyse aux valeurs du pH
rencontrées dans l'environnement. La faible valeur du coefficient de
partage entre l'octanol et l'eau (log Kow = 0,41) indique que la
capacité de bioaccumulation est faible. Le coefficient de sorption
( Koc) montre que la fixation aux particules n'est pas importante et
que le composé est par conséquent très mobile dans le sol.
Le 2-furaldéhyde subit une biodégradation aérobie rapide dans les
boues d'égout ainsi que dans les eaux superficielles. Cette
décomposition se produit également en anaérobiose et de nombreuses
bactéries et autres microorganismes sont capables d'utiliser ce
composé comme seule source de carbone. A forte concentration (>1000
mg/litre), le 2-furaldéhyde inhibe la croissance et l'activité
métabolique des cultures anaérobies non adaptées. En revanche, le
produit est plus facilement biodégradable par acclimatation des boues
anaérobies.
Les concentrations de 2-furaldéhyde les plus élevées qui aient
été constatées dans des eaux résiduaires industrielles provenaient de
condensats (environ 15 % des eaux résiduaires totales) rejetés par les
évaporateurs à bisulfite d'une usine de pâte à papier, avec une
moyenne de 274 mg/litre. On ne dispose que d'une seule étude donnant
les résultats de dosages du 2-furaldéhyde dans l'air intérieur et
extérieur; la valeur trouvée est d'environ 1 µg/m3; dans d'autres
études, on a mis en évidence la présence du composé mais sans procéder
à un dosage.
Le seuil de toxicité pour divers microorganismes se situe entre
0,6 et 31 mg/litre. Pour les poissons, la CL50 relative aux effets
toxiques aigus est comprise entre 16 et 32 mg/litre.
En se basant sur les concentrations auxquelles les rejets des
usines de pâte à papier sont susceptibles de donner lieu dans
l'environnement (cas le plus grave) et en prenant un facteur
d'incertitude de 1000 pour tenir compte de ce que les données de
toxicité aiguës sont limitées, on peut considérer que la libération de
2-furaldéhyde dans l'environnement représente un danger pour les
organismes aquatiques. On ne possède pas de données permettant
d'évaluer le risque pour les organismes terrestres, mais en tout état
de cause la décharge de 2-furaldéhyde au niveau du sol est
vraisemblablement peu importante.
RESUMEN DE ORIENTACION
El presente CICAD sobre el 2-furaldehído, preparado por la
Dirección de Salud y Seguridad del Reino Unido (Gregg et al., 1997),
se basa en un examen de los problemas relativos a la salud humana
(fundamentalmente ocupacionales), pero también contiene información
ambiental. Por consiguiente, este documento se concentra sobre todo en
la exposición a través de las rutas que son de interés para el entorno
ocupacional, pero incluye también una evaluación ambiental. En este
examen se han incorporado los datos identificados hasta enero de 1997.
Se realizó una ulterior búsqueda bibliográfica hasta diciembre de 1997
para localizar la nueva información que se hubiera publicado desde la
terminación del examen, pero no se encontraron estudios de interés. La
información acerca del carácter del examen colegiado del documento
original y su disponibilidad figura en el apéndice 1. La información
sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Washington, DC, Estados Unidos,
los días 8-11 de diciembre de 1998. La lista de participantes en esta
reunión figura en el apéndice 3. La Ficha internacional de seguridad
química (ICSC 0276) preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento.
El 2-furaldehído (C5H402) (CAS No 98-01-1) es un líquido de
olor acre "semejante al de la almendra". Se encuentra en cantidades
ínfimas en diversas fuentes de alimentos y se produce comercialmente
en digestores de lotes o continuos en los cuales se hidrolizan
pentosanos procedentes de residuos agrícolas a pentosas y éstas se
ciclodeshidratan a 2-furaldehído. Entre los usos industriales cabe
mencionar la producción de resinas, ruedas abrasivas, materiales
refractarios, refinado de los aceites lubricantes y recuperación de
disolventes. El 2-furaldehído se utiliza también, en cantidades muy
pequeñas, como aromatizante.
El 2-furaldehído está presente en numerosos alimentos, como
producto natural o como contaminante. Se ha notificado su presencia en
el agua potable y en la leche materna, pero las concentraciones no
fueron suficientes para su cuantificación.
Se dispone de mediciones de la exposición por inhalación en el
puesto de trabajo y también se han efectuado estimaciones utilizando
un sistema de computadora basado en los conocimientos, Estimación y
evaluación de la exposición a la sustancia (EASE). En general, la
exposición al compuesto en suspensión en el aire en todas las
industrias es inferior a 8 mg/m3 (2 ppm), con un promedio ponderado
por el tiempo (PPT) de ocho horas. No hay información suficiente para
pronosticar exposiciones PPT de 15 minutos para la mayoría de las
industrias; sin embargo, en la industria de los aromatizantes se ha
estimado una exposición PPT de 15 minutos de 1,2 a 6,8 mg/m3 (0,3 y
1,7 ppm). No se dispone de datos sobre mediciones de la exposición
cutánea. Se han calculado las exposiciones cutáneas basadas en la EASE
de 0,1 y 1 mg/cm2 al día para la mayoría de las industrias, con
exposiciones superiores, entre 1 y 5 mg/cm2 al día, para las
industrias de fabricación de materiales refractarios y ruedas
abrasivas.
Los datos toxicocinéticos son limitados, pero hay indicios de que
el 2-furaldehído se absorbe fácilmente por las vías de exposición por
inhalación y cutánea. Los estudios en animales demuestran que, tras la
administración oral a ratas, el 2-furaldehído se absorbe fácilmente y
excreta con rapidez sobre todo en la orina, aunque también se produce
alguna eliminación a través del anhídrido carbónico exhalado. El
metabolismo se caracteriza por la oxidación o la acetilación del grupo
aldehído, seguida de la conjugación de la glicina. La 2-furoilglicina
es el principal metabolito urinario; otros metabolitos secundarios son
el ácido furoico, el ácido furanacrílico y el ácido furanacrilúrico.
En el ser humano, se ha demostrado la absorción del vapor a
través de los pulmones y la piel. El metabolismo en las personas
parece ser semejante al de las ratas, excretándose la mayor parte de
la dosis retenida como 2-furoilglicina urinaria. Se han detectado
también como metabolitos secundarios el ácido furoico y el ácido
furanacrilúrico. Se ha observado asimismo absorción cutánea a partir
del 2-furaldehído líquido.
Los datos sobre la toxicidad aguda en los animales son variables;
en general, sin embargo, el 2-furaldehído es tóxico por inhalación y
por vía oral (CL50 a los cuatro horas, 940 mg/m3 [235 ppm]; DL50 por
vía oral, 120 mg/kg de peso corporal), no disponiéndose de información
clara en relación con la vía cutánea. Se ha observado sistemáticamente
irritación del sistema respiratorio y lesiones en los pulmones tras la
exposición por inhalación a una dosis única y repetida. También se ha
notificado irritación cutánea y ocular. Aparentemente no se detectó
irritación en la garganta o en los ojos en personas expuestas a 40
mg/m3 (10 ppm) durante ocho horas o a 80 mg/m3 (20 ppm) durante
cuatro horas. En animales se han determinado concentraciones sin
efectos adversos observados (NOAEL) de 80 mg/m3 (20 ppm) y 208 mg/m3
(52 ppm) en estudios de hasta 13 semanas de duración en hámsteres y
conejos, respectivamente, para los efectos no neoplásicos. Se han
observado tumores malignos y benignos en ratas y ratones tras la
exposición oral a 60 y 50 mg/kg de peso corporal, respectivamente,
durante 103 semanas. El 2-furaldehído es claramente genotóxico in
vitro en células de mamíferos; aunque no se puede llegar a una
conclusión definitiva sobre el potencial genotóxico del 2-furaldehído
in vivo, no se puede descartar la posibilidad de que la
genotoxicidad pueda contribuir al proceso carcinogénico. Estos
factores impiden determinar de manera fidedigna una NOAEL para el
2-furaldehído.
La falta de datos disponibles que sirvan de base para la
estimación de la exposición indirecta de las personas al 2-furaldehído
del medio ambiente general impiden la caracterización de los posibles
riesgos de cáncer para la población general.
En el entorno del puesto trabajo, existe un riesgo potencial de
efectos carcinogénicos y genotóxicos. El nivel de riesgo es incierto;
en consecuencia, existe el requisito permanente de reducir los niveles
de exposición todo lo que sea posible razonablemente con la tecnología
disponible en la actualidad.
No hay datos adecuados sobre los efectos en la reproducción o en
el desarrollo; por consiguiente, no es posible evaluar el riesgo para
la salud humana con respecto a estos efectos finales.
Las emisiones más altas notificadas de 2-furaldehído al medio
ambiente son las de la industria de la pasta de madera. El
2-furaldehído se libera a la atmósfera en la combustión natural y
antropogénica de madera.
No se prevén efectos atmosféricos, puesto que el 2-furaldehído se
destruye al reaccionar con los radicales hidroxilo, con una semivida
atmosférica calculada de 0,44 días. En el aire urbano, la reacción con
radicales nitrato puede representar un proceso de degradación
adicional. También se puede producir fotooxidación directa. Los bajos
valores de la presión de vapor y de la constante de Henry parecen
indicar que solamente se produce una volatilización lenta del
2-furaldehído de la superficie del agua y del suelo.
No se prevé que con el pH del medio ambiente se produzca
hidrólisis en el agua. El bajo coeficiente de reparto octanol/agua
(log Kow 0,41) deja entrever una escasa capacidad de
bioacumulación. Los coeficientes de sorción ( Koc) parecen indicar
una sorción escasa a partículas y una movilidad alta en el suelo.
El 2-furaldehído se biodegrada fácilmente en sistemas aerobios
utilizando fangos cloacales y en el agua superficial. También se
produce degradación en condiciones anaerobias, gracias a una serie de
bacterias y otros microorganismos capaces de utilizar el compuesto
como única fuente de carbono. A concentraciones altas (>1000
mg/litro), el 2-furaldehído inhibe el crecimiento y la actividad
metabólica de cultivos anaerobios no adaptados. Sin embargo, la
aclimatación aumenta la capacidad de degradación del compuesto en los
fangos anaerobios.
Las concentraciones más altas notificadas de 2-furaldehído en
aguas residuales industriales corresponden al líquido condensado de
los evaporadores de sulfito (alrededor del 15% de la corriente de
residuos de las fábricas de pasta de madera), con un promedio de
274 mg/litro. En un estudio aislado se cuantificó la concentración de
2-furaldehído en el aire de espacios cerrados y abiertos en alrededor
de 1 µg/m3; en otros estudios se ha detectado el compuesto, pero no
se ha cuantificado.
Se han notificado umbrales tóxicos para diversos microorganismos
en una gama de 0,6 a 31 mg/litro. Las CL50 aguda para los peces
oscila entre 16 y 32 mg/litro.
Teniendo cuenta las concentraciones pronosticadas estimadas en el
medio ambiente a partir de los residuos de la pasta de madera (se
considera que es el peor de los casos) y la aplicación de un factor de
incertidumbre de 1000 a los datos limitados de las pruebas de
toxicidad aguda, cabe prever que las emisiones de 2-furaldehído
plantearán un riesgo bajo para los organismos acuáticos. No se dispone
de datos sobre los cuales basar una evaluación del riesgo terrestre,
pero se supone que las emisiones a la tierra son bajas.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer concluded that
"There is inadequate evidence in humans for the carcinogenicity of
2-furaldehyde" and that "There is limited evidence in experimental
animals for the carcinogenicity of 2-furaldehyde." Its overall
evaluation is that "2-Furaldehyde is not classifiable as to its
carcinogenicity to humans" (IARC, 1995).
2-Furaldehyde has previously been evaluated by the Joint Expert
Committee on Food Additives (JECFA, 1999), whose conclusions are
summarized as follows:
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL [no-observed-effect level] was
identified for hepatotoxicity in rats, the Committee was unable
to allocate an ADI [acceptable daily intake]. Before reviewing
the substance again, the Committee would wish to review the
results of studies of DNA binding in mice and of a 90-day study
of toxicity in rats to identify a NOEL for hepatotoxicity.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0276) reproduced in this
document.
13.1 Health surveillance advice
An exposure surveillance program could include monitoring of
2-furoic acid in urine after alkaline hydrolysis of the main
metabolite of 2-furaldehyde, furoylglycine, immediately following
exposure.
13.2 Advice to physicians
In case of poisoning, treatment is supportive.
13.3 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from mixing with acids, bases, or oxidants
because of the risk of fire or explosion.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals, taken in a certain country, can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
2-Furaldehyde
INTERNATIONAL CHEMICAL SAFETY CARD
FURFURAL ICSC: 0276
November 1998
CAS # 98-01-1 2-Furancarboxyaldehyde
RTECS # LT7000000 2-Furaldehyde
UN # 1199 2-Furylmethanal
EC # 605-010-00-4 C5H4O2/C4H3OCHO
Molecular mass: 96.1
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID/FIRE
/EXPOSURE SYMPTOMS FIGHTING
FIRE Combustible. NO open flames. Powder, alcohol-resistant foam,
water spray, carbon dioxide.
EXPLOSION Above 60°C explosive vapour/air Above 60°C use a dosed system,
mixtures may be formed. ventilation, and explosion-proof
electrical equipment.
EXPOSURE
Inhalation Cough. Headache. Laboured Ventilation, local exhaust, or Fresh air, rest. Refer for
breathing. Shortness of breath. breathing protection. medical attention.
Sore throat.
Skin MAY BE ABSORBED! Dry skin. Protective clothing. Remove contaminated clothes.
Redness. Pain. Rinse skin with plenty of water
or shower. Refer for
medical attention.
Eyes Redness. Pain. Face shield. First rinse with plenty of water
for several minutes (remove contact
lenses if easily
possible), then take to a doctor.
Ingestion Abdominal pain. Diarrhoea. Do not eat, drink, or smoke Rinse mouth. Give plenty of water
Headache. Sore throat. during work. to drink. Refer for medical attention.
Vomiting.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Collect leaking liquid in sealable Do not transport with food and feedstuffs.
containers. Absorb remaining liquid in EU Classification
sand or inert absorbent and remove to safe place. Symbol: T
Do NOT let this chemical enter the environment. R: 21-23/25-36137-40
(Extra personal protection: self-contained S: (1/2-)26-36137/39-45
breathing apparatus). UN Classification
UN Hazard Class: 6.1
UN Subsidiary Risks: 3
UN Pack Group: II
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-84 Separated from strong bases, strong acids,
NFPA Code: H2; F2; R0; strong oxidants, food and feedstuffs. Keep
in the dark. Well closed. Ventilation
along the floor.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
COLOURLESS TO YELLOW LIQUID, WITH CHARACTERISTIC The substance can be absorbed Into the body
ODOUR. TURNS RED-BROWN ON EXPOSURE TO AIR AND by inhalation, through the skin and by
LIGHT. ingestion.
PHYSICAL DANGERS: EFFECTS OF SHORT-TERM EXPOSURE:
The vapour is heavier than air. The substance irritates the eyes, the skin
and the respiratory tract.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance polymerizes under the The liquid defats the skin. The substance may
influence of acids) or base(s) have effects on the liver.
with fire or explosion hazard. Reacts
violently with oxidants. Attacks
many plastics.
OCCUPATIONAL EXPOSURE LIMITS:
TLV (as TWA): 2 ppm; 7.9 mg/m3 (skin) (ACGIH 1998).
PHYSICAL PROPERTIES
Boiling point: 162°C Explosive limits, vol% in air: 2.1-19.3
Melting point: -36.5°C Octanol/water partition coefficient as log Pow: 0.41
Relative density (water = 1): 1.16
Solubility In water, g/100 ml at 20°C: 8.3
Vapour pressure, kPa at 20°C: 0.144
Relative vapour density (air = 1): 3.31
Flash point: 60°C (c.c.)
Auto-ignition temperature: 315°C
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should be given to water organisms.
NOTES
The odour warning when the exposure limit value is exceeded is insufficient.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on behalf of the CEC or the IPCS is responsible for the use
which might be made of this information
(c) IPCS, CEC 1999
REFERENCES
Agakishiyev D (1989) Skin irritation of laboratory animals caused by
single and combined applications of petroleum refinery (furfural and
D-11 mineral oil distillate). Vestnik Dermatologii i Venerologii,
65:51-56 (HSE Translation No. 14358A).
Agakishiyev D (1990) Changes in guinea-pig skin and visceral
morphology after multiple epicutaneous exposure to furfural and D-11
mineral oil distillate and to a combination of the two. Vestnik
Dermatologii i Venerologii, 66(12):16-20 (HSE Translation No.
14397A).
Apol A, Lucas J (1975) Health hazard evaluation/toxicity
determination report, Pacific Grinding Wheel Co., Marysville,
Washington. Cincinnati, OH, US Department of Health, Education and
Welfare, National Institute for Occupational Safety and Health (Report
No. 73-18-171).
Banerjee N, Bhatnagar R, Viswanathan L (1981) Inhibition of glycolysis
by furfural in Saccharomyces cerevisiae. European journal of applied
microbiology and biotechnology, 11:226-228.
Belay N, Boopathy R, Voskuilen G (1997) Anaerobic transformation of
furfural by Methanococcus deltae. Applied environmental
microbiology, 63:2092-2094.
Benjamin M, Woods S, Ferguson J (1984) Anaerobic toxicity and
biodegradability of pulp mill waste constituents. Water research,
18:601-607.
Bringmann G, Kuhn R (1976) Vergleichende Befunde der Schadwirkung
wassergefahrdender Stoffe gegen Bakterien ( Pseudomonas putida) und
Blaualgen ( Microcystis aeruginosa). GasWasserfach-Wasser Abwasser,
117:410-413.
Bringmann G, Kuhn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in the cell
multiplication test. Water research, 14:231-241.
Bringmann G, Kuhn R (1980b) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen II Backterienfressende
Ciliaten. Zeitschrift fuer Wasser und Abwasser Forschung, 13:26-31.
Bringmann G, Kuhn R (1980c) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen III Saprozoische
Flagellaten. Zeitschrift fuer Wasser und Abwasser Forschung,
13:170-173.
Brune G, Schoberth S, Sahm H (1983) Growth of a strictly anaerobic
bacterium on furfural (2-furaldehyde). Applied environmental
microbiology, 46:1187-1192.
Carter W, Winer A, Pitts J (1981) Effect of peroxyacetyl nitrate on
the initiation of photochemical smog. Environmental science and
technology, 15:829-831.
Castellino N, Elmino O, Rozera G (1963) Experimental research on
toxicity of furfural. Archives of environmental health, 7:574-582.
Dunlop AP, Peters FN (1953) The furans. New York, NY, Reinhold
Publishing Corporation (American Chemical Society Monograph Series
Vol. 119).
Espinosa-Mansilla A, Muno de la Pena A, Salinas F (1993) Semiautomatic
determination of furanic aldehydes in food and pharmaceutical samples
by a stopped-flow injection analysis method. Journal of the
Association of Official Analytical Chemists International,
76:1255-1261.
Ettinger M et al. (1954) In: Proceedings of the 8th Industrial Waste
Conference, Purdue University, West Lafayette, IN [cited in HSDB,
1998].
Feron V (1972) Respiratory tract tumours in hamsters after
intratracheal instillations of benzo(a)pyrene alone and with furfural.
Cancer research, 32:28-36.
Feron V, Kruysse A (1978) Effects of exposure to furfural vapour in
hamsters simultaneously treated with benzo(a)pyrene or
diethylnitrosamine. Toxicology, 11:127-144.
Feron V, Kruysse A, Dreef-van-der-Meulen H (1979) Repeated exposure to
furfural vapour: 13 week study in Syrian golden hamsters.
Zentralblatt fuer Bakteriologie, Parasitenkunde,
Infektionskrankheiten und Hygiene, Abteilung 1, Originale, Reihe B,
168:442-451.
Flek J, Sedivec V (1978a) Absorption, metabolism and excretion of
furfural in man. Prac-Lek, 30 (5):172-177 (HSE Translation No. 143
60B).
Flek J, Sedivec V (l978b) Absorption, metabolism and excretion of
furfural in man. International archives of occupational and
environmental health, 41:159-168.
Gardner H (1925) Physiological effects of vapours from a few
solvents used in paints, varnishes and lacquers. Scientific
Section, Educational Bureau, Paint Manufacturers' Association of the
U.S. (Circulation No. 250).
Gomez-Arroyo S, Souza V (1985) In vitro and occupational induction
of SCE in human lymphocytes with furfuryl alcohol and furfural.
Mutation research, 156:233-238.
Gregg C, Rajan R, Cocker J, Groves J (1997) 2-Furaldehyde. Sudbury,
Suffolk, UK, HSE Books (Risk Assessment Document EH72/6; ISBN
0-7176-1358-5).
Gupta G, Mishra A, Agarwal D (1991) Inhalation toxicology of furfural
vapours: an assessment of biochemical response in rat lungs.
Journal of applied toxicology, 11:343-347.
Hadi S, Rehman S (1989) Specificity of the interaction of furfural
with DNA. Mutation research, 225:101-106.
Hampton C, Pierson W, Harvey T, Updegrove W, Marano R (1982)
Hydrocarbon gases emitted from vehicles on the road. I. A qualitative
gas-chromatography mass-spectrometry survey. Environmental science
and technology, 16:287-298.
HSDB (1998) Hazardous substances data bank. Published by Micromedex
Inc. (Copyright 1998). Accessed via the CD-ROM version.
IARC (1995) Dry cleaning, some chlorinated solvents and other
industrial chemicals. IARC monographs on the evaluation of
carcinogenic risks to man, 63:409-429.
IPCS (1993) International Chemical Safety Card -- Furfural. Geneva,
World Health Organization, International Programme on Chemical Safety
(ICSC 0276).
Japan Environment Agency (1998) Chemicals in the environment, 1997
ed. Tokyo, Japan Environment Agency, Environmental Health and Safety
Division.
JECFA (1999) Toxicological evaluation of certain food additives.
Geneva, World Health Organization, International Programme on Chemical
Safety, Joint FAO/WHO Expert Committee on Food Additives, pp. 33-56
(WHO Food Additives Series 42).
Juttner F (1986) Analysis of organic-compounds (VOC) in the forest air
of the southern Black Forest. Chemosphere, 15:985-992.
Karickhoff S, Brown D, Scott T (1979) Sorption of hydrophobic
pollutants on natural sediments. Water research, 13:241-248.
Kato F, Araki A, Nozaki K, Matsushima T (1989) Mutagenicity of
aldehydes and diketones. Mutation research, 216:366-367.
Kawasaki M (1980) Experiences with the test scheme under the Chemical
Control Law of Japan: An approach to structure-activity correlations.
Ecotoxicology and environmental safety, 4:444-454.
Keith L (1974) Chemical characterization of industrial wastewaters by
gas chromatography-mass spectrometry. Science of the total
environment, 3:87-102.
Kim S, Hayase F, Kato H (1987) Desmutagenic effect of alpha-dicarbonyl
and alpha-hydroxycarbonyl compounds against mutagenic heterocyclic
amines. Mutation research, 177:9-15.
Kim S, Kim I, Yeum D, Park Y (1988) Desmutagenic action of sugar
degradation products. Korean journal of food science and technology,
20 (1):119-124.
Kim T, Hah Y, Hong S (1983) Toxic effects of furfural on
Pseudomonas fluorescens. Korean journal of microbiology, 21:149-155.
Kleindienst T, Shepson P, Edney E, Claxton L, Cupitt L (1986) Wood
smoke -- measurement of the mutagenic activity of its gas-phase and
particulate-phase photooxidation products. Environmental science and
technology, 20:493-501.
Kool HJ, van Kreijl CF, Zoetman BCJ (1982) Toxicological assessment of
organic compounds in drinking water. Critical reviews in toxicology,
12:307-357.
Laham S, Potvin M (1989) Metabolism of furfural in the Sprague-Dawley
rat. Toxicology and environmental chemistry, 24:35-47.
Lipari F, Dasch JM, Scruggs WF (1984) Aldehyde emissions from
wood-burning fireplaces. Environmental science and technology,
18:326-330.
Lo Coco F, Ceccon L, Valentini C, Novelli V (1992) High performance
liquid chromatographic determination of 2-furaldehyde in spirits.
Journal of chromatography, 590:235-240.
Loquet C, Toussaint G, LeTalaer J (1981) Studies on mutagenic
constituents of apple brandy and various alcoholic beverages collected
in Western France, a high incidence area for oesophageal cancer.
Mutation research, 88:155-164.
Maga J (1979) Furans in foods. Critical review. Food science and
nutrition, 4:355-399.
Marnett L, Hurd H, Hollstein M, Levin D, Esterbauer H, Ames B (1985)
Naturally occurring carbonyl compounds are mutagens in Salmonella
tester strain TA 104. Mutation research, 148:25-34.
Mattson V, Arthur J, Walbridge C (1976) Acute toxicity of selected
organic compounds to fathead minnows. Duluth, MN, US Environment
Protection Agency, Environmental Research Laboratory (Report No.
EPA/600/3-76-097).
McGregor D, Brown A, Cattanach P, Edwards I, McBride D, Caspary W
(1988) Responses of the L5l78Y tk+/tk- mouse lymphoma cell forward
mutation assay II: 18 coded chemicals. Environmental molecular
mutagenesis, 11:91-118.
McMahon R, Cline J, Thompson C (1979) Assay of 855 test chemicals in
ten tester strains using a new modification of the Ames test for
bacterial mutagens. Cancer research, 39:682-693.
Mishra A, Dwivedi P, Verma A, Sinha M, Mishra J, Lal K, Pandya K,
Dutta K (1991) Pathological and biochemical alterations induced by
inhalation of furfural vapour in rat lung. Bulletin of environmental
contamination and toxicology, 47:668-674.
Miyakama Y, Nishi Y, Kato K, Sato H, Takahashi M, Hayashi Y (1991)
Initiating activity of eight pyrolysates of carbohydrates in a two
stage mouse skin tumorigenesis model. Carcinogenesis, 12:1169-1173.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E (1986)
Salmonella mutagenicity tests 11: Results from the testing of 270
chemicals. Environmental mutagenesis, 8 (Suppl. 7):1-119.
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing
activity of chemical carcinogens and mutagens in Salmonella
typhimurium TA 1535/pSK1002. Examination of 151 chemicals.
Mutation research, 192:239-246.
Nazyrov G, Yampol'skaya Y (1969) The effect of furan resins on the
skin and skin allergies of persons employed in their manufacture.
Trudy Institut Gigiena Truda i Professionalyne Zabolevaniya, 10:
19-21 (HSE Translation No. 14480A).
NIOSH (1987) NIOSH Proficiency Analytical Testing (PAT) Program.
Cincinnati, OH, US Department of Health, Education and Welfare,
National Institute for Occupational Safety and Health (DHEW
Publication No. 77-173).
Nishi Y, Miyakama Y, Kato K (1989) Chromosome aberrations induced by
pyrolysates of carbohydrates in Chinese hamster V79 cells.
Mutation research, 227:117-123.
Nomier A, Silveira D, McComish M, Chadwick M (1992) Comparative
metabolism and disposition of furfural and furfural alcohol in rats.
Drug metabolism and disposition, 20:198-204.
NTP (1990) Toxicology and carcinogenesis studies of furfural in
F344/N rats and B6C3F1 mice (gavage studies). Research Triangle
Park, NC, US Department of Health and Human Services, Public Health
Service, National Institutes of Health, National Toxicology Program
(Technical Report Series No. 382; NIH Publication No. 90-2837).
Nutley B (1989) An analytical method for biological monitoring of
furfural exposure. Sheffield, UK, Health and Safety Executive,
Health and Safety Laboratories (Section Report IR/L/OT/89/1).
Parkash M, Caldwell J (1994) Metabolism and excretion of
[14C]furfural in the rat and mouse. Food and chemical toxicology,
32(10):887-895.
Patel S, Robertson S, Brown R (1988) Method validation for atmospheric
monitoring -- an illustration with furfural. In: Proceedings of the
Sixth Thermal Desorption Symposium, Anughaha, Windsor, UK.
Pawlowicz A, Droszcz W, Garlicki A, Kutyla J, Kowalczyk M (1984)
Effect of furfural on the respiratory system. Medycyna Pracy,
35:39-45 (HSE Translation No. 14361B).
Pellizzari E, Hartwell T, Harris B, Waddell R, Whitaker D, Erickson M
(1982) Purgeable organic compounds in mothers' milk. Bulletin of
environmental contamination and toxicology, 28:322-328.
Pitter P (1976) Determination of biological degradability of organic
substances. Water research, 10:231-235.
Reynolds S, Stower S, Patterson R, Maronpot R, Aaronson S, Anderson M
(1987) Activated oncogenes in B6C3F1 mouse liver tumours:
implications for risk assessment. Science, 237:1309-1316.
Rodriguez-Arnaiz R, Morales P, Zimmering S (1992) Evaluation in
Drosophila melanogaster of the mutagenic potential of furfural in
the mei-9a test for chromosome loss in germ-line cells and the wing
spot test for mutational activity in somatic cells.
Mutation research, 280:75-80.
Rowe E, Tullos L (1980) Lube solvents no threat to waste treatment.
Hydrocarbon processing, 59:63-65.
Ruus L (1964) A study of waste waters from the forest products
industry. 4. Composition of biochemical oxygen demand of condensate
from spent sulfite liquor evaporation. Svensk Papperstidning,
67:221-225.
Schafer E, Bowles W, Hurlbut J (1983) The acute oral toxicity,
repellency and hazard potential of 998 chemicals to one or more
species of wild and domestic birds. Archives of environmental
contamination and toxicology, 12:355-382.
Sedivec V, Flek J (1978) Biologic monitoring of persons exposed to
furfural vapours. International archives of occupational and
environmental health, 42:41-49.
Shane B, Troxclair A, McMillin D, Henry C (1988) Comparative
mutagenicity of 9 brands of coffee to Salmonella typhimurium TA100,
TA102, TA104. Environmental molecular mutagenesis, 11:195-206.
Shimizu A, Nakamura Y, Harada M, Ono T, Sato K, Inoue T, Kanisawa M
(1989) Positive foci of glutathione S-transferase placental form in
the liver of rats given furfural by oral administration.
Japanese journal of cancer research, 80:608-611.
Shinohara K, Omura H (1986) Furans as mutagens formed by
amino-carbonyl reactions. Developmental food science, 3:353-362.
Soska J, Koukalova B, Ebringer L (1981) Mutagenic activities of simple
nitrofuran derivatives. Comparison of related compounds in the phage
inductest, chloroplast-bleaching and bacterial-repair and mutagenicity
tests. Mutation research, 81:21-26.
SRI International (1982) The acute oral toxicity of furfural in
various vehicles in the rat. Menlo Park, California (SRI Project No.
LSC-4357; Compound Report No. 1, August 1982).
Stich H, Rosin M, Wu C, Powrie W (1981) Clastogenicity of furans found
in food. Cancer letters, 13:89-95.
Subramanyam S, Sailaja D, Rathnaprabha D (1989) Genotoxic assay of two
dietary furans by some in vivo cytogenetic parameters.
Environmental molecular mutagenesis, 14 (Suppl. 15):239.
Terrill J, Van Horn W, Robinson D, Thomas D (1989) Acute inhalation
toxicity of furan, 2-methyl furan, furfuryl alcohol and furfural in
the rat. American Industrial Hygiene Association journal,
50:A359-A361.
Turnbull H, DeMann J, Weston R (1954) Toxicity of various refinery
materials to fresh water fish. Industrial and engineering chemistry,
46:324-333.
US EPA (1998) Toxic Substances Control Act: Inventory update rule 51.
In: Federal register. Washington, DC, US Environmental Protection
Agency.
Wallen I, Greer W, Lasater R (1957) Toxicity to Gambusia affinis of
certain pure chemicals in turbid waters. Sewage and industrial
wastes, 29:695-711.
Wang P, Brenchley J, Humphrey A (1994) Screening microorganisms for
utilization of furfural and possible intermediates in its degradation
pathway. Biotechnology letters, 16:977-982.
Woodruff R, Mason J, Valencia R, Zimmering S (1985) Chemical
mutagenesis testing in Drosophila. V. Results of 53 coded compounds
tested for the National Toxicology Program. Environmental
mutagenesis, 7:677-702.
Woods L, Seevers M (1955) Toxicity of furfural. Unpublished report,
University of Michigan Medical School, Ann Arbor, MI. 28 March 1955.
Woods L, Seevers M (1956) Toxicity of furfural. Unpublished report,
University of Michigan Medical School, Ann Arbor, MI. February 1956.
Zdzienicka M, Tudek B (1978) Mutagenic activity of furfural in
Salmonella typhimurium TA 100. Mutation research, 58:205-209.
Zhang J, He Q, Lioy P (1994) Characteristics of aldehydes --
concentrations, sources and exposures for indoor and outdoor
residential microenvironments. Environmental science and technology,
28:146-152.
APPENDIX 1 -- SOURCE DOCUMENT
Gregg et al. (1997): 2-Furaldehyde (Risk Assessment Document EH72/6)
The authors' draft version of this Health and Safety Executive
report was initially reviewed internally by a group of approximately
10 Health and Safety Executive experts (mainly toxicologists, but also
scientists from other relevant disciplines, such as epidemiology and
occupational hygiene). The toxicology section of the amended draft was
then reviewed by toxicologists from the United Kingdom Department of
Health. Subsequently, the entire risk assessment document was reviewed
by a tripartite advisory committee to the United Kingdom Health and
Safety Commission, the Working Group for the Assessment of Toxic
Chemicals (WATCH). This committee is composed of experts in toxicology
and occupational health and hygiene from industry, trade unions, and
academia.
The members of the WATCH committee at the time of the peer review
were Mr Steve Bailey, Confederation of British Industries; Professor
Jim Bridges, University of Surrey; Dr Ian Guest, Confederation of
British Industries; Dr Alastair Hay, Trade Unions Congress; Dr Jenny
Leeser, Confederation of British Industries; Dr Len Levy, Institute of
Occupational Hygiene, Birmingham; Dr Mike Molyneux, Confederation of
British Industries; Mr Alan Moses, Confederation of British
Industries; Dr Ron Owen, Trade Unions Congress; Mr Jim Sanderson,
Independent Consultant; and Dr Mike Sharratt, University of Surrey.
APPENDIX 2 -- CICAD PEER REVIEW
The draft CICAD on 2-furaldehyde was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Chinese Academy of Preventive Medicine, Ministry of Health,
Beijing, People's Republic of China
Federal Institute for Health Protection of Consumers & Veterinary
Medicine, Berlin, Germany
National Institute of Health Sciences, Tokyo, Japan
National Institute of Public Health and Environmental Protection
(RIVM), Bilthoven, The Netherlands
Senatskommission der Deutschen Forschungsgemeinschaft, Bonn,
Germany
United States Department of Health and Human Services (National
Institute for Occupational Safety and Health, Cincinnati;
National Institute of Environmental Health Sciences, Research
Triangle Park), USA
United States Environmental Protection Agency (Region VIII;
National Center for Environmental Assessment, Washington, DC),
USA
World Health Organization/International Programme on Chemical
Safety, Montreal, Canada
APPENDIX 3 -- CICAD FINAL REVIEW BOARD
Washington, DC, USA, 8-11 December 1998
Members
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
( Vice-Chairperson)
Mr R. Cary, Toxicology Unit, Health Directorate, Health and Safety
Executive, Bootle, Merseyside, United Kingdom ( Rapporteur)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr O. Faroon, Agency for Toxic Substances and Disease Registry,
Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr G. Foureman, National Center for Environmental Assessment, US
Environmental Protection Agency, Research Triangle Park, NC, USA
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA ( Chairperson)
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Research, Hanover,
Germany
Dr A. Nishikawa, Division of Pathology, National Institute of Health
Sciences, Tokyo, Japan
Dr E.V. Ohanian, Office of Water/Office of Science and Technology,
Health and Ecological Criteria Division, US Environmental Protection
Agency, Washington, DC, USA
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute
of Health Sciences, Tokyo, Japan
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Ministry of Health, Beijing, People's Republic
of China
Observers
Dr K. Austin, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr I. Daly (ICCA representative), Regulatory and Technical Associates,
Lebanon, NJ, USA
Ms K.L. Lang (CEFIC, European Chemical Industry Council,
representative), Shell International, London, United Kingdom
Ms K. Roberts (ICCA representative), Chemical Self-funded Technical
Advocacy and Research (CHEMSTAR), Chemical Manufacturers Association,
Arlington, VA, USA
Dr W. Snellings (ICCA representative), Union Carbide Corporation,
Danbury, CN, USA
Dr M. Sweeney, Document Development Branch, National Institute for
Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Secretariat
Dr M. Baril, Institut de Recherches en Santé et Sécurité du Travail du
Québec (IRSST), Montreal, Quebec, Canada
Dr H. Galal-Gorchev, Chevy Chase, MD, USA
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Ms L. Regis, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
Mr A. Strawson, Health and Safety Executive, London, United Kingdom
Dr P. Toft, Programme for the Promotion of Chemical Safety, World
Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
Ce CICAD relatif au 2-furaldéhyde est basé sur une mise au point
rédigée par le Health and Safety Executive du Royaume-Uni au sujet des
effets que ce composé pourrait avoir sur la santé humaine,
principalement en milieu professionnel, mais aussi sur l'environnement
en général (Gregg et al., 1997). Ce document est donc principalement
consacré aux voies d'exposition qui existent sur lieux de travail,
mais il comporte également une évaluation d'ordre écologique. Les
données prises en compte vont jusqu'en janvier 1997. Une bibliographie
complémentaire a été effectuée jusqu'à décembre 1997 afin de relever
les données qui auraient pu être publiées après la première recherche
bibliographique, mais sans résultat. On trouvera à l'appendice 1 des
indications sur le mode d'examen par des pairs ainsi que sur les
sources documentaires utilisées. Les renseignements concernant
l'examen du CICAD par les pairs font l'objet de l'appendice 2. Ce
CICAD a été approuvé en tant qu'évaluation internationale lors de la
réunion du Comité d'évaluation finale qui s'est tenue à Washington du
8 au 11 décembre 1998. La liste des participants à cette réunion
figure à l'appendice 3. La fiche d'information internationale sur la
sécurité chimique (ICSC No 0276) relative au 2-furaldéhyde, établie
par le Programme internationale sur la sécurité chimique (IPCS, 1993),
est également reproduite dans ce document.
Le 2-furaldéhyde (C5H4O2) (No CAS 98-01-1) se présente sous la
forme d'un liquide à l'odeur piquante, rappelant celle des amandes. On
le trouve à l'état de traces dans diverses denrées alimentaires et il
est produit industriellement par digestion en continu ou en discontinu
des pentosanes contenus dans les déchets agricoles. Ces pentosanes
sont hydrolysés en pentoses qui subissent ensuite une
cyclodéshydratation en 2-furaldéhyde. On utilise ce composé dans
l'industrie pour la production de résines, de meules et de substances
réfractaires, pour le raffinage des huiles lubrifiantes et la
récupération des solvants. On l'emploie également en très petites
quantités comme aromatisant.
Le 2-furaldéhyde est présent dans de nombreuses denrées
alimentaires, soit naturellement soit comme contaminant. On en a
signalé la présence dans l'eau destinée à la boisson et le lait
maternel, mais en quantités non mesurables.
On dispose de mesures portant sur l'exposition professionnelle
par inhalation et des estimations ont été obtenues au moyen d'un
système expert appelé Estimation and Assessment of Substance Exposure
(EASE). En règle générale, dans l'ensemble des industries,
l'exposition au 2-furaldéhyde présent dans l'air est inférieure à 8
mg/m3 (2 ppm) en moyenne pondérée par rapport au temps calculée sur 8
h (TWA). Pour la plupart des industries, cette donnée ne permet pas de
prévoir l'exposition moyenne pondérée sur 15 minutes. Toutefois, on a
calculé que dans l'industrie des aromatisants, l'exposition moyenne
pondérée sur 15 min devait se situer entre 1,2 et 6,8 mg/m3
(c'est-à-dire entre 0,3 et 1,7 ppm). On ne dispose d'aucune mesure de
l'exposition par voie cutanée. Le système EASE donne une exposition
cutanée comprise entre 0,1 et 1 mg/cm2 j-1 pour la plupart des
industries, avec des valeurs plus élevées (entre 1 et 5 mg/cm2 j-1)
dans la fabrication de matériaux abrasifs et de meules.
Les données toxicocinétiques sont limitées, mais on est fondé à
penser que le 2-furaldéhyde est facilement résorbé par la voie
respiratoire et la voie percutanée. L'expérimentation animale montre
qu'après administration par voie orale à des rats, le composé est
facilement absorbé et rapidement excrété, principalement dans les
urines, mais une petite partie passe également dans l'air expiré sous
la forme de dioxyde de carbone. Le métabolisme du 2-furaldéhyde se
caractérise par l'oxydation ou l'acétylation du groupement aldéhyde,
suivie par une conjugaison avec la glycine. La 2-furoylglycine est le
principal métabolite urinaire à côté de l'acide furoïque, de l'acide
furanacrylique et de l'acide furanacrylurique, dont l'importance est
moindre.
Chez l'Homme, on a mis en évidence une résorption du
2-furaldéhyde gazeux au niveau des poumons et de la peau. L'organisme
humain métabolise ce composé de manière analogue à celui du rat, la
majeure partie de la dose absorbée se retrouvant dans les urines sous
la forme de 2-furoylglycine. L'acide furoïque et l'acide
furanacrylurique sont également présents en moindre quantité. On
observé une résorption du 2-furanaldéhyde par la voie percutanée.
Chez l'animal, la toxicité aiguë du composé est variable :
globalement, le 2-furaldéhyde est toxique par la voie respiratoire et
la voie orale (CL50 à 4 h, 940 mg/m3 [235 ppm]; DL50 par voie orale,
environ 120 mg/kg de poids corporel) et l'on ne possède pas de données
claire au sujet de la voie percutanée. Après exposition unique ou
répétée au produit par inhalation, on observe systématiquement une
irritation des voies respiratoires et des lésions pulmonaires. On a
également fait état d'irritation de la peau et des yeux. Il semble en
revanche qu'on n'ait pas noté d'irritation de la gorge ou des yeux
chez des sujets humains exposés à une concentration de 40 mg/m3 (10
ppm) pendant 8 h ou de 80 mg/m3 pendant 4 h. Chez l'animal, on a
obtenu, pour la dose sans effet nocif observable (NOAEL) relative aux
effets cancérogènes, une valeur de 80 mg/m3 (20 ppm) dans le cas du
hamster et de 208 mg/m3 (52 ppm) dans le cas du hamster, lors
d'études qui se sont prolongées jusqu'à 13 semaines. Après avoir fait
ingérer le composé à rats et des souris pendant 103 semaines, à des
doses respectivement égales à 60 et 50 mg/kg de poids corporel, on a
observé
des tumeurs malignes et bénignes. Le 2-furaldéhyde est indéniablement
génotoxique in vitro sur des cultures de mammifères; on ne peut
tirer de conclusions définitives quant à la génotoxicité de ce composé
in vivo, mais on ne peut pas exclure non plus que cette génotoxicité
soit réelle et qu'elle puisse contribuer au processus de
cancérisation. Dans ces conditions, il n'est pas possible de
déterminer avec certitude la valeur de la NOAEL.
Faute de données utilisables pour l'estimation de l'exposition
individuelle indirecte au 2-furaldéhyde présent dans l'environnement
général, il est impossible de préciser le risque cancérogène que ce
composé représente pour la population dans son ensemble.
En milieu professionnel, il existe un risque d'effets
cancérogènes et génotoxiques. Il y cependant incertitude quant au
degré de risque; il est donc nécessaire de continuer à réduire
l'exposition autant qu'il est techniquement possible de le faire.
On ne dispose pas de données suffisantes concernant les effets du
composé sur la reproduction et le développement; il n'est donc pas
possible de déterminer s'il existe des risques de cette nature pour
l'Homme.
C'est l'industrie de la pâte à papier qui rejette le plus de
2-furaldéhyde dans l'environnement. La combustion du bois, qu'elle
soit naturelle ou d'origine humaine, entraîne également la libération
de se composé dans l'atmosphère.
On ne prévoit pas d'effets atmosphériques étant donné que le
2-furaldéhyde est détruit par réaction sur les radicaux hydroxyle, sa
demi-vie calculée dans l'atmosphère étant égale à 0,44 jours. Dans
l'air des villes, il peut également être décomposé par réaction avec
des radicaux nitrate. Une photo-oxydation directe n'est pas non plus à
exclure. Etant donné la faible valeur de la tension de vapeur et de la
constante de Henry, sa volatilisation à partir de l'eau et du sol
devrait être lente.
Il ne devrait pas y avoir d'hydrolyse aux valeurs du pH
rencontrées dans l'environnement. La faible valeur du coefficient de
partage entre l'octanol et l'eau (log Kow = 0,41) indique que la
capacité de bioaccumulation est faible. Le coefficient de sorption
( Koc) montre que la fixation aux particules n'est pas importante et
que le composé est par conséquent très mobile dans le sol.
Le 2-furaldéhyde subit une biodégradation aérobie rapide dans les
boues d'égout ainsi que dans les eaux superficielles. Cette
décomposition se produit également en anaérobiose et de nombreuses
bactéries et autres microorganismes sont capables d'utiliser ce
composé comme seule source de carbone. A forte concentration (>1000
mg/litre), le 2-furaldéhyde inhibe la croissance et l'activité
métabolique des cultures anaérobies non adaptées. En revanche, le
produit est plus facilement biodégradable par acclimatation des boues
anaérobies.
Les concentrations de 2-furaldéhyde les plus élevées qui aient
été constatées dans des eaux résiduaires industrielles provenaient de
condensats (environ 15 % des eaux résiduaires totales) rejetés par les
évaporateurs à bisulfite d'une usine de pâte à papier, avec une
moyenne de 274 mg/litre. On ne dispose que d'une seule étude donnant
les résultats de dosages du 2-furaldéhyde dans l'air intérieur et
extérieur; la valeur trouvée est d'environ 1 µg/m3; dans d'autres
études, on a mis en évidence la présence du composé mais sans procéder
à un dosage.
Le seuil de toxicité pour divers microorganismes se situe entre
0,6 et 31 mg/litre. Pour les poissons, la CL50 relative aux effets
toxiques aigus est comprise entre 16 et 32 mg/litre.
En se basant sur les concentrations auxquelles les rejets des
usines de pâte à papier sont susceptibles de donner lieu dans
l'environnement (cas le plus grave) et en prenant un facteur
d'incertitude de 1000 pour tenir compte de ce que les données de
toxicité aiguës sont limitées, on peut considérer que la libération de
2-furaldéhyde dans l'environnement représente un danger pour les
organismes aquatiques. On ne possède pas de données permettant
d'évaluer le risque pour les organismes terrestres, mais en tout état
de cause la décharge de 2-furaldéhyde au niveau du sol est
vraisemblablement peu importante.
RESUMEN DE ORIENTACION
El presente CICAD sobre el 2-furaldehído, preparado por la
Dirección de Salud y Seguridad del Reino Unido (Gregg et al., 1997),
se basa en un examen de los problemas relativos a la salud humana
(fundamentalmente ocupacionales), pero también contiene información
ambiental. Por consiguiente, este documento se concentra sobre todo en
la exposición a través de las rutas que son de interés para el entorno
ocupacional, pero incluye también una evaluación ambiental. En este
examen se han incorporado los datos identificados hasta enero de 1997.
Se realizó una ulterior búsqueda bibliográfica hasta diciembre de 1997
para localizar la nueva información que se hubiera publicado desde la
terminación del examen, pero no se encontraron estudios de interés. La
información acerca del carácter del examen colegiado del documento
original y su disponibilidad figura en el apéndice 1. La información
sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este
CICAD se aprobó como evaluación internacional en una reunión de la
Junta de Evaluación Final celebrada en Washington, DC, Estados Unidos,
los días 8-11 de diciembre de 1998. La lista de participantes en esta
reunión figura en el apéndice 3. La Ficha internacional de seguridad
química (ICSC 0276) preparada por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993), también se
reproduce en el presente documento.
El 2-furaldehído (C5H402) (CAS No 98-01-1) es un líquido de
olor acre "semejante al de la almendra". Se encuentra en cantidades
ínfimas en diversas fuentes de alimentos y se produce comercialmente
en digestores de lotes o continuos en los cuales se hidrolizan
pentosanos procedentes de residuos agrícolas a pentosas y éstas se
ciclodeshidratan a 2-furaldehído. Entre los usos industriales cabe
mencionar la producción de resinas, ruedas abrasivas, materiales
refractarios, refinado de los aceites lubricantes y recuperación de
disolventes. El 2-furaldehído se utiliza también, en cantidades muy
pequeñas, como aromatizante.
El 2-furaldehído está presente en numerosos alimentos, como
producto natural o como contaminante. Se ha notificado su presencia en
el agua potable y en la leche materna, pero las concentraciones no
fueron suficientes para su cuantificación.
Se dispone de mediciones de la exposición por inhalación en el
puesto de trabajo y también se han efectuado estimaciones utilizando
un sistema de computadora basado en los conocimientos, Estimación y
evaluación de la exposición a la sustancia (EASE). En general, la
exposición al compuesto en suspensión en el aire en todas las
industrias es inferior a 8 mg/m3 (2 ppm), con un promedio ponderado
por el tiempo (PPT) de ocho horas. No hay información suficiente para
pronosticar exposiciones PPT de 15 minutos para la mayoría de las
industrias; sin embargo, en la industria de los aromatizantes se ha
estimado una exposición PPT de 15 minutos de 1,2 a 6,8 mg/m3 (0,3 y
1,7 ppm). No se dispone de datos sobre mediciones de la exposición
cutánea. Se han calculado las exposiciones cutáneas basadas en la EASE
de 0,1 y 1 mg/cm2 al día para la mayoría de las industrias, con
exposiciones superiores, entre 1 y 5 mg/cm2 al día, para las
industrias de fabricación de materiales refractarios y ruedas
abrasivas.
Los datos toxicocinéticos son limitados, pero hay indicios de que
el 2-furaldehído se absorbe fácilmente por las vías de exposición por
inhalación y cutánea. Los estudios en animales demuestran que, tras la
administración oral a ratas, el 2-furaldehído se absorbe fácilmente y
excreta con rapidez sobre todo en la orina, aunque también se produce
alguna eliminación a través del anhídrido carbónico exhalado. El
metabolismo se caracteriza por la oxidación o la acetilación del grupo
aldehído, seguida de la conjugación de la glicina. La 2-furoilglicina
es el principal metabolito urinario; otros metabolitos secundarios son
el ácido furoico, el ácido furanacrílico y el ácido furanacrilúrico.
En el ser humano, se ha demostrado la absorción del vapor a
través de los pulmones y la piel. El metabolismo en las personas
parece ser semejante al de las ratas, excretándose la mayor parte de
la dosis retenida como 2-furoilglicina urinaria. Se han detectado
también como metabolitos secundarios el ácido furoico y el ácido
furanacrilúrico. Se ha observado asimismo absorción cutánea a partir
del 2-furaldehído líquido.
Los datos sobre la toxicidad aguda en los animales son variables;
en general, sin embargo, el 2-furaldehído es tóxico por inhalación y
por vía oral (CL50 a los cuatro horas, 940 mg/m3 [235 ppm]; DL50 por
vía oral, 120 mg/kg de peso corporal), no disponiéndose de información
clara en relación con la vía cutánea. Se ha observado sistemáticamente
irritación del sistema respiratorio y lesiones en los pulmones tras la
exposición por inhalación a una dosis única y repetida. También se ha
notificado irritación cutánea y ocular. Aparentemente no se detectó
irritación en la garganta o en los ojos en personas expuestas a 40
mg/m3 (10 ppm) durante ocho horas o a 80 mg/m3 (20 ppm) durante
cuatro horas. En animales se han determinado concentraciones sin
efectos adversos observados (NOAEL) de 80 mg/m3 (20 ppm) y 208 mg/m3
(52 ppm) en estudios de hasta 13 semanas de duración en hámsteres y
conejos, respectivamente, para los efectos no neoplásicos. Se han
observado tumores malignos y benignos en ratas y ratones tras la
exposición oral a 60 y 50 mg/kg de peso corporal, respectivamente,
durante 103 semanas. El 2-furaldehído es claramente genotóxico in
vitro en células de mamíferos; aunque no se puede llegar a una
conclusión definitiva sobre el potencial genotóxico del 2-furaldehído
in vivo, no se puede descartar la posibilidad de que la
genotoxicidad pueda contribuir al proceso carcinogénico. Estos
factores impiden determinar de manera fidedigna una NOAEL para el
2-furaldehído.
La falta de datos disponibles que sirvan de base para la
estimación de la exposición indirecta de las personas al 2-furaldehído
del medio ambiente general impiden la caracterización de los posibles
riesgos de cáncer para la población general.
En el entorno del puesto trabajo, existe un riesgo potencial de
efectos carcinogénicos y genotóxicos. El nivel de riesgo es incierto;
en consecuencia, existe el requisito permanente de reducir los niveles
de exposición todo lo que sea posible razonablemente con la tecnología
disponible en la actualidad.
No hay datos adecuados sobre los efectos en la reproducción o en
el desarrollo; por consiguiente, no es posible evaluar el riesgo para
la salud humana con respecto a estos efectos finales.
Las emisiones más altas notificadas de 2-furaldehído al medio
ambiente son las de la industria de la pasta de madera. El
2-furaldehído se libera a la atmósfera en la combustión natural y
antropogénica de madera.
No se prevén efectos atmosféricos, puesto que el 2-furaldehído se
destruye al reaccionar con los radicales hidroxilo, con una semivida
atmosférica calculada de 0,44 días. En el aire urbano, la reacción con
radicales nitrato puede representar un proceso de degradación
adicional. También se puede producir fotooxidación directa. Los bajos
valores de la presión de vapor y de la constante de Henry parecen
indicar que solamente se produce una volatilización lenta del
2-furaldehído de la superficie del agua y del suelo.
No se prevé que con el pH del medio ambiente se produzca
hidrólisis en el agua. El bajo coeficiente de reparto octanol/agua
(log Kow 0,41) deja entrever una escasa capacidad de
bioacumulación. Los coeficientes de sorción ( Koc) parecen indicar
una sorción escasa a partículas y una movilidad alta en el suelo.
El 2-furaldehído se biodegrada fácilmente en sistemas aerobios
utilizando fangos cloacales y en el agua superficial. También se
produce degradación en condiciones anaerobias, gracias a una serie de
bacterias y otros microorganismos capaces de utilizar el compuesto
como única fuente de carbono. A concentraciones altas (>1000
mg/litro), el 2-furaldehído inhibe el crecimiento y la actividad
metabólica de cultivos anaerobios no adaptados. Sin embargo, la
aclimatación aumenta la capacidad de degradación del compuesto en los
fangos anaerobios.
Las concentraciones más altas notificadas de 2-furaldehído en
aguas residuales industriales corresponden al líquido condensado de
los evaporadores de sulfito (alrededor del 15% de la corriente de
residuos de las fábricas de pasta de madera), con un promedio de
274 mg/litro. En un estudio aislado se cuantificó la concentración de
2-furaldehído en el aire de espacios cerrados y abiertos en alrededor
de 1 µg/m3; en otros estudios se ha detectado el compuesto, pero no
se ha cuantificado.
Se han notificado umbrales tóxicos para diversos microorganismos
en una gama de 0,6 a 31 mg/litro. Las CL50 aguda para los peces
oscila entre 16 y 32 mg/litro.
Teniendo cuenta las concentraciones pronosticadas estimadas en el
medio ambiente a partir de los residuos de la pasta de madera (se
considera que es el peor de los casos) y la aplicación de un factor de
incertidumbre de 1000 a los datos limitados de las pruebas de
toxicidad aguda, cabe prever que las emisiones de 2-furaldehído
plantearán un riesgo bajo para los organismos acuáticos. No se dispone
de datos sobre los cuales basar una evaluación del riesgo terrestre,
pero se supone que las emisiones a la tierra son bajas.
 1. EXECUTIVE SUMMARY
This CICAD on 2-furaldehyde was based on a review of human health
concerns (primarily occupational) prepared by the United Kingdom's
Health and Safety Executive (Gregg et al., 1997), but it also contains
environmental information. Hence, this document focuses on exposures
via routes relevant to occupational settings but also includes an
environmental assessment. Data identified up to January 1997 were
covered in the review. A further literature search was performed up to
December 1997 to identify any new information published since the
review was completed, but no relevant studies were identified.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ISCS 0276) produced by the
International Programme on Chemical Safety (IPCS, 1993) has also been
reproduced in this document.
2-Furaldehyde (C5H4O2) (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on 2-furaldehyde was based on a review of human health
concerns (primarily occupational) prepared by the United Kingdom's
Health and Safety Executive (Gregg et al., 1997), but it also contains
environmental information. Hence, this document focuses on exposures
via routes relevant to occupational settings but also includes an
environmental assessment. Data identified up to January 1997 were
covered in the review. A further literature search was performed up to
December 1997 to identify any new information published since the
review was completed, but no relevant studies were identified.
Information on the nature of the peer review and availability of the
source document is presented in Appendix 1. Information on the peer
review of this CICAD is presented in Appendix 2. This CICAD was
approved as an international assessment at a meeting of the Final
Review Board, held in Washington, DC, USA, on 8-11 December 1998.
Participants at the Final Review Board meeting are listed in Appendix
3. The International Chemical Safety Card (ISCS 0276) produced by the
International Programme on Chemical Safety (IPCS, 1993) has also been
reproduced in this document.
2-Furaldehyde (C5H4O2) (CAS No.  The conversion factors for 2-furaldehyde in air at 20°C and 101.3
kPa are as follows:
1 ppm = 4.0 mg/m3
1 mg/m3 = 0.25 ppm
3. ANALYTICAL METHODS
Long- and short-term personal monitoring can be undertaken by
pumped sampling through XAD-2 resin impregnated with 2-(hydroxymethyl)
piperazine, desorbing with solvent (toluene), and analysing with gas
chromatography (NIOSH, 1987). The working range is 1.2-22 mg/m3
(0.3-5.5 ppm) for a 12-litre sample. Alternatively, a thermal
desorption tube may be used, in either the pumped or diffusive mode
(Patel et al., 1988). The working range for both is 4-40 mg/m3 (1-10
ppm) for a 10-litre sample. Screening measurements can be made with
colorimetric detector tubes, but these are unselective and not very
sensitive.
Biological monitoring of workers exposed to 2-furaldehyde is
possible by the analysis of 2-furoic acid (after alkaline hydrolysis
of the metabolite furoylglycine) in urine (Sedivec & Flek, 1978).
Although people not occupationally exposed to 2-furaldehyde have
background levels of 2-furoic acid in hydrolysed urine samples (from
dietary sources), these are low compared with those resulting from
occupational exposure (Nutley, 1989). The biological exposure index of
the American Conference of Government Industrial Hygienists is 200 mg
2-furoic acid/g creatinine (200 µmol/mmol creatinine), and a study in
the United Kingdom found that a 2-furoic acid concentration of over
160 mg/g creatinine (160 µmol/mmol creatinine) was likely to be due to
exposure to 2-furaldehyde at over 8 mg/m3 (2 ppm) (Nutley, 1989).
More sensitive analytical methods are required for monitoring in
food. Determination of the 2,4-dinitrophenylhydrazone derivative of
2-furaldehyde by high-performance liquid chromatography gives high
specificity, with a detection limit of 10 nmol/litre in spirits (Lo
Coco et al., 1992). A fast, semi-automatic, stopped-flow injection
analysis in which pretreatment of diversely coloured and turbid food
samples is not necessary has been developed (Espinosa-Mansilla et al.,
1993).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
2-Furaldehyde is found in a number of dietary sources. Because of
its formation during the thermal decomposition of carbohydrates,
2-furaldehyde is found in numerous processed foods and beverages,
including cocoa, coffee, tea, beer, wine, milk products, and bread
(Maga, 1979). It is also found in some fruits and vegetables, and it
is added as a flavouring agent to some foods. Industry in the United
Kingdom is voluntarily withdrawing 2-furaldehyde from addition to
food, although the chemical will still be present in food as a result
of its production during the thermal processing of anything containing
sugars. 2-Furaldehyde has also been found in the essential oils of
camphor, citronella, sassafras, lavender, and lime (Dunlop & Peters,
1953).
2-Furaldehyde is produced commercially in batch or continuous
digesters where pentosans from agricultural residues, including corn
cobs, oat hulls, rice hulls, and bagasse, are hydrolysed to pentoses
and the pentoses are subsequently cyclodehydrated to 2-furaldehyde.
2-Furaldehyde is shipped in steel tank cars, aluminium tank trucks,
and steel cans. When stored in bulk, the storage tanks normally have a
nitrogen blanket to prevent ingress of oxygen, which can cause
chemical degradation of the material.
Estimates of worldwide production are not available. US
production in 1983 was about 52 000 t (HSDB, 1998). The estimated
range of US production was 14 000-60 000 t in 1986, 2000-7000 t in
1990, and 11 000-45 000 t in 1994 (US EPA, 1998). Although
2-furaldehyde is not manufactured in the United Kingdom, it is
estimated that industry in the United Kingdom used approximately 3000
t in 1992. The official statistics indicate that imports into the
United Kingdom for the previous four years were 2800 t (1988), 3500 t
(1989), 3000 t (1990), and 3500 t (1991) (Gregg et al., 1997).
Industrial uses of 2-furaldehyde are summarized in Table 1. About
40% of 2-furaldehyde imported into the United Kingdom is used in the
production of resins, abrasive wheels, and refractories. The rest is
used in the refining of lubrication oils. Contacts with United Kingdom
industry in 1992 indicated that there was unlikely to be any
significant change in the pattern of use of 2-furaldehyde in the
foreseeable future.
Table 1: Industrial uses of 2-furaldehyde in the United Kingdom.
Industry Uses
1. Petrochemicals As a selective solvent in
the production of lubricating oils
2. Refractories As a reactive wetting agent in the
production of refractory components
3. Resin manufacture (a) Production of phenolic resins
(b) Production of cashew nutshell polymers
4. Abrasive materials As a reactive wetting agent for the resin
binder system in the production of abrasive wheels
5. Solvent recovery Steam distillation of spent 2-furaldehyde
for reuse by industry sectors 1, 2, and 4
6. Flavouring Used in very small quantities in
synthetic/natural oil blends
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The highest reported emissions of 2-furaldehyde to the
environment are from the wood pulp industry, with release to the
hydrosphere (section 6); release to water from other uses appears to
be substantially lower. The compound would be released to the
atmosphere from wood fires, a natural as well as anthropogenic source.
In the atmosphere, 2-furaldehyde will exist predominantly in the
vapour phase; the half-life for destruction by interaction with
hydroxyl radicals has been calculated at 0.44 days (HSDB, 1998).
Night-time destruction in the urban atmosphere may involve reaction
with nitrate radicals (Carter et al., 1981). Direct photo-oxidation
may occur in the atmosphere, based on experimental demonstration that
concentrations of 2-furaldehyde in wood smoke were reduced by
irradiation at sunlight and ultraviolet frequencies. Addition of
nitrogen oxides to wood smoke increased the rate of disappearance of
the compound (Kleindienst et al., 1986).
Virtually no degradation occurred in a solution of 2-furaldehyde
in distilled water over 30 days, suggesting that hydrolysis is not an
important process at environmental pH (Ettinger et al., 1954).
A Henry's law constant of 0.37 Pa.m3/mol at 25°C has been
calculated (HSDB, 1998), corresponding to a dimensionless Henry's law
constant (air/water partition coefficient) of 1.5 × 10-4. These
values indicate slow volatilization from water and damp soil surfaces;
an estimated half-life for volatilization from a model river of 9.9
days has been reported (HSDB, 1998).
2-Furaldehyde has a reported log Kow of 0.41, indicating low
capacity for bioaccumulation; calculated bioconcentration factors were
less than 1.2 (HSDB, 1998). The sorption coefficient ( Koc) can be
calculated as between 1.05 (Organisation for Economic Co-operation and
Development [OECD] Technical Guidance Manual) and 1.62 (Karickhoff et
al., 1979), indicating little sorption to particulates and a high
capacity for mobility in soil.
2-Furaldehyde was readily biodegraded in an aerobic batch culture
at 200 mg chemical oxygen demand (COD)/litre, with non-adapted sewage
sludge showing 96.3% degradation within 120 h and a degradation rate
of 37 mg COD/g per hour (Pitter, 1976). In a flow-through aerobic
laboratory bioreactor at an initial concentration of 300 mg/litre, 98%
degradation of 2-furaldehyde was reported using acclimated sewage
sludge. Degradation also occurred at a concentration of 1000 mg/litre
(Rowe & Tullos, 1980). 2-Furaldehyde was readily biodegradable in the
Japanese Ministry of International Trade and Industry (MITI) test
(Kawasaki, 1980). Wang et al. (1994) screened a range of
microorganisms for their ability to degrade 2-furaldehyde and reviewed
the earlier literature; some strains of the aerobic bacteria
Escherichia coli, Pseudomonas putida, and Rhodococcus
erythropolis and the yeast Hyphozyma rosoeniger were able to
degrade the compound to a greater or lesser degree. Inhibitory effects
of 2-furaldehyde on Pseudomonas putida were seen at concentrations
of 0.1% and above, with complete inhibition at 1% with exposure for 30
min (Kim et al., 1983).
2-Furaldehyde at 1 mg/litre was degraded completely in water from
the Great Miami, Little Miami, and Ohio rivers (USA) within 3 days
under aerobic conditions (Ettinger et al., 1954).
Under anaerobic conditions, 2-furaldehyde was completely degraded
to methane by unacclimated sewage sludge within 30 days at an initial
concentration of 580 mg/litre. The rate of methane production was
initially slower than in controls, indicating some interference of the
compound in the metabolism of the methanogenic bacteria. At an initial
concentration of 1160 mg/litre, gas production ceased after 5 days,
and none of the gas produced was methane; gas production did not
resume within the 28 days of the test, indicating that this
concentration was toxic to unacclimated microorganisms. Using
acclimated sludge (which had received 2-furaldehyde at 310 mg/litre
continuously for 8 months), full degradation occurred at 1160
mg/litre. At 2320 mg/litre, 2-furaldehyde was initially degraded
rapidly, but then gas production slowed for the remainder of the test
(Benjamin et al., 1984). A strictly anaerobic bacterium isolated from
a fermentor degrading sulfite evaporator condensate from wood pulp
production was able to use 2-furaldehyde as sole carbon source; the
organism was tentatively identified as Desulfovibrio sp. (Brune et
al., 1983). Methanococcus deltae was found to be able to use
2-furaldehyde as sole carbon source, degrading the compound to
furfuryl alcohol at initial concentrations of 5 and 10 mmol/litre (480
and 960 mg/litre); however, growth of the bacterium was inhibited at
concentrations of 20 and 25 mmol/litre (1920 and 2400 mg/litre). Other
methanogenic bacteria ( Methanobacterium thermoautotrophicum,
Methanosarcina barkeri, and Methanococcus thermolithotrophicus)
were unable to degrade the compound (Belay et al., 1997).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
2-Furaldehyde was detected in 1 out of 204 surface water samples
from heavily industrialized areas in the USA at 2 µg/litre (detection
limit 1 µg/litre) and in 1 out of 13 samples from the Lake Michigan
basin, USA, at 2 µg/litre in the late 1970s (HSDB, 1998).
2-Furaldehyde was not detected (detection limit 0.4 ng/litre) in
33 samples of surface waters in the 1996 monitoring of the general
environment in Japan (Japan Environment Agency, 1998). However, the
compound was detected in 6 out of 15 air samples (detection limit 40
ng/m3) in two cities (range 42-120 ng/m3). The analytical method was
not stated in the publication.
Levels of 2-furaldehyde in sulfite evaporator condensate, which
represents about 15% of the wastewater flow from pulp mills, have been
reported to range between 10 and 1280 mg/litre (Ruus, 1964) and
between 179 and 471 mg/litre (average 274 mg/litre) (Benjamin et al.,
1984). The 2-furaldehyde derives from pentoses in the wood pulp and is
formed during the waste treatment in the evaporator. 2-Furaldehyde was
detected in the wastewater of a synthetic rubber plant at 1.7 µg/litre
(Keith, 1974).
2-Furaldehyde was detected but not quantified in the air above
the Black Forest, Germany, in 1984-85 (Juttner, 1986). Although
2-furaldehyde has been identified in vehicle exhaust, it was not
detectable in air sampled from a road tunnel in the USA (Hampton et
al., 1982). The compound has been identified in smoke from burning
wood (Lipari et al., 1984; Kleindienst et al., 1986). Mean
concentrations of 2-furaldehyde measured in indoor and outdoor air in
suburban New Jersey, USA, in summer 1992 were 1.06 µg/m3 (0.27 ppb)
and 0.67 µg/m3 (0.17 ppb), respectively. The presence of the compound
in indoor air was presumed to be from emissions during cooking (Zhang
et al., 1994).
6.2 Human exposure
6.2.1 Oral exposure
2-Furaldehyde has been identified but not quantified as a major
flavour component in a range of food items, including beef, soy sauce,
roasted nuts, fried bacon, nectarines, baked potatoes, clove oil,
preserved mangoes, rum, roasted coffee, and blue cheese (HSDB, 1998).
Levels reported in food are as follows: non-alcoholic beverages, 4
mg/litre; alcoholic beverages, 10 mg/litre; ice creams, ices, etc., 13
mg/kg; candy, 12 mg/kg; baked goods (unspecified), 17 mg/kg; gelatins
and puddings, 0.8 mg/kg; chewing gum, 45 mg/kg; and syrups, 30
mg/litre (HSDB, 1998). 2-Furaldehyde was qualitatively identified in
two out of eight samples of mothers' milk from urban sites in the USA
(Pellizzari et al., 1982). Qualitative identification of 2-furaldehyde
in drinking-water has been reported from both the USA and Europe; no
quantification was reported (Kool et al., 1982).
6.2.2 Inhalation exposure
The remainder of the human exposure data available to the authors
of this CICAD are restricted to the occupational environment.
Measured inhalation exposure information was provided to the
United Kingdom's Health and Safety Executive by the petrochemical
industry. The Health and Safety Executive's National Exposure Data
Base contains information on exposure for the use of 2-furaldehyde in
the refractory industry and for a number of miscellaneous processes.
In all the exposure groupings below, when routine and breakdown
maintenance takes place and significant exposure to airborne
2-furaldehyde is anticipated, it is expected that appropriate
respiratory protective equipment would be used to minimize inhalation
exposure.
Exposures in the petrochemicals, resin and polymer manufacture,
and distillation industries are likely to be very similar. EASE
(Version 2) predictions seem to be in accord with the limited amount
of measured exposure information provided by the petrochemicals
industry (Gregg et al., 1997). The EASE predictions indicate that 8-h
TWA exposures will be somewhat below 8 mg/m3 (2 ppm). There is
insufficient information to predict 15-min TWA exposures for these
industries.
Exposures in the refractories and abrasive wheels manufacturing
industries are likely to be very similar. The high exposures measured
by the Health and Safety Executive in the refractories industry (20%
of 8-h TWA exposures are greater than 40 mg/m3 [10 ppm]) are likely
to be reduced following advice given about the application of
efficient local exhaust ventilation and the enclosure of the process
where possible (Gregg et al., 1997). The EASE-predicted 8-h TWA
exposures likely to result from these improvements will be between 2
and 12 mg/m3 (0.5 and 3 ppm) (Gregg et al., 1997). There is every
likelihood that exposures will actually be at the lower end of the
range -- i.e., less than 8 mg/m3 (2 ppm). There is insufficient
information to predict 15-min TWA exposures for these industries.
In the flavouring industry, very little 2-furaldehyde appears to
be used. The EASE model predicts exposures to be low: the 15-min TWA
exposure is between 1.2 and 6.8 mg/m3 (0.3 and 1.7 ppm), and the 8-h
TWA is between 0.04 and 0.2 mg/m3 (0.01 and 0.05 ppm) (Gregg et al.,
1997).
For oilseed residue application, no personal exposure was
detected. The extent of this industry is not known, but it seems that
8-h exposures to 2-furaldehyde are likely to be well below 8 mg/m3
(2 ppm) (Gregg et al., 1997). No comment can be made upon likely
short-term exposures.
Little is known about the extent of the use of 2-furaldehyde in
the glass reinforced plastics industry. It is very likely that the
application of appropriate controls would result in 8-h TWA exposures
to 2-furaldehyde being reduced to below 8 mg/m3 (2 ppm) (Gregg et
al., 1997). No comment can be made upon possible short-term exposures.
6.2.3 Dermal exposure
The industrial processes can be grouped in a similar manner to
that adopted for the general discussion of inhalation exposure above
(section 6.2.2). These predictions do not take account of personal
protective equipment that may be worn. Such equipment may
significantly reduce dermal exposure.
The EASE-predicted dermal exposures for the petrochemicals, resin
and polymer manufacture, and distillation industries are similar
-- namely, between 0.1 and 1 mg/cm2 per day (Gregg et al., 1997).
For the refractories and abrasive wheels manufacturing
industries, the EASE-predicted dermal exposures are between 1 and 5
mg/cm2 per day (Gregg et al., 1997). If improvements are made to the
process containment, opportunities for dermal contact will be reduced,
and predicted exposures will be reduced to between 0.1 and 1 mg/cm2
per day.
For the use of 2-furaldehyde in the flavourings industry, the
EASE prediction for dermal exposure is between 0 and 0.1 mg/cm2 per
day (Gregg et al., 1997). Insufficient information is provided for the
other minor uses to make predictions of dermal exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
No animal studies are available examining the toxicokinetics of
2-furaldehyde by the inhalation or dermal routes; however, signs of
systemic toxicity observed in animal studies (see section 8) indicate
that 2-furaldehyde is readily absorbed via both these routes of
exposure.
Animal studies demonstrate that, following oral administration in
rats, 2-furaldehyde is readily absorbed and rapidly excreted, with up
to 85% of the administered dose being detected in the urine within 24
h (Nomier et al., 1992). Some elimination via exhaled carbon dioxide
(7% of dose) also occurs. Metabolism is characterized by oxidation or
acetylation of the aldehyde group, followed by glycine conjugation.
2-Furoylglycine is the major urinary metabolite, accounting for
approximately 80% of the administered dose (Laham & Potvin, 1989;
Nomier et al., 1992; Parkash & Caldwell, 1994). Other minor
metabolites include furoic acid, furanacrylic acid, and furanacryluric
acid.
In humans, absorption of the vapour via both the lungs and skin
has been demonstrated (Flek & Sedivec, 1978a,b). Metabolism appears
similar to that in rats, with the majority of the retained dose being
excreted as urinary 2-furoylglycine. Furoic acid and furanacryluric
acid are also detected as minor metabolites. Dermal absorption from
liquid 2-furaldehyde has also been observed.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Unpublished or briefly reported papers indicate 1-, 4-, and 6-h
inhalation LC50 values in rats of 4148, 940, and 700 mg/m3 (1037,
235, and 175 ppm), respectively (Woods & Seevers, 1956; Terrill et
al., 1989). In contrast, an apparently well-conducted published study
indicated a 1-h LC50 value in rats of 756 mg/m3 (189 ppm) (Gupta et
al., 1991; Mishra et al., 1991). In the rat and other species,
respiratory tract irritation and lung damage were consistently
observed following inhalation exposure, with lung oedema and
congestion being reported in one study in rats immediately after
exposure to 380 mg/m3 (95 ppm) for 1 h. Oral LD50 values in rats
were in close agreement, ranging from 122 to 158 mg/kg body weight
(Woods & Seevers, 1955; SRI International, 1982). Toxic signs observed
indicated central nervous system depressant effects, with convulsions
also observed. The lungs also appeared to be a target organ via oral
dosing. No reliable information was available for the dermal route of
exposure.
8.2 Irritation and sensitization
No signs of skin irritation were noted in rabbits following a
single application of liquid 2-furaldehyde for 12 h, although mild
irritation was observed following a 48-h application (Woods & Seevers,
1955). Intense but reversible skin irritation was reported in
guinea-pigs after repeated application of undiluted liquid
2-furaldehyde (Agakishiyev, 1989, 1990).
A single instillation of liquid 2-furaldehyde produced gross
corneal opacities in rabbits (Woods & Seevers, 1955). 2-Furaldehyde
vapour produced eye irritation in several studies following repeated
exposure in different species (Gardner, 1925; Feron et al., 1979;
Gupta et al., 1991).
No information is available on the ability of 2-furaldehyde to
act as a skin or respiratory sensitizer.
8.3 Short-term exposure
The principal effects of repeated inhalation exposure were
similar to those seen in single-exposure experiments. Groups of rats
were exposed to 0 or 160 mg 2-furaldehyde/m3 (0 or 40 ppm) 1 h/day
for 5, 15, or 30 days (Gupta et al., 1991; Mishra et al., 1991), and
groups of rabbits were exposed to 208, 520, or 1040 mg/m3 (52, 130,
or 260 ppm) 4 h/day, 5 days/week, for at least 60 exposures
(Castellino et al., 1963). Respiratory irritation, hyperplasia and
degeneration of the olfactory epithelium, and lung congestion, oedema,
and inflammation were observed in rats following exposure to 160
mg/m3 (40 ppm) (Gupta et al., 1991; Mishra et al., 1991) and in
rabbits following exposure to 1040 mg/m3 (260 ppm) (Castellino et
al., 1963). There was also evidence of kidney damage seen
histopathologically and anaemia in rabbits following exposure to 520
mg 2-furaldehyde/m3 (130 ppm). A NOAEL of 208 mg/m3 (52 ppm) was
identified in rabbits; a NOAEL was not identified for rats.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
Groups of hamsters were exposed to 0, 80, 460, or 2208 mg/m3
(0, 20, 115, or 552 ppm) 6 h/day, 5 days/week, for 13 weeks (Feron et
al., 1979). Respiratory irritation, hyperplasia and degeneration of
the olfactory epithelium, and lung congestion, oedema, and
inflammation were observed in hamsters following exposure to 2208
mg/m3 (552 ppm) (Feron et al., 1979). Slight atrophy and hyperplasia
of the olfactory epithelium were also seen following exposure to 460
mg/m3 (115 ppm). A NOAEL of 80 mg/m3 (20 ppm) was identified.
Groups of 20 male and female rats received 0, 11, 22, 45, 90, or
180 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Mild to moderate centrilobular
vacuolation of hepatocytes was observed among all treated groups,
although there was no clear relationship between dose and incidence or
severity of the observed effect.
Groups of 20 male and 20 female mice received 0, 75, 150, 300,
600, or 1200 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Relative liver weights were
increased in a dose-related manner in all treated groups.
Centrilobular coagulative necrosis of hepatocytes was seen at
150 mg/kg body weight per day or more, and mild mononuclear
inflammatory cell infiltrate was seen in all treated groups.
8.4.2 Chronic exposure and carcinogenicity
No inhalation carcinogenicity studies in rats or mice were
available. An inhalation study in hamsters showed no evidence that
2-furaldehyde vapour had carcinogenic potential following 52 weeks'
exposure to 1000-1600 mg/m3 (250-400 ppm), 7 h/day, 5 days/week, by
this route (Feron & Kruysse, 1978). Nor was there any indication that
2-furaldehyde was carcinogenic to hamsters after 36 weekly
intratracheal instillations (Feron, 1972). However, both these studies
are not satisfactory negative studies, owing to their limited
duration.
In a gavage study, groups of 50 male and 50 female F344/N rats
were treated with 0, 30, or 60 mg 2-furaldehyde/kg body weight per
day, 5 days/week for 103 weeks (NTP, 1990). Animals were observed
twice daily, weighed monthly, and subjected to a thorough gross and
microscopic examination either at death or at the end of the study.
Mortality in males was not significantly affected by 2-furaldehyde
treatment throughout the study. Corresponding survival rates among
females were 18/50, 32/50, and 28/50. However, 19 of the 22 top-dose
deaths in females were attributed to accidental gavage errors. There
were no differences in body weight between test and control animals
throughout the study, and there were no treatment-related clinical
signs of toxicity. The main non-neoplastic changes observed in test
animals were minimal to mild centrilobular necrosis of the liver in
male rats (3/50, 9/50, 12/50) and biliary dysplasia with fibrosis in
two top-dose males.
Two cases of cholangiocarcinoma were seen in two other top-dose
males. The historical incidence of bile duct neoplasms in corn oil
vehicle control male F344/N rats in this laboratory was reported to be
3/2145 (0.1%). No other findings of biological significance were
observed. Hence, in this study, treatment by gavage with 2-furaldehyde
produced an increase in bile duct carcinoma in male rats only,
although a valid assessment of the carcinogenicity of 2-furaldehyde in
female rats cannot be made because of the low numbers of animals
surviving to the end of the study. Also, the high incidence of
accidental deaths casts doubt over the quality of the experimental
conditions.
Groups of 50 male and 50 female B6C3F1 mice were also dosed with
0, 50, 100, or 175 mg 2-furaldehyde/kg body weight per day by gavage,
5 days/week for 103 weeks, in the NTP study (NTP, 1990). Animals were
observed twice daily, weighed monthly, and subjected to a thorough
gross and microscopic examination either at death or at the end of the
study. No significant differences in survival were observed between
2-furaldehyde-treated and untreated animals throughout the study.
There were no differences in body weight gain between test and control
animals throughout the study, and there were no clinical signs of
treatment-related toxicity.
Non-neoplastic changes included chronic inflammation and
multifocal green-brown, granular pigmentation of the subserosa of the
liver in mid- and top-dose mice of each sex and forestomach
hyperplasia in 2-furaldehyde-treated female mice only. The principal
neoplastic changes observed were increases in hepatocellular adenomas
and hepatocellular carcinomas in males (adenoma: 9/50 [18%], 13/50
[26%], 11/49 [22%], 19/50 [38%]; carcinoma: 7/50 [14%], 12/50 [24%],
6/49 [12%], 21/50 [42%]; in control, low-, mid-, and top-dose animals,
respectively). Only adenomas were observed in females, the incidences
being dose-related: 1/50 (2%), 3/50 (6%), 5/50 (10%), 8/50 (16%).
Increases in adenomas and/or carcinomas were statistically significant
only in the top-dose animals of both sexes. An increase in squamous
cell papillomas of the forestomach was also seen in top-dose female
mice, although this increase was not statistically significant (1/50
[2%], 0/50 [0%], 1/50 [2%], 6/50 [12%] in control, low-, mid-, and
top-dose animals). No other findings of biological significance were
observed.
In an initiation/promotion study in mice using the dermal route,
an increased incidence of skin tumours was seen when 2-furaldehyde was
applied in combination with the promoting agent (5/20 compared with
1/20 in controls that received the promoting agent in combination with
dimethyl sulfoxide) (Miyakama et al., 1991). When 2-furaldehyde was
applied in combination with acetone, no skin tumours were seen.
Following the administration of an unstated amount of
2-furaldehyde to rats for 5 months, evidence of pre-neoplastic changes
(the formation of glutathione S-transferase placental form, GST-P,
foci) was seen in livers (Shimizu et al., 1989). Liver cirrhosis was
observed in treated rats; overall, however, it is impossible to reach
any conclusions about the possible mechanisms underlying cancer
induction from this study (e.g., to what extent cell proliferation or
direct mutagenicity may be involved in liver tumour formation).
8.5 Genotoxicity and related end-points
Experiments in cell-free systems have demonstrated that
2-furaldehyde causes DNA damage (Hadi & Rehman, 1989). Although
bacterial mutation studies with 2-furaldehyde have been largely
negative, they have in general been inadequately reported (Zdzienicka
& Tudek, 1978; McMahon et al., 1979; Loquet et al., 1981; Soska et
al., 1981; Marnett et al., 1985; Mortelmans et al., 1986; Shinohara &
Omura, 1986; Kim et al., 1987, 1988; Nakamura et al., 1987; Shane et
al., 1988; Kato et al., 1989). However, 2-furaldehyde is clearly
genotoxic in vitro in mammalian cell test systems, producing
chromosomal aberrations, gene mutations, and sister chromatid
exchanges (Stich et al., 1981; Gomez-Arroyo & Souza, 1985; McGregor et
al., 1988; Nishi et al., 1989; NTP, 1990). In addition, one positive
result and one negative result were obtained in Drosophila tests
(Woodruff et al., 1985; Rodriguez-Arnaiz et al., 1992).
The genotoxic potential of 2-furaldehyde in vivo is less
certain, with positive results being claimed in a briefly reported
cytogenetics study and negative results being reported in another
cytogenetics study and a sister chromatid exchange study (Subramanyam
et al., 1989; NTP, 1990). Further details of these studies are
provided in the source document to this CICAD (Gregg et al., 1997).
Overall, no firm conclusions can be drawn from these reports.
Point mutations in K-ras and H-ras genes were seen in the
2-furaldehyde-treated mouse livers taken from the NTP carcinogenicity
assay, demonstrating genotoxicity (possibly direct) at the tumour site
(Reynolds et al., 1987).
8.6 Reproductive and developmental toxicity
No studies specifically addressing these end-points are
available.
8.7 Immunological and neurological effects
No studies specifically addressing these end-points are
available.
9. EFFECTS ON HUMANS
The only effects reported following single exposure in humans
relate to irritation. Throat and eye irritation was apparently
experienced by a small group of workers exposed to 200 mg/m3 (50 ppm)
for at least 12 min.1 Apparently no irritation was noted with
40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h.
An unknown volume of 50% liquid 2-furaldehyde did not produce
skin irritation in workers (Nazyrov & Yampol'skaya, 1969).
Insufficient information is available on the ability of 2-furaldehyde
to act as a skin or respiratory sensitizer in humans.
A limited number of studies have investigated the effects of
repeated occupational exposure to 2-furaldehyde. One study suggested
that 2-furaldehyde led to eye, nose, and throat irritation in
situations where 10-min average 2-furaldehyde concentrations of 12-64
mg/m3 (3-16 ppm) were measured (Apol & Lucas, 1975). However, other
substances, including creosote and liquid resins, may have contributed
to the effects, and no firm conclusions with respect to the role of
2-furaldehyde can be drawn. In another study, no evidence of
respiratory effects in 2-furaldehyde-exposed workers was noted when
the highest average concentration was given as around 8 mg/m3 (2 ppm)
(Pawlowicz et al., 1984). However, no duration for this level of
exposure and no indication of peak concentrations were presented. No
conclusions can be drawn from other studies because of poor reporting
of exposure to 2-furaldehyde or because of the potential for
confounding factors to influence the findings.
No information was available on carcinogenicity in humans. The
only genotoxicity information available in humans comes from an
inadequate study that did not demonstrate any significant increase in
the incidence of sister chromatid exchanges in a group of only six
employees potentially exposed to unknown concentrations of
2-furaldehyde (Gomez-Arroyo & Souza, 1985).
No information is available on reproductive toxicity in humans.
1 QO Chemicals Inc. (1981) Summary of sensory response
criteria, furfural in air concentrations, Table 3.
Unpublished data.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic organisms
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
10.2 Terrestrial organisms
An estimated oral LD50 value of >98 mg/kg body weight has been
reported for the red-winged blackbird ( Agelaius phoeniceus)
(Schafer et al., 1983).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Acute toxicity data from animals are variable; overall, however,
2-furaldehyde is toxic by the inhalation and oral routes (4-h LC50,
940 mg/m3 [235 ppm]; oral LD50, about 120 mg/kg body weight), with
no clear information in relation to the dermal route.
Human data on effects of 2-furaldehyde are extremely limited and
of poor quality, although mucous membrane irritation has been
identified as the principal effect following exposure to
concentrations apparently as low as 12 mg/m3 (3 ppm) (10-min
average). However, peak exposure concentrations were not given, and
co-exposure with other substances was possible. In another study,
exposure to 40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h
apparently did not cause throat or eye irritancy.
From repeated-inhalation exposure studies in animals, the main
non-neoplastic effects are toxicity to the lung and respiratory tract.
NOAELs of 80 and 208 mg/m3 (20 and 52 ppm) have been identified in
hamsters and rabbits, respectively, from studies of up to 4-6 h/day, 5
days/week, for 13 weeks. Chronic oral exposure to 2-furaldehyde
produced increased incidences of bile duct carcinomas in male rats,
malignant and benign tumours in the liver in male mice, and benign
tumours in the liver and forestomach in female mice. The development
of the liver and forestomach tumours may be related to the chronic
inflammatory effects noted at the same organ sites.
The results of mutagenicity tests with bacteria were largely
negative, although 2-furaldehyde is clearly genotoxic in vitro in
mammalian cells, producing chromosomal aberrations, gene mutations,
and sister chromatid exchanges. In vivo genotoxicity has not been
adequately studied, and no firm conclusions can be drawn.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
11.1.2 Criteria for setting guidance values for 2-furaldehyde
2-Furaldehyde vapour and liquid are readily absorbed through the
skin, and experiments suggest that skin absorption of vapour may
account for a significant proportion of the total body burden. In view
of the potential for skin absorption, biological monitoring is an
important aspect of occupational exposure assessment. This is achieved
by measuring 2-furoic acid (after alkaline hydrolysis of the
metabolite furoylglycine) in urine.
Table 2: Acute toxicity of 2-furaldehyde to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold (16-h EC3, growth) 16 Bringmann & Kuhn (1976)
Microcystis aeruginosa Toxic threshold (8-day EC5, growth) 2.7 Bringmann & Kuhn (1976)
Yeast
Saccharomyces cerevisiae EC14, growth 1 Banerjee et al. (1981)
Algae
Scenedesmus quadricaudata Toxic threshold (7-day EC3, growth) 31 Bringmann & Kuhn (1980a)
Protozoa
Entosiphon sulcatum Toxic threshold (72-h EC5, growth) 0.6 Bringmann & Kuhn (1980a)
Uronema parduczi Toxic threshold (growth) 11 Bringmann & Kuhn (1980b)
Chilomonas paramaecium Toxic threshold (48-h EC5, growth) 3.9 Bringmann & Kuhn (1980c)
Fish
Mosquito fish 96-h LC50 2410 Wallen et al. (1957)
Gambusia affinis NOEC
Bluegill sunfish 96-h LC50 16 Turnbull et al. (1954)
Lepomis macrochirus
Fathead minnow 96-h LC50 32 Mattson et al. (1976)
Pimephales promelas
a ECn is the effective concentration inhibiting the stated end-point (growth) by n%.
The relative importance of genotoxicity and chronic inflammation
in tumorigenesis is uncertain; because of this uncertainty, it is not
possible to reliably identify a threshold below which exposure to
2-furaldehyde would not result in some risk to human health.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to 2-furaldehyde from the general
environment precludes the characterization of potential cancer risks
for the general population.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are a number of
models for characterizing potency, which may be of some benefit in
priority-setting schemes.
The scenario chosen here as an example is occupational exposure
in the United Kingdom. With respect to the irritant effects, in
general, in the industries where 2-furaldehyde is used, the measured
and predicted (using EASE modelling) exposure concentrations indicate
that there is little risk that irritation to the respiratory tract or
eyes will occur. However, in the manufacture of refractories and
abrasive wheels, current exposures in some parts of these industries
give rise to concern that there would be the risk of developing
irritation. Given the eye irritation potential of 2-furaldehyde, there
is a risk of developing eye irritation if appropriate personal
protective equipment is not employed to prevent eye contact with the
liquid.
With respect to the risk of genotoxicity and carcinogenicity, the
picture is unclear. Although the measured and predicted levels of
exposure to 2-furaldehyde generally are relatively low in these
industries, the risk of developing genetic damage at sites of initial
contact cannot be discounted, and thus there is cause for concern that
this may occur. The relative importance of genotoxicity and chronic
inflammation in tumorigenesis is uncertain, and a NOAEL cannot be
reliably identified because of this uncertainty. Consequently, it is
concluded that because of this toxicological picture, it is not
possible to identify a level of exposure at which one could be certain
that genetic damage leading to tumour formation would not occur; thus,
under contemporary occupational exposure conditions, there is cause
for concern for genotoxicity and carcinogenicity, although it is not
possible to reliably quantify this.
In view of the irritative effects reported in workers
occupationally exposed to low exposure concentrations of
2-furaldehyde, a short-term limit is likely to be appropriate in
addition to the 8-h TWA.
11.2 Evaluation of environmental effects
Although some emission to the atmosphere is expected from wood
burning, no atmospheric effects are expected given the short half-life
for reaction with hydroxyl and other radicals and possible
photodegradation of 2-furaldehyde. The low volatilization of the
compound from water and soil would not be expected to add
significantly to atmospheric levels.
The majority of 2-furaldehyde released to the environment will be
released to surface waters. Release from the wood pulp industry seems
to be the major source.
2-Furaldehyde has a low capacity for bioaccumulation. Binding to
particulates in soil and aquatic sediment is expected to be very low,
making 2-furaldehyde mobile in the environment.
2-Furaldehyde is readily biodegraded in aerobic sewage sludge and
has also been shown to degrade in anaerobic systems. Acclimation of
the sludge improves degradation. The compound is toxic to anaerobic
degrading bacteria at concentrations above 1000 mg/litre in
unacclimated sludges (these concentrations have been reported in wood
pulp waste), although acclimation allows full degradation at these
concentrations.
LC50s in fish range from 16 to 32 mg/litre, with a reported NOEC
in an acute test of 10 mg/litre. There are no test results for aquatic
invertebrates. Toxic thresholds (EC3 to EC5 for cell multiplication)
for bacteria, cyanobacteria, algae, and protozoa range from 0.6 to 31
mg/litre.
Only one estimated toxicity value is available for terrestrial
organisms, and little emission to land is expected; on this basis, no
quantitative risk assessment can be attempted for the terrestrial
environment.
11.2.1 Predicted environmental concentration
Very limited monitoring studies are available for surface waters,
with only two isolated measurements at 2 µg/litre reported. The sample
risk assessment will be based on emissions from the wood pulp
industry, the expected worst case. Reported concentrations in sulfite
evaporator condensate range up to 1280 mg/litre, with average
concentrations at 274 mg/litre in a more recent study. Since the
evaporator condensate represents 15% of the total wastewater flow, the
concentration in wastewater would be 41.1 mg/litre average.
Based on the average concentration and mainly default values from
the OECD Technical Guidance Manual, the initial concentration in
rivers receiving treated wastewater would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre),
calculated as Ceffluent = I × (100 - P)/100,
where:
I = input concentration to the wastewater treatment plant
(0.041 g/litre)
P = percent removal in the wastewater treatment plant (91%,
based on the "ready biodegradability" of the compound)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = foc(susp) × Koc, where:
foc(susp) = the fraction of organic carbon in suspended matter
(default 0.1)
Koc = the organic carbon/water partition coefficient
(1.05; see section 5)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (taken as 1000 minimum,
as the wood pulp process requires large water input and as plants
will normally be sited on moderate to large rivers)
Under these conservative conditions, PEClocal(water) = 3.7 µg/litre.
11.2.2 Predicted no-effect concentration
As no test results are available for aquatic invertebrates and no
long-term test results are available, an uncertainty factor of 1000
will be applied to the lowest reported acute LC50 of 16 mg/litre for
the bluegill sunfish ( Lepomis macrochirus) to give a predicted
no-effect concentration (PNEC) of 16 µg/litre. It is not considered
justifiable to base the PNEC on toxic threshold values.
11.2.3 Environmental risk factors
The risk factor (PEC/PNEC ratio) for aquatic organisms is,
therefore, 0.23, indicating low risk. The distribution of reported
toxicity test results against the worst-case PEC is plotted in Figure
1, illustrating the safety margin.
The conversion factors for 2-furaldehyde in air at 20°C and 101.3
kPa are as follows:
1 ppm = 4.0 mg/m3
1 mg/m3 = 0.25 ppm
3. ANALYTICAL METHODS
Long- and short-term personal monitoring can be undertaken by
pumped sampling through XAD-2 resin impregnated with 2-(hydroxymethyl)
piperazine, desorbing with solvent (toluene), and analysing with gas
chromatography (NIOSH, 1987). The working range is 1.2-22 mg/m3
(0.3-5.5 ppm) for a 12-litre sample. Alternatively, a thermal
desorption tube may be used, in either the pumped or diffusive mode
(Patel et al., 1988). The working range for both is 4-40 mg/m3 (1-10
ppm) for a 10-litre sample. Screening measurements can be made with
colorimetric detector tubes, but these are unselective and not very
sensitive.
Biological monitoring of workers exposed to 2-furaldehyde is
possible by the analysis of 2-furoic acid (after alkaline hydrolysis
of the metabolite furoylglycine) in urine (Sedivec & Flek, 1978).
Although people not occupationally exposed to 2-furaldehyde have
background levels of 2-furoic acid in hydrolysed urine samples (from
dietary sources), these are low compared with those resulting from
occupational exposure (Nutley, 1989). The biological exposure index of
the American Conference of Government Industrial Hygienists is 200 mg
2-furoic acid/g creatinine (200 µmol/mmol creatinine), and a study in
the United Kingdom found that a 2-furoic acid concentration of over
160 mg/g creatinine (160 µmol/mmol creatinine) was likely to be due to
exposure to 2-furaldehyde at over 8 mg/m3 (2 ppm) (Nutley, 1989).
More sensitive analytical methods are required for monitoring in
food. Determination of the 2,4-dinitrophenylhydrazone derivative of
2-furaldehyde by high-performance liquid chromatography gives high
specificity, with a detection limit of 10 nmol/litre in spirits (Lo
Coco et al., 1992). A fast, semi-automatic, stopped-flow injection
analysis in which pretreatment of diversely coloured and turbid food
samples is not necessary has been developed (Espinosa-Mansilla et al.,
1993).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
2-Furaldehyde is found in a number of dietary sources. Because of
its formation during the thermal decomposition of carbohydrates,
2-furaldehyde is found in numerous processed foods and beverages,
including cocoa, coffee, tea, beer, wine, milk products, and bread
(Maga, 1979). It is also found in some fruits and vegetables, and it
is added as a flavouring agent to some foods. Industry in the United
Kingdom is voluntarily withdrawing 2-furaldehyde from addition to
food, although the chemical will still be present in food as a result
of its production during the thermal processing of anything containing
sugars. 2-Furaldehyde has also been found in the essential oils of
camphor, citronella, sassafras, lavender, and lime (Dunlop & Peters,
1953).
2-Furaldehyde is produced commercially in batch or continuous
digesters where pentosans from agricultural residues, including corn
cobs, oat hulls, rice hulls, and bagasse, are hydrolysed to pentoses
and the pentoses are subsequently cyclodehydrated to 2-furaldehyde.
2-Furaldehyde is shipped in steel tank cars, aluminium tank trucks,
and steel cans. When stored in bulk, the storage tanks normally have a
nitrogen blanket to prevent ingress of oxygen, which can cause
chemical degradation of the material.
Estimates of worldwide production are not available. US
production in 1983 was about 52 000 t (HSDB, 1998). The estimated
range of US production was 14 000-60 000 t in 1986, 2000-7000 t in
1990, and 11 000-45 000 t in 1994 (US EPA, 1998). Although
2-furaldehyde is not manufactured in the United Kingdom, it is
estimated that industry in the United Kingdom used approximately 3000
t in 1992. The official statistics indicate that imports into the
United Kingdom for the previous four years were 2800 t (1988), 3500 t
(1989), 3000 t (1990), and 3500 t (1991) (Gregg et al., 1997).
Industrial uses of 2-furaldehyde are summarized in Table 1. About
40% of 2-furaldehyde imported into the United Kingdom is used in the
production of resins, abrasive wheels, and refractories. The rest is
used in the refining of lubrication oils. Contacts with United Kingdom
industry in 1992 indicated that there was unlikely to be any
significant change in the pattern of use of 2-furaldehyde in the
foreseeable future.
Table 1: Industrial uses of 2-furaldehyde in the United Kingdom.
Industry Uses
1. Petrochemicals As a selective solvent in
the production of lubricating oils
2. Refractories As a reactive wetting agent in the
production of refractory components
3. Resin manufacture (a) Production of phenolic resins
(b) Production of cashew nutshell polymers
4. Abrasive materials As a reactive wetting agent for the resin
binder system in the production of abrasive wheels
5. Solvent recovery Steam distillation of spent 2-furaldehyde
for reuse by industry sectors 1, 2, and 4
6. Flavouring Used in very small quantities in
synthetic/natural oil blends
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The highest reported emissions of 2-furaldehyde to the
environment are from the wood pulp industry, with release to the
hydrosphere (section 6); release to water from other uses appears to
be substantially lower. The compound would be released to the
atmosphere from wood fires, a natural as well as anthropogenic source.
In the atmosphere, 2-furaldehyde will exist predominantly in the
vapour phase; the half-life for destruction by interaction with
hydroxyl radicals has been calculated at 0.44 days (HSDB, 1998).
Night-time destruction in the urban atmosphere may involve reaction
with nitrate radicals (Carter et al., 1981). Direct photo-oxidation
may occur in the atmosphere, based on experimental demonstration that
concentrations of 2-furaldehyde in wood smoke were reduced by
irradiation at sunlight and ultraviolet frequencies. Addition of
nitrogen oxides to wood smoke increased the rate of disappearance of
the compound (Kleindienst et al., 1986).
Virtually no degradation occurred in a solution of 2-furaldehyde
in distilled water over 30 days, suggesting that hydrolysis is not an
important process at environmental pH (Ettinger et al., 1954).
A Henry's law constant of 0.37 Pa.m3/mol at 25°C has been
calculated (HSDB, 1998), corresponding to a dimensionless Henry's law
constant (air/water partition coefficient) of 1.5 × 10-4. These
values indicate slow volatilization from water and damp soil surfaces;
an estimated half-life for volatilization from a model river of 9.9
days has been reported (HSDB, 1998).
2-Furaldehyde has a reported log Kow of 0.41, indicating low
capacity for bioaccumulation; calculated bioconcentration factors were
less than 1.2 (HSDB, 1998). The sorption coefficient ( Koc) can be
calculated as between 1.05 (Organisation for Economic Co-operation and
Development [OECD] Technical Guidance Manual) and 1.62 (Karickhoff et
al., 1979), indicating little sorption to particulates and a high
capacity for mobility in soil.
2-Furaldehyde was readily biodegraded in an aerobic batch culture
at 200 mg chemical oxygen demand (COD)/litre, with non-adapted sewage
sludge showing 96.3% degradation within 120 h and a degradation rate
of 37 mg COD/g per hour (Pitter, 1976). In a flow-through aerobic
laboratory bioreactor at an initial concentration of 300 mg/litre, 98%
degradation of 2-furaldehyde was reported using acclimated sewage
sludge. Degradation also occurred at a concentration of 1000 mg/litre
(Rowe & Tullos, 1980). 2-Furaldehyde was readily biodegradable in the
Japanese Ministry of International Trade and Industry (MITI) test
(Kawasaki, 1980). Wang et al. (1994) screened a range of
microorganisms for their ability to degrade 2-furaldehyde and reviewed
the earlier literature; some strains of the aerobic bacteria
Escherichia coli, Pseudomonas putida, and Rhodococcus
erythropolis and the yeast Hyphozyma rosoeniger were able to
degrade the compound to a greater or lesser degree. Inhibitory effects
of 2-furaldehyde on Pseudomonas putida were seen at concentrations
of 0.1% and above, with complete inhibition at 1% with exposure for 30
min (Kim et al., 1983).
2-Furaldehyde at 1 mg/litre was degraded completely in water from
the Great Miami, Little Miami, and Ohio rivers (USA) within 3 days
under aerobic conditions (Ettinger et al., 1954).
Under anaerobic conditions, 2-furaldehyde was completely degraded
to methane by unacclimated sewage sludge within 30 days at an initial
concentration of 580 mg/litre. The rate of methane production was
initially slower than in controls, indicating some interference of the
compound in the metabolism of the methanogenic bacteria. At an initial
concentration of 1160 mg/litre, gas production ceased after 5 days,
and none of the gas produced was methane; gas production did not
resume within the 28 days of the test, indicating that this
concentration was toxic to unacclimated microorganisms. Using
acclimated sludge (which had received 2-furaldehyde at 310 mg/litre
continuously for 8 months), full degradation occurred at 1160
mg/litre. At 2320 mg/litre, 2-furaldehyde was initially degraded
rapidly, but then gas production slowed for the remainder of the test
(Benjamin et al., 1984). A strictly anaerobic bacterium isolated from
a fermentor degrading sulfite evaporator condensate from wood pulp
production was able to use 2-furaldehyde as sole carbon source; the
organism was tentatively identified as Desulfovibrio sp. (Brune et
al., 1983). Methanococcus deltae was found to be able to use
2-furaldehyde as sole carbon source, degrading the compound to
furfuryl alcohol at initial concentrations of 5 and 10 mmol/litre (480
and 960 mg/litre); however, growth of the bacterium was inhibited at
concentrations of 20 and 25 mmol/litre (1920 and 2400 mg/litre). Other
methanogenic bacteria ( Methanobacterium thermoautotrophicum,
Methanosarcina barkeri, and Methanococcus thermolithotrophicus)
were unable to degrade the compound (Belay et al., 1997).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
2-Furaldehyde was detected in 1 out of 204 surface water samples
from heavily industrialized areas in the USA at 2 µg/litre (detection
limit 1 µg/litre) and in 1 out of 13 samples from the Lake Michigan
basin, USA, at 2 µg/litre in the late 1970s (HSDB, 1998).
2-Furaldehyde was not detected (detection limit 0.4 ng/litre) in
33 samples of surface waters in the 1996 monitoring of the general
environment in Japan (Japan Environment Agency, 1998). However, the
compound was detected in 6 out of 15 air samples (detection limit 40
ng/m3) in two cities (range 42-120 ng/m3). The analytical method was
not stated in the publication.
Levels of 2-furaldehyde in sulfite evaporator condensate, which
represents about 15% of the wastewater flow from pulp mills, have been
reported to range between 10 and 1280 mg/litre (Ruus, 1964) and
between 179 and 471 mg/litre (average 274 mg/litre) (Benjamin et al.,
1984). The 2-furaldehyde derives from pentoses in the wood pulp and is
formed during the waste treatment in the evaporator. 2-Furaldehyde was
detected in the wastewater of a synthetic rubber plant at 1.7 µg/litre
(Keith, 1974).
2-Furaldehyde was detected but not quantified in the air above
the Black Forest, Germany, in 1984-85 (Juttner, 1986). Although
2-furaldehyde has been identified in vehicle exhaust, it was not
detectable in air sampled from a road tunnel in the USA (Hampton et
al., 1982). The compound has been identified in smoke from burning
wood (Lipari et al., 1984; Kleindienst et al., 1986). Mean
concentrations of 2-furaldehyde measured in indoor and outdoor air in
suburban New Jersey, USA, in summer 1992 were 1.06 µg/m3 (0.27 ppb)
and 0.67 µg/m3 (0.17 ppb), respectively. The presence of the compound
in indoor air was presumed to be from emissions during cooking (Zhang
et al., 1994).
6.2 Human exposure
6.2.1 Oral exposure
2-Furaldehyde has been identified but not quantified as a major
flavour component in a range of food items, including beef, soy sauce,
roasted nuts, fried bacon, nectarines, baked potatoes, clove oil,
preserved mangoes, rum, roasted coffee, and blue cheese (HSDB, 1998).
Levels reported in food are as follows: non-alcoholic beverages, 4
mg/litre; alcoholic beverages, 10 mg/litre; ice creams, ices, etc., 13
mg/kg; candy, 12 mg/kg; baked goods (unspecified), 17 mg/kg; gelatins
and puddings, 0.8 mg/kg; chewing gum, 45 mg/kg; and syrups, 30
mg/litre (HSDB, 1998). 2-Furaldehyde was qualitatively identified in
two out of eight samples of mothers' milk from urban sites in the USA
(Pellizzari et al., 1982). Qualitative identification of 2-furaldehyde
in drinking-water has been reported from both the USA and Europe; no
quantification was reported (Kool et al., 1982).
6.2.2 Inhalation exposure
The remainder of the human exposure data available to the authors
of this CICAD are restricted to the occupational environment.
Measured inhalation exposure information was provided to the
United Kingdom's Health and Safety Executive by the petrochemical
industry. The Health and Safety Executive's National Exposure Data
Base contains information on exposure for the use of 2-furaldehyde in
the refractory industry and for a number of miscellaneous processes.
In all the exposure groupings below, when routine and breakdown
maintenance takes place and significant exposure to airborne
2-furaldehyde is anticipated, it is expected that appropriate
respiratory protective equipment would be used to minimize inhalation
exposure.
Exposures in the petrochemicals, resin and polymer manufacture,
and distillation industries are likely to be very similar. EASE
(Version 2) predictions seem to be in accord with the limited amount
of measured exposure information provided by the petrochemicals
industry (Gregg et al., 1997). The EASE predictions indicate that 8-h
TWA exposures will be somewhat below 8 mg/m3 (2 ppm). There is
insufficient information to predict 15-min TWA exposures for these
industries.
Exposures in the refractories and abrasive wheels manufacturing
industries are likely to be very similar. The high exposures measured
by the Health and Safety Executive in the refractories industry (20%
of 8-h TWA exposures are greater than 40 mg/m3 [10 ppm]) are likely
to be reduced following advice given about the application of
efficient local exhaust ventilation and the enclosure of the process
where possible (Gregg et al., 1997). The EASE-predicted 8-h TWA
exposures likely to result from these improvements will be between 2
and 12 mg/m3 (0.5 and 3 ppm) (Gregg et al., 1997). There is every
likelihood that exposures will actually be at the lower end of the
range -- i.e., less than 8 mg/m3 (2 ppm). There is insufficient
information to predict 15-min TWA exposures for these industries.
In the flavouring industry, very little 2-furaldehyde appears to
be used. The EASE model predicts exposures to be low: the 15-min TWA
exposure is between 1.2 and 6.8 mg/m3 (0.3 and 1.7 ppm), and the 8-h
TWA is between 0.04 and 0.2 mg/m3 (0.01 and 0.05 ppm) (Gregg et al.,
1997).
For oilseed residue application, no personal exposure was
detected. The extent of this industry is not known, but it seems that
8-h exposures to 2-furaldehyde are likely to be well below 8 mg/m3
(2 ppm) (Gregg et al., 1997). No comment can be made upon likely
short-term exposures.
Little is known about the extent of the use of 2-furaldehyde in
the glass reinforced plastics industry. It is very likely that the
application of appropriate controls would result in 8-h TWA exposures
to 2-furaldehyde being reduced to below 8 mg/m3 (2 ppm) (Gregg et
al., 1997). No comment can be made upon possible short-term exposures.
6.2.3 Dermal exposure
The industrial processes can be grouped in a similar manner to
that adopted for the general discussion of inhalation exposure above
(section 6.2.2). These predictions do not take account of personal
protective equipment that may be worn. Such equipment may
significantly reduce dermal exposure.
The EASE-predicted dermal exposures for the petrochemicals, resin
and polymer manufacture, and distillation industries are similar
-- namely, between 0.1 and 1 mg/cm2 per day (Gregg et al., 1997).
For the refractories and abrasive wheels manufacturing
industries, the EASE-predicted dermal exposures are between 1 and 5
mg/cm2 per day (Gregg et al., 1997). If improvements are made to the
process containment, opportunities for dermal contact will be reduced,
and predicted exposures will be reduced to between 0.1 and 1 mg/cm2
per day.
For the use of 2-furaldehyde in the flavourings industry, the
EASE prediction for dermal exposure is between 0 and 0.1 mg/cm2 per
day (Gregg et al., 1997). Insufficient information is provided for the
other minor uses to make predictions of dermal exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
No animal studies are available examining the toxicokinetics of
2-furaldehyde by the inhalation or dermal routes; however, signs of
systemic toxicity observed in animal studies (see section 8) indicate
that 2-furaldehyde is readily absorbed via both these routes of
exposure.
Animal studies demonstrate that, following oral administration in
rats, 2-furaldehyde is readily absorbed and rapidly excreted, with up
to 85% of the administered dose being detected in the urine within 24
h (Nomier et al., 1992). Some elimination via exhaled carbon dioxide
(7% of dose) also occurs. Metabolism is characterized by oxidation or
acetylation of the aldehyde group, followed by glycine conjugation.
2-Furoylglycine is the major urinary metabolite, accounting for
approximately 80% of the administered dose (Laham & Potvin, 1989;
Nomier et al., 1992; Parkash & Caldwell, 1994). Other minor
metabolites include furoic acid, furanacrylic acid, and furanacryluric
acid.
In humans, absorption of the vapour via both the lungs and skin
has been demonstrated (Flek & Sedivec, 1978a,b). Metabolism appears
similar to that in rats, with the majority of the retained dose being
excreted as urinary 2-furoylglycine. Furoic acid and furanacryluric
acid are also detected as minor metabolites. Dermal absorption from
liquid 2-furaldehyde has also been observed.
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
Unpublished or briefly reported papers indicate 1-, 4-, and 6-h
inhalation LC50 values in rats of 4148, 940, and 700 mg/m3 (1037,
235, and 175 ppm), respectively (Woods & Seevers, 1956; Terrill et
al., 1989). In contrast, an apparently well-conducted published study
indicated a 1-h LC50 value in rats of 756 mg/m3 (189 ppm) (Gupta et
al., 1991; Mishra et al., 1991). In the rat and other species,
respiratory tract irritation and lung damage were consistently
observed following inhalation exposure, with lung oedema and
congestion being reported in one study in rats immediately after
exposure to 380 mg/m3 (95 ppm) for 1 h. Oral LD50 values in rats
were in close agreement, ranging from 122 to 158 mg/kg body weight
(Woods & Seevers, 1955; SRI International, 1982). Toxic signs observed
indicated central nervous system depressant effects, with convulsions
also observed. The lungs also appeared to be a target organ via oral
dosing. No reliable information was available for the dermal route of
exposure.
8.2 Irritation and sensitization
No signs of skin irritation were noted in rabbits following a
single application of liquid 2-furaldehyde for 12 h, although mild
irritation was observed following a 48-h application (Woods & Seevers,
1955). Intense but reversible skin irritation was reported in
guinea-pigs after repeated application of undiluted liquid
2-furaldehyde (Agakishiyev, 1989, 1990).
A single instillation of liquid 2-furaldehyde produced gross
corneal opacities in rabbits (Woods & Seevers, 1955). 2-Furaldehyde
vapour produced eye irritation in several studies following repeated
exposure in different species (Gardner, 1925; Feron et al., 1979;
Gupta et al., 1991).
No information is available on the ability of 2-furaldehyde to
act as a skin or respiratory sensitizer.
8.3 Short-term exposure
The principal effects of repeated inhalation exposure were
similar to those seen in single-exposure experiments. Groups of rats
were exposed to 0 or 160 mg 2-furaldehyde/m3 (0 or 40 ppm) 1 h/day
for 5, 15, or 30 days (Gupta et al., 1991; Mishra et al., 1991), and
groups of rabbits were exposed to 208, 520, or 1040 mg/m3 (52, 130,
or 260 ppm) 4 h/day, 5 days/week, for at least 60 exposures
(Castellino et al., 1963). Respiratory irritation, hyperplasia and
degeneration of the olfactory epithelium, and lung congestion, oedema,
and inflammation were observed in rats following exposure to 160
mg/m3 (40 ppm) (Gupta et al., 1991; Mishra et al., 1991) and in
rabbits following exposure to 1040 mg/m3 (260 ppm) (Castellino et
al., 1963). There was also evidence of kidney damage seen
histopathologically and anaemia in rabbits following exposure to 520
mg 2-furaldehyde/m3 (130 ppm). A NOAEL of 208 mg/m3 (52 ppm) was
identified in rabbits; a NOAEL was not identified for rats.
8.4 Long-term exposure
8.4.1 Subchronic exposuren
Groups of hamsters were exposed to 0, 80, 460, or 2208 mg/m3
(0, 20, 115, or 552 ppm) 6 h/day, 5 days/week, for 13 weeks (Feron et
al., 1979). Respiratory irritation, hyperplasia and degeneration of
the olfactory epithelium, and lung congestion, oedema, and
inflammation were observed in hamsters following exposure to 2208
mg/m3 (552 ppm) (Feron et al., 1979). Slight atrophy and hyperplasia
of the olfactory epithelium were also seen following exposure to 460
mg/m3 (115 ppm). A NOAEL of 80 mg/m3 (20 ppm) was identified.
Groups of 20 male and female rats received 0, 11, 22, 45, 90, or
180 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Mild to moderate centrilobular
vacuolation of hepatocytes was observed among all treated groups,
although there was no clear relationship between dose and incidence or
severity of the observed effect.
Groups of 20 male and 20 female mice received 0, 75, 150, 300,
600, or 1200 mg 2-furaldehyde/kg body weight per day by oral gavage,
5 days/week for 13 weeks (NTP, 1990). Relative liver weights were
increased in a dose-related manner in all treated groups.
Centrilobular coagulative necrosis of hepatocytes was seen at
150 mg/kg body weight per day or more, and mild mononuclear
inflammatory cell infiltrate was seen in all treated groups.
8.4.2 Chronic exposure and carcinogenicity
No inhalation carcinogenicity studies in rats or mice were
available. An inhalation study in hamsters showed no evidence that
2-furaldehyde vapour had carcinogenic potential following 52 weeks'
exposure to 1000-1600 mg/m3 (250-400 ppm), 7 h/day, 5 days/week, by
this route (Feron & Kruysse, 1978). Nor was there any indication that
2-furaldehyde was carcinogenic to hamsters after 36 weekly
intratracheal instillations (Feron, 1972). However, both these studies
are not satisfactory negative studies, owing to their limited
duration.
In a gavage study, groups of 50 male and 50 female F344/N rats
were treated with 0, 30, or 60 mg 2-furaldehyde/kg body weight per
day, 5 days/week for 103 weeks (NTP, 1990). Animals were observed
twice daily, weighed monthly, and subjected to a thorough gross and
microscopic examination either at death or at the end of the study.
Mortality in males was not significantly affected by 2-furaldehyde
treatment throughout the study. Corresponding survival rates among
females were 18/50, 32/50, and 28/50. However, 19 of the 22 top-dose
deaths in females were attributed to accidental gavage errors. There
were no differences in body weight between test and control animals
throughout the study, and there were no treatment-related clinical
signs of toxicity. The main non-neoplastic changes observed in test
animals were minimal to mild centrilobular necrosis of the liver in
male rats (3/50, 9/50, 12/50) and biliary dysplasia with fibrosis in
two top-dose males.
Two cases of cholangiocarcinoma were seen in two other top-dose
males. The historical incidence of bile duct neoplasms in corn oil
vehicle control male F344/N rats in this laboratory was reported to be
3/2145 (0.1%). No other findings of biological significance were
observed. Hence, in this study, treatment by gavage with 2-furaldehyde
produced an increase in bile duct carcinoma in male rats only,
although a valid assessment of the carcinogenicity of 2-furaldehyde in
female rats cannot be made because of the low numbers of animals
surviving to the end of the study. Also, the high incidence of
accidental deaths casts doubt over the quality of the experimental
conditions.
Groups of 50 male and 50 female B6C3F1 mice were also dosed with
0, 50, 100, or 175 mg 2-furaldehyde/kg body weight per day by gavage,
5 days/week for 103 weeks, in the NTP study (NTP, 1990). Animals were
observed twice daily, weighed monthly, and subjected to a thorough
gross and microscopic examination either at death or at the end of the
study. No significant differences in survival were observed between
2-furaldehyde-treated and untreated animals throughout the study.
There were no differences in body weight gain between test and control
animals throughout the study, and there were no clinical signs of
treatment-related toxicity.
Non-neoplastic changes included chronic inflammation and
multifocal green-brown, granular pigmentation of the subserosa of the
liver in mid- and top-dose mice of each sex and forestomach
hyperplasia in 2-furaldehyde-treated female mice only. The principal
neoplastic changes observed were increases in hepatocellular adenomas
and hepatocellular carcinomas in males (adenoma: 9/50 [18%], 13/50
[26%], 11/49 [22%], 19/50 [38%]; carcinoma: 7/50 [14%], 12/50 [24%],
6/49 [12%], 21/50 [42%]; in control, low-, mid-, and top-dose animals,
respectively). Only adenomas were observed in females, the incidences
being dose-related: 1/50 (2%), 3/50 (6%), 5/50 (10%), 8/50 (16%).
Increases in adenomas and/or carcinomas were statistically significant
only in the top-dose animals of both sexes. An increase in squamous
cell papillomas of the forestomach was also seen in top-dose female
mice, although this increase was not statistically significant (1/50
[2%], 0/50 [0%], 1/50 [2%], 6/50 [12%] in control, low-, mid-, and
top-dose animals). No other findings of biological significance were
observed.
In an initiation/promotion study in mice using the dermal route,
an increased incidence of skin tumours was seen when 2-furaldehyde was
applied in combination with the promoting agent (5/20 compared with
1/20 in controls that received the promoting agent in combination with
dimethyl sulfoxide) (Miyakama et al., 1991). When 2-furaldehyde was
applied in combination with acetone, no skin tumours were seen.
Following the administration of an unstated amount of
2-furaldehyde to rats for 5 months, evidence of pre-neoplastic changes
(the formation of glutathione S-transferase placental form, GST-P,
foci) was seen in livers (Shimizu et al., 1989). Liver cirrhosis was
observed in treated rats; overall, however, it is impossible to reach
any conclusions about the possible mechanisms underlying cancer
induction from this study (e.g., to what extent cell proliferation or
direct mutagenicity may be involved in liver tumour formation).
8.5 Genotoxicity and related end-points
Experiments in cell-free systems have demonstrated that
2-furaldehyde causes DNA damage (Hadi & Rehman, 1989). Although
bacterial mutation studies with 2-furaldehyde have been largely
negative, they have in general been inadequately reported (Zdzienicka
& Tudek, 1978; McMahon et al., 1979; Loquet et al., 1981; Soska et
al., 1981; Marnett et al., 1985; Mortelmans et al., 1986; Shinohara &
Omura, 1986; Kim et al., 1987, 1988; Nakamura et al., 1987; Shane et
al., 1988; Kato et al., 1989). However, 2-furaldehyde is clearly
genotoxic in vitro in mammalian cell test systems, producing
chromosomal aberrations, gene mutations, and sister chromatid
exchanges (Stich et al., 1981; Gomez-Arroyo & Souza, 1985; McGregor et
al., 1988; Nishi et al., 1989; NTP, 1990). In addition, one positive
result and one negative result were obtained in Drosophila tests
(Woodruff et al., 1985; Rodriguez-Arnaiz et al., 1992).
The genotoxic potential of 2-furaldehyde in vivo is less
certain, with positive results being claimed in a briefly reported
cytogenetics study and negative results being reported in another
cytogenetics study and a sister chromatid exchange study (Subramanyam
et al., 1989; NTP, 1990). Further details of these studies are
provided in the source document to this CICAD (Gregg et al., 1997).
Overall, no firm conclusions can be drawn from these reports.
Point mutations in K-ras and H-ras genes were seen in the
2-furaldehyde-treated mouse livers taken from the NTP carcinogenicity
assay, demonstrating genotoxicity (possibly direct) at the tumour site
(Reynolds et al., 1987).
8.6 Reproductive and developmental toxicity
No studies specifically addressing these end-points are
available.
8.7 Immunological and neurological effects
No studies specifically addressing these end-points are
available.
9. EFFECTS ON HUMANS
The only effects reported following single exposure in humans
relate to irritation. Throat and eye irritation was apparently
experienced by a small group of workers exposed to 200 mg/m3 (50 ppm)
for at least 12 min.1 Apparently no irritation was noted with
40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h.
An unknown volume of 50% liquid 2-furaldehyde did not produce
skin irritation in workers (Nazyrov & Yampol'skaya, 1969).
Insufficient information is available on the ability of 2-furaldehyde
to act as a skin or respiratory sensitizer in humans.
A limited number of studies have investigated the effects of
repeated occupational exposure to 2-furaldehyde. One study suggested
that 2-furaldehyde led to eye, nose, and throat irritation in
situations where 10-min average 2-furaldehyde concentrations of 12-64
mg/m3 (3-16 ppm) were measured (Apol & Lucas, 1975). However, other
substances, including creosote and liquid resins, may have contributed
to the effects, and no firm conclusions with respect to the role of
2-furaldehyde can be drawn. In another study, no evidence of
respiratory effects in 2-furaldehyde-exposed workers was noted when
the highest average concentration was given as around 8 mg/m3 (2 ppm)
(Pawlowicz et al., 1984). However, no duration for this level of
exposure and no indication of peak concentrations were presented. No
conclusions can be drawn from other studies because of poor reporting
of exposure to 2-furaldehyde or because of the potential for
confounding factors to influence the findings.
No information was available on carcinogenicity in humans. The
only genotoxicity information available in humans comes from an
inadequate study that did not demonstrate any significant increase in
the incidence of sister chromatid exchanges in a group of only six
employees potentially exposed to unknown concentrations of
2-furaldehyde (Gomez-Arroyo & Souza, 1985).
No information is available on reproductive toxicity in humans.
1 QO Chemicals Inc. (1981) Summary of sensory response
criteria, furfural in air concentrations, Table 3.
Unpublished data.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic organisms
Results of acute toxicity tests on aquatic organisms are
summarized in Table 2. All concentrations are nominal.
10.2 Terrestrial organisms
An estimated oral LD50 value of >98 mg/kg body weight has been
reported for the red-winged blackbird ( Agelaius phoeniceus)
(Schafer et al., 1983).
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Acute toxicity data from animals are variable; overall, however,
2-furaldehyde is toxic by the inhalation and oral routes (4-h LC50,
940 mg/m3 [235 ppm]; oral LD50, about 120 mg/kg body weight), with
no clear information in relation to the dermal route.
Human data on effects of 2-furaldehyde are extremely limited and
of poor quality, although mucous membrane irritation has been
identified as the principal effect following exposure to
concentrations apparently as low as 12 mg/m3 (3 ppm) (10-min
average). However, peak exposure concentrations were not given, and
co-exposure with other substances was possible. In another study,
exposure to 40 mg/m3 (10 ppm) for 8 h or 80 mg/m3 (20 ppm) for 4 h
apparently did not cause throat or eye irritancy.
From repeated-inhalation exposure studies in animals, the main
non-neoplastic effects are toxicity to the lung and respiratory tract.
NOAELs of 80 and 208 mg/m3 (20 and 52 ppm) have been identified in
hamsters and rabbits, respectively, from studies of up to 4-6 h/day, 5
days/week, for 13 weeks. Chronic oral exposure to 2-furaldehyde
produced increased incidences of bile duct carcinomas in male rats,
malignant and benign tumours in the liver in male mice, and benign
tumours in the liver and forestomach in female mice. The development
of the liver and forestomach tumours may be related to the chronic
inflammatory effects noted at the same organ sites.
The results of mutagenicity tests with bacteria were largely
negative, although 2-furaldehyde is clearly genotoxic in vitro in
mammalian cells, producing chromosomal aberrations, gene mutations,
and sister chromatid exchanges. In vivo genotoxicity has not been
adequately studied, and no firm conclusions can be drawn.
There are no adequate data available regarding reproductive or
developmental effects; hence, it is not possible to evaluate the risk
to human health for these end-points.
11.1.2 Criteria for setting guidance values for 2-furaldehyde
2-Furaldehyde vapour and liquid are readily absorbed through the
skin, and experiments suggest that skin absorption of vapour may
account for a significant proportion of the total body burden. In view
of the potential for skin absorption, biological monitoring is an
important aspect of occupational exposure assessment. This is achieved
by measuring 2-furoic acid (after alkaline hydrolysis of the
metabolite furoylglycine) in urine.
Table 2: Acute toxicity of 2-furaldehyde to aquatic organisms.
Organism End-point Concentration Reference
(mg/litre)
Bacteria and cyanobacteria
Pseudomonas putida Toxic threshold (16-h EC3, growth) 16 Bringmann & Kuhn (1976)
Microcystis aeruginosa Toxic threshold (8-day EC5, growth) 2.7 Bringmann & Kuhn (1976)
Yeast
Saccharomyces cerevisiae EC14, growth 1 Banerjee et al. (1981)
Algae
Scenedesmus quadricaudata Toxic threshold (7-day EC3, growth) 31 Bringmann & Kuhn (1980a)
Protozoa
Entosiphon sulcatum Toxic threshold (72-h EC5, growth) 0.6 Bringmann & Kuhn (1980a)
Uronema parduczi Toxic threshold (growth) 11 Bringmann & Kuhn (1980b)
Chilomonas paramaecium Toxic threshold (48-h EC5, growth) 3.9 Bringmann & Kuhn (1980c)
Fish
Mosquito fish 96-h LC50 2410 Wallen et al. (1957)
Gambusia affinis NOEC
Bluegill sunfish 96-h LC50 16 Turnbull et al. (1954)
Lepomis macrochirus
Fathead minnow 96-h LC50 32 Mattson et al. (1976)
Pimephales promelas
a ECn is the effective concentration inhibiting the stated end-point (growth) by n%.
The relative importance of genotoxicity and chronic inflammation
in tumorigenesis is uncertain; because of this uncertainty, it is not
possible to reliably identify a threshold below which exposure to
2-furaldehyde would not result in some risk to human health.
The lack of available data to serve as a basis for estimation of
indirect exposure of individuals to 2-furaldehyde from the general
environment precludes the characterization of potential cancer risks
for the general population.
11.1.3 Sample risk characterization
It is recognized that there are a number of different approaches
to assessing the risks to human health posed by genotoxic and
carcinogenic substances. In some jurisdictions, there are a number of
models for characterizing potency, which may be of some benefit in
priority-setting schemes.
The scenario chosen here as an example is occupational exposure
in the United Kingdom. With respect to the irritant effects, in
general, in the industries where 2-furaldehyde is used, the measured
and predicted (using EASE modelling) exposure concentrations indicate
that there is little risk that irritation to the respiratory tract or
eyes will occur. However, in the manufacture of refractories and
abrasive wheels, current exposures in some parts of these industries
give rise to concern that there would be the risk of developing
irritation. Given the eye irritation potential of 2-furaldehyde, there
is a risk of developing eye irritation if appropriate personal
protective equipment is not employed to prevent eye contact with the
liquid.
With respect to the risk of genotoxicity and carcinogenicity, the
picture is unclear. Although the measured and predicted levels of
exposure to 2-furaldehyde generally are relatively low in these
industries, the risk of developing genetic damage at sites of initial
contact cannot be discounted, and thus there is cause for concern that
this may occur. The relative importance of genotoxicity and chronic
inflammation in tumorigenesis is uncertain, and a NOAEL cannot be
reliably identified because of this uncertainty. Consequently, it is
concluded that because of this toxicological picture, it is not
possible to identify a level of exposure at which one could be certain
that genetic damage leading to tumour formation would not occur; thus,
under contemporary occupational exposure conditions, there is cause
for concern for genotoxicity and carcinogenicity, although it is not
possible to reliably quantify this.
In view of the irritative effects reported in workers
occupationally exposed to low exposure concentrations of
2-furaldehyde, a short-term limit is likely to be appropriate in
addition to the 8-h TWA.
11.2 Evaluation of environmental effects
Although some emission to the atmosphere is expected from wood
burning, no atmospheric effects are expected given the short half-life
for reaction with hydroxyl and other radicals and possible
photodegradation of 2-furaldehyde. The low volatilization of the
compound from water and soil would not be expected to add
significantly to atmospheric levels.
The majority of 2-furaldehyde released to the environment will be
released to surface waters. Release from the wood pulp industry seems
to be the major source.
2-Furaldehyde has a low capacity for bioaccumulation. Binding to
particulates in soil and aquatic sediment is expected to be very low,
making 2-furaldehyde mobile in the environment.
2-Furaldehyde is readily biodegraded in aerobic sewage sludge and
has also been shown to degrade in anaerobic systems. Acclimation of
the sludge improves degradation. The compound is toxic to anaerobic
degrading bacteria at concentrations above 1000 mg/litre in
unacclimated sludges (these concentrations have been reported in wood
pulp waste), although acclimation allows full degradation at these
concentrations.
LC50s in fish range from 16 to 32 mg/litre, with a reported NOEC
in an acute test of 10 mg/litre. There are no test results for aquatic
invertebrates. Toxic thresholds (EC3 to EC5 for cell multiplication)
for bacteria, cyanobacteria, algae, and protozoa range from 0.6 to 31
mg/litre.
Only one estimated toxicity value is available for terrestrial
organisms, and little emission to land is expected; on this basis, no
quantitative risk assessment can be attempted for the terrestrial
environment.
11.2.1 Predicted environmental concentration
Very limited monitoring studies are available for surface waters,
with only two isolated measurements at 2 µg/litre reported. The sample
risk assessment will be based on emissions from the wood pulp
industry, the expected worst case. Reported concentrations in sulfite
evaporator condensate range up to 1280 mg/litre, with average
concentrations at 274 mg/litre in a more recent study. Since the
evaporator condensate represents 15% of the total wastewater flow, the
concentration in wastewater would be 41.1 mg/litre average.
Based on the average concentration and mainly default values from
the OECD Technical Guidance Manual, the initial concentration in
rivers receiving treated wastewater would be as follows:
PEClocal (water) = Ceffluent/[(1 + Kp(susp) × C(susp)) × D]
where:
* PEClocal (water) is the predicted environmental concentration (g/litre)
* Ceffluent is the concentration of the chemical in the wastewater
treatment plant effluent (g/litre),
calculated as Ceffluent = I × (100 - P)/100,
where:
I = input concentration to the wastewater treatment plant
(0.041 g/litre)
P = percent removal in the wastewater treatment plant (91%,
based on the "ready biodegradability" of the compound)
* Kp(susp) is the suspended matter/water adsorption coefficient,
calculated as Kp(susp) = foc(susp) × Koc, where:
foc(susp) = the fraction of organic carbon in suspended matter
(default 0.1)
Koc = the organic carbon/water partition coefficient
(1.05; see section 5)
* C(susp) is the concentration of suspended matter in the river
water in kg/litre (default concentration 15 mg/litre)
* D is the dilution factor for river flow (taken as 1000 minimum,
as the wood pulp process requires large water input and as plants
will normally be sited on moderate to large rivers)
Under these conservative conditions, PEClocal(water) = 3.7 µg/litre.
11.2.2 Predicted no-effect concentration
As no test results are available for aquatic invertebrates and no
long-term test results are available, an uncertainty factor of 1000
will be applied to the lowest reported acute LC50 of 16 mg/litre for
the bluegill sunfish ( Lepomis macrochirus) to give a predicted
no-effect concentration (PNEC) of 16 µg/litre. It is not considered
justifiable to base the PNEC on toxic threshold values.
11.2.3 Environmental risk factors
The risk factor (PEC/PNEC ratio) for aquatic organisms is,
therefore, 0.23, indicating low risk. The distribution of reported
toxicity test results against the worst-case PEC is plotted in Figure
1, illustrating the safety margin.
 12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer concluded that
"There is inadequate evidence in humans for the carcinogenicity of
2-furaldehyde" and that "There is limited evidence in experimental
animals for the carcinogenicity of 2-furaldehyde." Its overall
evaluation is that "2-Furaldehyde is not classifiable as to its
carcinogenicity to humans" (IARC, 1995).
2-Furaldehyde has previously been evaluated by the Joint Expert
Committee on Food Additives (JECFA, 1999), whose conclusions are
summarized as follows:
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL [no-observed-effect level] was
identified for hepatotoxicity in rats, the Committee was unable
to allocate an ADI [acceptable daily intake]. Before reviewing
the substance again, the Committee would wish to review the
results of studies of DNA binding in mice and of a 90-day study
of toxicity in rats to identify a NOEL for hepatotoxicity.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0276) reproduced in this
document.
13.1 Health surveillance advice
An exposure surveillance program could include monitoring of
2-furoic acid in urine after alkaline hydrolysis of the main
metabolite of 2-furaldehyde, furoylglycine, immediately following
exposure.
13.2 Advice to physicians
In case of poisoning, treatment is supportive.
13.3 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from mixing with acids, bases, or oxidants
because of the risk of fire or explosion.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals, taken in a certain country, can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
2-Furaldehyde
INTERNATIONAL CHEMICAL SAFETY CARD
FURFURAL ICSC: 0276
November 1998
CAS #
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer concluded that
"There is inadequate evidence in humans for the carcinogenicity of
2-furaldehyde" and that "There is limited evidence in experimental
animals for the carcinogenicity of 2-furaldehyde." Its overall
evaluation is that "2-Furaldehyde is not classifiable as to its
carcinogenicity to humans" (IARC, 1995).
2-Furaldehyde has previously been evaluated by the Joint Expert
Committee on Food Additives (JECFA, 1999), whose conclusions are
summarized as follows:
Because of concern about the tumours observed in male mice given
furfural and the fact that no NOEL [no-observed-effect level] was
identified for hepatotoxicity in rats, the Committee was unable
to allocate an ADI [acceptable daily intake]. Before reviewing
the substance again, the Committee would wish to review the
results of studies of DNA binding in mice and of a 90-day study
of toxicity in rats to identify a NOEL for hepatotoxicity.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventive and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0276) reproduced in this
document.
13.1 Health surveillance advice
An exposure surveillance program could include monitoring of
2-furoic acid in urine after alkaline hydrolysis of the main
metabolite of 2-furaldehyde, furoylglycine, immediately following
exposure.
13.2 Advice to physicians
In case of poisoning, treatment is supportive.
13.3 Spillage
In the event of spillage, measures should be undertaken to
prevent this chemical from mixing with acids, bases, or oxidants
because of the risk of fire or explosion.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals, taken in a certain country, can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
2-Furaldehyde
INTERNATIONAL CHEMICAL SAFETY CARD
FURFURAL ICSC: 0276
November 1998
CAS # 